UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
☒ANNUAL REPORT UNDERPURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For fiscal year ended: December 31, 20202023
ORor
☐TRANSITION REPORT UNDERPURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _______________ to _______________
Commission file number: 001-39336
Aditx Therapeutics, Inc.
Aditxt, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 82-3204328 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (650) 870-1200
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol(s) | Name of Each Exchange on Which Registered | ||
| Common Stock, par value $0.001 per share | ADTX | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| Emerging growth company | ☒ | ||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in this filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant on June 30, 2020,2023, based on a closing price of $5.06$18.00 was approximately $20,079,659.$3,008,286.
As of March 24, 2020,April 12, 2024, the registrant had 14,225,3011,665,265 and 14,124,4981,665,214 shares of common stock, $0.001 par value per share, issued and outstanding, respectively.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive proxy statement relating to its 20212023 annual meeting of stockholders (the “2021“2023 Proxy Statement”) are incorporated by reference into Part III of this Annual Report on Form 10-K where indicated. The 20212023 Proxy Statement will be filed with the Securities and Exchange Commission (the “SEC”) within 120 days after the end of the fiscal year to which this report relates.
ADITX THERAPEUTICS,ADITXT, INC.
ANNUAL REPORT ON FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 20202023
TABLE OF CONTENTS
i
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 under Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements include statements with respect to our beliefs, plans, objectives, goals, expectations, anticipations, assumptions, estimates, intentions and future performance, and involve known and unknown risks, uncertainties and other factors, which may be beyond our control, and which may cause our actual results, performance or achievements to be materially different from future results, performance or achievements expressed or implied by such forward-looking statements. All statements other than statements of historical fact are statements that could be forward-looking statements. You can identify these forward-looking statements through our use of words such as “may,” “can,” “anticipate,” “assume,” “should,” “indicate,” “would,” “believe,” “contemplate,” “expect,” “seek,” “estimate,” “continue,” “plan,” “point to,” “project,” “predict,” “could,” “intend,” “target,” “potential” and other similar words and expressions of the future. The matters discussed in these forward-looking statements are subject to risks, uncertainties and other factors that could cause our actual results to differ materially from those projected, anticipated or implied in the forward-looking statements. As a result, you should not place undue reliance on any forward-looking statements. Except to the limited extent required by applicable law, we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
ii
RISK FACTOR SUMMARY
Our business is subject to numerous risks and uncertainties, including those highlighted in Section 1A titled “Risk Factors,” that represent challenges that we face in connection with the successful implementation of our strategy. The occurrence of one or more of the events or circumstances described in the section titled “Risk Factors,” alone or in combination with other events or circumstances, may have an adverse effect on our business, cash flows, financial condition and results of operations. Such risks include, but are not limited to:
| ● | we have generated no significant revenue from commercial sales to date and our future profitability is uncertain; |
| ● | if we fail to obtain the capital necessary to fund our operations, we will be unable to continue or complete our product development and you will likely lose your entire investment; |
| ● | our financial situation creates doubt whether we will continue as a going concern; |
| ● | we may need to raise additional funding, which may not be available on acceptable terms, or at all; |
| ● | even if we can raise additional funding, we may be required to do so on terms that are dilutive to you.; |
| ● | the regulatory approval process is expensive, time-consuming and uncertain and may prevent us from obtaining approvals for the commercialization of our future product candidates, if any; |
| ● | we may encounter substantial delays in completing our clinical studies which in turn will require additional costs, or we may fail to demonstrate adequate safety and efficacy to the satisfaction of applicable regulatory authorities; |
| ● | if our future pre-clinical development and future clinical Phase I/II studies are unsuccessful, we may be unable to obtain regulatory approval of, or commercialize, our product candidates on a timely basis or at all; |
| ● | even if we receive regulatory approval for any of our product candidates, we may not be able to successfully commercialize the product and the revenue that we generate from their sales, if any, may be limited; |
| ● | adverse events involving our products may lead the FDA or applicable foreign regulatory agency to delay or deny clearance for our products or result in product recalls that could harm our reputation, business and financial results; |
| ● |
| ● | if we were to lose our CLIA certification or state laboratory licenses, whether as a result of a revocation, suspension or limitation, we would no longer be able to offer our assays (including our AditxtScore™ platform), which would limit our revenues and harm our business. If we were to lose, or fail to obtain, a license in any other state where we are required to hold a license, we would not be able to test specimens from those states; |
| ● | our results of operations will be affected by the level of royalty and milestone payments that we are required to pay to third parties; |
| ● | we face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do; |
iii
| ● | our technologies and products under development, and our business, may fail if we are not able to successfully commercialize them and ultimately generate significant revenues as a result; |
| ● | customers may not adopt our products quickly, or at all; |
| ● |
| the failure to obtain or maintain patents, licensing agreements and other intellectual property could materially impact our ability to compete effectively; |
| ● | some of our intellectual property may be subject to “march-in” rights by the U.S. federal government; |
| ● | we do not expect to pay dividends in the foreseeable future; |
| ● |
| ● | future sales or issuances of substantial amounts of our common stock, including, potentially as a result of future acquisitions or strategic transactions, including the transaction with Cellvera Global, could result in significant dilution; |
| ● | while we have entered into a Share Exchange Agreement with Cellvera Global, we cannot assure you that the transaction contemplated by the Share Exchange Agreement will be consummated or, that if such transaction is consummated, that it will be accretive to stockholder value; |
| ● | we may engage in future acquisitions or strategic transactions, including the transaction with Cellvera Global, which may require us to seek additional financing or financial commitments, increase our expenses and/or present significant distractions to our management; |
| ● | we received the determination from Nasdaq that we regained compliance with the Nasdaq continued listing requirements, however, we remain subject to a panel monitor of our ongoing compliance until December 29, 2024 and if we fail to comply with such requirements during the panel monitor, it could result in the delisting of our securities by Nasdaq; and |
| ● | exclusive forum provisions in our amended and restated certificate of incorporation and amended and restated bylaws. |
iiiiv
PART I
Item 1. Business.
Overview and Mission
We believe the world needs—and deserves—a new approach to innovating that harnesses the power of large groups of stakeholders who work together to ensure that the most promising innovations make it into the hands of people who need them most.
We were incorporated in the State of Delaware on September 28, 2017, and our headquarters are a biotech innovationin Richmond, Virginia. The company was founded with a mission of bringing stakeholders together, to transform promising innovations into products and services that could address some of the most challenging needs. The socialization of innovation through engaging stakeholders in every aspect of it, is key to transforming more innovations, more rapidly, and more efficiently.
At inception, the first innovation we took on was an immune modulation technology titled ADI/Adimune with a focus on prolonging life and enhancing itslife quality by improvingof patients that have undergone organ transplants. Since then, we expanded our portfolio of innovations, and we continue to evaluate a variety of promising health innovations.
Our Model
Aditxt is not about a single idea or a single molecule. It is about making sure the right innovation is made possible. Our business model has three main components as follows:
| (1) | Securing an Innovation: Our process begins with identifying and securing innovations through licensing or acquisition of an innovation asset. Assets come from a variety of sources including research institutions, government agencies, and private organizations. |
| (2) | Growing an Innovation: Once an innovation is secured, we surround it with activation resources that take a systemized approach to bringing that idea to life. Our activation resources include innovation, operations, commercialization, finance, content and engagement, personnel, and administration. |
| (3) | Monetizing an Innovation: Our goal is for each innovation to become commercial-stage and financially and operationally self-sustainable, to create shareholder value. |
We engage various stakeholders for each of our programs on every level. This includes identifying researchers and research institution partners, such as Stanford University; leading health institutions to get critical trials underway, such as Mayo Clinic; manufacturing partners who enable us to take innovations from preclinical to clinical; municipalities and governments, such as the city of Richmond and the state of Virginia and public health agencies who work with us to launch our program, Pearsanta’s laboratory; and thousands of shareholders around the globe. We seek to enable promising innovation to become purposeful products that have the power to change lives.
Our Value Proposition
We believe that far too often, promising treatment or technology does not reach commercialization due to lack of expertise, key resources, or efficiency. As a result, potentially life-changing and lifesaving treatments are not available to the individuals who so desperately need them.
Aditxt seeks to bring the holistic concept of an efficient, socialized ecosystem for advancing and accelerating innovations. Our process: We seek to license or acquire promising innovations. We will then form and build out a subsidiary around each innovation and support the subsidiaries through innovation, operation, commercialization, content and engagement, finance, personnel, and administration to thrive and grow as a successful, monetizable business.
Since our inception, we have built infrastructure consisting of innovation, operation, commercialization, content and engagement, finance, personnel, and administration, to support the rapid transformation of untapped innovations. Each of the immune system.main components of our infrastructure has established global access to partnerships with industry leaders, top-rated research and medical institutions, universities, manufacturing and distribution companies, and critical infrastructure such as CLIA-certified state-of-the art labs and GMP manufacturing.
The Shifting Landscape of Innovation
Innovation in general, and health innovations specifically, require significant resources. The convergence of biotech, high-tech, and media offers new possibilities of accelerating breakthrough innovations faster and more efficiently. This approach reflects our mission of “Making Promising Innovations Possible, Together”.
People deserve innovative solutions, which have never been more within reach. We believe the best idea, best product and the best solution will come from creating an ecosystem where all stakeholders, such as vendors, customers, municipalities, and shareholders contribute. When we disrupt the way we’re innovating, through our collaborative model, we believe we can move faster and more efficiently to activate viable solutions that have the potential to make a measurable impact.
Our Growth Strategy
We are developing biotechnologies specifically focused on improvingbelieve that the era of precision and personalized medicine is here and that people around the globe would benefit from health diagnostics and treatments that more accurately pinpoint the problems and more precisely treat the condition. In addition to our current programs, Adimune and Pearsanta, we look to bring in future health innovations in the areas of the immune system through immune reprogrammingsoftware and monitoring. Our immune reprogrammingAI, medical devices, therapeutics, and other technologies are currently at the pre-clinical stagethat take a fundamentally different approach to health because they prioritize personalized precision medicine, timely disease root cause analysis, and are designed to retrain the immune system to induce tolerance with an objective of addressing rejection of transplanted organs, autoimmune diseases, and allergies. Our immune monitoring technologies are designed to provide a personalized comprehensive profile of the immune system andtargeted treatments.
Year over year, we plan to utilize themcontinue building our infrastructure and securing more personalized and precision health innovations that align with our mission. These opportunities may come in different forms such as IP, an early-stage company, or a late-stage company. We will continue to scale our upcoming reprogrammingsystemized approach to the innovation process, making large-scale automation and enterprise systems available to our portfolio companies at every stage of their growth. Specifically, certain subsidiaries will need to grow through further M&A activities, operational infrastructure implementation, and development or acquisition of critical technologies.
Our Team
Aditxt is led by an entrepreneurial team with passion for transforming promising innovations into successful businesses. Our leadership come from a variety of different industries, with collective expertise in founding startup innovation companies, developing and marketing biopharmaceutical and diagnostic products, designing clinical trials, manufacturing, and management of private and public companies. We have deep experience in identifying and accessing promising health innovations and developing them into products and services with the ability to monitor subjects’scale. We understand the capital markets, both public and private, as well as M&A and facilitating complex IPOs.
The following are profiles of three subsidiaries we have formed, including the terms of the intellectual property licenses that have been sublicensed from Aditxt to help build each of the businesses.
THE ADITXT PROGRAMS
ADIMUNE, INC.
Formed in January 2023, Adimune™, Inc. (“Adimune”) is focused on leading our immune response before, duringmodulation therapeutic programs. Adimune’s proprietary immune modulation product Apoptotic DNA Immunotherapy™, or ADI-100™, utilizes a novel approach that mimics the way our bodies naturally induce tolerance to our own tissues. It includes two DNA molecules designed to deliver signals to induce tolerance. ADI-100 has been successfully tested in several preclinical models (e.g., skin grafting, psoriasis, type 1 diabetes, multiple sclerosis).
In May 2023, Adimune entered into a clinical trial agreement with Mayo Clinic to advance clinical studies targeting autoimmune diseases of the central nervous system (“CNS”) with the initial focus on the rare, but debilitating, autoimmune disease Stiff Person Syndrome (“SPS”). According to the National Organization of Rare Diseases, the exact incidence and after drug administration.prevalence of SPS is unknown; however, one estimate places the incidence at approximately one in one million individuals in the general population.
Pending approval by the International Review Board and U.S. Food and Drug Administration, a human trial for SPS is expected get underway in 2024 with enrollment of 10-15 patients, some of whom may also have type 1 diabetes. ADI-100 will initially be tested for safety and efficacy. ADI-100 is designed to tolerize against an antigen known as glutamic acid decarboxylase (“GAD”), which is implicated in type-1 diabetes, psoriasis, and in many autoimmune diseases of the CNS. IND-enabling work is also near completion in support of a Clinical Trial Application submission to the Paul Ehrlich Institute, the regulatory agency in Germany, to initiate clinical trials in psoriasis and type 1 diabetes.
Immune Reprogramming
Background
The discovery of immunosuppressive (anti-rejection and monoclonal) drugs over 40 years ago has made possible life-saving organ transplantation procedures and blocking of unwanted immune responses in autoimmune diseases. However, immune suppression leads to significant undesirable side effects, such as increased susceptibility to life-threatening infections and cancers, because it indiscriminately and broadly suppresses immune function throughout the body. While the use of these drugs has been justifiable because they prevent or delay organ rejection, their use for treatment of autoimmune diseases and allergies may not be acceptable because of the above-mentionedaforementioned side effects. Furthermore, often transplanted organs often ultimately fail despite the use of immune suppression, and about 40% of transplanted organs survive no more than 5five years.
New, focused therapeutic approaches are needed that modulate onlyThrough Aditxt, Adimune has the small portionright of immune cells that are involved in rejection of the transplanted organ, as this approach can be safer for patients than indiscriminate immune suppression. Such approaches are referred to as immune tolerance, and when therapeutically induced, may be safer for patients and also potentially allow long-term survival of transplanted tissues and organs.
In the late 1990s, academic research on these approaches was conducted at the Transplant Center at Loma Linda University in connection with a project that secured initial grant funding from the U.S. Department of Defense. The focus of that project was for skin grafting for burn victims. Twenty years of research at LLU and an affiliated incubator led to a series of discoveries that have been translated into a large patent portfolio of therapeutic approaches that may be applieduse to the modulation of the immune system in order to induce tolerance to self and transplanted organs.
We have an exclusive worldwide license from LLU for commercializing thisADI nucleic acid-based technology (which is currently at the pre-clinical stage), called Apoptotic DNA Immunotherapy™ (ADi™), which utilizes from Loma Linda University. ADI uses a novel approach that mimics the way our bodiesthe body naturally induceinduces tolerance to our own tissues (“therapeutically induced immune tolerance”). While immune suppression requires continuous administration to prevent rejection of a transplanted organ, induction of tolerance has the potential to retrain the immune system to accept the organ for longer periods of time. Thus, ADi™ADI may allow patients to live with transplanted organs with significantly reduced immune suppression. ADi™ADI is a technology platform which we believe can be engineered to address a wide variety of indications.
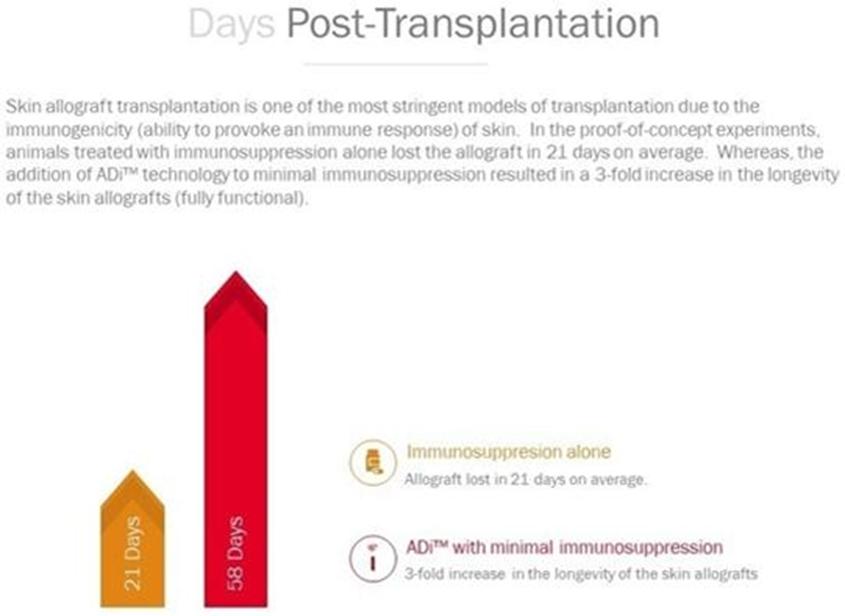
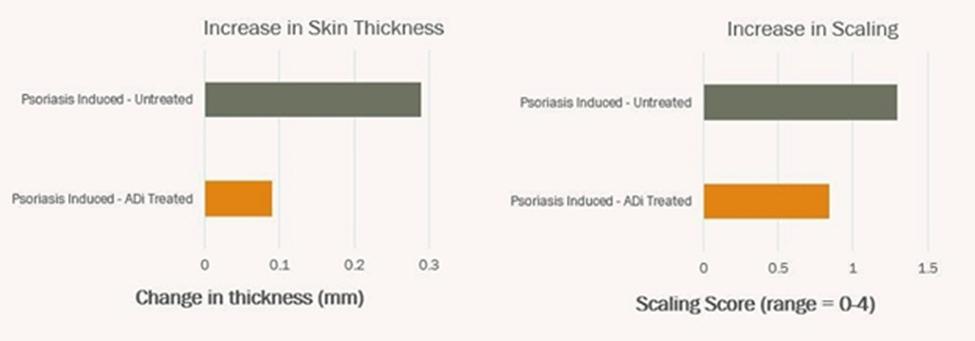
We are developing ADi™ products for organ transplantation including skin grafting, autoimmune diseases, and allergies, with the initial focus on skin allografts and psoriasis, as we believe these indications will be most efficient in providing safety and efficacy data in clinical trials. To submit a Biologics License Application (“BLA”) for a biopharmaceutical product, clinical safety and efficacy must be demonstrated in a series of clinical studies conducted with human subjects. For products in our class of drugs, the first-in-human trials will be a combination of Phase I (safety/tolerability) and Phase II (efficacy) in affected subjects. To obtain approval to initiate the Phase I/IIa studies, an Investigational New Drug Application will be submitted to compile non-clinical efficacy data as well as manufacturing and pre-clinical safety/toxicology data. To date, we have conducted non-clinical studies in a stringent model of skin transplantation using genetically mismatched donor and recipient animals demonstrating a 3-fold increase in the survival of the skin graft in animals that were tolerized with ADi™ compared to animals that receive immune suppression alone. Prolongation of graft life was observed despite discontinuation of immune suppression after the first 5 weeks. Additionally, in an induced non-clinical model for psoriasis, ADi™ treatment resulted in a 69% reduction in skin thickness and a 38% decrease in skin flaking (two clinical parameters for assessment of psoriasis skin lesions). The Phase I/IIa studies in psoriasis will evaluate the safety/tolerability of ADi™ in patients diagnosed with psoriasis. Since the drug will be administered in subjects diagnosed with psoriasis, effectiveness of the drug to improve psoriatic lesions will also be evaluated. In another Phase I/IIa study, patients requiring skin allografts will receive weekly intra-dermal injections of ADi™ in combination with standard immune suppression to assess safety/tolerability and possibility of reducing levels of immunosuppressive drugs as well as prolongation of graft life. Later phase trials are planned after successful completion of these studies in preparation for submission for a BLA to regulatory agencies.
Advantages
ADi™ Advantages
ADi™ADI™ is a nucleic acid-based technology (e.g., plasmid DNA-based), which we believe selectively suppresses only those immune cells involved in the rejection of tissueattacking or rejecting self and organ transplants.transplanted tissues and organs. It does so by tapping into the body’s natural process of cell death (apoptosis)turnover (i.e., apoptosis) to reprogramretrain the immune system to stop unwanted attacks on self or transplanted tissues. Apoptosis is a natural process of “immune tolerance” used by the body to clear dying cells and to allow recognition and tolerance to self-tissue. ADi™self-tissues. ADI triggers this process by enabling the naturalcells of the immune system cells to recognize the targeted tissues as “self”.“self.” Conceptually, it is designed to retrain the immune system to become accepting ofaccept the organtissues, similar to how natural apoptosis reminds our immune system to be tolerant to our own “self” tissues.
While efforts have been made by various groups to promotehave promoted tolerance through cell therapies and ex vivo manipulation of patient cells (takes(i.e., takes place outside the body typically requiring hospitalization)body), to our knowledge, we will be unique in our approach of using in-body induction of apoptosis to promote tolerance to specific tissues. In addition, ADi™ADI treatment itself will not require additional hospitalization but only an injection inof minute amounts of the therapeutic drug into the skin.
Reduce Chronic Rejection
While immunosuppressants control acute rejection during the early time-period after receiving an organ, chronic rejection of the organ that occurs one or more years after the transplant procedure continues to pose a major challenge for organ recipients.
Chronic rejection has been likened to autoimmunity (a misdirected immune response that occurs when the immune system goes awry), where specific tissues in the transplanted organ are attacked by the immune system. In other words, chronic rejection may not be caused just by differences between the donor and the recipient, but rather by an immune response by the recipient to specific tissues in the organ. Our pre-clinical studies suggest that ADi™ has the ability to tolerize to specific tissues in a transplanted organ, and conceivably, reducing incidences of chronic rejection.
Moreover, preclinical studies have demonstrated that ADi™ADI treatment significantly and substantially prolongs graft survival, in addition to successfully “reversing” other established immune-mediated inflammatory processes.
Reduce immune suppression
Studies in animal models have shown that conditioning/desensitizing the animals to receive the transplant, prolongs the survival of the transplanted tissue or organ. These studies have used repeated exposure to low doses of protein components in specific organs to reduce immunologic recognition and attack on the transplanted organ.
Based on some of our data, we believe that with ADi™ treatment, recipients can be conditioned/desensitized ahead of transplantation, thereby retraining the immune system to more readily accept the organ and also reduce the levels of immunosuppressive drugs needed post-transplantation.
Preformed Antibodies
Studies have shown that presence of preformed antibodies prior to transplantation procedures increases the rate of organ rejection. Preformed antibodies can develop in previously transplanted patients, patients who have given birth, and patients who have previously received blood transfusions. With more than 113,000 patients on transplant waiting lists in the U.S. alone, patients with pre-existing antibodies have much lower chances at qualifying to receive organs due to their increased risk of rejection – even with immune suppression.
Sadly, transplanted patients have a probability of needing re-transplantation at some point due to eventual chronic rejection of their transplanted organ, with the possible exception of some newborn recipients. With increased incidence of preformed antibodies, these patients may never have the opportunity to receive another organ. Based on experimental data, we believe that ADi™ may have the potential to address this issue providing these individuals better opportunities at receiving an organ transplantation.
ADi™ Key Differentiators
Ease of Delivery
Therapeutic products are typically administered systemically (i.e., by mouth in pill form or injected intramuscularly/intravenously). This requires repeated large doses of the drug to allow sufficient concentrations to reach the affected sites. ADi™ is a DNA-based product that can be injected directly into the skin where the target cells of the immune system reside, thereby significantly simplifying the delivery of the product and reducing the amount of product needed.
Repeat Dosing
DNA-based products are less likely to result in formation of neutralizing antibodies, which lend themselves to repeat dosing as may be required by ADi™ products.
Cost of Goods Advantage
ADi™ products are DNA-based and cost-effective to manufacture. Furthermore, DNA-based products are very stable and do not require adherence to cold chain (temperature-controlled) protocols for shipping. This also makes the product ideal for global distribution.
Simplified Therapy Delivery System
We believe that tolerance induction using ADi™ may potentially obviate the need for hospitalization because it can simply be injected into the skin. This approach reduces treatment costs and complexities in treatment delivery. The anticipated administration of ADi™ will include an initial priming regimen that will require injections administered once a week for several weeks. Thereafter, booster or maintenance doses will be provided on an individual basis as determined by immune and inflammation testing. ADi™ treatments will be significantly more convenient and comfortable for patients because they do not require removal of patient cells for ex vivo manipulation.
ADi™ Technology Platform
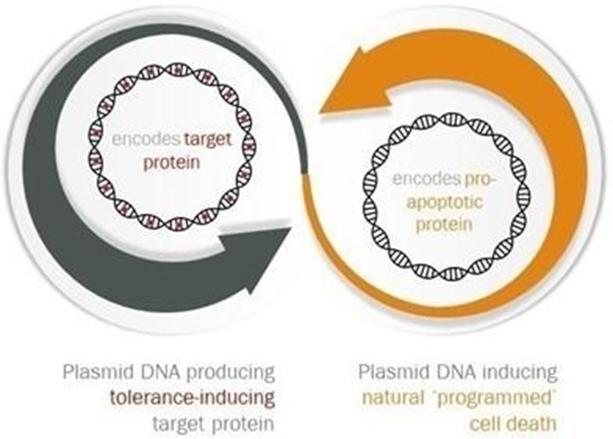
ADi™ utilizes a novel approach that mimics the way our bodies naturally induce tolerance to our own tissues. It is a technology platform which we believe can be engineered to address a wide variety of indications. ADi™ includes two DNA molecules which are designed to deliver signals to induce tolerance. The first DNA molecule encodes a pro-apoptotic protein, which induces ‘programmed’ cell death. This is a core component of the technology because it is intended to greatly increase the recruitment of dendritic cells, which are implicated in regulating the immune system. The second DNA molecule encodes the protein of interest (guiding antigen), which is modified to promote a path of tolerance. The guiding antigen is intended to result in tolerance induction specific to the tissue where the protein is found.
ADi™ has been successfully tested in several preclinical models (skin grafting, psoriaris, type 1 diabetes, alopecia areata) and its efficacy can be attributed to multiple factors:
Proof of Concept: Skin Grafting
Results shown are 5 weeks post-transplantation
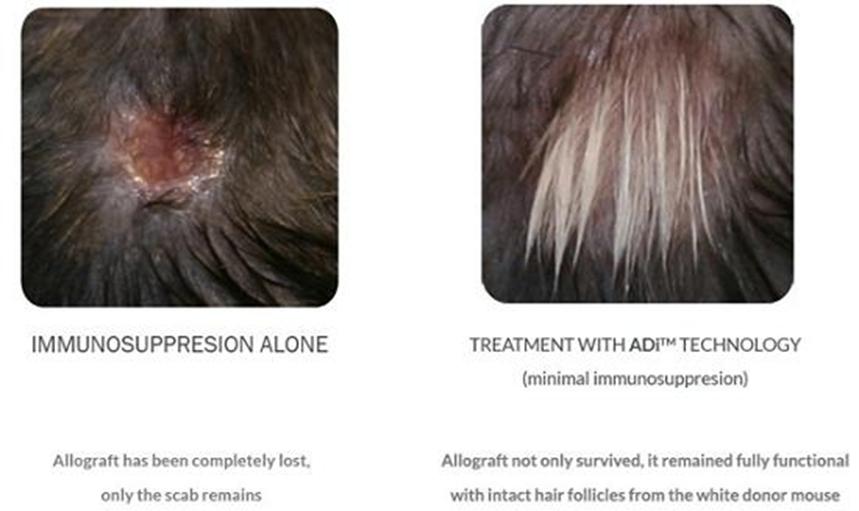
The proof of concept experiment performed in transplantation was a skin allograft transplantation procedure in which the donor skin was obtained from white BALB/c mice and transplanted to black C57BL/6 mice. The experiment was designed to address a more challenging scenario where the donor tissue was obtained from a donor which is genetically mismatched with the recipient. This is unlike clinical scenarios where the donor and recipient are genetically matched as much as possible. While these experiments were repeated in several separate experiments, the results shown here were obtained from a study conducted with 14 mice in the ADi™ treatment group and 7 mice in the control group. Prior to submission of an Investigational New Drug Application, additional non-clinical studies will be conducted in a pig model to establish the precise protocol (e.g. timing of vaccine administration, dosing, and appropriate immunosuppressive agents that will be used in combination with ADi™) that will be used in the clinical trials. In addition, IND-enabling safety/toxicology studies will be conducted by a GLP lab to ensure product safety for clinical testing.
Proof of Concept: Psoriasis
Proof of Concept: Type 1 Diabetes
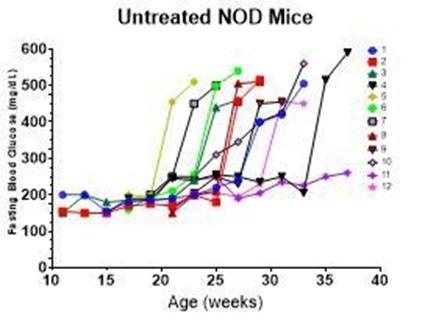
90% of female NOD mice developed spontaneous autoimmune diabetes. Disease progression may be different for individual animals.
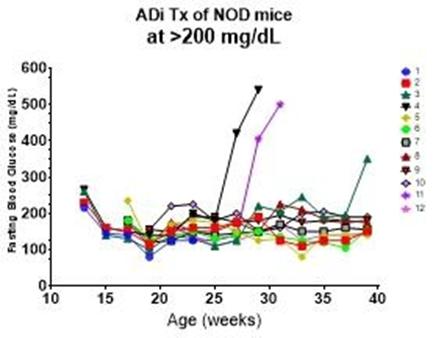
ADi™ was administered once a week for 8 weeks after each animal developed hyperglycemia. All animals responded with 80% showing durable response for the entire 40-week study period.
Proof of Concept: Alopecia Areata
ADi™ protects hair follicles from autoimmune attack
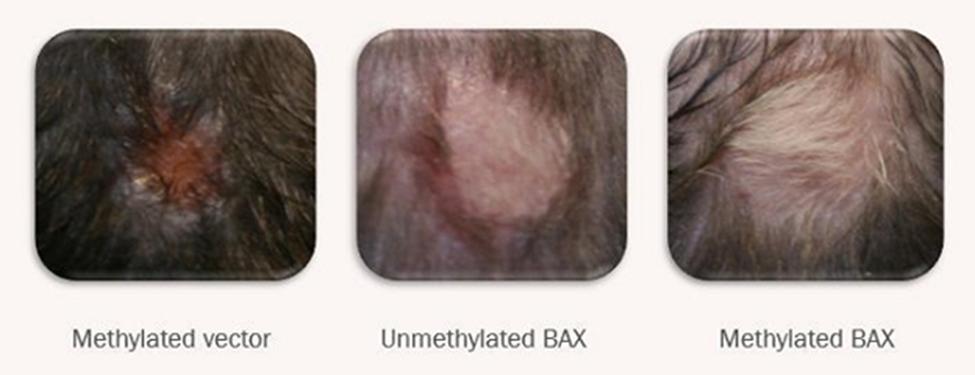
Immune Monitoring
We believe that understanding the status of an individual’s immune system is key to developing and administering immunotherapies such as ADi™. We have secured an exclusive worldwide license for commercializing a technology platform which provides a personalized comprehensive profile of the immune system. It is intended to be informative for individual immune responses to viruses, bacterial antigens, peptides, drugs, bone marrow and solid organ transplants, and cancer. It has broad applicability to many other agents of clinical interest impacting the immune system, including those not yet identified such as future infectious agents. We plan to brand this technology, and other future licensed and/or in-house developed monitoring technologies collectively as AditxtScore™.
AditxtScore™ is being designed to allow individuals to understand, manage and monitor their immune profiles in order to be informed about attacks on or by their immune system. We believe AditxtScore™ can also assist the medical community in anticipating possible immune responses and reactions to viruses, bacteria, allergens and transplanted organs. It can be useful in anticipating attacks on the body by having the ability to determine its potential response and for developing a plan to deal with an undesirable reaction by the immune system. Its advantages include the ability to provide a simple, rapid, accurate, high throughput, single platform assay that can be multiplexed to determine the immune status with respect to several factors simultaneously, in 3-16 hours, as well as detect antigen and antibody in a single test (i.e. infectious, recovered, immune). In addition, it can determine and differentiate between various types of cellular and humoral immune responses (T and B cells). It also provides for simultaneous monitoring of cell activation and levels of cytokine release (i.e., cytokine storms).
We plan to utilize AditxtScore™ in our upcoming clinical trials to monitor subjects’ immune response before, during and after ADi™ drug administration. We are working with regulatory consultants with the objective to obtain FDA approval for AditxtScore™ as a clinical assay. We are currently securing marketing and distribution partnerships for application of AditxtScore™ in the infectious diseases market. To obtain FDA approval to use AditxtScore™ as a clinical assay, we are performing validation studies to demonstrate AditxtScore™’s utility to evaluate various components of the immune system reproducibly. We believe that these data will show AditxtScore™’s ability to measure various components of the immune system (e.g. humoral and cell-mediated immune responses) to provide a broader view of the immune system and its status in health and disease. Our plan is to submit a 510(K) application to the FDA after compilation of these data. Beyond infectious diseases, we plan to develop AditxtScore™ for applications in additional markets such as organ rejection, allergies, drug/vaccine response, and disease susceptibility. The following are further descriptions of the applications of AditxtScoreTM:
(1) Organ Rejection
Typically, by the time a transplanted or a native organ shows signs of failure, the damage is already done, and reversal of the tissue injury becomes challenging. Access to early warning signs of damage would be invaluable to reverse or even prevent the damage. There are currently no practical, efficient assays available to measure cellular immune responses and available tools do not provide timely information for patients. AditxtScore™ can be used to provide a sensitive and rapid tool to determine T cell response and to differentiate between various types of cellular immune responses. It can be multiplexed providing information about the number of cells responding as well as quantifying the amounts of various cytokines released by the cells in a single assay. Determination of cellular response has valuable applications for prediction, monitoring, early detection, and treatment of disease, including organ failure/rejection, as well as treatment efficacy. It can also reveal dysfunction of the immune system potentially contributing to more severe disease.
(2) Allergies
Our immune system protects us by acting as a barrier against foreign substances and by eliminating them when they penetrate our bodies. Once the initial exposure has occurred, memory cells develop to prepare the body against a future exposure. This process is called immunity. In certain situations, however, instead of immunity, the immune system develops memory cells that result in a more severe reaction during a future exposure to the same substance. This type of response is called a hypersensitivity response, commonly known as an allergic response. AditxtScore™ can be used to develop multiplex assays each designed to test and monitor immune response to allergens. Based on the ability of this technology to run multiple tests in a single assay, 100 or more substances can potentially be tested for simultaneously.
(3) Drug/Vaccine Response
There are currently no effective assays to predict and easily assess responses to vaccination. To determine whether an individual has responded to a particular vaccine, antibody titers are measured. This process may take several days. Furthermore, for vaccines that require a series of injections, titers are not measured between injections and may not be known for months. AditxtScore™ can be used to determine whether a patient is a responder or non-responder. It can provide an effective and rapid tool for potentially determining beneficial responses to a vaccine and can be used to monitor titer development post vaccination. It can allow evaluation of multiple vaccines in a single test (for memory B cell detection). This application can be useful for vaccines, cancer therapeutics anti-rejection drugs, anti-viral drugs, among others.
(4) Disease Susceptibility
Disease susceptibility can vary from one individual to another and it can be a function of various factors, including genetic variability and differences in human leukocyte antigens (HLA) encoded by major histocompatibility complex (MHC) and responsible for regulation of the immune system in humans. People with certain HLA types may have higher or lower susceptibility to diseases. AditxtScore™ can be used to develop assays to evaluate differences in HLA types in individuals to help elucidate the relationship between certain HLA types and susceptibility to various diseases.
(5) Infectious Diseases
Infectious diseases can cause a major predicament for scientific and medical professionals, epidemiologists, and infectious disease specialists, among others, who need to determine how to treat patients in real time while efficacious therapies are still being developed. Proper decision making requires understanding why some affected individuals show minor or no symptoms, some recover, and others die. This is fundamental to creating effective targeted therapeutics which may differ depending on the underlying profile of the individual at risk for, or with, disease. The immune system plays a major role in how any given individual responds to the infectious agent. This response can be inadequate or too robust or appropriately effective. Regardless, the kinetics of the response by the cellular and humoral (antibody) immune systems to the infectious agent are often unknown. A basic critical question, then, is what do the dynamics of the immune response look like from exposure to and through the disease period and during convalescence for those who survive and those who don’t; and how might vaccines and therapies alter these profiles such that predictions of vaccine/drug efficacy could be inferred prior to vaccination/treatment and/or disease severity or progression be prognosticated. AditxtScore™ can be used to help address these questions with multiplex assays each designed to test and monitor the immune response to infectious agents. Based on the ability to run multiple tests in a single assay, 100 or more agents can potentially be tested for simultaneously.
License Agreement with Loma Linda University
On March 8, 2018, we entered into an Assignment Agreement (the “Assignment Agreement”) with Sekris Biomedical, Inc. (“Sekris”). Sekris was a party to a License Agreement with Loma Linda University (“LLU”), entered into and made effective on May 25, 2011, and amended on June 24, 2011, July 16, 2012 and December 27, 2012 (the “Original Agreement,” and together with the Assignment Agreement, the “Sekris Agreements”). Pursuant to the Assignment Agreement, Sekris transferred and assigned all of its rights and obligations in and to and liabilities under the Original Agreement, of whatever kind or nature, to us. In exchange, on March 8, 2018, we issued a warrant to Sekris to purchase up to 500,000 shares of our common stock (the “Sekris Warrant”). The warrant was immediately exercisable and has an exercise price of $4.00 per share. The expiration date of the warrant is March 8, 2023.
On March 15, 2018, aswe entered into a License Agreement with LLU, which was subsequently amended on July 1, 2020, we entered into a LLU License Agreement directly with Loma Linda University, which amends and restates the Sekris Agreements.
2020. Pursuant to the LLU License Agreement, we obtained the exclusive royalty-bearing worldwide license in and to all intellectual property, including patents, technical information, trade secrets, proprietary rights, technology, know-how, data, formulas, drawings, and specifications, owned or controlled by LLU and/or any of its affiliates (the “LLU Patent and Technology Rights”) and related to therapy for immune-mediated inflammatory diseases (the ADi™Adi™ technology). In consideration for the LLU License Agreement, we issued 25,000625 shares of Common Stock to LLU.
PEARSANTA, INC.
Formed in January 2023, our majority owned subsidiary Pearsanta™, Inc. (“Pearsanta”) seeks to take personalized medicine to a new level by delivering “Health by the Numbers.” On November 22, 2023, Pearsanta entered into an assignment agreement with FirstVitals LLC, an entity controlled by Pearsanta’s CEO, Ernie Lee (“FirstVitals”), pursuant to which FirstVitals assigned its rights in certain intellectual property and website domain to Pearsanta in consideration of the issuance of 500,000 shares of Pearsanta common stock to FirstVitals. On December 18, 2023, the board of directors of Pearsanta adopted the Pearsanta 2023 Omnibus Equity Incentive Plan (the “Pearsanta Omnibus Incentive Plan”), pursuant to which it reserved 15 million shares of common stock of Pearsanta for future issuance under the Pearsanta Omnibus Incentive Plan and the Pearsanta 2023 Parent Service Provider Equity Incentive Plan (the “Pearsanta Parent Service Provider Plan”) and approved the issuance of 9.32 million options, exercisable into shares of Pearsanta common stock under the Pearsanta Parent Service Provider Plan and the issuance of 4.0 million options, exercisable into shares of Pearsanta common stock, subject to LLU.vesting, and 1.0 million restricted common stock shares under the Pearsanta Omnibus Incentive Plan.
Pursuant
Since its founding, Pearsanta has been building the platform for enabling our vision of lab quality testing, anytime, anywhere. Our plan for Pearsanta’s platform is for it to be the LLU License Agreement, wetransactional backbone for sample collection, sample processing (on- and off-site), and reporting. This will require the development and convergence of multiple components developed by Pearsanta, or through transactions with third parties, including collection devices, “lab-on-a-chip” technologies, Lab Developed Test (LDT) assays, a data-driven analysis engine, and telemedicine. According to a comprehensive research report by Market Research Future, the clinical and consumer diagnostic market is estimated to hit $429.3 billion by 2030.
We believe that timely and personalized testing enables far more informed treatment decisions. Pearsanta’s platform is being developed as a seamless digital healthcare solution. This platform will integrate at-location sample collection, Point-of-Care (“POC”) and LDT assays, and an analytical reporting engine, with telemedicine-enabled visits with licensed physicians to review test results and, if necessary, order a prescription. Pearsanta’s goal of extending its platform to enable consumers to monitor their health more proactively as the goal is to provide a more complete picture about someone’s dynamic health status, factoring in genetic makeup and their response to medication. The POC component of Pearsanta would enable diagnostic testing at-home, at work, in pharmacies, and more to generate results quickly so that an individual can access necessary treatment faster. With certain infections, prescribing the most effective treatment according to one’s numbers can prevent hospital emergency room admissions and potentially life-threatening consequences.
Examples of indication-focused tests for the evaluation of advanced urinary tract infections (“UTIs”), COVID-19/flu/respiratory syncytial virus, sexually transmitted infections, gut health, pharmacogenomics (i.e., how your genes affect the way your body responds to certain therapeutics), and sepsis. We believe that these offerings are requirednovel and needed as the current standard of care using broad spectrum antibiotic treatment can be ineffective and potentially life-threatening. For example, improperly prescribed antibiotics may approach 50% of outpatient cases. Further, according to pay an annual license feearticle published in Physician’s Weekly, only 1% of board-certified critical care medicine physicians are trained in infectious disease.
Licensed Technologies – AditxtScoreTM
We issued Pearsanta an exclusive worldwide sub-license for commercializing the AditxtScore™ technology which provides a personalized comprehensive profile of the immune system. AditxtScore is intended to LLU. Also, we paid LLU $455,000detect individual immune responses to viruses, bacteria, peptides, drugs, supplements, bone marrow and solid organ transplants, and cancer. It has broad applicability to many other agents of clinical interest impacting the immune system, including those not yet identified such as emerging infectious agents.
AditxtScore is being designed to enable individuals and their healthcare providers to understand, manage and monitor their immune profiles and to stay informed about attacks on or by their immune system. We believe AditxtScore can also assist the medical community and individuals by being able to anticipate the immune system’s potential response to viruses, bacteria, allergens, and foreign tissues such as transplanted organs. This technology may be able to serve as a warning signal, thereby allowing for more time to respond appropriately. Its advantages include the ability to provide simple, rapid, accurate, high throughput assays that can be multiplexed to determine the immune status with respect to several factors simultaneously, in July 2020approximately 3-16 hours. In addition, it can determine and differentiate between distinct types of cellular and humoral immune responses (e.g., T and B cells and other cell types). It also provides for outstanding milestone paymentssimultaneous monitoring of cell activation and license fees. levels of cytokine release (i.e., cytokine storms).
We are also requiredactively involved in the regulatory approval process for AditxtScore assays for clinical use and securing manufacturing, marketing, and distribution partnerships for application in the various markets. To obtain regulatory approval to payuse AditxtScore as a clinical assay, we have conducted validation studies to LLU milestone paymentsevaluate its performance in connection with certain development milestones. Specifically, we are requireddetection of antibodies and plan to make the following milestone payments: $175,000 on March 31, 2022; $100,000 on March 31, 2024; $500,000 on March 31, 2026; and $500,000 on March 31, 2027. Additionally, as considerationcontinue conducting additional validation studies for prior expenses incurred by LLU to prosecute, maintain and defend the LLU Patent and Technology Rights, we made the following payments to LLU:, $70,000 due at the end of December 2018, and a final payment of $60,000 due at the end of March 2019. We are required to defend the LLU Patent and Technology Rights during the termnew applications in autoimmune diseases.
Advantages
The sophistication of the LLU License Agreement. Additionally, we will owe royalty payments of (i) 1.5% of Net Product Sales and Net Service Sales on any Licensed Products (defined as any finished pharmaceutical products which utilizesAditxtScore technology includes the LLU Patent and Technology Rights in its development, manufacture or supply), and (ii) 0.75% of Net Product Sales and Net Service Sales for Licensed Products and Licensed Services not covered by a valid patent claim for technology rights and know-how for a three (3) year period beyond the expiration of all valid patent claims. We also are required to produce a written progress report to LLU, discussing our development and commercialization efforts, within 45 days following the end of each year. All intellectual property rights in and to LLU Patent and Technology Rights shall remain with LLU (other than improvements developed by or on our behalf).following:
| ● | greater sensitivity/specificity. |
The LLU License Agreement shall terminate on the last day that a patent granted to us by LLU is valid and enforceable or the day that the last patent application licensed to us is abandoned. The LLU License Agreement may be terminated by mutual agreement or by us upon 90 days written notice to LLU. LLU may terminate the LLU License Agreement in the event of (i) non-payments or late payments of royalty, milestone and license maintenance fees not cured within 90 days after delivery of written notice by LLU, (ii) a breach of any non-payment provision (including the provision that requires us to meet certain deadlines for milestone events (each, a “Milestone Deadline”)) not cured within 90 days after delivery of written notice by LLU and (iii) LLU delivers notice to us of three or more actual breaches of the LLU License Agreement by us in any 12-month period. Additional Milestone Deadlines include: (i) the requirement to have regulatory approval of an IND application to initiate a first-in-human clinical trials on or before March 31, 2022, (ii) the completion of first-in-human (phase I/II) clinical trials by March 31, 2024, (iii) the completion of Phase III clinical trials by March 31, 2026 and (iv) biologic licensing approval by the FDA by March 31, 2027.
| ● | 20-fold higher dynamic range, greatly reducing signal to noise compared to conventional assays. |
| ● | ability to customize assays and multiplex a large number of analytes with speed and efficiency. |
| ● | ability to test for cellular immune responses (i.e., T and B cells and cytokines). |
| ● | proprietary reporting algorithm. |
License Agreement with Leland Stanford Junior University (“Stanford”)
On February 3, 2020, we entered into an exclusive license agreement (the “February 2020 License Agreement”) with Stanford with regard to a patent concerning a method for detection and measurement of specific cellular responses. Pursuant to the February 2020 License Agreement, we received an exclusive worldwide license to Stanford’s patent with regard to use, import, offer, and sale of Licensed Products (as defined in the agreement). The license to the patented technology is exclusive, including the right to sublicense, beginning on the effective date of the agreement, and ending when the patent expires. Under the exclusivity agreement, we acknowledged that Stanford had already granted a non-exclusive license in the Nonexclusive Field of Use, under the Licensed Patents in the Licensed Field of Use in the Licensed Territory (as those terms are defined in the February“February 2020 License Agreement”). However, Stanford agreed not to not grant further licenses under the Licensed Patents in the Licensed Field of Use in the Licensed Territory.
We were obligated On December 29, 2021, we entered into an amendment to pay and paid a fee of $25,000 to Stanford within 60 days of February 3, 2020. We also issued 18,750 shares of the Company’s common stock to Stanford. An annual licensing maintenance fee is payable by us on the first anniversary of the February 2020 License Agreement which extended our exclusive right to license the technology deployed in the amount of $40,000 for 2021 through 2024 AditxtScoreTM and $60,000 startingsecuring worldwide exclusivity in 2025 until the license expires upon the expiration of the patent. The Company is required to pay and has paid $25,000 for the issuances of certain patents. The Company will pay milestone fees of $50,000 on the first commercial sales of a licensed product and $25,000 at the beginning of any clinical study for regulatory clearance of an in vitro diagnostic product developed and a potential licensed product. We are also required to: (i) provide a listing of the management team or a schedule for the recruitment of key management positions by March 31, 2020 (which has been completed), (ii) provide a business plan covering projected product development, markets and sales forecasts, manufacturing and operations, and financial forecasts until at least $10,000,000 in revenue by June 30, 2020 (which has been completed), (iii) conduct validation studies by September 30, 2020 (which has been completed), (iv) hold a pre-submission meeting with the FDA by September 30, 2020 (which has been completed), (v) submit a 510(k) application to the FDA, Emergency Use Authorization (“EUA”), or a Laboratory Developed Test (“LDT”) by March 31, 2021, (vi) obtain FDA approval by December 31, 2021, (vii) complete a prototype assay kit by December 31, 2021 and (viii) have a written agreement with Stanford on further development and commercialization milestones for specificall fields of use by December 31, 2021.
of the licensed technology.
In addition
ADIVIR, INC.
Formed in April of 2023, Adivir™, Inc. is a wholly owned subsidiary, dedicated to the annual license maintenance fees outlined above,clinical and commercial development of innovative products, including anti-viral and other anti-infective products, for population health. These products have the potential to address a wide range of infectious diseases, including those that currently lack viable treatment options.
Background
On April 18, 2023, we entered into an Asset Purchase Agreement (the “Asset Purchase Agreement”) with Cellvera Global Holdings LLC (“Cellvera Global”), Cellvera Holdings Ltd. (“BVI Holdco”), Cellvera, Ltd. (“Cellvera Ltd.”), Cellvera Development LLC (“Cellvera Development” and together with Cellvera Global, BVI Holdco, Cellvera Ltd. and Cellvera Development (the “Sellers”), AiPharma Group Ltd. (“Seller Owner” and collectively with the Sellers, “Cellvera”), and the legal representative of Cellvera, pursuant to which, the Company will pay Stanford royalties on Net Sales (as such term ispurchase Cellvera’s 50% ownership interest in G Response Aid FZE (“GRA”), certain other intellectual property and all goodwill related thereto (the “Acquired Assets”). Unless expressly stated otherwise herein, capitalized terms used but not defined herein have the meanings ascribed to them in the February 2020 License Agreement) duringAsset Purchase Agreement. Pursuant to the Asset Purchase Agreement, the consideration for the Acquired Assets consists of (A) $24.5 million, comprised of: (i) the forgiveness of the Company’s $14.5 million loan to Cellvera Global, and (ii) approximately $10 million in cash, and (B) future revenue sharing payments for a term of seven years. GRA holds an exclusive, worldwide license for the agreementantiviral medication, Avigan® 200mg, excluding Japan, China and Russia. The other 50% interest in GRA is held by Agility, Inc. (“Agility”).
Additionally, upon the closing, the Share Exchange Agreement previously entered into as follows: 4% when Net Sales are below or equal to $5 million annually or 6% when Net Sales are above $5 million annually. of December 28, 2021, between Cellvera Global Holdings, LLC f/k/a AiPharma Global Holdings, LLC (together with other affiliates and subsidiaries) and the Company, and all other related agreements will be terminated.
The February 2020 License Agreement may be terminated upon our election on at least 30 days advance notice to Stanford, or by Stanford if we: (i) are delinquent on any report or payment; (ii) are not diligently developing and commercializing Licensed Product; (iii) miss certain performance milestones; (iv) are in breach of any provisionobligations of the February 2020 License Agreement;Company to consummate the Closing are subject to the satisfaction or (v) provide any false reportwaiver, at or prior to Stanford. Should any events in the preceding sentence occur, we have a thirty (30) day cure periodClosing of certain conditions, including but not limited to, remedy such violation.the following:
Plan of Operations – Immune Reprogramming
High-level objectives for skin allograft clinical program:
| (i) | |||
Our FIH clinical studies will combine Phase I (designed to test clinical safety) and Phase IIa (designed to obtain proof of effectiveness in human subjects), in subjects requiring skin and other organ and/or tissue allografts. We have selected this indication for several reasons, including:
| (ii) |
| (iii) |
| (iv) |
| (v) | Receipt by the Company of a release from Agility; |
| (vi) | Execution of an agreement acceptable to the Company with respect to the acquisition by the Company of certain intellectual property presently held by a third party; |
| (vii) | Execution of an amendment to an asset purchase agreement previously entered into by Cellvera with a third party that effectively grants the Company the rights to acquire the intellectual property from the third party under such agreement; |
| (viii) | Receipt of a fairness opinion by the Company with respect to the transactions contemplated by the Asset Purchase Agreement; and |
| (ix) | Receipt by the Company from the Seller Owner of written consent, whether through its official liquidator or the Board of Directors of Seller Owner, to the sale and purchase of the |
There can be no assurance that |
We have already identified a clinical trial center with adequate patients, which we believe will simplify and reduce the time required for patient recruitment. Upon approval by the FDA and/or the applicable regulatory agency, and once the exact protocol has been determined in the preclinical studies, clinical trialsconditions to closing will be initiated.
High-level objectives for psoriasis clinical program:
Our FIH clinical studies will combine Phase I (designed to test clinical safety) and Phase IIa (designed to obtain proof of effectiveness in human subjects), in psoriasis patients. We have selected this indication for several reasons, including:
Wesatisfied or that the proposed acquisition will be identifying clinical trial centers with adequate patients. Upon approval by the FDA and/completed as proposed or the applicable regulatory agency clinical trials will be initiated.at all.
We are developing
Our commitment to building our immune monitoring platforms with the objective of utilizing them as clinical assays in pre-clinicalantiviral portfolio is strategic and clinical studies. The multiplex technologies could potentially allow evaluation of more analytes with less tissue samples.
Drug Approval Process
In the United States, FDA approval is required before any new drugs can be introduced to the market. We currently have a product candidate for our first-in-human studies, but as of the date of report, we have not submitted an application to the regulatory agencies for approval.
We are working with a contract manufacturer who has the know-how, product ingredients including plasmid DNA molecules, and our patent-pending bacterial strain. Several batch runs have been successfully completed to demonstrate our ability to produce the DNA plasmids in a GMP facility. Based on validation studies, we are reasonably confident in our ability to produce clinical grade product candidates at larger scales. The contract manufacturer has provided a proposal for manufacturing of our clinical grade material, which will be signed and accepted once we are ready to initiate GMP manufacturing. We are not currently party to an agreement with this contract manufacturer.
The product candidate selected for clinical trials must be subjected to pre-clinical safety/toxicology studies by an independent GLP (Good Laboratory Practice) laboratory to demonstrate its suitability for clinical testing in human patients. Upon completion of manufacturing and safety/toxicology testing, an Investigational New Drug (IND) application will be prepared for submission to the regulatory agencies.
Upon receipt of clearance to initiate clinical testing, the ADi™ product can be tested in human patients. Our product will be tested in clinical trials, one in patients with psoriasis and one in patients who require skin allografting. Therefore, our first-in-human studies will be combined Phase I/Phase IIa studies in which safety and efficacy data will be obtained. We plan to start with in skin indications (psoriasis and skin allografting) because we believe these indications will be most efficient in providing safety and efficacy data in clinical trials. In parallel, we will continue to develop additional product formulations for other indications.
We are developing our immune monitoring platforms with the objective of utilizing them as clinical assays in pre-clinical and clinical studies. The multiplex technologies could potentially allow evaluation of more analytes with less tissue samples. In the U.S., FDA approval is required before any In Vitro Diagnostic (“IVD”) device can be introduced to the market for clinical use (excluding research purposes). This process does not require clinical trials, but it does require validation data demonstrating accuracy of the device.
Target Market
In the U.S. alone, there are over 36,000 patients who receive organ transplantations each year, with more than 113,000 on transplant waiting lists.
The field of organ transplantation has been made possible and continues to rely on broad-acting immunosuppressive drugs, high levels of which can result in a compromised immune system that renders organ recipients susceptible to cancer and potentially life-threatening infections including re-activation of latent viruses.
In addition, immunosuppressants control acute rejection during the early time-period after receiving an organ but chronic rejection of the organ remains an unmet challenge for surgeons and transplant recipients.
While efforts have been made by various groups to promote tolerance through cell therapies and ex vivo manipulation of patient cells, these procedures take place outside the body and typically require hospitalization.
Moreover, transplanted patients will need re-transplantation at some point, with the possible exception of some newborn recipients. With increased incidence of preformed antibodies, these patients may never have the opportunity to receive another organ. Preformed antibodies can develop in previously transplanted patients, patients who have given birth, and patients who have previously received blood transfusions. These patients have much lower chances at qualifying to receive organs due to their increased risk of rejection – even with immune suppression. The potential to reduce formation of preformed antibodies in these patients will provide better opportunities for them to receive another transplanted organ.
There are gaps between current approaches and what the market needs.timely. We believe that ADi™ addresses these gaps. ADi™ is easythere has never been a more important time to administer (does not require ex-vivo treatment of patient cells), it does not appear to suppressaddress the immune system, it may allow patients to live with transplanted organs with significantly reduced immune suppression, it may provide for long-term survival of transplanted tissues and organs, may be more effective because it does not rely on a single immune pathway/mechanism, and potentially provides patients with pre-existing antibodies a chance to qualify to receive organs.
While these advantages present opportunities for unmet medical needs in the field of organ transplantation, the industry in which we operate is highly competitive. A small company such as us will meet significant challenges including regulatory requirements for approval of a new class of therapeutic agents, challenges in large scale manufacturing and marketing, cost of developing a novel therapeutic agent, which may require co-development partners who may or may not be willing to work with us, and the willingness of transplant surgeons to adopt our therapeutic vaccines in their existing immune suppression protocols. These challenges pose risks that we may not be able to overcome.
Operational Advantages
Location
We lease laboratory space in Mountain View, CA, located near resources including Stanford University (“Stanford”).
Strategic Partners
Our plan is to work with strategic partners to leverage common resources to accomplish milestones over the next 3 years and potentially get access to expertise, materials, and infrastructure (such as laboratory space) which we believe can be advantageous to our development. We hope that this strategy will reduce costs by obviating thegrowing global need to duplicate resources.
Intellectual Property (IP)
We strive to protect and enhance the proprietary technology, inventions, and improvementsuncover new treatments or commercialize existing ones that are commercially important to our business, including seeking, maintaining and defending patent rights, whether developed internally or licensed from third parties. Our policy is to seek to protect our proprietary position by, among other methods, filing patent applications in the United States and in jurisdictions outside of the United States, to protect our proprietary technology, inventions, improvements and product candidates that are important to the development and implementation of our business. We also rely on trade secrets and know-how relating to our proprietary technology and product candidates, continuing innovation, and in-licensing opportunities to develop, strengthen and maintain our proprietary position in the field of immuno-therapy. We also plan to rely on data exclusivity, market exclusivity, and patent term extensions when available. Our commercial success will depend in part on our ability to obtain and maintain patent and other proprietary protection for our technology, inventions, and improvements; to preserve the confidentiality of our trade secrets; to obtain and maintain licenses to use intellectual property owned by third parties; to defend and enforce our proprietary rights, including any patents that we may own in the future; and to operate without infringing on the valid and enforceable patents and other proprietary rights of third parties.
The ADi™ technology and its various components are protected by multiple families of patents and patent applications, including several U.S. and non-U.S. issued patents. As of the date of this report, our patent portfolio licensed from LLU includes 8 U.S. patents, 3 U.S. pending patent applications, 87 foreign patents, and 14 foreign pending patent applications directed to ADi™ and related technologies. The ADi™ patents are broadly categorized into three groups, one for autoimmune diseases and type 1 diabetes; one for organ transplantation and a method of producing plasmid DNA that is mammalian-like to prevent immune activation; and one providing patent protection for a composition of matter for a tolerance delivery system for antigens of interest that would be relevant for various given indications. The third group is the basis for a platform allowing development of a new class of immunotherapeutics for various indications. The projected expiration dates for these ADi™ patents ranges from 2021 to 2034. The AditxtScore™ technology licensed from Stanford is protected by a U.S. patent which encompasses methods, systems and kits for detection and measurement of specific immune responses. The patent has been issued by the USPTO and expires on December 28, 2037. We also possess and/or in-license substantial know-how and trade secrets relating to the development and commercialization of our product candidates, including related manufacturing processes and technology. We plan to continue expanding and strengthening our IP portfolio with additional patent applications in the future.
In March 2021, Aditxt has signed an agreement with a regulatory consultant based in Munich, Germany, which will play a central role in navigating the first AditxtReprogrammingTM therapeutic program through the clinical trial and regulatory process. The firm will work with the Aditxt's AditxtReprogrammingTM team to submit an Investigational New Drug application (IND) to the regulatory agency in Germany. Psoriasis is the first indication being targeted for clinical trial in the AditxtReprogrammingTM therapeutics pipeline. Other candidates that are advancing toward clinical trials include ADi™ for type 1 diabetes and skin allografting.
Plan of Operations – Immune Monitoring
As previously announced on August 6, 2020, the initial application of the platform will be AditxtScore™ for COVID-19 which has been designed to provide a more complete assessment of an individual’s infection and immunity status with respect to the SARS-CoV-2 virus. Infection status will be determined by evaluating the presence or absence of the virus, and immunity status by measuring levels of antibodies againsttreat life-threatening global viral antigens and their ability to neutralize the virus. We will soon be expanding the panel to measure other components of the immune response such as cellular immunity.
In August 2020, we filed for an Emergency Use Authorization (EUA) with the FDA with the ultimate objective of filing a 510(K) application. We are in the process of filing an amended application to incorporate additional data and information about the use of the assay. In the meantime, we are providing AditxtScore™ as a service as a Laboratory Developed Test (LDT) to assess immunity status to COVID-19.
In early 2021, we established our AditxtScore™ Immune Monitoring Center in Richmond, Virginia (the “Center”). The Center operates as a Clinical Laboratory Improvement Amendments (CLIA) certified facility for the processing of our AditxtScore™ for COVID-19 Lab Developed Test (LDT) for our prospective channel partners, including labs and hospitals.infections.
Employees
We have forty-three (43)forty-seven (47) full time employees.employees as of December 31, 2023. We consider the relations with our employees to be good.
Item 1A. Risk Factors.
You should carefully consider the risks described below, as well as general economic and business risks and the other information in this Annual Report on Form 10-K. The occurrence of any of the events or circumstances described below or other adverse events could have a material adverse effect on our business, results of operations and financial condition and could cause the trading price of our common stock to decline. Additional risks or uncertainties not presently known to us or that we currently deem immaterial may also harm our business.
Risks Related to Our Financial Position and Need for Capital
We have generated no significant revenue from commercial sales to date and our future profitability is uncertain.
We were incorporated in September 2017 and have a limited operating history and our business is subject to all of the risks inherent in the establishment of a new business enterprise. Our likelihood of success must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered in connection with development and expansion of a new business enterprise. Since inception, we have incurred losses and expect to continue to operate at a net loss for at least the next several years as we commence our research and development efforts, conduct clinical trials and develop manufacturing, sales, marketing and distribution capabilities. Our net loss for the years ended December 31, 20202023 and 20192022 was $9,149,227$32,390,447 and $5,827,728,$27,549,876, respectively, and our accumulated deficit as of December 31, 20202023 was $20,879,178.$127,635,389. There can be no assurance that the products under development by us will be approved for sale in the U.S. or elsewhere. Furthermore, there can be no assurance that if such products are approved, they will be successfully commercialized, and the extent of our future losses and the timing of our profitability are highly uncertain. If we are unable to achieve profitability, we may be unable to continue our operations.
If we fail to obtain the capital necessary to fund our operations, we will be unable to continue or complete our product development and you will likely lose your entire investment.
We will need to continue to seek capital from time to time to continue development of our lead drug candidate beyond our initial combined Phase I/IIa clinical trial and to acquire and develop other product candidates. Once approved for commercialization, we cannot provide any assurances that any revenues it may generate in the future will be sufficient to fund our ongoing operations. Our current cash position, we expect to be sufficient to satisfy our capital requirements sufficient to fund our operations for the foreseeable future.
Our business or operations may change in a manner that would consume available funds more rapidly than anticipated and substantial additional funding may be required to maintain operations, fund expansion, develop new or enhance products, acquire complementary products, business or technologies or otherwise respond to competitive pressures and opportunities, such as a change in the regulatory environment or a change in preferred treatment modalities. In addition, we may need to accelerate the growth of our sales capabilities and distribution beyond what is currently envisioned, and this would require additional capital. However, we may not be able to secure funding when we need it or on favorable termsterms. We may not be able to raise sufficient funds to commercialize the product candidates we intend to develop.
If we cannot raise adequate funds to satisfy our capital requirements, we will have to delay, scale back or eliminate our research and development activities, clinical studies or future operations. We may also be required to obtain funds through arrangements with collaborators, which arrangements may require us to relinquish rights to certain technologies or products that we otherwise would not consider relinquishing, including rights to future product candidates or certain major geographic markets. This could result in sharing revenues which we might otherwise retain for ourselves. Any of these actions may harm our business, financial condition and results of operations.
The amount of capital we may need depends on many factors, including the progress, timing and scope of our product development programs; the progress, timing and scope of our preclinical studies and clinical trials; the time and cost necessary to obtain regulatory approvals; the time and cost necessary to further develop manufacturing processes and arrange for contract manufacturing; our ability to enter into and maintain collaborative, licensing and other commercial relationships; and our partners’ commitment of time and resources to the development and commercialization of our products.
Our financial situation creates doubt whether we will continue as a going concern.
The Company was incorporated on September 28, 2017 and through the date of this report has generated no significant revenues. For the years ended December 31, 20202023 and 2019,2022, the Company had a net loss of $9,149,227$32,390,447 and $5,827,728,$27,649,876, respectively. There can be no assurances that we will be able to achieve a level of revenues adequate to generate sufficient cash flow from operations or additional financing through private placements, public offerings and/or bank financing necessary to support our working capital requirements. To the extent that funds generated from any private placements, public offerings and/or bank financing are insufficient, we will have to raise additional working capital. No assurance can be given that additional financing will be available, or if available, will be on acceptable terms. These conditions raise substantial doubt about our ability to continue as a going concern. If adequate working capital is not available, we may be forced to discontinue operations, which would cause investors to lose their entire investment.
We may need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
We do not expect that our current cash position will be sufficient to fund our current operations for at least the next 1812 months. However, ourOur operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements or a combination of these approaches. In any event, we will require additional capital to obtain regulatory approval for, and to commercialize, our product candidates. Raising funds in the current economic environment may present additional challenges. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our shares to decline. The sale of additional equity or convertible securities may dilute our existing stockholders. The incurrence of indebtedness would result in increased fixed payment obligations and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable and we may be required to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any product candidate or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
Even if we can raise additional funding, we may be required to do so on terms that are dilutive to you.
The capital markets have been unpredictable in the past for unprofitable companies such as ours. In addition, it is generally difficult for development stage companies to raise capital under current market conditions. The amount of capital that a company such as ours is able to raise often depends on variables that are beyond our control. As a result, we may not be able to secure financing on terms attractive to us, or at all. If we are able to consummate a financing arrangement, the amount raised may not be sufficient to meet our future needs. If adequate funds are not available on acceptable terms, or at all, our business, including our results of operations, financial condition and our continued viability will be materially adversely affected.
Unstable market and economic conditions and adverse developments with respect to financial institutions and associated liquidity risk may have serious adverse consequences on our business, financial condition, and stock price.
The global credit and financial markets have recently experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, inflationary pressure, and interest rate changes, increases in unemployment rates and uncertainty about economic stability. The financial markets and the global economy may also be adversely affected by the current or anticipated impact of military conflict, including the conflict between Russia and Ukraine, terrorism, or other geopolitical events. Sanctions imposed by the United States and other countries in response to such conflicts, including the one in Ukraine, may also adversely impact the financial markets and the global economy, and any economic countermeasures by the affected countries or others could exacerbate market and economic instability. More recently, the closures of Silicon Valley Bank (“SVB”) and Signature Bank and their placement into receivership with the Federal Deposit Insurance Corporation (FDIC) created bank-specific and broader financial institution liquidity risk and concerns. Although the Department of the Treasury, the Federal Reserve, and the FDIC jointly released a statement that depositors at SVB and Signature Bank would have access to their funds, even those in excess of the standard FDIC insurance limits, under a systemic risk exception, future adverse developments with respect to specific financial institutions or the broader financial services industry may lead to market-wide liquidity shortages, impair the ability of companies to access near-term working capital needs, and create additional market and economic uncertainty. There can be no assurance that future credit and financial market instability and a deterioration in confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, liquidity shortages, volatile business environment or continued unpredictable and unstable market conditions. If the equity and credit markets deteriorate, or if adverse developments are experienced by financial institutions, it may cause short-term liquidity risk and also make any necessary debt or equity financing more difficult, more costly and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon clinical development plans. In addition, there is a risk that one or more of our current service providers, financial institutions, manufacturers and other partners may be adversely affected by the foregoing risks, which could directly affect our ability to attain our operating goals on schedule and on budget. The Company does not hold any deposits or securities or maintain any accounts at SVB or Signature Bank.
Risks Related to Product Development, Regulatory Approval, Manufacturing and Commercialization
The regulatory approval process is expensive, time-consuming and uncertain and may prevent us from obtaining approvals for the commercialization of our future product candidates, if any.
We will not be permitted to market our product candidates in the United States until we receive approval from the FDA, or in any foreign countries until we receive the requisite approval from corresponding agencies in such countries. The testing, manufacturing, labeling, approval, selling, marketing and distribution of health-health and life science-related products are subject to extensive regulation, which regulations differ from country to country.
Successfully completing our clinical program and obtaining approval of a Biologics License Application (“BLA”) is a complex, lengthy, expensive and uncertain process, and the FDA or other applicable foreign regulator may delay, limit or deny approval of our product candidates for many reasons, including, among others, because:
| ● | we may not be able to demonstrate that our product candidates are safe and effective in treating patients to the satisfaction of the FDA or foreign regulator; |
| ● | the results of our clinical trials may not meet the level of statistical or clinical significance required by the FDA or foreign regulator for marketing approval; |
| ● | the FDA or foreign regulator may disagree with the number, design, size, conduct or implementation of our clinical trials; |
| ● | the FDA or foreign regulator may require that we conduct additional clinical trials; |
| ● | the FDA or foreign regulator may not approve the formulation, labeling or specifications of our product candidates; |
| ● | the contract research organizations (CROs) and other contractors that we may retain to conduct our clinical trials may take actions outside of our control that materially adversely impact our clinical trials; |
| ● | the FDA or foreign regulator may find the data from preclinical studies and clinical trials insufficient to demonstrate that our product candidate(s) are safe and effective for their proposed indications; |
| ● | the FDA or foreign regulator may disagree with our interpretation of data from our preclinical studies and clinical trials; |
| ● | the FDA or foreign regulator may not accept data generated at our clinical trial sites or may disagree with us over whether to accept efficacy results from clinical trial sites outside the United States or outside the EU, as applicable, where the standard of care is potentially different from that in the United States or in the EU, as applicable; |
| ● | if and when our BLAs or foreign equivalents are submitted to the applicable regulatory authorities, such agencies may have difficulties scheduling the necessary review meetings in a timely manner, may recommend against approval of our application or may recommend or require, as a condition of approval, additional preclinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions; |
| ● | the FDA or foreign regulator may require development of a Risk Evaluation and Mitigation Strategy (REMS), which would use risk minimization strategies to ensure that the benefits of certain prescription drugs outweigh their risks, as a condition of approval or post-approval; |
| ● | the FDA or other applicable foreign regulatory agencies may not approve the manufacturing processes or facilities of third-party manufacturers with which we contract; or |
| ● | the FDA or the other applicable foreign regulatory agencies may change their approval policies or adopt new regulations. |
We may encounter substantial delays in completing our clinical studies which in turn will require additional costs, or we may fail to demonstrate adequate safety and efficacy to the satisfaction of applicable regulatory authorities.
It is difficult to predict if or when any of our product candidates, will prove safe or effective in humans or will receive regulatory approval. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct extensive clinical studies to demonstrate the safety and efficacy of the product candidates in humans. Clinical testing is expensive, time-consuming and uncertain as to outcome. We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical studies can occur at any stage of testing. Events that may prevent successful or timely completion of clinical development include:
| ● | delays in reaching, or failing to reach, a consensus with regulatory agencies on study design; |
| ● | delays in reaching, or failing to reach, agreement on acceptable terms with a sufficient number of prospective contract research organizations (“CROs”) and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; | |
| ● | delays in obtaining required Institutional Review Board (“IRB”) or Ethics Committee (“EC”) approval at each clinical study site; | |
| ● | delays in recruiting a sufficient number of suitable patients to participate in our clinical studies; | |
| ● | imposition of a clinical hold by regulatory agencies, after an inspection of our clinical study operations or study sites; |
| ● | failure by our CROs, other third parties or us to adhere to the clinical study, regulatory or legal requirements; | |
| ● | failure to perform in accordance with the FDA’s good clinical practices (“GCP”) or applicable regulatory guidelines in other countries; |
| ● | delays in the testing, validation, manufacturing and delivery of sufficient quantities of our product candidates to the clinical sites; | |
| ● | delays in having patients’ complete participation in a study or return for post-treatment follow-up; | |
| ● | clinical study sites or patients dropping out of a study; | |
| ● | delay or failure to address any patient safety concerns that arise during the course of a trial; | |
| ● | unanticipated costs or increases in costs of clinical trials of our product candidates; | |
| ● | occurrence of serious adverse events associated with the product candidates that are viewed to outweigh their potential benefits; or | |
| ● | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols. |
We could also encounter delays if a clinical trial is suspended or terminated by us, by the IRBs or ECs of the institutions in which such trials are being conducted, by an independent Safety Review Board (“SRB”) for such trial or by the FDA, European Medicines Agency (“EMA”), or other regulatory authorities. Such authorities may suspend or terminate a clinical trial due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA, EMA, or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenues from product sales, regulatory and commercialization milestones and royalties. In addition, if we make manufacturing or formulation changes to our product candidates, we may need to conduct additional studies to bridge our modified product candidates to earlier versions.
Clinical study delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may significantly harm our business, financial condition and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
The outcome of preclinical studies and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Further, preclinical and clinical data are often susceptible to various interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have, nonetheless, failed to obtain marketing approval. If the results of our clinical studies are inconclusive or if there are safety concerns or adverse events associated with our other product candidates, we may:
| ● | be delayed in obtaining marketing approval for our product candidates, if approved at all; |
| ● | obtain approval for indications or patient populations that are not as broad as intended or desired; | |
| ● | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
| ● | be required to change the way the product is administered; | |
| ● | be required to perform additional clinical studies to support approval or be subject to additional post-marketing testing requirements; | |
| ● | have regulatory authorities withdraw their approval of a product or impose restrictions on its distribution in the form of a modified risk evaluation and mitigation strategy; | |
| ● | be sued; or | |
| ● | experience damage to our reputation. |
Additionally, our product candidates could potentially cause other adverse events that have not yet been predicted. The inclusion of ill patients in our clinical studies may result in deaths or other adverse medical events due to other therapies or medications that such patients may be using. As described above, any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and impair our ability to commercialize our products.
If our future pre-clinical development and future clinical Phase I/II studies are unsuccessful, we may be unable to obtain regulatory approval of, or commercialize, our product candidates on a timely basis or at all.
The successful completion of pre-clinical development and multiple clinical trials is critical to the success of our future products. If the pre-clinical development and clinical trials are unsuccessful or produce inconsistent results or unanticipated adverse side effects, or if we are unable to collect reliable data, regulatory approval of our products could be delayed or not given and as a result we may be unable to commercialize our products. Generally, we expect to engage third parties such as consultants, universities or other collaboration partners to conduct clinical trials on our behalf. Incompatible practices or misapplication of our products by these third parties could impair the success of our clinical trials.
Even if we receive regulatory approval for any of our product candidates, we may not be able to successfully commercialize the product and the revenue that we generate from their sales, if any, may be limited.
If approved for marketing, the commercial success of our product candidates will depend upon each product’s acceptance by the medical community, including physicians, patients and health care payors. The degree of market acceptance for any of our product candidates will depend on a number of factors, including:
| ● | demonstration of clinical safety and efficacy; | |
| ● | relative convenience, dosing burden and ease of administration; | |
| ● | the prevalence and severity of any adverse effects; | |
| ● | the willingness of physicians to prescribe our product candidates, and the target patient population to try new therapies; |
| ● | efficacy of our product candidates compared to competing products; | |
| ● | the introduction of any new products that may in the future become available targeting indications for which our product candidates may be approved; | |
| ● | new procedures or therapies that may reduce the incidences of any of the indications in which our product candidates may show utility; |
| ● | pricing and cost-effectiveness; | |
| ● | the inclusion or omission of our product candidates in applicable therapeutic and vaccine guidelines; | |
| ● | the effectiveness of our own or any future collaborators’ sales and marketing strategies; |
| ● | limitations or warnings contained in approved labeling from regulatory authorities; | |
| ● | our ability to obtain and maintain sufficient third-party coverage or reimbursement from government health care programs, including Medicare and Medicaid, private health insurers and other third-party payors or to receive the necessary pricing approvals from government bodies regulating the pricing and usage of therapeutics; and | |
| ● | the willingness of patients to pay out-of-pocket in the absence of third-party coverage or reimbursement or government pricing approvals. |
If any of our product candidates are approved, but do not achieve an adequate level of acceptance by physicians, health care payors, and patients, we may not generate sufficient revenues and we may not be able to achieve or sustain profitability. Our efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may never be successful.
In addition, even if we obtain regulatory approvals, the timing or scope of any approvals may prohibit or reduce our ability to commercialize our product candidates successfully. For example, if the approval process takes too long, we may miss market opportunities and give other companies the ability to develop competing products or establish market dominance. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render our product candidates not commercially viable. For example, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve any of our product candidates with a label that does not include the labeling claims necessary or desirable for the successful commercialization for that indication. Further, the FDA or comparable foreign regulatory authorities may place conditions on approvals or require risk management plans or a Risk Evaluation and Mitigation Strategy (“REMS”) to assure the safe use of the drug. If the FDA or applicable foreign regulatory agency concludes a REMS is needed, the sponsor of the BLA must submit a proposed REMS; the regulatory agencies will not approve the BLA without an approved REMS, if required. A REMS could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The regulatory agencies may also require a REMS for an approved product when new safety information emerges. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of our product candidates. Moreover, product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following the initial marketing of the product. Any of the foregoing scenarios could materially harm the commercial success of our product candidates.
Adverse events involving our products may lead the FDA or applicable foreign regulatory agency to delay or deny clearance for our products or result in product recalls that could harm our reputation, business and financial results.
Once a product receives regulatory clearance or approval, the agency has the authority to require the recall of commercialized products in the event of adverse side effects, material deficiencies or defects in design or manufacture. The authority to require a recall must be based on a regulatory finding that there is a reasonable probability that the product would cause serious injury or death. Manufacturers may, under their own initiative, recall a product if any material deficiency in a product is found. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of adverse side effects, impurities or other product contamination, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. The regulatory agencies require that certain classifications of recalls be reported to them within ten (10) working days after the recall is initiated. Companies are required to maintain certain records of recalls, even if they are not reportable to the regulatory agency. We may initiate voluntary recalls involving our products in the future that we determine do not require notification of the regulatory agencies. If the regulatory agency disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the regulatory agency could take enforcement action for failing to report the recalls when they were conducted.
The in-licensing of technologies and the successful testing and early development of technologies in the laboratory may not be indicative of future results and may not result in commercially viable technologies or products. Further, our future products may have to be modified from their originally conceived versions in order to reach or be successful in the market.
Positive results from laboratory testing and early developmental successes, may not be predictive of future successful development, commercialization and sales results and should not be relied upon as evidence that products developed from our technologies will become commercially viable and successful. Further, the products we plan to develop in the future may have to be significantly modified from their originally conceived versions in order for us to control costs, compete with similar products, receive market acceptance, meet specific development and commercialization timeframes, avoid potential infringement of the proprietary rights of others, or otherwise succeed in developing our business and earning ongoing revenues. This can be a costly and resource draining activity. What appear to be promising technologies when we license them may not lead to viable technologies or products, or to commercial success.
Complying with numerous regulations pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties.
We are subject to the Clinical Laboratory Improvement Amendment of 1988, or CLIA, which is a federal law regulating clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease. Our clinical laboratory is located in Richmond, Virginia and must be certified under CLIA in order for us to perform testing on human specimens. CLIA is intended to ensure the quality and reliability of clinical laboratories in the United States by mandating specific standards in the areas of personnel qualifications, administration, and participation in proficiency testing, patient test management, quality control, quality assurance and inspections. We currently hold a CLIA certificate to perform high-complexity testing. Laboratories performing high complexity testing are required to meet more stringent requirements than laboratories performing less complex tests. CLIA regulations require clinical laboratories like ours to comply with various operational, personnel, facilities administration, quality, and proficiency testing requirements intended to ensure that testing services are accurate, reliable and timely. CLIA certification is a prerequisite for reimbursement eligibility for services provided to state and federal health care program beneficiaries. CLIA is user-fee funded. Therefore, all costs of administering the program must be covered by the regulated facilities, including certification and survey costs. To renew this certificate, we are subject to survey and inspection every two years. Moreover, CLIA inspectors may make periodic inspections of our clinical laboratory outside of the renewal process. The failure to comply with CLIA requirements can result in enforcement actions, including the revocation, suspension, or limitation of our CLIA certificate of compliance, as well as a directed plan of correction, state on-site monitoring, civil money penalties, civil injunctive suit and/or criminal penalties. We must maintain CLIA compliance and certification to be eligible to bill for assays provided to Medicare beneficiaries. If we were to be found out of compliance with CLIA program requirements and subjected to sanctions, our business and reputation could be harmed. Even if it were possible for us to bring our laboratory back into compliance, we could incur significant expenses and potentially lose revenue in doing so.
Additionally, certain states require laboratory licenses in order to test specimens from patients in those states or received from ordering physicians in those states. We may also be subject to regulation in foreign jurisdictions if we seek to expand international distribution of our assays outside the United States.
If we were to lose our CLIA certification or state laboratory licenses, whether as a result of a revocation, suspension or limitation, we would no longer be able to offer our assays (including our AditxtScore™ platform), which would limit our revenues and harm our business. If we were to lose, or fail to obtain, a license in any other state where we are required to hold a license, we would not be able to test specimens from those states.
Risks Related to the Company and our Business
Our technology isCertain technologies are subject to licenses from LLU and Stanford, each of which are revocable in certain circumstances, including in the event we do not achieve certain payments and milestone deadlines. Without these licenses, we may not be able to continue to develop our product candidates.
The LLU License Agreement may be terminated by LLU in the event of a breach by us of any non-payment provision (including the provision that requires us to meet certain deadlines for milestone events (each, a “Milestone Deadline”)) not cured within 90 days after delivery of written notice by LLU. Additional Milestone Deadlines include: (i) the requirement to have regulatory approval of an IND application to initiate first-in-human clinical trials on or before March 31, 2022,2023 (which has been extended to March 31, 2024 with a payment of a $100,000 extension fee), (ii) the completion of first-in-human (phase I/II) clinical trials by March 31, 2024, (iii) the completion of Phase III clinical trials by March 31, 2026 and (iv) biologic licensing approval (BLA) by the FDA by March 31, 2027. If the LLU License Agreement were to be terminated by LLU, we would lose our most significant asset and may no longer be able to develop our product candidates, which would have a material adverse effect on our operations.
The February 2020 License Agreement with Stanford may be terminated by Stanford if we (i) are delinquent on any report or payments; (ii) are not diligently developing and commercializing Licensed Product (as defined in the February 2020 License Agreement); (iii) miss a milestone described in the agreement; (iv) are in breach of any other provision of the agreement; or (v) if we provide a false report to Stanford. The Termination discussed above will take effect only upon 30 days written notice by Stanford unless we remedy the breach within a 30 day30-day cure period. If the February 2020 License Agreement were to be terminated by Stanford, we would lose a significant asset and may no longer be able to develop our product candidates, which would have a material adverse effect on our operations. The Company is current with its obligations and have submitted a year end report as well as provided additional milestone plans for research and development as well as commercialization.
Our results of operations will be affected by the level of royalty and milestone payments that we are required to pay to third parties.
The LLU License Agreement and February 2020 License Agreement with Stanford each require us to remit royalty payments and meet certain performance milestones related to in-licensed intellectual property. Any failure on our part to pay royalties owed or meet milestones could lead to us losing rights under our licenses and could thereby adversely affect our business. As our product sales increase, we may, from time-to-time, disagree with our third-party collaborators as to the appropriate royalties owed and the resolution of such disputes may be costly and may consume management’s time. Furthermore, we may enter into additional license agreements in the future, which may also include royalty payments.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of drugs is highly competitive. We compete with a variety of multinational pharmaceutical companies and specialized biotechnology companies, as well as products and processes being developed at universities and other research institutions. Our competitors have developed, are developing or will develop product candidates and processes competitive with our product candidates. Competitive therapeutic treatments include those that have already been approved and accepted by the medical community and any new treatments that may enter the market. We believe that a significant number of products are currently available, under development, and may become commercially available in the future, for the treatment of indications for which we may try to develop product candidates.
More established companies may have a competitive advantage over us due to their greater size, cash flows and institutional experience. Compared to us, many of our competitors may have significantly greater financial, technical and human resources. As a result of these factors, our competitors may have an advantage in marketing their approved products and may obtain regulatory approval of their product candidates before we are able to, which may limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are safer, more effective, more widely used and less expensive than ours, and may also be more successful than us in manufacturing and marketing their products.
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These companies compete with us in recruiting and retaining qualified scientific, management and commercial personnel, establishing clinical trial sites and subject registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Our technologies and products under development, and our business, may fail if we are not able to successfully commercialize them and ultimately generate significant revenues as a result.
Successful development of technologies and our product candidates will require significant additional investment, including costs associated with additional development, completing trials and obtaining regulatory approval, as well as the ability to manufacture or have others manufacture our products in sufficient quantities at acceptable costs while also preserving product quality. Difficulties often encountered in scaling up production include problems involving production yields, quality control and assurance, shortage of qualified personnel, production costs and process controls. In addition, we are subject to inherent risks associated with new technologies and products. These risks include the possibility that any of our technologies or future products may:
| ● | be found unsafe; |
| ● | be ineffective or less effective than anticipated; |
| ● | fail to receive necessary regulatory approvals; |
| ● | be difficult to competitively price relative to alternative solutions; |
| ● | be harmful to consumers or the environment; |
| ● | be difficult to manufacture on an economically viable scale; |
| ● | be subject to supply chain constraints for raw materials; |
| ● | fail to be developed and accepted by the market prior to the successful marketing of alternative products by competitors; |
| ● | be difficult to market because of infringement on the proprietary rights of third parties; or |
| ● | be too expensive for commercial use. |
Furthermore, we may be faced with lengthy market partner or distributor evaluation and approval processes. Consequently, we may incur substantial expenses and devote significant management effort in order to customize products for market partner or distributor acceptance, though there can be no assurance of such acceptance. As a result, we cannot accurately predict the volume or timing of any future sales.
Customers may not adopt our products quickly, or at all.
Customers in the sector in which we operate can be generally cautious in their adoption of new products and technologies. In addition, given the relative novelty of our future planned products (including our AditxtScore™ platform), customers of those products may require education regarding their utility and use, which may delay their adoption. There can be no assurance that customers will adopt our products quickly, or at all.
The significant level of competition in the markets for our products developed in the future may result in pricing pressure, reduced margins or the inability of our future products to achieve market acceptance.
The markets for our future products are intensely competitive and rapidly changing. We may be unable to compete successfully, which may result in price reductions, reduced margins and the inability to achieve market acceptance for our products.
Our competitors may have longer operating histories, significantly greater resources, greater brand recognition and large customer bases than we do. As a result, they may be able to devote greater resources to the manufacture, promotion or sale of their products, receive greater resources and support from market partners and independent distributors, initiate or withstand substantial price competition or more readily take advantage of acquisition or other opportunities.
We rely on third parties for the distribution of our current and future products, including our AditxtScore™ platform. If these parties do not distribute our products in a satisfactory or timely manner, in sufficient quantities or at an acceptable cost, our sales and development efforts could be delayed or otherwise negatively affected.
We rely on third parties for the distribution of our current and future products, including our AditxtScore™ platform. Our reliance on third parties to distribute products may present significant risks to us, including the risk that should any of these third parties fail to adequately distribute our products and services to end consumers and other market participants, our business may be materially harmed. Additionally, if we need to enter into agreements for the distribution of our future products with other third parties, there can be no assurance we will be able to do so on favorable terms, if at all.
We may rely on third parties for the production of our future products. If these parties do not produce our products at a satisfactory quality, in a timely manner, in sufficient quantities or at an acceptable cost, our sales and development efforts could be delayed or otherwise negatively affected.
We may rely on third parties for the manufacture of our future products. Our reliance on third parties to manufacture our future products may present significant risks to us, including the following:
| ● | reduced control over delivery schedules, yields and product reliability; |
| ● | price increases; |
| ● | manufacturing deviations from internal and regulatory specifications; |
| ● | the failure of a key manufacturer to perform as we require for technical, market or other reasons; |
| ● | difficulties in establishing additional manufacturer relationships if we are presented with the need to transfer our manufacturing process technologies to them; |
| ● | misappropriation of our intellectual property; and |
| ● | other risks in potentially meeting our product development schedule or satisfying the requirements of our market partners, distributors, direct customers and end users. |
If we need to enter into agreements for the manufacturing of our future products, there can be no assurance we will be able to do so on favorable terms, if at all.
If we are unable to establish successful relations with third-party market partners or distributors, or these market partners or distributors do not focus adequate resources on selling our products or are otherwise unsuccessful in selling them, sales of our products may not develop.
We anticipate relying on independent market partners and distributors to distribute and assist us with the marketing and sale of our products. Our future revenue generation and growth will depend in large part on our success in establishing and maintaining this sales and distribution channel. If our market partners and distributors are unable to sell our products, or receive negative feedback from end users, they may not continue to purchase or market our products. In addition, there can be no assurance that our market partners and distributors will focus adequate resources on selling our products to end users or will be successful in selling them. Many of our potential market partners and distributors are in the business of distributing and sometimes manufacturing other, possibly competing, products. As a result, these market partners and distributors may perceive our products as a threat to various product lines currently being distributed or manufactured by them. In addition, these market partners and distributors may earn higher margins by selling competing products or combinations of competing products. If we are unable to establish successful relationships with independent market partners and distributors, we will need to further develop our own sales and distribution capabilities, which would be expensive and time-consuming and might not be successful.
If we are not able to attract and retain highly skilled employees and contractors, we may not be able to implement our business model successfully.
We will rely upon employees and third-party consultant/contractors to effectively establish, manage and grow our business. Consequently, we believe that our future viability will depend largely on our ability to attract and retain highly skilled personnel. In order to do so, we may need to pay higher compensation, fees, and/or other incentives to our employees or consultants than we currently expect, and such higher compensation payments would have a negative effect on our operating results. Competition for experienced, high-quality employees, consultants and contractors is intense and we cannot assure that we will be able to recruit and retain such personnel. We may not be able to hire or retain the necessary personnel to implement our business strategy. Our failure to hire and retain such personnel could impair our ability to develop new products and manage our business effectively.
The loss of our management team or other key personnel would have an adverse impact on our future development and impair our ability to succeed.
In the early stages of development, our business will be significantly dependent on the Company’s management team and other key personnel. Our success will be particularly dependent upon our Chief Executive Officer, Mr. Amro Albanna and our Chief Innovation Officer, Dr. Shahrokh Shabahang. The loss of any one of these individuals or any other future key personnel could have a material adverse effect on the Company and our ability to further execute our intended business.
The use of our products may be limited by regulations, and we may be exposed to product liability and remediation claims.
The use of our planned products may be regulated by various local, state, federal and foreign regulators. Even if we are able to comply with all such regulations and obtain all necessary registrations, we cannot provide assurance that our future products will not cause injury to the environment, people, or animals and/or otherwise have unintended adverse consequences, under all circumstances. For example, our products may be improperly combined with other chemicals or, even when properly combined, our products may be blamed for damage caused by those other chemicals. The costs of remediation or products liability could materially adversely affect our results, financial condition and operations.
We may be held liable for, or incur costs to settle, liability and remediation claims if any products we develop, or any products that use or incorporate any of our technologies, cause injury or are found unsuitable during product testing, manufacturing, marketing, sale or use. These risks exist even with respect to products that have received, or may in the future receive, regulatory approval, registration or clearance for commercial use. We cannot guarantee that we will be able to avoid product liability exposure.
At the stage customary to do so, we expect to maintain product liability insurance at levels we believe are sufficient and consistent with industry standards for like companies and products. However, we cannot guarantee that our product liability insurance will be sufficient to help us avoid product liability-related losses. In the future, it is possible that meaningful insurance coverage may not be available on commercially reasonable terms or at all. In addition, a product liability claim could result in liability to us greater than our assets or insurance coverage. Moreover, even if we have adequate insurance coverage, product liability claims or recalls could result in negative publicity or force us to devote significant time and attention to these matters, which could harm our business.
There may be limitations on the effectiveness of our internal controls, and a failure of our control systems to prevent error or fraud may materially harm our Company.
We do not expect that internal control over financial accounting and disclosure, even if timely and well established, will prevent all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Failure of our control systems to prevent error or fraud could materially adversely affect our business.
COVID-19 may impact our operations.
On January 30, 2020 the World Health Organization declared the COVID-19 coronavirus outbreak a “Public Health Emergency of International Concern” and on March 10, 2020, declared it to be a pandemic. Actions taken around the world to help mitigate the spread of the coronavirus include restrictions on travel, and quarantines in certain areas, and forced closures for certain types of public places and businesses. The COVID-19 coronavirus and actions taken to mitigate it have had and are expected to continue to have an adverse impact on the economies and financial markets of many countries, including the geographical area in which the Company operates. While it is unknown how long these conditions will last and what the complete financial effect will be to the Company, capital raise efforts and additional development of our technologies may be negatively affected.
Risks Relating to Our Intellectual Property Rights
The failure to obtain or maintain patents, licensing agreements and other intellectual property could materially impact our ability to compete effectively.
In order for our business to be viable and to compete effectively, we need to develop and maintain, and we will heavily rely on, a proprietary position with respect to our technologies and intellectual property. However, there are significant risks associated with our actual or proposed intellectual property. The risks and uncertainties that we face with respect to our rights principally include the following:
| ● | pending patent applications we have filed or will file may not result in issued patents or may take longer than we expect to result in issued patents; |
| ● | we may be subject to interference proceedings; |
| ● | we may be subject to reexamination proceedings; |
| ● | we may be subject to post grant review proceedings; |
| ● | we may be subject to inter partes review proceedings; |
| ● | we may be subject to derivation proceedings; |
| ● | we may be subject to opposition proceedings in the U.S. or in foreign countries; |
| ● | any patents that are issued to us may not provide meaningful protection; |
| ● | we may not be able to develop additional proprietary technologies that are patentable; |
| ● | other companies may challenge patents licensed or issued to us; |
| ● | other companies may have independently developed and patented (or may in the future independently develop and patent) similar or alternative technologies, or duplicate our technologies; |
| ● | other companies may design around technologies we have licensed or developed; |
| ● | enforcement of patents is complex, uncertain and very expensive and we may not be able to secure, enforce and defend our patents; and |
| ● | in the event that we were to ever seek to enforce our patents in ligation, there is some risk that they could be deemed invalid, not infringed, or unenforceable. |
We cannot be certain that any patents will be issued as a result of any pending or future applications, or that any patents, once issued, will provide us with adequate protection from competing products. For example, issued patents may be circumvented or challenged, declared invalid or unenforceable, or narrowed in scope. In addition, since publication of discoveries in scientific or patent literature often lags behind actual discoveries, we cannot be certain that we or our licensors were the first to invent or to file patent applications covering them.
It is also possible that others may have or may obtain issued patents that could prevent us from commercializing our products or require us to obtain licenses requiring the payment of significant fees or royalties in order to enable us to conduct our business. There is no guarantee that such licenses will be available based on commercially reasonable terms. As to those patents that we have licensed, our rights depend on maintaining our obligations to the licensor under the applicable license agreement, and we may be unable to do so.
If we are unable to obtain and maintain patent protection for our products, or if the scope of the patent protection obtained is not sufficiently broad, competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our products could be impaired.
The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost, in a timely manner, or in all jurisdictions. It is also possible that we will fail to identify patentable aspects of our development output before it is too late to obtain patent protection.
The patent position of life science companies generally is highly uncertain, involves complex legal and factual questions and has in past years been the subject of much litigation. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States and we may fail to seek or obtain patent protection in all major markets. For example, unlike the U.S., European patent law restricts the patentability of methods of treatment of the human body. Our pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection, even post-grant.
Recent patent reform legislation has increased the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to United States patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The U.S. Patent and Trademark Office, or USPTO, recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
Moreover, we may be subject to a third-party pre-issuance submission of prior art to the USPTO, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights (whether licensed or otherwise held) or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights (whether licensed or otherwise held), allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications (whether licensed or otherwise held) is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Even if our patent applications (whether licensed or otherwise held) result in the issuance of patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our licensed or owned patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical products, or limit the duration of the patent protection of our products. Given the amount of time required for the development, testing and regulatory review of new life science product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our intellectual property rights portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may become involved in lawsuits to protect or enforce our intellectual property rights, which could be expensive, time-consuming and ultimately unsuccessful.
Competitors may infringe our intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their intellectual property or that our intellectual property is invalid or unenforceable. In addition, in a patent infringement proceeding, a court may decide that a licensed or owned patent of ours is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover that technology. Moreover, lawsuits to protect or enforce our intellectual property rights could be expensive, time-consuming and ultimately unsuccessful.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain.
Our commercial success depends upon our ability to develop, manufacture, market and sell our product candidates without infringing the proprietary rights of third parties. There is considerable intellectual property litigation in the life sciences industry. We cannot guarantee that our product candidates will not infringe third-party patents or other proprietary rights. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including inter partes review, interference, or derivation proceedings before the USPTO and similar bodies in other countries. Third parties may assert infringement claims against us based on existing intellectual property rights and intellectual property rights that may be granted in the future.
If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our own patent protection could be reduced or eliminated for noncompliance with these requirements.
Periodic maintenance fees and annuities on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter our markets, which could have a material adverse effect on our business.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property or claiming ownership of what we regard as our own intellectual property.
Certain of our employees and contractors were previously employed at universities or other companies, including potential competitors. Although we try to ensure that our employees and contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these employees or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims, and any such litigation could have an unfavorable outcome.
In addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our and their assignment agreements may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and adverse results, and be a distraction to management.
Some intellectual property which we own or have licensed may have been discovered through government funded programs such as, for example, the government funded programs referenced in intellectual property licensed under the LLU License Agreement, and thus may be subject to federal regulations such as “march-in” rights, certain reporting requirements, and a preference for United States industry. Compliance with such regulations may limit our exclusive rights, subject us to expenditure of resources with respect to reporting requirements, and limit our ability to contract with non-U.S. manufacturers.
Some of the intellectual property rights we own or have licensed have been generated through the use of United States government funding and may therefore be subject to certain federal regulations. As a result, the United States government may have certain rights to intellectual property embodied in our current or future products and product candidates pursuant to the Bayh-Dole Act of 1980. These United States government rights in certain inventions developed under a government-funded program include a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the United States government has the right to require us to grant exclusive, partially exclusive, or non-exclusive licenses to any of these inventions to a third party if it determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations (also referred to as “march-in rights”). The United States government also has the right to take title to these inventions if we fail to disclose the invention to the government and fail to file an application to register the intellectual property within specified time limits. In addition, the United States government may acquire title to these inventions in any country in which a patent application is not filed within specified time limits. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance with which may require us to expend substantial resources. In addition, the United States government requires that any products embodying the subject invention or produced through the use of the subject invention be manufactured substantially in the United States. The manufacturing preference requirement can be waived if the owner of the intellectual property can show that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible. This preference for United States manufacturers may limit our ability to contract with non-U.S. product manufacturers for products covered by such intellectual property. Any exercise by the government of any of the foregoing rights could harm our competitive position, business, financial condition, results of operations and prospects.
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have an adverse effect on the price of our common stock. Such litigation or proceedings could increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise our ability to compete in the marketplace.
We may spend considerable resources developing and maintaining patents, licensing agreements and other intellectual property that may later be abandoned or may otherwise never result in products brought to market.
Not all technologies and candidate products that initially show potential as the basis for future products ultimately meet the rigors of our development process and as a result may be abandoned and/or never otherwise result in products brought to market. In some cases, prior to abandonment we may be required to incur significant costs developing and maintaining intellectual property and/or maintaining license agreements and our business could be harmed by such costs.
We rely on information technology, and if we are unable to protect against service interruptions, data corruption, cyber-based attacks or network security breaches, our operations could be disrupted, and our business could be negatively affected.
We rely on information technology networks and systems to process, transmit and store electronic and financial information; to coordinate our business; and to communicate within our Company and with customers, suppliers, partners and other third-parties. These information technology systems may be susceptible to damage, disruptions or shutdowns, hardware or software failures, power outages, computer viruses, cyber-attacks, telecommunication failures, user errors or catastrophic events. If our information technology systems suffer severe damage, disruption or shutdown, and our business continuity plans do not effectively resolve the issues in a timely manner, our operations could be disrupted, and our business could be negatively affected. In addition, cyber-attacks could lead to potential unauthorized access and disclosure of confidential information, and data loss and corruption. There is no assurance that we will not experience these service interruptions or cyber-attacks in the future.
Risks Related to Our Common Stock
We are currently listed on Theunder a panel monitor from Nasdaq Capitalas we have historically failed to comply with certain listing requirements of the Nasdaq Stock Market, which could result in our Common Stock being delisted from the Nasdaq Stock Market.
On November 21, 2023, the Company received written notice from Nasdaq that it had regained compliance with the Public Float Rule. On December 29, 2023, the Company received written notice from Nasdaq that it had regained compliance with the Stockholders’ Equity Rule, but will be subject to a Mandatory Panel Monitor for a period of one year.
If we are unabledelisted from Nasdaq, our common stock may be eligible for trading on an over-the-counter market. If we are not able to maintainobtain a listing on another stock exchange or quotation service for our common stock, it may be extremely difficult or impossible for stockholders to sell their shares. We intend to monitor the closing bid price of our securitiescommon stock and may be required to seek approval from our stockholders to affect a reverse stock split of the issued and outstanding shares of our common stock. However, there can be no assurance that the reverse stock split would be approved by our stockholders. Further, there can be no assurance that the market price per new share of our common stock after the reverse stock split will remain unchanged or increase in proportion to the reduction in the number of old shares of our common stock outstanding before the reverse stock split. Even if the reverse stock split is approved by our stockholders, there can be no assurance that we will be able to regain compliance with the minimum bid price requirement or will otherwise be in compliance with other Nasdaq listing rules.
If we are delisted from Nasdaq, but obtain a substitute listing for our common stock, it will likely be on a market with less liquidity, and therefore experience potentially more price volatility than experienced on Nasdaq. Stockholders may not be able to sell their shares of common stock on any such substitute market in the quantities, at the times, or at the prices that could potentially be available on a more liquid trading market. As a result of these factors, if our common stock is delisted from Nasdaq, or any stock exchange, our stock price could be adversely affectedthe value and the liquidity of our common stock, warrants and pre-funded warrants would likely be significantly adversely affected. A delisting of our common stock from Nasdaq could also adversely affect our ability to obtain financing could be impaired and it may be more difficult for our stockholders to sell their securities.
Although our common stock is currently listed on The Nasdaq Capital Market, we may not be able to continue to meet the exchange’s minimum listing requirements operations and/or thoseresult in a loss of any other national exchange. If we are unable to maintain listing on Nasdaq confidence by investors, employees and/or if a liquid market for our common stock does not develop or is sustained, our common stock may remain thinly traded.
The listing rules of Nasdaq require listing issuers to comply with certain standards in order to remain listed on its exchange. If, for any reason, we should fail to maintain compliance with these listing standards and Nasdaq should delist our securities from trading on its exchange and we are unable to obtain listing on another national securities exchange, a reduction in some or all of the following may occur, each of which could have a material adverse effect on our stockholders:
business partners.
29
We do not expect to pay dividends in the foreseeable future.
We do not intend to declare dividends for the foreseeable future, as we anticipate that we will reinvest any and all future earnings in the development and growth of our business. Therefore, investors will not receive any funds unless they sell their securities, and holders may be unable to sell their securities on favorable terms or at all. We cannot assure you of a positive return on your investment or that you will not lose the entire amount of your investment.
Future sales or issuances of substantial amounts of our common stock, including, potentially, as a result of the future acquisitions or strategic transactions, including the transaction with Cellvera Global, could result in significant dilution.
On December 28, 2021, we entered into a Share Exchange Agreement with Cellvera Global f/k/a AiPharma Global, pursuant to which we (i) will acquire 9.5% of the issued and outstanding equity interests in Cellvera Global in exchange for the issuance of 61 shares of our common stock of Aditxt and a cash payment of $250,000, at an initial closing upon the satisfaction or waiver of certain conditions to closing; and (ii) acquire the remaining 90.5% of the issued and outstanding equity interests in Cellvera Global in exchange for the issuance of 500 shares of our common stock and a cash payment of $250,000 at a secondary closing upon the satisfaction or waiver of certain conditions to closing. Additionally, we may elect to raise additional capital due to market conditions or strategic considerations. If additional shares are issued in connection with the proposed acquisition transaction or additional capital is raised through the sale of equity or convertible debt securities, the issuance of those securities could result in further dilution to our stockholders.
While we have entered into a Share Exchange Agreement with Cellvera Global, we cannot assure you that the transactions contemplated by the Share Exchange Agreement will be consummated or, that if such transactions are consummated, they will be accretive to stockholder value.
The initial closing under the Share Exchange Agreement was expected to occur on or before January 31, 2022. We can provide no assurance that the conditions to the initial closing will be satisfied. Further, even if we are able to complete the initial closing following the satisfaction of such conditions, there is no guarantee that the conditions to the secondary closing, including but not limited to, the approval of the transaction by our stockholders, will be completed in the time frame or in the manner currently anticipated, or that we will recognize the anticipated benefits of the transaction.
In connection with the contemplated acquisition of Cellvera Global, we have provided secured loans to Cellvera Global in the aggregate principal amount of $14.5 million, which amounts came due on January 31, 2022. Although, we have agreed to forbear from exercising our rights and remedies against Cellvera Global while we continue to work towards an initial closing under the Share Exchange Agreement, if we are unable to complete the transactions contemplated by the Share Exchange Agreement, we cannot provide any assurance that we will be able to timely collect such amounts from Cellvera Global, if at all.
In connection with the contemplated acquisition of Cellvera Global, we entered into a Secured Credit Agreement with Cellvera Global, pursuant to which we have provided secured loans to Cellvera Global in the aggregate principal amount of $14.5 million, which amounts became due on January 31, 2022. On February 14, 2022, we entered into a Forbearance Agreement with Cellvera Global, pursuant to which we agreed to forbear from exercising our rights and remedies against Cellvera Global until the earlier of June 30, 2022 or the date of any default under the Forbearance Agreement. Under the Forbearance Agreement, the Company and the Borrower also agreed to certain amendments to the Credit Agreement, including, but not limited to: (i) the delivery by Cellvera Global of certain financial statements and forecasts, and (ii) certain regularly scheduled payments to be made by Cellvera Global to the Company during the forbearance period. If Cellvera Global defaults upon its obligations under the Forbearance Agreement or if we are otherwise unable to complete the contemplated acquisition of Cellvera Global under the Share Exchange Agreement, we cannot provide any assurance that we will be able to time collect the amounts due under the Secured Credit Agreement, if at all. The note receivable to Cellvera Global was deemed impaired and written down to zero as of December 31, 2021.
We may engage in future acquisitions or strategic transactions, which may require us to seek additional financing or financial commitments, increase our expenses and/or present significant distractions to our management.
As described herein, we entered into a Share Exchange Agreement with Cellvera Global in December 2021. We have also entered into other non-binding letters of intent. We may need to acquire additional financing to fund our obligations under the Share Exchange Agreement, the letter of intent or to fund other potential acquisitions or strategic transactions (particularly, if the acquired entity is not cash flow positive or does not have significant cash on hand). Obtaining financing through the issuance or sale of additional equity and/or debt securities, if possible, may not be at favorable terms and may result in additional dilution to our current stockholders. Additionally, any such transaction may require us to incur non-recurring or other charges, may increase our near and long-term expenditures and may pose significant integration challenges or disrupt our management or business, which could adversely affect our operations and financial results. For example, an acquisition or strategic transaction may entail numerous operational and financial risks, including the risks outlined above and additionally:
| ● | exposure to unknown liabilities; |
| ● | disruption of our business and diversion of our management’s time and attention in order to develop acquired products or technologies; |
| ● | higher than expected acquisition and integration costs; |
| ● | write-downs of assets or goodwill or impairment charges; |
| ● | increased amortization expenses; |
| ● | difficulty and cost in combining the operations and personnel of any acquired businesses with our operations and personnel; |
| ● | impairment of relationships with key suppliers or customers of any acquired businesses due to changes in management and ownership; and |
| ● | inability to retain key employees of any acquired businesses. |
Accordingly, although there can be no assurance that we will undertake or successfully complete any transactions of the nature described above, and any transactions that we do complete could have a material adverse effect on our business, results of operations, financial condition and prospects.
Upon dissolution of our Company, you may not recoup all or any portion of your investment.
In the event of a liquidation, dissolution or winding-up of our Company, whether voluntary or involuntary, our assets would be used to pay all of our debts and liabilities, and only thereafter would any remaining assets be distributed to our stockholders, subject to rights of the holders of the Preferred Stock, if any, on a pro rata basis. There can be no assurance that we will have assets available from which to pay any amounts to our stockholders upon such a liquidation, dissolution or winding-up. In such an event, you would lose all of your investment.
Limitation of Liability and Indemnification of Management.
The Delaware General Corporation Law and the Company’s Amended and Restated Certificate of Incorporation provide for the limitation of the liability of directors for monetary damages. Such provisions may discourage shareholders from bringing a lawsuit against directors for breaches of fiduciary duty and may also have the effect of reducing the likelihood of derivative litigation against directors and officers even though such action, if successful, might otherwise be a benefit to the Company’s shareholders. In addition, a shareholder’s investment in the Company may be adversely affected to the extent that costs of settlement and damage awards against the Company’s officers or directors are paid by the Company pursuant to such provisions. Additionally, in accordance with Delaware law and the Company’s Amended and Restated Certificate of Incorporation, the Company shall indemnify, hold harmless and provide advancement of expenses, to the fullest extent permitted by applicable law, directors, officers, employees, and agents that are made a party or threatened to be made a party to legal proceedings by reason of the fact that such parties were working at the request of the Company. We direct you to the Company’s Amended and Restated Certificate of Incorporation for more information.
Anti-takeover provisions under Delaware law could discourage, delay or prevent a change in control of our Company and could affect the trading price of our securities.
We are a Delaware corporation and the anti-takeover provisions of the Delaware General Corporation Law may discourage, delay or prevent a change in control by prohibiting us from engaging in a business combination with an interested stockholder for a period of three years after the person becomes an interested stockholder, even if a change in control would be beneficial to our existing stockholders.
Our management team is required to devote substantial time to public company compliance initiatives.
As a publicly reporting company, we incur significant legal, accounting and other expenses. Our management and other personnel devote a substantial amount of time to comply with our reporting obligations. Moreover, these reporting obligations increase our legal and financial compliance costs and make some activities more time-consuming and costly.
Failure to develop our internal controls over financial reporting as we grow could have an adverse impact on us.
As our Company matures, we will need to develop our current internal control systems and procedures to manage our growth. We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish appropriate controls, or any failure of those controls once established, could adversely impact our public disclosures regarding our business, financial condition or results of operations. In addition, management’s assessment of internal controls over financial reporting may identify weaknesses and conditions that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting, disclosure of management’s assessment of our internal controls over financial reporting or disclosure of our public accounting firm’s attestation to or report on management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our common stock.
We could issue “blank check” preferred stock without stockholder approval with the effect of diluting interests of then-current stockholders and impairing their voting rights, and provisions in our charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable.
Our Amended and Restated Certificate of Incorporation provides for the authorization to issue up to 3,000,000 shares of “blank check” preferred stock with designations, rights and preferences as may be determined from time to time by our board of directors. Our board of directors is empowered, without stockholder approval, to issue one or more series of preferred stock with dividend, liquidation, conversion, voting or other rights which could dilute the interest of, or impair the voting power of, our common stockholders. The issuance of a series of preferred stock could be used as a method of discouraging, delaying or preventing a change in control. For example, it would be possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of our company. In addition, advanced notice is required prior to stockholder proposals, which might further delay a change of control.
Our Amended and Restated Certificate of Incorporation provides that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for substantially all disputes between the Company and its stockholders, which could limit stockholders’ ability to obtain a favorable judicial forum for disputes with the Company or its directors, officers or employees.
Our Amended and Restated Certificate of Incorporation provides that unless the Company consents in writing to the selection of an alternative forum, the State of Delaware is the sole and exclusive forum for: (i) any derivative action or proceeding brought on behalf of the Company, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Company to the Company or the Company’s stockholders, (iii) any action asserting a claim against the Company, its directors, officers or employees arising pursuant to any provision of the Delaware General Corporation Law (the “DGCL”) or our Amended and Restated Certificate of Incorporation or the Company’s Amended and Restated Bylaws, or (iv) any action asserting a claim against the Company, its directors, officers, employees or agents governed by the internal affairs doctrine, except for, as to each of (i) through (iv) above, any claim as to which the Court of Chancery determines that there is an indispensable party not subject to the jurisdiction of the Court of Chancery (and the indispensable party does not consent to the personal jurisdiction of the Court of Chancery within ten days following such determination), which is vested in the exclusive jurisdiction of a court or forum other than the Court of Chancery, or for which the Court of Chancery does not have subject matter jurisdiction. This exclusive forum provision would not apply to suits brought to enforce any liability or duty created by the Securities Act or the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. To the extent that any such claims may be based upon federal law claims, Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder.
Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. However, our Amended and Restated Bylaws contain a federal forum provision which provides that unless the Company consents in writing to the selection of an alternative forum, the federal district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Any person or entity purchasing or otherwise acquiring any interest in shares of capital stock of the Corporation are deemed to have notice of and consented to this provision. The Supreme Court of Delaware has held that this type of exclusive federal forum provision is enforceable. There may be uncertainty, however, as to whether courts of other jurisdictions would enforce such a provision, if applicable.
These choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with the Company or its directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. Alternatively, if a court were to find our choice of forum provisions contained in either our Amended and Restated Certificate of Incorporation or Amended and Restated Bylaws to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations, and financial condition.
We are an “emerging growth company” and will be able to avail ourselves of reduced disclosure requirements applicable to emerging growth companies, which could make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. In addition, pursuant to Section 107 of the JOBS Act, as an “emerging growth company” we intend to take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act, for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. As a result, our financial statements may not be comparable to those of companies that comply with public company effective dates for complying with new or revised accounting standards.
We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.” We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of our initial public offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 2. Properties.
We lease property consisting of office and laboratory space located at 2569 Wyandotte, St., Suite 101 Mountain View, CA 94043. The lease expires on August 31, 2024, subject to extension. As of December 31, 2023 the Company is one month in arrears on our Mountain View lease.
We lease property consisting of office space located at 532 Broadhollow Road, Suite 118, Melville, NY 11747. The lease expires on December 31, 2025, subject to extension. As of December 31, 2023 the Company is one month in arrears on our Melville lease.
We lease property consisting of office and laboratory space located at 737 N. 5th Street Richmond, Virginia 23219. The lease expires on August 31, 2026, subject to extension. As of December 31, 2023 the Company is 1.75 months in arrears on our Richmond lease.
Item 3. Legal Proceedings.
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
On June 30, 2020, our common stock began trading on the Nasdaq Capital Market under the symbol “ADTX.” Prior to that time, there was no public market for our common stock.
Holders
As of April 12, 2024, there were approximately 168 record holders of our common stock. As of April 12, 2024, there were 5, 13, and 1 holder(s) of Series A-1 Convertible Preferred Stock, Series B-1 Convertible Preferred Stock, and Series B-2 Convertible Preferred Stock, respectively. The actual number of holders of our common stock is greater than this number of record holders, and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers or held by other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividend Policy
We have never paid or declared any cash dividends on our common stock, and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain all available funds and any future earnings to fund the development and expansion of our business. Any future determination to pay dividends will be at the discretion of our board of directors and will depend upon a number of factors, including our results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors that our board of directors deems relevant.
Recent Sales of Unregistered Securities
On March 17, 2023, the Company issued a consultant 4,675 shares of common stock for services rendered.
On December 19, 2023, the Company issued a consultant 70,000 shares of common stock for services rendered.
The issuances above were made pursuant to Section 4(a)(2) of the Securities Act.
Equity Compensation Plans
The information required by Item 5 of Form 10-K regarding equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report on Form 10-K.
Issuer Purchases of Equity Securities
We did not purchase any of our registered equity securities during the period covered by this Annual Report.
Use of Proceeds from Initial Public Offering
On July 2, 2020, we completed our initial public offering (“IPO”). In connection therewith, we issued 614 Units (the “IPO Units”), excluding the underwriters’ option to cover overallotments, at an offering price of $18,000.00 per IPO Unit, resulting in gross proceeds of approximately $11.0 million. The IPO Units issued in the IPO consisted of one share of common stock, one Series A warrant, and one Series B warrant. The Series A warrants originally had an exercise price of $18,000.00 and a term of 5 years. In addition, we issued a Unit Purchase Option at an exercise price of $22,500.00 per unit to the underwriters to purchase up to 34 units, with each unit consisting of (i) one share of common stock and (ii) one Series A Warrant. On August 19, 2020 we modified the exercise price of the Series A Warrants from $18,000.00 per share to $9,000.00 per share. The term of the Series A Warrants was not modified. The Series B warrants have an exercise price of $22,500.00 per share, a term of 5 years and contain a cashless exercise option upon certain criteria being met. As of December 31, 2020, substantially all of the Series B warrants issued in the IPO have been exercised pursuant to a cashless provision therein.
We received net proceeds of $8.5 million in the IPO, after deducting underwriting discounts and commissions and issuance expenses borne by us. No payments were made by us to directors, officers or persons owning ten percent or more of our common stock or to their associates, or to our affiliates, other than payments in the ordinary course of business to officers for salaries and to non-employee directors pursuant to our director compensation policy. Dawson James Securities, Inc. acted as lead book-running manager of the offering and as representative of the underwriters for the offering.
There has been no material change in the planned use of proceeds from our IPO from that described in the final prospectus related to the offering, dated June 29, 2020, as filed with the SEC.
Item 6. [Reserved]
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read together with our financial statements and the related notes and other financial information included elsewhere in this report. Some of the information contained in this discussion and analysis or set forth elsewhere in this report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. See “Cautionary Note Regarding Forward-Looking Statements.”
Overview and Mission
We believe the world needs—and deserves—a new approach to innovating that harnesses the power of large groups of stakeholders who work together to ensure that the most promising innovations make it into the hands of people who need them most.
We were incorporated in the State of Delaware on September 28, 2017, and our headquarters are in Richmond, Virginia. The company was founded with a mission of bringing stakeholders together, to transform promising innovations into products and services that could address some of the most challenging needs. The socialization of innovation through engaging stakeholders in every aspect of it, is key to transforming more innovations, more rapidly, and more efficiently.
At inception, the first innovation we took on was an immune modulation technology titled ADI/Adimune with a focus on prolonging life and enhancing life quality of patients that have undergone organ transplants. Since then, we expanded our portfolio of innovations, and we continue to evaluate a variety of promising health innovations.
ADIMUNE, INC.
Formed in January 2023, Adimune™, Inc. (“Adimune”) is focused on leading our immune modulation therapeutic programs. Adimune’s proprietary immune modulation product candidate, ADI-100™, based on the Apoptotic DNA Immunotherapy™ platform technology, utilizes a novel approach that mimics the way our bodies naturally induce tolerance to our own tissues. It includes two DNA molecules designed to deliver signals to induce tolerance. ADI-100 has been successfully tested in several preclinical models (e.g., skin grafting, psoriasis, type 1 diabetes, multiple sclerosis).
In May 2023, Adimune entered into a clinical trial agreement with Mayo Clinic to advance clinical studies targeting autoimmune diseases of the central nervous system (“CNS”) with the initial focus on the rare, but debilitating, autoimmune disease Stiff Person Syndrome (“SPS”). According to the National Organization of Rare Diseases, the exact incidence and prevalence of SPS is unknown; however, one estimate places the incidence at approximately one in one million individuals in the general population.
Pending approval by the International Review Board and U.S. Food and Drug Administration, a human trial for SPS is expected get underway in the first half of 2024 with enrollment of up to 20 patients, some of whom may also have type 1 diabetes. ADI-100 will initially be tested for safety and efficacy. ADI-100 is designed to tolerize against an antigen known as glutamic acid decarboxylase (“GAD”), which is implicated in type-1 diabetes, psoriasis, stiff person syndrome, and in many autoimmune diseases of the CNS. IND-enabling work is also near completion in support of a Clinical Trial Application submission to the Paul Ehrlich Institute, the regulatory agency in Germany, to initiate clinical trials in psoriasis and type 1 diabetes.
Background
The discovery of immunosuppressive (anti-rejection and monoclonal) drugs over 40 years ago has made possible life-saving organ transplantation procedures and blocking of unwanted immune responses in autoimmune diseases. However, immune suppression leads to significant undesirable side effects, such as increased susceptibility to life-threatening infections and cancers, because it indiscriminately and broadly suppresses immune function throughout the body. While the use of these drugs has been justifiable because they prevent or delay organ rejection, their use for treatment of autoimmune diseases and allergies may not be acceptable because of the aforementioned side effects. Furthermore, often transplanted organs ultimately fail despite the use of immune suppression, and about 40% of transplanted organs survive no more than five years.
Through Aditxt, Adimune has the right of use to the exclusive worldwide license for commercializing ADI nucleic acid-based technology (which is currently at the pre-clinical stage) from Loma Linda University. ADI uses a novel approach that mimics the way the body naturally induces tolerance to our own tissues (“therapeutically induced immune tolerance”). While immune suppression requires continuous administration to prevent rejection of a transplanted organ, induction of tolerance has the potential to retrain the immune system to accept the organ for longer periods of time. ADI may allow patients to live with transplanted organs with significantly reduced immune suppression. ADI is a technology platform which we believe can be engineered to address a wide variety of indications.
Advantages
ADI™ is a nucleic acid-based technology (e.g., DNA-based), which we believe selectively suppresses only those immune cells involved in attacking or rejecting self and transplanted tissues and organs. It does so by tapping into the body’s natural process of cell turnover (i.e., apoptosis) to retrain the immune system to stop unwanted attacks on self or transplanted tissues. Apoptosis is a natural process used by the body to clear dying cells and to allow recognition and tolerance to self-tissues. ADI triggers this process by enabling the cells of the immune system to recognize the targeted tissues as “self.” Conceptually, it is designed to retrain the immune system to accept the tissues, similar to how natural apoptosis reminds our immune system to be tolerant to our own “self” tissues.
While various groups have promoted tolerance through cell therapies and ex vivo manipulation of patient cells (i.e., takes place outside the body), to our knowledge, we will be unique in our approach of using in-body induction of apoptosis to promote tolerance to specific tissues. In addition, ADI treatment itself will not require additional hospitalization but only an injection of minute amounts of the therapeutic drug into the skin.
Moreover, preclinical studies have demonstrated that ADI treatment significantly and substantially prolongs graft survival, in addition to successfully “reversing” other established immune-mediated inflammatory processes.
License Agreement with Loma Linda University (“LLU”)
On March 15, 2018, we entered into a License Agreement with LLU, which was subsequently amended on July 1, 2020. Pursuant to the LLU License Agreement, we obtained the exclusive royalty-bearing worldwide license to all intellectual property, including patents, technical information, trade secrets, proprietary rights, technology, know-how, data, formulas, drawings, and specifications, owned or controlled by LLU and/or any of its affiliates (the “LLU Patent and Technology Rights”) and related to therapy for immune-mediated inflammatory diseases (the ADI™ technology). In consideration for the LLU License Agreement, we issued 13 shares of common stock to LLU.
PEARSANTA, INC.
Formed in January 2023, our subsidiary Pearsanta™, Inc. (“Pearsanta”) seeks to take personalized medicine to a whole new level by delivering “Health by the Numbers.” Since its founding, Pearsanta has been building the platform for enabling our vision of lab quality testing, anytime, anywhere. Our plan for Pearsanta’s platform is for it to be the transactional backbone for sample collection, sample processing (on- and off-site), and reporting. This will require the development and convergence of multiple components developed by Pearsanta, or through transactions with third parties, including collection devices, “lab-on-a-chip” technologies, Lab Developed Test (LDT) assays, a data-driven analysis engine, and telemedicine. According to a comprehensive research report by Market Research Future, the clinical and consumer diagnostic market is estimated to hit $429.3 billion by 2030.
We believe that timely and personalized testing enables far more informed treatment decisions. Pearsanta’s platform is being developed as a seamless digital healthcare solution. This platform will integrate at-location sample collection, Point-of-Care (“POC”) and LDT assays, and an analytical reporting engine, with telemedicine-enabled visits with licensed physicians to review test results and, if necessary, order a prescription. Pearsanta’s goal of extending its platform to enable consumers to monitor their health more proactively as the goal is to provide a more complete picture about someone’s dynamic health status, factoring in genetic makeup and their response to medication. The POC component of Pearsanta would enable diagnostic testing at-home, at work, in pharmacies, and more to generate results quickly so that an individual can access necessary treatment faster. With certain infections, prescribing the most effective treatment according to one’s numbers can prevent hospital emergency room admissions and potentially life-threatening consequences.
Examples of indication-focused tests for the Test2Treat platform will include the evaluation for advanced urinary tract infections (“UTIs”), COVID-19/flu/respiratory syncytial virus, sexually transmitted infections, gut health, pharmacogenomics (i.e., how your genes affect the way your body responds to certain therapeutics), and sepsis. We believe that these offerings are novel and needed as the current standard of care using broad spectrum antibiotic treatment can be ineffective and potentially life-threatening. For example, improperly prescribed antibiotics may approach 50% of outpatient cases. Further, according to an article published in Physician’s Weekly, only 1% of board-certified critical care medicine physicians are trained in infectious disease.
Licensed Technologies – AditxtScoreTM
We issued Pearsanta an exclusive worldwide sub-license for commercializing the AditxtScore™ technology which provides a personalized comprehensive profile of the immune system. AditxtScore is intended to detect individual immune responses to viruses, bacteria, peptides, drugs, supplements, bone marrow and solid organ transplants, and cancer. It has broad applicability to many other agents of clinical interest impacting the immune system, including those not yet identified such as emerging infectious agents.
AditxtScore is being designed to enable individuals and their healthcare providers to understand, manage and monitor their immune profiles and to stay informed about attacks on or by their immune system. We believe AditxtScore can also assist the medical community and individuals by being able to anticipate the immune system’s potential response to viruses, bacteria, allergens, and foreign tissues such as transplanted organs. This technology may be able to serve as a warning signal, thereby allowing for more time to respond appropriately. Its advantages include the ability to provide simple, rapid, accurate, high throughput assays that can be multiplexed to determine the immune status with respect to several factors simultaneously, in approximately 3-16 hours. In addition, it can determine and differentiate between distinct types of cellular and humoral immune responses (e.g., T and B cells and other cell types). It also provides for simultaneous monitoring of cell activation and levels of cytokine release (i.e., cytokine storms).
We are actively involved in the regulatory approval process for AditxtScore assays for clinical use and securing manufacturing, marketing, and distribution partnerships for application in the various markets. To obtain regulatory approval to use AditxtScore as a clinical assay, we have conducted validation studies to evaluate its performance in detection of antibodies and plan to continue conducting additional validation studies for new applications in autoimmune diseases.
Advantages
The sophistication of the AditxtScore technology includes the following:
| ● | greater sensitivity/specificity. |
| ● | 20-fold higher dynamic range, greatly reducing signal to noise compared to conventional assays. |
| ● | ability to customize assays and multiplex a large number of analytes with speed and efficiency. |
| ● | ability to test for cellular immune responses (i.e., T and B cells and cytokines). |
| ● | proprietary reporting algorithm. |
License Agreement with Leland Stanford Junior University (“Stanford”)
On February 3, 2020, we entered into an exclusive license agreement (the “February 2020 License Agreement”) with Stanford with regard to a patent concerning a method for detection and measurement of specific cellular responses. Pursuant to the February 2020 License Agreement, we received an exclusive worldwide license to Stanford’s patent with regard to use, import, offer, and sale of Licensed Products (as defined in the agreement). The license to the patented technology is exclusive, including the right to sublicense, beginning on the effective date of the agreement, and ending when the patent expires. Under the exclusivity agreement, we acknowledged that Stanford had already granted a non-exclusive license in the Nonexclusive Field of Use, under the Licensed Patents in the Licensed Field of Use in the Licensed Territory (as those terms are defined in the “February 2020 License Agreement”). However, Stanford agreed not to grant further licenses under the Licensed Patents in the Licensed Field of Use in the Licensed Territory. On December 29, 2021, we entered into an amendment to the February 2020 License Agreement which extended our exclusive right to license the technology deployed in AditxtScoreTM and securing worldwide exclusivity in all fields of use of the licensed technology.
ADIVIR, INC.
Formed in April of 2023, Adivir™, Inc. is a wholly owned subsidiary, dedicated to the clinical and commercial development efforts of innovative products for population health, including antiviral and other antimicrobial products, which have the potential to address a wide range of infectious diseases, including those that currently lack viable treatment options.
Background
On April 18, 2023, we entered into an Asset Purchase Agreement (the “Asset Purchase Agreement”) with Cellvera Global Holdings LLC (“Cellvera Global”), Cellvera Holdings Ltd. (“BVI Holdco”), Cellvera, Ltd. (“Cellvera Ltd.”), Cellvera Development LLC (“Cellvera Development” and together with Cellvera Global, BVI Holdco, Cellvera Ltd. and Cellvera Development (the “Sellers”), AiPharma Group Ltd. (“Seller Owner” and collectively with the Sellers, “Cellvera”), and the legal representative of Cellvera, pursuant to which, the Company will purchase Cellvera’s 50% ownership interest in G Response Aid FZE (“GRA”), certain other intellectual property and all goodwill related thereto (the “Acquired Assets”). Unless expressly stated otherwise herein, capitalized terms used but not defined herein have the meanings ascribed to them in the Asset Purchase Agreement. Pursuant to the Asset Purchase Agreement, the consideration for the Acquired Assets consists of (A) $24.5 million, comprised of: (i) the forgiveness of the Company’s $14.5 million loan to Cellvera Global, and (ii) approximately $10 million in cash, and (B) future revenue sharing payments for a term of seven years. GRA holds an exclusive, worldwide license for the antiviral medication, Avigan® 200mg, excluding Japan, China and Russia. The other 50% interest in GRA is held by Agility, Inc. (“Agility”).
Additionally, upon the closing, the Share Exchange Agreement previously entered into as of December 28, 2021, between Cellvera Global Holdings, LLC f/k/a AiPharma Global Holdings, LLC (together with other affiliates and subsidiaries) and the Company, and all other related agreements will be terminated.
The obligations of the Company to consummate the Closing under the Asset Purchase Agreement are subject to the satisfaction or waiver, at or prior to the Closing of certain conditions, including but not limited to, the following:
| (i) | Satisfactory completion of due diligence; |
| (ii) | Completion by the Company of financing sufficient to consummate the transactions contemplated by the Asset Purchase Agreement; |
| (iii) | Receipt by the Company of all required Consents from Governmental Bodies for the Acquisition, including but not limited to, any consents required to complete the transfer and assignment of Cellvera’s membership interests in GRA; |
| (iv) | Receipt of executed payoff letters reflecting the amount required to be fully pay all of each of Seller’s and Seller Owner’s Debt to be paid at Closing; |
| (v) | Receipt by the Company of a release from Agility; |
| (vi) | Execution of an agreement acceptable to the Company with respect to the acquisition by the Company of certain intellectual property presently held by a third party; |
| (vii) | Execution of an amendment to an asset purchase agreement previously entered into by Cellvera with a third party that effectively grants the Company the rights to acquire the intellectual property from the third party under such agreement; |
| (viii) | Receipt of a fairness opinion by the Company with respect to the transactions contemplated by the Asset Purchase Agreement; and |
| (ix) | Receipt by the Company from the Seller Owner of written consent, whether through its official liquidator or the Board of Directors of Seller Owner, to the sale and purchase of the Acquired Assets and Assumed Liabilities pursuant to the Assert Purchase Agreement. |
There can be no assurance that the conditions to closing will be satisfied or that the proposed acquisition will be completed as proposed or at all.
Our commitment to building our antiviral portfolio is strategic and timely. We believe that there has never has there been a more important time to address the growing global need to uncover new treatments or commercialize existing ones that treat life-threatening global viral infections.
Our Team
We have assembled a team of experts from a variety of scientific fields and commercial backgrounds, with many years of collective experience that ranges from founding startup biotech companies, to developing and marketing biopharmaceutical products, to designing clinical trials, and to management of private and public companies.
Going Concern
We were incorporated on September 28, 2017 and have not generated significant revenues to date. During the year ended and as of December 31, 2023, we had a net loss of $32,390,447 and cash of $97,102. We are currently over 90 days past due on a significant number of vendor obligations. The Company will require significant additional capital to operate in the normal course of business and fund clinical studies in the long-term. We believe our remaining funds on hand will not be sufficient to fund our operations for the next 12 months and such creates substantial doubt about our ability to continue as a going concern beyond one year.
Financial Results
We have a limited operating history. Therefore, there is limited historical financial information upon which to base an evaluation of our performance. Our prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies in their early stages of operations. Our financial statements as of December 31, 2023, show a net loss of $32,275,156. We expect to incur additional net expenses over the next several years as we continue to maintain and expand our existing operations. The amount of future losses and when, if ever, we will achieve profitability are uncertain.
Results of Operations
Results of operations for the years ended December 31, 2023 and 2022
We generated revenue of $645,176 and $933,715 for the years ended December 31, 2023 and 2022, respectively. Cost of sales for the years ended December 31, 2023 and 2022 was $756,836 and $766,779, respectively.
During the year ended December 31, 2023, we incurred a loss from operations of $26,062,425. This is due primarily to general and administrative expenses of $18,607,142. This includes approximately $9,641,000 in payroll expenses, $4,484,000 in professional fees, and $1,133,077 in stock-based compensation. Research and development expenses were $7,074,339 which includes $1,815,068 in consulting expenses and $262,154 in stock-based compensation. Sales and marketing expenses were $269,284, which includes $6,787 in stock-based compensation.
During the year ended December 31, 2022, we incurred a loss from operations of $25,480,098. This is due to general and administrative expenses of $15,985,552, which includes $1,516,805 in stock-based compensation, research and development of $7,268,084, which includes $591,518 in stock-based compensation, sales and marketing expenses of $1,849,460, which includes $1,023,045 in stock-based compensation and impairment on note receivable of $534,938. The $7,268,084 in research and development is mainly comprised of $2,145,382 in consulting expenses, and $3,375,757 in compensation offset by a one-time adjustment to research and development purchases. During the year, the Company transitioned from purchasing certain inventory items to internally manufacturing these items.
The decrease in expenses during the year ended December 31, 2023 compared to the year ended December 31, 2022 was due to decreased research and development spend and the termination of a sales and marketing vendor.
Liquidity and Capital Resources
We have incurred substantial operating losses since inception and expect to continue to incur significant operating losses for the foreseeable future and may never become profitable. As of December 31, 2023, we had an accumulated deficit of $127,635,389. We had working capital of $(18,976,866) as of December 31, 2023. During the year ended December 31, 2023, we purchased $14,407 in fixed assets, for which we made cash payments of $14,407. Of the $14,407, $12,356 of these purchased fixed assets were lab equipment and $2,051 was for computers.
Our consolidated financial statements have been prepared assuming that we will continue as a going concern.
We have funded our operations from proceeds from the sale of equity and debt securities. On July 2, 2020, we completed our IPO and raised approximately $9.5 million in net proceeds. At the time of the IPO, we believed that these funds would be sufficient to fund our operations for the foreseeable future.
On September 10, 2020, we completed a follow-on public offering. In connection therewith, we issued 1,200 units, or Follow-On Units, excluding the underwriters’ option to cover overallotments, at an offering price of $8,000.00 per Follow-On Unit, resulting in gross proceeds of approximately $9.6 million.
On January 25, 2021, the Company entered into a securities purchase agreement with an institutional accredited investor (the “Investor”) for the sale of a $6,000,000 senior secured convertible note (the “Convertible Note”). The Convertible Note had a term of 24 months, was originally convertible at a price of $8,000.00 per share and was issued at an original issuance discount of $1,000,000. On August 30, 2021, the Company entered into a defeasance and waiver agreement with the Investor, pursuant to which the Noteholder has agreed in exchange for (a) a cash payment by the Company to the Investor of $1.2 million (the Cash Payment”), (b) a waiver, in part of the conversion price adjustment provision such that the January 2021 Note shall be convertible into 2,401 shares of common stock (without giving effect to the conversion notice received by the company form the Noteholder prior to the date hereof totaling (503 shares) (the “Shares”), and (c) a voluntary and permanent reduction by the Company of the exercise price of the warrant to purchase 400 shares of the common stock of the Company (the “January 2021 Warrant”) to $5,060 per share. As of December 31, 2022, the outstanding principle of the convertible note had been converted to 2,401 shares of common stock.
On August 30, 2021, the Company completed a registered direct offering and raised approximately $10.1 million in net proceeds.
On October 20, 2021, the Company completed a public offering for net proceeds of $3.8 million. As part of this offering, we issued 1,417 shares of the Company’s common stock
On December 6, 2021, the Company completed a public offering for net proceeds of $16.0 million. As part of this offering, we issued 4,123 units consisting of shares of the Company’s common stock and warrant to purchase shares of the Company’s common stock and 4,164 pre-funded warrants. The warrant issued as part of the units had an exercise price of $2,300.00 and the prefunded warrants had an exercise price of $0.001.
On September 20, 2022, the Company completed a public offering for net proceeds of $18.1 million (the “September 2022 Offering”). As part of the September 2022 Offering, we issued 30,608 of shares of the Company’s common stock, pre-funded warrants to purchase 52,725 shares of the Company’s common stock and warrants to purchase 83,333 shares of the Company’s common stock. The warrants have an exercise price of $240.00 and the pre-funded warrants have an exercise price of $0.004.
On April 20, 2023, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with an institutional investor, pursuant to which the Company agreed to sell to such investor pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 39,634 shares of common stock of the Company (the “Common Stock”) at a purchase price of $48.76 per Pre-Funded Warrant. Concurrently with the sale of the Pre-Funded Warrants, pursuant to the Purchase Agreement in a concurrent private placement, for each Pre-Funded Warrant purchased by the investor, such investor received from the Company an unregistered warrant (the “Warrant”) to purchase two shares of Common Stock. The warrants have an exercise price of $34.40 per share and are exercisable for a three-year period. In addition, the Company issued a warrant to the placement agent to purchase up to 2,378 shares of common stock at an exercise price of $61.00 per share.
On August 31, 2023, the Company entered into a securities purchase agreement (the “August Purchase Agreement”) with an institutional investor for the issuance and sale in a private placement (the “Private Placement”) of (i) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 1,000,000 shares of the Company’s common stock at an exercise price of $0.001 per share, and (ii) warrants (the “Common Warrants”) to purchase up to 1,000,000 shares of the Company’s Common Stock at an exercise price of $10.00 per share. The Private Placement closed on September 6, 2023. The net proceeds to the Company from the Private Placement were approximately $9 million, after deducting placement agent fees and expenses and estimated offering expenses payable by the Company. The Company utilized net proceeds received from the Private Placement for (i) payment of approximately $3.1 million in outstanding obligations, (ii) repayment of approximately $0.4 million of outstanding debt, and (iii) continuing operating expenses and working capital.
On December 29, 2023, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with an institutional investor (“the “Purchaser”) for the issuance and sale in a private placement (the “Private Placement”) of (i) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 1,237,114 shares of the Company’s common stock, par value $0.001 (the “Common Stock”) at an exercise price of $0.001 per share, and (ii) warrants (the “Common Warrants”) to purchase up to 2,474,228 shares of the Company’s Common Stock, at a purchase price of $4.85 per share. The Private Placement closed on January 4, 2024. The net proceeds to the Company from the Private Placement are expected to be approximately $5.5 million, after deducting placement agent fees and expenses and estimated offering expenses payable by the Company. The Company intends to use the net proceeds received from the Private Placement for continuing operating expenses and working capital.
We will need significant additional capital to continue to fund our operations and the clinical trials for our product candidates. We may seek to sell common stock, preferred stock or convertible debt securities, enter into a credit facility or another form of third-party funding or seek other debt financing. In addition, we may seek to raise cash through collaborative agreements or from government grants. The sale of equity and convertible debt securities may result in dilution to our stockholders and certain of those securities may have rights senior to those of our common shares. If we raise additional funds through the issuance of preferred stock, convertible debt securities, or other debt financing, these securities or other debt could contain covenants that would restrict our operations. Any other third-party funding arrangement could require us to relinquish valuable rights.
The source, timing, and availability of any future financing will depend principally upon market conditions, and, more specifically, on the progress of our clinical development program. Funding may not be available when needed, at all, or on terms acceptable to us. Lack of necessary funds may require us to, among other things, delay, scale back or eliminate expenses including some or all our planned development, including our clinical trials. While we may need to raise funds in the future, we believe the current cash reserves should be sufficient to fund our operation for the foreseeable future. Because of these factors, we believe that this creates doubt about our ability to continue as a going concern.
Contractual Obligations
The following table shows our contractual obligations as of December 31, 2023:
| Payment Due by Year | ||||||||||||||||
| Total | 2024 | 2025 | 2026 | |||||||||||||
| Lease | $ | 2,139,458 | $ | 1,004,982 | $ | 710,546 | $ | 423,930 | ||||||||
Critical Accounting Polices and Estimates
Our consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States. The preparation of our consolidated financial statements and related disclosures requires us to make estimates, assumptions and judgments that affect the reported amount of assets, liabilities, revenue, costs and expenses, and related disclosures. We believe that our critical accounting policies described under the heading “Management’s Discussion and Analysis of Financial Condition and Plan of Operations—Critical Accounting Policies” in our Prospectus, dated September 1, 2020, filed with the SEC pursuant to Rule 424(b), are critical to fully understanding and evaluating our financial condition and results of operations. The following involve the most judgment and complexity:
| ● | Research and development |
| ● | Stock-based compensation expense |
| ● | Preferred Stock |
| ● | Investments |
Accordingly, we believe the policies set forth above are critical to fully understanding and evaluating our financial condition and results of operations. If actual results or events differ materially from the estimates, judgments and assumptions used by us in applying these policies, our reported financial condition and results of operations could be materially affected.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
JOBS Act
On April 5, 2012, the JOBS Act was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act, for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies.
When favorable, we have chosen to take advantage of the extended transition periods available to emerging growth companies under the JOBS Act for complying with new or revised accounting standards until those standards would otherwise apply to private companies provided under the JOBS Act.
We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we intend to rely on certain of these exemptions, including without limitation, (i) providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the Public Company Accounting Oversight Board (“PCAOB”) regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of our IPO (December 31, 2025); (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
Recently Issued and Adopted Accounting Pronouncements
See Note 3 - Summary of Significant Accounting Policies to the accompanying consolidated financial statements for a description of other accounting policies and recently issued accounting pronouncements.
Recent Developments
See Note 12 – Subsequent Event to the accompanying consolidated financial statements for a description of material recent developments.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are not required to provide the information required by this Item as it is a “smaller reporting company,” as defined in Rule 229.10(f)(1).
Item 8. Financial Statements and Supplementary Data.
See pages F-1 through F-39 following the Exhibit Index of this Annual Report on Form 10-K.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Assessment of the Effectiveness of Internal Controls over Financial Reporting
Disclosure Controls and Procedures
In accordance with Rules 13a-15(b) and 15d-15(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), we, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, carried out an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) and Rule 15d-15(e) of the Exchange Act) as of the end of the period covered by this Annual Report on Form 10-K. Based on the foregoing, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were (a) designed to ensure that the information we are required to disclose in our reports under the Exchange Act is recorded, processed, and reported in an accurate manner and on a timely basis and the information that we are required to disclose in our Exchange Act reports is accumulated and communicated to management to permit timely decisions with respect to required disclosure and (b) operating in a non-effective manner.
Change in Internal Control Over Financial Reporting
No change occurred in our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) of the Exchange Act) during the year ended December 31, 2023 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act.
A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our Company have been detected.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
Our independent registered accounting firm determined that we did not maintain effective internal controls over financial reporting and the following material weaknesses existed as of December 31, 2023:
| ● | We did not maintain adequate controls over the documentation of accounting and financial reporting policies and procedures. Specifically, we did not maintain policies and procedures to ensure account reconciliations were adequately prepared and reviewed by management. |
| ● | We did not retain individuals and/or entities with extensive knowledge to recognize and record technical and complex accounting issues. |
| ● | We did not maintain the sufficient procedures for the identification and cutoff of accounts payable. |
These material weaknesses resulted in material misstatements to the financial statements, which were corrected. There were no changes to previously released financial results. We are in the process of remediating these material weaknesses.
This report does not include an attestation report of our independent registered public accounting firm regarding our internal control over financial reporting in accordance with applicable SEC rules that permit us to provide only management´s report in this report.
Item 9B. Other Information.
In February 2023, the Company formed a wholly-owned subsidiary, Pearsanta, Inc. in order to accelerate the growth of the Company’s AditxtScore program through future strategic revenue and growth oriented transactions. In connection with the formation of Pearsanta and Corinne Pankovcin’s anticipated role in driving such strategic revenue and growth oriented transactions, Ms. Pankovcin’ s title was changed from President to Chief Commercialization Officer, effective April 12, 2023.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
N/A.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Executive Officers and Directors
Set forth below is certain information with respect to the individuals who are our directors and executive officers as of December 31, 2023:
| Name | Age | Positions | ||
| Amro Albanna | 54 | Chief Executive Officer, Director | ||
| Corinne Pankovcin | 57 | Chief Commercialization Officer | ||
| Shahrokh Shabahang, D.D.S., MS, Ph.D. | 61 | Chief Innovation Officer, Director | ||
| Rowena Albanna | 58 | Chief Operating Officer | ||
| Thomas J. Farley | 50 | Chief Financial Officer | ||
| Charles Nelson | 70 | Director | ||
| Brian Brady | 45 | Director | ||
| Jeffrey W. Runge, M.D. | 68 | Director |
Amro Albanna - Chief Executive Officer
Mr. Albanna has been our Chief Executive Officer and a Director since we were formed in 2017. He also served as our President from our inception through September 2021. In 2010, Mr. Albanna co-founded Innovation Economy Corporation (“IEC”), formed to license and commercialize innovations and create a group of life and health subsidiaries. From 2010 until 2017, Mr. Albanna was Chief Executive Officer and a Director of IEC and Olfactor Laboratories, Inc., a majority-owned subsidiary of IEC. From 2010 to August 2016, he was the Chief Executive Officer and a Director of Nano Engineered Applications, Inc., another majority-owned subsidiary of IEC. In 2003, Mr. Albanna founded Qmotions, Inc. (subsequently renamed Deal A Day Group Corp.). He served as its Chief Executive Officer and a Director until 2011. Qmotions used 3-D spatial tracking and pattern recognition technologies to develop motion-capturing video game controllers. In 2002, Mr. Albanna was a co-founder of Digital Angel Corporation - a company formed via the merger of three private companies (one being TTC below) into a fourth publicly traded company (American Stock Exchange) and was placed in charge of commercializing its GPS/wireless technologies. Around that time, Mr. Albanna co-founded an incubator for startups at the University of California, Riverside Research Park which was acquired in 2007. In 1997, he founded Timely Technology Corporation (“TTC”), which designed and developed e-commerce software for education, retail and finance. TTC was acquired in 2000 by a Nasdaq-listed company. Mr. Albanna graduated from California State University San Bernardino in 1991 with a B.S. in Business Administration with concentration in Computer Information Systems. He completed graduate coursework in Computer Science and Engineering at California State University, Long Beach from 1992 to 1993. In 2019, Mr. Albanna completed coursework in Immunology and Genetics at Harvard Medical School HMX online learning platform.
Corinne Pankovcin — Chief Commercialization Officer
Ms. Pankovcin has been our Chief Commercialization Officer since April 12, 2023. Ms. Pankovcin served as our President from September 2021 through April 2023. Ms. Pankovcin served as our Chief Financial Officer from July 2020 through August 2021. From December 2015 to July 2019, Ms. Pankovcin was the Chief Financial Officer and Managing Director and Treasurer of Business Development Corporation of America (“BDCA”), a business development company. Prior thereto, from January 2011 to August 2015, Ms. Pankovcin was the Chief Financial Officer and Treasurer of Blackrock Capital Investment Corporation (NASDAQ: BKCC), and a Managing Director of Finance at BlackRock Investment Management LLC. Prior to joining BlackRock, Ms. Pankovcin was a senior member of Finance & Accounting of Alternative Investments and served as Chief Financial Officer for the Global Emerging Markets products group at AIG Capital Partners. Ms. Pankovcin began her career with PricewaterhouseCoopers LLP, where she ultimately held the role of Senior Manager of Business Assurance for Consumer Products, Manufacturing, and Middle Market industries from 1991 to 2001. Ms. Pankovcin earned her B.S. in Accounting from Dowling College and her Master’s Degree in Business Administration from Hofstra University. She is a Certified Public Accountant.
Shahrokh Shabahang, D.D.S., MS, Ph.D. - Chief Innovation Officer
Dr. Shabahang has been our Chief Innovation Officer and Director since our inception. In 2009, Dr. Shabahang co-founded Sekris Biomedical Inc. to incubate immunotherapy technologies. He served as its Chairman of the board and Chief Executive Officer since its inception. In 2004, Dr. Shabahang joined Genelux Corporation to lead its clinical development program and to serve as board secretary. Genelux developed an oncolytic virus technology for treatment of cancer, co-invented by Dr. Shabahang. During his tenure from 2004-2007, Genelux raised $20M+ and obtained regulatory approval to initiate First-In-Human clinical studies in Europe with patients who had not responded to chemotherapy. In 2001, Dr. Shabahang became the Director of the Microbiology and Molecular Biology Lab at Loma Linda University (“LLU”). He led the research and development of an antimicrobial therapeutic agent for treatment of dental infections, which was licensed and marketed by one of the largest dental distribution companies. Dr. Shabahang attended the University of California, Santa Barbara from 1982 to 1984 and later received his DDS from the University of Pacific in 1987. He earned his PhD in Microbiology and Molecular Genetics at LLU in 2001. During the same year, he established his laboratory at LLU to study infectious diseases and host immune responses.
Rowena Albanna - Chief Operating Officer
Ms. Albanna has been our Chief Operating Officer since July 2020. From 2017 to immediately prior to her appointment as Chief Operating Officer, Ms. Albanna was an independent operations consultant for the Company. Prior thereto, from 2013 to 2017, Ms. Albanna was the Chief Operating Officer of Innovation Economy Corporation (“IEC”), formed to license and commercialize innovations and create a group of life and health subsidiaries. From 2010 to 2013, Ms. Albanna was Senior Vice President of IEC. From 2004 to 2009, Ms. Albanna was the founder and principal of Weezies, an online-based business focused on building and operating e-commerce stores and affiliate marketing sites. From 2003 to 2004, Ms. Albanna was the head of Product Development and Engineering of Qmotions Inc. Qmotions used 3-D spatial tracking and pattern recognition technologies to develop motion-capturing video game controllers. In 2002, Ms. Albanna was VP of Product Development at Digital Angel Systems where she led the development of devices which combined GPS, wireless, and biosensing. Prior to that, Ms. Albanna held multiple product development roles with increasing responsibilities for various technology companies in the areas of financial, medical, telecommunications, integrated circuit layout design, and defense. Ms. Albanna is a co-inventor of two patents related to systems for localizing, monitoring, and sensing objects. Ms. Albanna received a Bachelor of Science degree in Computer Science with a minor in Mathematics from California State University, San Bernardino in 1988. Ms. Albanna is the wife of Amro Albanna, our Chief Executive Officer.
Thomas J. Farley, CPA - Chief Financial Officer
Mr. Farley has been the Chief Financial Officer since September 2021. Prior to this, Mr. Farley was the Principal Accounting Officer and Controller from October of 2020 to September 2021. From December 2015 to June 2020, Mr. Farley was the Controller of Business Development Corporation of America (“BDCA”), a publicly listed business development company. Prior thereto, from January 2011 to August 2015, Mr. Farley was the Senior Controller of Blackrock Capital Investment Corporation (NASDAQ: BKCC). Prior to joining BlackRock Capital Investment Corporation, Mr. Farley was a Senior Controller for PineBridge Investments Emerging Markets practice. Mr. Farley was also an Accounting Manager for Bessemer Venture Partners prior to his tenure at PineBridge. Mr. Farley began his career with PricewaterhouseCoopers LLP, from 1996 to 2001. Mr. Farley earned his B.S. in Accounting from Long Island University and is a Certified Public Accountant.
Brian Brady - Director
Mr. Brady has served as a Director since December 1, 2018. Mr. Brady currently serves as President of a Family Office. Mr. Brady previously was the Director of Investments at a large hospital system from March 2016 through December 2022, where he was responsible for the management of investment activity related to the organization and personal investments of the family that owns that company. From December 2011 to March 2016, Mr. Brady was the Vice President/Portfolio Manager at a wealth advisory firm, where he served in an investment advisory role, including asset and portfolio management. Mr. Brady graduated in 2001 with a Bachelor’s degree in Finance from the University of Illinois at Chicago and in 2014 with a Master of Business Administration degree from the University of Chicago. We believe that Mr. Brady’s extensive experience with financial markets and management of investment activities qualifies him to serve as a director of our Company.
Charles Nelson - Director
Mr. Nelson has served as a director since November 2023. Prior to his appointment as a member of the Board, Mr. Nelson was a consultant to the Company from September 2020 through September 2023. He began his financial career as a market representative with American International Group and in 1979 joined Dean Witter Reynolds as a Financial Advisor, working with high net worth and institutional clients. In 1980, he joined Drexel Burnham and Lambert, and subsequently, at Ladenberg Thalmann and then at Auerbach Pollack and Richardson originating equity and investment banking transactions. Over the last 20 years, Mr. Nelson has been involved with financing companies in the fintech, healthcare and bio-pharma spaces through private equity and public financing including listings on the Nasdaq and the NYSE. We believe that Mr. Nelson’s extensive experience in capital markets qualifies him to serve as a director of our Company.
Jeffrey W. Runge, M.D - Director
Dr. Runge has served as a director since July 2020. From 2008 to the present, Dr. Runge has been the President and founder of Biologue, Inc., which provides consulting in biodefense, medical preparedness and injury control. From 2001 through August of 2008, Dr. Runge served in the Bush administration, first as the head of the National Highway Traffic Safety Administration, and, beginning in September 2005, as the Department of Homeland Security’s (DHS) first Chief Medical Officer. Dr. Runge founded the DHS Office of Health Affairs and was confirmed by the United States Senate as DHS’ first Assistant Secretary for Health Affairs in December of 2007. Dr. Runge also served as Acting DHS Undersecretary for Science and Technology from February through August 2006. In his role at DHS, Dr. Runge oversaw the operations of the department’s biodefense activities, medical preparedness and workforce health protection, as well as fulfilling DHS’ responsibilities in medical countermeasure development. Prior to his government service, Dr. Runge was Assistant Chairman and Director of Clinical Research in the Department of Emergency Medicine at Carolinas Medical Center in Charlotte, NC, from 1984 through 2001. Additionally, Dr. Runge is a Senior Advisor at The Chertoff Group, a firm providing advisory services in business risk management, security and homeland defense. Since 2010, Dr. Runge has served on the boards of two public companies, including their Audit and Compensation committees, both of which underwent strategic acquisitions. He has also served as President and CEO of a SEC-regulated startup company in the health sector. Dr. Runge earned his medical degree from the Medical University of South Carolina and his undergraduate degree from the University of the South. We believe that Dr. Runge’s experience in medicine, medical research, public service, business and his prior service on public corporate boards qualifies him to serve as a director of our Company.
Board Leadership Structure and Risk Oversight
The Board oversees our business and considers the risks associated with our business strategy and decisions. The Board currently implements its risk oversight function as a whole. Each of the Board committees, when established, will also provide risk oversight in respect of its areas of concentration and reports material risks to the Board for further consideration.
Term of Office
Officers hold office until his or her successor is elected and qualified. Directors are appointed to serve for one year until the meeting of the Board following the annual meeting of stockholders and until their successors have been elected and qualified.
Director Independence
We use the definition of “independence” of The Nasdaq Stock Exchange LLC (“Nasdaq”) listing rules to make this determination. Nasdaq listing rules provide that an “independent director” is one who the board “affirmatively determines” has no “material relationship” with the company “either directly or as a partner, shareholder or officer of an organization that has a relationship with the Company. Nasdaq listing rules provide that a director cannot be considered independent if:
| ● | the director is, or has been within the last three (3) years, an employee of the Company or an immediate family member of director is, or has been within the last three (3) years, an executive officer of the Company; |
| ● | the director has received, or has an immediate family member who is an executive officer of the Company and has received, during any twelve-month period within the last three (3) years, more than $120,000 compensation directly from the Company (not including compensation received for director service, pension plan payments or deferred compensation for prior service not contingent on continued service); |
| ● | the director or an immediate family member is a current partner of the Company’s internal or external auditor; the director is a current employee of the auditor; an immediate family member is a current employee of the auditor and personally works on the Company’s audit; or the director or an immediate family member was within the last three (3) years a partner or employee of the auditor and personally worked on the Company’s audit within that time; |
| ● | the director or an immediate family member is, or has been within the last three (3) years, employed as an executive officer of another company where any of the Company’s present executive officers at the same time serves or served on that company’s compensation committee; or |
| ● | the director is a current employee, or an immediate family member is a current executive officer, of an organization that has made to or received from the Company payments for property or services in an amount which, in any of the last three fiscal (3) years, exceeds greater of 2% of such other company’s consolidated gross revenues or $1 million. Charitable contributions not considered “payments” for purposes of this prohibition but contributions meeting these thresholds must be disclosed on the Company’s website or in its annual proxy statement or its Annual Report on Form 10-K. |
Under such definitions, we consider Mr. Nelson, Mr. Brady, and Dr. Runge to be “independent.” Nasdaq listing rules permits a phase-in period of up to one year for an issuer registering securities in an initial public offering to comply with its requirement that a majority of the board of directors be made up of independent directors. However, our common stock is not currently quoted or listed on any national exchange or interdealer quotation system with a requirement that a majority of our Board be independent and, therefore, the Company is not subject to any director independence requirements. We are subject to Nasdaq’s director independence requirements and are required to structure our board of directors accordingly.
Committees of the Board
Our board of directors has established three standing committees: Audit, Compensation, and Nominating and Corporate Governance. Each of these standing committees operate pursuant to its respective charter. The committee charters are reviewed annually by the Nominating and Corporate Governance Committee. If appropriate, and in consultation with the chairs of the other committees, the Nominating and Corporate Governance Committee may propose revisions to the charters. The responsibilities of each committee are described in more detail below.
Nasdaq listing rules permits a phase-in period for an issuer registering securities in an initial public offering to meet the Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee independence requirements. Under the initial public offering phase-in period, only one member of each committee is required to satisfy the heightened independence requirements at the time our registration statement becomes effective, a majority of the members of each committee must satisfy the heightened independence requirements within 90 days following the effectiveness of our registration statement, and all members of each committee must satisfy the heightened independence requirements within one year from the effectiveness of our registration statement.
The composition and functions of each committee are described below.
| Name | Independent | Audit | Nominating and Corporate Governance | Compensation | ||||||||||||
| Amro Albanna | ||||||||||||||||
| Shahrokh Shabahang, D.D.S., MS, Ph.D. | ||||||||||||||||
| Brian Brady | X | X | * | X | X | |||||||||||
| Charles Nelson | X | X | X | X | * | |||||||||||
| Jeffrey Runge, M.D. | X | X | X | * | X | |||||||||||
| * | Chairman of the committee |
Audit Committee
The Audit Committee, among other things, is responsible for:
| ● | appointing; approving the compensation of; overseeing the work of; and assessing the independence, qualifications, and performance of the independent auditor; |
| ● | reviewing the internal audit function, including its independence, plans, and budget; |
| ● | approving, in advance, audit and any permissible non-audit services performed by our independent auditor; |
| ● | reviewing our internal controls with the independent auditor, the internal auditor, and management; |
| ● | reviewing the adequacy of our accounting and financial controls as reported by the independent auditor, the internal auditor, and management; |
| ● | overseeing our financial compliance system; and |
| ● | overseeing our major risk exposures regarding the Company’s accounting and financial reporting policies, the activities of our internal audit function, and information technology. |
The Board has affirmatively determined that each member of the Audit Committee meets the additional independence criteria applicable to audit committee members under SEC rules and Nasdaq listing rules. The Board has adopted a written charter setting forth the authority and responsibilities of the Audit Committee. The Board has affirmatively determined that each member of the Audit Committee is financially literate, and that Mr. Brady meets the qualifications of an Audit Committee financial expert.
The Audit Committee consists of Mr. Brady, Mr. Nelson, and Dr. Runge. Mr. Brady chairs the Audit Committee.
Compensation Committee
The Compensation Committee is responsible for:
| ● | reviewing and making recommendations to the Board with respect to the compensation of our officers and directors, including the CEO; |
| ● | overseeing and administering the Company’s executive compensation plans, including equity-based awards; |
| ● | negotiating and overseeing employment agreements with officers and directors; and |
| ● | overseeing how the Company’s compensation policies and practices may affect the Company’s risk management practices and/or risk-taking incentives. |
The Board has adopted a written charter setting forth the authority and responsibilities of the Compensation Committee.
The Compensation Committee consists of Mr. Brady, Mr. Nelson, and Dr. Runge. Mr. Nelson serves as chairman of the Compensation Committee. The Board has affirmatively determined that each member of the Compensation Committee meets the independence criteria applicable to compensation committee members under SEC rules and Nasdaq listing rules.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee, among other things, is responsible for:
| ● | reviewing and assessing the development of the executive officers and considering and making recommendations to the Board regarding promotion and succession issues; |
| ● | evaluating and reporting to the Board on the performance and effectiveness of the directors, committees and the Board as a whole; |
| ● | working with the Board to determine the appropriate and desirable mix of characteristics, skills, expertise and experience, including diversity considerations, for the full Board and each committee; |
| ● | annually presenting to the Board a list of individuals recommended to be nominated for election to the Board; |
| ● | reviewing, evaluating, and recommending changes to the Company’s Corporate Governance Principles and Committee Charters; |
| ● | recommending to the Board individuals to be elected to fill vacancies and newly created directorships; |
| ● | overseeing the Company’s compliance program, including the Code of Conduct; and |
| ● | overseeing and evaluating how the Company’s corporate governance and legal and regulatory compliance policies and practices, including leadership, structure, and succession planning, may affect the Company’s major risk exposures. |
The Board of Directors has adopted a written charter setting forth the authority and responsibilities of the Nominating and Corporate Governance Committee.
The Nominating and Corporate Governance Committee consists of Dr. Runge, Mr. Brady, and Mr. Nelson. Dr. Runge serves as chairman of the Nominating and Corporate Governance Committee. The Company’s Board of Directors has determined that each member of the Nominating and Corporate Governance Committee is independent within the meaning of the independent director guidelines of Nasdaq listing rules.
Compensation Committee Interlocks and Insider Participation
None of the Company’s executive officers serves, or in the past has served, as a member of the board of directors or compensation committee, or other committee serving an equivalent function, of any entity that has one or more executive officers who serve as members of the Company’s board of directors or its compensation committee. None of the members of the Company’s compensation committee is, or has ever been, an officer or employee of the Company. There are no interlocking relationships as defined in the applicable SEC rules.
Code of Business Conduct and Ethics
The Company’s board of directors adopted a code of business conduct and ethics applicable to its employees, directors and officers, in accordance with applicable U.S. federal securities laws and the corporate governance rules of the Nasdaq Capital Market. The code of business conduct and ethics is publicly available on the Company’s website. Any substantive amendments or waivers of the code of business conduct and ethics or code of ethics for senior financial officers may be made only by the Company’s board of directors and will be promptly disclosed as required by applicable U.S. federal securities laws and the corporate governance rules of the Nasdaq Capital Market.
Corporate Governance Guidelines
The Company’s board of directors has adopted corporate governance guidelines in accordance with the corporate governance rules of the Nasdaq Capital Market.
Involvement in Certain Legal Proceedings
To our knowledge, none of our current directors or executive officers has, during the past ten years:
| ● | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| ● | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he or she was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| ● | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| ● | been found by a court of competent jurisdiction in a civil action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| ● | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| ● | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Securities Exchange Act of 1934, as amended (the Exchange Act)), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Except as set forth above and in our discussion below in “Certain Relationships and Related Transactions,” none of our directors or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Other than as set forth below, we are not currently a party to any legal proceedings, the adverse outcome of which, individually or in the aggregate, we believe will have a material adverse effect on our business, financial condition or operating results.
The Company, Amro Albanna, our President and Chief Executive Officer, and Dr. Shahrokh Shabahang, our Chief Innovation Officer, have been named as cross-defendants in a counterclaim filed by Christopher Sechrist in an action entitled Shahrokh Shabahang v. Christopher Sechrist, San Bernardino County Superior Court Case No. CIVDS1831323. In a cross-complaint, Mr. Sechrist contends that he was a partner in a dental practice with Dr. Shabahang, and that disputes arose as between those partners. Neither the Company nor Mr. Albanna were partners in, or otherwise have an interest in, the dental practice. Notwithstanding, and seemingly based solely on the fact that Dr. Shabahang became the Chief Innovation Officer for the Company, Mr. Sechrist has brought claims against the Company and Mr. Albanna. Both the Company and Mr. Albanna believe that the Counterclaims filed by Mr. Sechrist have no factual or legal merit, and they intend to vigorously defend themselves in the action and to seek a dismissal of the case as against them as soon as possible. On May 26, 2020, Mr. Sechrist filed a request for dismissal as to the Company and Mr. Albanna with the Superior Court of California, County of San Bernardino, San Bernardino District. The clerk of the court entered the dismissal with prejudice on May 26, 2020.
Our CEO,Chief Executive Officer, Amro Albanna, is a party to litigation matters unrelated to the Company or any of its properties. Such litigations relate to Innovation Economy Corporation (IEC), a company in which Mr. Albanna served as the CEO and a Director from 2010 until 2017, and its wholly-owned subsidiaries (Innovation Economy Corporation d/b/a ieCrowd). The first litigation (ieCrowd v. Kim, et. al, Superior Court, Riverside County) was originally commenced by IEC and its subsidiary after Mr. Albanna was no longer affiliated with IEC, against certain third-party defendants based upon claims related to their misconduct and mismanagement. Such defendants subsequently brought a countersuit against IEC and its subsidiary, in which they named Mr. Albanna and others as defendants, alleging that they were misled to invest in IEC and its subsidiary based upon misrepresentations by, among others, Mr. Albanna. The cases have now been consolidated. Mr. Albanna believes that the counteraction commenced by the third parties against him is without merit and intends to defend himself. The second matter (Calabria v. ieCrowd) was commenced by Calabria Ventures (the “Calabria Action”) more than 2 years after Mr. Albanna was no longer affiliated with IEC, related to uncollected rent. Mr. Albanna believes that the action commenced against him is without merit and intends to defend himself. IEC (either directly or through its Director and officer insurance policy) has covered all related legal costs to date. On August 5, 2020, the plaintiff in the Calabria Action filed a request for dismissal as to Mr. Albanna with the Superior Court of California, County of Riverside. The clerk of the court entered the dismissal without prejudice on August 5, 2020.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 11. Executive Compensation
The following table represents information regarding the total compensation for the named executive officers of the Company as of December 31, 2023 and 2022:
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Restricted Stock Units ($) | All Other Compensation ($)(4) | Total ($) | |||||||||||||||||||||||
| Amro Albanna | 2023 | 432,119 | - | - | 47,114 | - | 40,000 | 519,233 | |||||||||||||||||||||||
| Chief Executive Officer and Director | 2022 | 500,000 | - | - | - | - | 500,000 | ||||||||||||||||||||||||
| Shahrokh Shabahang, D.D.S., MS, Ph.D. | 2023 | 293,502 | - | - | 35,336 | - | 30,000 | 358,837 | |||||||||||||||||||||||
| Chief Innovation Officer | 2022 | 325,000 | - | - | - | - | - | 325,000 | |||||||||||||||||||||||
| Corinne Pankovcin | 2023 | 346,774 | - | - | 23,557 | - | 20,000 | 390,331 | |||||||||||||||||||||||
| Chief Commercialization Officer, Former President(1), Former Chief Financial Officer(2) | 2022 | 385,000 | - | - | - | - | - | 385,000 | |||||||||||||||||||||||
| Thomas J. Farley | 2023 | 337,894 | - | - | 23,557 | - | 20,000 | 381,451 | |||||||||||||||||||||||
| Chief Financial Officer | 2022 | 360,833 | - | - | - | - | 360,833 | ||||||||||||||||||||||||
| Matthew Shatzkes | 2023 | 198,670 | 890,893 | - | - | 34,076 | 1,123,639 | ||||||||||||||||||||||||
| Former Chief Legal Officer & General Counsel(3) | 2022 | 368,958 | 246,697 | - | - | 218,064 | - | 833,719 | |||||||||||||||||||||||
Option awards represent granted options at the fair market value as of the date of grant. Restricted stock units represent granted restricted stock units at the fair market value as of the date of grant.
| (1) | In February 2023, the Company formed a subsidiary, Pearsanta, Inc. in order to accelerate the growth of the Company’s AditxtScore program through future strategic revenue and growth oriented transactions. In connection with the formation of Pearsanta and Corinne Pankovcin’s anticipated role in driving such strategic revenue and growth oriented transactions, Ms. Pankovcin’ s title was changed from President to Chief Commercialization Officer, effective April 12, 2023. |
| (2) | Ms. Pankovcin served as the Company’s Chief Financial Officer from July 2020 through September 25, 2021. She was appointed as our President on September 25, 2021. Ms. Pankovcin’s title was changed from President to Chief Commercialization Officer effective April 12, 2023. |
| (3) | Mr. Shatzkes joined Aditxt in January of 2022. Mr. Shatzkes departed Aditxt in July of 2023. |
| (4) | All other compensation is inclusive of Pearsanta, Inc. option grants to Mr. Albanna, Dr. Shabahang, Ms. Pankovcin, and Mr. Farley. Mr. Shatzkes received consideration in connection with the Separation and General Release agreement. |
Employment Agreements
Amro Albanna, Chief Executive Officer
On November 14, 2021, the Company entered into an Amended and Restated Employment Agreement with Mr. Amro Albanna, the Chief Executive Officer of the Company (the “Amro Employment Agreement”). Pursuant to the Amro Employment Agreement, Mr. Albanna will receive (i) a base salary at the annual rate of $280,000 for Registrant’s Common Equity, Related Stockholder Mattersthe remainder of calendar year 2021, and Issuer Purchaseseffective January 1, 2022, $500,000 (prorated for any partial year) payable in bimonthly installments (ii) the opportunity to earn an annual bonus of Equity Securities.
Market Information
On June 30, 2020, our common stock began trading on2% of the Nasdaq Capital Market underCompany’s earnings before interest, taxes, depreciation, and amortization (EBITDA) with respect to an applicable year for which the symbol “ADTX.” Priorbonus is payable, provided that such bonus will not exceed two (2) times Mr. Albanna’s base salary, and (iii) eligible to that time, there was no public marketearn an annual discretionary bonus as determined by the Board or its Compensation Committee in their sole discretion. In addition, for our common stock.
Holders
As of March 24,calendar year 2021, there were approximately 135 record holders of our common stock and no holders of our preferred stock. The actual number of holders of our common stock is greater than this number of record holders, and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers or held by other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividend Policy
We have never paid or declared any cash dividends on our common stock, and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain all available funds and any future earnings to fund the development and expansion of our business. Any future determination to pay dividendsMr. Albanna will be ateligible to earn an additional discretionary bonus as determined by the discretion of our board of directors and will depend upon a number of factors, including our results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors that our board of directors deems relevant.Company.
Recent Sales of Unregistered Securities
On November 6, 2020 the Company issued warrants to a consultant to purchase up to 120,000 shares of common stock at an exercise price of $1.92 per share for services rendered.
On December 31, 2020, the Company issued a consultant 650,000 shares of common stock upon completion of certain milestones included in a consulting agreement.
Equity Compensation Plans
The information required by Item 5 of Form 10-K regarding equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report on Form 10-K.
Issuer Purchases of Equity Securities
We did not purchase any of our registered equity securities during the period covered by this Annual Report.
Use of Proceeds from Initial Public Offering
On July 2, 2020, we completed our initial public offering (“IPO”). In connection therewith, we issued 1,226,668 Units (the “Units”), excluding the underwriters’ option to cover overallotments, at an offering price of $9.00 per Unit, resulting in gross proceeds of approximately $11.0 million. The Units issued in the IPO consisted of one share of common stock, one Series A warrant, and one Series B warrant. The Series A warrants originally had an exercise price of $9.00 and a term of 5 years. In addition, we issued a Unit Purchase Option at an exercise price of $11.25 per unit to the underwriters to purchase up to 67,466 units, with each unit consisting of (i) one share of common stock and (ii) one Series A Warrant. On August 19, 2020 we modified the exercise price of the Series A Warrants from $9.00 per share to $4.50 per share. The term of Mr. Albanna’s engagement under the Series A Warrants was not modified. The Series B warrants have an exercise price of $11.25 per share, a term of 5 years and contain a cashless exercise option upon certain criteria being met. As of December 31, 2020, substantially allAmro Employment Agreement commences as of the Series B warrants issued in the IPO have been exercised pursuant to a cashless provision therein.
We received net proceeds of $8.5 million in the IPO, after deducting underwriting discounts and commissions and issuance expenses borne by us. No payments were made by us to directors, officers or persons owning ten percent or more of our common stock or to their associates, or to our affiliates, other than payments in the ordinary course of business to officers for salaries and to non-employee directors pursuant to our director compensation policy. Dawson James Securities, Inc. acted as lead book-running manager of the offering and as representative of the underwriters for the offering.
There has been no material change in the planned use of proceeds from our IPO from that described in the final prospectus related to the offering, dated June 29, 2020, as filed with the SEC.
Item 6. Selected Financial Data.
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read together with our financial statements and the related notes and other financial information included elsewhere in this report. Some of the information contained in this discussion and analysis or set forth elsewhere in this report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. See “Cautionary Note Regarding Forward-Looking Statements.”
Overview
We are a life sciences company with a mission of prolonging life and enhancing its quality by improving the health of the immune system. Our immune reprogramming technology is currently at the pre-clinical stage and designed to retrain the immune system to induce tolerance with an objective of addressing rejection of transplanted organs, autoimmune diseases, and allergies. Our immune monitoring technology is designed to provide a personalized comprehensive profile of the immune system and we plan to utilize it in our upcoming clinical trials to monitor subjects’ immune response before, during and after drug administration. We are also evaluating plans to obtain FDA approval for this monitoring tool’s use as a clinical assay.
Recent Developments
On January 25, 2021 (the “Closing Date”), we entered into a Securities Purchase Agreement (the “Purchase Agreement”) with an institutional accredited investor for the offering, sale, and issuance (the “Offering”) of a $6,000,000 Senior Secured Convertible Promissory Note (the “Note”). Concurrently with the sale of the Note, pursuant to the Purchase Agreement, we also issued a warrant “January 2021 Warrant”) to the investor to purchase up to 800,000 shares (the “January 2021 Warrant Shares”) of the Company’s common stock. As a result of the Offering, the Company received aggregate gross proceeds of $5,000,000.
The Note has a twenty-four month term and is convertible at the option of the investor at any time prior to maturity in shares of Common Stock (the “Conversion Shares”) at an initial conversion price of $4.00 per share, subject to adjustment under certain circumstances. The Note amortizes in nineteen (19) equal monthly installments (the “Installment Payments”) starting the first day of the sixth month after the ClosingEffective Date (each, an “Installment Date”). At the Company’s option, Installment Payments may be made in cash or in shares of the Company’s common stock. If the Company elects to repay in cash, the amount payable shall be 105% of the applicable Installment Payment. If the Company elects to repay in shares of common stock, the shares shall be priced at the lowest of (i) the Conversion Price then in effect, and (ii) the greater of (x) the Floor Price (as defined in the Note)Amro Employment Agreement) and (y)continues until November 14, 2023, unless earlier terminated in accordance with the lower of 90%terms of the lowest volume weighted average price (VWAP)Amro Employment Agreement. The term of Mr. Albanna’s Employment Agreement is automatically renewed for successive one (1) year periods until terminated by Mr. Albanna or the common stock for each of the five (5) Trading Days (as such term is defined in the Note) ending and including the Trading Day immediately prior to the applicable Installment Date.Company.
All Installment Payments are subject toUnder the investor’s right to (a) defer someAmro Employment Agreement, termination of Mr. Albanna by the Company for “Cause,” “Death,” or all of any Installment Payment to a subsequent Installment Date or (b) to convert an additional Installment Payment of the Note at the then-current Installment Price until the next Installment Date. Upon the occurrence of an Event of Default or a Change of Control“Disability,” (as such terms are defined in the Note)Amro Employment Agreement), or resignation by Mr. Albanna without “Good Reason” (as defined in the Note isAmro Employment Agreement), will not require the Company to pay severance to Mr. Albanna. Upon any such termination, Mr. Albanna will be entitled to receive any Accrued Compensation (as defined in the Amro Employment Agreement), which in the case of termination by the Company for Cause or resignation by Mr. Albanna for Good Reason will not include payment of pro rata bonus; provided, however, if termination of Mr. Albanna by the Company without “Cause” or resignation by Mr. Albanna for “Good Reason,” then under the Amro Employment Agreement will require the Company to pay severance to Mr. Albanna. Upon any such termination, Mr. Albanna will be entitled to receive any Accrued Compensation and, subject to redemptionMr. Albanna’s execution of an irrevocable release, receive (i) on the sixtieth day (60th) day following termination, a lump sum amount equal to twelve (12) months base salary then in effect as of the date of termination, less applicable taxes and withholdings; (ii) provide reimbursement to Mr. Albanna’s medical insurance premiums for a period of twelve (12) months following the date of termination; and (iii) cause any equity awards granted prior to the Effective Date (as defined in the Amro Employment Agreement), that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
Notwithstanding the foregoing, under the Amro Employment Agreement, termination of Mr. Albanna by the investor. The Company is prohibited from effectingwithout Cause or resignation by Mr. Albanna for Good Reason and a conversionChange of Control (as defined in the Amro Employment Agreement) of the NoteCompany occurs within six (6) months after such termination, or within twenty-four (24) months prior to such termination, the Company will pay severance to Mr. Albanna in connection to such termination. Upon such termination, Mr. Albanna will be entitled to receive any Accrued Compensation, and subject to Mr. Albanna’s execution of an irrevocable release, receive (i) on the sixtieth (60th) day of termination, a lump sum cash-payment equal to the extent that,product of three times Mr. Albanna’s salary then in effect as a result of such exercise, the investor, together with the its affiliates, would beneficially own more than 4.99% of the numberdate of sharestermination, less applicable taxes and withholdings; (ii) provide reimbursement to Mr. Albanna’s medical insurance premiums for a period of commontwenty-four (24) months following the date of termination; and (iii) notwithstanding any provision of any stock incentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to capital stock of the Company, outstanding immediately after giving effectcause any equity awards granted prior to the issuancethat are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable for twenty-four (24) months (but not later than when the award would otherwise expire).
The Amro Employment Agreement also contains customary non-solicitation and non-competition covenants, which covenants remain in effect for twelve (12) months following any cessation of employment with respect to Mr. Albanna. To the extent any of the such shares,payments or benefits provided for under the Amro Employment Agreement or any other agreement or arrangement between Mr. Albanna and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Mr. Albanna the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
Corinne Pankovcin, Chief Commercialization Officer
On November 14, 2021, Aditxt, Inc. (the “Company”) entered into a new employment agreement (the “Pankovcin Employment Agreement”) with the Company’s President, Corinne Pankovcin, pursuant to which beneficial ownership limitationMs. Pankovcin will continue to serve as the Company’s President and Secretary until the date upon which Ms. Pankovcin’s employment may be increasedterminated in accordance with the terms of the Pankovcin Employment Agreement.
The term of Ms. Pankovcin’s engagement under the Pankovcin Employment Agreement commences as of the Effective Date (as defined in the Pankovcin Employment Agreement) and continues until November 14, 2023, unless earlier terminated in accordance with the terms of the Pankovcin Employment Agreement. The term of Ms. Pankovcin’s Employment Agreement is automatically renewed for successive one (1) year periods until terminated by Ms. Pankovcin or the Company.
Pursuant to the Pankovcin Employment Agreement, Ms. Pankovcin will receive: (i) a base salary at the annual rate of $250,000 for the remainder of calendar year 2021, and effective January 1, 2022, $385,000 (prorated for any partial year) payable in bimonthly installments and (ii) eligible to earn an annual discretionary bonus with a target amount of 45% of Base Compensation, which is based on the achievement of performance objectives, which will be determined by the investor upBoard and Compensation Committee. In addition, for calendar year 2021, Ms. Pankovcin shall be eligible to butearn an additional discretionary bonus as determined by the Company.
Under the Pankovcin Employment Agreement, termination of Ms. Pankovcin by the Company for “Cause,” “Death,” or “Disability,” (as such terms are defined in the Pankovcin Employment Agreement), or resignation by Ms. Pankovcin for “Good Reason” (as defined in the Pankovcin Employment Agreement), will not exceeding, 9.99%.
The January 2021 Warrants are immediately exercisablerequire the Company to pay severance to Ms. Pankovcin. Upon any such termination, Ms. Pankovcin will be entitled to receive any Accrued Compensation (as defined in the Pankovcin Employment Agreement), which in the case of termination by the Company for Cause or resignation by Ms. Pankovcin for Good Reason will not include payment of pro rata bonus; provided, however, if termination of Ms. Pankovcin by the Company without “Cause” or resignation by Ms. Pankovcin for “Good Reason,” then under the Pankovcin Employment Agreement will require the Company to pay severance to Ms. Pankovcin. Upon any such termination, Ms. Pankovcin will be entitled to receive any Accrued Compensation and, subject to Ms. Pankovcin’s execution of an irrevocable release, receive: (i) on the sixtieth day (60th) day following termination, a lump sum amount equal to twelve (12) months base salary then in effect as of the date of termination, less applicable taxes and withholdings; (ii) provide reimbursement to Ms. Pankovcin’s medical insurance premiums for a period of three (3) years at an exercise pricetwelve (12) months following the date of $4.00 per share,termination; and (iii) cause any equity awards granted prior to the Effective Date (as defined in the Pankovcin Employment Agreement), that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
Notwithstanding the foregoing, under the Pankovcin Employment Agreement, termination of Ms. Pankovcin by the Company without Cause or resignation by Ms. Pankovcin for Good Reason and a Change of Control (as defined in the Pankovcin Employment Agreement) of the Company occurs within six (6) months after such termination, or within twenty-four (24) months prior to such termination, the Company will pay severance to Ms. Pankovcin in connection to such termination. Upon such termination, Ms. Pankovcin will be entitled to receive any Accrued Compensation, and subject to adjustment. AfterMs. Pankovcin’s execution of an irrevocable release, receive (i) on the sixtieth (60th) day of termination, a lump sum cash-payment equal to the sum of (A) the product of two times Ms. Pankovcin’s salary then in effect as of the date of termination, less applicable taxes and withholdings, and (B) the product of two times Ms. Pankovcin’s Target Bonus; (ii) provide reimbursement to Ms. Pankovcin’s medical insurance premiums for a period of one hundred eight (180) days, if a registration statement coveringtwenty-four (24) months following the resaledate of the sharestermination; and (iii) notwithstanding any provision of commonany stock underlying the January 2021 Warrants is not effective, the holder may exercise the January 2021 Warrant by means of a cashless exercise. The Company is prohibited from effecting an exercise of the January 2021 Warrantsincentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to the extent that, as a result of such exercise, the holder of the January 2021 Warrants together with the holder’s affiliates, would beneficially own more than 4.99% of the number of shares of commoncapital stock of the Company, outstanding immediately after giving effectcause any equity awards granted prior to the issuancethat are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable for twenty-four (24) months (but not later than when the award would otherwise expire).
The Pankovcin Employment Agreement also contains customary non-solicitation and non-competition covenants, which covenants remain in effect for twelve (12) months following any cessation of employment with respect to Ms. Pankovcin. To the extent any of the such shares,payments or benefits provided for under the Pankovcin Employment Agreement or any other agreement or arrangement between Ms. Pankovcin and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Ms. Pankovcin the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
Thomas J. Farley, Chief Financial Officer
On November 14, 2021, Aditxt, Inc. (the “Company”) entered into a new employment agreement (the “Farley Employment Agreement”) with the Company’s Chief Financial Officer, Thomas Farley, pursuant to which beneficial ownership limitationMr. Farley will continue to serve as the Company’s Chief Financial Officer until the date upon which Mr. Farley’s employment may be increasedterminated in accordance with the terms of the Farley Employment Agreement.
The term of Mr. Farley’s engagement under the Farley Employment Agreement commences as of the Effective Date (as defined in the Farley Employment Agreement) and continues until November 14, 2023, unless earlier terminated in accordance with the terms of the Farley Employment Agreement. The term of Mr. Farley’s Employment Agreement is automatically renewed for successive one (1) year periods until terminated by Mr. Farley or the Company.
Pursuant to the Farley Employment Agreement, Mr. Farley will receive: (i) a base salary at the annual rate of $225,000 for the remainder of calendar year 2021, and effective January 1, 2022, $355,000 (prorated for any partial year) payable in bimonthly installments and, (ii) eligible to earn an annual discretionary bonus with a target amount of 40% of Base Compensation, which is based on the achievement of performance objectives, which will be determined by the holder upBoard and Compensation Committee. In addition, for calendar year 2021, Mr. Farley will be eligible to butearn an additional discretionary bonus as determined by the Company.
Under the Farley Employment Agreement, termination of Mr. Farley by the Company for “Cause,” “Death,” or “Disability,” (as such terms are defined in the Farley Employment Agreement), or resignation by Mr. Farley without “Good Reason” (as defined in the Farley Employment Agreement), will not exceeding, 9.99%.require the Company to pay severance to Mr. Farley. Upon any such termination, Mr. Farley will be entitled to receive any Accrued Compensation (as defined in the Farley Employment Agreement which in the case of termination by the Company for Cause or resignation by Mr. Farley for Good Reason will not include payment of pro rata bonus; provided, however, if termination of Mr. Farley by the Company without “Cause” or resignation by Mr. Farley for “Good Reason,” then under the Farley Employment Agreement will require the Company to pay severance to Mr. Farley. Upon any such termination, Mr. Farley will be entitled to receive any Accrued Compensation and, subject to Mr. Farley’s execution of an irrevocable release, receive (i) on the sixtieth day (60th) day following termination, a lump sum cash-payment equal to the sum of (A) the product of two times Mr. Farley’s salary then in effect as of the date of termination, less applicable taxes and withholdings, and (B) the product of two times Mr. Farley’s Target Bonus (as defined in the Farley Employment Agreement); (ii) provide reimbursement to Mr. Farley’s medical insurance premiums for a period of twelve (12) months following the date of termination; and (iii) cause any equity awards granted prior to the Effective Date (as defined in the Farley Employment Agreement), that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
Additionally,
Notwithstanding the foregoing, under the Farley Employment Agreement, termination of Mr. Farley by the Company without Cause or resignation by Mr. Farley for Good Reason and a Change of Control (as defined in the Farley Employment Agreement) of the Company occurs within six (6) months after such termination, or within twenty-four (24) months prior to such termination, the Company will pay severance to Mr. Farley in connection to such termination. Upon such termination, Mr. Farley will be entitled to receive any Accrued Compensation, and subject to Mr. Farley’s execution of an irrevocable release, receive (i) on the sixtieth (60th) day of termination, a lump sum cash-payment equal to the product of two times Mr. Farley’s salary then in effect as of the date of termination, less applicable taxes and withholdings; (ii) provide reimbursement to Mr. Farley’s medical insurance premiums for a period of twelve (12) months following the date of termination; and (iii) notwithstanding any provision of any stock incentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to capital stock of the Company, cause any equity awards granted prior to the that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable (but not later than when the award would otherwise expire).
The Farley Employment Agreement also contains customary non-solicitation and non-competition covenants, which covenants remain in effect for twelve (12) months following any cessation of employment with respect to Mr. Farley. To the extent any of the payments or benefits provided for under the Farley Employment Agreement or any other agreement or arrangement between Mr. Farley and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Mr. Farley the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
Shahrokh Shabahang, Chief Innovation Officer
On November 14, 2021, Aditxt, Inc. (the “Company”) entered into a new employment agreement (the “Shabahang Employment Agreement”) with the Company’s Chief Innovation Officer, Shahrokh Shabahang, pursuant to which Mr. Shabahang will continue to serve as the Company’s Chief Innovation Officer until the earlierdate upon which Mr. Shabahang’s employment may be terminated in accordance with the terms of the Shabahang Employment Agreement.
The term of Mr. Shabahang’s engagement under the Shabahang Employment Agreement commences as of the Effective Date (as defined in the Shabahang Employment Agreement) and continues until November 14, 2023, unless earlier terminated in accordance with the terms of the Shabahang Employment Agreement. The term of Mr. Shabahang’s Employment Agreement is automatically renewed for successive one (1) year periods until terminated by Mr. Shabahang or the Company.
Pursuant to the Shabahang Employment Agreement, Mr. Shabahang will receive: (i) onea base salary at the annual rate of $210,000 for the remainder of calendar year anniversary the Closing Date,2021, and effective January 1, 2022, $325,000 (prorated for any partial year) payable in bimonthly installments, and (ii) eligible to earn an annual discretionary bonus with a target amount of 40% of Base Compensation, which is based on the achievement of performance objectives, which will be determined by the Board and Compensation Committee. In addition, for calendar year 2021, Mr. Shabahang will be eligible to earn an additional discretionary bonus as determined by the Company.
Under the Shabahang Employment Agreement, termination of Mr. Shabahang by the Company for “Cause,” “Death,” or “Disability,” (as such terms are defined in the Shabahang Employment Agreement), or resignation by Mr. Shabahang without “Good Reason” (as defined in the Shabahang Employment Agreement), will not require the Company to pay severance to Mr. Shabahang. Upon any such termination, Mr. Shabahang will be entitled to receive any Accrued Compensation (as defined in the Shabahang Employment Agreement), which in the case of termination by the Company for Cause or resignation by Mr. Shabahang for Good Reason will not include payment of pro rata bonus; provided, however, if termination of Mr. Shabahang by the Company without “Cause” or resignation by Mr. Shabahang for “Good Reason,” then under the Shabahang Employment Agreement will require the Company to pay severance to Mr. Shabahang. Upon any such termination, Mr. Shabahang will be entitled to receive any Accrued Compensation and, subject to Mr. Shabahang’s execution of an irrevocable release, receive: (i) on the sixtieth day (60th) day following termination, a lump sum cash-payment equal to the sum of (A) the product of two times Mr. Shabahangs’s salary then in effect as of the date of termination, less applicable taxes and withholdings, and (B) the product of two times Mr. Shabahang’s Target Bonus (as defined in the Shabahang Employment Agreement); (ii) provide reimbursement to Mr. Shabahang’s medical insurance premiums for a period of twelve (12) months following the date of termination; and (iii) cause any equity awards granted prior to the Effective Date (as defined in the Shabahang Employment Agreement), that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
Notwithstanding the foregoing, under the Shabahang Employment Agreement, termination of Mr. Shabahang by the Company for without Cause or resignation by Mr. Shabahang for Good Reason and a Change of Control (as defined in the Shabahang Employment Agreement) of the Company occurs within six (6) months after such termination, or within twenty-four (24) months prior to such termination, the Company will pay severance to Mr. Shabahang in connection to such termination. Upon such termination, Mr. Shabahang will be entitled to receive any Accrued Compensation, and subject to Mr. Shabahang’s execution of an irrevocable release, receive: (i) on the sixtieth (60th) day of termination, a lump sum cash-payment equal to the product of two times Mr. Shabahang’s salary then in effect as of the date of termination, less applicable taxes and withholdings; (ii) provide reimbursement to Mr. Shabahang’s medical insurance premiums for a period of twenty-four (24) months following the date of termination; and (iii) notwithstanding any provision of any stock incentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to capital stock of the Company, cause any equity awards granted prior to the that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable for twenty-four (24) months (but not later than when the award would otherwise expire).
The Shabahang Employment Agreement also contains customary non-solicitation and non-competition covenants, which covenants remain in effect for twelve (12) months following any cessation of employment with respect to Mr. Shabahang. To the extent any of the payments or benefits provided for under the Shabahang Employment Agreement or any other agreement or arrangement between Mr. Shabahang and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Mr. Shabahang the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
Rowena Albanna, Chief Operating Officer
On November 14, 2021, Aditxt, Inc. (the “Company”) entered into a new employment agreement (the “Rowena Employment Agreement”) with the Company’s Chief Operating Officer, Rowena Albanna, pursuant to which Ms. Albanna will continue to serve as the Company’s Chief Operating Officer until the date upon which Ms. Albanna’s employment may be terminated in accordance with the terms of the Rowena Employment Agreement.
The term of Ms. Albanna’s engagement under the Rowena Employment Agreement commences as of the Effective Date (as defined in the Rowena Employment Agreement) and continues until November 14, 2023, unless earlier terminated in accordance with the terms of the Rowena Employment Agreement. The term of Ms. Albanna’s Employment Agreement is automatically renewed for successive one (1) year periods until terminated by Ms. Albanna or the Company.
Pursuant to the Rowena Employment Agreement, Ms. Albanna will receive: (i) a base salary at the annual rate of $210,000 for the remainder of calendar year 2021 and effective January 1, 2022, $325,000 (prorated for any partial year) payable in bimonthly installments, and (ii) eligible to earn an annual discretionary bonus with a target amount of 40% of Base Compensation, which is based on the achievement of performance objectives, which will be determined by the Board and Compensation Committee. In addition, for calendar year 2021, Ms. Albanna will be eligible to earn an additional discretionary bonus as determined by the Company.
Under the Rowena Employment Agreement, termination of Ms. Albanna by the Company for “Cause,” “Death,” or “Disability,” (as such terms are defined in the Rowena Employment Agreement), or resignation by Ms. Albanna for “Good Reason” (as defined in the Rowena Employment Agreement), will not require the Company to pay severance to Ms. Albanna. Upon any such termination, Ms. Albanna will be entitled to receive any Accrued Compensation (as defined in the Rowena Employment Agreement), which in the case of termination by the Company for Cause or resignation by Ms. Albanna for Good Reason will not include payment of pro rata bonus; provided, however, if termination of Ms. Albanna by the Company without “Cause” or resignation by Ms. Albanna for “Good Reason” (as such terms are defined in the Rowena Employment Agreement), then under the Rowena Employment Agreement will require the Company to pay severance to Ms. Albanna. Upon any such termination, Ms. Albanna will be entitled to receive any Accrued Compensation and, subject to Ms. Albanna’s execution of an irrevocable release, receive: (i) on the sixtieth day (60th) day following termination, a lump sum amount equal to twelve (12) months base salary then in effect as of the date of termination, less applicable taxes and withholdings; (ii) provide reimbursement to Ms. Albanna’s medical insurance premiums for a period of twelve (12) months following the date of termination; and (iii) cause any equity awards granted prior to the Effective Date (as defined in the Rowena Employment Agreement), that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
Notwithstanding the foregoing, under the Rowena Employment Agreement, termination of Ms. Albanna by the Company without Cause or resignation by Ms. Albanna for Good Reason and a Change of Control (as defined in the Rowena Employment Agreement) of the Company occurs within six (6) months after such termination, or within twenty-four (24) months prior to such termination, the Company will pay severance to Ms. Albanna in connection to such termination. Upon such termination, Ms. Albanna will be entitled to receive any Accrued Compensation, and subject to Ms. Albanna’s execution of an irrevocable release, receive: (i) on the sixtieth (60th) day of termination, a lump sum cash-payment equal to the sum of (A) the product of two times Ms. Albanna’s salary then in effect as of the date of termination, less applicable taxes and withholdings, and (B) the product of two times Ms. Albanna’s Target Bonus; (ii) provide reimbursement to Ms. Albanna’s medical insurance premiums for a period of twenty-four (24) months following the date of termination; and (iii) notwithstanding any provision of any stock incentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to capital stock of the Company, cause any equity awards granted prior to the that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable for twenty-four (24) months (but not later than when the award would otherwise expire).
The Rowena Employment Agreement also contains customary non-solicitation and non-competition covenants, which covenants remain in effect for twelve (12) months following any cessation of employment with respect to Ms. Albanna. To the extent any of the payments or benefits provided for under the Rowena Employment Agreement or any other agreement or arrangement between Ms. Albanna and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Ms. Albanna the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
Matthew Shatzkes, Former Chief Legal Officer and General Counsel
On January 28, 2022, Aditxt, Inc. (the “Company”) entered into an employment agreement (the “Employment Agreement”) with Matthew Shatzkes, the Chief Legal Officer and General Counsel of the Company. Pursuant to the Employment Agreement, Mr. Shatzkes will (i) receive a base salary at the annual rate of $385,000 (the “Base Compensation”) payable in bimonthly installments, (ii) receive a one-time sign-on bonus (the “Sign-on Bonus”), (iii) a minimum 2022 quarterly bonus (the “Minimum 2022 Bonus”), and (iv) will be entitled to earn an annual discretionary bonus beginning in fiscal year 2022.
Following the first anniversary of the Employment Agreement (the “Anniversary Date”), in addition to Mr. Shatzkes’ Base Compensation, Mr. Shatzkes will be entitled to a minimum quarterly bonus (the “Subsequent Year Minimum Bonus”). Following the Anniversary Date, in addition to Mr. Shatzkes’ Base Compensation and Subsequent Year Minimum Bonus, Mr. Shatzkes will also be eligible to earn an annual discretionary bonus.
Under the Employment Agreement, Mr. Shatzkes will also receive (i) a restricted stock unit award that will entitle Mr. Shatzkes to receive 150,000 shares of the Company’s common stock which shall vest immediately, and (ii) a restricted stock unit award of an additional 330,000 shares of the Company’s common stock, which shall vest ratably over eight successive equal quarterly installments over a two-year period commencing on March 1, 2022 and ending on December 1, 2023.
The term of Mr. Shatzkes engagement under the Employment Agreement commences on the Effective Date (as defined in the Employment Agreement) and continues until January 16, 2024, unless earlier terminated in accordance with the terms of the Employment Agreement. The term of Mr. Shatzkes’ Employment Agreement is automatically renewed for successive one-year periods until terminated by Mr. Shatzkes or the Company.
Under the Employment Agreement, termination of Mr. Shatzkes by the Company for “Cause,” “Death,” or “Disability,” (as such terms are defined in the Employment Agreement), or resignation by Mr. Shatzkes without “Good Reason” (as defined in the Employment Agreement), will not require the Company to pay severance to Mr. Shatzkes. Upon any such termination, Mr. Shatzkes will be entitled to receive any Accrued Compensation (as defined in the Employment Agreement), which in the case of termination by the Company for Cause or resignation by Mr. Shatzkes for Good Reason will not include payment of pro rata bonus. If, however, termination of Mr. Shatzkes by the Company without “Cause”, resignation by Mr. Shatzkes for “Good Reason” or and a Change of Control (as defined in the Employment Agreement) event occurs, then the Employment Agreement will require the Company to pay severance to Mr. Shatzkes. Upon any such termination, Mr. Shatzkes will be entitled to receive any Accrued Compensation and, subject to Mr. Shatzkes’ execution of an irrevocable release, (i) on the sixtieth day following termination, a lump sum amount equal (a) twelve months of his Base Compensation, Sign-on Bonus and Minimum 2022 Bonus if his Employment Agreement is terminated prior to December 31, 2022, or (b) his Base Compensation and Subsequent Year Minimum Bonus if his Employment Agreement is terminated after December 31, 2022; (ii) provide reimbursement to Mr. Shatzkes’ medical insurance premiums for a period of twelve months following the date of termination; and (iii) notwithstanding any provision of any stock incentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to capital stock of the Company, cause any equity awards granted prior to that termination that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
To the extent any of the payments or benefits provided for under the Employment Agreement or any other agreement or arrangement between Mr. Shatzkes and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Mr. Shatzkes the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
On July 21, 2023, Matthew Shatzkes tendered his resignation as Chief Legal Officer, General Counsel and Corporate Secretary of the Company. In connection with his resignation, the Company entered into a Separation Agreement and General Release (the “Separation Agreement”). Pursuant to the Separation Agreement, Mr. Shatzkes employment with the Company terminated on August 4, 2023 (the “Termination Date”). In addition, the Company agreed to pay Mr. Shatzkes within seven days after the Termination Date: (i) $122,292, representing all accrued salary and wages (inclusive of Base Compensation and earned Subsequent Quarterly Bonus amounts, as those terms are defined in Mr. Shatzkes employment agreement), and (ii) $32,576, representing Mr. Shatzkes accrued, but unused paid time as less than $2 millionoff. The Company also agreed to pay Mr. Shatzkes: (i) $385,000, representing 12 months of aggregate Principal AmountMr. Shatzkes Base Compensation (as that term is defined in Mr. Shatzkes employment agreement), and (ii) $290,000, representing Mr. Shatzkes Subsequent Year Minimum Bonus (as such term is defined in Mr. Shatzkes employment agreement), on the Note)60th day following the Termination Date. In addition, the Company shall reimburse Mr. Shatzkes COBRA premium for a period of 12 months and shall cause any restricted stock units granted to Mr. Shatzkes to immediately vest as of the Note remains outstanding,Termination Date.
On August 15, 2023, the Company is prohibited from effectingentered into an Amendment to Separation Agreement and General Release with Mr. Shatzkes (the “Separation Agreement Amendment”). Pursuant to the Separation Agreement Amendment, the Company was required to pay Mr. Shatzkes, upon the earlier of (i) September 1, 2023 or (ii) two business days following the closing of a capital raise by the Company, an amount equal to $91,060.16, which amount represents the balance of Mr. Shatzkes’ Accrued Salary and Wages and Accrued PTO plus an additional $1,000 to serve as consideration for entering into the Separation Agreement Amendment. In addition, under the Separation Agreement Amendment, the Company was required to pay Mr. Shatzkes the Severance Base Compensation and the Severance Bonus upon the earlier of (i) the 60th day following the Termination Date or (ii) two business days following the closing of a capital raise by the Company.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth certain information regarding beneficial ownership of shares of our common stock as of February 12, 2024 based on 1,665,214 shares issued and outstanding by (i) each person known to beneficially own more than 5% of our outstanding common stock, (ii) each of our directors, (iii) our executive officers and (iv) all directors and executive officers as a group. Shares are beneficially owned when an agreementindividual has voting and/or investment power over the shares or could obtain voting and/or investment power over the shares within 60 days of February 12, 2024. Except as otherwise indicated, the persons named in the table have sole voting and investment power with respect to effectall shares beneficially owned, subject to community property laws, where applicable. Unless otherwise indicated, the address of each beneficial owner listed below is c/o Aditxt, Inc., 737 N. Fifth Street, Suite 200, Richmond, VA 23219.
| Number of shares of Common Stock Beneficially Owned | Percentage | |||||||
| Directors and Officers: | ||||||||
| Amro Albanna (1) | 10,103 | * | % | |||||
| Shahrokh Shabahang, D.D.S., MS, Ph.D. (2) | 7,780 | * | % | |||||
| Corinne Pankovcin (3) | 4,966 | * | % | |||||
| Rowena Albanna (4) | 4,956 | * | % | |||||
| Brian Brady (5) | 488 | * | % | |||||
| Jeffrey Runge, M.D. (6) | 483 | * | % | |||||
| Thomas J. Farley (7) | 4,812 | * | % | |||||
| Charles Nelson (8) | 731 | * | % | |||||
| All directors and executive officers as a group (9 persons) | 34,319 | 2.1 | % | |||||
| * | Less than 1% |
| (1) | Includes (i) 9,704 shares issuable pursuant to options that are fully vested; (ii) 228 shares beneficially owned by the Albanna Family Trust, of which Mr. Albanna is the Trustee; (iii) 151 shares directly owned by Mr. Albanna; and (iv) 20 Series A Warrants issued as part of the conversion of outstanding accrued compensation through March 31, 2020. Mr. Albanna may be deemed to beneficially own the securities held by his wife Rowena Albanna, the Company’s Chief Operating Officer. |
| (2) | Includes (i) 7,108 beneficially owned by Shabahang-Hatami Family Trust, of which Shahrokh Shabahang, D.D.S., MS, Ph.D. is the Trustee; (ii) warrants to purchase 111 shares, including 24 Series A Warrants issued as part of the conversion of outstanding accrued compensation through March 31, 2020, and 87 warrants beneficially owned by the Shabahang-Hatami Family Trust; (iii) 561 shares directly owned by Mr. Shabahang. |
| (3) | Includes (i) 86 shares held directly by Ms. Pankovcin; and (ii) 4,880 shares issuable pursuant to options that are fully vested. |
| (4) | Includes (i) 86 shares held directly by Ms. Albanna; (ii) 4,852 shares issuable pursuant to options that are fully vested; and (iii) 18 Series A Warrants issued as part of the conversion of outstanding accrued compensation through March 31, 2020. Ms. Albanna may be deemed to beneficially own the securities held by her husband Amro Albanna, the Company’s Chief Executive Officer. |
| (5) | Includes (i) 13 shares held directly by Mr. Brady; and (ii) 475 shares issuable pursuant to options that are fully vested. |
| (6) | Includes (i) 2 shares held by Biologue, Inc., over which Dr. Runge has voting and dispositive control; (ii) 6 shares held directly by Dr. Runge; and (iii) 475 shares issuable pursuant to options that are fully vested. |
| (7) | Includes (i) 80 shares held directly by Mr. Farley and (ii) 4,732 shares issuable pursuant to options that are fully vested. |
| (8) | Includes (i) 261 shares held by Siu Kim Athle International, LLC., over which Mr. Nelson has voting and dispositive control and (ii) 470 shares issuable pursuant to options that are fully vested. |
Item 13. Certain Relationships and Related Transactions, and Director Independence
Except as described below and except for employment arrangements which are described under “executive compensation,” since January 1, 2018, there has not been, nor is there currently proposed, other than described below, any issuancetransaction in which we are or were a participant, the amount involved exceeds the lesser of securities involving$120,000 or 1% of the average of the total assets at December 31, 2023 and 2022, and any of our directors, executive officers, holders of more than 5% of our Common Stock or any immediate family member of any of the foregoing had or will have a Variable Rate Transaction (as such termdirect or indirect material interest.
On February 29, 2024, Amro Albanna, the Chief Executive Officer of the Company, and Shahrokh Shabahang, the Chief Innovation Officer of the Company, loaned $117,000 and $115,000, respectively, to the Company. The loans were evidenced by an unsecured promissory note (the “February 29th Notes”). Pursuant to the terms of the February 29th Notes, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of August 29, 2024 or an event of default, as defined therein.
On February 15, 2024, Amro Albanna, the Chief Executive Officer of the Company loaned $205,000 to the Company. The loan was evidenced by an unsecured promissory note (the “February Note”). Pursuant to the terms of the February Note, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of August 15, 2024 or an event of default, as defined therein.
On February 7, 2024, Amro Albanna, the Chief Executive Officer of the Company loaned $30,000 to the Company. The loan was evidenced by an unsecured promissory note (the “February Note”). Pursuant to the terms of the February Note, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of August 7, 2024 or an event of default, as defined therein.
On December 20, 2023, Amro Albanna, the Chief Executive Officer of the Company loaned $165,000 to the Company. The loan was evidenced by an unsecured promissory note (the “Second December Note”). Pursuant to the terms of the December Note, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of June 20, 2024 or an event of default, as defined therein. As of December 31, 2023 this loan has been repaid.
On December 6, 2023, Amro Albanna, the Chief Executive Officer of the Company loaned $200,000 to the Company. The loan was evidenced by an unsecured promissory note (the “First December Note”). Pursuant to the terms of the December Note, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of June 6, 2024 or an event of default, as defined therein. As of December 31, 2023 this loan has been repaid.
On November 30, 2023, Amro Albanna, the Chief Executive Officer of the Company loaned $10,000 to the Company. The loan was evidenced by an unsecured promissory note (the “November Note”). Pursuant to the terms of the November Note, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of May 30, 2024 or an event of default, as defined therein. As of December 31, 2023 this loan has been repaid.
On June 12, 2023, Amro Albanna, the Chief Executive Officer of the Company and Shahrokh Shabahang, the Chief Innovation Officer of the Company, loaned $200,000 and $100,000, respectively, to the Company. The loans were evidenced by an unsecured promissory note (the “June Notes”). Pursuant to the terms of the June Notes, each of the June Notes will accrue interest at the Prime rate of eight and one-quarter percent (8.25%) per annum and is due on the earlier of December 12, 2023 or an event of default, as defined therein. As of December 31, 2023 this loan has been repaid.
On April 21, 2023, Amro Albanna, the Chief Executive Officer of the Company, and Shahrokh Shabahang, the Chief Innovation Officer of the Company, loaned $87,523 and $100,000, respectively, to the Company. The loans were each evidenced by an unsecured promissory note (the “April Note”). Pursuant to the terms each April Note, it will accrue interest at the Prime rate of eight percent (8.00%) per annum and is due on the earlier of October 21, 2023, or an event of default, as defined therein. As of September 30, 2023, the note was fully paid off.
On May 25, 2023, Amro Albanna, the Chief Executive Officer of the Company, loaned $200,000 to the Company. The loan was evidenced by an unsecured promissory note (the “May Note”). Pursuant to the terms of the May Note, it will accrue interest at a rate of eight and one-quarter percent (8.25%) per annum, the Prime rate on the date of signing, and is due on the earlier of November 25, 2023 or an event of default, as defined therein. As of September 30, 2023, the note was fully paid off.
On June 12, 2023, Amro Albanna, the Chief Executive Officer of the Company, and Shahrokh Shabahang, the Chief Innovation Officer of the Company, loaned $200,000 and $100,000, respectively, to the Company. The loans were evidenced by an unsecured promissory note (the “June Note”). Pursuant to the terms of the June Note, it will accrue interest at the Prime rate of eight and one-quarter percent (8.25%) per annum and is due on the earlier of December 12, 2023, or an event of default, as defined therein. As of September 30, 2023, the June Note was fully paid off.
On July 11, 2023, we entered into a Subscription and Investment Representation Agreement (the “Subscription Agreement”) with Amro Albanna, its Chief Executive Officer, who is an accredited investor (the “Purchaser”), pursuant to which the Company agreed to issue and sell one (1) share of the Company’s Series C Preferred Stock, par value $0.001 per share (the “Preferred Stock”), to the Purchaser for $1,000 in cash. The sale closed on July 11, 2023.
On July 19, 2022, we entered into a Subscription and Investment Representation Agreement (the “Subscription Agreement”) with Amro Albanna, its Chief Executive Officer, who is an accredited investor (the “Purchaser”), pursuant to which the Company agreed to issue and sell one (1) share of the Company’s Series B Preferred Stock, par value $0.001 per share (the “Preferred Stock”), to the Purchaser for $20,000 in cash. The sale closed on July 19, 2022. The one share of Series B Preferred Stock was redeemed by the Company on Pctober 7, 2022 for $20,000 following the approval of the 2022 reverse stock split.
During the years ended December 31, 2019 and 2018, Rowena Albanna, the wife of Amro Albanna, our Chief Executive Officer, provided the Company with operations consulting services. In July 2020, Ms. Albanna joined the Company as its Chief Operating Officer. As of December 31, 2018, $112,000 was accrued as compensation. An additional $180,000 was expensed as compensation during the year ended December 31, 2019, and $17,000 was paid on the accrued balance. As of December 31, 2019, $275,000 remained accrued and outstanding.
On January 22, 2018, the Company issued an unsecured promissory note to Sekris for $40,000 that accrued interest of 4% annually. The note was due on the earlier of July 22, 2018 or in the event of default, as defined in the Purchase Agreement).
Immune Reprogramming
The discovery of immunosuppressive (anti-rejection and monoclonal) drugs over 40 years ago has made possible life-saving organ transplantation procedures and blocking of unwanted immune responses in autoimmune diseases. However, immune suppression leads to significant undesirable side effects, such as increased susceptibility to life-threatening infections and cancers, because it indiscriminately and broadly suppresses immune function throughout the body. While the use of these drugsagreement. This note has been justifiable because they preventrepaid as of December 31, 2019.
On February 12, 2018, the Company issued an unsecured promissory note to Sekris for $50,000 that accrued interest of 4% annually. The note was due on the earlier of August 12, 2018 or delay organ rejection, their use for treatment of autoimmune diseases and allergies may not be acceptable because of the aforementioned side effects. Furthermore, transplanted organs often ultimately fail despite the use of immune suppression, and about 40% of transplanted organs survive no more than 5 years.
New, focused therapeutic approaches are needed that modulate only the small portion of immune cells that are involved in rejection of the transplanted organ, as this approach can be safer for patients than indiscriminate immune suppression. Such approaches are referred to as immune tolerance, and when therapeutically induced, may be safer for patients and also potentially allow long-term survival of transplanted tissues and organs.
In the late 1990s, academic research on these approaches was conducted at the Transplant Center in Loma Linda University (“LLU”) in connection with a project that secured initial grant funding from the U.S. Department of Defense. The focus of that project was for skin grafting for burn victims. Twenty years of research at LLU and an affiliated incubator led to a series of discoveries that have been translated into a large patent portfolio of therapeutic approaches that may be applied to the modulation of the immune system in order to induce tolerance to self and transplanted organs.
We have an exclusive worldwide license for commercializing this nucleic acid-based technology (which is currently at the pre-clinical stage), named Apoptotic DNA Immunotherapy™ (ADi™) from LLU, which utilizes a novel approach that mimics the way the body naturally induces tolerance to our own tissues (“therapeutically induced immune tolerance”). While immune suppression requires continuous administration to prevent rejection of a transplanted organ, induction of tolerance has the potential to retrain the immune system to accept the organ for longer periods of time. Thus, ADi™ may allow patients to live with transplanted organs with significantly reduced immune suppression. ADi™ is a technology platform which we believe can be engineered to address a wide variety of indications.
We are developing ADi™ products for organ transplantation including skin grafting, autoimmune diseases, and allergies, with the initial focus on skin allografts and psoriasis, as we believe these indications will be most efficient in providing safety and efficacy data in clinical trials. To submit a Biologics License Application (“BLA”) for a biopharmaceutical product, clinical safety and efficacy must be demonstrated in a series of clinical studies conducted with human subjects. For products in our class of drugs, the first-in-human trials will be a combination of Phase I (safety/tolerability) and Phase II (efficacy) in affected subjects. To obtain approval to initiate the Phase I/IIa studies, an Investigational New Drug Application will be submitted to compile non-clinical efficacy data as well as manufacturing and pre-clinical safety/toxicology data. To date, we have conducted non-clinical studies in a stringent model of skin transplantation using genetically mismatched donor and recipient animals demonstrating a 3-fold increase in the survivalevent of default, as defined in the skin graft in animalsagreement. This note has been repaid as of December 31, 2019.
On March 2, 2018, the Company issued an unsecured promissory note to Sekris for $10,000 that were tolerized with ADi™ compared to animals that receive immune suppression alone. Prolongationaccrued interest of graft life4% annually. The note was observed despite discontinuation of immune suppression after the first 5 weeks. Additionally, in an induced non-clinical model for psoriasis, ADi™ treatment resulted in a 69% reduction in skin thickness and a 38% decrease in skin flaking (two clinical parameters for assessment of psoriasis skin lesions). The Phase I/IIa studies in psoriasis will evaluate the safety/tolerability of ADi™ in patients diagnosed with psoriasis. Since the drug will be administered in subjects diagnosed with psoriasis, effectiveness of the drug to improve psoriatic lesions will also be evaluated. In another Phase I/IIa study, patients requiring skin allografts will receive weekly intra-dermal injections of ADi™ in combination with standard immune suppression to assess safety/tolerability and possibility of reducing levels of immunosuppressive drugs as well as prolongation of graft life. Later phase trials are planned after successful completion of these studies in preparation for submission for a BLA to regulatory agencies.
Immune Monitoring
We believe that understanding the status of an individual’s immune system is key to developing and administering immunotherapies such as ADi™. We have secured an exclusive worldwide license for commercializing a technology platform named AditxtScore™, which provides a personalized comprehensive profile of the immune system. It is intended to be informative for individual immune responses to viruses, bacterial antigens, peptides, drugs, bone marrow and solid organ transplants, and cancer. It has broad applicability to many other agents of clinical interest impacting the immune system, including those not yet identified such as future infectious agents.
AditxtScore™ is being designed to allow individuals to understand, manage and monitor their immune profiles in order to be informed about attacks on or by their immune system. We believe AditxtScore™ can also assist the medical community in anticipating possible immune responses and reactions to viruses, bacteria, allergens and transplanted organs. It can be useful in anticipating attacksdue on the body by having the ability to determine its potential response and for developing a plan to deal with an undesirable reaction by the immune system. Its advantages include the ability to provide a simple, rapid, accurate, high throughput, single platform assay that can be multiplexed to determine the immune status with respect to several factors simultaneously, in 3-16 hours, as well as detect antigen and antibody in a single test (i.e. infectious, recovered, immune). In addition, it can determine and differentiate between various typesearlier of cellular and humoral immune responses (T and B cells). It also provides for simultaneous monitoring of cell activation and levels of cytokine release (i.e., cytokine storms).
We plan to utilize AditxtScore™ in our upcoming clinical trials to monitor subjects’ immune response before, during and after ADi™ drug administration. We are also evaluating plans to obtain FDA approval for AditxtScore™’s use as a clinical assay and seeking to secure manufacturing, marketing and distribution partnerships for applicationSeptember 2, 2018 or in the Infectious Diseases market, by endevent of 2020. To obtain FDA approval to use AditxtScore™default, as a clinical assay, we plan to conduct validation studies comparing AditxtScore™ to other immunological tests to demonstrate reproducibility of data and to demonstratedefined in the sensitivity of the assays for use in different indications (e.g., detection of antigens present in infectious agents or antibodies against infectious agents). We believe that these data will show AditxtScore™’s ability to multiplex in two ways using a single assay: (i) evaluating the immune response to multiple antigens (from different infectious agents) and (ii) measuring quantities of multiple cytokines. Furthermore, we believe that the additional validation studies will demonstrate AditxtScore™’s ability to measure the presence of several antibody isotypes against several antigens in a single reaction. Our plan is to submit a 510(K) application to the FDA after successful completion of these studies. We have engaged consultants for our communications and submissions to the FDA. Beyond 2020, we plan to develop AditxtScore™for applications in additional markets such as Organ Rejection, Allergies, Drug/Vaccine Response, and Disease Susceptibility.
The initial application of the platform will be AditxtScore™ for COVID-19 whichagreement. This note has been designed to provide a more complete assessmentrepaid as of an individual’s infection and immunity status with respect to the SARS-CoV-2 virus. Infection status will be determined by evaluating the presence or absence of the virus, and immunity status by measuring levels of antibodies against viral antigens and their ability to neutralize the virus. We will soon be expanding the panel to measure other components of the immune response such as cellular immunity. In early 2021, we established our AditxtScore™ Immune Monitoring Center in Richmond, Virginia (the “Center”). The Center operates as a Clinical Laboratory Improvement Amendments (CLIA) certified facility for the processing of our AditxtScore™ for COVID-19 Lab Developed Test (LDT) for our prospective channel partners, including labs and hospitals.December 31, 2019.
License Agreement with Loma Linda University
On March 8, 2018, we entered into an Assignment Agreement (the “Assignment Agreement”) with Sekris Biomedical, Inc. (“Sekris”). Sekris was a party to aSekris. See “Summary — Overview — License Agreement with Loma Linda University (“LLU”), entered into and made effective on May 25, 2011, and amended on June 24, 2011, July 16, 2012 and December 27, 2012 (the “Original Agreement,University.” and together withDr. Shabahang, our Chief Innovative Officer, was the Assignment Agreement, the “Sekris Agreements”). Pursuant to the Assignment Agreement,Chief Executive Officer of Sekris. Sekris transferred and assigned all of its rights and obligationswas subsequently dissolved in and to the liabilities under the Original Agreement, of whatever kind or nature, to us. In exchange, on2019.
On March 8, 2018, we issued a warrant to Sekris to purchase up to 500,00010,000 shares of our common stock (the “Sekris Warrant”). The warrant was immediately exercisable and has an exercise price of $4.00 per share. The expiration date of the warrant is March 8, 2023.Common Stock to Sekris. On March 15,2, 2018, as amendedwe issued a 4% unsecured promissory note to Sekris in the principal amount of $10,000. Principal and interest was due on September 2, 2018 or immediately upon an event of default. On February 12, 2018, we issued a 4% unsecured promissory note to Sekris in the principal amount of $50,000. Principal and interest was due on August 12, 2018 or immediately upon an event of default. On January 22, 2018, we issued a 4% unsecured promissory note to Sekris in the principal amount of $40,000. Principal and interest was due on July 22, 2018 or immediately upon an event of default.
On June 18, 2018, the Company issued an unsecured promissory note to Sekris for $17,502 that accrued interest of 4% annually. The note was due on the earlier of December 18, 2018 or in the event of default, as defined in the agreement. This note has been repaid as of December 31, 2019.
On January 1, 2020,2019, we entered into a LLU License Agreement directlyconsulting agreement with Loma Linda University, which amends and restatesRowena Albanna, the Sekris Agreements.
Pursuantwife of Amro Albanna, our Chief Executive Officer, to perform operations consulting services. As part of this agreement, we pay Ms. Albanna $15,000 per month for her services. This agreement terminated on June 30, 2020. In July 2020, Ms. Albanna joined the LLU License Agreement, we obtained the exclusive royalty-bearing worldwide license in and to all intellectual property, including patents, technical information, trade secrets, proprietary rights, technology, know-how, data, formulas, drawings, and specifications, owned or controlled by LLU and/or any ofCompany as its affiliates (the “LLU Patent and Technology Rights”) and related to therapy for immune-mediated inflammatory diseases (the ADi™ technology). In consideration for the LLU License Agreement,Chief Operating Officer.
On March 21, 2019, we issued 25,000 shares of common stocka promissory note to LLU.
Pursuant to the LLU License Agreement, we are required to pay an annual license fee to LLU. Also, we paid LLU $455,000 in July 2020 in payment of outstanding milestone payments and license fees. We are also required to pay to LLU milestone payments in connection with certain development milestones. Specifically, we are required to make the following milestone payments: $175,000 on March 31, 2022; $100,000 on March 31, 2024; $500,000 on March 31, 2026; and $500,000 on March 31, 2027. Additionally, as consideration for prior expenses incurred by LLU to prosecute, maintain and defend the LLU Patent and Technology Rights, we were obligated to make the following payments to LLU:, $70,000 was paid at the end of December 2018, andDr. Shabahang, our Chief Innovative Officer. The note has a final payment of $60,000 due at the end of March 2019. We are required to defend the LLU Patent and Technology Rights during the term of the LLU License Agreement. Additionally, we will owe royalty payments of (i) 1.5% of Net Product Sales and Net Service Sales on any Licensed Products (defined as any finished pharmaceutical products which utilizes the LLU Patent and Technology Rights in its development, manufacture or supply), and (ii) 0.75% of Net Product Sales and Net Service Sales for Licensed Products and Licensed Services not covered by a valid patent claim for technology rights and know-how for a three (3) year period beyond the expiration of all valid patent claims. We also are required to produce a written progress report to LLU, discussing our development and commercialization efforts, within 45 days following the end of each year. All intellectual property rights in and to LLU Patent and Technology Rights shall remain with LLU (other than improvements developed by or on our behalf).
The LLU License Agreement shall terminate on the last day that a patent granted to us by LLU is valid and enforceable or the day that the last patent application licensed to us is abandoned. The LLU License Agreement may be terminated by mutual agreement or by us upon 90 days written notice to LLU. LLU may terminate the LLU License Agreement in the event of (i) non-payments or late payments of royalty, milestone and license maintenance fees not cured within 90 days after delivery of written notice by LLU, (ii) a breach of any non-payment provision (including the provision that requires us to meet certain deadlines for milestone events (each, a “Milestone Deadline”)) not cured within 90 days after delivery of written notice by LLU and (iii) LLU delivers notice to us of three or more actual breaches of the LLU License Agreement by us in any 12-month period. Additional Milestone Deadlines include: (i) the requirement to have regulatory approval of an IND application to initiate a first-in-human clinical trials on or before March 31, 2022, (ii) the completion of first-in-human (phase I/II) clinical trials by March 31, 2024, (iii) the completion of Phase III clinical trials by March 31, 2026 and (iv) biologic licensing approval by the FDA by March 31, 2027.
License Agreement with Leland Stanford Junior University (“Stanford”)
On February 3, 2020, we entered into an exclusive license agreement (the “February 2020 License Agreement”) with Stanford with regard to a patent concerning a method for detection and measurement of specific cellular responses. Pursuant to the February 2020 License Agreement, we received an exclusive worldwide license to Stanford’s patent with regard to use, import, offer, and sale of Licensed Products (as defined in the agreement). The license to the patented technology is exclusive, including the right to sublicense, beginning on the effective date of the agreement and ending when the patent expires. Under the exclusivity agreement, we acknowledged that Stanford had already granted a non-exclusive license in the Nonexclusive Field of Use, under the Licensed Patents in the Licensed Field of Use in the Licensed Territory (as those terms are defined in the February 2020 License Agreement”). However, Stanford agreed to not grant further licenses under the Licensed Patents in the Licensed Field of Use in the Licensed Territory.
We were obligated to pay and paid a fee of $25,000 to Stanford within 60 days of February 3, 2020. We also issued 18,750 shares of the Company’s common stock to Stanford. An annual licensing maintenance fee is payable by us on the first anniversary of the February 2020 License Agreement in theprincipal amount of $40,000 for 2021 through 2024 and $60,000 starting in 2025 until the license expires upon the expiration of the patent. The Company is required to pay and has paid $25,000 for the issuances of certain patents. The Company will pay milestone fees of $50,000 on the first commercial sales of a licensed product and $25,000 at the beginning of any clinical study for regulatory clearance of an in vitro diagnostic product developed and a potential licensed product. We are also required to: (i) provide a listing of the management team or a schedule for the recruitment of key management positions by March 31, 2020 (which has been completed), (ii) provide a business plan covering projected product development, markets and sales forecasts, manufacturing and operations, and financial forecasts until at least $10,000,000 in revenue by June 30, 2020 (which has been completed), (iii) conduct validation studies by September 30, 2020 (which has been completed), (iv) hold a pre-submission meeting with the FDA by September 30, 2020 (which has been completed), (v) submit a 510(k) application to the FDA, Emergency Use Authorization (“EUA”), or a Laboratory Developed Test (“LDT”) by March 31, 2021, (vi) obtain FDA approval by December 31, 2021, (vii) complete a prototype assay kit by December 31, 2021 and (viii) have a written agreement with Stanford on further development and commercialization milestones for specific fields of use by December 31, 2021.
In addition to the annual license maintenance fees outlined above, we will pay Stanford royalties on Net Sales (as such term is defined in the February 2020 License Agreement) during the of the term of the agreement as follows: 4% when Net Sales are below or equal to $5 million annually or 6% when Net Sales are above $5 million annually. The February 2020 License Agreement may be terminated upon our election on at least 30 days advance notice to Stanford, or by Stanford if we: (i) are delinquent on any report or payment; (ii) are not diligently developing and commercializing Licensed Product; (iii) miss certain performance milestones; (iv) are in breach of any provision of the February 2020 License Agreement; or (v) provide any false report to Stanford. Should any events in the preceding sentence occur, we have a thirty (30) day cure period to remedy such violation.
Our Team
We have assembled a team of experts from a variety of scientific fields and commercial backgrounds, with many years of collective experience that ranges from founding startup biotech companies, to developing and marketing biopharmaceutical products, to designing clinical trials, and to management of private and public companies.
Going Concern
We were incorporated$10,000, was due on September 28, 201721, 2019, and has not generated revenues to date. During the year ended December 31, 2020 we had a net lossbears an interest rate of $9,149,227 and will require significant additional capital in order to operate in the normal course of business and fund clinical studies in the long-term. As a result of the IPO and the September 2020 Offering, we received net proceeds of approximately $18 million during the period. We believe that the funds raised by the IPO and the September 2020 Offering will be sufficient to fund our operations for at least the next 12 months. As a result, these conditions have alleviated the doubt regarding our ability to continue as a going concern beyond one4% per year.
Financial Results
We have a limited operating history. Therefore, there is limited historical financial information upon which to base an evaluation of our performance. Our prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies in their early stages of operations. Our financial statements as of December 31, 2020, show a net loss of $9,149,227. We expect to incur additional net expenses over the next several years as we continue to maintain and expand our existing operations. The amount of future losses and when, if ever, we will achieve profitability are uncertain.
On July 2, 2020, we completed an IPO. In connection therewith, we issued 1,226,668 Units, excluding the underwriters’ overallotment, at an offering price of $9.00 per Unit, resulting in gross proceeds of approximately $11.0 million. The Units issued in the IPO consisted of one share of common stock, one Series A warrant, and one Series B warrant. The Series A warrants originally had an exercise price of $9.00 and a term of 5 years. In addition, the Company issued a Unit Purchase Option at an exercise price of $11.25 per unit to the underwriters to purchase up to 67,466 units, with each unit consisting of (i) one share of common stock and (ii) one Series A Warrant. On August 19, 2020 the Company modified the exercise price of the Series A Warrants from $9.00 per share to $4.50 per share. The term of the Series A Warrants was not modified. The Series B warrants have an exercise price of $11.25 per share and a term of 5 years. Substantially all of the Series B warrants issued in the IPO as part of the Units have been exercised pursuant to a cashless provision therein.
On September 10, 2020, we completed a follow-on public offering (“September 2020 Offering”). In connection therewith, we issued 2,400,000 units, or Follow-On Units, excluding the underwriters’ option to cover overallotments, at an offering price of $4.00 per Follow-On Unit, resulting in gross proceeds to the Company of approximately $9.6 million. Each of the Follow-On Units issued in the September 2020 Offering consisted of one share of common stock or Series A Preferred Stock for investors who would own more than 4.99% of the Company if they invested in common stock, one Series A-1 warrant, and one Series B-1 warrant. The Series A-1 warrants have an exercise price of $3.19 per share and a term of 5 years. The Series B-1 warrants have exercise price of $5.00 per share, a term of 5 years and contain a cashless exercise option upon certain criteria being met. In addition, the Company issued a warrant to the underwriters to purchase up to 60,000 shares of common stock at an exercise price of $5.00 per share. Subsequent to quarter end, substantially all of the Series B-1 warrants issued in the September 2020 Offering have been exercised pursuant to a cashless provision therein.
Results of Operations
Results of operations for the year ended December 31, 2020
During the year ended December 31, 2020, we incurred a loss from operations of $8,872,209. This is due to general and administrative expenses of $7,852,256, which includes $3,188,840 in stock-based compensation, research and development of $937,966, and sales and marketing expenses of $81,987. The $937,966 in research and development is comprised of $258,635 in licensing fees, $519,171 in product development, and $160,160 in other research and development expense.note remains outstanding.
During the year ended December 31, 2019, we incurredassumed an aggregate of $189,625 of liabilities from Sekris in exchange for the return of 94,813 shares of our Common Stock.
On January 20, 2020, we issued a loss from operationspromissory note to Brian Brady, a member of $5,870,798.our board of directors. The note has a principal amount of $50,000, was due on the earlier of April 19, 2020 or within 10 days of the closing of our initial public offering. This is duenote carried an original issue discount of $25,000. The note was amended on April 23, 2020 to general and administrative expenses of $5,694,806, which includes $4,221,733 in stock-based compensation, research and development of $175,441, which includes $10,000 in stock-based compensation, and sales and marketing expenses of $551. The $175,441 in research and development is comprised of $18,396 in licensing fees, $54,000 in product development and $103,045 in other research and development expense.
The increase in expenses duringextend the year ended December 31, 2020 comparedmaturity date to the year ended December 31, 2019earlier of June 30, 2020 or within 10 days of the closing of our initial public offering. This note was due to the Company beginning to execute its business planrepaid in July 2020.
Review, Approval and incur costsRatification of being a public company.
Related Party Transactions
Liquidity
Given our small size and Capital Resources
Welimited financial resources, we have incurred substantial operating losses since inceptionnot adopted formal policies and expect to continue to incur significant operating lossesprocedures for the foreseeable futurereview, approval or ratification of transactions, such as those described above, with our executive officer(s), Director(s) and may never become profitable. As of December 31, 2020, we had an accumulated deficit of $20,879,178.significant stockholders. We had working capital of $9,806,195 as of December 31, 2020. During the year ended December 31, 2020, we paid off outstanding notes payable with a principal totaling $715,600intend to establish formal policies and accrued interest totaling $5,842. During the year ended December 31, 2020, we paid $170,629 for the purchase of fixed assets and $58,475 on payments for financed assets. These fixed assets were purchased to furnish our new office and laboratory. Approximately $105,000 of these purchased fixed assets were lab equipment, approximately $55,000 was for office furniture and equipment, approximately $10,000 was for computers, and approximately $1,000 was for other fixed assets.
Our financial statements have been prepared assuming that we will continue as a going concern.
We have funded our operations from proceeds from the sale of equity and debt securities. On July 2, 2020, we completed our IPO and raised approximately $9.5 million in net proceeds. At the time of the IPO, we believed that these funds would be sufficient to fund our operations for the foreseeable future.
On September 10, 2020, we completed a follow-on public offering. In connection therewith, we issued 2,400,000 units, or Follow-On Units, excluding the underwriters’ option to cover overallotments, at an offering price of $4.00 per Follow-On Unit, resulting in gross proceeds of approximately $9.6 million.
We may need to raise significant additional capital to continue to fund our operations and the clinical trials for our product candidates. We may seek to sell common stock, preferred stock or convertible debt securities, enter into a credit facility or another form of third-party funding or seek other debt financing. In addition, we may seek to raise cash through collaborative agreements or from government grants. The sale of equity and convertible debt securities may result in dilution to our stockholders and certain of those securities may have rights senior to those of our common shares. If we raise additional funds through the issuance of preferred stock, convertible debt securities or other debt financing, these securities or other debt could contain covenants that would restrict our operations. Any other third-party funding arrangement could require us to relinquish valuable rights.
The source, timing and availability of any future financing will depend principally upon market conditions, and, more specifically, on the progress of our clinical development program. Funding may not be available when needed, at all, or on terms acceptable to us. Lack of necessary funds may require us to, among other things, delay, scale back or eliminate expenses including some or all of our planned development, including our clinical trials. While we may need to raise fundsprocedures in the future, once we believehave sufficient resources and have appointed additional Directors, so that such transactions will be subject to the current cash reserves should be sufficient to fund our operation for the foreseeable future. Because of these factors, we believe that this alleviates the issues about our ability to continue as a going concern.
Contractual Obligations
The following table shows our contractual obligations as of December 31, 2020:
| Payment Due by Year | ||||||||||||||||||||
| Total | 2021 | 2022 | 2023 | 2024 | ||||||||||||||||
| Lease | $ | 1,397,360 | $ | 406,640 | $ | 411,753 | $ | 363,416 | $ | 215,551 | ||||||||||
| Financed asset | 633,078 | 350,636 | 282,442 | - | - | |||||||||||||||
| Total contractual obligations | $ | 2,030,438 | $ | 757,276 | $ | 694,195 | $ | 363,416 | $ | 215,551 | ||||||||||
Critical Accounting Polices and Estimates
Our financial statements are prepared in accordance with generally accepted accounting principles in the United States. The preparationreview, approval or ratification of our financial statements andBoard of Directors, or an appropriate committee thereof. On a moving forward basis, our Directors will continue to approve any related disclosures requires us to make estimates, assumptions and judgments that affect the reported amount of assets, liabilities, revenue, costs and expenses, and related disclosures. We believe that of our critical accounting policies described under the heading “Management’s Discussion and Analysis of Financial Condition and Plan of Operations—Critical Accounting Policies” in our Prospectus, dated September 1, 2020, filed with the SEC pursuant to Rule 424(b), are critical to fully understanding and evaluating our financial condition and results of operations. The following involve the most judgment and complexity:party transaction.
Accordingly, we believe the policies set forth above are critical to fully understanding and evaluating our financial condition and results of operations. If actual results or events differ materially from the estimates, judgments and assumptions used by us in applying these policies, our reported financial condition and results of operations could be materially affected.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
JOBS Act
On April 5, 2012, the JOBS Act was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act, for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies.
We have chosen to take advantage of the extended transition periods available to emerging growth companies under the JOBS Act for complying with new or revised accounting standards until those standards would otherwise apply to private companies provided under the JOBS Act.
We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we intend to rely on certain of these exemptions, including without limitation, (i) providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the Public Company Accounting Oversight Board (“PCAOB”) regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of this offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission.
Recently Issued and Adopted Accounting Pronouncements
See Note 3 - Summary of Significant Accounting Policies to the accompanying financial statements for a description of other accounting policies and recently issued accounting pronouncements.
Item 14. Principal Accounting Fees and Services dbbmckennon acted as the Company’s independent registered public accounting firm for the years ended December 31, 2023 and 2022 and for the interim periods in such fiscal years. The following table shows the fees that were incurred by the Company for audit and other services provided by dbbmckennon for the years ended December 31, 2023 and 2022. PART IV Item 15. Exhibits, Financial Statement Schedules. All financial statement schedules have been omitted because they are not applicable, not required or the information required is shown in the financial statements or the notes thereto. EXHIBIT INDEX SIGNATURES Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized on this POWER OF ATTORNEY KNOW ALL BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Amro Albanna and Thomas J. Farley, and each of them, as his or her true and lawful attorneys-in-fact and agents, each with the full power of substitution, for him or her and in his or her name, place, or stead, in any and all capacities, to sign any and all amendments to this Report, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof. Pursuant to the requirements of the Securities Act of 1934, this annual report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated. ADITXT, INC. CONSOLIDATED FINANCIAL STATEMENTS FOR THE YEARS ENDED DECEMBER 31, REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM To the Board of Directors and Stockholders of Opinion on the Financial Statements We have audited the accompanying consolidated balance sheets of Going Concern The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company’s net losses and negative cash flow from operations, raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. Basis for Opinion These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB. We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion. /s/ We have served as the Company’s auditor since 2018. San Diego, California April 16, 2024 PART I - FINANCIAL INFORMATION Item 1. Financial Statements ADITXT, INC. CONSOLIDATED BALANCE SHEETS 22,277,211 11,173,772 (127,741,072 16,056,377 (9,608 See accompanying notes to the consolidated financial statements. ADITXT, INC. CONSOLIDATED STATEMENTS OF OPERATIONS (9,608 (32,380,839 (32,700,710 (27,687,553 (108.15 (597.12 See accompanying notes to the consolidated financial statements. ADITXT, INC. CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY Non- (32,380,839 (9,608 (127,741,072 (9,608 See accompanying notes to the consolidated financial statements. ADITXT, INC. CONSOLIDATED STATEMENTS OF YEARS ENDED DECEMBER 31, 2023 AND 2022 Non-Controlling Interest See accompanying notes to the consolidated financial statements. ADITXT, INC. CONSOLIDATED STATEMENTS OF CASH FLOWS 2,686,306 See accompanying notes to the consolidated financial statements. ADITXT, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS NOTE 1 – ORGANIZATION AND NATURE OF BUSINESS Company Background Overview We are an innovation company developing On On April 13, 2023, the Company formed Adivir, Inc., a Delaware wholly owned subsidiary. On August 24, 2023, the Company formed Adivue, Inc., a Delaware wholly owned subsidiary. On October 16, 2023, the Company formed Adicure, Inc., a Delaware wholly owned subsidiary. Reverse Stock Split On September 13, 2022, the Company effectuated a 1 for 50 reverse stock split (the “2022 Reverse Split”). The Company’s stock began trading on a split-adjusted basis effective on the Nasdaq Stock Market on September 14, 2022. There was no change to the number of authorized shares of the Company’s common stock. On August 17, 2023, the Company effectuated a 1 for 40 reverse stock split (the “2023 Reverse Split”). The Company’s stock began trading on a split-adjusted basis effective on the Nasdaq Stock Market on August 18, 2023. There was no change to the number of authorized shares of the Company’s common stock. All share amounts referenced in this report are adjusted to reflect the 2023 Reverse Split. Offerings On August 31, 2021, the Company completed On October 18, 2021, the Company entered into an underwriting agreement with Revere Securities LLC, relating to On December 6, 2021, the Company completed a public offering for net proceeds of $16.0 million (the “December 2021 Offering”). As part of the December 2021 Offering, we issued 4,123 units consisting of shares of the Company’s common stock and warrant to purchase shares of the Company’s common stock and 4,164 prefunded warrants. The warrant issued as part of the units had an exercise price of $2,300.00 and the prefunded warrants On September On April 20, 2023, the Company entered into a securities purchase agreement (the “April Purchase Agreement”) with an institutional investor, pursuant to which the Company agreed to sell to such investor pre-funded warrants (the “April Pre-Funded Warrants”) to purchase up to 39,634 shares of common stock of the Company (the “Common Stock”) at a On August 31, 2023, the “Company entered into a securities purchase agreement (the “August Purchase Agreement”) with an institutional investor for the issuance and sale in a private placement (the “Private Placement”) of (i) pre-funded warrants (the “August Pre-Funded Warrants”) to purchase up to 1,000,000 shares of the Company’s common stock at an exercise price of $0.001 per share, and (ii) warrants (the “Common Warrants”) to purchase up to 1,000,000 shares of the Company’s Common Stock at an exercise price of $10.00 per share. The Private Placement closed on September 6, 2023. The net proceeds to the Company from the Private Placement were approximately $9 million, after deducting placement agent fees and expenses and estimated offering expenses payable by the Company. The Company used the net proceeds received from the Private Placement for (i) the payment of approximately $3.1 million in outstanding obligations, (ii) the repayment of approximately $0.4 million of outstanding debt, and (iii) the balance for continuing operating expenses and working capital. On December 29, 2023, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with an institutional investor (“the “Purchaser”) for the issuance and sale in a private placement (the “Private Placement”) of (i) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 1,237,114 shares of the Company’s common stock, par value $0.001 (the “Common Stock”) at an exercise price of $0.001 per share, and (ii) warrants (the “Common Warrants”) to purchase up to 2,474,228 shares of the Company’s Common Stock, at a purchase price of $4.85 per share. The Private Placement closed and the funds were received on January 4, 2024. The net proceeds to the Company from the Private Placement were approximately $5.4 million, after deducting placement agent fees and expenses and estimated offering expenses payable by the Company. The Company intends to use the net proceeds received from the Private Placement for continuing operating expenses and working capital. Risks and Uncertainties The Company has a limited operating history and NOTE 2 – GOING CONCERN ANALYSIS Management Plans The Company was incorporated on September 28, 2017 and has not generated significant revenues to date. During the year ended December 31, As of December 31, 2023, the Company had approximately $1.8 million of availability to sell under its shelf registration statement on Form S-3. Upon the filing of the Company’s annual report on Form 10-K on April 17, 2023, the Company’s aggregate market value of the voting and non-voting equity held by non-affiliates was below $75.0 million. As a result, the maximum amount that the Company can sell under its shelf registration statement on Form S-3 during any 12 month period is equal to one-third of the aggregate market value of the voting and non-voting equity held by non-affiliates of the Company. On November 21, 2023, the Company received written notice from Nasdaq that we had regained compliance with the Public Float Rule. On December 29, 2023, the Company received written notice from Nasdaq that we had regained compliance with the Stockholders’ Equity Rule but will be subject to a Mandatory Panel Monitor for a period of one year. If we are delisted from Nasdaq, but obtain a substitute listing for our common stock, it will likely be on a market with less liquidity, and therefore experience potentially more price volatility than experienced on Nasdaq. Stockholders may not be able to sell their shares of common stock on any such substitute market in the quantities, at the times, or at the prices that could potentially be available on a more liquid trading market. As a result of these factors, if our common stock is delisted from Nasdaq, the value and liquidity of our common stock, warrants and pre-funded warrants would likely be significantly adversely affected. A delisting of our common stock from Nasdaq could also adversely affect our ability to obtain financing for our operations and/or result in a loss of confidence by investors, employees and/or business partners. The Company In addition, factors such as stock price, volatility, trading volume, market conditions, demand and regulatory requirements may adversely affect the Company’s ability to raise capital in an efficient manner. Because of these factors, the Company believes that this In addition to the shelf registration, the Company The financial statements included in this report do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the matters discussed herein. NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES Basis of Presentation The Company’s financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and the rules and regulations of the Securities and Exchange Commission (“SEC”). Principles of Consolidation The consolidated financial statements include the accounts of Aditxt, Inc., its wholly owned subsidiaries and, one majority owned subsidiary. All significant intercompany balances and transactions have been eliminated in the consolidated financial statements. Use of Estimates The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expense during the reporting period. Actual results could differ from those estimates. Significant estimates underlying the financial statements include the collectability of notes receivable, the reserve on insurance billing, value of preferred shares issued, our investments in preferred shares, estimation of discounts on non-interest bearing borrowing, and the fair value of stock options and warrants. Fair Value Measurements and Fair Value of Financial Instruments The Company adopted Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 820, Fair Value Measurements. ASC Topic 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows: The Company did not identify any assets or liabilities that are required to be presented on the balance sheets at fair value in accordance with ASC Topic 820. Due to the short-term nature of all financial assets and liabilities, their carrying value approximates their fair value as of the balance sheet dates. (See Note 9) Concentrations of Credit Risk Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable. The Company maintains its cash accounts at financial institutions which are insured by the Federal Deposit Insurance Corporation. At times, the Company may have deposits in excess of federally insured limits. Substantially all the Company’s accounts receivable are with companies in the healthcare industry, individuals, and the U.S. government. However, concentration of credit risk is mitigated due to the Company’s number of customers. In addition, for receivables due from U.S. government agencies, the Company does not believe the receivables represent a credit risk as these are related to healthcare programs funded by the U.S. government and payment is primarily dependent upon submitting the appropriate documentation. Cash and Cash Equivalents Cash and cash equivalents include short-term, liquid investments. Inventory Inventory consists of laboratory materials and supplies used in laboratory analysis. We capitalize inventory when purchased. Inventory is valued at the lower of cost or net realizable value on a first-in, first-out basis. We periodically perform obsolescence assessments and write off any inventory that is no longer usable. Fixed Assets Fixed assets are stated at cost less accumulated depreciation. Cost includes expenditures for furniture, office equipment, laboratory equipment, and other assets. Maintenance and repairs are charged to expense as incurred. When assets are sold, retired, or otherwise disposed of, the cost and accumulated depreciation are removed from the accounts and any resulting gain or loss is reflected in operations. The costs of fixed assets are depreciated using the straight-line method over the estimated useful lives or lease life of the related assets. Useful lives assigned to fixed assets Intangible Assets Intangible assets are stated at cost less accumulated amortization. For intangible assets that have The This investment is included in its own line item on the Company’s consolidated balance sheet. Non-marketable equity investments (for which we do not have significant influence or control) are investments without readily determinable fair values that are recorded based on initial cost minus impairment, if any, plus or minus adjustments resulting from observable price changes in orderly transactions for identical or similar securities, if any. All gains and losses on investments in non-marketable equity securities, realized and unrealized, are recognized in investment and other income (expense), net. We monitor equity method and non-marketable equity investments for events or circumstances that could indicate the investments are impaired, such as a deterioration in the investee’s financial condition and business forecasts and lower valuations in recently completed or anticipated financings, and recognize a charge to investment and other income (expense), net for the difference between the estimated fair value and the carrying value. For equity method investments, we record impairment losses in earnings only when impairments are considered other-than-temporary. Accounts Receivable and Allowance for Doubtful Accounts Accounts receivable are stated at the amount management expects to collect from outstanding balances. The Company generally does not require collateral to support customer receivables. The Company determines if receivables are past due based on days outstanding, and amounts are written off when determined to be uncollectible by management. As of December 31, 2023 and 2022, there was an allowance for doubtful accounts of zero and $18,634, respectively. Accounts receivable is made up on billed and unbilled of $236,605 and $171,721 as of December 31, 2023 and $527,961 and zero as of December 31, 2022, respectively. Income Taxes Deferred Offering Costs Offering costs incurred in connection with equity are recorded as a reduction of equity and offering costs incurred in connection with debt are recorded as a reduction of debt as a debt discount. Equity instruments issued as offering costs have zero net effect on the Company’s equity. Revenue Recognition In accordance with ASC 606 (Revenue From Contracts with Customers), revenue is recognized when a customer obtains control of promised services. The amount of revenue recognized reflects the consideration to which the Company expects to be entitled to receive in exchange for these services. To achieve this core principle, the Company applies the following five steps: Revenues reported from services relating to the The Company recognizes revenue in the following manner for the following types of customers: Client Payers: Client payers include physicians or other entities for which services are billed based on negotiated fee schedules. The Company principally estimates the Cash Pay: Customers are Insurance: Reimbursements from healthcare insurers are based on fee for service schedules. Net revenues recognized consist of amounts billed net of contractual allowances for differences between amounts billed and the Leases Under Topic 842 Leases with an initial term of twelve months or less are not recorded on the balance sheet. Stock-Based Compensation The Company accounts for stock-based compensation costs under the provisions of ASC 718, Compensation—Stock Compensation, which requires the measurement and recognition of compensation expense related to the fair value of stock-based compensation awards that are ultimately expected to vest. Patents The Company incurs fees from patent licenses, which are reflected in research and development expenses, and are expensed as incurred. During the Research and Development We incur research and development costs during the process of researching and developing our technologies and future offerings. Non-controlling Interest in Subsidiary Non-controlling interests represent the Company’s subsidiary’s cumulative results of operations and changes in deficit attributable to non-controlling shareholders. During the years ended December 31, 2023 and 2022, the Company recognized $9,608 and $0 in net loss attributable to non-controlling interest in Pearsanta. The Company owns approximately 97.5% of Pearsanta, Inc., as of December 31, 2023. Basic and Diluted Net Loss per Common Share Basic loss per common share is computed by dividing the net loss by the weighted average number of shares of common stock outstanding for each period. Diluted loss per share is computed by dividing the net loss attributable of common stockholders by the weighted average number of shares of common stock outstanding plus the dilutive effect of shares issuable through the common stock equivalents. The weighted-average number of common shares outstanding excludes common stock equivalents because their inclusion would be anti-dilutive. As of December 31, Recent Accounting Pronouncements The FASB issues ASUs to amend the authoritative literature in ASC. There have been several ASUs to date, including those above, that amend the original text of ASC. Management believes that those issued to date either (i) provide supplemental guidance, (ii) are technical corrections, (iii) are not applicable to us or (iv) are not expected to have a significant impact on our financial statements. NOTE 4 – FIXED ASSETS The Company’s fixed assets include the following on December 31, 2023: The Company’s fixed assets include the following on December 31, 2022: Depreciation expense was $435,027 and $428,977 for the years ended December 31, 2023 and 2022, respectively. As of December 31, 2023 and 2022, the fixed assets that serve as collateral subject to the financed asset liability have a carrying value of $1,316,830 and $1,359,091, respectively. Financed Assets: In October 2020, the Company purchased two pieces of lab equipment and financed them for a period of twenty-four months with a monthly payment of $19,487, with an interest rate of 8%. As of December 31, 2023, the Company has one payment in arrears. In January of 2021, the Company purchased one piece of lab equipment and financed it for a period of twenty-four months with a monthly payment of $9,733, with an interest rate of 8%. As of December 31, 2023, the Company has one payment in arrears. In March of 2021, the Company purchased five pieces of lab equipment and financed them for a period of twenty-four months with a monthly payment of $37,171, with an interest rate of 8%. As of December 31, 2023, the Company has four payments in arrears. As of December 31, 2023, all lab equipment financing agreements have matured and are in default status. NOTE The Company’s intangible assets include the following on December 31, 2023: The Company’s intangible assets include the following on December 31, 2022: Amortization expense was $107,556 and $107,000 for the years ended December 31, 2023 and 2022, respectively. The Company’s proprietary technology is being amortized over its estimated useful life of three years. NOTE 6 – RELATED PARTY TRANSACTIONS On January 28, 2022, the Company granted 9,600 restricted stock units to an officer of the Company pursuant to the Company’s 2021 Equity Incentive Plan. The On July 19, 2022, the Company On July 19, 2022, the Company filed a certificate of designation (the “Certificate of Designation”) with the Secretary of State of Delaware, effective as of the time of filing, designating the rights, preferences, privileges and restrictions of the share of Series B Preferred Stock. The Certificate of Designation provides that the share of Series B Preferred Stock will have 250,000,000 votes and will vote together with the outstanding shares of the Company’s common stock as a single class exclusively with respect to any proposal to amend the Company’s Restated Certificate of Incorporation to effect a reverse stock split of the Company’s common stock. The Series B Preferred Stock will be voted, without action by the holder, on any such proposal in the same proportion as shares of common stock The Series B Preferred Stock is not convertible into, or exchangeable for, shares of any other class or series of stock or other securities of the Company. The Series B Preferred Stock has no rights with respect to The outstanding share of Series B Preferred Stock shall be redeemed in whole, but not in part, at any time (i) if such redemption is ordered by the Board of Directors in its sole discretion or (ii) automatically upon the effectiveness of the amendment to the Certificate of Incorporation implementing a reverse stock split. Upon such redemption, the holder of the Series B Preferred Stock will receive consideration of $20,000 in cash. On September 13, 2022, the share was redeemed. On July 19, 2022, the Company filed a certificate of designation (the “Certificate of Designation”) with the Secretary of State of Delaware, effective as of the time of filing, designating the rights, preferences, privileges and restrictions of the share of Series B Preferred Stock. The Certificate of Designation provides that the share of Preferred Stock will have 250,000,000 votes and will vote together with the outstanding shares of the Company’s common stock as a single class exclusively with respect to any proposal to amend the Company’s Restated Certificate of Incorporation to effect a reverse stock split of the Company’s common stock. The Series B Preferred Stock will be voted, without action by the holder, on any such proposal in the same proportion as shares of common stock are voted. The Series B Preferred Stock otherwise has no voting rights except as otherwise required by the General Corporation Law of the State of Delaware. On July 21, 2022, the Chief Executive Officer loaned $80,000 to the Company. The loan was evidenced by an unsecured promissory note (the “July 2022 Promissory Note”). Pursuant to the terms of the July 2022 Promissory Note, it will accrue interest at a rate of four and three-quarters percent (4.75%) per annum, the Prime rate on the date of signing, and is due on the earlier of January 22, 2023, or an event of default. On October 7, 2022, the Company fully repaid the $80,000 July 2022 Promissory Note and $812 of accrued On May 25, 2023, Amro Albanna, the Chief Executive Officer of the Company, loaned $200,000 to the Company. The loan was evidenced by an unsecured promissory note (the “May Note”). Pursuant to the terms of the May Note, it will accrue interest at a rate of eight and On June 12, 2023, Amro Albanna, the Chief Executive Officer of the Company, On July 11, 2023, the Company entered into a Subscription and Investment Representation Agreement with the Purchaser, pursuant to which the Company agreed to issue and sell one (1) share of the Company’s Series C Preferred Stock (the “Series C Preferred Stock”), par value $0.001 per share, to the Purchaser for $1,000 in cash. On July 11, 2023, the Company filed a certificate of designation (the “Certificate of Designation”) with the Secretary of State of Delaware, effective as of the time of filing, designating the rights, preferences, privileges and restrictions of the share of Series C Preferred Stock. The Certificate of Designation provides that the share of Series C Preferred Stock will have 250,000,000 votes and will vote together with the outstanding shares of the Company’s common stock as a single class exclusively with respect to any proposal to amend the Company’s Restated Certificate of Incorporation to effect a reverse stock split of the Company’s common stock. The Series C Preferred Stock will be voted, without action by the holder, on any such proposal in the same proportion as shares of common stock The Series The outstanding share of Series C Preferred Stock shall be redeemed in On November 30, 2023, Amro Albanna, the Chief Executive Officer of the Company, loaned $10,000 to the Company. The loan was evidenced by an On December 6, 2023, Amro Albanna, the On December 20, 2023, Amro Albanna, the Chief Executive Officer of the Company, loaned $165,000 to the Company. The loan was evidenced by an unsecured promissory note (the “Second December Note”). Pursuant to the terms of the Second December Note, it will accrue interest at a rate of eight and a half percent (8.50%) per annum, the Prime rate on the date of signing, and is due on the earlier of June 20, 2024 or an event of default, as defined therein. As of December 31, 2023, there was a remaining principal balance of $165,000 on the Second December Loan and accrued interest of $423. See Note 12 for additional loans incurred or paid subsequent to December 31, 2023. NOTE 7 – NOTES PAYABLE On February 21, 2023, the Company entered into an agreement for the purchase and sale of future receipts (the “Future Receipts Agreement”) with a commercial funding source pursuant to which the Company agreed to sell to the funder certain future trade receipts in the aggregate amount of $2,160,000 (the “Future Receipts Purchased Amount” for gross proceeds to the Company of $1,500,000, less origination fees of $75,000. Pursuant to the Future Receipts Agreement, the Company granted the funder a security interest in all of the Company’s present and future accounts receivable in an amount not to exceed the Future Receipts Purchased Amount. The Future Receipts Purchased Amount shall be repaid by the Company in 28 weekly installments of approximately $77,000 with the final payment due on September 5, 2023. On May 30, 2023, the Company entered into the May Loan (as defined below) for gross proceeds to the Company of $2,000,000, less origination fees of $100,000 and less the full outstanding balance under the Future Receipts Agreement of $1,157,143, resulting in net proceeds to the Company of $742,857. On April 4, 2023, the Company entered into a Business Loan and Security Agreement (the “April Loan Agreement”) with a commercial funding source (the “April Lender”), pursuant to which the Company obtained a loan from the April Lender in the principal amount of $1,060,000, which includes origination fees of $60,000 (the “April Loan”). Pursuant to the April Loan Agreement, the Company granted the April Lender a continuing secondary security interest in; (i) any and all amounts owed to the Company now or in the future from any merchant processor processing charges made by customers of the Company via credit card or debit card transactions, and (ii) all other tangible and intangible property. The total amount of interest and fees payable by the Company to the April Lender under the April Loan (the “April Repayment Amount”) will be (i) $1,000,000 if paid prior to April 6, 2023, (ii) $1,219,000 if paid prior to April 10, 2023, or (iii) $1,590,000 if paid after April 10, 2023, and will be repaid in 20 weekly installments of $79,500 commencing on April 10, 2023 and ending on August 21, 2023. On April 24, 2023, the Company entered into the Loan Agreement (as defined below) for gross proceeds of $1,000,000, less the full outstanding balance under the April Loan Agreement of $139,500, resulting net proceeds to the Company of $860,500. On April 24, 2023, the Company entered into a Business Loan and Security Agreement (the “Loan Agreement”) with a commercial funding source (the “Lender”), pursuant to which the Company obtained a loan from the Lender in the principal amount of $1,060,000, which includes origination fees of $60,000 (the “Loan”). Pursuant to the Loan Agreement, the Company granted the Lender a continuing secondary security interest in; (i) any and all amounts owed to the Company now or in the future from any merchant processor processing charges made by customers of the Company via credit card or debit card transactions, and (ii) all other tangible and intangible property. The total amount of interest and fees payable by the Company to the Lender under the Loan (the “April Repayment Amount”) will be $1,590,000 and will be repaid in 20 weekly installments of $79,500. On August 23, 2023, the April Repayment Amount was restructured in connection with the August Loan Agreement, as defined below. On May 30, 2023, the Company entered into a Business Loan and Security Agreement (the “May Loan Agreement”) with a commercial funding source (the “May Lender”), pursuant to which the Company obtained a loan from the Lender in the principal amount of $2,000,000, which includes origination fees of $100,000 (the “May Loan”). Pursuant to the May Loan Agreement, the Company granted the May Lender a continuing secondary security interest in; (i) any and all amounts owed to the Company now or in the future from any merchant processor processing charges made by customers of the Company via credit card or debit card transactions, and (ii) all other tangible and intangible property. The total amount of interest and fees payable by the Company to the Lender under the Loan will be $2,880,000 (the “May Repayment Amount) and will be repaid in 28 weekly installments of $102,857. On October 5, 2023 the May Repayment Amount was restructured in connection with the October MCA Agreement (as defined below). On July 3, 2023, the Company entered into a Business Loan and Security Agreement (the “July Loan Agreement”) with a commercial funding source (the “July Lender’’), pursuant to which the Company obtained a loan from the Lender in the principal amount of $215,000, which includes origination fees of $10,750 (the “July Loan”). Pursuant to the July Loan Agreement, the Company granted the July Lender a continuing secondary security interest in certain collateral (as defined in the July Loan Agreement). The total amount of interest and fees payable by the Company to the Lender under the Loan (the “July Repayment Amount”) will be (i) $322,285 and will be repaid in 13 weekly installments of $24,500 with a final payment of $3,785 in the fourteenth week. As of December 31, 2023, the note was fully paid off. On August 23, 2023, the July Repayment Amount was restructured in connection with the August Loan Agreement, as defined below. On August 23, 2023, the Company entered into a Business Loan and Security Agreement (the “August Loan Agreement”) with a commercial funding source (the “August Lender’’), pursuant to which the Company obtained a loan from the Lender in the principal amount of $1,400,000, which includes origination fees of $70,000 (the “August Loan”). Pursuant to the August Loan Agreement, the Company granted the August Lender a continuing secondary security interest in certain collateral (as defined in the August Loan Agreement). The total amount of interest and fees payable by the Company to the Lender under the Loan (the “Repayment Amount”) will be (i) $2,079,000 (the “August Repayment Amount”) and will be repaid in 21 weekly installments of $99,000 On November 7, 2023 the August Repayment Amount was restructured in connection with the November Loan Agreement (as defined below). On October 5, 2023, the Company entered into an Agreement for the Purchase and Sale of Future Receipts (the “October MCA Agreement”) pursuant to which the existing funder (the “Funder”) increased the existing outstanding amount to $4,470,000 (the “October MCA Purchased Amount”) for gross proceeds to the Company of $3,000,000, less origination fees of $240,000 and the outstanding balance under the existing agreement of $1,234,461, resulting in net proceeds to the Company of $1,525,539. Pursuant to the October MCA Agreement, the Company granted the Funder a security interest in all of the Company’s present and future accounts receivable in an amount not to exceed the October MCA Purchased Amount. The October MCA Purchased Amount shall be repaid by the Company in 30 weekly installments of $149,000. The October Purchased Amount may be prepaid by the Company via a payment of $3,870,000 if repaid within 30 days, $4,110,000 if repaid within 60 days and $4,230,000 if repaid within 90 days. As of December 31, 2023 the October MCA Agreement has an outstanding principal balance of $2,498,245. The October MCA Agreement is currently in default status. On November 7, 2023, the Company entered into a Business Loan and Security Agreement (the “November Loan Agreement”) with the lender (the “Lender”), pursuant to which the Company obtained a loan from the Lender in the principal amount of $2,100,000, which satisfied the outstanding balance on the August Loan of $1,089,000 and includes origination fees of $140,000 (the “November Loan”). Pursuant to the November Loan Agreement, the Company granted the Lender a continuing secondary security interest in certain collateral (as defined in the November Loan Agreement). The total amount of interest and fees payable by us to the Lender under the November Loan will be $3,129,000, which will be repaid in 34 weekly installments ranging from $69,000 - $99,000. As of December 31, 2023 the November Loan has an outstanding principal balance of $1,990,699. The November Loan Agreement is currently in default status. On November 24, 2023, the Company entered into a loan with a principal of $53,099. The loan was evidenced by an unsecured promissory note (the “Second November Note”). Pursuant to the terms of the Second November Note, it will accrue interest at a rate of eight and a half percent (8.50%) per annum, the Prime rate on the date of signing, and is due on the earlier of May 24, 2024 or an event of default, as defined therein. As of December 31, 2023, there was a remaining principal balance of $53,099 on the Second December Loan and accrued interest of $458. Securities Purchase Agreement On July 3, 2023, the Company entered into a Securities Purchase Agreement (the “First Tranche Securities Purchase Agreement”) with an accredited investor pursuant to which the Company issued The Company recognized a total debt discount of $164,775 on the Note from the issuance of stock and original issuance discount. The First Tranche Note has a maturity date of December 31, 2023, and is In connection with the First Tranche Securities Purchase Agreement and the issuance of the First Tranche Note, the Company and certain of its subsidiaries also entered into a Security Agreement with the investor (the “First Tranche Security Agreement”) pursuant to On July 24, 2023, the Company entered into a Securities Purchase Agreement (the “Second Tranche Securities Purchase Agreement”) with an accredited investor pursuant to which the Company issued In connection with the Second Tranche Securities Purchase Agreement and the issuance of the Second Tranche Note, the Company and certain of its subsidiaries also entered into a Security Agreement with the investor (the “Second Tranche Security Agreement”) pursuant to which it granted the investor a security interest in certain Collateral (as defined in the Second Tranche Security Agreement) to secure its obligations under the Second Tranche Note. In addition, the Company entered into a registration rights agreement with the investor pursuant to which the Company agreed to prepare and file with the U.S. Securities and Exchange Commission a registration statement covering the resale of the Second Tranche Commitment Shares and any shares of the Company’s common stock Evofem Merger In connection with the Agreement and Plan of Merger (the “Merger Agreement”) with Adicure, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub”) and Evofem Biosciences, Inc., a Delaware corporation (“Evofem”), the Company, Evofem and the holders (the “Holders”) of certain senior indebtedness (the “Notes”) entered into an Assignment Agreement dated December 11, 2023 (the “Assignment Agreement”), pursuant to which The respective obligations of each of the Company, Merger Sub and Evofem to consummate the closing of the Merger (the “Closing”) are subject to the satisfaction or waiver, at or prior to the closing of certain conditions, including but not limited to, the following: a. waivers with respect to any fundamental transaction, change in control or other similar rights that such warrant holder may have under any such Evofem warrants, and (b) an agreement to such Evofem warrants to exchange such warrants for not more than an aggregate (for all holders of Evofem warrants) of 551 shares of Company Preferred Stock; The obligations of the Company and Merger Sub to consummate the Closing are subject to the satisfaction or waiver, at or prior to the Closing of certain conditions, including but not limited to, the following: The obligations of the Company to consummate the Closing are subject to the satisfaction or waiver, at or prior to the Closing of certain conditions, including but not limited to, the following: As the January 2024 Secured Notes and September 2024 Secured Notes did not contain a stated interest rate, the Company calculated an imputed interest rate of 26.7% based on the Company’s weighted average cost of capital for the period in which the January 2024 Secured Notes and September 2024 Secured Notes were outstanding. This amounted to approximately $1.8 million which was recorded as a discount to be amortized over the life of the January 2024 Secured Notes and September 2024 Secured Notes. See Note 12 for amendments entered into subsequent to year end. NOTE 8 – LEASES Our lease agreements generally do not provide an implicit borrowing rate; therefore, an internal incremental borrowing rate is determined based on information available at lease commencement date for purposes of determining the present value of lease payments. We used the incremental borrowing rate on December 31, 2023 and 2022 for all leases that commenced prior to that date. In determining this rate, which is used to determine the present value of future lease payments, we estimate the rate of interest we would pay on a collateralized basis, with similar payment terms as the lease and in a similar economic environment. Our corporate headquarters is located in Richmond, Virginia, where we lease approximately 25,000 square feet. The lease expires in August 31, 2026, subject to extension. As of December 31, 2023 the Company is 1.75 months in arrears on this lease. We also lease approximately 5,810 square feet of laboratory and office space in Mountain View, California. The lease expires in August 31, 2024, subject to extension. As of December 31, 2023 the Company is 1 month in arrears on this lease. Additionally, we lease approximately 3,150 square feet of office space in Melville, New York. The lease expires in December 31, 2025, subject to extension. As of December 31, 2023 the Company is 1 month in arrears on this lease. Lease Costs Lease Positions as of December 31, 2023 and 2022 ROU lease assets and lease liabilities for our operating leases are recorded on the balance sheet as follows: Lease Terms and Discount Rate as of December 31, 2023 Maturities of leases are as follows: Year Ended December 31, 2023 See Note 12 for additional disclosure regarding the Company’s leases. NOTE 9 – COMMITMENTS & CONTINGENCIES License Agreement with Loma Linda University On March 15, 2018, as amended on July 1, 2020, we entered into a LLU License Agreement directly with Loma Linda University. Pursuant to the LLU License Agreement, we obtained the exclusive royalty-bearing worldwide license in and to all intellectual property, including patents, technical information, trade secrets, proprietary rights, technology, know-how, data, formulas, drawings, and specifications, owned or controlled by LLU and/or any of its affiliates (the “LLU Patent and Technology Rights”) and related to therapy for immune-mediated inflammatory diseases (the ADI™ technology). In consideration for the LLU License Agreement, we issued 13 shares of common stock to LLU. Pursuant to the LLU License Agreement, we are required to pay an annual license fee to LLU. Also, we paid LLU $455,000 in July 2020 for outstanding milestone payments and license fees. We are also required to pay to LLU milestone payments in connection with certain development milestones. Specifically, we are required to make the following milestone payments to LLU: $175,000 on March 31, 2022; $100,000 on March 31, 2024; $500,000 on March 31, 2026; and $500,000 on March 31, 2027. In lieu of the $175,000 milestone payment due on March 31, 2023, the Company paid LLU an extension fee of $100,000. Upon payment of this extension fee, an additional year will be added for the March 31, 2023 milestone. Additionally, as consideration for prior expenses incurred by LLU to prosecute, maintain and defend the LLU Patent and Technology Rights, we made the following payments to LLU: $70,000 at the end of December 2018, and a final payment of $60,000 at the end of March 2019. We are required to defend the LLU Patent and Technology Rights during the term of the LLU License Agreement. Additionally, we will owe royalty payments of (i) 1.5% of Net Product Sales (as such terms are defined under the LLU License Agreement) and Net Service Sales on any Licensed Products (defined as any finished pharmaceutical products which utilizes the LLU Patent and Technology Rights in its development, manufacture or supply), and (ii) 0.75% of Net Product Sales and Net Service Sales for Licensed Products and Licensed Services (as such terms are defined under the LLU License Agreement) not covered by a valid patent claim for technology rights and know-how for a three (3) year period beyond the expiration of all valid patent claims. We also are required to produce a written progress report to LLU, discussing our development and commercialization efforts, within 45 days following the end of each year. All intellectual property rights in and to LLU Patent and Technology Rights shall remain with LLU (other than improvements developed by or on our behalf). The LLU License Agreement shall terminate on the last day that a patent granted to us by LLU is valid and enforceable or the day that the last patent application licensed to us is abandoned. The LLU License Agreement may be terminated by mutual agreement or by us upon 90 days written notice to LLU. LLU may terminate the LLU License Agreement in the event of (i) non-payments or late payments of royalty, milestone and license maintenance fees not cured within 90 days after delivery of written notice by LLU, (ii) a breach of any non-payment provision (including the provision that requires us to meet certain deadlines for milestone events (each, a “Milestone Deadline”)) not cured within 90 days after delivery of written notice by LLU and (iii) LLU delivers notice to us of three or more actual breaches of the LLU License Agreement by us in any 12-month period. Additional Milestone Deadlines include: (i) the requirement to have regulatory approval of an IND application to initiate first-in-human clinical trials on or before March 31, 2023, which will be extended to March 31, 2024 with a payment of a $100,000 extension fee, (ii) the completion of first-in-human (phase I/II) clinical trials by March 31, 2024, (iii) the completion of Phase III clinical trials by March 31, 2026 and (iv) biologic licensing approval by the FDA by March 31, 2027. License Agreement with Leland Stanford Junior University On February 3, 2020, we entered into an exclusive license agreement (the “February 2020 License Agreement”) with Stanford regarding a patent concerning a method for detection and measurement of specific cellular responses. Pursuant to the February 2020 License Agreement, we received an exclusive worldwide license to Stanford’s patent regarding use, import, offer, and sale of Licensed Products (as defined in the agreement). The license to the patented technology is exclusive, including the right to sublicense, beginning on the effective date of the agreement, and ending when the patent expires. Under the exclusivity agreement, we acknowledged that Stanford had already granted a non-exclusive license in the Nonexclusive Field of Use, under the Licensed Patents in the Licensed Field of Use in the Licensed Territory (as those terms are defined in the February 2020 License Agreement”). However, Stanford agreed to not grant further licenses under the Licensed Patents in the Licensed Field of Use in the Licensed Territory. On December 29, 2021, we entered into an amendment to the February 2020 License Agreement which extended our exclusive right to license the technology deployed in AditxtScoreTM and securing worldwide exclusivity in all fields of use of the licensed technology. We were obligated to pay and paid a fee of $25,000 to Stanford within 60 days of February 3, 2020. We also issued 10 shares of the Company’s common stock to Stanford. An annual licensing maintenance fee is payable by us on the first anniversary of the February 2020 License Agreement in the amount of $40,000 for 2021 through 2024 and $60,000 starting in 2025 until the license expires upon the expiration of the patent. The Company is required to pay and has paid $25,000 for the issuances of certain patents. The Company will pay milestone fees of $50,000 on the first commercial sales of a licensed product and $25,000 at the beginning of any clinical study for regulatory clearance of an in vitro diagnostic product developed and a potential licensed product. The Company paid a milestone fee for a clinical study for regulatory clearance of an in vitro diagnostic product developed and a potential licensed product of $25,000 in March of 2022. We are also required to: (i) provide a listing of the management team or a schedule for the recruitment of key management positions by March 31, 2020 (which has been completed), (ii) provide a business plan covering projected product development, markets and sales forecasts, manufacturing and operations, and financial forecasts until at least $10,000,000 in revenue by June 30, 2020 (which has been completed), (iii) conduct validation studies by September 30, 2020 (which has been completed), (iv) hold a pre-submission meeting with the FDA by September 30, 2020 (which has been completed), (iv) submit a 510(k) application to the FDA, Emergency Use Authorization (“EUA”), or a Laboratory Developed Test (“LDT”) by March 31, 2021 (which has been completed), (vi) develop a prototype assay for human profiling by December 31, 2021 (which has been completed), (vii) execute at least one partnership for use of the technology for transplant, autoimmunity, or infectious disease purposes by March 31, 2022 (which has been completed) and (viii) provided further development and commercialization milestones for specific fields of use in writing prior to December 31, 2022. In addition to the annual license maintenance fees outlined above, we will pay Stanford royalties on Net Sales (as such term is defined in the February 2020 License Agreement) during the of the term of the agreement as follows: 4% when Net Sales are below or equal to $5 million annually or 6% when Net Sales are above $5 million annually. The February 2020 License Agreement may be terminated upon our election on at least 30 days advance notice to Stanford, or by Stanford if we: (i) are delinquent on any report or payment; (ii) are not diligently developing and commercializing Licensed Product; (iii) miss certain performance milestones; (iv) are in breach of any provision of the February 2020 License Agreement; or (v) provide any false report to Stanford. Should any events in the preceding sentence occur, we have a thirty (30) day cure period to remedy such violation. Asset Purchase Agreement On April 18, 2023, the Company entered into an Asset Purchase Agreement (the “Asset Purchase Agreement”) with Cellvera Global Holdings LLC (“Cellvera Global”), Cellvera Holdings Ltd. (“BVI Holdco”), Cellvera, Ltd. (“Cellvera Ltd.”), Cellvera Development LLC (“Cellvera Development” and together with Cellvera Global, BVI Holdco, Cellvera Ltd. and Cellvera Development (the “Sellers”), AiPharma Group Ltd. (“Seller Owner” and collectively with the Sellers, “Cellvera”), and the legal representative of Cellvera, pursuant to which, the Company will purchase Cellvera’s 50% ownership interest in G Response Aid FZE (“GRA”), certain other intellectual property and all goodwill related thereto (the “Acquired Assets”). Unless expressly stated otherwise herein, capitalized terms used but not defined herein have the meanings ascribed to them in the Asset Purchase Agreement. Pursuant to the Asset Purchase Agreement, the consideration for the Acquired Assets consists of (A) $24.5 million, comprised of: (i) the forgiveness of the Company’s $14.5 million loan to Cellvera Global, and (ii) approximately $10 million in cash, and (B) future revenue sharing payments for a term of seven years. GRA holds an exclusive, worldwide license for the antiviral medication, Avigan® 200mg, excluding Japan, China and Russia. The other 50% interest in GRA is held by Agility, Inc. (“Agility”). Additionally, upon the closing, the Share Exchange Agreement previously entered into as of December 28, 2021, between Cellvera Global Holdings, LLC f/k/a AiPharma Global Holdings, LLC (together with other affiliates and subsidiaries) and the Company, and all other related agreements will be terminated. The obligations of the Company to consummate the closing are subject to the satisfaction or waiver, at or prior to the Closing of certain conditions, including but not limited to, the following: Departure of Officer On July 21, 2023, Matthew Shatzkes tendered his resignation as Chief Legal Officer, General Counsel and Corporate Secretary of the Company. In connection with his resignation, the Company entered into a Separation Agreement and General Release (the “Separation Agreement”) with Mr. Shatzkes. Pursuant to the Separation Agreement, Mr. Shatzkes’ employment with the Company terminated on August 4, 2023 (the “Termination Date”). In addition, the Company agreed to pay Mr. Shatzkes’ within seven days after the Termination Date: (i) $122,292, representing all accrued salary and wages (inclusive of Base Compensation and earned Subsequent Quarterly Bonus amounts, as those terms are defined in Mr. Shatzkes’ employment agreement), and (ii) $32,576, representing Mr. Shatzkes accrued, but unused paid time off (collectively, the “Initial Payment”). The Company also agreed to pay Mr. Shatzkes: (i) $385,000, representing 12 months of Mr. Shatzkes’ Base Compensation (as that term is defined in Mr. Shatzkes employment agreement), and (ii) $290,000, representing Mr. Shatzkes Subsequent Year Minimum Bonus (as such term is defined in Mr. Shatzkes employment agreement), on the 60th day following the Termination Date. In addition, the Company shall reimburse Mr. Shatzkes COBRA premium for a period of 12 months and shall cause any restricted stock units granted to Mr. Shatzkes to immediately vest as of the Termination Date. As of December 31, 2023, the Company has completed all obligations under the Separation Agreement. Contingent Liability On September 7, 2023, the Company received a demand letter from the holder of certain warrants issued by the Company in April 2023. The demand letter alleged that the investor suffered more than $2 million in damages as a result of the Company failing to register the shares of the Company’s common stock underlying the warrants as required under the securities purchase agreement. The Company denies the amount of the liability claimed by the investor and intends to defend itself vigorously against any such claims. The Company is engaged in ongoing discussions with the investor and, as a result, has accrued a loss of $1.6 million relating to the potential liability. This liability was settled subsequent to December 31, 2023. (See Note 12) Letter of Intent Termination On August 1, 2023, the Company and Natural State Genomics and Natural State Laboratories mutually agreed to terminate the Amended and Restated Non-Binding Letter of Intent dated June 12, 2023. EvoFem Merger Agreement On December 11, 2023 (the “Execution Date”), Aditxt, Inc., a Delaware corporation (the “Company”) entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Adicure, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub”) and Evofem Biosciences, Inc., a Delaware corporation (“Evofem”), pursuant to which, Merger Sub will be merged into and with Evofem (the “Merger”), with Evofem surviving the Merger as a wholly owned subsidiary of the Company. In connection with the Merger Agreement the Company assumed $13.0 million in notes payable held by Evofem (see Note 7) and assumed a payable for $154,480 (see Note 7). These items were capitalized on the Company’s balance sheet to deposit on acquisition as of December 31, 2023. The Company recognized a debt discount of $1,826,250. As of December 31, 2023, there was an unamortized discount of $1,633,389. Subject to the terms and conditions set forth in the Merger Agreement, at the effective time of the Merger (the “Effective Time”), (i) all issued and outstanding shares of common stock, par value $0.0001 per share of Evofem (“Evofem Common Stock”), other than any shares of Evofem Common Stock held by the Company or Merger Sub immediately prior to the Effective Time, will be converted into the right to receive an aggregate of 610,000 shares of the Company’s common stock, par value $0.001 per share (“Company Common Stock”); and (ii) all issued and outstanding shares of Series E-1 Preferred Stock, par value $0.0001 of Evofem (the “Evofem Unconverted Preferred Stock”), other than any shares of Evofem Unconverted Preferred Stock held by the Company or Merger Sub immediately prior to the Effective Time, will be converted into the right to receive an aggregate of 2,327 shares of Series A-1 Preferred Stock, par value $0.001 of the Company (the “Company Preferred Stock”), having such rights, powers, and preferences set forth in the form of Certificate of Designation of Series A-1 Preferred Stock. (See Note 10) Evofem Exchange Agreement On December 22, 2023, the Company entered into an Exchange Agreement (the “Exchange Agreement”) with the holders of an aggregate of 22,280 shares of Series F-1 Convertible Preferred Stock of Evofem (the “Evofem Series F-1 Preferred Stock”) agreed to exchange their respective shares of Evofem Series F-1 Preferred Stock for an aggregate of 22,280 shares of a new series of convertible preferred stock of the Company designated as Series A-1 Convertible Preferred Stock, $0.001 par value, (the “Series A-1 Preferred Stock”), having a total value of $22,277,233. (see Note 10) This investment has been recorded at cost in accordance with ASC 321. NOTE 10 – STOCKHOLDERS’ EQUITY Common Stock On May 24, 2021, the Company increased the number of authorized shares of the Company’s common stock, par value $0.001 per share, from 27,000,000 to 100,000,000 (the “Authorized Shares Increase”) by filing a Formed in January 2023, our majority owned subsidiary Pearsanta™, Inc. (“Pearsanta”) seeks to take personalized medicine to a new level by delivering “Health by the Numbers.” On November 22, 2023, Pearsanta entered into an assignment agreement with FirstVitals LLC, an entity controlled by Pearsanta’s CEO, Ernie Lee (“FirstVitals”), pursuant to which FirstVitals assigned its rights in certain intellectual property and website domain to Pearsanta in consideration of the issuance of 500,000 shares of Pearsanta common stock to FirstVitals. On December 18, 2023, the board of directors of Pearsanta adopted the Pearsanta 2023 Omnibus Equity Incentive Plan (the “Pearsanta Omnibus Incentive Plan”), pursuant to which it reserved 15 million shares of common stock of Pearsanta for future issuance under the Pearsanta Omnibus Incentive Plan and the Pearsanta 2023 Parent Service Provider Equity Incentive Plan (the “Pearsanta Parent Service Provider Plan”) and approved the issuance of 9.32 million options, exercisable into shares of Pearsanta common stock under the Pearsanta Parent Service Provider Plan and the issuance of 4.0 million options, exercisable into shares of Pearsanta common stock, subject to vesting, and 1.0 million restricted common stock shares under the Pearsanta Omnibus Incentive Plan. During the year ended December 31, 2023, the Company issued 74,675 shares of common stock and recognized expense of $484,525 in stock-based compensation for consulting services. The stock-based compensation for consulting services is calculated by the number of shares multiplied by the closing price on the effective date of the contract. The Company recognized expense of $308,479 in stock-based compensation related to the RSUs for the year ended December 31, 2023. The stock-based compensation for shares issued or RSUs granted during the period were valued based on the fair market value on the date of grant. During the year ended December 31, 2023, the Company issued 1,055,374 shares of common stock for the exercise of warrants. During the year ended December 31, 2022, the Company issued 3,707 shares of common stock and recognized expense of $507,558 in stock-based compensation for consulting services. The Company also granted 292 RSUs, 463 vested and resulted in the issuance of shares. As a result, the Company recognized expense of $1,209,906 in stock-based compensation. The stock-based compensation for shares issued or RSU’s granted during the period were valued based on the fair market value on the date of grant. During the year ended December 31, 2022, the Company issued 48,659 shares of common stock for the exercise of warrants. On December 20, 2022, the Company entered into an At The Market Offering Agreement (the “ATM”) with H.C. Wainwright & Co., LLC as agent (the “Agent”), pursuant to which the Company may offer and sell, from time to time through the Agent, shares of the Company’s common stock having an aggregate offering price of up to $50,000,000 (the “Shares”). The offer and sale of the Shares was made pursuant to a shelf registration statement on Form S-3 and the related prospectus (File No. 333-257645) filed by the Company with the SEC on July 2, 2021, amended on July 6, 2021 and declared effective by the SEC on July 13, 2021, under the Securities Act of 1933, as amended. For the year ended December 31, 2023, the Company sold 8,463 Shares at an average price of $62.05 per share On April 20, 2023, the Company entered into an amendment to Preferred Stock The Company is authorized to issue 3,000,000 shares of preferred stock, par value $0.001 per share. There were 24,905 and zero shares of preferred stock outstanding as of December 31, 2023 and 2022, respectively. Issuance of Series A-1 Preferred Stock: On December 11, 2023 (the “Execution Date”), the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Adicure, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub”) and Evofem Biosciences, Inc., a Delaware corporation (“Evofem”), pursuant to which, Merger Sub will be merged into and with Evofem (the “Merger”), with Evofem surviving the Merger as a wholly owned subsidiary of the Company. Subject to the terms and conditions set forth in the Merger Agreement, at the effective time of the Merger (the “Effective Time”), (i) all issued and outstanding shares of common stock, par value $0.0001 per share of Evofem (“Evofem Common Stock”), other than any shares of Evofem Common Stock held by the Company or Merger Sub immediately prior to the Effective Time, will be converted into the right to receive an aggregate of 610,000 shares of the Company’s common stock, par value $0.001 per share (“Company Common Stock”); and (ii) all issued and outstanding shares of Series E-1 Preferred Stock, par value $0.0001 of Evofem (the “Evofem Unconverted Preferred Stock”), other than any shares of Evofem Unconverted Preferred Stock held by the Company or Merger Sub immediately prior to the Effective Time, will be converted into the right to receive an aggregate of 2,327 shares of Series A-1 Preferred Stock, par value $0.001 of the Company (the “Company Preferred Stock”), having such rights, powers, and preferences set forth in the form of Certificate of Designation of Series A-1 Preferred Stock. See Series A-1 Preferred Stock certificate of designation incorporated by reference to this document. On December 22, 2023, the Company entered into an Exchange Agreement (the “Exchange Agreement”) with the holders (the “Holders”) of an aggregate of 22,280 shares of Series F-1 Convertible Preferred Stock of Evofem (the “Evofem Series F-1 Preferred Stock”) agreed to exchange their respective shares of Evofem Series F-1 Preferred Stock for an aggregate of 22,280 shares of a new series of convertible preferred stock of the Company designated as Series A-1 Convertible Preferred Stock, $0.001 par value, (the “Series A-1 Preferred Stock”). The following is only a summary of the Series A-1 Certificate of Designations, and is qualified in its entirety by reference to the full text of the Series A-1 Certificate of Designations, a copy of which is filed as Exhibit 3.1 to our Current Report on Form 8-K filed on December 26, 2023 and is incorporated by reference herein. Designation, Amount, and Par Value: The number of Series A-1 Preferred Stock designated is 22,280 shares. The shares of Series A-1 Preferred Stock have a par value of $0.001 per share and a stated value of $1,000 per share. Conversion Price: The Series A-1 Preferred Stock will be convertible into shares of Common Stock at an initial conversion price of $4.44 (subject to adjustment pursuant to the Series A-1 Certificate of Designations) (the “Conversion Price”). The Certificate of Designations also provides that in the event of certain Triggering Events (as defined below) any holder may, at any time, convert any or all of such holder’s Series A-1 Preferred Stock at an alternate conversion rate equal to the product of (i) the Alternate Conversion Price (as defined below) and (ii) the quotient of (x) the 25% redemption premium multiplied by (y) the amount of Series A-1 Preferred Stock subject to such conversion. “Triggering Events” include, among others, (i) a suspension of trading or the failure to be traded or listed on an eligible market for five consecutive days or more, (ii) the failure to remove restrictive legends when required, (iii) the Company’s default in payment of indebtedness in an aggregate amount of $500,000 or more (the Company is currently in default for payments greater than $500,000), (iv) proceedings for a bankruptcy, insolvency, reorganization or liquidation, which are not dismissed with 30 days, (v) commencement of a voluntary bankruptcy proceeding, and (viii) final judgments against the Company for the payment of money in excess of $100,000. “Alternate Conversion Price” means the lowest of (i) the applicable conversion price the in effect, (ii) the greater of (x) $0.888 (the “Floor Price”) and (y) 80% of the volume weighted average price (“VWAP”) of the Common Stock on the trading day immediately preceding the delivery of the applicable conversion notice. Further, the Series A-1 Certificate of Designations provides that if on any of the 90th and 180th day after each of the occurrence of any Stock Combination Event (as defined in the Series A-1 Certificate of Designations) and the Applicable Date (as defined in the Series A-1 Certificate of Designations), the conversion price then in effect is greater than the market price then in effect (the “Adjustment Price”), on such date then the conversion price shall automatically lower to the Adjustment Price. Dividends: Holders of the Series A-1 Preferred Stock shall be entitled to receive dividends when and as declared by the Board, from time to time, in its sole discretion, which Dividends shall be paid by the Company out of funds legally available therefor, payable, subject to the conditions and other terms hereof, in cash, in securities of the Company or any other entity, or using assets as determined by the Board on the Stated Value of such Preferred Share. Liquidation: In the event of a Liquidation Event (as defined in the Series A-1 Certificate of Designation), the holders the Series A-1 Preferred Stock shall be entitled to receive in cash out of the assets of the Company, before any amount shall be paid to the holders of any other shares of capital stock of the Company, equal to the greater of (A) 125% of the Conversion Amount (as defined in the Series A-1 Certificate of Designation) on the date of such payment and (B) the amount per share such holder of Series A-1 Preferred Stock would receive if they converted such share of Series A-1 Preferred Stock into Common Stock immediately prior to the date of such payment Company Redemption: The Company may redeem all, or any portion, of the Series A-1 Preferred Stock for cash, at a price per share of Series A-1 Preferred Stock equal to 115% of the greater of (i) the Conversion Amount (as defined in the Series A-1 Certificate of Designation)being redeemed as of the Company Optional Redemption Date (as defined in the Series A-1 Certificate of Designation) and (ii) the product of (1) the Conversion Rate (as defined in the Series A-1 Certificate of Designation) with respect to the Conversion Amount being redeemed as of the Company Optional Redemption Date multiplied by (2) the greatest Closing Sale Price (as defined in the Certificate of Designation) of the Common Stock on any Trading Day during the period commencing on the date immediately preceding such Company Optional Redemption Notice Date (as defined in the Certificate of Designation) and ending on the Trading Day immediately prior to the date the Company makes the entire payment required to be made under the Certification of Designation. Maximum Percentage: Holders of Series A-1 Preferred Stock are prohibited from converting shares of Series A-1 Preferred Stock into shares of Common Stock if, as a result of such conversion, such holder, together with its affiliates, would beneficially own in excess of 4.99% (the “Maximum Percentage”) of the total number of shares of Common Stock issued and outstanding immediately after giving effect to such conversion. Voting Rights: The holders of the Series A-1 Preferred Stock shall have no voting power and no right to vote on any matter at any time, either as a separate series or class or together with any other series or class of share of capital stock, and shall not be entitled to call a meeting of such holders for any purpose nor shall they be entitled to participate in any meeting of the holders of Common Stock, except as expressly provided in the Certificate of Designations and where required by the DGCL. Issuance of Series B Preferred Stock: On July 19, 2022, the Company entered into a Subscription and Investment Representation Agreement with its Chief Executive Officer (the “Purchaser”), pursuant to which the Company agreed to issue and sell one (1) share of the Company’s Series B Preferred Stock (the “Preferred Stock”), par value $0.001 per share, to the Purchaser for $20,000 in cash. On July 19, 2022, the Company filed a certificate of designation (the “Certificate of Designation”) with the Secretary of State of Delaware, effective as of the time of filing, designating the rights, preferences, privileges and restrictions of the share of Preferred Stock. The Certificate of Designation provides that the share of Preferred Stock will have 250,000,000 votes and will vote together with the outstanding shares of the Company’s common stock as a single class exclusively with respect to any proposal to amend the Company’s Restated Certificate of Incorporation to effect a reverse stock split of the Company’s common stock. The Preferred Stock will be voted, without action by the holder, on any such proposal in the same proportion as shares of common stock are voted. The Preferred Stock otherwise has no voting rights except as otherwise required by the General Corporation Law of the State of Delaware. The Preferred Stock is not convertible into, or exchangeable for, shares of any other class or series of stock or other securities of the Company. The Preferred Stock has no rights with respect to any distribution of assets of the Company, including upon a liquidation, bankruptcy, reorganization, merger, acquisition, sale, dissolution or winding up of the Company, whether voluntarily or involuntarily. The holder of the Preferred Stock will not be entitled to receive dividends of any kind. See Series B Preferred Stock certificate of designation incorporated by reference to this document. The outstanding share of Preferred Stock shall be redeemed in whole, but not in part, at any time (i) if such redemption is ordered by the Board of Directors in its sole discretion or (ii) automatically upon the effectiveness of the amendment to the Certificate of Incorporation implementing a reverse stock split. Upon such redemption, the holder of the Preferred Stock will receive consideration of $20,000 in cash. Redemption of Series B Preferred Stock On October 7, 2022, the Company paid $20,000 in consideration for the one share of Preferred Stock which was redeemed on September 13, 2022. Issuance of Series B-2 Preferred Stock: On December 29, 2023, the Company entered into an Exchange Agreement (the “Note Exchange Agreement”) with the Noteholder, pursuant to which the Noteholder agreed, subject to the terms and conditions set forth therein, to exchange the Note, including all accrued but unpaid interest thereon, for an aggregate of 2,625 shares of a new series of convertible preferred stock of the Company, designated as Series B-2 Convertible Preferred Stock, $0.001 par value (the “Series B-2 Preferred Stock”). See Series B-2 Preferred Stock certificate of designation incorporated by reference to this document. The following is only a summary of the Series B-2 Certificate of Designations, and is qualified in its entirety by reference to the full text of the Series B-2 Certificate of Designations, a copy of which is filed as an exhibit to our Current Report on Form 8-K filed with the SEC on January 2, 2024. Designation, Amount, and Par Value: The number of Series B-2 Preferred Stock designated is 2,625 shares. The shares of Series B-2 Preferred Stock have a par value of $0.001 per share and a stated value of $1,000 per share. Conversion Price: The Series B-2 Preferred Stock will be convertible into shares of Common Stock at an initial conversion price of $4.71 (subject to adjustment pursuant to the Series B-2 Certificate of Designations) (the “Conversion Price”). The Series B-2 Certificate of Designations also provides that in the event of certain Triggering Events (as defined below) any holder may, at any time, convert any or all of such holder’s Series B-2 Preferred Stock at an alternate conversion rate equal to the product of (i) the Alternate Conversion Price (as defined below) and (ii) the quotient of (x) the 125% redemption premium multiplied by (y) the amount of Series B-2 Preferred Stock subject to such conversion. “Triggering Events” include, among others, (i) a suspension of trading or the failure to be traded or listed on an eligible market for five consecutive days or more, (ii) the failure to remove restrictive legends when required, (iii) the Company’s default in payment of indebtedness in an aggregate amount of $500,000 or more(the Company is currently in default for payments greater than $500,000), (iv) proceedings for a bankruptcy, insolvency, reorganization or liquidation, which are not dismissed with 30 days, (v) commencement of a voluntary bankruptcy proceeding, and (viii) final judgments against the Company for the payment of money in excess of $500,000. “Alternate Conversion Price” means the lowest of (i) the applicable conversion price the in effect, (ii) the greater of (x) $0.9420 (the “Floor Price”) and (y) 80% of the lowest volume weighted average price (“VWAP”) of the Common Stock during the five consecutive trading day period ending and including the trading day immediately preceding the delivery of the applicable conversion notice. Further, the Series B-2 Certificate of Designations provides that if on any of the 90th and 180th day after each of the occurrence of any Stock Combination Event (as defined in the Series B-2 Certificate of Designations) and the Applicable Date (as defined in the Series B-2 Certificate of Designations), the conversion price then in effect is greater than the market price then in effect (the “Adjustment Price”), on such date then the conversion price shall automatically lower to the Adjustment Price. Dividends: Holders of the Series B-2 Preferred Stock shall be entitled to receive dividends when and as declared by the Board, from time to time, in its sole discretion, which Dividends shall be paid by the Company out of funds legally available therefor, payable, subject to the conditions and other terms hereof, in cash, in securities of the Company or any other entity, or using assets as determined by the Board on the Stated Value of such Preferred Share. Liquidation: In the event of a Liquidation Event (as defined in the Series B-2 Certificate of Designations), the holders the Series B-2 Preferred Stock shall be entitled to receive in cash out of the assets of the Company, before any amount shall be paid to the holders of any other shares of capital stock of the Company, equal to the greater of (A) 125% of the Conversion Amount (as defined in the Series B-2 Certificate of Designation) on the date of such payment and (B) the amount per share such holder of Series B-2 Preferred Stock would receive if they converted such share of Series B-2 Preferred Stock into Common Stock immediately prior to the date of such payment. Company Redemption: The Company may redeem all, or any portion, of the Series B-2 Preferred Stock for cash, at a price per share of Series B-2 Preferred Stock equal to 115% of the greater of (i) the Conversion Amount (as defined in the Series B-2 Certificate of Designations) being redeemed as of the Company Optional Redemption Date (as defined in the Series B-2 Certificate of Designations) and (ii) the product of (1) the Conversion Rate (as defined in the Series B-2 Certificate of Designations) with respect to the Conversion Amount being redeemed as of the Company Optional Redemption Date multiplied by (2) the greatest Closing Sale Price (as defined in the Series B-2 Certificate of Designations) of the Common Stock on any Trading Day during the period commencing on the date immediately preceding such Company Optional Redemption Notice Date (as defined in the Series B-2 Certificate of Designations) and ending on the Trading Day immediately prior to the date the Company makes the entire payment required to be made under the Certification of Designation. Maximum Percentage: Holders of Series B-2 Preferred Stock are prohibited from converting shares of Series B-2 Preferred Stock into shares of Common Stock if, as a result of such conversion, such holder, together with its affiliates, would beneficially own in excess of 4.99% (the “Maximum Percentage”) of the total number of shares of Common Stock issued and outstanding immediately after giving effect to such conversion. Voting Rights: The holders of the Series B-2 Preferred Stock shall have no voting power and no right to vote on any matter at any time, either as a separate series or class or together with any other series or class of share of capital stock, and shall not be entitled to call a meeting of such holders for any purpose nor shall they be entitled to participate in any meeting of the holders of Common Stock, except as expressly provided in the Series B-2 Certificate of Designations and where required by the DGCL. Series C Preferred Stock On July 11, 2023, the Company filed a certificate of designation (the “Certificate of Designation”) with the Secretary of State of Delaware, effective as of the time of filing, designating the rights, preferences, privileges and restrictions of the share of Preferred Stock. The Certificate of Designation provides that the share of Preferred Stock will have 250,000,000 votes and will vote together with the outstanding shares of the Company’s common stock as a single class exclusively with respect to any proposal to amend the Company’s Amended and Restated Certificate of Incorporation to effect a reverse stock split of the Company’s common stock. The Preferred Stock will be voted, without action by the holder, on any such proposal in the same proportion as shares of common stock are voted. The Preferred Stock otherwise has no voting rights except as otherwise required by the General Corporation Law of the State of Delaware. The Preferred Stock is not convertible into, or exchangeable for, shares of any other class or series of stock or other securities of the Company. The Preferred Stock has no rights with respect to any distribution of assets of the Company, including upon a liquidation, bankruptcy, reorganization, merger, acquisition, sale, dissolution or winding up of the Company, whether voluntarily or involuntarily. The holder of the Preferred Stock will not be entitled to receive dividends of any kind. The outstanding share of Preferred Stock shall be redeemed in whole, but not in part, at any time (i) if such redemption is ordered by the Board of Directors in its sole discretion or (ii) automatically upon the effectiveness of the amendment to the Certificate of Incorporation implementing a reverse stock split. Upon such redemption, the holder of the Preferred Stock will receive consideration of $1,000 in cash. As of December 31, 2023, the share has been redeemed and the consideration has been paid. On July 11, 2023, the Company entered into a Subscription and Investment Representation Agreement (the “Subscription Agreement”) with Amro Albanna, its Chief Executive Officer, who is an accredited investor (the “Purchaser”), pursuant to which the Company agreed to issue and sell one (1) share of the Company’s Series C Preferred Stock, par value $0.001 per share (the “Preferred Stock”), to the Purchaser for $1,000 in cash. The sale closed on July 11, 2023. The Subscription Agreement contains customary representations and warranties and certain indemnification rights and obligations of the parties. See Series C Preferred Stock certificate of designation incorporated by reference to this document. On August 17, 2023, the share was redeemed. Stock-Based Compensation In October 2017, our Board of Directors adopted the Aditx Therapeutics, Inc. 2017 Equity Incentive Plan (the “2017 Plan”). The 2017 Plan provides for the grant of equity awards to directors, employees, and consultants. On February 24, 2021, our Board of Directors adopted the Aditx Therapeutics, Inc. 2021 Omnibus Equity Incentive Plan (the “2021 Plan”). The 2021 Plan provides for grants of nonqualified stock options, incentive stock options, stock appreciation rights, restricted stock and restricted stock units, and other stock-based awards (collectively, the “Awards”). Eligible recipients of Awards include employees, directors or independent contractors of the Company or any affiliate of the Company. The Compensation Committee of the Board of Directors (the “Committee”) administers the 2021 Plan. A total of 60,000 shares of common stock, par value $0.001 per share, of the Company may be issued pursuant to Awards granted under the 2021 Plan. The exercise price per share for the shares to be issued pursuant to an exercise of a stock option will be no less than one hundred percent (100%) of the Fair Market Value (as defined in the 2021 Plan) of a share of Common Stock on the date of grant. The 2021 Plan was submitted and approved by the Company’s stockholders at the 2021 annual meeting of stockholders, held on May 19, 2021. During the years ended December 31, 2023 and 2022, the Company granted 44,445 and 0 new options. respectively. For the year ended December 31, The risk-free interest rate assumption for options granted is based upon observed interest rates on the United States The Company determined the expected volatility assumption for options granted using the historical volatility of comparable public companies’ common stock. The Company will continue to monitor peer companies and other relevant factors used to measure expected volatility for future The dividend yield assumption for The Company recognizes The following is an analysis of the stock option grant activity under the Plan: As of December 31, 2023 there were 45,572 exercisable options; these options had a weighted average exercise price $173.12. These options had a grant date fair value of $221,005. On December 18, 2023, our Board of Directors adopted the Pearsanta, Inc. 2023 Omnibus Equity Incentive Plan (the “Pearsanta 2023 Plan”) and the 2023 Parent Service Provider Equity Incentive Plan (the “Pearsanta Parent 2023 Plan”), collectively (the “Pearsanta Plans”). The Pearsanta Plans provides for grants of nonqualified stock options, incentive stock options, stock appreciation rights, restricted stock and restricted stock units, and other stock-based awards (collectively, the “Pearsanta Awards”). Eligible recipients of Pearsanta Awards include employees, directors or independent contractors of the Company or any affiliate of the Company. The Board of Directors administers the Pearsanta Plans. The Pearsanta 2023 Plan consists of a total of 15,000,000 shares of Pearsanta common stock, par value $0.001 per share, which may be issued pursuant to Pearsanta Awards granted under the Pearsanta 2023 Plan. The Pearsanta Parent 2023 Plan consists of a total of 9,320,000 shares of Pearsanta common stock, par value $0.001 per share, which may be issued pursuant to Pearsanta Awards granted under the Pearsanta Parent 2023 Plan. The exercise price per share for the shares to be issued pursuant to an exercise of a stock option will be no less than one hundred percent (100%) of the Fair Market Value (as defined in the Pearsanta Plans) of a share of Common Stock on the date of grant. During the years ended December 31, 2023 and 2022, Pearsanta granted 4,000,000 and 0 new options under the Pearsanta 2023 Plan, respectively. During the years ended December 31, 2023 and 2022, Pearsanta granted 9,320,000 and 0 new options under the Pearsanta Parent 2023 Plan, respectively. For the year ended December 31, 2023, the fair value of each option granted was estimated using the assumption and/or factors in the Black-Scholes Model as follows: The risk-free interest rate assumption for warrants granted is based upon observed interest rates on the United States Government Bond Equivalent Yield appropriate for the expected term of option. The Company determined the expected volatility assumption for options granted using the historical volatility of comparable public companies’ common stock. The Company will continue to monitor peer companies and other relevant factors used to measure expected volatility for future option grants, until such time that the Company’s common stock has enough market history to use historical volatility. The dividend yield assumption for option granted is based on the Company’s history and expectation of dividend payouts. The Company has never declared nor paid any cash dividends on its common stock, and the Company does not anticipate paying any cash dividends in the foreseeable future. The following is an analysis of the stock option grant activity under the Pearsanta Plans: As of December 31, 2023 there were 9,320,000 exercisable options; these options had a weighted average exercise price $0.02. These options had a grant date fair value of $265,929. The Company recognized stock-based compensation expense related to all options Warrants For the For the year ended December 31, 2022, the fair value of The risk-free interest rate assumption for warrants granted is based upon observed interest rates on the United States Government Bond Equivalent Yield appropriate for the The Company determined the The The Company recognizes warrant forfeitures as they occur, as there is insufficient historical data to A summary of warrant issuances are as follows: The Company recognized stock-based compensation expense related to warrants On April 20, 2023, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with an institutional investor, pursuant to which the Company agreed to sell to such investor pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 39,634 shares of common stock of the Company (the “Common Stock”) at a purchase price of $48.76 per Pre-Funded Warrant, resulting in proceeds of approximately $1.6 million after deducting approximately $291,000 in commissions and closing fees. Concurrently with the sale of the Pre-Funded Warrants, pursuant to the Purchase Agreement in a concurrent private placement, for each Pre-Funded Warrant purchased by the investor, such investor received from the Company an unregistered warrant (the “Warrant”) to purchase two shares of Common Stock. The warrants have an exercise price of $34.40 per share and are exercisable for a three year period. In addition, the Company issued a warrant to the placement agent to purchase up to 2,379 shares of common stock at an exercise price of $61.00 per share and were valued at $56,742 using a Black Scholes valuation model. As these warrants were considered offering costs, they had a zero net effect on the Company’s equity. On August 31, 2023, the Company entered into a securities purchase agreement (the “August Purchase Agreement”) with an institutional investor for the issuance and sale in a private placement (the “Private Placement”) of (i) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 1,000,000 shares of the Company’s common stock at an exercise price of $0.001 per share, and (ii) warrants (the “Common Warrants”) to purchase up to 1,000,000 shares of the Company’s Common Stock at an exercise price of $10.00 per share. 60,000 warrants were also issued to the placement agent. These warrants had an exercise price of $12.50 and a term of 5.5 years. The Common Warrants were valued at $32.3 million and the 60,000 warrants issued to the placement agents were valued at $1.9 million using a Black Scholes valuation model. As these warrants were considered offering costs, they had a zero net effect on the Company’s equity. The Private Placement closed on September 6, 2023. The net proceeds to the Company from the Private Placement were approximately $9 million, after deducting placement agent fees and expenses and estimated offering expenses payable by the Company. The Company used the net proceeds received from the Private Placement for (i) the payment of approximately $3.1 million in outstanding obligations, (ii) the repayment of approximately $0.4 million of outstanding debt, and (iii) the balance for continuing operating expenses and working capital. On December 29, 2023, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with an institutional investor (“the “Purchaser”) for the issuance and sale in a private placement (the “Private Placement”) of (i) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 1,237,114 shares of the Company’s common stock, par value $0.001 (the “Common Stock”) at an exercise price of $0.001 per share, and (ii) warrants (the “Common Warrants”) to purchase up to 2,474,228 shares of the Company’s Common Stock, at a purchase price of $4.85 per share. As of December 31, 2023, the Company had not received the funds from the Purchase Agreement resulting in a $5,444,628 receivable. These funds were received on January 4, 2024. The Common Warrants are exercisable immediately upon issuance at an exercise price of $4.60 per share and have a term of exercise equal to three years from the date of issuance. The Pre-Funded Warrants are exercisable immediately and may be exercised at any time until the Pre-Funded Warrants are exercised in full. A holder of Pre-Funded Warrants or Warrants (together with its affiliates) may not exercise any portion of a warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder 9.99%) of the Company’s outstanding common stock immediately after exercise. Pursuant to the Purchase Agreement, the Company agreed to reduce the exercise price of certain outstanding warrants to purchase Common Stock of the Company (“Outstanding Warrants”) held by the Purchaser to $4.60 per share in consideration for the cash payment by the Purchaser of $0.125 per share of Common Stock underlying the Outstanding Warrants, effective immediately. The Company issued a warrant to the placement agent to purchase up to 74,227 shares of common stock at an exercise price of $6.06 per share and were valued at $470,772 using a Black Scholes valuation model. As these warrants were considered offering costs, they had a zero net effect on the Company’s equity. Restricted Stock Units A summary of Restricted Stock Units (“RSUs”) issuances are as follows: The Company recognized stock-based compensation expense related to RSUs granted and vesting expense of $308,479 and $1,843,902 during the years ended December 31, 2023 and 2022, respectively. Of the $308,479, $242,915 is included in general and administrative, $58,777 is included in research and development, and $6,787 is included in sales and marketing in the accompanying Statements of Operations. Of the $1,843,902, $1,237,182 is included in general and administrative and $606,720 is included in research and development in the accompanying Statements of Operations. The remaining value to be expensed is $0 with a weighted average vesting term of During the year ended December 31, zero RSUs. During the year ended December 31, NOTE For the years ended December 31, A reconciliation of income tax expense (benefit) computed at the statutory federal income tax rate to income taxes as reflected in the financial statements is as follows: Deferred taxes are recognized for temporary differences between the basis of assets and liabilities for financial statement and income tax purposes. The significant components of the Company’s deferred tax assets and liabilities as of December 31, The Company has evaluated the positive and negative evidence bearing upon its ability to realize its deferred tax assets, which are comprised primarily of net operating loss carryforwards and tax credits. Management has considered the Company’s history of cumulative net losses in the United States, estimated future taxable income and prudent and feasible tax planning strategies and has concluded that it is more likely than not that the Company will not realize the benefits of its U.S. federal and state deferred tax assets. Accordingly, a full valuation allowance has been established against these net deferred tax assets as of December 31, As of December 31, As of December 31, As of December 31, Utilization of the U.S. federal and state net operating loss and research and development credit carryforwards may be subject to a substantial annual limitation under Section 382 and Section 383 of the Internal Revenue Code of 1986, as amended, and corresponding provisions of state law, due to ownership changes that have occurred previously or that could occur in the future. These ownership changes may limit the amount of net operating loss and research and development credit carryforwards that can be utilized annually to offset future taxable income and tax liabilities, respectively. The Company has not completed a study to assess whether a change of ownership has occurred, or whether there have been multiple ownership changes since its formation. Any limitation may result in expiration of a portion of the net operating loss carryforwards or research and development tax credit carryforwards before utilization. The Company has not, as of yet, conducted a study of research and development tax credit carryforwards. Such a study, once undertaken by the Company, may result in an adjustment to the research and development tax credit carryforwards; however, a full valuation allowance has been provided against the Company’s research and development tax credits and, if an adjustment is required, this adjustment would be offset by an adjustment to the valuation allowance. Thus, there would be no impact to the balance sheet or statement of operations if an adjustment is required. The Company files tax returns in the United States, California, Virginia, and New York. The Company is subject to U.S. federal and state tax examinations by tax authorities for the tax years NOTE On Pursuant to the Purchase Agreement, the Company agreed to reduce the exercise price of certain outstanding warrants to purchase Common Stock of the Company (“Certain Outstanding Warrants”) held by the Purchaser to $4.60 per share in consideration for the The In addition, the Company agreed to pay H.C. Wainwright & Co., LLC (“Wainwright”) certain expenses and issued to Wainwright or its designees warrants (the “December Placement Agent Warrants”) to purchase up to an aggregate of 74,227 shares of Common Stock at an exercise price equal to $6.0625 per share. The December Placement Agent Warrants are exercisable immediately upon issuance and have a term of Secured Notes Amendments and On January 2, 2024, the Company and certain holders of On January 5, 2024, the Company On January 31, 2024, the Company and the Holders entered into amendments to the January 2024 Secured Notes (“Amendment No. 3 to January 2024 Secured Notes”), pursuant to which the maturity date of the January 2024 Notes was extended to February 29, 2024. In addition, on January 31, 2024, the Company and the Holders entered into amendments to the September 2024 Secured Notes (“Amendment No. 2 to September 2024 Secured Notes”), pursuant to which the Company and the Holders agreed that in consideration of a principal payment in the aggregate amount of $1.25 million on the January 2024 Secured Notes and in increase in the aggregate principal balance of $300,000 on the September 2024 Secured Notes. Pursuant to Amendment No. 3 to the January 2024 Secured Notes, the Company was required to make the Additional Consideration payment no later than February 9, 2024. As a result of the Company’s As a On February 26, 2024, the Company and the Holders entered into an Settlement Agreement On January 3, 2024, the Company entered into a Closing of MDNA Transaction On January 4, 2024 (the “Closing Date”), the Company completed its acquisition of certain assets and issued to MDNA Lifesciences, Inc. (“MDNA”): the Company’s Common Stock, the Company’s Warrants, and the Pearsanta Preferred Stock. The Company expects to account for this transaction as an asset acquisition. On January 4, 2024, the Company, Pearsanta and MDNA entered into a First Amendment to Asset Purchase Agreement (the “First Amendment to Asset Purchase Agreement”), pursuant to which the parties agreed to: (i) the removal of an upfront working capital payment, (ii) the removal of a Closing Working Capital Payment (as defined in the Purchase Agreement”), and (iii) to increase the maximum amount of payments to be made by Aditxt under the Transition Services Agreement (as defined below) from $2.2 million to $3.2 million. On January 4, 2024, Pearsanta and MDNA entered into a Transition Services Agreement (the “Transition Services Agreement”), pursuant to which MDNA agreed that it would perform, or cause certain of its affiliates or third parties to perform, certain services as described in the Transition Services Agreement for a term of three months in consideration for the payment by Pearsanta of certain fees as provided in the Transition Services Agreement, in an amount not to exceed $3.2 million. Evofem Merger Agreement and Amendments As previously reported in a Current Report on Form 8-K filed by the Company, on December 11, 2023 the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Adicure, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub”) and Evofem Biosciences, Inc., a Delaware corporation (“Evofem”), pursuant to which, Merger Sub will be merged into and with Evofem (the “Merger”), with Evofem surviving the Merger as a wholly owned subsidiary of the Company. On January 8, 2024, the Company, Adicure, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub”), and Evofem Biosciences, Inc., a Delaware corporation (“Evofem”) entered into the First Amendment (the “First Amendment to Merger Agreement”), to the Agreement and Plan of Merger (the “Merger Agreement”) pursuant to which the parties agreed to extend the date by which the joint proxy statement would be filed with the SEC until February 14, 2024. On January 30, 2024, the Company, Adicure and Evofem entered into the Second Amendment to the Merger Agreement (the “Second Amendment to Merger Agreement”) to amend (i) the date of the Parent Loan (as defined in the Merger Agreement) to Evofem to be February 29, 2024, (ii) to change the date by which Evofem may terminate the Merger Agreement for failure to receive the Parent Loan to be February 29, 2024, and (iii) to change the filing date for the Joint Proxy Statement (as defined in the Merger Agreement) to April 1, 2024. On February 29, 2024, the Company, Adicure and Evofem entered into the Third Amendment to the Merger Agreement (the “Third Amendment to Merger Agreement”) in order to (i) make certain conforming changes to the Merger Agreement regarding the Notes, (ii) extend the date by which the Company and Evofem will file the joint proxy statement until April 30, 2024, and (iii) remove the requirement that the Company make the Parent Loan (as defined in the Merger Agreement) by February 29, 2024 and replace it with the requirement that the Company make an equity investment into Evofem consisting of (a) a purchase of 2,000 shares of Evofem Series F-1 Preferred Stock for an aggregate purchase price of $2.0 million on or prior to April 1, 2024, and (b) a purchase of 1,500 shares of Evofem Series F-1 Preferred Stock for an aggregate purchase price of $1.5 million on or prior to April 30, 2024. As of the date of this filing Business Loan Agreement On January 24, 2024, the Company entered into a Business Loan and Security Agreement (the “January Loan Agreement”) with a commercial funding source (the “Lender”), pursuant to which the Company obtained a loan from the Lender in the principal amount of $3,600,000, which includes origination fees of $252,000 (the “January Loan”). Pursuant to the January Loan Agreement, the Company granted the Lender a continuing secondary security interest in certain collateral (as defined in the January Loan Agreement). The total amount of interest and fees payable by the Company to the Lender under the January Loan will be $5,364,000, which will be repayable by the Company in 30 weekly installments of $178,800. The Company received net proceeds from the January Loan of $814,900 following repayment of the outstanding balance on the October Purchased Amount of $2,533,100. Brain Scientific Assignment Agreement On January 24, 2024, the Company entered into an Assignment and Assumption Agreement (the “Brain Assignment Agreement”) with the agent (the “Agent”) of certain secured creditors (the “Brain Creditors”) of Brain Scientific, Inc., a Nevada corporation (“Brain Scientific”) and Philip J. von Kahle (the “Brain Seller”), as assignee of Brain Scientific and certain affiliated entities (collectively, the “Brain Companies”) under an assignment for the benefit of creditors pursuant to Chapter 727 of the Florida Statutes. Pursuant to the Brain Assignment Agreement, the Agent assigned its rights under that certain Asset Purchase and Settlement Agreement dated October 31, 2023 between the Seller and the Agent (the “Brain Asset Purchase Agreement”) to the Company in consideration for the issuance by the Company of an aggregate of 6,000 shares of a new series of convertible preferred stock of the Company, designated as Series B-1 Convertible Preferred Stock, $0.001 par value (the “Series B-1 Preferred Stock”). The shares of Series B-1 Preferred Stock were issued pursuant to a Securities Purchase Agreement entered into by and between the Company and each of the purchasers signatory thereto (the “Brain Purchase Agreement”). In connection with the Brain Assignment Agreement, on January 24, 2024, the Company entered into a Patent Assignment with the Brain Seller (the “Brain Patent Assignment”), pursuant to which the Seller assigned all of its rights, titles and interests in certain patents and patent applications that were previously held by the Brain Companies to the Company. Series B-1 Preferred Stock Certificate of Designation On January 24, 2024, the Company filed a Certificate of Designations for its Series B-1 Preferred Stock with the Secretary of State of Delaware. See Series B-1 Preferred Stock certificate of designation incorporated by reference to this document. Officer Promissory Notes On January 8, 2024, the Company fully repaid the November Note, First December Note, and Second December Note to Amro Albanna, the Company’s Chief Executive Officer. On February 7, 2024, Amro Albanna, the Chief Executive Officer of the Company loaned $30,000 to the Company. The loan was evidenced by an unsecured promissory note (the “February 7th Note”). Pursuant to the terms of the February 7th Note, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of August 7, 2024 or an event of default, as defined therein. On February 15, 2024, Amro Albanna, the Chief Executive Officer of the Company loaned $205,000 to the Company. The loan was evidenced by an unsecured promissory note (the “February 15th Note”). Pursuant to the terms of the February 15th Note, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of August 15, 2024 or an event of default, as defined therein. On February 29, 2024, Amro Albanna, the Chief Executive Officer of the Company, and Shahrokh Shabahang, the Chief Innovation Officer of the Company, loaned $117,000 and $115,000, respectively, to the Company. The loans were evidenced by an unsecured promissory note (the “February 29th Notes”). Pursuant to the terms of the February 29th Notes, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of August 29, 2024 or an event of default, as defined therein. Engagement Letter with Dawson James Securities, Inc. On February 16, 2024, the “Company” entered into an engagement letter (the “Dawson Engagement Letter”) with Dawson James Securities, Inc.(“Dawson”), pursuant to which the Company engaged Dawson to serve as financial advisor with respect to one or more potential business combinations involving the Company for a term of twelve months. Pursuant to the Dawson Engagement Letter, the Company agreed to pay Dawson an initial fee of $1.85 million (the “Dawson Initial Fee”), which amount is payable on the later of (i) the closing of an offering resulting in gross proceeds to the Company of greater than $4.9 million, or (ii) five days after the execution of the Dawson Engagement Letter. At the Company’s option, the Dawson Initial Fee may be paid in securities of the Company. In addition, with respect to any business combination (i) that either is introduced to the Company by Dawson following the date of the Dawson Engagement Letter or (ii) that with respect to which the Company hereafter requests Dawson to provide M&A advisory services, the Company shall compensate Dawson in an amount equal to 5% of the Total Transaction Value (as defined in the Engagement Letter) with respect to the first $20.0 million in Total Transaction Value plus 10.0% of the Total Transaction Value that is in excess of $20.0 million (the “Transaction Fee”). The Transaction Fee is payable upon the closing of a business combination transaction. Lease Default On March 6, 2024, the Company received correspondence from 532 Realty Associates, LLC (the “Landlord”) that the Company is in default under that certain Agreement of Lease dated November 3, 2021 by and between the Landlord and the Company (the “New York Lease”) for failure to pay Basic Rent and Additional Rent (as each term is defined in the New York Lease) in the aggregate amount of $40,707 (the “Past Due Rent”). Promissory Note On March 7, 2024, Sixth Borough Capital Fund, LP loaned $300,000 to the Company. The loan was evidenced by an unsecured promissory note (the “Sixth Borough Note”). Pursuant to the terms of the Sixth Borough Note, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of March 31, 2024 or an event of default, as defined therein. Appili Arrangement Agreement On April 1, 2024 (the “Execution Date”), the Company, entered into an Arrangement Agreement (the “Arrangement Agreement”) with Adivir, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Adivir” or the “Buyer”), and Appili Therapeutics, Inc., a Canadian corporation (“Appili”), pursuant to which, Adivir will acquire all of the issued and outstanding Class A common shares of Appili (the “Appili Shares”) on the terms and subject to the conditions set forth therein. The acquisition of the Appili Shares (the “Arrangement”) will be completed by way of a statutory plan of arrangement under the Canada Business Corporation Act. At the effective time of the Arrangement (the “Effective Time”), each Appili Share outstanding immediately prior to the Effective Time (other than Appili Shares held by a registered holder of Appili Shares who has validly exercised such holder’s dissent rights) will be deemed to be assigned and transferred by the holder thereof to the Buyer in exchange for (i) $0.0467 in cash consideration per share for an aggregate cash payment of $5,668,222 (the “Cash Consideration”) and (ii) 0.002745004 of a share of common stock Promissory Note On April 10, 2024, Sixth Borough Capital Fund, LP loaned $230,000 to the Company. The loan was evidenced by an unsecured promissory note (the “April Sixth Borough Note”). Pursuant to the terms of the April Sixth Borough Note, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of April 19, 2024 or an event of default, as defined therein. F-39Item 7A. Quantitative and Qualitative Disclosures About Market Risk.We are not required to provide the information required by this Item as it is a “smaller reporting company,” as defined in Rule 229.10(f)(1).Item 8. Financial Statements and Supplementary Data.See pages F-1 through F-17 following the Exhibit Index of this Annual Report on Form 10-K.Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.None.Item 9A. Controls and Procedures.Assessment of the Effectiveness of Internal Controls over Financial ReportingDisclosure Controls and ProceduresIn accordance with Rules 13a-15(b) and 15d-15(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), we, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, carried out an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) and Rule 15d-15(e) of the Exchange Act) as of the end of the period covered by this Annual Report on Form 10-K. Based on the foregoing, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were (a) designed to ensure that the information we are required to disclose in our reports under the Exchange Act is recorded, processed, and reported in an accurate manner and on a timely basis and the information that we are required to disclose in our Exchange Act reports is accumulated and communicated to management to permit timely decisions with respect to required disclosure and (b) operating in an effective manner.Change in Internal Control Over Financial ReportingNo change occurred in our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) of the Exchange Act) during the year ended December 31, 2020 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.None.Item 10. Directors, Executive Officers and Corporate GovernanceThe information required by this Item is incorporated herein by reference to the information that will be contained in our definitive proxy statement related to the 2021 Annual Meeting of Stockholders, or the Proxy Statement, which we intend to file with the SEC within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.Item 11. Executive CompensationThe information required by this Item is incorporated herein by reference to the information that will be contained in our Proxy Statement, which we intend to file with the SEC within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder MattersThe information required by this Item is incorporated herein by reference to the information that will be contained in our Proxy Statement, which we intend to file with the SEC within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.Item 13. Certain Relationships and Related Transactions, and Director IndependenceThe information required by this Item is incorporated herein by reference to the information that will be contained in our Proxy Statement, which we intend to file with the SEC within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K. Year
Ended
December 31,
2023 Year
Ended
December 31,
2022 Audit Fees (a) $ 125,735 $ 111,250 Tax Fees (b) - - Other Fees (c) 33,325 7,400 Total $ 161,083 $ 120,672 (a) Audit fees represent fees for professional services provided in connection with the audit of the Company’s annual financial statements and the review of its financial statements included in the Company’s Quarterly Reports on Form 10-Q and services that are normally provided in connection with statutory or regulatory filings. The information required by this Item is incorporated herein by reference to the information that will be contained in our Proxy Statement, which we intend to file with the SEC within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.(b) Tax fees represent fees for professional services related to tax compliance, tax advice and tax planning. (c) Other fees represent fees related to our filing of certain Registration Statements. 42(a) (a)The following documents are filed as part of this report: (1) (1)Financial Statements: (2) (2)Financial Statement Schedules: (3) (3)Exhibits. 2516th day of March 2021.April 2024.Aditx Therapeutics,Aditxt, Inc.By: /s/ Amro Albanna Name: Amro Albanna Title: Chief Executive Officer Signature Title Date /s/ Amro Albanna Chief Executive Officer President, and Director March 25, 2021April 16, 2024Amro Albanna (Principal Executive Officer) /s/ Corinne PankovcinThomas J. Farley Chief Financial Officer March 25, 2021April 16, 2024Corinne PankovcinThomas J. Farley (Principal Financial and Accounting Officer) /s/ Thomas J. FarleyBrian Brady ControllerDirector March 25, 2021April 16, 2024Thomas J. Farley(Principal Accounting Officer)/s/ Laura E. AnthonyDirectorMarch 25, 2021Laura E. AnthonyBrian Brady /s/ Brian BradyCharles Nelson Director March 25, 2021April 16, 2024Brian BradyCharles Nelson /s/ Namvar KiaieJeffrey W. Runge, M.D. Director March 25, 2021April 16, 2024Namvar KiaieJeffrey W. Runge, M.D. /s/ Jeffrey W. Runge, M.D.DirectorMarch 25, 2021Jeffrey W. Runge, M.D./s/ Shahrokh Shabahang Chief Innovation Officer and Director March 25, 2021April 16, 2024Shahrokh Shabahang ADITX THERAPEUTICS,20202023 AND 20192022Aditx Therapeutics,Aditxt, Inc.Aditx Therapeutics,Aditxt, Inc. and its subsidiaries (the “Company”) as of December 31, 20202023 and 2019,2022, the related consolidated statements of operations, stockholders’ equity, (deficit), and cash flows, for the years ended December 31, 20202023 and 2019,2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 20202023 and 2019,2022, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.dbbmckennondbbmckennonNewport Beach, CaliforniaMarch 25, 2021 December 31, December 31, 2020 2019 ASSETS CURRENT ASSETS Cash and cash equivalents $ 10,500,826 $ 4,090 Prepaid expenses 147,642 - ROU asset - short term 384,685 - TOTAL CURRENT ASSETS 11,033,153 4,090 Fixed Assets 798,919 - Intangible Assets 321,000 - Deferred offering costs - 119,442 ROU asset - long term 871,136 - Deposits 72,296 - TOTAL ASSETS $ 13,096,504 $ 123,532 LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) CURRENT LIABILITIES Accounts payable and accrued expenses $ 241,613 $ 1,847,458 Accrued compensation to related parties - 962,651 Notes payable - related party - 10,000 Notes payable, net of discount - 155,600 Financing on fixed assets 587,588 - Lease liability - short term 391,221 - Other current assets 6,536 - TOTAL CURRENT LIABILITIES 1,226,958 2,975,709 Lease liability - long term 858,064 - TOTAL LIABILITIES 2,085,022 2,975,709 COMMITMENTS AND CONTINGENCIES STOCKHOLDERS’ EQUITY (DEFICIT) Preferred stock, $0.001 par value, 3,000,000 shares authorized, no shares issued and outstanding - - Common stock, $0.001 par value, 27,000,000 shares authorized, 13,074,495 and 3,915,900 shares issued and 12,973,692 and 3,821,088 shares outstanding, respectively 13,078 3,916 Treasury stock, 100,803 and 94,813 shares, respectively (201,605 ) (189,625 ) Additional paid-in capital 32,079,187 9,063,483 Accumulated deficit (20,879,178 ) (11,729,951 ) TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) 11,011,482 (2,852,177 ) TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) $ 13,096,504 $ 123,532 December 31, December 31, 2023 2022 ASSETS CURRENT ASSETS: Cash $ 97,102 $ 2,768,640 Accounts receivable, net 408,326 527,961 Inventory 745,502 950,093 Prepaid expenses 217,390 496,869 Subscription receivable 5,444,628 - TOTAL CURRENT ASSETS 6,912,948 4,743,563 Fixed assets, net 1,898,243 2,318,863 Intangible assets, net 9,444 107,000 Deposits 106,410 355,366 Right of use asset - long term 2,200,299 3,160,457 Deferred issuance costs - 50,000 Investment in Evofem - Deposit on acquisition - TOTAL ASSETS $ 44,578,327 $ 10,735,249 LIABILITIES AND STOCKHOLDERS’ EQUITY CURRENT LIABILITIES: Accounts payable and accrued expenses $ 8,554,959 $ 1,958,502 Notes payable - related party 375,000 - Notes payable, net of discount 15,653,477 - Financing on fixed assets 147,823 409,983 Deferred rent 158,612 188,581 Lease liability - current 999,943 1,086,658 TOTAL CURRENT LIABILITIES 25,889,814 3,643,724 Settlement liability 1,600,000 - Lease liability - long term 1,041,744 1,885,218 TOTAL LIABILITIES 28,531,558 5,528,942 STOCKHOLDERS’ EQUITY Preferred stock, $0.001 par value, 3,000,000 shares authorized, zero shares issued and outstanding, respectively - - Series A-1 Convertible Preferred stock, $0.001 par value, 22,280 shares authorized, 22,280 and zero shares issued and outstanding, respectively 22 - Series B Preferred stock, $0.001 par value, 1 share authorized, zero and zero shares issued and outstanding, respectively - - Series B-2 Convertible Preferred stock, $0.001 par value, 2,625 shares authorized, 2,625 and zero shares issued and outstanding, respectively 3 - Series C Preferred stock, $0.001 par value, 1 share authorized, zero and zero shares issued and outstanding, respectively - - Common stock, $0.001 par value, 100,000,000 shares authorized, 1,318,969 and 107,698 shares issued and 1,318,918 and 107,647 shares outstanding, respectively 1,319 108 Treasury stock, 51 and 51 shares, respectively (201,605 ) (201,605 ) Additional paid-in capital 143,997,710 100,448,166 Accumulated deficit ) (95,040,362 ) TOTAL ADITXT, INC. STOCKHOLDERS’ EQUITY 5,206,307 NON-CONTROLLING INTEREST ) - TOTAL STOCKHOLDERS’ EQUITY 16,046,769 5,206,307 TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY $ 44,578,327 $ 10,735,249 For the Year Ended For the Year Ended December 31,
2020 December 31,
2019 OPERATING EXPENSES General and administrative expenses, $3,188,840 and $4,221,733 in stock-based compensation $ 7,852,256 $ 5,694,806 Research and development, includes $0 and $10,000 in stock-based compensation 937,966 175,441 Sales and marketing $0 and $0 in stock-based compensation 81,987 551 Total Operating Expenses 8,872,209 5,870,798 LOSS FROM OPERATIONS (8,872,209 ) (5,870,798 ) OTHER INCOME (EXPENSE) Interest expense (10,081 ) (1,930 ) Interest income 563 - Gain on forgiveness of debt 32,500 45,000 Amortization of debt discount (300,000 ) - Total Other Income (Expense) (277,018 ) 43,070 NET LOSS $ (9,149,227 ) $ (5,827,728 ) Net loss per share - basic and diluted $ (1.33 ) $ (1.52 ) Weighted average number of shares outstanding during the period - basic and diluted 6,902,696 3,830,971 Year
Ended Year
Ended December 31,
2023 December 31,
2022 REVENUE Sales $ 645,176 $ 933,715 Cost of goods sold 756,836 766,779 Gross profit (loss) (111,660 ) 166,936 OPERATING EXPENSES General and administrative expenses $1,133,077, and $1,516,805 in stock-based compensation, respectively 18,607,142 15,985,552 Research and development, includes $262,154, and $591,518 in stock-based compensation, respectively 7,074,339 7,268,084 Sales and marketing $6,787, and $1,023,045 in stock-based compensation, respectively 269,284 1,849,460 Impairment on notes receivable - 543,938 Total operating expenses 25,950,765 25,647,034 NET LOSS FROM OPERATIONS (26,062,425 ) (25,480,098 ) OTHER EXPENSE Interest expense (4,195,127 ) (753,038 ) Interest income 10,166 57,348 Other income - 58,960 Amortization of debt discount (2,194,773 ) (1,533,048 ) Gain on note exchange agreement 51,712 - Total other expense (6,328,022 ) (2,169,778 ) Net loss before income taxes (32,390,447 ) (27,649,876 ) Income tax provision - - NET LOSS $ (32,390,447 ) $ (27,649,876 ) NET LOSS ATTRIBUTABLE TO NON-CONTROLLING INTEREST ) - NET LOSS ATTRIBUTABLE TO ADITXT, INC. & SUBSIDIARIES $ ) $ (27,649,876 ) Deemed Dividend (319,871 ) (37,667 ) NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS $ ) $ ) Net loss per share - basic and diluted $ ) $ ) Weighted average number of shares outstanding during the period - basic and diluted 302,356 46,369 (DEFICIT)FOR THE YEARS ENDED DECEMBER 31, 20202023 AND 20192022 Preferred Treasury Additional Total Common Preferred Shares Shares Paid-in Accumulated Stockholders’ Shares Par Shares Par Value Capital Deficit Deficit Balance December 31, 2018 3,763,925 $ 3,764 - $ - $ - $ 4,361,725 $ (5,902,223 ) $ (1,536,734 ) Issuance of shares for cash, net of issuance costs 131,475 131 - - - 470,046 - 470,177 Issuance of shares for services and licenses 20,500 21 - - - 81,979 - 82,000 Stock option and warrant compensation - - - - - 3,616,422 - 3,616,422 Warrants issued with notes - - - - - 533,311 - 533,311 Treasury stock (94,813 ) - - - (189,625 ) - - (189,625 ) Net loss - - - - - - (5,827,728 ) (5,827,728 ) Balance December 31, 2019 3,821,087 $ 3,916 - $ - $ (189,625 ) $ 9,063,483 $ (11,729,951 ) $ (2,852,177 ) Treasury stock (5,990 ) - - - (11,980 ) - - (11,980 ) Rounding adjustment from stock split (10 ) (1 ) - - - - - (1 ) Exercise of warrants 4,297,703 4,300 - - - 206,244 - 210,544 Stock option and warrant compensation - - - - - 711,406 - 711,406 Issuance of shares for services 874,916 876 - - - 2,476,558 - 2,477,434 Issuance of shares for intangible assets 150,000 150 - - - 320,850 - 321,000 Issuance of shares and warrants for the settlement of accrued compensation and accounts payable 146,818 147 - - - 1,221,878 - 1,222,025 Issuance of shares and warrants for IPO, net of issuance costs 1,226,668 1,227 - - - 9,429,455 - 9,430,682 Issuance of shares and warrants for follow-on offering, net of issuance costs 1,150,000 1,150 1,250,000 1,250 - 8,524,376 - 8,526,776 Issuance of shares and warrant for the settlement of debt 62,500 63 - - - 124,937 - 125,000 Exercise conversion of preferred shares 1,250,000 1,250 (1,250,000 ) (1,250 ) - - - - Net loss - - - - - - (9,149,227 ) (9,333,049 ) Balance December 31, 2020 12,973,692 $ 13,078 - $ - $ (201,605 ) $ 32,079,187 $ (20,879,178 ) $ 11,011,482 Preferred
Shares
Outstanding Preferred
Shares
Par Preferred A-1
Shares
Outstanding Preferred A-1
Shares
Par Preferred B
Shares
Outstanding Preferred B
Shares
Par Preferred B-2
Shares
Outstanding Preferred B-2
Shares
Par Preferred C
Shares
Outstanding Preferred C
Shares
Par Common
Shares
Outstanding Common
Shares
Par Treasury
Stock Additional
Paid-in
Capital Accumulated
Deficit
Controlling Interest Total
Stockholders’
Equity Balance December 31, 2022 - - $ - - - $ - - $ - - $ - 107,647 $ 108 $ (201,605 ) $ 100,448,166 $ (95,040,362 ) $ - $ 5,206,307 Stock option compensation - - - - - - - - - - - - - 589,014 - - 609,014 Restricted stock unit compensation - - - - - - - - - - - - - 308,479 - - 308,479 Issuance of restricted stock units for compensation - - - - - - - - - - 157 2 - (2 ) - - - Sale of common stock - - - - - - - - - - 8,463 9 - 507,007 - - 507,016 Issuance of shares for services - - - - - - - - - - 74,675 75 - 484,450 - - 484,525 Issuance of shares of Pearsanta Common Stock for IP - - - - - - - - - - - - - 10,000 - - 10,000 Warrants issued for cash, net of issuance costs - - - - - - - - - - - - - 1,581,467 - - 1,581,467 Exercise of warrants - - - - - - - - - - 1,055,374 1,057 - (57 ) - - 1,000 Sale of Series C Preferred shares to related party - - - - - - - - 1 - - - - 1,000 - - 1,000 Issuance of shares for debt issuance costs - - - - - - - - - - 31,251 32 - 354,806 - - 354,838 Issuance of warrants for offering, net of issuance costs - - - - - - - - - - - - - 14,411,028 - - 14,411,028 Modification of warrants - - - - - - - - - - - - - 319,871 (319,871 ) - - Redemption of Series C Preferred shares to related party - - - - - - - - (1 ) - - - - (1,000 ) - - (1,000 ) Series A-1 Preferred shares issued for exchange agreement - - 22,280 22 - - - - - - - 22,277,211 - - 22,277,233 Note exchange agreement - - - - - - 2,625 3 - - - - - 2,686,306 - - 2,686,309 Rounding from reverse stock split - - - - - - - - 41,351 36 - (36 ) - - - Net loss -- - - - - - - - - - - - - - ) ) (32,390,447 ) Balance December 31, 2023 - - $ 22,280 22 - $ - 2,625 $ 3 - $ - 1,318,918 $ 1,319 $ (201,605 ) $ 143,997,710 $ ) $ ) $ 16,046,769 CASH FLOWSSTOCKHOLDERS’ EQUITY For the Year Ended For the Year Ended December 31,
2020 December 31,
2019 CASH FLOWS FROM OPERATING ACTIVITIES: Net loss $ (9,149,227 ) $ (5,827,728 ) Adjustments to reconcile net loss to net cash used in operating activities Stock-based compensation 3,188,840 3,698,422 Depreciation expense 17,773 - Amortization of debt discount 300,000 - Modification of options - 533,311 Changes in operating assets and liabilities: Deposits (72,296 ) - Prepaid expenses (147,642 ) - Accounts payable and accrued expenses (1,483,180 ) 601,607 Accrued compensation to related parties 124,728 530,036 Net Cash Used In Operating Activities (7,221,004 ) (464,352 ) CASH FLOWS FROM INVESTING ACTIVITIES Fixed Assets (170,629 ) - Net Cash Used In Investing Activities (170,629 ) - CASH FLOWS FROM FINANCING ACTIVITIES: Proceeds from note payable - related party - 10,000 Proceeds from note payable 375,000 50,000 Repayments of note payable - related party (45,000 ) (42,502 ) Repayments of notes payable (670,600 ) (15,500 ) Common stock and warrants issued for cash, net of issuance costs 18,500,039 470,177 Offering costs (423,139 ) (119,442 ) Exercise of warrants 210,544 - Financed Asset (58,475 ) - Net Cash Provided By Financing Activities 17,888,369 352,733 NET INCREASE (DECREASE) IN CASH 10,496,736 (111,619 ) CASH AT BEGINNING OF PERIOD 4,090 115,709 CASH AT END OF PERIOD $ 10,500,826 $ 4,090 Supplemental cash flow information: Cash paid for income taxes $ - $ - Cash paid for interest expense $ 5,842 $ - NONCASH INVESTING AND FINANCING ACTIVITIES: Liabilities assumed for common stock $ 11,980 $ 189,625 Issuance of Units for the settlement of notes payable $ 125,000 $ - Issuance of units for the settlement of accrued compensation and accounts payable $ 1,222,025 $ - Original issuance discount on notes payable $ 300,000 $ - Lease liability recognized from right of use asset $ 1,191,985 $ - Liability recognized for financed assets $ 646,063 $ - Shares issued for intangible assets $ 321,000 $ - Conversion of preferred shares $ 1,250 $ - Preferred
Shares
Outstanding Preferred
Shares
Par Preferred A-1
Shares
Outstanding Preferred A-1
Shares
Par Preferred B
Shares
Outstanding Preferred B
Shares
Par Preferred B-2
Shares
Outstanding Preferred B-2
Shares
Par Preferred C
Shares
Outstanding Preferred C
Shares
Par Common
Shares
Outstanding Common
Shares
Par Treasury
Stock Additional
Paid-in
Capital Accumulated
Deficit Total
Stockholders’
Equity Balance December 31, 2021 - $ - - $ - - $ - - $ - - $ - 22,220 $ 22 $ (201,605 ) $ 77,735,165 $ (67,352,809 ) $ $ 10,180,773 Stock option and warrant compensation - - - - - - - - - - - - - 1,413,904 - - 1,413,904 Issuance of shares for vested restricted stock units - - - - - - - - - - 463 4 - 1,209,902 - - 1,209,906 Issuance of shares for services - - - - - - - - - - 3,707 5 - 507,553 - - 507,558 Exercise of warrants, modification of warrants, and issuance of warrants - - - - - - - - - - 4,486 5 - 1,203,764 - - 1,203,769 Sale of Series B Preferred shares to related party - - - - 1 - - - - - - - - 20,000 - - 20,000 Redemption of Series B Preferred shares to related party - - - - (1 ) - - - - - - - - (20,000 ) - - (20,000 ) Shares issued as inducement on loans, net of issuance costs - - - - - - - - - - 1,195 2 - 146,520 - - 146,522 Warrants issued with loans - - - - - - - - - - - - - 878,622 - - 878,622 Reset provision on warrants and modification of warrants - - - - - - - - - - - - - 37,677 (37,677 ) - - Issuance of shares for debt issuance costs - - - - - - - - - - 262 1 - 96,029 - - 96,030 Exercise of warrants - - - - - - - - - - 44,173 45 - (45 ) - - - Issuance of shares and warrants for offering, net of issuance costs - - - - - - - - - - 30,609 31 - 17,232,276 - - 17,232,307 Issuance costs related to exercise of warrants, modification of warrants, and issuance of warrants - - - - - - - - - - - - - (94,195 ) - - (94,195 ) Issuance of shares for settlement of AP - - - - - - - - - - 231 1 - 79,999 - - 80,000 Rounding from reverse stock split - - - - - - - - - - 301 (8 ) - (5 ) - - (13 ) Net loss - - - - - - - - - - - - - - (27,649,876 ) - (27,649,876 ) Balance December 31, 2022 - $ - - $ - - $ - - $ - - $ - 107,647 $ 108 $ (201,605 ) $ 100,448,166 $ (95,040,362 ) $ - $ 5,206,307 Year
Ended Year
Ended December 31,
2023 December 31,
2022 CASH FLOWS FROM OPERATING ACTIVITIES: Net loss $ (32,390,447 ) $ (27,649,876 ) Adjustments to reconcile net loss to net cash used in operating activities Stock-based compensation 1,402,018 3,131,368 Depreciation expense 435,027 428,977 Amortization of intangible assets 107,556 107,000 Amortization of debt discount 2,821,629 1,533,048 Impairment on notes receivable - 543,938 Disposal of fixed assets - 6,976 Gain on note exchange agreement (51,712 ) - Changes in operating assets and liabilities: Accounts receivable 119,635 (438,117 ) Prepaid expenses 279,479 (36,767 ) Deposits 248,956 23,884 Inventory 204,591 (455,396 ) Accounts payable and accrued expenses 6,646,457 412,959 Settlement liability 1,600,000 - Net cash used in operating activities (18,576,811 ) (22,392,006 ) CASH FLOWS FROM INVESTING ACTIVITIES: Purchase of fixed assets (14,407 ) (367,079 ) Tenant improvement allowance receivable - 125,161 Net cash used in investing activities (14,407 ) (241,918 ) CASH FLOWS FROM FINANCING ACTIVITIES: Proceeds from notes - related party 1,062,523 80,000 Proceeds from notes and convertible notes payable, net of offering costs 7,903,445 2,795,000 Repayments of note payable - related party (687,523 ) (80,000 ) Repayments of note payable (3,152,488 ) (3,206,887 ) Sale of Series B Preferred shares to related party - 20,000 Redemption of Series B Preferred shares to related party - (20,000 ) Common stock and warrants issued for cash, net of issuance costs 11,054,883 17,233,307 Sale of Series C Preferred shares to related party 1,000 - Redemption of Series C Preferred shares to related party (1,000 ) - Exercise of warrants, modification of warrants, and issuance of warrants 1,000 1,109,574 Payments on financing on fixed asset (262,160 ) (400,491 ) Net cash provided by financing activities 15,919,680 17,530,503 NET INCREASE (DECREASE) IN CASH (2,671,538 ) (5,103,421 ) CASH AT BEGINNING OF YEAR 2,768,640 7,872,061 CASH AT END OF YEAR $ 97,102 $ 2,768,640 Supplemental cash flow information: Cash paid for income taxes $ - $ - Cash paid for interest expense $ 2,726,020 $ 753,038 Issuance of shares for the settlement of accounts payable $ - $ 80,000 Debt discount from warrants issued with convertible note payable $ - $ 878,622 Debt discount from shares issued as inducement for note payable $ - $ 146,522 Shares issued for debt offering costs $ 354,838 $ 96,030 Warrant modification $ 319,871 $ 37,677 Deferred issuance costs $ - $ 50,000 Issuance of shares of Pearsanta Common Stock for IP $ 10,000 $ - Assumption of notes payable from Evofem merger agreement $ 11,173,750 $ - Series A-1 Preferred shares issued for exchange agreement $ 22,277,233 $ - Accrued intertest rolled into notes payable $ 701,315 $ - Series B-2 Preferred shares issued in note exchange agreement $ $ - Aditx Therapeutics, Inc. (“Aditxt” or the “Company”) was incorporated in the State of Delaware on September 28, 2017 and our headquartersWe are located in Mountain View, CA. The Company is a Biotech Innovationbiotech innovation company with a mission of prolonging life and enhancing its quality by improving the health of the immune system.biotechnologies specifically focusedand commercializing technologies with a focus on improving the health ofmonitoring and modulating the immune system through immune reprogramming and monitoring.system. Our immune reprogramming technologies are currently at the pre-clinical stage and are designed to retrain the immune system to induce tolerance with an objective of addressing rejection of transplanted organs, autoimmune diseases, and allergies. Our immune monitoring technologies are designed to provide a personalized comprehensive profile of the immune system and we plan to utilize them in our upcoming reprogramming clinical trials to monitor subjects’ immune response before, during and after drug administration.OfferingsOn January 1, 2023, the Company formed Adimune, Inc., a Delaware wholly owned subsidiary.July 2, 2020,January 1, 2023, the Company formed Pearsanta, Inc., a Delaware majority owned subsidiary.its initial publica registered direct offering (“IPO”August 2021 Offering”). In connection therewith, the Company issued 1,226,668 Units (the “Units”), excluding the underwriters’ option to cover overallotments,2,292 shares of common stock, at an offeringa purchase price of $9.00$4,800.00 per Unit,share, resulting in gross proceeds of approximately $11.0 million. The Units issued in the IPO consisted of one share of common stock, one Series A warrant, and one Series B warrant. The Series A warrants originally had an exercise price of $9.00 andIn a term of 5 years. In addition,concurrent private placement, the Company issued a Unit Purchase Option at an exercise price of $11.25 per unit to the underwriterswarrants to purchase up to 67,466 units, with each unit consisting of (i) one share of common stock and (ii) one Series A Warrant. On August 19, 2020 the Company modified the exercise price of the Series A Warrants from $9.00 per share to $4.50 per share.2,292 shares. The term of the Series A Warrants was not modified. The Series B warrants have an exercise price of $11.25 per share, a term of 5 years and contain a cashless exercise option upon certain criteria being met. As of December 31, 2020, substantially all of the Series B warrants issued in the IPO have been exercised pursuant to a cashless provision therein.On September 10, 2020, the Company completed a follow-on public offering (“September 2020 Offering”). In connection therewith, the Company issued 2,400,000 Units (the “Follow-On Units”), excluding the underwriters’ option to cover overallotments, at an offering price of $4.00 per Follow-On Unit, resulting in gross proceeds to the Company of approximately $9.6 million. The Follow-On Units issued in the September 2020 Offering consisted of one share of common stock (or Series A Preferred Stock for investors who would own more than 4.99% of the Company if they invested in common stock), one Series A-1 warrant, and one Series B-1 warrant. The Series A-1 warrants have an exercise price of $3.19$5,060.00 per share and are exercisable for a termfive-year period commencing months from the date of 5 years.issuance. The Series B-1 warrants have exercise price of $5.00 per share, a term of 5 years and contain a cashless exercise option upon certain criteria being met.was subsequently repriced to $3,000.00. In addition, the Company issued a warrant to the underwritersplacement agent to purchase up to 60,000115 shares of common stock at an exercise price of $5.00$6,000.00 per share. Subsequentquarter end, substantially allthe public offering (the “October 2021 Offering”) of 1,417 shares of the Series B-1Company’s common stock (the “Shares”) by the Company. The Shares were offered, issued, and sold at a price to the public of $3,000.00 per share under a prospectus supplement and accompanying prospectus filed with the SEC pursuant to an effective shelf registration statement filed with the SEC on Form S-3 (File No. 333-257645), which was declared effective by the SEC on July 13, 2021. The October 2021 Offering closed on October 20, 2021 for gross proceeds of $4.25 million. The Company utilized a portion of the proceeds, net of underwriting discounts of approximately $3.91 million from the October 2021 Offering to fund certain obligations of the Company.issuedhad an exercise price of $0.04. On June 15, 2022, the Company entered an agreement with a holder of certain warrants in the December 2021 Offering. (See Note 10)202020, 2022, the Company completed a public offering for net proceeds of $17.2 million (the “September 2022 Offering”). As part of the September 2022 Offering, have been exercisedwe issued 30,608 of shares of the Company’s common stock, pre-funded warrants to purchase 52,725 shares of common stock, and warrants to purchase 83,333 shares of the Company’s common stock. The warrants had an exercise price of $240.00 and the pre-funded warrants had an exercise price of $0.04.cashless provision therein.purchase price of $48.76 per April Pre-Funded Warrant. The April Pre-Funded Warrants (and shares of common stock underlying the April Pre-Funded Warrants) were offered by the Company pursuant to its shelf registration statement on Form S-3 (File No. 333-257645), which was declared effective by the Securities and Exchange Commission on July 13, 2021. Concurrently with the sale of the April Pre-Funded Warrants, pursuant to the Purchase Agreement in a concurrent private placement, for each April Pre-Funded Warrant purchased by the investor, such investor received from the Company an unregistered warrant (the “Warrant”) to purchase two shares of Common Stock. The warrants have an exercise price of $34.40 per share, and are exercisable for a three year period. In addition, the Company issued a warrant to the placement agent to purchase up to 2,378 shares of common stock at an exercise price of $61.00 per share. The closing of the sales of these securities under the April Purchase Agreement took place on April 24, 2023. The gross proceeds from the offering were approximately $1.9 million, prior to deducting placement agent’s fees and other offering expenses payable by the Company.has not generatedis in the very early stages of generating revenue from intended operations. The Company’s business and operations are sensitive to general business and economic conditions in the U.S. and worldwide along with local, state, and federal governmental policy decisions. A host of factors beyond the Company’s control could cause fluctuations in these conditions. Adverse conditions may include: changes in the biotechnology regulatory environment, technological advances that render our technologies obsolete, availability of resources for clinical trials, acceptance of technologies into the medical community, and competition from larger, more well-funded companies. These adverse conditions could affect the Company’s financial condition and the results of its operations.On January 30, 2020, the World Health Organization declared the COVID-19 novel coronavirus outbreak a “Public Health Emergency of International Concern” and on March 10, 2020, declared it to be a pandemic. Actions taken around the world to help mitigate the spread of the coronavirus include restrictions on travel, and quarantines in certain areas, and forced closures for certain types of public places and businesses. The COVID-19 coronavirus and actions taken to mitigate it have had and are expected to continue to have an adverse impact on the economies and financial markets of many countries, including the geographical area in which the Company operates. While it is unknown how long these conditions will last and what the financial impact will be to the Company, it is reasonably possible that future capital raise efforts and additional development of our technologies may be negatively affected.2020,2023, the Company had a net loss of $9,149,227$32,390,447 and negative cash flow from operating activities of $10,500,826. $18,576,811. As of December 31, 2023, the Company’s cash balance was $97,102.will be conducting medical research and development, andcontinues to actively pursue numerous capital raising transactions with the time at which the Company will begin generating revenue is unknown. The Company believes, however, that the funds raised by the IPO and the September 2020 Offering will beobjective of obtaining sufficient bridge funding to fundmeet the Company’s operation for at leastexisting capital needs as well as more substantial capital raises to meet the next 12 months.Company’s longer-term needs.alleviates issues in connectioncreates substantial doubt with the Company’s ability to continue as a going concern. The accompanying financial statements have been prepared assuming thatwill continue ashas the ability to raise capital from equity or debt through private placements or public offerings pursuant to a going concern.registration statement on Form S-1. We may also secure loans from related parties.While we believe in the viability of our strategy to generate sufficient revenue, control costs and raise additional funds when necessary, there can be no assurances to that effect. The Company’s ability to continue as a going concern is dependent upon the ability to complete clinical studies and implement the business plan, generate sufficient revenues and to control operating expenses. In addition, the Company is consistently focused on raising capital, strategic acquisitions and alliances, and other initiatives to strengthen the Company.Level 1 - Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. Level 2 - Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. Level 3 - Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. Level 1 - Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.Level 2 - Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data.Level 3 - Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. Depreciation expense was $17,773 for the year ended December 31, 2020 and zero for the year ended for December 31, 2019. Accumulated depreciation was $17,773 as of December 31, 2020 and zero as of December 31, 2019. None of the Company’sserveare as collateral against any loans as of December 31, 2020 and 2019, other than those subject to the financed asset liability.follows:Computers Three years to five years Lab Equipment Seven to ten years Office Furniture Five to ten years Other fixed assets Five to ten years Leasehold Improvements Shorter of estimated useful life or remaining lease term definitefinite lives, the assets are amortized using the straight-line method over the estimated useful lives of the related assets. For intangible assets with indefinite lives, the assets are tested periodically for impairment. Amortization expense was zero for the year ended December 31, 2020 and 2019.Offering CostsInvestmentsCompany accounts for offering costsfollowing table sets forth a summary of the changes in equity investments. This investment has been recorded at cost in accordance with ASC 340, Other Assets321. For the year
ended
December 31,
2023 As of December 31, 2022 - Purchase of equity investments 22,711,211 Unrealized gains - As of December 31, 2023 $ 22,711,221 Costs. Priortax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. At December 31, 2023 and December 31, 2022, the Company had a full valuation allowance against its deferred tax assets.1) Identify the contract with a customer 2) Identify the performance obligations in the contract 3) Determine the transaction price 4) Allocate the transaction price to performance obligations in the contract 5) Recognize revenue when or as the Company satisfies a performance obligation completionAditxtScore™ are recognized when the AditxtScoreTM report is delivered to the customer. The services performed include the analysis of an offering, offering costs were capitalized as deferred offering costsspecimens received in the Company’s CLIA laboratory and the generation of results which are then delivered upon completion.balance sheet. The deferred offering costsallowance for credit losses for client payers based on historical collection experience and the period of time the receivable has been outstanding.netted againstbilled based on established patient fee schedules or fees negotiated with physicians on behalf of their patients. Collection of billings is subject to credit risk and the proceedsability of the offering in stockholders’ equity (deficit) orpatients to pay.related debt, as applicable. Costs relatedestimated consideration the Company expects to unsuccessful offerings are expensed.receive from such payers, collection experience, and the terms of the Company’s contractual arrangements.adopted in 2020 with no impact related to adoption,(Leases), operating lease expense is generally recognized evenly over the term of the lease. The Company has operating leases consisting of office andspace, laboratory space, with remaining lease terms of 46 months. Rent and Lease costs were $154,263 and $30,362 for the year ended December 31, 2020 and 2019. There was no sublease rental income for the year ended December 31, 2020 and 2019.lab equipment.For lease agreements entered into or reassessed after the adoption of Topic 842, weWe combine the lease and non-lease components in determining the lease liabilities and right of use (“ROU”) assets.Our lease agreements generally do not provide an implicit borrowing rate, therefore an internal incremental borrowing rate is determined based on information available at lease commencement date for purposes of determining the present value of lease payments. We used the incremental borrowing rate on December 31, 2020 and 2019 for all leases that commenced prior to that date. In determining this rate, which is used to determine the present value of future lease payments, we estimate the rate of interest we would pay on a collateralized basis, with similar payment terms as the lease and in a similar economic environment.Lease Costs Year Ended
December 31,
2020 Year Ended
December 31,
2019 Components of total lease costs: Operating lease expense $ 154,263 $ - Total lease costs $ 154,263 $ - Lease Positions as of December 31, 2020ROU lease assets and lease liabilities for our operating leases were recorded in the balance sheet as follows: December 31,
2020 December 31,
2019 Assets Right of use asset – short term $ 384,685 $ - Right of use asset – long term 871,136 - Total assets $ 1,255,821 $ - Liabilities Operating lease liabilities – short term $ 391,221 $ - Operating lease liabilities – long term 858,064 - Total lease liability $ 1,249,285 $ - Lease Terms and Discount RateWeighted average remaining lease term (in years) – operating lease2.67Weighted average discount rate – operating lease8.00%The future annual minimum lease payments as of December 31, 2020 are as follows:2021 $ 406,640 2022 411,753 2023 363,416 2024 215,551 Total future minimum lease payments 1,397,360 Less: Lease imputed interest 148,075 Total $ 1,249,285 Stock basedStock-based compensation expense recognized includes the compensation cost for all stock-based payments granted to employees, officers, and directors based on the grant date fair value estimated in accordance with the provisions of ASC 718. ASC.ASC 718 is also applied to awards modified, repurchased, or cancelled during the periods reported. Stock-based compensation is recognized as expense over the employee’s requisite vesting period and over the nonemployee’s period of providing goods or services.yearyears ended December 31, 20202023 and 2019,2022, the Company had aincurred patent licensing fee for the patentsfees of $258,635$123,541 and $18,396,$263,273, respectively.Our research and development costs mainly consist of licensing costs. We expense these costs as incurred unless such costs qualify for capitalization under applicable guidance. During the years ended December 31, 2023 and 2022, the Company incurred research and development costs of $7,074,339 and $7,268,084, respectively.2020, 2,143,0002023, 45,572 stock options, 0 unvested restricted stock units, 5,047,451 warrants, 22,280 shares of preferred series A-1 stock, and 5,799,146 warrants2,625 shares of preferred series B-2 stock were excluded from dilutive earnings per share as their effects were anti-dilutive. As of December 31, 2019, 1,102,5002022, 1,105 stock options, 180 unvested restricted stock units, and 1,382,478127,251 warrants were excluded from dilutive earnings per share as their effects were anti-dilutive. Cost Basis Accumulated
Depreciation Net Computers $ 378,480 $ (320,473 ) $ 58,007 Lab Equipment 2,585,077 (859,612 ) 1,725,465 Office Furniture 56,656 (13,866 ) 42,790 Other Fixed Assets 8,605 (2,084 ) 6,521 Leasehold Improvements 120,440 (54,980 ) 65,460 Total Fixed Assets $ 3,149,258 $ (1,251,015 ) $ 1,898,243 Cost Basis Accumulated
Depreciation Net Computers $ 376,429 $ (197,907 ) $ 178,522 Lab Equipment 2,572,720 (579,015 ) 1,993,705 Office Furniture 56,656 (8,200 ) 48,456 Other Fixed Assets 8,605 (1,224 ) 7,381 Leasehold Improvements 120,440 (29,641 ) 90,799 Total Fixed Assets $ 3,134,850 $ (815,987 ) $ 2,318,863 45 – INTANGIBLE ASSETS Cost Basis Accumulated
Amortization Net Proprietary Technology $ 321,000 $ (321,000 ) $ - Intellectual property 10,000 556 9,444 Total Intangible Assets $ 321,000 $ (321,556 ) $ 9,444 Cost Basis Accumulated
Amortization Net Proprietary Technology $ 321,000 $ (214,000 ) $ 107,000 Total Intangible Assets $ 321,000 $ (214,000 ) $ 107,000 Company’s Chief Executive Officer (“CEO”) has provided certain periodsCompany recognized $146,613 in stock-based compensation for the issuance of service without payment. As of December 31, 2020these vested and 2019, the CEO is owed $0 and $309,500, respectively, related to compensation. Duringunvested restricted stock units during the year ended December 31, 2020,2022. (Note 11)issued 38,055 Units consistingentered into a Subscription and Investment Representation Agreement with its Chief Executive Officer (the “Purchaser”), pursuant to which the Company agreed to issue and sell one (1) share of onethe Company’s Series B Preferred Stock (the “Series B Preferred Stock”), par value $0.001 per share, to the Purchaser for $20,000 in cash.and one Series A warrant and oneare voted. The Series B warrantPreferred Stock otherwise has no voting rights except as otherwise required by the General Corporation Law of the State of Delaware.settle $342,500any distribution of assets of the Company, including upon a liquidation, bankruptcy, reorganization, merger, acquisition, sale, dissolution or winding up of the Company, whether voluntarily or involuntarily. The holder of the Series B Preferred Stock will not be entitled to receive dividends of any kind.compensation.interest to its Chief Executive Officer. The Chief Executive Officer and the Company entered the July 2022 Promissory Note on July 21, 2022.The Company’sOn April 21, 2023, Amro Albanna, the Chief Executive Officer of the Company, and Shahrokh Shabahang, the Chief Innovation Officer (“CIO”of the Company, loaned $87,523 and $100,000, respectively, to the Company. The loans were each evidenced by an unsecured promissory note (the “April Note”). Pursuant to the terms each April Note, it will accrue interest at the Prime rate of eight percent (8.00%) has provided certain periodsper annum and is due on the earlier of service without payment.October 21, 2023, or an event of default, as defined therein. As of December 31, 20202023, the note was fully paid off.2019,one-quarter percent (8.25%) per annum, the CIOPrime rate on the date of signing, and is owed $0 and $377,000, respectively, related to compensation. Duringdue on the year endedearlier of November 25, 2023 or an event of default, as defined therein. As of December 31, 2020,2023, the note was fully paid off.issued 47,222 Units consistingand Shahrokh Shabahang, the Chief Innovation Officer of the Company, loaned $200,000 and $100,000, respectively, to the Company. The loans were evidenced by an unsecured promissory note (the “June Note”). Pursuant to the terms of the June Note, it will accrue interest at the Prime rate of eight and one-quarter percent (8.25%) per annum and is due on the earlier of December 12, 2023, or an event of default, as defined therein. As of December 31, 2023, the June Note was fully paid off.oneare voted. The Series A warrant, and oneC Preferred Stock otherwise has no voting rights except as otherwise required by the General Corporation Law of the State of Delaware.B warrantC Preferred Stock is not convertible into, or exchangeable for, shares of any other class or series of stock or other securities of the Company. The Series C Preferred Stock has no rights with respect to settle $425,000any distribution of assets of the Company, including upon a liquidation, bankruptcy, reorganization, merger, acquisition, sale, dissolution or winding up of the Company, whether voluntarily or involuntarily. The holder of the Series C Preferred Stock will not be entitled to receive dividends of any kind.accrued compensation.Effective July 10, 2020,whole, but not in part, at any time (i) if such redemption is ordered by the Board of Directors appointedin its sole discretion or (ii) automatically upon the Company’s Chief Operating Officer (“COO”). Prioreffectiveness of the amendment to the appointment,Certificate of Incorporation implementing a reverse stock split. Upon such redemption, the COOholder of the Series C Preferred Stock will receive consideration of $1,000 in cash. On August 17, 2023, the share was redeemed.independent operations consultantunsecured promissory note (the “November Note”). Pursuant to the terms of the November Note, it will accrue interest at a rate of eight and had provided certain periodsa half percent (8.50%) per annum, the Prime rate on the date of service without payment.signing, and is due on the earlier of May 30, 2024 or an event of default, as defined therein. As of December 31, 20202023, there was a remaining principal balance of $10,000 on the November Loan and 2019,accrued interest of $72.COOChief Executive Officer of the Company, loaned $200,000 to the Company. The loan was owed $0evidenced by an unsecured promissory note (the “First December Note”). Pursuant to the terms of the First December Note, it will accrue interest at a rate of eight and $275,000, respectively, related to compensation. Duringa half percent (8.50%) per annum, the year endedPrime rate on the date of signing, and is due on the earlier of June 6, 2024 or an event of default, as defined therein. As of December 31, 2020,2023, there was a remaining principal balance of $200,000 on the First December Loan and accrued interest of $1,164.35,555 Units consistingand sold a secured promissory note in the principal amount of one share$375,000 (the “First Tranche Note”) resulting in gross proceeds to the Company of common stock, one Series A warrant, and one Series B warrant to settle $320,000 in accrued compensation.On March 21, 2019,$250,000. In connection with the issuance of the First Tranche Note, the Company issued 3,907 shares of its common stock (the “First Tranche Commitment Shares”) as a promissory notecommitment fee to a related party. The note had a principal of $10,000, a maturity date of September 21, 2019, and an interest rate of 4% per year. During the year ended December 31, 2020, this note was paid in full.Duringinvestor. Pursuant to the year ended December 31, 2020,First Tranche Securities Purchase Agreement, the Company assumed $11,980was obligated to and obtained approval of liabilities from a related partyits shareholders (“First Tranche Shareholder Approval”) with respect to the issuance of any securities in exchange forconnection with the returnFirst Tranche Securities Purchase Agreement and the First Tranche Note in excess of 5,99019.99% of the Company’s issued and outstanding shares on the closing date, which was equal to 33,792 shares of the Company’s common stock. NOTE 5 – STOCKHOLDERS’ EQUITY (DEFICIT)Common Stockauthorizedconvertible following First Tranche Shareholder Approval and the occurrence of an Event of Default (as defined in the July Note) at a conversion price of $18.00 per share.issue 27,000,000which it granted the investor a security interest in certain Collateral (as defined in the First Tranche Security Agreement) to secure its obligations under the First Tranche Note. In addition, the Company entered into a registration rights agreement with the investor pursuant to which the Company agreed to prepare and file with the U.S. Securities and Exchange Commission a registration statement covering the resale of the First Tranche Commitment Shares and any shares of the Company’s common stock par value $0.001 per share.Duringissuable upon conversion of the year endedFirst Tranche Note within 120 days of the closing date and to have such registration statement declared effective within 150 days of the closing date. As of December 31, 2020,2023, the First Tranche Note was fully paid off.874,916and sold a secured promissory note in the principal amount of $2,625,000 (the “Second Tranche Note”) resulting in gross proceeds to the Company of $1,750,000. In connection with the issuance of the Second Tranche Note, the Company agreed to issue a total of 27,344 shares of its common stock and recognized expense of $2,477,434 in stock compensation for consulting services. The Company issued 150,000 shares of common stock for intangible assets valued at $320,850. The Company also issued 4,297,703 shares of common stock for(the “Second Tranche Commitment Shares”) as a commitment fee to the exercise of warrants and received $210,544 forinvestor. At the exerciserequest of the warrants. The Company issued 1,250,000 shares of common stock for the exercise of 1,250,000 shares of Series A Preferred Stock. The Company issued 146,818 shares of common stock for the settlement of accounts payable and issued 62,500 shares of common stock for the settlement of debt. The Company issued 1,226,668 shares of common stock related to the IPO and issued 1,150,000 shares of common stock related to the September 2020 Offering. The stock compensation for the period was valued based on prior private placements or based on management’s estimates of value immediately prior to the IPO and the value of the shares based on public information post IPO.During the year ended December 31, 2019,investor, the Company issued 41,00017,278 Second Tranche Commitment Shares and will issue the remaining 10,066 Second Tranche Commitment Shares within 120 days, subject to the investor’s discretion. Pursuant to the Second Tranche Securities Purchase Agreement, the Company was obligated to and obtained approval of its shareholders (“Second Tranche Shareholder Approval”) with respect to the issuance of any securities in connection with the Second Tranche Securities Purchase Agreement and the Second Tranche Note in excess of 19.99% of the Company’s issued and outstanding shares on the closing date, which was equal to 38,026 shares of the Company’s common stock. The company recognized a total debt discount of $1.0 million on the Second Tranche Note from the issuance of stock and original issuance discount. The Note has a maturity date of December 31, 2023 and is convertible following Second Tranche Shareholder Approval and the occurrence of an Event of Default (as defined in the Second Tranche Note) at a conversion price of $15.60 per share.for servicesissuable upon conversion of the Second Tranche Note within 90 days of the closing date and recognized expenseto have such registration statement declared effective within 120 days of $82,000the closing date. As of December 31, 2023, $2,625,000 in stock compensation and license fees. These shares were valued basedoutstanding principal on the priceSecond Tranche Note and accrued interest of $113,021 was converted into 2,625 shares of the Company’s Series B-2 Preferred Stock (See Note 10).common shares were being soldthe Holders assigned the Notes to the Company in consideration for the private placement.issuance by the Company of (i) an aggregate principal amount of $5 million in secured notes of the Company due on January 2, 2024 (the “January 2024 Secured Notes”), (ii) an aggregate principal amount of $8 million in secured notes of the Company due on September 30, 2024 (the “September 2024 Secured Notes”), (iii) an aggregate principal amount of $5 million in ten-year unsecured notes (the “Unsecured Notes”), and (iv) payment of $154,480 in respect of net sales of Phexxi in respect of the calendar quarter ended September 30, 2023, which amount is due and payable on December 14, 2023. The January 2024 Secured Notes are secured by certain intellectual property assets of the Company and its subsidiaries pursuant to an Intellectual Property Security Agreement (the “IP Security Agreement”) entered into in connection with the Assignment Agreement. The September 2024 Secured Notes are secured by the Notes and certain associated security documents pursuant to a Security Agreement (the “Security Agreement”) entered into in connection with the Assignment Agreement. As of December 31, 2023, there was a remaining principal balance of $13,000,000 on the Notes.Reverse Stock SplitOn June 29, 2020,Subject to the Company effectuated a 1-for-2 reverse stock splitterms and conditions set forth in the Merger Agreement, at the effective time of itsthe Merger (the “Effective Time”), (i) all issued and outstanding shares of common stock, par value $0.0001 per share of Evofem (“Evofem Common Stock”), other than any shares of Evofem Common Stock held by the Company or Merger Sub immediately prior to the Effective Time, will be converted into the right to receive an aggregate of 610,000 shares of the Company’s common stock, par value $0.001 per share (“Company Common Stock”); and (ii) all issued and outstanding shares of Series E-1 Preferred Stock, par value $0.0001 of Evofem (the “Evofem Unconverted Preferred Stock”), other than any shares of Evofem Unconverted Preferred Stock held by the Company or Merger Sub immediately prior to the Effective Time, will be converted into the right to receive an aggregate of 2,327 shares of Series A-1 Preferred Stock, par value $0.001 of the Company (the “Company Preferred Stock”), having such rights, powers, and preferences set forth in the form of Certificate of Designation of Series A-1 Preferred Stock, the form of which is attached as Exhibit C to the Merger Agreement.(i) approval by the Company’s shareholders and Evofem shareholders; (ii) the registration statement on Form S-4 pursuant to which the shares of the Company Common Stock issuable in the Merger being declared effective by the U.S. Securities and Exchange Commission; (iii) the entry into a voting agreement by the Company and certain members of Evofem management; (iv) all preferred stock of Evofem other than the Evofem Unconverted Preferred Stock shall have been converted to Evofem Common Stock; (v) Evofem shall have received agreements (the “Evofem Warrant Holder Agreements”) from all holders of Evofem warrants which provide: (vi) Evofem shall have cashed out any other holder of Evofem warrants who has not provided an Evofem Warrant Holder Agreement; and (vii) Evofem shall have obtained waivers from the holders of the convertible notes of Evofem (the “Evofem Convertible Notes”) with respect to any fundamental transaction rights that such holder may have under the Evofem Convertible Notes, including any right to vote, consent, or otherwise approve or veto any of the transactions contemplated under the Merger Agreement. (i) the Company shall have obtained agreements from the holders of Evofem Convertible Notes and purchase rights they hold to exchange such Convertible Notes and purchase rights for not more than an aggregate (for all holders of Evofem Convertible Notes) of 86,153 shares of Company Preferred Stock; (ii) the Company shall have received waivers form the holders of certain of the Company’s securities which contain prohibitions on variable rate transactions; and (iii) the Company, Merger Sub and Evofem shall work together between the Execution Date and the Effective Time to determine the tax treatment of the Merger and the other transactions contemplated by the Merger Agreement. (i) the Company shall have regained compliance with the stockholders’ equity requirement in Nasdaq Listing Rule 5550(b)(1) and shall meet all other applicable criteria for continued listing, subject to any panel monitor imposed by Nasdaq. Year
Ended
December 31,
2023 Year
Ended
December 31,
2022 Components of total lease costs: Operating lease expense $ 1,140,949 $ 1,396,875 Total lease costs $ 1,140,949 $ 1,396,875 December 31,
2023 December 31,
2022 Assets Right of use asset – long term $ 2,200,299 $ 3,160,457 Total right of use asset $ 2,200,299 $ 3,160,457 Liabilities Operating lease liabilities – short term $ 999,943 $ 1,086,658 Operating lease liabilities – long term 1,041,744 1,885,218 Total lease liability $ 2,041,687 $ 2,971,876 Weighted average remaining lease term (in years) – operating leases 1.92 Weighted average discount rate – operating leases 8.00 % 2024 $ 1,004,982 2025 710,546 2026 423,930 Total lease payments $ 2,139,458 Less imputed interest (97,771 ) Less current portion (999,943 ) Total maturities, due beyond one year $ 1,041,744 (i) Satisfactory completion of due diligence; (ii) Completion by the Company of financing sufficient to consummate the transactions contemplated by the Asset Purchase Agreement; (iii) Receipt by the Company of all required Consents from Governmental Bodies for the Acquisition, including but not limited to, any consents required to complete the transfer and assignment of Cellvera’s membership interests in GRA; (iv) Receipt of executed payoff letters reflecting the amount required to be fully pay all of each of Seller’s and Seller Owner’s Debt to be paid at Closing; (v) Receipt by the Company of a release from Agility; (iv) Execution of an agreement acceptable to the Company with respect to the acquisition by the Company of certain intellectual property presently held by a third party; (v) Execution of an amendment to an asset purchase agreement previously entered into by Cellvera with a third party that effectively grants the Company the rights to acquire the intellectual property from the third party under such agreement; (vi) Receipt of a fairness opinion by the Company with respect to the transactions contemplated by the Asset Purchase Agreement; and (vii) Receipt by the Company from the Seller Owner of written consent, whether through its official liquidator or the Board of Directors of Seller Owner, to the sale and purchase of the Acquired Assets and Assumed Liabilities pursuant to the Assert Purchase Agreement. certificateCertificate of amendmentAmendment (the “Certificate of Amendment”) to its amendedAmended and restated certificateRestated Certificate of incorporationIncorporation with the Secretary of State of the State of Delaware. Accordingly, all shareIn accordance with the General Corporation Law of the State of Delaware, the Authorized Shares Increase and the Certificate of Amendment were approved by the stockholders of the Company at the Company’s Annual Meeting of Stockholders on May 19, 2021. On September 13, 2022, the Company effectuated a 1 for 50 reverse stock split (the “2022 Reverse Split”). The Company’s stock began trading at the 2022 Reverse Split price effective on the Nasdaq Stock Market on September 14, 2022. There was no change to the number of authorized shares of the Company’s common stock. On August 17, 2023, the Company effectuated a 1 for 40 reverse stock split (the “2023 Reverse Split”). The Company’s stock began trading at the 2023 Reverse Split price effective on the Nasdaq Stock Market on August 17, 2023. There was no change to the number of authorized shares of the Company’s common stock.amounts for all periods presented inunder the accompanying financial statementsATM. The sale of Shares generated net proceeds of $507,016 after paying commissions and notes thereto have been adjusted retroactively, where applicable,related fees.reflect this reverse stock split.the ATM, pursuant to which the Company and the Agent agreed to reduce the aggregate gross sales price of the Shares under the ATM from $50,000,000 to zero.UpThe Company is authorized to issue up to 2,500,000 shares of our common stock may be issued pursuant to awards granted under the 2017 Plan. The 2017 Plan is administered by our Board of Directors, and expires ten years after adoption, unless terminated earlier by the Board. Board of Directors. All shares of our common stock pursuant to awards under the 2017 Plan have been awarded.2020, the Company granted 880,500 stock options with an exercise price of $1.94, $1.92 or $11.00 per share, some of which vested immediately, and some of which vest between one and three years. The total grant date fair value was determined to be $1,668,997.During the year ended December 31, 2019, the Company granted 700,000 stock options with exercise prices of $4.00 per share vesting on issuance. The total grant date fair value was determined to be $2,495,556. For all periods presented,2023, the fair value of each stock option granted was estimated using the Black-Scholes assumption ranges and and/or factors in the Black-Scholes Model as follows: Exercise price $ 5.01 Expected dividend yield 0 % Risk free interest rate 4.49 % Expected life in years 10 Expected volatility 164 % Exercise price$1.92-11.00Expected dividend yield0%Risk free interest rate0.28%-2.65%Expected life in years2.70-10.00Expected volatility141-151%government securitiesGovernment Bond Equivalent Yield appropriate for the expected term of stock options.option.The expected term of stock options is calculated using either the simplified method for employee options which takes into consideration the contractual life and vesting terms of the options, unless the options are expected to vest in which case the contractual term of the options.stock option grants, until such time that the Company’s common stock has enough market history to use historical volatility.optionsoption granted is based on the Company’s history and expectation of dividend payouts. The Company has never declared ornor paid any cash dividends on its common stock, and the Company does not anticipate paying any cash dividends in the foreseeable future.Management estimated the fair value of common stock by looking at a market approach which takes into consideration past sales of stock to third parties and Company developments to date.stock option forfeitures as they occur, as there is insufficient historical data to accurately determine future forfeitures rates.Vested and Nonvested Stock Options Number Weighted
Average
Exercise
Price Weighted
Average
Remaining
Life Outstanding December 31, 2022 1,127 $ 6,802.93 5.74 Granted 44,445 5.01 9.86 Exercised - - - Expired or forfeited - - - Outstanding December 31, 2023 45,572 $ 173.12 9.74 Nonvested Stock Options Number Weighted-
Average
Exercise
Price Nonvested on December 31, 2022 55 $ 3,840 Granted 44,445 5.01 Vested (44,500 ) 9.75 Forfeited - - Nonvested on December 31, 2023 - $ - Stock Options Number Weighted
Average
Exercise
Price Weighted
Average
Remaining
Life Outstanding December 31, 2019 1,102,500 $ 4.00 7.77 Granted 1,045,500 2.32 8.91 Expired or forfeited (5,000 ) 1.92 - Outstanding December 31, 2020 2,143,000 $ 3.18 7.81 Exercise price $ 0.02 Expected dividend yield 0 % Risk free interest rate 3.95 % Expected life in years 10 Expected volatility 194 % Vested and Nonvested Stock Options Number Weighted
Average
Exercise
Price Weighted
Average
Remaining
Life Outstanding December 31, 2022 - $ - - Granted 13,320,000 0.02 9.97 Exercised - - - Expired or forfeited - - - Outstanding December 31, 2023 13,320,000 $ 0.02 9.97 Nonvested Stock Options Number Weighted-
Average
Exercise
Price Nonvested on December 31, 2022 - $ - Granted 13,320,000 0.02 Vested (9,320,000 ) 0.02 Forfeited - - Nonvested on December 31, 2023 4,000,000- $ 0.02- Nonvested Options Shares Weighted-
Average
Exercise
Price Nonvested at December 31, 2019 - $ - Granted 1,045,500 2.32 Vested (67,500 ) 2.93 Expired or forfeited (5,000 ) 1.92 Nonvested at December 31, 2020 973,000 $ 2.28 issuedgranted and vesting expense of $406,880$589,014 during the year ended December 31, 2020,2023, of which $385,640 is included in general and administrative expenses and $203,374 is included in research and development expenses in the accompanying statements of operations. The remaining value to be expensed is $1,781,485 with a$77,812 as of December 31, 2023. The weighted average vesting term of 2.40is 2.17 years as of December 31, 2020.2023. The Company recognized stock-based compensation expense related to all options issuedgranted and vesting expense of $2,513,826$791,187 during the year ended December 31, 2019,2022, of which $555,772 is included in general and administrative expenses and $235,415 is included in research and development expenses in the accompanying statements of operations.On October 6, 2020,Boardyear ended December 31, 2023, the fair value of Directors approvedeach warrant granted was estimated using the issuanceassumption and/or factors in the Black-Scholes Model as follows:Exercise price $ 300-2,300 Expected dividend yield 0 % Risk free interest rate 1.13%-3.47 % Expected life in years 5-5.50 Expected volatility 147-165 % an aggregate of 40,000 stock optionseach warrant granted was estimated using the assumption and/or factors in the Black-Scholes Model as compensationfollows:Exercise price $ 7.50-20.00 Expected dividend yield 0 % Risk free interest rate 2.55%-3.47 % Expected life in years 5.00-5.50 Expected volatility 147%-165 % non-employee membersexpected term of warrants.Boardexpected volatility assumption for warrants granted using the historical volatility of Directors undercomparable public companies’ common stock. The Company will continue to monitor peer companies and other relevant factors used to measure expected volatility for future warrant grants, until such time that the Company’s 2017 Equity Incentive Plan. common stock has enough market history to use historical volatility.options are subjectdividend yield assumption for warrants granted is based on the Company’s history and expectation of dividend payouts. The Company has never declared nor paid any cash dividends on its common stock, and the Company does not anticipate paying any cash dividends in the foreseeable future.certain vesting provisions.accurately determine future forfeitures rates.WarrantsWarrants Number Weighted
Average
Exercise
Price Weighted
Average
Remaining
Life Outstanding December 31, 2019 1,382,478 4.44 2.84 Vested and Nonvested Warrants Number Weighted
Average
Exercise
Price Weighted
Average
Remaining
Life Outstanding December 31, 2022 127,281 $ 514.97 4.54 Granted 8,906,381 6.24 5.00 5,975,936 3.92 2.72 Exercised (1,055,374 ) 0.24 - Expired or forfeited (190,810 ) 8.12 - (393 ) 8,249.36 - Exercised (4,298,903 ) 7.20 - Outstanding December 31, 2020 5,799,146 $ 5.05 4.00 Outstanding December 31, 2023 5,047,450 $ 14.11 2.73 Nonvested Warrants Shares Weighted-
Average
Exercise
Price Nonvested at December 31, 2019 200,000 4.00 Granted 8,906,381 6.24 Vested (4,296,668 ) 5.14 Expired or forfeited (4,489,713 ) 7.24 Nonvested at December 31, 2020 320,000 $ 3.69 The warrants granted for compensation are valued using similar inputs as notedOn September 1, 2023, the Company recognized a deemed dividend resulting in the stock options section above, with the exceptionissuance of the expected life9,086 warrants, 6,128 of which is the contractual life.were immediately exercised.Nonvested Warrants Number Weighted-
Average
Exercise
Price Nonvested on December 31, 2022 2,500 $ 300.00 Granted 5,975,936 3.92 Vested (5,978,436 ) 4.04 Forfeited - - Nonvested on December 31, 2023 - $ - issuedgranted and vesting expense of $304,526zero and $1,102,596$609,748 during the yearyears ended December 31, 20202023 and 2019,2022, respectively, of which $105,049 is included in general and administrative and $504,699 is included in sales and marketing in the accompanying Statements of Operations. The remaining value to be expensed is $294,948zero as of December 31, 2023. The weighted average vesting term is zero years as of December 31, 2023.Nonvested RSUs Number Weighted
Average
Price Nonvested December 31, 2022 187 $ 1,856,21 Granted - - Vested (170 ) 2,714.15 Forfeited (35 ) 1,345.77 Rounding for Reverse Split 18 - Nonvested December 31, 2023 - $ - 0.900 years as of December 31, 2020.2023.2020, 4,297,703 warrants were exercised for 4,298,903 shares2023, the Company granted a total of common stock. The Company recognized proceeds of $210,544 related to the exercises.2020,2023, 170 RSUs vested and the Company issued 60,000 warrants to the underwriters related to the September 2020 Offering. These warrants have an exercise price157 shares of $5.00, a term of five years, and become exercisable beginning on March 1, 2021. The value of these warrants were both an increase and decrease to additional paid in capital as a cost of the offering for net a zero impact on the financial statements.NOTE 6 – AGREEMENTSOn July 1, 2020, the Company entered into an amendment to patent and technology licensing agreement with Loma Linda University (“LLU”), dated March 15, 2018. Pursuant to the amendment, the Company paid LLU $455,000 within four days of the signing of such amendment. The amendment also updated the milestone payment dates to be $175,000 on March 31, 2022; $100,000 on March 31, 2024; $500,000 on March 31, 2026; and $500,000 on March 31, 2027.In October 2020, the Company entered into a 24-month financing agreement for lab equipment. The aggregate cost of this financing agreement will be $467,691. The financing agreement has an interest rate of 8% per year.In November 2020, the Company entered into an additional 24-month financing agreement for lab equipment. The aggregate cost of this financing agreement will be $215,192. The financing agreement has an interest rate of 8% per year.Salveo Consulting AgreementOn November 18, 2020, we entered into a Consulting Agreement (the “Salveo Consulting Agreement”) with Salveo Diagnostics, Inc., a Delaware corporation (“Salveo”). Pursuant to the Salveo Consulting Agreement, Salveo agreed to establish, setup and commence commercial operations of a licensed, College of American Pathologists accredited, and Clinical Laboratory Improvement Amendments (CLIA) certified, independent clinical and diagnostic laboratory for us and our AditxtScore™ immune monitoring technology (the “Salveo Services”).In considerationcommon stock for the Services, and upon the successful completion of certain milestones (the “Milestones”) described below, we issued Salveo 650,000 shares of our common stock (the “Salveo Shares”) in the aggregate. The Salveo Shares were issued to Salveo upon the completion of the following Milestones: (i) 150,000 shares upon the sale and transfer to the Company of certain code and interpretive commenting algorithms (the “Algorithms”) along with related testing protocols and all technology, codes and spreadsheets, know-how, any necessary information or tools to implement, use, and/or continue to improve or further refine the Algorithms, and other associated intellectual property; (ii) 250,000 shares upon securing temporary laboratory space and other related tasks in connection with the launch of the AditxtScore™ platform; and (iii) 250,000 shares upon satisfaction of tasks related to the establishment of a long-term AditxtScore™ center in Richmond, VA. We also pay Salveo at cost for Salveo’s reasonable and documented purchases, general operating costs and expenses incurred in connection with the Salveo Services.170 vested RSUs.NOTE 7 – NOTES PAYABLEPearsanta Restricted Stock AwardOn April 12, 2018, the Company issued an unsecured promissory note for $35,000 that accrued interest of 4% annually. The note was due on the earlier of November 12, 2018 or in the event of default, as defined in the agreement. During the year ended December 31, 2020, this note was paid in full.On July 10, 2018, the Company entered into a bridge loan with an investor for a principal amount of $15,600. The note was due on the earlier of October 8, 2018 or in the event of default, as defined in the agreement. During the year ended December 31, 2020, this note was paid in full.On July 18, 2018, the Company entered into a bridge loan with an investor for a principal amount of $130,000. The note was due on the earlier of October 16, 2018 or in the event of default, as defined in the agreement. During the year ended December 31, 2020, this note was paid in full.On November 1, 2019, the Company entered into a bridge loan with an investor for a principal amount of $50,000. This loan did not accrue any interest. The note was due on the earlier of April 28, 2020 or in the event of default, as defined in the agreement. The note was convertible into the same class of securities as those sold in the public offering with a conversion price of $2.00 per share. During the year ended December 31, 2020, the note was converted into securities of the Company in full.On January 10, 2020, the Company entered into a bridge loan with an investor for a principal amount of $75,000. This Note carried an original issue discount of $40,000. This loan did not accrue any interest. The note was due on the earlier of July 8, 2020 or in the event of default, as defined in the agreement, as amended. The note was convertible into the same class of securities as those sold in the public offering with a conversion price of $2.00 per share. During the year ended December 31, 2020, the note was converted into securities of the Company in full.During the first quarter of 2020, the Company entered into six bridge loans with investors for2023, Pearsanta granted a total principal amount of $600,000. These notes carried an aggregate original issue discount1,000,000 immediately vested restricted stock awards under the Pearsanta 2023 Plan. The Company recognized stock-based compensation expense related to the Pearsanta restricted stock awards of $300,000. The notes were due on the earlier of April 19, 2020 or ten days after the close of the Company’s IPO. During the year ended December 31, 2020, these notes were paid in full.$20,000.811 – INCOME TAXES20202023 and 2019,2022, the Company did not record a current or deferred income tax expense or benefit due to current and historical losses incurred by the Company. The Company’s losses before income taxes consist solely of losses from domestic operations.On March 27, 2020, the United States enacted the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”). The Cares Act includes provisions relating to refundable payroll tax credits, deferment of the employer portion of certain payroll taxes, net operating loss carryback periods, alternative minimum tax credit refunds, modifications to the net interest deduction limitations and technical corrections to tax depreciation methods for qualified improvement property. The CARES Act also established a Paycheck Protection Program whereby certain small businesses are eligible for a loan to fund payroll expenses, rent, and related costs.The Company considered the provisions under the CARES Act and elected not to take advantage of the provisions of CARES Act as the effect of such provisions was not expected to have a material impact on the Company’s results of operations, cash flows and financial statements. 2020 2019 2023 2022 Income taxes at U.S. statutory rate 21 % 21 % 21 % 21 % State income taxes 8.2 7.0 0.8 1.6 Tax Credits 1.3 - 0.5 1.0 Permanent Differences/Others 30.3 (20.4 ) (1.9 ) (10.5 ) Change in valuation allowance (60.8 ) (7.6 ) (20.5 ) (13.1 ) Total provision for income taxes 0 % 0 % 0 % 0 % 20202023 and 20192022 are comprised of the following: Year Ended December 31, Years Ended December 31, 2020 2019 2023 2022 Deferred tax assets Net operating loss carryforwards $ 3,651,932 $ 1,044,662 $ 18,555,428 $ 13,499,811 Tax credits carryforwards 116,949 - 796,320 430,468 Stock-based compensation 2,350,795 974,644 1,580,038 1,511,849 Lease liability 367,029 486,473 722,126 Section 174 Capitalization 2,207,611 1,547,343 Loss on impairment of debt 3,326,129 3,288,363 Other 1,920 - 92,704 114,973 Total deferred tax assets 6,488,625 2,019,306 27,044,703 21,114,933 Valuation allowance (6,109,685 ) (2,019,306 ) (26,414,533 ) (20,217,400 ) Net deferred tax assets 378,940 — 630,170 897,533 Deferred tax liabilities Right of use assets (368,950 ) (486,473 ) (722,127 ) Fixed Assets (9,990 ) Fixed assets (143,697 ) (175,406 ) Total deferred tax liabilities (378,940 ) — (630,170 ) (897,533 ) Net deferred taxes $ — $ — $ — $ — 20202023 and 2019,2022, respectively. The Company reevaluates the positive and negative evidence at each reporting period. The Company’s valuation allowance increased during 20202023 by approximately $4.1$6.2 million primarily due to the generation of net operating loss and tax credit carryforwards and stock based compensation.the capitalization of research and experimental expenditures. The Company’s valuation allowance increased during 2022 by approximately $3.5 million primarily due to the generation of net operating loss and tax credit carryforwards and the capitalization of research and experimental expenditures.20202023 and 2019,2022, the Company had U.S. federal net operating loss carryforwards of $12.6$75.2 million and $3.8$56.6 million, respectively, which may be available to offset future income tax liabilities. The 2017 Tax Cuts and Jobs Act (“ TCJA”) will generally allow losses incurred after 2017 to be carried over indefinitely, but will generally limit the net operating loss deduction to the lesser of the net operating loss carryover or 80% of a corporation’s taxable income (subject to Section 382 of the Internal Revenue Code of 1986, as amended). Also, there will be no carryback for losses incurred after 2017. Losses incurred prior to 2018 will generally be deductible to the extent of the lesser of a corporation’s net operating loss carryover or 100% of a corporation’s taxable income and be available for twenty years from the period the loss was generated. The Company has federal net operating losses generated following 2017 of $12.5$75.1 million, which do not expire. The federal net operating losses generated prior to 2018 of $0.1 million will expire at various dates through 2037. The CARES Act temporarily allows the Company to carryback net operating losses arising in 2018, 2019 and 2020 to the five prior tax years. In addition, net operating losses generated in these years could fully offset prior year taxable income without the 80% of the taxable income limitation under the TCJA which was enacted on December 22, 2017. The Company has been generating losses since its inception, as such the net operating loss carryback provision under the CARES Act is not applicable to the Company.20202023 and 2019,2022, the Company also had U.S. state net operating loss carryforwards (post-apportioned) of $15.2$28.2 million and $3.8$26.2 million, respectively, which may be available to offset future income tax liabilities and expire at various dates through 2040.2042.2020,2023, the Company had $0.1 million federal tax credit carryforwards of approximately $0.1 million, available to reduce future tax liabilities which expire at various dates through 2040.2042. As of December 31, 2020,2022, the Company had $0.1 federal tax credit carryforwards. As of December 31, 2023 and 2022, the Company had state research and development tax credit carryforwards of approximately $0.1$0.4 million and $0.2 million, respectively, which may be available to reduce future tax liabilities which expire at various dates through 2035. The Company did not have any federal and state tax credit carryforward as of December 31, 2019.can be carried over indefinitely.2017ended December 31, 2019 through present. As of December 31, 20202023 and 2019,2022, the Company has recorded no liability for unrecognized tax benefits, interest, or penalties related to federal and state income tax matters and there currently no pending tax examinations. The Company will recognize interest and penalties related to uncertain tax positions in income tax expenseexpense.912 – SUBSEQUENT EVENTSThe Company has evaluated subsequent events through the filingClosing of this Annual Report on Form 10-K and has determined that there have been no events that have occurred that would require adjustments to the Company’s disclosures in the financial statements, except for the following:Private PlacementJanuary 25, 2021,December 29, 2023, the Company entered into a Securitiessecurities purchase agreement (the “Purchase Agreement”) with an institutional investor (“the “December Purchaser”) for the issuance and sale in a private placement (the “December Private Placement”) of (i) pre-funded warrants (the “December Pre-Funded Warrants”) to purchase up to 1,237,114 shares of the Company’s Common Stock, par value $0.001 at an exercise price of $0.001 per share, and (ii) warrants (the “December Common Warrants”) to purchase up to 2,474,228 shares of the Company’s Common Stock, at a purchase price of $4.85 per share.salecash payment by the December Purchaser of $6,000,000 in Convertible Notes. $0.125 per share of Common Stock underlying the Certain Outstanding Warrants, effective immediately.Convertible NotesDecember Private Placement closed on January 4, 2024. The net proceeds to the Company from the December Private Placement were approximately $5.5 million, after deducting placement agent fees and expenses and estimated offering expenses payable by the Company.24 months, convert at $4.00 per share,exercise equal to three years from the date of issuance.have an original issuance discountAssignment$1,000,000. Thethe secured notes (the “Holders”) entered into amendments to the January 2024 Secured Notes (“Amendment No. 1 to January 2024 Secured Notes”), pursuant to which the maturity date of the January 2024 Notes was extended to January 5, 2024.also issued 800,000 warrantsand the Holders entered into amendments to purchase sharesthe January 2024 Secured Notes (“Amendment No. 2 to January 2024 Secured Notes”) and amendments to the September 2024 Secured Notes (“Amendment No. 1 to September 2024 Secured Notes”), pursuant to which the Company and the Holders agreed that in consideration of a principal payment in the aggregate amount of $1 million on the January 2024 Secured Notes and in increase in the aggregate principal balance of $250,000 on the September 2024 Secured Notes, that the maturity date of the January 2024 Secured Notes would be further extended to January 31, 2024.common stock. These warrants havefailure to make the Additional Consideration payment by February 9, 2023, the January 2024 Secured Notes and the September 2024 Secured Notes were in default and the entire principal balance of the January 2024 Secured Notes and the September 2024 Secured Notes, without demand or notice, were due and payable.termresult of three years, are immediately exercisable,the defaults on the January 2024 Secured Notes and havethe September 2024 Secured Notes, the Company was in default on the Business Loan and Security Agreement dated January 24, 2024 (the January Business Loan”), which has a current balance of approximately $5.2 million, and the Business Loan and Security Agreement dated November 7, 2023 (the “November Business Loan”) which had a current balance of approximately $2.7 million.exercise priceAssignment Agreement (the “February Assignment Agreement”), pursuant to which the Company assigned all remaining amounts due under the January 2024 Secured Notes, the September 2024 Secured Notes and the Unsecured Notes (collectively, the “Notes”) back to the Holders. In connection with the February Assignment Agreement, the Company and the Holders entered into a payoff letter (the “Payoff Letter”) and amendments to the January 2024 Secured Notes (“Amendment No. 4 to January 2024 Secured Notes”), pursuant to which the maturity date of $4.00.the January 2024 Secured Notes was extended to March 31, 2024 and the outstanding balance under the Notes, after giving effect to the transactions contemplated by the February Assignment Agreement as applied pursuant to the Payoff Letter, was adjusted to $250,000. On April 15, 2024, the Company has repaid the $250,000.During February 2021,24 month financingsettlement agreement for lab equipment.and general release with an investor (the “Settlement Agreement”), pursuant to which the Company and the investor agreed to settle an action filed in the United States District Court in the Southern District of New York by an investor against the Company (the “Action”) in consideration of the issuance by the Company of shares of the Company’s Common Stock (the “Settlement Shares”). The aggregate costnumber of this financing agreementSettlement Shares to be issued will be $892,094.equal to $1.6 million divided by the closing price of the Company’s Common Stock on the day prior to court approval of the joint motion. Following the issuance of the Settlement Shares, the Investor will file a dismissal stipulation in the Action.ThroughOn January 17, 2024, the Company issued 296,296 Settlement Shares to the investor. The Settlement Shares were issued pursuant to an exemption from registration pursuant to Section 3(a)(10) under the Securities Act of 1933, as amended.there were 1,142,306 warrants exercised forthe Company has not purchased the 2,000 shares of EvoFem Series F-1 Preferred Stock.resultingof Aditxt or an aggregate of 332,876 shares (the “Consideration Shares” and together with the Cash Consideration, the “Transaction Consideration”). In connection with the transaction, each outstanding option and warrant of Appili will be cashed-out based on the implied in-the-money value of the Transaction Consideration, which is expected to result in proceedsan additional aggregate cash payment of $3,643,956. approximately $341,000 (based on the number of issued and outstanding options and warrants and exchange rates as of the date of the Arrangement Agreement).F-17