UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
☒ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended AugustDecember 31, 20172022
or
☐TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission file number 000-50298001-35813
ORAMED PHARMACEUTICALS INC.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 98-0376008 | |
| (State or Other Jurisdiction of Incorporation or Organization) | (I.R.S. Employer Identification No.) | |
| (Address of Principal Executive Offices) | (Zip Code) |
+972-2-566-0001844-967-2633
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Exchange Act: None
| Title of each class | Trading symbol | Name of each exchange on which registered | ||
| Common Stock, par value $0.012 | ORMP | The Nasdaq Capital Market, Tel Aviv Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: Common Stock, $.012 par value per share
None.
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Date File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and"emerging “emerging growth company"company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |
| Non-accelerated filer | ☒ | Smaller reporting company | �� | |
| Emerging growth company | ☐ | |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes ☐ No ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates as of the last business day of the registrant’s most recently completed second fiscal quarter was $56,748,843,$169,594,349 based on a price of $6.05,$4.58, being the last price at which the shares of the registrant’s common stock were sold on The Nasdaq Capital Market prior to the end of the most recently completed second fiscal quarter.
IndicateAs of March 6, 2023, the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date: 14,306,100registrant had 39,783,813 shares of common stock issued and outstanding as of November 28, 2017.outstanding.
ORAMED PHARMACEUTICALS INC.
FORM 10-K(FOR THE FISCAL YEAR ENDED AUGUST 31, 2017)
TABLE OF CONTENTS
i
INTRODUCTION AND USE OF CERTAIN TERMS
On February 28, 2022, our Board of Directors, or our Board, approved a change of the Company’s fiscal year from the period beginning on September 1 and ending on August 31 to the period beginning on January 1 and ending on December 31. As a result, the Company filed a Transition Report on Form 10-Q with the Securities and Exchange Commission, or the SEC, on March 30, 2022 that included financial information for the transition period from September 1, 2021 through December 31, 2021, or the Transition Period. Subsequent to that report, the Company’s fiscal year now begins on January 1 and ends on December 31. This Annual Report on Form 10-K is the Company’s first annual report presenting its new fiscal year, and reports financial results for the 12 month period ended December 31, 2022.
As used in this Annual Report on Form 10-K, the terms “we,” “us,” “our,” the “Company,” and “Oramed” mean Oramed Pharmaceuticals Inc. and our wholly-owned Israeli subsidiary, Oramed Ltd.,subsidiaries, unless otherwise indicated. All dollar amounts refer to U.S. dollars unless otherwise indicated.
On AugustDecember 31, 2017,2022, the exchange rate between the New Israeli Shekel, or NIS, and the dollar, as quoted by the Bank of Israel, was NIS 3.5963.519 to $1.00. Unless indicated otherwise by the context, statements in this Annual Report on Form 10-K that provide the dollar equivalent of NIS amounts or provide the NIS equivalent of dollar amounts are based on such exchange rate.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
The statements contained in this Annual Report on Form 10-K that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws.laws and the Israeli securities law. Words such as “expects,” “anticipates,” “intends,” “plans,” “planned expenditures,” “believes,” “seeks,” “estimates” and similar expressions or variations of such words are intended to identify forward-looking statements, but are not deemed to represent an all-inclusive means of identifying forward-looking statements as denoted in this Annual Report on Form 10-K. Additionally, statements concerning future matters are forward-looking statements. We remind readers that forward-looking statements are merely predictions and therefore inherently subject to uncertainties and other factors and involve known and unknown risks that could cause the actual results, performance, levels of activity, or our achievements, or industry results, to be materially different from any future results, performance, levels of activity, or our achievements, or industry results, expressed or implied by such forward-looking statements. Such forward-looking statements appear in Item 1 - “Business”“Item 1. Business” and Item 7 - “Management's“Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations,” as well as elsewhere in this Annual Report on Form 10-K and include, among other statements, statements regarding the following:
| ● | our comprehensive analysis of data from our ORA-D-013-1 Phase 3 trial to understand if there is a path forward for our oral insulin candidate; |
| ● | our plan to evaluate potential strategic opportunities; |
| ● | our ability to enhance value for our stockholders; |
| ● | the expected development and potential benefits from our |
| ● | the prospects of entering into additional license agreements, or other partnerships or forms of cooperation with other companies or medical institutions; |
| ● | future milestones, conditions and royalties under our license agreements; |
| ● | expected timing of a clinical study for the |
| ● | our research and development plans, including pre-clinical and clinical trials plans and the timing of enrollment, obtaining results and conclusion of |
| ● | our belief that our technology has the potential to deliver medications and vaccines orally that today can only be delivered via injection; |
| ● | the competitive ability of our technology based on product efficacy, safety, patient convenience, reliability, value and patent position; |
ii
| ● | the potential market demand for our products; |
| ● | our ability to obtain patent protection for our intellectual property; |
| ● | our expectation that |
| ● | our expectations regarding our short- and long-term capital requirements; |
| ● | our outlook for the coming months and future periods, including but not limited to our expectations regarding future revenue and expenses; and |
| ● | information with respect to any other plans and strategies for our business. |
Although forward-looking statements in this Annual Report on Form 10-K reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by us.us at the time of such statements. Consequently, forward-looking statements are inherently subject to risks and uncertainties and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include, without limitation, those discussed herein, including those risks described in Item“Item 1A. "Risk Factors",Risk Factors,” and expressed from time to time in our other filings with the Securities and Exchange Commission, or SEC. In addition, historic results of scientific research, clinical and preclinical trials do not guarantee that the conclusions of future research or trials would not suggest different conclusions. Also, historic results referred to in this Annual Report on Form 10-K could be interpreted differently in light of additional research, clinical and preclinical trials results. Readers are urged not to place undue reliance on these forward-looking statements, which speak only as of the date of this Annual Report on Form 10-K. Except as required by law, we undertake no obligation to revise or update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this Annual Report on Form 10-K. Readers are urged to carefully review and consider the various disclosures made throughout the entirety of this Annual Report on Form 10-K which attempt to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
iii
PART I
DESCRIPTION OF BUSINESSDescription of Business
Research and Development
We are currently a pharmaceutical company currently engaged in the research and development of innovative pharmaceutical solutions including an oral insulin capsule to be usedwith a technology platform that allows for the treatment of individuals with diabetes, and the use of orally ingestible capsules or pills fororal delivery of other polypeptides.
Oral insulin: We are seeking to revolutionize the treatment of diabetes through our proprietary flagship product, an orally ingestible insulin capsule, or ORMD-0801. In August 2017, we had a call with the U.S. Food and Drug Administration, or FDA, regarding ORMD-0801. During the call, the FDA advised that the regulatory pathway for the submission of ORMD-0801 would be a Biologics License Application, or BLA. Such a pathway would grant us 12 years of marketing exclusivity for ORMD-0801, if approved, and an additional six months of exclusivity may be granted to us if the product also receives approval for use in pediatric patients. We plan to initiate in the first quarter of calendar year 2018, a clamp study on six type 1 diabetic patients and a three-month dose-ranging clinical trial on approximately 240 type 2 diabetic patients to assess the safety and evaluate the effect of ORMD-0801 on HbA1c, the main FDA registrational endpoint. In February 2017, we completed a Phase IIa dose finding clinical trial which was initiated in October 2016 in order to better define the optimal dosing of ORMD-0801. In April 2016, we completed a Phase IIb clinical trial on 180 type 2 adult diabetic patients that was initiated in June 2015 and conducted in 33 sites in the United States. This double-blind, randomized, 28-day dosing clinical trial was conducted under an Investigational New Drug application, or IND, with the FDA. The clinical trial, designed to assess the safety and efficacy of ORMD-0801, investigated ORMD-0801 over a 28 day treatment period and had statistical power to give us greater insight into the drug’s efficacy. The trial indicated a statistically significant lowering of blood glucose levels versus placebo across several endpoints, with no serious or severe adverse issues related to the drug. The trial successfully met all of its primary and most of its secondary and exploratory endpoints for both safety and efficacy. Prior to that trial, we completed Phase IIa clinical trials in patients with both type 1 and type 2 diabetes. Our technology allows insulin to travel from the gastrointestinal tract via the portal vein to the bloodstream, revolutionizing the manner in which insulin is delivered. It enables its passage in a more physiological manner than current delivery methods of insulin. Our technology is a platform that has the potential to deliver medications and vaccines orally that today can only be delivered via injection.
Oral Glucagon-like peptide-1: Glucagon-like peptide-1, or GLP-1, is an incretin hormone, which is a type of gastrointestinal hormone that stimulates the secretion of insulin from the pancreas. The incretin concept was hypothesized when it was noted that glucose ingested by mouth (oral) stimulated two to three times more insulin release than the same amount of glucose administered intravenously. In addition to stimulating insulin release, GLP-1 was found to suppress glucagon release (a hormone involved in the regulation of glucose) from the pancreas, slow gastric emptying to reduce the rate of absorption of nutrients into the blood stream, and increase satiety. Other important beneficial attributes of GLP-1 are its effects of increasing the number of beta cells (cells that manufacture and release insulin) in the pancreas and, possibly, protection of the heart. In addition to our flagship product, the ORMD-0801 insulin capsule, we are using our technology for an orally ingestible GLP-1 capsule, or ORMD-0901. In August 2015, we began a non-FDA clinical trial outside of the United States for our oral exenatide capsule on type 2 diabetic patients. The trial was completed during the second quarter of calendar year 2016 and indicated positive results as it showed ORMD-0901 to be safe and well tolerated and also demonstrated encouraging efficacy data. We completed a three-month pre-clinical toxicology study in March 2017, anticipate receiving the final report during the fourth quarter of calendar year 2017 and expect to file an IND and move directly into a pharmacokinetics study, followed by a large Phase II trial in the United States under an FDA IND.
Diabetes: Diabetes is a disease in which the body does not produce or properly use insulin. Insulin is a hormone that causes sugar to be absorbed into cells, where the sugar is converted into energy needed for daily life. The cause of diabetes is attributed both to genetics (type 1 diabetes) and, most often, to environmental factors such as obesity and lack of exercise (type 2 diabetes). According to the International Diabetes Federation, or IDF, an estimated 415 million adults worldwide suffered from diabetes in 2015 and the IDF projects this number will increase to 642 million by 2040. Also, according to the IDF, in 2015, an estimated 5.3 million people died from diabetes. According to the American Diabetes Association, or ADA, in the United States there were approximately 30.3 million people with diabetes, or 9.4% of the United States population in 2015. Diabetes is a leading cause of blindness, kidney failure, heart attack, stroke and amputation.
therapeutic proteins.
Intellectual property: We own a portfolio of patents and patent applications covering our technologies, and we are aggressively protecting these technology developments on a worldwide basis.
Management: We are led by a highly-experienced management team knowledgeable in the treatment of diabetes. Our Chief Scientific Officer, Miriam Kidron, PhD, is a world-recognized pharmacologist and a biochemist and the innovator primarily responsible for our oral insulin technology development and know-how.
Scientific Advisory Board: Our management team has access to our internationally recognized Scientific Advisory Board whose members are thought-leaders in their respective areas. The Scientific Advisory Board is comprised of Dr. Roy Eldor, Professor Ele Ferrannini, Professor Avram Hershko, Dr. Harold Jacob and Dr. Harvey L. Katzeff.
Strategy
Short Term Business Strategy
We plan to conduct further research and development on the technology covered by the patent application “Methods and Composition for Oral Administration of Proteins,” which we acquired from Hadasit Medical Research Services and Development Ltd. in 2006, and which is granted in various foreign jurisdictions, as well as the other patents we have filed in various foreign jurisdictions since then, as discussed below under“—Patents and Licenses” and below under“Item 1A. Risk Factors”.
Through our research and development efforts, we have successfully developed an oral dosage form that willintended to withstand the harsh environment of the stomach and intestines and will be effective in deliveringeffectively deliver active biological insulin or other proteins, such as exenatide, for the treatment of diabetes.proteins. The excipients that are added to the proteins in the formulation process mustare not intended to modify the proteins chemically or biologically, and the dosage form mustis designed to be safe to ingest. We plan to continue to conduct clinical trials to show the effectiveness of our technology.
On January 11, 2023, we announced that our Phase 3 trial, or the ORA-D-013-1 Phase 3 trial, did not meet its primary and secondary endpoints. As a result, we terminated both ORA-D-013-1 and ORA-D-013-2 Phase 3 clinical trials. In parallel, we have initiated a comprehensive analysis of the data to understand if there is a path forward for our oral insulin candidate. We originally filedare examining our existing pipeline and have commenced an INDevaluation process of potential strategic opportunities, with the FDA in December 2012goal of enhancing value for clearanceour stockholders.
Research and Development
Oral insulin
Type 2 Diabetes: We conducted the ORA-D-013-1 Phase 3 trial on patients with type 2 diabetes, or T2D, with inadequate glycaemic control who were on two or three oral glucose-lowering agents. The primary endpoint of the trial was to begin a Phase II clinical trialevaluate the efficacy of our oral insulin capsule, ORMD-0801, compared to placebo in order to evaluateimproving glycaemic control as assessed by HbA1c, with a secondary efficacy endpoint of assessing the safety, tolerability and efficacychange from baseline in type 2 diabetic volunteers. Because the identical formulation of ORMD-0801 had not yet been studied in humansfasting plasma glucose at bedtime, in February 2013, the FDA noted concerns about mitigating potential risks of severe hypoglycemia and requested that we perform a sub-study in a controlled in-patient setting for a one-week period prior to beginning the larger multi-centered Phase II trial. As a result, we withdrew the original IND and, in April 2013, we submitted a new IND for the Phase IIa study. Following the FDA’s clearance to proceed in May 2013, we began the Phase IIa study in July 2013. As26 weeks. On January 11, 2023, we announced in January 2014,that the ORA-D-013-1 Phase IIa study met all3 trial did not meet its primary and secondary endpoints. Specifically,Following the Phase IIa study evaluated the pharmacodynamic effects of ORMD-0801 on mean nighttime glucose (determined using a continuous glucose monitor). The results showed that ORMD-0801 exhibited a sound safety profile, led to reduced mean daytime and nighttime glucose readings and lowered fasting blood glucose concentrations, when compared to placebo. In addition, no serious adverse events occurred during this study, and the only adverse events that occurred were not drug related.
In light of these results, in June 2015, we initiated the Phase IIb clinical trial on 180 type 2 adult diabetic patients which was completed in April 2016. This double-blind, randomized, 28-day dosing clinical trial was designed to assess the safety and efficacy of ORMD-0801, and was conducted in 33 sites in the United States. The trial indicated a statistically significant lowering of blood glucose levels versus placebo across several endpoints, with no serious or severe adverse issues related to the drug. The trial successfully met all of its primary and most of its secondary and exploratory endpoints. The trial primarily evaluated the nighttime glucose lowering effect and safety of ORMD-0801 compared to a placebo. The results of the mean nighttime glucose showedORA-D-013-1 Phase 3 trial, we also terminated the ORA-D-013-2 Phase 3 trial, a significant difference in mean change from run-in versus placebo. ORMD-0801 oral insulin was safesecond Phase 3 trial that included T2D patients with inadequate glycaemic control who were attempting to manage their condition with either diet alone or with diet and well-tolerated for the dosing regimen in this trial. The trial further evaluated the effect of ORMD-0801 on mean 24-hour glucose, fasting glucose, and daytime glucose and the results showed a statistically significant difference in mean change from run-in versus placebo. Two examples of the data gleaned from this study are shown below:
metformin.
 | 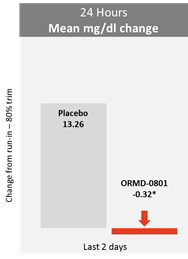 |
* Indicates Statistically Significant Difference from Placebo (p-Value<0.05)
No significant difference was shown in change in morning fasting serum insulin, C-Peptide, or triglycerides.
Following the significant results of the Phase IIb trial, we initiated in October 2016 an additional Phase IIa dose finding clinical trial which was completed in February 2017. This randomized, double-blind trial was conducted on 32 type 2 adult diabetic patients in order to better define the optimal dosing of ORMD-0801 moving forward. The results of the trial indicated a positive safety profile and potentially meaningful efficacy of ORMD-0801, as the efficacy data suggest ORMD-0801 improves glucose control.
NASH: In March 2017,December 2020, we initiated a six-month toxicology study to allowdouble blind, placebo controlled clinical trial of ORMD-0801 for the usetreatment of our oral insulin capsule for a longer period than previously performed,non-alcoholic steatohepatitis, or NASH, in preparation for our proposed upcoming three-month clinicalT2D. On September 13, 2022, we reported positive top line results from this trial, for type 2. We anticipate receiving the final report of this study in the first quarter of calendar year 2018.
In August 2017, we had a call with the FDA regarding ORMD-0801. During the call, the FDA advised that the regulatory pathway for submission of ORMD-0801 would be a BLA. Such a pathway would grant a full 12 years of marketing exclusivity for ORMD-0801, if approved. On top of this, an additional six months of exclusivity may be granted if the product also receives approval for use in pediatric patients. The FDA confirmed that the approach to nonclinical toxicology, chemistry manufacturing controls and qualification of excipients would be driven by their published guidance documents. We plan to initiate in the first quarter of calendar year 2018 a three-month dose-ranging clinical trial on approximately 240 type 2 diabetes patients to assess the safety and evaluate the effect of ORMD-0801 on HbA1c, the main FDA registrational endpoint. In addition, the FDA confirmed our ability to use insulin from different suppliers in a Phase III study.
In February 2014, we submitted a protocol to the FDA to initiate a Phase IIa trial of our oral insulin capsule for type 1 diabetes volunteers. The protocol was submitted under our existing IND to include both type 1 and type 2 diabetes indications. Beginning in March 2014, the double-blind, randomized, placebo controlled, seven-day treatment study design was carried out at an inpatient setting on 25 type 1 diabetic patients. As we announced in October 2014, the results showeddemonstrating that ORMD-0801 oral insulin given before meals appeared to be safe and well-tolerated for the dosing regimen in this study. Although the study was not powered to show statistical significance, there were internally consistent trends observed. Consistent with the timing of administration, the data showed a decrease in bolus insulin, a decrease in post-prandial glucose, a decrease in daytime glucose by continual glucose monitoring and an increase in post-prandial hypoglycemia in the active group, demonstrating the efficacy of ORMD-0801.
We also plan to conduct a glucose clamp study of our oral insulin capsule on six type 1 diabetic patients in the first quarter of calendar year 2018. The glucose clamp is a method for quantifying insulin absorption in order to measure a patient’s insulin sensitivity and how well a patient metabolizes glucose.
Should our Phase IIb three-month dosing clinical trial successfully meet its primary endpoints, we anticipate initiating two six-month Phase III clinical trials on both type 1 and type 2 diabetic patients, following which we expect to file a New Drug Application with a potential approval by the third quarter of calendar year 2023.
In September 2013, we submitted a pre-IND package to the FDA for ORMD-0901. In August 2015, we began a non-FDA clinical trial outside of the United States on type 2 diabetic patients. The trial was completed during the second quarter of calendar year 2016 and indicated positive results as it showed ORMD-0901 to be safe and well tolerated and demonstrated encouraging efficacy data. We completed a three-month pre-clinical toxicology studyat 8 mg twice daily dosing, meeting the primary endpoint of no difference in March 2017 and anticipate receiving the final report during the fourth quarter of calendar year 2017. We expect to file an IND during the first quarter of calendar year 2018 and move directly into a small pharmacokinetics study followed by a large Phase II trial in the United States under an FDA IND.
Clinical trials are planned in order to substantiate our results as well asadverse events for purposes of making future filings for drug approval. We also plan to conduct further research and development by deploying our proprietary drug delivery technology for the delivery of other polypeptides in addition to insulin, and to develop other innovative pharmaceutical products.
The table below gives an overview of our primary product pipeline (calendar quarters):
ORMD-0801
|  |
| |||
 |
| ||||
|  |
| |||
Another component of our business strategy is to partner with other companies or medical institutions in order to further develop our technology and commence pre-commercialization activities. On November 30, 2015, we, our Israeli subsidiary and HTIT entered into a Technology License Agreement, which was further amended, according to which we granted HTIT an exclusive commercialization license in the territory of the People's Republic of China, Macau and Hong Kong, or the Territory, related to our oral insulin capsule, ORMD-0801. Pursuant to this license agreement, HTIT will conduct, at its own expense, certain pre-commercialization and regulatory activities with respect to our technology related to the ORMD-0801 capsule, and will pay certain royalties and an aggregate of approximately $37.5 million (see “Out-Licensed Technology” below). We plan to seek additional partnerships or forms of cooperation with other companies or medical institutions. While our strategy is to partner with an appropriate party, no assurance can be given that we will in fact be able to reach an agreeable partnership with any third party. Under certain circumstances, we may determine to develop one or more of our oral dosage forms on our own, either world-wide or in select territories.
Long Term Business Strategy
If our oral insulin capsule or other drug delivery solutions show significant promise in clinical trials, we plan to ultimately seek a strategic commercial partner, or partners, with extensive experience in the development, commercialization, and marketing of insulin applications and/or other orally digestible drugs. We anticipate such partner or partners would be responsible for, or substantially support, late stage clinical trials (Phase III) to increase the likelihood of obtaining regulatory approvals and registrations in the appropriate markets in a timely manner. We further anticipate that such partner, or partners, would also be responsible for sales, marketing and support of our oral insulin capsule in these markets. Such planned strategic partnership, or partnerships, may provide a marketing and sales infrastructure for our products as well as financial and operational support for global clinical trials, post marketing studies, label expansions and other regulatory requirements concerning future clinical development in the United States and elsewhere. Any future strategic partner, or partners, may also provide capital and expertise that would enable the partnership to develop new oral dosage forms for other polypeptides. While our strategy is to partner with an appropriate party, no assurance can be given that we will in fact be able to reach an agreeable partnership with any third party. Under certain circumstances, we may determine to develop one or more of our oral dosage forms on our own, either world-wide or in select territories.
Other Planned Strategic Activities
In addition to developing our own oral dosage form drug portfolio, we are, on an on-going basis, considering in-licensing and other means of obtaining additional technologies to complement and/or expand our current product portfolio. Our goal is to create a well-balanced product portfolio that will enhance and complement our existing drug portfolio.
Product Development
Research and Development Summary
We devote the majority of our efforts to research and development, including clinical studies for our lead clinical product candidates, as described below.
Orally Ingestible Insulin
During the fiscal year ended August 31, 2007, we conducted several clinical studies of our orally ingestible insulin that were intended to assess both the safety/tolerability and absorption properties of our proprietary oral insulin. Based on the pharmacokinetic and pharmacologic outcomes of these trials, we decided to continue the development of our oral insulin product.
During the fiscal year ended August 31, 2008, we successfully completed animal studies and non-FDA approved clinical trials using our oral insulin capsule, including a Phase Ib clinical trial in healthy human volunteers with the intent of dose optimization; a Phase IIa study to evaluate the safety and efficacy of our oral insulin capsule in type 2 diabetic volunteers at Hadassah Medical Center in Jerusalem; and a Phase IIa study to evaluate the safety and efficacy of our oral insulin capsule on type 1 diabetic volunteers.
Our successful non-FDA clinical trials continued in the fiscal year ended August 31, 2009, or fiscal 2009, with a Phase IIb study in South Africa to evaluate the safety, tolerability and efficacy of our oral insulin capsule on type 2 diabetic volunteers.
In September 2010, we reported the successful results of an exploratory clinical trial testing the effectiveness of our oral insulin capsule in type 1 diabetes patients suffering from uncontrolled diabetes. Unstable or labile diabetes is characterized by recurrent, unpredictable and dramatic blood glucose swings often linked with irregular hyperglycemia and sometimes serious hypoglycemia affecting type 1 diabetes patients. This successfully completed exploratory study was a proof of concept study for defining a novel indication for ORMD-0801. We believe the encouraging results justify further clinical development of ORMD-0801 capsule application toward management of uncontrolled diabetes.
In March 2011, we reported that we successfully completed a comprehensive toxicity study for our oral insulin capsule. The study was completed under conditions prescribed by the FDA Good Laboratory Practices regulations.
We began FDA-approved clinical trials of ORMD-0801 in July 2013, with the Phase IIa study, which evaluated the pharmacodynamic effects of ORMD-0801 on mean nighttime glucose (determined using a continuous glucose monitor) on 30 volunteers with type 2 diabetes. As we announced in January 2014, the results showed that ORMD-0801 exhibited a sound safety profile, led to reduced mean daytime and nighttime glucose readings and lowered fasting blood glucose concentrations, when compared to placebo.
In March 2014, we began an FDA-approved Phase IIa trial of ORMD-0801 in volunteers with type 1 diabetes. As we announced in October 2014, the results showed that ORMD-0801 oral insulin given before meals appeared to be safe and well-tolerated for the dosing regimen in this study. Although the study was not powered to show statistical significance, there were internally consistent trends observed. Consistent with the timing of administration, the data showed a decrease in bolus insulin, a decrease in post-prandial glucose, a decrease in daytime glucose by continual glucose monitoring and an increase in post-prandial hypoglycemia in the active group, demonstrating the efficacy of ORMD-0801.
In June 2015, we initiated a Phase IIb clinical trial on 180 type 2 adult diabetic patients, which was completed in April 2016. This double-blind, randomized, 28-day dosing clinical trial was designed to assess the safety and efficacy of ORMD-0801 and was conducted in 33 sites in the United States. The trial indicated a statistically significant lowering of blood glucose levels versus placebo across several endpoints, with no serious or severe adverse issues related to the drug. The trial successfully met all of its primary and most of its secondary and exploratory endpoints for both safety and efficacy.
In October 2016, we initiated an additional Phase IIa, dose finding clinical trial which was completed in February 2017. This randomized, double-blind trial was conducted on 32 type 2 adult diabetic patients in order to better define the optimal dosing of ORMD-0801 moving forward. The results of the trial indicated a positive safety profile and potentially meaningful efficacy of ORMD-0801, as the efficacy data suggest ORMD-0801 improves glucose control.
In March 2017, we initiated a six-month toxicology study to allow for the use of our oral insulin capsule for a longer period than previously performed, in preparation for our proposed upcoming three-month clinical trial for type 2 diabetes. We anticipate receiving the final report of this study in the first quarter of calendar year 2018.
In August 2017, we had a call with the FDA regarding ORMD-0801. During the call, the FDA advised that the regulatory pathway for submission of ORMD-0801 would be a BLA. Such a pathway would grant a full 12 years of marketing exclusivity for ORMD-0801, if approved, and an additional six months of exclusivity may be granted to us if the product also receives approval for use in pediatric patients. We plan to initiate in the first quarter of calendar year 2018 a three-month dose-ranging clinical trial on approximately 240 type 2 diabetes patients to assess the safety and evaluate the effect of ORMD-0801 on HbA1c, the main FDA registrational endpoint.
We utilize Clinical Research Organizations, or CROs, to conduct our clinical studies.
GLP-1 Analog
During fiscal 2009, we completed pre-clinical trials of ORMD-0901, an analog for GLP-1, which suggested that the GLP-1 analog (exenatide-4), when combined with Oramed’s capsule technology, is absorbed through the gastrointestinal tract and retains its biological activity.
In December 2009, we completed non-FDA approved clinical trial in healthy, male volunteers conducted at Hadassah University Medical Center in Jerusalem. This study evaluated the safety and efficacy of ORMD-0901. The results of the study indicated that ORMD-0901 was well tolerated by all subjects and demonstrated physiological activity, as extrapolated from ensuing subject insulin levels when compared to those observed after treatment with placebo.
In January 2013, we began a clinical trial for our oral exenatide capsule on healthy volunteers and type 2 diabetic patients. Based on this study, we decided to make slight adjustments in the manufacturing of these capsules and have begun pre-toxicology studies on the new capsules.
In September 2013, we submitted a pre-IND package to the FDA for ORMD-0901.
In August 2015, we began a non-FDA clinical trial outside of the United States for ORMD-0901 on type 2 diabetic patients. The trial was completed during the second quarter of calendar year 2016 and indicated positive results as it showed ORMD-0901 to be safe and well tolerated and also demonstrated encouraging efficacy data.
We completed a three-month toxicology study in March 2017 and anticipate receiving the final report during the fourth quarter of calendar year 2017 and expect to file an IND and move directly into a pharmacokinetics study followed by a large Phase II trial in the United States under an FDA IND.
Combination Therapy
In June 2012, we presented an abstract, which reported the impact of our oral insulin capsule, ORMD-0801, delivered in combination with our oral exenatide capsule ORMD-0901. The work assessed the safety and effectiveness of a combination of oral insulin and oral exenatide treatments delivered to pigs prior to food intake. The drug combination resulted in significantly improved blood glucose regulation when compared to administration of each drug separately.
In the near term, we are focusing our efforts on the development of our flagship products, oral insulin and oral exenatide. Once these two products have progressed further in clinical trials, we intend to conduct additional studies with the oral combination therapy.
Feasibility study
In August 2015, we entered into an agreement with a large international pharmaceutical company, or the Pharma Company, pursuant to which we conducted a feasibility study, using one of the Pharma Company's propriety injectable compounds. The study used our proprietary technology in order to deliver the compound orally. Following the successful completion of the first stage of the study in July 2016, we continued to the second step of the study. The study will provide data required for decision making on whether to enter into a license agreement between the parties.
Other products
During the first quarter of calendar 2017, we began developing a new drug candidate, a weight loss treatment in the form of an oral leptin capsule, and in April 2017, Israel’s Ministry of Health approved our commencement of a proof of concept single dose study for our oral leptin drug candidate to evaluate its pharmacokinetic and pharmacodynamics (glucagon reduction) in 10 type 1 adult diabetic patients. The study is projected to initiate in calendar year 2018 and be completed during calendar year 2019.
In November 2017, Israel’s Ministry of Health approved us to initiate an exploratory clinical study of our oral insulin capsule, ORMD-0801, in patients with nonalcoholic steatohepatitis (NASH). The proposed three-month treatment study will assess the effectiveness of ORMD-0801 in reducing liver fat content inflammationover the 12-week treatment period by observing several independent measures. All the measurements showed a consistent clinically meaningful trend in favor of ORMD-0801. We are currently evaluating our path forward for ORMD-0801 for NASH.
Oral Vaccine
On March 18, 2021, we entered into a license agreement, or the Oravax License Agreement, with Oravax, our 63% owned joint venture, pursuant to which we granted to Oravax an exclusive, worldwide license of our rights in certain patents and fibrosis in patients with NASH. We expectrelated intellectual property relating to initiateour proprietary oral delivery technology to further develop, manufacture and commercialize oral vaccines for COVID-19 and other novel coronaviruses based on Premas Biotech Pvt. Ltd.’s, or Premas’s, proprietary vaccine technology involving a triple antigen virus like particle, or the study duringOravax product, which was previously owned by Cystron Biotech LLC, and later acquired by Akers Biosciences Inc., or Akers. Effective January 1, 2022, Oravax transferred its rights and obligations under the end of calendar year 2017 and complete it during calendar year 2019.Oravax License Agreement to its wholly-owned subsidiary, Oravax Medical Ltd. For further details regarding the Oravax License Agreement, see note 12 to our audited consolidated financial statements.
In October 2021, Oravax’s oral virus-like particle, or VLP, COVID-19 vaccine received clearance from the South African Health Products Regulatory Authority (SAPHRA) to initiate a Phase 1 trial. On December 14, 2021, Oravax screened and enrolled the first participant in this Phase 1 clinical trial. The trial protocol was divided into two cohorts each comprised of 12 participants, with a 42-day safety waiting period between cohorts. Due to several factors, including the fact that many volunteers did not qualify during screening due to prior asymptomatic COVID-19 infection and other conditions, the rate of enrollment was slower than anticipated and as a result, we added an additional clinical site. On October 11, 2022, Oravax reported positive preliminary Phase 1 data for Cohort A of this trial, meeting primary and secondary endpoints of safety and immunogenicity. These results included significant antibody response (2-6 fold over baseline) as measured by multiple markers of immune response to VLP vaccine antigens observed in the majority of the patients dosed, and no safety issues were observed, including mild symptoms. Cohort B completed dosing on January 5, 2023 and data is expected in the first half of 2023.
On December 29, 2021, Oravax signed a cooperation and purchase agreement for an initial pre-purchase of 10 million doses of oral COVID-19 vaccines with Tan Thanh Holdings to commercialize the vaccine in Southeast Asia.
Raw Materials
Our oral insulin capsule is currently manufactured by Swiss Caps AG.Fidelio Healthcare, a diversified European Contract Development and Manufacturing Organization (CDMO) in the pharmaceutical and healthcare industries.
One of our oral capsule ingredients is being developed and produced by an Indian company.
In July 2010, Oramed Ltd. entered into the Manufacturing and Supply Agreement or MSA, with Sanofi-Aventis Deutschland GMBH, or Sanofi-Aventis. According to the MSA,agreement, Sanofi-Aventis will supplysupplies Oramed Ltd. with specified quantities of recombinant human insulin to be used for clinical trials.
We purchase,have purchased, pursuant to separate agreements with third parties, the raw materials required for the manufacturing of our oral capsule. We generally depend upon a limited number of suppliers for the raw materials. Although alternative sources of supply for these materials are generally available, we could incur significant costs and disruptions if we would need to change suppliers. The termination of our relationships with our suppliers or the failure of these suppliers to meet our requirements for raw materials on a timely and cost-effective basis could have a material adverse effect on our business, prospects, financial condition and results of operations.
PatentsMarket Overview
Diabetes is a disease in which the body does not produce or properly use insulin. Insulin is a hormone that causes sugar to be absorbed into cells, where the sugar is converted into energy needed for daily life. The cause of diabetes is attributed both to genetics (type 1 diabetes, or T1D) and, Licensesmost often, to environmental factors such as obesity and lack of exercise (T2D). According to the International Diabetes Federation, or IDF, an estimated 537 million adults (20-79 years) worldwide suffered from diabetes in 2021 and the IDF projects this number will increase to 783 million by 2045. Also, according to the IDF, in 2021, an estimated 6.7 million people died from diabetes. According to the American Diabetes Association, or ADA, in the United States there were approximately 37.3 million people with diabetes, or 11.3% of the United States population in 2019. Diabetes is a leading cause of blindness, kidney failure, heart attack, stroke and amputation.
Impact of COVID-19
We do not expect any material impact on our development timeline and our liquidity due to COVID-19. However, we experienced approximately six months of delays in clinical trials due to slow-downs of recruitment for trials generally. On the other hand, Oravax continues to develop its oral vaccine, the demand for which may be reduced if COVID-19 continues to abate. We continue to assess the effect on our operations by monitoring the status of COVID-19.
Intellectual Property and Patents
We own a portfolio of patents and patent applications covering our technologies, and we are aggressively protecting these technology developments on a worldwide basis.
We maintain a proactive intellectual property strategy, which includes patent filings in multiple jurisdictions, including the United States and other commercially significant markets. We hold 2637 patent applications currently pending, with respect to various compositions, methods of production and oral administration of proteins and exenatide. Expiration dates for pending patents, if granted, will fall between 2026 and 2034.2039.
We hold 64112 patents, seventeentwenty of which were issued induring the fiscal 2017, fifteen of which were issued in September 2017 and two of which were allowed in Europe and Canada,year ended December 31, 2022, including patents issued by the United States, Swiss, German, French, U.K., Italian, Dutch,Netherlands, Swedish, Spanish, Australian, Israeli, Japanese, New Zealand, South African, Russian, Canadian, Hong Kong, Chinese, European and Indian patent offices that cover a part of our technology, which allows for the oral delivery of proteins; patents issued by the Australian, Canadian, European, Austrian, Belgian, French, German, Irish, Italian, Luxembourg, , Monaco, Dutch,Netherlands, Norwegian, Spanish, Swedish, Swiss, U.K., Israeli, New Zealand, South African, Russian, Brazilian and RussianJapanese patent offices that cover part of our technology for the oral delivery of exenatide; and patents issued by the European, Austrian, Belgian, Danish,Denmark, French, German, Irish, Italian, Luxembourg, , Monaco, Netherland,Netherlands, Norway, Spanish, Swedish, Swiss, U.K. and Japanese patent offices for treating diabetes.
Consistent with our strategy to seek protection in key markets worldwide, we have been and will continue to pursue the patent applications and corresponding foreign counterparts of such applications. We believe that our success will depend on our ability to obtain patent protection for our intellectual property.
Our patent strategy is as follows:
| ● | Aggressively protect all current and future technological developments to assure strong and broad protection by filing patents and/or continuations in part as appropriate, |
| ● | Protect technological developments at various levels, in a complementary manner, including the base technology, as well as specific applications of the technology, and |
| ● | Establish comprehensive coverage in the United States and in all relevant foreign markets in anticipation of future commercialization opportunities. |
Trademarks and Trade Secrets
We have trademark applications pending in Israel, with Corresponding international trademark applications in Australia, Brazil, Canada, China, Colombia, the European Union, India, Indonesia, Japan, Kazakhstan, Korea, Malaysia, Mexico, New Zealand, Norway, Oman, Philippines, Russia, Singapore, Switzerland, Thailand, Turkey, Ukraine, United Arab Emirates, United Kingdom, U.S.A., Uzbekistan and Vietnam.
We also rely on trade secrets and unpatentable know-how that we seek to protect, in part, by confidentiality agreements. Our policy is to require our employees, consultants, contractors, manufacturers, outside scientific collaborators and sponsored researchers, our board of directors, or our Board, technical review board and other advisors, to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific limited circumstances. We also require signed confidentiality or material transfer agreements from any company that is to receive our confidential information. In the case of employees, consultants and contractors, the agreements provide that all inventions conceived by the individual while rendering services to us shall be assigned to us as the exclusive property of ourthe Company. There can be no assurance, however, that all persons who we desire to sign such agreements will sign, or if they do, that these agreements will not be breached, that we would have adequate remedies for any breach, or that our trade secrets or unpatentable know-how will not otherwise become known or be independently developed by competitors.
Out-Licensed Technology
Entera Bio
In June 2010, our wholly-owned subsidiary, Oramed Ltd., entered into a joint venture agreement with DNA GROUP (T.R.) Ltd. (formerly D.N.A Biomedical Solutions Ltd.), or D.N.A,DNA, for the establishment of Entera Bio LTD,Ltd., or Entera.
Under the terms of a license agreement that was entered into between Oramed Ltd. and Entera in August 2010, we out-licensed technology to Entera, on an exclusive basis, for the development of oral delivery drugs for certain indications to be agreed upon between the parties. The out-licensed technology differs from our main delivery technology that is used for oral insulin and GLP-1 analog and is subject to different patent applications. Entera’s initial development effort is for an oral formulation for the treatment of osteoporosis. In March 2011, weOramed Ltd. sold shares of Entera to DNA, retaining 117,000 ordinary shares (after giving effect to a stock split by Entera in July 2018). In consideration for the shares sold to DNA, the Company received, among other payments, ordinary shares of DNA (see also note 3 to our audited consolidated financial statements).
As part of this agreement, Oramed Ltd. entered into a patent transfer agreement, to replaceor the original license agreement upon closing pursuantPatent Transfer Agreement, according to which Oramed Ltd. assigned to Entera all of its right, title and interest in andrights to a patent application related to the patent applicationoral administration of proteins that it hadhas licensed to Entera since August 2010, in August 2010. Under this agreement, Oramed Ltd. is entitled to receive from Enterareturn for royalties of 3% of Entera’s net revenues (as defined in the agreement) and a license back of that patent application for use in respect of diabetes and influenza.
In March As of December 31, 2022, Entera had not paid any royalties to Oramed Ltd. On December 11, 2018, Entera announced that it had entered into a research collaboration and license agreement with Amgen, Inc., or Amgen. To the extent that the license granted to Amgen results in net revenues as defined in the Patent Transfer Agreement, Oramed Ltd. will be entitled to the aforementioned royalties. As part of a consulting agreement with a third party dated February 15, 2011, we also consummated a transaction with D.N.A, whereby weOramed Ltd. is obliged to pay this third party royalties of 8% of the net royalties received in respect of the patent that was sold to D.N.A 47% of Entera’s outstanding share capital on an undiluted basis, retaining a 3% interest as ofEntera in March 2011. In consideration for the shares sold to D.N.A, the Company received, among other payments, 4,202,334 ordinary shares of D.N.A
The D.N.A ordinary shares are traded on the Tel Aviv Stock Exchange and have a quoted price, which is subject to market fluctuations, and may, at times, have a price below the value on the date we acquired such shares. In addition, the ordinary shares of D.N.A have historically experienced low trading volume; as a result, there is no guarantee that we will be able to resell the ordinary shares of D.N.A at the prevailing market prices. During the years ended AugustDecember 31, 2017, 20162022, 2021 and 2015,four month period ended December 2021, we did not sell any of the D.N.ADNA’s ordinary shares. As of AugustDecember 31, 2017,2022, we held approximately 7.9%1.4% of D.N.A’sDNA’s outstanding ordinary shares and approximately 0.4% of Entera’s outstanding ordinary shares.
In November 2017, Entera filed with the SEC a draft registration statement on Form F-1 for the initial public offering by Entera, a listing of its shares on the Nasdaq and for potential resale by certain selling stockholders of Entera's ordinary shares previously issued.HTIT
In June 2016, Entera announced that it had obtained orphan status from the European Medicines Agency, or EMA, for its oral treatment for hypoparathyroidism. EMA approval is in addition to the orphan status it obtained from the FDA for the same oral treatment in April 2014.
In July 2015, Entera announced it had completed a phase IIa study to assess the safety and efficacy of its oral treatment for hypoparathyroidism and that the goals of the study were achieved.
On November 30, 2015, we our Israeli subsidiary and HTIT entered into a Technology License Agreement, or TLA, with Hefei Tianhui Incubator of Technologies Co., Ltd., or HTIT, and on December 21, 2015, these parties entered into an Amended and Restated Technology License Agreement that was further amended by the parties on June 3, 2016 and July 24, 2016, or the HTIT License Agreement. According to the HTIT License Agreement, we granted HTIT an exclusive commercialization license in the Territory,territory of the People’s Republic of China, Macau and Hong Kong, related to our oral insulin capsule, ORMD-0801, or the Product. Pursuant to the HTIT License Agreement, HTIT will conduct, at its own expense, certain pre-commercialization and regulatory activities with respect to our technology and ORMD-0801 capsule, and will pay (i) royalties of 10% on net sales of the related commercialized products to be sold by HTIT in the Territory,territory, or Royalties, and (ii) an aggregate of $37.5 million, of which $3 million iswas payable immediately, $8 million will be paid subject to our entry into certain agreements with certain third parties, and $26.5 million will be payable upon achievement of certain milestones and conditions. In the event that we will not meet certain conditions, the Royalties rate may be reduced to a minimum of 8%. Following the final expiration of our patents covering the technology in the Territoryterritory in 2033, the Royalties rate may be reduced, under certain circumstances, to 5%.
The royalty payment obligation shall apply during the period of time beginning upon the first commercial sale of the Product in the Territory,territory, and ending upon the later of (i) the expiration of the last-to-expire licensed patents in the Territory;territory; and (ii) 15 years after the first commercial sale of the Product in the Territory, or the Royalty Term.
territory. The HTIT License Agreement shall remain in effect until the expiration of the Royalty Term.royalty term. The HTIT License Agreement contains customary termination provisions.
The initial payment of $3 million was Through December 31, 2022, we received in January 2016. Following the achievement of certain milestones, the second and thirdaggregate milestone payments of $6.5$20.5 million and $4 million, respectively, were received in July 2016 andout of the fourth milestone paymentaggregate amount of $4 million was received in October 2016.$37.5 million.
On August 21, 2020, we received a letter from HTIT, disputing certain pending payment obligations of HTIT under the TLA. We alsowholly dispute said claims and we are in discussions with HTIT in an attempt to reach a mutually agreeable solution. We are currently evaluating with HTIT a path forward to continue our collaboration, following the results of our ORA-D-013-1 Phase 3 trial.
Oravax License
In consideration for the grant of the license under the Oravax License Agreement, we will receive (i) royalties equal to 7.5% on net sales, as defined in the Oravax License Agreement, of each product commercialized by Oravax, its affiliates and permitted sublicensees related to the license during the term specified in the Oravax License Agreement, (ii) sublicensing fees equal to 15% of any non-sales-based consideration received by Oravax from a permitted sublicensee and (iii) other payments ranging between $25 million to $100 million, based on certain sales milestones being achieved by Oravax. The parties further agreed to establish a development and steering committee, which will consist of three members, of which two members will be appointed by us, that will oversee the ongoing research, development, clinical and regulatory activity with respect to the Oravax product. In addition, we agreed to buy and Oravax agreed to issue to us 1,890,000 shares of common stock of Oravax, representing 63% of the common stock of Oravax for the aggregate amount of $1.5 million. Akers contributed $1.5 million in cash to Oravax and a license agreement to the Oravax product. Nadav Kidron, the Company’s President and Chief Executive Officer, was one of the former members of Cystron. See note 12 to our audited consolidated financial statements.
Medicox License
On November 13, 2022, we entered into a separate securities purchasedistribution license agreement with HTIT,Medicox Co., Ltd., or Medicox. an emerging biotech company with a consortium of proven partnerships in the SPA, pursuantRepublic of Korea. The agreement grants Medicox the exclusive license to which HTIT invested $12apply for regulatory approval and distribute ORMD-0801 for ten years in the Republic of Korea. Medicox will comply with agreed distribution targets and will purchase ORMD-0801 at an agreed upon transfer price per capsule. In addition, Medicox will pay Oramed up to $15 million in usdevelopmental milestones, $2 million of which have already been received by Oramed to date, and up to 15% royalties on gross sales. Medicox will also be responsible for gaining regulatory approval in December 2015 (see – “Liquidity and capital resources” below). In connectionthe Republic of Korea.
We are currently evaluating with Medicox a path forward to continue our collaboration, following the License Agreement and the SPA, we received a non-refundable paymentresults of $500,000 as a no-shop fee.our ORA-D-013-1 Phase 3 trial.
Government Regulation
The Drug Development Process
Regulatory requirements for the approval of new drugs vary from one country to another. In order to obtain approval to market our drug portfolio, we need to go through a different regulatory process in each country in which we apply for such approval. In some cases, information gathered during the approval process in one country can be used as supporting information for the approval process in another country. As a strategic decision, we decided to first explore the FDA regulatory pathway. The following is a summary of the FDA’s requirements.
The FDA requires that pharmaceutical and certain other therapeutic products undergo significant clinical experimentation and clinical testing prior to their marketing or introduction to the general public. Clinical testing, known as clinical trials or clinical studies, is either conducted internally by life science, pharmaceutical or biotechnology companies or is conducted on behalf of these companies by CROs.
The process of conducting clinical studiestrials is highly regulated by the FDA, as well as by other governmental and professional bodies. Below we describe the principal framework in which clinical studiestrials are conducted, as well as describe a number of the parties involved in these studies.trials.
Protocols.Protocols. Before commencing human clinical studies,trials, the sponsor of a new drug or therapeutic product must submit an IND application to the FDA. The application contains, among other documents, what is known in the industry as a protocol. A protocol is the blueprint for each drug study. The protocol sets forth, among other things, the following:
| ● | Who must be recruited as qualified participants, |
| ● | How often to administer the drug or product, |
| ● | What tests to perform on the participants, and |
| ● | What dosage of the drug or amount of the product to give to the participants. |
Institutional Review Board.Board. An institutional review board is an independent committee of professionals and lay persons which reviews clinical research studiestrials involving human beings and is required to adhere to guidelines issued by the FDA. The institutional review board does not report to the FDA, but its records are audited by the FDA. Its members are not appointed by the FDA. All clinical studiestrials must be approved by an institutional review board. The institutional review board’s role is to protect the rights of the participants in the clinical studies.trials. It approves the protocols to be used, the advertisements which the company or CRO conducting the study proposes to use to recruit participants, and the form of consent which the participants will be required to sign prior to their participation in the clinical studies.
trials.
Clinical Trials.Trials. Human clinical studiestrials or testing of a potential product are generally done in three stages known as Phase I1 through Phase III3 testing. The names of the phases are derived from the regulations of the FDA. Generally, there are multiple studiestrials conducted in each phase.
Phase I.1. Phase I studies1 trials involve testing a drug or product on a limited number of healthy or patient participants, typically 24 to 100 people at a time. Phase I studies1 trials determine a product’s basic safety and how the product is absorbed by, and eliminated from, the body. This phase lasts an average of six months to a year.
Phase II.2. Phase II2 trials involve testing of no more than 300 participants at a time who may suffer from the targeted disease or condition. Phase II2 testing typically lasts an average of one to two years. In Phase II,2, the drug is tested to determine its safety and effectiveness for treating a specific illness or condition. Phase II2 testing also involves determining acceptable dosage levels of the drug. Phase II studies2 trials may be split into Phase IIa2a and Phase IIb sub-studies.2b sub-trials. Phase IIa studies2a trials may be conducted with patient volunteers and are exploratory (non-pivotal) studies,trials, typically designed to evaluate clinical efficacy or biological activity. Phase IIb studies2b trials are conducted with patients defined to evaluate definite dose range and evaluate efficacy. If Phase II studies2 trials show that a new drug has an acceptable range of safety risks and probable effectiveness, a company will generally continue to review the substance in Phase III studies.3 trials.
Phase III.3. Phase III studies3 trials involve testing large numbers of participants, typically several hundred to several thousand persons. The purpose is to verify effectiveness and long-term safety on a large scale. These studiestrials generally last two to three years. Phase III studies3 trials are conducted at multiple locations or sites. Like the other phases, Phase III3 requires the site to keep detailed records of data collected and procedures performed.
Biological License Application.Application. The results of the clinical trials for a biological product are submitted to the FDA as part of a Biological License Application, or BLA. Following the completion of Phase III studies,3 trials, assuming the sponsor of a potential product in the United States believes it has sufficient information to support the safety and effectiveness of its product, the sponsor will generally submit a BLA to the FDA requesting that the product be approved for marketing. The application is a comprehensive, multi-volume filing that includes the results of all clinical studies,trials, information about the drug’s composition, and the sponsor’s plans for producing, packaging and labeling the product. The FDA’s review of an application can take a few months to many years, with the average review lasting 18 months. Once approved, drugs and other products may be marketed in the United States, subject to any conditions imposed by the FDA. Approval of a BLA provides 12 years of exclusivity in the U.S. market.
Phase IV.4. The FDA may require that the sponsor conduct additional clinical trials following new drug approval. The purpose of these trials, known as Phase IV studies,4 trials, is to monitor long-term risks and benefits, study different dosage levels or evaluate safety and effectiveness. In recent years, the FDA has increased its reliance on these trials. Phase IV studies4 trials usually involve thousands of participants. Phase IV studies4 trials also may be initiated by the company sponsoring the new drug to gain broader market value for an approved drug.
European Regulation. Similar to the U.S., a European sponsor of a biological product may submit a Marketing Approval Application to the European Medicines Agency, or EMA, for the registration of the product. The approval process in Europe consists of several stages, which together are summed up to 210 days from the time of submission of the application (net, without periods in which the sponsor provides answers to questions raised by the agency) following which, a Marketing Approval may be granted. During the approval process, the sponsor'ssponsor’s manufacturing facilities will be audited in order to assess Good Manufacturing Practice compliance.
The drug approval process is time-consuming, involves substantial expenditures of resources, and depends upon a number of factors, including the severity of the illness in question, the availability of alternative treatments, and the risks and benefits demonstrated in the clinical trials.
Other Regulations
Various federal, state and local laws, regulations, and recommendations relating to safe working conditions, laboratory practices, the experimental use of animals, the environment and the purchase, storage, movement, import, export, use, and disposal of hazardous or potentially hazardous substances, including radioactive compounds and infectious disease agents, used in connection with our research are applicable to our activities. They include, among others, the U.S. Atomic Energy Act, the Clean Air Act, the Clean Water Act, the Occupational Safety and Health Act, the National Environmental Policy Act, the Toxic Substances Control Act, and Resources Conservation and Recovery Act, national restrictions on technology transfer, import, export, and customs regulations, and other present and possible future local, state, or federal regulation. The compliance with these and other laws, regulations and recommendations can be time-consuming and involve substantial costs. In addition, the extent of governmental regulation which might result from future legislation or administrative action cannot be accurately predicted and may have a material adverse effect on our business, financial condition, results of operations and prospects.
Competition
Competition in General
Competition in the area of biomedical and pharmaceutical research and development is intense and significantly depends on scientific and technological factors. These factors include the availability of patent and other protection for technology and products, the ability to commercialize technological developments and the ability to obtain regulatory approval for testing, manufacturing and marketing. Our competitors include major pharmaceutical, medical products, chemical and specialized biotechnology companies, many of which have financial, technical and marketing resources significantly greater than ours. In addition, many biotechnology companies have formed collaborations with large, established companies to support research, development and commercialization of products that may be competitive with ours. Academic institutions, governmental agencies and other public and private research organizations are also conducting research activities and seeking patent protection and may commercialize products on their own or through joint ventures. We are aware of certain other products manufactured or under development by competitors that are used for the treatment of the diseases and health conditions that we have targeted for product development. We can provide no assurance that developments by others will not render our technology obsolete or noncompetitive, that we will be able to keep pace with new technological developments or that our technology will be able to supplant established products and methodologies in the therapeutic areas that are targeted by us. The foregoing factors could have a material adverse effect on our business, prospects, financial condition and results of operations. These companies, as well as academic institutions, governmental agencies and private research organizations, also compete with us in recruiting and retaining highly qualified scientific personnel and consultants.
Competition within our sector is increasing, so we will encounter competition from existing firms that offer competitive solutions in diabetes treatment solutions. These competitive companies could develop products that are superior to, or have greater market acceptance, than the products being developed by us. We will have to compete against other biotechnology and pharmaceutical companies with greater market recognition and greater financial, marketing and other resources.
Our competition will be determined in part by the potential indications for which our technology is developed and ultimately approved by regulatory authorities. In addition, the first product to reach the market in a therapeutic or preventive area is often at a significant competitive advantage relative to later entrants to the market. Accordingly, the relative speed with which we, or our potential corporate partners, can develop products, complete the clinical trials and approval processes and supply commercial quantities of the products to the market are expected to be important competitive factors. Our competitive position will also depend on our ability to attract and retain qualified scientific and other personnel, develop effective proprietary products, develop and implement production and marketing plans, obtain and maintain patent protection and secure adequate capital resources. We expect our technology, if approved for sale, to compete primarily on the basis of product efficacy, safety, patient convenience, reliability, value and patent position.
Competition for Our Oral Insulin Capsule
We anticipate theanticipated that our oral insulin capsule towould be a competitive diabetes drug because of its anticipated efficacy and safety profile. The followingprofile; however, there are other treatment options for type 1T1D and type 2 diabetic patients:
Several entities who are actively developing oral insulin capsules and/or alternatives to insulin are thoughtor cause the body to be: Diabetology (UK), Biocon Limited (India) and Midatech (UK).
produce more insulin.
Scientific Advisory Board
We maintain a Scientific Advisory Board consisting of internationally recognized scientists who advise us on scientific and technical aspects of our business. The Scientific Advisory Board meets periodically to review specific projects and to assess the value of new technologies and developments to us. In addition, individual members of the Scientific Advisory Board meet with us periodically to provide advice in their particular areas of expertise. The Scientific Advisory Board consists of the following members, information with respect to whom is set forth below: Dr. Roy Eldor, Professor Ele Ferrannini, Dr. Alexander Fleming, Professor Avram Hershko, Dr. Harold Jacob, Dr. Julio Rosenstock, Dr. Jay Skyler and Dr. Harvey L. Katzeff.Anne Peters.
Dr. Roy Eldor,MD, PhD, joined the Oramed Scientific Advisory Board in July 2016. He is an endocrinologist, internist and researcher with over twenty years of clinical and scientific experience. He is currently Director of the Diabetes Unit at the Institute of Endocrinology, Metabolism & Hypertension at the Tel-Aviv Sourasky Medical Center. Prior to that, Dr. Eldor served as Principal Scientist at Merck Research Laboratories, Clinical Research -– Diabetes & Endocrinology, Rahway, New Jersey.Endocrinology. He has previously served as a senior physician in internal medicine at the Diabetes Unit in Hadassah Hebrew University Hospital in Jerusalem, Israel; and the Diabetes Division at the University of Texas Health Science Center in San Antonio, Texas (under the guidance of Dr. R.A. DeFronzo).Texas. Dr. Eldor is a recognized expert, with over 3550 peer reviewed papers and book chapters, and has been a guest speaker at numerous international forums.
Professor Ele Ferrannini,MD, joined the Oramed Scientific Advisory Board in February 2007. He is a past President to the European Association for the Study of Diabetes (EASD), which supports scientists, physicians and students from all over the world who are interested in diabetes and related subjects in Europe and performs functions similar to that of the ADAAmerican Diabetes Association in the United States. Professor Ferrannini has worked with various institutions including the Department of Clinical & Experimental Medicine at the University of Pisa School of Medicine, and CNR (National Research Council) Institute of Clinical Physiology in Pisa, Italy; and the Diabetes Division, Department of Medicine at the University of Texas Health Science Center atin San Antonio, Texas. He has also had extensive training in internal medicine and endocrinology, and has specialized in diabetes studies.trials. Professor Ferrannini has received a Certificate of the Educational Council for Foreign Medical Graduates from the University of Bologna, and with cum laude honors completed a subspecialty in Diabetes and Metabolic Diseases at the University of Torino.Torino, cum laude. He has published over 500 original papers and 50 book chapters and he is a “highly cited researcher,” according to the Institute for Scientific Information.
Dr. Alexander Fleming, MD, joined the Oramed Scientific Advisory Board in December 2019. Dr. Fleming, an endocrinologist, is Founder and Executive Chairman of Kinexum, a strategic advisory firm. From 1986 to 1998, he served at the FDA as a supervisory medical officer in the Division of Metabolism and Endocrine Drug Products and was responsible for landmark approvals of the first statin, metformin, and other endocrine and metabolic therapies. He also represented the FDA at the World Health Organization and on multiple expert working groups of the International Conference on Harmonization (ICH). Dr. Fleming coined the term, Metabesity, which refers to the constellation of major chronic diseases and the aging process itself, all which share common metabolic root causes and potential preventive therapies. He organized the first Congress on Metabesity in London in October 2017, followed by annual conferences. In 2020, Dr. Fleming founded the non-profit Kitalys Institute as a means of producing Metabesity conferences and advancing interventions of any kind that can improve health and healthspan.
Professor Avram Hershko, MD, PhD, joined the Oramed Scientific Advisory Board in July 2008. He earned his MD degree (1965) and PhD degree (1969) from the Hebrew University-Hadassah Medical School of Jerusalem. Professor Hershko served as a physician in the Israel Defense Forces from 1965 to 1967. After a post-doctoral fellowship with Gordon Tomkins at the University of San Francisco (1969-72),from 1969 to 1972, he joined the faculty of the Haifa Technion becoming a professor in 1980. He is now Distinguished Professor in the Unit of Biochemistry in the B. Rappaport Faculty of Medicine of the Technion.Technion in Haifa, Israel. Professor Hershko’s main research interests concern the mechanisms by which cellular proteins are degraded, a formerly neglected field of study. Professor Hershko and his colleagues showed that cellular proteins are degraded by a highly selective proteolytic system. This system tags proteins for destruction by linkage to a protein called ubiquitin, which had previously been identified in many tissues, but whose function was previously unknown. Subsequent work by Professor Hershko and many other laboratories has shown that the ubiquitin system has a vital role in controlling a wide range of cellular processes, such as the regulation of cell division, signal transduction and DNA repair. Professor Hershko was awarded the Nobel Prize in Chemistry (2004)in 2004, jointly with his former PhD student Aaron Ciechanover and their colleague Irwin Rose. His many honors include the Israel Prize for Biochemistry (1994), the Gairdner Award (1999), the Lasker Prize for Basic Medical Research (2000), the Wolf Prize for Medicine (2001) and the Louisa Gross Horwitz Award (2001). Professor Hershko is a member of the Israel Academy of Sciences (2000)since 2000 and a Foreign Associate of the U.S. Academy of Sciences (2003).
since 2003.
Dr. Harold Jacob, MD, joined the Oramed Scientific Advisory Board in November 2016. Since 1998, Dr. Jacob has served as the president of Medical Instrument Development Inc., a company which provides a range of support and consulting services to start-up and early stage companies as well as patenting its own proprietary medical devices. Since 2011, Dr. Jacob has also served as an attending physician at Hadassah University Medical Center in Jerusalem, Israel, where he has served as the director of the gastrointestinal endoscopy unit since September 2013. Dr. Jacob has advised a spectrum of companies in the past and he served as a consultant and then as the Director of Medical Affairs at Given Imaging Ltd., from 1997 to 2003, a company that developed the first swallowable wireless pill camera for inspection of the intestine. He has licensed patents to a number of companies including Kimberly-Clark Corporation. Since 2014, Dr. Jacob has served as the Chief Medical Officer and a director of NanoVibronix, Inc., a medical device company using surface acoustics to prevent catheter acquired infection as well as other applications, where he served as Chief Executive Officer from 2004 to 2014. He practiced clinical gastroenterology in New York and served as Chief of Gastroenterology at St. John’s Episcopal Hospital and South Nassau Communities Hospital from 1986 to 1995, and was a Clinical Assistant Professor of Medicine at SUNY from 1983 to 1990. Dr. Jacob founded and served as Editor in Chief of Endoscopy Review and has authored numerous publications in the field of gastroenterology.
Dr. Harvey L. Katzeff,Julio Rosenstock, MD, joined the Oramed Scientific Advisory Board in November 2016.January 2020. Dr. KatzeffRosenstock is an internationally recognized authority on diabetes with over 30 years’ experiencethe Senior Scientific Advisor and Director of Velocity Clinical Research at Medical City, Dallas, Texas, and a Clinical Professor of Medicine at the University of Texas Southwestern Medical Center in academic medicineDallas, Texas. He is board certified in Internal Medicine, Endocrinology and Metabolism. His clinical and basic research. He currently serves as Senior Directorresearch activities have focused on exploring novel agents and therapeutic strategies to improve glycemic control, particularly early combination therapies in Type 2 Diabetes. Over the last 30 years, he has participated in hundreds of clinical trials and has had an active role in the Cardiovascular, Metabolic, Endocrinologydevelopment of new oral agents, incretin-related therapies and Renalinsulin formulations, often acting as a lead clinical investigator and scientific advisor on the design and reporting of these clinical trials. Dr. Rosenstock has been the author or co-author of 360 peer-reviewed manuscripts (H-index 119) and several hundreds of scientific abstracts. He has also contributed to 13 book chapters on various topics in the field of diabetes and is considered a key opinion leader in Type 2 Diabetes.
Dr. Jay Skyler, MD, MCAP, FRCP, joined the Oramed Scientific Advisory Board in January 2020. Dr. Skyler is Professor of Medicine, Pediatrics and Psychology in the Division of Covance Inc.Endocrinology, Diabetes and Metabolism, Department of Medicine, University of Miami Leonard M. Miller School of Medicine. He previously was Executiveheld the position of Director of the Division of Endocrinology, Diabetes and GlobalMetabolism. In addition, Dr. Skyler is Deputy Director of Clinical Research and Academic Programs at the Diabetes Research Institute, and an Adjunct Professor of Pediatrics at the Barbara Davis Center for Scientific Affairs forChildhood Diabetes at Merck, and former Chiefthe University of Endocrinology and Metabolism at LIJ/North Shore Health System. He wasColorado in Denver. Dr. Skyler’s research focuses on the facultiesclinical aspects of Cornell Medical College and Rockefeller University,diabetes, specifically the conduct of randomized controlled clinical trials. From 1993 to 2015, he was PresidentChairman of the Eastern regionNational Institute of Health (NIDDK)-sponsored Diabetes Prevention Trial–- Type 1 (DPT-1) and its successor Type 1 Diabetes Trial Net, a nationwide and global network conducting clinical trials to prevent T1D.
Dr. Anne Peters, MD, joined the Oramed Scientific Advisory Board in June 2022. Dr. Peters is Professor of Medicine at the Keck School of Medicine of the AmericanUniversity of Southern California (USC) and Director of the USC Clinical Diabetes AssociationPrograms. Dr. Peters earned her medical degree from the Pritzker School of Medicine at the University of Chicago and performed an internal medicine residency at Stanford University and an endocrinology fellowship at Cedars-Sinai Medical Center. She previously directed the clinical diabetes programs at Cedars-Sinai Medical Center and UCLA in California. Her research has received numerous honorsfocused on testing new approaches for diagnosing and treating diabetes and developing systems of care to improve outcomes in diabetic populations. Dr. Peters is the chair of the Endocrine Society Committee on Diabetes Devices and is on the EASD/ADA Technology Safety Committee. Additionally, she is a member of the JDRF Panel on Management of Exercise in type 1 Diabetes and a member of the ABIM Endocrinology Subspecialty Board. Dr. Peters has consulted for many entities, including a National Institutesthe FDA, Optum Rx and CVS/Caremark to help guide the development and use of Health newtreatments for diabetes. In addition to being an investigator award.for more than 40 research studies, Dr. KatzeffPeters has published over 40 original reports, book chapters200 articles, has written four books, and reviews.has given more than 500 lectures locally, nationally, and internationally. She has been on multiple guideline writing committees for the treatment of both type 1 and type 2 diabetes. She was a recipient of the ADA Outstanding Physician Clinician Award, the Bernardo Houssay Award from the National Minority Quality Forum and received a 2021 Endocrine Society Laureate Award for Public Service.
Employees
We believe it is imperative to attract and retain top talent for all positions in the Company. We seek to make Oramed an inclusive, diverse and safe workplace, with meaningful compensation, benefits and wellness programs and opportunities.
We have been successful in retaining experienced personnel involved in our research and development program. In addition, we believe we have successfully recruited theprograms, as well as appropriate clinical/regulatory, quality assurance and other personnel needed to advance through clinical studiestrials or have engaged the services of experts in the field for these requirements. As of AugustDecember 31, 2017,2022, we have contracted with fourteenseventeen individuals for employment or consulting arrangements.arrangements, including employees of Oravax. Of our staff, six are senior management, threefour are engaged in research and development work, and the remaining fiveseven are involved in corporate and administration work.
We provide competitive compensation, health and retirement programs for our employees. We offer variable pay in the form of bonuses and stock-based compensation for eligible employees. We also provide our employees with additional benefits such as team-building and educational offsite activities and gym facilities. We believe that this provides a comprehensive package to engage, motivate and retain our employees as a cohesive unit unified in its goal to achieve the Company’s strategy and objectives.
Additional Information
Additional information about us is contained on our Internet website at www.oramed.com. Information on our website is not incorporated by reference into this report. On our website, under “Investors”, “SEC Filings”, we make available free of charge ourOur Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, are available free of charge on our website under “SEC Filings” as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Our reports filed with the SEC are also made available to read and copy at the SEC's Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. You may obtain information about the Public Reference Room by calling the SEC at 1-800-SEC-0330. Reports filed with the SEC are also made available on its website at www.sec.gov.www.sec.gov and are also available on the website of the Israeli Securities Authority at www.magna.isa.gov.il or on the website of the Tel Aviv Stock Exchange at www.tase.co.il. The following Corporate Governancecorporate governance documents are also posted on our website: Code of Ethics, Whistleblowing Policy and the Charterscharters for each of the Audit Committee, Compensation Committee and Nominating Committee of our Board.
An investment in our securities involves a high degree of risk. You should consider carefully the following information about these risks, together with the other information contained in this Annual Report on Form 10-K before making an investment decision. Our business, prospects, financial condition and results of operations may be materially and adversely affected as a result of any of the following risks. The value of our securities could decline as a result of any of these risks. You could lose all or part of your investment in our securities. Some of the statements in “Item 1A. Risk Factors” are forward-looking statements. The following risk factors are not the only risk factors facing ourthe Company. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our business, prospects, financial condition and results of operations.
Risks Related to Our Business
Our strategic review process may not be successful or timely.
Following the results of the ORA-D-013-1 Phase 3 trial, we have initiated a comprehensive analysis of the data to understand if there is a path forward for our oral insulin candidate. Concurrently, we are examining our existing pipeline and have commenced an evaluation process of potential strategic opportunities, including among others, continuation as a stand-alone business, capital raises, or one or more acquisitions, mergers or business combinations or other strategic transactions. Potential counterparties in a strategic transaction involving us may place minimal or no value on our assets. While we are devoting significant efforts to identify and evaluate potential strategic alternatives, there can be no assurance that this strategic review process will result in us pursuing any transaction or that any transaction, if pursued, will be completed on attractive terms or at all. Additionally, there can be no assurances that any particular course of action, business arrangement or transaction, or series of transactions, will be pursued, successfully consummated, or lead to any stockholder value. Any potential transaction would be dependent on a number of factors that may be beyond our control, including, among other things, market conditions, industry trends, the interest of third parties in a potential transaction with us, obtaining stockholder approval and the availability of financing to third parties in a potential transaction with us on reasonable terms. The process of reviewing alternative strategic paths may be time consuming, may involve the dedication of significant resources and may require us to incur significant costs and expenses. It could negatively impact our ability to attract, retain and motivate employees, and expose us to potential litigation in connection with this process or any resulting transaction. If we are not successful in setting forth a new strategic path for the Company, or if our plans are not executed in a timely fashion, this may cause reputational harm with our stockholders and other stakeholders and the value of our securities may be adversely impacted. In addition, speculation regarding any developments related to the review of strategic alternatives and perceived uncertainties related to the future of the Company could cause our stock price to fluctuate significantly. There can be no guarantee that the process of evaluating alternative strategic paths will result in our entering into or completing potential transactions within the anticipated timing or at all.
If we are successful in completing a strategic transaction, we may be exposed to other operational and financial risks.
Although there can be no assurance that a strategic transaction will result from the process we have undertaken to identify and evaluate strategic alternatives, the negotiation and consummation of any such transaction will require significant time on the part of our management and may disrupt our business. The negotiation and consummation of any such transaction may also require more time or greater cash resources than we anticipate and expose us to other operational and financial risks, including:
| ● | increased near-term and long-term expenditures; |
| ● | exposure to unknown liabilities; |
| ● | higher than expected acquisition or integration costs; |
| ● | incurrence of substantial debt or dilutive issuances of equity securities to fund future operations; |
| ● | write-downs of assets or goodwill or incurrence of non-recurring, impairment or other charges; |
| ● | increased amortization expenses; |
| ● | impairment of relationships with key suppliers of any acquired business due to changes in management and ownership; |
| ● | inability to retain our key employees ; and |
| ● | possibility of future litigation. |
Any of the above risks could have a material adverse effect on our business, financial condition, and prospects.
Our ability to consummate a strategic transaction depends on our ability to retain our employees required to consummate such transaction.
Our ability to consummate a strategic transaction depends upon our ability to retain our employees required to consummate such a transaction, the loss of whose services may adversely impact the ability to consummate such transaction. Our cash conservation activities may yield unintended consequences, such as attrition and reduced employee morale, which may cause remaining employees to seek alternative employment. Our ability to successfully complete a strategic transaction depends in large part on our ability to retain certain of our remaining personnel. If we are unable to successfully retain our remaining personnel, we are at risk of a disruption to our exploration and consummation of a strategic alternative as well as business operations.
We may become involved in securities and stockholder litigation that could divert management’s attention and harm the Company’s business, and insurance coverage may not be sufficient to cover all costs and damages.
In the past, securities and stockholder litigation has often followed certain significant business transactions, such as the sale of a company or announcement of any other strategic transaction, or the announcement of negative events, such as negative results from clinical trials. The market price of our common stock dropped substantially when we announced the results of the ORA-D-013-1 Phase 3 trial. We may be exposed to such litigation even if no wrongdoing occurred. Litigation is usually expensive and diverts management’s attention and resources, which could adversely affect our business and cash resources and our ability to consummate a potential strategic transaction or the ultimate value our stockholders receive in any such transaction.
We continue, and in the future expect, to incur losses.
Successful evaluation and completion of our remaining development programs and our transition to normal operations are dependent upon obtaining necessary regulatory approvals from the FDA prior to selling our products within the United States, and foreign regulatory approvals must be obtained to sell our products internationally. There can be no assurance that we will receive regulatory approval of any of our product candidates, and a substantial amount of time may pass before we achieve a level of revenues adequate to support our operations. We also expect to incur substantial expenditures in connection with our strategic evaluation process, as well as the regulatory approval process for each of our current or future product candidates during their respective developmental periods. Obtaining marketing approval will be directly dependent on our ability to implement the necessary regulatory steps required to obtain marketing approval in the United States and in other countries. We cannot predict the outcome of these activities.
Based on our current cash resources and commitments, we believe we will be able to maintain our current planned development activities and the corresponding level of expenditures for at least the next 12 months, and beyond, although no assurance can be given that we will not need additional funds prior to such time. If there are unexpected increases in our operating expenses, we may need to seek additional financing during the next 12 months.
We will need substantial additional capital in order to satisfy our business objectives.
To date, we have financed our operations principally through offerings of securities and we willmay require substantial additional financing at various intervals in order to implement any potential strategic alternative, to continue our remaining or potential future research and development programs, including significant requirements for operating expenses including intellectual property protection and enforcement, for pursuit of regulatory approvals, and for commercialization of our remaining or future products. We can provide no assurance that additional funding will be available on a timely basis, on terms acceptable to us, or at all. In the event that we are unable to obtain such financing, we may not be able to implement the actions we decide to take as part of our strategic review process, and we will not be able to fully develop and commercialize our technology or pursue new technology. Our future capital requirements will depend upon many factors, including:
| ● | ||
| ● | continued scientific progress in our research and development |
| ● | costs and timing of conducting clinical trials and seeking regulatory approvals and patent |
| ● | competing technological and market |
| ● | our ability to establish additional collaborative |
| ● | effects of commercialization activities and facility expansions if and as required. |
If we cannot secure adequate financing when needed, we may be required to delay, scale back or eliminate one or more of our existing or planned courses of action or research and development programs, or to enter into license or other arrangements with third parties to commercialize products or technologies that we would otherwise seek to develop ourselves and commercialize ourselves. In such event, our business, prospects, financial condition and results of operations may be adversely affected as we may be required to scale-back, eliminate, or delay development efforts or product introductions or enter into royalty, sales or other agreements with third parties in order to commercialize our products.
We have a history of losses and can provide no assurance as to our future operating results.
We do not have sufficient revenues from our research and development activities to fully support our operations. Consequently, we have incurred net losses and negative cash flows since inception. We currently have only licensing revenues and no product revenues, and may not succeed in developing or commercializing any products which could generate product revenues. We do not expect to have any products on the market for several years. In addition, development of our product candidates requires a process of pre-clinical and clinical testing, during which our products could fail. For example, in January 2023, the ORA-D-013-1 Phase 3 trial did not meet its primary and secondary endpoints. We may not be able to enter into agreements with one or more companies experienced in the manufacturing and marketing of therapeutic drugs and, to the extent that we are unable to do so, we will not be able to market our product candidates. Eventual profitability will depend on our success in developing, manufacturing, and marketing our product candidates.candidates or in pursuing a successful strategic alternative. As of AugustDecember 31, 2017, August 31, 20162022 and August 31, 2015,2021, we had working capital of $15,132,000, $27,609,000$151,363,000 and $15,883,000,$140,569,000, respectively, and stockholders’ equity of $19,238,000, $26,190,000$151,812,000 and $24,828,000,$166,453,000, respectively. During the 12year ended December 31, 2022, the four month periodsperiod ended December 31, 2021 and the year ended August 31, 2017, or fiscal 2017, and 2016, or fiscal 2016,2021, we generated revenues of $2,456,000$2,703,000, $904,000 and $641,000,$2,703,000, respectively. No revenues were generated in prior periods. For the period from our inception on April 12, 2002 through AugustDecember 31, 2017, fiscal 2017, fiscal 2016,2022, the year ended December 31, 2022, the four month period ended December 31, 2021 and the year ended August 31, 2015, or fiscal 2015,2021, we incurred net losses of $56,496,000, $10,480,000, $10,964,000$163,081,000, $126,520,000 and $7,232,000,$114,852,000, respectively. We may never achieve profitability and expect to incur net losses in the foreseeable future. See “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
We rely upon patents to protect our technology.
The patent position of biopharmaceutical and biotechnology firms is generally uncertain and involves complex legal and factual questions. We do not know whether any of our current or future patent applications will result in the issuance of any patents. Even issued patents may be challenged, invalidated or circumvented. Patents may not provide a competitive advantage or afford protection against competitors with similar technology. Competitors or potential competitors may have filed applications for, or may have received patents and may obtain additional and proprietary rights to compounds or processes used by or competitive with ours. In addition, laws of certain foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States.
Patent litigation is becoming widespread in the biopharmaceutical and biotechnology industry and we cannot predict how this will affect our efforts to form strategic alliances, conduct clinical testing or manufacture and market any products under development. If challenged, our patents may not be held valid. We could also become involved in interference proceedings in connection with one or more of our patents or patent applications to determine priority of invention. If we become involved in any litigation, interference or other administrative proceedings, we will likely incur substantial expenses and the efforts of our technical and management personnel will be significantly diverted. In addition, an adverse determination could subject us to significant liabilities or require us to seek licenses that may not be available on favorable terms, if at all. We may be restricted or prevented from manufacturing and selling our products in the event of an adverse determination in a judicial or administrative proceeding or if we fail to obtain necessary licenses.
We may be unable to protect our intellectual property rights and we may be liable for infringing the intellectual property rights of others.
Our ability to compete effectively will depend on our ability to maintain the proprietary nature of our technologies. We currently hold several pending patent applications in the United States, Canada, Brazil, Europe, India, Hong Kong, Japan and China for our technologies covering oral administration of insulin and other proteins and oral administration of exenatide and proteins two allowed patents in Europe and Canada and 62112 patents issued by the United States, Swiss, German, French, U.K., Italian, Netherlands, Swedish, Spanish, Australian, Canadian, Chinese, Israeli, Japanese, New Zealand, South African, Russian, European,Canadian, Hong Kong, Swiss, German, Spanish, French, United Kingdom, Italian,Chinese, European and Indian patent offices that cover a part of our technology, which allows for the oral delivery of proteins; patents issued by the Australian, Canadian, European, Austrian, Belgian, French, German, Irish, Italian, Luxembourg, Monaco, Netherlands, Norwegian, Spanish, Swedish, Danish, Luxembourg Swiss, U.K., Monaco, NorwayIsraeli, New Zealand, South African, Russian, Brazilian and DutchJapanese patent offices that cover part of our technology for our technologies coveringthe oral administrationdelivery of insulinexenatide; and other proteins, or for our technologies covering oral administration of exenatide, or for methodspatents issued by the European, Austrian, Belgian, Denmark, French, German, Irish, Italian, Luxembourg, Monaco, Netherlands, Norway, Spanish, Swedish, Swiss, U.K. and compositionsJapanese patent offices for treating diabetes. Further, we intend to rely on a combination of trade secrets and non-disclosure and other contractual agreements and technical measures to protect our rights in our technology. We intend to depend upon confidentiality agreements with our officers, directors, employees, consultants, and subcontractors, as well as collaborative partners, to maintain the proprietary nature of our technology. These measures may not afford us sufficient or complete protection, and others may independently develop technology similar to ours, otherwise avoid our confidentiality agreements, or produce patents that would materially and adversely affect our business, prospects, financial condition and results of operations. We believe that our technology is not subject to any infringement actions based upon the patents of any third parties; however, our technology may in the future be found to infringe upon the rights of others. Others may assert infringement claims against us or against companies to which we have licensed our technology, and if we should be found to infringe upon their patents, or otherwise impermissibly utilize their intellectual property, our ability to continue to use our technology could be materially restricted or prohibited. If this event occurs, we may be required to obtain licenses from the holders of this intellectual property, enter into royalty agreements, or redesign our products so as not to utilize this intellectual property, each of which may prove to be uneconomical or otherwise impossible. Licenses or royalty agreements required in order for us to use this technology may not be available on terms acceptable to us, or at all. These claims could result in litigation, which could materially adversely affect our business, prospects, financial condition and results of operations. Further, we may need to indemnify companies to which we licensed our technology in the event that such technology is found to infringe upon the rights of others.
Our commercial success will also depend significantly on our ability to operate without infringing the patents and other proprietary rights of third parties. Patent applications are, in many cases, maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications are filed. In the event of infringement or violation of another party’s patent, we may be prevented from pursuing product development or commercialization. See “Item 1. Business—Description of Business—Patents Intellectual Property and Licenses.Patents.”
At present, ourOur success dependswas primarily dependent on the successful commercialization of our oral insulin capsule.
The successful commercialization of our principal product, the oral insulin capsule, iswas crucial for our success. At present,On January 12, 2023, we announced top-line results from the phase 3 trial of our principal product is the oral insulin capsule. Our oral insulin capsule, which did not meet its primary or secondary endpoints, and indicated that we expect to discontinue oral insulin clinical activities for T2D. At present, following the results of the ORA-D-013-1 Phase 3 trial, we have initiated a comprehensive analysis of the data to understand if there is a path forward for our oral insulin candidate. Concurrently, we are examining our existing pipeline and have commenced an evaluation process of potential strategic opportunities. Even if our analysis results in a clinical development stage and facespath forward for our oral insulin capsule, there are a variety of risks and uncertainties.uncertainties related to its development. Principally, these risks include the following:
● Future clinical trial results may show that the oral insulin capsule is not well tolerated by recipients at its effective doses or is not efficacious as compared to placebo,
●
| ● | Future clinical trial results may show the same results as the ORA-D-013-1 Phase 3 trial; |
| ● | Future clinical trial results may be inconsistent with previous preliminary testing results and data from our earlier trials may be inconsistent with clinical data; |
| ● | Even if our oral insulin capsule is shown to be safe and effective for its intended purposes in future clinical trials, we may face significant or unforeseen difficulties in obtaining or manufacturing sufficient quantities or at reasonable prices; |
| ● | Our ability to complete the development and commercialization of the oral insulin capsule for our intended use is significantly dependent upon our ability to obtain and maintain experienced and committed partners to assist us with obtaining clinical and regulatory approvals for, and the manufacturing, marketing and distribution of, the oral insulin capsule on a worldwide basis; |
| ● | Even if our oral insulin capsule is successfully developed, commercially produced and receives all necessary regulatory approvals, there is no guarantee that there will be market acceptance of our product; and |
| ● | Our competitors may develop therapeutics or other treatments which are superior or less costly than our own with the result that our products, even if they are successfully developed, manufactured and approved, may not generate significant revenues. |
Our business may be inconsistent with previous preliminary testingseriously harmed if our analysis does not produce positive results, and data from our earlier studies may be inconsistent with clinical data,
● Even if we are unable to find a path forward to continue development of our oral insulin capsule, is shown to be safe and effective for its intended purposes, we may face significant or unforeseen difficulties in obtaining or manufacturing sufficient quantities or at reasonable prices,
● Our ability to complete the development and commercialization of the oral insulin capsule for our intended use is significantly dependent upon our ability to obtain and maintain experienced and committed partners to assist us with obtaining clinical and regulatory approvals for, and the manufacturing, marketing and distribution of, the oral insulin capsule on a worldwide basis,
● Even if our oral insulin capsule is successfully developed, commercially produced and receives all necessary regulatory approvals, there is no guarantee that there will be market acceptance of our product, and
● Our competitors may develop therapeutics or other treatments which are superior or less costly than our own with the result that our products, even if they are successfully developed, manufactured and approved, may not generate significant revenues.
If we are unsuccessful in realizing new strategic opportunities or dealing with any of these risks, or if we are unable to successfully commercialize our oral insulin capsule for some other reason, it would likely seriously harm our business.reason.
We have limited experience in conducting clinical trials.
Clinical trials must meet FDA and foreign regulatory requirements. We have limited experience in designing, conducting and managing the preclinical studiestrials and clinical trials necessary to obtain regulatory approval for our product candidates in any country. We have entered into agreements with Integrium LLC and other consultants to assist us in designing, conducting and managing our various clinical trials in the United States.States, Europe and Israel. Any failure of Integrium LLC or any other consultant to fulfill their obligations could result in significant additional costs as well as delays in designing, consulting and completing clinical trials on our products.
Our clinical trials may encounter delays, suspensions or other problems.
We may encounter problems in clinical trials that may cause us or the FDA or foreign regulatory agencies to delay, suspend or terminate our clinical trials at any phase. These problems could include the possibility that we may not be able to conduct clinical trials at our preferred sites, enroll a sufficient number of patients for our clinical trials at one or more sites or begin or successfully complete clinical trials in a timely fashion, if at all. For example, the rate of enrollment for our Phase 1 clinical trial for our oral COVID-19 vaccine in South Africa was slower than anticipated due to several factors, including the fact that many volunteers did not qualify during screening due to prior asymptomatic COVID-19 infection and other conditions, and as a result we had to add an additional clinical site. Furthermore, we, the FDA or foreign regulatory agencies may suspend clinical trials at any time if we or they believe the subjects participating in the trials are being exposed to unacceptable health risks or if we or they find deficiencies in the clinical trial process or conduct of the investigation. If clinical trials of any of the product candidates fail, we will not be able to market the product candidate which is the subject of the failed clinical trials. The FDA and foreign regulatory agencies could also require additional clinical trials, which would result in increased costs and significant development delays. Our failure to adequately demonstrate the safety and effectiveness of a pharmaceutical product candidate under development could delay or prevent regulatory approval of the product candidate and could have a material adverse effect on our business, prospects, financial condition and results of operations. For example, see “Item 1. Business—Description of Business— Research and Development During 2022” regarding the results of the ORA-D-013-1 Phase 3 trial that did not meet its primary and secondary endpoints. Finally, the COVID-19 pandemic has impacted clinical trials generally. However, we experienced approximately six months of delays in clinical trials due to slow-downs of recruitment for trials generally related to COVID-19. We may experience further delays in site initiation and patient enrollment, failures to comply with study protocols, delays in the manufacture of our product candidates for clinical testing and other difficulties in starting or competing our clinical trials.
Initial success in the completed and ongoing early-stage clinical trials does not ensure success in later stage trials, regulatory approval or commercial viability of a product.
Positive results in a clinical trial may not be replicated in subsequent or confirmatory trials. Additionally, success in preclinical work or early stage clinical trials does not ensure that later stage or larger scale clinical trials will be successful or that regulatory approval will be obtained. Any of our product’s failure to show sufficient efficacy in patients with the targeted indication, or if such studies are discontinued for any other reason, could negatively impact our development and commercialization goals for these products and our stock price could decline. Many companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials. As a result, preliminary and interim data should be viewed with caution until the final data are available. We have invested in clinical studies of medicines that have not met the primary clinical endpoints in their Phase 3 studies or have been discontinued for other reasons. For example, in January 2023, we reported that ORA-D-013-1 trial did not meet its primary or secondary endpoint. Even if later stage clinical trials are successful, regulatory authorities may delay or decline approval of our product candidates.
There are a number of factors that could cause a clinical study to fail or be delayed, including: (i) the clinical study may produce negative or inconclusive results; (ii) regulators may require that we hold, suspend or terminate clinical research for noncompliance with regulatory requirements; (iii) we, our partners, the FDA or foreign regulatory authorities could suspend or terminate a clinical study due to adverse side effects of a product on subjects or lack of efficacy in the trial; (iv) we, or our partners, may decide, or regulators may require us, to conduct additional preclinical testing or clinical studies; (v) change in rates of enrollment and dropout among clinical trial participants; (vi) differences in the size and type of the patient populations; (vii) changes in and adherence to the dosing regimen and other clinical trial protocols; and (viii) people who enroll in the clinical study may later drop out due to adverse events, a perception they are not benefiting from participating in the study, fatigue with the clinical study process or personal or other issues. The occurrence of any of these events could result in significant costs and expense, have an adverse effect on our business, financial condition and results of operations and/or cause our stock price to decline or experience periods of volatility.
Clinical trials of our products conducted by third parties may encounter delays, suspensions or other problems and are outside of our control.
Third parties who conduct clinical trials of our products may encounter problems that may cause delays, suspensions or other problems at any phase. These problems could include the possibility that they may not be able to conduct clinical trials at their preferred sites, enroll a sufficient number of patients for their clinical trials at one or more sites or begin or successfully complete clinical trials in a timely fashion, if at all. For example, the rate of enrollment for our Phase 1 clinical trial for our oral COVID-19 vaccine in South Africa was slower than anticipated due to several factors, including the fact that many volunteers did not qualify during screening due to prior asymptomatic COVID-19 infection and other conditions, and as a result we had to add an additional clinical site. In addition, these third parties are not controlled by us and may conduct these trials in a manner in which we disagree or which may prove to be unsuccessful. Furthermore, domestic or foreign regulatory agencies may suspend clinical trials at any time if they believe the subjects participating in the trials are being exposed to unacceptable health risks or if they find deficiencies in the clinical trial process or conduct of the investigation. If such clinical trials conducted by third parties fail, it could have a material adverse effect on our business, prospects, financial condition and results of operations.
We can provide no assurance that our products will obtain regulatory approval or that the results of clinical studiestrials will be favorable.
The testing, marketing and manufacturing of any of our products will require the approval of the FDA or regulatory agencies of other countries. We have completed certain non-FDA clinical trials and pre-clinical trials for our products. In addition, we have completed a Phase IIb clinical trial in patients with type 2 diabetes under an IND with the FDA and we have completed Phase IIa clinical trials of ORMD-0801 in patients with type 1 diabetes under an IND with the FDA. However, success in pre-clinical testing and early clinical trials does not ensure that later clinical trials will be successful. For example, a number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials.
We cannot predict with any certainty the amount of time necessary to obtain regulatory approvals, including from the FDA or other foreign regulatory authorities, and whether any such approvals will ultimately be granted. In any event, review and approval by the regulatory bodies is anticipated to take a number of years. Preclinical and clinical trials may reveal that one or more of our products are ineffective or unsafe, in which event further development of such products could be seriously delayed or terminated. For example, in January 2023, we announced that our ORA-D-013-1 Phase 3 trial did not meet its primary and secondary endpoints. As a result, we decided to terminate our ORA-D-013-2 Phase 3 trial and have initiated a comprehensive analysis of the data to understand if there is a path forward for our oral insulin candidate. Moreover, obtaining approval for certain products may require the testing on human subjects of substances whose effects on humans are not fully understood or documented. Delays in obtaining necessary regulatory approvals of any proposed product and failure to receive such approvals would have an adverse effect on the product’s potential commercial success and on our business, prospects, financial condition and results of operations. In addition, it is possible that a product may be found to be ineffective or unsafe due to conditions or facts which arise after development has been completed and regulatory approvals have been obtained. In this event we may be required to withdraw such product from the market. See “Item 1. Business—Description of Business—Government Regulation.”
We are dependent upon third party suppliers of our raw materials.materials and for other services.
We are dependent on outside vendors for our entire supply of the oral insulin and GLP-1 capsules and do not currently have any long-term agreements in place for the supply of oral insulin or GLP-1 capsules.capsules, which is still necessary if we decide to continue development of these projects. While we believe that there are numerous sources of supply available, if the third party suppliers were to cease production, or otherwise fail to supply us with quality raw materials in sufficient quantities on a timely basis and we were unable to contract on acceptable terms for these services with alternative suppliers, our ability to produce our products and to conduct testing and clinical trials would be materially adversely affected.
OurWe rely on suppliers, vendors, outsourcing partners, alliance partners and other third parties to research, develop, manufacture, commercialize, co-promote and sell our products, manage certain marketing, IT, data and other business unit and functional services and meet their contractual, regulatory and other obligations. Using these third parties poses a number of risks, such as: (i) they may not perform to our standards or legal requirements, for example, in relation to the outsourcing of significant clinical development activities for innovative medicines to some CROs; (ii) they may not produce reliable products; (iii) they may not perform in a timely manner; (iv) they may not maintain confidentiality of our proprietary information; (v) they may incur a significant cyberattack or business disruption; (vi) they may be subject to government orders or mandates that require them to give priority to the government and set aside pre-existing commercial orders; (vii) disputes may arise with respect to ownership of rights to technology developed with our partners; and (viii) disagreements could cause delays in, or termination of, the research, development or commercialization of the product or result in litigation or arbitration. The failure of any critical third party to meet its obligations; to adequately deploy business continuity plans in the event of a crisis; and/or to satisfactorily resolve significant disagreements with us or address other factors, could have a material adverse impact on our operations and results. In addition, if these third parties violate, or are alleged to have violated, any laws or regulations, including the local pharmaceutical code, the U.S. Foreign Corrupt Practice Act of 1977, the U.K. Bribery Act of 2010, the EU’s General Data Protection Regulations, and other similar laws and regulations, during the performance of their obligations for us, we could suffer financial and reputational harm or other negative outcomes, including possible legal consequences.
Any future revenues from HTIT are dependent upon third party suppliers and Chinese regulatory approvals.
OurAny future revenues from HTIT are dependent upon the achievement of certain milestones and conditions, and the success of HTIT to implement our technology and to manufacture the oral insulin capsule. OurAny future revenues from HTIT are also dependent upon the ability of third parties to scale-up one of our oral capsule ingredients and to scale-up the manufacturing process of our capsules. Our future revenues from royalties from HTIT are further dependent upon the granting of regulatory approvals in the Territory. Accordingly, if any of the foregoing does not occur, we may not be successful in receiving future revenues from HTIT and may not succeed with our business plans in China.
If we do not resolve our dispute with HTIT favorably, we may need to reverse deferred revenue of up to $2 million and may not receive an additional $4 million in royalties.
On August 21, 2020, we received a letter from HTIT, disputing certain pending payment obligations of HTIT under the TLA. We estimate this obligation to be between $2 million and $6 million. While we wholly dispute said claims and have been engaged in discussions and exchanges with HTIT in an attempt to clarify and resolve disagreements between the parties regarding milestone payments and work plan implementation, we may be subsequently required to repay to HTIT up to $2 million, which has been received and has been included in our deferred revenue in each of the consolidated balance sheets for the years ended December 31, 2022 and 2021. In addition, we may not receive an additional $4 million in Royalties if HTIT is entitled to the full disputed amount of $6 million.
We may not realize a return on the ordinary shares of DNA and Entera that we own.
DNA’s ordinary shares are traded on the Tel Aviv Stock Exchange and Entera’s ordinary shares are traded on the Nasdaq Stock Market, both of which are subject to market fluctuations, and may, at times, have a price below the value on the date we acquired such shares. In addition, the ordinary shares of DNA and Entera have historically experienced low trading volume. As a result, there is no guarantee that we will be able to resell the ordinary shares of DNA or Entera at the prevailing market prices or that we will realize a positive return on such shares.
We may not realize the full benefit from our distribution license agreement with Medicox.
Our distribution license agreement with Medicox provides that Medicox will comply with agreed distribution targets and will purchase ORMD-0801 at an agreed upon transfer price per capsule and pay us up to $15 million in developmental milestones, $2 million of which have already been received by us. Following the results of the ORA-D-013-1 Phase 3 trial, we are currently evaluating with Medicox a path forward to continue our collaboration. If we are not successful in finding a mutually agreed way to continue our collaboration, or if Medicox is not successful in independently advancing the oral insulin candidate, we may not realize the benefits from this collaboration.
We are highly dependent upon our ability to enter into agreements with collaborative partners to develop, commercialize and market our products.
Our long-term strategy is to ultimately seek a strategic commercial partner, or partners, such as large pharmaceutical companies, with extensive experience in the development, commercialization, and marketing of insulin applications and/or other orally digestible drugs. We anticipate such partner or partners would be responsible for, or substantially support, late stage clinical trials (Phase III) and sales and marketing of our oral insulin capsule and other products. Such planned strategic partnership, or partnerships, may provide a marketing and sales infrastructure for our products as well as financial and operational support for global clinical trials, post marketing studies,trials, label expansions and other regulatory requirements concerning future clinical development in the United States and elsewhere.
While our strategy is to partner with an appropriate party, no assurance can be given that any third party would be interested in partnering with us. We currently lack the resources to manufacture any of our product candidates on a large scale and we have no sales, marketing or distribution capabilities. In the event we are not able to enter into a collaborative agreement with a partner, or partners, on commercially reasonable terms, or at all, we may be unable to commercialize our products, which would have a material adverse effect upon our business, prospects, financial condition and results of operations.
The biotechnology and biopharmaceutical industries are characterized by rapid technological developments and a high degree of competition. We may be unable to compete with more substantial enterprises.
The biotechnology and biopharmaceutical industries are characterized by rapid technological developments and a high degree of competition.As a result, our products could become obsolete before we recoup any portion of our related research and development and commercialization expenses. These industries are highly competitive, and this competition comes both from biotechnology firms and from major pharmaceutical and chemical companies. Many of these companies have substantially greater financial, marketing and human resources than we do (including, in some cases, substantially greater experience in clinical testing, manufacturing and marketing of pharmaceutical products). We also experience competition in the development of our products from universities and other research institutions and compete with others in acquiring technology from such universities and institutions. We face the risk that new market entrants and existing competition may try to replicate our business model or introduce a more innovative offering that renders our services less competitive or obsolete. In addition, certain of our research and development efforts may target diseases and conditions for which there are existing therapies or therapies that are being developed by our competitors. Further, any products mayresulting from our research and development efforts might not be subjectable to competition from products developed using other technologies.compete successfully with others’ existing and future products. See “Item 1. Business—Description of Business—Competition.”
Our financial position or results could be negatively affected by product liability claims.
It is possible that we will be responsible for potential product liability stemming from product research, development or manufacturing and may face an even greater risk if any product candidate that we develop is commercialized. If we cannot successfully defend ourselves against claims that products we develop independently or with our partners caused injuries, we could incur substantial liabilities. Regardless of the merit or eventual outcome of such claims, any liability claims may result in, among other things, decreased demand for any product that we may develop, loss of revenues, significant time and costs to defend the related litigation, initiation of investigations by regulators and injury to our reputation and significant negative media attention. On occasion, large judgments have been awarded in class action lawsuits based on drugs or medical treatments that had unanticipated adverse effects. Our clinical trials are covered by liability insurance, but notwithstanding such coverage, our financial position or results could be negatively affected by product liability claims.
We have limited senior management resources and may be required to obtain more resources to manage our growth.
We expect the expansion of our business, as well as the activities we take as a result of our strategic review process, to place a significant strain on our limited managerial, operational and financial resources. We will be required to expand our operational and financial systems significantly and to expand, train and manage our work force in order to manage the expansion of our operations. Our failure to fully integrate our new employees into our operations could have a material adverse effect on our business, prospects, financial condition and results of operations. Our ability to attract and retain highly skilled personnel is critical to our operations and expansion. We face competition for these types of personnel from other technology companies and more established organizations, many of which have significantly larger operations and greater financial, technical, human and other resources than we have. We may not be successful in attracting and retaining qualified personnel on a timely basis, on competitive terms or at all. If we are not successful in attracting and retaining these personnel, our business, prospects, financial condition and results of operations will be materially adversely affected. See “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Item 1. Business—Description of Business—Strategy” and “—Employees.”
We depend upon our senior management and skilled personnel and their loss or unavailability could put us at a competitive disadvantage.
We currently depend upon the efforts and abilities of our senior executives, as well as the services of several key consultants and other key personnel, including Dr. Miriam Kidron, our Chief Scientific Officer. The loss or unavailability of the services of any of these individuals for any significant period of time could have a material adverse effect on our business, prospects, financial condition and results of operations. We do not maintain “key man” life insurance policies for any of our senior executives. In addition, recruiting and retaining qualified scientific personnel to perform future research and development work will be critical to our success. There is currently a shortage of employees with expertise in developing, manufacturing and commercialization of products and related clinical and regulatory affairs, and this shortage is likely to continue. Competition for skilled personnel is intense and turnover rates are high. Our ability to attract and retain qualified personnel may be limited. Our inability to attract and retain qualified skilled personnel would have a material adverse effect on our business, prospects, financial condition and results of operations.
Our existing and any future joint ventures may limit our flexibility with jointly owned investments and we may not realize the benefits we expect from these arrangements.
We are currently party to a joint venture, and we may in the future sell or contribute additional assets or acquire, develop or recapitalize assets to or in this joint venture or other joint ventures that we may enter.
Our participation in our existing joint venture is subject to risks, including the following:
| ● | We share approval rights over certain major decisions affecting the ownership or operation of the joint venture and any assets owned by the joint venture; |
| ● | We may need to contribute additional capital in order to preserve, maintain or grow the joint venture and its investments; |
| ● | Our joint venture investors may have economic or other business interests or goals that are inconsistent with our business interests or goals and that could affect our ability to fully benefit from the assets owned by the joint venture; |
| ● | Our joint venture investors may be subject to different laws or regulations than us, which could create conflicts of interest; |
| ● | Our joint venture has license and other agreements with other investors, which we are not party to and have no control over; |
| ● | Our ability to sell our interest in, or sell additional assets to, the joint venture or the joint venture’s ability to sell additional interests of, or assets owned by, the joint venture when we so desire are subject to the approval rights of the other joint venture investors under the terms of the agreements governing the joint venture; and |
| ● | Disagreements with our joint venture investors could result in litigation or arbitration that could be expensive and distracting to management and could delay important decisions. |
Any of the foregoing risks could have a material adverse effect on our business, financial condition and results of operations. Further, these, similar, enhanced or additional risks, including possible risks of the other joint venture investors having licensed assets to the joint venture, may apply to any future additional or amended joint ventures that we may enter into.
Healthcare policy changes, including pending legislation recently adopted and further proposals still pending to reform the U.S. healthcare system, may harm our future business.
Healthcare costs have risen significantly over the past decade. There have been and continue to be proposals by legislators, regulators and third-party payors to keep these costs down. Certain proposals, if passed, would impose limitations on the prices we will be able to charge for the products that we are developing, or the amounts of reimbursement available for these products from governmental agencies or third-party payors. These limitations could in turn reduce the amount of revenues that we will be able to generate in the future from sales of our products and licenses of our technology.
In 2010, the federal government enacted healthcare reform legislation that has significantly impacted the pharmaceutical industry. In addition to requiring most individuals to have health insurance and establishing new regulations on health plans, this legislation requires discounts under the Medicare drug benefit program and increased rebates on drugs covered by Medicaid. In addition, the legislation imposes an annual fee, which has increased annually, on sales by branded pharmaceutical manufacturers. There can be no assurance that our business will not be materially adversely affected by these increased rebates, fees and other provisions. In addition, these and other initiatives in the United States may continue the pressure on drug pricing, especially under the Medicare and Medicaid programs, and may also increase regulatory burdens and operating costs. The announcement or adoption of any such initiative could have an adverse effect on potential revenues from any product that we may successfully develop. An expansion in government’s role in the U.S. healthcare industry may lower the future revenues for the products we are developing and adversely affect our future business, possibly materially.
In September 2017, members of the U.S. Congress introduced legislation with the announced intention to repeal and replace major provisions of the Patient Protection and Affordable Care Act, or the ACA. AlthoughIn addition to those efforts, on October 12, 2017, an executive order was issued that modified certain aspects of the ACA. Following several years of litigation in the federal courts, in June 2021, the U.S. Supreme Court upheld the ACA when it is unclear whether such legislation will ultimately become law,dismissed a legal challenge to the ACA’s constitutionality. Further attempts to repeal or to repeal and replace the ACA will likelymay continue. In addition, various other healthcare reform proposals have also emerged at the federal and state level. We cannot predict what healthcare initiatives, if any, will be implemented at the federal or state level, or the effect any future legislation or regulation will have on us.
We are exposed to fluctuations in currency exchange rates.
A considerable amount of our expenses are generated in dollars or in dollar-linked currencies, but a significant portion of our expenses such as some clinical studiestrials and payroll costs are generated in other currencies such as NIS Euro and British pounds.Euro. Most of the time, our non-dollar assets are not totally offset by non-dollar liabilities. Due to the foregoing and to the fact that our financial results are measured in dollars, our results could be adversely affected as a result of a strengthening or weakening of the dollar compared to these other currencies. During the fiscal years ended AugustDecember 31, 2013, 20142017, 2019, 2020 and 2017,2021, the dollar depreciated in relation to the NIS, which raised the dollar cost of our Israeli based operations and adversely affected our financial results, while during fiscals 2015the year ended December 31, 2018 and 2016,2022, the dollar increased in relation to the NIS, which reduced the dollar cost of our Israeli based operations costs. In addition, our results could also be adversely affected if we are unable to guard against currency fluctuations in the future. Although we may in the future decide to undertake foreign exchange hedging transactions to cover a portion of our foreign currency exchange exposure, we currently do not hedge our exposure to foreign currency exchange risks. These transactions, however, may not adequately protect us from future currency fluctuations and, even if they do protect us, may involve operational or financing costs we would not otherwise incur.
The COVID-19 pandemic, or any other pandemic, epidemic or outbreak of an infectious disease, may materially and adversely affect our clinical trial operations, our business and operations.
The spread of COVID-19 may result in the inability of our suppliers to deliver supplies to us on a timely basis. In addition, health professionals may reduce staffing and reduce or postpone meetings with clients in response to the spread of an infectious disease. Though we have not yet experienced such events, if they would occur, they could result in a period of business disruption, and in reduced operations, any of which could materially affect our business, financial condition and results of operations.
However, we experienced approximately six months of delays in clinical trials due to slow-downs of recruitment for trials generally. Although we do not expect any material impact on our development timeline and our liquidity due to COVID-19, the ongoing development of the COVID-19 pandemic globally could adversely impact our clinical trial operations in the United States, Israel and in Europe, including our ability to recruit and retain patients and principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19 if an outbreak occurs in their geography or due to government or institutional quarantines or stay-at-home measures. We continue to assess the effect on our operations by monitoring the status of COVID-19.
We face uncertainties related to Oravax’s oral COVID-19 vaccine.
We face uncertainties related to Oravax’s oral COVID-19 vaccine, including uncertainties related to the risk that our continued development programs may not be successful, commercially viable or receive approval from regulatory authorities. Other companies may produce superior or competitive oral or other products that make Oravax’s oral COVID-19 vaccine not commercially worthwhile. Even if we succeed in developing the product, the demand for any product we may develop may no longer exist, given the fluid nature of the COVID-19 pandemic, including possible decreased demand for vaccines due to weaker strains, the need for different vaccines for new variants of the virus or an end of the pandemic that may render Oravax’s vaccine obsolete.
Risks Related to our Common Stock
Future sales of our common stock by our existing stockholders could adversely affect our stock price.
The market price of our common stock could decline as a result of sales of a large number of shares of our common stock in the market, or the perception that these sales could occur. We experienced a significant decline in the market price of our common stock and a significant increase in trading volume after announcing the results of our ORA-D-013-1 Phase 3 trial in January 2023. Any strategic decision we make as a result of our strategic review process may also negatively affect our common stock price or cause volatility in the market price of our common stock. Sales of large amounts of our securities or large variations in trading volume might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate. As of March 6, 2023, we had outstanding 39,783,813 shares of common stock, a large majority of which are freely tradable. Giving effect to the exercise in full of all of our outstanding warrants, options and restricted stock units, or RSUs, including those currently unexercisable or unvested, we would have outstanding 43,386,638 shares of common stock.
Our issuance of warrants, options and RSUs to investors, employees and consultants may have a negative effect on the trading prices of our common stock as well as a dilutive effect.
We have issued and may continue to issue warrants, options, RSUs and convertible notes at, above or below the current market price. As of March 6, 2023, we had outstanding warrants and options exercisable for 1,548,256 shares of common stock at a weighted average exercise price of $4.71. We also had outstanding RSUs exercisable for 265,302 shares of common stock at a total exercise price of $900. In addition to the dilutive effect of a large number of shares of common stock and a low exercise price for the warrants and options, there is a potential that a large number of underlying shares of common stock may be sold in the open market at any given time, which could place downward pressure on the trading of our common stock.
Because we will not pay cash dividends in the foreseeable future, investors may have to sell shares of our common stock in order to realize their investment.
We have not paid any cash dividends on our common stock and do not intend to pay cash dividends in the foreseeable future. We intend to retain future earnings, if any, for reinvestment in the development and expansion of our business. Any credit agreements which we may enter into with institutional lenders or otherwise may restrict our ability to pay dividends. Whether we pay cash dividends in the future will be at the discretion of our Board and will be dependent upon our financial condition, results of operations, capital requirements and any other factors that our Board decides is relevant.
Our failure to maintain compliance with the Nasdaq Capital Market’s continued listing requirements could result in the delisting of our common stock.
Our common stock is currently listed on The Nasdaq Capital Market. In order to maintain this listing, we must satisfy minimum financial and other requirements. Nasdaq Listing Rule 5550(a)(2) requires the minimum bid price of our common stock on the Nasdaq Capital Market to remain above $1.00. If the bid price of our common stock closes below $1.00 per share for 30 consecutive business days, we would be in violation of Nasdaq Listing Rule 5550(a)(2). In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we would have 180 calendar days to regain compliance with the minimum bid requirement to achieve compliance with the minimum bid price requirement.
While we intend to engage in efforts to maintain compliance, and thus maintain our listing, there can be no assurance that we will continue to meet all applicable Nasdaq Capital Market requirements in the future, especially in light of any strategic transaction we may choose to undertake. If our common stock were removed from listing with the Nasdaq Capital Market, it may be subject to the so-called “penny stock” rules. The SEC has adopted regulations that define a “penny stock” to be any equity security that has a market price per share of less than $5.00, subject to certain exceptions, such as any securities listed on a national securities exchange, which is the exception on which we currently rely. For any transaction involving a “penny stock,” unless exempt, the rules impose additional sales practice requirements on broker-dealers, subject to certain exceptions. If our common stock were delisted and determined to be a “penny stock,” a broker-dealer may find it more difficult to trade our common stock and an investor may find it more difficult to acquire or dispose of our common stock on the secondary market.
If our common stock is delisted and there is no longer an active trading market for our shares, it may, among other things:
| ● | cause stockholders difficulty in selling our shares without depressing the market price for the shares or selling our shares at all; |
| ● | substantially impair our ability to raise additional funds; |
| ● | result in a loss of institutional investor interest and fewer financing opportunities for us; and/or |
| ● | result in costly litigation, significant liabilities and diversion of our management’s time and attention and could have a material adverse effect on our financial condition, business and results of operations. |
A delisting would also reduce the value of our equity compensation plans, which could negatively impact our ability to retain employees.
As the market price of our common stock may fluctuate significantly, this may make it difficult for you to sell your shares of common stock when you want or at prices you find attractive.
The price of our common stock is currently listed on The Nasdaq Capital Market or Nasdaq, and on the Tel Aviv Stock Exchange and constantly changes. In recent years, the stock market in general has experienced extreme price and volume fluctuations. We expect that the market price of our common stock will continue to fluctuate. These fluctuations may result from a variety of factors, many of which are beyond our control. For example, we experienced a significant decline in the market price of our common stock after announcing the results of our ORA-D-013-1 Phase 3 trial in January 2023. These factors include:
| ● |
| ● | clinical trial results and the timing of the release of such |
| ● | the amount of cash resources and our ability to obtain additional |
| ● | announcements of research activities, business developments, technological innovations or new products by us or our |
| ● | entering into or terminating strategic |
| ● | changes in government |
| ● | departure of key |
| ● | disputes concerning patents or proprietary |
| ● | changes in expense |
| ● | future sales of our equity or equity-related |
| ● | public concern regarding the safety, efficacy or other aspects of the products or methodologies being |
| ● | activities of various interest groups or |
| ● | media coverage; and |
| ● | status of the investment markets. |
Future sales of common stock or the issuance of securities senior to our common stock or convertible into, or exchangeable or exercisable for, our common stock could materially adversely affect the trading price of our common stock, and our ability to raise funds in new equity offerings.
Future sales of substantial amounts of our common stock, including pursuant to any strategic opportunity, the Cantor Equity Distribution Agreement (as defined below), or other equity-related securities in the public market or privately, or the perception that such sales could occur, could adversely affect prevailing trading prices of our common stock and could impair our ability to raise capital through future offerings of equity or other equity-related securities. We anticipate that we will need to raise capital through offerings of equity and equity related securities. We can make no prediction as to the effect, if any, that future sales of shares of our common stock or equity-related securities, or the availability of shares of common stock for future sale, will have on the trading price of our common stock.
Our stockholders may experience significant dilution as a result of any additional financing using our equity securities.
To the extent that we raise additional funds by issuing equity securities, including in connection with any strategic opportunity or pursuant to the Cantor Equity Distribution Agreement, our stockholders may experience significant dilution.
Our management will have significant flexibility Additionally, we may, from time to time or in using the net proceeds of any offering of securities.
We intend generally to use the net proceeds from any offerings of our securities for expenses related to our clinical trials, research and product development activities, and for general corporate purposes, including general working capital purposes. Our management will have significant flexibility in applying the net proceeds of any such offering. The actual amounts and timing of expenditures will vary significantly depending onconnection with a number of factors, including the amount of cash used in our operations and our research and development efforts. Management’s failure to use these funds effectively would have an adverse effect on the value of our common stock and could make it more difficult and costly to raise funds in the future.
Future sales of our common stock by our existing stockholders could adversely affect our stock price.
The market price of our common stock could decline as a result of sales of a large number of shares of our common stock in the market, or the perception that these sales could occur. These sales also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate. As of November 28, 2017, we had outstanding 14,306,100 shares of common stock, a large majority of which are freely tradable. Giving effect to the exercise in full of all of our outstanding warrants, options and restricted stock units, or RSUs, including those currently unexercisable or unvested, we would have outstanding 15,722,651 shares of common stock.
Our issuance of warrants, options and RSUs to investors, employees and consultants may have a negative effect on the trading prices of our common stock as well as a dilutive effect.
We have issued and may continue tostrategic alternative, issue warrants, options, RSUs and convertible notes at, above or below the current market price. As of November 28, 2017, we had outstanding warrants and options exercisable for 1,221,855additional shares of common stock at a weighted average exercisediscount from the current trading price of $7.11. We also had outstanding RSUs exercisable for 164,636 shares of common stock at a total exercise price of $900. In addition to the dilutive effect of a large number of shares of common stock and a low exercise price for the warrants and options, there is a potential that a large number of underlying shares of common stock may be sold in the open market at any given time, which could place downward pressure on the trading of our common stock.
Delaware law could discourage As a change in control, or an acquisitionresult, our stockholders would experience immediate dilution upon the purchase of us by a third party, even if the acquisition would be favorable to you, and thereby adversely affect existing stockholders.
The Delaware General Corporation Law contains provisions that may have the effect of making more difficult or delaying attempts by others to obtain control of our Company, even when these attempts may be in the best interests of stockholders. Delaware law imposes conditions on certain business combination transactions with “interested stockholders.” These provisions and others that could be adopted in the future could deter unsolicited takeovers or delay or prevent changes in our control or management, including transactions in which stockholders might otherwise receive a premium for their shares of common stock over then current market prices. These provisions may also limit the ability of stockholders to approve transactions that they may deem to be in their best interests.
Because we will not pay cash dividends, investors may have to sellany shares of our common stock in order to realize their investment.
We have not paid any cash dividends on our common stock and do not intend to pay cash dividends in the foreseeable future. We intend to retain future earnings, if any, for reinvestment in the development and expansion of our business. Any credit agreements whichsold at such discount. In addition, as opportunities present themselves, we may enter into with institutional lendersfinancing or otherwise may restrict our ability to pay dividends. Whether we pay cash dividendssimilar arrangements in the future, will be atincluding the discretionissuance of our Board and will be dependent upon our financial condition, results of operations, capital requirements, and any other factors that our Board decides is relevant. See “Item 5. Market Price for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.”
Because certain of our stockholders control a significant number of shares ofconvertible debt securities, preferred stock or common stock. If we issue common stock or securities convertible into common stock, our common stock, they may have effective control over actions requiring stockholder approval.
��
As of November 28, 2017, our directors, executive officersstockholders would experience additional dilution and, principal affiliated stockholders beneficially own approximately 33.6% of our outstanding shares of common stock, excluding shares issuable upon the exercise of options, warrants and RSUs. Asas a result, these stockholders, should they act together,our stock price may have the ability to control the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. In addition, these stockholders, should they act together, may have the ability to control our management and affairs. Accordingly, this concentration of ownership might harm the market price of our common stock by:
decline.
Risks Related to Conducting Business in Israel
We are affected by the political, economic and military risks of locating our principalhaving operations in Israel.
OurWe have operations are located in the State of Israel, and we are directly affected by political, economic and security conditions in that country. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its Arab neighbors and a state of hostility, varying in degree and intensity, has led to security and economic problems for Israel. In addition, acts of terrorism, armed conflicts or political instability in the region could negatively affect local business conditions and harm our results of operations. We cannot predict the effect on the region of any diplomatic initiatives or political developments involving Israel or the Palestinians or other countries and territories in the Middle East. Recent political events, including political uprisings, social unrest and regime change, in various countries in the Middle East and North Africa have weakened the stability of those countries and territories, which could result in extremists coming to power. In addition, Iran has threatened to attack Israel and is widely believed to be developing nuclear weapons. Iran is also believed to have a strong influence among extremist groups in the region, such as Hamas in Gaza and Hezbollah in Lebanon. This situation has escalated in the past and may potentially escalate in the future to violent events which may affect Israel and us. Our business, prospects, financial condition and results of operations could be materially adversely affected if major hostilities involving Israel should occur or if trade between Israel and its current trading partners is interrupted or curtailed.
All adult male permanent residents of Israel, unless exempt, may be required to perform military reserve duty annually. Additionally, all such residents are subject to being called to active duty at any time under emergency circumstances. Some of our officers, directors and employees currently are or in the future may be obligated to perform annual military reserve duty. We can provide no assurance that such requirements will not have a material adverse effect on our business, prospects, financial condition and results of operations in the future, particularly if emergency circumstances occur.
Because we received grants from the Israel Innovation Authority of the Israeli Ministry of Economy & Industry we are subject to ongoing restrictions.
We received royalty-bearing grants from the Israel Innovation Authority or IIA, of the Israeli Ministry of Economy & Industry, Trade and Labor,or IIA, for research and development programs that meet specified criteria. We did not recognize any grants in fiscals 2017the year ended December 31, 2022, the four month period ended December 31,2021 and 2016, and recognized a grant in the amount of $49,000 in fiscal 2015.year ended August 31, 2021. We do not expect to receive further grants from the IIA in the future. The terms of the IIA grants limit our ability to transfer know-how developed under an approved research and development program outside of Israel, regardless of whether the royalties were fully paid.
It may be difficult to enforce a U.S. judgment against us or our officers and directors and to assert U.S. securities laws claims in Israel.
Almost all of our directors and officers are nationals and/or residents of countries other than the United States. As a result, service of process upon us, our Israeli subsidiary and our directors and officers, may be difficult to obtain within the United States. Furthermore, because the majority of our assets and investments, and most of our directors and officers are located outside the United States, it may be difficult for investors to enforce within the United States any judgments obtained against us or any such officers or directors. Additionally, it may be difficult to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on a violation of U.S. securities laws because Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to such claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law.
Subject to specified time limitations and legal procedures, under the rules of private international law currently prevailing in Israel, Israeli courts may enforce a U.S. judgment in a civil matter, including a judgment based upon the civil liability provisions of the U.S. securities laws, as well as a monetary or compensatory judgment in a non-civil matter, provided that the following key conditions are met:
| ● | subject to limited exceptions, the judgment is final and non-appealable; |
| ● | the judgment was given by a court competent under the laws of the state in which the court is located and is otherwise enforceable in such state; |
| ● | the judgment was rendered by a court competent under the rules of private international law applicable in Israel; |
| ● | the laws of the state in which the judgment was given provides for the enforcement of judgments of Israeli courts; |
| ● | adequate service of process has been effected and the defendant has had a reasonable opportunity to present |
| ● | the judgment and its enforcement are not contrary to the law, public policy, security or sovereignty of the State of Israel; |
| ● | the judgment was not obtained by fraud and does not conflict with any other valid judgment in the same matter between the same parties; and |
| ● | an action between the same parties in the same matter was not pending in any Israeli court at the time the lawsuit was instituted in the U.S. court. |
If any of these conditions are not met, Israeli courts will likely not enforce the applicable U.S. judgment.
General Risk Factors
Changes to tax laws could have a negative effect on us or our stockholders.
At any time, the U.S. federal or state income tax laws, or the administrative interpretations of those laws, may be amended. Federal and state tax laws are constantly under review by persons involved in the legislative process, the U.S. Internal Revenue Service, the U.S. Department of the Treasury and state taxing authorities. Changes to the tax laws, regulations and administrative interpretations, which may have retroactive application, could adversely affect us. Our stockholders are encouraged to consult with their tax advisors about the potential effects that changes in law may have on them and their ownership of our securities.
Our business and operations would suffer in the event of computer system failures, cyber-attacks or deficiencies in our cyber-security.
Despite the implementation of security measures, our internal computer systems, and those of third parties on which we rely, are vulnerable to damage from computer viruses, malware, natural disasters, terrorism, war, telecommunication and electrical failures, cyber-attacks or cyber-intrusions over the Internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our product development programs. For example, the loss of clinical trial data from completed or ongoing or planned clinical trials could result in delays in our clinical trial efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach was to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur material legal claims and liability, and damage to our reputation, and the further development of our product candidates could be delayed.
We also maintain compliance programs to address the potential applicability of restrictions against trading while in possession of material, nonpublic information generally and in connection with a cyber-security breach. However, a breakdown in existing controls and procedures around our cyber-security environment may prevent us from detecting, reporting or responding to cyber incidents in a timely manner and could have a material adverse effect on our financial position and value of our stock.
Our management will have significant flexibility in using the net proceeds of any offering of securities.
We intend generally to use the net proceeds from any offerings of our securities for expenses related to our clinical trials, research and product development activities, and for general corporate purposes, including general working capital purposes. Our management will have significant flexibility in applying the net proceeds of any such offering and we will necessarily be using our capital when we decide on new strategic initiatives. The actual amounts and timing of expenditures will vary significantly depending on a number of factors, including the amount of cash used in our operations and our research and development efforts. Management’s failure to use these funds effectively would have an adverse effect on the value of our common stock and could make it more difficult and costly to raise funds in the future.
Delaware law could discourage a change in control, or an acquisition of us by a third party, even if the acquisition would be favorable to you, and thereby adversely affect existing stockholders.
The Delaware General Corporation Law contains provisions that may have the effect of making more difficult or delaying attempts by others to obtain control of the Company, even when these attempts may be in the best interests of stockholders. Delaware law imposes conditions on certain business combination transactions with “interested stockholders.” These provisions and others that could be adopted in the future could deter unsolicited takeovers or delay or prevent changes in our control or management, including transactions in which stockholders might otherwise receive a premium for their shares of common stock over then current market prices. These provisions may also limit the ability of stockholders to approve transactions that they may deem to be in their best interests.
ITEM 1B.UNRESOLVED STAFF COMMENTS.
Not applicable.
Our principal executive offices are comprised of approximately 168 square meters of leased office space in Givat-Ram, Jerusalem, Israel. The current lease term is from October 1, 2016 until September 30, 2021. The aggregate annual base rent for this space is currently $33,000, linked to the increase in the Israeli consumer price index, and will be increased to $37,000 in October 2018. We believe that our existing facilities are suitable and adequate to meet our current business requirements. In the event that we should require additional or alternative facilities, we believe that such facilities can be obtained on short notice at competitive rates.
As security for our obligations under the lease agreement, we provided a bank guarantee in an amount equal to three monthly lease payments, valid until December 31, 2021.
From time to time we may become subject to litigation incidental to our business. We are not currently a party to any material legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES.
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market Price for our Common Stock
Our common stock is traded on the Nasdaq Capital Market and on the Tel Aviv Stock Exchange, in each case under the symbol “ORMP.” The quarterly high and low sales price on Nasdaq for the periods indicated are as follows:
| High | Low | |||||||
| Year Ended August 31, 2016 | ||||||||
| Three Months Ended November 30, 2015 | $ | 10.74 | $ | 5.40 | ||||
| Three Months Ended February 29, 2016 | $ | 9.95 | $ | 5.60 | ||||
| Three Months Ended May 31, 2016 | $ | 10.51 | $ | 6.06 | ||||
| Three Months Ended August 31, 2016 | $ | 8.82 | $ | 7.10 | ||||
| Year Ended August 31, 2017 | ||||||||
| Three Months Ended November 30, 2016 | $ | 8.01 | $ | 5.70 | ||||
| Three Months Ended February 28, 2017 | $ | 6.97 | $ | 5.82 | ||||
| Three Months Ended May 31, 2017 | $ | 8.94 | $ | 5.85 | ||||
| Three Months Ended August 31, 2017 | $ | 9.17 | $ | 7.08 | ||||
Holders
The last reported sale price per share of common stock as quoted on Nasdaq was $9.46 on November 28, 2017.
Holders
As of November 28, 2017,March 6, 2023, there were 14,306,10039,783,813 shares of our common stock issued and outstanding held of record by approximately 5034 registered stockholders. We believe that a significant number of stockholders hold their shares of our common stock in brokerage accounts and registered in the name of stock depositories and are therefore not included in the number of stockholders of record.
Dividend Policy
We have never paid any cash dividends on our capital stock and do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain future earnings to fund ongoing operations and future capital requirements of our business. Any future determination to pay cash dividends will be at the discretion of our Board and will be dependent upon our financial condition, results of operations, capital requirements and such other factors as our Board deems relevant.
Unregistered Sales of Equity Securities and Use of Proceeds
On August 1, 2017,During the three months ended December 31, 2022, we issued 2,500an aggregate amount of 3,000 unregistered shares of our common stock, valued at $20,000, in the aggregate, to Corporate Profile, LLC, or Corporate Profile, in payment of a portion of the consulting fee for investor relations services owed to Corporate Profile pursuant to a Letter Agreements, dated May 3, 2017, between us and Corporate Profile.
On August 8, 2017, we issued 5,631 shares of our common stock to an investor resulting from his exercisea service provider, as part of warrants purchased in connection with our 2012 private placementthe compensation for a total exercise price of $33,786.
These issuances and sales were exemptservices provided, pursuant to the exemption under Section 4(a)(2) of the Securities ActAct. 1,500 unregistered shares of 1933, as amended.
common stock were issued to the service provider on each of October 15, 2022 and December 15, 2022.
Comparative Stock Performance Graph
The following graph shows how an initial investment of $100 in our common stock would have compared to an equal investment in the Nasdaq Composite Index and the Nasdaq Biotechnology Index during the period from September 1, 2012 through August 31, 2017. The performance shown is not necessarily indicative of future price performance.
ITEM 6. SELECTED FINANCIAL DATA.[RESERVED]
The selected data presented below under the captions “Statements of Comprehensive Loss Data” and “Balance Sheet Data” for, and as of the end of, each of the fiscal years in the five-year period ended August 31, 2017, are derived from, and should be read in conjunction with, our audited consolidated financial statements.
The selected information contained in this table should also be read in conjunction with “Management's Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and related notes thereto included elsewhere in this Annual Report on Form 10-K. The selected consolidated statements of comprehensive loss data for fiscals 2017, 2016 and 2015 and the selected consolidated balance sheet data as of August 31, 2017 and 2016, are derived from the audited consolidated financial statements included elsewhere in this Annual Report. The statement of operations data for the years ended August 31, 2014 and 2013 and the balance sheet data as of August 31, 2015, 2014 and 2013 are derived from audited financial statements not included in this Annual Report. The historical results presented below are not necessarily indicative of future results.
| 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||||
| (in thousands of dollars except share and per share data) | ||||||||||||||||||||
| Statements of Comprehensive Loss: | ||||||||||||||||||||
| Revenues | $ | 2,456 | $ | 641 | $ | - | $ | - | $ | - | ||||||||||
| Cost of revenues | 187 | 490 | - | - | - | |||||||||||||||
| Research and development expenses, net | 10,281 | 7,709 | 4,781 | 3,277 | 2,272 | |||||||||||||||
| General and administrative expenses | 2,759 | 2,452 | 2,602 | 2,629 | 2,032 | |||||||||||||||
| Financial income | 792 | 474 | 168 | 225 | 180 | |||||||||||||||
| Financial expenses | 101 | 93 | 18 | 11 | 313 | |||||||||||||||
| Loss before taxes on income | 10,080 | 9,629 | 7,233 | 5,692 | 4,437 | |||||||||||||||
| Taxes on income (Tax benefit) | 400 | 1,335 | (1 | ) | 4 | (205 | ) | |||||||||||||
| Net loss for the year | $ | 10,480 | $ | 10,964 | $ | 7,232 | $ | 5,696 | $ | 4,232 | ||||||||||
| Loss per common share – basic and diluted | $ | 0.79 | $ | 0.87 | $ | 0.67 | $ | 0.62 | $ | 0.59 | ||||||||||
| Weighted average common shares outstanding | 13,296,633 | 12,624,356 | 10,820,465 | 9,244,059 | 7,209,283 | |||||||||||||||
| As of August 31, | ||||||||||||||||||||
| 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||||
| in thousands of dollars except share and per share data | ||||||||||||||||||||
| Balance Sheet Data: | ||||||||||||||||||||
| Cash, cash equivalents, short-term deposits, restricted cash and marketable securities | $ | 20,138 | $ | 31,032 | $ | 17,245 | $ | 21,306 | $ | 8,491 | ||||||||||
| Other current assets | 159 | 198 | 127 | 472 | 153 | |||||||||||||||
| Long-term deposits and other assets | 16,264 | 11,070 | 8,042 | 24 | 16 | |||||||||||||||
| Long-term marketable securities | 2,151 | 530 | 940 | - | - | |||||||||||||||
| Total assets | 38,712 | 42,830 | 26,354 | 21,802 | 8,660 | |||||||||||||||
| Current liabilities | 5,165 | 3,621 | 1,489 | 973 | 498 | |||||||||||||||
| Long-term liabilities | 14,309 | 13,019 | 37 | 36 | 31 | |||||||||||||||
| Stockholders’ equity | 19,238 | 26,190 | 24,828 | 20,793 | 8,131 | |||||||||||||||
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the audited consolidated financial statements and the related notes included elsewhere herein and in our audited consolidated financial statements.
In addition to our audited consolidated financial statements, the following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this Annual Report on Form 10-K, particularly in “Cautionary Statement Regarding Forward-Looking Statements” and “Item 1A. Risk Factors.”
Overview of Operations
We are currently a pharmaceutical company currently engaged in the research and development of innovative pharmaceutical solutions includingwith a technology platform that allows for the oral delivery of therapeutic proteins.
Through our research and development efforts, we have developed an orally ingestibleoral dosage form intended to withstand the harsh environment of the stomach and effectively deliver active biological insulin capsuleor other proteins. The excipients in the formulation are not intended to modify the proteins chemically or biologically, and the dosage form is designed to be used for the treatment of individuals with diabetes, and the use of orally ingestible capsules or pills for delivery of other polypeptides.
Oral Insulin:safe to ingest. We are seekingplan to revolutionize the treatment of diabetes through our proprietary flagship product, ORMD-0801, an orally ingestible insulin capsule. We completed a Phase IIb clinical trial in patients with type 2 diabetes under an IND with the FDA, following which we conducted a Phase IIa, dose finding clinical trialcontinue to better define the optimal dosing of ORMD-0801 moving forward. We also completed Phase IIaconduct clinical trials in patients with both type 1 and type 2 diabetes. During a call withto show the FDA regarding ORMD-0801,effectiveness of our technology.
On January 11, 2023, we were advisedannounced that the regulatory pathway for submissionORA-D-013-1 Phase 3 trial did not meet its primary and secondary endpoints. As a result, we have initiated a comprehensive analysis of ORMD-0801 would bethe data to understand if there is a BLA, and we plan to initiate a three-month trial in patients with type 2 diabetes to evaluate the effect of ORMD-0801 on HbA1c, the main FDA registrational endpoint.
GLP-1 Analog: Our second pipeline product, ORMD-0901, is an orally ingestible exenatide (GLP-1 analog) capsule, which aids in the balance of blood-sugar levels and decreases appetite. In January 2013, we began a clinical trialpath forward for our oral exenatide capsule on healthy volunteers and type 2 diabetic patients. Based on this study,insulin candidate. Concurrently, we decided to make slight adjustments in the manufacturing of these capsulesare examining our existing pipeline and have begun pre-clinical studiescommenced an evaluation process of potential strategic opportunities, with the goal of enhancing value for our stockholders.
Impact of COVID-19
We do not expect any material impact on the new capsules. In September 2013,our development timeline and our liquidity due to COVID-19. However, we submitted a pre-IND package to the FDA for ORMD-0901, our oral exenatide capsule. We completed during the second quarterexperienced approximately six months of calendar year 2016 a Phase Ib trial outside of the United States, which began in August 2015. We also completed a pre-clinical toxicology study in March 2017, anticipate receiving the final report during the fourth quarter of calendar year 2017 and expect to file an IND and move directly into a pharmacokinetics study followed by a large Phase II trial in the United States under an FDA IND.
Combination of Oral Insulin and GLP-1 Analog: Our third pipeline product is a combination of our two primary products, oral insulin and oral exenatide. In the near term, we are focusing our efforts on the development of the Company’s flagship products, oral insulin and oral exenatide. Once these two products have progressed furtherdelays in clinical trials we intenddue to slow-downs of recruitment for trials generally. On the other hand, Oravax continues to develop its oral vaccine, the demand for which may be reduced if COVID-19 continues to abate. We continue to assess the effect on running further studies withour operations by monitoring the oral combination therapy.status of COVID-19.
Results of Operations
Critical accounting policies
Our significant accounting policies are more fully described in the notes to our accompanying consolidated financial statements. We believeThe table and discussion that the accounting policies below are critical for one to fully understand and evaluate our financial condition and results of operations.
The discussion and analysisfollows includes a comparison of our financial condition and results of operations is based on our consolidated financial statements, which we prepared in accordance with U.S. generally accepted accounting principles, or GAAP. The preparation of our consolidated financial statements requires us to make estimates and assumptions that affectliquidity and capital resources for the reported amounts of assets and liabilitiesyear ended December 31, 2022 and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, we evaluate such estimates and judgments. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Valuation of options and warrants: We grant options to purchase shares of our common stock to employees and consultants and issue warrants in connection with some of our financings and to certain other consultants.
We account for share-based payments to employees and directors in accordance with the guidance that requires awards classified as equity awards to be accounted for using the grant-date fair value method. The fair value of share-based payment transactions is based on the Black Scholes option-pricing model or Monte Carlo model when appropriate, and is recognized as an expense over the requisite service period.
We elected to recognize compensation cost for awards to employees and directors that have a graded vesting schedule using the accelerated method based on the multiple-option award approach.
When stock options are granted as consideration for services provided by consultants and other non-employees, the transaction is accounted for based on the fair value of the consideration received or the fair value of the stock options issued, whichever is more reliably measurable. The fair value of the options granted is measured on each reporting date,year ended December 31, 2021 and the gains (losses) are recorded to earnings over the related service period using the straight-line method.
Revenue recognition: Revenue is recognized when delivery has occurred, evidence of an arrangement exists, title and risks and rewards for the products are transferred to the customer and collection is reasonably assured.
Given our continuing involvement through the expected product submission (June 2023), revenue from the License Agreement is recognized over the periods from which the Company is entitled to the respective payments (including milestones), and through the expected product submission date.
Comparison of Fiscal 2017 to Fiscal 2016 and Fiscal 2016 to Fiscal 2015
The following table summarizes certain statements of operations data for us for the twelvefour month periods ended December 31, 2021 and 2020. For a comparison of our results of operations and financial condition for the fiscal years ended August 31, 2017, 20162021 and 2015:
2020, see “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in our Annual Report on Form 10-K for the fiscal year ended August 31, 2021, filed with the SEC on November 24, 2021. For information regarding the change in the Company’s fiscal year from the period beginning on September 1 and ending on August 31 to the period beginning on January 1 and ending on December 31, see note 1 to our audited consolidated financial statements.
| Year ended August 31, | ||||||||||||
| Operating Data: | 2017 | 2016 | 2015 | |||||||||
| (dollar amounts in thousands) | ||||||||||||
| Revenues | $ | 2,456 | $ | 641 | $ | - | ||||||
| Cost of revenues | 187 | 490 | - | |||||||||
| Research and development expenses, net | 10,281 | 7,709 | 4,781 | |||||||||
| General and administrative expenses | 2,759 | 2,452 | 2,602 | |||||||||
| Financial income, net | 691 | 381 | 150 | |||||||||
| Loss before taxes on income | 10,080 | 9,629 | 7,233 | |||||||||
| Taxes on income (Tax benefit) | 400 | 1,335 | (1 | ) | ||||||||
| Net loss for the year | 10,480 | 10,964 | 7,232 | |||||||||
| Loss per common share – basic and diluted | $ | 0.79 | $ | 0.87 | $ | 0.67 | ||||||
| Weighted average common shares outstanding | 13,296,633 | 12,624,356 | 10,820,465 | |||||||||
| Year ended December 31, | Four months ended December 31, | Four months ended December 31, | Year ended August 31, | |||||||||||||||||
| 2022 | 2021 | 2021 | 2020 | 2021 | ||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||
| (dollar amounts in thousands, except share and per share data) | ||||||||||||||||||||
| Revenues | $ | 2,703 | $ | 2,703 | $ | 904 | $ | 904 | $ | 2,703 | ||||||||||
| Cost of revenues | - | - | - | - | - | |||||||||||||||
| Research and development expenses | 27,639 | 23,203 | 9,037 | 6,889 | 20,989 | |||||||||||||||
| Sales and marketing expenses | 1,851 | 898 | 898 | - | - | |||||||||||||||
| General and administrative expenses | 13,811 | 7,591 | 3,295 | 1,576 | 5,937 | |||||||||||||||
| Financial income (expense), net | 2,934 | 1,068 | 71 | 237 | 1,234 | |||||||||||||||
| Loss before taxes on income | 37,664 | 27,921 | 12,255 | 7,324 | 22,989 | |||||||||||||||
| Taxes on income | 100 | - | - | - | - | |||||||||||||||
| Net loss for the period | $ | 37,764 | $ | 27,921 | $ | 12,255 | $ | 7,324 | $ | 22,989 | ||||||||||
| Net loss attributable to Company’s stockholders | 36,561 | 26,583 | 11,668 | 7,324 | 22,238 | |||||||||||||||
| Net loss attributable to non-controlling interest | 1,203 | 1,338 | 587 | - | 751 | |||||||||||||||
| Net loss for the period | $ | 37,764 | $ | 27,921 | $ | 12,255 | $ | 7,324 | $ | 22,989 | ||||||||||
| Basic and diluted loss per share of common stock | $ | 0.94 | $ | 0.81 | $ | 0.31 | $ | 0.30 | $ | 0.78 | ||||||||||
| Weighted average shares of common stock outstanding used in computing basic and diluted loss per share of common stock | 38,997,649 | 32,641,288 | 37,113,137 | 24,394,010 | 28,469,068 | |||||||||||||||
Revenues
Revenues consist of proceeds related to the HTIT License Agreement that are recognized overon a cumulative basis when it is probable that a significant reversal in the termamount of cumulative revenue recognized will not occur, through the License Agreement throughexpected product submission date by HTIT of June 2023.2023, using the input method.
Revenues for fiscal 2017 increased by 283% to $2,456,000 from $641,000the years ended December 31, 2022 and 2021 were both $2,703.
Revenues for fiscal 2016. The increase is attributed to additional milestone payments received in connection with the License Agreement. No revenuesfour month periods ended December 31, 2021 and 2020 were recorded for fiscal 2015.both $904.
Cost of revenuesRevenues
Cost of revenues consists of royalties related to the HTIT License Agreement with HTIT that will be paid over the term of the HTIT License Agreement in accordance with the revenue recognition accounting and the Law for the Encouragement of Industrial Research, Development and Technological Innovation, 1984, as amended, including any regulations or tracks promulgated thereunder, or the R&D Law.
CostThere was no cost of revenues for fiscal 2017 decreased by 62% to $187,000 from $490,000 for fiscal 2016. The decrease reflects a decrease in the total proceeds related toyears ended December 31, 2022 and 2021 and the License Agreement received during the year. No cost of revenues was recorded for fiscal 2015.
four month periods ended December 31, 2021 and 2020.
Research and development expensesDevelopment Expenses
Research and development expenses include costs directly attributable to the conduct of research and development programs, including the cost of salaries, employee benefits, costs of materials, supplies, the cost of services provided by outside contractors, including services related to our clinical trials, clinical trial expenses, the full cost of manufacturing drugdrugs for use in research and preclinical development. All costs associated with research and development are expensed as incurred.
Clinical trial costs are a significant component of research and development expenses and include costs associated with third-party contractors. We outsource a substantial portion of our clinical trial activities, utilizing external entities such as CROs, independent clinical investigators and other third-party service providers to assist us with the execution of our clinical studies.trials.
Clinical activities which relate principally to clinical sites and other administrative functions to manage our clinical trials are performed primarily by CROs. CROs typically perform most of the start-up activities for our trials, including document preparation, site identification, screening and preparation, pre-study visits, training and program management.
Clinical trial and pre-clinical trial expenses include regulatory and scientific consultants’ compensation and fees, research expenses, purchase of materials, cost of manufacturing of the oral insulin and exenatide capsules, payments for patient recruitment and treatment, as well as salaries and related expenses of research and development staff.
From August 2009 to March 2014, Oramed Ltd. was awarded five government grants amounting to a total net amount of NIS 8 million (approximately $2,194,000) from the IIA. We used the funds to support further research and development and clinical studies of our oral insulin capsule and oral GLP-1 analog during the period from February 2009 to December 2014. The five grants are subject to repayment according to the terms determined by the IIA and applicable law. See “—Government grants” below.
Research and development expenses net, for fiscal 2017the year ended December 31, 2022 increased by 33%19% to $10,281,000 from $7,709,000$27,639,000, compared to $23,203,000 for fiscal 2016.the year ended December 31, 2021. The increase iswas mainly attributeddue to an increase in expenses related to our Phase 3 clinical trials and to stock-based compensation expenses. Stock-based compensation expenses for the year ended December 31, 2022, were $3,176,000, compared to $1,598,000 for the year ended December 31, 2021. The increase was mainly due to new grants in 2022.
Research and development expenses for the four month period ended December 31, 2021 increased by 31% to $9,037,000, compared to $6,889,000 for the four month period ended December 31, 2020. The increase was mainly due to an increase in expenses related to our Phase 3 and NASH clinical trials in addition to expenses related to in process research and development and production of our capsules andcosts related to Oravax. Stock-based compensation expenses for the required ingredients, progress in toxicology studies andfour month period ended December 31, 2021, were $649,000, compared to $171,000 for the four month period ended December 31, 2020. The increase in stock-based compensation costs, partially offset by a decrease in clinical trialswas mainly due to completionequity awards granted to a consultant and to new grants awarded in 2021.
Following the results of the ORA-D-013-1 Phase 3 trial, which did not meet its primary and secondary endpoints, we terminated both ORA-D-013-1 and ORA-D-013-2 Phase 3 clinical trials. In parallel, we have initiated a comprehensive analysis of the data to understand if there is a path forward for our Phase IIb clinical trial. During fiscal 2017, stock-based compensation costs totaled $1,134,000, as compared to $304,000 during fiscal 2016.oral insulin candidate. We are examining our existing pipeline and have commenced an evaluation process of potential strategic opportunities, with the goal of enhancing value for our stockholders.
Research and development expenses, net, for fiscal 2016 increased by 61% to $7,709,000 from $4,781,000 for fiscal 2015. The increase is attributed to expenses related to clinical trials and mainly our Phase IIb clinical trial. This increase was partially offset by a decrease in stock based compensation costs. During fiscal 2016, stock based compensation costs totaled $304,000, as compared to $616,000 during fiscal 2015.
Government grantsGrants
The Government of Israel encourages research and development projects through the IIA, pursuant to the R&D Law. Under the R&D Law, a research and development plan that meets specified criteria is generally eligible for a grant of up to 50% of certain approved research and development expenditures. Each plan must be approved by the IIA.
From August 2009 to March 2014, our subsidiary Oramed Ltd. was awarded five government grants amounting to a total net amount of NIS 8 million (approximately $2,194,000 during such period) from the IIA. We used these funds to support further research and development and clinical trials of our oral insulin capsule and oral GLP-1 analog candidate during the period from February 2009 to December 2014. The five grants are subject to repayment according to the terms determined by the IIA and applicable law.
In fiscals 2017the years ended December 31, 2022 and 2016,2021, the four month periods ended December 31, 2021 and 2020 and the year ended August 31, 2021, we did not recognize any research and development grants and in fiscal 2015, we recognized a research and development grant in an amount of $49,000.grants. As of AugustDecember 31, 2017,2022, we had incurred a liabilityliabilities to pay royalties to the IIAIsrael Innovation Authority of $533,000.the Israeli Ministry of Economy and Industry of $133,000.
Under the terms of the grants we received from the IIA, we are obligated to pay royalties of 3.5%3% on all revenues derived from the sale of the products developed pursuant to the funded plans, including revenues from licensed ancillary services. Royalties are generally payable up to a maximum amount equaling 100% of the grants received (dollar linked) with the addition of interest at an annual rate based on the LIBOR rate.
The R&D Law generally requires that a product developed under a program be manufactured in Israel. However, when applying for a grant, the applicant may declare that part of the manufacturing will be performed outside of Israel or by non-Israeli residents and if the IIA is convinced that performing some of the manufacturing abroad is essential for the execution of the program, it may still approve the grant. This declaration will be a significant factor in the determination of the IIA as to whether to approve a program and the amount and other terms of the benefits to be granted. If a company wants to increase the volume of manufacturing outside of Israel after the grant has been approved, it may transfer up to 10% of the company’s approved Israeli manufacturing volume, measured on an aggregate basis, outside of Israel after first notifying the IIA thereof (provided that the IIA does not object to such transfer within 30 days). In addition, upon the approval of the IIA, a portion greater than 10% of the manufacturing volume may be performed outside of Israel. In any case of transfer of manufacturing out of Israel, the grant recipient is required to pay royalties at an increased rate, which may be substantial, and the aggregate repayment amount is increased up to 120%, 150% or 300% of the grant, depending on the portion of the total manufacturing volume that is performed outside of Israel. The approval we received from the IIA for the License Agreement was subject to payment of increased royalties and an increased ceiling, all in accordance with the provisions of the R&D Law. The R&D Law further permits the IIA, among other things, to approve the transfer of manufacturing rights outside of Israel in exchange for the import of different manufacturing into Israel as a substitute, in lieu of the increased royalties.
The R&D Law also provides that know-how developed under an approved research and development program may not be transferred or licensed to third parties in Israel without the approval of the research committee. Such approval is not required for the sale or export of any products resulting from such research or development. The R&D Law further provides that the know-how developed under an approved research and development program may not be transferred or licensed to any third parties outside Israel absent IIA approval which may be granted in certain circumstances as follows: (a) the grant recipient pays to the IIA a portion of the sale or license price paid in consideration for the purchase or license of such IIA-funded know-how or the price paid in consideration for the sale of the grant recipient itself, as the case may be, in accordance with certain formulas included in the R&D Law; (b) the grant recipient receives know-how from a third party in exchange for its IIA-funded know-how; or (c) such transfer of IIA-funded know-how is made in the context of IIA approved research and development cooperation projects or consortia.
The R&D Law imposes reporting requirements with respect to certain changes in the ownership of a grant recipient. The R&D Law requires the grant recipient to notify the IIA of any change in control of the recipient or a change in the holdings of the means of control of the recipient that results in a non-Israeli entity becoming an interested party in the recipient, and requires the new non-Israeli interested party to undertake to the IIA to comply with the R&D Law. In addition, the rules of the IIA may require the provision of additional information or representations in respect of certain such events. For this purpose, “control” is defined as the ability to direct the activities of a company other than any ability arising solely from serving as an officer or director of the company. A person is presumed to have control if such person holds 50% or more of the means of control of a company. “Means of control” refers to voting rights or the right to appoint directors or the chief executive officer. An “interested party” of a company includes a holder of 5% or more of its outstanding share capital or voting rights, its chief executive officer and directors, someone who has the right to appoint its chief executive officer or at least one director, and a company with respect to which any of the foregoing interested parties holds 25% or more of the outstanding share capital or voting rights or has the right to appoint 25% or more of the directors.
Failure to meet the R&D Law’s requirements may subject us to mandatory repayment of grants received by us (together with interest and penalties), as well as expose us to criminal proceedings. In addition, the Israeli government may from time to time audit sales of products which it claims incorporate technology funded through IIA programs which may lead to additional royalties being payable on additional products.
Grants from Bio-JerusalemSales and Marketing Expenses
Sales and marketing expenses include the salaries and related expenses of our commercial functions, consulting costs and other general costs.
The Bio-Jerusalem fund was founded by the Jerusalem Development Authority in order to support the biomed industry in Jerusalem. We are committed to pay royalties to the Bio-Jerusalem fund on proceeds from future sales at a rate of 4%Sales and up to 100% ofmarketing expenses for the amount of the grants received by the Company (Israeli CPI linked) in the total aggregate amount of $65,000. We received no grants from the Bio-Jerusalem fund since the fiscal year ended AugustDecember 31, 2013. As of August2022 increased by 106% to $1,851,000, compared to $898,000 for the year ended December 31, 2017, we incurred a liability2021. The increase was mainly due to pay royaltiesstock-based compensation expenses, salary related expenses and consulting expenses, mainly resulting from hiring our Chief Commercial Officer. Stock-based compensation expenses for the year ended December 31, 2022 were $1,172,000, compared to $579,000 for the Bio-Jerusalem fund of $47,000.year ended December 31, 2021. The increase was mainly due to equity awards granted to an employee during 2022.
Sales and marketing expenses for the four month period ended December 31, 2021 were $898,000, compared to no expenses for the four month period ended December 31, 2020. The increase was mainly due to stock-based compensation expenses, salary related expenses and consulting expenses. Stock-based compensation costs for the four month period ended December 31, 2021 were $579,000, compared to no stock-based compensation expenses during the four month period ended December 31, 2020. The increase was mainly due to equity awards granted to an employee during 2021.
General and administrative expensesAdministrative Expenses
General and administrative expenses include the salaries and related expenses of our management, consulting costs, legal and professional fees, traveling,travel expenses, business development costs, insurance expenses and other general costs.
General and administrative expenses for the year ended December 31, 2022 increased by 12.5% from $2,452,00082% to $13,811,000, compared to $7,591,000 for fiscal 2016 to $2,759,000 for fiscal 2017.the year ended December 31, 2021. The increase was mainly due to higher stock-based compensation costs, an increase in costs incurred relatedlegal expenses and higher salary expenses due to generalthe recruitment of new employees in the year ended December 31, 2022, partially offset by lower bonuses in the year ended December 31, 2022. Stock-based compensation expenses for the year ended December 31, 2022 were $7,160,000, compared to $2,368,000 for the year ended December 31, 2021. The increase was mainly due to equity awards granted to employees during 2022.
General and administrative activities during fiscal 2017, reflectsexpenses for the four month period ended December 31, 2021 increased by 109% to $3,295,000, compared to $1,576,000 for the four month period ended December 31, 2020. The increase was mainly due to an increase in stock-based compensation costsexpenses and salariesprofessional fees as well as public relations and consultinginvestor relations expenses. During fiscal 2017, as part of our general and administrative expenses, we incurred $440,000 related to stock-basedStock-based compensation costs asfor the four month period ended December 31, 2021 were $1,034,000, compared to $329,000$242,000 during fiscal 2016.
General and administrative expenses decreased by 5.8% from $2,602,000 for fiscal 2015the four month period ended December 31, 2020. The increase was mainly due to $2,452,000 for fiscal 2016. The decrease in costs incurred related to general and administrative activities during fiscal 2016, reflects a decrease in stock-based compensation costs that was partially offset by an increase in salaries and consulting expenses resulting from cash bonusesequity awards granted to employees during the four month period ended December 31, 2021 and consultants paid in 2016. During fiscal 2016, as part of our general and administrative expenses, we incurred $329,000 related to stock-based compensation costs, as compared to $731,000new award grants during fiscal 2015.2021.
Financial income, netIncome (Expense), Net
Net financial income was $691,000$2,934,000 for fiscal 2017 asthe year ended December 31, 2022, compared to net financial income of $381,000$1,068,000 for fiscal 2016.the year ended December 31, 2021. The increase is mainly due to an increase in incomeinterest from short and long-term bank deposits, and held to maturity bonds as a resultpartially offset by loss from revaluation of the proceeds related to the License Agreementshares we hold in Entera and due to an increase in yield rates on investments.DNA.
Net financial income was $381,000$71,000 for fiscal 2016 asthe four month period ended December 31, 2021, compared to net financial income of $150,000$237,000 for fiscal 2015. The increase is mainly due to an increase in income from bank deposits and held to maturity bonds as a result of the increase in cash and investment balances.
Taxes on income / Tax benefit
We had taxes on income of $400,000 for fiscal 2017 as compared to $1,335,000 for fiscal 2016.four month period ended December 31, 2020. The decrease is mainly due to a decrease in withholding tax deducted from proceeds received related to the License Agreement, that resulted from a decrease in such proceeds. The Company estimates that withholding tax will not be utilized in the next five years, and therefore was deducted.
We had taxes on income of $1,335,000 for fiscal 2016 as compared to a tax benefit of $1,000 for fiscal 2015. The increase is due to withholding tax of $1,350,000 deducted from revenues received from the License Agreement, since according to the Company’s estimations, the withholding tax is not expected to be utilized in the next five years. This deduction is partially offset by a decrease in the accrual for an uncertain tax position in fiscal 2016.
Other comprehensive income
Unrealized gain on available for sale securities for fiscal 2017 of $295,000 resulted from the increase in fair value of our D.N.Athe ordinary shares.shares of Entera.
UnrealizedBasic and Diluted Loss Per Share of Common Stock
Basic and diluted loss on availableper share of common stock for sale securitiesthe year ended December 31, 2022 increased by 16% to $0.94, compared to $0.81 for fiscal 2016the year ended December 31, 2021. The increase in loss was mainly due to the higher net loss in the year ended December 31, 2022 compared to the year ended December 31, 2021.
Basic and diluted loss per share of $452,000 resulted fromcommon stock for the decreasefour month period ended December 31, 2021 increased by 3% to $0.31, compared to $0.30 for the four month period ended December 31, 2020. The increase in fair valueloss per share was due to a higher net loss and a higher number of our D.N.A ordinary shares.weighted average shares of common stock in the four month period ended December 31, 2021 compared to the four month period ended December 31, 2020.
Weighted Average Shares of Common Stock Outstanding
Weighted average shares of common stock outstanding for the year ended December 31, 2022 were 38,997,649, compared to 32,641,288 for the year ended December 31, 2021. The increase was mainly due to shares issued in connection with our controlled equity offering and registered direct offering.
Weighted average shares of common stock outstanding for the four month period ended December 31, 2021 were 37,113,137, compared to 24,394,010 for the four month period ended December 31, 2020. The increase was mainly due to shares issued in connection with our controlled equity offering and registered direct offering.
Liquidity and Capital Resources
From our inception through AugustDecember 31, 2017,2022, we have incurred losses in an aggregate amount of $56,496,000.$163,081,000. During that period and through December 31, 2022, we have financed our operations through several private placements of our common stock, as well as public offerings of our common stock, raising a total of $56,079,000,$252,946,000, net of transaction costs. During that period, we also received cash consideration of $4,880,000$28,001,000 from the exercise of warrants and options. We willexpect to seek to obtain additional financing through similar sources in the future, as needed. As of AugustDecember 31, 2017,2022, we had $3,969,000$40,464,000 of available cash, $29,525,000$111,513,000 of short term and long termshort-term bank deposits, and investment and $5,011,000$3,743,000 of marketable securities.securities and $2,700,000 of long-term investments.
From inception through December 31, 2022, we have not generated significant revenues from our operations. Management continues to evaluate various financing alternatives for funding new strategic activities, future research and development activities and general and administrative expenses through fundraising in the public or private equity markets. Although there is no assurance that we will be successful with those initiatives, management believes that it will be able to secure the necessary financing as a result of future third party investments. Based on our current cash resources and commitments, we believe we will be able to maintain our current planned development activities and the corresponding level of expenditures for at least the next 12 months, and beyond.although no assurance can be given that we will not need additional funds prior to such time.
If there are increases in our operating expenses, we may need to seek additional financing during the next 12 months. Successful completion of our development programs and our transition to normal operations is dependent upon obtaining necessary regulatory approvals from the FDA prior to selling our products within the United States, obtaining foreign regulatory approvals to sell our products internationally, or entering into licensing agreements with third parties. There can be no assurance that we will receive regulatory approval of any of our product candidates, and a substantial amount of time may pass before we achieve a level of revenues adequate to support our operations, if at all. We also expect to incur substantial expenditures in connection with the regulatory approval process for each of our product candidates during their respective developmental periods. Obtaining marketing approval will be directly dependent on our ability to implement the necessary regulatory steps required to obtain marketing approval in the United States and in other countries. We may also need additional funds to realize the decisions made as part of our strategic review process. We cannot predict the outcome of these activities.
As of AugustDecember 31, 2017,2022, our total current assets were $20,297,000$157,109,000 and our total current liabilities were $5,165,000.$5,746,000. On AugustDecember 31, 2017,2022, we had a working capital surplus of $15,132,000$151,363,000 and an accumulated loss of $56,496,000.$163,081,000. As of AugustDecember 31, 2016,2021, our total current assets were $31,230,000$147,937,000 and our total current liabilities were $3,621,000.$7,368,000. On AugustDecember 31, 2016,2021, we had a working capital surplus of $27,609,000$140,569,000 and an accumulated loss of $46,016,000.$126,520,000. The decreaseincrease in working capital surplus from AugustDecember 31, 20162021 to AugustDecember 31, 20172022 was primarilymainly due to the purchase of long-term bank depositsan increase in cash and due to the cash used in operating activities.
equivalents.
During fiscal 2017,the year ended December 31, 2022, cash and cash equivalents increaseddecreased to $3,969,000$40,464,000 from $3,907,000$77,245,000 as of August 31, 2016, which is2021. The decrease was mainly due to the reasons described below.
Operating activities used cash of $5,831,000$27,918,000 in fiscal 2017the year ended December 31, 2022, compared to $4,655,000 provided$21,181,000 used in fiscal 2016.the year ended August 31, 2021. Cash used in operating activities in fiscal 2017 primarily consisted mainly of net loss resulting from research and development, and general and administrative expenses, partially offset by changes in stock-based compensation expenses and deferred revenues, while cash provided by operating activities in fiscal 2016 primarily consisted of changes in deferred revenues due to the License Agreement partially offset by net loss resulting from researchsales and development and general and administrativemarketing expenses.
Investing activities provided cash of $4,302,000$30,211,000 in fiscal 2017, asthe year ended December 31, 2022, compared to $16,010,000cash used in fiscal 2016. Cash provided by investing activities of $23,764,000 in fiscal 2017 consisted primarily of the year ended August 31, 2021. Cash provided in investing activities is mainly due to proceeds from sale of short-term deposits and maturity of marketable securities,investments, partially offset by the purchase of bank deposits and marketable securities, while cash used for investing activities in fiscal 2016 consisted primarily of the purchaseacquisition of short-term and long-term bank deposits as well as the purchase of marketable securities.investments.
Financing activities provided cash of $1,586,000$10,779,000 in fiscal 2017 and $12,043,000the year ended December 31, 2022, compared to $102,892,000 in fiscal 2016.the year ended August 31, 2021. Cash provided by financing activities during both periods consisted mainly of proceeds from our issuance of common stock and proceeds from exercise of warrants and options. Our primary financing activities in fiscal 2017 and fiscal 2016since the beginning of the year ended December 31, 2022 were as follows:
| ● | During |
| ● | On |
During the four month period ended December 31, 2021, cash and cash equivalents decreased to $27,456,000 from the $77,245,000 reported as of August 31, 2021, which is due to the reasons described below.
Contractual ObligationsOperating activities used cash of $11,122,000 in the four month period ended December 31, 2021, compared to $8,263,000 used in the four month period ended December 31, 2020. Cash used in operating activities primarily consisted of research and development, sales and marketing and general and administrative expenses, as well as changes in deferred revenue due to the HTIT License Agreement, partially offset by changes in accounts payable and accrued expenses and stock-based compensation.
Investing activities used cash of $99,248,000 in the four month period ended December 31, 2021, compared to cash used in investing activities of $2,405,000 in the four month period ended December 31, 2020. Cash used in investing activities in the four month period ended December 31, 2021 consisted primarily of the purchase of short-term deposits. Cash used in investing activities in the four month period ended December 31, 2020 consisted primarily of the purchase of short-term deposits, offset by the proceeds from bonds held to maturity.
Financing activities provided cash of $60,572,000 in the four month period ended December 31, 2021, compared to $13,001,000 provided in the four month period ended December 31, 2020. Cash provided by financing activities consisted primarily of proceeds from the issuance of our common stock.
On November 3, 2021, we entered into a securities purchase agreement with several institutional and accredited investors, or the Purchasers, pursuant to which we agreed to sell, in a registered direct offering, or the Offering, an aggregate of 2,000,000 shares of our common stock to the Purchasers for an offering price of $25.00 per share. The following table summarizes our significant contractual obligationsclosing of the sale of the shares occurred on November 5, 2021. The net proceeds to us from the Offering, after deducting the placement agent’s fees and commercial commitments at August 31, 2017,expenses and the effects such obligationsCompany’s Offering expenses, were approximately $46,375,000.
Trend Information
Following the results of the Phase 3 trials for our oral insulin capsule candidate, ORMD-0801, we have initiated a comprehensive analysis of the data to understand if there is a path forward for our oral insulin candidate. Concurrently, we are expected toexamining our existing pipeline and have oncommenced an evaluation process of potential strategic opportunities, with the goal of enhancing value for our liquidity and cash flows in future periods (in thousands):
| Contractual Obligations | Total | Less than 1 year | 1-3 years | 3-5 years | Over 5 years | |||||||||||||||
| Clinical research study obligations | $ | 2,166 | $ | 2,166 | $ | - | $ | - | $ | - | ||||||||||
| Purchase and technology transfer obligations | 4,153 | 3,442 | 711 | - | - | |||||||||||||||
| Operating lease obligations | 168 | 47 | 81 | 40 | - | |||||||||||||||
| Royalty payment obligations | 579 | 137 | 154 | 154 | 134 | |||||||||||||||
| Accrued severance pay, net | 18 | - | - | - | 18 | |||||||||||||||
| Total | $ | 7,084 | $ | 5,792 | $ | 946 | $ | 194 | $ | 152 | ||||||||||
Off-Balance Sheet Arrangements
As of August 31, 2017,stockholders. At this time, we had no off balance sheet arrangements that have had or that we expect would be reasonably likely to have a future material effect oncannot foresee how these strategic decisions will impact our financial condition, changesresults and operations in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.2023.
Planned Expenditures
We invest heavily in research and development, and we expect that in the upcoming years our research and development expenses net, will continue to be our major operating expense. As of December 31, 2022, we had expected obligations with respect to an aggregate of approximately $21 million of clinical research obligations over the next three years.
Following the results of the Phase 3 trials for our oral insulin capsule candidate, ORMD-0801 and the current strategic review initiated by the Company, our obligations may change significantly.
Critical Accounting Policies
Our significant accounting policies are more fully described in the notes to our accompanying consolidated financial statements. We believe that the accounting policies below are critical for one to fully understand and evaluate our financial condition and results of operations.
The discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which we prepared in accordance with U.S. generally accepted accounting principles, or GAAP. The preparation of our consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, we evaluate such estimates and judgments. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Valuation of RSUs, options and warrants: We grant options to purchase shares of our common stock to employees and consultants and have and may in the future issue warrants in connection with some of our financings and to certain other consultants.
We account for share-based payments to employees, directors and consultants in accordance with the guidance that requires awards classified as equity awards to be accounted for using the grant-date fair value method. The fair value of share-based payment transactions is based on the Black Scholes option-pricing model or Monte Carlo model when appropriate and is recognized as an expense over the vesting period.
We elected to recognize compensation cost for awards to employees, directors and consultants that have a graded vesting schedule using the accelerated method based on the multiple-option award approach.
Revenue recognition: Revenue is recognized when delivery has occurred, evidence of an arrangement exists, title and risks and rewards for the products are transferred to the customer and collection is reasonably assured.
Under Accounting Standards Codification, or ASC, 605 (which was the authoritative revenue recognition guidance applied for all periods prior to September 1, 2018) given our continuing involvement through the expected product submission by HTIT in June 2023, amounts received relating to the HTIT License Agreement were recognized over the period from which we were entitled to the respective payment, and the expected product submission date using a time-based model approach over the periods that the fees were earned.
However, under ASC 606, we are required to recognize the total transaction price (which includes consideration related to milestones once the criteria for recognition have been satisfied) using the input method over the period the performance obligation is fulfilled. Accordingly, once the consideration associated with a milestone is included in the transaction price, incremental revenue is recognized immediately based on the period of time that has elapsed towards complete satisfaction of the performance obligation.
Since the customer benefits from the services as the entity performs, revenue is recognized over time through the expected product submission date by HTIT in June 2023, using the input method. The Company used the input method to measure the process for the purpose of recognizing revenue, which approximates the straight line attribution. The Company used significant judgment when it determined the product submission date.
Under ASC 606, the consideration that the Company would be entitled to upon the achievement of contractual milestones, which are contingent upon the occurrence of future events, are a form of variable consideration. When assessing the portion, if any, of such milestones-related consideration to be included in the transaction price, the Company first assesses the most likely outcome for each milestone and excludes the consideration related to milestones of which the occurrence is not considered the most likely outcome.
The Company then evaluates if any of the variable consideration determined in the first step is constrained by including in the transaction price variable consideration to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. The Company used significant judgment when it determined the first step of variable consideration.
On November 13, 2022, we entered into a distribution license agreement with Medicox, or the Medicox License Agreement. The Medicox License Agreement grants Medicox an exclusive license to apply for regulatory approval and distribute ORMD-0801 in the Republic of Korea.
Under ASC 606, we identified Medicox as a customer and the Medicox License Agreement as a contract with a customer.
We identified a performance obligation in the Medicox License Agreement to stand-ready and provide Medicox with support in its commercialization efforts in the Republic of Korea. This performance obligation includes a non-distinct distribution license for ORMD-0801, which we view a predominant item in the combined performance obligation. We concluded that the license is not distinct, as no party other than us is capable of providing related services to Medicox, and both the license and related services are necessary for the customer to obtain a regulatory approval in the Republic of Korea. In addition, the agreement covers the terms of future manufacturing services, that are contingent on the completion and success of the commercialization efforts.
The Medicox License Agreement contains a fixed consideration of $2 million, which was received by Oramed as of December 31, 2022 and is presented under long-term deferred revenues. It also contains variable consideration of contractual milestone payments and sales-based royalties.
Our obligation to stand-ready and support Medicox will be recognized on a straight-line basis over the period we expect to provide support to Medicox. As of December 31, 2022, this support has not commenced, and no revenue was recognized from the Medicox License Agreement.
If Medicox proceeds with the regulatory approval process in the Republic of Korea, we expect most of the revenue to be recognized in 2024, going forward. We note that our Phase 3 trial did not meet its primary and secondary endpoints. If Medicox chooses to terminate the agreement as a result of the outcome of the Phase 3 trials, we will accelerate revenue recognition and recognize it in 2023.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
We are exposed to a variety of risks, including changes in interest rates, foreign currency exchange rates, changes in the value of our marketable securities and inflation.
As of AugustDecember 31, 2017,2022, we had $4$40.5 million in cash and cash equivalents, $29.5$111.5 million in short term and long term bank deposits and restricted deposits and $5$3.7 million in marketable securities.
We aim to preserve our financial assets, maintain adequate liquidity and maximize return while minimizing exposure to market risks. Such policy further provides that we should hold most of our current assets in bank deposits. As of today, the currency of our financial assets is mainly in U.S. dollars.
Marketable securities
We own 10,208,1441,701,357 common shares of D.N.A,DNA and 117,000 ordinary shares of Entera, which are presented in our financial statements as marketable securities. Marketable securities are presented at fair value and their realization is subject to certain limitations if sold through the market, and we are therefore exposed to market risk. There is no assurance that at the time of sale of the marketable securities the price per share will be the same or higher, nor that we will be able to sell all of the securities at once given the volume of securities we hold. TheEntera shares are traded on Nasdaq in U.S. dollars, while DNA shares are traded on the Tel Aviv Stock Exchange and the shares' price is denominated in NIS. We are also exposed to changes in the market price of D.N.Athe Entera and DNA shares, as well as to exchange rates fluctuations in the NIS currency compared to the U.S. dollar.dollar with respect to the DNA shares.
Interest Rate Risk
We invest a major portion of our cash surplus in bank deposits in banks in Israel. Since the bank deposits typically carry fixed interest rates, financial income over the holding period is not sensitive to changes in interest rates, but only the fair value of these instruments. However, our interest gains from future deposits may decline in the future as a result of changes in the financial markets. In any event, given the historic low levels of the interest rate, we estimate that a further decline in the interest rate we are receiving will not result in a material adverse effect to our business.
Foreign Currency Exchange Risk and Inflation
A significant portion of our expenditures, including salaries, clinical research expenses, consultants'consultants’ fees and office expenses relate to our operations in Israel. The cost of those Israeli operations, as expressed in U.S. dollars, is influenced by the extent to which any increase in the rate of inflation in Israel is not offset (or is offset on a lagging basis) by a devaluation of the NIS in relation to the U.S. dollar. If the U.S. dollar declines in value in relation to the NIS, it will become more expensive for us to fund our operations in Israel. In addition, as of AugustDecember 31, 2017,2022, we own net balances in NIS of approximately $1,150,000.$1,854,000. Assuming a 10% appreciation of the NIS against the U.S. dollar, we would experience an exchange rate gain of approximately $128,000,$206,000, while assuming a 10% devaluation of the NIS against the U.S. dollar, we would experience an exchange rate loss of approximately $105,000.
$169,000.
The exchange rate of the U.S. dollar to the NIS, based on exchange rates published by the Bank of Israel, was as follows:
| Year Ended August 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Average rate for period | 3.697 | 3.864 | 3.851 | |||||||||
| Rate at period-end | 3.596 | 3.786 | 3.930 | |||||||||
| Year Ended December 31, | Year Ended December 31, | Four months ended December 31, | Four months ended December 31, | Year Ended August 31, | ||||||||||||||||
| 2022 | 2021 | 2021 | 2020 | 2021 | ||||||||||||||||
| Average rate for period | 3.358 | 3.229 | 3.165 | 3.533 | 3.292 | |||||||||||||||
| Rate at period-end | 3.519 | 3.11 | 3.11 | 3.215 | 3.207 | |||||||||||||||
We do not use any currency hedging transactions of options or forwards to decrease the risk of financial exposure from fluctuations in the exchange rate of the U.S. dollar against the NIS.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
See Item 15 of this Annual Report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
Disclosure Controls and Procedures
Our management, including our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of AugustDecember 31, 2017.2022. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures are effective.
Management’s Annual Report on Internal Control over Financial Reporting
Our management, under the supervision of our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over our financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act. The Company’s internal control over financial reporting is defined as a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP. Internal control over financial reporting includes policies and procedures that:
| ● | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and asset dispositions; |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit the preparation of our financial statements in accordance with GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| ● | provide reasonable assurance regarding the prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on our financial statements. |
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we evaluated the effectiveness of our internal control over financial reporting as of AugustDecember 31, 20172022 based on the current framework for Internal Control-Integrated Framework (2013) set forth by The Committee of Sponsoring Organizations of the Treadway Commission.
Based on this evaluation, our management concluded that the Company’s internal control over financial reporting was effective as of AugustDecember 31, 20172022 at a reasonable assurance level.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the quarter ended AugustDecember 31, 20172022 that have materially affected, or are reasonable likely to materially affect, our internal control over financial reporting.
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Set forth below is certain information with respect to the individuals who areDirectors and Executive Officers
The name and age of each of our directors and executive officers.officers, his or her position with us and the period during which such person has served as a director or executive officer of the Company are set forth below.
| Name | Age | Position | Serving Since | ||||
| Nadav Kidron | 48 | President, Chief Executive Officer, Director and Chairman (effective as of June 30, 2022) | 2006 | ||||
| Dr. Miriam Kidron | 82 | Chief Scientific Officer and Director | 2006 | ||||
| David Silberman | 39 | Chief Financial Officer and Treasurer | 2021 | ||||
| Joshua Hexter | 52 | Chief Operating & Business Officer | 2019 | ||||
| Michael Rabinowitz | 57 | Chief Commercial Officer | 2021 | ||||
| Netanel Derovan | 47 | Chief Legal Officer and Secretary | 2022 | ||||
| Dr. Arie Mayer | 66 | Director | 2019 | ||||
| Yadin Rozov | 45 | Director | 2022 | ||||
| Leonard Sank | 57 | Director | 2007 |
Dr. Miriam Kidron is Mr. Nadav Kidron’s mother. There are no other directors or officers of the Company who are related by blood or marriage.
Business Experience
The following is a brief account of the education and business experience during at least the past five years of each directorof our directors and of our executive officers who are not also directors, indicating the principal occupation during that period, and the name and principal business of the organization in which such occupation and employment were carried out.
Mr. Nadav Kidronwas appointedPresident, Chief Executive Officerand adirector in March 2006.2006, and Chairman of the Board effective as of June 30, 2022. He is also a director of Israel Advanced Technology Industries organization, and until 2016 was a director of Entera Bio Ltd. In 2009, he was a fellow at the Merage Foundation for U.S.-Israel Trade Programs for executives in the life sciences field. From 2003 to 2006, he was the managing director of the Institute of Advanced Jewish Studies at Bar Ilan University. From 2001 to 2003, he was a legal intern at Wine, Mishaiker & Ernstoff Law Offices in Jerusalem, Israel. Mr. Kidron holds an LL.B. and an International MBA from Bar Ilan University, Israel, and is a member of the Israel Bar Association.Israel.
We believe that Mr. Kidron’s qualifications to serve on our Board include his familiarity with the Company as its founder, his experience in capital markets, as well as his knowledge and familiarity with corporate management.
Dr. Miriam Kidronwas appointedChief Scientific Officerand adirector in March 2006. Dr. Kidron is a pharmacologist and a biochemist with a Ph.D. in biochemistry. From 1990 to 2007, Dr. Kidron was a senior researcher in the Diabetes Unit at Hadassah University Hospital in Jerusalem, Israel. During 2003 and 2004, Dr. Kidron served as a consultant to Emisphere Technologies Inc., a company that specializes in developing broad-based proprietary drug delivery platforms. Dr. Kidron was formerly a visiting professor at the Medical School at the University of Toronto (Canada), and is a member of the American, European and Israeli Diabetes Associations. Dr. Kidron is a recipient of the Bern Schlanger Award.
We believe that Dr. Kidron’s qualifications to serve on our Board include her expertise in the Company’s technology, as it is based on her research, as well as her experience and relevant education in the fields of pharmacology and diabetes.
Ms. Hilla EisenbergMr. David Silberman was appointedChief Financial Officer and Treasurer and Secretary effective August 2017.in July 2021. Prior to herhis appointment, Ms. Eisenbergfrom April 2018 to May 2021, Mr. Silberman served as a Corporate Financial Planning and Analysis associate director and director at Teva Pharmaceutical Industries Ltd., a global pharmaceutical company, committed to helping patients around the Company’s Finance Managerworld to access affordable medicines and benefit from March 2016 until July 2017. Before joining the Company, Ms. Eisenberginnovations to improve their health. From 2014 to 2018, Mr. Silberman served as Global Internal Audit senior manager at Teva Pharmaceutical Industries Ltd. From 2009 to 2014, Mr. Silberman provided internal audit and other accountingrisk management services atin the advisory department of Grant Thornton Fahn Kanne Control Management. From January 2009 until June 2009, Mr. Silberman worked in the audit department of KPMG, a certified public accounting firm in Israel. Prior to that, Ms. Eisenberg served as an auditor at PricewaterhouseCoopers in Israel, including a short secondment to PricewaterhouseCoopers in New York. Ms. Eisenbergfirm. Mr. Silberman holds a bachelor's degree in accountingDCG and economicsDSCG degrees from Tel-Aviv Universitythe French Ministry of Higher Study and Research and is a certified public accountant in Israel.
Mr. Joshua Hexter was appointedChief Operating & Business Officer in September 2019. Prior to his appointment, Mr. Hexter served as Chief Business Officer at BrainsWay Ltd. (Nasdaq/TASE: BWAY) from 2018 to 2019, a commercial stage medical device company focused on the development and sale of non-invasive neuromodulation products. From 2013 to 2018, Mr. Hexter served as Chief Operating Officer and VP Business Development in April 2013. From of the Company and from 2007 to 2013, Mr. Hexter was a Director or Executive Director inof BioLineRx Ltd. (Nasdaq/TASE: BLRX), or BioLineRx, a Tel-Aviv Stock Exchange-listed biopharmaceutical development company dedicated to identifying, in-licensing and developing innovative therapeutic candidates. Prior to his employment with BioLineRx, Mr. Hexter was a member of the Boardboard of Directorsdirectors and CEOChief Executive Officer of Biosensor Systems Design, Inc., a company developing market-driven biosensors. Mr. Hexter holds a bachelor’s degree from the University of Wisconsin and a master’s degree in management from Boston University.
Dr. Ronald LawMr. Michael Rabinowitz was appointedChief StrategyCommercial Officer in March 2017.August 2021. Prior to his appointment, Mr. Rabinowitz served for over 25 years, from 1993 to 2021, in various marketing, sales, business development, and financial leadership roles at the global biopharmaceutical company Merck & Co., where he launched and marketed products in over 30 countries across several disease areas, including launching billion-dollar oral agents in diabetes and managing a global business. Mr. Rabinowitz holds a bachelors’ degree summa cum laude from Northwestern University and a masters’ degree from The Carlson School of Management at the University of Minnesota. He has also participated in executive health care programs at the Harvard Business School and the Wharton School of the University of Pennsylvania.
Mr. Netanel Derovan was appointed Chief Legal Officer and Secretary in January 2022. Prior to his appointment, from 2012 to 2021, Mr. Derovan served as executive counsel, corporate and securities, in the legal department of Teva Pharmaceutical Industries Ltd. From 2004 to 2016, Dr. Law served in several leadership and strategic roles in Takeda Pharmaceuticals Company Limited and its business divisions, where his latest role was Vice President, New Frontier Science, Chief Medical and Scientific Officer in Takeda Pharmaceuticals International/Takeda Development Center, Americas. Dr. Law also2012, he served as senior counsel in the Consulting HeadInternational Corporate and Securities Department of Business DevelopmentGoldfarb Seligman & Co. From 2002 to 2004, he served as an associate attorney in PharmaIN Corporation in 2016 and currently servesthe International Corporate Department at Caspi & Co. From 2001 to 2002, he served as a consultant for other medical companies. Prior to joining Takeda Pharmaceuticals Company Limited, Dr. Law waslegal intern at Gornitzky & Co. Mr. Derovan holds an Associate Professor of Medicine in the Endocrinology Division,LLB degree from Bar Ilan University of California Los Angeles School of Medicine. Dr. Law received a Ph.D. in molecular biology from the University of California Los Angeles and a JD from the Whittier College School of Law. He is a member of the American Diabetes Association and the American HeartIsrael Bar Association.
Mr. Aviad FriedmanDr. Arie Mayer became adirector in August 2016. Mr. FriedmanDecember 2019. Dr. Mayer is an international businessman. Since 2007, he has been Chief Executive Officer of Most Properties 1998 Ltd.currently the Managing Director and the Chairman of the Board of Sigma-Aldrich Israel Association of Community Centers since 2013. Mr. Friedman was the first Director General of Israel's Ministry of Diaspora Affairs and served as personal advisor to Prime Minister Ariel Sharon from 1996 to 1999. Mr. Friedman served as Chief Operating Officer of one of Israel’s premier newspapers, Ma'ariv from 2003 to 2007,Ltd. and has more than 15 years of experience serving on boards of public and private companies including Maayan Ventures, Capital Point and Rosetta Greenheld that position since January 2010. Dr. Mayer has held various roles with Sigma-Aldrich Israel Ltd. Mr. Friedman additionally served as an investor and consultant at Rhythmia Medical Inc. from 2007,since 1995 and was actively involvedinstrumental in introducing and developing the sale of the company to Boston Scientific in 2012. Mr. FriedmanCell Culture and Molecular Biology business for Sigma Aldrich Israel Ltd. Dr. Mayer holds a bachelor’sBachelor of Science degree in chemistry from Hebrew University and master’s degree with honorsa Ph.D. in Public Administrationbiochemistry from Bar-Ilan University.Israel Institute of Technology.
We believe that Mr. Friedman’sDr. Mayer’s qualifications to serve on our Board include his experience in serving as a director of public and private companies as well as his knowledge and familiarity with corporate finance.
Ms. Xiaopeng Li became adirector in January 2016. Ms. Li currently serves on the board of directors in the Chairman’s Office in Hefei Tianmai Biotechnology Development Co. Ltd, or HTBT, where she has served as the head of financing and investment activities since 2013. Ms. Li also has served as Chief Financial Officer of Hi-Tech Brain Investment Company Limited, an affiliated company of HTBT, since 2015. Prior to that, she was a senior auditor in the Shanghai Branch of Ernst & Young Hua Ming LLP, where she served for four years. Ms. Li holds a bachelor’s degree from the College of Economics, Anhui University, a Master of Accounting degree from Monash University, Australia, and a Master of Management degree from Central Queensland University, Australia.
We believe that Ms. Li’s qualifications to serve on our Board include her experience and relevant education in the fields of finance, economics, capital markets and management, as well as her familiarity with the Eastern market.
Mr. Kevin Rakin became adirector in August 2016 and Chairman of the Board in July 2017. Mr. Rakin is a co-founder and partner at HighCape Partners, a growth equity life sciences fund where he has served since 2013. From June 2011 to November 2012, Mr. Rakin was the President of Regenerative Medicine at Shire plc, or Shire, a leading specialty biopharmaceutical company. Prior to joining Shire, Mr. Rakin served as the Chairman and Chief Executive Officer of Advanced BioHealing, Inc. from 2007 until its acquisition by Shire for $750 million in June 2011. Mr. Rakin currently serves on the board of Histogenics Corporation. Mr. Rakin holds an MBA from Columbia University and received his graduate and undergraduate degrees in Commerce from the University of Cape Town, South Africa.
We believe that Mr. Rakin’s qualifications to serve on our Board include his extensive experience as an executive in the biotechnology industry, as well as his service in positions in various companies as a chief executive officer, chief financial officerexperience and president and his involvement in public and private financings and mergers and acquisitionsrelevant education in the biotechnology industry.fields of chemistry and biochemistry.
Mr. Yadin Rozov became a director in April 2022. Mr. Rozov is the founder and managing partner of Terrace Edge Ventures LLC, a financial advisory firm, since January 2022. From 2019 to 2021, Mr. Rozov was a Partner of GoldenTree Asset Management LLC, a leading global credit asset management firm. From 2019 to 2021, Mr. Rozov also served as the Chief Executive Officer and President of Syncora Guarantee Inc. and from 2020 to 2021, as Chief Executive Officer of Financial Guaranty UK Ltd, each of which is a stand-alone specialty insurance company owned by GoldenTree. From 2009 to 2019, he was a Partner and Managing Director at Moelis & Company where he headed the Financial Institution Advisory group and was on the Management Committee of Moelis Asset Management. From 2014 to 2019, Mr. Rozov helped co-found College Avenue Student Loans LLC and served on its board and co-founded Chamonix Partners Capital Management LLC. From 2007 to 2009, Mr. Rozov was a Managing Director at UBS AG, where he was the Head of the Americas for the Repositioning Group. Mr. Rozov serves on the board of directors of Midwest Holding Inc. since June 2022, and on the board of directors of Neo Performance Materials Inc. since August 2022. Mr. Rozov holds an M.Sc. in data science from Columbia University and a bachelor’s degree with highest honors in physics and materials engineering from Rutgers University.
We believe Mr. Rozov’s qualifications to serve on the Board include his many years of experience in capital markets, corporate finance, investment banking and investment management, with substantial experience in corporate strategy and governance.
Mr. Leonard Sank became adirector in October 2007. Mr. Sank is a South African entrepreneur and businessman, whose interests lie in entrepreneurial endeavors and initiatives, with over 20 years'25 years’ experience of playing significant leadership roles in developing businesses. For the past seventeen years, Mr. Sank has servedserves on the boards of a few national businesses and local non-profit charity organizations in Cape Town, where he resides.
We believe that Mr. Sank’s qualifications to serve on our Board include his years of experience in development stage businesses, as well as his experience serving as a director of many entities.
Mr. David Slager became adirector in August 2016. Mr. Slager is the founder and Chairman of Regals Capital, a New York based private investment firm, and the Portfolio Manager of the fund. Prior to founding Regals Capital in 2012, Mr. Slager was the Chairman and the Portfolio Manager of Attara Capital. Prior to Attara Capital in 2009, Mr. Slager was the Vice Chairman of Atticus Capital LP, a global investment management firm he joined in 1998. Mr. Slager’s previous professional experience also includes having been in the Proprietary Equity Arbitrage Group at Goldman, Sachs & Co. in London and a financial analyst at Goldman, Sachs & Co. in New York and London. Mr. Slager holds a master’s degree in Legal Philosophy (Jurisprudence) from Oxford University.
We believe that Mr. Slager’s qualifications to serve on our Board include his years of experience in the capital markets as well as his management skills, his knowledge and familiarity with corporate finance and his familiarity with the Company given his history as a leading shareholder in the Company.
Board of Directors
There are no agreements with respect to the election of directors. Each director is currently elected for a period of one year at our annual meeting of stockholders and serves until the next such meeting and until his or her successor is duly elected or until his or her earlier resignation or removal. The Board may also appoint additional directors. A director so chosen or appointed will hold office until the next annual meeting of stockholders and until his or her successor is duly elected and qualified or until his or her earlier resignation or removal.
The Board has determined that Dr. Arie Mayer, Yadin Rozov and Leonard Sank David Slager, Kevin Rakin, Aviad Friedman and Xiaopeng Li are independent as defined under the rules promulgated by the Nasdaq. Other than Mr. Slager, Ms. Li and Mr. Friedman,Except for Dr. Arie Mayer, who serves on the Board of Directors of Oravax, a company 63% owned by us, none of the independent directors has any relationship with us besides serving on our Board. In connection with a private placement of our common stock in 2013, we have entered into a letter agreement with Mr. Slager pursuant to which we agreed not to issue stock options with an exercise price below $6.00 per share and not to grant more than 125,000 stock options in any calendar year without the consent of certain stockholders. Ms. Li was appointed to serve on our Board pursuant to the terms of the SPA dated November 30, 2015, but does not otherwise have any relationship with us. We had entered into a consulting agreement with Shikma A.M.R. Ltd., or Shikma, of which Mr. Friedman is the sole owner, pursuant to which Shikma was granted an option exercisable into shares of common stock of the Company as compensation for certain consulting services provided by Shikma to the Company. This consulting agreement was terminated in August 2016. The Board considered these relationships and determined that they would not interfere with Mr. Slager’s, Ms. Li’s or Mr. Friedman’s exercise of independent judgment in carrying out the responsibilities of a director.
We have determined that each of the directors is qualified to serve as a director of the Company based on a review of the experience, qualifications, attributes and skills of each director. In reaching this determination, we have considered a variety of criteria, including, among other things: character and integrity; ability to review critically, evaluate, question and discuss information provided, to exercise effective business judgment and to interact effectively with the other directors; and willingness and ability to commit the time necessary to perform the duties of a director.
Board Meeting Attendance
During the fiscal 2017,year ended December 31, 2022, our Board held 13six meetings and took actionsaction by written consent on twonine occasions. Ms. Xiaopeng LiDuring the Transition Period, our Board held five meetings and took action by written consent on one occasion. All of our directors attended fewer thanat least 75% of the aggregate of: (i) the total number of meetings of the Board (duringand the period for which such director served as a director); and (ii)committees that were held during the total number of meetings held by all committees of the Board on which such director served (during the period for which such director served on such committees).the Board. Board members are encouraged to attend our annual meetings of stockholders.
CommitteesBoard Evaluation Process
Our Board is committed to continuous improvement and conducts a board and committee evaluation process each year, to ensure that our Board maintains optimal composition and functions effectively.
As part of this process, the members of our Board complete a confidential written assessment of the performance, oversight and composition of the Board and its committees that is submitted to the Company secretary. The results are then reported back to the full Board. After the evaluations, the Board and management work to improve upon any issues presented during the evaluation process and to identify opportunities that may lead to further improvement.
Committees
Audit Committee and Audit Committee Financial Expert
The members of our Audit Committee are Aviad Friedman, David SlagerDr. Arie Mayer, Yadin Rozov and Kevin Rakin.Leonard Sank. Our Board has determined that Aviad FriedmanYadin Rozov is an “audit committee financial expert” as set forth in Item 407(d)(5) of Regulation S-K and that all members of the Audit Committee are “independent” as defined by the rules of the SEC and the Nasdaq rules and regulations. The Audit Committee operates under a written charter that is posted on the “Investors” section of our website, www.oramed.com. The primary responsibilities of our Audit Committee include:
| ● | Overseeing the accounting and financial reporting processes of the Company and the audits of the financial statements of the Company; | |
| ● | Appointing, compensating and retaining our registered independent public accounting firm; | |
| ● | Overseeing the work performed by any outside accounting firm; |
| ● | Assisting the Board in fulfilling its responsibilities by reviewing: (i) the financial reports provided by us to the SEC, our stockholders or to the general public and (ii) our internal financial and accounting controls; | |
| ● | Reviewing the Company’s policies with respect to cyber security risks and relevant contingent liabilities and risks that may be material to the Company; |
| ● | Recommending, establishing and monitoring procedures designed to improve the quality and reliability of the disclosure of our financial condition and results of | |
| ● | Reviewing major financial risk exposures and the steps management has taken to monitor and control such exposures, and discussing the guidelines and polices to govern the process by which risk assessment and management is undertaken. |
Our Audit Committee met six times and took action by written consent on four occasions during the fiscal year ended December 31, 2022. Our Audit Committee met three times and took action by written consent on two occasions during the Transition Period.
Compensation Committee
The members of our Compensation Committee are Dr. Arie Mayer, Leonard Sank Kevin Rakin and Aviad Friedman.Yadin Rozov. The Board has determined that all of the members of the Compensation Committee are “independent” as defined by the rules of the SEC and Nasdaq rules and regulations. The Compensation Committee operates under a written charter that is posted on the “Investors” section of our website, www.oramed.com. The primary responsibilities of our Compensation Committee include:
| ● | Reviewing, negotiating and approving, or recommending for approval by our Board the salaries and incentive compensation of our executive officers; |
| ● | Administering our equity based plans and making recommendations to our Board with respect to our incentive-compensation plans and equity-based plans; and |
| ● | Making recommendations to our Board with respect to director compensation. |
The Compensation Committee meets as often as it deems necessary, without the presence of any executive officer when approving compensation, except that the Company’s Chief Executive Officer, at the discretion of the Compensation Committee, may be present during the approval of, or deliberations with respect to, the compensation of other executive officers. The Compensation Committee may delegate any authority granted to it to one or more subcommittees of the Compensation Committee, in its sole discretion.
Our Compensation Committee met twice and took action by written consent on four occasions during the fiscal year ended December 31, 2022. Our Compensation Committee met once and took action by written consent on two occasions during the Transition Period.
Nominating Committee
The members of our Nominating Committee are Dr. Arie Mayer, Leonard Sank and Aviad Friedman.Yadin Rozov. The Board has determined that all of the members of the Nominating Committee are “independent” as defined by the rules of the SEC and Nasdaq rules and regulations. The Nominating Committee operates under a written charter that is posted on the “Investors” section of our website, www.oramed.com. The primary responsibilities of our Nominating Committee include:
| ● | Overseeing the composition and size of the Board, developing qualification criteria for Board members based on background, skills, experience and diversity, and actively seeking, interviewing and screening individuals qualified to become Board members for recommendation to the Board; |
| ● | Recommending the composition of the Board for each annual meeting of |
| ● | Reviewing periodically with the Chairman of the Board and the |
Our Nominating Committee took action by written consent on two occasions during the fiscal year ended December 31, 2022. Our Nominating Committee did not meet or take action by written consent during the Transition Period.
Delinquent Section 16(a) Beneficial Ownership Reporting ComplianceReports
Based solely upon a review of Forms 3, 4 and 5, and amendments thereto, furnished to us during the fiscal 2017,year ended December 31, 2022 and the Transition Period, we believe that during the fiscal 2017,year ended December 31, 2022 and the Transition Period, our executive officers, directors and all persons who own more than ten percent of a registered class of our equity securities complied with all Section 16(a) filing requirements, except: (a) Mr. Josh Hexter, our Chief Operating Officer, failed to timely file a Form 4 reporting his November 1, 2016 acquisition of 70,000 shares of our common stock. Mr. Hexter filed a Form 4 reporting this transaction on November 22, 2016, (b) Mr. Kevin Rakin,except that Dr. Arie Mayer, one of our directors, failed to timely file a Form 4 reporting his February 9, 2017 acquisitionNovember 1, 2021 sale of options to purchase 5,6973,000 shares of our common stock. Mr. RakinMayer filed a Form 4 reporting this transaction on February 16, 2017, (c) Mr. David Slager, one of our directors, failed to timely file a Form 4 reporting his February 9, 2017 acquisition of options to purchase 5,697 shares of our common stock. Mr. Slager filed a Form 4 reporting this transaction on February 16, 2017, (d) Mr. Aviad Friedman, one of our directors, failed to timely file a Form 4 reporting his February 9, 2017 acquisition of options to purchase 4,084 shares of our common stock. Mr. Friedman filed a Form 4 reporting this transaction on March 2, 2017 and (e) Ms. Xiaopeng Li, one of our directors, failed to timely file a Form 4 reporting her February 9, 2017 acquisition of options to purchase 12,253 shares of our common stock. Ms. Li filed a Form 4 reporting this transaction on March 2, 2017.May 10, 2022.
Code of Ethics
We have adopted a Code of Ethics and Business Conduct for our senior officers, directors and employees. A copy of the Code of Ethics and Business Conduct is located at our website at www.oramed.com. We intend to satisfy the disclosure requirement regarding any amendment to, or a waiver from, a provision of the Code of Ethics that applies to our Chief Executive Officer, or CEO, Chief Financial Officer or CFO, or controller, or persons performing similar functions and that relates to the Code of Ethics by posting such information on our website, www.oramed.com.
ITEM 11. EXECUTIVE COMPENSATION.
Compensation Discussion and Analysis
This section explains the policies and decisions that shape our executive compensation program, including its specific objectives and elements, as it relates to our “named executive officers,” or NEOs.
Our NEOs for fiscal 2017the year ended December 31, 2022 and the Transition Period are those three individuals listed in the “Summary Compensation Table” below. The Compensation Committee believes that our executive compensation is appropriately designed to incentivize our named executive officersNEOs to work for our long-term prosperity, is reasonable in comparison with the levels of compensation provided by comparable companies and reflects a reasonable cost. We believe our named executive officersNEOs are critical to the achievement of our corporate goals, through which we can drive stockholder value.
The Compensation Committee of our Board is comprised solely of independent directors as defined by NASDAQNasdaq and non-employee directors as defined by Rule 16b-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act. The Compensation Committee has the authority and responsibility to review and approve the compensation of our CEOPresident and Chief Executive Officer and other executive officers. Other information concerning the structure, roles and responsibilities of our Compensation Committee is set forth in "Board“Board Meetings and Committees—Compensation Committee"Committee” section.
Our executive compensation program and our NEOs’ compensation packages are designed around the following objectives:
| ● | attract, hire, and retain talented and experienced executives; |
| ● | motivate, reward and retain executives whose knowledge, skills and performance are critical to our success; |
| ● | ||
| ensure fairness among the executive management team via recognizing the contributions of each executive to our success; |
| ● | focus executive behavior on achievement of our corporate objectives and strategy; and |
| ● | align the interests of management and stockholders by providing management with longer-term incentives through equity ownership. |
The Compensation Committee reviews the allocation of compensation components regularly to ensure alignment with strategic and operating goals, competitive market practices and legislative changes. The Compensation Committee does not apply a specific formula to determine the allocation between cash and non-cash forms of compensation. Certain compensation components, such as base salaries, benefits and perquisites, are intended primarily to attract, hire, and retain well-qualified executives. Other compensation elements, such as long-term incentive opportunities, are designed to motivate and reward performance. Long-term incentives are intended to reward NEOs for our long-term performance and executing our business strategy, and to strongly align NEOs'NEOs’ interests with those of stockholders.
With respect to equity compensation, the Compensation Committee makes awards to executives under our Second Amended and Restated 2008 Stock2019 Incentive Plan, or 2008 Plan. Executive compensation is paid or granted based on such matters as the Compensation Committee deems appropriate, including our financial and operating performance and the alignment of the interests of the executive officers and our stockholders.
Elements of Compensation
Our executive officer compensation program is comprised of: (i) base salary or monthly compensation; (ii) discretionary bonus; (iii) long-term equity incentive compensation in the form of stock option and RSU grants; and (iv) benefits and perquisites.
In establishing overall executive compensation levels and making specific compensation decisions for our NEOs in fiscal 2017,the year ended December 31, 2022 and the Transition Period, the Compensation Committee considered a number of criteria, including the executive'sexecutive’s position, scope of responsibilities, prior base salary and annual incentive awards and expected contribution.
Generally, our Compensation Committee reviews and, as appropriate, approves compensation arrangements for the NEOs from time to time but not less than once each year. The Compensation Committee also takes into consideration the CEO'sPresident and Chief Executive Officer’s recommendations for executive compensation of the other three NEOs. The CEOPresident and Chief Executive Officer generally presents these recommendations at the time of our Compensation Committee'sCommittee’s review of executive compensation arrangements.
During the fiscal year ended December 31, 2022, the Compensation Committee received consulting services from Deloitte Israel & Co., or Deloitte, with regard to management compensation. The Compensation Committee engaged the consultant to review the Company’s current compensation plans for its management and collect and analyze data regarding management compensation at other companies comparable to the Company, in order to provide a competitive compensation benchmark. Deloitte collected SEC filings data regarding U.S. and Israeli compensation practices and developed a peer group of the following U.S. and Israeli companies: Theseus Pharmaceuticals Inc., Athira Pharma Inc., Zomedica Corp., Rallybio Corp., Verastem Inc., VistaGen Therapeutics Inc., Acumen Pharmaceuticals Inc., Enochian Biosciences Inc., Aldeyra Therapeutics Inc., Viking Therapeutics Inc., Eliem Therapeutics Inc., Werewolf Therapeutics Inc., Compugen Ltd., Urogen Pharma Ltd., Kamada Ltd., Gamida Cell Ltd., Sol Gel Technologies Ltd., Redhill Biopharma Ltd., Collplant Biotechnologies Ltd., Enlivex Therapeutics Ltd., Vascular Biogenics Ltd., PolyPid Ltd. and BioLine RX Ltd. Following its review, Deloitte provided recommendations for cash and equity compensation at various percentiles for the Compensation Committee’s consideration.
Base Salary
The Compensation Committee performs a review of base salaries and monthly compensation for our NEOs from time to time as appropriate. In determining salaries, the Compensation Committee members also take into consideration the scope of the NEOs'NEOs’ responsibilities and independent third partythird-party market data, such as compensation surveys to industry, individual experience and performance and contribution to our clinical, regulatory, commercial and operational performance. None of the factors above has a dominant weight in determining the compensation of our named executive officers,NEOs, and our Compensation Committee considers the factors as a whole when considering such compensation. In addition, our Compensation Committee uses comparative data regarding compensation paid by peer companies in order to obtain a general understanding of current trends in compensation practices and ranges of amounts being awarded by other public companies, and not as part of an analysis or a formula.
In fiscal 2017, for example, the Compensation Committee received consulting services from Aon Consulting, Inc., or Aon, through its Radford subdivision (part of Aon Hewitt), or Radford, with regard to management and Board compensation. The Compensation Committee engaged the consultant solely to collect and analyze data regarding management and Board compensation at other companies comparable to the Company. The consultant collected data regarding U.S. and Israeli practices, reviewed executive compensation against a market composite of peer proxy data and Radford survey data, determined the U.S. to Israeli discount and applied the discount to position specific U.S. data to arrive at Israeli market data.
The Israeli peer group consisted of the following companies: Alcobra Ltd., BioLine Rx Ltd., Can-Fite BioPharma Ltd., Foamix Pharmaceuticals Ltd., Galmed Pharmaceuticals Ltd., Intec Pharma Ltd., Kamada Ltd., MediWound Ltd., Pluristem Therapeutics Inc., Protalix BioTherapeutics Inc., RedHill Biopharma Ltd. and Vascular Biogenics Ltd. The U.S. peer group consisted of the following companies: Actinium Pharmaceuticals Inc., Athersys Inc., Capricor Therapeutics Inc., Cara Therapeutics Inc., Catabasis Pharmaceuticals Inc., Cymabay Therapeutics Inc., Eiger BioPharmaceuticals Inc., Eleven Biotherapeutics Inc., Endocyte Inc., Genocea Biosciences Inc., GlycoMimetics Inc., GTx Inc., Kura Oncology Inc., Ocera Therapeutics Inc., Stemline Therapeutics Inc., Tracon Pharmaceuticals Inc. and vTv Therapeutics Inc.
The Compensation Committee did not receive any executive compensation consulting services in fiscal 2016 and fiscal 2015.
We believe that a competitive base salary and monthly compensation is a necessary element of any compensation program that is designed to attract and retain talented and experienced executives. We also believe that attractive base salaries can motivate and reward executives for their overall performance. Base salary and monthly compensation are established in part based on the individual experience, skills and expected contributions to our performance, as well as such executive’s performance during the prior year. Generally, we believe that executives'executives’ base salaries should be targeted near the median of the range of salaries for executives in similar positions with similar responsibilities, experience and performance at comparable companies. Compensation adjustments are made occasionally based on changes in an executive'sexecutive’s level of responsibility, company progress or on changed local and specific executive employment market conditions.
In fiscal 2017,the year ended December 31, 2022, our Compensation Committee increased the base salariessalary of one of our NEOs by ten10% (effective January 1, 2023) as it deemed this to thirty three percentbe a reasonable rate based on, among other factors, such NEO’s responsibilities and the report from Deloitte, as it determined the salary was not in line with market compensation. In the Transition Period, our Compensation Committee increased the base salary of most of our NEOs by 15% based on the report from Aon,an independent compensation consultant, as it determined salaries were not in line with market and in fiscal 2016, our Compensation Committee increased the base salaries of our NEOs by ten to twenty percent, as it deemed this to be a reasonable rate in the biotechnology industry.compensation.
Performance Based Bonus
Our NEOs are eligible to receive discretionary annual bonuses based upon performance. The amount of annual bonus to our NEOs is based on various factors, including, among others, the achievement of scientific and business goals and our financial and operational performance. The Compensation Committee takes into account the overall performance of the individuals, as well as the overall performance of the Company over the period being reviewed and the recommendation of management. For any given year, the compensation objectives vary, but relate generally to strategic factors such as developments in our clinical path, the execution of a license agreement for the commercialization of product candidates, the establishment of key strategic collaborations, the build-up of our pipeline and financial factors such as capital raising. Bonuses are awarded generally based on corporate performance, with adjustments made within a range for individual performance, at the discretion of the Compensation Committee. The Compensation Committee determines, on a discretionary basis, the size of the entire bonus pool and the amount of the actual award to each NEO. The overall payment is also based on historic compensation of the NEOs.
We believe that annual bonuses payable based on the achievement of short-term corporate goals incentivize our NEOs to create stockholder value and attain short-term performance objectives.
Long-Term Equity Incentive Compensation
Long-term incentive compensation allows the NEOs to share in any appreciation in the value of our common stock. The Compensation Committee believes that stock participation aligns executive officers’ interests with those of our stockholders. Equity incentive awards are generally made at the commencement of employment and following a significant change in job responsibilities, or to meet other special retention or performance objectives. The amounts of the awards are designed to reward past performance and create incentives to meet long-term objectives. Awards are made at a level expected to be competitive within the biotechnology industry, as well as with Israeli-based companies. Awards are made on a discretionary basis and not pursuant to specific criteria set out in advance. In determining the amount of each grant, the Compensation Committee also takes into account the number of shares held by the executive prior to the grant. The vesting schedule for NEOs was based on monthly installmentsgenerally provides for periods of no longer than three years through the beginning of fiscal 2017; however, following consultation with Aon during fiscal 2017, the vesting schedule for NEOs was moved to annual installments for new grants.grants, though the Compensation Committee also utilizes quarterly vesting from time to time, as well as performance-based vesting. The Compensation Committee believes that time-based vesting encourages recipients to build stockholder value over a long period of time.
RSU awards provide our NEOs with the right to purchase shares of our common stock at a par value of $0.012, subject to continued employment with our company. In November 2014, the Compensation Committee awarded RSUs for the first time and again awarded RSUs in February 2015 and in November 2016. We chose to grant RSU awards and not options because RSU awards, once vested, always have an immediate financial value to the holder thereof, unlike options where the exercise price might be above the current market price of the shares and therefore not have any intrinsic value to the holder thereof. In addition, because vested RSU awards always have financial value, as opposed to options, we were able to limit the number of securities issued to our NEOs and other employees, directors and consultants. RSUs generally vest over a period of no longer than two years. In June 2017, following consultation with Aon, the Compensation Committee chose to grant options instead of RSUs and in addition granted to the CEO options with a market condition of our share price reaching a certain target, in order to further strengthen the alignment of our NEOs’ interests with those of our stockholders, as part of our efforts to increase the Company's market value. The Compensation Committee believes that time-basedperformance-based vesting encourages recipients to build stockholder value overachieve goals that benefit the Company.
As part of its engagement in the year ended December 31, 2022 described above, Deloitte also provided consulting services in connection with grants of equity awards to our executive officers. Deloitte reviewed annual long-term incentive grants at peer companies, as well as such grants made by companies in the broader market, based on a long periodblend of time.Black-Scholes valuations and grants as a percentage of the applicable company’s capitalization. Following such consultation, the Compensation Committee is considering alternative models and equity vehicles for future equity-based grants.
Benefits and Perquisites
Generally, benefits available to NEOs are available to all employees on similar terms and include welfare benefits, paid time-off, life and disability insurance and other customary or mandatory social benefits in Israel. We provide some of our NEOs with a cellular phone and a company car, which are customary benefits in Israel to managers and officers.
During fiscal 2017, the Compensation Committee approved the payment to Mr. Kidron of approximately $7,600 per month for a period beginning in August 2017, during which Mr. Kidron is in the United States. This payment replaced per diem payments for such business travel. The Compensation Committee determined that this amount reflects the difference in the cost of living between Israel and the United States.
We do not believe that the benefits and perquisites described above deviate materially from the customary practice for compensation of executive officers by other companies similar in size and stage of development in Israel. These benefits represent a relatively small portion of the executive officers'officers’ total compensation.
The Company paid for certain direct costs, related taxes and expenses incurred in connection with the relocation of our President and Chief Executive Officer to the United States. During the fiscal year ended December 31, 2022 and the Transition Period, such relocation expenses totaled approximately $331,000 and $109,000, respectively, and included mainly payments intended to reflect the difference in the cost of living between Israel and the United States, relocation expenses, accommodation allowances, education allowances, health insurance and related taxes.
Say-on-Pay Vote
Our stockholders approved, on an advisory basis, our executive compensation program at our 2016 Annual Meeting.annual meeting of stockholders held on June 30, 2022. We did not seek or receive any specific feedback from our stockholders concerning our executive compensation program during the past fiscal year. The Compensation Committee did not specifically rely on the results of the prior vote in making any compensation-related decisions during the fiscal 2017.
year ended December 31, 2022 and the Transition Period.
COMPENSATION COMMITTEE REPORT
The Compensation Committee has reviewed and discussed the foregoing Compensation Discussion and Analysis required by Item 402(b) of Regulation S-K with our management and, based on such review and discussions, the Compensation Committee recommended to our Board that the Compensation Discussion and Analysis be included in this Annual Report on Form 10-K and in our proxy statement relating to our next annual meeting of stockholders.
|
SUMMARY COMPENSATION TABLE
The following table sets forth the compensation earned by our NEOs for fiscals 2017, 2016the Transition Period and 2015.fiscal years ended December 31, 2022 and August 31, 2021.
| Name and Principal Position | Year (1) | Salary ($) (2) | Bonus ($) (2)(3) | Stock Awards ($) (4) | Option Awards ($) (5) | All Other Compensation ($) (2)(6) | Total ($) | |||||||||||||||||||
| Nadav Kidron | 2017 | 399,804 | 123,000 | - | 585,150 | 45,579 | 1,153,533 | |||||||||||||||||||
| President and CEO and | 2016 | 273,086 | 195,729 | - | - | 17,366 | 486,181 | |||||||||||||||||||
| director (7) | 2015 | 254,318 | 63,045 | 431,645 | - | 16,217 | 765,225 | |||||||||||||||||||
| Miriam Kidron | 2017 | 254,765 | 50,000 | 581,932 | 359,224 | 12,775 | 1,258,696 | |||||||||||||||||||
| Chief Scientific Officer | 2016 | 203,378 | 136,583 | - | - | 13,191 | 353,152 | |||||||||||||||||||
| and director (8) | 2015 | 188,466 | 50,436 | 431,645 | - | 13,592 | 684,139 | |||||||||||||||||||
| Joshua Hexter | 2017 | 148,499 | 33,000 | 463,400 | - | 46,408 | 691,307 | |||||||||||||||||||
| COO and VP Business | 2016 | 132,306 | 86,974 | - | - | 42,014 | 261,294 | |||||||||||||||||||
| Development | 2015 | 124,108 | 32,363 | - | - | 39,547 | 196,018 | |||||||||||||||||||
| Name and Principal Position | Year (1) | Salary ($) (2) | Bonus ($) (2)(3) | RSUs Awards ($) (4) | Option Awards ($) (4)(5) | All Other Compensation ($) (2)(6) | Total ($) | |||||||||||||||||||||
| Nadav Kidron | 2022 | 491,131 | 275,150 | 4,847,380 | 875,241 | 344,718 | 6,833,620 | |||||||||||||||||||||
| President, Chief Executive Officer and chairman(7) | Transition Period | 183,543 | 565,634 | - | - | 117,294 | 866,471 | |||||||||||||||||||||
| 2021 | 465,982 | 300,000 | 1,995,666 | 876,693 | 382,240 | 4,020,581 | ||||||||||||||||||||||
| Dr. Miriam Kidron | 2022 | 378,569 | 140,231 | 1,938,580 | 588,947 | 23,879 | 3,070,206 | |||||||||||||||||||||
| Chief Scientific Officer and director(8) | Transition Period | 134,505 | 285,273 | - | - | 5,327 | 425,105 | |||||||||||||||||||||
| 2021 | 319,868 | 86,000 | 1,330,451 | 584,462 | 14,193 | 2,334,974 | ||||||||||||||||||||||
| David Silberman | 2022 | 155,125 | 49,732 | 759,405 | 261,754 | 43,184 | 1,269,200 | |||||||||||||||||||||
| Chief Financial Officer(9) | Transition Period | 60,388 | 41,759 | 661,654 | 573,744 | 9,546 | 1,347,091 | |||||||||||||||||||||
| 2021 | 27,762 | - | - | - | 4,376 | 32,138 | ||||||||||||||||||||||
| (1) | The information is provided for |
| (2) | Amounts paid for Salary, Bonus and All Other Compensation that were originally denominated in NIS |
| (3) | Bonuses were granted at the discretion of the Compensation Committee. |
| (4) | For RSU awards, the amounts reflect the grant date fair value, as calculated pursuant to FASB ASC Topic 718. The assumptions used to determine the fair value of the RSU awards are set forth in |
| (5) | The amounts reflect the grant date fair value, as calculated pursuant to FASB ASC Topic 718, of these option awards. The assumptions used to determine the fair value of the option awards are set forth in |
| (6) | Amounts exclude the fair market value of the options that were re-granted on September 11, 2019, as it was offset by the negative amount created by the cancelled options (that is, it was accounted for as a modification under FASB ASC Topic 718, and no incremental compensation expense was recorded). For more information about the regrant see note 7a to our audited consolidated financial statements included in the Annual Report. |
| (6) | See “All Other Compensation Table” below. |
| (7) | Until November 1, 2022, Mr. Kidron |
| (8) | Dr. Kidron receives compensation from Oramed Ltd. through KNRY. See “—Employment and Consulting Agreements” below. |
| (9) | Mr. Silberman was appointed as Chief Financial Officer, effective July 5, 2021. |
All Other Compensation Table
The “All Other Compensation” amounts set forth in the Summary Compensation Table above consist of the following:
| Name | Year | Automobile- Related Expenses ($) | Manager’s Insurance* ($) | Education Fund* ($) | Business Travel** ($) | Total ($) | ||||||||||||||||
| Nadav Kidron | 2017 | 28,098 | - | - | 17,481 | 45,579 | ||||||||||||||||
| 2016 | 17,366 | - | - | - | 17,366 | |||||||||||||||||
| 2015 | 16,217 | - | - | - | 16,217 | |||||||||||||||||
| Miriam Kidron | 2017 | 12,775 | - | - | - | 12,775 | ||||||||||||||||
| 2016 | 13,191 | - | - | - | 13,191 | |||||||||||||||||
| 2015 | 13,592 | - | - | - | 13,592 | |||||||||||||||||
| Joshua Hexter | 2017 | 12,910 | 22,513 | 10,985 | - | 46,408 | ||||||||||||||||
| 2016 | 12,660 | 19,585 | 9,769 | - | 42,014 | |||||||||||||||||
| 2015 | 12,451 | 18,030 | 9,066 | - | 39,547 | |||||||||||||||||
| Name | Year (1) | Automobile- Related Expenses ($) | Manager’s Insurance (2)($) | Education Fund* ($) | Relocation Expenses (3)($) | Total ($) | ||||||||||||||||||
| Nadav Kidron | 2022 | 9,774 | 3,703 | 682 | 330,559 | 344,718 | ||||||||||||||||||
| Transition Period | 8,568 | - | - | 108,726 | 117,294 | |||||||||||||||||||
| 2021 | 4,926 | - | - | 377,314 | 382,240 | |||||||||||||||||||
| Dr. Miriam Kidron | 2022 | 23,879 | - | - | - | 23,879 | ||||||||||||||||||
| Transition Period | 5,327 | - | - | - | 5,327 | |||||||||||||||||||
| 2021 | 14,193 | - | - | - | 14,193 | |||||||||||||||||||
| David Silberman | 2022 | 16,095 | 21,835 | 5,254 | - | 43,184 | ||||||||||||||||||
| Transition Period | - | 7,677 | 1,869 | - | 9,546 | |||||||||||||||||||
| 2021 | - | 3,527 | 849 | - | 4,376 | |||||||||||||||||||
| (1) |
| (2) | Manager’s insurance and education funds are customary benefits provided to employees based in Israel. Manager’s insurance is a combination of severance savings (in accordance with Israeli law), defined contribution tax-qualified pension savings and disability insurance premiums. An education fund is a savings fund of pre-tax contributions to be used after a specified period of time for educational or other permitted purposes. |
| (3) | ||
Employment and Consulting Agreements
On July 1, 2008, Oramed Ltd. entered into a consulting agreement with KNRY, whereby Mr. Nadav Kidron, through KNRY, providesprovided services as President and Chief Executive Officer of both the Company and Oramed Ltd., or the Nadav Kidron Consulting Agreement. The Nadav Kidron Consulting Agreement was terminated, effective November 1, 2022, and replaced with the agreements as further described below. Additionally, on July 1, 2008, Oramed Ltd. entered into a consulting agreement with KNRY whereby Dr. Miriam Kidron, through KNRY, provides services as Chief Scientific Officer of both the Company and Oramed Ltd., or the Miriam Kidron Consulting Agreement. We refer to the
The Miriam Kidron Consulting Agreement and Nadav Kidron Consulting Agreement collectively as the Consulting Agreements.
The Consulting Agreements are bothis terminable by either party upon 60140 days prior written notice. The Consulting Agreements,agreement, as amended, provideprovides that KNRY will be reimbursed for reasonable expenses incurred in connection with performance of the Consulting Agreements and that Nadav Kidron receives a monthly consulting fee of NIS 127,570 and Miriam Kidron receives a monthly consulting fee of NIS 80,454.agreement. Pursuant to the Consulting Agreements,agreement, KNRY, Nadav Kidron andDr. Miriam Kidron each agreeagrees that during the term of the Consulting Agreementsagreement and for a 12 month12-month period thereafter, none of them will compete with Oramed Ltd. nor solicit employees of Oramed Ltd. Starting September 1, 2021, Dr. Miriam Kidron receives a monthly consulting fee of NIS 106,400.
We,The Nadav Kidron Consulting Agreement was terminable by either party upon 140 days prior written notice. The agreement, as amended, provided that KNRY will be reimbursed for reasonable expenses incurred in connection with performance of the agreement. Pursuant to the agreement, KNRY and Nadav Kidron each agreed that during the term of the agreement and for a 12-month period thereafter, none of them will compete with Oramed Ltd. nor solicit employees of Oramed Ltd. From September 1, 2021 until termination, Nadav Kidron received a monthly consulting fee of NIS 146,705.
Following the relocation of Nadav Kidron to the State of Israel, the Company entered into two agreements with Mr. Kidron, replacing the Nadav Kidron Consulting Agreement, substantially on the same terms, in order to allocate his time and services between the Company and Oramed Ltd.
Effective November 1, 2022, the Company entered into a consulting agreement with Shnida Ltd., whereby Nadav Kidron, through Shnida Ltd., provides services as President and Chief Executive Officer of the Company. The agreement is terminable by either party upon 140 days prior written notice. The agreement provides that Shnida Ltd. will be reimbursed for reasonable expenses incurred in connection with performance of the agreement and that Nadav Kidron will receive a monthly consulting fee of NIS 88,023. Pursuant to the agreement, Shnida Ltd. and Nadav Kidron each agree that during the term of the agreement and for a 12-month period thereafter, none of them will compete with the Company nor solicit employees of the Company.
In addition, we, through Oramed Ltd., have entered into an employment agreement with Joshua HexterNadav Kidron, effective as of April 14, 2013,November 1, 2022, pursuant to which Mr. Hexter was appointedKidron receives gross monthly salary of NIS 46,901 in consideration for his services as President and Chief OperatingExecutive Officer and VP Business Development of the Company and Oramed Ltd. In accordance with the employment agreement, as amended, Mr. Hexter’s current gross monthly salary is NIS 44,891. In addition, Mr. HexterKidron is provided with a cellular phone and a company car pursuant to the terms of his agreement.
We, through Oramed Ltd., have entered into a consulting agreement with Ronald Law as of March 1, 2017, pursuant to which Dr. Law was appointed as Chief Strategy Officer of the Company and Oramed Ltd., effective March 20, 2017. In accordance with the consulting agreement, Dr. Law’s current monthly consulting fee is $10,000. In addition, Dr. Law is entitled to a reimbursement of his communication expenses.
We, through Oramed Ltd., have entered into an employment agreement with Hilla EisenbergDavid Silberman as of July 20, 2017,May 23, 2021, pursuant to which Ms. EisenbergMr. Silberman was appointed as Chief Financial Officer, Treasurer and Secretary of the Company and Oramed Ltd., effective August 1, 2017.July 5, 2021. Mr. Silberman resigned as Secretary on January 9, 2022, upon the appointment of Mr. Netanel Derovan as Chief Legal Officer and Secretary. In accordance with the employment agreement, Ms. Eisenberg’sas amended, Mr. Silberman’s current gross monthly salary is NIS 34,000.47,438, effective January 1, 2023. In addition, Ms. EisenbergMr. Silberman is provided with a cellular phone and travel reimbursementa company car allowance pursuant to the terms of herhis agreement.
We have entered into indemnification agreements with our directors and officers pursuant to which we agreed to indemnify each director and officer for any liability he or she may incur by reason of the fact that he or she serves as our director or officer, to the maximum extent permitted by law.
Potential Payments upon Termination or Change-in-Control
We have no plans or arrangements in respect of remuneration received or that may be received by our named executive officersNEOs to compensate such officers in the event of termination of employment (as a result of resignation, retirement, change-in- control) or a change of responsibilities following a change-in-control.
Pension, Retirement or Similar Benefit Plans
We have no arrangements or plans under which we provide pension, retirement or similar benefits for directors or executive officers. Our directors and executive officers may receive stock options, RSUs or restricted shares at the discretion of our Compensation Committee in the future.
GRANTS OF PLAN-BASED AWARDS
The following table shows grants of plan-based equity awards made to our NEOs during fiscal 2017:
| Name | Grant Date | Options Awards: Number of Securities Underlying Options (#) | All Other Stock Awards: Number of Securities Underlying RSUs (#) | Grant Date Fair Value of Stock Awards ($) | ||||||||||
| Nadav Kidron(1) | 6/30/17 | 147,000 | - | 585,150 | ||||||||||
| Miriam Kidron(2) | 6/30/17 | 69,999 | - | 359,224 | ||||||||||
| Miriam Kidron(3) | 6/30/17 | - | 75,000 | 581,932 | ||||||||||
| Joshua Hexter(4) | 11/1/2017 | - | 70,000 | 463,400 | ||||||||||
|
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-ENDDECEMBER 31, 2022
The following table sets forth information concerning stock options and stock awards held by the NEOs as of AugustDecember 31, 2017.2022.
| Option Awards | Stock Awards | |||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price ($) | Option Expiration Date | Number of shares that have not vested (#) | Market value of shares that have not vested ($) | ||||||||||||||||
| Nadav Kidron | 72,000 | (1) | - | 6.48 | 5/7/18 | |||||||||||||||||
| 72,000 | (2) | - | 5.88 | 4/20/20 | ||||||||||||||||||
| 72,000 | (3) | - | 4.08 | 8/8/22 | ||||||||||||||||||
| 47,134 | (4) | - | 12.45 | 4/9/24 | ||||||||||||||||||
| - | 147,000 | (5) | 7.77 | 6/30/27 | ||||||||||||||||||
| - | (8)(9) | - | ||||||||||||||||||||
| Miriam Kidron | 72,000 | (1) | - | 6.48 | 5/7/18 | |||||||||||||||||
| 72,000 | (2) | - | 5.88 | 4/20/20 | ||||||||||||||||||
| 72,000 | (3) | - | 4.08 | 8/8/22 | ||||||||||||||||||
| 47,134 | (4) | - | 12.45 | 4/9/24 | ||||||||||||||||||
| 69,999 | (6) | 7.77 | 6/30/27 | |||||||||||||||||||
| - | (10) | - | ||||||||||||||||||||
| Joshua Hexter | 100,800 | (7) | - | 7.88 | 3/14/23 | |||||||||||||||||
| 29,000 | (11) | 250,850 | ||||||||||||||||||||
| Option Awards | Stock Awards | |||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price ($) | Option Expiration Date | Number of shares that have not vested (#) | Market value of shares that have not vested ($) | ||||||||||||||||||
| Nadav Kidron | 47,134 | (1) | - | 12.45 | 4/9/24 | |||||||||||||||||||
| 49,000 | (2) | - | 7.77 | 6/30/27 | ||||||||||||||||||||
| 97,000 | (3) | - | 8.14 | 1/31/28 | ||||||||||||||||||||
| 196,500 | (4)(5) | - | 3.16 | 2/26/29 | ||||||||||||||||||||
| 142,500 | (6) | 47,500 | (6) | 4.80 | 1/8/30 | |||||||||||||||||||
| 75,000 | (7) | 75,000 | (7) | 10.40 | 2/3/31 | |||||||||||||||||||
| - | 107,000 | (8) | 13.89 | 1/3/32 | ||||||||||||||||||||
| 58,063 | (9) | 58,064 | (9) | 3.91 | 9/17/32 | |||||||||||||||||||
| 452,000 | (10)(11)(12)(13)(14)(15) | 5,437,560 | ||||||||||||||||||||||
| Dr. Miriam Kidron | 47,134 | (1) | - | 12.45 | 4/9/24 | |||||||||||||||||||
| 69,999 | (16) | - | 7.77 | 6/30/27 | ||||||||||||||||||||
| 47,000 | (17) | - | 8.14 | 1/31/28 | ||||||||||||||||||||
| 104,000 | (18)(5) | - | 3.16 | 2/26/29 | ||||||||||||||||||||
| 75,000 | (19) | 25,000 | (19) | 4.80 | 1/8/30 | |||||||||||||||||||
| 50,000 | (20) | 50,000 | (20) | 10.40 | 2/3/31 | |||||||||||||||||||
| - | 72,000 | (21) | 13.89 | 1/3/32 | ||||||||||||||||||||
| 16,039 | (22) | 16,040 | (22) | 3.91 | 9/17/32 | |||||||||||||||||||
| 301,334 | (23)(24)(25)(26)(27) | 3,625,048 | ||||||||||||||||||||||
| David Silberman | 12,500 | (28) | 37,500 | (28) | 20.19 | 9/1/31 | ||||||||||||||||||
| - | 32,000 | (29) | 13.89 | 1/3/32 | ||||||||||||||||||||
| 4,747 | (30) | 4,748 | (30) | 3.91 | 9/17/32 | |||||||||||||||||||
| 127,500 | (31)(32)(33)(34) | 1,533,825 | ||||||||||||||||||||||
| (1) |
| On April 9, 2014, 47,134 options were granted to each of Nadav Kidron and Dr. Miriam Kidron under the 2008 Plan at an exercise price of $12.45 per share; 15,710 of such options vested on April 30, 2014 and the remainder vested in eight equal monthly installments, commencing on May 31, 2014. The options have an expiration date of April 9, 2024. |
| (2) | On June 30, 2017, 147,000 options were granted to Nadav Kidron under the 2008 Plan at an exercise price of $7.77 per share; |
| (3) | On |
| (4) | On |
| (5) | On September 11, 2019, these options were canceled and re-granted under the 2019 Incentive Plan in the same amounts and under the same terms as the original grants. |
| (6) | On January 8, 2020, 190,000 options were granted to Nadav Kidron under the 2019 Incentive Plan at an exercise price of $4.80 per share. 142,500 of the options vested in |
| (7) | On February 3, 2021, 150,000 options were granted to Nadav Kidron under the 2019 Incentive Plan at an exercise price of $10.40 per share. 75,000 of the options vested in two equal installments of 37,500 on each of December 31, 2021 and December 31, 2022, and the remainder shall vest in two equal installments of 37,500 on each of December 31, 2023 and December 31, 2024. The options expire on February 3, 2031. |
| (8) | On January 3, 2022, 107,000 options were granted to Nadav Kidron under the 2019 Incentive Plan at an exercise price of $13.89 per share. 107,000 of the options shall vest in four equal installments of 26,750 on each of January 1, 2023, January 1, 2024, January 1, 2025 and January 1, 2026. The options expire on January 3, 2032. |
| (9) | On September 18, 2022, 116,127 options were granted to Nadav Kidron under the Oravax Medical Inc. 2021 Long-Term Incentive Plan at an exercise price of $3.91 per share. 58,063 of the options vested in two installments on each of |
| (10) | On November 13, 2014, 9,788 RSUs, representing a right to receive shares of the Company’s common stock, were granted to Nadav Kidron. The RSUs vested in two equal installments, each of 4,894 shares, on November 30 and December 31, 2014. The shares of common stock underlying the RSUs will be issued upon request of the grantee. |
| (11) | On February 23, 2015, 79,848 RSUs, representing a right to receive shares of the Company’s common stock, were granted to Nadav Kidron. The RSUs vested in 23 installments consisting of one installment of 6,654 shares on February 28, 2015 and 22 equal monthly installments of 3,327 shares each, commencing March 31, 2015. The shares of common stock underlying the RSUs will be issued upon request of the grantee. |
| (12) |
| (13) | On January 3, 2022, 63,000 RSUs representing a right to receive shares of the Company’s common stock were granted to Nadav Kidron. 63,000 shall vest in four equal installments of 15,750 on each of January 1, 2023, January 1, 2024, January 1, 2025 and January 1, 2026. |
| (14) | On July 28, 2022, 126,000 RSUs representing a right to receive shares of the Company’s common stock were granted to Nadav Kidron. 126,000 shall vest in three equal installments of 42,000 on each of January 1, 2024, January 1, 2025 and January 1, 2026. |
| (15) | On July 28, 2022, 63,000 performance based RSUs representing a right to receive shares of the Company’s common stock were granted to Nadav Kidron. 42,000 were to vest upon receipt of positive topline data in the first oral insulin Phase 3 clinical trial and 21,000 were to vest upon completion of enrollment of the second oral insulin Phase 3 clinical trial by June 30,2023. Following the results of the ORA-D-013-1 Phase 3 trial and the termination of the ORA-D-013-2 Phase 3 trial, these performance goals have not been met and the RSUs did not vest. |
| (16) | On June 30, |
| (17) | On January 31, 2018, 47,000 options were granted to Dr. Miriam Kidron under the 2008 Plan at an exercise price of $8.14 per share; 47,000 of such options vested in four equal installments of 11,750 on each of January 1, 2019, January 1, 2020, January 1, 2021 and January 1, 2022. The options expire on January 31, 2028. |
| (18) | On February 26, 2019, 104,000 options were granted to Dr. Miriam Kidron under the 2008 Plan at an exercise price of $3.16 per share; 104,000 of such options vested in four equal installments of 26,000 on each of December 31, 2019, December 31, 2020, December 31, 2021 and December 31, 2022. The options expire on February 26, 2029. For additional information please see note 5 above. |
| (19) | On January 8, 2020, 100,000 options were granted to Dr. Miriam Kidron under the 2019 Incentive Plan at an exercise price of $4.80 per share. 75,000 of the options vested in three equal installments of 25,000 on each of December 31, 2020, December 31, 2021 and December 31, 2022 and the remaining 25,000 options shall vest on December 31, 2023. The options expire on January 8, 2030. |
| (20) | On February 3, 2021, 100,000 options were granted to Dr. Miriam Kidron under the 2019 Incentive Plan at an exercise price of $10.40 per share. 50,000 of such options vested in two equal installments of 25,000 on each of on each of December 31, 2021 and December 31, 2022 and the remaining 50,000 options shall vest in two equal installments of 25,000 on each of December 31, 2023 and December 31, 2024. The options expire on February 3, 2031. |
| (21) | On January 3, 2022, 72,000 options were granted to Dr. Miriam Kidron under the 2019 Incentive Plan at an exercise price of $13.89 per share. 72,000 of the options shall vest in four equal installments of 18,000 on each of January 1, 2023, January 1, 2024, January 1, 2025 and January 1, 2026. The options expire on January 3, 2032. |
| (22) | On September 18, 2022, 32,079 options were granted to Dr. Miriam Kidron under the Oravax Medical Inc. 2021 Long-Term Incentive Plan at an exercise price of $3.91 per share. 16,039 of the options vested in two installments on each of September 18, 2022 and December 31, 2022 and the remaining 16,040 options shall vest in two installments on each of December 31, 2023 and December 31, 2024. The options expire on September 17, 2032. |
| (23) | On June 30, 2017, 75,000 RSUs, representing a right to receive shares of the Company’s common stock, were granted to Dr. Miriam Kidron. The RSUs vested immediately, |
| (24) | On |
OPTIONS EXERCISED AND STOCK VESTED
certain business objectives. The following table sets forth information with respect to the NEOs concerning the vesting of RSUs during fiscal 2017. No options were exercised by the NEOs in fiscal 2017.
| Stock Awards | ||||||||
| Name | Number of Shares Acquired on Vesting (#) | Value Realized on Vesting ($) | ||||||
| Joshua Hexter | 41,000 | 298,430 | ||||||
| Nadav Kidron | 13,308 | (1) | 87,034 | (2) | ||||
| Miriam Kidron | 88,308 | (1) | 668,966 | (2) | ||||
| (25) |
Compensation Committee Interlocks and Insider Participation
During fiscal 2017, Mr. Aviad Friedman, Mr. Kevin Rakin and Mr. Leonard Sank served as the members of our Compensation Committee. None of the members of our Compensation Committee is, or has been, an officer or employee of ours.
| (26) | On July 28, 2022, 84,000 RSUs representing a right to receive shares of the Company’s common stock were granted to Dr. Miriam Kidron. 84,000 shall vest in three equal installments of 26,000 on each of January 1, 2024, January 1, 2025 and January 1, 2026. |
| (27) | On July 28, 2022, 42,000 performance based RSUs representing a right to receive shares of the Company’s common stock were granted to Dr. Miriam Kidron. 28,000 were to vest upon receipt of positive topline data in the first oral insulin Phase 3 clinical trial and 14,000 were to vest upon completion of enrollment of the second oral insulin Phase 3 clinical trial by June 30,2023. Following the results of the ORA-D-013-1 Phase 3 trial and the termination of the ORA-D-013-2 Phase 3 trial, these performance goals have not been met and the RSUs did not vest. |
During the last year, none of our NEOs served as: (1) a member of the compensation committee (or other committee of the Board performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers served on the compensation committee; (2) a director of another entity, one of whose executive officers served on the compensation committee; or (3) a member of the compensation committee (or other committee of the board of directors performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers served as a director on our Board.
| (28) | On September 1, 2021, 50,000 options were granted to David Silberman under the 2019 Incentive Plan at an exercise price of $20.19 per share. 12,500 options vested on June 27, 2022 and the remainder shall vest in three equal installments of 12,500 options on each of June 27, 2023, June 27, 2024 and June 27, 2025. The options expire on September 1, 2031. |
| (29) | On January 3, 2022, 32,000 options were granted to David Silberman under the 2019 Incentive Plan at an exercise price of $13.89 per share. 8,000 options vested on January 1, 2023 and the remainder shall vest in three equal installments of 8,000 on each of January 1, 2024, January 1, 2025 and January 1, 2026. The options expire on January 3, 2032. |
| (30) | On September 18, 2022, 9,495 options were granted to David Silberman under the Oravax Medical Inc. 2021 Long-Term Incentive Plan at an exercise price of $3.91 per share. 4,747 of the options vested in two installments on each of September 18, 2022 and December 31, 2022 and the remaining 4,748 options shall vest in two installments on each of December 31, 2023 and December 31, 2024. The options expire on September 17, 2032. |
| (31) | On September 1, 2021, 50,000 RSUs, representing a right to receive shares of the Company’s common stock, were granted to David Silberman. These RSUs vest as follows: (i) 33,333 shall vest upon our common stock achieving a price per share of $25 for at least 20 days out of any 30-day trading period and (a) if the first condition was met any time before June 27, 2022, then the RSUs would have vested in three equal installments (on June 27, 2022, June 27, 2023 and June 27, 2024), (b) if the first condition is met any time between June 27, 2022 and June 27, 2023, then 1/3 of the RSUs will vest immediately, and the remainder will vest in two equal installments (on June 27, 2023 and June 27, 2024), (c) if the first condition is met any time between June 27, 2023 and June 27, 2024, then 2/3 of the RSUs will vest immediately, and the remaining 1/3 will vest on June 27, 2024) and (d) if the first condition is met any time after June 27, 2024, then the RSUs will vest immediately; and (ii) 16,667 upon achievement of a certain licensing agreement as specified by the Board and (a) if the first condition was met any time before June 27, 2022, then the RSUs would have vested in three equal installments (on June 27, 2022, June 27, 2023 and June 27, 2024), (b) if the first condition is met any time between June 27, 2022 and June 27, 2023, then 1/3 of the RSUs will vest immediately, and the remainder will vest in two equal installments (on June 27, 2023 and June 27, 2024), (c) if the first condition is met any time between June 27, 2023 and June 27, 2024, then 2/3 of the RSUs will vest immediately, and the remaining 1/3 will vest on June 27, 2024) and (d) if the first condition is met any time after June 27, 2024, then the RSUs will vest immediately. |
| (32) | On January 3, 2022, 19,000 RSUs representing a right to receive shares of the Company’s common stock were granted to David Silberman. 19,000 shall vest in four equal installments of 4,750 on each of January 1, 2023, January 1, 2024, January 1, 2025 and January 1, 2026. |
| (33) | On July 28, 2022, 39,000 RSUs representing a right to receive shares of the Company’s common stock were granted to David Silberman. 30,000 shall vest in three equal installments of 13,000 on each of January 1, 2024, January 1, 2025 and January 1, 2026. |
| (34) | On July 28, 2022, 19,500 performance based RSUs representing a right to receive shares of the Company’s common stock were granted to David Silberman. 13,000 were to vest upon receipt of positive topline data in the first oral insulin Phase 3 clinical trial and 6,500 were to vest upon completion of enrollment of the second oral insulin Phase 3 clinical trial by June 30,2023. Following the results of the ORA-D-013-1 Phase 3 trial and the termination of the ORA-D-013-2 Phase 3 trial, these performance goals have not been met and the RSUs did not vest. |
DIRECTOR COMPENSATION
The following table provides information regarding compensation earned by, awarded or paid to each person for serving as a director who is not an executive officer during the fiscal 2017:year ended December 31, 2022:
| Name of Director | Fees Earned or Paid in Cash ($) | Stock Awards (2) ($) | Option Awards (3) ($) | All Other Compensation ($) | Total ($) | |||||||||||||||
| Nadav Kidron(1) | - | - | - | - | - | |||||||||||||||
| Miriam Kidron(1) | - | - | - | - | - | |||||||||||||||
| Leonard Sank | 20,000 | - | 86,076 | - | 106,076 | |||||||||||||||
| Xiaopeng Li | 20,000 | - | 153,909 | - | 173,909 | |||||||||||||||
| Aviad Friedman | 20,000 | - | 108,685 | - | 128,685 | |||||||||||||||
| Kevin Rakin | 20,000 | - | 316,406 | - | 336,406 | |||||||||||||||
| David Slager | 20,000 | - | 108,460 | - | 128,460 | |||||||||||||||
| Name of Director | Fees Earned or Paid in Cash ($) | Stock Awards (1)(2)($) | Option Awards (1)(2)($) | All Other Compensation ($) | Total ($) | |||||||||||||||
| Dr. Arie Mayer(4)(5) | 103,308 | 184,980 | 112,974 | - | 401,262 | |||||||||||||||
| Yadin Rozov | 30,094 | 124,770 | 24,061 | - | 178,925 | |||||||||||||||
| Leonard Sank | 39,188 | 184,980 | 81,798 | - | 305,966 | |||||||||||||||
| Aviad Friedman(3) | 15,000 | 83,340 | 81,798 | - | 180,138 | |||||||||||||||
| Kevin Rakin(3) | 34,688 | 83,340 | 81,798 | - | 199,826 | |||||||||||||||
(1) Please referThe following table provides information regarding compensation earned by, awarded or paid to each person for serving as a director who is not an executive officer during the Summary Compensation Table for executive compensation with respect to the named individual.Transition Period:
(2) As of August 31, 2017, our non-employee directors then in office held options and unvested RSUs to purchase shares of our common stock as follows:
| Name of Director | Fees Earned or Paid in Cash ($) | Stock Awards (2)($) | Option Awards (2)($) | All Other Compensation ($) | Total ($) | |||||||||||||||
| Aviad Friedman(3) | 8,344 | - | - | - | $ | 8,344 | ||||||||||||||
| Dr. Arie Mayer(4) | 70,561 | - | - | - | $ | 70,561 | ||||||||||||||
| Kevin Rakin(3) | 10,781 | - | - | - | $ | 10,781 | ||||||||||||||
| Leonard Sank | 7,875 | - | - | - | $ | 7,875 | ||||||||||||||
| As of |
| Name of Director | Aggregate Number of Shares Underlying Stock Awards | Aggregate Number of Shares Underlying Option Awards | ||||||
| Dr. Arie Mayer(5) | 18,000 | 45,398 | ||||||
| Yadin Rozov | 16,500 | 7,500 | ||||||
| Leonard Sank | 18,000 | 59,867 | ||||||
| (2) | The amounts reflect the grant date fair value, as calculated pursuant to FASB ASC Topic 718, of | |||
| The terms of each of Aviad Friedman and Kevin Rakin as directors expired on June 30, 2022. | ||||
| Includes $62,280 and $61,683 as remuneration for Dr. Mayer’s service as a member of the Board of Directors of Oravax during the Transition Period and during the year ended December 31, 2022, respectively. | ||||
| Includes 15,398 option awards granted by Oravax for Dr. Mayer’s service as a member of the Board of Directors of Oravax. | ||||
(3) The amounts reflect the grant date fair value, as calculated pursuant to FASB ASC Topic 718, of these option awards. The assumptions used to determine the fair value of the option awards are set forth in Note 8 to our audited consolidated financial statements included in this Annual Report on Form 10-K. Our directors will not realize the value of these awards in cash unless and until these awards are exercised and the underlying shares subsequently sold.
Our directors are entitled to reimbursement for reasonable travel and other out-of-pocket expenses incurred in connection with attendance at meetings of our Board. EachBased on a report provided to the Compensation Committee by Aon Consulting, Inc. during 2021, effective as of December 1, 2021, each independent director is entitled to receive as remuneration for his or her service as a member of the Board a sum equal to $20,000$30,000 per annum,annum. The Chairman of our Board is entitled to receive an additional sum equal to $25,500. The members of our Audit Committee are each entitled to receive an additional sum equal to $5,625. The members of our Compensation Committee are each entitled to receive an additional sum equal to $4,500. The members of our Nominating Committee are each entitled to receive an additional sum equal to $3,750. All remuneration is to be paid quarterly and shortly after the close of each quarter. Our executive officers did not receive additional compensation for service as directors. The Board may award special remuneration to any director undertaking any special services on behalf of us other than services ordinarily required of a director.
Other than as described above, we have no present formal plan for compensating our directors for their service in their capacity as directors. Other than indicated above, no director received and/or accrued any compensation for his services as a director, including committee participation and/or special assignments during fiscal 2017.the Transition Period and the year ended December 31, 2022.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Stock Option Plans
Our Board adopted the 2008 Plan and the 2019 Plan in order to attract and retain quality personnel.
The 2008 Plan, which is no longer utilized for new grants, provided for the grant of stock options, restricted stock, RSUs, and stock appreciation rights, collectively referred to as “awards.” Under the 2008 Plan, as amended, 2,400,000 shares were reserved for the grant of awards. As of December 31, 2022, options with respect to 2,287,989 shares had been granted, of which 275,673 had been forfeited, 308,804 had been exercised and 1,310,586 have expired. As of December 31, 2022, 525,824 RSUs had been granted, of which 164,636 have vested and the shares of common stock underlying those RSUs have not been issued and 34,118 have been forfeited.
The 2019 Plan provides for the grant of stock options, restricted stock, RSUs, and stock appreciation rights, collectively referred to as “awards.” Under the 2019 Plan, 1,000,000 shares were initially reserved for the grant of awards. On June 29, 2020, and August 3, 2020, respectively, our Board and stockholders approved to amend and restate the 2019 Plan, the principal change being an increase in the number of shares of common stock available under the 2019 Plan from 1,000,000 shares to 3,000,000 shares. On June 30, 2022, our Board and stockholders approved to amend and restate the 2019 Plan, the principal change being an increase in the number of shares of common stock available under the 2019 Plan from 3,000,000 shares to 7,500,000 shares. Stock options granted under the 20082019 Plan may be either incentive stock options under the provisions of Section 422 of the Internal Revenue Code, or non-qualified stock options. Under the 2008amended 2019 Plan, as amended, 2,400,0007,500,000 shares wereare reserved for the grant of awards, which may be issued at the discretion of our Board from time to time. The 2008 Plan permits awards to be based on performance-based criteria that will allow us to maximize its ability to pay deductible compensation for U.S. federal income tax purposes. As of AugustDecember 31, 2017,2022, options with respect to 1,870,8481,863,646 shares have been granted, of which 12,297 have100,918 had been forfeited, 119,224 have66,978 had been exercised and 475,292 havenone of them were expired. As of AugustDecember 31, 2017, 525,8242022, 1,881,600 RSUs havehad been granted, of which 294,300 have vested and the shares of common stock underlying RSUs were issued, 164,636100,666 have vested and the shares of common stock underlying those RSUs will behave not been issued upon requestand 85,334 have been forfeited. Since the Company had granted options during the time after the 2008 Plan allegedly terminated, and out of an abundance of caution, the Company canceled these grants and re-granted certain of the granteeoptions under 2019 Plan in the same amounts and 33,248 have been forfeited.under the same terms as the original grants.
The following table sets forth additional information with respect to our equity compensation plans (consisting solely of the 2008 Plan) as of AugustDecember 31, 2017:2022:
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weight- average exercise price of outstanding options, warrants and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | |||||||||
| Equity compensation plans approved by security holders | 1,001,041 | $ | 5.47 | 524,165 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 1,001,041 | $ | 5.47 | 524,165 | ||||||||
| Plan category | Number of securities to be issued upon exercise of outstanding options, RSUs and rights (a) | Weight- average exercise price of outstanding options, RSUs and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | |||||||||
| Equity compensation plans approved by security holders | 3,654,246 | $ | 4.79 | 4,106,898 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 3,654,246 | $ | 4.79 | 4,106,898 | ||||||||
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding the beneficial ownership of our common stock as of November 28, 2017March 6, 2023 by: (1) each person who is known by us to own beneficially more than 5% of our common stock; (2) each director;of our current directors; (3) each of our NEOs listed above under “Summary Compensation Table”;NEOs; and (4) all of our directors and executive officers as a group. On such date, we had 14,306,10039,783,813 shares of common stock outstanding.
As used in the table below and elsewhere in this form, the term “beneficial ownership” with respect to a security consists of sole or shared voting power, including the power to vote or direct the vote, and/or sole or shared investment power, including the power to dispose or direct the disposition, with respect to the security through any contract, arrangement, understanding, relationship or otherwise, including a right to acquire such power(s) during the next 60 days following November 28, 2017.March 6, 2023. Inclusion of shares in the table does not, however, constitute an admission that the named stockholder is a direct or indirect beneficial owner of those shares. Unless otherwise indicated, (1) each person or entity named in the table has sole voting power and investment power (or shares that power with that person’s spouse) with respect to all shares of common stock listed as owned by that person or entity and (2) the address of each of the individuals named below is: c/o Oramed Pharmaceuticals Inc., Hi-Tech Park 2/4 Givat Ram, PO Box 39098, Jerusalem 91390, Israel.
1185 Avenue of the Americas, Third Floor, New York, New York 10036.
| Name and Address of Beneficial Owner | Number of Shares | Percentage of Shares Beneficially Owned | ||||||
| Regals Fund LP | ||||||||
| 152 West 57th Street, 9th Floor | ||||||||
| New York, NY 10019 | 1,316,328 | (1) | 8.6 | % | ||||
| HTIT No. 199 Fanhua Road Economic and Technological Development Zone Heifei, Anhui Province, P.R. China, Zip Code: 230601 | 1,155,367 | (2) | 7.5 | % | ||||
| Nadav Kidron #+ | 2,631,999 | (3) | 17.3 | % | ||||
| Miriam Kidron #+ | 361,467 | (4) | 2.4 | % | ||||
| Joshua Hexter + | 147,800 | (5) | 1 | % | ||||
| Aviad Friedman # | 29,366 | (6) | * | |||||
| Xiaopeng Li # | 224,194 | (7) | * | |||||
| Kevin Rakin # | 36,349 | (8) | * | |||||
| Leonard Sank # | 574,861 | (9) | 3.8 | % | ||||
| David Slager # | 1,327,616 | (10) | 8.7 | % | ||||
| All current executive officers and directors, as a group (ten persons) | 5,151,949 | (11) | 33.6 | % | ||||
| Name and Address of Beneficial Owner | Number of Shares | Percentage of Shares Beneficially Owned | ||||||
| Nadav Kidron #+ | 1,735,966 | (1) | 4.3 | % | ||||
| Dr. Miriam Kidron #+ | 597,299 | (2) | 1.5 | % | ||||
| David Silberman+ | 25,250 | (3) | * | |||||
| Dr. Arie Mayer # | 24,000 | (4) | * | |||||
| Yadin Rozov # | - | * | ||||||
| Leonard Sank # | 64,563 | (5) | * | |||||
| All current executive officers and directors, as a group (nine persons) | 2,743,637 | (6) | 6.8 | % | ||||
| * | Less than 1% |
| # | ||
| + |
| (1) | Includes |
| (2) |
| Includes |
| (3) | Includes |
| (4) | Includes |
| (5) | Includes |
| (6) | Includes |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
During fiscal 2017the years ended December 31, 2022 and 2016,2021, except for compensation arrangements described elsewhere herein, we did not participate in any transaction, and we are not currently participating in any proposed transaction, or series of transactions, in which the amount involved exceeded the lesser of $120,000 or one percent of the average of our total assets at year end for the last two completed fiscal years, and in which, to our knowledge, any of our directors, officers, five percent beneficial security holders, or any member of the immediate family of the foregoing persons had, or will have, a direct or indirect material interest.
Our policy is to enter into transactions with related persons on terms that, on the whole, are no less favorable than those available from unaffiliated third parties. Based on our experience in the business sectors in which we operate and the terms of our transactions with unaffiliated third parties, we believe that all of the transactions described below met this policy standard at the time they occurred. All related person transactions are approved by our Board.
On November 30, 2015, we our Israeli subsidiary and HTIT entered into a Technology License Agreement,the TLA with HTIT, which was further amended according to which we grantedas the HTIT an exclusive commercialization license in the Territory related to our oral insulin capsule, ORMD-0801. Pursuant to this license agreement, HTIT will conduct certain pre-commercialization and regulatory activities with respect to our technology related to the ORMD-0801 capsule, and will pay certain royalties and an aggregateLicense Agreement. For further details, see “Item 1. Business—Description of approximately $37.5 million.Business— Out-Licensed Technology—HTIT.” On November 30, 2015, we also entered into a securities purchase agreementStock Purchase Agreement with HTIT, pursuant to which, among other things, Mr. Kidron willwould serve as proxy and attorney in fact of HTIT, with full power of substitution, to cast on behalf of HTIT all votes that HTIT is entitled to cast with respect to the Purchased Shares at any and all meetings of our shareholdersstockholders to consent or dissent to any action taken without a meeting and to vote all the Purchased Shares held by HTIT in any manner Mr. Kidron deemsdeemed appropriate except for matters related to our activities in the People’s Republic of China, on which Mr. Kidron willwould consult with HTIT before taking any action as proxy. On August 19, 2021, the proxy was revoked in accordance with its terms by HTIT.
The Board has determined that Dr. Arie Mayer, Yadin Rozov and Leonard Sank David Slager, Kevin Rakin, Aviad Friedman and Xiaopeng Li are independent as defined under the rules promulgated by Nasdaq.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
The aggregate fees billed by Kesselman & Kesselman, independent registered public accounting firm, and member firm of PricewaterhouseCoopers International Limited, for services rendered to us during fiscals 2017the fiscal year ended December 31, 2022, during the Transition Period and 2016:during the fiscal year ended August 31, 2021:
| 2017 | 2016 | |||||||
| Audit Fees(1) | $ | 109,000 | $ | 116,000 | ||||
| Audit-Related Fees | - | - | ||||||
| Tax Fees(2) | 4,000 | 32,000 | ||||||
| All Other Fees | - | - | ||||||
| Total Fees | $ | 113,000 | $ | 148,000 | ||||
| 2022 | Transition Period | 2021 | ||||||||||
| Audit Fees(1) | $ | 130,000 | $ | 39,000 | $ | 90,000 | ||||||
| Audit-Related Fees(2) | 45,000 | 42,000 | 47,500 | |||||||||
| Tax Fees(3) | 20,000 | - | 1,400 | |||||||||
| All Other Fees | - | - | - | |||||||||
| Total Fees | $ | 195,000 | $ | 81,000 | $ | 138,900 | ||||||
| (1) | Amount represents fees paid for professional services for the audit of our consolidated |
| (2) |
| (3) | Represents fees paid for tax consulting services. |
SEC rules require that before the independent registered public accounting firm are engaged by us to render any auditing or permitted non-audit related service, the engagement be: (1) pre-approved by our Audit Committee; or (2) entered into pursuant to pre-approval policies and procedures established by the Audit Committee, provided the policies and procedures are detailed as to the particular service, the Audit Committee is informed of each service, and such policies and procedures do not include delegation of the Audit Committee’s responsibilities to management.
The Audit Committee pre-approves all services provided by our independent registered public accounting firm. All of the above services and fees were reviewed and approved by the Audit Committee before the services were rendered.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
| (a) | Index to Financial Statements |
The following consolidated financial statements are filed as part of this Annual Report on Form 10-K:
| Page | ||
| REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM (PCAOB name: Kesselman & Kesselman C.P.A.s, PCAOB ID: 1309 and Auditor Location: Tel Aviv, Israel) | F-1 | |
| CONSOLIDATED FINANCIAL STATEMENTS: | ||
| Balance sheets | ||
| Statements of | ||
| Statements of changes in | ||
| Statements of cash flows | ||
| Notes to financial statements |
________________
__________________________
__________________________


REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Report of Independent Registered Public Accounting Firm
To the ShareholdersBoard of Directors and Stockholders of Oramed Pharmaceuticals Inc.
ORAMED PHARMACEUTICALS INC.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Oramed Pharmaceuticals Inc. and its subsidiarysubsidiaries (the “Company”) as of August 31, 2017December 2022 and 2016,2021, and the related consolidated statements of comprehensive loss, changes in stockholders’ equity and cash flows for eachthe year ended December 31, 2022, for the four-months ended December 2021 and for the year ended August 31,2021, including the related notes (collectively referred to as the” consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the three yearsCompany as of December 31, 2022 and 2021 and the results of its operations and its cash flows for the year ended December 31, 2022, for the four-months ended December 2021 and for the year ended August 31,2021, in conformity with accounting principles generally accepted in the period ended August 31, 2017. United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company'sCompany’s management. Our responsibility is to express an opinion on thesethe Company’s consolidated financial statements based on our audit.audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States).PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. Anmisstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit includesof its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence supportingregarding the amounts and disclosures in the consolidated financial statements. An auditOur audits also includes assessingincluded evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statement presentation.statements. We believe that our audit providesaudits provide a reasonable basis for our opinion.
In our opinion,Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the consolidated financial statements referredthat were communicated or required to above present fairly, in allbe communicated to the audit committee and that (i) relate to accounts or disclosures that are material respects,to the consolidated financial position ofOramed Pharmaceuticals Inc.statements and its subsidiary as ofAugust 31, 2017 and 2016, and the results of itsoperationsand itscash flows for each of the three yearsin the periodended August 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
(ii) involved our especially challenging, subjective, or complex judgments. We determined there are no critical audit matters.
/s/ Kesselman & Kesselman
Certified Public Accountants (Isr.)
A member firm of PricewaterhouseCoopers International Limited
Tel Aviv, Israel
March 6, 2023
We have served as the Company’s auditor since 2008.
Kesselman & Kesselman, Trade Tower, 25 Hamered Street, Tel-Aviv 6812508, Israel,
P.O Box 50005 Tel-Aviv 6150001 Telephone: +972 -3- 7954555, Fax:+972 -3- 7954556, www.pwc.com/il
ORAMED PHARMACEUTICALS INC.
CONSOLIDATED BALANCE SHEETS
U.S. Dollars inIn thousands (except share and per share data)
| August 31, | ||||||||
| 2017 | 2016 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 3,969 | $ | 3,907 | ||||
| Short-term deposits (note 2) | 13,293 | 24,254 | ||||||
| Marketable securities (note 3) | 2,860 | 2,855 | ||||||
| Restricted cash | 16 | 16 | ||||||
| Prepaid expenses and other current assets | 159 | 198 | ||||||
| Total current assets | 20,297 | 31,230 | ||||||
| LONG-TERM ASSETS: | ||||||||
| Long-term deposits and investment (note 4) | 16,232 | 11,043 | ||||||
| Marketable securities (note 3c) | 2,151 | 530 | ||||||
| Amounts funded in respect of employee rights upon retirement | 14 | 11 | ||||||
| Property and equipment, net | 18 | 16 | ||||||
| Total long-term assets | 18,415 | 11,600 | ||||||
| Total assets | $ | 38,712 | $ | 42,830 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable and accrued expenses | $ | 2,716 | $ | 1,411 | ||||
| Deferred revenues | 2,449 | 2,162 | ||||||
| Related parties (note 11c) | - | 48 | ||||||
| Total current liabilities | 5,165 | 3,621 | ||||||
| LONG-TERM LIABILITIES: | ||||||||
| Deferred revenues | 13,837 | 12,604 | ||||||
| Employee rights upon retirement | 18 | 14 | ||||||
| Provision for uncertain tax position (note 10e) | 11 | 11 | ||||||
| Other liabilities | 443 | 390 | ||||||
| Total long-term liabilities | 14,309 | 13,019 | ||||||
| COMMITMENTS (note 6) | ||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||
| Common stock, $ 0.012 par value (30,000,000 authorized shares as of August 31, 2017 and 2016; 13,668,530 and 13,183,425 shares issued and outstanding as of August 31, 2017 and 2016, respectively) | 163 | 157 | ||||||
| Additional paid-in capital | 75,170 | 71,943 | ||||||
| Accumulated other comprehensive income | 401 | 106 | ||||||
| Accumulated loss | (56,496 | ) | (46,016 | ) | ||||
| Total stockholders’ equity | 19,238 | 26,190 | ||||||
| Total liabilities and stockholders’ equity | $ | 38,712 | $ | 42,830 | ||||
| December 31, | ||||||||
| 2022 | 2021 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 40,464 | $ | 27,456 | ||||
| Short-term deposits (note 2) | 111,513 | 111,077 | ||||||
| Marketable securities (note 3) | 3,743 | 7,747 | ||||||
| Prepaid expenses and other current assets | 1,389 | 1,657 | ||||||
| Total current assets | 157,109 | 147,937 | ||||||
| LONG-TERM ASSETS: | ||||||||
| Long-term deposits (note 4) | 7 | 25,094 | ||||||
| Marketable securities (note 3) | - | 3,875 | ||||||
| Long-term investment (note 6j) | 2,700 | - | ||||||
| Amounts funded in respect of employee rights upon retirement | 24 | 26 | ||||||
| Property and equipment, net | 815 | 388 | ||||||
| Operating lease right of use assets | 987 | 500 | ||||||
| Total long-term assets | 4,533 | 29,883 | ||||||
| Total assets | $ | 161,642 | $ | 177,820 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable and accrued expenses (note 5) | $ | 4,158 | $ | 4,535 | ||||
| Deferred revenues | 1,340 | 2,703 | ||||||
| Payable to related parties (note 11b) | 1 | - | ||||||
| Operating lease liabilities | 247 | 130 | ||||||
| Total current liabilities | 5,746 | 7,368 | ||||||
| LONG-TERM LIABILITIES: | ||||||||
| Long-term deferred revenues | 4,000 | 3,340 | ||||||
| Employee rights upon retirement | 21 | 22 | ||||||
| Provision for uncertain tax position (note 10f) | 11 | 11 | ||||||
| Operating lease liabilities | 647 | 370 | ||||||
| Other liabilities | 61 | 99 | ||||||
| Total long-term liabilities | 4,740 | 3,842 | ||||||
| COMMITMENTS (note 6) | ||||||||
| EQUITY | ||||||||
| EQUITY ATTRIBUTABLE TO COMPANY’S STOCKHOLDERS’: | ||||||||
| Common stock, $ 0.012 par value (60,000,000 authorized shares as of December 31, 2022 and December 31, 2021; 39,563,888 and 38,158,792 shares issued and outstanding as of December 31, 2022 and December 31, 2021, respectively) | 476 | 459 | ||||||
| Additional paid-in capital | 314,417 | 292,514 | ||||||
| Accumulated deficit | (163,081 | ) | (126,520 | ) | ||||
| Total stockholders’ equity | 151,812 | 166,453 | ||||||
| Non-controlling interests | (656 | ) | 157 | |||||
| Total equity | 151,156 | 166,610 | ||||||
| Total liabilities and equity | $ | 161,642 | $ | 177,820 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
ORAMED PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
U.S. Dollars inIn thousands (except share and per share data)
| Year ended August 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| REVENUES | $ | 2,456 | $ | 641 | $ | - | ||||||
| COST OF REVENUES(notes 6j, 6k) | 187 | 490 | - | |||||||||
| RESEARCH AND DEVELOPMENT EXPENSES, NET | 10,281 | 7,709 | 4,781 | |||||||||
| GENERAL AND ADMINISTRATIVE EXPENSES | 2,759 | 2,452 | 2,602 | |||||||||
| OPERATING LOSS | 10,771 | 10,010 | 7,383 | |||||||||
| FINANCIAL INCOME(note 9a) | 792 | 474 | 168 | |||||||||
| FINANCIAL EXPENSES (note 9b) | 101 | 93 | 18 | |||||||||
| LOSS BEFORE TAXES ON INCOME | 10,080 | 9,629 | 7,233 | |||||||||
| TAXES ON INCOME (TAX BENEFIT) (note 10c) | 400 | 1,335 | (1 | ) | ||||||||
| NET LOSS FOR THE YEAR | $ | 10,480 | $ | 10,964 | $ | 7,232 | ||||||
| UNREALIZED LOSS (GAIN) ON AVAILABLE FOR SALE SECURITIES | (295 | ) | 452 | (106 | ) | |||||||
| TOTAL OTHER COMPREHENSIVE LOSS (INCOME) | (295 | ) | 452 | (106 | ) | |||||||
| TOTAL COMPREHENSIVE LOSS FOR THE PERIOD | $ | 10,185 | $ | 11,416 | $ | 7,126 | ||||||
| LOSS PER SHARE OF COMMON STOCK: | ||||||||||||
| BASIC AND DILUTED LOSS PER SHARE OF COMMON STOCK | $ | 0.79 | $ | 0.87 | $ | 0.67 | ||||||
| WEIGHTED AVERAGE NUMBER OF SHARES OF COMMON STOCK USED IN COMPUTING BASIC AND DILUTED LOSS PER SHARE OF COMMON STOCK | 13,309,372 | 12,624,356 | 10,820,465 | |||||||||
| Year ended December 31, | Four months ended December 31, | Year ended August 31, | ||||||||||
| 2022 | 2021 | 2021 | ||||||||||
| REVENUES | $ | 2,703 | $ | 904 | $ | 2,703 | ||||||
| RESEARCH AND DEVELOPMENT EXPENSES | 27,639 | 9,037 | 20,989 | |||||||||
| SALES AND MARKETING | 1,851 | 898 | - | |||||||||
| GENERAL AND ADMINISTRATIVE EXPENSES | 13,811 | 3,295 | 5,937 | |||||||||
| OPERATING LOSS | 40,598 | 12,326 | 24,223 | |||||||||
| FINANCIAL INCOME (note 9a) | 3,754 | 158 | 1,242 | |||||||||
| FINANCIAL EXPENSES (note 9b) | 820 | 87 | 8 | |||||||||
| LOSS BEFORE TAX EXPENSES | 37,664 | 12,255 | 22,989 | |||||||||
| TAX EXPENSES | 100 | - | - | |||||||||
| NET LOSS | $ | 37,764 | $ | 12,255 | $ | 22,989 | ||||||
| NET LOSS ATTRIBUTABLE TO: | ||||||||||||
| COMPANY’S STOCKHOLDERS | 36,561 | 11,668 | 22,238 | |||||||||
| NON-CONTROLLING INTERESTS | 1,203 | 587 | 751 | |||||||||
| NET LOSS | $ | 37,764 | $ | 12,255 | $ | 22,989 | ||||||
| BASIC AND DILUTED LOSS PER SHARE OF COMMON STOCK | $ | 0.94 | $ | 0.31 | $ | 0.78 | ||||||
| WEIGHTED AVERAGE NUMBER OF SHARES OF COMMON STOCK USED IN COMPUTING BASIC AND DILUTED LOSS PER SHARE OF COMMON STOCK | 38,997,649 | 37,113,137 | 28,469,068 | |||||||||
The accompanying notes are an integral part of the consolidated financial statements.
ORAMED PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
U.S. Dollars in thousands (except share data)
| Additional | Accumulated other | Total | ||||||||||||||||||||||
| Common Stock | paid-in | comprehensive | Accumulated | stockholders’ | ||||||||||||||||||||
| Shares | $ | capital | income | loss | equity | |||||||||||||||||||
| In thousands | ||||||||||||||||||||||||
| BALANCE AS OF AUGUST 31, 2014 | 10,103 | $ | 121 | $ | 48,040 | $ | 452 | $ | (27,820 | ) | $ | 20,793 | ||||||||||||
| SHARES, OPTIONS AND WARRANTS ISSUED FOR CASH, NET | 1,411 | 17 | 9,696 | - | - | 9,713 | ||||||||||||||||||
| SHARES ISSUED FOR SERVICES | 15 | * | 93 | - | - | 93 | ||||||||||||||||||
| EXERCISE OF OPTIONS | 1 | * | 8 | - | - | 8 | ||||||||||||||||||
| STOCK-BASED COMPENSATION | 33 | * | 1,347 | - | - | 1,347 | ||||||||||||||||||
| OTHER COMPREHENSIVE INCOME | - | - | - | 106 | - | 106 | ||||||||||||||||||
| NET LOSS | - | - | - | - | (7,232 | ) | (7,232 | ) | ||||||||||||||||
| BALANCE AS OF AUGUST 31, 2015 | 11,563 | 138 | 59,184 | 558 | (35,052 | ) | 24,828 | |||||||||||||||||
| ISSUANCE OF COMMON STOCK, NET | 1,155 | 14 | 10,580 | - | - | 10,594 | ||||||||||||||||||
| SHARES ISSUED FOR SERVICES | 14 | * | 101 | - | - | 101 | ||||||||||||||||||
| EXERCISE OF WARRANTS AND OPTIONS | 350 | 4 | 1,445 | - | - | 1,449 | ||||||||||||||||||
| STOCK-BASED COMPENSATION | 101 | 1 | 633 | - | - | 634 | ||||||||||||||||||
| OTHER COMPREHENSIVE LOSS | - | - | - | (452 | ) | - | (452 | ) | ||||||||||||||||
| NET LOSS | - | - | - | - | (10,964 | ) | (10,964 | ) | ||||||||||||||||
| BALANCE AS OF AUGUST 31, 2016 | 13,183 | 157 | 71,943 | 106 | (46,016 | ) | 26,190 | |||||||||||||||||
| SHARES ISSUED FOR SERVICES | 10 | * | 72 | - | - | 72 | ||||||||||||||||||
| ISSUANCE OF COMMON STOCK, NET | 3 | * | 25 | - | - | 25 | ||||||||||||||||||
| EXERCISE OF WARRANTS AND OPTIONS | 313 | 4 | 1,557 | - | - | 1,561 | ||||||||||||||||||
| STOCK-BASED COMPENSATION | 159 | 2 | 1,573 | - | - | 1,575 | ||||||||||||||||||
| OTHER COMPREHENSIVE INCOME | - | - | - | 295 | - | 295 | ||||||||||||||||||
| NET LOSS | - | - | - | - | (10,480 | ) | (10,480 | ) | ||||||||||||||||
| BALANCE AS OF AUGUST 31, 2017 | 13,668 | $ | 163 | $ | 75,170 | $ | 401 | $ | (56,496 | ) | $ | 19,238 | ||||||||||||
| Attributable to Company’s Stockholders | ||||||||||||||||||||||||||||
| Additional | Total | Non- | ||||||||||||||||||||||||||
| Common Stock | paid-in | Accumulated | stockholders’ | controlling | Total | |||||||||||||||||||||||
| Shares | $ | capital | deficit | equity | interests | Equity | ||||||||||||||||||||||
| In thousands | ||||||||||||||||||||||||||||
| BALANCE AS OF JANUARY 1, 2022 | 38,158 | 459 | 292,514 | (126,520 | ) | 166,453 | 157 | 166,610 | ||||||||||||||||||||
| SHARES ISSUED FOR SERVICES | 3 | * | 22 | - | 22 | - | 22 | |||||||||||||||||||||
| ISSUANCE OF COMMON STOCK, NET | 1,213 | 15 | 11,485 | - | 11,500 | - | 11,500 | |||||||||||||||||||||
| EXERCISE OF WARRANTS AND OPTIONS | 39 | * | 62 | - | 62 | - | 62 | |||||||||||||||||||||
| STOCK-BASED COMPENSATION | 151 | 2 | 11,117 | - | 11,119 | - | 11,119 | |||||||||||||||||||||
| STOCK-BASED COMPENSATION OF SUBSIDIARY | - | - | - | - | - | 390 | 390 | |||||||||||||||||||||
| TAX WITHHOLDINGS RELATED TO STOCK-BASED COMPENSATION SETTLEMENTS | - | - | (783 | ) | - | (783 | ) | - | (783 | ) | ||||||||||||||||||
| NET LOSS | - | - | - | (36,561 | ) | (36,561 | ) | (1,203 | ) | (37,764 | ) | |||||||||||||||||
| BALANCE AS OF DECEMBER 31, 2022 | 39,564 | 476 | 314,417 | (163,081 | ) | 151,812 | (656 | ) | 151,156 | |||||||||||||||||||
* Represents an amount of less than $1.
| * | Represents an amount of less than $1. |
| Attributable to Company’s Stockholders | ||||||||||||||||||||||||||||
| Additional | Total | Non- | ||||||||||||||||||||||||||
| Common Stock | paid-in | Accumulated | stockholders’ | controlling | Total | |||||||||||||||||||||||
| Shares | $ | capital | deficit | equity | interests | Equity | ||||||||||||||||||||||
| In thousands | ||||||||||||||||||||||||||||
| BALANCE AS OF SEPTEMBER 1, 2021 | 35,293 | 424 | 230,201 | (114,852 | ) | 115,773 | 744 | 116,517 | ||||||||||||||||||||
| ISSUANCE OF COMMON STOCK, NET | 2,631 | 32 | 59,901 | - | 59,933 | - | 59,933 | |||||||||||||||||||||
| EXERCISE OF WARRANTS AND OPTIONS | 92 | 1 | 638 | - | 639 | - | 639 | |||||||||||||||||||||
| STOCK-BASED COMPENSATION | 142 | 2 | 1,774 | - | 1,776 | - | 1,776 | |||||||||||||||||||||
| NET LOSS | - | - | - | (11,668 | ) | (11,668 | ) | (587 | ) | (12,255 | ) | |||||||||||||||||
| BALANCE AS OF DECEMBER 31, 2021 | 38,158 | 459 | 292,514 | (126,520 | ) | 166,453 | 157 | 166,610 | ||||||||||||||||||||
| Attributable to Company’s Stockholders | ||||||||||||||||||||||||||||
| Additional | Total | Non- | ||||||||||||||||||||||||||
| Common Stock | paid-in | Accumulated | stockholders’ | controlling | Total | |||||||||||||||||||||||
| Shares | $ | capital | deficit | equity | interests | Equity | ||||||||||||||||||||||
| In thousands | ||||||||||||||||||||||||||||
| BALANCE AS OF SEPTEMBER 1, 2020 | 23,675 | 284 | 125,209 | (92,614 | ) | 32,879 | - | 32,879 | ||||||||||||||||||||
| ISSUANCE OF COMMON STOCK, NET | 8,467 | 102 | 79,881 | - | 79,983 | - | 79,983 | |||||||||||||||||||||
| EXERCISE OF WARRANTS AND OPTIONS | 3,151 | 38 | 21,371 | - | 21,409 | - | 21,409 | |||||||||||||||||||||
| STOCK-BASED COMPENSATION | - | - | 2,695 | - | 2,695 | - | 2,695 | |||||||||||||||||||||
| ASSET ACQUISITION TRANSACTION | - | - | 1,045 | - | 1,045 | 1,495 | 2,540 | |||||||||||||||||||||
| NET LOSS | - | - | - | (22,238 | ) | (22,238 | ) | (751 | ) | (22,989 | ) | |||||||||||||||||
| BALANCE AS OF AUGUST 31, 2021 | 35,293 | 424 | 230,201 | (114,852 | ) | 115,773 | 744 | 116,517 | ||||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statementsstatements.
ORAMED PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. Dollars inIn thousands
| Year ended August 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | (10,480 | ) | $ | (10,964 | ) | $ | (7,232 | ) | |||
| Adjustments required to reconcile net loss to net cash provided by (used in) operating activities: | ||||||||||||
| Depreciation | 5 | 4 | 4 | |||||||||
| Exchange differences and interest on deposits and held to maturity bonds | 124 | (163 | ) | (20 | ) | |||||||
| Stock-based compensation | 1,575 | 634 | 1,347 | |||||||||
| Shares issued for services | 72 | 101 | 93 | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Prepaid expenses, other current assets and related parties | 39 | (71 | ) | 345 | ||||||||
| Accounts payable, accrued expenses and related parties | 1,257 | 470 | 16 | |||||||||
| Deferred revenue | 1,520 | 14,266 | 500 | |||||||||
| Liability for employee rights upon retirement | 4 | 3 | 2 | |||||||||
| Provision for uncertain tax position | - | (15 | ) | (1 | ) | |||||||
| Other liabilities | 53 | 390 | - | |||||||||
| Total net cash provided by (used in) operating activities | (5,831 | ) | 4,655 | (4,946 | ) | |||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||||||
| Purchase of property and equipment | (7 | ) | (9 | ) | (1 | ) | ||||||
| Purchase of short-term deposits | (3,557 | ) | (7,010 | ) | (3,673 | ) | ||||||
| Purchase of long-term deposits | (17,230 | ) | (22,274 | ) | (17,452 | ) | ||||||
| Purchase of held to maturity securities | (3,869 | ) | (1,775 | ) | (1,885 | ) | ||||||
| Proceeds from sale of short-term deposits | 26,551 | 14,160 | 19,701 | |||||||||
| Proceeds from maturity of held to maturity securities | 2,417 | 900 | - | |||||||||
| Funds in respect of employee rights upon retirement | (3 | ) | (2 | ) | (2 | ) | ||||||
| Total net cash provided by (used in) investing activities | 4,302 | (16,010 | ) | (3,312 | ) | |||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||
| Proceeds from issuance of common stock, options and warrants - net of issuance costs | 25 | 10,594 | 9,713 | |||||||||
| Proceeds from exercise of warrants and options | 1,561 | 1,449 | 8 | |||||||||
| Total net cash provided by financing activities | 1,586 | 12,043 | 9,721 | |||||||||
| EFFECT OF EXCHANGE RATE CHANGES ON CASH | 5 | 6 | (12 | ) | ||||||||
| INCREASE IN CASH AND CASH EQUIVALENTS | 62 | 694 | 1,451 | |||||||||
| CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 3,907 | 3,213 | 1,762 | |||||||||
| CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 3,969 | $ | 3,907 | $ | 3,213 | ||||||
| SUPPLEMENTARY DISCLOSURE ON CASH FLOWS - | ||||||||||||
| Interest received | $ | 833 | $ | 256 | $ | 115 | ||||||
| Year ended December 31, | Four months ended December 31, | Year ended August 31, | ||||||||||
| 2022 | 2021 | 2021 | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | (37,764 | ) | $ | (12,255 | ) | $ | (22,989 | ) | |||
| Adjustments required to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation | 58 | 18 | 77 | |||||||||
| Gain from selling fixed assets | (13 | ) | - | - | ||||||||
| Non-cash expense for acquired In-Process Research & Development (“IPR&D”) | - | - | 1,040 | |||||||||
| Exchange differences and interest on deposits and held to maturity bonds | (1,550 | ) | (34 | ) | 187 | |||||||
| Changes in fair value of investments | 763 | 72 | (876 | ) | ||||||||
| Stock-based compensation | 11,509 | 1,776 | 2,695 | |||||||||
| Shares issued for services | 22 | - | - | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Prepaid expenses and other current assets | 268 | (460 | ) | (586 | ) | |||||||
| Accounts payable, accrued expenses and related parties | (376 | ) | 689 | 2,060 | ||||||||
| Net changes in operating lease | (93 | ) | - | - | ||||||||
| Deferred revenues | (703 | ) | (904 | ) | (2,703 | ) | ||||||
| Liability for employee rights upon retirement | (1 | ) | 1 | 3 | ||||||||
| Other liabilities | (38 | ) | (25 | ) | (89 | ) | ||||||
| Total net cash used in operating activities | (27,918 | ) | (11,122 | ) | (21,181 | ) | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||||||
| Purchase of property and equipment | (496 | ) | (9 | ) | (375 | ) | ||||||
| Proceeds from selling fixed assets | 24 | - | - | |||||||||
| Investment in short-term deposits | (151,700 | ) | (100,000 | ) | (18,460 | ) | ||||||
| Investment in long-term deposits | (5 | ) | - | (25,000 | ) | |||||||
| Proceeds from sale of mutual funds | - | - | 3,765 | |||||||||
| Purchase of held to maturity securities | - | - | (10,362 | ) | ||||||||
| Proceeds from redemption of short-term deposits | 178,200 | - | 18,460 | |||||||||
| Proceeds from maturity of held to maturity securities | 6,886 | 761 | 8,209 | |||||||||
| Long-term investments | (2,700 | ) | - | - | ||||||||
| Funds in respect of employee rights upon retirement | 2 | - | (1 | ) | ||||||||
| Total net cash provided by (used in) investing activities | 30,211 | (99,248 | ) | (23,764 | ) | |||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||
| Proceeds from issuance of common stock, net of issuance costs | 11,500 | 59,933 | 79,983 | |||||||||
| Proceeds from exercise of warrants and options | 62 | 639 | 21,409 | |||||||||
| Tax withholdings related to stock-based compensation settlements | (783 | ) | - | - | ||||||||
| Transaction with non-controlling interests | - | - | 1,500 | |||||||||
| Total net cash provided by financing activities | 10,779 | 60,572 | 102,892 | |||||||||
| EFFECT OF EXCHANGE RATE CHANGES ON CASH AND CASH EQUIVALENTS | (64 | ) | 9 | 2 | ||||||||
| INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | 13,008 | (49,789 | ) | 57,949 | ||||||||
| CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR | 27,456 | 77,245 | 19,296 | |||||||||
| CASH AND CASH EQUIVALENTS AT END OF YEAR | $ | 40,464 | $ | 27,456 | $ | 77,245 | ||||||
| (A) SUPPLEMENTARY DISCLOSURE ON CASH FLOWS: | ||||||||||||
| Taxes paid | $ | 100 | $ | - | $ | - | ||||||
| Interest received | $ | 1,844 | $ | 128 | $ | 563 | ||||||
| (B) SUPPLEMENTAL DISCLOSURE OF NON-CASH ACTIVITIES: | ||||||||||||
| Recognition of operating lease right of use assets and liabilities | 730 | - | 582 | |||||||||
| (C) ASSET ACQUISITION TRANSACTION (see note 12): | ||||||||||||
| IPR&D | - | - | 1,040 | |||||||||
| Transaction with non-controlling interests | - | - | 1,500 | |||||||||
| Additional paid in capital | - | - | (1,045 | ) | ||||||||
| Non-controlling interests | - | - | (1,495 | ) | ||||||||
The accompanying notes are an integral part of the consolidated financial statements
ORAMED PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. Dollars inIn thousands (except share and per share data)
NOTE 1 - SIGNIFICANT ACCOUNTING POLICIES:
| a. | General |
| 1) | Incorporation and operations |
Oramed Pharmaceuticals Inc. (collectively with its subsidiary,subsidiaries, the “Company”,“Company,” unless the context indicates otherwise), a Delaware corporation, was incorporated on April 12, 2002, under the laws of the State of Nevada. From incorporation until March 3, 2006, the Company was an exploration stage company engaged in the acquisition and exploration of mineral properties. 2002.
On February 17, 2006, the Company entered into an agreement with Hadasit Medical Services and Development Ltd. (“Hadasit”) to acquire the provisional patent related to an orally ingestible insulin capsule to be used for the treatment of individuals with diabetes.
On May 14, 2007, the Company incorporated a wholly-owned subsidiary in Israel, Oramed Ltd. (the “Subsidiary”), which is engaged in research and development.
On July 30, 2019, the Subsidiary incorporated a wholly-owned subsidiary in Hong Kong, Oramed HK Limited (the “Hong Kong Subsidiary”). As of December 31, 2022, the Hong Kong Subsidiary has no operations.
On March 11, 2011, the Company was reincorporated from the State of Nevada to the State of Delaware.
On November 30, 2015,18, 2021, the Company entered into a Technologylicense agreement (the “Oravax License AgreementAgreement”) with Hefei Tianhui Incubation of Technologies Co. Ltd.Oravax Medical Inc. (“HTIT”Oravax”) and on December 21, 2015, the parties entered into an Amendeda stockholders agreement (the “Stockholders Agreement”) with Akers Biosciences Inc. (“Akers”), Premas Biotech Pvt. Ltd. (“Premas”), Cutter Mill Capital LLC (“Cutter Mill”) and Restated Technology License Agreement, that was further amended by the parties on June 3, 2016 and July 24, 2016 (the “License Agreement”Run Ridge LLC (“Run Ridge”). According to the LicenseStockholders Agreement, the Company granted HTIT an exclusive commercialization license in the territoryOravax issued 1,890,000 shares of the People's Republic of China, Macau and Hong Kong (the “Territory”), related to the Company’s oral insulin capsule, ORMD-0801 (the "Product"). Pursuant to the License Agreement, HTIT will conduct, at its own expense, certain pre-commercialization and regulatory activities with respect to the Subsidiary’s technology and ORMD-0801 capsule, and will pay to the Subsidiary (i) royalties of 10% on net sales of the related commercialized products to be sold by HTIT in the Territory (“Royalties”), and (ii) an aggregate of $37,500, of which $3,000 was payable immediately, $8,000 will be paid subjectcapital stock to the Company, entering into certain agreements with certain third parties,representing 63% of the issued and $26,500 will be paid upon achievementoutstanding share capital of certain milestonesOravax, on a fully diluted basis, as of the date of issuance. Consequently, Oramed consolidates Oravax in its consolidated financial statements since that time.
On November 23, 2021, Oravax incorporated a wholly-owned subsidiary in Israel, Oravax Medical Ltd., which is engaged in research and conditions. Indevelopment. Effective January 1, 2022, Oravax transferred its rights and obligations under the eventOravax License Agreement to Oravax Medical Ltd.
On January 11, 2023, the Company announced that the Company doesORA-D-013-1 Phase 3 trial did not meet certain conditions,its primary and secondary endpoints. The Company evaluated this subsequent event and determined that it was non-adjusting to the Royalties rate may be reduced toconsolidated financial statements for the year ended December 31, 2022, as it was not known or expected as of that date. The Company has initiated a minimum of 8%. Following the final expirationcomprehensive analysis of the Company's patents coveringdata to understand if there is a path forward for its oral insulin candidate. Concurrently, the technology inCompany is examining its existing pipeline and has commenced an evaluation process of potential strategic opportunities, with the Territory in 2033,goal of enhancing value for the Royalties rate may be reduced, under certain circumstances, to 5%.Company’s stockholders. See note 13.
The royalty payment obligation shall apply during the period of time beginning upon the first commercial sale of the Product in the Territory, and will end upon the later of (i) the expiration of the last-to-expire licensed patents in the Territory; and (ii) 15 years after the first commercial sale of the Product in the Territory (the "Royalty Term").
The License Agreement shall remain in effect until the expiration of the Royalty Term. The License Agreement contains customary termination provisions.
Among others, the Company's involvement through the product submission date will include consultancy for the pre-commercialization activities in the Territory, as well as advisory services to HTIT on an ongoing basis.
| Change in Fiscal Year |
On February 28, 2022, the Company’s Board of Directors (the “Board of Directors”) approved a change of the Company’s fiscal year from the period beginning on September 1 and ending on August 31 to the period beginning on January 1 and ending on December 31. As a result, the Company filed a Transition Report on Form 10-Q with the Securities and Exchange Commission (the “SEC”) on March 30, 2022 that included financial information for the transition period from September 1, 2021 through December 31, 2021. Subsequent to that report, the Company’s fiscal year now begins on January 1 and ends on December 31. This Annual Report on Form 10-K is the Company’s first annual report presenting its new fiscal year, and reports financial results for the 12 month period ended December 31, 2022.
ORAMED PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
U.S. Dollars inIn thousands (except share and per share data)
NOTE 1 - SIGNIFICANT ACCOUNTING POLICIES(continued):
The initial payment of $3,000 was received in January 2016. Following the achievement of certain milestones, the second and third payments of $6,500 and $4,000, respectively, were received in July 2016 and the fourth milestone payment of $4,000 was received in October 2016.
In addition, on November 30, 2015, the Company entered into a Stock Purchase Agreement with HTIT (the “SPA”). According to the SPA, the Company issued 1,155,367 shares of common stock to HTIT for $12,000. The transaction closed on December 28, 2015.
The License Agreement and the SPA were considered a single arrangement with multiple deliverables. The Company allocated the total consideration of $49,500 between the License Agreement and the SPA according to their fair value, as follows: $10,617 was allocated to the issuance of common stock (less issuance expenses of $23), based on the quoted price of the Company's shares on the closing date of the SPA on December 28, 2015, and $38,883 was allocated to the License Agreement. Given the Company's continuing involvement through the expected product submission (June 2023), amounts received relating to the License Agreement are recognized over the period from which the Company is entitled to the respective payment, and the expected product submission date using a time-based model approach over the periods that the fees are earned.
In July 2015, according to the letter of intent signed between the parties or their affiliates, HTIT's affiliate paid the Subsidiary a non-refundable amount of $500 as a no-shop fee. The no-shop fee was deferred and the related revenue is recognized over the estimated term of the License Agreement.Amounts that were allocated to the License Agreement as of August 31, 2017 aggregated $19,383, all of which were received through the balance sheet date. Through August 31, 2017, the Company recognized revenue in the amount of $3,097, and deferred the remaining amount of $16,286.
The following table summarizes the activities for deferred revenues for the years ended August 31, 2017 and 2016:
| August 31, | |||||||||
| 2017 | 2016 | ||||||||
| Deferred revenue at the beginning of period | $ | 14,766 | $ | - | |||||
| Amounts received | 4,000 | 15,383 | |||||||
| Amounts the Company was entitled to | (24 | ) | 24 | ||||||
| Revenue recognized | (2,456 | ) | (641 | ) | |||||
| Deferred revenue at the end of period | 16,286 | 14,766 | |||||||
| Less – current deferred revenue portion | (2,449 | ) | (2,162 | ) | |||||
| Non-current deferred revenue portion | $ | 13,837 | $ | 12,604 | |||||
| Development and liquidity risks |
The Company is engaged in research and development in the biotechnology field for innovative pharmaceutical solutions, including an orally ingestible insulin capsule to be used for the treatment of individuals with diabetes, and the use of orally ingestible capsules for delivery of other polypeptides, and has not generated significant revenues from its operations. Continued operation ofBased on the Company’s current cash resources and commitments, the Company is contingent upon obtaining sufficient funding untilbelieves it becomes profitable.
will be able to maintain its current planned development activities and the corresponding level of expenditures for at least the next 12 months, although no assurance can be given that the Company will not need additional funds prior to such time. If there are unexpected increases in its operating expenses, the Company may need to seek additional financing during the next 12 months. Successful completion of the Company’s development programs and its transition to normal operations is dependent upon obtaining necessary regulatory approvals from the U.S. Food and Drug Administration prior to selling its products within the United States, obtaining foreign regulatory approvals to sell its products internationally, or entering into licensing agreements with third parties. There can be no assurance that the Company will receive regulatory approval of any of its product candidates, and a substantial amount of time may pass before the Company achieves a level of revenues adequate to support its operations, if at all. The Company also expects to incur substantial expenditures in connection with the regulatory approval process for each of its product candidates during their respective developmental periods. Obtaining marketing approval will be directly dependent on the Company’s ability to implement the necessary regulatory steps required to obtain marketing approval in the United States and in other countries. The Company may also need additional funds to realize the decisions made as part of its strategic review process. The Company cannot predict the outcome of these activities.
In addition to the foregoing, based on the Company’s current assessment, the Company does not expect any material impact on its development timeline and its liquidity due to COVID-19. However, the Company experienced approximately six months of delays in clinical trials due to slow-downs of recruitment for trials generally. The Company continues to assess the effect on its operations by monitoring the status of COVID-19.