UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(MARK ONE)
|
| |
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE FISCAL YEAR ENDED DECEMBER 31, 20162019
OR
|
| |
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM TO
COMMISSION FILE NUMBER 000-52008
LUNA INNOVATIONS INCORPORATED
(Exact name of Registrant as Specified in its Charter)
|
| | |
| Delaware | | 54-1560050 |
| (State or Other Jurisdiction of Incorporation or Organization) | | (I.R.S. Employer Identification Number) |
301 1st St SW, Suite 200
Roanoke, VA 24011
(Address of Principal Executive Offices)
(540) 769-8400
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
|
| | |
Title of Each Classeach class | Trading Symbol | Name of Each Exchangeeach exchange on which Registeredregistered |
Common Stock, $0.001 par value $0.001 per share | LUNA | The NASDAQNasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer” andfiler,” “smaller reporting company”company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
| | |
Large accelerated filer ¨ | | Accelerated filer ¨x |
Non-accelerated filer ¨ (Do not check if a smaller reporting company) | | Smaller reporting company x |
Emerging growth company ¨ | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant on June 30, 2016,28, 2019 based upon the closing price of Common Stock on such date as reported by the NASDAQNasdaq Capital Market, was approximately $29.8$100.4 million.
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Section 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes x No ¨
Indicate the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date: As of March 15, 201711, 2020 there were 27,541,27730,391,879 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Specified portions of the registrant’s Proxy Statement with respect to its 20172020 Annual Meeting of stockholders, anticipated to be filed within 120 days after the end of its fiscal year ended December 31, 2016,2019, are incorporated by reference into Part III of this annual report on Form 10-K.
LUNA INNOVATIONS INCORPORATED
ANNUAL REPORT ON FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 20162019
TABLE OF CONTENTS
CAUTIONARY NOTE REGARDING FORWARD LOOKING STATEMENTS
This Annual Report on Form 10-K, including the “Management’s Discussion and Analysis of Financial Condition and Results of Operation” section in Item 7 of this report, and other materials accompanying this Annual Report on Form 10-K contain forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. All statements other than statements of historical facts are “forward-looking statements” for purposes of these provisions, including those relating to future events or our future financial performance. In some cases, you can identify these forward- looking statements by words such as “intends,” “will,” “plans,” “anticipates,” “expects,” “may,” “might,” “estimates,” “believes,” “should,” “projects,” “predicts,” “potential” or “continue,” or the negative of those words and other comparable words, and other words or terms of similar meaning in connection with any discussion of future operating or financial performance. Similarly, statements that describe our business strategy, goals, prospects, opportunities, outlook, objectives, plans or intentions are also forward-looking statements. These statements are only predictions and may relate to, but are not limited to, expectations of future operating results or financial performance, capital expenditures, introduction of new products, regulatory compliance, plans for growth and future operations, as well as assumptions relating to the foregoing.
These statements are based on current expectations and assumptions regarding future events and business performance and involve known and unknown risks, uncertainties and other factors that may cause actual events or results to be materially different from any future events or results expressed or implied by these statements. These factors include those set forth in the following discussion and within Item 1A “Risk Factors” of this Annual Report on Form 10-K and elsewhere within this report.
You should not place undue reliance on these forward-looking statements, which apply only as of the filing date of this Annual Report on Form 10-K. You should carefully review the risk factors described in other documents that we file from time to time with the U.S. Securities and Exchange Commission (“SEC”). Except as required by applicable law, including the rules and regulations of the SEC, we do not plan to publicly update or revise any forward-looking statements, whether as a result of any new information, future events or otherwise, other than through the filing of periodic reports in accordance with the Securities Exchange Act of 1934, as amended.
We have proprietary rights to a number of trademarks used in this Annual Report which are important to our business. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. All other trademarks, trade names and service marks appearing in this Annual Report are the property of their respective owners.
PART I
ITEM 1. BUSINESS
Company Overview and Business Model
Luna Innovations Incorporated ("we" or the "Company") is a leader in advanced optical technology, providing unique capabilities in high speed optoelectronics and high performance fiber optic test, measurement and control products for the telecommunications industryand photonics industries and distributed fiber optic sensing products for industries utilizing composite and other advanced materials, such as the automotive, aerospace, energy and automotiveinfrastructure industries. Our high-speed optical receiver ("HSOR") transmission products are deployed in the internet infrastructure to enable the high-speed bandwidth necessary to support video and data. Our distributed fiber optic sensing products provide criticalhelp designers and manufacturers more efficiently develop new and innovative products by providing valuable information such as highly detailed stress, strain, and temperature information to designers and manufacturers working with advanced materials. Our custom optoelectronicmeasurements of a new design or manufacturing process. In addition, our distributed fiber optic sensing products are soldused to scientificmonitor the structural integrity or industrial instrumentation manufacturers and to defense contractors for various applicationsoperational health of critical assets, including large civil structures such as metrology, missile guidance, flame monitoring,bridges. Our communications test and temperature sensing. In addition, wecontrol products accelerate the development of advanced fiber optic components and networks by providing fast and highly accurate characterization of components and networks. We also provide applied research services, typically under research programs funded by the U.S. government, in areas of sensing and instrumentation, advanced materials, sensing,optical technologies and healthcare applications.health sciences. Our business model is designed to accelerate the process of bringing new and innovative products to market. We use our in-house technical expertise across a range of technologies to perform applied research services for companies and for government funded projects. We continue to invest in product development and commercialization, which we anticipate will lead to increased product sales growth.
We are organized into two main businessreporting segments, our Products and Licensing segment and our Technology Development segment. Our Products and Licensing segment develops, manufactures and markets distributed fiber optic sensing products as well as test & measurement products, and also conducts applied research in the fiber optic sensing area for both corporate
communications test and government customers.control products. We are continuingcontinue to develop and commercialize our fiber optic technology for strain and temperature sensing applications for the aerospace, automotive, energy and energyinfrastructure as well as for test and measurement applications in the telecommunications and data communications industries. Our Products and Licensing segment revenues represented approximately 72%63% and 69%51% of our total revenues for the years ended December 31, 20162019 and 2015,2018, respectively. For 2016, ZTE Kangxun Telecom, Inc. accounted for approximately 16% of our Products and Licensing segment revenues and 11% of our total revenues.
Our Technology Development segment performs applied research principally in the areas of sensing &and instrumentation, advanced materials, optical technologies and health sciences. Our Technology Development segment comprised approximately 28%37% and 31%49% of our total revenues for the years ended December 31, 20162019 and 2015,2018, respectively. Most of the government funding for our Technology Development segment is derived from the Small Business Innovation Research ("SBIR") program coordinated by the U.S. Small Business Administration ("SBA").
Our SBIR research is focused on technological areas with commercial potential and we strive to commercialize any resulting scientific advancements. For the year ended December 31, 2016,2019, approximately 24%35% of our total revenues were generated under the SBIR program, compared to 26%44% for the year ended December 31, 2015.2018.
For the years ended December 31, 20162019 and 2015, 28%2018, 40% and 34%53%, respectively, of our total revenues were derived from the U.S. government.
Acquisition of General Photonics Corporation
MergerOn March 1, 2019, we acquired all of the outstanding stock of General Photonics Corporation ("GP"), a leading provider of innovative components, modules and test equipment focused on the generation, measurement and control of polarized light critical in fiber optic-based applications for aggregate consideration of $19.0 million with Advanced Photonix, Inc. an earn-out provision of up to $1.0 million. Of the purchase price, $17.1 million was paid at closing and $1.9 million was placed into escrow for possible working capital adjustments to the purchase price and potential satisfaction of certain post-closing indemnification obligations. As of December 31, 2019 we expect to pay, and have accrued, $1.0 million in additional cash consideration as a result of the successful completion of the earn-out provision.
Acquisition of Micron Optics, Inc.
On May 8, 2015,October 15, 2018, we completed a merger with Advanced Photonix,acquired substantially all of the assets, other than cash, of the U. S. operations of Micron Optics, Inc. ("API"MOI"), pursuant to the Agreement and Plan of Merger and Reorganization (the "Merger Agreement"), dated as of January 30, 2015, by and among Luna, API and API Merger Sub, Inc., our wholly owned subsidiary ("API Merger Sub"). In accordance with the terms of the Merger Agreement, upon the completion of the merger, API Merger Sub merged with and into API, with API surviving as our wholly-owned subsidiary. In the merger, former API stockholders received 0.31782 shares of our common stock for each share of API common stock they owned at the effective time of the merger.
API was a leading test & measurement companyprovider of innovative optical components and laser-based equipment that packaged optoelectronic semiconductors into high-speedadvance the quality of optical receivers, custom optoelectronic sub systemsmeasurements, allowing the sensing, imaging and Terahertz instrumentation, serving the test & measurement, telecommunications military/aerospace and medical markets. API had manufacturing facilities located in Camarillo, California and Ann Arbor, Michigan, bothindustries, to make critical measurements. We paid total cash consideration of which have remained in operation following the merger.
Our results of operations$5.5 million for the year ended December 31, 2015,acquisition of MOI. The acquisition of MOI expanded our technology and product portfolio to include optical sensors and sensing interrogators capable of a broader range of measurement capabilities, including higher speed measurements such as vibration, and the results associated withability to instrument larger structures over longer distances. In addition, the operationsMOI acquisition added a product suite of API from the closing of our merger on May 8, 2015 through December 31, 2015.tunable optical filters, optical sensors and swept lasers.
Products and Licensing
In addition to the operations of API acquired in May 2015, ourOur Products and Licensing segment includes the sale of fiber optic test, & measurement instruments.and control instruments and modules. We have been successful in developing and marketingprovide fiber optic test &and measurement products which provide solutions primarily for the telecommunications industry marketed under the Luna Technologies brand. In 2011, weWe also introducedmarket our ODiSIOptical Distributed Sensing Interrogator (“ODiSI”) platform of products for distributed and very high-resolution sensing of strain and temperature utilizing optical fiber. Following the acquisition of MOI, we also market our Hyperion platform of products for distributed fiber optic sensing that offer dynamic measurement capabilities over longer distances. Following our acquisition of GP, we also market solutions for the measurement, management and control of polarization and delay in fiber optic networks. We refer to the groups within our company who develop, manufacture, support and sell these products as our Lightwave division.
Our key initiativesinitiative for long term growth areis to become a leading provider of fiber optic straintest, measurement and temperaturecontrol equipment, including products for physical sensing systems and standard test methods based upon the ODiSI and Hyperion product platformplatforms and to be a leading supplierproducts for the characterization of HSOR products inhigh-speed fiber optic components and networks, including the expanding telecommunications markets, principally in Asia.growing silicon photonics market. Our primary product lines in our Products and Licensing segment are described in more detail below.
Test &and Measurement, Sensing and Instrumentation Products
Test &and Measurement Equipment for Fiber Optic Components and Sub-Assemblies
Our product lines in the optical test &and measurement domain include our Optical Vector Analyzer, our Optical Backscatter Reflectometer, and theour Phoenix family of tunable lasers.
Historically, ourOur optical test &and measurement products have primarily servedserve the telecommunications industry, as well as provide valuable applications in other fields. Our test &and measurement products test and monitor the integrity of fiber optic network components and sub-assemblies. These products are designed for manufacturers and suppliers of optical components and sub-assemblies and allowallowing them to reduce development, test and production costs and improve the quality of their products. Our products are particularly useful for characterizing and testing photonic integrated circuits, such as silicon photonics components, which are a critical technology enabling the growing worldwide demand for internet connectivity. Most manufacturers and suppliers of optical components and modules currently use a combination of different types of optical test equipment to
identify measure performance and measureidentify failures in optical networks, such as bad splices, bends, crimps and other reflective and non-reflective events that can cause defects and negatively impact product performance. Our optical test equipment products replaceeliminate the need to employ multiple test products by addressing all stages of the end user’s product development lifecycle, including design verification, component qualification, assembly process verification and failure analysis.
ODiSI Sensing Solution; Optical Distributed Sensor InterrogatorSolution
Our ODiSI products provide fully distributed strain and temperature measurements and deliverdelivering an extraordinary amount of data by using an optical fiber as a continuous sensor for up to 50 meters in length. Compared to traditional sensing methods, such as electrical strain gages, this technology provides greater insight into the performance, tolerances and failure mechanisms of composite structures and vehicles.vehicles and can be integrated into locations and environments not accessible with traditional sensors. We believe the technology canour ODiSI products provide exceptional value to the aerospace and automotive industries as they transition from steelcontinue to adopt electrification and aluminummove to lighter weight systems made of composite structures.
We have significant expertise in distributed sensing systems, such as ODiSI, which are products composedthat incorporate multiple channels of multiplefiber optic sensors whose inputs are integrated through a fiber optic networkan advanced measurement system and software. These products use fiber optic sensing technology with anour innovative monitoring system that allows several thousand sensors to be networked along a single optical fiber.
Hyperion Sensing Solution
Our Hyperion sensing products expand our capabilities in fiber optic sensing by providing distributed sensing using hundreds of Fiber-Bragg Grating ("FBG") or Extrinsic Fabry-Perot ("FP") sensors measured at sampling rates up to 5KHz. Hyperion enables rapid full-spectrum data acquisition and flexible peak detect algorithms of FBGs, Long Period FBGs and FP sensors with low-latency access to data for closed loop feedback applications. Our Hyperion products target fiber optic sensing applications that require more dynamic measurement capabilities or longer distances than provided by our ODiSI platform.
General Photonics
Our GP products include components, modules and instruments to measure, manage and control polarization and group delay in fiber optic networks. Our proprietary fiber optic squeezing technology ensures high performance polarization control. We also manufacture and sell fiber optic coils for use in gyroscopes.
Tunable Lasers
We have acquired the rights to manufacture a line of swept tunable lasers to allow us to compete more effectively in our existing fiber optic test & measurement as well as sensing markets. This technology is beingthat are integrated into current and new products to help us provide our customers with faster, more flexible and cost-effective test &and measurement products. TheOur laser has desirable properties in the quality of the laser light produced, the speed at which it can operate, the small size of the package, and the environmental conditions in which it can operate. We believe that these traits make it possible for us to move our fiber optic sensing capabilities out of the laboratory, and into more demanding environments such as aircraft structural health monitoring, automotive manufacturing, green energy and industrial applications.
High Speed Optical Receivers
Our HSOR transmission products include, among other things, 100G optical receivers and 10G avalanche photodiodes. These products are deployed in the internet communications equipment infrastructure to enable the high-speed bandwidth necessary to support video and data for television, computers, tablets or smart phones anytime and anywhere. Our communication test & measurement products are used to develop, manufacture and test optical communication components and equipment used in the telecom/datacom infrastructure.
Optoelectronic SolutionsTeraMetrix
Our optoelectronics TeraMetrix products are sold to a number of scientific instrumentation manufacturers and defense contractors for various applications such as metrology, missile guidance, flame monitoring, temperature sensing, particle detection, color sensing, infrared detection, and many other applications that can only be done through optical sensing.
Terahertz Sensing
Our THz systems are used to measure and verify physical properties on-line and in real-time to reduce raw materials and rework costs in manufacturing processes as well as to conductconducting quality control monitoring.monitoring utilizing terahertz ("THz") measurement technologies. THz is a region of the electromagnetic spectrum that lies between microwave and infrared waves and is in the early stages of adoption. While microwaves and infrared waves have been explored and commercialized
for decades, THz waves are in the early stages of being explored and commercialized due to the fact thatbecause they have historically been very difficult to generate and detect. Advances in femtosecond lasers and ultra-fast semiconductor and electro-optic devices combined with fiber-optic packaging technologies have enabled the development of practical THz instrumentation for the research market with increasing adoption in the industrial, military and aerospace markets. THz can beis used to "look" through and beneath materials with high two-dimensional and three-dimensional spatial resolution. It can also uniquely identify the chemical composition of many hidden or subsurface objects using non-ionizing radiation, which is not harmful to humans at the power levels commonly used today. We market our THz based products as our T-Ray product platforminstruments and sensors primarily through value added resellers. Our TeraMetrix THz solutions are aimed at two primary market opportunities: Non-Destructive Test (NDE) and Industrial Process Control. For both of these markets, our TeraMetrix products are used to measure layer thicknesses with very high resolutions for otherwise opaque materials, allowing our customers to manage critical coating and manufacturing processes.
Sales and Marketing
We primarily market our fiber optic test, & measurement and control products to telecommunications companies, defense agencies, government system integrators, researchers, original equipment manufacturers, distributors, testing labs and strategic partners
worldwide. We have a regional sales force that markets and sells our products through manufacturer representative organizations to customers in North America and through partner and distribution channels for other sales around the world.outside of North America. We have a dedicated sales force for direct marketing of our distributed sensing products, with an initial focus on customers in the automotive, aerospace, and energy industries.
We market our HSOR, optoelectronic, and THz productsinstruments primarily as components or sub-assemblies to original equipment manufacturers through a mix of technical sales engineers, value added resellers and independent sales representatives. We market these products and capabilities through industry specific channels, including the internet, industry trade shows and in print through trade journals.
We believe that we provide a high level of support in developing and maintaining our long-term relationships with our customers. Customer service and support are provided through our offices and those of our partners that are located throughout the world.
Technology Development
We provide applied research for customers in our primary areas of focus, including sensing and materials such as nanomaterials, coatings, adhesives, composites and bio-engineered materials. We generally compete to win contracts in these areas on a fee-for-service basis. Our Technology Development segment has a successful track record of evaluating innovative technologies to address the needs of our customers.
We seek to maximize the benefits we derive fromof our contract research business includingby generating revenue generation and identification ofidentifying promising technologies for further development.to develop. We focus primarily on opportunities in which we develop intellectual property rights in areas that we believe have commercialization potential.we can commercialize. We take a disciplined approach to contract research to try to ensure that the costs of any contractcontracts we undertake will be fully reimbursed. We believe that this model is cost-efficient and significantly reduces our development risk in that it enablesby enabling us to defray the development costs of higher risk technology development with third-party funding.
AlthoughWhile we conduct our applied research on a fee-for-service basis for third parties, we seek to retain full or partial rights to the technologies and patents developedwe develop under thosethese contracts and to continuously enlarge and strengthen our intellectual property portfolio. New technology that we develop may complement our existing technologies and enable us to develop applications and products that were previously not previously possible. In addition, the new technologies we develop may also be applicable tohave commercial markets beyond the scope of the applications originally contemplated in the contract research stage, and we endeavor to capture the value of those opportunities. Funded research and development within this business segment was $16.8$26.0 million and $13.6$21.0 million for the years ended December 31, 20162019 and 2015,2018, respectively.
Each year, U.S. government federal agencies and departments are required to set asideallocate a portion of their grant awards for SBIR-qualified organizations. SBIR contracts include Phase I feasibility contracts of up to $150,000$225,000 and Phase II proof-of-concept contracts, which can be as high as $1,000,000.$1,500,000. We have won three National Tibbetts Awards from the SBA for outstanding SBIR performance. We have also won research contracts outside the SBIR program from corporations and government entities. These contracts typically have a longer duration and higher value than SBIR grants. In the future, we will seek to derive a larger portion of our contract research revenues from contracts outside of the SBIR program.
Materials
We are actively developing a wide variety of materials. For example, we have developed a range of coatings, including both hydrophobic and superoleophobic coatings. These coatings are being evaluated for use in a number of applications. Other coatings under development include anti-corrosion and damage-indicating coatings.
We are also working on a variety of bioengineered materials for homeostatic agents and wound healing. These materials must be approved by the FDA or similar foreign regulatory agencies before they can be marketed, which we do not expect to occur for at least several years, if at all.
Sensing
Our Technology Development segment also performs a significant amount of applied research towards developing new sensors. This includes sensors for the purpose of corrosion, temperature, strain, pressure, structural health and chemical detection. Much of the work is directed to harsh environments and uses optics. Examples include measuring temperature and neutron flux in nuclear reactors, pressure and temperature in gas turbines and temperatures of cryogenic lines. The effort utilizes both discrete and distributed sensors. Our technology development work in this area is closely aligned with our Products and Licensing segment and is directed at advancing the technology and the development of new applications.
Intellectual Property
We seek patent protection on inventions that we consider important to the operations of our business. We rely on a combination of patent, trademark, copyright and trade secret laws in the United States and other jurisdictions, as well as confidentiality procedures and contractual provisions to protect our proprietary technology and our brand. We control access to our proprietary technology and enter into confidentiality and invention assignment agreements with our employees and consultants and confidentiality agreements with other third parties.
Our success depends in part on our ability to develop patentable products and obtain, maintain and enforce patent and trade secret protection for our products, as well as toincluding successfully defend thesedefending our patents against third-party challenges both in the United States and in other countries. We will only be able to protect our technologies from unauthorized use by third parties to the extent that we own or have licensed valid and enforceable patents or trade secrets that cover them. Furthermore, the degree of future protection of our proprietary rights is uncertain because we may not be able to obtain patent protection on some or all of our technology and because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage.
Currently, we own or license approximately 226400 U.S. and international patents and approximately 28250 U.S. and international patent applications, and we intend to file, or request that our licensors file, additional patent applications for patents covering our products.applications. Our issued patents generally have terms that are scheduled to expire between 20172020 and 2030.2037. The patents scheduled to expire in 20172020 are not expected to have a significant impact on our revenues or results of operations. However, patentsPatents may not be issued for any pending or future pending patent applications owned by or licensed to us. Claims allowed under any issued patent or future issued patent owned or licensed by us may not be valid or sufficiently broad to protect our technologies. Any issued patents owned by or licensed to us now or in the future may be challenged, invalidated or circumvented, and, in addition, the rights under such patents may not provide us with competitive advantages. In addition, competitors may design around our technology or develop competing technologies. IntellectualTo the extent we elect to pursue, intellectual property rights may also be unavailable or limited in some foreign countries, which could make it easier for competitors to capture or increase their market share with respect to related technologies.
A discussion of our material in-licensed patents and patent applications is set forth below.
NASA Patents
We have licensed, on a non-exclusive basis, four U.S. patents and related patents in Japan, Canada, Germany, France, Great Britain and Belgium from the National Aeronautics and Space Administration, an agency of the U.S. government (“NASA”), which patents concern the measurement of strain in optical fiber using Bragg gratings and Rayleigh scatter and the measurement of the properties of fiber-optic communications devices. These patents expire between February 2017March 2020 and SeptemberOctober 2020.
VTIP Patents
We have licensed, on an exclusive basis, two U.S. patents from Virginia Tech Intellectual Properties, Inc. (“VTIP”) to commercialize fullerenes and tri-metal nitride endohedral fullerene (“Trimetasphere”) nanomaterials for all fields of human endeavor. These patents expire in December 2019 and December 2022.
Coherent Patents
We have licensed, Under the license agreements, we pay NASA certain royalties based on a non-exclusive basis, several U.S.percentage of net sales of products covered by the patents. We incur a royalty obligation to NASA based upon a specified percentage of the revenue earned on each product sold utilizing these patents and other intellectual property rights owned or controlledsubject to combined minimum royalties of $220,000 per year under the license agreements. The term of the license agreements continues until the expiration of the last licensed patent. These license agreements may be terminated by Coherent, Inc., related tous on 90 days' notice. Either party may terminate the manufacturing, using, importing, selling and offeringlicense agreements for sale of Coherent’s “Iolon” brand of swept tunable lasers, which we market under our “Phoenix” brand. These patents expire between 2020 and 2025.cause upon certain conditions.
Shape Sensing Patents
As a part of our sale of assets associated with our fiber optic shape sensing technology in the medical field to Intuitive Surgical, Inc. ("Intuitive"), in 2014, we transferred our related patents to Intuitive. Also, as a part of this transaction, we entered into a revocable license agreement with Intuitive pursuant to which we have the right to use all of our transferred technology outside the field of medicine and in respect of our existing non-shape sensing products in certain non-robotic medical fields. Two U.S. patents that we now license back from Intuitive cover the use of optical frequency domain reflectometry and multiple, closely
spaced Bragg gratings for shape sensing, and the use of the inherent scatter as a strain sensor for shape sensing. These two patents expire in July 2025. We also have a license back from Intuitive for apatents and patent applicationapplications that coverscover certain refinements to the measurements covered in the firstforegoing two patents and related technologies, which are necessary in order to achieve the necessary accuracies for medical and other applications. ThisThese patent application wasapplications were filed in the United States, the European Patent Office, China, India, Russia, Brazil, Japan, Indonesia and Indonesia.elsewhere. These patents and patent applications can support other nonmedical applications of our fiber optic shape sensing technology.
Corporate History
We were incorporated in the Commonwealth of Virginia in 1990 and reincorporated in the State of Delaware in April 2003. We completed our initial public offering in June 2006. In May 2015, we merged with API. Our executive offices are located at 301 1st St SW, Suite 200, Roanoke, Virginia 24011 and our main telephone number is (540) 769-8400.
Material Agreements
Sale of Assets to Intuitive Surgical
On January 17, 2014, we entered into an Asset Purchase Agreement with Intuitive (the “Asset Purchase Agreement”). Under the Asset Purchase Agreement, effective as of 12:01 a.m. on January 21, 2014, we closed on the sale to Intuitive of substantially all of our assets related to our medical shape sensing business, including all of the patents and patent applications used or useful for our fiber optical shape sensing and localization technology, for $12 million, plus up to $8 million upon the accomplishment by Intuitive of certain technical specifications (the “Technical Specifications Payment”) and up to $10 million in potential future royalties (altogether, the “Transaction”). We had been engaged since 2007 in a development project for Intuitive developing a fiber optic-based shape sensing and position tracking system to be integrated into Intuitive’s products. Also as a part of the Transaction, Intuitive has hired certain of our employees, many of whom were historically engaged in this development project. In December 2015, we and Intuitive agreed to settle all remaining obligations related to the Technical Specifications Payment and royalties for a lump sum payment of $9 million, which we received in December 2015.
The Asset Purchase Agreement contains representations and warranties, covenants and indemnification provisions common to transactions of this nature, except that our indemnification obligations are only limited in time until no further payments are due from Intuitive. Any disputes between the parties will be handled by mediation and arbitration in Chicago, Illinois. All of the transfers of technology contemplated in the Transaction have been made subject to our existing licenses and related obligations to Hansen Medical Inc. ("Hansen") and Philips Medical Systems.
Also, in connection with the Transaction, we and Intuitive entered into a License Agreement of the same date under which we received a license back to all of our transferred technology outside the field of medicine and in respect of our existing non-shape sensing products in certain non-robotic medical fields. The license back to us outside the medical field is exclusive to us except that Intuitive retained certain non-sublicensable rights for itself. This license back to us is revocable if we were, after notice and certain time periods, (i) to challenge the validity or enforceability of the transferred patents and patent applications, (ii) to commercialize our fiber optical shape sensing and localization technology in the field of medicine (except to perform on a development and supply project for Hansen), (iii) to violate our obligations related to our ability to sublicense in the field of medicine or (iv) to violate our confidentiality obligations in a manner that advantages a competitor in the field of medicine and not cure such violation. As a part of the Transaction, we retained assets and rights necessary to perform on our development and supply project for Hansen if that project is re-started.
Also, as a part of the Transaction, for a period of 15 years after closing, we agreed to exit and not develop or commercialize our fiber optical shape sensing and localization technology in the field of medicine (except for Hansen as described above). For a period of 10 years after closing, Intuitive has agreed not to use any of the assets being acquired in the Transaction, including the key employees being hired, to compete with us outside the field of medicine for shape, strain and/or temperature sensing in the aerospace, automotive, and energy markets and for strain sensing in the civil structural monitoring and composite material markets.
Virginia Tech
Our nanomaterials activity is focused on Trimetasphere materials. The Trimetasphere nanomaterial is a carbon sphere with three metal atoms and a nitrogen atom enclosed. We have obtained an exclusive license from VTIP to commercialize Trimetasphere nanomaterials under two U.S. patents for all fields of human endeavor. The term of this license ends upon the
last expiration date of the underlying patents, which is December 2022. The license provides for certain royalties to be paid as a percentage of our net sales, certain percentages of amounts received from any of our sublicensees and certain milestone payments based on product development phases. We reimburse VTIP for patent costs incurred under the license. VTIP may terminate the license for cause. We may terminate the license at any time for any reason on 60 days' notice.
We primarily utilize the VTIP license in our ongoing research into the potential use of Trimetaspheres to improve the safety of contrast agents commonly utilized in magnetic resonance imaging (“MRI”) procedures. We believe that contrast agents utilizing our Trimetasphere nanomaterials may be able to provide a higher image contrast than existing contrast agents but with a lower risk of toxicity. Medical contrast agents for human use, such as our Trimetasphere nanomaterials, must be approved by the FDA or similar foreign regulatory agencies before they can be marketed, which we do not expect to occur for at least several years, if at all. As described below under “Government Regulation,” this approval process can involve significant time and expense and may delay or prevent our products from reaching the market.
Coherent
In December 2006, we entered into an asset transfer and license agreement with Coherent.Coherent, Inc. Under the agreement, we acquired the rights to manufacture Coherent’s “Iolon” brand of swept tunable lasers as well as certain manufacturing equipment and inventory previously used by Coherent to manufacture the lasers. We continue to enhance, produce and market these lasers under our “Phoenix” brand. Under this agreement, Coherent granted non-exclusive licenses to us for certain U.S. patents and other intellectual property rights owned or controlled by Coherent for making, having made, using, importing, selling and offering for sale the lasers. This agreement expired in 2016. However, the patent licenses became fully paid and perpetual, as we fulfilled our royalty obligations during the 10-year period and the license to the other intellectual property rights is perpetual. These U.S. patents expire between 2020 and 2025.2022. As consideration, we agreed to paypaid Coherent a total of $1.3 million over a period of two years and royalty paymentsin addition to paying royalties on net sales of products sold by us that incorporate the lasers or that are manufactured using the intellectual property covered by the licenses. We paid Coherent royalty fees of approximately $70,000 for both 2016 and 2015. We also agreed to sell Coherent a limited number of lasers each year.
The Phoenix laser is a miniaturized, external-cavity laser offering high performance in a compact footprint and is applicable to a range of fiber optic test and measurement, instrumentation, and sensing applications. These products employ frequency-tuned lasers to measure various aspects of the transmission properties of telecommunications fiber optic components and systems. Lasers are also used in fiber optic sensing applications such as distributed strain and temperature mapping, and distributed measurement of shape. We currently use these lasers within our ODiSI platform of products, our fiber optic shape sensing products and certain of our backscatter reflectometer products, and we also sell variations of the Phoenix laser as standalone products. Under our agreements related to our sale of assets to Intuitive, we have certain obligations to supply Intuitive with these lasers and Intuitive has certain rights to require us to transfer and assign this Coherent license to Intuitive, in which case Intuitive would be similarly required to supply us with lasers.
NASACorporate History
We have licensed, on a non-exclusive basis,were incorporated in the Commonwealth of Virginia in 1990 and reincorporated in the State of Delaware in April 2003. We completed our initial public offering in June 2006. Our executive offices are located at 301 1st St SW, Suite 200, Roanoke, Virginia 24011 and our main telephone number is (540) 769-8400.
Material Agreements
Sale of High-Speed Optical Receiver ("HSOR") Business
On August 9, 2017, we completed the sale of our HSOR business, which was part of our Products and Licensing segment, to an unaffiliated third party for an initial purchase price of $33.5 million, of which $29.5 million in cash has been received, and $4.0 million was placed into escrow until December 15, 2018 for possible working capital adjustments to the purchase price and potential satisfaction of certain patents from NASA under two license agreements. These patents concern the measurement of strain in optical fiber using Bragg gratings and Rayleigh scatter, and also the measurementpost-closing indemnification obligations. In December 2018, we received $1.5 million of the propertiesescrow amount. The remaining $2.5 million remains in escrow and is pending the resolution of fiber-optic communications devices. Undercertain indemnification claims which the license agreements, we pay NASA certain royalties based onbuyer has made and which are disputed by us. The HSOR business was a percentage of net sales of products covered by the patents. We incur a royalty obligation to NASA based upon a specified percentagecomponent of the revenue earned on each productoperations of Advanced Photonix, Inc., which we acquired in May 2015.
Sale of Luna Optoelectronics
In July 2018 we sold utilizing these patentssubstantially all of the assets associated with our custom optoelectronic components and sub-assemblies business for total cash consideration of $17.5 million, paid at closing, in addition to contingent consideration of up to $1.0 million. The contingent consideration is subject to combined minimum royaltiesthe optoelectronic business achieving specified revenue targets for
the 18-month period following the closing date. As of $220,000 per year under the license agreements. The termDecember 31, 2019, we are unsure if any of the license agreements continues until the expirationcontingent consideration will be received. The optoelectronic business was a component of the last licensed patent, which is September 2020. These license agreements may be terminated by us on 90 days' notice. Either party may terminate the license agreements for cause upon certain conditions.operations of Advanced Photonix, Inc.
Competition
We compete for government, university and corporate research contracts relating to a broad range of technologies. Competition for contract research is intense and the industry has few barriers to entry. We compete against a number of in-house research and development departments of major corporations, as well as a number of small, limited-service contract research providers and companies backed by large venture capital firms. The contract research industry continues to experience consolidation, which has resulted in greater competition for clients. Increased competition might lead to price and other forms of competition that could harm our operating results. We compete for contract research on the basis of a number of factors, including reliability, past performance, expertise and experience in specific areas, scope of service offerings, technological capabilities and price.
We also compete, or will compete with a variety of companies in several different product markets. The products that we have developed or are currently developing will compete with other technologically innovative products, as well as products incorporating conventional materials and technologies. We expect that we will compete with companies in a wide range of industries, including semiconductors, electronics, biotechnology, textiles, alternative energy, military, defense, healthcare, telecommunications, industrial measurement, security applications and consumer electronics. Although there can be no assurance that we will continue to do so, we believe that we compete favorably in these areas because our products leverage advanced technologies to offer superior performance. If we are unable to effectively compete in these areas in the future, we could lose business to our competitors, which could harm our operating results.
We also compete, or will compete, for government, university and corporate research contracts relating to a broad range of technologies. Competition for contract research is intense and the industry has few barriers to entry. We compete against a number of in-house research and development departments of major corporations, as well as a number of small, limited-service contract research providers and companies backed by large venture capital firms. The contract research industry continues to experience consolidation, which has resulted in greater competition for clients. Increased competition might lead to price and other forms of competition that could harm our operating results. We compete for contract research on the basis of a number of factors, including reliability, past performance, expertise and experience in specific areas, scope of service offerings, technological capabilities and price.
Government Regulation
Qualification for Small Business Innovation Research Grants
SBIR is a highly competitive program that encourages small businesses to explore their technological potential and provides them with incentives to commercialize their technologies by funding research that might otherwise be prohibitively expensive or risky for companies like us. As noted above, we presently derive a significant portion of our revenue from this program, but we must continue to qualify for the SBIR program in order to be eligible to receive future SBIR awards. The eligibility requirements are:
Ownership. The company must be more than 50 percent owned and controlled by U.S. citizens or permanent resident aliens, or owned by an entity that is itself more than 50 percent owned and controlled by U.S. citizens or permanent resident aliens; and
Size. The company, including its affiliates, cannot have more than 500 employees.
These requirements are set forth in the SBA’s regulations and are interpreted by the SBA’s Office of Hearings and Appeals. In determining whether we satisfy the more than 50% ownership requirement, agreements to merge, stock options, convertible debt and other similar instruments are given “present effect” by the SBA as though the underlying security were actually issued unless the exercisability or conversion of such securities is speculative, remote or beyond the control of the security holder. We therefore believe our outstanding options and warrants held by eligible individuals may be counted as outstanding equity for purposes of meeting the more than 50% equity ownership requirement. We believe that we are in compliance with the SBA ownership requirements.
In addition, to be eligible for SBIR contracts, the number of our employees, including those of any entities that are considered to be affiliated with us, cannot exceed 500. As of December 31, 2016,2019, we, including all of our divisions, had 245267 full- and part-time employees. In determining whether we have 500 or fewer employees, the SBA may count the number of employees of entities that are large stockholders who are “affiliated” or have the power to control us. In determining whether firms are affiliated, the SBA evaluates factors such as stock ownership and common management, but it ultimately may make its determination based on the totality of the circumstances. Eligibility protests can be raised to the SBA by a competitor or by the awarding contracting agency. If we grow larger, and if our ownership becomes more diversified, we may no longer qualify for the SBIR program, and we may be required to seek alternative sources and partnerships to fund some of our research and development costs. Additional information regarding these risks may be found below in “Risk Factors.”
Environmental, Health and Safety Regulation
Our facilities and current and proposed activities involve the use of a broad range of materials that are considered hazardous under applicable laws and regulations. Accordingly, we are subject to a number of domestic and foreign laws and regulations and other requirements relating to employee health and safety, protection of the environment, product labeling and product take back. Regulated activities include the storage, use, transportation and disposal of, and exposure to, hazardous or potentially hazardous materials and wastes. Our current and proposed activities also include potential exposure to physical hazards associated with work environment and equipment. We could incur costs, fines, civil and criminal penalties, personal injury and third-party property damage claims, or we could be required to incur substantial investigation or remediation costs, if we were to violate or become liable under environmental, health and safety laws and regulations or requirements. Liability under environmental, health and safety laws can be joint and several and without regard to fault. There can be no assurance that violations of environmental, health and safety laws will not occur in the future as a result of the inability to obtain permits in a timely manner, human error, equipment failure or other causes. Environmental, health and safety laws could also become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations, which could harm our business. Further, violations of present and future environmental, health and safety laws could restrict our
ability to expand facilities and pursue certain technologies, as well as require us to acquire costly equipment or to incur potentially significant costs to comply with environmental, health and safety regulations and other requirements.
We have made, and will continue to make, expenditures to comply with current and future environmental, health and safety laws. We anticipate that we could incur additional capital and operating costs in the future to comply with existing environmental, health and safety laws and new requirements arising from new or amended statutes and regulations. In addition, because the applicable regulatory agencies have not yet promulgated final standards for some existing environmental, health and safety programs, we cannot at this time reasonably estimate the cost for compliance with these additional requirements. The amount of any such compliance costs could be material. We cannot predict the impact that future regulations will impose upon our business.
Employees
As of December 31, 2016,2019, we had approximately 245267 total employees, including approximately 121130 in research, development and engineering positions, approximately 7872 in operations, approximately 1425 in sales and marketing, and approximately 3240 in administrative positions. None of our employees are covered by a collective bargaining agreement, and we consider our relationship with our employees to be good.
Backlog
Our backlog of purchase orders received for which the related goods have not been shipped or recognized as revenue within our Products and Licensing segment was $16.1 million and $5.8 millionat December 31, 2019 and 2018, respectively.
We have historically had a backlog of contracts, primarily within our Technology Development segment, for which work has been scheduled, but for which a specified portion of work has not yet been completed. The approximate value of our backlog was $17.6$31.3 million and $16.7$26.0 million at December 31, 20162019 and 2015,2018, respectively.
We define backlog as the dollar amount of obligations payable to us under negotiated contracts upon completion of a specified portion of work that has not yet been completed, exclusive of revenues previously recognized for work already performed under these contracts, if any. Total backlog includes funded backlog, which is the amount for which money has been directly authorized by the U.S. government or for which a purchase order has been received from a commercial customer, and unfunded backlog, which represents firm orders for which funding has not yet been appropriated. Unfunded backlog was $2.1$2.2 million and $3.3$4.5 million as of December 31, 20162019 and 2015,2018, respectively. Indefinite delivery and quantity contracts and unexercised options are not reported in total backlog. Our backlog is subject to delays or program cancellations that may be beyond our control.
Our backlog of purchase orders received for which the related goods have not been shipped or recognized as revenue within our Products and Licensing segment was $10.4 million and $10.7 million at December 31, 2016 and 2015, respectively.
Research, Development and Engineering
We incur research, development and engineering expenses that are not related to our contract performance. These expenses were $5.5 million and $4.3 million for the years ended December 31, 2016 and 2015, respectively. In addition, during these years, we spent $12.7 million and $10.4 million, respectively, on customer-sponsored research activities, which amounts are reimbursed as part of our performance of customer contracts.
Operating Segments and Geographic Areas
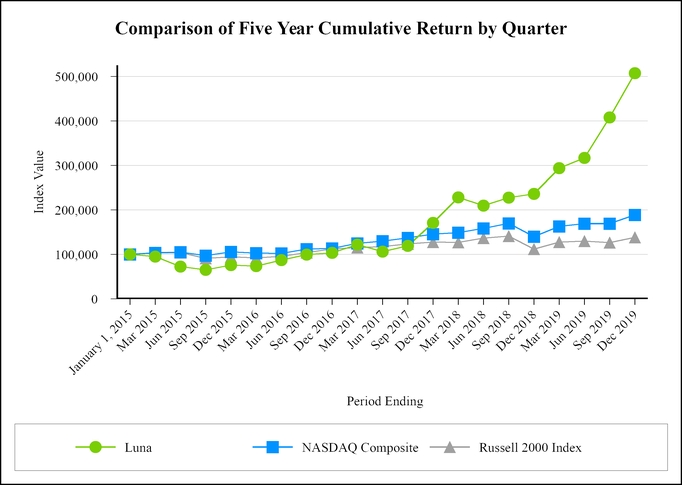
For information with respect to our operating segments and geographic markets, see Note 15 to our Consolidated Financial Statements in Part II, Item 8 of this Annual Report on Form 10-K.
Website Access to Reports
Our website address is www.lunainc.com. We make available, free of charge under “SEC Filings” on the Investor Relations portion of our website, access to our annual report on Form 10-K, our quarterly reports on Form 10-Q and our current reports on Form 8-K, as well as amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after such material is electronically filed with or furnished to the SEC. Information appearing on our website is not incorporated by reference in and is not a part of this annual report. A copy of this annual report, as well as our other periodic and current reports, may be obtained from the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding our filings at www.sec.gov.
ITEM 1A. RISK FACTORS
You should carefully consider the risks described below before deciding whether to invest in our common stock. The risks described below are not the only ones we face. Additional risks not presently known to us or that we currently believe are immaterial may also impair our business operations and financial results. If any of the following risks actually occurs, our business, financial condition or results of operations could be adversely affected. In such case, the trading price of our common stock could decline and you could lose all or part of your investment. Our filings with the Securities and Exchange Commission also contain forward-looking statements that involve risks or uncertainties. Our actual results could differ materially from those anticipated or contemplated by these forward-looking statements as a result of a number of factors, including the risks we face described below, as well as other variables that could affect our operating results. Past financial performance should not be considered to be a reliable indicator of future performance, and investors should not use historical trends to anticipate results or trends in future periods.
RISKS RELATING TO OUR BUSINESS GENERALLY
Our technology is subject to a license from Intuitive Surgical, Inc., which is revocable in certain circumstances. Without this license, we cannot continue to market, manufacture or sell certain of our fiber-optic products.
As a part of the sale of certain assets to Intuitive Surgical, Inc. ("Intuitive") in 2014, we entered into a license agreement with Intuitive pursuant to which we received rights to use all of our transferred technology outside the field of medicine and in respect of our existing non-shape sensing products in certain non-robotic medical fields. This license back to us is revocable if after notice and certain time periods, we were to (i) challenge the validity or enforceability of the transferred patents and patent applications, (ii) commercialize our fiber optical shape sensing and localization technology in the field of medicine (except to perform on a development and supply project for Hansen Medical, Inc.), (iii) violate our obligations related to our ability to sublicensesub-license in the field of medicine or (iv) violate our confidentiality obligations in a manner that advantages a competitor in the field of medicine and not cure such violation. Maintaining this license is necessary for us to conduct our fiber-optic products business, both for our telecom products and our ODiSI sensing products. If this license were to be revoked by Intuitive, we would no longer be able to market, manufacture or sell these products which could have a material adverse effect on our operations.
We depend on third-party vendors for specialized components in our manufacturing operations, making us vulnerable to supply shortages and price fluctuations that could harm our business.
We primarily rely on third-party vendors for the manufacture of the specialized components used in our products. The highly specialized nature of our supply requirements poses risks that we may not be able to locate additional sources of the specialized components required in our business. For example, there are few manufacturers who produce the special lasers used in our optical test equipment. Our reliance on these vendors subjects us to a number of risks that could negatively affect our ability to manufacture our products and harm our business, including interruption of supply. Although we are now manufacturing tunable lasers in low-rate initial production, we expect our overall reliance on third-party vendors to continue. Any significant delay or interruption in the supply of components, or our inability to obtain substitute components or materials from alternate sources at acceptable prices and in a timely manner could impair our ability to meet the demand of our customers and could harm our business.
We depend upon outside contract manufacturers for a portion of the manufacturing process for some of our products. Our operations and revenue related to these products could be adversely affected if we encounter problems with these contract manufacturers.
Many of our products are manufactured internally. However, we also rely upon contract manufacturers to produce the finished portion of some of our optoelectronic components and certain lasers. Our reliance on contract manufacturers for these products makes us vulnerable to possible capacity constraints and reduced control over delivery schedules, manufacturing yields, manufacturing quality control and costs. If the contract manufacturer for our products were unable or unwilling to manufacture our products in required volumes and at high quality levels or to continue our existing supply arrangement, we would have to identify, qualify and select an acceptable alternative contract manufacturer or move these manufacturing operations to internal manufacturing facilities. An alternative contract manufacturer may not be available to us when needed or may not be in a position to satisfy our quality or production requirements on commercially reasonable terms, including price. Any significant interruption in manufacturing our products would require us to reduce the supply of products to our customers, which in turn would reduce our revenue, harm our relationships with the customers of these products and cause us to forego potential revenue opportunities.
As a provider of contract research to the U.S. government, we are subject to federal rules, regulations, audits and investigations, the violation or failure of which could adversely affect our business.
We must comply with and are affected by laws and regulations relating to the award, administration and performance of U.S. government contracts. Government contract laws and regulations affect how we do business with our government customers and, in some instances, impose added costs on our business. A violation of a specific law or regulation could result in the imposition of fines and penalties, termination of our contracts or debarment from bidding on contracts. In some instances, these laws and regulations impose terms or rights that are more favorable to the government than those typically available to commercial parties in negotiated transactions. For example, the U.S. government may terminate any of our government contracts and, in general, subcontracts, at their convenience, as well as for default based on performance.
In addition, U.S. government agencies, including the Defense Contract Audit Agency and the Department of Labor, routinely audit and investigate government contractors. These agencies review a contractor’s performance under its contracts, cost structure and compliance with applicable laws, regulations and standards. The U.S. government also may review the adequacy of, and a contractor’s compliance with, its internal control systems and policies, including the contractor’s purchasing, property, estimating, compensation and management information systems. Any costs found to be improperly allocated to a specific contract will not be reimbursed, while such costs already reimbursed must be refunded. If an audit uncovers the inclusion of certain claimed costs deemed to be expressly unallowable, or improper or illegal activities, we may be subject to civil and criminal penalties and administrative sanctions, including termination of contracts, forfeiture of profits, suspension of payments, fines and suspension or prohibition from doing business with the U.S. government. In addition, our reputation could suffer serious harm if allegations of impropriety were made against us. In June 2015, we received a determination from the Defense Contract Management Agency ("DCMA") of expressly unallowable costs included in our claimed costs for the 2007 contract year. As a result of that determination, DCMA assessed us penalties, interest and over billings of $1.1 million. We have appealed that assessment, and our appeal is currently pending. Depending on the outcome of this appeal, we could be required to make payments that have a material adverse effect on our financial position.
In addition to the risk of government audits and investigations, U.S. government contracts and grants impose requirements on contractors and grantees relating to ethics and business practices, which carry civil and criminal penalties including monetary fines, assessments, loss of the ability to do business with the U.S. government and certain other criminal penalties.
We may also be prohibited from commercially selling certain products that we develop under our Technology Development segment or related products based on the same core technologies if the U.S. government determines that the commercial availability of those products could pose a risk to national security. For example, certain of our wireless technologies have been classified as secret by the U.S. government and as a result we cannot sell them commercially. Any of these determinations would limit our ability to generate product sales and license revenues.
We rely and will continue to rely on contracts and grants awarded under the SBIR program for a significant portion of our revenues. A finding by the SBA that we no longer qualify to receive SBIR awards could adversely affect our business.
We compete as a small business for some of our government contracts. Our revenues derived from the SBIR program account for a significant portion of our consolidated total revenues, and contract research, including SBIR contracts, will remain a significant portion of our consolidated total revenues for the foreseeable future. For the year ended December 31, 2016, 24%2019, 35% of our total revenues were generated under the SBIR program, compared to 26%44% in for the year ended December 31, 2015.2018.
We may not continue to qualify to participate in the SBIR program or to receive new SBIR awards from federal agencies. In order to qualify for SBIR contracts and grants, we must meet certain size and ownership eligibility criteria. These eligibility criteria are applied as of the time of the award of a contract or grant. A company can be declared ineligible for a contract award as a result of a size challenge filed with the SBA by a competitor or a federal agency.
In order to be eligible for SBIR contracts and grants, under current SBA rules we must be more than 50% owned and controlled by individuals who are U.S. citizens or permanent resident aliens, and/or other small business concerns (each of which is more than 50% owned and controlled by individuals who are U.S. citizens or permanent resident aliens) or certain qualified investment companies. In the event our institutional ownership significantly increases, either because of increased buying by institutions or selling by individuals, we could lose eligibility for new SBIR contracts and grants.
Also, in order to be eligible for SBIR contracts and grants, the number of our employees, including those of any entities that are considered to be affiliated with us, cannot exceed 500. As of December 31, 2016,2019, we had approximately 245267 full and part-time employees. In determining whether we are affiliated with any other entity, the SBA may analyze whether another entity controls or has the power to control us. Carilion Clinic is our largest institutional stockholder. Since early 2011, a formal size determination by the SBA that focused on whether or not Carilion is or was our affiliate has been outstanding. Although we
do not believe that Carilion has or had the power to control our company, we cannot assure you that the SBA will interpret its regulations in our favor on this question. If the SBA were to make a determination that we are or were affiliated with Carilion, we would exceed the size limitations, as Carilion has over 500 employees. In that case, we would lose eligibility for new SBIR contracts and grants and other awards that are set aside for small businesses based on the criterion of number of employees, and the relevant government agency would have the discretion to suspend performance on existing SBIR grants. The loss of our eligibility to receive SBIR awards would have a material adverse impact on our revenues, cash flows and our ability to fund our growth.
Moreover, as our business grows, it is foreseeable that we will eventually exceed the SBIR size limitations, in which case we may be required to seek alternative sources of revenues or capital.
A decline in government research contract awards or government funding for existing or future government research contracts, including SBIR contracts, could adversely affect our revenues, cash flows and ability to fund our growth.
Technology Development segment revenues, which consist primarily of government-funded research, accounted for 28%37% and 31%49% of our total revenues for the years ended December 31, 20162019 and 2015,2018, respectively. As a result, we are vulnerable to adverse changes in our revenues and cash flows if a significant number of our research contracts and subcontracts were to be simultaneously delayed or canceled for budgetary, performance or other reasons. For example, the U.S. government may cancel these contracts at any time without cause and without penalty or may change its requirements, programs or contract budget, any of which could reduce our revenues and cash flows from U.S. government research contracts.Our revenues and cash flows from U.S. government research contracts and subcontracts could also be reduced by declines or other changes in U.S. defense, homeland security and other federal agency budgets. In addition, we compete as a small business for some of these contracts, and in order to maintain our eligibility to compete as a small business, we, together with any affiliates, must continue to meet size and revenue limitations established by the U.S. government.
Our contract research customer base includes government agencies, corporations and academic institutions. Our customers are not obligated to extend their agreements with us and may elect not to do so. Also, our customers’ priorities regarding funding for certain projects may change and funding resources may no longer be available at previous levels.
In addition, the Budget Control Act commits the U.S. government to reduce the federal deficit by $1.2 trillion over ten years through a combination of automatic, across-the-board spending cuts and caps on discretionary spending. This “sequestration” under the Budget Control Act, which is split equally between defense and non-defense programs, went into effect on March 1, 2013. Any spending cuts required by “sequestration” could have a material adverse effect on our Technology Development segment revenues and, consequently, our results of operations. While the exact manner in which this “sequestration” may impact our business remains unclear, funding for programs in which we participate could be reduced, delayed or canceled. Our ability to obtain new contract awards also could be negatively affected.
In addition to contract cancellations and changes in agency budgets, our future financial results may be adversely affected by curtailment of or restrictions on the U.S. government’s use of contract research providers, including curtailment due to government budget reductions and related fiscal matters or any legislation or resolution limiting the number or amount of awards we may receive. These or other factors could cause U.S. defense and other federal agencies to conduct research internally rather than through commercial research organizations or direct awards to other organizations, to reduce their overall contract research requirements or to exercise their rights to terminate contracts. Alternatively, the U.S. government may discontinue the SBIR program or its funding altogether. Also, SBIR regulations permit increased competition for SBIR awards from companies that may not have previously been eligible, such as those backed by venture capital operating companies, hedge funds and private equity firms. Any of these developments could limit our ability to obtain new contract awards and adversely affect our revenues, cash flows and ability to fund our growth.
Our failure to attract, train and retain skilled employees or members of our senior management and to obtain necessary security clearances for such persons or maintain a facility security clearance would adversely affect our business and operating results.
The availability of highly trained and skilled technical and professional personnel is critical to our future growth and profitability. Competition for scientists, engineers, technicians and professional personnel is intense and our competitors aggressively recruit key employees. In the past, we have experienced difficulties in recruiting and hiring these personnel as a result of the tight labor market in certain fields. Any difficulty in hiring or retaining qualified employees, combined with our growth strategy and future needs for additional experienced personnel, particularly in highly specialized areas such as nanomaterial manufacturing and fiber optic sensing technologies, may make it more difficult to meet all of our needs for these employees in a timely manner. Although we intend to continue to devote significant resources to recruit, train and retain qualified employees, we may not be able to attract and retain these employees, especially in technical fields in which the supply of experienced qualified candidates is limited, or at the senior management level. Any failure to do so would have an adverse
effect on our business. Any loss of key personnel could have a material adverse effect on our ability to meet key operational objectives, such as timely and effective project milestones and product introductions, which in turn could adversely affect our business, results of operations and financial condition.
We provide certain services to the U.S. government that require us to maintain a facility security clearance and for certain of our employees and our board chairman to hold security clearances. In general, the failure for necessary persons to obtain or retain sufficient security clearances, any loss by us of a facility security clearance or any public reprimand related to security matters could result in a U.S. government customer terminating an existing contract or choosing not to renew a contract or prevent us from bidding on or winning certain new government contracts.
In addition, our future success depends in a large part upon the continued service of key members of our senior management team. We do not maintain any key-person life insurance policies on our officers. The loss of any members of our management team or other key personnel could seriously harm our business.
Our business is subject to the cyclical nature of the markets in which we compete, and any future downturn may reduce demand for our products and revenue.
Many factors beyond our control affect our business, including consumer confidence in the economy, interest rates, fuel prices and the general availability of credit. The overall economic climate and changes in Gross National Product growth have a direct impact on some of our customers and the demand for our products. We cannot be sure that our business will not be adversely affected as a result of an industry or general economic downturn.
Our customers may reduce capital expenditures and have difficulty satisfying liquidity needs because of continued turbulence in the U.S. and global economies, resulting in reduced sales of our products and harm to our financial condition and results of operations.
In particular, our historical results of operations have been subject to substantial fluctuations, and we may experience substantial period-to-period fluctuations in future results of operations. Any future downturn in the markets in which we compete could significantly reduce the demand for our products and therefore may result in a significant reduction in revenue or increase the volatility of the price of our common stock. Our revenue and results of operations may be adversely affected in the future due to changes in demand from customers or cyclical changes in the markets utilizing our products.
In addition, the telecommunications industry has, from time to time, experienced, and may again experience, a pronounced downturn. To respond to a downturn, many service providers may slow their capital expenditures, cancel or delay new developments, reduce their workforces and inventories and take a cautious approach to acquiring new equipment and technologies from original equipment manufacturers, which would have a negative impact on our business. Weakness in the global economy or a future downturn in the telecommunications industry may cause our results of operations to fluctuate from quarter-to-quarter and year-to-year, harm our business, and may increase the volatility of the price of our common stock.
Customer acceptance of our products is dependent on our ability to meet changing requirements, and any decrease in acceptance could adversely affect our revenue.
Customer acceptance of our products is significantly dependent on our ability to offer products that meet the changing requirements of our customers, including telecommunication, military, medical and industrial corporations, as well as government agencies. Any decrease in the level of customer acceptance of our products could harm our business.
Our products must meet exacting specifications, and defects and failures may occur, which may cause customers to return or stop buying our products.
Our customers generally establish demanding specifications for quality, performance and reliability that our products must meet. However, our products are highly complex and may contain defects and failures when they are first introduced or as new versions are released. Our products are also subject to roughharsh environments as they are integrated into our customer products for use by the end customers. If defects and failures occur in our products, we could experience lost revenue, increased costs, including warranty expense and costs associated with customer support, delays in or cancellations or rescheduling of orders or shipments, product returns or discounts, diversion of management resources or damage to our reputation and brand equity, and in some cases consequential damages, any of which would harm our operating results. In addition, delays in our ability to fill product orders as a result of quality control issues may negatively impact our relationship with our customers. We cannot assure you that we will have sufficient resources, including any available insurance, to satisfy any asserted claims.
Rapidly changing standards and regulations could make our products obsolete, which would cause our revenue and results of operations to suffer.
We design products to conform to our customers’ requirements and our customers’ systems may be subject to regulations established by governments or industry standards bodies worldwide. Because some of our products are designed to conform to current specific industry standards, if competing or new standards emerge that are preferred by our customers, we would have to make significant expenditures to develop new products. If our customers adopt new or competing industry standards with which our products are not compatible, or the industry groups adopt standards or governments issue regulations with which our products are not compatible, our existing products would become less desirable to our customers and our revenue and results of operations would suffer.
The markets for many of our products are characterized by changing technology which could cause obsolescence of our products, and we may incur substantial costs in delivering new products.
The markets for many of our products are characterized by changing technology, new product introductions and product enhancements, and evolving industry standards. The introduction or enhancement of products embodying new technology or the emergence of new industry standards could render existing products obsolete and result in a write down to the value of our inventory, or result in shortened product life cycles. Accordingly, our ability to compete is in part dependent on our ability to continually offer enhanced and improved products.
The success of our new product offerings will depend upon several factors, including our ability to:
accurately anticipate customer needs;
innovate and develop new technologies and applications;
successfully commercialize new technologies in a timely manner;
price products competitively and manufacture and deliver products in sufficient volumes and on time; and
differentiate our product offerings from those of our competitors.
Some of our products are used by our customers to develop, test and manufacture their products. We therefore must anticipate industry trends and develop products in advance of the commercialization of our customers’ products. In developing any new product, we may be required to make a substantial investment before we can determine the commercial viability of the new product. If we fail to accurately foresee our customers’ needs and future activities, we may invest heavily in research and development of products that do not lead to significant revenues.
Our inability to find new customers or retain existing customers could harm our business.
Our business is reliant on our ability to find new customers and retain existing customers. In particular, customers normally purchase the legacy APIcertain of our products and incorporate them into products that they, in turn, sell in their own markets on an ongoing basis. As a result, the historical sales orof these products have been dependent upon the success of our customers’ products and theour future performance of the legacy API business is dependent upon our success in finding new customers and receiving new orders from existing customers.
In several markets, the quality and reliability of our products are a major concern for our customers, not only upon the initial manufacture of the product, but for the life of the product. Many of our products are used in remote locations for higher value assembly, making servicing of our products unfeasible. Any failure of the quality or reliability of our products could harm our business.
If our customers do not qualify our products or if their customers do not qualify their products, our results of operations may suffer.
Most of our customers do not purchase our HSOR and optoelectronics products prior to qualification of the products and satisfactory completion of factory audits and vendor evaluation. Our existing products, as well as each new product, must pass through varying levels of qualification with our customers. In addition, because of the rapid technological changes in some markets, a customer may cancel or modify a design project before we begin large-scale manufacturing and receiving revenues from the customer. It is unlikely that we would be able to recover the expenses for cancelled or unutilized custom design projects. It is difficult to predict with any certainty whether our customers will delay or terminate product qualification or the frequency with which customers will cancel or modify their projects. Any such delay, cancellation or modification could have a negative effect on our results of operations.
In addition, once a customer qualifies a particular supplier’s product or component, these potential customers design the product into their system, which is known as a design-in win. Suppliers whose products or components are not designed in are
unlikely to make sales to that customer until at least the adoption of a future redesigned system. Even then, many customers may be reluctant to incorporate entirely new products into their new systems, as doing so could involve significant additional redesign efforts and increased costs. If we fail to achieve design-in wins in potential customers’ qualification processes, we will likely lose the opportunity for significant sales to those customers for a lengthy period of time.
If the end user customers that purchase systems from our customers fail to qualify or delay qualifications of any products sold by our customers that contain our products, our business could be harmed. The qualification and field testing of our customers’ systems by end user customers is long and unpredictable. This process is not under our control or that of our customers and, as a result, the timing of our sales may be unpredictable. Any unanticipated delay in qualification of one of our customers’ products could result in the delay or cancellation of orders from our customers for products included in their equipment, which could harm our results of operations.
Customer demand for our products is difficult to accurately forecast and, as a result, we may be unable to optimally match production with customer demand, which could adversely affect our business and financial results.
We make planning and spending decisions, including determining the levels of business that we will seek and accept, production schedules, inventory levels, component procurement commitments, personnel needs and other resource requirements, based on our estimates of customer requirements. The short-term nature of commitments by many of our customers and the possibility of unexpected changes in demand for their products reduce our ability to accurately estimate future customer requirements. On occasion, customers may require rapid increases in production, which can strain our resources, cause our manufacturing to be negatively impacted by materials shortages, necessitate higher or more restrictive procurement commitments, increase our manufacturing yield loss and scrapping of excess materials, and reduce our gross margin. We may not have sufficient capacity at any given time to meet the volume demands of our customers, or one or more of our suppliers may not have sufficient capacity at any given time to meet our volume demands. Conversely, a downturn in the markets in which our customers compete can cause, and in the past have caused, our customers to significantly reduce or delay the amount of products ordered or to cancel existing orders, leading to lower utilization of our facilities. Because many of our costs and operating expenses are relatively fixed, reduction in customer demand due to market downturns or other reasons would have a negative effect on our gross margin, operating income and cash flow.
Customer orders
Rapidly changing standards and forecasts areregulations could make our products obsolete, which would cause our revenue and results of operations to suffer.
We design products to conform to our customers’ requirements and our customers’ systems may be subject to cancellationregulations established by governments or modification at any time which could result in higher manufacturing costs.
Our salesindustry standards bodies worldwide. Because some of our products are made primarily pursuantdesigned to standard purchase orders for delivery of products. However, byconform to current specific industry practice, some orders may be canceledstandards, if competing or modified at any time. When a customer cancels an order, theynew standards emerge that are responsible for all finished goods, all costs, direct and indirect, incurred by us, as well as a reasonable allowance for anticipated profits. No assurance can be given that we will receive these amounts after cancellation. Furthermore, uncertainty in customer forecasts of their demands and other factors may lead to delays and disruptions in manufacturing, which could result in delays in product shipments to customers and could adversely affect our business.
Fluctuations and changes in customer demand are common in our business. Such fluctuations, as well as quality control problems experienced in manufacturing operations, may cause delays and disruptions in our manufacturing process and overall operations and reduce output capacity. As a result, product shipments could be delayed beyond the shipment schedules requestedpreferred by our customers, we would have to make significant expenditures to develop new products. If our customers adopt new or could be canceled,competing industry standards with which our products are not compatible, or the industry groups adopt standards or governments issue regulations with which our products are not compatible, our existing products would negatively affectbecome less desirable to our sales, operating income, strategic position at customers market share and reputation. In addition, disruptions, delays or cancellations could cause inefficient production which in turn could result in higher manufacturing costs, lower yieldsour revenue and potential excess and obsolete inventory or manufacturing equipment. In the past, we have experienced such delays, disruptions and cancellations.results of operations would suffer.
The results of our operations could be adversely affected by economic and political conditions and the effects of these conditions on our customers’ businesses and levels of business activity.
Global economic and political conditions affect our customers’ businesses and the markets they serve. A severe or prolonged economic downturn or a negative or uncertain political climate could adversely affect our customers’ financial conditions and the timing or levels of business activity of our customers and the industries we serve. This may reduce the demand for our products or depress pricing for our products and have a material adverse effect on our results of operations. Changes in global economic conditions could also shift demand to products or services for which we do not have competitive advantages, and this could negatively affect the amount of business we are able to obtain. In addition, if we are unable to successfully anticipate changing economic and political conditions, we may be unable to effectively plan for and respond to those changes, and our business could be negatively affected as a result.
We have a history ofexperienced net losses in the past, and because our strategy for expansion may be costly to implement, we may experience continuing losses and may never achieve ornot maintain profitability or positive cash flow.
We realized ahave experienced net loss from continuing operations of $2.4 million and $6.0 million forlosses in the years ended December 31, 2016 and 2015, respectively.past. We expect to continue to incur significant expenses as we pursue our strategic initiatives, including increased expenses for research and development, sales and marketing and manufacturing. We may also grow our business in part through acquisitions of additional companies and complementary technologies which could cause us to incur greater than anticipated transaction expenses, amortization or write-offs of intangible assets and other acquisition-related expenses. As a result, we may incur net losses forin the foreseeable future, and these losses could be substantial. At a certain level, continued net losses could impair our ability to comply with NASDAQNasdaq continued listing standards, as described further below.
Our ability to generate additional revenues and to becomeremain profitable will depend on our ability to execute our key growth initiative regarding the development, marketing and sale of HSOR and sensing products, develop and commercialize innovative technologies, expand our contract research capabilities and sell the products that result from those development initiatives. We are unable to predict when or if we will be able to achieve profitability. If our revenues do not increase, or if our expenses increase at a greater rate than our revenues, we will continue to experience losses. Even if we do achieve profitability, we may not be able to sustain or increase our profitability on a quarterly or annual basis.
We have obtained capital by borrowing money under a term loan and we mightmay require additional capital to support and expand our business; our term loan has various loan covenants with which we must comply.business.
We intend to continue to make investments to support our business growth, including developing new products, enhancing our existing products, obtaining important regulatory approvals, enhancing our operating infrastructure, completing our development activities and building our commercial scale manufacturing facilities. To the extent that we are unable to become or remain profitable and to finance our activities from our continuing operations, we may require additional funds to support these initiatives and to grow our business.
If we are successful in raising additional funds through issuances of equity or convertible debt securities, our existing stockholders could suffer significant dilution, including as the result of the issuance of warrants in connection with the financing, and any new equity securities we issue could have rights, preferences and privileges superior to those of our existing common stock. Furthermore, such financings may jeopardize our ability to apply for SBIR grants or qualify for SBIR contracts or grants, and our dependence on SBIR grants may restrict our ability to raise additional outside capital. If we raise additional funds through debt financings, these financings may involve significant cash payment obligations and covenants that restrict our ability to operate our business and make distributions to our stockholders.
We have a term loan with Silicon Valley Bank ("SVB"), which requires us to observe certain financial and operational covenants, including maintenance of a minimum cash balance of $5.0 million, protection and registration of intellectual property rights, and certain customary negative covenants, as well as other customary events of default. If any event of default occurs SVB may declare due immediately all borrowings under our term loan and foreclose on the collateral. Furthermore, an event of default would result in an increase in the interest rate on any amounts outstanding.
If we are unable to obtain adequate financing or financing terms satisfactory to us when we require it, our ability to continue to support our business growth and to respond to business challenges could be significantly limited.
Our nanotechnology-enabled products are new and may be, or may be perceived as being, harmful to human health or the environment.
While we believe that none of our current products contain chemicals known by us to be hazardous or subject to environmental regulation, it is possible that our current or future products, particularly carbon-based nanomaterials, may become subject to environmental or other regulation. We intend to develop and sell carbon-based nanomaterials as well as nanotechnology-enabled products, which are products that include nanomaterials as a component to enhance those products’ performance. Nanomaterials and nanotechnology-enabled products have a limited historical safety record. Because of their size or shape or because they may contain harmful elements, such as gadolinium and other rare-earth metals, our products could pose a safety risk to human health or the environment. These characteristics may also cause countries to adopt regulations in the future prohibiting or limiting the manufacture, distribution or use of nanomaterials or nanotechnology-enabled products. Such regulations may inhibit our ability to sell some products containing those materials and thereby harm our business or impair our ability to develop commercially viable products.
The subject of nanotechnology has received negative publicity and has aroused public debate. Government authorities could, for social or other purposes, prohibit or regulate the use of nanotechnology. Ethical and other concerns about
nanotechnology could adversely affect acceptance of our potential products or lead to government regulation of nanotechnology-enabled products.
We face and will face substantial competition in several different markets that may adversely affect our results of operations.
We face and will face substantial competition from a variety of companies in several different markets. As we focus on developing marketing and selling fiber optic sensing products, we may also face substantial and entrenched competition in that market.
Many of our competitors have longer operating histories, greater name recognition, larger customer bases and significantly greater financial, sales and marketing, manufacturing, distribution, technical and other resources than we do. These competitors may be able to adapt more quickly to new or emerging technologies and changes in customer requirements. In addition, current and potential competitors have established or may establish financial or strategic relationships among themselves or with existing or potential customers or other third parties. Accordingly, new competitors or alliances among competitors could emerge and rapidly acquire significant market share. We cannot assure you that we will be able to compete successfully against current or new competitors, in which case our revenues may fail to increase or may decline.
Intense competition in our markets could result in aggressive business tactics by our competitors, including aggressively pricing their products or selling older inventory at a discount. If our current or future competitors utilize aggressive business tactics, including those described above, demand for our products could decline, we could experience delays or cancellations of customer orders, or we could be required to reduce our sales prices.
Decreases in average selling prices of our products may increase operating losses and net losses, particularly if we are not able to reduce expenses commensurately.
The market for optical components and subsystems continues to be characterized by declining average selling prices resulting from factors such as increased price competition among optical component and subsystem manufacturers, excess capacity, the introduction of new products and increased unit volumes as manufacturers continue to deploy network and storage systems. In recent years, we have observed a significant decline of average selling prices, primarily in the telecommunications market. We anticipate that average selling prices will continue to decrease in the future in response to product introductions by competitors or by us, or in response to other factors, including price pressures from significant customers. In order to sustain profitable operations, we must, therefore, reduce the cost of our current designs or continue to develop and introduce new products on a timely basis that incorporate features that can be sold at higher average selling prices. Failure to do so could cause our sales to decline and operating losses to increase.
Our cost reduction efforts may not keep pace with competitive pricing pressures. To remain competitive, we must continually reduce the cost of manufacturing our products through design and engineering changes. We may not be successful in redesigning our products or delivering our products to market in a timely manner. We cannot assure you that any redesign will result in sufficient cost reductions enabling us to reduce the price of our products to remain competitive or positively contribute to operating results.
Shifts in product mix may result in declines in gross profit.
Our gross profit margins vary among our product platforms, and are generally highest on our test &and measurement instruments. Our overall gross profit may fluctuate from period to period as a result of a variety of factors including shifts in product mix, the introduction of new products, and decreases in average selling prices for older products. If our customers decide to buy more of our products with low gross profit margins or fewer of our products with high gross profit margins, our total gross profits could be harmed.
Risks Relating to our Operations and Business Strategy
If we are unable to successfully integrate acquired businesses, it could have an adverse effect on our future results and the market price of our common stock.
In the past, we have acquired businesses to support our growth strategy, including the acquisition of General Photonics Corporation in March 2019 and Micron Optics, Inc. in October 2018. In the future, we may continue to seek acquisition targets supporting our growth strategy. The success of an acquisition will depend, in large part, on sales of the acquired company's products and the realization of operating synergies. To realize these anticipated benefits, we must successfully integrate the acquired company's business into our existing business. Such integrations may be complex and time-consuming. The failure to successfully integrate and manage the challenges presented by the integration process may result in our failure to achieve some or all of the anticipated benefits of the acquisition. Potential difficulties that may be encountered in the integration process include the following:
lost sales and customers as a result of customers deciding not to do business with us;
complexities associated with managing the larger combined company with distant business locations;
integrating personnel while maintaining focus on providing consistent, high quality products;
loss of key employees;
potential unknown liabilities associated with the acquisition; and
performance shortfalls as a result of the division of management's attention caused by completing the acquisition and integrating operations.
If any of these events were to occur, our ability to maintain relationships with the customers, suppliers and employees or our ability to achieve the anticipated benefits of the acquisition could be adversely affected, or could reduce our future earnings or otherwise adversely affect our business and financial results and, as a result, adversely affect the market price of our common stock.
If we cannot successfully transition our revenue mix from contract research revenues to product sales and license revenues, we may not be able to fully execute our business model or grow our business.
Our business model and future growth depend on our ability to transition to a revenue mix that contains significantly larger product sales and revenues from the provision of services or from licensing. Product sales and these revenues potentially offer greater scalability than contract research revenues. Our current plan is to increase our sales of commercial products, our licensing revenues and our provision of non-research services to customers so as to represent a larger percentage of our total revenues. If we are unable to develop and grow our product sales and revenues from the provision of services or from licensing
to augment our contract research revenues, however, our ability to execute our business model or grow our business could suffer. There can be no assurance that we will be able to achieve increased revenues in this manner.
Failure to develop, introduce and sell new products or failure to develop and implement new technologies, could adversely impact our financial results.
Our success will depend on our ability to develop and introduce new products that customers choose to buy. The new products the market requires tend to be increasingly complex, incorporating more functions and operating at faster speeds than old products. If we fail to introduce new product designs or technologies in a timely manner or if customers do not successfully introduce new systems or products incorporating our products, our business, financial condition and results of operations could be materially harmed.
If we are unable to manage growth effectively, our revenues and net loss could be adversely affected.
We may need to expand our personnel resources to grow our business effectively. We believe that sustained growth at a higher rate will place a strain on our management as well as on our other human resources. To manage this growth, we must continue to attract and retain qualified management, professional, scientific and technical and operating personnel. If we are unable to recruit a sufficient number of qualified personnel, we may be unable to staff and manage projects adequately, which in turn may slow the rate of growth of our contract research revenues or our product development efforts.
We may not be successful in identifying market needs for new technologies or in developing new products.
Part of our business model depends on our ability to correctly identify market needs for new technologies. We intend to identify new market needs, but we may not always have success in doing so in part because our contract research largely
centers on identification and development of unproven technologies, often for new or emerging markets. Furthermore, we must identify the most promising technologies from a sizable pool of projects. If our commercialization strategy process fails to identify projects with commercial potential or if management does not ensure that such projects advance to the commercialization stage, we may not successfully commercialize new products and grow our revenues.
Our growth strategy requires that we also develop successful commercial products to address market needs. We face several challenges in developing successful new products. Many of our existing products and those currently under development are technologically innovative and require significant and lengthy product development efforts. These efforts include planning, designing, developing and testing at the technological, product and manufacturing-process levels. These activities require us to make significant investments. Although there are many potential applications for our technologies, our resource constraints require us to focus on specific products and to forgo other opportunities. We expect that one or more of the potential products we choose to develop will not be technologically feasible or will not achieve commercial acceptance, and we cannot predict which, if any, of our products we will successfully develop or commercialize. The technologies we research and develop are new and steadily changing and advancing. The products that are derived from these technologies may not be applicable or compatible with the state of technology or demands in existing markets. Our existing products and technologies may become uncompetitive or obsolete if our competitors adapt more quickly than we do to new technologies and changes in customers’ requirements. Furthermore, we may not be able to identify if and when new markets will open for our products given that future applications of any given product may not be readily determinable, and we cannot reasonably estimate the size of any markets that may develop. If we are not able to successfully develop new products, we may be unable to increase our product revenues.
We face risks associated with our international business.
We currently conduct business internationally and we might considerably expand our international activities in the future. Our international business operations are subject to a variety of risks associated with conducting business internationally, including:
having to comply with U.S. export control regulations and policies that restrict our ability to communicate with non-U.S. employees and supply foreign affiliates and customers;
changes in or interpretations of foreign regulations that may adversely affect our ability to sell our products, perform services or repatriate profits to the United States;
the imposition of tariffs;
hyperinflation or economic or political instability in foreign countries;
imposition of limitations on, or increase of withholding and other taxes on remittances and other payments by foreign subsidiaries or joint ventures;
conducting business in places where business practices and customs are unfamiliar and unknown;
the imposition of restrictive trade policies;
the imposition of inconsistent laws or regulations;
the imposition or increase of investment and other restrictions or requirements by foreign governments;
uncertainties relating to foreign laws and legal proceedings;
having to comply with a variety of U.S. laws, including the Foreign Corrupt Practices Act ("FCPA"); and
having to comply with licensing requirements.
We do not know the impact that these regulatory, geopolitical and other factors may have on our international business in the future. Further, the developing situation regarding the public health epidemic originating in China, has prompted precautionary government-imposed closures of certain travel and business. It is unknown whether and how global supply chains, may be affected if such an epidemic persists for an extended period of time. We may incur expenses or delays relating to such events outside of our control, or experience potential disruption of our ability to travel to customer sites and industry conferences important to the marketing and support of our products, any of which could have an adverse impact on our business, operating results and financial condition.
Legal, political and economic uncertainty surrounding the exit of the U.K., from the European Union may be a source of instability in international markets, create significant currency fluctuations, adversely affect our operations in the U.K. and pose additional risks to our business, revenue, financial condition and results of operations.
Following the result of a referendum in 2016, the U.K. left the EU on January 31, 2020, commonly referred to as Brexit. Pursuant to the formal withdrawal arrangements agreed between the U.K. and the EU, the U.K. will be subject to a transition period until December 31, 2020 (the "Transition Period"), during which the EU rules will continue to apply.
Negotiations between the U.K. and the EU are expected to continue in relation to the customs and trading relationship between the U.K. and the EU following the expiry of the Transition Period.
The uncertainty concerning the U.K.'s legal, political and economic relationship with the EU after the Transition Period may be a source of instability in the international markets, create significant currency fluctuations, and/or otherwise adversely affect trading agreements or similar cross-border cooperation arrangements (whether economic, tax, legal, regulatory or otherwise).
These developments, or the perception that any of them could occur, have had and may continue to have a significant adverse effect on global economic conditions and the stability of global financial markets, and could significantly reduce global market liquidity and limit the ability of key market participants to operate in certain financial markets. In particular, it could also lead to a period of considerable uncertainty in relation to the U.K. financial and banking markets, as well as on the regulatory process in Europe. Asset valuations, currency exchange rates and credit ratings may also be subject to increased market volatility.
If the U.K. and the EU are unable to negotiate acceptable trading and customs terms or if other EU Member States pursue withdrawal, barrier-free access between the U.K. and other EU Member States or among the European Economic Area overall could be diminished or eliminated. The long-term effects of Brexit will depend on any agreements (or lack thereof) between the U.K. and the EU and, in particular, any arrangements for the U.K. to retain access to EU markets after the Transition Period.
Such a withdrawal from the EU is unprecedented, and it is unclear how the U.K.'s access to the European single market for goods, capital, services and labor within the EU, or single market, and the wider commercial, legal and regulatory environment will impact our operations and customers. There may continue to be economic uncertainty surrounding the consequences of Brexit which could adversely impact customer confidence resulting in customers reducing their spending budgets, which could adversely affect our business, revenue, financial condition, and results of operations and could adversely affect the market price of our common stock.
We may dispose of or discontinue existing product lines and technology developments, which may adversely impact our future results.
On an ongoing basis, we evaluate our various product offerings and technology developments in order to determine whether any should be discontinued or, to the extent possible, divested. In addition, if we are unable to generate the amount of cash needed to fund the future operations of our business, we may be forced to sell one or more of our product lines or technology developments.
We cannot guarantee that we have correctly forecasted, or that we will correctly forecast in the future, the right product lines and technology developments to dispose or discontinue or that our decision to dispose of or discontinue various investments, productsproduct lines and technology developments is prudent if market conditions change. In addition, there are no assurances that the discontinuance of various product lines will reduce operating expenses or will not cause us to incur material charges associated with such decision. Furthermore, the discontinuance of existing product lines entails various risks, including the risk that we will not be able to find a purchaser for a product line or the purchase price obtained will not be equal to at least the book value of the net assets for the product line. Other risks include managing the expectations of, and maintaining good relations with, our historical customers who previously purchased products from a disposed or discontinued product line, which could prevent us from selling other products to them in the future. We may also incur other significant liabilities and costs associated with disposal or discontinuance of product lines, including employee severance costs and excess facilities costs.
We may be liable for damages based on product liability claims relating to defects in our products, which might be brought against us directly, or against our customers in their end-use markets. Such claims could result in a loss of customers in addition to substantial liability in damages.
Our products are complex and undergo quality testing as well as formal qualification, both by our customers and by us. However, defects may occur from time to time. Our customers’ testing procedures may be limited to evaluating our products under likely and foreseeable failure scenarios and over varying amounts of time. For various reasons, such as the occurrence of performance problems that are unforeseeable in testing or that are detected only when products age or are operated under peak stress conditions, our products may fail to perform as expected long after customer acceptance. Failures could result from faulty components or design, problems in manufacturing or other unforeseen reasons. As a result, we could incur significant costs to repair or replace defective products under warranty, particularly when such failures occur in installed systems. In addition, we may in certain circumstances honor warranty claims after the warranty has expired or for problems not covered by warranty in order to maintain customer relationships. Any significant product failure could result in lost future sales of the affected product and other products, as well as customer relations problems, litigation and damage to our reputation.
In addition, many of our products are embedded in, or deployed in conjunction with, our customers’ products, which incorporate a variety of components, modules and subsystems and may be expected to interoperate with modules produced by third parties. As a result, not all defects are immediately detectable, and, when problems occur, it may be difficult to identify the source of the problem. These problems may cause us to incur significant damages or warranty and repair costs, divert the attention of our engineering personnel from internal product development efforts and cause significant customer relations problems or loss of customers, all of which would harm our business.
Furthermore, many of our products may provide critical performance attributes to our customers’ products that will be sold to end users who could potentially bring product liability suits in which we could be named as a defendant. The sale of these products involves the risk of product liability claims. If a person were to bring a product liability suit against one of our customers, this customer may attempt to seek contribution from us. A person may also bring a product liability claim directly against us. A successful product liability claim or series of claims against us in excess of our insurance coverage for payments, for which we are not otherwise indemnified, could have a material adverse effect on our financial condition or results of operations.
We could be negatively affected by a security breach or other compromise, either through cyber-attack, cyber-intrusion or other significant disruption of our IT networks and related systems.
We face the risk, as does any company, of a security breach or other compromise, whether through cyber-attack or cyber-intrusion over the internet, malware, computer viruses, attachments to e-mails, persons inside our organization or persons with access to systems inside our organization, or other significant disruption of our IT networks and related systems. The risk of a security breach or disruption, particularly through cyber-attack or cyber-intrusion, including by computer hackers, foreign governments and cyber terrorists, has increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. We may also experience security breaches or compromises from unintentional or accidental actions by our employees, contractors, consultants, business partners, and/or other third parties. To the extent that any security breach or disruption were to result in a loss, destruction, unavailability, alteration or dissemination of, or damage to, our data or applications, or for it to be believed or reported that any of these occurred, we could incur liability and reputational damage.
As a technology company, and particularly as a government contractor, we may face a heightened risk of a security breach, compromise or disruption from threatsattempts to gain unauthorized access to our proprietary, confidential or classified information on our IT networks and related systems.systems via cyber-attacks or cyber-intrusions. These types of information and IT networks and related systems are critical to the operation of our business and essential to our ability to perform day-to-day operations, and, in some cases, are critical to theour operations of certainor those of our customers. Such critical information includes our proprietary software code, which we protect as a trade secret and is critical to the competitive advantage of many of our products, which could be adversely affected if this code were stolen in a cyber-intrusion or otherwise compromised. In addition, as certain of our technological capabilities become widely known, it is possible that we may be subjected to cyber-attack or cyber-intrusion as third parties seek to gain improper access to information regarding these capabilities and cyber-attacks or cyber-intrusion could compromise our confidential information or our IT networks and systems generally, as it is not practical as a business matter to isolate all of our confidential information and trade secrets from email and internet access. There can be no assurance that our security efforts and measures will be effective or that attempted security breaches or disruptions would not be successful or damaging.
A security breach, compromise or other significant disruption involving these types of information and IT networks and related systems could disrupt the proper functioning of these networks and systems and therefore our operations, compromise our confidential information and trade secrets, or damage our reputation among our customers and the public generally. We have not identified any significant security breaches or experienced other significant disruptions of these types to date. To date, we have not experienced a significant cyber-intrusion, cyber-attack or other similar disruption. There can be no assurance that our security efforts and measures will be effective or that attempted security breaches or disruptions would not be successful or damaging. Any of these developments in the future could have a negative impact on our results of operations, financial condition and cash flows.
We face risks related to health epidemics and other outbreaks, which could significantly disrupt our operations.
Our business could be adversely impacted by the effects of COVID-19 or other epidemics. Several of our customers and suppliers are located throughout Asia, and particularly in China and, consequently, we are susceptible to factors adversely affecting one or more of these locations. As a result, COVID-19, or any other epidemic, could impact our customers or suppliers in these regions, which could subject our business to disruptions, such as temporary closure of our offices or those of our customers or suppliers, or suspension of our services. Additionally, we are subject to risks that COVID-19 or other epidemic harms the Chinese economy in general. Any of such developments could materially and adversely affect our business, financial condition and results of operations.
Risks Relating to our Regulatory Environment
Our operations are subject to domestic and foreign laws, regulations and restrictions, and noncompliance with these laws, regulations and restrictions could expose us to fines, penalties, suspension or debarment, which could have a material adverse effect on our profitability and overall financial position.
Our operations, particularly our international sales, subject us to numerous U.S. and foreign laws and regulations, including, without limitation, regulations relating to imports, exports (including the Export Administration Regulations and the International Traffic in Arms Regulations), technology transfer restrictions, anti-boycott provisions, economic sanctions and the FCPA. The number of our various emerging technologies, the development of many of which has been funded by the Department of Defense, presents us with many regulatory challenges. Failure by us or our sales representatives or consultants to comply with these laws and regulations could result in administrative, civil, or criminal liabilities and could result in suspension of our export privileges, which could have a material adverse effect on our business. Changes in regulation or political environment may affect our ability to conduct business in foreign markets including investment, procurement and repatriation of earnings.
Environmental regulations could increase operating costs and additional capital expenditures and delay or interrupt operations.
The photonics industry, as well as the semiconductor industry, are subject to governmental regulations for the protection of the environment, including those relating to air and water quality, solid and hazardous waste handling, and the promotion of occupational safety. Various federal, state and local laws and regulations require that we maintain certain environmental permits. While we believe that we have obtained all necessary environmental permits required to conduct our manufacturing processes, if we are found to be in violation of these laws, we could be subject to governmental fines and liability for damages resulting from such violations.
Changes in the aforementioned laws and regulations or the enactment of new laws, regulations or policies could require increases in operating costs and additional capital expenditures and could possibly entail delays or interruptions of our operations.
If our manufacturing facilities do not meet Federal, state or foreign country manufacturing standards, we may be required to temporarily cease all or part of our manufacturing operations, which would result in product delivery delays and negatively impact revenues.
Our manufacturing facilities are subject to periodic inspection by regulatory authorities and our operations will continue to be regulated by the FDA for compliance with Good Manufacturing Practice requirements contained in the quality systems regulations. We are also required to comply with International Organization for Standardization ("ISO"), quality system standards in order to produce certain of our products for sale in Europe. If we fail to continue to comply with Good Manufacturing Practice requirements or ISO standards, we may be required to cease all or part of our operations until we comply with these regulations. Obtaining and maintaining such compliance is difficult and costly. We cannot be certain that our facilities will be found to comply with Good Manufacturing Practice requirements or ISO standards in future inspections and audits by regulatory authorities. In addition, if we cannot maintain or establish manufacturing facilities or operations that comply with such standards or do not meet the expectations of our customers, we may not be able to realize certain economic opportunities in our current or future supply arrangements.
Medical products are subject to various international regulatory processes and approval requirements. If we do not obtain and maintain the necessary international regulatory approvals for any such potential products, we may not be able to market and sell our medical products in foreign countries.
To be able to market and sell medical products in other countries, we must obtain regulatory approvals and comply with the regulations of those countries. These regulations, including the requirements for approvals and the time required for regulatory review, vary from country to country. Obtaining and maintaining foreign regulatory approvals are expensive, and we cannot be certain that we will have the resources to be able to pursue such approvals or whether we would receive regulatory approvals in any foreign country in which we plan to market our products. For example, the European Union requires that manufacturers of medical products obtain the right to affix the CE mark to their products before selling them in member countries of the European Union, which we have not yet obtained and may never obtain. If we fail to obtain regulatory approval in any foreign country in which we plan to market our products, our ability to generate revenues will be harmed.
We are subject to additional significant foreign and domestic government regulations, including environmental and health and safety regulations, and failure to comply with these regulations could harm our business.
Our facilities and current and proposed activities involve the use of a broad range of materials that are considered hazardous under applicable laws and regulations. Accordingly, we are subject to a number of foreign, federal, state and local laws and regulations relating to health and safety, protection of the environment and the storage, use, disposal of, and exposure to, hazardous materials and wastes. We could incur costs, fines and civil and criminal penalties, personal injury and third partythird-party property damage claims or could be required to incur substantial investigation or remediation costs, if we were to violate or become liable under environmental, health and safety laws. Moreover, a failure to comply with environmental laws could result in fines and the revocation of environmental permits, which could prevent us from conducting our business. Liability under environmental laws can be joint and several and without regard to fault. There can be no assurance that violations of environmental and health and safety laws will not occur in the future as a result of the inability to obtain permits, human error, equipment failure or other causes. Environmental laws could become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations, which could harm our business. Accordingly, violations of present and future environmental laws could restrict our ability to expand facilities, pursue certain technologies, and could require us to acquire costly equipment or incur potentially significant costs to comply with environmental regulations.
Compliance with foreign, federal, state and local environmental laws and regulations represents a small part of our present budget. If we fail to comply with any such laws or regulations, however, a government entity may levy a fine on us or require us to take costly measures to ensure compliance. Any such fine or expenditure may adversely affect our development. We cannot predict the extent to which future legislation and regulation could cause us to incur additional operating expenses, capital expenditures or restrictions and delays in the development of our products and properties.
We are or may become subject to a variety of privacy and data security laws, and our failure to comply with them could harm our business.
We maintain sensitive information, including confidential business and personal information in connection with our business customers and our employees, and may be subject to laws and regulations governing the privacy and security of such information. In the United States, there are numerous federal and state privacy and data security laws and regulations governing the collection, use, disclosure and protection of personal information. Each of these constantly evolving laws can be subject to varying interpretations.
In addition, states are constantly adopting new laws or amending existing laws, requiring attention to frequently changing regulatory requirements. For example, California enacted the California Consumer Privacy Act, or the CCPA, on June 28, 2018, which took effect on January 1, 2020 and has been dubbed the first “GDPR-like” law in the United States. The CCPA gives California residents expanded rights to access and delete their personal information, opt out of certain personal information sharing and receive detailed information about how their personal information is used by requiring covered companies to provide new disclosures to California consumers (as that term is broadly defined and can include any of our current or future employees who may be California residents) and provide such residents new ways to opt-out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. The CCPA may increase our compliance costs and potential liability. Some observers have noted that the CCPA could mark the beginning of a trend toward more stringent privacy legislation in the United States. Other states are beginning to pass similar laws.
A similar situation exists in the EU. In May 2018, a new privacy regime, the General Data Protection Regulation, the GDPR, took effect in the European Economic Area, the EEA. The GDPR governs the collection, use, disclosure, transfer or other processing of personal data of European data subjects. Among other things, the GDPR imposes requirements regarding the security of personal data and notification of data processing obligations to the competent national data processing authorities, changes the lawful bases on which personal data can be processed, and expands the definition of personal data. In addition, the GDPR increases the scrutiny of transfers of personal data from the EEA to the United States and other jurisdictions that the European Commission does not recognize as having “adequate” data protection laws, and imposes substantial fines for breaches and violations (up to the greater of €20 million or 4% of our consolidated annual worldwide gross revenue). The GDPR also confers a private right of action on data subjects and consumer associations to lodge complaints with supervisory authorities, seek judicial remedies and obtain compensation for damages resulting from violations of the GDPR. Compliance with these and any other applicable privacy and data security laws and regulations is a rigorous and time-intensive process, and we may be required to put in place additional mechanisms ensuring compliance with the new data protection rules. If we fail to comply with any such laws or regulations, we may face significant fines and penalties that could adversely affect our business, financial condition and results of operations. Furthermore, the laws are not consistent, and compliance in the event of a widespread data breach could be costly.
Risks Relating to our Intellectual Property
Our proprietary rights may not adequately protect our technologies.
Our commercial success will depend in part on our obtaining and maintaining patent, trade secret, copyright and trademark protection of our technologies in the United States and other jurisdictions as well as successfully enforcing this intellectual property and defending it against third-party challenges. We will only be able to protect our technologies from unauthorized use by third parties to the extent that valid and enforceable intellectual property protections, such as patents or trade secrets, cover them. In particular, we place considerable emphasis on obtaining patent and trade secret protection for significant new technologies, products and processes. The degree of future protection of our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. The degree of future protection of our proprietary rights is also uncertain for products that are currently
in the early stages of development because we cannot predict which of these products will ultimately reach the commercial market or whether the commercial versions of these products will incorporate proprietary technologies.
Our patent position is highly uncertain and involves complex legal and factual questions. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. For example:
we or our licensors might not have been the first to make the inventions covered by each of our pending patent applications and issued patents;
we or our licensors might not have been the first to file patent applications for these inventions;
others may independently develop similar or alternative technologies or duplicate any of our technologies;
it is possible that none of our pending patent applications or the pending patent applications of our licensors will result in issued patents;
patents may issue to third parties that cover how we might practice our technology;
our issued patents and issued patents of our licensors may not provide a basis for commercially viable technologies, may not provide us with any competitive advantages, or may be challenged and invalidated by third parties; and
we may not develop additional proprietary technologies that are patentable.
Patents may not be issued for any pending or future pending patent applications owned by or licensed to us, and claims allowed under any issued patent or future issued patent owned or licensed by us may not be valid or sufficiently broad to protect our technologies. Moreover, protection of certain of our intellectual property may be unavailable or limited in the United States or in foreign countries, and we have not sought to obtain foreign patent protection for certain of our products or technologies due to cost, concerns about enforceability or other reasons. Any issued patents owned by or licensed to us now or in the future may be challenged, invalidated, or circumvented, and the rights under such patents may not provide us with competitive advantages. In addition, competitors may design around our technology or develop competing technologies. Intellectual property rights may also be unavailable or limited in some foreign countries, and in the case of certain products no foreign patents were filed or can be filed. This could make it easier for competitors to capture or increase their market share with respect to related technologies. We could incur substantial costs to bring suits in which we may assert our patent rights against others or defend ourselves in suits brought against us. An unfavorable outcome of any litigation could have a material adverse effect on our business and results of operations.
We also rely on trade secrets to protect our technology, especially where we believe patent protection is not appropriate or obtainable. However, trade secrets are difficult to protect. We regularly attempt to obtain confidentiality agreements and contractual provisions with our collaborators, employees and consultants to protect our trade secrets and proprietary know-how. These agreements may be breached or may not have adequate remedies for such breach. While we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors or scientific and other advisors, or those of our strategic partners, may unintentionally or willfully disclose our information to competitors. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, our enforcement efforts would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside the United States are sometimes unwilling to protect trade secrets. Moreover, if our competitors independently develop equivalent knowledge, methods and know-how, it will be more difficult for us to enforce our rights and our business could be harmed.
If we are not able to defend the patent or trade secret protection position of our technologies, then we will not be able to exclude competitors from developing or marketing competing technologies and we may not generate enough revenues from product sales to justify the cost of developing our technologies and to achieve or maintain profitability.
We also rely on trademarks to establish a market identity for our company and our products. To maintain the value of our trademarks, we might have to file lawsuits against third parties to prevent them from using trademarks confusingly similar to or dilutive of our registered or unregistered trademarks. Also, we might not obtain registrations for our pending trademark applications, and we might have to defend our registered trademark and pending trademark applications from challenge by third parties. Enforcing or defending our registered and unregistered trademarks might result in significant litigation costs and damages, including the inability to continue using certain trademarks.
Third parties may claim that we infringe their intellectual property, and we could suffer significant litigation or licensing expense as a result.
Various U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in our technology areas. Such third parties may claim that we infringe their patents. Because patent applications can take several years to result in a patent issuance, there may be currently pending applications, unknown to us, which may later result in issued patents that our technologies may infringe. For example, we are aware of competitors with patents in technology areas applicable to our optical test equipment products. Such competitors may allege that we infringe these patents. There could also be existing patents of which we are not aware that our technologies may inadvertently infringe. We have from time to time
been, and may in the future be, contacted by third parties, including patent assertion entities or intellectual property advisors, about licensing opportunities that also contain claims that we are infringing on third party patent rights. If third parties assert these claims against us, we could incur extremely substantial costs and diversion of management resources in defending these claims, and the defense of these claims could have a material adverse effect on our business, financial condition and results of operations. Even if we believe we have not infringed on a third party’s patent rights, we may have to settle a claim on unfavorable terms because we cannot afford to litigate the claim. In addition, if third parties assert claims against us and we are unsuccessful in defending against these claims, these third parties may be awarded substantial damages as well as injunctive or other equitable relief against us, which could effectively block our ability to make, use, sell, distribute or market our products and services in the United States or abroad.
Commercial application of nanotechnologies in particular, or technologies involving nanomaterials, is new and the scope and breadth of patent protection is uncertain. Consequently, the patent positions of companies involved in nanotechnologies have not been tested, and there are complex legal and factual questions for which important legal principles will be developed or may remain unresolved. In addition, it is not clear whether such patents will be subject to interpretations or legal doctrines that differ from conventional patent law principles. Changes in either the patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our nanotechnology-related intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our nanotechnology-related patents or in third party patents. In the event that a claim relating to intellectual property is asserted against us, or third parties not affiliated with us hold pending or issued patents that relate to our products or technology, we may seek licenses to such intellectual property or challenge those patents. However, we may be unable to obtain these licenses on commercially reasonable terms, if at all, and our challenge of the patents may be unsuccessful. Our failure to obtain the necessary licenses or other rights could prevent the sale, manufacture or distribution of our products and, therefore, could have a material adverse effect on our business, financial condition and results of operations.
A substantial portion of our technology is subject to retained rights of our licensors, and we may not be able to prevent the loss of those rights or the grant of similar rights to third parties.
A substantial portion of our technology is licensed from academic institutions, corporations and government agencies. Under these licensing arrangements, a licensor may obtain rights over the technology, including the right to require us to grant a
license to one or more third parties selected by the licensor or that we provide licensed technology or material to third parties for non-commercial research. The grant of a license for any of our core technologies to a third party could have a material and adverse effect on our business. In addition, some of our licensors retain certain rights under the licenses, including the right to grant additional licenses to a substantial portion of our core technology to third parties for non-commercial academic and research use. It is difficult to monitor and enforce such non-commercial academic and research uses, and we cannot predict whether the third-party licensees would comply with the use restrictions of such licenses. We have incurred and could incur substantial expenses to enforce our rights against them. We also may not fully control the ability to assert or defend those patents or other intellectual property which we have licensed from other entities, or which we have licensed to other entities.
In addition, some of our licenses with academic institutions give us the right to use certain technology previously developed by researchers at these institutions. In certain cases, we also have the right to practice improvements on the licensed technology to the extent they are encompassed by the licensed patents and are within our field of use. Our licensors may currently own and may in the future obtain additional patents and patent applications that are necessary for the development, manufacture and commercial sale of our anticipated products. We may be unable to agree with one or more academic institutions from which we have obtained licenses whether certain intellectual property developed by researchers at these academic institutions is covered by our existing licenses. In the event that the new intellectual property is not covered by our existing licenses, we would be required to negotiate a new license agreement. We may not be able to reach agreement with current or future licensors on commercially reasonable terms, if at all, or the terms may not permit us to sell our products at a profit after payment of royalties, which could harm our business.
Some of our patents may cover inventions that were conceived or first reduced to practice under, or in connection with, U.S. government contracts or other federal funding agreements. With respect to inventions conceived or first reduced to practice under a federal funding agreement, the U.S. government may retain a non-exclusive, non-transferable, irrevocable, paid-up license to practice or have practiced for or on behalf of the United States the invention throughout the world. We may not succeed in our efforts to retain title in patents, maintain ownership of intellectual property or in limiting the U.S. government’s rights in our proprietary technologies and intellectual property when an issue exists as to whether such intellectual property was developed in the performance of a federal funding agreement or developed at private expense.
If we fail to obtain the right to use the intellectual property rights of others which are necessary to operate our business, and to protect their intellectual property, our business and results of operations will be adversely affected.
In the past, we have licensed certain technologies for use in our products. In the future, we may choose, or be required, to license technology or intellectual property from third parties in connection with the development of our products. We cannot assure you that third-party licenses will be available on commercially reasonable terms, if at all. Our competitors may be able to obtain licenses, or cross-license their technology, on better terms than we can, which could put us at a competitive disadvantage. Also, we often enter into confidentiality agreements with such third parties in which we agree to protect and maintain their proprietary and confidential information, including at times requiring our employees to enter into agreements protecting such information. There can be no assurance that the confidentiality agreements will not be breached by any of our employees or that such third parties will not make claims that their proprietary information has been disclosed.
RISKS RELATING TO OUR COMMON STOCK
If there are substantial sales of our common stock, or the perception that such sales may occur, our stock price could decline.
If any of our stockholders were to sell substantial amounts of our common stock, the market price of our common stock may decline, which might make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem appropriate. Substantial sales of our common stock, or the perception that such sales may occur, may have a material adverse effect on the prevailing market price of our common stock.
Carilion Clinic holds approximately 3.5 million shares of our common stock (including approximately 1.3 million shares issuable to Carilion upon conversion of shares of Series A Convertible Preferred Stock that Carilion holds). All of these shares have been registered for resale on a Form S-3 registration statement and, accordingly, may generally be freely sold by Carilion at any time. Any sales of these shares, or the perception that future sales of shares may occur by Carilion or any of our other significant stockholders, may have a material adverse effect on the market price of our stock. Any such continuing material adverse effect on the market price of our stock could impair our ability to comply with NASDAQ’s continuing listing standards in respect of our minimum stock price, as further described below.
We may become involved in securities class action litigation that could divert management’s attention and harm our business and our insurance coverage may not be sufficient to cover all costs and damages.
The stock market has from time to time experienced significant price and volume fluctuations that have affected the market prices for the common stock of technology companies. These broad market fluctuations may cause the market price of our common stock to decline. In the past, following periods of volatility in the market price of a particular company’s securities, securities class action litigation has often been brought against that company. Securities class litigation also often follows certain significant business transactions, such as the sale of a business division or a change in control transaction. We
may become involved in this type of litigation in the future. Litigation often is expensive and diverts management’s attention and resources, which could adversely affect our business.
We may not be able to comply with all applicable listing requirements or standards of The NASDAQ Capital Market and NASDAQ could delist our common stock.
Our common stock is listed on The NASDAQ Capital Market. In order to maintain that listing, we must satisfy minimum financial and other continued listing requirements and standards. One such requirement is that we maintain a minimum bid price of at least $1.00 per share for our common stock. Although we currently comply with the minimum bid requirement, in the recent past, our minimum bid price has fallen below $1.00 per share, and it could again do so in the future. If our bid price falls below $1.00 per share for 30 consecutive business days, we will receive a deficiency notice from NASDAQ advising us that we have 180 days to regain compliance by maintaining a minimum bid price of at least $1.00 for a minimum of ten consecutive business days. Under certain circumstances, NASDAQ could require that the minimum bid price exceed $1.00 for more than ten consecutive days before determining that a company complies.
In the event that our common stock is not eligible for continued listing on NASDAQ or another national securities exchange, trading of our common stock could be conducted in the over-the-counter market or on an electronic bulletin board established for unlisted securities such as the Pink Sheets or the OTC Bulletin Board. In such event, it could become more difficult to dispose of, or obtain accurate price quotations for, our common stock, and there would likely also be a reduction in our coverage by security analysts and the news media, which could cause the price of our common stock to decline further. Also, it may be difficult for us to raise additional capital if we are not listed on a major exchange.
Our common stock price has been volatile and we expect that the price of our common stock will fluctuate substantially in the future, which could cause you to lose all or a substantial part of your investment.
The public trading price for our common stock is volatile and may fluctuate significantly. Since January 1, 2009, our common stock has traded between a high of $5.00$9.32 per share and a low of $0.26 per share. Among the factors, many of which we cannot control, that could cause material fluctuations in the market price for our common stock are:
sales of our common stock by our significant stockholders, or the perception that such sales may occur;
changes in earnings estimates, investors’ perceptions, recommendations by securities analysts or our failure to achieve analysts’ earnings estimates;
changes in our status as an entity eligible to receive SBIR contracts and grants;
quarterly variations in our or our competitors’ results of operations;
challenges integrating our recent or future acquisitions, including the inability to realize any expected synergies;
general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors;
announcements by us, or by our competitors, of acquisitions, new products, significant contracts, commercial relationships or capital commitments;
pending or threatened litigation;
any major change in our board of directors or management or any competing proxy solicitations for director nominees;
changes in governmental regulations or in the status of our regulatory approvals;
announcements related to patents issued to us or our competitors;
a lack of, limited or negative industry or securities analyst coverage;
discussions of our company or our stock price by the financial and scientific press and online investor communities; and
general developments in our industry.
In addition, the stock prices of many technology companies have experienced wide fluctuations that have often been unrelated to the operating performance of those companies. These factors may materially and adversely affect the market price of our common stock.
If ourWe are obligated to develop and maintain proper and effective internal controlcontrols over financial reporting is found notand any failure to be effective or if we make disclosuremaintain the adequacy of existing or potential material weaknesses in thosethese internal controls investors could losemay adversely affect investor confidence in our financial reports,company and, as a result, the value of our stock price may be adversely affected.common stock.
We are required, pursuant to Section 404 of the Sarbanes-Oxley Act, of 2002 requires usor Section 404, to include an internal controlfurnish a report with our Annual Reportby management on, Form 10-K. That report must include management’s assessment ofamong other things, the effectiveness of our internal control over financial reporting ason an annual basis. This assessment includes disclosure of any material weaknesses identified by our management in our internal control over financial reporting.
During the endevaluation and testing process of the fiscal year.
We evaluate our existinginternal controls, if we identify one or more material weaknesses in our internal control over financial reporting, based on the framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. During the course ofwe will be unable to assert that our ongoing evaluation of the internal controls,control over financial reporting is effective. While we may identify areas requiring improvement, and may have to design enhanced processesestablished certain procedures and controls to address issues identified through this review. Remedying any deficiencies, significant deficiencies or material weaknesses thatover our financial reporting processes, we identify may require us to incur significant costs and expend significant time and management resources. We cannot assure you that these efforts will prevent restatements of our financial statements in the future. Our independent registered public accounting firm is also required, pursuant to Section 404, to attest to, and report on, management's assessment of our internal control over financial reporting, which report is included elsewhere in this Annual Report on Form 10-K. This assessment is required to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. For future reporting periods, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our controls are documented, designed or operating. We may not be able to remediate any future material weaknesses, or to complete our evaluation, testing and any required remediation in a timely fashion.
Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition or results of operations. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting, we could lose investor confidence in the measuresaccuracy and completeness of our financial reports, the market price of our common stock could decline, and we implementcould be subject to sanctions or investigations by the Nasdaq Stock Market, the SEC or other regulatory authorities. Failure to remedy any such deficiencies will effectively mitigate or remedy such deficiencies. Investors could lose confidence material weakness
in our internal control over financial reports,reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
If our estimates relating to our critical accounting policies are based on assumptions or judgments that change or prove to be incorrect, our operating results could fall below expectations of financial analysts and investors, resulting in a decline in our stock priceprice.
The preparation of financial statements in conformity with U.S. GAAP requires our management to make estimates, assumptions and judgments that affect the amounts reported in the consolidated financial statements and accompanying notes. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets, liabilities, equity, revenue and expenses that are not readily apparent from other sources. Our operating results may be adversely affected if our internal controlsassumptions change or if actual circumstances differ from those in our assumptions, which could cause our operating results to fall below the expectations of financial analysts and investors, resulting in a decline in our stock price. Significant assumptions and estimates used in preparing our consolidated financial statements include those related to revenue recognition, stock-based compensation and income taxes. Moreover, the revenue recognition guidance, ASC Topic 606, Revenue from Contracts with Customers, requires more judgment than did the prior guidance.
Our financial results may be adversely affected by changes in accounting principles applicable to us.
U.S. GAAP are subject to interpretation by the FASB, the SEC, and other bodies formed to promulgate and interpret appropriate accounting principles. For example, in May 2014, the FASB issued ASC Topic 606, Revenue from Contracts with Customers, which supersedes nearly all existing revenue recognition guidance under U.S. GAAP. We adopted this guidance as of January 1, 2018. The most significant impact relates to changing the revenue recognition for custom optoelectronics to an over time method. Before the adoption of this standard, we deferred the recognition of revenue until products were shipped to the customer. Any difficulties in implementing these pronouncements or adequately accounting after adoption could cause us to fail to meet our financial reporting are found not to be effective by management or if we make disclosure of existing or potential significant deficiencies or material weaknessesobligations, which could result in those controls.regulatory discipline and harm investors’ confidence in us.
Anti-takeover provisions in our amended and restated certificate of incorporation and bylaws and Delaware law could discourage or prevent a change in control, even if an acquisition would be beneficial to our stockholders, which could affect our stock price adversely and prevent attempts by our stockholders to replace or remove our current management.
Our amended and restated certificate of incorporation and bylaws and Delaware law contain provisions that might delay or prevent a change in control, discourage bids at a premium over the market price of our common stock and adversely affect the market price of our common stock and the voting and other rights of the holders of our common stock. These provisions include:
a classified board of directors serving staggered terms;
advance notice requirements to stockholders for matters to be brought at stockholder meetings;
a supermajority stockholder vote requirement for amending certain provisions of our amended and restated certificate of incorporation and bylaws; and
the right to issue preferred stock without stockholder approval, which could be used to dilute the stock ownership of a potential hostile acquirer.
We are also subject to provisions of the Delaware General Corporation law that, in general, prohibit any business combination with a beneficial owner of 15% or more of our common stock for three years unless the holder’s acquisition of our stock was approved in advance by our board of directors or certain other conditions are satisfied.
The existence of these provisions could adversely affect the voting power of holders of common stock and limit the price that investors might be willing to pay in the future for shares of our common stock.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 2. PROPERTIES
We lease approximately 4,400 square feet of office space in Roanoke, Virginia, which serves as our corporate headquarters and is used for general and administrative functions. This lease expires March 31, 2020.2021.
We lease approximately 42,000 square feet of space in Blacksburg, Virginia, near Virginia Tech, which is used by both our Technology Development segment and our Products and Licensing segment. This lease expires December 31, 2024.
We lease approximately 50,00011,000 square feet of space in Ann Arbor, Michigan, for research, development and manufacturing of our HSOR and THz product platforms.platform. This lease expires May 31,November 30, 2021.
We lease approximately 29,000 square feet of space in Camarillo, California, for development and manufacturing of our custom optoelectronic solutions operations. This lease expires March 31, 2019.
We lease approximately 16,00019,600 square feet of space in Charlottesville, Virginia, near the University of Virginia, for use by certain groups in our Technology Development segment. This lease expires December 31, 2020.
We lease approximately 4,00021,000 square feet of space in Montreal, CanadaAtlanta, Georgia, for researchuse by our Products and development of optoelectronic solutions.Licensing segment. This lease expires October 31, 2020.
We lease approximately 28,000 square feet of space in April 2017. We are currently evaluating whether to renew thisChino, California, for use by our Products and Licensing segment. This lease or relocate these operations to another location.expires October 31, 2020.
We own a 24,000 square foot facility in Danville, Virginia. OurVirginia for use by certain groups in our Technology Development segment primarily uses this facility for biomedical research and development and manufacturing.segment.
We believe that our existing facilities are adequate for our current needs and suitable additional or substitute space will be available as needed to accommodate expansion of our operations.
ITEM 3. LEGAL PROCEEDINGS
In June 2015,December 2018, we received a letternotice of final determinationclaim (the "Claim") from Macom Technology Solutions, Inc. ("Macom"), who acquired our HSOR business in August 2017 pursuant to an asset purchase agreement. Under the DCMA regardingasset purchase agreement, we agreed to indemnify Macom for certain matters, including, among other things, the allowabilitycollection of accounts receivable from certain costs we includedmajor customers, and placed $4.0 million of the purchase price into an escrow account for the potential settlement of any valid indemnity claims. The Claim received from Macom totaled $2.1 million under various indemnity provisions. We have disputed Macom's assertion of right to payment for the matters described in our billings under cost-plus type research contracts during 2007. In conjunctionthe Claim. It is uncertain what amount, if any, will be owed in settlement of the Claim. As of December 31, 2019, $1.5 million of the escrow balance had been received with the DCMA's determination of those costs as expressly unallowable underremaining $2.5 million in the provisionsescrow account pending resolution of the Federal Acquisition Regulations, the DCMA assessed penalties and interest to us totaling $1.1 million. In July 2015, we filed an appeal of the assessed penalties and interest with the Armed Services Board of Contract Appeals ("ASBCA"). A hearing was held with respect to this appeal in January 2017, and a decision has not yet been reached by ASBCA. Accordingly, the appeal remains outstanding.dispute.
Additionally, from time to time, we may become involved in litigation or claims arising out of our operations in the normal course of business. Management currently believes the amount of ultimate liability, if any, with respect to these actions will not materially affect our financial position, results of operations, or liquidity.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
PRICE RANGE OF COMMON STOCKSTOCKHOLDERS
Our common stock tradesis listed on The NASDAQthe Nasdaq Capital Market. The following table sets forthMarket under the high and low sales pricessymbol "LUNA." As of our common stock for each period indicated as reported by NASDAQ.
|
| | | | | | | | | | | | | | | | |
| | | 2016 | | 2015 |
| Fiscal Period | | High | | Low | | High | | Low |
| First Quarter | | $ | 1.25 |
| | $ | 0.74 |
| | $ | 1.85 |
| | $ | 1.27 |
|
| Second Quarter | | $ | 1.32 |
| | $ | 0.97 |
| | $ | 1.47 |
| | $ | 0.96 |
|
| Third Quarter | | $ | 1.69 |
| | $ | 1.08 |
| | $ | 1.30 |
| | $ | 0.88 |
|
| Fourth Quarter | | $ | 1.55 |
| | $ | 1.22 |
| | $ | 1.23 |
| | $ | 0.88 |
|
We have a single classMarch 11, 2020, we had 30,391,879 shares of common stock outstanding. Asoutstanding held by 100 holders of March 15, 2017 there were approximately 99record. The actual number of stockholders is greater than this number of record of our common stock. Theholders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. This number of holders of record of our common stockalso does not reflect the number of beneficial holdersinclude stockholders whose shares aremay be held in trust by depositories, brokers or other nominees.entities.
STOCK PERFORMANCE GRAPH
The graph set forth below compares the cumulative total stockholder return on our common stock for the previous five years, during which our common stock was traded on the NASDAQNasdaq Capital Market, as compared to the cumulative total return of the NASDAQNasdaq Composite Index and the Russell 2000 Index over the same period. This graph assumes the investment of $100,000 in our common stock at the closing price on January 1, 2012,2015, and an equivalent amount in the NASDAQNasdaq Composite Index and the Russell 2000 Index on that date, and assumes the reinvestment of dividends, if any. We have never paid dividends on our common stock and have no present plans to do so.
Since there is no published industry or line-of-business index for our business reflective of our performance, nor do we believe we can reasonably identify a peer group, we measure our performance against issuers with similar market capitalizations. We selected the Russell 2000 Index because it measures the performance of a broad range of companies with lower market capitalizations than those companies included in the S&P 500 Index.
The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock.
The preceding Stock Performance Graph is not deemed filed with the Securities and Exchange Commission and shall not be incorporated by reference in any of our filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
DIVIDEND POLICY
Since our inception, we have never declared or paid any cash dividends on our common stock. We currently expect to retain any future earnings for use in the operation and expansion of our business, and therefore do not anticipate paying any cash dividends in the foreseeable future. In addition, our debt facility with Silicon Valley Bank restricts us from paying cash dividends on our capital stock without the bank’s prior written consent.
Unregistered Sales of Equity Securities
Common Stock Dividend Payable to Carilion
We issued 1,321,514 shares of Series A Preferred Stock, par value $0.001 per share, to Carilion Clinic in January 2010, which shares were issued in reliance on the exemptions from registration under the Securities Act provided by Sections 3(a)(9) and 4 (a)(2) thereof. The Series A Preferred Stock accrues dividends at the rate of $0.2815 per share per annum, payable quarterly in arrears. Accrued dividends are payable in shares of our common stock, with the number of shares being equal to the quotient of (i) the cumulative aggregate balance of accrued but unpaid dividends on each share of Series A Preferred Stock divided by (ii) the conversion price of the Series A Preferred Stock, which is currently $4.69159 per share. For the period from January 12, 2010, the original issue date of the Series A Preferred Stock, through December 31, 2016, the Series A Preferred Stock issued to Carilion has accrued $1,013,442 in dividends. The accrued dividend as of December 31, 2016 will be paid by the issuance of 552,401 shares of our common stock, which we will issue at Carilion’s written request. As the Series A
Preferred Stock was issued in reliance on the exemption provided by Section 3(a)(9), the shares of common stock payable as dividends will also be exempt from registration in reliance on Section 3(a)(9) of the Securities Act.
Not applicable.
Purchases of Equity Securities by the Issuer and Affiliated PartiesParties-
The following table summarizes repurchases of our common stock during the three months ended December 31, 2016.
|
| | | | | | | | | | |
| Period | Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of a Publicly Announced Program (1) | Approximate Dollar Value of Shares that May Yet to be Purchased Under the Program |
| 10/1/2016-10/31/2016 | — |
| — |
| — |
| $ | 1,759,184 |
|
| 11/1/2016-11/30/2016 | 6,400 |
| $ | 1.25 |
| 6,400 |
| $ | 1,751,191 |
|
| 12/1/2016-12/31/2016 | — |
| $ | — |
| — |
| $ | 1,751,191 |
|
| Total | 6,400 |
| 1.25 |
| 6,400 |
| 1,751,191 |
|
(1) On June 9, 2016, we announced that our board of directors approved a stock repurchase program, authorizing the repurchase of up to $2.0 million of our common stock. Unless extended, the stock repurchase program expires on May 31, 2017.
Not applicable.
ITEM 6. SELECTED FINANCIAL DATA
The consolidated statement of operations data for each of the years ended December 31, 20162019 and 20152018 and the consolidated balance sheet data as of December 31, 20162019 and 20152018 have been derived from our audited consolidated financial statements appearing elsewhere in this report. The consolidated statement of operations data for the years ended December 31, 2014, 20132017, 2016 and 20122015 and the consolidated balance sheet data as of December 31, 2014, 20132017, 2016 and 20122015 have been derived from
our audited consolidated financial statements that do not appear in this report. The following selected consolidated financial data should be read in conjunction with our consolidated financial statements and the accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included at Part II, Item 7 in this Annual Report on Form 10-K. The selected data in this section is not intended to replace the consolidated financial statements, and the historical results are not necessarily indicative of the results to be expected in any future period.
|
| | | | | | | | | | | | | | | | | | | |
| | Years ended December 31, |
| In thousands, except share and per share data | 2019 | | 2018 | | 2017 | | 2016 | | 2015 |
| Consolidated Statement of Operations Data: | | | | | | | | | |
| Revenues: | | | | | | | | | |
| Products and licensing | $ | 44,491 |
| | $ | 21,950 |
| | $ | 14,505 |
| | $ | 13,323 |
| | $ | 12,975 |
|
| Technology development | 26,025 |
| | 20,968 |
| | 18,576 |
| | 16,281 |
| | 13,599 |
|
| Total revenues (1) | 70,516 |
| | 42,917 |
| | 33,082 |
| | 29,604 |
| | 26,574 |
|
| Cost of revenues: | | | | | | | | | |
| Products and licensing | 16,684 |
| | 8,079 |
| | 5,724 |
| | 5,417 |
| | 5,651 |
|
| Technology development | 18,649 |
| | 15,400 |
| | 13,988 |
| | 12,473 |
| | 10,379 |
|
| Total cost of revenues | 35,333 |
| | 23,479 |
| | 19,713 |
| | 17,890 |
| | 16,030 |
|
| Gross profit | 35,182 |
| | 19,438 |
| | 13,369 |
| | 11,714 |
| | 10,544 |
|
| Operating expense | 31,867 |
| | 18,560 |
| | 15,577 |
| | 15,840 |
| | 17,359 |
|
| Operating income/(loss) | 3,315 |
| | 878 |
| | (2,208 | ) | | (4,126 | ) | | (6,815 | ) |
| Other (expense)/income, net | (5 | ) | | (17 | ) | | 26 |
| | 28 |
| | (53 | ) |
| Interest income | 394 |
| | 550 |
| | — |
| | — |
| | — |
|
| Interest expense, net | (16 | ) | | (124 | ) | | (217 | ) | | (317 | ) | | (218 | ) |
| Income/(loss) from continuing operations before income taxes | 3,688 |
| | 1,286 |
| | (2,399 | ) | | (4,415 | ) | | (7,086 | ) |
| Income tax benefit/(expense) | 1,654 |
| | (48 | ) | | 1,149 |
| | 136 |
| | 602 |
|
| Net income/(loss) from continuing operations | 5,343 |
| | 1,238 |
| | (1,251 | ) | | (4,279 | ) | | (6,484 | ) |
| Income from discontinued operations, net of income taxes | — |
| | 9,766 |
| | 15,866 |
| | 1,909 |
| | 8,801 |
|
| Net income/(loss) | 5,343 |
| | 11,004 |
| | 14,615 |
| | (2,370 | ) | | 2,317 |
|
| Less: Preferred stock dividend | 285 |
| | 257 |
| | 147 |
| | 105 |
| | 85 |
|
| Net income/(loss) attributable to common stockholders | $ | 5,057 |
| | $ | 10,747 |
| | $ | 14,468 |
| | $ | (2,475 | ) | | $ | 2,232 |
|
| Net income/(loss) per share from continuing operations: | | | | | | | | | |
| Basic | $ | 0.19 |
| | $ | 0.04 |
| | $ | (0.05 | ) | | $ | (0.16 | ) | | $ | (0.28 | ) |
| Diluted | $ | 0.17 |
| | $ | 0.04 |
| | $ | (0.05 | ) | | $ | (0.16 | ) | | $ | (0.28 | ) |
| Net income per share from discontinued operations: | | | | | | | | | |
| Basic | $ | — |
| | $ | 0.35 |
| | $ | 0.58 |
| | $ | 0.07 |
| | $ | 0.38 |
|
| Diluted | $ | — |
| | $ | 0.30 |
| | $ | 0.58 |
| | $ | 0.07 |
| | $ | 0.38 |
|
| Net income/(loss) per share attributable to common stockholders: | | | | | | | | | |
| Basic | $ | 0.18 |
| | $ | 0.39 |
| | $ | 0.52 |
| | $ | (0.09 | ) | | $ | 0.10 |
|
| Diluted | $ | 0.16 |
| | $ | 0.33 |
| | $ | 0.52 |
| | $ | (0.09 | ) | | $ | 0.10 |
|
| Weighted-average shares: | | | | | | | | | |
| Basic | 28,688,867 |
| | 27,596,401 |
| | 27,579,988 |
| | 27,547,217 |
| | 23,026,494 |
|
| Diluted | 31,840,584 |
| | 32,452,228 |
| | 27,579,988 |
| | 27,547,217 |
| | 23,026,494 |
|
(1) The consolidated statement of operations for years ended December 31, 2019 and 2018 were recognized in accordance with ASC 606.
Years ended December 31, 2017 and prior were recognized under ASC 605.
|
| | | | | | | | | | | | | | | | | | | |
| | Years ended December 31, |
| In thousands, except share and per share data | 2016 | | 2015 | | 2014 | | 2013 | | 2012 |
| Consolidated Statement of Operations Data: | | | | | | | | | |
| Revenues: | | | | | | | | | |
| Technology development | $ | 16,825 |
| | $ | 13,599 |
| | $ | 12,206 |
| | $ | 11,422 |
| | $ | 15,127 |
|
| Products and licensing | 42,386 |
| | 30,421 |
| | 9,054 |
| | 6,912 |
| | 8,339 |
|
| Total revenues | 59,211 |
| | 44,020 |
| | 21,260 |
| | 18,334 |
| | 23,466 |
|
| Cost of revenues: | | | | | | | | | |
| Technology development | 12,711 |
| | 10,379 |
| | 9,376 |
| | 8,882 |
| | 10,749 |
|
| Products and licensing | 24,765 |
| | 17,141 |
| | 4,047 |
| | 3,403 |
| | 3,825 |
|
| Total cost of revenues | 37,476 |
| | 27,520 |
| | 13,423 |
| | 12,285 |
| | 14,574 |
|
| Gross profit | 21,735 |
| | 16,501 |
| | 7,837 |
| | 6,049 |
| | 8,892 |
|
| Operating expense | 23,672 |
| | 22,750 |
| | 12,342 |
| | 14,084 |
| | 13,022 |
|
| Operating loss | (1,937 | ) | | (6,250 | ) | | (4,505 | ) | | (8,035 | ) | | (4,130 | ) |
| Other (expense)/income, net | (36 | ) | | (10 | ) | | 111 |
| | 347 |
| | 108 |
|
| Interest expense, net | (321 | ) | | (220 | ) | | (96 | ) | | (208 | ) | | (312 | ) |
| Loss from continuing operations before income tax | (2,294 | ) | | (6,480 | ) | | (4,490 | ) | | (7,896 | ) | | (4,334 | ) |
| Income tax expense/(benefit) | 75 |
| | (471 | ) | | (1,137 | ) | | (2,387 | ) | | (1,107 | ) |
| Net loss from continuing operations | (2,369 | ) | | (6,009 | ) | | (3,353 | ) | | (5,508 | ) | | (3,227 | ) |
| Income from discontinued operations, net of income taxes | — |
| | 8,326 |
| | 9,347 |
| | 4,705 |
| | 1,843 |
|
| Net (loss)/income | (2,369 | ) | | 2,317 |
| | 5,995 |
| | (803 | ) | | (1,384 | ) |
| Preferred stock dividend | 105 |
| | 86 |
| | 112 |
| | 102 |
| | 120 |
|
| Net (loss)/income attributable to common stockholders | $ | (2,475 | ) | | $ | 2,231 |
| | $ | 5,883 |
| | $ | (905 | ) | | $ | (1,504 | ) |
| Net loss per share from continuing operations: | | | | | | | | | |
| Basic and diluted | $ | (0.09 | ) | | $ | (0.26 | ) | | $ | (0.23 | ) | | (0.38 | ) | | (0.23 | ) |
| Net income per share from discontinued operations: | | | | | | | | | |
| Basic and diluted | $ | — |
| | $ | 0.36 |
| | $ | 0.63 |
| | 0.33 |
| | 0.13 |
|
| Net (loss)/income per share attributable to common stockholders: | | | | | | | | | |
| Basic and diluted | $ | (0.09 | ) | | $ | 0.10 |
| | $ | 0.40 |
| | (0.06 | ) | | (0.11 | ) |
| Weighted-average shares: | | | | | | | | | |
| Basic and diluted | 27,547,217 |
| | 23,026,494 |
| | 14,880,697 |
| | 14,336,135 |
| | 13,930,267 |
|
| | | | As of December 31, | As of December 31, |
| | 2016 | | 2015 | | 2014 | | 2013 | | 2012 | |
| In thousands | | 2019 | | 2018 | | 2017 | | 2016 | | 2015 |
| Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | $ | 12,802 |
| | $ | 17,464 |
| | $ | 14,117 |
| | $ | 7,779 |
| | $ | 6,340 |
| $ | 25,006 |
| | $ | 42,460 |
| | $ | 36,982 |
| | $ | 17,464 |
| | $ | 14,117 |
|
Working capital | 21,129 |
| | 23,417 |
| | 15,413 |
| | 10,106 |
| | 10,509 |
| 41,072 |
| | 56,089 |
| | 43,975 |
| | 23,417 |
| | 15,413 |
|
Total assets | 54,997 |
| | 58,132 |
| | 27,584 |
| | 19,704 |
| | 20,458 |
| 86,524 |
| | 75,599 |
| | 66,223 |
| | 58,132 |
| | 27,584 |
|
Total current liabilities | 15,968 |
| | 15,334 |
| | 8,473 |
| | 7,206 |
| | 9,186 |
| 17,044 |
| | 12,139 |
| | 14,826 |
| | 15,334 |
| | 8,473 |
|
| Total debt | 4,253 |
| | 6,125 |
| | 625 |
| | 2,125 |
| | 3,625 |
| — |
| | 619 |
| | 2,436 |
| | 6,125 |
| | 625 |
|
(2) ROU assets and corresponding lease liabilities were recognized in the year ended December 31, 2019, in accordance with ASC 842. Years ended December 31, 2018 and prior were recognized under ASC 840.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the related notes to those statements included elsewhere in this report. In addition to historical financial information, the following discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results and timing of selected events may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those discussed under “Risk Factors” and elsewhere in this report.
Merger with Advanced Photonix, Inc.
On May 8, 2015, we completed a merger with Advanced Photonix, Inc. ("API"), pursuant to the Agreement and Plan of Merger and Reorganization (the "Merger Agreement"), dated as of January 30, 2015, by and among Luna, API and API Merger Sub, Inc., our wholly owned subsidiary ("API Merger Sub"). In accordance with the terms of the Merger Agreement, upon the completion of the merger, API Merger Sub merged with and into API, with API surviving as our wholly-owned subsidiary. In the merger, former API stockholders received 0.31782 shares of our common stock for each share of API common stock they owned at the effective time of the merger. The merger is being accounted for under the acquisition method of accounting in accordance with Financial Accounting Standards Board Accounting Standard Topic 805, Business Combinations ("ASC 805") with Luna treated as the accounting acquirer.Business Overview
We are a leader in advanced optical technology, providing unique capabilities in high speed optoelectronics and high performance fiber optic test, measurement and control products for the telecommunications industryand photonics industries and distributed fiber optic sensing products for industries utilizing composite and other advanced materials, such as the automotive, aerospace, energy and automotiveinfrastructure industries. Our high-speed optical receiver ("HSOR") transmission products are deployed in the internet infrastructure to enable the high-speed bandwidth necessary to support video and data. Our distributed fiber optic sensing products provide criticalhelp designers and manufacturers more efficiently develop new and innovative products by providing valuable information such as highly detailed stress, strain, and temperature information to designers and manufacturers working with advanced materials. Our custom optoelectronicmeasurements of a new design or manufacturing process. In addition, our distributed fiber optic sensing products are soldused to scientific instrumentation manufacturers for various applicationsmonitor the structural integrity or operational health of critical assets, including large civil structures such as metrology, missile guidance, flame monitoring,bridges. Our communications test and temperature sensing. In addition, wecontrol products accelerate the development of advanced fiber optic components and networks by providing fast and highly accurate characterization of components and networks. We also provide applied research services, typically under research programs funded by the U.S. government, in areas of sensing and instrumentation, advanced materials, sensing,optical technologies and healthcare applications.health sciences. Our business model is designed to accelerate the process of bringing new and innovative products to market. We use our in-house technical expertise across a range of technologies to perform applied research services for companies and for government funded projects. We continue to invest in product development and commercialization, which we anticipate will lead to increased product sales growth.
We are organized into two main businessreporting segments, our Products and Licensing segment and our Technology Development segment. Our Products and Licensing segment develops, manufactures and markets distributed fiber optic sensing products as well as test & measurement products, and also conducts applied research in the fiber optic sensing area for both corporatecommunications test and government customers.control products. We are continuingcontinue to develop and commercialize our fiber optic technology for strain and temperature sensing applications for the aerospace, automotive, energy and energyinfrastructure as well as for test and measurement applications in the telecommunications and data communications industries. Our Products and Licensing segment revenues represented approximately 72%63% and 69%51% of our total revenues for the years ended December 31, 20162019 and 2015,2018, respectively.
Our Technology Development segment performs applied research principally in the areas of sensing &and instrumentation, advanced materials and health sciences. Our Technology Development segment comprised approximately 28%37% and 31%49% of our total revenues for the years ended December 31, 20162019 and 2015,2018, respectively. Most of the government funding for our Technology Development segment is derived from the Small Business Innovation Research ("SBIR"), program coordinated by the U.S. Small Business Administration ("SBA").Administration. Our Technology Development segment revenues have historically accounted for a large portion of our total revenues, and we expect that they will continue to represent a significant portion of our total revenues for the foreseeable future. Within the Technology Development segment, we have historically had a backlog of contracts for which work has been scheduled, but for which a specified portion of work has not yet been completed. We define backlog as the dollar amount of obligations payable to us under negotiated contracts upon completion of a specified portion of work that has not yet been completed, exclusive of revenues previously recognized for work already performed under these contracts, if any. Total backlog includes funded backlog, which is the amount for which money has been directly authorized by the U.S. government and for which a purchase order has been received by a commercial customer, and unfunded backlog, representing firm orders for which funding has not yet been appropriated. Indefinite delivery and quantity contracts and unexercised options are not reported in total backlog. The approximate value of our Technology DevelopmentProducts and Licensing segment backlog was $17.6$16.1 million and $16.7
$5.8 million at December 31, 20162019 and 2015,2018, respectively. The approximate value of our Products and LicensingTechnology Development segment backlog was $10.4$31.3 million and $10.7$26.0 million at December 31, 20162019 and 2015,2018, respectively.
Revenues from product sales are mostly derived from the sales of our HSOR, optoelectronics, sensing and test, & measurement and control products that make use of light-transmitting optical fibers, or fiber optics. We continue to invest in product development and commercialization, which we anticipate will lead to increased product sales growth. Although we have been successful in licensing certain technologytechnologies in past years, we do not expect license revenues to represent a significant portion of future revenues. Over time however, we do intend to gradually increase such revenues. In the near term, we expect revenues from product sales to continue to be primarily in areas associated with our HSOR, optoelectronics,sensing and test, measurement and control fiber optic test & measurement and sensing platforms. In the long term, we expect that revenues from product sales will represent a larger portion of our total revenues and that asrevenues. As we develop and commercialize new products, theseour revenues will reflect a broader and more diversified mix of products.
We realized net lossincome attributable to common stockholders of approximately $2.5$5.1 million for the year ended December 31, 20162019 and net income attributable to common stockholders of approximately $2.2$10.7 million for the year ended December 31, 2015. Excluding the effects of our medical shape sensing business, which we sold in 2014, we incurred2018. We realized net lossesincome from continuing operations of approximately $2.4 million and $6.0$5.3 million for the yearsyear ended December 31, 20162019 and 2015, respectively.a net income from continuing operations of $1.2 million for the year ended December 31, 2018.
We may incur increasing expenses as we seek to expand our business, including expenses for research and development, sales and marketing and manufacturing capabilities. We may alsocontinue to grow our business in part through acquisitions of additional companies and complementary technologies, which could cause us to incur transaction expenses, amortization or write-offs of intangible assets and goodwill and other acquisition-related expenses. As a result, we may incur net losses for the foreseeablein future periods, and these losses could be substantial.
ReductionsAcquisition of General Photonics Corporation.
On March 1, 2019, we acquired all of the outstanding stock of General Photonics Corporation ("GP"), a leading provider of innovative components, modules and test equipment focused on the generation, measurement and control of polarized light critical in government spending may impactfiber optic-based applications for aggregate consideration of $19.0 million with an earn-out provision of up to $1.0 million.. Of the availabilitypurchase price, $17.1 million was paid at closing and $1.9 million was placed into escrow for possible working capital adjustments to the purchase price and potential satisfaction of new program awardscertain post-closing indemnification obligations. As of December 31, 2019, we expect to pay, and have accrued, $1.0 million in additional cash consideration as a result of the future. For example,successful completion of the Budget Control Act commitsearn-out provision.
Acquisition of Micron Optics, Inc.
On October 15, 2018, we acquired substantially all of the U.S. government to reduceassets, other than cash, of the federal deficit by $1.2 trillion over ten years through a combinationU. S. operations of automatic, across-the-board spending cuts and caps on discretionary spending, or sequestration. Automatic across-the-board cuts required by sequestration could have a material adverse effect on our Technology Development segment revenues and, consequently, our results of operations. While the exact manner in which sequestration will impact our business is unclear, funding for programs in which we participate could be reduced, delayed or canceled. Our ability to obtain new contract awards also could be negatively affected.
Sale of Medical Shape Sensing Business in 2014
In January 2014, we sold our assets associated with the development of fiber optic shape sensing and localization for the medical field to affiliates of Intuitive Surgical,Micron Optics, Inc. ("Intuitive"MOI") for, a leading provider of innovative optical components and laser-based equipment that advance the quality of optical measurements, allowing the sensing, imaging and telecommunications industries to make critical measurements. We paid total cash consideration of $5.5 million for the acquisition of MOI. The acquisition of MOI expanded our technology and product portfolio to include optical sensors and sensing interrogators capable of a broader range of measurement capabilities, including higher speed measurements such as vibration, and the ability to instrument larger structures over longer distances. In addition, the MOI acquisition added a product suite of tunable optical filters, optical sensors and swept lasers.
Sale of Luna Optoelectronics Business
On July 31, 2018, we sold the assets associated with our optoelectonic components business to an unaffiliated third party. The asset purchase agreement provides for additional consideration of up to $30$1.0 million including $6 million received at closing, $6 million received in April 2014 and up to $18 million in the future based oncontingent upon the achievement of certain technical milestonesa specified revenue level by the sold business during the 18 months following the sale. There have been no amounts recorded for the contingent consideration in the financial statements as of December 31, 2019, and royalties on system sales,it is uncertain what amount, if any. In December 2015, although the specified technical milestones had not yet been achieved, we and Intuitive agreed to settle all remaining potential amounts payable under the agreement for a single payment of $9 million, which weany, will be received in December 2015.
Description of Our Revenues, Costs and Expenses
Revenues
We generate revenues from technology development, product sales, and commercial product development and licensing and technology development activities. We derive Technology Development segment revenues from providing research and development services to third parties, including government entities, academic institutions and corporations, and from achieving milestones established by some of these contracts and in collaboration agreements. In general, we complete contracted research over periods ranging from six months to three years, and recognize these revenues over the life of the contract as costs are incurred. Our Technology Development segment revenues represented approximately 28% and 31% of our total revenues for the years ended December 31, 2016 and 2015, respectively.
Our Products and Licensing segment revenues reflect amounts that we receive from sales of our products or development of products for third parties and, to a lesser extent, fees paid to us in connection with licenses or sub-licenses of certain patents and other intellectual property. Products and licensing revenues represented approximately 72% and 69% of our total revenues for the years ended December 31, 2016 and 2015, respectively.
Cost of Revenues
Cost of revenues associated withWe derive Technology Development segment revenues consistsfrom providing research and development services to third parties, including government entities, academic institutions and corporations, and from achieving milestones established by
some of these contracts. In general, we complete contracted research over periods ranging from six months to three years and recognize these revenues over the life of the contract as costs associated with performing the related research activities including direct labor, amounts paid to subcontractors and overhead allocated to Technology Development segment activities.are incurred.
Cost of Revenues
Cost of revenues associated with Products and Licensing segment revenues consists of license fees for use of certain technologies, product manufacturing costs including all direct material and direct labor costs, amounts paid to our contract manufacturers, manufacturing, shipping and handling, provisions for product warranties and inventory obsolescence, as well as overhead allocated to each of these activities.
Cost of revenues associated with Technology Development segment revenues consists of costs associated with performing the related research activities including direct labor, amounts paid to subcontractors and overhead allocated to Technology Development segment activities.
Operating Expense
Operating expense consists of selling, general and administrative expenses,expense, as well as expenses related to research, development and engineering, depreciation of fixed assets and amortization of intangible assets. These expenses also include compensation for employees in executive and operational functions including certain non-cash charges related to expenses
from option grants,equity awards, facilities costs, professional fees, salaries, commissions, travel expense and related benefits of personnel engaged in sales, marketing, and administrative activities; costs of marketing programs and promotional materials; salaries, bonuses and related benefits of personnel engaged in our own research and development beyond the scope and activities of our Technology Development segment; product development activities not provided under contracts with third parties; and overhead costs related to these activities.
Investment Income
Investment income consists of amounts earned on our cash equivalents. We sweep on a daily basis a portion of our cash on hand into a fund invested in U.S. government obligations.
Interest Expense, Net
In February 2010, we entered into a lineInterest expense is composed of credit facility with Silicon Valley Bank ("SVB") with a borrowing capacity of $5.0 million. In May 2011, we entered into a loan modification agreement with SVBinterest paid under which we repaid the outstanding balance under the prior line of credit and obtained aour term loan in the amount of $6.0 million, along with a new $1.0 million line of credit. In May 2012, we entered into another loan modification agreement with SVB under which we extended the maturity date of the line of credit to May 2014 and adjusted certain covenants. On May 8, 2015, we entered into a loan modification agreement with SVB to borrow an additional $6 million, which we primarily used to repay the previously outstanding loans of API that existed at the time of our merger. On September 29, 2015, we entered into another loan modification with SVB under which we borrowed an additional $1 million to finance specified planned capital expenditures. On December 15, 2016, we entered into another loan modification with SVB, under which we and SVB adjusted specified covenants. At December 31, 2016, we had $4.3 million outstanding on the term loan.
During the years ended December 31, 2016 and 2015, interest expense primarily includedas well as interest accrued on our outstanding bank credit facilities and interest incurred with respect to our capitalfinance lease obligations.
Critical Accounting Policies and Estimates
Products and Licensing Revenues
To determine the proper revenue recognition method for Products and Licensing contracts, we evaluate whether two or more contracts should be combined and accounted for as one single contract and whether the combined or single contract should be accounted for as more than one performance obligation. We recognize revenue when the performance obligation has been satisfied by transferring the control of the product or service to the customer. For tangible products that contain software that is essential to the tangible product’s functionality, we consider the product and software to be a single performance obligation and recognize revenue accordingly. For contracts with multiple performance obligations, we allocate the contract’s transaction price to each performance obligation based on their relative stand-alone selling prices. In such circumstances, we use the observable price of goods or services which are sold separately in similar circumstances to similar customers. If these prices are not observable, then we will estimate the stand-alone selling price using information that is reasonably available. For the majority of our standard products and services, price list and discount structures related to customer type are available. For products and services that do not have price list and discount structures, we may use one or more of the following: (i) adjusted market assessment approach, (ii) expected cost plus a margin approach, and (iii) residual approach. The adjusted market approach requires us to evaluate the market in which we sell goods or services and estimate the price that a customer in that market would be willing to pay for those goods or services. The expected cost-plus margin approach requires us to forecast our expected costs of satisfying the performance obligation and then add a reasonable margin for that good or service. The residual approach decreases the total transaction price by the sum of the observable standalone selling prices if either the company sells the same good or services to different customers for a broad range of amounts or the company has not established a price for the good or service and that good or service has not been sold on a standalone basis. Shipping and handling activities primarily
occur after a customer obtains control and are considered fulfillment cost rather than separate performance obligations. Similarly, sales and similar taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction and collected by the entity from a customer are excluded from the measurement of the transaction price.
For standard products, we recognize revenue at a point in time when control passes to the customer. Absent substantial product acceptance clauses, this is based on the shipping terms. For custom products that require engineering and development based on customer requirements, we will recognize revenue over time using the output method for any items shipped and any finished goods or work in process that is produced for balances of open sales orders. For any finished goods or work in process that has been produced for the balance of open sales orders we recognize revenue by applying the average selling price for such open order to the lesser of the on-hand balance in finished goods or open sales order quantity which we present as a contract asset on the balance sheet. Cost of sales is recognized based on the standard cost of the finished goods and work in process associated with this revenue and inventory balances are reduced accordingly. For extended warranties and product rentals, revenue is recognized over time using the output method based on the time elapsed for the warranty or service period. In the case of warranties, we record a contract liability for amounts billed but that are not recognized until subsequent periods. A separate contract liability is recorded for the cost associated with warranty repairs based on our estimate of future expense. For testing services where we are performing testing on an asset the customer controls, revenue is recognized over time by the output method using the performance to date. For training, where the customer is receiving the benefit of training as it is occurring, and for repairs to a customer-controlled asset, revenue is recognized over time by the output method using the performance to date. For royalty revenue, we apply the practical expedient “royalty exception” recognizing revenue based on the royalty agreement which specifies an amount based on sales or minimum amount, whichever is greater.
In some product rental contracts, a customer may be offered a discount on the purchase of an item that would provide for a material right. When a material right has been provided to a customer, a separate performance obligation is established, and a portion of the rental revenue will be deferred until the future product is purchased or the option expires. This deferred revenue is recognized as a contract liability on the balance sheet.
Technology Development Revenues
We perform research and development for U.S. Federal government agencies, educational institutions and commercial organizations. We recognize revenue underaccount for a research contractscontract when a contract has been executed, the rights of the parties are identified, payment terms are identified, the contract price is fixed and determinable, delivery of services or products has occurred,commercial substance, and collectability of the contract price is considered reasonably assured and can be reasonably estimated.probable. Revenue is earned under cost reimbursable, time and materials and fixed price contracts. Direct contract costs are expensed as incurred.
Under cost reimbursable contracts, we are reimbursed for costs that are determined to be reasonable, allowable and allocable to the contract and paid a fixed fee representing the profit negotiated between us and the contracting agency. Revenue from cost reimbursable contracts is recognized as costs are incurred plus an estimate of applicable fees earned. We consider fixed fees under cost reimbursable contracts to be earned in proportion to the allowable costs incurred in performance of the contract.
Revenue from time and materials contracts is recognized based on direct labor hours expended at contract billing rates plus other billable direct costs.
Fixed price contracts may include either a product delivery or specific service performance throughout a period. For fixed price contracts that are based on the proportional performance method and involve a specified number of deliverables, we recognize revenue based on the proportion of the cost of the deliverables compared to the cost of all deliverables included in the contract as this method more accurately measures performance under these arrangements. For fixed price contracts that provide for the development and delivery of a specific prototype or product, revenue is recognized based upon the percentage of completion method.
Our contracts with agencies of the U.S. government are subject to periodic funding by the respective contracting agency. Funding for a contract may be provided in full at inception of the contract or ratably throughout the contract as the services are provided. In evaluating the probability of funding for purposes of assessing collectability of the contract price, we consider our previous experience with our customers, communication with our customers regarding funding status and our knowledge of available funding for the contract or program. If funding is not assessed as probable, revenue recognition is deferred until realization is reasonably assured.
Under the typical payment terms of our U.S. government contracts, the customer pays us either performance-based payments ("PBPs") or progress payments. PBPs, which are typically used in the firm fixed price contracts, are interim payments based on quantifiable measures of performance or on the achievement of specified events or milestones. Progress payments, which are typically used in our cost type contracts, are interim payments based on costs incurred as the work progresses. For our U.S. government cost-type contracts, the customer generally pays us during the performance period for 80%-90% of our actual costs incurred. Because the customer retains a small portion of the contract price until completion of the contract and audit of allowable costs, cost type contracts generally result in revenue recognized in excess of billings which we present as contract assets on the balance sheet. Amounts billed and due from our customers are classified as receivables on the balance sheet. For non-U.S. government contracts, we typically receive interim payments as work progresses, although for some contracts, we may be entitled to receive advance payments. We recognize a liability for these advance payments and PBPs paid in advance which are in excess of the revenue recognized and present these amounts as contract liabilities on the balance sheet.
To determine the proper revenue recognition method for research and development contracts, we evaluate whether two or more contracts should be combined and accounted for as one single contract and whether the combined or single contract
should be accounted for as more than one performance obligation. For instances where a contract has options that were bid with the initial contract and awarded at a later date, we combine the options with the original contract when options are awarded. For most of our contracts, the customer contracts for research with multiple milestones that are interdependent. Consequently, the entire contract is accounted for as one performance obligation. The effect of the combined or modified contract on the transaction price and measure of progress for the performance obligation to which it relates, is recognized as an adjustment to revenue (either as an increase in or a reduction of revenue) on a cumulative catch-up basis.
Contract revenue recognition inherently involves estimation, includingis measured over time as we perform because of continuous transfer of control to the contemplated levelcustomer. For U.S. government contracts which are typically subject to the Federal Acquisition Regulation, this continuous transfer of effortcontrol to accomplish the tasks undercustomer is supported by clauses in the contract that allow the customer to unilaterally terminate the contract for convenience, pay us for cost incurred plus a reasonable profit and take control of the effort and an ongoing assessment of progress toward completing the contract.any work in process. From time to time, as part of normal management processes, facts may change, causing revisions to estimated total costs or revenues expected. The cumulative impact of any revisions to estimates and the full impact of anticipated losses on any type of contract are recognized in the period in which they become known.
Because of control transfers over time, revenue is recognized over time based on the extent of progress towards completion of the performance obligation. The selection of the method to measure progress towards completion requires judgment and is based on the nature of the services to be provided. We generally use the input method, more specifically the cost-to-cost measure of progress for our contracts because it best depicts the transfer of control to the customer which occurs as we incur costs on our contracts. Under the cost-to-cost measure of progress, the extent of progress towards completion is measured based on the ratio of costs incurred to date to the total estimated costs at completion of the performance obligation. The underlying bases for estimating our contract research revenues are measurable expenses, such as labor, subcontractor costs and materials, and data that are updated on a regular basis for purposes of preparing our cost estimates. Our research contracts generally have a period of performance of six months to three years, and our estimates of contract costs have historically been consistent with actual results. Revisions in these estimates between accounting periods to reflect changing facts and circumstances have not had a material impact on our operating results, and we do not expect future changes in these estimates to be material. The cumulative impact of any revisions to estimates and the full impact of anticipated losses on any type of contract are recognized in the period in which they become known.
Under cost reimbursable contracts, we are reimbursed for costs that are determined to be reasonable, allowable and allocable to the contract and paid a fixed fee representing the profit negotiated between us and the contracting agency. Revenue from cost reimbursable contracts is recognized as costs are incurred plus an estimate of applicable fees earned. We consider fixed fees under cost reimbursable contracts to be earned in proportion to the allowable costs incurred in performance of the contract.
Revenue from time and materials contracts is recognized based on direct labor hours expended at contract billing rates plus other billable direct costs.
Fixed price contracts may include either a product delivery or specific service performance throughout a period. For fixed price contracts that are based on the proportional performance method and involve a specified number of deliverables, we recognize revenue based on the proportion of the cost of the deliverables compared to the cost of all deliverables included in the contract as this method more accurately measures performance under these arrangements. For fixed price contracts that provide for the development and delivery of a specific prototype or product, revenue is recognized based upon the percentage of completion method.
Whether certain costs under government contracts are allowable is subject to audit by the government. Certain indirect costs are charged to contracts using provisional or estimated indirect rates, which are subject to later revision based on government audits of those costs. Management is of the opinion that costs subsequently disallowed, if any, would not likely have a significant impact on revenues recognized for those contracts.
Products and Licensing Revenues
We recognize revenue relating to our product sales when persuasive evidence of an arrangement exists, delivery has occurred, the selling price is fixed or determinable and collectability of the resulting receivable is reasonably assured. For tangible products that contain software that is essential to the tangible product’s functionality, we consider the product and software to be a single unit of accounting and recognize revenue accordingly. We evaluate product sales that are a part of multiple-element revenue arrangements to determine whether separate units of accounting exist, and we follow appropriate revenue recognition policies for each separate unit. For multi-element arrangements we allocate revenue to all significant deliverables based on their relative selling prices. In such circumstances, we use a hierarchy to determine the selling price to be used for allocating revenue to deliverables: (i) vendor-specific objective evidence of fair value ("VSOE"), (ii) third-party evidence of selling price ("TPE"), and (iii) best estimate of the selling price ("ESP"). VSOE generally exists only when we sell the deliverable separately and is the price actually charged by us for that deliverable. Our product sales often include bundled products, options and services and therefore VSOE is not readily determinable. In addition, we believe that because of unique features of our products, TPE also is not available. ESPs reflect our best estimates of what the selling prices of elements would be if they were sold regularly on a stand-alone basis. We also sell extended warranties from time to time. In this case, we accrue warranty costs based on our estimate of future expense and defer revenue associated with the warranty. The deferred warranty revenue is recognized over the period of the warranty.
Our process for determining ESP for deliverables without VSOE or TPE considers multiple factors that may vary depending upon the unique facts and circumstances related to each deliverable. Key factors considered in developing the ESPs include prices charged by us for similar offerings, our historical pricing practices, the nature of the deliverables, and the relative ESP of all of the deliverables as compared to the total selling price of the product. We may also consider, when appropriate, the impact of other products and services, on selling price assumptions when developing and reviewing our ESPs.
Business Combinations
We apply the provisions of ASC 805 in the accounting for acquisitions. ASC 805 requires us to recognize separately from goodwill the assets acquired and the liabilities assumed at their acquisition date fair values. Goodwill as of the acquisition date is measured as the excess of consideration transferred over the net of the acquisition date fair values of the assets acquired and the liabilities assumed. While we use our best estimates and assumptions to accurately apply preliminary value to assets acquired and liabilities assumed at the acquisition date as well as contingent consideration, where applicable, these estimates are inherently uncertain and subject to refinement. As a result, during the measurement period, which may be up to one year from the acquisition date, we record adjustments to the assets acquired and liabilities assumed with the corresponding offset to goodwill. Upon the conclusion of the measurement period or final determination of the values of the assets acquired or liabilities assumed, whichever comes first, any subsequent adjustments are recorded in our Consolidated Statements of Operations. Accounting for business combinations requires management to make significant estimates and assumptions, especially at the acquisition date, including estimates for intangible assets, contractual obligations assumed, restructuring liabilities, pre-acquisition contingencies, and contingent consideration, where applicable. Although we believe the assumptions and estimates we have made have been reasonable and appropriate, they are based in part on historical experience and information obtained from management of the acquired companies and are inherently uncertain. Critical estimates in valuing certain of the intangible assets we have acquired include: future expected cash flows from product sales; customer contracts and acquired technologies; expected costs to develop in-process research and development into commercially viable products and estimated cash flows from the projects when completed; and discount rates. Unanticipated events and circumstances may occur that may affect the accuracy or validity of such assumptions, estimates, or actual results.
Income Taxes
We estimate our tax liability through calculating our current tax liability, together with assessing temporary differences resulting from the different treatment of items for tax and accounting purposes. These differences result in deferred tax assets and liabilities, which we record on our balance sheet. Management then assesses the likelihood that deferred tax assets will be recovered in future periods. In assessing the need for a valuation allowance against the net deferred tax asset, management considers factors such as future reversals of existing taxable temporary differences, taxable income in prior carry back years, whether carry back is permitted under the tax law, tax planning strategies and estimated future taxable income exclusive of
reversing temporary differences and carry forwards.carryforwards. To the extent that we cannot conclude that it is more likely than not that the benefit of such assets will be realized, we establish a valuation allowance to reduce their net carrying value.
As we assess our projections of future taxable income or other factors that may impact our ability to generate taxable income in future periods, our estimate of the required valuation allowance may change, which could have a material impact on future earnings or losses.
We recognize tax benefits from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by taxing authorities. While it is often difficult to predict the final outcome of timing of the resolution of any particular tax matter, we establish a liability at the time we determine it is probable we will be required to pay additional taxes related to certain matters. These liabilities are recorded in accrued liabilities in our consolidated balance sheets. We adjust this provision, including any impact on the related interest and penalties, in light of changing facts and circumstances, such as the progress of a tax audit. A number of years may elapse before a particular matter for which we have established a liability is audited and finally resolved. The number of years with open tax audits varies depending on the tax jurisdiction. Settlement of any particular issue would usually require the use of cash. We recognize favorable resolutions of tax matters for which we have previously established liabilities as a reduction to our income tax expense when the amounts involved become known.
Due to differences between federal and state tax law, and accounting principles generally accepted in the United States of America ("GAAP") certain items are included in the tax return at different times than when those items are reflected in the consolidated financial statements. Therefore, the annual tax rate reflected in our consolidated financial statements is different than that reported in our tax return. Some of these differences are permanent, such as expenses that are not deductible in our tax return. Some differences, such as depreciation expense, reverse over time and create deferred tax assets and liabilities. The tax rates used to determine deferred tax assets or liabilities are the enacted tax rates in effect for the year in which the differences are expected to reverse. Based on the evaluation of all available information, we recognize future tax benefits, such as net operating loss carry forwards,("NOL") carryforwards, to the extent that realizing these benefits is considered more likely than not.
As of December 31, 2019, we were no longer in a three-year cumulative loss position, which required additional analysis to be performed in order to determine the likelihood of realizing the deferred tax assets in the foreseeable future. After further analysis, it was determined that a portion of the December 31, 2019 balance of deferred tax assets will be realized. Because the NOLs carried over from API are limited under Section 382, the deferred tax asset of $1.2 million is expected to be realized over an extended period of time (with continued earnings realized ratably through 2034). The deferred tax asset related to the NOL carryovers of API Canada, Luna Analytics, Luna Nanomaterials, and Luna Quest will likely not be realized in the foreseeable future as these entities no longer have any activity.
Stock-Based Compensation
We recognize stock-based compensation expense based upon the fair value of the underlying equity award on the date of the grant. The calculation of the fair value of our awards requires certain inputs that are subjective and changes to the estimates used will cause the fair values of our stock awards and related stock-based compensation expense to vary. We have elected to use the Black-Scholes-Merton ("Black-Scholes") option pricing model to determine the fair value of stock options. The fair value of a stock option award is affected by our stock price on the date of the grant as well as other assumptions used as inputs in the valuation model including the estimated volatility of our stock price over the term of the awards, the estimate period of time that we expect employees to hold their stock options and the risk-free interest rate assumption. In addition, we are required to reduce stock-based compensation expense for the effects of estimatedactual forfeitures of unvested awards overin the expense recognition period. Although we estimate the rate of future forfeitures based on historical experience, actual forfeitures may differ.period they occur.
Long-lived and Intangible Assets
Long-lived assets and certain identifiable intangibles are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset might not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future un-discounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value, less cost to sell.
Goodwill
Goodwill is reviewed for impairment at least annually, or more frequently if events or circumstances indicate that goodwill might be impaired. We have established October 1 as our specified annual date for impairment testing.
Leases
In February 2016, the Financial Accounting Standards Board ("FASB") issued a new standard related to leases, Accounting Standards Update ("ASU") No. 2016-02, Leases (Topic 842) and subsequent amendments, which replaced existing GAAP and requires lessees to recognize right-of-use ("ROU") assets and corresponding lease liabilities that depict the rights and obligations arising from a lease agreement. We implemented ASU 2016-02 on January 1, 2019 and elected certain practical expedients available under the ASU. As a result of the adoption, we recognized ROU assets totaling $3.5 million and lease liabilities totaling $4.7 million as of the adoption date. For additional information related to the adoption of Topic 842, see Note 1 to our Consolidated Financial Statements elsewhere in this Annual Report on Form 10-K.
Business Combinations
We account for business combinations under the acquisition method of accounting, in accordance with ASC 805 - Business Combinations. Under ASC 805, the total estimated purchase consideration is allocated to the acquired tangible and intangible assets and assumed liabilities based on their estimated fair values as of the acquisition date. Any excess of the fair value of acquisition consideration over the fair value of identifiable assets acquired and liabilities assumed is recorded as goodwill.
Results of Operations
The following table shows information derived from our consolidated statements of operations expressed as a percentage of total revenues for the periods presented. | | | | Years ended December 31, | Years ended December 31, |
| | 2016 | | 2015 | 2019 | | 2018 |
| Revenues: | | | | | | |
| Products and licensing | | 63.1 | % | | 51.1 | % |
| Technology development | 28.4 | % | | 30.9 | % | 36.9 |
| | 48.9 |
|
| Products and licensing | 71.6 |
| | 69.1 |
| |
| Total revenues | 100.0 |
| | 100.0 |
| 100.0 |
| | 100.0 |
|
| Cost of revenues: | | | | | | |
| Products and licensing | | 23.7 |
| | 18.8 |
|
| Technology development | 21.5 |
| | 23.6 |
| 26.4 |
| | 35.9 |
|
| Products and licensing | 41.8 |
| | 38.9 |
| |
| Total cost of revenues | 63.3 |
| | 62.5 |
| 50.1 |
| | 54.7 |
|
| Gross profit | 36.7 |
| | 37.5 |
| 49.9 |
| | 45.3 |
|
| Operating expense | 40.0 |
| | 51.7 |
| 45.2 |
| | 43.2 |
|
| Operating loss | (3.3 | ) | | (14.2 | ) | |
| Total other expense | (0.6 | ) | | (0.5 | ) | |
| Loss from continuing operations before income taxes | (3.9 | ) | | (14.7 | ) | |
| Loss from continuing operations, net of income taxes | (4.0 | ) | | (13.7 | ) | |
| Operating income | | 4.7 |
| | 2.1 |
|
| Total other income | | 0.5 |
| | 1.0 |
|
| Income from continuing operations before income taxes | | 5.2 |
| | 3.1 |
|
| Income from continuing operations, net of income taxes | | 7.6 |
| | 2.9 |
|
| Income from discontinued operations, net of income taxes | — |
| | 18.9 |
| — |
| | 22.8 |
|
| Net (loss)/income | (4.0 | )% | | 5.2 | % | |
| Net income | | 7.6 | % | | 25.7 | % |
Year Ended December 31, 20162019 Compared to Year Ended December 31, 20152018
| | | | Years ended December 31, | | | | | Years ended December 31, | | | | |
| | 2016 | | 2015 | | $ Difference | | % Difference | 2019 | | 2018 | | $ Difference | | % Difference |
| Products and licensing revenues | | $ | 44,491,041 |
| | $ | 21,949,689 |
| | $ | 22,541,352 |
| | 102.7 | % |
| Technology development revenues | $ | 16,825,157 |
| | $ | 13,599,048 |
| | $ | 3,226,109 |
| | 23.7 | % | 26,024,674 |
| | 20,967,556 |
| | 5,057,118 |
| | 24.1 | % |
| Products and licensing revenues | 42,385,839 |
| | 30,421,310 |
| | 11,964,529 |
| | 39.3 | % | |
| Total revenues | $ | 59,210,996 |
| | $ | 44,020,358 |
| | $ | 15,190,638 |
| | 34.5 | % | $ | 70,515,715 |
| | $ | 42,917,245 |
| | $ | 27,598,470 |
| | 64.3 | % |
Our Products and Licensing segment included revenues from sales of test and measurement systems, primarily representing sales of our Optical Backscatter Reflectometer, ODiSI, and Optical Vector Analyzer platforms, optical components and sub-assemblies and sales of our Hyperion and Terahertz sensing platforms. Our Products and Licensing segment revenues increased $22.5 million to $44.5 million for the year ended December 31, 2019 compared to $21.9 million for the year ended December 31, 2018. The increase resulted primarily from $10.8 million of revenues realized from the legacy business of MOI
and $10.5 million of revenues realized from the legacy business of GP during the year ended December 31, 2019. Continued growth in sales of our fiber-optic sensing products, including our ODiSI products directed toward the expanding use of composite materials and the need for improved means of testing their structural integrity, and our communications test instruments also contributed to this increase.
Our Technology Development segment revenues increased $3.2$5.1 million to $16.8$26.0 million for the year ended December 31, 20162019 compared to $13.6$21.0 million for the year ended December 31, 2015. This2018. Revenues within this segment increased due to additional contract awards, including higher value Phase 2 SBIR contracts. The increase continues a growth trend experienced over the past two years largely driven by successes in Phase 2 SBIR awards. The increase was attributablerealized primarily to growth in our biomedical and intelligent systems, advanced materials, optical systems and terahertz research groups. These groups sawAs Phase 2 SBIR contracts generally have a significant increase in the numberperformance period of active contracts along with an increase in the average revenue per contract.
Our Products and Licensinga year or more, we currently expect Technology Development segment revenues increased $12.0 million to $42.4 millionremain at a similar level for the year ended December 31, 2016 compared to $30.4 million for the year ended December 31, 2015. This increase was due primarily to inclusion of product sales associated with API's legacy operations for the full year of 2016 compared to the period from the closing of our merger on May 8, 2015 through December 31, 2015 for 2015. The increase in product revenue from API's legacy operations due to inclusion of the full year for 2016 was approximately $10.0 million. Sales of HSOR products for the telecom market and instrumentation for measuring strain in composite materials contributed to the additional increase in product revenues.
near term.
Cost of Revenues
| | | | Years ended December 31, | | | | | Years ended December 31, | | | | |
| | 2016 | | 2015 | | $ Difference | | % Difference | 2019 | | 2018 | | $ Difference | | % Difference |
| Products and licensing costs | | $ | 16,684,172 |
| | $ | 8,078,870 |
| | $ | 8,605,302 |
| | 106.5 | % |
| Technology development costs | $ | 12,711,447 |
| | $ | 10,378,616 |
| | $ | 2,332,831 |
| | 22.5 | % | 18,649,161 |
| | 15,400,475 |
| | 3,248,686 |
| | 21.1 | % |
| Products and licensing costs | 24,764,788 |
| | 17,141,079 |
| | 7,623,709 |
| | 44.5 | % | |
| Total costs of revenues | $ | 37,476,235 |
| | $ | 27,519,695 |
| | $ | 9,956,540 |
| | 36.2 | % | $ | 35,333,333 |
| | $ | 23,479,345 |
| | $ | 11,853,988 |
| | 50.5 | % |
Our Products and Licensing segment costs increased $8.6 million to $16.7 million for the year ended December 31, 2019 compared to $8.1 million for the year ended December 31, 2018. This increase primarily resulted from $3.9 million of cost of revenues from the legacy business of MOI and $4.4 million of cost of revenues from the legacy business of GP during the year ended December 31, 2019, as well as an increase in sales volume.
Our Technology Development segment costs increased $2.3$3.2 million, to $12.7$18.6 million for the year ended December 31, 20162019 compared to $10.4$15.4 million for the year ended December 31, 2015.2018. The overall increase in Technology Development segment costs was driven by increases in direct labor and subcontractor costs consistent with the rate of growth in Technology Development segment revenues.
Our ProductsOperating Expense
|
| | | | | | | | | | | | | | |
| | Years ended December 31, | | | | |
| | 2019 | | 2018 | | $ Difference | | % Difference |
| Selling, general and administrative expense | $ | 24,371,349 |
| | $ | 14,794,205 |
| | $ | 9,577,144 |
| | 64.7 | % |
| Research, development and engineering expense | 7,496,012 |
| | 3,766,160 |
| | 3,729,852 |
| | 99.0 | % |
| Total operating expense | $ | 31,867,361 |
| | $ | 18,560,365 |
| | $ | 13,306,996 |
| | 71.7 | % |
Selling, general and Licensing segment costsadministrative expense increased $7.6$9.6 million to $24.8$24.4 million for the year ended December 31, 20162019 compared to $17.1$14.8 million for the year ended December 31, 2015. The overall increase in Products and Licensing segment costs was consistent with the rate of growth in the Products and Licensing segment revenues, when taking into account our revenue growth was driven by legacy API products. This product mix contributes to an overall lower margin for our product sales.
Operating Expense
|
| | | | | | | | | | | | | | |
| | Years ended December 31, | | | | |
| | 2016 | | 2015 | | $ Difference | | % Difference |
| Selling, general and administrative expense | $ | 18,139,966 |
| | $ | 18,481,270 |
| | $ | (341,304 | ) | | (1.8 | )% |
| Research, development and engineering expense | 5,532,130 |
| | 4,268,988 |
| | 1,263,142 |
| | 29.6 | % |
| Total operating expense | $ | 23,672,096 |
| | $ | 22,750,258 |
| | $ | 921,838 |
| | 4.1 | % |
2018. Selling, general and administrative expense increased primarily due to $4.4 million of expenses decreased $0.3associated with the legacy business of MOI and $2.0 million of expenses associated with the legacy business of GP , in addition to $1.0 million in transaction costs associated with the acquisition of GP, a $0.9 million increase in share-based compensation as a result of new awards, and a $1.1 million increase in expenses related to sales and marketing as a result of increased revenue.
Research, development and engineering expenses increased $3.7 million to $18.1$7.5 million for the year ended December 31, 2016 compared to $18.5 million for the year ended December 31, 2015. This decrease was due to $3.7 million in merger related expenses for the year ended December 31, 2015 not recurring in the year ended December 31, 2016. This decrease was partially offset by an increase of selling, general and administrative expenses of approximately $2.6 million due to the inclusion of expenses of API's legacy operations for the entire year ended December 31, 2016 compared to the period from the closing of our merger on May 8, 2015 through December 31, 2015. We also experienced an increase in costs related to annual employee salary increases and professional fees of approximately $0.8 million.
Research, development and engineering expenses increased $1.3 million to $5.5 million for the year ended December 31, 2016 compared to $4.3 million for the year ended December 31, 2015, primarily due to a $1.2 million increase resulting from the inclusion of expenses from API's legacy operations for the entire year ended December 31, 2016 compared to the period from the closing of our merger on May 8, 2015 through December 31, 2015.
Interest Expense, Net
Our net interest expense was approximately $0.3 million for the year ended December 31, 2016 compared to approximately $0.2 million for the year ended December 31, 2015. The average monthly loan balance for the year ended December 31, 2016 was $5.1 million as2019 compared to $3.8 million for the year ended December 31, 2015, resulting2018 primarily due to $1.1 million of research, development and engineering expense associated with the legacy business of MOI and $2.7 million of research, development and engineering expense associated with the legacy business of GP during the year ended December 31, 2019.
Interest Expense, Net
Our net interest expense was $15,878 for the year ended December 31, 2019 compared to $0.1 million for the year ended December 31, 2018, as a result of a decrease in thisdebt partially offset by the increase in interest expense.finance lease obligations.
Investment Income
Investment income was $0.4 million for the year ended December 31, 2019, compared to $0.5 million for the year ended December 31, 2018. During the years ended December 31, 2019 and 2018, we invested a portion of our cash in funds holding U.S. treasury securities.
Income Tax (Benefit)/Expense from Continuing Operations
For the year ended December 31, 2016,2019, we recorded income tax expensebenefit of $0.1$1.7 million, or 3.6%(44.9)% of our lossincome from continuing operations, compared to an income tax benefitexpense of $0.5 million,$47,818, or 7.3%3.8% of our lossincome from continuing operations for the year ended December 31, 2015.2018. The change from an income tax benefit in 2015 to income tax expense in 2016 resulted from the decrease in our loss from continuing operations. Certain expenses included in our loss from continuing operations are not deductible for determination of the federal alternative minimum taxable income or state income taxes, and the recognition of those book-tax differences, which in the aggregate exceeded our loss from continuing operations, resulted in a current tax expenserecognized for the year ended December 31, 2016.2019 was driven mostly by a partial release of our valuation allowances.
Net Income From Continuing Operations
For the year ended December 31, 2019, we recognized income from continuing operations before income taxes of $3.7 million, compared to $1.3 million for the year ended December 31, 2018. After tax, our net income from continuing operations was $5.3 million for the year ended December 31, 2019, compared to $1.2 million for the year ended December 31, 2018.
Income from Discontinued Operations, Net of Income Taxes
For the year ended December 31, 2016,2018, we did not recognize income from discontinued operations. For the year ended December 31, 2015, we recognized a net income from discontinued operations, net of income taxes, of $8.3$9.8 million. ThisNet income consisted of $9.0 million received from Intuitive indiscontinued operations for the year ended December 2015 in satisfaction of all remaining potential amounts payable, including future technological milestone payments and future royalty payments, associated with31, 2018, included an after tax gain recognized on the sale of our medical shape sensingoptoelectronics business for medical applicationsduring 2018 of $8.6 million in 2014, offset byaddition to $1.2 million of after-tax income tax expense of $0.7 million associated with the gain.operations of the optoelectronics business prior to its sale. There were no results from discontinued operations for the year ended December 31, 2019.
Preferred Stock Dividend
In January 2010, we issued 1,321,514 shares of our newly designated Series A Convertible Preferred Stock to Carilion. The Series A Convertible Preferred Stock carriescarried an annual cumulative dividend of 6%, or approximately 79,292 shares of common stock per year. During each of 20162019 and 2015,2018, we accrued $0.1$0.3 million and $0.3 million, respectively, for the dividends payable to Carilion. The dividends are not payable in cash, but rather in sharesDuring 2019, the total accrued dividend of our common stock, until liquidation event occurs. During each of 2016 and 2015, 79,292770,454 shares of common stock became issuablewere issued to Carilion as dividends and have been recordedshown on our consolidated statements of changes in the statement of stockholders’stockholders' equity.
Liquidity and Capital Resources
At December 31, 2016,2019, our total cash and cash equivalents were $12.8$25.0 million.
We have two term loans
On October 10, 2019, we entered into an Amended and Restated Loan and Security Agreement (the "Loan Agreement") with SVBSilicon Valley Bank ("SVB"), which at December 31, 2016, had an aggregate balance of $4.3 million. One term loan, with a balance of $0.7 millionamended and restated in its entirety our previous Loan and Security Agreement dated as of December 31, 2016, matures on December 1, 2018. The other term loan with a balance of $3.6 millionFebruary 18, 2010, as of December 31, 2016, matures on May 1, 2019.
We may prepay amounts due underamended. Under the term loans at any time, subjectLoan Agreement, SVB agreed to prepayment penalties ofmake advances available up to 2% of the amount of prepayment.
Amounts due$10.0 million (the "Revolving Line"). The Revolving Line terminates on October 10, 2020 unless earlier terminated by us. No amounts have been borrowed under the term loans are secured by substantially all of our assets, including intellectual property, personal property and bank accounts.
The term loans contain customary events of default, including nonpayment of principal, interest or other amounts, violation of covenants, material adverse change, an event of default under any subordinated debt documents, incorrectness of representations and warranties in any material respect, bankruptcy, judgments in excess of a threshold amount, and violations of other agreements in excess of a threshold amount. If any event of default occurs SVB may declare due immediately all borrowings under the credit facility and foreclose on the collateral. Furthermore, an event of default under the credit facility would result in an increase in the interest rate on any amounts outstanding. The credit facility requires us to comply with certain operational and financial covenants, including maintaining a minimum cash balance of at least $5.0 million. As of December 31, 2016, we were in compliance with all covenants under the credit facility.this Loan Agreement.
We maintain a letter-of-credit in the amount of $1.0 million as a condition of our lease on our Blacksburg office.
We believe that our cash balanceand cash equivalents as of December 31, 20162019 in addition to amounts available to us under our Revolving Line will provide adequate liquidity for us to meet our working capital needs over the next twelve months.months from the date of issuance of the consolidated financial statements included elsewhere in this Annual Report on Form 10-K. Additionally, we believe that should we have the need for increased capital spending to support our planned growth, we will be able to fund such growth through either third-party financing on competitive market terms or through our available cash.cash and cash equivalents.
| | | | Years ended December 31, | Years ended December 31, |
| | 2016 | | 2015 | 2019 | | 2018 |
| Net cash used in operating activities | $ | (399,837 | ) | | $ | (5,087,907 | ) | |
| Net cash provided by/(used in) operating activities | | $ | 4,798,201 |
| | $ | (3,308,826 | ) |
| Net cash (used in)/provided by investing activities | (2,000,184 | ) | | 8,294,714 |
| (19,814,991 | ) | | 10,037,123 |
|
| Net cash (used in)/provided by financing activities | (2,261,561 | ) | | 140,264 |
| |
| Net cash used in financing activities | | (2,437,560 | ) | | (1,249,564 | ) |
| Net (decrease)/increase in cash and cash equivalents | $ | (4,661,582 | ) | | $ | 3,347,071 |
| $ | (17,454,350 | ) | | $ | 5,478,733 |
|
During 2016, operations used $0.42019, the $4.8 million of net cash as compared to 2015, when operations used $5.1 millionprovided by operating activities consisted of net cash. In 2016, our net loss of $2.4 million included charges for depreciation and amortization of $3.7 million and stock-based
compensation of $0.9 million and bad debt of $0.3 million, which are non-cash expenses and do not impact cash flow for the period. Additionally, changes in working capital resulted in a net cash outflow of $2.9 million, principally driven by an increase in accounts receivable of $3.6 million, partially offset by an increase in accounts payable and accrued liabilities of $0.6 million.
In 2015, our net income of $2.3$5.3 million, and included a benefit of an $8.3 million after-tax gain associated with contingent consideration received in 2015 from the sale of our medical shape sensing business in 2014 and a tax benefit of $0.5 million. Absent the effects of that gain and the tax benefit, our pre-tax loss from continuing operations was $6.5 million. The pre-tax loss from continuing operations includednon-cash charges for depreciation and amortization of $2.5 million, and stock-based compensation of $1.1$1.5 million, eachoffset by a tax benefit from a partial release of which are non-cash items that do not impactthe valuation allowances of $3.3 million and a net cash flow foroutflow of $1.8 million from changes in working capital. The changes in working capital were principally driven by an increase in accounts receivable of $2.2 million, an increase in inventory of $0.7 million, an increase in contract assets of $0.4 million, and an increase in other assets of $0.2 million, all partially offset by decreases in accounts payable and accrued expenses of $0.6 million and contract liabilities of $1.2 million,
In 2018, the period. In 2015, our$3.3 million of net cash used in operating activities alsoconsisted of our net income of $11.0 million and included $3.7a gain recognized on the sale of our optoelectronic segment that was sold in July 2018 of $8.6 million in addition to non-cash charges for depreciation and amortization of expenses associated with completing our merger with API.$1.2 million and stock-based compensation of $0.6 million, offset by a net cash outflow of $7.6 million from changes in working capital. The changes in working capital were principally driven by an increase in inventory of $1.0 million, and increase in accounts receivable of $6.2 million, and increase in contract assets of $0.8 million, and an increase in accounts payable and accrued liabilities of $0.5 million, all partially offset by a $1.8 million decrease in other assets.
Cash used in investing activities in 20162019 consisted primarily of a cash outflowthe $19.0 million payment for our acquisition of $2.0GP, $0.5 million related to the purchase of propertyfixed asset additions and equipment to expand our manufacturing capability for HSOR products as well as$0.3 million of capitalized costs associated with securing intellectual property rights.costs.
Cash provided by investing activities in 20152018 consisted primarily of a cash inflow of $9.0 million related to a payment arisingthe proceeds from the sale of our medical shape sensing business in 2014, cashoptoelectronic segment of $15.8 million, partially offset by the $5.0 million payment for our acquisition of MOI, $0.4 million acquired in our merger with API, and cash outflows of $0.7 million for the purchase of equipmentfixed asset additions and $0.4 million in patent costs associated with certain intangible assets, primarily associated with our fiber optic platform and our THz sensing platform.of capitalized intellectual property rights.
Cash used in financing activities for the year ended December 31, 20162019 was $2.3$2.4 million, compared to cash provided by financing activities of $0.1$1.2 million in 2015.2018. During 2016,2019, we repaid $1.9$0.6 million on our term loans with SVB. We alsoSVB and used $0.3$2.2 million to repurchase our common stock under our stock repurchase program. These payments were partially offset by $0.4 million received from exercises of stock options and warrants. During 2015,2018, we repaid $6.7$1.8 million on our outstanding term loan with SVB and borrowed $7.0used $0.5 million to repurchase our common stock under a new term loan with SVB.our stock repurchase program. These payments were partially offset by $1.1 million received from exercises of stock options and warrants.
Summary of Contractual Obligations
The following table sets forth information concerning our known contractual obligations as of December 31, 20162019 that are fixed and determinable.
|
| | | | | | | | | | | | | | | | | | | |
| | Total | | Less than 1 year | | 1 - 3 years | | 3 - 5 years | | More than 5 years |
| Long-term debt obligations (1) | $ | 4,291,666 |
| | $ | 1,833,333 |
| | $ | 2,458,333 |
| | $ | — |
| | $ | — |
|
| Operating facility leases (2) | 8,244,602 |
| | 1,601,292 |
| | 3,054,013 |
| | 2,148,120 |
| | 1,441,177 |
|
| Other leases (3) | 186,501 |
| | 61,244 |
| | 85,737 |
| | 39,520 |
| | — |
|
| Purchase order obligation (4) | 1,522,642 |
| | 1,522,642 |
| | — |
| | — |
| | — |
|
| Other liabilities (5) | 880,000 |
| | 220,000 |
| | 440,000 |
| | 220,000 |
| | — |
|
| Total | $ | 15,125,411 |
| | $ | 5,238,511 |
| | $ | 6,038,083 |
| | $ | 2,407,640 |
| | $ | 1,441,177 |
|
|
| | | | | | | | | | | | | | | | | | | |
| | Total | | Less than 1 year | | 1 - 3 years | | 3 - 5 years | | More than 5 years |
| Operating facility leases (1) | $ | 3,742,613 |
| | $ | 1,467,701 |
| | $ | 1,185,504 |
| | $ | 1,089,408 |
| | $ | — |
|
| Finance leases (2) | 81,855 |
| | 56,019 |
| | 20,794 |
| | 5,042 |
| | — |
|
| Purchase order obligation (3) | 1,271,440 |
| | 1,271,440 |
| | — |
| | — |
| | — |
|
| Other liabilities (4) | 220,000 |
| | 220,000 |
| | — |
| | — |
| | — |
|
| Total | $ | 5,315,908 |
| | $ | 3,015,160 |
| | $ | 1,206,298 |
| | $ | 1,094,450 |
| | $ | — |
|
_________________________
| |
(1) | Amounts due under our debt obligations to SVB are payable in monthly installments, plus accrued interest, through May 2019. |
| |
(2)
| We lease our facilities in Blacksburg, Charlottesville and Roanoke, Virginia, Ann Arbor, Michigan, CamarilloChino, California and Montreal, CanadaAtlanta, Georgia under operating leases that as of December 31, 2016,2019, are scheduled to expire between April 2017October |
2020 and December 2024. Upon expiration of our office leases, we may exercise certain renewal options as specified in the leases. Rental payments associated with these option periods are not included in the table above.
| |
(2) | In January 2016, February 2018, and June 2018 we executed leases in the amounts of $207,000, $15,000, and $75,000, respectively, for office equipment. These equipment leases expire in 2021, 2021 and 2023, respectively. |
| |
(3) | Purchase order obligations included outstanding orders for inventory purchases. In August 2013 and January 2016, we executed leases in the amounts of $51,000, and $207,000, respectively, for office equipment. These equipment leases expire in 2018 and 2021, respectively. |
| |
(4)
| In 2016,2019, our Luna Technologies subsidiary executed two non-cancelable purchase orders in the amountsfor a total amount of $0.5 million and $1.0$1.9 million for multiple shipments of tunable lasers to be delivered over an 18-month period beginning in July 2016. In addition, in 2016 we issued two purchase orders for component parts and capital equipment for our HSOR products with an aggregate value of $0.8 million for delivery through the second quarter of 2017, of which $0.5 million was outstanding as of December 31, 2016.2019. |
| |
(5)(4)
| Other liabilities include remaining amounts payable for minimum royalty payments for certain licensed technologies payable over the remaining patent terms of the underlying technology. |
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements as defined in Regulation S-K, Item 303(a)(4)(ii).of December 31, 2019.
Inflation
We do not believe that inflation has had a material effect on our business, financial condition or results of operations.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. We do not hold or issue financial instruments for trading purposes or have any derivative financial instruments. Our exposure to market risk is limited to interest rate fluctuations due to changes in the general level of U.S. interest rates.
Interest Rate Risk
We do not use derivative financial instruments as a hedge against interest rate fluctuations, and, as a result, interest income earned on our cash and cash equivalents and short-term investments is subject to changes in interest rates. However, we believe that the impact of these fluctuations does not have a material effect on our financial position due to the immediate available liquidity or short-term nature of these financial instruments.
We are exposed to interest rate fluctuations as a result of our SVB debt facility having a variable interest rate. We do not currently use derivative instruments to alter the interest rate characteristics of our debt. The principal amount of $4.3 million outstanding under the term loan as of December 31, 2016, is scheduled to amortize in monthly installments through May 2019. A change of 1% in the applicable interest rate during 2017 would have an impact of approximately $33,000 in our annual interest expense under the SVB debt facility.
Foreign Currency Exchange Rate Risk
As of December 31, 2016,2019, all payments made under our research contracts have been denominated in U.S. dollars. Our product sales to foreign customers are also generally denominated in U.S. dollars, and we do not receive payments in foreign currency. As such, we are not directly exposed to significant currency gains or losses resulting from fluctuations in foreign exchange rates.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Luna Innovations Incorporated
Index to Consolidated Financial Statements
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Luna Innovations Incorporated
Opinion on the financial statements
We have audited the accompanying consolidated balance sheets of Luna Innovations Incorporated (a Delaware corporation) and subsidiaries (the “Company”) as of December 31, 20162019 and 2015, and2018, the related consolidated statements of operations, changes in stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2016. Our audits of2019, and the basic consolidated financial statements included therelated notes and financial statement schedule listedincluded under Item 15(a) (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the two years in the index appearing under Item 15(a)(2). These consolidatedperiod ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company’s internal control over financial reporting as of December 31, 2019, based on criteria established in the 2013 Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”), and our report dated March 13, 2020 expressed an unqualified opinion.
Change in accounting principle
As discussed in Note 1 to the financial statements, andthe Company has changed its method of accounting for leases as of January 1, 2019, in accordance with the adoption of Accounting Standards Codification (ASC) Topic 842, Leases.
Basis for opinion
These financial statement schedulestatements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidatedthe Company’s financial statements and financial statement schedule based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States).PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. We were not engagedmisstatement, whether due to perform an audit of the Company’s internal control over financial reporting.error or fraud. Our audits included considerationperforming procedures to assess the risks of internal control overmaterial misstatement of the financial reporting as a basis for designing auditstatements, whether due to error or fraud, and performing procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includesrespond to those risks. Such procedures included examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessingstatements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidatedpresentation of the financial statement presentation.statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ GRANT THORNTON LLP
We have served as the Company’s auditor since 2005.
Arlington, Virginia
March 13, 2020
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Luna Innovations Incorporated
Opinion on internal control over financial reporting
We have audited the internal control over financial reporting of Luna Innovations Incorporated (a Delaware corporation) and subsidiaries (the “Company”) as of December 31, 2019, based on criteria established in the 2013 Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). In our opinion, the consolidated financial statements referred to above present fairly,Company maintained, in all material respects, theeffective internal control over financial position of Luna Innovations Incorporated and subsidiariesreporting as of December 31, 2016 and 2015, and2019, based on criteria established in the results of their operations and their cash flows for each2013 Internal Control-Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the two years inPublic Company Accounting Oversight Board (United States) (“PCAOB”), the periodconsolidated financial statements of the Company as of and for the year ended December 31, 2016 in conformity with accounting principles generally accepted2019 and our report dated March 13, 2020 expressed an unqualified opinion on those financial statements.
Basis for opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the United States of America. Also inaccompanying Management’s Report on Internal Control over Financial Reporting (“Management’s Report”). Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our opinion,audit. We are a public accounting firm registered with the related financial statement schedule, when considered in relationPCAOB and are required to be independent with respect to the basic consolidatedCompany in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial statements taken as a whole, presents fairly,reporting was maintained in all material respects,respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the information set forth therein.risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Our audit of, and opinion on, the Company’s internal control over financial reporting does not include the internal control over financial reporting of General Photonics Corporation, a wholly-owned subsidiary, whose financial statements reflect total assets and revenues constituting 28 and 7 percent, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2019. As indicated in Management’s Report, General Photonics Corporation was acquired during 2019. Management’s assertion on the effectiveness of the Company’s internal control over financial reporting excluded internal control over financial reporting of General Photonics Corporation.
Definition and limitations of internal control over financial reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ GRANT THORNTON LLP
Arlington, Virginia
March 20, 201713, 2020
Luna Innovations Incorporated
CONSOLIDATED BALANCE SHEETSConsolidated Balance Sheets
| | | | December 31, 2016 | | December 31, 2015 | December 31, 2019 | | December 31, 2018 |
| Assets | | | | | | |
| Current assets: | | | | | | |
| Cash and cash equivalents | $ | 12,802,458 |
| | $ | 17,464,040 |
| $ | 25,005,917 |
| | $ | 42,460,267 |
|
| Accounts receivable, net | 14,297,725 |
| | 11,034,557 |
| 16,268,645 |
| | 13,037,068 |
|
| Receivable from sale of HSOR business | | 2,501,386 |
| | 2,500,000 |
|
| Contract assets | | 2,758,946 |
| | 2,422,495 |
|
| Inventory, net | 8,370,235 |
| | 8,863,167 |
| 10,294,431 |
| | 6,873,742 |
|
| Prepaid expenses and other current assets | 1,627,175 |
| | 1,388,439 |
| 1,286,968 |
| | 935,185 |
|
| Total current assets | 37,097,593 |
| | 38,750,203 |
| 58,116,293 |
| | 68,228,757 |
|
| Property and equipment, net | 6,780,838 |
| | 6,614,238 |
| 3,465,612 |
| | 3,627,886 |
|
| Intangible assets, net | 8,681,263 |
| | 10,404,312 |
| 10,194,477 |
| | 3,302,270 |
|
| Goodwill | 2,348,331 |
| | 2,274,112 |
| 10,541,676 |
| | 101,008 |
|
| Long term contract assets | | 449,260 |
| | 336,820 |
|
| Other assets | 88,948 |
| | 88,948 |
| 2,341,179 |
| | 1,995 |
|
| Deferred tax asset | | 1,415,563 |
| | — |
|
| Total assets | $ | 54,996,973 |
| | $ | 58,131,813 |
| $ | 86,524,060 |
| | $ | 75,598,736 |
|
| Liabilities and stockholders’ equity | | | | | | |
| Current liabilities: | | | | | | |
| Current portion of long term debt obligations | $ | 1,833,333 |
| | $ | 1,833,333 |
| |
| Current portion of long-term debt obligations | | $ | — |
| | $ | 619,315 |
|
| Current portion of capital lease obligations | 52,128 |
| | 31,459 |
| — |
| | 40,586 |
|
| Accounts payable | 4,466,192 |
| | 4,054,425 |
| 2,786,825 |
| | 2,395,984 |
|
| Accrued liabilities | 8,667,100 |
| | 8,304,686 |
| 10,369,545 |
| | 6,597,458 |
|
| Deferred revenue | 949,603 |
| | 1,109,759 |
| |
| Contract liabilities | | 3,887,685 |
| | 2,486,111 |
|
| Total current liabilities | 15,968,356 |
| | 15,333,662 |
| 17,044,055 |
| | 12,139,454 |
|
| Long-term portion of deferred rent | 1,403,957 |
| | 1,564,229 |
| — |
| | 1,035,974 |
|
| Long-term debt obligations | 2,420,032 |
| | 4,291,667 |
| |
| Other long-term liabilities | | 2,011,487 |
| | — |
|
| Long-term capital lease obligations | 114,940 |
| | 35,237 |
| — |
| | 68,978 |
|
| Total liabilities | 19,907,285 |
| | 21,224,795 |
| 19,055,542 |
| | 13,244,406 |
|
| Commitments and contingencies |
| |
| |
| Commitments and contingencies (Note 14) | |
| |
|
| Stockholders’ equity: | | | | | | |
| Preferred stock, par value $0.001, 1,321,514 shares authorized, issued and outstanding at December 31, 2016 and 2015 | 1,322 |
| | 1,322 |
| |
| Common stock, par value $0.001, 100,000,000 shares authorized, 27,988,104 and 27,644,833 shares issued, 27,541,277 and 27,477,181 shares outstanding at December 31, 2016 and 2015, respectively | 28,600 |
| | 28,178 |
| |
| Treasury stock at cost, 446,827 and 167,652 shares at December 31, 2016 and 2015, respectively | (517,987 | ) | | (184,934 | ) | |
| Preferred stock, par value $0.001, 1,321,514 shares authorized, 0 and 1,321,514 shares issued and outstanding at December 31, 2019 and 2018, respectively | | — |
| | 1,322 |
|
| Common stock, par value $0.001, 100,000,000 shares authorized, 31,788,896 and 29,209,506 shares issued, 30,149,105 and 27,956,401 shares outstanding at December 31, 2019 and 2018, respectively | | 31,849 |
| | 30,120 |
|
| Treasury stock at cost, 1,639,791 and 1,253,105 shares at December 31, 2019 and 2018, respectively | | (4,337,107 | ) | | (2,116,640 | ) |
| Additional paid-in capital | 82,451,958 |
| | 81,461,907 |
| 88,021,903 |
| | 85,744,750 |
|
| Accumulated deficit | (46,874,205 | ) | | (44,399,455 | ) | (16,248,127 | ) | | (21,305,222 | ) |
| Total stockholders’ equity | 35,089,688 |
| | 36,907,018 |
| 67,468,518 |
| | 62,354,330 |
|
| Total liabilities and stockholders’ equity | $ | 54,996,973 |
| | $ | 58,131,813 |
| $ | 86,524,060 |
| | $ | 75,598,736 |
|
The accompanying notes are an integral part of these consolidated financial statements.
Luna Innovations Incorporated
CONSOLIDATED STATEMENTS OF OPERATIONSConsolidated Statements of Operations
| | | | Years ended December 31, | Years ended December 31, |
| | 2016 | | 2015 | 2019 | | 2018 |
| Revenues: | | | | | | |
| Products and licensing | | $ | 44,491,041 |
| | $ | 21,949,689 |
|
| Technology development | $ | 16,825,157 |
| | $ | 13,599,048 |
| 26,024,674 |
| | 20,967,556 |
|
| Products and licensing | 42,385,839 |
| | 30,421,310 |
| |
| Total revenues | 59,210,996 |
| | 44,020,358 |
| 70,515,715 |
| | 42,917,245 |
|
| Cost of revenues: | | | | | | |
| Products and licensing | | 16,684,172 |
| | 8,078,870 |
|
| Technology development | 12,711,447 |
| | 10,378,616 |
| 18,649,161 |
| | 15,400,475 |
|
| Products and licensing | 24,764,788 |
| | 17,141,079 |
| |
| Total cost of revenues | 37,476,235 |
| | 27,519,695 |
| 35,333,333 |
| | 23,479,345 |
|
| Gross profit | 21,734,761 |
| | 16,500,663 |
| 35,182,382 |
| | 19,437,900 |
|
| Operating expense: | | | | | | |
| Selling, general and administrative | 18,139,966 |
| | 18,481,270 |
| 24,371,349 |
| | 14,794,205 |
|
| Research, development and engineering | 5,532,130 |
| | 4,268,988 |
| 7,496,012 |
| | 3,766,160 |
|
| Total operating expense | 23,672,096 |
| | 22,750,258 |
| 31,867,361 |
| | 18,560,365 |
|
| Operating loss | (1,937,335 | ) | | (6,249,595 | ) | |
| Other expense: | | | | |
| Operating income | | 3,315,021 |
| | 877,535 |
|
| Other income/(expense): | | | | |
| Other expense, net | (35,849 | ) | | (9,967 | ) | (4,504 | ) | | (17,143 | ) |
| Investment income | | 393,556 |
| | 549,580 |
|
| Interest expense, net | (320,942 | ) | | (220,403 | ) | (15,878 | ) | | (124,344 | ) |
| Total other expense | (356,791 | ) | | (230,370 | ) | |
| Loss from continuing operations before income taxes | (2,294,126 | ) | | (6,479,965 | ) | |
| Income tax expense/(benefit) | 75,366 |
| | (470,605 | ) | |
| Net (loss)/income from continuing operations | (2,369,492 | ) | | (6,009,360 | ) | |
| Income from discontinued operations, net of income taxes of $0 and $0.7 million | — |
| | 8,326,386 |
| |
| Net (loss)/income | (2,369,492 | ) | | 2,317,026 |
| |
| Preferred stock dividend | 105,258 |
| | 85,830 |
| |
| Net (loss)/income attributable to common stockholders | $ | (2,474,750 | ) | | $ | 2,231,196 |
| |
| Net loss per share from continuing operations: | | | | |
| Basic and diluted | $ | (0.09 | ) | | $ | (0.26 | ) | |
| Total other income | | 373,174 |
| | 408,093 |
|
| Income from continuing operations before income taxes | | 3,688,195 |
| | 1,285,628 |
|
| Income tax benefit/(expense) | | 1,654,350 |
| | (47,818 | ) |
| Net income from continuing operations | | 5,342,545 |
| | 1,237,810 |
|
| Operating income from discontinued operations, net of income tax expenses ($183,921) | | — |
| | 1,170,634 |
|
| Gain on sale, net of income tax expenses ($1,572,244) | | — |
| | 8,595,797 |
|
| Income from discontinued operations, net of income taxes | | — |
| | 9,766,431 |
|
| Net income | | 5,342,545 |
| | 11,004,241 |
|
| Less: Preferred stock dividend | | 285,450 |
| | 257,302 |
|
| Net income attributable to common stockholders | | $ | 5,057,095 |
| | $ | 10,746,939 |
|
| | | | | |
| Net income per share from continuing operations: | | | | |
| Basic | | $ | 0.19 |
| | $ | 0.04 |
|
| Diluted | | $ | 0.17 |
| | $ | 0.04 |
|
| Net income per share from discontinued operations: | | | | | | |
| Basic and diluted | $ | — |
| | $ | 0.36 |
| |
| Net (loss)/income per share attributable to common stockholders: | | | | |
| Basic and diluted | $ | (0.09 | ) | | $ | 0.10 |
| |
| Basic | | $ | — |
| | $ | 0.35 |
|
| Diluted | | $ | — |
| | $ | 0.30 |
|
| Net income per share attributable to common stockholders: | | | | |
| Basic | | $ | 0.18 |
| | $ | 0.39 |
|
| Diluted | | $ | 0.16 |
| | $ | 0.33 |
|
| Weighted average shares: | | | | | | |
| Basic and diluted | 27,547,217 |
| | 23,026,494 |
| |
| Basic | | 28,688,867 |
| | 27,596,401 |
|
| Diluted | | 31,840,584 |
| | 32,452,228 |
|
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITYLuna Innovations Incorporated
Consolidated Statements of Changes in Stockholders' Equity
| | | | Preferred Stock | | Common Stock | | Treasury Stock | Additional Paid in Capital | | Accumulated Deficit | | Total | Preferred Stock | | Common Stock | | Treasury Stock | Additional Paid in Capital | | Accumulated Deficit | | Total |
| | Shares | | $ | | Shares | | $ | | Shares | | $ | | $ | | $ | Shares | | $ | | Shares | | $ | | Shares | | $ | | $ | | $ |
| Balance—January 1, 2015 | 1,321,514 |
| | 1,322 |
| | 15,088,199 |
| | 15,541 |
| | 22,727 |
| | (32,221 | ) | 64,147,666 |
| | (46,630,651 | ) | | 17,501,657 |
| |
| Exercise of stock options | — |
| | — |
| | 233,704 |
| | 233 |
| | — |
| | — |
| 82,283 |
| | — |
| | 82,516 |
| |
| Stock-based compensation | — |
| | — |
| | 453,522 |
| | 453 |
| | — |
| | — |
| 1,018,224 |
| | — |
| | 1,018,677 |
| |
| Non-cash compensation | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| 280,202 |
| | — |
| | 280,202 |
| |
| Issuance of stock to former API stockholders | — |
| | — |
| | 11,872,557 |
| | 11,872 |
| | — |
| | — |
| 15,659,902 |
| | — |
| | 15,671,774 |
| |
| Options issued in exchange for API options | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| 187,879 |
| | — |
| | 187,879 |
| |
| Stock dividends (1) | — |
| | — |
| | — |
| | 79 |
| | — |
| | — |
| 85,751 |
| | (85,830 | ) | | — |
| |
| Forfeitures of restricted stock grants | — |
| | — |
| | (25,876 | ) | | — |
| | — |
| | — |
| — |
| | — |
| | — |
| |
| Purchase of treasury stock | — |
| | — |
| | (144,925 | ) | | — |
| | 144,925 |
| | (152,713 | ) | — |
| | — |
| | (152,713 | ) | |
| Net Income | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| — |
| | 2,317,026 |
| | 2,317,026 |
| |
| Balance—December 31, 2015 | 1,321,514 |
| | $ | 1,322 |
| | 27,477,181 |
| | $ | 28,178 |
| | 167,652 |
| | $ | (184,934 | ) | $ | 81,461,907 |
| | $ | (44,399,455 | ) | | $ | 36,907,018 |
| |
| Adjusted balance as of January 1, 2018 | | 1,321,514 |
| | $ | 1,322 |
| | 27,283,918 |
| | $ | 29,186 |
| | 1,070,904 |
| | $ | (1,649,746 | ) | $ | 83,563,208 |
| | $ | (32,052,161 | ) | | $ | 49,891,809 |
|
| Exercise of stock options and warrants | | — |
| | — |
| | 442,425 |
| | 441 |
| | — |
| | — |
| 1,096,592 |
| | — |
| | 1,097,033 |
|
| Stock-based compensation | — |
| | — |
| | 319,000 |
| | 319 |
| | — |
| | — |
| 859,896 |
| | — |
| | 860,215 |
| — |
| | — |
| | 282,394 |
| | 282 |
| | — |
| | — |
| 627,857 |
| | — |
| | 628,139 |
|
| Non-cash compensation | — |
| | — |
| | 24,271 |
| | 24 |
| | — |
| | — |
| 24,976 |
| | — |
| | 25,000 |
| — |
| | — |
| | 129,865 |
| | 131 |
| | — |
| | — |
| 199,871 |
| | — |
| | 200,002 |
|
| Stock dividends (1) | — |
| | — |
| | — |
| | 79 |
| | — |
| | — |
| 105,179 |
| | (105,258 | ) | | — |
| — |
| | — |
| | — |
| | 80 |
| | — |
| | — |
| 257,222 |
| | (257,302 | ) | | — |
|
| Purchase of treasury stock | — |
| | — |
| | (279,175 | ) | | — |
| | 279,175 |
| | (333,053 | ) | — |
| | — |
| | (333,053 | ) | — |
| | — |
| | (182,201 | ) | | — |
| | 182,201 |
| | (466,894 | ) | — |
| | — |
| | (466,894 | ) |
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| — |
| | (2,369,492 | ) | | (2,369,492 | ) | |
| Balance—December 31, 2016 | 1,321,514 |
| | $ | 1,322 |
| | 27,541,277 |
| | $ | 28,600 |
| | 446,827 |
| | $ | (517,987 | ) | $ | 82,451,958 |
| | $ | (46,874,205 | ) | | $ | 35,089,688 |
| |
| Net income | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| — |
| | 11,004,241 |
| | 11,004,241 |
|
| Balance—December 31, 2018 | | 1,321,514 |
| | $ | 1,322 |
|
| 27,956,401 |
|
| $ | 30,120 |
|
| 1,253,105 |
|
| $ | (2,116,640 | ) | $ | 85,744,750 |
|
| $ | (21,305,222 | ) |
| $ | 62,354,330 |
|
| Exercise of stock options and warrants | | — |
| | — |
| | 487,802 |
| | 488 |
| | — |
| | — |
| 447,466 |
| | — |
| | 447,954 |
|
| Stock-based compensation | | — |
| | — |
| | 16,286 |
| | 16 |
| | — |
| | — |
| 1,544,140 |
| | — |
| | 1,544,156 |
|
| Stock dividends (1) | | — |
| | — |
| | 770,454 |
| | 60 |
| | — |
| | — |
| 285,390 |
| | (285,450 | ) | | — |
|
| Preferred stock to common stock conversion | | (1,321,514 | ) | | (1,322 | ) | | 1,321,514 |
| | 1,322 |
| | — |
| | — |
| — |
| | — |
| | — |
|
| Forfeitures of restricted stock grants | | — |
| | — |
| | (16,666 | ) | | (157 | ) | | — |
| | — |
| 157 |
| | — |
| | — |
|
| Purchase of treasury stock | | — |
| | — |
| | (386,686 | ) | | — |
| | 386,686 |
| | (2,220,467 | ) | — |
| | — |
| | (2,220,467 | ) |
| Net income | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| — |
| | 5,342,545 |
| | 5,342,545 |
|
| Balance—December 31, 2019 | | — |
| | $ | — |
| | 30,149,105 |
| | $ | 31,849 |
| | 1,639,791 |
| | $ | (4,337,107 | ) | $ | 88,021,903 |
| | $ | (16,248,127 | ) | | $ | 67,468,518 |
|
| |
| (1) | The stock dividends payable in connection with the Series A Convertible Preferred Stock are issuable uponwere issued at the request of Carilion. See Note 11 - Stockholders' Equity for more information. |
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENTS OF CASH FLOWSLuna Innovations Incorporated
Consolidated Statements of Cash Flows
| | | | Years ended December 31, | Years ended December 31, |
| | 2016 | | 2015 | 2019 | | 2018 |
| Cash flows used in operating activities: | | | | |
| Net (loss)/income | $ | (2,369,492 | ) | | $ | 2,317,026 |
| |
| Adjustments to reconcile net income to net cash used in by operating activities: | | | | |
| Cash flows provided by/(used in) operating activities: | | | | |
| Net income | | $ | 5,342,545 |
| | $ | 11,004,241 |
|
| Adjustments to reconcile net income to net cash provided by/(used in) operating activities: | | | | |
| Depreciation and amortization | 3,713,879 |
| | 2,457,032 |
| 2,503,291 |
| | 1,218,559 |
|
| Stock-based compensation | 860,215 |
| | 1,124,379 |
| 1,544,156 |
| | 627,856 |
|
| Loss on disposal of fixed assets | | — |
| | (1,000 | ) |
| Gain on sale of discontinued operations, net of income taxes | — |
| | (8,326,386 | ) | — |
| | (8,595,798 | ) |
| Tax benefit from release of valuation allowance | | (3,349,032 | ) | | — |
|
| Bad debt | 305,593 |
| | 10,375 |
| 538,298 |
| | 6,000 |
|
| Tax benefit from utilization of loss from current year operations | — |
| | (510,772 | ) | |
| Changes in operating assets and liabilities: | | | | | | |
| Accounts receivable | (3,568,761 | ) | | (2,040,323 | ) | (2,248,925 | ) | | (6,240,377 | ) |
| Contract assets | | (448,891 | ) | | (761,714 | ) |
| Inventory | 492,932 |
| | (252,934 | ) | (722,690 | ) | | (967,797 | ) |
| Other assets | (238,736 | ) | | (131,411 | ) | (242,112 | ) | | 1,849,629 |
|
| Accounts payable and accrued liabilities | 564,689 |
| | 16,429 |
| |
| Deferred revenue | (160,156 | ) | | 248,678 |
| |
| Net cash used in operating activities | (399,837 | ) | | (5,087,907 | ) | |
| Other long-term assets | | 44,976 |
| | — |
|
| Accounts payable and accrued expenses | | 591,585 |
| | (461,927 | ) |
| Contract liabilities | | 1,245,000 |
| | (986,498 | ) |
| Net cash provided by/(used in) operating activities | | 4,798,201 |
| | (3,308,826 | ) |
| Cash flows (used in)/provided by investing activities: | | | | | | |
| Acquisition of property and equipment | (1,509,984 | ) | | (710,348 | ) | (540,635 | ) | | (386,890 | ) |
| Proceeds from sale of property and equipment | | — |
| | 1,000 |
|
| Intangible property costs | (490,200 | ) | | (367,050 | ) | (270,106 | ) | | (374,766 | ) |
| Cash acquired in business combination | — |
| | 374,517 |
| |
| Acquisition of Micron Optics, net | | — |
| | (5,001,750 | ) |
| Acquisition of General Photonics Corporation, net | | (19,004,250 | ) | | — |
|
| Proceeds from sale of discontinued operations, net | — |
| | 8,997,595 |
| — |
| | 15,799,529 |
|
| Net cash (used in)/provided by investing activities | (2,000,184 | ) | | 8,294,714 |
| (19,814,991 | ) | | 10,037,123 |
|
| Cash flows (used in)/provided by financing activities: | | | | |
| Cash flows used in financing activities: | | | | |
| Payments on debt obligations | (1,871,635 | ) | | (6,712,355 | ) | (625,000 | ) | | (1,833,333 | ) |
| Payments on capital lease obligations | (56,873 | ) | | (77,184 | ) | |
| Payments on finance lease obligations | | (40,047 | ) | | (46,653 | ) |
| Purchase of treasury stock | (333,053 | ) | | (152,713 | ) | (2,220,467 | ) | | (466,894 | ) |
| Borrowings under term loans | — |
| | 7,000,000 |
| |
| Proceeds from the exercise of options and warrants | — |
| | 82,516 |
| 447,954 |
| | 1,097,316 |
|
| Net cash (used in)/provided by financing activities | (2,261,561 | ) | | 140,264 |
| |
| Net cash used in financing activities | | (2,437,560 | ) | | (1,249,564 | ) |
| Net change in cash and cash equivalents | (4,661,582 | ) | | 3,347,071 |
| (17,454,350 | ) | | 5,478,733 |
|
| Cash and cash equivalents—beginning of period | 17,464,040 |
| | 14,116,969 |
| 42,460,267 |
| | 36,981,534 |
|
| Cash and cash equivalents—end of period | $ | 12,802,458 |
| | $ | 17,464,040 |
| $ | 25,005,917 |
| | $ | 42,460,267 |
|
| Supplemental disclosure of cash flow information | | | | | | |
| Cash paid for interest | $ | 308,116 |
| | $ | 187,017 |
| $ | 18,359 |
| | $ | 117,616 |
|
| Cash paid for income taxes | $ | 233,732 |
| | $ | 40,167 |
| $ | 1,160,276 |
| | $ | 1,828,203 |
|
| Cash received for income tax refunds | $ | 67,127 |
| | | |
| Value of common stock issued for business combination | $ | — |
| | $ | 15,485,187 |
| |
| Dividend on preferred stock, 79,292 shares of common stock issuable for each of the years ended December 31, 2016 and 2015 | $ | 105,258 |
| | $ | 85,830 |
| |
| Non-cash investing and financing activity: | |
|
| |
|
|
| Contingent liability for business combination | | $ | 900,000 |
| | $ | — |
|
| Common stock issued pursuant to restricted stock vesting | | $ | — |
| | $ | 200,202 |
|
| Dividend on preferred stock | | $ | 285,450 |
| | $ | 257,302 |
|
The accompanying notes are an integral part of these consolidated financial statements.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Summary of Significant Accounting Policies
Luna Innovations Incorporated (“we” or the "Company”), headquartered in Roanoke, Virginia, was incorporated in the Commonwealth of Virginia in 1990 and reincorporated in the State of Delaware in April 2003.
We are a leader in advanced optical technology, providing unique capabilities in high speed optoelectronics and high performance fiber optic test, measurement and control products for the telecommunications industryand photonics industries and distributed fiber optic sensing products for the aerospaceindustries utilizing composite and automotive industries. Our high-speed optical receiver ("HSOR") transmission products are deployed in the internet infrastructure to enable the high-speed bandwidth necessary to support video and data. Our distributed fiber optic sensing products provide critical stress, strain and temperature information to designers and manufacturers working withother advanced materials. Our custom optoelectronic products are sold to scientific instrumentation manufacturers for various applicationsmaterials, such as metrology, missile guidance, flame monitoring,the automotive, aerospace, energy and temperature sensing. In addition, we provide applied research services, typically under research programs funded by the U.S. government, in areas of advanced materials, sensing, and healthcare applications. Our business model is designed to accelerate the process of bringing new and innovative products to market. We use our in-house technical expertise to perform applied research services on government funded projects across a range of technologies and also for corporate customers in the fiber optic sensing area. We are organized into two business segments: our Technology Development segment and our Products and Licensing segment. Our Technology Development segment performs applied research principally on government-funded projects. Most of the government funding in our Technology Development segment is derived from the U.S. government’s Small Business Innovation Research ("SBIR") program coordinated by the U.S. Small Business Administration ("SBA"). Our Products and Licensing segment focuses on fiber optic test & measurement, sensing, and instrumentation products and also conducts applied research in the fiber optic sensing area to corporate and government customers.
We have a history of net losses and negative cash flow from operations. We have historically managed our liquidity through cost reduction initiatives, debt financings, capital markets transactions and the sale of assets.
Although there can be no guarantees, we believe that our current cash and cash equivalents balance, provides adequate liquidity for us to meet our working capital needs for the foreseeable future.infrastructure industries.
Consolidation Policy
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States ("GAAP") and include our accounts and the accounts of the Company and itsour wholly owned subsidiaries. We eliminate from our financial results all intercompany transactions.
Use of Estimates
The preparation of our consolidated financial statements in accordance with GAAP requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our consolidated financial statements and accompanying notes.
Although these estimates are based on our knowledge of current events and actions we may undertake in the future, actual results may differ from such estimates and assumptions.
Technology Development RevenuesRevenue Recognition
We perform researchProducts and development for U.S. government agencies, educational institutions and commercial organizations. We recognize revenues under research contracts when a contract has been executed, the contract price is fixed and determinable, delivery of services or products has occurred and collection of the contract price is considered reasonably assured. Revenue is earned under cost reimbursable, time and materials and fixed price contracts. Direct contract costs are expensed as incurred.
Under cost reimbursable contracts, we are reimbursed for costs that are determined to be reasonable, allowable and allocable to the contract and are paid a fixed fee representing the profit negotiated between us and the contracting agency. Revenue from cost reimbursable contracts is recognized as costs are incurred plus a portion of the fee earned. Revenue from time and materials contracts is recognized based on direct labor hours expended at contract billing rates plus other billable direct costs.
Revenue from fixed price research contracts that involve the delivery of services and a prototype model is recognized under the percentage of completion method. Revenue from fixed price arrangements are recognized under the percentage of
completion method by determining proportional performance based upon the ratio of costs incurred to achieve contract milestones to total estimated cost as this method more accurately measures performance under these arrangements. Losses on contracts, if any, are recognized in the period in which they become known and estimable.
Product SalesLicensing Revenues
Revenues from product sales are generated by the sale of commercial products and services under various sales programs to the end user and through distribution channels. We sell fiber optic test and sensing systems to end users for use in numerous fiber optic basedoptic-based measurement applications. Revenues are recorded net of applicable sales taxes collected from customers and payable to state or local governmental entities.
For Products and Licensing contracts, we evaluate whether two or more contracts should be combined and accounted for as one single contract and whether the combined or single contract should be accounted for as more than one performance obligation. We recognize revenue relating to our products when persuasive evidence of an arrangement exists, deliverythe performance obligation has occurred,been satisfied by transferring the selling price is fixed or determinable and collectabilitycontrol of the resulting receivable is reasonably assured.
product or service to the customer. For multi-element arrangements that include tangible products that contain software that is essential to the tangible product’s functionality, we consider the product and software to be a single performance obligation. For contracts with multiple performance obligations, we allocate revenuethe contract’s transaction price to all deliverableseach performance obligation based on their relative stand-alone selling prices. Other deliverables include extended warranty, training and various add-on products. In such circumstances, we use the observable price of goods or services which are sold separately in similar circumstances to similar customers. If these prices are not observable, then we will estimate the stand-alone selling price using information that is reasonably available. For the majority of our standard products and services, price list and discount structures related to customer type are available. For products and services that do not have price list and discount structures, we may use one or more of the following: (i) adjusted market assessment approach, (ii) expected cost-plus a hierarchymargin approach, and (iii) residual approach. The adjusted market approach requires us to evaluate the market in which we sell goods or services and estimate the price that a customer in that market would be willing to pay for those goods or services. The expected cost plus margin approach requires us to forecast our expected costs of satisfying the performance obligation and then add a reasonable margin for that good or service. The residual approach decreases the total transaction price by the sum of the observable standalone selling prices if either the company sells the same good or services to different customers for a broad range of amounts or the company has not established a price for the good or service and that good or service has not been sold on a standalone basis. Shipping and handling activities primarily occur after a customer obtains control and are considered fulfillment cost rather than separate performance obligations. Similarly, sales and similar taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction and collected by the entity from a customer are excluded from the measurement of the transaction price.
For standard products, we recognize revenue at a point in time when control passes to the customer. Absent substantial product acceptance clauses, this is based on the shipping terms. For custom products that require engineering and development based on customer requirements, we will recognize revenue over time using the output method for any items shipped and any
finished goods or work in process that is produced for balances of open sales orders. For any finished goods or work in process that has been produced for the balance of open sales orders we recognize revenue by applying the average selling price for such open order to the lesser of the on-hand balance in finished goods or open sales order quantity which we present as a contract asset on the balance sheet. Cost of sales is recognized based on the standard cost of the finished goods and work in process associated with this revenue and inventory balances are reduced accordingly. For extended warranties and product rentals, revenue is recognized over time using the output method based on the time elapsed for the warranty or service period. In the case of warranties, we record a contract liability for amounts billed but that are not recognized until subsequent periods. A separate contract liability is recorded for the cost associated with warranty repairs based on our estimate of future expense. For testing services where we are performing testing on an asset the customer controls, revenue is recognized over time by the output method using the performance to date. For training where the customer is receiving the benefit of training as it is occurring and for repairs to a customer-controlled asset, revenue is recognized over time by the output method using the performance to date. For royalty revenue, we apply the practical expedient “royalty exception” recognizing revenue based on the royalty agreement which specifies an amount based on sales or minimum amount, whichever is greater.
In some product rental contracts, a customer may be offered a discount on the purchase of an item that would provide for a material right. When a material right has been provided to a customer, a separate performance obligation is established, and a portion of the rental revenue will be deferred until the future product is purchased or the option expires. This deferred revenue is recognized as a contract liability on the balance sheet.
Technology Development Revenues
We perform research and development for U.S. Federal government agencies, educational institutions and commercial organizations. We account for a research contract when a contract has been executed, the rights of the parties are identified, payment terms are identified, the contract has commercial substance, and collectability of the contract price is considered probable. Revenue is earned under cost reimbursable, time and materials and fixed price contracts. Direct contract costs are expensed as incurred.
Our contracts with agencies of the U.S. government are subject to periodic funding by the respective contracting agency. Funding for a contract may be provided in full at inception of the contract or ratably throughout the contract as the services are provided. In evaluating the probability of funding for purposes of assessing collectability of the contract price, we consider our previous experience with our customers, communication with our customers regarding funding status and our knowledge of available funding for the contract or program. If funding is not assessed as probable, revenue recognition is deferred until realization is reasonably assured.
Under the typical payment terms of our U.S. government contracts, the customer pays us either performance-based payments ("PBPs") or progress payments. PBPs, which are typically used in the firm fixed price contracts, are interim payments based on quantifiable measures of performance or on the achievement of specified events or milestones. Progress payments, which are typically used in our cost type contracts, are interim payments based on costs incurred as the work progresses. For our U.S. government cost-type contracts, the customer generally pays us during the performance period for 80% to 90% of our actual costs incurred. Because the customer retains a small portion of the contract price until completion of the contract and audit of allowable costs, cost type contracts generally result in revenue recognized in excess of billings which we present as contract assets on the balance sheet. Amounts billed and due from our customers are classified as receivables on the balance sheet. For non-U.S. government contracts, we typically receive interim payments as work progresses, although for some contracts, we may be entitled to receive an advance payment. We recognize a liability for these advance payments and PBPs paid in advance which are in excess of the revenue recognized and present these amounts as contract liabilities on the balance sheet.
To determine the selling price toproper revenue recognition method for research and development contracts, we evaluate whether two or more contracts should be usedcombined and accounted for allocating revenue to deliverables: (i) vendor-specific objective evidenceas one single modified contract and whether the combined or single contract should be accounted for as more than one performance obligation. For instances where a contract has options that were bid with the initial contract and awarded at a later date, we combine the options with the original contract when options are awarded. For most of fair value ("VSOE"), (ii) third-party evidence of selling price ("TPE"), and (iii) best estimateour contracts, the customer contracts for research with multiple milestones that are interdependent. Consequently, the entire contract is accounted for as one performance obligation. The effect of the sellingcombined or modified contract on the transaction price ("ESP"). VSOE generally exists only whenand measure of progress for the performance obligation to which it relates, is recognized as an adjustment to revenue (either as an increase in or a reduction of revenue) on a cumulative catch-up basis.
Contract revenue recognition is measured over time as we sellperform because of continuous transfer of control to the deliverable separatelycustomer. For U.S. government contracts which are typically subject to the Federal Acquisition Regulation, this continuous
transfer of control to the customer is supported by clauses in the contract that allow the customer to unilaterally terminate the contract for convenience, pay us for cost incurred plus a reasonable profit and take control of any work in process. From time to time, as part of normal management processes, facts may change, causing revisions to estimated total costs or revenues expected. The cumulative impact of any revisions to estimates and the full impact of anticipated losses on any type of contract are recognized in the period in which they become known.
Because of control transferring over time, revenue is recognized based on the extent of progress towards completion of the performance obligation. The selection of the method to measure progress towards completion requires judgment and is the price actually charged by us for that deliverable. Due to the uniqueness of our products comparable third party evidence is generally not available. ESPs reflect our best estimates of what the selling prices of elements would be if they were sold regularlybased on a stand-alone basis.
Our process for determining our ESP for deliverables without VSOE or TPE considers multiple factors that may vary depending upon the unique facts and circumstances related to each deliverable. Key factors considered in developing the ESPs include prices charged by us for similar offerings, our historical pricing practices, the nature of the deliverables,services to be provided. We generally use the input method, more specifically the cost-to-cost measure of progress for our contracts because it best depicts the transfer of control to the customer, which occurs as we incur costs on our contracts. Under the cost-to-cost measure of progress, the extent of progress towards completion is measured based on the ratio of costs incurred to date to the total estimated costs at completion of the performance obligation. The underlying bases for estimating our contract research revenues are measurable expenses, such as labor, subcontractor costs and materials, and data that are updated on a regular basis for purposes of preparing our cost estimates. Our research contracts generally have a period of performance of six months to three years, and our estimates of contract costs have historically been consistent with actual results. Revisions in these estimates between accounting periods to reflect changing facts and circumstances have not had a material impact on our operating results, and we do not expect future changes in these estimates to be material. The cumulative impact of any revisions to estimates and the relative ESPfull impact of allanticipated losses on any type of contract are recognized in the period in which they become known.
Under cost reimbursable contracts, we are reimbursed for costs that are determined to be reasonable, allowable and allocable to the contract and paid a fixed fee representing the profit negotiated between us and the contracting agency. Revenue from cost reimbursable contracts is recognized as costs are incurred plus an estimate of applicable fees earned. We consider fixed fees under cost reimbursable contracts to be earned in proportion to the allowable costs incurred in performance of the contract.
Revenue from time and materials contracts is recognized based on direct labor hours expended at contract billing rates plus other billable direct costs.
Fixed price contracts may include either a product delivery or specific service performance throughout a period. For fixed price contracts that are based on the proportional performance method and involve a specified number of deliverables, we recognize revenue based on the proportion of the cost of the deliverables as compared to the total sellingcost of all deliverables included in the contract as this method more accurately measures performance under these arrangements. For fixed price contracts that provide for the development and delivery of a specific prototype or product, revenue is recognized based upon the percentage of completion method.
Whether certain costs under government contracts are allowable is subject to audit by the government. Certain indirect costs are charged to contracts using provisional or estimated indirect rates, which are subject to later revision based on government audits of those costs. Management is of the product. We may also consider, when appropriate, theopinion that costs subsequently disallowed, if any, would not likely have a significant impact of other products and services on selling price assumptions when developing and reviewing our ESPs.
Revenues from product sales that require no ongoing obligations arerevenues recognized as revenues when shipped to the customer, title has passed and collection is reasonably assured.for those contracts.
Allowance for Uncollectible Receivables
Accounts receivable are recorded at their face amount, less an allowance for doubtful accounts. We review the status of our uncollected receivables on a regular basis. In determining the need for an allowance for uncollectible receivables, we consider our customers’ financial stability, past payment history and other factors that bearbare on the ultimate collection of such amounts. The allowance was $252,639$0.9 million and $0.3 million at each of December 31, 20162019 and $134,811 at December 31, 2015.2018, respectively.
Cash Equivalents
We consider all highly liquid investments with maturities of three months or less when purchased to be cash equivalents. To date, we have not incurred losses related to cash and cash equivalents. Cash equivalents at December 31, 2019 and 2018 included $19.8 million and $38.3 million, respectively, invested in U.S. Treasury obligations through a sweep account with our bank. The full value of amounts invested through the sweep account are convertible to cash on a daily basis. Our cash transactions are processed through reputable commercial banks. We regularly maintain cash balances with financial institutions which exceed Federal Deposit Insurance Corporation (“FDIC”) insurance limits. At December 31, 20162019 and December 31, 2015,2018, we had approximately $12.6$5.0 million and $17.2$4.0 million, respectively, in excess of FDIC insured limits.
We have outstanding term loans that require us to comply with certain financial covenants, including maintaining a minimum cash balance of at least $5.0 million.
Fair Value Measurements
Our financial assets and liabilities are measured at fair value, which is defined as the price that would be received to sell an asset, or paid to transfer a liability, in an orderly transaction between market participants. Valuation techniques are based on observable or unobservable inputs. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect our market assumptions. These two types of inputs have created the following fair value hierarchy:
Level 1—Quoted prices for identical instruments in active markets.
Level 2—Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which significant value drivers are observable.
Level 3—Valuations derived from valuation techniques in which significant value drivers are unobservable.
The carrying values of cash and cash equivalents, accounts receivable, and accounts payable and accrued liabilities approximate fair value because of the short-term nature of these instruments. The carrying amount of lease liabilities approximate fair value because these financial instruments bear interest at rates that approximate current market rates for similar agreements with similar maturities and credit. We consider the terms of the Silicon Valley Bank ("SVB") debt facility including its interest rate of prime plus 2%1%, to be at market based upon similar instruments that would be available to us.
Property and Equipment, net
Property and equipment are stated at cost less accumulated depreciation. We record depreciation using the straight-line method over the following estimated useful lives:
|
| |
| Equipment | 3 – 7 years |
| Furniture and fixtures | 7 years |
| Software | 3 years |
| Leasehold improvements | Lesser of lease term or life of improvements |
Intangible Assets
Intangible assets consist of patents related to certain intellectual property that we have developed or acquired, and identifiable intangible assets recognized in connection with our merger with Advanced Photonix, Inc. ("API"), Micron Optics, Inc. ("MOI"), and General Photonics, Inc. ("GP"). (See Note 2) We amortize our patentsidentified intangible assets over their estimated useful life of fivelives ranging between one and fifteen years and analyze the reasonableness of the remaining useful life whenever events or circumstances indicate that the carrying amount may not be recoverable to determine whether their carrying value has been impaired.
Goodwill
Goodwill is tested annually for impairment in the fourth quarter (October 1st) and whenever events or changes in circumstances indicate the carrying value of goodwill may not be recoverable. Goodwill is tested for impairment at the reporting unit level. A qualitative assessment can be performed to determine whether it is more likely than not the fair value of the reporting unit is less than its carrying value. If the reporting unit does not pass the qualitative assessment, we compare the fair value of each reporting unit to its carrying value using a quantitative assessment. If the fair value of the reporting unit exceeds its carrying value, goodwill is considered not impaired. If the fair value of the reporting unit is less than the carrying value, the difference is recorded as an impairment loss.
For the quantitative assessment, we estimate the fair value of each reporting unit using a combination of an income approach using a discounted cash flow ("DCF") analysis and a market-based valuation approach based on comparable public company trading values. Determining the fair value of a reporting unit requires the exercise of significant management judgments, including the amount and timing of projected future revenues, earnings and cash flows after considering factors such as recent operating performance, general market and industry conditions, existing and expected future contracts, changes in working capital and long-term business plans and growth initiatives. The carrying value of each reporting unit includes the assets and liabilities employed in its operations and goodwill. There are no significant allocations of amounts held at the corporate level to the reporting units.
Based on our annual goodwill impairment analysis we performed in the fourth quarter of 2019, the fair value of our reporting units more likely than not exceed the carrying values.
Research, Development and Engineering
Research, development and engineering expensesexpense not related to contract performance are expensed as incurred. We expensed $5.5$7.5 million and $4.3$3.8 million of non-contract related research, development and engineering expensesexpense for the yearsyear ended December 31, 20162019 and 2015,2018, respectively.
ValuationImpairment of Long-Lived Assets
We review our long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets is measured by comparing the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds their fair value. Assets to be disposed of by sale are reflected at the lower of their carrying amount or fair value less cost to sell.
Inventory
Inventory consists of finished goods, work in process and raw materials valued at the lower of cost (determined on the first-in, first-out basis) or market.net realizable value.
Net (Loss)/Income per Share
Basic per share data is computed by dividing net (loss)/income attributable to common stockholders by the weighted average number of shares outstanding during the period. Diluted per share data is computed by dividing net (loss)/income attributable to common stockholders by the weighted average shares outstanding during the period increased to include, if dilutive, the number of additional common share equivalents that would have been outstanding if potential common shares had been issued using the treasury stock method. Diluted per share data would also include the potential common share equivalents relating to convertible securities by application of the if-converted method.
The effect of 5.53.2 million and 6.04.9 million common stock equivalents (which include outstanding warrants, preferred stock and stock options) are not included for the yearsdiluted per share data for the year ended December 31, 20162019 and 2015, respectively, as they2018, respectively. Accrued stock dividends and stock options are anti-dilutive to earnings per share due to us having a net loss from continuing operations.included in our common stock equivalents for the year ended December 31, 2019, while preferred stock is also included for the year ended December 31, 2018.
Stock-Based Compensation
We have two stock-based compensation plans, which are described further in Note 10.11. We recognize compensation expense based upon the fair value of the underlying equity award as of the date of grant. We have elected to use the Black-Scholes option pricing model to value any awardsstock options granted. Restricted stock and restricted stock units awarded are valued at the closing price of our common stock on the date of the award. We recognize stock-based compensation for such awards on a
straight-line method over the requisite service period of the awards taking into account the effects of the employees’ expected exercise and post-vesting employment termination behavior.
exercise. We recognizereduce stock-based compensation expense for equity instruments issued to non-employees based upon the fair value of the equity instruments issued.any forfeitures of unvested awards as such forfeitures occur.
Advertising
We expense the cost of advertising as incurred. Advertising expenses were $111,181 and $57,000$0.1 million for each of the years ended December 31, 20162019 and 2015, respectively.2018.
Income Taxes
We account for income taxes using the liability method. Deferred tax assets or liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities as measured by the enacted tax rates, which will be in effect when the differences reverse. A valuation allowance against net deferred tax assets is provided unless we conclude it is more likely than not that the deferred tax assets will be realized.
We recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates in effect for the year in which those temporary differences are expected to be recovered or settled. We evaluate our ability to benefit from all deferred tax assets and establish valuation allowances for amounts we believe are not more-likely-than-not to be realizable. For uncertain tax positions, we use a more-likely-than-not threshold, 51% or greater, based on the technical merits of the income tax position taken. Income tax positions that meet the more-likely-than-not recognition threshold are measured in order to determine the tax benefit recognized in the financial statements. Penalties, if probable and reasonably estimable, and interest expense related to uncertain tax positions are recognized as a component of the tax provision.
We account for income taxes using the liability method. Deferred tax assets or liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities as measured by the enacted tax rates which will be in effect when the differences reverse. A valuation allowance against net deferred tax assets is provided unless we conclude it is more likely than not that the deferred tax assets will be realized.
RecentRecently Issued Accounting Pronouncements
In January 2017,February 2016, the Financial Accounting Standards Board ("FASB") issued a new standard related to Leases, Accounting Standards Update ("ASU") No. 2017-04,2016-02, Intangibles—Goodwill Leases (Topic 842) and Othersubsequent amendments, based on previously defined GAAP, and requires lessees to recognize right-of-use ("ROU") assets and lease liabilities on the balance sheet for those leases classified as operating leases for greater transparency. We, using a modified retrospective adoption approach, are required to recognize and measure leases existing at the beginning of the adoption period, with certain practical expedients available.
We adopted the standard effective January 1, 2019. The standard allows a number of optional practical expedients to use for transition. We chose the certain practical expedients allowed under the transition guidance which permitted us to not to reassess any existing or expired contracts to determine if they contain embedded leases, to not reassess our lease classification on existing leases, to account for lease and non-lease components as a single lease component for equipment leases, and whether initial direct costs previously capitalized would qualify for capitalization under FASB ASC 842. The new standard also provides practical expedients and recognition exemptions for an entity's ongoing accounting policy elections. We have elected the short-term lease recognition for all leases that qualify, which means that we do not recognize a ROU asset and lease liability for any lease with a term of twelve months or less.
The most significant impact of adopting the standard was the recognition of ROU assets and lease liabilities for operating leases on our consolidated balance sheet but it did not have an impact on our consolidated statements of operations or consolidated statements of cash flows. Upon the adoption of the new lease standard on January 1, 2019, we recorded the following adjustments:
|
| | | | | | | | | | | |
| | Balance at | | Adjustment for | | Adjusted balance at |
| | December 31, 2018 | | ASC 842 | | January 1, 2019 |
| Assets: | | | | | |
| Property and equipment, net | $ | 3,627,886 |
| | $ | (90,494 | ) | | $ | 3,537,392 |
|
| Other assets, net | 1,995 |
| | 3,536,133 |
| | 3,538,128 |
|
| | | | | | |
| Liabilities: | | | | | |
| Accrued liabilities | 6,597,458 |
| | 1,242,669 |
| | 7,840,127 |
|
| Current portion of capital lease obligations | 40,586 |
| | (40,586 | ) | | — |
|
| Long-term deferred rent | 1,035,974 |
| | (1,035,974 | ) | | — |
|
| Long-term operating lease liability | — |
| | 3,271,705 |
| | 3,271,705 |
|
| Long-term capital lease obligations | 68,978 |
| | (68,978 | ) | | — |
|
| Long-term finance lease liability | — |
| | 76,803 |
| | 76,803 |
|
Recently Issued Pronouncements not yet adopted
In June 2016, the FASB issued ASU 2016-13: Financial Instruments - Credit Losses (Topic 350): 326) - Measurement of Credit Losses on Financial Instruments. The ASU requires companies to measure credit losses by using a methodology that reflects the expected credit losses based on historical information current economic conditions, and reasonable and supportable information. This standard was amended under ASU 2019-10 to allow an extension on the adoption date for entities that qualify as a small reporting company. We have elected this extension and the effective date for us to adopt this standard will be for fiscal years beginning after December 15, 2022. We do not expect the adoption of ASU 2016-13 to have a significant impact on our consolidated financial statements.
In January 2017, the FASB issued ASU 2017-04: Simplifying the Test for Goodwill Impairment. ImpairmentThis update,” which simplifies the subsequent measurement of goodwill.test for goodwill impairment. The guidance removes Step 2is effective for us beginning in the first quarter of fiscal year 2020. Early adoption is permitted for interim or annual goodwill impairments tests after January 1, 2017. We have assessed the goodwill impairment test, impact of adopting ASU 2017-04 and the adoption on January 1, 2020 should not have a significant impact on our consolidated financial statements.
In August 2018, the FASB issued ASU No. 2018-13 Fair Value Measurement (Topic 820): Changes to the Disclosure Requirements for Fair Value Measurement, which requires a hypothetical purchase price allocation. A goodwill impairment will now beamends the amountdisclosure requirements in ASC 820 by which a reporting unit's carrying value exceeds itsadding, changing, or
removing certain disclosures. The ASU applies to all entities that are required under this guidance to provide disclosures about recurring or nonrecurring fair value not to exceed the carrying amount of goodwill. The accounting standard will bemeasurements. These amendments are effective for reporting periodsus beginning after December 15, 2019.in the first quarter of fiscal year 2020. Early adoption is permitted. We dohave assessed the impact of adopting ASU 2018-13 and the adoption on January 1, 2020 should not expect ASU 2017-04 will have a materialsignificant impact on our consolidated financial statements.
In August 2018, the FASB issued ASU 2018-15 Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That is a Service Contract, which aligns the requirements for capitalizing implementation costs incurred in a hosting arrangement that is a service contract with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. The guidance is effective for us beginning in the first quarter of fiscal year 2020. Early adoption is permitted. We have assessed the impact of adopting ASU 2018-15 and the adoption on January 1, 2020 should not have a significant impact on our consolidated financial statements.
In December 2016,2019, the FASB issued ASU No. 2016-20,2019-12 Technical correctionsSimplifying the Accounting for Income Taxes, which removes certain exceptions to the general principles of the accounting for income taxes and improvements to Topic 606, Revenue from Contracts with Customers. This update provides additional clarificationalso improves consistent application of and implementationsimplification of other areas when accounting for income taxes. The guidance on the previously issued ASU 2014-09, Revenue from Contracts with Customers (Topic 606). ASU No. 2014-09 supersedes nearly all existing revenue recognition guidance under GAAP. The core principle of ASU 2014-09 is to recognize revenues when promised goods or services are transferred to customers in an amount that reflects the consideration to which an entity expects to be entitled for those goods or services. ASU 2014-09 defines a five step process to achieve this core principle and, in doing so, more judgment and estimates may be required within the revenue recognition process than are required under existing GAAP. The standard, as subsequently updated in July 2015, is effective for annual periodsus beginning after December 15, 2017, and interim periods therein, using either of the following transition methods: (i) a full retrospective approach reflecting the application of the standard in each prior reporting period with the option to elect certain practical expedients, or (ii) a retrospective approach with the cumulative effect of initially adopting ASU 2014-09 recognized at the date of adoption (which includes additional footnote disclosures). We are currently identifying our various revenue streams which may be subject to new recognition criteria under ASU 2014-09 and assessing the potential impacts on our financial statements. We plan to utilize a modified retrospective approach with the cumulative effect of initial adoption recognized at the date of adoption.
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230), which addresses eight specific cash flow issues with the objective of reducing the existing diversity in practice in how cash receipts and cash payments are presented in the statementfirst quarter of cash flows. ASU 2016-15fiscal year 2021. Early adoption is effective for public entities for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years, with early adoption permitted. The amendments should
be applied retrospectively to all periods presented. We do not expect ASU 2016-15 will have a material impact on our financial statements.
In March 2016, the FASB issued ASU No. 2016-09, Improvements to Employee Share-Based Payment Accounting. The amendments apply to several aspects of accounting for share-based compensation including the recognition of excess tax benefits and deficiencies and their related presentation in the statement of cash flows as well as accounting for forfeitures. ASU No. 2016-09 is effective for fiscal years beginning after December 15, 2016, including interim periods and allows for prospective, retrospective or modified retrospective adoption, depending on the area covered in the update, with early adoption permitted. We adopted ASU 2016-09 on January 1, 2017, and we do not expect the adoption toof ASU 2019-12 will have a materialsignificant impact on our financial statements.
In February 2016, the FASB issued ASU No. 2016-02, Leases, which requires a lessee to recognize in its statement of financial position an asset and liability for most leases with a term greater than 12 months. Lessees should recognize a liability to make lease payments and a right-of-use asset representing the lessee's right to use the underlying asset for the lease term. The amendment is effective for fiscal years ending after December 15, 2018, including interim periods within those fiscal years. We are currently evaluating the impact the adoption of this standard will have on our consolidated financial statements.
In September 2015, the FASB issued ASU No. 2015-16, Simplifying the Accounting for Measurement Period Adjustments, which requires that an acquirer recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined. The amendment is effective for fiscal years beginning after December 15, 2015 and requires acquirer to record, in the same period’s financial statements, the effect on earnings of changes in depreciation, amortization, or other income effects, if any, as a result of the change to the provisional amounts, calculated as if the accounting had been completed at the acquisition date. Additionally, an entity is to present separately on the face of the income statement or disclose in the notes the portion of the amount recorded in current-period earnings by line item that would have been recorded in previous reporting periods if the adjustment to the provisional amounts had been recognized as of the acquisition date. We adopted ASU 2015-16 on January 1, 2016.
2. Merger with Advanced Photonix,Business Acquisitions
Micron Optics, Inc.
On May 8, 2015,October 15, 2018, we completed our mergeracquired substantially all of the assets, other than cash, of the United States operations of Micron Optics, Inc. ("MOI") for cash consideration of $5.5 million. For the year ended December 31, 2019, we recognized revenue of $10.8 million and operating income of $1.8 million associated with API (the "Merger"the acquired operations of MOI. For the period from the closing of the MOI acquisition through December 31, 2018, we recognized revenue of $2.6 million and operating income of $1.1 million.
General Photonics Corporation
On March 1, 2019, we acquired the outstanding stock of General Photonics Corporation ("GP") pursuantfor cash consideration of $19.0 million. Of the purchase price, $17.1 million was paid at closing and $1.9 million was placed into escrow for possible working capital adjustments to the Agreementpurchase price and Planpotential satisfaction of Merger (the "Merger Agreement") for a total purchasecertain post-closing indemnification obligations. Additionally, we may become obligated to pay additional cash consideration of $15.9up to $1.0 million if certain revenue targets for the GP historical business are met for the twelve-month period following the closing. We currently estimate the fair value of the contingent obligation to be $1.0 million, which is shown in accrued liabilities on the consolidated balance sheet. The fair value of the contingent obligation was determined using the present value of estimated likely future payments.
For the period from the closing of the GP acquisition through December 31, 2019, we recognized revenue of $10.5 million and operating income of $1.4 million. In accordanceOperating income for the period from the closing of the acquisition through December 31, 2019 included $1.6 million in amortization expense for the acquired intangibles and step-up in value of acquired inventory associated with the termsacquisition of GP. Operating income for the Merger Agreement, API shareholders received 0.31782 sharesyear ended December 31, 2019 also included $0.9 million of costs associated with the acquisition of GP. The amortization expense for the acquired intangibles as well as the costs associated with the acquisition of GP are included in the cost of goods sold and selling, general and administrative expense in our common stock for each shareconsolidated statements of API common stock they owned. The Merger hasoperations.
These acquisitions have been accounted for under the acquisition method of accounting in accordance with ASC 805 with Luna treated as the accounting acquirer. We incurred approximately $3.7 million in Merger-related costs for the year ended December 31, 2015, which are included within selling, general and administrative expenses in the consolidated statement of operations. For the period from the closing of the Merger on May 8, 2015, through December 31, 2015, we recognized revenues of $20.6 million and income of $0.5 million associated with the operations of API.
The total purchase consideration of $15.9 million consisted of the following:
|
| | | |
| | Purchase Consideration |
| Fair value of Luna common stock Issued to API shareholders | $ | 15,671,775 |
|
| Fair value of vested API options assumed by Luna | 187,879 |
|
| Total purchase consideration | $ | 15,859,654 |
|
- Business Combinations. Under the acquisition method of accounting,ASC 805, the total estimated purchase consideration is allocated to the acquired tangible and intangible assets and assumed liabilities based on their estimated fair values as of the acquisition date. Any excess of the fair value of the acquisition consideration over the identifiable assets acquired and liabilities assumed over the fair value of the acquisition consideration is recognized as a gain by the acquirer.goodwill. We have completed the followingour allocation of the purchase consideration withfor the assistanceMOI and GP acquisitions.
The following table summarizes the allocation of a third-party valuation expert.
the purchase consideration of each acquisition:
| | | | Allocation of Purchase Consideration | | MOI | | GP |
| Cash | $ | 374,517 |
| |
| Accounts receivable | 3,314,994 |
| | $ | 1,742,693 |
| | $ | 1,520,950 |
|
| Inventory | 5,246,000 |
| | 1,435,606 |
| | 2,698,000 |
|
| Other current assets | 541,726 |
| | 69,951 |
| | 763,873 |
|
| Property and equipment | 3,601,850 |
| | 996,460 |
| | 286,000 |
|
| Identifiable intangible assets | 11,100,000 |
| | 1,650,000 |
| | 8,200,000 |
|
| Goodwill | 2,348,331 |
| | 29,760 |
| | 10,511,916 |
|
| Other assets | 86,953 |
| |
| Accounts payable and accrued expenses | (5,508,789 | ) | | (379,737 | ) | | (4,076,489 | ) |
| Debt | (5,212,355 | ) | |
| Other liabilities | (33,573 | ) | |
| Total purchase consideration | $ | 15,859,654 |
| | $ | 5,544,733 |
|
| $ | 19,904,250 |
|
| | | |
The preliminary identifiable intangible assets acquired and their estimated useful lives were as follows:
| | | | | | | | | Estimated | | Estimated Fair Value |
| Estimated Fair Value |
| Estimated Useful Life | | Useful Life | | MOI | | GP |
| Developed technology | $ | 4,500,000 |
|
| 2 - 10 years | | 5 - 8 years | | $ | 1,200,000 |
| | $ | 7,200,000 |
|
| In-process research and development | 3,900,000 |
|
| Indefinite | | 7 years | | 200,000 |
| | — |
|
| Trade names and trademarks | | | 3 years | | 150,000 |
| | 400,000 |
|
| Customer base | 1,300,000 |
|
| 9 - 11 years | | 7 - 15 years | | 100,000 |
| | 600,000 |
|
| Trade names | 1,000,000 |
|
| 10 years | |
| Backlog | 400,000 |
|
| 1 year | |
| $ | 11,100,000 |
|
| | $ | 1,650,000 |
| | $ | 8,200,000 |
|
Developed technologies acquired primarily consist of API's existingMOI's technologies related to HSOR products, optoelectronic systems,fiber optic sensing instruments, modules, and components and Terahertz solutions.GP's technologies relating to the measurement and control of the polarization of light. The developed technologies of API were valued using both the "relief-from-royalty" method and the "multi-period excess earnings" method, under the income approach. ThisThe multi-period excess earnings method reflects the present value of the projected cash flows that are expected to be generated by the developed technologies less charges representing the contribution of other assets to those cash flows. A discount rateDiscount rates of 32.5% was24.5% and 17% were used to discount thethese cash flows of MOI and GP, respectively, to the present value.
In-process research and development represents the estimated fair valuesvalue of an incomplete APIMOI research and development projectsproject that had not reached technological feasibility as of the closing date of the Merger. Asacquisition. In the fourth quarter of December 31, 2016, all2019, the fair value of this project at the closing date of the technologies associated with $3.9 million of in-process research and development were placed into service.acquisition will begin being amortized following the project's completion. The fair value of in-processin process research and development was determined using the multi-period excess earnings method. A discount rate of 37.5%29.5% was used to discount thethese cash flows to the present value.
Customer base represents the fair value of projected cash flows that will be derived from the sale of products to API's existing customers of MOI and GP as of the respective closing datedates of the Merger.their acquisitions. Customer relationships were valued utilizing both a multi-period excess earnings method andusing the "distributor" method, under the income approach. Under this premise, the margin of a distributor within the industry is deemed to be the margin attributable to customer relationships. This isolates the cash flows attributable to the customer relationships thatfor which a market participant would be willing to pay for. A discount ratepay. Discount rates of 32.5% was24.5% and 16% were used to discount thethese cash flows of MOI and GP, respectively, to the present value.
Trade names and trademarks are considered a type of guarantee of a certain level of quality or performance represented by the API brand.MOI and GP brands. Trade names and trademarks were valued using the "relief-from-royalty""relief from royalty" method of the income approach. This
method is based on the assumption that in lieu of ownership, a market participant would be willing to pay a royalty in order to exploit the related benefits of this asset. A discount rateDiscount rates of 24.5% was17% and 16% were used to discount thethese cash flows of MOI and GP, respectively, to the present value.
Backlog represents the fair value of projected cash flows that will be derived from the sale of products under existing contracts and customer orders as of the closing date of the Merger. The fair value of the customer backlog was determined using the multi-period excess earnings method. A discount rate of 21.5% was used to discount the cash flows to the present value.
Goodwill
Goodwill represents the excess of consideration transferred over the net of the acquisition date fair values of the assets acquired and the liabilities assumed in connection with the Merger. During 2015, we recognized various measurement period adjustmentsacquisition. Goodwill generated from our business acquisitions was primarily attributable to the value of assets acquired and liabilities assumed in the Merger. These adjustments primarily related to the estimated value of trade names acquired and certain deferred compensation liabilities assumed, with an offsetting increase to the recorded value of goodwill. During 2016, we recognized measurement period adjustments to the value of assets acquired and liabilities assumed in the Merger. These adjustments primarily related to the estimated value of accrued liabilities assumed, with an offsetting increase to the recorded value of goodwill. We performed a qualitative analysis of the goodwill balance as of October 1, 2016, and determined no impairment of goodwill is necessary.
|
| | | |
| Goodwill as of January 1, 2015 | $ | — |
|
| Goodwill recorded at acquisition date of API | 614,184 |
|
| Measurement period adjustments | 1,659,928 |
|
| Goodwill as of December 31, 2015 | 2,274,112 |
|
| Measurement period adjustments | 74,219 |
|
| Goodwill as of December 31, 2016 | $ | 2,348,331 |
|
expected synergies from future growth.
Pro Forma Consolidated Resultsforma consolidated results of Operationsoperations
The following unaudited pro forma financial information presents combined results of operations for each of the periods presented as if the Mergeracquisitions of MOI and GP had been completed on January 1, 2015.2018. The pro forma information includes adjustments to depreciation expense for property and equipment acquired toand amortization expense for the intangible assets acquired to interest expense forand the new debt facility, and to eliminate the Mergerelimination of transaction expenses recognized in each period. Transaction relatedTransaction-related expenses associated with the mergeracquisition and excluded from the pro forma lossincome from continuing operations were $1.0 million for the year ended December 31, 20152019. There were $4.7 million.no transaction-related expenses associated with the acquisition for the year ended December 31, 2018. The pro forma data are for informational purposes only and are not necessarily indicative of the consolidated results of operations ofor the combined business had the Merger actuallyacquisitions of MOI and GP occurred on January 1, 2015,2018, or the results of future operations of the combined business. For instance, planned or expected operational synergies following the Mergeracquisition are not reflected in the pro forma information. Consequently, actual results will differ from the unaudited pro forma information presented below.
| | | | | Years ended December 31, | | | | | | | | |
| | | 2016 | | 2015 | | Years Ended December 31, |
| | | (unaudited) | | 2019 | | 2018 |
| | | | | | | (unaudited) | | (unaudited) |
| Revenue | | $ | 59,210,996 |
| | $ | 52,887,000 |
| | $ | 72,576,902 |
| | $ | 60,249,896 |
|
| | | | | | | | | |
| Loss from continuing operations | | $ | (2,369,492 | ) | | $ | (4,641,000 | ) | |
| Income from continuing operations | | | $ | 6,912,802 |
| | $ | 1,559,008 |
|
3. Accounts Receivable, net
Accounts receivable, net consist of the following:
|
| | | | | | | |
| | December 31, |
| | 2019 | | 2018 |
| Billed | $ | 17,193,742 |
| | $ | 13,289,790 |
|
| Other | 5,182 |
| | 31,361 |
|
| | 17,198,924 |
| | 13,321,151 |
|
| Less: allowance for doubtful accounts | (930,279 | ) | | (284,083 | ) |
| Accounts receivable, net | $ | 16,268,645 |
| | $ | 13,037,068 |
|
4. Property and Equipment, net
Property and equipment, net, consists of the following:
|
| | | | | | | |
| | December 31, |
| | 2019 | | 2018 |
| Building | $ | 69,556 |
| | $ | 69,556 |
|
| Equipment | 9,564,426 |
| | 9,341,007 |
|
| Furniture and fixtures | 684,812 |
| | 640,890 |
|
| Software | 1,178,210 |
| | 1,122,231 |
|
| Leasehold improvements | 5,287,935 |
| | 4,950,510 |
|
| | 16,784,939 |
| | 16,124,194 |
|
| Less—accumulated depreciation | (13,319,327 | ) | | (12,496,308 | ) |
| Property and equipment, net | $ | 3,465,612 |
| | $ | 3,627,886 |
|
Depreciation for the years ended December 31, 2019 and 2018 was approximately $1.0 million and $0.5 million, respectively, and is included primarily in selling, general and administrative expense in our consolidated statements of operations.
5. Intangible Assets, net
Intangible assets, net consist of the following:
|
| | | | | | | | | |
| | | | December 31, |
| | Estimated Life | | 2019 | | 2018 |
| Patent costs | 1 - 18 years | | $ | 5,291,245 |
| | $ | 4,991,460 |
|
| Developed technology | 5 years | | 9,800,000 |
| | 2,600,000 |
|
| In-process research and development | N/A | | 1,580,000 |
| | 200,000 |
|
| Customer base | 7 years | | 700,000 |
| | 100,000 |
|
| Trade names | 3 years | | 550,000 |
| | 150,000 |
|
| | | | 17,921,245 |
| | 8,041,460 |
|
| Accumulated amortization | | | (7,726,768 | ) | | (4,739,190 | ) |
| Intangible assets, net | | | $ | 10,194,477 |
| | $ | 3,302,270 |
|
Amortization for the years ended December 31, 2019 and 2018 was approximately $1.6 million and $0.4 million, respectively, and is included primarily in selling, general and administrative expense in our consolidated statements of operations.
Estimated aggregate amortization, based on the net value of intangible assets at December 31, 2019, for each of the next five years and beyond is as follows:
|
| | | |
| Year Ending December 31, | |
| 2020 | $ | 1,640,851 |
|
| 2021 | 1,660,112 |
|
| 2022 | 1,509,418 |
|
| 2023 | 1,437,195 |
|
| 2024 | 1,247,195 |
|
| 2025 and beyond | 2,699,706 |
|
| $ | 10,194,477 |
|
We did not recognize any intangible asset impairment charges during the years ended December 31, 2019 or 2018.
3.6. Inventory
Inventory consists of finished goods, work-in-process and raw materials valued at the lower of cost (determined on the first-in, first-out basis) or market.net realizable value.
Components of inventory are as follows:
| | | | December 31, | December 31, |
| | 2016 | | 2015 | 2019 | | 2018 |
| Finished goods | $ | 1,993,543 |
| | $ | 1,938,466 |
| $ | 1,695,461 |
| | $ | 1,339,832 |
|
| Work-in-process | 1,098,173 |
| | 1,227,270 |
| 1,007,719 |
| | 643,420 |
|
| Raw materials | 5,278,519 |
| | 5,697,431 |
| 7,591,251 |
| | 4,890,490 |
|
| | $ | 8,370,235 |
| | $ | 8,863,167 |
| |
| Inventory, net | | $ | 10,294,431 |
| | $ | 6,873,742 |
|
4. Debt
Silicon Valley Bank Facility
We currently have a Loan and Security Agreement with SVB under which we have a term loan with an original borrowing amount of $6.0 million (the “Term Loan”). The Term Loan was to be repaid by us in 48 monthly installments, plus accrued interest payable monthly in arrears and matured on May 1, 2015. On May 8, 2015, in connection with the Merger with API, we entered into the Joinder, Consent and Sixth Loan Modification Agreement (the "2015 Term Loan") under which we borrowed $6.0 million and used the proceeds principally to repay the then outstanding debt of API at the time of the Merger. The 2015 Term Loan is to be repaid by us in 48 monthly installments, plus accrued interest payable monthly in arrears and matures at the earlier of May 1, 2019, or upon an event of a default under the loan agreement. The term loan carries a floating annual interest rate equal to prime rate then in effect, as published in the Wall Street Journal, plus 2%. We may prepay amounts due under the 2015 Term Loan at any time, subject to an early termination fee of up to 2% of the amount of prepayment.
On September 29, 2015, we entered into the Waiver and Seventh Loan Modification Agreement with SVB, under which we borrowed an additional $1.0 million in December 2015 to fund certain anticipated capital expenditures (the "2015 Equipment Term Loan"). The principal amount plus accrued interest of the 2015 Equipment Term Loan is to be repaid by us in 36 monthly installments. The 2015 Equipment Term Loan carries a floating annual interest rate equal to the prime rate then in effect, as published in the Wall Street Journal, plus 2%. We may prepay amounts due under the 2015 Equipment Term Loan at any time, subject to an early termination fee of up to 2% of the amount of prepayment.
In December 2016, we entered into the Eighth Loan Modification Agreement with SVB, under which the financial covenants were modified.
Amounts due under the 2015 Term Loan and the 2015 Equipment Term Loan (collectively, the "Term Loans") are secured by substantially all of our assets, including intellectual property, personal property and bank accounts.
In addition, the Term Loans contain customary events of default, including nonpayment of principal, interest or other amounts, violation of covenants, material adverse change, an event of default under any subordinated debt documents, incorrectness of representations and warranties in any material respect, bankruptcy, judgments in excess of a threshold amount, and violations of other agreements in excess of a threshold amount. The Term Loans require that we meet certain financial covenants, including a minimum cash balance of $5.0 million, and a minimum liquidity coverage ratio, each as defined in the 2015 Equipment Term Loan. If any event of default occurs, SVB may declare due immediately all borrowings under the Term Loans and foreclose on the collateral. Furthermore, an event of default under the Term Loans would result in an increase in the interest rate on any amounts outstanding. As of December 31, 2016, we were2019, goodwill has been allocated to the Products and Licensing segment. The changes in compliance with all covenants under the Term Loans.
The balance under the Term Loans at December 31, 2016 was $4.3 million,carrying value of which $1.8 million was classified as short-term. The effective rate of our Term Loans at December 31, 2016 was 5.75%.
The following table presents a summary of debt outstanding as of December 31, 2016 and 2015:
|
| | | | | | | |
| | December 31, |
| | 2016 | | 2015 |
| Silicon Valley Bank Term Loans | $ | 4,291,666 |
| | $ | 6,125,000 |
|
| Less: unamortized debt issuance costs | 38,301 |
| | — |
|
| Less: current portion | 1,833,333 |
| | 1,833,333 |
|
| Total long-term debt obligations | $ | 2,420,032 |
| | $ | 4,291,667 |
|
Debt issuance costs associated with the issuance of the SVB Term Loans totaled $55 thousand. Amortization of debt issuance costs is computed using the straight line method and is included in interest expense. Amortization of the debt issuance costs totaled $16 thousand forgoodwill during the year ended December 31, 2016.
Maturities on long-term debt are2019 were as follows:
|
| | | |
| Year | Amount |
| 2017 | $ | 1,833,333 |
|
| 2018 | 1,833,333 |
|
| 2019 | 625,000 |
|
| Total | $ | 4,291,666 |
|
Interest expense for the years ended December 31, 2016 and 2015 consisted of the following:
|
| | | | | | | | |
| | Years ended December 31, |
| | | 2016 | | 2015 |
| Interest expense on Term Loans | | $ | 287,491 |
| | $ | 202,994 |
|
| Amortization of debt issuance costs | | 16,308 |
| | 9,072 |
|
| Other interest expense | | 17,143 |
| | 8,337 |
|
| Total interest expense | | $ | 320,942 |
| | $ | 220,403 |
|
|
| | | |
| Balance as of December 31, 2018 | $ | 101,008 |
|
| Goodwill resulting from business combination - GP | 10,511,916 |
|
| Measurement Period Adjustment - MOI | (71,248 | ) |
| Balance as of December 31, 2019 | $ | 10,541,676 |
|
5. Accounts Receivable—Trade
Accounts receivable consistWe concluded the carrying value of the following:
|
| | | | | | | |
| | December 31, |
| | 2016 | | 2015 |
| Billed | $ | 12,299,453 |
| | $ | 9,661,387 |
|
| Unbilled | 2,231,432 |
| | 1,412,381 |
|
| Other | 19,479 |
| | 95,600 |
|
| | 14,550,364 |
| | 11,169,368 |
|
| Less: allowance for doubtful accounts | (252,639 | ) | | (134,811 | ) |
| | $ | 14,297,725 |
| | $ | 11,034,557 |
|
Unbilled receivables result from contract retainages and revenues that have been earned in advance of billing and can be invoiced at contractually defined intervals, milestones, or at completion of the contract. Unbilled amounts are expected to be billed in future periods and are classified as current assets in accordance with industry practice.
6. Property and Equipment
Property and equipment, net, consists of the following:
|
| | | | | | | |
| | December 31, |
| | 2016 | | 2015 |
| Building | $ | 69,556 |
| | $ | 69,556 |
|
| Equipment | 12,589,998 |
| | 11,055,886 |
|
| Furniture and fixtures | 656,769 |
| | 666,654 |
|
| Software | 1,225,992 |
| | 1,092,484 |
|
| Leasehold improvements | 5,267,349 |
| | 5,257,703 |
|
| | 19,809,664 |
| | 18,142,283 |
|
| Less—accumulated depreciation | (13,028,826 | ) | | (11,528,045 | ) |
| | $ | 6,780,838 |
| | $ | 6,614,238 |
|
Depreciation for the years ended December 31, 2016 and 2015goodwill was approximately $1.5 million and $1.2 million, respectively.
7. Intangible Assets
The following is a summary of intangible assets, net:
|
| | | | | | | |
| | December 31, |
| | 2016 | | 2015 |
| Patent costs | $ | 3,169,920 |
| | $ | 2,723,971 |
|
| Developed technology | 8,400,000 |
| | 6,100,000 |
|
| In-process research and development | — |
| | 2,300,000 |
|
| Customer base | 1,300,000 |
| | 1,300,000 |
|
| Trade names | 1,000,000 |
| | 1,000,000 |
|
| Backlog | 400,000 |
| | 400,000 |
|
| | 14,269,920 |
| | 13,823,971 |
|
| Accumulated amortization | (5,588,657 | ) | | (3,419,659 | ) |
| | $ | 8,681,263 |
| | $ | 10,404,312 |
|
During 2016, the development was completed for certain products that were in process at the time of the Merger, and those products began being marketed. Accordingly $2.3 million of in-process research and development recorded in the initial purchase price allocation has been reclassified to developed technologynot impaired as of December 31, 2016. Amortization for2019 as we determined that it was not more likely than not that the years ended December 31, 2016 and 2015 was approximately $2.2 million and $1.2 million, respectively. Estimated aggregate amortization, based on the netfair value of intangible assets at December 31, 2016, for each of the next five years and beyond is as follows:reporting units was less than its carrying value.
|
| | | |
| Year Ending December 31, | |
| 2017 | 1,754,967 |
|
| 2018 | 1,405,228 |
|
| 2019 | 894,325 |
|
| 2020 | 814,956 |
|
| 2021 | 807,043 |
|
| 2022 and beyond | 3,004,744 |
|
| $ | 8,681,263 |
|
8. Accrued Liabilities
Accrued liabilities consist of the following:
|
| | | | | | | | |
| | | December 31, |
| | | 2016 | | 2015 |
| | Accrued compensation | $ | 5,442,723 |
| | $ | 4,719,533 |
|
| | Accrued sub-contracts | 483,477 |
| | 351,847 |
|
| | Accrued professional fees | 67,719 |
| | 133,847 |
|
| | Accrued income tax | — |
| | 160,438 |
|
| | Deferred rent | 155,138 |
| | 137,889 |
|
| | Royalties | 345,895 |
| | 351,003 |
|
| | Warranty reserve | 185,125 |
| | 173,687 |
|
| | Claims reserve | 1,577,123 |
| | 1,752,904 |
|
| | Accrued liabilities - other | 409,900 |
| | 523,538 |
|
| Total accrued liabilities | $ | 8,667,100 |
| | $ | 8,304,686 |
|
|
| | | | | | | | |
| | | December 31, |
| | | 2019 | | 2018 |
| | Accrued compensation | $ | 6,416,163 |
| | $ | 4,467,587 |
|
| | Contingent consideration - GP | 1,000,000 |
| | — |
|
| | Accrued professional fees | 113,303 |
| | 198,062 |
|
| | Accrued income tax | 715,916 |
| | 236,636 |
|
| | Deferred rent | — |
| | 146,542 |
|
| | Current operating lease liability | 1,283,310 |
| | — |
|
| | Current finance lease liability | 50,307 |
| | — |
|
| | Accrued royalties | 364,951 |
| | 302,428 |
|
| | Accrued liabilities-other | 425,595 |
| | 404,752 |
|
| | Working capital payable - MOI | — |
| | 542,983 |
|
| | Customer deposits | — |
| | 298,468 |
|
| Total accrued liabilities | $ | 10,369,545 |
| | $ | 6,597,458 |
|
9. Debt
Silicon Valley Bank Facility
We maintained a Loan and Security Agreement with SVB (the "Credit Facility") under which we had a term loan with an original borrowing amount of $6.0 million (the “Original Term Loan”). The Original Term Loan carried a floating annual interest rate equal to SVB’s prime rate then in effect plus 2%. The Original Term Loan matured and was repaid in May 2019.
On October 10, 2019, we entered into an Amended and Restated Loan and Security Agreement (the “Loan Agreement”) with SVB, which amended and restated in its entirety our previous Credit Facility. Under the Loan Agreement, SVB agreed to make advances available up to $10.0 million (the “Revolving Line”). If we borrow from the Revolving Line, such borrowing would carry a floating annual interest rate equal to the greater of (i) the Prime Rate (as defined in the Loan Agreement) then in effect plus 1% or (ii) 6%. Amounts borrowed under the Revolving Line may be repaid and, prior to the Revolving Line Maturity Date (defined below), reborrowed. The Revolving Line terminates on October 10, 2020 (the “Revolving Line Maturity Date”), unless earlier terminated by us. No amounts have been borrowed under this Loan Agreement.
Amounts due under the Loan Agreement are secured by our assets, including all personal property, inventory and bank accounts; however, intellectual property is not secured under the Loan Agreement. The inventory used to secure the amount due does not include demo or loaner equipment with an aggregate book value up to $1.0 million. The Loan Agreement requires us to observe a number of financial and operational covenants, including maintenance of a specified Liquidity Coverage Ratio (as defined in the Loan Agreement), protection and registration of intellectual property rights and customary negative covenants. If any event of default occurs SVB may declare due immediately all borrowings under the Credit Facility and foreclose on the collateral. Furthermore, an event of default under the Credit Facility would result in an increase in the interest rate on any amounts outstanding. As of December 31, 2019, there were no events of default on the Credit Facility.
Interest expense, net for the years ended December 31, 2019 and 2018 consisted of the following:
|
| | | | | | | | |
| | Years ended December 31, |
| | | 2019 | | 2018 |
| Interest expense on Term Loans | | $ | 8,073 |
| | $ | 101,087 |
|
| Amortization of debt issuance costs | | 5,685 |
| | 16,308 |
|
| Other interest expense | | 2,120 |
| | 6,949 |
|
| Total interest expense, net | | $ | 15,878 |
| | $ | 124,344 |
|
9. Income Taxes10. Leases
Income tax expense/(benefit)We have operating leases for our facilities, which have remaining terms ranging from continuing operations consisted1 to 5 years. Our leases do not have an option to extend the lease period beyond the stated term unless the new term is agreed by both parties. They also do not have an early termination clause included. Our operating lease agreements do not contain any material restrictive covenants. Some of the followingour operating lease agreements contain variable payment provisions that provide for the periods indicated:
|
| | | | | | | |
| | Years ended December 31, |
| | 2016 | | 2015 |
| Current: | | | |
| Federal | $ | — |
| | $ | (510,772 | ) |
| State | 75,366 |
| | 40,167 |
|
| Deferred Federal | — |
| | — |
|
| Deferred State | — |
| | — |
|
| Income tax expense/(benefit) | $ | 75,366 |
| | $ | (470,605 | ) |
Deferred tax assets and liabilitiesWe also have finance leases for equipment which have remaining terms ranging from 1 to 4 years. These lease agreements are for general office equipment with a 5-year useful life. These lease agreements do not have an option to extend the lease beyond the stated terms nor do they have an early termination clause. These lease agreements do not have any variable payment provisions included. The finance lease costs consist of the following components:
|
| | | | | | | | | | | | | | | |
| | December 31, 2016 | | December 31, 2015 |
| | Current | | Long-Term | | Current | | Long-Term |
| Bad debt and inventory reserve | $ | 382,075 |
| | $ | — |
| | $ | 157,847 |
| | $ | — |
|
| Inventory adjustment | — |
| | 940,885 |
| | — |
| | 939,793 |
|
| UNICAP | — |
| | 46,593 |
| | — |
| | 40,752 |
|
| Deferred revenue | — |
| | 154,608 |
| | — |
| | 131,489 |
|
| Deferred rent | — |
| | 550,419 |
| | — |
| | 599,094 |
|
| Depreciation and amortization | — |
| | (3,490,869 | ) | | — |
| | (4,231,265 | ) |
| Charitable contributions | — |
| | 5,741 |
| | — |
| | 4,954 |
|
| Net operating loss carryforwards- Luna | — |
| | 4,779,976 |
| | — |
| | 5,106,166 |
|
| Net operating loss carryforwards- API | — |
| | 9,783,512 |
| | — |
| | 9,783,512 |
|
| Net operating loss carryforwards - state | — |
| | 281,799 |
| | — |
| | 323,557 |
|
| Net operating loss carryforwards- Canada | — |
| | 10,503 |
| | — |
| | 39,867 |
|
| Research and development credits | — |
| | 4,250,803 |
| | — |
| | 4,250,803 |
|
| California manufacturing credit | — |
| | 15,554 |
| | — |
| | 15,554 |
|
| Accrued liabilities | 1,067,019 |
| | | | 1,211,752 |
| | — |
|
| Deferred compensation | — |
| | 267,897 |
| | — |
| | 192,547 |
|
| Stock-based compensation | — |
| | 1,867,947 |
| | — |
| | 1,804,338 |
|
| AMT credit | — |
| | 395,083 |
| | — |
| | 388,342 |
|
| Total | 1,449,094 |
| | 19,860,451 |
| | 1,369,599 |
| | 19,389,503 |
|
| Valuation allowance | (1,449,094 | ) | | (19,860,451 | ) | | (1,369,599 | ) | | (19,389,503 | ) |
| Net deferred tax asset | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
|
The expense/(benefit) from income taxes from continuing operations differs fromdiscount rate for both our operating and finance leases was not readily determinable in the amount computed by applyingspecific lease agreements. As a result, our incremental borrowing rate was used as the federal statutory income taxdiscount rate towhen establishing the ROU assets and corresponding lease liabilities.
As of December 31, 2019, our loss from continuing operations before income taxeslease components included in the consolidated balance sheet were as follows for the periods indicated:follows:
|
| | | | | | |
| | | Years ended December 31, |
| | 2016 | | 2015 |
| Income tax expense at federal statutory rate | | 34.00 | % | | 34.00 | % |
| State taxes, net of federal tax effects | | (1.15 | )% | | 3.34 | % |
| Change in valuation allowance | | (23.99 | )% | | (10.50 | )% |
| Incentive stock options | | (11.08 | )% | | (4.84 | )% |
| Provision to return adjustments | | (1.01 | )% | | 0.18 | % |
| Meals and entertainment | | (0.78 | )% | | (0.25 | )% |
| Capitalized merger costs | | — | % | | (14.95 | )% |
| Windfall deduction | | — | % | | 0.26 | % |
| Other permanent differences | | 0.44 | % | | 0.01 | % |
| Income tax (expense)/benefit | | (3.57 | )% | | 7.25 | % |
|
| | | | |
| Lease component | Classification | December 31, 2019 |
| Assets | | |
| ROU assets - operating lease | Other assets | $ | 2,235,731 |
|
| ROU assets - finance lease | Other assets | 70,183 |
|
| Total ROU assets | | $ | 2,305,914 |
|
| | | |
| Liabilities | | |
| Current operating lease liability | Accrued liabilities | $ | 1,283,310 |
|
| Current finance lease liability | Accrued liabilities | 50,307 |
|
| Long-term operating lease liability | Other liabilities | 1,988,395 |
|
| Long-term finance lease liability | Other liabilities | 23,092 |
|
| Total lease liabilities | | $ | 3,345,104 |
|
The realization of our deferred income tax assetsRent expense is dependent upon sufficient taxable income in future periods. In assessing whether deferred tax assets may be realized, we consider whether it is more likely than not that some portion, or all,recognized on a straight-line basis over the life of the deferred tax asset will be realized. We consider scheduled reversalslease. Rent expense consists of deferred tax liabilities, projected future taxablethe following:
income and tax planning strategies that we can implement in making our assessment. We have U.S. federal income tax net |
| | | | |
| | | Year Ended |
| | | December 31, 2019 |
| Operating lease costs | | $ | 1,622,476 |
|
| Variable rent costs | | (146,542 | ) |
| Total rent expense | | $ | 1,475,934 |
|
Future minimum lease payments under non-cancelable operating loss carryforwards atleases were as follows as of December 31, 20162019:
|
| | | |
| Year Ending December 31, | |
| 2020 | $ | 1,467,701 |
|
| 2021 | 640,800 |
|
| 2022 | 544,704 |
|
| 2023 | 544,704 |
|
| 2024 and beyond | 544,704 |
|
| Total future minimum lease payments | 3,742,613 |
|
| Less: Interest | 470,908 |
|
| Total operating lease liabilities | $ | 3,271,705 |
|
| | |
| Current operating lease liability | $ | 1,283,310 |
|
| Long-term operating lease liability | 1,988,395 |
|
| Total operating lease liabilities | $ | 3,271,705 |
|
Future minimum lease payments under non-cancelable finance leases were as follows as of December 31, 2019:
|
| | | |
| Year Ending December 31, | |
| 2020 | $ | 56,020 |
|
| 2021 | 10,710 |
|
| 2022 | 10,083 |
|
| 2023 | 5,042 |
|
| 2024 and beyond | — |
|
| Total future minimum lease payments | 81,855 |
|
| Less: Interest | 8,456 |
|
| Total finance lease liabilities | $ | 73,399 |
|
| | |
| Current finance lease liability | $ | 50,307 |
|
| Long-term finance lease liability | 23,092 |
|
| Total finance lease liabilities | $ | 73,399 |
|
Other information related to leases is as follows:
|
| | | |
| | Year Ended |
| | December 31, 2019 |
| Finance lease cost: | |
| Amortization of right-of-use assets | $ | 46,498 |
|
| Interest on lease liabilities | 4,926 |
|
| Total finance lease cost | $ | 51,424 |
|
| | |
| Other information: | |
| Cash paid for amounts included in the measurement of lease liabilities: | |
| Operating cash flows from operating leases | $ | 1,622,476 |
|
| Finance cash flows from finance leases | $ | 40,047 |
|
| Right-of-use assets obtained in exchange for new finance lease liabilities | $ | 14,541 |
|
| Weighted-average remaining lease term (years) - operating leases | 3.7 |
|
| Weighted-average remaining lease term (years) - finance leases | 2.1 |
|
| Weighted-average discount rate - operating leases | 7 | % |
| Weighted-average discount rate - finance leases | 7 | % |
At December 31, 2019, we had no operating or finance leases that have not yet commenced.
Prior to the adoption of ASC 842, lease expense of approximately $12.6$1.0 million for Luna and net operating loss carryforwards of approximately $28.8 million for API expiring at varying dates through 2025. We have research and development tax credit carryforwards at December 31, 2016 of approximately $4.3 million, which expire at varying dates through 2024.
In 2015, we performed a formal section 382 study and determined that we do not have a limitation onwas recognized in our net operating loss available to offset future income for the Luna net operating losses. As a result of the acquisition of API, the API net operating loss carryover and research and development credits will be subject to the Section 382 limitation. A formal Section 382 study has not been prepared, however, based on our estimated calculations it is likely that that a portion of the net operating losses and research and development credits will expire unutilized. As there is a valuation allowance against the API deferred tax assets, there will not be aconsolidated statement of operations impact to any expiration of the net operating losses or research and development credits.
The U.S. federal statute of limitations remains open for the year 2007 and onward. We currently have no federal income tax returns under examination. U.S. state jurisdictions have statutes of limitation generally ranging from three to seven years. We currently have no state income or franchise tax returns under examination. We currently do not file tax returns in any foreign tax jurisdiction other than Canada.
We currently have no positions for which we expect that the amount of unrecognized tax benefit will increase or decrease significantly within twelve months of the reporting date or for which we believe there is significant risk of disallowance upon audit. We have no tax interest or penalties reported in either our statement of operations or statement of financial position for any year reported herein. Management believes it is not more likely than not that the deferred tax assets atended December 31, 2016 or December 31, 2015 will not be realized, and as a result a valuation allowance was established against all such deferred tax assets.2018.
Windfall equity-based compensation deductions are tracked, but will not be recorded to the balance sheet until management determines it is more likely than not that such amounts will be utilized. As of December 31, 2016, we had no windfall stock compensation deductions. If and when realized, the tax benefit associated with these deductions will be credited to additional paid-in capital. These excess benefit deductions are included in the total federal net operating losses disclosed above.
10.11. Stockholders’ Equity
Series A Convertible Preferred Stock
In January 2010, we entered into a transaction with Carilion, in which Carilion agreed to exchange all of its Senior Convertible Promissory Notes with an original principal amount of $5.0 million plus all accrued but unpaid interest, totaling $1.2 million, for 1,321,514 shares of our newly designated Series A Convertible Preferred Stock. The Series A Convertible
Preferred Stock is non-voting, carries a dividend of 6% payable in shares of common stock and maintains a liquidation preference up to $6.2 million. As of December 31, 2016,In September 2019, Carilion elected to convert the 552,4011,321,514 shares of commonpreferred stock were issuable to Carilion as dividends and have been recorded in the statementinto an equal number of stockholders’ equity. These dividends are issuable on demand. Each share of Series A Convertible Preferred Stock may be converted into one share of our common stock at the option of the holder. We recorded the fair value of the Series A Convertible Preferred Stock, determined based upon the conversion value immediately prior to the exchange, the fair value of the new warrant issued to Carilion, determined using the Black-Scholes valuation model, and the incremental fair value of the prior warrant due to the re-pricing and extension of maturity to stockholders’ equity.
Warrants
Carilion Clinic holds unexercised warrants for 366,000 shares of our common stock. The warrants have an exercise priceIn addition, we issued 770,454 shares of $2.50 and expireour common stock in satisfaction of the accrued dividends earned on December 31, 2020.the preferred stock prior to its conversion.
Equity Incentive Plans
In January 2006, we adopted our 2006 Equity Incentive Plan (the "2006 Plan"). Under the 2006 Plan, our Board of Directors was authorized to grant both incentive and non-statutory stock options to purchase common stock and restricted stock awards to our employees, directors, and consultants. The 2006 Plan expired in January 2016.
In April 2016, we adopted our 2016 Equity Incentive Plan (the "2016 Plan") as a successor to the 2006 Plan. Under the 2016 Plan, our Board of Directors is authorized to grant both incentive and non-statutory stock options to purchase common stock and restricted stock awards to our employees, directors, and consultants. The 2016 Plan provides for the issuance of
3,500,000 shares plus any amounts forfeited from grants under the 2006 Plan after the expiration date of the 2006 Plan. Options generally have a life of 10 years and exercise price equal to or greater than the fair market value of the Common Stock as determined by the Board of Directors.
Vesting for employees typically occurs over a four-year period.
The following table sets forth the activity of the options to purchase common stock under the 2006 Plan and the 2016 Plan. The prices represent the closing price of our Common Stock on the NASDAQNasdaq Capital Market on the respective dates.
| | | | Options Outstanding | | Options Exercisable | Options Outstanding | | Options Exercisable |
| | Number of Shares | | Price per Share Range | | Weighted Average Exercise Price | | Aggregate Intrinsic Value (1) | | Number of Shares | | Weighted Average Exercise Price | | Aggregate Intrinsic Value (1) | Number of
Shares | | Price per
Share Range | | Weighted
Average
Exercise
Price | | Aggregate
Intrinsic
Value (1) | | Number of Shares | | Weighted Average Exercise Price | | Aggregate Intrinsic Value (1) |
| Balance at January 1, 2015 | 4,289,631 |
| | $0.35 - 6.55 |
| | $ | 1.93 |
| | $ | 512,901 |
| | 3,111,199 |
| | $ | 2.11 |
| | $ | 453,032 |
| |
| Balance at January 1, 2018 | | 2,714,561 |
| | $0.61 - 6.55 | | $ | 1.88 |
| | $ | 2,098,195 |
| | 2,590,030 |
| | $ | 1.89 |
| | $ | 2,013,034 |
|
| Forfeited | (789,412 | ) | | $0.35 - 9.03 |
| | $ | 2.86 |
| | | | | | | | | (675,607 | ) | | $1.15 - 6.55 | | 1.90 |
| | | | | | | | |
| Exercised | (230,672 | ) | | $0.35 - 1.77 |
| | $ | 0.37 |
| | | | | | | | | (96,425 | ) | | $0.65 - 2.46 | | 2.07 |
| | | | | | | | |
| Granted | 65,500 |
| | $0.94 - 1.45 |
| | $ | 1.21 |
| | | | | | | | | 1,166,339 |
| | $2.67 - 3.53 | | 3.09 |
| | | | | | | | |
| Issued in exchange for API options | 465,681 |
| | $1.38 - 9.03 |
| | $ | 4.83 |
| | | | | | | | | |
| Balance at December 31, 2015 | 3,800,728 |
| | $0.61 - 8.43 |
| | $ | 2.17 |
| | $ | 111,314 |
| | 3,045,150 |
| | $ | 2.39 |
| | $ | 103,603 |
| |
| Balance at December 31, 2018 | | 3,108,868 |
| | $0.61 - 6.55 | | $ | 2.26 |
| | $ | 3,669,794 |
| | 1,986,740 |
| | $ | 1.81 |
| | $ | 3,314,494 |
|
| Forfeited | (963,614 | ) | | $1.18 - 8.43 |
| | $ | 2.99 |
| | | | | | | | | (14,707 | ) | | $1.47 - 3.37 | | 2.51 |
| | | | | | | | |
| Exercised | — |
| | — |
| | $ | — |
| | | | | | | | | (558,834 | ) | | $0.61 - 1.81 | | 1.21 |
| | | | | | | | |
| Granted | 20,000 |
| | $ | 1.15 |
| | $ | 1.15 |
| | | | | | | | | 625,070 |
| | $3.21 - 7.37 | | 3.63 |
| | | | | | | | |
| Balance at December 31, 2016 | 2,857,114 |
| | $0.61 - 6.83 |
| | $ | 1.89 |
| | $ | 107,063 |
| | 2,367,630 |
| | $ | 1.93 |
| | $ | 101,071 |
| |
| Balance at December 31, 2019 | | 3,160,397 |
| | $1.18 - 7.37 | | $ | 2.72 |
| | $ | 14,459,884 |
| | 1,835,799 |
| | $ | 2.28 |
| | $ | 9,197,775 |
|
| |
| (1) | The intrinsic value of an option represents the amount by which the market value of the stock exceeds the exercise price of the option of in-the-money options only. |
The fair value of each option granted is estimated as of the grant date using the Black-Scholes option pricing model with the following assumptions:
| | | | | Years ended December 31, | | Years ended December 31, |
| | | 2016 | | 2015 | | 2019 | | 2018 |
| Risk-free interest rate range | | 1.5% | | 1.88% – 1.95% | | 2.5% | | 3.04% |
| Expected life of option-years | | 7.5 | | 7.5 | | 7 | | 7 |
| Expected stock price volatility | | 73% | | 75% - 103% | | 65% | | 67% |
| Executive turnover rates | | —% | | —% | |
| Non-executive turnover rates | | 14.0% | | 40.0% | |
| Expected dividend yield | | —% | | —% | | —% | | —% |
The risk-free interest rate is based on U.S. Treasury interest rates, the terms of which are consistent with the expected life of the stock options. Expected volatility is based upon the average historical volatility of our common stock over the period commensurate with the expected term of the related instrument. The expected life and estimated post-employment termination behavior is based upon historical experience of homogeneous groups, executives and non-executes, within our company. We do not currently pay dividends on our common stock nor do we expect to in the foreseeable future.
|
| | | | | | | | | | | | | | | | | |
| | | | Options Outstanding | | Options Exercisable |
| | Range of Exercise Prices | | Options Outstanding | | Weighted Average Remaining Life in Years | | Weighted Average Exercise Price | | Options Exercisable | | Weighted Average Exercise Price of Options Exercisable |
| Year ended December 31, 2015 | $0.61 - $8.43 | | 3,800,728 |
| | 5.55 | | $ | 2.17 |
| | 3,045,150 |
| | $ | 2.39 |
|
| Year ended December 31, 2016 | $0.61- $6.83 | | 2,857,114 |
| | 5.09 | | $ | 1.89 |
| | 2,637,630 |
| | $ | 1.93 |
|
|
| | | | | | | | | | | | | | | |
| | | | Options Outstanding | | Options Exercisable |
| | Range of Exercise Prices | | Options Outstanding | | Weighted Average Remaining Life in Years | | Weighted Average Exercise Price | | Options Exercisable | | Weighted Average Remaining Life in Years | | Weighted Average Exercise Price of Options Exercisable |
| Year ended December 31, 2018 | $0.61 - 6.55 | | 3,108,868 |
| | 5.72 | | $2.26 | | 1,986,740 |
| | 3.46 | | $1.81 |
| Year ended December 31, 2019 | $1.18 - 7.37 | | 3,160,397 |
| | 6.24 | | $2.72 | | 1,835,799 |
| | 4.30 | | $2.28 |
|
| | | | | |
| | Total Intrinsic Value of Options Exercised | | Total Fair Value of Options Vested |
| Year ended December 31, 2015 | 198,013 |
| | 741,619 |
|
| Year ended December 31, 2016 | — |
| | 370,654 |
|
|
| | | | | | | |
| | Total Intrinsic Value of Options Exercised | | Total Fair Value of Options Vested |
| Year ended December 31, 2018 | $ | 112,213 |
| | $ | 2,980,110 |
|
| Year ended December 31, 2019 | $ | 1,641,687 |
| | $ | 3,267,672 |
|
For the years ended December 31, 20162019 and 2015,2018, the weighted average grant date fair value of options granted was $1.15$2.32 and $0.97, respectively,$2.07 per share.share, respectively. We estimate the fair value of options at the grant date using the Black-Scholes model. For all stock options granted through December 31, 2019, the weighted average remaining service period is 3.4 years.
Unamortized stock option expense at December 31, 2019 that will be amortized over the weighted-average remaining service period of 2.9 years totaled $2.8 million.
Restricted Stock Issuancesand Restricted Stock Units
In 2016 and 2015,Historically, we issued 319,000 and 334,000, respectively,have granted shares of restricted stock to certain employees that have vested in three equal annual installments on the anniversary dates of their grant. However, beginning in 2019, we altered our approach for these grants to replace the grant of restricted stock subject to time-based vesting with the grant of a combination of restricted stock units ("RSUs") subject to time-based vesting and performance-based vesting. Each RSU represents the contingent right to receive a single share of our common stock upon the vesting of the award. For the year ended December 31, 2019, we granted an aggregate of 280,000 RSUs to certain employees. These sharesOf the RSUs granted during 2019, 217,000 of such RSUs are subject to time-based vesting and are scheduled to vest in fourthree equal annual installments on the anniversary dates of the grant. The remaining 63,000 RSUs are performance-based awards that will vest based on our achievement of long-term performance goals, in particular, based on our levels of 2021 revenue and operating income. The 63,000 shares issuable upon vesting of the performance-based RSUs represent the maximum payout under our performance-based awards, based upon 150% of our target performance for 2021 revenue and operating income (the payout of such awards based on target performance for 2021 revenue and operating income would be 42,000 shares). In the case of the time-based and performance-based RSUs, vesting is also subject to the employee's continuous service with us through vesting. In 2018, we granted 280,000 shares of restricted stockto certain employees. Shares issued to employees vest in three equal annual installments on the anniversary dates of their grant. In 20162019 and 2015, 245,5832018, 194,333 and 168,750182,500 shares of restricted stock vested, respectively.
In addition, in conjunction with our 2018 and 2019 Annual Meetings of Stockholders, we granted RSUs to certain members of our Board of Directors in respect of the annual equity compensation under our non-employee director compensation policy (other members of our Board of Directors elected to receive their annual equity compensation for Board service in the form of stock units under our Deferred Compensation Plan as described below). In 2019 and 2018, we granted 11,600 and 16,286, respectively, RSUs to members of our Board of Directors in respect of the annual equity compensation under our non-employee director compensation policy. RSUs issued to our Board of Directors vest at the earlier of the one-year anniversary of their grant or the next annual stockholders' meeting. In 2019 and 2018, 16,286 and 129,865 RSUs, respectively, vested.
The following table summarizes our aggregate restricted stock awards:awards and RSU activity in 2019 and 2018:
| | | | Number of Unvested Shares | | Weighted Average Grant Date Fair Value | | Aggregate Value of Unvested Shares | Number of Unvested Shares | | Weighted Average Grant Date Fair Value | | Aggregate Grant Date Fair Value of Unvested Shares |
| Balance at January 1, 2015 | 530,250 |
| | $1.34 | | $ | 710,535 |
| |
| Balance at January 1, 2017 | | 489,698 |
| | $1.51 | | $ | 738,345 |
|
| Granted | 334,000 |
| | $1.12 | | $ | 374,080 |
| 296,287 |
| | $3.07 | | $ | 909,600 |
|
| Vested | (168,750 | ) | | $1.33 | | $ | (224,438 | ) | (312,365 | ) | | $1.45 | | $ | (454,339 | ) |
| Forfeitures | (25,875 | ) | | $1.35 | | $ | (35,018 | ) | (15,000 | ) | | $1.41 | | $ | (21,150 | ) |
| Balance at December 31, 2015 | 669,625 |
| | $1.23 | | $ | 825,159 |
| |
| Balance at December 31, 2018 | | 458,620 |
| | $2.56 | | $ | 1,172,456 |
|
| Granted | 319,000 |
| | $1.15 | | $ | 366,850 |
| 291,600 |
| | $3.75 | | $ | 1,094,430 |
|
| Vested | (245,583 | ) | | $1.23 | | $ | (303,245 | ) | (210,619 | ) | | $2.33 | | $ | (490,769 | ) |
| Forfeitures | — |
| | $ | — |
| | $ | — |
| (37,499 | ) | | $2.96 | | $ | (111,115 | ) |
| Balance at December 31, 2016 | 743,042 |
| | $1.20 | | $ | 888,764 |
| |
| Balance at December 31, 2019 | | 502,102 |
| | $3.32 | | $ | 1,665,002 |
|
We recognized $0.9$1.5 million and $1.1$0.6 million in stock-based compensation expense, which is recorded in selling, general and administrative expensesexpense on the consolidated statement of operations for the years ended December 31, 20162019 and 2015,2018, respectively, and we will recognize $0.9$4.0 million over the remaining requisite service period. For allperiod for unamortized restricted stock, options granted throughRSUs and stock options.
Unamortized restricted stock and RSUs expense at December 31, 2016,2019 that will be amortized over the weighted averageweighted-average remaining service period is 0.8 years.of 2 years totaled $1.2 million.
Restricted Stock UnitsNon-employee Director Deferred Compensation Plan
We issue restricted stock units, ("RSUs"maintain a non-employee director deferred compensation plan (the “Deferred Compensation Plan”), to that permits our non-employee directors to defer receipt of certain compensation that they receive for serving on our board and board committees. The Deferred Compensation Plan has historically permitted the participants to elect to defer cash fees to which they were entitled for board and committee service. For participating directors, in lieu of payment of cash fees, we credit their accounts under the Deferred Compensation Plan with a number of stock units based on the trading price of our common stock as of the date of the deferral. These stock units vest immediately, although the participating directors do not receive the shares represented by such units until a future qualifying event.
In December 2017, we amended and restated our Deferred Compensation Plan to also permit participants to defer the annual equity compensation for board service provided. Under(which would otherwise be issued in the form of restricted stock units) under our non-employee director compensation plan RSUs issued for annual retainer fees vestpolicy. For participating directors, we credit their accounts under the Deferred Compensation Plan with a number of stock units based on the trading price of our common stock as of the date of the deferral. These stock units are vested upon the earlier of the one yearone-year anniversary date of the
grant or next annual meeting of stockholders, although the following shareholder meeting. Amounts issued for quarterly fees vest immediately upon their issuance.participating directors do not receive the shares represented by such units until a future qualifying event. A summary of our RSUstock unit activity under the Deferred Compensation Plan for 20152019 and 20162018 is as follows.
|
| | | | | | | | | | |
| | Number of RSUs | | | | Intrinsic Value |
| | Issued | | Unvested | | Weighted Average Grant Date Fair Value per Share | | Outstanding | Unvested |
| Balance at January 1, 2015 | 329,524 |
| | — |
| | $1.53 | | | |
| Granted | 152,697 |
| | 72,813 | | $1.01 | | | |
| Vested | — |
| | — |
| | $0.00 | | | |
| Forfeitures | — |
| | — |
| | $0.00 | | | |
| Converted | (118,297 | ) | | — |
| | $1.46 | | | |
| Balance at December 31, 2015 | 363,924 |
| | 72,813 | | $1.33 | | | |
| Granted | 188,857 |
| | 86,956 | | $1.18 | | | |
| Vested | — |
| | (72,813 | ) | | $1.01 | | | |
| Forfeitures | — |
| | — |
| | $0.00 | | | |
| Converted | (24,271 | ) | | — |
| | $1.01 | | | |
| Balance at December 31, 2016 | 528,510 |
| | 86,956 | | $1.29 | | $776,910 | $127,825 |
|
| | | | | | | | |
| | Number of Stock Units | | Weighted Average Grant Date Fair Value per Share | | Intrinsic Value Outstanding |
| January 1, 2018 | 466,702 |
| | $1.40 | | $ | 1,134,086 |
|
| Granted | 40,588 |
| | $2.99 | | |
| December 31, 2018 | 507,290 |
| | $1.40 | | $ | 1,699,422 |
|
| Granted | 121,713 |
| | $4.41 | | |
| December 31, 2019 | 629,003 |
| | $2.09 | | $ | 4,585,432 |
|
As of December 31, 2019, 37,546 of the outstanding stock units had not yet vested.
Stock Repurchase Program
In May 2016,September 2017, our board of directors authorized us toa new stock repurchase program providing for the repurchase of up to $2,000,000$2.0 million of our common stock through May 31, 2017.September 19, 2018. Our stock repurchase program doesdid not obligate us to acquire any specific number of shares. Under the program, shares may be repurchased in privately negotiated or open market transactions, including under plans complying with Rule 10b5-1 under the Securities Exchange Act of 1934, as amended. As of December 31, 2016,September 19, 2018, we had repurchased a total of 205,500565,629 shares, respectively, for an aggregate purchase price of $1.1 million under this stock repurchase program. We currently maintain all repurchased shares under these stock repurchase programs as treasury stock.
In August 2019, our board of directors authorized a new stock repurchase program which allowed us to repurchase up to $2.0 million of our common stock through August 2020. As of September 30, 2019, we had repurchased a total of 333,953 shares for an aggregate purchase price of $0.2 million. $2.0 million under this new stock repurchase program, and accordingly the program expired. We currently maintain all repurchased shares under this stock repurchase program as treasury stock.
12. Revenue Recognition
Our operations are divided into two operating segments—“Products and Licensing” and “Technology Development”.
The following chartProducts and Licensing segment derives its revenues from product sales, funded product development and technology licenses.
The Technology Development segment provides applied research to customers in our areas of focus. Our engineers and scientists collaborate with our network of government, academic and industry experts to identify technologies and ideas with promising market potential. We then compete to win fee-for-service contracts from government agencies and industrial customers who seek innovative solutions to practical problems that require new technology. The Technology Development segment derives its revenues primarily from services.
Disaggregation of Revenue
We disaggregate our revenue from contracts with customers by geographic locations, customer-type, contract type, timing of recognition, and major categories for each of our segments, as we believe it best depicts how the nature, amount, timing and uncertainty of our revenue and cash flows are affected by economic factors.
The details our share repurchases during each month ofare listed in the quartertable below for the years ended December 31, 2016:2019 and 2018:
|
| | | | | | |
| | Total Number of Shares Repurchased | | Average Price Paid per Share |
| October 2016 | — |
| | $ | — |
|
| November 2016 | 6,400 |
| | $ | 1.25 |
|
| December 2016 | — |
| | $ | — |
|
|
| | | | | | | | | | | | | | | | | | | | |
| | | Years ended December 31, |
| | | 2019 | | 2018 |
| | | Products and Licensing | Technology Development | Total | | Products and Licensing | Technology Development | Total |
| Total Revenue by Geographic Location | | | | | |
| | United States | $ | 21,782,692 |
| $ | 26,024,674 |
| $ | 47,807,366 |
| | $ | 11,585,296 |
| $ | 20,967,556 |
| $ | 32,552,852 |
|
| | Asia | 13,669,304 |
| — |
| 13,669,304 |
| | 5,977,563 |
| — |
| 5,977,563 |
|
| | Europe | 7,277,234 |
| — |
| 7,277,234 |
| | 3,873,161 |
| — |
| 3,873,161 |
|
| | Canada, Central and South America | 1,432,082 |
| — |
| 1,432,082 |
| | 382,797 |
| — |
| 382,797 |
|
| | All Others | 329,729 |
| — |
| 329,729 |
| | 130,872 |
| — |
| 130,872 |
|
| | Total | $ | 44,491,041 |
| $ | 26,024,674 |
| $ | 70,515,715 |
| | $ | 21,949,689 |
| $ | 20,967,556 |
| $ | 42,917,245 |
|
| | | | |
| | | | |
| | | | |
| | | | |
| Total Revenue by Major Customer Type |
| | | | |
| | Sales to the U.S. government | $ | 2,601,069 |
| $ | 25,377,961 |
| $ | 27,979,030 |
| | $ | 1,834,289 |
| $ | 20,703,338 |
| $ | 22,537,627 |
|
| | U.S. direct commercial sales and other | 19,181,625 |
| 646,713 |
| 19,828,338 |
| | 9,737,720 |
| 264,218 |
| 10,001,938 |
|
| | Foreign commercial sales & other | 22,708,347 |
| — |
| 22,708,347 |
| | 10,377,680 |
| — |
| 10,377,680 |
|
| | Total | $ | 44,491,041 |
| $ | 26,024,674 |
| $ | 70,515,715 |
| | $ | 21,949,689 |
| $ | 20,967,556 |
| $ | 42,917,245 |
|
| | | | |
| | | | |
| | | | |
| | | | |
| Total Revenue by Contract Type |
| | | | |
| | Fixed-price contracts | $ | 44,491,041 |
| $ | 14,111,092 |
| $ | 58,602,133 |
| | $ | 21,949,689 |
| $ | 9,388,770 |
| $ | 31,338,459 |
|
| | Cost-type contracts | — |
| 11,913,582 |
| 11,913,582 |
| | — |
| 11,578,786 |
| 11,578,786 |
|
| | Total | $ | 44,491,041 |
| $ | 26,024,674 |
| $ | 70,515,715 |
| | $ | 21,949,689 |
| $ | 20,967,556 |
| $ | 42,917,245 |
|
| | | | |
| | | | |
| | | | |
| | | | |
| Total Revenue by Timing of Recognition |
| | | | |
| | Goods transferred at a point in time | $ | 43,129,361 |
| $ | — |
| $ | 43,129,361 |
| | $ | 21,330,000 |
| $ | — |
| $ | 21,330,000 |
|
| | Goods/services transferred over time | 1,361,680 |
| 26,024,674 |
| 27,386,354 |
| | 619,689 |
| 20,967,556 |
| 21,587,245 |
|
| | Total | $ | 44,491,041 |
| $ | 26,024,674 |
| $ | 70,515,715 |
| | $ | 21,949,689 |
| $ | 20,967,556 |
| $ | 42,917,245 |
|
| | | | | | | | | |
| | | | | | | | | |
| Total Revenue by Major Products/Services | | | | | |
| | Technology development | $ | — |
| $ | 26,024,674 |
| $ | 26,024,674 |
| | $ | — |
| $ | 20,967,556 |
| $ | 20,967,556 |
|
| | Test, measurement and sensing systems | 41,787,613 |
| — |
| 41,787,613 |
| | 19,641,434 |
| — |
| 19,641,434 |
|
| | Other | 2,703,428 |
| — |
| 2,703,428 |
| | 2,308,255 |
| — |
| 2,308,255 |
|
| | Total | $ | 44,491,041 |
| $ | 26,024,674 |
| $ | 70,515,715 |
| | $ | 21,949,689 |
| $ | 20,967,556 |
| $ | 42,917,245 |
|
Contract Balances
Our contract assets consist of unbilled amounts for technology development contracts as well as custom product contracts. Also included in contract assets are royalty revenue and carrying amounts of right of returned inventory. Long-term contract assets include the fee withholding on cost reimbursable contracts that will not be billed within a year. Contract liabilities include excess billings, subcontractor accruals, warranty expense, extended warranty revenue, right of return refund, and customer deposits. The net contract (liabilities)/assets changed by $1.0 million, due primarily to increased contract liabilities in addition to a slight increase in contract assets. The increase in contract liabilities is a result of the increased number of government research programs in addition to an increase in the number of our fixed-price contracts that have reached milestones as designated in their respective contracts, but revenue has not yet been recognized. The increase in contract assets is primarily driven by the unbilled fee required by our cost-reimbursable government contracts, which cannot be fully billed until after the specific contract is complete.
The following table shows the components of our contract balances as of December 31, 2019 and 2018:
|
| | | | | | | |
| | December 31, |
| | 2019 | | 2018 |
| Contract assets | $ | 3,208,206 |
| | $ | 2,759,315 |
|
| Contract liabilities | (3,887,685 | ) | | (2,486,111 | ) |
| Net contract assets | $ | (679,479 | ) | | $ | 273,204 |
|
Performance Obligations
Unfulfilled performance obligations represent amounts expected to be earned on executed contracts. Indefinite delivery and quantity contracts and unexercised options are not reported in total unfulfilled performance obligations. Unfulfilled performance obligations include funded obligations, which is the amount for which money has been directly authorized by the U.S. government and for which a purchase order has been received by a commercial customer, and unfunded obligations represent firm orders for which funding has not yet been appropriated. The approximate value of our Products and Licensing segment's unfulfilled performance obligations was $16.1 million at December 31, 2019. We currently maintain these repurchased shares as treasury stock.expect to satisfy 63% of the performance obligations in 2020, 13% in 2021 and the remainder by 2024. The approximate value of our Technology Development segment's unfulfilled performance obligations was $31.3 million at December 31, 2019. We expect to satisfy 70% of the performance obligations in 2020, 24% in 2021 and the remainder by 2022.
11.13. Income Taxes
Income tax (benefit)/expense from continuing operations consisted of the following for the periods indicated:
|
| | | | | | | |
| | Years ended December 31, |
| | 2019 | | 2018 |
| Current: | | | |
| Federal | $ | 1,466,770 |
| | $ | (44,727 | ) |
| State | 227,912 |
| | 92,545 |
|
| Deferred federal | (2,849,371 | ) | | — |
|
| Deferred state | (499,661 | ) | | — |
|
| Income tax (benefit)/expense | $ | (1,654,350 | ) | | $ | 47,818 |
|
Deferred tax assets and liabilities consist of the following components:
|
| | | | | | | | | |
| | December 31, 2019 | | December 31, 2018 |
| | | Long-Term | | | Long-Term |
| Bad debt and inventory reserve | | $ | 376,331 |
| | | $ | 332,721 |
|
| Inventory adjustment | | — |
| | | (21,785 | ) |
| UNICAP | | 4,828 |
| | | 2,804 |
|
| Deferred revenue | | 130,058 |
| | | 115,676 |
|
| ASC842 Lease Accounting (DTA) | | 796,864 |
| | | 288,017 |
|
| ASC842 Lease Accounting (DTL) | | (544,539 | ) | | | — |
|
| Depreciation and amortization | | (2,134,569 | ) | | | (838,540 | ) |
| Net operating loss carryforwards- Luna | | 349,421 |
| | | 349,421 |
|
| Net operating loss carryforwards- API | | 1,169,671 |
| | | 1,265,538 |
|
| Net operating loss carryforwards - state | | 150,050 |
| | | 179,149 |
|
| Net operating loss carryforwards- Canada | | 10,503 |
| | | 10,503 |
|
| Accrued liabilities | | 594,450 |
| | | 394,118 |
|
| Deferred compensation | | 294,190 |
| | | 216,944 |
|
| Stock-based compensation | | 373,658 |
| | | 803,757 |
|
| Restricted stock | | 102,741 |
| | | 60,681 |
|
| State bonus | | 33,791 |
| | | 44,861 |
|
| Performance based compensation | | 9,499 |
| | | — |
|
| Transaction costs | | 58,540 |
| | | 63,668 |
|
| Total | | 1,775,487 |
| | | 3,267,533 |
|
| Valuation allowance | | (359,924 | ) | | | (3,267,533 | ) |
| Net deferred tax asset | | $ | 1,415,563 |
| | | $ | — |
|
The net deferred tax asset is included in other non-current assets on the consolidated balance sheet.
The (benefit)/expense from income taxes from continuing operations differs from the amount computed by applying the federal statutory income tax rate to our loss from continuing operations before income taxes as follows for the periods indicated:
|
| | | | | | |
| | | Years ended December 31, |
| | 2019 | | 2018 |
| Income tax expense at federal statutory rate | | 21.00 | % | | 21.00 | % |
| State taxes, net of federal tax effects | | (8.67 | )% | | — | % |
| Change in valuation allowance | | (67.39 | )% | | (27.65 | )% |
| Incentive stock options | | (1.75 | )% | | (1.05 | )% |
| Provision to return adjustments | | 7.26 | % | | 21.24 | % |
| Meals and entertainment | | 0.50 | % | | 0.97 | % |
| AMT credit | | — | % | | (9.83 | )% |
| Other permanent differences | | 4.20 | % | | (0.88 | )% |
| Income tax (benefit)/expense | | (44.85 | )% | | 3.80 | % |
The realization of our deferred income tax assets is dependent upon sufficient taxable income in future periods. In assessing whether deferred tax assets may be realized, we consider whether it is more likely than not that some portion, or all,
of the deferred tax asset will be realized. We consider scheduled reversals of deferred tax liabilities, projected future taxable income and tax planning strategies that we can implement in making our assessment. We have net operating loss ("NOL") carryforwards of approximately $6.0 million for API expiring at varying dates through 2035.
In 2015, we performed a formal Section 382 study and determined that we do not have a limitation on our NOLs available to offset future income. As a result of the acquisition of API, the API NOL carryover and research and development credits will be subject to the Section 382 limitation. A formal Section 382 study was prepared in 2019, and it was determined that there was no ownership changes in 2019 resulting in a limitation on NOLs, but a portion of the NOLs will expire unutilized.
The U.S. federal statute of limitations remains open for the year 2016 and onward. We currently have no federal income tax returns under examination. U.S. state jurisdictions have statutes of limitation generally ranging from three to seven years. We currently have no state income or franchise tax returns under examination. We currently do not file tax returns in any foreign tax jurisdiction other than Canada.
As of December 31, 2019, we are no longer in a three-year cumulative loss position, which required additional analysis to be performed in order to determine the likelihood of realizing the deferred tax assets in the foreseeable future. After further analysis, it was determined that a portion of the December 31, 2019 balance of deferred tax assets will be realized. Because the NOLs carried over from API are limited under Section 382, the deferred tax asset of $1.2 million is expected to be realized over an extended period of time (with continued earnings realized ratably through 2034). The deferred tax asset related to the NOL carryovers of API Canada, Luna Analytics, Luna Nanomaterials, and Luna Quest will likely not be realized in the foreseeable future as these entities no longer have any activity. As of December 31, 2019, valuation allowances of $3.3 million were released and a tax benefit has been recognized in our consolidated statement of operations.
14. Commitments and Contingencies
Obligation under Operating Leases
We lease facilities in Virginia, Michigan, California, and Canada under operating leases that as of December 31, 2016 were scheduled to expire between April 2017 and December 2024. Certain of the leases are subject to fixed escalations and provide for possible termination prior to their expiration dates. We recognize rent expense on such leases on a straight-line basis over the lease term. The difference between the straight line method and cash paid is reflected in changes to the deferred rent balance in our consolidated balance sheets. Deferred rent primarily resulted from recognition of the value of certain leasehold improvements associated with our Blacksburg, Virginia facility at the inception of the lease. Rent expense under these leases recorded in selling, general and administrative expense on our statements of operations totaled approximately $1.5 million and $1.1 million, respectively, for the years ended December 31, 2016 and 2015.
Minimum future payments, as of December 31, 2016, under the aforementioned operating leases for each of the next five years and thereafter are:
|
| | | |
| 2017 | $ | 1,601,292 |
|
| 2018 | 1,602,932 |
|
| 2019 | 1,451,081 |
|
| 2020 | 1,341,255 |
|
| 2021 | 806,865 |
|
| Thereafter | 1,441,177 |
|
| | $ | 8,244,602 |
|
Purchase Commitment
In the third quarter of 2016 we executed two non-cancelable purchase orders totaling $1.5 million for multiple shipments of tunable lasers to be delivered over an 18-month period beginning in the third quarter of 2016. At December 31, 2016, approximately $1.0 million of this commitment remained under these purchase agreements. In 2016, we also executed two purchase orders for component parts and capital equipment related to our HSOR products in the aggregate amount of $0.7 million for delivery through the second quarter of 2017. As of December 31, 2016, approximately $0.5 million remained outstanding under these purchase orders.
Royalty Agreement
We have licensed certain third-party technologies from vendors for which we owe minimum royalties aggregating $0.9 million payable over the remaining patent terms of the underlying technology.
12. Employee Profit Sharing Plan
We maintain a salary reduction/profit-sharing plan under provisions of Section 401(k) of the Internal Revenue Code. The plan is offered to all permanent employees of Luna Innovations Incorporated and its wholly-owned subsidiaries. We contribute 25% of the salary deferral elected by each employee up to a maximum deferral of 10% of annual salary.
We contributed approximately $305,000 and $180,000 to the plan for the years ended December 31, 2016 and 2015, respectively.
13. Litigation and Other Contingenciesother contingencies
From time to time, we may become involved in litigation in relation to claims arising out of our operations in the normal course of business. While management currently believes it is not reasonably possible the amount of ultimate liability, if any, with respect to these actions will have a material adverse effect on our financial position, results of operations or liquidity, the ultimate outcome of any litigation is uncertain.
In September 2014,December 2018, we received a preliminary audit reportnotice of claim (the "Claim") from Macom Technology Solutions, Inc. ("Macom"), who acquired our HSOR business in August 2017 pursuant to an asset purchase agreement. Under the Defense Contract Audit Agencyasset purchase agreement, we agreed to indemnify Macom for certain matters, including, among other things, the collection of accounts receivable from certain major customers, and placed $4.0 million of the purchase price into an escrow account for the potential settlement of any valid indemnity claims. The Claim received from Macom totaled $2.1 million under various indemnity provisions. We have disputed Macom's assertion of right to payment for the matters described in the Claim. It is uncertain what amount, if any, will be owed in settlement of the Claim. As of December 31, 2019, $1.5 million of the escrow balance had been received with the remaining $2.5 million in the escrow account pending resolution of the dispute.
On July 31, 2018, we sold the assets associated with our optoelectronic components and sub-assemblies ("DCAA"Opto"), business to an unaffiliated third party. The asset purchase agreement provides for additional consideration of up to $1.0 million contingent upon the achievement of a specified revenue level by the sold business during the 18 months following the sale. There have been no amounts recorded in reference to the above matter in the financial statements as of December 31, 2019. It is uncertain what amount, if any, will be received with respect to our 2007 incurred cost submission and questioning $0.8 million of claimed costs that the DCAA believes are expressly unallowable under the Federal Acquisition Regulations and, therefore, subject tothis potential penalty. In June 2015, we received from the Defense Contract Management Agency (the "DCMA") a final determination and demand for payment of penalties, interest, and over billing in the aggregate amount of $1.1 million. In July 2015, we filed an appeal with the Armed Services Board of Contract Appeals ("ASBCA"). In January 2017, a hearing was held before ASBCA. No ruling has yet been issued with respect to our appeal. The appeals process remains ongoing, and we are unable to estimate the amount of loss, if any, that may be realized.
adjustment.
We have made, and will continue to make, efforts to comply with current and future environmental laws. We anticipate that we could incur additional capital and operating costs in the future to comply with existing environmental laws and new requirements arising from new or amended statutes and regulations. In addition, because the applicable regulatory agencies have not yet promulgated final standards for some existing environmental programs, we cannot at this time reasonably estimate the cost for compliance with these additional requirements. The amount of any such compliance costs could be material. We cannot predict the impact that future regulations will impose upon our business.
Obligation under Operating Leases
See Note 10 - Leases for discussion of our lease obligations.
Purchase Commitment
We executed a non-cancelable purchase order totaling $1.1 million in the first quarter of 2018 and a non-cancelable purchase order totaling $1.9 million in the third quarter of 2019 for multiple shipments of tunable lasers to be delivered over an 18-month period. At December 31, 2019, approximately $1.3 million of these commitments remained and is expected to be delivered by December 31, 2020.
Royalty Agreement
We have licensed certain third-party technologies from vendors for which we owe minimum royalties aggregating $0.2 million payable over the remaining patent terms of the underlying technology.
14.15. Employee Profit Sharing Plan
We maintain a salary reduction/profit-sharing plan under provisions of Section 401(k) of the Internal Revenue Code. The plan is offered to all permanent employees. We contribute 30% of the salary deferral elected by each employee up to a maximum deferral of 10% of annual salary.
We contributed approximately $0.4 million and $0.3 million to the plan for the years ended December 31, 2019 and December 31, 2018, respectively.
16. Relationship with Major Customers
During the years ended December 31, 20162019 and 2015,2018, approximately 28%40% and 34%53%, respectively, of our consolidated revenues were attributable to contracts with the U.S. government. In addition, we had one commercial customer whose revenues accounted for 11% of our consolidated revenues for 2016.
At December 31, 20162019 and 2015,2018, receivables with respect to contracts with the U.S. government represented 10%12% and 17%23% of total billed trade receivables, respectively. At December 31, 2016, receivables from the above noted commercial customer represented 19% of our billed trade receivables.
15.17. Financial Information About Segments
Our operations are divided into two operatingreportable segments: Products and Licensing and Technology Development. The Products and Licensing segment develops and sells products or licenses technologies based on commercially viable concepts developed by the Technology Development andsegment. The Products and Licensing. Licensing segment derives its revenue from product sales, funded product development and technology licenses.
Our engineers and scientists collaborate with our network of government, academic and industry experts to identify technologies and ideas with promising market potential. We then compete to win fee-for-service contracts from government agencies and industrial customers who seek innovative solutions to practical problems that require new technology. The Technology Development segment derives its revenue primarily from services. The Technology Development segment provides applied research to customers in our areas of focus.
The Products and Licensing segment develops and sells products or licenses technologies based on commercially viable concepts developed by the Technology Development segment. The Products and Licensing segment derives its revenue from product sales, funded product development and technology licenses.
Our President and Chief Executive Officer and his direct reports collectively represent our chief operating decision makers, and they evaluate segment performance based primarily on revenue and operating income or loss.
Information about the results of operations for each segment is set forth in the table below. There were no significant inter-segment sales during the years ended December 31, 20162019 and 2015.2018.
During the years ended December 31, 20162019 and 2015, 29%2018, 32% and 30%24%, respectively, of our total sales took place outside the United States. Revenues from customersCustomers in China represented approximately 16% and 9%11% of total revenues forin the yearsyear ended December 31, 2016 and 2015, respectively. No2019, while no other single country, outside of the United States, represented more than 10% of total revenues duringin the yearsyear ended December 31, 2016 or 2015.2018.
| | | | Years ended December 31, | Years ended December 31, |
| | | 2016 | | 2015 | | 2019 | | 2018 |
| Products and Licensing revenue | | | $ | 44,491,041 |
| | $ | 21,949,689 |
|
| Technology Development revenue | | $ | 16,825,157 |
| | $ | 13,599,048 |
| | 26,024,674 |
| | 20,967,556 |
|
| Products and Licensing revenue | | 42,385,839 |
| | 30,421,310 |
| |
| Total revenue | | 59,210,996 |
| | 44,020,358 |
| | $ | 70,515,715 |
| | $ | 42,917,245 |
|
| Technology Development operating loss | | (193,244 | ) | | (3,708,710 | ) | |
| Products and Licensing operating loss | | (1,744,091 | ) | | (2,540,885 | ) | |
| Total operating loss | | $ | (1,937,335 | ) | | $ | (6,249,595 | ) | |
| Products and Licensing operating income | | | $ | 1,807,616 |
| | $ | 499,323 |
|
| Technology Development operating income | | | 1,507,405 |
| | 378,212 |
|
| Total operating income | | | $ | 3,315,021 |
| | $ | 877,535 |
|
| Depreciation, Technology Development | | $ | 352,435 |
| | $ | 399,857 |
| | $ | 397,296 |
| | $ | 379,952 |
|
| Depreciation, Products and Licensing | | $ | 1,148,195 |
| | $ | 920,932 |
| | $ | 552,285 |
| | $ | 273,185 |
|
| Amortization, Technology Development | | $ | 195,619 |
| | $ | 88,423 |
| | $ | 91,185 |
| | $ | 130,765 |
|
| Amortization, Products and Licensing | | $ | 2,017,629 |
| | $ | 1,152,072 |
| | $ | 1,462,525 |
| | $ | 418,349 |
|
Additional segment information is as follows:
| | | | December 31, | December 31, |
| | 2016 | | 2015 | 2019 | | 2018 |
| Total segment assets: | | | | | | |
| Products and Licensing | | $ | 48,723,810 |
| | $ | 40,775,211 |
|
| Technology Development | $ | 16,923,090 |
| | $ | 21,203,211 |
| 37,800,250 |
| | 34,823,525 |
|
| Products and Licensing | 38,073,883 |
| | 36,928,602 |
| |
| Total | $ | 54,996,973 |
| | $ | 58,131,813 |
| $ | 86,524,060 |
| | $ | 75,598,736 |
|
| Property plant and equipment and intangible assets, Technology Development | $ | 2,602,803 |
| | $ | 2,917,448 |
| $ | 2,079,486 |
| | $ | 2,103,711 |
|
| Property plant and equipment and intangible assets, Products and Licensing | $ | 15,207,630 |
| | $ | 16,375,215 |
| $ | 22,122,279 |
| | $ | 4,927,453 |
|
16.18. Quarterly Results (unaudited)
The following table sets forth our unaudited historical revenues, operating loss(loss)/income and net (loss)/income by quarter during 20162019 and 2015.2018.
| | | | Quarter Ended | Three Months Ended |
(Dollars in thousands, except per share amounts) | March 31,
2016 | | June 30,
2016 | | September 30,
2016 | | December 31,
2016 | | March 31,
2015 | | June 30,
2015 | | September 30,
2015 | | December 31,
2015 | March 31,
2019 | | June 30,
2019 | | September 30,
2019 | | December 31,
2019 | | March 31,
2018 | | June 30,
2018 | | September 30,
2018 | | December 31,
2018 |
| Revenues: |
| |
| |
| |
| | | | | | | | |
| |
| |
| |
| | | | | | | | |
| Products and licensing | | $ | 8,192 |
| | $ | 11,373 |
| | $ | 11,894 |
| | $ | 13,032 |
| | $ | 4,131 |
| | $ | 4,457 |
| | $ | 5,371 |
| | $ | 7,990 |
|
| Technology development | $ | 3,723 |
| | $ | 4,137 |
| | $ | 4,312 |
| | $ | 4,653 |
| | $ | 2,876 |
| | $ | 3,728 |
| | $ | 3,277 |
| | $ | 3,718 |
| 6,641 |
| | 6,441 |
| | 6,495 |
| | 6,448 |
| | 4,637 |
| | 5,466 |
| | 5,316 |
| | 5,548 |
|
| Products and licensing | 10,264 |
| | 10,510 |
| | 10,307 |
| | 11,305 |
| | 2,463 |
| | 6,298 |
| | 9,928 |
| | 11,732 |
| |
| Total revenues | 13,987 |
| | 14,647 |
| | 14,619 |
| | 15,958 |
| | 5,339 |
| | 10,026 |
| | 13,205 |
| | 15,450 |
| 14,833 |
| | 17,814 |
| | 18,389 |
| | 19,480 |
| | 8,768 |
| | 9,923 |
| | 10,687 |
| | 13,538 |
|
| Gross margin | 4,844 |
| | 5,171 |
| | 5,609 |
| | 6,111 |
| | 2,289 |
| | 4,197 |
| | 4,979 |
| | 5,036 |
| 6,768 |
| | 8,752 |
| | 9,275 |
| | 10,388 |
| | 3,840 |
| | 4,231 |
| | 4,689 |
| | 6,572 |
|
| Operating (loss)/income | (1,352 | ) | | (652 | ) | | (362 | ) | | 427 |
| | (2,615 | ) | | (2,123 | ) | | (723 | ) | | (815 | ) | (898 | ) | | 1,014 |
| | 1,481 |
| | 1,718 |
| | (373 | ) | | 205 |
| | 581 |
| | 423 |
|
| Net (loss)/income from continuing operations | (1,460 | ) | | (771 | ) | | (445 | ) | | 306 |
| | (2,627 | ) | | (2,168 | ) | | (802 | ) | | (414 | ) | |
| Net income/(loss) from continuing operations | | 1,126 |
| | 840 |
| | 1,231 |
| | 2,146 |
| | (272 | ) | | 299 |
| | 1,293 |
| | (122 | ) |
| Income from discontinued operations net of income taxes | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 8,329 |
| — |
| | — |
| | — |
| | — |
| | 421 |
| | 768 |
| | 7,556 |
| | 1,062 |
|
| Net (loss)/income | (1,460 | ) | | (771 | ) | | (445 | ) | | 306 |
| | (2,627 | ) | | (2,168 | ) | | (802 | ) | | 7,914 |
| |
| Net (loss)/income attributable to common stockholders | $ | (1,481 | ) | | $ | (796 | ) | | $ | (474 | ) | | $ | 276 |
| | $ | (2,654 | ) | | $ | (2,189 | ) | | $ | (820 | ) | | $ | 7,893 |
| |
| Net (loss)/income per share from continuing operations: | | | | | | | | | | | | | | | | |
| Basic and diluted | $ | (0.05 | ) | | $ | (0.03 | ) | | $ | (0.02 | ) | | $ | 0.01 |
| | $ | (0.17 | ) | | $ | (0.10 | ) | | $ | (0.03 | ) | | $ | (0.02 | ) | |
| Net income | | 1,126 |
| | 840 |
| | 1,231 |
| | 2,146 |
| | 149 |
| | 1,067 |
| | 8,849 |
| | 940 |
|
| Net income attributable to common stockholders | | $ | 1,043 |
| | $ | 751 |
| | $ | 1,117 |
| | $ | 2,146 |
| | $ | 84 |
| | $ | 1,004 |
| | $ | 8,785 |
| | $ | 873 |
|
| Net income/(loss) per share from continuing operations: | | | | | | | | | | | | | | | | |
| Basic | | $ | 0.04 |
| | $ | 0.03 |
| | $ | 0.04 |
| | $ | 0.07 |
| | $ | (0.01 | ) | | $ | 0.01 |
| | $ | 0.05 |
| | $ | — |
|
| Diluted | | $ | 0.03 |
| | $ | 0.02 |
| | $ | 0.04 |
| | $ | 0.07 |
| | $ | (0.01 | ) | | $ | 0.01 |
| | $ | 0.04 |
| | $ | — |
|
| Net income per share from discontinued operations: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic and diluted | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | 0.30 |
| |
| Net (loss)/income attributable to common stockholders: | | | | | | | | | | | | | | | | |
| Basic and diluted | $ | (0.05 | ) | | $ | (0.03 | ) | | $ | (0.02 | ) | | $ | 0.01 |
| | $ | (0.18 | ) | | $ | (0.10 | ) | | $ | (0.03 | ) | | $ | 0.29 |
| |
| Basic | | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | 0.02 |
| | $ | 0.03 |
| | $ | 0.27 |
| | $ | 0.04 |
|
| Diluted | | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | 0.02 |
| | $ | 0.02 |
| | $ | 0.23 |
| | $ | 0.04 |
|
| Net income attributable to common stockholders: | | | | | | | | | | | | | | | | |
| Basic | | $ | 0.04 |
| | $ | 0.03 |
| | $ | 0.04 |
| | $ | 0.07 |
| | $ | — |
| | $ | 0.04 |
| | $ | 0.31 |
| | $ | 0.03 |
|
| Diluted | | $ | 0.03 |
| | $ | 0.02 |
| | $ | 0.03 |
| | $ | 0.07 |
| | $ | — |
| | $ | 0.03 |
| | $ | 0.27 |
| | $ | 0.03 |
|
| Weighted average shares: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic and diluted | 27,477,181 |
| | 27,557,960 |
| | 27,605,028 |
| | 27,538,606 |
| | 15,117,679 |
| | 21,997,768 |
| | 27,393,392 |
| | 27,464,993 |
| |
| Basic | | 28,039,080 |
| | 28,246,840 |
| | 28,291,297 |
| | 30,159,322 |
| | 27,204,989 |
| | 27,531,361 |
| | 27,901,631 |
| | 28,067,348 |
|
| Diluted | | 33,479,935 |
| | 33,650,790 |
| | 32,115,847 |
| | 32,211,847 |
| | 27,204,989 |
| | 31,506,745 |
| | 33,055,881 |
| | 28,067,348 |
|
17.19. Discontinued Operations
On January 21, 2014,July 31, 2018, we sold the assets and operations related to our assets associated with the developmentOpto business, which was part of fiber optic shape sensingour Products and localizationLicensing segment, to an unaffiliated third party for the medical field to affiliates of Intuitive Surgical, Inc. ("Intuitive"), for total cash consideration ofan initial purchase price up to $30$18.5 million, including $6of which $17.5 million was received at closing and $6 million to be received within 90 days of closing, andhas been properly recorded in the financial statements with the remaining purchase price adjustment up to $18$1.0 million that may be receivedwhich is contingent upon the attainment of specified revenue targets during the eighteen months following the closing of the sale. The Opto business was a component of the operations of API, which we acquired in the future based on the achievementMay 2015, and represented all of certain technical milestones and royalties on system sales, if any. In the transaction, we sold equipment and intellectual property associated with our shape sensing technology. Ten employees were
transferred to Intuitive. Included in the transaction were current assets of totaling approximately $0.2 million and long term assets with a net book value of approximately $0.2 million.
In December 2015, although the required technical milestones specifiedoperations in our agreement with Intuitive had not yet been achieved by Intuitive, weCamarillo, California and Intuitive agreed to settle all remaining future payment obligations related to the technical milestones and royalties on system sales for a lump-sum payment of $9 million, which we received in December 2015.Montreal, Quebec facilities.
We have reported the payment received from Intuitive in 2015results of operations of the Opto business as discontinued operations in our consolidated financial statements.statements for the year ended December 31, 2018. There was no income or loss from discontinued operations for the year ended December 31, 2019. We allocated a portion of the consolidated tax expense to discontinued operations based on the ratio of the discontinued groups’ income orbusiness's loss before allocations.
The following table presents a summary of the transactions related to the sales of Opto in the year ended December 31, 2018:
|
| | | |
| | Year Ended December 31, |
| | 2018 |
| Sale price | $ | 17,500,000 |
|
| Adjusted purchase price | 17,500,000 |
|
| | |
| Assets held for sale | (8,193,184 | ) |
| Liabilities held for sale | 989,453 |
|
| Transaction costs | (858,227 | ) |
| Return of working capital | 730,000 |
|
| Income tax expense | (1,572,245 | ) |
| Gain on sale of discontinued operations | $ | 8,595,797 |
|
The key components of net income from discontinued operations were as follows:
|
| | | | |
| | | Year ended December 31, |
| | | 2015 |
| Net revenues | | $ | — |
|
| Cost of revenues | | — |
|
| Operating expenses | | — |
|
| Loss before income taxes | | — |
|
| Allocated tax benefit | | — |
|
| Operating loss from discontinued operations | | — |
|
| Gain on sale, net of $0.7 million of related income taxes | | 8,326,386 |
|
| Income from discontinued operations, net of income taxes | | $ | 8,326,386 |
|
|
| | | |
| | Year Ended December 31, |
| | 2018 |
| Net revenues | $ | 8,363,606 |
|
| Cost of revenues | 5,294,268 |
|
| Operating expenses | 1,728,113 |
|
| Other (income)/expenses | (13,330 | ) |
| Income before income taxes | 1,354,555 |
|
| Allocated tax expense | 183,921 |
|
| Operating income from discontinued operations | 1,170,634 |
|
| Gain on sale, net of related income taxes | 8,595,797 |
|
| Net income from discontinued operations | $ | 9,766,431 |
|
For the year ended December 31, 2018, the acquisition of property plant and equipment was $0.1 million, intangible property costs were $0.01 million, and depreciation and amortization was $0.2 million, all related to discontinued operations. For the year ended December 31, 2018, proceeds from the sale of the Opto business which were included in cash flows from investing activities $16.0 million. The gain on sale of discontinued operations included in non-cash adjustments to cash flows from operating activities for 2018 was $8.6 million.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
We maintain “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which are controls and other procedures that are designed to provide reasonable assurance that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management
necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. In addition, the design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a control system, misstatements due to error or fraud may occur and not be detected.
Under the supervision and with the participation of our management, including our President and Chief Executive Officer and our Chief Financial Officer, we evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this report. Based on this evaluation, our President and Chief Executive Officer and our Chief Financial Officer have concluded that, as of December 31, 2016,2019, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
ThereThere have been no changes in our internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Securities Exchange Act Rule 13a-15(e) and Rule 15d-15(e) that occurred in the quarter ended December 31, 20162019 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is designed, under the supervision of our principal executive and principal financial officers, and effected by our board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America ("GAAP"). Our internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
There are inherent limitations in the effectiveness of any internal control over financial reporting, including the possibility of human error and the circumvention or overriding of controls. Accordingly, even effective internal control over financial reporting can provide only reasonable assurance with respect to financial statement preparation and may not prevent or detect all misstatements. Further, because of changes in conditions, effectiveness of internal control over financial reporting may vary over time. Our internal control system was designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP.
Under the supervision and with the participation of our management, including our President and Chief Executive Officer, and our Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2016.2019. This evaluation was based on the criteria established in the 2013 Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. As permitted by SEC rules, management's assessment and conclusion on the effectiveness of our internal control over financial reporting as of December 31, 2019, excludes an assessment of the internal control over financial reporting of General Photonics Corporation ("GP"), which were acquired on March 1, 2019. The total acquired assets, based on the preliminary purchase allocation is approximately 6% of our consolidated total assets, excluding the preliminary value of goodwill and intangible assets related to GP, at December 31, 2019. Revenues and income from continuing operations from GP for the period from March 2, 2019 through December 31, 2019, were approximately 7% and 26%, respectively, of our consolidated operations.
Based on our evaluation under the framework established in the 2013 Internal Control—Integrated Framework, our President and Chief Executive officer, and our Chief Financial Officer concluded that our internal control over financial reporting was effective as of December 31, 20162019 to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP.
This Annual Report on Form 10-K includes an attestation report of our independent registered public accounting firm regarding internal control over financial reporting, which appears in Part II, Item 8 of this Annual Report on Form 10-K.
ITEM 9B. OTHER INFORMATION.
None
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by Item 10 of Form 10-K will be included in the proxy statement related to our 20172020 Annual Meeting of Stockholders, (the "2017"2020 Proxy Statement"), anticipated to be filed with the SEC within 120 days after December 31, 2016,2019, and is incorporated into this report by reference.
ITEM 11. EXECUTIVE COMPENSATION.
The information required by Item 11 of Form 10-K is incorporated into this report by reference to the information to be provided in our 20172020 Proxy Statement.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Other than the information below relating to securities authorized for issuance under our equity compensation plans, theThe information required by Item 12 of Form 10-K is incorporated into this report by reference to the information to be provided in our 20172020 Proxy Statement.
EQUITY COMPENSATION PLANS
The following table summarizes our equity compensation plans as of December 31, 2016: |
| | | | | | | | | |
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | | Weighted- average exercise price of outstanding options, warrants and rights (b) | | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) |
| Equity compensation plans approved by security holders | 3,223,114 |
| | $ | 1.96 |
| | 3,749,619 |
|
| Total | 3,223,114 |
| | $ | 1.96 |
| | 3,749,619 |
|
Our 2016 Equity Incentive Plan allows for forfeited awards to be added back to our pool of available awards, including awards forfeited from the 2006 Plan after the expiration date of our 2006 Plan.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by Item 13 of Form 10-K is incorporated into this report by reference to the information to be provided in our 20172020 Proxy Statement.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by Item 14 of Form 10-K is incorporated into this report by reference to the information to be provided in our 20172020 Proxy Statement.
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULE
| |
| (a) | The following documents are filed as part of this Annual Report on Form 10-K: |
| |
| (1) | Financial Statements. See Index to Consolidated Financial Statements at Item 8 of this Report on Form 10-K. |
Schedule II
Luna Innovations Incorporated
Valuation and Qualifying Accounts
| | | Column A | Column B | | Column C | | Column D | | Column E | Column B | | Column C | | Column D | | Column E |
| | Balance at beginning of Period | | Additions | | Deductions | | Balance at end of period | Balance at beginning of Period | | Additions | | Deductions | | Balance at end of period |
| Year Ended December 31, 2015 | | | | | | | | |
| Year Ended December 31, 2018 | | | | | | | | |
| Reserves deducted from assets to which they apply: | | | | | | | | | | | | | | |
| Deferred tax valuation allowance | $ | 12,690,449 |
| | $ | 8,068,653 |
| | $ | — |
| | $ | 20,759,102 |
| $ | 5,020,744 |
| | $ | — |
| | $ | (1,753,211 | ) | | $ | 3,267,533 |
|
| Allowances for doubtful accounts | 134,811 |
| | 10,375 |
| | (10,375 | ) | | 134,811 |
| 286,717 |
| | 3,500 |
| | (6,134 | ) | | 284,083 |
|
| | $ | 12,825,260 |
| | $ | 8,079,028 |
| | $ | (10,375 | ) | | $ | 20,893,913 |
| $ | 5,307,461 |
| | $ | 3,500 |
| | $ | (1,759,345 | ) | | $ | 3,551,616 |
|
| Year Ended December 31, 2016 | | | | | | | | |
| Year Ended December 31, 2019 | | | | | | | | |
| Reserves deducted from assets to which they apply: | | | | | | | | | | | | | | |
| Deferred tax valuation allowance | $ | 20,759,102 |
| | $ | 550,444 |
| | $ | — |
| | $ | 21,309,546 |
| $ | 3,267,533 |
| | $ | — |
| | $ | (2,907,609 | ) | | $ | 359,924 |
|
| Allowances for doubtful accounts | 134,811 |
| | 305,593 |
| | (187,765 | ) | | 252,639 |
| 284,083 |
| | 646,196 |
| | — |
| | 930,279 |
|
| | $ | 20,893,913 |
| | $ | 856,037 |
| | $ | (187,765 | ) | | $ | 21,562,185 |
| $ | 3,551,616 |
| | $ | 646,196 |
| | $ | (2,907,609 | ) | | $ | 1,290,203 |
|
All other schedules are omitted as the required information is inapplicable or the information is presented in the Consolidated Financial Statements and notes thereto in Item 8 of Part II of this Annual Report on Form 10-K.
| |
| (3) | Exhibits. The exhibits filed as part of this report are listed under “Exhibits” at subsection (b) of this Item 15. |
EXHIBIT INDEX
|
| |
| Exhibit No. | Exhibit Document |
2.1(1)2.1# | Findings of Fact, Conclusions of Law, and Order under 11 U.S.C. §§ 1129(a) and (b) and Fed. R. Bankr. P. 3020 Confirming First Amended Joint Plan of Reorganization of Luna Innovations Incorporated and Luna Innovations, Inc., debtors and debtors-in-possession, dated January 12, 2010 (Exhibit 2.1) |
2.2(1) | First Amended Joint Plan of Reorganization of Luna Innovations Incorporated and Luna Technologies, Inc., dated December 18, 2009 (Exhibit 2.2) |
2.3(1) | First Amended Disclosure Statement in support of First Amended Joint Plan of Reorganization of Luna Innovations Incorporated, et al., under Chapter 11 of the Bankruptcy Code, dated December 18, 2009 (Exhibit 2.3) |
2.4(2)* | Asset Purchase Agreement, dated March 1, 2013, by and between Luna Innovations Incorporated and MacAulay-Brown, Inc. (Exhibit 2.4) |
2.5(3)* | Asset Purchase Agreement by and between Luna innovations Incorporated and Luna Technologies, Inc. and Intuitive Surgical Operations, Inc., and Intuitive Surgical International, Ltd., dated as of January 17, 2014 (Exhibit) 2.1) |
2.6(4) | |
3.1(5)2.2# | |
| 2.3# | |
| 2.4# | |
| 3.1 | |
3.2(6)3.2 | |
3.3(7)3.3 | |
3.4(8)3.4 | |
3.4(4)3.4 | |
4.1(9)4.1 | |
4.2(9)4.2 | |
4.3(7)4.3 | |
4.4(10)4.4 | Form of Performance Unit Award Agreement (Exhibit 4.5) |
4.5(39) | |
4.6(39)4.5 | |
4.7(39)4.6 | |
4.8(40)4.7 | |
10.1(11)4.8* | |
| 10.1 | |
10.2(12) | Commercial Lease, dated March 15, 2007, between Canvasback Real Estate & Investments LLC and Luna Innovations Incorporated (705 Dale Avenue, Charlottesville, Virginia) (Exhibit 10.1) |
10.3(9)*10.2** | |
|
10.4(9)* | |
| 10.3** | |
10.5(9)*10.4** | |
10.6(13)10.5 | |
10.7(14)*10.6** | |
10.8(15)10.7 | |
10.9(16)10.8 | Confidential Settlement Agreement, dated as of December 11, 2009, by and between Luna Innovations, Inc. and Luna Technologies, Inc. and Hansen Medical, Inc. (Exhibit 10.26) |
10.10(6) | |
(incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K (File No. 000-52008) filed on January 15, 2010).
|
| 10.9 | |
10.11(6) | Warrant No. 1 to Purchase Common Stock, dated January 13, 2010, issued to Carilion Clinic (Exhibit 10.2) |
10.12(6) | Warrant No. 2 to Purchase Common Stock, dated January 13, 2010, issued to Carilion Clinic (Exhibit 10.3) |
10.13(6) | |
10.14(17)10.10 | |
10.15(18)*10.11** | |
10.16(18)*10.12** | Development and Supply Agreement, effective January 12, 2010, by and among Luna Innovations Incorporated, Luna Technologies, Inc. and Hansen Medical, Inc., as amended on February 17, 2010 and April 2, 2010 (Exhibit 10.7) |
10.17(18)** | |
10.18(18)**10.13 | Amendments to Development and Supply Agreement, effective January 12, 2010 and April 27, 2010, by and between Luna Innovations Incorporated and Intuitive Surgical, Inc. (Exhibit 10.9) |
10.19(18) | Confidential Mutual Release, effective as of January 12, 2010, by and among Luna Innovations Incorporated, Luna Technologies, Inc. and Hansen Medical, Inc. (Exhibit 10.13) |
10.20(18) | Industrial Lease Agreement, dated as of March 21, 2006, by and between Luna Innovations Incorporated and the Economic Development Authority of Montgomery County, Virginia, as amended by a First Amendment effective as of May 11, 2006, a Second Amendment effective as of July 15, 2009 and a Third Amendment effective as of March 23, 2010 (Exhibit 10.14)(incorporated by reference to Exhibit 10.14 to Registrant's Quarterly Report on Form 10-Q (File No. 000-52008) filed on May 17, 2010). |
10.21(18)10.14 | Lease for Riverside Center, dated December 30, 2005, by and between Carilion Medical Center and Luna Innovations Incorporated, as amended by an Amended Lease dated July 20, 2006, a Second Amendment dated on or about October 5, 2007 and a Third Amendment effective as of April 1, 2010 (Exhibit 10.15) |
10.22(18) | Loan and Security Agreement, dated February 18, 2010, by and between Luna Innovations Incorporated, Luna Technologies, Inc. and Silicon Valley Bank (Exhibit 10.5) |
10.23(19) | |
10.24(20)**10.15 | Amendment No.3 to the Development Supply Agreement, dated as of September 2, 2010, by and between Luna Innovations Incorporated and Intuitive Surgical, Inc. (Exhibit 10.5) |
10.25(21) | First Loan Modification Agreement, dated as of March 7, 2011, by and between Luna Innovations Incorporated and Silicon Valley Bank (Exhibit 10.1) |
10.26(22)** | Amendment No. 4 to the Development and Supply Agreement, dated as of March 23, 2011, by and between Luna Innovations Incorporated and Intuitive Surgical, Inc. (Exhibit 10.2) |
10.27(22) | |
10.28(23)10.16 | Second Loan Modification Agreement, dated as of May 18, 2011, by and between Luna Innovations Incorporated, Luna Technologies, Inc. and Silicon Valley Bank (Exhibit 10.1) |
10.29(24) | |
10.30(24)10.17 | |
10.31(24) | Employment Agreement dated March 28, 2012, by and between Dale E. Messick and Luna Innovations Incorporated |
10.32(24) | Employment Agreement dated March 28, 2012,December 5, 2017, by and between Scott A. Graeff and Luna Innovations Incorporated (incorporated by reference to Exhibit 10.25 to the Registrant's Annual Report on Form 10-K (File No. 000-52008) filed on March 21, 2018). |
10.33(25)**10.18 | Amendment No. 5 to Development and Supply Agreement, dated as of March 22, 2012, by and between Luna Innovations Incorporated and Intuitive Surgical, Inc. (Exhibit 10.1) |
10.34(26) | |
10.35(27)10.19** | Development |
10.36(28) | Third Loan Modification Agreement, dated as of May 17, 2012, by and between Luna Innovations Incorporated, Luna Technologies, Inc. and Silicon Valley Bank (Exhibit 10.1) |
10.37(29) | Fourth Loan Modification Agreement, dated March 21, 2013, by and between Luna Innovations Incorporated, Luna Technologies,Intuitive Surgical Operations, Inc. and Silicon Valley Bank (Exhibit 10.1)Intuitive Surgical International, Ltd., dated as of January 17, 2014 (incorporated by reference to Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-Q (File No. 000-52008) filed on May 13, 2014). |
|
| |
10.38(29)10.20 | Fourth Amendment to Luna Innovations Lease of Riverside Center, dated March 21, 2013, by and between Carilion Clinic Properties, LLC and Luna Innovations Incorporated (Exhibit 10.2) |
10.39(30)** | Amendment No. 6 to the Intuitive-Luna Development and Supply Agreement, dated December 15, 2012, by and between Luna Innovations Incorporated and Intuitive Surgical Operations, Inc., a successor in interest to Intuitive Surgical, Inc. (Exhibit 10.2) |
10.40(30)** | Amendment No. 7 to the Intuitive-Luna Development and Supply Agreement, entered into on June 28, 2013, by and between Luna Innovations Incorporated and Intuitive Surgical Operations, Inc., a successor in interest to Intuitive Surgical, Inc. (Exhibit 10.3) |
10.41(31) | Fifth Amendment to Luna Innovations Lease of Riverside Center, dated December 13, 2013, by and between Carilion Clinic Properties, LLC and Luna Innovations Incorporated (Exhibit 10.44) |
10.42(3)** | Cross-License Agreement by and among Luna Innovations Incorporated and Luna Technologies, Inc., and Intuitive Surgical Operations, Inc., and Intuitive Surgical international, Ltd., dated as of January 17, 2014 (Exhibit 10.2) |
10.43(3)** | Consent, Release and Fifth Loan Modification Agreement between Luna Innovations incorporated and Silicon Valley Bank dated as of January 21, 2014 (Exhibit 10.3) |
10.44(32) | |
10.45(32)10.21 | |
10.46(32)10.22 | |
10.47(33)10.23 | Joinder, Consent, and Sixth Loan Modification Agreement between Luna Innovations Incorporated, Luna Technologies, Inc., Advanced Photonix, LLC and Silicon Valley Bank, dated as of May 8, 2015 (Exhibit 10.1) |
10.48(34) | Joinder, Consent, and Seventh Loan Modification Agreement between Luna Innovations Incorporated, Luna Technologies, Inc., Advanced Photonix, LLC and Silicon Valley Bank, dated as of September 29, 2015 (Exhibit 10.1) |
10.49(35) | |
10.50(35)10.24 | Sixth Amendment to Luna Innovations Lease of Riverside Center, dated January 20, 2015, by and between Carilion Clinic Properties, LLC and Luna Innovations Incorporated (Exhibit 10.4) |
10.51(36) | |
10.52(37)10.25 | |
10.53(38)10.26 | Addendum to Asset Purchase |
10.5410.27* | EighthAmended and Restated Loan Modificationand Security Agreement, dated December 15, 2016,October 10, 2019, by and between Luna Innovations Incorporated, Luna Technologies, Inc., Advanced Photonix,Former Luna Subsidiary, Inc., Terametrix, LLC, and General Photonics Corp. and Silicon Valley BankBank. |
21.1(10)10.28* | |
| 10.29* | |
| 10.30* | |
| 21.1 | |
| 23.1 | |
| 24.1 | Power of Attorney (see signature page) |
31.131.1* | |
31.231.2* | |
| 32.1*** | |
| 32.2*** | |
| 101 | The following materials from the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2016,2019, are formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets at December 31, 20162019 and 2015,2018, (ii) Consolidated Statements of Operations for the years ended December 31, 20162019 and 2015,2018, (iii) Consolidated Statements of Changes in Stockholder’s Equity for the years ended December 31, 20162019 and 20152018 (iv) Consolidated Statements of Cash Flows for the years ended December 31, 20162019 and 2015,2018, and (v) Notes to Audited Consolidated Financial Statements. |
| |
(1) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on January 15, 2010 (reporting under Items 1.03, 5.02 and 9.01). The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(2) | Incorporated by reference to the Registrant’s Annual Report on Form 10-K, Commission File No. 000-52008, filed on March 29, 2013. The number in parentheses indicates the corresponding exhibit number in such Form 10-K. |
| |
(3) | Incorporated by reference to the exhibit to the Registrant's Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on May 13, 2014. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(4) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on February 2, 2015. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(5) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on June 8, 2006. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(6) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on January 15, 2010 (reporting under Items 1.01, 3.02, 3.03, 5.03 and 9.01). The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(7) | Incorporated by reference to the exhibit to the Registrant’s Registration Statement on Form S-1, Commission File No. 333-131764, filed on February 10, 2006. The number given in parentheses indicates the corresponding exhibit number in such Form S-1. |
| |
(8) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed with the Securities and Exchange Commission on May 10, 2010. The number in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(9) | Incorporated by reference to the exhibit to Amendment No. 5 of the Registrant’s Registration Statement on Form S-1, Commission File No. 333-131764, filed on April 19, 2006. The number given in parentheses indicates the corresponding exhibit number in such Form S-1. |
| |
(10) | Incorporated by reference to the exhibit to the Registrant’s Annual Report on Form 10-K, Commission File No. 000-52008, filed on March 29, 2016. The number given in parentheses indicates the corresponding exhibit number in such Form 10-K. |
| |
(11) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on July 17, 2009 (reporting under Items 1.01, 5.02 and 9.01). The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(12) | Incorporated by reference to the exhibit to Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on May 15, 2007. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(13) | Incorporated by reference to the exhibit to Amendment No. 1 to Registrant’s Annual Report on Form 10-K, Commission File No. 000-52008, filed on April 6, 2007. The number given in parentheses indicates the corresponding exhibit number in such Form 10-K/A. |
| |
(14) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on June 14, 2007. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(15) | Incorporated by reference to the exhibit to Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on May 9, 2008. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(16) | Incorporated by reference to the exhibit to Registrant’s Annual Report on Form 10-K, Commission File No. 000-52008, filed on March 26, 2010. The number given in parentheses indicates the corresponding exhibit number in such Form 10-K. |
| |
(17) | Incorporated by reference to the exhibit to Registrant’s Annual Report on Form 10-K, Commission File No. 000-52008, filed on March 16, 2009. The number given in parentheses indicates the corresponding exhibit number in such Form 10-K. |
| |
(18) | Incorporated by reference to the exhibit to Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on May 17, 2010. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(19) | Incorporated by reference to the exhibit to Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on August 16, 2010. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(20) | Incorporated by reference to the exhibit to Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on November 15, 2010. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
* Filed herewith
| |
(21)# | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on March 9, 2011. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(22) | Incorporated by reference to the exhibit to Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on May 16, 2011. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(23) | Incorporated by reference to the exhibit to Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on August 12, 2011. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(24) | Incorporated by reference to the exhibit to the Registrant’s Annual Report on Form 10-K, Commission File No. 000-52008, filed on March 29, 2012. The number given in parentheses indicates the corresponding exhibit number in such Form 10-K. |
| |
(25) | Incorporated by reference to the exhibit to the Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on May 10, 2012. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(26) | Incorporated by reference to the exhibit to the Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on August 9, 2012. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(27) | Incorporated by reference to the exhibit to the Registrant’s Amendment No. 1 to Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on August 10, 2012. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(28) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on July 11, 2012. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(29) | Incorporated by reference to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on March 27, 2013. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(30) | Incorporated by reference to the exhibit to the Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on August 8, 2013. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(31) | Incorporated by reference to the exhibit to the Registrant's Annual Report on Form 10-K, Commission File No. 000-52008, filed on April 10, 2014. The number given in parentheses indicates the corresponding exhibit number in such Form 10-K. |
| |
(32) | Incorporated by reference to the exhibit to the Registrant's Annual Report on Form 10-K, Commission File No. 000-52008, filed on March 16, 2015. The number given in parentheses indicates the corresponding exhibit number in such Form 10-K. |
| |
(33) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on May 11, 2015. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(34) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on October 5, 2015. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(35) | Incorporated by reference to the exhibit to the Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on May 14, 2015. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(36) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on August 14, 2015. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(37) | Incorporated by reference to the exhibit to the Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on November 13, 2015. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
(38) | Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, Commission File No. 000-52008, filed on December 29, 2015. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| |
(39) | Incorporated by reference to the exhibit to the Registrant’s Registration Statement on Form S-8, Commission File No. 333-211802, filed on June 3, 2016. The number given in parentheses indicates the corresponding exhibit number in such Form S-8. |
| |
(40) | Incorporated by reference to the exhibit to the Registrant’s Quarterly Report on Form 10-Q, Commission File No. 000-52008, filed on August 10, 2016. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q. |
| |
* | Confidential treatment has been granted with respect to portions of this exhibit, indicated by asterisks, which has been filed separately with the Securities and Exchange Commission. Pursuant to Item 601(b)(2) of Regulation S-K, the schedules and exhibits to this agreement are omitted, but will be furnished to the Securities and Exchange Commission upon request. |
| |
| ** | Confidential treatment has been granted with respect to portions of this exhibit, indicated by asterisks, which has been filed separately with the Securities and Exchange Commission. |
| |
| *** | These certifications are being furnished solely to accompany this annual report pursuant to 18 U.S.C. Section 1350, and are not being filed for purposes of Section 18 of the Securities Exchange Act of 1934 and are not to be incorporated by reference into any filing of the registrant, whether made before or after the date hereof, regardless of any general incorporation language in such filing. |
| |
| ITEM 16. | FORM 10-K SUMMARY |
Not applicable.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
| | | |
| | LUNA INNOVATIONS INCORPORATED |
| | | | |
| | By: | | /s/ Dale E. MessickEugene J. Nestro |
| | | | Dale E. MessickEugene J. Nestro
Chief Financial Officer |
March 20, 201713, 2020
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Scott A. Graeff and Dale E. Messick,Eugene J. Nestro, and each of them acting individually, as his true and lawful attorneys-in-fact and agents, with full power of each to act alone, with full powers of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K with all exhibits thereto and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, with full power of each to act alone, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully for all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or his or their substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
| | | | |
| Signature | | Title | | Date |
| | | | | |
/s/ My E. ChungScott A. Graeff | | President, Chief Executive Officer and Director (Principal Executive Officer) | | March 20, 201713, 2020 |
My E. ChungScott A. Graeff | | |
/s/ Dale E. MessickEugene J. Nestro | | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | | March 20, 201713, 2020 |
Dale E. MessickEugene J. Nestro | | |
| /s/ Michael W. Wise | | Director | | March 20, 201713, 2020 |
| Michael W. Wise | | |
| /s/ Donald Pastor | | Director | | March 20, 201713, 2020 |
| Donald Pastor | | |
| /s/ John B. Williamson III | | Director | | March 20, 201713, 2020 |
| John B. Williamson III | | |
| /s/ N. Leigh Anderson | | Director | | March 13, 2020 |
| N. Leigh Anderson | | |
| /s/ Warren B. Phelps, III | | Director | | March 13, 2020 |
| Warren B. Phelps, III | | |
| /s/ Gary Spiegel | | Director | | March 20, 201713, 2020 |
| Gary Spiegel | | |
| /s/ Mary Beth Vitale | | Director | | March 13, 2020 |
| Mary Beth Vitale | | |
| /s/ Richard W. Roedel | | Chairman of the Board of Directors | | March 20, 201713, 2020 |
| Richard W. Roedel | | |