UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended MarchDecember 31, 2013
OR 15(d)
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ______________ to ______________
Commission File Number 000-54896
Intra-Cellular Therapies, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 36-4742850 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer | |
Identification No.) |
430 East 29th Street New York, New York 10016 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code (212) 923-3344 |
Title of each class | Name of each exchange on which registered | |
| Common Stock, $0.0001 Par Value Per Share | The NASDAQ Global Select Market |
Securities registered underpursuant to Section 12(g) of the Exchange Act:
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o ¨No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o ¨No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes xNo o¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website,Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes xNo o¨
Indicate by check mark if there is no disclosure of delinquent filers in responsepursuant to Item 405 of Regulation S-K (§229.405 of this chapter)is not contained herein, and no disclosure will not be contained, to the best of registrant'sregistrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large | ||||||
| accelerated filer | x | Accelerated filer | ¨ | |||
| Non-accelerated | ||||||
| Smaller reporting company | ¨ | |||||
Indicate by check mark whether the issuerregistrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes x ¨No ox
The aggregate market value of the registrant’s voting and non-voting common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate) computed by reference to the price at which the common stock was last sold as of the last business day of the registrant’s most recently completed second fiscal quarter was $750.7 million.
As of March 31, 2013, there were no non-affiliate holders of common stock ofFebruary 25, 2016, the Company.
| Page | ||||||
| 4 | ||||||
Item 1. | Business | 4 | ||||
Item 1A. | Risk Factors | 29 | ||||
Item 1B. | Unresolved Staff Comments | 52 | ||||
Item 2. | Properties | 52 | ||||
Item 3. | Legal Proceedings | 52 | ||||
Item 4. | Mine Safety Disclosures | 52 | ||||
| 53 | ||||||
Item 5. | 53 | |||||
Item 6. | Selected Financial Data | 55 | ||||
Item 7. | Management’s Discussion And Analysis Of Financial Condition And Results Of Operations | 56 | ||||
Item 7A. | Quantitative And Qualitative Disclosures About Market Risk | 71 | ||||
Item 8. | Financial Statements And Supplementary Data | 71 | ||||
Item 9. | Changes In And Disagreements With Accountants On Accounting And Financial Disclosure | 71 | ||||
Item 9A. | Controls And Procedures | 71 | ||||
Item 9B. | Other Information | 73 | ||||
| 74 | ||||||
Item 10. | Directors, Executive Officers And Corporate Governance | 74 | ||||
Item 11. | Executive Compensation | 74 | ||||
Item 12. | Security Ownership Of Certain Beneficial Owners And Management And Related Stockholder Matters | 74 | ||||
Item 13. | Certain Relationships And Related Transactions, And Director Independence | 74 | ||||
Item 14. | Principal Accounting Fees And Services | 74 | ||||
| 75 | ||||||
Item 15. | Exhibits, Financial Statement Schedules | 75 | ||||
| Signatures | 79 | |||||
DOCUMENTS INCORPORATED BY REFERENCE
The following documents (or parts thereof) are incorporated by reference into the following parts of this Form 10-K: Certain statements madeinformation required in Part III of this Annual Report on Form 10-K is incorporated by reference from the Registrant’s Proxy Statement for the 2016 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission.
All brand names or trademarks appearing in this report are “forward-looking statements” (within the meaningproperty of their respective holders. Use or display by us of other parties’ trademarks, trade dress, or products in this report is not intended to, and does not, imply a relationship with, or endorsements or sponsorship of, us by the Private Securities Litigation Reform Acttrademark or trade dress owners. Unless the context requires otherwise, references in this report to the “Company,” “we,” “us,” and “our” refer to Intra-Cellular Therapies, Inc. and its wholly-owned subsidiary, ITI, Inc.
| Item 1. | BUSINESS |
Overview
We are a biopharmaceutical company focused on the discovery and clinical development of 1995) regardinginnovative, small molecule drugs that address underserved medical needs in neuropsychiatric and neurological disorders by targeting intracellular signaling mechanisms within the planscentral nervous system, or CNS. ITI-007 is our lead drug development candidate with mechanisms of action that, we believe, may represent an effective treatment across multiple therapeutic indications. In our pre-clinical and objectivesclinical trials to date, ITI-007 combines potent serotonin 5-HT2A receptor antagonism, dopamine receptor phosphoprotein modulation, or DPPM, glutamatergic modulation, and serotonin reuptake inhibition into a single drug candidate for the treatment of managementacute and residual schizophrenia and the treatment of bipolar disorder, including bipolar depression. At dopamine D2 receptors, ITI-007 has been demonstrated to have dual properties and to act as both a pre-synaptic partial agonist and a post-synaptic antagonist. ITI-007 has also been demonstrated to have affinity for future operations. Such statements involve knowndopamine D1 receptors and unknown risks, uncertaintiesindirectly stimulate phosphorylation of glutamatergic NMDA GluN2B receptors in a mesolimbic specific manner. We believe that this regional selectivity in brain areas thought to mediate the efficacy of antipsychotic drugs, together with serotonergic, glutamatergic, and dopaminergic interactions, may result in efficacy for a broad array of symptoms associated with schizophrenia and bipolar disorder with improved psychosocial function. The serotonin reuptake inhibition potentially allows for antidepressant activity in the treatment of schizoaffective disorder, other disorders with co-morbid depression, and/or as a stand-alone treatment for major depressive disorder. We believe ITI-007 may also be useful for the treatment of other psychiatric and neurodegenerative disorders, particularly behavioral disturbances associated with dementia, autism, and other factors that may cause actualCNS diseases. ITI-007 is in Phase 3 clinical development as a novel treatment for schizophrenia and bipolar depression.
ITI-007 for the Treatment of Schizophrenia
In September 2015, we announced top-line clinical results performancefrom our first Phase 3 clinical trial of ITI-007 for the treatment of patients with schizophrenia. This randomized, double-blind, placebo-controlled Phase 3 clinical trial was conducted at 12 sites in the United States with 450 patients randomized (1:1:1) to receive either 60 mg of ITI-007, 40 mg of ITI-007 or achievementsplacebo once daily in the morning for 28 days. The pre-specified primary efficacy measure was change from baseline versus placebo at study endpoint (4 weeks) on the centrally rated Positive and Negative Syndrome Scale, or PANSS, total score. In this trial, the once-daily dose of Oneida Resources Corp. (the “Company”)60 mg of ITI-007 met the primary endpoint and demonstrated antipsychotic efficacy with statistically significant superiority over placebo at week 4 (study endpoint) with additional improvements observed in social function. Moreover, the 60 mg dose of ITI-007 showed significant antipsychotic efficacy as early as week 1, which was maintained at every time point throughout the entire study. ITI-007 showed a dose-related improvement in symptoms of schizophrenia with the 40 mg dose approximating the trajectory of improvement seen with the 60 mg dose, but the effect with 40 mg did not reach statistical significance on the primary endpoint. In addition, the 60 mg dose of ITI-007 met the key secondary endpoint of statistically significant improvement on the Clinical Global Impression Scale for Severity of Illness, or CGI-S. The 40 mg dose of ITI-007 also demonstrated a statistically significant improvement versus placebo on the CGI-S, though not formally tested against placebo as a key secondary endpoint since it did not separate on the primary endpoint. Consistent with previous studies, ITI-007 had a favorable safety and tolerability profile as evidenced by motoric, metabolic, and cardiovascular characteristics similar to be materially differentplacebo, and no clinically significant changes in akathisia, extrapyramidal symptoms, prolactin, body weight, glucose, insulin, or lipids.
In September 2015, we also announced top-line data from any future results, performancean open-label positron emission tomography, or achievements expressed or implied by such forward-looking statements. The forward-looking statements included herein are based on current expectations that involve numerous risksPET, study of ITI-007 examining brain occupancy of striatal D2 receptors. This study was conducted in patients diagnosed with schizophrenia who were otherwise healthy and uncertainties. The Company's plans and objectives are based, in part, on assumptions involving the continued expansion of business. Assumptions relating to the foregoing involve judgmentsstable with respect to amongtheir psychosis. After washout from their previous antipsychotic medication for at least two weeks, PET was used to determine target occupancy in brain regions at baseline (drug-free) and again after two weeks of once daily ITI-007 oral administration. In this trial, the 60 mg dose of ITI-007 was associated with a mean of approximately 40% striatal dopamine D2 receptor occupancy. As predicted by preclinical and earlier clinical data, ITI-007 demonstrated antipsychotic effect at relatively low striatal D2 receptor occupancy, lower than the occupancy range required by most other things, future economic, competitiveantipsychotic drugs. Unlike any existing schizophrenia treatment, this dopamine receptor phosphoprotein modulator, or DPPM, acts as a pre-synaptic partial agonist and market conditionspost-synaptic antagonist at D2 receptors. We believe this mechanism likely contributes to the favorable safety profile of ITI-007, with reduced risk for hyperprolactinemia, akathisia, extrapyramidal symptoms, and future business decisions, allother motoric side effects.
The top-line results from our first Phase 3 clinical trial of ITI-007 confirm the earlier Phase 2 results that we announced in December 2013, in which ITI-007 exhibited antipsychotic efficacy in a randomized, double-blind, placebo and active controlled clinical trial in patients with an acutely exacerbated episode of schizophrenia. In this Phase 2 trial, 335 patients were randomized to receive one of four treatments: 60 mg of ITI-007, 120 mg of ITI-007, 4 mg of risperidone (active control) or placebo in a 1:1:1:1 ratio, orally once daily for 28 days. The primary endpoint for this clinical trial was change from baseline to Day 28 on the PANSS total score. In this study, ITI-007 met the trial’s pre-specified primary endpoint, improving symptoms associated with schizophrenia as measured by a statistically significant and clinically meaningful decrease in the PANSS total score. The trial also met key secondary outcome measures related to efficacy on PANSS subscales and safety. We are difficultalso conducting a second Phase 3 clinical trial in schizophrenia that we initiated in the second quarter of 2015, with over 600 patients planned to be enrolled in the trial. In this trial, we are randomizing patients to two doses of ITI-007 (60 mg or impossible20 mg), risperidone (active control) or placebo over a 6-week treatment duration, and the primary outcome measure is change from baseline to predict accuratelyDay 42 on the PANSS total score. We expect patient enrollment will be completed in the second quarter of 2016. Subject to timely enrollment, we anticipate top-line results from the second Phase 3 clinical trial will be available in mid-2016.
In addition to our two Phase 3 clinical trials, we will need to complete other clinical and non-clinical trials and manufacturing and pre-commercialization activities necessary to support the submission of a planned New Drug Application, or NDA, for ITI-007 in schizophrenia, which we currently expect could occur in the first half of 2017.
ITI-007 for the Treatment of Depressive Episodes Associated with Bipolar Disorder (Bipolar Depression)
Our bipolar depression program consists of two Phase 3 multi-center, randomized, double-blind, placebo-controlled clinical trials: one to evaluate ITI-007 as a monotherapy and the other to evaluate ITI-007 as an adjunctive therapy with lithium or valproate. In each trial, approximately 550 patients with a clinical diagnosis of Bipolar I or Bipolar II disorder and who are experiencing a current major depressive episode will be randomized to receive one of three treatments: 60 mg ITI-007, 40 mg ITI-007, or placebo in a 1:1:1 ratio orally once daily for 6 weeks. In the ITI-007-401 trial, patients will receive ITI-007 or placebo as a monotherapy. In the ITI-007-402 trial, patients will receive ITI-007 or placebo adjunctive to their existing mood stabilizer lithium or valproate. We initiated our bipolar depression program in the third quarter of 2015.
The primary endpoint for both clinical trials is change from baseline at Day 42 on the Montgomery-Åsberg Depression Rating Scale, or MADRS, total score versus placebo. The MADRS is a well-validated 10-item checklist that measures the ability of a drug to reduce overall severity of depressive symptoms. Individual items are rated by an expert clinician on a scale of 0 to 6 in which a score of 6 represents the most depressed evaluation for each item assessed. The total score ranges from 0 to 60. Secondary endpoints include measures of social function and quality of life that may illustrate the differentiated clinical profile of ITI-007. Safety and tolerability are also assessed in both clinical trials.
Other Indications for ITI-007
In the fourth quarter of 2014, we announced the top-line data from ITI-007-200, a Phase 1/2 clinical trial designed to evaluate the safety, tolerability and pharmacokinetics of low doses of ITI-007 in healthy geriatric subjects and in patients with dementia, including Alzheimer’s disease. The completion of this study marks an important milestone in our strategy to develop low doses of ITI-007 for the treatment of behavioral disturbances associated with dementia and related disorders. The ITI-007-200 trial results to date indicate that ITI-007 is safe and well-tolerated across a range of low doses, has linear- and dose-related pharmacokinetics and improves cognition in the elderly. The most frequent adverse event was mild sedation at the higher doses. We believe these results further position ITI-007 as a development candidate for the treatment of behavioral disturbances in patients with dementia and other neuropsychiatric and neurological conditions. We plan to initiate late phase clinical programs evaluating ITI-007 in patients with behavioral disturbances associated with dementia and related disorders, including Alzheimer’s disease, in the first half of 2016.
We are also pursuing clinical development of ITI-007 for the treatment of additional CNS diseases and disorders. At the lowest doses, ITI-007 has been demonstrated to act primarily as a potent 5-HT2A serotonin receptor antagonist. As the dose is increased, additional benefits are derived from the engagement of additional drug targets, including modest dopamine receptor modulation and modest inhibition of serotonin transporters. We believe that combined interactions at these receptors may provide additional benefits above and beyond selective 5-HT2A antagonism for treating agitation, aggression and sleep disturbances in diseases that include dementia, Alzheimer’s disease, Huntington’s disease and autism spectrum disorders, while avoiding many of the side effects associated with more robust dopamine receptor antagonism. As the dose of ITI-007 is further increased, leading to moderate dopamine receptor modulation, inhibition of serotonin transporters, and indirect glutamate modulation, these actions complement the complete blockade of 5-HT2A serotonin receptors. At a dose of 60 mg, ITI-007 has been shown effective in treating the symptoms associated with schizophrenia, and we believe this higher dose range will be useful for the treatment of bipolar disorder, depressive disorders and other neuropsychiatric diseases.
Given the potential utility for ITI-007 and follow-on compounds to treat these additional indications, we may investigate, either on our own or with a partner, agitation, aggression and sleep disturbances in additional diseases that include autism spectrum disorders; depressive disorder; intermittent explosive disorder; non-motor symptoms and motor complications associated with Parkinson’s disease; and post-traumatic stress disorder. We hold exclusive, worldwide commercialization rights to ITI-007 and a family of compounds from Bristol-Myers Squibb Company pursuant to an exclusive license.
Other Product Candidates
We have a second major program called ITI-002 that has yielded a portfolio of compounds that selectively inhibits the enzyme phosphodiesterase type 1, or PDE1. We believe PDE1 helps regulate brain activity related to cognition, memory processes and movement/coordination. On February 25, 2011, we (through our wholly owned operating subsidiary, ITI) and Takeda Pharmaceutical Company Limited, or Takeda, entered into a license and collaboration agreement, or the Takeda License Agreement, under which are beyondwe agreed to collaborate to research, develop and commercialize our proprietary compound ITI-214 and other selected compounds that selectively inhibit PDE1 for use in the controlprevention and treatment of human diseases. On October 31, 2014, we entered into an agreement with Takeda terminating the Takeda License Agreement, or the Termination Agreement, pursuant to which all rights granted under the Takeda License Agreement were returned to us. On September 15, 2015, Takeda completed the transfer of the Company. AlthoughInvestigational New Drug application, or IND, for ITI-214 to us. ITI-214 is the Company believesfirst compound in its assumptions underlyingclass to successfully advance into Phase 1 clinical trials. We intend to pursue the forward-looking statements are reasonable, anydevelopment of our PDE program, including ITI-214 for the assumptions could prove inaccuratetreatment of several CNS and therefore, there can be no assurance the forward-looking statements includednon-CNS conditions, which may include cognition in this Report will prove to be accurate. In light of the significant uncertainties inherentParkinson’s disease, cognition in Alzheimer’s disease, cognition in schizophrenia and in other non-CNS indications. Other compounds in the forward-looking statements included herein,PDE portfolio are also being advanced for the inclusiontreatment of such information should not be regarded asvarious indications.
Our pipeline also includes pre-clinical programs that are focused on advancing drugs for the treatment of schizophrenia, Parkinson’s disease, Alzheimer’s disease and other neuropsychiatric and neurodegenerative disorders. We are also investigating the development of treatments for disease modification of neurodegenerative disorders and non-CNS diseases.
We have assembled a representation bymanagement team with significant industry experience to lead the Company or anydiscovery and development of our product candidates. We complement our management team with a group of scientific and clinical advisors that includes recognized experts in the fields of schizophrenia and other person that the objectives and plansCNS disorders, including Nobel laureate, Dr. Paul Greengard, one of the Company will be achieved.
We were originally incorporated in the State of Delaware in August 2012 under the name “Oneida Resources Corp.” Prior to a reverse merger that occurred on August 29, 2012 and maintains its principal executive office at c/o Samir Masri CPA Firm P.C., 175 Great Neck Road, Suite 403, Great Neck, NY 11021. Since inception,2013, or the Company has been engaged in organizational efforts and obtaining initial financing. The CompanyMerger, Oneida Resources Corp. was formed as a vehicle to investigate and, if such investigation warrants, acquire a target“shell” company or business seeking the perceived advantages of being a publicly held corporation. The Company filed a registration statement on Form 10 with the U.S. Securities and Exchange Commission (the “SEC”) on February 8, 2013, and since its effectiveness, the Company has focused on identifying a possible business combination. The Company selected March 31 as its fiscal year end.
Our corporate headquarters and laboratory are located at 430 East 29th Street, New York, New York 10016, and our telephone number is (646) 440-9333. We also have an office in Towson, Maryland. We maintain a website at www.intracellulartherapies.com, to which we regularly post copies of our press releases as well as additional information about us. Our filings with the SEC will be available free of charge through the Investor Relations section of our website as soon as reasonably practicable after being electronically filed with or furnished to the SEC. Information contained in our website does not constitute a part of this report or our other filings with the SEC.
Our Strategy
Our goal is to discover and develop novel small molecule therapeutics for the treatment of CNS diseases in order to improve the lives of people suffering from such illnesses. Using our key understanding of intracellular signaling, we seek to accomplish our goal, using our in-house expert drug discovery and clinical development teams, in two ways:
The key elements of our strategy are to:
Our Drug Discovery Platform and Capabilities
Based on the pioneering efforts of our co-founder and Nobel laureate, Dr. Paul Greengard, we have developed a detailed understanding of intracellular signaling pathways and intracellular targets. We have used that knowledge to develop several state of the art technology platforms, including one called CNSProfileTM. This technology monitors the phosphoprotein changes elicited by major psychotropic drug classes and subclasses, and generates a unique molecular signature for drug compounds. By monitoring how the levels of these phosphoproteins changein vivo, we identify intracellular signaling pathways through which several major drug classes operate. Along with what we believe to be state of the art drug discovery efforts, we have used, and may continue to use, this information as a tool to validate our selection of preclinical candidate molecules.
During the years ended December 31, 2015 and 2014, we incurred $87.7 million and $21.2 million in research and development expenses, respectively.
Given the nature of our research and development and business activities, we do not expect that compliance with federal, state and local environmental laws will result in material costs or have a significant negative effect on our operations.
Disease and Market Overview
Our programs for small molecule therapeutics are designed to address various CNS diseases that we believe are underserved or unmet by currently available therapies and that represent large potential commercial market opportunities for us. Background information on the CNS diseases and related commercial markets that may be addressed by our programs is set forth below.
Schizophrenia
Schizophrenia is a disabling and chronic mental illness that is characterized by multiple symptoms during an acute phase of the disorder that can include so-called “positive” symptoms, such as hearing voices, grandiose beliefs and suspiciousness or paranoia. These symptoms can be accompanied by additional, harder to treat symptoms, such as social withdrawal, blunted emotional response and speech deficits, collectively referred to as “negative” symptoms, difficulty concentrating and disorganized thoughts, or cognitive impairment, depression and insomnia. Such residual symptoms often persist even after the acute positive symptoms subside, and contribute substantially to the social and employment disability associated with schizophrenia. Current antipsychotic medications provide some relief for the symptoms associated with the acute phase of the disorder, but they do not effectively treat the residual phase symptoms associated with chronic schizophrenia. Currently available medications used to treat acute schizophrenia are limited in their use due to side effects that can include movement disorders, weight gain, metabolic disturbances, and cardiovascular disorders. Indeed, the side effects associated with current antipsychotic medications often make some of the residual phase symptoms, such as negative symptoms and social function, worse. There is an unmet medical need for new therapies that have improved side effect and efficacy profiles.
According to the National Institute of Mental Health, over 1% of the world’s population suffers from schizophrenia, and more than 2.5 million Americans suffer from the illness in any given year. Worldwide sales of antipsychotic drugs exceeded $14 billion in 2014. These drugs have been increasingly used by physicians to address a range of disorders in addition to schizophrenia, including bipolar disorder and a variety of psychoses and related conditions in elderly patients. Despite their commercial success, current antipsychotic drugs have substantial limitations, including inadequate efficacy and severe side effects.
The first-generation, or typical, antipsychotics that were introduced in the late-1950s block dopamine receptors. While typical antipsychotics are effective against positive symptoms of schizophrenia in many patients, these drugs often induce disabling motor disturbances, and they fail to address or worsen most of the negative symptoms and cognitive disturbances associated with schizophrenia.
Most schizophrenia patients in the United States are treated today with second-generation, or atypical, antipsychotics, which induce fewer motor disturbances than typical antipsychotics, but still fail to address most of the negative symptoms of schizophrenia and other symptoms associated with social function impairment. Many patients with schizophrenia have deficits in social function. Social function is the ability to recognize, understand, process and use external cues to solve problems, maintain work performance, and conduct interpersonal relationships. Deficits in social function often remain after positive symptoms, such as hallucinations and delusions, have resolved in these patients. In addition, currently prescribed treatments do not effectively address or may exacerbate cognitive disturbances associated with schizophrenia. It is believed that the efficacy of atypical antipsychotics is due to their interactions with dopamine and 5-HT2A receptors. The side effects induced by the atypical agents may include weight gain, non-insulin dependent (type II) diabetes, cardiovascular side effects, sleep disturbances, and motor disturbances. We believe that these side effects generally arise either from non-essential receptor interactions or from excessive dopamine blockade.
The limitations of currently available antipsychotics result in poor patient compliance. A landmark study funded by the National Institute of Mental Health, the Clinical Antipsychotic Trials of Intervention Effectiveness, also referred to as CATIE, which was published in The New England Journal of Medicine in September 2005, found that 74% of patients taking typical or atypical antipsychotics discontinued treatment within 18 months because of side effects or lack of efficacy. We believe there is a large underserved medical need for new therapies that have improved side effect and efficacy profiles.
Bipolar Disorder
Bipolar disorder, sometimes referred to as manic-depressive illness, is characterized by extreme shifts in mood. Individuals with bipolar disorder may experience intense feelings of over-excitement, irritability, and impulsivity with grandiose beliefs and racing thoughts, referred to as a manic episode. Symptoms of depression may include feeling tired, hopeless and sad, with difficulty concentrating and thoughts of suicide. Some people experience both types of symptoms in the same “mixed” episode. Severe symptoms of bipolar disorder can be associated with hallucinations or delusions, otherwise referred to as psychosis.
Bipolar disorder affects approximately 5.7 million adults in the United States in any given year, or about 2.6 percent of the adult U.S. population. In 2012, therapeutics used to treat bipolar disorder had global sales of approximately $6 billion.
Bipolar disorder is often treated with antipsychotic medications alone or in combination with mood stabilizers. The side effects and safety risks associated with antipsychotic drugs in patients with bipolar disorder are similar to those experienced by patients with schizophrenia. Moreover, a large national research program conducted from 1998 to 2005 called the Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, followed 4,360 patients with bipolar disorder long term and showed that about half of patients who were treated for bipolar disorder still experienced lingering and recurrent symptoms, indicating a clear need for improved treatments.
Behavioral Disturbances in Dementia, Including Alzheimer’s Disease
It has been estimated that 44.4 million people worldwide were living with dementia in 2013, including over 5.2 million patients with Alzheimer’s disease in the United States. This number is expected to increase to 75.6 million by 2030 and to increase to 135.5 million by 2050. While the diagnostic criteria for Alzheimer’s disease and other dementias mostly focus on the related cognitive deficits, it is often the behavioral and psychiatric symptoms that are most troublesome for caregivers and lead to poor quality of life for patients.
Several behavioral symptoms are quite prevalent in patients with dementia, including patients with Alzheimer’s disease. In view of the potential multiple effects of ITI-007 on aggression, agitation, sleep disorders and depression, and its safety profile to date, we believe that ITI-007 may provide a novel therapy for treating the behavioral disturbances accompanying dementia, including Alzheimer’s disease.
The FDA has not approved any drug to treat the behavioral symptoms of dementia, including Alzheimer’s disease. As symptoms progress and become more severe, physicians often resort to off-label use of antipsychotic medications in these patients. Current antipsychotic drugs are associated with a number of side effects, which can be problematic for elderly patients with dementia. In addition, antipsychotic drugs may exacerbate the cognitive disturbances associated with dementia. We believe there is a large unmet medical need for a safe and effective therapy to treat the behavioral symptoms in patients with dementia, including Alzheimer’s disease.
Alzheimer’s Disease
Alzheimer’s disease is a progressive neurodegenerative disorder that slowly destroys memory and thinking skills, and eventually even the ability to carry out simple tasks. Its symptoms include cognitive dysfunction, memory abnormalities, progressive impairment in activities of daily living, and a host of behavioral and neuropsychiatric symptoms. Alzheimer’s disease primarily affects older people and, in most cases, symptoms first appear after age 60. Alzheimer’s disease gets worse over time and is fatal.
The market for Alzheimer’s disease therapeutics is categorized into two segments: acetylcholinesterase inhibitors and NMDA receptor antagonists, which include Aricept®, Namenda®, Exelon® and Ebixa®. These two segments had total sales of $4.9 billion in 2013.
According to the Alzheimer’s Association, 5.2 million people in the United States are living with Alzheimer’s disease, and it is currently the fifth leading cause of death for people age 65 and older. It has been estimated that 44.4 million people worldwide were living with dementia in 2013. This number is expected to increase to 75.6 million by 2030 and to increase to 135.5 million by 2050. While the diagnostic criteria for Alzheimer’s disease mostly focus on the related cognitive deficits, it is often the behavioral and psychiatric symptoms that are most troublesome for caregivers and lead to poor quality of life for patients. These symptoms include agitation, aggressive behaviors, depression, sleep disorders, and psychosis. Studies have suggested that approximately 60% of patients with Alzheimer’s disease experience agitation/aggression, up to 87% of patients experience depression, approximately 60% of patients experience sleep disturbances, particularly as an increased likelihood of day-night reversal, and approximately 20% to 51% of Alzheimer’s disease patients may develop psychosis at some point in the disease process, commonly consisting of hallucinations and delusions. The diagnosis of Alzheimer’s disease psychosis is associated with more rapid cognitive and functional decline and institutionalization. Sleep disturbances increase the likelihood of day-night reversion, increased agitation and increased caregiver stress that strongly influences decisions for nursing home placement.
The FDA has not approved any drug to treat the behavioral symptoms of Alzheimer’s disease. As symptoms progress and become more severe, physicians often resort to off-label use of antipsychotic medications in these patients. Current antipsychotic drugs are associated with a number of side effects, which can be problematic for elderly patients with Alzheimer’s disease. In addition, antipsychotic drugs may exacerbate the cognitive disturbances associated with Alzheimer’s disease. Current antipsychotic drugs also have a boxed warning for use in elderly patients with dementia-related psychosis due to increased mortality and morbidity. There is a large unmet medical need for a safe and effective therapy to treat the behavioral symptoms in patients with Alzheimer’s disease.
Parkinson’s Disease
Parkinson’s disease is a chronic and progressive neurodegenerative disorder that involves malfunction and death of neurons in a region of the brain that controls movement. This neurodegeneration creates a shortage of an important brain signaling chemical, or neurotransmitter, known as dopamine, thereby rendering patients unable
to direct or control their movements in a normal manner. Parkinson’s disease is characterized by well-known motor symptoms, including tremors, limb stiffness, slowness of movements, and difficulties with posture and balance, as well as by non-motor symptoms, which include sleep disturbances, mood disorders, cognitive impairment and psychosis. Parkinson’s disease progresses slowly in most people and the severity of symptoms tends to worsen over time.
Parkinson’s disease is the second most common neurodegenerative disorder after Alzheimer’s disease. According to the National Parkinson Foundation, about 1 million people in the United States and from approximately 4 to 6 million people worldwide suffer from this disease. Parkinson’s disease is more common in people over 60 years of age, and the prevalence of this disease is expected to increase significantly as the average age of the population increases. Parkinson’s disease patients are commonly treated with dopamine replacement therapies, such as levodopa, commonly referred to as L-DOPA, which is metabolized to dopamine, and dopamine agonists, which are molecules that mimic the action of dopamine. Sales of therapeutics such as L-DOPA and dopamine agonists used to treat the motor symptoms of the disease reached $2.3 billion in 2013.
Non-motor symptoms can be particularly distressing and even more troublesome to patients with Parkinson’s disease than the primary motor disturbances. Non-motor symptoms substantially contribute to the burden of Parkinson’s disease and deeply affect the quality of life of patients and their caregivers. Non-motor symptoms of Parkinson’s disease are associated with increased caregiver stress and burden, nursing home placement, and increased morbidity and mortality.
Treatment of non-motor symptoms associated with Parkinson’s disease poses a challenge to physicians. Current dopamine replacement drugs used to treat the motor symptoms of Parkinson’s disease do not help, and sometimes worsen, the non-motor symptoms. No drugs are currently approved by the FDA for treating the broad non-motor symptoms associated with Parkinson’s disease, and this remains a large unmet medical need.
Depression
Major depressive disorder, or MDD, is a brain disorder that can be associated with symptoms of sadness, hopelessness, helplessness, feelings of guilt, irritability, loss of interest in formerly pleasurable activities, cognitive impairment, disturbed sleep patterns, and suicide ideation or behavior. Different people may experience different symptoms, but everyone with major depression experiences symptoms that are severe enough to interfere with everyday functioning, such as the ability to concentrate at work or school, social interactions, eating and sleeping. Sometimes the depressive episode can be so severe it is accompanied by psychosis (hallucinations and delusions). According to the National Institute of Mental Health, approximately 3% of teenagers and approximately 7% of adults experience MDD each year. Worldwide sales of antidepressant drugs reached $9.3 billion in 2013. The antidepressant market is primarily composed of selective serotonin reuptake inhibitors such as Lexapro® (marketed by Forest Laboratories and Lundbeck) and selective norepinephrine reuptake inhibitors, or SNRIs, such as Cymbalta® (marketed by Eli Lilly). Antipsychotics such as Seroquel® (marketed by Astrazeneca) and Abilify® (marketed jointly by Bristol-Myers Squibb and Otsuka Pharmaceutical) are also used as adjunctive treatments with antidepressant treatment. The National Institute of Mental Health-funded Sequenced Treatment Alternatives to Relieve Depression, or STAR*D, study showed that only one-third of treated patients experience complete remission of depressive symptoms. Nearly two-thirds of patients were considered treatment-resistant.
Our Clinical Programs
Our pipeline includes two product candidates in clinical development and two product candidates in advanced pre-clinical testing. We believe that our product candidates offer innovative therapeutic approaches and may provide significant advantages relative to current therapies. The following table summarizes our product candidates and programs:
OUR THERAPEUTIC PIPELINE
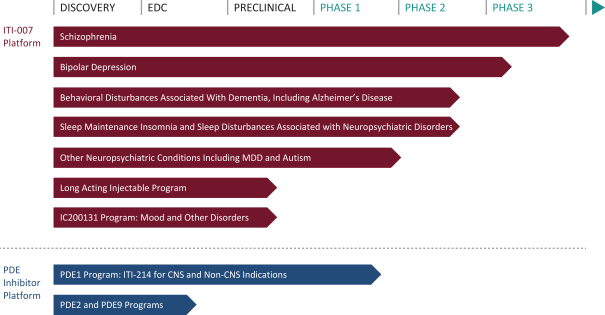
ITI-007 Program
Our lead product candidate, ITI-007, possesses mechanisms of action that we believe may represent an effective treatment across multiple therapeutic indications. ITI-007 is in Phase 3 clinical trials for the treatment of schizophrenia. In our pre-clinical and clinical trials to date, we have demonstrated that ITI-007 combines potent serotonin 5-HT2A receptor antagonism, dopamine receptor phosphoprotein modulation, or DPPM, glutamatergic modulation and serotonin reuptake inhibition into a single drug candidate for the treatment of acute and residual schizophrenia. At dopamine D2 receptors, ITI-007 has been demonstrated to have dual properties and to act as both a pre-synaptic partial agonist and a post-synaptic antagonist. ITI-007 has also been demonstrated to have affinity for dopamine D1 receptors and indirectly stimulate phosphorylation of glutamatergic NMDA NR2B, or GluN2B, receptors in a mesolimbic specific manner. We believe that this regional selectivity in brain areas thought to mediate the efficacy of antipsychotic drugs, together with serotonergic, glutamatergic, and dopaminergic interactions, may result in antipsychotic efficacy for positive, negative, affective and cognitive symptoms associated with schizophrenia. The serotonin reuptake inhibition could allow for antidepressant activity for the treatment of schizoaffective disorder, other disorders with co-morbid depression, and/or as a stand-alone treatment for major depressive disorder. We believe ITI-007 may also be useful for the treatment of bipolar disorder and other psychiatric and neurodegenerative disorders, particularly behavioral disturbances associated with dementia, autism and other CNS diseases.
We believe these features of ITI-007 may be able to improve the quality of life of patients with schizophrenia and enhance social function to allow them to integrate more fully into their families and their
workplaces. In addition, ITI-007 may be shown to treat disorders at either low-doses (e.g., sleep, aggression and agitation) or high-doses (e.g., acute exacerbated and residual schizophrenia, bipolar disorders, and mood disorders).
Phase 1 studies to support multiple clinical indications
We conducted a series of Phase 1 safety studies of ITI-007 in Europe and the United States during the period from 2007 to 2011. All of the studies conducted to date in the United States have been conducted under an Investigational New Drug application, or IND, filed in 2007 by ITI. Data from these studies are being used to support the clinical development of ITI-007 in multiple indications, including schizophrenia, sleep disorders in neuropsychiatric and neurodegenerative disease, major depressive disorders, bipolar disorders, behavioral disturbances in dementia and Alzheimer’s disease, autism, posttraumatic stress disorder, or PTSD, and intermittent explosive disorder, or IED. We have completed the following three Phase 1 trials in healthy volunteers:
We continued Phase 1 development of ITI-007 in patients with schizophrenia in order to advance ITI-007 in this target therapeutic indication. Specifically, we conducted the following additional studies:
ITI-007 for the treatment of exacerbated and residual schizophrenia
In multiple clinical trials of ITI-007 in patients with schizophrenia, the drug candidate has demonstrated clinical signals consistent with reductions in psychosis, depression and insomnia. Reductions in psychosis are consistent with the potential to treat acute schizophrenia, whereas reductions in depression and insomnia are
consistent with the potential to treat residual phase schizophrenia. ITI-007 has been shown to be safe and well-tolerated across a wide range of doses in these studies. Further, at doses that have demonstrated clinical activity, ITI-007 has caused fewer adverse effects than those typically associated with antipsychotic drug treatment, such as impaired motor function. These adverse side effects can be a major cause of patient noncompliance with current antipsychotic therapies and can lead to poorer social function.
Phase 2 Clinical Trial (ITI-007-005)
ITI-007 exhibited antipsychotic efficacy in ITI-007-005, a randomized, double-blind, placebo and active controlled Phase 2 clinical trial in patients with an acutely exacerbated episode of schizophrenia. In December 2013, we announced the clinical results from this Phase 2 trial. In this Phase 2 trial, 335 patients were randomized to receive one of four treatments: 60 mg of ITI-007, 120 mg of ITI-007, 4 mg of risperidone (active control) or placebo in a 1:1:1:1 ratio. Patients received study treatment orally once daily in the morning for 28 days. Of those randomized, 311 patients were included in the intent-to-treat primary analysis. Subject participation lasted approximately 7 to 8 weeks, including a one week screening period, a four week treatment period followed by stabilization on standard of care, and a safety follow up visit approximately two weeks after stabilization. The primary endpoint for this clinical trial was change from baseline to Day 28 on the PANSS total score. The PANSS is a well-validated 30-item rating scale that measures the ability of a drug to reduce schizophrenia symptom severity. The PANSS measures positive symptoms, such as delusions, suspiciousness, and hallucinations; negative symptoms, such as blunted affect, social and emotional withdrawal, and stereotyped thinking; and general psychopathology, such as anxiety, tension, depression, and active social avoidance.
Secondary endpoints in this trial included weekly assessments of the PANSS total score as well as its subscales (Positive Symptom Subscale, Negative Symptom Subscale, and General Psychopathology Subscale) and the Negative Symptom Factor (based on a subset of PANSS questions), individual item response on the PANSS, and the Calgary Depression Scale for Schizophrenia. Safety and tolerability were also assessed.
In December 2013, we announced that topline results from the ITI-007-005 study indicated that ITI-007 met the trial’s pre-specified primary endpoint, improving symptoms associated with schizophrenia as measured by a statistically significant and clinically meaningful decrease in the PANSS total score. The trial also met key secondary outcome measures related to efficacy on PANSS subscales and safety.
Many patients with schizophrenia have deficits in social function. Social function is the ability to recognize, understand, process and use external cues to solve problems, maintain work performance and conduct interpersonal relationships. Deficits in social function often remain after positive symptoms, such as hallucinations and delusions, have resolved in these patients. In the Phase 2 trial, ITI-007 exhibited a differentiating response profile across a broad range of symptoms that we believe is consistent with improvements in these social functioning deficits. The study also showed that ITI-007 was well-tolerated at the tested doses. ITI-007 demonstrated a favorable safety profile in the study without characteristic antipsychotic drug side effects or any serious adverse events.
ITI-007 at a dose of 60 mg demonstrated a statistically significant improvement in psychosis (p = 0.017) on the trial’s pre-specified primary endpoint, which was change from baseline on the PANSS total score, compared to placebo. The primary statistical analysis was pre-specified and used a Mixed-Effect Model Repeated Measure method for handling missing data in the intent-to-treat, or ITT, study population and a Bonferroni procedure to correct for multiple two-sided comparisons (each dose of ITI-007 compared to placebo). The trial’s pre-specified sensitivity analysis on the primary endpoint used the analysis of covariance, or ANCOVA, model and last observation carried forward, or LOCF, method for handling missing data for the ITT population and confirmed the positive outcome with statistically significant improvements compared to placebo in patients receiving the 60 mg dose of ITI-007 (p = 0.011). ITI-007 at a dose of 60 mg also significantly improved the positive symptom subscale (p < 0.05) and the general psychopathology subscale (p < 0.05) on the PANSS after 28 days of treatment using the ANCOVA-LOCF on the ITT population.
The improvement in the PANSS total score in the 120 mg dose group did not reach statistical significance. We believe that it is possible that sedation, the most frequent side effect in the 120 mg dose group, interfered with the ability to detect an efficacy signal at this dose administered once daily in the morning. Approximately 32.5% of subjects randomized to 120 mg of ITI-007 experienced sedation/somnolence, compared to 21% of subjects randomized to risperidone, 17% of subjects randomized to 60 mg of ITI-007, and 13% randomized to placebo. We believe that nighttime administration may be more appropriate for testing the effectiveness of the 120 mg dose of ITI-007 in this patient population. In the trial, the 60 mg dose of ITI-007 was effective when administered once daily in the morning.
Consistent with preliminary indications from the interim analysis and with the drug candidate’s pharmacological profile, ITI-007 at a dose of 60 mg significantly improved certain items on the negative symptom and general psychopathology subscales consistent with improved social function. The study was statistically powered only on the primary endpoint. ITI-007 did significantly improve many secondary endpoints, although the study was not designed for significance on secondary endpoints and was not powered to detect statistical differences in subgroup analyses.
A high percentage (74%) of randomized subjects completed trial participation. Only 19% of subjects discontinued from study treatment during the 28 day study treatment period, and an additional 7% of subjects completed study treatment but were lost to follow up.
In the Phase 2 trial, ITI-007 was well-tolerated. The most frequent AE was sedation, as described above. There were no serious adverse events related to ITI-007. There were no clinically meaningful changes in safety measures with ITI-007. Notably, ITI-007 demonstrated a favorable metabolic profile with no increase of blood levels of glucose, insulin, cholesterol or triglycerides over a four week treatment period. Moreover, in contrast to risperidone, 60 mg of ITI-007 was effective with no difference from placebo on weight change parameters, prolactin levels, extrapyramidal symptoms (EPS) or akathisia. ITI-007 was not associated with EPS as measured by the Simpson-Angus Scale, Barnes Akathisia Rating Scale, or Abnormal Involuntary Movement Scale. There was no increase in suicidal ideation or behavior with ITI-007.
Phase 3 Clinical Trials and Regulatory Plans
We are proceeding with Phase 3 development of ITI-007 for the treatment of schizophrenia. We are conducting two randomized, double-blind, placebo-controlled Phase 3 clinical trials of ITI-007 in patients with acutely exacerbated schizophrenia. In September 2015, we announced top-line clinical results from our first Phase 3 clinical trial of ITI-007 for the treatment of patients with schizophrenia. This randomized, double-blind, placebo-controlled Phase 3 clinical trial was conducted at 12 sites in the United States with 450 patients randomized (1:1:1) to receive either 60 mg of ITI-007, 40 mg of ITI-007 or placebo once daily in the morning for 28 days. The pre-specified primary efficacy measure was change from baseline versus placebo at study endpoint (4 weeks) on the centrally rated Positive and Negative Syndrome Scale, or PANSS, total score. In this trial, the once-daily dose of 60 mg of ITI-007 met the primary endpoint and demonstrated antipsychotic efficacy with statistically significant superiority over placebo at week 4 (study endpoint) with additional improvements observed in social function. Moreover, the 60 mg dose of ITI-007 showed significant antipsychotic efficacy as early as week 1, which was maintained at every time point throughout the entire study. ITI-007 showed a dose-related improvement in symptoms of schizophrenia with the 40 mg dose approximating the trajectory of improvement seen with the 60 mg dose, but the effect with 40 mg did not reach statistical significance on the primary endpoint. In addition, the 60 mg dose of ITI-007 met the key secondary endpoint of statistically significant improvement on the Clinical Global Impression Scale for Severity of Illness, or CGI-S. The 40 mg dose of ITI-007 also demonstrated a statistically significant improvement versus placebo on the CGI-S, though not formally tested against placebo since it did not separate on the primary endpoint. Consistent with previous studies, ITI-007 had a favorable safety and tolerability profile as evidenced by motoric, metabolic, and cardiovascular characteristics similar to placebo, and no clinically significant changes in akathisia, extrapyramidal symptoms, prolactin, body weight, glucose, insulin, or lipids. We are also conducting a second Phase 3 clinical trial in schizophrenia that we initiated in the second quarter of 2015, with over 600 patients
planned to be enrolled in the trial. In this trial, we are randomizing patients to two doses of ITI-007 (60 mg or 20 mg), risperidone (active control) or placebo over a 6-week treatment duration, and the primary outcome measure is change from baseline to Day 42 on the PANSS total score. We expect patient enrollment will be completed in the second quarter of 2016. Subject to timely enrollment, we anticipate top-line results from the second Phase 3 clinical trial will be available in mid-2016.
In addition to our Phase 3 clinical trials, we will need to complete other clinical and non-clinical trials and manufacturing and pre-commercialization activities necessary to support the submission of a planned NDA for ITI-007 in schizophrenia, which we currently expect could occur in the first half of 2017. Additional meetings with the FDA may be requested, as needed, to discuss in greater detail our plans for schizophrenia, and other elements of our regulatory strategy, including additional therapeutic indications, as the program progresses. Our clinical plans may change based on any discussions with the FDA, the relative success and cost of our research, preclinical and clinical development programs, whether we are able to enter into future collaborations, and any unforeseen delays or cash needs. If the FDA does not agree with our clinical development plans for ITI-007, our development of ITI-007 may be delayed and the costs of our development of ITI-007 could increase, which would have a material adverse effect on our business, financial condition and results of operations.
We are also developing long acting injectable formulations of ITI-007 for the treatment of schizophrenia. This is a pre-clinical stage development program.
PET study of ITI-007 in patients with stable schizophrenia
On September 16, 2015, we announced top-line data from an open-label positron emission tomography, or PET, study of ITI-007 examining brain occupancy of striatal D2 receptors. This study was conducted in patients diagnosed with schizophrenia who were otherwise healthy and stable with respect to their psychosis. After washout from their previous antipsychotic medication for at least two weeks, PET was used to determine target occupancy in brain regions at baseline (drug-free) and again after two weeks of once daily ITI-007 oral administration. In this trial, the 60 mg dose of ITI-007 was associated with a mean of approximately 40% striatal dopamine D2 receptor occupancy. As predicted by preclinical and earlier clinical data, ITI-007 demonstrated antipsychotic effect at relatively low striatal D2 receptor occupancy, lower than the occupancy range required by most other antipsychotic drugs. Unlike any existing schizophrenia treatment, this dopamine receptor phosphoprotein modulator, or DPPM, acts as a pre-synaptic partial agonist and post-synaptic antagonist at D2 receptors. We believe this mechanism likely contributes to the favorable safety profile of ITI-007, with reduced risk for hyperprolactinemia, akathisia, extrapyramidal symptoms, and other motoric side effects.
ITI-007 for the treatment of depressive episodes associated with bipolar disorder (bipolar depression)
The pharmacological profile of ITI-007 offers the potential to treat bipolar mania, depression, and mixed symptoms at doses similar to those targeted for the treatment of schizophrenia. We believe that ITI-007 may be effective alone or in combination with mood stabilizers. Given that many patients with bipolar disorder also experience disturbed sleep and cognitive impairment similar to that observed in schizophrenia, we believe that ITI-007 may treat a wide array of symptoms in patients with bipolar disorder, including improvement of cognition and sleep.
Our bipolar depression program consists of two Phase 3 multi-center, randomized, double-blind, placebo-controlled clinical trials: one to evaluate ITI-007 as a monotherapy and the other to evaluate ITI-007 as an adjunctive therapy with lithium or valproate. In each trial, approximately 550 patients with a clinical diagnosis of Bipolar I or Bipolar II disorder and who are experiencing a current major depressive episode will be randomized to receive one of three treatments: 60 mg ITI-007, 40 mg ITI-007, or placebo in a 1:1:1 ratio orally once daily for 6 weeks. In the ITI-007-401 trial, patients will receive ITI-007 or placebo as a monotherapy. In the ITI-007-402 trial, patients will receive ITI-007 or placebo adjunctive to their existing mood stabilizer lithium or valproate. We initiated our bipolar depression program in the third quarter of 2015.
The primary endpoint for both clinical trials is change from baseline at Day 42 on the Montgomery-Åsberg Depression Rating Scale (MADRS) total score versus placebo. The MADRS is a well-validated 10-item checklist that measures the ability of a drug to reduce overall severity of depressive symptoms. Individual items are rated
by an expert clinician on a scale of 0 to 6 in which a score of 6 represents the most depressed evaluation for each item assessed. The total score ranges from 0 to 60. Secondary endpoints include measures of social function and quality of life that may illustrate the differentiated clinical profile of ITI-007. Safety and tolerability are also assessed in both clinical trials.
ITI-007 for the treatment of behavioral disturbances associated with dementia, including Alzheimer’s disease
Behavioral disturbances are common in dementia and Alzheimer’s disease. These disturbances are a major component of the burden to caregivers, and often lead to institutionalization. Although currently available treatments for patients with dementia mainly address cognitive disturbances, behavioral disturbances are considerably more problematic and likely more amenable to drug treatment. Several behavioral symptoms are quite prevalent in patients with dementia, including patients with Alzheimer’s disease. In the fourth quarter of 2014, we announced the top-line data from ITI-007-200, a Phase 1/2 clinical trial designed to evaluate the safety, tolerability and pharmacokinetics of low doses of ITI-007 in healthy geriatric subjects and in patients with dementia, including Alzheimer’s disease. The ITI-007-200 clinical trial was conducted in two parts. Part 1 was a randomized, double-blind, placebo-controlled multiple ascending dose evaluation of ITI-007 in healthy geriatric subjects. In each of three cohorts in Part 1, approximately 10 subjects were randomized to receive ITI-007 (N=8) or placebo (N=2) orally once daily in the morning for seven days. Doses of ITI-007 up to and including 30 mg were evaluated in three cohorts in Part 1. In Part 2, eight patients with dementia were randomized to receive 9 mg ITI-007 (N=5) or placebo (N=3) orally once a day in the evening for seven days. The primary objectives of the study were to evaluate the safety, tolerability and pharmacokinetics of ITI-007 in the elderly and in the target dementia patient population. Secondary measures were included to explore the effects of ITI-007 on cognition and agitation. The Hopkins Verbal Learning Test-R, or HVLT-R, was used to assess cognition in healthy geriatric subjects and dementia patients. The results demonstrated impaired verbal learning and memory (recall and recognition memory) by dementia patients relative to healthy geriatric subjects. Moreover, the data indicated that healthy geriatric subjects treated with ITI-007 for approximately one week experienced an improvement in verbal learning and memory relative to placebo-treated subjects. Dementia patients treated with ITI-007 showed enhanced recognition memory, making fewer false positive errors (i.e., responding ‘yes’ to non-target words) than patients treated with placebo. Other secondary endpoints in the ITI-007-200 clinical trial included the assessment of agitation. However, none of the study participants experienced agitation at baseline or during the study, and therefore no signals on this behavioral endpoint could be assessed. The completion of this study marks an important milestone in our strategy to develop low doses of ITI-007 for the treatment of behavioral disturbances associated with dementia and related disorders. The ITI-007-200 trial results to date indicate that ITI-007 is safe and well-tolerated across a range of low doses, has linear- and dose-related pharmacokinetics and improves cognition in the elderly. The most frequent adverse event was mild sedation at the higher doses. We believe these results further position ITI-007 as a development candidate for the treatment of behavioral disturbances in patients with dementia and other neuropsychiatric and neurological conditions. We plan to initiate additional clinical programs evaluating ITI-007 in patients with behavioral disturbances associated with dementia and related disorders, including Alzheimer’s disease, in the first half of 2016.
ITI-007 for the treatment of sleep disturbances associated with neurologic and psychiatric disorders
A Phase 2 double-blind, placebo controlled cross-over clinical trial conducted in 19 patients with primary insomnia with disturbed sleep maintenance at low doses of ITI-007 was completed in 2008 in Europe. The primary outcome measure was slow wave sleep as determined by polysomnography. ITI-007 demonstrated a dose-related statistically significant increase in slow wave sleep. Secondary measures were consistent with improvement of sleep maintenance in patients with primary insomnia, indicated by decreased waking after sleep onset, increased total sleep time, and no increase in latency to sleep onset. At these low doses ITI-007 did not induce sleep, but rather helped maintain sleep once sleep had been initiated. In addition, ITI-007 was not associated with next day cognitive impairment, or “hang-over” effects. We believe that ITI-007 may be particularly useful in the treatment of sleep disorders that accompany neuropsychiatric and neurologic disorders, including schizophrenia, autism spectrum disorder, or ASD, Parkinson’s disease and dementia. Previous work
has suggested that selective 5-HT2A receptor antagonists increase deep, slow wave sleep in both humans and animals. We believe, however, that other neuropharmacological mechanisms, in addition to 5-HT2A receptor antagonism, such as engaging some dopamine modulation, may be beneficial for the successful treatment of sleep maintenance insomnia, or SMI, in humans. We believe that ITI-007 represents a new approach to the treatment of sleep maintenance insomnia because of its business planunique pharmacology and neuropharmacological interactions beyond selective 5-HT2A receptor antagonism. We believe that ITI-007 offers a potentially new approach to the treatment of sleep maintenance disorders, particularly in those disorders that accompany neuropsychiatric and neurologic disorders. Many of these disorders are accompanied by profound sleep deficits, which impair daytime functioning including cognition, exacerbate disease symptoms and increase the cost of care. We are presently exploring clinical designs to incorporate the examination of sleep disturbances in one or more of these indications. There is no assurance that any such design would be sufficient for an FDA approval for this indication.
ITI-007 for the treatment of sleep and behavioral disturbances associated with autism spectrum disorder
Sleep problems are common in patients with ASD and are not adequately treated by currently available interventions. Approximately two thirds of children and adolescents with ASD experience sleep problems, higher than the rate of sleep problems in age-matched developmentally typical children. Moreover, individuals with ASD suffer from behavioral disturbances, including aggression, irritability, anxiety and depression. With its multiple pathway mechanism of action, we believe that ITI-007 could address the multi-faceted behavioral symptoms associated with ASD. 5-HT2A receptor antagonism is predicted to mergeincrease slow wave sleep, improve sleep maintenance and reduce aggression. D2 receptor modulation is predicted to improve sleep maintenance and reduce irritability and aggression. Serotonin reuptake inhibition is predicted to reduce anxiety and depression. Accordingly, we believe that ITI-007 could improve sleep maintenance, reduce behavioral disturbances and enhance social interaction in patients with ASD. We believe that our completed Phase 1 studies support advancing ITI-007 into Phase 2 trials in this patient population, and we are presently exploring the feasibility of such trials.
ITI-007 for the treatment of depression and other mood disorders
As a potent 5-HT2A receptor antagonist and serotonin reuptake inhibitor, we believe that ITI-007 could improve symptoms of depression with fewer side effects than selective serotonin reuptake inhibitors, or SSRIs. Dopamine modulation by ITI-007 may reduce irritability and aggression that can accompany many mood disorders. As such, ITI-007 may be effective for the treatment of mood disorders including MDD, PTSD, and IED. We are presently exploring the feasibility of clinical studies in these indications.
ITI-002 (PDE1) Program
We have a second major program called ITI-002 that has yielded a portfolio of compounds that selectively inhibits the enzyme phosphodiesterase type 1, or PDE1. We believe PDE1 helps regulate brain activity related to cognition, memory processes and movement/coordination. In addition, PDE1 inhibitors may have utility in treating non-CNS disorders. On February 25, 2011, we (through our wholly owned operating subsidiary, ITI) and Takeda Pharmaceutical Company Limited, or Takeda, entered into a license and collaboration agreement, or the Takeda License Agreement, under which we agreed to collaborate to research, develop and commercialize our proprietary compound ITI-214 and other selected compounds that selectively inhibit PDE1 for use in the prevention and treatment of human diseases. Takeda conducted four Phase 1 studies. A single rising dose study was conducted in the U.S. in healthy male and female, Japanese and non-Japanese volunteers. In a second U.S. study, ITI-214 was administered once daily over 14 days to healthy volunteers and patients with stable schizophrenia. In a third study, conducted in Japan, ITI-214 was administered for seven days at multiple rising oral doses in both male and female healthy volunteers. A fourth study compared the relative bioavailability of oral formulations of ITI-214 used in all previous studies to an immediate-release tablet, either with or without food in healthy volunteers. In these studies, ITI-214 demonstrated a favorable safety profile and was generally
well-tolerated across a broad range of doses both in healthy volunteers and in patients with schizophrenia with a pharmacokinetic profile that supports once daily dosing. ITI-214 is the first compound in its class to successfully advance into Phase 1 clinical trials. On October 31, 2014, we entered into an agreement with Takeda terminating the Takeda License Agreement, or the Termination Agreement, pursuant to which all rights granted under the Takeda License Agreement were returned to us. On September 15, 2015, Takeda completed the transfer of the Investigational New Drug application, or IND, for ITI-214 to us. We intend to pursue the development of our PDE program, including ITI-214 for the treatment of several CNS and non-CNS conditions, which may include cognition in Parkinson’s disease, cognition in Alzheimer’s disease, cognition in schizophrenia and in other non-CNS indications. Other compounds in the PDE portfolio are also being advanced for the treatment of various indications.
Additional PDE Programs
There are multiple forms and isoforms of PDE with distinct roles in intracellular signaling. We have developed strong internal expertise in the design and synthesis of inhibitors specific for individual PDE isoforms. Based on our understanding of the expression and functions of these isoforms in the CNS, we have identified PDE2 and PDE9 as compelling targets for drug discovery. We believe that inhibitors of these PDEs may be useful in treating neurodegeneration and bioenergetic failure in a variety of CNS diseases.
Intellectual Property
Our Patent Portfolio
As of February 1, 2016, we owned or controlled approximately 70 patent families filed in the United States and other major markets worldwide, including approximately 54 issued or allowed U.S. patents, 41 pending U.S. patent applications, 214 issued foreign patents, and 194 pending foreign patent applications, directed to novel compounds, formulations, methods of treatment, synthetic methods, and platform technologies.
Our ITI-007 program on novel compounds for neuropsychiatric and neurodegenerative diseases includes patents exclusively in-licensed from Bristol-Myers Squibb on families of compounds, including the ITI-007 lead molecule. We have extensively characterized this lead and filed additional patent applications on polymorphs, formulations, additional indications, derivatives and additional compounds. The ITI-007 lead molecule has composition of matter protection through 2025 and additional Orange Book-listable protection to 2034. Additionally, we expect to have data exclusivity in the European Union for up to 11 years from commercial launch. We also have a follow-on program, directed to compounds structurally related to the ITI-007 lead, but having composition of matter protection beyond 2031.
Our program on PDE1 inhibitors for cognition and dopamine-mediated disorders, such as Parkinson’s disease, includes patent protection for the lead molecule, ITI-214, as well as a wide range of filings on other proprietary compounds and indications. The ITI-214 lead molecule has composition of matter protection to 2029, with possible extensions and additional Orange Book-listable protection to 2034. Additionally, we expect to have data exclusivity in the European Union for up to 11 years from commercial launch. We are also evaluating potential follow-on compounds for ITI-214 which would have patent protection beyond 2030.
We have also filed patent applications on novel proprietary targets and lead compounds for Alzheimer’s disease, which would provide compound protection beyond 2028 or beyond 2034, depending on which compound is ultimately selected for development.
License Agreement
The Bristol-Myers Squibb License Agreement
On May 31, 2005, we entered into a worldwide, exclusive License Agreement with Bristol-Myers Squibb Company, or BMS, pursuant to which we hold a license to certain patents and know-how of BMS relating to ITI-007 and other specified compounds. The agreement was amended on November 3, 2010. The licensed rights are
exclusive, except BMS retains rights in specified compounds in the fields of obesity, diabetes, metabolic syndrome and cardiovascular disease. However, BMS has no right to use, develop or commercialize ITI-007 and other specified compounds in any field of use. We have the right to grant sublicenses of the rights conveyed by BMS. We are obliged under the license to use commercially reasonable efforts to develop and commercialize the licensed technology. We are also prohibited from engaging in the clinical development or commercialization of specified competitive compounds.
Under the agreement, we made an upfront payment of $1.0 million to BMS, a milestone payment of $1.25 million in December 2013, and a milestone payment of $1.5 million in December 2014 following the initiation of our first Phase 3 clinical trial for ITI-007 for patients with exacerbated schizophrenia. Possible milestone payments remaining total $12.0 million. Under the agreement, we may be obliged to make other milestone payments to BMS for each licensed product of up to an aggregate of approximately $14.75 million. We are also obliged to make tiered single digit percentage royalty payments on sales of licensed products. We are obliged to pay to BMS a percentage of non-royalty payments made in consideration of any sublicense.
The agreement extends, and royalties are payable, on a country-by-country and product-by-product basis, through the later of ten years after first commercial sale of a licensed product in such country, expiration of the last licensed patent covering a licensed product, its method of manufacture or use, or the expiration of other government grants providing market exclusivity, subject to certain rights of the parties to terminate the agreement on the occurrence of certain events. On termination of the agreement, we may be obliged to convey to BMS rights in developments relating to a licensed compound or licensed product, including regulatory filings, research results and other intellectual property rights.
Collaboration Agreement
The Takeda Pharmaceutical License and Collaboration Agreement and Termination Agreement
On February 25, 2011, we entered into a license and collaboration agreement with Takeda Pharmaceutical Company Limited under which we agreed to collaborate to research, develop and commercialize our proprietary compound ITI-214 and other selected compounds that selectively inhibit PDE1 for use in the prevention and treatment of human diseases. As part of the agreement, we assigned to Takeda certain patents owned by us that claim ITI-214 and granted Takeda an exclusive license to develop and commercialize compounds identified in the conduct of the research program that satisfy specified criteria. However, we retained rights to all compounds that do not meet the specified criteria and we continue to develop PDE1 inhibitors outside the scope of the agreement. Upon execution of the agreement, Takeda made a nonrefundable payment to us.
Under the terms of the agreement, we conducted a research program with an unidentified company or companies." Under SEC Rule 12b-2initial term of three years to identify and characterize compounds that meet certain specified criteria sufficient for further development by Takeda. This research program ended in February 2014. We were responsible for our expenses incurred in the conduct of certain research activities specified in the research plan. Takeda agreed to reimburse us for expenses we incurred in conducting additional research activities.
On October 31, 2014, we entered into an agreement with Takeda terminating the Takeda License Agreement, pursuant to which all rights granted under the Exchange Act,Takeda License Agreement were returned to us. On September 15, 2015, Takeda completed the Companytransfer of the Investigational New Drug application, or IND, for ITI-214 to us. ITI-214 is the first compound in its class to successfully advance into Phase 1 clinical trials. We intend to pursue the development of our PDE program, including ITI-214 for the treatment of several CNS and non-CNS conditions, which may include cognition in Parkinson’s disease, cognition in Alzheimer’s disease, cognition in schizophrenia and in other non-CNS indications. Other compounds in the PDE portfolio are also qualifiesbeing advanced for the treatment of various indications.
Manufacturing
We do not own or operate manufacturing facilities for the production of any of our product candidates, nor do we have plans to develop our own manufacturing operations in the foreseeable future. We currently rely on third-party contract manufacturers for all of our required raw materials, active pharmaceutical ingredient, or API, and finished product for our preclinical research and clinical trials, including the Phase 3 trials for ITI-007 for the treatment of schizophrenia and the treatment of bipolar depression. We believe that we would be able to contract with other third-party contract manufacturers to obtain API if our existing sources of API were no longer available, but there is no assurance that API would be available from other third-party manufacturers on acceptable terms, on the timeframe that our business would require, or at all. We do not have long-term agreements with our existing third-party contract manufacturers. We also do not have any current contractual relationships for the manufacture of commercial supplies of any of our product candidates if they are approved. As ITI-007 and any of our other product candidates continue to progress towards potential regulatory approval, we intend to enter into agreements with a third-party contract manufacturer and one or more back-up manufacturers for the commercial production of those products. Development and commercial quantities of any products that we develop will need to be manufactured in facilities, and by processes, that comply with the requirements of the FDA and the regulatory agencies of other jurisdictions in which we are seeking approval. We currently employ internal resources to manage our manufacturing contractors.
Sales and Marketing
We currently have no marketing, sales or distribution capabilities. In order to commercialize any of our product candidates, we must develop these capabilities internally or through collaboration with third parties. In selected therapeutic areas where we feel that our product candidates can be commercialized by a specialty sales force that calls on a limited and focused group of physicians, we may plan to participate in the commercialization of our product candidates in the United States. In therapeutic areas that require a large sales force selling to a large and diverse prescribing population, we may elect to commercialize through, or in collaboration with, strategic partners. We may choose to commercialize our products in markets outside of the United States by establishing one or more strategic alliances in the future.
Competition
We face, and will continue to face, intense competition from pharmaceutical and biotechnology companies, as well as numerous academic and research institutions and governmental agencies, both in the United States and abroad. We compete, or will compete, with existing and new products being developed by our competitors. Some of these competitors are pursuing the development of pharmaceuticals that target the same diseases and conditions that our research and development programs target.
Even if we are successful in developing our product candidates, the resulting products would compete with a “shell company,” because it has novariety of established drugs in the areas of our targeted CNS therapeutic indications. Our potential products for the treatment of schizophrenia and bipolar disorder would compete with, among other branded products, Abilify®, marketed jointly by Bristol-Myers Squibb and Otsuka Pharmaceutical; Fanapt®, marketed by Novartis Pharmaceuticals; Seroquel XR®, marketed by AstraZeneca; Invega®, marketed by Janssen; VRAYLAR®, marketed by Allergan, Rexulti® marketed by Otsuka Pharmaceutical and Latuda®, marketed by Sunovion. In addition, our product candidates, if approved, will compete with, among other generic antipsychotic products, haloperidol, risperidone, quetiapine, olanzapine and clozapine.
In addition, the companies described above and other competitors may have a variety of drugs in development or nominal assets (otherawaiting FDA approval that could reach the market and become established before we have a product to sell. Our competitors may also develop alternative therapies that could further limit the market for any drugs that we may develop. Many of our competitors are using technologies or methods different or similar to
ours to identify and validate drug targets and to discover novel small molecule drugs. Many of our competitors and their collaborators have significantly greater experience than cash)we do in the following:
In addition, many of our competitors and their collaborators have substantially greater advantages in the following areas:
Smaller companies also may prove to be significant competitors, particularly through proprietary research discoveries and collaborative arrangements with large pharmaceutical and established biotechnology companies. Many of our competitors have products that have been approved by the FDA or nominal operations. Many states have enacted statutes, rulesare in advanced development. We face competition from other companies, academic institutions, governmental agencies and regulations limiting the sale of securities of "blank check"other public and private research organizations for collaborative arrangements with pharmaceutical and biotechnology companies, in recruiting and retaining highly qualified scientific and management personnel and for licenses to additional technologies. Our competitors, either alone or with their respective jurisdictions. Management does not intendcollaborators, may succeed in developing technologies or drugs that are more effective, safer, and more affordable or more easily administered than ours and may achieve patent protection or commercialize drugs sooner than us. Developments by others may render our product candidates or our technologies obsolete. Our failure to undertake any efforts to causecompete effectively could have a market to developmaterial adverse effect on our business.
Government Regulation
United States—FDA Process
The research, development, testing, manufacture, labeling, promotion, advertising, import and export, distribution and marketing, among other things, of drug products are extensively regulated by governmental authorities in our securities, either debtthe United States and other countries. In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or equity, until we have successfully concluded a business combination. The Company intendsthe FDCA, and its implementing regulations. Failure to comply with the periodic reportingapplicable U.S. requirements may subject us to administrative or judicial sanctions, such as FDA refusal to approve pending NDAs, warning letters, fines, civil penalties, product recalls, product seizures, total or partial suspension of production or distribution, injunctions and/or criminal prosecution.
Drug Approval Process. None of our drug product candidates may be marketed in the United States until the drug has received FDA approval. Such approval can take many years to obtain and may be rejected by the FDA at a number of steps. The steps required before a drug may be marketed in the United States generally include the following:
Pre-clinical tests include laboratory evaluation of product chemistry, toxicity and formulation, as it is subjectwell as animal studies. The conduct of the pre-clinical tests and formulation of the compounds for testing must comply with federal regulations and requirements. The results of the pre-clinical tests, together with manufacturing information and analytical data, are submitted to those requirements.
Clinical trials involve administration of the investigational drug to any specific business, industry or geographical location and, thus, may acquire any type of business.
Clinical trials necessary for product approval typically are conducted in three sequential phases, but the phases may overlap.
The FDA or an IRB may suspend clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. The FDA may approve an NDA for a product candidate, but require that the sponsor conduct additional clinical trials to further assess the drug after NDA approval under a post-approval commitment. Post-approval trials are typically referred to as Phase 4 clinical trials.
During the development of a new drug, sponsors are given an opportunity to meet with the FDA at certain points. These points may be prior to submission of an IND, at the end of Phase 2, and before an NDA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and the FDA to reach an agreement on the next phase of development. Sponsors typically use the end of Phase 2 meeting to discuss their Phase 2 clinical results and present their plans for the pivotal Phase 3 clinical trial that they believe will support approval of the new drug. A sponsor may request a Special Protocol Assessment, or SPA, to reach an agreement with the FDA that the protocol design, clinical endpoints, and statistical analyses are acceptable to support regulatory approval of the product candidate with respect to effectiveness in the indication studied. If such an agreement is reached, it will be documented and made part of the administrative record, and it will be binding on the FDA except in limited circumstances, such as if the FDA identifies a substantial scientific issue essential to determining the safety or effectiveness of the product after clinical studies begin, or if the sponsor fails to follow the protocol that was agreed upon with the FDA. There is no guarantee that a study will ultimately be adequate to support an approval even if the study is subject to an SPA.
Concurrent with clinical trials, companies usually complete additional animal safety studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the drug candidate and the manufacturer must develop methods for testing the quality, purity and potency of the final drugs. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its shelf life.
Assuming successful completion of the required clinical testing, the results of pre-clinical studies and of clinical studies, together with other detailed information, including information on the manufacture and composition of the drug, are submitted to the FDA in the form of an NDA requesting approval to market the product for one or more indications. An NDA must be accompanied by a significant user fee, which is waived for the first NDA submitted by a qualifying small business. The NDA is subject to a sixty day acceptance period, and if sufficiently complete to permit substantive review, will be filed by FDA at the end of that period. For NDAs assigned a standard review designation, the FDA’s goal is to complete its review 12 months from submission and for priority review NDAs, 8 months from submission. These goals can be extended by the FDA requests for additional information from the sponsor.
The testing and approval process requires substantial time, effort and financial resources. The FDA will review the NDA and may deem it to be inadequate to support approval, and we cannot be sure that any approval will be granted on a timely basis, if at all. The FDA may also refer the application to the appropriate advisory committee, typically a panel of clinicians, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendations of the advisory committee, but it typically follows such recommendations.
Before approving an NDA, the FDA inspects the facility or the facilities at which the drug and/or its active pharmaceutical ingredient is manufactured and will not approve the product unless the manufacturing is in compliance with cGMPs. If the FDA evaluates the NDA and the manufacturing facilities are deemed acceptable, the FDA may issue an approval letter, or in some cases a Complete Response Letter. The approval letter authorizes commercial marketing of the drug for specific indications. As a condition of NDA approval, the FDA may require post-marketing testing and surveillance to monitor the drug’s safety or efficacy, or impose other conditions. A Complete Response Letter indicates that the review cycle of the application is complete and the application is not ready for approval. A Complete Response Letter may require additional clinical data and/or additional clinical trial(s), and/or other significant, expensive and time-consuming requirements related to clinical trials, pre-clinical studies or manufacturing. Even if such additional information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data from clinical trials is not always conclusive and the FDA may interpret data differently than we or our collaborators interpret data.
Alternatively, the FDA could also approve the NDA with a Risk Evaluation and Mitigation Strategy to mitigate risks of the drug, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries or other risk minimization tools. Once the FDA approves a drug, the FDA may withdraw product approval if on-going regulatory requirements are not met or if safety problems occur after the product reaches the market. In addition, the FDA may require testing, including Phase 4 clinical trials, and surveillance programs to monitor the safety effects of approved products that have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs or other information.
Post-Approval Requirements. After a drug has been approved by the FDA for sale, the FDA may require that certain post-approval requirements be satisfied, including the conduct of additional clinical studies. In addition, certain changes to an approved product, such as adding new indications, making certain manufacturing changes, or making certain additional labeling claims, are subject to further FDA review and approval. Before a company can market products for additional indications, it must obtain additional approvals from the FDA, typically a new NDA. Obtaining approval for a new indication generally requires that additional clinical studies be conducted. A company cannot be sure that any additional approval for new indications for any product candidate will be approved on a timely basis, or at all.
If post-approval conditions are not satisfied, the FDA may withdraw its approval of the drug. In addition, holders of an approved NDA are required to (i) report certain adverse reactions to the FDA and maintain pharmacovigilance programs to proactively look for these adverse events; (ii) comply with certain requirements concerning advertising and promotional labeling for their products; and (iii) continue to have quality control and manufacturing procedures conform to cGMPs after approval. The FDA periodically inspects the sponsor’s records related to safety reporting and/or manufacturing facilities, which includes assessment of on-going compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance. We intend to use third-party manufacturers to produce our products in clinical and commercial quantities, and future FDA inspections may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of problems with a product after approval may result in restrictions on a product, manufacturer or holder of an approved NDA, including recall of the product from the market or withdrawal of approval of the NDA for that drug.
Patent Term Restoration and Marketing Exclusivity. Depending upon the timing, duration and specifics of FDA approval of the use of our drugs, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and the extension must be requested prior to expiration of the patent. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we intend to apply for restorations of patent term for some of our currently owned or licensed patents to add patent life beyond their current expiration date, depending on the expected length of clinical trials and other factors involved in the submission of the relevant NDA.
Data and market exclusivity provisions under the FDCA also can delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent data exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may
not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs or 505(b)(2) NDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct, or obtain a right of reference to all of the pre-clinical studies, adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of foreign countries before we can commence clinical trials and approval of foreign countries or economic areas, such as the European Union, before we may market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
Pricing and Reimbursement
In the United States and internationally, sales of products that we market in the future, and our ability to generate revenues on such sales, are dependent, in significant part, on the availability of adequate coverage and reimbursement from third-party payors, such as state and federal governments, managed care providers and private insurance plans. Private insurers, such as health maintenance organizations and managed care providers, have implemented cost-cutting and reimbursement initiatives and likely will continue to do so in the future. These include establishing formularies that govern the drugs and biologics that will be offered and the out-of-pocket obligations of member patients for such products. We may need to conduct pharmacoeconomic studies to demonstrate the cost-effectiveness of our products for formulary coverage and reimbursement. Even with such studies, our products may be considered less safe, less effective or less cost-effective than existing products, and third-party payors may not provide coverage and reimbursement for our product candidates, in whole or in part.
In addition, particularly in the United States and increasingly in other countries, we are required to provide discounts and pay rebates to state and federal governments and agencies in connection with purchases of our products that are reimbursed by such entities. It is possible that future legislation in the United States and other jurisdictions could be enacted to potentially impact reimbursement rates for the products we are developing and may develop in the future and could further impact the levels of discounts and rebates paid to federal and state government entities. Any legislation that impacts these areas could impact, in a significant way, our ability to generate revenues from sales of products that, if successfully developed, we bring to market.
Political, economic and regulatory influences are subjecting the health care industry in the United States to fundamental changes. There have been, and we expect there will continue to be, legislative and regulatory proposals to change the health care system in ways that could significantly affect our future business. For example, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively, the ACA, enacted in March 2010, substantially changes the way health care is financed by both governmental and private insurers. Among other cost containment measures, ACA establishes:
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted, which, among other things, reduce Medicare payments to providers by up to 2% per fiscal year.
Sales and Marketing
The FDA regulates all advertising and promotion activities for products under its jurisdiction prior to and after approval, including standards and regulations for direct-to-consumer advertising, dissemination of off-label information, industry-sponsored scientific and educational activities and promotional activities involving the Internet. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved label. Further, if there are any modifications to the drug, including changes in indications, labeling, or manufacturing processes or facilities, we may be required to submit and obtain FDA approval of a new or supplemental NDA, which may require us to collect additional data or conduct additional pre-clinical studies and clinical trials. Failure to comply with applicable FDA requirements may subject a company to adverse publicity, enforcement action by the FDA, corrective advertising, consent decrees and the full range of civil and criminal penalties available to the FDA.
Physicians may prescribe legally available drugs for uses that are not described in the drug’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties, and often reflect a physician’s belief that the off-label use is the best treatment for the patient. The FDA does not regulate the behavior of physicians in their choice of treatments, but FDA regulations do impose stringent restrictions on manufacturers’ communications regarding off-label uses. Failure to comply with applicable FDA requirements may subject a company to adverse publicity, enforcement action by the FDA, corrective advertising, consent decrees and the full range of civil and criminal penalties available to the FDA.
Outside the United States, our ability to market a product is contingent upon obtaining marketing authorization from the appropriate regulatory authorities. The requirements governing marketing authorization, pricing and reimbursement vary widely from country to country.
We may also be subject to various federal and state laws pertaining to health care “fraud and abuse,” including anti-kickback laws and false claims laws. Anti-kickback laws make it illegal for a prescription drug manufacturer to solicit, offer, receive, or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase or prescription of a particular drug. Due to the breadth of the statutory provisions, the absence of guidance in the form of regulations, and very few court decisions addressing industry practices, it is possible that our practices might be challenged under anti-kickback or similar laws. False claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented, for payment to third-party payors (including Medicare and Medicaid) claims for reimbursed drugs or services that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. Our activities relating to the sale and marketing of our products may be subject to scrutiny under these laws.
Violations of fraud and abuse laws may be punishable by criminal and/or civil sanctions, including fines and civil monetary penalties, the possibility of exclusion from federal health care programs (including Medicare and Medicaid) and corporate integrity agreements, which impose, among other things, rigorous operational and monitoring requirements on companies. Similar sanctions and penalties also may be imposed upon executive officers and employees, including criminal sanctions against executive officers under the so-called “responsible corporate officer” doctrine, even in situations where the executive officer did not intend to violate the law and
was unaware of any wrongdoing. Given the penalties that may be imposed on companies and directorindividuals if convicted, allegations of such violations often result in settlements even if the company or individual being investigated admits no wrongdoing. Settlements often include significant civil sanctions, including fines and civil monetary penalties, and corporate integrity agreements. If the government was to allege or convict us or our executive officers of violating these laws, our business could be harmed. In addition, private individuals have the ability to bring similar actions. Our activities could be subject to challenge for the reasons discussed above and due to the broad scope of these laws and the increasing attention being given to them by law enforcement authorities. Further, there are an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. Many of these laws contain ambiguities as to what is required to comply with the laws. Given the lack of clarity in laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state authorities.
Description of the Merger
Pursuant to an Agreement and Plan of Merger dated August 23, 2013, or the Merger Agreement, by and among Oneida Resources Corp., which we refer to as the Company, we, our and us; ITI, Inc., a Delaware corporation and wholly-owned subsidiary of the Company, or Merger Sub; and Intra-Cellular Therapies, Inc., a Delaware corporation, which we refer to as ITI; Merger Sub merged with and into ITI, with ITI remaining as the surviving entity and a wholly-owned operating subsidiary of the Company. This transaction is referred to throughout this report as the “Merger.” The Merger was effective on August 29, 2013, upon the filing of a Certificate of Merger with the Secretary of State of the State of Delaware. In connection with the Merger, ITI changed its name to ITI, Inc. and Oneida Resources Corp. assumed the name Intra-Cellular Therapies, Inc. The Merger was accounted for as a capital transaction. Upon the effectiveness of the Merger, the Company’s business became the operation of ITI and its business.
At the effective time of the Merger, or the Effective Time, the legal existence of Merger Sub ceased and each share of ITI common stock and each share of ITI preferred stock that was issued and outstanding immediately prior to the Effective Time was automatically exchanged for 0.5 shares of our common stock, which we refer to as the Exchange. Immediately following the Effective Time, we completed the closing of a redemption of 5,000,000 shares of our common stock, or the Redemption, from our then-current sole stockholder, which constituted all of the issued and outstanding shares of our capital stock, on a fully-diluted basis, immediately prior to the Merger. Upon completion of the Merger and the Redemption, the former stockholders of ITI held 100% of the outstanding shares of our capital stock. Unless otherwise indicated in this report, all share and per share figures reflect the exchange of each share of ITI common stock and each share of ITI preferred stock then outstanding for 0.5 shares of our common stock at the Effective Time.
Employees
As of this dateFebruary 15, 2016, we employed 39 employees, 37 of whom were full-time. We consider our relations with our employees to be good. To successfully develop our drug candidates, we must be able to attract and retain highly skilled personnel. We anticipate hiring additional employees for research and development, clinical and regulatory affairs and general and administrative activities over the Company has not entered into any definitive agreement with any party regarding business opportunitiesnext few years. In addition, we intend to use clinical research organizations and third parties to perform our clinical studies and manufacturing.
| Item 1A. | RISK FACTORS |
Except for the Company. The Company has unrestricted flexibilityhistorical information contained herein, this report contains forward-looking statements that involve risks and uncertainties. These statements include projections about our finances, plans and objectives for the future, future operating and economic performance and other statements regarding future performance. These statements are not guarantees of future performance or events. Our actual results could differ materially from those discussed in seeking, analyzing and participatingthis report. Factors that could cause or contribute to these differences include, but are not limited to, those discussed in potential business opportunities in that it may seek a business combination target located in any industry or location. In its efforts to analyze potential acquisition targets, the Company will consider the following kinds of factors:
You should consider carefully the following risk factors, together with all of the other information included or incorporated by reference in this report. If any of the following risks, either alone or taken together, or other risks not presently known to us or that we currently believe to not be significant, develop into actual events, then our business, financial condition, results of operations or prospects could be materially adversely affected. If that happens, the market price of our common stock could decline, and stockholders may lose all or part of their investment.
Risks Related to Our Business
We currently do not have, and may never have, any products that generate significant revenues.
We have a limited operating history on which to evaluate our business and prospects. To date, we have not generated any product revenues from our product candidates currently in development. We cannot guarantee that any of our product candidates currently in development will ever become marketable products.
We must demonstrate that our drug candidates satisfy rigorous standards of safety and efficacy for their intended uses before the FDA and other regulatory authorities in the European Union and elsewhere will approve them for commercialization. Significant additional research, preclinical testing and clinical testing is required before we can file applications with the FDA or other regulatory authorities for premarket approval of our drug candidates. In addition, to compete effectively, our drugs must be easy to administer, cost-effective and economical to manufacture on a commercial scale. We may not achieve any of these objectives. In September 2015, we announced top-line results from our first Phase 3 clinical trial of our most advanced drug candidate, ITI-007, in schizophrenia. We also initiated enrollment of our second Phase 3 clinical trial of ITI-007 in schizophrenia in the second quarter of 2015 and, subject to timely enrollment, we anticipate that top-line results from this trial will be available in mid-2016. In addition, all rights with respect to ITI-214, which has advanced into Phase 1 clinical trials that we previously granted to Takeda were recently returned to us in connection with the termination of the Takeda License Agreement. On September 15, 2015, Takeda completed the transfer of the IND for ITI-214 to us. We intend to pursue the development of our PDE program, including ITI-214 for the treatment of several CNS and non-CNS conditions, which may include cognition in Parkinson’s disease, cognition in Alzheimer’s disease, cognition in schizophrenia and in other non-CNS indications. We cannot be certain that the clinical development of these or any other drug candidates in preclinical testing or clinical development will be successful, that we will receive the regulatory approvals required to commercialize them or that any of our other research and drug discovery programs will yield a drug candidate suitable for investigation through clinical trials. Our commercial revenues from our product candidates currently in development, if any, will be derived from sales of drugs that will not become marketable for several years, if at all.
There is no guarantee that our planned clinical trials for ITI-007 in schizophrenia or in other indications will be successful.
In our Phase 1 and Phase 2 clinical trials, our lead product candidate, ITI-007, has demonstrated improved sleep maintenance, antipsychotic efficacy, and clinical signals consistent with reduction in negative symptoms associated with schizophrenia, depression and anxiety, and other symptoms associated with impaired social
function. In September 2015, we announced top-line clinical results from our first randomized, double-blind, placebo-controlled Phase 3 clinical trial in patients with an acutely exacerbated episode of schizophrenia. In this trial, a once-daily 60 mg dose of ITI-007 met the primary endpoint and demonstrated antipsychotic efficacy with statistically significant superiority over placebo at week 4 (study endpoint). In addition, the 60 mg dose of ITI-007 met the key secondary endpoint of statistically significant improvement on the CGI-S. We are currently planning confirmatory later-stage clinical trials and recently initiated enrollment of our second Phase 3 clinical trial of ITI-007 in schizophrenia in the second quarter of 2015. In addition, we initiated our bipolar depression program in the third quarter of 2015.
The historical rate of failures for product candidates in clinical development and late-stage clinical trials is high. While we plan to conduct further clinical trials in patients with schizophrenia and other indications, there is no guarantee that we will have the same level of success in these trials as we have had in our earlier clinical trials, or be successful at all.
In addition, although we believe that ITI-007 and follow-on compounds may also have clinical utility in indications other than schizophrenia, such as behavioral disturbances in dementia, bipolar disorder, intermittent explosive disorder, non-motor disorders associated with Parkinson’s disease, obsessive compulsive disorder and anxiety disorders and post-traumatic stress disorder, we have never tested ITI-007 in Phase 2 clinical trials in the patient population for these other indications, except for ITI-007-200, a Phase 1/2 clinical trial designed to evaluate the safety, tolerability and pharmacokinetics of low doses of ITI-007 in healthy geriatric subjects and in patients with dementia, including Alzheimer’s disease, for which we announced top-line data in the fourth quarter of 2014.
If we do not successfully complete clinical development of ITI-007, we will be unable to market and sell products derived from it and to generate product revenues. Even if we do successfully complete clinical trials for ITI-007 in patients with schizophrenia, those results are not necessarily predictive of results of future pivotal trials that may be needed before we may submit an NDA to the FDA for the initial or other future indications. Of the vast number of drugs in development, only a small percentage result in the submission of an NDA to the FDA, and even less result in the NDA ultimately being approved by the FDA for commercialization.
We have recently completed our first Phase 3 clinical trial of ITI-007 for the treatment of schizophrenia and are proceeding with our second Phase 3 clinical trial of ITI-007 for the treatment of schizophrenia. Although we have discussed our clinical development plans with the FDA, the agency may ultimately determine that our Phase 3 clinical trials and non-clinical studies, even if successfully completed, are not sufficient for regulatory approval. If we are required to conduct additional clinical trials and non-clinical studies, our development of ITI-007 for schizophrenia will be more time-consuming and costly than we presently anticipate, which would have a material adverse effect on our business, results of operations and financial condition.
In June 2014, we held our end-of-Phase 2 meeting with the FDA to discuss our plans for initiating Phase 3 clinical trials of ITI-007 in schizophrenia. Following this meeting, we proceeded with our Phase 3 development program, in which we recently completed the first of two randomized, double-blind, placebo-controlled Phase 3 clinical trials of ITI-007 in patients with acutely exacerbated schizophrenia, with 450 patients enrolled in the first Phase 3 clinical trial and over 600 patients planned to be enrolled in the second Phase 3 clinical trial. We completed enrollment of the first Phase 3 clinical trial in schizophrenia in the second quarter of 2015 and initiated enrollment of the second Phase 3 clinical trial in schizophrenia in the second quarter of 2015. In the first Phase 3 trial, we randomized patients to two doses of ITI-007 (60mg or 40mg) or placebo over a 4-week treatment duration, and the primary outcome measure is change from baseline to Day 28 on the PANSS total score. In the second Phase 3 trial, we are randomizing patients to receive one of four treatments: 60 mg ITI-007, 20 mg ITI-007, 4 mg risperidone (active control) or placebo in a 1:1:1:1 ratio. The second Phase 3 trial is being conducted for a 6-week treatment duration. We announced top-line results of the first Phase 3 clinical trial of ITI-007 in patients with schizophrenia in September 2015 and anticipate that the results of the second Phase 3 clinical trial will be available, subject to timely enrollment, in mid-2016. Even though we believe that our recently completed Phase 3 trial and our on-going Phase 3 clinical trial and ongoing and planned clinical and
non-clinical studies for ITI-007 in schizophrenia, if successful, will be sufficient to support our NDA, the FDA may not agree with one or more aspects of our clinical trial designs, including the duration of the trials, clinical endpoints, controls, dose ranges, collection of safety data, or adequacy of our non-clinical studies. If we submit an NDA and the FDA does not agree with our clinical and non-clinical designs, our development of ITI-007 in schizophrenia and other indications may be delayed, and we may incur additional costs and devote additional resources to address any concerns the FDA may have with our trial designs. In addition, we may be required to conduct additional clinical trials or studies, which could result in additional delays and costs. There is no assurance that we will complete the Phase 3 trials and other clinical and non-clinical studies within the industrytimeframes and the costs that we currently expect, or at all, or in a manner that is acceptable to the FDA. Any delays or unplanned costs resulting from our Phase 3 clinical trials of ITI-007 in schizophrenia may have a material adverse effect on our business, results of operations and financial condition. Even if we eventually complete Phase 3 clinical testing, submit an NDA and receive approval of ITI-007, the FDA may grant approval contingent on the performance of costly additional post-approval clinical trials. The FDA may also approve ITI-007 for a more limited indication or a narrower patient population than we originally requested, and the FDA may not approve the labeling that we believe is necessary or desirable for the successful commercialization of ITI-007 or our other product candidates. Any delay in obtaining, or inability to obtain, applicable regulatory approval for ITI-007 would delay or prevent commercialization of ITI-007 and would materially adversely impact our business, results of operations and financial condition.
We expect our net losses to continue for at least several years and are unable to predict the extent of future losses or when we will become profitable, if ever.
We have experienced significant net losses since our inception. As of December 31, 2015, we had an accumulated deficit of approximately $193.0 million. We expect to incur net losses over the next several years as we advance our programs and incur significant clinical development costs. We have not received, and do not expect to receive for at least the next several years, any revenues from the commercialization of our product candidates. Substantially all of our revenues to date were from our license and collaboration agreement with Takeda and our agreements with various U.S. governmental agencies and other parties, including our research and development grants. In October 2014, we entered into the Takeda Termination Agreement, which terminated our license and collaboration agreement with Takeda, pursuant to which all rights with respect to ITI-214 that we previously granted to Takeda were returned to us. We will not, therefore, receive any further milestone payments from Takeda and we cannot be certain that we will enter into additional collaboration agreements. To obtain revenues from our product candidates, we must succeed, either alone or with others, in developing, obtaining regulatory approval for, and manufacturing and marketing drugs with significant market potential. We may never succeed in these activities, and may never generate revenues that are significant enough to achieve profitability.
We will require substantial additional funding, which may not be available to us on acceptable terms, or at all, and, if not so available, may require us to delay, limit, reduce or cease our operations.
We have consumed substantial amounts of capital since our inception. Our cash, cash equivalents and investment securities totaled $475.2 million at December 31, 2015, which includes net proceeds of approximately $121.8 million from the public offering of shares of our common stock in March 2015 and approximately $327.4 million from the public offering of shares of our common stock in September 2015. While we believe that our existing cash, cash equivalents and investment securities, together with interest on cash balances, will be sufficient to fund our operating expenses and capital expenditure requirements through the end of 2018, the amount and timing of our actual expenditures will depend upon numerous factors, including the ongoing status of our second Phase 3 clinical trials of ITI-007 in patients with acute exacerbated schizophrenia and bipolar depression, the continued development of our PDE program, including ITI-214 for the treatment of several CNS and non-CNS conditions, and our other planned clinical and non-clinical trials. Furthermore, we anticipate that we will need to secure additional funding to obtain regulatory approval for ITI-007 in patients with dementia, including Alzheimer’s disease, for further development of ITI-007 in patients with bipolar disorder, depressive disorders and other indications, and for development of our other product candidates.
If the FDA requires that we perform additional preclinical studies or clinical trials, or we experience delays or other setbacks in our clinical trials, our expenses would further increase beyond what we currently expect and the anticipated timing of any potential NDA would likely be delayed.
With the remaining proceeds from our public offerings in February 2014, March 2015 and September 2015, we intend to fund the following: the completion of two Phase 3 clinical trials of ITI-007 in patients with acute exacerbated schizophrenia, one of which we announced top-line results in September 2015 and the second of which we initiated enrollment in the second quarter of 2015; the initiation of other planned clinical and non-clinical trials, including manufacturing, needed for anticipated regulatory approval of ITI-007 in patients with acute exacerbated schizophrenia and other potential additional indications; pre-launch activities for ITI-007 for the treatment of schizophrenia and, if it receives regulatory approval, to fund our initial commercialization efforts; the completion of our clinical trials of ITI-007 in bipolar disorder as a whole;
Our future capital requirements will depend on, and could increase significantly as a result of, many factors, including:
Until we can generate significant continuing revenues, we expect to satisfy our future cash needs through our existing cash, cash equivalents and investment securities, strategic collaborations, private or public sales of our securities, debt financings, grant funding, or by licensing all or a business combination transaction. The timeportion of our product candidates or technology. Turmoil and costsvolatility in the financial markets have adversely affected the market capitalizations of
many biotechnology companies, and generally made equity and debt financing more difficult to obtain. This, coupled with other factors, may limit our access to additional financing. This could have a material adverse effect on our ability to access sufficient funding. We cannot be certain that additional funding will be available to us on acceptable terms, or at all. If we do obtain additional funding through equity offerings, the ownership of our existing stockholders and purchasers of shares of our common stock in any such offering will be diluted, and the terms of any financing may adversely affect the rights of our stockholders. In addition, the issuance of additional shares by us, or the possibility of such issuance, may cause the market price of our shares to decline. If funds are not available, we will be required to delay, reduce the scope of, or eliminate one or more of our research or development programs or our commercialization efforts. We also could be required to seek funds through arrangements with collaboration partners or otherwise that may require us to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us.
Our management has broad discretion over the use of our cash and we may not use our cash effectively, which could adversely affect our results of operations.
Our management has significant flexibility in applying our cash resources, including the net proceeds from our public offerings completed in February 2014, March 2015 and September 2015, and could use these resources for corporate purposes that do not increase our market value, or in ways with which our stockholders may not agree. We may use our cash resources for corporate purposes that do not yield a significant return or any return at all for our stockholders, which could adversely affect our future growth prospects.
Our lead product candidate, ITI-007, is only part way through the clinical trials we anticipate needing to complete before we may be able to submit an NDA to the FDA. Clinical trials are long, expensive and unpredictable, and there is a business combination transactionhigh risk of failure.
Preclinical testing and clinical trials are long, expensive and unpredictable processes that can be ascertained oncesubject to delays. It may take several years to complete the preclinical testing and clinical development necessary to commercialize a drug, and delays or failure can occur at any stage. Interim results of clinical trials do not necessarily predict final results, and success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials, even after promising results in earlier trials.
In connection with clinical trials, we face risks that a product candidate may not prove to be efficacious; patients may die or suffer other adverse effects for reasons that may or may not be related to the product candidate being tested; the results may not confirm the positive results of our earlier preclinical studies and clinical trials; and the results may not meet the level of statistical significance required by the FDA or other regulatory agencies. If we do not successfully complete preclinical and clinical development, we will be unable to market and sell products derived from our product candidates and to generate product revenues. Even if we do successfully complete clinical trials, those results are not necessarily predictive of results of additional trials that may be needed before an NDA may be submitted to the FDA or the FDA may approve the NDA.
Delays, suspensions and terminations in our clinical trials could result in increased costs to us, delay our ability to generate product revenues and therefore may have a material adverse effect on our business, combination targetresults of operations and future growth prospects.
The commencement of clinical trials can be delayed for a variety of reasons, including delays in: demonstrating sufficient safety and efficacy to obtain regulatory approval to commence a clinical trial; reaching agreement on acceptable terms with prospective contract research organizations and clinical trial sites; manufacturing sufficient quantities of a product candidate; obtaining clearance from the FDA to commence clinical trials pursuant to an IND; obtaining institutional review board approval to conduct a clinical trial at a prospective clinical trial site; and patient enrollment, which is a function of many factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical trial sites, the availability of effective treatments for the relevant disease and the eligibility criteria for the clinical trial.
Once a clinical trial has been identified. Any costs incurredbegun, it may be delayed, suspended or terminated due to a number of factors, including: ongoing discussions with regulatory authorities regarding the scope or design of our clinical trials or requests by them for supplemental information with respect to evaluationour clinical trial results; failure to conduct clinical trials in accordance with regulatory requirements; lower than anticipated screening or retention rates of a prospective business combination that is not ultimately completed will resultpatients in a loss to us.
Many of these factors may place us atalso ultimately lead to denial of regulatory approval of a competitive disadvantagecurrent or potential product candidate. If we experience delays, suspensions or terminations in successfully negotiating a business combination.clinical trial, our costs will increase, the commercial prospects for the related product candidate will be harmed, and our ability to generate product revenues will be delayed.
Safety issues with our product candidates, or with product candidates or approved products of third parties that are similar to our product candidates, could give rise to delays in the regulatory approval process, restrictions on labeling or product withdrawal after approval.
Problems with product candidates or approved products marketed by third parties that utilize the same therapeutic target or that belong to the same therapeutic class as our product candidates could adversely affect the development, regulatory approval and commercialization of our product candidates. In 2012, the FDA released draft guidance recommending that prospective suicidality assessments be performed in clinical trials of any drug being developed for a psychiatric indication. Our management believes,development programs are focused on psychiatric indications. Our PDE program is a novel target and may have unexpected safety effects that do not appear until late in clinical development or after commercial approval. To date, none of our product candidates have experienced any serious and unexpected suspected adverse reactions that resulted in the submission of an IND safety report to the FDA; however, some approved products marketed by third parties for psychiatric indications that utilize different therapeutic targets or are in a different therapeutic class have experienced significant safety issues. As we continue the development and clinical trials of our product candidates, there can be no assurance that our statusproduct candidates will not experience significant safety issues.
Discovery of previously unknown class effect problems may prevent or delay clinical development and commercial approval of product candidates or result in restrictions on permissible uses after their approval, including withdrawal of the medicine from the market. Many drugs acting on the CNS include boxed warnings and precautions related to suicidal behavior or ideation, driving impairment, somnolence/sedation and dizziness, discontinuation, weight gain, non-insulin dependent (type II) diabetes, cardiovascular side effects, sleep disturbances, and motor disturbances. If we or others later identify undesirable side effects caused by the mechanisms of action or classes of our product candidates or specific product candidates:
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product or could substantially increase our commercialization costs and expenses, which, in turn, could delay or prevent us from generating significant revenues from its sale.
Finally, if the FDA determines that a drug may present a risk of substance abuse, it can recommend to the United States public equity markets may give us a competitive advantage over privately-held entities with aDrug Enforcement Administration that the drug be scheduled under the Controlled Substances Act. Any failure or delay in commencing or completing clinical trials or obtaining regulatory approvals for our product candidates would delay commercialization of our product candidates, and severely harm our business objective similar to ours to acquire a target business on favorable terms.
If we succeed in effectingseek to enter into strategic alliances for our drug candidates, but fail to enter into and maintain successful strategic alliances, we may have to reduce or delay our drug candidate development or increase our expenditures.
An important element of a business combination, there willbiotechnology company’s strategy for developing, manufacturing and commercializing its drug candidates may be in all likelihood, intense competition from competitors of the target business. Many of our target business’ competitors are likely to be significantly largerenter into strategic alliances with pharmaceutical companies or other industry participants to advance its programs and have far greaterenable it to maintain its financial and other resources thanoperational capacity. We may face significant competition in seeking appropriate alliances. If we will. Some of these competitors may be divisions or subsidiaries of large, diversified companies that have access to financial resources of their respective parent companies. Our target businessseek such alliances, we may not be able to compete effectively with these companies or maintain them as customers while competing with themnegotiate alliances on other projects.acceptable terms, if at all. In addition, it is likely that our target business will face significant competition from smaller companies that have specialized capabilities in similar areas. We cannot accurately predict how our target business’ competitive positionthese alliances may be affected by changing economic conditions, customer requirementsunsuccessful. On October 31, 2014, we entered into the Termination Agreement with Takeda, which terminated the Takeda License Agreement, pursuant to which all rights granted under the Takeda License Agreement were returned to us. If we seek such alliances and then fail to create and maintain suitable alliances, we may have to limit the size or technical developments. We cannot assure you that, subsequentscope of, or delay, one or more of our drug development or research programs. If we elect to a business combination,fund drug development or research programs on our own, we will have to increase our expenditures and will need to obtain additional funding, which may be unavailable or available only on unfavorable terms.
To the extent we are able to enter into collaborative arrangements or strategic alliances, we will be exposed to risks related to those collaborations and alliances.
Biotechnology companies at our stage of development sometimes become dependent upon collaborative arrangements or strategic alliances to complete the development and commercialization of drug candidates, particularly after the Phase 2 stage of clinical testing. If we elect to enter into collaborative arrangements or strategic alliances, these arrangements may place the development of our drug candidates outside our control, may require us to relinquish important rights or may otherwise be on terms unfavorable to us.
Dependence on collaborative arrangements or strategic alliances would subject us to a number of risks, including the risk that:
Preliminary and interim data from our clinical studies that we may announce or publish from time to time may change as more patient data become available.
From time to time, we may announce or publish preliminary or interim data from our clinical studies. Preliminary and interim results of a clinical trial are not necessarily predictive of final results. Preliminary and interim data are subject to the risk that one or more of the clinical outcomes may materially change as patient
enrollment continues and more patient data become available. As a result, preliminary and interim data should be viewed with caution until the final data are available. Material adverse changes in the final data compared to the interim data could significantly harm our business prospects.
We rely on third parties to conduct our clinical trials and perform data collection and analysis, which may result in costs and delays that prevent us from successfully commercializing our product candidates.
Although we design and manage our current preclinical studies and clinical trials, we do not now have the ability to conduct clinical trials for our product candidates on our own. In addition to our collaborators, we rely on contract research organizations, medical institutions, clinical investigators, and contract laboratories to perform data collection and analysis and other aspects of our clinical trials. In addition, we also rely on third parties to assist with our preclinical studies, including studies regarding biological activity, safety, absorption, metabolism, and excretion of product candidates.
Our preclinical activities or clinical trials may be delayed, suspended, or terminated if: the quality or accuracy of the data obtained by the third parties on whom we rely is compromised due to their failure to adhere to our clinical protocols or regulatory requirements or if for other reasons, these third parties do not successfully carry out their contractual duties or fail to meet regulatory obligations or expected deadlines, or these third parties need to be replaced.
If the third parties on whom we rely fail to perform, our development costs may increase, our ability to obtain regulatory approval, and consequently, to commercialize our product candidates may be delayed or prevented altogether. We currently use several contract research organizations to perform services for our preclinical studies and clinical trials. While we believe that there are numerous alternative sources to provide these services, in the event that we seek such alternative sources, we may not be able to enter into replacement arrangements without delays or incurring additional expenses.
Even if we successfully complete the clinical trials of one or more of our product candidates, the product candidates may fail for other reasons.
Even if we successfully complete the clinical trials for one or more of our product candidates, the product candidates may fail for other reasons, including the possibility that the product candidates will:
If we are unable to receive the required regulatory approvals, secure our intellectual property rights, minimize the incidence of Acquisitionany adverse side effects or fail to compete with our competitors’ products, our business, financial condition, and results of operations could be materially and adversely affected.
Following regulatory approval of any of our drug candidates, we will be subject to ongoing regulatory obligations and restrictions, which may result in significant expense and limit our ability to commercialize our potential products.
With regard to our drug candidates, if any, approved by the FDA or by another regulatory authority, we are held to extensive regulatory requirements over product manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion and record keeping. Regulatory approvals may also be subject to
significant limitations on the indicated uses or marketing of the drug candidates. Potentially costly follow-up or post-marketing clinical studies may be required as a condition of approval to further substantiate safety or efficacy, or to investigate specific issues of interest to the regulatory authority. Previously unknown problems with the drug candidate, including adverse events of unanticipated severity or frequency, may result in restrictions on the marketing of the drug, and could include withdrawal of the drug from the market.
In addition, the law or regulatory policies governing pharmaceuticals may change. New statutory requirements may be enacted or additional regulations may be enacted that could prevent or delay regulatory approval of our drug candidates. We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from future legislation or administrative action, either in the United States or elsewhere. If we are not able to maintain regulatory compliance, we might not be permitted to market our drugs and our business could suffer.
Our product candidates may not gain acceptance among physicians, patients, or the medical community, thereby limiting our potential to generate revenues, which will undermine our future growth prospects.
Even if our product candidates are approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of any approved product candidate by physicians, health care professionals and third-party payors, and our profitability and growth will depend on a number of factors, including:
pricing and cost effectiveness, which may be subject to regulatory control;
If any product candidate that we develop does not provide a treatment regimen that is at least as beneficial as the current standard of care or otherwise does not provide some additional patient benefit over the current standard of care, that product will not achieve market acceptance and we will not generate sufficient revenues to achieve profitability.
The mannerfailure to attract and retain skilled personnel and key relationships could impair our drug development and commercialization efforts.
We are highly dependent on our senior management and key clinical development, scientific and technical personnel. Competition for these types of personnel is intense. The loss of the services of any member of our senior management, clinical development, scientific or technical staff may significantly delay or prevent the achievement of drug development and other business objectives and could have a material adverse effect on our business, operating results and financial condition. We also rely on consultants and advisors to assist us in formulating our strategy. All of our consultants and advisors are either self-employed or employed by other organizations, and they may have conflicts of interest or other commitments, such as consulting or advisory contracts with other organizations, that may affect their ability to contribute to us. We intend to expand and develop new drug candidates, and will need additional funding to grow our business. We will need to hire additional employees in order to continue our research and clinical trials and to market our drugs when approved. This strategy will require us to recruit additional executive management and clinical development, regulatory, scientific, technical and sales and marketing personnel. There is currently intense competition for skilled executives and employees with relevant clinical development, scientific, technical and sales and marketing expertise, and this competition is likely to continue. The inability to attract and retain sufficient clinical development, scientific, technical and managerial personnel, due to intense competition and our limited
resources, would limit or delay our product development efforts, which would adversely affect the development of our drug candidates and commercialization of our potential drugs and growth of our business.
We may not be able to continue or fully exploit our partnerships with outside scientific and clinical advisors, which could impair the progress of our clinical trials and our research and development efforts.
We work with scientific and clinical advisors at academic and other institutions who are experts in the field of CNS disorders. They advise us with respect to our clinical trials. These advisors are not our employees and may have other commitments that would limit their future availability to us. If a conflict of interest arises between their work for us and their work for another entity, we may lose their services, which may impair our reputation in the industry and delay the development or commercialization of our product candidates.
Our product candidates have never been manufactured on a commercial scale, and there are risks associated with scaling up manufacturing to commercial scale. In particular, we will need to develop a larger scale manufacturing process that is more efficient and cost-effective to commercialize ITI-007 and other product candidates, which may not be successful, and which may require us to transfer our production to one or more other third-party manufacturers, potentially delaying regulatory approval and commercialization.
Our product candidates have never been manufactured on a commercial scale, and there are risks associated with scaling up manufacturing to commercial scale including, among others, cost overruns, potential problems with process scale-up, process reproducibility, stability issues, lot consistency and timely availability of raw materials. There is no assurance that our manufacturers will be successful in establishing a larger-scale commercial manufacturing process for ITI-007 which achieves our objectives for manufacturing capacity and cost of goods. Even if we could otherwise obtain regulatory approval for any product candidate, there is no assurance that our manufacturers will be able to manufacture the approved product to specifications acceptable to the FDA or other regulatory authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product or to meet potential future demand. If our manufacturers are unable to produce sufficient quantities of the approved product for commercialization, our commercialization efforts would be impaired, which would have an adverse effect on our business, financial condition, results of operations and growth prospects.
We rely on third-party manufacturers to manufacture and supply our product candidates for us. If one of our suppliers or manufacturers fails to perform adequately or fulfill our needs, we may be required to incur significant costs and devote significant efforts to find new suppliers or manufacturers. We may also face significant delays in our clinical trials, regulatory approvals and product introductions and commercialization.
We have no manufacturing facilities and have limited experience in the manufacturing of drugs or in designing drug-manufacturing processes. We have contracted with third-party manufacturers to produce, in collaboration with us, our product candidates, including ITI-007, for clinical trials. If any of our product candidates are approved by the FDA or other regulatory agencies for commercial sale, we may need to amend our contracts with our current manufacturers or contract with other third parties to manufacture them in larger quantities at commercial scale. While we believe that there are alternative sources available to manufacture our product candidates, in the event that we seek such alternative sources, we may not be able to enter into replacement arrangements without delays or additional expenditures. We cannot estimate these delays or costs with certainty but, if they were to occur, they could cause a delay in our development and commercialization efforts. We have not entered into a long-term agreement with our current third-party manufacturers or with any alternate suppliers. Although we intend to do so prior to any commercial launch of a product that is approved by the FDA in order to ensure that we maintain adequate supplies of commercial drug product, we may be unable to enter into such agreements or do so on commercially reasonable terms, which could delay a product launch or subject our commercialization efforts to significant supply risk.
Manufacturers of our product candidates are obliged to operate in accordance with FDA-mandated current good manufacturing practices, or cGMPs. The manufacture of pharmaceutical products in compliance with the cGMPs requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products often encounter difficulties in production, including difficulties with production costs and yields, quality control, including stability of the product candidate and quality assurance testing, shortages of qualified personnel, as well as compliance with strictly enforced cGMP requirements, other federal and state regulatory requirements and foreign regulations. If our manufacturers were to encounter any of these difficulties or otherwise fail to comply with their obligations to us or under applicable regulations, our ability to provide product candidates in our clinical trials would be jeopardized. Any delay or interruption in the supply of clinical trial materials could delay the completion of our clinical trials, increase the costs associated with maintaining our clinical trial programs and, depending upon the period of delay, require us to commence new clinical trials at significant additional expense or terminate the clinical trials completely.
In addition, the facilities used by our contract manufacturers or other third party manufacturers to manufacture our product candidates must be approved by the FDA pursuant to inspections that will be conducted after we request regulatory approval from the FDA. These requirements include, among other things, quality control, quality assurance and the maintenance of records and documentation. Manufacturers of our product candidates may be unable to comply with these cGMP requirements and with other FDA, state and foreign regulatory requirements. The FDA or similar foreign regulatory agencies may also implement new standards at any time, or change their interpretation and enforcement of existing standards for manufacture, packaging or testing of products. We have little control over our manufacturers’ compliance with these regulations and standards. A failure of any of our current or future contract manufacturers to establish and follow cGMPs and to document their adherence to such practices may lead to significant delays in clinical trials or in obtaining regulatory approval of product candidates or the ultimate launch of products based on our product candidates into the market. Failure by our current or future third-party manufacturers or us to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure of the government to grant pre-market approval of drugs, delays, suspension or withdrawal of approvals, seizures or recalls of products, operating restrictions, and criminal prosecutions. If the safety of any product supplied is compromised due to our manufacturers’ failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize our products and we may be held liable for any injuries sustained as a result. Any of these factors could cause a delay of clinical studies, regulatory submissions, approvals or commercialization of our product candidates, entail higher costs or impair our reputation.
We will need to continue to manage our organization and we may encounter difficulties with our staffing and any future transitions, which could adversely affect our results of operations.
We will need to manage our operations and facilities effectively in order to advance our drug development programs (including ITI-007 and ITI-214), facilitate any future collaborations, and pursue other development activities. It is possible that our infrastructure may be inadequate to support our future efforts and growth. In particular, we may have to develop internal sales, marketing, and distribution capabilities if we decide to market any drug that we may successfully develop. We may not successfully manage our operations and, accordingly, may not achieve our research, development, and commercialization goals.
Our ability to generate product revenues will be diminished if our products do not receive coverage from payors or sell for inadequate prices, or if patients are unable to obtain adequate levels of reimbursement.
Patients who are prescribed medicine for the treatment of their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their prescription drugs. Adequate coverage and reimbursement from governmental health care programs, such as Medicare and Medicaid, and commercial payors is critical to new product acceptance. Coverage decisions may depend upon clinical and economic standards that disfavor new drug products when more established or lower cost therapeutic alternatives are
already available or subsequently become available. Even if we obtain coverage for any approved products, the resulting reimbursement payment rates might not be adequate or may require co-payments that patients find unacceptably high. Patients are unlikely to use any products we may market unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of those products.
In addition, the market for any products for which we may receive regulatory approval will depend significantly on access to third-party payors’ drug formularies, or lists of medications for which third-party payors provide coverage and reimbursement. The industry competition to be included in such formularies often leads to downward pricing pressures on pharmaceutical companies. Also, third-party payors may refuse to include a particular branded drug in their formularies or otherwise restrict patient access to a branded drug when a less costly generic equivalent or other alternative is available.
Third-party payors, whether foreign or domestic, governmental or commercial, are developing increasingly sophisticated methods of controlling health care costs. In addition, in the United States, no uniform policy of coverage and reimbursement for drug products exists among third-party payors. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our product candidates to each payor separately, with no assurance that coverage will be obtained. If we are unable to obtain coverage of, and adequate payment levels for, our products from third-party payors, physicians may limit how much or under what circumstances they will prescribe or administer them and patients may decline to purchase them. This in turn could affect our ability to successfully commercialize any approved products and thereby adversely impact our profitability, results of operations, financial condition, and future success.
In the future, if we have products that are approved, health care legislation may make it more difficult to receive revenues from those products.
In both the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory proposals in recent years to change the health care system in ways that could impact our ability to sell our products profitably. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively, ACA, became law in the United States. The ACA substantially changed the way health care is financed by both governmental and private insurers and significantly affects the health care industry. Among the provisions of ACA of importance to our potential product candidates are the following:
Many of the Company anddetails regarding the promotersimplementation of the opportunity,ACA are yet to be determined and, at this time, it remains unclear what the relative negotiating strengthfull effect that the ACA will have on our business. At this time, it remains unclear whether there will be any further changes made to the ACA, whether in part or in its entirety.
In addition, in many foreign countries, particularly the countries of the CompanyEuropean Union, the pricing of prescription drugs is subject to government control. In some non-U.S. jurisdictions, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and such promoters.
We expect that the Company will acquire its participationACA, as well as other health care reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we may receive for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a business opportunity through the issuancesimilar reduction in payments from private payors. The implementation of common stockcost containment measures or other securitieshealth care reforms may prevent us from being able to generate revenue, attain profitability, or commercialize any products for which we receive regulatory approval.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market any products we may develop, we may not be able to generate product revenue.
We do not currently have an organization for the sales, marketing or distribution of pharmaceutical products. In order to market any products that may be approved by the Company. AlthoughFDA, we must build our sales, marketing, managerial, and related capabilities or make arrangements with third parties to perform these critical commercial services. There are risks involved with both establishing our own sales, marketing, managerial and related capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time-consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
We also may not be successful entering into arrangements with third parties to sell and market our product candidates or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our
products effectively, which could damage our reputation. If we do not establish adequate sales, marketing, and distribution capabilities, whether independently or in collaboration with third parties, we will not be successful in commercializing our product candidates, may not be able to generate product revenue and may not become profitable.
There are possible limitations on our use of net operating losses.
As of December 31, 2015, we had net operating loss carryforwards of approximately $174.8 million to reduce any such transaction cannotfuture federal and state taxable income through 2035. Since we had net operating loss carryforwards as of December 31, 2015, 2014 and 2013, no excess tax benefits for the tax deductions related to share-based awards were recognized in the statements of operations. The net operating loss carryforwards of approximately $174.8 million as of December 31, 2015 will begin to expire in the year 2030 if unused. The use of our net operating loss carryforwards may be predicted, it should be noted thatrestricted due to changes in certain circumstances the criteria for determining whether or not an acquisition isour ownership, including as a so-called "tax free" reorganization underresult of our public offerings in March 2015 and September 2015.
Under Section 368(a)(1)382 and 383 of the Internal Revenue Code of 1986, as amended, (the "Code") depends upon whetheror the ownersCode, substantial changes in our ownership may limit the amount of net operating loss carryforwards, or NOL’s, and tax credit carryforwards that could be utilized annually in the future to offset taxable income.
For the year ended December 31, 2015 we performed a Section 382 ownership analysis and determined that an ownership change occurred (within the meaning of Section 382 of the acquiredCode) in 2015. Based on the analysis performed, however, we do not believe that the Section 382 annual limitation will impact our ability to utilize the tax attributes that existed as of the date of the ownership change in a material manner. If we experience an ownership change in the future, the tax benefits related to the NOLs and tax credit carryforwards may be further limited or lost.
Security breaches, loss of data and other disruptions could compromise sensitive information related to our business, own 80%prevent us from accessing critical information or expose us to liability, which could adversely affect our business and our reputation.
In the ordinary course of our business, we, our clinical research organizations and other third parties on which we rely collect and store sensitive data, including legally protected patient health information, personally identifiable information about our employees, intellectual property, and proprietary business information. We manage and maintain our applications and data utilizing on-site systems. These applications and data encompass a wide variety of business critical information including research and development information and business and financial information.
The secure processing, storage, maintenance and transmission of this critical information is vital to our operations and business strategy, and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers, viruses, breaches, interruptions due to employee error, malfeasance or other disruptions, lapses in compliance with privacy and security mandates, or damage from natural disasters, terrorism, war and telecommunication and electrical failures. Any such event could compromise our networks and the information stored there could be accessed by unauthorized parties, publicly disclosed, lost or stolen. We have measures in place that are designed to detect and respond to such security incidents and breaches of privacy and security mandates. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as the Health Insurance Portability and Accountability Act of 1996, government enforcement actions and regulatory penalties. Unauthorized access, loss or dissemination could also disrupt our operations, including our ability to conduct research and development activities, process and prepare company financial information, manage various general and administrative aspects of our business and damage our reputation, any of which could adversely affect our business. For example, the loss of clinical trial data from completed or
ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. In addition, there can be no assurance that we will promptly detect any such disruption or security breach, if at all. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed.
Risks Related to Our Intellectual Property
Our ability to compete may be undermined if we do not adequately protect our proprietary rights.
Our commercial success depends on obtaining and maintaining proprietary rights to our product candidates and technologies and their uses, as well as successfully defending these rights against third-party challenges. We will only be able to protect our product candidates, proprietary technologies, and their uses from unauthorized use by third parties to the extent that valid and enforceable patents, or effectively protected trade secrets, cover them. We have patent rights under issued patents in many cases covering our ITI-007 and ITI-002 development programs. Nonetheless, the issued patents and patent applications covering our primary technology programs remain subject to uncertainty and continuous monitoring and action by us due to a number of factors, including:
Even if we have or obtain patents covering our product candidates or technologies, we may still be barred from making, using and selling our product candidates or technologies because of the patent rights of others. Others have or may have filed, and in the future are likely to file, patent applications covering compounds, assays, genes, gene products and therapeutic products that are similar or identical to ours. There are many issued U.S. and foreign patents relating to genes, nucleic acids, polypeptides, chemical compounds or therapeutic products, and some of these may encompass reagents utilized in the identification of candidate drug compounds or compounds that we desire to commercialize. Numerous U.S. and foreign issued patents and pending patent applications owned by others exist in the area of central nervous system disorders and the other fields in which we are developing products. These could materially affect our ability to develop our product candidates or sell our products. Because patent applications can take many years to issue, there may be currently pending
applications, unknown to us, that may later result in issued patents that our product candidates or technologies may infringe. These patent applications may have priority over patent applications filed by us.
We regularly conduct searches to identify patents or patent applications that may prevent us from obtaining patent protection for our proprietary compounds or that could limit the rights we have claimed in our patents and patent applications. Disputes may arise regarding the ownership or inventorship of our inventions. It is difficult to determine how such disputes would be resolved. Others may challenge the validity of our patents. If our patents are found to be invalid, we will lose the ability to exclude others from making, using or selling the inventions claimed in our patents.
Some of our academic institutional licensors, research collaborators and scientific advisors have rights to publish data and information to which we have rights. If we cannot maintain the confidentiality of our technology and other confidential information in connection with our collaborations, then our ability to receive patent protection or protect our proprietary information will be impaired. Additionally, any employee whose employment with us terminates, whether voluntarily by the employee or by us in connection with restructurings or otherwise, may seek future employment with our competitors. Although each of our employees is required to sign a confidentiality agreement with us at the time of hire, we cannot guarantee that the confidential nature of our proprietary information will be maintained in the course of such future employment. In addition, technology that we may license-in may become important to some aspects of our business. We generally will not control the patent prosecution, maintenance or enforcement of in-licensed technology.
Confidentiality agreements with employees and others may not adequately prevent disclosure of our trade secrets and other proprietary information and may not adequately protect our intellectual property, which could limit our ability to compete.
Because we operate in the highly technical field of drug discovery and development of small molecule drugs, we rely in part on trade secret protection in order to protect our proprietary technology and processes. However, trade secrets are difficult to protect. We enter into confidentiality and intellectual property assignment agreements with our corporate partners, employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors. These agreements generally require that the other party keep confidential and not disclose to third parties any confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. These agreements also generally provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to us. Enforcing a claim that a party illegally obtained and is using our trade secrets is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. The failure to obtain or maintain trade secret protection could adversely affect our competitive position.
A dispute concerning the infringement or misappropriation of our proprietary rights or the proprietary rights of others could be time consuming and costly, and an unfavorable outcome could harm our business.
There is significant litigation in our industry regarding patent and other intellectual property rights. While we are not currently subject to any pending intellectual property litigation, and are not aware of any such threatened litigation, we may be exposed to future litigation by third parties based on claims that our product candidates, technologies or activities infringe the intellectual property rights of others. If our drug development activities are found to infringe any such patents, we may have to pay significant damages or seek licenses to such patents. We may need to resort to litigation to enforce a patent issued to us, protect our trade secrets or determine the scope and validity of third-party proprietary rights. From time to time, we may hire scientific personnel formerly employed by other companies involved in one or more areas similar to the activities conducted by us. Either we or these individuals may be subject to allegations of trade secret misappropriation or other similar claims as a result of their prior affiliations. If we become involved in litigation, it could consume a substantial portion of our managerial and financial resources, regardless of whether we win or lose. We also may not be able to afford the costs of litigation.
The patent applications of pharmaceutical and biotechnology companies involve highly complex legal and factual questions, which, if determined adversely to us, could negatively impact our patent position.
The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions. The U.S. Patent and Trademark Office’s, or USPTO’s, standards are uncertain and could change in the future. Consequently, the issuance and scope of patents cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated or circumvented. U.S. patents and patent applications may also be subject to interference proceedings, and U.S. patents may be subject to reexamination proceedings in the USPTO (and foreign patents may be subject to opposition or comparable proceedings in the corresponding foreign patent office), which proceedings could result in either loss of the patent or denial of the patent application or loss or reduction in the scope of one or more of the voting stockclaims of the surviving entity.patent or patent application. Similarly, opposition or invalidity proceedings could result in loss of rights or reduction in the scope of one or more claims of a patent in foreign jurisdictions. In addition, such interference, reexamination and opposition proceedings may be costly. Accordingly, rights under any issued patents may not provide us with sufficient protection against competitive products or processes.
In addition, changes in or different interpretations of patent laws in the United States and foreign countries may permit others to use our discoveries or to develop and commercialize our technology and products without providing any compensation to us or may limit the number of patents or claims we can obtain. In particular, there have been proposals to shorten the exclusivity periods available under U.S. patent law that, if adopted, could substantially harm our business. The product candidates that we are developing are protected by intellectual property rights, including patents and patent applications. If any of our product candidates becomes a transaction were structuredmarketable product, we will rely on our exclusivity under patents to takesell the compound and recoup our investments in the research and development of the compound. If the exclusivity period for patents is shortened, then our ability to generate revenues without competition will be reduced and our business could be materially adversely impacted. The laws of some countries do not protect intellectual property rights to the same extent as U.S. laws, and those countries may lack adequate rules and procedures for defending our intellectual property rights. For example, some countries, including many in Europe, do not grant patent claims directed to methods of treating humans and, in these countries, patent protection may not be available at all to protect our product candidates. In addition, U.S. patent laws may change, which could prevent or limit us from filing patent applications or patent claims to protect our products and/or technologies or limit the exclusivity periods that are available to patent holders. For example, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was recently signed into law and includes a number of significant changes to U.S. patent law. These include changes to transition from a “first-to-invent” system to a “first-to-file” system and to the way issued patents are challenged. These changes may favor larger and more established companies that have more resources to devote to patent application filing and prosecution. The USPTO has been in the process of implementing regulations and procedures to administer the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act may affect our ability to obtain, enforce or defend our patents. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will ultimately have on the cost of prosecuting our patent applications, our ability to obtain patents based on our discoveries and our ability to enforce or defend our issued patents.
If we fail to obtain and maintain patent protection and trade secret protection of our product candidates, proprietary technologies and their uses, we could lose our competitive advantage and competition we face would increase, reducing our potential revenues and adversely affecting our ability to attain or maintain profitability.
Risks Related to Our Industry
We will be subject to stringent regulation in connection with the marketing of any products derived from our product candidates, which could delay the development and commercialization of our products.
The pharmaceutical industry is subject to stringent regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. Neither we nor our collaborators can market a pharmaceutical product in the United States until it has completed rigorous preclinical testing and clinical trials
and an extensive regulatory clearance process implemented by the FDA. Satisfaction of regulatory requirements typically takes many years, depends upon the type, complexity and novelty of the product, and requires substantial resources. Even if regulatory approval is obtained, it may impose significant restrictions on the indicated uses, conditions for use, labeling, advertising, promotion, and/or marketing of such products, and requirements for post-approval studies, including additional research and development and clinical trials. These limitations may limit the size of the market for the product or result in the incurrence of additional costs. Any delay or failure in obtaining required approvals could have a material adverse effect on our ability to generate revenues and continue our business.
Outside the United States, the ability to market a product is contingent upon receiving approval from the appropriate regulatory authorities. The requirements governing the conduct of clinical trials, marketing authorization, pricing, and reimbursement vary widely from country to country. Only after the appropriate regulatory authority is satisfied that adequate evidence of safety, quality, and efficacy has been presented will it grant a marketing authorization. Approval by the FDA does not automatically lead to the approval by regulatory authorities outside the United States and, similarly, approval by regulatory authorities outside the United States will not automatically lead to FDA approval.
Many of our competitors have greater resources and capital than us, putting us at a competitive disadvantage. If our competitors develop and market products that are more effective than our product candidates, they may reduce or eliminate our commercial opportunity.
Competition in the pharmaceutical and biotechnology industries is intense and increasing. We face competition from pharmaceutical and biotechnology companies, as well as numerous academic and research institutions and governmental agencies, both in the United States and abroad. Some of these provisions rathercompetitors have products or are pursuing the development of drugs that target the same diseases and conditions that are the focus of our drug development programs.
For example, our potential products for the treatment of schizophrenia would compete with, among other branded products, Abilify®, marketed jointly by Bristol-Myers Squibb and Otsuka Pharmaceutical, Fanapt®, marketed by Novartis Pharmaceuticals, Seroquel XR®, marketed by AstraZeneca, Invega®, marketed by Janssen, VRAYLAR®, marketed by Allergan, Rexulti® marketed by Otsuka Pharmaceutical and Latuda®, marketed by Sunovion. In addition, our product candidates, if approved, will compete with, among other generic antipsychotic drugs, haloperidol, risperidone, quetiapine, olanzapine and clozapine.
Many of our competitors and their collaborators have significantly greater experience than we do in the following:
In addition, many of our competitors and their collaborators have substantially greater capital and research and development resources, manufacturing, sales and marketing capabilities, and production facilities. Smaller companies also may prove to be significant competitors, particularly through proprietary research discoveries and collaboration arrangements with large pharmaceutical and established biotechnology companies. Many of our competitors have products that have been approved or are in advanced development and may develop superior technologies or methods to identify and validate drug targets and to discover novel small molecule drugs. Our competitors, either alone or with their collaborators, may succeed in developing drugs that are more effective, safer, more affordable, or more easily administered than ours and may achieve patent protection or
commercialize drugs sooner than us. Our competitors may also develop alternative therapies that could further limit the Code, all prior stockholdersmarket for any drugs that we may develop. Our failure to compete effectively could have a material adverse effect on our business.
Any claims relating to improper handling, storage, or disposal of biological, hazardous, and radioactive materials used in our business could be costly and delay our research and development efforts.
Our research and development activities involve the controlled use of potentially harmful hazardous materials, including volatile solvents, biological materials such as blood from patients that have the potential to transmit disease, chemicals that cause cancer, and various radioactive compounds. Our operations also produce hazardous waste products. We face the risk of contamination or injury from the use, storage, handling or disposal of these materials. We are subject to federal, state and local laws and regulations governing the use, storage, handling, and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations could be significant, and current or future environmental regulations may impair our research, development, or production efforts. If one of our employees were accidentally injured from the use, storage, handling, or disposal of these materials, the medical costs related to his or her treatment would be covered by our workers’ compensation insurance policy. However, we do not carry specific biological or hazardous waste insurance coverage and our general liability insurance policy specifically excludes coverage for damages and fines arising from biological or hazardous waste exposure or contamination. Accordingly, in such circumstances retain 20%the event of contamination or less of the total issuedinjury, we could be subject to criminal sanctions or fines or be held liable for damages, our operating licenses could be revoked, and outstanding shares of the surviving entity. Under other circumstances, depending upon the relative negotiating strength of the parties, prior stockholderswe could be required to suspend or modify our operations and our research and development efforts.
Consumers may retain substantially less than 20% of the total issued and outstanding shares of the surviving entity. Thissue us for product liability, which could result in substantial liabilities that exceed our available resources and damage our reputation.
Researching, developing, and commercializing drug products entail significant product liability risks. Liability claims may arise from our and our collaborators’ use of products in clinical trials and the commercial sale of those products. Consumers may make these claims directly and our collaborators or others selling these products may seek contribution from us if they receive claims from consumers. We have obtained limited product liability insurance coverage for our clinical trials. Our product liability insurance coverage for clinical trials is currently limited to an aggregate of $30 million. As such, our insurance coverage may not reimburse us or may not be sufficient to reimburse us for any expenses or losses we may suffer. Although we currently have product liability insurance that covers our clinical trials, we will need to increase and expand this coverage as we commence larger scale trials and if our product candidates are approved for commercial sale. This insurance may be prohibitively expensive or may not fully cover our potential liabilities. Inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of products that we or our collaborators develop. Product liability claims could have a material adverse effect on our business and results of operations. Our liability could exceed our total assets if we do not prevail in a lawsuit from any injury caused by our drug products.
Risks Related to Owning Our Common Stock
Numerous factors could result in substantial volatility in the trading price of our stock.
During the year ended December 31, 2015, the price per share of our common stock on the NASDAQ Global Select Market has ranged from a high of $60.79 to a low of $16.29. We have several stockholders, including affiliated stockholders, who hold substantial blocks of our stock. Sales of large numbers of shares by any of our large stockholders could adversely affect our trading price. If stockholders holding shares of our common stock sell, indicate an intention to sell, or if it is perceived that they will sell, substantial amounts of their common stock in the public market, the trading price of our common stock could decline.
In addition, the trading price of our common stock may be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. These factors include:
The stock market in general, and market prices for the securities of biotechnology companies like ours in particular, have from time to time experienced volatility that often has been unrelated to the operating performance of the underlying companies. These broad market and industry fluctuations may adversely affect the market price of our common stock, regardless of our operating performance. In several recent situations where the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders were to bring a lawsuit against us, the defense and disposition of the lawsuit could be costly and divert the time and attention of our management and harm our operating results.
Raising additional capital may cause dilution to existing stockholders, restrict our operations or require us to relinquish rights.
We will need to satisfy our future cash needs through public or private sales of our equity securities, sales of debt securities, the incurrence of debt from commercial lenders, strategic collaborations, licensing a portion or all of our product candidates and technology and, to a lesser extent, grant funding. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of those who wereour existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or grant licenses on terms that are not favorable to us.
The price of our common stock could be subject to volatility related or unrelated to our operations.
The market price of our common stock could fluctuate substantially due to a variety of factors, including market perception of our ability to meet our growth projections and expectations, quarterly operating results of other companies in the same industry, trading volume in our common stock, changes in general conditions in the economy and the financial markets or other developments affecting our business and the business of others in our industry. In addition, the stock market itself is subject to extreme price and volume fluctuations. This volatility has had a significant effect on the market price of securities issued by many companies for reasons related and unrelated to their operating performance and could have the same effect on our common stock.
We will incur increased costs and demands upon management as a result of complying with the laws and regulations affecting public companies, which could harm our operating results.
As a public company, we have incurred and will incur significant legal, accounting and other expenses, including costs associated with public company reporting requirements. We also have incurred and will incur costs associated with current corporate governance requirements, including requirements under Section 404 and other provisions of the Company priorSarbanes-Oxley Act, as well as rules implemented by the SEC or the NASDAQ Global Select Market or any other stock exchange or inter-dealer quotations system on which our common stock may be listed in the future. The expenses incurred by public companies for reporting and corporate governance purposes have increased dramatically in recent years.
If we fail to such reorganization.
We are required to comply with Section 404 of the Company will likely not have control of a majoritySarbanes-Oxley Act. Section 404 of the voting securitiesSarbanes-Oxley Act requires public companies to maintain effective internal control over financial reporting. In particular, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on the Company followingeffectiveness of our internal control over financial reporting. In addition, we are required to have our independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting beginning with this annual report on Form 10-K for the fiscal year ended December 31, 2015. Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a reorganization transaction. As part of suchtimely basis is a transaction, all or a majority of the Company's directors may resigncostly and one or more new directors may be appointed without any vote by stockholders.
Our ability to successfully implement our business plan and the negotiation, drafting and execution of relevant agreements, disclosure documents and other instruments will require substantial management time and attention and substantial cost for accountants, attorneys and others. The costs that will be incurred are difficult to determine at this time as the costs are expectedcomply with Section 404 requires us to be tiedable to the amount of time it takes to identifyprepare timely and complete a business combination transaction as well as the specific factors related to the business combination target that is chosen, including such factors as the location, size and complexity of the business of the target company. If a decision is made not to participate in a specific business opportunity, the costs theretofore incurred in the related investigation might not be recoverable. Furthermore, even if an agreement is reached for the participation in a specific business opportunity, the failure to consummate that transaction may result in the loss to the Registrant of the related costs incurred. The Company has not established a timeline with respect to the identification of a business combination target.
We are no longer an emerging growth company and are not able to devotetake advantage of the time requiredreduced disclosure requirements applicable to consummate a business combination transaction as necessary.
We expect no significant changes in the number of our employees other than such changes, if any, incident to a business combination.
accelerated filer and the reduced disclosure obligations of emerging growth companies that are not “emerging growth companies” including, but not limitedno longer available to not being requiredus. As a result, we will need to comply with the auditor attestation requirements of section 404(b) of the Sarbanes-Oxley Act, and exemptions from the requirements of Sections 14A(a) and (b) of the Securities Exchange Act of 1934 to hold a nonbinding advisory vote of shareholders on executive compensation and any golden parachute payments not previously approved.
If securities or industry analysts do not publish, or cease publishing, research or reports about us, our business or our market, or if they change their recommendations regarding our stock adversely, our stock price and trading volume could decline.
The trading market for our common stock is and will be influenced by whether industry or securities analysts publish or continue to publish research and reports about us, our business, our market or our competitors and, to the extent analysts do publish such reports, what they publish in those reports. We may not continue to have or to obtain analyst coverage in the future. Any analysts that do cover us may make adverse recommendations regarding our stock, adversely change their recommendations from time to time, and/or provide only two yearsmore favorable relative recommendations about our competitors. If any analyst who covers us or may cover us in the future were to cease coverage of auditedour company or fail to regularly publish reports on us, or if analysts fail to cover us or publish reports about us at all, we could lose, or never gain, visibility in the financial statements, insteadmarkets, which in turn could cause our stock price or trading volume to decline.
Provisions of three years.
The provisions of Delaware law and our restated certificate of incorporation and restated bylaws could discourage or make it more difficult to accomplish a proxy contest or other change in our management or the acquisition of control by a holder of a substantial amount of our voting stock. It is possible that these provisions could make it more difficult to accomplish, or could deter, transactions that stockholders may otherwise consider to be in their best interests or in our best interests. These provisions are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and in the policies formulated by the board of directors and to discourage certain types of transactions that may involve an actual or threatened change of our control. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal and to discourage certain tactics that may be used in proxy fights. Such provisions also may have the effect of preventing changes in our management.
We do not anticipate paying cash dividends in the foreseeable future.
We currently intend to retain any future earnings for funding growth. We do not anticipate paying any dividends in the foreseeable future. As a “smaller reportingresult, you should not rely on an investment in our securities if you require dividend income. Capital appreciation, if any, of our shares may be your sole source of gain for the foreseeable future. Moreover, you may not be able to re-sell your shares at or above the price you paid for them.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This report includes forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act that relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity,
performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Words such as, but not limited to, “believe,” “expect,” “anticipate,” “estimate,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “targets,” “likely,” “will,” “would,” “could,” “should,” “continue,” and similar expressions or phrases, or the negative of those expressions or phrases, are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Although we believe that we have a reasonable basis for each forward-looking statement contained in this report, we caution you that these statements are based on our projections of the future that are subject to known and unknown risks and uncertainties and other factors that may cause our actual results, level of activity, performance or achievements expressed or implied by these forward-looking statements, to differ. The description of our Business set forth in Item 1, the Risk Factors set forth in this Item 1A and our Management’s Discussion and Analysis of Financial Condition and Results of Operations set forth in Item 7 as well as other sections in this report, discuss some of the factors that could contribute to these differences. These forward-looking statements include, among other things, statements about:
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements
we make. We have included important cautionary statements in this report, particularly in the Risk Factors set forth in Item 1A of this Annual Report on Form 10-K, that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You should read this report and the documents that we reference in this report and have filed as defined by Item 10exhibits to this report completely and with the understanding that our actual future results may be materially different from what we expect. The forward-looking statements contained in this report are made as of Regulation S-K, the Company isdate of this report, and we do not requiredassume, and specifically disclaim, any obligation to provide this information.
| Item 1B. | UNRESOLVED STAFF COMMENTS |
None.
| Item 2. | PROPERTIES |
Our headquarters are located at 430 East 29th Street, New York, New York 10016, where we occupy approximately 16,753 square feet of useable office and laboratory space. The term of the lease, as defined by Item 10 of Regulation S-K, the Company is not required to provide this information.
| Item 3. | LEGAL PROCEEDINGS |
We are presently no pending legal proceedings to which the Company, any of its subsidiaries, any executive officer, any owner of record or beneficially of more than five percent of any class of voting securities isnot currently a party or as to which any of its property is subject, and no such proceedings are known to the Company to be threatened or contemplated against it.
| Item 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
From December 20, 2013 through January 30, 2014, our common stock was quoted on the OTC Markets—OTCQB tier, or OTCQB, under the symbol “ITCI.” On January 31, 2014, our common stock commenced trading on the NASDAQ Global Select Market under the symbol “ITCI” and ceased being quoted on the OTCQB. The high and low bid quotations per share of our common stock as reported by the OTCQB and the high and low sales prices per share of our common stock as reported by NASDAQ for Common Equity, Related Stockholder Mattersthe applicable periods when the common stock was quoted on the OTCBB or listed on the NASDAQ Global Select Market, as applicable, since the common stock commenced public trading are set forth below:
Year Ended December 31, 2015 | High | Low | ||||||
First Quarter | $ | 30.72 | $ | 16.29 | ||||
Second Quarter | $ | 35.45 | $ | 19.86 | ||||
Third Quarter | $ | 60.79 | $ | 21.19 | ||||
Fourth Quarter | $ | 59.96 | $ | 37.75 | ||||
Year Ended December 31, 2014 | High | Low | ||||||
First Quarter | $ | 21.26 | $ | 10.00 | ||||
Second Quarter | $ | 19.60 | $ | 14.53 | ||||
Third Quarter | $ | 19.77 | $ | 12.67 | ||||
Fourth Quarter | $ | 19.00 | $ | 13.37 | ||||
Stockholders
As of February 25, 2016, we had 43,202,709 outstanding shares of common stock and Small Business no outstanding shares of preferred stock. As of February 25, 2016, there were approximately 125 holders of record of our outstanding shares of common stock.
Dividends
We have never paid cash dividends on any of our capital stock and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. We do not intend to pay cash dividends to holders of our common stock in the foreseeable future.
Unregistered Sales of Securities
Not applicable.
Issuer Purchases of Equity Securities.
Not applicable.
Use of Incorporation authorizes the issuanceProceeds from Registered Securities
On February 5, 2014, we completed our initial public offering of up to 100,000,0007,063,300 shares of common stock, par value $.0001 per share (the “Common Stock”). The Common Stock is not listed on a publicly-traded market. As of the date of this filing, there was one (1) holder of record of the Common Stock.
Partners LLC and that any resale must be made pursuant to a registration or an available exemption from such registration.Cowen and Company, LLC acted as joint book-running managers for the offering and as representatives of the underwriters. Guggenheim Securities, LLC and JMP Securities LLC acted as co-managers for the offering. The offering commenced on January 24, 2014 and did not terminate until the sale of the foregoing securities was made without any form of general solicitation or advertising and all of the foregoing securities are deemed restricted securities for purposesshares offered.
We received aggregate net proceeds from the offering of approximately $115.4 million, after deducting approximately $7.4 million of underwriting discounts and commissions, and approximately $0.8 million of offering expenses payable by us. None of the Securities Act.
As of December 31, 2015, we have used the net proceeds of the offering primarily for working capital purposes, including recurring expenses and preclinical and clinical trial costs related to the development of ITI-007.
| Item 6. | SELECTED FINANCIAL DATA |
The following table sets forth consolidated financial data with respect to the Company is not required to providefor each of the five years in the period ended December 31, 2015. The selected financial data for each of the five years in the period ended December 31, 2015 have been derived from our audited consolidated financial statements. The consolidated balance sheets as of December 31, 2015 and 2014 and the related consolidated statements of operations, comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2015, and the report thereon, are included elsewhere in this information.
| 2015 | 2014 | 2013 | 2012 | 2011 | ||||||||||||||||
Statements of Operations: | ||||||||||||||||||||
Revenues: | ||||||||||||||||||||
License and collaboration revenue | $ | 30,659 | $ | 547,546 | $ | 2,737,002 | $ | 3,117,991 | $ | 22,327,464 | ||||||||||
Grant revenue | 60,705 | 29,755 | — | — | 1,034,495 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total Revenues | 91,364 | 577,301 | 2,737,002 | 3,117,991 | 23,361,959 | |||||||||||||||
Costs and expenses: | ||||||||||||||||||||
Research and development | 87,718,074 | 21,226,345 | 23,027,578 | 15,486,476 | 7,654,546 | |||||||||||||||
General and administrative | 18,187,286 | 10,337,679 | 5,976,276 | 4,034,925 | 4,612,450 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total costs and expenses | 105,905,360 | 31,564,024 | 29,003,854 | 19,521,401 | 12,266,996 | |||||||||||||||
(Loss) Income from operations | (105,813,996 | ) | (30,698,223 | ) | (26,266,852 | ) | (16,403,410 | ) | 11,094,963 | |||||||||||
Interest expense | — | (7,073 | ) | (612,963 | ) | (193,498 | ) | (15 | ) | |||||||||||
Interest income | 1,022,455 | 303,936 | 29,617 | 39,002 | 62,315 | |||||||||||||||
Income taxes | (1,600 | ) | (1,600 | ) | (18,000 | ) | (32,921 | ) | (64,834 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net (Loss) Income | $ | (104,793,141 | ) | $ | (30,691,460 | ) | $ | (26,868,198 | ) | $ | (16,590,827 | ) | $ | 11,092,429 | ||||||
Net (Loss) Income per common share: | ||||||||||||||||||||
Basic | $ | (2.91 | ) | $ | (1.07 | ) | $ | (1.56 | ) | $ | (2.96 | ) | $ | 1.98 | ||||||
Diluted | $ | (2.91 | ) | $ | (1.07 | ) | $ | (1.56 | ) | $ | (2.96 | ) | $ | 1.47 | ||||||
Weighted average number of common shares: | ||||||||||||||||||||
Basic | 36,069,237 | 28,650,067 | 17,260,768 | 5,607,539 | 5,601,495 | |||||||||||||||
Diluted | 36,069,237 | 28,650,067 | 17,260,768 | 5,607,539 | 7,558,150 | |||||||||||||||
| December 31, | ||||||||||||||||||||
| 2015 | 2014 | 2013 | 2012 | 2011 | ||||||||||||||||
Balance Sheet data: | ||||||||||||||||||||
Cash and cash equivalents | $ | 47,159,303 | $ | 61,325,044 | $ | 35,150,924 | $ | 15,645,528 | $ | 13,693,215 | ||||||||||
Total assets | 484,103,528 | 131,111,769 | 38,449,312 | 19,823,680 | 23,594,725 | |||||||||||||||
Total liabilities | 7,860,617 | 10,557,064 | 6,834,037 | 2,839,595 | 5,702,422 | |||||||||||||||
Accumulated deficit | (193,049,098 | ) | (88,255,957 | ) | (57,564,497 | ) | (30,696,299 | ) | (14,105,472 | ) | ||||||||||
Total stockholders’ equity | 476,242,911 | 120,554,705 | 31,615,275 | 16,984,085 | 17,892,303 | |||||||||||||||
| Item 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion of the financial condition and results of our operations and our wholly-owned subsidiary should be read in conjunction with the financial statements and the notes to those statements appearing elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should read the Risk Factors set forth in Item 7. Management’s Discussion1A of this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and Analysisanalysis.
Overview
We are a biopharmaceutical company focused on the discovery and clinical development of Financial Conditioninnovative, small molecule drugs that address underserved medical needs in neuropsychiatric and neurological disorders by targeting intracellular signaling mechanisms within the central nervous system, or CNS. ITI-007 is our lead drug development candidate with mechanisms of action that, we believe, may represent an effective treatment across multiple therapeutic indications. In our pre-clinical and clinical trials to date, ITI-007 combines potent serotonin 5-HT2A receptor antagonism, dopamine receptor phosphoprotein modulation, or DPPM, glutamatergic modulation, and serotonin reuptake inhibition into a single drug candidate for the treatment of acute and residual schizophrenia and for the treatment of bipolar disorder, including bipolar depression. At dopamine D2 receptors, ITI-007 has been demonstrated to have dual properties and to act as both a pre-synaptic partial agonist and a post-synaptic antagonist. ITI-007 has also been demonstrated to have affinity for dopamine D1 receptors and indirectly stimulate phosphorylation of glutamatergic NMDA GluN2B receptors in a mesolimbic specific manner. We believe that this regional selectivity in brain areas thought to mediate the efficacy of antipsychotic drugs, together with serotonergic, glutamatergic, and dopaminergic interactions, may result in efficacy for a broad array of symptoms associated with schizophrenia and bipolar disorder with improved psychosocial function. The serotonin reuptake inhibition potentially allows for antidepressant activity in the treatment of schizoaffective disorder, other disorders with co-morbid depression, and/or as a stand-alone treatment for major depressive disorder. We believe ITI-007 may also be useful for the treatment of other psychiatric and neurodegenerative disorders, particularly behavioral disturbances associated with dementia, autism, and other CNS diseases. ITI-007 is in Phase 3 clinical development as a novel treatment for schizophrenia and bipolar depression.
ITI-007 for the Treatment of Schizophrenia
In September 2015, we announced top-line clinical results from our first Phase 3 clinical trial of ITI-007 for the treatment of patients with schizophrenia. This randomized, double-blind, placebo-controlled Phase 3 clinical trial was conducted at 12 sites in the United States with 450 patients randomized (1:1:1) to receive either 60 mg of ITI-007, 40 mg of ITI-007 or placebo once daily in the morning for 28 days. The pre-specified primary efficacy measure was change from baseline versus placebo at study endpoint (4 weeks) on the centrally rated Positive and Negative Syndrome Scale, or PANSS, total score. In this trial, the once-daily dose of 60 mg of ITI-007 met the primary endpoint and demonstrated antipsychotic efficacy with statistically significant superiority over placebo at week 4 (study endpoint) with additional improvements observed in social function. Moreover, the 60 mg dose of ITI-007 showed significant antipsychotic efficacy as early as week 1, which was maintained at every time point throughout the entire study. ITI-007 showed a dose-related improvement in symptoms of schizophrenia with the 40 mg dose approximating the trajectory of improvement seen with the 60 mg dose, but the effect with 40 mg did not reach statistical significance on the primary endpoint. In addition, the 60 mg dose of ITI-007 met the key secondary endpoint of statistically significant improvement on the Clinical Global Impression Scale for Severity of Illness, or CGI-S. The 40 mg dose of ITI-007 also demonstrated a statistically significant improvement versus placebo on the CGI-S, though not formally tested against placebo as a key
secondary endpoint since it did not separate on the primary endpoint. Consistent with previous studies, ITI-007 had a favorable safety and tolerability profile as evidenced by motoric, metabolic, and cardiovascular characteristics similar to placebo, and no clinically significant changes in akathisia, extrapyramidal symptoms, prolactin, body weight, glucose, insulin, or lipids.
In September 2015, we also announced top-line data from an open-label positron emission tomography, or PET, study of ITI-007 examining brain occupancy of striatal D2 receptors. This study was conducted in patients diagnosed with schizophrenia who were otherwise healthy and stable with respect to their psychosis. After washout from their previous antipsychotic medication for at least two weeks, PET was used to determine target occupancy in brain regions at baseline (drug-free) and again after two weeks of once daily ITI-007 oral administration. In this trial, the 60 mg dose of ITI-007 was associated with a mean of approximately 40% striatal dopamine D2 receptor occupancy. As predicted by preclinical and earlier clinical data, ITI-007 demonstrated antipsychotic effect at relatively low striatal D2 receptor occupancy, lower than the occupancy range required by most other antipsychotic drugs. Unlike any existing schizophrenia treatment, this dopamine receptor phosphoprotein modulator, or DPPM, acts as a pre-synaptic partial agonist and post-synaptic antagonist at D2 receptors. We believe this mechanism likely contributes to the favorable safety profile of ITI-007, with reduced risk for hyperprolactinemia, akathisia, extrapyramidal symptoms, and other motoric side effects.
The top-line results from our first Phase 3 clinical trial of ITI-007 confirm the earlier Phase 2 results that we announced in December 2013, in which ITI-007 exhibited antipsychotic efficacy in a randomized, double-blind, placebo and active controlled clinical trial in patients with an acutely exacerbated episode of schizophrenia. In this Phase 2 trial, 335 patients were randomized to receive one of four treatments: 60 mg of ITI-007, 120 mg of ITI-007, 4 mg of risperidone (active control) or placebo in a 1:1:1:1 ratio, orally once daily for 28 days. The primary endpoint for this clinical trial was change from baseline to Day 28 on the PANSS total score. In this study, ITI-007 met the trial’s pre-specified primary endpoint, improving symptoms associated with schizophrenia as measured by a statistically significant and clinically meaningful decrease in the PANSS total score. The trial also met key secondary outcome measures related to efficacy on PANSS subscales and safety. We are also conducting a second Phase 3 clinical trial in schizophrenia that we initiated in the second quarter of 2015, with over 600 patients planned to be enrolled in the trial. In this trial, we are randomizing patients to two doses of ITI-007 (60 mg or 20 mg), risperidone (active control) or placebo over a 6-week treatment duration, and the primary outcome measure is change from baseline to Day 42 on the PANSS total score. We expect patient enrollment will be completed in the second quarter of 2016. Subject to timely enrollment, we anticipate top-line results from the second Phase 3 clinical trial will be available in mid-2016.
In addition to our two Phase 3 clinical trials, we will need to complete other clinical and non-clinical trials and manufacturing and pre-commercialization activities necessary to support the submission of a planned NDA for ITI-007 in schizophrenia, which we currently expect could occur in the first half of 2017.
ITI-007 for the Treatment of Depressive Episodes Associated with Bipolar Disorder (Bipolar Depression)
Our bipolar depression program consists of two Phase 3 multi-center, randomized, double-blind, placebo-controlled clinical trials: one to evaluate ITI-007 as a monotherapy and the other to evaluate ITI-007 as an adjunctive therapy with lithium or valproate. In each trial, approximately 550 patients with a clinical diagnosis of Bipolar I or Bipolar II disorder and who are experiencing a current major depressive episode will be randomized to receive one of three treatments: 60 mg ITI-007, 40 mg ITI-007, or placebo in a 1:1:1 ratio orally once daily for 6 weeks. In the ITI-007-401 trial, patients will receive ITI-007 or placebo as a monotherapy. In the ITI-007-402 trial, patients will receive ITI-007 or placebo adjunctive to their existing mood stabilizer lithium or valproate. We initiated our bipolar depression program in the third quarter of 2015.
The primary endpoint for both clinical trials is change from baseline at Day 42 on the Montgomery-Åsberg Depression Rating Scale, orMADRS, total score versus placebo. The MADRS is a well-validated 10-item checklist that measures the ability of a drug to reduce overall severity of depressive symptoms. Individual items
are rated by an expert clinician on a scale of 0 to 6 in which a score of 6 represents the most depressed evaluation for each item assessed. The total score ranges from 0 to 60. Secondary endpoints include measures of social function and quality of life that may illustrate the differentiated clinical profile of ITI-007. Safety and tolerability are also assessed in both clinical trials.
Other Indications for ITI-007
In the fourth quarter of 2014, we announced the top-line data from ITI-007-200, a Phase 1/2 clinical trial designed to evaluate the safety, tolerability and pharmacokinetics of low doses of ITI-007 in healthy geriatric subjects and in patients with dementia, including Alzheimer’s disease. The completion of this study marks an important milestone in our strategy to develop low doses of ITI-007 for the treatment of behavioral disturbances associated with dementia and related disorders. The ITI-007-200 trial results to date indicate that ITI-007 is safe and well-tolerated across a range of low doses, has linear- and dose-related pharmacokinetics and improves cognition in the elderly. The most frequent adverse event was mild sedation at the higher doses. We believe these results further position ITI-007 as a development candidate for the treatment of behavioral disturbances in patients with dementia and other neuropsychiatric and neurological conditions. We plan to initiate additional late phase clinical programs evaluating ITI-007 in patients with behavioral disturbances associated with dementia and related disorders, including Alzheimer’s disease, in the first half of 2016.
We are also pursuing clinical development of ITI-007 for the treatment of additional CNS diseases and disorders. At the lowest doses, ITI-007 has been demonstrated to act primarily as a potent 5-HT2A serotonin receptor antagonist. As the dose is increased, additional benefits are derived from the engagement of additional drug targets, including modest dopamine receptor modulation and modest inhibition of serotonin transporters. We believe that combined interactions at these receptors may provide additional benefits above and beyond selective 5-HT2A antagonism for treating agitation, aggression and sleep disturbances in diseases that include dementia, Alzheimer’s disease, Huntington’s disease and autism spectrum disorders, while avoiding many of the side effects associated with more robust dopamine receptor antagonism. As the dose of ITI-007 is further increased, leading to moderate dopamine receptor modulation, inhibition of serotonin transporters, and indirect glutamate modulation, these actions complement the complete blockade of 5-HT2A serotonin receptors. At a dose of 60 mg, ITI-007 has been shown effective in treating the symptoms associated with schizophrenia, and we believe this higher dose range will be useful for the treatment of bipolar disorder, depressive disorders and other neuropsychiatric diseases.
Given the potential utility for ITI-007 and follow-on compounds to treat these additional indications, we may investigate, either on our own or with a partner, agitation, aggression and sleep disturbances in additional diseases that include autism spectrum disorders; depressive disorder; intermittent explosive disorder; non-motor symptoms and motor complications associated with Parkinson’s disease; and post-traumatic stress disorder. We hold exclusive, worldwide commercialization rights to ITI-007 and a family of compounds from Bristol-Myers Squibb Company pursuant to an exclusive license.
Other Product Candidates
We have a second major program called ITI-002 that has yielded a portfolio of compounds that selectively inhibits the enzyme phosphodiesterase type 1, or PDE1. We believe PDE1 helps regulate brain activity related to cognition, memory processes and movement/coordination. On February 25, 2011, we (through our wholly owned operating subsidiary, ITI) and Takeda Pharmaceutical Company Limited, or Takeda, entered into a license and collaboration agreement, or the Takeda License Agreement, under which we agreed to collaborate to research, develop and commercialize our proprietary compound ITI-214 and other selected compounds that selectively inhibit PDE1 for use in the prevention and treatment of human diseases. On October 31, 2014, we entered into an agreement with Takeda terminating the Takeda License Agreement, or the Termination Agreement, pursuant to which all rights granted under the Takeda License Agreement were returned to us. On September 15, 2015, Takeda completed the transfer of the Investigational New Drug application, or IND, for ITI-214 to us. ITI-214 is
the first compound in its class to successfully advance into Phase 1 clinical trials. We intend to pursue the development of our PDE program, including ITI-214 for the treatment of several CNS and non-CNS conditions, which may include cognition in Parkinson’s disease, cognition in Alzheimer’s disease, cognition in schizophrenia and in other non-CNS indications. Other compounds in the PDE portfolio are also being advanced for the treatment of various indications.
Our pipeline also includes pre-clinical programs that are focused on advancing drugs for the treatment of schizophrenia, Parkinson’s disease, Alzheimer’s disease and other neuropsychiatric and neurodegenerative disorders. We are also investigating the development of treatments for disease modification of neurodegenerative disorders and non-CNS diseases.
We have assembled a management team with significant industry experience to lead the discovery and development of our product candidates. We complement our management team with a group of scientific and clinical advisors that includes recognized experts in the fields of schizophrenia and other CNS disorders, including Nobel laureate, Dr. Paul Greengard, one of our co-founders.
Since inception, we have devoted substantially all of our efforts and resources to our research and development activities. We have incurred significant net losses since inception. As of December 31, 2015, our accumulated deficit was $193.0 million. We expect to continue incurring substantial losses for the next several years as we continue to develop our clinical and pre-clinical drug candidates and programs. Our operating expenses are comprised of research and development expenses and general and administrative expenses.
Our corporate headquarters and laboratory are located in New York, New York.
Public Offerings in March 2015 and September 2015
On March 11, 2015, we completed a public offering of 5,411,481 shares of our common stock at a price of $24.00 per share for aggregate gross proceeds of approximately $129.9 million, and net proceeds of approximately $121.8 million. On September 28, 2015, we completed a public offering of 7,935,000 shares of our common stock at a price of $43.50 per share for aggregate gross proceeds of approximately $345.2 million and net proceeds of approximately $327.4 million.
Results of Operation
Revenues
The Companyfollowing discussion summarizes the key factors our management believes are necessary for an understanding of our financial statements.
We have not generated any revenue from product sales to date and we do not expect to generate revenues from product sales for at least the next several years. Our revenues for the year ended December 31, 2015 have been from a government grant and the residual reimbursement of expenses from the terminated Takeda License Agreement. Our revenues for the year ended December 31, 2014 have been from the terminated Takeda License Agreement and to a much lesser extent from a government grant. We will not receive any further revenue under the Takeda License Agreement, which was organizedterminated on October 31, 2014. We have received and may continue to receive grants from U.S. government agencies and foundations.
We do not expect any revenues that we may generate in the next several years to be significant enough to fund our operations.
Expenses
The process of researching and developing drugs for human use is lengthy, unpredictable and subject to many risks. We are unable with any certainty to estimate either the costs or the timelines in which those costs will be incurred. The clinical development of ITI-007 for the treatment of schizophrenia and for the treatment of bipolar depression consumes and will continue to consume a large portion of our current, as well as projected, resources. We intend to pursue other disease indications that ITI-007 may address, but there are significant costs associated with pursuing FDA approval for those indications, which would include the cost of additional clinical trials.
Our ITI-002 program has a vehiclecompound, ITI-214, in Phase 1 development. We intend to investigatepursue the development of our PDE program, including ITI-214 for the treatment of several CNS and non-CNS conditions, which may include cognition in Parkinson’s disease, cognition in Alzheimer’s disease, cognition in schizophrenia and in other non-CNS indications. Our other projects are still in the pre-clinical stages, and will require extensive funding not only to complete pre-clinical testing, but to enter into and complete clinical trials. Expenditures that we incur on these projects will be subject to availability of funding in addition to the funding required for the advancement of ITI-007. Any failure or delay in the advancement of ITI-007 could require us to re-allocate resources from our other projects to the advancement of ITI-007, which could have a significant material adverse impact on the advancement of these other projects and on our results of operations. Our operating expenses are comprised of (i) research and development expenses and (ii) general and administrative expenses. Our research and development costs are comprised of:
General and administrative expenses are incurred in three major categories:
We expect that research and development expenses will increase substantially as we proceed with our Phase 3 clinical trials for ITI-007 in patients with exacerbated schizophrenia and our Phase 3 clinical trials for ITI-007 in patients with bipolar disorder. We also expect that our general and administrative costs will increase substantially from prior periods primarily due to costs to perform pre-product commercialization activities and the increased costs associated with being a public reporting entity, which could include adding additional personnel. We granted options to purchase 884,703 shares of our common stock in 2015 and have granted options to purchase an additional 347,137 shares of our common stock in January 2016. We also granted restricted stock units for 5,272 and 78,806 shares of our common stock in December 2015 and January 2016, respectively. We will recognize expense associated with these restricted stock units and options over the next three years in both research and development expenses and general and administrative expenses. We expect this non-cash expense to be material and affect quarter to quarter and year to date comparisons in the upcoming year. We expect to continue to grant stock options and other stock-based awards in the future, which will increase our stock-based compensation expense in future periods.
The following table sets forth our revenues, operating expenses, interest income (expense) and income taxes expenses for the years ended December 31, 2015, 2014 and 2013 (in thousands):
| For the Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
Revenues | $ | 91 | $ | 577 | $ | 2,737 | ||||||
Expenses | ||||||||||||
Research and Development | 87,718 | 21,226 | 23,028 | |||||||||
General and Administrative | 18,187 | 10,338 | 5,976 | |||||||||
|
|
|
|
|
| |||||||
| 105,905 | 31,564 | 29,004 | ||||||||||
Interest Income (Expense) | 1,022 | 298 | (583 | ) | ||||||||
Income Taxes | (1 | ) | (2 | ) | (18 | ) | ||||||
|
|
|
|
|
| |||||||
Net Loss | $ | (104,793 | ) | $ | (30,691 | ) | $ | (26,868 | ) | |||
|
|
|
|
|
| |||||||
Comparison of Years Ended December 31, 2015 and December 31, 2014
Revenues
Revenues decreased for the year ended December 31, 2015 as compared to the year ended December 31, 2014 by approximately $485.9 thousand, or 84%, primarily due to reimbursable costs paid to us by Takeda in 2014 under the Takeda License Agreement which terminated on October 31, 2014 with limited reimbursements in 2015, offset by slightly higher revenue in 2015 from a government grant.
Research and Development Expenses
Research and development expenses increased for the year ended December 31, 2015 as compared to the year ended December 31, 2014 by approximately $66.5 million, or 313%. This change is due primarily to an increase of approximately $47.1 million of costs associated with outside clinical testing and an increase of approximately $14.9 million from nonclinical testing in the year ended December 31, 2015 over the year ended December 31, 2014. The vast majority of the increase is due to costs associated with conducting our ITI-007 Phase 3 clinical program in schizophrenia. In late 2014, we began a clinical trial of ITI-007 in patients with schizophrenia and incurred the majority of the costs for this trial in 2015. In addition, we started our second Phase 3 clinical trial of ITI-007 in patients with schizophrenia in June 2015 and incurred significant costs for this trial in the second half of 2015. In December 2015, we commenced our Phase 3 clinical trials in bipolar disorder and have incurred approximately $3.6 million in costs through December 31, 2015. In 2014, we did not incur significant costs related to clinical trials. Amounts paid to external parties comprise a large portion of our research and development costs. In the year ended December 31, 2015, we incurred approximately $76.8 million of costs to external parties who manufactured, tested and performed clinical trial related activities as compared to $16.3 million in the year ended December 31, 2014. Of these external costs, approximately $76.1 million in the year ended December 30, 2015 and $16.0 million in the year ended December 31, 2014 were for ITI-007 related projects. The remaining amounts for each of these periods were spent on other projects. Internal costs are comprised primarily of labor, fringe benefits, materials, supplies and facilities and maintenance costs and were approximately $10.9 million and $5.0 million for the years ended December 31, 2015 and 2014, respectively. The increase in these internal costs is due primarily to hiring additional research and development employees in 2015 in addition to increased stock based compensation expense.
As development of ITI-007 for the treatment of schizophrenia progresses, we anticipate costs for that program to increase considerably in the next several years as we continue to conduct Phase 3 and other clinical trials. We are also required to complete non-clinical testing to obtain FDA approval and manufacture material needed for clinical trial use, which includes non-clinical testing of the drug product and the creation of an inventory of drug product in anticipation of possible FDA approval. In addition we plan to spend increasing amounts to further our development of ITI-007 for other indications, including but not limited to, the treatment of
depressive episodes associated with bipolar disorder (bipolar depression), for treatment of behavioral disturbances associated with dementia and related disorders, and for treating agitation, aggression and sleep disturbances in diseases that include dementia, Alzheimer’s disease, Huntington’s disease and autism spectrum disorders, among other indications.
As of December 31, 2015, we employed 24 full time personnel in our research and development group as compared to 16 full time personnel at December 31, 2014. We expect to hire additional staff as we increase our development efforts and grow our business in the upcoming years.
We currently have several projects, in addition to ITI-007, that are in the research and development stages, including in the areas of cognitive dysfunction and the treatment of neurodegenerative diseases, including Alzheimer’s disease, among others. We have used internal resources and incurred expenses not only in relation to the development of ITI-007, but also in connection with these additional projects as well. We have not, however, reported these costs on a project by project basis, as these costs are broadly spread among these projects. The external costs for these projects have been minimal and are reflected in the amounts discussed in this section “—Research and Development Expenses.”
During previous years, we also incurred costs that were both reimbursable and non-reimbursable under the Takeda License Agreement. For the years ended December 31, 2015 and 2014, we incurred $30,700 and $14,000, respectively, of costs that were billable to Takeda pursuant to ongoing obligations under the termination agreement. We do not expect to incur material costs going forward under this agreement.
The research and development process necessary to develop a pharmaceutical product for commercialization is subject to extensive regulation by numerous governmental authorities in the United States and other countries. This process typically takes years to complete and requires the expenditure of substantial resources. The steps required before a drug may be marketed in the United States generally include the following:
The successful development of our product candidates and the approval process requires substantial time, effort and financial resources, and is uncertain and subject to a number of risks. We cannot be certain that any of our product candidates will prove to be safe and effective, will meet all of the applicable regulatory requirements needed to receive and maintain marketing approval, or will be granted marketing approval on a timely basis, if at all. Data from pre-clinical studies and clinical trials are susceptible to varying interpretations that could delay, limit or prevent regulatory approval or could result in label warnings related to or recalls of approved products.
We, the FDA, or other regulatory authorities may suspend clinical trials at any time if we or they believe that the subjects participating in such trials are being exposed to unacceptable risks or if such investigation warrants, acquireregulatory agencies find deficiencies in the conduct of the trials or other problems with our product candidates. Other risks associated with our product candidates are described in the section entitled “Risk Factors” in this Annual Report on Form 10-K.
General and Administrative Expenses
General and administrative expenses increased for the year ended December 31, 2015 as compared to the year ended December 31, 2014 by approximately $7.8 million, or 76%, primarily due to approximately $4.1 million of higher stock option expense and to a targetmuch lesser extent to increased bonus and labor costs and state and local franchise and capital taxes. Salaries, bonuses and related benefit costs for our executive, finance and administrative functions for the years ended December 31, 2015 and 2014 were approximately 59% and 49%, respectively, of our total general and administrative costs. Our other general and administrative expenses include patent costs, legal, accounting and other professional fees and, to a lesser extent, facilities and general office-related overhead.
We expect general and administrative costs to increase significantly as we hire additional staff, expand our operations, including initial preparation for potential commercial activities, and incur additional costs associated with the requirements of Section 404 of the Sarbanes-Oxley Act of 2002 in addition to being a public company and complying with exchange listing and SEC requirements, including the additional complexities and related costs of our transition at the end of 2015 from an “emerging growth company” to a “large accelerated filer” under the rules of the SEC. These increases could include higher legal fees, accounting fees and fees associated with investor relations activities, among others.
Interest Income
Interest income has increased to approximately $1.0 million from $304,000 for the year ended December 31, 2015 as compared to the year ended December 31, 2014. This increase is primarily a result of higher than average cash balances in 2015 as compared to 2014 which is due to the net offering proceeds of $121.8 million that we received in March 2015 and $327.4 million that we received in September 2015.
Comparison of Years Ended December 31, 2014 and December 31, 2013
Revenues
Revenue decreased for the year ended December 31, 2014 as compared to the year ended December 31, 2013 by approximately $2.2 million, or business seeking79%, due primarily to the perceived advantagesrecognition in 2013 of previously deferred revenue relating to the Takeda License Agreement and lower reimbursable costs payable to us by Takeda in 2014 under the Takeda License Agreement, offset to a much lesser extent by revenue from a government grant in 2014.
Research and Development Expenses
Research and development expenses decreased for the year ended December 31, 2014 as compared to the year ended December 31, 2013 by approximately $1.8 million, or 8%. This decrease is due primarily to costs associated with outside clinical testing for our ITI-007 Phase 2 clinical trial that was completed in late 2013 as compared to costs incurred in conducting our ITI-007 Phase 3 clinical trial, which began in the fourth quarter of 2014. Partially offsetting this decrease were expenses of approximately $6.6 million incurred in 2014 as compared to $1.9 million in 2013 related to the manufacturing and other clinical and non-clinical testing of our ITI-007 product candidate and expenses of approximately $1.9 million related to our ITI-007-200 Phase 1/2 clinical trial in healthy geriatric and dementia patients incurred only in 2014. In addition, stock option expense increased by $1.7 million for the year ended December 31, 2014 due primarily to stock options granted in 2014.
The research and development expenses incurred for amounts payable to external parties comprised a significant portion of our research and development expenses during the years ended December 31, 2014 and 2013. We incurred expenses of approximately $14.8 million and $18.8 million for the years ended December 31, 2014 and 2013, respectively, for amounts payable to external parties who manufactured, tested and performed clinical trial activities for all of our projects. We spent approximately $14.4 million and $18.3 million on external costs for the development of ITI-007 for the years ended December 31, 2014 and 2013, respectively. During the same periods, our internal research and development expenses for all projects were approximately $5.0 million and $3.0 million, respectively. The clinical development work related to ITI-007 requires the largest portion of our resources and, consequently, comprises the majority of our spending. For the years ended December 31, 2014 and 2013, we incurred total expenses of $18.8 million and $20.8 million, respectively, for all ITI-007 related projects and $2.4 million and $2.2 million, respectively, for all of our other projects. Total research and development expenses were approximately $21.2 million for the year ended December 31, 2014 as compared to $23.0 million for 2013.
During 2014 and in previous years, we also incurred costs that were both reimbursable and non-reimbursable under the Takeda License Agreement. For the year ended December 31, 2014, we incurred approximately $14,000 on direct costs that were billable to Takeda as compared to $97,000 for the year ended December 31, 2013.
General and Administrative Expenses
General and administrative expenses increased for the year ended December 31, 2014 as compared to the year ended December 31, 2013 by approximately $4.4 million, or 73%. This increase was primarily due to increased stock option expense of $1.7 million in 2014 related to options granted in 2014, and increased labor and related benefit costs of approximately $0.6 million, with the remainder comprised of higher professional fees, directors’ and officers’ insurance costs, and board of directors compensation fees, which are due to the activities associated with being a public company. We expect these costs to increase significantly as we expand our operations, including hiring of additional personnel, continue to be subject to the reporting requirements of being a publicly held corporation. Our principal business objectivepublic company and issue additional equity incentive awards.
Liquidity and Capital Resources
Through December 31, 2015, we provided funds for our operations by obtaining approximately $716.3 million of cash primarily through public and private offerings of our common stock and other securities, grants from government agencies and foundations and payments received under the terminated Takeda License Agreement. We do not believe that grant revenue will be a significant source of funding in the near future, and Takeda has limited ongoing funding obligations following the termination of the Takeda License Agreement on October 31, 2014. On March 11, 2015, we completed a public offering of 5,411,481 shares of our common stock for aggregate gross proceeds of approximately $129.9 million and net proceeds of approximately $121.8 million. On September 28, 2015, we completed an additional public offering of 7,935,000 shares of our common stock for aggregate gross proceeds of approximately $345.2 million and net proceeds of approximately $327.4 million.
As of December 31, 2015, we had a total of approximately $475.2 million in cash and cash equivalents and available-for-sale investment securities, and approximately $6.3 million of short-term liabilities consisting entirely of liabilities from operations. Excluding the increase in net cash of approximately $121.8 million and $327.4 million from the public offerings in March 2015 and September 2015, respectively, we spent approximately $103.1 million in cash for operations and equipment and we reduced working capital by approximately $92.7 million for the next 12 monthsyear ended December 31, 2015. This use of cash was primarily for conducting clinical trials and beyond such timenon-clinical testing, including manufacturing related activities and funding recurring operating expenses.
For the year 2016, we expect to spend between approximately $130 million and $160 million. We expect these expenditures to be due primarily to the development of ITI-007 in patients with schizophrenia, behavioral disturbances in dementia, bipolar disorder and depressive disorders, our ITI-007 long acting injectable development program through pre-clinical and early clinical development, research and preclinical development of our other product candidates, the continuation of manufacturing activities in connection with the development of ITI-007, recurring expenses and costs to produce, develop and validate materials to be used in clinical and non-clinical studies related to ITI-007, and expenses associated with our other development programs and general operations. We expect that cash expenditures will continue to increase after 2016 as we further expand the ITI-007 clinical stage programs, the ITI-007 long acting injectable development program through pre-clinical and early clinical development; research and preclinical development of our other product candidates; the continuation of manufacturing, pre-commercial activities in connection with the development of ITI-007 and the early stage pre-commercial launch activities for ITI-007. We believe that our existing cash and cash equivalents and investments will be sufficient to achieve long-term growthfund our operating expenses and capital expenditure requirements through the end of 2018.
We will require significant additional financing in the future to continue to fund our operations. We believe that we have the funding in place to complete the additional clinical and non-clinical trials, manufacturing and pre-commercialization activities needed for potential regulatory approval and commercialization of ITI-007 in patients with schizophrenia. With the remaining proceeds from our public offerings in March 2015 and September 2015, we believe that we have the funds to complete our proposed clinical trials of ITI-007 in bipolar disorder as a monotherapy and as an adjunctive therapy with lithium or valproate. We will also be funding clinical trials of ITI-007 for the treatment of behavioral disturbances in dementia; preclinical and clinical development of ITI-007 long acting injectable development program; additional clinical trials of ITI-007; continued clinical development of our PDE program, including ITI-214; research and preclinical development of our other product candidates; and the continuation of manufacturing activities in connection with the development of ITI-007. We anticipate requiring additional funds to obtain regulatory approval for ITI-007 in patients with dementia, including Alzheimer’s disease, for further development of ITI-007 in patients with bipolar disorder, depressive disorders and other indications, and for development of our other product candidates. We have incurred losses in every year since inception with the exception of 2011, when we received an up-front fee and a milestone payment related to the Takeda License Agreement. These losses have resulted in significant cash used in operations. For the year ended December 31, 2015, we used net cash in operating activities and purchases of equipment of approximately $103.1 million and expect to use additional cash of between approximately $130 and $160 million through a combination with a business rather than immediate, short-term earnings. The Companythe end of 2016. While we have several research and development programs underway, the ITI-007 program has advanced the furthest and will continue to consume increasing amounts of cash for conducting clinical trials and the testing and manufacturing of product material. As we continue to conduct the activities necessary to pursue FDA approval of ITI-007 and our other product candidates, we expect the amount of cash needed to fund operations to increase significantly over the next several years.
With the termination of the Takeda License Agreement in October 2014, we will not restrictreceive milestone payments and only limited expense reimbursements, including patent filing costs, from Takeda and will be responsible for the costs of developing ITI-214. On September 15, 2015, Takeda completed the transfer of the IND for ITI-214 to us. We intend to pursue the development of our potential candidate target companiesPDE1 program, including ITI-214 for the treatment of several CNS and non-CNS conditions, but we do not anticipate a significant increase in our operating expenses related to any specific business, industry or geographical locationour PDE development programs through at least the first half of 2016.
We seek to balance the level of cash, cash equivalents and thus, may acquire any typeinvestments on hand with our projected needs and to allow us to withstand periods of business.
We cannot be ablesure that future funding will be available to meet these costs through useus when we need it on terms that are acceptable to us, or at all. We sell securities and incur debt when the terms of funds in our treasury, through deferral of fees by certain service providerssuch transactions are deemed favorable to us and additional amounts, as necessary to fund our current and projected cash needs. The amount of funding we raise through sales of our common stock or other securities depends on many factors, including, but not limited to, the status and progress of our product development programs, projected cash needs, availability of funding from other sources, our stock price and the status of the capital markets. Due to the volatile nature of the financial markets, equity and debt financing may be loaneddifficult to obtain. In addition, any unfavorable development or investeddelay in the progress of our ITI-007 program could have a material adverse impact on our ability to raise additional capital.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through government or other third-party funding, marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
If adequate funds are not available to us on a timely basis, we may be required to: (1) delay, limit, reduce or terminate pre-clinical studies, clinical trials or other clinical development activities for one or more of our product candidates, including our lead product candidate ITI-007, ITI-214, and our other pre-clinical stage product candidates; (2) delay, limit, reduce or terminate our discovery research or pre-clinical development activities; or (3) enter into licenses or other arrangements with third parties on terms that may be unfavorable to us or sell, license or relinquish rights to develop or commercialize our product candidates, technologies or intellectual property at an earlier stage of development and on less favorable terms than we would otherwise agree.
Our cash is maintained in checking accounts, money market accounts, money market mutual funds, U.S. government agency securities, certificates of deposit, commercial paper, corporate notes and corporate bonds at major financial institutions. Due to the current low interest rates available for these instruments, we are earning limited interest income. We do not expect interest income to be a significant source of funding over the next several quarters. Our investment portfolio has not been adversely impacted by the problems in the credit markets that have existed over the last several years, but there can be no assurance that our investment portfolio will not be adversely affected in the future.
In 2014, we entered into a long-term lease, which was amended in December 2015, for 16,753 square feet of useable laboratory and office space located at 430 East 29th Street, New York, New York 10016. Due to the amortization of total lease payments, we have recognized $1.6 million of deferred rent in the year ended December 31, 2015. The deferred rent balance will incrementally increase over approximately the next year. We occupied these facilities as our headquarters in March 2015, replacing our previous laboratories and offices. The lease, as amended, has a term of 12 years. We expect that our facility related costs will increase moderately as a result of leasing this facility.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Contractual Obligations and Commitments
Total contractual obligations as of December 31, 2015 are summarized in the following table (in thousands):
| Payments Due By Period | ||||||||||||||||||||
| Total | Less than 1 Year | 1-3 Years | 4-5 Years | More than 5 Years | ||||||||||||||||
Operating Lease Obligations | $ | 16,256 | $ | 0 | $ | 4,257 | $ | 3,138 | $ | 8,861 | ||||||||||
The table of Contractual Obligations and Commitments does not reflect that, under the License Agreement with BMS, we may be obligated to make future milestone payments to BMS totaling $12 million; to make other future milestone payments to BMS for each licensed product of up to an aggregate of approximately $14.75 million; to make tiered single digit percentage royalty payments on sales of licensed products; and to pay BMS a percentage of non-royalty payments made in consideration of any sublicense.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP. The preparation of these financial statements requires management to make estimates and assumptions that affect reported amounts of assets and liabilities as of the date of the balance sheet and reported amounts of revenues and expenses for the periods presented. Judgments must also be made about the disclosure of contingent liabilities. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Management makes estimates and exercises judgment in revenue recognition and stock-based compensation. Actual results may differ from those estimates and under different assumptions or conditions.
We believe that the following critical accounting policies affect management’s more significant judgments and estimates used in the preparation of our financial statements:
Revenue Recognition
Revenue is recognized when all terms and conditions of the agreements have been met, including that persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable and collectability is reasonably assured. We are reimbursed for certain costs incurred on specified research projects under the terms and conditions of grants, collaboration agreements, and awards. We record the amount of reimbursement as revenues on a gross basis in accordance with ASC Topic 605-45,Revenue Recognition/Principal Agent Considerations. We are the primary obligor with respect to purchasing goods and services from third-party suppliers, are obligated to compensate the service provider for the work performed, and have discretion in selecting the supplier. Provisions for estimated losses on research grant projects and any other contracts are made in the period such losses are determined.
We have entered into arrangements involving the delivery of more than one element. Each required deliverable is evaluated to determine whether it qualifies as a separate unit of accounting. For us, this determination is generally based on whether the deliverable has “stand-alone value” to the customer. We adopted this accounting standard on a prospective basis for all Multiple-Deliverable Revenue Arrangements, or MDRAs, entered into on or after January 1, 2011, and for any MDRAs that were entered into prior to January 1, 2011, but materially modified on or after that date.
The adoption of this accounting standard did not have a material impact on our results of operations for the years ended December 31, 2015, 2014 and 2013, or on our financial positions as of those dates.
We have adopted ASC Topic 605-28,Milestone Method. Under this guidance, we recognize revenue contingent upon the achievement of a substantive milestone in its entirety in the period the milestone is achieved. Substantive milestone payments are recognized upon achievement of the milestone only if all of the following conditions are met:
Determination as to whether a payment meets the aforementioned conditions involves management’s judgment. If any of these conditions are not met, the resulting payment would not be considered a substantive milestone, and therefore, the resulting payment would be considered part of the consideration for the single unit of accounting and be recognized as revenue as such performance obligations are performed under either the proportional performance or straight-line methods, as applicable. In addition, the determination that one such payment was not a substantive milestone could prevent us from concluding that subsequent milestone payments were substantive milestones and, as a result, any additional milestone payments could also be considered part of the consideration for the single unit of accounting and would be recognized as revenue as such performance obligations are performed under either the proportional performance or straight-line methods, as applicable.
Research and Development
Except for payments made in advance of services, the Company expenses its research and development costs as incurred. For payments made in advance, the Company recognizes research and development expense as the services are rendered. Research and development costs primarily consist of salaries and related expenses for personnel and resources and the costs of clinical trials. Other research and development expenses include preclinical analytical testing, outside services, providers, materials and consulting fees.
Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as subject enrollment, clinical site activations or information provided to the Company by its vendors with respect to their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the financial statements as prepaid or accrued research and development expense, as the case may be.
As part of the process of preparing its financial statements, the Company is required to estimate its expenses resulting from its obligations under contracts with vendors, clinical research organizations and consultants and under clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment flows that do not match the periods over which materials or services are provided under such contracts. The Company’s objective is to reflect the appropriate trial expenses in its financial statements by matching those expenses with the period in which services are performed and efforts are expended. The Company accounts for these expenses according to the progress of the trial as measured by subject progression and the timing of various aspects of the trial. The
Company determines accrual estimates through financial models taking into account discussion with applicable personnel and outside service providers as to the progress or state of consummation of trials, or the services completed. During the course of a clinical trial, the Company adjusts its clinical expense recognition if actual results differ from its estimates. The Company makes estimates of its accrued expenses as of each balance sheet date based on the facts and circumstances known to it at that time. The Company’s clinical trial accruals are dependent upon the timely and accurate reporting of contract research organizations and other third-party vendors. Although the Company does not expect its estimates to be materially different from amounts actually incurred, its understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in it reporting amounts that are too high or too low for any particular period.
Stock-Based Compensation
Stock-based payments in the form of options are accounted for in accordance with the provisions of ASC Topic 718,Compensation—Stock Compensation. The fair value of share-based payments is estimated, on the date of grant, using the Black-Scholes-Merton option-pricing model, or the Black-Scholes model. The resulting fair value is recognized ratably over the requisite service period, which is generally the vesting period of the option. Restricted stock units are valued at the fair market value on the date of grant as determined by the closing stock price.
For all awards granted with time-based vesting conditions, expense is amortized using the straight-line attribution method. For awards that contain a performance-based vesting condition, expense is amortized using the accelerated attribution method. Share-based compensation expense recognized in the statements of operations for the years ended December 31, 2015, 2014 and 2013 is based on share-based awards ultimately expected to vest, and this amount has therefore been reduced for estimated forfeitures. ASC Topic 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Pre-vesting forfeitures for the fiscal years ended December 31, 2015, 2014 and 2013 were estimated based on our historical experience and have not been material.
We utilize the Black-Scholes model for estimating fair value of our stock options granted. Option valuation models, including the Black-Scholes model, require the input of subjective assumptions, and changes in the assumptions used can materially affect the grant date fair value of an award. These assumptions include the risk-free rate of interest, expected dividend yield, expected volatility and the expected life of the award. Restricted stock units are valued at the fair market value on the date of grant as determined by the closing stock price.
Expected volatility rates are based on historical volatility of the common stock of comparable publicly traded entities and other factors due to the limited historical information about our common stock. The expected life of stock options is the period of time for which the stock options are expected to be outstanding. Given the limited historical exercise data, the expected life is determined using the “simplified method,” which is defined as the midpoint between the vesting date and the end of the contractual term.
The risk-free interest rates are based on the U.S. Treasury yield for a period consistent with the expected term of the option in effect at the time of the grant. We have not paid dividends to our stockholders managementsince our inception and do not plan to pay cash dividends in the foreseeable future. Therefore, we have assumed an expected dividend rate of zero.
Prior to January 1, 2014, given that there was no active market for our common stock, the exercise price of the stock options on the date of grant was determined and approved by the board of directors using several factors, including progress and milestones achieved in our business development and performance, the price per share of our convertible preferred stock offerings and general industry and economic trends. In establishing the estimated fair value of our common stock, we considered the guidance set forth in American Institute of Certified
Public Accountants Practice Guide, “Valuation of Privately-Held-Company Equity Securities Issued as Compensation.” For stock options granted in 2014 and 2015, the exercise price was determined by using the closing market price of our common stock on the date of grant.
Under ASC Topic 718, the cumulative amount of compensation cost recognized for instruments classified as equity that ordinarily would result in a future tax deduction under existing tax law shall be considered to be a deductible difference in applying ASC Topic 740,Income Taxes. The deductible temporary difference is based on the compensation cost recognized for financial reporting purposes. However, these provisions currently do not impact us, as all the deferred tax assets have a full valuation allowance.
Since we had net operating loss carryforwards as of December 31, 2015, 2014 and 2013, no excess tax benefits for the tax deductions related to share-based awards were recognized in the statements of operations.
Equity instruments issued to non-employees are accounted for under the provisions of ASC Topic 718 and ASC Topic 505-50,Equity/Equity-Based Payments to Non-Employees. Accordingly, the estimated fair value of the equity instrument is recorded on the earlier of the performance commitment date or the date the services required are completed and are marked to market during the service period.
Recently Issued Accounting Pronouncements
We review new accounting standards to determine the expected financial impact, if any, that the adoption of each such standard will have.
In November 2015, the FASB issued Accounting Standards Update No. 2015-17, Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes. Current GAAP requires an entity to separate deferred income tax liabilities and assets into current and noncurrent amounts in a classified statement of financial position. To simplify the presentation of deferred income taxes, ASU 2015-17 requires that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. The current requirement that deferred tax liabilities and assets of a tax-paying component of an entity be offset and presented as a single amount is not affected by the amendments in this Update. The amendments in this Update are effective for financial statements issued for annual periods beginning after December 15, 2016, and interim periods within those annual periods. Earlier application is permitted for all entities as of the beginning of an interim or annual reporting period. We have elected to adopt this update as of the fourth quarter of 2015, retrospectively. The adoption of this standard did not have a material impact on our consolidated financial statements and accompanying notes.
In May 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update No. 2014-09 (ASU 2014-09), Revenue from Contracts with Customers. ASU 2014-09 will eliminate transaction-specific and industry-specific revenue recognition guidance under current GAAP and replace it with a principle-based approach for determining revenue recognition. ASU 2014-09 will require that companies recognize revenue based on the value of transferred goods or services as they occur in the contract. ASU 2014-09 also will require additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments and assets recognized from costs incurred to obtain or fulfill a contract. In July 2015, the FASB decided to defer the effective date of the standard from January 1, 2017, to January 1, 2018, with an option that permits companies to adopt the standard as early as the original effective date. Early application prior to the original effective date is not permitted. The standard permits the use of either the retrospective or cumulative effect transition method. Presently, we are assessing what effect the adoption of this standard will have on our consolidated financial statements and accompanying notes.
| Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Interest Rate Sensitivity. As of December 31, 2015, we had cash, cash equivalents and marketable securities of $475.2 million consisting of cash deposited in a highly rated financial institution in the United States and in a short-term U.S. Treasury money market fund, as well as high-grade corporate bonds and commercial paper. The primary objective of our investment activities is to preserve our capital for the purpose of funding operations and we do not enter into investments for trading or speculative purposes. We believe that we do not have material exposure to high-risk investments such as mortgage-backed securities, auction rate securities or other investors. Asspecial investment vehicles within our money-market fund investments. We believe that we do not have any material exposure to changes in fair value as a result of changes in interest rates, although the recent rise in interest rates has resulted in our unrealized loss on investments for the year ended December 31, 2015 and 2014 totaling approximately $0.5 million and $0.1 million, respectively. Since we plan on holding those investments to maturity, no recognition of impairment is required. Declines in interest rates, however, would reduce future investment income.
Capital Market Risk. We currently have no product revenues and depend on funds raised through other sources. One possible source of funding is through further equity offerings. Our ability to raise funds in this manner depends upon capital market forces affecting our stock price.
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
INTRA-CELLULAR THERAPIES, INC.
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
| Item 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
Our principal executive officer and principal financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of the dateend of the period covered by this report,Form 10-K, have concluded that, based on such evaluation, our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in the Company had $4,070reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in cash. There are no assurances that the Company will be able to secure any additional funding as needed. Currently, however our ability to continue as a going concern is dependent upon our ability to generate future profitable operations and/or to obtain the necessary financing to meet our obligationsSEC’s rules and repay our liabilities arising from normal business operations when they come due. Our ability to continue as a going concern is also dependent on our ability to find a suitable target company and enter into a possible reverse merger with such company. Management’s plan includes obtaining additional funds by equity financing through a reverse merger transaction and/or related party advances, however there is no assurance of additional funding being available.
Management’s Report our management believes that there are numerous firms seeking the perceived benefits of becoming a publicly traded corporation. Such perceived benefits of becoming a publicly traded corporation include, among other things, facilitating or improving the terms on which additional equity financing may be obtained, providing liquidity for the principals of and investors in a business, creating a means for providing incentive stock options or similar benefits to key employees, and offering greater flexibility in structuring acquisitions, joint ventures and the like through the issuance of stock. Potentially available business combinations may occur in many different industries and at various stages of development, all of which will make the task of comparative investigation and analysis of such business opportunities extremely difficult and complex.
For the Period from August 29, 2012 (Inception) to March 31, 2013 | ||||
| Net Cash (Used in) Operating Activities | $ | (5,930 | ) | |
| Net Cash (Used in) Investing Activities | $ | - | ||
| Net Cash Provided by Financing Activities | $ | 10,000 | ||
| Net Increase in Cash and Cash Equivalents | $ | 4,070 | ||
The Company’s management is responsible for theseestablishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act, as a process designed by, or under the supervision of, the Company’s principal executive and principal financial officers and effected by the Company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s internal control over financial reporting includes those policies and procedures that:
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2015. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (2013).
Based on our assessment, management believes that, as of December 31, 2015, the company’s internal control over financial reporting is effective based on those criteria.
Our independent registered public accounting firm has issued an audit report on our assessment of our internal control over financial reporting. This report appears further below in this Item 9A.
Changes in Internal Controls
There were no changes in our internal control over financial reporting, identified in connection with the evaluation of such internal control that occurred during the fourth quarter of our last fiscal year that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting other than our continued improvement of documentation and other existing internal controls. This improvement included but was not limited to hiring additional personnel, hiring an independent outside firm to aid in the controls testing and reassigning tasks to allow for greater segregation of duties.
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Intra-Cellular Therapies, Inc.
We have audited Intra-Cellular Therapies, Inc. and subsidiaries’ internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Intra-Cellular Therapies, Inc. and subsidiaries’ management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial
reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Intra-Cellular Therapies, Inc. and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Intra-Cellular Therapies, Inc. and subsidiaries as of December 31, 2015 and 2014, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2015 and our report dated February 25, 2016 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Baltimore, MD
February 25, 2016
| Item 9B. | OTHER INFORMATION |
Not applicable.
| Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Management and Corporate Governance,” “Section 16(a) Beneficial Ownership Reporting Compliance,” and “Code of Conduct and Ethics” in the Company’s Proxy Statement for the 2016 Annual Meeting of Stockholders.
| Item 11. | EXECUTIVE COMPENSATION |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Executive Officer and Director Compensation,” “Compensation Discussion and Analysis,” “Management and Corporate Governance – Compensation Committee Interlocks and Insider Participation,” “Compensation Committee Report” and “Compensation Practices and Policies Relating to Risk Management” in the Company’s Proxy Statement for the 2016 Annual Meeting of Stockholders.
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information” in the Company’s Proxy Statement for the 2016 Annual Meeting of Stockholders.
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Certain Relationships and Related Person Transactions” and “Management and Corporate Governance” in the Company’s Proxy Statement for the 2016 Annual Meeting of Stockholders.
| Item 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The response to this item is incorporated by reference from the discussion responsive thereto under the caption “Proposal 2: Ratification of Selection of Independent Registered Public Accounting Firm” in the Company’s Proxy Statement for the 2016 Annual Meeting of Stockholders.
| EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
Item 15(a). | The following documents are filed as part of this annual report on Form 10-K: |
Item 15(a)(1)and (2) | See “Index to Consolidated Financial Statements and Financial Statement Schedules” at Item 8 to this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto. |
Item 15(a)(3) | Exhibits |
The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
Exhibit | Exhibit Description | Filed Herewith | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Reg. Number | |||||||
| 2.1 | Agreement and Plan of Merger, dated as of August 23, 2013, by and among the Registrant, ITI, Inc. and Intra-Cellular Therapies, Inc. | 8-K (Exhibit 2.1) | 8/29/2013 | 000-54896 | ||||||||
| 2.2 | Agreement and Plan of Merger, dated as of August 29, 2013, by and between the Registrant and Intra-Cellular Therapies, Inc., relating to the name change of the Registrant. | 8-K (Exhibit 2.2) | 9/5/2013 | 000-54896 | ||||||||
| 3.1 | Restated Certificate of Incorporation of the Registrant, filed with the Secretary of State of the State of Delaware on November 7, 2013. | S-1/A (Exhibit 3.1) | 11/26/13 | 333-191238 | ||||||||
| 3.2 | Certificate of Merger relating to the Merger of ITI, Inc. with and into Intra-Cellular Therapies, Inc., filed with the Secretary of State of the State of Delaware on August 29, 2013. | 8-K (Exhibit 3.3) | 9/5/2013 | 000-54896 | ||||||||
| 3.3 | Certificate of Ownership and Merger relating to the Merger of Intra-Cellular Therapies, Inc. with and into the Registrant, filed with the Secretary of State of the State of Delaware on August 29, 2013, relating to the name change of the Registrant. | 8-K (Exhibit 3.4) | 9/5/2013 | 000-54896 | ||||||||
| 3.4 | Restated Bylaws of the Registrant. | 8-K (Exhibit 3.5) | 9/5/2013 | 000-54896 | ||||||||
| 4.1 | Form of common stock certificate. | 8-K (Exhibit 4.1) | 9/5/2013 | 000-54896 | ||||||||
| 4.2 | .1 | Warrant to Purchase Common Stock dated April 19, 2013 issued to Alzheimer Drug Discovery Foundation, Inc. | 8-K (Exhibit 4.2.1) | 9/5/2013 | 000-54896 | |||||||
Exhibit Exhibit Description (Exhibit 4.2.2) (Exhibit 10.1.1) (Exhibit 10.1.2) (Exhibit 10.2) (Exhibit 10.2.2) (Exhibit 10.3) (Exhibit 10.1) (Exhibit 10.4)
Number Filed
Herewith Incorporated
by Reference
herein from
Form or
Schedule Filing Date SEC File/
Reg. Number .2 Amendment dated August 29, 2013 to Warrant to Purchase Common Stock dated April 19, 2013 issued to Alzheimer Drug Discovery Foundation, Inc. 8-K 9/5/2013 000-54896 10.1 .1 License Agreement dated as of May 31, 2005 by and between Bristol-Meyers Squibb Company and Intra-Cellular Therapies, Inc.** 8-K/A 10/31/2013 000-54896 .2 Amendment No. 1 to License Agreement dated as of November 3, 2010 by and between Bristol-Meyers Squibb Company and Intra-Cellular Therapies, Inc. 8-K 9/5/2013 000-54896 10.2 .1 License and Collaboration Agreement dated as of February 25, 2011 by and between Takeda Pharmaceutical Company Limited and Intra-Cellular Therapies, Inc.** 8-K/A 10/31/2013 000-54896 .2 Termination Agreement dated as of October 31, 2014 by and between Takeda Pharmaceutical Company Limited and Intra-Cellular Therapies, Inc.† 10-K 3/12/2015 001-36274 10.3 Employment Agreement effective as of February 26, 2008 by and between Sharon Mates, Ph.D. and Intra-Cellular Therapies, Inc.* 8-K 9/5/2013 000-54896 10.4 Employment Agreement effective as of August 3, 2015 by and between Michael I. Halstead and Intra-Cellular Therapies, Inc..* 10-Q 11/5/2015 001-36274 10.5 Employment Agreement effective as of February 26, 2008 by and between Lawrence J. Hineline and Intra-Cellular Therapies, Inc.* 8-K 9/5/2013 000-54896 10.6 Employment Agreement effective as of November 4, 2015 by and between Robert Davis, Ph.D. and Intra-Cellular Therapies, Inc.* X 10.7 Employment Agreement effective as of November 5, 2015 by and between Kimberly Vanover, Ph.D. and Intra-Cellular Therapies, Inc.* X
Exhibit | Exhibit Description | Filed Herewith | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Reg. Number | |||||||||||||
| 10.8 | Employee Proprietary Information, Inventions, and Non-Competition Agreement effective as of September 1, 2003 by and between Sharon Mates, Ph.D. and Intra-Cellular Therapies, Inc.* | 8-K (Exhibit 10.8) | 9/5/2013 | 000-54896 | ||||||||||||||
| 10.9 | Employee Proprietary Information, Inventions, and Non-Competition Agreement effective as of July 29, 2014 by and between Michael Halstead and Intra-Cellular Therapies, Inc.* | 10-K (Exhibit 10.11) | 3/12/2015 | 001-36274 | ||||||||||||||
| 10.10 | Employee Proprietary Information, Inventions, and Non-Competition Agreement effective as of December 1, 2003 by and between Lawrence J. Hineline and Intra-Cellular Therapies, Inc.* | 8-K (Exhibit 10.9) | 9/5/2013 | 000-54896 | ||||||||||||||
| 10.11 | Employee Proprietary Information, Inventions, and Non-Competition Agreement effective as of November 4, 2015 by and between Robert Davis, Ph.D. and Intra-Cellular Therapies, Inc.* | X | ||||||||||||||||
| 10.12 | Employee Proprietary Information, Inventions, and Non-Competition Agreement effective as of March 5, 2007 by and between Kimberly E. Vanover, Ph.D. and Intra-Cellular Therapies, Inc.* | 8-K (Exhibit 10.12) | 9/5/2013 | 000-54896 | ||||||||||||||
| 10.13 | Form of Indemnification Agreement by and between the Company and its directors and executive officers.* | 8-K (Exhibit 10.13) | 9/5/2013 | 000-54896 | ||||||||||||||
| 10.14 | 2003 Equity Incentive Plan, as amended.* | 8-K (Exhibit 10.14) | 9/5/2013 | 000-54896 | ||||||||||||||
| 10.15 | Form of Stock Option Agreement under the 2003 Equity Incentive Plan, as amended.* | 8-K (Exhibit 10.15) | 9/5/2013 | 000-54896 | ||||||||||||||
| 10.16 | Amended and Restated 2013 Equity Incentive Plan.* | 8-K (Exhibit 10.1) | 6/18/2015 | 001-36274 | ||||||||||||||
| 10.17 | Form of Stock Option Agreement under the 2013 Equity Incentive Plan.* | 10-K (Exhibit 10.19) | 3/25/2014 | 001-36274 | ||||||||||||||
| 10.18 | Non-Employee Director Compensation Policy.* | 10-Q (Exhibit 10.1) | 8/12/2014 | 001-36274 | ||||||||||||||
Exhibit | Exhibit Description | Filed Herewith | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Reg. Number | |||||||||||
| 10.19 | Redemption Agreement dated as of August 29, 2013 by and between the Registrant and NLBDIT 2010 Services, LLC. | 8-K (Exhibit 10.17) | 9/5/2013 | 000-54896 | ||||||||||||
| 10.20 | Indemnity Agreement dated as of August 29, 2013 by and among the Registrant, Intra-Cellular Therapies, Inc. and Samir N. Masri. | 8-K (Exhibit 10.18) | 9/5/2013 | 000-54896 | ||||||||||||
| 10.21 | Registration Rights Agreement dated as of August 29, 2013 by and among Intra-Cellular Therapies, Inc., the stockholders named therein and the Registrant. | 8-K (Exhibit 10.19) | 9/5/2013 | 000-54896 | ||||||||||||
| 14.1 | Corporate Code of Conduct and Ethics and Whistleblower Policy. | X | ||||||||||||||
| 21.1 | Subsidiaries. | S-1 (Exhibit 21.1) | 9/18/13 | 333-191238 | ||||||||||||
| 23.1 | Consent of Ernst & Young LLP. | X | ||||||||||||||
| 31.1 | Certification of the Chief Executive Officer. | X | ||||||||||||||
| 31.2 | Certification of the Chief Financial Officer. | X | ||||||||||||||
| 32.1 | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | X | ||||||||||||||
| 101 | .INS | XBRL Instance Document. | X | |||||||||||||
| .SCH | XBRL Taxonomy Extension Schema Document. | X | ||||||||||||||
| .CAL | XBRL Taxonomy Extension Calculation Linkbase Document. | X | ||||||||||||||
| .DEF | XBRL Taxonomy Extension Definition. | X | ||||||||||||||
| .LAB | XBRL Taxonomy Extension Label Linkbase Document. | X | ||||||||||||||
| .PRE | XBRL Taxonomy Presentation Linkbase Document. | X | ||||||||||||||
| * | Management contract or compensatory plan or arrangement. |
| ** | Confidential treatment has been granted for portions of this Exhibit. Redacted portions filed separately with the Securities and Exchange Commission. |
| † | Confidential treatment is being requested for portions of this Exhibit. Redacted portions filed separately with the Securities and Exchange Commission. |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| INTRA-CELLULAR THERAPIES, INC. | ||||||
| Date: February 25, 2016 | By: | /s/ Sharon Mates, Ph.D. | ||||
| Sharon Mates, Ph.D. | ||||||
| Chairman, President and Chief Executive Officer | ||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated below and on the dates indicated.
Signatures | Title | Date | ||||
| By: | /s/ Sharon Mates, Ph.D. Sharon Mates, Ph.D. | Chairman, President and Chief Executive Officer (principal executive officer) | February 25, 2016 | |||
| By: | /s/ Lawrence J. Hineline Lawrence J. Hineline | Vice President of Finance and Chief Financial Officer (principal financial officer and principal accounting officer) | February 25, 2016 | |||
| By: | /s/ Christopher Alafi, Ph.D. Christopher Alafi, Ph.D. | Director | February 25, 2016 | |||
| By: | /s/ Richard Lerner, M.D. Richard Lerner, M.D. | Director | February 25, 2016 | |||
| By: | /s/ Joel S. Marcus Joel S. Marcus | Director | February 25, 2016 | |||
| By: | /s/ Rory B. Riggs Rory B. Riggs | Director | February 25, 2016 | |||
| By: | /s/ Robert L. Van Nostrand Robert L. Van Nostrand | Director | February 25, 2016 | |||
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Intra-Cellular Therapies, Inc.
We have audited the accompanying consolidated balance sheets of Intra-Cellular Therapies, Inc. and subsidiaries as of December 31, 2015 and 2014, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2015. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our auditaudits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements,statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our auditaudits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Oneida Resources Corp. as of MarchIntra-Cellular Therapies, Inc. and subsidiaries at December 31, 2013,2015 and 2014, and the consolidated results of itstheir operations and itstheir cash flows for each of the three years in the period from August 29, 2012 (inception) through Marchended December 31, 20132015, in conformity with accounting principlesU.S. generally accepted accounting principles.
We also have audited, in accordance with the United Statesstandards of America.
| ASSETS | ||||
| CURRENT ASSETS: | ||||
| Cash | $ | 4,070 | ||
| Total Current Assets | 4,070 | |||
| TOTAL ASSETS | $ | 4,070 | ||
| LIABILITIES AND STOCKHOLDER'S DEFICIENCY | ||||
| CURRENT LIABILITIES: | ||||
Accounts payable and accrued expenses | $ | 26,170 | ||
| Total Current Liabilities | $ | 26,170 | ||
| COMMITMENTS AND CONTINGENCIES | - | |||
| STOCKHOLDER'S DEFICIENCY: | ||||
| Preferred stock, $.0001 par value; 10,000,000 shares authorized; | ||||
| none issued and outstanding | - | |||
| Common stock, $.0001 par value; 100,000,000 shares authorized; | ||||
| 5,000,000 shares issued and outstanding | 500 | |||
| Additional paid-in capital | 9,500 | |||
| Accumulated deficit during the development stage | (32,100 | ) | ||
| Total Stockholder's Deficiency | (22,100 | ) | ||
TOTAL LIABILITIES AND STOCKHOLDER'S DEFICIENCY | $ | 4,070 | ||
/s/ Ernst & Young LLP
Baltimore, MD
February 25, 2016
Intra-Cellular Therapies, Inc.
| December 31, | ||||||||
| 2015 | 2014 | |||||||
Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 47,159,303 | $ | 61,325,044 | ||||
Investment securities, available-for-sale | 428,041,021 | 68,320,672 | ||||||
Accounts receivable | 30,660 | 51,603 | ||||||
Prepaid expenses and other current assets | 8,025,147 | 1,288,953 | ||||||
|
|
|
| |||||
Total current assets | 483,256,131 | 130,986,272 | ||||||
Property and equipment, net | 775,522 | 54,553 | ||||||
Other assets | 71,875 | 70,944 | ||||||
|
|
|
| |||||
Total assets | $ | 484,103,528 | $ | 131,111,769 | ||||
|
|
|
| |||||
Liabilities and stockholders’ equity | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 1,632,905 | $ | 2,052,765 | ||||
Accrued and other current liabilities | 3,423,464 | 7,529,241 | ||||||
Accrued employee benefits | 1,207,143 | 975,058 | ||||||
|
|
|
| |||||
Total current liabilities | 6,263,512 | 10,557,064 | ||||||
|
|
|
| |||||
Long-term deferred rent | 1,597,105 | — | ||||||
|
|
|
| |||||
Total liabilities | 7,860,617 | 10,557,064 | ||||||
Stockholders’ equity: | ||||||||
Common stock, $.0001 par value: 100,000,000 shares authorized; 43,155,875 and 29,499,059 shares issued and outstanding at December 31, 2015 and 2014, respectively | 4,316 | 2,950 | ||||||
Additional paid-in capital | 669,878,103 | 208,912,345 | ||||||
Accumulated deficit | (193,049,098 | ) | (88,255,957 | ) | ||||
Accumulated comprehensive loss | (590,410 | ) | (104,633 | ) | ||||
|
|
|
| |||||
Total stockholders’ equity | 476,242,911 | 120,554,705 | ||||||
|
|
|
| |||||
Total liabilities and stockholders’ equity | $ | 484,103,528 | $ | 131,111,769 | ||||
|
|
|
| |||||
See accompanying notes to theconsolidated financial statements.
| REVENUES | $ | - | ||
| GENERAL AND ADMINISTRATIVE EXPENSES | 32,100 | |||
| (LOSS) BEFORE BENEFIT FROM INCOME TAXES | (32,100 | ) | ||
| BENEFIT FROM INCOME TAXES | - | |||
| NET (LOSS) | $ | (32,100 | ) | |
| BASIC AND DILUTED LOSS PER SHARE | - | |||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES | ||||
| OUTSTANDING - BASIC AND DILUTED | 3,720,930 |
| Years Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
Revenues: | ||||||||||||
License and collaboration revenue | $ | 30,659 | $ | 547,546 | $ | 2,737,002 | ||||||
Grant Revenue | 60,705 | 29,755 | — | |||||||||
|
|
|
|
|
| |||||||
Total revenues | 91,364 | 577,301 | 2,737,002 | |||||||||
Costs and expenses: | ||||||||||||
Research and development | 87,718,074 | 21,226,345 | 23,027,578 | |||||||||
General and administrative | 18,187,286 | 10,337,679 | 5,976,276 | |||||||||
|
|
|
|
|
| |||||||
Total costs and expenses | 105,905,360 | 31,564,024 | 29,003,854 | |||||||||
Loss from operations | (105,813,996 | ) | (30,986,723 | ) | (26,266,852 | ) | ||||||
Interest income | 1,022,455 | 303,936 | 29,617 | |||||||||
Interest expense | — | (7,073 | ) | (612,963 | ) | |||||||
Income tax expense | (1,600 | ) | (1,600 | ) | (18,000 | ) | ||||||
|
|
|
|
|
| |||||||
Net loss | $ | (104,793,141 | ) | $ | (30,691,460 | ) | $ | (26,868,198 | ) | |||
|
|
|
|
|
| |||||||
Net loss per common share: | ||||||||||||
Basic & Diluted | $ | (2.91 | ) | $ | (1.07 | ) | $ | (1.56 | ) | |||
Weighted average number of common shares: | ||||||||||||
Basic & Diluted | 36,069,237 | 28,650,067 | 17,260,768 | |||||||||
See accompanying notes to theconsolidated financial statements.
| Accumulated | ||||||||||||||||||||||||||||
| Deficit | ||||||||||||||||||||||||||||
| Additional | During the | Total | ||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Paid-in | Development | Stockholder's | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Stage | Deficiency | ||||||||||||||||||||||
| Balance at August 29, 2012 (Inception) | - | $ | - | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||
| Common stock issuance | - | - | 5,000,000 | 500 | 9,500 | - | 10,000 | |||||||||||||||||||||
| Net (loss) | - | - | - | - | - | (32,100 | ) | (32,100 | ) | |||||||||||||||||||
| Balance at March 31, 2013 | - | $ | - | 5,000,000 | $ | 500 | $ | 9,500 | $ | (32,100 | ) | $ | (22,100 | ) | ||||||||||||||
Intra-Cellular Therapies, Inc.
Consolidated Statements of Comprehensive Loss
| Years Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
Net loss | $ | (104,793,141 | ) | $ | (30,691,460 | ) | $ | (26,868,198 | ) | |||
Other comprehensive loss: | ||||||||||||
Unrealized loss on investment securities | (485,777 | ) | (104,633 | ) | — | |||||||
|
|
|
|
|
| |||||||
Comprehensive loss | $ | (105,278,918 | ) | $ | (30,796,093 | ) | $ | (26,868,198 | ) | |||
|
|
|
|
|
| |||||||
See accompanying notes to theconsolidated financial statements.
| ONEIDA RESOURCES CORP. | ||||
| (A Development Stage Company) | ||||
| STATEMENT OF CASH FLOWS | ||||
| FOR THE PERIOD AUGUST 29, 2012 (INCEPTION) TO MARCH 31, 2013 | ||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||
| NET (LOSS) | $ | (32,100 | ) | |
| ADJUSTMENT TO RECONCILE NET LOSS TO NET | ||||
| CASH USED IN OPERATING ACTIVITIES: | ||||
| Increase in accounts payable | 26,170 | |||
| NET CASH USED IN OPERATING ACTIVITIES | (5,930 | ) | ||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||
| Proceeds from issuance of common stock | 10,000 | |||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 10,000 | |||
| NET INCREASE IN CASH | 4,070 | |||
| CASH, BEGINNING OF PERIOD | - | |||
| CASH, END OF PERIOD | $ | 4,070 | ||
Intra-Cellular Therapies, Inc.
Consolidated Statements of Stockholders’ Equity
| Common Stock | Additional Paid-in Capital | Accumulated Deficit | Accumulated Other Comprehensive Loss | Total Stockholders’ Equity | ||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
Balance at December 31, 2012 | 14,599,612 | 1,460 | $ | 47,678,924 | $ | (30,696,299 | ) | $ | — | $ | 16,984,085 | |||||||||||||
Conversion of convertible notes | 110,446 | 11 | 701,712 | — | — | 701,723 | ||||||||||||||||||
Private placement of common stock | 6,916,697 | 692 | 39,962,808 | — | — | 39,963,500 | ||||||||||||||||||
Exercise of stock options | 514,466 | 51 | 332,887 | — | — | 332,938 | ||||||||||||||||||
Stock subscription | 18,225 | 2 | 109,832 | — | — | 109,834 | ||||||||||||||||||
Share-based compensation | — | — | 391,393 | — | — | 391,393 | ||||||||||||||||||
Net loss | — | — | — | (26,868,198 | ) | — | (26,868,198 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balance at December 31, 2013 | 22,159,446 | $ | 2,216 | $ | 89,177,556 | $ | (57,564,497 | ) | $ | — | $ | 31,615,275 | ||||||||||||
Common shares issued February 5, 2014 | 7,063,300 | 706 | 115,442,041 | — | 115,442,747 | |||||||||||||||||||
Exercise of stock options | 247,165 | 25 | 162,955 | — | 162,980 | |||||||||||||||||||
Stock issued for services | 10,923 | 1 | 176,084 | 176,085 | ||||||||||||||||||||
Stock subscription | 18,225 | 2 | 109,831 | 109,833 | ||||||||||||||||||||
Share-based compensation | — | — | 3,843,878 | — | 3,843,878 | |||||||||||||||||||
Net loss | — | — | — | (30,691,460 | ) | (30,691,460 | ) | |||||||||||||||||
Other comprehensive loss | (104,633 | ) | (104,633 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balance at December 31, 2014 | 29,499,059 | $ | 2,950 | $ | 208,912,345 | $ | (88,255,957 | ) | $ | (104,633 | ) | $ | 120,554,705 | |||||||||||
Common shares issued March 11, 2015 | 5,411,481 | 541 | 121,803,828 | — | 121,804,369 | |||||||||||||||||||
Common shares issued September 28, 2015 | 7,935,000 | 793 | 327,435,412 | — | 327,436,205 | |||||||||||||||||||
Exercise of stock options | 305,005 | 31 | 653,015 | — | 653,046 | |||||||||||||||||||
Stock issued for services | 5,330 | 1 | 182,598 | 182,599 | ||||||||||||||||||||
Share-based compensation | — | — | 10,890,905 | — | 10,890,905 | |||||||||||||||||||
Net loss | — | — | — | (104,793,141 | ) | (104,793,141 | ) | |||||||||||||||||
Other comprehensive loss | (485,777 | ) | (485,777 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balance at December 31, 2015 | 43,155,875 | $ | 4,316 | $ | 669,878,103 | $ | (193,049,098 | ) | $ | (590,410 | ) | $ | 476,242,911 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
See accompanying notes to theconsolidated financial statements.
Intra-Cellular Therapies, Inc.
Consolidated Statements of Cash Flows
| Years Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
Operating activities | ||||||||||||
Net loss | $ | (104,793,141 | ) | $ | (30,691,460 | ) | $ | (26,868,198 | ) | |||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
Depreciation expense | 139,626 | 25,481 | 23,249 | |||||||||
Share-based compensation expense | 10,890,905 | 3,843,878 | 391,393 | |||||||||
Issuance of stock for services | 182,599 | 176,085 | — | |||||||||
Amortization of premiums on investment activities | 712,675 | 297,223 | — | |||||||||
Changes in operating assets and liabilities: | ||||||||||||
Accounts receivable | 20,943 | 284,715 | (35,889 | ) | ||||||||
Prepaid expenses and other assets | (6,737,125 | ) | (466,099 | ) | (574,341 | ) | ||||||
Accounts payable | (419,860 | ) | (1,342,302 | ) | 3,353,459 | |||||||
Accrued liabilities and employee benefits | (3,873,692 | ) | 5,065,329 | 2,785,736 | ||||||||
Deferred revenue | — | — | (1,666,674 | ) | ||||||||
Deferred rent | 1,597,105 | — | — | |||||||||
|
|
|
|
|
| |||||||
Net cash used in operating activities | (102,279,965 | ) | (22,807,150 | ) | (22,591,265 | ) | ||||||
Investing activities | ||||||||||||
Purchases of investments | (514,308,249 | ) | (103,601,836 | ) | — | |||||||
Maturities of investments | 153,389,448 | 36,879,308 | 1,500,000 | |||||||||
Purchase of property and equipment | (860,595 | ) | (11,762 | ) | (33,255 | ) | ||||||
|
|
|
|
|
| |||||||
Net cash (used in) provided by investing activities | (361,779,396 | ) | (66,734,290 | ) | 1,466,745 | |||||||
Financing activities | ||||||||||||
Proceeds from issuance of convertible promissory notes, net | — | — | 100,000 | |||||||||
Proceeds from stock option exercises | 653,046 | 162,980 | 332,938 | |||||||||
Proceeds from stock subscription | — | 109,833 | 109,834 | |||||||||
Proceeds of public offerings | 449,996,887 | 116,191,285 | 43,841,850 | |||||||||
Payment of costs of public offerings | (756,313 | ) | (748,538 | ) | (3,754,706 | ) | ||||||
|
|
|
|
|
| |||||||
Net cash provided by financing activities | 449,893,620 | 115,715,560 | 40,629,916 | |||||||||
Net (decrease) increase in cash and cash equivalents | (14,165,741 | ) | 26,174,120 | 19,505,396 | ||||||||
Cash and cash equivalents at beginning of period | 61,325,044 | 35,150,924 | 15,645,528 | |||||||||
|
|
|
|
|
| |||||||
Cash and cash equivalents at end of period | $ | 47,159,303 | $ | 61,325,044 | $ | 35,150,924 | ||||||
|
|
|
|
|
| |||||||
Cash paid for interest | $ | — | $ | 7,073 | $ | 11,320 | ||||||
|
|
|
|
|
| |||||||
See accompanying notes to consolidated financial statements.
Intra-Cellular Therapies, Inc.
Notes to Consolidated Financial Statements
December 31, 2015
1. Organization
Intra-Cellular Therapies, Inc. (the “Company”), through its wholly-owned operating subsidiary, ITI, Inc. (“ITI”), is a biopharmaceutical company focused on the discovery and Business
ITI was incorporated in the stateState of Delaware on May 22, 2001 under the name “Intra-Cellular Therapies, Inc.” and commenced operations in June 2002. ITI was founded to discover and develop drugs for the treatment of neurological and psychiatric disorders.
On August 29, 20122013, ITI completed a reverse merger (the “Merger”) with the objective to acquire, or merge with, an operating business.
In accordance with Financial Accounting Standards Board (“FASB”), Accounting Standards Codification (“ASC”) Topic 805,Business Combinations, ITI is considered the acquirer for accounting purposes, and has accounted for the transaction as a capital transaction, because ITI’s former stockholders received 100% of the voting rights in the combined entity and ITI’s senior management represented all of the senior management of the combined entity. Consequently, the assets and liabilities and the historical operations that are reflected in the Company’s consolidated financial statements are those of ITI and have been recorded at the historical cost basis of the Company. All share and per share amounts in the consolidated financial statements and related notes have been retrospectively adjusted to reflect the one-for-0.5 shares of capital stock exchange as well as the conversion of the Notes (defined below) and the Series A, B, and C redeemable convertible preferred stock of ITI.
Immediately prior to the Merger, on August 29, 2013, ITI sold to accredited investors approximately $60.0 million of its shares of common stock, or 18,889,307 shares at a price of $3.1764 per share (the “Private Placement”), which included $15.3 million in principal business objective overand $0.8 million in accrued interest from the next twelve monthsconversion of ITI’s then outstanding convertible promissory notes (the “Notes”).
On February 5, 2014, the Company completed a public offering of common stock in which the Company sold 7,063,300 shares of common stock, which included the exercise of the underwriters’ option to purchase an additional 921,300 shares, at an offering price of $17.50 per share. After deducting underwriting discounts, commissions and beyond will beoffering expenses, the net proceeds to achieve long-term growth potential throughthe Company were approximately $115.4 million.
1. Organization (continued)
On October 31, 2014, the Company entered into a combinationtermination agreement with a business rather than immediate short-term earnings.Takeda Pharmaceutical Company Limited (“Takeda”) terminating the worldwide license and collaboration agreement under which the Company and Takeda were jointly developing the Company’s proprietary compound ITI-214 and other selected compounds that selectively inhibit phosphodiesterase type 1 (“PDE1”) for use in the prevention and treatment of human diseases. Through December 31, 2015, the Company had received $29.0 million in total payments under the agreement and was eligible to receive milestone payments and royalties based on net sales. On September 15, 2015, Takeda completed the transfer of the Investigational New Drug application (“IND”) for ITI-214 to the Company. The Company will not restrictintends to explore the development of its potential target companies to any specific business, industry or geographical location. The analysisPDE program, including ITI-214 for the treatment of business opportunities will be undertaken by, or underseveral CNS and non-CNS conditions.
On March 11, 2015, the supervisionCompany completed a public offering of common stock in which the Company sold 5,411,481 shares of common stock, which included the exercise of the officersunderwriters’ option to purchase an additional 661,481 shares, at an offering price of $24.00 per share. After deducting underwriting discounts, commissions and directorsoffering expenses, the net proceeds to the Company were approximately $121.8 million.
On September 28, 2015, the Company completed a public offering of common stock in which the Company sold 7,935,000 shares of common stock, which included the exercise of the Company.
In order to further its research projects and support its collaborations, the Company will require additional financing until such time, if ever, that revenue streams are sufficient to generate consistent positive cash flow from operations. Possible sources of funds include public or private sales of the Company’s equity securities, sales of debt securities, the incurrence of debt from commercial lenders, strategic collaborations, licensing a portion or all of the Company’s product candidates and technology and, to a lesser extent, grant funding.
2. Summary of Significant Accounting Policies
Basis of Estimates
The preparation ofaccompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative United States generally accepted accounting principles as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”).
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported amounts of assetsin the financial statements and liabilities and disclosure of contingent assets and liabilities at the date of the balance sheet and the reported amounts of revenues and expenses during the reporting period. Actualaccompanying notes. Although actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid financial instruments purchasedinvestments with a maturity of three months or less from the date of purchase to be cash equivalents. There are noCash and cash equivalents atconsist of checking accounts, money market accounts, money market mutual funds, and certificates of deposit with a maturity date of three months or less. Their carrying values approximate the fair market value. Certificates of deposit, commercial paper, corporate notes and corporate bonds with a maturity date of more than three months are classified separately on the balance sheet date.
2. Summary of Significant Accounting Policies (continued)
Investment Securities
Investment securities may consist of investments in U.S. Treasuries, various U.S. governmental agency debt securities, corporate bonds, certificates of deposit, and other fixed income securities with an average maturity of twelve months or less. Management classifies the Company’s investments as available-for-sale. Such securities are carried at estimated fair value, with any unrealized holding gains or losses reported, net of any tax effects reported, as accumulated other comprehensive income, which is a separate component of stockholders’ equity. Realized gains and losses, and declines in value judged to be other-than-temporary, if any, are included in consolidated results of operations. A decline in the market value of any available-for-sale security below cost that is deemed to be other-than-temporary results in a reduction in fair value, which is charged to earnings in that period, and a new cost basis for the security is established. Dividend and interest income is recognized as interest income when earned. The cost of securities sold is calculated using the specific identification method.
Investment securities consisted of the following (in thousands):
| December 31, 2015 | ||||||||||||||||
| Amortized Cost | Unrealized Gains | Unrealized (Losses) | Estimated Fair Value | |||||||||||||
U.S. government agency securities | $ | 61,510 | $ | — | $ | (271 | ) | $ | 61,239 | |||||||
FDIC certificates of deposit (1) | 41,343 | 1 | (11 | ) | 41,333 | |||||||||||
Certificates of deposit | 219,500 | — | — | 219,500 | ||||||||||||
Commercial paper | 30,122 | — | (48 | ) | 30,074 | |||||||||||
Corporate bonds/notes | 76,157 | — | (262 | ) | 75,895 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
| $ | 428,632 | $ | 1 | $ | (592 | ) | $ | 428,041 | ||||||||
|
|
|
|
|
|
|
| |||||||||
| December 31, 2014 | ||||||||||||||||
| Amortized Cost | Unrealized Gains | Unrealized (Losses) | Estimated Fair Value | |||||||||||||
U.S. government agency securities | $ | 4,316 | $ | — | $ | (3 | ) | $ | 4,313 | |||||||
FDIC certificates of deposit (1) | 16,374 | — | (14 | ) | 16,360 | |||||||||||
Certificates of deposit | 2,000 | — | — | 2,000 | ||||||||||||
Commercial paper | 9,743 | 1 | — | 9,744 | ||||||||||||
Corporate bonds/notes | 35,992 | — | (89 | ) | 35,904 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
| $ | 68,425 | $ | 1 | $ | (106 | ) | $ | 68,321 | ||||||||
|
|
|
|
|
|
|
| |||||||||
| (1) | “FDIC certificates of deposit” consist of deposits that are less than $250,000. |
The Company utilizeshas classified all of its investment securities available-for-sale, including those with maturities beyond one year, as current assets on the accrual method of accounting for income taxes. Under the accrual method, deferred tax assets and liabilities are determinedconsolidated balance sheets based on the differences betweenhighly liquid nature of the financial reporting basisinvestment securities and because these investment securities are considered available for use in current operations. As of December 31, 2015 and December 31, 2014, the Company held $142.4 million and $31.8 million, respectively, of available-for-sale investment securities with contractual maturity dates more than one year and less than two years.
The Company monitors its investment portfolio for impairment quarterly or more frequently if circumstances warrant. In the event that the carrying value of an investment exceeds its fair value and the tax basisdecline in value is determined to be other-than-temporary, the Company records an impairment charge within earnings attributable to the estimated credit loss. In determining whether a decline in the value of an investment isother-than-temporary,the assetsCompany evaluates currently available factors that may include, among others: (1) general market conditions; (2) the duration and liabilitiesextent to which fair value has been less than the carrying
2. Summary of Significant Accounting Policies—(Continued)
value; (3) the investment issuer’s financial condition and are measured using enacted tax ratesbusiness outlook; and laws that will be in effect, when the differences are expected(4) our assessment as to reverse. An allowance against deferred tax assets is recognized, whenwhether it is more likely than not that we will be required to sell a security prior to recovery of its amortized cost basis.
As of December 31, 2015 the Company had approximately $9.2 million of investments that have been held for greater than one year which had a temporary impairment of approximately $19,000. As of December 31, 2014 the Company had no investments that had been held for greater than one year.
The Company attributes the unrealized losses on the available-for-sale securities as of December 31, 2015 and 2014, to the variability in related market interest rates. The Company does not intend to sell these securities, nor is it more likely than not that the Company will be required to sell them prior to the end of their contractual terms. Furthermore, the Company does not believe that these securities expose us to undue market risk or counterparty credit risk. As such, tax benefits willthe Company does not consider these securities to be realized.other-than-temporarily impaired.
Fair Value Measurements
The Company applies the fair value method under ASC Topic 820,Fair Value Measurements and Disclosures. ASC Topic 820 defines fair value, establishes a fair value hierarchy for assets and liabilities measured at fair value and requires expanded disclosures about fair value measurements. The ASC Topic 820 hierarchy ranks the quality and reliability of inputs, or assumptions, used in the determination of fair value and requires assets and liabilities carried at fair value to be classified and disclosed in one of the following categories based on the lowest level input used that is significant to a particular fair value measurement:
Level 2—Fair value is determined by using inputs other than Level 1 quoted prices that are directly or indirectly observable. Inputs can include quoted prices for similar assets and liabilities in active markets or quoted prices for identical assets and liabilities in inactive markets. Related inputs can also include those used in valuation or other pricing models, such as interest rates and yield curves that can be corroborated by observable market data.
The Company evaluates financial assets and liabilities subject to fair value measurements on a recurring basis to determine the appropriate level at which to classify them each reporting period. This determination requires the Company to make subjective judgments as to the significance of inputs used in determining fair value and where such inputs lie within the ASC Topic 820 hierarchy.
The Company has no assets or liabilities that were measured using quoted prices for significant unobservable inputs (Level 3 assets and liabilities) as of December 31, 2015 and December 31, 2014. The carrying value of cash held in money market funds of approximately $31.1 million as of December 31, 2015 and $8.5 million as of December 31, 2014, is included in cash and cash equivalents and approximates market value based on quoted market price or Level 1 inputs.
2. Summary of Significant Accounting Policies (cont’d.)
The fair value measurements of the Company’s cash equivalents and available-for-sale investment securities are identified in the following tables (in thousands):
| Fair Value Measurements at Reporting Date Using | ||||||||||||||||
| December 31, 2015 | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
Money market funds | $ | 31,114 | $ | 31,114 | $ | — | $ | — | ||||||||
U.S. government agency securities | 61,239 | — | 61,239 | — | ||||||||||||
FDIC certificates of deposit | 41,333 | — | 41,333 | — | ||||||||||||
Certificates of deposit | 219,500 | �� | — | 219,500 | — | |||||||||||
Commercial paper | 30,074 | — | 30,074 | — | ||||||||||||
Corporate bonds/notes | 75,895 | — | 75,895 | — | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
| $ | 459,155 | $ | 31,114 | $ | 428,041 | $ | — | |||||||||
|
|
|
|
|
|
|
| |||||||||
| Fair Value Measurements at Reporting Date Using | ||||||||||||||||
| December 31, 2014 | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
Money market funds | $ | 8,495 | $ | 8,495 | $ | — | $ | — | ||||||||
U.S. government agency securities | 4,313 | — | 4,313 | — | ||||||||||||
FDIC certificates of deposit | 16,360 | — | 16,360 | — | ||||||||||||
Certificates of deposit | 41,000 | — | 41,000 | — | ||||||||||||
Commercial paper | 9,744 | — | 9,744 | — | ||||||||||||
Corporate bonds/notes | 35,903 | — | 35,903 | — | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
| $ | 115,815 | $ | 8,495 | $ | 107,320 | $ | — | |||||||||
|
|
|
|
|
|
|
| |||||||||
Financial Instruments
The Company recognizesconsiders the recorded costs of its financial assets and liabilities, which consist of cash equivalents, accounts receivable, accounts payable and accrued liabilities, to approximate their fair value because of their relatively short maturities at December 31, 2015 and December 31, 2014. Management believes that the risks associated with its financial instruments are minimal as the counterparties are various corporations, financial institutions and government agencies of high credit standing.
Concentration of Credit Risk
Cash equivalents are held with major financial institutions in the United States. Certificates of deposit, cash and cash equivalents held with banks may exceed the amount of insurance provided on such deposits. Generally, these deposits may be redeemed upon demand and, therefore, bear minimal risk.
Accounts Receivable
Accounts receivable that management has the intent and ability to collect are reported in the balance sheets at outstanding amounts, less an allowance for doubtful accounts. The Company writes off uncollectible receivables when the likelihood of collection is remote.
2. Summary of Significant Accounting Policies (continued)
The Company evaluates the collectability of accounts receivable on a regular basis. The allowance, if any, is based upon various factors including the financial statement benefitcondition and payment history of customers, an overall review of collections experience on other accounts and economic factors or events expected to affect future collections experience. No allowance was recorded as of December 31, 2015 and 2014, as the Company has a history of collecting on all its accounts including government agencies and collaborations funding its research.
Property and Equipment
Property and equipment is stated at cost and depreciated on a straight-line basis over estimated useful lives ranging from three to five years. Leasehold improvements are amortized using the straight-line method over the shorter of the estimated useful life of the assets or the term of the related lease. Expenditures for maintenance and repairs are charged to operations as incurred.
When indicators of possible impairment are identified, the Company evaluates the recoverability of the carrying value of its long-lived assets based on the criteria established in ASC 360,Property, Plant and Equipment. The Company considers historical performance and anticipated future results in its evaluation of potential impairment. The Company evaluates the carrying value of those assets in relation to the operating performance of the business and undiscounted cash flows expected to result from the use of those assets. Impairment losses are recognized when carrying value exceeds the undiscounted cash flows, in which case management must determine the fair value of the underlying asset. No such impairment losses have been recognized to date.
Revenue Recognition
Revenue is recognized when all terms and conditions of the agreements have been met, including persuasive evidence of an uncertain tax position only after consideringarrangement, delivery has occurred or services have been rendered, price is fixed or determinable and collectability is reasonably assured. The Company is reimbursed for certain costs incurred on specified research projects under the probability that a tax authority would sustain the position in an examination.
The Company has entered into arrangements involving the delivery of more than one element. Each required deliverable is evaluated to determine whether it qualifies as a separate unit of accounting. For the Company, this determination is generally based on whether the deliverable has “stand-alone value” to the customer. The Company adopted this accounting standard on a prospective basis for all Multiple-Deliverable Revenue Arrangements (“MDRAs”) entered into on or after January 1, 2011, and for any MDRAs that were entered into prior to January 1, 2011, but materially modified on or after that date.
The Company has adopted ASC Topic 605-28,Milestone Method. Under this guidance, the Company recognizes revenue contingent upon the achievement of a substantive milestone in its entirety in the period the milestone is achieved. Substantive milestone payments are recognized upon achievement of the milestone only if all of the following conditions are met:
2. Summary of Significant Accounting Policies (continued)
Determination as to whether a payment meets the aforementioned conditions involves management’s judgment. If any of these conditions are not met, the resulting payment would not be considered a substantive milestone, and therefore, the resulting payment would be considered part of the consideration for the single unit of accounting and be recognized as revenue in accordance with the revenue models described above. In addition, the determination that one such payment was not a substantive milestone could prevent the Company from concluding that subsequent milestone payments were substantive milestones and, as a result, any additional milestone payments could also be considered part of the consideration for the single unit of accounting and would be recognized as revenue as such performance obligations are performed under either the proportional performance or straight-line methods, as applicable.
Research and Development
Except for payments made in advance of services, the Company expenses its research and development costs as incurred. For payments made in advance, the Company recognizes research and development expense as the services are rendered. Research and development costs primarily consist of salaries and related expenses for personnel and resources and the costs of clinical trials. Other research and development expenses include preclinical analytical testing, outside services, providers, materials and consulting fees.
Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as subject enrollment, clinical site activations or information provided to the Company by its vendors with respect to their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the financial statements as prepaid or accrued research and development expense, as the case may be.
As part of the process of preparing its financial statements, the Company is required to estimate its expenses resulting from its obligations under contracts with vendors, clinical research organizations and consultants and under clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment flows that do not match the benefitperiods over which materials or services are provided under such contracts. The Company’s objective is to reflect the appropriate trial expenses in its financial statements by matching those expenses with the period in which services are performed and efforts are expended. The Company accounts for these expenses according to the progress of the trial as measured by subject progression and the timing of various aspects of the trial. The Company determines accrual estimates through financial models taking into account discussion with applicable personnel and outside service providers as to the progress or state of consummation of trials, or the services completed. During the course of a clinical trial, the Company adjusts its clinical expense recognition if actual results differ from its estimates. The Company makes estimates of its accrued expenses as of each balance sheet date based on the facts and circumstances known to it at that time. The Company’s clinical trial accruals are dependent upon the timely and accurate reporting of contract research organizations and other third-party vendors. Although the Company does not expect its estimates to be materially different from amounts actually incurred, its understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in it reporting amounts that are too high or too low for any particular period. For the years ended December 31, 2015 and 2014, there were no material adjustments to the Company’s prior period estimates of accrued expenses for clinical trials.
Income Taxes
Income taxes are accounted for using the liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of
2. Summary of Significant Accounting Policies (continued)
existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the year in which those temporary differences are expected to be recovered or settled.
The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Valuation allowances are established when necessary to reduce net deferred tax assets to the amount expected to be realized. Income tax expense is the tax payable for the period and the change during the period in deferred tax assets and liabilities.
The Company accounts for uncertain tax positions pursuant to ASC Topic 740 (previously included in FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes—an Interpretation of FASB Statement No. 109). Financial statement recognition of a tax position taken or expected to be taken in a tax return is determined based on a more-likely-than-not threshold of that position being sustained. If the tax position meets this threshold, the benefit to be recognized is measured as the tax benefit having the highest likelihood of being realized upon ultimate settlement with the taxtaxing authority. For tax positions not meeting the threshold, no financial statement benefit is recognized. The Company recognizes interest accrued related to unrecognized tax benefits and penalties in the provision for income taxes.
Comprehensive Income (Loss)
All components of comprehensive income (loss), including net income (loss), are reported in the financial statements in the period in which they are incurred. Comprehensive income (loss) is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. In accordance with accounting guidance, the Company presents the impact of any unrealized gains or (losses) on its investment securities in a separate statement of comprehensive income (loss) for each period.
Share-Based Compensation
Share-based payments are accounted for in accordance with the provisions of ASC Topic 718,Compensation—Stock Compensation. The fair value of share-based payments is estimated, on the date of grant, using the Black-Scholes-Merton option-pricing model (the “Black-Scholes model”). The resulting fair value is recognized ratably over the requisite service period, which is generally the vesting period of the option.
For all awards granted with time-based vesting conditions, expense is amortized using the straight-line attribution method. For awards that contain a performance-based vesting condition, expense is amortized using the accelerated attribution method. Share-based compensation expense recognized in the statements of operations for the years ended December 31, 2015, 2014 and 2013 is based on share-based awards ultimately expected to vest, and this amount has therefore been reduced for estimated forfeitures. ASC Topic 718 requires forfeitures to be estimated at the time of grant and revised, if any, relatednecessary, in subsequent periods if actual forfeitures differ from those estimates. Pre-vesting forfeitures were estimated based on the Company’s historical experience and have not been material.
The Company utilizes the Black-Scholes model for estimating fair value of its stock options granted. Option valuation models, including the Black-Scholes model, require the input of subjective assumptions, and changes in the assumptions used can materially affect the grant date fair value of an award. These assumptions include the risk-free rate of interest, expected dividend yield, expected volatility and the expected life of the award. Restricted stock units are valued at the fair market value on the date of grant as determined by the closing stock price.
Expected volatility rates are based on historical volatility of the common stock of comparable publicly traded entities and other factors due to uncertain tax positionsthe limited historical information about the Company’s common stock. The expected life of stock options is the period of time for which the stock options are expected to be outstanding.
2. Summary of Significant Accounting Policies (continued)
Given the limited historical exercise data, the expected life is determined using the “simplified method,” which is defined as the midpoint between the vesting date and the end of the contractual term.
The risk-free interest rates are based on the U.S. Treasury yield for a period consistent with the expected term of the option in income tax expense. Aseffect at the time of March 31, 2013,the grant. The Company has not paid dividends to its stockholders since its inception and does not plan to pay cash dividends in the foreseeable future. Therefore, the Company has assumed an expected dividend rate of zero.
Prior to January 1, 2014, given that there was no accrued interest or penaltiesactive market for the Company’s common stock, the exercise price of the stock options on the date of grant was determined and approved by the board of directors using several factors, including progress and milestones achieved in the Company’s business development and performance, the price per share of its convertible preferred stock offerings and general industry and economic trends. In establishing the estimated fair value of the common stock, the Company considered the guidance set forth in American Institute of Certified Public Accountants Practice Guide,Valuation of Privately-Held-Company Equity Securities Issued as Compensation. For stock options granted in 2014 and 2015, the exercise price was determined by using the closing market price of the Company’s common stock on the date of grant.
Under ASC Topic 718, the cumulative amount of compensation cost recognized for instruments classified as equity that ordinarily would result in a future tax deduction under existing tax law shall be considered to be a deductible temporary difference in applying ASC Topic 740,Income Taxes. The deductible temporary difference is based on the compensation cost recognized for financial reporting purposes; however, these provisions currently do not impact the Company, as all the deferred tax assets have a full valuation allowance.
Since the Company had net operating loss carryforwards as of December 31, 2015, 2014 and 2013, no excess tax benefits for the tax deductions related to uncertain tax positions.
Equity instruments issued to consultants are accounted for under the provisions of ASC Topic 718 and ASC Topic 505-50,Equity/Equity-Based Payments to Non-Employees. Accordingly, the estimated fair value of the equity instrument is recorded on the earlier of the performance commitment date or the date the services required are completed and are marked to market during the service period.
Loss Per Common Share
Basic net loss per common share is calculated usingdetermined by dividing the net loss by the weighted-average number of common shares outstanding during each reporting period.the period, without consideration of common stock equivalents. Diluted net loss per share includes potentially dilutive securities such asis computed by dividing the net loss by the weighted-average number of common stock equivalents outstanding options and warrants, using various methods such asfor the treasury stock or modifiedperiod. The treasury stock method is used to determine the dilutive effect of the Company’s stock option grants.
The following common stock equivalents were excluded in the determinationcalculation of dilutive shares outstanding during eachdiluted loss per share because their effect would be anti-dilutive as applied to the loss from operations for the years ended December 31, 2015, 2014 and 2013:
| Years Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
Stock Equivalents | 1,322,311 | 958,712 | 898,982 | |||||||||
Recently Issued Accounting Standards
In November 2015, the FASB issued Accounting Standards Update No. 2015-17, Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes. Current GAAP requires an entity to separate deferred income tax liabilities and assets into current and noncurrent amounts in a classified statement of financial position. To simplify the presentation of deferred income taxes, ASU 2015-17 requires that deferred tax
2. Summary of Significant Accounting Policies (continued)
liabilities and assets be classified as noncurrent in a classified statement of financial position. The current requirement that deferred tax liabilities and assets of a tax-paying component of an entity be offset and presented as a single amount is not affected by the amendments in this Update. The amendments in this Update are effective for financial statements issued for annual periods beginning after December 15, 2016, and interim periods within those annual periods. Earlier application is permitted for all entities as of the beginning of an interim or annual reporting period. The Company does not have any potentially dilutive instruments for the period presented.
In May 2014, the FASB issued Accounting Standards Update No. 2014-09 (ASU 2014-09), Revenue from Contracts with Customers. ASU 2014-09 will eliminate transaction-specific and industry-specific revenue recognition guidance under current GAAP and replace it with a principle-based approach for determining revenue recognition. ASU 2014-09 will require that companies recognize revenue based on the value of transferred goods or services as they occur in the contract. ASU 2014-09 also will require additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments and assets recognized from costs incurred to delayobtain or fulfill a contract. In July 2015, the FASB decided to defer the effective date of the standard from January 1, 2017, to January 1, 2018, with an option that permits companies to adopt the standard as early as the original effective date. Early application prior to the original effective date is not permitted. The standard permits the use of either the retrospective or cumulative effect transition method. Presently, the Company is assessing what effect the adoption of this standard will have on our consolidated financial statements and accompanying notes.
3. Property and Equipment
Property and equipment consist of the following:
| December 31, | ||||||||
| 2015 | 2014 | |||||||
Computer equipment | $ | 42,064 | $ | 39,160 | ||||
Furniture and fixtures | 266,695 | 35,958 | ||||||
Scientific equipment | 2,823,601 | 2,207,848 | ||||||
|
|
|
| |||||
| 3,132,360 | 2,282,966 | |||||||
Less accumulated depreciation | (2,356,838 | ) | (2,228,413 | ) | ||||
|
|
|
| |||||
| $ | 775,522 | $ | 54,553 | |||||
|
|
|
| |||||
Depreciation expense for the years ended December 31, 2015, 2014 and 2013 was $139,626, $25,481 and $23,249, respectively. During 2015, the Company retired $11,201 of fully depreciated property and equipment. During 2014, in conjunction with its move in February 2015 to its new headquarters, the Company retired $319,553 of fully depreciated leasehold improvements and disposed of $709,518 of fully depreciated property and equipment.
4. Share-Based Compensation
The Company sponsors the Intra-Cellular Therapies, Inc. 2013 Equity Incentive Plan (the “2013 Plan”) to provide for the granting of stock-based awards, such as stock options, restricted common stock, restricted stock units and stock appreciation rights to employees, directors and consultants as determined by the Board of Directors. In August 2013, the Company assumed in the Merger the ITI 2003 Equity Incentive Plan, as amended (the “2003 Plan”), which expired by its terms in July 2013. As of December 31, 2015, there were options to
4. Share-Based Compensation (continued)
purchase 2,737,657 shares of common stock outstanding under the 2013 Plan. Effective in November 2013, the Company adopted the 2013 Plan. The Company initially reserved 2,850,000 shares of common stock for issuance under the 2013 Plan. In both January 2015 and 2014, the number of shares of common stock reserved for issuance under the 2013 Plan automatically increased by 800,000 pursuant to the evergreen provisions of the 2013 Plan. On June 16, 2015, the stockholders of the Company approved, at the Company’s 2015 Annual Meeting of Stockholders, an amendment to the 2013 Plan to increase the number of shares of common stock available for issuance under the plan by 3,100,000 shares, to increase by 100,000 shares the maximum number of shares available for the issuance of options, stock appreciation rights and other similar awards to any one participant in any calendar year for purposes of meeting the requirements for qualified performance-based compensation under Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Code”), and to eliminate the evergreen provisions of the 2013 Plan under which 800,000 shares were automatically added to the plan on each of January 1, 2014 and 2015.
Stock options granted under the 2013 Plan may be either incentive stock options (“ISOs”) as defined by the Code, or revised accounting standards that have different effective dates for publicnon-qualified stock options. The Board of Directors determines who will receive options, the vesting periods (which are generally two to three years) and private companies until those standards apply to private companies.
Total stock-based compensation expense related to all of the Company’s share-based awards, including stock options and restricted stock units to employees, directors and consultants recognized during the years ended December 31, 2015, 2014 and 2013, was comprised of the following:
| Years Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
Research and development | $ | 4,768,131 | $ | 1,842,828 | $ | 132,543 | ||||||
General and administrative | 6,122,774 | 2,001,050 | 258,850 | |||||||||
|
|
|
|
|
| |||||||
Total share-based compensation expense | $ | 10,890,905 | $ | 3,843,878 | $ | 391,393 | ||||||
|
|
|
|
|
| |||||||
The accompanying financial statements have been prepared assumingfollowing table describes the weighted-average assumptions used for calculating the value of options granted during the years ended December 31, 2015, 2014 and 2013:
| 2015 | 2014 | 2013 | ||||||||||
Dividend yield | 0 | % | 0 | % | 0 | % | ||||||
Expected volatility | 80.0 | % | 80.0 | % | 80.0 | % | ||||||
Weighted-average risk-free interest rate | 1.8 | % | 2.0 | % | 2.2 | % | ||||||
Expected term (in years) | 5.9 | 6.3 | 6.2 | |||||||||
Information regarding the stock options activity, including with respect to grants to employees, directors and consultants as of December 31, 2015, and changes during the period then ended, are summarized as follows:
| Number of Shares | Weighted- Average Exercise Price | Aggregate Intrinsic Value | Weighted- Average Contractual Life | |||||||||||||
Outstanding at December 31, 2014 | 2,233,460 | $ | 9.20 | $ | — | 7.3 years | ||||||||||
Options granted | 884,703 | $ | 21.00 | $ | — | 9.1 years | ||||||||||
Options exercised | (305,005 | ) | $ | 2.14 | $ | — | 2.2 years | |||||||||
Options canceled or expired | (75,501 | ) | $ | 12.33 | $ | — | 8.0 years | |||||||||
|
|
|
|
|
| |||||||||||
Outstanding at December 31, 2015 | 2,737,657 | $ | 13.72 | $ | 109,778,658 | 7.6 years | ||||||||||
|
|
|
|
|
| |||||||||||
Vested or expected to vest at December 31, 2015 | 2,737,657 | $ | 13.72 | $ | — | |||||||||||
|
|
|
|
|
| |||||||||||
Exercisable at December 31, 2015 | 1,485,918 | $ | 8.75 | $ | 66,920,372 | 6.7 years | ||||||||||
|
|
|
|
|
| |||||||||||
4. Share-Based Compensation (continued)
The weighted-average grant date fair value for awards granted during the years ended December 31, 2015, 2014, and 2013, was $21.00, $16.50, and $3.26 per share, respectively. Total intrinsic value of the options exercised during the years ended December 31, 2015, 2014, and 2013 was approximately $10,951,057, $3,696,775 and $580,623, respectively. The total fair value of shares vested in the years ended December 31, 2015, 2014 and 2013, was approximately $5,207,073, $3,703,000 and $278,000, respectively.
During 2015, 2014 and 2013, the Company will continue as a going concern, which contemplates the recoverability of assets and the satisfaction of liabilities in the normal course of business.
The unrecognized share-based compensation expense related to comply with all Section 16(a) filing requirements during such fiscalstock option awards at December 31, 2015, is $12,733,859 and will be recognized over a weighted-average period of 1.6 years.
5. Income Taxes
Total income tax expense for the years ended December 31 is allocated as follows:
| December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
Current | $ | 1,600 | $ | 1,600 | $ | 18,000 | ||||||
Deferred | (51,165,859 | ) | (14,655,320 | ) | (13,229,355 | ) | ||||||
Valuation allowance | 51,165,859 | 14,655,320 | 13,229,355 | |||||||||
|
|
|
|
|
| |||||||
Provision for income taxes | $ | 1,600 | $ | 1,600 | $ | 18,000 | ||||||
|
|
|
|
|
| |||||||
A reconciliation of Ethics
| December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
Income tax benefit at statutory federal rate | 35.00 | % | 35.00 | % | 35.00 | % | ||||||
Permanent differences | (0.56 | ) | 0.12 | (1.20 | ) | |||||||
Return-to-provision—R&D Credit | 0.00 | (0.05 | ) | 2.61 | ||||||||
R&D Credit—current year | 4.19 | 2.32 | 3.72 | |||||||||
Reserve for uncertain tax positions | 0.00 | (0.01 | ) | (6.53 | ) | |||||||
Change in effective state tax rates | (0.05 | ) | (0.14 | ) | 6.58 | |||||||
State income tax expense | 10.24 | 10.50 | 10.12 | |||||||||
Change in valuation allowance | (48.82 | ) | (47.75 | ) | (50.37 | ) | ||||||
|
|
|
|
|
| |||||||
Provision for income taxes | (0.00 | )% | (0.01 | )% | (0.07 | )% | ||||||
|
|
|
|
|
| |||||||
Deferred income taxes reflect the net tax effect of temporary differences that exist between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes, using enacted tax rates in effect for the year in which the differences are expected to reverse. As of December 31, 2015, the Company adopted a formal codehad $174.8 million of ethics statementfederal net operating loss carryforwards, which expire at various dates through 2035. The gross amount of the state net operating loss carryforwards is equal to or less than the federal net operating loss carryforwards and expires over various periods based on individual state tax law. In general, businesses with U.S. net operating losses (“NOLs”) are considered loss corporations for senior officers and directors (the “Code of Ethics”) that is designatedU.S. federal income tax purposes. Pursuant to deter wrongdoing and promote ethical conduct and full, fair, accurate, timely and understandable reports that the Company files or submits to the SEC and others. A formSection 382 of the Code, loss corporations that undergo an ownership change, as defined under the Code, may be subject to an annual limitation on the amount of Ethics is attached hereto as Exhibit 14.1,NOLs (and certain other tax attributes)
5. Income Taxes (continued)
available to offset taxable income earned after such ownership change. For the year ended December 31, 2015, the Company performed a Section 382 ownership analysis and incorporated herein by reference.
At December 31, 2015, the beneficial ownerCompany had $5.7 million in excess tax benefits related to stock-based compensation deductions, the benefit of more than 5%which will be recorded to additional paid-in-capital once the benefit is realized through a reduction of income taxes payable. The following summarizes the significant components of the outstanding sharesCompany’s deferred tax assets and liabilities as of Common Stock, (ii) each director, nomineeDecember 31, 2015 and executive officer of2014, respectively:
| December 31, | ||||||||
| 2015 | 2014 | |||||||
Deferred tax assets: | ||||||||
Net operating loss carryforwards | $ | 76,741,198 | $ | 34,870,169 | ||||
Accrued employee benefits | 543,466 | 443,371 | ||||||
Research and development credit | 6,962,731 | 2,570,540 | ||||||
Stock compensation | 5,641,993 | 1,563,785 | ||||||
Deferred rent | 725,195 | — | ||||||
Deferred tax liabilities: | ||||||||
Depreciation | (6,062 | ) | (5,204 | ) | ||||
|
|
|
| |||||
Net deferred tax asset | 90,608,521 | 39,442,661 | ||||||
Valuation allowance | (90,608,521 | ) | (39,442,661 | ) | ||||
|
|
|
| |||||
Net deferred tax asset | $ | — | $ | — | ||||
|
|
|
| |||||
Based upon the Company’s historical operating performance and the reported cumulative net losses to date, the Company and (iii) all officers and directors as a group.
| Name and Address | Amount and Nature of Beneficial Ownership | Percentage of Class | ||||||
NLBDIT 2010 Services, LLC c/o Sunrise Securities Corp 600 Lexington Avenue, 23rd Floor New York, NY 10022 | 5,000,000 | 100 | % | |||||
The Nathan Low 2008 Irrevocable Trust c/o Sunrise Securities Corp. 600 Lexington Avenue, 23rd Floor New York, NY 10022 | 5,000,000 | (1) | 100 | % | ||||
Nathan A. Low c/o Sunrise Securities Corp. 600 Lexington Avenue, 23rd Floor New York, NY 10022 | 5,000,000 | (2) | 100 | % | ||||
Samir N. Masri (3) 175 Great Neck Rd Suite 403 Great Neck, NY 11021 | 0 | (4) | 0 | % | ||||
All Directors and Officers as a Group (1 individual) | ||||||||
The total amount of 5,000,000 sharesunrecognized tax benefits as of Common StockDecember 31, 2015 and December 31, 2014 were $1.72 million and $1.72 million respectively. If recognized none of these tax benefits would affect the effective tax rate due to NLBDIT Services for an aggregate purchase price equal to $10,000, pursuant tovaluation allowances.
The following summarizes the termssignificant components of gross unrecognized tax benefits as of December 31, 2015 and conditions set forth in that certain common stock purchase agreement (the “Common Stock Purchase Agreement”). 2014, respectively:
| December 31, | ||||||||
| 2015 | 2014 | |||||||
Balance at January1, | $ | 1,717,635 | $ | 1,715,904 | ||||
Current Year Uncertain Tax Positions: | ||||||||
Gross Increases | 3,277 | 1,731 | ||||||
Prior Year Uncertain Tax Positions: | ||||||||
|
|
|
| |||||
Balance at December 31, | $ | 1,720,912 | $ | 1,717,635 | ||||
|
|
|
| |||||
6. Collaborations and License Agreements
The Bristol-Myers Squibb License Agreement
On October 23, 2012,May 31, 2005, the Company received payment of $10,000 for the shares of Common Stock issued to NLBDIT Services on October 15, 2012. The Low Trust owns 100% of the outstanding membership interests of NLBDIT Services and may be deemed to beneficially own the shares of Common Stock held of record by NLBDIT Services. Nathan Low is the family trustee of the Low Trust and has voting and dispositive control over any securities owned of record or beneficially by the Low Trust. Therefore, Mr. Low may be deemed to beneficially own the shares of Common Stock held of record by NLBDIT Services and beneficially by the Low Trust. Theentered into a worldwide, exclusive License Agreement with Bristol-Myers Squibb Company issued these shares of Common Stock under the exemption from registration provided by Section 4(2) of the Securities Act. The Common Stock Purchase Agreement is attached as Exhibit 10.1 to the Company’s Registration Statement on Form 10, as amended, filed with the SEC on March 22, 2013.
Under the agreement, the Company made an upfront payment of $1.0 million to BMS, a milestone payment of $1.25 million in December 2013, and a milestone payment of $1.5 million in December 2014 following the initiation of the Company’s first Phase 3 clinical trial for ITI-007 for patients with exacerbated schizophrenia. Possible milestone payments remaining total $12.0 million. Under the agreement, the Company may be obliged to make other milestone payments to BMS for each licensed product of up to an aggregate of approximately $14.75 million. The Company is also obliged to make tiered single digit percentage royalty payments on sales of licensed products. The Company is obliged to pay to BMS a percentage of non-royalty payments made in consideration of any sublicense.
The agreement extends, and all amounts that NLBDIT Enterprises may advanceroyalties are payable, on a country-by-country and product-by-product basis, through the later of ten years after first commercial sale of a licensed product in such country, expiration of the last licensed patent covering a licensed product, its method of manufacture or use, or the expiration of other government grants providing market exclusivity, subject to certain rights of the parties to terminate the agreement on the occurrence of certain events. On termination of the agreement, the Company onmay be obliged to convey to BMS rights in developments relating to a licensed compound or before the date thatlicensed product, including regulatory filings, research results and other intellectual property rights.
The Takeda Pharmaceutical License and Collaboration Agreement and Termination Agreement
On February 25, 2011, the Company consummatesentered into a business combinationlicense and collaboration agreement with a private company or reverse takeover transaction or other transaction afterTakeda Pharmaceutical Company Limited under which the Company would ceaseagreed to be a shell company (as definedcollaborate to research, develop and commercialize its proprietary compound ITI-214 and other selected compounds that selectively inhibit PDE1 for use in Rule 12b-2 under the Exchange Act). Although NLBDIT Enterprises has no obligation to advance funds toprevention and treatment of human diseases. As part of the agreement, the Company underassigned to Takeda certain patents owned by the Company that claim ITI-214 and granted Takeda an exclusive license to develop and commercialize compounds identified in the conduct of the research program that satisfy specified criteria. However, the Company retained rights to all compounds that do not meet the specified criteria and the Company continues to develop PDE1 inhibitors outside the scope of the agreement.
Under the terms of the NLBDIT Enterprises Note, it is anticipated that NLBDIT Enterprises will advance funds toagreement, the Company as feesconducted a research program with an initial term of three years to identify and characterize compounds that meet certain specified criteria sufficient for further development by Takeda. This research program ended in February 2014. The Company was responsible for its expenses are incurred in the future. As a result,conduct of certain research activities specified in the research plan. Takeda agreed to reimburse the Company issued NLBDIT Enterprises Notefor expenses the Company incurred in anticipation of such advances. NLBDIT Enterprises is wholly owned by The Nathan Low 2008 Irrevocable Trust ("Low Trust"). Nathan Low, a principal of our sole shareholder, is the family trusteeconducting additional research activities.
Upon execution of the Low Trust andagreement, Takeda made a principal of NLBDIT Enterprises. Interest shall accrue on the outstanding principal amount of the NLBDIT Enterprises Note on the basis of a 360-day year from the date of borrowing until paid in full at the rate of six percent (6%) per annum. As of March 31, 2013, there is outstanding balance under the NLBDIT Enterprises Note. The NLBDIT Enterprises Note is attached as Exhibit 4.1 to the Company’s Registration Statement on Form 10, as amended, filed with the SEC on March 22, 2013.
6. Collaborations and License Agreements (continued)
On October 31, 2014, the Company entered into an agreement with Takeda terminating the Takeda License Agreement, pursuant to which all rights granted under the Takeda License Agreement were returned to the Company. On September 15, 2015, Takeda completed the transfer of the IND for ITI-214 to the Company. ITI-214 is the founderfirst compound in its class to successfully advance into Phase 1 clinical trials. The Company intends to explore the development of its PDE program, including ITI-214 for the treatment of several CNS and Presidentnon-CNS conditions, which may include cognition in Parkinson’s disease, cognition in Alzheimer’s disease, cognition in schizophrenia and in other non-CNS indications. Other compounds in the PDE portfolio are also being advanced for the treatment of Samir Masri CPA Firm P.C.various indications.
Other License Agreement
In May 2002, ITI entered into a license agreement (the “License”) and research agreement with a university. Under the provisions of the License, ITI is entitled to use this organization’s patented technology and other intellectual property relating to diagnosis and treatment of central nervous system disorders.
The License expires upon expiration of the patent rights or 15 years subsequent to the first sale of products developed through this License. ITI is required to make future milestone payments for initiation of clinical trials and approval of a New Drug Application (“NDA”). Should ITI commercialize the technology related to this License, ITI would be required to make royalty payments, and would also be required to pay fees under any sublicense agreements with third parties.
In addition, ITI is required to use at least $1.0 million annually of its resources for the development and commercialization of the technology until ITI submits an NDA. ITI met its spending requirements in 2015. There were no other payments made or required under the License for the years ended December 31, 2015 and 2014.
7. Commitments and Contingencies
The Company currently has operating lease agreements with commitments for $16,255,927 for laboratory and office facilities through 2027.
At December 31, 2015, future minimum lease payments under leases having an initial or remaining non-cancellable lease term in excess of one year are set forth in the table below:
Year | ||||
2016 | $ | 0 | ||
2017 | 1,299,845 | |||
2018 | 1,457,008 | |||
2019 | 1,500,718 | |||
2020 | 1,545,740 | |||
Thereafter | 10,452,616 | |||
|
| |||
| $ | 16,255,927 | |||
|
| |||
Rent expense for the years ended December 31, 2015, 2014 and 2013 was $1,385,207, $853,504 and $827,479, respectively.
8. Employee Benefit Plan
The Company sponsors a defined contribution 401(k) plan covering all full-time employees. Participants may elect to contribute their annual pre-tax earnings up to the federally allowed maximum limits. The Company makes a matching contribution of 50% on the first 6% of contributions made by participants. Participant and Company contributions vest immediately. During the years ended December 31, 2015, 2014 and 2013, the Company recorded matching contribution expense of $109,963, $84,757 and $77,138, respectively.
9. Related Parties
In the first quarter of 2015, the Company moved its headquarters to 430 East 29th Street, New York, New York 10016. The Company has agreedentered into a long-term lease for approximately 16,753 square feet of useable laboratory and office space.The lease has a term of 12 years. Due to pay Samir Masri CPA Firm P.C. for services renderedthe amortization of total lease payments, the Company has recognized $1.6 million of deferred rent in connection with the preparation of12 months ended December 31, 2015. The deferred rent balance will incrementally increase over the financial statements required in this registration statement on Form 10 and the subsequent periodic reports during the first fiscal year in an aggregate amount equal to $10,000 to be paid as follows: (1) $5,000 upon the filingnext two years. A member of the Company’s registration statement, and (2) an additional $5,000 onboard of directors is the earlier of (i) March 31, 2013 or (ii) the date that the Company consummates a merger or similar transaction with an operating business. There are no written agreements in connection with this arrangement.
10. Unaudited Quarterly Financial Information
The tables herein set forth the Company’s unaudited condensed consolidated 2015 and 2014 quarterly statements of the Company.
The aggregate fees billed by Raich Ende Malter & Co. LLP for professional services renderedfollowing table sets for the auditCompany’s unaudited condensed consolidated statements of our annual financial statements and review of financial statements included in our quarterly reports on Form 10-Q or services that are normally provided in connection with statutory and regulatory filings were approximately $7,500operations for the fiscal year ended March 31, 2013.
2015 Quarter Ended | December 31, | September 30, | June 30, | March 31, | ||||||||||||
Revenue | $ | 30,659 | $ | — | $ | 57,390 | $ | 3,315 | ||||||||
Net loss | (28,834,516 | ) | (32,160,483 | ) | (21,511,318 | ) | (22,286,824 | ) | ||||||||
Basic and diluted net loss per share | $ | (0.67 | ) | $ | (0.91 | ) | $ | (0.61 | ) | $ | (0.72 | ) | ||||
The following table sets for assurance and related services that are reasonably related to the performance of the audit or review of the Company’s financialunaudited condensed consolidated statements of operations for the fiscal year ended March 31, 2013.
2014 Quarter Ended | December 31, | September 30, | June 30, | March 31, | ||||||||||||
Revenue | $ | 65,862 | $ | 124,414 | $ | 219,238 | $ | 167,787 | ||||||||
Net loss | (15,199,130 | ) | (6,415,507 | ) | (4,533,539 | ) | (4,543,284 | ) | ||||||||
Basic and diluted net loss per share | $ | (0.52 | ) | $ | (0.22 | ) | $ | (0.15 | ) | $ | (0.17 | ) | ||||
F-22