UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
___________________
FORM 10-K
ý[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 20152020
Commission file number 000-15746
Commission file number 0-15476
VIEWBIX INC.
EMERALD MEDICAL APPLICATIONS CORP.
(Exact Name Ofof Registrant As Specified In Its Charter)
| Delaware | 68-0080601 | |
| (State of Incorporation) | (I.R.S. Employer Identification No.) |
| 14 Aryeh Shenkar Street, Herzliya, Israel | ||
| (Address of Principal Executive Offices) | (ZIP Code) |
Registrant'sRegistrant’s Telephone Number, Including Area Code: +(972) 3-744-45051 (855) 879-8439
Securities Registered Pursuantregistered pursuant to Section 12(g)12(b) of Thethe Act: Common Stock, $0.0001
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, Par Value $0.0001 | VBIX | OTCQB |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] No [X]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes [ ] No [X]
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x[X] No ¨
[ ]
Indicate by check mark if disclosure of delinquent filerswhether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to ItemRule 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant's knowledge, in the definitive proxy or information statements incorporated by reference in Part IIIS-T (§ 232.405 of this Form 10-K or any amendment to this Form 10-K. ¨
On June 30, 2015,chapter) during the aggregate market value of the 5,713,985 common stock held by non-affiliates ofpreceding 12 months (or for such shorter period that the registrant was approximately $3,999,789 based on the closing price of $0.70 of the Registrants common stock on June 30, 2015. On March 31, 2016, the Registrant had 18,624,461 shares of common stock outstanding.required to submit such files). Yes [ ] No [X]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, (as defineda smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act) or a smaller reporting company.Act.
| Large accelerated filer | [ ] | Accelerated filer | [ ] | Non-Accelerated filer | [X] | Smaller reporting company | [X] |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. Yes [ ] No [X]
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes [ ] No [X]
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨[ ] No x
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was $344,778 as of June 30, 2020, based upon the closing price of the common stock on that date, which was $0.0170.
As of March 15, 2021, there were 34,753,669 shares of common stock outstanding.
TABLE OF CONTENTS
| 2 |
Cautionary Statement regarding Forward-Looking Statements
This Annual Report on Form 10-K includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The Registrant has based these forward-looking statements on its current expectations and projections about future events. These forward-looking statements are subject to known and unknown risks, uncertainties and assumptions about the Registrant that may cause its actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by such forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as "may," "will," "should," "could," "would," "expect," "plan," "anticipate," "believe," "estimate," "continue,"“may”, “will”, “should”, “could”, “would”, “expect”, “plan”, “anticipate”, “believe”, “estimate”, “continue”, or the negative of such terms or other similar expressions. Factors that might cause or contribute to such a discrepancy include, but are not limited to, those described in this Annual Report on Form 10-K and in the Registrant'sRegistrant’s other Securities and Exchange Commission filings.
PART I
| 3 |
ITEM 1. DESCRIPTION OF BUSINESSBack to Table of Contents
Overview
Our wholly-ownedOverview and recent developments
Viewbix Inc. (f/k/a Virtual Crypto Technologies, Inc., f/k/a Emerald Medical Applications Corp.) (the “Registrant” or the “Company”) was incorporated in the State of Ohio in 1989 under a predecessor name, Zaxis International, Inc. (“Zaxis”). On August 25, 1995, Zaxis merged with a subsidiary of The InFerGene Company, a Delaware corporation, which entity changed its name to Zaxis International, Inc. and the Company was reincorporated in Delaware under the name of Zaxis International, Inc. On December 30, 2014, Zaxis entered into an agreement with Emerald Medical Applications Ltd., wasa private limited liability company organized as a privately-owned company under the laws of the State of Israel (“Emerald Israel”).
On March 16, 2015, Zaxis and Emerald Israel executed a share exchange agreement, which closed on February 17, 2010.July 14, 2015, and Emerald is digital health startup companyIsrael became the Company’s wholly-owned subsidiary. Emerald Israel was engaged in the business of developing Emerald Israel’s DermaCompare technology and the development, sale and service of imaging solutions utilizing its proprietary DermaCompare software that it developed for use in derma imaging and analytics ("DermaCompare"). Emerald believes thatfor the detection of skin cancer. On January 29, 2018, the Company ceased the DermaCompare operations of its proprietary DermaCompare software represents an advancement in skin cancer screening that should enable physicians to more readily identify and monitor changes in their patients’ skin characteristics.former subsidiary.
Emerald’s DermaCompare solution allows dermatologists and other medical care professionals, usingOn January 17, 2018, the Company formed a setnew wholly-owned subsidiary under the laws of 25 total body photography ("TBP"the State of Israel, Virtual Crypto Technologies Ltd. (the “VCT Israel”), to capture setsdevelop and market software and hardware products facilitating and supporting the purchase and/or sale of skin lesion images with, among other devices, digital cameras, camera-equipped smart phones cryptocurrencies through ATMs, tablets, personal computers (“PCs”) and/or tablets. These images are then transmitted onlinemobile devices. On February 12, 2018, the Registrant filed a definitive information statement to change its name from Emerald Medical Applications Corp. to Virtual Crypto Technologies, Inc. to reflect its new operations and are remotely analyzed by professionals using our DermaCompare software.business focus, and, effective as of March 7, 2018, the Financial Industry Regulatory Authority (“FINRA”) approved the Registrant’s name change and its trading symbol was changed from “MRLA” to “VRCP” on the OTCQB.
Our sales and marketing plan, which has already commenced, is to sell licenses for our DermaCompare imaging software to: NHSs, HMOs, health insurance companies, hospitals and medical clinicsDuring the period commencing January 16, 2018 through distributers, health care channel partners or directlyMarch 23, 2018, the Registrant raised $1.9 million in equity capital through independent salespersons and/or web purchase to dermatologists and other physicians (GPs) that we expectthe offering of units (the “Unit Offering”) at a price of $0.07 per unit, each unit consisting of: (i) one (1) share of the Company’s common stock, par value $0.0001 (the “Shares”); (ii) one (1) warrant to purchase licenses based ona share of the numberCompany’s common stock, par value $0.0001 exercisable for a period of potential numberstwelve months at an exercise price of patients.$0.14 per Share (the “Class F Warrants”); and (iii) one (1) warrant to purchase a share of the Company’s common stock, par value $0.0001 exercisable for a period of twelve months at an exercise price of $0.28 per Share (the “Class G Warrants”). The proceeds of the Unit Offering were utilized by the Registrant to fund the operations of Virtual Crypto Israel including, but not limited to, the costs associated with the development of the products.
In furtherance of our business plan, which has resulted in us becoming an operating company, Emerald hasTransaction with Gix Internet Ltd.
On February 7, 2019, the Registrant entered into a series of agreementsshare exchange agreement (the “Share Exchange Agreement”) with unaffiliated third parties for the distribution of its DermaCompare Technology,Gix Internet Ltd., formerly known as follows:1. On August 12, 2013, Emerald entered into an exclusive distribution with Derma Italy Sri,Algomizer Ltd. (TASE:GIX), a company organized under the laws of the Italy ("Derma Italy"State of Israel (“Gix”), pursuant to which Derma Italyon July 25, 2019 (the “Closing Date”) Gix assigned, transferred and delivered its 99.83% holdings in Viewbix Ltd. (“Viewbix Israel”) to the Company in exchange for shares of restricted common stock, par value $0.0001 per share of the Company (the “Common Stock”), representing 65% of the issued and outstanding share capital of the Company on a fully diluted basis as of the Closing Date, following the conversion of certain convertible notes of the Company and excluding certain warrants to purchase shares of the Common Stock expiring in 2020 and additional warrants as further described below (the “Fully Diluted Share Capital”). In addition, upon the earlier of: (a) the launch of a live video product to an American consumer in the United States by Viewbix Israel, or (b) the launch of an interactive television product to an American consumer in the United States by Viewbix Israel, the Company will issue to Gix an additional 1,642,193 shares of restricted common stock of the Company representing 5% of the Fully Diluted Share Capital immediately following the Closing Date.
| 4 |
On July 24, 2019, the Company filed a Certificate of Amendment to its Certificate of Incorporation with the Secretary of State of Delaware reflecting its name change from Virtual Crypto Technologies, Inc. to Viewbix Inc. to reflect its new operations and business focus and, effective on August 7, 2019, FINRA approved the Registrant’s name change and its trading symbol was granted exclusive distribution rightschanged from “VRCP” to “VBIX” on the OTCQB.
On the Closing Date, (i) the Company issued 20,281,085 shares of its common stock to Gix in Italy;2. exchange for consideration consisting of 99.83% holdings in Viewbix Israel, and (ii) convertible notes representing 3,434,889 shares of Common Stock then currently issued to holders were converted. The shares of Common Stock were issued under Regulation S. The Company also issued a total of 7,298,636 warrants to Gix to purchase shares of Common Stock, whereby (i) 3,649,318 of such warrants were issued with an exercise price of $0.48, and (ii) 3,649,318 of such warrants were issued with an exercise price of $0.80.
Following the Closing Date, Viewbix Israel became a subsidiary of the Registrant. Viewbix Israel was incorporated in February 2006 in Israel.
On December 1, 2013, Emerald enteredJanuary 27, 2020, VCT Israel was sold to a third party for NIS 50,000 ($14,459).
Viewbix Business Overview
Viewbix is an interactive video technology and data platform that provides its client with deep insights into their video marketing performance as well as the effectiveness of its messaging. Viewbix allow companies to add a layer with interactive content on top of a video that allows viewers to engage and interact with the video. The platform measures exactly when a view takes an action while watching a video and collects and reports the results to the client.
Viewbix developed the interactive video platform based on Software as a Service (“SaaS”) business model with interactive elements, and the ability to collect and analyze information about each interactive action performed during the viewing of the video clip. The interactive elements and information gathered allows the client to analyze user viewing habits and optimize real-time throughout the campaign while increasing the effectiveness of online and live video marketing.
Viewbix has adapted its technology platform to work on most nonproprietary platforms on the Internet, including, but not limited to, online video campaigns, brand and image videos, online tutorials, live and real-time video streaming (e.g. music concerts and sporting events), video presentations, and more. Using the Viewbix platform, video creators can integrate advances features into their videos, specifically the inclusion of “click” buttons that trigger a particular action, into a distribution agreement with S. Bokhorst - Creatiekracht, organized undergiven video, like the lawsinsertion of a smart form for retrieving the contact information of the Netherlands, pursuantviewer. Viewbix then collects all the data around the cross section of the viewing data and engagement data and offers its clients the opportunity to which S. Bokhorst was granted exclusive distribution indownload and analyze the Netherlands;3. On February 6, 2014, Emerald entered intoresults. Viewbix also offers a distribution agreement with Medical Edge Pty Ltd, organized underfull service option where the laws of Australia ("Medical Edge"), pursuantViewbix account managers will analyze the data and report results and suggestions to which Medical Edge was granted exclusive distribution rights inits clients.
Notwithstanding the markets of Australia, New Zealand and Oceania;4.foregoing, the Company initiated certain cost reduction measures during fiscal year-ended December 31, 2020. On January 14, 2015, Emerald entered into a Project Agreement with Realize S.A. and Ubitech, entities engaged in IT related to medical technology in Greece, and MEDISP and MPUoP, academic and research institutes in Greece (collectively, the "Greek Partners"). Emerald and the Greek Partners anticipate imminent grants from the Office of Chief Scientist1, 2020, each of the State of IsraelCompany’s former Chief Executive Officer and the General Secretariat for Research and Technology of Greece, respectively, the proceeds of which will be used for development of enhanced smartphone applications for diagnosis of early stage Melanoma.
Utilizing capital raised prior to and subsequentChief Operating Officer tendered their resignations from their respective positions. Moreover, due to the closing ofCompany’s failure to meet predetermined sales targets set forth in the Share Exchange Agreement, Emerald completed the development of a commercial modelCompany determined to reduce the size of its DermaCompare Productsales team and, has commencedlikewise, the R&D team was replaced with a more cost-effective consultant. These decisions, and future decisions related to cost-reduction measures, may impact the Company’s ability to sell and support its products in the future and, accordingly, may materially impact the Company’s business operations.
Industry Overview
Video marketing efforts. Emerald is continuing to negotiate additional distribution agreements for territories including North America, Latin America, Southern Africa, Israel and elsewhere in Europe, among other countries and regions. We believe to generate revenues from our DermaCompare Technology during the first half of fiscal 2016. Emerald is continuing to work on developmentremains one of the "next generation" DermaCompare Technology,fastest growing industries, and, accordingly is increasingly crowded with enhanced features.competition. According to a study published by Cisco, by 2022 online videos will represent 82% of online consumer traffic. Globally, three trillion minutes (or five million years) of video content will cross the Internet each month by 2022, which is the equivalent of 1.1 million minutes of video streamed or downloaded every second.
Notwithstanding our belief that DermaCompare represents a significant advance on existing technologies,Competition
While there are a numbermany companies that offer hosting and streaming services, Viewbix focuses on providing expanded value to its clients that reaches beyond the hosting and streaming platforms. Viewbix has several direct competitors, including Hapyak, which operates primarily via websites, and Innovid, which focuses on advertisements. Additionally, video hosting companies, such as Wistia and Vidyard, both offer certain interactive elements similar to Viewbix. However, Viewbix’s proprietary component is its focus on interactivity and deep data, which results can thereafter be analyzed and applied.
| 5 |
Intellectual Property and Other Proprietary Rights
Our commercial success depends, in part, on obtaining and maintaining patent and other intellectual property protection, in the United States and internationally, for the technologies used in our products. We cannot be sure that any of potential difficultiesour patents will be commercially useful in protecting our technology. Our commercial success also depends in part on our non-infringement of the patents or proprietary rights of third parties. The patent positions can be highly uncertain and involve complex and evolving legal and factual questions.
We have four patents that have been granted to us in the U.S. which we might face,consider material to our business and operating success, including the following:
| ● | ||
| ● | ||
| ● | ||
| ● |
During the twelve months ended December 31, 2015, we raised $989,974We also protect our proprietary technology and processes, in equitypart, by confidentiality and debt capitalinvention assignment agreements with our employees, consultants, scientific advisors and other contractors. These agreements may be breached, and we may not have adequate remedies for any breach. We also rely on trade secrets to protect our product candidates. However, our trade secrets may otherwise become known or be expectedindependently discovered by competitors. To the extent that our employees, consultants, scientific advisors or other contractors use intellectual property owned by others in their work for us, disputes may arise as to require up to an additional $1.5 millionin capital during the next 12 months to fully implement our business planrights in related or resulting know-how and fund our operations.inventions.
Overview of MelanomaProduct Development
Melanoma is a type of skin cancer which forms from melanocytes (pigment-containing cells in the skin), is very aggressive cancer and, at present, there is no cure for Melanoma.

In women, the most common location is the legs. Melanomas in men are most commonly locatedViewbix has been focusing its R&D efforts on the back. It is particularly common among Caucasians, especially northern Europeansexpansion of its interactive live capabilities also collecting the engagement data for each session and northwestern Europeans, as well as those living in sunny climates. Melanoma rates are higher in Oceania, North America, Europe, Southern Africa,relating back to a live stream. This would greatly enhance our client’s feedback on its stream for both real time and Latin America. This geographic pattern reflects the primary cause of Melanoma, ultraviolet light (UV) exposure in conjunction with the amount of skin pigmentation in the population. Melanocytes produce the dark pigment, melanin, which is responsible for the color of skin. These cells predominantly occur in skin, but are also found in other parts of the body, including the bowel and the eye. Melanoma can originate in any part of the body that contains melanocytes.
The treatment includes surgical removal of the tumor. If Melanoma is detected early, while it is still relatively small and thin in depth, and provided that it is timely removed or otherwise treated, the cure rates are very high. The likelihood that the Melanoma will reoccur or spread depends on how deeply it has penetrated into the layers of the skin. For Melanomas that come back or spread, treatments include chemo- and immunotherapy and/or radiation therapy. According to National Cancer Institute statistics, the survival rates in the US after five years are is on average 91%.
While Melanoma is less common than other types of skin cancer, it is far more serious if it is not detected in its early stages. Melanoma causes the vast majority of deaths related to skin cancer. Globally, in 2012, that most recent year for which statistics have been reported, Melanoma occurred in 232,000 people and resulted in 55,000 deaths according to the World Cancer Report 2014 of the World Health Organization ("WHO").
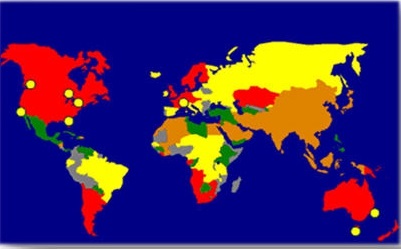
It is estimated that 420 million people across the globe are at high risk of Melanoma (See RED in Image).future stream optimizations.
The Dermatology Device Market
Various devices are used by dermatologists and surgeons to diagnose skin disorders and accurately determine the types of conditions and the treatments required. At present, the dermatology devices market consists of two segments:(i) diagnostic devices market; and (ii) treatment devices market. Our Product is part of the diagnostic device market aimed at increasing the speed and accuracy of skin disorder diagnosis at an early stage.
The respected research firm, "MarketsandMarkets.com," has forecast that the global market for dermatology devices to grow from $6.6 billion in 2014 to $11.3 billion by 2019 and the market in North America, a primary market that we hope to compete in, is expected to reach approximately $5.2 billion by 2019. The key factors expected to drive the forecasted growth are: (i) a rise in skin disorder incidence; (ii) an increase in awareness of available aesthetic procedures; (iii) advances in technology and rising prices; and (iv) the recognition by the population of the harmful effects of to exposure to the sun on skin. All of the forgoing are major contributing factors towards the increasing number of people that become more skin and health conscious.
The global dermatology devices market includes two distinct segments:
Based on the 2014 MarketsandMarkets.com report, imaging techniques accounted for the largest share of the diagnostic devices segment. Skin cancer diagnosis technologies represents the largest share of the device applications market.
The global dermatology devices market is expected to grow faster due to the increasing number of people suffering from skin-related disorders and the number of people opting for less invasive cosmetic surgeries. These are important factors contributing to the increasing demand of dermatology devices, which, in turn, is expected to contribute to demand for our DermaCompare software solution.
Dermatology devices and respective applications are rapidly gaining popularity not only due to their major role in aesthetic but also the rising numbers of skin disorders such as vascular and pigmented lesions, skin cancer, acne problems and others conditions that vary in different regions of the world.
Geographically, we plan to cover four major regions including North America, Europe, Australia and the increasing market in major Asian countries including China, India and Japan, among others. Rising occurrence of skin related ailments along with technological advancements and higher healthcare expenditures have resulted in North America being the largest market for dermatology devices. This trend is expected to continue. In Australia, Melanoma is the fourth most common cancer with 1 in 14 males and 1 in 23 females expected to develop melanomas during their life time. Its incidence has been increasing by approximately 16% in males and approximately 24% in females over the next decade, according to a report by National Health and Medical Research Council (NHMRC) and New Zealand Guidelines Group (NZGG). The Asian-Pacific market is anticipated to be most profitable due to highly untapped opportunities, rising public and physician awareness and improvement in healthcare infrastructure. Skin disorders such as acne, Melasma, dermatitis, skin warts, lesions and moles, especially in China and India, are projected to drive the Asian market.
Our Market Opportunity
The challenge for dermatologists is the detection of skin cancer in its early stages, which is crucial for patient survival. Approximately 60% of melanomas occur as a result of a new mole, while the remaining 40% are the result of a mole that has changed. Since the human body dynamically changes over time, dermatologists are still using manual techniques, which are time-consuming and, as a result, costly, often inaccurate and not readily available for population-wide screening. The most recent innovation in the skin cancer detection field is Total Body Photography ("TBP"), typically a set of 25 photos that cover the entire skin surface of the patient, and was adopted by dermatologists approximately fifteen years ago. At present, dermatologists recommend doing TBP on a yearly basis, comparing the photographs and detecting the key differences.
We believe that the most significant research in skin cancer detection over the last decade has been conducted principally in the state of Schleswig-Holstein, Germany. This has involved the use of manually taken TBP which, from an efficacy study performed for the early detection of skin cancer, found a 30% increase in the early discovery of skin cancer, resulting in approximately 90% of melanomas being diagnosed at an early stage and with mortality rates decreasing by approximately 50% of that expected five years after the study. As a result of the study, since 2008, the country has mandated a nationwide statutory plan for a bi-annual early screening of skin cancer for citizens aged 35.
Based on our estimates, there are approximately 420 million people, representing 7% of the total world population, that can be defined as within the Melanoma high risk group; the majority of which are living in the Western hemisphere. Melanoma patients are more likely to be found in countries with warm and sunny weather. The disease is, however, also prevalent in other regions such as China, India and elsewhere in the Far-East.
It is estimated that approximately 250,000 new cases of Melanoma are diagnosed worldwide each year. Based upon studies conducted by the National Institutes of Health ("NIH") and the Skin Cancer Foundation, an estimated 74,000 new cases of invasive Melanoma will be diagnosed in the US in 2015 with detection more frequently in male Caucasians. At present, Melanoma is the sixth leading cause of cancer mortality in men and the seventh leading cause of cancer fatalities in women. Based on these data, we believe that skin lesion imaging is expected to continue to be a growing market. We believe that current market potential is over $1 billion, although there can be no assurance that we will be able to commercially exploit this large and growing demand.
At present, the most conventional and widely-used visualization method is a standard photograph followed by manual image analysis and then comparing these images with previously taken photographic images to reach a diagnosis. This traditional method has several disadvantages, including the fact that only the outermost layer of skin is imaged and subjected to diagnosis, the visual comparison process is time-consuming, expensive, and often inaccurate because it is dependent on the dermatologists eyes only. The standard conventional photograph method, although inexpensive, is inefficient and laborious for examination purposes and limits the market to dermatologists and specialized physicians.
By revolutionizing the fundamental approach in which skin lesions and/or Melanoma is diagnosed, especially in the early stages, we reasonably expect that our DermaCompare product should be well-positioned to become one of the leading applications in the market, although there can be no such assurance. We hope that this will be achievable by replacing the need for manual photo image analysis with automated image analytics software using advanced algorithms of our DermaCompare process for anchoring, identifying and detecting changes in the shapes, color and sizes of skin lesions. We also plan to utilize available large data bases together with new "computer learning" and "artificial intelligence" techniques to learn from the "wisdom of the crowd" and, based on business analytic tools, we will use as a DSS (Decision Support System) for all range of physicians.
Our DermaCompare imaging software solution should provide several benefits including, but not limited to:
With the rise of the incidences of skin cancer, we believe that the medical community and the general population recognize that it is not only vital to monitor the skin on a regular basis, but it also important to have new means of diagnosing skin lesions more rapidly and accurately. One of the best early indicators of Melanoma is a new or changing mole. If detected in its early stage, Melanoma is almost always treatable. If left untreated for too long, skin Melanoma can become terminal and very difficult or virtually impossible to treat. In addition, if the skin is not monitored on a regular basis, it may be difficult for the patient or doctor to detect new moles or identify changes in existing ones.
Total body photography or TBP, which is part of the procedure used with our DermaCompare technology, is intended for use in detecting and monitoring skin moles and lesions, particularly for individuals considered at high risk for Melanoma. Early detection improves treatment and survival and increases the chance of a full recovery. Our DermaCompare application software is designed to assist dermatologists and other medical practitioners in diagnosing Melanoma quickly and with less effort.
Moreover, the use of computerized technologies with our DermaCompare provides an opportunity to compile, process and store data, thereby creating an extensive database for treating physicians as well as medical researchers. Availability of the data in Internet based SaaS and cloud networks can also provide cross linking between dermatologists, general physicians and/or oncologists.
We believe that this should help to alleviate the relative limited availability or even complete unavailability of suitable data in certain regions and for certain populations and may shed light on skin lesion development into Melanoma.
Our DermaCompare Solution
Our DermaCompare imaging solution is provided as a software platform aimed at early detection of Melanoma based on ABCD Rule for classification of dermatological lesions as published by the National Institute of Health ("NIH") for analysis of moles. The ABCD Rule is defined as follows:
Benign moles usually have a smaller diameter than malignant ones.Our software processes and analyzes derma images of skin lesions, moles or total body images. Our DermaCompare imaging software solution is able to read and extract data from those images and in essence turning digital camera, camera-equipped smart phones and tablets into virtual scanning devices.
Our imaging software can be installed on any desktop computer, smart phone or tablet with either iOS or Android operating systems. The software’s imaging capabilities include image recognition, repair and optimization, dynamic data extraction and several image-specific capabilities.
Our proprietary DermaCompare software combines our core image character recognition technology with advanced image processing capabilities that transform a color skin photograph or total body photograph into a digital image of various sizes and resolutions. Photographs taken by digital cameras or photographs of skin lesions captured by camera-equipped smart phones and tablets are exposed to variable lighting conditions and various angles and focal distances. Raw photos of skin lesions taken by a camera-equipped smart phone or tablet may be of an unknown size and resolution and may often be geometrically distorted, skewed or warped. As a result, an unedited mobile image of a skin lesion may be virtually unusable without the use of our DermaCompare imaging technology.
Our DermaCompare software solution uses advanced algorithms designed to identify and correct geometric and optical distortions and automatically correct each image, zoom in and manipulate both new and old images simultaneously in a corresponding manner to facilitate correct and timely diagnosis. In addition, our DermaCompare software is designed to enable dermatologists and other medical practitioners to review the skin lesion images and digital processing results in a graphical and analytic way.
These images can then be stored on our managed cloud-based servers and our licensee/users will be able to safely access their patients’ images via mobile access or Internet login. We believe that our central image storage solution insures that images and data are secured and kept confidential. We are compliant with HIPAA, the United States Health Insurance Portability and Accountability Act, sets the standard for protecting sensitive patient data. Any company that deals with protected health information must ensure that all the required physical, network, and process security measures are in place and followed.
This includes covered entities, anyone who provides treatment, payment and operations in healthcare, and business associates, anyone with access to patient information and provides support in treatment, payment or operations. Subcontractors, or business associates of business associates, must also be in compliance. Emerald has recently been as a HIPAA compliant company and also using the IBM SoftLayer cloud that already HIPAA compliance.
Practice and Pricing
Our pricing will be based on a fixed-price model, which fees will be charged directly by the App or collected either by the dermatologists, other physicians or medical centers. The process will start with the dermatologist or medical center charging the patient for the total body photography and upload the images through the Internet to a secure, company-owned server. We will invoice the dermatologist or medical center directly on a monthly, per-patient basis. If a patient is to be found to have Melanoma, our pricing model is to waive the fee for this particular patient. We believe that this should serve to incentivize physicians to use our DermaCompare software and encourage patient acceptance of its use.
Our physician/licensees can add new patient accounts to their online account and, at present, our pricing model contemplates that each patient registration will cost US$95 annually.
We will offer our dermatologist/licensees unlimited access to their patients’ images during the one-year period. Each registered patient will also receive a user and password to enable secure access to his/her images through the website or mobile access and enable any other physician to review the images with that patient’s consent ("2nd opinion" model).
We believe that our pricing strategy should make us competitive and is based on the fact that we do not plan on being directly engaged with the end-user and taking and transmitting images to the server. Our strategy is to provide imaging software as a service to dermatologists and medical centers that analyze their own patient’s images.
Maintenance and Product Support
We plan to provide ongoing software support services to assist our medical professional licensees with answers to technical questions and will also maintain customer service department for support with respect to DermaCompare software installation and system maintenance. The majority of the inquiries that we expect to receive will be handled by us via telephone and email. We will maintain our licensees’ software largely through online releases via the Internet that may be downloaded by our licensees with technology enhancements and updated software features. We plan to offer our licensees post-contract support. All of these services are expected to generate significant recurring revenues and shall be typically offered under contract on an annual basis.
Maintenance and support service fees will be deferred and recognized over the contract period on a straight-line basis. Costs incurred by us to provide maintenance and support services will be charged to cost of revenue as incurred.
Intellectual Property
Our success will in large part depend upon our ability to protect our proprietary DermaCompare technology. We plan to protect our intellectual property rights primarily through patents, copyrights, trademarks, trade secrets, employee and third party nondisclosure agreements and other measures.
If we are unable to protect our intellectual property or our intellectual property infringes, for any reason that we do not presently contemplate, on the intellectual property rights of a third party, our operating results would, in all likelihood be materially, adversely affected.
To date, we have not filed for domestic and international patents. Further, we have no registered trademarks, but will continue to evaluate advisability and the costs associated with the registration of trademarks as our management deems appropriate, from time to time.
Sales and Marketing Strategy
Our sales and marketing plan, which has already commenced, is to sell licenses for our DermaCompare imaging software to: NHSs, HMOs, health insurance companies, hospitals and medical clinics through distributers, health care channel partners or directly through independent salespersons and/or web purchase to dermatologists and other physicians (GPs) that we expect to purchase licenses based on the number of potential numbers of patients.
Initially, our marketing strategy for our product is based on a pilot program with worldwide leading dermatologists and medical centers in Israel and Europe in order to improve our DermaCompare software application further.
Subsequently, we plan to market our product worldwide through channel partners, via the Internet as well as through our direct sales force.
We intent to have an internal marketing group that develops our product marketing strategies and executes marketing plans with the support of external resources as needed. We will employ a technically oriented sales force that works with management to identify prospective customers.
Our indirect sales strategy concentrates on distributors and software solution companies that build, integrate and sell software solutions.
Our direct sales strategy will concentrate on health insurance companies, NHS, HMOs, medical centers, dermatologists and other physicians that want to provide our software to their patients. Our sales process will additionally be supported by a broad range of marketing programs, including trade shows, public relations and digital advertising.
In addition, we plan to utilize the following low-cost methods in order to maximize our marketing budget, such as:
Competition
The market for derma image processing software products is intensely competitive, subject to rapid change, and significantly affected by new product introductions and other market activities of industry participants. We face direct and indirect competition from a broad range of competitors who offer a variety of competitive products and solutions to our target markets. Our principal competition will come from: (i) manufacturers of custom-developed solutions; (ii) companies offering automated derma imaging processing systems; and (iii) companies offering competing technologies capable of recognizing and analyzing derma images. Many, if not all of these competing companies will have far greater financial and other resources, established name recognition and lengthy operating histories, any of which could make it difficult for us to compete effectively.
It is also possible that we will face competition from new industry participants and/or alternative technologies. Moreover, as the market for derma imaging software further evolves and develops, a number of companies with significantly greater resources than we have could attempt to enter or increase their presence in our industry, either independently or by acquiring or forming strategic alliances with our competitors, or otherwise increase their focus on the industry. In addition, current and potential competitors have established or may establish cooperative relationships among themselves or with third parties to increase the ability of their products to address the needs of our potential customers.
Our DermaCompare product competes, to various degrees, with products produced by a number of substantial competitors, many of which have far greater financial and other resources and established operating histories with name recognition. Competition among product providers in this market generally focuses on price, accuracy, reliability and technical support. We believe our primary competitive advantages in this market are: (i) flexibility resulting from the ability of our product to operate in Internet based web services environments; (ii) an architectural software design that allows our product to be more readily modified, improved with added functionality and configured for new products, thereby allowing our software to be easily upgraded ; and (iii) combined methodologies of "Big Data and wisdom of the crowd" (which meansanalyzing tens of thousands of electronic medical records, whereby investigators can uncover new risk factors, novel preventive measures and treatments that are the most effective for a range of diseases and conditions)with machine learning and artificial intelligence together with high end machine vision capabilities.Employees
As of December 31, 2020, Viewbix has two employees in management and finance in Israel. Additionally, Viewbix retains the services of two R&D service providers, and a result, we believe that our DermaCompare software Product should differ substantially from whatsales and marketing consultant which is currently availablebased in the market and heretofore has been known as "gold standard." Imaging and analytics is a major sector in the medical device industry and competition is expected to be broad-based and intense. Increased competition may result in price reductions, reduced gross margins and loss of market share, any of which could have a material adverse effect on our business, operating results and financial condition.UK.
The following list of competitors is not intended to be exhaustive, and there are other existing competitors and there likely will be new potential competitors in the future:
DermAlert:The DermAlert software, as presently constituted, is designed to compare images taken by digital camera obtained during a 6 to 12 month period in order to detect new or changing moles through total body photography, by monitoring a specific mole or moles. We believe that their software, at present, cannot define whether a mole is a new one or not.ITEM 1A. RISK FACTORS
Canfield Scientific: Canfield Scientific provides custom photographic systems, image monitoring and centralized analysis services for the pharmaceutical, biotechnology and cosmetics industries. Canfield software is a local based installation and is also expensive to purchase and for this reason is not truly competitive with our DermaCompare software.
DigitalDerm:DigitalDerm’s MoleMap CD technology is a baseline system for early Melanoma detection. Their technology is unique in that it combines total body photography and patented software into a CD-based imaging record that runs on any personal computer with a Windows-based operating system. DigitalDerm’s MoleMap CD applies 35 images as a baseline to compare new moles and moles that are changing or have changed and is based on a local DB, which is considered on older, conventional manually-based solution, not using the "wisdom of the crowd"
FotoFinder Systems:FotoFinder Systems’ Dermoscope is a system for digital dermoscopy, fluorescence diagnosis and standardized photo documentation in dermatology. We do not believe that any of these technologies are used by or are competitive with our DermaCompare software.
Notwithstanding our determination that the above-referenced companies are not actual competitors with our DermaCompare technology, they all have substantially far greater capital, marketing, personnel and other resources, and greater experience in commercializing products and services than we have.
Government Regulation
The Company’s DermaCompare software Product and systems are not subject to FDA or other governmental approval. Any change in current regulatory requirements or related interpretations by or the positions of, governmental agencies, federal or state officials where we plan to market out product could adversely affect our operations.
Employees
Mr. Lior Wyan, CEO and director, and Mr. Oded Gilboa, CFO, constitute our Management team. Mr. Yair Fudim is the Chairman of our board of directors. They are not obligated to contribute any specific number of hours per week to our operations and intend to devote only as much time as they deem necessary to the Company’s affairs until such time that we begin marketing our products and generate revenues. We have entered into employment agreements with Lior Wayn and with Oded Gilboa, Emerald’s CEO and CFO, respectively. Reference is made to the disclosure under Item 11. "Executive Compensation" which contains a summary of the material terms of the respective employment agreements.
At present, Emerald has 10 employees including its CEO, Lior Wayn.
ITEM 1A. RISK FACTORSBack to Table of Contents
The shares of our Common Stock are highly speculative in nature, involve a high degree of risk and should be purchased only by persons who can afford to lose their entire amount invested in the Common Stock. Accordingly, prospective investors should carefully consider, along with other matters referred to herein, the following risk factors in evaluating our business before purchasing any shares of Common Stocks.Stock. If any of the following risks actually occurs, our business, financial condition or operating results could be materially adversely affected. In such case, you may lose all or part of your investment. You should carefully consider the risks described below and the other information in this Prospectus before investing in our Common Stock.
Summary Risk Factors
Our business is subject to numerous risks and uncertainties, including those highlighted in the section titled “Risk Factors” immediately following this prospectus summary. These risks include, among others, the following:
● | We initiated certain cost-reduction measures during the previous fiscal year, which could have long-term adverse effects on our business and we may not realize the operational or financial benefits from such actions; | |
| ● | The COVID-19 pandemic has adversely affected, and will may continue to adversely affect, our business, financial condition, liquidity and results of operations; | |
| ● | Our success depends, in part, upon the continued demand of video as an integral part of corporate marketing and internal communications plans and the continued growth and acceptance of videos as effective alternatives to traditional online and offline marketing products and services; | |
| ● | Due to our evolving business model and rapid changes in the Internet and the nature of services, it is difficult to accurately predict our future performance and may be difficult to increase revenue or profitability; |
| 6 |
| ● | Our customers may reduce or terminate their business relationship with us at any time. If customers representing a significant portion of our revenue reduce or terminate their relationship with us, it could have a material adverse effect on our business, results of operations and financial condition; | |
| ● | Because we have sustained significant turnover to key management positions, we may not have the leadership and personnel with expertise to guide us to profitable operations; | |
| ● | Large and established internet and technology companies, such as Google and Facebook, play a substantial role in the digital advertising market and may significantly impair our ability to operate in this industry; | |
| ● | The advertising/marketing industry is highly competitive. If we cannot compete effectively in this market, our revenues are likely to decline; | |
| ● | If we cannot enforce and protect our intellectual property rights, our business could be adversely affected; | |
| ● | We may in the future be, subject to claims of intellectual property infringement that could adversely affect our business; | |
| ● | Patent terms may be inadequate to protect our competitive position for an adequate amount of time; | |
| ● | We may not be able to protect our systems, technology and infrastructure from cyberattacks; | |
| ● | Our business depends on our ability to collect and use data, and any limitation on the collection and use of this data could significantly diminish the value of our platform and cause us to lose customers and revenue; | |
| ● | Shares of Common Stock issuable upon the conversion of warrants may substantially increase the number of shares of Common Stock available for sale in the public market and depress the price of our Common Stock; | |
| ● | We are subject to compliance with securities law, which exposes us to potential liabilities, including potential rescission rights; | |
| ● | The availability of a large number of authorized but unissued shares of Common Stock may, upon their issuance, lead to dilution of existing stockholders; | |
| ● | We have never paid cash dividends and do not anticipate doing so in the foreseeable future; | |
| ● | Our Common Stock is subject to the “Penny Stock” rules of the SEC and the trading market in our stock is limited, which makes transactions in our stock cumbersome and may reduce the value of an investment; | |
| ● | Since our Common Stock is thinly traded, sale of your holding may take a considerable amount of time; | |
| ● | Shares of Common Stock eligible for future sale may adversely affect the market; | |
| ● | If we fail to maintain effective internal controls over financial reporting, the price of our Common Stock may be adversely affected; | |
| ● | We are required to comply with certain provisions of Section 404 of the Sarbanes-Oxley Act of 2002 and if we fail to comply in a timely manner, our business could be harmed and our stock price could decline; | |
| ● | Our annual and quarterly results may fluctuate, which may cause substantial fluctuations in our Common Stock price; |
| 7 |
| ● | Delaware law contains provisions that could discourage, delay or prevent a change in control of our company, prevent attempts to replace or remove current management and reduce the market price of our stock; | |
| ● | Political, economic and military instability in Israel may impede our ability to operate and harm our financial results; and | |
| ● | Exchange rate fluctuations between foreign currencies and the U.S. Dollar may negatively affect our earnings. |
Risks Associated Withwith Our Business and Industry
Our Independent Registered Public Accounting Firm has expressed substantial doubt as toWe initiated certain cost-reduction measures during the previous fiscal year, which could have long-term adverse effects on our business and we may not realize the operational or financial benefits from such actions.
We initiated certain cost-reduction measures during the previous fiscal year, and we may engage in similar activities in the future. This decision may distract management, could slow improvements in our platform and limit our ability to attract customers. It remains unclear how and to what extent this decision will impact our future business and operating success.
The COVID-19 pandemic has adversely affected, and may continue as a going concern.to adversely affect, our business, financial condition, liquidity and results of operations.
The auditedCOVID-19 pandemic has resulted in a widespread health crisis that has adversely affected businesses, economies and financial statementsmarkets worldwide, placed constraints on the operations of businesses, decreased consumer mobility and activity, and caused significant economic volatility in the United States, Israel and international capital markets. The extent to which COVID-19 impacts our results will depend on future developments, which are highly uncertain and cannot be predicted, including actions to contain COVID-19 or treat its impact and the efficacy and scale of the various vaccines currently deployed in Israel and across the world, among others. Our business has been affected in various ways, including in our operations, and we cannot predict the length and severity of the pandemic. We have been prepared assumingfollowed guidance by the U.S. and Israeli governments and the other local governments in which we operate to protect our employees and our operations during the pandemic and have implemented a remote environment for certain of our employees, and, as a result, may experience inefficiencies in our employees’ ability to collaborate. The COVID-19 pandemic could also affect the health of our consumers. In addition, the COVID-19 pandemic has caused an economic recession, high unemployment rates and other disruptions, both in the United States, Israel and the rest of the world. Any of these impacts, including the prolonged continuation of these impacts, could adversely affect our business.
We cannot predict the other potential impacts of the COVID-19 pandemic on our business or operations, and there is no guarantee that weany near-term trends in our results of operations will continue, as a going concern and do not include any adjustments that might resultparticularly if we cease to continue as a going concern. We believe that in order to continue as a going concern, including the costs of being a public company, we will need approximately $30,000 per year simply to cover the administrative, legal and accounting fees. We have funded these losses primarily through the sale of restricted shares of our Common StockCOVID-19 pandemic and the issuanceadverse consequences thereof continue for a long period of convertible notes, which have subsequently been converted into restricted sharestime. Additional waves of Common Stock.infections, a continuation of the current environment, or any further adverse impacts caused by the COVID-19 pandemic could further deteriorate employment rates and the economy, detrimentally affecting our consumer base and divert consumers’ discretionary income to other uses, including for essential items. These events could adversely impact our cash flows, results of operations and financial conditions and heighten many of the other risks described in these “Risk Factors.”
Our success depends, in part, upon the continued demand of video as an integral part of corporate marketing and internal communications plans and the continued growth and acceptance of videos as effective alternatives to traditional online and offline marketing products and services.
Based on our financial statements for the years ended December 31, 2015We provide a platform that allows companies to understand what messages are resonating with their video viewers and 2014, our independent registered public accounting firm has expressed substantial doubt ashow to our abilityleverage that data to continue asenrich and empower a going concern. To date we have not generated any revenue.
Notwithstanding our success in raising $989,974more effective video experience. Our revenues are derived from the sale of equity and debt securities duringour platform. If the year 2015, there can be no assurance that we will have adequate capital resources or be able todemand for video advertising does not continue to raise equitygrow or customers do not embrace our platform, this could have a material adverse effect on our business and financial condition.
| 8 |
Our success also depends, in part, on our ability to compete for a share of available video advertising/marketing expenditures as more traditional offline and emerging media companies continue to enter the online advertising/marketing market, as well as on the continued growth and acceptance of online advertising generally. If for any reason online advertising is not perceived as effective (relative to traditional advertising), web browsers, software programs and/or debt capitalother applications that limit or prevent advertising from being displayed become commonplace and/or the industry fails to fund planned operations or that any additional fundseffectively manage click fraud, the market for online advertising will be availablenegatively impacted. Any lack of growth in the market for online advertising/marketing (particularly for paid listings) could adversely affect our business, financial condition and results of operations.
Due to us when neededour evolving business model and rapid changes in the Internet and the nature of services, it is difficult to accurately predict our future performance and may be difficult to increase revenue or at all, or, if available, will be availableprofitability.
We developed our platform based on favorable terms orSaaS business model. We do not have an extensive history of ongoing operations in amounts required by us.using our business model from which to predict our future performance, and making such predictions, particularly with regard to the effect of our efforts to aggressively increase the distribution and profitability is very complex and challenging. If we are unable to obtain adequate capital resourcescontinuously improve our platform, this could have a negative effect on our competitiveness and ability to fund operations,service and attract customers. If we are unsuccessful in doing so in a timely fashion, we may not be requiredable to delay, scale backachieve revenue growth or eliminate someincrease our profitability.
Our customers may reduce or allterminate their business relationship with us at any time. If customers representing a significant portion of our plan of operations, which mayrevenue reduce or terminate their relationship with us, it could have a material adverse effect on our business, results of operations and financial condition.
We generally engage with two types of customers: small companies who change from time to time and a number of large companies with whom the engagement is for shorter periods of time. We do not enter into long-term contracts with our customers, and such customers do business with us on a non-exclusive basis. Accordingly, our business is highly vulnerable to adverse economic conditions, market evolution and development of new or more compelling offerings by our competitors, which could either lead to reduced advertising spend generally or motivate our current or potential customers to migrate to our competitors. Any reduction in spending by, or loss of, existing or potential customers would negatively impact our revenue and operating results.
Furthermore, the discretionary, non-exclusive nature of our relationships with customers subjects us to increased pricing pressure. Although we believe our rates are competitive, our competitors may be able to offer more favorable pricing or other advantageous terms. As a result, we may be compelled to reduce our rates or offer other incentives in order to maintain our current customers and attract new customers. If a significant number of customers are able to compel us to charge lower rates or provide rate concessions or incentives, there is no assurance that we would be able to compensate for such price reductions or conserve our profit margins.
Because we have sustained significant turnover to key management positions, we may not have the leadership and personnel with expertise to guide us to profitable operations.
We have sustained significant turnover to our management team. Our success depends in part upon our ability to retain the services of our executive officers and employees. The loss of the services of our executive officers or other employees would have a material adverse effect on our business, operating results and financial condition.
Risks Related to our Competition
Large and established internet and technology companies, such as Google and Facebook, play a substantial role in the digital advertising market and may significantly impair our ability to operate in this industry.
Google is a substantial player in the digital advertising market along with other players such as Microsoft. In addition, a going concern.small number of social network companies, such as Facebook, account for a large portion of digital advertising budgets. The high concentration of power among Google, Facebook and some other large market participants causes us to be subject to any unilateral changes they may make with respect to advertising on their respective platforms, which may be more lucrative than alternative methods of advertising or partnerships with other publishers that are not subject to such changes. Furthermore, we could have limited ability to respond to, and adjust for, changes implemented by large market participants.
| 9 |
These companies, along with other large and established Internet and technology companies, may also leverage their power to make changes to their web browsers, operating systems, platforms, networks or other products or services in a way that impacts the entire digital advertising marketplace.
Our limited operating history does not afford investors a sufficient history on whichThe advertising/marketing industry is highly competitive. If we cannot compete effectively in this market, our revenues are likely to base an investment decision.
decline.
Our wholly-owned subsidiary was incorporated under the laws of the State of Israel on February 17, 2010 and its DermaCompare was fully launched at the beginning of 2015 and has only recently commenced marketing DermaCompare. We are therefore in the very early stage of our marketing plan for Derma Compare. There can be no assurance at this time that we will be able to operate profitably or that we will have adequate working capital to meet our obligations as they become due. Investors must consider the risks and difficulties frequently encountered by early stage companies, particularly in rapidly evolving markets. Such risks include the following:
We cannot be certainface intense competition in the marketplace. We operate in a dynamic market that is subject to rapid development and introduction of new technologies, products and solutions, changing branding objectives, evolving customer demands and industry guidelines, all of which affect our business strategy will be successfulability to remain competitive. There are a large number of companies and advertising technology companies that offer products or services similar to ours and that compete with us for finite advertising budgets. There is also a large number of niche companies that are competitive with us, as they provide a subset of the services that we will successfully address these risks. In the eventprovide. Some of our existing and potential competitors may be better established, benefit from greater name recognition, may offer solutions and technologies that we do not successfully addressoffer or that are more evolved than ours, and may have significantly more financial, technical, sales and marketing resources than we do. In addition, some competitors, particularly those with a larger and more diversified revenue base and a broader offering, may have greater flexibility than we do to compete aggressively on the basis of price and other contract terms as well as respond to market changes. Additionally, companies that do not currently compete with us in this space may change their services to be competitive if there is a revenue opportunity, and new or stronger competitors may emerge through consolidations or acquisitions. If our platform is not perceived as competitively differentiated or we fail to develop adequately to meet market evolution, we could lose customers and market share or be compelled to reduce our prices and harm our operational results.
Risks Related to our Intellectual Property
If we cannot enforce and protect our intellectual property rights, our business could be adversely affected.
We rely on patents, copyright, trademark, domain name and trade secret laws in the United States and similar laws in other countries, as well as licenses and other agreements with our employees, and other parties, to establish and maintain our intellectual property rights in the technology, products and services used in our operations. These laws and agreements may not guarantee that our intellectual property rights will be protected and our intellectual property rights could be challenged or invalidated. Amendments to or interpretations of U.S. patent laws or new rulings around U.S. patent laws may adversely impact our ability to protect our new technologies, content, products and services and to defend against claims of patent infringement. In addition, such intellectual property rights may not be sufficient to permit us to take advantage of current industry trends or otherwise to provide competitive advantages, which could result in costly redesign efforts, discontinuance of offerings, decreased traffic and associated revenue or otherwise adversely affect our business.
We may in the future be, subject to claims of intellectual property infringement that could adversely affect our business.
Many companies (including patent holding companies) and individuals own patents, copyrights, trademarks, and trade secrets and frequently enter into litigation based on allegations of infringement or other violations of intellectual property rights. As we develop and offer our platform through various distribution channels we may experience an increase in the number of intellectual property claims against us. These claims, whether meritorious or not, may result in litigation, may be time-consuming and costly to resolve, and may require expensive changes in our methods of doing business. These intellectual property infringement claims may require us to enter into royalty or licensing agreements on unfavorable terms or to incur substantial monetary liability. Additionally, these risks,claims may result in our being enjoined preliminarily or permanently from further use of certain intellectual property or may require us to cease or significantly alter certain of our operations.
| 10 |
Some of our commercial agreements may require us to indemnify third parties against intellectual property infringement claims, which may require us to use substantial resources to defend against or settle such claims or, potentially, to pay damages. These third parties may also discontinue the use of our platform, as a result of injunctions or otherwise, which could result in loss of revenues and adversely impact our business. Additionally, we may be exposed to liability or substantially increased costs if a commercial partner does not honor its contractual obligation to indemnify us for intellectual property infringement claims made by third parties or if any amounts received are not adequate to cover our liabilities or the costs associated with defense of such claims. The occurrence of any of these events could adversely affect our business.
Patent terms may be inadequate to protect our competitive position for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional or international patent application filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our products are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
Risks Related to Cyber and Data Collection
We may not be able to protect our systems, technology and infrastructure from cyberattacks.
We may be under attack by perpetrators of malicious technology-related events, such as the use of botnets, malware or other destructive or disruptive software, distributed denial of service attacks, phishing, attempts to misappropriate user information and other similar malicious activities. The incidence of events of this nature (or any combination thereof) is on the rise worldwide. While we continuously develop and maintain systems designed to detect and prevent events of this nature from impacting our platform, we have invested (and continue to invest) heavily in these efforts. These efforts are costly and require ongoing monitoring and updating as technologies change and efforts to overcome preventative security measures become more sophisticated.
Any event of this nature that we experience could damage our systems, technology and infrastructure, prevent us from providing our services, compromise the integrity of our services, damage our reputation and/or be costly to remedy, as well as subject us to investigations by regulatory authorities, fines and/or litigation that could result in liability to third parties.
Our business depends on our ability to collect and use data, and any limitation on the collection and use of this data could significantly diminish the value of our platform and cause us to lose customers and revenue.
Our platform receives, collects, stores, processes, transfers and uses certain data about how viewers engaged with videos and helps companies to leverage that data to become a better story teller and optimize the videos. Our ability to access and utilize such data is crucial.
Our ability to either collect or use data could be restricted by new laws or regulations. We are subject to numerous federal, state, local, and international laws, directives and regulations regarding privacy, data protection, and data security and the collection, storing, sharing, use, processing, transfer, disclosure and protection of personal information and other data, the scope of which are changing, subject to differing interpretations, and may be inconsistent among jurisdictions or conflict with other legal and regulatory requirements. We are also subject to certain contractual obligations to third parties related to privacy, data protection and data security. We strive to comply with our applicable policies and applicable laws, regulations, contractual obligations and other legal obligations relating to privacy, data protection and data security to the extent possible. However, the regulatory framework for privacy, data protection and data security worldwide is, and is likely to remain for the foreseeable future, uncertain and complex, and it is possible that these or other actual or alleged obligations may be interpreted and applied in a manner that we do not anticipate or that is inconsistent from one jurisdiction to another and may conflict with other legal obligations or our practices. Further, any significant change to applicable laws, regulations or industry practices regarding the collection, use, retention, security or disclosure of data, or their interpretation, or any changes regarding the manner in which the consent of users or other data subjects for the collection, use, retention or disclosure of such data must be obtained, could increase our costs and require us to modify our services and features, possibly in a material manner, which we may be unable to complete, and may limit our ability to store and process user data or develop new services and features.
| 11 |
If we were found in violation of any applicable laws or regulations relating to privacy, data protection or security, our business may be materially and adversely affected and we would likely have to change our business practices and potentially the services and features available through our platform. In addition, these laws and regulations could impose significant costs on us and could constrain our ability to use and process data in manners that may be commercially desirable. In addition, if a breach of data security were to occur or to be alleged to have occurred, if any violation of laws and regulations relating to privacy, data protection or data security were to be alleged, or if we had any actual or alleged defect in our safeguards or practices relating to privacy, data protection, or data security, our solutions may be perceived as less desirable and our business, prospects, financial condition and results of operations could be materially and adversely affected.
DermaCompare may not be accepted in the marketplace.
Uncertainty exists as to whether our DermaCompare product will be accepted by the market without additional widespread doctor acceptance. A number of factors may limit the market acceptance of our DermaCompare product, including the availability of alternative products and the price of our DermaCompare product relative to alternative products. There is a riskWe also expect that dermatologists or other physicians will be encouraged to continue to use other products and/or methods instead of ours. We are assuming that, notwithstanding the fact that our DermaCompare product is new in the market, dermatologists or other physicians will elect to use DermaCompare because it will permit to safe valuable physician’s time and more subjective analysis. While we intend to continue to build and gather data to demonstrate the benefit of our DermaCompare product, this data gathering may not be conclusive or may be viewed as insufficient by potential users such as dermatologists and other physicians.
Patients have to be persuaded that a certain level of intense self-imaging is justified for the anticipated benefit, but there is no assurance that sufficient numbers of patients will be convinced to enable a successful market to develop for our product.
Our revenues will be dependent upon acceptance of our DermaCompare product by the market. The failure of such acceptance will cause us to curtail or cease operations.
Our revenues are expected to come from the sale of our one DermaCompare product. As a result, we will continue to incur operating losses untilbe new laws, regulations and industry standards concerning privacy, data protection and information security proposed and enacted in various jurisdictions. For example, the European Union’s (“EU”), data protection landscape is currently unstable, resulting in possible significant operational costs for internal compliance and risks to our business. The EU has adopted the General Data Protection Regulation (“GDPR”), which became effective in May 2018, and contains numerous requirements and changes from previously existing EU laws, including more robust obligations on data processors and heavier documentation requirements for data protection compliance programs by companies. Among other requirements, the GDPR regulates the transfer of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the United States. Failure to comply with the GDPR could result in penalties for noncompliance.
In addition to the GDPR, the European Commission has another draft regulation in the approval process that focuses on a person’s right to conduct a private life. The proposed legislation, known as the Regulation of Privacy and Electronic Communications (“ePrivacy Regulation”), would replace the current the current ePrivacy Directive. Originally planned to be adopted and implemented at the same time as sales of our DermaCompare product reaches a mature level and we are able to generate sufficient revenues from the sale of our DermaCompare product to meet our operating expenses. There can be no assurance that dermatologists or other physicians will adopt our DermaCompare product. InGDPR, the event that we are not able to market and significantly increase the number of dermatologists or other physicians that purchase our DermaCompare product, or if we are unable to charge the necessary prices, our financial condition and results of operations will be materially and adversely affected.ePrivacy Regulation is still being negotiated.
DefectsAdditionally, in June 2018, California passed the California Consumer Privacy Act (“CCPA”), which provides new data privacy rights for consumers and new operational requirements for companies. Specifically, the CCPA provides that covered companies must provide new disclosures to California consumers and afford such consumers new abilities to opt-out of certain sales of personal information. The CCPA became operative January 1, 2020. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. We cannot fully predict the impact of the CCPA on our business or malfunctionsoperations, but it may require us to modify our data practices and policies and to incur substantial costs and expenses in our productan effort to comply. Some observers have noted the CCPA could hurt our reputation, sales and profitability.
Our business andmark the levelbeginning of customer acceptance of our DermaCompare product depend upona trend toward more stringent privacy legislation in the effective and reliable operation of our one DermaCompare product. Our DermaCompare product is complex and is continually being modified and improved, and as such may contain undetected defects or errors when first introduced or as new versions are released. To the extent that defects or errors cause our DermaCompare product to malfunction and our customers’ use of our DermaCompare product is interrupted, our reputationUnited States, which could suffer andincrease our potential revenues could declineliability and adversely affect our business. Further in March 2017, the United Kingdom (“U.K.”) formally notified the European Council of its intention to leave the EU pursuant to Article 50 of the Treaty on European Union (“Brexit”). The U.K. ceased to be an EU Member State on January 31, 2020, but enacted, a Data Protection Act substantially implementing the GDPR, effective in May 2018, which was further amended to align more substantially with the GDPR following Brexit. It is unclear how U.K. data protection laws or be delayed while such defects are remedied. We may also be subject to liability for the defects and malfunctions.
There can be no assurance that, despite our testing, errorsregulations will not be found in our DermaCompare product or new releases, resulting in loss of future revenues or delay in market acceptance, diversion of development resources, damage to our reputation, adverse litigation, or increased service and warranty costs, any of which would have a material adverse effect upon our business, operating results and financial condition.
Software failures, breakdownsdevelop in the operations of our serversmedium to longer term and communications systemshow data transfers to and from the U.K. will be regulated. In addition, some countries are considering or the failure to implement system enhancements could harm our business.
Our success depends on the efficienthave enacted legislation requiring local storage and uninterrupted operation of our servers and communications systems. A failure of our network or data gathering procedures could impede the processing of data deliverythat could increase the cost and complexity of databasesdelivering our services.
In addition, failure to comply with the Israeli Privacy Protection Law 1981, and services, clientits regulations as well as the guidelines of the Israeli Privacy Protection Authority, may expose us to administrative fines, civil claims (including class actions) and in certain cases criminal liability. Current pending legislation may result in a change of the current enforcement measures and sanctions.
| 12 |
Any failure or perceived failure by us to comply with our posted privacy policies, our privacy-related obligations to users or other third parties, or any other legal obligations or regulatory requirements relating to privacy, data and day-to-day management of our businessprotection or data security may result in governmental investigations or enforcement actions, litigation, claims or public statements against us by consumer advocacy groups or others and could result in the corruption or loss of data. While all ofsignificant liability, cause our operations will have disaster recovery plansusers to lose trust in place, they might not adequately protect us. Despite any precautions we take, damage from fire, floods, hurricanes, power loss, telecommunications failures, computer viruses, break-ins and similar events at our computer facilities could result in interruptions in the flow of data to our servers and from our servers to our clients. In addition, any failure by our computer environment to provide our required data communications capacity could result in interruptions in our service. In the event of a server failure, we could be required to transfer our client data collection operations to an alternative provider of server hosting services. Such a transfer could result in delays in our ability to deliver our products and services to our clients.
Additionally, significant delays in the planned delivery of system enhancements, improvements and inadequate performance of the systems once they are completed could damage our reputation and harm our business. Long-term disruptions in the infrastructure caused by events such as natural disasters, the outbreak of war, the escalation of hostilities and acts of terrorism, particularly involving cities in which we have offices, could adversely affect our businesses. Although, we plan to carry property and business interruption insurance for our business operations, our coverage might not be adequate to compensate us for all losses that may occur.
We face risks related to the storage of customers’ and their end users’ confidential and proprietary information.
Our DermaCompare product is designed to maintain the confidentiality and security of our customers’ and their end users’ confidential and proprietary data that are stored on our server systems, which may include sensitive personal data. However, any accidental or willful security breaches or other unauthorized access to these data could expose us to liability for the loss of such information, time-consuming and expensive litigation and other possible liabilities as well as negative publicity. Techniques used to obtain unauthorized access or to sabotage systems change frequently and generally are difficult to recognize and react to. We may be unable to anticipate these techniques or implement adequate preventative or reactionary measures.
We might incur substantial expense to further develop our derma DermaCompare product that, once commercialized, may never become sufficiently successful.
Our growth strategy requires the successful launch of our DermaCompare product. Although management will take every precaution to ensure that our DermaCompare product will, with a high degree of likelihood, achieve commercial success, there can be no assurance that this will be the case. The causes for failure of our DermaCompare product once commercialized can be numerous, including:
Compliance with changing regulations concerning corporate governance and public disclosure may result in additional expenses.
In recent years, there have been several changes in laws, rules, regulations and standards relating to corporate governance and public disclosure, including the Dodd-Frank Wall Street Reform and Consumer Protection Act (the "Dodd-Frank Act"), the Sarbanes-Oxley Act of 2002 ("Sarbanes-Oxley") and various other new regulations promulgated by the SEC and rules promulgated by the national securities exchanges.
The Dodd-Frank Act, enacted in July 2010, expands federal regulation of corporate governance matters and imposes requirements on publicly-held companies, including us, to, among other things, provide stockholders with a periodic advisory vote on executive compensation and also adds compensation committee reforms and enhanced pay-for-performance disclosures. While some provisions of the Dodd-Frank Act were effective upon enactment, others will be implemented upon the SEC’s adoption of related rules and regulations. The scope and timing of the adoption of such rules and regulations is uncertain and accordingly, the cost of compliance with the Dodd-Frank Act is also uncertain.
In addition, Sarbanes-Oxley specifically requires, among other things, that we maintain effective internal control over financial reporting and disclosure of controls and procedures. In particular, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting, as required by Section 404 of Sarbanes-Oxley Act ("Section 404"), and our independent registered public accounting firm is required to attest to our internal control over financial reporting.
Our testing, or the subsequent testing by our independent registered public accounting firm may reveal deficiencies in our internal control over financial reporting that are deemed to be material weaknesses. Our compliance with Section 404 will require that we incur substantial accounting expenses and expend significant management efforts. We currently have limited internal audit capabilities and will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. Moreover, if we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identifies deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline, and we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources.
These and other new or changed laws, rules, regulations and standards are, or will be, subject to varying interpretations in many cases due to their lack of specificity. As a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies, which could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. Our efforts to comply with evolving laws, regulations and standards are likely to continue to result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities. Further, compliance with new and existing laws, rules, regulations and standards may make it more difficult and expensive for us to maintain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. Members of our board of directors and our principal executive officer and principal financial officer could face an increased risk of personal liability in connection with the performance of their duties. As a result, we may have difficulty attracting and retaining qualified directors and executive officers, which could harm our business. We continually evaluate and monitor regulatory developments and cannot estimate the timing or magnitude of additional costs we may incur as a result.
We cannot be certain that we will obtain patents for our DermaCompare product and technology or that such patents will protect us from competitors.
We believe that our success and competitive position will depend in part on our ability to obtain and maintain patents for our DermaCompare product, which is both costly and time consuming. We still are in the process to evaluate the patent potentials of our DermaCompare product. Patent Offices typically requires 12-24 months or more to process a patent application. There can be no assurance that any of our potential patent applications will be approved. However, we have decided to launch our DermaCompare product without patent protection. There can be no assurance that any potential patent issued or licensed to us will provide us with protection against competitive products, protect us against changes in industry trends which we have may not have anticipated or otherwise protect the commercial viability of our product, or that challenges will not be instituted against the validity or enforceability of any of our future patents or, if instituted, that such challenges will not be successful. The cost of litigation to uphold the validity of a patent and enforce it against infringement can be substantial. Even issued patents may later be modified or revoked by the Patent and Trademark Office or in legal proceedings. Patent applications in the United States are maintained in secrecy until the patent issues and, since publication of patents tends to lag behind actual discoveries, we cannot be certain that if we obtain patents for our product, we were the first creator of the inventions covered by a pending patent applications or the first to file patent applications on such inventions.
DermaCompare product liability is inherent in the medical devices industry and insurance is expensive and difficult to obtain, we may be exposed to large lawsuits.
Our business exposes us to potential product liability risks, which are inherent in the marketing and sale of medical devices. While we will take precautions we deem to be appropriate to avoid product liability suits against us, there can be no assurance that we will be able to avoid significant product liability exposure. DermaCompare product liability insurance for the medical products industry is generally expensive. We plan to obtain product liability professional indemnity insurance coverage for our DermaCompare product. There can be no assurance that we will be able to obtain such coverage on acceptable terms, or that any insurance policy will provide adequate protection against potential claims. A successful product liability claim brought against us may exceed any insurance coverage secured by us, and could have a material adverse effect on our results or ability to continue marketing our product.
We also plan to obtain Directors and Officers Liability Insurance and certain commercial and personal property insurance.
We may have to establish a reserve funds for potential warranty claims. If we experience warranty claims or if our repair and replacement costs associated with warranty claims will increase significantly, it would have a material adverse effect on our financial condition and results of operations.
We may need to raise additional capital to fund continuing operations and an inability to raise the necessary capital or to do so on acceptable terms could threaten the success of our business.
We currently anticipate that our available capital resources will be sufficient to meet our expected working capital and capital expenditure requirements for the twelve-month ended December 31, 2016. We anticipate that we will require an additional $1.5 million during the next twelve months to fulfill our business plan. However, such resources may not be sufficient to fund the long-term growth of our business. If we determine that it is necessary to raise additional funds, we may choose to do so through strategic collaborations, licensing arrangements, public or private equity or debt financing, a bank line of credit, or other arrangements.
We cannot be sure that any additional funding will be available on terms favorable to us or at all. Any additional equity financing may be dilutive to our stockholders, new equity securities may have rights, preferences or privileges senior to those of existing holders of our shares of common stock. Debt or equity financing may subject us to restrictive covenants and significant interest costs. If we obtain funding through a strategic collaboration or licensing arrangement, we may be required to relinquish our rights to our product or marketing territories. If we are unable to obtain the financing necessary to support our operations, we may be required to defer, reduce or eliminate certain planned expenditures or significantly curtail our operations.
We will need to increase the size of our organization, and may experience difficulties in managing growth.
At present, we are a small company. We expect to experience a period of expansion in headcount, infrastructure and overhead and anticipate that further expansion will be required to address potential growth and market opportunities. Future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate new managers. Our future financial performance and its ability to compete effectively will depend, in part, on its ability to manage any future growth effectively.
The loss of key personnel could adversely affect our business. We may not be able to hire and retain qualified personnel to support our growth.
Emerald’s success depends to a significant extent upon the efforts of Mr. Lior Wayn, its CEO, and other key senior employees and other key personnel. The loss of the services of such personnel could adversely affect our business and our ability to implement our growth plan. We cannot assure you that the services of the members of our management team will continue to be available to us, or that we will be able to find a suitable replacement for any of them. We do not have key man insurance on any members of our management team. If any member of our management team were to die and we are unable to replace either or both of them for a prolonged period of time, we may be unable to carry out our long term business plan and our future prospect for growth, and our business, may be harmed.
Our success is dependent upon our ability to attract, train, manage and retain sales, marketing and other qualified personnel. There is substantial competition for qualified personnel, and an inability to recruit or retain qualified personnel may impact our ability to implement our strategy to grow our business.
During 2015, we granted 534,400 options under our ESOP plan at an exercise price ranging between $0.01 and $0.40. As of December 31, 2015, we had 4,149,719 Class A Warrants, 2,500,000 Class B Warrants, 5,072,492 Class C Unit Warrants and 2,700,000 Class E Warrants outstanding. The Class B Warrants and Class C Unit Warrants were issued to Consultants for bona fide services to the Company as discussed in more detail under the subheading "Sales of Unregistered Securities" in "Market For Common Equity and Related Stockholder Matters" below.
If we are unable to adopt, implement and maintain equity compensation arrangements that provide sufficient incentives, we may be unable to retain our existing employees and attract additional qualified candidates. If we are unable to retain our existing employees, including qualified technical personnel, and attract additional qualified candidates, our business and results of operations could be adversely affected.
Some of our competitors are more established and better capitalized than we are and we may be unable to establish market share.
Some of our competitors are well known, more established and better capitalized than we are. As such, they may have at their disposal greater marketing strength and economies of scale and, as they may have additional products which they sell to the same customers, have greater presence with these customer. They may also have more resources to expend on research and development to create more innovative products in competition with ours. Competition will also likely increase as or when the cost benefits of the Company’s DermaCompare product are established and proven. Accordingly, we may not be successful in competing with them for market share.
We may license or collaborate with third parties in various potential markets.
We believe collaboration will allow us to leverage our resources and to access new markets while avoiding the cost of establishing or maintaining a direct sales force in each market. We may incur significant costs in the use of third parties to identify and assist in establishing relationships with potential collaborators. We currently have no direct sales force. We plan to sell our DermaCompare product first in the dermatology market in Israel, and we intend to slowly later expand geographically in the US and Europe.
To penetrate our target markets, we may need to enter into collaborative agreements to assist in the commercialization of our DermaCompare product. We may choose to license our DermaCompare product for distribution to a third party as opposed to pursuing commercialization ourselves. Establishing strategic collaborations is difficult and time-consuming. Potential collaborators may reject collaborations based upon their assessment of our financial or intellectual property position and our internal capabilities. Discussions with potential collaborators may not lead to the establishment of collaboration agreements on favorable terms and may have the potential to provide collaborators with access to our key intellectual property. We may have limited control over the amount and timing of resources that any future collaborators devote to our DermaCompare product. These collaborators may breach or terminate their agreements with us or otherwise fail to conduct their collaborative activities successfully and in a timely manner. By entering into collaboration, we may preclude opportunities to collaborate with other third parties who do not wish to associate with our existing third party strategic partners. Moreover, in the event of termination of a collaboration agreement, termination negotiations may result in less favorable terms.
Our future sales in international markets will subject us to foreign currency exchange and other risks and costs which could harm our business.
We expect that a substantial portion of our future revenues will be derived from outside Israel; primarily the US and Europe. We will be subject to the effects of exchange rate fluctuations. Our functional currency is the Israel Shekel. For the preparation of our consolidated financial statements, the financial results are translated into U.S. dollars using average exchange rates during the applicable period. If the U.S. dollar appreciates against the Shekel, as applicable, the revenues we recognize from sales will be adversely impacted. Foreign exchange gains or losses as a result of exchange rate fluctuations in any given period could harm our operating results and negatively impact our revenues. Additionally, if the effective price of our products were to increase as a result of fluctuations in foreign currency exchange rates, demand for our DermaCompare products could declinematerially and adversely affect our resultsreputation and business. Furthermore, the costs of operationscompliance with, and financial condition.
We intend not to use hedging strategies to help offsetother burdens imposed by, the effect of fluctuations in foreign currency exchange rates. Movements in foreign currency exchange rates could impact our financial results positively or negatively in one periodlaws, regulations, other obligations and not another, making it more difficult to compare our financial results from period to period.
The healthcare industry is subject to changing policies and procedures, we may find it difficult to continue to compete in an uncertain environment.
The health care industry is subject to changing political, economic and regulatory influences that may affect the procurement practices and operations of healthcare industry participants. During the past several years government regulation of the healthcare industry has changed significantly in several countries. Healthcare industry participants may react to new policies by curtailing or deferring use of new products, including our DermaCompare product. This could substantially impair our ability to successfully market our DermaCompare product, which would have a material adverse effect on our business prospects.
The market success of our DermaCompare product may be dependent in part upon third-party reimbursement policies that are often subjectapplicable to change.
Our ability to successfully penetrate the market with our DermaCompare product may, to some extent, depend on the availability of reimbursement to individuals for using our DermaCompare product from third-party payers, such as governmental programs, private insurance and private health plans. There is no guarantee that usersbusinesses of our DermaCompare product get reimbursed or that a change inusers may limit the futureadoption and use of, levels of reimbursement to individuals and hospitals, if any, will be high enough to allow us to charge a reasonable profit margin. If levels of reimbursement are decreased inreduce the future, theoverall demand for, our DermaCompare productplatform. Additionally, if third parties we work with violate applicable laws, regulations or contractual obligations, such violations may put our users’ data at risk, could diminishresult in governmental investigations or enforcement actions, fines, litigation, claims, or public statements against us by consumer advocacy groups or others and could result in significant liability, cause our abilityusers to selllose trust in us and otherwise materially and adversely affect our DermaCompare products on a profitable basis could be adversely affected.
We may not be ablereputation and business. Further, public scrutiny of, or complaints about, technology companies or their data handling or data protection practices, even if unrelated to successfully expand our business, through acquisitions.
We review corporateindustry or operations, may lead to increased scrutiny of technology companies, including us, and product line acquisition candidates as a part of our growth strategy. If we decidedmay cause government agencies to undertake an acquisition, we may not be ableenact additional regulatory requirements, or to successfully integrate it in order to realize the full benefit of such acquisition. Factorsmodify their enforcement or investigation activities, which may affectincrease our ability to grow successfully through acquisitions include:
costs and risks.
Risks Related to Our Common Stock
Shares of Common Stock issuable upon the conversion of warrants may substantially increase the number of Sharesshares of Common Stock available for sale in the public market and depress the price of our stock.Common Stock.
As of December 31, 2015,2020, we had outstanding: (i) Class AJ Warrants exercisable to purchase 4,149,7193,649,318 shares of Common Stock at an exercise price of $0.48 per share of Common Stock; and (ii) Class K Warrants exercisable to purchase 3,649,318 shares of Common Stock, at an exercise price of $0.80 per Share for two years; (ii) Class B Warrants exercisable to purchase 2,500,000 Shares at an exercise price of $0.40 per Share on a cashless basis for a period of two years; (iii) Class C Unit Warrants are exercisable to purchase 5,072,492 units at an exercise price of $0.40, each unit consisting of one share of Common Stock and one Class A Warrant at an exercise price of $0.80, for a period of ninety (90) days commencing ninety (90) days after the effective date of our Registration Statement; and (ii) Class E Warrants exercisable to purchase 2,700,000 Shares, in three equal tranches of 900,000 Shares, at an exercise price of $0.0001 per Share.Stock.
To the extent any of these Warrantswarrants are exercised and any additional warrants are grantedissued and subsequently exercised, there will be further dilution to our stockholders. Until the warrants expire, these warrant holders will have an opportunity to profit from any increase in the market price of our SharesCommon Stock without assuming the risks of ownership. Holders of options and warrants may exercise these securities at a time when we could obtain additional capital on terms more favorable.
The exercise price of the warrants will dilute the voting interest of the owners of presently outstanding shares of Common Stock by adding a substantial number of additional Sharesshares of our Common Stock. We have reserved Sharesshares of Common Stock for issuance upon the exercise of the warrants and may increase the Sharesshares reserved for these purposes in the future.
The Sharesshares of our Common Stock, which are issuable upon the exercise of any outstanding warrants may be sold in the public market pursuant to Rule 144, if applicable. The sale of our common stockCommon Stock issued or issuable upon the exercise of the warrants and options described above, or the perception that such sales could occur, may adversely affect the market price of our common stock.Common Stock.
We are subject to compliance with securities law, which exposes us to potential liabilities, including potential rescission rights.
We have offered and sold our Common Stock to investors pursuant to certain exemptions from the registration requirements of the Securities Act of 1933, as amended (the “Act”) as well as those of various state securities laws. The basis for relying on such exemptions is factual; that is, the applicability of such exemptions depends upon our conduct and that of those persons contacting prospective investors and making the offering. We have not received a legal opinion to the effect that any of our prior offerings were exempt from registration under any federal or state law. Instead, we have relied upon the operative facts as the basis for such exemptions, including information provided by investors themselves.
| 13 |
If any prior offering did not qualify for such exemption, an investor would have the right to rescind its purchase of the securities if it so desired. It is possible that if an investor should seek rescission, such investor would succeed. A similar situation prevails under state law in those states where the securities may be offered without registration in reliance on the partial preemption from the registration or qualification provisions of such state statutes. If investors were successful in seeking rescission, we would face severe financial demands that could adversely affect our business and operations. Additionally, if we did not in fact qualify for the exemptions upon which it has relied, we may become subject to significant fines and penalties imposed by the SECU.S. Securities and Exchange Commission (the “SEC”) and state securities agencies.
Our Executive Officers, Directors and the Chief Executive Officer of Emerald own over 29.4% of our common stock and may be able to influence the outcome of stockholder votes and their interests may differ from other stockholders.
As of March 31, 2016, our executive officers and directors beneficially own 7,002,868 Shares of our Common Stock representing approximately 37.6% of our outstanding Shares, excluding Shares underlying the Class E Warrants, the exercise of which are subject to certain Milestones which are not expected to be reached within 60 days. Subject to any fiduciary duties owed to our other stockholders under Delaware law, these stockholders may be able to exercise significant influence over matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions, and will have some control over our management and policies. Some of these persons may have interests that are different from yours. For example, these stockholders may support proposals and actions with which you may disagree. The concentration of ownership could delay or prevent a change in control of the Company or otherwise discourage a potential acquirer from attempting to obtain control of the Company, which in turn could reduce the price of our stock. In addition, these stockholders could use their voting influence to maintain our existing management and directors in office, delay or prevent changes in control of the Company, or support or reject other management and board proposals that are subject to stockholder approval, such as amendments to our employee stock plans and approvals of significant financing transactions.
The availability of a large number of authorized but unissued shares of Common Stock may, upon their issuance, lead to dilution of existing stockholders.
We are authorized to issue 490,000,000 shares of Common Stock, $0.0001 par value per share, of which, as of MarchDecember 31, 2016, 18,624,4612020, 34,753,669 shares of Common Stock were issued and outstanding. Additional shares of Common Stock may be issued by our board of directors without further stockholder approval. The issuance of large numbers of shares, possibly at below market prices, is likely to result in substantial dilution to the interests of other stockholders. In addition, issuances of large numbers of shares of Common Stock may adversely affect the market price of our Common Stock.
Our Certificate of Incorporation authorizes 10,000,000 shares of preferred stock, $0.0001 par value $0.0001 per share of which none were issued and outstanding as of the date of this registration statement.December 31, 2020. The board of directors is authorized to provide for the issuance of these unissued shares of preferred stock in one or more series, and to fix the number of shares and to determine the rights, preferences and privileges thereof. Accordingly, the board of directors may issue preferred stock which may convert into large numbers of shares of Common Stockcommon stock and consequently lead to further dilution of other shareholders.stockholders.
We have never paid cash dividends and do not anticipate doing so in the foreseeable future.
We have never declared or paid cash dividends on our common shares.Common Shares. We currently plan to retain any earnings to finance the growth of our business rather than to pay cash dividends. Payments of any cash dividends in the future will depend on our financial condition, results of operations and capital requirements, as well as other factors deemed relevant by our board of directors.
Our Common Stock is subject to the "Penny Stock"“Penny Stock” rules of the SEC and the trading market in our stock is limited, which makes transactions in our stock cumbersome and may reduce the value of an investment.
The Securities and Exchange CommissionSEC has adopted Rule 15g-9 which establishes the definition of a "penny“penny stock,"” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require:
| ● | That a broker or dealer approve a person’s account for transactions in penny stocks; and |
| ● | The broker or dealer receives from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased. |
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must:
| ● | Obtain financial information and investment experience objectives of the person; and |
| ● | Make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks. |
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the CommissionSEC relating to the penny stock market, which, in highlight form:
| 14 |
| ● | Sets forth the basis on which the broker or dealer made the suitability determination; and |
| ● | That the broker or dealer received a signed, written agreement from the investor prior to the transaction. |
Generally, brokers may be less willing to execute transactions in securities subject to the "penny stock"“penny stock” rules. This may make it more difficult for investors to dispose of our Common Stock and cause a decline in the market value of our stock.Common Stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Financial Industry Regulatory Authority, Inc. ("FINRA") sales practice requirements may limit a shareholder’s ability to buy and sellSince our Common Stock.
In addition to the "penny stock" rules described above, FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our Common Stock which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares.
Our stock is thinly traded, sale of your holding may take a considerable amount of time.
The shares of our Common Stock are thinly-traded on the OTCQB Market, meaning that the number of persons interested in purchasing our Common Stock at or near bid prices at any given time may be relatively small or non-existent. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our Common Stock will develop or be sustained, or that current trading levels will be sustained. Due to these conditions, we can give you no assurance that you will be able to sell your shares at or near bid prices or at all if you need money or otherwise desire to liquidate your shares.
Shares of Common Stock eligible for future sale may adversely affect the market.
From time to time, certain of our stockholders may be eligible to sell all or some of their shares of Common Stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144 promulgated under the Securities Act, subject to certain limitations. In general, pursuant to amended Rule 144, non-affiliate stockholders may sell freely after six months, subject only to the current public information requirement. Affiliates may sell after six months, subject to the Rule 144 volume, manner of sale (for equity securities), current public information and notice requirements. Any substantial sales of our Common Stockcommon stock pursuant to Rule 144 may have a material adverse effect on the market price of our Common Stock.
If we fail to maintain effective internal controls over financial reporting, the price of our Common Stock may be adversely affected.
We identified a material weakness in our period and our financial reporting process. Our internal control over financial reporting may have material weaknesses and conditions that could require correction or remediation, the disclosure of which may have an adverse impact on the price of our Common Stock. We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish those controls, or any failure of those controls once established, could adversely affect our public disclosures regarding our business, prospects, financial condition or results of operations. In addition, management’s assessment of internal controls over financial reporting may identify material weaknesses and conditions that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting or disclosure of management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our Common Stock.
We are required to comply with certain provisions of Section 404 of the Sarbanes-Oxley Act of 2002 and if we fail to comply in a timely manner, our business could be harmed and our stock price could decline.
Rules adopted by the SEC pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 require an annual assessment of internal controls over financial reporting, and for certain issuers an attestation of this assessment by the issuer’s independent registered public accounting firm. The standards that must be met for management to assess the internal controls over financial reporting as effective are evolving and complex, and require significant documentation, testing, and possible remediation to meet the detailed standards.
| 15 |
We expect to incur expenses and to devote resources to Section 404 compliance on an ongoing basis. It is difficult for us to predict how long it will take or costly it will be to complete the assessment of the effectiveness of our internal control over financial reporting for each year and to remediate any deficiencies in our internal control over financial reporting. As a result, we may not be able to complete the assessment and remediation process on a timely basis. In addition, although attestation requirements by our independent registered public accounting firm are not presently applicable to us, we could become subject to these requirements in the future and we may encounter problems or delays in completing the implementation of any resulting changes to internal controls over financial reporting. In the event that our Chief Executive Officer orand Chief Financial Officer, determinewhich currently is the same individual, determines that our internal control over financial reporting is not effective as defined under Section 404, we cannot predict how the market prices of our shares of Common Stock will be affected; however, we believe that there is a risk that investor confidence and share value may be negatively affected.
Our share price could be volatile and our trading volume may fluctuate substantially.
The price of our common shares has been and may in the future continue to be extremely volatile, with the sale price fluctuating from a low of $0.44 to a high of $2.24 since 2012. Many factors could have a significant impact on the future price of our common shares, including:
Our annual and quarterly results may fluctuate, which may cause substantial fluctuations in our common stockCommon Stock price.
Our annual and quarterly operating results may in the future fluctuate significantly depending on factors including the timing of purchase orders, new product releases by us and other companies, gain or loss of significant customers, price discounting of our product, the timing of expenditures, product delivery requirements and economic conditions. Revenues related to our product are required to be recognized upon satisfaction of all applicable revenue recognition criteria. The recognition of revenues from our product is dependent on a number of factors, including, but not limited to, the terms of any license agreement and the timing of implementation of our products by our customers.
Any unfavorable change in these or other factors could have a material adverse effect on our operating results for a particular quarter or year, which may cause downward pressure on our common stock price. We expect quarterly and annual fluctuations to continue for the foreseeable future.
Delaware law contains provisions that could discourage, delay or prevent a change in control of our company, prevent attempts to replace or remove current management and reduce the market price of our stock.
Provisions in our certificate of incorporation and bylaws may discourage, delay or prevent a merger or acquisition involving us that our stockholders may consider favorable. For example, our certificate of incorporation authorizes our board of directors to issue up to ten million shares of "blank check"“blank check” preferred stock. As a result, without further stockholder approval, the board of directors has the authority to attach special rights, including voting and dividend rights, to this preferred stock. With these rights, preferred stockholders could make it more difficult for a third party to acquire us.
We are also subject to the anti-takeover provisions of the DGCL.Delaware General Corporation Law (the “DGCL”). Under these provisions, if anyone becomes an "interested“interested stockholder,"” we may not enter into a "business combination"“business combination” with that person for three years without special approval, which could discourage a third party from making a takeover offer and could delay or prevent a change in control of us. An "interested stockholder"“interested stockholder” is, generally, a stockholder who owns 15% or more of our outstanding voting stock or an affiliate of ours who has owned 15% or more of our outstanding voting stock during the past three years, subject to certain exceptions as described in the DGCL.
| 16 |
Risks Related to our Operations in Israel
Political, economic and military instability in Israel may impede our ability to operate and harm our financial results.
Our offices and management team are located in the Tel-Aviv metropolitan area, Israel. Accordingly, political, economic, and military conditions in Israel and the surrounding region may directly affect our business and operations. In recent years, Israel has been engaged in sporadic armed conflicts with Hamas, an Islamist terrorist group that controls the Gaza Strip, with Hezbollah, an Islamist terrorist group that controls large portions of southern Lebanon, and with Iranian-backed military forces in Syria. In addition, Iran has threatened to attack Israel and may be developing nuclear weapons. Some of these hostilities were accompanied by missiles being fired from the Gaza Strip against civilian targets in various parts of Israel, including areas in which our employees and some of our consultants are located, and negatively affected business conditions in Israel. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its trading partners could adversely affect our operations and results of operations Our commercial insurance does not cover losses that may occur as a result of events associated with war and terrorism. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained or that it will sufficiently cover our potential damages. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts or political instability in the region would likely negatively affect business conditions and could harm our results of operations.
Further, in the past, the State of Israel and Israeli companies have been subjected to economic boycotts. Several countries still restrict business with the State of Israel and with Israeli companies. These restrictive laws and policies may have an adverse impact on our operating results, financial condition or the expansion of our business. A campaign of boycotts, divestment and sanctions has been undertaken against Israel, which could also adversely impact our business.
In addition, many Israeli citizens are obligated to perform several days, and in some cases more, of annual military reserve duty each year until they reach the age of 40 (or older, for reservists who are military officers or who have certain occupations) and, in the event of a military conflict, may be called to active duty. In response to increases in terrorist activity, there have been periods of significant call-ups of military reservists. It is possible that there will be military reserve duty call-ups in the future. Our operations could be disrupted by such call-ups, which may include the call-up of members of our management. Such disruption could materially adversely affect our business, prospects, financial condition and results of operations.
Exchange rate fluctuations between foreign currencies and the U.S. Dollar may negatively affect our earnings.
Our reporting and functional currency is the U.S. dollar. Our revenues are currently primarily payable in U.S. dollars and Euros and we expect our future revenues to be denominated primarily in U.S. dollars and Euros. However, certain amount of our expenses are in NIS and as a result, we are exposed to the currency fluctuation risks relating to the recording of our expenses in U.S. dollars. We may, in the future, decide to enter into currency hedging transactions. These measures, however, may not adequately protect us from material adverse effects.
ITEM 1B. UNRESOLVED STAFF COMMENTSBack to Table of Contents
None.
ITEM 2. DESCRIPTION OF PROPERTIESBack to Table of ContentsNone.
Our principal executive office is located at SOSA house, 12 Bar Yochayst, Tel Aviv 665320, Israel, Telephone: (972) 52-579-5082. This office consist of approximately 300 square feet of executive office space, which is provided to us on a rent-free basis. Our wholly-owned subsidiary has offices at the same address, which it leases from an unaffiliated third party for $1,600 per month. The Registrant believes that the office facilities are sufficient for the foreseeable future.
None.
ITEM 3. LEGAL PROCEEDINGBack
We are currently not involved in any litigation that we believe could have a material adverse effect on our financial condition or results of operations, except as set forth below. There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to Tablethe knowledge of Contentsthe executive officers of the Company, threatened against or affecting the Company, our Common Stock, our officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect, other than as set forth below.
| 17 |
On August 7, 2019, Viewbix Ltd. was named as a co-defendant in a civil lawsuit filed with the Jerusalem District Court (the “Jerusalem Court”) by three shareholders of Viewbix Ltd. (in this section, the “Shareholders”), alleging that they were entitled to receive certain preferred shares in Viewbix Ltd., pursuant to a certain 2007 loan agreement by and between Viewbix Ltd. and the petitioning Shareholders, following the sale of Viewbix Ltd. shares to Gix (the “Conversion”). The Shareholders sought declaratory recourse from the Jerusalem Court, pursuant to which the Shareholders demanded, inter alia, shares in Gix on a post-Conversion basis or in an alternative form of compensation. On February 27, 2020, the parties presented their respective arguments before the Jerusalem Court, and the Jerusalem Court determined that the Company is entitled to file a motion for dismissal of the claims by the Shareholders by March 31, 2020, which was subsequently postponed to August 9, 2020. On September 24, 2020, the parties submitted before the Jerusalem Court a settlement proposal, which was thereafter approved by the Jerusalem Court on the same date (the “Settlement”). The Settlement provided, inter alia, that in exchange for the voluntary waiver of claims held by the Shareholders, Gix instructed the trustee holding the instant shares of Gix, which were issued by Gix as part of the transaction where Gix acquired share capital of Viewbix in November 2018, to issue 63,350 shares of Common Stock owned by Gix to the Shareholders, whereby the remaining shares in the trust account will be used to indemnify Gix for any expenses related to the instant litigation. Since the aforementioned compensation was provided in the form of shares of Common Stock previously owned by Gix, the settlement has no effect on the Company’s financial statements.
None.
In June 2017, a lawsuit was filed with the Regional Labor Court in Tel Aviv (the “Tel Aviv Court”) against Emerald Israel, and other defendants, claiming certain damages in the total amount of approximately $225,000, under the assertion of wrongful termination by Emerald Israel. We believe these claims to be unsubstantiated and wholly without merit and accordingly filed our response with the Tel Aviv Court in October of 2017. The dispute was initially heard by the Tel Aviv Court on February 13, 2020. In a supplemental hearing on February 11, 2021, the plaintiff provided a certified confirmation of payment of approximately $14,668 by the National Insurance Institute of Israel for one month’s prior notice of termination, redemption of 16.8 days of vacation and severance pay. The plaintiff’s summaries were filed on March 11, 2021, and the defendant’s summaries will be filed within 30 days of receiving the plaintiff’s summaries.
ITEM 4. MINE SAFETY DISCLOSURESBack to Table of Contents
None.
PART IINone.
| 18 |
ITEM 5. MARKET FOR REGISTRANT'SREGISTRANT’S COMMON STOCK, AND RELATED STOCKHOLDER MATTERMATTERS AND ISSUER PURCHASE OF EQUITYBack to Table of Contents
Market Information
Our common stockCommon Stock is currently quoted on the OTCQB market under the symbol MRLA. ForVBIX. The following table, adjusted for a one-for-fifteen (1:15) reverse split that became effective on May 20, 2019, sets forth for the respective periods indicated the following table sets forthprices of our Common Stock in the highOTC Link Alternative Trading System. Such prices are based on inter-dealer bid and low bidasked prices, per share of common stock. The below prices, adjusted for a one-for-four (1:4) reverse split effective March 20, 2015, represent inter-dealer quotations without retail markup, markdown, commissions, or commissionadjustments and may not necessarily represent actual transactions.
Fiscal 2015 | Fiscal 2014 | Fiscal 2013 | ||||||||||||||||
High | Low | High | Low | High | Low | |||||||||||||
First Quarter ended March 31 | $ | 0.20 | $ | 0.11 | $ | 0.14 | $ | 0.11 | $ | 0.70 | $ | 0.12 | ||||||
Second Quarter ended June 30 | $ | 2.24 | $ | 0.45 | $ | 0.14 | $ | 0.14 | $ | 0.70 | $ | 0.12 | ||||||
Third Quarter ended September 30 | $ | 2.24 | $ | 1.00 | $ | 0.14 | $ | 0.14 | $ | 0.34 | $ | 0.12 | ||||||
Fourth Quarter ended December 31 | $ | 1.25 | $ | 1.00 | $ | 0.40 | $ | 0.14 | $ | 0.12 | $ | 0.11 | ||||||
During the fiscal year ended December 31, 2020 and the fiscal years ended December 31, 2019, we had the following trading history:
| Fiscal 2020 | Fiscal 2019 | ||||||||||||||||
| High | Low | High | Low | ||||||||||||||
| First Quarter ended March 31 | $ | 0.05 | $ | 0.02 | $ | 0.05 | 1 | $ | 0.03 | 1 | |||||||
| Second Quarter ended June 30 | $ | 0.03 | $ | 0.01 | $ | 1.12 | $ | 0.05 | |||||||||
| Third Quarter ended September 30 | $ | 0.03 | $ | 0.01 | $ | 0.86 | $ | 0.12 | |||||||||
| Fourth Quarter ended December 31 | $ | 0.02 | $ | 0.01 | $ | 0.12 | $ | 0.03 | |||||||||
| 1 | Adjusted for a one-for-fifteen (1:15) reverse split that became effective on May 20, 2019 (prior to the Recapitalization Transaction). |
Holders of Common Stock
As of December 31, 2015,2020, there were approximately 2,697 stockholders of record of our Common Stock and 34,753,669 shares of common stock were held by approximately 2,540 stockholders of record. Ourour Common Stock outstanding.
Our transfer agent is Transfer Online, 512 SE Salmon Street, Portland, OR 97214-3444, Phone: (503) 227-2950.
Dividends
Dividends
Holders of common stockCommon Stock are entitled to dividends if declared by the Boardour board of Directors,directors, out of funds legally available therefore. We have never declared cash dividends on our common stockCommon Stock and our Boardboard of Directorsdirectors does not anticipate paying cash dividends in the foreseeable future as it intends to retain future earnings to finance the growth of our businesses.
Rule 144 Shares
As of the date of this Registration Statement, we do not have any significant number of shares of our Common Stock that are currently available for sale to the public in accordance with the volume and trading limitations of Rule 144. This is due to the fact that shares of our Common Stock that were issued prior to the end of May 2015, at which time we ceased to be a shell company, as a result of our effective control of the business and financial operations and decisions of Emerald, were deemed to be a "shell" company as that term is defined under Rule 405 and Rule 144(i) promulgated by the SEC under the Act.
Option Grants
During 2015, we granted 534,400 options under our ESOP plan at an exercise price ranging between $0.01 and $0.40.
Outstanding Warrants
The following table summarizes information of outstanding warrants as of December 31, 2015:2020:
| Warrants | Warrant Term | Exercise Price | Exercisable | |||||||||
| Investors - Class A Warrants (1) | 4,149,719 | 2 years | $ | 0.80 | 4,149,719 | |||||||
| Investors - Class B Warrants (2) | 2,500,000 | 2 years | $ | 0.40 | 2,500,000 | |||||||
| Investors - Class C Warrants (3) | 5,072,492 | (3) | $ | (3) | 5,072,492 | |||||||
| Lior Wayn - Class E Warrants (4) | 2,700,000 | (4) | $ | 0.0001 | 2,700,000 | |||||||
| Warrants | Warrant Term | Exercise Price | Exercisable | |||||||||||
| Class J Warrants | 3,649,318 | July 2029 | $ | 0.48 | 3,649,318 | |||||||||
| Class K Warrants | 3,649,318 | July 2029 | $ | 0.80 | 3,649,318 | |||||||||
(1) The Class A Warrants were issued in connection with a private placement in reliance upon Regulation S, pursuant to which the Registrant sold a total of 4,149,719 units at a price of $0.40 per unit (the "Units"), each Unit comprised of one Share and one Class A Warrant exercisable at $0.80 per share with a term 24 months. While all of the Class A Warrants are exercisable within 60 days, in fact, none of these warrants will be exercised for the foreseeable future, based upon the exercise price of $0.80 per Share.(2) The Class B Warrants were issued to consultants for bona fide services to the Company and are exercise, on a cashless basis at a price of $0.40 per Share for a period of two years.(3) The Class C Unit Warrants were issued to consultants for bona fide services to the Company, and each Unit is exercisable at a price of $0.40 to purchase one Share of Common Stock and one Class A Warrant which, in turn, is exercisable to purchase one additional Share at a price of $0.80. The Class C Unit Warrants expire ninety (90) days after the effective date of this Registration Statement.(4) The Class E Warrants were issued by the Registrant to Lior Wayn inIn connection with the ClosingShare Exchange Agreement, upon the earlier of: (a) the launch of a live video product to an American consumer in the United States by Viewbix Israel, or (b) the launch of an interactive television product to an American consumer in the United States by Viewbix Israel, we will issue to Gix an additional 1,642,193 shares of restricted Common Stock of the Share Exchange Agreement. The Class E Warrants are exercisable to purchase a total of 2,700,000 Shares, in three equal tranches of 900,000 Shares each (the "Tranches") at an exercise price of $0.0001 per Share, subject to and within 45 days of the Registrant achieving the milestones defined in the table below (the "Milestones").Company.
| ||
Securities Authorized for Issuance Under Equity Compensation Plans
No equity compensation plan or agreements has been adopted
The following table summarizes information of outstanding options as of December 31, 2015.2020:
| Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance | ||||||||||
| Plan Category | ||||||||||||
| Equity compensation plans approved by security holders 2017 Employee Incentive Plan | - | - | 133,333 | |||||||||
| 19 |
SaleRecent Sales of Unregistered Securities
During the last three years, theRegistrantissued the following restricted shares which were not registered under the Act.
On December 16, 2014, the Registrant issued 4,125,000 restricted Shares to five holders of the Registrant’s convertible notes in the principal amount of $125,00018, 2020, we entered into a Stock Subscription Agreement (the "Notes"“Subscription”) upon their conversion the Notes. The table below sets forth the issuances of restricted Shares to note holders made in reliance on Regulation S promulgated by the SEC under the Act ("Reg S"with certain investors (the “Investors”).
| Name of Note Holder | Basis of Issuance | Total Notes Converted | Shares Issued (1) | |||||||
| Eli Yoresh | Conversion of Notes | $ | 12,500 | 412,500 | ||||||
| Kfir Silberman | Conversion of Notes | $ | 18,750 | 618,750 | ||||||
| Amir Uziel | Conversion of Notes | $ | 31,250 | 1,031,250 | ||||||
| Itschak Shrem | Conversion of Notes | $ | 31,250 | 1,031,250 | ||||||
| Lavi Krasney | Conversion of Notes | $ | 31,250 | 1,031,250 | ||||||
| Total | $ | 125,000 | 4,125,000 | |||||||
(1) Adjusted for the 1:4 reverse stock split effective in March 2015. No warrants were issued in connection with the conversionsale and issuance of these notes.
On June 18, 2015an aggregate of 3,000,000 shares of Common Stock, at a purchase price of US$0.01 per share, and July 21, 2015, afterfor an aggregate purchase price of US$30,000. In addition, and on the Company ceasedsame date, we entered into a Loan Agreement (the “Loan Agreement”) with the Investors, pursuant to be a shell company,which the Company issued and sold unregistered securities, as set forthInvestors lent an aggregate of $69,000 (the “Principal Amount”). In accordance with the terms of the Loan, we repaid the interest on the Principal Amount (8% compounded annually) to the Investors in the table below, in private offeringform of a totalan issuance of 2,762,500 units at a pricean aggregate of $0.40. Each Unit consisted of one Share and one Class A Warrant exercisable to purchase one additional Share552,000 shares of Common Stock, at a price per share of $0.80 (the "Units").$0.01. The sales were made without registration under the Act in reliance upon the exemptions provided in Section 4(2) of the Act and Reg S.
| Name of Subscriber | Bases for Issuance | Date of Issuance | Price Per Unit | Shares Issued | |||||
| Short Trade Ltd (1) | Subscription Agreement | 06/18/2015 | $ | 0.40 | 625,000 | ||||
| Prop Trade Ltd (2) | Subscription Agreement | 06/18/2015 | $ | 0.40 | 375,000 | ||||
| Dr. Ben Zion Weiner | Subscription Agreement | 06/18/2015 | $ | 0.40 | 125,000 | ||||
| RP Holdings (1992) Ltd. (3) | Subscription Agreement | 06/18/2015 | $ | 0.40 | 125,000 | ||||
| Dr. Tank Siak Khim | Subscription Agreement | 06/18/2015 | $ | 0.40 | 250,000 | ||||
| Yoel Yogev | Subscription Agreement | 06/18/2015 | $ | 0.40 | 200,000 | ||||
| Universal Link Ltd (4) | Subscription Agreement | 06/18/2015 | $ | 0.40 | 175,000 | ||||
| Avigdor Hakmon | Subscription Agreement | 06/18/2015 | $ | 0.40 | 62,500 | ||||
| Dr. Shmuel Pasternak | Subscription Agreement | 06/18/2015 | $ | 0.40 | 62,500 | ||||
| Liat Sidi | Subscription Agreement | 07/21/2015 | $ | 0.40 | 25,000 | ||||
| Tzvi Aharonson | Subscription Agreement | 07/21/2015 | $ | 0.40 | 137,500 | ||||
| Estory Giloz Ran | Subscription Agreement | 07/21/2015 | $ | 0.40 | 312,500 | ||||
| Malca Maimon | Subscription Agreement | 07/21/2015 | $ | 0.40 | 87,500 | ||||
| Ohad Cohen | Subscription Agreement | 07/21/2015 | $ | 0.40 | 150,000 | ||||
| Nissim Simhon | Subscription Agreement | 07/21/2015 | $ | 0.40 | 50,000 | ||||
| NE Solution Ltd (5) | Subscription Agreement | 07/21/2015 | $ | 0.40 | 162,500 | ||||
| Total | $ | 1,169,961 | 2,925,000 | ||||||
(1) Short Trade Ltd is controlled by Mr. Shlomo Noyman, a resident of Israel.
(2) Prop Trade Ltd is controlled by Mr. Andrew Philip Dings, a resident of Singapore.
(3) RP Holdings (1992) Ltd. is controlled by Mr. Rubin Zimerman, a resident of Israel.
(4) Universal Link Ltd is controlled by Mr. Ahmad Alimi, a resident of Israel.
(5) NE Solution Ltd is controlled by Mr. Lee Yang Tong, a resident of Singapore.
In July 2015, the persons listed in the table below, each a lender to Emerald on or before November 2014, converted their debt owed by Emerald into Units, each consisting of one restricted Share and one Class A Warrant, at a conversion price of $0.32. Each of the lenders was a resident of Israel and the issuance was without registration under the Act in reliance upon the exemptions provided in Section 4(2) of the Act and Reg S.
| Name of Note Holder | Bases of Issuance | Debt Converted | Shares Issued | |||||
| David Masasa | Conversion of Debt | $ | 8,788 | 27,463 | ||||
| Liron Carmel | Conversion of Debt | $ | 19,521 | 61,003 | ||||
| Yoseph Cohen | Conversion of Debt | $ | 15,632 | 48,850 | ||||
| Tzvi Aharonson | Conversion of Debt | $ | 43,969 | 137,403 | ||||
| Total | $ | 87,910 | 274,719 | |||||
On July 21, 2015, the Registrant issued 140,000 restricted Shares to Shira Brand Shiffer, a resident of Israel, at a price of $0.107 per Share, with no warrants attached. The issuance to Shira Brand Shiffer, without registration under the Act, was made in reliance upon Section 4(2) of the Act and Reg S.
On July 16, 2015, the Registrant issued 517,900 restricted shares of Common Stock were issued to Meyda Consulting Ltd, an entity organized under the laws of Israel controlled by Eliyahu Kirstein, a resident of Israel. The issuance of these shares was in consideration for services and was made without registration under the Act in reliance upon the exemptions provided in Section 4(2)Investors pursuant to Regulation S of the Securities Act and Reg S.of 1933, as amended.
On July 16, 2015, the Registrant issued Class B Warrants and Class C Unit Warrants to the following entities for bona fide services to the Registrant. The issuances of these Warrants was in consideration for services and was made without registration under the Act in reliance upon the exemptions provided in Section 4(2) of the Act and Reg S.
Basis of Issuance Class B Warrant Issued Class C Unit Warrants Issued Total Warrants Issued Yaad Consulting Ltd. (1) Services 625,000 $ 634,063 1,259,063 LA Pure Capital Ltd. (2) Services 375,000 $ 380,467 755,467 Amir Uziel Economic Consultant Ltd. (3) Services 625,000 $ 634,061 1,259,061 Capitalink Ltd. (4) Services 625,000 $ 634,061 1,259,061
(1) The control person of Yaad Consulting Ltd is Itschak Shrem, a resident of Israel.(2) The control person of LA Pure Capital Ltd is Kfir Silberman, a resident of Israel.(3) The control person of Amir Uziel Economic Consultant Ltd. is Amir Uziel, a resident of Israel.(4) The control person of Captalink Ltd is Lavi Krasney, a resident of Israel.
During November 2015, the Registrant issued and sold unregistered Shares as set forth on the table below:
(1) The issuance was pursuant to a Unit Subscription Agreement each consisting of 1 Share and 1 Class A Warrant exercisable for a period of 24 months to purchase 1 additional Share at $0.80.(2) Lyons Capital LLC is organized under the laws of Florida and its control person, Jason Lyons, is a resident of Florida.
The issuance and sale of Shares to Shirat Hahayim and Pnina Rosenbluem, residents of the State of Israel, and David Treves, resident of Australia, without registration under the Act, was made in reliance upon the exemptions provided in Section 4(2) of the Act and and Regulation S promulgated by the United States Securities and Exchange Commission (the "SEC") under the Act. The issuance of Shares to Lyons Capital LLC, without registration under the Act, was in reliance upon Section 4(2) and Regulation D promulgated by the SEC under the Act.
ITEM 6. SELECTED FINANCIAL DATABack to Table of Contents
None.
Not required for smaller reporting companies.
ITEM 7. MANAGEMENT'SMANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITIONS AND PLAN OF OPERATIONBack to Table of Contents
Overview
The following plan of operation provides information which management believes is relevant to an assessment and understanding of our results of operations and financial condition. The discussion should be read along with our consolidated financial statements and notes thereto. This section includes a number of forward-looking statements that reflect our current views with respect to future events and financial performance. Forward-looking statements are often identified by words like believe, expect, estimate, anticipate, intend, project and similar expressions, or words which refer to future events. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from our predictions.
PlanOrganizational Background
The Registrant was incorporated in the State of OperationsOhio in 1989 under a predecessor name, Zaxis International, Inc. On August 25, 1995, Zaxis International, Inc. merged with a subsidiary of The InFerGene Company, a Delaware corporation, which entity changed its name to Zaxis International, Inc. and the Company was reincorporated in Delaware under the name of Zaxis International, Inc. On December 30, 2014, Zaxis entered into an agreement with Emerald Medical Applications Ltd., a private limited liability company organized under the laws of the State of Israel.
We are
Emerald Medical Applications Ltd.
On March 16, 2015, Zaxis and Emerald Israel executed a digital health startup companyshare exchange agreement, which closed on July 14, 2015, and Emerald Israel became the Company’s wholly-owned subsidiary. Emerald Israel was engaged in the business of developing Emerald Israel’s DermaCompare technology and the development, sale and service of imaging solutions utilizing our proprietaryits DermaCompare software that we developed for use in derma imaging and analytics (our "DermaCompare" or "Product"). In our development of the DermaCompare technology, we utilized the knowledge learned from advanced military image processing and data analytics to improve the analysis of medical images for the benefit of patients and the medical community. We believe that our proprietary DermaCompare software represents an advancement in skin cancer screening that should enable physicians to more readily identify and monitor changes in their patients’ skin characteristics.
DermaCompare is Emerald’s first application of its technology, which we believe represents an advance in the early detection of skin cancer. On January 29, 2018, the Company ceased the DermaCompare is based on automated image analytics software using advanced algorithms for alignment, anchoring, identifying and detecting changes in the shapes, colors and sizesoperations of skin lesions, which could potentially become Melanoma. We apply our DermaCompare technology in image capture, correction and intelligent data extraction in the market for derma imaging products.its former subsidiary.
OurOn January 29, 2018, the Company ceased the DermaCompare solution allows dermatologistsoperations of Emerald Israel and other medical care professionals, usingon May 2, 2018, the District Court of Lod, Israel issued a set of 25 total body photography ("TBP"),winding-up order for Emerald Israel and appointed an Israeli attorney to capture sets of skin lesion images with, among other devices, digital cameras, camera-equipped smart phones or tablets. These images are then transmitted online and are remotely analyzed by professionals using our DermaCompare software.serve as special executor for Emerald Israel.
Our DermaCompare imaging software has 2 main modules:Virtual Crypto Technologies Ltd.
Our future plans also contemplateOn January 17, 2018, the useCompany formed VCT Israel to develop and market software and hardware products facilitating, allowing and supporting purchase and/or sale of wearable computing and imaging devices such as Google glasses cryptocurrencies through ATMs, tablets, personal computers (“PCs”) and/or other comparablemobile devices.
Our sales and marketing plan, which has already commenced, is to sell licenses for our DermaCompare imaging software to: NHSs, HMOs, health insurance companies, hospitals and medical clinics through distributers, health care channel partners or directly through independent salespersons and/or web purchase to dermatologists and other physicians (GPs) that we expect to purchase licenses based on the number of potential numbers of patients.
In furtherance of our business plan, which has resulted in us becoming an operating company, we haveOn January 24, 2018, VCT Israel entered into a seriesbinding term sheet (the “Chiron Term Sheet”) with Chiron Refineries Ltd. (“Chiron”), a public company listed on the Tel-Aviv Stock Exchange (TASE: CHR). Pursuant to the Chiron Term Sheet: (i) VCT Israel agreed to appoint a wholly-owned subsidiary of agreements with unaffiliated third parties for the distribution of its DermaCompare Technology, as follows:
1. On August 12, 2013, Emerald entered into an exclusive distribution with Derma Italy Sri,Chiron, to be organized under the laws of the Italy ("Derma Italy"Turkish Republic of Northern Cyprus (the “Distributor”), pursuantas the exclusive distributor of VCT Israel’s Products in Turkey, including the territory of Turkish Republic of Northern Cyprus (collectively, the “Territory”); and (ii) the Distributor shall have the right to which Derma Italy was granted exclusive distribution rights in Italy;2. On December 1, 2013, Emerald entered into a distribution agreement with S. Bokhorst - Creatiekracht, organized underappoint sub-distributors within the lawsTerritory. The appointment of the Netherlands, pursuantDistributor was subject to the payment by the distributor to VCT Israel of $250 thousand as an appointment fee, of which S. Bokhorst$150 thousand was to be deemed an advance payment by the distributor made on account of future purchases of the Company’s products.
| 20 |
VCT Israel further granted exclusive distribution in the Netherlands;3. On February 6, 2014, Emerald entered into a distribution agreement with Medical Edge Pty Ltd, organized underDistributor an option, exercisable by the laws of Australia ("Medical Edge"), pursuant to which Medical Edge was granted exclusive distribution rights in the markets of Australia, New Zealand and Oceania;4. On January 14, 2015, Emerald entered into a Project Agreement with Realize S.A. and Ubitech, entities engaged in IT related to medical technology in Greece, and MEDISP and MPUoP, academic and research institutes in Greece (collectively, the "Greek Partners"). Emerald and the Greek Partners anticipate imminent grantsDistributor within 12 months from the Office of Chief Scientistdate on which the ATM product, including the related software and hardware, was fully tested and ready for installation and operation, to be appointed as an exclusive distributor of the Stateproducts for the Federal Republic of Nigeria. If the option was exercised, the Distributor was required to pay VCT Israel an appointment fee not more than $250 thousand. In November 2018, Chiron reported that it had encountered financial difficulties and as such the General Secretariat for Research and Technology of Greece, respectively,Company will no longer pursue the proceeds of which will be used for development of enhanced smartphone applications for diagnosis of early stage Melanoma.transactions contemplated by the Chiron Term Sheet.
During the year 2018, $100 thousand was paid by the Distributor to VCT Israel, which has been recognized as revenues for the year ended December 31, 2015, we raised $989,974 through2018.
On January 27, 2020, VCT Israel was sold to a third party for NIS 50,000 ($14,459).
Transaction with Gix (the “Recapitalization Transaction”)
On February 7, 2019, the issuanceRegistrant entered into the Share Exchange Agreement with Gix, pursuant to which on the Closing Date Gix assigned, transferred and delivered its 99.83% holdings in Viewbix Israel to the Company in exchange for shares of equity debtrestricted common stock of the Company, representing 65% of the issued and we may be expectedoutstanding share capital of the Company on a fully diluted basis as of the Closing Date, following the conversion of certain convertible notes of the Company and excluding certain warrants to require uppurchase shares of the Common Stock expiring in 2020 and additional warrants as further described below (the “Fully Diluted Share Capital”). In addition, upon the earlier of: (a) the launch of a live video product to an American consumer in the United States by Viewbix Israel, or (b) the launch of an interactive television product to an American consumer in the United States by Viewbix Israel, the Company will issue to Gix an additional $1.5 million1,642,193 shares of restricted common stock of the Company representing 5% of the Fully Diluted Share Capital immediately following the Closing Date.
On July 24, 2019, the Company filed a Certificate of Amendment to its Certificate of Incorporation with the Secretary of State of Delaware reflecting its name change from Virtual Crypto Technologies, Inc. to Viewbix Inc. to reflect its new operations and business focus and, effective on August 7, 2019, FINRA approved the Registrant’s name change and its trading symbol was changed from “VRCP” to “VBIX” on the OTCQB.
On the Closing Date, the Company (i) issued 20,281,085 shares of its Common Stock to Gix in capitalexchange for consideration consisting of for its 99.83% holdings in Viewbix Israel, and (ii) convertible notes representing 3,434,889 shares of Common Stock then currently issued to holders were converted. The shares of common stock were issued under Regulation S. The Company also issued a total of 7,298,636 warrants to Gix to purchase the Company’s Common Stock, whereby (i) 3,649,318 of such warrants were issued with an exercise price of $0.48, and (ii) 3,649,318 of such warrants were issued with an exercise price of $0.80.
Following the Closing Date, and as a result of the Recapitalization Transaction, Viewbix Israel became a subsidiary of the Registrant.
As the shareholders of Viewbix Israel received the largest ownership interest in the Company, Viewbix Israel was determined to be the “accounting acquirer” in the Recapitalization Transaction. As a result, the historical financial statements of the Company were replaced with the historical financial statements of Viewbix Israel. The number of shares of Common Stock prior to the Recapitalization Transaction have been retroactively adjusted based on the equivalent number of shares of Common Stock received by the accounting acquirer in the Recapitalization Transaction.
Viewbix Israel was incorporated in Israel in February 2006. Viewbix Israel developed an interactive video platform based on SaaS business model with interactive elements, and the ability to collect and analyze information about each interactive action performed during the next 12 monthsviewing of the video clip. The interactive elements and information gathered, allowing the advertiser to fully implement our business plananalyze user viewing habits and fund our operations. optimize real-time throughout the campaign while increasing the effectiveness of online and live video advertising.
| 21 |
On July 25, 2019, the following changes were made to the Company’s management: (i) Mr. Eyal Ben Ami resigned from the Company’s board of directors; (ii) Mr. Alon Dayan resigned as the Company’s chief executive officer, however remained a member of the board of directors; (iii) Mr. Gadi Levin resigned as the Company’s chief financial officer, and transitioned to the role of senior accounting consultant; (iv) Mr. Noam Band was appointed to the board of directors; (v) Mr. Jonathan Stefansky was appointed as chief executive officer of the Company and elected as a member of the board of directors; (vi) Mr. Amihay Hadad was appointed as chief financial officer of the Company; and (vii) Mr. Hillel Scheinfeld was appointed as chief operating officer of the Company.
On July 25, 2019, the Company ceased the operations of VCT Israel.
Pursuant to a Tax Ruling issued by the Israeli Tax Authority, the Registrant ceased the options of VCT Israel on the Closing Date.
In connection with certain cost reduction measures that the Company is currently implementing, on January 1, 2020, Mr. Jonathan Stefansky tendered his resignation from the board of directors. On the same date, the Company and Mr. Stefansky reached a mutual understanding that Mr. Stefansky will step down as the Company’s chief executive officer, which entered into effect on March 1, 2020. Similarly, on January 1, 2020, the Company and Mr. Hillel Scheinfeld reached a similar mutual understanding and agreed Mr. Scheinfeld will step down as the Company’s chief operating officer, which also entered into effect on March 1, 2020.
On January 1, 2020, Mr. Amihay Hadad, the Company’s current chief financial officer, was appointed to serve as a member of the board of directors, and on February 20, 2020, Mr. Hadad was appointed to serve as chief executive officer of the Company.
No new compensatory arrangements were entered into in connection with the aforementioned leadership changes.
Results of Operations during the year ended December 31, 20152020 as compared to the year ended December 31, 20142019
We have had no revenues
Revenues for the years ended December 31, 2015 and 2014. We had operating expenses related to research and development and general and administrative expenses
During the year ended December 31, 2015, we incurred $8,756,267 in net loss2020 was $96 thousand as compared to $208 thousand for the year end December 31, 2019. The reason for the decrease during the fiscal year ended December 31, 2020 is due to $740,197 research and development expenses and $7,296,798 in general and administrative expenses, $6,494 depreciation expense, $30,604 interest expense, $678,027 lossthe Company’s cost-reduction measures implemented beginning on settlementJanuary 1, 2020.
Cost of debt and $4,147 loss from foreign currency translation.
Duringrevenues for the year ended December 31, 2014, we incurred $105,049 in net loss2020 was $5 thousand which is a slight increase to $2 thousand for the year end December 31, 2019.
Research and development costs for the year ended December 31, 2020 was $108 thousand as compared to $233 thousand for the year end December 31, 2019. The reason for the decrease during the fiscal year ended December 31, 2020 is due to $116,863 generalthe Company’s cost-reduction measures implemented beginning on January 1, 2020.
Sales and administrativemarketing expenses $66 depreciationfor the year ended December 31, 2020 was $8 thousand as compared to $257 thousand for the year end December 31, 2019. The reason for the decrease during the year ended December 31, 2020 is due to the Company’s cost-reduction measures implemented beginning on January 1, 2020.
General and Administration expenses for the year ended December 31, 2020 was $437 thousand as compared to $720 thousand for the year end December 31, 2019. The reason for the decrease in 2020 is due to certain cost reduction measures initiated by the Company as of the beginning of January 2020. Additionally, during the fiscal year-ended December 31, 2020, the Company was no longer obligated to pay recapitalization expenses in connection with the Recapitalization Transaction and the Share Exchange Agreement which were paid by the Company during the fiscal year-ended December 31, 2019.
| 22 |
Our net financial income was $13 thousand for the year ended December 31, 2020, compared to net financial expenses of $98 thousand for the year end December 31, 2019. The reason for the change is due to the US dollar exchange rate difference for the fiscal year ended December 31, 2020 as compared to the fiscal year end December 31, 2019.
Our tax on income was $2 thousand for the year ended December 31, 2020, as compared to $15 thousand for the year end December 31, 2019. The reason for the decrease is due to the fact that during the fiscal year ended December 31, 2019 the Company recognized a one-time tax expense $5,605 interest expense, a loss of $7,385 in fair value of derivative, $20,993 in income from grants and $3,877 gain from foreign currency translation.related to prior years.
Liquidity and Capital Resources
On
As of December 31, 2015,2020, we have had current assets of $141,246$225 thousand consisting of $115,449$148 thousand in cash and cash equivalents, $15 thousand in trade receivables, $20 thousand in other accounts receivables of $25,797. and, $42 thousand in prepaid expenses.
We had fixed assets, net of $21,120. We had $288,775$2,303 thousand in current liabilities consisting of $90,705$177 in other accounts payable and accrued liabilities, $22 trade payable, and $2,054 payable to our parent company.
As of December 31, 2019, we had current assets of $225 thousand consisting of $89 thousand in cash and cash equivalents and restricted cash, $119 thousand in other receivables and $17 thousand in prepaid expenses. We had $1,923 thousand in current liabilities, which consisted of $246 in accounts payable and accrued liabilities $3,480 in accountsand $66 trade payable, and $1,611 payable to related party, $25,612 employee payable, $19,285 in accrued interest, short-term note payable of $119,974 and $29,719 in convertible note payable.our parent company.
As of December 31, 2014, we had current assets of $40,128 consisting of cash of $14,411, due from related party of $18,999, other receivables of $6,718. We had fixed assets of $1,390. As of December 31, 2014, we had total current liabilities of $137,635 consisting of $2,577 in accounts payable and accrued liabilities, $4,439 accounts payable due to related party, $2,013 accrued interest, $19,521 short term notes payable due to related party, $20,164 in convertible notes payable, net of discount, $20,532 derivative liability and $68,389 in short term notes payable.
We had a negative working capital of $147,529$2,078 thousand and $97,507$1,698 thousand as of December 31, 20152020 and December 31, 2014,2019, respectively. The Company is assessing a number of options to increase its working capital to better sustain its operations.
Our total liabilities as of December 31, 20152020 were $288,775$2,303 thousand compared to $137,635 at$1,923 thousand as of December 31, 2014.2019.
During the fiscal year ended December 31, 2015,2020, we had negative cash flow from operations of $1,301,823$53 thousand which was mainly the result of a net loss of $8,756,267, $19,079 increase in other receivables and$443 thousand, depreciation expense of $5 thousand, offset by $6,626,619 in non-cash compensation, $678,027 loss on settlementgains from the sale of debt, $109,176 increase in accounts payablea subsidiary and accrued liabilities, $260 in decrease in related party payables, $24,913 increase in employee payable, $18,999 decrease in amounts due from related party, $6,494 depreciation expense, and $9,555 amortizationworking capital of debt discount.$385 thousand.
During the fiscal year ended December 31, 2014,2019, we had negative cash flow from operations of $110,694$135 thousand which was mainly the result of a net loss of $105,049, $18,999 increase in amounts due from related party and $6,718 increase in other receivables and$1,117 thousand, depreciation expense of $1 thousand, offset by $4,590 increasedecrease in accounts payable and accrued liabilities, $4,439 increase in related parties payable, $66 depreciation expense and $3,592 amortizationworking capital of debt discount and $7,385 change in fair value$981.
During the fiscal year ended December 31, 2020, we had a positive cash flow effect from investing activities of derivative liability.
During$13 thousand as compared to a negative cash flow effect from investing activities of $1 thousand as during the year ended December 31, 2015,2019.
During the fiscal year ended December 31, 2020, we offset our negativehad a positive cash flow from operations by $380,000 proceeds from salefinancing activities of common stock (net of issuance expenses), and $609,974 issuance of short-term payable. In addition, investing activities resulted in proceeds of $441,156 due to $467,380$99 thousand, which related to the reverse merger offset by $26,224 dueLoan Agreement and issuance of shares we have made during the fiscal year ended December 2020, compared to purchasea positive cash flow from financing activities of property$174 thousand during the fiscal year ended December 31, 2019, which related to the cash acquired in connection with the Recapitalization Transaction.
There are no limitations in the Company’s Certificate of Incorporation on the Company’s ability to borrow funds or raise funds through the issuance of shares of its common stock to affect a business combination. The Company’s limited resources and equipment.lack of having cash-generating business operations may make it difficult to borrow funds or raise capital. The Company’s limitations to borrow funds or raise funds through the issuance of restricted capital stock required to effect or facilitate a business combination may have a material adverse effect on the Company’s financial condition and future prospects, including the ability to complete a business combination.
During
| 23 |
Until such time as the Company can generate substantial revenues, the Company expects to finance its cash needs through a combination of the sale of its equity and/or convertible debt securities, debt financing and strategic alliances and collaborations. The Company does not have any committed external source of funds. To the extent that the Company raises additional capital through the sale of its equity and/or convertible debt securities, the ownership interest of its shareholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common shareholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. To the extent that debt financing ultimately proves to be available, any borrowing will subject us to various risks traditionally associated with indebtedness, including the risks of interest rate fluctuations and insufficiency of cash flow to pay principal and interest, including debt of an acquired business. If the Company raises funds through additional collaborations or strategic alliances with third parties, we may have to relinquish valuable rights to our future revenue streams and/or distribution arrangements. No assurance can be given that any future financing will be available or, if available, that it will be on terms that are satisfactory to the Company. If the Company is unable to raise additional funds through equity and/or debt financings when needed or on attractive terms, the Company may be required to delay, limit, reduce or terminate the operations of some or all of its business segments.
Going Concern
The Company has incurred $443 thousand in net losses for the year ended December 31, 2014, we offset our2020, has $2,078 thousand shareholders’ deficit as of December 31, 2020 and $1,693 thousand in total shareholders’ deficit as of December 31, 2019 and $53 thousand negative cash flowflows from operations by $117,629 issuance of short-term payable. In addition we hadfor the year ended December 31, 2020, and $135 thousand negative cash flowflows from investing activitiesoperations for the year ended December 31, 2019. Management expects the Company to continue to generate substantial operating losses and to continue to fund its operations primarily through utilization of $1,456 dueits current financial resources and through additional raises of capital.
Such conditions raise substantial doubts about the Company’s ability to purchasecontinue as a going concern. Management’s plan includes raising funds from outside potential investors. However, there is no assurance such funding will be available to the Company or that it will be obtained on terms favorable to the Company or will provide the Company with sufficient funds to meet its objectives. These financial statements do not include any adjustments relating to the recoverability and classification of propertyassets, carrying amounts or the amount and equipment.classification of liabilities that may be required should the Company be unable to continue as a going concern.
Availability of Additional Capital
Our potential financing transactions may include the issuance of equity and/or debt securities including convertible debt, obtaining credit facilities, or other financing mechanisms. In the event that we seek to raise funds through additional private placements of equity or convertible debt, the trading price of our common stock could be adversely effected.affected. Further, any adverse conditions in the financial markets could make it more difficult to obtain future financing through the issuance of equity or debt securities when and if needed. Even if we are able to raise a sufficient amount of funds that may be required, it is possible that we could incur unexpected costs and expenses or experience unexpected cash requirements that would force us to seek additional and/or alternative financing. Further, if we issue additional equity or debt securities, stockholders may experience additional dilution or the new equity securities may have rights, preferences or privileges senior to those of existing holders of our common stock. If additional financing is not available or is not available on acceptable terms, we may have to curtail our plan of operations.
The Company has only limited capital. Additional financing is necessary for the Company to continue as a going concern. Our independent auditors have issued an unqualified audit opinion for the year ended December 31, 20152020 with an explanatory paragraph on going concern.
In view of these matters, realization of a major portion of the assets in the accompanying balance sheet is dependent upon continued operations of the Company. Management believes that actions presently being taken to obtain additional equity financing will provide the opportunity to continue as a going concern.
| 24 |
Off-Balance Sheet Arrangements
As of December 31, 20152020, and 2014,2019, we did not have any off-balance sheet arrangements as defined in Item 303(a)(4)(ii) of Regulation S-K promulgated under the Securities Act of 1934.
Contractual Obligations and Commitments
As of December 31, 20152020, and 2014,2019, we did not have any contractual obligations.
Critical Accounting Policies
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the U.S. The preparation of our consolidated financial statements and disclosures requires us to make judgments, estimates, and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported revenue and expenses during the reporting periods. We base our estimates on historical experience, known trends and events and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions and conditions.
Our significant accounting policies are described in more detail in the notes to our audited consolidated financial statements for the year ended December 31, 2015, and are includedappearing elsewhere in this prospectus.Annual Report on Form 10-K.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISKBack to Table of Contents
We have not entered into, and do not expect to enter into, financial instruments
Not required for trading or hedging purposes.
smaller reporting companies.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATABack to Table of Contents
INDEX TO FINANCIAL STATEMENTS
| 25 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRMBack to Table of Contents
To the Shareholders and Board of DirectorsEmerald Medical Applications Corp. of
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Emerald Medical Applications Corp. ("the Company"Viewbix Inc. and its subsidiary (the “Company”) as of December 31, 20152020 and 2014,2019 and the related consolidated statements of operations, shareholders'comprehensive loss, stockholders’ deficit and cash flows for each of the two years then ended. in the period ended December 31, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company’s substantial net losses, shareholders’ deficit and negative cash flows from operations raise substantial doubt about its ability to continue as a going concern. Management’s plans concerning these matters are also described in Note 1 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of’ these uncertainties
Basis for Opinion
These financial statements are the responsibility of the Company'sCompany’s management. Our responsibility is to express an opinion on thesethe Company’s financial statements based on our audits.
We conducted our audit in accordanceare a public accounting firm registered with standards of the Public Company Accounting Oversight Board (United States). (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement.misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. OurAs part of our audits, included considerationwe are required to obtain an understanding of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company'sCompany’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence supportingregarding the amounts and disclosures in the financial statements. An auditOur audits also includes assessingincluded evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statement presentation.statements. We believe that our audits provide a reasonable basis for our opinion.
In our opinion,
Critical Audit Matter
Critical audit matters are matters arising from the financial statements referred to above present fairly, in all material respects, the financial position of Emerald Medical Applications Corp. at December 31, 2015 and 2014, and the results of its operations and cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 2current-period audit of the financial statements that were communicated or required to be communicated to the Company had incurred a loss, had negative cash flow from operating activitiesaudit committee and no revenue during the years ended December 31, 2015 and 2014. These matters raise substantial doubt about the Company's abilitythat (1) relate to continue as a going concern. Management's plan in regard to these matters is also described in Note 2accounts or disclosures that are material to the financial statements. The financial statements do not include any adjustmentsand (2) involved our especially challenging, subjective, or complex judgments. We determined that might result from the outcome of this uncertainty.there are no critical audit matters.
/s/ M&K CPAS, PLLCwww.mkacpas.comHouston, Texas
Brightman Almagor Zohar & Co.
Certified Public Accountants
A Firm in the Deloitte Global Network
Tel Aviv, Israel
March 31, 201616, 2021
We have served as the Company’s auditor since 2019

| 26 |
Emerald Medical Applications Corp. Balance Sheets As of December 31, 2015 and 2014 Back to Table of Contents December 31, 2015 December 31, 2014 Assets Current assets: Cash and cash equivalents $ 115,449 $ 14,411 Due from related party - 18,999 Other receivable 25,797 6,718 Total current assets 141,246 40,128 Fixed assets, net Fixed assets, net of accumulated depreciation of $6,536 and $66, respectively 21,120 1,390 Total assets $ 162,366 $ 41,518 Liabilities and Stockholders' Equity (Deficit) Current liabilities: Accounts payable and accrued liabilities $ 90,705 $ 2,577 Accounts payable - related party 3,480 4,439 Employee payable 25,612 - Accrued interest payable 19,285 2,013 Short term notes payable - related party - 19,521 Short term notes payable 119,974 68,389 Convertible note payable, net of discount of $0 and $9,555, respectively 29,719 20,164 Derivative liability - 20,532 Total current liabilities 288,775 137,635 Total liabilities 288,775 137,635 Stockholders' equity (deficit) Preferred stock, $0.0001 par value; 10,000,000 shares authorized; none issued. - - Common stock, $0.0001 par value; 490,000,000 shares authorized; 15,325,889 and 7,438,141 shares issued and outstanding at December 31, 2015 and 2014, respectively. 1,533 744 Accumulated other comprehensive income (19,337) 8,932 Additional paid-in capital 8,752,711 (744) Accumulated deficit (8,861,316) (105,049) Total stockholders' deficit (126,409) (96,117) Total liabilities and stockholders' equity (deficit) $ 162,366 $ 41,518 The accompanying notes are an integral part of these financial statements.
Consolidated Balance Sheets
U.S. dollars in thousands (except share and per share data)
As of December 31, | As of December 31 | |||||||||||
| Note | 2020 | 2019 | ||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS | ||||||||||||
| Cash and cash equivalents | $ | 148 | $ | 87 | ||||||||
| Restricted cash | - | 2 | ||||||||||
| Trade receivables | 15 | - | ||||||||||
| Other accounts receivable | 3 | 20 | 119 | |||||||||
| Prepaid expenses | 42 | 17 | ||||||||||
| Total current assets | $ | 225 | $ | 225 | ||||||||
| NON CURRENT ASSETS | ||||||||||||
| Property and equipment, net | 4 | $ | - | $ | 5 | |||||||
| Total assets | $ | 225 | $ | 230 | ||||||||
| LIABILITIES, TEMPORARY EQUITY AND STOCKHOLDERS’ DEFICIT | ||||||||||||
| CURRENT LIABILITIES | ||||||||||||
| Trade payables | $ | 22 | $ | 66 | ||||||||
| Other accounts payable and accrued liabilities | 5 | 177 | 246 | |||||||||
| Payable to parent company | 6 | 2,054 | 1,611 | |||||||||
| Short term loan | 7 | 50 | - | |||||||||
| Total current liabilities | $ | 2,303 | $ | 1,923 | ||||||||
| Commitments and contingencies | 9 | |||||||||||
| STOCKHOLDERS’ DEFICIT | 8 | |||||||||||
| Share Capital | ||||||||||||
| Common stock, $0.0001 par value; 490,000,000 shares authorized; 34,753,669 shares issued and outstanding at December 31, 2020 and 31,201,669 at December 31, 2019 | 3 | 3 | ||||||||||
| Additional paid-in capital | 7 | 13,073 | 13,015 | |||||||||
| Accumulated deficit | (15,154 | ) | (14,711 | ) | ||||||||
| Total stockholders’ deficit | $ | (2,078 | ) | $ | (1,693 | ) | ||||||
| Total liabilities, temporary equity and stockholders’ deficit | $ | 225 | $ | 230 | ||||||||
Emerald Medical Applications Corp. Statements of Operations For the Twelve Months Ended December 31, 2015 and 2014 Back to Table of Contents Twelve months Twelve months ended ended December 31, 2015 December 31, 2014 Revenues $ - $ - Expenses: Research and development (740,197) - General and administrative expenses (7,296,798) (116,863) Total operating expenses (8,036,995) (116,863) Loss from operations (8,036,995) (116,863) Other income (expense): Depreciation expense (6,494) (66) Interest expense (30,604) (5,605) Change in fair value of derivative - (7,385) Gain/(loss) from foreign currency (4,147) 3,877 Other income from grants - 20,993 Loss on settlement of debt (678,027) - Other income (expense) (719,272) 11,814 Total income (expense) (8,756,267) (105,049) Provision for income taxes - - Net loss $ (8,756,267) $ (105,049) Basic and diluted (net loss per share) $ (0.81) $ (0.01) Weighted average shares outstanding - basic and diluted 10,872,526 7,348,141 The accompanying notes are an integral part of these financial statements.
Emerald Medical Applications Corp. Statements of Comprehensive Income (Loss) For the Twelve Months Ended December 31, 2015 and 2014 Back to Table of Contents Twelve months Twelve months ended ended December 31, 2015 December 31, 2014 Net loss $ (8,756,267) $ (105,049) Change in unrealized foreign currency translation gain (loss) (28,269) 8,932 Total comprehensive income (loss) $ (8,784,536) $ (96,117) The accompanying notes are an integral part of these financial statements.
Emerald Medical Applications Corp. Statements of Cash Flows For the Twelve Months Ended December 31, 2015 and 2014 Back to Table of Contents Twelve months Twelve months ended ended December 31, 2015 December 31, 2014 Operating Activities: Net (loss) $ (8,756,267) $ (105,049) Depreciation expense 6,494 66 Amortization of debt discount 9,555 3,592 Change in fair value of derivative liabilities - 7,385 Shares issued for services 885,984 - Warrants issued for services 5,343,088 - Loss on settlement of debt 678,027 - Employee option expense 397,547 - Adjustments to reconcile net (loss) to net cash (used in) operating activities: Increase in accounts payable and accrued liabilities 109,176 4,590 Decrease in related parties payable (260) 4,439 Increase in employees payable 24,913 - Decrease in amounts due from related party 18,999 (18,999) Increase in other receivables (19,079) (6,718) Net cash used in operating activities (1,301,823) (110,694) Investing Activities: Purchase of property and equipment (26,224) (1,456) Effect of reverse merger 467,380 - Net cash provided by investing activities 441,156 (1,456) Financing Activities: Proceeds from sale of common stock (net of issuance expenses) 380,000 - Issuance of short-term payable 609,974 117,629 Net cash provided by financing activities 989,974 (117,629) Foreign currency adjustment (28,269) 8,932 Net increase (decrease) in cash 101,038 14,411 Cash and cash equivalents - beginning of period 14,411 - Cash and cash equivalents - end of period $ 115,449 $ 14,111 Non-cash transactions: Shares issued for reverse merger $ 547 $ - Debt settled with stock $ 91,687 $ - Stock receivable $ - $ 297 Discount on convertible note with embedded derivative $ - $ 13,147 Extinguishment on derivative $ 20,532 $ 13,147 The accompanying notes are an integral part of these financial statements.
Additional Paid-in 7,438,141 $ 744 $ (744) $ - - $ - $ - - - - - - (105,049) (105,049) 7,438,141 744 (744) - 8,932 (105,049) (96,117) - - - - - (8,756,267) (8,756,267) 15,325,889 $ 1,533 $ 8,752,711 $ - (19,337) $ (8,861,316) $ (126,409)Emerald Medical Applications Corp. Statement of Changes in Stockholders' Equity (Deficit) For the Years December 31, 2015 and 2014 Back to Table of Contents Other Total Common Stock Comprehensive Accumulated stockholders' Shares Amount Capital Payable Income Deficit equity Balance as of December 31, 2013 Other comprehensive income - - - - 8,932 - 8,932 Net loss for the year Balance as of December 31, 2014 Common stock issued for cash 1,252,500 125 459,875 (80,000) - - 380,000 Debt converted into shares 274,719 27 769,686 - - - 769,713 Shares issued for services 885,984 88 885,896 - - - 885,984 Class B and C warrants for services - - 5,343,088 - - - 5,343,088 ESOP options - - 397,547 - - - 397,547 Effect of reverse merger 5,474,545 547 876,833 80,000 - - 957,380 Other comprehensive income - - - - (28,269) - (28,269) Extinguishment on derivative - - 20,532 - - - 20,532 Net loss for the year Balance as of December 31, 2015 The accompanying notes are an integral part of these financial statements.
| 27 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Consolidated Statements of Comprehensive Loss
U.S. dollars in thousands (except share and per share data)
| Year ended | Year ended | |||||||||||
| December 31, | December 31, | |||||||||||
| Note | 2020 | 2019 | ||||||||||
| Revenues | 10 | 96 | 208 | |||||||||
| Cost of revenues | 5 | 2 | ||||||||||
| Gross profit | 91 | 206 | ||||||||||
| Expenses: | ||||||||||||
| Research and development | 11 | 108 | 233 | |||||||||
| Sales and marketing | 12 | 8 | 257 | |||||||||
| General and administrative | 13 | 437 | 720 | |||||||||
| Gain from sale of a subsidiary | (8 | ) | - | |||||||||
| Total operating expenses | 545 | 1,210 | ||||||||||
| Loss from operations | (454 | ) | (1,004 | ) | ||||||||
| Finance income | 14 | (20 | ) | (12 | ) | |||||||
| Finance expense | 14 | 7 | 110 | |||||||||
| Loss Before taxes on income | (441 | ) | (1,102 | ) | ||||||||
| Taxes on income | 15 | 2 | 15 | |||||||||
| Net Loss | (443 | ) | (1,117 | ) | ||||||||
| Basic and diluted net loss per share: | (0.014 | ) | (0.08 | ) | ||||||||
| Weighted average shares outstanding - basic and diluted | 16 | 31,201,669 | 13,746,064 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| 28 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Consolidated Statements of Changes in Stockholders’ Deficit
U.S. dollars in thousands (except share and per share data)
| Ordinary shares | Additional paid-in | Accumulated | Total shareholders’ | |||||||||||||||||
| Number | Amount | capital | deficit | deficit | ||||||||||||||||
| Balance as of January 1, 2020 | 31,201,669 | 3 | 13,015 | (14,711 | ) | (1,693 | ) | |||||||||||||
| Issuance of shares | 3,552,000 | 58 | 58 | |||||||||||||||||
| Net loss for the period | (443 | ) | (443 | ) | ||||||||||||||||
| Balance as of December 31, 2020 | 34,753,669 | 3 | 13,073 | (15,154 | ) | (2,078 | ) | |||||||||||||
| Total | Additional | Accumulated | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Preferred A-1 | Preferred A-2 | Preferred B | Preferred C | Preferred C-1 | Preferred C-2 | temporary | Ordinary shares | paid-in | shareholders’ | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Number | Amount | Number | Amount | Number | Amount | Number | Amount | Number | Amount | Number | Amount | equity | Number | Amount | capital | deficit | deficit | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance as of January 1, 2019 | 199,870 | * | 4,881,654 | 10 | 4,556,094 | 9 | 7,222,305 | 15 | 2,755,706 | 11 | 392,407 | 1 | 46 | 279,049 | 1 | 12,872 | (13,594 | ) | (721 | ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Effect of reverse recapitalization | (199,870 | ) | (*) | (4,881,654 | ) | (10 | ) | (4,556,094 | ) | (9 | ) | (7,222,305 | ) | (15 | ) | (2,755,708 | ) | (11 | ) | (392,407 | ) | (1 | ) | (46 | ) | 30,928,620 | 2 | 143 | - | 145 | ||||||||||||||||||||||||||||||||||||||||||
| Net loss for the period | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | (1,117 | ) | (1,117 | ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance as of December 21, 2019 | - | - | - | - | - | - | - | - | - | - | - | - | - | 31,201,669 | 3 | 13,015 | (14,711 | ) | (1,693 | ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
*) Represents an amount less than $1.
The accompanying notes are an integral part of these consolidated financial statements.
| 29 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Consolidated Statements of Cash Flows
U.S. dollars in thousands (except share and per share data)
For the year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Cash flows from operating activities | ||||||||
| Net loss for the period | (443 | ) | (1,117 | ) | ||||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | ||||||||
| Gain from sale of a subsidiary | (8 | ) | - | |||||
| Depreciation | 5 | 1 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Decrease (Increase) in trade receivables and prepaid expenses | (40 | ) | 5 | |||||
| Decrease (Increase) in other accounts receivable | 100 | (21 | ) | |||||
| Increase (decrease) in trade payables | (55 | ) | 36 | |||||
| Increase in payable to parent company (See Note 6) | 443 | 822 | ||||||
| Increase (decrease) in other accounts payables and accrued liabilities | (55 | ) | 139 | |||||
| Net cash used in operating activities | (53 | ) | (135 | ) | ||||
| Cash flows from investing activities | ||||||||
| Cash received from the sale of a subsidiary | 13 | - | ||||||
| Purchase of property and equipment | - | (2 | ) | |||||
| Proceeds from sale of property and equipment | - | 1 | ||||||
| Net cash used in investing activities | 13 | (1 | ) | |||||
| Cash flows from financing activities | ||||||||
| Cash acquired in connection with the reverse recapitalization | - | 174 | ||||||
| Issuance of shares | 49 | - | ||||||
| Short term loan received | 50 | - | ||||||
| Net cash provided by financing activities | 99 | 174 | ||||||
| Increase (decrease) in cash and cash equivalents and restricted cash | 59 | 38 | ||||||
| Cash and cash equivalents and restricted cash at the beginning of the period | 89 | 51 | ||||||
| Cash and cash equivalents and restricted cash at the end of the period | $ | 148 | $ | 89 | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
| 30 |
Viewbix Inc. (Formerly known as Virtual Crypto Technologies, Inc.)
Condensed Consolidated Statements of Cash Flows
U.S. dollars in thousands (except share and per share data)
(Unaudited)
Supplemental Cash Flow Information:
As of February 12, 2020 | ||||
| Current assets excluding cash and cash equivalents | 6 | |||
| Current liabilities | (1 | ) | ||
| Gain from sale of a subsidiary | 8 | |||
| Cash received from the sale of a subsidiary | 13 | |||
| Assets acquired (liabilities assumed): | As of July 25, 2019 | |||
| Current assets excluding cash and cash equivalents | 20 | |||
| Current liabilities | (95 | ) | ||
| Reverse recapitalization effect on equity | (99 | ) | ||
| Cash acquired in connection with Recapitalization Transaction | 174 | |||
The accompanying notes are an integral part of these consolidated financial statements.
| 31 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and per share data)
Note 1. General
| A. | Organizational Background | |
| Viewbix Inc. (formerly known as Virtual Crypto Technologies, Inc.) (the “Company”) was incorporated in the State of Ohio in 1989 under a predecessor name, Zaxis International, Inc. (“Zaxis”). On August 25, 1995, Zaxis merged with a subsidiary of The InFerGene Company, a Delaware corporation, which entity changed its name to Zaxis International, Inc. and the Company was reincorporated in Delaware under the name of Zaxis International, Inc. On December 30, 2014, Zaxis entered into an agreement with Emerald Medical Applications Ltd., a private limited liability company organized under the laws of the State of Israel (“Emerald Israel”). | ||
| B. | Emerald Medical Applications Ltd. | |
On March 16, 2015, Zaxis and Emerald Israel executed a share exchange agreement, which closed on July 14, 2015, and Emerald Israel became the Company’s wholly-owned subsidiary. Emerald Israel was engaged in the business of developing Emerald Israel’s DermaCompare technology and the development, sale and service of imaging solutions utilizing its DermaCompare software for use in derma imaging and analytics for the detection of skin cancer. On January 29, 2018, the Company ceased the DermaCompare operations of its former subsidiary. On May 2, 2018, the District Court of Lod, Israel issued a winding-up order for Emerald Israel and appointed an Israeli attorney as special executor for Emerald Israel. | ||
| C. | Virtual Crypto Technologies Ltd. | |
On January 17, 2018, the Company formed a new wholly-owned subsidiary under the laws of the State of Israel, Virtual Crypto Technologies Ltd. (the “VCT Israel”), to develop and market software and hardware products facilitating, allowing and supporting purchase and/or sale of cryptocurrencies through ATMs, tablets, personal computers (“PCs”) and/or mobile devices. VCT Israel ceased its business operation prior to consummation of the Recapitalization Transaction. On January 27, 2020, Virtual Crypto Israel was sold to a third party for NIS 50,000 ($14,459). |
| 32 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and per share data)
NOTE. 1 GENERAL (Cont.)
| D. | Transaction with Gix Internet Ltd. (formerly known as Algomizer Ltd.), or Gix (the “Recapitalization Transaction”) | |
| On June 6, 2020, Algomizer changed its name to Gix Internet Ltd., or Gix. | ||
| On February 7, 2019, the Company entered into a share exchange agreement (the “Share Exchange Agreement”) with Gix Internet Ltd. (TASE:ALMO), a company organized under the laws of the State of Israel (“Gix”), pursuant to which on July 25, 2019 (the “Closing Date”), Gix assigned, transferred and delivered its 99.83% holdings in Viewbix Ltd. (“Viewbix Israel”) to the Company in exchange for shares of restricted common stock of the Company, representing 65% of the issued and outstanding share capital of the Company on a fully diluted basis as of the Closing Date following the conversion of certain convertible notes of the Company and excluding certain warrants to purchase shares of the Common Stock expiring in 2020 and additional warrants as further described below (the “Fully Diluted Share Capital”). In addition, upon the earlier of: (a) the launch of a live video product to an American consumer in the United States by Viewbix Israel, or (b) the launch of an interactive television product to an American consumer in the United States by Viewbix Israel, the Company will issue to Gix an additional 1,642,193 shares of restricted common stock of the Company representing 5% of the Fully Diluted Share Capital immediately following the Closing Date. | ||
| On July 24, 2019, the Company filed a Certificate of Amendment to its Certificate of Incorporation with the Secretary of State of Delaware reflecting its name change from Virtual Crypto Technologies, Inc. to Viewbix Inc. to reflect its new operations and business focus and, effective on August 7, 2019, FINRA approved the Registrant’s name change and its trading symbol was changed from “VRCP” to “VBIX” on the OTCQB. | ||
| On the Closing Date, the Company (i) issued 20,281,085 shares of its common stock to Gix in exchange for consideration consisting of consideration for its 99.83% holdings in Viewbix Israel, and (ii) 3,434,889 shares of its common stock to holders of convertible notes, which were issued by the Company prior to the Reverse Recapitalization, and which were converted upon the Closing Date. The shares of common stock were issued under Regulation S. The Company also issued a total of 7,298,636 warrants to Gix to purchase the Company’s common stock, whereby (i) 3,649,318 of such warrants were issued with an exercise price of $0.48, and (ii) 3,649,318 of such warrants were issued with an exercise price of $0.80. |
| 33 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and per share data)
NOTE. 1 GENERAL (Cont.)
| D. | Transaction with Gix Internet Ltd. (formerly known as Algomizer Ltd.), or Gix (the “Recapitalization Transaction”): (Cont.) | |
| As a result of the Recapitalization Transaction, Viewbix Israel became a subsidiary of the Company. As the shareholders of Viewbix Israel received the largest ownership interest in the Company, Viewbix Israel was determined to be the “accounting acquirer” in the Recapitalization Transaction. As a result, the historical financial statements of the Company were replaced with the historical financial statements of Viewbix Israel. The number of shares prior to the reverse recapitalization have been retroactively adjusted based on the equivalent number of shares received by the accounting acquirer in the Recapitalization Transaction. | ||
| The Company and its subsidiaries are collectively referred to as the “Company”. Viewbix Israel was incorporated on February 2006 in Israel. The Company has developed an interactive video platform based on Software as a Service (“SaaS”) business model with interactive elements, and the ability to collect and analyze information about each interactive action performed during the viewing of the video clip. The interactive elements and information gathered, allowing the advertiser to analyze user viewing habits and optimize real-time throughout the campaign while increasing the effectiveness of online and live video advertising. | ||
| On January 1, 2020, the Company announced certain cost reduction measures due the Company not achieving certain revenues goals. | ||
| E. | Stock Subscription Agreement and Loan Agreement | |
| On December 18, 2020, the company entered into a Stock Subscription Agreement (the “Subscription”) with certain investors (the “Investors”) in connection with the sale and issuance of an aggregate of 3,000,000 shares of Common Stock, at a purchase price of $0.01 per share, and for an aggregate purchase price of $30,000. In addition, and on the same date, the company entered into a Loan Agreement (the “Loan”) with the Investors, pursuant to which the Investors lent an aggregate amount of $69,000 (the “Principal Amount”). In accordance with the terms of the Loan, the company repaid the interest on the Principal Amount (8% compounded annually) to the Investors as an issuance of 552,000 shares of Common Stock, at a price per share of $0.01. The shares of Common Stock were issued to the Investors pursuant to Regulation S of the Securities Act of 1933, as amended. |
| 34 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and per share data)
NOTE. 1 GENERAL (Cont.)
| F. | Going Concern | |
| The Company has incurred $ 443 in net loss for the year ended December 31, 2020, has $2,078 stockholders’ deficit as of December 31, 2020 and $1,693 in total stockholders’ deficit as of December 31, 2019 and $61 in negative cash flows from operations for the year ended December 31, 2020. On July 25, 2019, the Company ceased the operations of VCT Israel and since January 2020, the Company has significantly reduced its operations and expenses of Viewbix Israel. Management expects the Company to continue to generate substantial operating losses and to continue to fund its operations primarily through utilization of its current financial resources and through additional raises of capital. | ||
| Such conditions raise substantial doubts about the Company’s ability to continue as a going concern. Management’s plan includes raising funds from outside potential investors. However, there is no assurance such funding will be available to the Company or that it will be obtained on terms favorable to the Company or will provide the Company with sufficient funds to meet its objectives. These financial statements do not include any adjustments relating to the recoverability and classification of assets, carrying amounts or the amount and classification of liabilities that may be required should the Company be unable to continue as a going concern. |
NOTE. 2 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Emerald Medical Applications Corp.Notes to Financial StatementsDecember 31, 2015Back to TableThe significant accounting policies used in the preparation of Contentsthe financial statements are as follows:
Functional currency
Note 1.
The Company and Significant Accounting Policies.
Organizational Background:
Emerald Medical Applications Corp. ("the Company") (f/k/a Zaxis International Inc.) was incorporated in Ohio in 1989. On August 25, 1995, Zaxis merged with a subsidiaryfunctional currency of The InFerGene Company ("InFerGene") and InFerGene changed its name to Emerald Medical Applications Corp. InFerGene was incorporated in California in 1984 and subsequently changed its domicile in connection with the merger into Zaxis to Delaware in 1985. Operations ceased operations in 2002. In November 2002, the Company and its subsidiaries filed a petition for bankruptcysubsidiary is the US dollar, which is the currency of the primary economic environment in which it operates. In accordance with ASC 830, “Foreign Currency Matters” (ASC 830), balances denominated in or linked to foreign currency are stated on the basis of the exchange rates prevailing at the applicable balance sheet date. For foreign currency transactions included in the statement of operations, the exchange rates applicable on the relevant transaction dates are used. Gains or losses arising from changes in the exchange rates used in the translation of such transactions are carried as financing income or expenses.
| 35 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. Bankruptcy Court Northern Districtdollars in thousands (except share and per share data)
NOTE. 2 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
Principles of Ohio. On October 13, 2004, the Company emerged from bankruptcy.consolidation
The MOU provides that the Registrant and Emerald will enter into a reverse merger (the "Reverse Merger"), subject to the execution of a definitive agreement (the "Definitive Agreement"). The execution of Definitive Agreement and the closing of the Reverse Merger will be subject to the Registrant's raise of $800,000 from third party investors, including but not limited to the Registrant's existing stockholders (the "Investors"), at terms and conditions to be agreed upon by the Registrant and Emerald.
Upon the closing, the holders of Emerald's capital stock will receive in exchange a number of shares of the Registrant's common stock equal to 45% of the Registrant's issued and outstanding common stock on a fully-diluted basis as at immediately following the closing of the Reverse Merger, excluding Registrant's securities to be issued to the Investors upon exercise of warrants issued to the Investors within the framework of the Reverse Merger. In addition, Emerald's holders will be issued up to an additional 21% of the Registrant's common stock in three equal tranches of 7% of the Registrant's issued and outstanding common stock as at immediately following the closing of the Reverse Merger, subject to Emerald's achievement of certain milestones to be set forth in the Definitive Agreement.
On July 14, 2015 the closing of the Share Exchange Agreement was held (the "Closing") and as a result, Emerald Medical Applications Ltd. became a wholly-owned subsidiary of the Registrant.
Utilizing capital raised prior to and subsequent to the closing of the Share Exchange Agreement, Emerald completed the development of a commercial model of its DermaCompare Product and has commenced marketing efforts. Emerald is continuing to negotiate additional distribution agreements for territories including North America, Latin America, Southern Africa, Israel and elsewhere in Europe, among other countries and regions. Emerald expects to generate significant revenues from its DermaCompare Technology commencing in the first half of fiscal 2016. Emerald is continuing to work on development of the "next generation" DermaCompare Technology, with enhanced features.
Subsequently to the Closing Mr. Lior Wayn has been appointed as the Company's CEO, and has been granted considerable influence on the appointment of new directors thereby creating a new management structure for the company replacing the old management. Additionally Mr. Wayn is to receive additional shares in the future contingent on the Company achieving commercial milestone. Thus the new management, headed by Mr. Wayn, is considered to be in control of more than 50% of the company and with the ability to make all management decisions.
Emerald is a company organized under the laws of the State of Israel on February 17, 2010. Emerald is digital health Startup Company engaged in the development, sale and service of imaging solutions utilizing its proprietary DermaCompare software that it developed for use in derma imaging and analytics ("DermaCompare"). Emerald believes that its proprietary DermaCompare software represents an advancement in skin cancer screening that should enable physicians to more readily identify and monitor changes in their patients' skin characteristics.
Emerald's DermaCompare solution allows dermatologists and other medical care professionals, using a set of 25 total body photography ("TBP"), to capture sets of skin lesion images with, among other devices, digital cameras, camera-equipped smartphones or tablets. These images are then transmitted online and are remotely analyzed by professionals using our DermaCompare software.
Emerald's sales and marketing plan is to sell licenses for our imaging software to: NHSs, HMOs, health insurance companies, hospitals and medical clinics through distributers, health care channel partners or directly through independent salespersons and/or web purchase to dermatologists and other physicians (GPs) that we expect to purchase licenses based on the number of potential numbers of patients.
Basis of Presentation:
The accompanyingconsolidated financial statements have been prepared in conformity with accounting principles generally accepted ininclude the United States of America, which contemplate continuationaccounts of the Company as a going concern. The Company has not established any source of revenue to coverand its operating costs,subsidiary. All intercompany balances and as such, has incurred an operating loss since inception. Further, as of December 31, 2015, the cash resources of the Company were insufficient to meet its current business plan, and the Company had negative working capital. These and other factors raise substantial doubt about the Company's ability to continue as a going concern. The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the possible inability of the Company to continue as a going concern.transactions have been eliminated in consolidation.
Significant Accounting Policies
Use of Estimates:
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statement and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from the estimates.
Cash and Cash Equivalents:cash equivalents
For financial statement presentation purposes, the Company
The Group considers thoseall short-term investments, which are highly liquid investments with original maturities of three months or less at the date of purchase, to be cash or cash equivalents. There were no cash equivalents as of December 31, 2015 and December 31, 2014.
Property and Equipment:equipment
New property
1. Property and equipment are recordedstated at cost.cost, net of accumulated depreciation. Depreciation is computedcalculated using the straight-line method over the estimated useful lives of the assets, generally 5 years. Expenditures for renewalsassets. When an asset is retired or otherwise disposed of, the related carrying value and bettermentsaccumulated depreciation are capitalized. Expenditures for minor items, repairsremoved from the respective accounts and maintenance are charged to operations as incurred. Gain or loss upon sale or retirement due to obsolescencethe net difference less any amount realized from disposition is reflected in the operating results in the period the event takes place.statements of operations.
Valuation
2. Rates of Long-Lived Assets: depreciation:
We review the recoverability
| % | ||||
| Computers | 33 | |||
| Furniture and office equipment | 7-15 | |||
Impairment of ourlong-lived assets
The Company’s long-lived assets including equipment, goodwillare reviewed for impairment in accordance with ASC 360, “Property, Plant and other intangible assets, whenEquipment”, whenever events or changes in circumstances occur that indicate that the carrying valueamount of thean asset may not be recoverable. The assessmentRecoverability of possible impairmentassets to be held and used is based on our ability to recovermeasured by a comparison of the carrying valueamount of an asset to the future undiscounted cash flows expected to be generated by the asset. If such asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset fromexceeds its fair value. To date the expected future pre-tax cash flows (undiscountedGroup did not incur any material impairment losses.
| 36 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and without interest charges)per share data)
NOTE. 2 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
Share-based compensation
The Company applies ASC 718-10, “Share-Based Payment,” which requires the measurement and recognition of compensation expenses for all share-based payment awards made to employees and directors (including employee stock options under the related operations. If these cash flows are less than the carrying value of such asset, an impairment loss is recognized for the difference betweenCompany’s stock plans) based on estimated fair value and carrying value. Our primary measure of fair value is based on discounted cash flows. The measurement of impairmentvalues.
ASC 718-10 requires managementcompanies to make estimates of these cash flows related to long-lived assets, as well as other fair value determinations.
Stock Based Compensation:
Stock-based awards are accounted for using the fair value method in accordance with ASC 718, Share-Based Payments. Our primary type of share-based compensation consists of stock options. We use the Black-Scholes option pricing model in valuing options. The inputs for the valuation analysis of the options include the market value of the Company's common stock, the estimated volatility of the Company's common stock, the exercise price of the warrants and the risk free interest rate.
Accounting For Obligations And Instruments Potentially To Be Settled In The Company's Own Stock:
We account for obligations and instruments potentially to be settled in the Company's stock in accordance with FASB ASC 815, Accounting for Derivative Financial Instruments. This issue addresses the initial balance sheet classification and measurement of contracts that are indexed to, and potentially settled in, the Company's own stock.
Fair Value of Financial Instruments: FASB ASC 825, "Financial Instruments," requires entities to discloseestimate the fair value of financial instruments, both assets and liabilities recognized and not recognizedequity-based payment awards on the balance sheet,date of grant. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in the Company’s statement of operations.
The Company recognizes compensation expenses for which it is practicable to estimate fair value. FASB ASC 825 definesthe value of non-employee awards based on the straight-line method over the requisite service period of each award, net of estimated forfeitures.
The Company estimates the fair value of stock options granted as equity awards using a financial instrument as the amount atBlack-Scholes options pricing model. The option-pricing model requires a number of assumptions, of which the instrument could be exchangedmost significant are share price, expected volatility and the expected option term (the time from the grant date until the options are exercised or expire). Expected volatility is estimated based on volatility of similar companies in a current transaction between willing parties. At December 31, 2015 and 2014, the carrying value of certain financial instruments (cash and cash equivalents, accounts payable and accrued expenses.) approximates fair value due to the short-term nature of the instruments or interest rates, which are comparable with current rates.
Fair Value Measurements:
technology sector. The Company adopted the FASB standard relatedhas historically not paid dividends and has no foreseeable plans to fair value measurement at inception.issue dividends. The standard defines fair value, establishes a framework for measuring fair value and expands disclosure of fair value measurements. The standard applies under other accounting pronouncements that require or permit fair value measurements and, accordingly, does not require any new fair value measurements. The standard clarifies that fair valuerisk-free interest rate is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricingthe yield from governmental zero-coupon bonds with an asset or liability.
As a basisequivalent term. The expected option term is calculated for considering such assumptions,options granted to employees and directors using the standard established a three-tier fair value hierarchy, which prioritizes“simplified” method. Grants to non-employees are based on the inputs used in measuring fair value as follows.
Level 1. Observable inputs such as quoted prices in active markets;Level 2. Inputs, other than quoted prices in active markets, that are observable either directly or indirectly; andLevel 3. Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
The Company values its derivative instruments related to embedded conversion features and warrants from the issuance of convertible debentures in accordance with the Level 3 guidelines. For the twelve-month period ended December 31, 2015, 2015 and 2014, the following table reconciles the beginning and ending balances for financial instruments that are recognized at fair value in these consolidated financial statements. The fair value of embedded conversion features that have floating conversion features and tainted common stock equivalents (warrants and convertible debt) are estimated using a Binomial Lattice model. The key inputs to this valuation model as of December 31, 2014, were: Volatility of 143.9% for the twelve-period ended December 31, 2015 and 132.4% for the twelve months period ending December 31, 2014, inherent term of instruments equal to the remaining contractual term, quoted closing stock prices on valuation dates, and various settlement scenarios and probability percentages summing to 100%.
Fair Value Measurements at December 31, 2015
| Level 3 - Derivative liabilities from: | Balance at December 31, 2015 | New Issuances | Extinguishment | Change in Fair Value | Balance at December 31, 2015 | ||||||||||
| Convertible Note | $ | 20,532 | $ | - | $ | (20,532) | - | $ | - | ||||||
Fair Value Measurements at December 31, 2014
| Level 3 - Derivative liabilities from: | Balance at December 31, 2014 | New Issuances | Settlements | Change in Fair Value | Balance at December 31, 2014 | ||||||||||
| Convertible Note | $ | - | $ | 13,147 | $ | - | 7,385 | $ | 20,532 |
term. Changes in the unobservable input values would likely cause material changes indetermination of each of the inputs can affect the fair value of the Company's Level 3 financial instruments. The significant unobservable inputoptions granted and the results of operations of the Company.
Earnings per Common Share
Earnings or loss per share (“EPS”) is the amount of earnings attributable to each share of common stock. For convenience, the term is used to refer to either earnings or loss per share. EPS is computed pursuant to ASC 260-10-45. Pursuant to ASC 260-10-45-10 through 260-10-45-16 Basic EPS is computed by dividing income available to common stockholders (the numerator) by the weighted-average number of common shares outstanding (the denominator) during the period. Income available to common stockholders shall be computed by deducting both the dividends declared in the fair value measurementperiod on preferred stock (whether or not paid) from income from continuing operations (if that amount appears in the income statement) and also from net income. The computation of diluted EPS is similar to the estimation for probability percentages assignedcomputation of basic EPS except that the denominator is increased to future expected settlement possibilities. A significant increase (decrease) in this distributioninclude the number of percentagesadditional common shares that would result in a higher (lower) fair value measurement.have been outstanding if the dilutive potential common shares had been issued during the period to reflect the potential dilution that could occur from common shares issuable through contingent shares issuance arrangement, stock options or warrants.
Revenue recognition
The following table presents assetsCompany applies the provisions of Accounting Standards Codification (or “ASC”) 606, Revenue from Contracts with Customers (“ASC 606”). The Company adopted the provisions of ASC 606 effective January 1, 2018 using the modified retrospective application method for all uncompleted contracts as of that date. The adoption of ASC 606 did not have a material impact on the Company’s consolidated financial statements. In addition, the adoption of ASC 606 had no impact on the Company’s trade receivables, deferred revenues and liabilities that were measured and recognized at fair valueaccumulated deficit balances balance as of December 31, 2015 and December 31, 2014 and2018 or on the years then ended on a recurring basis:
Fair Value Measurements at December 31, 2015Company’s revenues, cost of revenues or its operating expenses during 2018, compared to ASC 605.
| Level 1 | Level 2 | Level 3 | Total Unrealized (Gain) Loss | ||||||||
| 12/31/15 Derivative Liability | $ | - | $ | - | $ | - | $ | - | |||
| 12/31/14 Derivative Liability | $ | - | $ | - | $ | 20,532 | $ | 7,385 | |||
The following schedule summarizes the valuation of financial instruments at fair value on a recurring basis in the balance sheets as of December 31, 2015 and December 31, 2014:
Fair Value Measurements at December 31, 2015
Fair Value Measurements at December 31, 2014
| Level 3 | ||
| Assets | ||
| Total Assets | $ | - |
| Liabilities | ||
| Derivative liability | $ | 20,532 |
| Total Liabilities | $ | 20,532 |
The fair values of our debts are deemed to approximate book value, and are considered Level 2 inputs as defined by ASC Topic 820-10-35.
There were no transfers of financial assets or liabilities between Level 1, Level 2 and Level 3 inputs for the years ended December 31, 2015 and 2014.
The Company had no other assets or liabilities valued at fair value ongenerates revenues primarily by granting customers the right to access software products through the Company’s cloud-based SaaS subscription offerings. Under a recurring or non-recurring basisSaaS subscription agreement, the customer receives a right to access the software for a specified period of time in an environment hosted, supported, and maintained by the Company. SaaS subscription services are a single performance obligation satisfied over time, and associated revenue is generally recognized ratably over the contract term once the software is made available to the customer. The SaaS subscription offerings are typically sold with one year subscription terms, generally invoiced in advance of each annual subscription period, and are non-cancelable during the committed subscription term.
Research and development expenses, net:
Research and development expenses are charged to the statement of operations as of December 31, 2015 or December 31, 2014.incurred.
| 37 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and per share data)
Note 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
Income Taxes:
We have adopted FASB
The Company accounts for income taxes in accordance with ASC 740, Accounting for Income Taxes. Pursuant to“Income Taxes”, and (“ASC 740”). ASC 740 we are required to computeprescribes the use of the asset and liability method whereby deferred tax asset benefits for net operating losses carried forward. The potential benefits of net operating losses have not been recognized in these financial statements because the Company cannot be assured it is more likely than not it will utilize the net operating losses carried forward in future years.
We must make certain estimates and judgments in determining income tax expense for financial statement purposes. These estimates and judgments occur in the calculation of certain tax assets and liabilities, which arise from differences in the timing of recognition of revenue and expense for tax and financial statement purposes.
Deferred tax assets and liabilitiesliability account balances are determined based on the differences between the financial reporting and the tax basisbases of assets and liabilities and for carry forward tax losses. Deferred taxes are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. ASC 740 provides for the recognition ofThe Company records a valuation allowance, if necessary, to reduce deferred tax assets to their estimated realizable value if realization of such assetsit is more likely than not to occur. Realization of our net deferred tax assets is dependent upon our generating sufficient taxable income in future years in appropriate tax jurisdictions to realize benefit from the reversal of temporary differences and from net operating loss,more-likely-than-not that some portion or NOL, carryforwards. We have determined it more likely than not that these timing differences will not materialize and have provided a valuation allowance against substantially all of our net deferred tax asset. Management will continue to evaluate the realizability of the deferred tax asset and its related valuation allowance. If our assessment of the deferred tax assets or the corresponding valuation allowance were to change, we would record the related adjustment to income during the period in which we make the determination. Our tax rate may also vary based on our results and the mix of income or loss in domestic and foreign tax jurisdictions in which we operate.will not be realized.
In addition, the calculation of our tax liabilities involves dealing with uncertainties in the application of complex tax regulations. We recognize liabilities for anticipated tax audit issues in the U.S. and other tax jurisdictions based on our estimate of whether, and to the extent to which, additional taxes will be due. If we ultimately determine that payment of these amounts is unnecessary, we will reverse the liability and recognize a tax benefit during the period in which we determine that the liability is no longer necessary. We will record an additional charge in our provision for taxes in the period in which we determine that the recorded tax liability is less than we expect the ultimate assessment to be.
ASC 740 which requiresprescribes a recognition of estimated income taxes payable or refundable on income tax returnsthreshold and measurement attribute for the current yearfinancial statement recognition and for the estimated future tax effect attributable to temporary differences and carry-forwards. Measurement of deferred income tax is based on enacted tax laws including tax rates, with the measurement of deferred income tax assets being reduced by available tax benefits not expected to be realized.
Uncertain Tax Positions:
When tax returns are filed, it is highly certain that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. In accordance with the guidance of FASB ASC 740-10, Accounting for Uncertain Income Tax Positions, the benefit of a tax position taken or expected to be taken in a tax return. The first step is recognizedto evaluate the tax position taken or expected to be taken in a tax return. This is done by determining if the financial statements in the period during which, based on allweight of available evidence management believesindicates that it is more likely than notmore-likely-than-not that, on an evaluation of the technical merits, the tax position will be sustained upon examination,on audit, including the resolution of any related appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meetprocesses. The second step is to measure the more-likely-than-not recognition threshold are measuredtax benefit as the largest amount of tax benefit that is more than 50 percent50% likely of beingto be realized upon settlement with the applicable taxing authority. ultimate settlement.
Contingencies
The portion of the benefits associated with tax positions takenCompany records accruals for loss contingencies arising from claims, litigation and other sources when it is probable that exceedsa liability has been incurred and the amount measuredcan be reasonably estimated. These accruals are adjusted periodically as described above should be reflectedassessments change or additional information becomes available. Legal costs incurred in connection with loss contingencies are expensed as a liability for unrecognized tax benefitsincurred.
| 38 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in the accompanying consolidated balance sheets along with any associated interestthousands (except share and penalties that would be payable to the taxing authorities upon examination.per share data)
Our federal and state income tax returns are open for fiscal years ending on or after December 31, 2011. We are not under examination by any jurisdiction for any tax year. At December 31, 2015 we had no material unrecognized tax benefits and no adjustments to liabilities or operations were required under FIN 48.
Note 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
Recent Accounting Pronouncements
Recently issued accounting pronouncements
Financial Instruments – Credit Losses
In September 2015,June 2016, the FASB issued ASU No. 2015-16, Business Combinations2016-13, “Financial Instruments—Credit Losses (Topic 805) ("ASU 2015-16")326). Topic 805” The guidance replaces the current incurred loss impairment methodology with a methodology that reflects expected credit losses and requires that an acquirer retrospectively adjust provisional amounts recognized inconsideration of a business combination, during the measurement period. To simplify the accounting for adjustments madebroader range of reasonable and supportable information to provisional amounts, the amendments in the Update require that the acquirer recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amount is determined.inform credit loss estimates. The acquirer is required to also record, in the same period's financial statements, the effect on earnings of changes in depreciation, amortization, or other income effects, if any, as a result of the change to the provisional amounts, calculated as if the accounting had been completed at the acquisition date. In addition an entity is required to present separately on the face of the income statement or disclose in the notes to the financial statements the portion of the amount recorded in current-period earnings by line item that would have been recorded in previous reporting periods if the adjustment to the provisional amounts had been recognized as of the acquisition date. ASU 2015-16 isguidance will be effective for the Company’s fiscal yearsyear beginning December 15, 2015. The adoption of ASU 2015-016 is not expected to have a material effect on the Company's consolidated financial statements.
In August 2015, the FASB issued ASU No. 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date ("ASU 2015-14"). The amendment in this ASU defers the effective date of ASU No. 2014-09 for all entities for one year. Public business entities, certain not-for-profit entities, and certain employee benefit plans should apply the guidance in ASU 2014-09 to annual reporting periods beginning December 15, 2017,January 1, 2023, including interim reporting periods within that reporting period. Earlier application is permitted only as of annual reporting periods beginning after December 31, 2016, including interim reporting periods with that reporting period.
In April 2015, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") No. 2015-03, Interest-Imputation of Interest (Subtopic 835-30) ("ASU 2015-03"), which changes the presentation of debt issuance costs in financial statements. ASU 2015-03 requires an entity to present such costs in the balance sheet as a direct deduction from the related debt liability rather than as an asset. Amortization of the costs will continue to be reported as interest expense. It is effective for annual reporting periods beginning after December 15, 2016. Early adoption is permitted. The new guidance will be applied retrospectively to each prior period presented.year. The Company is currently in the process of evaluating the impactpotential effect of the adoption of ASU 2015-032019-10 on its balance sheets.
On November 2014,our financial position and results of operations. The Financial Accounting Standards Board (FASB) issued Accounting Standard Update No. 2014-16-Derivatives and Hedging (Topic 815): Determining WhetherCompany does not expect the Host Contract in a Hybrid Financial Instrument Issued in the Form of a Share Is More Akin to Debt or to Equity (a consensus of the FASB Emerging Issues Task Force). The amendments in this Update do not change the current criteria in GAAP for determining when separation of certain embedded derivative features in a hybrid financial instrument is required. That is, an entity will continue to evaluate whether the economic characteristics and risks of the embedded derivative feature are clearly and closely related to those of the host contract, among other relevant criteria. The amendments clarify how current GAAP should be interpreted in evaluating the economic characteristics and risks of a host contract in a hybrid financial instrument that is issued in the form of a share. The effects of initially adopting the amendments in this Update should be applied on a modified retrospective basis to existing hybrid financial instruments issued in the form of a share as of the beginning of the fiscal year for which the amendments are effective. Retrospective application is permitted to all relevant prior periods.
On November 2014, The Financial Accounting Standards Board (FASB) issued Accounting Standard Update No. 2014-17-Business Combinations (Topic 805): Pushdown Accounting (a consensus of the FASB Emerging Issues Task Force). The amendments in this Update provide an acquired entity with an option to apply pushdown accounting in its separate financial statements upon occurrence of an event in which an acquirer obtains control of the acquired entity. The amendments in this Update are effective on November 18, 2014. After the effective date, an acquired entity can make an election to apply the guidance to future change-in-control events or to its most recent change-in-control event. However, if the financial statements for the period in which the most recent change-in-control event occurred already have been issued or made available to be issued, the applicationadoption of this guidance would be a change in accounting principle.
On August 2014, The Financial Accounting Standards Board (FASB) issued Accounting Standard Update No. 2014-15, Presentation of Financial Statements - Going Concerns (Subtopic 205-40): Disclosures of Uncertainties about an Entity's Ability to Continue as a Going Concern. The amendments require management to assess an entity's ability to continue as a going concern by incorporating and expanding upon certain principles that are currently in U.S. auditing standards. Specifically, the amendments (1) provide a definition of the term substantial doubt, (2) require an evaluation every reporting period including interim periods, (3) provide principles for considering the mitigating effect of management's plans, (4) require certain disclosures when substantial doubt is alleviated as a result of consideration of management's plans, (5) require an express statement and other disclosures when substantial doubt is not alleviated, and (6) require an assessment for a period of one year after the date that the financial statements are issued (or available to be issued). The amendments in this Update are effective for the annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. Early application is permitted.
In June 2014, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2014-10, "Development Stage Entities". The amendments in this update remove the definition of a development stage entity from the Master Glossary of the ASC thereby removing the financial reporting distinction between development stage entities and other reporting entities from U.S. GAAP. In addition, the amendments eliminate the requirements for development stage entities to (1) present inception-to-date information in the statements of income, cash flows, and shareholder equity, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. The amendments in this update are applied retrospectively. The adoption of ASU 2014-10 removed the development stage entity financial reporting requirements from the Company.
In June 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2014-12, Compensation - Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. The new guidance requires that share-based compensation that require a specific performance target to be achieved in order for employees to become eligible to vest in the awards and that could be achieved after an employee completes the requisite service period be treated as a performance condition. As such, the performance target should not be reflected in estimating the grant-date fair value of the award. Compensation costs should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. If the performance target becomes probable of being achieved before the end of the requisite service period, the remaining unrecognized compensation cost should be recognized prospectively over the remaining requisite service period. The total amount of compensation cost recognized during and after the requisite service period should reflect the number of awards that are expected to vest and should be adjusted to reflect those awards that ultimately vest. The requisite service period ends when the employee can cease rendering service and still be eligible to vest in the award if the performance target is achieved. This new guidance is effective for fiscal years and interim periods within those years beginning after December 15, 2015. Early adoption is permitted. Entities may apply the amendments in this Update either (a) prospectively to all awards granted or modified after the effective date or (b) retrospectively to all awards with performance targets that are outstanding as of the beginning of the earliest annual period presented in the financial statements and to all new or modified awards thereafter. The adoption of ASU 2014-12 is not expected to have a material impact on ourthe Company’s financial position or results of operations.statements.
Accounting for Income Taxes
In June 2014,December 2019, the FASB issued ASU No. 2014-10: Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendmenta new standard to Variable Interest Entities Guidance in Topic 810, Consolidation , to improve financial reporting by reducingsimplify the cost and complexity associated with the incremental reporting requirements of development stage entities.accounting for income taxes. The amendments in this update remove all incremental financial reporting requirements from U.S. GAAP for development stage entities, thereby improving financial reporting by eliminating the cost and complexity associated with providing that information. The amendments in this Update also eliminate an exception provided to development stage entities in Topic 810, Consolidation, for determining whether an entity is a variable interest entity on the basis of the amount of investment equity that is at risk. The amendments to eliminate that exception simplify U.S. GAAP by reducing avoidable complexity in existing accounting literature and improve the relevance of information provided to financial statement users by requiring the application of the same consolidation guidance by all reporting entities. The elimination of the exception may change the consolidation analysis, consolidation decision, and disclosure requirements for a reporting entity that has an interest in an entity in the development stage. The amendmentseliminates certain exceptions related to the elimination of inception-to-date informationapproach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period, and the other remaining disclosure requirementsrecognition of Topic 915 shoulddeferred tax liabilities for outside basis differences related to changes in ownership of equity method investments and foreign subsidiaries. The guidance also simplifies aspects of accounting for franchise taxes and enacted changes in tax laws or rates, and clarifies the accounting for transactions that result in a step-up in the tax basis of goodwill. The standard will be applied retrospectively except for the clarification to Topic 275, which shall be applied prospectively. For public companies, those amendments are effective for annual reporting periodsus beginning after December 15, 2014, and interim periods therein. EarlyJuly 1, 2021, with early adoption is permitted. The adoptionAdoption of ASU 2014-10 isthe standard will not expected to have a material impact on our consolidated financial position or results of operations.statements.
Note 2. Going Concern.
| 39 |
The accompanying financial statements have been preparedVIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern. The Company has not established any source of revenue to cover its operating costs,thousands (except share and as such, has incurred an operating loss since inception. Further, as of December 31, 2015 the cash resources of the company were insufficient to meet its current business plan and the company has negative working capital. These and other factors raise substantial doubt about the Company's ability to continue as a going concern. The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the possible inability of the Company to continue as a going concern.
Note 3. Stockholders' Equity.
On January 8, 2015 the shareholders approved a resolution to increase the authorized common shares from 100,000,000 to 490,000,000 shares. All other provisions of the common shares remain unchanged. Also on that date, the Company declared a reverse split of common stock at the ration of 1:4. The stock split was effective January 8, 2015 for holders of record as of that date. Except as otherwise noted, all share, option and warrant numbers have been restated to give retroactive effect to this split. All per share disclosures retroactively reflect shares outstanding or issuabledata)
| Note 3. | Other Accounts receivables |
Composition:
As of December 30 | As of December 31 | |||||||
| 2 0 2 0 | 2 0 1 9 | |||||||
| Government authorities | $ | 20 | $ | 118 | ||||
| Other | - | 1 | ||||||
| $ | 20 | $ | 119 | |||||
| Note 4. | Property and equipment |
Composition:
As of December 30 | As of December 31 | |||||||
| 2 0 2 0 | 2 0 1 9 | |||||||
| Cost: | ||||||||
| Computers and related equipment | $ | 34 | $ | 34 | ||||
| Office furniture and equipment | 9 | 9 | ||||||
| 43 | 43 | |||||||
| Accumulated depreciation | 43 | 38 | ||||||
| Net book value | $ | - | $ | 5 | ||||
| Note 5. | Other accounts payable and accrued liabilities |
Composition:
As of December 30 | As of December 31 | |||||||
| 2 0 2 0 | 2 0 1 9 | |||||||
| Other payables and deferred revenues | $ | 47 | $ | 91 | ||||
| Accrued liabilities | 130 | 149 | ||||||
| Other | - | 6 | ||||||
| $ | 177 | $ | 246 | |||||
| 40 |
VIEWBIX INC. (Formerly known as though the reverse split had occurred at January 1, 2012.Virtual Crypto Technologies, Inc.)
Recent Issuances of Common StockNotes to Consolidated Financial Statements
During the year ended December 31, 2014 we issued 4,125,000 shares of our common stock (16,500,000 pre-reverse stock split)U.S. dollars in exchange for converting $125,000 of promissory notes.
Between January 15, 2015thousands (except share and March 15, 2015 the Company sold a total of 2,052,000 units for cash consideration of $780,000 at a price of $0.40 (the "Units"), each unit comprised of one share of common stock and one Class A warrant exercisable at $0.80 per share with a term 24 month. The relative fair value of the stock with embedded warrants was $351,433 for the common stock and $428,567 for the class A warrants. The warrants were valued using the Black-Scholes model with 153% volatility and discount rates ranging between 0.44% to 0.7%. These units were issued as stock payable and the cash from sale of units was not received for the sale of stock pre-reverse merger.data)
Between April 1, 2015 and June 29, 2015 the Company sold a total of 1,012,500 units for cash consideration of $405,000 at a price of $0.40 (the "Units"), each unit comprised of one share of common stock and one Class A warrant exercisable at $0.80 per share with a term 24 month. The relative fair value of the stock with embedded warrants was $158,123 for the common stock and $246,877 for the class A warrants. The warrants were valued using the Black-Scholes model with volatility ranging between163% - 177% and discount rates ranging between 0.54% to 0.71%. These units were issued as stock payable and the cash from sale of units was not received for the sale of stock pre-reverse merger.
On July 21, 2015 the Company sold a total of 140,000 units for cash consideration of $15,000 at price of $0.107 (the "Units"), each unit comprised of one share of common stock and one Class A warrant exercisable at $0.80 per share with a term 24 month. The relative fair value of the stock with embedded warrants was $4,294 for the common stock and $10,706 for the class A warrants. The warrants were valued using the Black-Scholes model with volatility of 153% and discount rates of 0.61%. These units were issued as stock payable and the cash from sale of units was not received for the sale of stock pre-reverse merger.
Between July 1, 2015 and September 30, 2015 the Company sold a total of 862,500 units for cash consideration of $345,000 at price of $0.40 (the "Units"), each unit comprised of one share of common stock and one Class A warrant exercisable at $0.80 per share with a term 24 month. The relative fair value of the stock with embedded warrants was $118,415 for the common stock and $226,585 for the class A warrants. The warrants were valued using the Black-Scholes model with volatility ranging between 153% - 182% and discount rates ranging between 0.54% to 0.71%. Of these units $65,000 were issued as stock payable and the cash from sale of units was not received for the sale of stock pre-reverse merger and $280,000 cash was received subsequent to Closing of the reverse merger.
On July 31, 2015 and July 30, 2015 the Company issued 517,900 shares to one service provider and a total of 100,000 shares to two service providers, respectively, for services valued at a total value of $617,900, arrived at using the stock price on date of grant of $1.00 per Nasdaq.com.
On July 16, 2015 five Emerald debt holders in amount of $87,910 converted their debt into 274,719 units at a conversion price of $0.32 per unit, each unit comprised of one share of common stock and one Class A warrant exercisable at $0.80 per share with a term 24 month. The Loss on Settlement of Debt recorded is $678,027.
On July 14, 2015 the Company issued Emerald's CEO and founder, Lior Wayn, 5,474,545 shares as per the share purchase agreement valued at $877,380, valued on the date of grant for the price of common stock.
On July 16, 2015 consultants were issued 2,500,000 Class B Warrants exercisable for a two-year period to acquire one (1) share of Common Stock at a price of $0.40 per share; The fair value of these warrants is $2,199,507. The warrants were valued using the Black-Scholes model with volatility of 182% and discount rate of 0.67%. The Class B warrants are fully vested and were accordingly included in expenses as stock based compensation.
On July 16, 2015 consultants were issued 2,536,247 Class C Warrants exercisable for a 90 day period, commencing 90 days after the effective date of this Registration Statement, at an exercise price of $0.40 to acquire one (1) share of Common Stock and one (1) Class A Warrant at an exercise price of $0.80. The fair value of these warrants is $3,143,581. The warrants were valued using the Black-Scholes model with volatility of 182% and discount rate of 0.67%. The Class C warrants are fully vested and were accordingly included in expenses as stock based compensation.
On November 17, 2015, the Company sold 250,000 units for cash consideration of $100,000 at price of $0.40 (the "Units"), each unit comprised of one share of common stock and one Class A warrant exercisable at $0.80 per share with a term 24 month. The relative fair value of the stock with embedded warrants was $41,304 for the common stock and $58,696 for the class A warrants. The warrants were valued using the Black-Scholes model with volatility of 149% and a discount rate of 0.50%.
Between November 5, 2015 and November 16, 2015 the Company issued 268,084 shares to three service providers and for services valued at a total value of $268,084, arrived at using the stock price on date of grant of $1.00 per Nasdaq.com.
On October 1, 2015 the company granted a total of 534,400 stock options (the "Options") to three company employees. The options vest over 5 quarters and are exercisable at prices ranging from $0.01 to $0.40 per Share. The options were valued using the Black-Scholes model with 149% volatility and 0.67% discount rate for a total value of $528,857. Of this amount, $397,547 was expensed in Q4 2015 with the remaining balance to be expensed in 2016.
Recent Option Grants
During 2015, we granted 534,400 options at an exercise price ranging between $0.01 and $0.40.
(1) Options were granted to these employees, none of which have been exercised to date.
Note 4.
| Note 6. | Related Party Transactions. |
Balances:
| December 31, | December 31, | |||||||
| 2 0 2 0 | 2 0 1 9 | |||||||
| Gix – Parent Company Payable | $ | 2,054 | $ | 1,611 | ||||
As part of the agreement with Gix, the parties agreed to have the Company’s operations outsourced to Gix from the agreement date and until the acquisition is consummated. The following term were included in the agreement pursuant to the above:
| (a) | From May 2018 all of the Company’s employees will become employees of Gix. | |
| (b) | Between the periods of May 2018 to October 2018, Gix will pay the full expenses of the employees as well as other related expenses. | |
| (c) | From November 2018 until to the Closing Date, the employees transferred from the Company to Gix will dedicate half of their time to the Company’s operations and correspondingly 50% of the costs to be incurred by Gix in respect of these employees are to be charged to the Company. |
From the closing date, the actual of the expenses incurred by Gix that related to the Company will be charged to the Company.
No amounts were paid by the Company to Gix during 2020 and 2019.
| Note 7. | Short term loan and Issues of shares |
On March 25, 2014, our PresidentDecember 18, 2020, the company entered into a Loan Agreement (the “Loan”) and principal shareholder assigned accumulated advances and accruals totaling $124,229,Stock Subscription Agreement with certain Investors as described in note 1e, pursuant to which the Investors lent an unaffiliated third party. The advances carry no specificaggregate amount of $69,000 (the “Principal Amount”). In accordance with the terms of repayment. On December 15, 2014, $22,375the Loan, the company prepaid the interest on the Principal Amount of 8% compounded annually to the Investors as an issuance of 552,000 shares of Common Stock, at a price per share of $0.01. Under the Stock Subscription Agreement, the Investors transferred an amount of $ 30,587 to the company as consideration for the issued shares.
The Company allocated the total proceeds in respect of the then outstandingshares issued and the Loan extended based on its relative fair values. As a result of the allocation, a discount of $19 was recorded on the loan. The discount is amortized over the term of the loan as finance expense.
| 41 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and per share data)
| Note 7. | Short term loan and Issues of shares (Cont.) |
The allocation of the proceeds to the fair value distribution of the liability and equity components on the transactions date was as follows:
| Instrument | Fair Value | % of total fair | Allocated amount | |||||||||
| Loan | 55,200 | 49.45 | 49,246 | |||||||||
| Shares | 54,000 | 50.55 | 50,340 | |||||||||
| Total | 109,200 | 100 | 99,586 | |||||||||
The composition of short term loan balance was converted to a promissory note (see Note 4 below). A summaryas of transactionsthe transaction is as follows:
| December 31, 2015 | December 31, 2014 | |||
| Beginning balance | $ | - | $ | 161,729 |
| Increase due to payments made on behalf of the company | $ | - | $ | 21,625 |
| Less March 24, 2014 conversion to convertible note | $ | - | $ | (40,000) |
| Less December 15, 2014 conversion to promissory note | $ | - | $ | (22,375) |
| Obligation transferred to unrelated party | $ | - | $ | (120,979) |
| Total | - | - | ||
| Less current portion | - | - | ||
| Due after one year | $ | - | $ | - |
There was no
| Principal amount | 69 | |||
| Discount on Short term loan | (19 | ) | ||
| Short term loan, Net | 50 | |||
Note 8. Stockholders’ deficit.
Ordinary Shares:
Ordinary shares confer the right to participate in the general meetings, to one vote per share for any purpose, to an equal part, on share basis, in distribution of dividends and to equally participate, on share basis, in distribution of excess of assets and funds from the Company and they shall not confer other privileges unless stated term of interest associated with this obligation. Accordingly,hereunder or in the Companies Law otherwise. Some investors have standard anti-dilutive rights, registration rights, and information and representation rights.
On December 18, 2020, the company imputedentered into a Stock Subscription Agreement (the “Subscription”) with certain investors (the “Investors”) in connection with the sale and issuance of an aggregate of 3,000,000 shares of Common Stock, at a purchase price of $0.01 per share, and for an aggregate purchase price of $30,000. In addition, and on the same date, the company entered into a Loan Agreement (the “Loan”) with the Investors, pursuant to which the Investors lent an aggregate amount of $69,000 (the “Principal Amount”). In accordance with the terms of the Loan, the company repaid the interest on the Principal Amount of 8% compounded annually to the Investors as an issuance of 552,000 shares of Common Stock, at a price per share of $0.01. The shares of Common Stock were issued to the Investors pursuant to Regulation S of the Securities Act of 1933, as amended. For more details, please see note 1e.
| 42 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and per share data)
Note 8. Stockholders’ deficit. (Cont.)
Preferred shares (relating to Viewbix Ltd the prior to the Recapitalization Transaction):
Preferred shares may have been converted into ordinary shares of Viewbix Ltd at any time. The preferred shares would have automatically converted into ordinary shares if (a) the holders of at least (i) 67% (sixty seven percent) of the issued and outstanding Preferred C/C-1 shares, (ii) a majority of the issued and outstanding Preferred B shares, and (iii) a majority of the issued and outstanding Preferred A shares, so agree in writing; or (b) in the event of an appropriate rate estimatedIPO.
The conversion price for any class or series of preferred would have been subject to adjustment, as follows: at 8%any time, upon each issuance or deemed issuance by the Company of any new securities at a price per share less than the applicable conversion price in effect on the date of and immediately prior to the issuance of such new securities, the conversion price shall be reduced.
Preferred shares had priority in the distribution of dividends and upon liquidation in accordance with the Company’s Articles of Association (“AOA”). These rights may be changed if a meeting of the Company’s stockholders gather up and decides on a change of regulations in this context.
The preference mechanism for liquidation and the distribution of dividends gave priority to the most recent preferred stockholders.
The preferred shares were convertible into 16,199,520 ordinary shares of the Company.
Redemption
The Company’s AOA do not provide redemption rights to the holders of the preferred shares. In the event of a liquidation event, all the funds and assets of the Company available for distribution among all the stockholders shall be distributed based on a certain mechanism as prescribed under FASBdescribed in the Company’s AOA. Although the preferred shares are not redeemable, in the event of certain “deemed liquidation events” that are not solely within the Company’s control (including merger, acquisition, or sale of all or substantially all of the Company’s assets), the holders of the preferred shares would be entitled to preference amounts paid before distribution to other stockholders (as explained in the previous paragraph) and hence effectively redeeming the preference amount. In accordance with ASR 268 and ASC 835. For480 “Distinguishing Liabilities from Equity”, the period endingCompany’s preferred shares are classified outside of stockholders’ deficit as a result of these in-substance contingent redemption rights. As of December 31, 20142019 and 2018, the resultant chargeCompany did not adjust the carrying values of $11,210the convertible preferred shares to interest expensethe deemed liquidation values of such shares since a liquidation event was considerednot probable of occurring.
Share Exchange
As detailed in Note 1, as part of the Recapitalization Transaction in July 2019, the Company issued 30,928,620 common shares in exchange for 99.83% of the issued and outstanding ordinary shares and all the preferred shares of Viewbix Israel. The number of shares prior to the reverse capitalization have been retroactively adjusted based on the equivalent number of shares received by the accounting acquirer in the Recapitalization Transaction.
Warrants
The following table summarizes information of outstanding warrants as of December 31, 2020:
| Warrants | Warrant Term | Exercise Price | Exercisable | |||||||||||
| Class J Warrants | 3,649,318 | July 2029 | 0.48 | 3,649,318 | ||||||||||
| Class K Warrants | 3,649,318 | July 2029 | 0.80 | 3,649,318 | ||||||||||
Additionally, in connection with the Share Exchange Agreement, upon the earlier of: (a) the launch of a contributionlive video product to an American consumer in the United States by Viewbix Israel, or (b) the launch of capital.an interactive television product to an American consumer in the United States by Viewbix Israel, the Company will issue to Gix an additional 1,642,193 shares of restricted common stock of the Company. All of the Company’s warrants meet the US GAAP criteria for equity classification. During January and March 2020, 50,000 class H warrants expired. During January 2020, 38,095 class I warrants expired. During April 2020, 142,857 Class G warrants expired.
| 43 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and per share data)
Note 9. Commitments and Contingencies
During August 2019, a lawsuit was filed against the second quarter an agreementCompany and its parent Company, Gix. The plaintiffs claim that they were entitled to receive shares of the Company as a part of the consideration in Gix’s acquisition of the Company. In management’s opinion, the plaintiffs’ claims are based on incorrect assumptions that relate to the distribution of shares between the plaintiffs and other former shareholders of the Company prior to Gix’s acquisition which would have resulted in the receipt of shares in the acquisition transaction. During September 2020, a settlement was reached withbetween the holder of a $120,979 advance payable note to settleparties which was later approved by the full amount due for $30,000, and interest due.court. The settlement with all note holders resultedoutlines that in $528 loss on debt settlement dueexchange for the voluntary waiver of claims made by the plaintiffs, Gix will issue 63,350 shares of its common stock held in trust in favor of securing the transaction by which Gix acquired shares of ViewBix Ltd. in November 2018. The remaining shares in the trust account will be used to indemnify Gix for any expenses related to the payment being higher than principallitigation. Since the consideration was paid in Gix’s shares, and accrued interest as the claims relate to the distribution of shares between the plaintiffs and other former shareholders of the Company, the settlement date as well asdid not impact on the Company’s financial statements.
In June 2017, a charge of $90,979, thatlawsuit was consideredfiled by a contribution of capital due to the fact that note holder, IMWT, was a related party.
Formerformer CEO of the Company whom during November 2014 loaned amount to company of $19,521, an interest rate of 8% per annum convertedwith the balance to shares as describedTel Aviv District Court (the “Tel Aviv Court”) against the Company claiming certain damages in Note 3.
On July 16, 2015 five Emerald debt holders inthe total amount of $87,910 converted their debt into 274,719 units at a conversion price$225, under the assertion of $0.32 per unit, each unit comprised of one share of common stock and one Class A warrant exercisable at $0.80 per share with a term of 24 month. The Loss on Settlement of Debt recorded is $678,027, on the income statement.
On July 14, 2015wrongful termination by the Company issued Emerald'sand Emerald Israel. The Company believes these claims to be unsubstantiated and wholly without merit and accordingly filed its response with the Tel Aviv Court in October of 2017. The dispute was initially heard by the Tel Aviv Court on February 13, 2020. In a supplemental hearing on February 11, 2021 the former CEO provided data regarding his claims and founder, Lior Wayn, 5,474,545 shares as per the share purchase agreement valued at $877,380, valued on the date of grant using the closing price of common stockhis summaries were filed on that date.
day. The Company'sCompany’s summaries will be filed within 30 days of receiving the former CEO Lior Wayn was owed $3,480 and $0 payable as of December 31, 2015 and December 31, 2014, respectively.
Following the closing of the reverse merger, the $490,000 loan from Emerald Medical Applications Corp. to Emerald Medical Applications Ltd. was rendered an intercompany loan and as such was written off.
Note 5. Employee Payable.
For the periods ended December 31, 2015, the Company had a total of $29,092 of which $3,480 were related party and $25,612 were unrelated party employee payable related to the monthly wages payable to the Company's employees. For the periods ended December 31, 2014, the Company had $0 in employee payable related to the monthly wages payable to the Company's employees.
Note 6. Notes Payable.
Convertible Notes Payable
In accordance to ASC #815, Accounting for Derivative Instruments and Hedging Activities, we evaluated the holder's non-detachable conversion right provision and liquidated damages clause, contained in the terms governing the Note to determine whether the features qualify as an embedded derivative instruments at issuance. Such non-detachable conversion right provision and liquidated damages clause did not need to be accounted for as derivative financial instruments. Additionally, since the conversion price of the note was lower that the fair market value of the Company's common stock at the time of issuance, no beneficial conversion feature exists if the lender elected to convert. Based on that decision, no beneficial conversion feature was reflected in the financial statements.
On July 8, 2014, the Company issued a convertible promissory note to Axel Springer Plug & Play Accelerator GmbH (the "Holder"), in the amount of $29,719. During 2014 and 2015 we recorded $9,555 and $0, respectively, in amortization of debt discount.summaries. As of December 31, 20152020, the company’s management, in consultation with its legal advisors, believes that the former CEO’s claims will not be Successful.
Note 10. Revenues.
| Year ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Individual Subscriptions | 13 | 17 | ||||||
| Enterprise Subscriptions | 83 | 191 | ||||||
| 96 | 208 | |||||||
Note 11. Research and 2014, we had accrued interest of $2,013development expenses.
| Year ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Salaries and related expense | 55 | 219 | ||||||
| Others | 53 | 14 | ||||||
| 108 | 233 | |||||||
| 44 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and $19,285, respectively.per share data)
Note 12. Seles and marketing expenses.
| Year ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Salaries and related expense | 7 | 110 | ||||||
| Others | 1 | 147 | ||||||
| 8 | 257 | |||||||
Note 13. General and administrative expenses.
| Year ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Wages, salaries and related expenses | 214 | 276 | ||||||
| Professional fees | 176 | 213 | ||||||
| Depreciation | 5 | 1 | ||||||
| Recapitalization Transaction costs | - | 112 | ||||||
| Other | 42 | 118 | ||||||
| 437 | 720 | |||||||
Note 14. Financing (income) expenses, net
| Year ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Bank fees | 1 | 3 | ||||||
| Exchange rate differences | (14 | ) | 107 | |||||
| Other financial income | - | (12 | ) | |||||
| (13 | ) | 98 | ||||||
| 45 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and per share data)
Note 15. Income Taxes.
The Convertible NoteCompany is convertible atsubject to income taxes under the lessor of a market based discountedIsraeli and a fixed rate derived from a fixed market cap. The Holders haveU.S. tax laws:
Tax rates applicable to the right following the Date of Issuance, and until any time until the convertible Promissory Note is fully paid, to convert any outstanding and unpaid principal portionincome of the Convertible Promissory Note, and accrued interest, into fully paid and non-assessable shares of Common Stock. Holder was not issued warrants with the Convertible Promissory Note. See Note 7 for description of derivative testing.Company:
Note Payable - Not Convertible
Viewbix Inc. is taxed according to U.S. tax laws. On December 15, 2014, we issued a promissory note22, 2017, the U.S. enacted the Tax Cuts and Jobs Act (the “Act”), which among other provisions, reduced the U.S. corporate tax rate from 35% to 21%, effective January 1, 2018.
Viewbix Israel and Israeli subsidiaries are taxed according to Israeli tax laws. The Israeli corporate tax rate is 23% in the amountyears 2020, 2019 and onwards.
Deferred income taxes:
Deferred income taxes reflect the net tax effects of $22,375 to a related party in considerationtemporary differences between the carrying amounts of assets and liabilities for payments made on behalffinancial reporting purposes and the amounts used for income tax purposes. Significant components of the Company for service provided to the Company (the "December 2014 Note"). The December 2014 Note bears interest at the rate of 1% per annum, is due and payable on May 12, 2015. On April 21, 2015 this promissory note with interest due was repaid in full.Company’s deferred tax assets are as follows:
On January 14 and 16, 2015, we issued two promissory notes in the amount of $15,000 each to two different unrelated parties in consideration for cash transferred to the Company (the "January 2015 Notes"). The Notes were issued to unrelated parties and due to the low interest rate an imputed interest expense was calculated. The January 2015 Notes bears interest at the rate of 1% per annum, are due and payable on January 14 and 16, 2016 and are not convertible to common stock.
As of December 31 | As of December 31 | |||||||
| 2 0 2 0 | 2 0 1 9 | |||||||
| Deferred R&D expenses | $ | 114 | $ | 239 | ||||
| Operating loss carryforward | 32,256 | 32,443 | ||||||
| $ | 32,370 | $ | 32,682 | |||||
| Net deferred tax asset before valuation allowance | $ | 7,076 | $ | 7,149 | ||||
| Valuation allowance | (7,076 | ) | (7,149 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
One of the notes was repaid in full on March 3, 2015 with interest due waived the by the debtor, and the second note was repaid on April 22, 2015 with interest due waived the by the debtor.
During the second quarter an agreement was reached with the holder of a $120,979 advance payable note to settle the full amount due for $30,000, and interest due. The settlement with all note holders resulted in $528 loss on debt settlement due to the payment being higher than principal and accrued interest as of the settlement date as well as a charge of $90,979, that was considered a contribution of capital due to the fact that note holder, IMWT, was a related party.
We concluded that these notes have a stated rate of interest that is different from the rate of interest that is appropriate for this type of debt at the date of the transaction. Accordingly, the company imputed interest at an appropriate rate estimated at 8% as prescribed under FASB ASC 835. The resultant charge of $6,280 for the period ending December 31, 2014 and $4,113 for the period ending December 31, 2015 to interest expense was considered a contribution of capital and was recorded in additional paid in capital.
On November 16, 2014 four individuals loaned a total amount of $87,910 to the Company with maturity dates of November 16, 2015 and bearing an interest rate of 8% per annum. These notes were fully converted on July 16, 2015 to Company shares of commons stock and warrants as described in Note 3 resulting in a $678,027 loss on settlement of debt.
Between March 31, 2015 and December 31, 2015 the Chief Scientist Ministry of Israel loaned the company an amount of $119,974. The loan bears 17% interest and shall be due and payable when the company generates sales revenue from products in development.
For the periods ended December 31, 2015 and December 31, 2014 the Company has recognized $19,285 and $2,013, respectively, in accrued interest expense related to the stated interest rate on the notes. Interest expense for the periods ended December 31, 2015 and December 31, 2014, respectively, were $30,604 and $5,605 of which $9,555 and $0 is from the amortization of debt discount.
Note 7. Derivative Liabilities from Convertible Note.
On July 8, 2014 the Company issued a convertible promissory note to Axel Springer Plug & Play Accelerator GmbH (the "Holder"), in the amount of $29,719.
The Convertible note is convertible at the lessor of a market based discounted and a fixed rate derived from a fixed market cap. The Holder has the right following the Date of Issuance, and until any time until the convertible Promissory Note is fully paid, to convert any outstanding and unpaid principal portion of the Convertible Promissory Note, and accrued interest, into fully paid and non-assessable shares of Common Stock. The Holder was not issued warrants with the Convertible Promissory Note.
As of December 31, 2015 the note is no longer convertible since pursuant to the loan agreement prior to December 31,2015 (the "Maturity Date"),2020, the Company consummated a financing round led by unaffiliated investorshas provided valuation allowances of $7,076 in the amountrespect of at least 200,000 Euro, at a Company pre-money valuation on a fully diluted basis of at least 750,000 Euro (a "Qualified Round"), the Holder shall be entitled (but not obligated) to convert the entire loan amount into the most senior class of shares of the Company issued in such Qualified Round, based on a price per share equal to the lower of the price per share reflected by a Company pre-money valuation on a fully diluted basis calculated at the time of conversion equal to 1,500,000 Euro; or - price per share which reflects a 20% discount on the lowest price per share issued pursuant to such Qualified Rounddeferred tax assets resulting from tax loss carryforward and upon the occurrence of such event, the note holder elected not to convert upon receiving notice of such event and the loan became non-convertible.
The following shows the changes in the derivative liability measured on a recurring basis for the twelve months ended December 31, 2015, and year ended December 31, 2014.
| Level 3 | ||
| Derivative Liability at December 31, 2013 | $ | - |
| Additions to Derivative Liability related to Convertible Debt | 20,532 | |
| Derivative Liability at December 31, 2014 | $ | 20,532 |
| Extinguishment of Derivative Liability | (20,532) | |
| Derivative Liability at December 31, 2015 | $ | - |
As of December 31, 2014,other temporary differences. Management currently believes that because the Company has a $20,532 derivative liabilityhistory of losses, it is more likely than not that the deferred tax regarding the loss carryforward and a $20,164 convertible note payable, net of discount of $9,555. As of December 31, 2015, the Company has $0 derivative liability and $29,719 convertible note payable, net of discount of $0.
In accordance to ASC #815, Accounting for Derivative Instruments and Hedging Activities, we evaluated the holder's non-detachable conversion right provision and liquidated damages clause, containedother temporary differences will not be realized in the terms governing the Note to determine whether the features qualify as an embedded derivative instruments at issuance. Such non-detachable conversion right provision and liquidated damages clause did not need to be accounted for as derivative financial instruments. Additionally, since the conversion price of the notes represented the fair market value of the Company's common stock at the time of issuance, no beneficial conversion feature exists. No beneficial conversion feature was reflected in the financial statements because at the time of the agreement the FMV of the shares, if converted, were less than the original note amount and the $20,532 extinguishment of derivative was reflected in the equity and cash flow statements as a non-cash transfer from liabilities to equity.foreseeable future.
Note 8. Other Receivables.
Available carryforward tax losses:
As of December 31, 20152020, Viewbix Israel incurred operating losses in Israel of approximately $13,804 which may be carried forward and December 31, 2014offset against taxable income in the Company had other receivables of $25,797 and $6,718, respectively, which represent VAT refunds claimed resulting from excess VAT paid over VAT received from the Israeli government.future for an indefinite period.
Note 9. Accounts Payable and Accrued Liabilities.
As of December 31, 2015 and December 31, 20142020 the Company had accounts payable and accrued liabilities of $90,705 and $2,577, respectively, which mainly represent accrued expenses such as accrued vacation and deferred salary.
Note 10. Litigation Accruals.
On November 9, 2015, the Company received a notice of claim from Tomer Maharshak & Co., Israel, the Company's former attorneys, for legal fees allegedly owed by the Company and its wholly owned Israeli subsidiary, Emerald Medical Applications Ltd. On December 12, 2015 and December 23, 2015 the litigation was settled for cash payment and the Company recorded neither a gain nor a loss on the settlement. As of December 31, 2015 the litigation accrual balance is zero and there are currently no ongoing litigations against the Company.
Note 11. Income Taxes.
We have adopted ASC 740 which provides for the recognition of a deferred tax asset based upon the value the carry-forwards will have to reduce future income taxes and management's estimate of the probability of the realization of these tax benefits. Ourgenerated net operating loss carryovers incurred prior to 2014 consideredlosses in the U.S. of approximately $18,452 Net operating losses in the U.S. are available to reduce future income taxes were reduced or eliminated through our recent change2035. Utilization of control (I.R.C. Section 382(a)) and the continuity of business limitation of I.R.C. Section 382(c).
We have a currentU.S. net operating loss carry-forward of $1,941,140 resulting in deferred tax assets of $679,399. We have determined it more likely than not that these timing differences will not materialize and have provided a valuation allowance against substantially all our net deferred tax asset.
Future utilization of currently generated federal and state NOL and tax credit carry forwardslosses may be subject to a substantial annual limitation due to the ownership change limitations provided by“change in ownership” provisions of the Internal Revenue Code of 1986 as amended and similar state provisions. The annual limitation may result in the expiration of NOLnet operating losses before utilization.
| 46 |
VIEWBIX INC. (Formerly known as Virtual Crypto Technologies, Inc.)
Notes to Consolidated Financial Statements
U.S. dollars in thousands (except share and tax credit carry forwardsper share data)
Note 15. Income Taxes (cont.)
Loss (income) from continuing operations, before full utilization.taxes on income, consists of the following:
| December 31, 2015 | December 31, 2014 | |||
| Individual components giving rise to the deferred tax assets are as follows: | $ | $ | ||
| Future tax benefit arising from net operating loss carryovers | 679,399 | 35,510 | ||
| Less valuation allowance | (679,399) | (35,510) | ||
| Net deferred asset | $ | - | $ | - |
| For the year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| USA | $ | 65 | $ | 113 | ||||
| Israel | 376 | 989 | ||||||
| $ | 441 | $ | 1,102 | |||||
NOTE 16. LOSS PER SHARE-BASIC AND DILUTED
Composition:
| For the year ended December 31 | ||||||||
| 2 0 2 0 | 2 0 1 9 | |||||||
| Basic and diluted: | ||||||||
| Net loss attributable to ordinary stockholders | 443 | 1,117 | ||||||
| Weighted-average ordinary shares | 31,201,669 | 13,746,064 | ||||||
| Loss per share-basic and diluted | 0.014 | 0.08 | ||||||
NOTE 17. - COVID-19 PANDEMIC IMPLICATIONS
The COVID-19 pandemic, which originated in China in late 2019, has since spread across the globe and affected the economic condition of most, if not all, countries, including the United States, Israel and many countries in Europe. On March 11, 2020, the World Health Organization declared the outbreak a pandemic. While COVID-19 is still spreading and the final implications of the pandemic are difficult to estimate at this stage, it is clear that it has affected the lives of a large portion of the global population. As of December 31, 2020, the pandemic has caused repeated states of emergency to be declared in various countries, ongoing and extended travel restrictions have been imposed for several months, strict quarantines rules have been established and maintained for an extended period of time in a plethora of jurisdictions and various institutions and companies have been closed and rendered bankrupt. The Company is actively monitoring the pandemic and is taking any necessary measures to respond to the situation in cooperation with the various stakeholders. Due to the uncertainty surrounding the COVID-19 pandemic, the Company will continue to assess the situation, including government-imposed restrictions, market by market. It is not under examinationpossible at this time to estimate the full impact that the COVID-19 pandemic could have on the Company’s business, the continued spread of COVID-19, and any additional measures taken by any jurisdiction for any tax year. Our federalgovernments, health officials or by the Company in response to such spread, could have on the Company’s business, results of operations and state income tax returnsfinancial condition. The COVID-19 pandemic and mitigation measures have also negatively impacted global economic conditions, which, in turn, could adversely affect the Company’s business, results of operations and financial condition. The extent to which the COVID-19 outbreak continues to impact the Company’s financial condition will depend on future developments that are open for fiscal years ending onhighly uncertain and cannot be predicted, including new government actions or after December 31, 2011.
Note 12. Subsequent Events.
During Q1 2016 throughoutrestrictions, new information that may emerge concerning the dateseverity, longevity and impact of the filing of Form 10-K. the following subsequent events occurred:COVID-19 pandemic on economic activity.
On January 26, 2016 and after receiving conversion notices from Class B warrant holders, the Company's board of directors approved issuance of 1,928,572 shares for the cashless conversion of Class B warrants. On February 18, 2016, the Company's board of directors approved the issuance of 1,195,000 shares as compensation to three acting company directors: Mrs. Estery Giloz-Ran, Mr. Yair Fudim and Mr. Baruch Kfir. On February 17, 2016 and March 17, 2016, the Company board of directors approved the issuance of 175,000 shares for services to two investor relations service providers.
| 47 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSUREBack to Table of Contents
None.
ITEM 9A.9A. CONTROLS AND PROCEDURESBack to Table of Contents
Evaluation of Disclosure Controls and Procedures
Evaluation of disclosure
Disclosure controls and procedures. As of December 31, 2015,procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Company's chiefExchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, as appropriate, to allow timely decisions regarding required disclosure.
Our management, including our principal executive officer and chiefprincipal financial officer, conducted an evaluation regardingevaluated the effectiveness of the Company'sour disclosure controls and procedures (as defined inpursuant to Rules 13a-15(e) orand 15d-15(e) under the Exchange Act.Act as of December 31, 2020, the end of the period covered by this Annual Report on Form 10-K. Based uponon such evaluation, due to the evaluation of these controls and procedures,material weakness discussed below, our chiefprincipal executive officer and chiefprincipal financial officer concluded that our disclosure controls and procedures were ineffectivenot effective at a reasonable assurance level as of the end of the fiscal year 2015 under the COSO framework.December 31, 2020.
| 48 |
Management’s Annual Report on Internal Control Overover Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and for the assessment15d-15(f) of the effectiveness of those internal controls. As defined by the SEC,Exchange Act. The Company’s internal control over financial reporting is a process designed by our principal executive officer and principal financial officer, who is also the sole member of our Board of Directors, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the financial statements for external reporting purposes in accordance with U.S. generally accepted accounting principles.GAAP.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management has assessedevaluated the design and operating effectiveness of internal control over financial reporting based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO 2013”). Based on this evaluation, management concluded that our internal control over financial reporting as of December 31, 2015. 2019 was not effective due to the material weakness described below.
In making this assessment, management usedconnection with the criteria set forth bypreparation of our consolidated financial statements as of and for the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on our assessment and those criteria,year ended December 31, 2020, we have concluded thatidentified a material weakness in our internal control over financial reporting. The material weakness was identified in the period-end financial reporting process, and is associated with our history as a private company and a material weakness is a deficiency or combination of deficiencies in our internal control over financial reporting hadsuch that there is a reasonable possibility that a material weaknesses including lackmisstatement of sufficient internal accounting personnelour consolidated financial statements would not be prevented or detected on a timely basis. This deficiency could result in additional misstatements to our consolidated financial statements that would be material and would not be prevented or detected on a timely basis.
We are evaluating and implementing additional procedures in order to ensure complete documentationremediate this material weakness, however, we cannot assure you that these or other measures will fully remediate the material weakness in a timely manner.
Attestation Report of complex transactions and adequate financial reporting during the year ended December 31, 2015. Management has identified corrective actions for the weakness and will begin implementation during the second quarter of 2016.Registered Public Accounting Firm
This annual report on Form 10-K does not include an attestation report of the company'sCompany’s registered public accounting firm regarding internal control over financial reporting. Management'sManagement’s report was not subject to attestation by the Company'sCompany’s registered public accounting firm pursuant to temporary rules of the Securities and Exchange CommissionSEC that permit the Company, as a non-accelerated filer, to provide only Management'smanagement’s report in this annual report.report on Form 10-K.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting or in other factors identified in connection with the evaluation required by paragraph (d) of Exchange Act Rules 13a-15 or 15d-15 that occurred during the fourth quarter ended December 31, 20152020 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATIONBack to Table of Contents
None.
| 49 |
ITEM 10. DIRECTORS, AND EXECUTIVE OFFICERS OF THE REGISTRANT AND CORPORATE GOVERNANCEBack to Table of Contents
Our directors were elected to serve until the next annual meeting of shareholders and until his respective successors will have been elected and will have qualified. The following table sets forth the name, age and position held with respect to our present executive officers and directors:
| Name | Age | Title | ||
| 43 | ||||
| Director |
Lior Wayn, 43, the CEO and a director since August 28, 2015. Mr. Wayn founded Emerald Medical Applications Ltd. ("Emerald) on February 17, 2010, based upon his years of experience in the information and communications technologies (ICT) industry and his know how in mobilizing teams for large and complex projects. Before forming Emerald, Mr. Wayn served from 2006 through 2010 as head of the Business Development Division (VP) at Malam Team, a public company traded on the Tel-Aviv Stock Exchange, and the largest IT services group in Israel, providing a comprehensive range of computer services in the field of information technology. Mr. Wayn previously worked in sales and business development for several of Israel's leading ICT organizations including Ness Technologies, EIM and Michshuv group. Mr. Wayn received Lior received a BA degree in business administration from the Ruppin Academic Center, a leading Israeli university. He furthered his education in Human Resource Management at Bar-Iian University, in IT Management at College of Management, Rishon Lezion and in Hotel and Hospitality Management at Tadmor College, all in Israel.
Oded Gilboa, 42,Amihay Hadad a licensed CPA in the United States and Israel, has been the CFOserved as our chief executive officer since February 2015. Since December 2013, Mr. Gilboa has also been serving as CFO of BreedIt Corp., a reporting company under the Exchange Act. Mr. Gilboa has over 18 years of experience in finance20, 2020, chief financial officer since July 25, 2019, and public accounting, having servedwas appointed as a senior finance executive in the technology and biotech industries with responsibilities in corporate finance, accounting, strategic planning and operational and financial management.member of our board of directors on January 1, 2020. From 2010 through 2012,2011 until 2018, Mr. GilboaHadad served as the Revenue Accounting and Finance Managerchief financial officer of Mylan Specialty,Yedioth Internet. As of January 30, 2020, Mr. Hadad serves as the chief executive officer of Gix, a subsidiary of Mylan Inc. (NASDAQ: MYL), a company focused on the development, manufacturing and marketing of prescription drug products. From 2007 through 2009, Mr. Gilboa was the Executive Director of Finance and US Controller of Taro Pharmaceuticals (NASDAQ:TAROF), a global pharmaceutical company. From 1998 through 2007 Mr. Gilboa held various financial positions with IDT Corporation (NYSE:IDT), a world-wide provider of telecommunications and media services, where in his most recent role he served as Director of Finance. Mr. Gilboa began his career in public accounting, auditing both public and private companies and holds a B.A in Economics and Accounting and an M.B.A. from the Tel-Aviv University.
Yair Fudim, 66, Chairmancontrolling stockholder of the Registrant’s Board of Directors effective April 30, 2015, has also been servingCompany, in addition to his existing role as Chairman and CEO of Peregrine Industries, Inc.,Gix’s chief financial officer. Mr. Hadad holds both a public company (OTCQB: PGID) since July 2013. During the past five years, Mr. Fudim has also served as Chairman of Dolomite Holdings Ltd., a public company organized in Israel and listed on the Tel Aviv Stock Exchange ("TASE") since February 2013, prior to which he served as Dolomite's CEO from February 2010 until March 2013. From April 1991 through April 2013, Mr. Fudim served as CEO of Leader Holdings & Investments Ltd ("Leader Holdings"), a public company organized in Israel and listed on the TASE; Mr. Fudim also serves as Chairman of the Board of Leader Capital Markets Ltd., a TASE listed public company organized in Israel and a subsidiary of Leader Holdings. Mr. Fudim holds a B.A. in Economics and an MBA from the HebrewCollege of Management Academic Studies in Rishon LeZion, Israel, and an M.A. in law from Bar-Ilan University, of Jerusalem.Israel. Mr. Hadad is also a certified public accountant in Israel.
Baruch Kfir, 67,Alon Dayan has served as a directormember of our board of directors since September 6, 2015.March 14, 2018, and from January 24, 2018 until July 25, 2019, he served as our chief executive officer. From March 2010July 2014 to the present, Mr. Baruch KfirDayan served as the chief executive officer and founder of L1 Systems Ltd., an Israeli based company engaged in the business of providing the public and private sectors with advanced security solutions. Since July 2013, Mr. Dayan has served as Senior Wealth Manager for Pioneer International Ltd, a wealth management firm based in Israel. Mr. Kfir's duties have included both developingchief executive officer and maintaining financial planning for high-net-worth clients in Mexico and Venezuela and previously served as CEO of Pioneer, Venezuela. From October 2005 until February 2010, Mr. Kfir was the headfounder of the Latin-American Desk of the Israel Discount Bank, Switzerland (IDB), responsiblePolaris Star, an Israeli-based company which is engaged in providing advanced cyber security telecommunication for developing new clientsutilities world-wide. Mr. Dayan earned his B.Tech. degree in Argentina, Brazil, Venezuela and Mexico for IDB. Mr. Kfir has over 30 years of experience in international banking and finance, specialized in the Latin and South American markets, received his B.A.electronic engineering from HaifaAriel University Israel.
Dr. Estery Giloz-Ran, 41, a director since September 6, 2015, is a Certified Public Accountant, received a PhD in tax, accounting and finance from the Business Administration Department at the Ben-Gurion University in Beer Sheva, Israel in 2013. During the past five years, Dr. Giloz-Ran has served as Head of Accountancy at Peres Academic Center, a leading Israeli college located in Rehovot, Israel. Dr. Giloz-Ran presently serves as a director of: (i) Kamada Ltd, an Israeli biotech company listed on NASDAQ and Tel-Aviv Stock Exchange/TASE; (ii) Vaxil Bio Ltd, an Isreali biotech company listed on TASE, and (iii) Suny Electronic Inc. Ltd, an Israeli electronics company listed on TASE. Since 2006, Dr. Giloz-Ran has been lecturing at Ben-Gurion University at the Faculty of Business and Management - Department of Economics and Accounting. Dr. Giloz-Ran was a Visiting Assistant Professor of Finance at the Syms School of Business at Yeshiva University, New York and a Visiting Scholar at New York University - Leonard N. Stern School of Business, New York. Dr. Giloz-Ran served as a tax consultant and tax capital investment law adviser at Intel Corporation in Israel.
We do not compensate our directors. We do not have any standing committees at this time.
Involvement in Certain Legal Proceedings
Our director, officers or affiliates have not, within the past five years, filed any bankruptcy petition, been convicted in or been the subject of any pending criminal proceedings, or is any such person the subject or any order, judgment or decree involving the violation of any state or federal securities laws.
Family Relationships
There are no family relationships between or among any of our directors or executive officers.
Compliance with Section 16(a) Compliance.
Section 16(a) of the Securities and Exchange Act of 1934 requires that directors and executive officers, and persons who own beneficially more than ten percent (10%) of the Registrant’s Common Stock, to file reports of ownership and changes of ownership with the Securities and Exchange Commission. Copies of all filed reports are required to be furnished to the Registrant pursuant to Section 16(a). Based solely on the reports received by the RegistrantThe Registrant’s officers and on written representations from reporting persons, the Registrant was informed that our CEO has and the CFO and Chairman have not filed reports asdirectors are current in their filings are required under Section 16(a).
NASDAQ Rule 4200.The NASDAQ Rule 4200, which sets forth several tests to determine whether a director of a listed company is independent. Rule 4200 provides that a director would not be considered independent if the director or an immediate family member accepted any compensation from the listed company in excess of $120,000 during any period of 12 consecutive months within the three years preceding the determination of independence (excluding compensation for board or board committee service, compensation paid to an immediate family member as a non-executive employee, benefits paid under a tax-qualified retirement plan and non-discretionary compensation).
Director Independence.In determining whether or
The Company does not our directors are consideredcurrently have any independent the Company used the definition of independence as defined in NASDAQ Rule 4200. We therefore believe that only Yair Fudim is an independent director.directors.
Directors’ Term of Office.
Our directors are elected tofor a term of one year and serve until the next annual meeting of shareholders and until their respective successors will have beensuch director’s successor is duly elected and will have qualified. Each executive officer serves at the pleasure of the board.
| 50 |
Audit Committee and Financial Expert, Compensation Committee, Nominations Committee.
We do not have any of the above mentioned standing committees because our corporate financial affairs and corporate governance are simple in nature at this stage of development and each financial transaction is approved by our sole officerofficers or director.board of directors.
Potential Conflicts of Interest.Interest.
Since we do not have an audit or compensation committee comprised of independent Directors,directors, the functions that would have been performed by such committees are performed by our Boardboard of Directors.directors. Thus, there is a potential conflict of interest in that our Directorsdirectors have the authority to determine issues concerning management compensation, in essence their own, and audit issues that may affect management decisions. We are not aware of any other conflicts of interest with any of our Executivesexecutives or Directors.directors.
Board’s Role in Risk Oversight.The Board assesses
Our board of directors assess on an ongoing basis the risks faced by the Company. These risks include financial, technological, competitive, and operational risks. In addition, since the Company does not have an Audit Committee,audit committee, the Boardboard of directors is also responsible for the assessment and oversight of the Company’s financial risk exposures.
Involvement in Certain Legal Proceedings.
We are not aware of any material legal proceedings that have occurred within the past ten years concerning any Directordirector or control person which involved a criminal conviction, a pending criminal proceeding, a pending or concluded administrative or civil proceeding limiting one’s participation in the securities or banking industries, or a finding of securities or commodities law violations.
ITEM 11. EXECUTIVE COMPENSATIONBack
Any compensation received by our officers, directors, and management personnel will be determined from time to Tabletime by our Board of ContentsDirectors. Our officers, directors, and management personnel will be reimbursed for any out-of-pocket expenses incurred on our behalf.
The following table depictssets out the total compensation that we have paid or that has accrued on behalf of our chief executive officer and other executive officers duringfor the fiscal years endingended December 31, 2015, 20142020, 2019 and 2013.2018, as applicable, to the following Named Executive Officers:
| Annual Compensation | Long Term Compensation Awards | |||||||||||||
| Other | Restricted | Securities | ||||||||||||
| Annual | Stock | Underlying | All Other | |||||||||||
| Name and Principal | Salary | Bonus | Compensation | Award(s) | Options | Compensation | ||||||||
| Position | Year | ($) | ($) | ($) | ($) | ($) | ($) | |||||||
| Lior Wayn, CEO (1) | 2015 | 149,064 | — | — | — | — | — | |||||||
| Lior Wayn, CEO (1) | 2014 | 27,630 | — | — | — | — | — | |||||||
| Liron Carmel, former CEO (2) | 2015 | 2,500 | — | — | — | — | — | |||||||
| Liron Carmel, former CEO, CFO (2) | 2014 | — | — | — | — | — | — | |||||||
| Oded Gilboa, CFO (3) | 2015 | 79,353 | — | — | — | — | — | |||||||
| Ivo Heiden, former CEO and CFO (4) | 2013 | — | — | — | — | — | — | |||||||
| ● | Mr. Amihay Hadad, our current Chief Executive Officer and Chief Financial Officer; | |
| ● | Mr. Jonathan Stefansky, our former Chief Executive Officer, who resigned from such role on January 1, 2020; and |
| The table is in U.S. dollars Name and principal position | Year | Salary | Bonus | Stock Awards | Option Awards | All Other Compensation | Total | |||||||||||||||||||||
| Mr. Amihay Hadad | 2020 | 47,072 | - | - | - | - | 47,072 | |||||||||||||||||||||
| Current Chief Executive Officer, Chief Financial Officer | 2019 | - | - | - | - | - | - | |||||||||||||||||||||
| 2018 | - | - | - | - | - | - | ||||||||||||||||||||||
| Mr. Jonathan Stefansky | 2020 | 59,805 | 59,805 | |||||||||||||||||||||||||
| Former Chief Executive Officer | 2019 | 134,838 | - | - | - | - | 134,838 | |||||||||||||||||||||
| 2018 | 160,370 | - | - | - | - | 160,370 | ||||||||||||||||||||||
| 51 |
Executive Employment Agreements
Director’s Compensation
Emerald has entered into an employment agreements with Lior Wayn, its CEO, and Oded Gilboa its CFO.
(1) Emerald’sOur directors are not entitled to receive compensation for service rendered to us or for meeting(s) attended except for reimbursement of out-of-pocket expenses. There is no formal or informal arrangements or agreements to compensate employee directors for service provided as a director; however, compensation for new non-employee directors is determined on an ad hoc basis by the existing members of the board of directors at the time a director is elected.
Compensation Policies and Practices as They Relate to the Company’s Risk Management
We believe that our compensation policies and practices for all employees, including executive officers, do not create risks that are reasonably likely to have a material adverse effect on us.
Employment Contracts
We do not have any formal employment agreement with Lior Wayn, dated January 1, 2015, provides for a base annual salaryany of NIS46,000 which is equivalent to approximately $12,000 for which Mr. Wayn is required to devote 100% of his business time to the affairs of Emerald. In addition, Mr. Wayn’s agreement also provides for the payment of cash bonuses as follows: (i) a bonus equal to 5% of the monthly revenues of Emerald during the years ending December 31, 2015 and 2016, payable quarterly; and (ii) a cash bonus equal to 7 months base salary only in the event that the Registrant raises at least $1,150,000 from: (i) the sale of equity securities at a price of not less than $0.80 per Share; or (ii) the exercise of warrants at an exercise price of not less than $0.80 per Share.(2) Liron Carmel was the sole executive officer during 2014.
(3) The employment agreements between Mr. Gilboa and the Registrant and Emerald, dated March 22, 2015 and February 25, 2015, respectively, provide as follows: (i) the Registrant shall pay Mr. Gilboa cashour officers. Any future compensation for the initial two month period a total of $2,000 following which Mr. Gilboa will be paid atdetermined by the rateBoard of $3,000 per monthDirectors, and, as additionalappropriate, an employment agreement will be executed. We do not currently have plans to pay any compensation until such time as the Registrant issued Mr. Gilboa 125,000 restricted Shares; and (ii) Emerald shall pay Mr. GilboaCompany maintains a positive cash compensation of NIS13,200 which is equivalent to approximately $3,450 per month.(4) Ivo Heiden was the sole executive officer in 2013.flow.
Option GrantsOutstanding Equity Awards
There were no individual grantsequity awards outstanding as of the end the year ended December 31, 2020.
Option Grants
During the year ended December 31, 2020, the board of directors did not authorize the issuance of stock options to purchase our Common Stock made to the executive officers named in the Summary Compensation Table. However, the Registrant issued Class E Warrants to Lior Wayn at the Closing exercisableand directors to purchase 2,700,000 Shares in three equal tranchesshares of 900,000 Shares each, at a price of $0.0001 per Share.Common Stock.
Aggregated Option Exercises and Fiscal Year-End Option Value
There were no stock options exercised during periodthe year ending December 31, 20152020 by theour executive officers named in the Summary Compensation Table.officers.
Long-Term Incentive Plan ("LTIP"(“LTIP”) Awards
There were no awards made to a named executive officers in the last completed fiscal year under any LTIP.
Compensation of Directors
Directors are permitted to receive fixed fees and other compensation for their services as directors. The Board of Directors has the authority to fix the compensation of directors. No amounts have been paid to, or accrued to, directors in such capacity.
Outstanding Warrants
The following table summarizes information of outstanding warrants as of December 31, 2015:
| Warrants | Warrant Term | Exercise Price | Exercisable | |||||||||
| Investors - Class A Warrants (1) | 4,149,719 | 2 years | $ | 0.80 | 4,149,719 | |||||||
| Investors - Class B Warrants (2) | 2,500,000 | 2 years | $ | 0.40 | 2,500,000 | |||||||
| Investors - Class C Warrants (3) | 5,072,492 | (3) | (3) | 55,072,492 | ||||||||
| Lior Wayn - Class E Warrants (4) | 2,700,000 | (4) | 0.0001 | 2,700,000 | ||||||||
(1) The Class A Warrants were issued in connection with a private placement in reliance upon Regulation S, pursuant to which the Registrant sold a total of 4,149,719 units at a price of $0.40 per unit (the "Units"), each Unit comprised of one Share and one Class A Warrant exercisable at $0.80 per share with a term 24 months. While all of the Class A Warrants are exercisable within 60 days, in fact, none of these warrants will be exercised for the foreseeable future, based upon the exercise price of $0.80 per Share.
(2) The Class B Warrants were issued to consultants for bona fide services to the Company and are exercise, on a cashless basis at a price of $0.40 per Share for a period of two years.(3) The Class C Unit Warrants were issued to consultants for bona fide services to the Company, and each Unit is exercisable at a price of $0.40 to purchase one Share of Common Stock and one Class A Warrant which, in turn, is exercisable to purchase one additional Share at a price of $0.80. The Class C Unit Warrants expire ninety (90) days after the effective date of this Registration Statement.(4) The Class E Warrants were issued by the Registrant to Lior Wayn in connection with the Closing of the Share Exchange Agreement. The Class E Warrants are exercisable to purchase a total of 2,700,000 Shares, in three equal tranches of 900,000 Shares each (the "Tranches") at an exercise price of $0.0001 per Share, subject to and within 45 days of the Registrant achieving the milestones defined in the table below (the "Milestones").
Certain Relationships and Related Party Transactions and Director Independence
Mr. Ivo Heiden, our former CEO, CFO and sole director who resigned on March 24, 2014, provided securities compliance services valued at $5,250 in 2014 and $18,000 in 2013.
Indebtedness of Management
No officer, director or security holder known to us to own of record or beneficially more than 5% of our Common Stockcommon stock or any member of the immediate family or sharing the household (other than a tenant or employee) of any of the foregoing persons is indebted to us in the years 20152020 and 2014.2019.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED SHAREHOLDERSTOCKHOLDER MATTERSBack to Table of Contents
The following table depictsbelow provides information regarding the beneficial ownership of our common stockCommon Stock as of MarchDecember 31, 2016. The information provides2020, of (i) each of our current directors, (ii) each of the ownership information for:Named Executive Officers, (iii) all of our current directors and officers as a group, and (iv) each person or entity known byto us to be the beneficial owner ofwho owns more than 5% of our common stock; each of our directors; each of our executive officers; and our executive officers and directors as a group.stock.
| Name of Beneficial Owner | Common Stock Beneficially Owned (1) | Percentage of Common Stock Owned (1) | |||
| Lior Wayn, CEO (2) | 5,474,545 | 29.4% | |||
| 1 Emek Ayalon Street | |||||
| Modi’in, Israel | |||||
| Oded Gilboa, CFO | 125,000 | 0.70% | |||
| 10 Hayetsira Street | |||||
| Raanana, Israel | |||||
| Yair Fudim, Chairman | 482,000 | 2.60% | |||
| Zhitomir Street | |||||
| Tel-Aviv, Israel | |||||
| Baruch Kfir, Director | 231,000 | 1.20% | |||
| 7 Imber Street | |||||
| Petach Tivka, 4951141, Israel | |||||
| Dr. Estery Giloz-Ran, Director | 690,323 | 3.70% | |||
| 57/8 Borocov | |||||
| Givataim, Israel | |||||
| Amir Uziel (3) | 1,513,393 | 8.10% | |||
| 42 Ben Zvi Street | |||||
| Rmat Gan, Israel | |||||
| Itschak Shrem (4) | 1,367,429 | 7.30% | |||
| 42 Ben Zvi Street | |||||
| Rmat Gan, Israel | |||||
| Lavi Krasney (5) | 1,513,393 | 8.10% | |||
| 42 Ben Zvi Street | |||||
| Rmat Gan, Israel | |||||
| Directors and Officers (5 persons) | 7,002,868 | 40.25% | |||
| 52 |
(1) ApplicableThe percentage ownershipof Common Stock beneficially owned is based on 18,624,46134,753,669 shares of common stockCommon Stock outstanding as of MarchDecember 31, 2016. Beneficial ownership is determined in accordance with the rules2020. The number and percentage of the Securities and Exchange Commission and generally includes votingshares of Common Stock beneficially owned by a person or investment power with respect to securities. Sharesentity also include shares of common stockCommon Stock issuable upon exercise of warrants that are currently exercisable or will become exercisable within 60 days of MarchDecember 31, 20162020. However, these shares are not deemed to be beneficially owned by the person holding such securitiesoutstanding for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage ownershipshares beneficially owned of any other person.person or entity.
| Name and Address of Beneficial Owner | Title of Class | Amount and Nature of Beneficial Ownership(1) | Percent of Class | |||||||
| Gix Internet Ltd. | Common Stock | 27,579,721 | (2) | 79.36 | % | |||||
| Alon Dayan | Common Stock | 50,000 | 0.14 | % | ||||||
| L.I.A. Pure Capital Ltd. | Common Stock | 2,631,571 | (3) | 8.43 | % | |||||
| Directors and officers as a group (2 individuals) | Common Stock | 50,000 | 0.14 | % | ||||||
| (1) | Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Each of the beneficial owners named in the table have, to our knowledge, direct ownership of and sole voting and investment power with respect to the shares of Common Stock beneficially owned by them. |
| (2) | Includes (i) 20,281,085 of shares of Common Stock, (ii) warrants to purchase up to 3,649,318 shares of restricted Common Stock with an exercise price of $0.48 per share, and (iii) warrants to purchase up to 3,649,318 shares of restricted Common Stock with an exercise price of $0.80 per share, which are currently exercisable or will become exercisable within 60 days of December 31, 2020. |
| (3) | The number of shares shown as beneficially owned by this stockholder is based on its Schedule 13G filed on February 8, 2021. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTORS INDEPENDENCEBack to Table of Contents
Certain Related Party Transactions
Mr. Ivo Heiden, our former CEO, CFO and sole director who resigned on March 24, 2014, provided securities compliance services valued at $5,250 in 2014.
IndebtednessOn December 18, 2020, L.I.A. Pure Capital Ltd. (“Pure Capital”), together with other Investors, entered into the Stock Subscription Agreement, pursuant to which Pure Capital was issued 1,000,000 shares of Management
No officer, director or security holder known to us to own of record or beneficially more than 5% of our Common Stock or any memberin exchange for an investment of $10,000, at a purchase price of US$0.01 per share. Additionally, Pure Capital, together with other Investors, entered into the Loan Agreement, pursuant to which Pure Capital lent $23,000 and we repaid the interest on that amount in the form of an issuance of 184,000 shares of Common Stock to Pure Capital, at a price per share of $0.01. The shares of Common Stock were issued to the Investors pursuant to Regulation S of the immediate family or sharing the household (other than a tenant or employee)Securities Act of any of the foregoing persons is indebted to us in the years 2015 and 2014.1933, as amended.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICESBack to Table of Contents
Independent Public Accountants
The Registrant'sRegistrant’s Board of Directors has appointed M&K CPAS PLLCBrightman Almagor Zohar & Co. as independent public accountant for the fiscal years ended December 31, 2015 and 2014.
Principal Accounting Fees2020. Halperin had served as the Company’s independent registered public accounting firm for the fiscal periods beginning July 6, 2018 through November 7, 2019.
Principal Accounting Fees
The following table presents the fees for professional audit services rendered by M&K CPAS PLLC(a) Brightman Almagor Zohar & Co. for the audit of the Registrant'sRegistrant’s annual financial statements for the year ended December 31, 20152020; (b) professional audit services rendered by (i) Halperin Ilanit CPA and 2014 and(ii) Brightman Almagor Zohar & Co. for the audit of the Registrant’s annual financial statements for the year ended December 31, 2019; (c) fees billed for other services rendered by M&K CPAS PLLC during those periods.Brightman Almagor Zohar & Co. for the Registrant’s fiscal period beginning November 7, 2019 and ending December 31, 2019; and (c) the aggregate fees billed in each of the last two fiscal years as pertaining to, among others, tax compliance, tax advice and tax planning conferred to the Registrant.
| 53 |
| Year Ended | Year Ended | |||||||
December 31, 2020 | December 31, 2019 | |||||||
| Audit fees (1) | 50,000 | 42,500 | (2) | |||||
| Audit-related fees (3) | - | 60,000 | (4) | |||||
| Tax -related fees (5) | 2,948 | 20,500 | ||||||
| (1) | Audit fees consist of audit and review services, consents and review of documents filed with the SEC. |
| (2) | Audit Fees consists of $12,500 in connection with the services rendered by Halperin Ilanit CPA, and $30,000 in connection with the services rendered by Brightman Almagor Zohar & Co Audit-related fees. |
| (3) | Audit-related fees consist of assistance and discussion concerning financial accounting and reporting standards and other accounting issues in connection with the Share Exchange Agreement. |
| (4) | Consists of $2,000 in connection with the services rendered by Halperin Ilanit CPA, and $58,000 in connection with the services rendered by Brightman Almagor Zohar & Co. |
| (5) | Tax fees consist of, among other items, preparation of federal and state tax returns, review of quarterly estimated tax payments, Israeli tax rulings, consultation concerning tax compliance issues, and services rendered for purposes of a tax ruling filed with government agencies and institutions in connection with the Recapitalization Transaction. |
| 54 |
| Year Ended | Year Ended | ||||
| December 31, 2015 | December 31, 2014 | ||||
Audit fees (1) | $ | 6,500 | $ | 4,600 | |
Audit-related fees (2) | --- | --- | |||
Tax fees (3) | --- | --- | |||
All other fees | --- | --- | |||
| (1) Audit fees consist of audit and review services, consents and review of documents filed with the SEC. | |||||
| (2) Audit-related fees consist of assistance and discussion concerning financial accounting and reporting standards and other accounting issues. | |||||
| (3) Tax fees consist of preparation of federal and state tax returns, review of quarterly estimated tax payments, and consultation concerning tax compliance issues. | |||||
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULESBack to Table of Contents
(a) The following documents are filed as exhibits to this report on Form 10-K or incorporated by reference herein. Any document incorporated by reference is identified by a parenthetical reference to the SEC filing that included such document.
Exhibit No. Description
| * | Filed herewith. | |
| 55 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned.
EMERALD MEDICAL APPLICATIONS CORP.
| VIEWBIX INC. | ||
| Date: March 16, 2021 | By: | /s/ Amihay Hadad |
| Amihay Hadad | ||
| Chief Executive Officer | ||
By: /s/ Lior WaynLior WaynChief Executive Officer(Principal Executive Officer)Date: March 31, 2016By: /s/ Oded GilboaOded GilboaChief Financial Officer(Principal Financial and Principal Accounting Officer)Date: March 31, 2016
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below on this 16th day of March 2021 by the following persons on behalf of the registrant and in the capacities and onindicated, including a majority of the dates indicated.By: /s/ Yair FudimYair FudimChairmanDate: March 31, 2016
By: /s/ Lior WaynLior WaynDirectorDate: March 31, 2016directors.
| Signature | Title | |
| /s/ Amihay Hadad | Chief Executive Officer | |
| Amihay Hadad | (Principal Executive Officer) | |
| /s/ Amihay Hadad | Chief Financial Officer and Director | |
| Amihay Hadad | (Principal Financial and Accounting Officer) | |
| /s/ Amihay Hadad | Director | |
| Amihay Hadad | ||
| /s/ Alon Dayan | Director | |
| Alon Dayan |
| 56 |