| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
TORNIER001-35065
| The Netherlands | 98-0509600 | |
incorporation or organization) | (I.R.S. Employer Identification No.) | |
Prins Bernhardplein 200 1097 JB Amsterdam, The Netherlands | None (Zip code) | |
| (Address of | ||
Title of each class | Name of each exchange on which registered | |
| Ordinary shares, par value €0.03 per share | ||
| Contingent Value Rights | NASDAQ Stock Market LLC | |
(Check (Check one):
Large accelerated filerþ | Accelerated filer o | Non-accelerated filero | Smaller reporting company o | |||||
| (Do not check if a smaller reporting company) | ||||||||
None
Management’s Discussion and Analysis of Financial Condition and Results of Quantitative and Qualitative Disclosures About Market Changes in and Disagreements Directors, Executive Officers and Corporate Certain Relationships and Related Transactions, and Director corporate structure not liable for the underlying claims of the one particular entity, despite our deriving a significant portion of our revenues from operations in certain geographic markets that are subject to political, economic, and social instability, including in particular France, and risks and uncertainties involved in launching our products in certain new geographic fluctuations in foreign currency exchange rates; not successfully developing and marketing new products and technologies and implementing our business strategy; not successfully competing against our existing or potential competitors and the effect of significant recent consolidations amongst our competitors; the reliance of our business plan on certain market assumptions; our private label manufacturers failing to provide us with sufficient supply of their products, or failing to meet appropriate quality requirements; our inability to timely manufacture products or instrument sets to meet demand; our plans to increase our gross margins by taking certain actions designed to do so; the loss of key suppliers, which may result in our inability to meet customer orders for our products in a timely manner or within our budget; the incurrence of significant expenditures of resources to maintain relatively high levels of inventory, which could reduce our cash flows and increase the risk of inventory obsolescence, which could harm our operating results; consolidation in the healthcare industry that could lead to demands for price concessions or the exclusion of some suppliers from certain of our markets, which could have an adverse effect on our business, financial condition, or operating results; our clinical trials and their results and our reliance on third parties to conduct them; the compliance of our products with the laws and regulations of the countries in which they are marketed, which compliance may be costly and time-consuming; the use, misuse or off-label use of our products that may harm our image in the marketplace or result in injuries that may lead to product liability suits, which could be costly to our business or result in governmental sanctions; SEC. Business. product categories. We believe we are differentiated in the marketplace by our strategic focus on extremities and biologics, our full portfolio of upper and lower total ankle replacement or arthroplasty procedures. year ended December 31, 2014. Medical Education every market sector is dependent upon having a robust and compelling product offering, and equally as important, a dedicated, highly trained, focused sales organization to service our customers. We plan to continue to strategically focus on and invest in building a competitively superior U.S. sales organization by training and certifying our sales representatives on our innovative product portfolio, continuing to develop and implement strong performance management practices, and enhancing sales productivity. surgical instrumentation. We manufacture patient outcomes. We work closely with our suppliers to ensure continuity of supply while maintaining high quality and reliability. needs for the interim period until a new supplier is brought on line. notice. Our private-label distribution agreements do not, individually or in the aggregate, represent a material portion of our business and we are not substantially dependent on them. biologics products and expand our current product offerings and the markets in which they are offered. Our research and development teams are organized and aligned with our product marketing teams and are focused on improving clinical outcomes by designing innovative, clinically differentiated products with improved ease-of-use and by developing new product features and enhanced surgical techniques that can be leveraged across a broader base of surgeon customers. Our internal research and development teams work closely with external research and development consultants and a global network of physicians and medical personnel in hospitals and universities to ensure we have broad access to best-in-class ideas and technologies to drive our product development pipeline. We also AUGMENT® Bone Graft. operations could be harmed. (PMA) application. Most of our human subjects. During the trial, the sponsor must comply with the FDA’s IDE requirements including, for example, among others, the labeling and claims regulations, which require that promotion is truthful, not misleading, fairly balanced and provide adequate directions for use and that all claims are substantiated, and also prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; FDA guidance of off-label dissemination of information and responding to unsolicited requests for information; complaint handling regulations designed to track, monitor, and resolve complaints related to our products; Part 806 reporting of certain corrections, removals, enhancements, and recalls of products; obtaining or retaining business or other benefits, as well as similar anti-corruption laws of other countries, such as the UK Bribery Act. healthcare providers who use the products or therapies. Even though a new product may have been cleared for commercial distribution by the FDA, we may find limited demand for the In the United States, a uniform policy of coverage does not exist across all third-party payors relative to payment of claims for all products. Therefore, coverage and payment can be quite different from payor to payor, and from one region of the country to another. This is also true for foreign countries in that coverage and payment systems vary from country to country. Coverage also depends on our ability to demonstrate the short-term and long-term clinical effectiveness, and cost-effectiveness of our products. These supportive data are obtained from surgeon clinical experience, clinical trials, and literature reviews. We pursue and present these results at major scientific and medical meetings, and publish them in respected, peer-reviewed medical journals because data and evidence that can support coverage and payment are important to the successful commercialization and market access of our products. under recently enacted healthcare legislation. A key component in ensuring whether the appropriate payment amount is received for physician and other services, including an understanding of our business. 2006. Our principal executive offices are located at results. The risk factors described below may relate solely to one or more of the legal entities contained in our corporate structure and may not necessarily apply to Wright Medical Group N.V. or one or more of the other legal entities contained in our corporate structure. monitoring of new operations and associated increased costs and complexity. There can be no assurances that we will be successful or that we will realize the expected operating efficiencies, cost savings, and other benefits currently anticipated from the merger. results, results from discontinued operations, and financial condition. regulatory risks. These risks include the risk that our international distributors could engage in conduct violative of U.S. or local laws, including the U.S. Foreign Corrupt Practices Act (FCPA). Our U.S. operations, including those of our U.S. and we utilize a number of third-party sales representatives for whose actions we could be held liable under the FCPA. We inform our personnel and third-party sales representatives of the requirements of the FCPA and other Failure to comply with the FCPA or other anti-corruption laws could subject us to, among other things, penalties and legal expenses that could harm our reputation and have a material adverse effect on our business, financial condition, and operating results. condition. budget, which could adversely affect our sales and operating results. operations. IGNITE® products. future competitors. In addition, operating results. We business, financial condition, and operating results. In addition, we outstanding indebtedness. If we are expected tax treatment for us and adversely affect our financial results. and Jurisdiction of Incorporation widely due to factors beyond our control. variations in our announcements of new investments, acquisitions, strategic partnerships, or joint ventures; announcements of new announcements of divestitures or discontinuance of products or assets; additions or departures of key personnel; sales of our equity securities by our significant shareholders or management or sales of additional equity securities by our company; pending and potential litigation or regulatory investigations; and fluctuations in market prices for our products. or officers. U.S. federal securities laws. requiring our shareholders’ approval, including the Comments. Fiscal year 2012 First Quarter Second Quarter Third Quarter Fourth Quarter Fiscal year 2011 First Quarter (commencing on February 3, 2011) Second Quarter Third Quarter Fourth Quarter Tornier N.V. NASDAQ U.S. Companies Index NASDAQ Medical Equipment Companies Index 27, 2015. We are a global medical device company focused on available for sale in Canada for foot and ankle fusion indications and in Australia and New Zealand for hindfoot and ankle fusion indications. The AUGMENT® Bone Graft product line was acquired from BioMimetic Therapeutics, Inc. (BioMimetic) in March 2013. Our other principal biologics products include the GRAFTJACKET line of soft tissue repair Challenges. 31, Statements of Operations Data: Revenue Cost of goods sold Gross profit Selling, general and administrative Research and development Amortization of intangible assets Special charges Operating loss Interest income Interest expense Foreign currency transaction (loss) gain Loss on extinguishment of debt Other non-operating income Loss before income taxes Income tax benefit Consolidated net loss Net loss attributable to noncontrolling interest Net loss attributable to Tornier Accretion of noncontrolling interest Net loss attributable to ordinary shareholders year-over-year change: Upper extremity joints and trauma Lower extremity joints and trauma Sports medicine and biologics Total extremities Large joints and other Total United States International Total the Wright/Tornier merger. This step-up amortization will be recognized over a 14-month period subsequent to the Wright/Tornier merger. lower extremities market. Balance Sheet Cash and cash equivalents Working capital Total debt Lines of credit availability and debt issuances and through cash flow from operations. Cash Flow Consolidated net loss Cash provided by operating activities Cash used in investing activities Cash provided by financing activities Amounts reflected in consolidated balance sheet: Bank debt Shareholder loan Contingent consideration Capital leases Amounts not reflected in consolidated balance sheet: Interest on bank debt Interest on contingent consideration Interest on capital leases Operating leases Total dependent upon FDA clearance. Supplementary Data condition, changes in financial condition, or results of operations. include: ® line of products in connection with the marketing and distribution of KCI's soft tissue graft containment products used in the wound care field, subject to certain exceptions. License revenue under this agreement is being recognized over 12 years on a straight-line basis. allowances in future periods. We value our inventory at the lower of the actual cost to purchase and/or manufacture the inventory on a first-in, first-out We account for acquired businesses using the purchase method of accounting. Under the purchase method, our consolidated financial statements include the operating expenses as incurred. result in a triggering event for which we would test for impairment. surgical instrumentation. We depreciate our property, plant and equipment determinable, reducing income in that period. Our effective tax rate is based on income by tax jurisdiction, valuation allowances, statutory rates, and ongoing basis and provides valuation allowances to reduce net deferred tax assets to the amount that is more likely than not to be realized. See rate. are amortized on a straight-line basis over their respective requisite service periods, which are generally the vesting periods. revenue recognition practices. Subsequent Measurement of Debt Issuance Costs Associated With Line-of-Credit Arrangements ordinary shares. We believe that a 10% change in our share price is reasonably possible in the near term: Fluctuations foreign currency, we will receive less in U.S. dollars than we did before the rate increase went into effect. If we price our products in U.S. dollars and our competitors price their products in local currency, an increase in the relative strength of the U.S. dollar could result in our prices not being competitive in a market where business is transacted in the local currency. : : Committee of Sponsoring Organizations of the Treadway Commission (COSO). Assets Current assets: Cash and cash equivalents Accounts receivable (net of allowance of $4,846 and $2,486, respectively) Inventories Income taxes receivable Deferred income taxes Prepaid taxes Prepaid expenses Other current assets Total current assets Instruments, net Property, plant and equipment, net Goodwill Intangible assets, net Deferred income taxes Other assets Total assets Liabilities and shareholders’ equity Current liabilities: Short-term borrowing and current portion of long-term debt Accounts payable Accrued liabilities Income taxes payable Deferred income taxes Total current liabilities Long-term debt Deferred income taxes Contingent consideration Other non-current liabilities Total liabilities Shareholders’ equity: Ordinary shares, €0.03 par value; authorized 175,000,000 at December 30, 2012 and January 1, 2012, respectively; issued and outstanding 41,728,257 and 39,270,029 at December 30, 2012 and January 1, 2012, respectively Additional paid-in capital Accumulated deficit Accumulated other comprehensive income Total shareholders’ equity Total liabilities and shareholders’ equity Revenue Cost of goods sold Gross profit Operating expenses: Selling, general and administrative Research and development Amortization of intangible assets Special charges Total operating expenses Operating loss Other income (expense): Interest income Interest expense Foreign currency transaction (loss) gain Loss on extinguishment of debt Other non-operating income, net Loss before income taxes Income tax benefit Consolidated net loss Net loss attributable to non-controlling interest Net loss attributable to Tornier Accretion of non-controlling interest Net loss attributable to ordinary shareholders Net loss per share: Basic and diluted Weighted average shares outstanding: Basic and diluted Contents Consolidated net loss Unrealized loss on retirement plans, net of tax Foreign currency translation adjustments Comprehensive loss Comprehensive net loss attributable to non-controlling interest Comprehensive net loss attributable to Tornier Comprehensive Loss Cash flows from operating activities: Consolidated net loss Adjustments to reconcile consolidated net loss to cash provided by operating activities: Depreciation and amortization Impairment of fixed assets Lease termination costs Intangible impairment Non-cash foreign currency (gain) loss Deferred income taxes Tax benefit from reversal of valuation allowance Share-based compensation Non-cash interest expense and discount amortization Inventory obsolescence Loss on extinguishment of debt Change in fair value of warrant liability Incentive related to new facility lease Acquired inventory step-up Other non-cash items affecting earnings Changes in operating assets and liabilities, net of acquisitions: Accounts receivable Inventories Accounts payable and accruals Other current assets and liabilities Other non-current assets and liabilities Net cash provided by operating activities Cash flows from investing activities: Acquisition-related cash payments Purchases of intangible assets Additions of instruments Property, plant and equipment lease incentive Purchases of property, plant and equipment Proceeds from sale of property, plant and equipment Net cash used in investing activities Cash flows from financing activities: Change in short-term debt Repayments of long-term debt Repayment of notes payable Proceeds from issuance of long-term debt Deferred financing costs Issuance of ordinary shares Net cash provided by financing activities Effect of exchange rate changes on cash and cash equivalents (Decrease) increase in cash and cash equivalents Cash and cash equivalents: Beginning of period End of period Non cash investing and financing transactions: Fixed assets acquired pursuant to capital lease Supplemental disclosure: Income taxes paid (refunded) Interest paid following year. Prior to the merger, our fiscal year ended December 31 each year. December 31, 2013. The accompanying consolidated financial statements include have been eliminated in consolidation. The Credit Risk. Raw materials Work in process Finished goods Total Instruments Instruments in progress Accumulated depreciation Instruments, net handling costs associated with the shipment of goods to customers, independent distributors, and our subsidiaries. Amounts billed to customers for shipping and handling of products are included in net sales. Costs incurred related to shipping and handling of products to customers are included in selling, general and administrative expenses. All other shipping and handling costs are included in cost of sales. income is attributable to foreign currency translation. $25.0 million, $11.5 million, and $12.0 million during the years ended December 27, 2015 and December 31, 2014 and 2013, respectively, within results of continuing operations. The increase in expense in 2015 related to accelerated vesting of all unvested awards upon the closing of the Wright/Tornier merger. See Note 14 for further information regarding our share-based compensation assumptions and expenses. Level 3 Cash and cash equivalents Contingent consideration Derivative asset Total, net Cash and cash equivalents Total, net Goodwill Other intangible assets Tangible assets acquired and liabilities assumed: Accounts receivable Inventory Other assets Instruments, net Accounts payable and accrued liabilities Deferred income taxes Other long-term debt Total preliminary purchase price Developed technology In-process research and development Trademarks and trade names Non-compete agreements Total identifiable intangible assets Revenue Net Loss Basic and diluted net loss per share Land Building and improvements Machinery and equipment Furniture, fixtures and office equipment Software Construction in progress Accumulated depreciation Property, plant and equipment, net Balance at January 2, 2011 Contingent payment on acquisition Foreign currency translation Balance at January 1, 2012 Contingent payment on acquisition Goodwill acquired in acquisition Foreign currency translation Balance at December 30, 2012 Balances at December 30, 2012 Intangible assets subject to amortization: Developed technology Customer relationships Licenses In-process research and development Other Intangible assets not subject to amortization: Tradename Total Balances at January 1, 2012 Intangible assets subject to amortization: Developed technology Customer relationships Licenses Other Intangible assets not subject to amortization: Tradename Total 2013 2014 2015 2016 2017 Accrued payroll and related expenses Accrued royalties Other accrued liabilities Gross notes payable Discount to notes payable Net notes payable Lines of credit and overdraft arrangements Mortgages Bank term debt Shareholder debt Total debt Less current portion Long-term debt 2013 2014 2015 2016 2017 Thereafter Senior secured U.S. dollar term loan Senior secured Euro term loan Debt discount Total United States loss Rest of the world loss Loss before taxes Current (provision) benefit: United States Rest of the world Deferred benefit Total income tax benefit Income tax provision at U.S. statutory rate Release of valuation allowance Change in valuation allowance Non-taxed interest income on participating loan State and local taxes Tax deductible IPO costs Other foreign taxes Unrecognized interest deduction Impact of foreign income tax rates Non-deductible expenses Other Total Deferred tax assets: Net operating loss carryforwards Inventory Exchange rate changes Stock options Accruals and other provisions Total deferred tax assets Less: valuation allowance Total deferred tax assets after valuation allowance Deferred tax liabilities: Intangible assets Depreciation Total deferred tax liabilities Total net deferred tax liabilities $10 million. The total amount of net unrecognized tax benefits that, if recognized, would affect the tax rate was next twelve months. Gross unrecognized tax benefits at January 2, 2011 Increase for tax positions in prior years Decrease for tax positions in prior years Increase for tax positions in current year Foreign currency translation Gross unrecognized tax benefits at January 1, 2012 Increase for tax positions in prior years Decrease for tax positions in prior years Increase for tax positions in current year Foreign currency translation Gross unrecognized tax benefits at December 30, 2012 2015 has been accounted for as a “reverse acquisition” under US GAAP. As such, legacy Wright is considered the acquiring entity for accounting purposes; and therefore, legacy Wright’s historical results of operations replaced legacy Tornier’s historical results of operations for all periods prior to the merger. Additionally, each legacy Wright share was converted into the right to receive 1.0309 ordinary shares of the combined company and the par value was revised to reflect the €0.03 par value as compared to the legacy Wright par value of $0.01. These changes resulted in the restatement of the following to conform to the current presentation: Revenue by geographic region: United States France Other international Total Revenue by product type: Upper extremity joints and trauma Lower extremity joints and trauma Sports medicine and biologics Total extremities Large joints and other Total Long-lived assets: United States France Other international Total December 27, 2015, December 31, 2014, and 2013, respectively. Future minimum 2013 2014 2015 2016 2017 Thereafter Total 2013 2014 2015 2016 2017 Thereafter Total minimum lease payments Less amount representing interest Present value of minimum lease payments Current portion Long-term portion tables above based on their respective U.S. dollar exchange rates at also filed a counterclaim seeking declaratory judgment and indemnification and other damages in an unspecified amount from MicroPort. A scheduling order has not yet been entered in the lawsuit. Cost of goods sold Selling, general and administrative Research and development Total Risk-free interest rate Expected life in years Expected volatility Expected dividend yield Outstanding at December 27, 2009 Granted Exercised Forfeited or expired Outstanding at January 2, 2011 Granted Exercised Forfeited or expired Outstanding at January 1, 2012 Granted Exercised Forfeited or expired Outstanding at December 30, 2012 Exercisable at period end Outstanding at January 2, 2011 Granted Vested Cancelled Outstanding at January 1, 2012 Granted Vested Cancelled Outstanding at December 30, 2012 Restructuring costs Management exit costs Acquisition and integration costs Distribution channel change costs Increased allowances for uncollectible accounts in Italy Intangible asset impairments Other Total Employee termination benefits Impairment charges related to fixed assets Moving, professional fees and other initiative-related expenses Total facilities consolidation expenses Facility consolidation accrual balance as of January 2, 2012 Charges: Employee termination benefits Moving, professional fees and other initiative-related expenses Total charges Payments: Employee termination benefits Moving, professional fees and other initiative-related expenses Total payments Facilities consolidation accrual balance as of December 30, 2012 Revenue Gross profit Consolidated net loss Net loss per share: basic and diluted Revenue Gross profit Consolidated net loss Net loss attributable to ordinary shareholders Net loss per share: basic and diluted Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective as of December 27, 2015. legacy Wright and not legacy Tornier; and Name Position Kevin Julie B. Andrews public companies’ boards of directors, and his extensive business knowledge working with other public companies in the medical device industry. directors of GenMark Diagnostics, Inc., As of February 10, 2016, TMG beneficially owned 6.1% of our outstanding ordinary shares. Mr. Carney and Ms. Weatherman are our current directors who are designees of TMG. assisting our board of directors in monitoring the integrity of our financial statements, our compliance with legal and regulatory requirements insofar as they relate to our financial statements and financial reporting appointing, compensating, retaining, and overseeing the work of any independent registered public accounting firm engaged for the purpose of performing any audit, review, or attest services and providing a medium for consideration of matters relating to any audit issues; establishing procedures for the receipt, retention, and treatment of complaints received by reviewing and approving all related party transactions required to be disclosed under reviewing and approving corporate goals and objectives relevant to the compensation of our Chief Executive Officer and other executive officers, evaluating the performance of these officers in light of those goals and objectives, and setting compensation of these officers based on such evaluations; making recommendations to our board of directors with respect to incentive compensation and equity-based plans that are subject to board and shareholder approval, administering or overseeing all of our incentive compensation and equity-based plans, and discharging any responsibilities imposed on the committee by any of these plans; approving, or recommending to our board of directors for approval, the compensation programs, and the payouts for all programs, applying to our non-executive directors, including reviewing the competitiveness of our non-executive director compensation programs and reviewing the terms to make sure they are consistent with our board of directors compensation policy adopted by the general meeting of reviewing and discussing with our Chief Executive Officer and reporting periodically to our board of directors plans for development and corporate succession plans for our executive officers and other key employees. reviewing and making recommendations to our board of directors regarding the size and composition of our board of directors; identifying, reviewing, and recommending nominees for election as directors; making recommendations to our board of directors regarding corporate governance matters and practices, including any revisions to our internal rules for our board of directors; and overseeing our compliance efforts with respect to our legal, regulatory, and quality systems requirements and ethical programs, including our code of business conduct, Stevens. We have made no material changes to the procedures by which shareholders may recommend nominees to our board of directors as described in our most recent proxy statement. Robert J. Palmisano, who serves as our current President and Chief Executive Officer and an executive director, and prior to the Wright/Tornier merger, served as legacy Wright’s President and Chief Executive Officer; this CD&A. This CD&A should be read in conjunction with the accompanying compensation tables, corresponding notes and narrative discussion, as they provide additional information and context to our compensation disclosures. What We Do: Compensation Objectives and Philosophies Align the interests of our executives with the interests of our shareholders. Reward At least two-thirds of the CEO’s compensation and half of other executives’ compensation opportunity should be in the form of variable compensation that is tied to financial results and/or The portion of total compensation that is performance-based or at-risk should increase with an TORNIER Page Page Item 1.5 20 Item 1B.47 Item 2.47 Item 3.47Item 4.47Part IIItem 5. 4850OperationsOperations.RiskRisk.6769Withwith Accountants on Accounting and Financial DisclosureDisclosure.100 Item 9A.100 Item 9B.100 Item 10. GovernanceGovernance.102109143IndependenceIndependence.145 147 Item 15.149 152EX-10.42 EX-10.43 EX-10.48 EX-12.1 EX-21.1 EX-23.1 EX-31.1 EX-31.2 EX-32.1 EX-101 INSTANCE DOCUMENT EX-101 SCHEMA DOCUMENT EX-101 CALCULATION LINKBASE DOCUMENT EX-101 LABELS LINKBASE DOCUMENT EX-101 PRESENTATION LINKBASE DOCUMENT EX-101 DEFINITION LINKBASE DOCUMENT On January 28, 2011, Tornier B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) changed its legal form by converting to Tornier N.V., a public company with limited liability (naamloze vennootschap). This is referred to as the “conversion” in this report.References to “Tornier,” “Company,” “we,” “our” or “us” in this report refer to Tornier B.V. and its subsidiaries prior to the conversion and to Tornier N.V. and its subsidiaries upon and after the conversion, unless the context otherwise requires.This report contains references to, among others, our trademarks Aequalis®, Affiniti®, Ascend®, Ascend Flex™, BioFiber®, Piton®, Salto Talaris®, Simpliciti™, and Tornier®. All other trademarks or trade names referred to in this report are the propertyreportAnnual Report on Form 10-K contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933, as amended (Securities Act), and Section 21E of the Securities Exchange Act of 1934, as amended. Allamended (Exchange Act), and that are subject to the safe harbor created by those sections. These statements reflect management's current knowledge, assumptions, beliefs, estimates, and expectations and express management's current view of future performance, results, and trends. Forward looking statements may be identified by their use of terms such as anticipate, believe, could, estimate, expect, intend, may, plan, predict, project, will, and other thansimilar terms. Forward-looking statements are subject to a number of historical fact includedrisks and uncertainties that could cause actual results to materially differ from those described in the forward-looking statements. The reader should not place undue reliance on forward-looking statements. Such statements are made as of the date of this report, and we undertake no obligation to update such statements after this date. Risks and uncertainties that address activities, events or developments that we expect, believe or anticipate will or may occurcould cause our actual results to materially differ from those described in the future are forward-looking statements are discussed in our filings with the U.S. Securities and Exchange Commission (SEC) (including those described in "Part I. Item 1A. Risk Factors" of this report). By way of example and without implied limitation, such risks and uncertainties include:particular,seizures, injunctions, monetary sanctions, or criminal or civil liabilities;statements about our plans, objectives, strategiesrecently completed merger between Tornier N.V. (Tornier or legacy Tornier) and prospects regarding, among other things,Wright Medical Group, Inc. (WMG or legacy Wright), including the failure to realize intended benefits and anticipated synergies and cost-savings from the transaction or delay in realization thereof; cash costs associated with the transaction which may negatively impact our financial condition, operating results, and business. We have identified some of these forward-looking statements with words like “believe,” “may,” “will,” “should,” “could,” “expect,” “intend,” “plan,” “predict,” “anticipate,” “estimate”cash flow; our businesses may not be combined successfully, or “continue” other words and terms of similar meaning and the use of future dates. These forward-looking statements are based on current expectations about future events affecting us and are subjectsuch combination may take longer, be more difficult, time-consuming or costly to uncertainties and factors relating to our operationsaccomplish than expected; and business environment, alldisruption after the transaction, including adverse effects on employee retention, our sales and distribution channel, especially in light of which are difficult to predictanticipated territory transitions, and many of which are beyond our control and could cause our actual results to differ materially from those matters expressed or implied by our forward-looking statements. Forward-looking statements (including oral representations) are only predictions or statements of current plans and can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties, including, among other things, on business relationships with third parties;with:our historywith the recently completed divestiture of operating lossesthe U.S. rights to certain of legacy Tornier's ankle and negative cash flow;our recent acquisitionliability for product liability claims on hip/knee (OrthoRecon) products sold by legacy Wright prior to the divestiture of OrthoHelix Surgical Designs, Inc., and risks related thereto, including our inability to integrate successfully our commercial organizations, including in particular our distribution and sales representative arrangements, and our the OrthoRecon business;and synergiesfrom previous acquisitions or from the divestiture of the OrthoRecon business;business and operating results;reliance oncorporate structure which is intended to ring-fence liabilities;independent sales agencies and distributors to sell our products and the effect on our business and operating results of agency and distributor changes or transitions to direct selling models in certain geographies, including most recently in Canada, Belgium and Luxembourg andAUGMENT® Bone Graft in the United States, and possible ramificationsStates;such changes and transitionsothers;businessrelationships with customers and operating results;our recently completed facilities consolidation and its effect on our businessability to deliver timely and operating results;continuing weaknessthe possibility of private securities litigation or shareholder derivative suits;the global economy, which has been and may continue to be exacerbated by austerity measures taken by several countries, and automatic and discretionary governmental spending cuts,product reimbursements, which could reducegenerate downward pressure on our product pricing;availabilityconversion features of our convertible senior notes, or affordabilityrefinance our existing debt as it matures;private insurance or Medicare or other governmental reimbursement or may affect patient decision to undergo elective procedures,the commercial and could otherwise adversely affectcredit environment on us, our businesscustomers, and operating results;our suppliers;markets, including in particular Japan and China;disruption and turmoil in global credit and financial markets, which may be exacerbated by the inability of certain countries to continue to service their sovereign debt obligations;changes in our senior management, including our recent Chief Executive Officer and Chief Financial Officer changes;our credit agreement, senior secured term loans and revolving credit facility and risks related thereto;our reliance on sales of our upper extremity joints and trauma products, which generate a significant portion of our revenue;our patents and other intellectual property rights not adequately protecting our products or alleged claims of patent infringement by us, which may result in our loss of market share to our competitors and increased expenses;our inability to access our revolving credit facility or raise capital when needed, which could force us to delay, reduce, eliminate or abandon our commercialization efforts or product development programs;restrictive affirmative financial and other covenants in our credit agreement that may limit our operating flexibility;regulatory clearances or approvals and the extensive regulatory requirements to which we are subject;healthcare reform legislation, including the excise tax on U.S. sales of certain medical devices,pending and its future implementation, possible additional legislation, regulation and other governmental pressure in the United States and globally, which may affect utilization, pricing, reimbursement, taxation and rebate policies of governmental agencies and private payors,litigation, which could have an adverse effect on our business, financial condition, or operating results; andpending and future litigation.“Part I —“Part I. Item 1A. Risk Factors”.Factors” of this report. The risks and uncertainties described above and in the “Part I —“Part I. Item 1A. Risk Factors” sectionFactors” of this report are not exclusive and further information concerning us and our business, including factors that potentially could materially affect our financial results or condition, may emerge from time to time. We assume no obligation to update, amend, or clarify forward-looking statements to reflect actual results or changes in factors or assumptions affecting such forward-looking statements. We advise you, however, to consult any further disclosures we make on related subjects in our future annual reportsAnnual Reports on Form 10-K, quarterly reportsQuarterly Reports on Form 10-Q and current reportsCurrent Reports on Form 8-K we file with or furnish to the Securities and Exchange Commission.BusinessWe aresurgeons that treat musculoskeletal injuriesextremities and disordersbiologics products. We are committed to delivering innovative, value-added solutions improving quality of life for patients worldwide and are a recognized leader of surgical solutions for the shoulder,upper extremities (shoulder, elbow, wrist hand, ankle and foot. We refer to these surgeons as extremity specialists. We sell to this extremity specialist customer base a broad line of joint replacement, trauma, sports medicinehand), lower extremities (foot and biologic products to treat extremity joints. Our motto of “specialists serving specialists” encompasses this focus. In certain international markets, we also offer joint replacement products for the hip and knee. We currently sell approximately 100 product lines in approximately 40 countries.We have had a tradition of innovation, intense focus on surgeon education and commitment to advancement of orthopaedic technology since our founding over 70 years ago in France by René Tornier. Our history includes the introduction of the porous orthopaedic hip implant, the application of the Morse taper, which is a reliable means of joining modular orthopaedic implants, and, more recently, the introduction of the stemless shoulder both in Europe and in a U.S. clinical trial. This track record of innovation over the decades stems from our close collaboration with leading orthopaedic surgeons and thought leaders throughout the world.In 2006, an investor group led by Warburg Pincus (Bermuda) Private Equity IX, L.P. recognized Tornier’s reputation for innovation and its strong extremity joint portfolio as a platform upon which they could build a global company focused on the rapidly evolving upper and lower extremity specialties. The investor group contributed capital resources and a management team with a track record of success in the orthopaedic industry in an effort to expand our product offerings in extremities and accelerate our growth. In 2011, we became a U.S. public reporting company to provide additional access to capital and accelerate our plans to become the market leader in extremities. Since 2006, we have:created an extremity specialist focused sales channel in the United States primarily focused on our products;enhanced and broadened our global market leading portfolio of shoulder and ankle joint implants;significantly expanded our foot and ankle offering with the acquisition of OrthoHelix Surgical Designs, Inc. (OrthoHelix), with specialty implantable screw and plate systems for the repair of small bone fractures and deformities in the foot and ankle;entered the sports medicineankle) and biologics markets, through acquisitionsthree of the fastest growing segments in orthopaedics. We market our products in over 50 countries worldwide.licensing agreements andbiologics business. We believe our leadership will be further enhanced by the portfolio through internal development;improved our hiprecent U.S. Food and kneeDrug Administration (FDA) premarket approval of AUGMENT® Bone Graft, a biologic solution that adds additional depth to one of the most comprehensive extremities product offerings, helpingportfolios in the industry, as well as provides a platform technology for future new product development. The highly complementary nature of legacy Wright’s and legacy Tornier’s businesses has given us gain market share internationally;significant diversity andsignificantly increased investment in research scale across a range of geographies and development and expanded business development activities to build a pipeline of innovative new technologies.extremityextremities and biologics products, our extremity-focused sales organization and our strategic focus on extremities. We further believe that wespecialized and focused sales organization.well positioned to benefit fromlocated in Amsterdam, the opportunities in the extremity products marketplace as we are among the global leaders in the shoulder and ankle joint replacement markets and also the foot and ankle trauma market with our recent acquisition of OrthoHelix.Netherlands. We also have expandedsignificant operations located in Memphis, Tennessee (U.S. headquarters, research and development, sales and marketing administration, and administrative activities); Bloomington, Minnesota (upper extremities sales and marketing); Arlington, Tennessee (manufacturing and warehousing operations); Grenoble, France (manufacturing and research and development); and Macroom, Ireland (manufacturing). In addition, we have local sales and distribution offices in Canada, Australia, Asia, and throughout Europe. For purposes of this report, references to "international" or "foreign" relate to non-U.S. matters while references to "domestic" relate to U.S. matters.technology baseordinary shares changed from “TRNX” to “WMGI.” Because of these and product offering to include: new joint replacement products based on new materials; improved trauma products based on innovative designs;other facts and proprietary biologic materialscircumstances, the merger has been accounted for soft tissue repair. Inas a “reverse acquisition” under generally acceptable accounting principles in the United States (US GAAP), and as such, legacy Wright is considered the acquiring entity for accounting purposes.Therefore, legacy Wright’s historical results of operations replaced legacy Tornier’s historical results of operations for all periods prior to the merger. References in this section and certain other sections of Part I of this report to "we," "our" and "us" refer to Wright Medical Group N.V. and its subsidiaries after the Wright/Tornier merger and Wright Medical Group, Inc. and its subsidiaries before the merger.largest orthopaedic market, weindustry, each with approximately $2 billion or more in annual sales. The size of these companies often allows them to concentrate their marketing and research and development efforts on products they believe that our “specialists serving specialists” market approach is strategically aligned with what we believewill have a relatively high minimum threshold level of sales. As a result, there is an ongoing trendopportunity for a mid-sized orthopaedic company, such as Wright, to focus on less contested, higher-growth sectors of the orthopaedic market.specialize in certain parts of the anatomy or certain types of procedures.Our principal products are organized in four major categories: upper extremity joints and trauma, lower extremity joints and trauma, sports medicine and biologics, and large joints and other. Our upper extremity joints and trauma products include joint replacement and bone fixation devices for the shoulder, hand, wrist and elbow. Our lower extremity joints and trauma products, which include our OrthoHelix portfolio, include joint replacement and bone fixation devices for the foot and ankle. Our sports medicine and biologics product category includes products used across several anatomic sites to mechanically repair tissue-to-tissue or tissue-to-bone injuries,be over $3 billion in the case of sports medicine, or to support or induce remodeling and regeneration of tendons and ligaments, in the case of biologics. Our large joints and other products include hip and knee joint replacement implants and ancillary products.Our Business StrategiesOur goal is to achieve global leadershipUnited States. We believe major trends in the extremities market which we believe will require focusinclude procedure-specific and execution in the following key strategic elements:Leveraging our “specialists serving specialists” strategy: We believe our focus on and dedication to extremity specialists enables us to better understand and address the clinical needs of these surgeons. We believe that extremity specialists, who have emerged as a significant constituency in orthopaedics only in the last 10 to 15 years, have been underserved in terms of new technology and also inefficiently served by the current marketplace. We offer a comprehensive portfolio of extremity products, which has been further strengthened with our acquisition of OrthoHelix, and also serve our customers through a sales channel that is primarily dedicated to extremities, which we believe provides us with a significant competitive advantage, because our sales agencies and their representatives have both the knowledge and desire to comprehensively meet the needs of extremity specialists and their patients, without competing priorities. In 2013, we intend to further focus our channel to individually support and address the needs of upper extremity and lower extremity specialists. For example, in the U.S., we are pursuing dedicated sales representation aligned to upper and lower extremity specialists through leveraging the Tornier and OrthoHelix existing distribution channels, along with direct sales channels in certain markets.Introducing new products and technologies to address more of our extremity specialists’ clinical needs: Our goal is to continue to introduce new technologies for extremity joints that improve patient outcomes and thereby continue to expand our market opportunity and share. In addition, through our acquisition of OrthoHelix, we believe that we can leverage our strong arthroplasty position to further expand our opportunities in bone repair, thereby offering a more comprehensive foot and ankle portfolio. Since 2006, we have significantly increased our investment in research and development to accelerate the pace of new product introduction and with our acquisition of OrthoHelix in 2012, have expanded our capabilities. We have also been active in gaining access to new technologies through external partnerships, licensing agreements and acquisitions. We believe that our reputation for effective collaboration with industry thought leaders as well as our track record of effective new product development and introductions will allow us to continue to gain access to new ideas and technologies early in their development.Expanding our international business: We face a wide range of market dynamics that require our distribution channels to address both our local market positions and local market requirements. For example, in France, which is a more developed extremities market and where we have a diversified extremities, hip and knee business, we have two direct sales organizations. One is focused on products for upper extremities, and the other focused on hip and knee replacements and products for lower extremities. In other European markets, we utilize a combination of direct and distributor strategies that have evolved to support our expanding extremity business and also to support our knee and hip market positions. In international markets where the extremity market segment is relatively underdeveloped, the same sales channel may sell our hip and knee product portfolios and extremity joint products, which provides these sales channels sufficient product breadth and economic scale. We plan on expanding our international business by continuing to adapt our distribution channels to the unique characteristics of individual markets, which has included conversions to direct sales organizations in certain geographies. In addition, once we receive the necessary regulatory approvals, we will begin to introduce the innovative OrthoHelix product portfolio into select international markets and leverage our existing sales and distribution channels.Advancing scientific and clinical education:We believe our specialty focus, commitment to product innovation and culture of scientific advancement attract both thought leaders and up-and-coming surgeon specialists who share these values. We actively involve these specialists in the development of world-class training and education programs and encourage ongoing scientific study of our products. Specific initiatives include the Tornier Master’s Courses in shoulder and ankle joint replacement, The Fellows and Chief Residents Courses, and a number of clinical concepts courses. We also maintain a registry that many of our customers utilize to study and report on the outcomes of procedures in which our extremity products have been used. We believe our commitment to science and education also enables us to reach surgeons early in their careers and provide them access to a level of training in extremities that we believe is not easily accessible through traditional orthopaedic training.Our Surgeon CustomersWe estimate that there are over 80,000 orthopaedic and over 9,000 podiatric surgeons worldwide who specialize in surgical treatment of the musculoskeletal system, including bones, joints and soft tissues such as tendons and ligaments. In the United States and certain other developed markets, there has been a trend over the past two decades for these surgeons to specialize in certain parts of the anatomy or certain types of procedures. We believe that the trend toward specialization has been supported by the expansion of specialist professional societiesanatomy-specific devices, locking plates, and an increase in the number of fellowship programs. We focus on the following orthopaedic specialist groups:Extremity Specialists: Upper extremity specialists perform joint replacementextremities reconstruction involves implanting devices to replace, reconstruct, or fixate injured or diseased joints and trauma and soft tissue repair procedures forbones in the shoulder, elbow, wrist, and hand. It is estimated that approximately 60% of the upper extremities market is in total shoulder replacement or arthroplasty implants. We believe the evolution of this specialty has been driven by the unique requirements of these joints due to the relative importance of soft tissue to joint function and the increased complexity and breadth of technology available for use in these procedures. For this reason, in addition to joint replacement and trauma products, upper extremity specialists utilize a broad range of sports and biologic products. We believe upper extremity specialists now perform the majority of shoulder joint replacements that were previously performed by reconstructive and general orthopaedic surgeons.Lower Extremity Specialists: Lower extremity specialists perform a wide range of joint replacement, trauma, reconstruction and soft tissue repair procedures for the foot and ankle. This specialist group principally consists of orthopaedic surgeons who have received fellowship or other specialized training. Additionally, Doctors of Podiatric Medicine with special surgical training may perform certain foot and ankle surgical proceduresmajor trends in the United States, Canada and the United Kingdom.Sports Medicine Specialists:Sports medicine specialists are surgeons who useupper extremities market include minimally invasive surgical techniques, including arthroscopy, for thefracture repair of soft tissues. Arthroscopy is a minimally invasive surgical technique in which a surgeon creates several small incisions at the surgery site; inserts a fiber optic scope with a miniature video camera as well as surgical instruments through the incisionsdevices and next-generation joint arthroplasty systems.visualize, accessreplace, reconstruct, or fixate injured or diseased joints and conduct the procedure; and uses a video monitor to view the surgery itself. The sports medicine specialty is not just limited to treatment of athletes, but rather to all patients with orthopaedic soft tissue injuries or disease. The most common extremities sports medicine procedures are Achilles tendon repairs and rotator cuff repairs in the shoulder.Reconstructive and General Orthopaedic Surgeons: Reconstructive and general orthopaedic surgeons are important customers for us in selected European countries and other international markets. In these markets, orthopaedic surgeons may treat multiple areas of bone and joint disease and trauma, and commonly perform procedures involving extremity joints as well as hip and knee joint replacement. For these target customers, we are able to provide not only our broad product categories for extremity joint procedures, but also our hip and knee joint replacement products.Our Target MarketsWe compete on a worldwide basis providing upper and lower extremity specialist surgeons a wide range of products from several major segments of the orthopaedic market, including extremity joints, sports medicine, biologics and trauma. In addition, we compete in the hip and knee segments of certain international markets where we have a strong legacy presence such as in France, where participation in the local hip and knee market is important to our distributor partners, and in other international markets, where the market for our extremity focused products is still small.We believe our addressable portion of the market will grow at a faster rate than the overall orthopaedic market due to the introduction of new technologies with improved clinical outcomes, a growing number of extremity specialists, the aging of the general population and the desire for people to remain physically active as they grow older. Overviews of the major orthopaedic markets in which we compete, as well as our targeted participation in those markets, are as follows:Extremity Joints: The extremity joint market includes implantable devices used for the replacement of shoulder, elbow, hand, and foot and ankle joints. We believe this market has been under-served and underdeveloped by major orthopaedic companies, which have generally focused on the much larger hip, knee and spine markets. As a result, the growth of the extremity joint market is still benefiting from market-expanding design and materials technologies and from growth in the number of upper and lower extremity specialists. We believe that we are a leader in both the shoulder and ankle joint replacement portions of this market based upon revenue.Sports Medicine: Sports medicine refers to the repair of soft tissue injuries that often occur when people are engaged in physical activity, but that also result from age-related wear and tear. We believe market growth has been driven by both new technology and the continued acceptance of minimally invasive surgical techniques. The most common sports medicine procedures are Achilles tendon repairs and rotator cuff repairs in the shoulder. The primary sports medicine products include capital equipment and related disposables as well as bone anchors, which are implantable devices used to attach soft tissue to bone, sutures, or thread for soft tissue, and handheld instruments. We estimate that our products currently address only a portion of the sports medicine market, primarily bone anchors and other products utilized for rotator cuff repairs. The total sports medicine market also includes capital or powered equipment and related disposables, but we do not have any product offerings in these areas.Biologics:Biologics refer to products, both biologic and synthetic, that are utilized to stimulate hard and soft tissue healing following surgery for a wide range of orthopaedic injuries or disorders. We believe market growth is being driven by the application of an expanding biotechnology knowledge base to the development of products that can improve clinicaloutcomes by inducing tissue healing and regeneration. The primary product categories in the total biologics market are bone grafting materials, cell therapy systems, including growth factors, and tendon and ligament grafts. We currently offer tendon and ligament graft and scaffold products for extremities and platelet concentration systems.Trauma: The trauma market includes devices that are used to treat fractures, joint dislocations, severe arthritis and deformities that result from either acute injuries or chronic wear and tear. The major products in the trauma market include metal plates, screws, pins, wires and external fixation devices used to hold fractured bone fragments together until they heal properly. These devices are also utilized in the treatment of a wide range of non-traumatic surgical procedures, especiallybones in the foot and ankle. AsA large segment of the lower extremities market has transitioned from external casting performedis comprised of plating and screw systems for reconstructing and fusing joints or repairing bones after traumatic injury. We believe major trends in the emergency room,lower extremities market include the use of external fixation devices in diabetic patients, total ankle arthroplasty, advanced tissue fixation devices, and biologics. According to internal fixation performed on a scheduled basis in the operating room, our extremity specialist customers have expanded their role in treating trauma injuries. Our acquisition of OrthoHelix has significantly strengthened our product portfolio so thatvarious customer and market surveys, we are better able to address this importanta market segment.Knee Joints:Knee joint replacements are performed for patients who have developed an arthritic condition that compromises the joints’ articulating surfaces (articulating surfaces are bone segments connected by a joint). The knee joint replacement system has multiple components including a femoral component, a tibial component and a patella component (knee cap). We currently provide a broad line of knee joint replacement productsleader in selected international geographies. We do not currently address the knee joint market in the United States.Hip Joints:Hip joint replacements are performed for patients who have suffered a femoral fracture or suffer from severe arthritis or other conditions that have led to the degradation of the articular cartilage or bone structure residing between the femoral head and the acetabulum (hip socket). The hip joint replacement system generally includes both femoral and acetabular components. We currently provide a broad line of hip joint replacement products in selected international geographies. We do not currently address the hip joint market in the United States.Our Product PortfolioWe offer a broad product line designed to meet the needs of our extremity specialists and their patients. Although the industry traditionally organizes the orthopaedic market based on the mechanical features of the products, we organize our product categories in a way that aligns with the types of surgeons who use them. Therefore, we distinguish upper extremity joints and trauma from lower extremity joints and trauma, as opposed to viewing joint implants and trauma products as distinct product categories. Along these lines, our product offering is as follows:Product categoryTarget addressable geographyUpper extremity joints and traumaUnited States and InternationalLower extremity joints and traumaUnited States and InternationalSports medicine and biologicsUnited States and InternationalLarge joints and otherSelected International MarketsSee Fiscal Year Comparisons contained in the Management’s Discussion and Analysis of Financial Condition and Results of Operations section of this report for a three-year revenue history by product category.Upper Extremity Joints and TraumaThe upper extremity joints and trauma product category includes joint implants and bone fixation devices for the shoulder, hand, wrist and elbow. Our global revenue from this category for the year ended December 30, 2012 was $175.2 million, or 63% of total revenue, which represents growth of 7% over the prior year.Shoulder Joint Replacement and Trauma Implants: We expect the shoulder to continue to be the largest and most important product category for us for the foreseeable future. Our shoulder joint implants are used to treat painful shoulder conditions due to arthritis, irreparable rotator cuff tendon tears, bone disease, fractured humeral heads or failed previous shoulder replacement surgery. Our products are designed for the following:Our total joint replacement products have two components—a humeral implant consisting of a metal stem attached to a metal head, and a plastic implant for the glenoid (shoulder socket). Together, these two components mimic the function of a natural shoulder joint.Our hemi joint replacement products replace only the humeral head and allow it to articulate against the native glenoid.Our reversed implants are used in arthritic patients lacking rotator cuff function. The components are different from a traditional “total” shoulder in that the humeral implant has the plastic socket and the glenoid has themetal head. This design has the biomechanical impact of shifting the pivot point of the joint away from the body centerline and giving the deltoid muscles a mechanical advantage to enable the patient to elevate the arm.Our convertible implants are modular implants that can be converted from a total or hemi joint replacement to a reversed implant at a later date if the patient requires it.Our resurfacing implants are designed to minimize bone resection to preserve bone, which may benefit more active or younger patients with shoulder arthritis.Trauma devices, such as plates, screws and nails, are non-articulating implants used to help stabilize fractures of the humerus.Hand, Wrist and Elbow Joint Replacement and Trauma Implants: We offer joint replacement products that are used to treat arthritis in the hand, wrist and elbow. In addition, we offer trauma products including plates, screws and pins, to treat fractures of the hand, wrist and elbow. One of our distinctive product offerings for these smaller, non-load bearing joints are implants made from a biocompatible material called pyrolytic carbon (pyrocarbon), which has low joint surface friction and a high resistance to wear. We offer a wide range of pyrocarbon implants internationally and have begun to introduce some of these products into the United States.Lower Extremity Joints and TraumaOur global revenue from lower extremity joints and trauma for the year ended December 30, 2012 was $34.1 million, 12% of total revenue, which represents growth of 31% over the prior year. This included approximately $7.8 million of incremental revenue from our acquisition of OrthoHelix, which was completed on October 4, 2012.Ankle Joint Implants: Ankle arthritis is a painful condition that can be treated by fusing the ankle joint with plates or screws or by replacing the joint with an articulating multi-component implant. These joint implants may be mobile bearing, in which the plastic component is free to slide relative to the metal bearing surfaces, or fixed bearing, in which this component is constrained. We offer mobile bearing implants outside the United States and precision bearing implants globally, which are highly anatomic fixed bearing implants.Foot and Ankle Joint and Trauma Implants: Our products include a broad range of anatomically designed plates, screws and nails. These “trauma” products are used to stabilize and heal fractured bones, correct deformities and fuse arthritic joints of the foot and ankle.Other Foot and Ankle Joint and Trauma Implants: In addition to ankle joints, we offer a range of implants made of various materials to partially or fully replace the joints of the toes and correct deformities such as flatfoot.Sports Medicine and BiologicsOur revenue from sports medicine and biologics for the year ended December 30, 2012 was $15.5 million, or 6% of total revenue, which represents growth of 5% over the prior year.Sports Medicine: The sports medicine product category includes products used across several anatomic sites to mechanically repair tissue-to-tissue or tissue-to-bone injuries. Because of its close relationship to shoulder joint replacement, the sports medicine market is of critical strategic importance to us. Rotator cuff repair is the largest sub-segment in the sports medicine market. Other procedures relevant to extremities include shoulder instability treatment, Achilles tendon repair and soft tissue reconstruction of the foot and ankle surgical products. New technologies have been introduced into the lower extremities market in recent years, including next-generation total ankle replacement systems. Many of these technologies currently have low levels of market penetration. We believe that market adoption of total ankle replacement, which currently represents approximately 6% of the U.S. foot and several other soft tissue repair procedures.Biologics: ankle device market, will result in significant future growth in the lower extremities market.injuresinjuries and are complementary to many sports medicine applications, including rotator cuff tendon repair and Achilles tendon repair. Hard tissue biologics products are used in many bone fusion or trauma cases where healing potential may be compromised and additional biologic factors are desired to enhance healing, where the surgeon needs additional bone, stock and does not want to harvest a bone graft from another surgical site or in cases where the surgeon wishes to use materials that are naturally incorporated by the body over time. We estimate that the worldwide orthobiologics market to be over $3.5 billion, and with annual growth rates of 3-5%. Three multinational companies currently dominate the orthobiologics industry.• • contrastpre-clinical testing. Our other hard tissue repair products, including our IGNITE® Power Mix Injectable Stimulus, FUSIONFLEX™ demineralized moldable scaffold, ALLOMATRIX® injectable bone graft putty, OSTEOSET® bone graft substitute, MIIG® Injectable Graft, CANCELLO-PURE® bone wedge line, ALLOPURE® allograft bone wedge line and OSTEOCURE™ Resorbable Bead Kits.traditional metallic-basedmechanically repair tissue-to-tissue or tissue-to-bone injuries and other ancillary products. Because of its close relationship to extremities joint replacement and bone fixation, our sports medicine portfolio is comprised of products used to complement our upper and lower extremities product portfolios, providing surgeons a variety of products that may require later removal.We have a robust pipelinebe used in upper and lower extremities surgical procedures. Our global net sales from this product category for the year ended December 27, 2015 was $13 million or 3% of biologics products under development and are actively pursuing new product additions. We have in-licensed biologic materials such as BioFiber, an advanced high-strength resorbable polymer fiber produced using recombinant DNA technology as well as our F2A peptide, a synthetic versiontotal net sales compared to $10 million or 3% of total net sales for the natural human FGF-2 growth factor. and Other and other product category includes hip and knee joint replacement implants and ancillary products.implants. Hip and knee joint replacementsreplacement products are used to treat patients with painful arthritis in these larger joints.joints and to treat femoral fracture patients. We offer these products in France and select international geographies. Our global revenuenet sales from large joints and other productsthis product category for the year ended December 30, 201227, 2015 was $52.6$10 million, or 19%2% of total revenue, and was a result of the Wright/Tornier merger.represents a declinecould be significant. The financial results of the OrthoRecon business have been reflected within discontinued operations for all periods presented. See Note 4 to our consolidated financial statements contained in revenue of 7% over the prior year. This decline was primarily due“Item. 8. Financial Statements and Supplementary Data” for further information regarding this sale and our discontinued operations relating to the impact of foreign currency exchange rate fluctuationsOrthoRecon business and revenue decreases in certain Western European countries due primarilysee Note 16 to austerity measures. We generated nearly all of our revenue from this category outside of the United States, and a substantial majority of this revenue in France.We have continued to innovate in this area so that we may maintain or grow market share in select international markets where the extremity markets have not yet reached a size to permit the type of channel focus that we have in the United States or where extremities specialization is not as prevalent as in the United States. consolidated financial statements for further information regarding outstanding litigation involving our former OrthoRecon products.Distributiondeveloped our distribution channels to serve the needs of our customers, primarily extremity specialistcontractual relationships with these surgeon advisors, who help us train other surgeons in the United Statessafe and a mixeffective use of extremity specialistour products and generalhelp other surgeons perfect new surgical techniques. Together with these surgeon advisors, we provide surgeons extensive “hands on” orthopaedic training and education, including upper and lower extremities fellowships and masters courses that are not easily accessible through traditional medical training programs. We also offer clinical symposia and seminars, and publish advertisements and the results of clinical studies in industry publications. We believe that our history of innovation and focus on quality and improving clinical outcomes and “quality of life” for patients, along with our training programs, allow us to reach surgeons early in their careers and provide on-going value, which includes experiencing the clinical benefits of our products.international markets. orthopaedic device purchases. While we market our broad portfolio of products to surgeons, our revenue is generated from sales of our products to healthcare institutions and stocking distributors.have a broad offeringmarket and sell our full product portfolio, other than our products for the hip or knee, which we refer to as “large joints”. As of joint replacement and repair, sports and biologic products targeting extremity specialists through, historically, a single distribution channel, with variations based upon individual territories. As we integrate OrthoHelix, we plan to organizeDecember 27, 2015, our sales channels to focus on upper extremities and lower extremities to allow us to increase our selling opportunitiesdistribution system in the United States consisted of 65 geographic sales territories that are staffed by improving our overall procedure coverage, leveraging our entire product portfolio, and accessing new specialists and accounts. Internationally, we utilize several distribution approaches depending on individual market requirements. We utilize458 direct sales organizations in several mature European marketsrepresentatives and Australia,30 independent sales agencies or distributors. These sales representatives and independent sales agencies for most other international markets. In France, we have twoand distributors are generally aligned to selling either our upper extremities products or lower extremities products, but, in some cases, certain agencies or direct sales forces, one handlingrepresentatives sell products from both our upper extremity focused products and one handling our lower extremity focused products and our hip and knee portfolios. In emerging international geographies where extremity markets are still undeveloped, we utilize independent distributors who carry both our extremity-focused and our hip and knee portfolios.United StatesIn the United States, we sell upper extremity joints and trauma, lower extremity joints and trauma, sports medicine and biologics products. We do not actively market hip or knee replacement joints in the United States, although we have FDA clearance for selected large joint products. We have historically sold our products through a single distribution channel, with variations based upon individual territories. As we integrate OrthoHelix, in 2013 we plan to organize our sales channels to focus on upper extremities and lower extremities to allow us to increase our selling opportunities by improving our overall procedure coverage, leveraging our entire product portfolio, and accessing new specialists and accounts.portfolios in their territories. Our U.S. sales force consists of a network of approximately 65 independent commission-based sales agencies, and 4 direct sales organizations in certain territories. We believe a significant portion of our independent sales agencies’ commission revenue is generated by sales of our products. Our success depends largely upon our ability to motivate these sales agenciesrepresentatives, and their representatives to sell our products. Additionally, we depend on their sales and service expertise and relationships with the surgeons in the marketplace. Our independent sales agencies are not obligated to renew their contracts with us, may devote insufficient sales efforts to our products or may focus their sales efforts on other products that produce greater commissions for them. A failure to maintain our existing relationships with our independent sales agencies, and their representatives could have an adverse effect on our operations. We do not control our independent sales agencies and they may not be successful in implementing our marketing plans. As we begin to create focused upper extremity and lower extremity sales channels, and as we make other changes and transitions in our independent sales agency arrangements, our U.S. business may experience disruption due to these factors, but we believe that this strategy will be a significant competitive advantage longer term. Our field sales leadership team consists of five area sales directors from our legacy Tornier organization and three from our OrthoHelix organization, along with a team of upper and lower extremity technical specialists, to support our independent sales agencies and direct sales organizations and to drive focus, accountable performance, business leadership and technical expertise amongst our sales force. During the course of the year, we host numerousdistributors are provided opportunities for product training throughout the United States.year. We generated $156.8 million, or 56%, ofalso have working relationships with healthcare dealers, including group purchasing organizations, healthcare organizations, and integrated distribution networks. We believe our total revenuesuccess in the United States during the year ended December 30, 2012.Webiologicsother, and large joints, in select international markets. As we receivejoints. We utilize several distribution approaches that are tailored to the required regulatory approvals, we will begin to selectively introducethe OrthoHelix product portfolio into these markets. We believe our full rangeneeds and requirements of hip and knee products enable us to more effectively and efficiently service these markets where procedure or anatomic specialization is not as prevalent as in the United States and where extremities, sports medicine and biologics markets have not yet reached a size to permit the degree of channel focus we have in the United States.each individual market. Our international sales and distribution system currently consists of 1311 direct sales offices and approximately 3090 distributors that sell our products in approximately 40over 50 countries. Our largest international market is France, where weWe have a direct sales force of approximately 30 direct sales representatives. We also havesubsidiaries with direct sales offices and corporate subsidiaries in several countries, including Germany, Italy, Switzerland, the Netherlands, the United Kingdom, France, Germany, Italy, Denmark, Australia,Netherlands, Canada, Japan, and CanadaAustralia that employ direct sales employees, Additional European countries, as well asand in some cases, use independent sales representatives to sell our products in their respective markets. Our products are sold in other countries in Europe, Asia, Africa, and Latin America and Asia, are served byusing stocking distribution partners. Stocking distributors who purchase products directly from us for resale to their local customers, with product ownership generally passing to the distributor upon shipment. As partour strategy to grow internationally, we have selectively converted from distributors to direct sales representation in certain countries, as we did in the United Kingdom and Denmark in 2009, Belgium, Luxembourg and Japan in 2012 and Canada in 2013. We intend to focus on expanding our presence in underserved countries, such as China, where we signed an agreement in 2010 with Weigao for the exclusive distribution of our shoulder, hip and knee products for a four-year term. In our agreement with Weigao, purchase quotas and prices are set at the end of each year and the agreement may be terminated prior to its expiration in 2014 upon breach by either party, including Weigao’s failure to meet the purchase quota.We generated $120.8 million, or 44%, or our total revenue in international markets outside of the United States during the year ended December 30, 2012. Our total revenue in France was $52.7 million in 2012, $55.4 million in 2011 and $47.3 million in 2010. Our total revenue in the Netherlands was $5.3 million in 2012, $5.0 million in 2011 and $4.1 million in 2010.Research and DevelopmentWe are committed to a strong research and development program. Our research and development expenses were $22.5 million, $19.8 million and $17.9 million in 2012, 2011 and 2010, respectively. As of December 30, 2012, we had a research and development staff of over 130 people, or 14% of total employees, principally located in Montbonnot, France and Warsaw, Indiana, with additional staff in Grenoble, France; Medina, Ohio; and San Diego, CaliforniaWe have dedicated internal product development teams focused on continuous innovation and introduction of new products for extremity joint replacements, extremity joint trauma, soft tissue repair and large joint replacement. We also have an active business development team that seeks to in-license development-stage products, which our internal team assists in bringing to market. Our internal research and development teams work closely with external research and development consultantsmanufacturing and a global network of leading surgeon inventorsqualified outsourced manufacturing partners to ensure we have broad access to best-in-class ideasproduce our products and technology to drive our product development pipeline.Manufacturing and Supply substantially all of our internally-sourced products at three sites includingin four locations: Arlington, Tennessee; Montbonnot, andFrance; Grenoble, FranceFrance; and Macroom, Ireland. We lease the manufacturing facility in Arlington, Tennessee from the Industrial Development Board of the Town of Arlington. Our internal manufacturing operations are focused on product quality, continuous improvement, and efficiency. Our internal manufacturing operations have been practicing lean manufacturing concepts for many years with a philosophy focused on high productivity, flexibility, and capacity optimization. Our operations in France have a long history and deep experience with orthopaedic manufacturing and innovationprocess innovation. Additionally, we believe we are the only company to have vertically integrated operations for the manufacturing of pyrocarbon orthopaedic products. We believe that this capability gives us a competitive advantage in design for manufacturing and prototyping of this innovative material.have invested in facilities upgradesneed additional capacity. A significant portion of our lower extremities products and surgical instrumentation is produced to both expand our capabilities and establish incremental lean cellular manufacturing practices. Our Ireland location has been practicing lean cellular manufacturing conceptsspecifications by qualified subcontractors who serve medical device companies. We intend to look for many years with a philosophy focused on continuous operational improvement and optimization. In additionopportunities to optimize our internal manufacturing capabilities,capacity and insource manufacturing where we also use several outsource manufacturing partnersbelieve it makes sense to produce our products. These partners provide us with flexibilitydo so.capacity in our manufacturing operations. We continually evaluateISO 13485 and to the potential to in-source products currently purchased from outside vendors to internal production. Over time, we plan to conduct similar evaluations on our OrthoHelix product portfolio as it is currently completely outsourced to third party manufacturers.Canadian Medical Devices Conformity Assessment System (CMDCAS). We are continuously working on productaccredited by the American Association of Tissue Banks (AATB) and process improvement projectshave registrations with the FDA as a medical device establishment and as a tissue establishment. These certifications and registrations require periodic audits and inspections by various global regulatory entities to optimizedetermine if we have systems in place to ensure our manufacturingproducts are safe and effective for their intended use and that we are compliant with applicable regulatory requirements. Our quality system exists so that management has the proper oversight, designs are evaluated and tested, production processes are established and decrease product costsmaintained, and monitoring activities are in place to ensure products are safe, effective, and manufactured according to our specifications. Consequently, our quality system provides the way for us to ensure we design and build quality into our products while meeting global requirements. We are committed to meet or exceed customer needs as we strive to improve our profitability and cash flow. We believe that our manufacturing facilities and external vendor relationships will support our potential capacity needs for the foreseeable future.For example, we rely on one supplier for raw materials and select components in several of our products, including Poco Graphite, Inc., which supplies graphite for pyrocarbon on a purchase order basis; Heymark Metals Ltd., which supplies Cobalt Chrome used in certain of our hip, shoulder and elbow products on a purchase order basis; and CeramTec Group, which supplies ceramic for ceramic heads for hips on a purchase order basis.We believe we are the only vertically integrated manufacturer of pyrocarbon orthopaedic products with production equipment to enable production of larger-sized implants. While we rely on an external supplier to supply us with surgical grade substrate material, we control the remaining pyrocarbon manufacturing process, which we believe gives us a competitive advantage in design for manufacturing and prototyping of this innovative material. Towe have not experienced any significant difficultyand to use commercially reasonable efforts to assist us in locatingidentifying a new supplier and obtainingsupport the materials necessarytransfer of technology and supporting documentation to fulfillproduce this component. Our transition to a new supplier is well underway with full cooperation from the current as well as the new supplier. We believe the current supplier has produced sufficient product to more than meet our production requirements.the Tornier brandour brands for sale in certain fields of use and geographic territories. These agreements may be subject to minimum purchase or sales obligations.Our private-label distribution agreements expire between this yearobligations and 2015 and are renewable under certain conditions or by mutual agreement. These agreements are terminable by either party upon notice and such agreements include some or all of the following provisions allowing for termination under certain circumstances: (i) either party’s uncured material breach of the terms and conditions of the agreement; (ii) either party filing for bankruptcy, being bankrupt or becoming insolvent, suspending payments, dissolving or ceasing commercial activity; (iii) our inability to meet market development milestones and ongoing sales targets; (iv) termination without cause, provided that payments are made to the distributor; (v) a merger or acquisition of one of the parties by a third party; (vi) the enactment of a government law or regulation that restricts either party’s right to terminate or renew the contract or invalidates any provision of the agreement or (vii) the occurrence of a “force majeure,” including natural disaster, explosion or war.market for orthopaedic devices is highly competitive and subject to rapid and profound technological change. Our currently marketed products are, and any future products we commercialize will be, subject to intense competition. We believe that the principalprimary competitive factors in our marketsfacing us include price, quality, innovative design and technical capability, clinical results, breadth of product features and design,line, scale of operations, distribution capabilities, brand reputation, and strong customer service. OneOur ability to compete is affected by our ability to accomplish the following: factors to our future success, will be our ability to continue to introduce new products and improve existing products and technologies. In addition, we are committed to following the AdvaMed and Eucomed guidelines and codes of ethics in our interactions with customers and other healthcare professionals globally.We face competition from large diversified orthopaedic manufacturers, such as DePuy Orthopaedics, Inc., a Johnson & Johnson subsidiary, Biomet, Inc., Zimmer Corporation, and Stryker Corporation, and established mid-sized orthopaedic manufacturers, such as Arthrex, Inc., Wright Medical Group, Inc. and ArthroCare Corporation. Many of the companies developing or marketing competitive orthopaedic products enjoy several competitive advantages, including:greater financial and human resources for product development and sales and marketing;greater name recognition;established relationships with surgeons, hospitals and third-party payors;broader product lines and the ability to offer rebates or bundle products to offer greater discounts or incentives to gain a competitive advantage;established sales and marketing and distribution networks; andmore experience in conductingstrong research and development manufacturing, preparing regulatory submissionsprogram. The intent of our program is to develop new extremities and obtaining regulatory clearances or approvals for products.compete against smaller, entrepreneurial companieshave an active business development team that actively evaluates novel technologies and development stage products. In addition, our clinical and regulatory departments are devoted to verifying the safety and efficacy of our products according to regulatory standards enforced by the FDA and other international regulatory bodies. Our research and development expenses totaled $39.9 million, $25.0 million and $20.3 million in 2015, 2014 and 2013, respectively. Our research and development activities are principally located in Arlington, Tennessee; Montbonnot, France; and Warsaw, Indiana, with niche product lines. Our competitors may increase theiradditional staff in Grenoble, France; and Bloomington, Minnesota.market, which isprocedures and potential new applications for our primary strategic focus. Our competitors may develop and patent processes or products earlier than we can, obtain regulatory clearances or approvals for competing products more rapidly than we can or develop more effective or less expensive products or technologies that render our technology or products obsolete or non-competitive. We also compete with other organizations in recruiting and retaining qualified scientific and management personnel, as well as in acquiring technologies complementary to our products or advantageous to our business. If our competitors are more successful than us in these matters, we may be unable to compete successfully against our existing and future competitors.also rely uponcurrently own or have licenses to use more than 1,500 patents and pending patent applications throughout the world. We seek to aggressively protect technology, inventions, and improvements that we consider important through the use of patents and trade secrets know-how, continuing technological innovationin the United States and licensing opportunities to developsignificant foreign markets. We manufacture and maintain our competitive position. We protect our proprietary rights through a variety of methods, including confidentiality agreementsmarket products under both patents and proprietary informationlicense agreements with vendors, employees, consultantsother parties. These patents and others who maylicense agreements have accessa defined life and expire from time to proprietary information.Although we believetime. We are not materially dependent on any one or more of our patents. In addition to patents, are valuable, our knowledge and experience, our creative product development, and marketing staff and our trade secret information, with respect to manufacturing processes, materials and product design, have been equallyare as important as our patents in maintaining our proprietary product lines. As a condition of employment,generally require employeesbelieve that, in the aggregate, our patents are valuable, and patent protection is beneficial to execute a confidentiality agreement relatingour business and competitive positioning, our patent protection will not necessarily deter or prevent competitors from attempting to proprietary information and assigning patent rights to us. We cannotdevelop similar products. There can be assuredno assurances that our patents will provide competitive advantages for our products or that our competitors will not challenge or circumvent these rights. In addition, we cannotthere can be assuredno assurances that the United States Patent and Trademark Office or USPTO,(USPTO) or foreign patent offices will issue any of our pending patent applications. The USPTO andalso may rejectdeny or require a significant narrowing of the claims in our pending patent applications affectingand the patents issuing from the pending patentsuch applications. Any patents issuing from ourthe pending patent applications may not provide us with significant commercial protection. We could incur substantial costs in proceedings before the USPTO or foreign patent offices, including opposition and other post-grant proceedings. These proceedings could result in adverse decisions as to the validitypatentability, priority of our inventions.inventions, and the narrowing or invalidation of claims in issued patents. Additionally, the laws of some of the countries in which our products are or may be sold may not protect our products and intellectual property to the same extent as the laws in the United States or at all.third parties,others, we cannotare currently subject to patent infringement litigation and there can be assuredno assurances that we do not infringe upon any patents or other proprietary rights held by third parties.them. If our products were found to infringe upon any proprietary right of a thirdanother party, we could be required to pay significant damages or license fees to the thirdsuch party and/or cease production, marketing, and distribution of those products. Litigation also may be necessary to defend infringement claims of third parties or to enforce patent rights we hold or to protect trade secrets or techniques we own.The Leahy-Smith America Invents Act, or the Leahy-Smith Act, which was adopted in September 2011, includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. Under the Leahy-Smith Act, the U.S. will transition from a “first-to-invent” system to a “first-to-file” system for patent applications filed on or after March 16, 2013. The USPTO is currently developing regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act have recently become effective. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. also rely on trade secrets and other unpatented proprietary technology. We cannotThere can be assuredno assurances that we can meaningfully protect our rights in our unpatented proprietary technology or that others will not independently develop substantially equivalent proprietary products or processes or otherwise gain access to our proprietary technology. seek to protect our trade secretsproprietary rights through a variety of methods. As a condition of employment, we generally require employees to execute an agreement relating to the confidential nature of and company ownership of proprietary know-how,information and assigning intellectual property rights to us. We generally require confidentiality agreements with vendors, consultants, and others who may have access to proprietary information. We generally limit access to our facilities and review the release of company information in part, withadvance of public disclosure. There can be no assurances, however, that confidentiality agreements with employees, vendors, and consultants. We cannot be assured, however, that the agreementsconsultants will not be breached, that we will have adequate remedies for any breach would be available, or that our competitors will not discover or independently develop our trade secrets.Regulatory MattersFDA Litigation also may be necessary to protect trade secrets or techniques we own.Both beforeafter approval or clearance our productsJapan, government regulation is significant and, product candidateswe believe there is a general trend toward increased and more stringent regulation throughout the world. As a manufacturer and marketer of medical devices, we are subject to extensive regulation. Inregulation by the U.S. Food and Drug Administration, other federal governmental agencies, and state agencies in the United States we are regulated byand similar foreign governmental authorities in countries located outside the FDA underUnited States. These regulations generally govern the U.S. Federal Food, Drugintroduction of new medical devices; the observance of certain standards with respect to the design, manufacture, testing, labeling, promotion, and Cosmetic Act,sales of the devices; the maintenance of certain records; the ability to track devices; the reporting of potential product defects; the import and export of devices; as well as other regulatory bodies. Thesematters. In addition, as a participant in the healthcare industry, we are also subject to various other U.S. federal, state, and foreign laws.govern, amongand other things,requirements in the following activities in which wefuture could expose us to significant liability, including, but not limited to, exclusion from federal healthcare program participation, including Medicaid and our contract manufacturers, contract testing laboratoriesMedicare, potential prosecution, civil and suppliers are involved:product development;product testing;product clinical trial compliance;product manufacturing;product labeling;product safety;product safety reporting;product storage;product market clearancecriminal fines or approval;product modifications;product advertisingpenalties, as well as additional litigation cost and promotion;product import and export; andproduct sales and distribution.FailureWe strive to comply with regulatory requirements governing our products and operations and to conduct our affairs in an ethical manner. This practice is reflected in our Code of Business Conduct, various other compliance policies and through the Federal Food, Drugresponsibility of the nominating, corporate governance and Cosmetic Actcompliance committee of our board of directors, which oversees our corporate compliance program and compliance with legal and regulatory requirements as well as our ethical standards and policies. We devote significant time, effort, and expense to addressing the extensive government and regulatory requirements applicable to our business. Such regulatory requirements are subject to change and we cannot predict the effect, if any, that these changes might have on our business, financial condition, and results of operations. Governmental regulatory actions against us could result in among other things, warning letters, civil penalties, delays in approving or refusal to approve a product, candidate, productthe recall productor seizure interruption of our products, suspension or revocation of the authority necessary for the production operating restrictions, suspension on withdrawalor sale of product approval, injunctions orour products, litigation expense, andprosecution.FDA Approval or Clearancepenalties against us and our officers and employees. If we fail to comply with these regulatory requirements, our business, financial condition, and results of Medical Devicesdevicesdevice products. Our tissue-based products are subject to varying degreesFDA regulations, the National Organ Transplant Act (NOTA), and various state agency regulations. We are an accredited member of regulatory controlthe American Association of Tissue Banks and are classified in onean FDA-registered tissue establishment, which includes the packaging, processing, storage, labeling, and distribution of three classes depending on risktissue products regulated as medical devices and the extentstorage and distribution of controlstissue products regulated solely as human cell and tissue products. In addition, we maintain tissue bank licenses in Florida, Maryland, New York, California, and Oregon.determines are necessary to reasonably ensure their safety and efficacy. These classifications generally require the following:Class I: general controls, such as labeling and adherence to quality system regulations;Class II: general controls,must be obtained through either a premarket notification (510(k)) and special controls such as performance standards, patient registries and post-market surveillance; andClass III: general controls andunder Section 510(k) of the FDC Act or the approval of a de novo or premarket approval or a PMA.new products fall intoare FDA classificationscleared through the 510(k) premarket notification process. The FDA typically grants a 510(k) clearance if the applicant can establish that require the submissiondevice is substantially equivalent to a predicate device. It usually takes about three months from the date of a premarket notification (510(k))510(k) submission to the FDA. In theobtain clearance, but it may take longer, particularly if a clinical trial is required. The FDA may find that a 510(k) process, the FDA reviews a premarket notification and determines whether a proposed device is “substantially equivalent” to a previously cleared 510(k) devicenot appropriate or a device that was in commercial distribution before May 28, 1976, for which the FDAsubstantial equivalence has not yet called for the submission of been shown and, as a result, require a de novo or PMA application.referredmust be supported by valid scientific evidence to as a “predicate” device. In making this determination,demonstrate the FDA compares the proposed device to the predicate device. If the two devices are comparable in intended use and safety and effectiveness of the device, may be cleared for marketing. 510(k) submissions generally include, among other things,typically including the results of human clinical trials, bench tests, and laboratory and animal studies. The PMA application must also contain a complete description of the device and its manufacturing, device labeling, medical devicescomponents, and a detailed description of the methods, facilities, and controls used to whichmanufacture the device is substantially equivalent, safety and biocompatibility information anddevice. In addition, the results of performance testing. In some cases, a 510(k) submission must include data from human clinical studies. Marketing may commence only whenthe proposed labeling and any training materials. The PMA application process is expensive and generally takes significantly longer than the 510(k) process. Additionally, the FDA issues a clearance letter findingmay never approve the proposed device to be substantially equivalent to the predicate. After a device receives 510(k) clearance, any product modification that could significantly affect the safety or effectiveness of the product, or any product modification that would constitute a significant change in intended use, requires a new 510(k) clearance. If the device would no longer be substantially equivalent, it would require a PMA. If the FDA determines that the product does not qualify for 510(k) clearance, then the company must submit and the FDA must approve a PMA before marketing can begin.Other devices we may develop and market may be classified as Class III for which the FDA has implemented stringent clinical investigation and PMA requirements. The PMA process would require us to provide clinical and laboratory data that establishes that the new medical device is safe and effective in an absolute sense as opposed to in a comparative sense as with a 501(k). Information about the device and its components, device design, manufacturing and labeling, among other information, must also be included in the PMA.application. As part of the PMA application review process, the FDA generally will typically inspectconduct an inspection of the manufacturer’s facilities forto ensure compliance with applicable quality system regulation (QSR)regulatory requirements, which governinclude quality control testing, documentation control, documentation and other aspects of quality assurance with respect to manufacturing. The FDA will approve the new device for commercial distribution if it determines that the data and information in the PMA constitute valid scientific evidence and that there is reasonable assurance that the device is safe and effective for its intended use(s). Theprocedures. A PMA can include post-approval conditions including, among other things, restrictions on labeling, promotion, sale and distribution, data reporting (surveillance), or requirements to do additional clinical studies post-approval. Even after approval of a PMA, the FDA must grant subsequent approvals for a new PMA or a PMA supplement is required to authorize certain modifications to the device, its labeling, or its manufacturing process.All of our devices marketed in the United States have been listed, cleared or approved by the FDA. Some low-risk medical devices do not require FDA review and approval or clearance prior to commercial distribution, but are subject toFDA regulations and must be listed with the FDA. The FDA has the authority to: halt the distribution of certain medical devices; detain or seize adulterated or misbranded medical devices; or order the repair, replacement of or refund the costs of such devices. There are also requirements of state, local and foreign governments that we must comply with in the manufacture and marketing of our products. For example, some jurisdictions require compliance with the Pharmaceutical Research and Manufacturers of America’s Code on Interactions with Healthcare Professionals or its equivalent. Laws and regulations and the interpretation of those laws and regulations may change in the future. We cannot foresee what affect, if any, such changes may have on us.Specifically, FDA plans to issue new guides on several important topics in 2013. First, the interpretation and application of regulation regarding the custom device exemption has been a topic that both manufacturers and FDA have regarded with interest over recent years. FDA has taken action in the form of issuing 483’s to manufacturers that have applied the custom device exemption in a manner that FDA interprets to be in conflict with law. Tornier makes use of the custom device exemption in some limited circumstances, and in a manner Tornier believes to be in compliance with law. FDA plans to issue new guidance in 2013 that could affect the way we deliver these products to customers.FDA has also informed medical device manufacturers of new policies to be enacted in 2013 that could affect the ability to gain clearance for new products. Specifically, FDA will adopt through agency guidance new practices related to the acceptance of 510(k) applications which could place a high standard on data and evidence provided to the FDA. In addition, the FDA has expanded the pre-IDE process, encouraging manufacturers to request a meeting where 510k applications can be reviewed prior to submission. Finally, FDA has informed industry that a previous policy regarding questions and responses 510k applications will become more restricted, allowing fewer opportunities to respond to questions prior to automatic designation of devices to PMA.Clinical Trialsaremay be required to support a 510(k) application or a de novo submission and almost always are required to support a PMA application and are sometimes required to support a 510(k) submission.application. Clinical trials of unapproved or uncleared medical devices or devices being studied for uses for which they are not approved or cleared (investigational devices) must be conducted in compliance with FDA requirements. If an investigationalhuman clinical trials of a medical device could poseare required and the device presents a significant risk, to patients, the sponsor companyof the trial must submit an application forfile an investigational device exemption or IDE, to the FDA(IDE) application prior to initiation of thecommencing human clinical study. Antrials. The IDE application must be supported by appropriate data, such astypically including the results of animal andand/or laboratory test results, showing that ittesting. If the IDE application is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE will automatically become effective 30 days after receiptapproved by the FDA unlessand one or more institutional review boards (IRBs), human clinical trials may begin at a specific number of institutional investigational sites with the specific number of patients approved by the FDA. If the device presents a non-significant risk to the patient, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate approval from the FDA. Submission of an IDE does not give assurance that the FDA notifieswill approve the companyIDE. If an IDE is approved, there can be no assurance the FDA will determine that the investigationdata derived from the trials support the safety and effectiveness of the device or warrant the continuation of clinical trials. An IDE supplement must be submitted to and approved by the FDA before a sponsor or investigator may not begin. In addition, clinical trialsmake a change to the investigational plan in such a way that may affect its scientific soundness, study indication, or the rights, safety or welfare of investigational devices may not begin until an institutional review board, or IRB, has approved the study. for investigator selection, trial monitoring, adverse event reporting, and recordkeeping. The investigators must obtain patient informed consent, rigorously follow the investigational plan and trial protocol, control the disposition of investigational devices, and comply with reporting and recordkeeping requirements. We, the FDA and the IRB at each institution at which a clinical trial is being conducted may suspend a clinical trial at any time for various reasons, including a belief that the subjects are being exposed to an unacceptable risk. During the approval or clearance process, the FDA typically inspects the records relating to the conductWe are currently conducting a few clinical trials.apply.apply and we continue to be subject to inspection by the FDA to determine our compliance with these requirements, as do our suppliers, contract manufacturers, and contract testing laboratories. These requirements include, but are not limited to:QSR regulation,following:governs,govern, among other things, how manufacturers design, test, manufacture, modify, label, exercise quality control over and document manufacturing of their products;Part 11 compliance with FDA required records of documents in your quality system defined as “in scope”;the Medical Device Reporting (MDR) regulation, which requires reporting to the FDA certain adverse experiences associated with use of the product;results;results. Payment Act and various state laws on reporting remunerative relationships with health carehealthcare customers.We continue These laws impact the kinds of financial arrangements we may have with hospitals, surgeons or other potential purchasers of our products. They particularly impact how we structure our sales offerings, including discount practices, customer support, education and training programs, physician consulting, research grants and other arrangements. These laws are administered by, among others, the U.S. Department of Justice, the Office of Inspector General of the Department of Health and Human Services and state attorneys general. Many of these agencies have increased their enforcement activities with respect to medical device manufacturers in recent years. If our operations are found to be in violation of these laws, we may be subject to inspectionpenalties, including potentially significant criminal, civil and/or administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations.to determine our compliancein cooperation with U.S. Customs and Border Protection, administers controls over the import of medical devices into the United States. The U.S. Customs and Border Protection imposes its own regulatory requirements as doon the import of our suppliers, contract manufacturersproducts, including inspection and contract testing laboratories.In January 2013, our OrthoHelix facility located in Medina, Ohio waspossible sanctions for noncompliance. We are also subject to a routine FDA inspection. The inspection resulted inforeign trade controls administered by certain U.S. government agencies, including the issuanceBureau of a Form FDA-483 listing four inspectional observations. The FDA’s observations related to our documentationIndustry and Security within the Commerce Department and the Office of corrective and preventative actions, procedures for receiving, reviewing and evaluating complaints, procedures to control product that does not conform to specified requirements and procedures to ensure that all purchased or otherwise received product and services conform to specified requirements. We believeForeign Assets Control within the Treasury Department. have corrected all four of these observations.International RegulationWe are subject to regulations and product registration requirementsgovernment regulation in many foreignthe countries in which we mayoperate and sell our products, including in the areas of:design, development,products. We must comply with extensive regulations governing product approvals, product safety, quality, manufacturing, and testing;product standards;product safety;product safety reporting;marketing, sales and distribution;packaging and storage requirements;labeling requirements;content and language of instructions for use;clinical trials;record keeping procedures;advertising and promotion;recalls and field corrective actions;post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they werereimbursement processes in order to recur, could lead to death or serious injury;import and export restrictions; andtariff regulations, duties and tax requirementsregistration for reimbursementnecessity of testing performed in country by distributors for licenses.The time required to obtain clearance required by foreign countries may be longer or shorter than that required for FDA clearance, and requirements for licensing a product in a foreign country may differ significantly from FDA requirements.In many of the foreign countries in which we market our products we are subject to local regulations affecting, among other things, design and product standards, packaging requirements and labeling requirements. Manyin all major foreign markets. Although many of the regulations applicable to our devices and products in these countries are similar to those of the FDA.InFDA, these regulations vary significantly from country to country and with respect to the EEA,nature of the particular medical device. The time required to obtain foreign approvals to market our products may be longer or shorter than the time required in the United States, and requirements for such approvals may differ from FDA requirements.essential requirementsEuropean Medical Device Directives and to obtain CE mark certification. CE mark certification is the European symbol of adherence to quality assurance standards and compliance with applicable European Medical Device Directives. Under the EUEuropean Medical DevicesDevice Directives, (Council Directive 93/42/EEC of 14 June 1993 concerningall medical devices as amended, and Council Directive 90/385/EEC of 20 June 2009 relating to active implantable medical devices, as amended). Compliance with these requirements entitles us to affix themust qualify for CE conformity mark to our medical devices, without which they cannot be commercialized in the EEA. In order to demonstrate compliance with the essential requirements andmarking. To obtain the right to affix the CE conformity mark we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low-risk medical devices (Class I), where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the Medical Devices Directives, a conformity assessment procedure requires the intervention of a Notified Body, which is an organization accredited by a Member State of the EEA to conduct conformity assessments. The Notified Body would typically audit and examine the quality system for the manufacture, design and final inspection of our devices before issuing a certification demonstrating compliance with the essential requirements. Based on this certification we can draw up an EC Declaration of Conformity, which allows usauthorization to affix the CE mark to one of our products.Tornier anticipates that revised regulation of medical devices will be made applicable in 2014. We expect this revised regulationproducts, a recognized European Notified Body must assess our quality systems and the product’s conformity to include further controls and requirements on the following activities:High level of request for premarket clinical evidence for high risk devicesIncreased scrutiny of technical files for class IIb devicesMonitoring of notified bodies, by independent auditorsIncrease requirements regarding vigilance and product traceability (specifically related to labeling requirements)Increased regulation for non-traditional roles such as importer and distributor.U.S. Anti-Kickback and False Claims LawsIn the United States, there are federal and state anti-kickback laws that prohibit the payment or receipt of kickbacks, bribes or other remuneration intended to induce the purchase or recommendation of healthcare products and services. Violations of these laws can lead to civil and criminal penalties, including exclusion from participation in federal healthcare programs. These laws are potentially applicable to manufacturers of products regulated by the FDA, such as us, and hospitals, physicians and other potential purchasers of such products.In particular, the federal Anti-Kickback Law prohibits persons from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, to induce either the referral of an individual, or the furnishing, recommending, or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. The definition of “remuneration” has been broadly interpreted to include anything of value, including for example, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash, waivers of payments, ownership interests and providing anything at less than its fair market value. In addition, the recently enacted Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, collectively, the PPACA, among other things, amends the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claim statutes. The lack of uniform interpretation of the Anti-Kickback Law makes compliance with the law difficult. Thepenalties for violating the Anti-Kickback Law can be severe. These sanctions include criminal penalties and civil sanctions, including fines, imprisonment and possible exclusion from participation in federal healthcare programs.Recognizing that the Anti-Kickback Law is broad and may technically prohibit many innocuous or beneficial arrangements within the healthcare industry, the U.S. Department of Health and Human Services issued regulations in July1991, which the Department has referred to as “safe harbors.” These safe harbor regulations set forth certain provisions which, if met in form and substance, will assure medical device manufacturers, healthcare providers and other parties that they will not be prosecuted under the federal Anti-Kickback law. Additional safe harbor provisions providing similar protections have been published intermittently since 1991. Our arrangements with physicians, hospitals and other persons or entities who are in a position to refer may not fully meet the stringent criteria specified in the various safe harbors. Although full compliance with these provisions ensures against prosecution under the federal Anti-Kickback Law, the failure of a transaction or arrangement to fit within a specific safe harbor does not necessarily mean that the transaction or arrangement is illegal or that prosecution under the federal Anti-Kickback law will be pursued. Even though we continuously strive to comply with the requirements of the Anti-Kickback Law, liability under the Anti-Kickback Law may still arise because of the intentions or actions of the parties with whom we do business, including our independent distributors. While weEuropean Medical Device Directives. We are not aware of any such intentions or actions, we have only limited knowledge regarding the intentions or actions underlying those arrangements. Conduct and business arrangements that do not fully satisfy one of these safe harbor provisions may result in increased scrutiny by government enforcement authorities.The U.S. False Claims Act was enacted in 1865 during the Civil Warsubject to address government suppliers who would submit false claims for payment to the government. So if the government ordered and paid for a shipment of 5,000 blankets, guns or vials of medicine and only received 3,750 of each, the claim for payment was fraudulent. The Congress passed the False Claims Act (FCA) to address this issue and it is a civil statute that is today applied mostly to purchasesinspection by the Department of Defense andNotified Bodies for health care reimbursement. It is designed to penalize individuals who would seek reimbursement where none or a lesser amount is due. It cancompliance with these requirements. We also be turned into a criminal prosecution if the government pursues mail and wire fraud in the furtherance of the acts alleged to be false claims. For example, if a company were to illegally promote for an off-label use leading to an off-label prescription and an inappropriate reimbursement, that could trigger the FCA. In addition, the FCA seeks to prevent miscoding, stretched coding, the use of inappropriate modifiers, or seeking reimbursement for an inappropriate care setting (e.g. in-patient versus outpatient), or other forms of improper reimbursement. A company can be exposed to liability for the inappropriate provision of reimbursement services, reimbursement advice or promoting the inappropriate use of codes.The PPACA also includes new reporting and disclosure requirements on device and drug manufacturers for any “transfer of value” made or distributed to physicians, healthcare providers or hospitals, which are scheduled to become effective March 31, 2013 (known as the “Physician Sunshine Payment Act”). These provisions require, among other things, extensive tracking and maintenance of databases regarding the disclosure of relationships and payments to physicians, healthcare providers and hospitals. In addition, certain states have recently passed or are considering legislation restricting our interactions with health care providers and/or requiring disclosure of many payments to them. Failurerequired to comply with these new trackingregulations of other countries in which our products are sold, such as obtaining Ministry of Health Labor and reportingWelfare approval in Japan, Health Protection Branch approval in Canada and Therapeutic Goods Administration approval in Australia.could subject usand regulations, including those relating to significant civil monetary penalties.Other provisionsthe use, registration, handling, storage, disposal, recycling and human exposure to hazardous materials and discharges of state and federal law provide civil and criminal penalties for presenting, or causing to be presented, to third-party payors for reimbursement, claims that are false or fraudulent, or that are for items or services that were not provided as claimed. Although our business is structured to comply with these and other applicable laws, it is possible that some of our business practicessubstances in the future could beair, water and land. For example, in France, requirements known as the Installations Classées pour la Protection de l’Environnement regime provide for specific environmental standards related to industrial operations such as noise, water treatment, air quality, and energy consumption. In Ireland, our manufacturing facilities are likewise subject to scrutinylocal environmental regulations, such as related to water pollution and challengewater quality, which are administered by federal or state enforcement officials under these laws. This type of challenge could have a material adverse effect on our business, financial condition and results of operations.Many of these policies and regulations also apply to jurisdictionsthe Environmental Protection Agency.USA. Specifically,United States are subject to various other laws such as those regarding recordkeeping and privacy; laws regarding sanctioned countries, entities and persons; customs and import-export, and laws regarding transactions in foreign countries. We are also subject to the Physician Sunshine PaymentU.S. Foreign Corrupt Practices Act, is expectedwhich generally prohibits covered entities and their intermediaries from engaging in bribery or making other prohibited payments to be implemented in 2013 regardingforeign officials for the transparencypurpose of payments from industry to healthcare practitioners.Coverage and ReimbursementWe anticipate that sales will depend in large part on the availability of coverage and reimbursement from third-party payors. Third-party payors include governmental programs such as U.S. Medicare and Medicaid, private insurance plans, and workers’ compensation plans. These third-party payors may deny coverage or reimbursement for a product or therapyprocedure if they determine that the product or therapyprocedure was not medically appropriate or necessary. The third-partyThird-party payors also may place limitations on the types of physicians that can perform specific types of procedures.procedures or the care setting in which the procedure is performed, i.e., out-patient or in-hospital. Also, third-party payors are increasingly auditing and challenging the prices charged for medical products and services.services with concern for upcoding, miscoding, using inappropriate modifiers, or also approve coverage for new or innovative devices or therapiesprocedures before they will reimbursedeviceproduct until reimbursement approval has been obtained from governmental and private third-party payors.or CMS,(CMS), the agency responsible for administering the Medicare program, sets coverage and reimbursement policies for the Medicare program in the United States. CMS policies may alter coverage and payment related to our product portfolioproducts in the future. These changes may occur as the result of national coverage determinations issued by CMS or as the result of local coverage determinations by contractors under contract with CMS to review and make coverage and payment decisions. Medicaid programs are funded by both U.S. federal and state governments, may vary from state to state and from year to year and will likely play an even larger role in healthcare funding pursuant to the PPACA.those procedures using our products, is the existence of a Current Procedural Terminology or CPT,(CPT) code. To receive payment, health carehealthcare practitioners must submit claims to insurers using these codes for payment for medical services. CPT codes are assigned, maintained and annually updated by the American Medical Association and its CPT Editorial Board. If the CPT codes that apply to the procedures performed using our products are changed, reimbursement for performances of these procedures may be adversely affected.In the United States, some insured individuals enroll in managed care programs, which monitor and often require pre-approval of the services that a member will receive. Some managed care programs pay their providers on a per capita (patient) basis, which puts the providers at financial risk for the services provided to their patients by paying these providers a predetermined payment per member per month and, consequently, may limit the willingness of these providers to use our products.the governmentgovernments and private health insurance has led to, and will continue to lead to, increased pressures on the healthcare and medical device industry to reduce the costs of products and services. All third-party reimbursement programsThird-party payors are developing increasingly sophisticated methods of controlling healthcare costs through healthcare reform legislation and measures including, government-managed healthcare systems, health technology assessments, coverage with evidence development processes, quality initiatives, pay-for-performance, comparative effectiveness research, prospective reimbursement, and capitation programs, group purchasing, redesign of benefits,benefit offerings, requiring pre-approvals and second opinions prior to major surgery,procedures, careful review of bills, encouragement of healthier lifestyles and other preventative services, and exploration of more cost- effectivecost-effective methods of delivering healthcare. All of these types of programs can potentially impact market access for, and pricing structures of our products, which in turn, can impact our future sales. There can be no assurance that third-party reimbursement and coverage will be available or adequate, or that current and future legislation, regulation or reimbursement policies of third-party payors will not adversely affect the demand for our products or our ability to sell theseour products on a profitable basis. The unavailability or inadequacy of third-party payor coverage or reimbursement could have a material adverse effect on our business, operating results, and financial condition.In international markets,assuranceassurances that procedures using our products will be considered medically reasonable and necessary for a specific indication, that our products will be considered cost-effective by third-party payors, that an adequate level of reimbursement will be available, or that the third-party payors’ reimbursement policies will not adversely affect our ability to sell our products profitably.perceived continuing increase in request for clinical data for the supportbelieve our costs of registrationcomplying with current and reimbursement outside the USfuture environmental laws, regulations and Europe. Morepermits and more, local, product specific reimbursement law is applied as an overlayour liabilities arising from past or future releases of, or exposure to, medical device regulation, which has provided an additional layerhazardous substances will not materially adversely affect our business, results of clearance requirement. Specifically, Australia now requires clinical data for clearance and reimbursementoperations, or financial condition, although there can be no assurances of this.formthird quarter than throughout the rest of prospective, multi-center studies,the year as many of our products are used in elective procedures, which generally decline during June, July, and August. This typically results in our selling, general and administrative expenses and research and development expenses as a high bar not previously applied.percentage of our net sales that are higher during third quarter than throughout the rest of the year. In addition, our first quarter selling, general and administrative expenses include additional expenses that we incur in France, certainconnection with the annual meeting held by the American College of Foot and Ankle Surgeons (ACFAS) and the American Academy of Orthopaedic Surgeons (AAOS). During these three-day events, we display our most recent and innovative devices (such as some made from pyrolytic carbon from Tornier), have been identified as needingproducts.provide clinical evidencebe material to support a “mark-specific” reimbursement.30, 2012,27, 2015, we had 916 employees, including 344 in manufacturing and operations, 132 in research and development and2,295 employees. We believe that we have a good relationship with our employees.remaining in sales, marketing and related administrative support. Of our 916 worldwide employees, 295 employees were located in the United States and 621 employees were located outsidelaws of the United States, primarilyNetherlands. We were initially formed as a private company with limited liability (besloten vennootschap) in France and Ireland.Financial Information about Geographical AreasSee Note 13 to our consolidated financial statements for information regarding our revenues and long-lived assets by geographic area.Available InformationFred. Roeskestraat 123, 1076 EEPrins Bernhardplein 200, 1097 JB Amsterdam, Thethe Netherlands. Our telephone number at this address is (+ 31) 20 577 1177.675-4002. Our agent for service of process in the United States is CT Corporation, 1209 Orange St.,Street, Wilmington, Delaware 19801. Our corporate website is located at www.tornier.com.web site,corporate website, our annual reportsAnnual Reports on Form 10-K, quarterly reportsQuarterly Reports on Form 10-Q, current reportsCurrent Reports on Form 8-K, and any amendments to any such reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after wethey are electronically file such materialfiled with or furnish itfurnished to the Securities and Exchange Commission.The following is a discussionIn addition to the other information set forth in this report, careful consideration should be taken of the specific risks thatfactors described below, which could materially adversely affect our business, financial condition or operating results:Businessfuture success also will depend in part upon our ability to retain key employees. Competition for qualified personnel can be very intense. In addition, key employees may depart because of issues relating to the uncertainty or difficulty of integration or a desire not to remain with our company. Accordingly, no assurances can be given that we will retain key employees.Our Industrynegative cash flow and may never achieve or sustain profitability.30, 2012,27, 2015, we had an accumulated deficit of $235.7$774 million. Our ability to achieve profitability will be influenced by many factors, including, among others, the success of our recently completed Wright/Tornier merger; the extent and duration of our future operating losses,losses; the level and timing of future revenuenet sales and expenditures,expenditures; development, commercialization and market acceptance of new products,products; the results and scope of ongoing research and development projects,projects; competing technologies and market developments anddevelopments; regulatory requirements and delays.delays; anddo not successfully develop and market new products and technologies and implementare wrong, our business strategy, our business andfuture operating results, maycash flows, and prospects could be adversely affected.We may not be able to successfully implement our business strategy. To implement our business strategy we need to, among other things, develop and introduce new extremity joint products, find new applications for and improve our existing products, properly identify and anticipate our surgeons’ and their patients’ needs, obtain regulatory clearances or approvals for new products and applications and educate surgeons about the clinical and cost benefits of our products.We are continually engaged in product development and improvement programs, and we expect new products to account for a significant portion of our future growth. If we do not continue to introduce new products and technologies, or if those products and technologies are not accepted, we may not be successful. Moreover, research and development efforts may require a substantial investment of time and resources before we are adequately able to determine the commercial viability of a new product, technology, material or innovation. Demand for our products also could change in ways we may not anticipate due to evolving customer needs, changing demographics, slow industry growth rates, evolving surgical philosophies and evolving industry standards, among others. Additionally, our competitors’ new products and technologies may precede our products to market, may be more effective or less expensive than our products or may render our products obsolete. Our new products and technologies also could render our existing products obsolete and thus adversely affect sales of our existing products.Our targeted surgeons practice in areas such as shoulder, upper extremities, lower extremities, sports medicine and reconstructive and general orthopaedics, and our strategy of focusing exclusively on these surgeons may not be successful. In we are seeking to increase our international revenue and will need to increase our worldwide direct sales force and enter into distribution agreements with third parties in order to do so. All of this may result in additional or different foreign regulatory requirements, with which we may not be able to comply. Moreover, even if we successfully implement our business strategy, our operating results may not improve. We may decide to alter or discontinue aspects of our business strategy and may adopt different strategies due to business or competitive factors.We rely on our distributors, independent sales agencies and their representatives to market and sell our products in certain territories. A failure to maintain our existing relationships with or changes and transitions with respect to our distributors, independent sales agencies and their representatives could have an adverse effectbiologics product portfolio is AUGMENT® Bone Graft, which is based on our operating results.Inrecombinant human platelet-derived growth factor (rhPDGF-BB), a synthetic copy of one of the United States, we sell our products primarily through a sales channel consistingbody’s principal healing agents. We obtained FDA approval of mostly independent commission-based sales agencies, which utilize several sales representatives, with some direct sales organizations in certain territories. Internationally, we utilize several distribution approaches depending on the individual market requirements, including direct sales organizations in the largest European markets, Australia and Japan and independent distributors for most other international markets. Our distributors and sales agencies do not sell our products exclusively and may offer similar products from other orthopaedic companies. In 2012, no individual distributor or sales agency accounted for more than 7% of our global revenue. Our success depends largely upon our ability to motivate our distributors and sales agencies to sell our products. Additionally, we depend on their sales and service expertise and relationships with the surgeons in the marketplace. We also rely upon their compliance with federal laws and regulations such as with the advertising and promotion regulations under the federal Food, Drug and Cosmetic Act, the Anti-kickback Statute, the False Claims Act, the Physician Sunshine Payments Act and other applicable state laws. Our distributors and independent sales agencies may terminate their contracts with us, may devote insufficient sales efforts to our products or may focus their sales efforts on other products that produce greater commissions for them. If our relationship with any of our distributors or sales agencies terminated, we could enter into agreements with existing distributors and sales agencies to take on the related sales, contract with new distributors and new sales agencies, or hire direct sales representatives or a combination of these options. A failure to maintain our existing relationships with or changes and transitions with respect to our distributors and independent sales agencies and their representatives could have an adverse effect on our operations and operating results. We do not control our distributors or independent sales agencies and they may not be successful in implementing our marketing plans.During 2012, we terminated our sales relationships with a few independent sales agenciesAUGMENT® Bone Graft in the United States that were not performingfor ankle and/or hindfoot fusion indications during third quarter of 2015. AUGMENT® Bone Graft is currently available for sale as an alternative to our expectations. This resultedautograft in some disruption in our United States sales channel and adversely affected our revenues during 2012. During 2013, we may terminate our sales relationships with additional independent sales agencies and some of our distributors that are not performing to our expectations and it is possible that such actions will result in further disruption in our United States sales channel, disruption in certain countries outside the United States and adversely affect our revenues and other operating results during 2013. It is also possible that we may become subject to litigation and incur future charges and cash expenditures in connection with such independent sales agency and distributor changes and transitions, which charges and cash expenditures would adversely affect our operating results.In November 2012, Douglas W. Kohrs, our former President and Chief Executive Officer, resigned as a director, officer and employee of Tornier. Mr. Kohrs had built strong relationships with several of our key physicians, customers, distributors, sales representatives and employees. Accordingly, this change in our senior management may adversely affect our relationships with these individuals and have a material adverse effect on our business.Our recently completed facilities consolidation initiative may not result in anticipated operational efficiencies, expense savings and other benefits and could have an unintended adverse impact on our business.We recently implemented a facilities consolidation initiative pursuant to which we consolidated a number of our facilities in France, Ireland and the United States. The facilities consolidation initiative was driven by our strategy to drive operational productivity and to realize operating costs savings beginning in 2013. Under the initiative, we consolidated our Dunmanway, Ireland manufacturing facility into our Macroom, Ireland manufacturing facility and our St. Ismier, France manufacturing facility into our existing Montbonnot, France manufacturing facility. We also leased a new facility located in Bloomington, Minnesota to use as our U.S. business headquarters and consolidated our Minneapolis-based marketing, training, regulatory, clinical, supply chain and corporate functions with our Stafford, Texas-based distribution operations. In connection with the facilities consolidation, we recorded pre-tax charges, comprised of one-time employee termination costs; facility closure, moving and related expenses; fixed asset write-offs net of anticipated proceeds from the sale of facilities in Stafford, Texas and Dunmanway, Ireland; and other miscellaneous related charges during 2012, aggregating in $6.4 million of expense for 2012. Since the facilities consolidation is complete, we do not expect to record any significant additional expense related to the facilities consolidation during 2013. Although we continue to believe that the facilities consolidation will result in anticipated operational efficiencies, expense savings and other benefits that we believe should positively impact our business and operating results beginning in 2013, we may be incorrect. If the facilities consolidation results in unanticipated expenses and charges, including litigation expenses, and has unintended impacts on our business, including in particular our new product development efforts, or if does not produce the anticipated operational efficiencies, expense savings and other benefits, we may disappoint investors and it is possible that further restructuring activities might become necessary, resulting in additional future charges.We may be unable to compete successfully against our existing or potential competitors, in which case our revenue and operating results may be negatively affected and we may not grow.The market for orthopaedic devices is highly competitive and subject to rapid and profound technological change. Our success depends, in part, on our ability to maintain a competitive position in the development of technologies and products for use by our customers. We face competition from large diversified orthopaedic manufacturers, such as DePuyOrthopaedics, Inc., a Johnson & Johnson subsidiary, Zimmer Corporation and Stryker Corporation, and established mid-sized orthopaedic manufacturers, such as Arthrex, Inc., Wright Medical Group, Inc. and ArthroCare Corporation. Many of the companies developing or marketing competitive orthopaedic products enjoy several competitive advantages, including:greater financial and human resources for product development and sales and marketing;greater name recognition;established relationships with surgeons, hospitals and third-party payors;broader product lines and the ability to offer rebates or bundle products to offer greater discounts or incentives to gain a competitive advantage;established sales and marketing and distribution networks; andmore experience in conducting research and development, manufacturing, preparing regulatory submissions and obtaining regulatory clearances or approvals for products.We also compete against smaller, entrepreneurial companies with niche product lines. Our competitors may increase their focus on the extremities market, which is our primary strategic focus. Our competitors may develop and patent processes or products earlier than us, obtain regulatory clearances or approvals for competing products more rapidly than us and develop more effective or less expensive products or technologies that render our technology or products obsolete or non-competitive. We also compete with other organizations in recruiting and retaining qualified scientific and management personnel, as well as in acquiring technologies and technology licenses complementary to our products or advantageous to our business. If our competitors are more successful than us in these matters, we may be unable to compete successfully against our existing or future competitors.We derive a significant portion of our revenue from operations in international markets that are subject to political, economic and social instability.We derive a significant portion of our revenue from operations in international markets. Our international distribution system consists of 12 direct sales offices and approximately 30 distribution partners, who sell in approximately 40 countries. Most of these countries are, to some degree, subject to political, economic and social instability. For the year ended December 30, 2012, approximately 44% of our revenue was derived from our international operations, including 19% of our revenue from France. In 2012, we opened a direct sales office in Japan and we intend to further expand our international operations into key markets, such as Brazil and China. Our international sales operations expose us and our representatives, agents and distributors to risks inherent in operating in foreign jurisdictions. These risks include:the imposition of additional U.S. and foreign governmental controls or regulations on orthopaedic implants and biologics products;the imposition of costly and lengthy new export and import license requirements;the imposition of U.S. or international sanctions against a country, company, person or entity with whom we do business that would restrict or prohibit continued business with that country, company, person or entity;economic instability, including the European sovereign debt crisis and the austerity measures taken and to be taken by certain countries in response to such crisis, and the currency risk between the U.S. dollar and foreign currencies in our target markets;the imposition of restrictions on the activities of foreign agents, representatives and distributors;scrutiny of foreign tax authorities, which could result in significant fines, penalties and additional taxes being imposed upon us;a shortage of high-quality international salespeople and distributors;loss of any key personnel who possess proprietary knowledge or are otherwise important to our success in international markets;changes in third-party reimbursement policies that may require some of the patients who receive our products to directly absorb medical costs or that may require us to sell our products at lower prices;unexpected changes in foreign regulatory requirements;differing local product preferences and product requirements;changes in tariffs and other trade restrictions;work stoppages or strikes in the healthcare industry;difficulties in enforcing and defending intellectual property rights;foreign exchange controls that might prevent us from repatriating cash earned in countries outside the Netherlands;complex data privacy requirements and labor relations laws; andexposure to different legal and political standards.Not only are we subject to the laws of other jurisdictions, we also are subject to U.S. laws governing our activities in foreign countries, including various import-export laws, customs and import laws, anti-boycott laws and embargoes. For example, the FDA Export Reform and Enhancement Act of 1996 requires that, when exporting medical devices from the United States for saleankle and/or hindfoot fusion indications, in a foreign country, depending onCanada for foot and ankle fusion indications and in Australia and New Zealand for hindfoot and ankle fusion indications. We anticipate significant sales during 2016 and in future years from our AUGMENT® Bone Graft product. If we are wrong, our future operating results, cash flows, and prospects could be adversely affected. We acquired the type ofAUGMENT® Bone Graft product being exported, the regulatory status of the productline from BioMimetic Therapeutics, Inc. (BioMimetic) in March 2013 and the country to which the device is exported, we must ensure, among other things, that the device is produced in accordance with the specifications of the foreign purchaser; not in conflict with the laws of the country to which it is intended for export; labeled for export; and not offered for sale domestically. In addition, we must maintain records relevant to product export and, if requested by the foreign government, obtain a certificate of exportability. In some instances, prior notification to or approval from the FDA is required prior to export. The FDA can delay or deny export authorization if all applicable requirements are not satisfied. Imports of approved medical devices into the United States also are subject to requirements including registrationfuture milestone payments to the holders of establishment, listingthe contingent value rights issued in connection with that transaction. Therefore, even if we achieve significant sales of devices, manufacturingAUGMENT® Bone Graft, these sales will be offset in accordance with the quality system regulation, medical device reporting of adverse events, and premarket notification 510(k) clearance or premarket approval, or PMA, among others and if applicable. If our business activities were determined to violatepart by these laws, regulations or rules, we could suffer serious consequences.In addition, a portion of our international revenue is made through distributors. As a result, we are dependent upon the financial health of our distributors. milestone payment obligations.also dependent upon their compliance with foreign laws and the U.S. Foreign Corrupt Practices Act, or the FCPA, as it relatessubject to certain “facilitating” payments made to those employed by or acting on behalf of a foreignsubstantial government in the procurement, sale and prescription of medical devices. If a distributor were to go out of business, it would take substantial time, cost and resources to find a suitable replacement and the products held by such distributor may not be returned to us or to a subsequent distributor in a timely manner or at all.Any material decrease in our foreign revenue may negatively affect our profitability. We generate our international revenue primarily in Europe, where healthcare regulation and reimbursement for orthopaedic medical devices vary significantly from country to country. This changing environment could adversely affect our ability to sell our products in some European countries. In addition, many of the economies in Europe have undergone recessions which have threatened their ability to service their sovereign debt obligations. Several of these countries have implemented austerity measures, which have adversely affected our sales and may continue to adversely affect our sales.Disruption and turmoil in global credit and financial markets, which may be exacerbated by the inability of certain countries to continue to service their sovereign debt obligations and certain austerity measures countries have implemented, and the possible negative implications of such events to the global economy, may negatively impact our business, operating results and financial condition.A substantial portion of our revenue outside the United States is generated in Europe, including in particular France. The credit ratings of several European countries and the possibility that certain European Union member states will default on their debt obligations have contributed to significant uncertainty about the stability of global credit and financial markets. The credit and economic conditions within certain European Union countries in particular, including France, Greece, Ireland, Italy, Portugal and Spain, have contributed to the instability in global credit and financial markets. The possibility that such EU member states will default on their debt obligations, the continued uncertainty regarding international and the European Union’s financial support programs and the possibility that other EU member states may experience similar financial troubles could further disrupt global credit and financial markets. While the ultimate outcome of these events cannot be predicted, it is possible that such events could have a negative effect on the global economy as a whole, and our business, operating results and financial condition, in particular. For example, if the European sovereign debt crisis continues or worsens, the negative implications to the global economy and us could be significant. Since a significant amount of our trade receivables are with hospitals that are dependent upon governmental health care systems in many countries, repayment of such receivables is dependent upon the financial stability of the economies of those countries. A deterioration of economic conditions in such countries may increase the average length of time it takes for us to collect on our outstanding accounts receivable in these countries or even our ability to collect such receivables.In addition, if the European sovereign debt crisis continues or worsens, the value of the Euro could deteriorate or lead to the re-introduction of individual currencies in one or more Eurozone countries, or, in more extreme circumstances, the possible dissolution of the Euro currency entirely, all of which could negatively impact our business, operating results and financial condition in light of our substantial operations in and revenues derived from customers in the European Union. Should the Euro dissolve entirely, the legal and contractual consequences for holders of Euro-denominated obligations would be determined by laws in effect at such time. These potential developments, or market perceptions concerning these and related issues, could adversely affect the value of our Euro-denominated assets and obligations. In addition, concerns over the effect of this financial crisis on financial institutions in Europe and globally could lead to tightening of the credit andfinancial markets, which could negatively impact the ability of companies to borrow money from their existing lenders, obtain credit from other sources or raise financing to fund their operations. This could negatively impact our customers’ ability to purchase our products, our suppliers’ ability to provide us with materials and components and our ability, if needed, to finance our operations on commercially reasonable terms, or at all. We believe that European governmental austerity policies have reduced and may continue to reduce the amount of money available to purchase medical products, including our products. These austerity measures could negatively impact overall procedure volumes and result in increased pricing pressure for our products and the products of our competitors. Any or all of these events, as well as any additional austerity measures that may be taken which, among other things, could result in decreased utilization, pricing and reimbursement, could negatively impact our business, operating results and financial condition.Weakness in the global economy is likely to adversely affect our business until an economic recovery is underway.Many of our products are used in procedures covered by private insurance, and some of these procedures may be considered elective. We believe the global economic downturn may reduce the availability or affordability of private insurance or may affect patient decisions to undergo elective procedures. If current economic conditions do not continue to recover or worsen, we expect that increasing levels of unemployment and pressures to contain healthcare costs could adversely affect the global growth rate of procedure volume, which could have a material adverse effect on our revenuebusiness.operating results.Fluctuationsmarketing of our products and our ongoing research and development, pre-clinical testing, and clinical trial activities are subject to extensive regulation and review by numerous governmental authorities both in the United States and abroad. U.S. and foreign currency ratesregulations govern the testing, marketing, and registration of new medical devices, in addition to regulating manufacturing practices, reporting, labeling, relationships with healthcare professionals, and recordkeeping procedures. The regulatory process requires significant time, effort, and expenditures to bring our products to market, and we cannot be assured that any of our products will be approved. Our failure to comply with applicable regulatory requirements could result in declines ingovernmental authorities:reported revenueproducts;earnings.A substantial portionour officers and employees;revenue outsidenew products into the market;Europelight of the Wright/Tornier merger, there is still a risk we will be unable to replace the revenue and cash flow that the OrthoRecon business generated, or that the cost of such will be higher than expected. If we are unable to achieve our profit and growth objectives, such failure will be exacerbated by the loss of revenue and cash flow generated by the OrthoRecon business, and could result in a decline in our stock price.countriesrisks will not prevent us from realizing the expected benefits of these transactions.Latin Americaa number of product liability matters, including those relating to the OrthoRecon business, which legacy Wright divested to MicroPort in 2014. Legacy Wright remains responsible, as between it and AsiaMicroPort, for claims associated with products sold before divesting the OrthoRecon business to MicroPort.amounts are denominatedsettlement amount is reasonable relative to the risk and expense of litigation.currencies other than the U.S. dollar. For purposes of preparingNote 16 to our consolidated financial statements these amountsin "Item 8. Financial Statements and Supplementary Data" of this report. These matters are converted into U.S. dollars, the value of which varies with currency exchange rate fluctuations. For revenuesubject to many uncertainties and outcomes are not denominated in U.S. dollars, if there is an increase in the valuepredictable. Regardless of the U.S. dollar relativeoutcome of these matters, legal defenses are costly. We have incurred and expect to continue to incur substantial legal expenses in connection with the specified foreign currency, we will receive less in U.S. dollars than before the increase in the exchange rate, whichdefense of these matters. We could negatively impact our operating results. Although we address currency risk management through regular operating and financing activities, and more recently through hedging activities, those actions may not prove to be fully effective, and hedging activities involve additional risks.Our business plan relies on assumptions about the market forincur significant liabilities associated with adverse outcomes that exceed our products liability insurance coverage, which if incorrect, maycould adversely affect our revenue.We believe thatoperating results or results from discontinued operations and financial condition. The ultimate cost to us with respect to product liability claims could be materially different than the agingamount of the general populationcurrent estimates and increasingly active lifestyles will continueaccruals and that these trends will increasecould have a material adverse effect on our financial position, operating results or results from discontinued operations, and cash flows.need for our products.future, we may be subject to additional product liability claims. We anticipate that the market for our extremity productsalso could experience a material design or manufacturing failure in particular will continue to grow. The actual demand for our products, however, could differ materially from our projected demand if our assumptions regarding these trends and acceptance of our products by the medical community prove to be incorrecta quality system failure, other safety issues, or do not materialize, or if non-surgical treatments gain more widespread acceptance asheightened regulatory scrutiny that would warrant a viable alternative to our orthopaedic implants.Our upper extremity joints and trauma products, including in particular our shoulder products, generate a significant portion of our revenue. Accordingly, if revenue of these products were to decline, our operating results would be adversely affected.Our upper extremity joints and trauma products, which includes joint implants and bone fixation devices for the shoulder, hand, wrist and elbow, generate a significant portion of our revenue. During the year ended December 30, 2012, our upper extremity joints and trauma products generated approximately 63% of our revenue. We expect the shoulder to continue to be the largest and most important product category for us for the foreseeable future. A decline in revenue from these products as a result of increased competition, regulatory matters, intellectual property matters or any other reason would negatively impact our operating results.We obtain some of our products through private-label distribution agreements that subject us to minimum performance and other criteria. Our failure to satisfy those criteria could cause us to lose those rights of distribution.We have entered into private-label distribution agreements with manufacturersrecall of some of our products. These manufacturers brandProduct liability lawsuits and claims, safety alerts and product recalls, regardless of their ultimate outcome, could result in decreased demand for our products, accordinginjury to our specifications,reputation, significant litigation and other costs, substantial monetary awards to or costly settlements with patients, product recalls, loss of revenue, and the inability to commercialize new products or product candidates, and otherwise have a material adverse effect on our business and reputation and on our ability to attract and retain customers.maypresently are aware of only four lawsuits alleging personal injury related to cobalt chrome neck fractures (two in the United States and two outside the United States). However, if the number of product liability claims alleging personal injury from fractures of cobalt chrome modular necks we sold prior to the MicroPort transaction were to become significant, this could have exclusive rightsan adverse effect on our results from discontinued operations and financial condition. In addition, MicroPort filed a lawsuit against us seeking indemnification against losses arising from its recall and from the alleged fractures. We vigorously deny MicroPort’s claims; however, there can be no assurance we will be successful in certain fieldsdefending against them. An adverse outcome in this litigation could adversely affect our results from discontinued operations and financial condition.useits modular hip systems, and territoriesthe liability claims and adverse publicity which ensued, could generate copycat claims against modular hip systems legacy Wright sold.sellwhich it has been subject, and the general negative publicity surrounding “metal-on-metal” articulating surfaces (which do not involve modular hip stems), there remains a risk that, even in the absence of clinical evidence, claims for personal injury relating to sales of these products subject to minimum purchase, sales or other performance criteria. Though these agreements do not individually or inbefore divestiture of the aggregate represent a material portion of ourOrthoRecon business if we do not meet these performance criteria, or fail to renew these agreements, we may lose exclusivity in a field of use or territory or cease to have any rights to these products,could increase, which could have an adverse effect on our revenue. Furthermore, some of these manufacturersmay be smaller, undercapitalized companies that may not have sufficient resources to continue operations or to continue to supply us sufficient product without additional access to capital.If our private-label manufacturers fail to provide us with sufficient supply of their products, or if their supply fails to meet appropriate quality requirements, our business could suffer.Our private-label manufacturers are sole source suppliers of the products we purchase from them. Given the specialized nature of the products they provide, we may not be able to locate or establish additional or replacement manufacturers of these products. Moreover, these private-label manufacturers typically own the intellectual property associated with their products, and even if we could find a replacement manufacturer for the product, we may not have sufficient rights to enable the replacement party to manufacture the product. While we have entered into agreements with our private-label manufacturers that we believe will provide us sufficient quantities of products, we cannot assure you that they will do so, or that any products they do provide us will not contain defects in quality. Our private-label manufacturing agreements have terms expiring between this year and 2015 and are renewable under certain conditions or by mutual agreement. The agreements also include some or all of the following provisions allowing for termination under certain circumstances: (i) either party’s uncured material breach of the terms and conditions of the agreement, (ii) either party filing for bankruptcy, being bankrupt or becoming insolvent, suspending payments, dissolving or ceasing commercial activity, (iii) our inability to meet market development milestones and ongoing sales targets, (iv) termination without cause, provided that payments are made to the distributor, (v) a merger or acquisition of one of the parties by a third party, (vi) the enactment of a government law or regulation that restricts either party’s right to terminate or renew the contract or invalidates any provision of the agreement or (vii) the occurrence of a “force majeure,” including natural disaster, explosion or war.We also rely on these private-label manufacturers to comply with the regulations of the FDA, the competent authorities of the Member States of the European Economic Area, or EEA, or foreign regulatory authorities and their failure to comply with strictly enforced regulatory requirements could expose us to regulatory action including warning letters, product recalls, termination of distribution, product seizures or civil penalties. Any quality control problems that we experience with respect to products manufactured by our private-label manufacturers, any inability by us to provide our customers with sufficient supply of products or any investigations or enforcement actions by the FDA, the competent authorities of the Member States of the EEA or other foreign regulatory authorities could adversely affect our reputation or commercialization of our products and adversely and materially affect our business and operating results.We intend to continue to bring in-house the manufacturing of certain of our products that are currently manufactured by third parties. Should we encounter difficulties in manufacturing these or other products, it could adversely affect our business.We intend to continue our initiative to bring in-house the manufacturing of certain of our products, including in particular our Ascend and Simpliciti shoulder products. The technology and the manufacturing process for our shoulder products is highly complex, involving a large number of unique parts, and we may encounter difficulties in manufacturing these products in-house. There is no assurance that we will be able to meet the volume and quality requirements associated with our shoulder products. In addition, other products that we choose to bring in-house could encounter similar difficulties. Manufacturing and product quality issues may also arise as we increase the scale of our production. If our products do not consistently meet our customers’ performance expectations, our reputation may be harmed, and we may be unable to generate sufficient revenue to become profitable. Any delay or inability in bringing in-house the manufacturing of our products could diminish our ability to sell our products, which could result in lost revenue and seriously harm our business, financial condition and results from discontinued operations since legacy Wright retained responsibility, as between it and MicroPort, for these claims.results.conditioncondition.operating results.based subsidiary, Tornier, Inc.,operating subsidiaries, are currently subject to the U.S. Foreign Corrupt Practices Act. We are required to comply with the FCPA, which generally prohibits covered entities and their intermediaries from engaging in bribery or making other prohibited payments to foreign officials for the purpose of obtaining or retaining business or other benefits. In addition, the FCPA imposes accounting standards and requirements on publicly tradedpublicly-traded U.S. corporations and their foreign affiliates, which are intended to prevent the diversion of corporate funds to the payment of bribes and other improper payments, and to prevent the establishment of “off books” slush funds from which such improper payments can be made. We also are currently subject to similar anticorruptionanti-corruption legislation implemented in Europe under the Organization for Economic Co-operation and Development’s Convention on Combating Bribery of Foreign Public Officials in International Business Transactions. We either operate or plan to operate in a number of jurisdictions that pose a high risk of potential violations of the FCPA and other anticorruptionanti-corruption laws, such as China and Brazil,anticorruptionanti-corruption laws, including, but not limited to their reporting requirements. We also have developed and will continue to develop and implement systems for formalizinganticorruptionanti-corruption laws.TheRecent investigations of companies in our industry by the SEC is currentlyand the U.S. Department of Justice have focused on potential FCPA violations in connection with the midstsale of conducting an informal investigation of numerous medical device companies over potential violations of the FCPA. Although we do notdevices in foreign countries. We believe we have compliance systems, which enable us to prevent these behaviors. However, if despite our efforts we are currently anot successful in mitigating these risks, we could become the target anyof enforcement actions by U.S. or local authorities. Any investigation of any potential violations of the FCPA or other anticorruptionanti-corruption laws by U.S. or foreign authorities also could have ana material adverse impacteffect on our business, operating results, and financial condition and operating results.budget.usehave relied on a limited number of suppliers for raw materials and selectthe components that we need to manufactureused in our products. These suppliers must provide the materialsOur reconstructive joint devices are produced from various surgical grades of titanium, cobalt chrome, stainless steel, various grades of high-density polyethylenes and components to our standards for us to meet our quality and regulatory requirements.ceramics. We obtain some key raw materials and select components from a single source or a limited number of sources. For example, we relyhave relied on one supplier for raw materials and select components in several of our products, including Poco Graphite, Inc., which supplies graphite for our pyrocarbon products; CeramTec AG, or CeramTec, which supplies ceramic for ceramic heads for hips; and Heymark Metals Ltd., which supplies Cobalt Chrome used in certain of our hip, shoulder and elbow products. Establishing additional or replacement suppliers for these components, and obtaining regulatory clearances or approvals that may result from adding or replacing suppliers, could take a substantial amount of time, result in increased costs and impair our ability to produce our products, which would adversely impact our business and operating results. We do not have contracts with our sole source suppliers (other than a Quality Assurance Agreement and Secrecy Agreement with CeramTec, which only relate to quality and confidentiality obligations of the parties and do not govern the purchase and receipt of CeramTec products) and instead rely on purchase orders. As a result, those suppliers may elect not to supply us with product or to supply us with less product than we need, and we will have limited rights to cause them to do otherwise. In addition,a certain grade of cobalt chrome alloy, one supplier for the silicone elastomer used in some of our extremities products, one supplier for our pyrocarbon products, and one supplier to provide a key ingredient of AUGMENT® Bone Graft. The manufacture of our products is highly exacting and complex, and our business could suffer if a sole source supply arrangement is unexpectedly terminated or interrupted, and we are unable to obtain an acceptable new source of supply in a timely fashion.acquirepurchase from third parties, are highly technical and areNovartis purified bulk recombinant human platelet-derived growth factor (rhPDGF-BB), which is a key component of AUGMENT® Bone Graft. Our supplier was contractually required to meet exacting specifications,our supply requirements until the termination date, and any quality control problems that we experienceto use commercially reasonable efforts to assist us in identifying a new supplier and support the transfer of technology and supporting documentation to produce this component. Our transition to a new supplier is well underway with respect tofull cooperation from the products supplied by third parties could adversely and materially affect our reputation or commercialization of our products and adversely and materially affect our business, operating results and prospects. Furthermore, some of these suppliers are smaller companies. To the extent that any of these suppliers are, or become, undercapitalized and do not otherwise have sufficient resources to continue operations or to supply us sufficient product without additional access to capital, such a failure could adversely affect our business. We also may have difficulty obtaining similar components from other suppliers that are acceptable to the FDA, the competent authorities or notified bodies of the Member States of the EEA, or foreign regulatory authorities and the failure of our suppliers to comply with strictly enforced regulatory requirements could expose us to regulatory action including warning letters, product recalls, termination of distribution, product seizures or civil penalties. Furthermore, since many of these suppliers are located outside of the United States, we are subject to foreign export laws and U.S. import and customs regulations, which complicate and could delay shipments of components to us. For example, all foreign importers of medical devices are required to meet applicable FDA requirements, including registration of establishment, listing of devices, manufacturing in accordance with the quality system regulation, medical device reporting of adverse events, and premarket notification 510(k) clearance or PMA, if applicable. In addition, all imported medical devices also must meet Bureau of Customs and Border Protection requirements. While it is our policy to maintain sufficient inventory of materials and components so that our production will not be significantly disrupted even if a particular component or material is not available for a period of time, we remain at risk that we will not be able to qualify new components or materials quickly enough to prevent a disruption if one or more of our suppliers ceases production of important components or materials.Sales volumes may fluctuate depending on the season and our operating results may fluctuate over the course of the year.Our business is seasonal in nature. Historically, demand for our products has been the lowest in our third quarter as a result of the European holiday schedule during the summer months. We have experienced and expect to continue to experience meaningful variability in our revenue and gross profit among quarters,current as well as within each quarter,the new supplier. We believe the current supplier has produced sufficient product to meet our production needs for the interim period until a new supplier is brought on line.a result of a number of factors, including, among other things:the numberour source for demineralized bone matrix (DBM) and mix of products sold in the quartercancellous bone matrix (CBM), and the geographies in which they are sold;the demand for, and pricing of, our products and the products of our competitors;the timing of orany failure to obtain DBM and CBM from this source in a timely manner will deplete levels of on-hand raw materials inventory and could interfere with our ability to process and distribute allograft products. We rely on a single not-for-profit tissue bank to meet all of our DBM and CBM order requirements, a key component in the allograft products we currently produce, market, and distribute. In addition, we rely on a single supplier of soft tissue graft for BIOTAPE® XM.clearances or approvals for products;costs, benefits and timing of new product introductions;the level of competition;the timing and extent of promotional pricing or volume discounts;changes in average selling prices;the availability and cost of components and materials;the number of selling days;fluctuations in foreign currency exchange ratesthe timing of patients’ use ofaction impacting their calendar year medical insurance deductibles; andimpairment and other special charges.If product liability lawsuits are brought against us, our business may be harmed.The manufacture and sale of orthopaedic medical devices exposes us to significant risk of product liability claims. In the past, we have hadDBM, CBM and soft tissue graft for BIOTAPEproduct liability claims relatingsuppliers, if we cannot continue to obtain DBM, CBM, and soft tissue graft for BIOTAPE® XM from our products, none of which either individually, orcurrent sources in the aggregate, have resulted in a material negative impact onvolumes sufficient to meet our business. In the future, we may be subject to additional product liability claims, some of which may have a negative impact on our business. Such claims could divert our management from pursuing our business strategy and may be costly to defend. Regardless of the merit or eventual outcome, product liability claims may result in:decreased demand for our products;injury to our reputation;significant litigation costs;substantial monetary awards to or costly settlements with patients;product recalls;loss of revenue; andthe inability to commercialize new products or product candidates.Our existing product liability insurance coverage may be inadequate to protect us from any liabilities we might incur. If a product liability claim or series of claims is brought against us for uninsured liabilities or in excess of our insurance coverage, our business and operating results could suffer. In addition, a recall of some of our products, whether or not the result of a product liability claim, could result in significant costs and loss of customers.In addition,needs, we may not be able to maintain insurance coveragelocate replacement sources of DBM, CBM, and soft tissue graft for BIOTAPEa reasonable cost or in sufficient amounts or scope to protect us against losses. Any claims against us, regardless of their merit,all. This could severely harminterrupt our financial condition, strain our management and other resources andbusiness, which could adversely affect our sales.eliminatebe required, for reasons beyond our control to cease supplying raw materials and components to us. FDA regulations may require additional testing of any raw materials or components from new suppliers prior to our use of these materials or components, and in the prospects for commercialization or salescase of a productdevice with a PMA application, we may be required to obtain prior FDA permission, either of which could delay or product candidate which is the subjectprevent our access to or use of any such claim.Our inabilityraw materials or components.maintain adequate working relationships with external research and development consultants and surgeonsor unauthorized tampering of those systems could have a negative impactmaterial adverse effect on our business.markettimely ship and sell new products.We maintain professional working relationships with external researchtrack product orders, project inventory requirements, manage our supply chain, and development consultantsotherwise adequately service our customers, and leading surgeons and medical personnel in hospitals and universities who assist in product research and development and training. We continuelead to emphasize the development of proprietary products and product improvements to complement and expand our existing product lines. It is possible that U.S. federal and state laws requiring us to disclose payments or other transfers of value, such as free gifts or meals, to physiciansincreased costs and other healthcare providers could have a chilling effect on these relationships with individuals or entities that may, among other things, want to avoid public scrutiny of their financial relationships with us. Ifdifficulties. In the event we are unable to maintain these relationships, our ability to develop and sell new and improvedproducts could decrease, and our future operating results could be unfavorably affected.In November 2012, Douglas W. Kohrs, our former President and Chief Executive Officer, resignedexperience significant disruptions as a director, officerresult of the ERP implementation or otherwise, we may not be able to fix our systems in an efficient and employee of Tornier. Mr. Kohrs had built strong relationships with severaltimely manner. Accordingly, such events may disrupt or reduce the efficiency of our key research and development consultants and surgeons and medical personnel in hospitals and universities who assist in product research and development and training. Accordingly, this change in our senior management may adversely affect our relationships with these individualsentire operations and have a material adverse effect on our business.We incur significant expendituresoperating results and cash flows. In addition, if our systems are damaged or cease to function properly due to any number of resourcescauses, ranging from catastrophic events to maintain relatively high levels of inventorypower outages to security breaches, and instruments, which can reduce our cash flows.As a result of the need to maintain substantial levels of inventory and instruments, we are subject to the risk of obsolescence. The nature of our business requires us to maintain a substantial level of inventory and instruments. For example, our total consolidated inventory balance was $86.7 million at December 30, 2012 and our total consolidated instrument balance was $51.4 million at December 30, 2012. In order to marketcontinuity plans do not effectively we often must maintain and bring our customers instrument kits, back-up products and products of different sizes. In the event that a substantial portion of our inventory becomes obsolete, it could have a material adverse effect on our earnings and cash flows due to the resulting costs associated with the inventory impairment charges and costs required to replace such inventory.Our recent acquisition of OrthoHelix and any additional acquisitions and efforts to acquire and integrate other companies or product lines could adversely affect our operations and financial results.On October 4, 2012, we acquired OrthoHelix, a privately-held company focused on developing and marketing specialty implantable screw and plate systems for the repair of small bone fractures and deformities predominantly in the foot and ankle. In addition,compensate timely, we may pursue additional acquisitions of other companies or product lines. A successful acquisition depends onsuffer interruptions in our ability to identify, negotiate, complete and integrate such acquisition and to obtain any necessary financing. With respect to our recent acquisition of OrthoHelix and any future acquisitions, we may experience:difficulties in integrating OrthoHelix and its personnel and products, as well as the personnel and products of any other acquired companies, into our existing business;difficulties in integrating OrthoHelix’s and Tornier’s commercial organizations, including in particular distribution and sales representative arrangements;difficulties or delays in realizing the anticipated benefits of our acquisition of OrthoHelix or any additional acquired companies and their products;diversion of our management’s time and attention from other business concerns;challenges due to limited or no direct prior experience in new markets or countries we may enter;the potential loss of key employees, including in particular sales and research and development personnel, of our company, OrthoHelix or any other business we may acquire;the potential loss of key customers, distributors, representatives, vendors and other business partners who choose not to do business with our company post-acquisition;inability to effectively coordinate sales and marketing efforts to communicate our capabilities post-acquisition and coordinate sales organizations to sell our combined products;inability to successfully develop new products and services on a timely basis that address our new market opportunities post-acquisition;inability to compete effectively against companies already serving the broader market opportunities expected to be available to us post-acquisition;difficulties in the assimilation of different corporate cultures, practices and sales and distribution methodologies, as well as in the assimilation and retention of geographically dispersed, decentralized operations and personnel;unanticipated costs, litigation and other contingent liabilities;incurrence of acquisition and integration related costs, accounting charges, or amortization costs for acquired intangible assets;potential write-down of goodwill, acquired intangible assets and/or deferred tax assets;additional legal, financial and accounting challenges and complexities in areas such as intellectual property, tax planning, cash management and financial reporting andany unforeseen compliance risks and accompanying financial and reputational exposure/loss not uncovered in the due diligence process and which are imputed to Tornier such as compliance with federal laws andregulations the advertising and promotion regulations under the federal Food, Drug and Cosmetic Act, the Anti-kickback Statute, the False Claims Act, the Physician Sunshine Payments Act and other applicable state laws.In addition, we may have to incur debt or issue equity securities to pay for any future acquisition, the issuance of which could involve restrictive covenants or be dilutive to our existing shareholders. Acquisitions also could materially impair our operating results by requiring us to amortize acquired assets. For example, as a result of our acquisition of OrthoHelix, we incurred additional indebtedness, including two senior secured term loans in the aggregate principal amount of $115.0 million. The proceeds of the term loans were used to fund our acquisition of OrthoHelix and retire certain then existing indebtedness.In addition, effective internal controls are necessary for us to provide reliable and accurate financial reports and to effectively prevent fraud. The integration of acquired businesses is likely to result in our systems and controls becoming increasingly complex and more difficult to manage. We devote significant resources and time to comply with the internal control over financial reporting requirements of the Sarbanes-Oxley Act of 2002. However, we cannot be certain that these measures will ensure that we design, implement and maintain adequate control over our financial processes and reporting in the future, especially in the context of acquisitions of other businesses. Any difficulties in the assimilation of acquired businesses into our control system could harm our operating results or cause us to fail to meet our financial reporting obligations. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our stock and our access to capital.All of the risks described above may be exacerbated if we effect multiple acquisitions during a short period of time.If we do not achieve the contemplated benefits of our acquisition of OrthoHelix, our business and financial condition may be materially impaired.We may not achieve the desired benefits from our recent acquisition of OrthoHelix. For any of the reasons described above and elsewhere in this report and even if we are able to successfully operate OrthoHelix within our company, we may not be able to realize the revenue and other synergies and growth that we anticipate from the acquisition in the time frame that we currently expect, and the costs of achieving these benefits may be higher than what we currently expect, because of a number of risks, including, but not limited to:the possibility that the acquisition may not further our business strategy as we expected;the possibility that we may not be able to expand the reach and customer base for OrthoHelix’s products as expected;the possibility that we may not be able to expand the reach and customer base for our products as expected; andthe fact that the acquisition will substantially expand our lower extremity joints and trauma business, and we may not experience anticipated growth in that market.As a result of these risks, the OrthoHelix acquisition may not contribute to our earnings as expected, we may not achieve expected revenue synergies or our return on invested capital targets when expected, or at all, and we may not achieve the other anticipated strategic and financial benefits of the transaction.We have experienced recently certain changes in our senior management which could cause certain key employees to depart because of difficulties with change or a desire not to remain with our company. If we cannot retain our key personnel, we may not be able to manage and operate successfully, and we may not be able to meet our strategic objectives.In November 2012, Douglas W. Kohrs, our former President and Chief Executive Officer, resigned as a director, officer and employee of Tornier. Mr. Kohrs had built strong relationships with several of our key employees and other personnel. On November 12, 2012, we appointed David H. Mowry as Interim President and Chief Executive Officer, and in February 2013, Mr. Mowry was appointed President and Chief Executive Officer on a non-interim basis. In September 2012, our Chief Financial Officer joined Tornier after the former Global Chief Financial Officer resigned in July 2012. Our future success depends, in large part, upon our ability to retain and motivate our management team and key managerial, scientific, sales and technical personnel. Key personnel may depart because of difficulties with change or a desire not to remain with our company. Any unanticipated loss or interruption of services of our management team and our key personnel could significantly reduce our ability to meet our strategic objectives because it may not be possible for us to find appropriate replacement personnel should the need arise. We compete for such personnel with other companies, academic institutions, governmental entities and other organizations. There is no guarantee that we will be successful in retaining our current personnel or in hiring or retaining qualified personnel in the future. Loss of key personnel or the inability to hire or retainqualified personnel in the future could have a material adverse effect on our ability to operate successfully. Further, any inability on our part to enforce non-compete arrangements related to key personnel who have left the company could have a material adverse effect on our business.affected.impacted. Likewise, if any of our current insurance coverage should become unavailable to us or become economically impractical, we would be required to operate our business without indemnity from commercial insurance providers.If a natural or man-made disaster, including as a resultproperty and the disruption of our business from casualties, such insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms or at all.We may be unable to raise capital when needed, which would force us to delay, reduce, eliminate or abandon our commercialization efforts or product development programs.There is no guarantee that our anticipated cash flow from operations will be sufficient to meet all of our cash requirements. We intend to continue to make investments to support our business growth andmarketed devices may require additional funds to:expand the commercialization of our products;fund our operations and clinical trials;continue our research and development;defend, in litigation or otherwise, any claims that we infringe third-party patents or other intellectual property rights and enforce our patent and other intellectual property rights;commercialize our new products, if any such products receive regulatory clearance or approval for commercial sale; andacquire companies and in-license products or intellectual property.We believe that our cash and cash equivalents balance of $31.1 million as of December 30, 2012, anticipated cash receipts generated from revenue of our products and available credit under our $30.0 million senior secured revolving credit facility, will be sufficient to meet our anticipated cash requirements for 2013. However, our future funding requirements will depend on many factors, including:market acceptance of our products;the scope, rate of progress and cost of our clinical trials;the cost of our research and development activities;the cost of filing and prosecuting patent applications and defending and enforcing our patent and other intellectual property rights;the cost of defending, in litigation or otherwise, any claims that we infringe third-party patent or other intellectual property rights;the cost of defending any claims of product liability, or other claims against us, such as contract liabilities;the cost and timing of additional regulatory clearances or approvals;the cost and timing of expanding our sales, marketing and distribution capabilities;the cost and timing of expanding our product offering inventories;our ability to collect amounts receivable from customers;the effect of competing technological and market developments; andthe extent to which we acquire or invest in additional businesses, products and technologies, although we currently have no commitments or agreements relating to any of these types of transactions.In the event that we would require additional working capital to fund future operations, we could seek to acquire that through additional equity or debt financing arrangements which may or may not be available on favorable terms at such time. If we raise additional funds by issuing equity securities, our shareholders may experience dilution. Debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt, in addition to those under our existing credit facilities. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our shareholders. If we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our products or license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize. We also may have to reduce marketing, customer support or other resources devoted to our products or cease operations.Any lack of borrowing availability under our credit facility and our potential inability to obtain replacement sources of credit could materially affect our operations and financial condition.Although we currently have available credit under our $30.0 million senior secured revolving credit facility, our ability to draw on our credit facility may be limited by outstanding letters of credit or by operating and financial covenants under our the credit agreement. There can be no assurances that we will continue to have access to credit if our operating and financial performance do not satisfy these covenants. If we do not satisfy these criteria, and if we are unable to secure necessary waivers or other amendments from the lenders of our credit facility, we will not have access to this credit.Both the $30.0 million revolving credit facility and the aggregate $115.0 million of term loans under our credit agreement are secured by all of our assets (subject to certain exceptions) and except to the extent otherwise permitted under the terms of our credit agreement, our assets cannot be pledged as security for other indebtedness. These limits on our ability to offer collateral to other sources of financing could limit our ability to obtain other financing which could materially affect our operations and financial condition.Although we believe that our anticipated operating cash flows, on-hand cash levels and access to credit will give us the ability to meet our financing needs for at least the next 12 months, there can be no assurance that they will do so. Any lack of borrowing availability under our revolving credit facility and our potential inability to obtain replacement sources of credit could materially affect our operations and financial condition.We are leveraged financially, which could adversely affect our ability to adjust our business to respond to competitive pressures and to obtain sufficient funds to satisfy our future research and development needs, to protect and enforce our intellectual property and other needs.We have significant indebtedness. In connection with our acquisition of OrthoHelix, we obtained senior secured term loans in the aggregate principal amount of $115.0 million and a senior secured $30.0 million revolving line of credit. The degree to which we are leveraged could have important consequences, including, but not limited to, the following:our ability to obtain additional financing in the future for working capital, capital expenditures, acquisitions, litigation, general corporate or other purposes may be limited;a substantial portion of our cash flows from operations in the future will be dedicated to the payment of principal and interest on our indebtedness, including the requirement that certain excess cash flows and certain net proceeds of asset dispositions (including from condemnation or casualty) and certain new indebtedness be applied to prepayment of our senior secured terms loans; andwe may be more vulnerable to economic downturns, less able to withstand competitive pressures and less flexible in responding to changing business and economic conditions.A failure to comply with the covenants and other provisions of our credit agreement could result in events of default under such agreement, which could require the immediate repayment of our outstanding indebtedness. If we are at any time unable to generate sufficient cash flows from operations to service our indebtedness when payment is due, we may be required to attempt to renegotiate the terms of the agreements relating to the indebtedness, seek to refinance all or a portion of the indebtedness or obtain additional financing. There can be no assurance that we will be able to successfully renegotiate such terms, that any such refinancing would be possible or that any additional financing could be obtained on terms that are favorable or acceptable to us.Our credit agreement contains restrictive covenants that may limit our operating flexibility.The agreement relating to our senior secured term loans and senior secured revolving credit facility contains operating covenants limiting our ability to transfer or dispose of assets, merge with or acquire other companies, make investments, pay dividends, incur additional indebtedness and liens, make capital expenditures and conduct transactions with affiliates, and financial covenants requiring us to meet certain financial ratios. We, therefore, may not be able to engage in any of the foregoing transactions or in any that would cause us to breach these financial covenants until our current debt obligations are paid in full or we obtain the consent of the lenders. There is no guarantee that we will be able to generate sufficient cash flow or revenue to meet these operating and financial covenants or pay the principal and interest on our debt. Furthermore, there is no guarantee that future working capital, borrowings or equity financing will be available to repay or refinance any such debt.As a result of our acquisition of OrthoHelix, we may be required to make future earn-out payments of up to an aggregate of $20.0 million based upon our sales of lower extremity joints and trauma products during fiscal years 2013 and 2014, which payments may affect our liquidity and our operating results.In connection with our recent acquisition of OrthoHelix, we agreed to made additional earn-out payments of up to an aggregate of $20.0 million in cash based upon our sales of lower extremity joints and trauma products during fiscal years 2013 and 2014. A portion of the earn-out payments will be subject to certain rights of set-off for post-closing indemnification obligations of OrthoHelix’s equity holders. If we are required to make these payments, particularly at a time when we are experiencing financial difficulty, our liquidity, operating results and financial condition may be adversely affected.Our operating results could be negatively impacted by future changes in the allocation of income to each of the entities through which we operate and to each of the income tax jurisdictions in which we operate.We operate through multiple entities and in multiple income tax jurisdictions with different income tax rates both inside and outside the United States and the Netherlands. Accordingly, our management must determine the appropriate allocation of income to each such entity and each of these jurisdictions. Income tax audits associated with the allocation of this income and other complex issues, including inventory transfer pricing and cost sharing and product royalty arrangements, may require an extended period of time to resolve and may result in income tax adjustments if changes to the income allocation are required. Since income tax adjustments in certain jurisdictions can be significant, our future operating results could be negatively impacted by settlement of these matters.Future changes in technology or market conditions could result in adjustments to our recorded asset balance for intangible assets, including goodwill, resulting in additional charges that could significantly impact our operating results.Our consolidated balance sheet includes significant intangible assets, including $239.8 million in goodwill and $126.6 million in other acquired intangible assets, together representing 56% of our total assets. The determination of related estimated useful lives and whether these assets are impaired involves significant judgments. Our ability to accurately predict future cash flows related to these intangible assets may be adversely affected by unforeseen and uncontrollable events. In the highly competitive medical device industry, new technologies could impair the value of our intangible assets if they create market conditions that adversely affect the competitiveness of our products. We test our goodwill for impairment in the fourth quarter of each year, but we also test goodwill and other intangible assets for impairment at any time when there is a change in circumstances that indicates that the carrying value of these assets may be impaired. Any future determination that these assets are carried at greater than their fair value could result in substantial non-cash impairment charges, which could significantly impact our reported operating results.If reimbursement from third-party payors for our products becomes inadequate, surgeons and patients may be reluctant to use our products and our revenue may decline.In the United States, healthcare providers who purchase our products generally rely on third-party payors,principally federal Medicare, state Medicaid and private health insurance plans, to pay for all or a portion of the cost of joint reconstructive procedures and products utilized in those procedures. We may be unable to sell our products on a profitable basis if third-party payors deny coverage or reduce their current levels of reimbursement. Our revenue depends largely on governmental healthcare programs and private health insurers reimbursing patients’ medical expenses. As part of the Budget Control Act passed in August 2011 to extend the federal debt limit and reduce government spending, $1.2 trillion in automatic spending cuts (known as sequestration) over the next decade are due to go into effect, beginning in 2013, in the absence of further legislative action. Half of the automatic reductions would come from lowering the caps imposed on non-defense discretionary spending and cutting domestic entitlement programs, including reductions in payments to Medicare providers. Any such reductions in government healthcare spending could result in reduced demand for our products or additional pricing pressure.To contain costs of new technologies, third-party payors are increasingly scrutinizing new treatment modalities by requiring extensive evidence of clinical outcomes and cost-effectiveness. Currently, we are aware of several private insurers who have issued policies that classify procedures using our Salto Talaris Prosthesis and Conical Subtalar Implants as experimental or investigational and denied coverage and reimbursement for such procedures. Surgeons, hospitals and other healthcare providers may not purchase our products if they do not receive satisfactory reimbursement from these third-party payors for the cost of the procedures using our products. Payors continue to review their coverage policies carefully for existing and new therapies and can, without notice, deny coverage for treatments that include the use of our products. If we are not successful in reversing existing non-coverage policies or other private insurers issue similar policies, this could have a material adverse effect on our business and operations.In addition, some healthcare providers in the United States have adopted or are considering a managed care system in which the providers contract to provide comprehensive healthcare for a fixed cost per person. Healthcare providers may attempt to control costs by authorizing fewer elective surgical procedures, including joint reconstructive surgeries, or by requiring the use of the least expensive implant available. Additionally, there is a significant likelihood of reform of the U.S. healthcare system, and changes in reimbursement policies or healthcare cost containment initiatives that limit or restrict reimbursement for our products may cause our revenue to decline.If adequate levels of reimbursement from third-party payors outside of the United States are not obtained, international revenue of our products may decline. Outside of the United States, reimbursement systems vary significantly by country. Many foreign markets have government-managed healthcare systems that govern reimbursement for orthopaedic medical devices and procedures. Additionally, some foreign reimbursement systems provide for limited payments in a given period and therefore result in extended payment periods.Consolidation in the healthcare industry could lead to demands for price concessions or to the exclusion of some suppliers from certain of our markets, which could have an adverse effect on our business, financial condition or operating results.Because healthcare costs have risen significantly over the past decade, numerous initiatives and reforms initiated by legislators, regulators and third-party payors to curb these costs have resulted in a consolidation trend in the healthcare industry to create new companies with greater market power, including hospitals. As the healthcare industry consolidates, competition to provide products and services to industry participants has become and will continue to become more intense. This in turn has resulted and likely will continue to result in greater pricing pressures and the exclusion of certain suppliers from important market segments as group purchasing organizations, independent delivery networks and large single accounts continue to use their market power to consolidate purchasing decisions for some of our customers. We expect that market demand, government regulation, third-party reimbursement policies and societal pressures will continue to change the worldwide healthcare industry, resulting in further business consolidations and alliances among our customers, which may reduce competition, exert further downward pressure on the prices of our products and may adversely impact our business, financial condition or operating results.If we experience significant disruptions in our information technology systems, our business may be adversely affected.We depend on our information technology systems for the efficient functioning of our business, including accounting, data storage, purchasing and inventory management. Currently, we have a non-interconnected information technology system; however, we have undertaken planning for the implementation of an upgrade of our systems, which could include the implementation of a new global enterprise resource planning system (ERP). We expect that this upgrade will take two to three years to implement; however, when complete it should enable management to better and more efficiently conduct our operations and gather, analyze, and assess information across all of our business and geographic locations. This upgrade will require the investment of significant human and financial resources. We may experience difficulties in implementing this upgrade in our business operations, or difficulties in operating our business under this upgrade, either of which could disrupt our operations, including our ability to timely ship and track product orders, project inventoryrequirements, manage our supply chain, and otherwise adequately service our customers, and lead to increased costs and other difficulties. In the event we experience significant disruptions as a result of this implementation of an upgraded information technology system, we may not be able to fix our systems in an efficient and timely manner. Accordingly, such events may disrupt or reduce the efficiency of our entire operation and have a material adverse effect on our operating results and cash flows.Risks Related to Regulatory EnvironmentThe sale of our products is subject toFDA regulatory clearances or approvals and our business is subject to extensive regulatory requirements. If we fail to maintain regulatory clearances and approvals, or are unable to obtain, or experience significant delays in obtaining, FDA clearances or approvals for our future products or product enhancements, our ability to commercially distribute and market these products could suffer.Our medical device products and operations are subject to extensive regulation by the FDA and various other federal, state and foreign governmental authorities, such as those of the European Union and the competent authorities of the Member States of the EEA. Government regulation of medical devices is meant to assure their safety and effectiveness, and includes regulation of, among other things:design, development and manufacturing;testing, labeling, packaging, content and language of instructions for use, and storage;clinical trials;product safety;premarket clearance and approval;marketing, sales and distribution;advertising and promotion;recordkeeping procedures;recalls and field corrective actions;post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; andproduct import and export.Before a new medical device, or a new use of, or claim for, an existing product can be marketed in the United States, it must first receive either premarket clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act, or FDCA, ade novo approval or a PMA, from the FDA, unless an exemption applies. In the 510(k) clearance process, the FDA must determine that the proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, with respect to intended use, technology and safety and effectiveness to clear the proposed device for marketing. Clinical data is sometimes required to support substantial equivalence. Thede novo path is reserved for devices that are deemed by FDA to be Class II moderate risk devices for which there is no predicate device to which one can claim “substantial equivalence” as with a 510(k). These devices typically require more information for approval and take longer than a 510(k), but require less data and a shorter time period than a PMA approval. A device, once approved under thede novo path, becomes a 510(k) predicate for future devices seeking to call it a “predicate.” The PMA pathway requires an applicant to demonstrate the safety and effectiveness of the device for its intended use based, in part, on extensive data including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for devices that are deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices. Products that are approved through a PMA application generally need FDA approval before they can be modified. Similarly, some modifications made to products cleared through a 510(k) may require a new 510(k). Both the 510(k) and PMA processes can be expensive and lengthy and entail significant user fees, unless exempt. The FDA’s 510(k) clearance process usually takes from six to 18 months, but may take longer. The PMA pathway is much more costly and uncertain than the 510(k) clearance process and it generally takes from one to five years, or even longer, from the time the application is filed with the FDA until an approval is obtained. The process of obtaining regulatory clearances or approvals to market a medical device can be costly and time-consuming, and we may not be able to obtain these clearances or approvals on a timely basis, if at all.Most of our currently commercialized products have received premarket clearances under Section 510(k) of the FDCA. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, our product introductions or modifications could be delayed or canceled, which could cause our revenue to decline. In addition, the FDA may determine that future products will require the more costly, lengthy and uncertainde novo or PMA processes. Although we do not currently market any devices underde novo or PMA,we cannot assure you that the FDA will not demand that we obtain a PMA prior to marketing or that we will be able to obtain the 510(k) clearances with respect to future products.The FDA can delay, limit or deny clearance or approval of a device for many reasons, including:we may not be able to demonstrate to the FDA’s satisfaction that our products are safe and effective for their intended users;the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required;the manufacturing process or facilities we use may not meet applicable requirements; andchanges in FDA clearance or approval policies or the adoption of new regulations may require additional data.Any delay in, or failure to receive or maintain, clearances or approvals for our products under development could prevent us from generating revenue from these products or achieving profitability. Additionally, the FDA and other governmental authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could lead governmental authorities or a court to take action against us, including:issuing untitled (notice of violation) letters or public warning letters to us;imposing fines and penalties on us;obtaining an injunction preventing us from manufacturing or selling our products;seizing products to prevent sale or transport;bringing civil or criminal charges against us;recalling our products;detaining our products at U.S. Customs;delaying the introduction of our products into the market;delaying pending requests for clearance or approval of new uses or modifications to our existing products; orwithdrawing or denying approvals or clearances for our products.If we fail to obtain and maintain regulatory clearances or approvals, our ability to sell our products and generate revenue will be materially harmed.Outside of the United States, our medical devices must comply with the laws and regulations of the foreign countries in which they are marketed, and compliance may be costly and time-consuming.To market and sell our product in countries outside the United States, we must seek and obtain regulatory approvals, certifications or registrations and comply with the laws and regulations of those countries. These laws and regulations, including the requirements for approvals, certifications or registrations and the time required for regulatory review, vary from country to country. Obtaining and maintaining foreign regulatory approvals, certifications or registrations are expensive, and we cannot be certain that we will receive regulatory approvals, certifications or registrations in any foreign country in which we plan to market our products. If we fail to obtain or maintain regulatory approvals, certifications or registrations in any foreign country in which we plan to market our products, our ability to generate revenue will be harmed.In particular, in the EEA, which is composed of the 27 Member States of the EU plus Liechtenstein, Norway and Iceland, our medical devices must comply with the essential requirements of the EU Medical Devices Directives (Council Directive 93/42/EEC of 14 June 1993 concerning medical devices, as amended, and Council Directive 90/385/EEC of 20 June 2009 relating to active implantable medical devices, as amended). Compliance with these requirements entitles us to affix the CE conformity mark to our medical devices, without which they cannot be marketed in the EEA.Further, the advertising and promotion of our products is subject to EEA Member States laws implementing Directive 93/42/EEC concerning Medical Devices, or the EU Medical Devices Directive, Directive 2006/114/EC concerning misleading and comparative advertising, and Directive 2005/29/EC on unfair commercial practices, as well as other EEA Member State legislation governing the advertising and promotion of medical devices. These laws may limit or restrict the advertising and promotion of our products to the general public and may impose limitations on our promotional activities with healthcare professionals.Modifications to our marketed products may require new 510(k) clearances or PMAs, or may require us to cease marketing or recall the modified productsdevices until such additional clearances or approvals are obtained.a 510(k)-clearedan FDA approved or cleared device that couldwould significantly affect its safety or efficacy or that would constitute a major change in its intended use technology, materials, packaging and certain manufacturing processes, maywould require a new PMA or 510(k) clearance and could be considered misbranded if the modified device is commercialized and such additional approval or possibly, a PMA. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) clearance or PMA in the first instance, but the FDA may (and often does) review the manufacturer’s decision. The FDA maywas not agree with a manufacturer’s decision regarding whether a new clearance or approval is necessary for a modification, and may retroactively require the manufacturer to submit a premarket notification requesting 510(k) clearance or an application for PMA.obtained. We have made modifications to our products in the past and may make additional modifications in the future that we believe do not or will not require additional clearances or approvals. No assurance can be givencannot assure you that the FDA wouldwill agree with any of our decisions not to seek approvals or clearances for particular device modifications or that we will be successful in obtaining additional approvals or 510(k) clearances for modifications.PMA.efficacy of the device, and did not require new approvals or clearances. If the FDA disagrees with our decisions and requires us to obtain additional premarket approvals or 510(k) clearances for any modifications to our products and we fail to obtain such approvals or clearances or fail to secure approvals or clearances in a timely manner, we may be required to cease manufacturing and marketing andthe modified device or to recall thesuch modified device until we obtain a new 510(k)FDA approval or clearance or PMA, our business, financial condition, operating results and future growth prospects could be materially adversely affected. Further, our products couldwe may be subject to recallsignificant regulatory fines or penalties.FDA determines,United States Attorney’s Office for any reason,the District of New Jersey (USAO). On September 15, 2011, WMT reached an agreement with the USAO and the OIG-HHS under which WMT voluntarily agreed to extend the term of its the Deferred Prosecution Agreement for 12 months. On October 4, 2012, the USAO issued a press release announcing that the amended Deferred Prosecution Agreement expired on September 29, 2012, that the USAO had moved to dismiss the criminal complaint against WMT because WMT had fully complied with the terms of the Deferred Prosecution Agreement, and that the court had ordered dismissal of the complaint on October 4, 2012. On September 29, 2010, WMT also entered into a five-year Corporate Integrity Agreement with the Office of the Inspector General of the United States Department of Health and Human Services. The CIA was filed as Exhibit 10.2 to legacy Wright's Current Report on Form 8-K filed on September 30, 2010. The CIA expired on September 29, 2015 and on January 27, 2016, we received notification from the OIG-HHS that the term of the CIA has concluded. While the term of the CIA has concluded, our products are not safe or effective. Any recall or FDA requirement that we seek additional approvals or clearances could result in significant delays, fines, increased costs associatedfailure to continue to maintain compliance with modification of a product, loss of revenue and potential operating restrictions imposed by the FDA.Healthcare policy changes, including legislation to reform the U.S. healthcare system, may have a material adverse effect on us.The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, collectively, the PPACA, substantially changes the way health care is financed by both governmental and private insurers, encourages improvements in the quality of healthcare items and services, and significantly impacts the medical device industry. The PPACA includes, among other things, the following measures:an excise tax on any entity that manufactures or imports medical devices offered for sale in the United States;a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research;new reporting and disclosure requirements on device manufacturers for any “transfer of value” made or distributed to prescriberslaws, regulations and other healthcare providers, effective March 30, 2013 (referred to as the Physician Sunshine Payment Act), which reporting requirements will be difficult to define, track and report;payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models, beginning on or before January 1, 2013;an independent payment advisory board that will submit recommendations to reduce Medicare spending if projected Medicare spending exceeds a specified growth rate; anda new licensure framework for follow-on biologic products.We cannot predict what healthcare programs and regulations will be ultimately implemented at the federal or state level, or the effect of any future legislation or regulation. However, these provisions as adopted could meaningfully change the way healthcare is delivered and financed, and may materially impact numerous aspects of our business. In particular, any changes that lower reimbursements for our products or reduce medical procedure volumes could adversely affect our business and operating results.In addition, in the future there may continuecould expose us to besignificant liability, including, but not limited to, exclusion from federal healthcare program participation, including Medicaid and Medicare, potential prosecution, civil and criminal fines or penalties, as well as additional proposals relating to the reform of the U.S. healthcare system. Certain of these proposals could limit the prices we are able to charge for our products, or the amounts of reimbursement available for our products,litigation cost and could limit the acceptance and availability of our products. The adoption of some or all of these proposals couldexpense, which would have a material adverse effect on our financial position and operating results.Furthermore, initiatives sponsored by government agencies, legislative bodies and the private sector to limit the growth of healthcare costs, including price regulation and competitive pricing, are ongoing in markets where we do business. We could experience a negative impact on ourcondition, operating results dueand cash flows.increased pricing pressuremarket our products.United Statesprocess of implementing systems and certain other markets. Governments, hospitalsprocedures to control this activity in order to comply with these requirements, including establishing contractual relationships with the healthcare provider clinical study sites in accordance with our internal compliance requirements. We intend to obtain the needed clinical data to support our marketed products, but there can be no assurance that European regulators will accept the results. This could potentially impact business performance. In addition, changes to the certification and other third-party payorsoversight responsibilities of notified bodies presently under consideration by the European Commission, if implemented, could reduceresult in more stringent notified body oversight requirements, require additional resources to maintain compliance, and increase the amountrisk of approved reimbursementsnegative audit observations.our products. Reductions in reimbursement levels or coverage or other cost-containment measures could unfavorably affect our future operating results.Our financial performance mayallograft-based, tissue-containing products, which are principally derived from cadaveric tissue. The framework developed by the FDA establishes risk-based criteria for determining whether a particular human tissue-based product will be adversely affected by medical device tax provisions in the health care reform laws.The PPACA imposes a deductible excise tax equal to 2.3% of the price ofclassified as human tissue, a medical device, on any entity that manufactures or imports medical devices offered for sale in the United States, with limited exceptions, beginning in 2013. Under these provisions, the total cost to the medical device industry is estimated to be approximately $20 billion over 10 years. These taxes would result in a significant increase in the tax burden on our industry, which could have a material, negative impact on our operating results and our cash flows. The tax could create a risk up to 2.3% of our United States revenue.The use, misuse or off-label use of our products may harm our image in the marketplace or result in injuries that lead to product liability suits, which could be costly to our business or result in FDA sanctions if we are deemed to have engaged in improper promotion of our products.Our currently marketed products have been cleared by the FDA’s 510(k) clearance process for use under specific circumstances. Our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition on the promotion of a medical device for a use that has not been cleared or approved by the FDA. Use of a device outside of its cleared or approved indication is known as “off-label” use. We cannot prevent a surgeon from using our products or procedure for off-label use, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. However, if the FDA determines that our promotional materials or training constitute promotion of an off-label use, the FDA could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties. Other federal, state or foreign governmental authorities also might take action if they consider our promotion or training materials to constitute promotion of an uncleared or unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of the products would be impaired. Although we train our sales force not to promote our products for off-label uses, and our instructions for use in all markets specify that our products are not intended for use outside of those indications cleared for use, the FDA or another regulatory agency could conclude that we have engaged in off-label promotion.In addition, there may be increased risk of injury if surgeons attempt to use our products off-label. Furthermore, the use of our products for indications other than those indications for which our products have been cleared by the FDA may not effectively treat such conditions, which could harm our reputation in the marketplace among surgeons and patients. Surgeons also may misuse our products or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability. Product liability claims are expensive to defend and could divert our management’s attention and result in substantial damage awards against us. Any of these events could harm our business and operating results.If our marketed medical devices are defective or otherwise pose safety risks, the FDA and similar foreign governmental authorities could require their recall, or we may initiate a recall of our products voluntarily.The FDA and similar foreign governmental authorities may require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture or in the event that a product poses an unacceptable risk to health. Manufacturers, on their own initiative, may recall a product if any material deficiency in a device is found. In the past we have initiated voluntary product recalls. For example, in 2008, we recalled a small number of medical devices due to a mislabeled product. We requested FDA closure of the recall in January 2010. A government-mandated or voluntary recall by us or one of our sales agencies could occur as a result of an unacceptable risk to health, component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and operating results. Any recall could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We also may be required to bear other costs or take other actions that may have a negative impact on our future revenue and our ability to generate profits. We may initiate voluntary recalls involving our products in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our revenue. In addition, the FDA could take enforcement action for failing to report the recalls when they were conducted.In the EEA we must comply with the EU Medical Device Vigilance System, the purpose of which is to improve the protection of health and safety of patients, users and others by reducing the likelihood of reoccurrence of incidents related to the use of a medical device. Under this system, incidents must be reported to the competent authorities of the Member States of the EEA. An incident is defined as any malfunction or deterioration in the characteristics and/or performance of a device, as well as any inadequacy in the labeling or the instructions for use which, directly or indirectly, might lead to or might haveled to the death of a patient or user or of other persons or to a serious deterioration in their state of health. Incidents are evaluated by the EEA competent authorities to whom they have been reported, and where appropriate, information is disseminated between them in the form of National Competent Authority Reports, or NCARs. The Medical Device Vigilance System is further intended to facilitate a direct, early and harmonized implementation of Field Safety Corrective Actions, or FSCAs across the Member States of the EEA where the device is in use. An FSCA is an action taken by a manufacturer to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. An FSCA may include the recall, modification, exchange, destruction or retrofitting of the device. FSCAs must be communicated by the manufacturer or its legal representative to its customers and/or to the end users of the device through Field Safety Notices.If our products cause or contribute to a death or a serious injury, or malfunction in certain ways, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.Under the FDA medical device reporting regulations, or MDR, we are required to report to the FDA any incident in which our product has or may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. If we fail to report these events to the FDA within the required timeframes, or at all, the FDA could take enforcement action against us. Any adverse event involving our products could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection, mandatory recall or other enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.Our manufacturing operations require us to comply with the FDA’s and other governmental authorities’ laws and regulations regarding the manufacture and production of medical devices, which is costly and could subject us to enforcement action.We and certain of our third-party manufacturers are required to comply with the FDA’s Quality System Regulation, or QSR, which covers the methods of documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our products. We and certain of our suppliers also are subject to the regulations of foreign jurisdictions regarding the manufacturing process for our products marketed outside of the United States. The FDA enforces the QSR through periodic announced and unannounced inspections of manufacturing facilities. In January 2013, our OrthoHelix facility located in Medina, Ohio was subject to a routine FDA inspection. The inspection resulted in the issuance of a Form FDA-483 listing four inspectional observations. The FDA’s observations related to our documentation of corrective and preventative actions, procedures for receiving, reviewing and evaluating complaints, procedures to control product that does not conform to specified requirements and procedures to ensure that all purchased or otherwise received product and services conform to specified requirements. Although we believe we have corrected all four of these observations, the FDA could disagree with our conclusion and corrective and remedial measures. The failure by us or one of our suppliers to comply with applicable statutes and regulations administered by the FDA and other regulatory bodies, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in, among other things, any of the following enforcement actions:untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;customer notifications or repair, replacement, refunds, recall, detention or seizure of our products;operating restrictions or partial suspension or total shutdown of production;refusing or delaying our requests forbiologic drug requiring 510(k) clearance or PMA of new products or modified products;withdrawing 510(k) clearances or PMAs that have already been granted;refusal to grant export approval for our products; orcriminal prosecution.Any of these actions could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We also may be required to bear other costs or take other actions that may have a negative impact on our future revenue and our ability to generate profits. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.We are subject to substantial post-market government regulation that could have a material adverse effect on our business.The production and marketing of ourapproval. All tissue-based products are subject to extensive FDA regulation, including establishment of registration requirements, product listing requirements, good tissue practice requirements for manufacturing, and screening requirements that ensure that diseases are not transmitted to tissuebyof safety and efficacy is not required before the tissue can be marketed. However, if tissue is considered a medical device or biologic drug, then FDA andnumerous other governmental authorities both inclearance or approval is required.United Statesprocurement and abroad. For example, in additiontransplantation of allograft tissue, which is subject to other state regulatory requirements, Massachusetts, California and Arizona require compliance with the standards in industry codes such as the Code of Ethics on Interactions with Health Care Professionals issued by the Advanced Medical Technology Association (commonly known as AdvaMed), the Code on Interactions with Healthcare Professionals issued by MEDEC, the national association of Canada’s medical technology companies, and international equivalents. Many of these standards simply create industry standards of conduct, others tie into compliance with the advertising and promotion regulationsfederal regulation under the Food, Drug & CosmeticNational Organ Transplant Act the Anti-kickback Statute, the False Claims Act, HIPAA and the Physician Sunshine Payment Act. The failure by us or one of our suppliers to comply with applicable legal and regulatory requirements could result in, among other things, the FDA or other governmental authorities:imposing fines and penalties on us;preventing us from manufacturing or selling our products;delaying the introduction of our new products into the market;recalling, seizing or enjoining(NOTA). NOTA prohibits the sale of our products;withdrawing, delaying or denying approvals or clearances for our products;issuing warning letters or untitled letters;imposing operating restrictions;imposing injunctions; andcommencing criminal prosecutions.Failure to comply with applicable regulatory requirements also could result in civil actions against ushuman organs, including bone and other unanticipated expenditures. If anyhuman tissue, for valuable consideration within the meaning of NOTA. NOTA permits the payment of reasonable expenses associated with the transportation, processing, preservation, quality control, and storage of human tissue. We currently charge our customers for these actions wereexpenses. In the future, if NOTA is amended or reinterpreted, we may not be able to occur it would harmcharge these expenses to our reputationcustomers, and, causeas a result, our product revenue to sufferbusiness could be adversely affected.may prevent us from generating revenue.pre-clinicalclinical development do not perform as contractually required or expected, we may not be able to obtain, or in some cases, maintain regulatory clearance or approval for or commercialize our products.thetheir failure to adhere to our clinical protocols or regulatory requirements, or for other reasons, our pre-clinical and clinical development activities or clinical trials may be extended, delayed, suspended, or terminated, and we may not be able to obtain or, in some cases maintain, regulatory clearance or approval for, or successfully commercialize, our products on a timely basis, if at all, and our business, operating results, and prospects may be adversely affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control.Future regulatory actionsadversely affectbe negatively affected, and we may not achieve future growth.sellmaintain a competitive position in the development of technologies and products for use by our customers. Many of the companies developing or marketing competitive products profitably.From timeenjoy several competitive advantages over us, including greater financial and human resources for product development and sales and marketing; greater name recognition; established relationships with surgeons, hospitals and third-party payors; broader product lines and the ability to time, legislation is draftedoffer rebates or bundle products to offer greater discounts or incentives to gain a competitive advantage; and introduced that could significantly change the statutory provisions governing the clearance or approval, manufactureestablished sales and marketing and distribution networks. Some of a medical device. In addition, FDAour competitors have indicated an increased focus on the extremities and other regulationsbiologics markets, which are our primary strategic focus. Our competitors may develop and guidance are often revisedpatent processes or reinterpreted in waysproducts earlier than us, obtain regulatory clearances or approvals for competing products more rapidly than us, develop moremay significantly affectrender our businesstechnology or products obsolete or non-competitive or acquire technologies and our products. It is impossibletechnology licenses complementary to predict whether legislative changes will be enacted or regulations, guidance or interpretations changed, andwhat the impact of such changes, if any, may be.We may be subject to or otherwise affected by federal and state healthcare laws, including fraud and abuse and health information privacy and security laws, and could face substantial penalties if we are unable to fully comply with such laws.Although we do not provide healthcare services, submit claims for third-party reimbursement, or receive payments directly from Medicare, Medicaid or other third-party payors for our products or the procedures in which our products are used, healthcare regulation by federal and state governments could significantly impact our business. Healthcare fraud and abuse and health information privacy and security laws potentially applicableadvantageous to our operations include:the federal Anti-Kickback Law,business, which constrains our marketing practices and those of our independent sales agencies, educational programs, pricing, bundling and rebate policies, grants for physician-initiated trials and CME, and other remunerative relationships with healthcare providers, by prohibiting, among other things, soliciting, receiving, offering or providing remuneration, intended to induce the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare or Medicaid programs;federal false claims laws which prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent;the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, and its implementing regulations, which created federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain regulatory and contractual requirements regarding the privacy, security and transmission of individually identifiable health information; andstate laws analogous to each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy and security of certain health information, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.If our past or present operations, or those of our independent sales agencies, are found to be in violation of any of such laws or any other governmental regulations that may apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from federal healthcare programs and the curtailment or restructuring of our operations. Similarly, if the healthcare providers or entities with whom we do business are found to be non-compliant with applicable laws, they may be subject to sanctions, which could also have a negative impact on us. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. The risk of our company being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Further, the recently enacted PPACA, among other things, amends the intent requirement of the federal anti-kickback and criminal health care fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. Any action against us for violation of these laws, even if we successfully defend against them, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.The PPACA also includes a number of provisions that impact medical device manufacturers, including new reporting and disclosure requirements on device and drug manufacturers for any “transfer of value” made or distributed to prescribers and other healthcare providers, effective March 30, 2013 (known as the Physician Sunshine Payment Act). Such information will be made publicly available in a searchable format beginning September 30, 2013. In addition, device and drug manufacturers also will be required to report and disclose any investment interests held by physicians and their immediate family members during the preceding calendar year. Failure to submit required information may result in civil monetary penalties of up to an aggregate of $150,000 per year (and up to an aggregate of $1 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests not reported in an annual submission. The PPACA also imposes excise taxes on medical device manufacturers, permits the use of comparative effectiveness research to make Medicare coverage determinations in certain circumstances, creates an Independent Medicare Advisory Board charged with recommending ways to reduce the rate of Medicare spending and changes payment methodologies under the Medicare and Medicaid programs. All of these changes could adversely affect our business and financialoperating results.Governments and regulatory authorities have increased their enforcement of these healthcare fraud and abuse laws in recent years. For example, in 2007 five competitors in the orthopaedics industry settled a Department of Justiceinvestigation into the financial relationships and consulting agreements between the companies and surgeons for a combined fine of $311.0 million. The companies agreed to new corporate compliance procedures and federal monitoring. At issue were alleged financial inducements designed to encourage physicians to use the payor company’s products exclusively and the failure of physicians to disclose these relationships to hospitals and patients. Individual states also may be investigating the relationship between healthcare providers and companies in the orthopaedics industry. Many states have their own regulations governing the relationship between companies and healthcare providers. While we have not been the target of any investigations, we cannot guarantee that we will not be investigated in the future. If investigated we cannot assure that the costs of defending or resolving those investigations or proceedings would not have a material adverse effect on our financial condition, operating results and cash flows.Failure to obtain and maintain regulatory approvals in jurisdictions outside the United States will prevent us from marketing our products in such jurisdictions.We currently market, and intend to continue to market, our products outside the United States. Outside the United States, we can market a product only if we receive a marketing authorization and, in some cases, pricing approval, from the appropriate regulatory authorities. The approval procedure varies among countries and can involve additional testing, and the time required to obtain approval may differ from that required to obtain FDA clearance or approval. The regulatory approval process outside the United States may include Not all of the risks associated with obtaining FDA clearance or approval in addition to other risks. For example, in order to market our products in the Member States of the EEA, our devices are required to comply with the essential requirements of the EU Medical Devices Directives (Council Directive 93/42/EEC of 14 June 1993 concerning medical devices, as amended, and Council Directive 90/385/EEC of 20 June 2009 relating to active implantable medical devices, as amended). Compliance with these requirements entitles us to affix the CE conformity mark to our medical devices, without which they cannot be commercialized in the EEA. In order to demonstrate compliance with the essential requirements and obtain the right to affix the CE conformity mark we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low risk medical devices (Class I), where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the Medical Devices Directives, a conformity assessment procedure requires the intervention of a Notified Body, which is an organization accredited by a Member State of the EEA to conduct conformity assessments. The Notified Body would typically audit and examine the quality system for the manufacture, design and final inspection of our devices before issuing a certification demonstrating compliance with the essential requirements. Based on this certification we can draw up an EC Declaration of Conformity, which allows us to affix the CE mark to our products.We may not obtain regulatory approvals or certifications outside the United States on a timely basis, if at all. Clearance or approval by the FDA does not ensure approval or certification by regulatory authorities or Notified Bodies in other countries, and approval or certification by one foreign regulatory authority or Notified Body does not ensure approval by regulatory authorities in other countries or by the FDA. We may be required to perform additional pre-clinical or clinical studies even if FDA clearance or approval, or the right to bear the CE mark, has been obtained. If we fail to receive necessary approvals to commercialize our products in jurisdictions outside the United States on a timely basis, or at all, our business, financial condition and operating results could be adversely affected.Our existing xenograft-based biologics business is and any future biologics products we pursue would be subject to emerging governmental regulations that could materially affect our business.Some of our products are xenograft, or animal-based, tissue products. Our principal xenograft-based biologics offering is Conexa reconstructive tissue matrix. All of our current xenograft tissue-based products are regulated as medical devices and are subject to the FDA’s medical device regulations.We currently are planning to offer products based on human tissue. The FDA has statutory authority to regulate human cells, tissues and cellular and tissue-based products, or HCT/Ps. An HCT/P is a product containing or consisting of human cells or tissue intended for transplantation into a human patient, including allograft-based products. The FDA, EU and Health Canada have been working to establish more comprehensive regulatory frameworks for allograft-based, tissue-containing products, which are principally derived from cadaveric tissue.Section 361 of the Public Health Service Act, or PHSA, authorizes the FDA to issue regulations to prevent the introduction, transmission or spread of communicable disease. HCT/Ps regulated as 361 HCT/Ps are subject to requirements relating to: registering facilities and listing products with the FDA; screening and testing for tissue donor eligibility; Good Tissue Practice, or GTP, when processing, storing, labeling and distributing HCT/Ps, including required labeling information; stringent recordkeeping; and adverse event reporting. The FDA has also proposed extensive additional requirements that address sub-contracted tissue services, tracking to the recipient/patient, and donor records review. If a tissue-based product isconsidered human tissue, the FDA requirements focus on preventing the introduction, transmission and spread of communicable diseases to recipients. A product regulated solely as a 361 HCT/P is not required to undergo premarket clearance or approval.The FDA may inspect facilities engaged in manufacturing 361 HCT/Ps and may issue untitled letters, warning letters, or otherwise authorize orders of retention, recall, destruction and cessation of manufacturing if the FDA has reasonable grounds to believe that an HCT/P or the facilities where it is manufactured are in violation of applicable regulations. There also are requirements relating to the import of HCT/Ps that allow the FDA to make a decision as to the HCT/Ps’ admissibility into the United States.An HCT/P is eligible for regulation solely as a 361 HCT/P if it is: minimally manipulated; intended for homologous use as determined by labeling, advertising or other indications of the manufacturer’s objective intent for a homologous use; the manufacture does not involve combination with another article, except for water, crystalloids or a sterilizing, preserving, or storage agent (not raising new clinical safety concerns for the HCT/P); and it does not have a systemic effect and is not dependent upon the metabolic activity of living cells for its primary function or, if it has such an effect, it is intended for autologous use or allogenetic use in close relatives or for reproductive use. If any of these requirements are not met, then the HCT/P is also subject to applicable biologic, device, or drug regulation under the FDCA or the PHSA. These biologic, device or drug HCT/Ps must comply both with the requirements exclusively applicable to 361 HCT/Ps and, in addition, with requirements applicable to biologics under the PHSA, or devices or drugs under the FDCA, including premarket licensure, clearance or approval.Title VII of the PPACA, the Biologics Price Competition and Innovation Act of 2009, or BPCIA, creates a new licensure framework for follow-on biologic products, which could ultimately subject our biologics business to competition to so-called “biosimilars.” Under the BPCIA, a manufacturer may submit an application for licensure of a biologic product that is “biosimilar to” or “interchangeable with” a referenced, branded biologic product. Previously, there had been no licensure pathway for such a follow-on product. While we do not anticipate that the FDA will license a follow-on biologic for several years, given the need to generate data sufficient to demonstrate “biosimilarity” to or “interchangeability” with the branded biologic according to criteria set forth in the BPCIA, as well as the need for the FDA to implement the BPCIA’s provisions with respect to particular classes of biologic products, we cannot guarantee that our biologics will not eventually become subject to direct competition by a licensed “biosimilar.”Procurement of certain human organs and tissue for transplantation, including allograft tissue we may use in future products, is subject to federal regulation under the National Organ Transplant Act, or NOTA. NOTA prohibits the acquisition, receipt, or other transfer of certain human organs, including bonesales and other human tissue, for valuable consideration within the meaning of NOTA. NOTA permits the payment of reasonable expenses associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human organs. For any future products implicating NOTA’s requirements, we would reimburse tissue banks for their expenses associated with the recovery, storage and transportation of donated human tissue that they would provide to us. NOTA payment allowances may be interpreted to limit the amount of costs and expenses that we may recover in our pricing for our services, thereby negatively impacting our future revenue and profitability. If we were to be found topersonnel have violated NOTA’s prohibition on the sale or transfer of human tissue for valuable consideration, we would potentially be subject to criminal enforcement sanctions, which could materially and adversely affect our operating results. Further, in the future, if NOTA is amended or reinterpreted, we may not be able to pass these expenses on to our customers and, as a result, our business could be adversely affected.Our operations involve the use of hazardous materials, and we must comply with environmental health and safety laws and regulations, which can be expensive and may affect our business and operating results.We are subject to a variety of laws and regulations of the countries in which we operate and distribute products, such as the European Union, or EU, France, Ireland, other European nations and the United States, relating to the use, registration, handling, storage, disposal, recycling and human exposure to hazardous materials. Liability under environmental laws can be joint and several and without regard to comparative fault, and environmental, health and safety laws could become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations, which could harm our business. In the EU, where our manufacturing facilities are located, we and our suppliers are subject to EU environmental requirements such as the Registration, Evaluation, Authorization and Restriction of Chemicals, or REACH, regulation. In addition, we are subject to the environmental, health and safety requirements of individual European countries in which we operate such as France and Ireland. For example, in France, requirements known as the Installations Classées pour la Protection de l’Environnement regime provide for specific environmental standards related to industrial operations such as noise, water treatment, air quality and energy consumption. In Ireland, our manufacturing facilities are likewise subject to local environmental regulations, such as related to water pollution and water quality, that are administered by the Environmental Protection Agency. We believe that we are in material compliance with all applicable environmental, healthand safety requirements in the countries in which we operate and do not have reason to believe that we are responsible for any cleanup liabilities. In addition, certain hazardous materials are present at some of our facilities, such as asbestos, that we believe are managed in compliance with all applicable laws.non-compete agreements. We also are subject to greenhouse gas regulationscompete with other organizations in the EUrecruiting and elsewhere and we believe that we are in compliance based on present emissions levels at our facilities. Although we believe that our activities conform in all material respects with applicable environmental, health and safety laws, we cannot assure you that violations of such laws will not arise as a result of human error, accident, equipment failure, presently unknown conditions or other causes. The failure to comply with past, present or future laws, including potential laws relating to climate control initiatives, could result in the imposition of fines, third-party property damage and personal injury claims, investigation and remediation costs, the suspension of production or a cessation of operations. We also expect that our operations will be affected by other new environmental and health and safety laws, including laws relating to climate control initiatives, on an ongoing basis. Although we cannot predict the ultimate impact of any such new laws, they could result in additional costs and may require us to change how we design, manufacture or distribute our products, which could have a material adverse effect on our business.Our business is subject to evolving corporate governance and public disclosure regulations that have increased both our compliance costs and the risk of noncompliance, which could have an adverse effect on our stock price.We are subject to changing rules and regulations promulgated by a number of governmental and self-regulated organizations, including the SEC, the NASDAQ Stock Market, and the FASB. These rules and regulations continue to evolve in scope and complexity and many new requirements have been created in response to laws enacted by Congress, making compliance more difficult and uncertain. For example, our efforts to comply with the Dodd-Frank Wall Street Reform and Consumer Protection Act and other new regulations have resulted in, and are likely to continue to result in, increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities.Risks Related to Our Intellectual PropertyIf our patents and other intellectual property rights do not adequately protect our products, we may lose market share to our competitors.We rely on patents, trade secrets, copyrights, know-how, trademarks, license agreements and contractual provisions to establish our intellectual property rights and protect our products. These legal means, however, afford only limited protection and may not adequately protect our rights. The patents we own may not be of sufficient scope or strength to provide us with any meaningful protection or commercial advantage, and competitors may be able to design around our patents or develop products that provide outcomes that are similar to ours. In addition, we cannot be certain that any of our pending patent applications will be issued. The USPTO may reject or require a significant narrowing of the claims in our pending patent applications affecting the patents issuing from such applications. Any patents issuing from the pending patent applications may not provide us with significant commercial protection. We could incur substantial costs in proceedings before the USPTO and the proceedings may be time-consuming, which may cause significant diversion of effort by our technicalretaining qualified scientific, sales, and management personnel. These proceedings could resultIf our competitors are more successful than us in adverse decisions as to the validity of our inventions and may result in the narrowing or cancellation of claims in issued patents. In addition, the laws of some of the countries in which our products are or may be sold may not protect our intellectual property to the same extent as U.S. laws or at all. We alsothese matters, we may be unable to protectcompete successfully against our rights in trade secrets and unpatented proprietary technology in these countries.In the event a competitor infringes our patentexisting or other intellectual property rights, enforcing those rights may be costly, difficult and time-consuming. Even if successful, litigation to enforce our intellectual property rights or to defend our patents against challenge could be expensive and time-consuming and could divert our management’s attention. We may not have sufficient resources to enforce our intellectual property rights or to defend our patents or other intellectual property rights against a challenge. If we are unsuccessful in enforcing and protecting our intellectual property rights and protecting our products, it could harm our business and operating results.there are numerous recent changesthe orthopaedic industry has been subject to increasing consolidation recently and over the U.S. patent lawslast few years. Consolidation in our industry not involving our company could result in existing competitors increasing their market share through business combinations and proposed changes to the rules of the USPTO, which may have a significant impact on our ability to obtain and enforce intellectual property rights. For example, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was adoptedresult in September 2011. The Leahy-Smith Act includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. Under the Leahy-Smith Act, the U.S. will transition from a “first-to-invent” system to a “first-to-file” system for patent applications filed on or after March 16, 2013. With respect to patent applications filed on or after March 16, 2013, if we are the first to invent but not the first to file a patent application, we may not be able to fully protect our intellectual property rights and may be found to have violated the intellectual property rights of others if we continue to operate in the absence of a patent issued to us. The USPTO is currently developing regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associatedwith the Leahy-Smith Act have recently become effective. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all ofstronger competitors, which could have a material adverse effect on our business, financial condition, and financial condition.rely onmay be unable to compete successfully in an increasingly consolidated industry and cannot predict with certainty how industry consolidation will affect our trademarks, trade namescompetitors or us.brand namessocial instability and expose us to distinguish our products fromadditional risks.productsUnited States accounted for approximately 28% of our competitors, and have registered or applied to register many of these trademarks. However,net sales for our trademark applications may not be approved. Third parties may also oppose our trademark applications or otherwise challenge our usefiscal year ended December 27, 2015. Our operations outside of the trademarks. InUnited States are accompanied by certain financial and other risks. We intend to continue to pursue growth opportunities in sales outside the eventUnited States, especially in emerging markets, which could expose us to greater risks associated with international sales operations. Our international sales operations expose us and our representatives, agents, and distributors to risks inherent in operating in foreign jurisdictions. These risks include:trademarks are successfully challenged, we could be forced to rebrand our products,target markets;brand recognitionany key personnel who possess proprietary knowledge or are otherwise important to our success in international markets;requiresuffer serious consequences.devote resourcespay our indebtedness, including payments of principal upon conversion of outstanding Notes or on their respective maturity dates or in connection with a transaction involving us that constitutes a fundamental change under the respective indenture governing the Notes, or to advertisingfund our liquidity needs, we may be forced to refinance all or a portion of our indebtedness, including the Notes, on or before the maturity dates thereof, sell assets, reduce or delay capital expenditures, seek to raise additional capital, or take other similar actions. We may not be able to execute any of these actions on commercially reasonable terms or at all. Our ability to refinance our indebtedness will depend on our financial condition at the time, the restrictions in the instruments governing our indebtedness, and marketing these new brands. Further,other factors, including market conditions. In addition, in the event of a default under the Notes, the holders and/or the trustee under the indentures governing the Notes may accelerate payment obligations under the Notes, which could have a material adverse effect on our business, financial condition, and operating results. Our inability to generate sufficient cash flow to satisfy our debt service obligations, or to refinance or restructure our obligations on commercially reasonable terms or at all, would likely have an adverse effect, which could be material, on our business, financial condition, and operating results.may infringewho have less debt; andtrademarks,ability to borrow additional amounts for working capital, capital expenditures, contractual obligations, research and development efforts, acquisitions, debt service requirements, execution of our business strategy, or we may not have adequate resources to enforceother purposes.trademarks.hold licenses from third partiesmay incur additional indebtedness, and if we do, the risks related to our business and our ability to service our indebtedness would increase.are necessarywould otherwise be beneficial to our security holders.designcovenants and manufacturingother provisions of somethe indentures governing the Notes could result in events of default under such indentures, which could require the immediate repayment of our products. The loss of such licenses would prevent us from manufacturing, marketing and selling these products, which could harm our business.In addition to patents, we seek to protect our trade secrets, know-how and other unpatented technology, in part, with confidentiality agreements with our vendors, employees, consultants and others who may have access to proprietary information. We cannot be certain, however, that these agreements will not be breached, adequate remedies for any breach would be available or our trade secrets, know-how and other unpatented proprietary technology will not otherwise become known to or be independently developed by our competitors.subjectat any time unable to any future intellectual property lawsuits, a court could require usgenerate sufficient cash flows from operations to pay significant damages or prevent us from sellingservice our products.The orthopaedic medical device industryindebtedness when payment is litigious with respect to patents and other intellectual property rights. Companies in the orthopaedic medical device industry have used intellectual property litigation to gain a competitive advantage. In the future, we may become a party to lawsuits involving patents or other intellectual property. A legal proceeding, regardless of outcome, could drain our financial resources and divert the time and effort of our management. A patent infringement suit or other infringement or misappropriation claim brought against us or any of our licensees may force us or any of our licensees to stop or delay developing, manufacturing or selling potential products that are claimed to infringe a third party’s intellectual property, unless that party grants us or any licensees rights to use its intellectual property. In such cases,due, we may be required to attempt to renegotiate the terms of the indentures and other agreements relating to the indebtedness, seek to refinance all or a portion of the indebtedness, or obtain licensesadditional financing. There can be no assurance that we will be able to patentssuccessfully renegotiate such terms, that any such refinancing would be possible, or proprietary rightsthat any additional financing could be obtained on terms that are favorable or acceptable to us.orderaddition to those under our existing indentures. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our shareholders. If we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our products or scale back our operations.commercializedevelop and market new products and technologies, we may experience a decrease in demand for our products. However,products, or our products could become obsolete, and our business would suffer.obtaindetermine the commercial viability of a new product, technology, material, or innovation. Demand for our products also could change in ways we may not anticipate due to evolving customer needs, changing demographics, slow industry growth rates, declines in the extremities and biologics market, the introduction of new products and technologies, evolving surgical philosophies, and evolving industry standards, among others. Additionally, our competitors’ new products and technologies may beat our products to market, may be more effective or less expensive than our products, or may render our products obsolete. Our new products and technologies also could render our existing products obsolete and thus adversely affect sales of our existing products and lead to increased expense for excess and obsolete inventory.licenses required under any patentsinability on our part to enforce non-compete or proprietary rightsnon-solicitation arrangements related to key personnel who have left the business could have a material adverse effect on our business.third partiestime, and our sales could be disrupted. Evenour licensees were ablefailure to obtain rightsregulatory clearances or approvals for products;third party’s intellectual property,amount and timing of excess and obsolete inventory charges, commodity prices, and manufacturing variances;rights mayevents can vary dramatically due to a number of factors, including the risk factors described in this report. As a result, there can be nonexclusive, thereby givingno assurance that we will succeed in achieving our competitors accessprojected goals and objectives in the time periods that we anticipate or announce publicly. The failure to achieve such projected goals and objectives in the same intellectual property. Ultimately,time periods that we anticipate or announce publicly could have an adverse effect on our business, disappoint investors and analysts, and cause the market price of our ordinary shares to decline.commercialize somemaintain competitive global cash management and a competitive effective corporate tax rate.our potential products oramong other things, uncertainty regarding the tax policies of the jurisdictions where we operate and uncertainty regarding the level of net income that we will earn in those jurisdictions in the future. Our actual effective tax rate may have to cease somevary from this expectation and that variance may be material. Additionally, the tax laws of our business operations asthe Netherlands and other jurisdictions in which we operate could change in the future, and such changes could cause a result of patent infringement claims, which could severely harm our business.In any infringement lawsuit, a third party could seek to enjoin, or prevent, us from commercializing our existing or future products, or may seek damages from us, and any such lawsuit would likely be expensive for us to defend against. If we lose one of these proceedings, a court or a similar foreign governing body could require us to pay significant damages to third parties, seek licenses from third parties, pay ongoing royalties, redesign our products so that they do not infringe or prevent us from manufacturing, using or selling our products. In addition to being costly, protracted litigation to defend or prosecute our intellectual property rights could resultmaterial change in our customers or potential customers deferring or limiting their purchase or useeffective tax rate.affected products until resolutionavailability of benefits under tax treaties, the litigation.From timerates of taxes payable in respect of that income, and withholding taxes on dividends paid from one jurisdiction to time,the next. We will enter into many transactions and arrangements in the ordinary course of business in respect of which the tax treatment is not entirely certain. We will, therefore, make estimates and judgments based on our knowledge and understanding of applicable tax laws and tax treaties, and the application of those tax laws and tax treaties to our business, in determining our consolidated tax provision. For example, certain countries could seek to tax a greater share of income than will be provided for by us. The final outcome of any audits by taxation authorities may differ from the estimates and assumptions we receive noticesmay use in determining our consolidated tax provisions and accruals. This could result in a material adverse effect on our consolidated income tax provision, financial condition, and the net income for the period in which such determinations are made.third parties alleging infringementour U.S. affiliates to certain of our non-U.S. subsidiaries may be subject to U.S. withholding tax at a rate of 30% unless the entity receiving such payments can demonstrate that it qualifies for reduction or misappropriationelimination of the patent, trademarkU.S. withholding tax under the income tax treaty (if any) between the United States and the jurisdiction in which the entity is organized or other intellectual property rights of third parties by us or our customers in connection with the useis a tax resident. In certain cases, treaty qualification may depend on whether at least 50% of our products or we otherwise may become aware of possible infringement claims against us. We routinely analyze such claims and determine how best to respond in lightultimate beneficial owners are qualified residents of the circumstances existingUnited States or the treaty jurisdiction within the meaning of the applicable treaty. There can be no assurance that we will satisfy this beneficial ownership requirement at the time includingwhen such dividends, distributions, or other payments are made. Moreover, the importanceU.S. Internal Revenue Service (IRS) may challenge our determination that the beneficial ownership requirement is satisfied. If we do not satisfy the beneficial ownership requirement, such dividends, distributions, or other payments may be subject to 30% U.S. withholding tax.intellectual property rightInternal Revenue Code of 1986, as amended (Code) can limit the ability of the acquired U.S. corporation and its U.S. affiliates to utilize U.S. tax attributes such as net operating losses and certain tax credits to offset U.S. taxable income resulting from certain transactions. Based on the limited guidance available, we currently expect that this limitation likely will not apply to us and as a result, our U.S. affiliates likely will not be limited by Section 7874 of the third party,Code in their ability to utilize their U.S. tax attributes to offset their U.S. taxable income, if any, resulting from certain specified taxable transactions. However, no assurances can be given in this regard. If, however, Section 7874 of the relative strengthCode were to apply to the Wright/Tornier merger and if our U.S. affiliates engage in transactions that would generate U.S. taxable income subject to this limitation in the future, it could take us longer to use our net operating losses and tax credits and, thus, we could pay U.S. federal income tax sooner than we otherwise would have. Additionally, if the limitation were to apply and if we do not generate taxable income consistent with our expectations, it isnon-infringement or non-misappropriationU.S. corporate tax residence, limit the ability of foreign-owned corporations to deduct interest expense, and make other changes in the taxation of multinational corporations.productOrganization for Economic Co-operation and Development have focused on issues related to the taxation of multinational corporations. One example is in the area of “base erosion and profit shifting,” where payments are made between affiliates from a jurisdiction with high tax rates to a jurisdiction with lower tax rates. As a result, the tax laws in the United States, the Netherlands and other countries in which we and our affiliates do business could change on a prospective or products incorporatingretroactive basis, and any such changes could impact the intellectual property right at issue.Since our initial public offering in February 2011,During 2015, the sale price of our ordinary shares has ranged from $14.53 per share$18.03 to $29.93 per share, as reported by$27.06. Such volatility may be the NASDAQ Global Select Market. This may happen becauseresult of broad market and industry factors, like the performance and fluctuation of the market prices of other companies with business operations located mainly in Europe that have listed their securities in the United States.factors. In addition to market and industry factors, the price and trading volume for our ordinary shares may be highly volatile for factors specific to our own operations, including the following:revenue,net sales, earnings, and cash flow;services and expansionsproducts by us or our competitors;In the past, shareholdersShareholders of a public company often broughtsometimes bring securities class action suits against the company following periods of instability in the market price of that company’s securities. If we were involved in a class action suit, it could divert a significant amount of our management’s attention and other resources from our business and operations, which could harm our operating results and require us to incur significant expenses to defend the suit. Any such class action suit, whether or not successful, could harm our reputation and restrict our ability to raise capital in the future. In addition, if a claim is successfully made against us, we may be required to pay significant damages, which could have a material adverse effect on our financial condition and operating results.We have in the past and may in the future experience deficiencies, including material weaknesses, in our internal control over financial reporting. Our business and our share price may be adversely affected if we do not remediate these material weaknesses or if we have other weaknesses in our internal controls.Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with U.S. GAAP. A material weakness, as defined in the standards established by the Public Company Accounting Oversight Board, is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. In connection with the audit of our financial statements for 2009, we identified a material weakness in our internal control over financial reporting relating to our audited financial statements for 2007 and 2008. Specifically, in our case, management and our independent registered accounting firm determined that internal controls over identifying, evaluating and documenting accounting analysis and conclusions over complex non-routine transactions, including related-party transactions, required strengthening. Although we remediated this material weakness, additional control deficiencies may be identified by management or our independent registered public accounting firm, and such control deficiencies also could represent one or more material weaknesses. A report by us of a material weakness may cause investors to lose confidence in our financial statements, and the trading price of our ordinary shares may decline. If we fail to remedy any material weakness, our financial statements may be inaccurate, our access to the capital markets may be restricted and the trading price of our ordinary shares may decline.Your percentage of ownership in us may be diluted in the future.As with any publicly-traded company, your percentage ownership in us may be diluted in the future because of equity issuances for acquisitions, capital market transactions or otherwise, including equity awards that we expect will be granted to our directors, officers and employees.An entity affiliated with Alain Tornier, one of our directors, has recently sold a portion of its Tornier share holdings. We cannot predict what effect, if any, market sales of securities held by our significant shareholders or any other shareholder or the availability of these securities for future sale will have on the market price of our ordinary shares.certain of our shareholders and officers, including TMG Holdings Coöperatief U.A. (TMG), or TMG, Vertical Fund I, L.P., Vertical Fund II, L.P., entities affiliated with Alain Tornier, Douglas W. Kohrs and certain former shareholders of OrthoHelix, which requires us to register ordinary shares held by these personsTMG under the U.S. Securities Act of 1933, as amended, subject to certain limitations, restrictions and conditions. The market price of our ordinary shares could decline as a result of the registration and sale of or the perception that registration and sales may occur of a large number of our ordinary shares.NetherlandsDutch public company with limited liability (naamloze vennootschap). Our corporate affairs and the rights of holders of our ordinary shares are governed by Dutch law and our articles of association. The rights of our shareholders and the responsibilities of members of our board of directors may be different from those in companies governed by the laws of U.S. jurisdictions. For example, Dutch law does not provide for a shareholder derivative action. In addition, in the performance of its duties, our board of directors is required by Dutch law to act in the interest of our company and itour affiliated business, and to consider the interests of our company, our shareholders, our employees, and other stakeholders, in all cases with reasonableness and fairness. It is possible that some of these parties will have interests that are different from, or in addition to, interests of our shareholders.difficult for youable to obtain or enforce judgments obtained in U.S. courts in civil and commercial matters against us or our executive officers, somemembers of our board of directors and some of our named experts in the United States.were formedare organized under the laws of the Netherlands, and, as such, the rights of holders of our ordinary shares and the civil liability of our directors are governed by Dutchthe laws of the Netherlands and our articles of association. The rights of shareholders under the laws of the Netherlands may differ from the rights of shareholders of companies incorporated in other jurisdictions. Certain of our directors and executive officers and mostA substantial portion of our assets and some of the assets of our directors are located outside of the United States. As a result, youit may not be abledifficult for investors to serveeffect service of process within the United States on us, or on such persons into enforce outside the United States or obtain or enforceany judgments fromobtained against us in U.S. courts against them or us based onin any action, including actions predicated upon the civil liability provisions of the U.S. federal securities laws oflaws. In addition, it may be difficult for investors to enforce rights predicated upon the United States. There is doubt as to whether Dutch courts would enforce certain civil liabilities under U.S. federal securities laws in original actions brought in courts in jurisdictions located outside the United States (including the Netherlands) or enforce claims for punitive damages.Under our articlesassociation, we indemnifyjudgments in civil and hold our directors harmless against all claims and suits brought against them, subject to limited exceptions. Although there is doubt as to whether U.S. courts would enforce such provision in an action broughtcommercial matters (other than arbitral awards). A final judgment for the payment of money rendered by any federal or state court in the United States underwhich is enforceable in the United States, whether or not predicated solely upon U.S. federal securities laws, such provision could make enforcing judgments obtained outsidewould not automatically be recognized or enforceable in the Netherlands. In order to obtain a judgment which is enforceable in the Netherlands, the party in whose favor a final and conclusive judgment of the U.S. court has been rendered will be required to file its claim with a court of competent jurisdiction in the Netherlands. Such party may submit to a Dutch court the final judgment rendered by the U.S. court. If and to the extent that the Dutch court finds that the jurisdiction of the U.S. court has been based on grounds which are internationally acceptable and that proper legal procedures have been observed, the Dutch court will generally tend to give binding effect to the judgment of the court of the United States without substantive re-examination or re-litigation on the merits of the subject matter, unless the judgment contravenes principles of public policy of the Netherlands.more difficult to enforce against our assets inor countries other than the Netherlands or jurisdictions that would apply Dutch law.Rights of a holder of ordinary shares are governed by Dutch law and differ from the rights of shareholders under U.S. law.We are a public limited liability company incorporated under Dutch law. The rights of holders of ordinary shares are governed by Dutch law and our articles of association. These rights differ from the typical rights of shareholdersUnited States any judgments obtained in U.S. corporations. For example, Dutch law significantly limitscourts in civil and commercial matters, including judgments under the circumstances under which shareholders of Dutch companies may bring an action on behalf of a company. Our boardapproximately 44.3%6.1% of our outstanding ordinary shares, and this concentration of ownershipcontrol may have an effect on transactions that are otherwise favorable to our shareholders.or Warburg Pincus,(Warburg Pincus), beneficially own, in the aggregate, approximately 44.3%6.1% of our outstanding ordinary shares. These shareholdersThis concentration of ownership could have an effect on matterselectionappointment of directors. This concentration of ownership also may delay, deter or prevent a change in control, and may make some transactions more difficult or impossible to complete without the support of these shareholders,Warburg Pincus, regardless of the impact of this transaction on our other shareholders. In addition, our securityholders’ agreement as amended on August 27, 2010, gives TMG Holdings Coöperatief U.A., an affiliate of Warburg Pincus, the right to designate three of the eight directors to be nominated to our board of directors for so long as TMG beneficially owns at least 25% of theour outstanding ordinary shares, two of the eight directors for so long as TMG beneficially owns at least 10% but less than 25% of theour outstanding ordinary shares and one of the eight directorsdirector for so long as TMG beneficially owns at least 5% but less than 10% of theour outstanding ordinary shares, and we have agreed to use our reasonable best efforts to cause the TMG designees to be elected.Commentswherethat we conduct our principal executive, sales and marketing, administrative activities, U.S. distribution and customer service operations. This facility is leasedlease through 2022. Our OrthoHelixU.S. manufacturing operations which includeconsist of a state of the art manufacturing facility in Arlington, Tennessee. We lease the manufacturing facility from the Industrial Development Board of the Town of Arlington. At this facility, we produce primarily orthopaedic implants and some related surgical instrumentation while utilizing lean manufacturing philosophies. We also have research and development distribution, customer service and administrative functions, are located in Medina Ohio. Our primary U.S. research and development operations are based in a 12,200 square foot leased facility in Warsaw, Indiana, with small satellite quality, marketing and research and development offices in Medina, Ohio and San Diego, California.Our global corporate headquarters are located in Amsterdam, the Netherlands. Indiana.such as purchasing and engineering,activities, sales and marketing, research and development, quality and regulatory assurance, distribution and administrative functions. In our 84,700 square foot Macroom facilities,facility, we conduct manufacturing operations and manufacturing support, such as purchasing, engineering, and quality assurance functions. Our pyrocarbon manufacturing is performed at our 9,900 square foot facility in Grenoble, France. In addition, we maintain subsidiary sales offices and distribution warehouses in various countries, including France, Germany, Italy, the Netherlands, Denmark, Switzerland, United Kingdom, Belgium, Japan, Canada, and Australia. We have an international research and development facility in Costa Rica.Entity CityState/Country State/Country Owned orLeasedOccupancyMemphis OccupancySquareFootageLeaseExpirationDateTornier, Inc.BloomingtonMinnesota,LeasedOffices/Warehouse/Distribution56,00001/01/2022Tornier, IncEdinaMinnesota, United StatesLeasedOffices19,10012/31/2015Tornier, Inc.WarsawIndiana, United States Leased Offices/R&D Arlington 12,200 Leased 02/28/2015Bloomington OrthoHelix Surgical Designs, Inc Leased Warsaw Leased Offices/R&D Medina Leased Offices/Warehouse/R&D 19,50005/31/2014Tornier SASMontbonnot France Leased Offices15,10005/31/2022Offices/ Tornier SASMontbonnot France Leased 19,50005/31/2022Tornier SASMontbonnot France Leased Offices/R&D 25,50005/31/2022Tornier SASMontbonnot France Owned 51% 51,70009/03/2018Tornier SASGrenoble France Leased 9,90007/22/2012Tornier Orthopedics Ireland LimitedDunmanwayIrelandOwnedManufacturing/Offices15,200N/ATornier Orthopedics Ireland LimitedMacroom Ireland Leased 84,70012/01/2028A descriptionNote 19the ordinary course of our consolidated financial statements included in this report is incorporated herein by reference.business.StockholderShareholder Matters and Issuer Purchases of Equity Securities.“TRNX.“WMGI.” OurPrior to the completion of the Wright/Tornier merger on October 1, 2015, legacy Tornier ordinary shares have traded onunder the NASDAQ Global Select Market sincesymbol "TRNX" while legacy Wright ordinary shares traded under the datesymbol "WMGI." Due to the "reverse acquisition" nature of our initial public offering on February 3, 2011. the Wright/Tornier merger, historical information below reflects the high and low prices of legacy Tornier.fiscal quartersperiods indicated, the high and low daily per share sales prices for our ordinary shares as reported by the NASDAQ Global Select Market. High Low $ 25.84 $ 17.25 $ 25.91 $ 19.21 $ 23.02 $ 17.15 $ 20.49 $ 14.53 $ 20.28 $ 17.80 $ 29.38 $ 18.31 $ 29.93 $ 19.58 $ 24.42 $ 16.69 High Low Fiscal Year 2015 First Quarter $ 26.98 $ 23.32 Second Quarter $ 27.06 $ 24.45 Third Quarter $ 26.13 $ 21.43 Fourth Quarter $ 23.86 $ 18.03 Fiscal Year 2014 First Quarter $ 21.17 $ 17.77 Second Quarter $ 24.35 $ 16.68 Third Quarter $ 25.11 $ 19.28 Fourth Quarter $ 28.53 $ 21.64 24, 201310, 2016, there were 111437 holders of record of our ordinary shares. The credit agreement relating to our senior secured term loans and senior secured revolving credit facility contains covenants limiting our ability to pay cash dividends.None.Recent SalesUnregistered SecuritiesDuringour company during the fourth fiscal quarter ended December 30, 2012, we27, 2015.except forduring the issuancefourth fiscal quarter ended December 27, 2015.StockholderShareholder Returnsourlegacy Tornier's initial public offering, to December 30, 2012, forOctober 1, 2015, the date of the Wright/Tornier merger, and our combined company ordinary shares from October 1, 2015 to December 27, 2015 (our fiscal year-end). The graph also reflects cumulative total shareholder returns from an index composed of U.S. companies whose stock is listed on the NASDAQ Global Select Market (the( NASDAQ U.S. CompaniesComposite Index), and an index consisting of NASDAQ-listed companies in the surgical, medical and dental instruments and supplies industry (the( NASDAQ Medical Equipment CompaniesSubsector), as well as an index of companies with the SIC Code 384 - Surgical, Medical, and Dental Instruments Supplies (Surgical, Medical, and Dental Instruments Index). Total returns for the indices are weighted based on the market capitalization of the companies included therein. In addition, due the "reverse acquisition" nature of the Wright/Tornier merger and the fact that the historical financial statements of legacy Wright have replaced the historical financial statements of legacy Tornier, the graph below also includes the cumulative total shareholder returns for WMG common stock from February 3, 2011 to October 1, 2015, the date of the Wright/Tornier merger.ourlegacy Tornier/Wright Medical Group N.V. ordinary shares, legacy Wright common stock, the NASDAQ U.S. CompaniesComposite Index, and the NASDAQ Medical Equipment CompaniesSubsector, and the Surgical, Medical, and Dental Instruments Supplies Index, and that all dividends were reinvested. Total returns for the two NASDAQ indices are weighted based on the market capitalization of the companies included therein. stock price performance of our ordinary shares is not indicative of future stockshare price performance. We do not make or endorse any prediction as to future share price performance.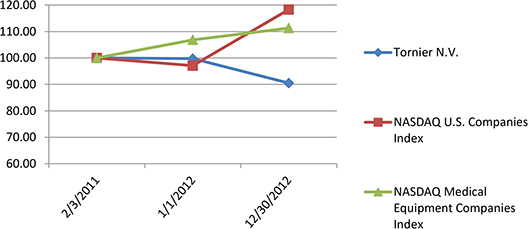
February 3,
2011 January 1,
2012 December 30,
2012 100.00 99.72 90.50 100.00 97.07 118.30 100.00 106.85 111.32 The above stock performance graph shall not be deemed to be “filed” 2/3/2011 2011 2012 2013 2014 2015 Legacy Tornier / Wright Medical Group N.V. $ 100.00 $ 99.72 $ 90.25 $ 101.33 $ 141.05 $ 130.53 Legacy Wright 100.00 109.27 134.11 199.47 175.96 139.21 NASDAQ Stock Market (US Companies) 100.00 96.90 112.41 159.02 186.95 199.95 NASDAQ Medical Equipment Index 100.00 106.18 115.96 137.82 161.79 189.90 SIC Code 384 - Surgical, Medical, and Dental Instruments and Supplies 100.00 103.99 113.11 135.59 156.93 170.26 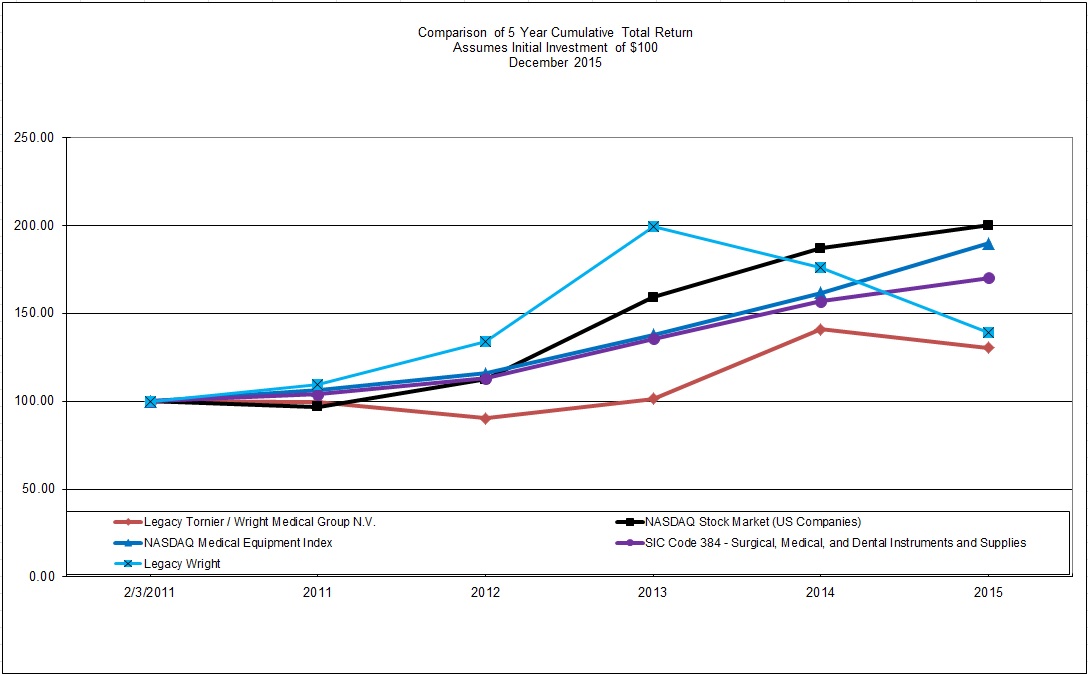
the Securities and Exchange Commission or subject to the liabilitiespermission. All rights reserved. Copyright 1980-2016The selected consolidated financial data was derived from our consolidated financial statements. The audited consolidatedDue to the "reverse acquisition" nature of the Wright/Tornier merger, the historical financial statements as of December 30, 2012 and January 1, 2012, and forlegacy Wright have replaced the three years in the period ended December 30, 2012 are included elsewhere in this report. The audited consolidatedhistorical financial statements as of January 2, 2011, December 27, 2009 and December 28, 2008 and for the years ended December 27, 2009 and December 28, 2008 are not included in this report.legacy Tornier. Historical results are not necessarily indicative of the results to be expected for any future period. These tables are presented in thousands, except per share data.Our fiscal year-end is generally determined on a 52-week basis and always falls on the Sunday nearest to December 31. Every few years, it is necessary to add an extra week to the year making it a 53-week period in order to have our year end fall on the Sunday nearest to December 31. For example, the year ended January 2, 2011 includes an extra week Fiscal year ended December 27, December 31, December 31, December 31, December 31, 2015 2014 2013 2012 2011 Consolidated Statement of Operations: Net sales $ 415,461 $ 298,027 $ 242,330 $ 214,105 $ 210,753 119,255 73,223 59,721 48,239 56,762 — — — — 667 Gross profit 296,206 224,804 182,609 165,866 153,324 Operating expenses: 429,398 289,620 230,785 150,296 131,611 39,855 24,963 20,305 13,905 15,422 Amortization of intangible assets 16,922 10,027 7,476 4,417 2,412 BioMimetic impairment charges — — 206,249 — — — — — (15,000 ) — — — — 431 4,613 Total operating expenses 486,175 324,610 464,815 154,049 154,058 (189,969 ) (99,806 ) (282,206 ) 11,817 (734 ) Interest expense, net 41,358 17,398 16,040 10,113 6,381 10,884 129,626 (67,843 ) 5,089 4,241 Loss before income taxes (242,211 ) (246,830 ) (230,403 ) (3,385 ) (11,356 ) (3,851 ) (6,334 ) 49,765 2 (3,961 ) Net loss from continuing operations $ (238,360 ) $ (240,496 ) $ (280,168 ) $ (3,387 ) $ (7,395 ) (Loss) income from discontinued operations, net of tax $ (60,341 ) $ (19,187 ) $ 6,223 $ 8,671 $ 2,252 Net (loss) income $ (298,701 ) $ (259,683 ) $ (273,945 ) $ 5,284 $ (5,143 ) Net loss from continuing operations per share: $ (3.68 ) $ (4.69 ) $ (5.82 ) $ (0.08 ) $ (0.19 ) $ (3.68 ) $ (4.69 ) $ (5.82 ) $ (0.08 ) $ (0.19 ) 64,808 51,293 48,103 39,967 39,462 64,808 51,293 48.103 39.967 39.462 December 27, December 31, December 31, December 31, December 31, 2015 2014 2013 2012 2011 Consolidated Balance Sheet Data: Cash and cash equivalents $ 139,804 $ 227,326 $ 168,534 $ 320,360 $ 153,642 Marketable securities — 2,575 14,548 12,646 18,099 352,946 249,958 375,901 545,611 383,799 2,089,675 890,073 996,789 945,301 742,991 827,711 424,209 417,011 343,440 198,549 Shareholders’ equity 1,055,026 278,803 459,714 523,441 468,464 Fiscal year ended December 27, December 31, December 31, December 31, December 31, 2015 2014 2013 2012 2011 Other Data: Cash flow provided by (used in) operating activities $ (195,870 ) $ (116,002 ) $ (36,601 ) $ 68,822 $ 61,441 Cash flow provided by (used in) investing activities (15,970 ) 145,630 (121,317 ) (1,048 ) (30,560 ) Cash flow provided by (used in) financing activities 126,862 33,051 6,257 98,721 (30,050 ) Depreciation 29,481 18,582 26,296 38,275 40,227 Share-based compensation expense 24,964 11,487 15,368 10,974 9,108 43,666 48,603 37,530 19,323 46,957 (1) These line items include the following amounts of non-cash, share-based compensation expense for the periods indicated: Fiscal year ended December 27, December 31, December 31, December 31, December 31, 2015 2014 2013 2012 2011 Cost of sales $ 287 $ 254 $ 503 $ 704 $ 735 Selling, general and administrative 22,777 10,149 10,675 6,767 4,875 Research and development 1,900 1,084 780 368 320 Discontinued operations — — 3,410 3,135 3,178 (2) During the years ended December 31, 2012 and 2011, we recorded pre-tax charges associated with the cost improvement restructuring efforts totaling $0.4 million and $5.3 million, respectively. (3) During the year ended December 31, 2012, we recorded income of $15 million related to a sale and license back transaction for intellectual property. (4) During the year ended December 27, 2015, we recognized $91.1 million in costs for due diligence, transaction, and transition costs related to the Wright/Tornier merger, $14.2 million of share-based compensation acceleration, and $11.4 million of inventory step-up amortization. During the year ended December 31, 2014, we recognized: (a) $2.1 million in costs associated with distributor conversions and non-competes; (b) $14.1 million in costs for due diligence, transaction, and transition costs related to the Biotech, Solana, and OrthoPro acquisitions, (c) $11.9 million in charges related to the Wright/Tornier merger; (d) $5.9 million in transition costs related to the OrthoRecon divestiture; (e) $1.2 million in costs associated with management changes; and (f) $0.9 million in costs associated with a patent dispute settlement. During the year ended December 31, 2013, we recognized: (a) $3.7 million in costs associated with distributor conversions and non-competes; (b) $12.9 million in due diligence and transaction costs related to the BioMimetic and Biotech acquisitions; (c) $21.6 million in transaction costs for the OrthoRecon divestiture; and (d) $206.2 million in BioMimetic impairment charges. (5) During the year ended December 27, 2015, we recognized a $7.6 million gain from mark-to-market adjustments on the Contingent Value Rights (CVRs) issued in connection with the BioMimetic acquisition and $9.8 million of charges for the mark-to-market adjustment of our derivative instruments. During the year ended December 31, 2014, we recognized approximately $125 million from mark-to-market adjustments on the CVRs issued in connection with the BioMimetic acquisition, $2.0 million of charges for the mark-to-market adjustment of our derivative instruments, and $1.8 million of charges due to the fair value adjustment to contingent consideration associated with our acquisition of WG Healthcare. During the year ended December 31, 2013, we recognized a $7.8 million gain related to the previously held investment in BioMimetic. During the year ended December 31, 2012, we recognized $2.7 million for the write-off of unamortized deferred financing fees associated with the termination of our senior credit facility and the redemption of approximately $25 million of our 2014 convertible notes. Additionally, we recognized $1.1 million of charges for the mark-to-market adjustment of our derivative 30, 2012 include31, 2011, we recognized $4.1 million for the resultswrite-off of OrthoHelix Surgical Designs Inc. from October 4, 2012 (datepro-rata unamortized deferred financing fees and transaction costs associated with the tender offer for our convertible notes completed during 2011.(6) During the year ended December 31, 2013, we recognized a $119.6 million tax valuation allowance recorded against deferred tax assets in our U.S. jurisdiction due to recent operating losses. (7) (8) The prior year deferred tax balances were reclassified to account for early adoption of ASU 2015-17. (9) During the year ended December 31, 2014, our capital expenditures included $9.4 million related to the expansion of our manufacturing facility in Arlington, Tennessee. You should read the our financial condition and results of operations togetherdescribes the principal factors affecting the results of our operations, financial condition, and changes in financial condition, as well as our critical accounting estimates.notes thereto included elsewheremerger also include legacy Tornier and its subsidiaries.report,section to "we," "our" and other financial information included"us" refer to Wright Medical Group N.V. and its subsidiaries after the Wright/Tornier merger and Wright Medical Group, Inc. and its subsidiaries before the merger. Beginning in this report. The following discussion may contain predictions, estimates2015 as a result of the Wright/Tornier merger, our fiscal year runs from the Monday nearest to the 31st of December of a year, and other forward-looking statements that involve a number of risks and uncertainties, including those discussed under “Special Note Regarding Forward Looking Statements,” “Part 1, Item 1A. Risk Factors” and elsewhere in this report. These risks could cause our actual results to differ materially from any future performance suggested below.Basis of PresentationOur fiscal year-end is generally determined on a 52-week basis and always fallsends on the Sunday nearest to the 31st of December of the following year. Prior to the Wright/Tornier merger, our fiscal year end was December 31. Every few years, it is necessaryReferences in this report to add an extra weeka particular year generally refer to the year making it a 53-week period in orderapplicable fiscal year. Accordingly, references to have our year end fall on the Sunday nearest to December 31. For example, the“2015” or “the year ended January 2, 2011 (2010) includes an extra week of operations relative toDecember 27, 2015” mean the yearsfiscal year ended December 30, 2012 (2012) and January 1, 2012 (2011). The extra week was added in the first quarter of the year ended January 2, 2011, making the first quarter 14 weeks in length, as opposed to 13 weeks in length.OverviewCompany Description.surgeons that treat musculoskeletal injuriesextremities and disordersbiologics products. We are committed to delivering innovative, value-added solutions improving quality of life for patients worldwide, and are a recognized leader of surgical solutions for the upper extremities (shoulder, elbow, wrist and hand), lower extremities (foot and ankle) and biologics markets, three of the shoulder, elbow, wrist, hand, ankle and foot. We refer to these surgeons as extremity specialists. We sell to this extremity specialist customer base a broad line of joint replacement, trauma, sports medicine and biologic products to treat extremity joints. fastest growing segments in orthopaedics.motto of “specialists serving specialists” encompasses this focus. In certain international markets, we also offer joint replacement products forglobal corporate headquarters are located in Amsterdam, the hip and knee. We currently sell approximately 100 product lines in approximately 40 countries.We believe we are differentiated by our full portfolio of upper and lower extremity products, our focused extremity-focused sales organization and our strategic focus on extremities. We further believe that we are well positioned to benefit from the opportunities in the extremity products marketplace, primarily in the shoulder and ankle joint replacement markets and also the foot and ankle trauma market with our acquisition of OrthoHelix.Netherlands. We also have expandedsignificant operations located in Memphis, Tennessee (U.S. headquarters, research and development, sales and marketing administration, and administrative activities); Bloomington, Minnesota (upper extremities sales and marketing); Arlington, Tennessee (manufacturing and warehousing operations); Grenoble, France (manufacturing and research and development); and Macroom, Ireland (manufacturing). In addition, we have local sales and distribution offices in Canada, Australia, Asia, and throughout Europe.technology basesurgeon customers, with the goal of improving clinical outcomes and the “quality of life” for their patients. Our product offering to include: new joint replacement products based on new materials; improved trauma products based on innovative designs; and proprietary biologic materials for soft tissue repair. In the United States, which is the largest orthopaedic market, we believe that our “specialists serving specialists” market approach is strategically aligned with what we believe is an ongoing trend in orthopaedics for surgeons to specialize in certain partsportfolio consists of the anatomy or certain types of procedures.Our principal products are organized in four majorfollowing product categories: upper extremity joints and trauma, lower extremity joints and trauma, sports medicine and biologics, and large joints and other. Our upper extremity joints and trauma productsreplacementimplants and bone fixation devices for the shoulder, hand,elbow, wrist, and elbow. Our lower extremity joints and trauma products,hand;our OrthoHelix portfolio, include joint replacementimplants and bone fixation devices for the foot and ankle. Our sportsankle;biologics product category includesother, which include products used across several anatomic sites to mechanically repair tissue-to-tissue or tissue-to-bone injuries in the case of sports medicine, or to support or induce remodeling and regeneration of tendons and ligaments, in the case of biologics. Our large joints and other productsancillary products; andjoint replacement implantsimplants.ancillary products.Indistribution system in the United States wecurrently consists of 65 geographic sales territories that are staffed by 458 direct sales representatives and 30 independent sales agencies or distributors. These sales representatives and independent sales agencies and distributors are generally aligned to selling either our upper extremities products or lower extremities products, but, in some cases, certain agencies or direct sales representatives sell products from both our upper extremity joints and trauma, lower extremity joints and trauma, and sports medicine and biologics product categories; we do not currently market large joints in the United States. While we market our products to extremity specialists, our revenue is generated from sales to healthcare institutions and distributors. In the United States, we currently sell through our legacy Tornier and OrthoHelix sales channels, which both consist of independent commission-based sales agencies, with variations based upon individual territories. As we integrate OrthoHelix, we plan to organize our sales channels to focus on upper extremities and lower extremities to allow us to increase our selling opportunities by improving our overall procedure coverage, leveraging our entire product portfolio, and accessing new specialists and accounts. Although this may resultportfolios in some disruption our U.S. distribution channels, we believe that this strategy will be a significant competitive advantage longer term.their territories. Internationally, we sell our full product portfolio, including upper extremity joints and trauma, lower extremity joints and trauma, sports medicine and biologics and large joints. We utilize several distribution approaches depending onthat are tailored to the needs and requirements of each individual market requirements, includingmarket. Our international sales and distribution system currently consists of 11 direct sales organizationsoffices and approximately 90 distributors that sell our products in over 50 countries, with principal markets outside the United States in Europe, Asia, Canada, Australia, and Latin America. Our U.S. sales accounted for 72% of total net sales in 2015.largest Europeanextremities and biologics markets. We believe a more active and aging patient population with higher expectations regarding “quality of life,” an increasing global awareness of extremities and biologics solutions, improved clinical outcomes as a result of the use of such products, technological advances resulting in specific designs for such products that simplify procedures and address unmet needs for early interventions, and the growing need for revisions and revision related solutions will drive the market for extremities and biologics products.Australia, Japan and Canada and independent distributors for most other international markets. As we receive required regulatory approvals, we will begin to selectively introduce the OrthoHelix product portfoliointo select international markets. In 2012, we generated revenuea couple of $277.5 million, of which 56%years, but was just commercially launched by legacy Tornier on a limited focused basis in the United States late in the second quarter of 2015, after receipt of FDA 510(k) clearance in March 2015. We believe SIMPLICITI® allows us to expand the market to include younger patients that historically have deferred these procedures. Other principal upper extremities products include the EVOLVE44%the MICRONAIL® intramedullary wrist fracture repair system.international.OrthoHelix Acquisition®4, 2012,1, 2015, we acquired OrthoHelix Surgical Designs, Inc (OrthoHelix). Incompleted the transaction, we paid consideration consisting of $100.4 million cashWright/Tornier merger, as previously described. The merger created a mid-sized growth company uniquely positioned with leading technologies and 1,941,270 of our ordinary shares (which was determined to be equal to $35 million divided by the average closing sale price per ordinary share during the five trading days immediately prior to and after the date of our initial public announcementspecialized sales forces in three of the merger agreement). In addition,fastest growing areas of orthopaedics – upper extremities, lower extremities, and biologics. The highly complementary nature of the two legacy businesses has provided us significant diversity and scale across a range of geographies and product categories. Legacy Wright is a recognized leader of surgical solutions for the lower extremities market and legacy Tornier has an impressive upper extremities product portfolio, including in particular, shoulder replacement products. Together, we agreedintend to make additional earn-out paymentscontinue to leverage the global strengths of both product brands as a pure-play extremities-biologics business.cashthe United States for ankle and/or hindfoot fusion indications was obtained, and we commercially launched the product in the United States shortly thereafter.upthe Inspector General of the United States Department of Health and Human Services expired, and on January 27, 2016, we received notification from the OIG-HHS that the term of the CIA has concluded.an aggregateoffer a total ankle replacement system that addresses the continuum of $20.0 million based uponcare for end-stage ankle arthritis patients.salesglobal extremities business. Legacy Wright acquired 100% of lower extremity joints and trauma products during fiscal years 2013 and 2014. Of the transaction consideration, $10.0outstanding equity of Solana for approximately $48 million in cash remainsand $41.4 million of WMG common stock. Legacy Wright acquired 100% of OrthoPro's outstanding equity for approximately $32.5 million in an escrow accountcash.fund payment obligations with respect to post-closing indemnification obligations of OrthoHelix’s former equity holders. In addition, a portionMicroPort. The financial results of the earn-out payments are subjectOrthoRecon business have been reflected within discontinued operations for all periods presented and, unless otherwise noted, the discussion below is on a continuing operations basis.certain rights$298.0 million in 2014, driven by a $57 million increase in upper extremities sales primarily resulting from the Wright/Tornier merger and $43 million in growth from our lower extremities business.set-off for post-closing indemnification obligationsour Total Ankle Replacement products, as well as growth in our core foot and ankle plating systems. OrthoHelix’s equity holders.In addition, and as part of$240.5 million in 2014. This decrease in net loss from continuing operations was primarily driven by:OrthoHelix transaction, on October 4, 2012, we and our U.S. operating subsidiary, Tornier, Inc. (Tornier USA), entered into a credit agreement with Bank of America, N.A., as administrative agent, SG Americas Securities, LLC, as syndication agent, BMO Capital Markets and JPMorgan Chase Bank, N.A., as co-documentation agents, Merrill Lynch, Pierce, Fenner & Smith Incorporated and SG Americas Securities, LLC, as joint lead arrangers and joint bookrunners, and the other lenders party thereto. The credit agreement provides for an aggregate credit commitment to Tornier USA, as borrower, of $145.0 million, consisting of: (1) a senior secured term loan facility to Tornier USA denominated in dollars in an aggregate principal amount of up to $75.0 million; (2) a senior secured term loan facility to Tornier USA denominated in euros in an aggregate principal amount of up to the U.S. dollar equivalent of $40.0 million; and (3) a senior secured revolving credit facility to Tornier USA denominated at the election of Tornier USA, in U.S. dollars, euros, pounds sterling and yen in an aggregate principal amount of up to the U.S. dollar equivalent of $30.0 million. Funds available under the revolving credit facility may be used for general corporate purposes. The borrowings under the credit facility were used to pay the consideration for the OrthoHelix acquisition, and fees, costs and expenses incurredContingent Value Rights (CVRs) issued in connection with the OrthoHelixBioMimetic acquisitioncredit agreement and to repay prior existing indebtedness. The credit agreement contains customary covenants, including financial covenants which require us to maintain minimum interest coverage, annual capital expenditure limits and maximum total net leverage ratios, as well as customary eventsWright/Tornier merger;default. The obligations undercharges for the credit agreement are guaranteed by us, Tornier USA and certain othermark-to-market adjustment of our subsidiaries,derivative instruments;subject2020 convertible notes;certain exceptions, are secured by a first priority security interestthe write-off of unamortized debt discount and deferred financing costs associated with the settlement of the 2017 convertible notes;substantially all2015 associated with the accelerated vesting of our assetslegacy Wright's unvested awards outstanding upon the closing of the Wright/Tornier merger; andassets of certain of our existingWright/Tornier merger.future subsidiaries of Tornier.Facilities Consolidation InitiativeWe recently implemented a facilities consolidation initiative pursuant to which we consolidated a number of our facilities in France, Ireland and the United States. The facilities consolidation initiative was driven by our strategy to drive operational productivity and to realize operating costs savings beginning in 2013. Under the initiative, we consolidated our Dunmanway, Ireland manufacturing facility into our Macroom, Ireland manufacturing facility and our St. Ismier, France manufacturing facility into our existing Montbonnot, France manufacturing facility. We also leased a new facility located in Bloomington, Minnesota to use as our U.S. business headquarters and consolidated our Minneapolis-based marketing, training, regulatory, clinical, supply chain and corporate functions with our Stafford, Texas-based distribution operations. With the completion of the U.S. consolidationWright/Tornier merger, we believe we are now well positioned and committed to accelerating growth in our extremities and biologics business. We intend to leverage the global strengths of both the legacy Wright and legacy Tornier product brands as a pure-play extremities and biologics business. We believe our leadership will be further enhanced by the recent FDA premarket approval of AUGMENT® Bone Graft, a biologic solution that adds additional depth to one of the most comprehensive extremities product portfolios in the fourth quarterindustry, as well as provides a platform technology for future new product development. The highly complementary nature of 2012,legacy Wright’s and legacy Tornier’s businesses has given us significant diversity and scale across a range of geographies and product categories. We believe we are differentiated in the global facilities consolidation initiativemarketplace by our strategic focus on extremities and biologics, our full portfolio of upper and lower extremities and biologics products, and our specialized and focused sales organization.concluded. For"Do no harm." Although we recognize that we will have revenue dis-synergies during the integration period, we believe we have an excellent opportunity to improve efficiency and leverage fixed costs in our business going forward.30, 2012, we recognized a pretax charge of $6.4 million related27, 2015 to the global facilities consolidation initiative. Any remaining payments related to the charges taken as part of the initiative will be paid in 2013. For further discussion, please refer to Note 18 to our consolidated financial statements.Medical Device TaxAn excise tax of 2.3% on the sale, lease, rental or use of certain medical devices was mandated by the 2010 health care reform legislation and went into effect January 1, 2013. The excise tax applies to manufacturers, producers and importers of taxable medical devices. The excise tax generally is based on the medical device’s wholesale price and is imposed on the manufacturer or importer when the taxable device is first sold, leased, rented or used by the manufacturer or importer. A taxable device generally is considered sold, for purposes of the excise tax, when title passes from the manufacturer to the purchaser. The tax could create a risk up to 2.3% of our United States revenue.Foreign Currency Exchange RatesA substantial portion of our business is located outside the United States and as a result we generate revenue andincur expenses denominated in currencies other than the U.S. dollar. As a result, fluctuations in the value of foreign currencies relative to the U.S. Dollar can impact our operating results. The majority of our operations denominated in currencies other than the U.S. dollar are denominated in Euros. In the yearsyear ended December 30, 2012 and January 1, 2012, approximately 44% and 46% of our revenue was denominated in foreign currencies. As a result, our revenue can be significantly impacted by fluctuations in foreign currency exchange rates. We expect that foreign currencies will continue to represent a similarly significant percentage of our revenue in the future. Selling, marketing and administrative costs related to these sales are largely denominated in the same foreign currencies, thereby limiting our foreign currency transaction risk exposure. In addition, we also have significant levels of other selling, general and administrative expenses and research and development expenses denominated in foreign currencies. We, therefore, believe that the risk of a significant impact on our earnings from foreign currency fluctuations is mitigated to some extent.A substantial portion of the products we sell in the United States are manufactured in countries where costs are incurred in Euros. Fluctuations in the Euro to U.S. dollar exchange rate will have an impact on the cost of the products we manufacture in those countries, but we would not likely be able to change our U.S. dollar selling prices of those same products in the United States in response to those cost fluctuations. As a result, fluctuations in the Euro to U.S. dollar exchange rates could have a significant impact on our gross profit in future periods in which that inventory is sold. Impacts associated with fluctuations in foreign currency exchange rates are discussed in more detail under “Item 7A. Quantitative and Qualitative Disclosures about Market Risk.”We evaluate our results of operations on both an as reported and a constant currency basis. The constant currency presentation is a non-GAAP financial measure, which excludes the impact of fluctuations in foreign currency exchange rates. We believe providing constant currency information provides valuable supplemental information regarding our results of operations, consistent with how we evaluate our performance. We calculate constant currency percentages by converting our current-period local currency financial results using the prior-period foreign currency exchange rates and comparing these adjusted amounts to our prior-period reported results. This calculation may differ from similarly-titled measures used by others; and, accordingly, the constant currency presentation is not meant to be a substitution for recorded amounts presented in conformity with GAAP nor should such amounts be considered in isolation.Revenue and Expense Components2014The following is a description of the primary components of revenue and expenses.RevenueWe derive our revenue from the sale of medical devices that are used by orthopaedic surgeons who treat diseases and disorders of extremity joints including the shoulder, elbow, wrist, hand, ankle and foot, and large joint, including the hip and knee. We report our sales in four primary product categories: upper extremity joints and trauma, lower extremity joints and trauma, sports medicine and biologics, and large joints and other. Our revenue is generated from sales to two types of customers: healthcare institutions and distributors, with sales to healthcare institutions representing a majority of our revenue. In the United States, we sell through a focused sales channel consisting of a network of mostly independent commission-based sales agencies, with some direct sales organizations in certain territories. Internationally, in select markets, we sell our full product portfolio, including upper extremity joints and trauma, lower extremity joints and trauma, sports medicine and biologics and large joints. Revenue from sales to healthcare institutions is recognized at the time of surgical implantation. We generally record revenue from sales to our distributors at the time the product is shipped to the distributor. These distributors, who sell the products to their customers, take title to the products and assume all risks of ownership at the time of shipment. Our distributors are obligated to pay within specified terms regardless of when, if ever, they sell the products. We charge our customers for shipping and record shipping revenue as part of revenue.Cost of Goods SoldWe manufacture a majority of the products that we sell. Our cost of goods sold consists primarily of direct labor, manufacturing overhead, raw materials and components, and excludes amortization of intangible assets, which is presented as a separate component of operating expenses. A portion of the products we sell are manufactured by third parties, and our cost of goods sold for those products consists primarily of the price invoiced to us by our third-party vendors. Cost of goods sold also includes share-based compensation expenses related to individuals whose salaries are also included within this category. All of our internal manufacturing facilities are located in Europe and the related manufacturing costs are incurred in Euro. As a result, the cost of goods sold for our products manufactured in Europe and then sold in the United States and other geographies with functional currencies other than the Euro is subject to foreign currency exchange rate fluctuations, which can impact our gross profits.Selling, General and Administrative ExpensesOur selling, general and administrative expenses consist of both variable components, which fluctuate based on our revenues, and non-variable components, which do not fluctuate based on our revenues. Our variable selling costs consist primarily of commissions paid to our independent sales agencies used in the United States and some other countries to generate sales, royalties based on certain product sales and freight expense we pay to ship our products to customers. Our non-variable sales costs consist primarily of salaries, personnel costs, including share-based compensation and other support costs related to the selling, marketing and support of our products as well as trade shows, promotions and physician training. Selling, general and administrative expenses also include the cost of distributing our products, which includes the operating costs and certain administrative costs related to our various worldwide sales and distribution operations. We provide surgical instrumentation to our customers for use during procedures involving our products. There are no contractual arrangements related to our customers’ use of our surgical instrumentation and we do not charge a fee for providing access to the related instrumentation. We record surgical instrumentation on our balance sheet as a long-lived asset. The depreciation expense related to our surgical instrumentation is included in sales, general and administrative expenses. Additionally, expenses for our executive, finance, legal, compliance, administrative, information technology and human resource departments are included in selling, general and administrative expenses, as well as the U.S. Medical Device Excise tax which was effective on January 1, 2013.Research and Development ExpensesResearch and development expenses include costs associated with the design, development, testing, deployment and enhancement of products and certain regulatory costs. This category also includes costs associated with the design and execution of our clinical trials and regulatory submissions. Research and development expenses also include share-based compensation related to individuals within our research and development groups.Amortization of Intangible AssetsOur intangible assets include developed technology, customer relationships, trademarks and trade names and other identified intangibles as a result of business acquisitions. In addition, intangible assets also include purchases of intellectual property including patents, license rights and customer lists, among other things. The amortization expenses related to these items is recorded in amortization of intangible assets within operating expenses.Special ChargesSpecial charges are recorded as a separate line item within our operating expenses on the consolidated statement of operations and include operating expenses directly related to business combinations and related integration activities, restructuring initiatives, management exit costs, and certain other items that are typically infrequent in nature and that affect the comparability and trend of operating results.Interest IncomeInterest income reflects interest associated with both our cash held at financial institutions and highly liquid investments with maturities of three months or less.Interest ExpenseInterest expense reflects interest associated with our senior secured term loans, revolving line of credit, mortgage-related debt and capital leases. We also accrete interest expense on our earnout liabilities related to previous business acquisitions.Foreign Currency Transaction (Loss) GainForeign currency transaction (loss) gain consists primarily of foreign currency gains and losses on transactions denominated in a currency other than the functional currency of the related entity. Our foreign currency transactions primarily consist of foreign currency denominated cash, liabilities and intercompany receivables and payables.Other Non-Operating Income (Expense)Other non-operating income (expense) primarily relates to the results of transactions that are not directly associated with the results of our ongoing primary operating activities.Income Tax BenefitIncome tax benefit includes federal income taxes, income taxes in foreign jurisdictions, state income taxes and changes to our deferred taxes and deferred tax valuation allowance.Results of OperationsFiscal Year Comparisonscertain items from our consolidated statementsresults of operations expressed as dollar amounts (in thousands) and as percentages of net sales: Fiscal year ended December 27, 2015 December 31, 2014 Amount % of sales Amount % of sales Net sales $ 415,461 100.0 % $ 298,027 100.0 % 119,255 28.7 % 73,223 24.6 % Gross profit 296,206 71.3 % 224,804 75.4 % Operating expenses: 429,398 103.4 % 289,620 97.2 % 39,855 9.6 % 24,963 8.4 % Amortization of intangible assets 16,922 4.1 % 10,027 3.4 % Total operating expenses 486,175 117.0 % 324,610 108.9 % Operating loss (189,969 ) (45.7 )% (99,806 ) (33.5 )% Interest expense, net 41,358 10.0 % 17,398 5.8 % Other expense (income), net 10,884 2.6 % 129,626 43.5 % Loss from continuing operations before income taxes (242,211 ) (58.3 )% (246,830 ) (82.8 )% (Benefit) provision for income taxes (3,851 ) (0.9 )% (6,334 ) (2.1 )% Net loss from continuing operations $ (238,360 ) (57.4 )% $ (240,496 ) (80.7 )% (60,341 ) (19,187 ) Net loss $ (298,701 ) $ (259,683 ) 1 These line items include the following amounts of non-cash, share-based compensation expense for the periods indicated: Fiscal year ended December 27, 2015 % of sales December 31, 2014 % of sales Cost of sales $ 287 0.1 % $ 254 0.1 % Selling, general and administrative 22,777 5.5 % 10,149 3.4 % Research and development 1,900 0.5 % 1,084 0.4 % Income from discontinued operations, net of tax — n/a — n/a revenue that such items represent for the periods shown. Year ended December 30,
2012 January 1,
2012 January 2,
2011 $ 277,520 100 % $ 261,191 100 % $ 227,378 100 % 81,918 30 74,882 29 63,437 28 195,602 70 186,309 71 163,941 72 170,447 61 161,448 62 149,175 66 22,524 8 19,839 8 17,896 8 11,721 4 11,282 4 11,492 5 19,244 7 892 0 306 0 (28,334 ) (10 ) (7,152 ) (3 ) (14,928 ) (7 ) 338 0 550 0 223 0 (3,733 ) (1 ) (4,326 ) (2 ) (21,805 ) (10 ) (473 ) (0 ) 193 0 (8,163 ) (4 ) (593 ) (0 ) (29,475 ) (11 ) — — 116 0 1,330 1 43 0 (32,679 ) (12 ) (38,880 ) (15 ) (44,630 ) (20 ) 10,935 4 8,424 3 5,121 2 (21,744 ) (8 ) (30,456 ) (12 ) (39,509 ) (17 ) — — — — (695 ) 0 (21,744 ) (8 ) (30,456 ) (12 ) (38,814 ) (17 ) — — — — (679 ) 0 $ (21,744 ) (8 )% $ (30,456 ) (12 )% $ (39,493 ) (17 )% The following tables set forth, for the periods indicated, our revenue by product category and geography expressed as dollar amounts and the changes in revenue between the specified periods expressed as percentages: Fiscal year ended December 27, December 31, % 2015 2014 change U.S. Lower extremities 187,096 148,631 25.9 % Upper extremities 58,756 15,311 283.8 % Biologics 50,583 45,494 11.2 % Sports med & other 3,388 2,641 28.3 % Total extremities & biologics 299,823 212,077 41.4 % Large joint 18 — N/A Total U.S. $ 299,841 $ 212,077 41.4 % International Lower extremities 51,200 47,001 8.9 % Upper extremities 24,789 11,312 119.1 % Biologics 19,652 20,590 (4.6 )% Sports med & other 9,862 7,047 39.9 % Total extremities & biologics 105,503 85,950 22.7 % Large joint 10,117 — N/A Total International $ 115,620 $ 85,950 34.5 % Total Sales $ 415,461 $ 298,027 39.4 % Revenue by Product CategoryU.S. Sales. Year ended Percent change December 30,
2012 January 1,
2012 January 2,
2011 2012/
2011 2011/
2010 2012/
2011 2011/
2010 ($ in thousands) (as stated) (constant
currency) $ 175,242 $ 164,064 $ 139,175 7 % 18 % 9 % 16 % 34,109 26,033 23,629 31 10 33 8 15,526 14,779 13,210 5 12 7 10 224,877 204,876 176,014 10 16 12 14 52,643 56,315 51,364 (7 ) 10 1 4 $ 277,520 $ 261,191 $ 227,378 6 % 15 % 9 % 12 % Revenue by Geography Year ended Percent change January 1,
2012 January 1,
2012 January 2,
2011 2012/
2011 2011/
2010 2012/
2011 2011/
2010 ($ in thousands) (as stated) (constant
currency) $ 156,750 $ 141,496 $ 127,762 11 % 11 % 11 % 11 % 120,770 119,695 99,616 1 20 8 14 $ 277,520 $ 261,191 $ 227,378 6 % 15 % 9 % 12 % Year Ended December 30, 2012 (2012) Compared to Year Ended January 1, 2012 (2011)Revenue. Revenue increased by 6% to $277.5U.S. net sales totaled $299.8 million in 20122015, a 41% increase from $261.2$212.1 million in 2011 as a result2014, representing approximately 72% of increasedtotal net sales in all2015 and 71% of total net sales in 2014. Products acquired as part of the Wright/Tornier merger contributed sales of $51.6 million in 2015, which accounted for 24 percentage points of the increase from 2014.categories, partially offset byproducts in our international regions totaled $105.5 million in 2015, a decrease23% increase from $86.0 million in 2014. Products acquired as part of the Wright/Tornier mergerlarge joints and other due primarily to$21.7 million in 2015, which accounted for 25 percentage points of the negativeincrease from 2014. Our 2015 international extremities sales included an unfavorable foreign currency impact of approximately $10.5 million when compared to 2014 net sales, which had a 12 percentage point unfavorable impact on the growth rate.exchange rates. Theimpact which had a 13 percentage point unfavorable impact on the growth experiencedrate. Sales in 2015 included $2.5 million from products acquired from the extremities categories was driven primarily by increased demand, product expansion and our acquisition of OrthoHelix. Excluding the negative impacts of foreign currency exchange rate fluctuations of approximately $8.1 million, principally due to the performanceWright/Tornier merger, which accounted for 5 percentage points of the U.S. dollar against the Euro, on a constant currency basis, our revenue grew by 9%.Revenue by product category. Revenue in upper extremity joints and trauma increased by 7% to $175.2 million in 2012 from $164.1 million in 2011, primarily as a result of the continued increase in sales of our Aequalis reversed and Aequalis Ascend shoulder products, and to a lesser degree, our Simpliciti shoulder products. We believe that increased sales of our Aequalis reversed shoulder products resulted from continued market growth in shoulder replacement procedures and continued market movement towards reversed shoulder replacement procedures. We also saw an increase in sales of our Aequalis Ascend shoulder products which continued to gain share in the shoulder replacement market. Included in the upper extremity joints and trauma revenue for 2012 was $0.2 million of incremental revenue from our acquisition of OrthoHelix. Offsetting these increases was the negative impact of foreign currency exchange rate fluctuations of $3.4 million. Excluding the impacts of foreign currency exchange rates, revenue in upper extremity joints and trauma increased by 9%. Revenue in our lower extremity joints and trauma increased by 31% to $34.1 million in 2012 from $26.0 million in 2011, primarily due to $7.8 million in incremental revenue from our acquisition of OrthoHelix. Revenue in sports medicine and biologics increased by 5% to $15.5 million in 2012 from $14.8 million in 2011. This increase was primarily attributable to increased sales of our anchor and suture products internationally, partially offset by a decrease in revenue of our biologics products. Biologics revenue primarily consisted of sales of our Conexa product. The market for this type of biologic product had been declining recently due to difficulties in reimbursement and a lack of supporting clinical data. We have been focused on providing the market additional clinical information related to our Conexa product and have seen revenue growth in recent periods. Revenue from large joints and other decreased by 7% to $52.6 million in 2012 from $56.3 million in 2011 primarily related to negative foreign currency exchange rate fluctuations of $4.0 million. Excluding2015. Before the impact of foreign currency exchange rate fluctuations,and acquired products, the 17% increase was driven by an 8% increase in sales in our direct markets in Europe, a 50% increase in sales in Australia and a 30% increase in sales in Canada. joints and other product revenue increased 1% on a constant currency basis in 2012 comparedjoint net sales of $10.1 million are wholly attributable to 2011. products acquired from the Wright/Tornier merger.believeanticipate that our 2016 net sales will show significant growth from 2015 due to the market growthimpact of large bonesthe acquired products, is lower than extremities products and would expect this trend to continueparticularly in the near future.Revenueupper extremities product line, which we expect to be partially offset by geography. Revenueanticipated sales dis-synergies due to sales force integrations. Additionally, we anticipate higher levels of growth in our U.S. biologics net sales due to the ongoing launch of AUGMENT® Bone Graft in the United States increased by 11%States.$156.8$73.2 million or 24.6% of sales in 2012 from $141.5 million in 2011. While the United States revenue was negatively affected by certain distribution channel changes made during 2012, overall United States revenue increased2014, representing an increase of 4.1 percentage points as a resultpercentage of the incremental revenue from our OrthoHelix acquisition and increases in sales in upper extremity joints and trauma products. Included in the United States revenue was $8.0 million in incremental revenue from our OrthoHelix acquisition. International revenue increased slightly to $120.8 million in 2012 from $119.7 million in 2011. International revenue was negatively impacted by foreign currency exchange rate fluctuations of approximately $8.1 million, principally due to the performance of the U.S. dollar against the Euro. Excluding the impact of foreign currency exchange rate fluctuations, our international revenue grew by 8% on a constant currency basis.net sales. This increase was primarily due todriven by $11.4 million (2.2% of net sales) of inventory step-up amortization in 2015 associated with inventory acquired from the Wright/Tornier merger, as well as increased revenue in France, Australia, the United Kingdom and the Netherlands as a result of increased demand.Cost of goods sold. Our cost of goods sold increased by 9% to $81.9 million in 2012 from $74.9 million in 2011. As a percentage of revenue, cost of goods sold increased to 30% in 2012 from 29% in 2011, primarily due to a higher level ofprovisions for excess and obsolete inventory charges including $3.0 million related to product rationalization initiatives as a result of our acquisition of OrthoHelix. Additionally, cost of goods sold in 2012 included approximately $2.0 million in fair value adjustments related toand inventory acquired in our acquisitions of OrthoHelix and our exclusive stocking distributor in Belgium and Luxembourg. losses.goods soldsales and corresponding gross profit as a percentage of revenuepercentages can be expected to fluctuate in future periods depending upon certain factors, including, among others, changes in our product sales mix and prices, distribution channels and geographies, manufacturing yields, plans for insourcing some previously outsourcedproduction activities, inventory reserves required,period expenses, levels of production volume, and fluctuating inventory costs due to changes in foreign currency exchange rates since the period they were manufactured. In addition,rates. Additionally, we expect an increase over the next year in the level of ouranticipate that cost of goods sold from the sell through ofsales in 2016 will be unfavorably impacted by inventory step-up amortization associated with inventory acquired from business combinations.administrative. Ouradministrativeby 6% to $170.4103.4% in 2015, compared to 97.2% in 2014. Selling, general and administrative expense included $75.9 million (18.3% of net sales) and $31.9 million (10.7% of net sales) of due diligence, transition, and transaction costs associated with the Wright/Tornier merger and other recent acquisitions in 2012 from $161.42015 and 2014, respectively. For 2015, selling, general and administrative expense also included a $13.1 million in 2011. As(3.2% of net sales) share-based compensation charge for accelerated vesting of outstanding unvested awards upon closing of the Wright/Tornier merger. For 2014, selling, general and administrative expense also included $1.2 million of costs related to management changes (0.4% of net sales) and $0.9 million of costs related to a percentagepatent dispute settlement (0.3% of revenue,net sales). The remaining selling, general and administrative expenses remained consistentdecrease as a percentage of sales was driven primarily by leveraging general and administrative expenses over increased net sales.62%December 27, 2015, we expect to amortize approximately $25.2 million in 20122016, $24.6 million in 2017, $20.8 million in 2018, $19.2 million in 2019, and 2011. The$18.5 million in 2020. Year ended December 31, 2014 2013 Amount % of sales Amount % of sales Net sales $ 298,027 100.0 % $ 242,330 100.0 % 73,223 24.6 % $ 59,721 24.6 % Gross profit 224,804 75.4 % 182,609 75.4 % Operating expenses: 289,620 97.2 % 230,785 95.2 % 24,963 8.4 % 20,305 8.4 % Amortization of intangible assets 10,027 3.4 % 7,476 3.1 % BioMimetic impairment charges — — % 206,249 85.1 % Total operating expenses 324,610 108.9 % 464,815 191.8 % Operating loss (99,806 ) (33.5 )% (282,206 ) (116.5 )% Interest expense, net 17,398 5.8 % 16,040 6.6 % Other expense, net 129,626 43.5 % (67,843 ) (28.0 )% Loss from continuing operations before income taxes (246,830 ) (82.8 )% (230,403 ) (95.1 )% Provision for income taxes (6,334 ) (2.1 )% 49,765 20.5 % Net loss from continuing operations $ (240,496 ) (80.7 )% $ (280,168 ) (115.6 )% (19,187 ) 6,223 Net loss $ (259,683 ) $ (273,945 ) 1 These line items include the following amounts of non-cash, share-based compensation expense for the periods indicated: Year Ended December 31, 2014 % of sales 2013 % of sales Cost of sales $ 254 0.1 % $ 503 0.2 % Selling, general and administrative 10,149 3.4 % 10,675 4.4 % Research and development 1,084 0.4 % 780 0.3 % Loss from discontinued operations, net of tax — n/a 3,410 n/a Fiscal year ended December 31, December 31, % 2014 2013 change U.S. Lower extremities 148,631 115,642 28.5 % Upper extremities 15,311 17,423 (12.1 )% Biologics 45,494 42,561 6.9 % Sports med & other 2,641 2,022 30.6 % Total extremities & biologics 212,077 177,648 19.4 % Large joint — — N/A Total U.S. $ 212,077 $ 177,648 19.4 % International Lower extremities 47,001 35,020 34.2 % Upper extremities 11,312 7,240 56.2 % Biologics 20,590 17,231 19.5 % Sports med & other 7,047 5,191 35.8 % Total extremities & biologics 85,950 64,682 32.9 % Large joint — — N/A Total International $ 85,950 $ 64,682 32.9 % Total Sales $ 298,027 $ 242,330 23.0 % was primarily a resultincreased to 97.2% in 2014, compared to 95.2% in 2013. For 2014, selling, general and administrative expense included $14.1 million transition and transaction costs associated with acquired businesses (4.7% of $3.4net sales), $11.9 million of additional variable selling expenses including commissions, royaltiesWright/Tornier merger costs (4.0% of net sales), $5.8 million of transition costs associated with the sale of the OrthoRecon business (2.0% of net sales), $1.2 million of costs related to management changes (0.4% of net sales), and freight expenses due$0.9 million of costs related to increased revenue.a patent dispute settlement (0.3% of net sales). For 2013, Selling, general and administrative expenses also increased compared to 2011 as a resultexpense included $21.6 million of increased instrument depreciation, sales managementtransition costs associated with the sale of our OrthoRecon business (8.9% of net sales), $12.9 million of due diligence, transition, and transaction costs related to information technology, partially offset by a decrease in expensesour acquisitions of BioMimetic and Biotech (5.3% of net sales), and $0.9 million of cost related to certain management incentives. These items were partially offset by the favorable impactdistributor transition agreements (0.4% of foreign currency exchange rate fluctuations of $6.1 million. We expect annet sales). The remaining increase in our 2013 selling, general and administrative expenses as a result of the U.S. Medical Device Excise tax, which was effective on January 1, 2013.Research and development. Research and development expenses increased by 14% to $22.5 million in 2012 from $19.8 million in 2011. As a percentage of revenue, research and development expenses remained consistent with the prior year period at 8%. The increasenet sales was driven by investment in total research and development expense of $2.7 million was primarily due to increased clinical study related expenses, an increased level of expenses on certain shoulder related development projects including the Ascend Flex convertible shoulder, certain biologics related development projects and increased personnel related expenses. These items were partially offset by the favorable impact of foreign currency exchange rate fluctuations of $0.8 million and a decrease in expenses related to certain management incentives. We anticipate that research and development expense will not remain at our current levels.Amortization of intangible assets. Amortization of intangible assets increased by 4% to $11.7 million in 2012 from $11.3 million in 2011, primarilyinternational growth opportunities, dis-synergies as a result of the sale of the OrthoRecon business in certain corporate and international expenses, and short-term expense dis-synergies associated with the acquired Solana and OrthoPro businesses, which were partially offset by lower levels of expense for cash incentive compensation. The dis-synergies as a result of the sale of the OrthoRecon business included expenses associated with our information technology support, a new corporate headquarters, and international employees and facilities.recorded through our acquisitiontotaled $10.0 million in 2014, compared to $7.5 million in 2013. This increase was driven by intangible assets acquired during the first quarter of OrthoHelix2014 and the acquisitionfourth quarter of our exclusive stocking distributor2013. (See further discussion in Belgium and Luxembourg in 2012, partially offset by the complete amortization of certain license related intangible assets that were fully amortized in 2011.Special charges. Special charges were $19.2 million in 2012 compared to $0.9 million in 2011. Special charges in 2012 included approximately $6.4 million of expense related to our facilities consolidation initiative. The $6.4 million is comprised of employee-benefit related expenses including severance and retention of terminated employees in the U.S., moving and transportation expenses, impairment charges on fixed assets related to the impacted facilities of Stafford, Texas and Dunmanway, Ireland, lease termination costs related to the Edina, Minnesota facility, professional fees and other expenses. Also included in special charges in 2012 is approximately $2.0 million of bad debt expense related to the termination of a distributor and worsening general economic conditions in Italy, $1.4 million of expense related to certain distribution changes in the U.S. and internationally, $3.5 million of integration costs related to our acquisitions of OrthoHelix and our exclusive stocking distributor in Belgium and Luxembourg, $1.2 million of expense related to management exit costs including the departures of our former Chief Executive Officer and Global Chief Financial Officer and $4.7 million of intangible impairment charges. For 2011, the $0.9 million of special charges were primarily related to severance costs from management organizational changes in 2011. See Note 133 to our consolidated financial statements contained in “Item 8. Financial Statements and Supplementary Data.”). This increase was partially offset by a decrease in amortization expense associated with certain distributor non-compete agreements that became fully amortized during 2014.further detailsAUGMENT® Bone Graft for use as an alternative to autograft in hindfoot and ankle fusion procedures, and we were required to evaluate assets associated with the BioMimetic acquisition for impairment. As a result of this evaluation, we recorded an intangible impairment charge of approximately $88.1 million and a goodwill impairment charge of $115.0 million, as well as the recognition of a $3.2 million charge for non-cancelable inventory commitments for the raw materials used in the manufacture of AUGMENT® Bone Graft, which we estimated would expire unused.facilities consolidation initiativeCVRs issued in connection with the BioMimetic acquisition, $1.8 million for the fair value adjustment for contingent consideration associated with the WG Healthcare acquisition, and an unrealized loss of $2.0 million for net mark-to-market adjustments onspecial charges. expense (income), net included a $61.1 million unrealized gain on the CVRs issued in connection with the BioMimetic acquisition and a $7.8 million realized gain on our previously held investment in BioMimetic, partially offset by an unrealized loss of $1.0 million for net mark-to-market adjustments on our derivative asset and liability.expectrecorded a tax benefit of $6.3 million in 2014 and tax expense of $49.8 million in 2013. During 2014, our effective tax rate was approximately 2.6% as compared to continue to record special charges(21.6)% in 2013 aggregating to approximately $10 million to $12 million,2013. Our relatively low effective tax rate in 2014 was primarily related to inventory step-up, the integration of OrthoHelix and transitions withinvaluation allowance on our U.S. distribution channel.Interest income.Our interest income decreased by 38%net deferred tax assets, resulting in the inability to $0.3 millionrecognize a tax benefit for pre-tax losses in 2012 from $0.6 million in 2011, primarily as a result of lower average levels of cash held and decreased average interest rates in 2012 compared to 2011.Interest expense. Our interest expense decreased by 14% to $3.7 million in 2012 from $4.3 million in 2011 due primarilythe United States, except to the repaymentextent to which we recognize a gain in discontinued operations. Our 2014 tax benefit, therefore, included $5.5 million of our notes payablebenefit recorded in February 2011. Our interest expense for 2012 related primarily to the interest paid on our term loans, mortgages, and prior lines of credit and overdraft arrangements. We anticipate an increase in our interest expense in future periodscontinuing operations as a result of the establishment of a new credit facilitygain realized in late 2012, which was used to fund our acquisition of OrthoHelix, that significantly increased our total debt. In addition, we expect to accrete additional interest expense on the OrthoHelix earn-out liabilities.Foreign currency transaction (loss) gain. We recognized $0.5 million of foreign currency transaction losses in 2012 compared to $0.2 million of foreign currency transaction gains in 2011. Foreign currency gains and losses are recognized when a transaction is denominated in a currency other than the subsidiary’s functional currency and are primarily attributable to foreign currency exchange rate fluctuations on foreign currency denominated intercompany payables and receivables.Loss on extinguishment of debt.We recognized a $0.6 million loss on the extinguishment of debt in 2012 as a result of penalties incurred upon repayment of certain portions of our European debt. We were required to pay-off all existing debt prior to entering into the senior secured term loans that were used to finance our acquisition of OrthoHelix. See Note 8 to our consolidated financial statements for further details. In 2011, we recognized a $29.5 million loss on extinguishment of debt due to the repayment of our notes payable.discontinued operations. Our notes payable were issued in 2008 and 2009 together with warrants to purchase ordinary shares of our company. At the time of issuance, we recognized the estimated fair value of the warrants as a warrant liability with an offsetting debt discount to reduce the carrying value of the notes payable to the estimated fair value at the time of issuance. This debt discount was then amortized as additional interest expense over the term of the notes. At the time of repayment in the first quarter of 2011, we recognized the remaining unamortized portion of the discount as a loss on the extinguishment of debt. See Note 7 to our consolidated financial statements for further discussion of the accounting treatment of the notes payable and related warrants.Other non-operating income. Our other non-operating income decreased to $0.1 million in 2012 from $1.3 million in 2011. The $1.3 million related to 2011 was primarily due to the recognition of a gain related to the resolution of our contingent liability recorded as a part of our acquisition of C2M Medical Inc.Income tax benefit.Our effective tax rate was 33.5% in 2012 and 21.6% in 2011, respectively. The change in our effective tax rate from 2011 to 2012 primarily relates to the relative percentage of our pre-tax income from operations in countries with related income2013 tax expense comparedincluded a $119.6 million provision to operations in countries in which we have pre-tax losses but for which we record a valuation allowance against our deferred tax assets and thus, cannot recognize income tax benefits. In connectionprimarily associated with net operating losses in the acquisition of OrthoHelix in 2012, we recorded deferred tax liabilities of $11.9 million, which included $10.7 million related to amortizable intangible assets and $1.2 million related to indefinite-lived acquired in-process research and development. The deferred tax liabilities of $10.7 million related to the amortizable intangibles reduces our net deferred tax assets by a like amount and in a manner that provides predictable future taxable income over the asset amortization period. As a result, we reduced our pre-acquisition deferred tax asset valuation allowance in 2012 by $10.7 million, which has been reflected as an income tax benefit in our consolidated statements of operations. Although the deferred tax liability of $1.2 million related to acquired in-process research and development also reduces our net deferred tax assets by a like amount, it does so in a manner that does not provide predictable future taxable income because the related asset is indefinite-lived. Therefore, the deferred tax asset valuation allowance was not reducedUnited States as a result of this item. As a result, our income tax benefit increased to $10.9 million for the year ended December 30, 2012 compared to an income tax benefit of $8.4 million in 2011. Given our history ofrecent cumulative operating losses we do not generally record a provision for income taxes in the United States and certain of our European geographies.Year Ended January 1, 2012 (2011) Compared to Year Ended January 2, 2011 (2010)Revenue. Revenue increased by 15% to $261.2 million in 2011 from $227.4 million in 2010 as a result of increased sales in each of our product categories, with the most significant dollar increase occurring in our upper extremity joints and trauma category. The growth across all product categories was due primarily to increased demand and product expansion. Our overall revenue growth of 15% consisted of 11% growth in the United States and 20% growth in our international geographies. Our revenue was positively impacted by foreign currency exchange rate fluctuations of approximately $6.6 million during 2011. Our global revenue growth, excluding the impact of foreign currency exchange rate fluctuations for 2011, was 12%.Revenue by product category. Revenue in upper extremity joints and trauma product category increased by 18% to $164.1 million in 2011 from $139.2 million in 2010 primarily as a result ofU.S. tax jurisdiction, which had an increase in sales of our Aequalis Reverse and Aequalis Ascend shoulder products. We believe that increased sales of our Aequalis shoulder products resulted from market growth in shoulder replacement procedures and market movement towards reversed shoulder replacement procedures. We also saw an increase in sales of our Ascend shoulder product which gained share in the shoulder replacement market. Revenue in our lower extremity joints and trauma increased by 10% to $26.0 million for 2011 from $23.6 million for 2010, primarily due to increased sales in our ankle replacement products in both the United States and internationally and increased sales of our ankle fusion product in the United States due to expanded instrument set availability. Revenue in sports medicine and biologics increased by 12% to $14.8 million for 2011 from $13.2 million for 2010. This increase was attributable to an increase in international sales of our sports medicine product lines. Revenue from large joints and other increased by 10% to $56.3 million for 2011 from $51.4 million for 2010. Our large joint and other revenue increase was primarily due to an increased level of sales in our hip and knee product lines in France, in addition to $2.7 million of favorable impact from changes in foreign currency exchange rates.Revenue by geography. Revenue in the United States increased by 11% to $141.5 million in 2011 from $127.8 million in 2010, primarily driven by an increase in sales in upper extremities joints and trauma products. International revenue increased by 20% to $119.7 million in 2011 from $99.6 million in 2010. Our international revenue was positivelyimpacted by approximately $6.6 million in 2011 as a result of foreign currency exchange rate fluctuations, principally due to the performance of the U.S. dollar against the Euro. Excluding the impact of foreign currency exchange rate fluctuations, our international revenue increased by 14% in 2011, primarily due to increased revenue in France, Australia and Germany; however, nearly all of our international markets experienced constant currency revenue growth during 2011.Cost of goods sold. Our cost of goods sold increased by 18% to $74.9 million in 2011 from $63.4 million in 2010. As aunfavorable 51.9 percentage of revenue, cost of goods sold increased to 29% in 2011 from 28% in 2010, primarily due to the impact of manufacturing variances in 2011 as compared to 2010 resulting from lower inventory growth offset partially by a lower level of inventory obsolescence expense. Our geographic revenue mix can also have anpoint impact on our cost2013 effective tax rate.goodstaxa percentagewell as the $24.3 million gain on the sale of revenue because our international revenue generally resultsthe OrthoRecon business.a higher levelthe third quarter than throughout the rest of cost of goods soldthe year as a percentage of revenue than our United States revenue due to the differences in selling pricesmany of our products are used in various countries. Our international revenue represented 46% of total revenueelective procedures, which generally decline during 2011 compared to 44% during 2010. However, the majority of the increasesummer months. This typically results in international revenue mix for the full year was in countries with relatively stronger pricing. The fourth quarter of 2010 included a higher level of revenue to our European distributor business than the fourth quarter of 2011. As a result, our shift in geographic mix for the full year of 2011 had a limited impact on our total gross profit as a percentage of revenue.Selling, general and administrative. Our selling, general and administrative expenses increased by 8% to $161.4 million in 2011 from $149.2 million in 2010. Asand research and development expenses as a percentage of revenue,net sales that are higher during this period than throughout the rest of the year. In addition, our first quarter selling, general and administrative expenses decreased to 62% during 2011 compared to 66% during 2010 due primarily to leverageinclude additional expenses that we incur in connection with the annual meetings held by the American College of Foot and Ankle Surgeons and the American Academy of Orthopaedic Surgeons. During these three-day events, we display our existing salesmost recent and marketing infrastructure. The increase in total expense is primarily a result of $4.0 million related to foreign currency exchange rate fluctuations, $5.4 million of additional variable selling related expenses such as commissions, royalties and freight on our higher revenue base, and $0.8 million of increased instrument depreciation and maintenance costs from a higher level of instrumentsinnovative products in the field. In addition, we incurred increased legal, audit and administrative fees as a result of being a U.S. public reporting company as well as increased stock-based compensation expense in 2011.Research and development. Research and development expenses increased by 11% to $19.8 million in 2011 from $17.9 million in 2010. As a percentage of revenue, research and development expenses remained at 8% during 2011 and 2010. The increase in total expenses was the result of increased clinical study related expenses and certain biologic related development projects as well as $0.4 million related to foreign currency exchange rate fluctuations.Amortization of intangible assets. Amortization of intangible assets decreased by 2% to $11.3 million in 2011 from $11.5 million in 2010, primarily as a result of the impairment of an intangible that was abandoned in 2011.Special charges. Special charges increased to $0.9 million for 2011 compared to $0.3 million for 2010. This increase was primarily related to severance costs from management organizational changes in 2011.Interest income.Our interest income increased 147% to $0.5 million in 2011 from $0.2 million in 2010 as a result of an increase in cash from the net proceeds from our initial public offering in February 2011.Interest expense. Our interest expense decreased by 80% to $4.3 million in 2011 from $21.8 million in 2010 as a result of the repayment of our notes payable in February 2011. Our notes payable carried an 8% stated interest rate and were recorded at a discount because they were issued together with warrants. The discount on our notes payable was previously also amortized as additional interest expense. As a result, the existence of our notes payable in prior periods caused a much higher level of interest expense. Other interest expense related to the interest paid on our term loans, mortgages, and existing lines of credit.Foreign currency transaction gain (loss). We recorded a foreign currency transaction gain of $0.2 million in 2011 and a foreign currency transaction loss of $8.2 million in 2010. Our foreign currency transaction gains and losses recognized during the year relate to various foreign currency denominated intercompany balances between our various global operating entities. The primary driver of our foreign currency transaction loss in 2010 was related to the revaluation of our warrant liability which was denominated in a currency other than the functional currency of our parent legal entity. We settled our warrant liability in May 2010 by exchanging all the outstanding warrants for our ordinary shares.Loss on extinguishment of debt.We recognized a $29.5 million loss on extinguishment of debt in 2011 due to the repayment of our notes payable. Our notes payable were issued in 2008 and 2009 together with warrants to purchase ordinary shares of the company. At the time of issuance, we recognized the estimated fair value of the warrants as a warrant liability with an offsetting debt discount to reduce the carrying value of the notes payable to the estimated fair value at the time of issuance. This debt discount was then amortized as additional interest expense over the term of the notes. At the time of repayment in the first quarter of 2011, we wrote-off the remaining unamortized portion of the discount as a loss on theextinguishment of debt. See Note 7 to our consolidated financial statements for further discussion of the accounting treatment of the notes payable and related warrants.Other non-operating income. We recorded other non-operating income of $1.3 million in 2011 and less than $0.1 million in 2010. The income in 2011 was primarily related to recognition of a gain on the resolution of a contingent liability recorded from the prior consolidation and acquisition of C2M Medical, Inc. The contingent liability related to then remaining earnout payments on sales of our Piton products. This earnout period ended during the third quarter of 2011 and the remaining liability was reversed and recognized as a gain in the same quarter.Income tax benefit. Our income tax benefit increased $3.3 million to $8.4 million in 2011 compared to $5.1 million in 2010. Our effective tax rate for 2011 and 2010 was 22% and 11%, respectively. During 2011, we recognized $7.5 million of deferred tax benefit related to the $29.5 million loss on extinguishment of debt previously discussed. This benefit was the result of reversing the remaining deferred tax liability related to the unamortized debt discount on our notes payable at the time of repayment. The remaining income tax benefit recognized during 2011 related primarily to pre-tax losses of certain of our international subsidiaries. Our income tax benefit in 2010 primarily related to a tax benefit recorded related to our French subsidiaries as well as deferred tax benefit on the amortization of the debt discount recognized on our notes payable.Seasonality and Quarterly FluctuationsOur business is seasonal in nature. Historically, demand for our products has been the lowest in our third quarter as a result of the European holiday schedule during the summer months.revenuenet sales and gross profitcost of sales as a percentage of net sales among quarters, as well as within each quarter, as a result of a number of factors including, among others,other things, the number and mix of products sold in the quarter and the geographies in which they are sold; the demand for, and pricing of our products and the products of our competitors; the timing of or failure to obtain regulatory clearances or approvals for products; costs, benefits, and timing of new product introductions; the level of competition; the timing and extent of promotional pricing or volume discounts; changes in average selling prices; the availability and cost of components and materials; number of selling days; fluctuations in foreign currency exchange rates; the timing of patients’ use of their calendar year medical insurance deductibles; and impairment and other special charges.Since inception, we have generated significant operating losses. These, combined with significant charges not related to cash from operations, which have included amortization of acquired intangible assets, fair value adjustments to a warrant liability and accretion of noncontrolling interests, have resulted in an accumulated deficit of $235.7 million as of December 30, 2012. Historically, our liquidity needs have been met through a combination of sales of our equity securities together with issuances of debt, including term loans, notes payable and warrants, and other bank related debt. In February 2011, we completed an initial public offering from which we received net proceeds of approximately $162.0 million after underwriters’ discounts, commissions and offering expenses. Certain notes payable were repaid in full during the first quarter of 2011 using $116.1 million of these proceeds.measures: As of December 30,
2012 January 1,
2012 January 2,
2011 ($ in thousands) $ 31,108 $ 54,706 $ 24,838 136,692 133,398 96,965 120,052 39,911 138,120 29,000 23,796 11,252 On October 4, 2012,measures (in thousands): December 27, December 31, 2015 2014 Cash and cash equivalents $ 139,804 $ 227,326 Short-term marketable securities — 2,575 Working capital 352,946 249,958 OrthoHelix Surgical Designs, Inc. In$30 million of cash as a result of the Wright/Tornier merger since this merger was an all-stock transaction, and we paid consideration consisting of $100.4$4.9 million cash (including a final working capital adjustment of $0.3 million) and 1,941,270 of our ordinary shares (which was determined to be equal to $35.0 million divided byfor the average closing sale price per ordinary share during the five trading days immediately prior to and after the date of our initial public announcementacquisition of the merger agreement).Surgical Specialties sales and distribution business. In addition,2014, we agreed to make additional earn-out payments in cash of up topaid an aggregate of $20.0 million based upon our sales of lower extremity joints and trauma products during fiscal years 2013 and 2014. Of the transaction consideration, $10.0$81 million in cash, remainsnet of cash acquired, for the Solana and OrthoPro acquisitions.an escrow account2014 and $6.3 million in 2013. Cash provided by financing activities in 2015 resulted primarily from proceeds received from the issuance of the 2020 Notes, and to funda lesser extent, proceeds from the issuance of the related warrants and proceeds from settling the 2017 Notes hedge option. These amounts were partially offset by amounts used to redeem some of the 2017 Notes, repurchase all of the warrants related to the 2017 Notes, enter into hedges in connection with the 2020 Notes, repay legacy Tornier debt, and pay contingent consideration. See Notes 6 and 9 of our consolidated financial statements contained in “Item 8. Financial Statements and Supplementary Data” for additional information regarding our derivative and debt activity, respectively.obligationsassociated with respect post-closing indemnification obligationsthe BioMimetic acquisition upon FDA approval of OrthoHelix’s former equity holders. In addition, aAUGMENT® Bone Graft. This portion of the earn-outpayment represents the value originally assigned as part of the purchase price allocation. Payments due by periods Total Less than 1 year 1-3 years 3-5 years More than 5 years Amounts reflected in consolidated balance sheet: $ 17,659 $ 1,989 $ 3,643 $ 3,299 $ 8,728 697,238 835 61,100 632,930 2,376 Amounts not reflected in consolidated balance sheet: Operating leases 37,659 10,001 9,945 6,999 10,714 55,009 13,952 26,227 14,830 — Total contractual cash obligations $ 807,565 $ 26,777 $ 100,915 $ 658,058 $ 21,818 (1) Payments include amounts representing interest. (2) (3) rights property and equipment. The present value of set-offthe minimum lease payments are recorded in our balance sheet at December 27, 2015. The minimum lease payments related to these leases are discussed further in post-closing indemnificationoperating leases represent future minimum lease payments under non-cancelable operating leases primarily for certain equipment and office space. In accordance with US GAAP, our operating leases are not recognized on our consolidated balance sheets; however, the minimum lease payments related to these agreements are disclosed in Note 16 to our consolidated financial statements contained in “Item 8. Financial Statements and Supplementary Data.”OrthoHelix’sup to $84 million may be paid upon reaching certain revenue milestones related to the BioMimetic acquisition. In addition, contingent consideration of up to $1.5 million and $0.6 million may be paid upon achieving revenue milestones related to the acquisitions of Surgical Specialties Australia Pty and WG Healthcare, respectively. These potential additional cash payments are based on the future financial performance of the acquired assets. The estimated fair value of these liabilities has been recorded on our consolidated balance sheets within Accrued expenses and other current liabilities and Other long-term liabilities.holders.our acquisitionthe offering of OrthoHelix,the 2020 Notes, WMG entered into convertible note hedging transactions with three counterparties. WMG also entered into warrant transactions in which WMG sold warrants for an aggregate of 20,489,142 shares of WMG common stock to these three counterparties. WMG used approximately $58 million of the net proceeds from the offering to pay the cost of the convertible note hedging transactions (after such cost was partially offset by the proceeds we received from the sale of the warrants). WMG also used approximately $292 million of the net proceeds from the offering to repurchase approximately $240 million aggregate principal amount of outstanding 2017 Notes in privately negotiated transactions. On November 24, 2015, we entered into a new credit agreement. Undersupplemental indenture to the credit agreement, we borrowed $145.0 million, consisting of: (1)indenture governing the 2020 Notes which provided for, among other things, our full and unconditional guarantee, on a senior secured term loan facility to Tornier USA denominated in dollars in an aggregate principal amountunsecured basis, of up to U.S. $75.0 million (referred to as the USD term loan facility); (2) a senior secured term loan facility to Tornier USA denominated in euros in an aggregate principal amountall of upWMG's obligations relating to the U.S. dollar equivalent of U.S. $40.0 million (referred2010 Notes and to as the EUR term loan facility); and (3) a senior secured revolving credit facility to Tornier USA denominated at the election of Tornier USA, in U.S. dollars, euros, pounds, sterling and yen in an aggregate principal amount of upmake certain other adjustments to the U.S. dollar equivalentterms of U.S. $30.0 million. Funds available under the revolving credit facility may be used for general corporate purposes.The borrowings underindenture to give effect to the term loan facilities were used to payWright/Tornier merger. Also on November 24, 2015, we assumed the consideration for our OrthoHelix acquisition, and fees, costs and expenses incurredwarrants initially issued by WMG in connection with the acquisition and the credit agreement and to repay prior existing indebtedness of us and our subsidiaries. The credit agreement contains customary covenants, including financial covenants which require2020 Note offering.maintain minimum interest coverage and maximum total net leverage ratios, and customary events of default. The obligations under the credit agreement are guaranteed by us, Tornier USA and certain other ofpredict our subsidiaries, and subject to certain exceptions, are secured by a first priority security interest in substantially all of our assets and the assets of certain of our existing and future subsidiaries of Tornier.Loans under our USD term facility bear interest at (a) the alternate base rate (if denominated in U.S. dollars), equal to the greatest of (i) the prime rate in effect on such day, (ii) the federal funds rate in effect on such day plus 1/2 of 1%, and (iii) the adjusted LIBO rate, with a floor of 1% (as defined in our new credit agreement) plus 1%, plus in the case of each of (i)-(iii) above, an applicable rate of 2.00% or 2.25% (depending on our total net leverage ratio as defined in our credit agreement), or (b) in the case of a eurocurrency loan (as defined in our credit agreement), at the applicable adjusted LIBO rate for the relevant interest period plus an applicable rate of 3.00% or 3.25% (depending on our total net leverage ratio), plus the mandatory cost (as defined in our credit agreement) if such loan is made in a currency other than U.S. dollars or from a lending office in the United Kingdom or a participating member state (as defined in our credit agreement). Under the EUR term facility, (a) alternate base rate loans bear interest at the alternate base rate plus the applicable rate, which is 3.00% or 3.25% (depending on our total net leverage ratio) and (b) eurocurrency loans bear interest at the adjusted LIBO rate for the relevant interest period, plus an applicable rate, which is 4.00% or 4.25% (depending on our total net leverage ratio), plus the mandatory cost, if applicable. Loans under our revolving credit facility bear interest at (a) the alternate base rate (if denominated in U.S. dollars), equal to the greatest of (i) the prime rate in effect on such day, (ii) the federal funds rate in effect on such day plus 1/2 of 1%, and (iii) the adjusted LIBO rate plus 1%, plus in the case of each of (i)-(iii) above, an applicable rate of 2.00% or 2.25% (depending on our total net leverage ratio as defined in our credit agreement), or (b) in the case of a eurocurrency loan (as defined in our credit agreement), at the applicable adjusted LIBO rate for the relevant interest period plus an applicable rate of 3.00% or 3.25% (depending on our total net leverage ratio), plus the mandatory cost (as defined in our credit agreement) if such loan is made in a currency other than U.S. dollars or from a lending office in the United Kingdom or a participating member state (as defined in our credit agreement).Weliquidity requirements, we believe that our cash and cash equivalents balance for the combined business of approximately $31.1$139.8 million as of December 30, 2012 along with available credit under our revolving credit facility of $29.0 million27, 2015 will be sufficient for the next 12 months to fund our working capital requirements and operations, and permit anticipated capital expenditures during 2013. in 2016 of approximately $43 million, and meet our anticipated contractual cash obligations in 2016. However, our future funding requirements will depend on many factors, including our future net sales and expenses.or for other needs, we could seek to acquire that through additional issuances of equity or to a lesser extent due to limitations in place as a result of our credit facility, debt financing arrangements which may or may not be available on favorable terms at such time.The following table sets forth, for the periods indicated, certain cash flow measures: As of December 30,
2012 January 1,
2012 January 2,
2011 ($ in thousands) (21,744 ) (30,456 ) (39,509 ) 14,431 23,166 2,889 (125,795 ) (29,475 ) (22,853 ) 86,666 39,110 7,427 Operating activities. Net cash provided If we raise additional funds by operating activities decreased by $8.8 millionissuing equity securities, our shareholders may experience dilution. Debt financing, if available, may involve covenants restricting our operations or our ability to $14.4 millionincur additional debt, in 2012 comparedaddition to $23.2 million in 2011. The primary driver of this decrease was the increase in the portionthose under our existing indentures. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our shareholders. If we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our consolidatednet loss that wasproducts or scale back our operations.related. Our 2011 consolidated net loss of $30.5 million included a $29.5 million non-cash loss on the extinguishment of debt, while our 2012 consolidated net loss of $21.7 million included significant cash charges for our facilities consolidation initiative, the acquisitionbalance and integration of OrthoHelix,any additional financing to fund transaction and certain senior management exit costs, among other items.Net cash provided by operating activities was $23.2 million in 2011 compared to net cash provided by operating activities of $2.9 million in 2010. The increase was primarily driven by improvement in our consolidated net loss adjusted for non-cash items and a reduced use of cash for working capital during 2011 as compared to 2010.Investing activities. Net cash used in investing activities totaled $125.8 million, $29.5 million and $22.9 million in 2012, 2011 and 2010, respectively. The increase in net cash used in investing activities in 2012 compared to 2011 was primarily driven by cash paid for acquisitions. In 2012, our acquisition of OrthoHelix included cash consideration of $100.4 million, while the acquisition of our exclusive stocking distributor in Belgium and Luxembourg was a $2.2 million all-cash transaction. Our industry is capital intensive, particularly as it relates to surgical instrumentation. Historically, our capital expenditures have consisted principally of purchased manufacturing equipment, research and testing equipment, computer systems, office furniture and equipment and surgical instruments. Expenditures related to property, plant and equipment increased in 2012 as compared to 2011 due to investments made related to our facilities consolidation initiative. Total capital expenditures were $11.3 million, $6.6 million and $6.7 million in 2012, 2011 and 2010, respectively. Lastly, these items were partially offset by a year-over-year decrease in instrument additions in 2012. Our instrument additions in 2011 were higher than both 2012 and 2010 due to the allocation of a portion of our 2011 initial public offering proceeds to make additional investments in instrumentation to support anticipated future revenue growth. Instrument additions in 2012, 2011 and 2010 were $12.0 million, $19.7 million and $13.8 million, respectively. We anticipate that capital expenditures in 2013 will approximate their historical levels.Financing activities. Net cash provided by financing activities totaled $86.7 million, $39.1 million and $7.4 million in 2012, 2011 and 2010, respectively. The increase in net cash provided by financing activities in 2012 compared to 2011 was due to proceeds received from the issuance of long-term debt of $115.0 million related to our acquisition of OrthoHelix. This increase in cash provided by financing activities was partially offset by the repayment of a majority of our previously existing debt, which was a requirement of the new credit agreement, and the incurrence of debt issuancetransition costs associated with the new credit agreement.The increase in net cash provided by financing activities in 2011 comparedWright/Tornier merger, to 2010 was duefund growth opportunities for our extremities and biologics business, and to the receipt of approximately $168.8 million from the completion of our initial public offering and subsequent exercisepay certain retained liabilities of the underwriters’ overallotment option, netOrthoRecon business.underwriters’ discountsthe acquisition date, which included AUGMENT® Bone Graft, which was undergoing the FDA approval process, and commissions and offering expenses. ThisAUGMENT® Injectable Bone Graft. FDA approval of AUGMENT® Bone Graft in the United States for ankle and/or hindfoot fusion indications was offset in part byobtained during the repaymentthird quarter of our previously existing notes payable2015.$116.1 million. We also used cashthe IPRD technology was $27.1 million for AUGMENT® Injectable Bone Graft. The fair value of the IPRD technology was reduced to reduce our short-term borrowings under various lines of credit by $10.5 million and our long-term borrowing arrangements net of newly issued long-term debt by $3.1 million.Contractual Obligations and CommitmentsThe following table summarizes our outstanding contractual obligations$0 as of December 30, 2012 for31, 2014, which reflects the categories set forth below, assuming only scheduled amortizations and repayment at maturity: Payment Due By Period Contractual Obligations Total Less than
1 Year 1 - 3 Years 3 - 5 Years More than
5 Years ($ in thousands) $ 116,312 $ 3,971 $ 7,957 $ 104,015 $ 369 2,198 — — — 2,198 15,265 360 14,905 — — 1,542 623 720 199 — 22,401 5,318 10,101 6,925 57 1,989 995 994 — — 156 80 65 11 — 31,920 5,489 9,098 72,14 10,119 $ 191,783 $ 16,836 $ 43,840 $ 118,364 $ 12,743 Off-Balance Sheet ArrangementsWe do not have any off-balance sheet arrangements, as defined by the rules and regulationsimpairment charges recognized in 2013 after receipt of the SEC,not approvable letter from the FDA in response to a PMA application for AUGMENT• oran anticipated completion date in 2017 and the cost to complete the project is estimated to be less than $1 million. However, the risks and uncertainties associated with completion are reasonably likelydependent upon FDA clearance.• a material effect on our financial condition, changesan anticipated completion date in financial condition, revenue or expenses, results of operations, liquidity, capital expenditures or capital resources. As a result, we2016 and project cost to complete is estimated to be less than $1 million. However, the risks and uncertainties associated with completion are not materially exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in these arrangements.PoliciesOur consolidated financial statementsEstimatesrelated financial informationestimates are based on the application of U.S. GAAP. The preparation ofdescribed in Note 2 to our consolidated financial statements contained in conformity with U.S. GAAP requires us to make estimates“Item 8. Financial Statements and assumptions that affect the amounts reported in our consolidated financial statements and accompanying notes.policiesestimates require the application of significant judgment by management in selecting the appropriate assumptions for calculating financial estimates.in determining the estimate. By their nature, these judgments are subject to an inherent degree of uncertainty. TheseWe develop these judgments are based on our historical experience, terms of existing contracts, our observance of trends in the industry, information provided by our physician customers, and information available from other outside sources, as appropriate. ChangesDifferent, reasonable estimates could have been used in the current period. Additionally, changes in accounting estimates are reasonably likely to occur from period to period. Changes inBoth of these estimates and changes in our businessfactors could have a material impact on the presentation of our consolidated financial statements.accounting policiesfinancial estimates are both important to the portrayal of our financial condition and results of operations and require subjective or complex judgments. Further, we believe that the items discussed below are properly recognizedrecorded in our consolidated financial statements for all periods presented. ManagementOur management has discussed the development, selection, and disclosure of our most critical financial estimates with the audit committee andof our board of directors.directors and with our independent auditors. The judgments about those financial estimates are based on information available as of the date of our consolidated financial statements. Our critical accounting policies andThose financial estimates are described below:Revenue RecognitionDiscontinued operations.We derive our revenue fromOn January 9, 2014, legacy Wright completed the sale of medical devices that are used by orthopaedic surgeons who treat diseases and disordersthe OrthoRecon business, which consists of extremity joints, including the shoulder, elbow, wrist, hand, ankle and foot, and large joints, including thelegacy Wright's hip and knee.knee product implants, to MicroPort. We determined that this transaction meets the criteria for classification as discontinued operations under the provisions of FASB ASC 205-20. As such, all historical operating results for the OrthoRecon business are reflected within discontinued operations in our consolidated statements of operations. In addition, costs incurred in 2013 associated with corporate employees and infrastructure transferred as a part of the sale have been included in discontinued operations. As this sale occurred in early 2014, costs for 2014 and 2015 primarily relate to product liability claims, including legal defense, settlements and judgments, and changes in contingent liabilities net of product liability insurance recoveries. Further, all assets and associated liabilities transferred to MicroPort were classified as assets and liabilities held for sale on our consolidated balance sheet, in accordance with FASB ASC 360.revenue isrevenues are primarily generated from sales tothrough two types of customers: healthcare institutionscustomers, hospitals and distributors. Sales to healthcare institutions representsurgery centers and stocking distributors, with the majority of our revenue. Inrevenue derived from sales to hospitals and surgery centers. Our products are sold through a network of employee and independent sales representatives in the United States we sell through a focused sales channel primarily consisting of a network of mostly independent commission-based sales agencies, with some direct sales organizations in certain territories. Internationally, we utilizeand by a combination of directemployee sales organizations,representatives, independent sales representatives, and distributors. Generally, revenuestocking distributors outside the United States. We record revenues from sales to healthcare institutionshospitals and surgery centers when they take title to the product, which is generally when the product is surgically implanted in a patient.surgical implantation. approximately $3 million in the quarter ended December 27, 2015.generally record revenuerevenues from sales to our stocking distributors at the time the product is shipped to the distributor. Distributors,Our stocking distributors, who sell the products to their customers, take title to the products and assume all risks of ownership at time of shipment. We do not have any arrangements with distributors that allow for retroactive pricing adjustments.ownership. Our stocking distributors are obligated to pay us within specified terms regardless of when, if ever, they sell the products. In certain circumstances, we may accept sales returns fromgeneral, our stocking distributors and in certain situations in which the rightdo not have any rights of return exists,or exchange; however, in limited situations, we estimatehave repurchase agreements with certain stocking distributors. Those certain agreements require us to repurchase a reserve forspecified percentage of the inventory purchased by the distributor within a specified period of time prior to the expiration of the contract. During those specified periods, we defer the applicable percentage of the sales. An insignificant amount of sales related to these types of agreements were deferred and not yet recognized as revenue as of December 27, 2015 and December 31, 2014.and recognize the reserve as a reduction ofrelated to current period product revenue. We base our estimate for sales returns on historical sales and product return information, including historical experience and trend information. Our reserve for sales returns has historically been immaterial. We charge our customers for shipping and handling and recognize these amounts as part of revenue.AllowanceIn 2011, we entered into a trademark license agreement with KCI Medical Resources, a subsidiary of Kinetic Concepts, Inc. (KCI). In exchange for Doubtful Accounts$8.5 million, of which $5.5 million was received immediately and $3 million was received in January 2012, this license agreement provides KCI with a non-transferable license to use our trademarks associated with our GRAFTJACKETmaintain anexperience credit losses on our accounts receivable; and accordingly, we must make estimates related to the ultimate collection of our accounts receivable. Specifically, we analyze our accounts receivable, historical bad debt experience, customer concentrations, customer creditworthiness, and current economic trends when evaluating the adequacy of our allowance for doubtful accounts for estimated losses in the collection of accounts receivable. We make estimates regarding the future ability of our customers to make required payments based on historical credit experience, delinquency and expected future trends. accounts.receivablesaccounts receivable are due from healthcare institutions,hospitals, many of which are government funded. Accordingly, our collection history with this class of customer has been favorablefavorable. Historically, we have experienced minimal bad debts from our hospital customers and has resulted inmore significant bad debts from certain international stocking distributors, typically as a low levelresult of historical write-offs.specific financial difficulty or geo-political factors. We write off accounts receivable when we determine that the accounts receivable are uncollectible, typically upon customer bankruptcy or the customer’s non-response to continuedrepeated collection efforts.historically has been ana historically appropriate estimate of the amount of accounts receivable that isare ultimately not collected. While we believe that our allowance for doubtful accounts is adequate, the financial condition of our customers and the geopoliticalgeo-political factors that impact reimbursement under individual countries’ healthcare systems can change rapidly, which maywould necessitate additionalFor example, in 2012, we recorded a $2.0 million reserve for certain specific customer accounts in Italy, primarily due to the impact of the ongoing economic challenges in Italy and the termination of an agreement with one distributor. Our allowanceallowances for doubtful accounts was $4.8were $1.2 million and $2.5$0.9 million, at December 30, 201227, 2015 and January 1, 2012,December 31, 2014, respectively.Obsolete Inventoryobsolete inventories.or FIFO,(FIFO) basis or its net realizable value. We regularly review inventory quantities on hand for excess and obsolete inventory (which can include charges for product expirations) and, when circumstances indicate, we incur charges to write down inventories to their net realizable value. Our review of inventory for excess and obsolete quantities is based primarily on an analysis of historical product sales together with our forecast of future product demand and production requirements.requirements for the next 36 months. A significant decrease in demand could result in an increase in the amount of excess inventory quantities on hand. Additionally, our industry is characterized by regular new product developmentsdevelopment that could result in an increase in the amount of obsolete inventory quantities on hand due to cannibalization of existing products. Also, our estimates of future product demand may prove to be inaccurate in which case we may be required to incur charges for excess and obsolete inventory. In the future, if additional inventory write-downs are required, we would recognize additional cost of goods sold at the time of such determination. Regardless of changes in our estimates of future product demand, we do not increase the value of our inventory above its adjusted cost basis. Therefore, although we make every effort to ensure the accuracy of our forecasts of future product demand, significant unanticipated decreases in demand or technological developments could have a significant impact on the value of our inventory and our reported operating results. Charges incurred$8.2approximately $14.2 million, $5.0$4.0 million, and $5.2$4.7 million for the years ended 2012, 2011,December 27, 2015 and 2010,December 31, 2014 and 2013, respectively. The $8.2 million of charges incurred in 2012 included a $3.0 million charge related to the rationalization of products associated with the integration of OrthoHelix into our company.InstrumentsInstruments are surgical tools used by orthopaedic surgeons during joint replacement and other surgical procedures to facilitate the implantation of our products. There are no contractual terms with respect to the usage of our instruments by our customers. Surgeons are under no contractual commitment to use our instruments. We maintain ownership of these instruments and, when requested, we allow the surgeons to use the instruments to facilitate implantation of our related products. We currently do not charge for the use of our instruments and there are no minimum purchase commitments relating to our products. As our surgical instrumentation is used numerous times over several years, often by many different customers, instruments are recognized as long-lived assets once they have been placed in service. Instruments and instrument parts that have not been placed in service are carried at cost and are included as instruments in progress within instruments, net of allowances for excess and obsolete instruments, on our consolidated balance sheets. Once placed in service, instruments are carried at cost, less accumulated depreciation. Instrument parts used to maintain the functionality of instruments but do not extend the life of the instruments are expensed as they are consumed and recorded as part of selling, general and administrative expense. Depreciation is computed using the straight-line method based on average estimated useful lives. Estimated useful lives are determined principally in reference to associated product life cycles, and average five years. As instruments are used as tools to assist surgeons, depreciation of instruments is recognized as a selling, general and administrative expense. Instrument depreciation expense was $12.4 million, $11.0 million, and $9.4 million during 2012, 2011, and 2010, respectively.We review instruments for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. An impairment loss would be recognized when estimated future undiscounted cash flows relating to the assets are less than the asset’s carrying amount. An impairment loss is measured as the amount by which the carrying amount of an asset exceeds its fair value.Business Combinations, Goodwill and Long-Lived Assetslong-lived assets.operationsfinancial results of an acquired business starting fromcompletion ofdate the acquisition.acquisition is completed. In addition, the assets acquired, liabilities assumed, and any contingent consideration must be recorded at the date of acquisition at their respective estimated fair values, with any excess of the purchase price over the estimated fair values of the net assets acquired recorded as goodwill. Significant judgment is required in estimating the fair value of contingent consideration and intangible assets and in assigning their respective useful lives. Accordingly, we typically obtain the assistance of third-party valuation specialists for significant acquisitions. The fair value estimates are based on available historical information and on future expectations and assumptions deemed reasonable by management, but are inherently uncertain.ChangesContingent consideration is adjusted based on experience in subsequent periods and the valueimpact of the contingent considerationchanges related to assumptions are accreted through interest expenserecorded in the consolidated statement of operations.occur that could affect the accuracy or validity of the estimates and assumptions.Certain intangibles are expected to have indefinite lives based on their history and our plans to continue to support and build the acquired brands. Other acquired intangible assets (e.g., certain trademarks or brands, customer relationships, patents and technologies) are expected to have finite useful lives. Our assessment as to trademarks and brands that have an indefinite life and those that have a finite life is based on a number of factors including competitive environment, market share, trademark and/or brand history, underlying product life cycles, operating plans, and the macroeconomic environment of the countries in which the trademarks or brands are sold. Our estimates of the useful lives of finite-lived intangibles are primarily based on these same factors. All of our acquired technology and customer-related intangibles are expected to have finite useful lives.We have$239.8$876.3 million of goodwill recorded as a result of the acquisition of businesses. Goodwill is tested for impairment annually, or more frequently if changes in circumstances or the occurrence of events suggest that impairment exists. Based on our single business approach to decision-making, planningThe annual evaluation of goodwill impairment may require the use of estimates and resource allocation, we have determined that we have one reporting unit for purposes of evaluating goodwill for impairment. We use widely accepted valuation techniquesassumptions to determine the fair value of our reporting unit used in our annual goodwill impairment analysis. Our valuation is primarily based on quantitative assessments regardingunits using projections of future cash flows. Unless circumstances otherwise dictate, the fair value of the reporting unit relative to the carrying value. We also use a market approach to evaluate the reasonableness of the income approach. We performed our annual impairment test is performed on October 1 each year.first day of the fourth quarter of 2012Wright/Tornier merger, and determined that it is not more likely than not that the respective carrying values of our pre-merger reporting units (U.S., International and BioMimetic) exceeded their fair value, of our reporting unit significantly exceeded its carrying value and, therefore, no impairment chargeindicating that goodwill was necessary.The impairment evaluation relatednot impaired.goodwill requires the use of considerable management judgment to determine discounted future cash flows, including estimates and assumptions regarding the amount and timing of cash flows, cost of capital and growth rates. Cash flow assumptions used in the assessment are estimated using assumptions in our annual operating plan as well as our five-year strategic plan. Our annual operating plan and strategic plan contain revenue assumptions that are derived from existing technology as well as future revenues attributed to in-process technologies and the associated launch, growth and decline assumptions normal for the life cycle of those technologies. In addition, management considers relevant market information, peer company data and historical financial information. We also considered our historical operating losses in assessing the risk related to our future cash flow estimates and attempted to reflect that risk in the development of our weighted average cost of capital. and instruments and amortize our intangible assets based upon our estimate of the respective asset’s useful life. Our estimate of the useful life of an asset requires us to make judgments about future events, such as product life cycles, new product development, product cannibalization, and technological obsolescence, as well as other competitive factors beyond our control. We account for the impairment of finite, long-lived assets in accordance with Financial Accounting Standards Board (FASB) Accounting Standard Codification (ASC)the FASB ASC Section 360, Equipment (FASB ASC 360).Equipment. Accordingly, when indicators of impairment exist, we evaluate impairments of our property, plant and equipment and instruments based upon an analysis of estimated undiscounted future cash flows. If we determine that a change is required in the useful life of an asset, future depreciation and amortization is adjusted accordingly. Alternatively, if we determine that an asset has been impaired, an adjustment would be charged to earningsincome based on the asset’s fair market value, or discounted cash flows if the fair market value is not readily determinable.Income Taxesincome taxes.tax-savingtax saving initiatives available to us in the various jurisdictions in which we operate. Significant judgment is required in determining our effective tax rate and evaluating our tax positions. This process includes assessing temporary differences resulting from differing recognition of items for income tax and financial reportingaccounting purposes. These differences result in deferred tax assets and liabilities, which are included within our consolidated balance sheet. Realization of deferred tax assets in each taxable jurisdiction is dependent on our ability to generate future taxable income sufficient to realize the benefits. Management evaluates deferred tax assets on an$30.0$336.1 million and $29.8$171.4 million as of December 30, 201227, 2015 and January 1, 2012,December 31, 2014, respectively, due to uncertainties related to our ability to realize, before expiration, somecertain of our deferred tax assets for both U.S. and foreign income tax purposes. During 2013, we recognized a $119.6 million valuation allowance against our U.S. deferred tax assets due to recent operating losses in the U.S. tax jurisdiction, which resulted in the determination that our U.S. deferred tax assets were not more likely than not to be utilized in the foreseeable future. These deferred tax assets primarily consist of the carryforward of certain tax basis net operating losses and general business tax credits.We recognize See benefits when they are more likely than not to be realized. assets and the associated valuation allowance.liability for unrecognized tax benefits totaled $2.6$9.9 million and $1.9$4.4 million as of December 30, 201227, 2015 and January 1, 2012,December 31, 2014, respectively.Share-Based Compensation to our consolidated financial statements contained in “For purposesItem 8. Financial Statements and Supplementary Data” for further discussion of calculating share-basedour unrecognized tax benefits. we estimate. We calculate the grant date fair value of non-vested shares as the closing sales price on the trading day immediately prior to the grant date. We use the Black-Scholes option pricing model to determine the fair value of stock options using a Black-Scholes option pricing model.and employee stock purchase plan shares. The determination of the fair value of these share-based payment awards utilizing this Black-Scholeson the date of grant using an option-pricing model is affected by our ordinary sharestock price andas well as assumptions regarding a number of assumptions, including expected volatility,complex and subjective variables, which include the expected life of the award, the expected stock price volatility over the expected life of the awards, expected dividend yield, and risk-free interest rate and expected dividends. The estimated fair value of share-based awards exchanged for employee and non-employee director services are expensed over the requisite service period. Option awards issued to non-employees (excluding non-employee directors) are recorded at their fair value as determined in accordance with authoritative guidance, are periodically revalued as the options vest and are recognized as expense over the related service period. do not have information available which is indicative of future exercise and post-vesting behavior to estimate the expected term. As a result, we adoptedlife of options evaluating the historical activity as required by FASB ASC Topic 718, Compensation — Stock Compensation. Prior to the Wright/Tornier merger, the expected life of options was estimated based on historical option exercise and employee termination data. Post merger, the expected life of options was estimated based on the simplified method due to a lack of estimatingcomparable, historic option exercise, and employee termination data for the combined company. The expected term of a stock option, as permitted by the Staff Accounting Bulletin No. 107. Under this method, the expected term is presumed to be the mid-point between the vesting date and the contractual end of the term of our share-based awards. As a non-public entity prior to February 2011, historicprice volatility assumption was not available for our ordinary shares. As a result, we estimated volatility based on a peer group of companies that we believe collectively provides a reasonable basis for estimating volatility. We intend to continue to consistently use the same group of publicly traded peer companies to determine volatility in the future until sufficient information regardingupon historical volatility of our ordinary share price becomes available or the selected companies are no longer suitableshares for this purpose.both legacy Wright and legacy Tornier prior to October 1, 2015. The risk-free interest rate is based on the implied yield available ondetermined using U.S. Treasury zero-coupon issuesrates where the term is consistent with a remaining term approximately equal to the expected life of ourthe stock options. Expected dividend yield is not considered as we have never paid dividends and have no plans of doing so in the future.estimated pre-vesting forfeiture rateBlack-Scholes option-pricing model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable, characteristics not present in our option grants and employee stock purchase plan shares. Existing valuation models, including the Black-Scholes and lattice binomial models, may not provide reliable measures of the fair values of our share-based compensation. Consequently, there is based ona risk that our historical experience together with estimates of future employee turnover. We do not expectthe fair values of our share-based compensation awards on the grant dates may bear little resemblance to declare cash dividendsthe actual values realized upon the exercise, expiration, early termination, or forfeiture of those share-based payments in the foreseeable future. ForCertain share-based payments, such as employee stock options, may expire worthless or otherwise result in zero intrinsic value as compared to the fair valuessummarymarket-based mechanism or other practical application to verify the reliability and accuracy of the estimates stemming from these valuation models.relatedonly for those awards that are expected to vest. All share-based awards see Note 16 of our consolidated financial statements.past. If there iscurrent period and could materially affect our operating income, net income, and net income per share. A change in assumptions may also result in a difference betweenlack of comparability with other companies that use different models, methods, and assumptions.assumptions usedprovisions of FASB ASC 360. We record severance-related expenses once they are both probable and estimable in determining share-based compensationaccordance with the provisions of FASB ASC Section 712, Compensation-Nonretirement Postemployment Benefits, for severance provided under an ongoing benefit arrangement. One-time termination benefit arrangements and other costs associated with exit activities are accounted for under the provisions of FASB ASC Section 420, Exit or Disposal Cost Obligations. We estimated the expense for our restructuring initiatives by accumulating detailed estimates of costs, including the estimated costs of employee severance and related termination benefits, impairment of property, plant and equipment, contract termination payments for leases, and any other qualifying exit costs. Such costs represented management’s best estimates, which were evaluated periodically to determine if an adjustment was required.actual factors which become known over time, specifically with respectamount of revenue recognized reflects the consideration that a company expects to anticipated forfeitures, we may changereceive for the input factors used in determining share-based compensation costsgoods and services provided. The ASU will be effective for future grants. These changes, if any, may materially impact our results of operationsus fiscal year 2018. We are in the period such changesinitial phases of our adoption plans and; accordingly, we are made. We expectunable to continue to grant stock options and other share-based awards in the future, and to the extent that we do,estimate any effect this may have on our actual share-based compensation expense recognized in future periods will likely increase.Recent Accounting PronouncementsIn June 2011, the Financial Accounting Standards Board issued Accounting Standards Update (ASU) 2011-05,Comprehensive Income (Topic 220), Presentation of Comprehensive Income, which converges the presentation of other comprehensive income (OCI) in financial statements prepared under US GAAP and International Financial Reporting Standards (IFRS). This guidance would require disclosure of reclassification adjustments from OCI to net income. In December 2011,On April 7, 2015, the FASB issued ASU 2011-12,Comprehensive Income (Topic 220), Deferral of the Effective Date for Amendments to2015-03, Simplifying the Presentation of ReclassificationsDebt Issuance Costs, as part of Items Outits simplification initiative. The ASU changes the presentation of Accumulated Other Comprehensive Incomedebt issuance costs in Accounting Standards Update No. 2011-05financial statements. Under current guidance (i.e., which deferredASC 835-30-45-3 before the effective date of this guidance to fiscal years beginning after December 15, 2011, with early election permitted. We adopted the provisions of ASU 2011-05ASU), an entity reports debt issuance costs in the first quarterbalance sheet as deferred charges (i.e., as an asset). Under the ASU, an entity presents such costs in the balance sheet as a direct deduction from the related debt liability rather than as an asset. Amortization of 2012. As the adoption was only related to disclosures, the impact of adoption was immaterial to our consolidated financial results.In September 2011,costs is reported as interest expense. Further, on August 16, 2015, the FASB issued ASU 2011-08, Goodwill2015-15 Presentation and Other (ASC Topic 350), Testing Goodwill forImpairment, which simplifiedto clarify the requirements related toSEC staff’s position on presenting and measuring debt issuance costs incurred in connection with line-of-credit arrangements given the annual goodwill impairment test. Companies now have the option to first assess qualitative factors to determine whether it is more likely than not that the fair valuelack of a reporting unit is less than its carrying amount. If the assessment indicatesguidance on this topic in ASU 2015-03. The SEC staff has announced that it iswould not more likely than not thatobject to an entity deferring and presenting debt issuance costs as an asset and subsequently amortizing the fair valuedeferred debt issuance costs ratably over the term of a reporting unit is less than its carrying amount, the Company no longer has to perform the two-step impairment test.line-of-credit arrangement. The ASU 2011-08 waswill be effective for us fiscal years beginning after December 15, 2011 with early adoption permitted.year 2016. We adopteddo not expect this guidance beginning in the first quarter of 2012. Thechange to significantly impact of adoption did not have a material impact on our consolidated financial position or operating results.statements.In July 2012,On September 25, 2015, the FASB issued ASU 2012-02,Intangibles — Goodwill and Other (Topic 350),Testing Indefinite-Lived Intangible Assets2015-16, Simplifying the Accounting for Impairment.Measurement-Period Adjustments to simplify the accounting for measurement-period adjustments. The ASU, adds an optional qualitative assessment for determining whether an indefinite-lived intangible assetwhich is impaired. Companies have the option to first perform a qualitative assessment to determine whether it is more likely than not (a likelihood of more than 50%) that an indefinite-lived intangible asset is impaired. If a company determines that it is more likely than not that the fair value of such an asset exceeds its carrying amount, it would not need to calculate the fair valuepart of the assetFASB’s simplification initiative, was issued in response to stakeholder feedback that year. However, ifrestatements of prior periods to reflect adjustments made to provisional amounts recognized in a company concludes otherwise, it must calculatebusiness combination increase the fair valuecost and complexity of financial reporting but do not significantly improve the usefulness of the asset, compare that value with its carrying amount and record an impairment charge, if any.information. The guidance isASU will be effective for annualus fiscal year 2016. As detailed in Note 3, purchase price allocations for the Wright/Tornier merger are subject to adjustment during the measurement period. Under this ASU, an acquirer must recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined and interim impairment tests performed for fiscal years beginning after September 15, 2012 withmust present these amounts separately on the face of the income statement or disclose in the notes, the portion of the amount recorded in current-period earnings by line item that would have been recorded in previous reporting periods if the adjustment to the provisional amounts had been recognized as of the acquisition date.adoption permitted.adoption. We adoptedhave elected to early adopt this guidance infor the fourth quarter of 2012. The impact of adoptionyear ended December 27, 2015 and retrospectively applied this guidance to the 2014 tax balances. We noted that this change did not have a materialsignificantly impact on our consolidated financial position or operating results.statements.aboutAbout Market Risk.We are exposedvarious market risks, which may result in potential losses arisinginterest rate risk arises principally from adverse changes in market rates and prices, such asthe interest rates and foreign currency exchange rate fluctuations. We do not enter into derivatives or other financial instruments for trading or speculative purposes. We believe we are not exposed to a material market riskassociated with respect to our invested cash balances. On December 27, 2015, we had invested short-term cash and cash equivalents.Interest Rate RiskBorrowings under our senior secured term loans have variableequivalents of approximately $140 million for the combined business. We believe that a 10 basis point change in interest rates as detailed below. As of December 30, 2012, we had $1.0 million in borrowings under our revolving lines of credit and $113.1 million in borrowings under our senior secured term loans. Based upon this debt level, a 25 basis point increaseis reasonably possible in the annualnear term. Based on our current level of investment, an increase or decrease of 10 basis points in interest rate on such borrowingsrates would have an annual impact of $0.3 million onapproximately $140,000 to our interest expense.Atincome.optionprice of Tornier USA, loans under our revolving credit facilityordinary shares and USD term facility, both of which were completed on October 4, 2012, bear interest at (a) the alternate base rate (if denominated in U.S. dollars), equalprior to the greatestWright/Tornier merger, WMG common stock, at conversion or at maturity of (i) the prime ratenotes. On February 13, 2015, WMG issued $632.5 million of the 2020 Notes, which generated net proceeds of approximately $613 million. Approximately $292 million of the net proceeds from the offering were used to repurchase approximately $240 million aggregate principal amount of the 2017 Notes in effect on such day, (ii)privately negotiated transactions. In addition, all of the federal funds effective rate2017 Notes Hedges were settled and all of the warrants associated with the 2017 Notes were repurchased, generating net proceeds of approximately $10 million. As of December 27, 2015, we had approximately $60 million in effect on such day plus 1/2outstanding debt under the 2017 Notes. The following table shows the amount of 1%,cash that we would be required to provide holders of the 2017 Notes upon maturity assuming various closing prices of our ordinary shares at the date of maturity:Share price Cash payment in excess of principal (in thousands) $27.98 (10% greater than conversion price) $ 6,001 $30.53 (20% greater than conversion price) $ 12,002 $33.07 (30% greater than conversion price) $ 18,003 $35.62 (40% greater than conversion price) $ 24,004 $38.16 (50% greater than conversion price) $ 30,004 (iii)prior to the adjusted LIBO rate (as defined inWright/Tornier merger, WMG common stock. The following table presents the fair values of our credit agreement) plus 1%) plus in the case of each of (i)-(iii) above, an applicable rate of 2.00% or 2.25% (depending on our total net leverage ratio (as defined in our credit agreement)), or (b) in the case2017 Notes Conversion Derivative as a result of a eurocurrency loan (as defined in our credit agreement), at the applicable adjusted LIBO rate for the relevant interest period plus an applicable rate of 3.00% or 3.25% (depending on our total net leverage ratio), plus the mandatory cost (as defined in our credit agreement) if such loan is made in a currency other than dollars of any lender our new credit agreement (other than a lender to our credit agreement on October 4, 2012) from a lending office in the United Kingdom or a participating member state (as defined in our credit agreement). Under the EUR term facility, (a) alternate base rate loans bear interest at the alternate base rate plus the applicable rate, which is 3.00% or 3.25% (depending on our total net leverage ratio)hypothetical 10% increase and (b) eurocurrency loans bear interest at the adjusted LIBO rate for the relevant interest period, plus an applicable rate, which is 4.00% or 4.25% (depending on our total net leverage ratio), plus the mandatory cost, if applicable.At December 30, 2012 our cash and cash equivalents were $31.1 million. Based on our annualized average interest rate, a 10% decrease in the annual interest rateprice of our ordinary shares. We believe that a 10% change in our share price is reasonably possible in the near term:(in thousands) Fair value of security given a 10% decrease in share price Fair value of security as of December 27, 2015 Fair value of security given a 10% increase in share price 2017 Notes Conversion Derivative (Liability) 7,282 10,440 14,079 such balancesthe price of our ordinary shares at conversion or at maturity of the notes. In addition, the hedges and warrants associated with these convertible notes also include settlement provisions that are based on the price of our ordinary shares. The amount of cash we may be required to pay, or the number of shares we may be required to provide to note holders at conversion or maturity of these notes, is determined by the price of our ordinary shares. The amount of cash that we may receive from hedge counterparties in connection with the related hedges and the number of shares that we may be required to provide warrant counterparties in connection with the related warrants are also determined by the price of our ordinary shares.Share price Shares (in thousands) $44.00 (10% greater than strike price) 1,863 $48.00 (20% greater than strike price) 3,415 $52.00 (30% greater than strike price) 4,728 $56.00 (40% greater than strike price) 5,854 $60.00 (50% greater than strike price) 6,830 an immaterial impact onthe price of our interest income on an annual basis.(in thousands) Fair value of security given a 10% decrease in share price Fair value of security as of December 27, 2015 Fair value of security given a 10% increase in share price 2020 Notes Hedges (Asset) $101,688 $127,758 $155,911 2020 Notes Conversion Derivative (Liability) $99,942 $129,107 $160,910 Risk rate between the U.S. dollar and foreign currencies could adversely affect our financial results. In 2012Approximately 30% and 2011, approximately 44% and 46%, respectively,21% of our revenuesnet sales from continuing operations were denominated in foreign currencies. Wecurrencies during the years ended December 27, 2015 and December 31, 2014, respectively, and we expect that foreign currencies will continue to represent a similarly significant percentage of our revenuesnet sales in the future. Operating expensesCost of sales related to these revenuessales are primarily denominated in U.S. dollars; however, operating costs related to these sales are largely denominated in the same respective currency,currencies, thereby partially limiting our transaction risk exposure, to some extent. However, for revenuesexposure. For sales not denominated in U.S. dollars, if there is an increase in the rate at which a foreign currency is exchanged for U.S. dollars it will require more of the foreign currency to equal a specified amount of U.S. dollars than before the rate increase. In such cases, and if we price our products in thethe year ended December 30, 2012,2015, approximately 79%97% of our revenuesnet sales denominated in foreign currencies wereare derived from EUEuropean Union countries, and werewhich are denominated in Euros.the Euro; from the United Kingdom, which are denominated in the British pound; from Australia which are denominated in Australian dollar; and from Canada, which are denominated in the Canadian dollar. Additionally, we have significant intercompany receivables, payables, and debt with certain Europeanfrom our foreign subsidiaries whichthat are denominated in foreign currencies, principally the Euro.Euro, the Japanese yen, the British pound, the Australian dollar, and the Canadian dollar. Our principal exchange rate risk, therefore, exists between the U.S. dollar and the Euro.Euro, British pound, Australian dollar, and the Canadian dollar. Fluctuations from the beginning to the end of any given reporting period result in the remeasurementrevaluation of our foreign currency-denominated cash,intercompany receivables, payables, and debt generating currency transactiontranslation gains or losses that impact our non-operating income/income and expense levels in the respective periodperiod.reporteddesigned to mitigate our exposure to currency fluctuations in our intercompany balances denominated currently in Euros, British pounds, and Canadian dollars. Any change in the fair value of these forward contracts as a result of a fluctuation in a currency exchange rate is expected to be offset by a change in the value of the intercompany balance. These contracts are effectively closed at the end of each reporting period.transaction gain (loss)exchange rates does not factor in a potential change in sales levels or local currency prices, which can also be affected by the change in exchange rates.statements. In 2012, we began to economically hedge our exposure to fluctuationsstatement risk associated with changes in interest rates. However, the fair value of these instruments fluctuates when interest rates change, and in the Euro by entering into foreign exchange forward contracts. In future periods, we may hedge other foreign currency exposures.case of our 2017 and 2020 Notes, when the market price of our ordinary shares fluctuates. We do not carry the 2017 and 2020 Notes at fair value, but present the fair value of the principal amount of our 2017 and 2020 Notes for disclosure purposes.Index to Consolidated Financial StatementsPage 70Consolidated Financial Statements 727373747576of Tornier and SubsidiariesTornierWright Medical Group N.V. and subsidiaries (the Company) as of December 30, 2012,27, 2015 and January 1, 2012,December 31, 2014, and the related consolidated statements of operations, comprehensive loss, cash flows, and changes in shareholders’ equity and cash flows for each of the three fiscal years in the period ended December 30, 2012. Our audits also included the27, 2015, December 31, 2014 and December 31, 2013. These consolidated financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and schedule based on our audits.consolidated financial position of Tornier N.V.the Company as of December 27, 2015 and subsidiaries at December 30, 2012 and January 1, 2012,31, 2014, and the consolidated results of theirits operations and theirits cash flows for each of the three fiscal years in the period ended December 30, 2012,27, 2015, December 31, 2014 and December 31, 2013, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, present fairly in all material respects the information set forth therein.Tornier N.V.’sthe Company’s internal control over financial reporting as of December 30, 2012,27, 2015, based on criteria established in Internal Control-IntegratedControl - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 1, 2013,February 23, 2016 expressed an unqualified opinion thereon./s/ Ernst & Youngon the effectiveness of the Company’s internal control over financial reporting.Minneapolis, MinnesotaMarch 1, 2013of Tornier and SubsidiariesTornierWright Medical Group N.V. and subsidiaries’s (the Company) internal control over financial reporting as of December 30, 2012,27, 2015, based on criteria established in Internal Control—Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria)(COSO). Tornier Wright Medical Group N.V. and subsidiaries’’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’sCompany’s internal control over financial reporting based on our audit.risk, andrisk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.As indicated in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting, management’s assessment of and conclusion on the effectiveness of internal control over financial reporting did not include the internal controls of OrthoHelix Surgical Designs, Inc., which is included in the 2012 consolidated financial statements of Tornier N.V. and subsidiaries and constituted less than 5% of total assets as of December 30, 2012 and less than 3% of revenues for the year then ended. Our audit of internal control over financial reporting also did not include an evaluation of the internal control over financial reporting of OrthoHelix Surgical Designs, Inc.TornierWright Medical Group N.V. and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of December 30, 2012,27, 2015, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO criteria.TornierWright Medical Group N.V. and subsidiaries as of December 30, 201227, 2015 and January 1, 2012,December 31, 2014, and the related consolidated statements of operations, comprehensive loss, cash flows, and shareholders’ equity and cash flows for each of the three fiscal years in the period ended December 30, 201227, 2015, December 31, 2014 and December 31, 2013, and our report dated March 1, 2013February 23, 2016 expressed an unqualified opinion thereon./s/ Ernst & Youngon those consolidated financial statementsMinneapolis, MinnesotaMarch 1, 2013TORNIER N.V. AND SUBSIDIARIESFebruary 23, 2016Consolidated Balance Sheets(in thousands except share and per share data) December 30,
2012 January 1,
2012 $ 31,108 $ 54,706 54,192 45,908 86,697 79,883 382 — 2,734 620 �� 14,752 12,417 2,998 2,225 4,455 3,113 197,318 198,872 51,394 49,347 37,151 33,353 239,804 130,544 126,594 97,665 159 69 1,807 1,850 $ 654,227 $ 511,700 $ 4,595 $ 18,011 11,526 12,020 44,410 34,445 83 917 12 81 60,626 65,474 115,457 21,900 20,284 16,966 15,265 — 6,516 5,900 218,148 110,240 1,655 1,560 660,968 608,772 (235,732 ) (213,988 ) 9,188 5,116 436,079 401,460 $ 654,227 $ 511,700 December 27, 2015 December 31, 2014 Assets: Current assets: Cash and cash equivalents $ 139,804 $ 227,326 Marketable securities — 2,575 Accounts receivable, net 131,050 57,190 Inventories 229,109 88,412 Prepaid expenses 15,002 11,161 Other current assets 44,919 50,355 559,884 437,019 Property, plant and equipment, net 240,769 104,235 Goodwill 876,344 190,966 Intangible assets, net 256,743 69,025 2,580 1,649 Other assets 153,355 87,179 $ 2,089,675 $ 890,073 Liabilities and Shareholders’ Equity: Current liabilities: Accounts payable $ 30,904 $ 16,729 173,863 169,614 Current portion of long-term obligations 2,171 718 206,938 187,061 Long-term debt and capital lease obligations 577,382 280,612 41,755 9,553 Other liabilities 208,574 134,044 1,034,649 611,270 Shareholders’ equity: 3,790 2,101 1,835,586 749,469 Accumulated other comprehensive (loss) income (10,484 ) 2,398 Accumulated deficit (773,866 ) (475,165 ) Total shareholders’ equity 1,055,026 278,803 $ 2,089,675 $ 890,073 The prior year deferred tax balances were reclassified to account for early adoption of ASU 2015-17. thethese consolidated financial statements.TORNIER N.V. AND SUBSIDIARIESConsolidated StatementsOperations(in thousands except per share data) Fiscal Year Ended December 30,
2012 January 1,
2012 January 2,
2011 $ 277,520 $ 261,191 $ 227,378 81,918 74,882 63,437 195,602 186,309 163,941 170,447 161,448 149,175 22,524 19,839 17,896 11,721 11,282 11,492 19,244 892 306 223,936 193,461 178,869 (28,334 ) (7,152 ) (14,928 ) 338 550 223 (3,733 ) (4,326 ) (21,805 ) (473 ) 193 (8,163 ) (593 ) (29,475 ) — 116 1,330 43 (32,679 ) (38,880 ) (44,630 ) 10,935 8,424 5,121 (21,744 ) (30,456 ) (39,509 ) — — (695 ) (21,744 ) (30,456 ) (38,814 ) — — (679 ) $ (21,744 ) $ (30,456 ) $ (39,493 ) $ (0.54 ) $ (0.80 ) $ (1.42 ) 40,064 38,227 27,770 TORNIER N.V. AND SUBSIDIARIESConsolidated Statements of Comprehensive Loss(in thousands) Fiscal year ended December 30,
2012 January 1,
2012 January 2,
2011 $ (21,744 ) $ (30,456 ) $ (39,509 ) (866 ) (32 ) (32 ) 4,938 (10,160 ) (4,149 ) $ (17,672 ) $ (40,648 ) $ (43,690 ) — — (695 ) $ (17,672 ) $ (40,648 ) $ (42,995 ) Fiscal year ended December 27, 2015 December 31, 2014 December 31, 2013 Net sales $ 415,461 $ 298,027 $ 242,330 119,255 73,223 59,721 Gross profit 296,206 224,804 182,609 Operating expenses: 429,398 289,620 230,785 39,855 24,963 20,305 Amortization of intangible assets 16,922 10,027 7,476 BioMimetic impairment charges — — 206,249 Total operating expenses 486,175 324,610 464,815 Operating loss (189,969 ) (99,806 ) (282,206 ) Interest expense, net 41,358 17,398 16,040 Other expense (income), net 10,884 129,626 (67,843 ) Loss from continuing operations before income taxes (242,211 ) (246,830 ) (230,403 ) (Benefit) provision for income taxes (3,851 ) (6,334 ) 49,765 Net loss from continuing operations $ (238,360 ) $ (240,496 ) $ (280,168 ) $ (60,341 ) $ (19,187 ) $ 6,223 Net loss $ (298,701 ) $ (259,683 ) $ (273,945 ) Basic $ (3.68 ) $ (4.69 ) $ (5.82 ) Diluted $ (3.68 ) $ (4.69 ) $ (5.82 ) Basic $ (4.61 ) $ (5.06 ) $ (5.69 ) Diluted $ (4.61 ) $ (5.06 ) $ (5.69 ) 64,808 51,293 48,103 64,808 51,293 48,103 These line items include the following amounts of non-cash, share-based compensation expense for the periods indicated: Fiscal year ended December 27, 2015 December 31, 2014 December 31, 2013 Cost of sales $ 287 $ 254 $ 503 Selling, general and administrative 22,777 10,149 10,675 Research and development 1,900 1,084 780 Discontinued operations — — 3,410 thethese consolidated financial statements.TORNIERCash FlowsinIn thousands) Fiscal Year Ended December 30,
2012 January 1,
2012 January 2,
2011 $ (21,744 ) $ (30,456 ) $ (39,509 ) 30,232 28,107 27,038 2,041 — — 731 — — 4,737 210 — (495 ) 298 7,143 (4,506 ) (11,619 ) (6,548 ) (10,700 ) — — 6,830 6,547 5,630 524 2,040 19,612 8,171 4,996 5,212 — 29,475 — — — (172 ) 1,400 — — 1,993 — — 1,836 (186 ) 1,871 (2,188 ) (4,673 ) (3,790 ) (3,057 ) (7,939 ) (17,349 ) 87 2,573 2,348 (1,526 ) 3,987 (307 ) 65 (194 ) 1,710 14,431 23,166 2,889 (102,612 ) — — (1,410 ) (3,142 ) (2,328 ) (11,999 ) (19,734 ) (13,838 ) (1,400 ) — — (9,891 ) (6,599 ) (6,687 ) 1,517 — — (125,795 ) (29,475 ) (22,853 ) (8,009 ) (10,513 ) 6,468 (28,684 ) (8,147 ) (7,687 ) — (116,108 ) — 121,045 5,032 11,361 (5,396 ) (2,731 ) (3,534 ) 7,710 171,577 819 86,666 39,110 7,427 1,100 (2,933 ) (594 ) (23,598 ) 29,868 (13,131 ) 54,706 24,838 37,969 $ 31,108 $ 54,706 $ 24,838 $ 560 $ 640 $ 614 2,937 $ 1,119 $ 999 $ 2,084 $ 2,235 $ 2,193 Fiscal year ended December 27, December 31, December 31, 2015 2014 2013 Net loss $ (298,701 ) $ (259,683 ) $ (273,945 ) Other comprehensive income (loss), net of tax: Changes in foreign currency translation (12,882 ) (17,840 ) (1,381 ) Reclassification of gain on equity securities, net of taxes $1 and $3,041, respectively — 1 (4,757 ) Unrealized gain on marketable securities, net of taxes $987 — — 1,543 Reclassification of currency translation adjustment (CTA) write-off to earnings related to liquidation of Japanese subsidiary — 2,628 — Reclassification of minimum pension liability to earnings — (344 ) 14 Other comprehensive loss (12,882 ) (15,555 ) (4,581 ) Comprehensive loss $ (311,583 ) $ (275,238 ) $ (278,526 ) thethese consolidated financial statements.TORNIER N.V. AND SUBSIDIARIESConsolidated Statements Fiscal year ended December 27, December 31, December 31, 2015 2014 2013 Operating activities: Net loss $ (298,701 ) $ (259,683 ) $ (273,945 ) Adjustments to reconcile net loss to net cash used in operating activities: Depreciation 29,481 18,582 26,296 Share-based compensation expense 24,964 11,487 15,368 Amortization of intangible assets 16,922 10,027 8,345 Amortization of deferred financing costs and debt discount 27,600 10,969 10,288 (3,087 ) (396 ) 51,958 14,218 3,967 4,688 Write-off of deferred financing costs 25,101 — — Excess tax benefit from share-based compensation arrangements — (59 ) (804 ) Amortization of inventory step-up 11,356 — — Non-cash adjustment to derivative fair value (10,045 ) 2,000 1,000 — — (7,798 ) — (24,277 ) — BioMimetic goodwill and intangible impairment charge — — 203,081 (7,571 ) 125,012 (61,151 ) Reduction of insurance receivable 25,000 — — Other 4,780 2,582 (2,788 ) Changes in assets and liabilities (net of acquisitions): Accounts receivable (13,078 ) (11,970 ) (3,477 ) (24,695 ) (25,317 ) 2,686 Prepaid expenses and other current assets (10,471 ) 30,531 (21,945 ) Accounts payable (2,919 ) 12,907 (1,334 ) Accrued expenses and other liabilities 23,258 (22,364 ) 12,931 CVR payment in excess of value assigned as part of PPA (27,983 ) — — Net cash used in operating activities (195,870 ) (116,002 ) (36,601 ) Investing activities: Capital expenditures (43,666 ) (48,603 ) (37,530 ) Acquisition of businesses (4,905 ) (80,556 ) (95,409 ) Purchase of intangible assets (82 ) (11,693 ) (4,291 ) Cash acquired from merger with Tornier 30,117 — — Sales and maturities of available-for-sale marketable securities 2,566 11,795 27,332 Investment in available-for-sale marketable securities — — (20,719 ) Proceeds from sale of assets — 274,687 9,300 Net cash (used in) provided by investing activities (15,970 ) 145,630 (121,317 ) Financing activities: Issuance of ordinary shares 3,513 37,201 6,328 Proceeds from 2020 Warrants 87,072 — — Payment of 2020 Notes hedge option (144,843 ) — — Repurchase of 2017 Warrants (59,803 ) — — Payment of 2017 Notes Premium (49,152 ) — — Proceeds from 2017 Notes hedge option 69,764 — — Maturity/redemption of 2014 convertible senior notes — (3,768 ) — Payment of debt acquired from merger with Tornier (81,367 ) — — Proceeds from convertible 2020 notes 632,500 — — Redemption of convertible 2017 notes (240,000 ) — — Payments of deferred financing costs and equity issuance costs (20,081 ) — (16 ) Payment of contingent consideration (70,120 ) — — Payments of capital leases (621 ) (441 ) (859 ) Fiscal year ended December 27, December 31, December 31, 2015 2014 2013 Excess tax benefit from share-based compensation arrangements — 59 804 Net cash provided by financing activities 126,862 33,051 6,257 Effect of exchange rates on cash and cash equivalents (2,544 ) (4,088 ) 36 Net (decrease) increase in cash and cash equivalents (87,522 ) 58,591 (151,625 ) Cash and cash equivalents, beginning of year 227,326 168,735 320,360 Cash and cash equivalents, end of year $ 139,804 $ 227,326 $ 168,735 The prior year balances were revised to show separate presentation related to provision for excess and obsolete inventory. thethese consolidated financial statements. Ordinary shares Retained earnings/ (accumulated deficit) Accumulated other comprehensive income Total shareholders' equity Balance at December 31, 2012 40,930,191 $ 1,620 $ 440,824 $ 58,463 $ 22,534 $ 523,441 2013 Activity: Net loss — — — (273,945 ) — (273,945 ) Foreign currency translation — — — — (1,381 ) (1,381 ) Reclassification of gain on equity securities, net of taxes $3,041 — — — — (4,757 ) (4,757 ) Unrealized gain (loss) on marketable securities, net of taxes $987 — — — — 1,543 1,543 Minimum pension liability adjustment — — — — 14 14 Issuances of ordinary shares 317,040 12 6,316 — — 6,328 Ordinary shares issued in connection with BioMimetic acquisition 7,171,847 279 168,482 — — 168,761 Ordinary shares issued in connection with Biotech acquisition 765,046 31 20,933 — — 20,964 Grant of non-vested shares of ordinary shares 290,193 — — — — — Forfeitures of non-vested shares of ordinary shares (40,695 ) — — — — — Vesting of stock-settled phantom stock and restricted stock units 43,116 14 (14 ) — — — Tax deficits realized from share-based compensation arrangements, net — — (1,045 ) — — (1,045 ) Share-based compensation — — 19,687 — — 19,687 Equity issuance costs associated with BioMimetic acquisition — — 104 — — 104 Balance at December 31, 2013 49,476,738 $ 1,956 $ 655,287 $ (215,482 ) $ 17,953 $ 459,714 2014 Activity: Net loss — — — (259,683 ) — (259,683 ) Foreign currency translation — — — — (17,840 ) (17,840 ) Reclassification of gain on equity securities, net of taxes $1 — — — — 1 1 — — — — (344 ) (344 ) — — — — 2,628 2,628 Issuances of ordinary shares 1,718,100 68 37,132 — — 37,200 Ordinary shares issued in connection with Solana acquisition 1,406,799 57 41,387 — — 41,444 Grant of non-vested shares of ordinary shares 252,477 — — — — — Forfeitures of non-vested shares of ordinary shares (24,051 ) — — — — — Vesting of stock-settled phantom stock and restricted stock units 83,030 20 (20 ) — — — Share-based compensation — — 15,683 — — 15,683 Balance at December 31, 2014 52,913,093 $ 2,101 $ 749,469 $ (475,165 ) $ 2,398 $ 278,803 Ordinary shares Retained earnings/ (accumulated deficit) Accumulated other comprehensive income Total shareholders' equity 2015 Activity: Net loss — $ — $ — $ (298,701 ) $ — $ (298,701 ) Foreign currency translation — $ — $ — $ — $ (12,882 ) $ (12,882 ) Issuances of ordinary shares 160,306 $ 6 $ 3,514 $ — $ — $ 3,520 Ordinary shares issued in connection with Tornier merger 49,569,007 $ 1,666 $ 1,032,570 $ — $ — $ 1,034,236 Grant of non-vested shares of ordinary shares 5,246 $ — $ — $ — $ — $ — Forfeitures of non-vested shares of ordinary shares (5,869 ) $ — $ — $ — $ — $ — Vesting of stock-settled phantom stock and restricted stock units 30,895 $ 17 $ (17 ) $ — $ — $ — Share-based compensation — $ — $ 24,803 $ — $ — $ 24,803 Issuance of stock warrants, net of equity issuance costs — $ — $ 25,247 $ — $ — $ 25,247 Balance at December 27, 2015 102,672,678 $ 3,790 $ 1,835,586 $ (773,866 ) $ (10,484 ) $ 1,055,026 The balances of CTA and minimum pension liability adjustment within AOCI were written-off in 2014 following the liquidation of our former Japanese subsidiary as part of the sale of our OrthoRecon business. This was recorded within the gain on the sale of the OrthoRecon business within results of discontinued operations. TORNIERWright Medical Group N.V. AND SUBSIDIARIESNotes to the Consolidated Financial Statements1. Business DescriptionTornier N.V. (Tornier(Wright or the Company)we) is a global medical device company focused on orthopaedic surgeons that treat musculoskeletal injuriesextremities and disordersbiologics products. We are committed to delivering innovative, value-added solutions improving quality of life for patients worldwide and are a recognized leader of surgical solutions for the upper extremities (shoulder, elbow, wrist and hand), lower extremities (foot and ankle) and biologics markets, three of the shoulder, elbow, wrist, hand, ankle and foot. Tornier refers to these surgeons as extremity specialists. Tornier sells to this extremity specialist customer base a broad line of joint replacement, trauma, sports medicine and biologicfastest growing segments in orthopaedics. We market our products to treat extremity joints. The Company’s motto of “specialists serving specialists” encompasses this focus. In certain international markets, Tornier also offers joint replacement products for the hip and knee. Tornier currently sells approximately 100 product lines in approximately 40 countries.The Company’sover50 countries worldwide.The Company’s U.S.We also have significant operations located in Memphis, Tennessee (U.S. headquarters, and its U.S. sales and distribution operations are in Bloomington, Minnesota. The Company conducts manufacturing, research and development, sales and distributionmarketing administration, and administrative activities inactivities); Bloomington, Minnesota (upper extremities sales and marketing); Arlington, Tennessee (manufacturing and warehousing operations); Grenoble, France (manufacturing and also has manufacturing operations in Ireland. The Company has otherresearch and development); and Macroom, Ireland (manufacturing). In addition, we have local sales and distribution operationsoffices in Canada, Australia, Germany, Italy,Asia, and throughout Europe. For purposes of this report, references to "international" or "foreign" relate to non-U.S. matters while references to "domestic" relate to U.S. matters.Netherlands, Belgium,merger between legacy Wright and legacy Tornier (the Wright/Tornier merger or merger), Robert J. Palmisano, former President and Chief Executive Officer (CEO) of legacy Wright, became President and CEO of the combined company. David H. Mowry, former President and CEO of legacy Tornier, became Executive Vice President and Chief Operating Officer, and Lance A. Berry, former Senior Vice President (SVP) and Chief Financial Officer (CFO) of legacy Wright, became SVP and CFO. Our board of directors is comprised of five representatives from legacy Wright’s board of directors and five representatives from legacy Tornier’s board of directors, including Mr. Palmisano and Mr. Mowry. Immediately upon completion of the merger, legacy Wright shareholders owned approximately 52% of the combined company and legacy Tornier shareholders owned approximately 48%. In connection with the merger, the trading symbol for our ordinary shares changed from “TRNX” to “WMGI.” Because of these and other facts and circumstances, the merger has been accounted for as a “reverse acquisition” under generally acceptable accounting principles in the United Kingdom, Scandinavia, JapanStates (US GAAP), and Switzerlandas such, legacy Wright is considered the acquiring entity for accounting purposes. Therefore, legacy Wright’s historical results of operations replaced legacy Tornier’s historical results of operations for all periods prior to the merger. More specifically, the accompanying consolidated financial statements for periods prior to the merger are those of legacy Wright and has other researchits subsidiaries, and developmentfor periods subsequent to the merger also include legacy Tornier and qualityits subsidiaries.regulatory functions located in Warsaw, Indiana, and San Diego, California. The operationsends on the Sunday nearest to the thirty-first of OrthoHelix Surgical Designs, Inc. (OrthoHelix), which was acquired inDecember of the fourth quarter of 2012, are located in Medina, Ohio.theour consolidated results of the Company for each of the fiscal years in the three-year period ended December 30, 2012, January 1, 201227, 2015, December 31, 2014, and January 2, 2011.On January 28, 2011, the Company executed a 3-to-1 reverse stock split of the Company’s ordinary shares. The consolidated financial statements as of January 2, 2011 and for the year ended January 2, 2011 give retroactive effect to the reverse stock split.On January 28, 2011, the Company made a change to its legal form by converting from Tornier B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) to Tornier N.V., a public company with limited liability (naamloze vennootschap).In February 2011, the Company completed an initial public offering of 8,750,000 ordinary shares at an offering price of $19.00 per share (before underwriters’ discounts and commissions). The Company received proceeds of approximately $149.2 million (after underwriters’ discounts and commissions of approximately $10.8 million and additional offering related costs of $6.2 million). Net proceeds were used for the retirement of debt, working capital and other general corporate purposes. Additionally, on March 7, 2011, the Company issued an additional 721,274 ordinary shares at an offering price of $19.00 per share (before underwriters’ discounts and commissions) due to the exercise of the underwriters’ overallotment option. The Company received additional net proceeds of approximately $12.8 million (after underwriters’ discounts and commissions of approximately $0.9 million).Dollar (“$”dollar ($), except where expressly stated as being in other currencies, e.g., Euros (“€”(€).Consolidationtheour accounts and those of the Company and all of its wholly and majority ownedour wholly-owned subsidiaries. In consolidation, all material intercompanyIntercompany accounts and transactions are eliminated.EstimatesEstimates.consolidatedpreparation of financial statements are prepared in conformity with United States generally accepted accounting principles (U.S. GAAP) and include amounts that are based on management’s bestUS GAAP requires management to make estimates and judgments.assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.Basis The most significant areas requiring the use of PresentationThe Company’s fiscal year-end is generally determined on a 52-week basismanagement estimates relate to discontinued operations, revenue recognition, the determination of allowances for doubtful accounts and always falls onexcess and obsolete inventories, accounting for business combinations and the Sunday nearest to December 31. Every few years, it is necessary to add an extra weekevaluation of goodwill and long-lived assets, product liability claims and other litigation, income taxes, share-based compensation, and accounting for restructuring charges.year making it a 53-week period in order previously disclosed Asset Purchase Agreement, dated as of June 18, 2013 (the MicroPort Agreement), by and among us and MicroPort Scientific Corporation (MicroPort), we completed our divesture and sale of our business operations operating under our prior OrthoRecon operating segment (the OrthoRecon Business)have the year end fall on the Sunday nearest to December 31. For example, the year ended January 2, 2011 (2010) includes an extra week of operations relativeMicroPort. Pursuant to the years ended December 30, 2012 (2012) and January 1, 2012 (2011). The extra week was addedterms of the MicroPort Agreement, the purchase price (as defined in the first quarteragreement) for the OrthoRecon Business was approximately $283 million (including a working capital adjustment), which MicroPort paid in cash.year ended January 2, 2011, making this quarter 14 weekssale, are reflected within discontinued operations in length.ReclassificationsCertain prior year amounts have been reclassified to conform with the presentation used in 2012. These reclassifications did not have a material impact in the presentation of prior year amounts.Foreign Currency TranslationThe functional currencies for the Company and all of the Company’s wholly owned subsidiaries are their local currencies. The reporting currency of the Company is the United States dollar. Accordingly, the consolidated financial statements of the Company and its international subsidiaries are translated into United States dollars using current exchange rates for the consolidated balance sheets and average exchange rates for the consolidated statements of operations. Further, all assets and associated liabilities to be transferred to MicroPort were classified as assets and liabilities held for sale on our consolidated balance sheet as of December 31, 2013. See flows. Unrealized translation gainsequivalents include all cash balances and lossesshort-term investments with original maturities of three months or less. Any such investments are readily convertible into known amounts of cash, and are so near their maturity that they present insignificant risk of changes in value because of interest rate variation.accumulated other comprehensive income (loss) in shareholders’ equity. When a transaction is denominated in a currency other than the subsidiary’s functional currency, the Company recognizes a transaction gain or loss in net earnings. Foreign currency transaction (losses) gains included in net earnings“Cost of sales” were $(0.5)approximately $14.2 million, $0.2$4.0 million, and $(8.2) million during the years ended December 30, 2012, January 1, 2012, and January 2, 2011, respectively.Revenue RecognitionThe Company derives its revenue from the sale of medical devices that are used by orthopaedic surgeons who treat diseases and disorders of extremity joints, including the shoulder, elbow, wrist, hand, ankle and foot, and large joints, including the hip and knee. The Company’s revenue is generated from sales to two types of customers: healthcare institutions and distributors. Sales to healthcare institutions represent the majority of the Company’s revenue. The Company utilizes a network of independent commission based sales agencies for sales in the United States, with variations based upon individual territories, and a combination of direct sales organizations, independent sales representatives and distributors for sales outside the United States. Generally, revenue from sales to healthcare institutions is recognized at the time of surgical implantation. The Company generally records revenue from sales to its distributors at the time the product is shipped to the distributor. Distributors, who sell the products to their customers, take title to the products and assume all risks of ownership at time of shipment. The Company does not have any arrangements with distributors that allow for retroactive pricing adjustments. The Company’s distributors are obligated to pay within specified terms regardless of when, if ever, they sell the products. In certain circumstances, the Company may accept sales returns from distributors and in certain situations in which the right of return exists, the Company estimates a reserve for sales returns and recognizes the reserve as a reduction of revenue. The Company bases its estimate for sales returns on historical sales and product return information including historical experience and trend information. The Company’s reserve for sales returns has historically been immaterial.Shipping and HandlingAmounts billed to customers for shipping and handling of products are reflected in revenue and are not considered significant. Costs related to shipping and handling of products are expensed as incurred, are included in selling, general and administrative expense, and were $5.1 million, $5.2 million, and $4.3$4.7 million for the years ended December 30, 2012, January 1, 2012,27, 2015 and January 2, 2011,December 31, 2014 and 2013, respectively.CashProduct Liability Claims, Product Liability Insurance Recoveries, and Cash EquivalentsOther Litigation. Cash equivalentsWe are highly liquid investmentsinvolved in legal proceedingsinvolving product liability claims as well as contract, patent protection, and other matters. See Note 16 for additional information regarding product liability claims, product liability insurance recoveries, and other litigation.original maturity of three months or less. The carrying amount reportedexpense in the consolidated balance sheetsperiod incurred. These expenses are reflected in either continuing or discontinued operations depending on the product associated with the claim.Land improvements 15 to 25 years Buildings 10 to 33 years Machinery and equipment 3 to 14 years Furniture, fixtures and office equipment 1 to 14 years Surgical instruments 6 years equivalentsflows. If it is cost,determined that a change is required in the useful life of an asset, future depreciation and amortization is adjusted accordingly. Alternatively, should we determine that an asset is impaired, an adjustment would be charged to income based on the difference between the asset’s fair market value and the asset's carrying value.approximates fair value.Accounts ReceivableAccountsare government funded. Accordingly, our collection history with this class of customer has been favorable. Historically, we have experienced minimal bad debts from our hospital customers and more significant bad debts from certain international stocking distributors, typically as a result of specific financial difficulty or geo-political factors. We write off accounts receivable consist of tradewhen we determine that the accounts receivable are uncollectible, typically upon customer receivables. The Company maintains anbankruptcy or the customer’s non-response to continued collection efforts. Our allowance for doubtful accounts for estimated losses in the collection of accounts receivable. The Company makes estimates regarding the futureability of its customers to make required payments based on historical credit experience, delinquency and expected future trends. The majority of the Company’s receivables are from health care institutions, many of which are government-funded. The Company’s allowance for doubtful accounts was $4.8 million, $2.5totaled $1.2 million and $2.5$0.9 million at 30, 2012, January 1, 201227, 2015 and January 2, 2011,December 31, 2014, respectively. The increase in the allowance for doubtful accounts in 2012 was primarily due to additional reserves recorded related to specific customers and the impactgeneral economic conditions in Italy.the Companyus to concentrations of credit risk consist principally of accounts receivable. Management attempts to minimize credit risk by reviewing customers’ credit history before extending credit and by monitoring credit exposure on a regular basis. TheAn allowance for doubtfulpossible losses on accounts receivable is established based upon factors surrounding the credit risk of specific customers, historical trends, and other information. Collateral or other security is generally not required for accounts receivable. As30, 2012, there were no customers that2013, this supplier notified us of its intent to terminate the supply agreement in December 2015. This supplier was contractually required to meet our supply requirements until the termination date, and to use commercially reasonable efforts to assist us in identifying a new supplier and support the transfer of technology and supporting documentation to produce this component. Our transition to a new supplier is underway with full cooperation from both the current and the new supplier. Management believes the current supplier has produced sufficient product to meet our production needs for the interim period until the new supplier is on line. See Item 1A, Risk Factors, for further information on our suppliers.more than 10% of accounts receivable.RoyaltiesThe Company pays royalties to certain individuals and companies that have developed and retain the legal rightspursuant to the technology or have assisted the Companyprovisions of FASB ASC Section 740, developmentvarious jurisdictions in which we operate. Significant judgment is required in determining our effective tax rate and evaluating our tax positions. This process includes assessing temporary differences resulting from differing recognition of technology or new products.items for income tax and financial accounting purposes. These royalties are based on sales and are reflected as selling, general and administrative expensesdifferences result in the consolidated statements of operations.InventoriesInventories, net of reserves for obsolete and slow-moving goods, are stated at the lower of cost or market value. Cost is determined on a first-in, first-out (FIFO) basis. Inventory is held both within the Company and by third-party distributors on a consignment basis. Inventories consist of raw materials, work-in-process and finished goods. Finished goods inventories are held primarily in the United States, Europe and Australia and consist primarily of joint implants and related products. Inventory balances consist of the following (in thousands): December 30,
2012 January 1,
2012 $ 5,696 $ 5,986 4,933 4,766 76,068 69,131 $ 86,697 $ 79,883 The Company regularly reviews inventory quantities on-hand for excess and obsolete inventory and, when circumstances indicate, incurs charges to write down inventories to their net realizable value. The Company’s review of inventory for excess and obsolete quantities is based primarily on the estimated forecast of future product demand, production requirements, and introduction of new products. The Company recognized $8.2 million, $5.0 million and $5.2 million of expense for excess or obsolete inventory in earnings during the years ended December 30, 2012, January 1, 2012 and January 2, 2011, respectively. The increase in the earnings charges in 2012 included a $3.0 million charge related to the rationalization of products associated with the integration of OrthoHelix into Tornier. Additionally, the Company had $29.3 million and $20.1 million in inventory held on consignment at December 30, 2012 and January 1, 2012, respectively. The increase in inventory held on consignment was due to inventory acquired as a result of the acquisition of OrthoHelix.Property, Plant and EquipmentProperty, plant and equipment are carried at cost less accumulated depreciation. Depreciation is computed using the straight-line method based on estimated useful lives of five to thirty-nine years for buildings and improvements and two to eight years for machinery and equipment. The cost of maintenance and repairs is expensed as incurred. The Company reviews property, plant and equipment for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. An impairment loss would be recognized when estimated future undiscounted cash flows relating to the asset are less than the asset’s carrying amount. An impairment loss is measured as the amount by which the carrying amount of an asset exceeds its fair value. As a result of the facilities consolidation initiative, the Company recorded several fixed asset impairments during fiscal 2012 related to the Company’s facilities in St. Ismier, France, Dunmanway, Ireland, and Stafford, Texas in the aggregate amount of $0.9 million for the year ended December 30, 2012. No impairment losses were recognized during the years ended January 1, 2012 and January 2, 2011.InstrumentsInstruments are surgical tools used by surgeons during joint replacement and other surgical procedures to facilitate the implantation of the Company’s products. Instruments are recognized as long-lived assets. Instruments and instrument parts that have not been placed in service are carried at cost, and are included as instruments in progress within instruments, net on the consolidated balance sheets. Once placed in service, instruments are carried at cost, less accumulated depreciation. Depreciation is computed using the straight-line method based on average estimated useful lives. Estimated useful lives are determined principally in reference to associated product life cycles, and average five years. Instrument parts used to maintain the functionality of instruments but do not extend the life of the instruments are expensed as they are consumed and recorded as part of selling, general and administrative expense. The Company reviews instruments for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. An impairment loss would be recognized when estimated future undiscounted cash flows relating to the asset are less than the asset’s carrying amount. An impairment loss is measured as the amount by which the carrying amount of an asset exceeds its fair value. The Company recorded an impairment charge of $1.0 million for the year ended December 30, 2012 related to instrument sets and components that were impaired as a result of revisions to existing product lines. No impairment losses were recognized during years ended January 1, 2012 or January 2, 2011. Instruments included in long-term assets on the consolidated balance sheets are as follows (in thousands): December 30,
2012 January 1,
2012 $ 85,869 $ 72,971 18,171 18,024 (52,646 ) (41,648 ) $ 51,394 $ 49,347 The Company provides instruments to surgeons for use in surgeries and retains title to the instruments throughout the implantation process. The increase in gross instruments in 2012 is a result of the acquisition of OrthoHelix. As instruments are used as tools to assist surgeons, depreciation of instruments is recognized as a selling, general and administrative expense. Instrument depreciation expense was $12.4 million, $11.0 million and $9.4 million during the years ended December 30, 2012, January 1, 2012 and January 2, 2011, respectively.Business CombinationsFor all business combinations, the Company records all assets and liabilities of the acquired business, including goodwill and other identified intangible assets, generally at their fair values. Contingent consideration, if any, is recognized at its fair value on the acquisition date and changes in fair value are recognized in earnings until settlement. Acquisition-related transaction costs are expensed as incurred rather than treated as part of the cost of acquisition.GoodwillGoodwill is recognized as the excess of the purchase price over the fair value of net assets of businesses acquired. Goodwill is not amortized, but is subject to impairment tests. Based on the Company’s single business approach to decision-making, planning and resource allocation, management has determined that the Company has one reporting unit for the purpose of evaluating goodwill for impairment. The Company performs its annual goodwill impairment test as of the first day of the fourth quarter of its fiscal year or more frequently if changes in circumstances or the occurrence of events suggest that an impairment exists. Impairment tests are done by comparing the reporting unit’s fair value to its carrying amount to determine if there is potential impairment. If the fair value of the reporting unit is less than its carrying value, an impairment loss is recorded to the extent that the implied fair value of the reporting unit’s goodwill is less than the carrying value of the reporting unit’s goodwill. The fair value of the reporting unit and the implied fair value of goodwill are determined based on widely accepted valuation techniques. No goodwill impairment losses were recorded during the years ended December 30, 2012, January 1, 2012 and January 2, 2011 as the fair value of the reporting unit significantly exceeded its carrying value.Intangible AssetsIntangible assets with an indefinite life, including certain trademarks and trade names, are not amortized. The useful lives of indefinite-life intangible assets are assessed annually to determine whether events and circumstances continue to support an indefinite life. Intangible assets with an indefinite life are tested for impairment annually or whenever events or circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognized if the carrying amount exceeds the estimated fair value of the asset. The amount of the impairment loss to be recorded would be determinedbased upon the excess of the asset’s carrying value over its fair value. No impairment losses were recorded during the years ended December 30, 2012, January 1, 2012 and January 2, 2011.Intangible assets with a finite life, including developed technology, customer relationships, and patents and licenses, are amortized on a straight-line basis over their estimated useful lives, ranging from one to twenty years. Costs incurred to extend or renew license arrangements are capitalized as incurred and amortized over the shorter of the life of the extension or renewal, or the remaining useful life of the underlying product being licensed. Intangible assets with a finite life are tested for impairment whenever events or circumstances indicate that the carrying amount may not be recoverable. An impairment loss would be recognized when estimated future undiscounted cash flows relating to the asset are less than the asset’s carrying amount. An impairment loss is measured as the amount by which the carrying amount of an asset exceeds its fair value. For the year ended December 30, 2012, the Company recognized an impairment charge of $4.7 million dollars related to intangibles where the carrying value was greater than the fair value of the intangibles. This impairment related to developed technology and customer relationship intangibles that were recognized as part of acquisitions that occurred in 2007. The fair value of the intangibles was determined using a discounted cash flow analysis. For the year ended January 1, 2012, the Company recognized an impairment charge of $0.2 million related to developed technology from acquired entities that is no longer being used. No impairment charges were recognized during the year ended January 2, 2011. For the year ended December 30, 2012, intangible asset impairments are included in special charges on the consolidated statement of operations. For the year ended January 1, 2012 and January 2, 2011 intangible asset impairments are included in amortization of intangible assets in the consolidated statements of operations.Derivative Financial InstrumentsAll of the Company’s derivative instruments are recorded in the accompanying consolidated balance sheets as either an asset or liability and are measured at fair value. The changes in the derivative’s fair value are recognized currently in current period earnings.Changes to the fair value of foreign currency derivative instrument economic hedges are recognized in foreign currency transaction gain (loss) on the consolidated statement of operations. Any related derivative assets or liabilities are recorded as other current assets or other current liabilities, respectively, in the consolidated balance sheets.The Company also issued warrants in 2008 and 2009 for ordinary shares that were recognized as warrant liabilities on the consolidated balance sheets. Changes in the fair value of these warrants resulted in other non-operating gain of $0.4 million for the year ended January 2, 2011. See Note 7 for additional information on these warrants.Research and DevelopmentAll research and development costs are expensed as incurred.Income TaxesDeferreddeferred tax assets and liabilities, which are determined based on differences between the financial reporting and tax basesincluded within ourare recognizedis reduced by a valuation allowance if, based upon available evidence, it is more likely than not that some component or all of the benefits of deferred tax assets will not be realized.The Company accrues interestpenalties related toliabilities, and the associated valuation allowance.Company’s provisionUnited States and by a combination of employee sales representatives, independent sales representatives, and stocking distributors outside the United States. Revenues from sales to hospitals are recorded when the hospital takes title to the product, which is generally when the product is surgically implanted in a patient.income taxes. Atthis as a change in estimate and have recorded additional revenue of approximately $3 million in the quarter ended December 27, 2015.20122014.January 1, 2012, accrued interestestimates made in connection with establishing the allowance for sales returns in any accounting period. Our reserve for sales returns has historically been immaterial.penalties were $0.2 millionHandling Costs. We incur shipping and $0.2 million, respectively.Other Comprehensive Income (Loss)Research and Development Costs.Other comprehensive income (loss) refers Research and development costs are charged to expense as incurred.that under U.S. GAAPresulting from transactions denominated in a currency other than the local functional currency are included in comprehensive income (loss) but are excluded from net earnings, as these amounts are recorded directly as an adjustment to shareholders’ equity. Other comprehensive income (loss) is comprised mainly of foreign currency translation adjustments and unrealized gains (losses) on retirement plans. These amounts are presented“Other expense, net” in theour consolidated statements of operations.loss.CompensationCompensation.The Company accounts We account for share-based compensation in accordance with FASB ASC TopicSection 718, formerly StatementCompensation — Stock Compensation (FASB ASC 718). Under the fair value recognition provisions of Financial Accounting Standards (SFAS) No. 123(R),Share-Based Payments—Revised, which requiresFASB ASC 718, share-based compensation cost to beis measured at the grant date based on the fair value of the award and is recognized as expense on a straight-line basis over the requisite service period, which is the vesting period. The determination of the fair value of share-based payment awards, such as options, on the date of grant using an option-pricing model is affected by the Company’s shareour stock price, as well as assumptions regarding asharestock price volatility over the expected life of the award,awards, expected dividend yield, and risk-free interest rate. Fiscal year ended December 27, December 31, December 31, 2015 2014 2013 Interest $ 11,198 $ 6,518 $ 5,904 Income taxes $ 1,051 $ 1,525 $ 1,634 Fair value of ordinary shares effectively transferred to Tornier shareholders $ 1,005,468 Fair value of ordinary shares effectively transferred to Tornier share award holders 8,091 Fair value of ordinary shares effectively issued to Tornier stock option holders 20,676 Fair value of total consideration $ 1,034,235 Cash and cash equivalents 30,117 Accounts receivable 63,510 Inventories 140,715 Other current assets 9,256 Property, plant and equipment, net 123,099 Intangible assets, net 204,200 Deferred income taxes 1,399 Other assets 8,658 Total assets acquired 580,954 Current liabilities (105,500 ) Long-term debt (79,554 ) Deferred income taxes (36,544 ) Other non-current liabilities (8,434 ) Total liabilities assumed (230,032 ) Net assets acquired 350,922 Goodwill 683,313 Total preliminary purchase consideration $ 1,034,235 Net sales 656,417 627,435 Net loss from continuing operations (293,419 ) (330,231 ) Cash and cash equivalents $ 416 Accounts receivable 2,366 Inventory 2,244 Prepaid and other current assets 372 Property, plant and equipment 360 Intangible assets 21,584 Accounts payable and accrued liabilities (2,196 ) Total net assets acquired $ 25,146 Goodwill 64,326 Total purchase consideration $ 89,472 Cash and cash equivalents $ 98 Accounts receivable 1,308 Inventory 2,156 Prepaid and other current assets 49 Property, plant and equipment 1,801 Intangible assets 7,772 Accounts payable and accrued liabilities (949 ) Total net assets acquired $ 12,235 Goodwill 20,801 Total purchase consideration $ 33,036 Cash and cash equivalents $ 252 Accounts receivable 4,364 Inventory 5,188 Prepaid and other current assets 303 Deferred tax asset - current 501 Property, plant and equipment 2,573 Intangible assets 17,800 Accounts payable and accrued liabilities (2,552 ) Deferred tax liability - noncurrent (4,228 ) Net assets acquired 24,201 Goodwill 51,836 Total purchase consideration $ 76,037 Fiscal year ended December 27, December 31, December 31, 2015 2014 2013 Revenue $ — $ 3,056 $ 231,865 (Loss) income before tax (60,341 ) (13,521 ) 9,489 Income tax provision — 5,666 3,266 (Loss) income from discontinued operations, net of tax (60,341 ) (19,187 ) 6,223 December 27, December 31, 2015 2014 Raw materials $ 18,057 $ 6,910 Work-in-process 27,946 13,849 Finished goods 183,106 67,653 $ 229,109 $ 88,412 The Company applies Accounting Standards Codification (ASC) Topic 820, and Derivativesa framework for measuringaccounting and reporting standards requiring that derivative instruments be recorded on the balance sheet as either an asset or liability measured at fair value. Additionally, changes in the derivative’s fair value shall be recognized currently in earnings unless specific hedge accounting criteria are met.clarifies the definition of fair value within that framework. The Company measures certain assets and liabilities at fair value on a recurring or non-recurring basis. U.S. GAAPDisclosures requires fair value measurements to be classified and disclosed in one of the following three categories:Level 1—AssetsLevel 1: Financial instruments with unadjusted, quoted prices listed on active market exchanges. Level 2: Financial instruments determined using prices for recently traded financial instruments with similar underlying terms as well as directly or indirectly observable inputs, such as interest rates and yield curves that are observable at commonly quoted intervals. Level 3: Financial instruments that are not actively traded on a market exchange. This category includes situations where there is little, if any, market activity for the financial instrument. The prices are determined using significant unobservable inputs or valuation techniques. liabilitiesNotes Hedgingunadjusted, quoted prices listed on active market exchanges.Level 2—AssetsASC Topic 815, and liabilities determinedis accounted for as a derivative liability. The fair value of the 2017 Notes Conversion Derivative at the time of issuance of the 2017 Notes was $48.1 million. Location on consolidated balance sheet December 27, 2015 December 31, 2014 2017 Notes Hedges Other assets $ — $ 80,000 2017 Notes Conversion Derivative Other liabilities $ 10,440 $ 76,000 prices for recently traded assets and liabilities with similar underlying terms, as well as directly or indirectly observable inputs, such as interest rates and yield curves that are observable at commonly quoted intervals.—Assets and liabilities that inputs. These instruments are not actively traded and are valued using an option pricing model that uses observable and unobservable market data for inputs. December 27, December 31, 2015 2014 2017 Notes Hedges $ (10,236 ) $ (38,000 ) 2017 Notes Conversion Derivative 16,408 36,000 Net gain/(loss) on changes in fair value $ 6,172 $ (2,000 ) Location on condensed consolidated balance sheet December 27, 2015 December 31, 2014 2020 Notes Hedges Other assets $ 127,758 $ — 2020 Notes Conversion Derivative Other liabilities $ 129,107 $ — exchange.data for inputs. December 27, December 31, 2015 2014 2020 Notes Hedges $ (17,085 ) $ — 2020 Notes Conversion Derivative 20,677 — Net gain on changes in fair value $ 3,592 $ — category includes situationslattice generates a distribution of stock prices at the maturity date and throughout the life of the 2017 Notes and 2020 Notes. Using this stock price lattice, a convertible note lattice was created where there is little, if any, market activitythe value of the embedded conversion option was estimated by comparing the value produced in a convertible note lattice with the option to convert against the value without the ability to convert. In each case, the convertible note lattice first calculates the possible convertible note values at the maturity date, using the distribution of stock prices, which equals to the maximum of (x) the remaining bond cash flows and (y) stock price times the conversion price. The values of the 2017 Notes Conversion Derivative and 2020 Notes Conversion Derivative at the valuation date was estimated using the values at the maturity date and moving back in time on the lattices (both for the assetlattice with the conversion option and without the conversion option). Specifically, at each node, if the 2017 Notes or liability. 2020 Notes are eligible for early conversion, the value at this node is the maximum of (i) converting to stock, which is the stock price times the conversion price, and (ii) holding onto the 2017 Notes and 2020 Notes, which is the discounted and probability-weighted value from the three possible outcomes at the future nodes plus any accrued but unpaid coupons that are not considered at the future nodes. If the 2017 Notes or 2020 Notes are not eligible for early conversion, the value of the conversion option at this node equals to (ii). In the lattice, a credit adjustment was applied to the discount for each cash flow in the model as the embedded conversion option, as well as the coupon and notional payments, is settled with cash instead of shares.pricesfollowing assumptions were used in the fair market valuations of the 2017 Notes Conversion Derivatives and 2020 Notes Conversion Derivatives and the 2020 Notes Hedge as of December 27, 2015: 2017 Notes Conversion Derivative 2020 Notes Conversion Derivative Stock Price Volatility (1) 43.21% 43.21% 43.21% Credit Spread for Wright (2) 6.54% 5.4% NA Credit Spread for Deutsche Bank AG (3) N/A N/A 0.82% Credit Spread for Wells Fargo Securities, LLC (3) N/A N/A 0.43% Credit Spread for JPMorgan Chase Bank (3) N/A N/A 0.62% (1) Volatility selected based on historical and implied volatility of ordinary shares of Wright Medical Group N.V. (2) Credit spread implied from traded price. (3) Credit spread of each bank is estimated using CDS curves. Source: Bloomberg. significant unobservable inputs or valuation techniques.A summarya discounted cash flow model and probability adjusted estimates of the future earnings and is classified in Level 3. Changes in the fair value of contingent consideration are recorded in “Other (income) expense, net” in our consolidated statements of operations. Total At December 27, 2015 Assets Cash and cash equivalents $ 139,804 $ 139,804 $ — $ — Available-for-sale marketable securities U.S. agency debt securities — — — — Certificate of deposit — — — — Corporate debt securities — — — — U.S. government debt securities — — — — Total available-for-sale marketable securities — — — — 2020 Notes Hedges 127,758 — — 127,758 Total $ 267,562 $ 139,804 $ — $ 127,758 Liabilities 2017 Notes Conversion Derivative $ 10,440 $ — $ — $ 10,440 2020 Notes Conversion Derivative 129,107 — — 129,107 Contingent consideration 2,340 — — 2,340 Contingent consideration (CVRs) 28,310 28,310 — — Total $ 170,197 $ 28,310 $ — $ 141,887 Total At December 31, 2014 Assets Cash and cash equivalents $ 227,326 $ 227,326 $ — $ — Available-for-sale marketable securities U.S. agency debt securities — — — — Certificates of deposits — — — — Corporate debt securities 566 — 566 — U.S. government debt securities 2,009 2,009 — — Total available-for-sale marketable securities 2,575 2,009 566 — 2017 Notes Hedges 80,000 — — 80,000 Total $ 309,901 $ 229,335 $ 566 $ 80,000 Liabilities 2017 Notes Conversion Derivative $ 76,000 $ — $ — $ 76,000 Contingent consideration 1,705 — — 1,705 Contingent consideration (CVRs) 133,981 133,981 — — Total $ 211,686 $ 133,981 $ — $ 77,705 that are measured at fair value on a recurring basis atusing unobservable inputs (Level 3): Balance at December 31, 2014 Additions Transfers into Level 3 Gain/(Loss) included in Earnings Settlements Currency Balance at December 27, 2015 2017 Notes Hedges 80,000 — — (10,236 ) (69,764 ) — — 2017 Notes Conversion Derivative (76,000 ) — — 16,408 49,152 — (10,440 ) 2020 Notes Hedges — 144,843 — (17,085 ) — — 127,758 2020 Notes Conversion Derivative — (149,784 ) — 20,677 — — (129,107 ) Contingent consideration (1,705 ) (1,546 ) — 171 656 84 (2,340 ) December 27, December 31, 2015 2014 Land and land improvements $ 1,986 $ 520 Buildings 36,746 26,887 Machinery and equipment 40,251 24,265 Furniture, fixtures and office equipment 98,521 59,885 Construction in progress 21,505 14,178 Surgical instruments 149,960 65,359 348,969 191,094 Less: Accumulated depreciation (108,200 ) (86,859 ) $ 240,769 $ 104,235 December 27, December 31, 2015 2014 Buildings $ 12,408 $ 8,471 Machinery and equipment 3,302 477 Furniture, fixtures and office equipment — 59 15,710 9,007 Less: Accumulated depreciation (3,052 ) (862 ) $ 12,658 $ 8,145 30, 201227, 2015 and January 1, 2012 are as follows: December 30, 2012 Quoted Prices in
Active Markets
(Level 1) Significant Other
Observable Inputs
(Level 2) Significant
Unobservable
Inputs (Level 3) $ 31,108 $ 31,108 $ — $ — (15,265 ) — — (15,265 ) 274 — 274 — $ 16,117 $ 31,108 $ 274 $ (15,265 ) January 1, 2012 Quoted Prices in
Active Markets
(Level 1) Significant Other
Observable Inputs
(Level 2) Significant
Unobservable
Inputs (Level 3) $ 54,706 $ 54,706 $ — $ — $ 54,706 $ 54,706 $ — $ — AsDecember 31, 2014 and 2013, respectively, and included depreciation of December 30, 2012,assets under capital leases.Company had a derivative asset with recurring Level 2 fair value measurements. The derivatives are foreign exchange forward contracts and their fair values are based on pricing for similar recently executed transactions. The contracts were first entered into in 2012. Thecarrying amount of loss recognized in foreign exchange loss forgoodwill occurring during the year ended December 30, 2012 related to this derivative27, 2015, are as follows (in thousands): Total Goodwill at December 31, 2014 $ 190,966 Goodwill associated with Tornier N.V. merger $ 683,313 Goodwill associated with Surgical Specialties acquisition $ 6,158 Foreign currency translation $ (4,093 ) Goodwill at December 27, 2015 $ 876,344 approximately $0.3 million. Included in Level 3 fair value measurements as of December 30, 2012 is a $0.7 million contingent consideration liability related to potential earnout paymentsrecognized for the acquisitionexcess of the Company’s exclusive distributor in Belgium and Luxembourg that was completed in May 2012, and a $14.5 million contingent consideration liability related to potential earnout payments for the acquisition of OrthoHelix that was completed in October 2012. Earn-out liabilities are included in contingent consideration on the consolidated balance sheet. The earn-out liabilities are carried at fair value, which is determined based on a discounted cash flow analysis that included revenue estimates and a discount rate, which are considered significant unobservable inputs as of December 30, 2012. The revenue estimates were based on current management expectations for these businesses and the discount rate used as of December 30, 2012 was 8% and was based on the Company’s estimated weighted average cost of capital. To the extent that these assumptions were to change,purchase price over the fair value of the earn-out liabilities could change significantly. For the year ended December 30, 2012, the Company recognized $0.3 million in interest expense on the mark-to-marketnet assets of the earn-out liabilities. The Company had no Level 3 fair value measurements asbusinesses acquired. Goodwill is required to be tested for impairment at least annually. On October 1, 2015, we performed a qualitative assessment of January 1, 2012. Therewere no transfers into or out of Level 3 fair value measurements in the period.The Company also has some assets and liabilities that are measured at fair value on a non-recurring basis. The Company reviews the carrying amount of its long-lived assets other thanlegacy Wright’s goodwill for potential impairment, whenever events or changesbased upon our reporting units in circumstances indicate that their carrying values may not be recoverable. During the year ended December 30, 2012, the Company recognized an intangible impairment of $4.7 million. The impairment was determined using a discounted cash flow analysis. Key inputs into the analysis included estimated future revenues and expenses and a discount rate. The discount rate of 8% was based on the Company’s weighted average cost of capital. These inputs are considered to be significant unobservable inputs and are considered Level 3 fair value measurements.During the year ended December 30, 2012, the Company initiated and completed a facilities consolidation initiative that included the closure and consolidation of certain facilities in France, Ireland and the U.S., which resulted in the recognition of a $0.9 million impairment charge to write down certain fixed assets to their estimated fair values. The fair value calculations were performed using a cost-to-sell analysis and are considered Level 2 fair value measurements as the key inputs into the calculations included estimated market values of the facilities, which are considered indirect observable inputs. In addition, the Company recorded $0.7 million of lease termination costs for the year ended December 30, 2012 relatedeffect prior to the facilities consolidation initiative. The termination costs wereWright/Tornier merger, and determined using a discounted cash flow analysis that included a discount rate assumption, which is based on the credit adjusted risk free interest rate input, and an assumption related to the timing and amount of sublease income. The timing of the sublease income is a significant unobservable input and thus is considered a Level 3 fair value measurement.As of December 30, 2012, the Company had short-term and long-term debt of $120.1 million, the vast majority of which was variable rate debt. The fair value of the Company’s debt obligations approximates carrying value as a result of its variable rate term and would be considered a Level 2 measurement.Recent Accounting PronouncementsIn June 2011, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2011-05,Comprehensive Income (Topic 220), Presentation of Comprehensive Income. The guidance requires an entity to present components of net income and other comprehensive income in one continuous statement, referred to as the statement of comprehensive income, or in two separate, but consecutive statements. Companies will no longer be permitted to present components of other comprehensive income solely in the statements of stockholders’ equity. The Company adopted ASU 2011-05 beginning in the quarter ended April 1, 2012 and has made the appropriate disclosures in the consolidated financial statements.In September 2011, the FASB issued ASU 2011-08,Goodwill and Other (ASC Topic 350), Testing Goodwill for Impairment, which simplified the requirements related to the annual goodwill impairment test. Companies now have the option to first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If the assessment indicates that it is not more likely than not that the carrying value exceeded fair value, indicating that goodwill was not impaired.a reporting unit is less than its carrying amount, the Company no longer has to performWright/Tornier merger, our management began managing our operations as one reportable segment, orthopaedic products, which includes the two-step impairment test. ASU 2011-08 was effective for fiscal years beginning after December 15, 2011 with early adoption permitted. The Company adopted this guidance beginning in the first quarterdesign, manufacture, marketing, and sales of 2012. The impact of adoption did not have a material impactextremities and biologics products. Based on the Company’s consolidated financial position or operating results.In July 2012, the FASB issued ASU 2012-02,Intangibles — Goodwill and Other (Topic 350), Testing Indefinite-Lived Intangible Assets for Impairment, which adds an optional qualitative assessment for determining whether an indefinite-lived intangible asset is impaired, similarabove, the fair valuation analysis performed in conjunction with the Wright/Tornier merger and the close proximity of the merger to the goodwill guidance issued in ASU 2011-08. Companies have the option to first perform a qualitative assessment to determine whether it isyear-end, we believe that no event has occurred that would more likely than not (a likelihood of more than 50%) that an indefinite-lived intangible asset is impaired. If a company determines that it is more likely than not thatreduce the fair value of such an asset exceedsbelow its carrying amount it would not need to calculate the fair value of the asset inand that year. However, if a company concludes otherwise, it must perform the annual quantitative impairment tests. The Company adopted this guidance beginning in 2012. The impacttest is unnecessary between October 1, 2015 and December 27, 2015.adoption did not have a material impact on the Company’s consolidated financial position or operating results.3. Business CombinationsOn October 4, 2012, the Company completedSurgical Specialties Australia Pty. Ltd. Prior to the acquisition, of 100% of the outstanding common stock of OrthoHelix Surgical Designs, Inc. OrthoHelix is an innovative, high-growth company that is focused on developing and marketing specialty implantable screw and plate systems for the repair of small bone fractures and deformities predominantlySpecialties was our exclusive sales agent in the foot and ankle. Under the terms of the agreement, the Company acquired the assets and assumed certain liabilities ofOrthoHelix for an aggregate purchase price of $152.6 million, including $100.4 million in cash, the equivalent of $38.0 million in Tornier ordinary shares based on the closing share price on the date of acquisition, and $14.2 million related to the fair value of additional contingent consideration of up to $20.0 million. The contingent consideration is payable in future periods based on growth of the lower extremity joints and trauma revenue category.The OrthoHelix acquisition was accounted for as an acquisition ofAustralia. As a business; and, accordingly, the results have been included in the Company’s consolidated results of operations from the date of acquisition. The allocation of the total purchase price of to the net tangible and identifiable intangible assets was based on their estimated fair values asresult of the acquisition, date.we now have a direct employee sales force in Australia. We will not record any incremental revenue as a result of the acquisition as we have historically directly billed the end customer and paid Surgical Specialties a commission. The excessasset purchase agreement included a $4.9 million cash payment and estimated future payments of $5.3 million, primarily related to non-competition and meeting certain financial milestones. As part of the purchase price overallocation, we acquired $5.3 million of intangible assets related to customer relationships, non-competition, and settlement of the identifiable intangiblepre-existing agreement and net tangible assets in the amount of $105.9$6.2 million was allocated to goodwill, which is not deductible for tax purposes. Qualitatively, the three largest components of goodwill, include: (1) expansion into international markets; (2)offset by a $1.4 million deferred tax liability recorded as part of the relationships betweentransaction. Company’s sales representatives and physicians; and (3) the development of new product lines and technology. The purchase price allocation, is preliminary pending the final determinationwe acquired $683.3 million of the fair valuegoodwill and $204.2 million of certain acquired assets and assumed liabilities.The following represents the preliminary allocation of the purchase price: Purchase Price
Allocation
(In Thousands) $ 105,904 40,600 4,330 12,033 776 4,475 (3,606 ) (11,900 ) (16 ) $ 152,596 Acquired identifiable intangible assets are amortized on a straight-line basis over their estimated useful lives. The following table represents components of these identifiable intangible assets and their estimated useful lives at the acquisition date: Fair Value
(In Thousands) Estimated Useful
Life
(In Years) $ 35,500 10 3,500 N/A 1,500 3 100 3 $ 40,600 The preliminary estimated fair value of the intangible assets acquired was determined by the Company with the assistance of a third-party valuation expert. Tornier used an income approachrelated to measure the fair value of the developedcustomer relationships, completed technology, and in-process research and development based on the multi-period excess earnings method, whereby the fair value is estimated based upon the present valuetechnology, and trade names. See the trademarks based upon the relief from royalty method, whereby the fair value is estimated based upon discounting the royalty savings as well as any tax benefits related to ownership to a present value. Tornier used an income approach to measure the fair value of non-compete agreements, based on the incremental income method, whereby value is estimated by discounting the cash flow differential as well as any tax benefits related to ownership to a present value. These fair value measurements were based on significant inputs not observable in the market and thus represent Level 3 measurements under the fair value hierarchy. The significant unobservable inputs include the discount rate of 8% which was based on the Company’s estimate of its weighted cost of capital.Pro forma results of operations (unaudited) of the Company for the years ended December 30, 2012 and January 1, 2012, as if the acquisition had occurred on January 3, 2011, are as follows: Year Ended
December 30,
2012 Year Ended
January 1, 2012 $ 298,051 $ 283,370 (31,390 ) (43,155 ) $ (0.75 ) $ (1.07 ) Pro formaour identifiable intangible assets, net income for the year ended December 30, 2012 was adjusted to exclude all acquisition-related costs. Total acquisition costs related to OrthoHelix were $1.2 million and included in special charges on the consolidated statement of operations. The pro forma results of operations are not necessarily indicative of future operating results. Included in the consolidated statement of operations is approximately $8.0 million of revenue and $1.8 million of net loss related to operations of OrthoHelix subsequent to the transaction closing.4. Property, Plant and EquipmentProperty, plant and equipment balances are as follows (in thousands): December 30,
2012 January 1,
2012 $ 1,830 $ 2,138 12,908 12,501 25,767 20,335 26,541 24,255 4,729 4,110 2,148 — 73,923 63,339 (36,772 ) (29,986 ) $ 37,151 $ 33,353 December 27, 2015 December 31, 2014 Cost Cost Indefinite life intangibles: IPRD technology $ 15,290 $ 4,266 Trademarks — 4,004 Total indefinite life intangibles 15,290 8,270 Finite life intangibles: Distribution channels 250 $ 219 250 $ 194 Completed technology 124,388 14,877 33,253 9,185 Licenses 4,868 703 8,234 1,637 Customer relationships 119,235 7,966 27,946 4,636 Trademarks 14,861 3,464 2,798 1,850 Non-compete agreements 7,521 2,917 8,508 3,397 Other 527 51 771 106 Total finite life intangibles 271,650 $ 30,197 81,760 $ 21,005 Total intangibles 286,940 90,030 Less: Accumulated amortization (30,197 ) (21,005 ) Intangible assets, net $ 256,743 $ 69,025 a resultsuch, the only indefinite life intangible as of the facilities consolidation initiative, the Company recorded several fixed asset impairments during 2012December 27, 2015 related to the Company’s facilities in St. Ismier, France, Dunmanway, Ireland, and Stafford, Texas inIPRD acquired from the aggregate amount of $0.9 million for year ended December 30, 2012. Additionally,Wright/Tornier merger.Company recorded $0.1 million in impairments related to certain distribution channel changes in Europe. These changes are reflected in related fixed asset categories above. These impairments were recorded in special charges, a component of operating expenses, in the consolidated statements of operations for the year ended December 30, 2012. See Note 18 for further description of the facilities consolidation initiative.Depreciation expense recorded on property, plant and equipment was $6.1 million, $6.0 million and $6.1 million during the years ended December 30, 2012, January 1, 2012 and January 2, 2011, respectively.5. Goodwill and Other Intangible AssetsThe following table summarizes the changes in the carrying amount of goodwill for the years ended December 30, 2012 and January 1, 2012 (in thousands): $ 131,830 1,099 (2,385 ) $ 130,544 1,193 106,654 1,413 $ 239,804 The goodwill balancetotal finite life intangible assets held at December 30, 2012 contains $11.427, 2015, we expect to amortize approximately of goodwill that qualifies for future tax deductions.The components of identifiable intangible assets are as follows (in thousands): Gross Value Accumulated
Amortization Net Value $ 107,974 $ (34,109 ) $ 73,865 59,212 (24,634 ) 34,578 5,525 (2,927 ) 2,598 3,500 (5 ) 3,495 3,923 (1,357 ) 2,566 9,492 — 9,492 $ 189,626 $ (63,032 ) $ 126,594 Gross Value Accumulated
Amortization Net Value $ 75,106 $ (29,313 ) $ 45,793 60,399 (21,821 ) 38,578 4,882 (2,061 ) 2,821 1,930 (1,056 ) 874 9,599 — 9,599 $ 151,916 $ (54,251 ) $ 97,665 During the year ended December 30, 2012, the Company acquired its exclusive distributorBelgium2016, $24.6 million in 2017, $20.8 million in 2018, $19.2 million in 2019, and Luxembourg for $3.5$18.5 million which included a $1.0 million earn-out. The preliminary purchase accounting for this transaction resulted in an increase in intangible assets of $3.0 million2020.goodwill of $0.8 million for the year ended December 30, 2012. Additionally, the Company acquired OrthoHelix on October 4, 2012 which resulted in the recording of additional goodwill of $105.9 millionCapital Lease Obligationsadditional intangible assets of $40.6 million for the year ended December 30, 2012. See Note 3 for further details on the acquisition of OrthoHelix.For the year ended December 30, 2012, the Company recognized an impairment charge of $4.7 million related to intangibles where the carrying value was greater than the fair value of the intangibles due to a reduction in forecasted revenue from these products due to cannibalization as a result of acquiring similar products as part of the OrthoHelix acquisition. For the year ended January 1, 2012, the Company recognized an impairment charge of $0.2 million related to developed technology from acquired entities that is no longer being used. No impairment charges were recognized during the yearended January 2, 2011. For the year ended December 30, 2012, intangible asset impairments are included in special charges on the consolidated statement of operations. For the year ended January 1, 2012, intangible asset impairments are included in amortization of intangible assets in the consolidated statements of operations.All finite-lived intangible assets have been assigned an estimated useful life and are amortized on a straight-line basis over the number of years that approximates the assets’ respective useful lives (ranging from one to twenty years). Included in other intangibles are non-compete agreements and patents. The weighted-average amortization periods, by major intangible asset class, are as follows:Weighted-AverageAmortization Period(In Years)Developed technology12Customer relationships13Licenses5In-process research and development5Other4Total amortization expense for finite-lived intangible assets was $11.6 million, $11.3 million and $11.5 million during the years ended December 30, 2012, January 1, 2012 and January 2, 2011, respectively. Amortization expense is recorded as amortization of intangible assets in the consolidated statements of operations. Estimated annual amortization expense for fiscal years ending 2013 through 2017 is as follows (in thousands): Amortization Expense $ 14,588 14,283 14,098 13,112 12,505 6. Accrued LiabilitiesAccrued liabilitiescapital lease obligations consist of the following (in thousands): December 30, 2012 January 1, 2012 $ 16,521 $ 14,596 9,001 7,771 18,888 12,078 $ 44,410 $ 34,445 7. December 27, 2015 December 31, 2014 Capital lease obligations $ 13,763 $ 8,678 2017 Notes 56,505 272,652 2020 Notes 504,547 — Mortgages 2,740 — Shareholder debt 1,998 — 579,553 281,330 Less: current portion (2,171 ) (718 ) $ 577,382 $ 280,612 PayableWarrantsThe Bank of New York Mellon Trust Company, N.A., as Trustee. The 2020 Notes require interest to Issue Ordinary SharesIn April 2009,be paid semi-annually on each February 15 and August 15 at an annual rate of 2.00%, and mature on February 15, 2020 unless earlier converted or repurchased. The 2020 Notes are convertible at the Company issued notes payableoption of the holder, during certain periods and subject to certain conditions described below, solely into cash at an initial conversion rate of 32.3939 shares of WMG common stock per $1,000 principal amount of the 2020 Notes, subject to adjustment upon the occurrence of certain events, which representsaggregate2020 Notes Indenture) for the 2020 Notes was adjusted to an initial conversion rate of 33.39487 ordinary shares (subject to adjustment as provided in the 2020 Notes Indenture) per $1,000 principal amount of €37the 2020 Notes (subject to, and in accordance with, the settlement provisions of the 2020 Notes Indenture). The 2020 Notes may not be redeemed by WMG prior to the maturity date, and no “sinking fund” is available for the 2020 Notes, which means that WMG is not required to redeem or retire the 2020 Notes periodically.(approximately $49.3 million)which are being amortized over the term of the 2020 Notes using the effective interest method. Fiscal year ended December 27, 2015 Principal amount of 2020 Notes 632,500 Unamortized debt discount (127,953 ) Net carrying amount of 2020 Notes $ 504,547 grouptermination event as described above, such as the announcement of investorsa third-party tender offer for more than 10% of our ordinary shares or that included then existing shareholders, new investors and managementmay have a material economic effect on the warrant transactions. Any such adjustment may also reduce the effectiveness of the Company.2020 Note Hedges or increase WMG's obligations under the warrant transactions. The notes carriedaggregate cost of the 2020 Notes Hedges was $145 million and is accounted for as a fixedderivative asset in accordance with ASC Topic 815. See Note 6 of the condensed consolidated financial statements for additional information regarding the 2020 Notes Hedges and the 2020 Notes Conversion Derivative. interest rate of 8.0% with2.00%. WMG may not redeem the 2017 Notes prior to the maturity date, and no “sinking fund” is available for the 2017 Notes, which means that WMG is not required to redeem or retire the 2017 Notes periodically. The 2017 Notes are convertible at the option of the holder, during certain periods and subject to certain conditions as described below, solely into cash at an initial conversion rate of 39.3140 shares per $1,000 principal amount of the 2017 Notes, subject to adjustment upon the occurrence of specified events, which represents an initial conversion price of $25.44 per share. The holder of the 2017 Notes may convert their notes at any time prior to February 15, 2017 only under the following circumstances: (1) during any calendar quarter commencing after the calendar quarter ending December 31, 2012 (and only during such calendar quarter), if the last reported sale price of our ordinary shares for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending on the last trading day of the immediately preceding calendar quarter is greater than or equal to 130% of the conversion price on eachkind semi-annually.the indenture relating to the 2017 Notes. In addition, following certain corporate transactions, WMG, under certain circumstances, will pay a cash make-whole premium by increasing the applicable conversion rate for a holder that elects to convert its 2017 Notes in connection with such corporate transaction. The notes were set2017 Notes are senior unsecured obligations that rank: (i) senior in right of payment to matureany of WMG's indebtedness that is expressly subordinated in March 2014. right of payment to the 2017 Notes; (ii) equal in right of payment to any of WMG's unsecured indebtedness that is not so subordinated; (iii) effectively junior in right of payment to any secured indebtedness to the extent of the value of the assets securing such indebtedness; and (iv) structurally junior to all indebtedness and other liabilities (including trade payables) of WMG's subsidiaries. As a result of this transaction, we recognized deferred financing charges of approximately $8.8 million, which are being amortized over the term of the 2017 Notes using the effective interest method.note agreement,issuance of the Company also issued warrants to purchase an2020 Notes, on February 13, 2015, WMG repurchased and extinguished $240 million aggregate principal amount of 2.9 million ordinary shares at an exercise pricethe 2017 Notes and settled all of $16.98 per share. The Company recordedthe 2017 Notes Hedges (receiving $70 million) and repurchased all of the warrants as liabilities(paying $60 million) associated with an offsettingthe 2017 Notes. As a result of the repurchase, we recognized approximately $25.1 million for the write-off of related pro-rata unamortized deferred financing fees and debt discount recorded as a reductionwithin "Other expense (income), net" in our condensed consolidated statements of operations. As of December 27, 2015, $60 million aggregate principal amount of the carrying2017 Notes remained outstanding and is included within long-term obligations on the consolidated balance sheet. December 27, 2015 December 31, 2014 Principal amount of 2017 Notes $ 60,000 $ 300,000 Unamortized debt discount (3,495 ) (27,348 ) Net carrying amount of 2017 Notes $ 56,505 $ 272,652 notes. The debt discount2017 Notes was being amortized as additional interest expense over the lifeapproximately $68 million at December 27, 2015, based on a quoted price in an active market (Level 1).the notes. The Company executed agreements in May 2010 where 100%Certain Indebtedness and Termination of the warrants were exchanged for ordinary shares.In February 2008, the Company issued notes payable in the aggregate amount of €34.5 million (approximately $52.4 million) to a group of investors that included then existing shareholders and management of the Company. The notes carried a fixed annual interest rate of 8.0% with interest payments accrued in-kind. The notes were set to mature on February 28, 2013. Also,Credit Facility2008 note agreement, the Company issued warrants to purchase an aggregate of 3.1 million ordinary shares at a price of $16.98 per share. The Company had recorded the warrants as liabilities with an offsetting debt discount recorded as a reductionconsummation of the carrying value of the notes. TheWright/Tornier merger, we acquired certain mortgages, shareholder debt, discount was being amortized as additional interest expense over the life of the notes. The Company executed agreements in May 2010 where 100% of the warrants were exchanged for ordinary shares.In February 2011, the Company used approximately $116.1 million (€86.4 million) of the net proceeds from its initial public offering to repay all of the outstanding indebtedness under the notes payable, including accrued interest thereon. At the time of repayment, the Company recognizedterm debt, and a loss on debt extinguishment of $29.5 million and related deferred tax benefit of $7.5 million to recognize the remaining balance of unamortized discount on the notes and to reverse the related deferred tax liability.Notes payable balances prior to the repayment in February 2011 were as follows: February 14,
2011
(Time of
Repayment) January 2,
2011 $ 116,109 $ 114,357 (29,352 ) (30,096 ) $ 86,757 $ 84,261 8. Long-Term DebtA summary of other long-term debt is as follows (in thousands): December 30,
2012 January 1,
2012 $ 1,000 $ 9,989 3,719 5,508 113,135 22,262 2,198 2,152 120,052 39,911 (4,595 ) (18,011 ) $ 115,457 $ 21,900 Aggregate maturities of debt for the next five years are as follows (in thousands): $ 3,574 2,968 5,710 5,605 98,609 2,566 Lines of CreditOn October 4, 2012, the Company, and its U.S. operating subsidiary, Tornier, Inc. (Tornier USA), entered into a credit agreement with Bank of America, N.A., as Administrative Agent, SG Americas Securities, LLC, as Syndication Agent, BMO Capital Markets and JPMorgan Chase Bank, N.A., as Co-Documentation Agents, Merrill Lynch, Pierce, Fenner & Smith Incorporated and SG Americas Securities, LLC, as Joint Lead Arrangers and Joint Bookrunners, and the other lenders party thereto. The credit facility included a senior secured revolving credit facility to Tornier USA denominated at the election of Tornier USA, in U.S. dollars, Euros, pounds, sterling and yen in an aggregate principal amount of up to the U.S. dollar equivalent of $30.0 million. Funds available under the revolving credit facility may be used for general corporate purposes. Loans under our revolving credit facility bear interest at (a) the alternate base rate (if denominated in U.S. dollars), equal to the greatest of (i) the prime rate in effect on such day, (ii) the federal funds rate in effect on such day plus 1/2 of 1%, and (iii) the adjusted LIBO rate plus 1%, plus in the case of each of (i)-(iii) above, an applicable rate of 2.00% or 2.25% (depending on our total net leverage ratio as defined in our credit agreement), or (b) in the case of a eurocurrency loan (as defined in our credit agreement), at the applicable adjusted LIBO rate for the relevant interest period plus an applicable rate of 3.00% or 3.25% (depending on our total net leverage ratio), plus the mandatory cost (as defined in our credit agreement) if such loan is made in a currency other than U.S. dollars or from a lending office in the United Kingdom or a participating member state (as defined in our credit agreement). The total amount outstanding as of December 30, 2012 related to this line of credit was $1.0 million. credit.debt matures in October 2017.Tornier USA had established a $10.0 million secured line of credit at January 1, 2012. The line was secured by working capital and equipment. As of January 1, 2012, there was no outstanding balance under the line. Borrowings underthe line of credit beared annual interest at a 30-day LIBOR plus 2.25%, with a minimum interest rate of 5%. This facility was terminated in 2012.The Company’s European subsidiaries had established unsecured bank overdraft arrangements which allowed for available credit totaling $23.8 million at January 1, 2012. Borrowings under these overdraft arrangements were $10.0 million at January 1, 2012 and had variable annual interest rates based on the Euro Overnight Index Average plus 1.3% or a three-month Euro plus 0.5%-3.0%. This debt was paid off in 2012.MortgagesThe Company had a mortgagemortgages acquired are secured by an office building in Stafford, Texas. This building was sold in December 2012 and the outstanding mortgage balance was paid off. This mortgage had an outstanding amount of $1.2 million at January 1, 2012 and beared a variable annual interest rate of LIBOR plus 2%.The Company also has a mortgage secured by an office building in Grenoble,Montbonnot, France. This mortgageThese mortgages had an outstanding balance of $3.7 million and $4.3$2.7 million at December 30, 201227, 2015 and January 1, 2012, respectively. This mortgage bears a fixed annual interest rate of 4.9%.Bank Term DebtIn addition to the senior secured revolving credit facility discussed above, the credit agreement entered into on October 4, 2012 also provided for an aggregate credit commitment to Tornier USA of $145.0 million, consisting of: (1) a senior secured term loan facility to Tornier USA denominated in dollars in an aggregate principal amount of up to $75.0 million; (2) a senior secured term loan facility to Tornier USA denominated in Euros in an aggregate principal amount of up to the U.S. dollar equivalent of $40.0 million. The borrowings under the term loan facilities were used to pay the cash consideration for the OrthoHelix acquisition, and fees, costs and expenses incurred in connection with the acquisition and the credit agreement and to repay prior existing indebtedness of the Company and its subsidiaries. The debt matures in October 2017. Borrowings under these facilities within the credit agreement as of December 30, 2012 were as follows: $ 75,000 40,772 (5,138 ) $ 110,634 The USD term facility bears interest at (a) the alternate base rate (if denominated in U.S. dollars), equal to the greatest of (i) the prime rate in effect on such day, (ii) the federal funds rate in effect on such day plus 1/2 of 1%, and (iii) the adjusted LIBO rate, with a floor of 1% (as defined in our new credit agreement) plus 1%, plus in the case of each of (i)-(iii) above, an applicable rate of 2.00% or 2.25% (depending on our total net leverage ratio as defined in our credit agreement), or (b) in the case of a eurocurrency loan (as defined in our credit agreement), at the applicable adjusted LIBO rate for the relevant interest period plus an applicable rate of 3.00% or 3.25% (depending on our total net leverage ratio), plus the mandatory cost (as defined in our credit agreement) if such loan is made in a currency other than U.S. dollars or from a lending office in the United Kingdom or a participating member state (as defined in our credit agreement). Under the EUR term facility, (a) alternate base rate loans bear interest at the alternate base rate plus the applicable rate, which is 3.00% or 3.25% (depending on our total net leverage ratio) and (b) eurocurrency loans bear interest at the adjusted LIBO rate for the relevant interest period, plus an applicable rate, which is 4.00% or 4.25% (depending on our total net leverage ratio), plus the mandatory cost, if applicable.The credit agreement, including the term loans and the revolving line of credit, contains customary covenants, including financial covenants which require the Company to maintain minimum interest coverage, annual capital expenditure limits and maximum total net leverage ratios, and customary events of default. The obligations under the credit agreement are guaranteed by the Company, Tornier USA and certain other specified subsidiaries of the Company, and subject to certain exceptions, are secured by a first priority security interest in substantially all of the assets of the Company and certain specified existing and future subsidiaries of the Company. The Company was in compliance with all covenants as of December 30, 2012.As a result of entering into the credit agreement, the Company used a portion of the proceeds to repay several of its previous outstanding debt balances.The Company’s international subsidiaries had other long-term secured and unsecured notes totaling $1.3 million and $22.3 million at December 30, 2012 and January 1, 2012, respectively, with initial maturities ranging from three to ten years. A portion of these notes have fixed annual interest rates that range from 3.7% to 8.5%. A majority of 2.55%-4.9%.notes outstanding in 2011 were paid off in 2012. At the timeresult of repayment, the Company recognized a loss on debt extinguishment of $0.6 million related to penalties and fees for prepayment.Also included in term debt is $1.5 million related to capital leases. See Note 14 for further details.Shareholder DebtIn 2008 one of the Company’stransaction where a 51%-owned and consolidated subsidiariessubsidiary of legacy Tornier borrowed $2.2 million from a then-current member of the Company’slegacy Tornier board of directors, who iswas also a 49% owner of the consolidated subsidiary. This loan was used to partially fund the purchase of real estate in Grenoble, France, to be used asvariable basedvariable-based on the three-month Euro Libor rate plus 0.5%. and has no stated term. The outstanding balance on this debt was $2.2$2 million as of December 30, 201227, 2015. See Note 17 of the condensed consolidated financial statements for additional information regarding this related party transaction.January 1, 2012, respectively. $7 million in a line of credit under a pre-existing credit agreement. Upon completion of the Wright/Tornier merger, we terminated all commitments under this credit agreement and repaid approximately $81 million in outstanding indebtedness. We did not incur any early termination penalties in connection with such repayment and termination.2016 835 2017 60,589 2018 509 2019 212 2020 632,717 Thereafter 2,376 $ 697,238 2016 $ 1,989 2017 1,842 2018 1,801 2019 1,718 2020 1,581 Thereafter 8,728 Total minimum payments 17,659 Less amount representing interest (3,896 ) Present value of minimum lease payments 13,763 Current portion (1,341 ) Long-term portion $ 12,422 Currency translation adjustment Total Balance December 31, 2013 $ 17,610 $ (1 ) $ 344 $ 17,953 Other comprehensive income loss, net of tax (17,840 ) 1 — (17,839 ) 2,628 — (344 ) 2,284 Balance December 31, 2014 $ 2,398 $ — $ — $ 2,398 Other comprehensive income loss, net of tax (12,882 ) — — (12,882 ) Balance December 27, 2015 $ (10,484 ) $ — $ — $ (10,484 ) The balances of CTA and minimum pension liability adjustment within AOCI were written-off following the liquidation of our former Japanese subsidiary as part of the sale of our OrthoRecon business. This was recorded within the gain on the sale of the OrthoRecon business within results of discontinued operations. non-controlling interestcomponents of our loss before income taxes are as follows (in thousands): Fiscal year ended December 27, 2015 December 31, 2014 December 31, 2013 U.S. $ (225,473 ) $ (242,998 ) $ (230,975 ) Foreign (16,738 ) (3,832 ) 572 Loss before income taxes $ (242,211 ) $ (246,830 ) $ (230,403 ) Fiscal year ended December 27, 2015 December 31, 2014 December 31, 2013 Current (benefit) provision: U.S.: Federal $ — $ (48 ) $ 296 State 255 198 85 Foreign 608 1,674 180 Total current (benefit) provision 863 1,824 561 Deferred provision (benefit): U.S.: Federal (1,450 ) (3,164 ) 48,257 State (166 ) (1,411 ) 884 Foreign (3,098 ) (3,583 ) 63 Total deferred provision (benefit) (4,714 ) (8,158 ) 49,204 Total provision (benefit) for income taxes $ (3,851 ) $ (6,334 ) $ 49,765 Fiscal year ended December 27, 2015 December 31, 2014 December 31, 2013 Income tax provision at statutory rate 35.0 % 35.0 % 35.0 % State income taxes 3.7 % 1.8 % 3.2 % Change in valuation allowance (36.5 )% (15.9 )% (51.9 )% CVR fair market value adjustment 1.1 % (17.7 )% 9.3 % Goodwill impairment — % — % (17.5 )% Other, net (1.7 )% (0.6 )% 0.3 % Total 1.6 % 2.6 % (21.6 )% Fiscal year ended December 27, 2015 December 31, 2014 Deferred tax assets: Net operating loss carryforwards $ 289,715 $ 131,986 General business credit carryforward 6,121 3,696 Reserves and allowances 52,482 27,334 Share-based compensation expense 18,423 7,942 Convertible debt notes and conversion option 46,631 31,491 Other 6,720 7,418 Valuation allowance (336,060 ) (171,392 ) Total deferred tax assets 84,032 38,475 Deferred tax liabilities: Depreciation 8,455 1,915 Intangible assets 58,266 9,977 Convertible note bond hedge 49,826 31,200 Other 6,660 3,287 Total deferred tax liabilities 123,207 46,379 Net deferred tax liabilities $ (39,175 ) $ (7,904 ) this subsidiary is deemed immaterial2016 and extend through 2035. State net operating losses carryforwards at December 27, 2015 totaled approximately $537 million, which begin to expire in 2016 and extend through 2035. Additionally, we had general business credit carryforwards of approximately $6 million, which begin to expire in 2016 and extend through 2035. At December 27, 2015, we had foreign net operating loss carryforwards of approximately $101 million, $45 million of which do not expire and $56 million which begin to expire in 2016 and extend through 2028.consolidated financial statements.9. Retirement and Postretirement Benefit PlansThe Company’s French subsidiary is required by French government regulations to offer a plan to its employees that provides certain lump-sum retirement benefits. This plan qualifies as a defined benefit retirement plan. The French regulations do not require fundingmerger with Tornier. We recognized income tax expense for valuation allowance increase of this liability in advance and as a result there are no plan assets associated with this defined-benefit plan. The Company has a liability of $2.5$109 million and $1.5 million recorded at December 30, 2012 and January 1, 2012, respectively, for future obligations under the plan. The increase in the liability was driven by a decrease in the government mandated discount rate from 4.7% to 2.8%. The change in the discount rate resulted in a $0.9 million unrealized loss recorded as a component of other comprehensive loss. The related periodic benefit expense was immaterial in all periods presented.10. Derivative InstrumentsThe Company’s operations outside the United States are significant. As a result, the Company has foreign exchange exposure on transactions denominated in currencies that are different than the functional currency in certain legal entities. Starting in 2012, the Company began entering into forward contracts to manage their exposure to foreign currency transaction gains (losses). As it relates to the Company’s U.S. operating entity, Tornier Inc., the Company has entered into forward contracts to manage the foreign currency exposures to the Euro. As it relates to the Company’s French operating entity, Tornier SAS, the Company has entered into forward contracts to manage the foreign currency exposure to the Australian Dollar, British Pound, Japanese Yen, Swiss Franc and U.S. Dollar. Forward contracts are recorded on the consolidated balance sheet at fair value. At December 30, 2012, the Company had foreign currency forward contracts outstanding with a fair value of $0.3 million. These contracts are accounted for as economic hedges and accordingly, changes in fair value are recognized in earnings. The net loss on foreign exchange forward contracts is recognized in foreign currency transaction gain (loss). Forduring the year ended December 30, 2012, the Company recognized gains of $0.3 million27, 2015, primarily related to these forward currency contracts.11. Income TaxesThe components of earnings (loss) before taxes for the years ended December 30, 2012, January 1, 2012 and January 2, 2011, consist of the following (in thousands): December 30,
2012 January 1, 2012 January 2, 2011 $ (19,858 ) $ (2,631 ) $ (6,526 ) (12,821 ) (36,249 ) (38,104 ) $ (32,679 ) $ (38,880 ) $ (44,630 ) The income tax benefit (provision) for the years ended December 30, 2012, January 1, 2012 and January 2, 2011, consists of the following (in thousands): December 30,
2012 January 1,
2012 January 2,
2011 $ (150 ) $ (327 ) $ (433 ) (2,523 ) (3,140 ) 539 13,608 11,891 5,015 $ 10,935 $ 8,424 $ 5,121 A reconciliation ofadditional net operating losses incurred in the United States statutory income tax rate toStates. Management believes it is more likely than not that the Company’s effective tax rate for the years ended December 30, 2012, January 1, 2012 and January 2, 2011, is as follows: December
30, 2012 January 1,
2012 January 2,
2011 34.0 % 34.0 % 34.0 % 32.8 — — (33.4 ) (10.1 ) (11.9 ) 1.7 6.4 0.3 2.6 (0.4 ) (0.1 ) 1.7 — — (3.5 ) (2.0 ) (6.0 ) — (0.5 ) (2.5 ) (2.5 ) (6.9 ) (5.8 ) (1.8 ) (0.6 ) (0.4 ) 1.9 1.7 3.9 33.5 % 21.6 % 11.5 % Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The Company has established valuation allowances forremaining deferred tax assets whenwill be fully realized.expected future taxable income is not likely to support the use of the deduction or credit.The components of deferred taxes for the years ended December 30, 2012 and January 1, 2012, consist of the following (in thousands): December 30,
2012 January 1,
2012 $ 27,924 $ 26,219 4,960 1,769 223 156 9,715 8,289 5,067 987 47,889 37,420 (30,011 ) (29,817 ) 17,878 7,603 (33,248 ) (22,209 ) (2,033 ) (1,752 ) (35,281 ) (23,961 ) $ (17,403 ) $ (16,358 ) In connection with the acquisition of OrthoHelix, the Company recorded deferred tax liabilities of $11.9 million, which included $10.7 million related to amortizable intangible assets and $1.2 million related to indefinite-lived acquired in-process research and development. The deferred tax liabilities of $10.7 million related to the amortizable intangibles reduces the Company’s net deferred tax assets by a like amount and in a manner that provides predictable future taxable income over the asset amortization period. As a result, the Company reduced its pre-acquisition deferred tax asset valuation allowance in 2012 by $10.7 million, which has been reflected as an income tax benefit in the consolidated statements of operations. Although theunrecognized deferred tax liability on these undistributed earnings is not practicable.$1.2 million related to acquired in-process research and development also reduces the net deferredDecember 27, 2015, our unrecognized tax assets by a like amount, it does so in a manner that does not provide predictable future taxable income because the related asset is indefinite-lived. Therefore, the deferred tax asset valuation allowance was not reduced as a result of this item.The Company had $30.0 million, $29.8 million and $26.9 million of valuation allowance recorded at December 30, 2012, January 1, 2012 and January 2, 2011, respectively. If any amounts reverse, the reversals would be recognized in the income tax provision in the period of reversal. The Company recognized $0.2 million ($10.9 million of increase valuation allowance netted against the $10.7 million of reversal from the OrthoHelix acquisition), $2.9 million and $5.2 million of the valuation allowance as a tax expense during the years ended December 30, 2012, January 1, 2012, and January 2, 2011, respectively.Net operating loss carryforwards totalingbenefits totaled approximately $106 million at December 31, 2012, $53 million which relates to the United States and $53 million related to jurisdictions outside the United States. are available to reduce future taxable earnings of the Company’s consolidated U.S. subsidiaries and certain European subsidiaries, respectively. These net operating loss carryforwards include $14 million with no expiration date; the remaining carryforwards have expiration dates between 2015 and 2030.The Company has recorded a long-term liability of approximately $2.6 million and $1.9 million at December 30, 2012 and January 1, 2012, respectively, which represents the Company’s best estimate of the potential additional tax liability related to certain tax positions from unclosed tax years in certain of its subsidiaries. To the extent that the results of any future tax audits differ from the Company’s estimate, changes to tax uncertainties will be reported as adjustments to income tax expense.$7.9approximately $5 million at December 30, 2012. The Company files27, 2015. Our 2009-2013 U.S. federal income tax returns in the U.S. federal jurisdiction and in various U.S. state and foreign jurisdictions. The Company is notare currently under examination by any U.S. federal, statethe Internal Revenue Service. While we believe that we are adequately accrued for possible adjustments, the final resolution of this examination cannot be determined at this time and local, or non-U.S. incomecould result in a final settlement that differs from current estimates. It is, therefore, possible that our unrecognized tax examinations by tax authorities. If any examinations were initiated,benefits could change in the Company would not expect the results of these examinations to have a material impact on its consolidated financial statements in future years.balances of the total amountsamount of unrecognized tax benefits is as followsfollows:Balance at January 1, 2015 $ 4,439 Additions from mergers 5,618 Additions for tax positions related to current year 344 Additions for tax positions of prior years — Reductions for tax positions of prior years (206 ) Settlements — Foreign currency translation (254 ) Balance at December 27, 2015 $ 9,941 $ 4,707 246 (24 ) 453 (150 ) $ 5,232 2,282 — 306 89 $ 7,909 12. December 27, December 31, 2015 2014 13,990 6,050 139,547 76,000 3,263 3,689 29,858 36,549 Other 21,916 11,756 $ 208,574 $ 134,044 December 27, December 31, 2015 2014 Employee bonus $ 27,515 $ 2,557 Other employee benefits 22,816 5,968 Royalties 12,918 3,220 Taxes other than income 18,895 5,782 Commissions 15,196 6,857 Professional and legal fees 21,048 13,822 792 99,137 16,630 10,262 Other 38,053 22,009 $ 173,863 $ 169,614 The Company41.7 million102,672,678 and 39.3 million52,913,093 ordinary shares issued and outstanding as of December 30, 201227, 2015 and JanuaryDecember 31, 2014, respectively. As discussed in Note 3, the Wright/Tornier merger completed on October 1, 2012, respectively.• Companydilutive effect of the stock options, non-vested shares of ordinary shares, stock-settled phantom stock units, restricted stock units, and warrants is calculated using the treasury-stock method. The dilutive effect of the 2014 Notes is calculated by applying the “if-converted” method. This assumes an add-back of interest, net of income taxes, to net income as if the securities were converted at the beginning of the period. The 2014 Notes matured on December 1, 2014. Net-share settled warrants on the 2017 Notes and 2020 Notes were anti-dilutive for the years ended December 27, 2015 and December 31, 2014.3.8 million, 4.2 million and 3.7 million9,866,666 ordinary shares at December 30, 2012, January 1, 2012 and January 2, 2011, respectively. The Company also had 0.4 million and 0.2 million1,133,295 restricted stock units outstanding at December 30, 201227, 2015, 4,309,062 ordinary shares and January 1, 2012. Outstanding options to purchase ordinary shares,282,674 restricted stock units and warrants representing an aggregaterestricted stock awards at December 31, 2014, and 3,472,561 ordinary shares and 129,353 restricted stock units and restricted stock awards at December 31, 2013. None of 4.2 million, 4.4 million and 3.7 million shares are notthe options, restricted stock units, or restricted stock awards were included in diluted earnings per share for the years ended December 30, 2012, January 1, 201227, 2015, December 31, 2014, and January 2, 2011, respectively,December 31, 2013 because the Companywe recorded a net loss infor all periodsperiods; and therefore, including these instruments would be anti-dilutive.13. SegmentGeographic DataThe Company has one reportable segment, orthopaedic products, which includes the design, manufacture and marketing of joint implants and other related products. The Company’s geographic regions consist of the United States, France and other areas. Long-lived assets are those assets located in each region. Revenues attributed to each region are based on the location in which the products were sold.Revenue by geographic regiondiluted earnings per share purposes is as follows (in thousands): Year Ended December 30, 2012 January 1, 2012 January 2, 2011 $ 156,750 $ 141,496 $ 127,762 52,737 55,438 47,324 68,033 64,257 52,292 $ 277,520 $ 261,191 $ 227,378 Revenue Fiscal year ended December 27, 2015 December 31, 2014 December 31, 2013 64,808 51,293 48,103 Ordinary share equivalents — — — 64,808 51,293 48,103 The prior year balances were converted to meet post-merger valuations as described above. Fiscal year ended December 27, 2015 December 31, 2014 December 31, 2013 Total cost of share-based payment plans $ 24,716 $ 11,287 $ 11,912 Amounts capitalized as inventory (51 ) (66 ) (467 ) Amortization of capitalized amounts 299 266 513 Charged against income before income taxes 24,964 11,487 11,958 Amount of related income tax benefit recognized in income — — (3,945 ) Impact to net loss from continuing operations $ 24,964 $ 11,487 $ 8,013 Impact to net income from discontinued operations — 8,845 2,320 Impact to net (loss) income $ 24,964 $ 20,332 $ 10,333 $ 0.39 $ 0.22 $ 0.17 $ 0.39 $ 0.40 $ 0.21 $ 0.39 $ 0.22 $ 0.17 $ 0.39 $ 0.40 $ 0.21 product categoryemployees assigned to MicroPort to accelerate vesting for unvested share-based compensation awards, as an incentive to induce each employee to accept and continue employment with MicroPort, contingent upon the closing of the sale. On January 12, 2014, all unvested share-based compensation awards held by these former 65 employees were vested, which was comprised of approximately 500,000 non-vested options with a weighted-average exercise price of $22.50 per share and 266,000 non-vested Fiscal year ended December 27, 2015 December 31, 2014 December 31, 2013 Risk-free interest rate 1.4% - 1.6% 1.5% - 1.8% 0.1% - 1.4% Expected option life 6 years 6 years 6 years Expected price volatility 33% 31% 36% Outstanding at December 31, 2014 3,517 $ 24.22 Exercised (134) 23.13 Forfeited or expired (87) 26.26 Incremental shares upon conversion 99 23.49 Assumed awards in merger 2,476 20.43 Granted post-merger 3,135 20.63 Exercised post-merger (22) 19.01 Forfeited or expired post-merger (34) 20.26 Outstanding at December 27, 2015 8,950 $ 21.66 7.45 $ 17,945 Exercisable at December 27, 2015 5,826 $ 22.21 6.19 $ 7,871 * (in(shares in thousands): Year Ended December 30, 2012 January 1, 2012 January 2, 2011 $ 175,242 $ 164,064 $ 139,175 34,109 26,033 23,629 15,526 14,779 13,210 224,877 204,876 176,014 52,643 56,315 51,364 $ 277,520 $ 261,191 $ 227,378 Long-lived tangible assets, including instruments, property, plant Options outstanding Options exercisable Range of exercise prices Number outstanding Weighted-average remaining
contractual life Weighted-average exercise
price Number exercisable Weighted-average exercise
price$2.00 — $16.00 441 3.8 $ 13.54 441 $ 13.54 $16.01 — $24.00 7,117 7.8 20.86 3,993 21.05 $24.01 — $35.87 1,392 6.8 28.28 1,392 28.28 8,950 7.4 $ 21.66 5,826 $ 22.21 equipmentstock-settled phantom stock units Non-vested at December 31, 2014 493 $ 26.23 Vested (213 ) 25.11 Forfeited (6 ) 29.59 Incremental shares upon conversion 9 $ 26.30 Acceleration upon merger (283 ) $ 26.30 Granted post-merger 1,139 $ 20.60 Vested post-merger (2 ) $ 20.62 Forfeited post-merger (4 ) $ 10.87 Non-vested at December 27, 2015 1,133 $ 20.63 $ 26,700 * Outstanding at December 31, 2014 890 $ 17.21 Granted — — Exercised — — Forfeited or expired — — Incremental shares upon conversion 27 16.69 Outstanding at December 27, 2015 917 16.69 6 $ 6,300 Exercisable at December 27, 2015 917 $ 16.69 6 $ 6,300 * The aggregate intrinsic value is calculated as the difference between the market value of ordinary shares as of December 27, 2015 and the exercise price of the shares. The market value as of December 27, 2015 was 23.56 per share, which is the closing sale price of our ordinary shares on December 24, 2015, the last trading day prior to December 27, 2015, as reported by the NASDAQ Global Select Market. (in(shares in thousands): December 30,
2012 January 1,
2012 $ 31,342 $ 25,221 39,764 40,564 17,439 16,915 $ 88,545 $ 82,700 Options outstanding Options exercisable Range of exercise prices Number outstanding Weighted-average remaining
contractual life Weighted-average exercise
price Number exercisable Weighted-average exercise
price$2.00 — $16.00 696 5.76 $ 15.57 696 $ 15.57 $16.01 — $35.87 221 6.76 20.22 221 20.22 917 6.00 $ 16.69 917 $ 16.69 Fiscal year ended December 31, 2014 December 31, 2013 Risk-free interest rate 0.3% - 0.6% 0.1% - 0.4% Expected option life 6 months 6 months Expected price volatility 31% 36% 14.Legacy Wright sponsored a defined contribution plan under Section 401(k) of the Internal Revenue Code of 1986, as amended (Code), which covered U.S. employees who are 21 years of age and over. Under this plan, legacy Wright matched voluntary employee contributions at a rate of 100% for the first 2% of an employee’s annual compensation and at a rate of 50% for the next 2% of an employee’s annual compensation. Employees vest in company contributions after three years of service. The expense related to this plan recognized within our results from continuing operations was $2.5 million in 2015, $1.6 million in 2014, and $1.2 million in 2013.rental commitmentspayments, by year and in the aggregate, under non-cancelable operating leases in effect aswith initial or remaining lease terms of December 30, 2012one year or more, are as follows at December 27, 2015(in thousands): $ 5,489 4,923 4,175 3,645 3,568 10,120 $ 31,920 Operating leases include copiers, automobiles and property leases and have maturity dates between 2013 and 2022. Total rent expense2016 $ 10,001 2017 5,608 2018 4,337 2019 3,717 2020 3,282 Thereafter 10,714 $ 37,659 the years ended December 30, 2012, January 1, 2012 and January 2, 2011 was $4.3 million, $3.5 million and $3.3 million, respectively.Future lease payments under capitaloperating leases are as follows (in thousands): $ 691 475 272 123 77 — 1,638 (153 ) 1,485 (622 ) $ 863 Fixed assets that are recorded as capital lease assets consist of machinerydenominated in foreign currencies and equipment, and have a carrying value of $2.6 million ($3.4 million gross value, less $0.8 million accumulated depreciation) and $1.7 million ($2.7 million gross value, less $1.0 million accumulated depreciation) at December 30, 2012 and January 1, 2012, respectively. Amortization of capital lease assets is included in depreciation expensewere translated in the consolidated financial statements.15. Certain RelationshipsDecember 27, 2015. These future payments are subject to foreign currency exchange rate risk.Related-Party TransactionsThe Company leases all of its approximately 55,000 square feet of manufacturing facilities and approximately 52,000 square feet of office space located in Grenoble, France, from Alain Tornier (Mr. Tornier), who is a current shareholder and member of the Company’s board of directors. Annual lease payments to Mr. Tornier amounted to $1.6 million, $1.9 million and $1.7$2.0 million during the years ended December 27, 2015 and December 31, 2014 under those supply agreements. During 2015, we entered into a supply agreement which includes minimum purchase obligations of $0.4 million, $1.5 million, and $3 million for 2016, 2017, and 2018, respectively.1, 20124, 2016, we filed an answer to the complaint and January 2, 2011, respectively.the CompanyTornier SAS, a subsidiary of legacy Tornier, formed a real estate holding company (SCI Calyx) together with Mr. Tornier.Alain Tornier (Mr. Tornier). SCI Calyx is owned 51% by the CompanyTornier SAS and 49% by Mr. Tornier. SCI Calyx was initially capitalized by a contribution of capital of €10,000 funded 51% by the CompanyTornier SAS and 49% by Mr. Tornier. SCI Calyx then acquired a combined manufacturing and office facility in Montbonnot, France, for approximately $6.1 million. The manufacturing and office facility acquired was to be used to support the manufacture of certain of the Company’slegacy Tornier’s current products and house certain operations already located in Montbonnot, France. This real estate purchase was funded through mortgage borrowings of $4.1 million and $2.0 million cash borrowed from the two current shareholders of SCI Calyx. The $2.0 million cash borrowed from the SCI Calyx shareholders originally consisted of a $1.0 million note due to Mr. Tornier and a $1.0 million note due to Tornier SAS, which is the Company’s wholly owned French operating subsidiary.SAS. Both of the notes issued by SCI Calyx bear annual interest at the three-month Euro Libor rate plus 0.5% and have no stated term. During 2010, SCI Calyx borrowed approximately $1.4 million from Mr. Tornier in order to fund on-going leasehold improvements necessary to prepare the Montbonnot facility for its intended use. This cash was borrowed under the same terms as the original notes. On September 3, 2008, Tornier SAS the Company’s French operating subsidiary, entered into a lease agreement with SCI Calyx relating to these facilities. The agreement, which terminates in 2015,2018, provides for an annual rent payment of €440,000, which has subsequently been increased and is currently €888,583€965,655 annually. Annual lease payments to SCI Calyx amounted to $2.2 million during the year ended December 27, 2015, $0.6 million of which is reflected in our consolidated financial statements in light of the timing of the Wright/Tornier merger. As of December 30, 2012,27, 2015, future minimum payments under this lease were €4.4$12.3 million in the aggregate. As of December 30, 2012,27, 2015, SCI Calyx had related-party debt outstanding to Mr. Tornier of $2.2$2.0 million. The SCI Calyx entity is consolidated by the Company,us, and the related real estate and liabilities are included in theon our consolidated balance sheets.May 30, 2006, Tornier SAS entered into two lease agreements with Mr. Tornier and his sister, Colette Tornier, relating to the Company’s former facilities in Saint-Ismier, France. The agreements provided for annual rent payments of €104,393 and €28,500, respectively, which were increased to €121,731 and €33,233 annually, respectively. On June 26, 2012, Tornier SAS entered into an amendment to these lease agreements to terminate them effective as of September 30, 2012. No early termination payments were made by Tornier SAS pursuant to the terms of the amendment. During 2012, Tornier SAS paid an aggregate of €2.7 million to an entity affiliated with Mr. Tornier and his sister, Colette Tornier, as rent for the Company’s former facility located in Saint-Ismier, France. On December 29, 2007, Tornier SAS entered into a lease agreement with Cymaise SCI, relating to the Company’s former facilities in Saint-Ismier, France. The agreement provides for a term through May 30, 2015 and an annual rent payment of €480,000, which was subsequently increased to €531,243 annually. Cymaise SCI is wholly owned by Mr. Tornier and his sister, Colette Tornier. On December 29, 2007, Tornier SAS entered into a lease agreement with Animus SCI, relating to the Company’sour facilities in Montbonnot Saint Martin, France. On August 18, 2012, the parties amended the lease agreement to extend the term until May 31, 2022 and reduce the annual rent. The amended agreement provides for an initial annual rent payment of €279,506, annually.which was subsequently increased to €296,861. Animus SCI is wholly owned by Mr. Tornier. On February 6, 2008, Tornier SAS entered into a lease agreement with Balux SCI, effective as of May 22, 2006, relating to the Company’sour facilities in Montbonnot Saint Martin, France. On August 18, 2012, the parties amended the lease agreement to extend the term until May 31, 2022 and reduce the annual rent. The amended agreement provides for an initial annual rent payment of €252,545.€252,254, which was subsequently increased to €564,229. Balux SCI is wholly ownedwholly-owned by Mr. Tornier and his sister, Colette Tornier. As of December 30, 2012,27, 2015, future minimum payments under all of these agreements were €279,506$6.0 million in the aggregate.16. Share-Based CompensationShare-based awards are granted under the Tornier N.V. 2010 Incentive Plan, as amended and restated (2010 Plan). This plan allows for the issuanceup to 7.7 million ordinary shares in connection with the grant of a combination of potential share-based awards, including stock options, restricted stock units, stock appreciation rights and other types of awards as deemed appropriate. To date, only options to purchase ordinary shares (options) and restricted stock units (RSUs) have been awarded. Both types of awards generally have graded vesting periods of four years and the options expire ten years after the grant date. Options are granted with exercise prices equal to the fair value of the Company’s ordinary shares on the date of grant.The Company recognizes compensation expense for these awards on a straight-line basis over the vesting period. Share-based compensation expense is included in cost of goods sold, selling, general and administrative, and research and development expenses on the consolidated statements of operations.Below is a summary of the allocation of share-based compensation (in thousands): Year ended December 30,
2012 January 1,
2012 January 2,
2011 $ 864 $ 841 $ 536 5,477 5,263 4,661 489 443 433 $ 6,830 $ 6,547 $ 5,630 The Company recognizes the fair value of share-based awards granted in exchange for employee services as a cost of those services. Total compensation cost included in the consolidated statements of operations for employee share-based payment arrangements was $6.5 million, $6.2 million and $5.1 million during the years ended December 30, 2012, January 1, 2012 and January 2, 2011, respectively. The amount of expense related to non-employee options was $0.3 million, $0.3 million and $0.5 million for the years ended December 30, 2012, January 1, 2012 and January 2, 2011, respectively. Additionally, $0.3 million and $0.6 million were included in inventory as a capitalized cost as of December 31, 2012 and January 1, 2012, respectively.Stock Option AwardsThe Company estimates the fair value of stock options using the Black-Scholes option pricing model. The Black-Scholes option pricing model requires the input of estimates, including the expected life of stock options, expected stock price volatility, the risk-free interest rate and the expected dividend yield. The Company calculates the expected life of stock options using the SEC’s allowed short-cut method. The expected stock price volatility assumption was estimated based upon historical volatility of the common stock of a group of the Company’s peers that are publicly traded. The risk-free interest rate was determined using U.S. Treasury rates with terms consistent with the expected life of the stock options. Expected dividend yield is not considered, as the Company has never paid dividends and currently has no plans of doing so during the term of the options. The Company estimates forfeitures at the time of grant and revises those estimates in subsequent periods if actual forfeitures differ from those estimates. The Company uses historical data when available to estimate pre-vesting option forfeitures, and records share-based compensation expense only for those awards that are expected to vest. The weighted-average fair value of the Company’s options granted to employees was $8.55, $12.06 and $11.03 per share, in 2012, 2011 and 2010, respectively. The fair value of each option grant is estimated on the date of grant using the Black-Scholes option pricing model using the following weighted-average assumptions: Years ended December 30,
2012 January 1,
2012 January 2,
2011 0.9 % 2.1 % 2.3 % 6.1 6.1 5.8 48.1 % 48.6 % 49.8 % 0.0 % 0.0 % 0.0 % As of December 30, 2012, the Company had $9.8 million of total unrecognized compensation cost related to unvested share-based compensation arrangements granted to employees under the 2010 Plan and the Company’s prior stock option plan. That cost is expected to be recognized over a weighted-average service period of 2.7 years. Shares reserved for future compensation grants were 2.5 million and 0.4 million at December 30, 2012 and January 1, 2012, respectively. Exercise prices per share for options outstanding at December 30, 2012, ranged from $13.39 to $27.31.A summary of the Company’s employee stock option activity is as follows: Ordinary Shares
(In Thousands) Weighted-Average
Exercise Price Weighted-Average
Remaining
Contractual Life
(In Years) Aggregate Intrinsic
Value (in Millions) 2,651 15.06 7.6 5.0 992 22.50 (32 ) 15.32 (79 ) 15.81 3,532 17.02 7.4 19.4 647 24.76 (210 ) 15.02 (73 ) 20.96 3,896 18.32 6.9 (3.8 ) 626 18.45 (426 ) 16.56 (314 ) 22.33 3,782 18.23 6.4 (7.3 ) 2,567 16.93 5.3 (1.7 ) The Company did not grant options to purchase ordinary shares to non-employees in the year ended December 30, 2012. During the years ended January 1, 2012 and January 2, 2011, the Company granted options to purchase 74,667 and 21,000 ordinary shares, respectively, to non-employees in exchange for consulting services. The options granted in 2011 and 2010 had weighted-average exercise prices of $19.39 and $22.50 per share, respectively. 197,773 of these non-employee options were exercisable at December 30, 2012. 31,060 of these options were exercised in 2012. These options have vesting periods of either two or four years and expire 10 years after the grant date. The measurement date for options granted to non-employees is often after the grant date, which often requires updates to the estimate of fair value until the services are performed. The weighted-average per share fair value on the date of grant of each non-employee option granted was $9.74 in 2011.Total compensation expense related to stock options, including employees and non-employees, recognized in the consolidated statements of operations was approximately $5.0 million, $5.8 million and $5.6 million for the years ended December 30, 2012, January 1, 2012 and January 2, 2011, respectively.Restricted Stock Units AwardsThe Company began to grant RSUs in 2011 under the 2010 Plan. Vesting of these awards typically occurs over a four-year period and the grant date fair value of the awards is recognized as expense over the vesting period. Total compensation expense recognized in the consolidated statements of operations related to RSUs was $1.8 million and $0.7 million for the years ended December 30, 2012 and January 1, 2012, respectively.A summary of the Company’s activity related to the RSUs is as follows: Shares
(In Thousands) Weighted-Average
Grant Date Fair
Value Per Share — — 221 25.06 (7 ) 23.61 (7 ) 25.52 207 25.10 305 18.51 (55 ) 20.21 (35 ) 24.01 422 20.57 17. Other Non-Operating IncomeDuring the year ended December 30, 2012, the Company recognized $0.1 million in other non-operating income. During the year ended January 1, 2012, the Company recognized $1.3 million in non-operating income, which included a $1.0 million gain on settlement of a contingent liability and a $0.3 million gain related to the sale of certain non-operating real estate in France. During the year ended January 2, 2011, the Company recognized $0.4 million of non-operating gains related to the mark-to-market of the warrant liability. These warrants were converted into ordinary shares later in 2011.18. Special ChargesSpecial charges are recorded as a separate line item within operating expenses on the consolidated statement of operations and primarily include operating expenses directly related to business combinations and related integration activities, restructuring initiatives (including the facilities consolidation initiative), management exit costs and certain other items that are typically infrequent in nature and that affect the comparability and trend of operating results. The table below summarizes amounts included in special charges for the related periods: Year ended December 30,
2012 January 1,
2012 January 2,
2011 $ 6,357 $ — $ — 1,229 632 — 3,546 — — 1,374 — — 2,001 — — 4,737 — — — 260 306 $ 19,244 $ 892 $ 306 Included in special charges for the year ended December 30, 2012, were $6.4 million of restructuring costs related to the Company’s facilities consolidation initiative. See below for further details on this initiative. Also included in special charges were management exit costs of $1.2 million which included severance related to the Company’s former Chief Executive Officer and Global Chief Financial Officer; acquisition and integration costs of $3.5 million which included costs related to the Company’s acquisition of OrthoHelix and the Company’s exclusive distributor in Belgium and Luxembourg; distribution channel change costs of $1.4 million which included termination costs related to certain strategic business decisions made related to the Company’s U.S. and international distribution channels; Increased allowances of uncollectible accounts in Italy of $2.0 million which related to bad debt expense related to the termination of a distributor and general economic conditions in Italy; and intangible impairments of $4.7 million as the Company made certain strategic decisions related to previously acquired intangibles which was determined to be impaired as a result of the acquisition of OrthoHelix. Included in special charges on the consolidated statement of operations for the year ended January 1, 2012 are $0.6 million in management termination costs and $0.3 million of charges related to the closure of the Company’s Beverly, Massachusetts facility. Included in special charges for the year ended January 2, 2011 are $0.3 million in costs incurred related to commissions paid in the United Kingdom related to the termination of the relationship with a former distributor and expenses related to the Company’s consolidation of its U.S. operations.On April 13, 2012, the Company announced a facilities consolidation initiative, stating that it planned to consolidate several of its facilities to drive operational productivity and to reduce annual operating expenses by $2.3 million to $2.8 million beginning in 2013. Under the initiative, the Company consolidated its Dunmanway, Ireland manufacturing facility into its Macroom, Ireland manufacturing facility in the second quarter of 2012 and, in the third quarter of 2012, the Company consolidated its St. Ismier, France manufacturing facility into its Montbonnot, France manufacturing facility. In addition, the Company leased a new facility in Bloomington, Minnesota to use its U.S. business headquarters and consolidated its Minneapolis-based marketing, training, regulatory, supply chain, and corporate functions with it Stafford, Texas-based distribution operations. This initiative was completed in the fourth quarter of 2012.Charges incurred in connection with the facilities consolidation initiative during the year ended December 30, 2012 are presented in the following table (in thousands). All of the following amounts were recognized within special charges in the Company’s consolidated statements of operations for the year ended December 30, 2012. Fiscal Year Ended December 30, 2012 $ 1,180 872 4,305 $ 6,357 The $1.2 million of employee termination benefits includes severance and retention related to approximately 65 employees impacted by the facilities consolidation initiative in the U.S. The $0.9 million of impairment charges related to fixed assets include charges for closing the impacted facilities in the U.S., France and Ireland. The $4.3 million of moving, professional fees and other initiative-related expenses include moving and transportation expenses, lease termination costs, professional fees and other expenses that were incurred to execute the facilities consolidation initiative.Included in accrued liabilities on the consolidated balance sheet as of December 30, 2012 is an accrual related to the facilities consolidation initiative. Activity in the facilities consolidation accrual is presented in the following table (in thousands): $ — 1,180 4,305 5,485 (620 ) (4,191 ) (4,811 ) $ 674 The Company anticipates that substantially all of this accrual will be paid in the first quarter of 2013.19. LitigationOn October 25, 2007, two of the Company’s former sales agents filed a complaint in the U.S. District Court for the Southern District of Illinois, alleging that the Company had breached their agency agreements and committed fraudulent and negligent misrepresentations. The jury rendered a verdict on July 31, 2009, awarding the plaintiffs a total of $2.6 million in actual damages and $4.0 million in punitive damages. While the court struck the award of punitive damages on March 31, 2010, it denied the Company’s motion to set aside the verdict or order a new trial. The Company timely filed a notice of appeal with the U.S. Court of Appeals for the Seventh Circuit in respect of the remaining actual damages. On August 24, 2011, the U.S. Court of Appeals for the Seventh Circuit issued its decision affirming the order of the lower court setting aside the award of punitive damages. In addition, the appellate court affirmed the lower court’s finding of liability against the Company, but vacated the lower court’s damages award of $2.6 million in compensatory damages as being not supported by the record and being too speculative. The case was then remanded to the lower court for a recalculation of damages that is consistent with the appellate court’s decision. On May 17, 2012, the lower court ordered a new trial on the issue of damages. It is anticipated that the new trial will be conducted during the first half of 2013.The Company has considered the facts of the case, the related case law and the decision of the U.S. Court of Appeals for the Seventh Circuit and, based on this information, believes that the verdict rendered on July 31, 2009 was inappropriate given the related facts and supporting legal arguments. The Company has considered the progress of the case, the views of legal counsel, the facts and arguments presented at the original jury trial, and the decision of the U.S. Court of Appeals for the Seventh Circuit and the fact that the Company intends to continue to vigorously defend its position through the remand proceedings in assessing the probability of a loss occurring for this matter. The Company has determined that aloss is reasonably possible. The Company estimates the high end of the range to be $2.6 million, the amount of the initial jury verdict, minus the punitive damage award. The Company believes it continues to have a strong defense against these claims and is vigorously contesting these allegations. After assessing all relevant information, the Company has accrued an amount at the low end of the range, which is deemed immaterial.In addition to the item noted above, the Company is subject to various other legal proceedings, product liability claims and other matters which arise in the ordinary course of business. In the opinion of management, the amount of liability, if any, with respect to these matters will not materially affect the Company’s consolidated financial statements or liquidity.20. Selected Quarterly InformationOperations (unaudited):the Company’sour unaudited quarterly operating results for each of the four quarters in 20122015 and 2011,2014, respectively (in thousands). This information was derived from unaudited interim financial statements that, in the opinion of management, have been prepared on a basis consistent with the financial statements contained elsewhere in this reportfiling and include all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentationstatement of such information when read in conjunction with the Company’sour audited financial statements and related notes. The operating results for any quarter are not necessarily indicative of results for any future period. Year ended December 30, 2012 Fourth
Quarter Third
Quarter Second
Quarter First
Quarter (in thousands, except per share data) $ 79,033 $ 58,015 $ 66,014 $ 74,458 52,059 42,285 47,916 53,342 (4,803 ) (11,681 ) (5,084 ) (176 ) $ (0.12 ) $ (0.29 ) $ (0.13 ) $ (0.00 ) Year ended January 1, 2012 Fourth
Quarter Third
Quarter Second
Quarter First
Quarter (in thousands, except per share data) $ 69,042 $ 57,556 $ 65,158 $ 69,435 48,868 40,906 47,141 49,394 (1,981 ) (1,637 ) (2,869 ) (23,969 ) (1,981 ) (1,637 ) (2,869 ) (23,969 ) $ (0.05 ) $ (0.04 ) $ (0.07 ) $ (0.68 ) 2015 Net sales $ 77,934 $ 80,420 $ 80,139 $ 176,968 Cost of sales 19,125 21,635 23,052 55,443 Gross profit 58,809 58,785 57,087 121,525 Operating expenses: Selling, general and administrative 82,199 82,605 85,997 178,596 Research and development 7,117 7,957 9,570 15,211 Amortization of intangible assets 2,614 2,565 2,562 9,181 Total operating expenses 91,930 93,127 98,129 202,988 Operating loss $ (33,121 ) $ (34,342 ) $ (41,042 ) $ (81,463 ) Net loss from continuing operations, net of tax $ (46,248 ) $ (37,306 ) $ (62,650 ) $ (92,155 ) Income (loss) from discontinued operations, net of tax $ (3,500 ) $ (7,009 ) $ (36,211 ) $ (13,621 ) Net income (loss) $ (49,748 ) $ (44,315 ) $ (98,861 ) $ (105,776 ) (0.88 ) (0.71 ) (1.19 ) (0.90 ) (0.88 ) (0.71 ) (1.19 ) (0.90 ) $ (0.95 ) $ (0.84 ) $ (1.87 ) $ (1.03 ) $ (0.95 ) $ (0.84 ) $ (1.87 ) $ (1.03 ) year ended December 30, 2012 include costs incurredfourth quarter of 2015 associated with the accelerated vesting of legacy Wright's unvested awards outstanding upon the closing of the Wright/Tornier merger; andspecial charges that are not included inthe write-off of unamortized debt discount and deferred financing costs associated with the settlement of 2017 Convertible Notes during the first quarter of 2012. The fourth quarter for 2015;year ended December 30, 2012 also includes the impactafter-tax effects of the OrthoHelix acquisition. The first quarter of the year ended January 1, 2012 includes a $22.0 million loss, net of $7.5 million of tax benefit, on the extinguishment of the Company’s notes payable, as well as $2.1 million ofnon-cash interest expense related to the amortization of the debt discount on our 2017 Convertible Notes and 8% interest2020 Convertible Notes totaling $4.5 million, $6.6 million, $6.8 million, and $6.9 million during the first, second, third, and fourth quarters of 2015, respectively; 2014 First
quarter Second
quarter Third
quarter Fourth
quarterNet sales $ 71,062 $ 72,364 $ 71,307 $ 83,294 Cost of sales 17,417 20,006 16,703 19,097 Gross profit 53,645 52,358 54,604 64,197 Operating expenses: Selling, general and administrative 68,648 72,055 66,926 81,991 Research and development 5,856 6,799 5,948 6,360 Amortization of intangible assets 2,187 2,675 2,379 2,786 BioMimetic impairment charges — — — — Total operating expenses 76,691 81,529 75,253 91,137 Operating income (loss) $ (23,046 ) $ (29,171 ) $ (20,649 ) $ (26,940 ) Net income (loss), continuing operations, net of tax $ (30,298 ) $ (53,583 ) $ (49,647 ) $ (106,968 ) Net income (loss), discontinued operations, net of tax $ (122 ) $ (2,643 ) $ (12,160 ) $ (4,262 ) Net income (loss) $ (30,420 ) $ (56,226 ) $ (61,807 ) $ (111,230 ) $ (0.60 ) $ (1.05 ) $ (0.96 ) $ (2.05 ) $ (0.60 ) $ (1.05 ) $ (0.96 ) $ (2.05 ) $ (0.61 ) $ (1.10 ) $ (1.20 ) $ (2.13 ) $ (0.61 ) $ (1.10 ) $ (1.20 ) $ (2.13 ) notes payable, which is not includedBiotech, Solana, and OrthoPro acquisitions totaling $5.2 million, $4.6 million, $1.9 million, and $2.5 million during the first, second, third, and fourth quarters of 2014, respectively;otherthird quarter of 2014;2011.2014, respectively; and Fiscal year ended December 27, December 31, December 31, 2015 2014 2013 U.S. Lower extremities $ 187,096 $ 148,631 $ 115,642 Upper extremities 58,756 15,311 17,423 Biologics 50,583 45,494 42,561 Sports med & other 3,388 2,641 2,022 Total extremities & biologics 299,823 212,077 177,648 Large joint 18 — — Total U.S. $ 299,841 $ 212,077 $ 177,648 International Lower extremities $ 51,200 $ 47,001 $ 35,020 Upper extremities 24,789 11,312 7,240 Biologics 19,652 20,590 17,231 Sports med & other 9,862 7,047 5,191 Total extremities & biologics 105,503 85,950 64,682 Large joint 10,117 — — Total International $ 115,620 $ 85,950 $ 64,682 Total $ 415,461 $ 298,027 $ 242,330 Fiscal year ended December 27, December 31, December 31, Net sales by geographic region: 2015 2014 2013 United States $ 299,841 $ 212,077 $ 177,648 Europe 72,779 48,991 31,210 Other 42,841 36,959 33,472 Total $ 415,461 $ 298,027 $ 242,330 Fiscal year ended December 27, December 31, December 31, Long-Lived Assets: 2015 2014 2013 United States $ 160,989 $ 92,822 $ 61,179 Europe 72,643 8,065 6,581 Other 7,137 3,348 2,755 Total $ 240,769 $ 104,235 $ 70,515 Withwith Accountants on Accounting and Financial Disclosure.Our President and Chief Executive Officer and Chief Financial Officer, referred to collectively herein as the Certifying Officers, are responsible for establishing and maintaining ourProceduresprocedures. The Certifying Officers have reviewedprocedures, as such term is defined in Rule 13a-15(e) under the Securities Exchange Act of 1934. Our disclosure controls and evaluatedprocedures are designed to ensure that material information relating to us, including our consolidated subsidiaries, is made known to our principal executive officer and principal financial officer by others within our organization. Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rules 240.13a-15(e) and 240.15d-15(e) promulgated under the Securities Exchange Act of 1934, as amended) as of December 30, 2012. Based on27, 2015 to ensure that review and evaluation, which included inquiries made to certain of our other employees, the Certifying Officers have concluded that, as of the end of the period covered by this report, our disclosure controls and procedures, as designed and implemented, are effective in ensuring that information relating to Tornier required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934 as amended, is recorded, processed, summarized, and reported within the time periods specified in the Securities and Exchange Commission’sSEC’s rules and forms, including ensuringforms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that such information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934 is accumulated and communicated to our management, including the Certifying Officers,our principal executive officer and principal financial officer as appropriate, to allow timely decisions regarding required disclosure.Presidentprincipal executive officer and Chief Executive Officer and Chief Financial Officer,principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 30, 2012,27, 2015, based on the criteria established in Internal Control — Integrated Framework issuedset forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). in Internal Control - Integrated Framework (2013) issued. Based on this evaluation, our management concluded that our internal control over financial reporting was effective as of December 30, 2012. This evaluation did not include the27, 2015. Our internal controls related to OrthoHelix, the acquisition of which was completed during 2012. OrthoHelix is a wholly owned subsidiary whose total assets and revenues represent 4.7% and 2.9%, respectively, of the related consolidatedcontrol over financial statement amountsreporting as of and for the year ended December 30, 2012 (see Note 3). The report of Ernst &Young27, 2015 has been audited by KPMG LLP, ouran independent registered public accounting firm, regardingas stated in their report, which is included herein.“Part II. Item 8, Financial Statementsparticular the fact that our principal executive officer, principal financial officer and Supplementary Data” under “Reportprincipal accounting officer are the principal executive officer, principal financial officer and principal accounting officer of Independent Registered Public Accounting Firm.”three monthsfourth quarter of the fiscal year ended December 30, 2012,27, 2015, there were no changes in our internal control over financial reporting that materially affected, or that are reasonably likely to materially affect, our internal control over financial reporting, except for the factchanges that we are currently inmade to begin to incorporate the processinternal control over financial reporting of evaluatinglegacy Tornier with and integrating OrthoHelix’sinto our internal controls into ours.control over financial reporting.On February 12, 2013, our board25, 2013,10, 2016, certain information concerning our current directors and executive officers. No family relationships exist among any of our directors or executive officers. Age David H. Mowry 5071 President and Chief Executive Officer and Executive Director Shawn T McCormick 53 Executive Vice President and Chief Operating Officer and Executive Director Lance A. Berry 43 Senior Vice President and Chief Financial Officer Robert P. Burrows 69 Senior Vice President, Supply Chain James A. Lightman 58 Senior Vice President, General Counsel and Secretary Gregory Morrison 52 Senior Vice President, Human Resources J. Wesley Porter 46 Senior Vice President and Chief Compliance Officer Julie D. Tracy 54 Senior Vice President and Chief Communications Officer Jennifer S. Walker 48 Chief Financial OfficerSenior Vice President, Process ImprovementStéphan Epinette 4248 Vice President, International Commercial OperationsUpper ExtremitiesM. Klemz 5150 Vice President, Chief Legal OfficerLower Extremities and SecretaryBiologicsGregory Morrison 4950 Global Vice President, Human ResourcesInternationalTerry M. Rich 4567 Senior Vice President U.S. Commercial Operationsand General Manager, BioMimetic44 Vice President and Chief Accounting Officer SeanDavid D. CarneyStevens(1)(2) 4362 Chairman and Non-Executive Director Kevin C. O’Boyle(2)(3)56Interim Vice Chairman, Non-Executive DirectorRichard B. EmmittGary D. Blackford(3) 6858 Non-Executive Director Alain Tornier 6646 Non-Executive Director Richard F. WallmanJohn L. Miclot(1)(3)(4) 6156 Non-Executive Director 59 Non-Executive Director 64 Non-Executive Director 64 Non-Executive Director 5255 Non-Executive Director (1)Member of the compensation committee.(2)(1)Member of the nominating, corporate governance and compliance committee. (2) Member of the strategic transactions committee. (3) Member of the audit committee. (4) Member of the compensation committee. officers:usTornier in July 2011 as Chief Operating Officer and inOfficer. In November 2012, he was appointed Interim President and Chief Executive Officer, on an interim basis, and effective as ofin February 21, 2013, he was appointed President and Chief Executive Officer on a non-interim basis. HeMr. Mowry has over 24 years of experience in the medical device industry. Prior to joining us, Mr. MowryTornier, he served from July 2010 to July 2011 as the President of the Global Neurovascular Division of Covidien plc, a global provider of healthcare products.products company, from July 2010 to July 2011. From January 2010 to July 2010, Mr. Mowryhe served as Senior Vice President and President, Worldwide Neurovascular of ev3 Inc., a global endovascular device company acquired by a wholly owned subsidiary of Covidien Group S.a.r.l in July 2010. From August 2007 to January 2010, Mr. Mowryhe served as Senior Vice President of Worldwide Operations of ev3. Prior to this position, Mr. Mowry wasev3 and as Vice President of Operations forof ev3 Neurovascular from November 2006 to October 2007. Before joining ev3, Mr. Mowry served as Vice President of Operations and Logistics at the Zimmer Spine division of Zimmer Holdings Inc., a reconstructive and spinal implants, trauma, and related orthopaedic surgical products company, from February 2002 to November 2006. Prior to Zimmer, Mr. Mowry was the President and Chief Operating Officer of HeartStent Corp., a medical device company. Mr. Mowry iscurrently serves on the board of directors of EndoChoice Holdings, Inc., a graduate of the United States Military Academy in West Point, New York with a degree in Engineering and Mathematics.Shawn T McCormick joined us in September 2012 as Chief Financial Officer. He has over 20 years of experience in thepublicly held medical device industry. Priorcompany. Mr. Mowry’s qualifications to joining us, he servedsit on our board of directors include his extensive knowledge of our company and day-to-day operations in light of his current position as Executive Vice President and Chief Operating Officer and former position as President and Chief Executive Officer of Lutonix, Inc., a medical device company acquired by C. R. Bard, Inc.Tornier.December 2011, from April 2011 to February 2012. From January 2009 to July 2010,October 2015 in connection with the Wright/Tornier merger. Mr. McCormickBerry has served as Senior Vice President and Chief Financial Officer of ev3Wright Medical Group, Inc., a global medical device company acquired by Covidien plc since 2009. He joined Wright in July 2010. Prior to joining ev3, Mr. McCormick2002, and, until his appointment as Chief Financial Officer, served as Vice President and Corporate DevelopmentController. Prior to joining Wright, Mr. Berry served as audit manager with the Memphis, Tennessee office of Arthur Andersen LLP from 1995 to 2002. Mr. Berry is a certified public accountant, inactive.Medtronic,On-Point, Mr. Burrows led over 40 client engagements, most recently as an operations consultant overseeing the transition and expansion of Wright’s extremities and biologics manufacturing., a global medical device company, where he was responsible for leading Medtronic’s worldwide business development activities in December 2011 as Senior Vice President, General Counsel and previously hadSecretary. Prior to joining Wright, Mr. Lightman served in key corporatevarious legal and divisional financial leadership roles withinexecutive positions with Bausch & Lomb Incorporated, a privately held eye contact company. From February 2008 to November 2009, Mr. Lightman served as Vice President and Assistant General Counsel of Bausch & Lomb, and most recently held the Medtronic organization. Mr. McCormick joined Medtronic in July 1992 and held various finance and leadership positions during his tenure.position of Vice President, Global Sales Operations until August 2011. From JulyJune 2007 to MayFebruary 2008, he served as Vice President Corporate Technology and New VenturesGeneral Counsel of Medtronic. From July 2002 to July 2007, he was Vice President, Finance for Medtronic’s Spinal, Biologics and Navigation business. Prior to that, Mr. McCormick held various other positions with Medtronic, including Corporate Development Director, Principal Corporate Development Associate, Manager, Financial Analysis, Senior Financial Analyst and Senior Auditor.Eyeonics, Inc. Prior to joining Medtronic, he spent almost four years with the public accounting firm KPMG Peat Marwick.Eyeonics, Mr. McCormick earned his Master of Business Administration from the University of Minnesota’s Carlson School of Management and his Bachelor of Science in Accounting from Arizona State University. He is a Certified Public Accountant.Stéphan Epinette joined us in December 2008 and leads our international commercial operations (primarily Europe, Asia Pacific and Latin America) and large joints business as Vice President of International Commercial Operations. Mr. Epinette has over 19 years of experience in the orthopaedic medical device industry. Prior to joining us, he served in various leadership roles with Stryker Corporation, a medical device and equipment company, in its MedSurg and Orthopaedic divisions in France, the United States and Switzerland from 1993 to December 2008, including as Business Unit Director France from 2005 to 2008. His past functions at Stryker Corporation also included Marketing Director MedSurg EMEA, Assistant to the EMEA President and Director of Business Development & Market Intelligence EMEA. Mr. Epinette earned a Master’s Degree in Health Economics from Sciences Politiques, Paris, a Master’s Degree in International Business from Paris University XII and a Bachelor of Arts from EBMS Barcelona. He also attended the INSEAD executive course in Finance and in Marketing.Kevin M. Klemz joined us in September 2010 as Vice President, Chief Legal Officer and Secretary. Prior to joining us, Mr. KlemzLightman served as Senior Vice President Secretary and Chief Legal Officer at ev3 Inc.General Counsel of IntraLase Corp. from August 2007February 2005 to August 2010, and asApril 2007.SecretaryHuman Resources in October 2015 in connection with the Wright/Tornier merger. Mr. Morrison served as Senior Vice President, Global Human Resources and Chief Legal Officer at ev3 Inc.HPMS (High Performance Management System) of Tornier from January 20072014 to August 2007. Prior to joining ev3 Inc., Mr. Klemz was a partner in the law firm Oppenheimer Wolff & Donnelly LLP, where he was a corporate lawyer for approximately 20 years. Mr. Klemz has a Bachelor of Arts in Business Administration from Hamline UniversityOctober 2015 and a Juris Doctor from William Mitchell College of Law.Gregory Morrison joined us in December 2010served as Global Vice President, Human Resources.Resources from December 2010 to January 2014. Prior to joining us,Tornier, Mr. Morrison served as Senior Vice President, Human Resources atof ev3 Inc., a global endovascular device company acquired by Covidien plc in July 2010, from August 2007 to December 2010, and as Vice President, Human Resources from May 2002 to August 2007. Prior to joining ev3, Inc., Mr. Morrison served as Vice President of Organizational Effectiveness forof Thomson Legal & Regulatory from March 1999 to February 2002 and Vice President of Global Human Resources forof Schneider Worldwide, which was acquired by Boston Scientific Corporation, from 1988 to March 1999.Morrison hasPorter joined Wright Medical Group, Inc. in July 2014 as Vice President, Compliance and became Senior Vice President and Chief Compliance Officer in October 2014. Prior to joining Wright, Mr. Porter served as Vice President, Deputy Compliance Officer of Allergan, Inc. from September 2012 to February 2014, Vice President, Ethics and Compliance of CareFusion Corp. from June 2009 to September 2012, and Senior Corporate Counsel, Compliance, HIPAA and Reimbursement of Smith & Nephew, Inc. from April 2006 to May 2009.Bachelorpublicly held company that sold physician platforms for clinical content, practice tools and health industry engagement, from March 2011 to October 2011. From January 2008 to July 2010, Ms. Tracy was Senior Vice President and Chief Communications Officer of Artsev3 Inc. Prior to ev3, Ms. Tracy held marketing and investor relations positions at Kyphon Inc. from January 2003 to November 2007 and Thoratec Corporation from January 1998 to January 2003. Ms. Tracy currently serves as a member of the Board of Directors for the National Investor Relations Institute, the professional association of corporate officers and investor relations consultants responsible for communication among corporate management, shareholders, securities analysts and other financial community constituents.EnglishOctober 2015 in connection with the Wright/Tornier merger. Ms. Walker served as Senior Vice President, Process Improvement of Wright Medical Group, Inc. from December 2011 to October 2015 and CommunicationsVice President and Corporate Controller from North Adams State CollegeDecember 2009 to December 2011. Since joining Wright’s financial organization in 1993, she served as Assistant Controller, Director, Financial Reporting & Risk Management, Director, Corporate Tax & Risk Management, and Tax Manager of Wright. Prior to joining Wright, Ms. Walker was a Master of Arts in Corporate Communications from Fairfield University.senior tax accountant with Arthur Andersen LLP. Ms. Walker is a certified public accountant. joined us was appointed our President, Upper Extremities in March 2012October 2015 in connection with the Wright/Tornier merger. Mr. Rich served as Senior Vice President, U.S. Commercial Operations.Operations of Tornier from March 2012 to October 2015. Prior to joining us,Tornier, Mr. Rich served as Senior Vice President of Sales –- West of NuVasive, Inc., a medical device company focused on developing minimally disruptive surgical products and procedures for the spine. Prior to such position, Mr. Rich served as Area Vice President, Sales Director and Area Business Manager of NuVasive Inc. from December 2005. Prior to joining NuVasive, Mr. Rich served as Partner/Area Sales Manager of Bay Area Spine of DePuy Spine, Inc., a spine company and subsidiary of Johnson & Johnson, from July 2004 to December 2005.RichCordell served as President, U.S. Extremities of Wright Medical Group, Inc. from September 2014 to October 2015. Prior to joining Wright, Mr. Cordell served as Vice President of Sales for the GI Solutions business at Covidien plc, a global healthcare products company, from May 2012 to September 2014. While at Covidien, he served as Vice President of Sales and Global Marketing for its Peripheral Vascular business from July 2010 to May 2012. He joined Covidien in July 2010 through the acquisition of ev3 Inc., a global endovascular device company, where he served as Vice President of U.S. Sales from January 2009 to July 2010. Prior to ev3, Mr. Cordell served as Vice President, Global Sales of FoxHollow Technologies, Inc. from March 2007 to October 2007. Earlier in his career, Mr. Cordell held various positions of increasing responsibility for Johnson & Johnson’s Cordis Cardiology and Centocor companies. Mr. Cordell serves on the board of directors of TissueGen, Inc., a privately-held developer of biodegradable polymer technology for implantable drug delivery.Bachelornon-executive director in October 2015 in connection with the Wright/Tornier merger. Mr. Stevens serves as our Chairman of Labor Relationsthe Board. Mr. Stevens was a member of the board of directors of Wright Medical Group, Inc. from Rutgers College, Rutgers University.2004 to 2015 and served as Chairman of the Board from 2009 to October 2015 and interim Chief Executive Officer of Wright from April 2011 to September 2011. He has been a private investor since 2006. Mr. Stevens served as Chief Executive Officer of Accredo Health Group, Inc., a subsidiary of Medco Health Solutions, Inc., from 2005 to 2006. He was Chairman of the Board and Chief Executive Officer of Accredo Health, Inc. from 1996 to 2005, and was President and Chief Operating Officer of the predecessor companies of Accredo Health from their inception in 1983 until 1996. He serves on the board of directors of Allscripts Healthcare Solutions, Inc., a publicly held company. He previously served on the board of directors of Viasystems Group, Inc., a publicly held company, from 2012 until May 2015 when it was acquired by TTM Technologies, Inc., Medco Health Solutions, Inc., a publicly held company, from 2006 until 2012 when it was acquired by Express Scripts Holding Company, and Thomas & Betts Corporation, a publicly held company, from 2004 to 2012 when it was acquired by ABB Ltd. Mr. Steven’s qualifications to serve on our board of directors include his extensive experience serving as a chief executive officer, including as interim chief executive officer of Wright, his close familiarity with our business, and his prior experience as a director of Wright. is one of our directors and has served as a non-executive director and member of our board of directors since July 2006. Mr. Carney servesserved as our Chairman.Chairman of the Board of Tornier from May 2010 to October 2015. Mr. Carney was appointed as a director of Tornier in connection with the securityholders’ agreement that weTornier entered into with certain holders of our securities. Mr. Carney became Chairman of our board of directors in May 2010.its shareholders. For more information regarding the securityholders’ agreement, please refer to the discussion below under “—“-Board Structure and Composition.Composition.” Since 1996, Mr. Carney has been employed by Warburg Pincus LLC, a private equity firm, and has served as a Member and Managing Director of Warburg Pincus LLC and a General Partner of Warburg Pincus & Co. since January 2001. Warburg Pincus LLC and Warburg Pincus & Co. are part of the Warburg Pincus entities collectively referred to elsewhere in this report as Warburg Pincus, a principal stockholdershareholder that owns approximately 44.3%6.1% of our outstanding ordinary shares as of February 15, 2013.10, 2016. Prior to joining Warburg Pincus, Mr. Carney formerly served on the boardwas a consultant at McKinsey & Company, Inc., a He is also a member of the board of directors of Bausch & Lomb Incorporated and several other private companies. During the past five years, Mr. Carney previously served on the board of directors of DexCom, Inc., a publicly held medical device company.company, Arch Capital Group Ltd., a publicly held company, MBIA Inc., a publicly held company, and several privately held companies. Mr. Carney receivedCarney’s qualifications to serve as a Mastermember of Business Administration from Harvard Business School and a Bachelorour board of Arts from Harvard College. Mr. Carney’sdirectors include his substantial experience as an investor and director in medical device companies, his experience as a public company director, and his experience evaluating financial results have ledresults.conclusionboard of directors of LinguaFlex, Inc., a medical device company focused on treatment of sleep disordered breathing, since August 2015. From December 2011 to December 2014, he served as Chief Executive Officer and a member of the board of directors of Tengion Inc., a publicly held company that focused on organ and cell regeneration. Prior to joining Tengion, Mr. Miclot was an Executive-in Residence at Warburg Pincus, LLC. From 2008 to 2010, he should servewas President and Chief Executive Officer of CCS Medical, Inc., a provider of products and services for patients with chronic diseases. From 2003 until 2008, he served as President and Chief Executive Officer of Respironics, Inc., a provider of sleep and respiratory products, and prior to such time, served in various positions at Respironics, Inc. from 1998 to 2003, including Chief Strategic Officer and President of the Homecare Division. From 1995 to 1998, he served as Senior Vice President, Sales and Marketing of Healthdyne Technologies, Inc., a medical device company that was acquired by Respironics, Inc. in 1998. Mr. Miclot spent the early part of his medical career at DeRoyal Industries, Inc., Baxter International Inc., Ohmeda Medical, Inc. and Medix Inc. Mr. Miclot serves on the board of directors of Dentsply International, a publicly held company, and serves as Chairman and a member of the board of directors of Breathe Technologies, Inc., a privately held company. Mr. Miclot also serves as a director at this timeof the Pittsburgh Zoo and PPG Aquarium, charitable and educational institutions, serves on the University of Iowa Tippie College of Business board of advisors and serves as an industrial advisor to EQT Partners, an investment company. Mr. Miclot previously served on the board of directors of ev3 Inc., a global endovascular device company, prior to the sale of the company in light2010. Mr. Miclot’s qualifications to serve on our board of our businessdirectors include his substantial experience as a chief executive officer of several medical device companies, his deep knowledge of the medical device industry, and structure.his prior experience as a director of Wright. is one of our directors and has served as a non-executive director and member of our board of directors since June 2010. In November 2012, Mr. O’Boyle was appointed as Interim Vice Chairman.Chairman of Tornier, a position he held for about a year. From December 2010 to October 2011, Mr. O’Boyle served as Senior Vice President and Chief Financial Officer of Advanced BioHealing Inc., a medical device company which was acquired by Shire PLCplc in May 2011, and from June 2011 to May 2012, served as Senior Vice President of Business Operations.2011. From January 2003 until December 2009, Mr. O’Boyle served as the Chief Financial Officer of NuVasive, Inc., a medical device orthopedics company that completed its initial public offeringspecializing in May 2004.spinal disorders. Prior to that time, Mr. O’Boyle served in various positions during his six years with ChromaVision Medical Systems, Inc., a publicly held medical device company specializing in the oncology market, including as its Chief Financial Officer and Chief Operating Officer. Mr. O’Boyle also held various positions during his seven years with Albert Fisher North America, Inc., a publicly held international food company, including Chief Financial Officer and Senior Vice President of Operations. Mr. O’Boyle currently serves on the board ofZeltiqZELTIQ Aesthetics, Inc., and Durata Therapeutics,Sientra, Inc., all publicly tradedheld companies. Mr. O’Boyle is a Certified Public Accountant and received a Bachelorpreviously served on the board of Sciencedirectors of Durata Therapeutics, Inc. until its acquisition by Actavis plc in Accounting from the Rochester InstituteNovember 2014. Mr. O’Boyle’s qualifications to serve on our board of Technology and successfully completed the Executive Management Program at the University of California Los Angeles, John E. Anderson Graduate Business School. Mr. O’Boyle’sdirectors includes his executive experience in the healthcare industry, his experience with companies during their transition from being privately held to publicly reportingheld, and his financial and accounting expertise have ledexpertise.to the conclusion that Mr. O’Boyle should serve as a non-executive director and on our audit committee at this time in light of our business and structure.Richard B. Emmitt is one of our directors and has served as a director since July 2006. Mr. Emmitt was appointed as a directorOctober 2015 in connection with the securityholders’ agreement that we entered intoWright/Tornier merger. Ms. Paul was a member of the board of directors of Wright Medical Group, Inc. from 2008 to 2015. Ms. Paul retired in 2008 following a 26-year career with certain holders of our securities. For more information regarding the securityholders’ agreement, please refer to the discussion below under “—Board Structure and Composition.” Mr. Emmitt served asC.R. Bard, Inc., a General Partner of The Vertical Group L.P., an investment management and venture capital firm focused on the medical device company, most recently serving as the Group Vice President-International since 2003. She served in various positions at C.R. Bard, Inc. from 1982 to 2003, including President of Bard Access Systems, Inc., President of Bard Endoscopic Technologies, Vice President and biotechnology industries, from its inception in 1989 through December 2007. Commencing in January 2008, Mr. Emmitt has been a Member andBusiness Manager of The Vertical Group G.P.Bard Ventures, Vice President of Marketing of Bard Cardiopulmonary Division, Marketing Manager for Davol Inc., LLC, which controls The Vertical Group L.P. Mr. Emmitt currentlyand Senior Product Manager for Davol Inc. Ms. Paul serves on the board of directors of several privatelyDerma Sciences, Inc., a publicly held companies. During the past five years, Mr. Emmittcompany. Ms. Paul previously served on the board of directors of ev3Viking Systems, Inc., a publicly held company, until October 2012 when it was acquired by Conmed Corporation, and American Medical Systems Holdings, Inc. Mr. Emmitt holdswas a Mastercommissioner of Business Administrationthe Northwest Commission on Colleges and Universities from 2010 to 2013. Ms. Paul serves on the Rutgers School of Business and a Bachelor of Arts from Bucknell University. Mr. Emmitt’s substantial experience as an investor and board member of numerous medical device companies ranging from development stage private companiesPresident’s Innovation Network at Westminster College. Ms. Paul’s qualifications to public companies with substantial revenues has ledserve on our board of directors toinclude her over three decades of experience in the conclusionmedical device industry, including having served in various executive roles with responsibilities that he should serveinclude international and divisional operations asat this timeof another public company in lightthe healthcare industry, and her prior experience as a director of our business and structure.Wright.Alain Tornier is one of our directors and has served as a non-executive director since May 1976. Mr. Tornier assumed a leadership role in our predecessor entity in 1976, following the deathand member of his father, René Tornier, our founder. Mr. Tornier later served as our President and Chief Executive Officer until our acquisition by an investor group in September 2006, when he retired. Mr. Tornier holds a Master of Sciences degree from Grenoble University. Mr. Tornier’s significant experience in the global orthopaedics industry and deep understanding of our company’s history and operations have led our board of directors to the conclusion that he should serve as a director at this time in light of our business and structure.Richard F. Wallman is one of our directors and has served as a director since December 2008. From 1995 through his retirement in 2003, Mr. Wallman served as the Senior Vice President and Chief Financial Officer of Honeywell International, Inc., a diversified technology company, and AlliedSignal, Inc., a diversified technology company (prior to its merger with Honeywell International, Inc.). Prior to joining AlliedSignal, Inc. as Chief Financial Officer,, Mr. Wallman served as Controller of International Business Machines Corporation. In addition to serving as one of our directors, Mr. Wallman is also a member ofserves on the board of directors of Charles River Laboratories International, Inc., Convergys Corporation, Dana Holding CorporationExtended Stay America, Inc. and its wholly subsidiary ESH Hospitality, Inc., and Roper Industries,Technologies, Inc., all publicly held companies. During the past five years, Mr. Wallman previously served on the board of directors of Ariba, Inc., ExpressJet Holdings Inc. and Avaya Inc., as well as auto suppliers LearDana Holding Corporation, and Hayes Lemmerz International, Inc., allboth publicly held companies. Mr. Wallman holds a MasterWallman’s qualifications to serve on our board of Business Administration from the University of Chicago Booth School of Business with concentrations in finance and accounting and a Bachelor of Science in Electrical Engineering from Vanderbilt University. Mr. Wallman’sdirectors include his prior public company experience, including as Chief Financial Officer of Honeywell, his significant public company director experience, and his financial experience and expertise, have ledexpertise. to the conclusion that he should serve as a director at this time in light of our business and structure.Elizabeth H. Weatherman is one of our directors and has served as a director since July 2006. Ms. Weatherman was appointed as a director of Tornier in connection with the securityholders’ agreement that weTornier entered into with certain holders of our securities.shareholders. For more information regarding the securityholders’ agreement, please refer to the discussion below under “—Board Structure and Composition.Composition.” Ms. Weatherman is a General Partner of Warburg Pincus & Co., a private equity firm, a Managing Director of Warburg Pincus LLC and a member of the firm’s Executive Management Group. Ms. Weatherman joined Warburg Pincus in 1988 and is currently responsible forprimarily focused on the firm’s U.S. healthcare investment activities. Warburg Pincus LLC and Warburg Pincus & Co. are part of the Warburg Pincus entities collectively referred to elsewhere in this report as Warburg Pincus, a principal stockholdershareholder that owns approximately 44.3%6.1% of our outstanding ordinary shares as of February 15, 2013.10, 2016. Ms. Weatherman currently serves on the board of directors of Bausch & Lomb Incorporated and several other privately held companies. During the past five years, Ms. Weatherman previously served on the boards of directors of several publicly held companies, primarily in the medical device industry, including ev3 Inc., Wright Medical Group, Inc., and Kyphon Inc., and several privately held companies. Ms. Weatherman’s qualifications to serve on our board of directors of ev3 Inc., American Medical Systems Holdings, Inc. and Kyphon, Inc. Ms. Weatherman earned a Master of Business Administration from the Stanford Graduate School of Business and a Bachelor of Arts from Mount Holyoke College. Ms. Weatherman’sinclude her extensive experience as a director of several public and private companies in the medical device industry has led our board of directors to the conclusion that she should serve as a director at this time in light of our business and structure.industry.members of our board of directors will be determined by our board of directors, provided that at all times our board of directors shallwill be comprised of at least one executive director and two non-executive directors. Our board of directors currently consists of sixten directors, alltwo of whom are executive directors and eight of whom are non-executive directors. As a result of resignation of Douglas W. Kohrs, our former President, Chief Executive Officer and Executive Director, in November 2012, the executive director position is currently vacant. Under applicable Dutch law, the vacancy can only be filled by a resolution of the general meeting of shareholders from a binding nomination drawn up by the board of directors. In November 2012, upon the resignation of Mr. Kohrs, the board of directors delegated to our then interim President and Chief Executive Officer, David H. Mowry, the duties and responsibilities of our executive director. Prior to our next annual general meeting of shareholders, the board of directors, upon a recommendation of our nominating, corporate governance and compliance committee, intends to make a binding nomination of an individual, who likely will be Mr. Mowry, to fill the open vacant executive director position. except Mr. Tornier, are “independent directors” under the Listing Rules of the NASDAQ Global Select Stock Market. Therefore, five of our current six directors are independent directors. Independence requirements for service on our audit committee are discussed below under “—Board Committees—Audit Committee.Committee” and independence requirements for service on our compensation committee are discussed below under “—Compensation Committee.” All of our non-executive directors, other than Mr. WallmanCarney and Mr. O’BoyleMs. Weatherman, are independent under the independence definition in the Dutch Corporate Governance Code. Because we currently comply with the NASDAQ corporate governance requirements, the Dutch Corporate Governance Code requirement that a majority of our directors be independent within the meaning of the Dutch Corporate Governance Code does not apply provided we explain such deviation in our annual report.Our board of directors and our shareholders each have approved that our board of directors be divided into three classes, as nearly equal in number as possible, with each director serving a three-year term and one class being elected at each year’s annual general meeting of shareholders. Messrs. Wallman and O’Boyle are in the class of directors whose term expires at the 2013 annual general meeting of our shareholders. Mr. Tornier and Ms. Weatherman are in the class of directors whose term expires at the 2014 annual general meeting of our shareholders. Messrs. Carney and Emmitt are in the class of directors whose term expires at the 2015 annual general meeting of our shareholders. At each annual general meeting of our shareholders, successors to the class of directors whose term expires at such meeting will be elected to serve for three-year terms or until their respective successors are elected and qualified.theour board of directors in accordance with the relevant provisions of the Dutch Civil Code. Our board of directors will makemakes the binding nomination based on a recommendation of our nominating, corporate governance and compliance committee. A nominee is deemedIf the list of candidates contains one candidate for each open position to be filled, such candidate shall be appointed unlessby the general meeting of shareholders opposesunless the usebinding nature of the nominations by our board of directors is set aside by the general meeting of shareholders. The binding nomination procedurenature of nomination(s) by our board of directors can only be set aside by a resolution passed with the affirmative vote of at least two-thirds majority of the votes cast which votes also representat an annual or extraordinary general meeting of shareholders, provided such two-thirds vote constitutes more than 50%one-half of our issued share capital. In such case, a new meeting is called to fill the vacancies forat which the binding nominations were initially made. Nominees for appointment are presented by the board of directors. These nominations are not binding. The resolution for appointment in such meetingof a member of our board of directors shall require the affirmative votea majority of at least two-thirds majority of the votes cast representing more than 50%one-half of our issued share capital.If our board of directors fails to use its right to submit a binding nomination, the general meeting of shareholders may appoint members of our board of directors with a resolution passed with the affirmative vote of at least a two-thirds majority of the votes cast, representing more than 50% of our issued share capital. 50%one-half of our issued share capital.Pursuantdated July 18, 2006, byamong our company and among Tornier N.V., formerly known as TMG B.V.,certain of our shareholders, including TMG Holdings Coöperatief V.A.U.A. (TMG), Vertical Fund I, L.P., Vertical Fund II, L.P., KCH Stockholm AB, Mr. Tornier, WP Bermuda and certain other shareholders at the time, and by subsequent joinder agreements, additional shareholders, which agreement was amended on August 27, 2010, TMG has the right to designate three directors to be nominated to our board of directors for so long as TMG beneficially owns at least 25% of our outstanding ordinary shares, two directors for so long as TMG beneficially owns at least 10% but less than 25% of our outstanding ordinary shares and one director for so long as TMG beneficially owns at least 5% but less than 10% of our outstanding ordinary shares, and we haveshares. We agreed to use our reasonable best efforts to cause the TMG designees to be elected.theour board of directors are collectively responsible for theour management, general, and financial affairs and policy and strategy ofour company.strategy. Our executive director historically has been our Chief Executive Officer, who isdirectors are primarily responsible for managing our day-to-day affairs as well as other responsibilities that have been delegated to theour executive directordirectors in accordance with our articles of association and our internal rules for the board of directors. In November 2012, upon the resignation of our former President and Chief Executive Officer, the board of directors delegated to our then interim President and Chief Executive Officer, Mr. Mowry, the duties and responsibilities of our executive director. We intend to nominate an individual to fill the vacant executive director position prior to our next annual general meeting of shareholders. Our non-executive directors supervise our Chief Executive Officerexecutive directors and our general affairs and provide general advice to our Chief Executive Officer.them. In performing their duties, our non-executive directors are guided by the interests of our company and, shall, within the boundaries set by relevant Dutch law, must take into account the relevant interests of our stakeholders. The internal affairs of theour board of directors are governed by our internal rules for the board of directors, a copy of which is available on the Investor Relations—Corporate Information—Governance Documents & Charters section of our corporate website atwww.tornier.comwww.wright.com.CarneyStevens serves as Chairman and Mr. O’Boyle serves as Interim Viceour Chairman. The duties and responsibilities of the Chairman include, among others: determining the agenda and chairing the meetings of theour board of directors, managing theour board of directors to ensure that it operates effectively, ensuring that the members of theour board of directors receive accurate, timely and clear information, encouraging active engagement by all the members of theour board of directors, promoting effective relationships and open communication between non-executive directors and the executive directordirectors, and monitoring effective implementation of our board of directors decisions. The duties and responsibilities of the Interim Vice Chairman include, among others, serving as liaison between our President and Chief Executive Officer and the non-executive directors, coordinating a board evaluation of the performance of the President and Chief Executive Officer, coordinating feedback among the non-executive directors and the President and Chief Executive Officer, and in the absence of the Chairman, performing the duties and responsibilities of the Chairman. The duties of the Interim Vice Chairman also included leading, together with the nominating, corporate governance and compliance committee, the search process for a non-interim President and Chief Executive Officer and coordinating such process with the other members of the nominating, corporate governance and compliance committee.However, asAs required by Dutch law, our articles of association provide that when one or more members of our board of directors is absent or prevented from acting, the remaining members of our board of directors will be entrusted with the management of our company. The intent of this provision is to satisfy certain requirements under Dutch law and provide that, in rare circumstances, when a director is incapacitated, severely ill, or similarly absent or prevented from acting, the remaining members of our board of directors (or, in the event there are no such remaining members, a person appointed by our shareholders at a general meeting) will be entitled to act on behalf of our board of directors in the management of our company, notwithstanding the general requirement that otherwise requires a majority of our board of directors be present. In these limited circumstances, our articles of association permit our board of directors to pass resolutions even if a majority of the directors is not present at the meeting.a majorityall of the directors in office. Pursuant toUnder Dutch law, members of the internal rules for our board of directors a director may not participate in discussions orthe deliberation and the decision-making process on a transactionsubject or subjecttransaction in relation to which he or she has a direct or indirect personal interest that conflicts with the interest of our company and business enterprise. If all directors are conflicted and in the absence of a supervisory board, the resolution shall be adopted by the general meeting of shareholders, except if the articles of association prescribe otherwise. Our articles of association provide that a director shall not take part in any vote on a subject or transaction in relation to which he or she has a direct or indirect personal interest that conflicts with the interest of our company and business enterprise. In such event, the other directors shall be authorized to adopt the resolution. If all directors have a conflict of interest with us. Resolutions to enter into such transactions mustas mentioned above, the resolution shall be approvedadopted by a majoritythe non-executive directors.anfour standing board committees: audit committee, a compensation committee, and a nominating, corporate governance and compliance committee, eachand strategic transactions committee. Each of whichthese committees has the responsibilities and composition described in the table below and the responsibilities described in the sections below. Our board of directors has adopted a written charter for each committee of our board of directors, whichdirectors. These charters are available on the Investor Relations—Corporate Information—Governance Documents & Charters section of our corporate website atwww.tornier.comwww.wright.com. Our board of directors from time to time may establish other committees.Director Audit Compensation Nominating, corporate governance and compliance Strategic transactions Robert J. Palmisano — — — — David H. Mowry — — — — Gary D. Blackford √ — — — Sean D. Carney — Chair √ — Kevin C. O’Boyle √ — — — John L. Miclot — √ — — Amy S. Paul — — Chair — David D. Stevens — — √ √ Richard F. Wallman Chair — — √ Elizabeth H. Weatherman — √ — Chair Ourourthe audit committee include:obligations and any accounting, internal accounting controls or auditing matters, our independent auditor’s qualifications and independence and the performance of our internal audit function and independent auditors;for dealing directly with any such accounting firm;our companyus regarding accounting, internal accounting controls, or auditing matters, and for the confidential, anonymous submission by our employees of concerns regarding questionable accounting or auditing matters; andItem 404 of SEC Regulation S-K.OurOurEmmittBlackford, and Mr. O’Boyle. We believe that the composition of ourthe audit committee complies with the applicable rules of the SEC and the NASDAQ Global Select Stock Market. Our board of directors has determined that each of Mr. Wallman, Mr. EmmittBlackford, and Mr. O’Boyle is an “audit committee financial expert,” as defined in the SEC rules, and satisfies the financial sophistication requirements of the NASDAQTheOur board of directors also has determined that each of Messrs.Mr. Wallman, EmmittMr. Blackford, and Mr. O’Boyle meets the more stringent independence requirements for audit committee members of Rule 10A-3(b)(1) under the Exchange Act and the Listing Rules of the NASDAQ Global Select Stock Market, and each of Messrs.Mr. Wallman, Mr. Blackford, and Mr. O’Boyle is independent under the Dutch Corporate Governance Code.ourthe compensation committee, which are within the scope of the board of directors compensation policy adopted by the general meeting of our shareholders, include:“Compensation“Compensation Discussion and Analysis”Analysis” section of this report and based on such discussions, recommending to our board of directors whether the “Compensation“Compensation Discussion and Analysis”Analysis” section should be included in this report;our shareholders; andOurOurWallmanMiclot, and Ms. Weatherman. We believe that the composition of the compensation committee complies with the applicable rules of the SEC and the NASDAQ Global Select Stock Market. Our board of directors has determined that each of Mr. Carney, Mr. Miclot, and Ms. Weatherman meets the more stringent independence requirements for compensation committee members of Rule 10C-1 under the Exchange Act and the Listing Rules of the NASDAQ Global Select Stock Market. None of our executive officers has served as a member of the board of directors or compensation committee of any entity that has an executive officer serving as a member of our board of directors.ourthe nominating, corporate governance and compliance committee include: and ethics, other than with respect to matters relating to our financial statements and financial reporting obligations and any accounting, internal accounting controls or auditing matters, which are within the purview of the audit committee.OurOur(Chair) and Mr. O’Boyle.OurThe nominating, corporate governance and compliance committee considers all candidates recommended by our shareholders pursuant to those specific minimum qualifications that the nominating, corporate governance and compliance committee believes must be met by a recommended nominee for a position on our board of directors, which qualifications are described in the nominating, corporate governance and compliance committee’s charter, a copy of which is available on the Investor Relations—Corporate Information—Governance Documents & Charters section of our corporate websitewww.tornier.comwww.wright.com. and Ethics and ethics, which applies to all of our directors, officers, and employees. OurThe code of business conduct and ethics is available on the Investor Relations—Corporate Information—Governance Documents & Charters section of our corporate website atwww.tornier.comwww.wright.com. Any person may request a copy free of charge by writing to us at Tornier, Inc.James A. Lightman, Senior Vice President, General Counsel and Secretary, Wright Medical Group N.V., 10801 Nesbitt Ave South, Bloomington, Minnesota 55437.Prins Bernhardplein 200, 1097 JB Amsterdam, the Netherlands. We intend to disclose on our website any amendment to, or waiver from, a provision of our code of business conduct and ethics that applies to directors and executive officers and that is required to be disclosed pursuant to the rules of the SEC and the NASDAQ Global Select Stock Market. and executive officers, and all persons who beneficially own more than 10% of our outstanding ordinary shares to file with the SEC initial reports of ownership and reports of changes in ownership of our ordinary shares. Directors, executive officers, and greater than 10% beneficial owners also are required to furnish us with copies of all Section 16(a) forms they file. To our knowledge, based on review of the copies of such reports and amendments to such reports furnished to us with respect to the year ended December 30, 2012,27, 2015, and based on written representations by our directors and executive officers, all required Section 16 reports under the Exchange Act for our directors, executive officers, and beneficial owners of greater than 10% of our ordinary shares were filed on a timely basis during the year ended December 30, 2012.27, 2015.or CD&A,(CD&A), we describe the key principles and approaches we use to determine elements of compensation paid to, awarded to and earned by the following named executive officers, whose compensation is set forth in the Summary Compensation Table found later in this report:under “Executive Compensation Tables and Narratives—Summary Compensation Information”:currently serves as our current Executive Vice President and Chief Operating Officer and an executive director, and prior to the Wright/Tornier merger, served as legacy Tornier’s former President and Chief Executive Officer;and during 2012 served as our Interim President and Chief Executive Officer and, prior to such position, Chief Operating Officer;Douglas W. Kohrs, who served as our President, Chief Executive Officer and Executive Director until his resignation on November 12, 2012;Shawn T McCormick, who joined Tornier“CEO” in September 2012 and currently serves as our Chief Financial Officer;Carmen L. Diersen, who served as our Global Chief Financial Officer Director until her resignation on July 17, 2012;Terry M. Rich, who joined Tornier in March 2012 and currently serves as our Senior Vice President, U.S. Commercial Operations;Stéphan Epinette, who currently serves as our Vice President, International Commercial Operations; andKevin M. Klemz, who currently serves as our Vice President, Chief Legal Officer and Secretary.performance.performance by aligning the financial interests of our executives with those of our shareholders and by emphasizing pay for performance in our compensation programs. We typically strive to accomplish this objective primarily through the operation of our employeeannual performance incentive compensation plan, which compensates our executive officersexecutives for achieving annual corporate financial performanceand other goals and, in the case of some executives, individual goals. Although the performance goals under our performance incentive plan for the first half of 2015 were primarily financial related, our executive officers, divisionalsecond half of 2015 performance goals were broader and intended to motivate our combined company to achieve short-term common goals determined after completion of the merger to be critically important in positioning our combined company for a successful 2016.goals and individual performance goals.In 2012, we experienced increased revenues compared to 2011 and believe we made strideshad the following impact on our pay programs in restructuring certain aspects of our business and realigning our management structure. Our acquisition of OrthoHelix Surgical Designs, Inc. in October 2012, in particular, was important in expanding our lower extremity product portfolio and positioning us for future2015:growth in ourtotal extremities products. However, our financial performance, as measured by certain key performance indicators for 2012 including revenue, gross margin as a percentage of revenue, EBITDA, revenue from new products and free cash flow, in each case as adjusted, for certain items, such as effects fromlegacy Tornier for the first half of 2015 were between threshold and target goals or between target and maximum goals, resulting in first half of 2015 performance incentive plan bonuses to our OrthoHelix acquisition, was belownamed executive officers who were executives of legacy Tornier during that time of 96.4% of target for our internal expectations set atcorporate performance goals.beginningfirst half of the year. As a result, there were no 2012 payouts for2015 substantially exceeded target goals, resulting in first half of 2015 performance incentive plan corporate portion bonuses to legacy Wright named executive officers of 144% of target.divisional financiallegacy Tornier global upper extremities revenue and other performance goals under ourfor the second half of 2015 substantially exceeded target goals, resulting in second half of 2015 performance incentive compensation plan for executive officers. There were, however, payouts for achievement of individual performance goals by our executive officers, generally ranging from 100% to 105% of target. However, we place primary emphasis on overall corporate and divisional performance goals rather than individual performance goals as evidenced by the fact that 80% to 90% of our executives’ 2012 payouts were determined based on the achievement of corporate and divisional performance goals and only 10% a 20% based on achievement of individual performance goals. Thus, the overall 2012 incentive plan payoutsbonuses for our named executive officers ranged between only 10%of 150% of target.22%legacy Tornier’s stock-based compensation plans, all unvested equity awards of target. Since mostlegacy Wright and legacy Tornier outstanding as of our executives’ pay is variable compensation tied to financial results or share price, and not fixed compensation, these low performance incentive compensation payoutsthe merger automatically vested. While this automatic vesting resulted in actual totaladditional compensation for our executives substantially belowfor 2015, we believe it served its intended purpose of retaining and motivating the legacy Wright and legacy Tornier executive teams through the completion of the merger.targeted range of 50th to 75th percentile of a group of similarly sized peer companies for 2012.Key 2012 Compensation-Related ActionsDuring 2012, we took a number of actions that supported ourprincipal executive compensation philosophy of ensuring that ourofficer, principal financial officer, and several other executive pay program reinforces our corporate mission, vision and values and is reflectiveofficer positions during 2015. Because the departures of our performance, market competitive to attractformer legacy Tornier executives were in connection with a “change in control,” these executives received “change in control” severance payments and retain key employeesbenefits, which resulted in additional compensation for 2015. While these payments resulted in higher compensation for these executives than in prior years, we believe these payments served their intended purpose of retaining and aligned withmotivating these executives through the interests of our shareholders, including the following:Our compensation committee reviewed and revised our formal compensation objectives and principles to guide executive pay decisions, which are described in more detail below.Our compensation committee engaged an independent compensation consultant, Mercer (US) Inc., to provide executive pay advice to our compensation committee. During 2012, at the requestcompletion of the compensation committee, Mercer recommended a peer groupmerger.companies, collected relevant market data from these companies to allow the compensation committee to compare elements ofmerger, we entered into an employment with our pay program to those of our peers, provided information on executive pay trends and implications for our company and made other recommendations to our compensation committee regarding our executive compensation program.Our board of directors, upon recommendation of our compensation committee, adopted long-term incentive grant guidelines for the grant of equity awards to our employees under the Tornier N.V. 2010 Incentive Plan.Our board of directors, upon recommendation of our compensation committee, approved, and our shareholders approved at our 2012 annual general meeting of shareholders, an amendment to the Tornier N.V. 2010 Incentive Plan to increase the number of ordinary shares available for issuance under the plan by 2.7 million. The share increase was intended to provide a sufficient number of shares for at least a couple of years; and thus, we do not anticipate submitting another plan amendment to increase the number of shares available for issuance under the plan this year.In November 2012, we promoted David H. Mowry to Interim President and Chief Executive Officer uponand separation pay agreements with our other named executive officers who were continuing as officers of the resignationcombined company. We also entered into confidentiality, non-competition, non-solicitation and intellectual property rights agreements with our executives. The terms of Douglas W. Kohrs,these agreements are substantially identical to prior agreements with legacy Wright. We also entered into a service agreement with our former President and Chief Executive Officer and Executive Director who resigned on November 12, 2012. In February 2013, we appointed Mr. MowryVice President and Chief ExecutiveOperating Officer, onwhich deal with certain Dutch law matters relating to their roles as executive directors, and under which we allocate a non-interim basis. In connection with Mr. Kohrs departure,portion of their annual base salary to their service as executive directors.entered into a severance arrangement with Mr. Kohrs, which included a six-month transition consulting arrangement.We hired a new Chief Financial Officer, Shawn T McCormick, who commenced employment with us on September 4, 2012,granted special one-time “re-up” equity awards to replace Carmen L. Diersen,several of our former Global Chief Financial Officer, who resigned on July 17, 2012. In connection with her departure, we entered into a severance arrangement with Ms. Diersen, which included a one-year transition consulting arrangement. Mr. Kohrs served askey executives, including many of our principal financial officer from July 17, 2012 until September 4, 2012.We realigned and streamlined ournamed executive management structure by reducing the number of direct reportsofficers, in addition to our President and Chief Executive Officer.We honoreddesire of a significant portionmerger. These equity awards resulted in some of our shareholders, who at our 2011 annual general meeting of shareholders supported a “say-on-pay” vote every three years,named executive officers receiving higher stock-based compensation in 2015 than in prior years.accordingly, did not submit a “say-on-pay” proposal to our shareholders during 2012. At our 2011 annual general meeting of shareholders, over 99% of the votes cast by our shareholders were in favor of our “say-on-pay” proposal. Accordingly, ourBest Practicescommittee generally believes that such results affirmed shareholder support of our approach to executive compensation and did not believe it was necessary to make, and therefore have not made, any significant changes to our executive pay program solely in response to that vote. We intend to submit a “say-on-pay” proposal to our shareholders again at our 2014 annual general meeting of shareholders.Compensation Best PracticesWe maintain certainpractices include many best pay practices whichthat support our executive compensation objectives and principles, and benefit our shareholders. These practices include the following:performance.and other performance metrics. Our annual cashperformance incentive plan typically pays out with respect to each performance measure only if certain minimum threshold levels of financial performance are met, and even if maximum levels ofmet. are exceeded, our annual cash incentive plan payoutsbonuses are capped at 200% of target and legacy Tornier's plan bonuses were capped at 150% of target.target for the first half of 2015.executives’executive compensation is “performance-based” or “at risk.” For 2012, 71% and 62% of target total direct compensation was performance-based for our current and former CEOs, respectively, and at least 57% of target total direct compensation for our other named executive officers was performance-based, assuming grant date fair values for equity awards.executives’executive compensation is “equity-based” and in the form of stock-based incentive awards, comprising of 48%awards.44% of target total direct compensationadopt long-term incentive guidelines for the executives who served asgrant of equity awards under our CEO during 2012 and at least 39% of target total direct compensation for our other named executive officers in 2012, assuming grant date fair values for equity awards.stock incentive plan. our long-term equity-based incentive awards is tied to three-year to four-year vesting and any value received by executives from stock option grants is contingent upon long-term stock price performance in that stock options have value only if the pricemarket value of our ordinary shares exceeds the exercise price of the options. our executives engaged in certain conduct adverse to the company’sour interests. In addition, our performance incentive plan also contains a clawback provision.“gross up”“gross-up” payments to our executives, other than certain limited tax gross-up payments to our CEO as required under the terms of his employment agreement.employment agreementssecurities.any other compensation, benefits or perquisites provided to our executives.2017 annual general meeting of shareholders.We provide only limited modest perquisites to our executives.Principlesprofitability,financial performance, and thus shareholder value, by aligning the financial interests of our executives with those of our shareholders and by emphasizing pay for performance.pay-for-performance. Specifically, our executive compensation programs are designed to:company and the creation of value for our shareholders.company.Reinforce our corporate mission, vision and values. our executives for progress toward our corporate mission and vision, the achievement of company performance objectives, the creation of shareholder value in the short- and long-term, and their general contributions to the success of our company.ourthe compensation committee makes executive compensation decisions based on the following principles:philosophies:• withinto be near the range of the 50th to 7567th percentile of a group of similarly sizedsimilarly-sized peer companies. However, the specific competitiveness of any individual executive’s salary and compensation will be determined considering factors like the executive’s skills and capabilities, contributions as a member of the executive management team, and contributions to our overall performance. Pay levels will also reflectperformance, and the sufficiency of total compensation potential and structure to ensure the retention of an executive when considering the executive’s compensation potential that may be available elsewhere.share price.executives’executive’s overall responsibilities, job level, and compensation. However, compensation programs should not encourage excessive risk-taking by executives.A primaryPrimary emphasis should be placed on company performance as measured against goals approved by ourthe compensation committee rather than on individual performance.
At least half of the CEO’s compensation and one-third of other executives’ compensation opportunity should be in the form of stock-based incentive awards.
Role of Compensation Committee and Board. The responsibilities of ourthe compensation committee include reviewing and approving corporate goals and objectives relevant to the compensation of our executive officers, evaluating each executive’s performance in light of those goals and objectives and, either as a committee or together with the other directors, determining and approving each executive’s compensation, including performance-based compensation based on these evaluations (and, in the case of the executives, other than the CEO, the CEO’s evaluation of such executive’s individual performance). Consistent with our shareholder-approved board of directors compensation policy, the compensation packagepackages for our CEO isand Executive Vice President and Chief Operating Officer, who also serve as executive directors of our company, are determined by theour non-executive members of our board in accordance with such policy,directors, based upon recommendations from the compensation committee.
each executive’s position within the company and the level of responsibility;
the ability of the executive to impact key business initiatives;
the executive’s individual experience and qualifications;
compensation paid to executives of comparable positions by companies similar to our company;
company performance, as compared to specific pre-established objectives;
individual performance, generally and as compared to specific pre-established objectives;
the executive’s current and historical compensation levels;
advancement potential and succession planning considerations;
an assessment of the risk that the executive would leave our companyus and the harm to our company’s business initiatives if the executive left;
the retention value of executive equity holdings, including outstanding stock options and restricted stock unit (RSU) awards;
the dilutive effect on the interests of our shareholders of long-term equity-based incentive awards; and
anticipated share-based compensation expense as determined under applicable accounting rules.
directors.
considers in setting executive compensation, including in particular the results of each executive’s annual performance review and the executive’s achievement of his or her individual management performance objectives established in connection with our annual cashperformance incentive plan, described below. Our GlobalThe Senior Vice President, Human Resources assists ourthe compensation committee primarily by gathering compensation related data regarding our executives and coordinating the exchange of suchthis information and other executive compensation information among the members of ourthe compensation committee, ourthe compensation committee’s compensation consultant and management in anticipation of compensation committee meetings. OurThe Senior Vice President, Chief Legal OfficerGeneral Counsel and Secretary assists ourthe compensation committee primarily by ensuring compliance with legal and regulatory requirements and educating the committee on executive compensation trends and best practices from a corporate governance perspective. Final deliberations and decisions regarding the compensation to be paid to each of our executives,executive, however, are made by our board of directors or compensation committee without the presence of suchthe executive.
The compensation committee believes that compensation paid by peer group companies is more representative of the compensation required to attract, retain, and motivate our executive talent than broader survey data. The compensation committee believesdata and that the compensation paid by the peer companies whichthat are in the same business,industry, with similar products and operations, and with revenues in a range similar to oursus, generally provides more relevant comparisons.
| Merit Medical Systems, Inc. | ||||
| Natus Medical Incorporated | ||||
| NxStage Medical, Inc. | Thoratec Corporation | |||
| Integra LifeSciences Holdings Corporation | ||||
| Annual revenue (in millions) | Market capitalization (in millions) | |||||||
25th percentile | $ | 217 | $ | 629 | ||||
Median | 333 | 863 | ||||||
75th percentile | 437 | 996 | ||||||
Tornier | 267 | 752 | ||||||
Percentile rank | 31 | % | 43 | % | ||||
that the compensation committee used in connection with its recommendations and decisions regarding executive compensation for 2015:
Trailing 12-month revenue (in millions) | Three-year revenue growth | Trailing 12-month EBIT | Market capitalization (in millions) | |||||
25th percentile | $478 | 25% | $69 | $1,325 | ||||
50th percentile | 688 | 34% | 93 | 2,171 | ||||
75th percentile | 928 | 42% | 143 | 2,299 | ||||
| Tornier + Wright | N/A | N/A | N/A | 3,300 | ||||
| Percentile rank | 51% | N/A | N/A | N/A | 78% | |||
base salary;
short-term cash incentive compensation;
long-term equity-based incentive compensation, in the form of stock options and stockRSU awards; and
other compensation arrangements, such as benefits made generally available to our other employees, limited and modest executive benefits and perquisites, and severance and change in control arrangements.
In addition, our objective is that at least two-thirds of the CEO’s compensation and one-half of other executives’ compensation opportunity be in the form of variable compensation that is tied to financial results or share price and that at least half of the CEO’s compensation and one-third of other executives’ compensation opportunity be in the form of stock-based incentive awards.
Overview. We provide a base salary for our named executive officers which,that, unlike some of the other elements of our executive compensation program, is not subject to company or individual performance risk. We recognize the need for
most executives to receive at least a portion of their total compensation in the form of a guaranteed base salary that is paid in cash regularly throughout the year. Base salary amountssalaries are established under each executive’s employment agreement,upon hiring an executive, and are subject to subsequent annual upward adjustments by our compensation committee, or in the case of any executive who is also a director, our board of directors, upon recommendation of our compensation committee.
adjustments.
new U.S. corporate headquarters in Memphis, Tennessee from our former U.S. corporate headquarters in Bloomington, Minnesota.
| Name | 2014 base salary (actual/notional) ($) | 2015 base salary ($) | 2015 base salary % increase compared to 2014 actual and notional base salary(1)(2) | 2015 base salary compared to peer group percentile | ||||
| Robert J. Palmisano | $ 750,000/836,200 | $886,200 | 18.2%/6.0% | Above 75th | ||||
| David H. Mowry | 550,000 | 622,000 | 13.0% | Above 75th | ||||
| Lance A. Berry | 375,000 | 397,500 | 6.0% | Above 50th | ||||
| Shawn T McCormick | 365,456 | 377,333 | 3.0% | At 50th | ||||
| Gregory Morrison | 300,002 | 365,000 | 21.7% | Above 50th | ||||
| Terry M. Rich | 369,694 | 384,482 | 4.0% | Above 75th | ||||
| James A. Lightman | 310,000/352,000 | 373,100 | 20.4%/6.0% | Above 50th | ||||
| Gordon W. Van Ummersen | 356,122 | 365,025 | 2.5% | Above 50th | ||||
| (1) | Percentage increase compared to 2014 base salary reflects any base salary increase received effective October 1, 2015 and, in the case of the legacy Tornier executives, any base salary increase received effective February 1, 2015. |
| (2) | In the case of the legacy Wright executives who previously elected to receive legacy Wright equity in lieu of prior base salary increases, the percentage increase is compared to both their 2014 actual base salary and 2014 notional base salary. |
The merit increasessalaries for 2016 for our named executive officers who were executives at the timeeffective April 1, 2016: Mr. Palmisano ($921,648), Mr. Mowry ($646,880), Mr. Berry ($413,400), Mr. Morrison ($379,600), Mr. Rich ($399,861), and Mr. Lightman $388,024). The 2016 base salaries represent merit increases of the increase in February 2012 ranged from 2.5% to 3.5%4% over 2011their respective 2015 base salaries. 2012 base salaries (effective as of February 1, 2012), the percentage increases compared to 2011 base salaries, and the 2012 base salaries compared to the peer 50th percentile are provided in the table below for each of our named executive officers whoNo upward market adjustments were executives at the time of the merit increase:made.
Name | 2012 base salary ($) | 2012 base salary % increase compared to 2011 | 2012 base peer group | |||||||
David H. Mowry(1) | 335,563 | 3.25 | % | 13% below | ||||||
Douglas W. Kohrs | 518,490 | 2.89 | % | 6% below | ||||||
Carmen L. Diersen | 342,286 | 2.50 | % | 8% above | ||||||
Stéphan Epinette(2) | 286,866 | 3.50 | % | 10% below | ||||||
Kevin M. Klemz | 286,441 | 3.25 | % | 5% below | ||||||
Whether an executive received a 2.5% to 3.5% merit increase was based primarily on the results of the executive’s performance review for 2011. In evaluating the performance of Mr. Kohrs and the amount of his 2012 merit increase, the compensation committee reviewed Mr. Kohrs’s self-review, discussed his performance and sought the input from the non-executive directors. In assessing the performance of Mr. Kohrs, the compensation committee evaluated primarily his ability to achieve his goals and objectives and lead the company.
Salary Increases in Connection with Promotions. We typically increase the base salaries of our named executive officers in connection with promotions to compensate them for their assumption of increased roles and responsibilities and to bring their base compensation closer to those of executives in comparable positions at similar companies. In connection with his promotion to Interim President and Chief Executive Officer in November 2012, Mr. Mowry received a base salary increase of $49,437. This increase was intended to compensate Mr. Mowry for his increased responsibilities and to bring his
base salary closer to the 50th percentile for Chief Operating Officers in our peer group with the understanding that the amount of Mr. Mowry’s base salary would be revisited if he is appointed President and Chief Executive Officer on a non-interim basis. In February 2013, Mr. Mowry was appointed President and Chief Executive Officer on a non-interim basis and his base salary was increased to $450,000 to bring his base salary closer to the 50th percentile for CEOs in our peer group. Mr. Mowry’s base salary of $450,000 is still below the 25th percentile for CEOs in our peer group. The compensation committee believes this market positioning is appropriate in light of Mr. Mowry’s prior base salary and that this will be his first full year serving as President and Chief Executive Officer.
Employee Performance Incentive Compensation Plan. Annual cash bonuses under our employee performance incentive compensation plan areis intended to compensate executives, as well as other employees, for achieving annual corporate financial and other performance goals and, in some cases, divisional financial goals, and, in most cases, individual performance goals.
| Name | First half of 2015 percentage of base salary | Second half of 2015 percentage of base salary | ||
| Robert J. Palmisano | 100% | 100% | ||
| David H. Mowry | 80% | 80% | ||
| Lance A. Berry | 60% | 65% | ||
| Shawn T McCormick | 50% | 50% | ||
| Gregory Morrison | 40% | 50% | ||
| Terry M. Rich | 75% | 55% | ||
| James A. Lightman | 50% | 50% | ||
| Gordon W. Van Ummersen | 50% | 50% | ||
For 2012, payouts under our employee incentive compensation plan to our named executive officersVan Ummersen, were based upon achievement of corporate performance goals for all executives, divisional performance goals for two executives andplus individual performance goals for all executives, except our former PresidentMessrs. Mowry and Chief Executive Officer whose payout wasRich.
| Named executive officer | Percentage based upon corporate performance goals | Percentage based upon individual performance goals | ||
| David H. Mowry | 100% | 0% | ||
| Shawn T McCormick | 90% | 10% | ||
| Gregory Morrison | 80% | 20% | ||
| Terry M. Rich | 100% | 0% | ||
| Gordon W. Van Ummersen | 90% | 10% | ||
Named executive officer | Percentage based upon corporate performance goals | Percentage based upon divisional performance goals | Percentage based upon individual performance goals | |||||||||
David H. Mowry | 90 | % | 0 | % | 10 | % | ||||||
Douglas W. Kohrs | 100 | % | 0 | % | 0 | % | ||||||
Shawn T McCormick | 90 | % | 0 | % | 10 | % | ||||||
Carmen L. Diersen | 90 | % | 0 | % | 10 | % | ||||||
Terry Rich | 20 | % | 70 | % | 10 | % | ||||||
Stéphan Epinette | 20 | % | 70 | % | 10 | % | ||||||
Kevin M. Klemz | 80 | % | 0 | % | 20 | % | ||||||
Consistent with the design for the 2012 payout for our former President2015 as evaluated by management and Chief Executive Officer, the payout under our 2013 employee performance incentive compensation plan for Mr. Mowry is based 100% upon achievement of corporate performance goals, with no divisional performance or individual performance components. Otherwise, the percentage split among corporate performance goals, divisional performance goals and individual performance goals is the same for our other named executive officers for 2013.
For 2012,analysts. Extremities revenue was weighted most heavily since that was intended to be legacy Tornier’s greatest focus in 2015.
| First half of 2015 corporate performance metric | Weighting | |
| Adjusted extremities revenue | 50% | |
| Adjusted EBITDA | 20% | |
| Adjusted free cash flow | 20% | |
| Adjusted total revenue | 10% | |
revenue, adjusted EBITDA and adjusted free cash flow, and the weightings will be 60%, 20% and 20%, respectively. These three corporate performance goals were selected for 2013 because they were determined to be the three most important key indicatorsrevenues of our financial performance for 2013. Revenue is weighted more heavily since that is intended to be our greatest focus in 2013.
The table below sets forth the corporate performance metrics and goals for 2012SALTO® ankle products, which were established by our board of directors, upon recommendation of our compensation committee,divested in connection with the range of possible payouts, and the actual payout percentage for our named executive officers based on the actual performance achieved.Wright/Tornier merger. If performance achieved falls below the threshold level, there is no payout for such performance metric. If performance achieved falls between the threshold, target and maximum levels, actual payout percentages are determined on a sliding scale basis, with payouts for each performance metric starting at 50% of target for threshold performance achievement and capped at 150% of target for maximum achievement. For 2012,the first half of 2015, the total weighted-averageweighted‑average payout percentage applicable to the portion of the 2012first half of 2015 annual cashperformance incentive bonus tied to corporate performance goals was zero, as detailed in96.4% of target. Actual performance exceeded target for the table below, resulting in no payout based on the corporateadjusted EBITDA and free cash flow performance goals and was just below target for 2012.
| Level of fiscal 2012 payout | ||||||||||||||||||||||||||||||||||||
| Performance goals(1) | Payout percentage | 2012 performance(2) | ||||||||||||||||||||||||||||||||||
Performance metric | Weighting | Threshold | Target | Maximum | Threshold | Target | Maximum | |||||||||||||||||||||||||||||
Adjusted revenue(3) | 40 | % | $ | 287.3 mil. | $ | 290.0 mil. | $ | 295.2 mil. | 50 | % | 100 | % | 150 | % | $ | 277.6 mil. | 0.0 | % | ||||||||||||||||||
Adjusted gross margin % of revenue(4) | 15 | % | 72.0 | % | 72.4 | % | 73.3 | % | 50 | % | 100 | % | 150 | % | 71.8 | % | 0.0 | % | ||||||||||||||||||
Adjusted EBITDA(5) | 15 | % | $ | 40.3 mil. | $ | 42.1 mil. | $ | 46.3 mil. | 50 | % | 100 | % | 150 | % | $ | 31.6 mil. | 0.0 | % | ||||||||||||||||||
Adjusted revenue from new products(6) | 15 | % | $ | 14.1 mil. | $ | 15.7 mil. | $ | 17.3 mil. | 50 | % | 100 | % | 150 | % | $ | 9.4 mil. | 0.0 | % | ||||||||||||||||||
Adjusted free cash flow(7) | 15 | % | $ | 8.6 mil. | $ | 9.1 mil. | $ | 10.5 mil. | 50 | % | 100 | % | 150 | % | $ | 0.2 mil. | 0.0 | % | ||||||||||||||||||
Performance goals(1) | ||||||||||
| Performance metric | Threshold (50% payout) | Target (100% payout) | Maximum (150% payout) | First half of 2015 performance(2) | First half of 2015 bonus | |||||
Adjusted extremities revenue(3) | $153.3 mil. | $157.3 mil. | $161.3 mil. | $156.1 mil. | 85.3% | |||||
Adjusted EBITDA(4) | 16.8 mil. | 17.8 mil. | 19.6 mil. | 18.7 mil. | 125% | |||||
Adjusted free cash flow(5) | (14.8) mil. | (11.8) mil. | (9.8) mil. | (11.3) mil. | 112.5% | |||||
Adjusted total revenue(6) | 184.9 mil. | 189.9 mil. | 194.9 mil. | 186.2 mil. | 62.7% | |||||
| (1) | The performance goals were |
| (2) | The compensation committee determined |
| (3) | “Adjusted extremities revenue” means |
| (4) | “Adjusted EBITDA” means |
| “Adjusted free cash flow” means legacy Tornier’s net cash flow |
For 2012, payouts under our employee incentive compensation plan for two
| (6) | “Adjusted total revenue” means legacy Tornier’s total revenue for the six months ended June 28, 2015, as adjusted for changes to foreign currency exchange rates and revenue related to legacy Tornier’s SALTO® ankle products which legacy Tornier divested in connection with the Wright/Tornier merger. |
| First half of 2015 corporate performance metric | Weighting | |
Adjusted revenue from continuing operations(1) | 67% | |
Adjusted gross margin from continuing operations(2) | 33% | |
| (1) | This performance measure was calculated using a non-GAAP financial measure, which we believe provides meaningful supplemental information regarding our core operational performance. Adjusted revenue from continuing operations was calculated by excluding (a) the difference in foreign currency to a plan rate and (b) AUGMENT® Bone Graft revenues. |
| (2) | This performance measure was calculated using a non-GAAP financial measure, which we believe provides meaningful supplemental information regarding our core operational performance. Adjusted gross margin from continuing operations was calculated by excluding (a) the difference in foreign currency to a plan rate; (b) AUGMENT® Bone Graft revenues; and (c) non-cash inventory step-up amortization. |
| Performance level | Percent of target bonus earned | |
| Minimum | 0% | |
| Threshold (50% payout) | 50.1% to 99.9% | |
| Target (100% payout) | 100% | |
| Above target (150% payout) | 100.1% to 150% | |
| High (200% payout) | 150.1% to 200% | |
| Performance level | Adjusted revenue from continuing operations | Adjusted gross margin from continuing operations | ||
| Minimum | <$138,400,000 | <74.30% | ||
| Threshold (50% payout) | $138,400,001 to $150,399,999 | 74.30% to 75.79% | ||
| Target (100% payout) | $150,400,000 | 75.8% | ||
| Above target (150% payout) | $150,400,001 to $155,000,000 | 75.81% to 76.80% | ||
| High (200% payout) | $155,000,001 to $158,000,000 | 76.81% to 77.80% | ||
| 1. | 2016 Annual Operating Plan: Complete our 2016 annual operating plan and workforce planning by February 2016 board of directors meeting. |
| 2. | HPMS - Total Alignment: Complete High Performance Management System (HPMS) success tree to include the new mission, vision, values, and vital few initiatives for the combined company. |
| 3. | Continue Driving Core Business While Executing Integration: Achieved combined revenue growth of legacy Wright’s U.S. lower extremity and legacy Tornier’s global upper extremity products at 1.5x or greater of market. |
| 4. | Rapid AUGMENT® Adoption: Completed training of greater than 150 foot and ankle surgeons on AUGMENT® Bone Graft. |
150% of target.
| Named executive officer | First half of 2015 | Second half of 2015 | Total | |||||||||
| Robert J. Palmisano | $ | 602,004 | $ | 645,651 | $ | 1,247,655 | ||||||
| David H. Mowry | 220,870 | 358,200 | 579,070 | |||||||||
| Lance A. Berry | 162,113 | 181,266 | 343,379 | |||||||||
| Shawn T McCormick | 90,724 | 141,500 | 232,224 | |||||||||
| Gregory Morrison | 59,709 | 115,238 | 174,947 | |||||||||
| Terry M. Rich | 128,874 | 187,435 | 316,309 | |||||||||
| James A. Lightman | 117,084 | 135,931 | 253,015 | |||||||||
| Gordon W. Van Ummersen | 87,885 | 136,884 | 224,769 | |||||||||
For 2012, the payoutincentive plan for 2016. The 2016 target bonus percentages attributable to corporate and divisional performance represented between 20% and 90% and individual performance represented 10% or 20% of the overall annual cash incentive bonus payouts for thoseour named executive officers that received 2012 bonuses, which resulted in payouts at approximately the following aggregate percentages: (i) Mr. Mowry – 10.38%, (ii) Mr. McCormick – 10.00%, (iii) Mr. Rich – 10.00%, (iv) Mr. Epinette – 21.58%, and (v) Mr. Klemz – 20.00%. As a result of a termination of their employment during 2012, Mr. Kohrs and Ms. Diersen were not eligible to receive and did not receivechange from their second half of 2015 levels. Consistent with the design for the second half of 2015 plan, the annual cash incentive bonus payouts for 2012.
The table below sets forth, with respect to each named executive officer, the maximum potential bonus opportunity as a percentage of base salary and the actual bonus paid under the employee performance incentive compensation plan for 2012, both in amount and as a percentage of 2012 base earnings:
Name Maximum potential David H. Mowry Douglas W. Kohrs Shawn T McCormick(1) Carmen L. Diersen Terry M. Rich(1) Stéphan Epinette(2) Kevin M. Klemz
bonus as percentage of
base salary Actual
bonus paid
($) Actual bonus paid as a
percentage of
2012 base earnings 75% (150% of 50%) 17,666 5.2 % 120% (150% of 80%) 0 0 % 75% (150% of 50%) 5,710 5.0 % 75% (150% of 50%) 0 0 % 113% (150% of 75%) 21,185 7.5 % 60% (150% of 40%) 25,395 8.6 % 60% (150% of 40%) 22,825 8.0 %
For 2013, thereour CEO will be no payouts attributable to individual performance for our executive officers and certain other employees if a threshold level of performance for the corporate performance goal, adjusted EBITDA, is not met.
French Incentive Compensation Scheme.In addition to participating in our employee performance incentive compensation plan, Mr. Epinette participates in an incentive compensation schemebased 100% on the same basis as other employees of our French operating subsidiary. This scheme enables our French operating subsidiary to provide its employees with a form of compensation that is efficient with respect to income tax and mandated social contributions in France. The payments made under the French incentive compensation scheme, which receives preferential tax treatment, are exempted from social security contributions. Under the French incentive compensation scheme, employees of our French operating subsidiary may receive an annual incentive cash payment equal to a specified percentage of their base salary, up to certain statutory limits. In 2012, employees were eligible to receive up to 16% of base salary, up to a statutory limit of €17,676. For 2012, annual incentive payments were dependent on the achievement of performance goals relating to adjusted revenue, adjusted EBITDA, adjusted revenue over net value of implants and instruments and on-time delivery to market of certain new products. In each case these amounts are adjusted for certain items similar to the adjustments that apply to the corporate performance goals, established underwith no individual performance components. Bonuses for our employeeother named executive officers will be based 100% on achievement of corporate performance incentive compensation plan.
goals for Messrs. Mowry and Berry and 80% on achievement of corporate performance goals and 20% on achievement of individual goals for Messrs. Morrison and Lightman. Mr. Rich's 2016 bonus will be based 40% on corporate performance goals and 60% on divisional goals. The table below sets forth the 2012 financialcorporate performance metricsmeasures for the French incentive compensation scheme, the range of possible payouts for Mr. Epinette, and the estimated actual payout percentage for Mr. Epinette2016 will be based on our adjusted net sales, adjusted net sales of AUGMENT® Bone Graft, adjusted EBITDA, and adjusted free cash flow.
| Payout | ||||||||||||||||||||||||||||
| Performance goals(1) | Threshold (% of base salary) | Target/max. (% of base salary) | 2012 performance(2) | Level for 2012 payment | ||||||||||||||||||||||||
Performance metric | Weighting | Threshold | Target/max.(3) | |||||||||||||||||||||||||
Adjusted revenue(4) | 25 | % | $ | 249.6 million | $ | 293.7 million | 0.25 | % | 4 | % | $ | 278.2 million | 2.5 | % | ||||||||||||||
Adjusted EBITDA(5) | 25 | % | $ | 39.4 million | $ | 46.4 million | 0.25 | % | 4 | % | $ | 31.6 million | 0.0 | % | ||||||||||||||
Adjusted revenue/net value of implants and instruments(6) | 25 | % | 1.79 | 2.10 | 0.25 | % | 4 | % | 2.09 | 3.8 | % | |||||||||||||||||
On-time delivery to market of new products(7) | 25 | % | N/A | N/A | 0.25 | % | 4 | % | 100 | % | 4.0 | % | ||||||||||||||||
|
certain toe products.
Generally. OurThe compensation committee’s primary objectives with respect to long-term equity-based incentives are to align the interests of our executives with the long-term interests of our shareholders, promote stock ownership, and create significant incentives for executive retention. Long-term equity-based incentives typically comprise a significant portion of each named executive officer’s compensation package, consistent with our executive compensation philosophy that at least half of the CEO’s compensation and one-third of other executives’ compensation opportunity should be in the form of stock-based incentive awards. For 2012, equity-based compensation comprised 49% and 61% of
Before our initial public offering in February 2011, we granted stock options under our prior stock option plan, which is now the TornierWright Medical Group N.V. Amended and Restated Stock Option Plan and referred to in this report as our prior stock option plan. As of February 2, 2011, we ceased making grants under our prior stock option plan and subsequently have granted stock options and other equity-based awards under our new stock incentive plan, the Tornier N.V. 2010 Incentive Plan, referredwhich we refer to in this report as our stock incentive plan. Both our board of directors and shareholders have approved our stock incentive plan, under which our named executive officers (as well as other executives and key employees) are eligible to receive equity-based incentive awards. For more information on the terms of our stock incentive plan, see “Executive Compensation—“-—Grants of Plan-Based Awards— TornierAwards- Wright Medical Group N.V. Amended and Restated 2010 Incentive Plan.Plan.” All equity-based incentive awards granted to our named executive officers during 20122015 were made under our stock incentive plan.
To assist our board of directors in granting, and our compensation committee and management in recommending the grant of, equity-based incentive awards, our compensation committee, on recommendation of Mercer, in February 2012, adopted long-term incentive grant guidelines. In addition to our long-term incentive grant guidelines, our board of directors has adopted a stock grant policy document, which includes policies that our board of directors and compensation committee follows in connection with granting equity-based incentive awards, including the long-term incentive grant guidelines.
2015 Equity Awards.”
Once the target total long-term equity value is determined for each executive based on the executive’s relevant percentage of base salary, half of the value is provided in stock options and the other half is provided in stockRSU awards. The reasons we use stock options and stock awards are described below under the headings “—Stock Options” and “—Stock Awards.” The target dollar value to be delivered in stock options (50% of the target total long-term equity value) is divided by the Black-Scholes value of one ordinary share to determine the number of stock options, which may then be rounded to the nearest whole number or in some cases multiple of 100. The number of stock awards is calculated using the intended dollar value (50% of the target total long-term equity value) divided by the 10-trading day average closing sale price of our ordinary shares as reported by the NASDAQ Global Select Market and as determined one week before the date of anticipated corporate approval of the award, which number may then be rounded to the nearest whole number or in some cases multiple of 100. Typically, the number of ordinary shares subject to stock awards is fewer than the number of ordinary shares that would have been covered by a stock option of equivalent target value. The actual number of stock options and stock awards granted may then be pared back so that the estimated run rate dilution under our stock incentive plan is acceptable to our compensation committee (i.e., approximately 2.75% for 2012). The CEO next reviews the preliminary individual awards and may make recommended discretionary adjustments. Such proposed individual awards are then presented to the compensation committee, which also may make discretionary adjustments before recommending awards to our board of directors for approval. After board approval, awards are issued,Consistent with the exercise price of the stock options equal to the closing price of our ordinary shares on the grant date. In determining the number of stock options or stock awards to make to an executive as part of a performance recognition grant, previous awards, whether vested or unvested, granted to such individual have no impact.
The table below describes our long-term incentive grant guidelines for annual performance recognition grantsprinciple that applied to our named executive officers for 2012. Mr. McCormick and Ms. Diersen are not listed in the table because they did not receive annual performance recognition grants for 2012. As described below under “—2012 Equity Awards,” because of the disappointing financial performance of the company, the compensation committee approved equity awards for each of these named executive officers with dollar values less than the incentive grant guidelines amount.
Named executive officer | Grade level | Incentive grant guideline expressed as % of base salary for grade level | Incentive grant guideline dollar value of long-term incentives ($) | |||||||||
David H. Mowry | 9 | 175 | % | 587,235 | ||||||||
Douglas W. Kohrs | 11 | 275 | % | 1,425,848 | ||||||||
Terry M. Rich | 8 | 125 | % | 437,500 | ||||||||
Stéphan Epinette(1) | 8 | 125 | % | 339,274 | ||||||||
Kevin M. Klemz | 8 | 125 | % | 358,051 | ||||||||
We seek to align the interests of our executives should be aligned with those of our shareholders by providing a significant portion of compensation in equity-based awards. Consistent with this principle,and that the portion of an executive’s total compensation that varies with performance and is at risk should increase with the executive’s level of responsibility. Thus,responsibility, incentive grants, expressed as a percentage of base salary and dollar values, increase as an executive’s level of responsibility increases. The incentive grant guidelines were benchmarked by Mercer against our peer group.
Talent acquisition
| Named executive officer | Grade level | Incentive grant guideline expressed as % of base salary | Dollar value of incentive grant guideline (1) ($) | |||||
| Robert J. Palmisano | 13 | 400% | $ | 3,477,600 | ||||
| David H. Mowry | 12 | 250% | 1,555,000 | |||||
| Lance A. Berry | 11 | 175% | 682,500 | |||||
| Shawn T McCormick | N/A | N/A | N/A | |||||
| Gregory Morrison | 10 | 125% | 456,250 | |||||
| Terry M. Rich | 10 | 100% | 384,500 | |||||
| James A. Lightman | 10 | 125% | 457,625 | |||||
| Gordon W. Van Ummersen | N/A | N/A | N/A | |||||
| (1) | The dollar value of the incentive grant guideline that applied for the 2015 equity grants to the legacy Wright executives was based on a base salary that reflected a 4% merit increase rather than the 6% merit increase that they received. |
executive’s compensation package at the time of hire (with the grant date and exercise price delayed until the hire date)date or the first open window period after board approval of the grant). As with our performance recognition grants, the size of our talent acquisition grants is determined by dollar amount (as opposed to number of underlying shares), and under our long-term incentive grant guidelines, is generally two times the long-term incentive grant guidelines for annual performance recognition grants. We have set talent acquisition grants, at two times the long-term incentive grant guidelines for annual performance recognition grants, upon recommendationas recommended by Mercer. We recognize that higher initial grants often are necessary to attract a new executive, especially one who may have accumulated a substantial amount of equity-based long-term incentive awards at a previous employer that would typically be forfeited upon acceptance of employment with us. In some cases, we may need to further increase a talent acquisition grant to attract an executive.
Our compensation committee Although no talent acquisitions grants were made during 2015, we made special one-time “re-up” grants to all of our named executive officers other than Messrs. McCormick and Van Ummersen in October 2015 together with the annual performance recognition grants. The purpose of these “re-up” grants was to encourage these executives to stay with our combined company after completion of the Wright/Tornier merger by partially restoring their unvested equity retention value and, in some cases, to facilitate the transition of executives into new roles. The amount of these “re-up” grants was based on what we typically grant in connection with talent acquisition grants and was benchmarked by Mercer and found to be aligned with market practice of award sizes for new hires who would similarly have no unvested equity awards.
for retention or other purposes. Such grants may vest based on the passage of time and/or the achievement of certain performance goals.
To comply with Dutch insider trading laws, we
laws which prohibit option grants when we are aware of material nonpublic information.
The specific terms of vesting of a stockan RSU award dependdepends on whether the award is a performance recognition grant or talent acquisition grant. Performance recognition grants of stockRSU awards are made mid-year and vest in four annual installments on June 1st1st of each year. Talent acquisition grants of stockRSU awards to new hires vest in a similar manner, except that the first installment is often pro-rated, depending on the grant date. Due
2012 Equity Awards. Our board of directors, on recommendation of the compensation committee, made annual performance recognition grants and talent acquisition grants to one or more of our named executive officers during 2012.
The table below describes the actual performance recognition grants made to our named executive officers in 2012 and the applicable long-term incentive grant guideline for such performance recognition grants for these executives. Since neither2015. Neither Mr. McCormick nor Ms. Diersen was employed at the time of the performance recognition grants, neitherMr. Van Ummersen received a grant for 2012 and they are not listed in the table below. Because of the disappointing financial performance of the company, the compensation committee approved equity awards for each of these named executive officers with dollar values less thanduring 2015 since they were leaving the incentive grant guidelines.
Named executive officer David H. Mowry Douglas W. Kohrs Terry M. Rich Stéphan Epinette(2) Kevin M. Klemz Stock
options Stock
awards Actual award value per
long-term incentive
grant guideline(1)
($) Difference between actual
award value per long-
term incentive grant
guideline and guideline(1)
($) 23,365 10,678 452,961 (134,274 ) 51,987 23,758 1,007,814 (418,034 ) 14,443 6,601 280,014 (157,486 ) 17,501 7,998 339,274 0 15,515 7,090 300,758 (57,293 )
Since Mr. McCormick and Mr. Rich joined Tornier as new executives in 2012, they received talent acquisition grants in 2012. The table below describes the 2012 talent acquisition grants made to Mr. McCormick and Mr. Rich and the long-term incentive grant guidelines for these grants:
Named executive officer | Stock options | Stock awards | Value of long-term incentive grant guideline(1) ($) | |||||||||
Shawn T McCormick | 42,645 | 19,531 | 875,000 | |||||||||
Terry M. Rich | 55,690 | 21,220 | 875,000 | |||||||||
company.
| Annual performance recognition grants | Special one-time re-up grants | |||||||
| Named executive officer | Stock options | RSU awards | Stock options | RSU awards | ||||
| Robert J. Palmisano | 239,481 | 82,761 | 598,702 | 206,901 | ||||
| David H. Mowry | 107,083 | 37,006 | 214,167 | 74,012 | ||||
| Lance A. Berry | 47,000 | 16,242 | 70,499 | 24,363 | ||||
| Shawn T McCormick | N/A | N/A | N/A | N/A | ||||
| Gregory Morrison | 31,419 | 10,858 | 47,129 | 16,287 | ||||
| Terry M. Rich | 26,478 | 9,150 | 39,717 | 13,726 | ||||
| James A. Lightman | 31,514 | 10,891 | 47,271 | 16,336 | ||||
| Gordon W. Van Ummersen | N/A | N/A | N/A | N/A | ||||
under the heading “
Executive Compensation Tables and Narratives.”Contingent Sign-On Bonus. Under Mr. McCormick’s employment agreement, we agreed to pay him a $75,000 sign-on bonus, contingent on his employment for at least one year. We believe that this payment assisted in our ability to hire Mr. McCormick.
Perquisites and Other Benefits.Our named executive officers receive other benefits, which also are received by our other employees, including the opportunity to purchase our ordinary shares at a discount with payroll deductions under our tax-qualified employee stock purchase plan, and health, dental and life insurance benefits. We provide limited additional modest perquisites provided to our named executive officers only on a case-by-case basis, including the housing stipend for Mowry described above2015 can be found under “Executive Compensation Tables and an automobile allowanceNarratives—All Other Compensation for Mr. Epinette. We provided Mr. Epinette with an automobile allowance on the same basis as other key employees of our French operating subsidiary pursuant to our company policy, which we believe is necessary in light of the competitive market for talent in our industry.2015-Supplemental
.”
basis by reviewing a tally sheet for each executive that summarizes the change in control and severance benefits potentially payable to each executive. We also believe that the form and amount of such benefits are reasonable in light of those provided to executives by companies in our peer group and other companies with which we compete for executive talent and the amount of time typically required to find executive employment opportunities. We, thus, believe we must continue to offer such protections in order to remain competitive in attracting and retaining executive talent.
| Named executive officer | Stock ownership target as a multiple of base salary | In compliance (yes/no) | ||
| Robert J. Palmisano | 4x | Yes | ||
| David H. Mowry | 2x | Yes | ||
| Lance A. Berry | 2x | Yes | ||
| Gregory Morrison | 2x | Yes | ||
| Terry M. Rich | 2x | Yes | ||
| James A. Lightman | 2x | Yes | ||
achieve a result that the compensation committee determines to be appropriate.
Our
This report is dated February 11, 2013.
10-K for the year ended December 27, 2015.
Richard W. Wallman
Tables and Narratives
Information
of which they served as an executive officer.
Name and principal position | Year | Salary(1) ($) | Bonus(2) ($) | Stock awards(3) ($) | Option awards(4) ($) | Non-equity incentive plan compensation(5) ($) | All other compen- sation(6) ($) | Total ($) | ||||||||||||||||||||||||
David H. Mowry(7) President and Chief Executive Officer |
| 2012 2011 |
|
| 341,591 143,844 |
|
| 0 0 |
|
| 192,630 436,313 |
|
| 195,481 539,650 |
|
| 17,666 46,627 |
|
| 42,251 35,706 |
|
| 789,619 1,202,140 |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Douglas W. Kohrs(8) Former President, Chief Executive Officer and Executive Director |
| 2012 2011 2010 |
|
| 421,688 503,422 490,333 |
|
| 0 0 0 |
|
| 428,592 830,340 0 |
|
| 434,944 1,067,336 913,625 |
|
| 0 209,134 236,994 |
|
| 127,541 0 0 |
|
| 1,412,765 2,610,232 1,640,952 |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Shawn T McCormick(9) Chief Financial Officer | 2012 | 114,198 | 75,000 | 354,488 | 357,207 | 5,710 | 0 | 906,603 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Carmen L. Diersen(10) Former Global Chief |
| 2012 2011 2010 |
|
| 182,342 333,193 172,500 |
|
| 0 0 0 |
|
| 0 249,984 0 |
|
| 0 321,509 1,711,935 |
|
| 0 106,888 70,691 |
|
| 186,263 7,350 184,866 |
|
| 368,605 1,018,924 2,139,992 |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Terry M. Rich(11) Senior Vice President, U.S. Commercial Operations | 2012 | 282,468 | 0 | 614,993 | 735,654 | 21,185 | 0 | 1,654,300 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Stéphan Epinette(12) Vice President, International Commercial Operations |
| 2012 2011 2010 |
|
| 297,688 299,620 275,303 |
|
| 0 28,636 0 |
|
| 143,323 186,186 0 |
|
| 145,192 236,519 365,450 |
|
| 48,962 81,960 92,843 |
|
| 87,988 99,002 98,715 |
|
| 723,153 931,923 832,311 |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Kevin M. Klemz(13) Vice President, Chief Legal Officer and Secretary |
| 2012 2011 2010 |
|
| 285,690 276,806 81,865 |
|
| 0 0 0 |
|
| 127,903 166,320 0 |
|
| 129,805 213,640 899,925 |
|
| 22,825 74,730 26,839 |
|
| 7,350 7,350 0 |
|
| 573,573 738,846 1,008,629 |
| ||||||||
| Name and principal position | Year | Salary(1) ($) | Bonus(2) ($) | Stock awards(3) ($) | Option awards(4) ($) | Non-equity incentive plan compensation(5) ($) | All other compen-sation(6) ($) | Total ($) | |||||||||||||||
Robert J. Palmisano(7) President and Chief Executive Officer and Executive Director | 2015 | 222,068 | — | 5,972,830 | 5,914,722 | 1,247,655 | 1,668,463 | 15,025,738 | |||||||||||||||
David H. Mowry(8) Executive Vice President and Chief Operating Officer and Executive Director | 2015 | 544,527 | — | 2,289,191 | 2,266,933 | 579,070 | 947,471 | 6,627,192 | |||||||||||||||
| 2014 | 548,613 | — | 649,995 | 655,281 | 568,632 | 7,350 | 2,375,238 | ||||||||||||||||
| 2013 | 444,334 | — | 687,758 | 689,921 | 513,999 | 27,673 | 1,955,971 | ||||||||||||||||
Lance A. Berry(9) Senior Vice President and Chief Financial Officer | 2015 | 105,894 | — | 837,275 | 829,143 | 343,379 | 253,346 | 2,369,037 | |||||||||||||||
Shawn T McCormick(10) Former Chief Financial Officer | 2015 | 368,935 | — | — | — | 232,224 | 1,144,672 | 1,745,831 | |||||||||||||||
| 2014 | 364,433 | — | 456,450 | 217,703 | 211,098 | 4,773 | 1,254,457 | ||||||||||||||||
| 2013 | 354,411 | — | 240,848 | 241,636 | 47,686 | 3,707 | 888,288 | ||||||||||||||||
Gregory Morrison(11) Senior Vice President, Human Resources | 2015 | 316,467 | — | 559,730 | 554,282 | 174,947 | 566,958 | 2,172,384 | |||||||||||||||
| 2014 | 297,730 | — | 658,265 | 178,716 | 137,194 | 6,954 | 1,278,859 | ||||||||||||||||
Terry M. Rich(12) Senior Vice President, U.S. Commercial Operations | 2015 | 363,097 | — | 471,703 | 467,112 | 316,309 | 475,419 | 2,093,640 | |||||||||||||||
| 2014 | 368,726 | — | 458,941 | 220,230 | 380,525 | — | 1,428,422 | ||||||||||||||||
| 2013 | 358,823 | — | 244,116 | 244,915 | 16,093 | — | 863,947 | ||||||||||||||||
James A. Lightman(13) Senior Vice President, General Counsel and Secretary | 2015 | 97,295 | — | 561,420 | 555,955 | 253,015 | 285,730 | 1,753,415 | |||||||||||||||
Gordon W. Van Ummersen(14) Former Senior Vice President, Global Product Delivery | 2015 | 357,149 | — | — | — | 324,769 | 1,107,650 | 1,789,568 | |||||||||||||||
| 2014 | 325,533 | — | 408,842 | 169,712 | 207,951 | 37,350 | 1,149,388 | ||||||||||||||||
| 2013 | 196,314 | 80,000 | 475,161 | 476,721 | 26,414 | 21,510 | 1,276,120 | ||||||||||||||||
| (1) |
| (2) | We generally do not pay any discretionary bonuses or bonuses that are subjectively determined and did not pay any such bonuses to any named executive officers in |
| (3) |
| (4) |
Grant date | Grant date fair value per share ($) | Risk free interest rate | Expected life | Expected volatility | Expected dividend yield | |||||||||||||||
03/12/2012 | 11.04 | 1.20 | % | 6.11 years | 48.65 | % | 0 | |||||||||||||
08/10/2012 | 8.37 | 0.93 | % | 6.11 years | 48.14 | % | 0 | |||||||||||||
08/28/2012 | 8.30 | 0.95 | % | 6.25 years | 47.94 | % | 0 | |||||||||||||
09/04/2012 | 8.38 | 0.85 | % | 6.11 years | 48.03 | % | 0 | |||||||||||||
08/12/2011 | 11.13 | 1.29 | % | 6.11 years | 48.33 | % | 0 | |||||||||||||
05/12/2011 | 12.34 | 2.26 | % | 6.11 years | 48.60 | % | 0 | |||||||||||||
06/21/2010 | 11.41 | 2.42 | % | 6.11 years | 50.57 | % | 0 | |||||||||||||