2023

| Delaware | 34-1940305 | ||||
|
| ||||
State or other jurisdiction of incorporation or organization | (I.R.S. Employer Identification Number) | ||||
7555 Innovation Way, Mason, OH | 45040 | ||||
(Address of principal executive offices) | (Zip Code) | ||||
Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||
Common Stock, $.001 | ATRC | NASDAQ Global Market | ||||||
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
| Accelerated Filer ☐ | Non-Accelerated Filer ☐ |
| Emerging Growth Company ☐ | ||||||||||||||||||||||
As of February 23, 2018, there were 34,560,275 shares of Common Stock, $.001 par value per share, outstanding.
| Class | Outstanding February 13, 2024 | ||||
| Common Stock, $.001 par value | 47,587,966 | ||||
(Dollar
Physicians have adopted our radiofrequency (RF) ablation and cryoablation systems to treat Afib in over 250,000 patients since 2004, and we believe that we are currently the market leader in the surgical treatment of Afib. Our products are used by physicians during both open-heart and minimally invasive surgical procedures, either on a concomitant or standalone basis. During a concomitant procedure, the physician ablates cardiac tissue and/or occludes the LAA, secondary, or concomitant, to a primary structural heart procedure such as a valve repair or replacement or coronary artery bypass graft (CABG). Our Isolator Synergy System is approved by FDA for the treatment of persistent and long-standing persistent Afib concomitant to other open-heart surgical procedures. Our other ablation devices are all cleared for sale under FDA 510(k) clearances, including our other RF and cryoablation products, which are indicated for the ablation of cardiac tissue and/or the treatment of cardiac arrhythmias. In addition, our cryoICE probe is cleared for managing pain by temporarily ablating peripheral nerves. Our AtriClip products are 510(k)-cleared with an indication for the occlusion of the heart’s LAA, in conjunction with other cardiac surgical procedures. We also have a line of reusable surgical instruments typically used in cardiac valve replacement or repair. We anticipate that substantially all of our revenue for the foreseeable future will relate to products we currently sell, or are in the process of developing, which are used to ablate cardiac tissue, to occlude the left atrial appendage, to support mitral and aortic valve replacement and repair and/or to ablate peripheral nerves during cardiothoracic surgery.
Afib affects approximately 1% of the population in the United States.epidemic. It is the most common cardiac arrhythmia or irregular heartbeat, encountered in clinical practice and accountsresults in high utilization of healthcare services and significant cost burden. Patients often progress from being in Afib intermittently (paroxysmal) to being in Afib continuously. The continuous Afib patient population includes early persistent Afib, which lasts seven days to 6 months, persistent Afib, which lasts 6 months to one year, and long-standing persistent Afib, which lasts longer than one year. It is estimated that over 3.5 million people in the United States currently suffer from long-standing persistent Afib. Afib often occurs in conjunction with other cardiovascular diseases, including hypertension, congestive heart failure, left ventricular dysfunction, coronary artery disease and valvular disease.
In the United States, we sell our products to medical centers through our direct sales force. In certain international markets, such as Germany, France, the United Kingdom and the Benelux region, sales are also made directly to medical centers, while other international sales are made to distributors who in turn sell our products to end users. Our business is primarily transacted in U.S. Dollars with the exception of transactions with our European subsidiaries, which are transacted in Euros or British Pounds.
1
Market Overview
Afib is the most commonly diagnosed sustained cardiac arrhythmia, and affects more than 30 million people worldwide, including more than five millionin the United States. It is estimated that the incidence of Afib doubles with each decade of an adult’s life. At age 40, remaining lifetime risk for Afib is 26% for men and 23% for women. Afib is an under-diagnosed condition due in large part to the fact that patients with Afib, often have mild or no symptoms and their Afib is only diagnosed when they seek treatment for an associated condition, such as a structural heart disease or stroke.significant percentage of those clots can form inside of the LAA. We believe that increasing awareness of Afib and improved diagnostic screening will result in an increased number of patients diagnosed with Afib. Recently, there have been several new diagnostic technologies introduced in the United States that allow for less invasive screening options, which should assist patients with more compliant and proactive identification of Afib.Afib over time. Also, since the prevalence of Afib increases with age, there will likely be an increase in the number of diagnosed Afib patients in the United Statesglobally as the world population ages. We believe that the same trends in the United States apply globally, as in many geographies the incidence of Afib is increasing as the population ages.
surgical ablation. In addition, Afib is thought to be responsible for approximately 15% to 20% of the estimated 700,000800,000 strokes that occur annually in the United States. According to the American Heart Association, the risk of stroke is five times higher in people with Afib. Studies have also suggested that 90% of clots that cause strokes in patients who have Afib originate from within the LAA. Recently, a large independent international randomized trial, Left Atrial Appendage Occlusion Study (LAAOS) III, demonstrated a significant reduction in strokes when the LAA was managed during cardiac surgery. Afib accounts for billions of dollars in hospitalization-related and office visit costs in the United States each year. Indirect costs, such as the management of Afib-related strokes, are also believed to be significant. Because of the risk of stroke and the significant cost burden on the healthcare system, more and more surgeons are routinely addressing the LAA.LAA, both in patients who have Afib and in those who do not have Afib but may be at increased risk of developing the disease in the future. We believe that our AtriClip system is safer, more effective and easier to use than other products and techniques for occludingexcluding the LAA and, because of this,during cardiac surgery. Therefore, we believe that the market for our ablation products and the AtriClip system represent significant growth opportunities.
Cardiothoracicopportunity for the Company.
2
Clinicalapproaches. We have completed, and continue to invest in, clinical studies for the use of our ablation and LAAM products to treat Afib are ongoing.and reduce stroke. Leading cardiothoracic surgeons and electrophysiologists, including those who serve or who have served as consultants to us, have published results of initialpreclinical and clinical studies utilizing our Isolator Synergy System.devices. The results of these studies are to evaluatehave assessed efficacy, ease of use and safety.
We have two primary product linessafety endpoints.
Product linesthe treatment of persistent and long-standing persistent Afib concomitant to other open-heart surgical procedures. Certain products of our Isolator Synergy clamps bear the CE mark and may be commercially distributed throughout the member states of the European Union and other countries that comply with or mirror the Medical Device Directive. These products are available for sale in a number of other countries globally.
|
|
| •Multifunctional Pens and Linear Ablation Devices. These devices are single-use disposable RF products that come in multiple configurations. The MAX Pen devices enable surgeons to evaluate cardiac arrhythmias, perform temporary cardiac pacing, sensing and stimulation and ablate cardiac tissue with the same device. Surgeons can readily toggle back and forth between these functions. The device comes in multiple configurations that have unique tissue contacting and shaft lengths. The Coolrail® device enables the user to make longer linear lines of ablation. Surgeons generally use one or more of our pen and linear devices in combination with Isolator Synergy clamps. |
|
|
|
|
|
|
Our linear ablation devices are cleared for sale in the United States under FDA 510(k) clearances, with indications for the ablation of cardiac tissue and/or the treatment of cardiac arrhythmias. Our Isolator Synergy pens bear the CE mark, and most configurations may be commercially distributed throughout the member states of the European Union and other countries that comply with or mirror the Medical Device Directive. These products are available for sale in a number of other countries globally.
|
|
Product line for left atrial appendage management:
|
|
3
Product line
|
|
In addition to the above product lines we also sell enabling technologies including ourthat hold 510(k) approvals and/or bear the CE mark. The LARIAT® System is a solution for soft-tissue closure that includes a suture loop coupled to a single-use disposable applier. The Lumitip™ dissectors, the Fusion Magnetic Retriever System and a line of reusable cardiac surgery (valve) instruments. The Lumitip dissector is used by surgeons to separate tissues to provide access to key anatomical structures that are targeted for ablation. The Fusion Magnetic Retriever System™ allowsOther enabling technologies include our Glidepath™ guides for placement of our clamps, Subtle™ Cannula’s to support access around key anatomical structuresfor our EPi-Sense catheters and facilitates positioninga line of reusable cardiac surgery instruments.
Current Afib Treatment Alternatives
Physicians usually begin treating Afib patients with a variety of drugs intended to prevent blood clots, control heart rate or restore the heart to normal sinus rhythm. If a patient’s Afib cannot be adequately controlled with drug therapy, doctors may perform one of several procedures that vary depending on the severity of the Afib symptoms and whether or not the patient suffers from other forms of heart disease.
Alternative treatments to open-heart and minimally invasive procedures include:
|
|
|
|
|
|
With the exception of the Isolator Synergy System, which may be promoted according to its FDA-approved indication for patients with persistent and long-standing persistent Afib undergoing certain open-heart procedures, we do not promote our products specifically for Afib treatment in the United States. Nevertheless, some physicians have adopted our products for use in open-heart and minimally invasive procedures for the treatment of Afib. During elective open-heart surgical procedures, such as bypass or valve surgery, cardiothoracic surgeons use our ablation systems to treat patients with a pre-existing history of Afib. Surgeons use our products to perform cardiac procedures that may vary depending on the length of time a patient has been diagnosed with Afib and whether the patient’s Afib is intermittent, known as paroxysmal, or more continuous (non-paroxysmal), which is typically further classified as persistent, long-standing persistent or permanent. Patients who have been diagnosed with Afib for a longer duration and have non-paroxysmal forms of Afib generally receive more extensive ablation procedures than patients who have been diagnosed with Afib for a shorter duration or who have paroxysmal Afib. Additionally, during an open-heart procedure, physicians may use our AtriClip system to occlude the left atrial appendage.
For those patients with Afib who do not require a concomitant open-heart surgical procedure, surgeons have used our products for minimally invasive Afib treatment procedures. These procedures have generally been performed through minimally invasive incisions without the need to place patients on a heart-lung bypass machine.
Additionally, some physicians are performing various minimally invasive stand-alone procedures which combine epicardial (surgical) ablation (ablation on the outside of the heart) with endocardial ablation and mapping techniques (from the inside of the heart). These combination procedures are often referred to as “hybrid” or “multi-disciplinary” approaches, in that both surgical ablation and catheter ablations are performed. Sometimes, both procedures are performed on the same day or in the same hospital stay, where other times they are performed days or weeks apart. Physician preference as well as hospital logistics and procedural room availability plays into the decision whether to perform hybrid ablations in a single or a staged setting. Physicians are reporting that they are performing these procedures utilizing certain of our products to primarily treat patients who have non-paroxysmal forms of Afib.
4
Business Strategy
pain after surgery. Our missionstrategy is to expand the treatment options for patients who suffer from Afib, or have a high risk of stroke, or who suffer from post-operative pain, through the continued development of our technologies and expansion of our product offerings, clinical science investments and global commercial expansion and clinical science investments.expansion. The key elements of our strategy include:
Invest
efforts and expand the body of clinical evidence. We believe publication of additional scientific evidence, in addition to robust ongoing research activities, will ultimately create an increased demand for our products.
In addition, we
Expand Adoption of Our Minimally Invasive Products. We believe that the catalysts for expanded adoption of our minimally invasive products include procedural advancements, such as the hybrid or multi-disciplinary procedure, and the publication of peer-reviewed articles, which we believe will help validate the successful, long-term use of our products for patients with Afib. We believe that ongoing research activities, including prospective clinical trials, new procedural techniques and anticipated presentations and publications will create an increased demand for our minimally invasive products.
Clinical Trials
criteria.
CONVERGE. We
2023, results from our CEASE-AF trial were presented at the European Heart Rhythm Association meeting and subsequently published in July 2023. CEASE-AF is a prospective, multi-center randomized control trial that demonstrated superior freedom from atrial arrhythmias for staged hybrid ablation compared to endocardial catheter ablation. During the fourth quarter of 2023, the 12-month follow-up results of enrolled patients from the DEEP AF Pivotal Study.study were presented at the American Heart Association meeting. The DEEP AF IDE pivotal trial evaluatesevaluated the safety and efficacy of the Isolator SynergyAtriCure Bipolar System when used in a staged approach where a minimally invasive surgical ablation procedure is first performed and theperformed. The patient undergoes the intracardiacendocardial catheter procedure approximately 90-12091-120 days later. We have FDA approvalThe results from this single arm study demonstrated superior freedom from atrial arrhythmias for staged hybrid ablation compared to enroll up to 220 patients at 23 domestic medical centers and two international medical centers. Enrollment was temporarily suspended beginninga pre-specified performance goal. The Company is in May 2016 while we
5
evaluated changes to the trial protocol with FDA. We received FDA approval on the updated trial protocol during the third quarter of 2017. Six domestic medical centers have resumed participation in the study and five patients have been enrolled. Total enrollment in the trial is currently 47 patients.
ATLAS. The ATLAS study is a non-IDE randomized pilot study evaluating outcomes of patients with risk factors for developing postoperative Afib as well as risk of bleeding on oral anticoagulation. There are two types of patients subject to this study: those with a postoperative Afib diagnosis and receiving prophylactic exclusion of the left atrial appendage with the AtriClip device concomitant to cardiac surgery and those with a postoperative Afib diagnosis who are medically managed. The study provides for enrollment up to 2,000 patients at up to 40 medical centers. Enrollment began in February 2016, and there are currently 517 patients enrolled and twenty-three medical centers initiated. Enrollment is expected to end in 2018.
FROST. We are conducting a cryoanalgesia study, which is a non-IDE randomized pilot study evaluating whether intraoperative intercostal cryoanalgesia in conjunction with standard of care provides improved analgesic efficacy in patients undergoing unilateral thoracotomy cardiac procedures as compared to the current standard of care. The study involves treatment arm patients who receive intercostal cryoanalgesia in conjunction with standard post-operative pain management and control arm patients who receive standard post-operative pain management only. The study provides for enrollment of up to 100 patients at five medical centers. We began enrollment in June 2016, and there are currently 61 patients enrolled and four medical centers initiated.
CEASE AF. We are also pursuing a non-IDE trial in Europe to compare staged hybrid ablation treatment (minimally invasive surgical ablation procedure is first performed and the patient undergoes the intracardiac catheter procedure approximately 91-180 days later) versus catheter ablation alone. We expect the study to have an enrollment of approximately 210 patients at twelve sites. There are currently 90 patients enrolled and eleven medical centers initiated.
Our
To compete effectively, we strive to demonstrate that our products are an attractive alternative to other treatments by differentiating our products on the basis of safety, efficacy, performance, ease of use, reputation, service and price. We have encountered and expect to continue to encounter potential customersUnited States who prefer products offered by our competitors.
market their devices for a similar therapy.
Payment
6
may follow the coverage and payment policies for Medicare, Medicare’s coding, coverage and payment policies for cardiothoracic surgical procedures are significant to our business.
products even when multiple procedures are performed. Similar to surgical ablation for Afib or surgical LAAM, cryoablation performed for post-operative pain
post operative pain control.
used or pursuing specific reimbursement for utilization of our devices.
countries worldwide.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
total product lifecycle from early design, development and testing, to manufacturing and commercialization activities, as well as post-market surveillance and reporting, including corrective actions, removals and recalls. Unless an exemption applies, most medical devices distributed commercially in the United States require either 510(k) clearance or PMA from FDA.
7
510(k) Clearance Pathway.Pathway. To obtain 510(k) clearance, we must submit a notification to FDA demonstrating that our proposed device is substantially equivalent to a predicate device, i.e., a previously cleared and legally marketed 510(k) device or a device that was in commercial distribution before May 28, 1976, for which FDA has not yet called for the submission of a PMA. Any modification to a 510(k)-cleared device that would constitute a major change in its intended use, or a change in its design or manufacture that could significantly affect the safety or effectiveness of the device, requires a new 510(k) clearance or, possibly, in connection with safety and effectiveness, approval of a PMA. FDA requires every manufacturer to make the determination regarding a new 510(k) submission in the first instance, but FDA may review any manufacturer’s decision.
clearance. device. Accountability Act (HIPAA). In addition to FDA regulation, the advertising and promotion of certain medical devices are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. health care program. The different, but the general trend is toward increasing regulation and greater requirements for the manufacturer to provide more bench testing and clinical evidence. In addition, regulatory agencies and authorities can halt distribution within the country or otherwise take action in accordance with local laws. payments are made. equipment on an ongoing basis. Order quantities and lead times for components purchased from outside suppliers are based on our forecasts derived from historical demand and anticipated future demand. Lead times may vary significantly depending on the size of the order, time required to fabricate and test the components, specific supplier requirements and current market demand for the components and Our attrition rate is historically lower than the industry average. AtriCure has a strong company culture, which is reflected in our employee engagement and overall success. manufacturing operations are highly centralized and disruption could harm our business. develop competing products, procedures and/or clinical solutions. There are few barriers to prevent new entrants or existing competitors from developing products to compete directly with ours. Companies also compete with us to attract qualified scientific, technical and business. We may experience unfavorable publicity relating to our business We rely upon single and limited source third-party suppliers and third-party Identifying and qualifying additional or replacement suppliers or sterilizers for any of the components used in our products or business, customer relations and financial condition. We spend considerable time and money complying with federal, state and foreign regulations in addition to FDA regulations, and, if we attention. We Due to the global nature of our business, we may be exposed to liabilities under the Foreign Corrupt Practices Act and various other anti-corruption laws, and any allegation or determination that we violated these laws could have a material adverse effect on our business. Credit Agreement. harm our ability to grow our business. threats. The The Company believes that its existing facilities are adequate to meet its immediate needs and that suitable additional space will be available in the future on commercially reasonable terms as needed. Price Range High Low 2017 First Quarter $ $ Second Quarter $ $ Third Quarter $ $ Fourth Quarter $ $ Price Range High Low 2016 First Quarter $ $ Second Quarter $ $ Third Quarter $ $ Fourth Quarter $ $ As of February Performance Graph 2023. 12/31/2013 12/31/2014 12/31/2015 12/31/2016 12/31/2017 AtriCure, Inc. $ $ $ $ $ NASDAQ Composite $ $ $ $ $ NASDAQ Medical Equipment $ $ $ $ $ Year Ended December 31, 2017 2016 2015 (1) 2014 2013 (2) (in thousands, except per share data) Operating Results: Revenue $ $ $ $ $ Gross profit Gross margin Net loss Basic and diluted net loss per share Weighted average shares outstanding Financial Position: Cash, cash equivalents and investments $ $ $ $ $ Working capital Total assets Long-term debt and capital leases Stockholders’ equity 2022 Year Ended December 31, 2017 2016 % of % of Amount Revenue Amount Revenue (dollars in thousands) Revenue $ % $ % Cost of revenue Gross profit Operating expenses: Research and development expenses Selling, general and administrative expenses Total operating expenses Loss from operations Other income (expense): Interest expense Interest income Other Other expense Loss before income tax expense Income tax expense — — Net loss $ % $ % major geographic regions. mix. domestic and international regulatory submissions drove a $2,389 increase in spending. second quarter of 2023. See Note 10 – Commitments and Contingencies for further information. Year Ended December 31, 2016 2015 % of % of Amount Revenue Amount Revenue (dollars in thousands) Revenue $ % $ % Cost of revenue Gross profit Operating expenses: Research and development expenses Selling, general and administrative expenses Total operating expenses Loss from operations Other income (expense): Interest expense Interest income Other Other income (expense) Loss before income tax expense Income tax expense — — Net loss $ % $ % Uses of liquidity and capital resources. Our executive officers and Board of Directors review our funding sources and future capital requirements in connection with our annual operating plan and periodic updates to the plan. Our principal cash requirements include costs of operations, capital expenditures, debt service costs and other contractual obligations. Our future capital requirements depend on a number of factors, including, SentreHEART acquisition. Subject to the terms and conditions of the SentreHEART merger agreement, such contingent consideration would be paid in AtriCure common stock and cash, up to a specified maximum number of shares. As of December 31, 2023, we believe the likelihood of payment is remote, and the estimated fair value of the contingent consideration is $0. See Note 2 – Fair Value. foreseeable future. Historical Cash Flow Activity.The following table Less than More than Contractual Obligations Total 1 year 1-3 years 3-5 years 5 years Long-term debt(1) $ $ — $ $ $ Capital leases(2) Operating leases(3) — Royalty obligations(4) — — — Restricted grants — — — Total contractual obligations $ $ $ $ $ Inflation has impacted our operating costs throughout 2023 and 2022. Continued increases in our cost of revenue may affect our ability to maintain our gross margin if the selling prices of our products do not increase commensurately, while continued increases in our operating expenses may adversely affect our operating results and the on historical experience, current conditions and other reasonable factors. Actual results could differ from those estimates under different assumptions or conditions. customers. We account for revenue in accordance with FASB ASC physically disposed. prior periods will be reversed. future periods. 16, 2024 2022 2017 2016 Assets Current assets: Cash and cash equivalents $ $ Short-term investments Accounts receivable, less allowance for doubtful accounts of $32 and $246 Inventories Other current assets Total current assets Property and equipment, net Long-term investments — Intangible assets, net Goodwill Other noncurrent assets Total Assets $ $ Liabilities and Stockholders’ Equity Current liabilities: Accounts payable $ $ Accrued liabilities Other current liabilities and current maturities of capital leases and long-term debt Total current liabilities Capital leases Long-term debt Other noncurrent liabilities Total Liabilities Commitments and contingencies (Note 10) Stockholders’ Equity: Common stock, $0.001 par value, 90,000 shares authorized and 34,586 and 33,342 issued and Additional paid-in capital Accumulated other comprehensive income (loss) Accumulated deficit Total Stockholders’ Equity Total Liabilities and Stockholders’ Equity $ $ (LOSS) INCOME 2021 2017 2016 2015 Revenue $ $ $ Cost of revenue Gross profit Operating expenses: Research and development expenses Selling, general and administrative expenses Total operating expenses Loss from operations Other income (expense): Interest expense Interest income Other Loss before income tax expense Income tax expense Net loss $ $ $ Basic and diluted net loss per share $ $ $ Weighted average shares outstanding – basic and diluted Comprehensive loss: Unrealized gain on investments $ $ $ Foreign currency translation adjustment Other comprehensive income (loss) Net loss Comprehensive loss, net of tax $ $ $ 2021 Accumulated Additional Other Total Common Stock Paid-in Accumulated Comprehensive Stockholders’ Shares Amount Capital Deficit Income (Loss) Equity Balance—December 31, 2014 Issuance of common stock through public offering — — Issuance of common stock under equity incentive �� — — Issuance of common stock under employee stock — — — Reclassification of non-employee option liability — — — — Share-based employee compensation expense — — — — Other comprehensive loss — — — — Net loss — — — — Balance—December 31, 2015 $ $ $ $ $ Issuance of common stock under equity incentive — — Issuance of common stock under employee stock — — — Share-based employee compensation expense — — — — Other comprehensive income — — — — Net loss — — — — Balance—December 31, 2016 $ $ $ $ $ Issuance of common stock under equity incentive — — Issuance of common stock under employee stock — — — Share-based employee compensation expense — — — — Other comprehensive income — — — — Net loss — — — — Balance—December 31, 2017 $ $ $ $ $ 2021 2017 2016 2015 Cash flows from operating activities: Net loss $ $ $ Adjustments to reconcile net loss to net cash used in operating activities: Share-based compensation expense Depreciation Amortization of intangible assets Amortization of deferred financing costs Loss on disposal of property and equipment and impairment of assets Realized (gain) loss from foreign exchange on intercompany transactions Amortization/accretion on investments Change in allowance for doubtful accounts Change in fair value of contingent consideration — Changes in operating assets and liabilities, net of amounts acquired: Accounts receivable Inventories Other current assets Accounts payable Accrued liabilities Other noncurrent assets and liabilities Net cash used in operating activities Cash flows from investing activities: Purchases of available-for-sale securities Sales and maturities of available-for-sale securities Purchases of property and equipment Proceeds from sale of property and equipment — — Increases in property under build-to-suit obligation — — Cash paid for nContact business combination — — Net cash provided by (used in) investing activities Cash flows from financing activities: Proceeds from debt borrowings — — Payments on debt and capital leases Proceeds from build-to-suit obligation — — Proceeds from economic incentive loan — — Payment of debt fees Proceeds from stock option exercises Shares repurchased for payment of taxes on stock awards Proceeds from issuance of common stock under employee stock purchase plan Payment of stock issuance fees — — Net cash provided by financing activities Effect of exchange rate changes on cash and cash equivalents Net (decrease) increase in cash and cash equivalents Cash and cash equivalents—beginning of period Cash and cash equivalents—end of period $ $ $ Supplemental cash flow information: Cash paid for interest $ $ $ Cash paid for income taxes Non-cash investing and financing activities: Accrued purchases of property and equipment Assets acquired through capital lease Capital lease asset early termination — — Stock issuance in business combinations — — Contingent consideration in business combinations — — STATEMENTS—(Continued) include demand deposits and money market funds with financial institutions. products. Revenue includes shipping and handling revenue of The Company does not maintain any post-shipping obligations to customers; no installation, calibration or testing of products is performed subsequent to shipment in order to render products operational. The Company expects to be entitled to the total consideration for the products ordered as product pricing is fixed, and there are no adjustments for a significant financing component as payment terms fall within one year. The Company excludes taxes assessed by governmental authorities on revenue-producing transactions from the measurement of the transaction price. liabilities. Recoveries are recognized when received as a reduction to the allowance for credit losses by decreasing bad debt expense. The following depreciated from three to seven years. The Company’s repair costs are expensed as incurred. The Company retires assets no longer in use. revenue. The Company reviews intangible assets for impairment at least annually or more often if impairment indicators are present using its best estimates based on reasonable and supportable assumptions and projections. lease term. See Note 9 – Leases for further discussion. Pounds, Australian Dollars and Canadian Dollars. 2017 2016 2015 Total accumulated other comprehensive loss at beginning of period $ $ $ Unrealized losses on investments Balance at beginning of period $ $ $ Other comprehensive income before reclassifications Amounts reclassified from accumulated other comprehensive income (loss) — — — Balance at end of period $ $ $ Foreign currency translation adjustment Balance at beginning of period $ $ $ Other comprehensive income before reclassifications Amounts reclassified from accumulated other comprehensive income (loss) Balance at end of period $ $ $ Total accumulated other comprehensive income (loss) at end of period $ $ $ Research and Development Costs—Research and development activities, as well as amortization of technology assets. Research and development costs are expensed as incurred. Clinical trial costs and other development costs incurred by third parties are expensed as contracted work is performed or over the expected service period. 2021. Effective January 1, 2023, the Company's policy was amended to account for forfeitures as they occur rather than estimating at the time of grant, and the effect on income from continuing operations and retained earnings is not significant. statements. Quoted Prices in Active Significant Significant Markets for Other Other Identical Observable Unobservable Assets Inputs Inputs (Level 1) (Level 2) (Level 3) Total Assets: Money market funds $ — $ $ — $ Commercial paper — — U.S. government agencies and securities — — Corporate bonds — — Total assets $ $ $ — $ Liabilities: Acquisition-related contingent consideration $ — $ — $ $ Total liabilities $ — $ — $ $ The following table represents the Company’s fair value hierarchy for its financial assets and liabilities measured at fair value on a recurring basis as of December 31, Quoted Prices in Active Significant Significant Markets for Other Other Identical Observable Unobservable Assets Inputs Inputs (Level 1) (Level 2) (Level 3) Total Assets: Money market funds $ — $ $ — $ Commercial paper — — U.S. government agencies and securities — Corporate bonds — — Total assets $ $ $ — $ Liabilities: Acquisition-related contingent consideration $ — $ — $ $ Total liabilities $ — $ — $ $ 2017 2016 2015 Beginning Balance $ — $ — $ Total loss included in earnings — — Exercises — — Ending Balance $ — $ — $ — 2022. 2017 2016 2015 Beginning Balance $ $ $ — Amounts acquired — — Changes in fair value included in earnings — Ending Balance $ $ $ Unrealized Gains Cost Basis (Losses) Fair Value Corporate bonds $ $ $ U.S. government agencies and securities Commercial paper — Total $ $ $ Unrealized Gains Cost Basis (Losses) Fair Value Corporate bonds $ $ $ U.S. government agencies and securities Commercial paper — Total $ $ $ 2017 2016 Estimated Accumulated Accumulated Useful Life Cost Amortization Cost Amortization Fusion technology 10 years $ $ $ $ Clamp & probe technology 3 years SUBTLE access technology 5 years IPR&D — — Total $ $ $ $ 2021. The following table summarizes the allocation of amortization expense of intangible assets: 2018 $ 2019 2020 2021 2022 2023 and thereafter Total $ 2017 2016 Raw materials $ $ Work in process Finished goods Inventories $ $ Estimated Useful Life 2017 2016 Generators and other capital equipment 1 - 3 years $ $ Building under capital lease 15 years Computer and other office equipment 3 years Machinery, equipment and vehicles 3 - 7 years Furniture and fixtures 3 - 7 years Leasehold improvements 5 - 15 years Construction in progress N/A Equipment under capital leases 3 - 5 years Total Less accumulated depreciation Property and equipment, net $ $ 2017 2016 Accrued commissions $ $ Accrued bonus Accrued payroll and employee-related expenses Sales returns and allowances Other accrued liabilities Accrued taxes and value-added taxes payable Accrued royalties Total $ $ approximately $28,750. 2018 $ 2019 2020 2021 2022 2023 and thereafter Total payments $ Imputed interest on capital lease obligations Net debt obligations, of which $561 is current and $36,861 is noncurrent $ 2018 $ 2019 2020 2021 2022 2023 and thereafter — Total $ Purchase Agreements. The Company enters into standard purchase agreements with business, generally with terms that allow cancellation. License Agreement, and that the Company did not provide related notices required under the License Agreement. The Company filed its Answering Statement and Counterclaims to the allegations in September 2022, denying each claim and counterclaiming for breach of contract, correction of inventorship, declaratory judgment, patent prosecution and legal fees. In May 2023, the Company entered into an Assignment and Agreement Regarding IDx and CCF Intellectual Property (Assignment Agreement) with Clinic and IDx. Pursuant to the Assignment Agreement, during the second quarter of 2023, the Company made a one-time payment of $33,400 to Clinic and IDx for the acquisition of patents and other intellectual property. The Assignment Agreement also required dismissal of the arbitration and release of payment for royalty obligations due to Clinic and IDx under the License Agreement after March 31, 2023. The amount paid, together with transaction costs, was allocated between the acquired intangible asset, the release of payment for royalty obligations and the settlement of the dispute. The intangible asset was assigned a value of $30,000 and is being amortized over an estimated useful life of 5 years. The release of the royalty obligations was valued at $432. The remaining $3,088 was allocated to the settlement and is included in selling, general and administrative expenses for the twelve months ended December 31, 2023. 2017 2016 Deferred tax assets (liabilities): Net operating loss carryforward $ $ Research and development and AMT credit carryforwards, net Equity compensation Accruals and reserves Inventories Intangible assets Property and equipment, net Other, net Subtotal Less valuation allowance Total $ $ — The Company’s provision for income taxes for each of the years ended December 31 is as follows: 2017 2016 2015 Current Tax Expense Federal $ — $ — $ State Foreign — Total current tax expense Deferred Tax Expense Federal $ $ $ State Foreign Change in valuation allowance Total deferred tax expense — — Total tax expense $ $ $ 2017 2016 2015 Federal tax at statutory rate % $ % $ % $ Federal tax rate change Federal R&D credit Federal NOL adjustment for ASU Valuation allowance State income taxes Foreign NOL rate change Foreign tax rate differential Permanent differences and other Effective tax rate % $ % $ % $ 2017 2016 2015 Balance at the beginning of the year $ $ $ Increases (decreases) for prior year tax positions — Increases (decreases) for current year tax positions — — — Increases (decreases) related to settlements — — — Decreases related to statute lapse — — — Balance at the end of the year $ $ $ 13. EMPLOYEE BENEFIT PLANS 2021. Stockholders approved the 2023 Plan at the 2023 Annual Meeting of Stockholders. Pursuant to its terms, the 2023 Plan supersedes and replaces the 2014 Stock Incentive Plan (Prior Plan). Activity under the plans during Weighted Weighted Average Number of Average Remaining Aggregate Shares Exercise Contractual Intrinsic Time-Based Stock Options Outstanding Price Term Value Outstanding at January 1, 2017 $ Granted Exercised Cancelled Outstanding at December 31, 2017 $ $ Vested and expected to vest $ $ Exercisable at December 31, 2017 $ $ Weighted Number of Average Shares Grant Date Restricted Stock Outstanding Fair Value Outstanding at January 1, 2017 $ Awarded Released Forfeited Outstanding at December 31, 2017 $ Weighted Weighted Average Number of Average Remaining Aggregate Shares Exercise Contractual Intrinsic Performance Stock Options Outstanding Price Term Value Outstanding at January 1, 2017 $ Granted — — Exercised — — Cancelled — — Outstanding at December 31, 2017 $ $ Exercisable at December 31, 2017 $ $ Weighted Weighted Average Number of Average Remaining Aggregate Shares Exercise Contractual Intrinsic Time-Based Stock Options Outstanding Price Term Value Outstanding at January 1, 2016 $ Granted Exercised Cancelled Outstanding at December 31, 2016 $ $ Vested and expected to vest $ $ Exercisable at December 31, 2016 $ $ Weighted Number of Average Shares Grant Date Restricted Stock Outstanding Fair Value Outstanding at January 1, 2016 $ Awarded Forfeited Released Outstanding at December 31, 2016 $ Weighted Weighted Average Number of Average Remaining Aggregate Shares Exercise Contractual Intrinsic Performance Stock Options Outstanding Price Term Value Outstanding at January 1, 2016 $ Granted — — Exercised — — Cancelled — — Outstanding at December 31, 2016 $ $ Exercisable at December 31, 2016 $ $ The total intrinsic value of options exercised during the years ended December 31, Employee Stock Purchase Plan 2017 2016 2015 Cost of revenue $ $ $ Research and development expenses Selling, general and administrative expenses Total $ $ $ 2017 2016 2015 Risk-free interest rate 1.75 - 2.12 % 1.06 - 2.02 % 1.30 - 1.96 % Expected life of option (years) 5.21 to 5.76 5.27 to 7.10 5.20 to 6.89 Expected volatility of stock 43.00 - 48.00 % 46.00 - 51.00 % 46.00 - 67.00 % Weighted-average volatility % % % Dividend yield % % % 2017 2016 2015 Stock options $ $ $ Restricted stock Price Fair Value of Fair Value of Target 2012 Grant 2014 Grant Tranche 1 $ $ $ Tranche 2 Tranche 3 Tranche 4 Tranche 5 Tranche 6 Tranche 7 Tranche 8 Tranche 9 2017 2016 2015 United States $ $ $ Europe Asia Other international Total international Total revenue $ $ $ 2017 2016 2015 Open-heart ablation $ $ $ Minimally invasive ablation AtriClip devices Total ablation and AtriClip devices Valve tools Total United States $ $ $ 2017 2016 2015 Open-heart ablation $ $ $ Minimally invasive ablation AtriClip devices Total ablation and AtriClip devices Valve tools Total international $ $ $ For the Three Months Ended March 31, June 30, September 30, December 31, 2017 2016 2017 2016 2017 2016 2017 2016 Operating Results: Revenue $ $ $ $ $ $ $ $ Gross profit Loss from operations Net loss Net loss per share (basic and diluted) $ $ $ $ $ $ $ $ Beginning Ending Balance Additions Deductions Balance Reserve for sales returns and allowances Year ended December 31, 2017 $ $ $ $ Year ended December 31, 2016 Year ended December 31, 2015 Allowance for inventory valuation Year ended December 31, 2017 $ $ $ $ Year ended December 31, 2016 Year ended December 31, 2015 Valuation allowance for deferred tax assets Year ended December 31, 2017 $ $ — $ $ Year ended December 31, 2016 — Year ended December 31, 2015 — 2023. is incorporated herein by reference. Statement under the heading “Corporate Governance Guidelines—Code of Conduct” and is incorporated herein by reference. Number of securities Weighted-average Number of securities remaining Plan Category (a) (b) (c) Equity compensation plans approved by $ Equity compensation plans not approved by — — — Total $ 3.2 4.1 23.1 31.1 31.2 32.1 32.2 101.INS XBRL Instance Document 101.SCH XBRL Taxonomy Extension Schema Document 101.CAL XBRL Taxonomy Extension Calculation Linkbase Document 101.DEF XBRL Taxonomy Definition Linkbase Document 101.LAB XBRL Taxonomy Extension Label Linkbase Document 101.PRE XBRL Taxonomy Extension Presentation Linkbase Document Date: February /s/ Michael H. Carrel Michael H. Carrel President and Chief Executive Officer (Principal Executive Officer) Date: February /s/ (Principal Accounting and Financial Officer) /s/ Michael H. Carrel Michael H. Carrel Michael H. Carrel Director, President and Chief Executive Officer (Principal Executive Officer) /s/ Regina E. Groves Regina E. Groves Regina E. Groves Director Director /s/ Sven A. Wehrwein Sven A. Wehrwein Sven A. Wehrwein Director /s/ Robert S. White Robert S. White Robert S. White DirectorPathway.Pathway. A PMA must be submitted to FDA if the device cannot be cleared through the 510(k) process and is not otherwise exempt. A PMA must be supported by extensive data, including but not limited to technical, preclinical, clinical, real-world data, manufacturing and labeling, to demonstrate the safety and effectiveness of the device for its intended use. AfterA PMA supplement is required for changes affecting the safety or effectiveness of a PMA is submitted and FDA has determined that the application is sufficiently completePMA-approved device, including but not limited to permitnew indications for use, a substantive review, FDA will accept the application for filing. During the review period, FDA may request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside FDA may be convened to review and evaluate the application and provide recommendations to FDA as to the approvability of the device. In addition, FDA will conduct a preapproval inspection of thedifferent manufacturing facility, to ensure compliance with quality system regulations. Any approvals we receive may be limitedor changes in scope or may be contingent upon further post-approval study commitments or other conditions. New PMAs or PMA supplements are required for significant modification to the device, including indicated use, manufacturing process, labeling, andor design of a device that is approved through the premarket approval process. PMA supplements often require submissionspecifications or components of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel.Trials.Trials. Clinical trials are required to support a PMA and are sometimes required for 510(k) clearance. Clinical trials are subject to extensive recordkeeping and reporting requirements. Our clinical trials must be conducted under the oversight of an Institutional Review Board (IRB) for the relevant clinical trial sites and must comply with FDA regulations, including, but not limited to, those relating to current good clinical practices. We are also required to obtain the written informed consent of patients in form and substance that complies with both FDA requirements and other humanhuman subject protection regulations. Similarly, in Europe, the clinical study must be approved by a local ethics committee and, in some cases, including studies with high-risk devices, by the ministry of health in the applicable country.Grants.Grants. FDA regulates manufacturers of medical devices and, in particular, the promotion of medical devices by manufacturers.manufacturers and prohibits the promotion by manufacturers of uses that are not within the approved or cleared labeling of the device. FDA does not regulate the practice of medicine or the conduct or content of medical education conducted by third parties.parties, which may include uses that are not within approved or cleared device labeling. Manufacturers may provide unrestricted financial support for suchindependent third-party medical education programs in the form of educational grants intended to offset the cost of such programs. If the manufacturer controls or unduly influences the content of such programs, FDA considers those programs to be promotional activities by the manufacturer and thus subject to FDA regulation including promotional restrictions. We seek to ensure that the activities we support pursuant to our educational grants program areis conducted in accordance with FDA criteria for independent educational activities. However, we cannot provide an assurance that FDA or other government authorities would view the third-party programs we have supported as being independent.Regulation.Regulation. There are numerous regulatory requirements that apply after a product is cleared or approved. These include:·FDA’s Quality System Regulation (QSR) which requires manufacturers, including third-party manufacturers,approved by FDA, including, but not limited to: annual establishment registration and product listing; current good manufacturing practice for devices (GMP); labeling requirements and advertising and promotion guidelines; assessing the significance of any changes to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process;·labeling regulations and FDA prohibitions against the false or misleading promotion or the promotion of products for uncleared, unapproved or off-label use or indication;·requirements to obtain clearance or approval of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use;·medical device reporting regulations which require that manufacturers comply with reporting requirements of FDA and report if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur;·post-approval restrictions or conditions, including post-approval study commitments;·post-market surveillance regulations which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; and·requirements to issue notices of correction or removal, or conduct market withdrawals or recalls where quality or other issues arise.Under FDA’s MedWatch regulation, we must submit a Medical Device Report (MDR)device; monitoring and reporting serious and adverse events and certain device malfunctions; and reporting certain device corrections and removals. Our manufacturing facilities and processes are also subject to FDA within 30 days whenever we receive information that reasonably suggests that one of our products may have caused or contributedinspections to a death or serious injury, or that one of our products malfunctioned in a manner which, if the malfunction were to recur, could cause or contribute to a death or8serious injury. Our products are often used to treat very ill patients in highly complex surgeries, only a small portion of which may involve our products, and it is frequently difficult to determine whether our products caused or contributed to a patient injury or death that occurred during or after the procedure. If we are able to determine that our product caused or potentially contributed to a death or serious injury in the particular case, or that a malfunction of the type reported would cause death or serious injury, we submit an MDR on the case. Other incidents, including serious injuries or deaths, which occurred during procedures utilizing our products and that are not the subject of MDRs, may occur either because we are not aware of those incidents or because our investigation determined that the incident did not involve a malfunction of an AtriCure device and/or that an AtriCure device did not cause or contribute to a serious injury or death.Recently, someOn occasion, promotional activities for FDA-regulated products have beencan be the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the Federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims.We have registered with FDA as a medical device manufacturer and listed our devices. FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by FDA and our Notified Body to determine our compliance with the QSR, the European Union’s Medical Device Directive (MDD) and other regulations. Such inspections may include the manufacturing facilities of our suppliers.Claims.Claims. We are directly and indirectly subject to various federal and state laws governing our relationship with healthcare providers and pertaining to healthcare fraud and abuse, including anti-kickback laws.providers. In particular, the federal healthcare program Anti-Kickback Statute is a federal criminal law that applies broadly and prohibits persons from knowinglythe knowing and willfully soliciting, offering, receivingwillful offer or providingpayment of remuneration directly or indirectly, in exchange for or to induce either the referral of an individual,or reward patient referrals or the furnishing, arranging for or recommending a goodgeneration of business involving any item or service for which payment may be made in whole or part underpayable by a federal healthcare programs, such as the Medicare and Medicaid programs.Federalfederal False Claims Act (FCA) imposes civil liability on any person or entity that submits, or causes the submission of, a false or fraudulent claim to the United States Government.government. Damages under the FCA consist of the imposition of fines and penalties and can be significant. The FCA also allows a private individual or entity with knowledge of past or present fraud against the federal government to sue on behalf of the government to recover the civil penalties and treble damages. The U.S. DepartmentJustice (DOJ), on behalf of the government, has previously alleged that the marketing and promotional practices of pharmaceutical and medical device manufacturers included the off-label promotion of products or the payment of prohibited kickbacks to doctors violated the FCA resulting in the submission of improper claims to federal and state healthcare entitlement programs such as Medicaid. In certain cases, manufacturers have entered into criminal and civil settlements with the federal government under which they entered into plea agreements, paid substantial monetary amounts and entered into corporate integrity agreements that require, among other things, substantial reporting and remedial actions going forward.The Advanced Medical Technology Association (AdvaMed) is one of the primary, a voluntary United States trade associationsassociation for medical device manufacturers. This association has established guidelines and protocols for medical device manufacturers in their relationships with healthcare professionals on matters including research and development, product training and education, grants and charitable contributions, support of third-party educational conferences and consulting arrangements. Adoption of the AdvaMed Code of Ethics for Interactions with Healthcare Professionals (the “AdvaMed Code”) by a medical device manufacturer is voluntary, and while the Office of the Inspector General and other federal and state healthcare regulatory agencies encourage its adoption and may look to the AdvaMed Code, they do not view adoption of the AdvaMed Code as proof of compliance with applicable laws. We have adopted the AdvaMed Code and incorporated its principles in our standard operating procedures, sales forceemployee training programs and relationships with medical professionals. In addition, we have conducted training sessions for employees on these principles.States.States:different. and voluntary standards regulate the design, manufacture and labeling of medical devices. Devices may only be placed on the marketdevices, and more stringent conformity assessment requirements have been put in the European Union if they complyplace with the essential requirements of a relevant directive and bear the CE mark. Manufacturers must demonstrate that their devices comply with the relevant essential requirements through a conformity assessment procedure.2017 Medical Device Regulation, effective May 26, 2021. The method for assessing conformity varies depending on the type and class of the product, but normallytypically involves a combination of self-assessment by the manufacturerquality system assessment and a third-party product conformitywill includeincludes a review of documentation relatingrelated to the device andthat may consist of an audit ofbe as extensive as the documentation requirements that the United States FDA requires for higher risk products. The notified body also audits the manufacturer’s quality system and specificperforms a detailed review of the testing of the manufacturer’s device. Successful completion of a conformity assessment procedure allows a manufacturer to issue a declaration of conformity with the requirements of the relevant directive and affix the CE mark to the device. Devices that bear the CE mark may be commercially distributed throughout the member states of the European Union and other countries that comply with or mirror the medical device directives. Aregulations.has granted us a certificatefor CE marking, including, but not limited to: labeling, advertising and promotion, reporting of device modifications, monitoring the safety of the product and performing corrections and removals when necessary, maintaining “state of the art” requirements for the devices through compliance with the International Organization for9Standardization, (ISO) 13485:2003 Quality Management System. Compliance with this standard establishes the presumption that our quality system conformsand individual device certificates on a periodic basis.essential requirementslaws of the jurisdictions where those physicians reside or practice, or where the relevant directive. We have successfully completed the conformity assessment procedure and affixed the CE mark to our Isolator Synergy clamps, Isolator Synergy pens, Coolrail linear pen, cryosurgery devices, AtriClip LAA Exclusion System, COBRA Fusion Ablation System, Numeris System and the EPi-Sense Guided Coagulation System with VisiTrax technology.patentsknow-how and related technology of importance to the commercialization of our products. For example, toTo continue developing and commercializing our current and future products, we may license intellectual property from commercial or academic entities to obtain the rights to technology that is required for our research, development and commercialization activities.facilityfacilities in Ohio, and our products are sterilized by third parties. Purchased components are generallyoften sourced from a single supplier, but alternatives to critical suppliers are available from more than one supplier. However, some products, such as our RF generatorsin the event this would be needed.Fusionraw materials specific to the respective part or device. We assess tooling and EPi-Sense products, are critical components of our RF ablation lines and there are relatively few alternative sources of supply available.subassemblies.raw materials. To date, we have not experienced significant delays inproduct availability or delay issues directly related to obtaining any of our components.ISOInternational Organization of Standardization (ISO) standards. We are an FDA-registered medical device manufacturer and certified to ISO 13485:2003.2016. We routinely conduct internal audits of our quality systems in accordance with various international standards. In addition, we have successfully participated in the Medical Device Single Audit Program (MDSAP) and have been certified accordingly. The MDSAP program is recognized in Australia, Brazil, Canada, Europe, Japan and the United States.Consulting Relationshipshave developed consulting relationships with scientistscontinuously evaluate, modify and physicians throughout the worldenhance our internal processes to increase employee engagement, productivity and efficiency, as well as to recruit new employees to support our growth. Recognizing the significance of our employees to our success, in 2022 we introduced a “people objective” to our annual incentive plan focused on attraction, development and retention of talent, in addition to strategic Diversity, Equity and Inclusion (DE&I) initiatives.development, clinical trials through healthcare partnershipstrainingethnically diverse groups, while collaborating with our partners to engage communities to promote heart health awarenesseducation programs.programs by fostering employee understanding, intentionality and measurable processes. This commitment is also reflected in the current makeup of our Board of Directors, which helps to set the “tone at the top” for our DE&I initiatives.work closely with these thought leadersare committed to understand unmet needsregularly analyzing and emerging applications forevaluating the treatmenteffectiveness of Afib. Our physician consulting agreementsour compensation and benefit programs and benchmarking our programs against the market and our industry peers. Annual pay increases and other forms of incentive compensation are intendedbased on performance and market evaluation. Performance expectations are communicated to satisfyemployees at the requirementstime of the personal services “Safe Harbor” regulationhiring, as well as the AdvaMedupon internal transfer or promotion, and Eucomed Codes. As such, they providedocumented through our annual performance management process.payment ofeligible U.S.-based employees include medical, dental and vision insurance; paid leave for vacation, illness and volunteer time; parental leave, fertility and adoption assistance; a fair market value fee only for legitimate services rendered401(k) retirement plan that includes a company matching contribution; a stock purchase plan enabling employees to us.purchase AtriCure stock at a reduced price; and life and disability insurance. Our international employee benefits vary due to local regulations and offerings. We do not expect or require the consultant to utilize or promote our products,ensure compliance with all statutory and consultants are required to disclose their relationship with us as appropriate,mandatory benefits which vary by country, such as when publishing an article in which onemedical, disability, retirement/pension, workers compensation, accident, social benefits and paid leave. None of our products is discussed. Amounts paid to physicians in the United Statesemployees are disclosed by us in annual reports submitted to CMS under the federal “Open Payments” law.10EmployeesWe had approximately 570 full-time employees as of January 31, 2018. None of the employees were represented by a labor union, or covered by a collective bargaining agreement. Weand we have never experienced any employment-related work stoppages andstoppages. We consider our employee relations to be in good standing. Relating To Businesswill depend,depends in large part on the medical community’s acceptance of our principal products in the United States, which is the largest revenue market in the world for medical devices. Our ablation and LAAM product sales in the United States generate the majority of our revenue. We expect that sales of these products will continue to account for a majority of our revenue for the foreseeable future and that our future revenue will depend on the increasing acceptance by the medical community of our products as standard of care for treating Afib, managing the LAA and managing pain with Cryo Nerve Block therapy. The U.S. medical community’s acceptance of our products will depend upon our ability to demonstrate the safety and efficacy, advantages, long-term clinical performance and cost-effectiveness of our products as compared to other products. In addition, acceptance of products for the treatment of Afib is dependent upon, among other factors, the level of screening for Afib general awareness and education of the medical community about the surgical treatment of Afib and the existence, effectiveness and safety of our products. Market acceptance and adoption of our products for the treatment of Afib also depends on the level of health insurer (including Medicare) reimbursement to physicians and hospitals for the use ofprocedures using our products.We cannot predict whether the U.S. medical community will accept our products or, if accepted, the extent of their use. Negative publicity resulting from incidents involving our products, otheror similar products related to those we sell or products or procedures subject to our clinical trials could have a significant adverse effect on the overall acceptance of our products. If we encounter difficulties growing the market for our products in the U.S., we may not be able to increase our revenue enough to achieve or sustain profitability, and our business and operating results will be seriously harmed.We rely onablation-related andimplantable devices, other surgical ablation devices, other products or techniques to occlude the left atrial appendage managementor other products as our primary sources of revenue. If we are not successful in selling these products, or if these products become obsolete, our operating results will be harmed.Our ablation and ablation-related products, along with our left atrial appendage management products, generate a large majority of our revenue. We expect that sales of these products will continuetechniques to account for a majority of our revenue for the foreseeable future and that our future revenue will depend on the increasing acceptance by the medical community of our products as a standard surgical treatment of Afib. We may not be able to maintain or increase market acceptance of our products for a number of additional reasons, including those set forth elsewhere in this “Risk Factors” section. In addition, ourmanage post-operative pain. Our products may become obsolete prior to the end of their anticipated useful lives, or we may introduce new products or next-generation products prior to the end of the useful life of a prior generation,our current products, either of which may require us to dispose of existing inventory and related capital equipment and/or write off their value or accelerate their depreciation. Since we believe that physicians are using our ablation and ablation-related products largely for the surgical treatment of Afib, if physicians do not use our products to treat Afib, we would lose substantially all of our revenue.Competition from existing and new products and procedures may decrease our market share and cause our revenue to decline.The medical device industry, including the market for the treatment of Afib, is highly competitive, subject to rapid technological change and significantly affected by new product introductions and promotional activities of its participants. There is no assurance that our products will compete effectively against drugs, catheter-based ablation, implantable devices, other ablation systems,In addition, other products or techniques to exclude the left atrial appendage, or other surgical Afib treatments, which may be more well-established among doctors and hospitals. In addition, such other products or techniques may be sold or implemented at lower prices. Due to the size of the Afib and LAA exclusionour markets, and the unmet need for an Afib cure, we anticipate that new or existing competitors may11technicalcommercial personnel as well as funding. SomeMost of our competitors and potential competitors have greater financial, manufacturing, marketing and research and development capabilities than we have, and may obtain FDA approval or clearance for their products before we do.products. In 2023, Medtronic announced the FDA clearance of the Penditureof our competitors obtaining FDA approvals or clearances, such as Medtronic's Penditure device, may result in price reductions, reduced margins, loss of market share, or may render our products obsolete, which could adversely affect our revenue and future profitability.Worldwide economic conditionsreduce demand for procedures usingnot be positive, and our current and planned clinical trials may not satisfy the requirements of the FDA or other regulatory authorities.otherwise result in the failure of the clinical trial. Conducting successful clinical studies may require the enrollment of large numbers of clinical sites and patients, and suitable patients may be difficult to identify and recruit. Patient enrollment in clinical trials and completion of patient participation and follow-up depends on many factors, including the size of the patient population; the nature of the trial protocol; the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects; the availability of appropriate clinical trial investigators, support staff, and proximity of patients to clinical sites; and the ability to comply with the eligibility and exclusion criteria for participation in the clinical trial and patient compliance.implicationseffect on our business, operatingoperations. Turnover among our independent distributors, even if replaced, may adversely affect our short-term financial results while we transition to new independent distributors or direct sales personnel. The ability of these independent distributors to market and sell our products could also be adversely affected by unexpected events, including, but not limited to, power failures, nuclear events, local economic and political conditions, natural or other disasters and war or terrorist activities. In addition, the ability of our independent distributors to borrow money from their existing lenders or to obtain credit from other sources to purchase our products may be impaired or our independent distributors could experience a significant change in their liquidity or financial condition.General worldwidecondition, all of which could impair their ability to distribute our products and eventually lead to distributor turnover, and may adversely impact our sales.conditions may deteriorate due todownturn as a result of the collateral effects of among other developments, general credit market crises, collateral effects on the finance and banking industries, concerns about inflation,inflationary pressures, increases in interest rates, slower economic activity, whicha future outbreak of COVID-19 or a similar infectious disease, among other factors, may be caused by many factors, including natural disasters or other catastrophes, decreased consumer confidence, reduced corporate profits and capital spending, adverse business conditions and liquidity concerns. We are unable to predict the extent to which current or future worldwide economic conditions mayadversely impact our business. Specifically, because many procedures usingimpacts to procedure volumes and hospital staffing may result in reductions of our revenue and materially and adversely affect our results of operations and cash flows. Geopolitical issues around the world have impacted the global supply chain and could materially adversely affect global economic growth, disrupt discretionary spending habits and generally decrease demand for our products are elective, they can be deferred by patients. In addition, patients may not be as willing under current or future economic conditions to take time off from work or spend their money on deductibles and co-payments often required in connection with the procedures that use our products.Beyond patient demand, any current or future deterioration in worldwide economic conditions, including in particular their effects on the credit and capital markets, may have other adverse implications for our business. For example, ourservices. Our customers’ ability to borrow money from their existing lenders or to obtain credit from other sources to purchase our products may be impaired,Although we maintain allowances for estimated losses resultingWe may experience diversion of healthcare resources away from the inabilityconduct of clinical trials, including the diversion of hospitals serving as our customersclinical trial sites. We may also encounter interruption or delays in the operations of FDA or other regulatory authorities, which may impact review and approval timelines. We are unable to make required payments, we cannot guarantee that we will accurately predict the loss rates we will experience, especially given any continuing turmoil in theextent to which current or future worldwide economy. A significant change in the liquidity or financial condition ofeconomic conditions may impact our customers could cause unfavorable trends in our receivable collections and additional allowances may be required, which could adversely affect our operating results. Further, given the economic and political challenges facing Eurozone countries, concerns have been raised regarding the stability and suitability of the Euro as a single currency. The failure of the Euro as a single currency could adversely affect our operating results.We face significant uncertaintythe industry duegovernmental and third-party payors’ policies toward coverage and reimbursement for surgical procedures would harm our ability to government healthcare reform. The U.S. Patient Protectionpromote and Affordable Care Act (PPACA), as amended, and other healthcare reform have a significant impact onsell our business. The impact of the PPACA on the healthcare industry is extensive and includes, among other things, the federal government assuming a larger role in the healthcare system, expanding healthcare coverage of United States citizens and mandating basic healthcare benefits. The PPACA impacted our business by requiring an excise tax on all U.S. medical device sales beginning in January 2013. In December 2015, the U.S. government approved the suspension of the excise taxproducts.sales beginning January 1, 2016 through December 31, 2017. Then,companies to reduce their prices. Even to the extent that the use of our products is reimbursed by private payors and governmental payors, adverse changes in January 2018,payors’ policies toward coverage and reimbursement for surgical procedures would also harm our ability to promote and sell our products. Payors continue to review their policies and can, without notice, deny coverage for treatments that include the U.S. government approved an additional suspensionuse of the excise tax on medical device sales from January 1, 2018 to December 31, 2019. When in effect, the increased tax burden from the PPACA significantly impacts our results of operationsproducts. Because each third-party payor individually approves coverage and cash flows.It is possible that legislation willreimbursement, obtaining these approvals may be introducedtime-consuming and passed by the Republican-controlled Congress repealing the PPACA in whole or in part and signed into law. Because of the continued uncertainty about the implementation or continued effectiveness of the PPACA, including the potential for further legal challenges or repeal of that legislation, we cannot quantify or predict with any certainty the likely impact of the PPACA or its repeal on our business model, prospects, financial condition or results of operations.Any healthcare reforms enacted in the futurecostly. In addition, third-party payors may like the PPACA, be phased in over a number of years but, if enacted, could reduce our revenue, increase our costs or require us to reviseprovide scientific and clinical support for the waysuse of our products. Adverse changes in coverage and reimbursement for surgical procedures could harm our business and reduce our revenue.conduct business or put us at risk for losssell our products, and these efforts are expected to continue. To the extent that the use of12business. In addition, our results of operations, financial position and cash flows could be materially adversely affected by changesdevices has historically received reimbursement under a foreign healthcare payment system, such reimbursement, if any, has typically been significantly less than the PPACA and changes under any federal or state legislation adoptedreimbursement provided in the future. We sellUnited States. If coverage and adequate levels of reimbursement from governmental and third-party payors outside of the United States are not obtained and maintained, sales of our products outside of the United States may decrease, and we are subjectmay fail to various regulatory and other risks relating to international operations, which could harm our revenue and profitability.Doing businessachieve or maintain significant sales outside of the United States exposes us to risks distinct from those we face in our domestic operations. For example, our operations outside of the United States are subject to different regulatory requirements in each jurisdiction where we operate or have sales. Our failure, or the failure of our distributors, to comply with current or future foreign regulatory requirements, or the assertion by foreign authorities that we or our distributors have failed to comply, could result in adverse consequences, including enforcement actions, fines and penalties, recalls, cessation of sales, civil and criminal prosecution, and the consequences could be disproportionate to the relative contribution of our international operations to our results of operations. Moreover, if political or economic conditions deteriorate in these countries, or if any of these countries are affected by a natural disaster or other catastrophe, our ability to conduct our international operations or collect on international accounts receivable could be limited and our costs could be increased, which could negatively affect our operating results. Engaging in business outside of the United States inherently involves a number of other difficulties and risks, including, but not limited to:
States.·export restrictions and controls relating to technology;
Operational Risks·pricing pressure that we may experience internationally;·difficulties in enforcing agreements and collecting receivables through certain foreign legal systems;·political and economic instability;·consequences arising from natural disasters and other similar catastrophes, such as hurricanes, tornados, earthquakes, floods and tsunamis;·potentially adverse tax consequences, tariffs and other trade barriers;·the need to hire additional personnel to promote our products outside of the United States;·international terrorism and anti-American sentiment;·fluctuations in exchange rates for future sales denominated in foreign currency, which represent a majority of our sales outside of the United States; and·difficulty in obtaining and enforcing intellectual property rights.In addition, our business practices in foreign countries must comply with U.S. laws, including the Foreign Corrupt Practices Act (FCPA). We have a compliance program in place designed to reduce the likelihood of potential violations of the FCPA and other U.S. and foreign anti-bribery and anti-corruption laws. If violations were to occur, they could subject us to fines and other penalties as well as increased compliance costs.Our exposure to each of these risks may increase our costs and require significant management attention. We cannot assure you that one or more of these factors will not harm our business.Our quarterly financial results are likely to fluctuate significantly because our sales prospects are uncertain.Due to current worldwide economic conditions, natural disasters and other factors discussed in this “Risk Factors” section which may impact our sales results, our quarterly operating results are difficult to predict and may fluctuate significantly from quarter to quarter or from prior year to current year periods, particularly because our sales prospects are uncertain. These fluctuations may also affect our annual operating results and may cause those results to fluctuate unexpectedly from year to year.Restrictions in our ability to train surgeons in the use of our products could reduce the market acceptance of our products or result in injuries to patients or other adverse events that could possibly lead to litigation that could harm us or could reduce our revenue.It is critical to the success of our sales efforts to ensure that there are a sufficient number of surgeons familiar with, trained on and proficient in the use of our products. While we train providers in the safe and effective use of our products, we do not train them to use any of our products specifically to treat Afib unless the product is FDA-approved specifically for the treatment of Afib. Our Isolator Synergy System is approved for the treatment of persistent and long standing persistent forms of Afib concomitant to open heart bypass graft or valve replacement surgery. The procedure using our Isolator Synergy System in this manner is known as the MAZE IV™ procedure. Following FDA approval, we instituted a program to train all new and existing users of the Isolator Synergy System in the MAZE IV procedure. We also make available training on the safe and effective use of our other products consistent with their FDA approved or cleared indications, but we cannot provide assurance that a sufficient number of surgeons will become aware of training programs.13Surgeons may not commit enough time to sufficiently learn our products. In order for surgeons to learn to use our products, they must attend structured training sessions in order to familiarize themselves with the products and they must be committed to learning the technology. Further, surgeons must utilize the technology on a regular basis to ensure they maintain the skill set necessary to use the products. Continued market acceptance could be delayed by lack of surgeon willingness to attend training sessions, by the time required to complete this training or by state or institutional restrictions on our ability to provide training. An inability to train a sufficient number of surgeons to generate adequate demand for our products could have a material adverse impact on our financial condition and cash flow. Our marketing strategy is dependent on collaboration with physician “thought leaders.” Our research and development efforts and our marketing strategy depend heavily on obtaining support, physician training assistance and collaboration from highly-regarded physicians at leading commercial and research hospitals, particularly in the U.S. and Europe. If we are unable to gain and/or maintain such support, training services and collaboration, or if the reputation or standing of these physicians is impaired or otherwise adversely affected, our ability to market our products and, as a result, our financial condition, results of operations and cash flow, could be materially and adversely affected.Unless and until we obtain additional FDA approval for our products, we will not be able to promote many of them to treat Afib or to prevent stroke, and our ability to maintain and grow our business could be harmed.Although our Isolator Synergy System received FDA approval for the treatment of some forms of Afib in certain procedures, we have not received FDA clearance or approval to promote our other products for the treatment of Afib or the prevention of stroke. See “Business—Government Regulation.” Unless and until we obtain FDA clearance or approval for the use of our products to treat Afib or prevent stroke, we, and others acting on our behalf, may not claim in the U.S. that our products are safe and effective for such uses or otherwise promote them for such uses. Similar restrictions exist outside of the U.S. There is no assurance that future clearances or approvals of our products will be granted or that current or future clearances or approvals will not be withdrawn. Failure to obtain a clearance or approval or loss of an existing clearance or approval, could hurt our ability to maintain and grow our business.In order to obtain additional FDA approvals to promote our products for the treatment of Afib or reduction in stroke risk, we will need to demonstrate in clinical trials that our products are safe and effective for such use. Development of sufficient and appropriate clinical protocols to demonstrate quality, safety and efficacy may be required and we may not adequately develop such protocols to support approval. We cannot assure you that any of our clinical trials will be completed in a timely manner or successfully or that the results obtained will be acceptable to FDA. We, FDA or the IRB may suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. In addition, if the results obtained from our clinical trials, any other clinical studies, or clinical or commercial experience indicate that any of our products are not safe or effective, or not as safe or effective as other treatment options, FDA may not approve our products for the treatment of Afib or reduction in stroke risk, and the adoption of the use of our products may suffer and our business would be harmed.Our clinical trials are typically time consuming, expensive and the outcome uncertain. Delays in patient enrollment or failure of patients to consent or continue to participate in a clinical trial may cause an increase in costs and delays in the approval and attempted commercialization of our products or result in the failure of the clinical trial. Conducting successful clinical studies may require the enrollment of large numbers of clinical sites and patients, and suitable patients may be difficult to identify and recruit. Patient enrollment in clinical trials and completion of patient participation and follow-up depends on many factors, including the size of the patient population, the nature of the trial protocol, the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects, the availability of appropriate clinical trial investigators, support staff, and proximity of patients to clinical sites and ability to comply with the eligibility and exclusion criteria for participation in the clinical trial and patient compliance. For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures or follow-up to assess the safety and effectiveness of our products or if they determine that the treatments received under the trial protocols are not attractive or involve unacceptable risks or discomforts. Patients may also not participate in our clinical trials if they choose to participate in contemporaneous clinical trials of competitive products or they can obtain the treatment without participating in our trial.andor our industry. This publicity could have a negative impact on our sales, our ability to attract and retain customers, our sales, clinical studies involving our products, our reputation and our stock price.certain of our consultants who are involved with clinical studies and the publication of articles concerning our products.consultants. We believe that such publicity would potentially have a negative clinical studies, business, results of operations and financial condition and our clinical studies, or cause other adverse effects, including a decline in the price of our stock.14We may be subject to fines, penalties, injunctions and other sanctions if we are deemed to be promoting the use of our products for unapproved, or off-label, uses.Our business and future growth depend on the continued use of our products for the treatment of Afib or prevention of stroke. Unless the products are approved or cleared by FDA specifically for the treatment of Afib or prevention of stroke, we may not make claims about the safety or effectiveness of our products for such uses.These limitations present a material risk that FDA or other federal or state law enforcement authorities could determine that the nature and scope of our sales, marketing and/or support activities, though designed to comply with all FDA requirements, constitute the promotion of our products for an unapproved use in violation of the FDCA. We also face the risk that the FDA or other governmental authorities might pursue enforcement based on past activities that we have discontinued or changed, including sales activities, arrangements with institutions and doctors, educational and training programs and other activities. Investigations concerning the promotion of unapproved uses and related issues, are typically expensive, disruptive and burdensome and generate negative publicity. If our promotional activities are found to be in violation of the law, we may face significant fines and penalties and may be required to substantially change our sales, promotion, grant and educational activities. There is also a possibility that we could be enjoined from selling some or all of our products for any unapproved use. In addition, as a result of an enforcement action against us or our executive officers, we could be excluded from participation in government healthcare programs such as Medicare and Medicaid.The use of products we sell may result in injuries or other adverse events that lead to product liability suits, which could be costly to our business or our customers’ businesses.The use of products we sell may result in a variety of serious complications, including damage to the heart, internal bleeding, death or other adverse events, potentially leading to product liability claims. Serious complications are commonly encountered in connection with surgical procedures. If products we sell are defectively designed, manufactured or labeled, contain inadequate warnings, contain defective components, are misused or are associated with serious injuries or deaths, we may become subject to costly litigation by our customers or their patients. We carry product liability insurance that is limited in scope and amount and may not be adequate to fully protect us against product liability claims. We could be required to pay damages that exceed our insurance coverage. Any product liability claim, with or without merit, could result in an increase in our product insurance rates or our inability to secure coverage on reasonable terms, if at all. Even in the absence of a claim, our insurance rates may rise in the future. Any product liability claim, even a meritless or unsuccessful one, would be time-consuming and expensive to defend and could result in the diversion of our management’s attention from our business and result in adverse publicity, withdrawal of clinical trial participants, injury to our reputation and loss of revenue. Any of these events could negatively affect our earnings and financial condition.Our intellectual property rights may not provide meaningful commercial protection for our products, which could enable third parties to use our technology or methods, or very similar technology or methods, and could reduce our ability to compete.Our success depends significantly on our ability to protect our proprietary rights to the technologies used in our products. We rely on patent protection, as well as a combination of copyright, trade secret and trademark laws and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Our patent applications may not issue as patents at all or in a form that will be advantageous to us. Our issued patents and those that may be issued in the future may be challenged, invalidated or circumvented, which could limit our ability to stop competitors from marketing related products. Although we have taken steps to protect our intellectual property and proprietary technology, we cannot assure you that third parties will not be able to design around our patents or, if they do infringe upon our technology, that we will be successful in or will have sufficient resources to pursue a claim of infringement against those third parties. We believe that third parties may have developed or are developing products that could infringe upon our patent rights. Any pursuit of an infringement claim by us may involve substantial expense or diversion of management attention. In addition, although we have generally entered into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, investigators and advisors, such agreements may be breached, may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements. Additionally, as is common in the medical device industry, some of these individuals were previously employed at other medical equipment or biotechnology companies, including our competitors. Although no claims are currently pending against us, we may be subject to claims that these individuals or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Furthermore, the laws of foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States. Foreign countries generally do not allow patents to cover methods for performing surgical procedures. If our intellectual property does not provide significant protection against foreign or domestic competition, our competitors could compete more directly with us, which could result in a decrease in our market share. All of these factors may harm our competitive position.15The medical device industry is characterized by extensive litigation and administrative proceedings over patent and other intellectual property rights and any litigation or claim against us may cause us to incur substantial costs, could place a significant strain on our financial resources, divert the attention of management from our business and harm our reputation.Despite measures taken to protect our intellectual property, unauthorized parties might copy aspects of our products or obtain and use information that we regard as proprietary. Whether a product infringes a patent involves complex legal and factual issues, the determination of which is often uncertain. Any patent dispute, even one without merit or an unsuccessful one, would be time-consuming and expensive to defend and could result in the diversion of our management’s attention from our business and result in adverse publicity, the disruption of development and marketing efforts, injury to our reputation and loss of revenue. Litigation also puts our patent applications at risk of being rejected and our patents at risk of being invalidated or interpreted narrowly, and may provoke third parties to assert claims against us. Any of these events could negatively affect our earnings and financial condition.In the event of a patent dispute, if a third party’s patents were upheld as valid and enforceable and we were found to be infringing, or found to be inducing infringement by others, we could be prevented from selling our products unless we were able to obtain a license to use technology or ideas covered by such patent or are able to redesign our system to avoid infringement, or we may be ordered to pay substantial damages to the patent holders. A license may not be available at all or on terms acceptable to us, and we may not be able to redesign our products to avoid any infringement. Modification of our products or development of new products could require us to conduct additional clinical trials and to revise our filings with the FDA and other regulatory bodies, which would be time-consuming and expensive. If we are not successful in obtaining a license or redesigning our products, we may be unable to sell our products and our business could suffer.The increase in cost of medical malpractice premiums to doctors and hospitals or the lack of malpractice insurance coverage due to the use of our products by doctors for an off-label indication may cause certain doctors or hospitals to decide not to use our products and may damage our ability to grow and maintain the market for our products. Insurance carriers have been raising premiums charged for medical malpractice insurance due, at least in part, to increased risks associated with off-label procedures, including higher damage awards for successful plaintiffs. Insurance carriers may continue to raise premiums or they may deny malpractice coverage for procedures performed using products such as ours on an off-label basis. If this trend continues or worsens, our revenue may fall as doctors or hospitals decide against purchasing our products due to the cost or unavailability of insurance coverage.We have a history of net losses and we may never become profitable.We have incurred net losses each year since our inception, including, most recently, net losses of $26,892 in 2017, $33,338 in 2016 and $27,212 in 2015. As of December 31, 2017, we had an accumulated deficit of $225,866. Our net losses have resulted principally from costs and expenses relating to sales, training and promotional efforts, research and development, seeking regulatory clearances and approvals and general operating expenses. We expect to continue to incur substantial expenditures and to potentially incur additional operating losses in the future as we further develop and commercialize our products, including completing clinical trials and seeking regulatory clearances and approvals. If sales of our products do not continue to grow as we anticipate, we will not be able to achieve profitability. Our expansion efforts may prove to be more expensive than we currently anticipate, and we may not succeed in increasing our revenue sufficiently to offset these higher expenses. Our losses have had, and are expected to continue to have, an adverse impact on our working capital, total assets and accumulated deficit. Our capital needs after the next twelve months are uncertain and we may need to raise additional funds in the future and such funds may not be available on acceptable terms, if at all.We believe that our current cash, cash equivalents and investments, along with the cash we expect to generate or use for operations or access via our term loan and revolving line of credit will be sufficient to meet our projected capital requirements for at least the next 12 months. Our Loan and Security Agreement with Silicon Valley Bank (SVB), as amended and restated effective February 23, 2018, provides for a $40,000 term loan and $20,000 revolving line of credit, with an option to increase the revolving line of credit by an additional $20,000. The term loan and revolving credit facility both mature in February 2023. According to the Loan and Security Agreement, principal payments on the term loan are to be made ratably commencing eighteen months after the inception of the loan through the loan’s maturity date. If we meet certain conditions, as specified by the agreement, the commencement of term loan principal payments may be deferred by an additional six months. The term loan accrues interest at the greater of the Prime Rate plus 3.75% or 8.25% and is subject to an additional 3.50% fee on the original $40,000 term loan principal amount at maturity. Borrowing availability under the revolving credit facility is based on the lesser of $20,000 or a borrowing base calculation as defined by the Loan and Security Agreement. The applicable borrowing rate on advances outstanding under the revolving credit facility is the greater of the Prime Rate and 4.50%. The Loan and Security Agreement also provides for certain prepayment and early termination fees, as well as establishes a covenant related to sales growth and includes other customary terms and conditions. As of December 31, 2017, we had no borrowings under the revolving credit facility, and we had borrowing availability of $15,000. The nContact acquisition provided for contingent consideration to be paid upon attaining specified regulatory approvals and clinical and revenue milestones over the next three years. Subject to the terms and conditions of the nContact merger agreement, such16contingent consideration is paid in AtriCure common stock and cash, with a requirement to make payments in AtriCure common stock first, up to a specified maximum number of shares. Over the next twelve months, we do not expect our cash requirements to include significant payments of contingent consideration based on terms of the acquisition agreement and related milestones. However, we do expect to issue shares in the amount of $7,500 as payment of contingent consideration related to the completion of the trial enrollment milestone in 2018. Significant changes to the estimated consideration to be paid could result in a substantial increase in liabilities for contingent consideration and our accumulated deficit, and reduce our net income or increase our net loss for the year in which the changes occur, which could contribute to difficulty in raising additional funds. The issuance of our stock to nContact shareholders to settle contingent consideration obligations would dilute the holdings of our existing stockholders. We believe we have adhered to the nContact contract provisions that provide for contingent consideration if the conditions described above are met. It is possible that nContact representatives may dispute our adherence to the contract and pursue a claim for non-adherence which could involve complex legal and factual issues, the determination of which is often uncertain. Any such claim, even one without merit or an unsuccessful one, would be time-consuming and expensive to defend and could result in the diversion of our management’s attention from our business and result in adverse publicity, the disruption of development and marketing efforts, injury to our reputation and loss of revenue.If we need to raise additional funds, we cannot be certain that such funds will be available to us on acceptable terms, if at all. Furthermore, if we issue equity securities to raise additional funds, our existing stockholders will experience dilution, and if we issue equity or debt securities, such securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, if we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish potentially valuable rights to our future products or proprietary technologies, or grant licenses on terms that are not favorable to us. If we cannot raise funds on acceptable terms, we may not be able to expand our operations, develop new products, take advantage of future opportunities or respond to competitive pressures or unanticipated customer requirements.We may be unable to comply with the covenants of our Loan Agreement.Our Loan Agreement with SVB contains a covenant related to sales growth and other customary terms and conditions. The occurrence of an event of default could result in an increase to the applicable interest rate by 3.0%, an acceleration of all obligations, an obligation to repay all obligations in full and a right by SVB to exercise all remedies available to them. If we are unable to pay those amounts, SVB could proceed against the collateral granted to it pursuant to the Loan Agreement, and we may in turn lose access to our current source of borrowing availability.Our federal tax net operating loss (NOL) and general business credit carryforwards generated or acquired may expire or will be limited because we experienced an ownership change of more than 50 percent, which could result in greater future income tax expense and adversely impact future cash flows.On June 30, 2001, we experienced an ownership change as defined by Section 382 of the Internal Revenue Code of 1986. Section 382 imposes limitations (Section 382 limitation) on a company’s ability to use net operating loss and general business credit carryforwards if a company experiences a more-than-50-percent ownership change over a three-year testing period. Additionally, in connection with acquisitions, additional acquired NOLs are also subject to Section 382 limitation. The Section 382 limitations could limit the availability of our net operating loss and general business credit carryforwards to offset any future taxable income, which may increase our future income tax expense and adversely impact future cash flows. Net operating losses generated prior to 2018 are also subject to expiration under current IRS regulations. We have total federal income tax net operating loss and research and development credit carryforwards that, if not used to reduce our taxable income, will begin to expire in 2021. We have generated or acquired available net operating loss and research and development credit carryforwards of $240,286 and $6,392. logisticsservice providers, making us vulnerable to supply problems and price fluctuations which could harm our business.several of our RF generators,generator, as well as separate vendors to manufacture our COBRA Fusion Surgical Ablation Systems, EPi-Sense Guided Coagulation System with VisiTrax technology, nContactand related RF generator and ORLab™ System.generator. It would be a time consuming and lengthy process to secure these products from an alternative supplier. In addition, in some cases thereWe have significant concentrations with a limited number of vendors. Additionally, our devices are relatively few alternative sourcessterilized prior to use using ethylene oxide at third-party sterilizers. Recently, certain sterilization facilities have experienced mandated temporary closures due to concerns over the impact of supply for certain other components that are critical to our products.emissions of ethylene oxide from such facilities, and the Environmental Protection Agency has proposed regulations aimed at reducing hazardous air pollutants. We also rely on a third partyparties to handle our warehousing and logistics functions for European and Middle Easternseveral other international markets on our behalf.·we may not be able to obtain adequate supply in a timely manner or on commercially reasonable terms;
•we may not be able to obtain adequate supply in a timely manner or on commercially reasonable terms;·we may have difficulty timely locating and qualifying alternative suppliers;
•we may have difficulty timely locating and qualifying alternative suppliers or sterilizers;·switching components may require product redesign and new submissions to FDA which could significantly delay production or, if FDA refuses to approve the changes, completely eliminate our ability to manufacture or sell our products;17Contentscustomers, and fluctuations in demand for the products those suppliers manufacture for others may affect their ability to deliver components to us in a timely manner; and
•our suppliers may encounter financial hardships unrelated to our demand for components, which could inhibit their ability to fulfill our orders and meet our requirements.·our suppliers manufacture products for a range of customers, and fluctuations in demand for the products those suppliers manufacture for others may affect their ability to deliver components to us in a timely manner; and·our suppliers may encounter financial hardships unrelated to our demand for components, which could inhibit their ability to fulfill our orders and meet our requirements.a replacement of warehousing and logistics provider,providers, if required, may not be accomplished quickly and could involve significant additional costs. Any interruption or delay in the supply of components, materials, sterilization or warehousing and logistics, or our inability to obtain components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our customers and cause them to cancel orders or switch to competitive products and could therefore have a material adverse effect on our business, financial condition and results of operations.Ifgoodwill or other intangible assets become impaired, itmanufacturing facilities could materially reduce the value of our assets and increase our net loss for the year in which the impairment occurs.As of December 31, 2017, we had $105,257 in goodwill related to acquisitions, which represents the purchase price we paid in excess of the fair value of the net assets we acquired. The Financial Accounting Standards Board’s (FASB) Accounting Standards Codification (ASC) 350, “Goodwillexpenses and Other Intangible Assets” requires that goodwill be tested for impairment at least annually (absent any impairment indicators). The testing includes comparing the fair value of each reporting unit with its carrying value. We estimate fair value using several valuation methods, including discounted cash flows, market multiples and market capitalization. Impairment adjustments, if any,decrease our revenue.required to be recognized as operating expenses. We may have future impairment adjustmentshighly centralized to our recorded goodwill. Any finding that the value of our goodwill has been impaired would require us to record an impairment charge which could materially reduce the value of our assets and reduce our net income or increase our net losscorporate headquarters. While we take precautions, such as qualifying a second building for the year in which the write off occurs and increase our accumulated deficit, which could contribute to difficulty in raising additional funds. In Process Research and Development (IPR&D) valued at $44,021 was recorded as an intangible asset in connection with the nContact acquisition. Ifmanufacturing, we do not obtain the regulatory approvals that would confirm the technological feasibility of the IPR&D project, or if the IPR&D project is abandoned for any other reason, we would have an impairment adjustment to this asset that would require us to write it off. Additionally, and similar to goodwill, if the IPR&D asset is deemed to be impaired (asmaintain a result of the estimated fair value being less than carrying value), we would be required to write off the impaired portion of the IPR&D asset. This would materially reduce the valuebackup manufacturing facility outside of our assetsOhio campus, making us dependent on the current facilities and reduce our net income or increase our net lossproduction workers for the year in which the write off occurs and increase our accumulated deficit, which could contribute to difficulty in raising additional funds. An inability to forecast future revenue or estimate life cycles of products may result in inventory-related charges that would negatively affect our gross margins and results of operations.To mitigate the risk of supply interruptions, we may choose to maintain excess inventorycontinued operation of our productsbusiness. A natural or component parts. Managing our inventory levels is important to our cash position and results of operations and is challenging in the current economic environment. As we grow and expand our product offerings, managing our inventory levels becomes more difficult, particularly as we expand into new product areas and bring product enhancements to market. While we rely on our personnel and information technology systems for inventory management to effectively manage accounting and financial functions, our personnel and information technology systems may fail to adequately perform these functionsother disaster could damage or may experience an interruption. An excessive amount of inventory reduces our cash available for operations and may result in excess or obsolete materials. Conversely, inadequate inventory levels may make it difficult for us to meet customer product demand, resulting in decreased revenue. An inability to forecast future revenue or estimated life cycles of products may result in inventory-related charges that would negatively affect our gross margins and results of operations and increase our accumulated deficit, any of which could contribute to difficulty in raising additional funds.If we or our third-party vendors fail to comply with extensive FDA regulations relating to the manufacturing of our products or any component part, we may be subject to fines, injunctions and penalties, and our ability to commercially distribute and sell our products may be hurt.Our manufacturing facility and the manufacturing facility of any of our third-party component manufacturers, critical suppliers or third-party sterilization facility are required to comply with FDA’s Quality System Regulation (QSR) which sets forth minimum standards for the procedures, execution and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of the products we sell. FDA may evaluate our compliance with the QSR, among other ways, through periodic announced or unannounced inspections which could disrupt our operations and interrupt our manufacturing. If in conducting an inspection ofdestroy our manufacturing facility or the manufacturing facility of any of our third-party component manufacturers, critical suppliers or third-party sterilization facility, an FDA investigator observes conditions or practices believed to violate the QSR, the investigator may document their observations on a Form FDA-483 that is issued at the conclusion of the inspection. A manufacturer that receives an FDA-483 may respondequipment and cause substantial delays in writing and explain any corrective actions taken in response to the inspectional observations. FDA will typically review the facility’s written response and may re-inspect to determine the facility’s compliance with the QSR and other applicable regulatory requirements. Failure to take adequate and timely corrective actions to18remedy objectionable conditions listed on an FDA-483 could result in FDA taking administrative or enforcement actions. Among these may be FDA’s issuance of a Warning Letter to a manufacturer, which informs it that FDA considers the observed violations to be of “regulatory significance” that, if not corrected, could result in further enforcement action. FDA enforcement actions, which include seizure, injunction and criminal prosecution, could result in total or partial suspension of a facility’s production and/or distribution, product recalls, fines, suspension of FDA’s review of product applications and FDA’s issuance of adverse publicity. Thus, an adverse inspection could force a shutdown of our manufacturing operations, or a recall of our products. Adverse inspections could also delay FDA approval of our products and could have an adverse effect on our production, sales and profitability.We and any of our third-party vendors may also encounter other problems during manufacturing including failure to follow specific protocols and procedures, equipment malfunction and environmental factors, any of which could delaylead to additional expense and decreased revenue due to lack of supply. The insurance we maintain may not be adequate to cover our losses. With or impedewithout insurance, damage to our abilityfacilities or our other property due to meet demand. The manufacture of our product also subjects us to risks thata natural disaster or casualty event could harm our business, including problems relating to the sterilization of our products or facilities and errors in manufacturing components that could negatively affect the efficacy or safety of our products or cause delays in shipment of our products. Any interruption or delay in the manufacture of the product or any of its components could impair our ability to meet the demand of our customers and cause them to cancel orders or switch to competitive products and could, therefore, have a material adverse effect on our business, financial condition and results of operations.comply with the extensive FDA regulations relating toproperly manage our anticipated growth, our business wecould suffer.be subjectexperience periods of rapid growth and expansion, which could place a significant strain on our personnel, information technology systems and other resources. In particular, the increase in our direct sales force requires significant management and other supporting resources. Any failure by us to fines, injunctions and penalties, andmanage our growth effectively could have an adverse effect on our ability to commercially distributeachieve our development and promotecommercialization goals.may be hurt.Ourand our operations and the limited pool of people with relevant experience in the medical device field, the loss of service of one or more of these individuals could significantly affect our ability to operate and manage our business. The announcement of the loss of one or more of our key personnel could negatively affect our stock price.are classifiedand further develop products, while managing anticipated growth by FDA as medical devicesimplementing effective planning, manufacturing and as such, are subjectoperating processes. Managing this growth will require us to extensive regulationattract and retain additional management and technical personnel. We rely primarily on direct sales employees to sell our products in the United States by FDA and numerous other federal, statein Europe, and foreign governmental authorities. FDA regulations, guidance, notices and other issuances specificfailure to medical devices are broad and regulate numerous aspects of our business. Compliance with FDA, state and other regulations can be complex, expensive and time-consuming. FDA and other authorities have broad enforcement powers. Furthermore, changesadequately train them in the applicable governmental regulations could prevent further commercializationuse and benefits of our products will prevent us from achieving our market share and technologiesrevenue growth goals. We have key relationships with physicians that involve procedure, product, market and clinical development and training. Our business could materiallybe negatively impacted if any of these physicians end their relationship with us. We cannot assure you that we will be able to attract and retain the personnel and physician relationships necessary to grow and expand our business and operations. If we fail to identify, attract, retain and motivate these highly skilled personnel and physicians, we may be unable to continue our development and sales activities.business.serioussecurity breach or cyber-attack or other disruption from occurring, then we could incur losses or damage to our data, or inappropriate disclosure of our confidential information or that of others. We have cyber-insurance coverage that may not cover all possible events, and this insurance is subject to deductibles and coverage limitations. We could sustain damage to our reputation and customer and employee relationships, suffer disruptions to our business and incur increased operating costs including costs to mitigate any damage caused and protect against future damage, and be exposed toregulatory requirements was determined, itlaws and regulations could result in enforcement action by FDA or other state or federal agencies, including the DOJ, which may include any of the following sanctions, among others: ·warning letters, fines, injunctions, consent decrees and civil penalties;·repair, replacement, refunds, recall or seizure ofbe materially impaired. Any such impairment could have a material adverse effect on our products;·operating restrictions, partial suspension or total shutdown of production;·suspension or termination of our clinical trials;·refusing or delaying our pending requests for 510(k) clearance or PMAs, new intended uses or modifications to existing products;·withdrawing 510(k) clearance or PMAs that have already been granted; and·criminal prosecution.If any of these events were to occur, we could lose customers and our production, product sales, business, results of operations, and financial condition wouldand the timeliness with which we report our operating results.harmed.Wecustomary for our industry. The coverage provided by such insurance may not be adequate for claims we may make or may be contested by our insurance carriers. If our insurance is not adequate or available to pay liabilities associated with our operations, or if we are also subjectunable to medical device reporting regulations that require us to file reports with FDA if our products may have caused or contributed to a death or serious injury or,purchase adequate insurance at reasonable rates in the eventfuture, our business, financial condition, results of product malfunction, that if such malfunction were to recur, would likely causeoperations or contribute to a death or serious injury. There have been incidents, including patient deaths, which have occurred during or following procedures using our products that we have not, and believe were not required tocash flows may be reported to FDA because we determined that our products did not cause or contribute to the outcomes in these incidents. If FDA disagrees with us, however, and determines that we should have submitted reports for these adverse events, we could be subject to significant regulatory fines or other penalties. In addition, the number of medical device reports we make, or the magnitude of the problems reported, could cause FDA or us to terminate or modify our clinical trials or recall or cease the sale of our products, and could hurt commercial acceptance of our products and harm our reputation with customers. Modifications to our products may require new clearances or approvals or may require us to cease promoting or to recall the modified products until such clearances or approvals are obtained and FDA may not agree with our conclusions regarding whether new clearances or approvals were required.Any modification to a 510(k)-cleared device that would constitute a change in its intended use, design or manufacture could require a new or supplemental 510(k) clearance or, possibly, submission and FDA approval of a PMA. FDA requires every medical device company to make the determination as to whether a 510(k) must be filed, but FDA may review any medical device company’s decision. We have made modifications to our products and concluded that such modifications did not require us to submit a 510(k). FDA may not agree with our decisions regarding whether submissions were required.19If FDA were to disagree with us and require us to submit a 510(k), PMA or a PMA supplement for then-existing modifications, we could be required to cease promoting or to recall the modified product until we obtain clearance or approval. In addition, we could be subject to significant regulatory fines or other penalties. Furthermore, our products could be subject to recall if FDA determines, for any reason, that our products are not safe or effective or that appropriate regulatory submissions were not made. Delays in receipt or failure to receive clearances or approvals, the loss of previously received clearances or approvals or the failure to comply with existing or future regulatory requirements could reduce our sales, profitability and future growth prospects.are unable todo not fully comply with such regulations, we could face substantial penalties.·state consumer protection, fraud and business practice laws;
•the Federal Anti-Kickback Statute, which prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual, or furnishing or arranging for a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid Programs;·the Federal Anti-Kickback Statute, which prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual, or furnishing or arranging for a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid Programs;
•the Federal False Claims Act, which prohibits submitting a false claim or causing the submission of a false claim to the government;·the Federal False Claims Act, which prohibits submitting a false claim or causing of the submission of a false claim to the government;
•Medicare laws and regulations that prescribe the requirements for coverage and payment, including the amount of such payment, and laws prohibiting false claims for reimbursement under Medicare and Medicaid;·Medicare laws and regulations that prescribe the requirements for coverage and payment, including the amount of such payment, and laws prohibiting false claims for reimbursement under Medicare and Medicaid;
•state consumer protection, fraud and business practice laws, including the California Consumer Privacy Act (“CCPA”), which among other things, requires disclosures to California consumers and provides consumers new abilities to opt out of certain sales of personal information;·state laws that prohibit the practice of medicine by non-doctors and by doctors not licensed in a particular state, and fee-splitting arrangements between doctors and non-doctors, as well as state law equivalents to the Anti-Kickback Statute and the Stark Law, which may not be limited to government-reimbursed items;
•state laws that prohibit the practice of medicine by non-doctors and by doctors not licensed in a particular state, and fee-splitting arrangements between doctors and non-doctors, as well as state law equivalents to the Anti-Kickback Statute and the Stark Law, which may not be limited to government-reimbursed items;·federal and state healthcare fraud and abuse laws or laws protecting the privacy of patient medical information, including the Health Insurance Portability and Accountability Act (HIPAA) which protects medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting reasonably necessary to accomplish the intended purpose;
•federal and state healthcare fraud and abuse laws or laws protecting the privacy of patient medical information, including the Health Insurance Portability and Accountability Act (HIPAA) which protects medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting reasonably necessary to accomplish the intended purpose;·the Federal Trade Commission Act and similar laws regulating advertising and consumer protection; and
•laws and regulations, such as the General Data Protection Regulation in the European Union, that govern collection, use, disclosure, transfer and storage of personal data that we may collect from our employees, consultants or in conjunction with clinical trials;·similar and other regulations outside the United States. For example, if we were found to be in violation of the Federal False Claims Act, we would likely face significant fines and penalties and would likely be required to change substantially our sales, promotion, grant and educational activities. There is also a possibility that we could face an injunction that would prohibit in whole or in part our current business activities, and, as a result of enforcement actions against us or our senior officers, we could be excluded from participation in government healthcare programs such as Medicare and Medicaid. If there is a change in law, regulation or administrative or judicial interpretations, we may have to change our business practices or our existing business practices could be challenged as unlawful, which could have a material adverse effect on our business, financial condition and results of operations. past or present operations are found to be in violation of any of the laws described above or the other governmental regulations to which we, our distributors or our customers are subject, we may be subject to the applicable penalty associated with the violation, including civil and criminal penalties, damages, fines, exclusion from Medicare, Medicaid and other government programs and the curtailment or restructuring of our operations. If we are required to obtain permits or licensure under these laws that we do not already possess, we may become subject to substantial additional regulation or incur significant expense. Any penalties, damages, fines, curtailment or restructuring of our operations would adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully or clearly interpreted by the regulatory authorities or the courts, and their provisions are subject to a variety of interpretations and additional legal or regulatory change. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and damage our reputation.Adverse changes in payors’ policies toward coveragereimbursement for surgical procedures would harmpenalties, and our ability to commercially distribute and promote our products may be hurt.sellas such, are subject to extensive regulation by FDA and numerous other federal, state and foreign governmental authorities. FDA regulations, guidance, notices and other issuances specific to medical devices are broad and regulate numerous aspects of our products.Third-party payorsbusiness. Compliance with FDA, state and other regulations can be complex, expensive and time-consuming. FDA and other authorities have broad enforcement powers. Furthermore, changes in the applicable governmental regulations could prevent further commercialization of our products and technologies and could materially harm our business.increasingly exerting pressure onalso subject to medical device companiesreporting regulations that require us to reduce their prices. Evenfile reports with FDA if our products may have caused or contributed to a death or serious injury or, in the event of product malfunction, that if such malfunction were to recur, would likely cause or contribute to a death or serious injury. There have been incidents, including patient deaths, which have occurred during or following procedures using our products that we have not reported to FDA because we determined that our products did not malfunction and did not cause or contribute to the extentoutcomes in these incidents. If FDA disagrees with us, however, and determines that we should have submitted reports for these adverse events, we could be subject to significant regulatory fines or other penalties. In addition, the number of medical device reports we make, or the magnitude of the problems reported, could cause us or FDA to terminate or modify our clinical trials or recall or cease the sale of our products, and could hurt commercial acceptance of our products and harm our reputation with customers.is reimbursed by private payorsfor unapproved, or off-label, uses.governmental payors, adverse changes in payors’ policies toward coverage and reimbursement for surgical procedures would also harm our ability to promote and sell our products. Payors continue to review their policies and can, without notice, deny coverage for treatments that includefuture growth depend on the continued use of our products. Because each third-party20payor individually approves coverageAfib. Unless the products are approved or cleared by FDA specifically for the treatment of Afib or prevention of stroke, we may not make claims about the safety or effectiveness of our products for such uses. In order to obtain additional FDA approvals to promote our products for the treatment of Afib or reduction in stroke risk, we will need to demonstrate in clinical trials that our products are safe and reimbursement, obtaining these approvalseffective for such use. Development of sufficient and appropriate clinical protocols to demonstrate quality, safety and efficacy may be time-consumingrequired and costly. In addition, third-party payorswe may require usnot adequately develop such protocols to provide scientificsupport approval. We cannot assure you that any of our clinical trials will be completed in a timely manner or successfully or that the results obtained will be acceptable to FDA. We, FDA or the IRB may suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits.clinicalscope of our sales, marketing and/or support activities, though designed to comply with all FDA requirements, constitute the promotion of our products for an unapproved use in violation of the FDCA. We also face the risk that FDA or other governmental authorities might pursue enforcement based on past activities that we have discontinued or changed, including sales activities, arrangements with institutions and doctors, educational and training programs and other activities. Investigations concerning the promotion of unapproved uses and related issues, are typically expensive, disruptive and burdensome and generate negative publicity. If our promotional activities are found to be in violation of the law, we may face significant fines and penalties and may be required to substantially change our sales, promotion, grant and educational activities. There is also a possibility that we could be enjoined from selling some or all of our products for any unapproved use.usetreatment of some forms of Afib in certain procedures, we have not received FDA clearance or approval to promote our products. Adverse changes in coverage and reimbursement for surgical procedures could harm our business and reduce our revenue.FDA does not regulate the practice of medicine. Physicians may use ourother products in circumstances where they deem it medically appropriate, such as for the treatment of Afib or the reduction in stroke risk, even thoughprevention of stroke. Unless and until we obtain FDA may not have approvedclearance or cleared our products to be marketed specificallyapproval for those indications. Some payors may deem the use of our other products to treat Afib or prevent stroke, we, and others acting on our behalf, may not claim in the U.S. that such products are safe and effective for indications not specifically approvedsuch uses or cleared by FDA to be experimental and, asotherwise promote them for such may deny coverage or payment. Often times, these denials can be overcome through an appeals process, but thereuses. Similar restrictions also exist outside of the U.S. There is no guarantee of success in these cases.We have traditionally had limited long-term clinical data regarding the safety and efficacy of our products. Any long-term dataassurance that is generated may not be positivefuture clearances or consistent with our limited short-term data, which would affect the rate at which our products are adopted by the medical community.Important factors upon which the efficacyapprovals of our products will be measured include long-term data ongranted or that current or future clearances or approvals will not be withdrawn. Failure to obtain a clearance or approval or loss of an existing clearance or approval, could hurt our ability to maintain and grow our business.numbermodified products until such clearances or approvals are obtained and FDA may not agree with our conclusions regarding whether new clearances or approvals were required.patients that experience Afiba PMA application or stroke following treatment withPMA supplement. FDA requires every medical device company to make the determination as to whether a 510(k) must be filed, but FDA may review any medical device company’s decision. We have made modifications to our products and concluded that such modifications did not require us to submit a new or supplemental 510(k). FDA may not agree with our decisions regarding whether submissions were required.numbermodified product until we obtain clearance or approval. In addition, we could be subject to significant regulatory fines or other penalties. Furthermore, our products could be subject to recall if FDA determines, for any reason, that our products are not safe or effective or that appropriate regulatory submissions were not made. Delays in receipt or failure to receive clearances or approvals, the loss of patients that have serious complications resulting from ablationspreviously received clearances or LAA occlusion usingapprovals or the failure to comply with existing or future regulatory requirements could reduce our products. Whilesales, profitability and future growth prospects.believe we are now well-positionedor our third-party vendors fail to provide sufficient long-term data regardingcomply with extensive FDA regulations relating to the safety and efficacymanufacturing of our products such dataor component parts, we may be subject to fines, injunctions and penalties, and our ability to commercially distribute and sell our products may be hurt.identify unexpected safety issues. We cannot providedisrupt our operations and interrupt our manufacturing. If in conducting an inspection of our manufacturing facilities or the manufacturing facilities of any assuranceof our third-party component manufacturers, critical suppliers or third-party sterilization facilities, an FDA investigator observes conditions or practices believed to violate the QSR, the investigator may document their observations on a Form FDA-483 that is issued at the data collected during our clinical trials will be compellingconclusion of the inspection. A manufacturer that receives an FDA-483 may respond in writing and explain any corrective actions taken in response to the medical community because it may not be scientifically meaningfulinspection observations. FDA will typically review the facility’s written response and may re-inspect to determine the facility’s compliance with the QSR and other applicable regulatory requirements. Failure to take adequate and timely corrective actions to remedy objectionable conditions listed on an FDA-483 could result in FDA taking administrative or enforcement actions. Among these may be FDA’s issuance of a Warning Letter to a manufacturer, which informs the manufacturer that FDA considers the observed violations to be of “regulatory significance” that, if not demonstrate that procedures utilizingcorrected, could result in further enforcement action. FDA enforcement actions, which include seizure, injunction and criminal prosecution, could result in total or partial suspension of a facility’s production and/or distribution, product recalls, fines, suspension of FDA’s review of product applications and FDA’s issuance of adverse publicity. Thus, an adverse inspection could force a shutdown of our products are an attractive option when compared against data from alternative procedures andmanufacturing operations or a recall of our products. Negative long-term data would affect the useAdverse inspections could also delay FDA approval of our products and could have an adverse effect on our production, sales and financial condition.and prospects.Fluctuations in foreign currency exchange rates could result in declines in our reported sales and results of operations.Because someincluding problems relating to the sterilization of our international sales are denominatedproducts or facilities and errors in local currencies and notmanufacturing components that could negatively affect the efficacy or safety of our products or cause delays in U.S. Dollars,shipment of our reported sales and earnings are subject to fluctuationsproducts. Interruption or delay in foreign currency exchange rates, primarily the Euro and British Pound. We translate results of transactions denominated in local currencies into U.S. Dollars using market conversion rates applicable to the period in which the transaction is reported. As a result, changes in exchange rates during a period can unpredictably and adversely affect our consolidated operating results and our asset and liability balances, even if the underlying valuemanufacture of the item inproduct or any of its original currency has not changed. At present, we do not hedgecomponents could impair our exposureability to foreign currency fluctuations. As a result, sales and expenses occurring inmeet the future that are denominated in foreign currencies may be translated into U.S. Dollars at less favorable rates, resulting in reduced revenues and earnings.Our manufacturing operations are primarily conducted at a single location, and any disruption at our manufacturing facility could increase our expenses and decrease our revenue.Our manufacturing operations are conducted at a single location in Ohio. While we take precautions at this location, we do not maintain a backup manufacturing facility, making us dependent on our current facility for the continued operationdemand of our business. A natural or other disaster could damage or destroy our manufacturing equipmentcustomers and cause substantial delays in our manufacturing operations, whichthem to cancel orders or switch to competitive products and could, lead to additional expense and decreased revenue due to lack of supply. The insurance we maintain may not be adequate to cover our losses in any particular case. With or without insurance, damage to our facility or our other property due to a natural disaster or casualty event couldtherefore, have a material adverse effect on our business, financial condition and results of operations.relyare currently defending against a lawsuit brought under the False Claims Act, and any adverse finding, judgement, or enforcement action could materially and adversely affect our business, financial condition or results of operations.independent distributorsDecember 11, 2017, the Company received a Civil Investigative Demand (CID) from the US Department of Justice (USDOJ) stating that it was investigating the Company to marketdetermine whether the Company has violated the False Claims Act, relating to the promotion of certain medical devices related to the treatment of atrial fibrillation for off-label use and sell our products insubmitted or caused to be submitted false claims to certain markets outsidefederal and state health care programs for medically unnecessary healthcare services related to the treatment of Afib. The Company provided the USDOJ with documents and answers to the written interrogatories, and cooperated with the investigation. In 2021, USDOJ informed the Company that the investigation resulted from a lawsuit by a private individual, or "relator", brought on behalf of the United States and various state and local governments under the qui tam provisions of the federal and similar state and local laws. Although the USDOJ and all of the state and local governments declined to intervene, the relator continues to pursue the lawsuit. During the third quarter of 2022, the relator filed a failureFourth Amended Complaint, which dropped allegations of off-label promotion and now alleges that the Company paid illegal kickbacks to healthcare providers in exchange for using or referring the Company’s products, in violation of the federal Anti-Kickback Statute and various comparable state and local laws. While the Company is contesting the case, it is not possible to predict when the lawsuitindependent distributorsproducts may result in a variety of serious complications, including damage to successfullythe heart, nerves, internal bleeding, death, paralysis or other adverse events. Serious complications are commonly encountered in connection with surgical procedures. If products we sell are defectively designed, manufactured or labeled, contain inadequate warnings, contain defective components, are misused or are associated with serious injuries or deaths, we may become subject to costly litigation by our customers or their patients. We carry product liability insurance that is limited in scope and amount and may not be adequate to fully protect us against product liability claims. We could be required to pay damages that exceed our insurance coverage, and such amounts could be significant. Any product liability claim, with or without merit, could also result in an increase in our insurance rates or our inability to secure coverage on reasonable terms, if at all. Even in the absence of a claim, our insurance rates may rise in the future. Any product liability claim, even a meritless or unsuccessful one, would be time-consuming and expensive to defend and could result in the diversion of our management’s attention from our business and result in adverse publicity, withdrawal of clinical trial participants, injury to our reputation and loss of revenue. Any of these events could negatively affect our financial condition.anyobtain and use information that we regard as proprietary. Whether a product infringes a patent involves complex legal and factual issues, the determination of which is often uncertain. Any patent dispute, even one without merit or an unsuccessful one, would be time-consuming and expensive to defend and could result in the diversion of our management’s attention from our business and result in adverse publicity, the disruption in their abilityof development and marketing efforts, injury to do soour reputation and loss of revenue. Litigation also puts our patent applications at risk of being rejected and our patents at risk of being invalidated or interpreted narrowly and may adversely impactprovoke third parties to assert claims against us. Any of these events could negatively affect our sales.We depend on third-party distributorsfinancial condition.sellbe infringing, or found to be inducing infringement by others, we could be prevented from selling our products unless we were able to obtain a license to use technology or ideas covered by such patent or are able to redesign our system to avoid infringement, or we may be ordered to pay substantial damages to the patent holders. A license may not be available at all or on terms acceptable to us, and we may not be able to redesign our products to avoid any infringement. Modification of our products or development of new products could require us to conduct additional clinical trials and to revise our filings with FDA and other regulatory bodies, which would be time-consuming and expensive. If we are not successful in certain markets outside of the United States, and if these distributors do not perform,obtaining a license or redesigning our products, we may be unable to maintain or increase our level of international revenue. Over the long term, we intend to continue to grow our business outside of the United States. To do so we will need to attract additional distributors or hire direct sales personnel to expand the territories in which we sell our products. Independent distributors may terminate their relationship with us or devote insufficient sales efforts to our products. We are not able to control our independent distributors, and they may not be successful in implementing our marketing plans. In addition, many of our independent distributors outside of the United States initially obtain and maintain foreign regulatory approval for sale of our products in their respective countries. Our failure to maintain our relationships with our independent distributors outside of the United States, or our failure to recruit and retain additional skilled independent distributors in these locations, could have an adverse effect on our operations. Turnover among our independent distributors, even if replaced, may adversely affect our short-term financial results while we transition to new independent distributors or direct sales personnel. Fluctuations in foreign currency exchange rates including any strengthening of the U.S. dollar may cause our independent sales distributors to seek longer payment terms to offset the higher prices they are paying in local currency for our products. The ability of these third-party distributors to market and sell our products and our business could also be adversely affected by unexpected events, including, but not limited to, power failures, nuclear events, natural or other disasters and war or terrorist activities. In addition, in light of the worldwide economic crisis, the ability of our distributors to borrow money from their existing lenders or to obtain credit from other sources to purchase our products may be impaired or our distributors could experience a significant change insuffer.21their liquidity or financial condition, all of which could impair their ability to distribute our products and eventually lead to distributor turnover.If coverage and adequate levels of reimbursement from governmental and third-party payors outside of the United States are not obtained and maintained, sales ofWe sell our products outside of the United States, may decrease and we may failare subject to achieve or maintain significant sales outside of the United States.Ourvarious regulatory and other risks relating to international operations, which could harm our revenue generated from salesand profitability.is also dependent upon the availability of coverage and reimbursement within prevailing foreign healthcare payment systems. Foreign healthcare payors generally do not provide the same level of reimbursement for sole-therapy minimally invasive procedures utilizing ablation devices and related products as payors in the United States. In addition, healthcare cost containment efforts similarexposes us to risks distinct from those we face in the United States are prevalent in many of the other countries in which we sell our products, and these efforts are expected to continue. To the extent that the use of ablation devices such asdomestic operations. For example, our Isolator Synergy system has historically received reimbursement under a foreign healthcare payment system, such reimbursement, if any, has typically been significantly less than the reimbursement provided in the United States. If coverage and adequate levels of reimbursement from governmental and third-party payorsoperations outside of the United States are subject to different regulatory requirements in each jurisdiction where we operate or have sales. Our failure, or the failure of our distributors, to comply with current or future foreign regulatory requirements, or the assertion by foreign authorities that we or our distributors have failed to comply, could result in adverse consequences, including enforcement actions, fines and penalties, recalls, cessation of sales, civil and criminal prosecution, and the consequences could be disproportionate to the relative contribution of our international operations to our results of operations. Moreover, if political or economic conditions deteriorate in these countries, or if any of these countries are affected by a natural disaster or other catastrophe, our ability to conduct our international operations or collect on international accounts receivable could be limited and our costs could be increased, which could negatively affect our operating results. Engaging in business outside of the United States inherently involves a number of other difficulties and risks, including, but not obtainedlimited to:maintained, sales ofcontrols relating to technology;States may decreaseStates;we may fail to achieve or maintain significantanti-American sentiment;States.If we failStates; andproperly manageeach of these risks may increase our anticipated growth, our business could suffer.We may experience periods of rapid growthcosts and expansion, which could place a significant strain on our personnel, information technology systems and other resources. In particular, the increase in our direct sales force requiresrequire significant management and other supporting resources. Any failure by us to manage our growth effectively could have an adverse effect on our ability to achieve our development and commercialization goals.To achieve our revenue goals, we must successfully increase production output as required by customer demand. In the future, we may experience difficulties in increasing production, including problems with production yields and quality control, component supply and shortages of qualified personnel. These problems could result in delays in product availability and increases in expenses. Any such delay or increased expense could adversely affect our ability to generate revenues.Future growth will also impose significant added responsibilities on management, including the need to identify, recruit, train and integrate additional employees. In addition, rapid and significant growth will place a strain on our administrative and operational infrastructure.In order to manage our operations and growth, we will need to continue to improve our operational and management controls, reporting and information technology systems and financial internal control procedures. If we are unable to manage our growth effectively, it may be difficult for us to execute our business strategy and our operating results and business could suffer.depend on our officers and other skilled and experienced personnel to operate our business effectively. If we are not able to retain our current employees or recruit additional qualified personnel, our business will suffer and our future revenue and profitability will be impaired.We are highly dependent on the skills and experience of our President and Chief Executive Officer, Michael H. Carrel, and certain other officers and key employees. We do not have any insurance in the event of the death or disability of our key personnel. Our officers and key employees, with the exception of our President and Chief Executive Officer and Senior Vice President, Operations, do not have employment agreements and they may terminate their employment and work elsewhere without notice and without cause or good reason. Currently we have non-compete agreements with our officers and other employees. Due to the specialized knowledge of each of our officers with respect to our products and our operations and the limited pool of people with relevant experience in the medical device field, the loss of service ofcannot assure you that one or more of these individuals could significantly affectfactors will not harm our ability to operate and manage our business. The announcement of the loss of one or more of our key personnel could negatively affect our stock price.We depend on our scientific and technical personnel for successful product development and innovation, which are critical to the success of our business. In addition, to succeed in the implementation of our business strategy, our management team must rapidly execute our sales strategy, obtain expanded FDA clearances and approvals, achieve market acceptance for our products and further develop products, while managing anticipated growth by implementing effective planning, manufacturing and operating processes. Managing this growth will require us to attract and retain additional management and technical personnel. We rely primarily on direct sales employees to sell our products in the United States and failure to adequately train them in the use and benefits of our products will prevent us from achieving our market share and revenue growth goals. We have key relationships with physicians that involve procedure, product, market and clinical development. If any of these physicians end their relationship with us, our business could be negatively impacted. We cannot assure you that we will be able to attract and retain the personnel and physician relationships necessary to grow and expand our business and operations. If we fail to identify, attract, retain and motivate these highly skilled personnel and physicians, we may be unable to continue our development and sales activities.22Our business growth strategy involves the potential for significant acquisitions, which involve risks and difficulties in integrating potential acquisitions and may adversely affect our business, results of operations and financial condition.All acquisitions involve inherent uncertainties, which may include, among other things, our ability to:·successfully identify targets for acquisition;·negotiate reasonable terms;·properly perform due diligence and determine all the significant risks associated with a particular acquisition;·properly evaluate target company management capabilities; and·successfully transition and integrate the acquired company into our business and achieve the desired performance.We may acquire businesses with unknown liabilities, contingent liabilities or internal control deficiencies. We have plans and procedures in place to conduct reviews of potential acquisition candidates for compliance with applicable regulations and laws prior to acquisition. Despite these efforts, realization of any of these liabilities or deficiencies may increase our expenses, adversely affect our financial position through the initiation, pendency or outcome of litigation or otherwise, or cause us to fail to meet our public financial reporting obligations.We have consummated two significant acquisitions in the past five years and in the future may continue to invest a substantial amount of capital in acquisitions. We continue to evaluate potential acquisition opportunities to support, strengthen and grow our business. There can be no assurance that we will be able to locate suitable acquisition candidates, acquire possible acquisition candidates, acquire such candidates on commercially reasonable terms, or integrate acquired businesses successfully in the future. In addition, any governmental review or investigation of our proposed acquisitions, such as by the Federal Trade Commission, may impede, limit or prevent us from proceeding with an acquisition. Future acquisitions may require us to incur additional debt and contingent liabilities, which may adversely affect our business, results of operations and financial condition. The process of integrating acquired businesses into our existing operations may result in operating, contract and supply chain difficulties, such as the failure to retain customers or management personnel. Such difficulties may divert significant financial, operational and managerial resources from our existing operations and make it more difficult to achieve our operating and strategic objectives.Disruptions of critical information systems or material breaches in the security of our systems could harm our business, customer relations and financial condition.In the ordinary course of our business, we collect and store sensitive data, including intellectual property, our proprietary business information and that of our customers, suppliers and business partners, and personally identifiable information of our customers and employees in our data centers and on our networks. The secure processing, maintenance and transmission of this information is critical to our operations and business strategy. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Any such breach could compromise our networks and the information stored there could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, regulatory penalties, disrupt our operations and the services we provide to customers, and damage our reputation and cause a loss of confidence in our products and services, which could adversely affect our business, operating margins, revenues and competitive position.We also rely in part on information technology to store information, interface with customers, maintain financial accuracy, secure our data and accurately produce our financial statements. If our information technology systems do not effectively and securely collect, store, process and report relevant data for the operation of our business, whether due to equipment malfunction or constraints, software deficiencies, human error or cyber incident, our ability to effectively plan, forecast and execute our business plan and comply with applicable laws and regulations would be materially impaired. Any such impairment could have a material adverse effect on our results of operations, financial condition and the timeliness with which we report our operating results.We are subject to credit risk from our accounts receivable related to our product sales, which include sales to countries outside the United States that may experience economic turmoil.The majority of our accounts receivable arise from product sales in the United States. However, we also have significant receivable balances from customers within the European Union, Asia and other regions. Our accounts receivable in the United States are primarily due from public and private hospitals. Our accounts receivable outside the United States are primarily due from public and private hospitals and from independent distributors. Our historical write-offs of accounts receivable have not been significant. We monitor the financial performance and credit worthiness of our customers so that we can properly assess and respond to changes in their credit profile. Our independent distributors and sub-dealers operate in certain countries where economic conditions continue to present challenges to their businesses, and, thus, could place in risk the amounts due to us from them. These distributors are owed amounts from public hospitals that are funded by their governments. Adverse financial conditions in these countries may negatively affect the length of time that it will take us to collect associated accounts receivable or impact the likelihood of ultimate collection.23Compliance with environmental laws and regulations may be expensive. Failure to comply with environmental laws and regulations could subject us to significant liability.Our manufacturing operations and research and development activities involve the use of biological materials and hazardous substances and are subject to a variety of federal, state and local environmental laws and regulations relating to the storage, use, discharge, disposal, remediation of and human exposure to hazardous substances. Our research and development and manufacturing operations may produce biological waste materials, such as animal tissues and certain chemical waste. These operations are permitted by regulatory authorities, and the resultant waste materials are disposed of in compliance with environmental laws and regulations. Compliance with these laws and regulations may be expensive, and non-compliance could result in substantial liabilities. In addition, we cannot completely eliminate the risk of accidental contamination or injury to third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed any applicable insurance coverage we may have. Our manufacturing operations may result in the release, discharge, emission or disposal of hazardous substances that could cause us to incur substantial liabilities, including costs for investigation and remediation. We believe we are in compliance with such laws and regulations. The results of the United Kingdom’s referendum on withdrawal from the European Union may have a negative effect on global economic conditions, financial markets and our business.In June 2016, a majority of voters in the United Kingdom elected to withdraw from the European Union, or the EU, in a national referendum. The referendum was advisory, and the terms of any withdrawal are subject to a negotiation period that could last at least two years after the government of the United Kingdom formally initiates a withdrawal process. Nevertheless, the referendum has created significant uncertainty about the future relationship between the United Kingdom and the EU, including with respect to the laws and regulations that will apply as the United Kingdom determines which EU laws to replace or replicate in the event of a withdrawal. The referendum has also given rise to calls for the governments of other EU member states to consider withdrawal. These developments, or the perception that any of them could occur, have had and may continue to have a material adverse effect on global economic conditions and the stability of global financial markets, and may significantly reduce global market liquidity and restrict the ability of key market participants to operate in certain financial markets. Any of these factors could depress economic activity and restrict our access to capital, which could have a material adverse effect on our business, financial condition and results of operations and reduce the price of our common stock.Our effective income tax rate may fluctuate, which may adversely affect our operations, earnings and earnings per share.Our effective income tax rate is influenced by our projected profitability in the various taxing jurisdictions in which we operate. The global nature of our business increases our tax risks. In addition, revenue authorities in many of the jurisdictions in which we operate are known to have become more active in their tax collection activities. Changes in the distribution of profits and losses among taxing jurisdictions may have a significant impact on our effective income tax rate, which in turn could have an adverse effect on our net income and earnings per share. The application of tax laws in various taxing jurisdictions, including the United States, is subject to interpretation, and tax authorities in various jurisdictions may have diverging and sometimes conflicting interpretations of the application of tax laws. Changes in tax laws or tax rulings, in the United States or other tax jurisdictions in which we operate, could materially impact our effective tax rate.Factors that may affect our effective income tax rate include, but are not limited to:·the requirement to exclude from our quarterly worldwide effective income tax calculations losses in jurisdictions where no income tax benefit can be recognized;·actual and projected full year pre-tax income, including differences between actual and anticipated income before taxes in various jurisdictions;·changes in tax laws, or in the interpretation or application of tax laws, in various taxing jurisdictions;·changes in the relative mix and staffing levels in various tax jurisdictions;·audits or other challenges by taxing authorities; and·the establishment of valuation allowances against a portion or all of certain deferred income tax assets if we determined that it is more likely than not that future income tax benefits will not be realized.These changes may cause fluctuations in our effective income tax rate that could adversely affect our results of operations and cause fluctuations in our earnings and earnings per share.Governmental authorities may question our intercompany transfer pricing policies or change their laws in a manner that could increase our effective tax rate or otherwise harm our business.As a U.S. company doing business in international markets through subsidiaries, we are subject to foreign tax and intercompany pricing laws, including those relating to the flow of funds between the parent and subsidiaries. Tax authorities in the United States and24in foreign markets closely monitor our corporate structure and how we account for intercompany fund transfers. If tax authorities challenge our corporate structure, transfer pricing mechanisms or intercompany transfers, our operations may be negatively impacted and our effective tax rate may increase. Tax rates vary from country to country and if regulators determine that our profits in one jurisdiction should be increased, we might not be able to fully utilize all foreign tax credits that are generated, which would increase our effective tax rate. Additionally, the Organization for Economic Cooperation and Development, or OECD, has issued certain proposed guidelines regarding base erosion and profit sharing. Once these guidelines are formally adopted by the OECD, it is possible that separate taxing jurisdictions may also adopt some form of these guidelines. In such case, we may need to change our approach to intercompany transfer pricing in order to maintain compliance under the new rules. Our effective tax rate may increase or decrease depending on the current location of global operations at the time of the change. Finally, we might not always be in compliance with all applicable customs, exchange control, Value Added Tax and transfer pricing laws despite our efforts to be aware of and to comply with such laws. In such case, we may need to adjust our operating procedures and our business could be adversely affected.We are required toFCPA,Foreign Corrupt Practices Act (FCPA), the UK BriberyAnti-Bribery Act of 2010 and other U.S. and foreign anti-corruption laws, which prohibit companies from engaging in bribery including corruptly or improperly offering, promising, or providing money or anything else of value to foreign officials and certain other recipients. In addition, the FCPA imposes certain books, records and accounting control obligations on public companies and other issuers. We operate in parts of the world in which corruption can be common and compliance with anti-bribery laws may conflict with local customs and practices. Our global operations face the risk of unauthorized payments or offers being made by employees, consultants, sales agents and other business partners outside of our control or without our authorization.Our insurancenot cover alllimit our ability to maintain sales of our indemnification obligationsproducts in European markets or to introduce new products into European markets.liabilities associatedthings, stricter controls of medical devices, including strengthening of the conformity assessment procedures, increased expectations with our operations.We maintain insurance designedrespect to provide coverageclinical data for ordinary risks associateddevices and pre-market regulatory review of high-risk devices. The MDR also envisages greater control over notified bodies and their standards, increased transparency, more robust device vigilance requirements and clarification of the rules for clinical investigations. Under transitional provisions, medical devices with our operations and our ordinary indemnification obligations, which we believenotified body certificates issued under the MDD prior to May 26, 2021, may continue to be customary for our industry. The coverage providedplaced on the market until 2027 or 2028, depending on device classification, as long as those devices meet the requirements of 2017/745 as amended by such insuranceEU 2023/607. After the expiry of any applicable transitional period, only devices that have been CE marked under the MDR may be placed on the market in the EU. If we fail to comply with the new MDR, we may not be adequateable to continue to sell existing products in the EU or introduce new products for all claimssale in the EU, either of which could materially harm our results of operations and financial condition.make or may be contested by our insurance carriers. If our insurance is not adequate or availablenever become profitable.pay liabilities associated with our operations, or if we are unablesales, training and promotional efforts, research and development, clinical trials, seeking regulatory clearances and approvals and general operating expenses. We expect to purchase adequate insurance at reasonable ratescontinue to incur substantial expenditures and to potentially incur additional operating losses in the future as we further develop and commercialize our products. If sales of our products do not continue to grow as we anticipate, we may not be able to achieve profitability. Our expansion efforts may prove to be more expensive than we currently anticipate, and we may not succeed in increasing our revenue sufficiently to offset these higher expenses. Our losses have had, and are expected to continue to have, an adverse impact on our working capital, total assets and accumulated deficit.financial condition,could be adversely affected.or cash flows may be materially adversely impacted.Risks Relating To Our Common StockThe price and trading volume ofis challenging in the current economic environment. As we grow and expand our common stock may experience extreme fluctuationsproduct offerings, managing our inventory levels becomes more difficult, particularly as we expand into new product areas and bring product enhancements to market. While we rely on our stockholders could lose some or all of their investment.Because we operate within the medical device segment of the healthcare industry,personnel and information technology systems for inventory management, our stock price is likely to be volatile. The market price of our common stock may havepersonnel and has had a history of substantial fluctuation due to a variety of factors, including, but not limited to: ·variations in our quarterly financial and operating results;·physician and patient acceptance of the surgical treatment of Afib or exclusion of the LAA using our products;·adverse regulatory developments with respect to our products, such as recalls, new regulatory requirements, changes in regulatory requirements or guidance and timing of regulatory clearances and approvals for new products;·coverage and reimbursement determinations for our products and the related procedures;·the timing of orders received;·delays or interruptions in manufacturing or shipping of our products;·pricing of our products;25
information technology systems may fail to adequately perform these functions or may experience an interruption. An excessive amount of inventory reduces our cash available for operations and may result in excess or obsolete materials. Conversely, inadequate inventory levels may make it difficult for us to meet customer product demand, resulting in decreased revenue. An inability to forecast future revenue or estimated life cycles of products may result in inventory-related charges that would negatively affect our gross margins and results of operations and increase our accumulated deficit.·media reports, publications or announcements about products or new innovations that could compete with our products or about the medical device product segment in general;
We are subject to credit risk from our accounts receivable related to our sales, which include sales into countries outside the United States that may experience economic turmoil.·investigations, claims or allegations by regulatory agencies, such as the Department of Justice and Financial Industry Regulatory Authority; ·market conditions or trends related to the medical device and healthcare industries or the market in general;·additions to or departures of our key personnel;·disputes, litigation or other developments relating to proprietary rights, including patents, and our ability to obtain patent protection for our technologies;·changes in financial estimates, investors’ perceptions or recommendations by securities analysts;·failure to achieve or maintain an effective healthcare compliance environment;·changes in accounting principles; and·failure to achieve and maintain an effective internal control environment.These factors, some of which are not within our control, may cause the priceThe majority of our stock to fluctuate substantially. If our quarterly or annual operating results fail to meet or exceedaccounts receivable arise from sales in the expectationsUnited States. However, we also have significant receivable balances from customers within the European Union and Asia. Our accounts receivable in the United States are primarily due from public and private hospitals. Our accounts receivable outside the United States are primarily due from public and private hospitals and from independent distributors. Our historical write-offs of securities analysts or investors, our stock price could drop suddenlyaccounts receivable have not been significant. We monitor the financial performance and significantly. We believe the quarterly and annual comparisonscredit worthiness of our customers so that we can properly assess and respond to changes in their credit profile. Our independent distributors operate in certain countries where economic conditions continue to present challenges to their businesses, and, thus, could place the amounts due to us at risk. These distributors are owed amounts from public hospitals that are funded by their governments. Adverse financial results are not necessarily meaningful and should notconditions in these countries may negatively affect the length of time that it will take us to collect associated accounts receivable or impact the likelihood of ultimate collection.relied upon as an indicationunable to comply with the covenants of our future performance.market pricesCredit Agreement entered into on January 5, 2024, contains specific financial covenants and a minimum liquidity requirement, along with other terms restricting indebtedness, liens, investments and acquisitions, asset dispositions, certain payments and other customary representations and warranties. The Credit Agreement contains mandatory prepayment provisions which require prepayment of amounts outstanding (i) upon the securitiesreceipt of medical device companies, particularly companies like ours without consistent product revenueproceeds from the issuance of any non-permitted indebtedness and earnings, have been highly volatile and are likely to remain highly volatile in the future. This volatility has often been unrelated to the operating performance(ii) when there is an Availability shortfall, as defined. The occurrence of these particular companies. In the past, companies that experience volatility in the market pricean event of their securities have often faced securities class action litigation. Whether or not meritorious, litigation brought against usdefault could result in substantial costs, divertan obligation to repay all obligations in full and a right by our management’s attention and resources and harm our abilitylenders to grow our business.We may be obligatedexercise all remedies available to issue additional shares of our common stock to the former stockholders of nContact as a result of our satisfaction of certain milestones set forth in the merger agreement with nContact and the other parties thereto, resulting in stock ownership dilution.Under the terms of the merger agreement with nContact and the other parties thereto, we agreed to issue additional shares of our common stock, or make payments in cash, to the former stockholders of nContact as contingent consideration upon our satisfaction of milestones described in the merger agreement. Issuing additional shares of our common stock to the former stockholders of nContact in satisfaction of contingent consideration dilutes the ownership interests of holders of our common stock on the dates of such issuances.them. If we are unable to realizepay those amounts, our lenders could proceed against the strategic, operationalcollateral granted to it pursuant to the Credit Agreement, and financial benefits anticipated fromwe may in turn lose access to both our acquisitioncollateral and our current source of nContact,borrowing availability.stockholders may experience dilution of their ownership interests inpublicly announced guidance or other expectations about our company upon any suchbusiness and future issuances of shares of our common stock without receiving any commensurate benefit.The sale of material amounts of common stockoperating results, which could encourage short sales by third parties and depress the price of our common stock. As a result, our stockholders may lose all or part of their investment.The downward pressure on our stock price caused by the sale of a significant number of shares of our common stock or the perception that such sales could occur by any of our significant stockholders could cause our stock price to decline, thus allowing short sellers of our stock an opportunity to take advantage of any decrease in the value of our stock. The presence of short sellers in our common stock may further depress the price of our common stock.Sales of common stock by us in a capital raising transaction may dilute stockholder ownership of common stock and cause a decline in the market priceour stock price.common stock.Weproducts, projected hiring to support our growth, continued increase of our market share, potential impact from competitive devices and therapies, and stability of the macro-economic environment in our key markets. Furthermore, analysts and investors may needdevelop and publish their own projections of our business, which may form a consensus about our future performance. Our business results may vary significantly from such guidance or that consensus due to raise capital in the future to funda number of factors, many of which are outside of our control and could adversely affect our operations and operating results. Furthermore, if we make downward revisions of our previously announced guidance, or new initiatives or reduce or pay in fullif our indebtedness. If we raise funds by issuing equity securities, our stock price may decline and our existing stockholders may experience significant dilution. Furthermore, we may enter into capital raising transactions at prices that represent a substantial discountpublicly announced guidance of future operating results fails to market price. A negative reaction by investors andmeet expectations of securities analysts, to any sale of our equity securities could result in a decline in the trading price of our common stock.Anti-takeover provisions in our amended and restated certificate of incorporation and amended and restated bylaws and under Delaware law could inhibit a change in controlinvestors, or a change in management that stockholders consider favorable. Provisions in our certificate of incorporation and bylaws could delay or prevent a change of control or change in management that would provide a premium to the market price of common stock. These provisions include those:·authorizing the issuance without further approval of “blank check” preferred stock that could be issued by our board of directors to increase the number of outstanding shares and thwart a takeover attempt;26·prohibiting cumulative voting in the election of directors, which would otherwise allow less than a majority of stockholders to elect director candidates;·limiting the ability of stockholders to call special meetings of stockholders;·prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of stockholders; and·establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon by stockholders at stockholder meetings.In addition, Section 203 of the Delaware General Corporation Law limits business combination transactions with 15% stockholders that have not been approved by our board of directors. These provisions and others could make it difficult for a third party to acquire us, or for members of our board of directors to be replaced, even if doing so would be beneficial to our stockholders. Because our board of directors is responsible for appointing the members of our management team, these provisions could, in turn, affect any attempt to replace the current management team. If a change of control or change in management is delayed or prevented, stockholders may lose an opportunity to realize a premium on shares of common stock orother interested parties, the market price of our common stock could decline.We do not expect to pay dividends in the foreseeable future. As a result, stockholders must rely on stock appreciation for any return on investment.We do not anticipate paying cash dividends on our common stock in the foreseeable future. Any payment of cash dividends will also depend on our financial condition, results of operations, capital requirements and other factors and will be at the discretion of our board of directors. Accordingly, stockholders will have to rely on capital appreciation, if any, to earn a return on investment in our common stock. Furthermore, pursuant to our credit facility, we are currently subject to restrictions on our ability to pay dividends and we may in the future become subject to other contractual restrictions on, or prohibitions against, the payment of dividends.business.markets. If sufficient securities analysts do not cover our common stock, the lack of research coverage may adversely affect the market price of our common stock. It may be difficult for companies such as ours, with a smaller market capitalizations,capitalization, to attract andrequirementsprice and trading volume of beingour common stock may experience extreme fluctuations and our stockholders could lose some or all of their investment.public companyvariety of factors, including, but not limited to those risk factors described in the “Risk Factors” section herein. These factors, some of which are not within our control, may straincause the price of our stock to fluctuate substantially. We believe the quarterly and annual comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.distract management.public company, we are subject toresult, our stockholders may lose all or part of their investment.reporting requirementssale of the Securities Exchange Acta significant number of 1934, as amended (Exchange Act), and the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley Act). We are also subject to certain provisions of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 (Dodd-Frank Act). The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal controls over financial reporting. In order to maintain and improve the effectivenessshares of our disclosure controls and procedures and internal control over financial reporting,common stock or the perception that such sales could occur by any of our significant resources and management oversight is required.stockholders could cause our stock price to decline, thus allowing short sellers of our stock an opportunity to take advantage of any decrease in the value of our stock. The Dodd-Frank Act requirespresence of short sellers in our common stock may further depress the SEC to adopt certain rules and regulations relating toprice of our public disclosures, corporate governancecommon stock. Some of our directors and executive compensation, among other things, and such rules and regulations require significant attention from management. Compliance with allofficers have entered into, or may enter into, Rule 10b5-1 trading plans pursuant to which they may sell shares of these laws, rules and regulations mayour stock from time to time divert management’s attention fromin the future. Actual or potential sales by these insiders, including those under a prearranged Rule 10b5-1 trading plan, could be interpreted by the market as an indication that the insider has lost confidence in our stock and adversely impact the market price of our stock.business concerns,factors and will be at the discretion of our board of directors. Accordingly, stockholders will have to rely on capital appreciation, if any, to earn a return on investment in our common stock. Furthermore, pursuant to our credit facility, we are currently subject to restrictions on our ability to pay dividends and we may in the future become subject to other contractual restrictions on, or prohibitions against, the payment of dividends.materialmaterially adverse effect on our business, financial condition, results, revenues and competitive position. See “Part I—Item 1A. Risk Factors” for further discussion of operationsthese risks.cash flows.SECBoard has adopted rules regardingdelegated certain information security and data privacy oversight to the disclosureAudit Committee and the Compliance, Quality and Risk Committee (CQRC) of the useBoard. The CQRC oversees compliance with information security and data privacy laws, while the Audit Committee has oversight responsibility for cybersecurity risks related to accounting, audit and financial matters. The CQRC, Audit Committee and management report to the Board on a periodic basis regarding our information security and data privacy functions, including any cybersecurity threats.conflict minerals (commonly referred toour cybersecurity policy, procedures and risk mitigation. Our information technology (IT) leadership briefs the CQRC on a periodic basis on information security matters, including the current cybersecurity landscape, progress on information security initiatives and accomplishments, and reports on material cybersecurity incidents, as tantalum, tin, tungstenneeded. Our enterprise risk management team reports address the Company’s cybersecurity risk management processes. Our Chief Legal Officer oversees the management of our ERM program and gold) which are mined from the Democratic Republichas over a decade of experience in risk management. The Chair of the Congo (DRC)CQRC is an expert in enterprise risk assessment and neighboring countries. Under the rules, we are required to disclose the procedures we employ to determine the sourcing of such mineralsmitigation and metals produced from those minerals. holds a CERT Certificate in Cybersecurity Oversight.requirements require due diligence effortsAudit Committee is responsible for reviewing our disclosures on cybersecurity risk management, strategy and could affect the sourcing of components usedgovernance in our products. IfAnnual Report on Form 10-K. The Audit Committee assists in determining materiality for timely reporting of cybersecurity incidents and is notified immediately if the conflict minerals includedincident response team has assessed that a material event may have occurred that may require filing an SEC Current Report on Form 8-K.our products are found to be sourced from the DRC or surrounding countries, we may take actions to change materials or product designs to reduce the possibility that our purchasevarious roles in information technology and information security for over 20 years. Our information security team has an aggregate of conflict minerals may fund armed groupsmore than 60 years of experience in information technology roles across several industries.region. These actions could add engineering and other costs to the manufacture of our products. We expect to continue to incur costs in the investigation of the origin of the conflict minerals used in our products and in the reporting of the findings of our investigation.following principal locations:27Our reputation may suffer if we have included conflict minerals in our products that are found to have funded armed groups in the DRC region.ITEM 1B. UNRESOLVED STAFF COMMENTS None. The Company maintains its headquarters•AtriCure Corporate Headquarters Campus; Mason, Ohio – This campus encompasses three locations in Mason, Ohio, in a leasedincluding our global headquarters facility totaling approximately 92,000 square feet. The facilitythat contains the Company’sCompany's administrative, clinical, regulatory, engineering, product development, distributionquality and some manufacturing functions. The monthly rentheadquarters facility is approximately 106,000 square feet. The Mason Distribution Warehouse is primarily used for thiswarehousing and distribution activities and is approximately 40,000 square feet. The Mason Manufacturing Building is approximately 37,000 square feet and is used for manufacturing, quality and engineering activities.$117. The initial lease term expires in September 2030. The Company also maintains the following locations:
approximately 9,000 square feet.·Minneapolis, Minnesota – This location includes both administrative and product development space. The office is approximately 22,300 square feet with monthly rent of $22. The lease will expire in October 2022.
•Hertogenbosch, Netherlands – This location is used for service activities and is approximately 19,000 square feet.·San Ramon, California – This location is primarily used for research and development activities and is approximately 3,800 square feet with monthly rent of $8. The lease will expire in December 2019.·Amsterdam, Netherlands – This location is primarily for the administration of our European subsidiaries and is approximately 7,500 square feet. The monthly rent for this space is $17, and the lease will expire in January 2021.·Hong Kong – This location is for the administration of business throughout Asia. Monthly rent under this lease, which expires in June 2018, is approximately $5. ·Beijing, China – This location is for the administration of business in China. Monthly rent under this lease, which expires in July 2018, is approximately $3. The Company is not party to any material pending or threatened litigation. applicableapplicable.28The following table sets forth the high and low closing sales price of our common stock for 2017 and 2016:19.20 14.94 24.65 18.54 24.88 20.00 23.44 17.69 21.47 15.29 17.75 13.57 17.19 13.54 20.06 15.20 23, 2018,13, 2024, the closing price of our common stock on the NASDAQ Global Market was $18.00$31.71 per share, and the number of stockholders of record was 86.Dividend PolicyThe Company has not declared or paid any dividends on its capital stock since incorporation. Furthermore, pursuant to the revolving credit facility terms, the Company is subject to certain restrictions on its ability to pay dividends. The Company currently expects to retain future earnings, if any, for use in the operation and expansion of the business and does not anticipate paying any cash dividends in the foreseeable future.29Medical EquipmentHealth Care Index (“NASDAQ Health Care”) for the period beginning on January 1, 2013December 31, 2018, and ending on December 31, 2017.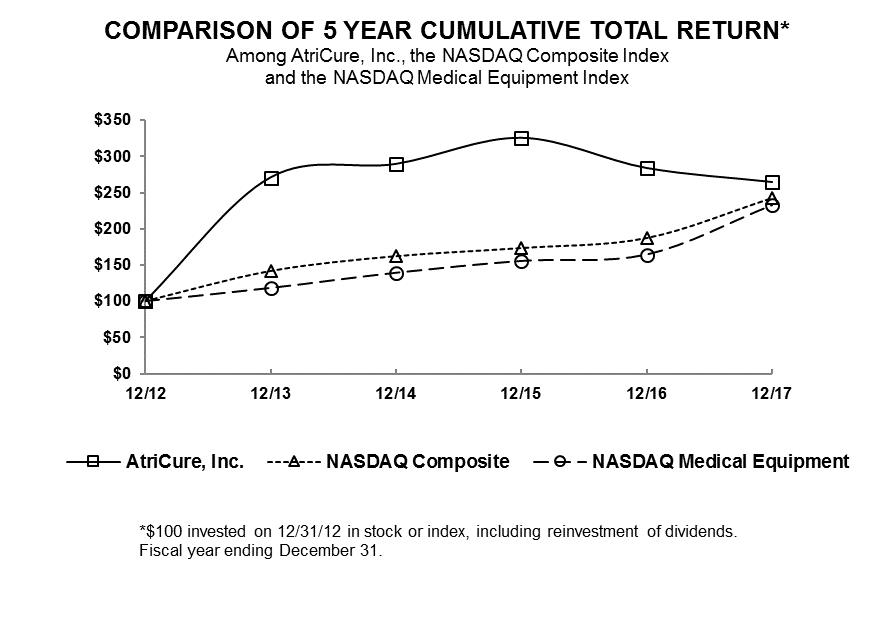

20122018, in our common stock, the NASDAQ Composite Index and the NASDAQ Medical EquipmentHealth Care Index, and that all dividends are reinvested. No dividends have been declared or paid on our common stock. Stock performance shown in the above chart for our common stock is historical and should not be considered indicative of future price performance.270.72 289.28 325.22 283.62 264.35 141.63 162.09 173.33 187.19 242.29 118.21 139.19 155.48 164.37 232.47 30 12/31/2018 12/31/2019 12/31/2020 12/31/2021 12/31/2022 12/31/2023 AtriCure, Inc. $ 100.00 $ 106.24 $ 181.93 $ 227.22 $ 145.03 $ 116.63 NASDAQ Composite $ 100.00 $ 136.69 $ 198.10 $ 242.03 $ 163.28 $ 236.17 NASDAQ Health Care $ 100.00 $ 110.75 $ 140.85 $ 126.71 $ 95.29 $ 96.06 ITEMSELECTED FINANCIAL DATAThe following table reflects selected financial data derived from our Consolidated Financial Statements for each of the last five years. The operating results data for the years ended December 31, 2017, 2016 and 2015 and the financial position data as of December 31, 2017 and 2016 are derived from our audited financial statements included in this Form 10-K. The operating results data for the years ended December 31, 2014 and 2013 and the financial position data as of December 31, 2015, 2014 and 2013 are derived from our audited financial statements not included in this Form 10-K. Historical results are not necessarily indicative of future results. The selected financial data set forth below should be read in conjunction with our financial statements, the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this Form 10-K.174,716 155,109 129,755 107,454 81,889 126,163 111,101 92,875 75,750 59,563 72.2% 71.6% 71.6% 70.5% 72.7% (26,892) (33,338) (27,212) (16,211) (11,462) (0.83) (1.05) (0.97) (0.61) (0.56) 32,387 31,609 28,058 26,374 20,431 34,451 47,009 42,284 68,543 34,125 50,355 56,889 43,164 67,865 25,774 267,704 276,421 273,092 158,404 111,947 36,861 37,205 13,710 74 4,412 161,166 168,442 186,685 132,538 72,604 _________________________
[RESERVED](1)We acquired nContact for $116.8 million on October 13, 2015. The acquisition is included in our Consolidated Balance Sheets beginning October 13, 2015, and the results of operations are included in our Consolidated Statements of Operations and Comprehensive Loss beginning with the period October 14, 2015 through December 31, 2015.(2)We acquired Estech for $39.7 million on December 31, 2013. The acquisition is included in our Consolidated Balance Sheets as of December 31, 2013.31ITEMITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSconsolidated financial statementsConsolidated Financial Statements and notes thereto contained in Item 8, “Financial Statements and Supplementary Data,” to provide an understanding of our results of operations, financial condition and cash flows. This section of this Form 10-K generally discusses 2023 and 2022 items and year-to-year comparisons between 2023 and 2022. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. The actual results may differ from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those set forth under Item 1A “Risk Factors,” the cautionary statement regarding forward-looking statements at the beginning of Part I and elsewhere in this Form 10-K.(LAA) management. We have several product lines for the ablation of cardiac tissue, including our Isolator Synergy Ablation System, the first and only surgical device approved by the United States Food and Drug Administration (FDA) for the treatment of persistent and longstanding persistent forms of Afib in patients undergoing certain open concomitant procedures. We also offer a variety of minimally invasive ablation devices and access tools to facilitate the growing trend in less invasive cardiac and thoracic surgery. Our cryoICE cryosurgery product line offers a variety of cryoablation devices for use in various types of cardiothoracic surgery. Our AtriClip Left Atrial Appendage Exclusion System is a device specifically designed to occlude the heart’s left atrial appendage.Physicians have adopted our radiofrequency (RF) ablation and cryoablation systems to treat Afib, and we believe that we are currently the market leader in the surgical treatment of Afib. Ourmanagement (LAAM) products are used by physicians during both open-heart and minimally invasive surgicalprocedures. In open-heart procedures, either on a concomitant or standalone basis. During a concomitant procedure, the physician ablates cardiac tissue and/or occludes the LAA, secondary, or concomitant, to a primary structuralis performing heart procedure such as a valve repair or replacement or coronary artery bypass graft (CABG). Our Isolator Synergy System is approved by FDAsurgery for the treatment of persistentother conditions, and long-standing persistent Afib concomitant to other open-heart surgical procedures. All of our other ablation devices are cleared for sale under FDA 510(k) clearances, including our other RF and cryoablation products, which are indicated for the ablation of cardiac tissue and/or treatment of cardiac arrhythmias. In addition, our cryoICE probe is cleared for managing pain by temporarily ablating peripheral nerves. Our AtriClip products are 510(k)-cleared with an indication for the occlusion of the heart’s LAA, performed under direct visualization andused in conjunction with other cardiac(or “concomitant” to) such a procedure. Minimally invasive procedures are performed on a standalone basis, and often include multi-disciplinary or “hybrid” approaches, combining surgical procedures. Direct visualization, in this context, requires that the surgeon is ableprocedures using AtriCure ablation and LAAM products with catheter ablation procedures performed by electrophysiologists. Our pain management devices are used by physicians to see the heart directly, withfreeze nerves during cardiothoracic or without assistance from a camera, endoscope or other appropriate viewing technologies. We also have a line of reusablethoracic surgical instruments typically used for cardiac valve replacement or repair.procedures. We anticipate that substantially all of our revenue for the foreseeable future will relate to products we currently sell or are in the process of developing, which are used to ablate cardiac tissue, to occlude the left atrial appendage, to perform mitral and aortic valve replacement and repair and/or to ablate peripheral nerves during cardiothoracic surgery.In the United States, wedeveloping.force. In certain international markets, such asforce in the United States, Germany, France, the United Kingdom, and the Benelux region, sales areAustralia and Canada. We also made directly to medical centers, while other international sales are madesell our products to distributors who in turn sell our products to end users.medical centers in other markets. Our business is primarily transacted in U.S. Dollars with the exception ofDollars; direct international sales transactions with our European subsidiaries, which are transacted in Euros, British Pounds, Australian Dollars or British Pounds.Canadian Dollars.3220172023 compared to December 31, 2016Year Ended December 31, Year Ended December 31, 2023 2023 2022 % of % of Amount Amount Revenue Amount Revenue 174,716 100.0 155,109 100.0 Revenue $ 399,245 100.0 100.0 % 330,379 100.0 % 48,553 27.8 44,008 28.4 126,163 72.2 111,101 71.6 Operating expense (benefit): Research and development expenses Research and development expenses 34,144 19.5 35,824 23.1 116,998 67.0 106,415 68.6 Total operating expenses Total operating expenses 151,142 86.5 142,239 91.7 (24,979) (14.3) (31,138) (20.1) (2,264) (1.3) (1,801) (1.2) 227 0.1 227 0.1 138 0.1 (586) (0.4) (1,899) (1.1) (2,160) (1.4) Other expense, net (26,878) (15.4) (33,298) (21.5) 14 40 (26,892) (15.4) (33,338) (21.5) Net loss $ (30,438) (7.6) (7.6) % $ (46,466) (14.1) (14.1) % Total The following table sets forth, for the periods indicated, our revenue by product type and geography expressed as dollar amounts and the corresponding change in such revenues between periods, in both dollars and percentages:Year Ended December 31, Change 2023 2022 Amount % Open ablation $ 105,287 $ 86,119 $ 19,168 22.3 % Minimally invasive ablation 44,577 38,553 6,024 15.6 % Pain management 49,199 39,974 9,225 23.1 % Appendage management 134,481 112,555 21,926 19.5 % Total United States $ 333,544 $ 277,201 $ 56,343 20.3 % Total International 65,701 53,178 12,523 23.5 % Total Revenue $ 399,245 $ 330,379 $ 68,866 20.8 % 12.6% (12.4%20.8% (20.6% on a constant currency basis). Revenue from customersIn the United States, we experienced growth in all key product lines as a result of deepening market penetration and expanding physician adoption. Key products contributing to the increase in revenue in the United States were:$16,002, or 13.1%, and revenue from international customers increased $3,605, or 11.0% (9.6%23.5% (22.1% on a constant currency basis). Sales in the United States grew, across several key product categories. Ablation-related open-heart sales increased $6,467, or 11%, primarily due to growth in our cryo products line, including the impact of the cryoFORM® product which launched in the second quarter of 2016. Ablation-related minimally invasive (MIS) sales increased $3,252, or 10%, reflecting strong growth in our EPi-Sense product line which was offset partially by a decline in legacy MIS product sales. Growth in EPi-Sense product resulted from both an increase in volume of procedures in existing accounts as well as the addition of new customer accounts. Legacy MIS product sales in the United States were impacted throughout 2017 by various disruptions to key accounts such as physician movementall franchises and wildfires in California. AtriClip sales increased $6,960, or 23%, due to increased volume and pricing. AtriClip sales reflect the positive impact of the AtriClip PRO2® and AtriClip PRO·V LAA Exclusion System devices, which launched in the second quarter of 2016 and late third quarter of 2017, respectively. International revenue grew primarily in Asia, Germany, France, Turkey, Austria and the Benelux region as a result of increased volumes in AtriClip and cryo product sales.and is calculated by applying previous period foreign currency (Euro) exchange rates, which are determined by the average daily exchange rate, to each of the comparable periods. Revenue is analyzed on a constant currency basis to better measure the comparability of results between periods. Because changes in foreign currency exchange rates have a non-operating impact on revenue, the Company believeswe believe that evaluating growth in revenue on a constant currency basis provides an additional and meaningful assessment of revenue to both management and the company’s investors.$4,545 and$14,436 primarily reflecting higher sales volumes. The gross margin increased 0.6% from 71.6% in 2016 to 72.2% in 2017. While 2017 includes heavier capital equipment sales, this factor isincrease of 80 basis points was driven by favorable production efficiencies, partially offset by a slight increase in the percentage of total revenue from customers in the United States,less favorable geographic and product mix and lower inventory obsolescence charges in 2017.decreased $1,680,increased $16,578, or 4.7%28.9%. The decrease in expense was primarily due to lower expenseExpansion of $1,887 related to product development projects resulting from the timing of project activities, $474 related to regulatory filing fees, $339 related to clinical trials and grants and $276 related to amortization expense. These decreases in expense were partially offset by higher expense of $1,115 related to product development, regulatory and clinical teams resulted in $7,413 of additional personnel costs, resulting fromincluding variable compensation and share-based compensation. Clinical trial expenses increased headcount$6,667 due to strong enrollment activity in the LeAAPS clinical trial throughout the year. Additionally, our expanding product pipeline and $227 related to share-based compensation expense.$10,583,$21,866, or 9.9%, primarily due to higher expense9.5%. Personnel costs increased $26,971 as a result of $9,136 related to personnelgrowth in headcount, variable compensation and relatedshare-based compensation. Trade shows and marketing activities increased $1,538 and other administrative and operating expenses suchincreased $2,274 as travel costs, resulting from increased headcount, $2,563 related to professional education, marketing and tradeshow expenses, $2,501 related to share-based compensation expense, $1,405 related to legal expenses and $530 related to product samples, largely relatedcompared to the September 2017 launch of the33AtriClip PRO·V LAA Exclusion System. These increases in expense wereprior year. This increase was offset by a $5,047 reduction$4,019 decrease in expense relatedtraining costs as a result of growing efficiencies and enhancements to the contingent consideration adjustment (see Note 3 – Fair Value to the Consolidated Financial Statements)our global training programs and lower expenses related to consulting and professional services.Net interest expense. Net interest expense was $2,037 for 2017 and $1,574 for 2016. Interest expense associated with outstanding amounts on our term loan and capital lease obligations, as well as the amortizationa net gain of financing costs, are included in net interest expense. Also included in net interest expense is interest income$4,412 from investments, including gains and losses on investments soldnon-recurring legal settlements during the period. The increase in interest expense was drivenfirst half of 2023. Legal settlement activity included a $7,500 gain from proceeds on a legal matter settled during the first quarter of 2023, partially offset by a full year$3,088 charge for settlement of expense incurred on borrowings underan intellectual property matter during the term loan in 2017, which was effective April 2016.gains and losses. Year Ended December 31, 2016 compared to December 31, 2015The following table sets forth, for the periods indicated, our results of operations expressed as dollar amounts and as percentages of total revenue:155,109 100.0 129,755 100.0 44,008 28.4 36,880 28.4 111,101 71.6 92,875 71.6 35,824 23.1 25,742 19.8 106,415 68.6 93,853 72.3 142,239 91.7 119,595 92.2 (31,138) (20.1) (26,720) (20.6) (1,801) (1.2) (292) (0.2) 227 0.1 190 0.1 (586) (0.4) (354) (0.3) (2,160) (1.4) (456) (0.4) (33,298) (21.5) (27,176) (21.0) 40 36 (33,338) (21.5) (27,212) (21.0) Revenue. Total revenue increased 19.5% (19.6% on a constant currency basis), from $129,755 in 2015 to $155,109 in 2016. Constant currency basis amounts are calculated by applying previous period foreign currency exchange rates to each of the comparable periods. Revenue from sales to customers in the United States increased $20,173, or 19.7%, and revenue from sales to international customers increased $5,181, or 18.8% (18.9% on a constant currency basis). The increase in sales to customers in the United States was primarily due to increased sales of ablation-related open-heart products of $4,509, increased sales of ablation-related minimally invasive (MIS) products of $9,605 and increased sales of the AtriClip system of $5,944. The increase in MIS sales was largely influenced by the nContact acquisition, which closed in the fourth quarter of 2015. Increases in both ablation-related open-heart product and AtriClip system revenues were positively impacted by new product launches in 2016, which include the cryoFORM cryoablation probe and AtriClip PRO2™ device. While U.S. MIS and AtriClip sales were consistently strong throughout 2016, U.S. open-heart sales were up 11% through the third quarter but slowed to 1% growth during the fourth quarter. The Company is working to improve focus on the open-chest procedures and take advantage of recent guideline changes related to Afib from a U.S. cardiothoracic surgeon society. The increase in international revenue was primarily due to an increase in sales in Japan, China, Italy, Germany and France. Unlike domestic sales, international revenue growth was primarily the result of increased volumes, rather than new product launches or the nContact acquisition.Cost of revenue and gross margin. Cost of revenue increased $7,128, from $36,880 in 2015 to $44,008 in 2016. As a percentage of revenue, cost of revenue was 28.4% for 2015 and 2016. Gross margin for 2015 and 2016 was 71.6%. Favorable impacts on gross margin in 2016 include the suspension of the medical device excise tax, as well as product mix, which was affected by the nContact acquisition and new product launches. These increases in gross margin were offset by increased loaner generator depreciation and increased costs related to moving into a larger and more modern facility. While certain scrap and obsolescence charges were incurred in both 2016 and 2015, we expect such amounts to be significantly reduced in future years.34Research and development expenses. Research and development expenses increased $10,082, or 39.2%, from $25,742 in 2015 to $35,824 in 2016. The increase in expense was primarily due to a $3,147 increase in product development, regulatory and clinical personnel expense, a $2,599 increase in product development project expense, a $1,334 increase in clinical trial spending, a $443 increase in regulatory filing expense, a $452 increase in share-based compensation and a $348 increase in amortization expense. The nContact acquisition directly impacted personnel, clinical trial, regulatory filing and amortization expenses.Selling, general and administrative expenses. Selling, general and administrative expenses increased $12,562, or 13.4%, from $93,853 in 2015 to $106,415 in 2016. The increase was primarily due to a $6,560 increase in personnel and related expenses, such as travel costs, a $2,244 increase in share-based compensation expense and a $2,196 increase in marketing, tradeshows, training and related expenses, partially offset by a $2,489 decrease in expense due to transaction costs recorded in connection with the acquisition of nContact during 2015.Net interest income (expense). Net interest expense was $1,574$3,133 for 20162023 and $102$2,992 for 2015. Interest expense associated with outstanding amounts on our term loan and capital lease obligations, as well as the amortization2022.2017, the Company2023, we had cash, cash equivalents and investments of $34,451$137,285 and outstanding debt of $24,100. We had unused borrowing capacity of $15,000approximately $28,750 under our revolvingthe SVB Credit Facility. As a result of the new asset-based credit facility. Mostagreement with JPMorgan Chase Bank, N.A. entered into on January 5, 2024, unused borrowing availability increased to approximately $61,885 (see Note 8 – Indebtedness for related discussion). All cash equivalents and investments and most of our operating cash and all cash equivalents and investments are held byin United States financial institutions. A minor portion of our cash is held in foreign banks to support our international operations. We had net working capital of $50,355$191,677 and an accumulated deficit of $225,866$357,057 as of December 31, 2017.Cash flows used in operating activities. Net cash used in operating activities was $8,944 during 2017. The primary net uses of cash for operating activities were as follows:
2023.·the net loss of $26,892, offset by $19,950 of non-cash expenses, including $14,615 in share-based compensation, $9,128 in depreciation and amortization and a change in fair value of contingent consideration of $4,078; and·a net decrease in cash used related to changes in operating assets and liabilities of $2,002, due primarily to the following:·an increase in accounts receivable of $1,464, due primarily to increased sales and the timing of collections; ·an increase in inventory of $4,477, due primarily to additional products in inventory, as well as increased inventory levels in support of anticipated revenue growth; and·a $3,518 increase in accounts payable and accrued liabilities due primarily to an increase in accrued variable compensation payments.Cash flows provided by investing activities. Net cash provided by investing activities was $3,761 during 2017. The primary source of cash from investing activities was $26,600 related to sales and maturities of available-for-sale securities to fund operations. This source of cash was offset by $6,384 related to the purchase of property and equipment, which included the placement of our RF and cryo generators with our customers, and $16,455 related to the purchase of available-for-sale securities.Cash flows provided by financing activities. Net cash provided by financing activities during 2017 was $2,760, which was primarily due to proceeds from stock option exercises of $4,402 and proceeds from the issuance of common stock under our employee stock purchase plan of $2,110, partially offset by shares repurchased for payment of taxes on stock awards of $2,013 and debt and capital lease payments of $1,689.Credit facility. The Company’s Loan and Security Agreement with Silicon Valley Bank (SVB), as amended, restated, and modified effective April 25, 2016 (Loan Agreement) provides for a $25,000 term loan and a revolving credit facility under which we may borrow a maximum of $15,000. The term loan and revolving credit facility both mature in April 2021. According to the Loan Agreement, principal payments on the term loan are to be made ratably commencing eighteen months after the inception of the loan (November 2017) through the loan’s maturity date. The term loan accrues interest at the Prime Rate and is subject to an additional 4.0% fee on the original $25,000 term loan principal amount at maturity or prepayment of the term loan. Borrowing availability under the revolving credit facility is based on the lesser of $15,000 or a borrowing base calculation as defined by the Loan Agreement. As of December 31, 2017, we had no borrowings under the revolving credit facility, and we had borrowing availability of $15,000. The revolving line of credit is subject to an annual commitment fee of $50, and any borrowings bear interest at the Prime Rate. The Loan Agreement also provides for certain prepayment and early termination fees and includes other customary terms and conditions.35The Loan Agreement establishes covenants related to maintaining a minimum liquidity ratio, achieving a minimum sales growth measured over a trailing twelve-month period and maintaining a minimum cash balance. Additional covenants include, among others, covenants that limit our ability to dispose of assets, enter into mergers or acquisitions, incur indebtedness, incur liens, pay dividends or make distributions on our capital stock, make investments or loans and enter into certain affiliate transactions, in each case subject to customary exceptions for a credit facility of this size and type. Certain covenants apply when we have outstanding borrowings under the revolving credit facility or when we hold less than $20,000 in cash and investments with SVB. Further, a minimum fixed charge ratio applies when specific covenant milestones are achieved. The occurrence of an event of default could result in an increase to the applicable interest rate by 3.0%, an acceleration of all obligations under the Loan Agreement, an obligation to repay all obligations in full and a right by SVB to exercise all remedies available to it under the Loan Agreement and related agreements including the Guaranty and Security Agreement. Specified assets have been pledged as collateral. We are in compliance with the covenants of the Loan Agreement as of December 31, 2017.Effective February 23, 2018, the Company and SVB entered into a Loan and Security Agreement which amends and restates the Company’s credit facility with SVB. The agreement provides for a $40,000 term loan and $20,000 revolving line of credit, with an option to increase the revolving line of credit by an additional $20,000. The Loan and Security Agreement credit facility has a five-year term, expiring February 2023. Principal payments of the term loan are to be made ratably commencing eighteen months after the inception of the loan through the loan’s maturity date. If the Company meets certain conditions, as specified by the agreement, the commencement of term loan principal payments may be deferred by an additional six months. The term loan accrues interest at the greater of the Prime Rate plus 3.75% or 8.25% and is subject to an additional 3.50% fee on the original $40,000 term loan principal amount at maturity. The revolving line of credit is subject to an annual facility fee of 0.33% of the revolving line of credit, and any borrowings bear interest at the greater of the Prime Rate and 4.50%. The Loan and Security Agreement also provides for certain prepayment and early termination fees, as well as establishes covenants related to sales growth, along with other customary terms and conditions similar to those in the Company’s current agreement with SVB. The proceeds from the agreement are expected to fund current and future operations of the Company.In connection with the terms of our corporate headquarters lease agreement, a letter of credit in the amount of $1,250 was issued to the landlord in October 2015. The letter of credit is renewed annually and remains outstanding as of December 31, 2017.the rate ofwithout limitation: market acceptance of our current and future products, the resources we devoteproducts; investments in working capital; costs to developingdevelop and supportingsupport our products, future expensesincluding professional training; costs to expand and support our sales and marketing efforts,efforts; operating and filing costs relating to changes in regulatory policies or laws that affect our operations andlaws; costs of filings, costs associated withfor clinical trials and securingto secure regulatory approval for new products,products; costs associated with acquiringto prosecute, defend and integrating businesses, costs associated with prosecuting, defending and enforcingenforce our intellectual property rightsrights; maintenance and enhancements to our information systems and security; and possible acquisitions and joint ventures. Global economic turmoilventures, including potential business integration costs. We continue to evaluate additional measures to maintain financial flexibility, and we will continue to closely monitor macroeconomic conditions including, but not limited to, inflationary pressures, rising interest rates, and fluctuations in currency exchange rates that may adversely impact our revenue,liquidity and access to capital resources.capital markets or future demandPrime Rate plus 1.25% and is subject to an additional 3.00% fee on the term loan principal amount at maturity. We had unused borrowing capacity of approximately $28,750 under our revolving credit facility.our products.We have on filea $125,000 asset-based revolving credit facility (ABL Facility), with an option to increase the SECrevolving commitment by an additional $40,000. A portion of the ABL facility, limited to $5,000, is available for the issuance of letters of credit. The Credit Agreement has a shelf registration statementthree-year term and expires January 5, 2027. Amounts available to be drawn from time to time under the ABL Facility are determined by calculating the applicable borrowing base, which allows us to sell any combinationis based upon applicable percentages of senior or subordinated debt securities, common stock, preferred stock, warrants, depositary shares and units in one or more offerings should we choose to do sothe values of eligible accounts receivable, eligible inventory, eligible liquid assets, less reserves as determined by the Administrative Agent, all as specified in the future.Credit Agreement. The borrowings bear interest at a rate per annum equal to, at the Company's election: (i) an alternate base rate (ABR) plus an applicable margin or (ii) an adjusted term secured overnight financing rate (SOFR) plus an applicable margin. At the time of closing, the Company borrowed $61,865 and had unused borrowing availability of approximately $61,885. The proceeds of the ABL Facility were used to terminate the Company’s indebtedness under the SVB Loan Agreement. As a result of the new Credit Agreement, the $60,000 borrowings outstanding under the SVB Loan Agreement as of December 31, 2023 are classified as noncurrent in the Consolidated Balance Sheet.maintaindisburse between $14,000 and $17,000 of fixed andeffectivenessnext twelve months.this shelf registration statementprincipal and interest payments related to our Mason, Ohio headquarters building. As of December 31, 2023, we have current finance lease obligations of $1,086 and long-term obligations of $8,061. Our operating leases for office and warehouse space includes current obligations of $1,447 and long-term obligations of $3,307. For additional information, see Note 9 – Leases.foreseeable future.term loan and revolving line of credit,Credit Agreement, will be sufficient to meet our anticipated cash needs for working capital and capital expenditures for at least the next twelve months. The nContact transaction provides for contingent considerationHowever, we have on file with the SEC a shelf registration statement which allows us to be paid upon attaining specified regulatory approvalssell any combination of debt securities, common stock, preferred stock, warrants, depository shares and clinical and revenue milestones overunits in one or more offerings should we choose to do so in the next three years. Subjectfuture. We expect to maintain the terms and conditionseffectiveness of the nContact merger agreement, such contingent consideration will be paid in AtriCure common stock and cash, with a requirement to make payments in AtriCure common stock first, up to a specified maximum number of shares. Overshelf registration statement for the next twelve months, we do not expect our cash requirements to include significant payments of contingent consideration based on terms of the acquisition agreement and related milestones. However, we do expect to issue shares in the amount of $7,500 as payment of contingent consideration related to the completion of the trial enrollment milestone in 2018.couldwould have rights senior to those associated with our common stock and could contain covenants that would restrict our operations. Finally, our term loan agreement and revolving line of credit requireCredit Agreement requires compliance with certain financial and other covenants. If we are unable to maintain these financing arrangements, we may be required to reduce the scope of our planned research and development, clinical activities and selling, training, education and marketing efforts.36Contractual Obligations and Commitments sets forthsummarizes our approximate aggregate obligations at December 31, 2017consolidated cash flow activities:Years Ended December 31, 2023 2022 Change Net cash provided by (used in) operating activities $ 4,484 $ (22,141) $ 26,625 Net cash provided by investing activities 21,817 44,006 (22,189) Net cash used in financing activities (32) (7,059) 7,027 futureworking capital remained relatively flat year over year, with increased investment in inventories largely offset by increased accruals for annual variable compensation payments under contractsdue to improved operating performance.other contingent commitments:24,100 9,070 13,606 1,424 20,451 1,468 2,990 3,038 12,955 3,348 965 1,664 719 2,379 2,379 804 804 51,082 5,616 13,724 17,363 14,379 _________________________
equipment following our 2022 manufacturing facilities expansion and $2,928 increase in net maturities of available-for-sale securities.(1)Long-term debt represents principal repayment related to our term loan. Obligations under the term loan reflect the impact of the refinancing transaction consummated in February 2018, which delays the payment of principal amounts under the term loan for 18 months, after which principal payments are made ratably until maturity in February 2023. Interest on the term loan accrues at the Prime Rate and is payable monthly over the term of the loan. In addition, we have a contractual obligation to pay interest on amounts drawn on the revolving credit facility.(2)Capital leases consist of principal and interest payments related to a building and computer equipment. The rent payments related to our corporate headquarters building in Mason, Ohio are also included in these amounts. See Note 9 – Indebtedness to our Consolidated Financial Statements.(3)Represents lease commitments under various operating leases.(4)Represents obligations for royalty agreements ranging from 3% to 5% of specified product sales estimated using 2017 sales. See Note 10 – Commitments and Contingencies to our Consolidated Financial Statements.We have contractual obligations for contingent consideration payments related to the nContact acquisition. Subject to the terms and conditions of the nContact merger agreement, such contingent consideration will be paid in AtriCure common stock and cash, with a requirement to make payments in AtriCure common stock first, up to a specified maximum number of shares.Off-Balance-Sheet ArrangementsAs of December 31, 2017, we had operating lease agreements that were not recorded on the Consolidated Balance Sheets. Operating leases areCash flows used in financing activities. Net cash from financing activities increased by $7,027 in 2023 compared to 2022, reflecting savings of $5,644 due to fewer shares repurchased at a lower value for payment of taxes on stock awards and an increase of $1,536 of proceeds from the normal course of business.InflationInflation has not had a significant impact on our historical operationsemployee stock purchase plan and we do not expect it tostock option exercise activity.a significantan adverse impact on our results of operations or financial condition in the foreseeable future.consolidated financial statements,Consolidated Financial Statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of consolidated financial statements requires management to make estimates and judgments that affect the reported amounts of assets and liabilities, revenue and expenses, and disclosures of contingent assets and liabilities at the date of the financial statements. On a periodic basis, we evaluate our estimates, including those related to sales returns and allowances, accounts receivable, inventories and share-based compensation. We useusing authoritative pronouncements, historical experience and other assumptions as the basis for making estimates. Actual results could differ from those estimates under different assumptions or conditions. We have described our significant accounting policies in Note 1 – Description of Business and Summary of Significant Accounting Policies to our consolidated financial statementsConsolidated Financial Statements included in this Form 10-K.affectinvolve a significant level of estimation uncertainty and judgments that are reasonably likely to have a material impact on our more significantConsolidated Financial Statements. We base our judgments and estimates used in the preparation of our consolidated financial statements. primarily from the sale of our disposable surgicalmedical devices. PursuantWe recognize revenue in an amount that reflects the consideration we expect to our standard termsbe entitled to in exchange for those devices when control of sale, revenuepromised devices is recognized when titletransferred to the goods and risk of loss transfers to customers and there are no remaining obligations that will affect customers’ final acceptance of the sale. Generally, our standard terms of sale define the transfer of title and risk of loss to occur upon shipment to the respective customer. We generally do not maintain any post-shipping obligations to the recipients of the products. No installation, calibration or testing of this equipment is performed by AtriCure subsequent to shipment to the customer in order to render it operational. Shipping and handling revenues and cost of freight for shipments made to customers is included in revenue and cost of revenue. Sales and other value-added taxes collected from customers and remitted to governmental37authorities are excluded from revenue. We sell our products through a direct sales force, distributors outside of the U.S. and through a wholly-owned subsidiary, AtriCure Europe, B.V. Terms of sale are generally consistent for both end-users and distributors except that payment terms are generally net 30 days for end-users and net 60 days for distributors, with limited exceptions.605,606, “Revenue Recognition” (ASC 605)from Contracts with Customers”. We determineSignificant judgments and estimates involved in the timingCompany’s recognition of revenue recognition based upon factors such as passageinclude the estimation of title, payment terms and ability to return products.a provision for returns. We recognize revenue when all ofestimate the following criteria are met: (i) there is persuasive evidence that an arrangement exists; (ii) delivery of the products and/or services has occurred; (iii) the selling price is fixed or determinable; and (iv) collectability is reasonably assured.We maintain a provision for sales returns and allowances to account for potentialusing the expected value method based on historical experience and other factors that we believe could impact our expected returns, ofincluding defective or damaged products products shipped in error and invoice adjustments,adjustments. In the normal course of business, we are not obligated to accept product returns unless a product is defective as well as current deferrals of revenue. We adjustmanufactured, and we do not provide customers with the provision quarterly usingright to a combination of specific identification and an estimated general reserve based on historical experience.Allowance for Doubtful Accounts Receivable—We evaluate the collectability of accounts receivable to determine the appropriate reserve for doubtful accounts. In determining the amount of the reserve, we consider the aging of account balances, historical credit losses, customer-specific information and other relevant factors. We review accounts receivable and adjust the allowance based on current circumstances and charge off uncollectible receivables against the allowance when all attempts to collect the receivable have failed. Our history of write-offs against the allowance has not been significant.Inventories—refund.usesales all impact excess and obsolete inventory. We estimate and record an inventory reservereserves for excess, slow movingobsolete and obsoleteexpired products. An increase to inventory onreserves results in a quarterly basis.Property and Equipment—We state property and equipment at cost less accumulated depreciation. Depreciation is computed using the straight-line method for financial reporting purposes and applied over the estimated useful lives of the assets. Included in property and equipment are generators and other capital equipment (such as our RF and cryo generators) that are placed with direct customers that use our disposable products. These generators and other capital equipment are depreciated over a period of one to three years, which approximates their useful lives, and such depreciation is includedcorresponding increase in cost of revenue. We estimateInventories are written off against the useful lives of this equipment based on anticipated usage by our customers and the timing and impact of our expected new technology rollouts. To the extent we experience changes in the usage of this equipment or the introductions of new technologies, the estimated useful lives of this equipment may change in a future period.Intangible Assets—Intangible assets with determinable useful livesreserve when they are amortized on a straight-line basis over the estimated periods benefitted. Included in intangible assets is In Process Research and Development (IPR&D), which represents the value of acquired technology which has not yet reached technological feasibility. The primary basis for determining the technological feasibility is obtaining specific regulatory approvals. IPR&D is accounted for as an indefinite-lived intangible asset until completion or abandonment of the IPR&D project. Upon completion of the development project, the IPR&D will be amortized over its estimated useful life. If the IPR&D project is abandoned or regulatory approvals are not obtained, the related IPR&D asset would be written off. We review intangible assets for impairment using our best estimates based on reasonable and supportable assumptions and projections.Goodwill— Goodwill represents the excess of purchase price over the fair value of the net assets acquired in business combinations. We test goodwill for impairment annually on November 30, or more often if impairment indicators are present. Our goodwill is accounted for in a single reporting unit representing the Company as a whole.We account for share-based compensation for all share-based payment awards, including stock options, restricted stock, and stock purchases related to an employee stock purchase plan, based on their estimated fair values. We estimate the fair value of time-based optionsperformance share awards with a performance condition initially based on the closing stock price on the date of grant usingassuming the Black-Scholes option pricing model (Black-Scholes model). Our determination of fair value of share-based paymentperformance goal will be achieved. Such performance share awards is affected by our stock price, as well as assumptions regarding a number of subjective variables. These variables include but are not limited to our expected stock price volatility overhave specified performance targets based on the term of the awards and actual and projected employee stock option exercise behaviors. The fair valuecompound annual growth rate (CAGR) of our market-basedrevenue over a three-year performance option grants is estimated at the date of grant using a Monte-Carlo simulation. The value of the portion of theperiod. With respect to these performance share awards, that is ultimately expected to vest is recognized as expense over the requisite service periods in our Consolidated Statements of Operations and Comprehensive Loss.We estimate the fair value of restricted stock awards based upon the grant date closing market price of our common stock.We also have an employee stock purchase plan (ESPP) which is available to all eligible employees as defined by the plan document. Under the ESPP, shares of our common stock may be purchased at a discount. We estimate the number of shares that vest and are issued to be purchased under the ESPP at the beginning of the purchase period and calculate estimated compensation expense using the Black-Scholes modelrecipient is based upon revenue performance over the fair valueperformance period. We may adjust the expense over the performance period based on changes to estimates of performance target achievement. If such goals are not met or service is not rendered for the stock at the beginning of the purchase period. Compensation expenserequisite service period, no compensation cost is recognized, over each purchase period, and expense is adjusted at the time of stock purchase.38Acquisition-Related Contingent Consideration—Contingent consideration arrangements obligate the Company to pay former shareholders of an acquired entity certain amounts if specified future events occur or conditions are met, such as the achievement of certain technological milestones or the achievement of targeted revenue milestones. We measure such liabilities using unobservable inputs by applying an income approach, such as the discounted cash flow technique or the probability-weighted scenario method. Various key assumptions, such as the probability of achievement of the agreed milestones, projected revenuesany recognized compensation cost from acquisitions and the discount rate, are used in the determination of fair value of contingent consideration arrangements and are not observable in the market. Subsequent revisions to key assumptions, which impact the estimated fair value of contingent consideration liabilities, are reflected in the Consolidated Statements of Operations and Comprehensive Loss.a changechanges in tax rates is recognized in the period that includes the enactment date. us to make significant estimates and judgments about our future operating results. Deferred income tax assets are reduced by valuation allowances if, based on the consideration of all available evidence, it is more-likely-than-not that some portion of thea deferred tax asset will not be realized. Significant weight is given to evidence that can be objectively verified. We evaluate deferred income tax assets on an annual basis to determine if valuation allowances are required by considering all available evidence. Deferred income tax assets are realized by having sufficient future taxable income to allow the related tax benefits to reduce taxes otherwise payable. The sources of taxable income that may be available to realize the benefit of deferred tax assets are future taxable income, future reversals of existing taxable temporary differences, future taxable income, exclusive of reversing temporary differences and carryforwards, taxable income in carry-backprior carryforward years and tax planning strategies that are both prudent and feasible. In evaluating whether to recordthe need for a valuation allowance, the applicable accounting standards deem that the existence of cumulative losses in recent years is a piece of objectively verifiable negative evidence that must be overcome by objectively verifiableobjectively-verifiable positive evidence to avoid the need to recordfor a valuation allowance.We believe our critical accounting policies regarding revenue recognition, Our valuation allowance for uncollectible accounts receivable, inventories, property and equipment, intangibleoffsets substantially all net deferred income tax assets goodwill, share-based employee compensation, acquisition-related contingent consideration and taxes affect our more significant judgments and estimates usedas it is more-likely-than-not that the benefit of such deferred income tax assets will not be recognized in the preparation of our consolidated financial statements. We base our judgments and estimates on historical experience, current conditions and other reasonable factors.21 – RecentDescription of Business and Summary of Significant Accounting PronouncementsPolicies to ourthe Consolidated Financial Statements in Item 8 of Part II for further information.more information regarding recent accounting pronouncements.39ITEMITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISKonRate Riskterm loanCompany believes it has invested in a conservative manner, with preservation being the primary investment objective, the value of the securities held will fluctuate with changes in financial markets including, among other things, changes in interest rates, credit quality and revolvinggeneral volatility. This risk is managed by investing in high quality investment grade securities to maintain liquidity and preserve principal without significantly increasing risk.facility accruerisk consist of cash equivalents and investments in corporate bonds. The Company maintains deposit accounts in federally insured financial institutions in excess of federally insured limits. Cash held in financial institutions in foreign countries is not significant. Although these depository accounts may exceed government insured depository limits, we have evaluated the credit worthiness of these applicable financial institutions and determined the risk of material financial loss due to the exposure of such credit risk to be minimal. The Company also maintains investments in money market funds that are not federally insured.Rate. For the years ended December 31, 2017NYFRB Rate plus 0.50% and 2016,Adjusted Term SOFR Rate plus 1.00%. The applicable margin spread is 1.50% to 2.75%, as determined by the average excess availability of the aggregate revolving commitment. All swingline loans bear interest at a rate per annum equal to the ABR plus the applicable margin under the Credit Agreement. Interest periods for SOFR Term Benchmark borrowings range from one month, three months or six months, at the Company's election. Interest rate risk is highly sensitive due to many factors, including United States monetary and tax policies and United states and international economic factors beyond our control.12.5%9.4% and 12.7%9.0% of the Company’s total revenue. Since such revenue was primarily denominated in Euros,for 2023 and 2022. Accordingly, the Company is exposed to exchange rate fluctuations between the Euro and the U.S. Dollar as well as exchange rate fluctuationsand between the British Pound and the Euro. To a lesser extent, the Company is also exposed to exchange rate fluctuations between the Australian and Canadian Dollars to the U.S. Dollar. For the years ended December 31, 20172023 and 2016,2022, foreign currency transaction gains (losses)losses of $138$(101) and ($586)$(559) were recorded primarily in connection with partial settlements of the intercompany receivable balance with the subsidiarybalances and invoices transacted in British Pounds. For revenue denominated in Euros, if there is an increase in the rate at which Euros are exchanged for U.S. Dollars, it willand if products are priced in Euros, the Company will receive less in U.S. Dollars than was received before the rate increase went into effect. If products are priced in U.S. Dollars and competitors price their products in Euros, an increase in the relative strength of the U.S. Dollar could result in the Company’s price not being competitive in a market where business is transacted in Euros. The Euro to U.S. Dollar conversion rate fluctuations may impact our reported revenue and expenses.Company invests its cash primarily in money market accounts, U.S. government agencies and securities, corporate bonds and commercial paper. Although the Company believes its cash to be invested in a conservative manner, with cash preservation being the primary investment objective, the valueterms of the securities heldagreement require we make fixed milestone payments upon achievement of various enrollment and project milestones over the estimated ten-year term. Additional variable costs, including pass through costs incurred at clinical trial sites, will fluctuate with changesbe billed to us by the contracted party. Fixed milestone payments are denominated in Canadian Dollars, while variable pass-through fees incurred at clinical trial sites outside the United States may be billed in U.S. Dollars or other local currencies. Fluctuations in the financial markets including, among other things, changes in interestconversation rates credit quality and general volatility. This risk is managed by investing in high quality investment grade securities with short-term maturities.Financial instruments that potentially subject the Company to credit risk consist of cash and cash equivalent balances and investments in corporate bonds. Certain of AtriCure’s cash and cash equivalents balances exceed FDIC insured limits or are invested in money market accounts with investment banks that are not FDIC-insured. The Company places its cash and cash equivalents in what it believes to be credit-worthy financial institutions. As of December 31, 2017, $21,360 of the U.S. Dollar to the Canadian Dollar and local currencies of international trial sites may impact the cash outlay required for future milestone payments and cash equivalents balance was in excess of FDIC limits. 40Page Financial Statements: PageFinancial Statements:42 43 44 45 46 47 Financial Statement Schedule:66 41Mason, Ohio20172023 and 2016,2022, the related consolidated statements of operations and comprehensive loss,(loss) income, stockholders’ equity, and cash flows, for each of the three years in the period ended December 31, 2017,2023, and the related notes and the schedule listed in the Index at Item 15 (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 20172023 and 2016,2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2017,2023, in conformity withaccounting principles generally accepted in the United States of America.2017,2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 28, 2018,16, 2024, expressed an unqualified opinion on the Company's internal control over financial reporting. /s/28, 20184220172023 and 201621,809 24,208 12,642 19,801 23,083 21,094 22,451 17,660 2,273 2,954 82,258 85,717 28,749 29,995 3,000 50,764 52,131 105,257 105,257 676 321 267,704 276,421 12,431 10,673 18,911 16,467 561 1,688 31,903 28,828 12,761 13,319 24,100 23,886 37,774 41,946 106,538 107,979
outstanding35 33 386,963 367,851 34 (468) (225,866) (198,974) 161,166 168,442 267,704 276,421 2023 2022 Assets Current assets: Cash and cash equivalents $ 84,310 $ 58,099 Short-term investments 52,975 63,014 Accounts receivable, less allowance for credit losses of $500 and $230 52,501 42,693 Inventories 67,897 45,931 Prepaid and other current assets 8,563 5,477 Total current assets 266,246 215,214 Long-term investments — 51,509 Property and equipment, net 42,435 38,833 Operating lease right-of-use assets 4,324 3,787 Intangible assets, net 63,986 39,339 Goodwill 234,781 234,781 Other noncurrent assets 2,160 1,985 Total Assets $ 613,932 $ 585,448 Liabilities and Stockholders’ Equity Current liabilities: Accounts payable $ 27,354 $ 19,898 Accrued liabilities 44,682 33,022 Current maturities of debt and leases 2,533 5,472 Total current liabilities 74,569 58,392 Long-term debt 60,593 56,834 Finance lease liabilities 8,061 9,147 Operating lease liabilities 3,307 3,095 Other noncurrent liabilities 1,234 1,226 Total Liabilities 147,764 128,694 Commitments and contingencies (Note 10) Stockholders’ Equity: Common stock, $0.001 par value, 90,000 shares authorized; 47,526 and 46,563 issued and outstanding 48 47 Additional paid-in capital 824,170 787,422 Accumulated other comprehensive loss (993) (4,096) Accumulated deficit (357,057) (326,619) Total Stockholders’ Equity 466,168 456,754 Total Liabilities and Stockholders’ Equity $ 613,932 $ 585,448 43LOSS2017, 20162023, 2022 and 2015174,716 155,109 129,755 48,553 44,008 36,880 126,163 111,101 92,875 34,144 35,824 25,742 116,998 106,415 93,853 151,142 142,239 119,595 (24,979) (31,138) (26,720) (2,264) (1,801) (292) 227 227 190 138 (586) (354) (26,878) (33,298) (27,176) 14 40 36 (26,892) (33,338) (27,212) (0.83) (1.05) (0.97) 32,387 31,609 28,058 15 18 15 487 125 (278) 502 143 (263) (26,892) (33,338) (27,212) (26,390) (33,195) (27,475) 2023 2022 2021 Revenue $ 399,245 $ 330,379 $ 274,329 Cost of revenue 98,875 84,439 68,469 Gross profit 300,370 245,940 205,860 Operating expenses (benefit): Research and development expenses 73,915 57,337 48,506 Selling, general and administrative expenses 253,138 231,272 204,649 Change in fair value of contingent consideration (Note 2) — — (184,800) Intangible asset impairment (Note 4) — — 82,300 Total operating expenses 327,053 288,609 150,655 (Loss) income from operations (26,683) (42,669) 55,205 Other income (expense): Interest expense (6,925) (4,986) (4,918) Interest income 3,792 1,994 466 Other (31) (537) (366) (Loss) income before income tax expense (29,847) (46,198) 50,387 Income tax expense 591 268 188 Net (loss) income $ (30,438) $ (46,466) $ 50,199 Net (loss) income per share: Basic net (loss) income per share $ (0.66) $ (1.02) $ 1.11 Diluted net (loss) income per share $ (0.66) $ (1.02) $ 1.09 Weighted average shares outstanding: Basic 46,309 45,740 45,066 Diluted 46,309 45,740 46,039 Comprehensive (loss) income: Unrealized gain (loss) on investments $ 2,898 $ (2,811) $ (941) Foreign currency translation adjustment 205 (337) (319) Other comprehensive income (loss) 3,103 (3,148) (1,260) Net (loss) income (30,438) (46,466) 50,199 Comprehensive (loss) income, net of tax $ (27,335) $ (49,614) $ 48,939 442017, 2016,2023, 2022, and 201527,580 28 271,282 (138,424) (348) 132,538 3,757 3 68,985 68,988
plans850 1 1,920 1,921
purchase plan87 1,539 1,539 177 177 8,997 8,997 (263) (263) (27,212) (27,212) 32,274 32 352,900 (165,636) (611) 186,685
plans934 1 1,636 1,637
purchase plan134 1,618 1,618 11,697 11,697 143 143 (33,338) (33,338) 33,342 33 367,851 (198,974) (468) 168,442
plans1,112 2 2,387 2,389
purchase plan132 2,110 2,110 14,615 14,615 502 502 (26,892) (26,892) 34,586 35 386,963 (225,866) 34 161,166 Balance—December 31, 2020 45,346 $ 45 $ 742,389 $ (330,352) $ 312 $ 412,394 Issuance of common stock under equity incentive plans 589 1 (9,837) — — (9,836) Issuance of common stock under employee stock purchase plan 81 — 4,181 — — 4,181 Share-based employee compensation expense — — 28,078 — — 28,078 Other comprehensive loss — — — — (1,260) (1,260) Net income — — — 50,199 — 50,199 Balance—December 31, 2021 46,016 $ 46 $ 764,811 $ (280,153) $ (948) $ 483,756 Issuance of common stock under equity incentive plans 426 1 (10,385) — — (10,384) Issuance of common stock under employee stock purchase plan 121 — 4,225 — — 4,225 Share-based employee compensation expense — — 28,771 — — 28,771 Other comprehensive loss — — — — (3,148) (3,148) Net loss — — — (46,466) — (46,466) Balance—December 31, 2022 46,563 $ 47 $ 787,422 $ (326,619) $ (4,096) $ 456,754 Issuance of common stock under equity incentive plans 811 1 (4,241) — — (4,240) Issuance of common stock under employee stock purchase plan 152 — 5,261 — — 5,261 Share-based employee compensation expense — — 35,728 — — 35,728 Other comprehensive income — — — — 3,103 3,103 Net loss — — — (30,438) — (30,438) Balance—December 31, 2023 47,526 $ 48 $ 824,170 $ (357,057) $ (993) $ 466,168 452017, 20162023, 2022 and 2015 (26,892) (33,338) (27,212) 14,615 11,697 8,997 7,761 7,655 4,975 1,367 1,644 1,303 264 218 61 336 433 276 (173) 407 434 30 126 577 (172) 149 144 (4,078) 969 (1,464) (1,982) (900) (4,477) (79) (2,950) 829 122 (928) 1,290 (1,072) 4,013 2,228 (1,915) 3,070 (408) (153) 298 (8,944) (15,119) (7,842) (16,455) (28,592) (19,525) 26,600 24,202 40,602 (6,384) (7,692) (13,445) 3 (10,552) (7,581) 3,761 (12,079) (10,501) 25,000 (1,689) (439) (263) 10,552 340 (50) (120) (62) 4,402 3,337 2,703 (2,013) (1,701) (782) 2,110 1,618 1,539 (66) 2,760 27,695 13,961 24 (53) (238) (2,399) 444 (4,620) 24,208 23,764 28,384 21,809 24,208 23,764 2,002 1,506 232 37 30 20 650 340 1,277 2 152 50 37 69,054 40,207 462023 2022 2021 Cash flows from operating activities: Net (loss) income $ (30,438) $ (46,466) $ 50,199 Adjustments to reconcile net (loss) income to net cash used in operating activities: Share-based compensation expense 35,728 28,771 28,078 Depreciation 9,460 8,057 7,534 Amortization of intangible assets 5,353 3,653 2,907 Amortization of deferred financing costs 486 507 759 Amortization of investments 632 1,478 2,482 Change in fair value of contingent consideration — — (184,800) Intangible asset impairment — — 82,300 Other non-cash adjustments 1,503 739 1,607 Changes in operating assets and liabilities: Accounts receivable (9,872) (8,989) (10,087) Inventories (21,830) (7,305) (4,274) Other current assets (3,084) (515) (700) Accounts payable 6,177 2,677 4,710 Accrued liabilities 11,562 (2,966) 8,271 Other noncurrent assets and liabilities (1,193) (1,782) (2,766) Net cash provided by (used in) operating activities 4,484 (22,141) (13,780) Cash flows from investing activities: Purchases of available-for-sale securities — (24,637) (173,105) Sales and maturities of available-for-sale securities 63,815 85,524 206,362 Purchases of property and equipment (11,998) (16,881) (9,753) Acquisition of intellectual property (30,000) — — Net cash provided by investing activities 21,817 44,006 23,504 Cash flows from financing activities: Proceeds from debt borrowings — — 5,000 Payments on debt and finance leases (992) (899) (5,816) Payment of debt fees (60) — (1,171) Proceeds from stock option exercises 2,316 1,816 8,175 Shares repurchased for payment of taxes on stock awards (6,557) (12,201) (18,011) Proceeds from issuance of common stock under employee stock purchase plan 5,261 4,225 4,181 Net cash used in financing activities (32) (7,059) (7,642) Effect of exchange rate changes on cash and cash equivalents (58) (361) (372) Net increase in cash and cash equivalents 26,211 14,445 1,710 Cash and cash equivalents—beginning of period 58,099 43,654 41,944 Cash and cash equivalents—end of period $ 84,310 $ 58,099 $ 43,654 Supplemental cash flow information: Cash paid for interest $ 6,376 $ 4,270 $ 4,223 Cash paid for income taxes, net of refunds 395 192 190 Non-cash investing and financing activities: Accrued purchases of property and equipment 1,427 272 1,552 STATEMENTS and, left atrial appendage (LAA) management and itpost-operative pain management, and sells its products to medical centers globally through its direct sales force and distributors.the Company, AtriCure, LLC, Endoscopic Technologies, LLCInc. and nContact Surgical, LLC, the Company’sits wholly-owned subsidiaries, all organized in the State of Delaware; AtriCure Europe B.V. (AtriCure Europe), the Company’s wholly-owned subsidiary incorporated in the Netherlands; AtriCure Spain, S.L., AtriCure Europe’s wholly-owned subsidiary incorporated in Spain, AtriCure Germany GmbH, AtriCure Europe’s wholly-owned subsidiary incorporated in Germany, and AtriCure Hong Kong Limited, the Company’s wholly-owned subsidiary incorporated in Hong Kong.subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.acquisitionpurchase as cash equivalents. Cash equivalents in the accompanying Consolidated Financial Statements.places its investmentsinvests primarily in U.S. Government agenciesgovernment and securities,agency obligations, corporate bonds, and commercial paper and asset-backed securities and classifies all investments as available-for-sale. Investments with maturities ofmaturing in less than one year are classified as short-term investments. Investments are recorded at fair value, with unrealized gains and losses recorded as accumulated other comprehensive income (loss). Gains and losses are recognized using the specific identification method when securities are sold and are included in interest income or expense inincome.Consolidated Statementssale of Operationsmedical devices. Sales of devices are categorized based on the type of product as follows: open ablation, minimally invasive ablation, pain management and Comprehensive Loss.Revenue Recognition—The Company accounts for revenue in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) 605, “Revenue Recognition” (ASC 605).appendage management. The Company recognizes revenue when allcontrol of promised devices is transferred to customers in an amount that reflects the following criteria are met: (i) thereconsideration the Company expects to be entitled to in exchange for those devices. Revenue is persuasive evidence that an arrangement exists, (ii)recognized at a point in time upon shipment or delivery of the products and/or services has occurred, (iii) the selling price is fixed or determinable,products. Shipping and (iv) collectability is reasonably assured.Pursuant to the Company’s standard terms of sale, revenue is recognized when title to the goods and risk of losshandling activities performed after control transfers to customers and there are no remaining obligations that will affectconsidered activities to fulfill the customers’ final acceptance ofpromise to transfer the sale. Generally, the Company’s standard terms of sale define the transfer of title and risk of loss to occur upon shipment to the respective customer. The Company does not maintain any post-shipping obligations to customers. No installation, calibration or testing of products is performed by the Company subsequent to shipment to the customer in order to render products operational.$1,090, $1,266$1,860, $1,496 and $1,056$1,354 in 2017, 2016the years ended December 31, 2023, 2022 and 2015. Cost of freight for shipments to customers is included in cost of revenue. Sales and other value-added taxes collected from customers and remitted to governmental authorities2021.excluded from revenue. The Company sells its productssold primarily through a direct sales force with sales madeand through distributors in selectcertain international markets. Terms of sale are generally consistent for both end-users and distributors, except that payment terms are generally net 30 days for end-users and net 60 days for distributors, with limitedsome exceptions. for sales returns and allowances to account for potential returns of defective or damaged products, products shipped in error and invoice adjustments, as well as current deferrals of revenue.adjustments. The Company adjusts the provision quarterly using a combination of specific identification and an estimated general reservethe expected value method based on historical experience. Increases to the provision result in a reduction of revenue. Thereduce revenue, and the provision is included in accrued liabilities in the Consolidated Balance Sheets.DoubtfulCredit Losses on Accounts Receivable—Receivable—The Company evaluates the collectability ofexpected credit losses on accounts receivable, to determine the appropriate reserve for doubtful accounts. In determining the amount of the reserve, the Company considers aging of account balances,considering historical credit losses, current customer-specific information and other relevant factors.factors when determining the allowance. An increase to the allowance for doubtful accountscredit losses results in a corresponding increase in selling, general and administrative expense.expenses. The Company reviews accounts receivable and adjusts the allowance based on current circumstances and charges off uncollectible receivables against the allowance when all attempts to collect the receivable have failed. The Company’s history of write-offs has not been significant.Year Ended December 31, 2023 2022 2021 Beginning balance - January 1 $ 230 $ 1,096 $ 1,096 Provision for expected credit losses 270 190 65 Recovery — (1,056) (65) Ending balance - December 31 $ 500 $ 230 $ 1,096 productregulatory approvals, variability in product launch strategies and variation in product usesales all impact inventory reserves for excess, obsolete and obsoleteexpired products. An estimated inventory reserve for excess, slow moving and obsolete inventory is recorded quarterly. An increase to inventory reserves results in a corresponding increase in cost of revenue. Inventories are written off against the reserve when they are physically disposed.computeddetermined using the straight-line method over the estimated useful liveslife. The estimated useful life of assets (see Note 7).leasehold improvements is the shorter of the estimated life or the lease term. The Company reassesses theestimated useful lives of47ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)property buildings is 15 to 20 years, while furniture, fixtures, computers and office equipment annually and retires assets if they are no longer in service. Maintenance and repair costs are expensed as incurred.RFradiofrequency and cryocryothermic generators are generally placed with customers that usepurchase the Company’s disposable products. The estimated useful lives of this equipmentgenerators are based on anticipated usage by customers and the timing and impact of expected new technology rollouts by the Company and may change in a future period if the Company experiencesperiods with changes in the usage or introduction of the equipment or introduces new technologies.technology. Depreciation related to generators and other capital equipment is recorded in cost of revenue in the Consolidated Statements of Operationsover three years. Maintenance and Comprehensive Loss.reviewsassesses the useful lives of property and equipment for impairment using its best estimates based on reasonableat least annually and supportable assumptions and projections of expected future cash flows. Property and equipment impairments recorded by the Company have not been significant.IntangibleTechnology intangible assets with determinable useful lives are amortized on a straight-line basis over the estimated periods benefited (see Note 5).Includedfifteen year period benefited. Patent intangible assets with determinable useful lives are amortized over the estimated useful life of five years in a pattern reflecting the estimated economic benefit of the asset to the Company. Amortization of technology intangible assets is In Process Researchrecorded in research and Development (IPR&D). The Company defines IPR&D as the valuedevelopment expense, while amortization of acquired technology which has not yet reached technological feasibility. The primary basis for determining the technological feasibilitypatent intangible assets is obtaining specific regulatory approvals. IPR&D is accounted for as an indefinite-lived intangible asset until completion or abandonmentrecorded in cost of the IPR&D project. Upon completion of the development project, the IPR&D will be amortized over its estimated useful life. If the IPR&D project is abandoned, the related IPR&D asset would be written off. The IPR&D asset represents an estimate of the fair value of the pre-market approval (PMA) that could result from the CONVERGE IDE clinical trial.Company tests goodwill for impairment annually on November 30, or more often if impairment indicators are present. The Company’s goodwill is accounted for in a single reporting unit representing the Company as a whole.Other Noncurrent Liabilities—Other noncurrent The Company performs impairment testing annually on October 1 or more often if impairment indicators are present.consistrepresent the obligation to make payments under the lease. Lease assets and liabilities are measured and recorded at the commencement date based on the present value of contingent consideration recorded in business combinations, deferred revenuespayments over the lease term.other contractual obligations. Althoughliabilities include lease incentives and options to extend or terminate when it is reasonably certain the Company expectswill exercise that option. The Company uses the implicit rate when readily determinable; however, as most leases do not provide an implicit rate, the Company generally uses its incremental borrowing rate. The Company also applies the short-term lease recognition exemption, recognizing lease payments in profit or loss, for lease terms of 12 months or less at commencement and with no option to settleextend the lease whose exercise is reasonably certain. The Company accounts for the lease and non-lease components as a portionsingle lease component. Additionally, the portfolio approach is applied for operating leases based on the terms of the contingent consideration liability within the following year, the balance isunderlying leases.noncurrentoperating lease right-of-use (ROU) assets and operating lease liabilities, as such settlementwhile finance leases are included in property and equipment and finance lease liabilities. The short-term portions of lease liabilities are included in other current liabilities and current maturities of debt and leases. Operating lease expense is both required and expected to be made in shares ofrecognized on a straight-line basis over the Company’s common stock pursuant to the nContact merger agreement.Pounds. existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities from a change in tax rates is recognized in the period that includes the enactment date.it to make significant estimates and judgments about its future operating results. Deferred income tax assets are reduced by valuation allowances if, based on the consideration of all available evidence, it is more-likely-than-not that some portion of thea deferred income tax asset will not be realized. Significant weight is given to evidence that can be objectively verified. The Company evaluates deferred income tax income assets on an annual basis to determine if valuation allowances are required by considering all available evidence. Deferred income tax assets are realized by having sufficient future taxable income to allow the related tax benefits to reduce taxes otherwise payable. The sources of taxable income that may be available to realize the benefit of deferred income tax assets are future taxable income, future reversals of existing taxable temporary differences, future taxable income, exclusive of reversing temporary differences and carryforwards, taxable income in carry-backprior carryforward years and tax planning strategies that are both prudent and feasible. In evaluating whether to recordthe need for a valuation allowance, the applicable accounting standards deem that the existence of cumulative losses in recent years is significant objectively verifiableobjectively-verifiable negative evidence that must be overcome by objectively verifiableobjectively-verifiable positive evidence to avoid the need to recordfor a valuation allowance. The Company has recorded a fullCompany's valuation allowance againstoffsets substantially all net deferred income tax assets as it is more-likely-than-not that the benefit of the deferred income tax assets will not be recognized in future periods.A provision of The Patient Protection and Affordable Care Act enacted in 2010, as amended (PPACA), requires manufacturers of medical devices to pay an exciseCompany has not reclassified income tax on all U.S. medical device sales. In December 2015, the U.S. government approved the suspensioneffects of the excise tax on medical device sales beginning January 1, 2016 through December 31, 2017. Then, in January 2018,48ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, ExceptEarnings Per Share Amounts)the U.S. government approved an additional suspension of the excise tax on medical device sales from January 1, 2018 to December 31, 2019. The Company’s expense related to the medical device excise tax, which was recorded in cost of revenue, was $667 for the year ended December 31, 2015.Net Loss Per Share—Basic and diluted net lossearnings per share is computed in accordance with FASB ASC 260 “Earnings Per Share” (ASC 260) by dividing net (loss) income available to common stockholders by the weighted average number of shares of common stock outstanding during the period. Diluted earnings per share reflects net lossincome available to common stockholders divided by the weighted average number of common shares outstanding during the period. Sinceperiod and any dilutive common share equivalents, including shares issuable upon the Company has experienced net losses for all periods presented, net loss per share excludes the effectvesting of 4,321, 4,320 and 4,255restricted stock optionsawards and restricted stock units, exercise of stock options as well as shares asissuable under the Company's employee stock purchase plan (ESPP).Year Ended December 31, 2023 2022 2021 Net (loss) income available to common stockholders $ (30,438) $ (46,466) $ 50,199 Basic weighted average common shares outstanding 46,309 45,740 45,066 Effect of dilutive securities — — 973 Diluted weighted average common shares outstanding 46,309 45,740 46,039 Basic net (loss) income per common share $ (0.66) $ (1.02) $ 1.11 Diluted net (loss) income per common share $ (0.66) $ (1.02) $ 1.09 2017, 20162023 and 2015 because they are anti-dilutive. Therefore,2022, the number of shares calculated for basic net loss per share is also used for the diluted net loss per share calculation.Comprehensive Income (Loss)calculation, and Accumulated Other Comprehensive Income (Loss)—In addition to net losses,loss per share excludes the comprehensive loss includes foreign currency translation adjustmentseffect of 1,668 and unrealized gains and losses on investments.Accumulated other comprehensive income (loss) consisted1,292 shares because the effect would be anti-dilutive. The computation of diluted earnings per share in the following (net of tax):(468) (611) (348) (21) (39) (54) 15 18 15
to other income (loss)(6) (21) (39) (447) (572) (294) 660 532 156
to other income (loss)(173) (407) (434) 40 (447) (572) 34 (468) (611) costs are expensed as incurred. These costs include compensation and other internal and external costs associated with the development of and research related toof new and existing products or concepts, preclinical studies, clinical trials healthcare compliance and studies, and related regulatory affairs.$900, $625$1,695, $1,233 and $476$907 during the years ended December 31, 2017, 20162023, 2022 and 2015.follows FASB ASC 718 “Compensation-Stock Compensation” (ASC 718) to recordrecognizes share-based compensation expense for all share-based payment awards, including stock options, restricted stock awards, restricted stock units, performance share awards (PSAs) and stock purchases related to an employee stock purchase plan, based on estimated fair values. The Company’s share-based compensation expense recognized under ASC 718 for the years ended December 31, 2017, 2016 and 2015 was $14,615, $11,697 and $8,997.ASC 718 requires companies to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model. The value of the portion of thean award that is ultimately expected to vest is recognized as expense over the requisite service periods inperiod. Prior to January 1, 2023, the Company’s Consolidated Statements of Operations and Comprehensive Loss. The expense has been reduced forCompany estimated forfeitures. The Company estimates forfeitures at the time of grant and revisesrevised them, ifas necessary, in subsequent periods ifas actual forfeitures differ from those estimates.assumptions regarding several subjective variables. These variables include, but are not limited to,assumptions, such as the Company’s expected stock price volatility over the term of the awards and actual and projected employee stock option exercise behaviors. The fair value of market-based performance option grants is estimated at the date of grant using a Monte-Carlo simulation. The value of the portion of the awards that is ultimately expected to vest is recognized as expense over the requisite service periods in the Consolidated Statements of Operations and Comprehensive Loss.49ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)The Company estimates the fair value of restricted stock awards and restricted stock units based upon the grant date closing market price of the Company’s common stock.which is available tocovering substantially all eligibleU.S. employees as defined byof the plan document.Company. Under the ESPP, shares of the Company’s common stock may be purchased at a discount. The Company estimates the number of shares to be purchased under the ESPP at the beginning of each purchase period based upon themanagement to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure ofincluding intangible assets, contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expense during the reporting period. Estimates are based on historical experience, where applicable, and other assumptions believed to be reasonable by management. Actual results could differ from those estimates.and investments in U.S. government agencies and securities as Level 1 within the fair value hierarchy. Accountsagency obligations, accounts receivable, short-term other current assets, and accounts payable and accrued liabilities are also classified as Level 1. The carrying amounts of these assets and liabilities approximate their fair value due to their relatively short-term nature. Cash equivalents and investments in corporate bonds, and commercial paper and asset-backed securities are classified as Level 2 within the fair value hierarchy. The fair value of fixed term debt is estimated by calculating the net present value of future debt payments at current market interest rates and is classified as Level 2. The book value of the Company’s fixed term debt approximates its fair value.value because the interest rate varies with market rates. Significant unobservable inputs with respect to the fair value measurementmeasurements of the Level 3 contingent consideration liabilityliabilities are developed using Company data. When an input is changed, the corresponding valuation models are updated and the results are analyzed for reasonableness. See Note 32 – Fair Value for further information on fair value measurements.2. RECENT ACCOUNTING PRONOUNCEMENTSMay 2014 the FASB issued Accounting Standards Update (ASU) 2014-09, “Revenue from Contracts with Customers” (ASU 2014-09), which requires an entity to recognize revenue for the transfer of goods or services equal to the amount that it expects to be entitled in exchange for those goods or services. ASU 2014-09 supersedes most current revenue recognition guidance. In July 2015 the FASB deferred the effective date of ASU 2014-09 for entities reporting under U.S. GAAP from interim and annual reporting periods beginning after December 15, 2016 to interim and annual reporting periods beginning after December 15, 2017. A full retrospective or modified retrospective approach may be taken to adopt the guidance in the ASU. FASB ASU 2016-08, “Revenue from Contracts with Customers (Topic 606): Principal Versus Agent Considerations (Reporting Revenue Gross Versus Net)”, FASB ASU 2016-10, “Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing”, FASB ASU 2016-12, “Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients” and FASB ASU 2017-13, “Revenue Recognition (Topic 605), Revenue from Contracts with Customers (Topic 606), Leases (Topic 840), and Leases (Topic 842)” were issued to further refine the guidance in ASU 2014-09.The Company performed a comprehensive review of the requirements of ASU 2014-09. This review identified customer contracts and associated revenue streams within the scope of the new guidance by applying the five-step model of the new standard and comparing the results to current accounting to identify potential differences that would result from applying the requirements of the new standard. The Company’s revenue recognition related to product sales will remain substantially unchanged since the majority of the Company’s revenue arrangements consist of a single performance obligation related to the transfer of a promised good to a customer that allows the Company to recognize revenue at a point in time.The Company will adopt the new guidance as of January 1, 2018 using the modified retrospective adoption method. The adoption of ASU 2014-09 will not have a material impact on the amount and timing of revenue recognized in the consolidated financial statements.In February 2016November 2023, the FASB issued ASU 2016-02, “Leases”(ASU 2016-02) which requires lessees2023-07, “Segment Reporting (Topic 280): Improvements to record most leases onto their balance sheet but recognize expenses on their income statement inReportable Segment Disclosures”. This guidance provides new segment disclosure requirements for entities with a manner similar to today’s accounting.single reportable segment and modifies certain reportable segment disclosure requirements. The guidance is effective for fiscal years beginning after December 15, 2018, including2023, and interim periods within those fiscal years. Entities are required to use a modified retrospective approach for leases that exist or are entered intoyears beginning after the beginning of the earliest comparative period in the financial statements. Full retrospective application is prohibited.December 15, 2024, with early adoption permitted. The Company is evaluatingin the provisionsprocess of ASU 2016-02 to determineassessing the impact of the adoption of this guidance; however, adoption is not expected to have a material impact on itsthe Company’s consolidated financial position, results of operations and related disclosures. May 2017December 2023, the FASB issued ASU 2017-09, “Compensation — Stock Compensation2023-09, “Income Taxes (Topic 718), Scope740): Improvements to Income Tax Disclosures”. This guidance requires disclosure of Modification Accounting” (ASU 2017-09), which amends the scope of modification accounting for share-based payment arrangements. ASU 2017-09 provides guidance on the types of changes to the terms or conditions of share-based payment awards to which an entity would be required to apply modification accounting under ASC 718. Specifically, an entity would not apply modification accounting if the fair value, vesting conditions, and classification of the awards are the same immediately before and after the modification. The new guidance also clarifies that a modification to an award could be significant and therefore require disclosure, even if modification50ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)accounting is not required. ASU 2017-09 is effective for annual reporting periods, including interim periods within those annual reporting periods, beginning after December 15, 2017. Early adoption is permitted, including adoption in any interim period. The Company will consider the new guidance in its accounting and financial reporting for modifications if and when they occur.In February 2018, the FASB issued ASU 2018-02, “Reclassification of Certain Tax Effects From Accumulated Other Comprehensive Income (AOCI)”(ASU 2018-02) to address industry concerns related to the application of ASC 740, “Income Taxes” to certain provisions of the new tax reform legislation. Upon adopting ASU 2018-02, an entity is required to disclose (1) its accounting policy related to releasing income tax effects from AOCI, (2) whether it has elected to reclassify, to retained earningsspecific categories in the statement of stockholders’ equity, the stranded tax effects in AOCI related to the new tax reform legislationrate reconciliation and (3) if it has elected to reclassify to retained earnings the stranded tax effects in AOCI related to the new tax reform legislation, what the reclassification encompasses.provide additional information for reconciling items that meet a specified quantitative threshold. The guidance is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. Early2024, with early adoption is permitted. An entity will apply this guidance to each period in which the effect of the new tax reform legislation (or portion thereof) is recorded and may apply it either (1) retrospectively as of the date of enactment or (2) as of the beginning of the period of adoption. The Company is evaluatingin the provisionsprocess of ASU 2018-02 to determineassessing the impact of the adoption of this guidance; however, adoption is not expected to have a material impact on itsthe Company’s consolidated financial position, results of operations and related disclosures.3.statements. (ASC 820), defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value hierarchy is based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value:·Level 1—Quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date. An active market for the asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis. The valuation under this approach does not entail a significant degree of judgment.
•Level 1—Quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date. An active market for the asset or liability is a market in which transactions for the asset or·Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. The valuation technique for the Company’s Level 2 assets is based on quoted market prices for similar assets from observable pricing sources at the reporting date.·Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Unobservable inputs shall be used to measure fair value to the extent that observable inputs are not available, thereby allowing for situations in which there is little, if any, market activity for the asset or liability at the measurement date.2017:2023:12,774 12,774 7,472 7,472 2,999 2,999 2,920 2,920 2,999 23,166 26,165 37,098 37,098 37,098 37,098 Quoted Prices
in Active
Markets for
Identical
Assets
(Level 1)Significant
Other
Observable
Inputs
(Level 2)Significant
Other
Unobservable
Inputs
(Level 3)Total Assets: Money market funds $ — $ 77,864 $ — $ 77,864 Government and agency obligations 12,711 — — 12,711 Corporate bonds — 38,033 — 38,033 Asset-backed securities — 2,231 — 2,231 Total assets $ 12,711 $ 118,128 $ — $ 130,839 51ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)2016:Quoted Prices
in Active
Markets for
Identical
Assets
(Level 1)Quoted Prices
in Active
Markets for
Identical
Assets
(Level 1)Significant
Other
Observable
Inputs
(Level 2)Significant
Other
Unobservable
Inputs
(Level 3)Total 17,085 17,085 Money market funds Money market funds $ — $ 54,414 $ — $ 54,414 5,996 5,996 Commercial paper — 11,935 — 11,935 7,000 1,529 8,529 Government and agency obligations Government and agency obligations 32,637 — 32,637 8,276 8,276 Corporate bonds — 67,598 — 67,598 Asset-backed securities Asset-backed securities — 2,353 — 2,353 7,000 32,886 39,886 Total assets $ 32,637 $ 136,300 $ — $ 168,937 41,176 41,176 41,176 41,176 20172023 and 2016.Derivative Instruments. Vested non-employee options historically issued by the Company were accounted for as derivative liabilities and remeasured at fair value through earnings at each reporting period until exercised or forfeited. All vested non-employee options were exercised as of December 31, 2015. The following table represents the Company’s Level 3 fair value measurements using significant other unobservable inputs for derivative instruments for each of the years ended December 31:120 57 (177) Acquisition-Related Contingent Consideration. ContingentThe Company's contingent consideration arrangements underarising from the nContact merger agreementSentreHEART acquisition obligate the Company to pay certain defined amounts to former shareholders of nContactSentreHEART if specified milestones are met related to the aMAZE IDE clinical trial, including PMA approval and reimbursement for the followingtherapy involving SentreHEART's devices. The achievement periods for the PMA approval and reimbursement milestones if achieved:·Trial Enrollment Milestone – $7,500 upon completion of patient enrollment in the CONVERGE IDE clinical trial. Such payment is due within 30 days following enrollment of the final patient.·Regulatory Milestone – up to $42,500 upon the completion of the CONVERGE IDE clinical trial and receiving a PMA from FDA for the EPi-Sense AF Guided Coagulation System and/or any other nContact product with an indication for symptomatic persistent Afib or similar or related indication.expire on December 31, 2023 and December 31, 2026, respectively. The full contingent consideration amount of $42,500 is only earned if such regulatory approvals are received on or before January 1, 2020. The potential contingent consideration is reduced by 8.33% (or one-twelfth) each month following January 2020, and is reduced to zero if the regulatory milestone is achieved after December 31, 2020. Any payment of the regulatory milestone contingent consideration is due within 30 days following the receipt of the related PMA approval.·Commercial Milestone – for calendar years 2016 through 2019, nContact revenues in excess of specified target revenue amounts will result in contingent consideration equal to 1.5 times the revenues in excess of target. Payments of contingent consideration when the commercial milestone is achieved are due within 65 days of each calendar year end.Subject to the terms and conditions of the merger agreement, all contingent consideration must be paid first in shares of AtriCure common stock. The merger agreement limits the total number of shares of AtriCure common stock issued in connection with the acquisition to 5,660, of which 3,757 shares were issued at closing of the nContact acquisition on October 13, 2015. Since the acquisition, no payments of contingent consideration have been required or made. As of December 31, 2017 and 2016, contingent consideration is recorded in other noncurrent liabilities in the Consolidated Balance Sheets.52The Company measures contingent consideration liabilities by applying an income approach, such as the discounted cash flow technique or the probability-weighted scenario method. Various key assumptions, such as the probability of achievement of the agreed milestones, projected revenues from acquisitions and the discount rate, are used in the determination of fair value of contingent consideration arrangements and are not observable in the market, thus representing a Level 3 measurement within the fair value hierarchy. Subsequent revisionsDuring 2021, the Company was informed that data from the aMAZE clinical trial did not achieve statistical superiority, and the Company assessed the projected probability of payment to key assumptions, which impactbe remote. The Company recorded a credit to operating expenses of $184,800 reflecting the estimated fair value of contingent consideration liabilities, are reflectedchange in the Consolidated Statements of Operations and Comprehensive Loss.The fair value of the nContact contingent consideration was remeasuredconsideration. The Company continues to assess the projected probability of payment during the contractual achievement periods to be remote, resulting in no fair value as of December 31, 2017, resulting in a decrease in fair value of $4,078. This decrease in fair value is due primarily to changes in estimates related to the timing of achievement of the regulatory milestone as a result of actual enrollment in the CONVERGE IDE clinical trial in 2017, offset partially by an increase in forecasted revenues for 20182023 and 2019 under the commercial milestone payment. The fair value of contingent consideration increased $969 during the year ended December 31, 2016 due primarily to a reduction in the discount period. Adjustments to fair value are recorded in selling, general and administrative expenses in the accompanying Consolidated Statements of Operations and Comprehensive Loss. company’sCompany’s Level 3 fair value measurements using significant other unobservable inputs for acquisition-related contingent consideration for each of the years ended December 31:41,176 40,207 40,207 (4,078) 969 37,098 41,176 40,207 2023 2022 2021 Beginning Balance – January 1 $ — $ — $ 184,800 Amounts acquired — — — Changes in fair value of contingent consideration — — (184,800) Ending Balance – December 31 $ — $ — $ — 4.20172023 consisted of the following: Cost Basis Unrealized
LossesFair Value 2,925 (5) 2,920 Corporate bonds $ 38,514 $ (481) $ 38,033 3,000 (1) 2,999 6,723 6,723 Government and agency obligations Government and agency obligations 12,998 (287) 12,711 Asset-backed securities Asset-backed securities Asset-backed securities 2,263 (32) 2,231 12,648 (6) 12,642 Total $ 53,775 $ (800) $ 52,975 20162022 consisted of the following:Cost Basis Cost Basis Unrealized
LossesFair Value 8,284 (8) 8,276 Corporate bonds $ 69,832 $ (2,234) $ 67,598 8,542 (13) 8,529 Government and agency obligations Government and agency obligations 33,971 (1,334) 32,637 5,996 5,996 Commercial paper 11,935 — 11,935 Asset-backed securities Asset-backed securities 2,483 (130) 2,353 22,822 (21) 22,801 Total $ 118,221 $ (3,698) $ 114,523 Company has not experienced any significantgross realized gains or losses on itsfrom sales of available-for-sale investments were not material in the years ended December 31, 2023, 2022 and 2021.the periods presented in the Consolidated Statementsdebt securities, by contractual maturity, as of Operations and Comprehensive Loss. Long term investments held by the Company at December 31, 2016 had2023 were as follows:Available-for-sale Amortized Cost Fair Value Due in 1 year or less $ 51,512 $ 50,744 Instruments not due at a single maturity date 2,263 2,231 Total $ 53,775 $ 52,975 between one and two years.may differ from the contractual maturities due to call or prepayment rights.535.9,242 3,697 9,242 2,773 829 829 829 829 2,179 981 2,179 538 44,021 44,021 56,271 5,507 56,271 4,140 Amortization expense related 2023 2022 Cost Accumulated Amortization Cost Accumulated Amortization Technology $ 46,470 $ 10,084 $ 46,470 $ 7,131 Patents 30,000 2,400 $ — $ — Total $ 76,470 $ 12,484 $ 46,470 $ 7,131 intangible assets with definite lives, which excludes the Company. See Note 10 – Commitments and Contingencies for further information on the patent acquisition. During 2021, the Company recorded an impairment charge of $82,300 to reduce the carrying value of the aMAZE IPR&D asset to $0 as of December 31, 2021 resulting from the aMAZE clinical trial not achieving statistical superiority.$1,367, $1,644$5,353, $3,653 and $1,303$2,907 for the years ended December 31, 2017, 20162023, 2022 and 2015.2023 2022 2021 Cost of revenues $ 2,400 $ — $ — Selling, general and administrative expenses 2,953 3,653 2,907 Total $ 5,353 $ 3,653 $ 2,907 related to intangible assets with definite lives is projected as follows:1,367 1,367 1,235 924 925 925 6,743 2024 $ 7,453 2025 8,353 2026 9,553 2027 10,453 2028 6,553 2029 and thereafter 21,621 Total $ 63,986 Net carrying amount as of December 31, 2015$105,257 Additions (Impairments) —Net carrying amount as of December 31, 2016105,257 Additions (Impairments) —Net carrying amount as of December 31, 2017$105,257 6.Net carrying amount as of December 31, 2021 $ 234,781 Additions (Impairment) — Net carrying amount as of December 31, 2022 234,781 Additions (Impairment) — Net carrying amount as of December 31, 2023 $ 234,781 7,755 5,719 1,299 1,221 13,397 10,720 22,451 17,660 54 2023 2022 Raw materials $ 36,751 $ 19,880 Work in process 3,582 2,959 Finished goods 27,564 23,092 Inventories $ 67,897 $ 45,931 ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)7.15,754 13,087 14,250 14,250 5,873 5,321 4,576 3,731 4,366 3,676 3,636 3,319 1,810 539 221 215 50,486 44,138 (21,737) (14,143) 28,749 29,995 2023 2022 Buildings and improvements $ 29,193 $ 28,947 Generators 23,407 21,354 Machinery and office equipment 24,076 20,184 Computer equipment and software 9,845 10,251 Construction in progress 7,332 3,909 Land 1,006 1,006 Total 94,859 85,651 Less accumulated depreciation (52,424) (46,818) Property and equipment, net $ 42,435 $ 38,833 $7,761, $7,655$9,460, $8,057 and $4,975$7,534 for the years ended December 31, 2017, 20162023, 2022 and 2015. Depreciation related to generators and other capital equipment was $3,574, $3,591 and $2,944 in 2017, 2016 and 2015.2021. As of December 31, 20172023 and 2016,2022, the net carrying value of generators was $4,912 and other capital equipment was $4,656 and $5,692.8.$4,447.6,964 5,737 4,726 2,871 4,097 4,326 1,169 834 695 929 634 1,289 626 481 18,911 16,467 2023 2022 Accrued compensation and employee-related expenses $ 39,425 $ 26,924 Other accrued liabilities 2,503 3,301 Sales returns and allowances 2,754 2,797 Total $ 44,682 $ 33,022 9.Credit Facility. The(Loan Agreement)as amended and modified effective February 8, 2021 and as further amended November 1, 2021 with Silicon Valley Bank (SVB) (SVB Loan Agreement). The SVB Loan Agreement as amended, restated and modified, includes a $25,000$60,000 term loan, with an option to make available an additional $30,000 in term loan borrowings, and $15,000a $30,000 revolving line of credit. The SVB Loan Agreement has a five-year term, expiring November 2026.both which mature in April 2021.is subject to an annual facility fee of 0.20% of the revolving line of credit, and any borrowings thereunder bear interest at the Prime Rate. Borrowing availability under the revolving credit facility is based on the lesser of $15,000$30,000 or a borrowing base calculation as defined by the SVB Loan Agreement. As of December 31, 2017, the Company had no borrowings under the revolving credit facility and had borrowing availability of $15,000. The revolving line of credit is subject to an annual commitment fee of $50, and any borrowings thereunder bear interest at the Prime Rate. Financing costs related to the revolving line of credit are included in other assets in the Consolidated Balance Sheets and amortized ratably over the termtwelve-month period of the Loan Agreement.The term loan has a five-year term, with principal payments made ratably commencing eighteen months after the inception of the loan (November 2017) through the loan’s maturity date. The term loan accrues interest at the Prime Rate and is subject to an additional 4.0% fee on the original $25,000 principal amount at maturity or prepayment of the term loan. The Company is accruing the 4.0% fee over the term of the Loan Agreement.annual fee. As of December 31, 2017,2023, the Company has accrued $337had no borrowings under the revolving credit facility and had borrowing availability of this fee and included it in the outstanding loan balance in the Consolidated Balance Sheets. Other financing costs related to the term loan are net against the outstanding loan balance in the Consolidated Balance Sheets and amortized ratably over the term of the Loan Agreement.covenants related to liquidity, sales growth and a minimum cash balance,liquidity covenant and includesdividend restrictions, along with other customary terms and conditions. Specified assets have been pledged as collateral.55ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)Effective February 23, 2018,New Credit Agreement. On January 5, 2024, the Company and SVB entered into an asset-based credit agreement (Credit Agreement) among the Borrowers, JPMorgan Chase Bank, N.A., as administrative agent, and JPMorgan Chase Bank, N.A., as bookrunner and lead arranger (JPMCB), and Silicon Valley Bank, a LoanDivision of First-Citizen Bank & Trust Company, as Joint Lead Arrangers and SecurityJoint Bookrunners, and the lenders party thereto (Lenders). The Credit Agreement which amends and restates the Company’sprovides for an asset based revolving credit facility with SVB.(ABL Facility) in an amount of up to $125,000. The agreement provides for a $40,000 term loan, and $20,000 revolving line of credit withCompany may request an option to increase in the revolving linecommitment by up to $40,000 (not to exceed a total of $165,000). Borrowing availability under the ABL Facility is based on the lesser of $125,000 or a borrowing base calculation as defined by the Credit Agreement. A portion of the ABL Facility, limited to $5,000, is available for the issuance of letters of credit by an additional $20,000.JPMCB or other financial institutions. JPMCB in its sole discretion, may create swingline loans by advancing floating rate revolving loans requested. Any such swingline loans will reduce availability under the ABL Facility on a dollar-for-dollar basis. The Loan and SecurityCredit Agreement credit facility has a five-yearthree-year term, expiring February 2023. Principal payments of the term loan are to be made ratably commencing eighteen months after the inception of the loan through the loan’s maturity date. If the Company meets certain conditions, as specified by the agreement, the commencement of term loan principal payments may be deferred by an additional six months. January 5, 2027.term loan accrues interest at the greater of the Prime Rate plus 3.75% or 8.25% andABL facility is subject to an additional 3.50% fee on the original $40,000 term loan principal amount at maturity. The revolving line of credit is subject to an annuala facility fee of 0.33%0.37% per annum of the daily available revolving line of credit,commitment and any borrowingspaid on a quarterly basis. Outstanding amounts under the Credit Agreement bear interest at a rate per annum equal to, at the greaterCompany's election: (i) an alternate base rate (ABR) plus an applicable margin or (ii) an adjusted term secured overnight financing rate (SOFR) plus an applicable margin. All swingline loans bear interest at a rate per annum equal to the ABR plus the applicable margin under the Credit Agreement. Alternate base rate is equal to the greatest of Prime, the NYFRB Rate plus 0.50% and Adjusted Term SOFR Rate plus 1.00%. The applicable margin on borrowings will adjust ranging 1.50% to 1.75% per annum for ABR borrowings and from 2.50% to 2.75% per annum for SOFR term borrowings determined by the average historical excess availability. Participation and fronting fees are accrued and paid on a quarterly basis. At time of closing, the Company borrowed $61,865 and had $61,885 of available borrowing capacity under the ABL facility. The proceeds of the Prime RateABL Facility were used to terminate the Company’s indebtedness under the SVB Loan Agreement. The SVB Loan Agreement terminated on January 5, 2024 and 4.50%. The Loan and Security Agreement also provides for certainwas treated as a debt extinguishment. Certain prepayment and early termination fees as well as establishes covenants related to sales growth, along with other customary terms and conditions similar to thoseunder the SVB Loan Agreement were waived at termination. The resulting loss on debt extinguishment in the Company’s current agreement with SVB. The proceeds from the agreement are expected to fund current and future operations of the Company.2024 is not significant. As a result of the refinancing,new Credit Agreement, borrowings outstanding under the existing term loan agreementSVB Loan Agreement have been classified as long-term in the Consolidated Balance Sheet as of December 31, 2017.Capital Lease Obligations. As2023.December 31, 2017,the Credit Agreement in January 5, 2027. Through January 2025, the Company's required minimum utilization of the ABL facility is 40% of the aggregate revolving commitment or $50,000. Subject to customary exceptions and restrictions, the Company had capital leases for its corporate headquarters building and computer equipment that expiremay voluntarily prepay outstanding amounts under the ABL Facility at various terms through 2030. Capital leaseany time thereafter without premium or penalty. Any voluntary prepayments made will not reduce commitments under the ABL Facility. The Credit Agreement contains mandatory prepayment provisions which require prepayment of amounts outstanding under the ABL Facility upon specified events or shortfall. are depreciated over their estimated useful lives. As of December 31, 2017, the cost of the leased assets, both building and computer equipment, was $14,471. Accumulated amortization onCompany, whether consisting of personal, tangible or intangible property, including specified all of the capital lease assets was $2,249. In connection with the termsoutstanding equity interests of the Company’s corporate headquartersdirect subsidiaries, subject to limitations specified in the Credit Agreement. The Credit Agreement contains customary representations and warranties, events of default and financial, affirmative and negative covenants for facilities of this type, including but not limited to financial covenants relating to a fixed charge coverage ratio, a minimum liquidity requirement and a minimum excess availability requirement, and restrictions on indebtedness, liens, investments and acquisitions, asset dispositions, specified agreements, restricted payments and prepayment of certain indebtedness.2024 $ — 2025 — 2026 — 2027 61,865 2028 — Total long-term debt, of which $0 is current and $61,865 is noncurrent $ 61,865 aterms of one to nine years. Options to renew or extend leases beyond their initial term have been excluded from measurement of the ROU assets and lease liabilities as exercise is not reasonably certain.As of As of As of December 31, 2023 December 31, 2022 December 31, 2021 Operating Leases Weighted average remaining lease term (years) 4.8 4.4 3.6 Weighted average discount rate 5.75 % 4.60 % 4.69 % Finance Leases Weighted average remaining lease term (years) 6.7 7.6 8.6 Weighted average discount rate 6.93 % 6.92 % 6.91 % in the amount offor $1,250 was issued to the landlordlessor of the Company's corporate headquarters building in October 2015. The letterat inception of credit wasthe lease and is renewed in June 2017annually and remains outstanding as of December 31, 2017.Future maturities on capital2023.obligationsexpense are as follows: Year Ended Year Ended Year Ended December 31, 2023 December 31, 2022 December 31, 2021 Operating lease cost $ 1,284 $ 1,133 $ 1,052 Finance lease cost: Amortization of right-of-use assets 1,020 1,016 1,019 Interest on lease liabilities 673 735 792 Total finance lease cost $ 1,693 $ 1,751 $ 1,811 debt, after consideration2021.1,468 3,755 8,305 8,309 8,335 14,379 44,551 (7,129) 37,422 Year Ended Year Ended Year Ended December 31, 2023 December 31, 2022 December 31, 2021 Cash paid for amounts included in the measurement of lease liabilities: Operating cash flows for operating leases $ 1,235 $ 845 $ 998 Operating cash flows for finance leases 673 735 620 Financing cash flows for finance leases 992 899 792 Right-of-use assets obtained in exchange for lease obligations: Operating Leases 1,509 — 3,752 Finance Leases — 62 — As of December 31, 2023 As of December 31, 2022 Operating Leases Operating lease right-of-use assets $ 4,324 $ 3,787 Other current liabilities and current maturities of debt and leases 1,447 1,147 Operating lease liabilities 3,307 3,095 Total operating lease liabilities $ 4,754 $ 4,242 Finance Leases Property and equipment, at cost $ 14,620 $ 14,645 Accumulated depreciation (8,105) (7,109) Property and equipment, net $ 6,515 $ 7,536 Other current liabilities and current maturities of debt and leases $ 1,086 $ 992 Finance lease liabilities 8,061 9,147 Total finance lease liabilities $ 9,147 $ 10,139 Operating Leases Finance Leases 2024 $ 1,449 $ 1,689 2025 1,188 1,638 2026 848 1,671 2027 842 1,703 2028 458 1,725 2029 and thereafter 767 3,099 Total payments $ 5,552 $ 11,525 Less imputed interest (798) (2,378) Total lease liabilities $ 4,754 $ 9,147 Lease Commitments. leases certain office and warehouse facilities andhad been party to a vehicle under noncancelable operating leaseslicense agreement that expire at various terms through 2022. Future minimum leaserequired payments under non-cancelable operating leases are projected as follows:965 927 737 413 306 3,348 Rent expense was approximately $850, $1,250 and $1,515 in 2017, 2016, and 2015. Royalty Agreements. The Company has certain royalty agreements in place with terms that include payment of royalties based on product revenue from sales of specified current products. The current royalty agreements have effective dates as early as 2003 and terms ranging from eighteen years to at least twenty years. The royalties range from 3% to 5% of specified product sales. PartiesIn May 2023, the Company entered into an agreement that terminated the license agreement and the Company's obligations to themake royalty agreements have the right at any time to terminate the agreement immediatelypayments. See cause.additional information. Royalty expense of $2,323, $1,895was $1,333, $3,264 and $1,799 was recorded as part of cost of revenue$3,124 for the years ended December 31, 2017, 20162023, 2022 and 2015. 2021.56ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)certain vendorssuppliers in the ordinary course of business. Outstanding commitments at December 31, 2017 were not significant.WhenA liability is established once management has assessed thatdetermines a loss is probable and an amount can be reasonably estimated, theestimated. The Company recordsrecognizes income from a liability in the Consolidated Financial Statements. Costs associated withfavorable resolution of legal proceedings could have a material adverse effect onwhen the Company’s future consolidated results of operations, financial position,associated cash or cash flows.On December 11, 2017, theassets are received.1, 2010 to the presentDecember 2017 and requiresrequired the production of documents and answers to written interrogatories. The Company had no knowledge of the investigation prior to receipt of the CID. The Company maintains rigorous policies and procedures to promote compliance with the False Claims Act and other applicable regulatory requirements,requirements. The Company provided the USDOJ with documents and is working with the U.S. Department of Justice to promptly respondanswers to the CID. However,written interrogatories. In March 2021, USDOJ informed the Company cannotthat its investigation was based on a lawsuit brought on behalf of the United States and various state and local governments under the qui tam provisions of federal and certain state and local False Claims Acts. Although the USDOJ and all of the state and local governments declined to intervene, the relator continues to pursue the case. During the third quarter of 2022, the relator filed a Fourth Amended Complaint, which dropped allegations of off-label promotion and alleges that the Company paid illegal kickbacks to healthcare providers in exchange for using or referring the Company’s products, in violation of the federal Anti-Kickback Statute and various comparable state and local laws. While the Company is contesting the case, it is not possible to predict when the investigation willthis matter may be resolved or what impact, if any, the outcome of this matter might have on our consolidated financial position, results of operations or cash flows.investigation orCleveland Clinic Foundation (Clinic) and IDx Medical, Ltd. (IDx) filed a Demand for Arbitration against the Company with the American Arbitration Association (AAA), alleging that the Company breached certain provisions of the License Agreement dated December 9, 2003, among the Company, Clinic and IDx (License Agreement). Clinic and IDx allege the Company did not include the revenues from sales of certain products in its potential impact oncalculation of royalty payments due under the Company. 2023 2022 2021 Open ablation $ 105,287 $ 86,119 $ 72,396 Minimally invasive ablation 44,577 38,553 39,380 Pain management 49,199 39,974 22,787 Total ablation $ 199,063 $ 164,646 $ 134,563 Appendage management 134,481 112,555 94,568 Total United States $ 333,544 $ 277,201 $ 229,131
Revenue attributed to customer geographic locations is as follows: 2023 2022 2021 Open ablation $ 31,483 $ 26,809 $ 23,194 Minimally invasive ablation 6,670 5,986 6,409 Pain management 2,013 558 61 Total ablation $ 40,166 $ 33,353 $ 29,664 Appendage management 25,535 19,825 15,534 Total International $ 65,701 $ 53,178 $ 45,198 2023 2022 2021 United States $ 333,544 $ 277,201 $ 229,131 Europe 38,469 30,428 27,931 Asia-Pacific 24,526 20,734 16,077 Other International 2,706 2,016 1,190 Total International 65,701 53,178 45,198 Total Revenue $ 399,245 $ 330,379 $ 274,329 local and foreign income tax returns in jurisdictions with varying statutes of limitations. Income taxes are computed usingThe Company uses the asset and liability method in accordance with FASB ASC 740, “Income Taxes”, under which deferred income taxes are provided for the temporary differences between the financial reporting basis and the tax basis of the Company’s assets and liabilities. Deferred taxes are measured using provisions of currently enacted tax laws. A valuation allowance against deferred tax assets is recorded when it is more likely than not that such assets will not be fully realized. The Company has recorded a fullCompany's valuation allowance againstoffsets substantially all its net deferred tax assets as it is more likely than not that the benefit of the deferred tax assets will not be recognized in future periods. Tax credits are accounted for as a reduction of income taxes in the year in which the credit originates.On December 22, 2017, H.R.1, “An Act to Provide for Reconciliation Pursuant to Titles II and V of the Concurrent Resolution on the Budget for Fiscal Year 2018” (the Tax Reform Act) was enacted, and amends the Internal Revenue Code to reduce tax rates and modify policies, credits, and deductions for individuals and businesses. For businesses, U.S. GAAP requires resulting tax effects of accounting for the Tax Reform Act to be recorded in the reporting period of enactment. Also on December 22, 2017, the SEC staff issued Staff Accounting Bulletin No. 118 (SAB 118) to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed in reasonable detail to complete the accounting for certain income tax effects of the Tax Reform Act.We have not completed our accounting for the tax effects of enactment of the Tax Reform Act. However, we have made a reasonable estimate of the effects on our existing deferred tax balances where possible, and accounted for material provisions of the Tax Reform Act as follows:Reduction of US federal corporate tax rate: The Tax Reform Act reduces the corporate tax rate from 34 to 21 percent, effective January 1, 2018. Consequently, the Company has recorded a reduction to its federal deferred tax assets of $29,480 with an offsetting reduction in its valuation allowance at December 31, 2017. In addition, the Company’s state deferred tax assets and corresponding valuation allowance have been adjusted to account for the impact of the federal rate change on state deferred taxes.Deemed Repatriation Transition Tax: The Tax Reform Act provides for a one-time "deemed repatriation" of accumulated foreign earnings for the year ended December 31, 2017. The Company does not anticipate a tax on the deemed repatriation as a result of its foreign deficits.Compensation and Shared-Based Payment Awards: The Tax Reform Act modifies the deductibility of covered employees’ compensation and eliminates the exclusion of performance-based compensation under IRC § 162(m), prospectively. The Tax Reform Act includes a transition rule that permits the continued exclusion of performance-based compensation paid pursuant to a written, binding contract which was in effect on November 2, 2017, and which was not modified in any material respect on or after such date. The Company has not completed its analysis of all of its relevant equity compensation agreements to determine if the transition rule will apply and the deferred tax implications of this provision. 57Corporate Alternative Minimum Tax (AMT): The repeal of AMT provides companies with the ability to obtain refunds of historic AMT credits. The Company has recorded a deferred tax benefit of $102 associated with release of its valuation allowance on its AMT credits.Bonus Depreciation: The Tax Reform Act provides for 100 percent bonus depreciation on personal tangible property expenditures beginning September 27, 2017 through 2022. The bonus depreciation percentage is phased down from 100 percent beginning in 2023 through 2026. The Company is continuing to evaluate its bonus depreciation election based on an analysis of property eligible for 100 percent bonus depreciation and its net operating loss carryforwards. The Company expects to complete the accounting for the Tax Reform Act when the 2017 U.S. corporate income tax return is filed in 2018. The ultimate impact may differ materially from these provisional amounts due to additional analysis, changes in interpretations and assumptions the Company has made, additional regulatory guidance that may be issued, and actions the Company may take as a result of the Tax Reform Act.The detail of deferred tax assets and liabilities at December 31 is as follows:64,776 84,056 5,339 5,446 6,955 8,406 874 914 588 1,503 (11,297) (16,922) (339) (1,487) 179 66 67,075 81,982 (66,973) (81,982) 102 2 44 32 34 72 8 116 40 36 18,485 (7,333) (7,154) (1,337) 210 (398) (2,241) (1,177) (955) (15,009) 8,300 8,507 (102) 14 40 36 2023 2022 2021 Current tax expense Federal $ — $ — $ — State 389 142 42 Foreign 217 118 125 Total current tax expense 606 260 167 Deferred tax expense Federal $ (2,972) $ (8,351) $ (30,925) State (928) (459) (4,803) Foreign (3,671) (1,636) (826) Change in valuation allowance 7,556 10,454 36,575 Total deferred tax expense (15) 8 21 Total tax expense $ 591 $ 268 $ 188 2023 2022 Deferred tax assets: Net operating loss carryforwards $ 129,744 $ 138,263 Research and development credit carryforwards 15,171 13,205 Research and experimental expenditures 20,193 10,104 Equity compensation 10,599 8,287 Finance and operating lease liabilities 3,083 3,395 Deferred interest — 2,411 Inventories 2,822 1,896 Accruals and reserves 1,131 1,332 Property and equipment 219 (2,568) Total deferred tax assets 182,962 176,325 Deferred tax liabilities: Intangible assets (8,568) (9,278) Right-of-use assets (2,160) (2,626) Other (444) 506 Total deferred tax liabilities (11,172) (11,398) Valuation allowance (171,766) (164,918) Net deferred tax assets $ 24 $ 9 $240,286$276,866 which expire between 2024 and 2037 and $175,758 which have expirations between 2021no expiration. The Company has state and 2038 and statelocal net operating loss carryforwards of $147,841 with varying expirations from 2018$301,639 which expire between 2024 to 2038. At December 31, 2016, there were $2,816 of unrecognized deferred tax assets that arose from tax deductions for equity compensation in excess of compensation recognized for financial reporting during years when net operating losses were created. On January 1, 2017, the Company adopted ASU 2016-09, “Improvements to Employee Share-Based Payment Accounting” and recognized $2,816 of previously unrecognized deferred tax assets with a corresponding increase in its valuation allowance.2043. A portion of the Company’s federal and state net operating loss carryforwards are subject to certain limitations under Internal Revenue Code Sections 382 and 383. The Company has federal research and development credit carryforwards of $6,392$15,171 which have expirationsexpire between 20222024 and 2038.2043. Additionally, the Company has foreign net operating loss carryforwards of approximately $30,501$75,355 which have expirations between 2018 and 2027.no expiration.582017, 20162023, 2022 and 20152021 effective income tax rates differ from the federal statutory rate as follows:34.00 (9,139) 34.00 (11,322) 34.00 (9,240) (109.68) 29,480 (0.40) 107 2.89 (962) 3.23 (878) 10.48 (2,816) 55.84 (15,009) (24.93) 8,300 (31.30) 8,507 4.81 (1,292) (0.69) 231 1.38 (375) 1.30 (348) (1.36) 452 (2.02) 549 (2.45) 658 (1.62) 539 (1.99) 542 6.05 (1,627) (8.41) 2,802 (3.43) 931 (0.05) 14 (0.12) 40 (0.13) 36 2023 2022 2021 Federal tax at statutory rate 21.0 % $ (6,268) 21.0 % $ (9,701) 21.0 % $ 10,580 Permanent differences (10.4) 3,092 (1.9) 876 (80.3) (40,439) Valuation allowance (25.3) 7,556 (22.6) 10,454 72.6 36,575 State income taxes 1.8 (539) 0.7 (317) (9.4) (4,760) Federal R&D credit 6.6 (1,966) 4.2 (1,936) (3.7) (1,878) Foreign income taxes 3.4 (1,012) (0.5) 215 0.7 344 Federal deferred adjustments 0.9 (272) (1.5) 677 (0.5) (234) Effective tax rate (2.0) % $ 591 (0.6) % $ 268 0.4 % $ 188 loss(loss) income for domestic and international operations respectively, was $(19,409)$(17,822) and $(7,469)$(12,025) for 2017, ($27,271)2023, $(38,008) and ($6,027)$(8,190) for 20162022, and ($21,157)$55,666 and ($6,019)$(5,279) for 2015. 2021.$107$444 at December 31, 2017.2023. The Company does not consider these earnings as permanently reinvested and thus has recognized appropriate U.S.determined that no current and deferred taxes are required on such amounts.20152020 are open for examination. Generally, state and foreign income tax returns for periods beginning in 20142019 are open for examination. However, taxing authorities have the ability to adjustaudit net operating loss and tax credit carryforwards from years prior to these periods. The Company has not recognized certain tax benefits because of the uncertainty of realizing the entire value of the tax position taken on income tax returns upon review by the taxing authorities.2017, 20162023, 2022 and 20152021 is presented below:3,175 1,982 1,982 (2,018) 1,193 1,157 3,175 1,982 2023 2022 2021 Balance at the beginning of the year $ 1,762 $ 1,798 $ 1,798 Increases (decreases) for prior year tax positions (90) (36) — Increases (decreases) for current year tax positions — — — Increases (decreases) related to settlements — — — Decreases related to statute lapse — — — Balance at the end of the year $ 1,672 $ 1,762 $ 1,798 Internal Revenue Service completed its review of the Company’s 2014 federal income tax return in February 2017. In 2017, the Company also completed a detailed analysis of R&D credit carryforwards for the tax years 2008 through 2016. As a result of this analysis, as well as completion of the IRS audit of the 2014 credit, the Company has reduced both the R&D credit carryforward and related unrecognized tax benefits by $2,018. The Company has not had to accrue any interest and penalties related to unrecognized income tax benefits as a result of offsetting of net operating losses. However, if the situation occurs, the Company will recognize interest and penalties within the income tax expense line in the Consolidated Statements of Operations and Comprehensive Loss and within the related tax liability line in the Consolidated Balance Sheets.There are no amounts included in the balance of unrecognized tax benefits at December 31, 2017, 20162023, 2022 and 2015 that, if recognized, would affect the effective tax rate. Included in the balance of unrecognized tax benefits at December 31, 2017 are $1,1572021 includes $1,672, $1,762 and $1,798 of tax benefits that, if recognized, would result in adjustments to other tax accounts, primarily deferred taxes and valuation allowance. The Company does not expect that its unrecognized tax benefits for research credits will significantly change within twelve months of December 31, 2017. 12. CONCENTRATIONSDuring 2017, 2016 and 2015, approximately 13.2%, 14.4% and 12.9%, of the Company’s total net revenue was derived from its top ten customers. During 2017, 2016 and 2015 no individual customer accounted for more than 10% of the Company’s revenue.As of December 31, 2017 and 2016, 19.7% and 19.9% of the Company’s total accounts receivable balance was derived from its top ten customers. No individual customer accounted for more than 10% of the Company’s accounts receivable as of December 31, 2017 and 2016.59ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)The Company maintains cash and cash equivalents balances at financial institutions which at times exceed FDIC limits. As of December 31, 2017, $21,360 of the cash and cash equivalents balance was in excess of the FDIC limits.2017, 2016the years ended December 31, 2023 and 20152022, the Company made matchingmatched contributions of 50% on the first 8% of employee contributions to the 401(k) Plan. During the year ended December 31, 2021, the Company matched contributions of 50% on the first 6% of employee contributions to the 401(k) Plan. The Company’s matching contributions expensed during 2017, 2016in 2023, 2022 and 20152021 were $1,367, $1,222$4,949, $4,447 and $1,007.$2,651. Additional amounts may be contributed to the 401(k) Plan at the discretion of the Company’s Board of Directors,Directors; however, no such discretionary contributions were made during 2017, 2016in 2023, 2022 or 2015.2021. The Company also provides retirement benefits forAtriCure Europe and otherits foreign subsidiaries. Total contributions to foreign retirement plans for these employees were $205, $101$503, $446 and $133$349 in 2017, 20162023, 2022 and 2015.20142023 Stock Incentive Plan (2014(2023 Plan) and the 20082018 Employee Stock Purchase Plan (ESPP).20142023 Plan, the Board of Directors may grant incentiverestricted stock options to Company employees and may grantawards or restricted stock units (collectively RSAs), nonstatutory stock options, restricted stockperformance share awards (PSAs) or stock appreciation rights to Company employees, directors and consultants.consultants, and may grant incentive stock options to Company employees. The administrator (currently the Compensation Committee of the Board of Directors)Directors, as the administrator of the 2023 Plan, has the authority to determine the terms of any awards, including the number of shares subject to each award, the exercisability of the awards and the form of consideration. As of December 31, 2017, 10,2492023, 2,287 shares of common stock had been reserved for issuance under the 20142023 Plan and 1,1282,238 shares were available for future grants. OptionsThe Company issues registered shares of common stock for stock option exercises, restricted stock grants and performance award grants. 2023 2022 2021 Cost of revenue $ 1,817 $ 1,868 $ 2,243 Research and development expenses 5,802 4,544 4,206 Selling, general and administrative expenses 28,109 22,359 21,629 Total $ 35,728 $ 28,771 $ 28,078 2014 Planplans during 2023 was as follows:Performance Share Awards Number of Shares Outstanding Outstanding at January 1, 2023 213 $ 90.70 Awarded 236 46.16 Vested (96) 89.36 Forfeited — — Outstanding at December 31, 2023 353 $ 61.09 2023 2022 2021 Stock price $ 38.81 $39.94 - $69.59 $ 66.31 Expected term (years) 2.8 2.6 to 2.8 2.8 Company volatility 44.80% 43.50 - 46.90% 42.10% Market index average volatility 91.00% 90.30 - 92.00% 91.00% Market index average correlation 32.20% 33.50 - 35.40% 31.50% Risk-free interest rate 4.60% 1.40 - 2.70% 0.20% Dividend yield 0.00% 0.00% 0.00% 2023 2022 2021 Weighted average estimated grant date fair value $ 46.16 $ 91.05 $ 89.36 Expense 11,417 8,731 8,095 Restricted Stock Awards Outstanding at January 1, 2023 598 $ 60.00 Awarded 751 39.21 Released (338) 54.08 Forfeited (29) 50.25 Outstanding at December 31, 2023 982 $ 46.43 2023 2022 2021 Weighted average estimated grant date fair value $ 39.21 $ 63.14 $ 67.51 Expense 21,797 17,621 17,746 grant and generally vest at a rate of 25% on the first anniversary date of the grant and ratably each month thereafter over the following three years. Restricted stock awards granted under the 2014 Plan vest between one and four years from the date of grant.60ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)20172023 was as follows:2,454 12.51 65 20.22 (458) 9.61 (35) 19.08 2,026 13.30 5.62 11,730 2,004 13.23 5.58 11,717 1,766 12.48 5.20 11,471 1,416 17.40 771 19.38 (331) 17.43 (11) 18.52 1,845 18.22 450 13.48 450 13.48 5.45 2,774 250 13.48 5.45 1,541 Time-Based Stock Options Outstanding at January 1, 2023 481 $ 29.34 Granted — — Exercised (137) 16.93 Forfeited (12) 64.93 Outstanding at December 31, 2023 332 $ 33.20 4.0 $ 3,584 Vested and expected to vest 332 $ 33.15 4.0 $ 3,584 Exercisable at December 31, 2023 308 $ 30.22 3.7 $ 3,584 61ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)Activity under the plans during 2016 was as follows:2,734 11.75 215 16.74 (394) 8.47 (101) 16.66 2,454 12.51 6.02 18,295 2,420 12.42 5.99 18,228 1,914 11.01 5.40 16,897 1,071 17.30 710 16.35 (70) 17.31 (295) 14.49 1,416 17.40 450 13.48 450 13.48 6.45 3,074 250 13.48 6.45 1,708 2017, 20162023, 2022 and 20152021 was $5,121, $3,550$2,982, $5,565 and $2,740.$27,318. As a result of the Company’s full valuation allowance on its net deferred tax assets, no tax benefit was recognized related to the stock option exercises. The exercise price per share of each option is equal to the fair market value of the underlying share on the date of grant. For 2017, 20162023, 2022 and 2015, $4,402, $3,3372021, $2,316, $1,816 and $2,703$8,175 in cash proceeds from the exercise of stock options were included in the Company’s Consolidated Statements of Cash Flows as a result of the exercise of stock options. Flows. total fair value of restrictedoptions is estimated on the grant date using the Black-Scholes model. No options were granted during 2023 or 2022.2021 Range of risk-free interest rate 0.43 - 1.22% Range of expected life of stock options (years) 5.3 to 5.7 Range of expected volatility of stock 40.00 - 43.00% Weighted-average volatility 41.84% Dividend yield 0.00% vested during 2017, 2016 and 2015 was $6,235, $5,102 and $2,767.price over the expected option life. The risk-free interest rate assumption is based upon the U.S. treasury yield curve at the time of grant for the expected option life. The Company issues registered sharesestimates the expected terms of common stock to satisfy stock option exercisesoptions using historical employee exercise behavior. Based on the assumptions noted above, the weighted average estimated grant date fair value per share and restricted stock grants. The Company recognized expense related to time-based stock options and restricted stock for 2017, 2016, and 2015 of $13,908, $10,872 and $8,072. was as follows: 2023 2022 2021 Weighted average estimated grant date fair value $ — $ — $ 27.31 Expense 765 1,012 981 2017 there was $23,3312023, $287 of unrecognized compensation costs related to non-vested stock option and restricted stock arrangements ($2,197 relating to stock options and $21,134 relating to restricted stock). This cost isare expected to be recognized over a weighted-average period of 2.2 years for stock options and 2.2 years for restricted stock.The Company has awarded 450 performance options to its President and Chief Executive Officer. The options expire ten years from the date of grant and vest in increments of 25 shares when the volume adjusted weighted average closing price of the common stock of the Company as reported by NASDAQ (or any other exchange on which the common stock of the Company is listed) for 30 consecutive days equals or exceeds each of $10.00 per share, $12.50 per share, $15.00 per share, $17.50 per share, $20.00 per share, $25.00 per share, $30.00 per share, $35.00 per share and $40.00 per share. In accordance with FASB ASC 718, a Monte Carlo simulation was performed to estimate the fair values, vesting terms and vesting probabilities for each tranche of options. Expense calculated using these estimates is being recorded over the estimated vesting terms. The Company recognized expense related to the62ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)performance options during 2017, 2016 and 2015 of $43, $269 and $546. As of December 31, 2017, compensation costs related to non-vested performance options were fully recognized. The ESPP is available to eligible employees as defined in the plan document. (currently 15%(15%) ofto the lesser of the closing price of the Company’s common stock on the first trading day or the last trading day of the offering period. The offering period (currently six months) and the offering price are subject to change. Participants may not purchase more than $25 of the Company’s common stock in a calendar year and may not purchase a value of more than 3 shares during an offering period. On the first day of each fiscal year during the term of the ESPP, the number of shares available for sale under the ESPP may be increased by the lesser of (i) two percent (2%) of the Company’s outstanding shares of common stock as of the close of business on the last business day of the prior calendar year, not to exceed 600 shares, or (ii) a lesser amount determined by the Board of Directors. Shares have not been added to the ESPP since 2011. As of December 31, 2017, there were 2252023, 782 shares are available for future issuance under the ESPP. Share-based compensation expense with respect to the ESPP was $664, $556 and $379 for 2017, 2016 and 2015.Valuation and Expense Information Under FASB ASC 718The following table summarizes share-based compensation expense related to employees, directors and consultants under FASB ASC 718 for 2017, 2016 and 2015. The expense was allocated as follows:610 420 416 2,052 1,825 1,373 11,953 9,452 7,208 14,615 11,697 8,997 calculating compensation expense, the fair valueaddition to net (loss) income, comprehensive (loss) income includes foreign currency translation adjustments and unrealized losses on investments. Accumulated other comprehensive income (loss) consisted of the options is estimated on the grant date using the Black-Scholes model including the following, assumptions:44.50 48.87 54.75 0.00 0.00 0.00 The Company’s estimatenet of volatility is based solely on the Company’s trading history over the expected option life. The risk-free interest rate assumption is based upon the U.S. treasury yield curve at the time of grant for the expected option life. The Company estimates the expected terms of options using historical employee exercise behavior.The fair value of restricted stock awards is based on the market value of the Company’s stock on the date of the awards.Based on the assumptions noted above, the weighted average estimated grant date fair value per share of the stock options and restricted stock granted for 2017, 2016 and 2015 was as follows:8.60 8.25 11.12 19.38 16.35 17.82 2023 2022 2021 Total accumulated other comprehensive (loss) income at beginning of period $ (4,096) $ (948) $ 312 Unrealized (losses) gains on investments Balance at beginning of period $ (3,698) $ (887) $ 54 Other comprehensive income (loss) before reclassifications 2,898 (2,739) (941) Amounts reclassified from accumulated other comprehensive income (loss) to interest income — (72) — Balance at end of period $ (800) $ (3,698) $ (887) Foreign currency translation adjustment Balance at beginning of period $ (398) $ (61) $ 258 Other comprehensive income (loss) before reclassifications 154 (774) (768) Amounts reclassified from accumulated other comprehensive (loss) income to other (expense) income 51 437 449 Balance at end of period $ (193) $ (398) $ (61) Total accumulated other comprehensive loss at end of period $ (993) $ (4,096) $ (948) In calculating compensation expense for performance options, the fair value of the options was estimated on the grant dates using a Monte Carlo simulation including strike prices of $5.91 and $21.04, contractual terms of 10 years, expected volatility of 69.60% and 60.50% and interest rates of 1.75% and 2.73%. The contractual term assumes that the performance options issued to the CEO of the Company will be held until expiration. Expected volatility was estimated based on the Company’s trading history over the expected option life. The expected rate of return assumption was based upon the U.S. treasury yield curve at the time of grant for the expected option life.63ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)Based on the assumptions noted above, the estimated grant date fair value per share of the performance options granted were as follows:10.00 4.32 14.74 12.50 4.30 14.74 15.00 4.27 14.74 17.50 4.23 14.74 20.00 4.19 14.73 25.00 4.10 14.73 30.00 4.01 14.71 35.00 3.92 14.67 40.00 3.83 14.61 15. SEGMENT AND GEOGRAPHIC INFORMATIONThe Company evaluates reporting segments in accordance with FASB ASC 280, “Segment Reporting”. The Company develops, manufactures, and sells devices designed primarily for the surgical ablation of cardiac tissue and systems designed for the exclusion of the left atrial appendage. These devices are developed and marketed to a broad base of medical centers globally. Management considers all such sales to be part of a single operating segment. Revenue attributed to geographic areas is based on the location of the customers to whom products are sold.Revenue by geographic area was as follows:138,387 122,385 102,212 21,901 19,772 17,180 13,616 12,223 9,510 812 729 853 36,329 32,724 27,543 174,716 155,109 129,755 United States revenue by product type was as follows:64,517 58,050 53,541 34,421 31,169 21,564 37,281 30,321 24,377 136,219 119,540 99,482 2,168 2,845 2,730 138,387 122,385 102,212 International revenue by product type was as follows:20,718 20,189 16,287 8,007 8,065 7,964 7,251 3,986 2,868 35,976 32,240 27,119 353 484 424 36,329 32,724 27,543 64ATRICURE, INC. AND SUBSIDIARIESNOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)(In Thousands, Except Per Share Amounts)The Company’s long-lived assets are located primarily in the United States, except for $957 as of December 31, 2017 and $931 as of December 31, 2016, which are located primarily in Europe.16. SELECTED QUARTERLY FINANCIAL DATA (UNAUDITED)41,273 35,940 45,231 39,672 42,150 38,340 46,062 41,157 30,008 25,914 32,554 28,818 30,918 27,472 32,683 28,897 (9,642) (9,419) (6,355) (7,738) (6,847) (6,286) (2,135) (7,695) (10,183) (9,724) (6,883) (8,206) (7,246) (6,783) (2,580) (8,625) (0.32) (0.31) (0.21) (0.26) (0.22) (0.21) (0.08) (0.27) Amounts may not sum to consolidated totals for the full year due to rounding. Basic and diluted net loss per share is computed independently for each of the quarters presented. Therefore, the sum of the quarterly per share amounts will not necessarily equal the total for the year.65VALUATION AND QUALIFYING ACCOUNTS834 441 106 1,169 207 634 7 834 135 78 6 207 1,080 1,004 1,195 889 843 1,692 1,455 1,080 522 720 399 843 81,982 15,009 66,973 73,682 8,300 81,982 59,554 14,128 73,682 66ITEMITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSUREAs defined in Rules 13(a)-15(e) and 15(d) -15(e) of the Securities Exchange Act of 1934 (Exchange Act), the and Senior Vice President and Chief Financial Officer (the Principal Accounting and Financial Officer), has evaluated the effectiveness of the Company’s disclosure controls and procedures as defined in Rule 13(a) – 15(e) of the Securities Exchange Act of 1934 (Exchange Act), as of the end of the period covered by this report. Based on this evaluation, we concluded that, as of the end of the period covered by this report, our disclosure controls and procedures were effective in providing reasonable assurance that information required to be disclosed by us in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s forms and rules, and the material information relating to the Company is accumulated and communicated to management, including the President and Chief Executive Officer and the Senior Vice President and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosures.20172023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.2017.2023. No matter how well designed, because of inherent limitations in all control systems, internal control over financial reporting may not prevent or detect misstatements should they occur. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the control procedures may deteriorate. In making this assessment, the Company’s management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013). Based on such assessment, management has concluded that the Company’s internal control over financial reporting was effective as of December 31, 2017. consolidated financial statementsConsolidated Financial Statements included in this Annual Report on Form 10-K and, as part of its audit, has issued an attestation report on the effectiveness of the Company’s internal control over financial reporting. The attestation report can be found on the following page as part of this Item 9A.67Mason, Ohio2017,2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017,2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.2017,2023, of the Company and our report dated February 28, 2018,16, 2024, expressed an unqualified opinion on those financial statements.overOver Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.28, 201816, 202468Itemitem with respect to the Company’s Directors is incorporated by reference to thecontained in our definitive proxy statement (the “Proxy Statement”) for our 20182024 Annual Meeting of Stockholders to be filed withunder the Securitiesheading “Proposal One—Election of Directors” and Exchange Commission within 120 days after the end of 2017 (the “Proxy Statement”).ITEM 11. EXECUTIVE COMPENSATION Itemitem with respect to the Company’s Executive Officers is contained in the Proxy Statement under the heading “Management” and is incorporated herein by referencereference.Statement.2017.2023.
to be issued upon
exercise of
outstanding options,
warrants and rights (1)
exercise price of
outstanding options,
warrants and rights (2)
available for future issuance
under equity compensation
plans (excluding securities
reflected in column (a))
security holders (3)4,320,704 13 1,127,972
security holders4,320,704 13 1,127,972 _________________________(1)Represents outstanding stock options and restricted stock as of December 31, 2017. Plan Category (a) (c) 1,667,626 $ 33.20 2,237,742 Equity compensation plans not approved by security holders — — — Total 1,667,626 $ 33.20 2,237,742 (2)The weighted average exercise price is calculated without taking into account restricted stock that will become issuable, without any cash consideration or other payment, as vesting requirements are achieved.
(1)Represents outstanding stock options, restricted stock awards and performance shares as of December 31, 2023.(3)Amounts include awards under our 2005 Equity Incentive Plan and 2014 Stock Incentive Plan but exclude shares purchased under our 2008 Employee Stock Purchase Plan.remainingweighted average exercise price is calculated without taking into account restricted stock that will become issuable, without any cash consideration or other payment, as vesting requirements are achieved.Itemitem with respect to security ownership of certain beneficial owners and management is contained in the Proxy Statement under the heading “Security Ownership of Certain Beneficial Owners and Management” and is incorporated herein by reference to the Proxy Statement.Itemitem with respect to director independence is contained in the Proxy Statement under the heading “Corporate Governance and Board Matters – Independence of the Board” and is incorporated herein by reference to the Proxy Statement.Itemitem with respect to audit fees, tax fees and the Audit Committee’s pre-approval policies and procedures are contained in the Proxy Statement under the heading “Proposal Two-Ratification of Appointment of Independent Registered Public Accounting Firm” and is incorporated herein by reference to the Proxy Statement.reference.69Exhibit No. Description Exhibit No.Description 3.110.1#10.2#10.3#10.4#10.5#10.6#10.7#10.4# 10.8#10.6# 10.910.1010.11#10.12#10.13#10.14#10.1510.13 10.14 70Exhibit No.Description10.1610.15 10.16 Exhibit No. Description 10.17 12.12110.20# 10.21# 10.22# 10.23# 10.24# 14 19 21 97 104 Cover Page Interactive Data File providedprovided.71AtriCure, Inc. AtriCure, Inc.(REGISTRANT)28, 201828, 2018M. Andrew WadeM. Andrew WadeSenior Vice President and Chief Financial OfficerMENWOMEN AND WOMENMEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Michael H. Carrel and Angela L. Wirick, her or his attorney-in-fact, with the power of substitution, for her or him in any and all capacities, to sign any and all amendments to this Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the U.S. Securities and Exchange Commission, granting unto said attorneys-in-fact, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as she or he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact, and any of them or her or his substitute or substitutes, may do or cause to be done by virtue thereof.28, 2018. 16, 2024.Signature Title(s) Signature/s/ B. Kristine Johnson Title(s)B. Kristine Johnson /s/ Richard M. JohnstonRichard M. JohnstonRichardM.JohnstonChairmanChair of the Board/s/ M. Andrew WadeM. Andrew WadeM. Andrew WadeSenior Vice President and Chief Financial Officer(Principal Accounting and Financial Officer)/s/ Mark A. CollarMark A. CollarMark A. CollarDirector/s/ Scott W. DrakeScott W. DrakeScott W. DrakeDirector/s/ Shlomo Nachman Shlomo Nachman /s/ B. Kristine JohnsonB. Kristine JohnsonB. Kristine Johnson/s/ Karen N. Prange DirectorKaren N. Prange Director /s/ Elizabeth D. KrellDeborah H. TelmanElizabeth D. KrellElizabeth D. Krell/s/ Mark R. LanningMark R. LanningMark R. LanningDirector/s/ Maggie Yuen Maggie Yuen Maggie Yuen Director 72