UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| | | | | |
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 20222023
or
| | | | | |
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-37507
IMMUNITYBIO, INC.
(Exact name of registrant as specified in its charter)
| | | | | |
| Delaware | 43-1979754 |
(State or other jurisdiction of
incorporation or organization) | (I.R.S. Employer
Identification No.) |
| |
3530 John Hopkins Court San Diego, California | 92121 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (858) 633-0300(844) 696-5235
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share | | IBRX | | The Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes þ No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
| Large accelerated filer | ¨ | | Accelerated filer | ¨ |
| | | | |
| Non-accelerated filer | þ | | Smaller reporting company | þ |
| | | | |
| | | Emerging growth company | ¨ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. þ¨
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ¨
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No þ
The aggregate market value of the registrant’s voting and non-voting common equity held by non-affiliates, computed based on the closing price of shares of common stock on the Nasdaq Global Select Market on June 30, 20222023 was approximately $302.1$315.9 million.
The number of shares of the registrant’s common stock outstanding as of February 24, 2023March 14, 2024 was 435,835,583673,952,278 (excluding 163,800 shares held by a majority owned subsidiary of ours that are treated as treasury shares for accounting purposes).
DOCUMENTS INCORPORATED BY REFERENCE
As noted herein, the information called for by Part III of this Annual Report on Form 10-K is incorporated by reference to specified portions of the registrant’s definitive proxy statement to be filed in conjunction with the registrant’s 20232024 Annual Meeting of Stockholders, which is expected to be filed not later than 120 days after the registrant’s fiscal year ended December 31, 2022.2023.
IMMUNITYBIO, INC.
ANNUAL REPORT ON FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 20222023
TABLE OF CONTENTS
| | | | | | | | |
| | Page |
| PART I |
| Item 1. | | |
| Item 1A. | | |
| Item 1B. | | |
| Item 1C. | | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| | |
| PART II |
| Item 5. | | |
| Item 6. | | |
| Item 7. | | |
| Item 7A. | | |
| Item 8. | | |
| Item 9. | | |
| Item 9A. | | |
| Item 9B. | | |
| Item 9C. | | |
| | |
| PART III |
| Item 10. | | |
| Item 11. | | |
| Item 12. | | |
| Item 13. | | |
| Item 14. | | |
| | |
| PART IV |
| | |
| Item 15. | | |
| Item 16. | | |
| |
Defined Terms
Unless expressly indicated or the context required otherwise, the terms “ImmunityBio,” “the company,” “the combined company,” “we,” “us,” and “our” in this Annual Report refer to ImmunityBio, Inc., a Delaware corporation, and, where appropriate, its subsidiaries. We have also used several other terms in this Report, the consolidated financial statements and accompanying notes included herein, most of which are defined below:
| | | | | | | | |
| Term | | Definition |
| | |
| 2014 Plan | | NantKwest, Inc. 2014 Equity Incentive Plan |
| 2015 Plan | | ImmunityBio, Inc. 2015 Equity Incentive Plan |
| 2015 Share Repurchase Program | | Board of Directors-approved share repurchase program |
| 3M IPC | | 3M Innovative Properties Company |
| 401(k) Plan | | 401(k) retirement and savings plan |
| AAHI | | Access to Advanced Health Institute |
| ACA | | Affordable Care Act |
| ADCC | | antibody-dependent cellular cytotoxicity |
| Altor | | Altor BioScience, LLC |
| America Invents Act | | Leahy-Smith America Invents Act |
| AML | | acute myeloid leukemia |
| Amyris | | Amyris, Inc. |
Anktiva® | | Proposed proprietary name for N-803, our novel antibody-cytokine fusion protein (nogapendekin alfa inbakicept-pmln) in the non-muscle invasive bladder cancer indication. The FDA has not yet approved this proposed proprietary name for such use, and this proposed proprietary name will be approved at the time the BLA is approved, if at all. |
| Annual Report | | Annual Report on Form 10-K |
| ART | | anti-retroviral therapy |
| ASC | | Accounting Standards Codification |
| ASCO | | American Society of Clinical Oncology |
| ASU | | Accounting Standards Update |
| Athenex | | Athenex, Inc. |
| ATM | | “at-the-market” sales agreement |
| ATRA | | American Taxpayer Relief Act of 2012 |
| BARDA | | Biomedical Advanced Research and Development Authority |
| BCG | | bacillus Calmette-Guérin |
| Beike | | Shenzhen Beike Biotechnology Co. Ltd. |
| BICR | | blinded independent central review |
| BLA | | Biologics License Application |
| BPCIA | | Biologics Price Competition and Innovation Act of 2009 |
| bNAbs | | broadly-neutralizing antibodies |
| Brink | | Brink Biologics, Inc. |
| Cambridge | | Cambridge Equities, LP |
| CAR | | chimeric antigen receptor |
| CCPA | | California Consumer Privacy Act of 2018 |
| CEO | | chief executive officer |
| CFO | | chief financial officer |
| cGMP | | current Good Manufacturing Practice |
| China | | when used in connection with the RIPA, People’s Republic of China, Hong Kong and any territories controlled by the People’s Republic of China |
| | | | | | | | |
| Term | | Definition |
| | |
| CI | | confidence interval |
| CIO | | chief information officer |
| CIS | | carcinoma in situ |
| Clinic | | Immuno-Oncology Clinic, Inc. |
| Closing Date | | when used in connection with the RIPA, December 29, 2023 |
| CMC | | Chemistry, Manufacturing and Controls |
| CMO | | contract manufacturing organization |
| CMS | | Centers for Medicare & Medicaid Services |
| Code | | Internal Revenue Code of 1986, as amended |
| Company Common Stock | | common stock, par value $0.0001 per share, of the company |
| CPRA | | California Privacy Rights Act of 2020 |
| CR | | complete response |
| CRADA | | Cooperative Research and Development Agreement |
| CRL | | complete response letter |
| CRO | | contract research organization |
| CVR | | contingent value right |
Cynviloq™ | | IG-101 (paclitaxel nanoparticle polymeric micelle) |
| | |
| DGCL | | Delaware General Corporation Law |
| DSCSA | | Drug Supply Chain Security Act |
| Duley Road | | Duley Road, LLC |
| Dunkirk Facility | | a leasehold interest in a cGMP ISO Class 5 pharmaceutical manufacturing space in
western New York |
| EGFR | | epidermal growth factor receptor |
| EMA | | European Medicines Agency |
| ERM | | Enterprise Risk Management |
| Etubics | | Etubics Corporation |
| EU | | European Union |
| EUA | | emergency use authorization |
| Exchange Act | | Securities Exchange Act of 1934, as amended |
| Exchange Ratio | | a right to receive 0.8190 newly issued shares of Company Common Stock |
| Exyte | | Exyte U.S., Inc. |
| FASB | | Financial Accounting Standards Board |
| FCA | | False Claims Act |
| FCPA | | U.S. Foreign Corrupt Practices Act |
| FD&C Act | | Federal Food, Drug, and Cosmetic Act |
| FDA | | U.S. Food and Drug Administration |
| FDASIA | | Food and Drug Administration Safety and Innovation Act of 2012 |
| FSMC | | Fort Schuyler Management Corporation, a not-for-profit corporation affiliated with the
state of New York |
| FTC | | Federal Trade Commission |
| FTO | | freedom-to-operate |
| FVO | | fair value option |
| GBM | | glioblastoma multiforme |
| | | | | | | | |
| Term | | Definition |
| | |
| GCP | | Good Clinical Practice |
| GDPR | | General Data Protection Regulation |
| GlobeImmune | | GlobeImmune, Inc. |
| GLP | | Good Laboratory Practice |
| GMP | | Good Manufacturing Practice |
| hAd5 | | human adenovirus serotype 5 |
| Hatch-Waxman Act | | Drug Price Competition and Patent Term Restoration Act of 1984 |
| HCW | | HCW Biologics, Inc. |
| HHS | | U.S. Department of Health and Human Services |
| HIPAA | | Health Insurance Portability and Accountability Act of 1996 |
| HITECH | | Health Information Technology for Economic and Clinical Health Act |
| HIV | | human immunodeficiency virus |
| ICB | | immune checkpoint blockade |
| IDE | | Investigational Device Exemption |
| IDRI | | Infectious Disease Research Institute |
| IgDraSol | | IgDraSol, Inc., a subsidiary of the company |
| IL-15 | | novel interleukin 15 |
| IND | | investigational new drug |
| Infinity | | Infinity SA LLC, as purchaser agent for affiliates of Oberland |
| IPR&D | | In-process research and development |
| IRA | | Inflation Reduction Act of 2022 |
| IRB | | Institutional review boards |
| IRS | | Internal Revenue Service |
| LadRx | | LadRx Corporation |
| LMIC | | low- and middle-income countries |
| Lung-MAP | | Lung Cancer Master Protocol |
| M-ceNK | | memory-like cytokine-enhanced NK cells |
| mAbs | | monoclonal antibodies |
| MDSC | | myeloid-derived suppressor cells |
| MHC | | majors histocompatability |
| MNC | | mononuclear cells |
| Nant Capital | | Nant Capital, LLC |
| NantBio | | NantBio, Inc. |
| NantCell | | NantCell, Inc., a subsidiary of the company |
| NANTibody | | Immunotherapy NANTibody, LLC, a subsidiary of the company |
| NantKwest | | NantKwest, Inc. |
| NantMobile | | NantMobile, LLC |
| NantPharma | | NantPharma, LLC |
| NantWorks | | NantWorks, LLC, a related-party |
| NC 2015 Plan | | NantCell, Inc. 2015 Stock Incentive Plan |
| NCI | | National Cancer Institute |
| NCSC | | NantCancerStemCell, LLC |
| NCTN | | National Clinical Trials Network |
| | | | | | | | |
| Term | | Definition |
| | |
| NDA | | New Drug Application |
| NEO | | named executive officer |
| NHP | | non-human primate |
| NIAID | | National Institute of Allergy and Infectious Diseases |
| NIDCD | | National Institute on Deafness and Other Communication Disorders |
| NIH | | National Institutes of Health |
| NIH Guidelines | | NIH Guidelines for Research Involving Recombinant DNA Molecules |
| NK | | natural killer |
| NLC | | nanostructured lipid carrier |
| NMIBC | | non-muscle invasive bladder cancer |
| NOL | | net operating loss |
| NSCLC | | non-small cell lung cancer |
| OBA | | NIH Office of Biotechnology Activities |
| Oberland | | Oberland Capital Management LLC and its affiliates (including Purchasers as
defined in the RIPA) |
| OFAC | | U.S. Treasury Department’s Office of Foreign Assets Control |
| OSHA | | Occupational Safety and Health Administration |
| OWS | | Operation Warp Speed |
| PCAOB | | Public Company Accounting Oversight Board (United States) |
| PDMA | | U.S. Prescription Drug Marketing Act |
| PDUFA date | | user fee goal date |
| PF | | physical function |
| PHI | | Protected Health Information |
| PHSA | | Public Health Service Act |
| PIK | | paid-in-kind |
| PMA | | premarket approval |
| PREA | | Pediatric Research Equity Act |
| PRO | | Patient Recorded Outcomes |
| Proxy Statement | | Schedule 14A in connection with our 2024 Annual Meeting of Stockholders |
| QMSR | | Quality Management System Regulation |
| QSR | | Quality System Regulation |
| R&D | | research and development |
| R&E | | research and experimental expenditures |
| RAC | | Recombinant DNA Advisory Committee |
| RECIST | | response evaluation criteria in solid tumors |
| REMS | | Risk Evaluation and Mitigation Strategy |
| RIPA | | Revenue Interest Purchase Agreement |
| Riptide | | Riptide Bioscience, Inc. |
| RSU | | restricted stock unit |
| Sarbanes-Oxley | | Sarbanes-Oxley Act of 2002 |
| saRNA | | self-amplifying RNA |
| SARS-CoV-2 | | novel strain of the coronavirus (COVID-19) |
| | | | | | | | |
| Term | | Definition |
| | |
| SBRT | | stereotactic body radiotherapy |
| SEC | | U.S. Securities and Exchange Commission |
| Section 404 | | Section 404 of the Sarbanes-Oxley Act of 2002 |
| Securities Act | | Securities Act of 1933, as amended |
| SCLC | | small cell lung cancer |
| Sorrento | | Sorrento Therapeutics, Inc. |
| SPOA | | Stock Purchase and Option Agreement |
| SRLY | | separate return limitation year |
| SWOG | | SWOG Cancer Research Network |
| TAA | | tumor-associated antigen |
| TCJA | | Tax Cuts and Jobs Act of 2017 |
| Term SOFR | | Term Secured Overnight Financing Rate |
| Test Date | | when used in connection with the RIPA, December 31, 2029 |
| TLR | | toll-like receptor |
| Treg | | Regulatory T cells |
| U.S. GAAP | | accounting principles generally accepted in the United States of America |
| USPTO | | U.S. Patent and Trademark Office |
| | |
| VBC Holdings | | VBC Holdings, LLC, a subsidiary of the company |
| VIE | | variable interest entity |
| Viracta | | Viracta Therapeutics, Inc. |
| VivaBioCell | | VivaBioCell, S.p.A., a wholly-owned subsidiary of VBC Holdings |
PART I
ITEM 1. BUSINESS.
Forward-Looking Statements
This Annual Report on Form 10-K (Annual Report) contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (Securities Act), and Section 21E of the Securities Exchange Act of 1934, as amended (Exchange Act), that are based on our management’s beliefs and assumptions and on information currently available to our management. Forward-looking statements may include, but are not limited to:
•our ability to develop next-generation therapies and vaccines that complement, harness, and amplify the immune system to defeat cancers and infectious diseases;
•our ability to obtain funding foradditional financing to fund our operations including funding necessary toand complete furtherthe development and any commercialization of our various product candidates;
•whether or not the FDA will ultimately determine that the BLA resubmission and related actions successfully address and resolve the issues identified in the CRL;
•our ability, and the ability of our third-party CMOs, to adequately address the issues raised in the FDA’s CRL;
•whether the FDA approval milestone after which Oberland may purchase $100.0 million in Revenue Interests will be achieved;
•our ability to meet our payment obligations under the RIPA and to service the interest on our related-party promissory notes and repay such notes, to the extent required;
•our ability to comply with the terms, conditions, covenants, restrictions, and obligations set forth in the RIPA and related transaction documents;
•our expectations regarding the potential benefits of our strategy and technology;
•our ability to forecast operating results and make period-to-period comparisons predictive of future performance due to fluctuations in warrant values;
•our expectations regarding the operation and effectiveness of our product candidates and related benefits;
•our ability to utilize multiple modes to induce cell death;
•our beliefs regarding the benefits and perceived limitations of competing approaches, and the future of competing technologies and our industry;
•details regarding our strategic vision and planned product candidate pipeline, including that we eventually plan to advance vaccines and therapies for virally-induced infectious diseases;pipeline;
•our beliefs regarding the success, cost and timing of our product candidate development activities and current and future clinical trials and studies, including study design and the enrollment of patients;
•the timing of the development and commercialization of our product candidates;
•our expectations regarding our ability to utilize the Phase 1/2I/II aNK and haNK® clinical trials data to support the development of our product candidates, including our haNK, taNK, t‑haNK™, MSC, and M-ceNK™ product candidates;
•our expectations regarding the development, application, commercialization, marketing, prospects and use generally of our product candidates, including Anktiva,™ (N-803), saRNA, hAd5 and yeastsaRNA constructs, recombinant subunit proteins, toll-like receptor-activating adjuvants, and aldoxorubicin;PD-L1 t‑haNK and M-ceNK;
•the timing or likelihood of regulatory filings or other actions and related regulatory authority responses, including any planned investigational new drug (IND), Biologics License Application (BLA)IND, BLA or New Drug Application (NDA)NDA filings or pursuit of accelerated regulatory approval pathways or orphan drug status and Breakthrough Therapy designations;
•our ability to implement and support our SARS-CoV-2 (COVID‑19) vaccine and therapeutic programs;
•our ability to implement an integrated discovery ecosystem and the operation of that planned ecosystem, including being able to regularly add neoepitopes and subsequently formulate new product candidates;
•the ability and willingness of strategic collaborators to share our vision and effectively work with us to achieve our goals;
•the ability and willingness of various third parties to engage in research and developmentR&D activities involving our product candidates, and our ability to leverage those activities;
•our ability to attract additional third-party collaborators;
•our expectations regarding the ease of administration associated with our product candidates;
•our expectations regarding patient compatibility associated with our product candidates;
•our beliefs regarding the potential markets for our product candidates and our ability to serve those markets;
•our expectations regarding the timing of enrollment and submission of our clinical trials, and protocols related to such trials;
•our ability to produce an antibody cytokineantibody-cytokine fusion protein, a DNA, RNA, or recombinant protein vaccine, a toll-like receptor-activating adjuvant, an NK-cell or T-cell therapy, or a damage-associated molecular patterns (DAMP) inducercell therapy;
•our beliefs regarding the potential manufacturing and distribution benefits associated with our product candidates, and our abilitythird-party CMOs’ abilities to follow cGMP standards to scale up the production of our product candidates;
•our plans regarding our manufacturing facilities and our belief that our manufacturing is capable of being conducted in‑house;
•our belief in the potential of our antibody cytokineantibody-cytokine fusion proteins, DNA, RNA, or recombinant protein vaccines, toll-like receptor-activating adjuvants, NK-cellor cell therapies, or DAMP inducer platforms, and the fact that our business is based upon the success individually and collectively of these platforms;
•our belief regarding the magnitude or duration for additional clinical testing of our antibody cytokineantibody-cytokine fusion proteins, DNA, RNA or recombinant protein vaccines, toll-like receptor-activating adjuvants, NK-cellor cell therapies, or DAMP inducers along with other product candidate families;
•even if we successfully develop and commercialize specific product candidates like our N-803 or PD-L1 t‑haNK, our ability to develop and commercialize our other product candidates either alone or in combination with other therapeutic agents;
•the ability to obtain and maintain regulatory approval of any of our product candidates, and any related restrictions, limitations and/or warnings in the label of any approved product candidate;
•our ability to commercialize any approved products;
•the rate and degree of market acceptance of any approved products;
•our ability to attract and retain key personnel;
•the accuracy of our estimates regarding our future revenue, as well as our future operating expenses, capital requirements and needs for additional financing;
•our ability to obtain, maintain, protect, and enforce intellectual propertypatent protection and other proprietary rights for our product candidates and technology and not infringe upon, misappropriate or otherwise violate the intellectual property of others;technologies;
•the terms and conditions of licenses granted to us and our ability to license additional intellectual property relating to our product candidates and technology;
•any government shutdown, which could adversely affect the U.S. and global economies, and materially and adversely affect our business and/or our BLA submission;
•the impact on us, if any, if the contingent value rights (CVRs)CVRs held by former Altor BioScience Corporation (Altor) stockholders become due and payable in accordance with their terms; and
•regulatory developments in the United States (U.S.)U.S. and foreign countries; andcountries.
•Table ofContentsany impact of the coronavirus pandemic, or responses to the pandemic, on our business, clinical trials or personnel.Forward-looking statements include statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “continues,” “goal,” “could,” “seeks,“estimates,” “estimates,“scheduled,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “indicate,” “projects,” “seeks,” “should,” “will,” “would,” “strategy,” and variations of such words or similar expressionsexpressions. and the negatives of those terms. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. Statements of past performance, efforts, or results of our preclinical and clinical trials, about which inferences or assumptions may be made, can also be forward-looking statements and are not indicative of future performance or results. These statements are based upon information available to us as of the date of this Annual Report, and although we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted a thorough inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and investors are cautioned not to unduly rely upon these statements.
This Annual Report also contains estimates, projections and other information concerning our industry, our business, and the markets for certain diseases, including data regarding the estimated size of those markets, and the incidence and prevalence of certain medical conditions. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market, and other data from reports, research surveys, studies, and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data, and similar sources.
Forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. We discuss these risks in greater detail in Item 1A. “Risk Factors” of this Annual Report. Given these uncertainties, you should not place undue reliance on these forward-looking statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame. Also, forward-looking statements represent our management’s beliefs and assumptions only as of the date of this Annual Report. Except as required by law, we assume no obligation to update these forward-looking statements, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future. You should read this Annual Report completely and with the understanding that our actual future results may be materially different from what we expect.
Anktiva, ceNK, Conkwest, GlobeImmune, GlobeImmune (logo), haNK, haNK (Chinese characters), ImmunityBio, NantKwest, Anktiva, VesAnktiva, ThAnktiva, NK-92, ceNK, M-ceNK, haNK, taNK, t-haNK, GlobeImmune, Tarmogen, VivaBioCell, Nant001, NantXL, Nant Cancer Vaccine, QUILT, IPRT, Outsmart your disease, taNK, Tarmogen, VesAnktiva,Your Disease, Smart Therapies for Difficult Diseases, and VivaBioCellNature’s First Responder are trademarks or registered trademarks of ImmunityBio, Inc., its subsidiaries, or its affiliates.
Our product candidates, including N-803, are investigational.investigational agents that are restricted by federal law to investigational use only. Safety and efficacy have not been established by any agency, including the U.S. Food and Drug Administration (FDA).FDA.
This Annual Report contains references to our trademarks and trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this Annual Report, including logos, artwork, and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us, by any other companies.
In this Annual Report, “ImmunityBio,” “the company,” “the combined company,” “we,” “us,” and “our” refer to ImmunityBio, Inc. and its subsidiaries.
Our Business
ImmunityBio, Inc. is aWe are an integrated clinical-stage biotechnology company discovering, developing, and commercializing next-generation immuno- and cellular therapies that bolster the natural immune system to drive and sustain an immune response. Using our proprietary platforms that amplify both the innate and adaptive branches of the immune system, our teams of clinical, scientific, and manufacturing experts, advance novel therapies and vaccines that complement, harness,aimed at defeating urologic and amplify the immune system to defeatother cancers, andas well as infectious diseases. We striveAlthough such designations may not lead to be a vertically-integrated immunotherapy company designingfaster development process or regulatory review and manufacturingmay not increase the likelihood that a product candidate will receive approval, N-803 (Anktiva), our products so they are more effective, accessible, more conveniently stored,lead biologic commercial product candidate, has received Breakthrough Therapy and more easily administered to patients.Fast Track designations and is currently under review by the FDA for treatment in combination with BCG of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease and has a new user fee goal date (PDUFA date) of April 23, 2024.
Our broad immunotherapyplatforms and cell therapy platformstheir associated product candidates are designed to attack cancer and infectious pathogens by activating both the innate immune system—natural killer (NK)system, including—NK cells, dendritic cells, and macrophages—and macrophages, as well as—the adaptive immune system—system comprising—B cells and T cells—cells,—in an orchestrated manner. The goal of this potentially best-in-class approach is to generate immunogenic cell death thereby eliminating rogue cells from the body whether they are cancerous or virally infected.virally-infected. Our ultimate goal is to employovercome the limitations of current treatments, such as checkpoint inhibitors, and/or reduce the need for standard high-dose chemotherapy in cancer by employing this coordinated approach to establish an “immunological memory” that confers long-term benefit for the patient.
Our business is based on the foundation of multiple platforms that collectively act on the entire immune response with the goal of targeted, durable, coordinated,biologic product candidates include: (i) antibody-cytokine fusion proteins, (ii) DNA, RNA, and safe immunity against disease.recombinant protein vaccines, and (iii) cell therapies. These platforms and their associated product candidates are designed to overcome the limitations of the current standards of care in oncology and infectious diseases, such as checkpoint inhibitors and antiretroviral therapies. Our portfolio includes:
Our platforms includehave generated 9 first-in-humannovel therapeutic agents thatfor which clinical trials are currently being studiedeither underway or planned in 26solid and liquid tumors. Specifically, our clinical trials—17 offocus includes bladder, lung, and colorectal cancers and GBM, which are in Phase 2 or 3 development—across 12 indications in liquid and solid tumors, including bladder, pancreatic and lung cancers. These are among the most frequent and lethal cancer types, for whichand where there are high failure rates for existing standards of care or in some cases, no available effective treatment. In infectious diseases, our pipeline currently targets such pathogens as the novel strain of the coronavirus (SARS-CoV-2) and human immunodeficiency virus (HIV). We believe SARS-CoV-2 currently lacks a vaccine that provides long-term protection against the virus, particularly its variants, while HIV affects tens of millions of people globally and currently has no known cure.
We believe that our innovative approach to orchestrate and combine therapies for optimal immune system response will become a therapeutic foundation across multiple clinical indications. Additionally, we believe that data from multiple clinical trials indicates N-803 has broad potential to enhance the activity of therapeutic monoclonal antibodies (mAbs), including checkpoint inhibitors (e.g., Keytruda), across a wide range of tumor types. N-803 is currently being studied in 21 clinical trials (both ImmunityBio and investigator-sponsored) across 12 indications. Although such designations may notOur lead to a faster development process or regulatory review and may not increase the likelihood that abiologic commercial product candidate will receive approval, Anktiva ImmunityBio’s novel antibody cytokineis an IL-15 superagonist antibody-cytokine fusion protein, has received Breakthrough Therapy and Fast Track designations from the FDA in combination with bacillus Calmette-Guérin (BCG) for the treatment of patients with BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without Ta or T1 disease.protein. In May 2022, we announced the submission of a Biologics License Application (BLA)BLA to the FDA for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In JulyOn May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. The deficiencies in the CRL related to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolution of the observations noted at the pre-license inspection would be required before the BLA could be approved. At the time, the FDA further provided recommendations specific to additional CMC issues and assays to be resolved. The CRL did not request new preclinical studies or Phase III clinical trials to evaluate safety or efficacy. The FDA requested that the company provide updated duration of response data for the efficacy population as identified by the FDA in the company’s resubmission, as well as a safety update.
On October 23, 2023, we announced that we had completed the resubmission of the BLA addressing the issues in the CRL. As part of our resubmission, we provided an update of the duration of response regarding the responders identified by the FDA in the efficacy population for BCG unresponsive subjects with high-risk CIS disease. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a target Prescription Drug User Fee Act (PDUFA) actionnew user fee goal date (PDUFA date) of MayApril 23, 2023.2024. While we believe the BLA resubmission addresses the issues identified in the CRL, there is no guarantee that the FDA will ultimately agree that such issues have been successfully addressed and resolved. It is unclear when the FDA will approve our BLA, if at all.
Further late-stage efforts for Anktiva are in development within the broader bladder cancer space, including BCG-naïve NMIBC. In addition, data from multiple clinical trials suggest N-803 has potential to enhance the activity of therapeutic mAbs, including checkpoint inhibitors (e.g., pembrolizumab/Keytruda), across a wide range of tumor types. We believe there is potential for N-803 to become a therapeutic foundation across all phases of treatment, including in adjunctive therapy, to amplify, reactivate or extend the efficacy of standard of care. In addition to N-803, we have active clinical programs evaluating therapeutic candidates from our DNA and RNA vaccine technology platforms and our NK cell-based therapy platforms in oncology and infectious disease indications.
We have established Good Manufacturing Practice (GMP) manufacturing capacityOn December 29, 2023, we entered into the RIPA with Infinity and Oberland. Pursuant to the RIPA, Oberland acquired certain Revenue Interests (as defined in the RIPA) from us for a gross purchase price of $200.0 million paid on closing, less certain transaction expenses. In addition, Oberland may purchase additional Revenue Interests from us in exchange for the $100.0 million Second Payment upon satisfaction of certain conditions specified in the RIPA, including following the receipt of approval by the FDA of the company’s BLA for N-803 in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease on or before June 30, 2024 (the Second Payment). Under the RIPA, Oberland has the right to receive quarterly payments from us based on, among other things, a certain percentage of our worldwide net sales, excluding those in China, during such quarter.
Also, on December 29, 2023 and in connection with the RIPA, we entered into an SPOA with Oberland pursuant to which we sold an aggregate of approximately $10.0 million of our common stock at scale with cutting-edge cell manufacturing expertise and ready-to-scale facilities, as well as extensive and seasoned research and development (R&D), clinical trial, and regulatory operations and development teams.$4.1103 per share in a private placement. Oberland also has an option to purchase up to an additional $10.0 million of our common stock, at a price per share to be determined by reference to the 30-day trailing volume weighted-average price of our common stock calculated from the date of exercise.
I.Our Strategy
We seek to become thea leading global immunological therapeutics company by creating the next generation of immunotherapiesnext-generation immuno- and cell therapies to address serious unmet needs within oncologyurologic and other cancers as well as infectious diseases. To achieve this goal, the key elements of our strategy include:
•advancing the approval and commercialization of our lead antibody cytokineIL-15 superagonist antibody-cytokine fusion protein, N-803, as an integral component of immunotherapy combinations, including those with checkpoint inhibitors;
•continuously scrutinizing our clinical pipeline and assessing our strategic priorities to maximize opportunities for regulatory approval and to meet unmet medical needs;pipeline;
•accelerating product candidates generated from our immunotherapy platform and product candidatesplatforms with registrational intent to address difficult-to-treat oncological and infectious disease indications;
•continuing to prospect, license, and acquire technologies to complement and strengthen our platforms and product candidates, both as single agent and combination therapies, in order to activate and coordinateoptimize responses of the innate and adaptive immune systemsystems to generate cellular memory against multiple tumor types and infectious diseases;
•optimizing investmentinvesting in our discovery, development, and manufacturing capabilities for our next-generation targeted antibody cytokine fusion and recombinant proteins and vaccine candidates, as well as for cell therapies;
•advancing our formulations and delivery mechanisms to make our promising biotechnology product candidates available to the broadest population possible;in both oncology and infectious disease; and
•cultivating new and expanding existing collaborations for our multi-stage pipeline to efficientlyreach global scale globally.efficiently.
II.Our Next-Generation Platforms
Antibody-Cytokine Fusion Proteins
1.Antibody Cytokine Fusion Proteins
Antibody cytokineAntibody-cytokine fusion proteins, such as N-803, are a novel class of biopharmaceuticalsbiologics that enhance the therapeutic potential of cytokines, and promote lymphocyte infiltration at a site of disease, improving immune response. N-803 is a novel interleukin-15 (IL-15)an IL-15 superagonist fusion protein consisting of high-affinity mutant IL-15N72D fused to the IL-15 receptor α sushi subunit and linked to the Fc portion of IgG1 Fc.Fc that exerts its effects via enhanced IL-2 receptor The novel antibody cytokine fusion proteinβ site binding. N-803 specifically increases the activityproliferation and activation of two critical aspectscell types of the immune system—system – NK cells and cytotoxic (tumor cell killing) CD8 T-cells—and exerts its effects via enhanced IL-2 receptor bindingCD8+T-cells – but not immunosuppressive T-reg cells, leading to the β site resulting in generationestablishment of Killer T cells and not the immunosuppressive regulatory T-cell (T-reg) cells. This superagonist IL-15 activity is the first in class to activate and proliferate NK cells, CD8+ Killer T cells, and Memorymemory T cells. Anktiva has received Breakthrough Therapy and Fast Track designations by the FDA for the treatment of BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease as well as Fast Track designation for BCG-unresponsive NMIBC papillary and BCG-naïve NMIBC with CIS. We believe this fusion protein combined with our natural killer cell therapy platform and our DNA/RNA vector delivery platforms places ImmunityBio at the leading edge to successfully deliver a cancer vaccine for durable complete responses across multiple tumor types. Althoughnote such designations may not lead to a faster development process or regulatory review and may not increase the likelihood that a product candidate will receive approval Anktiva has received Breakthrough Therapy and Fast Track designations by the FDA for the treatment of BCG-unresponsive NMIBC CIS with or without Ta or T1 disease as well as Fast Track designation for BCG-unresponsive NMIBC papillary and BCG-naïve NMIBC CIS..
In May 2022, we announced the submission of a BLA to the FDA for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In July 2022,As described above, on October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a target PDUFA actionnew user fee goal date (PDUFA date) of MayApril 23, 2023.2024. It is unclear when the FDA will approve our BLA, if at all.
We believe that other N-803 indications with registration potential include BCG-unresponsive papillary, bladder cancer,BCG-naïve CIS and BCG-naïve papillary, lung cancer, pancreatic cancer, and glioblastoma multiforme (GBM)colorectal cancers, and GBM in oncology and HIV in infectious diseases.
N-803
In addition to N-803, we are developing bi-specific fusion proteins targeting CD20, PD-L1, IL-12, and TGF-ß to further enhance NK and T-cell activation directed to the infectious disease or tumor microenvironment, and to modulate the systemic and local immune response to accelerate immunogenic cell death. Prioritized product candidates in preclinical development include antibody cytokineantibody-cytokine fusion proteins N-820 (targeting CD20), N-809 (targeting PD-L1), N-812 (delivering IL-12 to necrotic tumor cells), and N-830 (delivering a TGF-ß Trap to necrotic tumor cells).
2.DNA, RNA, and Recombinant Protein Vaccine TechnologiesVaccines
We have developed andand/or acquired rights to multiple vaccine delivery technologies for oncology to deliver common TAAs, and neoepitopes (expressed only by cancer cells) and for infectious diseases to target key viruses, including SARS-CoV-2, and for oncology to deliver common tumor-associated antigens (TAAs), and neoepitopes (expressed only by cancer cells).SARS-CoV-2. These technologies can deliver DNA, self-amplifying RNA (saRNA),saRNA, and subunit proteins to induce B- and T-cell memory due to the activation of both CD4+ and CD8+ T cells along with antibody (humoral) responses. In SARS-CoV-2, we believe
Our key vaccine delivery technologies include:
a.Second-generation hAd5 vector
Adenovirus is a well-established viral vector and can be utilized as a vaccine platform to stimulate the focus on targeting a highly mutable S-protein and the associated antigenic drift with limited memory T-cell response is leading to a potential for emergence of resistance to the current generation of vaccines. A booster vaccine targeting the N protein, which is highly conservedimmune system. Our hAd5 technology has unique deletions in the coronavirus genusearly 1, (E1), early 2 (E2b) and is oneearly 3 (E3) regions (hAd5 [E1-, E2b-, E3-]), which allows it to be effective in the presence of pre-existing adenovirus immunity and lowers the most abundant structural proteinsrisk of generating de novo vector-directed immunity. We have developed several hAd5 product candidates that have been studied in virus-infected cells, could provide maximal immune protection against currentmultiple clinical trials as potential vaccines for the treatment of infectious diseases and future COVID-19 variants such as Deltacertain cancers. Importantly, these product candidates have shown an ability to overcome previous adenovirus immunity in preclinical models and Omicron and their associated sub-lineage variants of concern.in cancer patients. In oncology, we are exploring the delivery of N-803 in combination with hAd5 TAAs like E6/E7, CEA, MUC1, Brachyury, [E6/E7] and PSA, which we believe could yield immunological memory.Our key vaccine delivery technologies include:
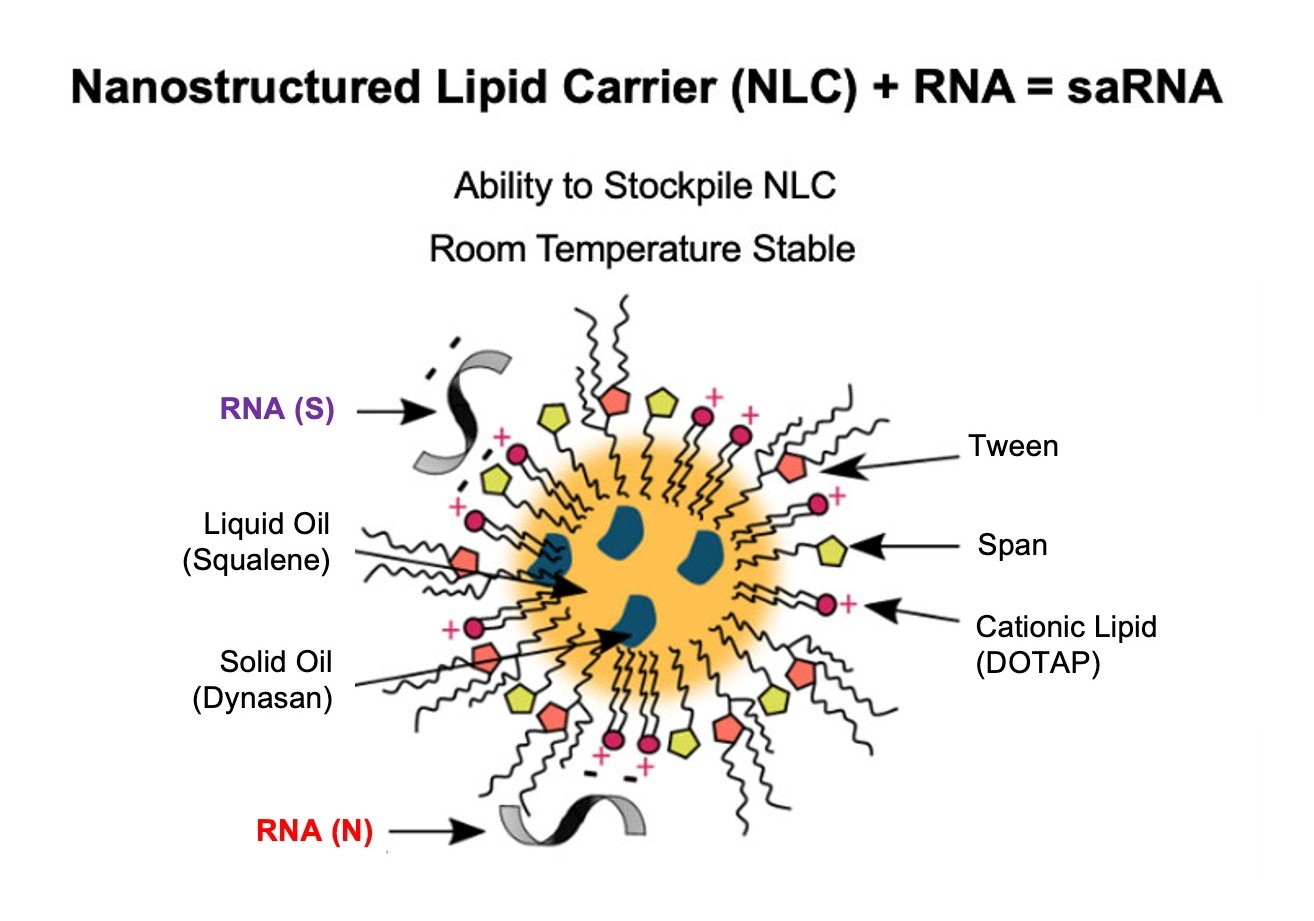
a.b.Self-amplifying RNAsaRNA in an NLC formulation
Synthetic RNA technology has quickly emergedcome to the forefront for use in prophylactic and therapeutic vaccines in part because it allows for rapid, scalable, and cell-free manufacturing as evidenced by the adoption of SARS-CoV-2 RNA vaccines. We believe ourOur saRNA technology (licensed from the Access to Advanced Health Institute (AAHI) (formerlyAAHI, formerly known as the Infectious Disease Research Institute, or IDRI), both directlyincludes an NLC formulation that protects the saRNA cargo and in the case of SARS-CoV-2, through our joint venture formed with Amyris, Inc. (Amyris) in December 2021) represents a significant improvement over existing RNA technologies. Our saRNA constructs include a nanostructured lipid carrier (NLC) formulationis important for thermal stability and facilitating thestability. The platform technology facilitates substitution of genetic sequences to generate novel vaccines and have demonstratedhas an ability to vaccinate with multivalent strains. The self-replicating capability allows for increased potency by maintaining auto-replicative activity derived from the RNA virus vector, while the self-amplifying capability may increase the duration and breadth of immunity. Preclinical studies in small animal and non-human primate (NHP)NHP models have shown that theNLC saRNA delivery vehicle results inelicits potent humoral and cell-based immunogenicity. Phase 1I first-in-human trials of saRNA for COVID-19 began in 2022 and continues in the first quarter of 2023.2022. Results to date have demonstrated limited adverse events of the saRNA S construct and the trial is currently evaluating the safety and immunogenicity of our saRNA N construct in a similar dose escalation manner. Once Phase 1 is complete, the results will be usedare being analyzed to inform the Phase 2 design for optimal immunogenicity.
b.Second-generation adenovirus hAd5 vector
Adenovirus is a well-established viral vectorfuture studies in oncology and can be utilized as a vaccine to stimulate the immune system. Our human adenovirus serotype 5 (hAd5) technology has unique deletions in the early 1, (E1), early 2 (E2b) and early 3 (E3) regions (hAd5 [E1-, E2b-, E3-]), which allows it to be effective in the presence of pre-existing adenovirus immunity and lowers the risk of generating de novo vector-directed immunity. We have developed several hAd5 product candidates, which have been studied in multiple clinical trials as potential vaccines for the treatment of infectious diseases and certain cancers. Importantly, these product candidates have shown an ability to overcome previous adenovirus immunity in preclinical models and in cancer patients.
In oncology, we are exploring the delivery of N-803 in combination with hAd5 TAAs like HPV E6/E7, CEA, MUC1, Brachyury, and PSA, which we believe could yield immunological memory.disease.
c.Recombinant protein vaccine platforms
Our yeast vaccine platforms have been studied in both oncology and infectious diseases:
(i)diseases, including our Tarmogen platform (licensed from our subsidiary GlobeImmune, Inc. (GlobeImmune))GlobeImmune), which has been administered to over 400 patients with cancer or infectious diseases in FDA-regulated clinical trials. This platform technology consists of a heat-killed, recombinant S cerevisiae yeast-based vaccine engineered to express immunogens such as TAAs, pathogen antigens, and tumor-specific neoepitopes. Immunization with this platform elicits CD4+ and CD8+ T cell responses capable of eliminating tumor cells or pathogen-infected cells.
(ii)our pichia platform was used to produce RBD antigens (licensed from Baylor College of Medicine (BCM) (which was developed at the Texas Children’s Hospital Center for Vaccine Development)), which, when combined with the 3M-052/Alum adjuvants and related technology (licensed from AAHI and 3M Company (3M) and affiliates), had been shown to provide protection against SARS-CoV-1, SARS-CoV-2 (and variants of concern), and animal coronaviruses in preclinical models.
3.Toll-Like Receptor Activating Adjuvants
Adjuvants are either synthetic or naturally occurring moleculesWe believe that activate toll-like receptors (TLRs) thereby enhancing the humoral and cell-mediated immune response of vaccines. There are 10 human TLRs expressed either on the inside or outside of the immune cell and their function is to recognize foreign substances expressed by pathogens. Once activated, these TLRs stimulate danger signals to the immune cells initiating an immune response. Wewe have licensed adjuvants and related technology from AAHI and 3M and its affiliates to incorporate with the vaccine delivery platforms described above and have access to multiple toll receptor activators, including TLR 4, TLR 7, and TLR 8. The synthetic imidazoquinolinone 3M-052 is structurally similar to resiquimod. The 3M-052/Alum adjuvant formulation is in Phase 1 trials in the U.S. with an HIV antigen and has been well-tolerated and immunogenic.
4.NK Cell Therapy
ImmunityBio has one of the most comprehensive clinical-stage natural killer cellcell-based platforms which hasin development. Our engineered NK cells have demonstrated the ability to induce cell death in cancers and virally-infected cells through a variety of concurrent mechanisms including innate killing, antibody-mediated killing, chimeric antigen receptor (CAR)-directedCAR-directed killing, and a combination of both antibody-mediated and CAR-directed killing.the latter.
a.Off-the-shelf natural killer (NK)NK cells
Natural killerNK cells (NK cells) are a type of cytotoxic lymphocyte critical to the innate immune system. NK cells show spontaneous cytolytic activity against cells under stress such as tumor cells and virally-infected cells. After activation, NK cells also secrete several cytokines such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), granulocyte macrophage colony-stimulating factor (GM-CSF)(GM‑CSF), and chemokines that can modulate the function of other innate and adaptive immune cells. CytotoxicOur NK-92 cytotoxic cell lines (including our NK-92) have beenline was established from patientsa patient with clonal NK-cell lymphoma. ThoseThese NK cells can be expanded in culture in the presence of cytokines (IL-2, IL-15). Our “off-the-shelf” NKaNK cell platform has been molecularly engineered in a variety of ways to boost its killing capabilities against cancers and virally-infected cells. Unlike normal natural killerNK cells, our aNK cells do not express the key inhibitory receptors that diseased cells often exploit to turn off the killing function of NK cells and escape elimination. Further, wWee have genetically engineered our aNK cell platform to overexpress high-affinity CD16 receptors that bind to antibodies. These antibody-targeted haNK cells are designed to directly bind to IgG1-type antibodies, such as avelumab, trastuzumab, cetuximab, and rituximab, with the intention of enhancing the cancer-killing efficacy of these antibodies by boosting the population of competent natural killerNK cells that can kill cancer cells through antibody dependent cellular cytotoxicity (ADCC).ADCC.
Our most advanced line of off-the-shelf product candidatesNK cell, t-haNK, is an innovative, bioengineered combinationcell line that incorporates all the features of our haNK platform together with a CAR, (t-haNK).such as programmed PD-L1. Product candidates under this platform have three modes of killing: innate, antibody-mediated, and CAR-directed killing. These product candidates also include one or more additional expression elements such as functional cytokines, chemokines, and trafficking factors. These product candidates are intended to be combined with commercially availablecommercially-available therapeutic antibodies to effectively target either two different epitopes of the same cancer-specific protein or two entirely different cancer specificcancer-specific proteins. Trials studyingClinical trials to assess our t-haNKt‑haNK product candidates were initiated—PD-L1 t-haNK in a Phase 1I trial in triple negativetriple-negative breast cancer, (TNBC), and a Phase 2II trial in pancreatic cancer—and CD19 t-haNK has been cleared to commence Phase 1I testing.
InFindings from a preclinical study performed in collaboration with the National Cancer Institute (NCI),NCI demonstrated our PD-L1 t-haNK was reported to be acells exert potent cell therapy agentantitumor effects against myeloid-derived suppressor cells (MDSC)MDSC and overcome T cell escape in multiple types of resistant tumors (Fabian et al 2020). Resultstumors. The contribution of the QUILT 88 trial of patients with advanced pancreatic cancerPD-L1 t‑haNK to antitumor efficacy is further evidenced by data reported at the American Society of Clinical Oncology (ASCO)ASCO meeting in June 2022 and updated at ASCO GI in January 2023 showedfrom the QUILT 88 trial of patients with advanced pancreatic cancer who were administered PD-L1 t-haNK, aldoxorubicin and N-803. The multi-modal therapy resulted in a median overall survival of 6.3 months (95% CI: 5.0, 7.2 months) in patients who had progressed after two prior lines of therapy, more than doubling the historical survival.survival rate.
We believe we have aour pipeline ofthat includes t-haNK cells engineered to express other prominent CARs, for t-haNK, including epidermal growth factor receptor (those targeting EGFR),EGFR, which is advancing through clinical-enabling studies, which will enable usfacilitate our ability to potentially address an even broader range of cancers as part of a chemotherapy-free combination regimen.
b.Autologous and allogenic memory-like cytokine-enhanced NK cells (M-ceNK)allogeneic M-ceNK
Memory-like cytokine-enhanced NKM-ceNK cells are a unique set ofgenerated from lymphocytes collected from donors that differentiate after a brief pre-activation with interleukin-12are then pre-activated ex-vivo by exposure to interleukins-12 (IL-12), IL-15-15 and IL-18-18, which results in differentiation and exhibitacquisition of enhanced responses to cytokine re-stimulation that includere-stimulation. M-ceNK have increased antitumor characteristics, including enhanced IFN-γ production and cytotoxicity against leukemic cell lines. TheseM-ceNK cells have been isolated and characterizedare further distinguished by their unique cell-surface marker profile and their highly desirable feature of immune-memory, marked by their pronounced anti-cancer activity for weeks to months in duration, which has made these cells a research focus for more than a decade. We have developed a unique ability to generate a portfolio of distinct M-ceNK cell products through the application of our proprietary technology and cytokines and our proprietary methods and overall expertise in scale manufacturing of NK cell-based products. A Phase 1I first-in-human trial is open and actively enrolling patients in a first-in-human trial to study the M-ceNK platform in solid tumors (QUILT 3.076)3076).
5.Damage-Associated Molecular Patterns Inducers
DAMPs are releasedWe anticipate commencement of Phase II trials of M-ceNK plus N-803 in response to cell stresspatients with AML (QUILT 102) and death, and elicit potent sterile inflammation. Recent evidence suggests that DAMPs may also have a key roleplatinum-resistant ovarian cancer (QUILT 108) in the development of cancer as well as in the host response to cytotoxic anti-tumor therapy. DAMPs may play a protective role by alerting the immune system to the existence of dying tumor cells, thereby triggering immunogenic tumor cell death. Albumin-bound immune modulators and tumor associated antigen regulators target delivery of the chemotherapy agent to the tumor microenvironment, activate tumor killing macrophages and/or enable a tumor suppressive microenvironment.near term.
a.Aldoxorubicin
Aldoxorubicin is an albumin-associated anthracycline designed to target immune evasion in cancer. Aldoxorubicin has the same cytotoxic mechanism of action as doxorubicin, which is currently approved for use in 14 indications, including breast cancer, Hodgkin’s lymphoma and small-cell lung cancer (SCLC), but also has unique pharmacological properties resulting in lower cardiotoxicity as shown in Phase 2 and Phase 3 clinical trials for soft tissue sarcoma previously conducted by LadRx Corporation (LadRx, formerly CytRx Corporation). LadRx out-licensed global development, manufacturing, and commercialization rights for aldoxorubicin to us in 2017. The investigative therapeutic is currently in a Phase 2 trial in metastatic pancreatic cancer (QUILT 88) of DAMP inducers combined with N-803and PD-L1 t-haNK to evaluate the safety and efficacy of the combination.
b.Nanatinostat
VRx-3996 (nanatinostat), an orally available histone deacetylase (HDAC) inhibitor, is being developed by Viracta. Nanatinostat is selective for specific isoforms of Class I HDACs, which is key to inducing viral genes that are epigenetically silenced in Epstein-Barr virus-associated malignancies. In preclinical studies, nanatinostat has been shown to reactivate silenced transgenes in tumor cells thereby turning them into preferential targets for NK cell killing, while also serving to broadly stimulate a patient’s immune system, offering the potential for improved clinical responses in cancer patients. The activity of HDAC inhibitors are believed to be based on the upregulation of natural killer group 2D (NKG2D) ligand expression on cancer cells, which serve as “eat-me” signals for NK cells and can drive NK proliferation, activation and cancer cell killing. We entered into an agreement with Viracta under which we were granted exclusive worldwide rights in patents and know-how related to nanatinostat for use in combination with our platform of NK cell therapies.
III.Our Pipeline
OurAs of March 2024, our platforms includehave generated 9 first-in-human therapeutic agents that are currently being or planned to be studied in 2624 clinical trials—17 of which are in Phase 2 or 3 development—trials across 12 indications in liquid and solid tumors, including bladder, pancreaticlung and lung cancers.colorectal cancers, and GBM. These indications are among the most frequent and lethal cancer types for which there are high failure rates for existing standards of care or, in some cases, no available effective treatment. We are constantly monitoring and deciding which trials to initiate or continueprioritizing clinical development based upon the availability of our resources and the efficacy and market developments of our and our competitors’, products and product candidates, among other factors.
1.Non-Muscle Invasive Bladder Cancer
In the U.S., bladder cancer is the fourth most commonly-diagnosed solid malignancy in men and twelfth in women.men. The American Cancer Society estimates there will be 82,29083,190 new cases and 16,71016,840 deaths from bladder cancer in 2023.2024. There is an urgent, unmet need to treat NMIBC and avoid radical cystectomy of the bladder in an attempt to control the disease. Although such designations may not lead to a faster development process or regulatory review and may not increase the likelihood that a product candidate will receive approval, Anktiva has received Breakthrough Therapy and Fast Track designations by the FDA for the treatment of BCG-unresponsive NMIBC with CIS (Cohort A) with or without Ta or T1 disease as well as Fast Track designation for BCG-unresponsive NMIBC papillary (Cohort B) and BCG-naïve NMIBC with CIS. In our QUILT 3.0323032 trial, the company reported in November 2022, as published in NEJM Evidence, that the primary end points were met for both BCG-unresponsive NMIBC with CIS with a complete response rate of 71%, and BCG-unresponsive NMIBC papillary with a 12-month disease-free rate of 55%. As presented at ASCO 2022, the combination of BCG plus AnktivaN-803 (as measured in BCG-unresponsive NMIBC patients, Cohorts A and B combined) was well-tolerated with 1% treatment-related serious adverse events, 0% immune-related serious adverse events, and 100% bladder cancer-specific overall survival at 24 months. Low-grade treatment-related adverse events include dysuria (22%), pollakiuria (20%), hematuria (17%), fatigue (16%), and urgency (12%), and all other treatment-related adverse events were seen at 7% or less. Seminal patents covering intravesical administration of BCG and AnktivaN-803 were issued providing term coverage until 2035.
BCG-UnresponsiveBCG Unresponsive NMIBC CIS (QUILT 3.032)(Cohort A) – QUILT 3032
In our Phase 2/3II/III open-label multi-center trial of BCG-unresponsive high grade NMIBC patients, the patients are receiving BCG plus AnktivaN-803 weekly for six consecutive weeks during induction. The patients also receive additional treatment including three weekly maintenance instillations every three months for up to 12 months and then at month 18. Patients with no disease or low-grade Ta disease at months 24, 30, and 36 are eligible for continued BCG plus AnktivaN-803 (Cohort A) or AnktivaN-803 alone (Cohort C) treatment (3 weekly instillations), at the principal investigators’ discretion.
The primary endpoint of the BCG-unresponsive NMIBC with CIS trial is a CRcomplete response rate at any time equal to or greater than 30% and the lower bound of the 95% confidence intervalCI must be greater than or equal to 20% for success. Complete response, or the disappearance of measurable disease in response to treatment, is evaluated at three months or six months following initial administration of BCG plus AnktivaN-803 (and every three months thereafter until 24 months). This endpoint would be achieved once at least 24 of the 80 patients in the trial achieve a complete response.
All patients enrolled in Cohort A have been treated with the recommended number of full-strength doses of BCG on study during our trial. We have enrolled patients who have received a lower dosage of BCG therapy before enrollment in our trial as a result of BCG shortages. The mean and median number of prior BCG doses in Cohort A is 16.5 and 12.0, respectively, which are consistent with the FDA definition of BCG-unresponsive CIS. The FDA allowed our modification of the study design to allow enrollment of such patients, and definition of these patients may require further discussions with the FDA upon review. A published meta-analysis (Zeng 2015) of six relevant randomized controlled trials and two quasi-randomized controlled trials in NMIBC concluded that low-dose BCG instillation significantly reduces the incidence of overall side effects, especially severe and systemic symptoms in patients with NMIBC, while the oncological control efficacy of low-dose BCG is not inferior to standard-dose BCG. There can be no assurance that the FDA will agree with this conclusion.
A data cutoff occurred in January 2022, which provided a median follow-up in Cohort A of approximately 24 months. Data as published in NEJM Evidence in November 2022 showed a complete response in 58 of 82 patients with a 71% CRcomplete response rate (95% CI: 59.6, 80.3) and a median duration of CR of 26.6 months (95% CI: 9.9, [upper bound not reached]). At 24 months in patients with a complete response, the probability of avoiding cystectomy and disease-specific survival was 91.4% and 100%, respectively. Also, at 24 months in all patients in Cohort A, the probability of avoiding cystectomy and of disease-specific survival was 84.1% and 100%, respectively.
As part of planned analyses, BCG-unresponsive patients in the QUILT 3032 trial of N-803 plus BCG completed PRO questionnaires, which revealed stability of both mean physical function and global health from baseline to 24 months on-study for those participants who had reached the 24-month assessment. Further, at month 6, Cohort A (CIS disease) patients that achieved a complete response reported higher physical function scores than those without a CR (P = 0.0659). Summary scores for the NMIBC-specific questionnaire also remained stable. These PROs, taken together with efficacy findings from both the NEJM Evidence report and subsequent follow-up, suggest a favorable risk-benefit ratio for the novel therapeutic combination.
In May 2022, we announced the submission ofsubmitted a BLA to the FDA for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In July for which we received a target PDUFA action date of May 23, 2023. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. The deficiencies in the CRL related to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolution of the observations noted at the pre-license inspection would be required before the BLA could be approved. At the time, the FDA further provided recommendations specific to additional CMC issues and assays to be resolved. The CRL did not request new preclinical studies or clinical trials to evaluate safety or efficacy. The FDA requested that the company provide updated duration of response data for the efficacy population as identified by the FDA in the company’s resubmission, as well as a safety update.
On October 23, 2023, we announced that we had completed the resubmission of the BLA addressing the issues in the CRL. As part of our resubmission, we provided an update of the duration of response regarding the responders identified by the FDA in the efficacy population for BCG-unresponsive subjects with high-risk CIS disease. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a target PDUFA actionnew user fee goal date (PDUFA date) of MayApril 23, 2023.2024. While we believe the BLA resubmission addresses the issues identified in the CRL, there is no guarantee that the FDA will ultimately agree that such issues have been successfully addressed and resolved. It is unclear when the FDA will approve our BLA, if at all.
BCG-UnresponsiveBCG Unresponsive NMIBC Papillary (QUILT 3.032)(Cohort B) – QUILT 3032
In our Phase 2,II, open-label multi-center trial of BCG-unresponsive high grade NMIBC papillary patients (Cohort B), the patients are receiving BCG plus N-803 weekly for six consecutive weeks during induction. The patients also receive additional treatment including three weekly maintenance instillations every three months for up to 12 months and then every nine months for up to 24 months. The primary endpoint of the trial is a 12-month disease free rate greater than or equal to 30% and the lower bound of the 95% confidence intervalCI must be greater than or equal to 20% for success. To meet the primary endpoint, 24 out of 80 patients must be disease free at 12 months.
A data cutoff occurred in January 2022, which provided a median follow-up in Cohort B of approximately 21 months. Data as published in NEJM Evidence in November 2022 showed a 12-month disease-free survival rate of 55% (95% CI: 42.0, 66.8), with median disease-free survival of 19.3 months (95% CI: 7.4, [upper bound not reached]). At the cutoff date 67 of 72 patients, 93.1%, had not progressed to radical cystectomy and the 24 month24-month disease-free survival rate was 97.7%.
We met with the FDA in December 2022, and the FDA advised us to conductthat when there is a time-to-event endpoint a randomized trial in localizedis required. We are continuing to evaluate trial designs while we work with the FDA on BCG-unresponsive NMIBC papillary disease.
BCG-Naïve (QUILT 2.005)– QUILT 2005
As discussed above, N-803Anktiva has been awarded Fast Track designation by the FDA for the treatment of BCG-naïve NMIBC with CIS. We are currently enrolling patients in our Phase 2bIIb blinded, randomized, two-cohort, open-label, multi-center trial of intravesical BCG plus AnktivaN-803 versus BCG alone, in BCG naïBCG-naïve patients with high-grade NMIBC with CIS (Cohort A) and NMIBC papillary (Cohort B). Planned enrollment for Cohort A (CIS) and Cohort B (papillary) is 366 patients and 230 patients, respectively. As part of our October 2023 BLA resubmission for Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, we provided an update on the long-term follow-up (QUILT 205) of BCG-naïve subjects in QUILT 2005 receiving N-803 plus BCG for CIS± Ta/T1 in the Phase Ib trial, examining the survival of the 9 subjects who entered the trial since 2014. As initially reported in 2021, all 9 subjects (100%) achieved a complete response, and in an 8-year follow up, the 6 evaluable patients remain disease-free (two were deceased from causes other than bladder cancer and one was lost to follow-up) with bladder preservation over a median survival period of 8.8 years.
2.Lung Cancer
According to the American Cancer Society, lung cancer is the second most common cancer in the U.S. In 2023,2024, it is estimated that 238,340234,580 new cases of lung cancer will be diagnosed in the U.S. and 127,070125,070 deaths will be attributed to the disease. Non-small cell lung cancer (NSCLC)NSCLC accounts for about 80% to 85% of all lung cancers diagnoses and there are very few successful treatment options for these patients once the cancer spreads beyond the lungs. The development of checkpoint inhibitors in NSCLC has been revolutionary, doubling the median overall survival in some settings; however, patient response may be short lived, due to late response and/or progression after achieving an initial response. As with bladder cancer, N-803 enhances the proliferation and activation of NK and T cells critical for targeting and killing lung cancer cells. There is therefore a strong rationale to evaluate N-803 in addition to an anti-PD-1 or anti-PD-L1 checkpoint inhibitor for patients with NSCLC who have relapsed after achieving an initial response to PD-1 or PD-L1 checkpoint inhibitor therapy. SCLC accounts for about 10% to 15% of all lung cancers. About two-thirds of patients with SCLC are diagnosed with extensive-stage disease, which is associated with especially poor prognosis, and a median survival of 10–13 months.
Analysis of pooled data from a Phase 1/2I/II trial conducted from January 2016 to June 2017 in 23 patients, and a subsequent investigator-initiated Phase 2II trial conducted by the Medical University of South Carolina, yielded confirmation of activity of the combination of checkpoint inhibitors and N-803 in relapsed NSCLC. In 15 patients with PD-L1 greater than 50%, the overall response rate was 38% and the median overall survival rate was 17.1 months. These preliminary findings were favorable relative to the historical response rate seen in this patient population in the first-line setting with checkpoint inhibitor therapy.
Non-Small Cell Lung Cancer – QUILT 3055
On the basis of these findings discussed above, we initiated a single-arm Phase 2bIIb multi-cohort basket trial (QUILT 3.055) of N-803 and checkpoint inhibitor combinations in patients who have previously received treatment with PD-1/PD-L1 immune checkpoint inhibitors per an FDA-approved indication. Patients enrolled in this trial were eligible if actively progressing on checkpoint inhibitor therapy. Upon enrollment, patients continued on the same checkpoint inhibitor but with the addition of N-803. Despite progressing on checkpoint inhibitor therapy upon entry into the trial, the majority of patients reverted to stable disease and demonstrated durability of stable disease, some extending as long as nine months. Data presented at the ASCO Annual Meeting in 2021 showed that despite the patients’ prior progression on checkpoint inhibitor therapy alone, upon entry into the trial the majority of patients experienced clinical benefit either as stable disease (49%) or a partial response (9%).
Among 140 patients enrolled in QUILT 3.055,3055, the common N-803 attributed grade 1 and 2 adverse events included: injection siteinjection-site reaction (71%), chills (34%), fatigue (27%), pyrexia (24%), flu-like illness (13%), and decreased appetite (10%). A total of 18 grade 3 and 4 adverse events attributed to N-803 have been reported among 16 patients (12%) in the trial as of February 2021. All reported grade 3 and 4 adverse events occurred at a frequency of 5% or less; two patients reported increased alanine amino transferase, increased aspartate amino transferase or increased blood alkaline phosphatase, anemia, injection-site reaction, or injection-site pain. All other occurrences of grade 3 or 4 adverse events that the clinical trial site investigators reported as suspected as being due to N-803 include: decreased lymphocyte count; weight loss; influenza-like illness; injection siteinjection-site pruritus; cellulitis; injection-site cellulitis; sepsis; deep vein thrombosis; hypovolemic shock; colitis; diarrhea; delirium; respiratory failure; and maculopapular rash. Although further studies are warranted, based on this relatively well-tolerated adverse event profile, coupled with NK and CD8+ T cell stimulatory effects, we believe that N-803 has the potential to become a standard in combination with other immunotherapies for multiple indications.
In March 2023, we reviewed the updated QUILT 3055 data, through February 5, 2023, from several cohorts of NSCLC patients who have progressed after check point inhibitor therapy. These cohorts are:
•Cohort 1a – NSCLC patients with initial response on single-agent checkpoint inhibitor therapy and subsequently progressed on or after that therapy.
•Cohort 2 – NSCLC patients having high PD-L1 expression (tumor proportion score ≥50%) and disease progression on a PD-1/PD-L1 checkpoint inhibitor after experiencing an initial response when received checkpoint inhibitor as a single-agent for first-line treatment.
•Cohort 3 – NSCLC patients with initial response but subsequently relapsed on maintenance PD-1/PD-L1 checkpoint inhibitor therapy when initially received checkpoint inhibitor therapy in combination with chemotherapy as first-line treatment.
•Cohort 4 – NSCLC patients currently receiving PD-1/PD-L1 checkpoint inhibitor therapy that progressed after experiencing stable disease for at least 6 months during previous treatment with PD-1/PD-L1 checkpoint inhibitor therapy.
The results are listed in the table below:
QUILT 3055: Overall Survival – NSCLC Subjects Safety Population
| | | | | | | | | | | | | | | | | |
| Variable | Cohort 1a
(N=19) | Cohort 2
(N=9) | Cohort 3
(N=20) | Cohort 4
(N=38) | All Subjects (N=86) |
| | | | | |
| Number of Deaths | 13 (68%) | 6 (67%) | 15 (75%) | 21 (55%) | 55 (64%) |
| Median Survival (months) | 14.1 | 18.5 | 15.8 | 12.6 | 13.9 |
| 95% CI for Median Survival | 11.7, 28.7 | 6.1, – | 7.5, 24.9 | 8.8, 25.5 | 11.7, 17.4 |
These results show a median overall survival of 13.9 months in the 86 patients in the pooled analysis. This is in contrast to the overall survival of 6.1 months reported by Freeman et al. for patients who received any therapy post-checkpoint inhibitor therapy progression or an overall survival of 7.5 months, as reported by Brueckl et al., for patients who received docetaxel plus ramucirumab after initial failure of first-line chemotherapy plus checkpoint inhibitor.
Small Cell Lung Cancer – QUILT 211
Recently approved therapeutics for SCLC include checkpoint inhibitors in combination with platinum-based chemotherapy as first-line and lurbinectedin for second-line therapy. Nonetheless, only a subset of patients respond, a limit attributed to low MHC class I expression in SCLC. NK cells do not require such expression and preclinical studies have demonstrated M-ceNK are effective in killing SCLC cells of all subtypes as well as neuroendocrine prostate cancer. Based on these and similar findings, a Phase III, open-label, randomized clinical trial of N-803 and M-ceNK in combination with standard of care versus standard of care alone for previously treated patients with extensive-stage SCLC was designed, with enrollment expected to commence in 2024. The primary endpoint is the objective response rate with secondary endpoints of progression-free survival, overall survival, duration of response and disease control rate based on RECIST 1.1 criteria, and quality of life based on PROs.
Non-small Cell LungColorectal Cancer (Lung-Map)
In October 2021, we announcedAccording to the American Cancer Society, colorectal cancer is the third-leading cause of cancer-related deaths in the U.S. in men and the fourth-leading cause in women, but it is the second most common cause of cancer deaths when numbers for men and women are combined. Colorectal cancer is expected to cause about 53,010 deaths during 2024.
Lynch syndrome is the most common cause of hereditary colorectal cancer. People with this syndrome are at high risk of developing colorectal cancer. Lynch syndrome causes about 4,300 colorectal cancers per year. These cancers are more likely to develop at earlier ages, often before the age of 50. If someone has Lynch syndrome, it means that N-803 had been chosentheir close relatives (parents, siblings, and children) have a 50% chance of having the mutation that causes it too.
Lynch Syndrome (NCI) – QUILT 5015
The Lynch syndrome trial, sponsored by Lung Cancer Master Protocol (Lung-MAP), a public-private partnership—which includes the NCI, focuses on the National Clinical Trials Network (NCTN) Cooperative Groups (SWOG, ECOG-ACRIN, Alliance, and NRG), Friendsability of Cancer Research, and the Foundation for the National InstitutesTri-Ad5–a combination of Health (FNIH)—to study N-803 in the Lung-MAP trial.
The trial design is a randomized comparison of N-803three vaccines targeting different TAAs–used in combination with Keytruda® (pembrolizumab) comparedN-803 to standardreduce the incidence of care chemotherapy optionsonset of cancer. People with Lynch syndrome harbor mutations in mismatch repair genes that put them at high risk for development of cancer, particularly colon cancer. Recently, we announced full accrual of participants in the first two separate cohorts:
•Primary Resistance (Cohort 1). Disease progression during or after anti-PD-1 or anti-PD-L1 therapy that occurred less than or equal to (≤) 84 days following initiation (Cycle 1 Day 1)open-label phases of anti-PD-1 or PD-L1 therapy (combination or monotherapy).
•Acquired Resistance (Cohort 2). Disease progression during or after anti-PD-1 or anti-PD-L1 therapy that occurred more than (>) 84 days following initiation (Cycle 1 Day 1) of anti-PD-1 or PD-L1 therapy (combination or monotherapy).
the Lynch syndrome trial. The trial will includeplans to enroll up to 478 second-line patients with tumors that are not targetable with a drug, which accounts for186 participants, and the majorityrandomized controlled portion of NSCLC cases. In April 2022, we announced that the first participants had been enrolled in the trial.
Non-small Cell Lung Cancer (QUILT 2.023)
On the basis of earlier trials in NSCLC, and our belief that N-803 enhances the proliferation and activation of NK and T cells, we enrolled patients in a randomized Phase 3 trial to evaluate N-803 plus checkpoint inhibitor combinations versus other checkpoint inhibitor combinations in the first-line setting for NSCLC in the cohorts described below:
•Immunotherapy for either squamous or non-squamous NSCLC with PD-L1 TPS >1% (Cohort A). Combination of N-803 with Keytruda versus Keytruda as the standard of care control arm in this randomized trial.
•Chemoimmunotherapy for squamous NSCLC (Cohort B). Combination of N-803 with Carboplatin, Abraxane® and Keytruda versus Carboplatin, Abraxane / paclitaxel and Keytruda as the standard of care control arm in this randomized trial.
•Chemoimmunotherapy for non-squamous NSCLC (Cohort C). Combination of N-803 with Cisplatin / Carboplatin, Keytruda and Pemetrexed versus Cisplatin / Carboplatin, Keytruda and Pemetrexed as the standard of care control arm in this randomized trial.
The trial is currently ongoing.now recruiting.
3.Pancreatic Cancer
According to ASCO, in the U.S. pancreatic cancer is the fourth leading cause of cancer-related death in both men and women and has one of the highest mortality rates of all major cancers, taking nearly 50,000 lives every year, with a five-year survival rate for late-stage cases of only 3%.
Advanced Pancreatic Cancer (QUILT 88)
Exploratory Phase 1b/2 trials in patients with second-line or greater metastatic pancreatic cancer in which N-803 and aldoxorubicin were combined with off-the-shelf NK (haNK) cells, other agents, and stereotactic body radiotherapy (SBRT) showed encouraging results in patients with advanced disease. The primary endpoints of the Phase 1b and 2 portions of the trials were safety and objective response rate, respectively. In aggregate, 82% of patients (14/17) with advanced pancreatic cancer achieved disease control following combination therapy including N-803 and aldoxorubicin. There were no N-803-related grade 3 or 4 adverse events reported.
On the basis of these exploratory trials, together with the preclinical findings that PD-L1 t-haNK is as active as haNK + anti-PD-L1 mAbs, we initiated a first- through third-line pancreatic cancer clinical trial that uses PD-L1 t-haNK as described below.
•First-line advanced pancreatic cancer (Cohort A). Combination of N-803 with aldoxorubicin and low-dose chemotherapy plus SBRT with or without PD-L1 t-haNK versus gemcitabine/Abraxane® as the standard of care control arm in this randomized trial.
•Second-line advanced pancreatic cancer (Cohort B). Combination of N-803 with aldoxorubicin and low-dose chemotherapy plus SBRT + PD-L1 t-haNK versus 5FU/Onivyde® as the standard of care control arm in this randomized trial.
•Third-line and beyond (Cohort C). Combination of N-803 with aldoxorubicin and low-dose chemotherapy plus SBRT + PD-L1 t-haNK in a single arm cohort of this trial with a primary endpoint of overall survival.
On October 13, 2021, we announced that the trial’s Cohort C was fully enrolled. Based on the strength of earlier data and the significant unmet medical need, we submitted an amendment to the FDA to increase enrollment in Cohort C. As of January 2023 as reported at ASCO GI, the median overall survival in this highly advanced group of patients (who failed two to six prior lines of treatment) is 5.8 months (95% CI: 4.9, 6.4 months) exceeding the approximately two- to three-month historical median overall survival. Of the 83 patients, 41 (49.4%) had progressed after two prior lines of therapy. Median overall survival in this group was 6.3 months (95% CI: 5.0, 7.2 months), more than doubling the historical overall survival (survival of three months as reported by Manax et al ASCO GI 2019). In Cohort C, grade 3 or greater treatment-related adverse events included anemia (32%), neutropenia (25%), thrombocytopenia (13%) and fatigue (7%) while all other grade 3 or greater treatment-related adverse events occurred at a frequency of less than 5%.
We met with the FDA in December 2022 to obtain guidance toward a registration pathway in metastatic pancreatic cancer with combination immunotherapy and NK cell therapy, and the FDA advised us to conduct a randomized trial in late-stage metastatic pancreatic cancer.
4.Glioblastoma Multiforme
According to the American Association of Neurological Surgeons, GBM is the most common malignant brain tumor accounting for approximately 48% of all primary brain tumors withtumors. GBM has a low survival rate of approximately 40% in the first year after diagnosis and only 17% in the second year. In a preclinical settings,study, we evaluated the preclinical activity of N-803 in a murine GL261-luc glioblastoma model. We showed that N-803, as a single-agent treatment as well asalone and in combination with an anti-PD-1 antibody or stereotactic radiosurgery in a murine GL261-luc GBM model and demonstrated that N-803 as mono-or combination therapy exhibits a robust antitumor immune response resulting in prolonged survival including complete remission in tumor bearing mice. N-803-treated mice had decreased tumor volume and increased median survival compared to control. In addition, N-803 treatment resulted in long-term immune memory against glioblastomaGBM tumor rechallenge.
Recurrent or Progressive GBM – QUILT 3078
A new randomized, multi-center, open-label Phase 3II/III trial is currently beinghas been developed to evaluate the safety and efficacy of the combinations ofcombination therapy with N-803, PD-L1 t-haNK, and bevacizumab in patients with recurrent or progressive GBM. A pilot phase will evaluate theIn Phase II, safety of thisthe combination will be assessed prior to thePhase III wherein participants will be randomized Phase 3 portion versusto either combination therapy or bevacizumab monotherapy as the current standard of care.
•Pilot (Part A). Enrollment will initiate with a single-arm study of 10 patients to receive N-803, PD-L1 t-haNK, and bevacizumab combination therapy. Continued development of the experimental arm in Part B will be based on the overall risk/benefit of the combined treatment regimen observed in Part A.
•Randomized Comparison of Combination Therapy versus Bevacizumab Monotherapy (Part B). Part B will enroll 336 patients to be randomly assigned (1:1) to the experimental arm or to the control arm.
Ovarian Cancer
According to estimates from the American Cancer Society, in 2024 about 19,680 women will receive a new diagnosis of ovarian cancer and about 12,740 women will die from ovarian cancer. A woman’s risk of getting ovarian cancer in her lifetime is about 1 in 87 and her chance of dying from ovarian cancer is about 1 in 130.
Planned Platinum-Resistant Ovarian Cancer – QUILT 108
An open-label Phase II trial of M-ceNK plus N-803 in patients with platinum-resistant ovarian cancer has been designed. In this trial, patients with platinum-resistant high-grade ovarian cancer will receive M-ceNK adoptive cell therapy in combination with N-803 and gemcitabine or investigator’s choice chemotherapy. The study consists of two arms, with participants in the control arm receiving investigator’s choice chemotherapy such as pegylated liposomal doxorubicin or gemcitabine, and participants in the experimental arm receiving M-ceNK adoptive cell therapy in combination with N-803 and gemcitabine. Only
5.Human Papillomavirus-Associated Tumorsthe experimental arm participants will undergo mononuclear cell collection for M-ceNK generation, day 8 induction and every 28-day (if M-ceNK are available) maintenance dosing, along with N-803. The goal for enrollment has not been established, and is pending statistician recommendation. The primary endpoint is tumor response by BICR using RECIST 1.1 criteria and secondary endpoints are overall survival, overall response rate, duration of response, disease control rate, and CA-125 levels, with safety endpoints of adverse events, treatment-emergent adverse events and significant adverse events, graded using NCI Common Terminology Criteria for Adverse Events Version 5.0.
Acute Myeloid Leukemia
According to the Centers for Disease Control and Prevention, every year about 19,400 women and 12,100 men experience cancers caused by the human papillomavirus (HPV), which is the most common sexually transmitted infection in the U.S. According to ASCO, head and neck cancers account for nearly 4% of all cancers in the U.S. It is estimated that more than 66,470 men and women in the U.S. will be diagnosed with head and neck cancers in 2023, and 15,050 will dieestimates from the disease.
HPV Associated Cancer in Lower Genitourinary, Cervical, and Head & Neck
Backed by data from preclinical animal models, we are exploring IBRX-042 that leverages our hAd5-based vaccine platform to target tumors caused by HPV infection (which includes most types of lower genitourinary (GU), cervical, and some types of head/neck cancers). Our second-generation hAd5 vector includes enhancements with the E1, E2b, E3 regions of the hAd5 deleted (E1-, E2b-, E3-), decreasing the risk for vector-directed immune responses that may reduce efficacy, while maintaining high target antigen production. The platform has previously been used to generate vaccines against TAAs such as CEA, MUC1 and Brachyury. In IBRX-042, hAd5 encodes HPV-16 E6 and E7 tumor-associated proteins (oncotarget antigens). E6 and E7 play a role in early infection and, importantly, transformation of cells into rapidly-dividing tumor cells. Specifically, E6 promotes degradation of p53—a factor that typically prevents excess cell division—indirectly activates telomerase, and disrupts the function of the cellular phosphatase tumor suppressor PTPN13. E7 inactivates pRb (which plays a role similar to the p53) and activates Mi2b and is also implicated in transformation. Together, these oncogenic alterations drive rapid cellular proliferation, suppress or downregulate key tumor suppressor proteins, and lead to cellular immortality. In addition, E6 and E7 expression is required to maintain a malignant transformed phenotype. IBRX-042 has demonstrated the ability to induce immune responses against HPV16 antigens that induced significant anti-tumor effects in vivo in HPV+ mouse models. To support clinical studies, we have successfully completed the GMP production of GMP IBRX-042.
We are planning a Phase 1/2 open-label trial to evaluate the safety and efficacy of subcutaneous IBRX-042 vaccination combined with N-803 (to enhance immune responses) in combination with standard of care in patients with HPV-associated tumors.
6.Solid Tumors
Autologous M-ceNK in Locally Advanced or Metastatic Solid Tumors (QUILT 3.076)
An initial study conducted by the NCI showed that N-803-enhanced NK cell anti-tumor activity against all variants of small-cell lung cancer (Fousek 2022). Based on this data and other preclinical analysis, we initiated a two-part, open-label Phase 1 trial to evaluate safety and preliminary efficacy of cryopreserved M-ceNK cells, in combination with N-803 for subcutaneous administration, in patients with locally advanced or metastatic solid tumors. The trial will compare the quantity and quality of the M-ceNK cells collected and manufactured from newly diagnosed patients who have not received prior treatment or who have received prior first-line treatment (prior to the M-ceNK cells collected and manufactured) to the quantity and quality of the M-ceNK cells collected and manufactured from patients who have progressive disease and who have received at least two prior treatments for their cancer. The trial consists of two cohorts and there will be up to 80 participants total with up to 30 enrolled in Cohort 1 and up to 50 enrolled in Cohort 2.
•Newly diagnosed or no more than one line of treatment (Cohort 1). Includes patients with newly diagnosed high-risk solid tumors who have not received prior treatment for high-risk tumors or who have received prior first-line treatment. Patients in Cohort 1 will participate in apheresis collection of lymphocytes (Part A) and will not receive any investigational therapy unless they subsequently enroll in Cohort 2 (Part B) if they have progressive disease after ≥ 2 prior therapies or if they have progressive disease within 12 months of receiving neoadjuvant or adjuvant chemotherapy. They must also meet the inclusion criteria to participate in the treatment phase (Part B).
•Progressive disease with at least two prior lines of treatment (Cohort 2). Includes patients with r/r solid tumors who have progressive disease after receiving at least two prior therapies.
◦Apheresis collection (Part A). Patients in Cohort 2 will undergo an apheresis collection of lymphocytes prior to receiving four weeks of disease-specific therapy per their oncologists’ recommendations.
◦Treatment with M-ceNK and N-803 (Part B). If an adequate number of M-ceNK cells can be manufactured from the autologous apheresis product and the patients are eligible to participate in the treatment phase (Part B), M-ceNK will be infused in combination with subcutaneous N-803 on monthly repeatable cycles, subject to adequate doses of M-ceNK being available.
7.Non-Hodgkin Lymphoma
According to the American Cancer Society, in 2023 it is estimated that 80,5502024 there will be about 20,800 new cases of AML (mostly in adults) and about 11,220 people will bedie from AML (almost all in adults). AML is one of the most common types of leukemia in adults but is fairly rare overall, accounting for only about 1% of all cancers. The average age of people when they are first diagnosed with non-Hodgkin lymphoma (NHL) in the U.S. and 20,180 deaths will be attributedis about 68. People above 60 years of age generally don’t respond as well as younger people to the disease. Aconventional treatment as they often have trouble tolerating intensive treatment.
Planned AML – QUILT 102
An open-label Phase 1II trial evaluatingof M-ceNK plus N-803 in combination with rituximab, an anti-CD20 mAb therapy, in patients with indolent non-Hodgkin lymphoma (iNHL), who had relapsedAML has been designed. In this trial, patients either between 2- and 15-years of age (Cohort 1) or were patients 16 years or older (Cohort 2) with relapsed/refractory after two lines of therapy, was published in the American Association for Cancer Research journal, Clinical Cancer Research, in 2021. The combination regimen ofAML will receive subcutaneous N-803 and Rituxan® was well tolerated with a single reported grade 4 adverse event and no reported grade 5 adverse events. For patients with anti-CD20 mAb sensitive disease, the overall response rate in the SQ cohort was 78% (7M-ceNK following hematopoietic stem cell transplant. N-803 will be given both before collection of 9) with 7 of 7 (100%) responses in the SQ cohorts were complete remissions (CRs).
Most B-cell malignancies express high levels of CD19, including the majority of NHLs, such as diffuse large B Cell lymphoma (DLBCL), follicular lymphoma (FL), and mantle cell lymphoma. In addition, many leukemias express high levels of CD19, including B-cell precursor acute lymphoblastic leukemia, chronic lymphocytic leukemia (CLL), and hairy cell leukemia. As such, two CD19‑directed CAR-T cell therapeutics are FDA-approved for use in a variety of indications including NHL and ALL. Like T cells, NK cells can be genetically modified to express CARs that recognize tumor‑associated cell-surface antigens and mediate specific recognition and lysis of cancer cells. A number of aNK cell lines that express CARs have been developed and importantly, aNK cells engineered with CARs targeting CD19 and CD20 have shown to be effective against in vitro/in vivo models of lymphoblastic leukemia and lymphoma (Boissel 2013, Muller 2008, Romanski 2016). Derived from aNK, the haNK cell line is engineered to express the high-affinity variant of the Fcγ receptor (FcγRIIIa/CD16a 158V) as well as endoplasmic reticulum-retained IL-2, and has demonstrated enhanced ADCC-mediated antitumor activity (Jochems 2016). The CD19 t-haNK cell line combines the engineered enhancements of haNK cells with the expression of a CAR targeting CD19, and thus has potential to demonstrate robust ADCC antitumor activity against cancerous Bmononuclear cells and provide clinical benefitM-ceNK generation, and after M-ceNK infusion to patients with r/r NHL.activate NK/M-ceNK cells. Participants will also receive standard graft versus host disease prophylaxis. Up to 200 participants will be enrolled in each cohort. The primary endpoints are overall survival and leukemia-free survival rates, and incidence of relapse in those that achieve a complete response. Secondary endpoints are incidence of significant adverse events and overall adverse events.
Relapsed/Refractory Non-Hodgkin Lymphoma (QUILT 3.092)
We are currently developing an open-label, Phase 1, first-in-human, open-label trial to study the safety of CD19 t-haNK as a single agent and the safety and preliminary efficacy of CD19 t-haNK in combination rituximab only (Cohort A) and in combination with N-803 and rituximab (Cohort B) in patients with r/r NHL.
8.Other OncologyInfectious Disease Indications
In addition to the trials listed above in oncology, we are exploring or pursuing several other both company-sponsored and investigator-initiated studies of our product candidates in infectious diseases, including in colon cancer (N-803, hAd5 CEA), prostate cancer (N-803, hAd5 PSA), Lynch Syndrome (N-803, hAd5 CEA, hAd5 MUC1, hAd5 Brachyury), and head and neck cancer (N-803, hAd5 CEA, hAd5 MUC1, hAd5 Brachyury) among others.HIV.
1.SARS-CoV-2
Since the inception of the pandemic, we have believed that a highly mutable S-only focused, antibody-based vaccine approach that could require boosters approximately every six months, would prove insufficient to overcome viral evolution. We have believed T cell response is critical to clearing the virus and long-term immunity and so our focus is on vaccine candidates that also target the more highly conserved N protein. With mass adoption of first-generation S-only vaccines, our data from our prior NHP Challenge Study and early trials have formed the basis of our design of a universal T cell boost vaccine candidate and the associated set of trials currently underway in South Africa.
THEMBA saRNA Vaccine Boost—South Africa (COVID 4.015)
The THEMBA trials are the first studies of our joint venture saRNA technology with next-generation NLCs. In South Africa in 2022, we initiated a Phase 1/2 trial assessing the safety, reactogenicity, and immunogenicity of saRNA-based vaccines against SARS-CoV-2 as boost vaccines in participants that have been previously vaccinated against or previously infected with COVID-19. We are testing both an saRNA S and saRNA N construct. The Phase 1 portion of the trial is ongoing in the first quarter of 2023. The Phase 1 trial, for up to 60 previously vaccinated/infected participants, will be enrolled as 6 separate cohorts to receive a single vaccine boost consisting of different doses of either saRNA encoding the Spike protein (AAHI-SC2 vaccine) or saRNA encoding the nucleocapsid protein (AAHI-SC3 vaccine) delivered by NLC. We expect the Phase 2 trial, of up to 120 previously vaccinated/infected additional participants, to be enrolled with a randomized 1:1:1:1 design to receive Janssen, Moderna or Pfizer-BioNTech vaccines (control arm), the AAHI-SC2 vaccine (experimental arm 1), or the AHHI-SC3 (experimental arms 2 and 3).
2.HIV
HIV affects tens of millions of people globally and while ART has increased survival of infected HIV individuals, there is currently has no known cure. One current strategy for curing HIV is known as the “kick and kill” approach. The “kick” is to induce HIV out of its latent resting state in T cells, revealing infected cells to the immune system, and the “kill” is to remove or killeliminate the infected cells via an immune response or immunotherapy. We believe N-803 is a promising molecule capable of bothto elicit “kick and kill” in this strategy because of its ability to activate viral transcription in CD4+ T cells (“kick”) while strongly activating CD8+ effector memory cells and NK cells important for recognizing and killing HIV infected cells (“kill”), as well as directing these cells to sites of viral reservoirs.
HIV Cure StudyStudies
In June 2021, we announced the opening of a studyclinical trial sponsored by the AIDS Clinical Trials Group (ACTG) and the NIAID (the “HIVHIV Cure Study”)Study) that will evaluate whether N-803 alone or together with broadly neutralizing antibodiesbNAbs can control HIV following interruption of antiretroviral therapy (ART).ART. The Phase 1I open-label, randomized trial will enroll 46 people living with HIV whose virus has been suppressed by ART for approximately two years, including at least 30% cisgender women or transgender men.
these trials are actively enrolling as of January 2024. Thai Red Cross and the U.S. Military HIV Research Program
In April 2021, we announced the launch of a Phase 2II trial sponsored by the Thai Red Cross and the U.S. Military HIV Research Program. The trial is enrolling 15enrolled 14 patients and iswas designed to investigate the safety, tolerability and immunostimulatory effects of administering N-803 during acute HIV infection. N-803 will bewas administered subcutaneously at weeks zero, three and six (for a total of three doses) and will bewas initiated together with antiretroviral therapy in order to determine if the immunostimulatory
effects of N-803 will reduce the amount of HIV present during acute infection. The trial duration for individual participants will bewas approximately 12 weeks. It is hypothesized that N-803 initiated with anti-retroviral therapy during acute HIV infection will not result in complications or additional toxicities compared with anti-retroviral therapy alone, and may result in a reduced viral load in these patients by inhibiting early establishment of HIV reservoirs in infected individuals. The trial was recently completed and data analyses are ongoing.
NIAID University of Minnesota TrialTrials
ABased on the hypothesis that in HIV-infected individuals treated with N-803,CD8+ T cells will migrate to B cell follicles and reduce the frequency of cells with an inducible HIV provirus, a Phase 1b,I proof-of-concept non-randomized, open-label dose-escalation clinical trial sponsored by the University of Minnesota in collaboration with the National InstituteNIAID was conducted. The primary assessment is the safety of Allergy and Infectious Diseases (NIAID) investigatedN-803 in ART-suppressed people living with HIV, along with exploratory analysis of effects on the effectHIV reservoir. In a 2022 report, no significant laboratory adverse events attributable to N-803 were recorded and N-803 was associated with proliferation and/or activation of N-803 on B cell follicles in antiretroviral treated HIV disease. This trial included 10 HIV-infected adults on effective antiretroviral therapy who received N-803 to intensively investigate the immunological effects of treatment. The hypothesis is that in HIV-infected subjects treated with N-803,CD4+ and CD8+ T cells will migrate to and increase in number in B cell follicles that will result inNK cells, with a reductionsmall but significant decrease in the frequency of peripheral blood mononuclear cells with an inducible HIV provirus. The trial results are complete and a publication is in data analysis mode with all subjects having completed the trial.expected during late 2024.
In additionA separate small HIV Cure Phase I trial evaluating N-803 in combination with haploidentical NK cells in HIV-infected patients was completed in 2023. Reported data from the study validate the hypothesis that N-803 combined with NK cells has the potential to reduce viral load in people living with HIV, showing a marked decrease in HIV-producing cells in lymph nodes. The approach was well tolerated with no unexpected adverse events. The trial is complete and results were published online in January 2024, with print edition expected in March 2024. A follow-on trial is being planned to further investigate the studiesabove regimen in additional patients.
Other Infectious Diseases
We previously developed COVID-19 vaccine candidates based on our hAd5 and NLC-saRNA platforms that delivered DNA or RNA, respectively, for SARS-CoV-2 spike (S) and nucleocapsid (N) proteins that underwent early clinical testing in the U.S. and South Africa. These trials listed above,demonstrated the tolerability of the platforms, which elicited no severe adverse events, and provided evidence of effective antigen delivery. Given the development and adoption of other approved vaccine candidates, we are exploring an additional HIV trialhave prioritized our pipeline to focus on delivery of TAAs with our Thai and U.S. Military collaborators using N-803 plus broadly neutralizing antibodies (bNAbs) and HIV vaccines.these platforms for cancer indications. Our latest TAA vaccine is currently being tested with the NCI as discussed above.
Our Large-Scale GMP Biologic Manufacturing Capabilitiesand Distribution
ImmunityBio hasWe have adopted a strategic position to be vertically integrated and develop itsour products according to the FDA’s GMP standards for large-scale manufacturing, even during Phase 2II clinical trial development. Biological upstream and downstream manufacturing capabilities, with its attendant know-how and regulatory compliance for approval, have long lead times. We have adopted an approach for preparedness to provide our vaccine, immunotherapy, and cell therapy products at a global scale. As such, we have established our own plants and have access to facilities on a global basis.
Our ability to create an efficient manufacturing process and supply chain will be important in enabling us to develop novel therapies. Our strategy is to anticipate the needs of our early-stage research and development initiatives for preclinical and eventual clinical product candidates with a focus on rapid capability to produce at scale fusion proteins, hAd5, saRNA, subunit protein,proteins, toll receptor activator,activators, and NK cell products. In addition, our pipeline for development of synthetic small molecules and immunomodulatory peptides uses innovative technology to derive new therapies. We believe members of our management team, many of whom have experience in both nanoparticle commercialization and large-scale injectable drug production, are capable of constructing the processes and commissioning the facilities necessary to meet our development and commercialization goals. For well-known processes, we currently work, and plan to continue working, with established contract manufacturing organizations (CMOs)third-party CMOs to produce drug substance and drug products. In addition, we plan to further enhance our in-house manufacturing capabilities for drug substances, drug products, and labeling and packaging.
Overview of our Manufacturing Model
Our manufacturing capabilities include advanced technology facilities to produce and test various drug substances and drug products. Our experienced operations and quality team focuses on internal manufacturing and testing with a constant endeavor to create robust, high quality, efficient and consistent supply that meets target product profiles. Our Phase 1I manufacturing process is designed to seamlesslyefficiently scale-up through all phases of clinical development to commercial manufacturing to drive successful commercialization.
Commercial cGMP Production
For our N-803 product candidate, we have contracted with amultiple multi-national biologics manufacturermanufacturers with multipleseveral cGMP-compliant facilities in the U.S., Europe and Asia for our current clinical trials and future commercial sales, if approved. TheWe believe the facilities have robust process development and validation and quality oversight with high-capacity production suites operating multiple 2,000-20,000L production bioreactors.bioreactors and high-capacity fill lines. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form. The deficiencies in the CRL related to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolution of the observations noted at the pre-license inspection would be required before the BLA could be approved. At the time, the FDA further provided recommendations specific to additional CMC issues and assays to be resolved. On October 23, 2023, we announced that we had completed the resubmission of the BLA addressing the issues in the CRL. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a new user fee goal date (PDUFA date) of April 23, 2024. While we believe the BLA resubmission addresses the issues identified in the CRL, there is no guarantee that the FDA will ultimately agree that such issues have been successfully addressed and resolved. It is unclear when the FDA will approve our BLA, if at all.
Clinical Trial GMP Antibody and Fusion Protein Production
We are establishing a cGMP-compliant multi-platform facility in California, which includes a large space for the production of antibodies and fusion proteins (including N-803) to treat cancers and infectious diseases. This facility will include fully integrated biologic upstream and downstream production suites and a quality assurance/quality control release laboratory for high-capacity antibody and fusion protein production.
Clinical Trial GMP saRNA, Adenovirus, and Yeast Production
We have established other cGMP-compliant facilities for saRNA, adenovirus, and yeast production in multiple sites in California and a site in Colorado for oncology and infectious diseases. One of our sites in California is dedicated to adenovirus product candidates for the production of vaccine candidates to treat infectious diseases and oncology TAAs. These facilities generally have fully-integrated biologic upstream and downstream production suites and quality assurance/quality control release laboratories for high capacity, continuous, or personalized just-in-time vaccine production.
Clinical Trial GMP NK Cell Therapy Production
We have established other cGMP-compliant facilities for NK cell therapy product production in multiple sites in California for oncology. One of our sites in California is dedicated to our off-the-shelf product candidates (including PD-L1 t‑t‑haNK), while another is primarily focused on our M-ceNK product candidates, including a training lab for our second-generation offerings.
cGMP ISO Class 5 Manufacturing Facility
On February 14, 2022, we acquired a leasehold interest in approximately 409,000 rentable square feet of cGMP ISO Class 5 pharmaceutical manufacturing space in western New York (the Dunkirk Facility). In September 2022, we initiated a workforce reduction at the Dunkirk FacilityFacility. This facility has construction needs that may require an additional 12 to 18 months to complete in order for it to be used as intended, and which needs remain as a result of upcoming construction atan ongoing dispute with the project, whichDunkirk Facility’s general contractor and a stay in resolving the dispute related to Athenex’s ongoing bankruptcy proceedings. See Item 1A. Risk Factors “We are party to a public-private partnership regarding our manufacturing facility in Dunkirk, NewYork, and if we believe may take approximately 12or our counterparties fail to 18 months.meet the obligations of those agreements, it could materially impact our development, operations and prospects” for more information. We believe this facility will provide us with a state-of-the-art biotech production center that will substantially expand and diversify our existing manufacturing capacity in the U.S. and the ability to scale production across allassociated with certain of our key platforms.product candidates.
Manufacture of Platform Product Candidates
ImmunityBio’s diverse product candidate portfolio and pipeline requires a broad knowledge of various manufacturing and quality assurance methods. We have invested heavily in the processes, systems, and technology to build an extensive range of manufacturing programs spanning various levels of development from IND-enablement through BLA preparation of our first commercial product.
We believe our plan to selectively use third-party CMOs for certain of our assets at various stages, coupled with internal development, will give us assurance that any products will have backup manufacturing options.
TableIf and when our lead product candidate, or another one of Contentsour product candidates is approved for commercial sale, we plan to work with a leading third-party logistic provider in a title model while we finish establishing direct licenses in the remaining states where we cannot receive a license until after our first approval. We also plan on contracting with the large specialty distributors to make our product available across relevant clinics, hospitals, infusion centers, and government entities.Competition
We face potential competition from many different sources, including major and specialty pharmaceutical and biotechnology companies, academic research institutions, governmental agencies, and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with current therapies and new therapies that may become available in the future. We believe that the key competitive factors affecting the success of any of our product candidates will include efficacy, safety profile, efficacy, convenience, cost, market access, level of promotional activity devoted to them, competitive intensity, and intellectual property protection.
We have focused our efforts on urologic and other oncological and infectious disease indications that are difficult to treat and with large unmet needs, and we believe our platform will be broadly applicable across multiple tumor types and infections. Based on the breadth and depth of our platforms, we believe our competitors will range from large pharmaceutical companies to emerging novel biotechnology companies.
Oncology
•Antibody CytokineAntibody-Cytokine Fusion and Recombinant Proteins. This platform primarily competes with large pharmaceutical companies marketing checkpoint inhibitors. However, the potential exists for some of these large pharmaceutical companies to seek collaboration for combination of N-803 with their marketed checkpoint inhibitor. This platform will also compete with immunotherapy fusion protein companies developing similar approaches, including Nektar Therapeutics, Neoleukin Therapeutics, Inc., Novartis International AG (Novartis), F. Hoffmann-La Roche AG (Roche), Xencor, Inc., Sanofi, S.A. (Sanofi), Xencor, Inc., and in the context of NMIBC, CG Oncology Inc., Ferring Pharmaceuticals, Janssen Pharmaceuticals, Inc. (Janssen)/Johnson & Johnson, and Merck & Co., Inc. (Merck), and CG Oncology Inc..
•DNA, RNA, and Recombinant Protein Vaccine TechnologiesVaccines. This platform and the associated product candidates will likely compete with other cancer vaccines. Other potential cancer vaccine competitors include Achilles Therapeutics, Roche, BioNTech SE (BioNTech), Merck, Genocea Biosciences, Inc., Geneos Therapeutics, Inc., Hangzhou Neoantigen Therapeutics, Inc., and Gritstone Bio, Inc. There is currently one approved dendritic cell-based cancer vaccine, which is marketed by Dendron Pharmaceuticals, LLC for the treatment of metastatic castration-resistant prostate cancer. Competitor companies focused on dendritic cell-based approaches include Argos Therapeutics,, Merck, Moderna, Inc., Merck, Inovio Pharmaceuticals, Inc., Precigen Corporation, Inc., Medigene AG, and Northwest Biotherapeutics, Inc. (Northwest).Roche.
•Toll-Like Receptor Activators. This platform competes with companies offering other TLR agonist-based approaches, including Dynavax Technologies Corporation, Panacela Labs LLC, Primmune Therapeutics, Inc., and Statera BioPharma, Inc.
•NK Cell Therapy.Therapies. This platform’s product candidates (haNK, taNK, t‑haNK and M-ceNK) face competition from several companies focused on NK cell-based approaches, including Artiva Biotherapeutics Inc./Merck, Catamaran Bio Inc., Celularity, Inc. (Celularity), Century Therapeutics, Inc., Fate Therapeutics, Inc., Gamida Cell, Ltd., INmune Bio Inc., Nkarta Therapeutics, Inc., NKGen Biotech, Inc., Artiva Biotherapeutics Inc./Merck, Sanofi, Shoreline Biosciences, Inc., and Takeda Pharmaceutical Company Limited (Takeda). In addition, our NK cell product candidates compete with other cell and molecule-based immunotherapy approaches using or targeting natural killerNK cells, NKT cells, T cells, macrophages, and dendritic cells. There are currently six approved T cell-based treatments marketed by Novartis,Bristol-Myers Squibb Company (BMS) (two marketed products), Gilead Sciences, Inc. (Gilead)/Kite Pharma (two marketed products), Bristol-Myers Squibb Company (BMS) (two marketed products), and Janssen Pharmaceuticals, Inc. (Janssen)/Janssen/Johnson & Johnson.Johnson, and Novartis. Additional companies focused on CAR‑CAR T-related treatment approaches include Allogene Therapeutics, Inc., BMS, Cellectis SA, Celularity, Gilead, Janssen, Novartis, Pfizer, Inc. (Pfizer), Cellectis SA, Poseida, Therapeutics, Inc., Janssen, Celularity, Takeda, and Gilead.Takeda. Competitor companies focused on other T cell-based approaches include Adaptimmune Ltd., Adicet Bio, Inc., Autolus Therapeutics, plc, Beam Therapeutics Inc., BioNTech, GlaxoSmithKline plc. (GSK), Precision Biosciences, Inc., Beam Therapeutics Inc., BioNTech, Sensei Biotherapeutics, Inc., Senti Biosciences, Inc., and TCR² Therapeutics Inc.
•DAMP Inducers. This platform competes with companies offering various chemotherapeutic agents, including Abraxane® (BMS), doxorubicin and paclitaxel/Taxol, as well as an antibody drug conjugate produced by Immunomedics, Inc. (acquired by Gilead).
Other potential immunotherapy competitors in oncology include Affimed GmbH, AgenTusMiNK Therapeutics, Inc., Appia Bio, Inc., Codiak Biosciences, Compass Therapeutics, Inc., Glycostem Therapeutics BV, Kuur Therapeutics Limited, GammaDelta Therapeutics Ltd. (Takeda)GT Biopharma, Inc., and Lyell Immunopharma, Inc., and GT Biopharma, Inc.
Infectious Diseases
Currently, our infectious disease product candidates are primarily focused on SARS-CoV-2 and HIV. Competitor companies focused on COVID-19 vaccines (adenovirus, mRNA, and other approaches) include AstraZeneca, Johnson & Johnson/Janssen, Merck, Moderna Therapeutics, Inc., Novavax, Inc., and Pfizer/BioNTech. In addition, a very large number of companies, government agencies and academic centers around the world are developing or have developed COVID-19 vaccines and therapeutics. In the HIVthis space, we have product candidates that use N-803 that will likely compete with companies who have approved therapeutics for HIV, including Abbott LaboratoriesGilead Sciences, ViiV Healthcare Limited (a joint venture between GSK, Pfizer, and Shionogi, Inc.), Merck & Co., BMS, Gilead, and GSK.Janssen Pharmaceuticals (a subsidiary of Johnson & Johnson).
Intellectual Property
We strive to protect and enhance the proprietary technology, inventions, and improvements that are commercially important to our business, including seeking, maintaining, and defending patent rights, whether developed internally or licensed from third parties. Our policy is to seek to protect our proprietary position by, among other methods, filing patent applications in the U.S. and in jurisdictions outside of the U.S. related to our proprietary technology, inventions, improvements, and product candidates that are important to the development and implementation of our business. We also rely on trade secrets and know-how relating to our proprietary technology and product candidates, continuing innovation, and in-licensing opportunities to develop, strengthen, and maintain our proprietary position in the field of cancer therapeutics and immunotherapy. We expect to rely on data exclusivity, market exclusivity, patent term adjustment and patent term extensions when available, as well as on regulatory protection afforded through orphan drug designations. Our commercial success will depend in part on our ability to obtain and maintain patent and other proprietary protection for our product candidates, technology, inventions, and improvements; to preserve the confidentiality of our trade secrets; to maintain our licenses to use intellectual property owned by third parties; to defend and enforce our proprietary rights, including our patents; and to operate without infringing, misappropriating or otherwise violating the valid and enforceable patents and other proprietary rights of third parties.
We have developed, acquired, and in-licensed patents and patent applications across platforms as previously described for: (1) activated NK and T cells; (2) memory T cell activation; and (3) activated tumoricidal macrophages. With respect to activated NK and T cells, we have developed N-803, an N72D variant IL-15 complexed to a dimeric IL-15Ra/Fc fusion protein; with respect to memory T cell activation, we have developed adenoviral and yeast immunotherapies expressing tumor antigens such as CEA, MUC1, and Brachyury, and in-licensed saRNA technologies; and with respect to activated tumoricidal macrophages, we have in-licensed intellectual property licensed to aldoxorubicin, a tumor-targeted doxorubicin conjugate, from LadRx.
We own patents and patent applications related to the development and commercialization of N-803. As of December 31, 2022,2023, our owned patent portfolio directed to N-803, methods of use of N-803, and combinations with additional therapeutics consists of approximately 1927 issued U.S. patents and 814 pending U.S. patent applications, as well as approximately 7782 patents issued in jurisdictions outside of the U.S., including Europe, China, Japan, Canada, and Australia. Excluding any patent term adjustment and patent term extension, the issued U.S. patents directed to N-803, methods of use of N-803 and combinations with additional therapeutics are expected to expire from 2028 to 2039.2040. Excluding any applicable extensions, the issued foreign
patents are expected to expire from 2028 to 2038.2039. If patents issue from our pending U.S. patent applications, excluding any patent term adjustment and patent term extension, such patents will be expected to expire from 2031 to 2039.
one of our European patents, EP Patent No. 3601363, is being challenged in an opposition proceeding. This patent is directed to methods of using N-803-based combination therapy with anti-CD38 antibodies to treat cancer, which does not directly relate to any of our current programs. We intend to defend our patent and believe we have meritorious defenses against this opposition. For example, these patents and patent applications include claims directed to:
•N-803 compositions of matter;
•uses of N-803 in methods of treating cancers;
•uses of N-803 in treating HIV; and
•combination treatments using N-803 and additional therapeutics.
We own, co-own, and in-license patents and patent applications related to the development and commercialization of cell-based therapies. As of December 31, 2022,2023, our owned and co-owned patent portfolio directed to NK, haNK, and t-haNK cell lines, methods of use of these cells, and combinations with additional therapeutics consists of approximately 1519 issued U.S. patents and 2423 pending U.S. patent applications, as well as approximately 3258 patents issued in jurisdictions outside of the U.S., including Europe, China, Japan, and Australia. As of December 31, 2022,2023, our in-licensed patent portfolio directed to NK, haNK, and t-haNK lines, methods of use of these cells, and combinations with additional therapeutics consists of approximately 4 issued U.S. patents, as well as approximately 41 patents issued in jurisdictions outside of the U.S., including Europe, Canada, and Australia. Excluding any patent term adjustment and patent term extension, the issued U.S. patents directed to these cell therapies, methods of use, and combinations with additional therapeutics are expected to expire from 2025 to 2040. Excluding any applicable extensions, the issued foreign patents are expected to expire from 2025 to 2040. If patents issue from our pending U.S. patent applications, excluding any patent term adjustment and patent term extension, such patents will be expected to expire from 2034 to 2040.2042. For example, these patents and patent applications include claims directed to:
•NK cells;
•haNK cells;
•EGFR t-haNK cells;
•CD19 t-haNK cells;
•HER2 t-haNK cells; and
•PD-L1 t-haNK cells.
We own patents and patent applications related to development and commercialization of our preclinical assets N-820, N-809, N-812, and N-809.N-830. As of December 31, 2022,2023, our owned patent portfolio directed to N-820, N-809, N-812, and N-809N-830 and methods of use of N-820, N-809, N-812, and N-809N-830 consists of approximately 715 issued U.S. patents and 13 pending U.S. patent application,applications, as well as approximately 4857 patents issued in jurisdictions outside of the U.S. Excluding any patent term adjustment and patent term extension, the issued U.S. patents directed to N-820, N-809, N-812, and N-809N-830 are expected to expire from 2028 to 2039. If a patent issuespatents issue from our pending U.S. patent application,applications, excluding any patent term adjustment and patent term extension, this patentthese patents will be expected to expire in 2028.from 2028 to 2038. For example, these patents and patent applications include claims directed to fusions of checkpoint inhibitor and TAA antibodies and binding molecules with IL-15/IL-15Ra/Fc fusion proteins complexes.
We co-own and exclusively in-license patents and patent applications from LadRx related to the development and commercialization of aldoxorubicin. As of December 31, 2022,2023, our licensed patent portfolio directed to aldoxorubicin and methods of use of aldoxorubicin consists of approximately 34 issued U.S. patents, as well as approximately 2223 patents issued in jurisdictions outside of the U.S., including Europe, Japan, Korea, and Australia. Excluding any patent term adjustment and patent term extension, the issued U.S. patents directed to aldoxorubicin are expected to expire from 20332030 to 2034. Excluding any applicable extensions, the issued foreign patents are expected to expire from 2033 to 2034. For example, these patents and this patent application include claims directed to:
•Aldoxorubicin formulations; and
•Aldoxorubicin formulations for use in treating cancer.
We exclusively own, and co-own with and in-license from the U.S. Department of Health and Human Services (HHS),HHS, patents and patent applications related to the development and commercialization of adenovirus-based cancer and viral immunotherapies. As of December 31, 2022,2023, our patent portfolio directed to adenovirus and methods of use of adenovirus in treating or preventing cancer and viral diseases consists of approximately 2730 issued U.S. patents and approximately 68 pending U.S. patent applications, as well as approximately 7088 patents issued in jurisdictions outside of the U.S. Excluding any patent term adjustment and patent term extension, the issued U.S. patents directed to adenovirus-based cancer and viral immunotherapies are expected to expire from 2024 to 2038.2039. If patents issue from our pending U.S. patent applications, excluding any patent term adjustment and patent term extension, such patents will be expected to expire from 20302028 to 2037.2041. For example, these patents and patent applications include claims directed to:
•Adenovirus vectors and virus particles comprising TAAs; and
•uses of adenovirus vectors and virus particles in methods of treating cancers.
We own, co-own with HHS and in-license from HHS and the University of Colorado, patents and patent applications related to the development and commercialization of yeast-based cancer and viral immunotherapies. As of December 31, 2022,2023, our patent portfolio directed to yeast-based cancer and viral immunotherapies and methods of use of yeast-based cancer and viral immunotherapies in treating or preventing cancer and viral diseases consists of approximately 2421 issued U.S. patents and approximately 4 pending U.S. patent applications, as well as approximately 214155 patents issued in jurisdictions outside of the U.S. Excluding any patent term adjustment and patent term extension, the issued U.S. patents directed to yeast-based cancer and viral immunotherapies are expected to expire from 20232027 to 2036. If any patents issue from our pending U.S. patent applications, excluding any patent term adjustment and patent term extension, such patents will be expected to expire from 20322030 to 2039. For example, these patents and patent applications include claims directed to:
•yeast and yeast vehicles expressing TAAs and neoepitopes; and
•uses of yeast and yeast vehicles expressing TAAs and neoepitopes in methods of treating cancers.
We own 5approximately 2 issued U.S. non-provisionalpatents and 3 pending U.S. patent applications and 2 patent cooperation treaty (PCT) applications directed to therapeutics for COVID-19. Some of these patent applications are directed to the use of our adenovirus and yeast technologies for a COVID-19 vaccine. Excluding any patent term adjustment and patent term extension, the issued U.S. patents directed to therapeutics for COVID-19 expire in 2040. If any patents issue from our pending U.S. patent applications, excluding any patent term adjustment and patent term extension, such patents will be expected to expire infrom 2040 andto 2042.
We in-license patents and patent applications from AAHI related to the development and commercialization of adjuvant formulations and saRNA based vaccines. As of December 31, 2022,2023, our licensed patent portfolio directed to adjuvant formulations and saRNA vaccine platforms consists of approximately 45 issued U.S. patents and approximately 45 pending U.S. patent applications, as well as 1 issued patent in jurisdictions outside of the U.S. Excluding any patent term adjustment and patent term extension, the issued U.S. patents directed to adjuvant formulations and saRNA-based vaccines are expected to expire from 2027 to 2038. If any patents issue from our pending U.S. patent applications, excluding any patent term adjustment and patent term extension, such patents will be expected to expire from 20342033 to 2038. TheIn November 2023, the validity of one of our in-licensed issued European patents, (EPEP Patent No. 2068918) is being2068918, previously challenged in an opposition proceeding.proceeding was upheld upon appeal. This patent is directed to vaccine compositions comprising certain lipid adjuvants. We believe AAHI has meritorious defenses against the opposition.
We own patents and patent applications related to the development and commercialization of GMP-in-a-Box. As of December 31, 2022,2023, our patent portfolio directed to GMP-in-a-Box consists of approximately 78 issued U.S. patents and approximately 2 pending U.S. patent applications as well as approximately 6265 patents issued in jurisdictions outside of the U.S. Excluding any patent term adjustment and patent term extension, the issued U.S. patents directed to GMP-in-a-Box are expected to expire in 2030 and 2037. If patents issue from our pending patent applications, excluding any patent term adjustment and patent term extension, such patents will be expected to expire in 2035 and 2039. For example, these patents and patent applications include claims directed to methods, bioreactors, and apparatuses for monitoring and culturing cells.
The termterms of individual patents extend for varying periods of time, depending upon the date of filing of the patent application, the date of patent issuance, and the legal term of patents in the countries in which they are obtained. Generally, patents issued for applications filed in the U.S. are effective for 20 years from the earliest effective filing date of a non-provisional patent application. The patent term may be adjusted to compensate for delayed patent issuance when such delays are caused by the United States Patent and Trademark Office (USPTO)USPTO or successful appeals against USPTO actions. There is no statutory limit on this patent term adjustment, which is generally the length of any such delays caused by the USPTO. In addition, in certain instances, a patent term can be extended to
recapture a portion of the term effectively lost as a result of the FDA regulatory review period. The restoration period cannot be longer than five years, the total patent term, including the restoration period, must not exceed 14 years following FDA approval, only one patent applicable to an approved drug may be extended and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. While we plan to seek such patent term adjustments and extensions where applicable, there is no guarantee that the USPTO and/or FDA will agree with our assessment of whether such adjustments or extensions should be granted, and if granted, the length of such adjustments or extensions. The duration of patents outside of the U.S. varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective filing date. However, the actual protection afforded by a patent varies on a product-by-product and country-to-country basis and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country, and the validity and enforceability of the patent.
The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. No consistent policy regarding the scope of claims allowable in patents in the field of immunotherapy has emerged in the U.S. The patent situation outside of the U.S. is even more uncertain. Changes in either the patent laws or their interpretation in the U.S. and other countries may diminish our ability to protect our inventions and enforce our intellectual property rights, and more generally could affect the value of our intellectual property. In particular, our ability to stop third parties from making, using, selling, offering to sell, or importing products that infringe our intellectual property will depend in part on our success in obtaining and enforcing patent claims that cover our technology, inventions, and improvements. With respect to both licensed and owned intellectual property, we cannot be sure that patents will be granted with respect to any current pending patent applications or with respect to any patent applications filed in the future, nor can we be sure that any existing patents or any patents that may be granted in the future will be commercially useful in protecting our product candidates and the methods used to manufacture those product candidates. Moreover, even our issued patents do not guarantee us the right to practice our technology in relation to the commercialization of our product candidates. The area of patent and other intellectual property rights in biotechnology is an evolving one with many risks and uncertainties, and third parties may have blocking patents that could be used to prevent us from commercializing our product candidates and practicing our technology. Our issued patents and those that may issue in the future may be challenged, invalidated, or circumvented, which could limit our ability to stop competitors from marketing related products or limit the length of the term of patent protection that we may have for our product candidates. In addition, the rights granted under any issued patents may not provide us with protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may independently develop similar technologies. For these reasons, we may have competition for our product candidates. Moreover, because of the extensive time required for development, testing and regulatory review of a potential product candidate, it is possible that, before any particular product candidate can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent.
Our registered trademark portfolio currently contains approximately 1623 registered trademarks in the U.S., approximately 119217 registered trademarks in foreign jurisdictions, approximately 2942 pending trademark applications in the U.S., and approximately 5264 pending trademark applications in foreign jurisdictions. We may also rely, in some circumstances, on trade secrets to protect our technology. However, trade secrets are difficult to protect. We seek to protect our trade secrets and other proprietary information, in part, by entering into confidentiality agreements with those who have access to our confidential information, including our employees, contractors, consultants, collaborators, and advisors. We also seek to preserve the integrity and confidentiality of our proprietary technology and processes by maintaining physical security of our premises and physical and electronic security of our information technology systems. Although we have confidence in these individuals, organizations, and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or may be independently discovered by competitors. To the extent that our employees, contractors, consultants, collaborators, or advisors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. ForSee Item 1A. Risk Factors “Risks Related to Intellectual Property” and Item 3. “Legal Proceedings” of this Annual Report for risks related to our proprietary technology, inventions, improvements, and products, see Part I, Item 1A., “Risk Factors—Risks Related to Intellectual Property” and Item 3., “Legal Proceedings” of this Annual Report.products.
Collaboration and License Agreements
We anticipate that strategic collaborations will continue to be an integral part of our operations, providing opportunities to leverage our partners’ expertise and capabilities to gain access to new technologies and further expand the potential of our technologies and product candidates across relevant platforms. We believe we are well positioned to become a leader in immunotherapy due to our broad and vertically-integrated platforms and through complementary strategic partnerships. Agreements shown below have been arranged in alphabetical order.
The following description of certain of our collaboration and license agreements is not a comprehensive listing of all such agreements to which we are a party, and the inclusion of a description of any collaboration or license agreement is not an indication that we consider such agreement(s) to be material to our business and operations as a whole, which is a dynamic and evolving analysis and may change over time.
Collaboration Agreements
Amyris Joint Venture
In December 2021, Immunity Bio and Amyris entered into a 50:50 joint venture arrangement and formed a new limited liability company to conduct the business of the joint venture. The purpose of the joint venture iswas to accelerate commercialization of a next-generation COVID-19 vaccine utilizing an RNA vaccine platform license. As part of the limited liability agreement, Amyris agreed to contribute, in part, rights to its license agreement with AAHI for an RNA platform for the field of COVID-19, and ImmunityBio agreed to contribute, in part, priority access to its manufacturing capacity for the joint venture product. In August 2023, Amyris announced that it filed for Chapter 11 bankruptcy protection. The Amyris bankruptcy case remains ongoing, and there can be no assurance that we will receive any recovery on account of our claims against Amyris, including for Amyris’ portion of expenses incurred by the joint venture. As of December 31, 2023, the carrying amount of our equity investment in the joint venture was zero.
National Cancer Institute
The company and its subsidiaries began their relationship with HHS, as represented by the NCI of the National Institutes of Health (NIH)NIH in 2015. Pursuant to the Cooperative Research and Development Agreement (CRADA),CRADAs, the NCI provides scientific staff and other support necessary to conduct research and related activities as described in the CRADA.CRADAs. During the term of the initial and amended CRADAs, we collaborated with the NCI on the preclinical and clinical development of an adenovirus technology expressing TAAs for cancer immunotherapy, the preclinical and clinical development of our proprietary yeast-based Tarmogens expressing TAAs, and the proprietary adenovirus technology expressing TAASTAAs for cancer immunotherapy.
In 2021, the CRADA was amended and the research plan was modified to include the preclinical and clinical development of ImmunityBio’s proprietary adenovirus platform expressing TAAs; proprietary yeast platform expressing TAAs; proprietary agent N-803 and derivatives, agent N-809 and derivatives, and/or TxM product candidates; proprietary recombinant NK cells and mAbs; proprietary RNA vaccines and adjuvants; and other proprietary agents owned or controlled by ImmunityBio for cancer immunotherapy. The term of the CRADA was extended through May 2026. Under this agreement, we agreed to pay NCI funding totaling $1.3 million per year, payable in semi-annual installments each year through 2025.
License Agreements
3M Innovative Properties Company (3M IPC)IPC and AAHI License Agreement
We have licensed rights to 3M-052, a synthetic TLR7/8 agonist, 3M-052 formulations and related technology from 3M IPC and its affiliates and AAHI. In November 2021 we obtained nonexclusive rights in the field of SARS-CoV-2 and in June 2022 we modified those rights and expanded the scope of the license to include (1) SARS-CoV-2 and other infectious diseases including malaria, HIV, tuberculosis, hookworm and varicella zoster on an exclusive basis in countries other than low- and middle-income countries (LMIC),LMIC, and (2) oncology applications, when used in combination with our proprietary technology and/or IL-15 agonists. Adjuvants are either synthetic or naturally occurring molecules that activate TLRs thereby enhancing the humoral and cell-mediated immune response of vaccines. There are 10 human TLRs expressed either on the inside or outside of the immune cell and their function is to recognize foreign substances expressed by pathogens. Once activated, these TLRs stimulate danger signals to the immune cells
initiating an immune response. The synthetic imidazoquinolinone 3M-052 is structurally similar to resiquimod. The 3M-052/Alum adjuvant formulation is in Phase I trials in the U.S. with an HIV antigen and has been well-tolerated and immunogenic. In consideration for the license, we agreed to make certain periodic license payments, including $2.25 million each year through June 2025. We have also agreed to make payments upon the achievement of certain regulatory milestone events and tiered royalties ranging from the low to high single-digits as a percentage of net sales. Beginning in April 2026, the annual minimum licensing payment is $1.0 million, which can be credited against any royalty payments due under this agreement. We may terminate this license for any reason after providing 3M and AAHI sixty (60) days written notice.
AAHI License Agreements
In May 2021, we entered into two license agreements with the AAHI pursuant to which we received a license to certain patents and know-how relating to AAHI’s (i) adjuvant formulations for the treatment, prevention and/or diagnosis of SARS-CoV-2 (the AAHI Adjuvant Formulation License Agreement) and (ii) RNA vaccine platform as further described below (the AAHI RNA License Agreement). Under both agreements, we were obligated to pay one-time, non-creditable, non-refundable upfront cash payments totaling $2.0 million. In addition, under the AAHI Adjuvant Formulation License Agreement we owe milestone payments to a total of up to $2.5 million based on the achievement of certain development and regulatory milestones for the first licensed product and royalties on annual net sales of licensed products on a country-by-country and product-by-product basis of a low-single digit percentage, subject to certain royalty-reduction provisions.
In September 2021, we amended and restated the AAHI RNA License Agreement, pursuant to which AAHI granted us an exclusive, worldwide, sublicensable license to AAHI’s rights to an RNA vaccine platform for the development and commercialization of certain therapeutic, diagnostic, or prophylactic products for the prevention, treatment or diagnosis of any indication, other than those subject to pre-existing third-party license grants, including, without limitation, SARS-CoV-2. Pursuant to the terms of the amended and restated AAHI RNA License Agreement, we made an additional one-time, non-creditable, non-refundable, upfront payment to AAHI of $1.5 million. We paid a license fee of $3.0 million in 2022 and $5.5 million in 2023. We are also required to pay a license maintenance feesfee to AAHI as follows: $3.0 million paid in 2022 andof $5.5 million annually from 20232024 through 2030. The company may terminate the restated agreement without cause by paying AAHI a $10.0 million one-time early termination fee. In addition, the milestone payments to AAHI based on the achievement of certain development and regulatory milestones for the first licensed product were amended to a total of up to $4.0 million. We are required to pay royalties on annual net sales of licensed products on a country-by-country and product-by-product basis of a low- to mid-single digit percentage.
In connection with the license agreements, in May 2021 we also entered into a sponsored research agreement (SRA) with AAHI pursuant to which we will fund continued research of at least $2.0 million per year, payable in four equal quarterly installments each year until May 2024, or such year of earlier termination.
GlobeImmune, Inc.
In 2020, we entered into an exclusive licensing agreement with GlobeImmune, a consolidated entity of the company, pursuant to which we obtained worldwide, exclusive licenses under certain patents, know-how, and other intellectual property to use, research, develop and commercialize products with GlobeImmune’s COVID-19 vaccine program, other Tarmogen-based programs, and neoepitopes programs in exchange for a license fee for the first two years of the agreement totaling $1.2 million, up to $345.0 million in milestone payments related to the successful completion of clinical and regulatory milestones and up to $240.0 million in total milestone payments based on licensed product net sales milestones, and a royalty on net sales of licensed products, on a product-by-product basis ranging in percentage from the mid-single digits to the mid-teens. We may terminate this agreement, in whole or on a licensed-product-by-licensed-product and/or country-by-country basis, at any time upon sixty (60) days written notice to GlobeImmune.
LadRx Corporation
In 2017, we entered into an exclusive license agreement with LadRx pursuant to which we obtained a royalty-bearing, exclusive, worldwide license, with the right to sublicense, LadRx’s applicable intellectual property to research, develop and commercialize aldoxorubicin for all indications. Under the terms of the license agreement, LadRx is entitled to receive milestone payments of up to $345.7 million related to regulatory approvals and commercial milestones for aldoxorubicin. In addition, LadRx will receive
increasing low double-digit percentage royalties on net sales of aldoxorubicin for the treatment of soft tissue sarcomas and mid-to-high single-digit percentage royalties on net sales of aldoxorubicin for all other indications. We may terminate the agreement in its entirety at any time upon twelve (12) months written notice to LadRx. Upon termination of the agreement, any licenses granted to us under the agreement are terminated, and we must cease the development, manufacture, and commercialization of aldoxorubicin.
Sanford Health
In 2017, and as amended in November 2021, we entered into a license agreement with Sanford pursuant to which we obtained a worldwide, exclusive license under Sanford’s applicable patent and know-how rights to use, make, have made, sell, offer to sell, export and import products for all uses and applications of polynucleotides encoding mutant E16 antigen (mutant HPV16 E6 antigen + mutant HPV16 E7 antigen) and the encoded mutant E16 antigen, in exchange for consideration that includes the amount equal to the patent prosecution costs incurred by Sanford for the prosecution of the licensed patent rights, milestone payments payable upon the achievement of certain contractual and regulatory milestones of up to $2.0 million, a low single-digit percentage royalty on net sales of the resulting licensed products, and a low to high-teen percentage share of non-royalty sublicensing revenue. Our obligation to pay royalties continues, on a licensed product-by-licensed product and country-by-country basis, until the date on which such licensed product is no longer covered by a valid claim of a patent licensed pursuant to the agreement in such country. We must use commercially reasonable efforts to develop and commercialize the licensed products. Sanford is responsible for the prosecution and maintenance of the patents licensed pursuant to the agreement. We are required to use commercially reasonable efforts to develop and make available the licensed products, which include achieving certain regulatory objectives within certain specific time periods. We have the first right to enforce the patents licensed pursuant to the agreement, subject to Sanford’s ability to exercise such right if we fail to do so. We may terminate this agreement at any time upon 60 days’ written notice to Sanford. Sanford may terminate the agreement in the event of an uncured material breach by us.
In June 2023, we filed an IND for QUILT 3100 exploring the use of an hAd5 [E6/E7] construct known as IBRX-042 in a Phase I open-label trial to evaluate safety and determine the maximum tolerated dose in subjects with HPV-associated tumors. During the second half of 2023, we received correspondence from the FDA that it was placing the IND on clinical hold, and requesting additional toxicology studies. The company submitted a complete response to the hold letter in 2024.
Shenzhen Beike Biotechnology Co. Ltd.
In 2014, Altor entered into a license, development and commercialization agreement with Beike, which agreement was amended and restated in 2017, pursuant to which Altor granted to Beike an exclusive license under certain of its intellectual property rights in order to use, research, develop and commercialize products based on N-803 in China for human therapeutic uses, in exchange for consideration that includes up to $195.5 million in total milestone payments based on the successful completion of regulatory and sales milestones for each resulting product, and a royalty on net sales of licensed products, on a product-by-product basis ranging in percentage from the mid-single digits to the mid-teens. Beike’s obligation to pay royalties continues, on a licensed product-by-licensed product basis, until the later of (i) the date on which such licensed product is no longer covered by a valid claim of a patent licensed pursuant to the agreement in China and (ii) ten years after the first commercial sale of such licensed product in China. Altor has the sole right to prosecute and maintain the patents licensed pursuant to the agreement. Altor has the first right to enforce the patents licensed pursuant to the agreement, subject to Beike’s ability to exercise such right if Altor fails to do so. Altor and Beike each have the right to terminate the agreement in the event of a material breach by the other party.
Sorrento Therapeutics, Inc.
In 2015,2020, we received a Request for Arbitration before the International Chamber of Commerce, International Court of Arbitration, served by Beike asserting breach of contract under our subsidiary NantCell, Inc. and Sorrento established NANTibody as a stand-alone biotechnology company with $100.0 million in initial joint funding. We own 60% of the equity interests and Sorrento owns 40% of the equity interests in NANTibody, which focuses on accelerating the development of multiple therapeutic product candidates that are being developed as standalone treatments as well as in combination with other therapies as part of an immune-oncology treatment regimen.
In 2015, NantCell entered into an exclusiveAltor’s license agreement with
Sorrento pursuant to which we obtained an exclusive license under certain patent rights and antibody materials, including antibody sequences and complementary DNA (cDNA) and clones and a non-exclusive license under certain know-how, in each case to use, research and develop certain antibodies and anti-body drug conjugates (ADCs) includingthem. See Item 3. “Legal Proceedings” for neoepitopes, which are epitopes resulting from mutations specific to an individual’s cancer cells, and to commercialize the resulting licensed products, in exchange for consideration that included an upfront cash payment of $10 million, equity consideration with a valuation of $100 million, and mid-single digit percentage royalties on net sales of the resulting licensed products. Our obligation to pay royalties was, on a licensed product-by-licensed product and country-by-country basis, until the later of (i) the date on which such licensed product is no longer covered by a valid claim of a patent licensed pursuant to the agreement in such country and (ii) ten years after the first commercial sale of such licensed product in such country. In addition, the agreement provided us with the right to negotiate an exclusive license from Sorrento for two CAR-T/NK cell products to be mutually determined on terms substantially similar to the terms of the license agreement. NantCell was to use commercially reasonable efforts to develop and commercialize the licensed products. Subject, as applicable, to the licenses granted by Sorrento to us, each party was to own all inventions and other developments it creates or develops in the course of activities conducted pursuant to the agreement.
In 2015, NANTibody entered into an exclusive license agreement with Sorrento pursuant to which NANTibody obtained a royalty-free exclusive license under certain patent rights and materials, including antibody sequences and cDNA, and clones and a non-exclusive license under certain know-how, in each case related to up to 75 immuno-oncology antibodies, immune-check point antibodies, bi-specific antibodies and/or ADCs from Sorrento’s G-MAB library to be mutually identified by the parties (21 of which were already identified at the time of signing the agreement), to use, research, develop and commercialize the resulting licensed products.
In 2019, we filed cross-claims against Sorrento in the Superior Court of California, Los Angeles County, alleging that Sorrento had breached the exclusive license agreement with us; these claims were pursued in arbitration. Sorrento filed counterclaims against the company and NANTibody in the arbitration.
On December 2, 2022, the arbitrator issued a final award finding that Sorrento had breached the two exclusive license agreements with NantCell and NANTibody. In addition, the arbitrator determined that our license agreement remains in full force and effect with respect to ImmunityBio’s PD-L1 NK-cell. The arbitrator ruled that Sorrento, NantCell, and NANTibody have no further rights or obligations under either license agreement with respect to other targets.Sorrento has no further obligation to contribute materials or know how with respect to the PD-LI antibody, and NantCell and NANTibody are not required to return any materials or know-how received from Sorrento. For more information, see Note 7, Commitments and Contingencies—Sorrento Therapeutics, Inc. Litigation, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report.information.Viracta Therapeutics, Inc.
In 2017, we entered into an agreement with Viracta under which we were granted exclusive worldwide rights to Viracta’s Phase 2II drug candidate, nanatinostat, for use in combination with our platform of NK cell therapies. In consideration for the license, we are obligated to pay Viracta mid-single digit percentage royalties on net sales of licensed products for therapeutic use and milestone payments ranging from $10.0 million to $25.0 million up to an aggregate maximum of $100.0 million for various regulatory approvals and cumulative net sales levels. We may terminate the agreement, at our sole discretion, in whole or on a product-by-product and/or country-by-country basis, at any time upon 90 days’ prior written notice. In addition, either party may terminate the agreement in the event of a material breach or for bankruptcy of the other party.
Government Regulation
In the U.S., the FDA regulates biopharmaceuticals under the Food, Drug and CosmeticFD&C Act (FDCA) and the Public Health Service Act (PHSA).PHSA. Biopharmaceuticals also are subject to other federal, state, and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or post-market may subject an applicant to administrative or judicial sanctions. These sanctions could include, among other actions, the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, untitled or warning letters, product recalls or market withdrawals, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement and civil or criminal penalties. Any agencyFDA or judicial enforcement action could have a material adverse effect on us. Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including warning letters, the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations and civil and criminal penalties. Contract manufacturers often encounter difficulties involving production yields, quality control and quality assurance, as well as shortages of qualified personnel. Any of these actions or events could have a material impact on the availability of our product candidates.
The FDA and other regulatory authorities at federal, state, and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring, and post-approval reporting of small molecule and biologics such as those we are developing. We, along with third-party contractors, will be required to navigate the various preclinical, clinical, and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval or licensure of our product candidates. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources.
The process required by the FDA before biopharmaceutical product candidates may be marketed in the U.S. generally involves the following:
•completion of preclinical laboratory tests and animal studies performed in accordance with the FDA’s GLP requirements;guidelines;
•submission to the FDA of an IND, which must become effective before clinical trials may begin and must be updated annually or when significant changes are made;
•approval by an independent IRB or ethics committee for each clinical site before the clinical trial is begun;
•performance of adequate and well-controlled human clinical trials to establish the safety, purity, and potency of the proposed biologic product candidate for its intended purpose;
•preparation of and submission to the FDA of a BLA or NDA, after completion of all required clinical trials;
•a determination by the FDA within 60 days of its receipt of a BLA/NDA to file the application for review;
•satisfactory completion of an FDA Advisory Committee review, if applicable;
•satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the proposed product is produced to assess compliance with cGMP and to assure that the facilities, methods, and controls are adequate to preserve the product candidates’ continued safety, quality, purity and potency or efficacy, and of selected clinical investigational sites to assess compliance with GCP requirements;guidelines;
•FDA review and approval of the BLA or NDA to permit commercial marketing of the product for particular indications for use in the U.S.; and
•compliance with any post-approval requirements, including the potential requirement to implement a REMS, and the potential requirement to conduct post-approval studies.
The testing and approval process requires substantial time, effort, and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all. Prior to beginning the first clinical trial with a product candidate, we must submit an IND to the FDA. An IND is a request for authorization from the FDA to administer an IND product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for clinical trials. The IND also includes results of animal and in vitro studies assessing the toxicology, pharmacokinetics, pharmacology, and pharmacodynamic characteristics of the product; chemistry, manufacturing, and controls information; and any available human data or literature to support the use of the investigational product. An IND must become effective before human clinical trials may begin. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises safety concerns or questions about the proposed clinical trial. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before the clinical trial can begin. Submission of an IND therefore may or may not result in FDA authorization to begin a clinical trial.
When a clinical trial using genetically engineered cells is conducted at, or sponsored by, institutions receiving NIH funding for wild-type DNA research, prior to the submission of an IND to the FDA, a protocol and related documentation is submitted to and the study is registered with the NIH Office of Biotechnology Activities (OBA)OBA pursuant to the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines).Guidelines. Compliance with the NIH Guidelines is mandatory for investigators at institutions receiving NIH funds for research involving wild-type DNA, and many companies and other institutions not otherwise subject to the NIH Guidelines voluntarily follow them. The NIH is responsible for convening the Recombinant DNA Advisory Committee (RAC),RAC, a federal advisory committee that discusses protocols that raise novel or particularly important scientific, safety, or ethical considerations at one of its quarterly public meetings. The OBA will notify the FDA of the RAC’s decision regarding the necessity for full public review of a protocol. RAC proceedings and reports are posted to the OBA web site and may be accessed by the public. If the FDA allows the IND to proceed, but the RAC decides that full public review of the protocol is warranted, the FDA will request at the completion of its IND review that sponsors delay initiation of the protocol until after completion of the RAC review process.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCP requirement,guidelines, which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development and for any subsequent protocol amendments. Furthermore, an independent IRB, for each site proposing to conduct the clinical trial, must review and approve the plan for any clinical trial and its informed consent form before the clinical trial begins at that site and must monitor the study until completed. Regulatory authorities, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk or that the trial is unlikely to meet its stated objectives. Some studies also include oversight by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain data from the study and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. There are also requirements governing the reporting of ongoing clinical trials and clinical trial results to public registries.
For purposes of BLA or NDA approval, human clinical trials are typically conducted in three sequential phases that may overlap.overlap:
•Phase 1.I. The investigational product is initially introduced into healthy human subjects and tested for safety. In the case of some products for severe or life-threatening diseases, the initial human testing is often conducted in patients.
•Phase 2.II. The investigational product is evaluated in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy or potency of the product for specific targeted diseases and to determine dosage tolerance, optimal dosage, and dosing schedule.
•Phase 3.III. Clinical trials are undertaken to further evaluate dosage, clinical efficacy or potency, and safety in an expanded patient population at geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk to benefit ratio of the product and provide an adequate basis for product labeling.
•Phase 4.IV. Companies may voluntarily pursue additional clinical trials after a product is approved to gain more information about the product for that approved indication.
In some cases, the FDA may require an additional trial after a product is approved, and these so-called Phase 4IV trials may be a condition to approval of the BLA or NDA.
Phase 1,I, Phase 2II, and Phase 3III testing may not be completed successfully within a specified period, if at all, and there can be no assurance that the data collected will support FDA approval or licensure of the product. Concurrent with clinical trials, companies may complete additional animal studies and develop additional information about the biological characteristics of the product candidate and must finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. To help reduce the risk of the introduction of adventitious agents with use of biological products, the Public Health Service ActPHSA emphasizes the importance of manufacturing control for products whose attributes cannot be precisely defined. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final product, or for biologics, the safety, purity and potency. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
We may be required to develop and implement additional clinical trial policies and procedures designed to help protect subjects from the COVID-19 virus. Since March 2020, the FDA has issued various COVID-19 related guidance for sponsors and manufacturers, including guidance on, on conducting clinical trials during the pandemic, and Good Manufacturing Practice considerations for responding to COVID-19 infection in employees in drug and biological products manufacturing, among others. The extent to which the COVID-19 pandemic impacts our business, preclinical studies and clinical trials will depend on future developments, which are highly uncertain and cannot be predicted with confidence. Recently, President Biden announced that the administration intends to end the COVID-19 national and public health emergencies on May 11, 2023. The full impact of the termination of the public health emergencies on FDA and other regulatory policies and operations are unclear.
BLA/NDA Submission and Review by the FDA
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, nonclinical studies and clinical trials are submitted to the FDA as part of a BLA for a biologic product candidate or an NDA for a small molecule product candidate requesting approval to market the product for one or more indications. Unless agreed to in advance with the FDA, the BLA/NDA must include all data available from pertinent preclinical and clinical trials, including negative or ambiguous results, as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls, and proposed labeling, among other things. Data can come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of the product or from a number of alternative sources, including studies initiated by investigators.investigators, including government agencies (e.g., NIH). The submission of a BLA/NDA requires payment of a substantial user fee to the FDA, and the sponsor of an approved BLA/NDA is subject to annual product and establishment user fees. These fees typically increase annually. A waiver of user fees may be obtained under certain limited circumstances.
Within 60 days following submission of the application, the FDA reviews a BLA/NDA to determine if it is substantially complete before the agency accepts it for filing. The FDA may refuse to file any BLA or NDA that it deems incomplete or not properly reviewable at the time of submission and may request additional information. In this event, the BLA/NDA must be resubmitted with the additional information. Once a BLA/NDA has been filed,submitted, the FDA’s goal is to review the application within ten months after it accepts the application for filing, or, if the application relates to an unmet medical need in a serious or life-threatening indication, the FDA may review the application six months after the FDA accepts the application for filing. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA reviews a BLA/NDA to determine, among other things, whether a product is safe and effective, or safe, pure, and potent for the proposed indication(s) and the facility in which it is manufactured, processed, packed or held meets standards designed to assure the product’s continued safety, purity and potency or efficacy. The FDA may convene an advisory committee to provide clinical insight on application review questions. Before approving a BLA/NDA, the FDA will typically inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities comply with cGMP requirements and are adequate to assure consistent production of the product within required specifications. If applicable, FDA regulations also require tissue establishments to register and list their human cells, tissues, and cellular and tissue-based products with the FDA and to evaluate donors through screening and testing. Additionally, before approving a BLA/NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP.GCP guidelines. If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The testing and approval process require substantial time, effort, and financial resources, and each may take several years to complete. The FDA may not grant approval on a timely basis, or at all, and we may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our product candidates. After the FDA evaluates a BLA/NDA and conducts inspections of manufacturing facilities where the investigational product and/or its drug substance will be produced, the FDA may issue an approval letter or a complete response letter.CRL. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A complete response letterCRL indicates that the review cycle of the application is complete, and the application is not ready for approval. A complete response letterCRL may request additional information or clarification. The FDA may delay or refuse approval of a BLA/NDA if applicable regulatory criteria are not satisfied, require additional testing or information and/or require post-marketing testing and surveillance to monitor safety or efficacy of a product.
If regulatory approval of a product is granted, such approval may entail limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the BLA/NDA with a REMS plan to mitigate risks, which could include medication guides, physician communication plans, or other restrictions to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing regulatory standards is not maintained or if problems occur after the product reaches the marketplace. The FDA may require one or more Phase 4IV post-market trials and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization and may limit further marketing of the product based on the results of these post-marketing studies. In addition, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our product candidates under development.
A sponsor may seek approval of its product candidate under programs designed to accelerate the FDA’s review and approval of new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. For a Fast Track product, the FDA may consider sections of the BLA/NDA for review on a rolling basis before the complete application is submitted if relevant criteria are met. A Fast Track-designated product candidate may also qualify for priority review. Priority review is granted when there is evidence that the proposed product would be a significant improvement in the safety or effectiveness of the treatment, diagnosis, or prevention of a serious condition. If criteria are not met for priority review, the application is subject to the standard FDA review period. Priority review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval.
Under the accelerated approval program, the FDA may approve a BLA/NDA on the basis of either a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. Post-marketing studies or completion of ongoing studies after marketing approval are generally required to verify the product’s clinical benefit in relationship to the surrogate endpoint or ultimate outcome in relationship to the clinical benefit. In December 2022, the Consolidated Appropriations Act, 2023, including theThe Food and Drug Omnibus Reform Act (FDORA), was signed into law. FDORA made several changes to the FDA’s authorities and its regulatory framework, including, among other changes, reforms to the accelerated approval pathway, such as requiring the FDA to specify conditions for post-approval study requirements and setting forth procedures for the FDA to withdraw a product on an expedited basis for non-compliance with post-approval requirements.
In addition, the Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA)FDASIA established Breakthrough Therapy designation. A sponsor may seek FDA designation of its product candidate as a Breakthrough Therapy if the product candidate is intended, alone or in combination with one or more other drugs or biologics, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the therapy may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Sponsors may request the FDA to designate a Breakthrough Therapy at the time of, or any time after, the submission of an IND, but ideally before an end-of-Phase 2II meeting with the FDA. If the FDA designates a Breakthrough Therapy, it may take actions appropriate to expedite the development and review of the application, which may include holding meetings with the sponsor and the review team throughout the development of the therapy; providing timely advice to, and interactive communication with, the sponsor regarding the development of the product
candidate to ensure that the development program to gather the nonclinical and clinical data necessary for approval is as efficient as practicable; involving senior managers and experienced review staff, as appropriate, in a collaborative, cross-disciplinary review; assigning a cross-disciplinary project lead for the FDA review team to facilitate an efficient review of the development program and to serve as a scientific liaison between the review team and the sponsor; and considering alternative clinical trial designs when scientifically appropriate, which may result in smaller or more efficient clinical trials that require less time to complete and may minimize the number of patients exposed to a potentially less efficacious treatment. Breakthrough Therapy designation also allows the sponsor to file sections of the BLA/NDA for review on a rolling basis. We may seek designation as a Breakthrough Therapy for some or all of our product candidates.
Breakthrough Therapy and/or Fast Track designations and priority review do not change the standards for approval. The receipt of such designations may not lead to a faster development process or regulatory review and may not increase the likelihood that a product candidate will receive approval.
In addition, the Pediatric Research Equity Act (PREA)PREA requires a sponsor to conduct pediatric clinical trials for certain drugs and biological products, for a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. Under PREA, original NDAs/BLAs and supplements must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must evaluate the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The sponsor or FDA may request a deferral of pediatric clinical trials for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that the product candidate is ready for approval for use in adults before pediatric clinical trials are complete or that additional safety or effectiveness data needs to be collected before the pediatric clinical trials begin. The FDA must send a non-compliance letter to any sponsor that fails to submit the required assessment, keep a deferral current or fails to submit a request for approval of a pediatric formulation.
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, defined as a disease or condition with a patient population of fewer than 200,000 individuals in the U.S., or a patient population greater than 200,000 individuals in the U.S. and when there is no reasonable expectation that the cost of developing and making available the drug or biologic in the U.S. will be recovered from sales in the U.S. for that drug or biologic. Orphan drug designation must be requested before submitting a BLA or NDA. After the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA.
If a product that has orphan drug designation subsequently receives the first FDA approval for a particular active ingredient for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full BLA or NDA, to market the same biologic or drug product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or if the FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Orphan drug exclusivity does not prevent the FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the BLA application user fee.
A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the U.S. may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Emergency Use Authorization
Operation Warp Speed (OWS) aimed to deliver 300 million doses of a safe, effective vaccine for COVID-19 by January 2021, and was part of a broader government strategy to accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics. OWS was a partnership among components of HHS, including the Centers for Disease Control and Prevention, the FDA, the NIH, and the Biomedical Advanced Research and Development Authority (BARDA) and the Department of Defense; engaged with private firms and other federal agencies; and coordinated existing HHS-wide efforts, including the NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines partnership, NIH’s Rapid Acceleration of Diagnostics initiative, and work by BARDA. A COVID-19 vaccine product may be approved initially under an emergency use authorization (EUA) followed by a full BLA approval when more data are available and submitted to FDA for approval.
On February 4, 2020, the Secretary of HHS determined that the COVID-19 pandemic is a public health emergency that has a significant potential to affect national security or the health and security of U.S. citizens. On the basis of such determination, on March 27, 2020, the Secretary of HHS declared that circumstances exist justifying the authorization of emergency use of drugs and biologics during the COVID-19 outbreak, pursuant to Section 564 of the FDCA, which permits the FDA Commissioner to allow unapproved medical products or unapproved uses of approved medical products to be used in the COVID-19 public health emergency. FDA has created the Coronavirus Treatment Acceleration Program, a new program designed to expedite the development of potential COVID-19 therapies by using every tool at the agency’s disposal to determine if the therapies are safe and effective for their intended uses. In issuing an emergency use authorization, FDA will consider the totality of scientific evidence available to FDA regarding safety, efficacy and known and potential risks of such products and availability of alternatives to the emergency use products, among others. Emergency Use Authorizations issued by FDA will specify the scope authorization and conditions of authorization, including limitations on distribution and conditions related to product advertising and promotion. Once granted, an EUA is effective will be effective until the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biologics for prevention and treatment of COVID-19 is terminated under Section 564(b)(2) of the FDCA or the EUA is revoked under Section 564(g) of the FDCA, after which the product must be approved by FDA under a traditional pathway in order to remain on the market or to continue commercialization of the product.
Post-Approval Requirements
Any products manufactured or distributed by us pursuant to FDA approvalsapproval are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to record keeping, reporting of adverse experiences, periodic reporting, distribution, and advertising and promotion of the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are
continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data. Biopharmaceutical manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP, which impose certain procedural and documentation requirements upon us and any third-party manufacturers that we may decide to use. Changes to the manufacturing process are strictly regulated and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting requirements upon us, and any third-party manufacturers, that we may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP regulations and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may, among other things, halt our clinical trials, require us to recall a product from distribution, or withdraw approval of the BLA or NDA.
In the U.S., once a drug is approved, its manufacture is subject to comprehensive and continuing regulation by the FDA. FDA regulations require that drugs be manufactured in specific facilities per the BLA or NDA approval and in accordance with cGMP. Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance. These regulations also impose certain organizational, procedural, and documentation requirements with respect to manufacturing and quality assurance activities. BLA or NDA holders using contract manufacturers, laboratories or packagers are responsible for the selection and monitoring of qualified firms, and, in certain circumstances, qualified suppliers to these firms. These firms and, where applicable, their suppliers are subject to inspections by the FDA at any time, and the discovery of violative conditions, including failure to conform to cGMP, could result in enforcement actions that interrupt the operation of any such facilities or the ability to distribute drugs manufactured, processed, or tested by them.
Future FDA and state inspections may identify compliance issues at our facilities or at the facilities of contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of previously unknown problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer, or holder of an approved BLA or NDA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial action that could delay or prohibit further marketing.
Post-Marketing Requirements
Following approval of a new drug, a biopharmaceutical company and the approved drug are subject to continuing regulation by the FDA, including, among other things, establishment registration and drug listing, monitoring and recordkeeping activities, reporting to the applicable regulatory authorities of adverse experiences with the drug, providing the regulatory authorities with updated safety and efficacy information, drug sampling and distribution requirements, and complying with promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting drugs for uses or in patient populations that are not described in the drug’s approved labeling, limitations on industry-sponsored scientific and educational activities, and requirements for promotional activities involving the internet.
In the U.S., once a drug is approved, its manufacture is subject to comprehensive and continuing regulation by the FDA. FDA regulations require that drugs be manufactured in specific facilities per the NDA approval and in accordance with cGMP. We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of its drugs in accordance with cGMP regulations. cGMP regulations require among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation and the obligation to investigate and correct any deviations from cGMP.
Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance. These regulations also impose certain organizational, procedural, and documentation requirements with respect to manufacturing and quality assurance activities. NDA holders using contract manufacturers, laboratories or packagers are responsible for the selection and monitoring of qualified firms, and, in certain circumstances, qualified suppliers to these firms. These firms and, where applicable, their suppliers are subject to inspections by the FDA at any time, and the discovery of violative conditions, including failure to conform to cGMP, could result in enforcement actions that interrupt the operation of any such facilities or the ability to distribute drugs manufactured, processed, or tested by them. Discovery of problems with a drug after approval may result in restrictions on a drug, manufacturer, or holder of an approved NDA, including, among other things, recall or withdrawal of the drug from the market, and may require substantial resources to correct.
The FDA may also require post-approval testing, sometimes referred to as Phase 4IV testing, risk minimization action plans, and post-marketing surveillance to monitor the effects of an approved drug or place conditions on an approval that could restrict the distribution or use of the drug. The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, among other things:
•restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
•fines, warning letters or holds on post-approval clinical trials;
•refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals;
•product seizure or detention, or refusal to permit the import or export of products; or
•injunctions or the imposition of civil or criminal penalties.
The FDA closely regulates the marketing, labeling, advertising and promotion of biologics or drug products. A company can make only those claims relating to safety and efficacy, purity and potency that are approved by the FDA and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. Failure to comply with these requirements can result in, among other things, adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties, and exclusion from participation in governmental health programs, like Medicare and Medicaid. Physicians may prescribe legally available products for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturer’s communications on the subject of off-label use of their products. The federal government has levied large civil and criminal fines against companies for alleged improper promotion of off-label use and has enjoined companies from engaging in off-label promotion. The FDA and other regulatory agencies have also required that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. However, companies may share truthful and not misleading information that is otherwise consistent with a product’s FDA-approved labelling. Modifications or enhancements to the drug or its labeling or changes of the site or process of manufacture are often subject to the approval of the FDA and other regulators, which may or may not be received or may result in a lengthy review process.
Prescription drug advertising is subject to federal, state, and foreign regulations. In the U.S., the FDA regulates prescription drug promotion, including direct-to-consumer advertising. Prescription drug promotional materials must be submitted to the FDA in conjunction with their first use. Any distribution of prescription drugs and pharmaceutical samples must comply with the U.S. Prescription Drug Marketing Act (PDMA),PDMA, a part of the FDCA.FD&C Act. The Drug Supply Chain Security Act (DSCSA),DSCSA, enacted in 2013, aims to build an electronic system to identify and
trace certain prescription drugs distributed in the U.S. The DSCSA mandates phased-in and resource-intensive obligations for pharmaceutical manufacturers, wholesale distributors, and dispensers. The law’s requirements include the quarantine and prompt investigation of a suspect product to determine if it is illegitimate and notifying trading partners and the FDA of any illegitimate product. Drug manufacturers and their collaborators are also required to place a unique product identifier on prescription drug packages.
Premarket Clearance and Approval Requirements for Medical Devices
Each medical device we seek to commercially distribute in the U.S., including our bioreactors, will require either a prior 510(k) clearance, unless it is exempt (PMA)has received a PMA from the FDA. Generally, if a new device has a predicate that is already on the market under a 510(k) clearance, the FDA will allow that new device to be marketed under a 510(k) clearance; otherwise, a PMA is required. Medical devices are classified into one of three classes: Class 1, Class 2, or Class 3, depending on the degree of risk associated with each medical device and the extent of control needed to provide reasonable assurance of safety and effectiveness. Class 1 devices are deemed to be low risk and are subject to the general controls of the FDCA,FD&C Act, such as provisions that relate to: adulteration; misbranding; registration and listing; notification, including repair, replacement, or refund; records and reports; and good manufacturing practices. Most Class 1 devices are classified as exempt from pre-marketpremarket notification under section 510(k) of the FDCA,FD&C Act, and therefore may be commercially distributed without obtaining 510(k) clearance from the FDA. Class 2 devices are subject to both general controls and special controls to provide reasonable assurance of safety and effectiveness. Special controls include performance standards, post market surveillance, patient registries and guidance documents. A manufacturer may be required to submit to the FDA a pre-marketpremarket notification requesting permission to commercially distribute some Class 2 devices. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously cleared 510(k) device, are placed in Class 3. A Class 3 device cannot be marketed in the U.S. unless the FDA approves the device after submission of a PMA. However, there are some Class 3 devices for which FDA has not yet called for a PMA. For these devices, the manufacturer must submit a pre-marketpremarket notification and obtain 510(k) clearance in orders to commercially distribute these devices. The FDA can also impose sales, marketing, or other restrictions on devices in order to assure that they are used in a safe and effective manner.
510(k) Clearance Pathway
When a 510(k) clearance is required, we must submit a pre-marketpremarket notification to the FDA demonstrating that our proposed device is substantially equivalent to a predicate device, which is a previously cleared and legally marketed 510(k) device or a device that was in commercial distribution before May 28, 1976. By regulation, a pre-marketpremarket notification must be submitted to the FDA at least 90 days before we intend to distribute a device. As a practical matter, clearance often takes significantly longer. To demonstrate substantial equivalence, the manufacturer must show that the proposed device has the same intended use as the predicate device, and it either has the same technological characteristics, or different technological characteristics and the information in the pre-marketpremarket notification demonstrates that the device is equally safe and effective and does not raise different questions of safety and effectiveness. The FDA may require further information, including clinical data, to make a determination regarding substantial equivalence. If the FDA determines that the device, or its intended use, is not substantially equivalent to a previously cleared device or use, the FDA will place the device into Class 3.
There are three types of 510(k)s: traditional; special; and abbreviated. Special 510(k)s are for devices that are modified and the modification needs a new 510(k) but does not affect the intended use or alter the fundamental scientific technology of the device. Abbreviated 510(k)s are for devices that conform to a recognized standard. The special and abbreviated 510(k)s are intended to streamline review, and the FDA intends to process special 510(k)s within 30 days of receipt.
De Novo Classification
Medical device types that the FDA has not previously classified as Class 1, 2 or 3 are automatically classified into Class 3 regardless of the level of risk they pose. The Food and Drug Administration Modernization Act of 1997 established a new route to market for low to moderate risk medical devices that are automatically placed into Class 3 due to the absence of a predicate device, called the Request for Evaluation of Automatic Class 3 Designation (or the De Novo Classification Process).
This procedure allows a manufacturer whose novel device is automatically classified into Class 3 to request down-classification of its medical device into Class 1 or Class 2 on the basis that the device presents low or moderate risk, rather than requiring the submission and approval of a PMA application. Prior to the enactment of the FDASIA, a medical device could only be eligible for de novoDe Novo classification if the manufacturer first submitted a 510(k) pre-marketpremarket notification and received a determination from the FDA that the device was not substantially equivalent. The FDASIA streamlined the de novoDe Novo classification pathway by permitting manufacturers to request de novoDe Novo classification directly without first submitting a 510(k) pre-marketpremarket notification to the FDA and receiving a not substantially equivalent determination. Under the FDASIA, the FDA is required to classify the device within 120 days following receipt of the de novoDe Novo application. If the manufacturer seeks reclassification into Class 2, the manufacturer must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. In addition, the FDA may reject the reclassification petition if it identifies a legally marketed predicate device that would be appropriate for a 510(k) or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and special controls cannot be developed.
Pre-marketPremarket Approval Pathway
A PMA application must be submitted to the FDA for Class 3 devices for which the FDA has required a PMA. The PMA application process is much more demanding than the 510(k) pre-marketpremarket notification process. A PMA application must be supported by extensive data, including but not limited to technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to the FDA’s satisfaction reasonable evidence of safety and effectiveness of the device.
After a PMA application is submitted, the FDA has 45 days to determine whether the application is sufficiently complete to permit a substantive review and thus whether the FDA will file the application for review. The FDA has 180 days to review a filed PMA application, although the review of an application generally occurs over a significantly longer period of time and can take up to several years. During this review period, the FDA may request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device.
Although the FDA is not bound by the advisory panel decision, the panel’s recommendations are important to the FDA’s overall decision making process. In addition, the FDA may conduct a preapproval inspection of the manufacturing facility to ensure compliance with the Quality System Regulation (QSR).QSR. The agency also may inspect one or more clinical sites to assure compliance with FDA’s regulations.
Upon completion of the PMA review, the FDA may: (i) approve the PMA application which authorizes commercial marketing with specific prescribing information for one or more indications, which can be more limited than those originally sought; (ii) issue an approvable letter which indicates the FDA’s belief that the PMA application is approvable and states what additional information the FDA requires or the post-approval commitments that must be agreed to prior to approval; (iii)issue a not approvable letter which outlines steps required for approval, but which are typically more onerous than those in an approvable letter, and may require additional clinical trials that are often expensive and time consuming and can delay approval for months or even years; or (iv) deny the application. If the FDA issues an approvable or not approvable letter, the applicant has 180 days to respond, after which the FDA’s review clock is reset.
Clinical trials are almost always required to support PMA and are sometimes required for 510(k) clearance. In the U.S., for significant risk devices, these trials require submission of an application for an Investigational Device Exemption (IDE)IDE to the FDA. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE must be approved in advance by the FDA for a specific number of patients at specified trial sites. During the trial, the sponsor must comply with the FDA’s IDE requirements for investigator selection, trial monitoring, reporting and recordkeeping. The investigators must obtain patient informed consent, rigorously follow the investigational plan, and trial protocol, control the disposition of investigational devices and comply with all reporting and recordkeeping requirements. Clinical trials for significant risk devices may not begin until the
IDE application is approved by the FDA and the appropriate IRBs at the clinical trial sites. An IRB is an appropriately constituted group that has been formally designated to review and monitor medical research involving subjects and which has the authority to approve, require modifications in, or disapprove research to protect the rights, safety, and welfare of human research subjects. A nonsignificant
risk device does not require FDA approval of an IDE; however, the clinical trial must still be conducted in compliance with various requirements of FDA’s IDE regulations and be approved by an IRB at the clinical trial sites. The FDA or the IRB at each site at which a clinical trial is being performed may withdraw approval of a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the benefits or a failure to comply with FDA or IRB requirements. Even if a trial is completed, the results of clinical testing may not demonstrate the safety and effectiveness of the device, may be equivocal or may otherwise not be sufficient to obtain approval or clearance of the product.
Sponsors of clinical trials of devices are required to register with clinical trials.gov, a public database of clinical trial information. Information related to the device, patient population, phase of investigation, study sites and investigators and other aspects of the clinical trial is made public as part of the registration.
Ongoing Medical Device Regulation by the FDA
Even after a device receives clearance or approval and is placed on the market, numerous regulatory requirements apply. These include:
•establishment registration and device listing;
•the QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation, and other quality assurance procedures during all aspects of the manufacturing process;
•labeling regulations and the FDA prohibitions against the promotion of products for uncleared, unapproved or off-label uses and other requirements related to promotional activities;
•medical device reporting regulations, which require that manufactures report to the FDA if their device may have caused or contributed to a death or serious injury, or if their device malfunctioned and the device or a similar device marketed by the manufacturer would be likely to cause or contribute to a death or serious injury if the malfunction were to recur;
•corrections and removal reporting regulations, which require that manufactures report to the FDA field corrections or removals if undertaken to reduce a risk to health posed by a device or to remedy a violation of the FDCAFD&C Act that may present a risk to health; and
•post market surveillance regulations, which apply to certain Class 2 or 3 devices when necessary to protect the public health or to provide additional safety and effectiveness data for the device.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, will require a new clearance or possibly a PMA. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacture’s determination. If the FDA disagrees with our determination not to seek a new 510(k) clearance, the FDA may retroactively require us to seek 510(k) clearance or possibly a PMA. The FDA could also require manufacturer to cease marketing and distribution and/or recall the modified device until 510(k) clearance or PMA is obtained. Also, in these circumstances, the manufacturer may be subject to significant regulatory fines and penalties.
Some changes to an approved PMA device, including changes in indications, labeling or manufacturing processes or facilities, require submission and FDA approval of a new PMA application or PMA supplement, as appropriate, before the change can be implemented. Supplements to a PMA application often require the submission of the same type of information required for an original PMA application, except that the supplement is generally limited to that information needed to support the proposed change from the device covered by the original PMA. The FDA uses the same procedures and actions in reviewing PMA supplements as it does in reviewing original PMA applications.
FDA regulations require us to register as a medical device manufacturer with the FDA. Additionally, some states require us to register as a medical device manufacturer within the state. Because of this, the FDA and similar state agencies may inspect us on a routine basis for compliance with the QSR. In February 2024, the FDA issued a final rule replacing the QSR with the QMSR, which incorporates by reference the quality management system requirements of ISO 13485:2016. The FDA has stated
that the standards contained in ISO 13485:216 are substantially similar to those set forth in the existing QSR. This final rule does not go into effect until February 2026. These regulations require that the manufacturer maintain proper documentation in a prescribed manner with respect to manufacturing, testing and control activities. Further, the FDA requires medical device manufacturers to comply with various FDA regulations regarding labeling.
Failure by us or by our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or state authorities, which may include any of the following sanctions:
•warning or untitled letters, fines, injunctions, consent decrees and civil penalties;
•customer notifications, voluntary or mandatory recall or seizure of our medical device product candidates;
•operating restrictions, partial suspension, or total shutdown of production;
•delay in processing submissions or applications for new products or modifications to existing products;
•withdrawing approvals that have already been granted; and
•criminal prosecution.
The Medical Device Reporting laws and regulations require medical device manufacturers to provide information to the FDA when they receive or otherwise become aware of information that reasonably suggests the device may have caused or contributed to a death or serious injury as well as a device malfunction that likely would cause or contribute to death or serious injury if the malfunction were to recur. In addition, the FDA prohibits an approved device from being marketed for off-label use. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability, including substantial monetary penalties and criminal prosecution.
Newly discovered or developed safety or effectiveness data may require changes to a product’s labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory clearance or approval of our medical device product candidates under development. Medical device manufacturers are also subject to other federal, state, and local laws and regulations relating to safe working conditions, laboratory and manufacturing practices.
Other Healthcare Laws and Compliance Requirements
Manufacturing, sales, promotion, and other activities following drug approval are also subject to regulation by numerous regulatory authorities in addition to the FDA, including, in the U.S., CMS, other divisions of HHS, the Drug Enforcement Administration for controlled substances, the Consumer Product Safety Commission, the FTC, Occupational Safety and Health Administration, the Environmental Protection Agency, and state and local governments. In the U.S., sales, marketing, and scientific/educational programs must also comply with state and federal fraud and abuse laws. Pricing and rebate programs must comply with the Medicaid rebate requirements of the U.S. Omnibus Budget Reconciliation Act of 1990 and more recent requirements in the Patient Protection and Affordable Care Act as amended by the ACA. If drugs are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. The handling of any controlled substances must comply with the U.S. Controlled Substances Act and Controlled Substances Import and Export Act. Drugs must meet applicable child-resistant packaging requirements under the U.S. Poison Prevention Packaging Act. Manufacturing, sales, promotion, and other activities are also potentially subject to federal and state consumer protection and unfair competition laws.
We are subject to numerous foreign, federal, state, and local environmental, health, and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment, and disposal of hazardous materials and wastes. In addition, our leasing and operation of real property may subject us to liability pursuant to certain U.S. environmental laws and regulations, under which current or previous owners or operators of real property and entities that disposed or arranged for the disposal of hazardous substances may be held strictly, jointly, and severally liable for the cost of investigating or remediating contamination caused by hazardous substance releases, even if they did not know of and were not responsible for the releases.
The distribution of pharmaceutical drugs is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage, and security requirements intended to prevent the unauthorized sale of pharmaceutical drugs. The failure to comply with regulatory requirements subjects firms to possible legal or regulatory action. Depending on the circumstances, failure to meet applicable regulatory requirements can result in criminal prosecution, fines, or other penalties, injunctions, voluntary recall, or seizure of drugs, total or partial suspension of production, denial or withdrawal of product approvals, or refusal to allow a firm to enter into supply contracts, including government contracts. In addition, even if a firm complies with FDA and other requirements, new information regarding the safety or efficacy of a product could lead the FDA to modify or withdraw product approval. Prohibitions or restrictions on sales or withdrawal of future products marketed by us could materially affect our business in an adverse way.
existing regulations could impact our business in the future by requiring, for example: (i) changes to our manufacturing arrangements; (ii) additions or modifications to product labeling; (iii) the recall or discontinuation of our products; or (iv) additional record-keeping requirements. If any such changes were to be imposed, they could adversely affect the operation of our business. Our sales, promotion, medical education, clinical research, and other activities following product approval will be subject to regulation by numerous regulatory and law enforcement authorities in the U.S. in addition to the FDA, including potentially the Federal Trade Commission,FTC, the Department of Justice, the CMS, other divisions of HHS and state and local governments. Our promotional and scientific/educational programs must comply with the AKS, the FCA, physician payment transparency laws, privacy laws, security laws, and additional federal and state laws similar to the foregoing. If drugs are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. Drugs must meet applicable child-resistant packaging requirements under the U.S. Poison Prevention Packaging Act. Manufacturing, sales, promotion, and other activities are also potentially subject to federal and state consumer protection and unfair competition laws. Changes in regulations, statutes, or the interpretation of existing regulations could impact our business in the future by requiring, for example: (i) changes to our manufacturing arrangements; (ii) additions or modifications to product labeling; (iii) the recall or discontinuation of our products; or (iv) additional record-keeping requirements. If any such changes were to be imposed, they could adversely affect the operation of our business.
The AKS prohibits, among other things, the knowing and willing, direct or indirect offer, receipt, solicitation, or payment of remuneration in exchange for or to induce the referral of patients, including the purchase, order or lease of any good, facility, item or service that would be paid for in whole or part by Medicare, Medicaid or other federal health care programs. Remuneration has been broadly defined to include anything of value, including cash, improper discounts, and free or reduced-price items and services. The AKS has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, formulary managers, and beneficiaries on the other. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the AKS. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the AKS has been violated. The government has enforced the AKS to reach large settlements with healthcare companies based on sham research or consulting and other financial arrangements with physicians. Further, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the AKS constitutes a false or fraudulent claim for purposes of the FCA. Many states have similar laws that apply to their state health care programs, as well as private payors.
Federal false claims and false statement laws, including the FCA, imposes liability on persons or entities that, among other things, knowingly present or cause to be presented claims that are false or fraudulent or not provided as claimed for payment or approval by a federal health care program. The FCA has been used to prosecute persons or entities that “cause”cause the submission of claims for payment that are inaccurate or fraudulent, by, for example, providing inaccurate billing or coding information to customers, promoting a product off-label, submitting claims for services not provided as claimed, or submitting claims for services that were provided but not medically necessary. Actions under the FCA may be brought by the Attorney General, or as a
qui tam action by a private individual, in the name of the government. Violations of the FCA can result in significant monetary penalties and treble damages. The federal government is using the FCA, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical and biotechnology companies throughout the country, for example, in connection with the promotion of products for unapproved uses and other illegal sales and marketing practices. The government has obtained multi-million and multi-billion dollar settlements under the FCA in addition to individual criminal convictions under applicable criminal statutes. In addition, certain companies that were found to be in violation of the FCA have been forced to implement extensive corrective action plans and have often become subject to consent decrees or corporate integrity agreements, restricting the manner in which they conduct their business.
The Health Insurance Portability and Accountability Act (HIPAA)HIPAA created additional federal criminal statutes that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors; knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services; and willfully obstructing a criminal investigation of a healthcare offense. Like the AKS, the ACA amended the intent standard for certain healthcare fraud statutes under HIPAA such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws. In addition, many states have similar fraud and abuse statutes or regulations that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs. Additionally, to the extent that our product candidates, once commercialized, are sold in a foreign country, we may be subject to similar foreign laws.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians and certain other healthcare providers and teaching hospitals. The ACA, among other things, under the federal Physician Payment Sunshine Act, imposed reporting requirements on certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, for payments or other transfers of value made by them to certain covered recipients, including physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Effective January 1, 2022, these reporting obligations with respect to covered recipients have been extended to include payments and transfers of value made to non-physician providers such as physician assistants and nurse practitioners. Covered manufacturers are required to collect and report detailed payment data and submit legal attestation to the accuracy of such data to the government each year. Failure to submit required information may result in civil monetary penalties for all payments, transfers of value or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission. Additionally, entities that do not comply with mandatory reporting requirements may be subject to a corporate integrity agreement. Certain states also mandate implementation of commercial compliance programs, impose restrictions on covered manufacturers’ marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to physicians and other healthcare professionals.
We may also be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA, as amended by the Health Information Technology and Clinical Health Act of 2009 (HITECH)HITECH and their respective implementing regulations, impose specified requirements on certain health care providers, plans, and clearinghouses, or collectively, covered entities, and their “businessbusiness associates,” relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s security standards directly applicable to “businessbusiness associates,” which includes independent contractors or agents of covered entities that create, receive, maintain, or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce HIPAA and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, certain states have their own laws that govern the privacy and security of health information in certain circumstances, many of which differ from each other and/or HIPAA in significant ways and may not have the same effect, thus complicating compliance efforts.
If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs, imprisonment, contractual damages, reputational harm, and diminished profits and future earnings, any of which could adversely affect our ability to operate our business and our financial results. If any of the physicians or other providers, independent contractors, or entities with whom we do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs and imprisonment, which could affect our ability to operate our business. Further, defending against any such actions can be costly, time-consuming and may require significant personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired.
In addition to the foregoing health care laws, we are also subject to the U.S. Foreign Corrupt Practices Act (FCPA)FCPA and similar worldwide anti-bribery laws, which generally prohibit companies and their intermediaries from making improper payments to government officials or private-sector recipients for the purpose of obtaining or retaining business. The anti-corruption policy mandates compliance with the FCPA and similar anti-bribery laws applicable to our business throughout the world. However, we cannot assure you that such a policy or procedures implemented to enforce such a policy will protect us from intentional, reckless, or negligent acts committed by our employees, distributors, partners, collaborators or agents. Violations of these laws, or allegations of such violations, could result in fines, penalties or prosecution and have a negative impact on our business, results of operations and reputation.
Coverage and Reimbursement
Sales of pharmaceutical products depend significantly on the extent to which coverage and adequate reimbursement are provided by third-party payors. Third-party payors include state and federal government health care programs, managed care providers, private health insurers and other organizations. Although we currently believe that third-party payors will provide coverage and reimbursement for our product candidates, if approved, we cannot be certain of this. Third-party payors are increasingly challenging the price, examining the cost-effectiveness, and reducing reimbursement for medical products and services. In the U.S., no uniform policy of coverage and reimbursement for drugs or biological products exists, and one payor’s determination to provide coverage and adequate reimbursement for a product does not assure that other payors will make a similar determination. In the U.S., the principal decisions about reimbursement for new medicines are typically made by the CMS, an agency within the HHS, as the CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private third-party payors tend to follow Medicare coverage and reimbursement limitations to a substantial degree, but also have their own methods and approval process apart from Medicare determinations. Significant uncertainty exists as to the reimbursement status of newly approved healthcare products.
The U.S. government, state legislatures and foreign governments have continued implementing cost containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Adoption of price controls and cost containment measures and adoption of more restrictive policies in jurisdictions with existing controls and measures could further limit our revenues and results. We may need to conduct expensive clinical trials to demonstrate the comparative cost-effectiveness of our product candidates. The product candidates that we develop may not be considered cost-effective and thus may not be covered or sufficiently reimbursed. It is time consuming and expensive for us to seek coverage and reimbursement from third-party payors, as each payor will make its own determination as to whether to cover a product and at what level of reimbursement. Thus, one payor’s decision to provide coverage and adequate reimbursement for a product does not assure that another payor will provide coverage or that the reimbursement levels will be adequate. Moreover, a payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Reimbursement may not be available or sufficient to allow us to sell our product candidates on a competitive and profitable basis.
Healthcare Reform
The U.S. and some foreign jurisdictions are considering or have enacted a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our product candidates profitably. Among policy makers and payors in the U.S. and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the U.S., the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives.
By way of example, in March 2010, the ACA was signed into law, intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Among the provisions of the ACA of importance to our potential product candidates are:
•an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs;
•an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13.0% of the average manufacturer price for branded and generic drugs, respectively;
•a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected;
•a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 70% (increased pursuant to the Bipartisan Budget Act of 2018, effective as of 2019) point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for a manufacturer’s outpatient drugs to be covered under Medicare Part D;
•extension of a manufacturer’s Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations;
•expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing a manufacturer’s Medicaid rebate liability;
•expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; and
•a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes include, among others, the Budget Control Act of 2011, which mandates aggregate reductions to Medicare payments to providers of up to 2% per fiscal year effective April 1, 2013, and, due to subsequent legislative amendments, will remain in effect through 2031, with the exception of a temporary suspension implemented under various COVID-19 relief legislation from May 1, 2020 through March 31, 2022. Under current legislation, the actual reduction in Medicare payments will vary from 1% in 2022 to up to 4% in the final fiscal year of this sequester.2032. In January 2013, President Obama signed into law the ARTA,ATRA, which, among other things, further reduced Medicare payments to several providers, including hospitals and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for our product candidates, if approved, and, accordingly, our financial operations.
Under the American Rescue Plan Act of 2021, effective January 1, 2024, the statutory cap on Medicaid Drug Rebate Program rebates that manufacturers pay to state Medicaid programs will bewas eliminated. Elimination of this cap may require pharmaceutical manufacturers to pay more in rebates than it receives on the sale of products, which could have a material impact on our business. Further, in July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at increasing competition for prescription drugs. In August 2022, Congress passed the Inflation Reduction Act of 2022, which includes prescription drug provisions that have significant implications for the pharmaceutical industry and Medicare beneficiaries, including allowing the federal government to negotiate a maximum fair price for certain high-priced single source Medicare drugs, imposing penalties and excise tax for manufacturers that fail to comply with the drug price negotiation requirements, requiring inflation rebates for all Medicare Part B and Part D drugs, with limited exceptions, if their drug prices increase faster than inflation, and redesigning Medicare Part D to reduce out-of-pocket prescription drug costs for beneficiaries, among other changes. Various industry stakeholders have initiated lawsuits against the U.S. government asserting that the price negotiation provisions of the IRA are unconstitutional. The impact of these judicial challenges, legislative, executive, and administrative actions and any future healthcare measures and agency rules implemented by the Biden administration on us and the pharmaceutical industry as a whole is unclear. The impact of these regulations and any future
healthcare measures and agency rules implemented by the Biden administration on us and the pharmaceutical industry as a whole is currently unknown. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our product candidates if approved. Complying with any new legislation and regulatory changes could be time-intensive and expensive, resulting in a material adverse effect on our business.
Since the enactment of the ACA, there have been judicial and Congressional challenges to certain aspects of the ACA. In June 2021, the United States Supreme Court held that Texas and other challengers had no legal standing to challenge the ACA, dismissing the case without specifically ruling on the constitutionality of the ACA. Accordingly, the ACA remains in effect in its current form. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear how additional challenges and healthcare reform measures of the Biden administration will impact the ACA. Complying with any new legislation or changes in healthcare regulation could be time-intensive and expensive, resulting in material adverse effect on our business.
At the state level, individual states are increasingly passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including discounts, restrictions on certain product access and marketing cost disclosure and transparency measures. For example, a number of states are considering or have recently enacted state drug price transparency and reporting laws that could substantially increase our compliance burdens and expose us to greater liability under such state laws once we begin commercialization after obtaining regulatory approval for any of our products. For example, the FDA recently authorized the state of Florida to import certain prescription drugs from Canada for a period of two years to help reduce drug costs, provided that Florida’s Agency for Health Care Administration meets the requirements set forth by the FDA. Other states may follow Florida. These measures could reduce the demand for our products, if approved, or impose additional pricing pressures on how much we can charge for our products if approved.
We expect that the ACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and lower reimbursement and in additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government-funded programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our product candidates. Furthermore, the current presidential administration and Congress may continue to attempt broad sweeping changes to the current health care laws. We face uncertainties that might result from modifications or repeal of any of the provisions of the ACA, including as a result of current and future executive orders and legislative actions. The impact of those changes on us and potential effect on the pharmaceutical and biotechnology industries as a whole is currently unknown. However, any changes to the ACA are likely to have an impact on our results of operations and may have a material adverse effect on our results of operations. We cannot predict what other healthcare programs and regulations will ultimately be implemented at the federal or state level or the effect any future legislation or regulation in the U.S. may have on our business.
Foreign Regulation
In addition to regulations in the U.S., we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our product candidates to the extent we choose to develop or sell any product candidates outside of the U.S. The approval process varies from country to country and the time may be longer or shorter than that required to obtain FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Human Capital
Our Human Capital talent strategy relies on attracting, retaining and developing top talent that align with our culture and mission to “outsmart your disease.“Outsmart Your Disease.” We promote a culture that is focused on delivering treatments utilizing natural immunities, and we seek to harness our science first focus to deliver solutions to patients and families. As of December 31, 2022,2023, we had 725628 employees located across the U.S. and Italy. Among our employees, 23%40% are focused on research and development, 14% on clinical development and regulatory, 40%34% on manufacturing and quality, and 23%26% on general and administrative functions. We have not been subject to labor action or union activities, and our management considers its relationships with employees to be good.
We believe that fostering a workplace that celebrates differences and strengths creates an environment that supports the inclusion and value of diverse thoughts, backgrounds and perspectives. A well rounded culture allows for ongoing dialogue and discussions that challenge the status quo and create a learning environment that supports diversity, equity and inclusion. As part of our commitment, we continue to encourage a culture where employees can freely ask questions and raise concerns. Our annual performance review process helps support our commitment to develop and retain top talent by providing an opportunity to have open dialogue, establish goals, discuss milestones and continue to engage in opportunities to develop and cultivate the talent. Additionally, our management team makes themselves available to all employees including 1:1s, Department Meetings and Town Hall events.
Our ongoing success will continue to depend on our ability to attract, engage and retain top talent in an ever growing competitive market. We offer a competitive compensation package to help meet the needs of our employees. In addition to salaries, these programs include the potential to receive annual bonuses stockand equity awards, and a 401(k) plan, healthcare and insurance benefits, flexible spending accounts, paid time off, family leave, flexible work schedules, and an employee assistance program, among others. We work to ensure pay equity by assessing our compensation practices and working with external benchmarks and compensation consultants to design and benchmark our programs.
Our ongoing response to the COVID-19 pandemic, which complies with government orders in all the states and counties where we operate, focuses on employee health and wellness. We implemented a number of health-related measures over the past three years that included work from home policies, on-site access restrictions, and increased cleaning and sanitizing procedures.
Organization and Development of ImmunityBio, Inc.
ImmunityBio, Inc. was established following a series of mergers and name changes. We were incorporated in Illinois on October 7, 2002 under the name ZelleRx Corporation. Our name was later changed to Conkwest, Inc., and we were reincorporated in the state of Delaware in March 2014. On July 10, 2015, we changed our name to NantKwest, Inc. (NantKwest).NantKwest.
NantCell, LLC was originally organized as a Delaware limited liability company in November 2014. In April 2015, it was converted to a Delaware corporation, NantCell, Inc., and in May 2019 changed its name to ImmunityBio, Inc. (a private company) (NantCell).
On December 21, 2020, NantKwest and NantCell entered into an Agreement and Plan of Merger (the Merger Agreement), pursuant to which NantKwest and NantCell agreed to combine their businesses. The Merger Agreement provided that a wholly-owned subsidiary of the company would merge with and into NantCell (the Merger), with NantCell surviving the Merger as a wholly-owned subsidiary of the company. We believe that the Merger, which closed on March 9, 2021, combined two companies to create a clinical-stage biotechnology company developing next-generation therapies and vaccines that complement, harness, and amplify the immune system to defeat cancers and infectious diseases.
ImmunityBio is incorporated in Delaware and its principal executive offices are located in San Diego, California.
Available Information
Financial and other information about our company is available on our website at https://www.immunitybio.com. We make available on our website, free of charge, copies of our Annual Report, on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it, to the U.S. Securities and Exchange Commission (the SEC).SEC. All reports we file with the SEC are available free of charge via EDGAR through the SEC website at https://www.sec.gov. We have included the web addresses of ImmunityBio and the SEC as inactive textual references only. Except as specifically incorporated by reference into this Annual Report, information on these websites is not part of this filing.
ITEM 1A. RISK FACTORS.
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, any of which may be relevant to decisions regarding an investment in or ownership of our stock. The occurrence of any of these risks could have a significant adverse effect on our reputation, business, financial condition, results of operations, growth, and ability to accomplish our strategic objectives. We have organized the description of these risks into groupings in an effort to enhance readability, but many of the risks interrelate or could be grouped or ordered in other ways, so no special significance should be attributed to the groupings or order below.
Risk Factor Summary
Risks Related to Our Limited Operating History, Financial Condition and Capital Requirements
•We anticipate needing additional financing to fund our operations and complete the development and commercialization of our various product candidates, and if we are unable to obtain such financing when needed, or on acceptable terms, we may be unable to complete the development and commercialization of our product candidates.
•Our debt could adversely affect our cash flows and limit our flexibility to raise additional capital.
•Conversion of certain related-party notes may dilute the ownership interest of existing stockholders or may otherwise depress the price of our common stock.
•The accounting method for convertible debt securities could have a material effect on our reported financial results.
•The value of our warrants outstanding is subject to potentially material increases and decreases based on fluctuations in the price of our common stock, which may affect our results of operations and financial position and could adversely affect our stock price.
•We are a clinical-stage biotechnology company with a limited operating history and no products approved for commercial sale. We have a history of operating losses, and we expect to continue to incur losses and may never be profitable, which together with our limited operating history, makes it difficult to assess our future viability.
•We anticipate needing additional financing to fund our operations and complete the development and commercialization of our various product candidates, and if we are unable to obtain such financing when needed, or on acceptable terms, we may be unable to complete the development and commercialization of our product candidates.
•The RIPA imposes Revenue Interest Payments (as defined in the RIPA) obligations, which may adversely affect our financial position and results of operations, as well as affirmative and negative covenants, which restrict our business operations. The breach of our obligations under the RIPA could give rise to a default, triggering a Put Option (as defined in the RIPA) and/or foreclosure on our assets.
•Our debt and revenue interest liability could adversely affect our cash flows and limit our flexibility to raise additional capital.
•The value of our warrants outstanding and the revenue interest liability are subject to potentially material increases and decreases based on fluctuations in the price of our common stock or projected sales and the probability of specific events, which may affect our results of operations and financial position and could adversely affect our stock price.
Risks Related to the Discovery, Development and Commercialization of our Product Candidates
•We will be substantially dependent on the success of our product candidates and cannot guarantee that these product candidates will successfully complete development, receive regulatory approval or be successfully commercialized.
•The CRL we received from the FDA for the BLA has delayed, and may decrease the likelihood of, ultimate approval and successful commercialization of our lead product candidate in the U.S. and potentially other markets.
•We are developing product candidates in combination with other therapies, which exposes us to additional risks.
•We may choose to expend our limited resources on programs that do not yield successful product candidates as opposed to indications that may be more profitable or for which there is a greater likelihood of success.
•Our projections regarding the market opportunities for our product candidates may not be accurate, and the actual market for our products, if approved, may be smaller than we estimate.
•Our clinical trials may fail to adequately demonstrate the safety and efficacy of our product candidates, which would prevent or delay regulatory approval and commercialization. If our trials are not successful, we will be unable to commercialize our product candidates.
Risks Related to Reliance on Third Parties
•We have limited experience conducting clinical trials and have relied and will continue to rely on third parties and related parties to conduct many of our preclinical studies and clinical trials, to manufacture products, and to perform many essential services for any products that we commercialize, including services related to distribution, government price reporting, customer service, accounts receivable management, cash collection and adverse event reporting.commercialize. Any failure by a third party, related party, or by us to perform as expected, to comply with legal and regulatory requirements or to conduct the clinical trials according to Good Clinical Practice (GCP) regulations,GCP guidelines, and in a timely manner, may delay or prevent our ability to seek or obtain regulatory approval for or commercialization of our product candidates and our ability to commercialize our current or future product candidates will be significantly impacted and we may be subject us to regulatory sanctions.
•If third-party manufacturers, wholesalers and distributors fail to perform as expected, or fail to devote sufficient time and resources to our product candidates, our clinical development may be delayed, our costs may be higher than expected or our product candidates may fail to be approved, or we may fail to commercialize any product candidates if approved.
•We use the Immuno-Oncology Clinic, Inc. (the Clinic), a related party, in some of our clinical trials which may expose us to significant regulatory risks. If our data for this site is not sufficiently robust or if there are any data integrity issues, we may be required to repeat such studies or required to contract with other clinical trial sites, andwhich could delay and/or increase the cost of our clinical development plans will be significantly delayed, and we will incur additional costs.plans.
•We have formed, and may in the future form or seek, strategic alliances or enter into collaborations with third parties or additional licensing arrangements, in the future, and we may not realize the benefits of such alliances or licensing arrangements. If we fail to enter into such strategic alliances, collaborations or licensing arrangements, or such strategic alliances, collaborations or licensing arrangements are not successful, weConflicts may not be able to capitalize on the market potential of our product candidates.
•If conflicts arise between us and our collaborators or strategic partners, these partiesand such strategic alliances, collaborations or licensing arrangements may act in a manner adverse to us and could limit our ability to implement our strategies.not be successful.
Risks Related to Healthcare and Other Government Regulations
•We may be unable to obtain U.S. or foreign regulatory approval and, as a result, be unable to commercialize our product candidates. We are, and if we receive regulatory approval of our product candidates, will continue to be subject to ongoing extensive regulation, regulatory obligations and continued regulatory review, which may result in significant additional expense.
•Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions.
•Even if we receive regulatory approval for our lead product candidate Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, or any other product candidates, theywe will continue to be subject to ongoing regulatory requirements, which may result in significant additional expenses. Additionally, our product candidates, if approved, could be subject to labeling and other restrictions, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our product candidates.
•If we are unable to adequately establish sales, marketing and distribution capabilities, we may not be successful commercializing our product candidates if and when they are approved.
•Problems related to large scalelarge-scale commercial manufacturing could cause delays in product launches, an increase in costs, product recalls or shortages of product candidates.shortages.
Risks Related to Intellectual Property
•If we are unable to obtain, maintain, protect and enforce patent protection and other proprietary rights for our product candidates and technologies, we may not be able to compete effectively or operate profitably and our ability to prevent our competitors from commercializing similar or identical technology and product candidates would be adversely affected.
•If any of our owned or in-licensed patent applications do not issue as patents in any jurisdiction, we may not be able to compete effectively.
•We or our licensors, collaborators, or any future strategic partners may become subject to third-party claims or litigation alleging infringement of patents or other proprietary rights or seeking to invalidate patents or other proprietary rights, and we may need to resort to litigation to protect or enforce our patents or other intellectual property or the patents or other intellectual property of our licensors, all of which could be expensive, time-consuming and unsuccessful, may delay or prevent the development and commercialization of our product candidates, or may put our patents and other proprietary rights at risk.
•The use of our technology and product candidates could potentially conflict with the rights of others, and third-party claims of intellectual property infringement, misappropriation or other violation against us, our licensors or our collaborators may prevent or delay the development and commercialization of our product candidates and technologies.
•Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
Risks Related to Our Common Stock and CVRs
•Dr. Soon-Shiong, our Executive Chairman, Global Chief Scientific and Medical Officer and our principal stockholder, has significant interests in other companies which may conflict with our interests.
•Dr. Soon-Shiong, through his voting control of the company, has the ability to control actions that require stockholder approval.
•Conversion of certain related-party promissory notes, exercise of outstanding warrants and options to purchase our common stock, the achievement of the milestone under our outstanding CVRs, and potential additional equity issuances may dilute the ownership interest of existing stockholders or may otherwise depress the price of our common stock.
•The market price of our common stock has been and may continue to be volatile, and investors may have difficulty selling their shares.
Risks Related to Our Limited Operating History, Financial Condition and Capital Requirements
We will need additional financing to fund our operations and complete the development and commercialization of our various product candidates, and if we are unable to obtain such financing when needed, or on acceptable terms, we may be unable to complete the development and commercialization of our product candidates.
The development of biopharmaceutical products, including conducting preclinical studies and clinical trials, is a very time-consuming, expensive and uncertain process that takes years to complete. Our operations have consumed substantial amounts of cash since inception. A significant portion of our funding had been in the form of promissory notes totaling $737.4 million in indebtedness (consisting of related-party promissory notes and accrued and unpaid interest) as of December 31, 2022 held by entities affiliated with Dr. Soon-Shiong.
As of December 31, 2022, we held cash, cash equivalents and marketable securities totaling $108.0 million. We will need to obtain additional financing to fund our future operations, including completing the development and commercialization of our product candidates. Changing circumstances may cause us to increase our spending significantly faster than we currently anticipate and we may need to raise additional funds sooner than we presently anticipate. Moreover, research and development and our operating costs and fixed expenses such as rent and other contractual commitments, including those for our research collaborations, are substantial and are expected to increase in the future.
Unless and until we can generate a sufficient amount of revenues, we may finance future cash needs through public or private equity offerings, license agreements, debt financings, collaborations, strategic alliances and marketing or distribution arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms, or at all.
To the extent that we raise additional capital through the sale of equity or equity-linked securities (including warrants), convertible debt, or through an at-the-market (the ATM) or other offerings, or if any of our current debt is converted into equity, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. The incurrence of additional indebtedness would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms unfavorable to us. We have no committed source of additional capital and if we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may be required to delay or reduce the scope of or eliminate one or more of our research or development programs or our commercialization efforts. Our current license and collaboration agreements may also be terminated if we are unable to meet the payment obligations under those agreements. As a result, we may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time.
Our debt could adversely affect our cash flows and limit our flexibility to raise additional capital.
We have a significant amount of debt and may need to incur additional debt to support our growth. As of December 31, 2022, our indebtedness totals $737.4 million, (consisting of related-party promissory notes and accrued and unpaid interest), held by entities affiliated with Dr. Soon-Shiong.
Our substantial amount of debt could have important consequences and could:
•require us to dedicate a substantial portion of our cash and cash equivalents to make interest and principal payments on our debt, reducing the availability of our cash and cash equivalents and cash flow from operations to fund future capital expenditures, working capital, execution of our strategy and other general corporate requirements;
•increase our cost of borrowing and even limit our ability to access additional debt to fund future growth;
•increase our vulnerability to general adverse economic and industry conditions and adverse changes in governmental regulations;
•limit our flexibility in planning for, or reacting to, changes in our business and industry, which may place us at a disadvantage compared with our competitors; and
•limit our ability to borrow additional funds, even when necessary to maintain adequate liquidity, which would also limit our ability to further expand our business.
The occurrence of any of the foregoing factors could have a material adverse effect on our business, results of operations and financial condition.
Further, the company’s ability to make scheduled payments of the principal of, to pay interest on, or to refinance any current or future indebtedness, including the related-party promissory notes, depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate sufficient cash flows from operations in the future to service our indebtedness and make necessary capital expenditures. If we are unable to generate such cash flows, we may be required to adopt one or more alternatives, such as selling assets, restructuring indebtedness or obtaining additional equity capital on terms that may be onerous or highly dilutive. Our ability to refinance our indebtedness, at maturity or otherwise, will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
There can be no assurance that we can refinance these promissory notes or what terms will be available in the market at the time of refinancing. Furthermore, if prevailing interest rates or other factors at the time of refinancing result in higher interest rates upon refinancing, then the interest expense relating to the refinanced indebtedness would increase. These risks could materially adversely affect our financial condition, cash flows and results of operations.
Conversion of certain related-party notes may dilute the ownership interest of existing stockholders or may otherwise depress the price of our common stock.
As of December 31, 2022, the company currently has promissory notes totaling an aggregate of $737.4 million, (consisting of related-party promissory notes and accrued and unpaid interest) held by entities affiliated with Dr. Soon-Shiong (some of which are convertible under certain circumstances), including variable-rate promissory notes in an aggregate amount of $475.0 million that become due and payable on December 31, 2023, and fixed-rate promissory notes in an aggregate amount of $262.4 million (consisting of principal and accrued and unpaid interest) that become due and payable on September 30, 2025.
In the event of a default on the $300.0 million loan (as defined in the promissory note), including if the company does not repay the loan at maturity, the company has the right, at its sole option, to convert the outstanding principal amount of accrued and unpaid interest due under this note into shares of the company’s common stock at a price of $5.67 per share. The terms of the fixed-rate promissory notes were amended and restated on August 31, 2022 to include a conversion feature that gives each lender the right at any time, including upon notice of prepayment, at its sole option, to convert the entire outstanding principal amount and accrued and unpaid interest due under each note at the time of conversion into shares of the company’s common stock at a price of $5.67 per share.
The conversion of some or all of the aforementioned promissory notes, to the extent we deliver shares upon conversion, at a time when the company’s common stock is valued at greater than $5.67 per share would dilute the ownership interests of existing stockholders. Any sales in the public market of the promissory notes or our common stock issuable upon conversion of the promissory notes could adversely affect prevailing market prices of our common stock.
The accounting method for convertible debt securities could have a material effect on our reported financial results.
In accordance with ASC 470-50, Debt – Modifications and Extinguishments, we recorded the amendments to our related-party promissory notes entered into on August 31, 2022 under the extinguishment accounting model, as the amendments to the fixed-rate promissory notes added a substantive conversion option to the debt. Under this model, the company calculated a gain on extinguishment of $82.9 million, representing the difference between the fair value of the new and amended promissory notes and the carrying value of the extinguished debt, net of any unamortized related-party notes discounts plus the cash proceeds from the new promissory note. Since the debt was obtained from entities under common control, such gain was recorded in additional paid-in capital, on the consolidated statement of stockholders’ deficit for the year ended December 31, 2022. Also, the difference between the principal and accrued interest outstanding as of the date of amendment and the fair value of the new and amended promissory notes was recorded as a debt discount to be amortized as interest expense over the remaining term (or until conversion). As a result, we will be required to record a greater amount of non-cash interest expense in current periods presented as a result of the amortization of the discounted carrying value of the promissory notes to their face amount over the term of such notes. We will report lower net income in our consolidated financial results because ASC 470-20, Debt with Conversion and Other Options, requires interest to include both the current period’s amortization of the debt discount and the instrument’s coupon interest, which could adversely affect our reported or future financial results and the trading price of our common stock.
In August 2020, the FASB issued ASU 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20), to reduce complexity in applying U.S. GAAP to certain financial instruments with characteristics of liability and equity. In addition, the new guidance requires diluted earnings per share (diluted EPS) calculations be prepared using the if-converted method instead of the treasury stock method. Under the if-converted method, the denominator of the diluted EPS calculation is adjusted to reflect the full number of common shares issuable upon conversion, assuming the effect is dilutive, while the numerator is adjusted to add back interest expense (after-tax) for the period. Also, the new guidance eliminated the ability to overcome the presumption of share settlement. The company adopted this statement effective January 1, 2022.
The value of our warrants outstanding is subject to potentially material increases and decreases based on fluctuations in the price of our common stock, which may affect our results of operations and financial position and could adversely affect our stock price.
In December 2022, we completed a registered direct offering of 9,090,909 shares, raising approximately $47.0 million in net proceeds. In connection with the sale of our common stock, we entered into a warrant agreement that offers the purchase of up to 9,090,909 shares at an exercise price of $6.60 per share. The warrants will become immediately exercisable after the issuance date and expire two years after the initial issuance date.
We account for the warrants as a derivative instrument, and changes in the fair value of the warrants are included in other income (expense), net, in the company’s consolidated statement of operations for each reporting period. At December 31, 2022, the fair value of the warrant liability included in the company’s consolidated balance sheet was $21.6 million. We use the Black-Scholes option pricing model to determine the fair value of the warrants. As a result, the valuation of this derivative instrument is subjective, and the Black-Scholes option pricing model requires the input of highly subjective assumptions, including the expected stock price volatility and probability of a fundamental transaction (a strategic merger or sale). Changes in these assumptions can materially affect the fair value estimate. We could, at any point in time, ultimately incur amounts different than the carrying value, which could have a significant impact on our results of operations and financial position.
The fluctuations of warrant value and changes in the assumptions and factors used in the model may impact our operating results, making it difficult to forecast our operating results and making period-to-period comparisons less predictive of future performance. In one or more future quarters, our results of operations may fall below the expectations of securities analysts and investors. In that event, the market price of our common stock could decline. In addition, the market price of our common stock may fluctuate or decline regardless of our operating performance.
We are a clinical-stage biotechnology company with a limited operating history and no products approved for commercial sale. We have a history of operating losses, and we expect to continue to incur losses and may never be profitable, which together with our limited operating history, makes it difficult to assess our future viability.
We are a clinical-stage biotechnology company with a limited operating history upon which you can evaluate our business and prospects, and we have a broad portfolio of product candidates at various stages of development. None of our products have been approved for commercial sale, and we have not generated any revenue from product sales, although we have generated revenues from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables and grant programs. In addition, we have limited experience and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biotechnology industry, including in connection with obtaining marketing approvals, manufacturing a commercial-scale product or arranging for a third party to do so on our behalf or conducting sales and marketing activities necessary for successful product commercialization. Because of the numerous risks and uncertainties associated with our product development efforts, we are unable to predict when we may become profitable, if at all.
Since the commencement of our operations, we have incurred significant losses each year, and, as of December 31, 20222023, we had an accumulated deficit of $2.4$3.0 billion. We expect to continue to incur significant expenses as we seek to expand our business, including in connection with conducting research and development across multiple therapeutic areas, participating in clinical trial activities, continuing to acquire or in-license technologies, maintaining, protecting and expanding our intellectual property, seeking regulatory approvals, increasing our manufacturing capabilities and, upon successful receipt of FDA approval, commercializing our products. Moreover, if our lead product candidate is not approved, we do not expect to have significant product sales or revenue in the near term, if ever. Furthermore, even if approved, the timing and magnitude of product sales and revenue remain uncertain, and may take a significant amount of time to materialize, if ever.
If we are required by the FDA or any equivalent foreign regulatory authority to perform clinical trials or studies in addition to those we currently expect to conduct, or if there are any delays in completing the clinical trials of our product candidates, our expenses could increase substantially. Although weWe have submitted a BLA for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, which was accepted bydisease. On May 9, 2023, the FDA for review, settingdelivered a target PDUFA action date ofCRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised.
On October 23, 2023, we may not receive approval byannounced that we had completed the target PDUFA actionresubmission of the BLA addressing the issues in the CRL. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a new user fee goal date (PDUFA date) of April 23, 2024. While we believe the BLA resubmission addresses the issues identified in the CRL, there is no guarantee that the FDA will ultimately agree that such issues have been successfully addressed and resolved. It is unclear when the FDA will approve our BLA, if at all, for commercialization and even if approved, the resulting revenue may not enable us to achieve profitability. Even if we obtain regulatory approval to market a product candidate, our future revenues will depend upon the size of any markets in which our product candidates have received approval, and our ability to achieve sufficient market acceptance, reimbursement from third-party payors and adequate market share for our product candidates in those markets.
We expect our expenses and net losses to increase significantly as we prepare to potentially commercialize our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, if approved by the FDA, continue our development of, and seek regulatory approvals for, our other product candidates, and begin to commercialize other approved products, if any, as well as hire additional personnel, protect our intellectual property and incur additional costs associated with operating as a public company. Our net losses may fluctuate significantly from quarter to quarter and year to year, depending on the timing of our clinical studies and trials, associated manufacturing needs, commercialization activities if our product candidates are approved and our expenditures on other research and development activities.
If our research and development efforts are successful, we may also face the risks associated with the shift from development to commercialization of new products based on innovative technologies. Our ability to achieve profitability, if ever, is dependent upon, among other things, obtaining regulatory approvals for our product candidates and successfully commercializing our product candidates alone or with third parties. However, our operations may not be profitable even if one or more of our product candidates under development are successfully developed and produced and thereafter commercialized. Even if we do become profitable, we may not be able to sustain or increase our profitability on a quarterly or annual basis. As a result, it may be more difficult for you to assess our future viability than it could be if we had a longer operating history.
We anticipate needing additional financing to fund our operations and complete the development and commercialization of our various product candidates, and if we are unable to obtain such financing when needed, or on acceptable terms, we may be unable to complete the development and commercialization of our product candidates.
The development of biopharmaceutical products, including conducting preclinical studies and clinical trials, is a very time-consuming, expensive and uncertain process that takes years to complete. Our operations have consumed substantial amounts of cash since inception. A significant portion of our funding had been in the form of promissory notes totaling $735.0 million in indebtedness (consisting of related-party promissory notes and accrued and unpaid interest) outstanding as of December 31, 2023 held by entities affiliated with Dr. Soon-Shiong.
As of December 31, 2023, we held cash, cash equivalents and marketable securities totaling $267.4 million. We will need to obtain additional financing to fund our future operations, including completing the development and commercialization of our product candidates. Changing circumstances may cause us to increase our spending significantly faster than we currently anticipate and we may need to raise additional funds sooner than we presently anticipate. Moreover, research and development and our operating costs and fixed expenses such as rent and other contractual commitments, including those for our research collaborations, are substantial and are expected to increase in the future.
Unless and until we can generate a sufficient amount of revenue, we may finance future cash needs through public or private equity offerings, license agreements, debt financings, collaborations, strategic alliances, marketing or distribution arrangements, or exchange additional future Revenue Interests for the Second Payment in the RIPA agreement. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms, or at all.
To the extent that we raise additional capital through the sale of equity or equity-linked securities (including warrants), convertible debt or under the ATM, our shelf registration statement, or other offerings, or if any of our current debt is converted into equity or if our existing warrants are exercised, your ownership interest will be diluted, and the liquidation or other preferences may adversely affect your rights as a stockholder. If we incur additional indebtedness, our fixed payment obligations will increase and we may have to comply with certain restrictive covenants that are similar to those associated with the revenue interest liability, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms unfavorable to us, or exercise our Call Option (as defined in the RIPA) to purchase the outstanding revenue interest liability which will require us to generate a significant amount of cash flow to offset these outflows. Absent the Second Payment under the RIPA, subject to satisfaction of certain conditions specified in the RIPA, we have no committed source of additional capital and if we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may be required to delay or reduce the scope of or eliminate one or more of our research or development programs or our commercialization efforts. See “—Our payment obligations under the RIPA may adversely affect our financial position and results of operations and our ability to raise additional capital which in turn may increase our vulnerability to adverse regulatory developments or economic or business downturns”and Note 9, Revenue InterestPurchase Agreement, of the “Notes to the Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information. Our current license and collaboration agreements may also be terminated if we are unable to meet the payment obligations under those agreements. As a result, we may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time.
Our payment obligations under the RIPA may adversely affect our financial position and results of operations and our ability to raise additional capital which in turn may increase our vulnerability to adverse regulatory developments or economic or business downturns.
On December 29, 2023, we entered into the RIPA with Infinity and Oberland. Pursuant to the RIPA, Oberland acquired certain Revenue Interests from us for a gross purchase price of $200.0 million paid on closing, less certain transaction expenses. In addition, Oberland may purchase additional Revenue Interests from us in exchange for the $100.0 million Second Payment subject to certain conditions specified in the RIPA, including following the receipt of approval by the FDA of the company’s BLA for Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 diseaseon or before June 30, 2024. As consideration for the aforementioned payments, Oberland has the right to receive quarterly Revenue Interest Payments from us based on, among other things, our worldwide net sales, excluding those in China, which will be tiered payments initially ranging from 3.00% to 7.00% (or after funding of the Second Payment, 4.50% to 10.00%), subject to increase or decrease, following December 31, 2029 depending on whether the aggregate payments made to Oberland as of that date met or exceeded the Cumulative Purchaser Payments (as defined in the RIPA). In addition, if the aggregate payments to Oberland as of December 31, 2029 do not equal or exceed the amount of the Cumulative Purchaser Payments, then we are obligated to make a one-time payment to Oberland in an amount equal to 100% of the Cumulative Purchaser Payments, less the aggregate amount of our previous payments to Oberland as of December 31, 2029 (the True-Up Payment, as defined in the RIPA). See Note 9, Revenue Interest Purchase Agreement, of the “Notes to the Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information. The RIPA and our payment obligations to Oberland could have important negative consequences to holders of our securities. For example, a portion of our cash flow from operations will be needed to make required payments to Oberland and will not be available to fund future operations.
Payment requirements under the RIPA will increase our cash outflows. Our future operating performance is subject to market conditions and business factors that are beyond our control. If our cash inflows and capital resources are insufficient to allow us to make required payments, we may have to reduce or delay capital expenditures, sell assets or seek additional capital. If we raise funds by selling additional equity, such sale would result in dilution to our stockholders. There is no assurance that if we are required to secure funding we can do so on terms acceptable to us, or at all. Failure to pay amounts owed to Oberland when due would result in a default under the RIPA and could result in foreclosure on all or substantially all of our assets, which would have a material adverse effect.
The RIPA contains affirmative and negative operational covenants and events of default, which may prevent us from capitalizing on business opportunities and taking some corporate actions, and gives rise to a Put Option in favor of Oberland, which could have a material adverse effect on our financial condition and business operations.
The RIPA contains affirmative and negative covenants and events of default, including covenants and restrictions that, among other things, restrict our ability to incur liens, incur additional indebtedness, make loans and investments, enter into transactions with affiliates, engage in mergers and acquisitions, engage in asset sales and exclusive licensing arrangements, and declare dividends to our stockholders, in each case, subject to certain exceptions set forth in the RIPA. Additionally, Oberland has a Put Option enabling them to terminate the RIPA and to require the company to repurchase the Revenue Interests upon enumerated events such as a bankruptcy event, failure to make a payment, an uncured material breach, default on certain third-party agreements, a breach or default under any subordination agreements with respect to indebtedness to existing stockholders, any right to repurchase or accelerate debt instruments like permitted convertible notes, existing stockholder indebtedness, or subordinated notes during certain time periods, judgments in excess of certain amounts against us, a material adverse effect, the loss of regulatory approval of our product candidates or a change of control. The triggering of the Put Option, including by our failure to comply with these covenants, would permit Oberland to declare certain amounts to be immediately due and payable. If we were to default under the terms of the RIPA, including by failure to make such accelerated payments, Oberland could exercise remedies, including initiating foreclosure proceedings against all or substantially all of our assets. Oberland’s right to repayment is senior to the rights of the holders of our common stock. Any triggering of the Put Option or other declaration by Oberland of an event of default under the RIPA could significantly harm our financial condition, business and prospects and could cause the price of our common stock to decline.
Our debt and revenue interest liability could adversely affect our cash flows and limit our flexibility to raise additional capital.
We have a significant amount of debt and revenue interest liability and may need to incur additional debt to support our growth. As of December 31, 2023, our indebtedness totaled $735.0 million, (consisting of related-party promissory notes and accrued and unpaid interest), held by entities affiliated with Dr. Soon-Shiong along with a $200.0 million revenue interest liability with Oberland.
Our substantial amount of debt could have important consequences and could:
•require us to dedicate a substantial portion of our cash and cash equivalents to make interest and principal payments on our debt and revenue interest liability payments, reducing the availability of our cash and cash equivalents and cash flow from operations to fund future capital expenditures, working capital, execution of our strategy and other general corporate requirements;
•increase our cost of borrowing and even limit our ability to access additional debt to fund future growth;
•increase our vulnerability to general adverse economic and industry conditions and adverse changes in governmental regulations;
•limit our flexibility in planning for, or reacting to, changes in our business and industry, which may place us at a disadvantage compared with our competitors; and
•limit our ability to borrow additional funds, even when necessary to maintain adequate liquidity, which would also limit our ability to further expand our business.
The occurrence of any of the foregoing factors could have a material adverse effect on our business, results of operations and financial condition.
Further, the company’s ability to make scheduled payments of the principal of, potential Test Date payments of, to pay interest or royalties on, or to refinance any current or future indebtedness, including the related-party promissory notes or the revenue interest liability, depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate sufficient cash flows from operations in the future to service our indebtedness, pay the revenue interest liability, and make necessary capital expenditures. If we are unable to generate such cash flows, we may be required to adopt one or more alternatives, such as selling assets, restructuring indebtedness, including the revenue interest liability, or obtaining additional equity or equity-linked capital on terms that may be onerous or highly dilutive. Our ability to refinance our indebtedness, including the revenue interest liability, at maturity or otherwise, will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
There can be no assurance that we can refinance the related-party promissory notes or revenue interest liability or what terms will be available in the market at the time of refinancing. Furthermore, if prevailing interest rates or other factors at the time of refinancing result in higher interest rates upon refinancing, then the interest expense relating to the refinancing would increase. These risks could materially adversely affect our financial condition, cash flows and results of operations.
The value of our warrants outstanding and the revenue interest liability are subject to potentially material increases and decreases based on fluctuations in the price of our common stock or projected sales and the probability of specific events, which may affect our results of operations and financial position and could adversely affect our stock price.
In connection with our registered direct offerings, during the years ended December 31, 2023 and 2022 we entered into warrant agreements with certain institutional investors that allows such investors to purchase up to an aggregate total of 37,732,820 shares of our common stock at exercise prices ranging from $3.2946 per share to $6.60 share. As of December 31, 2023, all of the warrants were exercisable and had expiration dates ranging from December 12, 2024 to July 24, 2026.
We account for the warrants as derivative instruments, and changes in the fair value of the warrants are included in other expense, net, on the consolidated statement of operations for each reporting period. At December 31, 2023, the fair value of warrant liabilities included in the consolidated balance sheet was $118.8 million. We use the Black-Scholes option pricing model
to determine the fair value of the warrants. As a result, the valuation of these derivative instruments is subjective, and the Black-Scholes option pricing model requires the input of subjective assumptions, including the expected stock price volatility and probability of a fundamental transaction (a strategic merger or sale). Changes in these assumptions can materially affect the fair value estimate. We could, at any point in time, ultimately incur amounts different than the carrying value, which could have a significant impact on our results of operations and financial position.
We account for the revenue interest liability as a liability, net of a debt discount comprised of deferred issuance costs, the fair value of a freestanding option agreement related to the SPOA, and the fair value of embedded derivatives requiring bifurcation on the consolidated balance sheet. The company imputes interest expense associated with this liability using the effective interest rate method. The effective interest rate is calculated based on the rate that would enable the debt to be repaid in full over the anticipated life of the arrangement. Interest expense is recognized over the estimated term on the consolidated statement of operations. The interest rate on the liability and the underlying value of the bifurcated embedded derivative may vary during the term of the agreement depending on a number of factors, including the level of actual and forecasted net sales, and in the case of the derivative, specific probabilities associated with RIPA Put/Call events or Test Date payments underlying our Monte Carlo analysis. The company evaluates the interest rate quarterly based on actual and forecasted net sales utilizing the prospective method. A significant increase or decrease in actual or forecasted net sales will materially impact the revenue interest liability and/or the bifurcated embedded derivative, interest expense, and the time period for repayment.
Fluctuations in warrant, revenue interest liability, and derivative values and changes in the assumptions and factors used in the model may impact our operating results, making it difficult to forecast our operating results and making period-to-period comparisons less predictive of future performance. In one or more future quarters, our results of operations may fall below the expectations of securities analysts and investors. In that event, the market price of our common stock could decline. In addition, the market price of our common stock may fluctuate or decline regardless of our operating performance.
The accounting method for convertible debt securities could have a material effect on our reported financial results.
In accordance with FASB ASC Topic 470-50, Debt – Modifications and Extinguishments (ASC 470-50), we recorded amendments to our related-party promissory notes entered into on September 11, 2023 under the debt modification accounting model, as the amendments were not substantially different than the terms of the promissory notes prior to the amendment. Under this model, the unamortized debt discounts from the promissory notes are amortized as an adjustment of interest expense over the remaining term of modified promissory notes using the effective interest rate method. Also, the increase in fair value of the embedded conversion feature from the debt modification was accounted for as a debt discount to the $200.0 million convertible note that is not recorded at fair value with a corresponding increase in additional paid-in capital. In addition, we recorded amendments to our related-party promissory notes entered into on December 29, 2023 under the debt extinguishment model, and as a result recognized a total net gain on extinguishment of $36.1 million, which was recorded in additional paid-in capital, on the consolidated statement of stockholders’ deficit, as the debt was acquired from entities under common control. As a result of the debt amendments, we will be required to record a greater amount of non-cash interest expense in current periods presented as a result of the amortization of the discount associated with certain promissory notes. We will either report lower net income or a higher net loss in our consolidated financial results because FASB ASC Topic 470-20, Debt with Conversion and Other Options, requires interest to include both the current period’s amortization of the debt discount and the instrument’s coupon interest, which could adversely affect our reported or future financial results and the trading price of our common stock.
We invest our cash on hand in various financial instruments which are subject to risks that could adversely affect our business, results of operations, liquidity and financial condition.
We investhave typically invested our cash in a variety of financial instruments, principally commercial paper,including investment-grade short- to intermediate-term corporate debt securities, government-sponsored securities and governmentEuropean bonds; however, after our entry into the RIPA, we can no longer invest our excess funds in corporate or European bonds. AllCertain of theseour investments are subject to credit, liquidity, market, and interest rate risk. Such risks, including the failure or severe financial distress of the financial institutions that hold our cash, cash equivalents and investments, may result in a loss of liquidity, impairment to our investments, realization of substantial future losses, or a complete loss of the investments in the long-term, which may have a material adverse effect on our business, results of operations, liquidity and financial condition. In order toTo manage the risk to our investments, we maintain an investment policy that, among other things, limits the amount that we may invest in any one issue or any single issuer and requires us to only invest in high credit quality securities to preserve liquidity.
Our ability to use NOLs and research and development credits to offset future taxable income may be subject to certain limitations.
In general, under Sections 382 and 383 of the Code, a corporation that undergoes an “ownership change”ownership change is subject to limitations on its ability to utilize its pre-change NOLs or credits, to offset future taxable income or taxes. For these purposes, an ownership change generally occurs where the aggregate stock ownership of one or more stockholders or groups of stockholders who owns at least 5% of a corporation’s stock increases its ownership by more than 50 percentage points over its lowest ownership percentage within a specified testing period. We have not conducted a complete study to assess whether a change of control has occurred or whether there have been multiple changes of control since inception due to the significant complexity and cost associated with such a study. If we have experienced a change of control, as defined by Section 382, at any time since inception (including as a result of the Merger)March 2021 merger which pursuant to which NantKwest and NantCell combined their businesses), utilization of the NOL carryforwards or research and development tax credit carryforwards would be subject to an annual limitation under Section 382. Any limitation may result in expiration of a portion of the NOL carryforwards or research and development tax credit carryforwards before utilization. In addition, our NOLs or credits may also be impaired under state law. Accordingly, we may not be able to utilize a material portion of our NOLs or credits.
Since we will need to raise substantial additional funding to finance our operations, we may experience further ownership changes in the future, some of which may be outside of our control. Limits on our ability to use our pre-change NOLs or credits to offset U.S. federal taxable income could potentially result in increased future tax liability to us if we earn net taxable income in the future. In addition, under the legislation commonly referred to as the Tax Cuts and Jobs Act of 2017 (TCJA),TCJA, as modified by the Coronavirus Aid, Relief, and Economic Security Act, (CARES Act), the amount of NOLs generated in taxable periods beginning after December 31, 2017, that we are permitted to deduct in any taxable year beginning after December 31, 2020 is limited to 80% of our taxable income in such year, where taxable income is determined without regard to the NOL deduction itself. The TCJA allows post-2017 unused NOLs to be carried forward indefinitely. Similar rules may apply under state tax laws.
Our transfer pricing policies may be subject to challenge by the Internal Revenue ServiceIRS or other taxing authorities.
Our intercompany relationships are subject to complex transfer pricing regulations administered by taxing authorities in various jurisdictions. The relevant taxing authorities may disagree with our determinations as to the value of assets sold or acquired or income and expenses attributable to specific jurisdictions. If such a disagreement were to occur, and our position were not sustained, we could be required to pay additional taxes, interest and penalties, which could result in one-time tax charges, higher effective tax rates, reduced cash flows, and lower overall profitability of our operations. We believe that our consolidated financial statements reflect adequate reserves to cover such a contingency, but there can be no assurances in that regard.
Unanticipated changes in effective tax rates or adverse outcomes resulting from examination of our income or other tax returns could expose us to greater than anticipated tax liabilities.
The tax laws applicable to our business, including the laws of the U.S. and other jurisdictions, are subject to interpretation and certain jurisdictions may aggressively interpret their laws in an effort to raise additional tax revenue. It is possible that tax authorities may disagree with certain positions we have taken, are currently taking or will take, and any adverse outcome of such a review or audit could have a negative effect on our financial position and results of operations. Further, the determination of our provision for income taxes and other tax liabilities requires significant judgment by management, and there are transactions where the ultimate tax determination is uncertain. Although we believe that our estimates are reasonable, the ultimate tax outcome may differ from the amounts recorded on the consolidated financial statements and may materially affect our financial results in the period or periods for which such determination is made.
In addition, tax laws are dynamic and subject to change as new laws are passed and new interpretations of the law are issued or applied. For example, in August 2022, the U.S. enacted the IRA, which imposes a 15% minimum tax on the adjusted financial statement income of certain large corporations, as well as a 1% percent excise tax on corporate stock repurchases by publicly-traded companies. Additionally, for taxable years beginning on or after January 1, 2022, the Code eliminated the right to deduct research and development expenditures currently and requires taxpayers to capitalize and amortize U.S. and foreign research and development expenditures over 5 and 15 tax years, respectively. These updates, as well as any other changes to tax laws that are enacted, could adversely affect our tax liability.
Risks Related to the Discovery, Development and Commercialization of our Product Candidates
We will be substantially dependent on the success of our product candidates and cannot guarantee that these product candidates will successfully complete development, receive regulatory approval or be successfully commercialized.
From inception through the date of this Annual Report, we have generated minimal revenue from non-exclusive license agreements related to our cell lines, and the sale of our bioreactors and related consumables.consumables, and grant programs. We have no clinical products approved for commercial sale and have not generated any revenue from therapeutic and vaccine product candidates that are under development. In May 2022, we announced the submission of a BLA to the FDA for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In JulyOn May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. On October 23, 2023, we announced that we had completed the resubmission of the BLA addressing the issues in the CRL. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a target PDUFA actionnew user fee goal date (PDUFA date) of MayApril 23, 2023.2024. While we believe the BLA resubmission addresses the issues identified in the CRL, there is no guarantee that the FDA will ultimately agree that such issues have been successfully addressed and resolved. It is unclear when the FDA will approve our BLA, if at all.
We have invested a significant portion of our efforts and financial resources in the development of our main product candidates, N-803, our novel antibody cytokineantibody-cytokine fusion protein, saRNAsecond-generation hAd5 and second-generation hAd5saRNA vaccine candidates, and aldoxorubicin, some of which are used in combination with our NK cell therapy
candidates. Our product candidates will require additional clinical and non-clinical development, regulatory approval, commercial manufacturing arrangements, establishmentenhancement of aour commercial organization and service providers, significant marketing efforts, and further investment before we can generate any revenues from product sales. We expect to invest heavily in these product candidates as well as in our other existing product candidates and in any future product candidates that we may develop. Our product candidates are susceptible to the risks of failure inherent at any stage of product development, including the appearance of unexpected adverse events or failure to achieve primary endpoints in clinical trials. Furthermore, we cannot assure you that we will meet our timelines for current or future clinical trials, which may be delayed or not completed for a number of reasons. Additionally, our ability to generate revenues from our combination therapy products will also depend on the availability of the other therapies, including BCG, with which our products are intended to be used. We currently generate no meaningful revenues from the sale of any product candidates, and we may never be able to develop or commercialize a product.
The CRL we received from the FDA for the BLA has delayed, and may decrease the likelihood of, ultimate approval and successful commercialization of our lead product candidate in the U.S. and potentially other markets.
In May 2022, we announced the submission of a BLA to the FDA for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In July 2022, we announced that the FDA had accepted our BLA for review and set a target PDUFA action date of May 23, 2023. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that it had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. On October 23, 2023, we announced that we had completed the resubmission of the BLA addressing the issues in the CRL. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a new user fee goal date (PDUFA date) of April 23, 2024.
There can be no assurance that the FDA will ultimately agree that the issues raised in the CRL have been successfully addressed and resolved in the BLA resubmission. Further, any inability to successfully work with the FDA to find a satisfactory solution to address any concerns in a timely manner or at all during the review process for the BLA, including any inability to provide the FDA with data, analysis or other information sufficient to support an approval of the BLA, may adversely impact the prospects for FDA approval. In addition, the results from any FDA re-inspection of any facility including those of our third-party CMOs may further delay, or adversely impact, potential approval. It is unclear when the FDA will approve our BLA, if at all. The CRL and any subsequent resulting delay in the development and approval of such product candidate may prevent, decrease and/or delay expected revenue. Any of these risks could have a material impact on our business, operating results, and financial condition.
We are developing product candidates in combination with other therapies, which exposes us to additional risks.
We are developing product candidates in combination with one or more other therapies. We are studying N-803 therapy along with other products and product candidates, such as BCG, PD-L1 t-haNK, hAd5 and yeast TAAs, and aldoxorubicin. If we choose to develop a product candidate for use in combination with an approved therapy, we are subject to the risk that the FDA, EMA or comparable foreign regulatory authorities in other jurisdictions could revoke approval of, or that safety, efficacy, manufacturing or supply issues could arise with the therapy used in combination with our product candidate. The FDA may require us to use more complex clinical trial designs in order to evaluate the contribution of each product and product candidate to any observed effects. To the extent that we do not have rights to already approved products, this may require us to work with another company to satisfy such a requirement or increase our cost of development. It is possible that the results of these trials could show that any positive results are attributable to the already approved product. Following product approval, the FDA may require that products used in conjunction with each other be cross labeled for combined use. If the therapies we use in combination with our product candidates are replaced as the standard of care for the indications we choose for any of our product candidates, the FDA or comparable foreign regulatory authorities may require us to conduct additional clinical trials. The occurrence of any of these risks could result in our own products, if approved, being removed from the market or being less successful commercially.
In addition, unapproved therapies face the same risks described with respect to our product candidates currently in development and clinical trials, including the potential for serious adverse effects, delays in clinical trials and lack of FDA approval. If the FDA or comparable foreign regulatory authorities do not approve or revoke their approval of these other therapies, or if safety, efficacy, quality, manufacturing or supply issues arise with, the therapies we choose to evaluate in combination with any of our product candidates, we may be unable to obtain approval of or market such combination therapy.
We may choose to expend our limited resources on programs that do not yield successful product candidates as opposed to indications that may be more profitable or for which there is a greater likelihood of success.
We do not have sufficient resources to pursue development of all or even a substantial portion of the potential opportunities that we believe will be afforded to us by our product candidates. Because we have limited resources and access to capital to fund our operations, our management must make strategic decisions as to which product candidates and indications to pursue and how much of our resources to allocate to each. Our management must also evaluate the benefits of developing in‑licensed or jointly owned technologies, which in some circumstances we may be contractually obligated to pursue, relative to developing other product candidates, indications or programs. Our management has broad discretion to suspend, scale down, or discontinue any or all of these development efforts, or to initiate new programs to treat other diseases. If we select and commit resources to opportunities that we are unable to successfully develop, or we forego more promising opportunities, our business, financial condition and results of operations will be adversely affected.
Our projections regarding the market opportunities for our product candidates may not be accurate, and the actual market for our products, if approved, may be smaller than we estimate.
Since our current product candidates and any future product candidates will represent novel approaches to treating various conditions, it may be difficult, in any event, to accurately estimate the potential revenues from these product candidates. Accordingly, we may spend significant capital trying to obtain approval for product candidates that have an uncertain commercial market. Our projections of addressable patient populations that may benefit from treatment with our product candidates are based on our beliefs and estimates. These estimates, which have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations, or market research by third parties, may prove to be incorrect. Further, new studies or approvals of new therapeutics may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for our product candidates may be limited or may not be amenable to treatment with our product candidates and may also be limited by the cost of our treatments and the reimbursement of those treatment costs by third-party payors. Even if we obtain significant market share for our product candidates, because the potential target populations may be small, we may never achieve profitability without obtaining regulatory approval for additional indications.
Our clinical trials may fail to adequately demonstrate the safety and efficacy of our product candidates, which would prevent or delay regulatory approval and commercialization. If our trials are not successful, we will be unable to commercialize our product candidates.
Our research and development programs are at various stages of development. The clinical trials of our product candidates as well as the manufacturing and marketing of our product candidates will be subject to extensive and rigorous review and regulation by numerous government authorities in the U.S. and in other countries where we intend to test and market our product candidates. Before obtaining regulatory approvals for the commercial sale of any of our product candidates, we must demonstrate through lengthy, complex and expensive preclinical testing and clinical trials that our product candidates are safe, pure, and potent for use in their target indications. Each product candidate must demonstrate an adequate risk versus benefit profile in its intended patient population and for its intended use. The risk/benefit profile required for product licensure will vary depending on these factors and may include not only the ability to show tumor shrinkage, but also adequate duration of response, a delay in the progression of the disease, and/or an improvement in survival. For example, response rates from the use of our product candidates or their contribution of effect, may not be sufficient to obtain regulatory approval unless we can also show an adequate duration of response. The clinical trials for our product candidates under development may not be completed on schedule and regulatory authorities may ultimately disagree with our chosen endpoints or may find that our studies or study results do not support product approval and we cannot guarantee that the FDA or foreign regulatory authorities will interpret the results as we do or accept the therapeutic effects as valid endpoints in clinical trials necessary for market approval or they may find that our clinical trial design or conduct does not meet the applicable approval requirement and more trials could be required before we submit our product candidates for approval. Success in early clinical trials does not ensure that large-scale clinical trials will be successful, nor does it predict final results. Product candidates in later stages of clinical trials may fail to show the desired safety, tolerability and efficacy traits despite having progressed through preclinical studies and initial clinical trials and after reviewing test results, we or our collaborators may abandon projects that we might previously have believed to be promising.
In addition, we do not have data on possible harmful long-term effects of our product candidates and do not expect to have this data in the near future. As a result, our ability to generate clinical safety and effectiveness data sufficient to support submission of a marketing application or commercialization of our product candidates is uncertain and is subject to significant risk.
The ongoing shortage of BCG may adversely impact market uptake of our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, if such product candidate receives regulatory approval and it may delay our ability to execute our clinical trials or seek new approvals.
There is an ongoing shortage of BCG, which may adversely impact market uptake of our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, if such product candidate receives regulatory approval. The BCG shortage may impact the number of patients who are treated with BCG for NMIBC with CIS with or without Ta or T1 disease, therefore limiting the pool of BCG-unresponsive patients who may be candidates for our product, if approved. In addition, the BCG shortage may also constrain the number of patients we can treat with our product since our product, if approved, will be administered along with BCG. In addition, Anktiva was awarded Fast Track designation by the FDA for the treatment of BCG-naïve NMIBC with CIS. We are currently enrolling patients in our Phase IIb blinded, randomized, two-cohort, open-label, multi-center trial of intravesical BCG plus Anktiva versus BCG alone, in BCG-naïve patients with high-grade NMIBC with CIS (Cohort A) and NMIBC papillary (Cohort B), which is impacted by the availability of BGC. If we do not complete new trials timely, our ability to generate clinical safety and effectiveness data sufficient to support submission of a marketing application or commercialization of our product candidates in new indications could harm our business, operating results, prospects or financial condition.
We may choose to expend our limited resources on programs that do not yield successful product candidates as opposed to indications that may be more profitable or for which there is a greater likelihood of success.
We do not have sufficient resources to pursue development of all or even a substantial portion of the potential opportunities that we believe will be afforded to us by our product candidates. Because we have limited resources and access to capital to fund our operations, our management must make strategic decisions as to which product candidates and indications to pursue and how much of our resources to allocate to each. Our management must also evaluate the benefits of developing in‑licensed or jointly-owned technologies, which in some circumstances we may be contractually obligated to pursue, relative to developing other product candidates, indications or programs. Our management has broad discretion to suspend, scale down, or discontinue any or all of these development efforts, or to initiate new programs to treat other diseases. If we select and commit resources to opportunities that we are unable to successfully develop, or we forego more promising opportunities, our business, financial condition and results of operations will be adversely affected.
Our projections regarding the market opportunities for our product candidates may not be accurate, and the actual market for our products, if approved, may be smaller than we estimate.
Since our current product candidates and any future product candidates will represent novel approaches to treating various conditions, it may be difficult, in any event, to accurately estimate the potential revenues from these product candidates. Accordingly, we may spend significant capital trying to obtain approval for product candidates that have an uncertain commercial market. Our projections of addressable patient populations that may benefit from treatment with our product candidates are based on our beliefs and estimates, and estimates of the therapeutic benefit and adverse event profile of our product candidates. These estimates, which have been derived from a variety of sources, including scientific literature, preclinical and clinical studies, surveys of clinics, patient foundations, or market research by third parties, may prove to be incorrect. Further, new studies or approvals of new therapeutics may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for our product candidates may be limited or may not be amenable to treatment with our product candidates and may also be limited by the cost of our treatments and the reimbursement of those treatment costs by third-party payors. Even if we obtain significant market share for our product candidates, because the potential target populations may be small, we may never achieve profitability without obtaining regulatory approval for additional indications.
There can be no assurance that we will complete a strategic partnership transaction on acceptable terms in accordance with our anticipated timeline, or at all.
Following our BLA resubmission, we continue to explore potential global strategic partnership transactions for commercialization of N-803 for certain indications. There is a risk that our ability to enter into such a strategic partnership transaction may be impacted by the CRL and BLA resubmission and associated review process. Further, there are other factors that may impact our ability, or decision, to enter into such a strategic partnership, including without limitation the put/call features of the RIPA that may be triggered by entry into a strategic partnership depending on its scope and terms, and ultimately there can be no assurance that we will complete a transaction on acceptable terms, or at all. If we do not execute a strategic partnership transaction in the near-term, it would eliminate a potential source of near-term funding, and may impact our ability to raise additional funds to meet our business needs. In addition, there are significant risks involved with building and managing a commercial infrastructure on a stand-alone basis, which could materialize in the event we do not execute a strategic partnership transaction, or depending on the geographic scope of any executed transaction.
Interim, initial, “top-line”top-line and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publicly disclose preliminary, interim or top-line data from our preclinical studies and clinical trials, which are based on preliminary analyses of then-available data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available or as patients from our clinical trials continue other treatments for their disease.disease, or as inclusion and exclusion criteria is discussed with regulators. We also may make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the interim, top-line or preliminary results that we report may differ from future results of the same studies, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Top-line data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, top-line data should be viewed with caution until the final data are available. Adverse differences between preliminary or interim data and final data could significantly harm our business prospects. Further, disclosure of interim data by us or by our competitors could result in volatility in the price of our common stock.
In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is typically selected from a more extensive amount of available information, and you or others may not agree with what we determine is material or otherwise appropriate information to include in our disclosure. If the interim, top-line or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our product candidates may be harmed, which could harm our business, operating results, prospects or financial condition.
Our clinical trials may not be initiated or completed when we expect, or at all, they may take longer and cost more to complete than we project, our clinical trial costs may be higher than for more conventional therapeutic technologies or drug products, and we may be required to conduct additional clinical trials or modify current or future clinical trials based on feedback we receive from the FDA.
We cannot guarantee that any current or future clinical trials will be conducted as planned or completed on schedule, if at all, or that any of our product candidates will receive regulatory approval. A failure of one or more clinical trials can occur at any stage of the clinical trial process, other events may cause us to temporarily or permanently stop a clinical trial temporarily or permanently, and our future clinical trials may not be successful.
Because our product candidates include, and we expect our future product candidates to include, candidates based on advanced therapy technologies, we expect that they will require extensive research and development and have substantial manufacturing costs. In addition, costs to treat patients and to treat potential side effects that may result from our product
candidates can be significant. Some clinical trial sites may not bill, or obtain coverage from Medicare, Medicaid, or other third-party payors for some or all of these costs for patients enrolled in our clinical trials, and clinical trial sites outside of the U.S. may not reimburse for costs typically covered by third-party payors in the U.S., and as a result we may be required by those trial sites to pay such costs. Accordingly, our clinical trial costs are likely to be significantly higher per patient than those of more conventional therapeutic technologies or drug products.
Collaborations with other entities may be subject to additional delays because of the management of the trials, contract negotiations, the need to obtain agreement from multiple parties and the necessity of obtaining additional approvals for therapeutics used in the combination trials. These combination therapies will require additional testing and clinical trials will require additional FDA regulatory approval and will increase our future costs.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us, slow down our product development and approval process or impair our ability to commence product sales and generate revenues. In addition, if we make manufacturing changes to our product candidates, we may be required to, or we may elect to, conduct additional trials to bridge our modified product candidates to earlier versions. These changes may require FDA approval or notification and may not have their desired effect. The FDA may also not accept data from prior versions of the product to support an application, delaying our clinical trials or programs or necessitating additional clinical trials or preclinical studies. We may find that this change has unintended consequences that necessitates additional development and manufacturing work, additional clinical and preclinical studies, or that results in refusal to file or non-approval of a BLA and/or NDA.
Clinical trial delays could shorten any periods during which our product candidates have patent protection and may allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates and may harm our business and results of operations. In addition, we have in the past experienced clinical holds imposed upon certain of our or investigator-initiatedinvestigator-led clinical trials for various reasons, and we may experience further clinical trial holds in the future. If we fail to commence or complete, or experience delays in, any of our planned clinical trials, our stock price and our ability to conduct our business as currently planned could be harmed.
Even if one of our product candidates is approved and commercialized, we may not become profitable.
If approved for marketing by applicable regulatory authorities, our ability to generate revenues from our product candidates will depend on our ability to:
•price our product candidates competitively such that third-party and government reimbursement leads to broad product adoption;
•prepare a broad network of clinical sites for administration of our product;
•create market demand for our product candidates through our own or our partner’s marketing and sales activities, and any other arrangements to promote these product candidates that we may otherwise establish;
•receive regulatory approval for the targeted patient population(s) and claims that are necessary or desirable for successful marketing;
•manufacture product candidates through third-party CMOs or in our own or our affiliates’, manufacturing facilities or facilities owned by entities affiliated with Dr. Soon-Shiong in sufficient quantities and at acceptable quality and manufacturing cost to meet regulatory requirements and commercial demand at launch and thereafter;
•establish and maintain agreements with wholesalers, distributors, pharmacies, and group purchasing organizations on commercially reasonable terms;
•obtain, maintain, protect and enforce patent and other intellectual property protection and regulatory exclusivity for our product candidates;
•successfully commercialize any of our product candidates that receive regulatory approval;
•maintain compliance with applicable laws, regulations, and guidance specific to commercialization including interactions with health care professionals, patient advocacy groups, and communication of health care economic information to payors and formularies;
•achieve market acceptance of our product candidates by patients, the medical community, and third-party payors;
•achieve appropriate reimbursement for our product candidates;
•maintain a distribution and logistics network capable of product storage within our specifications and regulatory guidelines, and further capable of timely product delivery to commercial clinical sites;
•effectively compete with other therapies or competitors; and
•following launch, assureensure that our product will be used as directed and that additional unexpected safety risks will not arise.
Even if the FDA approves N-803 for certain indications or in combination with other therapeutic products, and even if we obtain significant market share for it, because the potential target population may be small, we may never achieve profitability without obtaining regulatory approval for additional indications. The FDA often approves new therapies initially only for use in patients with r/rrelapsed and/or refractory metastatic disease, which may limit our patient population. Additionally, we may not be able to obtain the labeling claims necessary or desirable for the promotion of our product candidates.
In connection with our 2017 acquisition of Altor, we issued CVRs under which we agreed to pay the prior stockholders of Altor approximately $304.0 million of contingent consideration upon the successful regulatory approval of a BLA by the FDA, or foreign equivalent, for N-803 by December 31, 2022, and approximately $304.0 million of contingent consideration upon calendar-year worldwide net sales of N-803 exceeding $1.0exceeding $1.0 billion prior to December 31, 2026 with amounts payable in cash or shares of our common stock or a combination thereof.
With respect to the regulatory milestone CVR agreement, in May 2022 we announced the submission of a BLA to the FDA for our product candidate, Anktiva (N-803) in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In July 2022 we announced that the FDA had accepted our BLA for review and set a target PDUFA action date of May 23, 2023. It is unclear when the FDA will approve our BLA, if at all. The FDA did not approve our BLA on or before December 31, 2022, and therefore the regulatory milestone was not met, and the regulatory milestone CVR agreement terminated in accordance with its terms.
With respect to the net sales milestone CVR agreement, as As of December 31, 2022,2023, Dr. Soon-Shiong and his related party hold approximately $139.8 million of net sales CVRs, and they have both irrevocably agreed to receive shares of the company’s common stock in satisfaction of their CVRs. We may be required to pay the other prior Altor stockholders up to $164.2 million for their net sales CVRs should they choose to have their CVRs paid in cash instead of common stock. If this were to occur, we may need to seek additional sources of capital and any such financing activities may be restricted by the covenants included in the terms of the RIPA. As such, we may face difficulties raising additional capital and may have to accept unfavorable terms and as a result, we may not be able to achieve profitability or positive cash flow.
We planIn connection with our financing in December 2023, we entered into the RIPA with Infinity and Oberland. Oberland has the right to collaborate with governmental, academicreceive quarterly Revenue Interest Payments from us based on, among other things, our worldwide net sales, excluding those in China, which will be tiered payments initially ranging from 3.00% to 7.00% (or after funding of the Second Payment, 4.50% to 10.00%), subject to increase or decrease, following the Test Date depending on whether our aggregate payments made to Oberland as of the Test Date have met or exceeded the Cumulative Purchaser Payments. In addition, if our aggregate payments made as of the Test Date to Oberland do not equal or exceed the amount of the Cumulative Purchaser Payments as of such date, then we are obligated to make a one-time payment True-Up Payment as described above. In addition to other considerations of the RIPA and corporate partners, including affiliates,the associated impact to improveour profitability and develop N-803, hAd5cash flow, if we were required to make a True-Up Payment, we may need to seek additional sources of capital, and other therapies for new indications for use in combination with other therapies and to improve and develop other product candidates, which may expose us to additional risks, or we may not realize the benefits of such collaborations.be able to achieve profitability or positive cash flow.
If we encounter delays or difficulties enrolling and/or maintaining patients in our clinical trials, our clinical development activities and receipt of necessary marketing approvals could be delayed or otherwise adversely affected.
The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the trial until its conclusion. We may experience difficulties or delays in patient enrollment and retention in our clinical trials for a variety of reasons.
Because the number of qualified clinical investigators is limited, we may need to conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials at such clinical trial sites. In addition, in the past we have engaged, and we intend to continue to engage, in clinical trial efforts outside of the U.S., which gives rise to additional potential complexity and challenges, and further reliance upon third parties in foreign jurisdictions. Moreover, because our product candidates represent a departure from more commonly used methods for cancer and/or viral disease treatment, potential patients and their doctors may be inclined to use conventional therapies, such as chemotherapy and approved immunotherapies that have established safety and efficacy profiles, rather than enroll patients in any future clinical trial.
Delays or failures in planned patient enrollment or retention may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our product candidates or could render further development impossible.
Our product candidates may cause undesirable side effects or have other properties that could halt their clinical development, delay or prevent their regulatory approval, limit their commercial potential or result in significant negative consequences.
Results of our trials could reveal a high and unacceptable severity and prevalence of side effects, adverse events or unexpected characteristics. Combination immunotherapy that includes our current product candidates may be associated with more frequent adverse events or additional adverse events. Undesirable side effects or unacceptable toxicities caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials or order our clinical trials to be placed on clinical hold, and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications. The FDA or comparable foreign regulatory authorities may also require additional data, clinical trials, or preclinical studies should unacceptable toxicities arise. We may need to abandon development or limit development of that product candidate to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk/benefit perspective. Toxicities associated with our clinical trials and product candidates may also negatively impact our ability to conduct clinical trials using tumor-infiltrating lymphocyte therapy in larger patient populations, such as in patients that have not yet been treated with other therapies or have not yet progressed on other therapies. Even if we were to receive product approval, such approval could be contingent on inclusion of unfavorable information in our product labeling, such as limitations on the indicated uses for which the products may be marketed or distributed, a label with significant safety warnings, including boxed warnings, contraindications, and precautions, a label without statements necessary or desirable for successful commercialization, or requirements for costly post marketing testing and surveillance, or other requirements, including a Risk Evaluation and Mitigation Strategy (REMS)REMS to monitor the safety or efficacy of the products, and in turn prevent us from commercializing and generating revenues from the sale of our current or future product candidates. In addition, these serious adverse effects may not be appropriately recognized or managed by the treating medical staff, as toxicities resulting from our product candidates are not normally encountered in the general patient population and by medical personnel. They may have difficulty observing patients and treating toxicities, which may be more challenging due to personnel changes, shift changes, house staff coverage or related issues. This could lead to more severe or prolonged toxicities or even patient deaths, which could result in us or the FDA delaying, suspending or terminating one or more of our clinical trials and which could jeopardize regulatory approval. Any of these occurrences may materially harm our business, financial condition and prospects.
The manufacture of our product candidates is complex, and we may encounter difficulties in production, particularly with respect to process development, quality control, or scaling-up of our manufacturing capabilities. If we or our related parties, or any of our third-party manufacturers encounter such difficulties, our ability to gain approval, or to provide adequate supply of our product candidates for clinical trials or our products for patients, if approved, could be delayed or stopped, or we may be unable to maintain a commercially viable cost structure.
The manufacture of our product candidates involves complex processes, especially for our biologics, vectors and cell therapy product candidates, which are complex, highly regulated and subject to multiple risks. As a result of the complexities, the cost to manufacture biologics, vectors and cell therapies is generally higher than traditional small molecule chemical compounds, and the manufacturing process is less reliable and is more difficult to reproduce. The manufacture of fusion proteins, DNA and RNA constructs, and cell therapy products requiresrequire significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of cell therapy products often encounter difficulties in production, particularly in scaling up initial production. These problems include difficulties with production costs and yields, quality control, including stability of the product candidate and quality assurance testing, shortages of qualified personnel and compliance with strictly enforced federal, state, local and foreign regulations. We may also find that the manufacture of our product candidates is more difficult than anticipated, resulting in an inability to produce a sufficient amount of our product candidates for our clinical trials or, if approved, commercial supply. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects, and other supply disruptions. Currently, ourOur product candidates are manufactured using processes developed or modified by us, our affiliates or by our third-party research institution collaborators that we may not utilize for more advanced clinical trials or commercialization.
Currently we manufacture our product candidates in our own manufacturing facilities, facilities owned by entities affiliated with Dr. Soon-Shiong, or we may usethrough third-party CMOs or some of our related parties to manufacture our product candidates.CMOs. Our clinical trials will need to be conducted with product candidates and materials that were produced under cGMP and/or Good Tissue Practice regulations, which are enforced by regulatory authorities. Our product candidates may compete with other products and product candidates for access to manufacturing facilities. Moreover, because of the complexity and novelty of our manufacturing process, there are only a limited number of manufacturers that operate under cGMP regulations and that are both capable of manufacturing our product candidates
for us and willing to do so. If our third-party CMOs should cease manufacturing for us, we would experience delays in obtaining sufficient quantities of our product candidates for clinical trials and, if approved, commercial supply. Further, our third-party CMOs may breach, terminate, or not renew our agreements with them. If we were to need to find alternative manufacturing facilities or transfer between existing facilities it may take us significant time to find a replacement, if we are able to find a replacement at all and it would significantly impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved. The commercial terms of any new arrangement could be less favorable than our existing arrangements and the expenses relating to the transfer of necessary technology and processes could be significant.
Our failure to comply or our third-party CMOs’ failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approvalreview process. We may not be able to demonstrate sufficient comparability between products manufactured in different runs at the same or at different facilities to allow for inclusion of the clinical results from patients treated with products from these different facilities,runs, in our product registrations. registrations or to assure a cGMP process to qualify our product candidates. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. The deficiencies in the CRL related to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolution of the observations noted at the pre-license inspection would be required before the BLA could be approved.
We also are required to register certain clinical trials and post the results of certain completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within specified timeframes. Failure to do so could result in enforcement actions and adverse publicity.
Reliance on third-party manufacturers entails exposure to risks to which we would not be subject if we manufactured the product candidate ourselves, including:
•inability to negotiate manufacturing and quality agreements with third parties under commercially reasonable terms;
•reduced day-to-day control over the manufacturing process for our product candidates as a result of using third-party manufacturers for all aspects of manufacturing activities;
•reduced control over the protection of our trade secrets, know-how and other proprietary information from misappropriation or inadvertent disclosure or from being used in such a way as to expose us to potential litigation;
•termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that may be costly or damaging to us or result in delays in the development or commercialization of our product candidates; and
•disruptions to the operations of our third-party manufacturers or suppliers caused by conditions unrelated to our business or operations, including the bankruptcy ofor personnel turnover at the manufacturer or supplier.
Moreover, any problems or delays we or our third-party CMOs experience in preparing for commercial scale manufacturing of a product candidate may result in a delay in the FDA approval of the product candidate or may impair our ability to manufacture commercial quantities or such quantities at an acceptable cost, which could result in the delay, prevention, or impairment of clinical development and commercialization of our product candidates and could adversely affect our business. Furthermore, if we or our third-party CMOs fail to deliver the required commercial quantities of our product candidates on a timely basis and at reasonable costs, we would likely be unable to meet demand for our products and we would lose potential revenues. We may ultimately be unable to reduce the cost of goods for our product candidates to levels that will allow for an attractive return on investment if and when those product candidates are commercialized.
In addition, the manufacturing process and facilities for any products that we may develop are subject to FDA and foreign regulatory authority approval processes, and we or our third-party CMOs will need to meet all applicable FDA and foreign regulatory authority requirements, including cGMP, on an ongoing basis. The cGMP requirements include quality control, quality assurance and the maintenance of records and documentation. The FDA and other regulatory authorities enforce these requirements through facility inspections. Manufacturing facilities must submit to pre-approval inspections by the FDA that will be conducted after we submit our marketing applications, including BLAs and NDAs, to the FDA. Manufacturers are also subject to continuing FDA and other regulatory authority inspections following marketing approval. Further, we and our third-party CMOs must supply all necessary Chemistry, Manufacturing and Controls (CMC)CMC documentation in support of a BLA or NDA on a timely basis. Our or our third-party CMOs’ manufacturing facilities may be unable to comply with our specifications, cGMP, and with other FDA, state, and
foreign regulatory requirements, and there is no guarantee that we or our third-party CMOs will be able to successfully pass all aspects of a pre-approval inspection by the FDA or other foreign regulatory authorities. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. The deficiencies in the CRL related to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolution of the observations noted at the pre-license inspection would be required before the BLA could be approved.
On October 23, 2023, we announced that we had completed the resubmission of the BLA. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a new user fee goal date (PDUFA date) of April 23, 2024. While we believe the BLA resubmission addresses the issues identified in the CRL, there is no guarantee that the FDA will ultimately agree that such issues have been successfully addressed and resolved. Further, the results from any FDA re-inspection of any facility, including those of our third-party CMOs, may further delay, or adversely impact, potential approval. It is unclear when the FDA will approve our BLA, if at all.
Poor control of production processes can lead to the introduction of adventitious agents or other contaminants, or to inadvertent changes in the properties or stability of product candidates that may not be detectable in final product testing. If microbial, viral, environmental or other contaminants are discovered in our product candidates or in the manufacturing facilities in which our product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination which could delay clinical trials and adversely harm our business. If we or our third-party CMOs are unable to reliably produce products to specifications acceptable to the FDA or other regulatory authorities, or in accordance with the strict regulatory requirements, we may not obtain or maintain the approvals we need to commercialize such products. Even if we obtain regulatory approval for any of our product candidates, there is no assurance that either we or our third-party CMOs will be able to manufacture the approved product to specifications acceptable to the FDA or other regulatory authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product, or to meet potential future demand. Deviations from manufacturing requirements may further require remedial measures that may be costly and/or time-consuming for us or a third party to implement and may include the temporary or permanent suspension of a clinical trial or commercial sales or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business.
As product candidates progress through preclinical and clinical trials to marketing approval and commercialization, it is common that various aspects of the development program, such as manufacturing methods and formulation, are altered along the way in an effort to optimize yield and manufacturing batch size, minimize costs and achieve consistent quality and results. Such changes carry the risk that they will not achieve these intended objectives. Any of these changes could cause our product candidates to perform differently and affect the results of planned clinical trials or other future clinical trials conducted with the altered materials. This could delay completion of clinical trials, require the conduct of bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our product candidates and jeopardize our ability to commercialize our product candidates, if approved, and generate revenues.
To the extent we use third-party CMOs, we are ultimately responsible for the manufacture of our products, if approved, and product candidates. A failure to comply with these requirements may result in regulatory enforcement actions against our manufacturers or us, including fines and civil and criminal penalties, which could result in imprisonment, suspension or restrictions of production, injunctions, delay or denial of product approval or supplements to approved products, clinical holds or termination of clinical trials, warning or untitled letters, regulatory authority communications warning the public about safety issues with the biologic, refusal to permit the import or export of the products, product seizure, detention, or recall, operating restrictions, suits under the federal civil False Claims Act (FCA),FCA, corporate integrity agreements, consent decrees, or withdrawal of product approval.
Any of these challenges could delay completion of clinical trials, require bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our product candidate, impair commercialization efforts, increase our cost of goods, and have a material adverse effect on our business, financial condition, results of operations and growth prospects.
We may not be successful in managing the build-out of our manufacturing facilities and associated costs or satisfying manufacturing relatedmanufacturing-related regulatory requirements.
We have entered into facility leases for our planned manufacturing operations and related activities under which we are responsible for the build-out of the facility space and associated costs. The build-out of these facilities and related equipment purchases are complex and specialized and will involve substantial capital expenditure, and it could take longer, and cost more, than currently expected. Significant delays and/or cost overruns would result in higher expenditures and could be disruptive ofto operations, any of which could have a negative impact on our financial condition or results of operations. For example, during the first quarter of 2022 we acquired a leasehold interest in the 409,000 square foot Dunkirk Facility as described below. While we believe that governmental funding will assist in funding a small portion of the further build-out of the Dunkirk Facility, we will need to plan and fund most of the additional build-out of, and purchase additional equipment for, the Dunkirk Facility in connection with our planned full operations. In addition, it is possible that, once built, the leased facilities may prove to be less conducive to our operations than is currently anticipated, resulting in operational inefficiencies or similar difficulties that could prove difficult or impossible to remediate and result in an adverse impact on our financial condition or results of operations. We also may not successfully realize the anticipated benefits from the capital expenditure at such facilities based on factors such as delays and uncertainties regarding development, regulatory approval and commercialization of our product candidates, as well as the potential to lose access to the leased facilities.
Further, in the future if we transition from our current third-party CMOs to our own manufacturing facilities, or to alternative third-party CMOs, for one or more of our product candidates, including our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG‑unresponsive NMIBC with CIS with or without Ta or T1 disease, for which we submitted a BLA in May 2022, we may need to conduct additional preclinical, analytical or clinical trials and obtain FDA approval before such manufacturing changes are implemented. If we are unsuccessful in demonstrating the comparability of supplies before and after a manufacturing change, such manufacturing change can result in a delay or disruption in our clinical development plan or our ability to commercialize any approved product. Any production shortfall that impairs the supply of our product candidates could negatively impact our ability to complete clinical trials, obtain regulatory approval and commercialize our product candidates. If our product candidates receive approval, a product shortfall could have a material adverse effect on our business, financial condition and results of operations and adversely affect our ability to satisfy demand for our product candidates, which could materially and adversely affect our revenue and results of operations.
In addition, our planned operations, including our development, testing and future manufacturing activities, are subject to numerous environmental, health and safety laws and regulations. These laws and regulations govern, among other things, the controlled use, handling, release and disposal of and the maintenance of a registry for, hazardous materials and biological materials, such as chemical solvents, human cells, carcinogenic compounds, mutagenic compounds and compounds that may have a toxic effect on reproduction, laboratory procedures and exposure to blood-borne pathogens. If we fail to comply with such laws and regulations, we could be subject to fines or other sanctions. Failure to successfully complete our build-outs and successfully operate our planned manufacturing facilities and satisfy manufacturing-related regulatory requirements could adversely affect the commercial viability of our product candidates and our business.
Cell-based therapies and biologics rely on the availability of reagents, specialized equipment and other specialty materials, which may not be available to us on acceptable terms or at all. For some of these reagents, equipment and materials, we rely or may rely on sole source vendors or a limited number of vendors, which could impair our ability to manufacture and supply our products, if approved.
We currently depend on a small number of suppliers for some of the materials used in, and processes required to develop, our product candidates. For some of these reagents, equipment and materials used in the manufacture of our product candidates, we rely, and we may in the future rely, on sole source vendors or a limited number of vendors. Some of these suppliers may not have the capacity to support clinical trials and commercial products manufactured under cGMP by biopharmaceutical firms or may otherwise be ill-equipped to support our needs. We also do not have supply contracts with many of these suppliers and may not be able to obtain supply contracts with them on acceptable terms or at all. Accordingly, we may experience delays in receiving key materials and equipment to support clinical or commercial manufacturing. An inability to continue to source product from any of these suppliers could adversely affect our ability to satisfy demand for our product candidates, which could adversely and materially affect our product sales and operating results or our ability to conduct clinical trials, either of which could significantly harm our business.
As we seek to develop and scale our manufacturing process, we expect that we will need to obtain rights to and supplies of certain materials and equipment to be used as part of that process. We may not be able to obtain rights to such materials on commercially reasonable terms, or at all, and if we are unable to alter our process in a commercially viable manner to avoid the use of such materials or find a suitable substitute, it would have a material adverse effect on our business. Even if we are able to alter our process so as to use other materials or equipment, such a change may lead to a delay in our clinical development and/or commercialization plans. If such a change occurs for a product candidate that is already in clinical testing, the change may require us to perform both ex vivo comparability studies and to collect additional data from patients prior to undertaking more advanced clinical trials.
Because our current product candidates represent, and our other potential product candidates will represent, novel approaches to the treatment of disease, there are many uncertainties regarding the development, market acceptance, public opinion, third-party reimbursement coverage and the commercial potential of our product candidates, which may impact public perception of us and our product candidates and which may adversely affect our ability to conduct our business and implement our business plans.
Human immunotherapy products are a new category of therapeutics. We use relatively novel technologies involving N-803, hAd5, saRNA hAd5 and yeast technologies, aldoxorubicin,constructs, and cell-based therapies, and our NK cell platform utilizes a relatively novel technology involving the genetic modification of human cells and utilization of those modified cells in other individuals. Because this is a relatively new and expanding area of novel therapeutic interventions, there are many uncertainties related to development, marketing, reimbursement and the commercial potential for our product candidates. There can be no assurance as to the length of the trial period, the number of patients the FDA will require to be enrolled in the trials in order to establish the safety, efficacy, purity and potency of immunotherapy products, or that the data generated in these trials will be acceptable to the FDA to support marketing approval. Adverse public attitudes may adversely impact our ability to enroll patients in clinical trials. The FDA may take longer than usual to come to a decision on any BLA and/or NDA that we submit and may ultimately determine that there is not enough data, information, or experience with our product candidates to support an approval decision. The FDA may also require that we conduct additional post-marketing studies or implement risk management programs, such as REMS, until more experience with our product candidates is obtained. Finally, after increased usage, we may find that our product candidates do not have the intended effect, do not work with other combination therapies or have unanticipated side effects, potentially jeopardizing initial or continuing regulatory approval and commercial prospects. More restrictive government regulations or negative public opinion could have an adverse effect on our business or financial condition and may delay or impair the development and commercialization of our product candidates or demand for any products we may develop. Adverse events in our clinical trials, even if not ultimately attributable to our product candidates, and the resulting publicity could result in increased governmental regulation, unfavorable public perception, potential regulatory delays in the testing or approval of our potential product candidates, stricter labeling requirements for those product candidates that are approved and a decrease in demand for any such product candidates.
There is no assurance that the approaches offered by our product candidates will gain broad acceptance among doctors or patients or that governmental agencies or third-party medical insurers will be willing to provide reimbursement coverage for our proposed product candidates. Public perception may be influenced by claims, such as claims that our technologies are unsafe, unethical or immoral and, consequently, our approach may not gain the acceptance of the public or the medical community. Negative public reaction to cell-based immunotherapy in general could result in greater government regulation and stricter labeling requirements of immunotherapy products, including our product candidates, and could cause a decrease in the demand for any products we may develop. Moreover, our success will depend upon physicians specializing in the treatment of those diseases that our product candidates target prescribing, and their patients being willing to receive treatments that involve the use of our product candidates in lieu of, or in addition to, existing treatments they are already familiar with and for which greater clinical data may be available. The market for any products that we successfully develop will also depend on the cost of the product. WeAside from our lead product candidate, where costs could materially change, we do not yet have sufficient information to reliably estimate what it will cost to commercially manufacture our current product candidates, and the actual cost to manufacture these products could materially and adversely affect the commercial viability of these products. Our goal is to reduce the cost of manufacturing and providing our therapies. However, unless we can
reduce those costs to an acceptable amount, we may never be able to develop a commercially viable product. If we do not successfully develop and commercialize products based upon our approach or find suitable and economical sources for materials used in the production of our potential products, we will not
become profitable, which would materially and adversely affect the value of our common stock. Our N-803 therapies and our other therapies may be provided to patients in combination with other agents provided by third parties or our affiliates. The cost of such combination therapy may increase the overall cost of therapy and may result in issues regarding the allocation of reimbursements between our therapy and the other agents, all of which may affect our ability to obtain reimbursement coverage for the combination therapy from governmental or private third-party medical insurers.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability as a result of the clinical development, testing and manufacturing of our product candidates and will face an even greater risk if we commercialize any products. For example, we may be sued if our product candidates cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. Large judgements have been awarded in class action lawsuits based on therapeutics that had unanticipated side effects. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in a regulatory investigation of the safety and effectiveness of our products, our third-party manufacturer’s manufacturing processes and facilities or our marketing programs and potentially a recall of our products or more serious enforcement action, including limitations on the approved indications for which our product candidates may be used or suspension or withdrawal of approvals, decreased demand for our products, injury to our reputation, costs to defend the related litigation, a diversion of management’s time and our resources, substantial monetary awards to trial participants or patients and a decline in our stock price.
Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we may develop, alone or with corporate collaborators. Our insurance policies may also have various exclusions, and we may be subject to product liability claims for which we have no coverage. While we have obtained clinical trial insurance for our clinical trials, we may have to pay amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. Even if our agreements with any future corporate collaborators entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
We will face significant competition from other biotechnology and pharmaceutical companies and from non-profit institutions.
Competition in the field of cancer and viral infectious disease therapy is intense and is accentuated by the rapid pace of technological development. We compete with a variety of multi-national biopharmaceutical companies and specialized biotechnology companies, as well as technology being developed at universities and other research institutions. These competitors have developed, may develop and are developing product candidates and processes competitive with our product candidates. Research and discoveries by others may result in breakthroughs which may render our product candidates obsolete even before they generate any revenues. We believe that a significant number of products are currently under development, and may become commercially available in the future, for the treatment of conditions for which we are developing product candidates. Many of our competitors have several therapeutic products that have already been developed, approved and successfully commercialized, or are in the process of obtaining regulatory approval for their therapeutic products in the U.S. and internationally. Many of our
competitors, either alone or with their strategic partners, have substantially greater financial, technical, and human resources than we do, as well as significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of treatments and commercializing those treatments. Accordingly, our competitors may be more successful in obtaining approval of treatments and achieving widespread market acceptance, rendering our treatments obsolete or non-competitive, possibly even before we are able to enter the market. Accelerated merger and acquisition activity in the biotechnology and biopharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Even if we obtain regulatory approval for our product candidates, the availability and price of our competitors’ products could limit the demand and the price we are able to charge for our therapies. The level of generic competition and the availability of reimbursement from government and other third-party payors will also significantly affect the pricing and competitiveness of our products.
A large number of companies, government agencies and academic centers around the world are developing COVID‑19 vaccines, and many of these entities are in more advanced stages of development than we are, including some that have started Phase 2 and/or 3 clinical trials or have already obtained emergency regulatory approval in the U.S. and internationally. Even if one of our COVID‑19 vaccine candidates is ultimately approved for marketing, the value of our opportunity will be adversely impacted by other COVID‑19 vaccines that have obtained emergency regulatory approval, obtain full regulatory approval, or demonstrate better safety or efficacy than our COVID‑19 vaccine candidate.
We may not be able to implement our business plan if the acceptance of our product candidates is inhibited by price competition or the reluctance of physicians to switch from other methods of treatment to our product, or if physicians switch to other new therapies, drugs or biologic products or choose to reserve our product candidates for use in limited circumstances. We may be adversely impacted if any of these competitors gain market share as a result of new technologies, commercialization strategies or otherwise.
We may seek orphan drug status or Breakthrough Therapy or Fast Track or Breakthrough Therapy designations or other designation for one or more of our product candidates, but even if any such designation or status is granted, it may not lead to a faster development process or regulatory review and may not increase the likelihood that our product candidates will receive marketing approval, and we may be unable to maintain any benefits associated with such designations or status, including market exclusivity.
In 2012, the FDA established a Breakthrough Therapy designation, which is intended to expedite, although there is no guarantee, the development and review of products that treat serious or life-threatening conditions. We have been awarded, and may seek in the future, Fast TrackBreakthrough Therapy or Breakthrough TherapyFast Track designation for current or future product candidates. Receipt of a designation to facilitate product candidate development is within the discretion of the FDA. Accordingly, even if we believe one of our product candidates meets the criteria for a designation, the FDA may disagree. In any event, the receipt of such a designation for a product candidate may not result in a faster development process, review or approval compared to product candidates considered for approval under conventional FDA procedures and does not assure ultimate marketing approval by the FDA. In addition, the FDA may later decide that the product candidates no longer meet the designation conditions.
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition or for which there is no reasonable expectation that the cost of developing and making available the drug or biologic will be recovered from sales in the U.S. If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full BLA to market the same drug or biologic for the same indication for seven years, except in limited circumstances. We may seek orphan drug status for one or more of our product candidates, but exclusive marketing rights in the U.S. may be lost if we seek approval for an indication broader than the orphan designated indication and may be lost if the FDA later determines that the request for designation was materially defective or if we are unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
In response to TableCatalyst Pharms., Inc. v. Becerra, 14 F.4th 1299 (11th Cir. 2021), the FDA clarified in a January 2023 notice that it intends to continue to apply its longstanding interpretation of Contentsthe regulations to matters outside of the scope of the Catalyst order – that is, the agency will continue tying the scope of orphan-drug exclusivity to the uses or indications for which a drug is approved, which permits other sponsors to obtain approval of a drug for new uses or indications within the same orphan designated disease or condition that have not yet been approved. It is unclear how future litigation, legislation, agency decisions, and administrative actions will impact the scope of the orphan drug exclusivity.As a condition of approval, the FDA may require that we implement various post-marketing requirements and conduct post-marketing studies, any of which would require a substantial investment of time, effort, and money, and which may limit our commercial prospects.
As a condition of biologic licensing, the FDA is authorized to require that sponsors of approved BLAs implement various post-market requirements, including REMS and Phase 4IV trials. For example, in connection with FDA approval of another company’s drug, the FDA required significant post-marketing commitments, including a Phase 4IV trial, revalidation of a test method, and a substantial REMS program that included, among other requirements, the certification of hospitals and their associated clinics that dispensed the drug, including the implementation of a training program and limited distribution only to certified hospitals and their associated clinics. If we receive approval of our product candidates, the FDA may determine that similar or additional or more burdensome post-approval requirements are necessary to ensure that our product candidates are safe, pure and potent. To the extent that we are required to establish and implement any post-approval requirements, we will likely need to invest a significant amount of time, effort and money. Such post-approval requirements may also limit the commercial prospects of our product candidates.
We have never commercialized a product candidate before, and we may lack the necessary expertise, personnel and resources to successfully commercialize any products on our own or together with suitable collaborators. We may be unable to establish effective marketing and sales capabilities or enter into agreements with third parties or related parties to market and sell our product candidates, if they are approved, and as a result, we may be unable to generate product revenues.
We have little to no prior experience in, and currently have a limited commercial infrastructure for, the marketing, sale and distribution of biopharmaceutical products. To achieve commercial success for the product candidates, which we may license to others, we will rely on the assistance and guidance of those collaborators. For product candidates for which we retain commercialization rights and marketing approval, if approved, in order to commercialize our product candidates, we must continue to build out our marketing, sales and distribution capabilities, including a comprehensive healthcare compliance program, and/or arrange with third parties to perform these services, which will continue to take time and require significant financial expenditures and could delay any product launch and we may not be successful in doing so. There are significant risks involved with building and managing a commercial infrastructure. We, or our collaborators and third-party contractors, will have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train, manage and retain medical affairs, marketing, sales and commercial support personnel. Recruiting, training and retaining a sales force is expensive and time-consuming and could delay any product launch. If the commercial launch of a product candidate for which we or our third-party contractors recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have incurred these commercialization expenses prematurely or unnecessarily. These efforts may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel. In the event we are unable to develop a commercial infrastructure, we may not be able to commercialize our current or future product candidates, which would limit our ability to generate product revenues. Even if we and/or our third-party contractors are able to effectively establish a sales force and develop a marketing and sales infrastructure, oursuch sales force and marketing teams may not be successful in commercializing our current or future product candidates. To the extent we rely on third parties to commercialize any products for which we obtain regulatory approval, which we intend to do to a certain extent in connection with our initial product launch, if approved, we would have less control over their sales efforts and could be held liable if they failed to comply with applicable legal or regulatory requirements.
If our product candidates do not achieve broad market acceptance, the revenues that we generate from their sales will be limited.
We have not commercialized a product candidate for any indication. Even if our product candidates are approved by the appropriate regulatory authorities for marketing and sale, they may not gain acceptance among physicians, patients, third-party payors and others in the medical community. If any product candidate for which we obtain regulatory approval does not gain an adequate level of market acceptance, we may not generate significant product revenues or become profitable. Market acceptance of our product candidates by the medical community, patients and third-party payors will depend on a number of factors, some of which are beyond our control. For example, physicians are often reluctant to switch their patients, and patients may be reluctant to switch from existing therapies even when new and potentially more effective or safer treatments enter the market. Efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may not be successful. Even if the medical community accepts that our product candidates are safe and effective for their approved indications, physicians and patients may not immediately be receptive to such product candidates and may be slow to adopt them as an accepted treatment of the approved indications. If any of our product candidates is approved but does not achieve an adequate level of market acceptance, we may not generate significant revenues and we may not become profitable. The degree of market acceptance of any of our product candidates will depend on a number of factors, including:
•the continued safety and efficacy of our product candidates;
•the prevalence and severity of adverse events associated with such product candidates;
•the clinical indications for which the products are approved and the approved claims that we may make for the products;
•limitations or warnings contained in the product’s FDA-approved labeling, including potential limitations or warnings for such products that may be more restrictive than other competitive products or distribution and use restrictions imposed by the FDA with respect to such product candidates or to which we agree as part of a mandatory REMS or voluntary risk management plan;
•changes in the standard of care for the targeted indications for such product candidates;
•the relative difficulty of administration of such product candidates;
•our ability to offer such product candidates for sale at competitive prices, including the cost of treatment versus economic and clinical benefit in relation to alternative treatments or therapies;
•the availability of adequate coverage or reimbursement by third parties, such as insurance companies and other healthcare payors, and by government healthcare programs, including Medicare and Medicaid;
•the extent and strength of our marketing and distribution of such product candidates;
•the safety, efficacy and other potential advantages over, and availability of, alternative treatments already used or that may later be approved for any of our intended indications;
•the timing of market introduction of such product candidates, as well as competitive products;
•the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
•the extent and strength of our third-party manufacturer and supplier support;
•adverse publicity about the product or favorable publicity about competitive products; and
•potential product liability claims.
If any product candidate we commercialize fails to achieve market acceptance, it could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Our product candidates may face competition sooner than anticipated.
The enactment of the Biologics Price Competition and Innovation Act of 2009 (BPCIA)BPCIA created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. Under the BPCIA, the FDA cannot make an approval of an application for a biosimilar product effective until 12 years after the original branded product was approved under a BLA. Certain changes, however, and supplements to an approved BLA, and subsequent applications filed by the same sponsor, manufacturer, licensor, predecessor in interest or other related entity do not qualify for the 12-year exclusivity period.
Our product candidates may qualify for the BPCIA’s 12-year period of exclusivity. There is a risk that any product candidates we may develop that are approved as a biological product under a BLA would not qualify for the 12-year period of exclusivity or that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider any product candidates we may develop to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Additionally, this period of regulatory exclusivity does not block companies pursuing regulatory approval via their own traditional BLA, rather than via the abbreviated pathway. Even if we receive a period of BPCIA exclusivity for our first licensed product, if subsequent products do not include a modification to the structure of the product that impacts safety, purity, or potency, we may not receive additional periods of exclusivity for those products. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference product candidates in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing. Medicare Part B encourages use of biosimilars by paying the provider the same percentage of the reference product average sale price as a mark-up, regardless of which product is reimbursed. It is also possible that payors will give reimbursement preference to biosimilars even over reference biologics absent a determination of interchangeability.
For our small molecular product candidates, if qualified, the regulatory exclusivity period is less than for our biologic product candidates. The Federal Food, Drug, and CosmeticFD&C Act (FDCA) provides a five-year period of non-patent marketing exclusivity within the U.S. to the first applicant to gain approval of an NDA for a drug where the FDA has not previously approved any other new drug containing the same active molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated NDA or a 505(b)(2) NDA submitted by another company for a generic version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCAFD&C Act also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, thatwhich were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, new indications, dosages or strengths of an existing drug. As such, we may face competition from generic versions of our small molecule product candidates, which will negatively impact our long-term business prospects and marketing opportunities.
We will need to obtain FDA approval of any proposed branded product names, and any failure or delay associated with such approval may adversely affect our business.
Any name we intend to use for our product candidates in the U.S. will require approval from the FDA regardless of whether we have secured a formal trademark registration from the U.S. Patent and Trademark Office (USPTO).USPTO including, without limitation, Anktiva. The FDA typically conducts a review of proposed product names, including an evaluation of the potential for confusion with other product names. The FDA may also object to a product name if it believes the name inappropriately implies medical claims or contributes to an overstatement of efficacy. If the FDA objects to any of our proposed product names, we may be required to adopt alternative names for our product candidates. If we adopt alternative names, we wouldwill lose the benefit of any existing trademark applications for such product candidate and may be required to expend significant additional resources in an effort to identify a suitable product name that would qualify under applicable trademark laws, not infringe or otherwise violate the existing rights of third parties, and be acceptable to the FDA. We may be unable to build a successful brand identity for a new product name in a timely manner or at all, which would limit our ability to commercialize our product candidates.
Our internal computer systems, infrastructure or data, or those used by our CROs, CMOs, clinical sites or other contractors or consultants, may or may be perceived to fail or suffer security breaches. A breakdown,a cyberattack, or information security breach couldor other incident, including a breakdown or compromise of the confidentiality, integrity and availability of our information technology systems, network-connected control systems and/networks or our data, interruptwhich could adversely affect the operation of our business and/or affect ourand reputation.
We are and will be dependent upon information technology systems, infrastructure and data. In the ordinary course of our business, we will directly or indirectly collect, store, transmit, and transmitotherwise process sensitive data,and confidential information, including intellectual property, confidential information, preclinical and clinical trial data, proprietary business information, personal data and personally identifiable healthpersonal information of our clinical trial subjectspatients and employees, including in our data centers and on our systems and networks or on those of third parties. The secure maintenance, transmission, and processing maintenance and transmission of this informationdata is critical to our operations. The multitude and complexity of our computer systems and those of our contract research organizations (CROs),CROs, CMOs, clinical sites or other contractors or consultants makemay subject them inherently vulnerable to servicevarious threats, including interruption, or destruction, malicious intrusion, and random attack. Data privacyPrivacy or security breaches or other incidents, including by third parties, employees, contractors or others, may pose a risk that sensitive data,or confidential information, including our intellectual property, trade secrets or personal information of our employees, patients, or other business partners may be exposed to unauthorized persons or to the public. Further, as many of our employees are workingwork remotely, our reliance on our and third-party information technology systems has increased substantially and is expected to continue to increase.
Despite the implementation of security measures, our internal computer systems, infrastructure and data, and those of our CROs, CMOs, clinical sites and other contractors and consultants, are vulnerablesubject to failurerisks relating to cyberattacks, security breaches, or damage from computerother incidents, including through viruses and other malware, employee error, unauthorized and authorized access, or other cybersecurity attacks, natural disasters, terrorism, war, fire, and telecommunication and electrical failures.failures, denial of service attacks, social engineering (including phishing attacks) and other means. As the cyberthreat landscape evolves, these cyberattacks are increasing in their frequency, sophistication and intensity and are becoming increasingly difficult to detect. The techniques used by cybercriminals change frequently, may not be recognized until launched and can originate from a wide variety of sources, including outside groups such as external service providers, organized crime affiliates, terrorist organizations or hostile foreign governments or agencies. Cyberattacks could include the deployment of harmful malware, denial-of-service, social engineering and other means to affect service reliability and threaten data confidentiality, integrity and availability. While we and our shared services partner, NantWorks, LLC (NantWorks), have invested, and continue to invest, in the protection of our data, systems, and information technology infrastructure, there can be no assurance that our efforts, or the efforts of our partners, vendors, CROs, CMOs, clinical sites and other contractors and consultants, will be successful. Any failure or perceived failure in our systems, infrastructure or data, or to identify or prevent cyberattacks, security breaches or other incidents, including service interruptions or identify breaches in our or their systems, that could adversely affect our business and operations, and/or result in the loss, unavailability, misuse, unauthorized use or acquisition, or other unauthorized processing of critical or sensitive information, which couldand result in financial, legal, business or reputational harm to us.harm. In addition, our liability insurance may not be sufficient in type or amount to cover us against claims related to any such failures, security breaches, cyberattacks andor other related breaches.incidents.
If any such event were to occur, andit could also cause interruptions in our operations, it could result inincluding a disruption of our product development programs. For example, the loss of clinical trial data from completed or ongoing clinical trials for a product candidate could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data or may limit our ability to effectively execute a product recall, if required. To the extent that any disruption or security breach were toAny such event could result in a lossliability, delays in
the further development and commercialization of any product candidates, could be delayed. Any such event could also result in legal claims, demands or proceedings liability underinitiated by regulatory authorities or private parties, violations of laws, including laws that protect the privacy or security of personal information, and significant liabilities, including regulatory penalties, and damage to our reputation and a loss of confidence in us and our ability to conduct clinical trials.
an infectious disease, such as COVID-19, or the perception of its effects, may materially and adversely affect our business, operations and financial condition. OurPublic health outbreaks, such as epidemics or pandemics may significantly disrupt our business. Such outbreaks pose the risk that we or our employees, contractors, suppliers, and other partners may be prevented from conducting business could be adversely affected by the effectsactivities for an indefinite period of health epidemics, pandemics or contagious diseases, including the recent COVID‑19 pandemic and the public and governmental efforttime due to mitigate against the spread of the disease, in regions where wedue to shutdowns that may be requested or third partiesmandated by federal, state, and local governmental authorities or certain employers, or due to the economic consequences associated with the pandemic. Business disruptions could include disruptions or restrictions on which we rely have significant manufacturingour ability to travel, as well as temporary closures of our facilities concentrationsand the facilities of our partners, clinical trial sites, service providers, suppliers, or other business operations, and may havecontract manufacturers. For example, the COVID-19 pandemic caused a material adverse effect, ontemporary disruption in our ability to recruit participants for our clinical trials operations, supply chains, distribution systems, product development, businessin the calendar year 2020 and resultsthe first quarter of operations.
Outbreaks of2021. While it is not possible to predict whether another pandemic, epidemic, pandemic or contagious diseases, such as the ongoing COVID‑19 pandemic, andinfectious disease outbreak similar to COVID-19 will materialize, any measures taken in response by governments and businesses worldwidelocal authorities in response to contain its spreadsuch future health crises have the potential to disrupt and delay the initiation of new clinical trials, the progress of our ongoing clinical trials and our preclinical activities, and potentially the manufacture or shipment of both drug substance and finished drug product of our product candidates for preclinical testing and clinical trials, and commercial product, if approved, as well as adversely impacted and may continue to significantly disrupt our operations and adversely affectimpact our business, financial condition, and results of operations. Many countries including the U.S. implemented measures such as quarantine, shelter-in-place, curfew, travel and activity restrictions and similar isolation measures, including government orders and other restrictions on the conduct of business operations. The continued spread of this pandemic has caused significant volatility and uncertainty in the U.S. and international markets and has resulted in increased risks to our operations. The COVID‑19 pandemic and any actions we have taken in response, are affecting and could materially affect our operations, including at our headquarters and at our manufacturing facilities, which have been and may in the future be subject to state executive orders and shelter-in-place orders, and at our clinical trial sites, as well as the business or operations of our CROs, CMOs, clinical sites or other third parties with whom we conduct business. Any such epidemic or pandemic may heighten the risk that a significant portion of our workforce could suffer illness or otherwise not be permitted or be unable to work, and may require that certain of our employees work remotely, which heightens certain risks, including but not limited to, those associated with an increased demand for information technology resources, increased risk of cybersecurity attacks (including social engineering attacks), risks related to internal controls and increased risk of unauthorized dissemination of sensitive personal information or our proprietary or confidential information.
The rapid development and fluidity of the pandemic preclude any prediction as to the ultimate effect of COVID-19 on us. While the U.S. and other countries have reopened their economies to varying degrees, the extent to which COVID-19 will impact our future operations will depend on many factors which cannot be predicted with confidence, including the duration of the outbreak. Any resurgence in COVID-19 infections could result in the imposition of new mandates and prolonged restrictive measures implemented in order to control the spread of the disease.
U.S. President Biden has issued an Executive Order requiring federal employees and covered contractors to be vaccinated against COVID-19. Additionally, on November 4, 2021, the U.S. Department of Labor’s Occupational Safety and Health Administration (OSHA) issued a COVID-19 Vaccination and Testing Emergency Temporary Standard requiring all employers with 100 or more employees to ensure that their employees are fully vaccinated or tested for COVID-19 on at least a weekly basis. On January 20, 2022, The U.S. Supreme Court invalidated this requirement. However additional vaccine and testing mandates may be announced in other jurisdictions in which we operate our business. While it is not currently possible to predict with any certainty the exact impact the new regulations would have on us and our suppliers, the implementation of such government mandated vaccination or testing mandates may impact our ability to retain current employees and attract new employees and result in labor disruptions.
We are monitoring a number of risks related to this pandemic, including the following:
•Financial: We expect to continue spending on research and development during the year ending December 31, 2022 and beyond, and we could also have unexpected expenses related to the pandemic. The short-term continued expenses, as well as the overall uncertainty and disruption caused by the pandemic, will likely cause a delay in our ability to commercialize a product and adversely impact our financialoperating results.
•Manufacturing: The pandemic has impacted, and may continue to impact, our manufacturing locations, including through the effects of facility closures, reductions in operating hours and other social distancing efforts.
•Supply Chain: As the pandemic continues to progress, it has resulted and could continue to result in significant disruptions in our respective supply chains and distribution channels in the future. In addition, there may be unfavorable changes in the availability or cost of raw materials, intermediates and other materials necessary for production, which may result in disruptions in our supply chain and adversely affect our ability to have manufactured certain product candidates for clinical supply.
•Clinical Trials: This pandemic may adversely affect certain of our clinical trials, including our ability to initiate and complete our clinical trials within the anticipated timelines. Due to site and participant availability during the pandemic, new subject enrollment has slowed and is expected to continue to slow, at least in the short-term, for most of our clinical trials. For ongoing trials, we have seen, and expect to continue to see an increasing number of clinical trial sites imposing restrictions on patient visits to limit risks of possible COVID‑19 exposure, and we may experience issues with participant compliance with clinical trial protocols as a result of quarantines, travel restrictions and interruptions to healthcare services. The current pressures on medical systems and the prioritization of healthcare resources toward the COVID‑19 pandemic have also resulted, and may continue to result, in interruptions in data collection and submissions for certain clinical trials and delayed starts for certain planned studies. As a result, our anticipated filing and marketing timelines may be adversely impacted.
•Overall Economic and Capital Markets Environment: The continued spread of COVID‑19 has led to and could continue to lead to severe disruption and volatility in the U.S. and global capital markets, which could result in a decline in stock price, high inflation, increase our cost of capital and adversely affect our ability to access the capital markets in the future even after local conditions improve. In addition, trading prices on the public stock market have been highly volatile as a result of the COVID‑19 pandemic.
•Regulatory Reviews: The operations of the FDA or other regulatory agencies may be adversely affected. The legislative and regulatory environment governing our businesses is dynamic and changing frequently in response to COVID‑19. In response to COVID‑19, federal, state and local governments are issuing new rules, regulations, orders and advisories on a regular basis. These government actions can impact us, our members and our suppliers. There is also the possibility that we may experience delays with obtaining approvals for our IND applications, BLAs, and/or NDAs. The pandemic may also result in greater regulatory uncertainty.
Risks Related to Reliance on Third Parties
We have limited experience conducting clinical trials and have relied and will continue to rely on third parties and related parties to conduct many of our preclinical studies and clinical trials, to manufacture products, and to perform many essential services for any products that we commercialize, including services related to distribution, government price reporting, customer service, accounts receivable management, cash collection and adverse event reporting. Any failure by a third party, related party, or by us to perform as expected, to comply with legal and regulatory requirements or to conduct the clinical trials according to GCP regulations,guidelines, and in a timely manner, may delay or prevent our ability to seek or obtain regulatory approval for or commercialization of our product candidates and our ability to commercialize our current or future product candidates will be significantly impacted and we may be subject to regulatory sanctions.
We have relied and will continue to rely on third parties and related parties to conduct many of our preclinical studies and clinical trials, manufacture products, and perform many essential services for any products that we commercialize. Any failure by a third party, related party, or by us to perform as expected, to comply with legal and regulatory requirements or to conduct the clinical trials according to GCP guidelines, and in a timely manner, may delay or prevent our ability to seek or obtain regulatory approval for or commercialization of our product candidates and subject us to regulatory sanctions.
Large-scale clinical trials require significant financial and management resources. We expect to be heavily reliant on third and related parties, including medical institutions, academic institutions, clinical investigators or CROs to conduct, supervise or monitor some or all aspects of our clinical trials, and in some cases, third-party CMOs to manufacture products, which may force us to encounter delays and challenges that are outside of our control. Nevertheless, we are responsible for ensuring that each of our trials is conducted in accordance with the applicable trial protocol and legal, regulatory and scientific standards, and our reliance on CROs, clinical trial sites, and other third parties does not relieve us of these responsibilities. Our CROs and other third parties must communicate and coordinate with one another in order for our trials to be successful. We have a limited history of conducting clinical trials and have nolimited experience as a company in filingsubmitting and supporting the applications necessary to gain marketing approvals. Our relative lack of experience conducting clinical trials may contribute to our planned clinical trials not beginning or completing on time, if at all. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities and clinical trial sites by, applicable regulatory authorities.
For example, we will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial and for ensuring that our preclinical studies are conducted in accordance with Good Laboratory Practice (GLP) regulations,GLP guidelines, as appropriate. Moreover, the FDA and comparable foreign regulatory authorities require us and the third parties upon which we intend to rely for conducting our clinical trials to comply with GCP guidelines for conducting, recording, and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity, and confidentiality of trial participants are protected. Regulatory authorities enforce these requirements through periodic inspections (including pre-approval inspections once a BLA or NDA is filed with the FDA) of trial sponsors, clinical investigators, trial sites and certain third parties including CMOs. If we, our CROs, clinical trial sites, or other third parties fail to comply with applicable GCP guidelines or other regulatory requirements, we or they may be subject to enforcement or other legal actions, the clinical data generated in our clinical trials may be deemed unreliable and have to be repeated, and our submission of marketing applications may be delayed or the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP regulations.guidelines.
We rely on third parties to manufacture, package, label and ship some of our product candidates for the clinical trials that we conduct. Any performance failure on the part of these third parties could delay clinical development or marketing approval of our product candidates or commercialization of our product candidates, if approved, producing additional losses and depriving us of potential product revenues.
Our CROs, clinical trial sites and other third parties may also have relationships with other entities, some of which may be our competitors, for whom they may also be conducting clinical trials or other therapeutic development activities that could harm our competitive position. In addition, these third parties are not our employees, and except for remedies available to us under our agreements with them, we cannot control whether or not they devote sufficient time and resources to our ongoing clinical and preclinical programs. If these third parties conducting our clinical trials (i) do not successfully carry out their contractual duties, (ii) do not meet expected deadlines, (iii) experience work stoppages, (iv) do not conduct our clinical trials in accordance with regulatory requirements or our stated protocols, (v) need to be replaced, (vi) experience financial hardships or (vii) terminate their agreements with us or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical trial protocols, GCP guidelines or other regulatory requirements or for other reasons, our trials may need to be repeated, extended, delayed or terminated, we may not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates, we will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates or we or they may be subject to regulatory enforcement actions. Additionally, we may need to conduct additional clinical trials or enter into new arrangements with alternative CROs, clinical investigators or other third parties, which we may not be able to do on commercially reasonable terms, or at all and which may involve additional cost and time and require management time and focus. As a result, delays could occur, which could compromise our ability to meet our desired development timelines. Furthermore, if any of the third parties conducting our clinical trials experience any financial hardships due to difficulties relating to the operation of their business, it could damage our business, financial condition, results of operations and prospects. In addition, if an agreement with any of our collaborators terminates, our access to technology and intellectual property licensed to us by that collaborator may be restricted or terminate entirely, which may delay the continued development of our product candidates using the collaborator’s technology or intellectual property or require us to stop development of those product candidates completely. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed. To the extent we are unable to successfully identify and manage the performance of third-party service providers in the future, our business may be materially and adversely affected.
We expect to retain third-party service providers to perform a variety of functions related to the sale of our current or future product candidates, key aspects of which will be out of our direct control. These service providers may provide key services related to sales, market access, distribution, customer service, accounts receivable management, state reporting, compliance support, and cash collection. If we retain a service provider, we wouldwill substantially rely on it as well as other third-party providers that perform services for us, including entrusting our inventories of products to their care and handling. If these third-party service providers fail to comply with applicable laws and regulations, fail to meet expected deadlines or otherwise do not carry out their contractual duties to us, or encounter physical or natural damage at their facilities, our ability to deliver product to meet commercial demand would be significantly impaired and we may be subject to regulatory enforcement action.
In addition, we may engage in the future with third parties to perform various other services for us relating to adverse event reporting, safety database management, fulfillment of requests for medical information regarding our product candidates and related services. If the quality or accuracy of the data maintained by these service providers is insufficient, or these third parties otherwise fail to comply with regulatory requirements related to adverse event reporting, we could be subject to regulatory sanctions.
Additionally, we may contract in the future with a third party to calculate and report pricing information mandated by various government programs. If a third party fails to timely report or adjust prices as required or errs in calculating government pricing information from transactional data in our financial records, it could impact our discount and rebate liability, and potentially subject us to regulatory sanctions or FCA lawsuits.
Our reliance on third and related parties can also present intellectual property-related risks. For example, collaborators may not properly obtain, maintain, enforce or defend intellectual property or proprietary rights relating to our product candidates or technology or may use our proprietary information in such a way as to expose us to potential litigation or other intellectual property-related proceedings, including proceedings challenging the scope, ownership, validity and enforceability of our intellectual property. Collaborators may also own or co-own intellectual property covering our product candidates or technology that results from our collaboration with them, and in such cases, we may not have the exclusive right to commercialize such intellectual property or such product candidates or technology. Collaborators may also gain access to our trade secrets or formulations and impact our ability to commercialize proprietary technology. We may also need the cooperation of our collaborators to enforce or defend any intellectual property we contribute to or that arises out of our collaborations, which may not be provided to us.
We also anticipate that part of our strategy for pursuing the wide range of indications potentially addressed by N-803 will involve further investigator-initiatedinvestigator-led clinical trials. While these trials generally provide us with valuable clinical data that can inform our future development strategy, we generally have less control over not only the conduct but also the design of these clinical trials. Third-party investigators may design clinical trials involving our product candidates with clinical endpoints that are more difficult to achieve or in other ways that increase the risk of negative clinical trial results compared to clinical trials we may design on our own. Negative results from investigator-initiatedinvestigator-led clinical trials, regardless of how the clinical trial was designed or conducted, could have a material adverse effect on our business and the perception of our product candidates.
Moreover, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services.
If third-party manufacturers, wholesalers and distributors fail to perform as expected, or fail to devote sufficient time and resources to our product candidates, our clinical development may be delayed, our costs may be higher than expected or our product candidates may fail to be approved, or we may fail to commercialize any product candidates if approved.
Our reliance on third-party manufacturers, wholesalers and distributors exposes us to the following risks, any of which could delay FDA approval of our product candidates and commercialization of our product candidates if approved, result in higher costs, or deprive us of potential product revenues:
•our CMOs, or other third parties we rely on, may encounter difficulties in achieving the volume of production needed to satisfy commercial demand, may experience technical issues that impact quality or compliance with applicable and strictly enforced regulations governing the manufacture of pharmaceutical products, and may experience shortages of qualified personnel to adequately staff production operations;
•our wholesalers and distributors could become unable to sell and deliver our product candidates for regulatory, compliance and other reasons;
•our CMOs, wholesalers and distributors could breach or default on their agreements with us to meet our requirements for commercialization of our product candidates;
•our CMOs, wholesalers and distributors may not perform as agreed or may not remain in business for the time required to successfully produce, store, sell and distribute our product candidates and we may incur additional cost;
•our CMOs, wholesalers and distributors may misappropriate our proprietary information; and
•if our CMOs, wholesalers and distributors were to terminate our arrangements or fail to meet their contractual obligations, we may be forced to delay our commercial programs.
Our reliance on third parties reduces our control over our product candidate development activities but does not relieve us of our responsibility to ensure compliance with all required legal, regulatory and industry standards. For example, the FDA and other regulatory authorities require that our product candidates and any products that we may eventually commercialize be manufactured according to cGMP requirements. Any failure by our third-party manufacturers to comply with cGMP or maintain a compliance status acceptable to the FDA or other regulatory authorities or failure to scale up manufacturing processes, including any failure to deliver sufficient quantities of product candidates in a timely manner, could lead to a delay in, or failure to obtain, regulatory approval of any of our product candidates. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. The deficiencies in the CRL related to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolution of the observations noted at the pre-license inspection would be required before the BLA could be approved.
On October 23, 2023, we announced that we had completed the resubmission of the BLA. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a new user fee goal date (PDUFA date) of April 23, 2024. While we believe the BLA resubmission addresses the issues identified in the CRL, there is no guarantee that the FDA will ultimately agree that such issues have been successfully addressed and resolved. It is unclear when the FDA will approve our BLA, if at all. In addition, our third-party manufacturers will be subject to periodic inspections by the FDA and other regulatory authorities, and failure to comply with cGMP could be the basis for the FDA to issue a warning or untitled letter, withdraw approvals for product candidates previously granted to us, or take other regulatory or legal action, including a request to recall or seize product candidates, total or partial suspension of production, suspension of clinical trials, refusal to approve pending applications or supplemental applications, detention of product, refusal to permit the import or export of product candidates, injunction, imposing civil penalties or pursuing criminal prosecution.
Additionally, as we scale up manufacturing of our product candidates and conduct required stability testing, we may encounter additional challenges or cGMP issues. These issues may require refinement or resolution in order to proceed with commercial marketing of our product candidates if approved. In addition, quality issues may arise during scale-up and validation of commercial manufacturing processes. Any issues in our manufacturing process could result in increased scrutiny by regulatory authorities, delays in our regulatory approvalreview process, increases in our operating expenses, or failure to obtain or maintain approval for our product candidates. If such issues relate to an approved product, we may not be able to commercialize the approved product as we planned or fail to meet commercial demand, any of which can materially and adversely affect our position in the market.
We use the Clinic, a related party, in some of our clinical trials which may expose us to significant regulatory risks. If our data for this site is not sufficiently robust or if there are any data integrity issues, we may be required to repeat such studies or required to contract with other clinical trial sites, andwhich could delay and/or increase the cost of our clinical development plans will be significantly delayed, and we will incur additional costs.plans.
The Clinic has conducted, is currently conducting, and in the future may conduct, clinical trials involving our product candidates. The Clinic is a related party as it is owned by an officer of the company and additionally, NantWorks manages the administrative operations of the Clinic. Prior to June 30, 2019, one of the company’s officers was an investigator or sub‑investigator for certain of the company’s trials conducted at the Clinic. NantWorks, which is wholly owned by our Executive Chairman and Global Chief Scientific and Medical Officer, Dr. Soon‑Shiong, provides certain administrative services (and has loaned money) to the Clinic. Under certain circumstances, we may be required to report some of these relationships to the FDA.
Relying on a related-party clinical site to develop data that is used as the basis to support regulatory approval can expose us to significant regulatory risks. The FDA may conclude that a financial relationship between us, the Clinic and/or a principal investigator has created a conflict of interest or otherwise affected interpretation of the study. The FDA or comparable regulatory authorities may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. If any data integrity, or regulatory non-compliance issues occur during the study, we may not be able to use the data for our regulatory approval. This could result in a delay in approval, or rejection, of our marketing applications by the FDA and may ultimately lead to the denial of regulatory approval of one or more of our product candidates.
We have formed, and may in the future form or seek, strategic alliances or enter into collaborations with third parties or additional licensing arrangements, in the future, and we may not realize the benefits of such alliances or licensing arrangements. If we fail to enter intoConflicts may arise between us and our collaborators or strategic partners, and such strategic alliances, collaborations or licensing arrangements or such strategic alliances, collaborations or licensing arrangements are not successful, we may not be able to capitalize on the market potential of our product candidates.successful.
We have formed, and may in the future form or seek, strategic alliances, create joint ventures or collaborations or enter into collaborations with third parties or additional licensing arrangements with third and related parties that we believe will complement or augment our development and commercialization efforts with respect to our product candidates and any future product candidates that we may develop. We plan to collaborate with governmental, academic and corporate partners, including affiliates, to improve and develop N-803, hAd5, saRNA hAd5 and yeast technologies,constructs, and other cell therapies for new indications for use in combination with other therapies and to improve and develop other product candidates, which may expose us to additional risks, or we may not realize the benefits of such collaborations.
Because some of our collaborations are conducted at outside laboratories, and we do not have complete control over how the studies are conducted or reported, or over the manufacturing methods used to manufacture our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, the results of such studies, which we may use as the basis for our conclusions, projections or decisions with respect to our current or future product candidates, may be incorrect or unreliable, or may have a negative impact on us if the results of such studies are imputed to our product candidates or proposed indications, even if such imputation is improper. Additionally, we may use third‑party data to analyze, reach conclusions or make predictions or decisions with respect to our product candidates that may be incomplete, inaccurate or otherwise unreliable.
Further,We also plan to collaborate with governmental, academic and corporate partners, including affiliates, to improve and develop N-803, hAd5, saRNA and yeast constructs, cell therapies and other therapies for new indications for use in combination with other therapies and to improve and develop other product candidates, which may expose us to additional risks, or we may not realize the benefits of such collaborations.
Furthermore, conflicts may arise between us and our collaborators or strategic partners, and such strategic alliances, collaborations or licensing arrangements may result in litigation. For example, in 2019 Sorrento, with which we jointly established a new entity called NANTibody as a stand-alone biotechnology company, commenced litigation against us and certain of our officers and directors, alleging that we improperly caused NANTibody to acquire IgDraSol. Additionally, in 2020 we received a Request for Arbitration before the International Chamber of Commerce, International Court of Arbitration, served by Beike asserting breach of contract under our subsidiary Altor’s license agreement with them. See Item 3. “Legal Proceedings” for more information regarding these disputes. Any such developments could harm our product development efforts. In addition, collaborations involving our product candidates will be subject to numerous risks, which may include the following:
•collaborators, including their related or affiliated companies, may be entitled to receive exclusive rights for or involving our products;
•collaborators have significant discretion in determining the efforts and resources that they will apply to a collaboration;
•collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization of our product candidates based on clinical trial results, changes in their strategic focus due to the acquisition of competitive products, availability of funding or other external factors, such as a business combination that diverts resources or creates competing priorities;
•collaborators may delay clinical trials, provide insufficient funding for a clinical trial, stop a clinical trial, abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing;
•collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates;
•a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to their marketing and distribution;
•collaborators may not properly maintain, defend or enforce our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability;
•disputes may arise between us and a collaborator that cause the delay or termination of the research, development or commercialization of our product candidates, or that result in costly litigation or arbitration that diverts management attention and resources;
•collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates;
•if an agreement with any collaborator terminates, our access to technology and intellectual property licensed to us by that collaborator may be restricted or terminate entirely, which may delay our continued development of our product candidates using the collaborator’s technology or intellectual property or require us to stop development of those product candidates completely; and
•collaborators may own or co-own intellectual property covering our product candidates or technology that results from our collaborating with them, and in such cases, we may not have the exclusive right to commercialize such intellectual property.
As a result, if we enter into collaboration agreements and strategic partnerships or license our product candidates, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture, which could delay our timelines or otherwise adversely affect our business. Additionally, exclusive rights that we may grant in connection with collaboration agreements may limit our ability to enter into new or additional collaboration agreements or strategic partnerships if we experience issues with existing collaborations. We also cannot be certain that, following a strategic transaction or license, we will achieve the revenues or specific net income that justifies such transaction. Any delays in entering into new collaborations or strategic partnership agreements related to our product candidates could delay the development and commercialization of our product candidates in certain geographies for certain indications, which would harm our business prospects, financial condition and results of operations.
Any of these relationships may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for our product candidates because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy.
If conflicts arise between us and our collaborators or strategic partners, these parties may act in a manner adverse to us and could limit our ability to implement our strategies.
If conflicts arise between our corporate or academic collaborators or strategic partners and us, the other party may act in a manner adverse to us and could limit our ability to implement our strategies. Some of our existing academic collaborators and strategic partners are conducting multiple product development efforts. Such current or future collaborators or strategic partners could become our competitors in the future and could develop competing products, preclude us from entering into collaborations with their competitors, fail to obtain timely regulatory approvals, terminate their agreements with us prematurely or fail to devote sufficient resources to the development and commercialization of our product candidates. Competing product candidates, either developed by the collaborators or strategic partners or to which the collaborators or strategic partners have rights, may result in the withdrawal of our collaborator’s or partner’s support for our product candidates.
For example, in 2019, Sorrento Therapeutics, Inc. with which we jointly established a new entity called Immunotherapy NANTibody, LLC as a stand-alone biotechnology company, commenced litigation against us and certain of our officers and directors, alleging that we improperly caused NANTibody to acquire IgDraSol, Inc. Additionally, in 2020, we received a Request for Arbitration before the International Chamber of Commerce, International Court of Arbitration, served by Shenzhen Beike Biotechnology Co. Ltd. asserting breach of contract under our subsidiary Altor’s license agreement with them. For more information regarding these disputes, see Note 7, Commitments and Contingencies—Litigation, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report. Any of these developments could harm our product development efforts.
Our use of joint ventures, strategic partnerships and alliances may expose us to risks associated with jointly owned investments.
We may operate parts of our business through joint ventures, strategic partnerships and/or alliances with other companies. While such arrangements may, in some cases, give us access to technologies that we may not otherwise have or may give us access to capital, they involve risks not otherwise present in our own investments, including: (i) we may not control the venture, and it may divert management time and resources; (ii) the partner(s) may not agree to distributions that we believe are appropriate; (iii) we may experience impasses or disputes with such partner(s) on certain decisions, which could require us to expend additional resources to resolve such impasses or disputes, including litigation or arbitration; (iv) our partner(s) may become insolvent or bankrupt, fail to fund their share of required capital contributions or fail to fulfil their obligations as a venture partner; (v) the arrangements governing these relationships may contain certain conditions or milestone events that may never be satisfied or achieved; (vi) our partner(s) may have business or economic interests that are inconsistent with our interests and may
take actions contrary to our interests; (vii) we may suffer losses as a result of actions taken by the partner(s); and (viii) it may be difficult for us to exit if an impasse arises or if we desire to sell our interest for any reason. For example, in December 2021 we have established a joint venture relationship with Amyris. However, in August 2023, Amyris and thereannounced that it had filed for Chapter 11 bankruptcy protection. As of December 31, 2023, the carrying amount of our equity investment in the joint venture was zero. There can be no guarantee that itthe strategic partnerships that we currently have or may enter into will be successful. In addition,Furthermore, we may, in certain circumstances, be liable for the actions of our partners. Any of the foregoing risks could have a material adverse effect on our business, financial condition and results of operations.
We are heavily dependent on our senior management, particularly Dr. Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer, and a loss of a member of our senior management team in the future, even if only temporary, could harm our business.
Our operations will be dependent upon the services of our executives and our employees who are engaged in research and development. If we lose the services of members of our senior management, particularly Dr. Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer, for a short or an extended time, for any reason, we may not be able to find appropriate replacements on a timely basis, and our business, financial condition and results of operations could be materially adversely affected. Our existing operations and our future development depend to a significant extent upon the performance and active participation of certain key individuals, particularly Dr. Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer.Soon-Shiong. Although Dr. Soon-Shiong focuses heavily on our matters and is highly active in our management, he does devote a significant amount of his time to a number of different endeavors and companies, including NantHealth, Inc., NantMedia Holdings, LLC (which operates the Los Angeles Times and the San Diego Union-Tribune)Times) and NantWorks, which is a collection of multiple companies in the healthcare and technology space. The risks related to our dependence upon Dr. Soon-Shiong are particularly acute given his ownership percentage, the commercial and other relationships that we have with entities affiliated with him, his role in our company and his public reputation. We may also be dependent on additional funding from Dr. Soon-Shiong and his affiliates, which may not be available when needed and which he is under no obligation to provide.
To induce valuable employees to remain at our company, in addition to salary and cash incentives, we have provided, and plan to continue providing, equity incentive awards that vest over time. The value to employees of equity incentive awards that vest over time may be significantly affected by movements in our stock price that are beyond our control and may at any time be insufficient to counteract more lucrative offers from other companies. Despite our efforts to retain valuable employees, members of our management, scientific and development teams may terminate their employment with us on short notice. We do not have employment agreements with our NEOs and do not maintain “key man” insurance policies on the lives of allmost of these individuals or the lives of anymembers of our other employees.
We will need to grow the size and capabilities of our organization, and we may experience difficulties in managing this growth.
Our future financial performance and our ability to commercialize our product candidates will depend, in part, on our ability to effectively manage any future growth, and our management may also have to divert a disproportionate amount of their attention away from day-to-day activities in order to devote a substantial amount of time to managing these growth activities. In order to develop our business in accordance with our business plan, we will have to hire additional qualified personnel, including in the areas of research, manufacturing, clinical trials management, regulatory affairs, and sales and marketing. We are continuing our efforts to recruit and hire the necessary employees to support our planned operations in the near term. However, competition for qualified personnel in the biotechnology and pharmaceuticals industry is intense due to the limited number of individuals who possess the skills and experience required, and no assurance can be given that we will be able attract, hire, retain and motivate the highly skilled employees that we need, on acceptable terms or at all. Future growth will impose significant added responsibilities on members of management, including:
•identifying, recruiting, integrating, maintaining, and motivating additional employees;
•managing our internal development efforts effectively, including the clinical and FDA review process for our product candidates, while complying with our contractual obligations to contractors and other third parties; and
•improving our operational, financial and management controls, reporting systems, and procedures.
We currently rely, and for the foreseeable future we expect to rely, in substantial part, on certain independent organizations, advisors and consultants to provide certain services. There can be no assurance that the services of these independent organizations, advisors and consultants will continue to be available to us on a timely basis when needed, or that we can find qualified replacements on economically reasonable terms, or at all. In addition, if we are unable to effectively manage our outsourced activities or if the quality, compliance or accuracy of the services provided by consultants is compromised for any reason, our clinical trials may be extended, delayed, or terminated, and we may not be able to obtain regulatory approval of our product candidates or otherwise advance our business.
If we are not able to effectively expand our organization by hiring new employees and expanding our groups of consultants and contractors, we may not be able to successfully implement the tasks necessary to further develop and commercialize our product candidates and, accordingly, may not achieve our research, development, and commercialization goals on a timely basis, or at all.
If we engage in future acquisitions or strategic partnerships, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities, and subject us to other risks.
We may evaluate various acquisitions and strategic partnerships, including licensing or acquiring complementary products, intellectual property rights, technologies, or businesses. Any potential acquisition or strategic partnership may entail numerous risks, including:
•assimilation of operations, intellectual property, and products of an acquired company or product, including difficulties associated with integrating new personnel;
•the diversion of our managements’ attention from our existing product programs and initiatives in pursuing such a strategic merger or acquisition;
•retention of key employees, the loss of key personnel, and uncertainties in our ability to maintain key business relationships;
•significant upfront milestone and/or royalty payments from which we may not realize the anticipated benefits;
•risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or product candidates and regulatory approvals; and
•our inability to generate revenues from acquired technology and/or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs.
Depending on the size and nature of future strategic acquisitions, we may acquire assets or businesses that require us to raise additional capital or to operate or manage businesses in which we have limited experience. Making larger acquisitions that require us to raise additional capital to fund the acquisition will expose us to the risks associated with capital raising activities. Acquiring and thereafter operating larger new businesses will also increase our management, operating and reporting costs and burdens (including increased cash requirements). In addition, if we undertake acquisitions, we may issue dilutive equity securities, assume or incur additional debt obligations or contingent liabilities, incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense. Moreover, we may not be able to locate suitable acquisition opportunities and this inability could impair our ability to grow or obtain access to technology or products that may be important to the development of our business.
A variety of risks associated with marketing our product candidates internationally could materially adversely affect our business.
We plan to seek regulatory approval of our product candidates outside of the U.S. and, accordingly, we expect that we will be subject to additional risks related to operating in foreign countries if we obtain the necessary approvals, including:
•differing regulatory requirements in foreign countries;
•unexpected changes in tariffs, trade barriers, price and exchange controls and other regulatory requirements;
•economic weakness, including inflation, or political instability in particular foreign economies and markets;
•compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
•foreign taxes, including withholding of payroll taxes;
•foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country;
•difficulties staffing and managing foreign operations;
•workforce uncertainty in countries where labor unrest is more common than in the U.S.;
•differing payor reimbursement regimes, governmental payors or patient self-pay systems, and price controls;
•potential liability under the FCPA or comparable foreign regulations;
•challenges enforcing our contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the U.S.;
•production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad;
•the impact of public health epidemics on the global economy, such as the coronavirus pandemic currently having an impact throughout the world;pandemic; and
•business interruptions resulting from geopolitical actions, including war and terrorism.
These and other risks associated with international operations may materially adversely affect our ability to attain or maintain profitable operations.
We are party to a public-private partnership regarding our manufacturing facility in Dunkirk, New York, and if we or our counterparties fail to meet the obligations of those agreements, it could materially impact our development, operations and prospects.
On February 14, 2022, we acquired a leasehold interest in the Dunkirk Facility from Athenex. The facility is expected to beAthenex with a lease term that commenced on October 1, 2021 (the Commencement Date), which we believe will provide us with a state-of-the-art biotech production center that we believe will substantially expand and diversify our existing manufacturing capacity in the U.S. and the ability to scale production associated with certain of our product candidates.
We paid approximately $40.0 million to Athenex, and the leasehold interest in the Dunkirk Facility was transferred to us. Our annual lease payment will be $2.00 per year for an initial 10-year term, with an option to renew the lease under substantially the same terms and conditions for an additional 10-year term. As part of the transaction, we assumed obligations under various third-party agreements, and committed to spend $1.52 billion on operational expenses during the initial term, and an additional $1.50 billion on operational expenses if we elect to renew the lease for the additional 10-year term. We also committed to hiring 450 employees at the Dunkirk Facility within the first 5five years of operations,following the Commencement Date, with 300 such employees to be hired within the first 2.5 years of operation.following the Commencement Date. We are eligible for certain sales-tax exemption savings during the development of the Dunkirk Facility, and certain property tax savings over the next 20 years, subject to certain terms and conditions, including performance of certain of the obligations described above.
In addition, we believe that the Dunkirk Facility has construction needs that may require approximately 12 to 18 months to complete in order for it to be used as intended.intended, and which needs remain as a result of an ongoing dispute with the Dunkirk Facility’s general contractor and stay related to Athenex’s ongoing bankruptcy proceedings, as described below. Consequently, during the third quarter of 2022, we determined to conduct a reduction-in-force of a significant portion of the then-current employees at the Dunkirk Facility, which became effective in late December 2022. The construction period and reduction-in-force may adversely affect our ability to satisfy certain operational obligations described above. In addition, while we believe we are in compliance with all applicable laws and agreements implicated by the reduction-in-force, we could become subject to litigation in connection with these measures.
Failure to satisfy the obligations over the lease term, including the milestones we have committed to achieve, may give rise to certain rights and remedies of the lessor and other governmental authorities including, for example, termination of the lease agreement and other related agreements and potential recoupment of a percentage of the grant funding received by the SellerAthenex for construction of the Dunkirk Facility and other benefits received, subject to the terms and conditions of the applicable agreements. If we lose access to the Dunkirk Facility and related leased equipment, it could disrupt our operations and manufacturing
activities, cause us to divert resources to finding alternative facilities, which would not have any subsidies, and could have a significant impact on our operations and financial performance. We may also be subject to lawsuits or claims for damages against us if we are unable to comply with our obligations under these arrangements or in connection with other aspects of the Dunkirk Facility, which could materially and adversely affect our business, results of operations and financial condition. For example, we were named as a defendant in a lawsuit filed during the fourth quarter of 2022 by Exyte, U.S., Inc. (Exyte)the general contractor for the Dunkirk Facility, in New York state court arising from a construction agreement Exyte entered with Athenex pertaining to construction of the Dunkirk Facility. We believe we are entitled to defense costs and indemnification and, accordingly, we have provided notice to Athenex. On May 14, 2023, Athenex, together with certain of its subsidiaries, filed voluntary petitions for relief under Chapter 11 of the United States Bankruptcy Court for the Southern District of Texas. The lawsuit with Exyte has remained stayed as a result of Athenex’s bankruptcy proceedings and the construction needs of the Dunkirk Facility remain. The extent of the impact of the Athenex Proceedings and its automatic stay will have on any continuing obligations Athenex may have under the purchase agreement remain unclear. We further believe Exyte’s claims against us are without merit, and we intend to defend the claims vigorously. Furthermore, there is no guarantee that the counterparties to our public-private partnerships will comply with the terms of the agreements, including that their ability to fund their capital commitments under the agreements may be subject to their ability to raise additional capital and that further construction or operational timetables may not be met. Public-private partnerships are also subject to risks associated with government and government agency counterparties, including risks related to government relations compliance, sovereign immunity, shifts in the political environment, changing economic and legal conditions and social dynamics.
Our contractors and subcontractors may place liens on our projects, and if they then successfully foreclose on such projects, we may not be able to use such assets for our business.
all labor and material furnished to the project. A valid lien holder could, after the lien is perfected, institute a collection suit, according to the lien, and if it were successful in obtaining a judgment, the real property and the equipment thereon could be foreclosed upon. If a contractor were to successfully foreclose on such liens, we may not then be able to use such assets to manufacture our products, and our business could be materially harmed. Risks Related to Healthcare and Other Government Regulations
We may be unable to obtain U.S. or foreign regulatory approval and, as a result, unable to commercialize our product candidates. We are, and if we receive regulatory approval of our product candidates, will continue to be subject to ongoing extensive regulation, regulatory obligations and continued regulatory review, which may result in significant additional expense.
Our product candidates are subject to extensive governmental regulations relating to, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting of drugs and therapeutic biologics. Rigorous preclinical testing and clinical trials and an extensive regulatory approvalreview process are required to be successfully completed in the U.S. and in many foreign jurisdictions before a new drug or therapeutic biologic can be marketed. Satisfaction of these and other regulatory requirements is costly, lengthy, time-consuming, uncertain and subject to unanticipated delays and can vary substantially based upon the type, complexity and novelty of the products involved. In May 2022, we announced the submission of a BLA to the FDA for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In JulyOn May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. The deficiencies in the CRL related to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolution of the observations noted at the pre-license inspection is required before the BLA would be approved. At the time, the FDA further provided recommendations specific to additional CMC issues and assays to be resolved.
On October 23, 2023, we announced that we had completed the resubmission of the BLA addressing the issues in the CRL. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a target PDUFA actionnew user fee goal date (PDUFA date) of MayApril 23, 2023. It is unclear when2024. There can be no assurance that the FDA will approve our BLA, if at all. If the FDA requires additional data, findsultimately agree that the CMC informationissues raised in the CRL have been successfully addressed and resolved in the BLA is deficient, disagreesresubmission. Further, any inability to successfully work with our interpretationthe FDA to find a satisfactory solution to address any
concerns in a timely manner or at all during the review process for the BLA, including any inability to provide the FDA with data, analysis of clinical data, identifies any deficiency in our clinical data, or finds deficiencies in our pre-approval inspection, we may failother information sufficient to obtainsupport an approval of the BLA, may adversely impact the prospects for FDA approval. In addition, to the extent the FDA requires a re-inspection of any facility including those of our third-party CMOs or otherwise may further delay, or adversely impact, potential approval. Further, the FDA may not accept the data and results as included in our BLA resubmission at levels consistent with the published results, or at all. Accordingly, even if we receive FDA approval for the BLA, which we may not, it is possible that any data accepted for use on any approved label will differ from data previously published in peer review publications or announced by the company in press releases or other forms of communication, which may have an adverse impact on the commercial prospects for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, or approval may be delayed. candidate.
We have not submitted any other marketing or drug approval applications to the FDA or comparable foreign authorities, for any other product candidate, and we may never receive such regulatory approval for any of our product candidates or regulatory approval that will allow us to successfully commercialize our product candidates. In addition, regulatory agencies may lack experience with our technologies and products, which may lengthen the regulatory review process, increase our development costs and delay or prevent their commercialization.
Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical studies, clinical trials or other research. The number and types of preclinical studies and clinical trials that will be required for regulatory approval also vary depending on the product candidate, the disease or condition that the product candidate is designed to address and the regulations applicable to any particular product candidate. Approval policies, regulations or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates.
Any delay in completing development or obtaining, or failing to obtain, required approvals would have a material and adverse effect on our ability to generate revenue from the particular product candidate for which we are developing and seeking approval. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, be subject to other regulatory enforcement action, and we may not achieve or sustain profitability.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, however a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approvalreview process in others. Approval policies, procedures and requirements may vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the U.S., including additional preclinical studies or clinical trials as clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. In many jurisdictions outside the U.S., a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our product candidates is also subject to approval.
Obtaining foreign regulatory approvals and establishing and maintaining compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our product candidates in certain countries. If we fail to comply with the regulatory requirements in international markets and/or fail to receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be harmed.
Even if we receive regulatory approval for our lead product candidate Anktiva in combination with BCG for the treatment of patients with NMIBC with CIS with or without Ta or T1 disease, or any other product candidates, theywe will continue to be subject to ongoing regulatory requirements, which may result in significant additional expenses. Additionally, our product candidates, if approved, could be subject to labeling and other restrictions, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our product candidates.
Any regulatory approvals that we receive for our product candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed, or to conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor safety and efficacy. In addition, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for any approved product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, including reporting of certain adverse events as well as continued compliance with cGMP for the drug products, and GCP guidelines for any clinical trials that we conduct post-approval.
Later discovery of previously unknown problems with an approved product, including adverse events of unanticipated severity or frequency, or with manufacturing operations or processes, or failure to comply with regulatory requirements, may result in, among other things:
•holds on clinical trials;
•restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls;
•imposition of a REMS, which may include distribution or use restrictions;
•requirements to conduct additional post-market clinical trials to assess the safety of the product;
•revisions to the labeling, including limitation on approved uses or the addition of additional warnings, contraindications or other safety information, including boxed warnings;
•manufacturing delays and supply disruptions where regulatory inspections identify observations of noncompliance requiring remediation;
•fines, warning or untitled letters;
•refusal by the FDA to approve pending applications or supplements to approved applications submitted by us, or withdrawal of product approvals;
•product seizure or detention, or refusal to permit the import or export of product candidates; and
•injunctions or the imposition of civil or criminal penalties.
The FDA’s policies may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the U.S. or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or are not able to maintain regulatory compliance, we may lose any marketing approval that may have been obtained and we may not achieve or sustain profitability, which would adversely affect our business.
If we are unable to establish sales, marketing and distribution capabilities, we may not be successful commercializing our product candidates if and when they are approved.
We are in the process of implementing our sales and marketing personnel hiring plan and building out key commercialization infrastructure. To achieve commercial success for any product for which we have obtained marketing approval, we will need to establish a sales and marketing team.
We expect to build a focused sales and marketing infrastructure to market our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, and potentially other product candidates in the U.S., if and when they are approved.approved, including by partnering with experienced third party contractors. There are risks involved with establishing our own sales, marketing and distribution capabilities and entering
into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, including failure to receive marketing approval from the FDA, we would have prematurely or unnecessarily incurred these commercialization expenses. For example, we had hired sales and marketing personnel for a launch of our lead product candidate, but we received a CRL from the FDA in May 2023. We may also inaccurately estimate the number of representatives needed to build our sales force, which may result in unnecessary expense or the inability to scale as quickly as needed. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our product candidates, if approved, on our own include:
•our inability to recruit, train and retain adequate numbers of effective sales, marketing, reimbursement, customer service, medical affairs, and other support personnel;
•the inability of sales personnel to obtain access to physicians or increase market acceptable of our approved product;
•the inability of reimbursement professionals to negotiate arrangements for coverage or adequate reimbursement by payors for our approved products;
•the inability to price our product candidates at a sufficient price point to ensure an adequate and attractive level of profitability;
•restricted or closed distribution channels that make it difficult to distribute our product candidates to segments of the patient population; and
•unforeseen costs and expenses associated with creating an independent commercialization organization.
If we do not establish sales, marketing and distribution capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
Problems related to large-scale commercial manufacturing could cause delays in product launches, an increase in product reclass or costs, product recalls or shortages of product candidates.shortages.
Manufacturing finished drug products, especially in large quantities, is complex. If our product candidates receive regulatory approval, they will require several manufacturing steps and may involve complex techniques to assureensure quality and sufficient quantity, especially as the manufacturing scale increases. Our product candidates will need to be made consistently and in compliance with a clearly defined manufacturing process pursuant to FDA regulations. Accordingly, it will be essential to be able to validate and control the manufacturing process to assure that it is reproducible. Slight deviations anywhere in the manufacturing process, including obtaining materials, filling, labeling, packaging, storage, shipping, quality control and testing, may result in lot failures, delay in the release of lots, product recalls or spoilage. Success rates can vary dramatically at different stages of the manufacturing process, which can lower yields and increase costs. We may experience deviations in the manufacturing process that may take significant time and resources to resolve and, if unresolved, may affect manufacturing output and cause us to fail to satisfy contractual commitments, cause recalls, lead to delays in our clinical trials or result in litigation or regulatory action. Such actions would hinder our ability to meet contractual obligations and could cause material adverse consequences for our business.
If we fail to comply with U.S. and foreign regulatory requirements, regulatory authorities could limit or withdraw any marketing or commercialization approvals we may receive and subject us to other penalties that could materially harm our business. For example, our GMP-in-a-Box willmay be regulated by the FDA as a medical device, and regulatory compliance for medical devices is expensive, complex and uncertain, and a failure to comply could lead to enforcement actions against us and other negative consequences for our business.
The FDA and similar agencies regulate medical devices. All of our potential medical device products and material modifications will be subject to extensive regulation and clearance or approval from the FDA and non-U.S. regulatory agencies prior to commercial sale and distribution as well as after clearance or approval. Complying with these regulations is costly, time‑consuming, complex and uncertain. For instance, before a new medical device, or a new intended use for an existing device, can be marketed in the U.S., a company must first submit a premarket submission, such as a premarket notification (510(k)), De Novo request, or PMA, and receive either 510(k) clearance, De Novo grant, or pre-marketing approval from the FDA, unless an exemption applies.
Any regulatory approvals that we receive for our drug product candidates will require surveillance to monitor the safety and efficacy of the product candidate. The FDA and similar agencies have significant pre- and post-market authority, including requirements related to product design, development, testing, laboratory and preclinical studies, clinical trials and preclinical studies approval, manufacturing processes and quality (including suppliers), labeling, packaging, distribution, adverse event and deviation reporting, storage, shipping, pre-marketpremarket clearance or approval, advertising, marketing, promotion, sale, import, export, product change, recalls, submissions of safety and effectiveness, post-market surveillance and reporting of deaths or serious injuries and certain malfunctions, and other post-marketing information and reports such as deviation reports, registration, product listing, annual user fees, and recordkeeping for our product candidates. The FDA may also require a REMS to approve our product candidates, which may impose further requirements or restrictions on the distribution or use of an approved drug or therapeutic biologic. The FDA may also require post-approval Phase 4 trials. Moreover, the FDA and comparable foreign regulatory authorities will continue to closely monitor the safety profile of any product even after approval.
Medical devices regulated by the FDA are subject to general controls which include: registration with the FDA; listing commercially distributed products with the FDA; complying with cGMP under Quality Systems Regulations;QSR; filing reports with the FDA of and keeping records relative to certain types of adverse events associated with devices under the medical device reporting regulation; assuring that device labeling complies with device labeling requirements; and reporting certain device field removals and corrections to the FDA; and obtaining pre-market notification 510(k) clearance for devices prior to marketing. Some devices known as 510(k)-exempt devices can be marketed without prior marketing-clearance or approval from the FDA. In addition to the general controls, some Class 2 medical devices are also subject to special controls,controls. Most medical devices that require premarket review by the FDA, including adherence tomost Class 2 medical devices, require the submission of a particular guidance document510(k) or a De Novo request and compliance with the performance standard. Instead of obtaining 510(k) clearance mostor De Novo grant prior to marketing the device. Some devices known as 510(k)-exempt devices can be marketed without prior clearance or approval from the FDA. Most Class 3 devices are subject to premarket approval (PMA).the FDA’s PMA requirement. Further, in February 2024, the FDA issued a final rule replacing the QSR with the QMSR, which incorporates by reference the quality management system requirements of ISO 13485:2016. The FDA has stated that the standards contained in ISO 13485:216 are substantially similar to those set forth in the existing QSR. This final rule does not go into effect until February 2026.
The FDA can also refuse to clear or approve pre-market applicationspremarket submissions for any medical device we develop. We may not be able to obtain the necessary clearances or approvals or may be unduly delayed in doing so, for any medical device products we develop, which could harm our business. Furthermore, even if we are granted regulatory clearances or approvals for any medical device products, they may include significant limitations on the indicated uses for the product, which may limit the market for the product.
In addition, we, our contractors, and our collaborators are and will remain responsible for FDA compliance. We and any of our collaborators, including our contract manufacturers, could be subject to periodic unannounced inspections by the FDA to monitor and ensure compliance with regulatory requirements. Application holders must further notify the FDA, and depending on the nature of the change, obtain FDA pre-approval for product and manufacturing changes. The cost of compliance with post-approval regulations may have a negative effect on our operating results and financial condition.
If the FDA or comparable foreign regulatory authorities become aware of new safety information or previously unknown problems after approval of any of our product candidates, including: (i) adverse events of unanticipated severity or frequency, (ii) that the product is less effective than previously thought, (iii) problems with our third-party manufacturers or manufacturing processes or (iv) failure to comply with regulatory requirements, or if we violate regulatory requirements at any stage, whether before or after marketing approval is obtained, we may face a number of regulatory consequences, including fines, warnings or untitled letters, holds on clinical trials, delay of approval or refusal by the FDA to approve pending applications or supplements to approved applications, suspension or withdrawal of regulatory approval, product recalls and seizures, administrative detention of products, refusal to permit the import or export of products, operating restrictions or partial suspension or total shutdown of production, injunctions, consent decrees, civil penalties and criminal prosecution, among other consequences. Additionally, we may face unanticipated expenditures to address or defend such actions and customer notifications for repair, replacement or refunds. Any such restrictions could limit sales of the product. Any of these events could further have other material and adverse effects on our operations and business and could adversely impact our stock price and could significantly harm our business, financial condition, results of operations, and prospects.
The FDA also regulates the advertising and promotion of medical devices to ensure that the claims are consistent with their regulatory clearances or approvals, that there are adequate and reasonable data to substantiate the claims and that the promotional labeling and advertising is neither false nor misleading in any respect. If the FDA determines that any of our advertising or promotional claims are misleading, not substantiated or not permissible, we may be subject to enforcement actions, including warning letters, and we may be required to revise our promotional claims and make other corrections or restitutions. Failure to comply with applicable U.S. requirements regarding, for example, promoting, manufacturing, or labeling our medical device products, may subject us to a variety of administrative or judicial actions and sanctions, such as Form 483 observations, warning letters, untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution. If any of our medical device products cause or contribute to a death or a serious injury or malfunction in certain ways, we will be required to report under applicable medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
If any of these events were to occur, it would have a material and adverse effect on our business, financial condition and results of operations.
The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting pre-approval promotion and the promotion of off-label uses.
The FDA prohibits the pre-approval promotion of drugs as safe and effective for the purposes for which they are under investigation. Similarly, the FDA prohibits the promotion of approved drugs for new or unapproved indications. If the FDA finds that we have engaged in pre-approval promotion of our future product candidates, or if any of our future product candidates are approved and we are found to have improperly promoted off-label uses of those products, we may become subject to significant liability. The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products, such as our future product candidates, if approved. In particular, an approved product may not be promoted for uses that are not approved by the FDA or such other regulatory agencies as reflected in the product’s approved labeling. If we receive marketing approval for a product candidate, physicians may nevertheless prescribe it to their patients in a manner that is inconsistent with the approved label, which is within their purview as part of their practice of medicine. If we are found to have promoted such off-label uses, however, we may become subject to significant liability. The U.S. federal government has levied large civil and criminal fines against companies for alleged improper promotion of off-label use and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. The FDA may also issue a public warning letter or untitled letter to the company. If we cannot successfully manage the promotion of our future approved products, we could become subject to significant liability, which would materially adversely affect our business and financial condition.
Results for any patient who receives compassionate use access to our product candidates should not be viewed as representative of how the product candidate will perform in a well-controlled clinical trial and cannot be used to establish safety or efficacy for regulatory approval.
We often receive requests for compassionate use access to our investigational drugs by patients that do not meet the entry criteria for enrollment into our clinical trials. Generally, patients requesting compassionate use have no other treatment alternatives for life threateninglife-threatening conditions. We evaluate each compassionate use request on an individual basis, and in some cases grant access to our investigational product candidates outside of our sponsored clinical trials if a physician certifies that the patient receiving treatment is critically ill and does not meet the entry criteria for one of our open clinical trials. Individual patient results from compassionate use access may not be used to support submission of a regulatory application, may not support approval of a product candidate, and should not be considered to be indicative of results from any on-goingongoing or future well-controlled clinical trial. Before we can seek regulatory approval for any of our product candidates, we must demonstrate in well-controlled clinical trials statistically significant evidence that the product candidate is both safe and effective for the indication for which we are seeking approval. The results of our compassionate use program may not be used to establish safety or efficacy or regulatory approval.
We are and will be subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal and/or civil liability and other serious consequences for violations, which can harm our business.
Our product candidates will be subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations and various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls,OFAC, the FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. §201, the U.S. Travel Act, the USA PATRIOT Act and possibly other state and national anti-bribery and anti-money laundering laws in countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, third-party intermediaries, joint venture partners and collaborators from authorizing, promising, offering or providing, directly or indirectly, improper payments or benefits to recipients in the public or private sector. We use CROs abroad for clinical trials. In addition, we may engage third-party intermediaries to sell our product candidates and solutions abroad once we enter a commercialization phase for our product candidates and/or to obtain necessary permits, licenses, and other regulatory approvals. We or our third-party intermediaries may have direct or indirect interactions with officials and employees of government agencies or state-owned or affiliated entities. We can be held liable for the corrupt or other illegal activities of these third-party intermediaries, our employees, representatives, contractors, partners and agents, even if we do not explicitly authorize or have actual knowledge of such activities. ifIf we fail to comply with these laws and regulations, we and certain of our employees could be subject to substantial civil or criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm and other consequences.
We have adopted an anti-corruption policy, which mandates compliance with the FCPA and other anti-corruption laws applicable to our business throughout the world. However, there can be no assurance that our employees and third-party intermediaries will comply with this policy or such anti-corruption laws. Non-compliance with anti-corruption and anti-money laundering laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, other investigations, or other enforcement actions. If such actions are launched, or governmental or other sanctions are imposed, or if we do not prevail in any possible civil or criminal litigation, our business, results of operations and financial condition could be materially harmed. In addition, responding to any action will likely result in a materially significant diversion of management’s attention and resources and significant defense and compliance costs and other professional fees. In certain cases, enforcement authorities may even cause us to appoint an independent compliance monitor, which can result in added costs and administrative burdens.
There is currently significant uncertainty about the future relationship between the U.S. and various other countries, most significantly China, with respect to trade policies, treaties, tariffs, taxes, and other limitations on cross-border operations. The U.S. government has made and continues to make significant additional changes in U.S. trade policy and may continue to take future actions that could negatively impact U.S. trade. For example, legislation has been introduced in Congress to limit certain U.S. biotechnology companies from using equipment or services produced or provided by select Chinese biotechnology companies, and others in Congress have advocated for the use of existing executive branch authorities to limit those Chinese service providers’ ability to engage in business in the U.S. We cannot predict what actions may ultimately be taken with respect to trade relations between the U.S. and China or other countries, what products and services may be subject to such actions or what actions may be taken by the other countries in retaliation. If we are unable to obtain or use services from existing service providers or become unable to export or sell our products to any of our customers, our business, liquidity, financial condition, and/or results of operations would be materially and adversely affected.
Our failure to comply with state, national and/or international data protectionprivacy and security laws and regulations could lead to government enforcement actions and significant penalties against us, and adversely impact our operating results.
There are numerous laws and legislative and regulatory initiativesregulations at the federal and state levels addressing privacy and security concerns, and some state privacy laws apply more broadly than HIPAA and associated regulations. For example, California recently enacted legislation—the California Consumer Privacy Act of 2018 (CCPA)—CCPA, which went into effect on January 1, 2020. The CCPA,2020, provides, among other things, creates new data privacy and security obligations for covered companies and provides new privacy rights to California consumers, including the right to opt out of certain disclosuressales of their personal information. The CCPA also provides for civil penalties as well as a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. Although the lawCCPA includes limited exceptions, including for certain personal information collected as part of certain clinical trials as specified in the law,or other biomedical research studies, it may regulate or impact our
processing of personal information depending on the context. Additionally, a new privacy law, the California Privacy Rights Act (CPRA),CPRA was approved by California voters in November 2020 and went into effect in most material respects on January 1, 2023.2020. The CPRA significantly modifiedmodifies the CCPA, which may require us to modify our practices and policies and may further increase our compliance costs and potential liability. Certain other statestates have also enacted or proposed privacy laws impose similar privacy obligations,governing health information, including for example, Washington’s My Health, My Data Act and Nevada’s Senate Bill 370, and all 50 states have enacted laws includingimposing obligations to provide notification of certain security breaches of computer databases that contain personal informationinformation. Additionally, several states have enacted or proposed laws similar to affected individuals, state officers and others. For example, the CCPA, has prompted the enactment of several new state laws or amendments of existing state laws, such as in New York, Nevada, Virginia, Colorado, Utah, Connecticut, Iowa, Indiana, Montana, Tennessee, Oregon, Florida, Delaware, and Colorado.Texas. These laws could mark the beginning of a further trend toward more stringent privacy legislationlaws in otherthe U.S. states and have prompted a number of proposals for new federal and state-level privacy legislation. Tolaws. We cannot yet determine the extentimpact these state laws as well as other federal and state privacy laws, including new laws andor changes in existing laws, apply tomay have on our business and operations, but anticipate they could increase our compliance costs and potential liability, with respectimpair our ability to collect, use or otherwise process personal information, we collect could expose us to greatgreater liability and increase compliance costs.
require us to modify our practices and policies in an effort to comply. There are also various laws and regulations in other jurisdictions relating to privacy and security. For example, European Union (EU)EU member states and other foreign jurisdictions, including the UK and Switzerland, have adopted data protection laws and regulations which impose significant compliance obligations on us. The collection, use, and useother processing of personal data, including patient or health data, in the EU, ismay be governed by the EU General Data Protection Regulation (GDPR).GDPR. The GDPR, which is wide-ranging in scope and applies extraterritorially, imposes, severalamong other things, requirements relating to the consent of the individuals to whom the personal data relates, the informationnotices provided to such individuals, the security and confidentiality of the personal data, data breach notification, the adoption of appropriate privacy governance, including policies, procedures, training and audits, and the use of third-party processors in connection with the processing of personal data. The GDPR also imposes strict rules on the transfer of personal data out of the EU, including to the U.S., provides andata protection authorities with enforcement authority and imposes large penalties for noncompliance, including the potential for fines of up to €20 million or up to 4% of the total worldwide annual global revenues of the noncompliant entity, whichever is greater. The GDPR requirements apply not only to third-party transactions,personal data transfers, but also to transfers of informationpersonal data between us and our subsidiaries, including employee information. In addition, in January 2021, following its exit from the EU, the UK transposed the GDPR into its domestic law with its own version of the GDPR (combining the GDPR and the UK Data Protection Act of 2018) (UK GDPR), which currently imposes the same obligations as the GDPR in most material respects and provides for fines of up £17.5 million or up to 4% of the total worldwide annual global revenues of the noncompliant entity, whichever is greater.
Complying with these numerous, complex, and often changing laws and regulations is expensive and difficult, anddifficult. Any actual or alleged failure to comply with any privacy laws or data security lawslaw or anyregulation, or security breach or other incident, or breachincluding those involving the misappropriation, loss, or other unauthorized processing, use, disclosure or disclosureother processing of sensitive or confidential patient, consumer or other personal information, whether by us, one of our CROs or business associates or another third party, could adversely affect our business, financial condition, and results of operations, including but not limited to: investigation costs;and could subject us to investigations, litigation, and other proceedings, material fines and penalties;penalties, compensatory, special, punitive and statutory damages; litigation;damages, consent orders regarding our privacy and security practices;practices, requirements that we provide notices, credit monitoring services and/or credit restoration services or other relevant services to impacted individuals;individuals, adverse actions against our licenses to do business;business, reputational damage;damage, and injunctive relief. The recent implementationenactment of, the CCPA, GDPR and UK GDPR haschanges to, privacy and security laws and regulations have increased our responsibility and potential liability, including in relation to the personal data that we process including inand our clinical trials, and we may in the future be required to put in place additional mechanisms in an effort to ensure compliancecomply with the CCPA, GDPR, UK GDPR and other applicable laws and regulations, which could divert management’s attention and increase our cost of doing business. In addition, any new law or regulation or legislative actions regarding datarelating to privacy and security, (together withor any applicable industry standards)standard, may increase our costs of doing business. In this regard, we expect that there will continue to be new proposed laws, regulations and industry standards relating to privacy and data protectionsecurity in the U.S., the United Kingdom,UK, the EU, and other jurisdictions, and we cannot determine the impact such future laws, regulations and standards may have on our business.
We cannot assure you that our CROs or other third-party service providers with access to our or our customers’, suppliers’, trial patients’ and employees’ personally identifiable andpersonal information or other sensitive or confidential information in relation to which we are responsible will not breach applicable laws or regulations or contractual obligations imposed by us, or that they will not experience data security breaches or incidents, which could have a corresponding effect on our business, including putting us in breach of our obligations under privacy and security laws and regulations and/or which could in turn adversely affect our business, results of operations and financial condition. We cannot assure you that our contractualthe measures and our own privacy and security-related safeguards we have taken will protect us from the foregoing risks, associated with the third-party processing, use, storage and transmissionany of such information. Any of the foregoingwhich could have a material adverse effect on our business, financial condition, results of operations and prospects.
We and our third-party contractors must comply with environmental, health and safety laws and regulations. A failure to comply with these laws and regulations could expose us to significant costs or liabilities.
We and any of our third-party contract manufacturers or suppliers are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, generation, manufacture, storage, treatment and disposal of hazardous materials and wastes. Hazardous chemicals, including flammable and biological materials, are involved in certain aspects of our business, and we cannot eliminate the risk of injury or contamination from the use, generation, manufacture, distribution, storage, handling, treatment or disposal of hazardous materials and wastes. In the event of contamination or injury, or failure to comply with such environmental, health and safety laws and regulations, we could be held liable for any resulting damages, fines and penalties associated with such liability, which could exceed our assets and resources.
Although we will maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of biological or hazardous materials or wastes arising out of and in the course of employment, this insurance may not provide adequate coverage against potential liabilities. We do not maintain comprehensive insurance coverage for liabilities arising from medical or hazardous materials, environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials.
Environmental, health and safety laws and regulations are becoming increasingly more stringent. We may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts, which could harm our business, prospects, financial condition or results of operations. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Coverage and reimbursement may be limited or unavailable in certain market segments for our product candidates, which could make it difficult for us to sell our product candidates profitably.
In both domestic and foreign markets, sales of our product candidates, if approved, depend on the availability of coverage and adequate reimbursement from third-party payors. Third-party payors, whether domestic or foreign, or governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. Regulatory authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications, which could affect our ability or that of our collaborators to sell our product candidates profitably. In addition, third‑party payors are requiring higher levels of evidence of the benefits and clinical outcomes of new technologies and are challenging the prices charged. Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Patients are unlikely to use our product candidates unless coverage is provided, and reimbursement is adequate to cover a significant portion of the cost of our product candidates. Such third-party payors include government health programs such as Medicare and Medicaid, managed care providers, private health insurers and other organizations. Obtaining coverage and adequate reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors is critical to new product acceptance. In addition, because our product candidates represent new approaches to the treatment of cancer, we cannot accurately estimate the potential revenues from our product candidates.
Government authorities and third-party payors decide which drugs and treatments they will cover and the amount of reimbursement. Coverage decisions may depend upon clinical and economic standards that disfavor new drug products when more established or lower cost therapeutic alternatives are already available or subsequently become available. These payors may not view our products, if any, as cost-effective, and coverage and reimbursement may not be available to our customers, or those of our collaborators, or may not be sufficient to allow our products, if any, to be marketed on a competitive basis. If reimbursement is not available, or is available only to limited levels, our product candidates may be competitively disadvantaged, and we, or our collaborators, may not be able to successfully commercialize our product candidates. Alternatively, securing favorable reimbursement terms may require us to compromise pricing and prevent us from realizing an adequate margin over cost. Reimbursement by a third-party payor may depend upon a number of factors, including, but not limited to, the third-party payor’s determination that use of a product is:
•a covered benefit under its health plan;
•safe, effective and medically necessary;
•appropriate for the specific patient;
•cost-effective; and
•neither experimental nor investigational.
In the U.S., no uniform policy of coverage and reimbursement for products exists among third-party payors. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our product candidates to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained. Moreover, the factors noted above have continued to be the focus of policy and regulatory debate that has, thus far, shown the potential for movement towards permanent policy changes; this trend is likely to continue, and may result in more or less favorable impacts on pricing. The recent and ongoing series of congressional hearings relating to drug pricing has presented heightened attention to the biopharmaceutical industry, creating the potential for political and public pressure, while the potential for resulting legislative or policy changes presents uncertainty. Congress is consideringhas considered and may continue to consider legislation that, if passed, could have significant impact on prices of prescription drugs covered by Medicare, including limitations on drug price increases. The impact of these regulations and any future healthcare measures and agency rules implemented by the Biden administration on us and the pharmaceutical industry as a whole is currently unknown. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our product candidates if approved. Complying with any new legislation and regulatory changes could be time-intensive and expensive, resulting in a material adverse effect on our business.
Prices paid for a drug also vary depending on the class of trade. Prices charged to government customers are subject to price controls, including ceilings, and private institutions obtain discounts through group purchasing organizations. Net prices for drugs may be further reduced by mandatory discounts or rebates required by government healthcare programs and demanded by private payors. It is also not uncommon for market conditions to warrant multiple discounts to different customers on the same unit, such as purchase discounts to institutional care providers and rebates to the health plans that pay them, which reduces the net realization on the original sale.
In addition, federal programs impose penalties on manufacturers of drugs marketed under a BLA or NDA, in the form of mandatory additional rebates and/or discounts if commercial prices increase at a rate greater than the Consumer Price Index-Urban, and these rebates and/or discounts, which can be substantial, may impact our ability to raise commercial prices. For example, under the American Rescue Plan Act of 2021, effective January 1, 2024, the statutory cap on Medicaid Drug Rebate Program rebates that manufacturers pay to state Medicaid programs will be eliminated. Elimination of this cap may require pharmaceutical manufacturers to pay more in rebates than it receives on the sale of products, which could have a material impact on our business. In August 2022, Congress passed the Inflation Reduction Act of 2022,IRA, which includes prescription drug provisions that have significant implications for the pharmaceutical industry and Medicare beneficiaries, including allowing the federal government to negotiate a maximum fair price for certain high-priced single source Medicare drugs, imposing penalties and excise tax for manufacturers that fail to comply with the drug price negotiation requirements, requiring inflation rebates for all Medicare Part B and Part D drugs, with limited exceptions, if their drug prices increase faster than inflation, and redesigning Medicare Part D to reduce out-of-pocket prescription drug costs for beneficiaries, among other changes. Various industry stakeholders, including certain pharmaceutical companies and the Pharmaceutical Research and Manufacturers of America, have initiated lawsuits against the federal government asserting that the price negotiation provisions of the IRA are unconstitutional. The impact of these judicial challenges, legislative, executive, and administrative actions and any future healthcare measures and agency rules implemented by the Biden administration on us and the pharmaceutical industry as a whole is unclear. Cost control initiatives could cause us, or our collaborators, to decrease, discount, or rebate a portion of the price we, or they, might establish for products, which could result in lower than anticipated product revenues. If the realized prices for our product candidates, if any, decrease or if governmental and other third-party payors do not provide adequate coverage or reimbursement, our prospects for revenues and profitability will suffer.
Even if we obtain coverage for a given product, the resulting approved reimbursement payment rates might not be high enough to allow us to establish or maintain a market share sufficient to realize a sufficient return on our or their investments or achieve or sustain profitability or may require co-payments that patients find unacceptably high. If payors subject our product candidates to maximum payment amounts or impose limitations that make it difficult to obtain reimbursement, providers may
choose to use therapies which are less expensive when compared to our product candidates. Additionally, if payors require high co-payments, beneficiaries may decline prescriptionsour therapies and seek alternative therapies. We may need to conduct post-marketing studies in order to demonstrate the cost-effectiveness of any future products to the satisfaction of hospitalsphysicians and other target customers and their third-party payors. Such studies might require us to commit a significant amount of management time and financial and other resources. Our future products might not ultimately be considered cost-effective. Adequate third-party coverage and reimbursement might not be available to enable us to maintain price levels sufficient to realize an appropriate return on investment in product development.
We, and our collaborators, cannot be sure that coverage will be available for any product candidate that we, or they, commercialize and, if available, that the reimbursement rates will be adequate. Further, the net reimbursement for drug products may be subject to additional reductions if there are changes to laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. An inability to promptly obtain coverage and adequate payment rates from both government-funded and private payors for any of our product candidates for which we obtain marketing approval could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products, and our overall financial condition.
There have been, and likely will continue to be, legislative and regulatory proposals at the federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare and/or impose price controls may adversely affect:
•the demand for our product candidates, if we obtain regulatory approval;
•our ability to set a price that we believe is fair for our product candidates;
•our ability to generate revenues and achieve or maintain profitability;
•the level of taxes that we are required to pay; and
•the availability of capital.
Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors, which may adversely affect our future profitability. A particularpossible challenge for our product candidates arises from the fact that they will primarilymay potentially be used in an inpatient setting. Inpatient reimbursement generally relies on stringent packaging rules that may mean that there is no separate payment for our product candidates. Additionally, data used to set the payment rates for inpatient admissions is usually several years old and would not take into account all of the additional therapy costs associated with the administration of our product candidates. If special rules are not created for reimbursement for immunotherapy treatments such as our product candidates, hospitals might not receive enough reimbursement to cover their costs of treatment, which will have a negative effect on their adoption of our product candidates.
We may face difficulties from changes to current regulations and future legislation.
In the U.S. and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities, and affect our ability, or the ability of our collaborators, to profitably sell any products for which we obtain marketing approval. We expect that current laws, as well as other federal and state healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria, increased regulatory burdens and operating costs, decreased revenues from our biopharmaceutical product candidates, decreased potential returns from our development efforts, and additional downward pressure on the price that we, or our collaborators, may receive for any approved products.
Since enactment of the Affordable Care Act (ACA)ACA in 2010, in both the U.S. and certain foreign jurisdictions, there have been a number of legislative and regulatory changes to the health care system that could impact our ability to sell our product candidates profitably. These changes included aggregate reductions of Medicare payments to providers of up to 2% per fiscal year, effective April 1, 2013, which, due to subsequent legislative amendments, will stay in effect through 2031,2032, with the exception of a temporary suspension implemented under various COVID‑19 relief legislation from May 1, 2020 through March 31, 2022. Under current legislation, the actual reduction in Medicare payments can vary from 1% in 2022 to up to 4% in the final fiscal year of this sequester.legislation. In January 2013, the American Taxpayer Relief Act of 2012 (ATRA)ATRA was approved which, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the
government to recover overpayments to providers from three to five years. These laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for our product candidates, if approved, and accordingly, our financial operations.
Since its enactment, various portions of the ACA have been subject to judicial and constitutional challenges. In June 2021, the U.S. Supreme Court held that Texas and other challengers had no legal standing to challenge the ACA, dismissing the case without specifically ruling on the constitutionality of the ACA. Accordingly, the ACA remains in effect in its current form. It is unclear how this Supreme Court decision, future litigation, or healthcare measures promulgated by the Biden administration will impact our business, financial condition and results of operations. Complying with any new legislation or reversing changes implemented under the ACA could be time-intensive and expensive, resulting in a material adverse effect on our business.
Any reduction in reimbursement from Medicare or other government healthcare programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenues, attain profitability or commercialize our product candidates.
Legislative and regulatory proposals may also be made to expand post-approval requirements and restrict sales and promotional activities for drugs. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance, or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements. Further, if the Supreme Court reverses or curtails the Chevron doctrine, which gives deference to regulatory agencies in litigation against the FDA and other agencies, more companies may bring lawsuits against the FDA to challenge longstanding decisions and policies of the FDA, which could undermine the FDA’s authority, lead to uncertainties in the industry, and disrupt FDA’s normal operations, which could delay the FDA’s review of our marketing applications.
In addition, there have been increasing legislative efforts and enforcement interest in the U.S. with respect to drug pricing practices, including Congressional inquiries and proposed federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. As discussed above, in August 2022, Congress passed the Inflation Reduction Act of 2022,IRA, which includes prescription drug provisions that have significant implications for the pharmaceutical industry and Medicare beneficiaries, including allowing the federal government to negotiate a maximum fair price for certain high-priced single source Medicare drugs, imposing penalties and excise tax for manufacturers that fail to comply with the drug price negotiation requirements, requiring inflation rebates for all Medicare Part B and Part D drugs, with limited exceptions, if their drug prices increase faster than inflation, and redesigning Medicare Part D to reduce out-of-pocket prescription drug costs for beneficiaries, among other changes. Various stakeholders have initiated lawsuits against the federal government asserting that the price negotiation provisions of the IRA are unconstitutional. The impact of these judicial challenges, legislative, executive, and administrative actions and any future healthcare measures and agency rules implemented by the Biden administration on us and the pharmaceutical industry as a whole is unclear. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. For example, the FDA recently authorized the state of Florida to import certain prescription drugs from Canada for a period of two years to help reduce drug costs, provided that Florida’s Agency for Health Care Administration meets the requirements set forth by the FDA. Other states may follow Florida.
We are unable to predict the future course of federal or state healthcare legislation in the U.S. directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. The ACA and any further changes in the law or regulatory framework that reduce our revenues or increase our costs could also have a material and adverse effect on our business, financial condition and results of operations. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our current product candidates and any future product candidates or additional pricing pressures.
Governments outside the U.S. tend to impose strict price controls, which may adversely affect our revenues, if any.
In international markets, reimbursement and health care payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies. In some countries, particularly the countries of the EU, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain coverage and reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost‑effectiveness of our product candidate to other available therapies. There can be no assurance that our product candidates will be considered cost-effective by third-party payors, that an adequate level of reimbursement will be available, or that the third-party payors’ reimbursement policies will not adversely affect our ability to sell our product candidates profitably. If reimbursement of our product candidates is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed, possibly materially.
Our employees, independent contractors, consultants, commercial partners, principal investigators, CROs, suppliers and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of employee fraud or other illegal activity by our employees, independent contractors, consultants, commercial partners, principal investigators, CROs, suppliers and vendors. Misconduct by these parties could include intentional, reckless and/or negligent conduct that fails to: comply with the laws of the FDA and other similar foreign regulatory bodies, provide true, complete and accurate information to the FDA and other similar foreign regulatory bodies, comply with manufacturing standards we have established, comply with healthcare fraud and abuse laws in the U.S. and similar foreign fraudulent misconduct laws, or report financial information or data accurately or to disclose unauthorized activities to us. If we obtain FDA approval of any of our product candidates and begin commercializing those product candidates in the U.S., our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws and regulations designed to prevent fraud, kickbacks, self‑dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials, which could result in regulatory sanctions and serious harm to our reputation.
It is not always possible to identify and deter misconduct or other improper activities by our employees or third parties that we engage for our business operations and the precautions we take to detect and prevent inappropriate conduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a material adverse effect on our business, financial condition, results of operations and prospects, including the imposition of significant fines or other sanctions, including exclusion from government healthcare programs, and serious harm to our reputation. In addition, the approval and commercialization of any of our product candidates outside the U.S. will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws. Efforts to ensure that our business arrangements will comply with applicable healthcare laws may involve substantial costs.
Our relationships with health care professionals, institutional providers, principal investigators, consultants, potential customers and third-party payors are, and will continue to be, subject, directly and indirectly, to federal and state health care fraud and abuse, false claims, marketing expenditure tracking and disclosure, government price reporting, and privacy and data security laws. If we are unable to comply, or have not fully complied, with such laws, we could face significant penalties and liabilities.
Our business operations and activities may be directly or indirectly subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act.FCA. If we obtain FDA approval for any of our product candidates and begin commercializing those product candidates in the U.S., our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. Our current and future arrangements with healthcare professionals, clinical investigators, CROs, third-party payors and customers may expose us
to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. In addition, we may be subject to laws of the federal government and state governments in which we conduct our business relating to privacy and data security with respect to patient information.or health data. The laws that may affect our ability to operate include, but are not limited to:
•the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual for a healthcare item or service, or the purchasing or ordering of an item or service, for which payment may be made under a federal healthcare program such as Medicare or Medicaid;
•the U.S. federal false claims and civil monetary penalties laws, including the federal civil False Claims Act,FCA, which prohibit, among other things, individuals or entities from knowingly presenting or causing to be presented, claims for payment by government funded programs such as Medicare or Medicaid that are false or fraudulent, and which may apply to us by virtue of statements and representations made to customers or third parties;
•HIPAA, which created additional federal criminal statutes that prohibit, among other things, knowingly and willfully executing or attempting to execute a scheme to defraud healthcare programs;programs, as well as;
•HIPAA, as amended by HITECH, which imposes requirements on certain types of people and entities relating to the privacy, security, and transmission of individually identifiable PHI, and requires notification to affected individuals and regulatory authorities of certain breaches of the privacy or security of PHI;PHI, and other U.S. laws and foreign laws that govern the privacy or security of health or patient data;
•the federal Physician Payment Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, to report annually to the Centers for Medicare & Medicaid Services (CMS)CMS information related to payments and other transfers of value to covered recipients, including physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), other healthcare providers (such as physician assistants and nurse practitioners) and teaching hospitals, and ownership and investment interests held by physicians and their immediate family members, which is published in a searchable form on an annual basis;
•federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making, or causing to be made, false statements relating to healthcare matters;
•the federal Civil Monetary Penalties Law, which prohibits, among other things, offering or transferring remuneration to a federal healthcare beneficiary that a person knows or should know is likely to influence the beneficiary’s decision to order or receive items or services reimbursable by the government from a particular provider or supplier;
•the FCPA, the U.K. Bribery Act of 2010, and other local anti-corruption laws that apply to our international activities; and
•state laws comparable to each of the above federal laws, such as, for example, anti-kickback and false claims laws that may be broader in scope and also apply to commercial insurers and other non-federal payors, requirements for mandatory corporate regulatory compliance programs, and laws relating to patient or health data, privacy andor security. Other state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government;government, and require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.expenditures.
We expect to incur increased costs of compliance with such laws and regulations as they continue to evolve. If we or our contractors are unable to comply, or have not fully complied, with such laws, we could face penalties, including, without limitation, civil, criminal, and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal and state health care programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations. Any of these could adversely affect our business, financial condition, and results of operations.
If we were to grow our business and expand our sales organization or rely on distributors outside of the U.S., we would be at increased risk of violating these laws or our internal policies and procedures. The risk of us being found in violation of these or other laws and regulations is further increased by the fact that many have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
Disruptions at the FDA, the SEC and other government agencies caused by funding shortages could hinder their ability to hire and retain key leadership and other personnel, prevent new products from being developed or commercialized in a timely manner, or otherwise prevent those agencies from performing normal business functions, which could negatively impact our business and the approval of our BLA submission, as well as adversely affect the U.S. and global economy and our liquidity, financial condition and earnings.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels and related government shutdowns, the ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely is subject to the impacts of political events, which are inherently fluid and unpredictable.
Disruptions at the FDA and other agencies, including disruptions due to public health concerns, resurgence of COVID-19 cases, travel restrictions, or staffing shortages, may slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which could adversely affect our business. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. If a prolonged government shutdown occurs in the future, including as a result of any failure by the U.S. federal government to increase the debt ceiling, it could significantly impact the ability of the FDA and the SEC to timely review and process our submissions, as well as cause interest rates and borrowing costs to further increase, which may negatively impact our ability to access the debt markets, including the corporate bond markets, on favorable terms, which could have a material adverse effect on our business, financial condition and results of operations and/or our BLA submission.
Risks Related to Intellectual Property
If we are unable to obtain, maintain, protect and enforce patent protection and other proprietary rights for our product candidates and technologies, we may not be able to compete effectively or operate profitably and our ability to prevent our competitors from commercializing similar or identical technology and product candidates would be adversely affected.
Our success is dependent in large part on our obtaining, maintaining, protecting and enforcing patents and other proprietary rights in the U.S. and other countries with respect to our product candidates and technology and on our ability to avoid infringing the intellectual property and other proprietary rights of others. Certain of our intellectual property rights are licensed from other entities, and as such the preparation and prosecution of any such patents and patent applications was not performed by us or under our control. Furthermore, patent law relating to the scope of claims in the biotechnology field in which we operate is still evolving and, consequently, patent positions in our industry may not be as strong as in other more well-established fields. The patent positions of biotechnology and pharmaceutical companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved and has been the subject of much litigation in recent years. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date. As a result, the issuance, scope, validity, enforceability, or commercial value of our patent rights remain highly uncertain.
Any future patents we obtain may not be sufficiently broad to prevent others from using our technology or from developing competing therapeutics and technology. There is no guarantee that any of our pending patent applications will result in issued or granted patents, any of our issued or granted patents will not later be found to be invalid or unenforceable, or any issued or granted patents will include claims sufficiently broad to cover our product candidates and technology, or to provide meaningful protection from our competitors. Our owned or in-licensed pending and future patent applications may not result in patents being issued that protect our N-803, hAd5, saRNA hAd5 and yeast technologies and constructs, cell-based therapies, aldoxorubicin or other product candidates and technologies or that effectively prevent others from commercializing competitive technologies and product candidates.
Moreover, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Even if patent applications we license or own currently or in the future issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors or other third parties from competing with us, or otherwise provide us with any competitive advantage. Any patents that we own or in-license may be challenged, narrowed, circumvented, or invalidated by third parties. Consequently, we do not know whether our N-803, hAd5, saRNA hAd5 and yeast technologies and constructs, cell-based therapies or other product candidates and technologies will be protectable or remain protected by valid and enforceable patents. Our competitors or other third parties may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner which could materially adversely affect our business, financial condition, results of operations and growth prospects.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability and it is uncertain how much protection, if any, will be provided by our patents, including if they are challenged in the courts or patent offices or in other proceedings, such as re-examinations or oppositions, which may be brought in the U.S. or foreign jurisdictions to challenge the validity of a patent. A third party may challenge the validity or enforceability of a patent after its issuance. It is possible that a competitor may successfully challenge our patents or that a challenge will result in limiting their coverage. Moreover, it is possible that competitors may infringe our patents or successfully avoid the patented technology through design innovation. To counter infringement or other unauthorized use, we may be required to file infringement claims, which can be expensive and time‑consuming, even if we were successful in stopping the violation of our patent rights.
We or our licensors may be subject to a third-party preissuance submission of prior art to the USPTO, or become involved in opposition, derivation, revocation, reexamination, post-grant and inter partes review, or interference proceedings or other similar proceedings challenging our owned or licensed patent rights. Should third parties file patent applications, or be issued patents claiming technology also used or claimed by our licensor(s) or by us in any future patent application, we, or one of our licensors, may be required to participate in interference proceedings in the USPTO to determine priority of invention for those patents or patent applications that are subject to the first-to-invent law in the U.S., or may be required to participate in derivation proceedings in the USPTO for those patents or patent applications that are subject to the first-inventor-to-file law in the U.S. We may be required to participate in such interference or derivation proceedings involving our issued patents and pending applications. We may also be required to participate in post-grant challenge proceedings, such as oppositions in a foreign patent office, thatwhich challenge our or our licensor’s priority of invention or other features of patentability with respect to our owned or in‑licensed patents and patent applications. Such challenges may result in loss of patent rights, loss of exclusivity, or in patent claims being narrowed, invalidated, or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our N-803, hAd5, saRNA hAd5 and yeast technologies and constructs, cell-based therapies or other product candidates and technologies. For example, the validity of one of our European patents, EP Patent No. 3601363, is being challenged in an opposition proceeding. This patent is directed to methods of using N-803-based combination therapy with anti-CD38 antibodies to treat cancer, which does not directly relate to any of our current programs. We intend to defend our patent and believe we have meritorious defenses against this opposition. An adverse determination in any such submission,of the type of submissions described above, proceeding or litigation could reduce the scope of, or invalidate or render unenforceable, our owned or in‑licensed patent rights, allow third parties to commercialize our N-803, hAd5, saRNA hAd5 and yeast technologies and constructs, cell-based therapies or other product candidates or technologies and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights.
If we or our collaborators are unsuccessful in any such proceeding or other priority or inventorship dispute, we may be required to cease using the technology or to obtain and maintain license rights from prevailing third parties, including parties involved in any such interference proceedings or other priority or inventorship disputes. A prevailing party in that case may not offer us a license on commercially acceptable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. If we are unable to obtain and maintain such licenses, we may need to cease the development, manufacture, and commercialization of one or more of the product candidates we may develop. The loss of exclusivity or the narrowing of our owned and licensed patent claims could limit our ability to stop others from using or commercializing similar or identical technology and products. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
In addition, given the amount of time required for the development, testing, and regulatory review of new product candidates, patents protecting such product candidates might expire before or shortly after such product candidates are commercialized. As a result, our intellectual property may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
Some of our owned and in-licensed patents and patent applications are, and may in the future be, co-owned with third parties. In addition, certain of our licensors co-own the patents and patent applications we in-license with other third parties with whom we do not have a direct relationship. Our exclusive rights to certain of these patents and patent applications are dependent, in part, on inter-institutional or other operating agreements between the joint owners of such patents and patent applications, who are not parties to our license agreements. If our licensors do not have exclusive control of the grant of licenses under any such third-party co-owners’ interest in such patents or patent applications or we are otherwise unable to secure such exclusive rights, such co-owners may be able to license their rights to other third parties, including our competitors, and our competitors could market competing products and technology. In addition, we may need the cooperation of any such co-owners of our patents in order to enforce such patents against third parties, and such cooperation may not be provided to us. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations and growth prospects.
If any of our owned or in-licensed patent applications do not issue as patents in any jurisdiction, we may not be able to compete effectively.
Changes in either the patent laws or their interpretation in the U.S. and other countries may diminish our ability to protect our inventions, obtain, maintain, and enforce our intellectual property rights and, more generally, could affect the value of our intellectual property or narrow the scope of our owned and licensed patents. With respect to both in-licensed and owned intellectual property, we cannot predict whether the patent applications we and our licensors are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient protection from competitors or other third parties. The patent prosecution process is expensive, time-consuming, and complex, and we may not be able to file, prosecute, maintain, enforce, or license all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output in time to obtain patent protection. Although we enter into nondisclosure and confidentiality agreements with parties who have access to confidential or patentable aspects of our research and development output, such as our employees, corporate collaborators, outside scientific collaborators, CROs, CMOs, consultants, advisors, and other third parties, any of these parties may breach the agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection. In addition, our ability to obtain and maintain valid and enforceable patents depends on whether the differences between our inventions and the prior art allow our inventions to be patentable over the prior art. Furthermore, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we or our licensors were the first to make the inventions claimed in any of our owned or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions.
We or our licensors, collaborators, or any future strategic partners may become subject to third-party claims or litigation alleging infringement of patents or other proprietary rights or seeking to invalidate patents or other proprietary rights, and we may need to resort to litigation to protect or enforce our patents or other intellectual property or the patents or other intellectual property of our licensors, all of which could be expensive, time-consuming and unsuccessful, may delay or prevent the development and commercialization of our product candidates, or may put our patents and other proprietary rights at risk.
If we or one of our licensors initiate legal proceedings against a third party to enforce a patent covering one of our product candidates or other technologies, the defendant could counterclaim that the patent is invalid and/or unenforceable or that we infringe their patents. In patent litigation in the U.S., defendant counterclaims alleging invalidity and/or unenforceability are commonplace, and there are numerous grounds upon which a third party can assert invalidity or unenforceability of a patent. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or other applicable body, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the U.S. or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, inter partes review, interference proceedings, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings).
With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we, our licensor, our or our licensor’s patent counsel and the patent examiner were unaware during prosecution. Moreover, even if our patents were to survive such a litigation challenge to their validity, the patents might still be held to be valid but unenforceable if a court were to decide that the patents are being enforced in a manner inconsistent with the antitrust laws, or that the patents were obtained through deceit during patent office examination or other such failure of sufficient candor to the patent office. If a third party were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection could have a material adverse impact on our business, financial condition, results of operations and prospects. The validity of one of our European patents, EP Patent No. 3601363, is being challenged in an opposition proceeding. We intend to defend our patent and believe we have meritorious defenses against this opposition.
The cost to us of any litigation or other proceeding relating to intellectual property rights, even if resolved in our favor, could be substantial. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources, including our scientists and management, from our business.
An adverse result in any litigation or defense proceeding could put one or more of our owned or licensed patents at risk of being invalidated, held unenforceable, or interpreted narrowly, and could put our patent applications at risk of not issuing.being issued. Such proceedings could result in revocation or cancellation of, or amendment to, our patents in such a way that they no longer cover our product candidates or technologies. If the outcome of litigation is adverse to us, third parties may be able to use our patented invention without payment to us. In addition, in an infringement proceeding, there is a risk that a court may decide that one or more of our patents is not valid or is unenforceable and that we do not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of our patents were upheld, a court would refuse to stop the other party on the grounds that its activities are not covered by, that is, do not infringe, our patents. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be better able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. The outcome following legal assertions of invalidity and unenforceability is unpredictable. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
The use of our technology and product candidates could potentially conflict with the rights of others, and third-party claims of intellectual property infringement, misappropriation or other violation against us, our licensors or our collaborators may prevent or delay the development and commercialization of our product candidates and technologies.
Our commercial success depends in part on our, our licensors’ and our collaborators’ ability to avoid infringing, misappropriating and otherwise violating the patents and other intellectual property rights of third parties. There is a substantial amount of complex litigation involving patents and other intellectual property rights in the biopharmaceutical industry. Our potential competitors or other parties may have, develop or acquire patent or other intellectual property rights that they could assert against us. If they do so, then we may be required to alter our product candidates, pay licensing fees or cease our development and commercialization activities with respect to the applicable product candidates or technologies. If our product candidates conflict with patent or other intellectual property rights of others, such parties could bring legal actions against us or our collaborators, licensees, suppliers or customers, claiming damages and seeking to enjoin manufacturing, use and marketing of the affected products.
Although we have conducted freedom-to-operate (FTO)FTO analyses of the patent landscape with respect to our lead product candidates and continue to undertake FTO analyses of our manufacturing processes, our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, and contemplated future processes and products, because patent applications do not publish for 18 months, and because the claims of patent applications
can change over time, no FTO analysis can be considered exhaustive. We may not be aware of patents that have already been issued and that a competitor or other third party might assert are infringed by our current or future product candidates or technologies. It is also possible that we could be found to have infringed patents owned by third parties of which we are aware, but which we do not believe are relevant to our product candidates or technologies. In addition, because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our product candidates or technologies may infringe. Furthermore, patent and other intellectual property rights in biotechnology remains an evolving area with many risks and uncertainties. As such, we may not be able to ensure that we can market our product candidates without conflict with the rights of others.
If intellectual property-related legal actions asserted against us are successful, in addition to any potential liability for damages (including treble damages and attorneys’ fees for willful infringement), we could be enjoined from, or required to obtain a license to continue, manufacturing, promoting the use of or marketing the affected products. We may not prevail in any legal action and a required license under the applicable patent or other intellectual property may not be available on acceptable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. We also could be required to redesign our infringing products, which may be impossible or require substantial time and monetary expenditure.
Defense of infringement claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and other employee resources from our business, and may impact our reputation. Some of our competitors may be able to sustain the costs of litigation or administrative proceedings more effectively than we can because of greater financial resources. In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. The occurrence of any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other immunotherapy and biopharmaceutical companies, our success is dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involveinvolves both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain. In addition, the U.S. has recently enacted and is currently implementing wide-ranging patent reform legislation. Assuming that other requirements for patentability are met, prior to March 2013, in the U.S., the first to invent the claimed invention was entitled to the patent, while outside the U.S., the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America Invents Act (the America Invents Act) enacted in September 2011, the U.S. transitioned to a first-to-file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party that files a patent application in the USPTO after March 2013, but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party.party made it. This will require us to be cognizant of the time from invention to filing of a patent application. Since patent applications in the U.S. and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we or our licensors were the first to either file any patent application related to our product candidates or other technologies or invent any of the inventions claimed in our or our licensor’s patents or patent applications. The America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO-administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Therefore, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our owned or in-licensed patent applications and the enforcement or defense of our owned or in-licensed issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Additionally, U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. While we do not believe that any of the patents owned or licensed by us will be found invalid based on the foregoing, we cannot predict how future decisions by Congress, the federal courts or the USPTO may impact the value of our patents.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees, and various other government fees on patents and patent applications are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of a patent. The USPTO and various foreign governmental patent agencies require compliance with several procedural, documentary, fee payment and other similar provisions during the patent application process. In certain circumstances, we rely on our licensors to pay these fees and take the necessary actions to comply with these requirements. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter the market with similar or identical products or technology, which would have a material adverse impact on our business, financial condition, results of operations and prospects.
Our rights to develop and commercialize our product candidates and technologies are subject, in part, to the terms and conditions of licenses granted to us by others.
We will rely on licenses to certain patent rights and proprietary technology from third parties that are important or necessary to the development of aldoxorubicin as well as products enabled by our adenoviral, saRNA and yeast including Tarmogen,(including Tarmogen), vaccine technologies, and saRNA technology.technologies.
License agreements may not provide exclusive rights to use certain licensed intellectual property and technology in all relevant fields of use and in all territories in which we may wish to develop or commercialize our technology and product candidates in the future. As a result, we may not be able to prevent competitors or other third parties from developing and commercializing competitive products that also utilizesutilize technology that we have in-licensed.
In addition, subject to the terms of any such license agreements, we do not have the right to control the preparation, filing, prosecution and maintenance, and we may not have the right to control the enforcement, and defense of patents and patent applications covering the technology that we license from third parties. We cannot be certain that our in-licensed or out-licensed patents and patent applications that are controlled by our licensors or licensees will be prepared, filed, prosecuted, maintained, enforced, and defended in a manner consistent with the best interests of our business. If our licensors or licensees fail to prosecute, maintain, enforce, and defend such patents, or lose rights to those patents or patent applications, the rights we have licensed may be reduced or eliminated, our right to develop and commercialize N-803 and any of our product candidates that are subject of such licensed rights could be adversely affected, and we may not be able to prevent competitors from making, using and selling competing products. In addition, even where we have the right to control patent prosecution of patents and patent applications we have licensed to and from third parties, we may still be adversely affected or prejudiced by actions or inactions of our licensees, our licensors and their counsel that took place prior to the date upon which we assumed control over patent prosecution.
Furthermore, our owned and in-licensed patents may be subject to a reservation of rights by one or more third parties. For example, certain of our in-licensed intellectual property was funded in part by the U.S. government. As a result, the U.S. government may have certain rights to such intellectual property. When new technologies are developed with
U.S. government funding, the U.S. government generally obtains certain rights in any resulting patents, including a non-exclusive license authorizing the U.S. government to use the invention or to have others use the invention on its behalf. The U.S. government’s rights may also permit it to disclose the funded inventions and technology to third parties and to exercise march-in rights to use or allow third parties to use the technology we have licensed that was developed using U.S. government funding. The U.S. government may exercise its march-in rights if it determines that action is necessary because we fail to achieve practical application of the government-funded technology, or because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations, or to give preference to U.S. industry. In addition, our rights in such inventions may be subject to certain requirements to manufacture products embodying such inventions in the U.S. in certain circumstances if this requirement is not waived. Any exercise by the U.S. government of such rights or by any third party of its reserved rights could have a material adverse effect on our competitive position, business, financial condition, results of operations and growth prospects.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we may be required to pay damages and we could lose license rights that are important to our business.
We have entered into license agreements with third parties and may need to obtain additional licenses from others to advance our research or allow commercialization of our product candidates. We may be unable to obtain certain additional licenses at a reasonable cost or on reasonable terms, if at all. In that event, we may be required to expend significant time and resources to redesign our technology, product candidates, or the methods for manufacturing them or to develop or license replacement technology, all of which may not be feasible on a technical or commercial basis. If we are unable to do so, we may be unable to develop or commercialize the affected product candidates or continue to utilize our existing technology, which could harm our business, financial condition, results of operations and growth prospects significantly. We cannot provide any assurances that third-party patents do not exist which might be enforced against our current technology, manufacturing methods, product candidates, or future methods or products resulting in either an injunction prohibiting our manufacture or future sales, or, with respect to our future sales, an obligation on our part to pay royalties and/or other forms of compensation to third parties, which could be significant.
In addition, each of our license agreements, and we expect our future agreements, will impose various development, diligence, commercialization, and other obligations on us. Certain of our license agreements also require us to meet development timelines, or to exercise commercially reasonable efforts to develop and commercialize licensed products, in order to maintain the licenses. In spite of our efforts, our licensors might conclude that we have materially breached our obligations under such license agreements and might therefore terminate the license agreements, thereby removing or limiting our ability to develop and commercialize products and technology covered by these license agreements. If these in-licenses are terminated, or if the underlying patents fail to provide the intended exclusivity, competitors or other third parties would have the freedom to seek regulatory approval of, and to market, products identical to ours and we may be required to cease our development and commercialization of certain of our product candidates or of N-803. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations and growth prospects.
Moreover, disputes may arise regarding intellectual property subject to a licensing agreement, including:
•the scope of rights granted under the license agreement and other interpretation-related issues;
•the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
•the sublicensing of patent and other rights under our collaborative development relationships;
•our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
•the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and
•the priority of invention of patented technology.
In addition, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations and growth prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates, which could have a material adverse effect on our business, financial conditions, results of operations and growth prospects.
We have limited foreign intellectual property rights and may not be able to protect our intellectual property rights in various jurisdictions throughout the world.
We have limited intellectual property rights outside the U.S. Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the U.S. can be less extensive than those in the U.S. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the U.S. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the U.S., or from selling or importing products made using our inventions in and into the U.S. or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the U.S. These products may compete with our product candidates and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biopharmaceutical products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in
violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations and prospects may be adversely affected.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed trade secrets or other confidential information of third parties or claims asserting ownership of what we regard as our own intellectual property.
We have received confidential and proprietary information from third parties and their employees and contractors. In addition, we plan to employ and contract with individuals who were previously employed at other biotechnology or pharmaceutical companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed the trade secrets or other confidential information of these third parties or our employees’ former employers. Litigation may be necessary to defend against or pursue these claims. Even if we are successful in resolving these claims, litigation could result in substantial cost and be a distraction to our management and employees.
In addition, while it is our policy to require our employees, consultants and independent contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own. The assignment of intellectual property rights may not be self-executing, or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations, and prospects.
We may not be able to license or acquire new or necessary intellectual property rights or technology from third parties.
An element of our intellectual property strategy is to license intellectual property rights and technologies from third parties and/or our affiliates. Other parties, including our competitors or our affiliates, may have patents relevant to our business, may have already filed patent applications relevant to our business, and are likely filing patent applications potentially relevant to our business. In order to avoid infringing these patents, we may find it necessary or prudent to obtain licenses to such patents from such parties. In addition, with respect to any patents we co-own with other parties, including our affiliates, we may require licenses to such co-owners’ interest to such patents. The licensing or acquisition of intellectual property rights is a competitive area, and other more established companies may pursue strategies to license or acquire third-party intellectual property rights that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, capital resources, and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment or at all. No assurance can be given that we will be successful in licensing any additional rights or technologies from third parties and/or our affiliates. Our inability to license the rights and technologies that we have identified, or that we may in the future identify, could have a material adverse impact on our ability to complete the development of our product candidates or to develop additional product candidates. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. Failure to obtain any necessary rights or licenses may detrimentally affect our planned development of our current or future additional product candidates and could increase the cost, and extend the timelines associated with our development, of such other products, and we may have to abandon development of the relevant program or product candidate. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
If we do not obtain patent term extension and data exclusivity for any product candidates we may develop, our business may be materially harmed.
Depending upon the timing, duration and specifics of any FDA marketing approval of any product candidates we may develop, one or more of our owned or in-licensed U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984 (the Hatch-Waxman Act).Act. The Hatch-Waxman Act permits a patent term extension of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval. Only one patent may be extended per new drug, and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. Similar extensions as compensation for patent term lost during regulatory review processes are also available in certain foreign countries and territories, such as in Europe under a Supplementary Patent Certificate. However, we may not be granted an extension in the U.S. and/or foreign countries and territories because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents, or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is shorter than what we request, our competitors may obtain approval of competing products following our patent expiration, and our business, financial condition, results of operations and growth prospects could be materially harmed.
We may be subject to claims challenging rights in our patents and other intellectual property.
We or our licensors may be subject to claims that former employees, collaborators or other third parties have an interest in our owned or in-licensed patents, trade secrets, or other intellectual property, including as an inventor or co-inventor. For example, we or our licensors may have disputes arisearising from conflicting obligations of employees, consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging
inventorship, or our or our licensors’ ownership of our owned or in-licensed patents, trade secrets or other intellectual property. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of or right to use valuable intellectual property. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for N-803, hAd5, saRNA hAd5 and yeast technologies and constructs, cell therapies, and other product candidates and technologies, we also rely on trade secrets and confidentiality agreements to protect our unpatented know-how, technology, and other proprietary information and to maintain our competitive position. Trade secrets and know-how can be difficult to protect. We expect our trade secrets and know-how will over time be disseminated within the industry through independent development, the publication of journal articles describing the methodology, and the movement of personnel from academic to industry scientific positions.
We seek to protect these trade secrets and other proprietary technology, in part, by entering into nondisclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, CROs, CMOs, consultants, advisors, and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants as well as train our employees not to bring or use proprietary information or technology from former employers to us or in their work and remind former employees when they leave their employment of their confidentiality obligations. We cannot guarantee that we have entered into such agreements with each party that may have or have had access to our trade secrets or proprietary technology and processes. Despite our efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside
the U.S. are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor or other third party, our competitive position would be materially and adversely harmed.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential partners or customers in our markets of interest. At times, competitors or other third parties may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively, and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect our business, financial condition, results of operations and growth prospects.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
•others may be able to make products that are similar to our product candidates or utilize similar technology but that are not covered by the claims of the patents that we license or may own;
•we, or our current or future licensors or collaborators, might not have been the first to make the inventions covered by the issued patent or pending patent application that we license or own now or in the future;
•we, or our current or future licensors or collaborators, might not have been the first to file patent applications covering certain of our or their inventions;
•others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our owned or licensed intellectual property rights;
•it is possible that our current or future pending owned or licensed patent applications will not lead to issued patents;
•issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors or other third parties;
•our competitors or other third parties might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
•we may not develop additional proprietary technologies that are patentable;
•the patents of others may harm our business; and
•we may choose not to file a patent in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property.
Should any of these events occur, they could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Risks Related to Our Common Stock and CVRs
Dr. Soon-Shiong, our Executive Chairman, Global Chief Scientific and Medical Officer and our principal stockholder, has significant interests in other companies which may conflict with our interests.
Our Executive Chairman, Global Chief Scientific and Medical Officer and our principal stockholder, Dr. Soon-Shiong, is the founder of NantWorks. The various NantWorks companies are currently exploring opportunities in the immunotherapy, oncology, infectious disease and inflammatory disease fields. In particular, we have agreements with a number of related parties that provide services, technology and equipment for use in their efforts to develop their product pipelines. Dr. Soon-Shiong holds a controlling interest, either directly or indirectly, in these entities. Consequently, Dr. Soon-Shiong’s interests may not be aligned with our other stockholders, and he may from time to time be incentivized to take certain actions that benefit his other interests and that our other stockholders do not view as being in their interest as investors in our company. In addition, other companies affiliated with Dr. Soon-Shiong may compete with us for business opportunities or, in the future, develop products that are competitive with ours (including products in other therapeutic fields which we may target in the future). Moreover, even if they do not directly relate to us, actions taken by Dr. Soon-Shiong and the companies with which he is involved could impact us.
We are also pursuing supply arrangements for various investigational agents controlled by affiliates to be used in their clinical trials. If Dr. Soon-Shiong were to cease his affiliation with us or NantWorks, these entities may be unwilling to continue these relationships with us on commercially reasonable terms, or at all, and as a result may impede our ability to control the supply chain for our combination therapies. These collaboration agreements do not typically specify how sales will be apportioned between the parties upon successful commercialization of the product. As a result, we cannot guarantee that we will receive a percentage of the revenues that is at least proportional to the costs that we will incur in commercializing the product candidate.
We have entered into shared services agreements with NantWorks, pursuant to which NantWorks and its affiliates provide corporate, general and administrative and other support services to us. If Dr. Soon-Shiong was to cease his affiliation with us or with NantWorks, we may be unable to establish or maintain this relationship with NantWorks on a commercially reasonable basis, if at all. As a result, we could experience a lack of business continuity due to loss of historical and institutional knowledge and a lack of familiarity of new employees and/or new service providers with business processes, operating requirements, policies and procedures, and we may incur additional costs as new employees and/or service providers gain necessary experience. In addition, the loss of the services of NantWorks might significantly delay or prevent the development of our product candidates or achievement of other business objectives by diverting management’s attention to transition matters and identification of suitable replacements, if any, and could have a material adverse effect on our business and results of operations.
Dr. Soon-Shiong, through his voting control of the company, has the ability to control actions that require stockholder approval.
Dr. Soon-Shiong, through his direct and indirect ownership of the company’s common stock, has voting control of the company. As of December 31, 2022,2023, Dr. Soon-Shiong and his affiliates own approximately 76.7%79.4% of the company’s common stock outstanding.
As of December 31, 2022,existing stockholders or may otherwise depress the company has a $300.0 million promissory note with an entity affiliated with Dr. Soon-Shiong that is due and payable on December 31, 2023. In the event of a default on the loan (as defined in the promissory note), including if the company does not repay the loan at maturity, the company has the right, at its sole option, to convert the outstanding principal amount and accrued and unpaid interest due under this note into shares of the company’s common stock at price of $5.67 per share. In addition, entities affiliated with Dr. Soon-Shiong hold fixed-rate promissory notes representing $262.4 million in indebtedness (including principal and accrued and unpaid interest) as of December 31, 2022. These notes include a conversion feature that gives each lender the right at any time, including upon notice of prepayment, at its sole option, to convert the entire outstanding principal amount and accrued and unpaid interest due under each note at the time of conversion into shares of the company’sour common stock at a price of $5.67 per share.
Dr. Soon-Shiong also has a total of 1,626,064 stock options outstanding as of December 31, 2022, of which 926,064 are exercisable and 700,000 are unvested and unexercisable.” below.Dr. Soon-Shiong is in a position to control the outcome of corporate actions that require, or may be accomplished by, stockholder approval, including amending the bylaws of the company, the election or removal of directors and transactions involving a change of control. Dr. Soon-Shiong’s controlling ownership could limit the ability of the remaining stockholders of the company to influence corporate matters, and the interests of Dr. Soon-Shiong may not coincide with the company’s interests or the interests of its remaining stockholders.
In addition, pursuant to the Nominating Agreement between us and Cambridge, Equities, LP (Cambridge), an entity that Dr. Soon-Shiong controls, Cambridge has the ability to designate one director to be nominated for election to the Board of Directors for as long as Cambridge continues to hold at least 20% of the issued and outstanding shares of our common stock. Dr. Soon-Shiong was selected by Cambridge to hold this board seat. Dr. Soon-Shiong and his affiliates will therefore have significant influence over management and significant control over matters requiring stockholder approval, including the annual election of directors and significant corporate transactions, such as a merger or other sale of our company or its assets, for the foreseeable future. This control will limit stockholders’ ability to influence corporate matters and, as a result, we may take actions that our stockholders do not view as beneficial. As a result, the market price of our common stock could be adversely affected.
Conversion of certain related-party promissory notes, exercise of outstanding warrants and options to purchase our common stock, the achievement of the milestone under our outstanding CVRs, and potential additional equity issuances may dilute the ownership interest of existing stockholders or may otherwise depress the price of our common stock.
As of December 31, 2023, the company had outstanding promissory notes representing an aggregate of $610.0 million principal amount held by entities affiliated with Dr. Soon-Shiong that are convertible into shares of our common stock under certain circumstances, including the following:
•a $380.0 million principal amount of Tranche 2 of our convertible promissory note due December 31, 2025 bearing interest at 3-month Term SOFR plus 7.5% per annum, which provides that the noteholder has the sole option to convert all (but not less than all) of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $8.2690 per share (subject to appropriate adjustment from time to time for any stock dividend, stock split, combination of shares, reorganization, recapitalization, reclassification or other similar event);
•a $200.0 million principal amount convertible promissory note due September 11, 2026 bearing interest at 1-month Term SOFR plus 8.0% per annum provides that the noteholder has the sole option to convert all (but not less than all) of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $1.9350 per share (subject to appropriate adjustment from time to time for any stock dividend, stock split, combination of shares, reorganization, recapitalization, reclassification or other similar event); and
•a $30.0 million principal amount convertible promissory note due December 31, 2025 bearing interest at 3-month Term SOFR plus 8.0% per annum, which provides that the noteholder has the sole option to convert all (but not less than all) of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $2.28 per share (subject to appropriate adjustment from time to time for any stock dividend, stock split, combination of shares, reorganization, recapitalization, reclassification or other similar event).
In addition, as of December 31, 2023, we had outstanding warrants and stock options covering the sale of up to:
•9,090,909 shares of our common stock at an exercise price of $6.60 per share, which are currently exercisable with an expiration date of December 12, 2024 (these warrants were issued to certain institutional investors);
•28,641,911 shares of our common stock at an exercise price of $3.2946 per share, which are currently exercisable with an expiration date of July 24, 2026 (these warrants were issued to certain institutional investors);
•$10.0 million of the company’s common stock at a price per share to be determined by the 30-day trailing volume weighted-average price of our common stock, calculated from the date of exercise. The option is exercisable by Oberland any time after the closing of the SPOA, until the earliest of (i) December 29, 2028, (ii) a change of control of the company, or (iii) a sale of substantially all of the company’s assets;
•1,626,064 stock options issued to Dr. Soon-Shiong that are outstanding as of December 31, 2023, of which 1,159,398 are vested and exercisable and 466,666 are unvested and unexercisable; and
•1,638,000 shares of our common stock at an exercise price of $3.24 per share exercisable from the 30th day following the achievement of a performance-based vesting condition pertaining to building manufacturing capacity to support supply requirements for one of our product candidates (which has not yet been satisfied) with an expiration date on the tenth anniversary of such initial exercise date (this warrant was issued to an affiliate of Dr. Soon-Shiong).
In addition, as of December 31, 2023, we had outstanding an aggregate of approximately $300.6 million of CVRs issued to the former stockholders of Altor, including Dr. Soon-Shiong and certain affiliates, which such stockholders may choose to receive either in cash or shares of our common stock based upon an average of closing prices on a 20-trading day trailing period, upon the first calendar year prior to December 31, 2026 in which worldwide net sales of N-803 exceed $1.0 billion. N-803 is not currently approved for commercial sale, and there can be no assurance that such sales milestone will be achieved. Dr. Soon-Shiong and his related party hold approximately $139.8 million of such CVRs, and have irrevocably agreed to receive shares of the company’s common stock in satisfaction of their CVRs.
The conversion or exchange of some or all of our outstanding promissory notes into shares of our common stock, the exercise of any of our outstanding warrants and stock options, and the decision of the holders of our CVRs to receive shares of our common stock could dilute the ownership interests of existing stockholders. Any sales in the public market of our outstanding promissory notes or warrants, or our common stock issuable upon conversion of the promissory notes or exercise of the warrants, could adversely affect prevailing market prices of our common stock.
The market price of our common stock has been and may continue to be volatile, and investors may have difficulty selling their shares.
Although our common stock is listed on the Nasdaq Global Select Market, the market for our shares has demonstrated varying levels of trading activity. You may not be able to sell your shares quickly or at the market price if trading in shares of our common stock is not active. Further, an inactive market may also impair our ability to raise capital by selling shares of our common stock and warrants and may impair our ability to enter into strategic partnerships or acquire companies or products by using our shares of common stock as consideration.
The stock market in general and the market for biopharmaceutical companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. The market price of our common stock has been and may continue to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, including:
•the commencement, enrollment or results of the planned clinical trials of our product candidates or any future clinical trials we may conduct, or changes in the development status of our product candidates;
•any delay in our regulatory filingssubmissions for our product candidates and any adverse development or perceived adverse development with respect to the applicable regulatory authority’s review of such filings,submissions, including without limitation the FDA’s issuance of a CRL or a “refusal to file” letter or a request for additional information;
•adverse results or delays in clinical trials;
•our decision to initiate a clinical trial, not to initiate a clinical trial or to terminate an existing clinical trial;
•adverse regulatory decisions, including failure to receive regulatory approval of our product candidates;
•changes in laws or regulations applicable to our products, including but not limited to clinical trial requirements for approvals;
•our failure to commercialize our product candidates;
•additions or departures of key scientific or management personnel;
•unanticipated serious safety concerns related to the use of our product candidates;
•announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments;
•our ability to effectively manage our growth;
•variations in our quarterly operating results;results, including those driven by liability accounting associated with embedded derivatives;
•our liquidity position, RIPA liability covenants and the amount and nature of any debt we may incur;
•announcements that our revenue or income are below or that costs or losses are greater than analysts’ expectations;
•publication of research reports about us or our industry, or immunotherapy in particular, or positive or negative recommendations or withdrawal of research coverage by securities analysts;
•changes in the market valuations of similar companies;
•sales of large blocks of our common stock;
•fluctuations in stock market prices and volumes;
•disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies;
•significant lawsuits, including patent or stockholder litigation;
•the perception of our clinical trial results by retail investors, which investors may be subject to the influence of information provided by third party investor websites and independent authors distributing information on the internet;
•general economic slowdowns;
•government-imposed lockdowns, supply chain disruptions, and adverse economic effects from the ongoing COVID-19a potential pandemic, epidemic, or outbreak of an infectious disease, in the U.S. and abroad;
•geopolitical tensions and war, including the war in Ukraine;Ukraine and ongoing conflicts in Gaza and Yemen;
•coordinated actions by independent third-party actors to affect the price of certain stocks, coordinated via the Internetinternet and otherwise; and
•other factors described in this “Risk Factors” section.
In the past, securities class action litigation has often been instituted against companies following periods of volatility in the market price of a company’s securities. This type of litigation could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, operating results or financial condition.
We are currently subject to securities class action litigation and may be subject to similar or other litigation in the future, all of which will require significant management time and attention, result in significant legal expenses and may result in unfavorable outcomes, which may have a material adverse effect on our business, operating results and financial condition, and negatively affect the price of our common stock.
We are, and may in the future become, subject to various legal proceedings and claims that arise in or outside the ordinary course of business. For example, on June 30, 2023, a putative securities class action complaint, captioned Salzman v. ImmunityBio, Inc. et al., No. 3:23-cv-01216-BEN-WVG, was filed in the U.S. District Court for the Southern District of California against the company and three of its officers and/or directors, asserting violations of Sections 10(b) and 20(a) of the
Exchange Act stemming from the company’s disclosure on May 11, 2023 that it had received an FDA CRL stating, among other things, that it could not approve the company’s original BLA submission for its product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, in its initial form due to deficiencies related to its pre-license inspection of the company’s third-party CMOs. The complaint alleges that the defendants had previously made materially false and misleading statements and/or omitted material adverse facts regarding its third-party clinical manufacturing organizations and the prospects for regulatory approval of the BLA. On September 27, 2023, the court appointed a lead plaintiff, approved their selection of lead counsel, and re-captioned the case In re. ImmunityBio, Inc. Securities Litigation, No. 3:23-cv-01216. On November 17, 2023, lead plaintiff filed an amended complaint, which named the same defendants and asserted the same claims as the previous complaint. On January 8, 2024, defendants filed a motion to dismiss the amended complaint. A hearing on the motion is currently scheduled for April 23, 2024.
The results of the securities class action lawsuit, and any future legal proceedings cannot be predicted with certainty. Also, our insurance coverage may be insufficient, our assets may be insufficient to cover any amounts that exceed our insurance coverage, and we may have to pay damage awards or otherwise may enter into a settlement arrangement in connection with such claim. Any such payments or settlement arrangements in current or future litigation could have a material adverse effect on our business, operating results or financial condition. Even if the plaintiffs’ claims are not successful, current or future litigation could result in substantial costs and significantly and adversely impact our reputation and divert management’s attention and resources, which could have a material adverse effect on our business, operating results and financial condition, and negatively affect the price of our common stock. In addition, such lawsuits may make it more difficult to finance our operations.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plan, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. If our stockholders sell, or the market perceives that our stockholders intend to sell substantial amounts of our common stock in the public market, including shares obtained from the conversion or exchange of our convertible promissory notes, exercise of our warrants, satisfaction of our CVRs, or the exercise or settlement of our equity incentive awards, the market price of our common stock could decline significantly. In addition, our Executive Chairman and Global Chief Scientific and Medical Officer, Dr. Soon-Shiong, and his affiliates currently ownowned approximately 76.7%79.4% of our outstanding shares of common stock as of December 31, 2022.2023. Sales of stock by Dr. Soon-Shiong and his affiliates could have an adverse effect on the trading price of our common stock.
Certain holders of our common stock are entitled to certain rights with respect to the registration of their shares under the Securities Act.Act, including the shares purchased by affiliates of Oberland in connection with our entry into the RIPA. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by our affiliates as defined in Rule 144 under the Securities Act. Any sales of securities by these stockholders could have an adverse effect on the market price of our common stock.
In addition, we expect that additional capital may be needed in the future to continue our planned operations, including conducting clinical trials, regulatory approval efforts, pre-commercialization and commercialization efforts,activities, expanded research and development activities and costs associated with operating as a public company. To raise capital, we may sell common stock, including as part of the ATM, convertible securities or other equity securities (including warrants) in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, including through the ATM, convertible securities or other equity securities, (including warrants),existing investors may be materially diluted, and new investors could gain rights, preferences and privileges senior to the holders of our common stock. The issuance of additional shares of common stock or warrants to purchase common stock, perception that such issuances may occur, or the exercise of outstanding warrants or other equity securities will have a material dilutive impact on existing stockholders and could have a material negative effect on the market price of our common stock.
We have incurred and will continue to incur costs as a result of operating as a public company and our management has been and will be required to devote substantial time to compliance initiatives and corporate governance practices, including maintaining an effective system of internal control over financial reporting.
As a public company listed in the U.S., we have incurred and will continue to incur significant additional legal, accounting and other expenses as a result of operating as a public company. In addition, changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 (Sarbanes Oxley) and regulations implemented by the SEC and
Nasdaq, may increase legal and financial compliance costs and make some activities more time consuming. These laws, regulations and standards are subject to varying interpretations and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. We intend to invest resources to create a larger finance function with additional personnel to comply with evolving laws, regulations and standards, and this investment will result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If, notwithstanding our efforts to comply with new laws, regulations and standards, we fail to comply, regulatory authorities may initiate legal proceedings against us, and our business may be harmed.
As a public company in the U.S., we are required, pursuant to Section 404 of Sarbanes-Oxley (Section 404) to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. The controls and other procedures are designed to ensure that information required to be disclosed by us in the reports that we file with the SEC is disclosed accurately and is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms.
In the normal course of business our controls and procedures may become inadequate because of changes in conditions or the degree of compliance with these policies or procedures may deteriorate and material weaknesses in our internal control over financial reporting may be discovered. We may err in the design or operation of our controls, and all internal control systems, no matter how well designed and operated, can provide only reasonable assurance that the objectives of the control system are met. Because there are inherent limitations in all control systems, there can be no absolute assurance that all control issues have been or will be detected. If we are unable, or are perceived as unable, to produce reliable financial reports due to internal control deficiencies, investors could lose confidence in our reported financial information and operating results, which could result in a negative market reaction.
To fully comply with Section 404, we will need to retain additional employees to supplement our current finance staff, and we may not be able to do so in a timely manner, or at all. In addition, in the process of evaluating our internal control over financial reporting, we expect that certain of our internal control practices will need to be updated to comply with the requirements of Section 404 and the regulations promulgated thereunder, and we may not be able to do so on a timely basis, or at all. In the event that we are not able to demonstrate compliance with Section 404 in a timely manner, or are unable to produce timely or accurate financial statements, we may be subject to sanctions or investigations by regulatory authorities, such as the SEC or Nasdaq, and investors may lose confidence in our operating results and the price of our common stock could decline. Furthermore, if we are unable to certify that our internal control over financial reporting is effective and in compliance with Section 404, we may be subject to sanctions or investigations by regulatory authorities, such as the SEC or stock exchanges, and investors could lose confidence in the accuracy and completeness of our financial reports, which could hurt our business, the price of our common stock and our ability to access the capital markets.
Operating as a public company makes it more expensive for us to obtain directors’ and officers’ liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified persons to serve on the Board of Directors, on committees of the Board of Directors, or as members of senior management.
If a restatement of our consolidated financial statements were to occur, our stockholders’ confidence in the company’s financial reporting in the future may be affected, which could in turn have a material adverse effect on our business and stock price.
If any material weaknesses in our internal control over financial reporting are discovered or occur in the future, our consolidated financial statements may contain material misstatements, and we could be required to restate our financial results. In addition, if we are unable to successfully remediate any future material weaknesses in our internal controls or if we are unable to produce accurate and timely financial statements, our stock price may be adversely affected, and we may be unable to maintain compliance with applicable stock exchange listing requirements.
We have not paid cash dividends in the past and do not expect to pay dividends in the future. Any return on investment may be limited to the value of our common stock.
We have never paid cash dividends on our common stock and do not anticipate paying cash dividends for the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition and other business and economic factors affecting us at such time as the Board of Directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
Because we are relying on the exemptions from corporate governance requirements as a result of being a “controlled company” within the meaning of the Nasdaq listing standards, you do not have the same protections afforded to stockholders of companies that are subject to such requirements.
Our Executive Chairman and Global Chief Scientific and Medical Officer, Dr. Soon-Shiong, and entities affiliated with him, control a majority of our common stock. As a result, we are a “controlled company”controlled company within the meaning of the Nasdaq listing standards. Under these rules, a company of which more than 50% of the voting power is held by an individual, a group or another company is a “controlled company”controlled company and may elect not to comply with certain Nasdaq corporate governance requirements, including (1) the requirement that a majority of the Board of Directors consist of independent directors, and (2) the requirement that we have a Nominating and Corporate Governance Committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities. Accordingly, you do not have the same protections afforded to stockholders of companies that are subject to all of the Nasdaq corporate governance requirements. However, our Board of Directors is currently comprised of a majority of independent directors, and we currently have a Nominating and Corporate Governance Committee and the majority of the members of such committee are independent directors.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common stock and the value of our warrants will depend on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will cover us or provide favorable coverage. If one or more of the analysts who cover us downgrade our stock or change their opinion of our stock, our share price and the value of our warrants would likely decline. If one or more of these analysts’ cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
Holders of our CVRs that are payable contingent upon us achieving certain milestones may not receive any further consideration.
In connection with our 2017 acquisition of Altor, we issued CVRs under which we agreed to pay the prior stockholders of Altor approximately $304.0 million of contingent consideration upon the successful regulatory approval of a BLA by the FDA, or foreign equivalent, for N-803 by December 31, 2022, and approximately $304.0 million of contingent consideration upon calendar-year worldwide sales of N-803 exceeding $1.0 billion prior to December 31, 2026.
With respect to the regulatory milestone CVR agreement, in May 2022 we announced the submission of a BLA to the FDA for our product candidate, Anktiva (N-803) in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In July 2022 we announced that the FDA had accepted our BLA for review and set a target PDUFA action date of May 23, 2023. It is unclear when the FDA will approve our BLA, if at all. The FDA did not approve our BLA on or before December 31, 2022, and therefore the regulatory milestone was not met, and the regulatory milestone CVR agreement terminated in accordance with its terms. With respect to the sales milestone CVR agreement, N-803 is not currently approved for commercial sale, and there can be no assurance that such sales milestone will be achieved. Accordingly, holders of our CVRs that are payable contingent upon us achieving the aforementioned milestones may not receive any further consideration.
We are not subject to the provisions of Section 203 of the Delaware General Corporation Law (DGCL),DGCL, which could negatively affect your investment.
We elected in our amendedAmended and restated certificateRestated Certificate of incorporationIncorporation to not be subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination”business combination with an “interested stockholder”interested stockholder for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination”business combination includes a merger, asset sale or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder”interested stockholder is a
person who, together with affiliates and associates, owns (or, in certain cases, within three years prior, did own) 15% or more of the corporation’s voting stock. Our decision not to be subject to Section 203 will allow, for example, our Executive Chairman and Global Chief Scientific and Medical Officer (who, with members of his immediate family and entities affiliated with him, currently own,owned, in the aggregate, approximately 76.7%79.4% of our common stock as of December 31, 2022)2023) to transfer shares in excess of 15% of our voting stock to a third-party free of the restrictions imposed by Section 203. This may make us more vulnerable to takeovers that are completed without the approval of our Board of Directors and/or without giving us the ability to prohibit or delay such takeovers as effectively.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders.
These provisions include:
•a requirement that special meetings of stockholders be called only by the board of directors, president or chief executive officer;
•advance notice requirements for stockholder proposals and nominations for election to the board of directors; and
•the authority of the board of directors to issue preferred stock on terms determined by the board of directors without stockholder approval and which preferred stock may include rights superior to the rights of the holders of common stock.
These anti-takeover provisions and other provisions in our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws could make it more difficult for stockholders or potential acquirers to obtain control of our Board of Directors or initiate actions that are opposed by the then-current Board of Directors and could also delay or impede a merger, tender offer or proxy contest involving our company. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing or cause us to take other corporate actions you desire. Any delay or prevention of a change of control transaction or changes in our Board of Directors could cause the market price of our common stock to decline.
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to us.
Our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws provide that we will indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. In addition, as permitted by Section 145 of the DGCL, our Amended and Restated Bylaws and our indemnification agreements that we have entered into with our directors and officers provide that:
•We will indemnify our directors and officers for serving us in those capacities or for serving other business enterprises at our request, to the fullest extent permitted by Delaware law. Delaware law provides that a corporation may indemnify such person if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the registrant and, with respect to any criminal proceeding, had no reasonable cause to believe such person’s conduct was unlawful.
•We may, in our discretion, indemnify employees and agents in those circumstances where indemnification is permitted by applicable law.
•We are required to advance expenses, as incurred, to our directors and officers in connection with defending a proceeding, except that such directors or officers shall undertake to repay such advances if it is ultimately determined that such person is not entitled to indemnification.
•We are not obligated pursuant to our Amended and Restated Bylaws to indemnify a person with respect to proceedings initiated by that person against us or our other indemnitees except with respect to proceedings authorized by our Board of Directors or brought to enforce a right to indemnification.
•The rights conferred in our Amended and Restated Bylaws are not exclusive, and we are authorized to enter into indemnification agreements with our directors, officers, employees and agents and to obtain insurance to indemnify such persons.
•We may not retroactively amend our bylaw provisions to reduce our indemnification obligations to directors, officers, employees and agents.
To the extent that a claim for indemnification is brought by any of our directors or officers, it would reduce the amount of funds available for use in our business.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
ITEM 1C. CYBERSECURITY.
Risk Assessment and Management
We regularly assess risks from cybersecurity threats; monitor our information systems for potential vulnerabilities; and test those systems pursuant to our cybersecurity policies, processes, and practices, which are integrated into our overall risk management program. To protect our information systems from cybersecurity threats, we use various security tools that are designed to help identify, escalate, investigate, resolve, and recover from security incidents in a timely manner.
The company has an ERM program to identify, evaluate, and manage risks. Cybersecurity risks are evaluated alongside other critical business risks under the ERM program to align cybersecurity efforts with the company’s broader business goals and objectives. We believe that integrating cybersecurity risks into our ERM program fosters a proactive and holistic approach to cybersecurity, which helps safeguard the company’s operations, financial condition, and reputation in an ever-evolving threat landscape.
The company maintains a cybersecurity program that is designed to identify, protect from, detect, respond to, and recover from cybersecurity threats and risks, and protect the confidentiality, integrity, and availability of its information systems, including the information residing on such systems
Cybersecurity threats, including those resulting from any previous cybersecurity incidents, had not materially affected the company, including our business strategy, results of operations, or financial condition. We do not believe that cybersecurity threats resulting from any previous cybersecurity incidents of which we are aware are reasonably likely to materially affect our company. See Item 1A. Risk Factors “—Our systems, infrastructure or data, or those used by our CROs, CMOs, clinical sites or other contractors or consultants, may or may be perceived to fail or suffer a cyberattack, security breach or other incident, including a breakdown or compromise of the confidentiality, integrity and availability of our systems, networks or data, which could adversely affect the operation of our business and reputation” for more information regarding cybersecurity risks and the potential impact on the company. Incident Response
The company has a dedicated incident management team responsible for managing and coordinating its cybersecurity incident response efforts. This team also collaborates closely with other teams in identifying, protecting from, detecting, responding to, and recovering from cybersecurity incidents. Cybersecurity incidents that meet certain thresholds are escalated to the executive incident team and cross-functional teams on an as-needed basis for support and guidance. Additionally, this team tracks cybersecurity incidents to help identify and analyze them. The company’s incident response team partners with other key stakeholders as appropriate to respond to cybersecurity incidents. The company maintains a cybersecurity and incident response program to prepare for and respond to cybersecurity incidents, which includes standard processes for reporting and escalating cybersecurity incidents to the executive incident team. Additionally, the company conducts at least one incident response test exercise on an annual basis, where members of a cross-functional team engage in a simulated incident scenario.
Governance
Board Oversight Role
Our Board of Directors oversees our risk management process, including as it pertains to cybersecurity risks, directly and through its committees. The Audit Committee (the Committee) of the Board of Directors oversees our cybersecurity and data privacy. The Committee meets periodically to review and discuss with management risks relating to significant cybersecurity matters and concerns involving the company, including information security, data privacy, backup of information systems and related regulatory matters and compliance. The Committee regularly reports to the Board of Directors with respect to the Committee’s activities and recommendations, including those relating to cybersecurity matters and concerns. The CIO provides quarterly reports to the Committee on information security matters, including the adequacy and effectiveness of the company’s information security policies and practices and the internal controls regarding information security, and notifies the chairperson of the Committee as soon as practicable of significant information security matters and concerns as they arise.
Management’s Role
The company has a dedicated cybersecurity organization within its technology department that focuses on current and emerging cybersecurity matters. The company’s cybersecurity function is led by the company’s director of information security, who reports to the CIO. The director of information security and CIO (collectively, the Cybersecurity Leaders) are actively involved in assessing and managing cybersecurity risks, and are responsible for implementing cybersecurity policies, programs, procedures, and strategies. The responsibilities and relevant experience of each of the Cybersecurity Leaders are as follows:
The CIO provides leadership for the company’s technology department. She holds a Bachelor of Science, Communication from Arizona State University, has served in various roles in information technology for over 25 years, holds multiple certifications, and has been actively involved in the information security and cybersecurity domains for over 20 years.
The director of information security is responsible for all aspects of cybersecurity across the company’s locations and data centers, including security engineering, security operations, incident response, threat intelligence, risk and compliance, and vulnerability management. He holds a Bachelor of Science in Business Administration, Management Information Systems from the University of Arizona, holds various certifications, and has served in various roles in information technology for over 25 years at numerous technology companies and consulting firms.
The company’s cybersecurity department is comprised of teams that engage in a range of cybersecurity activities such as threat intelligence, security architecture, and incident response. These teams conduct vulnerability management and penetration testing to identify, classify, prioritize, remediate, and mitigate vulnerabilities. Leaders from each team regularly meet with the Cybersecurity Leaders to provide visibility to major issues and seek alignment with strategy. As discussed above, the company’s cybersecurity incident response plan includes standard processes for reporting and escalating cybersecurity incidents to senior management. Cybersecurity incidents that meet certain thresholds are escalated to the Cybersecurity Leaders and cross-functional teams on an as-needed basis for support and guidance. The company’s incident response team also coordinates with external legal advisors, communication specialists, and other key stakeholders.
Use of Third Parties
Cybersecurity Service Providers and Third-Party Consultants
The company engages cybersecurity consultants and other third parties to assess and enhance its cybersecurity practices. These third parties conduct assessments and penetration testing to identify weaknesses and recommend improvements. Additionally, the company leverages a number of third-party tools and technologies as part of its efforts to enhance cybersecurity functions, including a managed security service provider to augment the company’s internal resources, an endpoint detection and response system for continuous monitoring, detection, and response capabilities, and a security information and event management solution to automate real-time threat detection, investigation, and prioritization of high-fidelity alerts.
Oversight of Third-Party Service Providers
The company also uses third-party service providers to support its operations and many of its technology initiatives, and evaluates its third-party service providers from a cybersecurity risk perspective, which may include an assessment of that service provider’s cybersecurity posture or a recommendation of specific mitigation controls. Following such evaluation, the company determines and prioritizes service provider risk based on the potential threat impact and likelihood, and such risk determination drives the level of due diligence and ongoing compliance monitoring required for each service provider.
ITEM 2. PROPERTIES.
We lease property in multiple facilities across the U.S. and Italy, including facilities located in El Segundo and Culver City, CA that are leased from related parties. See Note 1011, Related-Party Agreements, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for additionalmore information about our related-party leases. The following table summarizes our principal properties under lease as of December 31, 2022:2023:
| | Location | Location | | Expiration
Year (1) | | Approximate
Rentable
Square Feet (2) | | Primary Function(s) | Location | | Expiration
Year (1) | | Approximate
Rentable
Square Feet (2) | | Primary Function(s) |
| | United States | United States | |
| United States | |
| United States | |
| Dunkirk, NY | |
| Dunkirk, NY | |
| Dunkirk, NY | Dunkirk, NY | | 2031 | | 409,000 | | | Future Manufacturing Facility | | 2031 | | 409,000 | | | Future Manufacturing Facility | | Future Manufacturing Facility |
| El Segundo, CA | El Segundo, CA | | 2023 – 2028 | | 179,401 | | | Laboratory – Research & Manufacturing | El Segundo, CA | | 2026 – 2029 | | 132,136 | | | Laboratory – Research & Manufacturing | | Laboratory – Research & Manufacturing |
| Louisville, CO | Louisville, CO | | 2025 | | 50,838 | | | Laboratory – Research | Louisville, CO | | 2025 | | 50,838 | | | Laboratory – Research | | Laboratory – Research |
| Culver City, CA | Culver City, CA | | Month to Month | | 46,330 | | | Laboratory – Research & Manufacturing | Culver City, CA | | Month to Month | | 46,330 | | | Laboratory – Research & Manufacturing | | Laboratory – Research & Manufacturing |
| San Diego, CA | San Diego, CA | | 2030 | | 44,681 | | | Laboratory – Research & Corporate Office | San Diego, CA | | 2030 | | 44,681 | | | Laboratory – Research & Corporate Office | | Laboratory – Research & Corporate Office |
| Woburn, MA | Woburn, MA | | 2025 | | 8,153 | | | Laboratory – Research & Office | Woburn, MA | | 2025 | | 8,153 | | | Laboratory – Research & Office | | Laboratory – Research & Office |
| Seattle, WA | Seattle, WA | | 2025 | | 5,527 | | | Laboratory – Research | Seattle, WA | | 2025 | | 5,527 | | | Laboratory – Research | | Laboratory – Research |
| Miramar, FL | | 2025 | | 2,571 | | | Clinical Affairs & Office |
| International | International | |
| Italy | Italy | | 2024 – 2028 | | 15,748 | | | Laboratory – Research & Office |
| Italy | |
| Italy | | | 2024 – 2028 | | 15,748 | | | Laboratory – Research & Office |
_______________
| | | | | | | | | | | | | | |
| (1) | Expiration years shown are per the lease agreements in effect as of December 31, 20222023 and do not reflect contractual options to extend the term of the lease available to us under the lease agreements. For locations with multiple leases, the first and last expiration year are shown. |
| (2) | Amounts shown represent the total approximate rentable square feet for all buildings located in each city. |
We believe that our existing facilities are adequate to meet our current and future needs and that we will be able to renew existing leases and obtain additional commercial space as needed.
ITEM 3. LEGAL PROCEEDINGS.
From time to time, we may be involved in various claims and legal proceedings relating to claims arising out of our operations. We are not currently a party to any legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business. If we are served with any such complaints, we will assess at that time any contingencies for which we may need to reserve. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
Altor BioScience, LLC Litigation
In 2017, NantCell announced it had entered into a definitive merger agreement to acquire Altor BioScience Corporation. An action captioned Gray v. Soon-Shiong, et al. was filed in Delaware Chancery Court by plaintiffs Clayland Boyden Gray (Gray) and Adam R. Waldman. The plaintiffs, two minority stockholders, asserted claims against the company and other defendants for (1) breach of fiduciary duty and (2) aiding and abetting breach of fiduciary duty and filed a motion to enjoin the merger. The court denied the motion and permitted the merger to close.
Subsequent to the close of the merger, in 2017 the plaintiffs (joined by two additional minority stockholders, Barbara Sturm Waldman and Douglas E. Henderson (Henderson)) filed a second amended complaint, including appraisal claims, and which the defendants subsequently moved to dismiss. In a second action, Dyad Pharmaceutical Corporation (Dyad) filed a petition in Delaware Chancery Court for appraisal in connection with the merger. The defendants moved to dismiss the appraisal petition in 2018. The court issued an oral ruling in 2019 that dismissed certain claims and dismissed Altor BioScience from the action. The following claims remained: (a) the appraisal claims by all plaintiffs and Dyad (against Altor BioScience, LLC), and (b) Henderson’s claims for breach of fiduciary duty and aiding and abetting breach of fiduciary duty.
In 2019, the court issued a written order implementing its ruling on the defendants’ motions (the Implementing Order). In the Implementing Order, the court confirmed that all fiduciary duty claims brought by Gray, both individually and as trustee of the Gordon Gray Trust f/b/o C. Boyden Gray, were dismissed. The plaintiffs then moved for leave to file a third amended complaint to add two former Altor stockholders as plaintiffs and a fiduciary duty claim on behalf of a purported class of former Altor stockholders, which the defendants opposed.
In 2020, the court granted the plaintiffs’ motion, and the plaintiffs filed the third amended complaint. In 2020, the defendants answered the third amended complaint and asserted counterclaims against the plaintiffs. The defendants sought damages for attorneys’ fees and costs incurred as a result of the breaches of “standstill” agreements and of stockholder releases. The plaintiffs filed an answer denying the counterclaims and asserting defenses.
The shares of the former Altor stockholders seeking appraisal met the definition of dissenting shares under the merger agreement and were not entitled to receive any portion of the merger consideration at the closing date, given that those shares were the subject of the above-described appraisal claims.
In late March 2022, the company agreed to the terms of a settlement with the appraisal petitioners, without any admission of liability or fault. The settlement provided that in exchange for complete releases, the appraisal petitioners, who as a group held 3,167,565 dissenting Altor shares, collectively would receive an aggregate of 2,229,296 shares of the company’s common stock issued in a private placement, plus an aggregate of $21.13 in cash in lieu of fractional shares. The company’s Board of Directors approved the settlement and stock issuance in April 2022, and the court approved the settlement and dismissed the appraisal petitioners’ claims on July 9, 2022. On July 9, 2022, the company issued 2,229,296 shares of its common stock with an aggregate market value of $10.7 million, based on the closing price of its common stock on the Nasdaq as of July 8, 2022, to the appraisal petitioners pursuant to the court-approved settlement agreement. As of December 31, 2021, we had accrued $7.1 million related to the dissenting share obligation.
In late April 2022, the company also agreed to the terms of a settlement with the putative class plaintiffs without any admission of liability or fault. In exchange for class-wide releases, the company committed to make a settlement payment of $5.0 million in cash by December 31, 2022. On December 8, 2022, the Delaware Court of Chancery entered a final judgment approving the settlement, and the company timely made the $5.0 million settlement payment.
Sorrento Therapeutics, Inc. Litigation
Sorrento Therapeutics, Inc. (Sorrento), derivatively on behalf of NANTibody, filed an action in the Superior Court of California, Los Angeles County (the Superior Court) against the company’s subsidiary NantCell, Dr. Soon-Shiong, and Charles Kim. The action alleged that the defendants improperly caused NANTibody to acquire IgDraSol, Inc. from NantPharma, LLC (NantPharma) and sought to have the transaction undone and the purchase amount returned to NANTibody. In 2019, we filed a demurrer to several causes of action alleged in the Superior Court action, and Sorrento filed an amended complaint, eliminating Mr. Kim as a defendant and dropping the causes of action we had challenged in our demurrer. The company believes the case is without merit and intends to vigorously defend against the claims asserted. Trial has been set to commence in Sorrento’s Superior Court action on July 17, 2023.
Sorrento filed a related arbitration proceeding (the Cynviloq arbitration) against Dr. Soon-Shiong and NantPharma; the company was not named in the Cynviloq arbitration. In 2020, the Superior Court granted Dr. Soon-Shiong’s request for a preliminary injunction barring Sorrento from pursuing claims against him in the Cynviloq arbitration. Sorrento then filed the claims it had previously asserted in arbitration against Dr. Soon-Shiong in the Superior Court, and at Sorrento’s request, the arbitrator entered an order dismissing Sorrento’s claims against Dr. Soon-Shiong in the Cynviloq arbitration. The hearing in the Cynviloq arbitration commenced in June 2021, and continued with breaks until early October 2021. The parties completed post-hearing briefing in early May 2022, and summations were heard on September 8, 2022. On December 20, 2022, the parties received the arbitrator’s final award; the award is in favor of Sorrento and against NantPharma in the amount of approximately $125 million. On December 28, 2022, Sorrento submitted an application for modification of the final award to include prejudgment interest in excess of $83 million; on January 20, 2023, Sorrento’s application was denied. On February 2, 2023, Sorrento filed a petition to confirm the NantPharma award, and on February 13, 2023, NantPharma responded with a motion to vacate; both remain pending. The company was not a party to the Cynviloq arbitration and we believe that neither the company nor any of its subsidiaries has any obligations with respect to the award against NantPharma, and we intend to defend vigorously against attempts by Sorrento to pursue any such theory.
Also in 2019, the company and Dr. Soon-Shiong filed cross-claims in the Superior Court action against Sorrento and its Chief Executive Officer Henry Ji, asserting claims for fraud, breach of contract, breach of the covenant of good faith and fair dealing, tortious interference with contract, unjust enrichment, and declaratory relief. Our claims alleged that Dr. Ji and Sorrento breached the terms of an exclusive license agreement between the company and Sorrento related to Sorrento’s antibody library and that Sorrento did not perform its obligations under the exclusive license agreement. The Superior Court ruled that the company’s claims should be pursued in arbitration and that Dr. Soon-Shiong’s claims could be pursued in Superior Court.
In 2019, the company, along with NANTibody, filed an arbitration against Sorrento and Dr. Ji asserting our claims relating to the exclusive license agreement. Sorrento filed counterclaims against the company and NANTibody in the arbitration. The hearings in the NANTibody arbitration commenced in April 2021 and concluded in early August 2021. After post-hearing briefing was concluded, the parties were notified on November 30, 2021 that the arbitrator in the NANTibody arbitration had passed away. A substitute arbitrator was appointed on February 25, 2022, and the parties have worked with the substitute arbitrator to conclude the proceedings. Additional hearing sessions were held in May and July 2022, and summations took place on August 2, 2022.
On December 2, 2022, the arbitrator issued a final award finding that Sorrento had breached the two exclusive license agreements with NantCell and NANTibody. The arbitrator awarded NantCell approximately $156.8 million and NANTibody approximately $16.7 million, plus post-award interest accruing at a daily rate. On December 21, 2022, NantCell and NANTibody filed petitions in the Superior Court to confirm the arbitration award; on January 16, 2023, Sorrento filed a response to the petitions and moved to vacate the award. On February 7, 2023, after a hearing, the Superior Court entered orders confirming the arbitration award and denying Sorrento’s motion to vacate. The Superior Court entered judgments against Sorrento in the aggregate amount of approximately $176.4 million plus 10% post-judgment interest, of which approximately $159.4 million is payable to NantCell, and the remainder of which is payable to NANTibody. On February 13, 2023, Sorrento informed counsel to the company that it had filed a Chapter 11 proceeding in the U.S. District Court for the Southern District of Texas, In re: Sorrento Therapeutics, Inc., et al., Case No. 23-bk-90085 (Bankr. S.D. Tex.) (DRJ). The company intends to continue to pursue vigorously, consistent with its rights in light of Sorrento’s Chapter 11 filing, the collection of the judgments and 10% post-judgment interest from Sorrento, but we make no assurances that we will receive the full amount or with respect to the timing of our receipt of any funds.
The Superior Court actions remain pending, and it remains to be determined how, if at all, the awards in the arbitrations will affect the Superior Court actions. A July 17, 2023 trial date has been set in the first-filed Superior Court action. An estimate of the possible loss or range of loss resulting from the Superior Court litigation cannot be made at this time.
Shenzhen Beike Biotechnology Co. Ltd. Arbitration
In 2020, we received a Request for Arbitration before the International Chamber of Commerce, International Court of Arbitration. The arbitration relates to a license, development, and commercialization agreement that Altor entered into with Beike in 2014, which agreement was amended and restated in 2017, pursuant to which Altor granted to Beike an exclusive license to use, research, develop and commercialize products based on N-803 in China for human therapeutic uses. In the arbitration, Beike is asserting a claim for breach of contract under the license agreement. Among other things, Beike alleges that we failed to use commercially reasonable efforts to deliver to Beike materials and data related to N-803. Beike is seeking specific performance or in the alternative, damagesand declaratory relief for the alleged breaches. On September 25, 2020, the parties entered into a standstill and tolling agreement (standstill agreement) under which, among other things, the parties affirmed they will perform certain of their obligations under
the license agreement by specified dates and agreed that all deadlines in the arbitration are indefinitely extended. The standstill agreement maycould be terminated by any party on ten calendar days’ notice, and upon termination, the parties will havehad the right to pursue claims arising from the license agreement in any appropriate tribunal. On March 20, 2023, we terminated the standstill agreement, and on April 11, 2023, Beike served an amended Request for Arbitration. We served an Answer and Counterclaims on May 19, 2023. Beike served a Reply to our counterclaims on June 21, 2023. Beike’s Statement of Claim is due on March 22, 2024, and the company’s Statement of Defense and Counterclaim is due on June 21, 2024. The parties have been providing periodic updateshearing in the arbitration is scheduled to the International Chamber of Commerce confirming a stay of all proceedings during the standstill.begin on June 9, 2025. Given that this action remains at the pleading stage and no discovery has occurred, it remains too early to evaluate the likely outcome of the case or to estimate any range of potential loss. We believe the claims asserted against the company lack merit and intend to defend the case, vigorously and that we may have counterclaims.to pursue our counterclaims, vigorously.
Litigation Related to the Merger withSecurities Class Action
On June 30, 2023, a putative securities class action complaint, captioned Salzman v. ImmunityBio, Inc.
In connection with the Merger with NantCell, Inc. (formerly known as ImmunityBio, Inc. et al., a private company), a Delaware corporation, via a wholly-owned subsidiary of NantKwest, several complaints wereNo. 3:23-cv-01216-BEN-WVG, was filed as individual actions in the United StatesU.S. District Courts,Court for the Southern District of California against the company and subsequently were voluntarily dismissed (the Merger Actions). The Merger Actions generally alleged that the Definitive Proxy Statement filed with the SEC on February 2, 2021 misrepresentedthree of its officers and/or omitted certain purportedly material information relating to financial projections, analysis performed by the financial advisor to NantKwest’s Special Committee, alleged past engagements of the Special Committee’s financial advisor and industry consultant, and the terms of the engagement of such consultant. The Merger Actions asserteddirectors, asserting violations of Section 14(a) of the Exchange ActSections 10(b) and Rule 14a-9 promulgated thereunder against all defendants and violations of Section 20(a) of the Exchange Act against NantKwest’s directors. The Merger Actions sought,Act. Stemming from the company’s disclosure on May 11, 2023 that it had received an FDA CRL stating, among other things, that it could not approve the company’s BLA for its product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, in its present form due to deficiencies related to its pre-license inspection of the company’s third-party CMOs, the complaint alleges that the defendants had previously made materially false and misleading statements and/or omitted material adverse facts regarding its third-party clinical manufacturing organizations and the prospects for regulatory approval of the BLA. On September 27, 2023, the court appointed a lead plaintiff, approved their selection of lead counsel, and re-captioned the case In re. ImmunityBio, Inc. Securities Litigation, No. 3:23-cv-01216. On November 17, 2023, lead plaintiff filed an injunction enjoiningamended complaint, which named the stockholder votesame defendants and asserted the same claims as the previous complaint. On January 8, 2024, defendants filed a motion to dismiss the amended complaint. A hearing on the Mergermotion is currently scheduled for April 23, 2024. The company believes the lawsuit is without merit and intends to defend the consummationcase vigorously. The company is unable to estimate a range of loss, if any, that could result were there to be an adverse final decision in this action. If an unfavorable outcome were to occur, it is possible that the Merger unlessimpact could be material to the company’s results of operations in the period(s) in which any such outcome becomes probable and until certain additional information was disclosed to NantKwest’s stockholders, costs of the action, including plaintiffs’ attorneys’ fees and experts’ fees, and other relief the Court may deem just and proper. Neither the stockholder vote on the Merger nor the Merger were enjoined and both occurred on March 8 and March 9, 2021, respectively. The Merger Actions were voluntarily dismissed on March 25, 2022.estimable.
Altor BioScience, LLC, and NantCell, Inc. Matters Against Dr. Hing Wong and HCW Biologics, Inc.
On December 23, 2022, Altor and NantCell filed an arbitration demand against Dr. Hing Wong, former CEO of Altor and NantCell. The demand asserts claims for breach of Dr. Wong’s contracts with the companies, breach of the covenant of good faith and fair dealing, conversion, fraudulent concealment, unjust enrichment, breach of fiduciary duty, and replevin. The same day, Dr. Wong filed an arbitration demand seeking a declaratory judgment finding that Dr. Wong is not liable to Altor or NantCell for any of their claims. The parties have agreed to consolidate the arbitration filings in one proceeding, and on January 23, 2023, Dr. Wong filed an Answering Statement denying the claims.
Also, on December 23, 2022 Altor and NantCell filed a complaint in the United States District Court for the Southern District of Florida against HCW, Biologics, Inc. (HCW Biologics), Dr. Wong’s new company. Altor’s and NantCell’s complaint asserts claims for misappropriation of trade secrets under both Florida and federal law, inducement of breach of contract, tortious interference with contractual relations, inducement of breach of fiduciary duty, conversion, unjust enrichment, replevin, specific performancerequest for assignment of patents and patent applications, and establishment of a constructive trust. On January 31, 2023, HCW Biologics filed motions to compel arbitration of Altor’s and NantCell’s claims, or in the alternative to stay or dismiss them. Altor and NantCell filed an opposition to the motions on February 14, 2023, and HCW Biologics filed reply papers on February 21, 2023. The motions remain pendingAt a hearing on April 18, 2023, the court heard argument and norequested supplemental briefing. After the hearing, date has been set.
Stipulation of Settlement
In 2019, following approval bythe parties reached an agreement to consolidate all claims in a single arbitration proceeding. On May 1, 2023, we filed our Board of Directors, we entered into a settlement agreement (the Stipulation of Settlement) with three stockholders ofarbitration demand asserting the company, each of whom had submitted a stockholder demand for the Board of Directors to take action to remedy purported harm to the company resulting from certain alleged wrongful conduct concerning, among other things, disclosures about Dr. Soon-Shiong’s compensation and a related-party lease agreement. The Stipulation of Settlement called for us to adopt certain governance changes, and for the three stockholders to file a stockholder derivative actionsame claims against HCW that were asserted in the Superior Court offederal court complaint. On May 15, 2023, HCW filed an Answering Statement denying the State of California, County of San Diego, followed by an application for court approval ofclaims. The parties are currently engaged in discovery. The hearing in the Stipulation of Settlement. The court entered an order preliminarily approving the Stipulation of Settlement. Pursuantconsolidated arbitration is scheduled to the Stipulation of Settlement, we provided stockholders with notice of the settlement and the final settlement hearing.begin on May 20, 2024.
ITEM 4. MINE SAFETY DISCLOSURES.
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market Information
Our common stock is traded under the ticker symbol “IBRX” on the Nasdaq Global Select Market.
Holders of Record
As of February 24, 2023,March 14, 2024, there were approximately 8691 stockholders of record of our common stock. The actual number of stockholders is greater than the number of record holders and includes stockholders who are beneficial owners but whose shares are held in “street name” by brokers and other nominees. The number of stockholders of record also does not include stockholders whose shares may be held in trust or by other entities.
Dividend Policy
We have never paid cash dividends on our common stock and do not anticipate paying cash dividends on our common stock for the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition and other business and economic factors affecting us at such time as the Board of Directors may consider relevant.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table sets forth information regardingrequired by this item will be contained in our equity compensation plansdefinitive proxy statement to be filed with the SEC on Schedule 14A in effect asconnection with our Proxy Statement, which is expected to be filed not later than 120 days after the end of our fiscal year ended December 31, 2022 (including upon the exercise of stock options2023, and the vesting of restricted stock units (RSUs)):
| | | | | | | | | | | | | | | | | | | | |
| | Equity Compensation Plan Information |
| | Number of
Securities to be
Issued upon
Exercise of
Outstanding
Options,
Warrants and
Rights | | Weighted-average
Exercise Price of
Outstanding
Options,
Warrants and
Rights | | Number of
Securities
Remaining
Available for
Future Issuance
under Equity
Compensation
Plans (Excluding
Securities
Reflected in
Column (a)) |
| Plan Category | | (a) | | (b) | | (c) |
| | | | | | |
| Equity compensation plans approved by security holders (1), (2), (3), (4) | | 15,814,314 | | $ | 9.87 | | | 18,382,213 |
| Equity compensation plan not approved by security holders | | — | | | | — |
| Total | | 15,814,314 | | | | 18,382,213 |
_______________
(1)The equity compensation plans approvedis incorporated herein by security holders are the 2014 Equity Incentive Plan (2014 Plan) and the 2015 Equity Incentive Plan (2015 Plan). The 2014 Plan has terminated as to future grants. The amount shown in Column (a) with respect to the 2014 Plan includes 503,493 shares issuable upon the exercise of vested stock options. The amount shown in Column (a) with respect to the 2015 Plan includes 8,334,489 shares issuable upon the exercise of vested stock options and 2,615,574 shares issuable upon the vesting of RSUs.
(2)The Amended and Restated ImmunityBio, Inc. 2015 Stock Incentive Plan (2015 NC Plan) was approved by security holders in conjunction with the Merger. The 2015 NC Plan has terminated as to future grants. The amount shown in Column (a) with respect to this plan includes 424,944 shares issuable upon the exercise of vested stock options and 3,935,814 shares issuable upon the vesting of RSUs.
(3)The amount shown in Column (b) is the weighted average exercise price for stock options outstanding.
(4)The amount shown in Column (c) is the number of shares available for future grants under the 2015 Plan.
Stock Performance Graph
The following graph compares the cumulative total return on our common stock, the Russell 2000 Index, and the NASDAQNasdaq Biotechnology Index over the five-year period ending December 31, 2022.2023. The graph assumes that $100 was invested on December 31, 20172018 in our common stock or the comparative indices, including reinvestment of dividends. The returns shown are based on historical results and are not indicative of, or intended to forecast, future performance of our common stock or the comparative indices. This performance graph shall not be deemed “filed”filed for purposes of Section 18 of the Exchange Act or incorporated by reference into any filing of ImmunityBio, Inc. under the Securities Act.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among ImmunityBio, Inc., the Russell 2000 Index
and the NASDAQNasdaq Biotechnology Index
Recent Sales of Unregistered Securities
None.
Issuer Purchases of Equity Securities
No shares of our common stock were repurchased during the three months ended December 31, 20222023 under the 2015 Share Repurchase Program. As of December 31, 2022,2023, $18.3 million remained authorized to use for share repurchases under the program. For additional information regarding the 2015 Share Repurchase Program, seeSee Note 1213, Stockholders’ Deficit, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report.Report for more information regarding the 2015 Share Repurchase Program. ITEM 6. RESERVED
ITEM 6. RESERVED
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The following discussion and analysis of our financial condition and results of operations should be read together with the description of our business appearingthat appears in Part I, Item 1. “Business”“Business” and the consolidated financial statements and related notes thereto in Part II, Item 8. “Financial“Financial Statements and Supplementary Data”Data” of this Annual Report. This discussion contains forward-looking statements as a result of many factors, including those set forth under Part I, Item 1. “Business—Forward-Looking Statements”Statements” and Item 1A. “Risk“Risk Factors,” and elsewhere in this Annual Report. These statements are based on the current expectationsour management’s beliefs and assumptions of management that are subjectand on information currently available to risks and uncertainties.our management. Actual results could differ materially from those discussed in or implied by such forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this Annual Report, particularly in Part I, Item 1A. “Risk Factors.“Risk Factors.” Except as required by law, we do not undertake any responsibility to update any of these factors or to announce publicly any revisions to any of the forward-looking statements contained in this or any document, whether as a result of new information, future events, or otherwise. Our Business
ImmunityBio, Inc. is aWe are an integrated clinical-stage biotechnology company discovering, developing, and commercializing next-generation immuno- and cellular therapies that bolster the natural immune system to drive and sustain an immune response. Using our proprietary platforms that amplify both the innate and adaptive branches of the immune system, our teams of clinical, scientific, and manufacturing experts, advance novel therapies and vaccines that complement, harness,aimed at defeating urologic and amplify the immune system to defeatother cancers, andas well as infectious diseases. We striveAlthough such designations may not lead to be a vertically-integrated immunotherapy company designingfaster development process or regulatory review and manufacturingmay not increase the likelihood that a product candidate will receive approval, N-803 (Anktiva), our products so they are more effective, accessible, more conveniently stored,lead biologic commercial product candidate, has received Breakthrough Therapy and more easily administered to patients.Fast Track designations and is currently under review by the FDA for treatment in combination with BCG of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease and has a new user fee goal date (PDUFA date) of April 23, 2024.
Our broad immunotherapyplatforms and cell therapy platformstheir associated product candidates are designed to attack cancer and infectious pathogens by activating both the innate immune system—system, including—NK cells, dendritic cells, and macrophages—and macrophages, as well as—the adaptive immune system—system comprising—B cells and T cells—cells,—in an orchestrated manner. The goal of this potentially best-in-class approach is to generate immunogenic cell death thereby eliminating rogue cells from the body whether they are cancerous or virally infected andvirally-infected. Our ultimate goal is to ultimatelyovercome the limitations of current treatments, such as checkpoint inhibitors, and/or reduce the need for standard high-dose chemotherapy in cancer by employing this coordinated approach to establish an “immunological memory” that confers long-term benefit for the patient.
Our business is based on the foundation of multiple platforms that collectively act on the entire immune response with the goal of targeted, durable, coordinated,biologic product candidates include: (i) antibody-cytokine fusion proteins, (ii) DNA, RNA, and safe immunity against disease.recombinant protein vaccines, and (iii) cell therapies. These platforms and their associated product candidates are designed to overcome the limitations of the current standards of care in oncology and infectious diseases, such as checkpoint inhibitors and antiretroviral therapies. Our portfolio includes:

Our platforms includehave generated 9 first-in-humannovel therapeutic agents thatfor which clinical trials are currently being studiedeither underway or planned in 26solid and liquid tumors. Specifically, our clinical trials—17 offocus includes bladder, lung, and colorectal cancers and GBM, which are in Phase 2 or 3 development—across 12 indications in liquid and solid tumors, including bladder, pancreatic and lung cancers. These are among the most frequent and lethal cancer types, for whichand where there are high failure rates for existing standards of care or in some cases, no available effective treatment. In infectious diseases, our pipeline currently targets such pathogens as SARS-CoV-2 and HIV. We believe SARS-CoV-2 currently lacks a vaccine that provides long-term protection against the virus, particularly its variants, while HIV affects tens of millions of people globally and currently has no known cure.
We believe that our innovative approach to orchestrate and combine therapies for optimal immune system response will become a therapeutic foundation across multiple clinical indications. Additionally, we believe that data from multiple clinical trials indicates N-803 has broad potential to enhance the activity of therapeutic mAbs, including checkpoint inhibitors (e.g., Keytruda®), across a wide range of tumor types. N-803 is currently being studied in 21 clinical trials (both ImmunityBio and investigator-sponsored) across 12 indications. Although such designations may notOur lead to a faster development process or regulatory review and may not increase the likelihood that abiologic commercial product candidate will receive approval, Anktiva ImmunityBio’s novel antibody cytokineis an IL-15 superagonist antibody-cytokine fusion protein, has received Breakthrough Therapy and Fast Track designations in combination with BCG from the FDA for the treatment of patients with BCG-unresponsive NMIBC CIS with or without Ta or T1 disease. In protein. In May 2022, we announced the submission of a BLA to the FDA for our product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. The deficiencies in the CRL related to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolution of the observations noted at the pre-license inspection would be required before the BLA could be approved. At the time, the FDA further provided recommendations specific to additional CMC issues and assays to be resolved. The CRL did not request new preclinical studies or Phase III clinical trials to evaluate safety or efficacy. The FDA requested that the company provide updated duration of response data for the efficacy population as identified by the FDA in the company’s resubmission, as well as a safety update.
On October 23, 2023, we announced that we had completed the resubmission of the BLA addressing the issues in the CRL. As part of our resubmission, we provided an update of the duration of response regarding the responders identified by the FDA in the efficacy population for BCG unresponsive subjects with high-risk CIS disease. In July 2022,On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a target PDUFA actionnew user fee goal date (PDUFA date) of MayApril 23, 2023.2024. While we believe the BLA resubmission addresses the issues identified in the CRL, there is no guarantee that the FDA will ultimately agree that such issues have been successfully addressed and resolved. It is unclear when the FDA will approve our BLA, if at all.
Further late-stage efforts for Anktiva are in development within the broader bladder cancer space, including BCG-naïve NMIBC. In addition, data from multiple clinical trials suggests N-803 has potential to enhance the activity of therapeutic mAbs, including checkpoint inhibitors (e.g., pembrolizumab/Keytruda), across a wide range of tumor types. We believe there is potential for N-803 to become a therapeutic foundation across all phases of treatment, including in adjunctive therapy, to amplify, reactivate or extend the efficacy of standard of care. In addition to N-803, we have active clinical programs evaluating therapeutic candidates from our DNA and RNA vaccine technology platforms and our NK cell-based therapy platforms in oncology and infectious disease indications.
On December 29, 2023, we entered into the RIPA with Infinity and Oberland. Pursuant to the RIPA, Oberland acquired certain Revenue Interests from us for a gross purchase price of $200.0 million paid on closing, less certain transaction expenses. In addition, Oberland may purchase additional Revenue Interests from us in exchange for the $100.0 million Second Payment upon satisfaction of certain conditions specified in the RIPA, including following the receipt of approval by the FDA of the company’s BLA for N-803 in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease on or before June 30, 2024. Under the RIPA, Oberland has the right to receive quarterly payments from us based on, among other things, a certain percentage of our worldwide net sales, excluding those in China, during such quarter.
Also, on December 29, 2023 and in connection with the RIPA, we entered into an SPOA with Oberland pursuant to which we sold an aggregate of approximately $10.0 million of our common stock at $4.1103 per share in a private placement. Oberland also has an option to purchase up to an additional $10.0 million of our common stock, at a price per share to be determined by reference to the 30-day trailing volume weighted-average price of our common stock calculated from the date of exercise.
We have established GMP manufacturing capacity at scale with cutting-edge cell manufacturing expertise and ready-to-scale facilities, as well as extensive and seasoned R&D, clinical trial, and regulatory operations, and development teams.
The Merger
On December 21, 2020, NantKwest and NantCell entered into the Merger Agreement, pursuant to which NantKwest and NantCell agreed to combine their businesses. The Merger Agreement provided that a wholly-owned subsidiary of the company would merge with and into NantCell, with NantCell surviving the Merger as a wholly-owned subsidiary of the company.
On March 9, 2021, we completed the Merger pursuant to the terms of the Merger Agreement. Under the terms of the Merger Agreement, at the effective time of the Merger (the Effective Time), each share of NantCell common stock, par value $0.001 per share, issued and outstanding immediately prior to the Effective Time, subject to certain exceptions as set forth in the Merger Agreement, was converted automatically into a right to receive 0.8190 (theusing the Exchange Ratio)Ratio into newly issued shares of common stock, par value $0.0001 per share, of the company (CompanyCompany Common Stock),Stock, with cash paid in lieu of any fractional shares. At the Effective Time, each share of the company’s common stock issued and outstanding immediately prior to the Effective Time, remained an issued and outstanding share of the combined company. At the Effective Time, each outstanding option, RSU or warrant to purchase NantCell common stock was converted using the Exchange Ratio into an option, RSU or warrant, respectively, on the same terms and conditions immediately prior to the Effective Time, to purchase shares of Company Common Stock.
Immediately following the Effective Time, the former stockholders of NantCell held approximately 71.5% of the outstanding shares of Company Common Stock and the stockholders of NantKwest as of immediately prior to the Merger held approximately 28.5% of the outstanding shares of Company Common Stock. As a result of the Merger and immediately following the Effective Time, Dr. Patrick Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer, and his affiliates beneficially owned, in the aggregate, approximately 81.8% of the outstanding shares of Company Common Stock. Following the consummation of the Merger, the symbol for shares of the company’s common stock was changed to “IBRX.”
During the year ended December 31, 2021, we recorded $13.0 million of costs totaling $23.3 million in in connection with the Merger consisting of financial advisory, legal and other professional fees, of which $13.0 million and $10.3 million were recorded for the years endedDecember 31, 2021 and 2020, respectively.fees.
Accounting Treatment of the Merger
The Merger represents a business combination pursuant to Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC)FASB ASC Topic 805-50, Mergers (ASC 805-50), which was accounted for as a transaction between entities under common control as Dr. Soon‑Shiong and his affiliates were the controlling stockholders of both the company and NantCell for all of the periods presented in this report. As a result, all of the assets and liabilities of NantCell were combined with ours at their historical carrying amounts on the closing date of the Merger. We recast our prior period financial statements for the yearsyear ended December 31, 2021 and 2020 to reflect the conveyance of NantCell’s common shares as if the Merger had occurred as of the earliest date of the consolidated financial statements presented in Part II, Item 8. “Financial“Financial Statements and Supplementary Data”Data” of this Annual Report. All material intercompany accounts and transactions have been eliminated in consolidation. COVID-19 Pandemic
The COVID-19 pandemic continues to present a substantial public health and economic challenge around the world. Through the date of this Annual Report, we have not seen a material adverse impact to our business from the pandemic. However, given the unprecedented and continuously evolving nature of the pandemic, we cannot at this time predict the specific extent, duration, or full impact that this pandemic may have on our financial condition and results of operations, including ongoing and planned clinical trials. More specifically, the pandemic may result in prolonged impacts that we cannot predict at this time and we expect that such uncertainties will continue to exist for the foreseeable future. The impact of the pandemic on our financial performance will depend on future developments, including the duration and spread of the outbreak, impact of potential variants and the related governmental advisories and restrictions. These developments and the impact of the ongoing pandemic on the financial markets and the overall economy are highly uncertain. If the financial markets and/or the overall economy are impacted for an extended period, our results may be adversely affected. In addition, we anticipate that enrollment of patients in certain studies will likely take longer than previously forecasted and that our clinical trials may require additional time to complete which would in turn impact the timeline of BLA submissions of our product candidates and subsequent revenue generation.
These factors have been accounted for in the company’s anticipated upcoming milestones. During any such delays in our clinical trials, we will continue to incur fixed costs such as selling, general and administrative expenses and operating expenses related to our laboratory, GMP manufacturing, and office facilities.
Many of our office-based employees have been working from home since mid-March 2020. Essential staffing levels for our research and development operations remain in place, including maintaining key personnel in our laboratory and GMP manufacturing facilities. It is likely that the pandemic and resulting mitigation efforts could have an impact in the future on our third-party suppliers who manufacture laboratory supplies required for our in-house manufacturing process, which in turn could have an impact on having sufficient clinical product supply available for our clinical trials. We have addressed this in part by ensuring that we have sufficient supplies on hand to weather interruptions in our supply chain.
We continue to monitor the impact of the COVID-19 pandemic on our business, including our clinical trials, manufacturing facilities and capabilities, and ability to access necessary resources. For a discussion of the risks presented by the COVID-19 pandemic to our results of operations and financial condition, see Part I, Item 1A. “Risk“Risk Factors.” Operating Results
From inception through the date of this Annual Report, we have generated minimal revenue from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables, and grant programs. We have no clinical products approved for commercial sale and have not generated any revenue from therapeutic and vaccine product candidates that are under development. We have incurred net losses in each year since our inception and, as of December 31, 2022,2023, we had an accumulated deficit of $2.4$3.0 billion. OurDuring the years ended December 31, 2023, 2022 and 2021, net losses attributable to ImmunityBio common stockholders were $583.2 million, $416.6 million, and $346.8 million, and $221.9 million for the years ended December 31, 2022, 2021 and 2020, respectively. Substantially all of our net losses resulted principally from costs incurred in connection with our ongoing clinical trials and operations, our research and development programs, and from selling, general and administrative costs associated with our operations, including stock-based compensation expense.
As of December 31, 2022,2023, we had 725628 employees. Personnel of related companies who provide corporate, general and administrative, certain research and development, and other support services under our shared services agreement with NantWorks are not included in this number. For additional information, seeSee Note 1011, Related-Party Agreements, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report.Report for more information. In anticipation of the commercialization of select drug candidates, we expect to continue to incur significant expenses and increasing operating losses for the foreseeable future, which may fluctuate significantly from quarter-to-quarter and year-to-year. See “—Future Funding Requirements” below for a discussion of our anticipated expenditures and sources of capital we expect to access to fund these expenditures. Collaboration Agreements
We anticipate that strategic collaborations will continue to be an integral part of our operations, providing opportunities to leverage our partners’ expertise and capabilities to gain access to new technologies and further expand the potential of our technologies and product candidates across relevant platforms. We believe we are well positioned to become a leader in immunotherapy due to our broad and vertically-integrated platforms and through complementary strategic partnerships.
We believe that our innovative approach to orchestrate and combine therapies for optimal immune system response will become a therapeutic foundation across multiple clinical indications. Additionally, we believe that data from multiple clinical trials indicates N-803 has broad potential to enhance the activity of therapeutic mAbs, including checkpoint inhibitors, (e.g., Keytruda), across a wide range of tumor types. N-803 is currently being studied in 21 clinical trials (both ImmunityBio and investigator-sponsored) across 12 indications. We may also enter into supply arrangements for various investigational agents to be used in our clinical trials. See Note 6, Collaboration and License Agreements and Acquisition, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for a more detailed discussioninformation regarding our collaboration and license agreements.
Agreements with Related Parties
Our Executive Chairman, Global Chief Scientific and Medical Officer and our principal stockholder, founded and has a controlling interest in NantWorks, which is a collection of companies in the healthcare and technology space. We have entered into arrangements with NantWorks, and certain affiliates of NantWorks. Affiliates of NantWorks are also affiliates of the company due to the common control by and/or common ownership interest of our Executive Chairman and Global Chief Scientific and Medical Officer.
Related-Party Debt
$50.0 million Variable-RateRelated-Party Convertible Promissory NoteNotes
On December 12, 2022,September 11, 2023, the company entered into a stock purchase agreement with Nant Capital, NantMobile and NCSC (collectively, the “related-party purchasers”), which are entities affiliated with Dr. Soon-Shiong, pursuant to which the related-party purchasers exchanged outstanding fixed-rate promissory notes held by such related-party purchasers, representing approximately $270.0 million in aggregate principal amount and accrued and unpaid interest, in exchange for an aggregate of 209,291,936 shares of common stock at an exchange price of $1.29 per share. As a result of the exchange, the company is forever released and discharged from all its obligations and liabilities under these notes.
On September 11, 2023, the company executed a $50.0$200.0 million convertible promissory note with Nant Capital, an entity affiliated with Dr. Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer. ThisCapital. The note bears interest at Term Secured Overnight Financing Rate (Term SOFR)SOFR plus 8.0% per annum. The accrued interest on this note shall be payable quarterly on the last business day of March, June, September and December,monthly commencing on DecemberSeptember 30, 2022.2023. The outstanding principal amount and any accrued and unpaid interest are due on December 31, 2023. The companythe earlier of (i) September 11, 2026 or (ii) upon the occurrence and during the continuance of an “event of default” (as defined in the note). We may prepay the outstanding principal amount, together with any accrued interest, at any time, in whole or in part, without premium or penalty. The company must prepay the outstanding principal amount, together with any accrued interest, if requested by the holder, following the successful closing of a strategic collaboration transaction with a large biopharmaceutical company.
The company received net proceeds of $49.7 million, net of a $0.3 million origination fee paidpenalty upon five (5) days’ written notice to the lender, whichnoteholder. The noteholder has the company intends to use for pre-commercialization efforts and clinical development programs, other research and development activities, capital expenditures, and other general corporate purposes.
Conversion of Fixed-Rate Promissory Note due 2025
On December 12, 2022, the company received written notice from NantWorks, the holder of the existing convertible promissory note of NantCell, Inc., a wholly-owned subsidiary of the company (the Existing Note), of its electionsole option to convert the entire outstanding principal and accrued interest under the Existing Note into sharesall (but not less than all) of the company’s common stock. As of such date, the entire outstanding principal amount and accrued andbut unpaid interest due under the Existing Note was approximately $56.6 million, which were converted into 9,986,920 shares of the company’s common stock at a conversion price of $5.67$1.9350 per shareshare. We received net proceeds of approximately $199.0 million from this financing, net of a $1.0 million origination fee paid to the noteholder. On December 29, 2023 in accordanceconnection with the terms ofRIPA, the Existing Note.company and Nant Capital entered into a letter amendment whereby this promissory note became subordinated to the RIPA payment obligations.
$125.0 million Variable-Rate PromissoryRelated-Party Convertible Note at Fair Value
On AugustMarch 31, 2022,2023, the company executed a $125.0$30.0 million promissory note with Nant Capital thatCapital. This note bears interest at Term SOFR plus 8.0% per annum. The accrued interest is payable quarterly on the last business day of March, June, September and December, commencing on September 30, 2022. The outstanding principal amount and any accrued and unpaid interest are due on the earlier of December 31, 2023 or upon the occurrence and continuation of an event of default (as defined in the note). The interest on this note is paid on a quarterly basis beginning on June 30, 2023. The company may prepay the outstanding principal amount and any accrued and unpaid interest of the promissory note, at any time, in whole or in part, without penalty. Upon receipt of a written notice of prepayment from the company, the noteholder has the right, at its option, to convert the outstanding principal amount to be prepaid and the accrued and unpaid interest thereon (as specified in the notice of prepayment) into shares of the company’s common stock at a price of $2.28 per share. Additionally, the whole promissory note and any accrued interest may be converted into shares of the company’s common stock at a conversion price of $2.28 per share (subject to appropriate adjustment from time to time for any stock dividend, stock split, combination of shares, reorganization, recapitalization, reclassification or other similar event), at the option of the noteholder.
The company received net proceeds of approximately $29.9 million from this financing, net of a $0.1 million origination fee paid to the noteholder, which we used, together with other available funds, to progress our pre-commercialization efforts and clinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes.
On September 11, 2023, the company and Nant Capital entered into a letter agreement pursuant to which the maturity date of this promissory note was extended from December 31, 2023 to December 31, 2024. On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into a letter amendment pursuant to which the maturity date of this promissory note was extended to December 31, 2025, and the note became subordinated to the RIPA payment obligations.
This note was accounted for under the ASC 825-10-15-4 FVO election until it was amended on December 29, 2023. Under the FVO election, the note was initially measured at its issuance date estimated fair value and subsequently remeasured at estimated fair value on a recurring basis at each reporting period date. With each such remeasurement, the note was adjusted to fair value, with the change in fair value recorded in other expense, net, on the consolidated statement of operations.
Related-Party Nonconvertible Notes
On June 13, 2023, the company executed a $30.0 million promissory note with Nant Capital. The company was able to request up to three (3) advances of $10.0 million each pursuant to the note. The principal amount of each advance bears interest at Term SOFR plus 8.0% per annum. The accrued interest on any advance is payable quarterly on the last business day of June, September and December. The outstanding principal amount and any accrued and unpaid interest on advances were due on the earlier of (i) December 31, 2023 or (ii) upon the occurrence and during the continuance of an event of default (as defined in the note). We may prepay the outstanding principal amount, together with any accrued interest, aton any time, in whole or in part, without premium or penalty.
The company received net proceeds of $124.4 million, net of a $0.6 million origination fee paid to the lender, which the company intends to use for pre-commercialization efforts and clinical development programs, working capital and other general corporate purposes.
$300.0 million Variable-Rate Promissory Note
As of December 31, 2022, the company has a $300.0 million variable-rate promissory note with Nant Capital. On August 31, 2022, the terms of this promissory note were amended and restated to extend the maturity date of the loan from December 17, 2022 to December 31, 2023, increase the spread on the loan from Term SOFR plus 5.4% per annum to Term SOFR plus 8.0% per annum, and reset the quarterly interest payment date from the 17th of the month to the last business day of March, June, September and December, commencing on September 30, 2022. No other material terms or conditions of this variable-rate promissory note were modified as part of this amendment and restatement. In the event of a default on the loan (as defined in the promissory note), including if the company does not repay the loan at maturity, the company has the right, at its sole option, to convert the outstanding principal amount and accrued and unpaid interest due under this note into shares of the company’s common stock at a price of $5.67 per share.
There can be no assurance that the company can refinance the variable-rate promissory notes described above or what terms will be available in the market at the time of refinancing. Furthermore, if prevailing interest rates or other factors at the time of refinancing result in higher interest rates upon refinancing, then the interest expense relating to the refinanced indebtedness would increase. These risks could materially adversely affect the company’s financial condition, cash flows and results of operations.
Fixed-Rate Convertible Promissory Notes
As of December 31, 2022, the company has outstanding fixed-rate promissory notes with entities affiliated with Dr. Soon-Shiong in an aggregate amount of $262.4 million (consisting of principal and accrued and unpaid interest). These notes bear interest at a per annum rate ranging from 3.0% to 6.0%, provide that the outstanding principal is due and payable on September 30, 2025, and accrued and unpaid interest is payable on either upon maturity or, with respect to one of the notes, on a quarterly basis. The company may prepay the outstanding amount of any advance under such notes, together with accrued and unpaid interest,then-outstanding advances, at any time, in whole or in part, without premium or penalty, now subjectand without the prior consent of the noteholder, upon five (5) days written notice to an advance notice periodthe noteholder.
The company received net proceeds of at least five business days, duringapproximately $29.9 million from this financing, net of a $0.1 million origination fee paid to Nant Capital, which we used, together with other available funds, to progress our regulatory approval efforts, pre-commercialization activities and clinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes.
On September 11, 2023, the company and Nant Capital entered into a series of letter agreements pursuant to which the lender can convertmaturity date of related-party nonconvertible promissory notes totaling $505.0 million in principal were extended from December 31, 2023 to December 31, 2024.
On December 29, 2023 in connection with the amount requestedRIPA, the company and Nant Capital entered into an amended and restated promissory note pursuant to which the existing $30.0 million promissory note, along with other existing related-party nonconvertible promissory notes, was amended to be prepaid byincluded in the company into shares of company common stock, as partTranche 2 principal amount of the amendmentamended $505.0 million December 2023 promissory note. The Tranche 2 of the promissory note has a principal amount of $380.0 million, a maturity date of December 31, 2025, and restatement described below.
On August 31, 2022,an interest rate of Term SOFR plus 7.5% per annum. Based on the terms of each fixed-rate promissory note werethe amended and restated to include a conversion feature that gives each lenderpromissory note, the right at any time, including upon notice of prepayment,noteholder, at its sole option, todiscretion, may convert all of the entire outstanding$380.0 million principal amount and accrued and unpaid interest due under each note at the time of conversion into shares of the company’s common stock at a conversion price of $5.67$8.2690 per share. No other material terms or conditionsThe new note became subordinated to the RIPA payment obligations.
See Note 10, Related-Party Debt, of these fixed-rate promissory notes were modified as partthe “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of these amendments.this Annual Report for more information regarding our related-party debt. Immuno-Oncology Clinic, Inc.
We have entered into multiple agreements with the Clinic to conduct clinical trials related to certain of our product candidates. The Clinic is a related party as it is owned by an officer of the company and NantWorks manages the administrative operations of the Clinic.
In 2021, we completed a review of alternative structures that could support our more complex clinical trial requirements and made a decision to explore a potential transition of clinical trials at the Clinic to a new structure (including contracting with a new, non-affiliated professional corporation) to be determined and agreed upon by all parties. WeWhile we have not yet finalized the potential transition, we continue productive negotiationsdiscussions with potential partners around such alternative structures and expect to completestructures. During the process during the year ending December 31, 2023, but there can be no assurance that we will be successful. For the yearyears ended December 31, 2023, 2022 and 2021, we incurredrecorded $2.2 million, $2.4 million and $1.6 million, respectively, in research and development expense, on the consolidated statementstatements of operations related to clinical trial and transition services provided by the Clinic.
Related-Party Leases
NantWorks605 Doug St, LLC
On May 6, 2022,In June 2023, we amended our facility licenseexercised the option to extend the lease agreement with NantWorks to expand the licensed premises to605 Doug St, LLC, an aggregate total of 46,330 rentable square feet.entity owned by our Executive Chairman and Global Chief Scientific and Medical Officer, for one additional three-year term through July 2026.
23 Alaska, LLC
On May 6, 2022, we entered into a lease agreement with 23 Alaska, LLC for a 47,265 rentable square foot facility located at 2335 Alaska Ave., El Segundo, California, to be used primarily for quality control and process science activities.
557 Doug St, LLC
Effective September 27, 2021, we entered into a lease agreement with Nant Capital under which we leased 557 South Douglas Street in El Segundo, California. Effective MayAugust 31, 2022,2023, we executed a lease termination agreement with Nant Capital under which23 Alaska, LLC, an entity owned by our Executive Chairman and Global Chief Scientific and Medical Officer.
Duley Road, LLC
Effective October 3, 2023, we received a full refund ofexercised the first month’s rentoption to extend the lease agreement with Duley Road, a related party that is indirectly controlled by our Executive Chairman and security deposit totaling $0.2 million that we paid upon execution of the lease.Global Chief Scientific and Medical Officer, for one additional five-year term through October 31, 2029.
See Note 9, Related-Party Debt, and Note 1011, Related-Party Agreements, of the “Notes to Consolidated Financial Statements” that appearappears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for a more detailed discussioninformation regarding our related-party agreements. Components of our Results of Operations
Revenue
From inception through the date of this Annual Report, we have generated minimal revenue from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables, and grant programs. We have no clinical products approved for commercial sale and have not generated any revenue from therapeutic and vaccine product candidates that are under development. If we fail to complete the development of our product candidates in a timely manner or fail to obtain regulatory approval for them, we may never be able to generate substantial future revenue.
Operating Expenses
We generally classify our operating expenses into research and development, and selling, general and administrative expenses. Personnel costs, including salaries, benefits, bonuses, and stock-based compensation expense comprise a significant component of our research and development, and selling, general and administrative expense categories. We allocate expenses associated with our facilities and information technology costs between these two categories, primarily based on the nature of each cost.
Research and Development
Research and development expense consists of expenses incurred while performing research and development activities to discover and develop our technology and product candidates. This includes conducting preclinical studies and clinical trials, manufacturing development efforts and activities related to regulatory filingssubmissions for product candidates. We recognize research and development expenses as they are incurred.
Our research and development expenses primarily consist of:
•clinical trial and regulatory-related costs;
•expenses incurred under agreements with investigative sites and consultants that conduct our clinical trials;
•expenses incurred under collaborative agreements;
•manufacturing and testing costs and related supplies and materials;
•employee-related expenses, including salaries, benefits, travel and stock-based compensation; and
•facility expenses dedicated to research and development.
The company classifies its research and development expenses as either external or internal. The company’s external research and development expenses support its various preclinical and clinical programs. The company’s internal research and development expenses include payroll and benefits expenses, facilities and equipment expense, and other indirect research and development expenses incurred in support of its research and development activities. The company’s external and internal resources are not directly tied to any one research or drug discovery program and are typically deployed across multiple programs and are not allocated to specific product candidates or development programs.
We expect our research and development expense to continueexpenses to increase significantly for the foreseeable future as we advancecontinue to invest in research and development activities related to developing our product candidates through clinical development, including the conduct ofand conducting our ongoing and any futureplanned clinical trials.trials.
The process of conducting clinical trials necessary to obtain regulatory approval is costly and time consuming. The successful development of product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing or costs required to complete the remaining development of any product candidates. This is due to the numerous risks and uncertainties associated with the development of product candidates.
The costs of clinical trials may vary significantly over the life of a project owing to, but not limited to, the following:
•per patient trial costs;
•the number of sites included in the clinical trials;
•the countries in which the clinical trials are conducted;
•the length of time required to enroll eligible patients;
•the number of patients that participate in the clinical trials;
•the number of doses that patients receive;
•the cost of comparative agents used in clinical trials;
•the drop-out or discontinuation rates of patients;
•potential additional safety monitoring or other studies requested by regulatory agencies;
•the duration of patient follow-up; and
•the safety profile and efficacy of the product candidate.
We have only one product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, for which we submitted a BLA to the FDA in May 2022. In JulyOn May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. On October 23, 2023, we announced that we had completed the resubmission of the BLA addressing the issues in the CRL. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a
complete response to the CRL. The FDA has set a target PDUFA actionnew user fee goal date (PDUFA date) of MayApril 23, 2023. However, there2024. There can be no assurance that our product candidate will be approved for commercial sale by the target PDUFA action date,FDA in the near term, if ever. We do not expect any of our other product candidates to be commercially available for the foreseeable future, if ever.
Selling, General and Administrative
Selling, general and administrative expense consists primarily of salaries and personnel-related costs, including employee benefits and any stock-based compensation, for employees performing functions other than research and development. This includes personnel in executive, finance, human resources, information technology, legal, and administrative support functions. Other selling, general and administrative expenses include facility-related costs not otherwise allocated to research and development expense, professional fees for auditing, tax and legal services, advertising costs, expenses associated with strategic business transactions and business development efforts, obtaining and maintaining patents, consulting costs, royalties and licensing costs, and costs of our information systems.
We expect that our selling, general and administrative expense will increase for the foreseeable future as we expand operations, build out information systems and increase our headcount to support continued research activities and the development of our clinical programs. We have incurred and expect that we will continue to incur in the future, additional costs associated with operating as a public company, including costs to comply with stock exchange listing and SEC requirements, future funding efforts, corporate governance, internal controls, investor relations, disclosure and similar requirements applicable to public companies. Additionally, if and when we believe that a regulatory approval of a product candidate appears likely, we expect to incur significant increases in our selling, general and administrative expense relating to the sales and marketing of the approved product candidate.
Other Income and Expense, Net
Other income and expense, net consists primarily of interest and investment income, interest expense (including amortization of debt discounts), unrealized gains and losses onfrom investments in equity securities and equity-method investments, changes in fair value of warrants, derivative liabilities, and a convertible note, realized gains and losses on both debt and equity securities, and gains and losses on foreign currency transactions.
Income Taxes
We are subject to U.S. federal income tax, as well as income tax in Italy, South Korea, California and other states. From inception through December 31, 2022,2023, we have not been required to pay U.S. federal and state income taxes because of current losses from operations.and accumulated NOLs.
Discussion of Consolidated Results of Operations
Comparison of the Years Ended December 31, 20222023 and 20212022
| | | Year Ended December 31, | |
| | 2023 | |
| | 2023 | |
| | 2023 | | | 2022 | | $ Change | | % Change |
| | Year Ended December 31, | |
| | ($ in thousands) | |
| | ($ in thousands) | |
| | ($ in thousands) | |
| | 2022 | | 2021 | | $ Change | | % Change |
| Revenue | |
| | ($ in thousands) | |
| Revenue | |
| | Revenue | Revenue | | $ | 240 | | | $ | 934 | | | $ | (694) | | | (74) | % | | $ | 622 | | | $ | | $ | 240 | | | $ | | $ | 382 | | | 159 | | 159 | % |
| Operating expenses: | Operating expenses: | |
Research and development (including amounts
with related parties) | |
Research and development (including amounts
with related parties) | |
Research and development (including amounts
with related parties) | Research and development (including amounts
with related parties) | | 248,149 | | | 195,958 | | | 52,191 | | | 27 | % | | 232,366 | | | 248,149 | | 248,149 | | | (15,783) | | (15,783) | | | (6) | | (6) | % |
Selling, general and administrative (including amounts
with related parties) | Selling, general and administrative (including amounts
with related parties) | | 102,708 | | | 135,256 | | | (32,548) | | | (24) | % | Selling, general and administrative (including amounts
with related parties) | | 129,620 | | | 102,708 | | 102,708 | | | 26,912 | | 26,912 | | | 26 | | 26 | % |
| Impairment of intangible assets | Impairment of intangible assets | | 681 | | | — | | | 681 | | | — | % | Impairment of intangible assets | | 886 | | | 681 | | 681 | | | 205 | | 205 | | | 30 | | 30 | % |
| Total operating expenses | Total operating expenses | | 351,538 | | | 331,214 | | | 20,324 | | | 6 | % | Total operating expenses | | 362,872 | | | 351,538 | | 351,538 | | | 11,334 | | 11,334 | | | 3 | | 3 | % |
| Loss from operations | Loss from operations | | (351,298) | | | (330,280) | | | (21,018) | | | 6 | % | Loss from operations | | (362,250) | | | (351,298) | | (351,298) | | | (10,952) | | (10,952) | | | 3 | | 3 | % |
| Other expense, net: | Other expense, net: | |
| Interest and investment (loss) income, net | | (3,090) | | | (4,100) | | | 1,010 | | | (25) | % |
| Interest and investment income (loss), net | |
| Interest and investment income (loss), net | |
| Interest and investment income (loss), net | | | 1,131 | | | (3,090) | | | 4,221 | | | (137) | % |
| Interest expense (including amounts with related parties) | Interest expense (including amounts with related parties) | | (63,515) | | | (14,849) | | | (48,666) | | | 328 | % | Interest expense (including amounts with related parties) | | (129,198) | | | (63,515) | | (63,515) | | | (65,683) | | (65,683) | | | 103 | | 103 | % |
| Loss on equity method investment | Loss on equity method investment | | (12,107) | | | (803) | | | (11,304) | | | 1408 | % | Loss on equity method investment | | (7,549) | | | (12,107) | | (12,107) | | | 4,558 | | 4,558 | | | (38) | | (38) | % |
| Change in fair value of warrant liability | | 13,460 | | | — | | | 13,460 | | | — | % |
Other (loss) income, net (including amounts
with related parties) | | (736) | | | 193 | | | (929) | | | (481) | % |
| Change in fair value of warrant liabilities | | Change in fair value of warrant liabilities | | (47,600) | | | 13,460 | | | (61,060) | | | (454) | % |
| Change in fair value of convertible note | | Change in fair value of convertible note | | (36,203) | | | — | | | (36,203) | | | — | % |
Other expense, net (including amounts
with related parties) | | Other expense, net (including amounts
with related parties) | | (2,223) | | | (736) | | | (1,487) | | | 202 | % |
| Total other expense, net | Total other expense, net | | (65,988) | | | (19,559) | | | (46,429) | | | 237 | % | Total other expense, net | | (221,642) | | | (65,988) | | (65,988) | | | (155,654) | | (155,654) | | | 236 | | 236 | % |
| Loss before income taxes and noncontrolling interests | Loss before income taxes and noncontrolling interests | | (417,286) | | | (349,839) | | | (67,447) | | | 19 | % | Loss before income taxes and noncontrolling interests | | (583,892) | | | (417,286) | | (417,286) | | | (166,606) | | (166,606) | | | 40 | | 40 | % |
| Income tax (expense) benefit | | (34) | | | (9) | | | (25) | | | 278 | % |
| Income tax benefit (expense) | | Income tax benefit (expense) | | 40 | | | (34) | | | 74 | | | (218) | % |
| Net loss | Net loss | | $ | (417,320) | | | $ | (349,848) | | | $ | (67,472) | | | 19 | % | Net loss | | $ | (583,852) | | | $ | | $ | (417,320) | | | $ | | $ | (166,532) | | | 40 | | 40 | % |
Revenue
Revenue decreased $0.7increased $0.4 million forduring the year ended December 31, 2022,2023, as compared to the year ended December 31, 2021.2022. The decreaseincrease was primarily driven by less bioreactor and royaltyhigher grant revenue received in 2022.2023.
Research and Development Expense
Research and development expenses were $248.1expense decreased $15.8 million forduring the year ended December 31, 2022,2023, as compared to $196.0 million for the year ended December 31, 2021.2022. The following table summarizes our research and development expenses forduring the years ended December 31, 20222023 and 2021,2022, together with the changes in those items (in thousands):
| | Year Ended December 31, | |
| 2022 | | 2021 | | $ Change |
| | Year Ended December 31, | |
| | 2023 | |
| | 2023 | |
| | 2023 | | | 2022 | | $ Change |
| | External research and development expenses | External research and development expenses | | $ | 59,993 | | | $ | 36,985 | | | $ | 23,008 | |
| External research and development expenses | |
| External research and development expenses | |
| Internal research and development expenses: | Internal research and development expenses: | | — | |
| Personnel-related costs | |
| Personnel-related costs | |
| Personnel-related costs | Personnel-related costs | | 96,357 | | | 82,546 | | | 13,811 | |
| Equipment, depreciation, and facility costs | Equipment, depreciation, and facility costs | | 53,920 | | | 35,631 | | | 18,289 | |
| Other research and development costs | Other research and development costs | | 37,879 | | | 40,796 | | | (2,917) | |
| Total internal research and development expenses | Total internal research and development expenses | | 188,156 | | | 158,973 | | | 29,183 | |
| Total research and development expenses | Total research and development expenses | | $ | 248,149 | | | $ | 195,958 | | | $ | 52,191 | |
Research and development expense increased $52.2decreased $15.8 million primarily attributable to the following:
•A $23.0$5.1 million increase in external research and development expenses that was primarily due to a rise in CMOhigher qualification and testing costs, pre-licensing inspection costs, and outside consulting fees and drug materials purchased used in manufacturing, an increase inrelating to regulatory and compliance costs resulting fromservices for the resubmission of the BLA, partially offset by a decrease in clinical trial costs and initial BLA submission for our product candidate Anktiva, and higher equipment validation and qualification costs;fees;
•A $13.8$7.3 million increasedecrease in personnel-related costs that was primarily due to the resultwrite off of higheraccrued discretionary bonuses for 2022 not paid and a reduction in headcount, additions for personnel involvedpartially offset by an increase in our quality control, clinical operations and drug discovery and development activities;stock-based compensation costs associated with new retention awards granted to existing employees;
•An $18.3A $2.1 million increasedecrease in equipment, depreciation, and facility costs that was primarily due to a reduction in activities related to facility expansion that occurred in the expansion of our manufacturing facilities in California and New York,prior year, which resulted in increases in lease expense,lower expenditures for equipment qualification and certification, as well as lower expenses for sanitary services, partially offset by higher equipment maintenance costs, and depreciation expense;costs; and
•A $2.9An $11.5 million decrease in other research and development costs, that was primarily attributable to greater nonclinical collaboration expensesreductions in laboratory and test supplies and material supplies used for internal manufacturing, and no impairment costs from long-lived assets in the current periodyear compared to the prior year, as well as an increase in collaboration costs allocated to oura joint venture,venture. Additionally, the write down of right-of-use assets from a lease used for R&D contributed to an additional decrease in other R&D expenses. The reduction in costs were partially offset by an increase in applicationhigher R&D activities and increased costs associated with submitting the BLA for our product candidate Anktiva, an increase in impairment costs associated with a group of long-lived assets, and an increase in license fees.related to R&D licenses.
We expect our research and development expenses to increase significantly for the foreseeable future as we continue to invest in research and development activities related to developing our product candidates and conductconducting our ongoing and planned clinical trials.trials.
Selling, General and Administrative Expense
Selling, general and administrative expense decreased $32.5increased $26.9 million forduring the year ended December 31, 2022,2023, as compared to the year ended December 31, 2021. 2022. The reductionrise in selling, general and administrative expenseexpenses was primarily driven bydue to a $29.5$28.9 million net declineincrease in legal expenses, which was driven by higher defense costs and general legal services, as a result of greater well as fewer legal insurance reimbursements received in the current period that offset current-period legal expenses, as well as $11.0 million in lower general consulting costs and insurance costs primarily duecompared to Merger-related expenses incurred in the prior period.year. Additionally, there was a $1.1 million increase in personnel-related costs resulting from higher stock-based compensation expenses and travel costs, a $0.6 million increase in IT and security license fees, and a $0.2 million increase in marketing costs for pre-commercialization activities. These decreasesincreases were partially offset by an increase of $4.7a $2.7 million reduction in personnel costs, including sharedconsulting services costsfrom ERP implementations and stock compensation costs asprofessional fees from accounting and tax services, and a result of a rise$1.2 million reduction in headcount, recruiting costs and travel-relatedinsurance expenses as well as an increase of $1.8 million in software license fees and an increase of $1.5 million in facility costs and other general administrative expenses.operating costs.
Impairment of Intangible Assets
Impairment of intangible assets increased $0.7$0.2 million forduring the year ended December 31, 2022,2023, compared to the year ended December 31, 2021,2022, due to an impairment charge of $0.9 million related to indefinite-lived intangible assets associated with the Tarmogen platform as the research and development program was limited in the current year. The $0.7 million write off of the organized workforce with definite lives during 2022 was as a result of the workforce reduction at the Dunkirk Facility.
Other Expense, Net
Other expense, net increased $46.4$155.7 million during the year ended December 31, 2022,2023, as compared to the year ended December 31, 2021. The increase was2022, due to a $48.7$65.7 million increase in interest expense driven by higher related-party borrowings, an increase in relatedthe‑party borrowings alongTerm SOFR rate, and higher amortization of related-party notes discounts. Additionally, there was a total increase in expense of $97.3 million driven by the change in the fair value of warrant liabilities and the change in the fair value of a related-party convertible note, and a $1.5 million increase in expense due to higher issuance costs associated with our registered direct offerings, and an increase in SOFR rates and amortization of debt discounts related to a gain on debt extinguishments, as well as an $11.3 million loss on our equity method investment in a joint venture, and an additional $0.9 million increase in other costs as a result of issuance costs allocated to the warrant liability.expenses. These increaseschanges were partially offset by a $13.5$4.6 million decrease in the fair value of our warrant liabilitylower loss on equity method investments and a $1.0$4.2 million decreaseincrease in net interest and investment losses as a resultincome.
Comparison of the Years Ended December 31, 20212022 to 20202021
See Part II, Item 7,7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Results of Operations” of our Annual Report on Form 10-K filed with the SEC on March 1, 20222023 for a discussion of the company’s results of operations forduring the year ended December 31, 20212022 compared to the year ended December 31, 2020.2021.
Financial Condition, Liquidity and Capital Resources
Sources of Liquidity
Our principal sourcesSince our inception, we have funded our operations primarily through proceeds from the issuance of liquidity arerelated-party promissory notes and sales of our existingcommon stock under the ATM and our shelf registration statement, and through registered direct offerings.
On December 29, 2023, we entered into the RIPA to support the commercialization of Anktiva for the treatment of BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, the further development of our other product platforms, and to provide for other working capital needs.
Cash and Marketable Securities on Hand
As of December 31, 2023, we had cash and cash equivalents, and marketable securities.securities of $267.4 million compared to $108.0 million as of December 31, 2022. We have historicallytypically invested our cash primarily in investment grade short- to intermediate-term corporate debt securities, commercial paper, government-sponsored securities, U.S. treasury securities,a variety of financial instruments and foreign government bonds and classifyclassified these investments as available-for-sale. However, after our entry into the RIPA we can no longer invest our excess funds in corporate or European bonds. Certain of theseour investments are subject to general credit, liquidity, market, and other marketinterest-rate risks. The general condition of the financial markets and the economy may increase those risks and may affect the value and liquidity of investments and restrict our ability to access the capital markets.
Cash and Marketable Securities on Hand
As of December 31, 2022, we had cash and cash equivalents, and marketable securities of $108.0 million compared to $317.9 million as of December 31, 2021. On April 30, 2021, we entered into an open market sale agreement (the Sale Agreement) with respect to an ATM offering program under which we may offer and sell, from time to time at our sole discretion, shares of our common stock, having an aggregate offering price of up to $500.0 million through our sales agent, which was subsequently reduced by $92.0 million during December 2022 in connection with a sale of our common stock. As of December 31, 2022, we had $225.4 million available for future stock issuances under the ATM. See Note 12, Stockholders’ Deficit, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report.Proceeds from the ATM
ForDuring the year ended December 31, 2022,2023, we received net proceeds totaling $13.1of approximately $16.1 million from the issuance of 2,051,8945,605,323 shares under the ATM. We currently intend to use the net proceeds from this offering for pre-commercialization efforts and clinical development programs, other research and development activities, capital expenditures, and other general corporate purposes.
Proceeds from Registered Direct Offerings
On December 12, 2022, we entered into a securities purchase agreement with an institutional investor for the sale of 9,090,909 shares of our common stock, as well as warrants to purchase an additional 9,090,909 shares of common stock at an exercise price of $6.60 per share, for a purchase price of $5.50 per share and accompanying warrant, generating net proceeds of approximately $47.0 million, after deducting placement agent fees and other offering costs. We currently intend to useused the net proceeds from this offering, together with other available funds, to progress our regulatory approval efforts, pre-commercialization effortsactivities and clinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes. As of December 31, 2023, we had $208.8 million available for future stock issuances under the ATM.
Shelf Registration Statement
During February 2023, we filed a $750.0 million shelf registration statement with the SEC on Form S-3 for the offering and sale of equity and equity-linked securities, including common stock, preferred stock, debt securities, depositary shares, warrants to purchase common stock, preferred stock or debt securities, subscription rights, purchase contracts, and units. During the year ended December 31, 2023, we sold shares of our common stock and warrants valued at $184.4 million under the shelf. As of December 31, 2023, we had $565.6 million available for use under the shelf. This available shelf is in addition to the ATM.
Proceeds from Registered Direct Offerings
On February 15, 2023, we entered into a securities purchase agreement with certain institutional investors for the purchase and sale of 14,072,615 shares of our common stock, as well as warrants (the February 2023 Warrants) that allow such investors to purchase an additional 14,072,615 shares of common stock at an exercise price of $4.2636 per share, for a purchase price of $3.5530 per share and accompanying warrant, generatingwarrant. This transaction generated net proceeds of approximately $47.0 million, after deducting placement agent fees and other estimated offering costs. The closingPursuant to the terms of a letter agreement dated July 25, 2023, the company and the investors agreed to reduce the exercise price of the outstanding February 2023 Warrants from $4.2636 per share to $3.2946 per share and extend the expiration date of the warrants until July 24, 2026.
On July 20, 2023, we entered into a securities purchase agreement with certain institutional investors for the purchase and sale of 14,569,296 shares of our common stock, as well as warrants that allow such investors to purchase an additional 14,569,296 shares of common stock at an exercise price of $3.2946 per share, for a purchase price of $2.7455 per share and accompanying warrant. This transaction generated net proceeds of approximately $37.5 million, after deducting placement agent fees and other offering occurred on February 17, 2023. costs.
We currently intend to useused the net proceeds from this offering,these registered direct offerings, together with other available funds, to progress our regulatory approval efforts, pre-commercialization effortsactivities and clinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes. We may also use
Related-Party Promissory Notes
On September 11, 2023, the company entered into a portionstock purchase agreement with Nant Capital, NantMobile and NCSC pursuant to which the noteholders exchanged promissory notes totaling approximately $270.0 million in aggregate principal amount and accrued and unpaid interest for an aggregate of 209,291,936 shares of common stock at an exchange price of $1.29 per share. As a result of the net proceedsexchange, the company was forever released and discharged from all its obligations and liabilities under the notes.
On September 11, 2023, the company and Nant Capital entered into a series of letter agreements pursuant to license intellectual propertywhich the maturity date of the related-party nonconvertible notes described above totaling $505.0 million in principal was extended from December 31, 2023 to December 31, 2024. In addition, the company entered into a $200.0 million September 2023 promissory note with Nant Capital.
On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into an amended and restated promissory note and a letter amendment for the following outstanding promissory notes. Pursuant to the terms of the amended and restated promissory note, the amended $505.0 million December 2023 promissory note is comprised of a Tranche 1 principal amount of $125.0 million, which was previously the $125.0 million August 2022 promissory note before amendment, and a Tranche 2 with principal amount of $380.0 million, which is made up of the previous $300.0 million December 2021 promissory note, $50.0 million December 2022 promissory note, and $30.0 million June 2023 promissory note. In addition, the amendment allows Nant Capital, in its sole discretion, to convert all the Tranche 2 principal amount of $380.0 million of the amended promissory note into shares of the company’s common stock. The conversion price was determined subsequently at $8.2690 per share based on the agreement. The maturity date of the amended promissory note is December 31, 2025. Pursuant to the terms of the letter amendment, the maturity date of the $30.0 million March 2023 promissory note was extended from December 31, 2024 to December 31, 2025. Also, in connection with the RIPA transaction, all outstanding related-party promissory notes became subordinated to the RIPA payment obligations. After the amendment, the interest rates for the Tranche 1 principal amount and Tranche 2 principal amount of the $505.0 million December 2023 promissory note were Term SOFR plus 8.0% and Term SOFR plus 7.5%, respectively.
See Note 10, Related-Party Debt, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding our related-party debt. Revenue Interest Purchase Agreement
On December 29, 2023, we entered into the RIPA with Infinity and Oberland. Pursuant to the RIPA, Oberland acquired certain Revenue Interests from us for a gross purchase price of $200.0 million paid on closing, less certain transaction expenses. In addition, Oberland may purchase additional Revenue Interests from us in exchange for the $100.0 million Second Payment upon satisfaction of certain conditions specified in the RIPA, including following the receipt of approval by the FDA of the company’s BLA for N-803 in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or
without Ta or T1 disease on or before June 30, 2024. Under the RIPA, Oberland has the right to
make acquisitions or investments. See “Subsequent Event” for further information. In order to complete the developmentreceive quarterly payments from us based on, among other things, a certain percentage of our current product candidates,worldwide net sales, excluding those in China, during such quarter.
Also, on December 29, 2023 and implementin connection with the RIPA, we entered into an SPOA with Oberland pursuant to which we sold an aggregate of approximately $10.0 million of our business plan, we will require substantialcommon stock at $4.1103 per share in a private placement. Oberland also has an option to purchase up to an additional funding. Furthermore, changing circumstances may cause us$10.0 million of our common stock, at a price per share to increasebe determined by reference to the 30-day trailing volume weighted-average price of our spending significantly faster than we currently anticipate,common stock calculated from the date of exercise.
For more information, see Note9, Revenue Interest Purchase Agreement, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and we may need to raise even greater amountsSupplementary Data” of funds sooner if we choose to expand more rapidly than we presently anticipate. Moreover, our fixed expenses such as rent and other contractual commitments are substantial and are expected to increase in the future.this Annual Report.
Uses of Liquidity
In addition to the cash used to fund our operating activities discussed in “—Future Funding Requirements” below, we will require cash to settle the following obligations: •As of December 31, 2022,2023, our indebtedness payable at maturity totals $737.4totaled $735.0 million (excluding unamortized related-party notes discounts), held by entities affiliated with Dr. Soon-Shiong.
Of this amount, $475.0 In connection with the RIPA, all of our related-party promissory notes are now general unsecured obligations of the company that are subordinated in right of payment to indebtedness, obligations and other liabilities under the RIPA, the Revenue Interests issued pursuant to such agreement, and refinancing of the foregoing. In addition, the terms of promissory notes totaling $535.0 million is duewere amended and payable onrestated to extend the maturity date by one year to December 31, 2023. In2025. The remaining $200.0 million promissory note and any accrued and unpaid interest are due on the earlier of (i) September 11, 2026 or (ii) upon the occurrence and during the continuance of an event of a default on the $300.0 million loan (as defined in the note).
◦Pursuant to the terms of the amended and restated promissory note), including if we do not repaynote, the loanamended $505.0 million December 2023 promissory note is comprised of the Tranche 1 principal amount of $125.0 million and the Tranche 2 principal amount of $380.0 million. All Tranche 2 principal amount of $380.0 million and any accrued and unpaid interest can be converted into shares of the company’s stock at maturity,$8.2690 per share at the companyoption of the noteholder. The interest rates for the Tranche 1 principal amount and Tranche 2 principal amount of the $505.0 million December 2023 promissory note were Term SOFR plus 8.0% per annum and Term SOFR plus 7.5% per annum, respectively.
◦The $200.0 million convertible promissory note bears interest at Term SOFR plus 8.0% per annum. The noteholder has the right, at its sole option to convert all of the outstanding principal amount and accrued andbut unpaid interest due under this note into shares of the company’s common stock at a conversion price of $5.67$1.9350 per share.
◦The $30.0 million convertible promissory note bears interest at Term SOFR plus 8.0% per annum. The noteholder has the sole option to convert all of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $2.28 per share.
There can be no assurance that the company can refinance these promissory notes or what terms will be available in the market at the time of refinancing. Furthermore, if prevailing interest rates or other factors at the time of refinancing result in higher interest rates upon refinancing, then the interest expense relating to the refinanced indebtedness would increase. These risks could materially adversely affect the company’s financial condition, cash flows and results of operations.
The remaining $262.4 million is due•On December 29, 2023, we entered into the RIPA with Infinity and payableOberland. Oberland has the right to receive quarterly Revenue Interest Payments from us based on, September 30, 2025, including any accrued and unpaid interest. The company can prepay the outstanding principal (together with accrued and unpaid interest),among other things, our worldwide net sales, excluding those in whole or in part, at any time without premium or penalty and without the prior consentChina, which will be tiered payments initially ranging from 3.00% to 7.00% (or after funding of the lender. On AugustSecond Payment, 4.50% to 10.00%), subject to increase or decrease, following December 31, 2022, the terms of each fixed-rate promissory note were amended and restated2029 (the Test Date) depending on whether our aggregate payments made to include a conversion feature that gives each lender the right at any time, including upon notice of prepayment, at its sole option, to convert the entire outstanding principal amount and accrued and unpaid interest due under each note at the time of conversion into sharesOberland as of the company’s common stock atTest Date have met or exceeded the Cumulative Purchaser Payments. In addition, if our aggregate payments made as of the Test Date to Oberland do not equal or exceed the amount of the Cumulative Purchaser Payments as of such date, then we are obligated to make a priceone-time True-Up Payment to Oberland in an amount equal to 100% of $5.67 per share.the Cumulative Purchaser Payments as of the Test Date, less the aggregate amount of our previous payments to Oberland as of the Test Date. See Note 9, Revenue Interest Purchase Agreement, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding the RIPA.•In connection with our 2017 acquisition of Altor, we issued CVRs under which we agreed to pay the prior stockholders of Altor approximately $304.0 million of contingent consideration upon the successful regulatory approval of a BLA by the FDA, or foreign equivalent, for N-803 by December 31, 2022, and approximately $304.0 million of contingent consideration upon calendar-year worldwide net sales of N-803 exceeding $1.0 billion prior to December 31, 2026, with amounts payable in cash or shares of our common stock or a combination thereof.
With respect to the regulatory milestone CVR agreement, in May 2022 we announced the submission of a BLA to the FDA for our product candidate, Anktiva (N-803) in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In July 2022 we announced that the FDA had accepted our BLA for review and set a target PDUFA action date of May 23, 2023. It is unclear when the FDA will approve our BLA, if at all. The FDA did not approve our BLA on or before December 31, 2022, and therefore the regulatory milestone was not met, and the regulatory milestone CVR agreement terminated in accordance with its terms.
With respect to the net sales milestone CVR agreement, as As of December 31, 2022,2023, Dr. Soon-Shiong and his related party hold approximately $139.8 million of net sales CVRs and they have both irrevocably agreed to receive shares of the company’s common stock in satisfaction of their CVRs. We may be required to pay the other prior Altor stockholders up to $164.2 million for their net sales CVRs should they choose to have their CVRs paid in cash instead of common stock. We may need to seek additional sources of capital to satisfy the CVR obligations if they are achieved.
•In connection with our acquisition of VivaBioCell, we are obligated to pay the former owners approximately $2.1$2.2 million of contingent consideration upon the achievement of a regulatory milestone relating to the GMP-in-a-Box technology.
Discussion of Consolidated Cash Flows
The following discussion of ImmunityBio’s cash flows is based on the consolidated statements of cash flows in Part II, Item 8. “Financial Statements and Supplementary Data” and is not meant to be an all‑inclusive discussion of the changes in its cash flows for the periodsyears presented below.
The following table sets forth our primary sources and uses of cash for the years indicated (in thousands):
| | | Year Ended December 31, | | | | Year Ended December 31, |
| | 2023 | | | | 2023 | | 2022 |
| | Year Ended December 31, |
| 2022 | | 2021 |
| | Cash (used in) provided by: | |
| Cash provided by (used in): | |
| Cash provided by (used in): | |
| Cash provided by (used in): | |
| Operating activities | |
| Operating activities | |
| Operating activities | Operating activities | | $ | (337,509) | | | $ | (274,419) | |
| Investing activities | Investing activities | | 27,297 | | | (84,886) | |
| Financing activities | Financing activities | | 233,613 | | | 505,443 | |
Effects of exchange rate changes on cash, cash equivalents,
and restricted cash | Effects of exchange rate changes on cash, cash equivalents,
and restricted cash | | 284 | | | 48 | |
| Net change in cash, cash equivalents, and restricted cash | Net change in cash, cash equivalents, and restricted cash | | $ | (76,315) | | | $ | 146,186 | |
Operating Activities
ForDuring the year ended December 31, 2023, net cash used in operating activities of $366.8 million consisted of a net loss of $583.9 million, partially offset by $213.1 million in adjustments for non-cash items and $3.9 million of cash provided by net working capital. Adjustments for non-cash items primarily consisted of $49.2 million in stock-based compensation expense, $47.6 million in change in fair value of warrant liabilities, $42.4 million in amortization of debt issuance costs and accretion of discounts, $36.2 million in change in fair value of convertible notes, $18.5 million in depreciation and amortization expense, $9.2 million in non-cash interest primarily related to related-party promissory notes, $6.1 million in non-cash lease expense related to operating lease right‑of‑use assets, $2.0 million of transaction costs allocable to warrant liabilities, $1.6 million in unrealized losses on equity securities driven by a decrease in the value of our investments, $0.9 million in loss on impairment of intangible assets, partially offset by $0.5 million in other items, and a decrease of $0.1 million in amortization of premiums, net of discounts on marketable debt securities. The changes in net working capital consisted primarily of an increase of $6.7 million in accrued expenses, a decrease of $5.9 million in prepaid expenses and other current assets, and a decrease of $1.9 million in other assets, partially offset by a decrease of $6.5 million in accounts payable, a decrease of $3.0 million in operating lease liabilities, and a decrease of $1.1 million in due to related parties.
During the year ended December 31, 2022, net cash used in operating activities of $337.5 million consisted of a net loss of $417.3 million and $8.0 million of cash used in net working capital, partially offset by $87.8 million in adjustments for non-cash items. The changes in net working capital consisted primarily of an increase of $16.6 million in prepaid expenses and other current assets, and decreases of $4.3 million in operating lease liabilities and $1.2 million with related parties, partially offset by an increase of $8.0 million in accounts payable, an increase of $4.1 million in accrued expenses and other liabilities, and a decrease of $2.0 million in investment and other assets. Adjustments for non-cash items primarily consisted of $40.2 million in stock-based compensation expense, $18.3 million in depreciation and amortization expense, $16.3 million in amortization of debt issuance costs and accretion of discounts, $11.7 million in non-cash interest primarily related to related-party promissory notes, $5.9 million in non-cashnon‑cash lease expense related to operating lease right‑of‑useright-of-use assets, $4.2 million in unrealized losslosses on equity securities driven by a decrease in the value of our investments, $1.3 million in loss on impairment of fixed assets, $1.3 million in amortization of premiums, net of discounts, on marketable debt securities, $1.1 million in transaction costs allocable to a warrant liability, a non-cash $0.7 million loss on the impairment of intangible assets, and $0.3 million in other non-cash items, reduced by a $13.5 million change in fair value of a warrant liability.
For the year ended December 31, 2021, net cash used in operating activitiesTable of $274.4 million consisted of a net loss of $349.8 million and $19.1 million of cash used in net working capital, partially offset by $94.5 million in adjustments for non-cash items. The change in net working capital consisted primarily of decreases of $10.2 million with related parties and $4.2 million in operating lease liabilities, an increase of $4.0 million in investment and other assets, a decrease of $3.7 million in accounts payable, and an increase of $2.2 million in prepaid and other current assets, partially offset by an increase of $5.2 million in accrued expenses and other liabilities. Adjustments for non-cash items primarily consisted of $57.2 million in stock-based compensation expense, $14.2 million in depreciation and amortization, $12.4 million in non-cash interest primarily related to related-party loans, $4.9 million in nonContents‑cash lease expense related to operating lease right-of-use assets, $4.6 million in unrealized losses on equity securities driven primarily by a decrease in the value of our investments, $0.8 million in other non-cash items, and $0.4 million in amortization of premiums, net of discounts, on marketable debt securities.We have historically experienced negative cash flows from operating activities, with such negative cash flows likely to continue for the foreseeable future.
Investing Activities
ForDuring the year ended December 31, 2023, net cash used in investing activities was $30.5 million, which included cash outflows of $30.6 million in purchases of property, plant and equipment (including construction in progress and the completion of a warehouse facility), and $10.4 million in purchases of marketable debt securities, partially offset by cash inflows of $10.5 million from maturities and sales of marketable debt and equity securities. Our investments in property, plant and equipment are primarily related to acquisitions of equipment that will be used for the manufacturing of our product candidates and expenditures related to the build out of our manufacturing facilities.
During the year ended December 31, 2022, net cash provided by investing activities was $27.3 million, which included cash inflows of $162.0 million from maturities and sales of marketable debt and equity securities, partially offset by $78.2 million of purchases of property, plant and equipment (including construction in processprogress and depreciable property acquired in the Dunkirk acquisition), $34.3 million of purchases of marketable debt securities, $21.2 million for purchase of intangible assets (related to the Dunkirk acquisition), and a $1.0 million investment in a joint venture. Our investments in property, plant and equipment are primarily related to acquisitions of equipment that will be used for the manufacturing of our product candidates and expenditures related to the build out of our manufacturing facilities.
For the year ended December 31, 2021, net cash used in investing activities was $84.9 million, which included $141.8 million in purchases of marketable debt securities and $33.6 million of purchases of property, plant and equipment, partially offset by cash inflows of $70.0 million from maturities and sales of marketable debt and equity securities and $20.5 million in proceeds from the sale of 557 Doug St, LLC that were allocated to the book value of the property. Our investments in property, plant and equipment are primarily related to acquisitions of equipment that will be used for the manufacturing of our product candidates and expenditures related to the build out of our manufacturing facilities.
We expect to accelerate our capital spending as we scale our GMPcGMP manufacturing capabilities, which will require significant capital for the foreseeable future.
Financing Activities
ForDuring the year ended December 31, 2023, net cash provided by financing activities was $558.3 million, which consisted of $258.7 million in net proceeds from issuances of related-party promissory notes, $192.8 million in net proceeds from payments received pursuant to the RIPA, $84.4 million in net proceeds from registered direct offerings, $16.1 million in net proceeds from the ATM offering, $9.5 million in net proceeds from issuance of common stock in connection with the RIPA, and $0.3 million in proceeds from the exercise of stock options, partially offset by $3.4 million used for payment of payroll tax withholding in connection with the net share settlement of vested RSUs, and $0.1 million used for principal payments of finance leases.
During the year ended December 31, 2022, net cash provided by financing activities was $233.6 million, which consisted of $174.1 million in net proceeds from issuances of related-party promissory notes, $47.3 million in net proceeds from a registered direct offering, and $13.1 million in net proceeds from the ATM offering, partially offset by $0.6 million related to net share settlement of vested RSUs for payment of payroll tax withholding and a $0.3 million used in other financing activities.
For the year ended December 31, 2021, netWe will need to raise additional capital to fund our future cash provided by financing activities was $505.4 million, which consisted of $338.5 million in net proceeds from issuances of related-party promissory notes, $164.5 million in net proceeds from the ATM offering, $5.5 million in proceeds from exercises of stock options and a $1.4 million capital contribution representing excess proceeds received over book value from the sale of 557 Doug St, LLC to an entity under common control. Net cash used in financing activities consisted of $4.1 million related to net share settlement of vested RSUs for payment of payroll tax withholding and a $0.4 million payment for contingent consideration.needs.
Comparison of the Years Ended December 31, 20212022 to 20202021
See Part II, Item 7,7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Results of Operations” of our Annual Report on Form 10-K filed with the SEC on March 1, 20222023 for a discussion of the company’s consolidated cash flows for the year ended December 31, 20212022 compared to the year ended December 31, 2020.2021.
Future Funding Requirements
From inception through the date of this Annual Report we have generated minimal revenue, we have no clinical products approved for commercial sale, and we have not generated any revenue from therapeutic and vaccine product candidates that are under development. We do not expect to generate significant revenue unless and until we obtain regulatory approval of and commercialize any of our product candidates, and we do not know when, or if, this will occur. In addition, we expect our operating expenses to significantly increase in connection with our ongoing development activities, particularly as we continue the research, development and clinical trials of, and seek regulatory approval for, our product candidates. We have also incurred and expect that we will continue to incur in the future additional costs associated with operating as a public company as well as costs related to future fundraising efforts. In addition, subject to obtaining regulatory approval of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. We anticipate that we will need substantial additional funding in connection with our continuing operations.
We expect that our operating expenses will increase substantially if and as we:
•continue research and development, including preclinical and clinical development of our existing product candidates;
•potentially seek regulatory approval for our product candidates;
•seek to discover and develop additional product candidates;
•establish a commercialization infrastructure and scale up our manufacturing and distribution capabilities to commercialize any of our product candidates for which we may obtain regulatory approval;
•seek to comply with regulatory standards and laws;
•maintain, leverage and expand our intellectual property portfolio;
•hire clinical, manufacturing, scientific and other personnel to support our product candidates’ development and future commercialization efforts;
•add operational, financial and management information systems and personnel; and
•incur additional legal, accounting and other expenses in operating as a public company.
As a result of continuing anticipated operating cash outflows, we believe that substantial doubt exists regarding our ability to continue as a going concern without additional funding or financial support. However, we believe our existing cash, cash equivalents, and investments in marketable securities, together with capital to be raised through equity offerings (including but not limited to the ATM)offering, issuance and sale by us of our common stock that may be issued and sold under the ATM, of which we had $208.8 million available for future issuance as of December 31, 2023, and a February 2023 shelf registration statement, of which we had $565.6 million available for future offerings as of December 31, 2023), and our potential ability to borrow from affiliated entities, will be sufficient to fund our operations through at least the next 12 months following the issuance date of the consolidated financial statements based primarily upon our Executive Chairman and Global Chief Scientific and Medical Officer’s intent and ability to support our operations with additional funds, including loans from affiliated entities, as required, which we believe alleviates such doubt. We may also seek to sell additional equity, through one or more follow-on public offerings, or in separate financings, or obtain a credit facility.facility, issue other debt in compliance with the terms of the RIPA, or engage in strategic partnership transactions. However, we may not be able to secure such external financing in a timely manner or on favorable terms.terms, if at all. Without additional funds, we may choose to delay or reduce our operating or investment expenditures. Further, because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we may need additional funds to meet our needs sooner than planned.
We will need to obtain additional financing to fund our future operations, including completing the development and commercialization of our product candidates. Changing circumstances may cause us to increase our spending significantly faster than we currently anticipate and we may need to raise additional funds sooner than we presently anticipate. Moreover, research and development and our operating costs and fixed expenses such as rent and other contractual commitments, including those for our research collaborations, are substantial and are expected to increase in the future.
Our future funding requirements will depend on many factors, including, but not limited to:
•progress, timing, number, scope and costs of researching and developing our product candidates and our ongoing, planned and potential clinical trials;
•time and cost of regulatory approvals;
•our ability to successfully commercialize any product candidates, if approved and the costs of such commercialization activities;
•revenue from product candidates that we may commercialize, if any, including the selling prices for such potential products and the availability of adequate third-party coverage and reimbursement for patients;
•interest and principal payments on our related-party promissory notes, and payment of Revenue Interests, the Second Payment and Test Date payments due under the RIPA;
•cost of building, staffing and validating our own manufacturing facilities in the U.S., including having a product candidate successfully manufactured consistent with FDA and European Medicines AgencyEMA regulations;
•terms, timing and costs of our current and any potential future collaborations, business or product acquisitions, CVRs, milestones, royalties, licensing or other arrangements that we have established or may establish;
•time and cost necessary to respond to technological, regulatory, political and market developments; and
•costs of filing, prosecuting, maintaining, defending and enforcing any patent claims and other intellectual property rights.
Unless and until we can generate a sufficient amount of revenues, we may finance future cash needs through public or private equity offerings, license agreements, debt financings, collaborations, strategic alliances and marketing or distribution arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms, or at all, including but not limited to the offering, issuance and sale by us of up to a maximum aggregate amount of $500.0 million of our common stock that may be issued and sold under the ATM, which was subsequently reduced by $92.0 million during December 2022 in connection with a sale of our common stock. As of December 31, 2022, we had $225.4 million available for future stock issuances under the ATM. See Note 12, Stockholders’ Deficit, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report. To the extent that we raise additional capital through the sale of equity or equity-linked securities including(including warrants), convertible debt or throughunder the ATM, our shelf registration statement, or other offerings, or if any of our current debt is converted into equity or if our existing warrants are exercised, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. The incurrence of additional indebtedness would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms unfavorable to us. We have no committed source of additional capital and if we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may be required to delay or reduce the scope of or eliminate one or more of our research or development programs or our commercialization efforts. Our current license and collaboration agreements may also be terminated if we are unable to meet the payment obligations under those agreements. As a result, we may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time.
Contractual Obligations
We have material cash requirements to pay related-party affiliates and third parties under various contractual obligations discussed below:
•We are obligated to make payments to several related-party affiliates under written agreements and other informal arrangements. We are also obligated to pay interest and to repay principal under our related-party notes payable.promissory notes. For information regarding our financing obligations, see Note 910, Related-Party Debt, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report. •We are obligated to make payments to Oberland associated with our revenue interest liability, which do not have a fixed repayment schedule. Oberland’s right to receive payments under the RIPA shall terminate when Oberland has received maximum payments (including any True-Up Payment) equal to 195.0% of the then Cumulative Purchaser Payments unless the RIPA is terminated prior to such date.
Under the terms of the agreement, prior to the Test Date, every $100.0 million of worldwide net sales, excluding those in China, of less than or equal to $600.0 million in a calendar year would result in a tiered Revenue Interest Payment of approximately $7.0 million or 7.0% (or $10.0 million or 10.0% after funding of the Second Payment). Worldwide net sales, excluding those in China, for a calendar year exceeding $600.0 million would result in a tiered Revenue Interest Payment of approximately $3.0 million or 3.0% (or $4.5 million or 4.5% after funding of the Second Payment) for every $100.0 million of worldwide net sales, excluding those in China, above the threshold.
In the future, cumulative worldwide net sales, excluding those in China, levels up to the Test Date will determine whether or not we are required to make a True-Up Payment and implement modified payment rates. The amount of the obligation and timing of payment is likely to change. See Note 9, Revenue Interest Purchase Agreement, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding the RIPA. •We are obligated to make payments under our operating leases, which primarily consist of facility leases. For information regarding our lease obligations, seeSee Note 8, Lease Arrangements, and Note 1011, Related-Party Agreements, of the “Notes to Consolidated Financial Statements” that appear in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report.Report for information regarding our lease obligations. •In connection with the acquisitions of Altor and VivaBioCell, we are obligated to pay contingent consideration upon the achievement of certain milestones. For information regarding our contingent consideration obligations, seeSee Note 7, Commitments and Contingencies—Contingent Consideration Related to Business Combinations, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report.Report for information regarding our contingent consideration obligations. •We have contractual obligations to make payments to related-party affiliates and third parties under unconditional purchase arrangements. For information on these unconditional purchase obligations, seeSee Note 7, Commitments and Contingencies—Unconditional Purchase Obligations, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report.Report for information on these unconditional purchase obligations. •We have certain contractual commitments that are expected to be paid within onein fiscal year 2024, depending on the progress of build outs, completion of services, and the realization of milestones associated with third-party agreements. This amountamounts totals $121.1$60.8 million and is primarily related to capital expenditures, open purchase orders as of December 31, 20222023 for the acquisition of goods and services in the ordinary course of business, and near termnear-term upfront milestone payments to third parties.
•In addition, we have contractual commitments that are expected to be paid in fiscal year 20242025 and beyond based on the achievement of various development, regulatory and commercial milestones for agreements with third parties. These payments may not be realized or may be modified and are contingent upon the occurrence of various future events, substantially all of which have a high degree of uncertainty of occurring. As of December 31, 2022,2023, the maximum amount that may be payable related to these commitments is $780.3$769.3 million.
•In connection with our leasehold interest in the Dunkirk Facility, we committed to spend an aggregate of $1.52 billion on operational expenses during the initial 10-year term, and an additional $1.50 billion on operational expenses if we elect to renew the lease for the additional 10-year term. These amounts are not included in the discussion above. See Note 6, Collaboration and License Agreements and Acquisition, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report.Report for more information on these obligations.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of consolidated financial statements requires management to make certain estimates and assumptions that affect the reported amounts of assets, and liabilities and expenses and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, we evaluate our estimates, including those related to the valuation of equity-based awards, deferred income taxes and related valuation allowances, revenue interest liability, preclinical and clinical trial accruals, impairment assessments, contingent value rightCVR measurement and assessments, the measurement of right-of-use assets and lease liabilities, useful lives of long-lived assets, loss contingencies, fair value calculation of warrants and convertible promissory notes, fair value measurements, asset acquisition, and the assessment of our ability to fund our operations for at least the next 12 months from the date of issuance of these consolidated financial statements. We base our estimates on historical experience and on various other market-specific and relevant assumptions that we believe to be reasonable under the circumstances. Estimates are assessed each period and updated to reflect current information, such as the economic considerations related to the impact that theany ongoing coronavirus pandemic could have on our significant accounting estimates. Actual results could differ from those estimates.
While our significant accounting policies are more fully described in the notes accompanying our consolidated financial statements that appear in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report, we believe the following accounting policies to be the most critical in understanding the judgments and estimates we use in preparing our consolidated financial statements:
Revenue Recognition
We have primarily generated revenues from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables and grant programs. The nonexclusive license agreements with a limited number of pharmaceutical and biotechnology companies grant them the right to use our cell lines and intellectual property for non-clinical use. These agreements generally include upfront fees and annual research license fees for such use, as well as commercial license fees for sales of the licensee products developed or manufactured using our intellectual property and cell lines. We have generated revenues from product sales of our proprietary GMP-in-a-Box bioreactors and related consumables, primarily to related parties. Additionally, we also generated revenues from grant programs.
A performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account in ASC 606.customer. A contract’s transaction price is allocated to each distinct performance obligation based on relative standalone selling price and recognized as revenue when, or as, the performance obligation is satisfied.
Under our license agreements with customers, we typically promise to provide a license to use certain cell lines and related patents, the related know-how, and future research and development data that affect the license. We have concluded that these promises represent one performance obligation due to the highly interrelated nature of the promises. We provide the cell lines and know-how immediately upon entering into the contracts. Research and development data are provided throughout the term of the contract when and if available.
The license agreements may include non-refundable upfront payments, event-based milestone payments, sales-based royalty payments, or some combination of these. The event-based milestone payments represent variable consideration and we use the most likely amount method to estimate this variable consideration. Given the high degree of uncertainly around the achievement of these milestones, we do not recognize revenue from these milestone payments until the uncertainty associated
with these payments is resolved. We currently estimate variable consideration related to milestone payments to be zero and, as such, no revenue has been recognized for milestone payments. We recognize revenue from sales-based royalty payments when or as the sales occur. On a quarterly basis, we re-evaluate our estimate of milestone variable consideration to determine whether any amount should be included in the transaction price and recorded in revenue prospectively.
We also have sold our proprietary GMP-in-a-Box bioreactors and related consumables to affiliated companies. The arrangements typically include delivery of bioreactors, consumables, and providing installation service and perpetual software licenses for using the equipment. We recognize revenue when customers obtain control and can benefit from the promised goods or services, generally upon installation of the bioreactors, in an amount that reflects the consideration that we expect to receive in exchange for those goods or services. Upfront payments and fees are recorded as deferred revenue upon receipt and recognized as revenue when we satisfy our performance obligations under these arrangements.
Grant revenue is typically paid for reimbursable costs incurred over the duration of the associated research project or clinical trial and is recognized either when expenses reimbursable under the grants have been incurred and payments under the grants become contractually due.due or when cash is received, depending on the certainty of payment and other factors specific to each grant.
WarrantsRevenue Interest Liability
The company accountsOn December 29, 2023, we entered into the RIPA with Infinity and Oberland. Pursuant to the RIPA, Oberland acquired certain Revenue Interests from us for warrants as either equity-classified or liability-classified instrumentsa gross purchase price of $200.0 million paid on closing. In addition, Oberland may purchase additional Revenue Interests from us in exchange for the $100.0 million Second Payment upon satisfaction of certain conditions specified in the RIPA. Under the RIPA, Oberland has the right to receive quarterly payments from us based on, an assessmentamong other things, a certain percentage of the warrant’s specific termsour worldwide net sales, excluding those in China, during such quarter. The RIPA is considered a sale of future revenues and applicable authoritative guidance in ASC Topic 480, Distinguishing Liabilities from Equity (ASC 480), and ASC Topic 815, Derivatives and Hedging (ASC 815). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definitionis accounted for as a liability net of a liability pursuant to ASC 480, and whetherdebt discount comprised of deferred issuance costs, the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the company’s own stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For warrants that meet all criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and on each balance sheet date thereafter. Changes in the estimated fair value of a freestanding option agreement related to the warrants areSPOA, and the fair value of embedded derivatives requiring bifurcation on the consolidated balance sheet. The company imputes interest expense associated with this liability using the effective interest rate method. The effective interest rate is calculated based on the rate that would enable the debt to be repaid in full over the anticipated life of the arrangement. Interest expense is recognized as a non-cash gain or loss in other income (expense), net,over the estimated term on the consolidated statement of operations. The fair valueinterest rate on this liability may vary during the term of the warrants was estimated usingagreement depending on a number of factors, including the Black-Scholes option pricing model.level of actual and forecasted net sales. The company evaluates the interest rate quarterly based on actual and forecasted net sales utilizing the prospective method. A significant increase or decrease in actual or forecasted net sales will materially impact the revenue interest liability, interest expense, and the time period for repayment.
Business CombinationsDerivative Liabilities
Business combinationsEmbedded derivatives that are required to be bifurcated from the underlying debt instrument that do not meet the derivative scope exception and equity classification criteria are accounted for usingand valued as separate financial instruments. The terms of an embedded derivative related to a contingent exercisable prepayment feature of a convertible note have been evaluated and deemed to require bifurcation. This embedded derivative will be initially measured at fair value and will be remeasured to fair value at each reporting date until the acquisition methodderivative is settled.
In addition, the RIPA contains certain features that meet the definition of accounting in accordance with ASC Topic 805, Business Combinations (ASC 805). These standards require thatbeing an embedded derivative requiring bifurcation as a separate compound financial instrument apart from the total cost of acquisition be allocatedRIPA. The derivative liability is initially measured at fair value upon issuance and is subject to the tangible and intangible assets acquired and liabilities assumed based on their respective fair valuesremeasurement at the date of acquisition, with the excess purchase price recorded as goodwill. The allocation of the purchase price is dependent upon certain valuations and other studies. Acquisition costs are expensed as incurred.
Contingent consideration incurred in connection with a business combination are recorded at their fair values on the acquisition date and re-measured at their fair values each subsequent reporting period until the related contingencies are resolved. The resultingwith changes in fair values are recorded as research and developmentvalue recognized in other expense, net, on the consolidated statementsstatement of operations and comprehensive income (loss). Changes in fair values reflect changes to our assumptions regarding probabilities of successful achievement of related milestones, the timing in which the milestones are expected to be achieved, and the discount rate used to estimate the fair value of the obligation.
Preclinical and Clinical Trial Accruals
As part of the process of preparing the consolidated financial statements, we are required to estimate expenses resulting from obligations under contracts with vendors, clinical research organizations and consultants. The financial terms of these contracts vary and may result in payment flows that do not match the periods over which materials or services are provided under such contracts.
We estimate clinical trial and research agreement-related expenses based on the services performed, pursuant to contracts with research institutions and clinical research organizations and other vendors that conduct clinical trials and research on our behalf. In accruing clinical and research-related fees, we estimate the time period over which services will be performed and activity expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, we will adjust the accrual accordingly. Payments made under these arrangements in advance of the receipt of the related services are recorded as prepaid expenses until the services are rendered.
Research and Development Costs
Major components of research and development costs include cash compensation and other personnel-related expenses, stock-based compensation, depreciation and amortization expense on research and development property and equipment and intangible assets, costs of preclinical studies, clinical trials costs, including CROs and related clinical manufacturing, including third-party CMOs, costs of drug development, costs of materials and supplies, facilities cost, overhead costs, regulatory and compliance costs, and fees paid to consultants and other entities that conduct certain research and development activities on our behalf. Costs incurred in research and development are expensed as incurred.
The company classifies its research and development expenses as either external or internal. The company’s external research and development expenses support its various preclinical and clinical programs. The company’s internal research and development expenses include payroll and benefits expenses, facilities and equipment expense, and other indirect research and development expenses incurred in support of its research and development activities. The company’s external and internal resources are not directly tied to any one research or drug discovery program and are typically deployed across multiple programs and are not allocated to specific product candidates or development programs.
Included in research and development costs are clinical trial and research expenses based on the services performed pursuant to contracts with research institutions and clinical research organizations and other vendors that conduct clinical trials and research on our behalf. We record accruals for estimated costs under these contracts. When evaluating the adequacy of the accrued liabilities, we analyze the progress of the preclinical studies or clinical trials, including the phase or completion of events, invoices received, contracted costs and purchase orders. Significant judgments and estimates are made in determining the accrued balances at the end of any reporting period based on the facts and circumstances known at that time. Although we do not expect the estimates to be materially different from the amounts actually incurred, if the estimates of the status and timing of services performed differ from the actual status and timing of services performed, we may report amounts that are too high or too low in any particular period. Actual results could differ from our estimates. We adjust the accruals in the period when actual costs become known.
Stock-Based Compensation
We account for stock-based compensation under the provisions of ASC Topic 718, Compensation—Stock Compensation (ASC 718). We estimate fair value of each stock option award on the date of grant using the Black-Scholes option pricing model. The Black-Scholes option pricing model requires the use of highly subjective assumptions, including, but not limited to, expected stock price volatility over the term of the awards and the expected term of the stock options. We measure the fair value of an equity-classified award at the grant date and recognize the stock-based compensation expense over the period of vesting on the straight-line basis for our outstanding share awards that do not contain a performance condition. For awards subject to performance-based vesting conditions, we assess the probability of the individual milestones under the award being achieved and stock-based compensation expense is recognized over the service period using the graded vesting method once management believes the performance criteria is probable of being met. If factors change and we employ different assumptions, share-based compensation expense may differ significantly from what we have recorded in the past. For awards with service or performance conditions, we recognize the effect of forfeitures in compensation cost in the period that the award was forfeited.
Contingencies
We record accruals for loss contingencies to the extent that we conclude it is probable that a liability has been incurred and the amount of the related loss can be reasonably estimated. We accrue for the best estimate of a loss within a range; however, if no estimate in the range is better than any other, then we accrue the minimum amount in the range. If we determine that a material loss is reasonably possible, we disclose the possible loss or range of loss, or that the amount of loss cannot be estimated at this time.
Because of the inherent uncertainty and unpredictability related to these matters, accruals are based on what we believe to be the best information available at the time of our assessment, including the legal facts and circumstances of the case, status of the proceedings, applicable law and the views of legal counsel. Upon the final resolution of such matters, it is possible that there may be a loss in excess of the amount recorded, and such amounts could have a material adverse effect on our results of operations, cash flows or financial position. We evaluate, on a quarterly basis, developments in legal proceedings and other matters that could cause a change in the potential amount of the liability recorded or the range of potential losses disclosed. Moreover, we record gain contingencies only when they are realizable and the amount is known. Additionally, we record our rights to insurance recoveries, limited to the extent of incurred or probable losses, as a receivable when such recoveries have been agreed to with our third-party insurers and when receipt is deemed probable. This includes instances when our third-party insurers have agreed to pay, on our behalf, certain legal defense costs and settlement amounts directly to applicable law firms and a settlement fund.
Warrants
See further informationThe company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC Topic 480, Distinguishing Liabilities from Equity (ASC 480) and FASB ASC Topic 815, Derivatives and Hedging (ASC 815). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are
indexed to the company’s own stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For warrants that meet all criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital, on the consolidated statement of stockholders’ deficit at the time of issuance. For warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and on each balance sheet date thereafter. Changes in the estimated fair value of the warrants are recognized as a non-cash gain or loss in other expense, net, on the consolidated statement of operations. The fair value of the warrants was estimated using the Black-Scholes option pricing model.
Fair Value Option Election
The company accounted for a convertible note issued on March 31, 2023 under the FVO election of ASC 825 until it was amended and restated on December 29, 2023. Prior to its extinguishment on December 29, 2023, the convertible note was a debt host financial instrument containing embedded features wherein the entire financial instrument was initially measured at its issuance-date estimated fair value and then subsequently remeasured at estimated fair value on a recurring basis at each reporting period date.
Changes in the estimated fair value of this convertible note were recorded in Note 7other expense, net, on the consolidated statement of operations, except that changes in estimated fair value caused by changes in the instrument-specific credit risk were recorded in other comprehensive (loss) income. In accordance with ASC 470-50, when the convertible note was extinguished on December 29, 2023, the cumulative amount previously recorded in Commitmentsother comprehensive (loss) income resulting from changes in the instrument-specific credit risk were reclassified and Contingencies,reported in current earnings on the consolidated statement of operations. Debt Modification and Extinguishment
The company evaluates amendments to its debt instruments in accordance with ASC 470-50. This evaluation includes comparing (1) if applicable, the net present value of future cash flows of the “Notesamended debt to Consolidated Financial Statements” that appearsof the original debt and (2) the change in Part II, Item 8. “Financial Statementsfair value of an embedded conversion feature to that of the carrying amount of the debt immediately prior to amendment to determine, in each case, if a change greater than 10% occurred. In instances where the net present value of future cash flows or the fair value of an embedded conversion feature, if any, changed more than 10%, the company applies extinguishment accounting. In instances where the net present value of future cash flows and Supplementary Data”the fair value of this Annual Report.an embedded conversion feature, if any, changed less than 10%, the company accounts for the amendment to the debt as a debt modification. Gains and losses on debt amendments that are considered extinguishments are recognized in current earnings or in additional paid-in capital if the transactions are with entities under common control. Debt amendments that are considered debt modifications are accounted for prospectively through yield adjustments, based on the revised terms. The increase in fair value of the embedded conversion feature from the debt modification was accounted for as an increase in debt discount with a corresponding increase in additional paid-in capital. Legal fees and other costs incurred with third parties that are directly related to debt modifications are expensed as incurred. Amounts paid by the company to the lenders, are reflected as additional debt discount and amortized as an adjustment of interest expense over remaining term of modified debt using the effective interest rate method.
Stock-Based Compensation
We account for stock-based compensation under the provisions of FASB ASC Topic 718, Compensation—Stock Compensation (ASC 718). We estimate fair value of each stock option award on the date of grant using the Black-Scholes option pricing model. The Black-Scholes option pricing model requires the use of subjective assumptions, including, but not limited to, expected stock price volatility over the term of the awards and the expected term of the stock options. We measure the fair value of an equity-classified award at the grant date and recognize the stock-based compensation expense over the period of vesting on the straight-line basis for our outstanding share awards that do not contain a performance condition. For awards subject to performance-based vesting conditions, we assess the probability of the individual milestones under the award being achieved and stock-based compensation expense is recognized over the service period using the graded vesting method once management believes the performance criteria is probable of being met. For awards with service or performance conditions, we recognize the effect of forfeitures in compensation cost in the period that the award was forfeited.
Business Combinations
Business combinations are accounted for using the acquisition method of accounting in accordance with ASC 805-50. These standards require that the total cost of acquisition be allocated to the tangible and intangible assets acquired and liabilities assumed based on their respective fair values at the date of acquisition, with the excess purchase price recorded as goodwill. The allocation of the purchase price is dependent upon certain valuations and other studies. Acquisition costs are expensed as incurred.
Contingent consideration incurred in connection with a business combination are recorded at their fair values on the acquisition date and re-measured at their fair values each subsequent reporting period until the related contingencies are resolved. The resulting changes in fair values are recorded as research and development expense, on the consolidated statements of operations and comprehensive income (loss). Changes in fair values reflect changes to our assumptions regarding probabilities of successful achievement of related milestones, the timing in which the milestones are expected to be achieved, and the discount rate used to estimate the fair value of the obligation.
Recent Accounting Pronouncements
Refer toSee Note 2, Summary of Significant Accounting Policies, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for a discussion ofmore information regarding recent accounting pronouncements or changes in accounting pronouncements that are of significance, or potential significance, to us.Subsequent Event
Registered Direct Offering
On February 15, 2023, we entered into a securities purchase agreement with certain institutional investors for the sale of 14,072,615 shares of our common stock, as well as warrants to purchase an additional 14,072,615 shares of common stock at an exercise price of $4.2636 per share, for a purchase price of $3.5530 per share and accompanying warrant, generating net proceeds of approximately $47.0 million, after deducting placement agent fees and other estimated offering costs. The warrants are immediately exercisable after the issuance date and expire two years after the initial issuance date. The closing of the offering occurred on February 17, 2023. We currently intend to use the net proceeds from this offering, together with other available funds, to progress our pre-commercialization efforts and clinical development programs, fund other research and development activities, for capital expenditures, and for other general corporate purposes. We may also use a portion of the net proceeds to license intellectual property or to make acquisitions or investments.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Our exposure to market risk for changes in interest rates relates primarily to interest earned on our cash equivalents, investments and variable interest rate debt. The primary objective of our investment activities is to preserve our capital to fund operations. A secondary objective is to maximize income from our investments without assuming significant risk. Our investment policy provides for investments in low-risk, investment-grade debt instruments. As of December 31, 2022,2023, we had $104.6$265.5 million in cash and cash equivalents and $3.4$1.9 million in our investment portfolio. Our cash equivalents are short-term investments with maturities of 90 days or less at the time of purchase. We maintain cash deposits in FDIC insured financial institutions in excess of federally insured limits. However, we believe that we are not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. As of December 31, 2022,2023, our investment portfolio was comprised of available-for-sale securities, and we did not hold or issue financial instruments for trading purposes.
Interest Rate Risk – Cash
With the cash discussed above, our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S interest rates. However, we do not believe a sudden change in the interest rates would have a material impact on our financial condition or results of operations due to the short-term maturities onof our cash equivalents. A hypothetical 100 basis point change in interest rates during any of the periods presented would not have had a material impact on our consolidated financial statements.
Interest Rate Risk – Cash Equivalents and Investment Portfolio
We invest a portion of our cash in a number of diversified fixed and floating ratefixed-and floating-rate securities, consisting of marketable debt securities and debt funds that are subject to interest rate risk. Changes in the general level of interest rates can affect the fair value of our investment portfolio. If interest rates in the general economy were to rise, our holdings could lose value. At December 31, 2022,2023, a hypothetical increase in interest rates of 100 basis points across the entire yield curve on our holdings would not have resulted in a material impact on the fair value of our portfolio.
Interest Rate Risk – Variable-Rate Debt
Our use of variable-rate debt exposes us to interest rate risk as changes in interest rates would affect interest expense. As of December 31, 20222023, we have $475.0 million of variable-rate loans outstanding that maturetotaling $735.0 million, of which:
•$380.0 million matures on December 31, 2023. These loans bear2025 and bears interest at 3-month Term SOFR +plus 7.5% per annum;
•$155.0 million matures on December 31, 2025 and bears interest at 3-month Term SOFR plus 8.0%. per annum; and
•$200.0 million matures on September 11, 2026 and bears interest at 1-month Term SOFR plus 8.0% per annum.
As of December 31, 20222023, the weighted-average interest rate on these loans was 12.59%13.09%. A hypothetical 100-basis point increase in the Term SOFR rate as of December 31, 20222023 would increase our future interest paymentpayments by $4.8 million.$16.1 million. Similarly, a hypothetical 100-basis point decrease in the Term SOFR rate as of December 31, 20222023 would decrease our future interest payment by $16.1 million.
Interest Rate Risk – RIPA
We have entered into a revenue interest purchase agreement. Our primary exposure to market risk is that the interest rate on the revenue interest liability may vary during the term of the agreement depending on a number of factors, including the level of forecasted net sales. A significant increase or decrease in actual or forecasted net sales will materially impact the revenue interest liability, interest expense, and the time period for repayment. See $4.8 millionNote9., “Revenue Interest Purchase Agreement,” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding the terms of the RIPA. Foreign Currency Exchange Risk
We are exposed to foreign currency exchange rate risk inherent in conducting business globally in numerous currencies. We contract with clinical research organizations, investigational sites and suppliers in foreign countries. We are, therefore, subject to fluctuations in foreign currency rates in connection with these agreements. We have not entered into any material foreign currency hedging contracts although we may do so in the future. From inception through the date of this Annual Report, we have not incurred any material effects from foreign currency changes on these contracts. The effect of a 10% adverse change in exchange rates on foreign currency denominated cash and payables as of December 31, 2022 2023 would not have been material.material. However, fluctuations in currency exchange rates could harm our business in the future.
We are also exposed to foreign currency fluctuations related to the operations of our subsidiary in Italy whose financial statements are denominated in the Euro. We translate all assets and liabilities denominated in foreign currency into U.S. dollars using the exchange rate as of the end of the reporting period, while the operating results are translated into U.S. dollars using the average exchange rates for the reporting periods. Gains and losses resulting from translating the financial statements from our subsidiary’s functional currency to U.S. dollars are recognized as a component of other comprehensive (loss) income (loss), on the consolidated statement of comprehensive loss. Foreign currency exchange rate fluctuations affect our reported net loss and can make comparisons from period to period more difficult. Our foreign operations are not material to our operations as a whole. As such, we currently do not enter into currency forward exchange or option contracts to hedge foreign currency exposures.
Market Risk
As of December 31, 2022, 9,090,9092023, 37,732,820 warrants from theour direct registered offeringofferings remained outstanding at a fair value of $21.6$118.8 million. The fair value of thisthe warrant liabilityliabilities is determined using the Black-Scholes option pricing model and is therefore sensitive to changes in the market price and volatility of our common stock among other factors. In the event of a hypothetical 10% increase in the market price of our common stock ($5.585.52 based on the $5.07$5.02 market price of our stock at December 31, 2022)2023) on which the December 31, 20222023 valuation was based, the fair value of the warrant liabilityliabilities would have increased by $3.3$15.6 million. Similarly, based on the fair value of the warrants outstanding as of December 31, 2022,2023, a hypothetical decrease of 10% in the market price of our common stock would have the fair value of the warrant liabilityliabilities decreased by $3.315.5 million. Such increase or decrease would have been reflected as a change in fair value of warrant liabilityliabilities in other income (expense),expense, net, in ouron the consolidated statement of operations.
Inflation Risk
Inflation may affect us by increasing our cost of labor, clinical trial, and other costs. We do not believe that inflation has had a material effect on our business, financial condition or results of operations for any period presented herein.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of ImmunityBio, Inc. and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of ImmunityBio, Inc. and Subsidiaries (the Company) as of December 31, 20222023 and 2021,2022, the related consolidated statements of operations, comprehensive loss, stockholders’ deficit and cash flows for each of the three years in the period ended December 31, 2022,2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 20222023 and 2021,2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2022,2023, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated March 1, 2023 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOBPublic Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accountaccounts or disclosuredisclosures to which it relates.
| | | | | |
| Related party transactions and disclosures |
| |
Description of the
Matter | As described in Notes 910 and 1011 to the consolidated financial statements, the Company’s Executive Chairman, Global Chief Scientific and Medical Officer and principal stockholder Dr. Patrick Soon-Shiong has a controlling interest in certain entities with which the Company has entered into material transactions including related party debt transactions with Nant Capital, LLC, NantWorks, NantMobile, LLC and NantCancerStemCell. Affiliates of such entities are also affiliates of the Company due to the common control of the Company’s Executive Chairman, Global Chief Scientific and Medical Officer and principal stockholder. Assessing the sufficiency of procedures performed to identify related parties and significant related party transactions and determining the identified significant related party transactions were properly recorded, presented and disclosed was challenging due to the nature, volume and the significance of related party transactions. |
| |
How We Addressed
the Matter in Our
Audit | We obtained an understanding, evaluated the design, and tested the operating effectiveness of internal controls over the Company’s related party process. This included testing controls over management’s identification, review, recognition and disclosure of significant related party transactions. The audit procedures we performed included, among others, testing the completeness and accuracy of the listing of related parties identified and significant related party transactions provided by management, and testing the manner in which significant related party transactions were recorded, presented and disclosed. We performed journal entry searches of identified related parties to verify completeness and accuracy of the Company’s significant related party transactions. We inquired of management, and members of the Company’s audit committee, and the Company’s related party transaction committee chair regarding the completeness of the significant related party transactions identified. We also inspected questionnaires received from the Company’s directors and officers, read minutes of the meetings of Board of Directors and its various Committees, read employment and compensation contracts, proxy statements and other relevant filings with the Securities and Exchange Commission that relate to the Company’s financial relationships and transactions with the Company’s executive officers and with other entities controlled by the Company’s Executive Chairman, Global Chief Scientific and Medical Officer and principal stockholder. We confirmed the significant transactions and/or balances, as applicable, with the related parties. We also obtained the underlying agreements for significant related party transactions and assessed the associated accounting and recognition. We involved a valuation specialistspecialists to assist in evaluating the appropriateness of the valuation methodologies and reasonableness of the assumptions used in the valuation of the related party debt including underlying conversion features and embedded derivatives, as deemed necessary, as described in Note 910 to the consolidated financial statements. |
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2016.
Los Angeles, California
March 1, 202319, 2024
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of ImmunityBio, Inc. and Subsidiaries
Opinion on Internal Control Over Financial Reporting
We have audited ImmunityBio, Inc. and Subsidiaries’ internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, ImmunityBio, Inc. and Subsidiaries (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2022, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2022 and 2021, the related consolidated statements of operations, comprehensive loss, stockholders’ deficit and cash flows for each of the three years in the period ended December 31, 2022, and the related notes and our report dated March 1, 2023 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Los Angeles, California
March 1, 2023
ImmunityBio, Inc. and Subsidiaries
Consolidated Balance Sheets
(in thousands, except share and per share amounts)
| | As of December 31, |
| 2022 | | 2021 |
| As of December 31, | | | As of December 31, |
| 2023 | | | 2023 | | 2022 |
| ASSETS | ASSETS | | | | ASSETS | | | |
| Current assets: | Current assets: | | | | Current assets: | | | |
| Cash and cash equivalents | Cash and cash equivalents | $ | 104,641 | | | $ | 181,101 | |
| Marketable securities | Marketable securities | 2,543 | | | 136,015 | |
| Due from related parties | Due from related parties | 1,890 | | | 1,333 | |
| Prepaid expenses and other current assets (including amounts with related parties) | Prepaid expenses and other current assets (including amounts with related parties) | 31,503 | | | 15,898 | |
| Total current assets | Total current assets | 140,577 | | | 334,347 | |
| Marketable securities, noncurrent | Marketable securities, noncurrent | 840 | | | 822 | |
| Property, plant and equipment, net | Property, plant and equipment, net | 143,659 | | | 82,863 | |
| Intangible asset, net | 20,003 | | | 1,420 | |
| Intangible assets, net | |
| Convertible note receivable | Convertible note receivable | 6,629 | | | 6,379 | |
| Operating lease right-of-use assets, net (including amounts with related parties) | Operating lease right-of-use assets, net (including amounts with related parties) | 45,788 | | | 36,304 | |
| Investment and other assets (including amounts with related parties) | 4,860 | | | 6,775 | |
| Other assets (including amounts with related parties) | |
| Total assets | Total assets | $ | 362,356 | | | $ | 468,910 | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | |
| Current liabilities: | Current liabilities: | | | | Current liabilities: | | | |
| Accounts payable | Accounts payable | $ | 21,016 | | | $ | 11,418 | |
| Accrued expenses and other liabilities | Accrued expenses and other liabilities | 41,825 | | | 51,387 | |
| Related-party promissory notes, net of discounts and deferred issuance costs | 431,901 | | | 299,236 | |
Related-party promissory notes, net of discounts and deferred issuance costs (Note 10) | |
| | Due to related parties | |
| Due to related parties | |
| Due to related parties | Due to related parties | 3,469 | | | 3,943 | |
| Operating lease liabilities (including amounts with related parties) | Operating lease liabilities (including amounts with related parties) | 2,650 | | | 3,011 | |
| Total current liabilities | Total current liabilities | 500,861 | | | 368,995 | |
Related-party promissory notes, less current portion (Note 9) | — | | | 306,349 | |
Related-party convertible notes and accrued interest, net of discount, less current portion (Note 9) | 241,271 | | | — | |
Related-party nonconvertible note, net of discount (Note 10) | |
Related-party convertible notes and accrued interest, net of discount (Note 10) | |
| Revenue interest liability (Note 9) | |
Revenue interest liability (Note 9) | |
Revenue interest liability (Note 9) | |
| |
| Operating lease liabilities, less current portion (including amounts with related parties) | Operating lease liabilities, less current portion (including amounts with related parties) | 47,951 | | | 37,068 | |
| | Warrant liability | 21,636 | | | — | |
| Warrant liabilities | |
| Warrant liabilities | |
| Warrant liabilities | |
| Other liabilities | Other liabilities | 457 | | | 411 | |
| Total liabilities | Total liabilities | 812,176 | | | 712,823 | |
Commitments and contingencies (Note 7) | Commitments and contingencies (Note 7) | | | | Commitments and contingencies (Note 7) | | | |
| Stockholders’ deficit: | Stockholders’ deficit: | | | | Stockholders’ deficit: | | | |
Common stock, $0.0001 par value; 900,000,000 and 500,000,000 shares authorized as of December 31, 2022 and 2021, respectively; 421,569,115 and 397,830,044 shares issued and outstanding as of December 31, 2022 and 2021, respectively; excluding treasury stock, 163,800 shares outstanding as of December 31, 2022 and 2021, respectively | 42 | | | 40 | |
Common stock, $0.0001 par value; 1,350,000,000 and 900,000,000 shares authorized as of December 31, 2023 and 2022, respectively; 670,867,344 and 421,569,115 shares issued and outstanding as of December 31, 2023 and 2022, respectively; excluding 163,800 treasury stock shares outstanding as of December 31, 2023 and 2022 | |
| Additional paid-in capital | Additional paid-in capital | 1,930,936 | | | 1,719,704 | |
| Accumulated deficit | Accumulated deficit | (2,378,488) | | | (1,961,921) | |
| Accumulated other comprehensive income | Accumulated other comprehensive income | 183 | | | 4 | |
| Total ImmunityBio stockholders’ deficit | Total ImmunityBio stockholders’ deficit | (447,327) | | | (242,173) | |
| Noncontrolling interests | Noncontrolling interests | (2,493) | | | (1,740) | |
| Total stockholders’ deficit | Total stockholders’ deficit | (449,820) | | | (243,913) | |
| Total liabilities and stockholders’ deficit | Total liabilities and stockholders’ deficit | $ | 362,356 | | | $ | 468,910 | |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
Consolidated Statements of Operations
(in thousands, except share and per share amounts)
| | Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| Revenue | Revenue | $ | 240 | | | $ | 934 | | | $ | 605 | |
| Operating expenses: | Operating expenses: | |
Research and development (including amounts
with related parties) | |
Research and development (including amounts
with related parties) | |
Research and development (including amounts
with related parties) | Research and development (including amounts
with related parties) | 248,149 | | | 195,958 | | | 139,507 | |
Selling, general and administrative (including amounts
with related parties) | Selling, general and administrative (including amounts
with related parties) | 102,708 | | | 135,256 | | | 71,318 | |
| Impairment of intangible assets | Impairment of intangible assets | 681 | | | — | | | 10,660 | |
| Total operating expenses | Total operating expenses | 351,538 | | | 331,214 | | | 221,485 | |
| Loss from operations | Loss from operations | (351,298) | | | (330,280) | | | (220,880) | |
| Other expense, net: | Other expense, net: | | | | | |
| Interest and investment (loss) income, net | (3,090) | | | (4,100) | | | 2,435 | |
| Interest and investment income (loss), net | |
| Interest and investment income (loss), net | |
| Interest and investment income (loss), net | |
| Interest expense (including amounts with related parties) | Interest expense (including amounts with related parties) | (63,515) | | | (14,849) | | | (9,074) | |
| Loss on equity method investment | Loss on equity method investment | (12,107) | | | (803) | | | — | |
| Change in fair value of warrant liability | 13,460 | | | — | | | — | |
| Change in fair value of warrant liabilities | |
| Change in fair value of related-party convertible note | |
Other (expense) income, net (including amounts
with related parties) | Other (expense) income, net (including amounts
with related parties) | (736) | | | 193 | | | 1,486 | |
| Total other expense, net | Total other expense, net | (65,988) | | | (19,559) | | | (5,153) | |
| Loss before income taxes and noncontrolling interests | Loss before income taxes and noncontrolling interests | (417,286) | | | (349,839) | | | (226,033) | |
| Income tax (expense) benefit | (34) | | | (9) | | | 1,846 | |
| Income tax benefit (expense) | |
| Net loss | Net loss | (417,320) | | | (349,848) | | | (224,187) | |
| Net loss attributable to noncontrolling interests, net of tax | Net loss attributable to noncontrolling interests, net of tax | (753) | | | (3,058) | | | (2,336) | |
| Net loss attributable to ImmunityBio common stockholders | Net loss attributable to ImmunityBio common stockholders | $ | (416,567) | | | $ | (346,790) | | | $ | (221,851) | |
| | Net loss per ImmunityBio common share – basic and diluted | Net loss per ImmunityBio common share – basic and diluted | $ | (1.04) | | | $ | (0.89) | | | $ | (0.59) | |
| Net loss per ImmunityBio common share – basic and diluted | |
| Net loss per ImmunityBio common share – basic and diluted | |
Weighted-average number of common shares used in computing
net loss per share – basic and diluted | Weighted-average number of common shares used in computing
net loss per share – basic and diluted | 399,900,374 | | 389,234,156 | | 377,067,527 | Weighted-average number of common shares used in computing
net loss per share – basic and diluted | 508,635,592 | | 399,900,374 | | 389,234,156 |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
Consolidated StatementsThe Merger
On December 21, 2020, NantKwest and NantCell entered into the Merger Agreement, pursuant to which NantKwest and NantCell agreed to combine their businesses. The Merger Agreement provided that a wholly-owned subsidiary of Comprehensive Loss
(in thousands)the company would merge with and into NantCell, with NantCell surviving the Merger as a wholly-owned subsidiary of the company.
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Net loss | $ | (417,320) | | | $ | (349,848) | | | $ | (224,187) | |
| Other comprehensive income (loss), net of income taxes: | | | | | |
| Net unrealized (losses) gains on available-for-sale securities | (183) | | | (13) | | 140 | |
Reclassification of net realized gains on
available-for-sale securities included in net loss | 124 | | | — | | 9 | |
| Foreign currency translation adjustments | 238 | | | (105) | | 60 | |
| Total other comprehensive income (loss) | 179 | | | (118) | | | 209 | |
| Comprehensive loss | (417,141) | | | (349,966) | | | (223,978) | |
| Less: Comprehensive loss attributable to noncontrolling interests | (753) | | | (3,058) | | | (2,336) | |
Comprehensive loss attributable to ImmunityBio
common stockholders | $ | (416,388) | | | $ | (346,908) | | | $ | (221,642) | |
The accompanying notes are an integral partImmediately following the Effective Time, the former stockholders of these consolidated financial statements.NantCell held approximately 71.5% of the outstanding shares of Company Common Stock and the stockholders of NantKwest as of immediately prior to the Merger held approximately 28.5% of the outstanding shares of Company Common Stock. As a result of the Merger and immediately following the Effective Time, Dr. Patrick Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer, and his affiliates beneficially owned, in the aggregate, approximately 81.8% of the outstanding shares of Company Common Stock. Following the consummation of the Merger, the symbol for shares of the company’s common stock was changed to “IBRX.”
ImmunityBio, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ Deficit
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Additional
Paid-in
Capital | | Accumulated
Deficit | | Accumulated
Other
Comprehensive
(Loss)
Income | | Total
ImmunityBio
Stockholders’
Equity
(Deficit) | | Noncontrolling
Interests | | Total
Stockholders’
Equity
(Deficit) |
| | Shares | | Amount | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Balance as of December 31, 2019 | | 371,976,995 | | | $ | 37 | | | $ | 1,406,002 | | | $ | (1,393,280) | | | $ | (87) | | | $ | 12,672 | | | $ | 3,654 | | | $ | 16,326 | |
Issuance of common stock, net of offering costs of $4,373 | | 8,521,500 | | | 1 | | | 86,301 | | | — | | | — | | | 86,302 | | | — | | | 86,302 | |
| Stock-based compensation expense | | — | | | — | | | 2,187 | | | — | | | — | | | 2,187 | | | — | | | 2,187 | |
| Exercise of stock options | | 1,272,273 | | | — | | | 1,176 | | | — | | | — | | | 1,176 | | | — | | | 1,176 | |
| Vesting of restricted stock units (RSUs) | | 648,336 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Net share settlement for RSUs vesting | | (175,962) | | | — | | | (503) | | | — | | | — | | | (503) | | | — | | | (503) | |
| Other comprehensive income (loss), net of tax | | — | | | — | | | — | | | — | | | 209 | | | 209 | | | — | | | 209 | |
| Net loss | | — | | | — | | | — | | | (221,851) | | | — | | | (221,851) | | | (2,336) | | | (224,187) | |
| Balance as of December 31, 2020 | | 382,243,142 | | | 38 | | | 1,495,163 | | | (1,615,131) | | | 122 | | | (119,808) | | | 1,318 | | | (118,490) | |
Issuance of common stock “at-the-market” offering, net of commissions and offering costs of $4,674 | | 13,295,817 | | | 2 | | | 164,528 | | | — | | | — | | | 164,530 | | | — | | | 164,530 | |
| Stock-based compensation expense | | — | | | — | | | 57,181 | | | — | | | — | | | 57,181 | | | — | | | 57,181 | |
| Exercise of stock options | | 1,695,638 | | | — | | | 5,461 | | | — | | | — | | | 5,461 | | | — | | | 5,461 | |
| Vesting of RSUs | | 873,058 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Net share settlement for RSUs vesting | | (277,611) | | | — | | | (4,064) | | | — | | | — | | | (4,064) | | | — | | | (4,064) | |
| Sales of assets to an entity under common control | | — | | | — | | | 1,435 | | | — | | | — | | | 1,435 | | | — | | | 1,435 | |
| Other comprehensive income (loss), net of tax | | — | | | — | | | — | | | — | | | (118) | | | (118) | | | — | | | (118) | |
| Net loss | | — | | | — | | | — | | | (346,790) | | | — | | | (346,790) | | | (3,058) | | | (349,848) | |
| Balance as of December 31, 2021 | | 397,830,044 | | | $ | 40 | | | 1,719,704 | | | (1,961,921) | | | 4 | | | (242,173) | | | (1,740) | | | (243,913) | |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc.During the year ended December 31, 2021, we recorded $13.0 million of costs in connection with the Merger consisting of financial advisory, legal and Subsidiariesother professional fees.
Consolidated StatementsAccounting Treatment of Stockholders’ Deficit (Continued)
(in thousands, except share amounts)the Merger
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Additional
Paid-in
Capital | | Accumulated
Deficit | | Accumulated
Other
Comprehensive
(Loss)
Income | | Total
ImmunityBio
Stockholders’
Equity
(Deficit) | | Noncontrolling
Interests | | Total
Stockholders’
Equity
(Deficit) |
| | Shares | | Amount | | | | | | |
Conversion of related-party convertible note and
accrued interest, net of unamortized debt discount,
into equity | | 9,986,920 | | | 1 | | | 51,946 | | | — | | | — | | | 51,947 | | | — | | | 51,947 | |
Issuance of shares in a registered direct offering, net of discount and offering costs of $1,897 and value ascribed to associated warrants (Note 11) | | 9,090,909 | | | 1 | | | 13,006 | | | — | | | — | | | 13,007 | | | — | | | 13,007 | |
Issuance of common stock “at-the market” offering, net of commissions and offering costs of $302 | | 2,051,894 | | | — | | | 13,129 | | | — | | | — | | | 13,129 | | | — | | | 13,129 | |
| Stock-based compensation expense | | — | | | — | | | 40,179 | | | — | | | — | | | 40,179 | | | — | | | 40,179 | |
| Exercise of stock options | | 14,767 | | | — | | | 74 | | | — | | | — | | | 74 | | | — | | | 74 | |
| Vesting of RSUs | | 521,296 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Net share settlement for RSUs vesting | | (156,011) | | | — | | | (616) | | | — | | | — | | | (616) | | | — | | | (616) | |
| Shares issued pursuant to litigation settlement | | 2,229,296 | | | — | | | 10,656 | | | — | | | — | | | 10,656 | | | — | | | 10,656 | |
Gain on extinguishment of debt with related parties
under common control | | — | | | — | | | 82,858 | | | — | | | — | | | 82,858 | | | — | | | 82,858 | |
| Other comprehensive income (loss), net of tax | | — | | | — | | | — | | | — | | | 179 | | | 179 | | | — | | | 179 | |
| Net loss | | — | | | — | | | — | | | (416,567) | | | — | | | (416,567) | | | (753) | | | (417,320) | |
| Balance as of December 31, 2022 | | 421,569,115 | | | $ | 42 | | | $ | 1,930,936 | | | $ | (2,378,488) | | | $ | 183 | | | $ | (447,327) | | | $ | (2,493) | | | $ | (449,820) | |
COVID-19 Pandemic
The accompanying notesCOVID-19 pandemic continues to present a public health and economic challenge around the world. Through the date of this Annual Report, we have not seen a material adverse impact to our business from the pandemic. However, given the continuously evolving nature of the pandemic, we cannot at this time predict the specific extent, duration, or full impact that this pandemic may have on our financial condition and results of operations, including ongoing and planned clinical trials. More specifically, the pandemic may result in prolonged impacts that we cannot predict at this time and we expect that such uncertainties will continue to exist for the foreseeable future.
We continue to monitor the impact of COVID-19 on our business, including our clinical trials, manufacturing facilities and capabilities, and ability to access necessary resources. For a discussion of the risks presented by the COVID-19 pandemic to our results of operations and financial condition, see Part I, Item 1A. “Risk Factors.” Operating Results
From inception through the date of this Annual Report, we have generated minimal revenue from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables, and grant programs. We have no clinical products approved for commercial sale and have not generated any revenue from therapeutic and vaccine product candidates that are under development. We have incurred net losses in each year since our inception and, as of December 31, 2023, we had an accumulated deficit of $3.0 billion. During the years ended December 31, 2023, 2022 and 2021, net losses attributable to ImmunityBio common stockholders were $583.2 million, $416.6 million, and $346.8 million, respectively. Substantially all of our net losses resulted principally from costs incurred in connection with our ongoing clinical trials and operations, our research and development programs, and from selling, general and administrative costs associated with our operations, including stock-based compensation expense.
As of December 31, 2023, we had 628 employees. Personnel of related companies who provide corporate, general and administrative, certain research and development, and other support services under our shared services agreement with NantWorks are not included in this number. See Note 11, Related-Party Agreements, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information. In anticipation of the commercialization of select drug candidates, we expect to continue to incur significant expenses and increasing operating losses for the foreseeable future, which may fluctuate significantly from quarter-to-quarter and year-to-year. See “—Future Funding Requirements” below for a discussion of our anticipated expenditures and sources of capital we expect to access to fund these expenditures. Collaboration Agreements
We anticipate that strategic collaborations will continue to be an integral part of these consolidated financial statements.our operations, providing opportunities to leverage our partners’ expertise and capabilities to gain access to new technologies and further expand the potential of our technologies and product candidates across relevant platforms. We believe we are well positioned to become a leader in immunotherapy due to our broad and vertically-integrated platforms and through complementary strategic partnerships.
ImmunityBio, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(in thousands)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Operating activities: | | | | | |
| Net loss | $ | (417,320) | | | $ | (349,848) | | | $ | (224,187) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Stock-based compensation expense | 40,179 | | | 57,181 | | | 2,187 | |
| Change in fair value of warrant liability | (13,460) | | | — | | | — | |
| Transaction costs allocated to warrant liability | 1,082 | | | — | | | — | |
| Depreciation and amortization | 18,260 | | | 14,238 | | | 12,739 | |
| Non-cash interest items, net (including amounts with related parties) | 11,746 | | | 12,417 | | | 8,531 | |
| Amortization of related-party notes discounts | 16,282 | | | 62 | | | — | |
| Non-cash lease expense related to operating lease right-of-use assets | 5,932 | | | 4,884 | | | 5,155 | |
Amortization of premiums, net of discounts, on marketable
debt securities | 1,318 | | | 403 | | | 794 | |
| Unrealized losses (gains) on equity securities | 4,190 | | | 4,615 | | | (2,876) | |
| Unrealized loss on non-marketable equity investment | — | | | — | | | 1,405 | |
| Impairment of intangible assets | 681 | | | — | | | 10,660 | |
| Impairment of fixed assets | 1,333 | | | — | | | — | |
| | | | | |
| Deferred tax | (4) | | | (8) | | | (2,938) | |
| Other | 273 | | | 749 | | | 446 | |
| Changes in operating assets and liabilities: | | | | | |
| Prepaid expenses and other current assets | (16,557) | | | (2,249) | | | 4,208 | |
| Investment and other assets | 1,998 | | | (3,977) | | | (684) | |
| Accounts payable | 8,000 | | | (3,717) | | | 2,570 | |
| Accrued expenses and other liabilities | 4,102 | | | 5,182 | | | 12,495 | |
| Related parties | (1,225) | | | (10,187) | | | 3,378 | |
| Operating lease liabilities | (4,319) | | | (4,164) | | | (5,607) | |
| Net cash used in operating activities | (337,509) | | | (274,419) | | | (171,724) | |
| Investing activities: | | | | | |
| Purchases of property, plant and equipment | (78,162) | | | (33,563) | | | (1,669) | |
| Purchase of intangible assets | (21,229) | | | — | | | — | |
| Proceeds from sales of property, plant and equipment | — | | | 20,498 | | | — | |
| Purchases of marketable debt securities, available-for-sale | (34,312) | | | (141,750) | | | (91,765) | |
| Maturities of marketable debt securities, available for sale | 128,188 | | | 56,166 | | | 65,350 | |
| Proceeds from sales of marketable debt and equity securities | 33,812 | | | 13,763 | | | 8,272 | |
| Investment in joint venture – an equity method investment | (1,000) | | | — | | | — | |
| Net cash provided by (used in) investing activities | 27,297 | | | (84,886) | | | (19,812) | |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc.We believe that our innovative approach to orchestrate and Subsidiariescombine therapies for optimal immune system response will become a therapeutic foundation across multiple indications. Additionally, we believe that data from multiple clinical trials indicates N-803 has broad potential to enhance the activity of therapeutic mAbs, including checkpoint inhibitors, across a wide range of tumor types. We may also enter into supply arrangements for various investigational agents to be used in our clinical trials. See Note 6, Collaboration and License Agreements and Acquisition, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding our collaboration and license agreements.Consolidated Statements
Agreements with Related Parties
Our Executive Chairman, Global Chief Scientific and Medical Officer and principal stockholder, founded and has a controlling interest in NantWorks, which is a collection of Cash Flows (Continued)companies in the healthcare and technology space. We have entered into arrangements with NantWorks, and certain affiliates of NantWorks. Affiliates of NantWorks are also affiliates of the company due to the common control by and/or common ownership interest of our Executive Chairman and Global Chief Scientific and Medical Officer.
(Related-Party Debt
The following discussion of the company’s related-party promissory notes does not purport to be complete and is qualified in thousands)its entirety by reference to the full text of the notes, copies of which are incorporated by reference in Part IV, Item 15. “Exhibits and Financial Statement Schedules” of this Annual Report. | | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Financing activities: | | | | | |
Proceeds from issuance of related-party promissory notes,
net of issuance costs paid | $ | 174,125 | | | $ | 338,500 | | | $ | 63,700 | |
Proceeds from equity offerings,
net of discounts and issuance costs | 60,427 | | | 164,530 | | | 86,302 | |
| Proceeds from exercises of stock options | 74 | | | 5,461 | | | 1,176 | |
| Sale of assets to an entity under common control | — | | | 1,435 | | | — | |
| Net share settlement for RSUs vesting | (616) | | | (4,064) | | | (503) | |
| Principal payments of finance leases | (58) | | | — | | | — | |
| Payment for contingent consideration | (339) | | | (419) | | | — | |
| Net cash provided by financing activities | 233,613 | | | 505,443 | | | 150,675 | |
Effect of exchange rate changes on cash, cash equivalents, and
restricted cash | 284 | | | 48 | | | (25) | |
| Net change in cash, cash equivalents, and restricted cash | (76,315) | | | 146,186 | | | (40,886) | |
| Cash, cash equivalents, and restricted cash, beginning of year | 181,280 | | | 35,094 | | | 75,980 | |
| Cash, cash equivalents, and restricted cash, end of year | $ | 104,965 | | | $ | 181,280 | | | $ | 35,094 | |
On September 11, 2023, the company entered into a stock purchase agreement with Nant Capital, NantMobile and NCSC (collectively, the “related-party purchasers”), which are entities affiliated with Dr. Soon-Shiong, pursuant to which the related-party purchasers exchanged outstanding fixed-rate promissory notes held by such related-party purchasers, representing approximately $270.0 million in aggregate principal amount and accrued and unpaid interest, in exchange for an aggregate of 209,291,936 shares of common stock at an exchange price of $1.29 per share. As a result of the exchange, the company is forever released and discharged from all its obligations and liabilities under these notes.
On September 11, 2023, the company executed a $200.0 million convertible promissory note with Nant Capital. The accompanying notesnote bears interest at Term SOFR plus 8.0% per annum. The accrued interest shall be payable monthly commencing on September 30, 2023. The outstanding principal amount and any accrued and unpaid interest are due on the earlier of (i) September 11, 2026 or (ii) upon the occurrence and during the continuance of an integral“event of default” (as defined in the note). We may prepay the outstanding principal amount, together with any accrued interest, at any time, in whole or in part, without premium or penalty upon five (5) days’ written notice to the noteholder. The noteholder has the sole option to convert all (but not less than all) of these consolidated financial statements.the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $1.9350 per share. We received net proceeds of approximately $199.0 million from this financing, net of a $1.0 million origination fee paid to the noteholder. On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into a letter amendment whereby this promissory note became subordinated to the RIPA payment obligations.
Related-Party Convertible Note at Fair Value
On March 31, 2023, the company executed a $30.0 million promissory note with Nant Capital. This note bears interest at Term SOFR plus 8.0% per annum. The outstanding principal amount and any accrued and unpaid interest are due on the earlier of December 31, 2023 or upon the occurrence and continuation of an event of default (as defined in the note). The interest on this note is paid on a quarterly basis beginning on June 30, 2023. The company may prepay the outstanding principal amount and any accrued and unpaid interest of the promissory note, at any time, in whole or in part, without penalty. Upon receipt of a written notice of prepayment from the company, the noteholder has the right, at its option, to convert the outstanding principal amount to be prepaid and the accrued and unpaid interest thereon (as specified in the notice of prepayment) into shares of the company’s common stock at a price of $2.28 per share. Additionally, the whole promissory note and any accrued interest may be converted into shares of the company’s common stock at a conversion price of $2.28 per share (subject to appropriate adjustment from time to time for any stock dividend, stock split, combination of shares, reorganization, recapitalization, reclassification or other similar event), at the option of the noteholder.
ImmunityBio, Inc.The company received net proceeds of approximately $29.9 million from this financing, net of a $0.1 million origination fee paid to the noteholder, which we used, together with other available funds, to progress our pre-commercialization efforts and Subsidiariesclinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes.
Consolidated StatementsOn September 11, 2023, the company and Nant Capital entered into a letter agreement pursuant to which the maturity date of Cash Flows (Continued)this promissory note was extended from December 31, 2023 to December 31, 2024. On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into a letter amendment pursuant to which the maturity date of this promissory note was extended to December 31, 2025, and the note became subordinated to the RIPA payment obligations.
(This note was accounted for under the ASC 825-10-15-4 FVO election until it was amended on December 29, 2023. Under the FVO election, the note was initially measured at its issuance date estimated fair value and subsequently remeasured at estimated fair value on a recurring basis at each reporting period date. With each such remeasurement, the note was adjusted to fair value, with the change in thousands)fair value recorded in other expense, net, on the consolidated statement of operations.
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
Reconciliation of cash, cash equivalents, and restricted cash,
end of year: | | | | | |
| Cash and cash equivalents | $ | 104,641 | | | $ | 181,101 | | | $ | 34,915 | |
| 324 | | | 179 | | | 179 | |
| Cash, cash equivalents, and restricted cash, end of year | $ | 104,965 | | | $ | 181,280 | | | $ | 35,094 | |
| | | | | |
| Supplemental disclosure of cash flow information: | | | | | |
| Cash paid during the year for: | | | | | |
| Interest | $ | 35,442 | | | $ | 2,106 | | | $ | 40 | |
| Income taxes | 2 | | | 9 | | | 8 | |
| | | | | |
| Supplemental disclosure of non-cash activities: | | | | | |
Gain on extinguishment of debt with related parties under
common control | $ | 82,858 | | | $ | — | | | $ | — | |
Conversion of related-party convertible note and accrued interest,
net of unamortized discount, into equity | 51,947 | | | — | | | — | |
Initial measurement of warrants issued in connection with the
registered direct offering accounted for as liabilities | 35,096 | | | — | | | — | |
Right-of-use assets obtained in exchange for operating
lease liabilities | 14,798 | | | 23,069 | | | 2,394 | |
Property and equipment purchases included in accounts payable,
accrued expenses and due to related parties | 12,693 | | | 11,654 | | | 220 | |
| Common stock issued pursuant to litigation settlement | 10,656 | | | — | | | — | |
Right-of-use assets obtained in exchange for finance
lease liabilities | 199 | | | — | | | — | |
| Unrealized losses on marketable debt securities, net | (59) | | | (13) | | | (17) | |
| Cashless exercise of stock options | — | | | 1,035 | | | 1,233 | |
| Accrued investment in joint venture | — | | | 1,000 | | | — | |
On June 13, 2023, the company executed a $30.0 million promissory note with Nant Capital. The company was able to request up to three (3) advances of $10.0 million each pursuant to the note. The principal amount of each advance bears interest at Term SOFR plus 8.0% per annum. The accrued interest on any advance is payable quarterly on the last business day of June, September and December. The outstanding principal amount and any accrued and unpaid interest on advances were due on the earlier of (i) December 31, 2023 or (ii) upon the occurrence and during the continuance of an event of default (as defined in the note). We may prepay the outstanding principal amount, together with any accrued interest, on any then-outstanding advances, at any time, in whole or in part, without premium or penalty, and without the prior consent of the noteholder, upon five (5) days written notice to the noteholder.
The accompanyingcompany received net proceeds of approximately $29.9 million from this financing, net of a $0.1 million origination fee paid to Nant Capital, which we used, together with other available funds, to progress our regulatory approval efforts, pre-commercialization activities and clinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes.
On September 11, 2023, the company and Nant Capital entered into a series of letter agreements pursuant to which the maturity date of related-party nonconvertible promissory notes aretotaling $505.0 million in principal were extended from December 31, 2023 to December 31, 2024.
On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into an integral partamended and restated promissory note pursuant to which the existing $30.0 million promissory note, along with other existing related-party nonconvertible promissory notes, was amended to be included in the Tranche 2 principal amount of these consolidated financial statements.the amended $505.0 million December 2023 promissory note. The Tranche 2 of the promissory note has a principal amount of $380.0 million, a maturity date of December 31, 2025, and an interest rate of Term SOFR plus 7.5% per annum. Based on the terms of the amended and restated promissory note, the noteholder, at its sole discretion, may convert all of the $380.0 million principal amount into shares of common stock at a conversion price of $8.2690 per share. The new note became subordinated to the RIPA payment obligations.
See Note 10, Related-Party Debt, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding our related-party debt. Immuno-Oncology Clinic, Inc.
We have entered into multiple agreements with the Clinic to conduct clinical trials related to certain of our product candidates. The Clinic is a related party as it is owned by an officer of the company and NantWorks manages the administrative operations of the Clinic.
ImmunityBio, Inc.In 2021, we completed a review of alternative structures that could support our more complex clinical trial requirements and Subsidiariesmade a decision to explore a potential transition of clinical trials at the Clinic to a new structure (including contracting with a new, non-affiliated professional corporation) to be determined and agreed upon by all parties. While we have not yet finalized the potential transition, we continue discussions with potential partners around such alternative structures. During the years ended December 31, 2023, 2022 and 2021, we recorded $2.2 million, $2.4 million and $1.6 million, respectively, in research and development expense, on the consolidated statements of operations related to clinical trial and transition services provided by the Clinic.
NotesRelated-Party Leases
605 Doug St, LLC
In June 2023, we exercised the option to extend the lease agreement with 605 Doug St, LLC, an entity owned by our Executive Chairman and Global Chief Scientific and Medical Officer, for one additional three-year term through July 2026.
23 Alaska, LLC
Effective August 31, 2023, we executed a lease termination agreement with 23 Alaska, LLC, an entity owned by our Executive Chairman and Global Chief Scientific and Medical Officer.
Duley Road, LLC
Effective October 3, 2023, we exercised the first option to extend the lease agreement with Duley Road, a related party that is indirectly controlled by our Executive Chairman and Global Chief Scientific and Medical Officer, for one additional five-year term through October 31, 2029.
See Note 11, Related-Party Agreements, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding our related-party agreements. 1. DescriptionComponents of Businessour Results of Operations
InRevenue
From inception through the date of this Annual Report, we have generated minimal revenue from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables, and grant programs. We have no clinical products approved for commercial sale and have not generated any revenue from therapeutic and vaccine product candidates that are under development. If we fail to complete the development of our product candidates in a timely manner or fail to obtain regulatory approval for them, we may never be able to generate substantial future revenue.
Operating Expenses
We generally classify our operating expenses into research and development, and selling, general and administrative expenses. Personnel costs, including salaries, benefits, bonuses, and stock-based compensation expense comprise a significant component of our research and development, and selling, general and administrative expense categories. We allocate expenses associated with our facilities and information technology costs between these notestwo categories, primarily based on the nature of each cost.
Research and Development
Research and development expense consists of expenses incurred while performing research and development activities to discover and develop our technology and product candidates. This includes conducting preclinical studies and clinical trials, manufacturing development efforts and activities related to regulatory submissions for product candidates. We recognize research and development expenses as they are incurred.
Our research and development expenses primarily consist of:
•clinical trial and regulatory-related costs;
•expenses incurred under agreements with investigative sites and consultants that conduct our clinical trials;
•expenses incurred under collaborative agreements;
•manufacturing and testing costs and related supplies and materials;
•employee-related expenses, including salaries, benefits, travel and stock-based compensation; and
•facility expenses dedicated to research and development.
The company classifies its research and development expenses as either external or internal. The company’s external research and development expenses support its various preclinical and clinical programs. The company’s internal research and development expenses include payroll and benefits expenses, facilities and equipment expense, and other indirect research and development expenses incurred in support of its research and development activities. The company’s external and internal resources are not directly tied to any one research or drug discovery program and are typically deployed across multiple programs and are not allocated to specific product candidates or development programs.
We expect our research and development expenses to increase significantly for the foreseeable future as we continue to invest in research and development activities related to developing our product candidates and conducting our ongoing and planned clinical trials.
The process of conducting clinical trials necessary to obtain regulatory approval is costly and time consuming. The successful development of product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing or costs required to complete the remaining development of any product candidates. This is due to the consolidated financial statements,numerous risks and uncertainties associated with the terms “ImmunityBio,” “the company,” “the combined company,” “we,” “us,” and “our” refer to ImmunityBio and subsidiaries.development of product candidates.
Our Business
ImmunityBio, Inc. is a clinical-stage biotechnology company developing next-generation therapies and vaccines that complement, harness, and amplifyThe costs of clinical trials may vary significantly over the immune system to defeat cancers and infectious diseases. We strive to be a vertically-integrated immunotherapy company designing and manufacturing our products so they are more effective, accessible, more conveniently stored, and more easily administered to patients.
Our broad immunotherapy and cell therapy platforms are designed to attack cancer and infectious pathogens by activating both the innate immune system—natural killer (NK) cells, dendritic cells, and macrophages—and the adaptive immune system—B cells and T cells—in an orchestrated manner. The goal of this potentially best-in-class approach is to generate immunogenic cell death thereby eliminating rogue cells from the body whether they are cancerous or virally infected and to ultimately establish an “immunological memory” that confers long-term benefit for the patient.
Although such designations may not lead to a faster development process or regulatory review and may not increase the likelihood that a product candidate will receive approval, Anktiva™(N-803), our novel antibody cytokine fusion protein, has received Breakthrough Therapy and Fast Track designations in combination with BCG from the U.S. Food and Drug Administration (FDA) for bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS). In May 2022, we announced the submissionlife of a Biologics License Application (BLA)project owing to, but not limited to, the FDA for ourfollowing:
•per patient trial costs;
•the number of sites included in the clinical trials;
•the countries in which the clinical trials are conducted;
•the length of time required to enroll eligible patients;
•the number of patients that participate in the clinical trials;
•the number of doses that patients receive;
•the cost of comparative agents used in clinical trials;
•the drop-out or discontinuation rates of patients;
•potential additional safety monitoring or other studies requested by regulatory agencies;
•the duration of patient follow-up; and
•the safety profile and efficacy of the product candidate.
We have only one product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In Julydisease, for which we submitted a BLA to the FDA in May 2022. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. On October 23, 2023, we announced that we had completed the resubmission of the BLA addressing the issues in the CRL. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a
complete response to the CRL. The FDA has set a target Prescription Drug User Fee Act (PDUFA) actionnew user fee goal date (PDUFA date) of April 23, 2024. There can be no assurance that our product candidate will be approved for commercial sale by the FDA in the near term, if ever. We do not expect any of our other product candidates to be commercially available for the foreseeable future, if ever.
Selling, General and Administrative
Selling, general and administrative expense consists primarily of salaries and personnel-related costs, including employee benefits and any stock-based compensation, for employees performing functions other than research and development. This includes personnel in executive, finance, human resources, information technology, legal, and administrative support functions. Other selling, general and administrative expenses include facility-related costs not otherwise allocated to research and development expense, professional fees for auditing, tax and legal services, advertising costs, expenses associated with strategic business transactions and business development efforts, obtaining and maintaining patents, consulting costs, royalties and licensing costs, and costs of our information systems.
We expect that our selling, general and administrative expense will increase for the foreseeable future as we expand operations, build out information systems and increase our headcount to support continued research activities and the development of our clinical programs. We have incurred and expect that we will continue to incur in the future, additional costs associated with operating as a public company, including costs to comply with stock exchange listing and SEC requirements, future funding efforts, corporate governance, internal controls, investor relations, disclosure and similar requirements applicable to public companies. Additionally, if and when we believe that a regulatory approval of a product candidate appears likely, we expect to incur significant increases in our selling, general and administrative expense relating to the sales and marketing of the approved product candidate.
Other Expense, Net
Other expense, net consists primarily of interest and investment income, interest expense (including amortization of debt discounts), unrealized gains and losses from investments in equity securities and equity-method investments, changes in fair value of warrants, derivative liabilities, and a convertible note, realized gains and losses on debt and equity securities, and gains and losses on foreign currency transactions.
Income Taxes
We are subject to U.S. federal income tax, as well as income tax in Italy, South Korea, California and other states. From inception through December 31, 2023, we have not been required to pay U.S. federal and state income taxes because of current and accumulated NOLs.
Discussion of Consolidated Results of Operations
Comparison of the Years Ended December 31, 2023 and 2022
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | | | |
| | 2023 | | 2022 | | $ Change | | % Change |
| | | | | | | | |
| | ($ in thousands) | | |
| | | | | | | | |
| Revenue | | $ | 622 | | | $ | 240 | | | $ | 382 | | | 159 | % |
| Operating expenses: | | | | | | | | |
Research and development (including amounts
with related parties) | | 232,366 | | | 248,149 | | | (15,783) | | | (6) | % |
Selling, general and administrative (including amounts
with related parties) | | 129,620 | | | 102,708 | | | 26,912 | | | 26 | % |
| Impairment of intangible assets | | 886 | | | 681 | | | 205 | | | 30 | % |
| Total operating expenses | | 362,872 | | | 351,538 | | | 11,334 | | | 3 | % |
| Loss from operations | | (362,250) | | | (351,298) | | | (10,952) | | | 3 | % |
| Other expense, net: | | | | | | | | |
| Interest and investment income (loss), net | | 1,131 | | | (3,090) | | | 4,221 | | | (137) | % |
| Interest expense (including amounts with related parties) | | (129,198) | | | (63,515) | | | (65,683) | | | 103 | % |
| Loss on equity method investment | | (7,549) | | | (12,107) | | | 4,558 | | | (38) | % |
| Change in fair value of warrant liabilities | | (47,600) | | | 13,460 | | | (61,060) | | | (454) | % |
| Change in fair value of convertible note | | (36,203) | | | — | | | (36,203) | | | — | % |
Other expense, net (including amounts
with related parties) | | (2,223) | | | (736) | | | (1,487) | | | 202 | % |
| Total other expense, net | | (221,642) | | | (65,988) | | | (155,654) | | | 236 | % |
| Loss before income taxes and noncontrolling interests | | (583,892) | | | (417,286) | | | (166,606) | | | 40 | % |
| Income tax benefit (expense) | | 40 | | | (34) | | | 74 | | | (218) | % |
| Net loss | | $ | (583,852) | | | $ | (417,320) | | | $ | (166,532) | | | 40 | % |
Revenue
Revenue increased $0.4 million during the year ended December 31, 2023, as compared to the year ended December 31, 2022. The increase was primarily driven by higher grant revenue received in 2023.
Research and Development Expense
Research and development expense decreased $15.8 million during the year ended December 31, 2023, as compared to the year ended December 31, 2022. The following table summarizes our research and development expenses during the years ended December 31, 2023 and 2022, together with the changes in those items (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | |
| | 2023 | | 2022 | | $ Change |
| | | | | | |
| External research and development expenses | | $ | 68,212 | | | $ | 63,113 | | | $ | 5,099 | |
| Internal research and development expenses: | | | | | | |
| Personnel-related costs | | 89,085 | | | 96,357 | | | (7,272) | |
| Equipment, depreciation, and facility costs | | 51,810 | | | 53,920 | | | (2,110) | |
| Other research and development costs | | 23,259 | | | 34,759 | | | (11,500) | |
| Total internal research and development expenses | | 164,154 | | | 185,036 | | | (20,882) | |
| Total research and development expenses | | $ | 232,366 | | | $ | 248,149 | | | $ | (15,783) | |
Research and development expense decreased $15.8 million primarily attributable to the following:
•A $5.1 million increase in external research and development expenses that was primarily due to higher qualification and testing costs, pre-licensing inspection costs, and outside consulting fees relating to regulatory and compliance services for the resubmission of the BLA, partially offset by a decrease in clinical trial costs and initial BLA submission fees;
•A $7.3 million decrease in personnel-related costs that was primarily due to the write off of accrued discretionary bonuses for 2022 not paid and a reduction in headcount, partially offset by an increase in stock-based compensation costs associated with new retention awards granted to existing employees;
•A $2.1 million decrease in equipment, depreciation, and facility costs that was primarily due to a reduction in activities related to facility expansion that occurred in the prior year, which resulted in lower expenditures for equipment qualification and certification, as well as lower expenses for sanitary services, partially offset by higher equipment maintenance costs; and
•An $11.5 million decrease in other research and development costs, primarily attributable to reductions in laboratory and test supplies and material supplies used for internal manufacturing, and no impairment costs from long-lived assets in the current year compared to the prior year, as well as an increase in collaboration costs allocated to a joint venture. Additionally, the write down of right-of-use assets from a lease used for R&D contributed to an additional decrease in other R&D expenses. The reduction in costs were partially offset by higher R&D activities and increased costs related to R&D licenses.
We expect our research and development expenses to increase significantly for the foreseeable future as we continue to invest in research and development activities related to developing our product candidates and conducting our ongoing and planned clinical trials.
Selling, General and Administrative Expense
Selling, general and administrative expense increased $26.9 million during the year ended December 31, 2023, as compared to the year ended December 31, 2022. The rise in expenses was primarily due to a $28.9 million increase in legal expenses, which was driven by higher defense costs and general legal services, as well as fewer legal insurance reimbursements received compared to the prior year. Additionally, there was a $1.1 million increase in personnel-related costs resulting from higher stock-based compensation expenses and travel costs, a $0.6 million increase in IT and security license fees, and a $0.2 million increase in marketing costs for pre-commercialization activities. These increases were partially offset by a $2.7 million reduction in consulting services from ERP implementations and professional fees from accounting and tax services, and a $1.2 million reduction in insurance expenses and other operating costs.
Impairment of Intangible Assets
Impairment of intangible assets increased $0.2 million during the year ended December 31, 2023, compared to the year ended December 31, 2022, due to an impairment charge of $0.9 million related to indefinite-lived intangible assets associated with the Tarmogen platform as the research and development program was limited in the current year. The $0.7 million write off of the organized workforce with definite lives during 2022 was as a result of the workforce reduction at the Dunkirk Facility.
Other Expense, Net
Other expense, net increased $155.7 million during the year ended December 31, 2023, as compared to the year ended December 31, 2022, due to a $65.7 million increase in interest expense driven by higher related-party borrowings, an increase in theTerm SOFR rate, and higher amortization of related-party notes discounts. Additionally, there was a total increase in expense of $97.3 million driven by the change in the fair value of warrant liabilities and the change in the fair value of a related-party convertible note, and a $1.5 million increase in expense due to higher issuance costs associated with our registered direct offerings, and an increase in other expenses. These changes were partially offset by a $4.6 million lower loss on equity method investments and a $4.2 million increase in investment income.
Comparison of the Years Ended December 31, 2022 to 2021
See Part II, Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Results of Operations” of our Annual Report filed with the SEC on March 1, 2023 for a discussion of the company’s results of operations during the year ended December 31, 2022 compared to the year ended December 31, 2021.
Financial Condition, Liquidity and Capital Resources
Sources of Liquidity
Since our inception, we have funded our operations primarily through proceeds from the issuance of related-party promissory notes and sales of our common stock under the ATM and our shelf registration statement, and through registered direct offerings.
On December 29, 2023, we entered into the RIPA to support the commercialization of Anktiva for the treatment of BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, the further development of our other product platforms, and to provide for other working capital needs.
Cash and Marketable Securities on Hand
As of December 31, 2023, we had cash and cash equivalents, and marketable securities of $267.4 million compared to $108.0 million as of December 31, 2022. We have typically invested our cash in a variety of financial instruments and classified these investments as available-for-sale. However, after our entry into the RIPA we can no longer invest our excess funds in corporate or European bonds. Certain of our investments are subject to credit, liquidity, market, and interest-rate risks. The general condition of the financial markets and the economy may increase those risks and may affect the value and liquidity of investments and restrict our ability to access the capital markets.
Proceeds from the ATM
During the year ended December 31, 2023, we received net proceeds of approximately $16.1 million from the issuance of 5,605,323 shares under the ATM. We used the net proceeds from this offering, together with other available funds, to progress our regulatory approval efforts, pre-commercialization activities and clinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes. As of December 31, 2023, we had $208.8 million available for future stock issuances under the ATM.
Shelf Registration Statement
During February 2023, we filed a $750.0 million shelf registration statement with the SEC on Form S-3 for the offering and sale of equity and equity-linked securities, including common stock, preferred stock, debt securities, depositary shares, warrants to purchase common stock, preferred stock or debt securities, subscription rights, purchase contracts, and units. During the year ended December 31, 2023, we sold shares of our common stock and warrants valued at $184.4 million under the shelf. As of December 31, 2023, we had $565.6 million available for use under the shelf. This available shelf is in addition to the ATM.
Proceeds from Registered Direct Offerings
On February 15, 2023, we entered into a securities purchase agreement with certain institutional investors for the purchase and sale of 14,072,615 shares of our common stock, as well as warrants (the February 2023 Warrants) that allow such investors to purchase an additional 14,072,615 shares of common stock at an exercise price of $4.2636 per share, for a purchase price of $3.5530 per share and accompanying warrant. This transaction generated net proceeds of approximately $47.0 million, after deducting placement agent fees and other offering costs. Pursuant to the terms of a letter agreement dated July 25, 2023, the company and the investors agreed to reduce the exercise price of the outstanding February 2023 Warrants from $4.2636 per share to $3.2946 per share and extend the expiration date of May 23, 2023. Itthe warrants until July 24, 2026.
On July 20, 2023, we entered into a securities purchase agreement with certain institutional investors for the purchase and sale of 14,569,296 shares of our common stock, as well as warrants that allow such investors to purchase an additional 14,569,296 shares of common stock at an exercise price of $3.2946 per share, for a purchase price of $2.7455 per share and accompanying warrant. This transaction generated net proceeds of approximately $37.5 million, after deducting placement agent fees and other offering costs.
We used the net proceeds from these registered direct offerings, together with other available funds, to progress our regulatory approval efforts, pre-commercialization activities and clinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes.
Related-Party Promissory Notes
On September 11, 2023, the company entered into a stock purchase agreement with Nant Capital, NantMobile and NCSC pursuant to which the noteholders exchanged promissory notes totaling approximately $270.0 million in aggregate principal amount and accrued and unpaid interest for an aggregate of 209,291,936 shares of common stock at an exchange price of $1.29 per share. As a result of the exchange, the company was forever released and discharged from all its obligations and liabilities under the notes.
On September 11, 2023, the company and Nant Capital entered into a series of letter agreements pursuant to which the maturity date of the related-party nonconvertible notes described above totaling $505.0 million in principal was extended from December 31, 2023 to December 31, 2024. In addition, the company entered into a $200.0 million September 2023 promissory note with Nant Capital.
On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into an amended and restated promissory note and a letter amendment for the following outstanding promissory notes. Pursuant to the terms of the amended and restated promissory note, the amended $505.0 million December 2023 promissory note is unclear whencomprised of a Tranche 1 principal amount of $125.0 million, which was previously the $125.0 million August 2022 promissory note before amendment, and a Tranche 2 with principal amount of $380.0 million, which is made up of the previous $300.0 million December 2021 promissory note, $50.0 million December 2022 promissory note, and $30.0 million June 2023 promissory note. In addition, the amendment allows Nant Capital, in its sole discretion, to convert all the Tranche 2 principal amount of $380.0 million of the amended promissory note into shares of the company’s common stock. The conversion price was determined subsequently at $8.2690 per share based on the agreement. The maturity date of the amended promissory note is December 31, 2025. Pursuant to the terms of the letter amendment, the maturity date of the $30.0 million March 2023 promissory note was extended from December 31, 2024 to December 31, 2025. Also, in connection with the RIPA transaction, all outstanding related-party promissory notes became subordinated to the RIPA payment obligations. After the amendment, the interest rates for the Tranche 1 principal amount and Tranche 2 principal amount of the $505.0 million December 2023 promissory note were Term SOFR plus 8.0% and Term SOFR plus 7.5%, respectively.
See Note 10, Related-Party Debt, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding our related-party debt. Revenue Interest Purchase Agreement
On December 29, 2023, we entered into the RIPA with Infinity and Oberland. Pursuant to the RIPA, Oberland acquired certain Revenue Interests from us for a gross purchase price of $200.0 million paid on closing, less certain transaction expenses. In addition, Oberland may purchase additional Revenue Interests from us in exchange for the $100.0 million Second Payment upon satisfaction of certain conditions specified in the RIPA, including following the receipt of approval by the FDA of the company’s BLA for N-803 in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease on or before June 30, 2024. Under the RIPA, Oberland has the right to receive quarterly payments from us based on, among other things, a certain percentage of our worldwide net sales, excluding those in China, during such quarter.
Also, on December 29, 2023 and in connection with the RIPA, we entered into an SPOA with Oberland pursuant to which we sold an aggregate of approximately $10.0 million of our common stock at $4.1103 per share in a private placement. Oberland also has an option to purchase up to an additional $10.0 million of our common stock, at a price per share to be determined by reference to the 30-day trailing volume weighted-average price of our common stock calculated from the date of exercise.
For more information, see Note9, Revenue Interest Purchase Agreement, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report.
Uses of Liquidity
In addition to the cash used to fund our operating activities discussed in “—Future Funding Requirements” below, we will approverequire cash to settle the following obligations: •As of December 31, 2023, our BLA,indebtedness payable at maturity totaled $735.0 million (excluding unamortized related-party notes discounts), held by entities affiliated with Dr. Soon-Shiong. In connection with the RIPA, all of our related-party promissory notes are now general unsecured obligations of the company that are subordinated in right of payment to indebtedness, obligations and other liabilities under the RIPA, the Revenue Interests issued pursuant to such agreement, and refinancing of the foregoing. In addition, the terms of promissory notes totaling $535.0 million were amended and restated to extend the maturity date by one year to December 31, 2025. The remaining $200.0 million promissory note and any accrued and unpaid interest are due on the earlier of (i) September 11, 2026 or (ii) upon the occurrence and during the continuance of an event of default (as defined in the note).
◦Pursuant to the terms of the amended and restated promissory note, the amended $505.0 million December 2023 promissory note is comprised of the Tranche 1 principal amount of $125.0 million and the Tranche 2 principal amount of $380.0 million. All Tranche 2 principal amount of $380.0 million and any accrued and unpaid interest can be converted into shares of the company’s stock at $8.2690 per share at the option of the noteholder. The interest rates for the Tranche 1 principal amount and Tranche 2 principal amount of the $505.0 million December 2023 promissory note were Term SOFR plus 8.0% per annum and Term SOFR plus 7.5% per annum, respectively.
◦The $200.0 million convertible promissory note bears interest at Term SOFR plus 8.0% per annum. The noteholder has the sole option to convert all of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $1.9350 per share.
◦The $30.0 million convertible promissory note bears interest at Term SOFR plus 8.0% per annum. The noteholder has the sole option to convert all of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $2.28 per share.
There can be no assurance that the company can refinance these promissory notes or what terms will be available in the market at the time of refinancing. Furthermore, if prevailing interest rates or other factors at the time of refinancing result in higher interest rates upon refinancing, then the interest expense relating to the refinanced indebtedness would increase. These risks could materially adversely affect the company’s financial condition, cash flows and results of operations.
•On December 29, 2023, we entered into the RIPA with Infinity and Oberland. Oberland has the right to receive quarterly Revenue Interest Payments from us based on, among other things, our worldwide net sales, excluding those in China, which will be tiered payments initially ranging from 3.00% to 7.00% (or after funding of the Second Payment, 4.50% to 10.00%), subject to increase or decrease, following December 31, 2029 (the Test Date) depending on whether our aggregate payments made to Oberland as of the Test Date have met or exceeded the Cumulative Purchaser Payments. In addition, if our aggregate payments made as of the Test Date to Oberland do not equal or exceed the amount of the Cumulative Purchaser Payments as of such date, then we are obligated to make a one-time True-Up Payment to Oberland in an amount equal to 100% of the Cumulative Purchaser Payments as of the Test Date, less the aggregate amount of our previous payments to Oberland as of the Test Date. See Note 9, Revenue Interest Purchase Agreement, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding the RIPA. •In connection with our 2017 acquisition of Altor, we issued CVRs under which we agreed to pay the prior stockholders of Altor approximately $304.0 million of contingent consideration upon calendar-year worldwide net sales of N-803 exceeding $1.0 billion prior to December 31, 2026, with amounts payable in cash or shares of our common stock or a combination thereof. As of December 31, 2023, Dr. Soon-Shiong and his related party hold approximately $139.8 million of net sales CVRs and they have both irrevocably agreed to receive shares of the company’s common stock in satisfaction of their CVRs. We may be required to pay the other prior Altor stockholders up to $164.2 million for their net sales CVRs should they choose to have their CVRs paid in cash instead of common stock. We may need to seek additional sources of capital to satisfy the CVR obligations if they are achieved.
•In connection with our acquisition of VivaBioCell, we are obligated to pay the former owners approximately $2.2 million of contingent consideration upon the achievement of a regulatory milestone relating to the GMP-in-a-Box technology.
Discussion of Consolidated Cash Flows
The following discussion of ImmunityBio’s cash flows is based on the consolidated statements of cash flows in Part II, Item 8. “Financial Statements and Supplementary Data” and is not meant to be an all‑inclusive discussion of the changes in its cash flows for the years presented below.
The following table sets forth our primary sources and uses of cash for the years indicated (in thousands):
| | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | 2022 |
| | | | |
| Cash provided by (used in): | | | | |
| Operating activities | | $ | (366,757) | | | $ | (337,509) | |
| Investing activities | | (30,470) | | | 27,297 | |
| Financing activities | | 558,341 | | | 233,613 | |
Effects of exchange rate changes on cash, cash equivalents,
and restricted cash | | (292) | | | 284 | |
| Net change in cash, cash equivalents, and restricted cash | | $ | 160,822 | | | $ | (76,315) | |
Operating Activities
During the year ended December 31, 2023, net cash used in operating activities of $366.8 million consisted of a net loss of $583.9 million, partially offset by $213.1 million in adjustments for non-cash items and $3.9 million of cash provided by net working capital. Adjustments for non-cash items primarily consisted of $49.2 million in stock-based compensation expense, $47.6 million in change in fair value of warrant liabilities, $42.4 million in amortization of debt issuance costs and accretion of discounts, $36.2 million in change in fair value of convertible notes, $18.5 million in depreciation and amortization expense, $9.2 million in non-cash interest primarily related to related-party promissory notes, $6.1 million in non-cash lease expense related to operating lease right‑of‑use assets, $2.0 million of transaction costs allocable to warrant liabilities, $1.6 million in unrealized losses on equity securities driven by a decrease in the value of our investments, $0.9 million in loss on impairment of intangible assets, partially offset by $0.5 million in other items, and a decrease of $0.1 million in amortization of premiums, net of discounts on marketable debt securities. The changes in net working capital consisted primarily of an increase of $6.7 million in accrued expenses, a decrease of $5.9 million in prepaid expenses and other current assets, and a decrease of $1.9 million in other assets, partially offset by a decrease of $6.5 million in accounts payable, a decrease of $3.0 million in operating lease liabilities, and a decrease of $1.1 million in due to related parties.
During the year ended December 31, 2022, net cash used in operating activities of $337.5 million consisted of a net loss of $417.3 million and $8.0 million of cash used in net working capital, partially offset by $87.8 million in adjustments for non-cash items. The changes in net working capital consisted primarily of an increase of $16.6 million in prepaid and other current assets, and decreases of $4.3 million in operating lease liabilities and $1.2 million with related parties, partially offset by an increase of $8.0 million in accounts payable, an increase of $4.1 million in accrued expenses and other liabilities, and a decrease of $2.0 million in investment and other assets. Adjustments for non-cash items primarily consisted of $40.2 million in stock-based compensation expense, $18.3 million in depreciation and amortization expense, $16.3 million in amortization of debt issuance costs and accretion of discounts, $11.7 million in non-cash interest primarily related to related-party promissory notes, $5.9 million in non‑cash lease expense related to operating lease right-of-use assets, $4.2 million in unrealized losses on equity securities driven by a decrease in the value of our investments, $1.3 million in loss on impairment of fixed assets, $1.3 million in amortization of premiums, net of discounts, on marketable debt securities, $1.1 million in transaction costs allocable to a warrant liability, a non-cash $0.7 million loss on the impairment of intangible assets, and $0.3 million in other non-cash items, reduced by a $13.5 million change in fair value of a warrant liability.
We have historically experienced negative cash flows from operating activities, with such negative cash flows likely to continue for the foreseeable future.
Investing Activities
During the year ended December 31, 2023, net cash used in investing activities was $30.5 million, which included cash outflows of $30.6 million in purchases of property, plant and equipment (including construction in progress and the completion of a warehouse facility), and $10.4 million in purchases of marketable debt securities, partially offset by cash inflows of $10.5 million from maturities and sales of marketable debt and equity securities. Our investments in property, plant and equipment are primarily related to acquisitions of equipment that will be used for the manufacturing of our product candidates and expenditures related to the build out of our manufacturing facilities.
During the year ended December 31, 2022, net cash provided by investing activities was $27.3 million, which included cash inflows of $162.0 million from maturities and sales of marketable debt and equity securities, partially offset by $78.2 million of purchases of property, plant and equipment (including construction in progress and depreciable property acquired in the Dunkirk acquisition), $34.3 million of purchases of marketable debt securities, $21.2 million for purchase of intangible assets (related to the Dunkirk acquisition), and a $1.0 million investment in a joint venture. Our investments in property, plant and equipment are primarily related to acquisitions of equipment that will be used for the manufacturing of our product candidates and expenditures related to the build out of our manufacturing facilities.
We expect to accelerate our capital spending as we scale our cGMP manufacturing capabilities, which will require significant capital for the foreseeable future.
Financing Activities
During the year ended December 31, 2023, net cash provided by financing activities was $558.3 million, which consisted of $258.7 million in net proceeds from issuances of related-party promissory notes, $192.8 million in net proceeds from payments received pursuant to the RIPA, $84.4 million in net proceeds from registered direct offerings, $16.1 million in net proceeds from the ATM offering, $9.5 million in net proceeds from issuance of common stock in connection with the RIPA, and $0.3 million in proceeds from the exercise of stock options, partially offset by $3.4 million used for payment of payroll tax withholding in connection with the net share settlement of vested RSUs, and $0.1 million used for principal payments of finance leases.
During the year ended December 31, 2022, net cash provided by financing activities was $233.6 million, which consisted of $174.1 million in net proceeds from issuances of related-party promissory notes, $47.3 million in net proceeds from a registered direct offering, and $13.1 million in net proceeds from the ATM offering, partially offset by $0.6 million related to net share settlement of vested RSUs for payment of payroll tax withholding and a $0.3 million used in other financing activities.
We will need to raise additional capital to fund our future cash needs.
Comparison of the Years Ended December 31, 2022 to 2021
See Part II, Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Results of Operations” of our Annual Report filed with the SEC on March 1, 2023 for a discussion of the company’s consolidated cash flows for the year ended December 31, 2022 compared to the year ended December 31, 2021.
Future Funding Requirements
From inception through the date of this Annual Report we have generated minimal revenue, we have no clinical products approved for commercial sale, and we have not generated any revenue from therapeutic and vaccine product candidates that are under development. We do not expect to generate significant revenue unless and until we obtain regulatory approval of and commercialize any of our product candidates, and we do not know when, or if, this will occur. In addition, we expect our operating expenses to significantly increase in connection with our ongoing development activities, particularly as we continue the research, development and clinical trials of, and seek regulatory approval for, our product candidates. We have also incurred and expect that we will continue to incur in the future additional costs associated with operating as a public company as well as costs related to future fundraising efforts. In addition, subject to obtaining regulatory approval of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. We anticipate that we will need substantial additional funding in connection with our continuing operations.
We expect that our operating expenses will increase substantially if and as we:
•continue research and development, including preclinical and clinical development of our existing product candidates;
•potentially seek regulatory approval for our product candidates;
•seek to discover and develop additional product candidates;
•establish a commercialization infrastructure and scale up our manufacturing and distribution capabilities to commercialize any of our product candidates for which we may obtain regulatory approval;
•seek to comply with regulatory standards and laws;
•maintain, leverage and expand our intellectual property portfolio;
•hire clinical, manufacturing, scientific and other personnel to support our product candidates’ development and future commercialization efforts;
•add operational, financial and management information systems and personnel; and
•incur additional legal, accounting and other expenses in operating as a public company.
As a result of continuing anticipated operating cash outflows, we believe that substantial doubt exists regarding our ability to continue as a going concern without additional funding or financial support. However, we believe our existing cash, cash equivalents, and investments in marketable securities, together with capital to be raised through equity offerings (including but not limited to the offering, issuance and sale by us of our common stock that may be issued and sold under the ATM, of which we had $208.8 million available for future issuance as of December 31, 2023, and a February 2023 shelf registration statement, of which we had $565.6 million available for future offerings as of December 31, 2023), and our potential ability to borrow from affiliated entities, will be sufficient to fund our operations through at least the next 12 months following the issuance date of the consolidated financial statements based primarily upon our Executive Chairman and Global Chief Scientific and Medical Officer’s intent and ability to support our operations with additional funds, including loans from affiliated entities, as required, which we believe alleviates such doubt. We may also seek to sell additional equity, through one or more follow-on public offerings, or in separate financings, or obtain a credit facility, issue other debt in compliance with the terms of the RIPA, or engage in strategic partnership transactions. However, we may not be able to secure such external financing in a timely manner or on favorable terms, if at all. Without additional funds, we may choose to delay or reduce our operating or investment expenditures. Further, because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we may need additional funds to meet our needs sooner than planned.
We will need to obtain additional financing to fund our future operations, including completing the development and commercialization of our product candidates. Changing circumstances may cause us to increase our spending significantly faster than we currently anticipate and we may need to raise additional funds sooner than we presently anticipate. Moreover, research and development and our operating costs and fixed expenses such as rent and other contractual commitments, including those for our research collaborations, are substantial and are expected to increase in the future.
Our platforms, whichfuture funding requirements will depend on many factors, including, but not limited to:
•progress, timing, number, scope and costs of researching and developing our product candidates and our ongoing, planned and potential clinical trials;
•time and cost of regulatory approvals;
•our ability to successfully commercialize any product candidates, if approved and the costs of such commercialization activities;
•revenue from product candidates that we may commercialize, if any, including the selling prices for such potential products and the availability of adequate third-party coverage and reimbursement for patients;
•interest and principal payments on our related-party promissory notes, and payment of Revenue Interests, the Second Payment and Test Date payments due under the RIPA;
•cost of building, staffing and validating our own manufacturing facilities in the U.S., including having a product candidate successfully manufactured consistent with FDA and EMA regulations;
•terms, timing and costs of our current and any potential future collaborations, business or product acquisitions, CVRs, milestones, royalties, licensing or other arrangements that we have established or may establish;
•time and cost necessary to respond to technological, regulatory, political and market developments; and
•costs of filing, prosecuting, maintaining, defending and enforcing any patent claims and other intellectual property rights.
Unless and until we can generate a sufficient amount of revenues, we may finance future cash needs through public or private equity offerings, license agreements, debt financings, collaborations, strategic alliances and marketing or distribution arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms, or at all, including but not limited to the offering, issuance and sale by us of our common stock that may be issued and sold under the ATM.
To the extent that we raise additional capital through the sale of equity or equity-linked securities (including warrants), convertible debt or under the ATM, our shelf registration statement, or other offerings, or if any of our current debt is converted into equity or if our existing warrants are exercised, your ownership interest will be diluted, and the terms may include 9 first-in-human therapeutic agents,liquidation or other preferences that adversely affect your rights as a stockholder. The incurrence of additional indebtedness would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms unfavorable to us. We have no committed source of additional capital and if we are being studiedunable to raise additional capital in 26 actively recruiting clinical trials—17sufficient amounts or on terms acceptable to us, we may be required to delay or reduce the scope of whichor eliminate one or more of our research or development programs or our commercialization efforts. Our current license and collaboration agreements may also be terminated if we are in Phase 2unable to meet the payment obligations under those agreements. As a result, we may seek to access the public or 3 development—across 12 indications in liquid and solid tumors, including bladder, pancreatic and lung cancers. Theseprivate capital markets whenever conditions are among the most frequent and lethal cancer typesfavorable, even if we do not have an immediate need for which there are high failure rates for existing standardsadditional capital at that time.
Contractual Obligations
We have established Good Manufacturing Process (GMP) manufacturing capacitymaterial cash requirements to pay related-party affiliates and third parties under various contractual obligations discussed below:
•We are obligated to make payments to several related-party affiliates under written agreements and other informal arrangements. We are also obligated to pay interest and to repay principal under our related-party promissory notes. For information regarding our financing obligations, see Note 10, Related-Party Debt, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report. •We are obligated to make payments to Oberland associated with our revenue interest liability, which do not have a fixed repayment schedule. Oberland’s right to receive payments under the RIPA shall terminate when Oberland has received maximum payments (including any True-Up Payment) equal to 195.0% of the then Cumulative Purchaser Payments unless the RIPA is terminated prior to such date.
Under the terms of the agreement, prior to the Test Date, every $100.0 million of worldwide net sales, excluding those in China, of less than or equal to $600.0 million in a calendar year would result in a tiered Revenue Interest Payment of approximately $7.0 million or 7.0% (or $10.0 million or 10.0% after funding of the Second Payment). Worldwide net sales, excluding those in China, for a calendar year exceeding $600.0 million would result in a tiered Revenue Interest Payment of approximately $3.0 million or 3.0% (or $4.5 million or 4.5% after funding of the Second Payment) for every $100.0 million of worldwide net sales, excluding those in China, above the threshold.
In the future, cumulative worldwide net sales, excluding those in China, levels up to the Test Date will determine whether or not we are required to make a True-Up Payment and implement modified payment rates. The amount of the obligation and timing of payment is likely to change. See Note 9, Revenue Interest Purchase Agreement, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding the RIPA. •We are obligated to make payments under our operating leases, which primarily consist of facility leases. See Note 8, Lease Arrangements, and Note 11, Related-Party Agreements, of the “Notes to Consolidated Financial Statements” that appear in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for information regarding our lease obligations. •In connection with the acquisitions of Altor and VivaBioCell, we are obligated to pay contingent consideration upon the achievement of certain milestones. See Note 7, Commitments and Contingencies—Contingent Consideration Related to Business Combinations, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for information regarding our contingent consideration obligations. •We have contractual obligations to make payments to related-party affiliates and third parties under unconditional purchase arrangements. See Note 7, Commitments and Contingencies—Unconditional Purchase Obligations, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for information on these unconditional purchase obligations. •We have certain contractual commitments that are expected to be paid in fiscal year 2024, depending on the progress of build outs, completion of services, and the realization of milestones associated with third-party agreements. This amounts totals $60.8 million and is primarily related to capital expenditures, open purchase orders as of December 31, 2023 for the acquisition of goods and services in the ordinary course of business, and near-term upfront milestone payments to third parties.
•In addition, we have contractual commitments that are expected to be paid in fiscal year 2025 and beyond based on the achievement of various development, regulatory and commercial milestones for agreements with third parties. These payments may not be realized or may be modified and are contingent upon the occurrence of various future events, substantially all of which have a high degree of uncertainty of occurring. As of December 31, 2023, the maximum amount that may be payable related to these commitments is $769.3 million.
•In connection with our leasehold interest in the Dunkirk Facility, we committed to spend an aggregate of $1.52 billion on operational expenses during the initial 10-year term, and an additional $1.50 billion on operational expenses if we elect to renew the lease for the additional 10-year term. These amounts are not included in the discussion above. See Note 6, Collaboration and License Agreements and Acquisition, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information on these obligations. Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of consolidated financial statements requires management to make certain estimates and assumptions that affect the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities at scalethe date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, we evaluate our estimates, including those related to the valuation of equity-based awards, deferred income taxes and related valuation allowances, revenue interest liability, preclinical and clinical trial accruals, impairment assessments, CVR measurement and assessments, the measurement of right-of-use assets and lease liabilities, useful lives of long-lived assets, loss contingencies, fair value calculation of warrants and convertible promissory notes, fair value measurements, asset acquisition, and the assessment of our ability to fund our operations for at least the next 12 months from the date of issuance of these consolidated financial statements. We base our estimates on historical experience and on various other market-specific and relevant assumptions that we believe to be reasonable under the circumstances. Estimates are assessed each period and updated to reflect current information, such as the economic considerations related to the impact that any ongoing pandemic could have on our significant accounting estimates. Actual results could differ from those estimates.
While our significant accounting policies are more fully described in the notes accompanying our consolidated financial statements that appear in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report, we believe the following accounting policies to be the most critical in understanding the judgments and estimates we use in preparing our consolidated financial statements:
Revenue Recognition
We have primarily generated revenues from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables and grant programs. The nonexclusive license agreements with cutting-edgea limited number of pharmaceutical and biotechnology companies grant them the right to use our cell manufacturing expertiselines and ready-to-scale facilities,intellectual property for non-clinical use. These agreements generally include upfront fees and annual research license fees for such use, as well as extensivecommercial license fees for sales of the licensee products developed or manufactured using our intellectual property and seasonedcell lines. We have generated revenues from product sales of our proprietary GMP-in-a-Box bioreactors and related consumables, primarily to related parties. Additionally, we also generated revenues from grant programs.
A performance obligation is a promise in a contract to transfer a distinct good or service to the customer. A contract’s transaction price is allocated to each distinct performance obligation based on relative standalone selling price and recognized as revenue when, or as, the performance obligation is satisfied.
Under our license agreements with customers, we typically promise to provide a license to use certain cell lines and related patents, the related know-how, and future research and development (R&D),data that affect the license. We have concluded that these promises represent one performance obligation due to the highly interrelated nature of the promises. We provide the cell lines and know-how immediately upon entering into the contracts. Research and development data are provided throughout the term of the contract when and if available.
The license agreements may include non-refundable upfront payments, event-based milestone payments, sales-based royalty payments, or some combination of these. The event-based milestone payments represent variable consideration and we use the most likely amount method to estimate this variable consideration. Given the high degree of uncertainly around the achievement of these milestones, we do not recognize revenue from these milestone payments until the uncertainty associated
with these payments is resolved. We currently estimate variable consideration related to milestone payments to be zero and, as such, no revenue has been recognized for milestone payments. We recognize revenue from sales-based royalty payments when or as sales occur. On a quarterly basis, we re-evaluate our estimate of milestone variable consideration to determine whether any amount should be included in the transaction price and recorded in revenue prospectively.
We also have sold our proprietary GMP-in-a-Box bioreactors and related consumables to affiliated companies. The arrangements typically include delivery of bioreactors, consumables, and providing installation service and perpetual software licenses for using the equipment. We recognize revenue when customers obtain control and can benefit from the promised goods or services, generally upon installation of the bioreactors, in an amount that reflects the consideration that we expect to receive in exchange for those goods or services. Upfront payments and fees are recorded as deferred revenue upon receipt and recognized as revenue when we satisfy our performance obligations under these arrangements.
Grant revenue is typically paid for reimbursable costs incurred over the duration of the associated research project or clinical trial and is recognized either when expenses reimbursable under the grants have been incurred and payments under the grants become contractually due or when cash is received, depending on the certainty of payment and other factors specific to each grant.
Revenue Interest Liability
On December 29, 2023, we entered into the RIPA with Infinity and Oberland. Pursuant to the RIPA, Oberland acquired certain Revenue Interests from us for a gross purchase price of $200.0 million paid on closing. In addition, Oberland may purchase additional Revenue Interests from us in exchange for the $100.0 million Second Payment upon satisfaction of certain conditions specified in the RIPA. Under the RIPA, Oberland has the right to receive quarterly payments from us based on, among other things, a certain percentage of our worldwide net sales, excluding those in China, during such quarter. The RIPA is considered a sale of future revenues and is accounted for as a liability net of a debt discount comprised of deferred issuance costs, the fair value of a freestanding option agreement related to the SPOA, and the fair value of embedded derivatives requiring bifurcation on the consolidated balance sheet. The company imputes interest expense associated with this liability using the effective interest rate method. The effective interest rate is calculated based on the rate that would enable the debt to be repaid in full over the anticipated life of the arrangement. Interest expense is recognized over the estimated term on the consolidated statement of operations. The interest rate on this liability may vary during the term of the agreement depending on a number of factors, including the level of actual and forecasted net sales. The company evaluates the interest rate quarterly based on actual and forecasted net sales utilizing the prospective method. A significant increase or decrease in actual or forecasted net sales will materially impact the revenue interest liability, interest expense, and the time period for repayment.
Derivative Liabilities
Embedded derivatives that are required to be bifurcated from the underlying debt instrument that do not meet the derivative scope exception and equity classification criteria are accounted for and valued as separate financial instruments. The terms of an embedded derivative related to a contingent exercisable prepayment feature of a convertible note have been evaluated and deemed to require bifurcation. This embedded derivative will be initially measured at fair value and will be remeasured to fair value at each reporting date until the derivative is settled.
In addition, the RIPA contains certain features that meet the definition of being an embedded derivative requiring bifurcation as a separate compound financial instrument apart from the RIPA. The derivative liability is initially measured at fair value upon issuance and is subject to remeasurement at each reporting period with changes in fair value recognized in other expense, net, on the consolidated statement of operations.
Preclinical and Clinical Trial Accruals
As part of the process of preparing the consolidated financial statements, we are required to estimate expenses resulting from obligations under contracts with vendors, clinical research organizations and consultants. The financial terms of these contracts vary and may result in payment flows that do not match the periods over which materials or services are provided under such contracts.
We estimate clinical trial and research agreement-related expenses based on the services performed, pursuant to contracts with research institutions and clinical research organizations and other vendors that conduct clinical trials and research on our behalf. In accruing clinical and research-related fees, we estimate the time period over which services will be performed and activity expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, we will adjust the accrual accordingly. Payments made under these arrangements in advance of the receipt of the related services are recorded as prepaid expenses until the services are rendered.
Research and Development Costs
Major components of research and development costs include cash compensation and other personnel-related expenses, stock-based compensation, depreciation and amortization expense on research and development property and equipment and intangible assets, costs of preclinical studies, clinical trials costs, including CROs and related clinical manufacturing, including third-party CMOs, costs of drug development, costs of materials and supplies, facilities cost, overhead costs, regulatory and compliance costs, and fees paid to consultants and other entities that conduct certain research and development activities on our behalf. Costs incurred in research and development are expensed as incurred.
The company classifies its research and development expenses as either external or internal. The company’s external research and development expenses support its various preclinical and clinical programs. The company’s internal research and development expenses include payroll and benefits expenses, facilities and equipment expense, and other indirect research and development expenses incurred in support of its research and development activities. The company’s external and internal resources are not directly tied to any one research or drug discovery program and are typically deployed across multiple programs and are not allocated to specific product candidates or development programs.
Included in research and development costs are clinical trial and research expenses based on the services performed pursuant to contracts with research institutions and clinical research organizations and other vendors that conduct clinical trials and research on our behalf. We record accruals for estimated costs under these contracts. When evaluating the adequacy of the accrued liabilities, we analyze the progress of the preclinical studies or clinical trials, including the phase or completion of events, invoices received, contracted costs and purchase orders. Significant judgments and estimates are made in determining the accrued balances at the end of any reporting period based on the facts and circumstances known at that time. Although we do not expect the estimates to be materially different from the amounts actually incurred, if the estimates of the status and timing of services performed differ from the actual status and timing of services performed, we may report amounts that are too high or too low in any particular period. Actual results could differ from our estimates. We adjust the accruals in the period when actual costs become known.
Contingencies
We record accruals for loss contingencies to the extent that we conclude it is probable that a liability has been incurred and the amount of the related loss can be reasonably estimated. We accrue for the best estimate of a loss within a range; however, if no estimate in the range is better than any other, then we accrue the minimum amount in the range. If we determine that a material loss is reasonably possible, we disclose the possible loss or range of loss, or that the amount of loss cannot be estimated at this time. We evaluate, on a quarterly basis, developments in legal proceedings and other matters that could cause a change in the potential amount of the liability recorded or the range of potential losses disclosed. Moreover, we record gain contingencies only when they are realizable and the amount is known. Additionally, we record our rights to insurance recoveries, limited to the extent of incurred or probable losses, as a receivable when such recoveries have been agreed to with our third-party insurers and when receipt is deemed probable. This includes instances when our third-party insurers have agreed to pay, on our behalf, certain legal defense costs and settlement amounts directly to applicable law firms and a settlement fund.
Warrants
The company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC Topic 480, Distinguishing Liabilities from Equity (ASC 480) and FASB ASC Topic 815, Derivatives and Hedging (ASC 815). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are
indexed to the company’s own stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For warrants that meet all criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital, on the consolidated statement of stockholders’ deficit at the time of issuance. For warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and on each balance sheet date thereafter. Changes in the estimated fair value of the warrants are recognized as a non-cash gain or loss in other expense, net, on the consolidated statement of operations. The fair value of the warrants was estimated using the Black-Scholes option pricing model.
Fair Value Option Election
The company accounted for a convertible note issued on March 31, 2023 under the FVO election of ASC 825 until it was amended and restated on December 29, 2023. Prior to its extinguishment on December 29, 2023, the convertible note was a debt host financial instrument containing embedded features wherein the entire financial instrument was initially measured at its issuance-date estimated fair value and then subsequently remeasured at estimated fair value on a recurring basis at each reporting period date.
Changes in the estimated fair value of this convertible note were recorded in other expense, net, on the consolidated statement of operations, except that changes in estimated fair value caused by changes in the instrument-specific credit risk were recorded in other comprehensive (loss) income. In accordance with ASC 470-50, when the convertible note was extinguished on December 29, 2023, the cumulative amount previously recorded in other comprehensive (loss) income resulting from changes in the instrument-specific credit risk were reclassified and reported in current earnings on the consolidated statement of operations.
Debt Modification and Extinguishment
The company evaluates amendments to its debt instruments in accordance with ASC 470-50. This evaluation includes comparing (1) if applicable, the net present value of future cash flows of the amended debt to that of the original debt and (2) the change in fair value of an embedded conversion feature to that of the carrying amount of the debt immediately prior to amendment to determine, in each case, if a change greater than 10% occurred. In instances where the net present value of future cash flows or the fair value of an embedded conversion feature, if any, changed more than 10%, the company applies extinguishment accounting. In instances where the net present value of future cash flows and the fair value of an embedded conversion feature, if any, changed less than 10%, the company accounts for the amendment to the debt as a debt modification. Gains and losses on debt amendments that are considered extinguishments are recognized in current earnings or in additional paid-in capital if the transactions are with entities under common control. Debt amendments that are considered debt modifications are accounted for prospectively through yield adjustments, based on the revised terms. The increase in fair value of the embedded conversion feature from the debt modification was accounted for as an increase in debt discount with a corresponding increase in additional paid-in capital. Legal fees and other costs incurred with third parties that are directly related to debt modifications are expensed as incurred. Amounts paid by the company to the lenders, are reflected as additional debt discount and amortized as an adjustment of interest expense over remaining term of modified debt using the effective interest rate method.
Stock-Based Compensation
We account for stock-based compensation under the provisions of FASB ASC Topic 718, Compensation—Stock Compensation (ASC 718). We estimate fair value of each stock option award on the date of grant using the Black-Scholes option pricing model. The Black-Scholes option pricing model requires the use of subjective assumptions, including, but not limited to, expected stock price volatility over the term of the awards and the expected term of the stock options. We measure the fair value of an equity-classified award at the grant date and recognize the stock-based compensation expense over the period of vesting on the straight-line basis for our outstanding share awards that do not contain a performance condition. For awards subject to performance-based vesting conditions, we assess the probability of the individual milestones under the award being achieved and stock-based compensation expense is recognized over the service period using the graded vesting method once management believes the performance criteria is probable of being met. For awards with service or performance conditions, we recognize the effect of forfeitures in compensation cost in the period that the award was forfeited.
Business Combinations
Business combinations are accounted for using the acquisition method of accounting in accordance with ASC 805-50. These standards require that the total cost of acquisition be allocated to the tangible and intangible assets acquired and liabilities assumed based on their respective fair values at the date of acquisition, with the excess purchase price recorded as goodwill. The allocation of the purchase price is dependent upon certain valuations and other studies. Acquisition costs are expensed as incurred.
Contingent consideration incurred in connection with a business combination are recorded at their fair values on the acquisition date and re-measured at their fair values each subsequent reporting period until the related contingencies are resolved. The resulting changes in fair values are recorded as research and development expense, on the consolidated statements of operations and development teams.comprehensive income (loss). Changes in fair values reflect changes to our assumptions regarding probabilities of successful achievement of related milestones, the timing in which the milestones are expected to be achieved, and the discount rate used to estimate the fair value of the obligation.
Recent Accounting Pronouncements
See Note 2, Summary of Significant Accounting Policies, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding recent accounting pronouncements or changes in accounting pronouncements that are of significance, or potential significance, to us. ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Our exposure to market risk for changes in interest rates relates primarily to interest earned on our cash equivalents, investments and variable interest rate debt. The primary objective of our investment activities is to preserve our capital to fund operations. A secondary objective is to maximize income from our investments without assuming significant risk. Our investment policy provides for investments in low-risk, investment-grade debt instruments. As of December 31, 2023, we had $265.5 million in cash and cash equivalents and $1.9 million in our investment portfolio. Our cash equivalents are short-term investments with maturities of 90 days or less at the time of purchase. We maintain cash deposits in FDIC insured financial institutions in excess of federally insured limits. However, we believe that we are not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. As of December 31, 2023, our investment portfolio was comprised of available-for-sale securities, and we did not hold or issue financial instruments for trading purposes.
Interest Rate Risk – Cash
With the cash discussed above, our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S interest rates. However, we do not believe a sudden change in the interest rates would have a material impact on our financial condition or results of operations due to the short-term maturities of our cash equivalents. A hypothetical 100 basis point change in interest rates during any of the periods presented would not have had a material impact on our consolidated financial statements.
Interest Rate Risk – Cash Equivalents and Investment Portfolio
We invest a portion of our cash in a number of diversified fixed-and floating-rate securities, consisting of marketable debt securities and debt funds that are subject to interest rate risk. Changes in the general level of interest rates can affect the fair value of our investment portfolio. If interest rates in the general economy were to rise, our holdings could lose value. At December 31, 2023, a hypothetical increase in interest rates of 100 basis points across the entire yield curve on our holdings would not have resulted in a material impact on the fair value of our portfolio.
Interest Rate Risk – Variable-Rate Debt
Our use of variable-rate debt exposes us to interest rate risk as changes in interest rates would affect interest expense. As of December 31, 2023, we have variable-rate loans outstanding totaling $735.0 million, of which:
•$380.0 million matures on December 31, 2025 and bears interest at 3-month Term SOFR plus 7.5% per annum;
•$155.0 million matures on December 31, 2025 and bears interest at 3-month Term SOFR plus 8.0% per annum; and
•$200.0 million matures on September 11, 2026 and bears interest at 1-month Term SOFR plus 8.0% per annum.
As of December 31, 2023, the weighted-average interest rate on these loans was 13.09%. A hypothetical 100-basis point increase in the Term SOFR rate as of December 31, 2023 would increase our future interest payments by $16.1 million. Similarly, a hypothetical 100-basis point decrease in the Term SOFR rate as of December 31, 2023 would decrease our future interest payment by $16.1 million.
Interest Rate Risk – RIPA
We have entered into a revenue interest purchase agreement. Our primary exposure to market risk is that the interest rate on the revenue interest liability may vary during the term of the agreement depending on a number of factors, including the level of forecasted net sales. A significant increase or decrease in actual or forecasted net sales will materially impact the revenue interest liability, interest expense, and the time period for repayment. See Note9, “Revenue Interest Purchase Agreement,” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding the terms of the RIPA. Foreign Currency Exchange Risk
We are exposed to foreign currency exchange rate risk inherent in conducting business globally in numerous currencies. We contract with clinical research organizations, investigational sites and suppliers in foreign countries. We are, therefore, subject to fluctuations in foreign currency rates in connection with these agreements. We have not entered into any material foreign currency hedging contracts although we may do so in the future. From inception through the date of this Annual Report, we have not incurred any material effects from foreign currency changes on these contracts. The effect of a 10% adverse change in exchange rates on foreign currency denominated cash and payables as of December 31, 2023 would not have been material. However, fluctuations in currency exchange rates could harm our business in the future.
We are also exposed to foreign currency fluctuations related to the operations of our subsidiary in Italy whose financial statements are denominated in the Euro. We translate all assets and liabilities denominated in foreign currency into U.S. dollars using the exchange rate as of the end of the reporting period, while the operating results are translated into U.S. dollars using the average exchange rates for the reporting periods. Gains and losses resulting from translating the financial statements from our subsidiary’s functional currency to U.S. dollars are recognized as a component of other comprehensive (loss) income, on the consolidated statement of comprehensive loss. Foreign currency exchange rate fluctuations affect our reported net loss and can make comparisons from period to period more difficult. Our foreign operations are not material to our operations as a whole. As such, we currently do not enter into currency forward exchange or option contracts to hedge foreign currency exposures.
Market Risk
As of December 31, 2023, 37,732,820 warrants from our direct registered offerings remained outstanding at a fair value of $118.8 million. The fair value of the warrant liabilities is determined using the Black-Scholes option pricing model and is therefore sensitive to changes in the market price and volatility of our common stock among other factors. In the event of a hypothetical 10% increase in the market price of our common stock ($5.52 based on the $5.02 market price of our stock at December 31, 2023) on which the December 31, 2023 valuation was based, the fair value of the warrant liabilities would have increased by $15.6 million. Similarly, based on the fair value of the warrants outstanding as of December 31, 2023, a hypothetical decrease of 10% in the market price of our common stock would have the fair value of the warrant liabilities decreased by $15.5 million. Such increase or decrease would have been reflected as a change in fair value of warrant liabilities in other expense, net, on the consolidated statement of operations.
Inflation Risk
Inflation may affect us by increasing our cost of labor, clinical trial, and other costs. We do not believe that inflation has had a material effect on our business, financial condition or results of operations for any period presented herein.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of ImmunityBio, Inc. and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of ImmunityBio, Inc. and Subsidiaries (the Company) as of December 31, 2023 and 2022, the related consolidated statements of operations, comprehensive loss, stockholders’ deficit and cash flows for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
| | | | | |
| Related party transactions and disclosures |
| |
Description of the
Matter | As described in Notes 10 and 11 to the consolidated financial statements, the Company’s Executive Chairman, Global Chief Scientific and Medical Officer and principal stockholder Dr. Patrick Soon-Shiong has a controlling interest in certain entities with which the Company has entered into material transactions including related party debt transactions with Nant Capital, LLC, NantWorks, NantMobile, LLC and NantCancerStemCell. Affiliates of such entities are also affiliates of the Company due to the common control of the Company’s Executive Chairman, Global Chief Scientific and Medical Officer and principal stockholder. Assessing the sufficiency of procedures performed to identify related parties and significant related party transactions and determining the identified significant related party transactions were properly recorded, presented and disclosed was challenging due to the nature, volume and the significance of related party transactions. |
| |
How We Addressed
the Matter in Our
Audit | We obtained an understanding, evaluated the design, and tested the operating effectiveness of internal controls over the Company’s related party process. This included testing controls over management’s identification, review, recognition and disclosure of significant related party transactions. The audit procedures we performed included, among others, testing the completeness and accuracy of the listing of related parties identified and significant related party transactions provided by management, and testing the manner in which significant related party transactions were recorded, presented and disclosed. We performed journal entry searches of identified related parties to verify completeness and accuracy of the Company’s significant related party transactions. We inquired of management, members of the Company’s audit committee, and the Company’s related party transaction committee chair regarding the completeness of the significant related party transactions identified. We also inspected questionnaires received from the Company’s directors and officers, read minutes of the meetings of Board of Directors and its various Committees, read employment and compensation contracts, proxy statements and other relevant filings with the Securities and Exchange Commission that relate to the Company’s financial relationships and transactions with the Company’s executive officers and with other entities controlled by the Company’s Executive Chairman, Global Chief Scientific and Medical Officer and principal stockholder. We confirmed the significant transactions and/or balances, as applicable, with the related parties. We also obtained the underlying agreements for significant related party transactions and assessed the associated accounting and recognition. We involved valuation specialists to assist in evaluating the appropriateness of the valuation methodologies and reasonableness of the assumptions used in the valuation of the related party debt including underlying conversion features and embedded derivatives, as deemed necessary, as described in Note 10 to the consolidated financial statements. |
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2016.
Los Angeles, California
March 19, 2024
ImmunityBio, Inc. and Subsidiaries
Consolidated Balance Sheets
(in thousands, except share and per share amounts)
| | | | | | | | | | | |
| As of December 31, |
| 2023 | | 2022 |
| ASSETS | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 265,453 | | | $ | 104,641 | |
| Marketable securities | 1,009 | | | 2,543 | |
| Due from related parties | 2,019 | | | 1,890 | |
| Prepaid expenses and other current assets (including amounts with related parties) | 25,603 | | | 31,503 | |
| Total current assets | 294,084 | | | 140,577 | |
| Marketable securities, noncurrent | 891 | | | 840 | |
| Property, plant and equipment, net | 146,082 | | | 143,659 | |
| Intangible assets, net | 17,093 | | | 20,003 | |
| Convertible note receivable | 6,879 | | | 6,629 | |
| Operating lease right-of-use assets, net (including amounts with related parties) | 36,543 | | | 45,788 | |
| Other assets (including amounts with related parties) | 2,880 | | | 4,860 | |
| Total assets | $ | 504,452 | | | $ | 362,356 | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 9,195 | | | $ | 21,016 | |
| Accrued expenses and other liabilities | 42,708 | | | 41,825 | |
Related-party promissory notes, net of discounts and deferred issuance costs (Note 10) | — | | | 431,901 | |
| | | |
| Due to related parties | 1,136 | | | 3,469 | |
| Operating lease liabilities (including amounts with related parties) | 5,244 | | | 2,650 | |
| Total current liabilities | 58,283 | | | 500,861 | |
Related-party nonconvertible note, net of discount (Note 10) | 104,586 | | | — | |
Related-party convertible notes and accrued interest, net of discount (Note 10) | 576,951 | | | 241,271 | |
| | | |
Revenue interest liability (Note 9) | 155,415 | | | — | |
| 35,333 | | | — | |
| Operating lease liabilities, less current portion (including amounts with related parties) | 39,942 | | | 47,951 | |
| | | |
| Warrant liabilities | 118,770 | | | 21,636 | |
| Other liabilities | 1,109 | | | 457 | |
| Total liabilities | 1,090,389 | | | 812,176 | |
Commitments and contingencies (Note 7) | | | |
| Stockholders’ deficit: | | | |
Common stock, $0.0001 par value; 1,350,000,000 and 900,000,000 shares authorized as of December 31, 2023 and 2022, respectively; 670,867,344 and 421,569,115 shares issued and outstanding as of December 31, 2023 and 2022, respectively; excluding 163,800 treasury stock shares outstanding as of December 31, 2023 and 2022 | 67 | | | 42 | |
| Additional paid-in capital | 2,374,620 | | | 1,930,936 | |
| Accumulated deficit | (2,961,684) | | | (2,378,488) | |
| Accumulated other comprehensive income | 10 | | | 183 | |
| Total ImmunityBio stockholders’ deficit | (586,987) | | | (447,327) | |
| Noncontrolling interests | 1,050 | | | (2,493) | |
| Total stockholders’ deficit | (585,937) | | | (449,820) | |
| Total liabilities and stockholders’ deficit | $ | 504,452 | | | $ | 362,356 | |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
Consolidated Statements of Operations
(in thousands, except share and per share amounts)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Revenue | $ | 622 | | | $ | 240 | | | $ | 934 | |
| Operating expenses: | | | | | |
Research and development (including amounts
with related parties) | 232,366 | | | 248,149 | | | 195,958 | |
Selling, general and administrative (including amounts
with related parties) | 129,620 | | | 102,708 | | | 135,256 | |
| Impairment of intangible assets | 886 | | | 681 | | | — | |
| Total operating expenses | 362,872 | | | 351,538 | | | 331,214 | |
| Loss from operations | (362,250) | | | (351,298) | | | (330,280) | |
| Other expense, net: | | | | | |
| Interest and investment income (loss), net | 1,131 | | | (3,090) | | | (4,100) | |
| Interest expense (including amounts with related parties) | (129,198) | | | (63,515) | | | (14,849) | |
| Loss on equity method investment | (7,549) | | | (12,107) | | | (803) | |
| Change in fair value of warrant liabilities | (47,600) | | | 13,460 | | | — | |
| Change in fair value of related-party convertible note | (36,203) | | | — | | | — | |
Other (expense) income, net (including amounts
with related parties) | (2,223) | | | (736) | | | 193 | |
| Total other expense, net | (221,642) | | | (65,988) | | | (19,559) | |
| Loss before income taxes and noncontrolling interests | (583,892) | | | (417,286) | | | (349,839) | |
| Income tax benefit (expense) | 40 | | | (34) | | | (9) | |
| Net loss | (583,852) | | | (417,320) | | | (349,848) | |
| Net loss attributable to noncontrolling interests, net of tax | (656) | | | (753) | | | (3,058) | |
| Net loss attributable to ImmunityBio common stockholders | $ | (583,196) | | | $ | (416,567) | | | $ | (346,790) | |
| | | | | |
| Net loss per ImmunityBio common share – basic and diluted | $ | (1.15) | | | $ | (1.04) | | | $ | (0.89) | |
Weighted-average number of common shares used in computing
net loss per share – basic and diluted | 508,635,592 | | 399,900,374 | | 389,234,156 |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
The Merger
On December 21, 2020, NantKwest and NantCell entered into the Merger Agreement, pursuant to which NantKwest and NantCell agreed to combine their businesses. The Merger Agreement provided that a wholly-owned subsidiary of the company would merge with and into NantCell, with NantCell surviving the Merger as a wholly-owned subsidiary of the company.
On March 9, 2021, we completed the Merger pursuant to the terms of the Merger Agreement. Under the terms of the Merger Agreement, at the effective time of the Merger (the Effective Time), each share of NantCell common stock, par value $0.001 per share, issued and outstanding immediately prior to the Effective Time, subject to certain exceptions as set forth in the Merger Agreement, was converted automatically using the Exchange Ratio into newly issued shares of Company Common Stock, with cash paid in lieu of any fractional shares. At the Effective Time, each share of the company’s common stock issued and outstanding immediately prior to the Effective Time, remained an issued and outstanding share of the combined company. At the Effective Time, each outstanding option, RSU or warrant to purchase NantCell common stock was converted using the Exchange Ratio into an option, RSU or warrant, respectively, on the same terms and conditions immediately prior to the Effective Time, to purchase shares of Company Common Stock.
Immediately following the Effective Time, the former stockholders of NantCell held approximately 71.5% of the outstanding shares of Company Common Stock and the stockholders of NantKwest as of immediately prior to the Merger held approximately 28.5% of the outstanding shares of Company Common Stock. As a result of the Merger and immediately following the Effective Time, Dr. Patrick Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer, and his affiliates beneficially owned, in the aggregate, approximately 81.8% of the outstanding shares of Company Common Stock. Following the consummation of the Merger, the symbol for shares of the company’s common stock was changed to “IBRX.”
During the year ended December 31, 2021, we recorded $13.0 million of costs in connection with the Merger consisting of financial advisory, legal and other professional fees.
Accounting Treatment of the Merger
The Merger represents a business combination pursuant to FASB ASC Topic 805-50, Mergers (ASC 805-50), which was accounted for as a transaction between entities under common control as Dr. Soon‑Shiong and his affiliates were the controlling stockholders of both the company and NantCell for all of the periods presented in this report. As a result, all of the assets and liabilities of NantCell were combined with ours at their historical carrying amounts on the closing date of the Merger. We recast our prior period financial statements for the year ended December 31, 2021 to reflect the conveyance of NantCell’s common shares as if the Merger had occurred as of the earliest date of the consolidated financial statements presented in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report. All material intercompany accounts and transactions have been eliminated in consolidation. COVID-19 Pandemic
The COVID-19 pandemic continues to present a public health and economic challenge around the world. Through the date of this Annual Report, we have not seen a material adverse impact to our business from the pandemic. However, given the continuously evolving nature of the pandemic, we cannot at this time predict the specific extent, duration, or full impact that this pandemic may have on our financial condition and results of operations, including ongoing and planned clinical trials. More specifically, the pandemic may result in prolonged impacts that we cannot predict at this time and we expect that such uncertainties will continue to exist for the foreseeable future.
We continue to monitor the impact of COVID-19 on our business, including our clinical trials, manufacturing facilities and capabilities, and ability to access necessary resources. For a discussion of the risks presented by the COVID-19 pandemic to our results of operations and financial condition, see Part I, Item 1A. “Risk Factors.” Operating Results
From inception through the date of this Annual Report, we have generated minimal revenue from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables, and grant programs. We have no clinical products approved for commercial sale and have not generated any revenue from therapeutic and vaccine product candidates that are under development. We have incurred net losses in each year since our inception and, as of December 31, 2023, we had an accumulated deficit of $3.0 billion. During the years ended December 31, 2023, 2022 and 2021, net losses attributable to ImmunityBio common stockholders were $583.2 million, $416.6 million, and $346.8 million, respectively. Substantially all of our net losses resulted principally from costs incurred in connection with our ongoing clinical trials and operations, our research and development programs, and from selling, general and administrative costs associated with our operations, including stock-based compensation expense.
As of December 31, 2023, we had 628 employees. Personnel of related companies who provide corporate, general and administrative, certain research and development, and other support services under our shared services agreement with NantWorks are not included in this number. See Note 11, Related-Party Agreements, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information. In anticipation of the commercialization of select drug candidates, we expect to continue to incur significant expenses and increasing operating losses for the foreseeable future, which may fluctuate significantly from quarter-to-quarter and year-to-year. See “—Future Funding Requirements” below for a discussion of our anticipated expenditures and sources of capital we expect to access to fund these expenditures. Collaboration Agreements
We anticipate that strategic collaborations will continue to be an integral part of our operations, providing opportunities to leverage our partners’ expertise and capabilities to gain access to new technologies and further expand the potential of our technologies and product candidates across relevant platforms. We believe we are well positioned to become a leader in immunotherapy due to our broad and vertically-integrated platforms and through complementary strategic partnerships.
We believe that our innovative approach to orchestrate and combine therapies for optimal immune system response will become a therapeutic foundation across multiple indications. Additionally, we believe that data from multiple clinical trials indicates N-803 has broad potential to enhance the activity of therapeutic mAbs, including checkpoint inhibitors, across a wide range of tumor types. We may also enter into supply arrangements for various investigational agents to be used in our clinical trials. See Note 6, Collaboration and License Agreements and Acquisition, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding our collaboration and license agreements. Agreements with Related Parties
Our Executive Chairman, Global Chief Scientific and Medical Officer and principal stockholder, founded and has a controlling interest in NantWorks, which is a collection of companies in the healthcare and technology space. We have entered into arrangements with NantWorks, and certain affiliates of NantWorks. Affiliates of NantWorks are also affiliates of the company due to the common control by and/or common ownership interest of our Executive Chairman and Global Chief Scientific and Medical Officer.
Related-Party Debt
The following discussion of the company’s related-party promissory notes does not purport to be complete and is qualified in its entirety by reference to the full text of the notes, copies of which are incorporated by reference in Part IV, Item 15. “Exhibits and Financial Statement Schedules” of this Annual Report. Related-Party Convertible Promissory Notes
On September 11, 2023, the company entered into a stock purchase agreement with Nant Capital, NantMobile and NCSC (collectively, the “related-party purchasers”), which are entities affiliated with Dr. Soon-Shiong, pursuant to which the related-party purchasers exchanged outstanding fixed-rate promissory notes held by such related-party purchasers, representing approximately $270.0 million in aggregate principal amount and accrued and unpaid interest, in exchange for an aggregate of 209,291,936 shares of common stock at an exchange price of $1.29 per share. As a result of the exchange, the company is forever released and discharged from all its obligations and liabilities under these notes.
On September 11, 2023, the company executed a $200.0 million convertible promissory note with Nant Capital. The note bears interest at Term SOFR plus 8.0% per annum. The accrued interest shall be payable monthly commencing on September 30, 2023. The outstanding principal amount and any accrued and unpaid interest are due on the earlier of (i) September 11, 2026 or (ii) upon the occurrence and during the continuance of an “event of default” (as defined in the note). We may prepay the outstanding principal amount, together with any accrued interest, at any time, in whole or in part, without premium or penalty upon five (5) days’ written notice to the noteholder. The noteholder has the sole option to convert all (but not less than all) of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $1.9350 per share. We received net proceeds of approximately $199.0 million from this financing, net of a $1.0 million origination fee paid to the noteholder. On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into a letter amendment whereby this promissory note became subordinated to the RIPA payment obligations.
Related-Party Convertible Note at Fair Value
On March 31, 2023, the company executed a $30.0 million promissory note with Nant Capital. This note bears interest at Term SOFR plus 8.0% per annum. The outstanding principal amount and any accrued and unpaid interest are due on the earlier of December 31, 2023 or upon the occurrence and continuation of an event of default (as defined in the note). The interest on this note is paid on a quarterly basis beginning on June 30, 2023. The company may prepay the outstanding principal amount and any accrued and unpaid interest of the promissory note, at any time, in whole or in part, without penalty. Upon receipt of a written notice of prepayment from the company, the noteholder has the right, at its option, to convert the outstanding principal amount to be prepaid and the accrued and unpaid interest thereon (as specified in the notice of prepayment) into shares of the company’s common stock at a price of $2.28 per share. Additionally, the whole promissory note and any accrued interest may be converted into shares of the company’s common stock at a conversion price of $2.28 per share (subject to appropriate adjustment from time to time for any stock dividend, stock split, combination of shares, reorganization, recapitalization, reclassification or other similar event), at the option of the noteholder.
The company received net proceeds of approximately $29.9 million from this financing, net of a $0.1 million origination fee paid to the noteholder, which we used, together with other available funds, to progress our pre-commercialization efforts and clinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes.
On September 11, 2023, the company and Nant Capital entered into a letter agreement pursuant to which the maturity date of this promissory note was extended from December 31, 2023 to December 31, 2024. On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into a letter amendment pursuant to which the maturity date of this promissory note was extended to December 31, 2025, and the note became subordinated to the RIPA payment obligations.
This note was accounted for under the ASC 825-10-15-4 FVO election until it was amended on December 29, 2023. Under the FVO election, the note was initially measured at its issuance date estimated fair value and subsequently remeasured at estimated fair value on a recurring basis at each reporting period date. With each such remeasurement, the note was adjusted to fair value, with the change in fair value recorded in other expense, net, on the consolidated statement of operations.
Related-Party Nonconvertible Notes
On June 13, 2023, the company executed a $30.0 million promissory note with Nant Capital. The company was able to request up to three (3) advances of $10.0 million each pursuant to the note. The principal amount of each advance bears interest at Term SOFR plus 8.0% per annum. The accrued interest on any advance is payable quarterly on the last business day of June, September and December. The outstanding principal amount and any accrued and unpaid interest on advances were due on the earlier of (i) December 31, 2023 or (ii) upon the occurrence and during the continuance of an event of default (as defined in the note). We may prepay the outstanding principal amount, together with any accrued interest, on any then-outstanding advances, at any time, in whole or in part, without premium or penalty, and without the prior consent of the noteholder, upon five (5) days written notice to the noteholder.
The company received net proceeds of approximately $29.9 million from this financing, net of a $0.1 million origination fee paid to Nant Capital, which we used, together with other available funds, to progress our regulatory approval efforts, pre-commercialization activities and clinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes.
On September 11, 2023, the company and Nant Capital entered into a series of letter agreements pursuant to which the maturity date of related-party nonconvertible promissory notes totaling $505.0 million in principal were extended from December 31, 2023 to December 31, 2024.
On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into an amended and restated promissory note pursuant to which the existing $30.0 million promissory note, along with other existing related-party nonconvertible promissory notes, was amended to be included in the Tranche 2 principal amount of the amended $505.0 million December 2023 promissory note. The Tranche 2 of the promissory note has a principal amount of $380.0 million, a maturity date of December 31, 2025, and an interest rate of Term SOFR plus 7.5% per annum. Based on the terms of the amended and restated promissory note, the noteholder, at its sole discretion, may convert all of the $380.0 million principal amount into shares of common stock at a conversion price of $8.2690 per share. The new note became subordinated to the RIPA payment obligations.
See Note 10, Related-Party Debt, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding our related-party debt. Immuno-Oncology Clinic, Inc.
We have entered into multiple agreements with the Clinic to conduct clinical trials related to certain of our product candidates. The Clinic is a related party as it is owned by an officer of the company and NantWorks manages the administrative operations of the Clinic.
In 2021, we completed a review of alternative structures that could support our more complex clinical trial requirements and made a decision to explore a potential transition of clinical trials at the Clinic to a new structure (including contracting with a new, non-affiliated professional corporation) to be determined and agreed upon by all parties. While we have not yet finalized the potential transition, we continue discussions with potential partners around such alternative structures. During the years ended December 31, 2023, 2022 and 2021, we recorded $2.2 million, $2.4 million and $1.6 million, respectively, in research and development expense, on the consolidated statements of operations related to clinical trial and transition services provided by the Clinic.
Related-Party Leases
605 Doug St, LLC
In June 2023, we exercised the option to extend the lease agreement with 605 Doug St, LLC, an entity owned by our Executive Chairman and Global Chief Scientific and Medical Officer, for one additional three-year term through July 2026.
23 Alaska, LLC
Effective August 31, 2023, we executed a lease termination agreement with 23 Alaska, LLC, an entity owned by our Executive Chairman and Global Chief Scientific and Medical Officer.
Duley Road, LLC
Effective October 3, 2023, we exercised the first option to extend the lease agreement with Duley Road, a related party that is indirectly controlled by our Executive Chairman and Global Chief Scientific and Medical Officer, for one additional five-year term through October 31, 2029.
See Note 11, Related-Party Agreements, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding our related-party agreements. Components of our Results of Operations
Revenue
From inception through the date of this Annual Report, we have generated minimal revenue from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables, and grant programs. We have no clinical products approved for commercial sale and have not generated any revenue from therapeutic and vaccine product candidates that are under development. If we fail to complete the development of our product candidates in a timely manner or fail to obtain regulatory approval for them, we may never be able to generate substantial future revenue.
Operating Expenses
We generally classify our operating expenses into research and development, and selling, general and administrative expenses. Personnel costs, including salaries, benefits, bonuses, and stock-based compensation expense comprise a significant component of our research and development, and selling, general and administrative expense categories. We allocate expenses associated with our facilities and information technology costs between these two categories, primarily based on the nature of each cost.
Research and Development
Research and development expense consists of expenses incurred while performing research and development activities to discover and develop our technology and product candidates. This includes conducting preclinical studies and clinical trials, manufacturing development efforts and activities related to regulatory submissions for product candidates. We recognize research and development expenses as they are incurred.
Our research and development expenses primarily consist of:
•clinical trial and regulatory-related costs;
•expenses incurred under agreements with investigative sites and consultants that conduct our clinical trials;
•expenses incurred under collaborative agreements;
•manufacturing and testing costs and related supplies and materials;
•employee-related expenses, including salaries, benefits, travel and stock-based compensation; and
•facility expenses dedicated to research and development.
The company classifies its research and development expenses as either external or internal. The company’s external research and development expenses support its various preclinical and clinical programs. The company’s internal research and development expenses include payroll and benefits expenses, facilities and equipment expense, and other indirect research and development expenses incurred in support of its research and development activities. The company’s external and internal resources are not directly tied to any one research or drug discovery program and are typically deployed across multiple programs and are not allocated to specific product candidates or development programs.
We expect our research and development expenses to increase significantly for the foreseeable future as we continue to invest in research and development activities related to developing our product candidates and conducting our ongoing and planned clinical trials.
The process of conducting clinical trials necessary to obtain regulatory approval is costly and time consuming. The successful development of product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing or costs required to complete the remaining development of any product candidates. This is due to the numerous risks and uncertainties associated with the development of product candidates.
The costs of clinical trials may vary significantly over the life of a project owing to, but not limited to, the following:
•per patient trial costs;
•the number of sites included in the clinical trials;
•the countries in which the clinical trials are conducted;
•the length of time required to enroll eligible patients;
•the number of patients that participate in the clinical trials;
•the number of doses that patients receive;
•the cost of comparative agents used in clinical trials;
•the drop-out or discontinuation rates of patients;
•potential additional safety monitoring or other studies requested by regulatory agencies;
•the duration of patient follow-up; and
•the safety profile and efficacy of the product candidate.
We have only one product candidate, Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, for which we submitted a BLA to the FDA in May 2022. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. On October 23, 2023, we announced that we had completed the resubmission of the BLA addressing the issues in the CRL. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a
complete response to the CRL. The FDA has set a new user fee goal date (PDUFA date) of April 23, 2024. There can be no assurance that our product candidate will be approved for commercial sale by the FDA in the near term, if ever. We do not expect any of our other product candidates to be commercially available for the foreseeable future, if ever.
Selling, General and Administrative
Selling, general and administrative expense consists primarily of salaries and personnel-related costs, including employee benefits and any stock-based compensation, for employees performing functions other than research and development. This includes personnel in executive, finance, human resources, information technology, legal, and administrative support functions. Other selling, general and administrative expenses include facility-related costs not otherwise allocated to research and development expense, professional fees for auditing, tax and legal services, advertising costs, expenses associated with strategic business transactions and business development efforts, obtaining and maintaining patents, consulting costs, royalties and licensing costs, and costs of our information systems.
We expect that our selling, general and administrative expense will increase for the foreseeable future as we expand operations, build out information systems and increase our headcount to support continued research activities and the development of our clinical programs. We have incurred and expect that we will continue to incur in the future, additional costs associated with operating as a public company, including costs to comply with stock exchange listing and SEC requirements, future funding efforts, corporate governance, internal controls, investor relations, disclosure and similar requirements applicable to public companies. Additionally, if and when we believe that a regulatory approval of a product candidate appears likely, we expect to incur significant increases in our selling, general and administrative expense relating to the sales and marketing of the approved product candidate.
Other Expense, Net
Other expense, net consists primarily of interest and investment income, interest expense (including amortization of debt discounts), unrealized gains and losses from investments in equity securities and equity-method investments, changes in fair value of warrants, derivative liabilities, and a convertible note, realized gains and losses on debt and equity securities, and gains and losses on foreign currency transactions.
Income Taxes
We are subject to U.S. federal income tax, as well as income tax in Italy, South Korea, California and other states. From inception through December 31, 2023, we have not been required to pay U.S. federal and state income taxes because of current and accumulated NOLs.
Discussion of Consolidated Results of Operations
Comparison of the Years Ended December 31, 2023 and 2022
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | | | |
| | 2023 | | 2022 | | $ Change | | % Change |
| | | | | | | | |
| | ($ in thousands) | | |
| | | | | | | | |
| Revenue | | $ | 622 | | | $ | 240 | | | $ | 382 | | | 159 | % |
| Operating expenses: | | | | | | | | |
Research and development (including amounts
with related parties) | | 232,366 | | | 248,149 | | | (15,783) | | | (6) | % |
Selling, general and administrative (including amounts
with related parties) | | 129,620 | | | 102,708 | | | 26,912 | | | 26 | % |
| Impairment of intangible assets | | 886 | | | 681 | | | 205 | | | 30 | % |
| Total operating expenses | | 362,872 | | | 351,538 | | | 11,334 | | | 3 | % |
| Loss from operations | | (362,250) | | | (351,298) | | | (10,952) | | | 3 | % |
| Other expense, net: | | | | | | | | |
| Interest and investment income (loss), net | | 1,131 | | | (3,090) | | | 4,221 | | | (137) | % |
| Interest expense (including amounts with related parties) | | (129,198) | | | (63,515) | | | (65,683) | | | 103 | % |
| Loss on equity method investment | | (7,549) | | | (12,107) | | | 4,558 | | | (38) | % |
| Change in fair value of warrant liabilities | | (47,600) | | | 13,460 | | | (61,060) | | | (454) | % |
| Change in fair value of convertible note | | (36,203) | | | — | | | (36,203) | | | — | % |
Other expense, net (including amounts
with related parties) | | (2,223) | | | (736) | | | (1,487) | | | 202 | % |
| Total other expense, net | | (221,642) | | | (65,988) | | | (155,654) | | | 236 | % |
| Loss before income taxes and noncontrolling interests | | (583,892) | | | (417,286) | | | (166,606) | | | 40 | % |
| Income tax benefit (expense) | | 40 | | | (34) | | | 74 | | | (218) | % |
| Net loss | | $ | (583,852) | | | $ | (417,320) | | | $ | (166,532) | | | 40 | % |
Revenue
Revenue increased $0.4 million during the year ended December 31, 2023, as compared to the year ended December 31, 2022. The increase was primarily driven by higher grant revenue received in 2023.
Research and Development Expense
Research and development expense decreased $15.8 million during the year ended December 31, 2023, as compared to the year ended December 31, 2022. The following table summarizes our research and development expenses during the years ended December 31, 2023 and 2022, together with the changes in those items (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | |
| | 2023 | | 2022 | | $ Change |
| | | | | | |
| External research and development expenses | | $ | 68,212 | | | $ | 63,113 | | | $ | 5,099 | |
| Internal research and development expenses: | | | | | | |
| Personnel-related costs | | 89,085 | | | 96,357 | | | (7,272) | |
| Equipment, depreciation, and facility costs | | 51,810 | | | 53,920 | | | (2,110) | |
| Other research and development costs | | 23,259 | | | 34,759 | | | (11,500) | |
| Total internal research and development expenses | | 164,154 | | | 185,036 | | | (20,882) | |
| Total research and development expenses | | $ | 232,366 | | | $ | 248,149 | | | $ | (15,783) | |
Research and development expense decreased $15.8 million primarily attributable to the following:
•A $5.1 million increase in external research and development expenses that was primarily due to higher qualification and testing costs, pre-licensing inspection costs, and outside consulting fees relating to regulatory and compliance services for the resubmission of the BLA, partially offset by a decrease in clinical trial costs and initial BLA submission fees;
•A $7.3 million decrease in personnel-related costs that was primarily due to the write off of accrued discretionary bonuses for 2022 not paid and a reduction in headcount, partially offset by an increase in stock-based compensation costs associated with new retention awards granted to existing employees;
•A $2.1 million decrease in equipment, depreciation, and facility costs that was primarily due to a reduction in activities related to facility expansion that occurred in the prior year, which resulted in lower expenditures for equipment qualification and certification, as well as lower expenses for sanitary services, partially offset by higher equipment maintenance costs; and
•An $11.5 million decrease in other research and development costs, primarily attributable to reductions in laboratory and test supplies and material supplies used for internal manufacturing, and no impairment costs from long-lived assets in the current year compared to the prior year, as well as an increase in collaboration costs allocated to a joint venture. Additionally, the write down of right-of-use assets from a lease used for R&D contributed to an additional decrease in other R&D expenses. The reduction in costs were partially offset by higher R&D activities and increased costs related to R&D licenses.
We expect our research and development expenses to increase significantly for the foreseeable future as we continue to invest in research and development activities related to developing our product candidates and conducting our ongoing and planned clinical trials.
Selling, General and Administrative Expense
Selling, general and administrative expense increased $26.9 million during the year ended December 31, 2023, as compared to the year ended December 31, 2022. The rise in expenses was primarily due to a $28.9 million increase in legal expenses, which was driven by higher defense costs and general legal services, as well as fewer legal insurance reimbursements received compared to the prior year. Additionally, there was a $1.1 million increase in personnel-related costs resulting from higher stock-based compensation expenses and travel costs, a $0.6 million increase in IT and security license fees, and a $0.2 million increase in marketing costs for pre-commercialization activities. These increases were partially offset by a $2.7 million reduction in consulting services from ERP implementations and professional fees from accounting and tax services, and a $1.2 million reduction in insurance expenses and other operating costs.
Impairment of Intangible Assets
Impairment of intangible assets increased $0.2 million during the year ended December 31, 2023, compared to the year ended December 31, 2022, due to an impairment charge of $0.9 million related to indefinite-lived intangible assets associated with the Tarmogen platform as the research and development program was limited in the current year. The $0.7 million write off of the organized workforce with definite lives during 2022 was as a result of the workforce reduction at the Dunkirk Facility.
Other Expense, Net
Other expense, net increased $155.7 million during the year ended December 31, 2023, as compared to the year ended December 31, 2022, due to a $65.7 million increase in interest expense driven by higher related-party borrowings, an increase in theTerm SOFR rate, and higher amortization of related-party notes discounts. Additionally, there was a total increase in expense of $97.3 million driven by the change in the fair value of warrant liabilities and the change in the fair value of a related-party convertible note, and a $1.5 million increase in expense due to higher issuance costs associated with our registered direct offerings, and an increase in other expenses. These changes were partially offset by a $4.6 million lower loss on equity method investments and a $4.2 million increase in investment income.
Comparison of the Years Ended December 31, 2022 to 2021
See Part II, Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Results of Operations” of our Annual Report filed with the SEC on March 1, 2023 for a discussion of the company’s results of operations during the year ended December 31, 2022 compared to the year ended December 31, 2021.
Financial Condition, Liquidity and Capital Resources
Sources of Liquidity
Since our inception, we have funded our operations primarily through proceeds from the issuance of related-party promissory notes and sales of our common stock under the ATM and our shelf registration statement, and through registered direct offerings.
On December 29, 2023, we entered into the RIPA to support the commercialization of Anktiva for the treatment of BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, the further development of our other product platforms, and to provide for other working capital needs.
Cash and Marketable Securities on Hand
As of December 31, 2023, we had cash and cash equivalents, and marketable securities of $267.4 million compared to $108.0 million as of December 31, 2022. We have typically invested our cash in a variety of financial instruments and classified these investments as available-for-sale. However, after our entry into the RIPA we can no longer invest our excess funds in corporate or European bonds. Certain of our investments are subject to credit, liquidity, market, and interest-rate risks. The general condition of the financial markets and the economy may increase those risks and may affect the value and liquidity of investments and restrict our ability to access the capital markets.
Proceeds from the ATM
During the year ended December 31, 2023, we received net proceeds of approximately $16.1 million from the issuance of 5,605,323 shares under the ATM. We used the net proceeds from this offering, together with other available funds, to progress our regulatory approval efforts, pre-commercialization activities and clinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes. As of December 31, 2023, we had $208.8 million available for future stock issuances under the ATM.
Shelf Registration Statement
During February 2023, we filed a $750.0 million shelf registration statement with the SEC on Form S-3 for the offering and sale of equity and equity-linked securities, including common stock, preferred stock, debt securities, depositary shares, warrants to purchase common stock, preferred stock or debt securities, subscription rights, purchase contracts, and units. During the year ended December 31, 2023, we sold shares of our common stock and warrants valued at $184.4 million under the shelf. As of December 31, 2023, we had $565.6 million available for use under the shelf. This available shelf is in addition to the ATM.
Proceeds from Registered Direct Offerings
On February 15, 2023, we entered into a securities purchase agreement with certain institutional investors for the purchase and sale of 14,072,615 shares of our common stock, as well as warrants (the February 2023 Warrants) that allow such investors to purchase an additional 14,072,615 shares of common stock at an exercise price of $4.2636 per share, for a purchase price of $3.5530 per share and accompanying warrant. This transaction generated net proceeds of approximately $47.0 million, after deducting placement agent fees and other offering costs. Pursuant to the terms of a letter agreement dated July 25, 2023, the company and the investors agreed to reduce the exercise price of the outstanding February 2023 Warrants from $4.2636 per share to $3.2946 per share and extend the expiration date of the warrants until July 24, 2026.
On July 20, 2023, we entered into a securities purchase agreement with certain institutional investors for the purchase and sale of 14,569,296 shares of our common stock, as well as warrants that allow such investors to purchase an additional 14,569,296 shares of common stock at an exercise price of $3.2946 per share, for a purchase price of $2.7455 per share and accompanying warrant. This transaction generated net proceeds of approximately $37.5 million, after deducting placement agent fees and other offering costs.
We used the net proceeds from these registered direct offerings, together with other available funds, to progress our regulatory approval efforts, pre-commercialization activities and clinical development programs, to fund other research and development activities, for capital expenditures, and for other general corporate purposes.
Related-Party Promissory Notes
On September 11, 2023, the company entered into a stock purchase agreement with Nant Capital, NantMobile and NCSC pursuant to which the noteholders exchanged promissory notes totaling approximately $270.0 million in aggregate principal amount and accrued and unpaid interest for an aggregate of 209,291,936 shares of common stock at an exchange price of $1.29 per share. As a result of the exchange, the company was forever released and discharged from all its obligations and liabilities under the notes.
On September 11, 2023, the company and Nant Capital entered into a series of letter agreements pursuant to which the maturity date of the related-party nonconvertible notes described above totaling $505.0 million in principal was extended from December 31, 2023 to December 31, 2024. In addition, the company entered into a $200.0 million September 2023 promissory note with Nant Capital.
On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into an amended and restated promissory note and a letter amendment for the following outstanding promissory notes. Pursuant to the terms of the amended and restated promissory note, the amended $505.0 million December 2023 promissory note is comprised of a Tranche 1 principal amount of $125.0 million, which was previously the $125.0 million August 2022 promissory note before amendment, and a Tranche 2 with principal amount of $380.0 million, which is made up of the previous $300.0 million December 2021 promissory note, $50.0 million December 2022 promissory note, and $30.0 million June 2023 promissory note. In addition, the amendment allows Nant Capital, in its sole discretion, to convert all the Tranche 2 principal amount of $380.0 million of the amended promissory note into shares of the company’s common stock. The conversion price was determined subsequently at $8.2690 per share based on the agreement. The maturity date of the amended promissory note is December 31, 2025. Pursuant to the terms of the letter amendment, the maturity date of the $30.0 million March 2023 promissory note was extended from December 31, 2024 to December 31, 2025. Also, in connection with the RIPA transaction, all outstanding related-party promissory notes became subordinated to the RIPA payment obligations. After the amendment, the interest rates for the Tranche 1 principal amount and Tranche 2 principal amount of the $505.0 million December 2023 promissory note were Term SOFR plus 8.0% and Term SOFR plus 7.5%, respectively.
See Note 10, Related-Party Debt, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding our related-party debt. Revenue Interest Purchase Agreement
On December 29, 2023, we entered into the RIPA with Infinity and Oberland. Pursuant to the RIPA, Oberland acquired certain Revenue Interests from us for a gross purchase price of $200.0 million paid on closing, less certain transaction expenses. In addition, Oberland may purchase additional Revenue Interests from us in exchange for the $100.0 million Second Payment upon satisfaction of certain conditions specified in the RIPA, including following the receipt of approval by the FDA of the company’s BLA for N-803 in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease on or before June 30, 2024. Under the RIPA, Oberland has the right to receive quarterly payments from us based on, among other things, a certain percentage of our worldwide net sales, excluding those in China, during such quarter.
Also, on December 29, 2023 and in connection with the RIPA, we entered into an SPOA with Oberland pursuant to which we sold an aggregate of approximately $10.0 million of our common stock at $4.1103 per share in a private placement. Oberland also has an option to purchase up to an additional $10.0 million of our common stock, at a price per share to be determined by reference to the 30-day trailing volume weighted-average price of our common stock calculated from the date of exercise.
For more information, see Note9, Revenue Interest Purchase Agreement, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report.
Uses of Liquidity
In addition to the cash used to fund our operating activities discussed in “—Future Funding Requirements” below, we will require cash to settle the following obligations: •As of December 31, 2023, our indebtedness payable at maturity totaled $735.0 million (excluding unamortized related-party notes discounts), held by entities affiliated with Dr. Soon-Shiong. In connection with the RIPA, all of our related-party promissory notes are now general unsecured obligations of the company that are subordinated in right of payment to indebtedness, obligations and other liabilities under the RIPA, the Revenue Interests issued pursuant to such agreement, and refinancing of the foregoing. In addition, the terms of promissory notes totaling $535.0 million were amended and restated to extend the maturity date by one year to December 31, 2025. The remaining $200.0 million promissory note and any accrued and unpaid interest are due on the earlier of (i) September 11, 2026 or (ii) upon the occurrence and during the continuance of an event of default (as defined in the note).
◦Pursuant to the terms of the amended and restated promissory note, the amended $505.0 million December 2023 promissory note is comprised of the Tranche 1 principal amount of $125.0 million and the Tranche 2 principal amount of $380.0 million. All Tranche 2 principal amount of $380.0 million and any accrued and unpaid interest can be converted into shares of the company’s stock at $8.2690 per share at the option of the noteholder. The interest rates for the Tranche 1 principal amount and Tranche 2 principal amount of the $505.0 million December 2023 promissory note were Term SOFR plus 8.0% per annum and Term SOFR plus 7.5% per annum, respectively.
◦The $200.0 million convertible promissory note bears interest at Term SOFR plus 8.0% per annum. The noteholder has the sole option to convert all of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $1.9350 per share.
◦The $30.0 million convertible promissory note bears interest at Term SOFR plus 8.0% per annum. The noteholder has the sole option to convert all of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $2.28 per share.
There can be no assurance that the company can refinance these promissory notes or what terms will be available in the market at the time of refinancing. Furthermore, if prevailing interest rates or other factors at the time of refinancing result in higher interest rates upon refinancing, then the interest expense relating to the refinanced indebtedness would increase. These risks could materially adversely affect the company’s financial condition, cash flows and results of operations.
•On December 29, 2023, we entered into the RIPA with Infinity and Oberland. Oberland has the right to receive quarterly Revenue Interest Payments from us based on, among other things, our worldwide net sales, excluding those in China, which will be tiered payments initially ranging from 3.00% to 7.00% (or after funding of the Second Payment, 4.50% to 10.00%), subject to increase or decrease, following December 31, 2029 (the Test Date) depending on whether our aggregate payments made to Oberland as of the Test Date have met or exceeded the Cumulative Purchaser Payments. In addition, if our aggregate payments made as of the Test Date to Oberland do not equal or exceed the amount of the Cumulative Purchaser Payments as of such date, then we are obligated to make a one-time True-Up Payment to Oberland in an amount equal to 100% of the Cumulative Purchaser Payments as of the Test Date, less the aggregate amount of our previous payments to Oberland as of the Test Date. See Note 9, Revenue Interest Purchase Agreement, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding the RIPA. •In connection with our 2017 acquisition of Altor, we issued CVRs under which we agreed to pay the prior stockholders of Altor approximately $304.0 million of contingent consideration upon calendar-year worldwide net sales of N-803 exceeding $1.0 billion prior to December 31, 2026, with amounts payable in cash or shares of our common stock or a combination thereof. As of December 31, 2023, Dr. Soon-Shiong and his related party hold approximately $139.8 million of net sales CVRs and they have both irrevocably agreed to receive shares of the company’s common stock in satisfaction of their CVRs. We may be required to pay the other prior Altor stockholders up to $164.2 million for their net sales CVRs should they choose to have their CVRs paid in cash instead of common stock. We may need to seek additional sources of capital to satisfy the CVR obligations if they are achieved.
•In connection with our acquisition of VivaBioCell, we are obligated to pay the former owners approximately $2.2 million of contingent consideration upon the achievement of a regulatory milestone relating to the GMP-in-a-Box technology.
Discussion of Consolidated Cash Flows
The following discussion of ImmunityBio’s cash flows is based on the consolidated statements of cash flows in Part II, Item 8. “Financial Statements and Supplementary Data” and is not meant to be an all‑inclusive discussion of the changes in its cash flows for the years presented below.
The following table sets forth our primary sources and uses of cash for the years indicated (in thousands):
| | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | 2022 |
| | | | |
| Cash provided by (used in): | | | | |
| Operating activities | | $ | (366,757) | | | $ | (337,509) | |
| Investing activities | | (30,470) | | | 27,297 | |
| Financing activities | | 558,341 | | | 233,613 | |
Effects of exchange rate changes on cash, cash equivalents,
and restricted cash | | (292) | | | 284 | |
| Net change in cash, cash equivalents, and restricted cash | | $ | 160,822 | | | $ | (76,315) | |
Operating Activities
During the year ended December 31, 2023, net cash used in operating activities of $366.8 million consisted of a net loss of $583.9 million, partially offset by $213.1 million in adjustments for non-cash items and $3.9 million of cash provided by net working capital. Adjustments for non-cash items primarily consisted of $49.2 million in stock-based compensation expense, $47.6 million in change in fair value of warrant liabilities, $42.4 million in amortization of debt issuance costs and accretion of discounts, $36.2 million in change in fair value of convertible notes, $18.5 million in depreciation and amortization expense, $9.2 million in non-cash interest primarily related to related-party promissory notes, $6.1 million in non-cash lease expense related to operating lease right‑of‑use assets, $2.0 million of transaction costs allocable to warrant liabilities, $1.6 million in unrealized losses on equity securities driven by a decrease in the value of our investments, $0.9 million in loss on impairment of intangible assets, partially offset by $0.5 million in other items, and a decrease of $0.1 million in amortization of premiums, net of discounts on marketable debt securities. The changes in net working capital consisted primarily of an increase of $6.7 million in accrued expenses, a decrease of $5.9 million in prepaid expenses and other current assets, and a decrease of $1.9 million in other assets, partially offset by a decrease of $6.5 million in accounts payable, a decrease of $3.0 million in operating lease liabilities, and a decrease of $1.1 million in due to related parties.
During the year ended December 31, 2022, net cash used in operating activities of $337.5 million consisted of a net loss of $417.3 million and $8.0 million of cash used in net working capital, partially offset by $87.8 million in adjustments for non-cash items. The changes in net working capital consisted primarily of an increase of $16.6 million in prepaid and other current assets, and decreases of $4.3 million in operating lease liabilities and $1.2 million with related parties, partially offset by an increase of $8.0 million in accounts payable, an increase of $4.1 million in accrued expenses and other liabilities, and a decrease of $2.0 million in investment and other assets. Adjustments for non-cash items primarily consisted of $40.2 million in stock-based compensation expense, $18.3 million in depreciation and amortization expense, $16.3 million in amortization of debt issuance costs and accretion of discounts, $11.7 million in non-cash interest primarily related to related-party promissory notes, $5.9 million in non‑cash lease expense related to operating lease right-of-use assets, $4.2 million in unrealized losses on equity securities driven by a decrease in the value of our investments, $1.3 million in loss on impairment of fixed assets, $1.3 million in amortization of premiums, net of discounts, on marketable debt securities, $1.1 million in transaction costs allocable to a warrant liability, a non-cash $0.7 million loss on the impairment of intangible assets, and $0.3 million in other non-cash items, reduced by a $13.5 million change in fair value of a warrant liability.
We have historically experienced negative cash flows from operating activities, with such negative cash flows likely to continue for the foreseeable future.
Investing Activities
During the year ended December 31, 2023, net cash used in investing activities was $30.5 million, which included cash outflows of $30.6 million in purchases of property, plant and equipment (including construction in progress and the completion of a warehouse facility), and $10.4 million in purchases of marketable debt securities, partially offset by cash inflows of $10.5 million from maturities and sales of marketable debt and equity securities. Our investments in property, plant and equipment are primarily related to acquisitions of equipment that will be used for the manufacturing of our product candidates and expenditures related to the build out of our manufacturing facilities.
During the year ended December 31, 2022, net cash provided by investing activities was $27.3 million, which included cash inflows of $162.0 million from maturities and sales of marketable debt and equity securities, partially offset by $78.2 million of purchases of property, plant and equipment (including construction in progress and depreciable property acquired in the Dunkirk acquisition), $34.3 million of purchases of marketable debt securities, $21.2 million for purchase of intangible assets (related to the Dunkirk acquisition), and a $1.0 million investment in a joint venture. Our investments in property, plant and equipment are primarily related to acquisitions of equipment that will be used for the manufacturing of our product candidates and expenditures related to the build out of our manufacturing facilities.
We expect to accelerate our capital spending as we scale our cGMP manufacturing capabilities, which will require significant capital for the foreseeable future.
Financing Activities
During the year ended December 31, 2023, net cash provided by financing activities was $558.3 million, which consisted of $258.7 million in net proceeds from issuances of related-party promissory notes, $192.8 million in net proceeds from payments received pursuant to the RIPA, $84.4 million in net proceeds from registered direct offerings, $16.1 million in net proceeds from the ATM offering, $9.5 million in net proceeds from issuance of common stock in connection with the RIPA, and $0.3 million in proceeds from the exercise of stock options, partially offset by $3.4 million used for payment of payroll tax withholding in connection with the net share settlement of vested RSUs, and $0.1 million used for principal payments of finance leases.
During the year ended December 31, 2022, net cash provided by financing activities was $233.6 million, which consisted of $174.1 million in net proceeds from issuances of related-party promissory notes, $47.3 million in net proceeds from a registered direct offering, and $13.1 million in net proceeds from the ATM offering, partially offset by $0.6 million related to net share settlement of vested RSUs for payment of payroll tax withholding and a $0.3 million used in other financing activities.
We will need to raise additional capital to fund our future cash needs.
Comparison of the Years Ended December 31, 2022 to 2021
See Part II, Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Results of Operations” of our Annual Report filed with the SEC on March 1, 2023 for a discussion of the company’s consolidated cash flows for the year ended December 31, 2022 compared to the year ended December 31, 2021.
Future Funding Requirements
From inception through the date of this Annual Report we have generated minimal revenue, we have no clinical products approved for commercial sale, and we have not generated any revenue from therapeutic and vaccine product candidates that are under development. We do not expect to generate significant revenue unless and until we obtain regulatory approval of and commercialize any of our product candidates, and we do not know when, or if, this will occur. In addition, we expect our operating expenses to significantly increase in connection with our ongoing development activities, particularly as we continue the research, development and clinical trials of, and seek regulatory approval for, our product candidates. We have also incurred and expect that we will continue to incur in the future additional costs associated with operating as a public company as well as costs related to future fundraising efforts. In addition, subject to obtaining regulatory approval of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. We anticipate that we will need substantial additional funding in connection with our continuing operations.
We expect that our operating expenses will increase substantially if and as we:
•continue research and development, including preclinical and clinical development of our existing product candidates;
•potentially seek regulatory approval for our product candidates;
•seek to discover and develop additional product candidates;
•establish a commercialization infrastructure and scale up our manufacturing and distribution capabilities to commercialize any of our product candidates for which we may obtain regulatory approval;
•seek to comply with regulatory standards and laws;
•maintain, leverage and expand our intellectual property portfolio;
•hire clinical, manufacturing, scientific and other personnel to support our product candidates’ development and future commercialization efforts;
•add operational, financial and management information systems and personnel; and
•incur additional legal, accounting and other expenses in operating as a public company.
As a result of continuing anticipated operating cash outflows, we believe that substantial doubt exists regarding our ability to continue as a going concern without additional funding or financial support. However, we believe our existing cash, cash equivalents, and investments in marketable securities, together with capital to be raised through equity offerings (including but not limited to the offering, issuance and sale by us of our common stock that may be issued and sold under the ATM, of which we had $208.8 million available for future issuance as of December 31, 2023, and a February 2023 shelf registration statement, of which we had $565.6 million available for future offerings as of December 31, 2023), and our potential ability to borrow from affiliated entities, will be sufficient to fund our operations through at least the next 12 months following the issuance date of the consolidated financial statements based primarily upon our Executive Chairman and Global Chief Scientific and Medical Officer’s intent and ability to support our operations with additional funds, including loans from affiliated entities, as required, which we believe alleviates such doubt. We may also seek to sell additional equity, through one or more follow-on public offerings, or in separate financings, or obtain a credit facility, issue other debt in compliance with the terms of the RIPA, or engage in strategic partnership transactions. However, we may not be able to secure such external financing in a timely manner or on favorable terms, if at all. Without additional funds, we may choose to delay or reduce our operating or investment expenditures. Further, because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we may need additional funds to meet our needs sooner than planned.
We will need to obtain additional financing to fund our future operations, including completing the development and commercialization of our product candidates. Changing circumstances may cause us to increase our spending significantly faster than we currently anticipate and we may need to raise additional funds sooner than we presently anticipate. Moreover, research and development and our operating costs and fixed expenses such as rent and other contractual commitments, including those for our research collaborations, are substantial and are expected to increase in the future.
Our future funding requirements will depend on many factors, including, but not limited to:
•progress, timing, number, scope and costs of researching and developing our product candidates and our ongoing, planned and potential clinical trials;
•time and cost of regulatory approvals;
•our ability to successfully commercialize any product candidates, if approved and the costs of such commercialization activities;
•revenue from product candidates that we may commercialize, if any, including the selling prices for such potential products and the availability of adequate third-party coverage and reimbursement for patients;
•interest and principal payments on our related-party promissory notes, and payment of Revenue Interests, the Second Payment and Test Date payments due under the RIPA;
•cost of building, staffing and validating our own manufacturing facilities in the U.S., including having a product candidate successfully manufactured consistent with FDA and EMA regulations;
•terms, timing and costs of our current and any potential future collaborations, business or product acquisitions, CVRs, milestones, royalties, licensing or other arrangements that we have established or may establish;
•time and cost necessary to respond to technological, regulatory, political and market developments; and
•costs of filing, prosecuting, maintaining, defending and enforcing any patent claims and other intellectual property rights.
Unless and until we can generate a sufficient amount of revenues, we may finance future cash needs through public or private equity offerings, license agreements, debt financings, collaborations, strategic alliances and marketing or distribution arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms, or at all, including but not limited to the offering, issuance and sale by us of our common stock that may be issued and sold under the ATM.
To the extent that we raise additional capital through the sale of equity or equity-linked securities (including warrants), convertible debt or under the ATM, our shelf registration statement, or other offerings, or if any of our current debt is converted into equity or if our existing warrants are exercised, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. The incurrence of additional indebtedness would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms unfavorable to us. We have no committed source of additional capital and if we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may be required to delay or reduce the scope of or eliminate one or more of our research or development programs or our commercialization efforts. Our current license and collaboration agreements may also be terminated if we are unable to meet the payment obligations under those agreements. As a result, we may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time.
Contractual Obligations
We have material cash requirements to pay related-party affiliates and third parties under various contractual obligations discussed below:
•We are obligated to make payments to several related-party affiliates under written agreements and other informal arrangements. We are also obligated to pay interest and to repay principal under our related-party promissory notes. For information regarding our financing obligations, see Note 10, Related-Party Debt, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report. •We are obligated to make payments to Oberland associated with our revenue interest liability, which do not have a fixed repayment schedule. Oberland’s right to receive payments under the RIPA shall terminate when Oberland has received maximum payments (including any True-Up Payment) equal to 195.0% of the then Cumulative Purchaser Payments unless the RIPA is terminated prior to such date.
Under the terms of the agreement, prior to the Test Date, every $100.0 million of worldwide net sales, excluding those in China, of less than or equal to $600.0 million in a calendar year would result in a tiered Revenue Interest Payment of approximately $7.0 million or 7.0% (or $10.0 million or 10.0% after funding of the Second Payment). Worldwide net sales, excluding those in China, for a calendar year exceeding $600.0 million would result in a tiered Revenue Interest Payment of approximately $3.0 million or 3.0% (or $4.5 million or 4.5% after funding of the Second Payment) for every $100.0 million of worldwide net sales, excluding those in China, above the threshold.
In the future, cumulative worldwide net sales, excluding those in China, levels up to the Test Date will determine whether or not we are required to make a True-Up Payment and implement modified payment rates. The amount of the obligation and timing of payment is likely to change. See Note 9, Revenue Interest Purchase Agreement, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding the RIPA. •We are obligated to make payments under our operating leases, which primarily consist of facility leases. See Note 8, Lease Arrangements, and Note 11, Related-Party Agreements, of the “Notes to Consolidated Financial Statements” that appear in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for information regarding our lease obligations. •In connection with the acquisitions of Altor and VivaBioCell, we are obligated to pay contingent consideration upon the achievement of certain milestones. See Note 7, Commitments and Contingencies—Contingent Consideration Related to Business Combinations, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for information regarding our contingent consideration obligations. •We have contractual obligations to make payments to related-party affiliates and third parties under unconditional purchase arrangements. See Note 7, Commitments and Contingencies—Unconditional Purchase Obligations, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for information on these unconditional purchase obligations. •We have certain contractual commitments that are expected to be paid in fiscal year 2024, depending on the progress of build outs, completion of services, and the realization of milestones associated with third-party agreements. This amounts totals $60.8 million and is primarily related to capital expenditures, open purchase orders as of December 31, 2023 for the acquisition of goods and services in the ordinary course of business, and near-term upfront milestone payments to third parties.
•In addition, we have contractual commitments that are expected to be paid in fiscal year 2025 and beyond based on the achievement of various development, regulatory and commercial milestones for agreements with third parties. These payments may not be realized or may be modified and are contingent upon the occurrence of various future events, substantially all of which have a high degree of uncertainty of occurring. As of December 31, 2023, the maximum amount that may be payable related to these commitments is $769.3 million.
•In connection with our leasehold interest in the Dunkirk Facility, we committed to spend an aggregate of $1.52 billion on operational expenses during the initial 10-year term, and an additional $1.50 billion on operational expenses if we elect to renew the lease for the additional 10-year term. These amounts are not included in the discussion above. See Note 6, Collaboration and License Agreements and Acquisition, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information on these obligations. Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of consolidated financial statements requires management to make certain estimates and assumptions that affect the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, we evaluate our estimates, including those related to the valuation of equity-based awards, deferred income taxes and related valuation allowances, revenue interest liability, preclinical and clinical trial accruals, impairment assessments, CVR measurement and assessments, the measurement of right-of-use assets and lease liabilities, useful lives of long-lived assets, loss contingencies, fair value calculation of warrants and convertible promissory notes, fair value measurements, asset acquisition, and the assessment of our ability to fund our operations for at least the next 12 months from the date of issuance of these consolidated financial statements. We base our estimates on historical experience and on various other market-specific and relevant assumptions that we believe to be reasonable under the circumstances. Estimates are assessed each period and updated to reflect current information, such as the economic considerations related to the impact that any ongoing pandemic could have on our significant accounting estimates. Actual results could differ from those estimates.
While our significant accounting policies are more fully described in the notes accompanying our consolidated financial statements that appear in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report, we believe the following accounting policies to be the most critical in understanding the judgments and estimates we use in preparing our consolidated financial statements:
Revenue Recognition
We have primarily generated revenues from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables and grant programs. The nonexclusive license agreements with a limited number of pharmaceutical and biotechnology companies grant them the right to use our cell lines and intellectual property for non-clinical use. These agreements generally include upfront fees and annual research license fees for such use, as well as commercial license fees for sales of the licensee products developed or manufactured using our intellectual property and cell lines. We have generated revenues from product sales of our proprietary GMP-in-a-Box bioreactors and related consumables, primarily to related parties. Additionally, we also generated revenues from grant programs.
A performance obligation is a promise in a contract to transfer a distinct good or service to the customer. A contract’s transaction price is allocated to each distinct performance obligation based on relative standalone selling price and recognized as revenue when, or as, the performance obligation is satisfied.
Under our license agreements with customers, we typically promise to provide a license to use certain cell lines and related patents, the related know-how, and future research and development data that affect the license. We have concluded that these promises represent one performance obligation due to the highly interrelated nature of the promises. We provide the cell lines and know-how immediately upon entering into the contracts. Research and development data are provided throughout the term of the contract when and if available.
The license agreements may include non-refundable upfront payments, event-based milestone payments, sales-based royalty payments, or some combination of these. The event-based milestone payments represent variable consideration and we use the most likely amount method to estimate this variable consideration. Given the high degree of uncertainly around the achievement of these milestones, we do not recognize revenue from these milestone payments until the uncertainty associated
with these payments is resolved. We currently estimate variable consideration related to milestone payments to be zero and, as such, no revenue has been recognized for milestone payments. We recognize revenue from sales-based royalty payments when or as sales occur. On a quarterly basis, we re-evaluate our estimate of milestone variable consideration to determine whether any amount should be included in the transaction price and recorded in revenue prospectively.
We also have sold our proprietary GMP-in-a-Box bioreactors and related consumables to affiliated companies. The arrangements typically include delivery of bioreactors, consumables, and providing installation service and perpetual software licenses for using the equipment. We recognize revenue when customers obtain control and can benefit from the promised goods or services, generally upon installation of the bioreactors, in an amount that reflects the consideration that we expect to receive in exchange for those goods or services. Upfront payments and fees are recorded as deferred revenue upon receipt and recognized as revenue when we satisfy our performance obligations under these arrangements.
Grant revenue is typically paid for reimbursable costs incurred over the duration of the associated research project or clinical trial and is recognized either when expenses reimbursable under the grants have been incurred and payments under the grants become contractually due or when cash is received, depending on the certainty of payment and other factors specific to each grant.
Revenue Interest Liability
On December 29, 2023, we entered into the RIPA with Infinity and Oberland. Pursuant to the RIPA, Oberland acquired certain Revenue Interests from us for a gross purchase price of $200.0 million paid on closing. In addition, Oberland may purchase additional Revenue Interests from us in exchange for the $100.0 million Second Payment upon satisfaction of certain conditions specified in the RIPA. Under the RIPA, Oberland has the right to receive quarterly payments from us based on, among other things, a certain percentage of our worldwide net sales, excluding those in China, during such quarter. The RIPA is considered a sale of future revenues and is accounted for as a liability net of a debt discount comprised of deferred issuance costs, the fair value of a freestanding option agreement related to the SPOA, and the fair value of embedded derivatives requiring bifurcation on the consolidated balance sheet. The company imputes interest expense associated with this liability using the effective interest rate method. The effective interest rate is calculated based on the rate that would enable the debt to be repaid in full over the anticipated life of the arrangement. Interest expense is recognized over the estimated term on the consolidated statement of operations. The interest rate on this liability may vary during the term of the agreement depending on a number of factors, including the level of actual and forecasted net sales. The company evaluates the interest rate quarterly based on actual and forecasted net sales utilizing the prospective method. A significant increase or decrease in actual or forecasted net sales will materially impact the revenue interest liability, interest expense, and the time period for repayment.
Derivative Liabilities
Embedded derivatives that are required to be bifurcated from the underlying debt instrument that do not meet the derivative scope exception and equity classification criteria are accounted for and valued as separate financial instruments. The terms of an embedded derivative related to a contingent exercisable prepayment feature of a convertible note have been evaluated and deemed to require bifurcation. This embedded derivative will be initially measured at fair value and will be remeasured to fair value at each reporting date until the derivative is settled.
In addition, the RIPA contains certain features that meet the definition of being an embedded derivative requiring bifurcation as a separate compound financial instrument apart from the RIPA. The derivative liability is initially measured at fair value upon issuance and is subject to remeasurement at each reporting period with changes in fair value recognized in other expense, net, on the consolidated statement of operations.
Preclinical and Clinical Trial Accruals
As part of the process of preparing the consolidated financial statements, we are required to estimate expenses resulting from obligations under contracts with vendors, clinical research organizations and consultants. The financial terms of these contracts vary and may result in payment flows that do not match the periods over which materials or services are provided under such contracts.
We estimate clinical trial and research agreement-related expenses based on the services performed, pursuant to contracts with research institutions and clinical research organizations and other vendors that conduct clinical trials and research on our behalf. In accruing clinical and research-related fees, we estimate the time period over which services will be performed and activity expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, we will adjust the accrual accordingly. Payments made under these arrangements in advance of the receipt of the related services are recorded as prepaid expenses until the services are rendered.
Research and Development Costs
Major components of research and development costs include cash compensation and other personnel-related expenses, stock-based compensation, depreciation and amortization expense on research and development property and equipment and intangible assets, costs of preclinical studies, clinical trials costs, including CROs and related clinical manufacturing, including third-party CMOs, costs of drug development, costs of materials and supplies, facilities cost, overhead costs, regulatory and compliance costs, and fees paid to consultants and other entities that conduct certain research and development activities on our behalf. Costs incurred in research and development are expensed as incurred.
The company classifies its research and development expenses as either external or internal. The company’s external research and development expenses support its various preclinical and clinical programs. The company’s internal research and development expenses include payroll and benefits expenses, facilities and equipment expense, and other indirect research and development expenses incurred in support of its research and development activities. The company’s external and internal resources are not directly tied to any one research or drug discovery program and are typically deployed across multiple programs and are not allocated to specific product candidates or development programs.
Included in research and development costs are clinical trial and research expenses based on the services performed pursuant to contracts with research institutions and clinical research organizations and other vendors that conduct clinical trials and research on our behalf. We record accruals for estimated costs under these contracts. When evaluating the adequacy of the accrued liabilities, we analyze the progress of the preclinical studies or clinical trials, including the phase or completion of events, invoices received, contracted costs and purchase orders. Significant judgments and estimates are made in determining the accrued balances at the end of any reporting period based on the facts and circumstances known at that time. Although we do not expect the estimates to be materially different from the amounts actually incurred, if the estimates of the status and timing of services performed differ from the actual status and timing of services performed, we may report amounts that are too high or too low in any particular period. Actual results could differ from our estimates. We adjust the accruals in the period when actual costs become known.
Contingencies
We record accruals for loss contingencies to the extent that we conclude it is probable that a liability has been incurred and the amount of the related loss can be reasonably estimated. We accrue for the best estimate of a loss within a range; however, if no estimate in the range is better than any other, then we accrue the minimum amount in the range. If we determine that a material loss is reasonably possible, we disclose the possible loss or range of loss, or that the amount of loss cannot be estimated at this time. We evaluate, on a quarterly basis, developments in legal proceedings and other matters that could cause a change in the potential amount of the liability recorded or the range of potential losses disclosed. Moreover, we record gain contingencies only when they are realizable and the amount is known. Additionally, we record our rights to insurance recoveries, limited to the extent of incurred or probable losses, as a receivable when such recoveries have been agreed to with our third-party insurers and when receipt is deemed probable. This includes instances when our third-party insurers have agreed to pay, on our behalf, certain legal defense costs and settlement amounts directly to applicable law firms and a settlement fund.
Warrants
The company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC Topic 480, Distinguishing Liabilities from Equity (ASC 480) and FASB ASC Topic 815, Derivatives and Hedging (ASC 815). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are
indexed to the company’s own stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For warrants that meet all criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital, on the consolidated statement of stockholders’ deficit at the time of issuance. For warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and on each balance sheet date thereafter. Changes in the estimated fair value of the warrants are recognized as a non-cash gain or loss in other expense, net, on the consolidated statement of operations. The fair value of the warrants was estimated using the Black-Scholes option pricing model.
Fair Value Option Election
The company accounted for a convertible note issued on March 31, 2023 under the FVO election of ASC 825 until it was amended and restated on December 29, 2023. Prior to its extinguishment on December 29, 2023, the convertible note was a debt host financial instrument containing embedded features wherein the entire financial instrument was initially measured at its issuance-date estimated fair value and then subsequently remeasured at estimated fair value on a recurring basis at each reporting period date.
Changes in the estimated fair value of this convertible note were recorded in other expense, net, on the consolidated statement of operations, except that changes in estimated fair value caused by changes in the instrument-specific credit risk were recorded in other comprehensive (loss) income. In accordance with ASC 470-50, when the convertible note was extinguished on December 29, 2023, the cumulative amount previously recorded in other comprehensive (loss) income resulting from changes in the instrument-specific credit risk were reclassified and reported in current earnings on the consolidated statement of operations.
Debt Modification and Extinguishment
The company evaluates amendments to its debt instruments in accordance with ASC 470-50. This evaluation includes comparing (1) if applicable, the net present value of future cash flows of the amended debt to that of the original debt and (2) the change in fair value of an embedded conversion feature to that of the carrying amount of the debt immediately prior to amendment to determine, in each case, if a change greater than 10% occurred. In instances where the net present value of future cash flows or the fair value of an embedded conversion feature, if any, changed more than 10%, the company applies extinguishment accounting. In instances where the net present value of future cash flows and the fair value of an embedded conversion feature, if any, changed less than 10%, the company accounts for the amendment to the debt as a debt modification. Gains and losses on debt amendments that are considered extinguishments are recognized in current earnings or in additional paid-in capital if the transactions are with entities under common control. Debt amendments that are considered debt modifications are accounted for prospectively through yield adjustments, based on the revised terms. The increase in fair value of the embedded conversion feature from the debt modification was accounted for as an increase in debt discount with a corresponding increase in additional paid-in capital. Legal fees and other costs incurred with third parties that are directly related to debt modifications are expensed as incurred. Amounts paid by the company to the lenders, are reflected as additional debt discount and amortized as an adjustment of interest expense over remaining term of modified debt using the effective interest rate method.
Stock-Based Compensation
We account for stock-based compensation under the provisions of FASB ASC Topic 718, Compensation—Stock Compensation (ASC 718). We estimate fair value of each stock option award on the date of grant using the Black-Scholes option pricing model. The Black-Scholes option pricing model requires the use of subjective assumptions, including, but not limited to, expected stock price volatility over the term of the awards and the expected term of the stock options. We measure the fair value of an equity-classified award at the grant date and recognize the stock-based compensation expense over the period of vesting on the straight-line basis for our outstanding share awards that do not contain a performance condition. For awards subject to performance-based vesting conditions, we assess the probability of the individual milestones under the award being achieved and stock-based compensation expense is recognized over the service period using the graded vesting method once management believes the performance criteria is probable of being met. For awards with service or performance conditions, we recognize the effect of forfeitures in compensation cost in the period that the award was forfeited.
Business Combinations
Business combinations are accounted for using the acquisition method of accounting in accordance with ASC 805-50. These standards require that the total cost of acquisition be allocated to the tangible and intangible assets acquired and liabilities assumed based on their respective fair values at the date of acquisition, with the excess purchase price recorded as goodwill. The allocation of the purchase price is dependent upon certain valuations and other studies. Acquisition costs are expensed as incurred.
Contingent consideration incurred in connection with a business combination are recorded at their fair values on the acquisition date and re-measured at their fair values each subsequent reporting period until the related contingencies are resolved. The resulting changes in fair values are recorded as research and development expense, on the consolidated statements of operations and comprehensive income (loss). Changes in fair values reflect changes to our assumptions regarding probabilities of successful achievement of related milestones, the timing in which the milestones are expected to be achieved, and the discount rate used to estimate the fair value of the obligation.
Recent Accounting Pronouncements
See Note 2, Summary of Significant Accounting Policies, of the “Notes to Consolidated Financial Statements” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding recent accounting pronouncements or changes in accounting pronouncements that are of significance, or potential significance, to us. ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Our exposure to market risk for changes in interest rates relates primarily to interest earned on our cash equivalents, investments and variable interest rate debt. The primary objective of our investment activities is to preserve our capital to fund operations. A secondary objective is to maximize income from our investments without assuming significant risk. Our investment policy provides for investments in low-risk, investment-grade debt instruments. As of December 31, 2023, we had $265.5 million in cash and cash equivalents and $1.9 million in our investment portfolio. Our cash equivalents are short-term investments with maturities of 90 days or less at the time of purchase. We maintain cash deposits in FDIC insured financial institutions in excess of federally insured limits. However, we believe that we are not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. As of December 31, 2023, our investment portfolio was comprised of available-for-sale securities, and we did not hold or issue financial instruments for trading purposes.
Interest Rate Risk – Cash
With the cash discussed above, our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S interest rates. However, we do not believe a sudden change in the interest rates would have a material impact on our financial condition or results of operations due to the short-term maturities of our cash equivalents. A hypothetical 100 basis point change in interest rates during any of the periods presented would not have had a material impact on our consolidated financial statements.
Interest Rate Risk – Cash Equivalents and Investment Portfolio
We invest a portion of our cash in a number of diversified fixed-and floating-rate securities, consisting of marketable debt securities and debt funds that are subject to interest rate risk. Changes in the general level of interest rates can affect the fair value of our investment portfolio. If interest rates in the general economy were to rise, our holdings could lose value. At December 31, 2023, a hypothetical increase in interest rates of 100 basis points across the entire yield curve on our holdings would not have resulted in a material impact on the fair value of our portfolio.
Interest Rate Risk – Variable-Rate Debt
Our use of variable-rate debt exposes us to interest rate risk as changes in interest rates would affect interest expense. As of December 31, 2023, we have variable-rate loans outstanding totaling $735.0 million, of which:
•$380.0 million matures on December 31, 2025 and bears interest at 3-month Term SOFR plus 7.5% per annum;
•$155.0 million matures on December 31, 2025 and bears interest at 3-month Term SOFR plus 8.0% per annum; and
•$200.0 million matures on September 11, 2026 and bears interest at 1-month Term SOFR plus 8.0% per annum.
As of December 31, 2023, the weighted-average interest rate on these loans was 13.09%. A hypothetical 100-basis point increase in the Term SOFR rate as of December 31, 2023 would increase our future interest payments by $16.1 million. Similarly, a hypothetical 100-basis point decrease in the Term SOFR rate as of December 31, 2023 would decrease our future interest payment by $16.1 million.
Interest Rate Risk – RIPA
We have entered into a revenue interest purchase agreement. Our primary exposure to market risk is that the interest rate on the revenue interest liability may vary during the term of the agreement depending on a number of factors, including the level of forecasted net sales. A significant increase or decrease in actual or forecasted net sales will materially impact the revenue interest liability, interest expense, and the time period for repayment. See Note9, “Revenue Interest Purchase Agreement,” that appears in Part II, Item 8. “Financial Statements and Supplementary Data” of this Annual Report for more information regarding the terms of the RIPA. Foreign Currency Exchange Risk
We are exposed to foreign currency exchange rate risk inherent in conducting business globally in numerous currencies. We contract with clinical research organizations, investigational sites and suppliers in foreign countries. We are, therefore, subject to fluctuations in foreign currency rates in connection with these agreements. We have not entered into any material foreign currency hedging contracts although we may do so in the future. From inception through the date of this Annual Report, we have not incurred any material effects from foreign currency changes on these contracts. The effect of a 10% adverse change in exchange rates on foreign currency denominated cash and payables as of December 31, 2023 would not have been material. However, fluctuations in currency exchange rates could harm our business in the future.
We are also exposed to foreign currency fluctuations related to the operations of our subsidiary in Italy whose financial statements are denominated in the Euro. We translate all assets and liabilities denominated in foreign currency into U.S. dollars using the exchange rate as of the end of the reporting period, while the operating results are translated into U.S. dollars using the average exchange rates for the reporting periods. Gains and losses resulting from translating the financial statements from our subsidiary’s functional currency to U.S. dollars are recognized as a component of other comprehensive (loss) income, on the consolidated statement of comprehensive loss. Foreign currency exchange rate fluctuations affect our reported net loss and can make comparisons from period to period more difficult. Our foreign operations are not material to our operations as a whole. As such, we currently do not enter into currency forward exchange or option contracts to hedge foreign currency exposures.
Market Risk
As of December 31, 2023, 37,732,820 warrants from our direct registered offerings remained outstanding at a fair value of $118.8 million. The fair value of the warrant liabilities is determined using the Black-Scholes option pricing model and is therefore sensitive to changes in the market price and volatility of our common stock among other factors. In the event of a hypothetical 10% increase in the market price of our common stock ($5.52 based on the $5.02 market price of our stock at December 31, 2023) on which the December 31, 2023 valuation was based, the fair value of the warrant liabilities would have increased by $15.6 million. Similarly, based on the fair value of the warrants outstanding as of December 31, 2023, a hypothetical decrease of 10% in the market price of our common stock would have the fair value of the warrant liabilities decreased by $15.5 million. Such increase or decrease would have been reflected as a change in fair value of warrant liabilities in other expense, net, on the consolidated statement of operations.
Inflation Risk
Inflation may affect us by increasing our cost of labor, clinical trial, and other costs. We do not believe that inflation has had a material effect on our business, financial condition or results of operations for any period presented herein.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of ImmunityBio, Inc. (NantKwest)and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of ImmunityBio, Inc. and Subsidiaries (the Company) as of December 31, 2023 and 2022, the related consolidated statements of operations, comprehensive loss, stockholders’ deficit and cash flows for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
| | | | | |
| Related party transactions and disclosures |
| |
Description of the
Matter | As described in Notes 10 and 11 to the consolidated financial statements, the Company’s Executive Chairman, Global Chief Scientific and Medical Officer and principal stockholder Dr. Patrick Soon-Shiong has a controlling interest in certain entities with which the Company has entered into material transactions including related party debt transactions with Nant Capital, LLC, NantWorks, NantMobile, LLC and NantCancerStemCell. Affiliates of such entities are also affiliates of the Company due to the common control of the Company’s Executive Chairman, Global Chief Scientific and Medical Officer and principal stockholder. Assessing the sufficiency of procedures performed to identify related parties and significant related party transactions and determining the identified significant related party transactions were properly recorded, presented and disclosed was challenging due to the nature, volume and the significance of related party transactions. |
| |
How We Addressed
the Matter in Our
Audit | We obtained an understanding, evaluated the design, and tested the operating effectiveness of internal controls over the Company’s related party process. This included testing controls over management’s identification, review, recognition and disclosure of significant related party transactions. The audit procedures we performed included, among others, testing the completeness and accuracy of the listing of related parties identified and significant related party transactions provided by management, and testing the manner in which significant related party transactions were recorded, presented and disclosed. We performed journal entry searches of identified related parties to verify completeness and accuracy of the Company’s significant related party transactions. We inquired of management, members of the Company’s audit committee, and the Company’s related party transaction committee chair regarding the completeness of the significant related party transactions identified. We also inspected questionnaires received from the Company’s directors and officers, read minutes of the meetings of Board of Directors and its various Committees, read employment and compensation contracts, proxy statements and other relevant filings with the Securities and Exchange Commission that relate to the Company’s financial relationships and transactions with the Company’s executive officers and with other entities controlled by the Company’s Executive Chairman, Global Chief Scientific and Medical Officer and principal stockholder. We confirmed the significant transactions and/or balances, as applicable, with the related parties. We also obtained the underlying agreements for significant related party transactions and assessed the associated accounting and recognition. We involved valuation specialists to assist in evaluating the appropriateness of the valuation methodologies and reasonableness of the assumptions used in the valuation of the related party debt including underlying conversion features and embedded derivatives, as deemed necessary, as described in Note 10 to the consolidated financial statements. |
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2016.
Los Angeles, California
March 19, 2024
ImmunityBio, Inc. and Subsidiaries
Consolidated Balance Sheets
(in thousands, except share and per share amounts)
| | | | | | | | | | | |
| As of December 31, |
| 2023 | | 2022 |
| ASSETS | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 265,453 | | | $ | 104,641 | |
| Marketable securities | 1,009 | | | 2,543 | |
| Due from related parties | 2,019 | | | 1,890 | |
| Prepaid expenses and other current assets (including amounts with related parties) | 25,603 | | | 31,503 | |
| Total current assets | 294,084 | | | 140,577 | |
| Marketable securities, noncurrent | 891 | | | 840 | |
| Property, plant and equipment, net | 146,082 | | | 143,659 | |
| Intangible assets, net | 17,093 | | | 20,003 | |
| Convertible note receivable | 6,879 | | | 6,629 | |
| Operating lease right-of-use assets, net (including amounts with related parties) | 36,543 | | | 45,788 | |
| Other assets (including amounts with related parties) | 2,880 | | | 4,860 | |
| Total assets | $ | 504,452 | | | $ | 362,356 | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 9,195 | | | $ | 21,016 | |
| Accrued expenses and other liabilities | 42,708 | | | 41,825 | |
Related-party promissory notes, net of discounts and deferred issuance costs (Note 10) | — | | | 431,901 | |
| | | |
| Due to related parties | 1,136 | | | 3,469 | |
| Operating lease liabilities (including amounts with related parties) | 5,244 | | | 2,650 | |
| Total current liabilities | 58,283 | | | 500,861 | |
Related-party nonconvertible note, net of discount (Note 10) | 104,586 | | | — | |
Related-party convertible notes and accrued interest, net of discount (Note 10) | 576,951 | | | 241,271 | |
| | | |
Revenue interest liability (Note 9) | 155,415 | | | — | |
| 35,333 | | | — | |
| Operating lease liabilities, less current portion (including amounts with related parties) | 39,942 | | | 47,951 | |
| | | |
| Warrant liabilities | 118,770 | | | 21,636 | |
| Other liabilities | 1,109 | | | 457 | |
| Total liabilities | 1,090,389 | | | 812,176 | |
Commitments and contingencies (Note 7) | | | |
| Stockholders’ deficit: | | | |
Common stock, $0.0001 par value; 1,350,000,000 and 900,000,000 shares authorized as of December 31, 2023 and 2022, respectively; 670,867,344 and 421,569,115 shares issued and outstanding as of December 31, 2023 and 2022, respectively; excluding 163,800 treasury stock shares outstanding as of December 31, 2023 and 2022 | 67 | | | 42 | |
| Additional paid-in capital | 2,374,620 | | | 1,930,936 | |
| Accumulated deficit | (2,961,684) | | | (2,378,488) | |
| Accumulated other comprehensive income | 10 | | | 183 | |
| Total ImmunityBio stockholders’ deficit | (586,987) | | | (447,327) | |
| Noncontrolling interests | 1,050 | | | (2,493) | |
| Total stockholders’ deficit | (585,937) | | | (449,820) | |
| Total liabilities and stockholders’ deficit | $ | 504,452 | | | $ | 362,356 | |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
Consolidated Statements of Operations
(in thousands, except share and per share amounts)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Revenue | $ | 622 | | | $ | 240 | | | $ | 934 | |
| Operating expenses: | | | | | |
Research and development (including amounts
with related parties) | 232,366 | | | 248,149 | | | 195,958 | |
Selling, general and administrative (including amounts
with related parties) | 129,620 | | | 102,708 | | | 135,256 | |
| Impairment of intangible assets | 886 | | | 681 | | | — | |
| Total operating expenses | 362,872 | | | 351,538 | | | 331,214 | |
| Loss from operations | (362,250) | | | (351,298) | | | (330,280) | |
| Other expense, net: | | | | | |
| Interest and investment income (loss), net | 1,131 | | | (3,090) | | | (4,100) | |
| Interest expense (including amounts with related parties) | (129,198) | | | (63,515) | | | (14,849) | |
| Loss on equity method investment | (7,549) | | | (12,107) | | | (803) | |
| Change in fair value of warrant liabilities | (47,600) | | | 13,460 | | | — | |
| Change in fair value of related-party convertible note | (36,203) | | | — | | | — | |
Other (expense) income, net (including amounts
with related parties) | (2,223) | | | (736) | | | 193 | |
| Total other expense, net | (221,642) | | | (65,988) | | | (19,559) | |
| Loss before income taxes and noncontrolling interests | (583,892) | | | (417,286) | | | (349,839) | |
| Income tax benefit (expense) | 40 | | | (34) | | | (9) | |
| Net loss | (583,852) | | | (417,320) | | | (349,848) | |
| Net loss attributable to noncontrolling interests, net of tax | (656) | | | (753) | | | (3,058) | |
| Net loss attributable to ImmunityBio common stockholders | $ | (583,196) | | | $ | (416,567) | | | $ | (346,790) | |
| | | | | |
| Net loss per ImmunityBio common share – basic and diluted | $ | (1.15) | | | $ | (1.04) | | | $ | (0.89) | |
Weighted-average number of common shares used in computing
net loss per share – basic and diluted | 508,635,592 | | 399,900,374 | | 389,234,156 |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
Consolidated Statements of Comprehensive Loss
(in thousands)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Net loss | $ | (583,852) | | | $ | (417,320) | | | $ | (349,848) | |
| Other comprehensive (loss) income, net of income taxes: | | | | | |
| Net unrealized gains (losses) on available-for-sale securities | 60 | | | (183) | | (13) | |
Reclassification of net realized gains (losses) on
available-for-sale securities included in net loss | (15) | | | 124 | | — | |
| Foreign currency translation adjustments | (218) | | | 238 | | (105) | |
| | | | | |
| Total other comprehensive (loss) income | (173) | | | 179 | | | (118) | |
| Comprehensive loss | (584,025) | | | (417,141) | | | (349,966) | |
| Less: Comprehensive loss attributable to noncontrolling interests | 656 | | | 753 | | | 3,058 | |
Comprehensive loss attributable to ImmunityBio
common stockholders | $ | (583,369) | | | $ | (416,388) | | | $ | (346,908) | |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ Deficit
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Additional
Paid-in
Capital | | Accumulated
Deficit | | Accumulated
Other
Comprehensive
Income | | Total
ImmunityBio
Stockholders’
Deficit | | Noncontrolling
Interests | | Total
Stockholders’
Deficit |
| | Shares | | Amount | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Balance as of December 31, 2020 | | 382,243,142 | | | $ | 38 | | | $ | 1,495,163 | | | $ | (1,615,131) | | | $ | 122 | | | $ | (119,808) | | | $ | 1,318 | | | $ | (118,490) | |
Issuance of common stock “at-the-market” offering, net of commissions and offering costs of $4,674 (Note 13) | | 13,295,817 | | | 2 | | | 164,528 | | | — | | | — | | | 164,530 | | | — | | | 164,530 | |
| Stock-based compensation expense | | — | | | — | | | 57,181 | | | — | | | — | | | 57,181 | | | — | | | 57,181 | |
| Exercise of stock options | | 1,695,638 | | | — | | | 5,461 | | | — | | | — | | | 5,461 | | | — | | | 5,461 | |
| Vesting of RSUs | | 873,058 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Net share settlement for RSUs vesting | | (277,611) | | | — | | | (4,064) | | | — | | | — | | | (4,064) | | | — | | | (4,064) | |
| Sales of assets to an entity under common control | | — | | | — | | | 1,435 | | | — | | | — | | | 1,435 | | | — | | | 1,435 | |
| Other comprehensive (loss) income, net of tax | | — | | | — | | | — | | | — | | | (118) | | | (118) | | | — | | | (118) | |
| Net loss | | — | | | — | | | — | | | (346,790) | | | — | | | (346,790) | | | (3,058) | | | (349,848) | |
| Balance as of December 31, 2021 | | 397,830,044 | | | 40 | | | 1,719,704 | | | (1,961,921) | | | 4 | | | (242,173) | | | (1,740) | | | (243,913) | |
Issuance of common stock “at-the-market” offering, net of commissions and offering costs of $302 (Note 13) | | 2,051,894 | | | — | | | 13,129 | | | — | | | — | | | 13,129 | | | — | | | 13,129 | |
Conversion of related-party convertible note and accrued interest, net of unamortized debt discount, | | 9,986,920 | | | 1 | | | 51,946 | | | — | | | — | | | 51,947 | | | — | | | 51,947 | |
Issuance of shares in a registered direct offering, net of discount and offering costs of $1,897 and value ascribed to associated warrants (Note 12) | | 9,090,909 | | | 1 | | | 13,006 | | | — | | | — | | | 13,007 | | | — | | | 13,007 | |
| Stock-based compensation expense | | — | | | — | | | 40,179 | | | — | | | — | | | 40,179 | | | — | | | 40,179 | |
| Exercise of stock options | | 14,767 | | | — | | | 74 | | | — | | | — | | | 74 | | | — | | | 74 | |
| Vesting of RSUs | | 521,296 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Net share settlement for RSUs vesting | | (156,011) | | | — | | | (616) | | | — | | | — | | | (616) | | | — | | | (616) | |
| Shares issued pursuant to litigation settlement | | 2,229,296 | | | — | | | 10,656 | | | — | | | — | | | 10,656 | | | — | | | 10,656 | |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ Deficit (Continued)
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Additional
Paid-in
Capital | | Accumulated
Deficit | | Accumulated
Other
Comprehensive
Income | | Total
ImmunityBio
Stockholders’
Deficit | | Noncontrolling
Interests | | Total
Stockholders’
Deficit |
| | Shares | | Amount | | | | | | |
Gain on extinguishment of debt with related parties
under common control | | — | | | — | | | 82,858 | | | — | | | — | | | 82,858 | | | — | | | 82,858 | |
| Other comprehensive income (loss), net of tax | | — | | | — | | | — | | | — | | | 179 | | | 179 | | | — | | | 179 | |
| Net loss | | — | | | — | | | — | | | (416,567) | | | — | | | (416,567) | | | (753) | | | (417,320) | |
| Balance as of December 31, 2022 | | 421,569,115 | | | 42 | | | 1,930,936 | | | (2,378,488) | | | 183 | | | (447,327) | | | (2,493) | | | (449,820) | |
Issuance of common stock “at-the market” offering, net of commissions and | | 5,605,323 | | | 1 | | | 16,105 | | | — | | | — | | | 16,106 | | | — | | | 16,106 | |
Issuance of common stock in exchange for notes | | 209,291,936 | | | 21 | | | 269,966 | | | — | | | — | | | 269,987 | | | — | | | 269,987 | |
Issuance of shares in a registered direct offerings, net of discounts and offering costs of $3,535 and value ascribed to associated warrants (Note 12) | | 28,641,911 | | | 3 | | | 36,928 | | | — | | | — | | | 36,931 | | | — | | | 36,931 | |
Issuance of common stock in connection with the RIPA, net of transaction costs of $473 (Note 13) | | 2,432,894 | | | — | | | 11,581 | | | — | | | — | | | 11,581 | | | — | | | 11,581 | |
Gain on debt extinguishment with related-parties | | — | | | — | | | 36,110 | | | — | | | — | | | 36,110 | | | — | | | 36,110 | |
Increase in fair value of embedded conversion feature from debt modification with entities under common | | — | | | — | | | 31,179 | | | — | | | — | | | 31,179 | | | — | | | 31,179 | |
| Stock-based compensation expense | | — | | | — | | | 49,163 | | | — | | | — | | | 49,163 | | | — | | | 49,163 | |
| Exercise of stock options | | 184,362 | | | — | | | 294 | | | — | | | — | | | 294 | | | — | | | 294 | |
| Vesting of RSUs | | 4,545,644 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Net share settlement for RSUs vesting | | (1,403,841) | | | — | | | (3,443) | | | — | | | — | | | (3,443) | | | — | | | (3,443) | |
Change in ownership interest in a joint venture due to | | — | | | — | | | (4,199) | | | — | | | — | | | (4,199) | | | 4,199 | | | — | |
| Other comprehensive income (loss), net of tax | | — | | | — | | | — | | | — | | | (173) | | | (173) | | | — | | | (173) | |
| Net loss | | — | | | — | | | — | | | (583,196) | | | — | | | (583,196) | | | (656) | | | (583,852) | |
| Balance as of December 31, 2023 | | 670,867,344 | | | $ | 67 | | | $ | 2,374,620 | | | $ | (2,961,684) | | | $ | 10 | | | $ | (586,987) | | | $ | 1,050 | | | $ | (585,937) | |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(in thousands)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Operating activities: | | | | | |
| Net loss | $ | (583,852) | | | $ | (417,320) | | | $ | (349,848) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Stock-based compensation expense | 49,163 | | | 40,179 | | | 57,181 | |
| Change in fair value of warrant liabilities | 47,600 | | | (13,460) | | | — | |
| Amortization of related-party notes discounts | 42,396 | | | 16,282 | | | 62 | |
| Change in fair value of convertible note | 36,203 | | | — | | | — | |
| Depreciation and amortization | 18,512 | | | 18,260 | | | 14,238 | |
| Non-cash interest items, net (including amounts with related parties) | 9,189 | | | 11,746 | | | 12,417 | |
| Non-cash lease expense related to operating lease right-of-use assets | 6,112 | | | 5,932 | | | 4,884 | |
| Transaction costs allocated to warrant liabilities | 2,010 | | | 1,082 | | | — | |
| Unrealized losses on equity securities | 1,591 | | | 4,190 | | | 4,615 | |
| Impairment of intangible assets | 886 | | | 681 | | | — | |
Amortization of premiums, net of discounts, on marketable
debt securities | (137) | | | 1,318 | | | 403 | |
| Impairment of fixed assets | — | | | 1,333 | | | — | |
| | | | | |
| | | | | |
| Other | (407) | | | 269 | | | 741 | |
| Changes in operating assets and liabilities: | | | | | |
| Prepaid expenses and other current assets | 5,958 | | | (16,557) | | | (2,249) | |
| Other assets | 1,913 | | | 1,998 | | | (3,977) | |
| Accounts payable | (6,476) | | | 8,000 | | | (3,717) | |
| Accrued expenses and other liabilities | 6,689 | | | 4,102 | | | 5,182 | |
| Related parties | (1,129) | | | (1,225) | | | (10,187) | |
| Operating lease liabilities | (2,978) | | | (4,319) | | | (4,164) | |
| Net cash used in operating activities | (366,757) | | | (337,509) | | | (274,419) | |
| Investing activities: | | | | | |
| Purchases of property, plant and equipment | (30,584) | | | (78,162) | | | (33,563) | |
| Purchase of intangible assets | — | | | (21,229) | | | — | |
| Proceeds from sales of property, plant and equipment | — | | | — | | | 20,498 | |
| Purchases of marketable debt securities, available-for-sale | (10,358) | | | (34,312) | | | (141,750) | |
| Maturities of marketable debt securities, available for sale | 10,100 | | | 128,188 | | | 56,166 | |
| Proceeds from sales of marketable debt and equity securities | 372 | | | 33,812 | | | 13,763 | |
| Investment in joint venture – an equity method investment | — | | | (1,000) | | | — | |
| Net cash (used in) provided by investing activities | (30,470) | | | 27,297 | | | (84,886) | |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
Consolidated Statements of Cash Flows (Continued)
(in thousands)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Financing activities: | | | | | |
Proceeds from issuance of related-party promissory notes
net of issuance costs paid | $ | 258,700 | | | $ | 174,125 | | | $ | 338,500 | |
| Proceeds from the RIPA, net of transaction costs | 192,764 | | | — | | | — | |
Proceeds from equity offerings,
net of discounts and issuance costs | 100,561 | | | 60,427 | | | 164,530 | |
Proceeds from stock issuance in connection with the RIPA,
net of transaction costs | 9,542 | | | — | | | — | |
| Proceeds from exercises of stock options | 294 | | | 74 | | | 5,461 | |
| Net share settlement for RSUs vesting | (3,443) | | | (616) | | | (4,064) | |
| Principal payments of finance leases | (77) | | | (58) | | | — | |
| Sale of assets to an entity under common control | — | | | — | | | 1,435 | |
| Payment for contingent consideration | — | | | (339) | | | (419) | |
| Net cash provided by financing activities | 558,341 | | | 233,613 | | | 505,443 | |
Effect of exchange rate changes on cash, cash equivalents, and
restricted cash | (292) | | | 284 | | | 48 | |
| Net change in cash, cash equivalents, and restricted cash | 160,822 | | | (76,315) | | | 146,186 | |
| Cash, cash equivalents, and restricted cash, beginning of year | 104,965 | | | 181,280 | | | 35,094 | |
| Cash, cash equivalents, and restricted cash, end of year | $ | 265,787 | | | $ | 104,965 | | | $ | 181,280 | |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
Consolidated Statements of Cash Flows (Continued)
(in thousands)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
Reconciliation of cash, cash equivalents, and restricted cash,
end of year: | | | | | |
| Cash and cash equivalents | $ | 265,453 | | | $ | 104,641 | | | $ | 181,101 | |
| 334 | | | 324 | | | 179 | |
| Cash, cash equivalents, and restricted cash, end of year | $ | 265,787 | | | $ | 104,965 | | | $ | 181,280 | |
| | | | | |
| Supplemental disclosure of cash flow information: | | | | | |
| Cash paid during the year for: | | | | | |
| Interest | $ | 77,192 | | | $ | 35,442 | | | $ | 2,106 | |
| Income taxes | $ | 8 | | | $ | 2 | | | $ | 9 | |
| | | | | |
| Supplemental disclosure of non-cash activities: | | | | | |
Conversion of related-party convertible notes and accrued interest,
net of unamortized discount, into equity | $ | 269,987 | | | $ | 51,947 | | | $ | — | |
Initial measurement of warrants issued in connection with
registered direct offerings accounted for as liabilities | $ | 49,534 | | | $ | 35,096 | | | $ | — | |
Gain on extinguishment of debt with related parties under
common control | $ | 36,110 | | | $ | 82,858 | | | $ | — | |
Increase in fair value of embedded conversion feature from
debt modification | $ | 31,179 | | | $ | — | | | $ | — | |
| | | | | |
| Common stock issuance discount related to the revenue interest liability | $ | 2,039 | | | $ | — | | | $ | — | |
Property and equipment purchases included in accounts payable,
accrued expenses and due to related parties | $ | 1,156 | | | $ | 12,693 | | | $ | 11,654 | |
| | | | | |
Right-of-use assets obtained in exchange for
operating lease liabilities | $ | — | | | $ | 14,798 | | | $ | 23,069 | |
Right-of-use assets disposed in exchange for
operating lease liabilities | $ | (3,777) | | | $ | — | | | $ | — | |
| Common stock issued pursuant to litigation settlement | $ | — | | | $ | 10,656 | | | $ | — | |
Unpaid offering and transaction costs included in accounts payable
and accrued expenses | $ | 255 | | | $ | 277 | | | $ | — | |
| | | | | |
| | | | | |
| | | | | |
| Cashless exercise of stock options | $ | — | | | $ | — | | | $ | 1,035 | |
| Accrued investment in joint venture | $ | — | | | $ | — | | | $ | 1,000 | |
The accompanying notes are an integral part of these consolidated financial statements.
ImmunityBio, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
1. Description of Business
In these notes to the consolidated financial statements, the terms “ImmunityBio,” “the company,” “the combined company,” “we,” “us,” and “our” refer to ImmunityBio and subsidiaries.
Our Business
We are an integrated clinical-stage biotechnology company discovering, developing, and commercializing next-generation immuno- and cellular therapies that bolster the natural immune system to drive and sustain an immune response. Using our proprietary platforms that amplify both the innate and adaptive branches of the immune system, our teams of clinical, scientific, and manufacturing experts, advance novel therapies and vaccines aimed at defeating urologic and other cancers, as well as infectious diseases. Although such designations may not lead to a faster development process or regulatory review and may not increase the likelihood that a product candidate will receive approval, N-803 (Anktiva), our lead biologic commercial product candidate, has received Breakthrough Therapy and Fast Track designations and is currently under review by the FDA for treatment in combination with BCG of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease and has a new user fee goal date (PDUFA date) of April 23, 2024.
Our platforms and their associated product candidates are designed to attack cancer and infectious pathogens by activating both the innate immune system, including—NK cells, dendritic cells, and macrophages, as well as—the adaptive immune system comprising—B and T cells,—in an orchestrated manner. The goal of this potentially best-in-class approach is to generate immunogenic cell death thereby eliminating rogue cells from the body whether they are cancerous or virally-infected. Our ultimate goal is to overcome the limitations of current treatments, such as checkpoint inhibitors, and/or reduce the need for standard high-dose chemotherapy in cancer by employing this coordinated approach to establish “immunological memory” that confers long-term benefit for the patient.
Our proprietary platforms for the development of biologic product candidates include: (i) antibody-cytokine fusion proteins, (ii) DNA, RNA, and recombinant protein vaccines, and (iii) cell therapies. These platforms have generated 9 novel therapeutic agents for which clinical trials are either underway or planned in solid and liquid tumors. Specifically, our clinical focus includes bladder, lung, and colorectal cancers and GBM, which are among the most frequent and lethal cancer types, and where there are high failure rates for existing standards of care or no available effective treatment.
Our lead biologic commercial product candidate Anktiva is an IL-15 superagonist antibody-cytokine fusion protein. In May 2022, we announced the submission of a BLA to the FDA for Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. On May 9, 2023, the FDA delivered a CRL to us regarding the BLA filed in May 2022, indicating that the FDA had determined that it could not approve the original BLA submission in its initial form, and the FDA made recommendations to address the issues raised. The deficiencies in the CRL related to the FDA’s pre-license inspection of the company’s third-party CMOs, among other items. Satisfactory resolution of the observations noted at the pre-license inspection would be required before the BLA could be approved. At the time, the FDA further provided recommendations specific to additional CMC issues and assays to be resolved. The CRL did not request new preclinical studies or Phase III clinical trials to evaluate safety or efficacy. The FDA requested that the company provide updated duration of response data for the efficacy population as identified by the FDA in the company’s resubmission, as well as a safety update.
On October 23, 2023, we announced that we had completed the resubmission of the BLA addressing the issues in the CRL. As part of our resubmission, we provided an update of the duration of response regarding the responders identified by the FDA in the efficacy population for BCG unresponsive subjects with high-risk CIS disease. On October 26, 2023, we announced that the FDA had accepted our BLA resubmission for review and considered it as a complete response to the CRL. The FDA has set a new user fee goal date (PDUFA date) of April 23, 2024. While we believe the BLA resubmission addresses the issues identified in the CRL, there is no guarantee that the FDA will ultimately agree that such issues have been successfully addressed and resolved. It is unclear when the FDA will approve our BLA, if at all.
The Merger
On December 21, 2020, NantKwest and NantCell, Inc. (formerly known as ImmunityBio, Inc., a private company) (NantCell) entered into an Agreement and Plan of Merger (the Merger Agreement), pursuant to which NantKwest and NantCell agreed to combine their businesses. The Merger Agreement provided that a wholly-owned subsidiary of the company would merge with and into NantCell (the Merger), with NantCell surviving the Merger as a wholly-owned subsidiary of the company.
On March 9, 2021, we completed the Merger pursuant to the terms of the Merger Agreement. Under the terms of the Merger Agreement, at the effective time of the Merger (the Effective Time), each share of NantCell common stock, par value $0.001 per share, issued and outstanding immediately prior to the Effective Time, subject to certain exceptions as set forth in the Merger Agreement, was converted automatically using the Exchange Ratio into a right to receive 0.8190 (the Exchange Ratio) newly issued shares of common stock, par value $0.0001 per share, of the company (CompanyCompany Common Stock),Stock, with cash paid in lieu of any fractional shares. At the Effective Time, each share of the company’s common stock issued and outstanding immediately prior to the Effective Time, remained an issued and outstanding share of the combined company. At the Effective Time, each outstanding option, RSU or warrant to purchase NantCell common stock was converted using the Exchange Ratio into an option, RSU or warrant, respectively, on the same terms and conditions immediately prior to the Effective Time, to purchase shares of Company Common Stock.
Immediately following the Effective Time, the former stockholders of NantCell held approximately 71.5% of the outstanding shares of Company Common Stock and the stockholders of NantKwest as of immediately prior to the Merger held approximately 28.5% of the outstanding shares of Company Common Stock. As a result of the Merger and immediately following the Effective Time, Dr. Patrick Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer, and his affiliates beneficially owned, in the aggregate, approximately 81.8% of the outstanding shares of Company Common Stock. Following the consummation of the Merger, the symbol for shares of the company’s common stock was changed to “IBRX.”
We incurredDuring the year ended December 31, 2021, we recorded $13.0 million of costs totaling $23.3 million inin connection with the Merger consisting of financial advisory, legal and other professional fees, of which $13.0 million and $10.3 million were recorded for the years ended December 31, 2021 and 2020, respectively. Merger-related costs are reported in selling, general and administrative expense, on the consolidated statementsstatement of operations.operations, consisting of financial advisory, legal and other professional fees.
Accounting Treatment of the Merger
The Merger represents a business combination pursuant to Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) TopicASC 805-50,Mergers, which was accounted for as a transaction between entities under common control as Dr. Soon-Shiong and his affiliates were the controlling stockholders of both the company and NantCell for all of the periods presented in this report. As a result, all of the assets and liabilities of NantCell were combined with ours at their historical carrying amounts on the closing date of the Merger. We recast our prior period financial statements for the yearsyear ended December 31, 2021 and 2020 to reflect the conveyance of NantCell’s common shares as if the Merger had occurred as of the earliest date of the consolidated financial statements presented. All material intercompany accounts and transactions have been eliminated in consolidation.
The following tables providetable provides the impact of the change in reporting entity on our unaudited condensed consolidated statement of operations for the three months ended March 31, 2021 and our consolidated statement of operations for the year ended December 31, 2021 (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
March 31, 2021 |
| (Unaudited) |
| NantCell | | NantKwest | | Intercompany
Eliminations | | ImmunityBio,
Inc. |
| | | | | | | |
| Revenue | $ | 183 | | | $ | — | | | $ | (44) | | | $ | 139 | |
| Operating expenses: | | | | | | | |
Research and development (including amounts
with related parties) | 21,509 | | | 19,725 | | | (106) | | | 41,128 | |
Selling, general and administrative (including amounts
with related parties) | 24,382 | | | 20,903 | | | (10) | | | 45,275 | |
| Loss from operations | (45,708) | | | (40,628) | | | 72 | | | (86,264) | |
Other (expense) income, net (including amounts
with related parties) | (848) | | | 6,637 | | | — | | | 5,789 | |
| Income tax expense | — | | | (6) | | | — | | | (6) | |
| Net loss | $ | (46,556) | | | $ | (33,997) | | | $ | 72 | | | $ | (80,481) | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, 2020 |
| (Unaudited) |
| NantCell | | NantKwest | | Intercompany
Eliminations | | ImmunityBio,
Inc. |
| | | | | | | |
| Revenue | $ | 1,695 | | | $ | 111 | | | $ | (1,201) | | | $ | 605 | |
| Operating expenses: | | | | | | | |
Research and development (including amounts
with related parties) | 75,763 | | | 64,483 | | | (739) | | | 139,507 | |
Selling, general and administrative (including amounts
with related parties) | 44,099 | | | 27,254 | | | (35) | | | 71,318 | |
| Impairment of intangible assets | 10,660 | | | — | | | — | | | 10,660 | |
| Loss from operations | (128,827) | | | (91,626) | | | (427) | | | (220,880) | |
Other (expense) income, net (including amounts
with related parties) | (4,401) | | | (752) | | | — | | | (5,153) | |
| Income tax benefit (expense) | 1,851 | | | (5) | | | — | | | 1,846 | |
| Net loss | $ | (131,377) | | | $ | (92,383) | | | $ | (427) | | | $ | (224,187) | |
2. Summary of Significant Accounting Policies
Basis of Presentation
The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (U.S. GAAP)U.S. GAAP and pursuant to the rules and regulations of the SEC. Certain items in the prior years’ consolidated financial statements have been reclassified to conform to the current presentation. These reclassifications had no effect on the reported results of operations. The consolidated financial statements reflect all adjustments which are, in the opinion of management, necessary for a fair presentation of our financial position and results of operations.
Principles of Consolidation
The consolidated financial statements include the accounts of the company, its wholly-owned subsidiaries, and its subsidiaries ina VIE for which the company has a controlling financial interest. Theis the primary beneficiary. Any material intercompany transactions and balances have been eliminated upon consolidation. For consolidated financial statements also include certain variable interest entities in which we are the primary beneficiary (as described in more detail below). For consolidated entities where we have less than 100% of ownership, we record net loss attributable to noncontrolling interestinterests, net of tax, on the consolidated statement of operations equal to the percentage of the ownership interest retained in such entities by the respective noncontrolling parties. Any material intercompany transactions and balances have been eliminated upon consolidation.
We assess whether we are the primary beneficiary of a VIE at the inception of the arrangement and at each reporting date. This assessment is based on our power to direct the activities of the VIE that most significantly impactimpacts the VIE’s economic performance and our obligation to absorb losses or the right to receive benefits from the VIE that could potentially be significant to the VIE.
If the entity is within the scope of the variable interest model and meets the definition of a variable interest entity (VIE),VIE, we consider whether we must consolidate the VIE or provide additional disclosures regarding our involvement with the VIE. If we determine that we are the primary beneficiary of the VIE, we will consolidate the VIE. This analysis is performed at the initial investment in the entity or upon any reconsideration event.
For entities we hold as an equity investment that are not consolidated under the VIE model, we consider whether our investment constitutes a controlling financial interest in the entity and therefore should be considered for consolidation under the voting interest model.
Liquidity
As of December 31, 2022,2023, the company had an accumulated deficit of $2.4$3.0 billion. We also had negative cash flows from operations of $337.5$366.8 million forduring the year ended December 31, 2022.2023. The company will likely need additional capital to further fund the development of, and to seek regulatory approvals for, our product candidates, and to begin to commercialize any approved products.
The consolidated financial statements have been prepared assuming the company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business, and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or amounts and classification of liabilities that may result from the outcome of the uncertainty of our ability to continue as a going concern. As a result of continuing anticipated operating cash outflows, we believe that substantial doubt exists regarding our ability to continue as a going concern without additional funding or financial support. However, we believe our existing cash, cash equivalents, and investments in marketable securities, together with capital to be raised through equity offerings (including but not limited to the offering, issuance and sale by us of our common stock that may be issued and sold under an “at-the-market” sales agreement with Jefferies LLC (the ATM),the ATM, of which we had $225.4$208.8 million available for future issuance as of December 31, 2022)2023, and a February 2023 shelf registration statement, of which we had $565.6 million available for future offerings as of December 31, 2023), a potential $100.0 million Second Payment upon satisfaction of certain conditions specified in the RIPA, including upon approval of N-803 before June 30, 2024, and our potential ability to borrow from affiliated entities, will be sufficient to fund our operations through at least the next 12 months following the issuance date of the consolidated financial statements based primarily upon our Executive Chairman and Global Chief Scientific and Medical Officer’s intent and ability to support our operations with additional funds, including loans from affiliated entities, as required, which we believe alleviates such doubt.
We may also seek to sell additional equity, through one or more follow-on offerings, or in separate financings, or obtain a credit facility.incremental subordinated debt in compliance with our existing revenue interest liability. However, we may not be able to secure such external financing in a timely manner or on favorable terms. Without additional funds, we may choose to delay or reduce our operating or investment expenditures. Further, because of the risk and uncertainties associated with the potential commercialization of our product candidates in development, we may need additional funds to meet our needs sooner than planned.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, we evaluate our estimates, including those related to the valuation of equity-based awards, deferred income taxes and related valuation allowances, preclinical and clinical trial accruals, impairment assessments, contingent value rightCVR measurement and assessments, the measurement of right-of-use assets and lease liabilities, useful lives of long-lived assets, loss contingencies, fair value calculation of warrants, stock options, derivative liabilities, and convertible promissory notes, fair value measurements, revenue interest liability, and the assessment of our ability to fund our operations for at least the next 12 months from the date of issuance of these consolidated financial statements. We base our estimates on historical experience and on various other market-specific and relevant assumptions that we believe to be reasonable under the circumstances. Estimates are assessed each period and updated to reflect current information, such as the economic considerations related to the impact that the ongoing coronavirus pandemic could have on our significant accounting estimates.information. Actual results could differ from those estimates.
Acquisitions
We make certain judgments to determine whether transactions should be accounted for as acquisitions of assets or as business combinations. If it is determined that substantially all of the fair value of gross assets acquired in a transaction is concentrated in a single asset (or a group of similar assets), the transaction is treated as an acquisition of assets. We evaluate the inputs, processes, and outputs associated with the acquired set of activities and assets. If the assets in a transaction include an input and a substantive process that together significantly contribute to the ability to create outputs, the transaction is treated as an acquisition of a business.
We account for business combinations using the acquisition method of accounting, which requires that assets acquired and liabilities assumed generally be recorded at their fair values as of the acquisition date. Excess of consideration over the fair value of net assets acquired is recorded as goodwill. Estimating fair value requires us to make significant judgments and assumptions. We perform impairment testing of goodwill annually or more frequently if events or changes in circumstances indicate that it is more likely than not that the asset is impaired.
In transactions accounted for as asset acquisitions, the cost of an asset acquisition, including transaction costs, are allocated to identifiable assets acquired and liabilities assumed based on a relative fair value basis. Goodwill is not recognized in an asset acquisition. Any difference between the cost of an asset acquisition and the fair value of the net assets acquired is allocated to the non-monetary identifiable assets based on their relative fair values. In an asset acquisition, upfront payments allocated to in-process research and development projects at the acquisition date are expensed unless there is an alternative future use. In addition, product development milestones are expensed upon achievement. Any contingent consideration, such as payments upon achievement of various developmental, regulatory, and commercial milestones, generally is not recognized at the acquisition date.
Contingencies
We record accruals for loss contingencies to the extent that we conclude it is probable that a liability has been incurred and the amount of the related loss can be reasonably estimated. We accrue for the best estimate of a loss within a range; however, if no estimate in the range is better than any other, then we accrue the minimum amount in the range. If we determine that a material loss is reasonably possible, we disclose the possible loss or range of loss, or that the amount of loss cannot be estimated at this time. We evaluate, on a quarterly basis, developments in legal proceedings and other matters that could cause a change in the potential amount of the liability recorded or the range of potential losses disclosed. Moreover, we record gain contingencies only when they are realizable and the amount is known. Additionally, we record our rights to insurance recoveries, limited to the extent of incurred or probable losses, as a receivable when such recoveries have been agreed to with our third-party insurers and when receipt is deemed probable. This includes instances when our third-party insurers have agreed to pay, on our behalf, certain legal defense costs and settlement amounts directly to applicable law firms and a settlement fund.
Concentration of Credit Risk and Other Risks and Uncertainties
Financial instruments that potentially subject us to concentrations of risk consist principally of cash and cash equivalents, marketable securities, and a convertible note receivable.
We attempt to minimize credit risk associated with our cash and cash equivalents by periodically evaluating the credit quality of our primary financial institutions. Our investment portfolio is maintained in accordance with our investment policy. While we maintain cash deposits in FDIC insured financial institutions in excess of federally insured limits, we do not believe that we are exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. We have not experienced any losses on such accounts.
We also monitor the creditworthiness of the borrower of the convertible promissory note. We believe that any concentration of credit risk in its convertible note receivable was mitigated in part by our ability to convert, if necessary, at the qualifying financing event or upon a payment default into shares of the senior class of equity securities of the borrower.
Product candidates developed by us will require approvals or clearances from the FDA or international regulatory agencies prior to commercial sales. There can be no assurance that any of our product candidates will receive any of the required approvals or clearances. If we were to be denied approval or clearance or any such approval or clearance was to be delayed, it would have a material adverse impact on us.
Cash, Cash Equivalents and Restricted Cash
Cash equivalents include highly liquid investments with an original maturity of three months or less from the date of purchase.
Restricted cash includes a certificate of deposit held as a substitute letter of credit for one of our leased properties. This certificate of deposit is included in other assets, on the consolidated balance sheet as the landlord is the beneficiary of the account and we are not able to access the funds during the term of the lease.
A reconciliation of cash, cash equivalents, and restricted cash is included on the consolidated statements of cash flows as of December 31, 2023, 2022 2021 and 2020.
Marketable Securities and Other Investments
Marketable Debt Securities
We investhave typically invested our excess fundscash in investment gradea variety of financial instruments, including investment-grade short- to intermediate-term corporate debt securities, government-sponsored securities and foreign government bondsEuropean bonds; however, after our entry into the RIPA, we can no longer invest our excess funds in corporate or European bonds. Certain of our investments are subject to credit, liquidity, market, and classify theseinterest-rate risks. The general condition of the financial markets and the economy may increase those risks and may affect the value and liquidity of investments as available-for-sale.and restrict our ability to access the capital markets. Marketable debt securities with remaining maturities of 12 months or less are classified as short-term and marketable securities with remaining maturities greater than 12 months are classified as long-term. All marketable debt securities are reported at fair value and any unrealized gains and losses are reported as a component of accumulated other comprehensive loss,income, on the consolidated statement of stockholders’ deficit, with the exception of unrealized losses believed to be other-than-temporary, which are recorded in interest and investment income (loss), net, on the consolidated statement of operations. Realized gains and losses from sales of securities and the amounts, net of tax, reclassified out of accumulated other comprehensive lossincome, if any, are determined on a specific identification basis.
Marketable Equity Securities
Investments in mutual funds and equity securities, other than equity method investments, are recorded at fair market value, if fair value is readily determinable and any unrealized gains and losses are included in other income (expense),expense, net, on the consolidated statement of operations. Realized gains and losses from the sale of the securities are determined on a specific identification basis and the amounts are included in other income (expense),expense, net, on the consolidated statement of operations.
Evaluating Investments for Other-than-Temporary Impairments
We periodically evaluate whether declines in fair values of our investments below their book value are other-than-temporary. This evaluation consists of several qualitative and quantitative factors regarding the severity and duration of the unrealized loss, as well as our ability and intent to hold the investment until a forecasted recovery occurs. Additionally, we assess whether we have plans to sell the security or whether it is more likely than not we will be required to sell any investment before recovery of its amortized cost basis. Factors considered include quoted market prices, recent financial results and operating trends, implied values from any recent transactions or offers of investee securities, credit quality of debt instrument issuers, other publicly available information that may affect the value of our investments, duration and severity of the decline in value, and our strategy and intentions for holding the investment. There were no other-than-temporary impairments recorded during the years ended December 31, 2023, 2022 2021 and 2020.2021.
Equity Method of Accounting
In circumstances where we have the ability to exercise significant influence over the operating and financial policies of a company in which we have an investment, we utilize the equity method of accounting for recording investment activity. In assessing whether we exercise significant influence, we consider the nature and magnitude of our investment, the voting and protective rights we hold, any participation in the governance of the other company and other relevant factors such as the presence of a collaborative or other business relationship. Under the equity method of accounting, we record our share of the income or loss of the other company as gain (loss)loss on equity method investment, in our consolidated statement of operations.
Property, Plant and Equipment, Net
Property, plant and equipment are stated at historical cost less accumulated depreciation. Historical cost includes expenditures that are directly attributable to the acquisition of the items. All repairs and maintenance are charged to net lossoperating expenses during the financial period in which they are incurred. Depreciation of property, plant and equipment is calculated using the straight-line method over the estimated useful lives of the assets, as follows:
| | | | | | | | |
| Buildings | | 39 years |
| Software | | 3 years |
| Laboratory equipment | | 5 to 7 years |
| Furniture & fixtures | | 5 years |
| IT equipment | | 3 years |
| Leasehold improvements | | The lesser of the lease term or life of the asset |
Upon disposal of property, plant and equipment, the cost and related accumulated depreciation are removed from the consolidated financial statements and the net amount, less any proceeds, is included in other income (expense),expense, net, on the consolidated statement of operations.
We review impairment of property, plant and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability is measured by a comparison of the carrying amount to the future net cash flows that the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the projected undiscounted future cash flows arising from the assets using a discount rate determined by management to be commensurate with the risk inherent to our current business model.
Business Combinations
Business combinations are accounted for using the acquisition method of accounting in accordance with ASC Topic 805, Business Combinations (ASC 805).805-50. These standards require that the total cost of acquisition be allocated to the tangible and intangible assets acquired and liabilities assumed based on their respective fair values at the date of acquisition, with the excess purchase price recorded as goodwill. The allocation of the purchase price is dependent upon certain valuations and other studies. Acquisition costs are expensed as incurred.
Contingent consideration incurred in connection with a business combination are recorded at their fair values on the acquisition date and re-measured at their fair values each subsequent reporting period until the related contingencies are resolved. The resulting changes in fair value are recorded as research and development expense, on the consolidated statements of operations and comprehensive loss. Changes in fair value reflect changes to our assumptions regarding probabilities of successful achievement of related milestones, the timing in which the milestones are expected to be achieved, and the discount rate used to estimate the fair value of the obligation.
Common Control Transactions
Transactions between us and entities where Dr. Soon-Shiong and his affiliates are the controlling stockholders are accounted for as common control transactions whereby the net assets acquired or transferred are accounted at their carrying value. Any difference between the carrying value and consideration recognized is treated as a capital transaction. Cash consideration up to the carrying value of the net assets acquired or transferred is presented as an investing activity in ouron the consolidated statement of cash flows. Cash consideration in excess of the carrying value of the net assets acquired or transferred is presented as a financing activity in ouron the consolidated statement of cash flows.
Intangible Assets, Net
Intangible assets acquired in a business combination or an asset acquisition are initially recognized at their fair value on the acquisition date. Acquired indefinite-lived assets, such as in-process research and development (IPR&D),IPR&D, are not amortized until they become definite-lived assets, upon the successful completion of the associated research and development effort. At that time, we evaluate whether the recorded
amounts are impaired and make any necessary adjustments, and then determine the useful life of the asset and begin amortization. If the associated research and development effort is abandoned, the related IPR&D assets is written-off and an impairment charge recorded.
Acquired definite-lived intangible assets are amortized using the straight-line method over their respective estimated useful lives. The amortization of these intangible assets is included in amortizationselling, general and administrative expense, on the consolidated statement of operations. Intangible assets are tested for impairment at least annually or more frequently if indicators of potential impairment exist. In connection with a workforce reduction at the Dunkirk Facility, we wrote off the remaining unamortized organized workforce intangible asset totaling $0.7 million during the year ended December 31, 2022 in impairment of intangible assets, on the consolidated statement of operations. See Note 6, Collaboration and License Agreements and Acquisition, for further information. Patents
Patent costs, including related legal costs, are expensed as incurred and recorded in selling, general and administrative expense on the consolidated statement of operations.
Fair Value of Financial InstrumentsMeasurements
Fair value is defined as an exit price that would be received from the sale of an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. We use a three-tier fair value hierarchy to classify and disclose all assets and liabilities measured at fair value on a recurring basis, as well as assets and liabilities measured at fair value on a non-recurring basis, in periods subsequent to their initial measurement. The hierarchy requires us to use observable inputs when available, and to minimize the use of unobservable inputs, when determining fair value.
The three tiers are defined as follows:
•Level 1—1—Observable inputs that reflect quoted market prices (unadjusted) for identical assets or liabilities in active markets at the measurement date. Since valuations are based on quoted prices that are readily and regularly available in an active market, the valuation of these products does not entail a significant degree of judgment. Our Level 1 assets consist of bank deposits, money market funds, and marketable equity securities.
•Level 2—2—Observable inputs other than quoted prices in active markets that are observable either directly or indirectly in the marketplace for identical or similar assets and liabilities. Our Level 2 assets consist of corporate debt securities including commercial paper, government-sponsored securities, and corporate bonds, as well as foreign municipal securities.
•Level 3—3—Valuations based on inputs that are unobservable and significant to the overall fair value measurement.
We utilize a third-party pricing service to assist in obtaining fair value pricing for our investments in marketable debt securities. Inputs are documented in accordance with the fair value disclosure hierarchy. The fair values of financial instruments other than marketable securities and cash and cash equivalents are determined through a combination of management estimates and third-party valuations.
During the years ended December 31, 2023, 2022 2021 and 2020,2021, no transfers were made into or out of the Level 1, 2 or 3 categories. We will continue to review the fair value inputs on a quarterly basis.
Collaboration Arrangements
We analyze our collaboration arrangements to assess whether they are within the scope of FASB ASC Topic 808, Collaborative Arrangements (ASC 808). A collaborative arrangement is a contractual arrangement that involves a joint operating activity. These arrangements involve two or more parties who are active participants in the activity, and are exposed to significant risks and rewards dependent on the commercial success of the activity. This assessment is performed throughout the life of the arrangement based on changes in the responsibilities of all parties in the arrangement. To the extent the collaboration agreement is within the scope of ASC 808, we also assess whether the arrangement contains multiple elements that are within the scope of other accounting literature. If we conclude that some or all aspects of the agreement are distinct and represent a transaction with a customer, we account for those aspects of the arrangement within the scope of FASB ASC Topic 606, Revenue from Contracts with Customers (ASC(ASC 606). Amounts that are owed by collaboration partners within the scope of ASC 808 are recognized as an offset to research and development expense as such amounts are incurred by the collaboration partner. The amounts owed to a collaboration partner are classified as research and development expense.
Our collaboration arrangements require us to acquire certain equipment for exclusive use in the joint operating activities. These equipment purchases do not have an alternative use and are therefore expensed as incurred within research and development expense.
Our collaboration arrangements are further discussed in Note 6, Collaboration and License Agreements and Acquisition. Preclinical and Clinical Trial Accruals
As part of the process of preparing the consolidated financial statements, we are required to estimate expenses resulting from obligations under contracts with vendors, clinical research organizations and consultants. The financial terms of these contracts vary and may result in payment flows that do not match the periods over which materials or services are provided under such contracts.
We estimate clinical trial and research agreement-related expenses based on the services performed, pursuant to contracts with research institutions and clinical research organizations and other vendors that conduct clinical trials and research on our behalf. In accruing clinical and research-related fees, we estimate the time period over which services will be performed and activity expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, we will adjust the accrual accordingly. Payments made under these arrangements in advance of the receipt of the related services are recorded as prepaid expenses until the services are rendered.
Transactions with Related Parties
As outlined in Note9 10, Related-Party Debt, and Note 1011, Related-Party Agreements, we have various agreements with related parties. These arrangements can be billed and settled in cash monthly, billed quarterly and settled in cash the following month, or estimated in advance and collected or paid upfront based on expected utilization. Monthly accruals are made for all quarterly billing arrangements. Lease Obligations
For all leases other than short-term leases, at the lease commencement date, a right-of-use asset and a lease liability are recognized and included in operating lease right-of-use assets, net, and current and non-current operating lease liabilities, respectively, on the consolidated balance sheet.recognized. The right-of-use asset represents the right to use the leased asset for the lease term. At the commencement date, operating lease right-of-use assets and operating lease liabilities are determined based on the present value of lease payments to be made over the lease term. Leases are classified as either finance leases or operating leases. We do not currently have any leases classified as finance leases.
As the rate implicit in lease contracts are not readily determinable, we utilize its incremental borrowing rate as a discount rate for purposes of determining the present value of lease payments, which is based on the estimated interest rate at which we could borrow on a collateralized basis the amount of the lease payments in the same currency, for a similar term, in a similar economic environment. Prospectively, we will remeasure the lease liability at the net present value of the remaining lease payments using the same incremental borrowing rate that was in effect as of the lease commencement or transition date.
Operating lease right-of-use assets also include any rent paid prior to the commencement date, less any lease incentives received, and initial direct costs incurred. Lease expense for minimum lease payments is recognized on a straight-line basis over the lease term. We determine the lease term by assuming the exercise of renewal options that are reasonably assured. The exercise of lease renewal options is at our sole discretion. Several of our leases have renewal options, however, the exercise of renewal is only assured for five of our current Good Manufacturing Practices (cGMP)cGMP facilities where we have made significant improvements or extended the lease.
We combine our lease components (e.g., fixed payments including rent, real estate taxes and insurance costs) with non-lease components (e.g., common-area maintenance costs and equipment maintenance costs) and as such, we account for lease and non-lease components as a single component. Lease expense also includes amounts relating to variable lease payments. Variable lease payments include amounts relating to common area maintenance and real estate taxes.
We do not recognize right-of-use assets and lease liabilities for qualifying short-term leases with an initial lease term of 12 months or less at lease inception. Such leases are expensed on a straight-line basis over the lease term. The lease term includes the non-cancellable period of the lease and any additional periods covered by either options to renew or not to terminate when the company is reasonably certain to exercise.
The depreciable life of operating right-of-use-assets and leasehold improvements is limited by the expected lease term.
Warrants
The company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in ASC Topic 480Distinguishing Liabilities from Equity (ASC 480), and ASC Topic 815, Derivatives and Hedging (ASC 815).815. The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the company’s own stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For warrants that meet all criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital, on the consolidated statement of stockholders’ deficit at the time of issuance. For warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and on each balance sheet date thereafter. Changes in the estimated fair value of the warrants are recognized as a non-cash gain or loss in other income (expense),expense, net, on the consolidated statement of operations. The fair value of the warrants was estimated using the Black-Scholes option pricing model.
Fair Value Option Election
The company accounted for a convertible note issued on March 31, 2023 under the FVO election of ASC 825 until it was amended and restated on December 29, 2023. Prior to its extinguishment on December 29, 2023, the convertible note was a debt host financial instrument containing embedded features wherein the entire financial instrument was initially measured at its issuance-date estimated fair value and then subsequently remeasured at estimated fair value on a recurring basis at each reporting period date.
Changes in the estimated fair value of this convertible note were recorded in other expense, net, on the consolidated statement of operations, except that changes in estimated fair value caused by changes in the instrument-specific credit risk were recorded in other comprehensive (loss) income. In accordance with ASC 470-50, when the convertible note was extinguished on December 29, 2023, the cumulative amount previously recorded in other comprehensive (loss) income resulting from changes in the instrument-specific credit risk were reclassified and reported in current earnings on the consolidated statement of operations. See Note 10, Related-Party Debt, for more information. Debt Modification and Extinguishment
The company evaluates amendments to its debt instruments in accordance with ASC 470-50. This evaluation includes comparing (1) if applicable, the net present value of future cash flows of the amended debt to that of the original debt and (2) the change in fair value of an embedded conversion feature to that of the carrying amount of the debt immediately prior to amendment to determine, in each case, if a change greater than 10% occurred. In instances where the net present value of future cash flows or the fair value of an embedded conversion feature, if any, changed more than 10%, the company applies extinguishment accounting. In instances where the net present value of future cash flows and the fair value of an embedded conversion feature, if any, changed less than 10%, the company accounts for the amendment to the debt as a debt modification. Gains and losses on debt amendments that are considered extinguishments are recognized in current earnings or in additional paid-in capital if the transactions are with entities under common control. Debt amendments that are considered debt modifications are accounted for prospectively through yield adjustments, based on the revised terms. The increase in fair value of the embedded conversion feature from the debt modification was accounted for as an increase in debt discount with a corresponding increase in additional paid-in capital. Legal fees and other costs incurred with third parties that are directly related to debt modifications are expensed as incurred. Amounts paid by the company to the lenders, are reflected as additional debt discount and amortized as an adjustment of interest expense over remaining term of modified debt using the effective interest rate method.
Income Taxes
We recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the financial reporting and tax basis of assets and liabilities, as well as for operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using the tax rates that are expected to apply to taxable income for the years in which those tax assets and liabilities are expected to be realized or settled. We record valuation allowances to reduce deferred tax assets to the amount we believe is more likely than not to be realized.
We recognize uncertain tax positions when the position will more likely than not be upheld on examination by the taxing authorities based solely upon the technical merits of the positions. We recognize interest and penalties, if any, related to unrecognized income tax uncertainties in income tax benefit (expense) benefit,, on the consolidated statement of operations. We did not have any accrued interest or penalties associated with uncertain tax positions as of December 31, 20222023 and 2021.2022.
Stock Repurchases
In 2015, the Board of Directors approved a share repurchase program (thethe 2015 Share Repurchase Program).Program. As it is our intent for the repurchased shares to be retired, we have elected to account for the shares repurchased using the constructive retirement method. For shares repurchased in excess of par, we record the purchase price in excess of par value in accumulated deficit, on the consolidated balance sheet.
Revenue Interest Liability
On December 29, 2023, we entered into the RIPA with Infinity and Oberland. Pursuant to the RIPA, Oberland acquired certain Revenue Interests from us for a gross purchase price of $200.0 million paid on closing. In addition, Oberland may purchase additional Revenue Interests from us in exchange for the $100.0 million Second Payment upon satisfaction of certain conditions specified in the RIPA. Under the RIPA, Oberland has the right to receive quarterly payments from us based on, among other things, a certain percentage of our worldwide net sales, excluding those in China, during such quarter. The RIPA is considered a sale of future revenues and is accounted for as a liability net of a debt discount comprised of deferred issuance costs, the fair value of a freestanding option agreement related to the SPOA, and the fair value of embedded derivatives requiring bifurcation on the consolidated balance sheet. The company imputes interest expense associated with this liability using the effective interest rate method. The effective interest rate is calculated based on the rate that would enable the debt to be repaid in full over the anticipated life of the arrangement. Interest expense is recognized over the estimated term on the consolidated statement of operations. The interest rate on this liability may vary during the term of the agreement depending on a number of factors, including the level of actual and forecasted net sales. The company evaluates the interest rate quarterly based on actual and forecasted net sales utilizing the prospective method. A significant increase or decrease in actual or forecasted net sales will materially impact the revenue interest liability, interest expense, and the time period for repayment.
Derivative Liabilities
Embedded derivatives that are required to be bifurcated from the underlying debt instrument that do not meet the derivative scope exception and equity classification criteria are accounted for and valued as separate financial instruments. The terms of an embedded derivative related to a contingent exercisable prepayment feature of a convertible note have been evaluated and deemed to require bifurcation. This embedded derivative will be initially measured at fair value and will be remeasured to fair value at each reporting date until the derivative is settled.
In addition, the RIPA contains certain features that meet the definition of being an embedded derivative requiring bifurcation as a separate compound financial instrument apart from the RIPA. The derivative liability is initially measured at fair value upon issuance and is subject to remeasurement at each reporting period with changes in fair value recognized in other expense, net, on the consolidated statement of operations.
Revenue Recognition
We have primarily generated revenues from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables and grant programs. The nonexclusive license agreements with a limited number of pharmaceutical and biotechnology companies grant them the right to use our cell lines and intellectual property for non-clinical use. These agreements generally include upfront fees and annual research license fees for such use, as well as commercial license fees for sales of the licensee products developed or manufactured using our intellectual property and cell lines. We have generated revenues from product sales of our proprietary GMP-in-a-Box bioreactors and related consumables, primarily to related parties. Additionally, we also generated revenues from grant programs.
A performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account in ASC 606.customer. A contract’s transaction price is allocated to each distinct performance obligation based on relative standalone selling price and recognized as revenue when, or as, the performance obligation is satisfied.
Under our license agreements with customers, we typically promise to provide a license to use certain cell lines and related patents, the related know-how, and future research and development data that affect the license. We have concluded that these promises represent one performance obligation due to the highly interrelated nature of the promises. We provide the cell lines and know-how immediately upon entering into the contracts. Research and development data are provided throughout the term of the contract when and if available.
The license agreements may include non-refundable upfront payments, event-based milestone payments, sales-based royalty payments, or some combination of these. The event-based milestone payments represent variable consideration and we use the most likely amount method to estimate this variable consideration. Given the high degree of uncertainly around the achievement of these milestones, we do not recognize revenue from these milestone payments until the uncertainty associated with these payments is resolved. We currently estimate variable consideration related to milestone payments to be zero and, as such, no revenue has been recognized for milestone payments. We recognize revenue from sales-based royalty payments when or as the sales occur. On a quarterly basis, we re-evaluate our estimate of milestone variable consideration to determine whether any amount should be included in the transaction price and recorded in revenue prospectively.
We also have sold our proprietary GMP-in-a-Box bioreactors and related consumables to affiliated companies. The arrangements typically include delivery of bioreactors, consumables, and providing installation service and perpetual software licenses for using the equipment. We recognize revenue when customers obtain control and can benefit from the promised goods or services, generally upon installation of the bioreactors, in an amount that reflects the consideration that we expect to receive in exchange for those goods or services. Upfront payments and fees are recorded as deferred revenue upon receipt and recognized as revenue when we satisfy our performance obligations under these arrangements.
Grant revenue is typically paid for reimbursable costs incurred over the duration of the associated research project or clinical trial and is recognized either when expenses reimbursable under the grants have been incurred and payments under the grants become contractually due.due or when cash is received, depending on the certainty of payment and other factors specific to each grant.
From inception through December 31, 2022,2023, we have generated minimal revenue from non-exclusive license agreements related to our cell lines, the sale of our bioreactors and related consumables, and grant programs. We have no clinical products approved for commercial sale and have not generated any revenue from therapeutic and vaccine product candidates that are under development.
Research and Development Costs
Major components of research and development costs include cash compensation and other personnel-related expenses, stock-based compensation, depreciation and amortization expense on research and development property and equipment and intangible assets, costs of preclinical studies, clinical trials costs, including contract research organizations (CROs)CROs and related clinical manufacturing, including contract manufacturing organizations (CMOs),third-party CMOs, costs of drug development, costs of materials and supplies, facilities cost, overhead costs, regulatory and compliance costs, and fees paid to consultants and other entities that conduct certain research and development activities on our behalf. Costs incurred in research and development are expensed as incurred.
The company classifies its research and development expenses as either external or internal. The company’s external research and development expenses support its various preclinical and clinical programs. The company’s internal research and development expenses include payroll and benefits expenses, facilities and equipment expense, and other indirect research and development expenses incurred in support of its research and development activities. The company’s external and internal resources are not directly tied to any one research or drug discovery program and are typically deployed across multiple programs and are not allocated to specific product candidates or development programs.
Included in research and development costs are clinical trial and research expenses based on the services performed pursuant to contracts with research institutions and clinical research organizations and other vendors that conduct clinical trials and research on our behalf. We record accruals for estimated costs under these contracts. When evaluating the adequacy of the accrued liabilities, we analyze the progress of the preclinical studies or clinical trials, including the phase or completion of events, invoices received, contracted costs and purchase orders. Significant judgments and estimates are made in determining the accrued balances at the end of any reporting period based on the facts and circumstances known at that time. Although we do not expect the estimates to be materially different from the amounts actually incurred, if the estimates of the status and timing of services performed differ from the actual status and timing of services performed, we may report amounts that are too high or too low in any particular period. Actual results could differ from our estimates. We adjust the accruals in the period when actual costs become known.
Stock-Based Compensation
We account for stock-based compensation under the provisions of ASC Topic 718, Compensation—Stock Compensation (ASC 718).718. We estimate fair value of each stock option award on the date of grant using the Black-Scholes option pricing model. The Black-Scholes option pricing model requires the use of highly subjective assumptions, including, but not limited to, expected stock price volatility over the term of the awards and the expected term of the stock options. We measure the fair value of an equity-classified award at the grant date and recognize the stock-based compensation expense over the period of vesting on the straight-line basis for our outstanding share awards that do not contain a performance condition. For awards subject to performance-based vesting conditions, we assess the probability of the individual milestones under the award being achieved and stock-based compensation expense is recognized over the service period using the graded vesting method once management believes the performance criteria is probable of being met. For awards with service or performance conditions, we recognize the effect of forfeitures in compensation cost in the period that the award was forfeited. See Note 1314, Stock-Based Compensation., for more information.
Sale-Leaseback Transaction
A sale-leaseback transaction occurs when an entity sells an asset it owns and immediately leases the asset back from the buyer. The seller then becomes the lessee and the buyer becomes the lessor. When entering into a sale-leaseback transaction as a seller-lessee, the requirements in ASC Topic 606,Revenue from Contracts with Customers, and all related accounting standards updatesASUs to such Topic are applied in determining whether the transfer of an asset shall be accounted for as a sale of the asset by assessing whether it satisfies a performance obligation under the contract by transferring control of an asset. If the company transfers control of an asset to the buyer-lessor, the transfer is accounted for as a sale and the company derecognizes the transferred asset. The subsequent leaseback of the asset is accounted for in accordance with FASB ASC Topic 842, Leases, in the same manner as any third-party lease. If the company does not transfer control of an asset to the buyer-lessor, the sale-leaseback transaction is accounted for as a financing arrangement.
In September 2021, we entered into a sale transaction with Nant Capital, LLC (Nant Capital), a related party, for a building located at 557 South Douglas Street, El Segundo, California. We subsequently leased back the building for an initial seven-year lease term with an option to extend the lease for two additional seven-year periods. There was no purchase option at the end of the lease term. Since we transferred the legal title and all benefits and risks incidental to the ownership of the property to Nant Capital, we accounted for the transfer as a sale. We have classified the leaseback of the building as an operating lease and accordingly, a right-of-use asset and an operating lease liability were established on the lease commencement date that will be amortized through the end of the lease term. Effective May 31, 2022, we executed a termination agreement on this lease. See Note 10, Related-Party Agreements, for further information.Comprehensive LossComprehensive Income (Loss)
Comprehensive income (loss)loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. Comprehensive income or loss is composed of net (loss) income (loss) and other comprehensive income (loss). income. Our other comprehensive income or loss consists of net unrealized gains (losses) on marketable debt securities classified as available-for-sale, net of income taxes and foreign currency translation adjustments.
Noncontrolling Interests
Noncontrolling interests are recorded for the entities that we consolidate but are not wholly-owned by the company. Noncontrolling interests are classified as a separate component of equity on the consolidated balance sheet and consolidated statement of stockholders’ deficit. Additionally, net loss attributable to noncontrolling interests is reflected separately from consolidated net loss on the consolidated statement of operations and the consolidated statement of stockholders’ deficit.
We record the noncontrolling interests’ share of loss based on the percentage of ownership interest retained by the respective noncontrolling interest holders. Noncontrolling interests recorded on the consolidated financial statements result from the company’s share of GlobeImmune, Inc. (GlobeImmune), of which we controlled 69.1%, as of December 31, 2023, 2022 and Immunotherapy2021, respectively, and NANTibody, LLC (NANTibody), of which we controlled 100.0% as of December 31, 2023 and 60.0% as of December 31, 2022 and 2021, and 2020.respectively. Noncontrolling interest stockholders are common stockholders.
GlobeImmune was determined to be a VIE as it does not have sufficient equity investment at risk to finance its operations without additional subordinated financial support and we are deemed the primary beneficiary of GlobeImmune and, accordingly, consolidatesconsolidate GlobeImmune into the company’s consolidated financial statements under the VIE model. The company also supports GlobeImmune through a promissory note agreement, in which the company provides advances to GlobeImmune from time to time up to $6.0 million with a per annum interest rate of five percent (5%). As of December 31, 20222023 and 2021,2022, there were no outstanding advances due from GlobeImmune under the promissory note agreement.
GlobeImmune recognized no revenue forDuring the years ended December 31, 2023, 2022, and 2021, and 2020,GlobeImmune recognized no revenue, respectively, and recognized $0.3 million, $0.5 million, $0.7 million and $2.0$0.7 million of operating expenses, forrespectively, during the years ended December 31, 2023, 2022 2021 and 2020, respectively. The2021. As of both December 31, 2023 and 2022, the consolidated balance sheets includeincluded approximately $1.4 million and $0.8 million of total assets that can only be used to settle obligations of GlobeImmune, and no liabilities, as of December 31, 2022 and 2021, respectively, related to GlobeImmune.
GlobeImmune do not have recourse to the general credit of the primary beneficiary. Foreign Currencies
We have operations and hold assets in Italy and South Korea. The functional currency of the subsidiary in Italy is the Euro, based on the nature of the transactions occurring within this entity, and accordingly, assets and liabilities of this subsidiary are translated into U.S. dollars at exchange rates prevailing as of the balance sheet dates, while the operating results are translated into U.S. dollars using the average exchange rates for the period correlating with those operating results. Adjustments resulting from translating the financial statements of the foreign subsidiary into U.S. dollars are recorded as a component of other comprehensive (loss) income (loss), on the consolidated statement of comprehensive loss. Transaction gains and losses are recorded in other income (expense),expense, net, on the consolidated statement of operations.
Basic and Diluted Net Loss per Share of Common Stock
Basic net loss per share is calculated by dividing the net loss attributable to ImmunityBio common stockholders by the weighted-average number of common shares outstanding for the period. Diluted loss per share is computed by dividing net loss attributable to ImmunityBio common stockholders by the weighted-average number of common shares, including the number of additional shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive.
For all periods presented, potentially dilutive securities are excluded from the computation of fully diluted loss per share as their effect is anti-dilutive. The following table details those securities that have been excluded from the computation of potentially dilutive securities:
| | As of December 31, | | | As of December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| | As of December 31, |
| 2022 | | 2021 | | 2020 |
| | Related-party convertible notes | |
| Related-party convertible notes | |
| Related-party convertible notes | | 162,471,837 | | 46,274,965 | | — |
| Outstanding third-party warrants | | Outstanding third-party warrants | 37,732,820 | | 9,090,909 | | — |
| Outstanding stock options | Outstanding stock options | 9,262,926 | | 4,124,930 | | 4,996,284 | Outstanding stock options | 9,820,435 | | 9,262,926 | | 4,124,930 |
| Outstanding third-party warrants | 9,090,909 | | — | | — |
| Outstanding RSUs | Outstanding RSUs | 6,551,388 | | 6,515,889 | | 466,842 | Outstanding RSUs | 7,503,979 | | 6,551,388 | | 6,515,889 |
| Outstanding related-party warrants | Outstanding related-party warrants | 1,638,000 | | 1,638,000 | | 1,638,000 | Outstanding related-party warrants | 1,638,000 | | 1,638,000 |
| Total | Total | 26,543,223 | | 12,278,819 | | 7,101,126 | Total | 219,167,071 | | 72,818,188 | | 12,278,819 |
Amounts inThe dilutive securities exclude the table above reflectoption to purchase up to $10.0 million of the company’s common stock equivalents of the noted instruments, including awards issued under the NantKwest 2015 Equity Incentive Plan (the 2015 Plan), the NantKwest 2014 Equity Incentive Plan (the 2014 Plan), and awards issued under the NantCell, Inc. 2015 Stock Incentive Plan (the NC 2015 Plan) that, in the case of December 31, 2021, were outstanding immediately priorpursuant to the Effective Time ofSPOA entered in connection with the Merger and inRIPA, as the case of December 31, 2020 have been adjusted to include the combined NC 2015 Plan and NantCell warrants then outstanding (in both cases adjusted using the Exchange Ratio of 0.8190).exercise price is not yet determined. See Note13, Stock-Based CompensationStockholders’ Deficit,, for furthermore information.Segment and Geographic Information
We operate in one reporting segment focused on creating the next generation of immunotherapies to address serious unmet needs within oncology and infectious diseases. Our chief executive officer (CEO)CEO is the chief operating decision-maker (CODM) of the company, and manages and allocates resources to our operations on a company-wide basis. Consistent with this decision-making process, our CEO uses consolidated, single-segment financial information for purposes of evaluating performance, forecasting future-period financial results, allocating resources, and setting incentive targets.
We generate a portion of our revenues from outside of the U.S. Information about our revenues by geographic region is as follows (in thousands):
| | Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| | Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| | United States | |
| United States | |
| United States | United States | $ | 42 | | | $ | 373 | | | $ | 513 | |
| Europe | Europe | 198 | | | 561 | | | 92 | |
| Total segment revenue | Total segment revenue | $ | 240 | | | $ | 934 | | | $ | 605 | |
Recent Accounting Pronouncements
Application of New or Revised Accounting Standards – Adopted
In May 2021, the FASB issued Accounting Standards Update (ASU) 2021-04, Earnings Per Share (Topic 260), Debt—Modifications and Extinguishments (Subtopic 470-50), Compensation—Stock Compensation (Topic 718), and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40). This update provides guidance to clarify and reduce diversity in an accounting for modifications or exchanges of freestanding equity-classified written call options (for example, warrants) that is not within the scope of another Topic. An entity should treat a modification of the terms or conditions or an exchange of a freestanding equity-classified written call option that remains equity classified after modification or exchange as an exchange of the original instrument for a new instrument. This update additionally provides further guidance on measuring the effect of a modification or an exchange of a freestanding equity-classified written call option that remains equity classified after modification or exchange on the basis of the substance of the transaction, in the same manner as if cash had been paid as consideration. This guidance is effective for the fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. The company adopted this guidance on January 1, 2022 on a prospective basis.
In August 2020, the FASB issued ASU 2020-06, Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, which simplifies and clarifies certain calculation and presentation matters related to convertible equity and debt instruments. Specifically, ASU 2020-06 removes requirements to separately account for conversion features as a derivative under ASC Topic 815 and removes the requirement to account for beneficial conversion features on such instruments. In addition, ASU 2020-06 eliminates the treasury stock method when calculating diluted earnings per share for convertible instruments that can be settled in whole or in part with equity and requires the use of the if-converted method. The guidance is effective for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. The company adopted this guidance on January 1, 2022 on a modified prospective basis.
Application of New or Revised Accounting Standards – Not Yet Adopted
In June 2022,December 2023, the FASB issued ASU 2022-03,2023-09, Fair Value MeasurementIncome Taxes (Topic 820)(Topic 740): Improvements to Income Tax DisclosuresFair Value Measurement, to improve its income tax disclosure requirements. Under the ASU, entities must annually (1) disclose specific categories in the rate reconciliation, (2) provide additional information for reconciling items that meet a quantitative threshold, and (3) disclose more detailed information about income taxes paid, including by jurisdiction; pretax income (or loss) from continuing operations; and income tax expense (or benefit). The ASU is effective for fiscal years beginning after December 15, 2024, and interim periods beginning after December 15, 2025, with early adoption permitted. We are currently evaluating the impact of Equity Securities Subjectthis standard on our disclosures.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting(Topic 280): Improvements to Contractual Sale RestrictionsReportable Segment Disclosures, which amendsrequires disclosure of incremental segment information on an annual and interim basis. This ASU is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024 on a retrospective basis. We are currently evaluating the guidance in Topic 820,impact of this standard on our disclosures.
In August 2023, the FASB issued ASU 2023-05, Fair ValueBusiness Combinations-Joint Venture Formations (Subtopic 805-60): Recognition and Initial Measurement, which requires a joint venture to clarify that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The amendments also clarify that an entity cannot, as a separate unit of account, recognize andinitially measure a contractual sale restriction. In addition, ASU 2022-03 introduces new disclosure requirements for equity securities subject to contractual sale restrictions that are measuredall contributions received upon its formation at fair value. This ASU 2022-03is applicable to joint venture entities with a formation date on or after January 1, 2025 on a prospective basis. We will apply this guidance prospectively in future reporting periods after the guidance is effective to any future arrangements meeting the definition of a joint venture.
In March 2023, the FASB issued ASU 2023-01, Leases (Topic 842): Common Control Arrangements, which requires that leasehold improvements associated with common control leases be amortized over the useful life of the leasehold improvements to the common control group, regardless of the lease term. This ASU is effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. We are currently evaluating the impact of this standard on our consolidated financial statements.statements and related disclosures.
Other recent authoritative guidance issued by the FASB (including technical corrections to the ASC), the American Institute of Certified Public Accountants, and the SEC during the year ended December 31, 20222023 did not, or are not expected to, have a material effect on our consolidated financial statements.
3. Financial Statement Details
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist of the following (in thousands):
| | As of December 31, | | | As of December 31, |
| 2023 | | | 2023 | | 2022 |
| | Prepaid research and development costs | |
| Prepaid research and development costs | |
| Prepaid research and development costs | |
| Prepaid services | |
| Prepaid insurance | |
| Prepaid software license fees | |
| Insurance premium financing asset | |
| | Insurance claims receivable | |
| | Insurance claims receivable | |
| | Insurance claims receivable | |
| Prepaid equipment maintenance | |
| ERP system implementation cost | |
| | | As of December 31, |
| | 2022 | | 2021 |
| | Prepaid research and development costs | $ | 11,704 | | | $ | 692 | |
| Prepaid services | 8,013 | | | 6,274 | |
| | Prepaid insurance | 2,282 | | | 2,266 | |
| Prepaid software license fees | 2,195 | | | 1,111 | |
| Prepaid supplies | Prepaid supplies | 2,160 | | | — | |
| Insurance premium financing asset | 1,417 | | | 2,598 | |
| | Prepaid supplies | |
| | Prepaid supplies | |
| Other | Other | 3,732 | | | 2,957 | |
| Prepaid expenses and other current assets | Prepaid expenses and other current assets | $ | 31,503 | | | $ | 15,898 | |
Property, Plant and Equipment, Net
Property, plant and equipment, net, consist of the following (in thousands):
| | As of December 31, | | | As of December 31, |
| 2023 | | | 2023 | | 2022 |
| | As of December 31, |
| 2022 | | 2021 |
| | Leasehold improvements | |
| Leasehold improvements | |
| Leasehold improvements | Leasehold improvements | $ | 68,710 | | | $ | 62,482 | |
| Equipment | Equipment | 67,945 | | | 54,284 | |
| Construction in progress | Construction in progress | 72,693 | | | 16,575 | |
| Software | Software | 1,657 | | | 1,544 | |
| Furniture & fixtures | Furniture & fixtures | 1,906 | | | 1,052 | |
| Gross property, plant and equipment | Gross property, plant and equipment | 212,911 | | | 135,937 | |
| Less: Accumulated depreciation and amortization | Less: Accumulated depreciation and amortization | 69,252 | | | 53,074 | |
| Property, plant and equipment, net | Property, plant and equipment, net | $ | 143,659 | | | $ | 82,863 | |
DepreciationDuring the years ended December 31, 2023, 2022 and 2021, depreciation expense related to property, plant and equipment totaled $16.5 million, $16.3 million and $14.2 million, and $12.7 million for the years ended December 31, 2022, 2021 and 2020, respectively.
Intangible Assets, Net
The gross carrying amounts and accumulated amortization of intangible assets are as follows at the dates indicated (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2022 |
| Weighted-
Average
Life
(in years) | | Gross Carrying
Amount | | Accumulated
Amortization | | Impairment | | Net Carrying
Amount |
| | | | | | | | | |
| Favorable leasehold rights | 9.1 | | $ | 20,398 | | | $ | (1,785) | | | $ | — | | | $ | 18,613 | |
| Organized workforce | | | 831 | | | (150) | | | (681) | | | — | |
| Total definite-lived intangible assets | | | 21,229 | | | (1,935) | | | (681) | | | 18,613 | |
| Indefinite-lived: IPR&D | | | 1,390 | | | — | | | — | | | 1,390 | |
| Total intangible assets | | | $ | 22,619 | | | $ | (1,935) | | | $ | (681) | | | $ | 20,003 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2023 |
| Weighted-
Average
Life
(in years) | | Gross Carrying
Amount | | Accumulated
Amortization | | Impairment | | Net Carrying
Amount |
| | | | | | | | | |
| Definite-lived: Favorable leasehold rights | 8.1 | | $ | 20,398 | | | $ | (3,825) | | | $ | — | | | $ | 16,573 | |
| Indefinite-lived: IPR&D | | | 1,406 | | | — | | | (886) | | | 520 | |
| Total intangible assets | | | $ | 21,804 | | | $ | (3,825) | | | $ | (886) | | | $ | 17,093 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2022 |
| Weighted-
Average
Life
(in years) | | Gross Carrying
Amount | | Accumulated
Amortization | | Impairment | | Net Carrying
Amount |
| | | | | | | | | |
| Favorable leasehold rights | 9.1 | | $ | 20,398 | | | $ | (1,785) | | | $ | — | | | $ | 18,613 | |
| Organized workforce | | | 831 | | | (150) | | | (681) | | | — | |
| Total definite-lived intangible assets | | | 21,229 | | | (1,935) | | | (681) | | | 18,613 | |
| Indefinite-lived: IPR&D | | | 1,390 | | | — | | | — | | | 1,390 | |
| Total intangible assets | | | $ | 22,619 | | | $ | (1,935) | | | $ | (681) | | | $ | 20,003 | |
Definite-Lived IntangiblesIntangible Assets
Definite-lived intangible assets subject to amortization includeIn connection with the acquisition of the Dunkirk Facility in 2022, we acquired definite-lived intangibles consisting of favorable leasehold rights and an organized workforce acquired from the asset acquisition of the Dunkirk facility in February 2022.workforce. During the year ended December 31, 2022, we wrote off the entire unamortized organized workforce intangible asset totaling $0.7 million in impairment of intangible assets, on the consolidated statement of operations. See Note 6, Collaboration and License Agreements and Acquisition, for furthermore information.WeDuring the years ended December 31, 2023 and 2022, we recorded amortization expense of definite-lived intangible assets totaling $2.0 million and $1.9 million, respectively, in research and development expense, on the consolidated statementstatements of operations for the year ended December 31, 2022. Future amortization expense for the favorable leasehold rights is as follows: $2.0 million for each of the years from 2023 to 2027 and $8.4 million thereafter.operations.
Indefinite-Lived IntangiblesIntangible Assets
During the year ended December 31, 2020,2023, we determined to discontinuediscontinued the LMP1research and LMP/IPS programsdevelopment program associated with the Tarmogen platform based on results gathered from preclinicalclinical data. AsWe recorded a result, the carrying valuecharge totaling $0.9 million in impairment of the IPR&D relating to the LMP1 and LMP/IPS program was written down to zero and we recorded an impairment charge of $10.7 millionintangible assets, on the consolidated statement of operations.operations in connection with the writedown of the carrying value of Tarmogen to zero. No such impairments were recorded during the years ended December 31, 2022 and 2021. As of December 31, 20222023 and 2021,2022, the company had indefinite-lived IPR&D intangible assets of $0.5 million and $1.4 million, respectively, which were obtained from business acquisitions.
Future amortization expense associated with our definite-lived intangible assets is as follows (in thousands):
| | | | | | | | |
| Years ending December 31: | | Definite-lived Intangible Assets |
| | |
| 2024 | | $ | 2,040 | |
| 2025 | | 2,040 | |
| 2026 | | 2,040 | |
| 2027 | | 2,040 | |
| 2028 | | 2,040 | |
| Thereafter | | 6,373 | |
| Total | | $ | 16,573 | |
Convertible Note Receivable
In 2016, we executed a convertible promissory note with Riptide Bioscience, Inc., or Riptide, andpursuant to which we advanced Riptide a principal amount of $5.0 million. The note bears interest at a per annum rate of five percent (5%). The original term of the promissory note requires that the entire unpaid principal amount and all unpaid accrued interest shall become fully due and payable upon the earlier of (i) the three (3) year anniversary of the issuance date, and (ii) when we accelerate the maturity of the note upon the occurrence of an event of default. In the event of qualified financing, the outstanding principal amount and unpaid accrued interest automatically convert into the most senior class of preferred stock sold in such qualified financing at a 25% discount to the price per share paid for such preferred stock. In addition, in the event of a change in control, we have the option to be paid in cash or to convert, immediately prior to the closing of such transaction, the outstanding indebtedness into Riptide’s most senior class of equity securities at a 25% discount to the price per share paid for such equity securities in such transaction.
Concurrent with the transaction, we entered into an exclusive license agreement with Riptide to obtain worldwide exclusive rights, with the right to sublicense, certain know-how related to RP-182, RP-233 and RP-183. We are required to pay a single-digit royalty on net sales of the licensed products on a country-by-country basis. Pursuant to the license agreement, we are also required to make cash milestone payments upon successful completion of certain clinical, regulatory and commercial milestones up to an aggregate amount of $47.0 million for the first three indications of the licensed product with a maximum payment amount of $100.0 million.
In 2019, we and Riptide entered into a first amendment to the convertible promissory note.note with Riptide. Under the agreement, we extended the maturity of the promissory note to the earlier of a)(a) the later of i)(i) the completion of non-clinical IND enabling studies by the company, or ii)(ii) December 31, 2020; and b)(b) when we accelerate the maturity of the note upon the occurrence of an event of default. No other terms and conditions of the promissory note were modified. Concurrently, we also entered into a first amendment to the exclusive license agreement with Riptide and extended the achievement dates for certain clinical trial milestones related to the licensed products. This option for receiving a 25% discount was determined to have an immaterial value at inception and life to datelife-to-date of the note, as the probability of a future qualifying event is remote. All other terms and conditions of the license agreement continued in full force and effect. This promissory note is still outstanding as of December 31, 2022. The convertible note receivable balance was $6.6$6.9 million and $6.4$6.6 million, which included accrued interest of $1.6$1.9 million and $1.4$1.6 million as of December 31, 20222023 and 2021,2022, respectively.
Accrued Expenses and Other Liabilities
Accrued expenses and other liabilities consist of the following (in thousands):
| | As of December 31, |
| As of December 31, | | | As of December 31, |
| 2023 | | | 2023 | | 2022 |
| | 2022 | | 2021 |
| | Accrued bonus | Accrued bonus | $ | 12,068 | | | $ | 8,316 | |
| Accrued construction costs | 7,072 | | | 8,145 | |
| | Accrued bonus | |
| | Accrued bonus | |
| Accrued professional and service fees | Accrued professional and service fees | 6,685 | | | 6,909 | |
| Accrued research and development costs | |
| Accrued compensation | Accrued compensation | 6,040 | | | 5,613 | |
| Accrued preclinical and clinical trial costs | Accrued preclinical and clinical trial costs | 4,985 | | | 5,842 | |
| Accrued research and development costs | 1,930 | | | 2,107 | |
| Financing obligation – current portion | Financing obligation – current portion | 1,417 | | | 2,598 | |
| Accrued construction costs | |
| | Accrued laboratory equipment, supplies and related services | 303 | | | 2,144 | |
Accrued dissenting shares (Note 7) | — | | | 7,118 | |
| | Other | |
| | Other | |
| | Other | Other | 1,325 | | | 2,595 | |
| Accrued expenses and other liabilities | Accrued expenses and other liabilities | $ | 41,825 | | | $ | 51,387 | |
Interest and Investment Income (Loss) Income,, Net
Interest and investment income (loss) income,, net consists of the following (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| | | | | |
| Unrealized (losses) gains from equity securities | $ | (4,190) | | | $ | (4,615) | | | $ | 1,577 | |
| Interest income | 2,708 | | | 836 | | | 1,725 | |
| Investment (amortization expense) accretion income, net | (1,486) | | | (488) | | | (858) | |
| Net realized (losses) gains on investments | (122) | | | 167 | | | (9) | |
| Interest and investment (loss) income, net | $ | (3,090) | | | $ | (4,100) | | | $ | 2,435 | |
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
| | | | | |
| Unrealized losses from equity securities | $ | (1,591) | | | $ | (4,190) | | | $ | (4,615) | |
| Interest income | 863 | | | 2,708 | | | 836 | |
| Investment accretion income (amortization expense), net | 1,844 | | | (1,486) | | | (488) | |
| Net realized gains (losses) on investments | 15 | | | (122) | | | 167 | |
| Interest and investment income (loss), net | $ | 1,131 | | | $ | (3,090) | | | $ | (4,100) | |
Interest income includes interest from marketable securities, convertible notes receivable, other assets, and on bank deposits.
Interest expense
Interest expense consists of the following (in thousands):
| | Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| | Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| | Interest expense on related-party notes payable | |
| Interest expense on related-party notes payable | |
| Interest expense on related-party notes payable | Interest expense on related-party notes payable | $ | (47,145) | | | $ | (14,695) | | | $ | (9,033) | |
| Amortization of related-party notes discounts | Amortization of related-party notes discounts | (16,282) | | | (62) | | | — | |
| Other interest expense | Other interest expense | (88) | | | (92) | | | (41) | |
| Interest expense | Interest expense | $ | (63,515) | | | $ | (14,849) | | | $ | (9,074) | |
4. Financial Instruments
Investments in Marketable Debt Securities
As of December 31, 2023, the weighted-average remaining contractual life, amortized cost, gross unrealized gains, gross unrealized losses, and fair value of marketable debt securities, which were considered as available-for-sale, by type of security were as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2023 |
| Weighted-
Average
Remaining
Contractual Life
(in years) | | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair
Value |
| | | | | | | | | |
| Current: | | | | | | | | | |
| | | | | | | | | |
| Foreign bonds | 0.8 | | $ | 54 | | | $ | — | | | $ | — | | | $ | 54 | |
| Mutual funds | | | 40 | | | — | | | (1) | | | 39 | |
| Current portion | | | 94 | | | — | | | (1) | | | 93 | |
| Noncurrent: | | | | | | | | | |
| Foreign bonds | 3.3 | | 939 | | | — | | | (48) | | | 891 | |
| | | | | | | | | |
| Total | | | $ | 1,033 | | | $ | — | | | $ | (49) | | | $ | 984 | |
As of December 31, 2022, the weighted-average remaining contractual life, amortized cost, gross unrealized gains, gross unrealized losses, and fair value of marketable debt securities, which were considered as available-for-sale, by type of security were as follows (in thousands):
| | December 31, 2022 | | | December 31, 2022 |
| Weighted-
Average
Remaining
Contractual Life
(in years) | | | Weighted-
Average
Remaining
Contractual Life
(in years) | | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair
Value |
| | | December 31, 2022 |
| Weighted-
Average
Remaining
Contractual Life
(in years) | | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair
Value |
| | Current: | |
| Current: | |
| Current: | Current: | |
| | Mutual funds | Mutual funds | | $ | 38 | | | $ | — | | | $ | (2) | | | $ | 36 | |
| | | Mutual funds | |
| | Mutual funds | |
| | Noncurrent: | Noncurrent: | |
| Noncurrent: | |
| Noncurrent: | |
| Foreign bonds | |
| Foreign bonds | |
| Foreign bonds | Foreign bonds | 4.5 | | 932 | | | — | | | (92) | | | 840 | |
| | Total | Total | | $ | 970 | | | $ | — | | | $ | (94) | | | $ | 876 | |
| Total | |
| Total | |
As of December 31, 2021,2023, 12 of the amortized cost, grosssecurities were in an unrealized gains, gross unrealized losses and fair value of marketable debt securities, which were considered as available-for-sale, by type of security were as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2021 |
| Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair
Value |
| | | | | | | |
| Current: | | | | | | | |
| Corporate debt securities | $ | 129,190 | | | $ | 10 | | | $ | (36) | | | $ | 129,164 | |
| Foreign bonds | 116 | | | — | | | (1) | | | 115 | |
| Mutual funds | 35 | | | 3 | | | — | | | 38 | |
| Current portion | 129,341 | | | 13 | | | (37) | | | 129,317 | |
| Noncurrent: | | | | | | | |
| Foreign bonds | 719 | | | 103 | | | — | | | 822 | |
| Noncurrent portion | 719 | | | 103 | | | — | | | 822 | |
| Total | $ | 130,060 | | | $ | 116 | | | $ | (37) | | | $ | 130,139 | |
loss position. Accumulated unrealized losses on marketable debt securities that have been in a continuous loss position for less than 12 months and more than 12 months were as follows (in thousands):
| | December 31, 2023 | | | December 31, 2023 |
| Less than 12 months | | | Less than 12 months | | More than 12 months |
| Estimated
Fair
Value | | | Estimated
Fair
Value | | Gross
Unrealized
Losses | | Estimated
Fair
Value | | Gross
Unrealized
Losses |
| | | December 31, 2022 |
| Mutual funds | |
| | Less than 12 months | | More than 12 months |
| Estimated
Fair
Value | | Gross
Unrealized
Losses | | Estimated
Fair
Value | | Gross
Unrealized
Losses |
| | Mutual funds | |
| | Mutual funds | Mutual funds | $ | — | | | $ | — | | | $ | 36 | | | $ | (2) | |
| Foreign bonds | Foreign bonds | — | | | — | | | 840 | | | (92) | |
| Total | Total | $ | — | | | $ | — | | | $ | 876 | | | $ | (94) | |
| | December 31, 2022 | | | December 31, 2022 |
| Less than 12 months | | | Less than 12 months | | More than 12 months |
| Estimated
Fair
Value | | | Estimated
Fair
Value | | Gross
Unrealized
Losses | | Estimated
Fair
Value | | Gross
Unrealized
Losses |
| | | December 31, 2021 |
| Mutual funds | |
| | Less than 12 months | | More than 12 months |
| Mutual funds | |
| | Estimated
Fair
Value | | Gross
Unrealized
Losses | | Estimated
Fair
Value | | Gross
Unrealized
Losses |
| | Corporate debt securities | $ | 86,158 | | | $ | (36) | | | $ | — | | | $ | — | |
| Mutual funds | Mutual funds | — | | | — | | | 34 | | | (2) | |
| Foreign bonds | Foreign bonds | 115 | | | (1) | | | 113 | | | (1) | |
| Total | Total | $ | 86,273 | | | $ | (37) | | | $ | 147 | | | $ | (3) | |
WeDuring the years ended December 31, 2023, 2022 and 2021, we evaluated our securities for other-than-temporary impairment and we did not recognize any other-than-temporary impairment losses forlosses.
Marketable Equity Securities
As of December 31, 2023 and 2022, we held investments in marketable equity securities with readily determinable fair values of $0.9 million and $2.5 million, respectively. During the years ended December 31, 2023, 2022 and 2021, unrealized losses recorded on these securities totaled $1.6 million, $4.2 million, and 2020.$4.6 million, respectively, in interest and investment income (loss), net, on the consolidated statements of operations.
5. Fair Value Measurements
Recurring Valuations
Financial assets and liabilities measured at fair value on a recurring basis are summarized below (in thousands):
| | Fair Value Measurements at December 31, 2023 | | | Fair Value Measurements at December 31, 2023 |
| Total | | | Total | | | Level 1 | | Level 2 | | Level 3 |
| | Fair Value Measurements at December 31, 2022 |
| Total | | Level 1 | | Level 2 | | Level 3 |
| | Assets: | |
| Assets at Fair Value: | |
| Assets at Fair Value: | |
| Assets at Fair Value: | |
| Current: | Current: | |
| Current: | |
| Current: | |
| Cash and cash equivalents | |
| Cash and cash equivalents | |
| Cash and cash equivalents | Cash and cash equivalents | $ | 104,641 | | (1) | | $ | 63,860 | | | $ | 40,781 | | | $ | — | |
| Equity securities | Equity securities | 2,507 | | | 2,507 | | | — | | | — | |
| | Foreign bonds | |
| Foreign bonds | |
| Foreign bonds | |
| Mutual funds | Mutual funds | 36 | | | 36 | | | — | | | — | |
| Noncurrent: | Noncurrent: | |
| Foreign bonds | Foreign bonds | 840 | | | — | | | 840 | | | — | |
| Foreign bonds | |
| Foreign bonds | |
| Total assets measured at fair value | Total assets measured at fair value | $ | 108,024 | | | $ | 66,403 | | | $ | 41,621 | | | $ | — | |
| Liabilities: | | | | | | | |
| Liabilities at Fair Value: | |
| Current: | Current: | |
| Contingent consideration obligations | $ | (19) | | (2) | | $ | — | | | $ | — | | | $ | (19) | |
| Current: | |
| Current: | |
| Contingent consideration | |
| Contingent consideration | |
| Contingent consideration | |
| Noncurrent: | Noncurrent: | |
| Warrant liability | (21,636) | | (3) | | — | | | — | | | (21,636) | |
| Stock option purchase liability | |
| Stock option purchase liability | |
| Stock option purchase liability | |
| Derivative liabilities | |
| Warrant liabilities | |
| Total liabilities measured at fair value | Total liabilities measured at fair value | $ | (21,655) | | | $ | — | | | $ | — | | | $ | (21,655) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Fair Value Measurements at December 31, 2022 |
| Total | | | Level 1 | | Level 2 | | Level 3 |
| | | | | | | | |
| Assets at Fair Value: | | | | | | | | |
| Current: | | | | | | | | |
| Cash and cash equivalents | $ | 104,641 | | (5) | | $ | 63,860 | | | $ | 40,781 | | | $ | — | |
| Equity securities | 2,507 | | | | 2,507 | | | — | | | — | |
| | | | | | | | |
| | | | | | | | |
| Mutual funds | 36 | | | | 36 | | | — | | | — | |
| Noncurrent: | | | | | | | | |
| Foreign bonds | 840 | | | | — | | | 840 | | | — | |
| Total assets measured at fair value | $ | 108,024 | | | | $ | 66,403 | | | $ | 41,621 | | | $ | — | |
| Liabilities at Fair Value: | | | | | | | | |
| Current: | | | | | | | | |
| Contingent consideration | $ | (19) | | (1) | | $ | — | | | $ | — | | | $ | (19) | |
| Noncurrent: | | | | | | | | |
| Warrant liability | (21,636) | | (4) | | — | | | — | | | (21,636) | |
| Total liabilities measured at fair value | $ | (21,655) | | | | $ | — | | | $ | — | | | $ | (21,655) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Fair Value Measurements at December 31, 2021 |
| Total | | | Level 1 | | Level 2 | | Level 3 |
| | | | | | | | |
| Assets: | | | | | | | | |
| Current: | | | | | | | | |
| Cash and cash equivalents | $ | 181,101 | | (1) | | $ | 51,421 | | | $ | 129,680 | | | $ | — | |
| Equity securities | 6,698 | | | | 6,698 | | | — | | | — | |
| Corporate debt securities | 129,164 | | | | — | | | 129,164 | | | — | |
| Foreign bonds | 115 | | | | 115 | | | — | | | — | |
| Mutual funds | 38 | | | | 38 | | | — | | | — | |
| Noncurrent: | | | | | | | | |
| Foreign bonds | 822 | | | | 822 | | | — | | | — | |
| Total assets measured at fair value | $ | 317,938 | | | | $ | 59,094 | | | $ | 258,844 | | | $ | — | |
| Liabilities: | | | | | | | | |
| Contingent consideration | $ | (409) | | (2) | | $ | (388) | | | $ | — | | | $ | (21) | |
_______________
| | | | | |
| (1) | Amounts shown as a Level 2 measurement include government-sponsored securities of $32.0 million and $75.0 million, corporate debt securities of $8.8 million and $54.2 million, and no commercial paper and $0.5 million of commercial paper, with original maturities of less than 90 days, as of December 31, 2022 and 2021, respectively. |
| |
(2) | Contingent consideration is recorded at estimated fair value and revalued each reporting period until the related contingency is resolved. The fair value measurement is based on inputs that are unobservable and significant to the overall fair value measurement (i.e., a Level 3 measurement within the fair value hierarchy) and are reviewed periodically by management. See ConsiderationNote 7, Commitments and Contingencies—Contingent Consideration Related to Business Combinations, for further |
Contingent consideration is recorded at estimated fair value and revalued each reporting period until the related contingency is resolved. The fair value measurement is based on inputs that are unobservable and significant to the overall fair value measurement (i.e., a Level 3 measurement within the fair value hierarchy) and are reviewed periodically by management. See Note 7, Commitments and Contingencies—Contingent Consideration Related to Business Combinations, for more information. Changes in the carrying amount of contingent consideration were as follows (in thousands):
| | Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| | Fair value, beginning of year | Fair value, beginning of year | $ | (409) | | | $ | (972) | | | $ | (1,725) | |
| Fair value, beginning of year | |
| Fair value, beginning of year | |
| Consideration paid | Consideration paid | 339 | | | 419 | | | — | |
| Net decrease in fair value | 51 | | | 144 | | | 753 | |
| Net (increase) decrease in fair value | |
| Fair value, end of year | Fair value, end of year | $ | (19) | | | $ | (409) | | | $ | (972) | |
| | | | | |
| (2) | Stock Option Purchase Liability |
In connection with the RIPA, we entered into an SPOA pursuant to which Oberland has an option to purchase up to an additional $10.0 million of our common stock, at a price to be determined by reference to the 30-day trailing volume weighted-average price of our common stock calculated from the date of exercise. This stock purchase option was classified as a liability at its fair value upon issuance. The fair value was estimated using probability-weighted scenarios over the likelihood of this option being exercised. As of December 31, 2023, the stock purchase option was outstanding. The fair value of the stock purchase option was estimated at $0.8 million at issuance. Due to the proximity of the inception date of the SPOA to December 31, 2023, the change in fair value was immaterial. See Note 9, Revenue Interest Purchase Agreement, for more information. | | | | | |
| (3) | On December 12, 2022, we issued a total of 9,090,909 warrants for a period of two years with an exercise price of $6.60 per share in connection with a registered direct offering of common stock. See Note 11, Warrant Liability, for further information. The warrants were classified as a liability at its fair value upon the issuance. As of December 31, 2022, all warrants were outstanding. We utilized the Black-Scholes option pricing model to value warrants with the following assumptions: |
| | | | | | | | |
| | Year Ended |
| | December 31, 2022 |
| | |
Expected term | | 1.96 years |
Expected average volatility | | 99.4 | % |
Expected dividend yield | | — | |
Risk-free interest rate | | 4.4 | %Derivative Liabilities |
The debt incurred pursuant to the RIPA entered on December 29, 2023 contains embedded derivatives requiring bifurcation as a single compound derivative instrument. The company estimated the fair value of the derivative liability using a “with-and-without” method. The with-and-without methodology involves valuing the whole instrument on an as-is basis and then valuing the instrument without the individual embedded derivative. The difference between the entire instrument with the embedded derivative compared to the instrument without the embedded derivative is the fair value of the derivative liability, which is estimated at $34.5 million as of December 31, 2023. The estimated probability and timing of underlying events triggering the exercisability of the Put Option contained in the RIPA, forecasted cash flows and the discount rate are significant unobservable inputs used to determine the estimated fair value of the entire instrument with the embedded derivative. As of December 31, 2023, the discount rate used for valuation of the derivative liability was 12.1%. See Note9, Revenue Interest Purchase Agreement, for more information. In connection with the December 2023 debt extinguishment, the company identified an embedded derivative related to a contingently exercisable prepayment feature of the amended $505.0 million December 2023 promissory note, which allows the noteholder to request up to a $50.0 million prepayment and accrued interest upon occurrence of a specified transaction (defined in the promissory note). This embedded derivative is recorded as a derivative liability on the consolidated balance sheet and is measured at fair value. Changes in the fair value of the derivative liability are reported as change in fair value of derivative, on the consolidated statement of operations. The fair value of the derivative liability is determined at each period end using a with and without method, which assesses the likelihood and timing of a specified transaction that if triggered could result in a repayment. The fair value of the embedded derivative is estimated at $0.8 million as of December 31, 2023, and will be remeasured to fair value at each reporting date until the derivative is settled.
Changes | | | | | |
| (4) | Third-Party Warrant Liabilities |
December 2022 Warrants
In connection with the December 12, 2022 registered direct offering of common stock, the company issued 9,090,909 warrants (December 2022 Warrants). The warrants were classified as a liability at their fair value upon issuance. As of December 31, 2023 and 2022, all warrants were outstanding. The estimated fair value of the warrants was computed using the Black-Scholes option pricing model with the following unobservable assumptions at the following dates:
| | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 |
| | | |
| Exercise price per share | $6.60 | | $6.60 |
| Expected term | 1.0 years | | 2.0 years |
| Expected average volatility | 119.0 | % | | 99.4 | % |
| Expected dividend yield | — | % | | — | % |
| Risk-free interest rate | 4.7 | % | | 4.4 | % |
February 2023 Warrants
In connection with the February 15, 2023 registered direct offering of common stock, the company issued 14,072,615 warrants. The warrants were classified as a liability at their fair value upon issuance. As of December 31, 2023, all warrants were outstanding. The estimated fair value of the warrants was computed using the Black-Scholes option pricing model with the following unobservable assumptions at the following dates:
| | | | | | | | | | | |
| December 31,
2023 | | Issuance Date February 17,
2023 |
| | | |
| Exercise price per share | $3.2946 | | $4.2636 |
| Expected term | 2.6 years | | 2.0 years |
| Expected average volatility | 107.3 | % | | 97.0 | % |
| Expected dividend yield | — | % | | — | % |
| Risk-free interest rate | 4.1 | % | | 4.6 | % |
On July 25, 2023, the company reduced the exercise price of the outstanding February 2023 Warrants from $4.2636 per share to $3.2946 per share and extended the expiration date of the warrants until July 24, 2026. The effect of amending the terms of the warrants is reflected in a change in the fair value of warrant liability of $7.3 million.
July 2023 Warrants
In connection with the July 20, 2023 registered direct offering of common stock, the company issued 14,569,296 warrants (July 2023 Warrants). The warrants were classified as a liability at their fair value upon issuance. As of December 31, 2023, all warrants were outstanding. The estimated fair value of the warrants was computed using the Black-Scholes option pricing model with the following unobservable assumptions at the following dates:
| | | | | | | | | | | |
| December 31,
2023 | | Issuance Date July 25,
2023 |
| | | |
| Exercise price per share | $3.2946 | | $3.2946 |
| Expected term | 2.6 years | | 3.0 years |
| Expected average volatility | 107.3 | % | | 121.0 | % |
| Expected dividend yield | — | % | | — | % |
| Risk-free interest rate | 4.1 | % | | 4.5 | % |
The change in the carrying amount of the warrant liability in connection with the issuance of equity instrument wereliabilities was as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Total | | December 2022
Warrants | | February 2023
Warrants | | July 2023
Warrants |
| | | |
| | | | | | | |
| Fair value, December 31, 2022 | $ | 21,636 | | | $ | 21,636 | | | $ | — | | | $ | — | |
| Fair value at issuance | 49,534 | | | — | | | 23,698 | | | 25,836 | |
| Change in fair value | 47,600 | | | (4,545) | | | 26,260 | | | 25,885 | |
| Fair value, December 31, 2023 | $ | 118,770 | | | $ | 17,091 | | | $ | 49,958 | | | $ | 51,721 | |
| | | | | | | | |
| | Year EndedDecember 2022
Warrants |
| | December 31, 2022 |
| | |
| Fair value at issuance, December 12, 2022 | | $ | 35,096 | |
Net decreaseChange in fair value | | (13,460) | |
Fair value, end of yearDecember 31, 2022 | | $ | 21,636 | |
| | | | | |
| (5) | As of December 31, 2022, the Level 2 measurements include $32.0 million in U.S. government agency securities and $8.8 million in corporate debt securities with original maturities of less than 90 days. |
Non-Recurring Valuations
Non-financial assets and liabilities are recognized at fair value subsequent to initial recognition when they are deemed to be other-than-temporarily impaired. ThereExcept for the impairments discussed in Note 3, Financial Statement Details—Intangible Assets, Net, there were no other material non-financial assets andor liabilities deemed to be other-than-temporarily impaired and measured at fair value on a non-recurring basis forduring the years ended December 31, 2023, 2022 2021 and 2020.2021. We measured the fair value of the fixed-rate promissory notes and variable-rate promissory notes before and after amendments that were entered on December 29, 2023 and August 31, 2022, as they were accounted for under the debt extinguishment accounting model. We used the discounted cash flow analyses for promissory notes without a holder conversion option and used a binomial lattice convertible note modelmodels for the fixed-rate promissory notes with a holder conversion option. Since certain of the factors analyzed are considered to be unobservable inputs, both the discounted cash flow model and the lattice model are considered to be a Level 3 valuation. See Note9 10, Related-Party Debt, for additionalmore information. 6. Collaboration and License Agreements and Acquisition
Collaboration Agreements
National Cancer Institute
2015 NCI CRADA
In May 2015, Etubics Corporation (Etubics) entered into a Cooperative Research and Development Agreement (CRADA)CRADA with the U.S. Department of Health and Human Services (HHS)HHS as represented by the National Cancer Institute (NCI)NCI of the National Institutes of Health (NIH)NIH to collaborate on the preclinical and clinical development of an adenovirus technology expressing tumor-associated antigens (TAAs)TAAs for cancer immunotherapy. In January 2016, we acquired all of the outstanding equity interests in Etubics and Etubics became a wholly-owned subsidiary.
Effective January 2018, our subsidiary NantCell assumed the CRADA and it was amended to cover a collaboration for the preclinical and clinical development of our proprietary yeast-based Tarmogens expressing TAAs and proprietary adenovirus technology expressing TAAs for cancer immunotherapy. Pursuant to the CRADA, the NCI provides scientific staff and other support necessary to conduct research and related activities as described in the CRADA. During the term of the CRADA, we were required to make annual payments of $0.6 million to the NCI for support of research activities.
In November 2021, NantCell entered into a third amendment to the CRADA, which was effective as of March 16, 2021. The principal changes effected by the third amendment are the following: (i) assignment of the CRADA from NantCell to ImmunityBio; (ii) modification of the research plan; (iii) extension of the CRADA term through May 2026; and (iv) an increase in funding for a total of $1.3 million per year, payable in semi-annual installments from 2022 through 2025.
During the years ended December 31, 2023, 2022 and 2021, we recorded R&D expense of $1.3 million, $1.2 million, and $1.1 million, and $0.6 millionrespectively, in research and development expense on the consolidated statements of operations for the year ended December 31, 2022, 2021 and 2020, respectively.operations.
Pursuant to the updated CRADA research plan, NCI and ImmunityBio will collaborate on the preclinical and clinical development of ImmunityBio’s proprietary adenovirus platform expressing TAAs; proprietary yeast platform expressing TAAs; proprietary agent N-803 and derivatives, agent N-809 and derivatives, and/or TxM product candidates; proprietary recombinant NK cells and mAbs; proprietary RNA vaccines and adjuvants; and other proprietary agents owned or controlled by ImmunityBio as contemplated in the research plan, for cancer immunotherapy.
Under any of the CRADAs, any party may unilaterally terminate the agreement by providing timely advance written notice to the other party before the desired termination date.
Pursuant to the terms of the CRADAs, we have an option to elect to negotiate an exclusive or non-exclusive commercialization license to any inventions discovered in the performance of any of the CRADAs. The parties jointly own any inventions and materials that are jointly produced by employees of both parties in the course of performing activities under the CRADAs.
Amyris Joint Venture
In December 2021, ImmunityBio and Amyris Inc. (Amyris) entered into a 50:50 joint venture arrangement and formed a new limited liability company to conduct the business of the joint venture. The purpose of the joint venture is to accelerate commercialization of a next-generation COVID-19 vaccine utilizing an RNA vaccine-platform license.vaccine-platform. As part of the limited liability agreement, Amyris contributed $1.0 million in cash and rights to its license agreement with AAHI for an RNA platform for the field of COVID-19. ImmunityBio contributed $1.0 million in cash and priority access to ourits manufacturing capacity for the joint venture product. Both parties agreed to enter into a separate manufacturing and supply agreement and a sublicense agreement following the execution of the joint venture agreement.
The joint venture agreement stipulates the initial terms for equal representation in the management of the newly-formed joint venture. The joint venture is managed by a board of directors consisting of four directors: two appointed by the company and two appointed by Amyris. Both parties agreed to make additional capital contributions in cash, in proportion to their respective interests, as determined by the board of directors of the joint venture.
We considered the joint venture entity as a VIE and determined that we are not the primary beneficiary of the VIE. In February 2022, we made a cash investment totaling $1.0 million in the joint venture’s common stock. We account for our investment in the joint venture using the equity method of accounting,accounting. During the years ended December 31, 2023, 2022 and 2021, we recorded our 50% share of the net losslosses from the joint venture totaling $7.5 million, $12.1 million, and $0.8 million, respectively, in other expense, net, on the consolidated statements of operations foroperations. During the years ended December 31, 2023 and 2022, and 2021, respectively. Suchsuch losses includeincurred included $7.5 million and $11.9 million, ofrespectively, attributable to expenses incurred by us on behalf of the joint venture during the year ended December 31, 2022.venture. We are not obligated to fund the joint venture’s potential future losses,losses. In August 2023, Amyris announced that it filed for Chapter 11 bankruptcy protection. The Amyris bankruptcy case remains ongoing, and thereforethere can be no assurance that we will not record additional equity method losses that would result inreceive any recovery on account of our equity investment inclaims against Amyris, including for Amyris’ portion of expenses incurred by the joint venture to be reduced to below zero.venture. As of December 31, 2022,2023, the carrying amount of our equity investment in the joint venture was zero.
License Agreements
3M Innovative Properties Company (3M IPC)IPC and the Access to Advanced Health Institute (AAHI)AAHI License Agreement
We have licensed rights to 3M-052, a synthetic TLR7/8 agonist, 3M-052 formulations and related technology from 3M IPC and its affiliates and AAHI. In November 2021 we obtained nonexclusive rights in the field of SARS-CoV-2 and in June 2022 we modified those rights and expanded the scope of the license to include (1) SARS-CoV-2 and other infectious diseases including malaria, HIV, tuberculosis, hookworm and varicella zoster on an exclusive basis in countries other than low- and middle-income countries (LMIC),LMIC, and (2) oncology applications, when used in combination with our proprietary technology and/or IL-15 agonists. In consideration for the license, we agreed to make certain periodic license payments, including $2.25 million each year through June 2025, with the June 2022 payment being partially offset by the $0.5 million previously paid under the initial November 2021 license agreement.2025. We have also agreed to make payments upon the achievement of certain regulatory milestone events and tiered royalties ranging from the low to high single-digits as a percentage of net sales. Beginning in April 2026, the annual minimum licensing payment is $1.0 million, which can be credited against any royalty payments due under this agreement.
In June 2023 and 2022, we made a payment of $1.75 million for the annual license maintenance fee. Wefee payments of $2.25 million and $1.75 million, respectively. During the years ended December 31, 2023, 2022 and 2021, we expensed $2.0 million, $1.0 million, and $0.5 million, respectively, in research and development expense, on the consolidated statements of operations, for the years ended December 31, 2022 and 2021, respectively.operations.
AAHI License Agreements
In May 2021, we entered into two license agreements with AAHI pursuant to which we received a license to certain patents and know-how relating to AAHI’s (i) adjuvant formulations for the treatment, prevention and/or diagnosis of SARS-CoV-2 (the AAHI Adjuvant Formulation License Agreement) and (ii) RNA vaccine platform as further described below (the AAHI RNA License Agreement). Under both agreements, we were obligated to pay one-time, non-creditable, non-refundable upfront cash payments totaling $2.0 million. In addition, under the AAHI Adjuvant Formulation License Agreement we owe milestone payments to a total of up to $2.5 million based on the achievement of certain development and regulatory milestones for the first licensed product and royalties on annual net sales of licensed products on a country-by-country and product-by-product basis of a low-single digit percentage, subject to certain royalty-reduction provisions. NoDuring the years ended December 31, 2023 and 2022, no milestone fees were incurred for the year ended December 31, 2022.incurred.
In September 2021, we amended and restated the AAHI RNA License Agreement, pursuant to which AAHI granted us an exclusive, worldwide, sublicensable license to AAHI’s rights to an RNA vaccine platform for the development and commercialization of certain therapeutic, diagnostic, or prophylactic products for the prevention, treatment or diagnosis of any indication, other than those subject to pre-existing third-party license grants, including, without limitation, SARS-CoV-2. Pursuant to the terms of the amended and restated AAHI RNA License Agreement, we made an additionala one-time, non-creditable, non-refundable, upfront payment to AAHI of $1.5 million.million and a license maintenance fee of $3.0 million in June 2022. The company is also required to pay license maintenance fees to AAHI as follows: $3.0 million in 2022 andof $5.5 million annually from 2023 through 2030. The company may terminate the restated agreement without cause by paying AAHI a $10.0 million one-time early termination fee. In addition, the milestone payments to AAHI based on the achievement of certain development and regulatory milestones for the first licensed product were amended to a total of up to $4.0 million. We are required to pay royalties on annual net sales of licensed products on a country-by-country and product-by-product basis of a low to mid-single digit percentage. In JuneDuring the years ended December 31, 2023, 2022 and 2021, we made a payment of $3.0recorded $4.5 million, for the annual license maintenance fee. We recorded $1.8 million, and $1.5 million, respectively, in research and development expense, on the consolidated statements of operations during the years ended December 31, 2022 and 2021, respectively.operations.
In connection with the license agreements, in May 2021 we also entered into a sponsored research agreement with the AAHI pursuant to which we will fund continued research of at least $2.0 million per year, payable in four equal quarterly installments each year until May 2024, or such year of earlier termination. As of December 31, 2023, $0.5 million payable to AAHI in connection with the sponsored research agreement was recorded in accrued expenses and other liabilities, on the consolidated balance sheet. During the year ended December 31, 2023, we recorded $1.2 million in research and development expense, and $3.7 million in loss on equity method investment, during the year ended December 31, 2022, respectively, on the consolidated statements of operations related to the sponsored research agreement.
Viracta License Agreement
In 2017, we entered into an agreement with Viracta under which we were granted exclusive worldwide rights to Viracta’s Phase 2II drug candidate, VRx-3996 (nanatinostat), for use in combination with our platform of NK cell therapies. In consideration for the license, we are obligated to pay Viracta mid-single digit percentage royalties on net sales of licensed products for therapeutic use and milestone payments ranging from $10.0 million to $25.0 million up to an aggregate maximum of $100.0 million for various regulatory approvals and cumulative net sales levels. We may terminate the agreement, at our sole discretion, in whole or on a product by product and/or country by country basis, at any time upon 90 days’ prior written notice. In addition, either party may terminate the agreement in the event of a material breach or for bankruptcy of the other party. To date, we have not had incurred any royalty or milestone payment obligations under this agreement, including during the years ended December 31, 2023, 2022 2021 and 2020.2021.
Acquisition
Dunkirk Facility Leasehold Interest
On February 14, 2022, we completed the acquisition of a leasehold interest in approximatelythe Dunkirk Facility (approximately 409,000 rentable square feet of current Good Manufacturing Practice (cGMP) ISO Class 5 pharmaceutical manufacturing space in western New York (the Dunkirk Facility)feet) from Athenex, Inc. (the Seller). Wewhich we believe this facility will provide us with a state-of-the-art biotech production center that will substantially expand and diversify our existing manufacturing capacity in the U.S. and the ability to scale production across allassociated with certain of our key platforms.product candidates. The company accounted for the transaction as an asset acquisition because the Dunkirk Facility’s integrated set of assets and activities does not meet the definition of a business.
The total consideration for the acquisition was approximately $40.5 million, including a cash payment of $40.0 million, and transaction costs of approximately $0.5 million. The following table summarizes the fair value of assets acquired as of the acquisition date (in thousands):
| | | | | |
| Construction in progress | $ | 10,043 | |
| Leasehold improvements | 6,253 | |
Definite-lived intangible assets (1) | 21,229 | |
| Other depreciable assets and prepaid expenses | 2,983 | |
| Total consideration | $ | 40,508 | |
_______________
(1)Definite-lived intangible assets consist of favorable leasehold rights totaling $20.4 million and organized workforce totaling $0.8 million as of the acquisition date.
Upon the closing of the Dunkirk transaction, the company became the tenant of the Dunkirk Facility under the Fort Schuyler Management Corporation Lease, dated October 1, 2021 and as amended as of the February 14, 2022 closing date (as amended, the Dunkirk Lease), with Fort Schuyler Management Corporation, a not-for-profit corporation affiliated with the State of New York (FSMC)FSMC as landlord. The Dunkirk Facility, as well as certain equipment, is owned by the FSMC and is leased to us under the Dunkirk Lease. Our annual lease payment will be $2.00 per year for an initial 10-year term, with anone option to renew the lease under substantially the same terms and conditions for an additional 10-year term. As part of the transaction, we assumed certain of the Seller’sAthenex’s obligations under various third-party agreements (the Facility Agreements), subject to the terms and conditions of the purchase agreement by and between the company and SellerAthenex dated as of January 7, 2022, and committed to spend an aggregate of $1.52 billion on operational expenses during the initial term, and an additional $1.50 billion on operational expenses if we elect to renew the lease for theone additional 10-year term. We also committed to hiring 450 employees at the Dunkirk Facility within the first 5five years of operations,following the Commencement Date, with 300 such employees to be hired within the first 2.5 years of operation.following the Commencement Date. We are eligible for certain sales-tax exemption savings during the development of the Dunkirk Facility, and certain property tax savings over the next 20 years, subject to certain terms and conditions, including performance of certain of the obligations described above. Failure to satisfy the obligations over the lease term may give rise to certain rights and remedies of governmental authorities including, for example, termination of the Dunkirk Lease and other Facility Agreements and potential recoupment of a percentage of the grant funding received by the SellerAthenex for construction of the facility and other benefits received, subject to the terms and conditions of the applicable agreements.
In connection with the ongoing partnership with the State of New York (the State) to construct the Dunkirk Facility, we received funds from the State as reimbursement for certain expenses incurred related to such construction totaling $1.1 million for the year ended December 31, 2022. Although we believe that governmental funding will assist in funding a portion of the further build-out of the Dunkirk Facility, which we estimate to be approximately $8.0 million to $10.0 million of governmental funding remaining available as of December 31, 2022,2023, there can be no assurance as to the final acceptance and timing of the requests for governmental funding that we submit, and we will need to plan and fund most of the additional build-out of, and purchase additional equipment for, the Dunkirk Facility in connection with our planned full operations. In addition, any future governmental funding will be subject to the eligibility of submitted expenses, as well as our compliance with the obligations that we are subject to pursuant to the agreements with parties regarding the Dunkirk Facility as described above. Further, on May 14, 2023, Athenex, together with certain of its subsidiaries, filed voluntary petitions for relief under Chapter 11 of the United States Bankruptcy Court for the Southern District of Texas (the Athenex Proceedings). We do not know what, if any, impact the Athenex Proceedings will have on any portion of the potential governmental funding remaining for the Dunkirk Facility.
Dunkirk Facility Workforce Reduction
In September 2022, the company initiated a workforce reduction at the Dunkirk Facility as a result of upcoming construction at the project, which we believe may take approximately 12 to 18 months. In connection with the workforce reduction, weand recorded severance and retention benefits for the terminated employees totaling $1.0 million during the year ended December 31, 2022 in selling, general and administrative expense, on the consolidated statement of operations. The terminated employees were not required to render service through their termination date in December 2022 to receive these benefits.
7. Commitments and Contingencies
Contingent Consideration Related to Business Combinations
VivaBioCell, S.p.A.
In April 2015, NantWorks, LLC (NantWorks), a related party, acquired a 100% interest in VivaBioCell S.p.A. (VivaBioCell) through its wholly-owned subsidiary, VBC Holdings LLC, (VBC Holdings) for $0.7 million, less working capital adjustments. In June 2015, NantWorks contributed its equity interest in VBC Holdings to the company, in exchange for cash consideration equal to its cost basis in the investment. VivaBioCell develops bioreactors and products based on cell culture and tissue engineering in Italy.
In connection with our acquisition of VBC, we are obligated to pay the former owners contingent consideration upon the achievement of certain milestones related to the GMP-in-a-Box technology. A clinical milestone totaling $0.8 million was earned by the former owners of VivaBioCell, of which $0.4 million was paid during the year ended December 31, 2021. The2021, with the remaining $0.3 million clinical obligation, net of foreign exchange adjustment, was paidsettled during the year ended December 31, 2022. If a government agency unconditionally approves the GMP-in-a-Box technology for commercial sale (the regulatory milestone) in the future, we will be obligated to pay an additional approximately $2.1$2.2 million to the former owners.
Altor BioScience, CorporationLLC
In connection with our 2017 acquisition of Altor, we issued CVRs under which we agreed to pay the prior stockholders of Altor approximately $304.0 million of contingent consideration upon the successful regulatory approval of a BLA by the FDA, or foreign equivalent, for N-803 by December 31, 2022 and approximately $304.0 million of contingent consideration upon calendar-year worldwide net sales of N-803 exceeding $1.0 billion prior to December 31, 2026, with amounts payable in cash or shares of our common stock or a combination thereof. As the transaction was recorded as an asset acquisition, future CVR payments will be recorded when the corresponding events are probable of achievement or the consideration becomes payable.
With respect to the regulatory milestone CVR agreement, in May 2022 we announced the submission of a BLA to the FDA for our product candidate, Anktiva (N-803) in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease. In July 2022 we announced that the FDA had accepted our BLA for review and set a target PDUFA action date of May 23, 2023. It is unclear if the FDA will approve our BLA, if at all. The FDA did not approve our BLA on or before December 31, 2022, and therefore the regulatory milestone was not met, and the regulatory milestone CVR agreement terminated in accordance with its terms.
With respect to the net sales milestone CVR agreement, as As of December 31, 2022,2023, Dr. Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer, and his related party hold approximately $139.8 million of net sales CVRs and they have both irrevocably agreed to receive shares of the company’s common stock in satisfaction of their CVRs. We may be required to pay the other prior Altor stockholders up to $164.2 million for their net sales CVRs should they choose to have their CVRs paid in cash instead of common stock.
Litigation
From time to time, we may be involved in various claims and legal proceedings relating to claims arising out of our operations. We are not currently a party to any legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business. If we are served with any such complaints, we will assess at that time any contingencies for which we may need to reserve. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
Altor BioScience, LLC Litigation
In 2017, NantCell announced it had entered into a definitive merger agreement to acquire Altor BioScience Corporation.Altor. An action captioned Gray v. Soon-Shiong, et al. was filed in Delaware Chancery Court by plaintiffs Clayland Boyden Gray (Gray) and Adam R. Waldman. The plaintiffs, two minority stockholders, asserted claims against the company and other defendants for (1) breach of fiduciary duty and (2) aiding and abetting breach of fiduciary duty and filed a motion to enjoin the merger. The court denied the motion and permitted the merger to close.
Subsequent to the close of the merger, in 2017 the plaintiffs (joined by two additional minority stockholders, Barbara Sturm Waldman and Douglas E. Henderson (Henderson)) filed a second amended complaint, including appraisal claims, and which the defendants subsequently moved to dismiss. In a second action, Dyad Pharmaceutical Corporation (Dyad) filed a petition in Delaware Chancery Court for appraisal in connection with the merger. The defendants moved to dismiss the appraisal petition in 2018. The court issued an oral ruling in 2019 that dismissed certain claims and dismissed Altor BioScience from the action. The following claims remained: (a) the appraisal claims by all plaintiffs and Dyad (against Altor BioScience, LLC), and (b) Henderson’s claims for breach of fiduciary duty and aiding and abetting breach of fiduciary duty.
In 2019, the court issued a written order implementing its ruling on the defendants’ motions (the Implementing Order). In the Implementing Order, the court confirmed that all fiduciary duty claims brought by Gray, both individually and as trustee of the Gordon Gray Trust f/b/o C. Boyden Gray, were dismissed. The plaintiffs then moved for leave to file a third amended complaint to add two former Altor stockholders as plaintiffs and a fiduciary duty claim on behalf of a purported class of former Altor stockholders, which the defendants opposed.
In 2020, the court granted the plaintiffs’ motion, and the plaintiffs filed the third amended complaint. In 2020, the defendants answered the third amended complaint and asserted counterclaims against the plaintiffs. The defendants sought damages for attorneys’ fees and costs incurred as a result of the breaches of “standstill” agreements and of stockholder releases. The plaintiffs filed an answer denying the counterclaims and asserting defenses.
The shares of the former Altor stockholders seeking appraisal met the definition of dissenting shares under the merger agreement and were not entitled to receive any portion of the merger consideration at the closing date, given that those shares were the subject of the above-described appraisal claims.
In late March 2022, the company agreed to the terms of a settlement with the appraisal petitioners, without any admission of liability or fault. The settlement provided that in exchange for complete releases, the appraisal petitioners, who as a group held 3,167,565 dissenting Altor shares, collectively would receive an aggregate of 2,229,296 shares of the company’s common stock issued in a private placement, plus an aggregate of $21.13 in cash in lieu of fractional shares. The company’s Board of Directors approved the settlement and stock issuance in April 2022, and the court approved the settlement and dismissed the appraisal petitioners’ claims on July 9, 2022. On July 9, 2022, the company issued 2,229,296 shares of its common stock with an aggregate market value of $10.7 million, based on the closing price of its common stock on the Nasdaq as of July 8, 2022, to the appraisal petitioners pursuant to the court-approved settlement agreement. As of December 31, 2021, we had accrued $7.1 million related to the dissenting share obligation.
In late April 2022, the company also agreed to the terms of a settlement with the putative class plaintiffs without any admission of liability or fault. In exchange for class-wide releases, the company committed to make a settlement payment of $5.0 million in cash by December 31, 2022. On December 8, 2022, the Delaware Court of Chancery entered a final judgment approving the settlement, and the company timely made the $5.0 million settlement payment.
Sorrento Therapeutics, Inc. Litigation
Sorrento, Therapeutics, Inc. (Sorrento), derivatively on behalf of NANTibody, filed an action in the Superior Court of California, Los Angeles County (the Superior Court) against the company’s subsidiary NantCell, Dr. Soon-Shiong, and Charles Kim. The action alleged that the defendants improperly caused NANTibody to acquire IgDraSol Inc. from NantPharma LLC (NantPharma) and sought to have the transaction undone and the purchase amount returned to NANTibody. In 2019, we filed a demurrer to several causes of action alleged in the Superior Court action, and Sorrento filed an amended complaint, eliminating Mr. Kim as a defendant and dropping the causes of action we had challenged in our demurrer. The company believes the case is without merit and intends to vigorously defend against the claims asserted. Trial hashad been set to commence in Sorrento’s Superior Court action on August 7, 2023, but on July 17, 2023.24, 2023 the Superior Court vacated the August 7, 2023 trial date at the parties’ request in light of the pending settlement discussed below.
Also in 2019, the company and Dr. Soon-Shiong filed cross-claims in the Superior Court action against Sorrento and its Chief Executive Officer Henry Ji, asserting claims for fraud, breach of contract, breach of the covenant of good faith and fair dealing, tortious interference with contract, unjust enrichment, and declaratory relief. Our claims alleged that Dr. Ji and Sorrento breached the terms of an exclusive license agreement between the company and Sorrento related to Sorrento’s antibody library and that Sorrento did not perform its obligations under the exclusive license agreement. The Superior Court ruled that the company’s claims should be pursued in arbitration and that Dr. Soon-Shiong’s claims could be pursued in Superior Court.
In 2019, the company, along with NANTibody, filed an arbitration against Sorrento and Dr. Ji asserting our claims relating to the exclusive license agreement. Sorrento filed counterclaims against the company and NANTibody in the arbitration. The hearings in the NANTibody arbitration commenced in April 2021 and concluded in early August 2021. After post-hearing briefing was concluded, the parties were notified on November 30, 2021 that the arbitrator in the NANTibody arbitration had passed away. A substitute arbitrator was appointed on February 25, 2022, and the parties have worked with the substitute arbitrator to conclude the proceedings. Additional hearing sessions were held in May and July 2022, and summations took place on August 2, 2022.
On December 2, 2022, the arbitrator issued a final award finding that Sorrento had breached the two exclusive license agreements with NantCell and NANTibody. The arbitrator awarded NantCell approximately $156.8 million and NANTibody approximately $16.7 million, plus post-award interest accruing at a daily rate. On December 21, 2022, NantCell and NANTibody filed petitions in the Superior Court to confirm the arbitration award; on January 16, 2023, Sorrento filed a response to the petitions and moved to vacate the award. On February 7, 2023, after a hearing, the Superior Court entered orders confirming the arbitration award and denying Sorrento’s motion to vacate. The Superior Court entered judgments against Sorrento in the aggregate amount of approximately $176.4 million plus 10% post-judgment interest, of which approximately $159.4 million iswas payable to NantCell, and the remainder of which iswas payable to NANTibody. On February 13, 2023, Sorrento informed counsel to the company that it had filed a Chapter 11 proceeding in the U.S. District Court for the Southern District of Texas, In re: Sorrento Therapeutics, Inc., et al., Case No. 23-bk-90085 (Bankr. S.D. Tex.)23-90085 (DRJ). The company intends to continue to pursue vigorously, consistent with, Docket Entry 810.
On June 6, 2023, Sorrento filed a motion in its rights in light of Sorrento’s Chapter 11 filing,proceeding for entry of an order approving and implementing a mediation settlement reached with the collection ofcompany and other entities. The settlement involved two possible scenarios: Either, if Sorrento were to raise an amount needed to pay its debtor in possession lender and its unsecured creditors by August 31, 2023, Sorrento would pay those obligations, including the judgments held by NantCell and 10% post-judgment interest from Sorrento, but we make no assurancesNANTibody, by 2:00 p.m. ET on August 31, 2023 and be free to proceed with pending litigation; or, failing that, we will receive the full amount or with respect tojudgments would be released, the timing of our receipt of any funds.
The Superior Court actions remain pending, and it remains tolitigation claims would be determined how, if at all, the awards in the arbitrations will affectreleased, including, without limitation, the Superior Court actions. A July 17, 2023 trial date has been setaction discussed above, Sorrento would relinquish its interests in NANTibody and certain other entities, Sorrento would forfeit its rights to any payments from NantCell arising out of its antibody exclusive license agreement with NantCell (rights to PD-L1), and certain other provisions not impacting the company would be implemented as described in the first-filed Superiormotion. On August 14, 2023, the United States Bankruptcy Court action. An estimatefor the Southern District of Texas issued an order approving the settlement described above, such that the settlement became binding on the parties. As of 2:00 p.m. ET on August 31, 2023, Sorrento had not paid the judgments held by NantCell and NANTibody. Accordingly, in relevant part to the company and NantCell, a mutual release of claims became effective such that the aforementioned judgments were released, the litigation claims were released including, without limitation, the derivative litigation against NantCell described above, Sorrento relinquished its interests in NANTibody, and Sorrento forfeited its rights to any payments from NantCell arising out of its antibody exclusive license agreement with NantCell, including any royalties associated with the company’s engineered NK cell therapy in Phase II clinical trials, PD-L1 t-haNK. As a result of the possible loss or rangesettlement, the parties filed dismissals of loss resultingthe litigation matters discussed above. After the settlement, the company’s ownership in NANTibody increased from 60% to 100%, and, as a result, the Superior Court litigation cannot be made at this time.
additional paid-in capital attributable to the company, on the consolidated statement of stockholders’ deficit. Shenzhen Beike Biotechnology Co. Ltd. Arbitration
In 2020, we received a Request for Arbitration before the International Chamber of Commerce, International Court of Arbitration. The arbitration relates to a license, development, and commercialization agreement that Altor entered into with Beike in 2014, which agreement was amended and restated in 2017, pursuant to which Altor granted to Beike an exclusive license to use, research, develop and commercialize products based on N-803 in China for human therapeutic uses. In the arbitration, Beike is asserting a claim for breach of contract under the license agreement. Among other things, Beike alleges that we failed to use commercially reasonable efforts to deliver to Beike materials and data related to N-803. Beike is seeking specific performance or in the alternative, damagesand declaratory relief for the alleged breaches. On September 25, 2020, the parties entered into a standstill and tolling agreement under which, among other things, the parties affirmed they willwould perform certain of their obligations under the license agreement by specified dates and agreed that all deadlines in the arbitration arewere indefinitely extended. The standstill agreement maycould be terminated by any party on ten calendar days’ notice, and upon termination, the parties will havehad the right to pursue claims arising from the license agreement in any appropriate tribunal. On March 20, 2023, we terminated the standstill agreement, and on
April 11, 2023, Beike served an amended Request for Arbitration. We served an Answer and Counterclaims on May 19, 2023. Beike served a Reply to our counterclaims on June 21, 2023. Beike’s Statement of Claim is due on March 22, 2024, and the company’s Statement of Defense and Counterclaim is due on June 21, 2024. The parties have been providing periodic updateshearing in the arbitration is scheduled to the International Chamber of Commerce confirming a stay of all proceedings during the standstill.begin on June 9, 2025. Given that this action remains at the pleading stage and no discovery has occurred, it remains too early to evaluate the likely outcome of the case or to estimate any range of potential loss. We believe the claims asserted against the company lack merit and intend to defend the case, vigorously and that we may have counterclaims.to pursue or counterclaims, vigorously.
Litigation Related to the Merger withSecurities Class Action
On June 30, 2023, a putative securities class action complaint, captioned Salzman v. ImmunityBio, Inc.
In connection with the Merger with NantCell, Inc. (formerly known as ImmunityBio, Inc. et al., a private company), a Delaware corporation, via a wholly-owned subsidiary of NantKwest, several complaints wereNo. 3:23-cv-01216-BEN-WVG, was filed as individual actions in the United StatesU.S. District Courts,Court for the Southern District of California against the company and subsequently were voluntarily dismissed (the Merger Actions). The Merger Actions generally alleged that the Definitive Proxy Statement filed with the SEC on February 2, 2021 misrepresentedthree of its officers and/or omitted certain purportedly material information relating to financial projections, analysis performed by the financial advisor to NantKwest’s Special Committee, alleged past engagements of the Special Committee’s financial advisor and industry consultant, and the terms of the engagement of such consultant. The Merger Actions asserteddirectors, asserting violations of Section 14(a) of the Securities Exchange Act of 1934, as amended (the Exchange Act),Sections 10(b) and Rule 14a-9 promulgated thereunder against all defendants and violations of Section 20(a) of the Exchange Act against NantKwest’s directors. The Merger Actions sought,Act. Stemming from the company’s disclosure on May 11, 2023 that it had received an FDA CRL stating, among other things, an injunction enjoiningthat it could not approve the stockholder vote oncompany’s BLA for its product candidate, Anktiva in combination with BCG for the Mergertreatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 disease, in its present form due to deficiencies related to its pre-license inspection of the company’s third-party CMOs, the complaint alleges that the defendants had previously made materially false and misleading statements and/or omitted material adverse facts regarding its third-party clinical manufacturing organizations and the consummation of the Merger unless and until certain additional information was disclosed to NantKwest’s stockholders, costs of the action, including plaintiffs’ attorneys’ fees and experts’ fees, and other relief the Court may deem just and proper. Neither the stockholder vote on the Merger nor the Merger were enjoined and both occurred on March 8 and March 9, 2021, respectively. The Merger Actions were voluntarily dismissed on March 25, 2022.
Stipulation of Settlement
In 2019, following approval by our Board of Directors, we entered into a settlement agreement (the Stipulation of Settlement) with three stockholders of the company, each of whom had submitted a stockholder demandprospects for the Board of Directors to take action to remedy purported harm to the company resulting from certain alleged wrongful conduct concerning, among other things, disclosures about Dr. Soon-Shiong’s compensation and a related-party lease agreement. The Stipulation of Settlement called for us to adopt certain governance changes, and for the three stockholders to file a stockholder derivative action in the Superior Court of the State of California, County of San Diego, followed by an application for courtregulatory approval of the StipulationBLA. On September 27, 2023, the court appointed a lead plaintiff, approved their selection of Settlement.lead counsel, and re-captioned the case In re. ImmunityBio, Inc. Securities Litigation, No. 3:23-cv-01216. On November 17, 2023, lead plaintiff filed an amended complaint, which named the same defendants and asserted the same claims as the previous complaint. On January 8, 2024, defendants filed a motion to dismiss the amended complaint. A hearing on the motion is currently scheduled for April 23, 2024.The company believes the lawsuit is without merit and intends to defend the case vigorously. The court enteredcompany is unable to estimate a range of loss, if any, that could result were there to be an order preliminarily approvingadverse final decision in this action. If an unfavorable outcome were to occur, it is possible that the Stipulation of Settlement. Pursuantimpact could be material to the Stipulationcompany’s results of Settlement, we provided stockholders with notice ofoperations in the settlementperiod(s) in which any such outcome becomes probable and the final settlement hearing.estimable.
Unconditional Purchase Obligations
Unconditional purchase obligations are defined as an agreement to purchase goods or services that are enforceable and legally binding (non-cancelable, or cancelable only in certain circumstances). In the normal course of business, we enter into unconditional purchase obligation arrangements with a third-party CMO to reserve manufacturing slots in its cGMP manufacturing facility for the manufacture and supply of cGMP batches per FDA and European Medicines Agency (EMA)EMA regulations for commercial use. The total amount of future non-cancelable purchase commitments related to the manufacture of cGMP batches is $19.5$2.0 million and $5.6$0.9 million for the years ending December 31, 20232024 and 2024,2025, respectively.
December 31, 2023. Payments by year are estimated as follows: 2024 ($1.6 million), 2025 ($1.8 million) and 2026 ($1.8 million). Additionally, our total unconditional purchase obligation for laboratory cleanroom service costs are estimated at $1.9 million as of December 31, 2023. Payments by year are estimated as follows: 2024 ($0.5 million) and 2025 ($1.4 million). The purchase obligation amounts do not represent the entire anticipated purchases in the future, but represent only those items for which we are contractually obligated. The majority of our goods and services are purchased as needed, with no unconditional commitment. For this reason, these amounts do not provide an indication of our expected future cash outflows related to purchases. Commitments
WeDuring the year ended December 31, 2023, we did not enter into any significant contracts or material unconditional purchase commitments, during the year ended December 31, 2022, other than those disclosed in these consolidated financial statements.
8. Lease Arrangements
We lease property in multiple facilities across the U.S. (including the Dunkirk Facility in upstate New York) and Italy, including facilities located in El Segundo, CA which are leased from related parties.and the Dunkirk Facility in upstate New York. Substantially all of our operating lease right-of-use assets and operating lease liabilities relate to facilities leases. All of our finance leases are related to equipment rentalrentals at the Dunkirk Facility. See Note 1011, Related-Party Agreements, for additionalmore information about our related-party leases. Our leases generally have initial terms ranging from two to ten years and often include one or more options to renew. These renewal terms can extend the lease term from one to ten years, and are included in the lease term when it is reasonably certain that we will exercise the option.
Supplemental balance sheet information related to our leases is as follows (in thousands):
| | As of December 31, |
| Classification | | 2022 | | 2021 |
| | | As of December 31, | | | | | As of December 31, |
| Classification | | | Classification | | 2023 | | 2022 |
| | Assets | Assets | | |
| Assets | |
| Assets | |
| Operating lease assets | |
| Operating lease assets | |
| Operating lease assets | Operating lease assets | Operating lease right-of-use assets | | $ | 45,788 | | | $ | 36,304 | |
| Finance lease assets | Finance lease assets | Other assets | | 135 | | | — | |
| Total lease assets | Total lease assets | | $ | 45,923 | | | $ | 36,304 | |
| | Liabilities | Liabilities | |
| Current | |
| Liabilities | |
| Liabilities | |
| Current: | |
| Current: | |
| Current: | |
| Operating lease liabilities | |
| Operating lease liabilities | |
| Operating lease liabilities | Operating lease liabilities | Operating lease liabilities | | $ | 2,650 | | | $ | 3,011 | |
| Finance lease liabilities | Finance lease liabilities | Accrued expenses and other liabilities | | 77 | | | — | |
| Non-current | |
| Non-current: | |
| Operating lease liabilities | |
| Operating lease liabilities | |
| Operating lease liabilities | Operating lease liabilities | Operating lease liabilities, less current portion | | 47,951 | | | 37,068 | |
| Finance lease liabilities | Finance lease liabilities | Other liabilities | | 64 | | | — | |
| Total lease liabilities | Total lease liabilities | | $ | 50,742 | | | $ | 40,079 | |
Information regarding our lease terms is as follows:
| | Year Ended December 31, |
| 2022 | | 2021 |
| Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 |
| | Weighted-average remaining lease term: | Weighted-average remaining lease term: | | | |
| Weighted-average remaining lease term: | |
| Weighted-average remaining lease term: | |
| Operating leases | |
| Operating leases | |
| Operating leases | |
| Operating leases | |
| Operating leases | |
| Operating leases | Operating leases | 6.6 years | | 7.8 years | 6.2 years | | 6.6 years |
| Finance leases | Finance leases | 1.8 years | | — | | Finance leases | 0.8 years | | 1.8 years |
| Weighted-average discount rate: | Weighted-average discount rate: | | | |
| Operating leases | Operating leases | 10.5 | % | | 9.6 | % |
| Operating leases | |
| Operating leases | |
| Operating leases | |
| Operating leases | |
| Operating leases | | 10.9 | % | | 10.5 | % |
| Finance leases | Finance leases | 11.7 | % | | — | | Finance leases | 11.7 | % | | 11.7 | % |
The components of lease expense consist of the following (in thousands):
| | Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| | Operating lease costs | Operating lease costs | $ | 11,093 | | | $ | 7,977 | | | $ | 5,668 | |
| Operating lease costs | |
| Operating lease costs | |
| Short-term lease costs | Short-term lease costs | 3,060 | | | — | | | — | |
Finance lease costs (including amortization and
interest costs) | 80 | | | — | | | — | |
Finance lease costs (including right-of-use asset
amortization and interest expense) | |
| Variable lease costs | Variable lease costs | 3,880 | | | 2,862 | | | 3,564 | |
| Total lease costs | $ | 18,113 | | | $ | 10,839 | | | $ | 9,232 | |
| Total lease expense | |
Cash paid for amounts included in the measurement of lease liabilities is as follows (in thousands):
| | Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| | Year Ended December 31, |
| 2022 | | 2021 |
| | Cash paid for operating leases (excluding variable lease costs) | |
| Cash paid for operating leases (excluding variable lease costs) | |
| Cash paid for operating leases (excluding variable lease costs) | Cash paid for operating leases (excluding variable lease costs) | $ | 10,241 | | | $ | 9,034 | |
| Financing cash flow from finance leases | Financing cash flow from finance leases | 58 | | | — | |
| Operating cash flow from finance leases | Operating cash flow from finance leases | 15 | | | — | |
Future minimum lease payments as of December 31, 2022,2023, including $14.8$11.5 million related to options to extend lease terms that are reasonably certain of being exercised, are presented in the following table (in thousands). Common area maintenance costs and taxes are not included in these payments.
| | | | | | | | | | | | | | | | | | | | |
| Years ending December 31: | | Operating Leases | | Finance
Leases | | Total |
| | | | | | |
| 2023 | | $ | 9,539 | | | $ | 88 | | | $ | 9,627 | |
| 2024 | | 12,118 | | | 66 | | | 12,184 | |
| 2025 | | 12,145 | | | — | | | 12,145 | |
| 2026 | | 10,289 | | | — | | | 10,289 | |
| 2027 | | 8,209 | | | — | | | 8,209 | |
| Thereafter | | 23,247 | | | — | | | 23,247 | |
| Total future minimum lease payments | | 75,547 | | | 154 | | | 75,701 | |
| Less: Interest | | 22,005 | | | 13 | | | 22,018 | |
| Less: Tenant improvement allowance receivable | | 2,941 | | | — | | | 2,941 | |
| Present value of operating lease liabilities | | $ | 50,601 | | | $ | 141 | | | $ | 50,742 | |
3530 John Hopkins Court | | | | | | | | | | | | | | | | | | | | |
| Years ending December 31: | | Operating Leases | | Finance
Leases | | Total |
| | | | | | |
| 2024 | | $ | 10,354 | | | $ | 66 | | | $ | 10,420 | |
| 2025 | | 10,864 | | | — | | | 10,864 | |
| 2026 | | 8,983 | | | — | | | 8,983 | |
| 2027 | | 8,220 | | | — | | | 8,220 | |
| 2028 | | 8,465 | | | — | | | 8,465 | |
| Thereafter | | 16,056 | | | — | | | 16,056 | |
| Total future minimum lease payments | | 62,942 | | | 66 | | | 63,008 | |
| Less: Interest | | 17,086 | | | 2 | | | 17,088 | |
| Less: Tenant improvement allowance receivable | | 670 | | | — | | | 670 | |
| Present value of operating lease liabilities | | $ | 45,186 | | | $ | 64 | | | $ | 45,250 | |
In April 2022,During the year ended December 31, 2023, we extended oursome existing lease for 44,681 rentable square feet at 3530 John Hopkins Court in San Diego, California from July 31, 2023 to July 31, 2030 (the Extended Lease Term). This facility is used primarily as a research laboratory and our corporate offices. The Extended Lease Term will commence on August 1, 2023, and includes an option to extend the lease for one five-year term through July 31, 2035. The base rent effective during the Extended Lease Term will be approximately $323,937 per month with an annual increase of 3% beginning on August 1, 2024. At the beginning of the option term, the initial monthly base rent will be adjusted to market rent (as defined in the lease agreement). We will receive a rent abatement for the first seven months of the Extended Lease Term beginning on August 1, 2023, and a tenant improvement allowance of $0.7 million from the landlord for costs and expenses associated with the construction of tenant improvements that can be used during the 12-month period ending on August 1, 2024.
In addition to the lease described above, we also acquired a leasehold interest at the Dunkirk Facility discussed in Note 6, Collaboration and License Agreements and Acquisition, and entered into new related-party leases and terminated an existing related-party lease discussed inlease. See Note10 11, Related-Party Agreements, for more information regarding our related-party leases.
9. Revenue Interest Purchase Agreement
On December 29, 2023, we entered into the RIPA with Infinity and Oberland, to support the commercialization of Anktiva, our lead product candidate, to further the development of our product platforms, and to provide for other working capital needs. Pursuant to the RIPA, Oberland acquired certain Revenue Interests from us for a gross purchase price of $200.0 million paid on closing, less certain transaction expenses. In addition, Oberland may purchase additional Revenue Interests from us in exchange for the $100.0 million Second Payment upon satisfaction of certain conditions specified in the RIPA, including following the receipt of approval by the FDA of the company’s BLA for Anktiva in combination with BCG for the treatment of patients with BCG-unresponsive NMIBC with CIS with or without Ta or T1 diseaseon or before June 30, 2024.
As consideration for the aforementioned payments, Oberland has the right to receive quarterly Revenue Interest Payments from us based on, among other things, a certain percentage of our net sales during such quarter, which will be tiered payments initially ranging from 3.00% to 7.00% (or after funding of the Second Payment, 4.50% to 10.00%) of the company’s worldwide net sales, excluding those in China.
If the aggregate Revenue Interest Payments made to Oberland as of December 31, 2029 equal or exceed the Cumulative Purchaser Payments as of that date, the initially tiered revenue interest rate will be decreased to a single rate of 1.50% (or after the funding of the Second Payment, 2.25%) of the company’s worldwide net sales, excluding those in China. If the aggregate Revenue Interest Payments made to Oberland as of the Test Date are less than the aggregate amount of Cumulative Purchaser Payments as of the Test Date, then following the Test Date the initially tiered revenue interest rate will increase to a rate that, had such increased rate applied during the period from December 29, 2023 through December 31, 2029, it would have resulted in Oberland receiving aggregate Revenue Interest Payments (excluding certain payments detailed in the RIPA) equal to the Cumulative Purchaser Payments as of the Test Date. In addition, if aggregate Revenue Interest Payments made to Oberland as of the Test Date are less than the aggregate amount of Cumulative Purchaser Payments as of the Test Date, then the company must make the True-Up Payment.
Oberland’s rights to receive Revenue Interest Payments under the RIPA shall terminate when Oberland has received payments (including any True-Up Payment) equal to 195.0% of the then Cumulative Purchaser Payments unless the RIPA is terminated prior to such date. If Oberland has not received total payments (including any True-Up Payment) equal to 195.0% of the then Cumulative Purchaser Payments on or before the twelfth anniversary of the RIPA, then the company shall be obligated to pay to Oberland an amount equal to 195.0% of the then Cumulative Purchaser Payments less the aggregate payments (including any True-Up Payments) made as of such date.
Under the RIPA, the company has a Call Option to terminate the RIPA and repurchase the Revenue Interests at any time upon advance written notice, subject to certain limitations set forth in the RIPA. Additionally, Oberland has a Put Option enabling them to terminate the RIPA and to require the company to repurchase the Revenue Interests upon enumerated events, such as a bankruptcy event, failure to make a payment, an uncured material breach, default in certain third-party agreements, a breach or default under any subordination agreements with respect to indebtedness to existing stockholders, or subordinated notes during certain time periods, judgments in excess of certain amounts against the company, a material adverse effect, the loss of regulatory approval of our product candidates or a change of control. The required purchase price with respect to the Call Option and/or Put Option, as applicable, shall be (a) 120.0% of the Cumulative Purchaser Payments as of such date, if Oberland exercises the Put Option (other than in connection with a change of control) on or prior to the first anniversary the Closing Date, (b) 135.0% of the Cumulative Purchaser Payments as of such date, if the Put Option or the Call Option is exercised in connection with a change of control on or prior to the date that is eighteen (18) months after the Closing Date, and (c) in all other cases, (i) 175.0% of the Cumulative Purchaser Payments as of such date, if the Put Option or the Call Option is exercised no later than the date that is thirty six (36) months after the Closing Date, and (ii) 195.0% of the Cumulative Purchaser Payments as of such date, if the Put Option or the Call Option is exercised later than the date that is thirty six (36) months after the Closing Date, minus, in each case, the total payments made to Oberland on or prior to such date.
The company’s obligations under the RIPA are guaranteed by certain of its subsidiaries meeting materiality thresholds set forth in the RIPA. To secure the company’s obligations under the RIPA and the subsidiary guarantors’ obligations under the guarantees, each of the company and the subsidiary guarantors has granted a security interest in substantially all its assets, subject to certain exceptions and limitations.
The RIPA contains affirmative and negative covenants and events of default, including covenants and restrictions that, among other things, restrict our ability to incur additional liens, incur additional indebtedness, make loans and investments, enter into transactions with affiliates, engage in mergers and acquisitions, engage in asset sales and exclusive licensing arrangements, and declare dividends to our stockholders, in each case, subject to certain exceptions set forth in the RIPA. As of December 31, 2023, the company was in compliance with all covenants.
The RIPA is considered a sale of future revenues and accounted for as long-term debt recorded at amortized cost using the effective interest rate method.
Also, on December 29, 2023 and in connection with the RIPA, we entered into an SPOA with Oberland pursuant to which we sold an aggregate of approximately $10.0 million of our common stock at $4.1103 per share in a private placement. Oberland also has an option to purchase up to an additional $10.0 million of our common stock, at a price per share to be determined by reference to the 30-day trailing volume weighted-average price of our common stock calculated from the date of exercise. This stock purchase option was classified as a liability estimated at fair value at issuance. The $200.0 million received pursuant to the RIPA and $10.0 million received pursuant to the SPOA were allocated among the resulting financial instruments on a relative fair value basis, with $197.1 million allocated to the debt under the RIPA, $12.0 million allocated to the common stock issued under the SPOA, and $0.8 million allocated to the stock purchase option.
The Put Option under the RIPA that is exercisable by Oberland upon certain contingent events and the Call Option that is exercisable by the company upon a change of control were determined to be embedded derivatives requiring bifurcation and separately accounted for as a single compound derivative instrument. The company recorded the initial fair value of the derivative liability of $34.5 million as a debt discount, which will be amortized to interest expense over the expected term of the debt using the effective interest rate method.
In connection with the RIPA, as of December 31, 2023 $155.4 million was recorded as revenue interest liability, on the consolidated balance sheet. The company imputes interest expense associated with this liability using the effective interest rate method. The effective interest rate is calculated based on the rate that would enable the debt to be repaid in full over the anticipated life of the arrangement. The interest rate on this liability may vary during the term of the agreement depending on a number of factors, including the level of forecasted net sales. The company evaluates the interest rate quarterly based on its current net sales forecasts utilizing the prospective method. A significant increase or decrease in actual or forecasted net sales may materially impact the revenue interest liability, interest expense, other income, and the time period for repayment. During the year ended December 31, 2023, we recorded $0.3 million of interest expense related to this arrangement.
The company incurred $7.5 million of issuance costs in connection with the RIPA, which will be amortized to interest expense over the estimated term of the debt.
The following table summarizes the revenue interest liability activity during the year ended December 31, 2022.2023 (in thousands):
| | | | | |
| Revenue interest liability at inception | $ | — | |
| Proceeds from the RIPA, gross | 200,000 | |
| Less: Issuance costs | 7,491 | |
| Proceeds from the RIPA, net | 192,509 | |
| Debt discount from: | |
| Embedded contingent derivative liability | (34,500) | |
| Issuance of common stock | (2,039) | |
| Fair value of stock purchase option | (819) | |
| Interest expense recognized | 264 | |
| Revenue interest liability as of December 31, 2023 | $ | 155,415 | |
10. Related-Party Debt
Our related-party debt is summarized below (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances as of December 31, 2023 |
| Maturity
Year | | Interest
Rate | | Principal
Amount | | Less:
Unamortized
Discounts | | Total |
| | | | | | | | | |
| Related-Party Nonconvertible Note: | | | | | | | | | |
$505 million December 2023 Promissory Note – Tranche 1 (1), (2), (3) | 2025 | | Term SOFR +8.0% | | $ | 125,000 | | | $ | 20,414 | | | $ | 104,586 | |
| | | | | | | | | |
| Related-Party Convertible Notes: | | | | | | | | | |
$300 million December 2021 Promissory Note (1), (2), (3) | | | | | $ | 300,000 | | | $ | 26,091 | | | $ | 273,909 | |
$50 million December 2022 Promissory Note (1), (2), (3) | | | | | 50,000 | | | 4,349 | | | 45,651 | |
$30 million June 2023 Promissory Note (2), (3) | | | | | 30,000 | | | 2,609 | | | 27,391 | |
| $505 million December 2023 Promissory Note Tranche 2 | 2025 | | Term SOFR +7.5% | | 380,000 | | | 33,049 | | | 346,951 | |
$30 million March 2023 Promissory Note (2), (3) | 2025 | | Term SOFR +8.0% | | 30,000 | | | — | | | 30,000 | |
$200 million September 2023 Promissory Note (2), (3) | 2026 | | Term SOFR +8.0% | | 200,000 | | | — | | | 200,000 | |
| Total related-party convertible notes | | | | | $ | 610,000 | | | $ | 33,049 | | | $ | 576,951 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances as of December 31, 2022 |
| Maturity
Year | | Interest
Rate | | Principal
Amount | | Accrued PIK
Interest
Added to
Note | | Less:
Unamortized
Discounts | | Total |
| | | | | | | | | | | |
| Related-Party Nonconvertible Notes: | | | | | | | | | | | |
$300 million December 2021 Promissory Note (1), (2), (3) | 2023 | | Term SOFR +8.0% | | $ | 300,000 | | | $ | — | | | $ | 35,822 | | | $ | 264,178 | |
$125 million August 2022 Promissory Note (1), (2), (3) | 2023 | | Term SOFR +8.0% | | 125,000 | | | — | | | 7,039 | | | 117,961 | |
$50 million December 2022 Promissory Note (1), (2), (3) | 2023 | | Term SOFR +8.0% | | 50,000 | | | — | | | 238 | | | 49,762 | |
| Total related-party nonconvertible notes | | | | | $ | 475,000 | | | $ | — | | | $ | 43,099 | | | $ | 431,901 | |
| | | | | | | | | | | |
| Related-Party Convertible Notes: | | | | | | | | | | | |
Nant Capital 2015 Note Payable (1), (2) | 2025 | | 5.0% | | $ | 55,226 | | | $ | 9,320 | | | $ | 5,188 | | | $ | 59,358 | |
Nant Capital 2020 Note Payable (1), (2) | 2025 | | 6.0% | | 50,000 | | | 7,039 | | | 4,068 | | | 52,971 | |
Nant Capital 2021 Note Payable (1), (2) | 2025 | | 6.0% | | 40,000 | | | — | | | 2,580 | | | 37,420 | |
NantMobile Note Payable (1), (2) | 2025 | | 3.0% | | 55,000 | | | 5,110 | | | 5,978 | | | 54,132 | |
NCSC Note Payable (1), (2) | 2025 | | 5.0% | | 33,000 | | | 7,684 | | | 3,294 | | | 37,390 | |
| Total related-party convertible notes | | | | | $ | 233,226 | | | $ | 29,153 | | | $ | 21,108 | | | $ | 241,271 | |
$125.0300.0 million Variable-RateDecember 2021 Promissory Note
On August 31, 2022,December 17, 2021, the company executed a $125.0$300.0 million promissory note with NantNant Capital, an affiliatedaffiliated entity of the company due to theunder common control of our Executive Chairman, and Global Chief Scientific and Medical Officer. ThisOfficer, and principal stockholder. The note bearsbore an interest atrate of Term Secured Overnight Financing Rate (Term SOFR)SOFR plus 8.0%5.4% per annum. The accrued interestannum, payable on this note shall be payablea quarterly on the last business day of March, June, September and December, commencing on September 30, 2022.basis. The outstanding principal amount and any accrued and unpaid interest areon advances were due on December 31, 2023. The company may prepay this note at any time, in whole or in part, without premium or penalty.
The company received net proceeds of $124.4 million, net of a $0.6 million origination fee paid to the lender, which the company intends to use for pre-commercialization efforts and clinical development programs, working capital, and other general corporate purposes. The interest paid in cash amounted to $5.2 million for the year ended December 31,17, 2022.
$300.0 million Variable-Rate Promissory Note
On August 31, 2022, the company amended and restated its $300.0 million variable-rate promissory note with Nant Capital. Prior to the amendment and restatement, the outstanding balance due under the promissory note was due and payable on December 17, 2022, the loan bore interest at Term SOFR + 5.4%, which was payable quarterly commencing on March 17, 2022, and the company could and can continue to prepay the outstanding principal (together with accrued and unpaid interest), in whole or in part, upon five business days’ prior written notice to the lender.
The terms of this promissory note were amended and restated to extend the maturity date of the loan to December 31, 2023, increase the interest rate on the loan to Term SOFR + 8.0% per annum, and reset the quarterly interest payment date from the 17th of the month to the last business day of March, June, September and December, commencing on September 30, 2022. No other material terms or conditions of this variable-rate promissory note were modified as part of the August 31, 2022 amendment and restatement. The interest paid in cash amounted to $27.4 million for the year ended December 31, 2022.
In the event of a default on the loan (as defined in both the original and amended and restated promissory notes), including if the company does not repay the loan at maturity, the company had and continues to have the right, at its sole option, to convert the outstanding principal amount and accrued and unpaid interest due under this note into fully paid and non-assessable shares of the company’s common stock at a price of $5.67 per share equal to $5.67.
Fixed-Rate Convertible Promissory Notes
On August 31, 2022, the company alsoand Nant Capital entered into an amended and restated promissory note, pursuant to which the maturity date of the promissory note was extended to December 31, 2023 and the interest rate was amended to Term SOFR plus 8.0% per annum.
On September 11, 2023, the company and Nant Capital entered into a letter amendment pursuant to which the maturity date of the promissory note was further extended to December 31, 2024.
On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into an aggregateamended and restated promissory note pursuant to which this existing promissory note was amended to be included in the Tranche 2 principal amount of $315.1the amended $505.0 million (includingDecember 2023 promissory note, with a maturity date of December 31, 2025, and an interest rate of Term SOFR plus 7.5% per annum. Based on the terms of the amended and restated promissory note, the noteholder, at its sole discretion, may convert all of the Tranche 2 $380.0 millionprincipal amount into shares of common stock at a conversion price of $8.2690 per share.
$125.0 million August 2022 Promissory Note
On August 31, 2022, the company executed a $125.0 million promissory note with Nant Capital. The note bears an interest rate of Term SOFR plus 8.0% per annum, payable on a quarterly basis. The company may prepay the outstanding promissory note, at any time, in whole or in part, without penalty.
On September 11, 2023, the company and Nant Capital entered into a letter amendment pursuant to which the maturity date of the promissory note was extended to December 31, 2024.
On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into an amended and restated promissory note letter agreement pursuant to which the existing promissory note was amended to be included in the Tranche 1 principal amount of the amended $505.0 million December 2023 promissory note, with a maturity date of December 31, 2025 and an interest rate of Term SOFR plus 8.0% per annum.
$50.0 million December 2022 Promissory Note
On December 12, 2022, the company executed a $50.0 million promissory note with Nant Capital. The note bore an interest rate of Term SOFR plus 8.0% per annum, payable on a quarterly basis. The company may prepay the outstanding promissory note, at any time, in whole or in part, without penalty. In the event of a specified transaction (as defined in the note), the noteholder can request the outstanding principal and interest due on the loan to be repaid in full upon consummation of such specified transaction.
On September 11, 2023, the company and Nant Capital entered into a letter amendment pursuant to which the maturity date of the promissory note was further extended to December 31, 2024.
On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into an amended and restated promissory note pursuant to which the existing promissory note was amended to be included in the Tranche 2 principal amount of the amended $505.0 million December 2023 promissory note, with a maturity date of December 31, 2025, and an interest rate of Term SOFR plus 7.5% per annum. Pursuant to the terms of the amended and restated promissory note, the investor, in its sole discretion may convert all of the outstanding Tranche 2 principal amount into shares of common stock at a conversion price of $8.2690 per share. The noteholder can request up to $50.0 million of the Tranche 2 principal amount and accrued interest to be repaid upon consummation of such specified transaction.
$30.0 million March 2023 Promissory Note
On March 31, 2023, the company executed a $30.0 million promissory note with Nant Capital. This note bears interest at Term SOFR plus 8.0% per annum, payable on a quarterly basis. The outstanding principal amount and any accrued and unpaid interest)interest was originally due on December 31, 2023. The company may prepay the outstanding promissory note, at any time, in whole or in part, without penalty. Upon receipt of a written notice of prepayment from the company, the noteholder may choose to convert the outstanding principal amount to be prepaid and the accrued and unpaid interest thereon into shares of the company’s common stock at a price of $2.28 per share. Additionally, the noteholder may at its option convert the entire outstanding principal amount of the promissory note and accrued interest into shares of the company’s common stock at a conversion price of $2.28 per share, at the option of the noteholder.
The company received net proceeds of approximately $29.9 million from this financing, net of a $0.1 million origination fee paid to the noteholder.
On September 11, 2023, the company and Nant Capital entered into a letter agreement pursuant to which the maturity date of the $30.0 million promissory note described above was extended from December 31, 2023 to December 31, 2024.
On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into a letter agreement pursuant to which the maturity date of this promissory note was extended to December 31, 2025.
$30.0 million June 2023 Promissory Note
On June 13, 2023, the company executed a $30.0 million promissory note with Nant Capital, pursuant to which, the company could request up to three (3) advances of $10.0 million each. The principal amount of each advance bore interest rate at Term SOFR plus 8.0% per annum, payable on a quarterly basis. The outstanding principal amount and any accrued and unpaid interest on advances was originally due on December 31, 2023. We may prepay the outstanding principal amount, together with any accrued interest, on any then-outstanding advances, at any time, in whole or in part, without premium or penalty, and without the prior consent of the noteholder upon five (5) days written notice to the noteholder.
We received net proceeds of approximately $29.9 million from this promissory note, net of a $0.1 million origination fee paid to Nant Capital.
On September 11, 2023, the company and Nant Capital entered into a letter amendment pursuant to which the maturity date of the promissory note was further extended to December 31, 2024.
On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into an amended and restated promissory note pursuant to which the existing promissory note was amended to be included in the Tranche 2 principal amount of the amended $505.0 million December 2023 promissory note, with a maturity date of December 31, 2025, and an interest rate of Term SOFR plus 7.5% per annum. Pursuant to the terms of the amended and restated promissory note the investor, in its sole discretion may convert all of the Tranche 2 $380.0 million principal amount into shares of common stock at a conversion price of $8.2690 per share.
$200.0 million September 2023 Promissory Note
On September 11, 2023, the company executed a $200.0 million convertible promissory note with Nant Capital. The note bears interest at Term SOFR plus 8.0% per annum, payable on a monthly basis. The outstanding principal amount and any accrued and unpaid interest are due on September 11, 2026. We may prepay the outstanding principal amount, together with any accrued interest, at any time, in whole or in part, without premium or penalty upon five (5) days written notice to the noteholder. The noteholder has the sole option to convert all (but not less than all) of the outstanding principal amount and accrued but unpaid interest into shares of the company’s common stock at a conversion price of $1.9350 per share. The company received net proceeds of approximately $199.0 million from this financing, net of a $1.0 million origination fee paid to the noteholder.
Footnotes to Related-Party Debt Tables
Debt Modification and Debt Extinguishments
(1)August 2022 Debt Extinguishment
On August 31, 2022, the company amended and restated the above fixed-rate promissory notes payables held by Nant Capital, NantWorks, NantMobile and NCSC, which are entities affiliated with Dr. Soon-Shiong. Prior to the amendments and restatements, these notes bore and continuethereafter continued to bear interest at a per annum rate ranging from 3.0% to 6.0%, provideprovided that the outstanding principal was and continuesthereafter continued to be due and payable on September 30, 2025, and accrued and unpaid interest was or continuescontinued to be payable either upon maturity or, with respect to one of the notes, on a quarterly basis. Prior to the amendments and restatements, the company could and can continuethereafter continued to be able to prepay the outstanding principal (together with accrued and unpaid interest), either in whole or in part, at any time without premium or penalty and without the prior consent of the lender, nownoteholder, subject to an advance notice period of at least five business days during which the lender cannoteholder could convert the amount requested to be prepaid by the company into shares of the company’s common stock, as part of the amendment and restatement described below.restatement.
The terms of these fixed-rate promissory notes were amended and restated to include a conversion feature that givesgave each lender the rightnoteholder, at its sole option, at any time including upon(other than when the noteholder is in receipt of a written notice of prepayment at its sole option,from the borrower), the right to convert the entire outstanding principal amount and accrued and unpaid interest due under each note at the time of conversion into shares of the company’s common stock at a price of $5.67 per share. No other material terms or conditions of these fixed-rate promissory notes were modified as part of the August 31, 2022 amendments and restatements.
Since all of the above promissory notes were entered into or amended at the same time and with entities under common control, the company determined that the promissory notes were required to be evaluated collectively to accurately capture the economics of the transactions entered in contemplation of each other and contemporaneously. ASC 470-50Debt –Modifications and Extinguishments, provides that a modification or an exchange that adds or eliminates a substantive conversion option as of the conversion date would always be considered substantial and require extinguishment accounting. Accordingly, as a result of the addition of the conversion feature to the fixed-rate promissory notes, the fixed-rate promissory notes and the variable-rate promissory notes were determined to be extinguished given the contemporaneous nature of the amendments. The company performed a valuation of the fixed-rate promissory notes and variable-rate promissory notes before and after amendments. Under this model, the company calculated a gain on extinguishment of $82.9 million, representing the difference between the fair value of the new and amended promissory notes and the carrying value of the extinguished debt, net of any unamortized related-party notes discounts plus the cash proceeds from the new promissory note. Since the debt was obtained from entities under common control, such gain was recorded in additional paid-in capital, on the consolidated statement of stockholders’ deficit forduring the year ended December 31, 2022. Also, the difference between the face values of the new and amended promissory notes (and accrued interest on the date of the amendment) and the fair values of the new and restated promissory notes was recorded as a debt discount to be amortized asto interest expense over the remaining term (or until conversion in the case of fixed-rate promissory notes) of the respective promissory notes. The companyDuring the year ended December 31, 2022, we recorded amortization of related-party notes discounts totaling $16.3 million in interest expense, on the consolidated statement of operations during the year ended December 31, 2022.operations.
The fair values of the promissory notes without a holder conversion option were estimated using discounted cash flow analyses, based on market rates available to the company for similar debt at issuance after consideration of default and credit risk and the level of subordination. The fair values of the fixed-rate promissory notes, which were each modified to include a holder conversion option, were determined based on a binomial lattice convertible note model. The analysis involved the construction of various intermediate lattices: stock price tree, conversion value tree, conversion probability tree, and discount rate tree. Since certain of the factors analyzed are considered to be unobservable inputs, both the discounted cash flow model and the lattice model are considered to be Level 3 valuations. Significant unobservable inputs used for the discounted cash flow analysis included market yields from 18.0% to 24.8% and a risk freerisk-free rate of 4.1%, and the significant unobservable inputs used for the binomial lattice model included a volatility of 84.9%, a market yield of 17.4% and a risk freerisk-free rate of 3.5%.
$50.0 million Variable-Rate Promissory Note
On December 12, 2022, the company executed a $50.0 million promissory note with Nant Capital. This note bears interest at Term SOFR plus 8.0% per annum. The accrued interest on this note shall be payable quarterly on the last business day of March, June, September and December, commencing on December 30, 2022. The outstanding principal amount and any accrued and unpaid interest are due on December 31, 2023. The company may prepay the outstanding principal amount, together with any accrued interest at any time, in whole or in part, without premium or penalty. The company must prepay the outstanding principal amount, together with any accrued interest, if requested by the holder, following the successful closing of a strategic collaboration transaction with a large biopharmaceutical company.
The company received net proceeds of $49.7 million, net of a $0.3 million origination fee paid to the lender, which the company intends to use for pre-commercialization efforts and clinical development programs, other research and development activities, capital expenditures, and other general corporate purposes. The interest paid in cash amounted to $0.3 million for the year ended December 31, 2022.
Our related-party debt is summarized below (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances at December 31, 2022 |
| Maturity
Year | | Interest
Rate | | Outstanding
Advances | | Accrued
Interest
Added to
Note | | Less:
Unamortized
Discounts | | Total |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Related-Party Notes: | | | | | | | | | | | |
| Nant Capital (1) | 2023 | | Term SOFR + 8.0% | | $ | 475,000 | | | $ | — | | | $ | 43,099 | | | $ | 431,901 | |
| Related-Party Convertible Notes: | | | | | | | | | | | |
| Nant Capital | 2025 | | 5.0% | | 55,226 | | | 9,320 | | | 5,188 | | | 59,358 | |
| Nant Capital | 2025 | | 6.0% | | 50,000 | | | 7,039 | | | 4,068 | | | 52,971 | |
| Nant Capital | 2025 | | 6.0% | | 40,000 | | | — | | | 2,580 | | | 37,420 | |
| NantMobile, LLC | 2025 | | 3.0% | | 55,000 | | | 5,110 | | | 5,978 | | | 54,132 | |
| NantCancerStemCell, LLC | 2025 | | 5.0% | | 33,000 | | | 7,684 | | | 3,294 | | | 37,390 | |
| Total related-party convertible notes | | | | | 233,226 | | | 29,153 | | | 21,108 | | | 241,271 | |
| Total related-party debt | | | | | $ | 708,226 | | | $ | 29,153 | | | $ | 64,207 | | | $ | 673,172 | |
_______________
| | | | | |
(1) | The interest rate on our related-party variable-rate notes as of December 31, 2022 was 12.59%. |
Conversion of Fixed-Rate Promissory Note due 2025
On December 12, 2022, the company received written notice from NantWorks, the holder of thean existing convertible promissory note of NantCell, Inc., a wholly-owned subsidiary of the company, (the Existing Note), of its election to convert the entire outstanding principal and accrued interest under the Existing NoteNantCell promissory note into shares of the company’s common stock. As of such date, the holder of the NantCell note converted the entire $56.6 million of outstanding principal amount and accrued and unpaid interest due under the Existing Note, net of unamortized discount, was approximately $51.9 million, which were convertednote into 9,986,920 shares of the company’s common stock at a price of $5.67 per share in accordance with the terms of the Existing Note.promissory note.
(2)September 11, 2023 Debt Modification
On September 11, 2023, the company entered into a stock purchase agreement with Nant Capital, NantMobile and NCSC pursuant to which the holders exchanged promissory notes totaling approximately $270.0 million in aggregate principal amount and accrued and unpaid interest for an aggregate of 209,291,936 shares of common stock at an exchange price of $1.29 per share. As a result of the exchange, the company was forever released and discharged from all its obligations and liabilities under the notes.
On September 11, 2023, the company and Nant Capital entered into a series of letter agreements pursuant to which the maturity date of the related-party nonconvertible notes described above totaling $505.0 million in principal was extended from December 31, 2023 to December 31, 2024. In addition, the company entered into the $200.0 million September 2023 promissory note with Nant Capital. No other material terms or conditions of these promissory notes were modified.
Since all of the above promissory notes were entered into or amended at the same time on September 11, 2023 and with entities under common control, the company determined that the promissory notes were required to be evaluated collectively to accurately capture the economics of the transactions entered in contemplation of each other and contemporaneously. Pursuant to ASC 470-50, as the terms of the amendment were not substantially different than the terms of the promissory notes prior to the amendment, the amendment was accounted for as a debt modification. The unamortized debt discounts from the promissory notes are being amortized as an adjustment to interest expense over the remaining term of modified promissory notes that are not recorded at fair value using the effective interest rate method. Also, a $29.6 million increase in fair value of the embedded conversion feature from the debt modification was accounted for as a debt discount to the $200.0 million convertible note that is not recorded at fair value, and a $1.6 million increase in fair value of the embedded conversion feature related to the promissory note recorded at fair value was accounted for as interest expense during the year ended December 31, 2023. Such increase in fair values of the embedded conversion features totaling $31.2 million has been recorded with a corresponding increase in additional paid-in capital,on the consolidated statement of stockholders’ deficit.
(3)December 29, 2023 Debt Extinguishment
On December 29, 2023 in connection with the RIPA, the company and Nant Capital entered into an amended and restated promissory note and a letter amendment for the following outstanding promissory notes. Pursuant to the terms of the amended and restated promissory note, the amended $505.0 million December 2023 promissory note is comprised of a Tranche 1 with principal amount of $125.0 million which was previously the $125.0 million August 2022 promissory note before amendment, and a Tranche 2 with principal amount of $380.0 million, which is made up of the previous $300.0 million December 2021 promissory note, $50.0 million December 2022 promissory note and $30.0 million June 2023 promissory note. In addition, the amendment allows Nant Capital, in its sole discretion, to convert all the Tranche 2 principal amount of $380.0 million of the amended promissory note into shares of common stock. The conversion price was subsequently determined at $8.2690 per share based on the agreement. The maturity date of the amended promissory note is December 31, 2025. Pursuant to the terms of the letter amendment, the maturity date of the $30.0 million March 2023 promissory note was extended from December 31, 2024 to December 31, 2025. Also, in connection with the RIPA transaction, all outstanding related-party promissory notes became subordinated to the RIPA payment obligations.
The following table summarizes the Nant Capital promissory notes before the amendments on December 29, 2023 (principal amount in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Principal
Amount | | Maturity
Date | | Conversion
Price | | Interest
Rate |
| | | | | | | |
| Related-Party Nonconvertible Note: | | | | | | | |
| $125 million August 2022 Promissory Note | $ | 125,000 | | | 12/31/2024 | | | | Term SOFR +8.0% |
| | | | | | | |
| Related-Party Convertible Notes: | | | | | | | |
| $300 million December 2021 Promissory Note | $ | 300,000 | | | 12/31/2024 | | | | Term SOFR +8.0% |
| $50 million December 2022 Promissory Note | 50,000 | | | 12/31/2024 | | | | Term SOFR +8.0% |
| $30 million June 2023 Promissory Note | 30,000 | | | 12/31/2024 | | | | Term SOFR +8.0% |
| $30 million March 2023 Promissory Note | 30,000 | | | 12/31/2024 | | $2.2800 | | Term SOFR +8.0% |
| $200 million September 2023 Promissory Note | 200,000 | | | 9/11/2026 | | $1.9350 | | |
Total related-party promissory notes
before amendments | $ | 735,000 | | | | | | | |
The following table summarizes the Nant Capital promissory notes after the amendments on December 29, 2023 (principal amount in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Principal
Amount | | Maturity
Date | | Conversion
Price | | Interest
Rate |
| | | | | | | |
| Related-Party Nonconvertible Note: | | | | | | | |
$505 million December 2023 Promissory Note – Tranche 1 | $ | 125,000 | | | 12/31/2025 | | | | Term SOFR +8.0% |
| | | | | | | |
| Related-Party Convertible Notes: | | | | | | | |
| $300 million December 2021 Promissory Note | $ | 300,000 | | | | | | | |
$50 million December 2022 Promissory Note | 50,000 | | | | | | | |
| $30 million June 2023 Promissory Note | 30,000 | | | | | | | |
$505 million December 2023 Promissory Note – Tranche 2 | 380,000 | | | 12/31/2025 | | $8.2690 | | Term SOFR +7.5% |
| Total $505 million December 2023 Promissory Note | 505,000 | | | | | | | |
| $30 million March 2023 Promissory Note | 30,000 | | | 12/31/2025 | | $2.2800 | | Term SOFR +8.0% |
| $200 million September 2023 Promissory Note | 200,000 | | | 9/11/2026 | | $1.9350 | | Term SOFR +8.0% |
Total related-party promissory notes
after amendments | $ | 735,000 | | | | | | | |
Since all of the above outstanding promissory notes were amended at the same time, with entities under common control, the company determined that the promissory notes were required to be evaluated collectively to accurately capture the economics of the transactions entered in contemplation of each other and contemporaneously. Also, in accordance with ASC 470-50 the company used the debt terms that existed before the September 11, 2023 modification to determine whether the current modification is substantially different, as the September 11, 2023 modification was within a year of the current transaction, and the promissory notes, at that time, had been modified without being deemed to be substantially different. As the modifications (September 11, 2023 and December 29, 2023 on a cumulative basis) added a substantive conversion feature to the promissory notes, these promissory notes were determined to be extinguished given the contemporaneous nature of the amendments. The company performed a valuation of all the promissory notes before and after amendments. Under this model, the company calculated a loss on extinguishment of $318.8 million, representing the difference between the fair value of the amended promissory notes and the carrying value of the extinguished debt, net of any unamortized related-party notes discounts. Since the debt was obtained from entities under common control, such loss was recorded in additional paid-in capital. In addition, a debt premium totaling $354.9 million, calculated as the difference between the fair values of certain promissory notes after modifications and their respective face values, was also recorded in additional paid-in capital. Collectively, net gain on debt extinguishment of $36.1 million was recorded in additional paid-in capital, on the consolidated statement of stockholders’ deficit for the year ended December 31, 2023. Also, the difference between face values of certain new and amended promissory notes and their respective fair values of $53.1 million was recorded as a debt discount to be amortized as interest expense over the remaining term. During the year ended December 31, 2023, we recorded amortization of related-party notes discounts totaling $0.5 million in interest expense, on the consolidated statement of operations related to the new and amended promissory notes.
In regard to the Tranche 2 principal amount of the $505.0 million December 2023 promissory note, the company identified an embedded derivative related to a contingent exercisable prepayment feature of the promissory note, which allows the noteholder to request up to a $50.0 million prepayment of the promissory note and accrued interest upon the occurrence of a specified transaction. After the debt extinguishment, the company concluded that this promissory note was issued at a substantial discount, so the embedded derivative that is contingent exercisable was required to be bifurcated and accounted separately from the debt host instrument. The fair value of the embedded derivative was estimated at $0.8 million as of December 31, 2023, using a with and without method, which assesses the likelihood and timing of the specified transaction to be triggered and result in a repayment. Significant unobservable inputs used for the valuation included a volatility of 118.0%, a market yield of 23.2% and a risk-free rate of 4.8%.
The fair value of Tranche 1 principal amount of the amended $505.0 million December 2023 promissory note, which had no noteholder conversion option, was estimated using discounted cash flow analyses, based on market rates available to the company for similar debt at issuance after consideration of default and credit risk and the level of subordination. The fair value of Tranche 2 of the amended promissory note, which was modified to include a noteholder conversion option, was determined based on a binomial lattice convertible note model. The analysis involved the construction of various intermediate lattices: stock price tree, conversion value tree, conversion probability tree, and discount rate tree. Since certain of the factors analyzed were considered to be unobservable inputs, both the discounted cash flow model and the lattice model are considered to be Level 3 valuations. Significant unobservable inputs used for the discounted cash flow analysis included a market yield of 23.2% and the significant unobservable inputs used for the binomial lattice model included a volatility of 118.0%, a market yield of 23.2% and a risk-free rate of 4.4%. The effective unamortized debt discount rate of the amended Tranche 1 and Tranche 2 principal amount of the $505.0 million December 2023 promissory note is 23.65% and 18.04%, respectively.
The fair value of the $200.0 million September 2023 promissory note was determined using the binomial lattice model. Significant unobservable inputs used for the valuation included a volatility of 119.3%, a market yield of 23.3% and a risk-free rate of 5.2%.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances at December 31, 2021 |
| Maturity
Year | | Interest
Rate | | Outstanding
Advances | | Accrued
Interest
Added to
Note | | Less:
Unamortized
Debt Issuance
Costs | | Total |
| | | | | | | | | | | |
| Related-Party Note: | | | | | | | | | | | |
| Nant Capital (1) | 2022 | | Term SOFR + 5.4% | | $ | 300,000 | | | $ | 674 | | | $ | 1,438 | | | $ | 299,236 | |
| | | | | | | | | | | |
| Related-Party Convertible Notes: | | | | | | | | | | | |
| Nant Capital | 2025 | | 5.0% | | 55,226 | | | 6,141 | | | — | | | 61,367 | |
| Nant Capital | 2025 | | 6.0% | | 50,000 | | | 3,810 | | | — | | | 53,810 | |
| Nant Capital | 2025 | | 6.0% | | 40,000 | | | — | | | — | | | 40,000 | |
| NantMobile | 2025 | | 3.0% | | 55,000 | | | 3,359 | | | — | | | 58,359 | |
| NantWorks | 2025 | | 5.0% | | 43,418 | | | 10,649 | | | — | | | 54,067 | |
| NCSC | 2025 | | 5.0% | | 33,000 | | | 5,746 | | | — | | | 38,746 | |
| Total related-party convertible notes | | | | | 276,644 | | | 29,705 | | | — | | | 306,349 | |
| | | | | | | | | | | |
| Total related-party debt | | | | | $ | 576,644 | | | $ | 30,379 | | | $ | 1,438 | | | $ | 605,585 | |
| | | | | |
| Expected market yield | 23.5 | % |
| Expected volatility | 138.0 | % |
| Risk-free rate | 5.2 | % |
The change in the carrying value of this note was as follows (in thousands):
| | | | | |
| |
| |
| Fair value at issuance date, March 31, 2023 | $ | 29,850 | |
| Change in fair value | 36,203 | |
| Gain on debt extinguishment with entities under common control | (36,053) | |
| Carrying value, December 29, 2023 | $ | 30,000 | |
The following table summarizes the interest expense for our related-party promissory notes during the year ended December 31, 2023 (in thousands):
| | | | | | | | | | | | | | |
| | Year Ended December 31, 2023 |
| | Interest
Expense | | Debt
Discount
Amortization |
| | | | |
$300 million December 2021 Promissory Note (1) | | $ | 39,653 | | | $ | 27,967 | |
$125 million August 2022 Promissory Note (1) | | 16,521 | | | 5,962 | |
$50 million December 2022 Promissory Note (1) | | 6,609 | | | 478 | |
$30 million March 2023 Promissory Note (1) | | 4,590 | | | — | |
| $30 million June 2023 Promissory Note | | 2,096 | | | 258 | |
| $200 million September 2023 Promissory Note | | 8,185 | | | 2,586 | |
| Related-Party Fixed-Rate Promissory Notes | | 8,799 | | | 5,145 | |
| Total | | $ | 86,453 | | | $ | 42,396 | |
_______________
| | | | | |
| (1) | Balances include the amortization of debt discount totaling $0.5 million recorded during the period from December 29, 2023 to December 31, 2023. The interest rate on our related-party variable-rate note as of December 31, 2021expense recorded during this period was 5.47%.$0.4 million. |
The following table summarizes the estimated future contractual obligations for our related-party debt as of December 31, 20222023 (in thousands):
| | Principal Payments | |
| Convertible
Notes | |
| Convertible
Notes | |
| Convertible
Notes | | | Nonconvertible
Notes | | Convertible
Notes | | Nonconvertible
Notes | | Total |
| | Principal Payments | | Interest Payments (1) | |
| Convertible
Notes | | Non-convertible
Notes | | Convertible
Notes | | Non-convertible
Notes | | Total |
| | 2023 | | $ | — | | | $ | 475,000 | | | $ | 2,400 | | | $ | 59,622 | | | $ | 537,022 | |
| 2024 | |
| 2024 | |
| 2024 | 2024 | | — | | | — | | | 2,407 | | | — | | | 2,407 | |
| 2025 | 2025 | | 233,226 | | | — | | | 61,050 | | | — | | | 294,276 | |
Total principal and estimated interest
due on related-party debt | | $ | 233,226 | | | $ | 475,000 | | | $ | 65,857 | | | $ | 59,622 | | | $ | 833,705 | |
| 2026 | |
| Total | |
| | | | | | | | | | | | | | |
| (1) | Interest payments on our fixed-rate convertible notes are calculated based on contractual interest rates and scheduled maturity dates. Interest payments on our variable-ratepromissory notes are calculated based on Term SOFR plus the contractual spread per the loan agreements. The weighted-average interest rate on our variable-ratepromissory notes as of December 31, 20222023 was 12.59%13.09%. |
10.11. Related-Party Agreements
We conduct business with several affiliates under written agreements and informal arrangements. Below is a summary of outstanding balances and a description of significant relationships (in thousands):
| | | | | | | | | | | |
| As of December 31, |
| 2022 | | 2021 |
| | | |
| Due from related party–NantBio, Inc. | $ | 1,294 | | | $ | 1,294 | |
| | | |
| Due from related party–Brink Biologics | 271 | | | — | |
| Due from related parties–Various | 325 | | | 39 | |
| Total due from related parties | $ | 1,890 | | | $ | 1,333 | |
| | | |
| Due to related party–Duley Road, LLC | $ | 1,431 | | | $ | 1,380 | |
| Due to related party–NantWorks | 986 | | | 1,113 | |
| Due to related party–NantBio, Inc. | 943 | | | 943 | |
| | | |
| Due to related party–Immuno-Oncology Clinic, Inc. | 109 | | | 507 | |
| | | |
| Total due to related parties | $ | 3,469 | | | $ | 3,943 | |
| | | | | | | | | | | |
| As of December 31, |
| 2023 | | 2022 |
| | | |
| Due from related party–NantBio | $ | 1,294 | | | $ | 1,294 | |
| Due from related party–NantWorks | 541 | | | — | |
| Due from related party–Brink | 62 | | | 271 | |
| Due from related parties–Various | 122 | | | 325 | |
| Total due from related parties | $ | 2,019 | | | $ | 1,890 | |
| | | |
| Due to related party–NantBio | $ | 943 | | | $ | 943 | |
| Due to related party–Duley Road | 136 | | | 1,431 | |
| Due to related party–the Clinic | 57 | | | 109 | |
| Due to related party–NantWorks | — | | | 986 | |
| | | |
| Total due to related parties | $ | 1,136 | | | $ | 3,469 | |
Our Executive Chairman, Global Chief Scientific and Medical Officer, and principal stockholder founded and has a controlling interest in NantWorks, which is a collection of companies in the healthcare and technology space. As described below, we have entered into arrangements with NantWorks, and certain affiliates of NantWorks, including NantBio, Inc., Duley Road, LLC, and 605 Nash, LLC.NantWorks. Affiliates of NantWorks are also affiliates of the company due to the common control by and/or common ownership interest of our Executive Chairman, Global Chief Scientific and Medical Officer, and principal stockholder.
NantWorks, LLC
Shared Services Agreement
Under the amended and restated shared services agreement with NantWorks dated as of June 2016, but effective as of August 2015, NantWorks, a related party, provides corporate, general and administrative, certain research and development, and other support services. We are charged for thethese services at cost plus reasonable allocations of employee benefits, facilities and other direct or fairly allocated indirect costs that relate to the employees providing the services. ForDuring the years ended December 31, 2023, 2022 2021 and 2020,2021, we recorded $3.3 million, $3.8 million, $4.4 million and $6.0$4.4 million, respectively, in selling, general and administrative expense, and $2.2 million, $0.9 million, $0.4 million and $2.0$0.4 million, respectively, of expense reimbursements under this arrangement in research and development expense, on the consolidated statements of operations. These amounts exclude certain general and administrative expenses provided by third-party vendors directly for our benefit, which were reimbursed to NantWorks based on those vendors’ invoiced amounts without markup by NantWorks.
As of December 31, 2023 and 2022, we had a receivable of $0.5 million and 2021, we owed NantWorks net amountsa payable of $1.0 million and $1.1 million, respectively, for all agreements between the two affiliates,with NantWorks, which are included in due from/due to related parties, on the consolidated balance sheet.sheets. We also recorded $2.0$1.0 million and $2.2$2.0 million of prepaid expenses for services that have been passed through to the company from NantWorks as of December 31, 20222023 and 2021,2022, respectively, which are included in prepaid expenses and other current assets, on the consolidated balance sheets.
Facility License Agreement
In November 2015, we entered into a facility license agreement with NantWorks for approximately 9,500 rentable square feet of office space in Culver City, California, which was converted to a research and development laboratory and a cGMP manufacturing facility. The initial license was effective from May 2015 through December 2020. The base rent for the initial lease term was $47,000 per month, with annual increases of 3% beginning in January 2017. In September 2020, we amended this agreement to extend the term of this leaselicense agreement through December 31, 2021. Commencing January 1, 2022, the license fee increased by 3% to approximately $56,120 per month.
On May 6, 2022, we amended our facility license agreement with NantWorks to expand the licensed premises by 36,830 rentable square feet to an aggregate total of 46,330 rentable square feet. Effective May 1, 2022, the license fee iswas approximately $273,700 per month which isand was subject to a 3% increase commencing on January 1 of each year. The space continues to be rented on a month-to-month basis, which can be terminated by either party with at least 30 days’ prior written notice to the other party. LicenseDuring the years ended December 31, 2023, 2022 and 2021, we recorded license fee expense for this facility totaling $3.4 million, $2.4 million, and $0.7 million, and $0.6 million for the years ended December 31, 2022, 2021 and 2020, respectively, was recorded in research and development expense, on the consolidated statements of operations.
Immuno-Oncology Clinic, Inc.
We have entered into multiple agreements with Immuno-Oncology Clinic, Inc.the (the Clinic)Clinic to conduct clinical trials related to certain of our product candidates. The Clinic is a related party as it is owned by an officer of the company and NantWorks manages the administrative operations of the Clinic. Pursuant to the terms of the Clinic agreement (as amended), we made payments totaling $5.6 million in consideration of future services to be performed by the Clinic.
In 2021, we completed a review of alternative structures that could support our more complex clinical trial requirements and made a decision to explore a potential transition of clinical trials at the Clinic to a new structure (including contracting with a new, non-affiliated professional corporation) to be determined and agreed upon by all parties. Based on this decision to explore a potential transition, we determined that it was more likely than not that thea previously recorded prepaid asset would not result in the collection of fees for services performed by the Clinic as contemplated in the original agreements. As a result, during the year endedDecember 31, 2021, we wrote down the remaining value of our prepaid asset and recorded approximately $4.4 million in research and development expense, on the consolidated statement of operations foroperations. While we have not yet finalized the year ended December 31, 2021. Wepotential transition, we continue productive negotiationsdiscussions with potential partners around such alternative structures and expect to complete the process during the year ending December 31, 2023, but there can be no assurance that we will be successful.structures.
ForDuring the years ended December 31, 2022,2023, 2022 and 2021, and 2020, we incurredrecorded $2.2 million, $2.4 million, and $1.6 million, and $0.9 millionrespectively, in research and development expense, on the consolidated statements of operations related to clinical trial and transition services provided by the Clinic Agreement.Clinic. As of December 31, 20222023 and 2021,2022, we owed the Clinic $0.1 million, and $0.5 million, respectively, for services excluded fromwhich are included in due to related parties, on the Clinic Agreement.consolidated balance sheets.
Brink Biologics, Inc.
In 2015, we entered into an agreement with Brink Biologics, Inc. (Brink) whereby we granted to Brink worldwide exclusive licenses tofor the use of certain cell lines and intellectual property forin non-clinical laboratory testing. Brink is a related party as our Executive Chairman, Global Chief Scientific and Medical Officer and our principal stockholder, and our Chief Corporate Affairs Officer and member of our boardBoard of directors,Directors, collectively own more than 50% of Brink’s outstanding shares. WeDuring the years ended December 31, 2023 and 2022, we recognized revenue of an immaterial amount, respectively, and $0.4 million and $0.1 million forduring the year ended December 31, 2022, 2021 and 2020, respectively, related to this license.
NantBio, Inc.
In August 2018, we entered into a supply agreement with NantCancerStemCell, LLC (NCSC),NCSC, a 60%100% owned subsidiary of NantBio (with the other 40% owned by Sorrento).NantBio. Under this agreement, we agreed to supply VivaBioCell’s proprietary GMP-in-a-Box bioreactors and related consumables, made according to specifications mutually agreed to with both companies. The agreement hashad an initial term of five years and renews automatically for successive one-year terms unless terminated by either party in the event of material default upon prior written notice of such default and the failure of the defaulting party to remedy the default within 30 days of the delivery of such notice, or upon 90 days’ prior written notice by NCSC. We recognized no revenue for
During the years ended December 31, 20222023 and 20222020,, we recognized no revenue, respectively, and $0.3 million of revenue forduring the year ended December 31, 2021. WeAs of December 31, 2023 and 2022, we recorded $0.1 million and $0.1 million of deferred revenue for bioreactors that were delivered but not installed as ofin December 31, 2022accrued expenses and other liabilities and 2021, respectively., on the consolidated balance sheets. As of December 31, 20222023 and 2021,2022, we recorded $0.9 million, respectively, in due to related parties,, on the consolidated balance sheets related to this agreement.
In 2018, we entered into a shared service agreement pursuant to which we are charged for services at cost, without mark-up or profit by NantBio, but including reasonable allocations of employee benefits related to the employees providing the services. In April 2019, we agreed with NantBio to transfer certain NantBio employees and associated research and development projects comprising the majority of NantBio’s business, to the company. After the transfer, we settled certain employee bonuses and benefits that were accrued by NantBio for 2018. As of December 31, 20222023 and 2021,2022, we recorded a net receivable from NantBio of $1.3 million which included $1.0 millionin due from related parties on the consolidated balance sheets for employee bonuses and $0.3 million for vendor costsamounts we paid on behalf of NantBio.NantBio during the year ended December 31, 2019.
605 Doug St, LLC
In September 2016, we entered into a lease agreement with 605 Doug St, LLC, an entity owned by our Executive Chairman and Global Chief Scientific and Medical Officer, for approximately 24,250 rentable square feet in El Segundo, California, which has been converted to a research and development laboratory and a cGMP manufacturing facility. The lease runsterm was from July 2016 through July 2023. We haveIn June 2023, we exercised the option to extend the lease for one additional three-year term through July 2026. We have included the first option to extend the lease term for three years as part of the initial lease term as it is reasonably certain that we will exercise the option, which implies lease expiration in July 2026. The base rent is approximately $72,385 per month, with annual increases of 3% that began in July 2017. Lease expense for this facility totaling $0.9 million forDuring the years ended December 31, 2022,2023, 2022 and 2021, and 2020,we recorded lease expense for this facility of $0.9 million, respectively, was recorded in research and development expense, on the consolidated statements of operations.
Duley Road, LLC
In February 2017, Altor BioScience Corporation (succeeded by our wholly-owned subsidiary Altor BioScience, LLC), through its wholly-owned subsidiary,we entered into a lease agreement with Duley Road, a related party that is indirectly controlled by our Executive Chairman and Global Chief Scientific and Medical Officer, for approximately 11,980 rentable square feet of office and cGMP manufacturing facility space in El Segundo, California. The lease term is from February 2017 through October 2024. We have the option to extend the initial term for two consecutive five-year periods through October 2034. The base rent is approximately $40,700 per month, with annual increases of 3% that began in November 2018. Effective October 3, 2023, we exercised the first option to extend the lease for one additional five-year term through October 31, 2029.
Effective in January 2019, we entered into two lease agreements with Duley Road for a second building located in El Segundo, California. The first lease is for the first floor of the building with approximately 5,650 rentable square feet. The lease has a seven-year term commencingthat commenced in September 2019. The second lease is for the second floor of the building with approximately 6,488 rentable square feet. The lease has a seven-year term commencingthat commenced in July 2019. Both floors of the building are used for research and development and office space. We have options to extend the initial terms of both leases for two consecutive five-year periods through 2036. The base rent for the two leases is approximately $35,800 per month, thatwith annual increases at a rate of 3% per year.
As of December 31, 2022 and 2021, we recorded $0.9 million and $0.9 million of leasehold improvement payables, respectively, and $0.6 million and $0.5 million of lease-related payables to Duley Road, which were included in due to related parties, on the consolidated balance sheets. ForDuring the years ended December 31, 2022,2023, 20212022 and 2020,2021, we recorded $0.8 million, $1.0 million and $0.8 million of rent expense for the twothese leases totaling $0.9 million, $0.8 million, and $1.0 million, respectively, which was included in research and development expense, on the consolidated statements of operations. As of December 31, 2023 and 2022, we recorded $0.1 million and $1.4 million of lease-related payables to Duley Road, respectively, in due to related parties, on the consolidated balance sheets.
605 Nash, LLC
In February 2021, but effective on January 1, 2021, we entered into a lease agreement with 605 Nash, a related party, whereby we leased approximately 6,883 rentable square feet (the Initial Premises) in a two story mixed use building containing approximately 64,643 rentable square feet onat 605-607 Nash Street in El Segundo, California. This facility is used primarily for pharmaceutical development and manufacturing purposes. The lease term commenced in January 2021 and expires in December 2027, and includes an option to extend the lease for one three-year term through December 2030. The base rent is approximately $20,300 per month with an annual increase of 3% on January 1 of each year during the leaseinitial term, and if applicable, during the option term. In addition, under the agreement, we are required to pay our share of estimated property taxes and operating expenses. We have included the first option to extend the lease term for three years as part
In May 2021, but effective on April 1, 2021, we entered into an amendment to our Initial Premises lease with 605 Nash. The amendment expanded the leased square feet by approximately 57,760 rentable square feet (the Expansion Premises). The lease term of the Expansion Premises commenced in April 2021 and expires in March 2028, whereby the company has theone option to extend the initial term for three years. Per the terms of the amendment, the term of the Initial Premises lease was extended for an additional three months and now expires inon March 2031.31, 2028. Base rent for the Expansion Premises is approximately $170,400 per month with annual increases of 3% on April 1 of each year. We are responsible for the build out of the facility space and associated costs. The amended lease provides for a rent abatement for the first seven months, and for a tenant improvement allowance of approximately $2.6 million for costs and expenses related to improvements made by us to the Expansion Premises.
We have included the options to extend the lease term of both the Initial and Expansion Premises for three years as part of the initial term of the leases as it is reasonably certain that we will exercise the options, which implies expiration of both leases in December 2030. During the years ended December 31, 2023, 2022, and 2021, we recorded rent expense for the Initial and Expansion Premises leases totaling $2.2 million, and $1.7 million for the years ended December 31, 2022 and 2021, respectively, in research and development expense, on the consolidated statements of operations. The terms of initial and amended leases provided for tenant improvement allowances totaling $2.9 million for costs and expenses related to improvements made by us to the Initial and Expansion Premises, which were received from the landlord during the year ended December 31, 2023.
557 Doug St, LLC
On September 27, 2021, we entered into a Membership Interest Purchase Agreement with Nant Capital (the Purchase Agreement). Nant Capital is a related party controlled by Dr. Soon-Shiong. The Purchase Agreement transferred all outstanding membership interests in 557 Doug St, LLC from the company to Nant Capital. The only asset owned by 557 Doug St, LLC is the improved property located at 557 South Douglas Street, El Segundo, California with a building area of approximately 36,434 rentable square feet (the Douglas Property).
The purchase price under the Purchase Agreementpurchase agreement was $22.0 million, and after the offset prorated property taxes of $0.1 million, the net proceeds from the sale were $21.9 million. An independent appraisal of the Douglas Property (the Appraisal) assigned the Douglas Property a value of $22.0 million. The net carrying value of the property was $20.5 million as of the closing date. We accounted for the transfer as a sale of an asset to an entity under common control, recorded the transfer at book value and recognized the excess of net consideration over carrying book value of $1.4 million as a capital contribution received from Nant Capital in additional paid-in capital, on the consolidated statement of stockholders’ deficit forduring the year ended December 31, 2022.
In September 2021, we entered into a lease agreement with Nant Capital under which we leased back 557 South Douglas Street for an initial lease term of seven years, which commenced on September 27, 2021. The monthly base rent under the lease iswas approximately $81,976 per month with an annual increase of 3% on October 1 of each year beginning in 2022 during the initial term and, if applicable, during the option term. For the first two years under the lease, we willwould not be charged rent; we willwould begin paying rent on October 1, 2023 at the current monthly base rent. We prepaid the first month rent and security deposit totaling $0.2 million upon the execution of the lease. We have an option to extend the lease for two additional seven-year periods when the prior term expires. We have included the first option to extend the lease term for seven years as part of the initial lease term as it is reasonably certain that we will exercise the option, which implies lease expiration on September 30, 2035. The lease iswas classified as an operating lease. ForDuring the year ended December 31, 2021, we recorded $0.3 million of rent expense for the lease, which was included in research and development expense, on the consolidated statement of operations.
Effective May 31, 2022, we executed a lease termination agreement with Nant Capital under which we received a full refund of the first month’s rent and security deposit totaling $0.2 million that we paid upon execution of the lease. WePrior to the termination of the lease, we recorded year-to-date rent expense of $0.4 million prior toduring the termination of the lease,year ended December 31, 2022 in research and development expense, on the consolidated statement of operations. WeDuring the year ended December 31, 2022, we recognized a gain of $0.6 million on the disposal of this lease for the year ended December 31, 2022 in other income,expense, net, on the consolidated statement of operations.
420 Nash, LLC
On September 27, 2021, we entered into a lease agreement with 420 Nash, LLC, a related party, whereby we leased an approximately 19,125 rentable square foot property located at 420 Nash Street, El Segundo, California, to be used primarily for the warehousing and storage of drug manufacturing supplies, products and equipment and ancillary office space.
Under the terms of the lease agreement, the lease term began on October 1, 2021 and expires on September 30, 2026. The base rent is approximately $38,250 per month with an annual increase of 3% on October 1 of each year beginning in 2022 during the initial term. The company is responsible for the payment of real property taxes, repairs and maintenance, improvements, insurance, and operating expenses during the term of the lease. We will receivereceived a rent abatement for the first month of the lease, and a one-time improvement allowance of $15,000 from the landlord that will bewas credited against base rent obligations for the second month of the lease.
The company has options to extend the lease term for two additional consecutive periods of five years each. At the beginning of each option term, the initial monthly base rent will be adjusted to market rent (as defined in the lease agreement) with an annual increase of 3% during the option term. We have included the first option to extend the lease term for five years as part of the initial term of the lease as it is reasonably certain that we will exercise the option, which implies lease expiration in September 2031. ForDuring the years ended December 31, 20222023 and 2021,2022, we recorded rent expense for this lease totaling $0.5 million, respectively, and $0.1 million of rent expense forduring the lease, respectively, which was includedyear ended December 31, 2021 in research and development expense, on the consolidated statements of operations.
23 Alaska, LLC
On May 6, 2022, we entered into a lease agreement with 23 Alaska, LLC, a related party, for a 47,265 rentable square foot facility located at 2335 Alaska Ave., El Segundo, California, to be used primarily for pharmaceutical development and manufacturing, research and development, and office space.
Under the terms of the agreement, the lease term beginsbegan on May 1, 2022 and expireswas to expire on April 30, 2027. The base rent iswas approximately $139,400 per month with an annual increase of 3% on May 1 of each year beginning in 2023 during the initial term. We will receive a rent abatement for the second through sixth month of the lease. We arewere also required to pay $7,600 per month for parking during the initial term and extension term, if exercised.term. The company iswas responsible for the payment of real property taxes, repairs and maintenance, improvements, insurance, and operating expenses during the term of the lease.
The company iswas responsible for the costs associated with the build-out of the premises and will receivedwas to receive a one-time tenant improvement allowance of approximately $0.9 million from the landlord. As of December 31, 2022, we re-evaluated plan ofplans for the future development of the facility and deemed it unlikely to claim any of the allowance during the reimbursement time frame. As such, during the year ended December 31, 2022 we wrote off the entire allowance receivable of $0.9 million.
Effective August 31, 2023, we executed a lease termination agreement with the lessor under which we received a full refund of the security deposit totaling $0.1 million duringthat we paid upon execution of the yearlease. During the years ended December 31, 2022.
The company has an option to extend the lease term for one additional consecutive five-year period. At the beginning of the option term, the initial monthly base rent will be adjusted to market rent (as defined in the lease agreement) with an annual increase of 3% during the option term. We2023 and 2022, we recorded $1.2 million of rent expense for this lease, forrespectively, in research and development expense, on the consolidated statements of operations. During the year ended December 31, 20222023, we recognized a gain of $0.6 million on the disposal of this lease in research and development expense, on the consolidated statement of operations.
11.12. Warrant LiabilityLiabilities
December 2022 Warrants
On December 12, 2022, in connection with the sale of 9,090,909 shares of our common stock to an institutional investor, we entered into a warrant agreement that offersallows such investor to purchase of up to 9,090,909 shares at an exercise price of $6.60 per share. The warrants are accounted for in accordance with ASC 815-40. Such guidance provides that because the warrants do not meet the criteria for equity treatment thereunder, such warrant must be recorded as a liability. The warrant agreement contains a provision that in the event of a “fundamental transaction” (as defined in the warrant agreement), in certain circumstances the holder of warrants may choose to require the company, or its successor, to redeem the warrants for cash at a value based on the Black-Scholes option pricing model obtained from the Bloomberg Financial Markets with inputs as defined in the warrant agreement.
We determined that the defined input in the warrant agreement for the exercise price per share in the Black-Scholes option pricing model will result in a settlement value which may not be considered a fair value, and therefore the warrants do not meet the criteria for equity treatment. We classified the warrants as a liability at their fair value determined using the Black-Scholes option pricing model, andmodel. The fair value of the warrants were allocated a portion of the proceeds fromwas estimated at $35.1 million at the issuance ofdate. Of the common stock equal toplacement agent fees and other offering costs totaling $35.13.0 million, on the date of the transaction. The $1.1$1.1 million of transaction costswas allocated to the warrant liability was recognizedwarrants and recorded in other expense, net, on the consolidated statement of operations foron the date of the transaction.
As of December 31, 2023 and 2022, all 9,090,909 underlying warrants were outstanding. As of December 31, 2023 and 2022, the estimated fair value of the warrants were $17.1 million and $21.6 million, respectively. During the year ended December 31, 2022. The warrant liability is subject to remeasurement at each balance sheet date. With each such remeasurement, the warrant liability will be adjusted to fair value, with the change2023, a decrease in fair value recognizedof $4.5 million was recorded in other expense, net, on the consolidated statement of operations.
February 2023 Warrants
On February 15, 2023, in connection with the sale of 14,072,615 shares of our common stock to certain institutional investors, we entered into a warrant agreement that allows such investors to purchase up to 14,072,615 shares at an exercise price of $4.2636 per share.
December 31, 2022, there were 9,090,909 third-party warrants outstanding, withWe classified the warrants as a liability at their fair value determined using the Black-Scholes option pricing model. The fair value of $21.6warrants was estimated at $23.7 million at the issuance date. Of the placement agent fees and other offering costs totaling $3.0 million, $1.0 million was allocated to the warrants and recorded in other expense, net, on the consolidated statement of operations on the date of the transaction.
On July 25, 2023, the company amended the terms of the warrant, reducing the exercise price of the warrants from $4.2636 per share to $3.2946 per share and extending the expiration date of the warrants until July 24, 2026. Since the warrants were classified as liabilities and measured at fair value, we recognized the change of $7.3 million in fair value of warrant liability in earnings due to the warrant modification.
As of December 31, 2023, all 14,072,615 underlying warrants were outstanding, with an estimated fair value of $50.0 million. During the year ended December 31, 2023, an increase in fair value of $26.3 million was recorded in other expense, net, on the consolidated statement of operations.
July 2023 Warrants
On July 20, 2023, in connection with the sale of 14,569,296 shares of our common stock to certain institutional investors, we entered into a warrant agreement that allows such investors to purchase up to 14,569,296 shares at an exercise price of $3.2946 per share.
We classified the warrants as a liability at their fair value determined using the Black-Scholes option pricing model. The fair value of warrants was estimated at $25.8 million at the issuance date. Of the placement agent fees and other offering costs totaling $2.5 million, $1.0 million was allocated to the warrants and recorded in other expense, net, on the consolidated statement of operations on the date of the transaction.
As of December 31, 2023, all 14,569,296 underlying warrants were outstanding, with an estimated fair value of $51.7 million. During the year ended December 31, 2023, an increase in fair value of $25.9 million was recorded in other expense, net, on the consolidated statement of operations.
12.13. Stockholders’ Deficit
Stock Authorized for Issuance
As of December 31, 2022,2023, the company was authorized to issue up to 900,000,0001,350,000,000 shares of its common stock, par value $0.0001 per share, and 20,000,000 shares of our preferred stock, par value $0.0001 per share. As of December 31, 2022,2023, there were 421,569,115670,867,344 shares of our common stock outstanding (excluding 163,800 shares held by a majority owned subsidiary of the company that are treated as treasury shares for accounting purposes).
Effective February 1, 2022, ImmunityBioOctober 18, 2023, we amended and restated itsour Amended and Restated Certificate of Incorporation to increase the number of shares of common stock that the company is authorized to issue from 500,000,000900,000,000 shares of common stock, $0.0001 par value per share, to 900,000,0001,350,000,000 shares of common stock, $0.0001 par value per share. The number of shares of preferred stock, $0.0001 par value per share, that the company is authorized to issue remainsremained unchanged at 20,000,000 shares.
Common Stock Issued in Connection with the Merger
Under the terms of the Merger Agreement, at the Effective Time of the Merger, each share of NantCell common stock, par value $0.001 per share, issued and outstanding immediately prior to the Effective Time, subject to certain exceptions as set forth in the Merger Agreement, was converted automatically into a right to receive 0.8190 newly issued shares of common stock, par value $0.0001 per share, resulting in the issuance of approximately 273.7 million shares of Company Common Stock. From and after the Effective Time, all of such NantCell shares ceased to be outstanding, were canceled and ceased to exist. At the Effective Time, each share of our common stock issued and outstanding immediately prior to the Effective Time, remained an issued and outstanding share of the combined company.
Since the Merger was accounted for as a transaction between entities under common control, the outstanding shares presented on the consolidated financial statements assume that NantCell outstanding common stock was converted into shares of Company Common Stock forduring the periods endingperiod ended December 31, 2021, and 2020, and in connection with the conversion, those shares of common stock were recorded at the company’s par value of $0.0001 per share.
Stock Repurchases
2015 Share Repurchase Program
In 2015, the Board of Directors approved the 2015 Share Repurchase Program, which allows our CEO or chief financial officer (CFO),CFO, to repurchase on behalf of the company, from time to time in the open market or in privately negotiated transactions, up to $50.0 million of our outstanding shares of common stock, exclusive of any commissions, markups, or expenses. The timing and amounts of any purchases were and will continue to be based on market conditions and other factors, including price, regulatory requirements, and other corporate considerations. The 2015 Share Repurchase Program does not require the purchase of any minimum number of shares and may be suspended, modified, or discontinued at any time without prior notice. We have financed, and expect to continue to finance, the purchases with existing cash balances. Shares repurchased under this program are formally retired through approval of the Board of Directors upon repurchase.
NoDuring the years ended December 31, 2023, 2022 and 2021, no shares of our common stock were repurchased during the years ended December 31, 2022, 2021 and 2020 under the program. Since the plan’s inception, we have repurchased a total of 6,403,489 shares at a total cost of $31.7 million. As of December 31, 2022,2023, $18.3 million remained authorized to use for share repurchases under the program.
Shelf Registration Statement
During February 2023, we filed a $750.0 million shelf registration statement with the SEC on Form S-3 for the offering and sale of equity and equity-linked securities, including common stock, preferred stock, debt securities, depositary shares, warrants to purchase common stock, preferred stock or debt securities, subscription rights, purchase contracts, and units. During the year ended December 31, 2023, we sold shares of our common stock and warrants valued at $184.4 million under the shelf. As of December 31, 2023, we had $565.6 million available for use under the shelf. This available shelf is in addition to the Open Market Sale Agreement described below.
Open Market Sale Agreement
OnIn April 30, 2021, we entered into an open market sale agreement (the Sale Agreement) with respect to anthe ATM offering program under which we may offer and sell, from time to time at our sole discretion, shares of our common stock having an initial aggregate offering price of up to $500.0 million through our sales agent, which was subsequently reduced by $92.0 million during December 2022 in connection with a sale of our common stock described below. See “—Other Sales of Common Stock.”agent. We pay our sales agent a commission of up to 3.0% of the gross sales proceeds of any shares of our common stock sold through them under the Sale Agreement,ATM, and also have provided them with customary indemnification and contribution rights. As of December 31, 2022, we had $225.4 million available for future stock issuances under
During the ATM.
For the yearyears ended December 31, 2023, 2022 we received net proceeds totaling $13.1 million from the issuance of 2,051,894 shares under the ATM. We currently intend to use the net proceeds from this offering, together with other available funds, to progress our pre-commercialization efforts and clinical development programs, fund other research and development activities, for capital expenditures, and for other general corporate purposes. We may also use a portion of the net proceeds to license intellectual property or to make acquisitions or investments.
During the year ended December 31, 2021, we received net proceeds totalingof approximately $16.1 million, $13.1 million, and $164.5 million, respectively, from the issuance of 13,295,817 shares under the ATM, which were usedATM. As of December 31, 2023, we had $208.8 million available for general corporate purposes, including to progress our clinical development programs, fund other research and development activities, make capital expenditures and fund working capital.future issuances under the ATM.
We are not obligated to sell any shares and may at any time suspend solicitation and offers under the Sale Agreement. The Sale Agreement may be terminated by us at any time given written notice to the sales agent for any reason or by the sales agent at any time by giving written notice to us for any reason or immediately under certain circumstances and shall automatically terminate upon the issuance and sale of all of the shares.
Registered Direct Offerings
2023 Offerings
February 2023
On February 15, 2023, we entered into a securities purchase agreement with certain institutional investors for the purchase and sale of 14,072,615 shares of our common stock, as well as warrants that allow such investors to purchase an additional 14,072,615 shares of common stock at an exercise price of $4.2636 per share, for a purchase price of $3.5530 per share and accompanying warrant. This transaction generated net proceeds of approximately $47.0 million, after deducting placement agent fees and other offering costs of $3.0 million, of which $2.0 million was allocated to the sale of our common stock and recorded in additional paid-in capital, on the consolidated statement of stockholders’ deficit during the year ended December 31, 2023. The warrants became immediately exercisable on February 17, 2023.
On July 25, 2023, pursuant to the terms of a letter amendment, the company and the investors agreed to reduce the exercise price of the outstanding February 2023 Warrants from $4.2636 per share to $3.2946 per share and extend the expiration date of the warrants to July 24, 2026. See Note 12, Warrant Liabilities,for more information. July 2023
On July 20, 2023, we entered into a securities purchase agreement with certain institutional investors for the purchase and sale of 14,569,296 shares of our common stock, as well as warrants that allow such investors to purchase an additional 14,569,296 shares of common stock at an exercise price of $3.2946 per share, for a purchase price of $2.7455 per share and accompanying warrant. This transaction generated net proceeds of approximately $37.5 million, after deducting placement agent fees and other estimated offering costs of $2.5 million, of which $12.7 million was allocated to the sale of our common stock and recorded in additional paid-in capital, on the consolidated statement of stockholders’ deficit during the year ended December 31, 2023. The warrants became immediately exercisable on July 25, 2023 and expire on July 24, 2026.
2022 Offering
On December 12, 2022, we entered into a securities purchase agreement with an institutional investor for the sale of 9,090,909 shares of our common stock, as well as warrants that allow such investor to purchase an additional 9,090,909 shares of common stock at an exercise price of $6.60 per share, for a purchase price of $5.50 per share and accompanying warrant, generatingwarrant. This transaction generated net proceeds of approximately $47.0 million, after deducting placement agent fees and other offering costs of $3.0 million, of which $1.9 million was allocated to the sale of our common stock and recognized asrecorded in additional-paid-in capital, on the consolidated statement of stockholders’ deficit forduring the year ended December 31, 2022.
Stock Purchase and Option Agreement
On December 29, 2023 and in connection with the RIPA, we entered into an SPOA with Oberland pursuant to which we sold an aggregate of approximately $10.0 million of our common stock at $4.1103 per share in a private placement, which resulted in the issuance of 2,432,894 shares of common stock. We currently intend to use thereceived net proceeds from thisof approximately $9.5 million, after deducting offering together with other available funds,costs of $0.5 million, which were allocated to progressthe sale of our pre-commercialization effortscommon stock and clinical development programs, fund other research and development activities, forrecorded in additional-paid-in capital, expenditures, and for other general corporate purposes. See Note 11, Warrant Liability, for further information. Underwritten Public Offering
During on the consolidated statement of stockholders’ deficit during the year ended December 31, 2020, we closed2023.
Oberland also has an underwritten public offeringoption to purchase up to an additional $10.0 million of our common stock, at a price per share to be determined by reference to the 30-day trailing volume weighted-average price of our common stock, calculated from the date of exercise. The option is exercisable by Oberland any time after the closing of the SPOA, until the earliest of
(i) December 29, 2028, (ii) a change of control of the company, or (iii) a sale of substantially all of the company’s assets. Among other limitations, the option may only be exercised to the extent that the common stock issuable pursuant to such exercise would not exceed 19.9% of the common stock outstanding immediately after giving effect to such exercise. We have agreed to file a shelf registration statement allowing for the resale of the common stock acquired under the SPOA and to cause the registration statement to be effective no later than May 15, 2024, or if the SEC decides to review such registration statement, no later than June 30, 2024.
Conversion of Promissory Notes into Common Stock
On September 11, 2023, the company entered into a stock purchase agreement with Nant Capital, NantMobile and NCSC pursuant to which the related-party purchasers exchanged all such fixed-rate promissory notes, representing approximately $270.0 million in aggregate principal amount and accrued and unpaid interest, in exchange for an aggregate of 8,521,500209,291,936 shares of common stock which included 4,811,500 shares issued to the public at aan exchange price of $9.50$1.29 per share (including 1,111,500 shares sold to the public upon full exercise of the underwriters’ option to purchase additional shares at a public offering price of $9.50 per share), less underwriting discounts and commissions, and 3,710,000 shares issued to Dr. Soon-Shiong, our Executive Chairman and Global Chief Scientific and Medical Officer, at a price of $12.12 per share, less underwriting discounts and commissions. All of the shares were offered by the company. Including the underwriters’ option exercise, the aggregate gross proceeds from the offering were $90.7 million, before deducting underwriting discounts, commissions and other offering costs of $4.4 million.
Conversion of Fixed-Rate Promissory Note into Common Stockshare.
On December 12, 2022, the company received written notice from NantWorks, the holder of the Existing Note,existing note, of its election to convert the entire outstanding principal and accrued interest under the Existing Noteexisting note into shares of the company’s common stock. As of such date, the entire outstanding principal amount and accrued and unpaid interest due under the Existing Noteexisting note of approximately $56.6 million and an unamortized debt discount of $4.7 million were converted into 9,986,920 shares of the company’s common stock at a price of $5.67 per share in accordance with the terms of the Existing Note. We recorded a net increase of $51.9 million in additional paid-in capital, on the consolidated balance sheet related to this transaction.
13.14. Stock-Based Compensation
2015 Equity Incentive Plan
In 2015, the Board of Directors adopted, and our stockholders approved, the 2015 Plan. The 2015 Plan, as amended, permits the grant of incentive stock options to the company’s employees, and the grant of non-statutory stock options, restricted stock, RSUs, stock appreciation rights, performance units and performance shares to the company’s employees, directors, and consultants. In addition, the number of shares reserved for future grant under the 2015 Plan include shares subject to stock options granted under the 2014 Plan that expire or terminate without having been exercised in full and shares issued pursuant to awards granted under the 2014 Plan that are forfeited to or repurchased by us (provided that the maximum number of shares that may be added to the 2015 Plan pursuant to this provision is approximately 503,493346,840 shares as of December 31, 2022)2023). Pursuant to the Merger, we assumed 7,121,110 RSUs (adjusted for the Exchange Ratio of 0.8190)Ratio) issued under the NC 2015 NC Plan.
As of December 31, 2022,2023, the 2015 Plan is the only equity plan available for grant of equity awards to employees, directors, and consultants of the company. At the company’s 2022 Annual Meeting of Stockholders held on June 14, 2022, stockholders approved an amendment to increase the number of shares of common stock authorized for issuance under the 2015 Plan by 19,900,000 shares. As of December 31, 2022,2023, approximately 18.413.1 million shares were available for future grants under the 2015 Plan.
Stock-Based Compensation
The following table presents stock-based compensation included on the consolidated statements of operations (in thousands):
| | Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| | Stock-based compensation expense: | Stock-based compensation expense: | |
| Stock-based compensation expense: | |
| Stock-based compensation expense: | |
| Stock options | |
| Stock options | |
| Stock options | Stock options | $ | 13,280 | | | $ | 11,623 | | | $ | 1,426 | |
| RSUs | RSUs | 26,899 | | | 45,558 | | | 761 | |
| $ | 40,179 | | | $ | 57,181 | | | $ | 2,187 | |
| $ | |
| Stock-based compensation expense in operating expenses: | Stock-based compensation expense in operating expenses: | | | | | |
| Research and development | Research and development | $ | 11,669 | | | $ | 18,819 | | | $ | 261 | |
| Research and development | |
| Research and development | |
| Selling, general and administrative | Selling, general and administrative | 28,510 | | | 38,362 | | | 1,926 | |
| $ | 40,179 | | | $ | 57,181 | | | $ | 2,187 | |
| $ | |
Stock Options
The following table summarizes stock option activity and related information for the year ended December 31, 2022:2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Number of Options | | Weighted- Average Exercise Price | | Aggregate Intrinsic Value (in thousands) | | Weighted- Average Remaining Contractual Life (in years) |
| | | | | | | |
| Outstanding at December 31, 2021 | 4,124,930 | | | $ | 15.62 | | | $ | 4,178 | | | 5.3 |
| Granted | 5,736,256 | | | $ | 5.33 | | | | | |
| Exercised | (14,767) | | | $ | 5.07 | | | | | |
| Expired/forfeited | (583,493) | | | $ | 5.91 | | | | | |
| Outstanding at December 31, 2022 | 9,262,926 | | | $ | 9.87 | | | $ | 4,848 | | | 7.2 |
| Vested and exercisable at December 31, 2022 | 3,445,499 | | | $ | 14.75 | | | $ | 2,742 | | | 3.8 |
On March 23, 2022, the Compensation Committee of the Board of Directors granted option awards to purchase a total of 4,728,634 shares of our common stock pursuant to the 2015 Plan at an exercise price of $5.83 per share, the closing price reported on the Nasdaq on the date of grant.
Of the option awards granted, 3,903,634 shares subject to such option awards were awarded to employees of the company (of which 825,000 options were awarded to the company’s named executive officers (NEOs)). The shares subject to the option shall vest in equal annual installments of 1/3rd on each of the first, second and third anniversaries of March 23, 2022 (the “vesting commencement date”), such that all shares shall be fully vested on the third anniversary of the vesting commencement date, subject to the recipient continuing to be a “service provider” as defined in the 2015 Plan through each applicable vesting date.
The remaining 825,000 shares subject to such option awards were awarded to the company’s NEOs with a performance condition. Subject to the company’s attainment of a financial goal for the fiscal year ending December 31, 2022, 1/3rd of the shares subject to the option shall vest in equal annual installments on each of the first, second and third anniversaries of the vesting commencement date, such that all shares shall be fully vested on the third anniversary of the vesting commencement date, subject to the recipient continuing to be a “service provider” through each applicable vesting date.
| | | | | | | | | | | | | | | | | | | | | | | |
| | Number of Options | | Weighted- Average Exercise Price | | Aggregate Intrinsic Value (in thousands) | | Weighted- Average Remaining Contractual Life (in years) |
| | | | | | | |
| Outstanding as of December 31, 2022 | 9,262,926 | | | $ | 9.87 | | | $ | 4,848 | | | 7.2 |
| Granted | 949,578 | | | $ | 2.99 | | | | | |
| Exercised | (184,362) | | | $ | 1.59 | | | | | |
| Expired/forfeited | (207,707) | | | $ | 5.53 | | | | | |
| Outstanding as of December 31, 2023 | 9,820,435 | | | $ | 9.46 | | | $ | 6,046 | | | 6.6 |
| Vested and exercisable as of December 31, 2023 | 5,867,252 | | | $ | 11.54 | | | $ | 4,118 | | | 5.4 |
As of December 31, 2022,2023, the unrecognized compensation cost related to outstanding stock options was $20.4$8.4 million, which is expected to be recognized over a remaining weighted-average period of 1.81.0 years.
TheDuring the year ended December 31, 2023, the total intrinsic value of stock options exercised duringwas $0.3 million. During the yearyears ended December 31, 2023, 2022 was immaterial. Cashand 2021, cash proceeds received from stock option exercises during the year ended December 31, 2022 waswere $0.3 million, $0.1 million.million, and $5.4 million, respectively.
As of December 31, 2021,2022, a total of 3,038,3223,445,499 vested and exercisable stock options were outstanding.
The fair value of stock options issued was estimated at the date of grant using the Black-Scholes option pricing model with the following weighted-average assumptions:
| | Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| | Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| | Expected term | |
| Expected term | |
| Expected term | Expected term | 5.69 years | | 5.90 years | | 5.50 years | 5.50 years | | 5.69 years | | 5.90 years |
| Risk-free interest rate | Risk-free interest rate | 2.6 | % | | 0.7 | % | | 0.4 | % | Risk-free interest rate | 4.0 | % | | 2.6 | % | | 0.7 | % |
| Expected volatility | Expected volatility | 101.8 | % | | 101.0 | % | | 96.8 | % | Expected volatility | 116.2 | % | | 101.8 | % | | 101.0 | % |
| Dividend yield | Dividend yield | 0.0 | % | | 0.0 | % | | 0.0 | % | Dividend yield | — | % | | — | % | | — | % |
| Weighted-average grant date fair value | Weighted-average grant date fair value | $ | 4.20 | | | $ | 16.80 | | | $ | 4.64 | | Weighted-average grant date fair value | $2.53 | | $4.20 | | $16.80 |
The expected term was estimated using the average of the contractual term and the weighted-average vesting term of the options. The risk-free interest rate was based on the U.S. Treasury’s rates for U.S. Treasury zero-coupon bonds with maturities similar to those of the expected term of the award being valued. The expected volatility was estimated based on the historical volatility of our common stock. The assumed dividend yield was based on our expectation of not paying dividends for the foreseeable future.
Restricted Stock Units
The following table summarizes RSU activity during the year ended December 31, 2022:2023:
| | Number of Units | | | Number of Units | | Weighted- Average Grant Date Fair Value |
| | Number of Units | | Weighted- Average Grant Date Fair Value |
| | Nonvested balance at December 31, 2021 | 6,515,889 | | | $ | 21.88 | |
| Nonvested balance as of December 31, 2022 | |
| Nonvested balance as of December 31, 2022 | |
| Nonvested balance as of December 31, 2022 | |
| Granted | Granted | 1,772,562 | | | $ | 4.29 | |
| Vested | Vested | (521,296) | | | $ | 17.32 | |
| Forfeited/canceled | Forfeited/canceled | (1,215,767) | | | $ | 17.60 | |
| Nonvested balance at December 31, 2022 | 6,551,388 | | | $ | 18.27 | |
| Nonvested balance as of December 31, 2023 | |
As of December 31, 2022,2023, there was $70.1$38.2 million of unrecognized stock-based compensation expense related to RSUs that is expected to be recognized over a weighted-average period of 2.72.2 years. TheDuring the year ended December 31, 2023, the total intrinsic value of RSUs vested duringwas $11.1 million.
Effective as of August 25, 2023, the year ended December 31, 2022 was $2.6 million.
We may grantCompensation Committee of the Board of Directors granted 5,727,159 RSUs to botheligible employees and directors of the company, and towith the intention of retaining such employees of related parties that provide shared services(the “retention award”). The retention award vests according to the company under our shared services agreement with NantWorks as discussedfollowing schedule: ½ (one-half) was to vest and vested on September 1, 2023 and ½ (one-half) will vest on January 31, 2024, subject to the recipients continuing to be a “service provider” (as defined in Note 10, Related-Party Agreements. Thethe 2015 Plan) through each applicable vesting date. With respect to RSUs granted, compensation cost was measured using the grant date fair value of an RSU equals$1.65 per share, the closing price of ourthe company’s common stock on the date of grant.
RSUs awarded to employees and consultants of affiliated companies are accounted for as stock-based compensation in accordance with FASB ASU 2018-07, Compensation—Stock Compensation (Topic 718), as the compensation was in exchange for continued support or services expected to be provided to the company over the vesting periods under the NantWorks shared services agreement discussed in Note10 11, Related-Party Agreements. We have evaluated the associated benefit of these awards to the affiliated companies under common control and determined that the benefit is limited to the retention of their employees. We estimated such benefit at the grant date fair value of $4.0 millionmillion. During the years ended December 31, 2023 and 2022, we recorded $0.4 million, respectively, and $0.9 million of deemed dividends forduring the yearsyear ended December 31, 2022 and 2021 in additional paid-in capital, on the consolidated balance sheets, with a corresponding credit to stock-based compensation expense. Related-Party Warrants
In connection with the Merger, warrants issued to NantWorks, a related party, in connection with NantCell’s acquisition of Altor were assumed by the company. After applying the Exchange Ratio at the Effective Time of the Merger, a total of 1,638,000 warrants with an exercise price of $3.24 per share were outstanding as of December 31, 2022.2023. The fair value of $18.0 million assigned to the warrants will be recognized in equity upon achievement of a performance-based vesting condition pertaining to building manufacturing capacity to support supply requirements for one of our product candidates.
14.
15. Income Taxes
We are subject to U.S. federal income tax, as well as income tax in Italy, South Korea, California, and other states. From inception through December 31, 2022,2023, we have not been required to pay U.S. federal and state income taxes because of current and accumulated net operating losses (NOLs).NOLs. Our federal returns for tax years 20192020 through 20212022 remain open to examination, and our state returns remain subject to examination for tax years 20182019 through 2021.2022. The Italian and South KoreaKorean returns for tax years 20172018 through 20212022 remain open to examination.
Carryforward attributes that were generated in years where the statute of limitations is closed may still be adjusted upon examination by the Internal Revenue Service (IRS)IRS or other respective tax authorities. No income tax returns are currently under examination by taxing authorities. There are no cumulative earnings in our Italian and South Korean subsidiaries as of December 31, 20222023 that would be subject to U.S. income tax or foreign withholding tax. We plan to indefinitely reinvest any future earnings of our foreign subsidiaries.
On March 9, 2021, the company completed the Merger with NantCell. The Merger is accounted for as a transaction between entities under common control, and is considered a nontaxable transaction for U.S. income tax purposes, as it is intended to qualify as a “reorganization” within(within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended (the Code).
Our loss before income taxes is as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| | | | | |
| U.S. loss before income taxes | $ | (413,653) | | | $ | (347,226) | | | $ | (223,519) | |
| Foreign loss before income taxes | (3,633) | | | (2,613) | | | (2,514) | |
| Loss before income taxes | $ | (417,286) | | | $ | (349,839) | | | $ | (226,033) | |
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
| | | | | |
| U.S. loss before income taxes | $ | (581,136) | | | $ | (413,653) | | | $ | (347,226) | |
| Foreign loss before income taxes | (2,756) | | | (3,633) | | | (2,613) | |
| Loss before income taxes | $ | (583,892) | | | $ | (417,286) | | | $ | (349,839) | |
Income tax benefit (expense) benefit consists of the following (in thousands):
| | Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| | Current: | Current: | |
| Current: | |
| Current: | |
| Federal | |
| Federal | |
| Federal | Federal | $ | — | | | $ | — | | | $ | — | |
| State | State | (38) | | | (9) | | | (5) | |
| Foreign | Foreign | — | | | — | | | — | |
| Total current | Total current | (38) | | | (9) | | | (5) | |
| Deferred: | Deferred: | | | | | |
| Federal | Federal | 2 | | | — | | | 1,187 | |
| Federal | |
| Federal | |
| State | State | 2 | | | — | | | 664 | |
| Foreign | Foreign | — | | | — | | | — | |
| Total deferred | Total deferred | 4 | | | — | | | 1,851 | |
| Total income tax (expense) benefit | $ | (34) | | | $ | (9) | | | $ | 1,846 | |
| Total income tax benefit (expense) | |
The components that comprise our net deferred tax liabilityliabilities consist of the following (in thousands):
| | | | | | | | | | | |
| As of December 31, |
| 2022 | | 2021 |
| | | |
| Deferred tax assets: | | | |
| Net operating loss carryforwards | $ | 362,360 | | | $ | 314,612 | |
| Section 174 R&E capitalization | 50,571 | | | — | |
| Research and development credits | 40,954 | | | 17,716 | |
| Stock-based compensation | 20,927 | | | 23,116 | |
| Interest expense | 19,974 | | | 8,531 | |
| Operating lease liabilities | 12,986 | | | 10,316 | |
| Investments | 5,886 | | | 3,227 | |
| Amortization | 3,969 | | | 4,407 | |
| Accrued compensation | 3,398 | | | 2,525 | |
| Other accrued liabilities | 1,640 | | | 905 | |
| Other | 698 | | | 2,055 | |
| Total deferred tax assets | 523,363 | | | 387,410 | |
| Deferred tax liabilities: | | | |
| Debt discount | (16,527) | | | — | |
| Operating lease right-of-use assets | (11,747) | | | (9,345) | |
| Depreciation | (3,300) | | | (2,905) | |
| Indefinite-lived intangible assets | (192) | | | (162) | |
| Total deferred tax liabilities | (31,766) | | | (12,412) | |
| Net deferred tax assets | 491,597 | | | 374,998 | |
| Valuation allowance | (491,755) | | | (375,160) | |
| Net deferred tax liability | $ | (158) | | | $ | (162) | |
| | | | | | | | | | | |
| As of December 31, |
| 2023 | | 2022 |
| | | |
| Deferred tax assets: | | | |
| Net operating loss carryforwards | $ | 420,782 | | | $ | 362,360 | |
| Section 174 R&E capitalization | 87,721 | | | 50,571 | |
| Research and development credits | 56,610 | | | 40,954 | |
| Interest expense | 39,859 | | | 19,974 | |
| Stock-based compensation | 22,554 | | | 20,927 | |
| Capital loss | 16,192 | | | — | |
| Operating lease liabilities | 11,605 | | | 12,986 | |
| Valuation discount | 9,095 | | | — | |
| Investments | 7,544 | | | 5,886 | |
| Amortization | 3,715 | | | 3,969 | |
| Accrued compensation | 3,262 | | | 3,398 | |
| Other accrued liabilities | 2,944 | | | 1,640 | |
| Other | 839 | | | 698 | |
| Total deferred tax assets | 682,722 | | | 523,363 | |
| | | |
| Deferred tax liabilities: | | | |
| Debt discount | (23,365) | | | (16,527) | |
| Operating lease right-of-use assets | (9,380) | | | (11,747) | |
| Depreciation | (1,533) | | | (3,300) | |
| Indefinite-lived intangible assets | (148) | | | (192) | |
| Total deferred tax liabilities | (34,426) | | | (31,766) | |
| Net deferred tax assets | 648,296 | | | 491,597 | |
| Valuation allowance | (648,444) | | | (491,755) | |
| Net deferred tax liabilities | $ | (148) | | | $ | (158) | |
As of December 31, 2022,2023, we have federal net operating losses (NOLs)NOLs of $1.4$1.6 billion, state NOLs of $1.5$2.0 billion, and foreign NOLs of $10.1$14.3 million. Of the $1.4$1.6 billion in federal NOLs, $967.3 million$1.2 billion do not expire and will be able to be used to offset 80% of taxable income in future years. Of the $1.5$2.0 billion in state NOLs, $49.7$50.7 million do not expire and will be able to be used to offset 80% of taxable income in future years. The remaining federal NOL carryforwards expire beginning in 2023,2024, the remaining state NOL carryforwards expire beginning in 2023,2024, the South Korean NOL carryforwards expire beginning in 20232024, and the Italian NOLs do not expire.
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some or all of our deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. Based on the level of historical operating results and the uncertainty of economic conditions, we have recorded a valuation allowance of $491.8$648.4 million and $375.2$491.8 million as of December 31, 2023 and 2022, and 2021, respectively. The change in the valuation allowance forDuring the years ended December 31, 2023 and 2022, and 2021 were increases of $116.6the valuation allowance increased by $156.6 million and $113.7$116.6 million, respectively, which werewas mainly driven by losses from which we cannot benefit. The portion of the valuation allowance for deferred tax assets for which subsequently recognized tax benefits will be credited directly to contributed capital is $0.2 million.
A reconciliation of the federal statutory income tax rate to our effective income tax rate is as follows:
| | Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| | Federal statutory tax rate | Federal statutory tax rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| Federal statutory tax rate | |
| Federal statutory tax rate | | 21.0 | % | | 21.0 | % | | 21.0 | % |
| State income taxes, net of federal tax benefit | State income taxes, net of federal tax benefit | 9.5 | % | | 7.0 | % | | 7.2 | % | State income taxes, net of federal tax benefit | 6.8 | % | | 9.5 | % | | 7.0 | % |
| Change in fair value of warrants | | Change in fair value of warrants | (1.8) | % | | 0.5 | % | | — | % |
| Change in fair value of convertible notes | | Change in fair value of convertible notes | (1.3) | % | | — | % | | — | % |
| Investment loss | | Investment loss | 2.1 | % | | — | % | | — | % |
| Other permanent items | Other permanent items | 0.4 | % | | (0.1) | % | | (0.1) | % | Other permanent items | — | % | | (0.1) | % | | (0.1) | % |
| Tax rate adjustment | Tax rate adjustment | (0.4) | % | | 1.5 | % | | (0.3) | % | Tax rate adjustment | 1.4 | % | | (0.4) | % | | 1.5 | % |
| Research and development credits | Research and development credits | 3.6 | % | | 3.7 | % | | 0.1 | % | Research and development credits | 3.1 | % | | 3.6 | % | | 3.7 | % |
| Stock-based compensation | Stock-based compensation | (0.5) | % | | 0.5 | % | | 1.3 | % | Stock-based compensation | (1.0) | % | | (0.5) | % | | 0.5 | % |
| Section 162(m) limitation | Section 162(m) limitation | (2.1) | % | | — | % | | — | % | Section 162(m) limitation | (0.4) | % | | (2.1) | % | | — | % |
| Other | Other | 1.5 | % | | (0.9) | % | | (0.2) | % | Other | 0.3 | % | | 1.5 | % | | (0.9) | % |
| Valuation allowance | Valuation allowance | (33.0) | % | | (32.7) | % | | (28.2) | % | Valuation allowance | (30.2) | % | | (33.0) | % | | (32.7) | % |
| Effective income tax rate | Effective income tax rate | — | % | | — | % | | 0.8 | % | Effective income tax rate | — | % | | — | % | | — | % |
Pursuant to Code Sections 382 and 383 of the Code, annual use of our net operating loss and research and development credit carryforwards may be limited in the event a cumulative change in ownership of more than 50% occurs within a three-year period. We have not recognized the deferred tax assets for federal and state NOLs and credits of $274.2$274.1 million from our deferred tax asset schedules as of December 31, 20222023 due to Section 382/383 limitations. There is no impact to tax expense for the derecognition of net operating losses, and federal and state research and development credits due to the valuation allowance recorded against our deferred tax assets.
As of December 31, 2022,2023, we also had federal research tax credit carryforwards of $36.0$47.7 million and state research tax credits of $22.5$29.6 million. The federal research tax credit carryforwards expire beginning in 2032 and certain state research tax credit carryforwards expire beginning in 2030. Our California research tax credits can be carried forward indefinitely. As of December 31, 2023, we also had federal other tax credits carryforwards of $1.3 million and the tax credit carryforwards expire beginning in 2036
Net operating losses and tax credits also are limited when there is a separate return limitation year (SRLY).SRLY. These rules generally limit the use of the acquired or departing members’ net operating loss and tax credit carryovers to the amount of taxable income such entity contributes to consolidated taxable income. The 80% limitation also applies to SRLY NOL carryovers and tax credits. Therefore, any SRLY NOLs and tax credits will be subject to this limitation, as well as Section 382 and 383 limitations.
As of December 31, 20222023 and 2021,2022, we have $77.6$164.6 million and $33.1$77.6 million of interest, respectively, that is temporarily disallowed pursuant to IRC Section 163(j). of the Code. This interest can be carried forward indefinitely and will be deductible when the company generates sufficient adjusted taxable income.
A summary of changes to the amount of unrecognized tax benefits is as follows (in thousands):
| | Year Ended December 31, | |
| Year Ended December 31, | |
| Year Ended December 31, | |
| 2023 | |
| 2023 | |
| 2023 | |
| | Year Ended December 31, | |
| Unrecognized tax benefits, beginning of year | |
| | 2022 | | 2021 | | 2020 | |
| Unrecognized tax benefits, beginning of year | |
| | Unrecognized tax benefits, beginning of year | Unrecognized tax benefits, beginning of year | $ | 13,504 | | | $ | 20,413 | | | $ | 15,656 | | |
| Additions based on tax positions related to the current year | Additions based on tax positions related to the current year | 1,710 | | | 536 | | | 4,763 | | |
| Additions based on tax positions related to the current year | |
| Additions based on tax positions related to the current year | |
| | Additions based on tax positions related to prior years | |
| | Additions based on tax positions related to prior years | |
| | Additions based on tax positions related to prior years | Additions based on tax positions related to prior years | 1,038 | | | — | | | — | | |
| Reductions for tax positions of prior years | Reductions for tax positions of prior years | — | | | (7,445) | | | (6) | | |
| Reductions for tax positions of prior years | |
| Reductions for tax positions of prior years | |
| | Unrecognized tax benefits, end of year | Unrecognized tax benefits, end of year | $ | 16,252 | | | $ | 13,504 | | | $ | 20,413 | | |
| | Unrecognized tax benefits, end of year | |
| | Unrecognized tax benefits, end of year | |
Included in the balance of unrecognized tax benefits as of December 31, 20222023 is $13.8$15.1 million that, if recognized, would not impact our income tax benefit or effective tax rate as long as the deferred tax asset remains subject to a full valuation allowance. We do not expect that the unrecognized tax benefits will change within 12 months of December 31, 2022.2023. Due to the existence of the valuation allowance, future changes in our unrecognized tax benefits will not impact our effective tax rate. We have not incurred any material interest or penalties as of the current reporting date with respect to income tax matters.
Inflation Reduction Act of 2022
The Inflation Reduction Act 2022, which incorporates a Corporate Alternative Minimum Tax (CAMT),corporate alternative minimum tax, was signed on August 16, 2022. The changes are effective for the tax years beginning after December 31, 2022. The new tax will require companies to compute two separate calculations for federal income tax purposes and pay the greater of the new minimum tax or their regular tax liability. The company will be monitoring the impact of the act to determine if it will have an impact on the company for years beginning after December 31, 2022. We currently do not expect this act will have a material effect on our consolidated financial statements.
Coronavirus Aid, Relief and Economic Security Act
On March 27, 2020, the U.S. enacted the Coronavirus Aid, Relief and Economic Security Act (the CARES Act). The Cares Act is an emergency economic stimulus package that includes spending and tax breaks to strengthen the U.S. economy and fund a nationwide effort to curtail the effect of COVID-19. The CARES Act provides sweeping tax changes in response to the COVID-19 pandemic. Under the CARES Act, some of the more significant provisions are NOL carrybacks for five years to offset previous years’ income, or can be carried forward indefinitely to offset 100% of taxable income for the tax year beginning before 2021 and 80% of taxable income for tax years 2021 and thereafter, increasing the ability to deduct interest expense, as well as amending certain provisions of the previously enacted Tax Cuts and Jobs Act. During the years ended December 31, 2022, 2021, and 2020, we did not record any income tax (expense) benefit resulting from the CARES Act, mainly due to our history of net operating losses generated and the maintenance of a full valuation allowance against our net deferred tax assets. There was no material impact from the provisions of the Cares Act for the years ended December 31, 2022 and 2021.
State of California Assembly Bill No. 85
On June 29, 2020, the state of California enacted Assembly Bill No. 85 (AB 85) suspending California NOL utilization and imposing a cap on the amount of business incentive tax credits companies can utilize, effective for tax years 2020, 2021 and 2022. There was no material impact from the provisions of AB 85 for the years ended December 31, 2022, 2021 and 2020. On February 9, 2022, Senate Bill No. 113 was enacted that removed the limitations on the use of NOLs and the cap on the business incentive tax credits that were suspended in accordance with AB 85 effective for tax year 2022.
15.16. Employee Benefits
Defined Contribution Benefit Plan
In December 2015, we adopted a 401(k) retirement and savings plan (the 401(k) Plan)Plan covering all employees. The 401(k) Plan allows employees to make pre- and post-tax contributions up to the maximum allowable amount set by the IRS. The company, at its discretion, may make certain contributions to the 401(k) Plan. We made contributions totaling $2.7 million, $1.7 million and $1.1 million to the 401(k) Plan forDuring the years ended December 31, 2023, 2022 and 2021, we made contributions totaling $2.8 million, $2.7 million, and 2020, respectively.$1.7 million, respectively, to the 401(k) Plan.
Compensated Absences
Under our vacation policy, salaried employees are provided unlimited vacation leave. Therefore, we do not record an accrual for paid leave related to these employees since we are unable to reasonably estimate the compensated absences that these employees will take.
16. Subsequent Event
Registered Direct Offering
On February 15, 2023, we entered into a securities purchase agreement with certain institutional investors for the sale of 14,072,615 shares of our common stock, as well as warrants to purchase an additional 14,072,615 shares of common stock at an exercise price of $4.2636 per share, for a purchase price of $3.5530 per share and accompanying warrant, generating net proceeds of approximately $47.0 million, after deducting placement agent fees and other estimated offering costs. The warrants are immediately exercisable after the issuance date and expire two years after the initial issuance date. The closing of the offering occurred on February 17, 2023. We currently intend to use the net proceeds from this offering, together with other available funds, to progress our pre-commercialization efforts and clinical development programs, fund other research and development activities, for capital expenditures, and for other general corporate purposes. We may also use a portion of the net proceeds to license intellectual property or to make acquisitions or investments.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
Our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives of ensuring that information we are required to disclose in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our chief executive officer (CEO)CEO and chief financial officer (CFO),CFO, as appropriate, to allow timely decisions regarding required disclosures, and is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. There is no assurance that our disclosure controls and procedures will operate effectively under all circumstances.
Management, with the participation of our CEO and CFO, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2022.2023. The term “disclosure controls and procedures,” asprocedures” (as defined in Rule 13a-15(e) of the Exchange ActAct) means controls and other procedures of a company that are designed to provide reasonable assurance that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to provide reasonable assurance that information required to be disclosed is accumulated and communicated to our management, including our CEO and CFO, as appropriate, to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their desired control objectives, and management necessarily is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2022,2023, our CEO and CFO have concluded that, as of December 31, 2022,2023, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as such term is defined in Rule 13a-15(f) under the Exchange Act. Internal control over financial reporting is a process designed under the supervision and with the participation of our management, including our CEO and CFO, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; and (ii) provide reasonable assurance (a) that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, (b) that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (c) regarding the prevention or timely detection of the unauthorized acquisition, use or disposition of assets that could have a material effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As of December 31, 2022,2023, our management conducted an evaluation of the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control - Integrated Framework (2013). Based on this evaluation, our management concluded that, as of December 31, 2022,2023, our internal control over financial reporting was effective.
The effectivenessThis Annual Report does not include an attestation report of the company’s internal control over financial reporting has been audited by Ernst & Young LLP, our independent registered public accounting firm as stated in its attestation report appearing on page 147 of this Annual Report under the heading “Report of Independent Registered Public Accounting Firm,” which expresses an unqualified opinion concerning the effectiveness of the company’sregarding internal control over financial reporting asreporting. Management’s report was not subject to attestation by our registered independent public accounting firm pursuant to the rules of December 31, 2022.the SEC that permit us to provide only management’s report in this Annual Report.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) during the fiscal quarter ended December 31, 2022,2023, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Management recognizes that a control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud or error, if any, have been detected. These inherent limitations include the realities that judgments in decision making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by management override of the controls. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
ITEM 9B. OTHER INFORMATION.
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
The information required by this item will be contained in our definitive proxy statement to be filed with the SEC on Schedule 14A in connection with our 2023 Annual Meeting of Stockholders (the Proxy Statement),Statement, which is expected to be filed not later than 120 days after the end of our fiscal year ended December 31, 2022,2023, and is incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION.
The information required by this item will be contained in the Proxy Statement under the headings “Executive Compensation,” “Executive Compensation Tables,” and “Director Compensation,” and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The information required by this item will be contained in the Proxy Statement under the headings “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information,” and is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
The information required by this item will be contained in the Proxy Statement under the headings “Certain Relationships and Related-Party Transactions” and “Corporate Governance,” and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
The information required by this item will be contained in the Proxy Statement under the heading “Fees Paid to Independent Registered Public Accounting Firm” and is incorporated herein by reference.
PART IV
ITEM 15. EXHIBIT AND FINANCIAL STATEMENT SCHEDULES.
The consolidated financial statements, financial statement schedules and exhibits filed as part of this Annual Report are as follows:
(1)Financial Statements
(2)Financial Statement Schedules for the Years Ended December 31, 2023, 2022 2021 and 20202021
(3)Exhibits
The documents listed below are incorporated by reference or filed or furnished with this Annual Report, in each case as indicated therein (numbered in accordance with Item 601 of Regulation S-K).
| Exhibit
Number | Exhibit
Number | | Incorporated by Reference Herein | Exhibit
Number | | | | Incorporated by Reference Herein |
| Description | | Form | | File No. | | Exhibit No. | | Filing Date | | Description | | Form | | File No. | | Exhibit No. | | Filing Date |
| | 2.1† | | | | 8-K | | 001-37507 | | 2.1 | | December 22, 2020 |
| 2.1+ | |
| 2.1+ | |
| 2.1+ | | | | | 8-K | | 001-37507 | | 2.1 | | December 22, 2020 |
| | 3.1 | |
| 3.1 | |
| 3.1 | 3.1 | | | | 8-K | | 001-37507 | | 3.1 | | August 4, 2015 | | | | 8-K | | 001-37507 | | 3.1 | | August 4, 2015 |
| | 3.2 | 3.2 | | | | 8-K | | 001-37507 | | 3.1 | | March 10, 2021 |
| 3.2 | |
| 3.2 | | | | | 8-K | | 001-37507 | | 3.1 | | March 10, 2021 |
| | 3.3 | |
| 3.3 | |
| 3.3 | 3.3 | | | | POSASR | | 333-255699 | | 3.3 | | March 1, 2022 | | | | POSASR | | 333-255699 | | 3.3 | | March 1, 2022 |
| | 3.4 | 3.4 | | | | 10-Q | | 001-37507 | | 3.2 | | August 12, 2021 |
| 3.4 | |
| 3.4 | | | | | 10-Q | | 001-37507 | | 3.4 | | November 9, 2023 |
| | 3.5 | |
| 3.5 | |
| 3.5 | | | | | 10-Q | | 001-37507 | | 3.2 | | August 12, 2021 |
| | 4.1 | |
| 4.1 | |
| 4.1 | 4.1 | | | | S-1 | | 333-205124 | | 4.1 | | June 19, 2015 | | | | S-1 | | 333-205124 | | 4.1 | | June 19, 2015 |
| | 4.2 | 4.2 | | | | S-1 | | 333-205124 | | 4.2 | | June 19, 2015 |
| 4.2 | |
| 4.2 | | | | | S-1 | | 333-205124 | | 4.2 | | June 19, 2015 |
| | 4.3 | 4.3 | | | | S-1 | | 333-205124 | | 4.3 | | June 19, 2015 |
| 4.3 | |
| 4.3 | | | | | S-1 | | 333-205124 | | 4.3 | | June 19, 2015 |
|
| Exhibit
Number | Exhibit
Number | | Incorporated by Reference Herein | Exhibit
Number | | | | Incorporated by Reference Herein |
| Description | | Form | | File No. | | Exhibit No. | | Filing Date | | Description | | Form | | File No. | | Exhibit No. | | Filing Date |
| | 4.4 | 4.4 | | | | S-1 | | 333-205124 | | 4.5 | | June 19, 2015 |
| 4.4 | |
| 4.4 | | | | | S-1 | | 333-205124 | | 4.5 | | June 19, 2015 |
| | 4.5 | |
| 4.5 | |
| 4.5 | 4.5 | | | | S-1 | | 333-205124 | | 4.6 | | June 19, 2015 | | | | S-1 | | 333-205124 | | 4.6 | | June 19, 2015 |
| | 4.6 | 4.6 | | | | S-8 POS | | 333-252232 | | 4.1 | | May 21, 2021 |
| 4.6 | |
| 4.6 | | | | | S-8 POS | | 333-252232 | | 4.1 | | May 21, 2021 |
| | 4.7 | | | | 10-K | | 001-37507 | | 4.7 | | March 1, 2022 |
| 4.7* | |
| 4.7* | |
| 4.7* | |
| | 4.8+ | |
| | 4.8+ | |
| | 4.8+ | | | | | S-4 | | 333-252232 | | 10.13 | | January 19, 2021 |
| | 4.9 | |
| 4.9 | |
| 4.9 | | | | | 8-K | | 001-37507 | | 4.1 | | December 12, 2022 |
| | 4.10 | |
| 4.10 | |
| 4.10 | | | | | 10-Q | | 001-37507 | | 4.1 | | November 9, 2023 |
| | 4.11 | |
| 4.11 | |
| 4.11 | | | | | 8-K | | 001-37507 | | 4.1 | | July 21, 2023 |
| | 10.1 | |
| 10.1 | |
| 10.1 | 10.1 | | | | 8-K | | 001-37507 | | 10.1 | | December 22, 2020 | | | | 8-K | | 001-37507 | | 10.1 | | December 22, 2020 |
| | 10.2 | 10.2 | | | | 8-K | | 001-37507 | | 10.2 | | December 22, 2020 |
| 10.2 | |
| 10.2 | | | | | 8-K | | 001-37507 | | 10.2 | | December 22, 2020 |
| | 10.3+ | | | | S-4 | | 333-252232 | | 10.13 | | January 19, 2021 |
| 10.3+* | |
| 10.3+* | |
| 10.3+* | |
| | 10.4 | | | | 8-K | | 001-37507 | | 4.1 | | December 12, 2022 |
| 10.4+* | |
| | 10.5† | | | | S-4 | | 333-252232 | | 10.30 | | January 19, 2021 |
| 10.4+* | |
| | 10.4+* | |
| | 10.5+* | |
| | 10.5+* | |
| | 10.5+* | |
| | 10.6 | |
| | 10.6 | |
| | 10.6 | 10.6 | | | | 8-K | | 001-37507 | | 10.1 | | December 12, 2022 | | Stock Purchase Agreement, dated September 11, 2023, by and among ImmunityBio, Inc., NantCell, Inc., Nant Capital, LLC, NantMobile, LLC, and NantCancerStemCell, LLC. | | 10-Q | | 001-37507 | | 10.3 | | November 9, 2023 |
| | 10.7 | 10.7 | | | | 8-K | | 001-37507 | | 10.1 | | May 3, 2021 |
| 10.7 | |
| 10.7 | | | | | 8-K | | 001-37507 | | 10.1 | | July 21, 2023 |
| | 10.8† | | | | S-4 | | 333-252232 | | 10.4 | | January 19, 2021 |
| 10.8 | |
| 10.8 | |
| 10.8 | | | | | 8-K | | 001-37507 | | 10.1 | | February 15, 2023 |
| | 10.9 | | | | S-4 | | 333-252232 | | 10.11 | | January 19, 2021 |
| | 10.10 | | | | S-4 | | 333-252232 | | 10.12 | | January 19, 2021 |
|
| Exhibit
Number | Exhibit
Number | | Incorporated by Reference Herein | Exhibit
Number | | | | Incorporated by Reference Herein |
| Description | | Form | | File No. | | Exhibit No. | | Filing Date | | Description | | Form | | File No. | | Exhibit No. | | Filing Date |
| | 10.11† | | | | S-4 | | 333-252232 | | 10.5 | | January 19, 2021 |
| 10.9 | |
| 10.9 | |
| 10.9 | | | | | 8-K | | 001-37507 | | 10.1 | | December 12, 2022 |
| | 10.10 | |
| 10.10 | |
| 10.10 | | | | | 8-K | | 001-37507 | | 10.1 | | May 3, 2021 |
| | 10.11+ | |
| 10.11+ | |
| 10.11+ | | | | | S-4 | | 333-252232 | | 10.4 | | January 19, 2021 |
| | 10.12 | 10.12 | | | | 8-K | | 001-37507 | | 10.2 | | December 12, 2022 |
| 10.12 | |
| 10.12 | | | | | S-4 | | 333-252232 | | 10.12 | | January 19, 2021 |
| | 10.13 | | | | 10-Q | | 001-37507 | | 10.1 | | November 9, 2022 |
| 10.13* | |
| 10.13* | |
| 10.13* | |
| | 10.14 | |
| | 10.14 | |
| | 10.14 | 10.14 | | | | 10-Q | | 001-37507 | | 10.2 | | November 9, 2022 | | | | 10-Q | | 001-37507 | | 10.4 | | November 9, 2023 |
| | 10.15 | 10.15 | | | | 10-Q | | 001-37507 | | 10.3 | | November 9, 2022 |
| 10.15 | |
| 10.15 | | | | | 10-Q | | 001-37507 | | 10.8 | | November 9, 2023 |
| | 10.16 | 10.16 | | | | 10-Q | | 001-37507 | | 10.4 | | November 9, 2022 |
| 10.16 | |
| 10.16 | | | | | 8-K | | 001-37507 | | 10.1 | | March 31, 2023 |
| | 10.17 | | | | 10-Q | | 001-37507 | | 10.5 | | November 9, 2022 |
| 10.17* | |
| 10.17* | |
| 10.17* | |
| | 10.18 | |
| | 10.18 | |
| | 10.18 | 10.18 | | | | 10-Q | | 001-37507 | | 10.6 | | November 9, 2022 | | | | 10-Q | | 001-37507 | | 10.7 | | November 9, 2023 |
| | 10.19 | 10.19 | | | | 10-Q | | 001-37507 | | 10.7 | | November 9, 2022 |
| 10.19 | |
| 10.19 | | | | | 8-K | | 001-37507 | | 10.2 | | December 12, 2022 |
| | 10.20 | |
| 10.20 | |
| 10.20 | 10.20 | | | | 10-Q | | 001-37507 | | 10.8 | | November 9, 2022 | | | | 10-Q | | 001-37507 | | 10.6 | | November 9, 2023 |
| | 10.21 | 10.21 | | | | S-1/A | | 333-205124 | | 10.19 | | July 27, 2015 |
| 10.21 | |
| 10.21 | | | | | 10-Q | | 001-37507 | | 10.1 | | November 9, 2022 |
| | 10.22 | 10.22 | | | | 10-Q | | 001-37507 | | 10.6 | | August 8, 2022 |
| 10.22 | |
| 10.22 | | | | | 10-Q | | 001-37507 | | 10.9 | | November 9, 2023 |
| | 10.23 | | | | 10-Q | | 001-37507 | | 10.7 | | August 8, 2022 |
|
| Exhibit
Number | Exhibit
Number | | Incorporated by Reference Herein | Exhibit
Number | | | | Incorporated by Reference Herein |
| Description | | Form | | File No. | | Exhibit No. | | Filing Date | | Description | | Form | | File No. | | Exhibit No. | | Filing Date |
| | 10.23 | |
| 10.23 | |
| 10.23 | | | | | 8-K | | 001-37507 | | 10.1 | | June 14, 2023 |
| | 10.24 | |
| 10.24 | |
| 10.24 | 10.24 | | | | 10-Q | | 001-37507 | | 10.8 | | August 8, 2022 | | | | 10-Q | | 001-37507 | | 10.5 | | November 9, 2023 |
| | 10.25 | 10.25 | | | | 10-K | | 001-37507 | | 10.23 | | March 30, 2016 |
| 10.25 | |
| 10.25 | | | | | 10-Q | | 001-37507 | | 10.2 | | November 9, 2022 |
| | 10.26 | |
| 10.26 | |
| 10.26 | 10.26 | | | | 10-Q | | 001-37507 | | 10.2 | | November 9, 2020 | | | | 10-Q | | 001-37507 | | 10.3 | | November 9, 2022 |
| | 10.27 | 10.27 | | | | 10-Q | | 001-37507 | | 10.4 | | August 8, 2022 |
| 10.27 | |
| 10.27 | | | | | 10-Q | | 001-37507 | | 10.4 | | November 9, 2022 |
| | 10.28 | 10.28 | | | | 10-Q | | 001-37507 | | 10.1 | | November 10, 2016 |
| 10.28 | |
| 10.28 | | | | | 10-Q | | 001-37507 | | 10.5 | | November 9, 2022 |
| | 10.29+ | | | | S-4/A | | 333-252232 | | 10.27 | | January 19, 2021 |
| 10.29 | |
| 10.29 | |
| 10.29 | | | | | 10-Q | | 001-37507 | | 10.7 | | November 9, 2022 |
| | 10.30+ | | | | S-4/A | | 333-252232 | | 10.28 | | January 19, 2021 |
| 10.30 | |
| 10.30 | |
| 10.30 | | | | | 10-Q | | 001-37507 | | 10.8 | | November 9, 2022 |
| | 10.31+ | | | | S-4/A | | 333-252232 | | 10.29 | | January 19, 2021 |
| 10.31 | |
| 10.31 | |
| 10.31 | | | | | S-1/A | | 333-205124 | | 10.19 | | July 27, 2015 |
| | 10.32 | |
| 10.32 | |
| 10.32 | 10.32 | | | | 10-Q | | 001-37507 | | 10.3 | | November 9, 2020 | | | | 10-Q | | 001-37507 | | 10.6 | | August 8, 2022 |
| | 10.33 | 10.33 | | | | 10-K | | 001-37507 | | 10.35 | | March 4, 2021 |
| 10.33 | |
| 10.33 | | | | | 10-Q | | 001-37507 | | 10.7 | | August 8, 2022 |
| | 10.34 | |
| 10.34 | |
| 10.34 | 10.34 | | | | 10-Q | | 001-37507 | | 10.1 | | August 12, 2021 | | | | 10-Q | | 001-37507 | | 10.8 | | August 8, 2022 |
| | 10.35 | 10.35 | | | | 10-Q | | 001-37507 | | 10.2 | | November 12, 2021 |
| 10.35 | |
| 10.35 | | | | | 10-K | | 001-37507 | | 10.23 | | March 30, 2016 |
| | 10.36 | | | | 10-Q | | 001-37507 | | 10.3 | | November 12, 2021 |
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | | | Incorporated by Reference Herein |
| Description | | Form | | File No. | | Exhibit No. | | Filing Date |
| | | | | | | | | | |
| 10.37 | | | | 10-Q | | 001-37507 | | 10.9 | | August 8, 2022 |
| | | | | | | | | | |
| 10.38 | | | | 10-Q | | 001-37507 | | 10.4 | | November 12, 2021 |
| | | | | | | | | | |
| 10.39 | | | | 10-Q | | 001-37507 | | 10.2 | | May 10, 2022 |
| | | | | | | | | | |
| 10.40 | | | | 10-Q | | 001-37507 | | 10.3 | | May 10, 2022 |
| | | | | | | | | | |
| 10.41 | | | | 10-Q | | 001-37507 | | 10.5 | | August 8, 2022 |
| | | | | | | | | | |
| 10.42† | | | | 8-K | | 001-37507 | | 10.1 | | January 12, 2022 |
| | | | | | | | | | |
| 10.43+ | | | | 10-Q | | 001-37507 | | 10.4 | | August 6, 2019 |
| | | | | | | | | | |
| 10.44 | | | | 10-Q | | 001-37507 | | 10.1 | | May 11, 2020 |
| | | | | | | | | | |
| 10.45 | | | | 10-Q | | 001-37507 | | 10.1 | | August 15, 2016 |
| | | | | | | | | | |
| 10.46#^ | | | | 10-Q | | 001-37507 | | 10.4 | | November 9, 2020 |
| | | | | | | | | | |
| 10.47#^ | | | | S-4 | | 333-252232 | | 10.31 | | January 19, 2021 |
| | | | | | | | | | |
| 10.48 | | | | S-1 | | 333-205124 | | 10.1 | | June 19, 2015 |
| | | | | | | | | | |
| 10.49# | | | | S-1/A | | 333-205124 | | 10.4 | | July 15, 2015 |
| | | | | | | | | | |
| 10.50# | | | | S-1 | | 333-205124 | | 10.2 | | June 19, 2015 |
| | | | | | | | | | |
| 10.51# | | | | S-8 | | 333-265599 | | 10.1 | | June 14, 2022 |
| | | | | | | | | | |
| 10.52# | | | | S-4 | | 333-252332 | | 10.14 | | January 19, 2021 |
| | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | | | Incorporated by Reference Herein |
| Description | | Form | | File No. | | Exhibit No. | | Filing Date |
| | | | | | | | | | |
| 10.36 | | | | 10-Q | | 001-37507 | | 10.2 | | November 9, 2020 |
| | | | | | | | | | |
| 10.37 | | | | 10-Q | | 001-37507 | | 10.4 | | August 8, 2022 |
| | | | | | | | | | |
| 10.38 | | | | 10-Q | | 001-37507 | | 10.1 | | November 10, 2016 |
| | | | | | | | | | |
| 10.39 | | | | 10-Q | | 001-37507 | | 10.2 | | August 8, 2023 |
| | | | | | | | | | |
| 10.40+ | | | | S-4/A | | 333-252232 | | 10.27 | | January 19, 2021 |
| | | | | | | | | | |
| 10.41* | | | | | | | | | | |
| | | | | | | | | | |
| 10.42+ | | | | S-4/A | | 333-252232 | | 10.28 | | January 19, 2021 |
| | | | | | | | | | |
| 10.43+ | | | | S-4/A | | 333-252232 | | 10.29 | | January 19, 2021 |
| | | | | | | | | | |
| 10.44 | | | | 10-Q | | 001-37507 | | 10.3 | | November 9, 2020 |
| | | | | | | | | | |
| 10.45 | | | | 10-K | | 001-37507 | | 10.35 | | March 4, 2021 |
| | | | | | | | | | |
| 10.46 | | | | 10-Q | | 001-37507 | | 10.1 | | August 12, 2021 |
| | | | | | | | | | |
| 10.47 | | | | 10-Q | | 001-37507 | | 10.2 | | November 12, 2021 |
| | | | | | | | | | |
| 10.48 | | | | 10-Q | | 001-37507 | | 10.3 | | November 12, 2021 |
| | | | | | | | | | |
| 10.49 | | | | 10-Q | | 001-37507 | | 10.9 | | August 8, 2022 |
| | | | | | | | | | |
| 10.50 | | | | 10-Q | | 001-37507 | | 10.4 | | November 12, 2021 |
| | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | | | Incorporated by Reference Herein |
| Description | | Form | | File No. | | Exhibit No. | | Filing Date |
| | | | | | | | | | |
| 10.53 | | | | S-4 | | 333-252332 | | 10.25 | | January 19, 2021 |
| | | | | | | | | | |
| 10.54 | | | | S-4 | | 333-252332 | | 10.26 | | January 19, 2021 |
| | | | | | | | | | |
| 10.55 | | | | 8-K | | 001-37507 | | 99.1 | | June 10, 2019 |
| | | | | | | | | | |
| 10.56 | | | | 8-K | | 001-37507 | | 99.2 | | June 10, 2019 |
| | | | | | | | | | |
| 21.1* | | | | | | | | | | |
| | | | | | | | | | |
| 23.1* | | | | | | | | | | |
| | | | | | | | | | |
| 24.1* | | | | | | | | | | |
| | | | | | | | | | |
| 31.1* | | | | | | | | | | |
| | | | | | | | | | |
| 31.2* | | | | | | | | | | |
| | | | | | | | | | |
| 32.1** | | | | | | | | | | |
| | | | | | | | | | |
| 32.2** | | | | | | | | | | |
| | | | | | | | | | |
| 101.INS | | Inline XBRL Instance Document (the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document). | | | | | | | | |
| | | | | | | | | | |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document. | | | | | | | | |
| | | | | | | | | | |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document. | | | | | | | | |
| | | | | | | | | | |
| 101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document. | | | | | | | | |
| | | | | | | | | | |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document. | | | | | | | | |
| | | | | | | | | | |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document. | | | | | | | | |
| | | | | | | | | | |
| 104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | | | Incorporated by Reference Herein |
| Description | | Form | | File No. | | Exhibit No. | | Filing Date |
| | | | | | | | | | |
| 10.51 | | | | 10-Q | | 001-37507 | | 10.2 | | May 10, 2022 |
| | | | | | | | | | |
| 10.52 | | | | 10-Q | | 001-37507 | | 10.3 | | May 10, 2022 |
| | | | | | | | | | |
| 10.53 | | | | 10-Q | | 001-37507 | | 10.5 | | August 8, 2022 |
| | | | | | | | | | |
| 10.54 | | | | 10-Q | | 001-37507 | | 10.2 | | November 9, 2023 |
| | | | | | | | | | |
| 10.55+ | | | | 8-K | | 001-37507 | | 10.1 | | January 12, 2022 |
| | | | | | | | | | |
| 10.56+ | | | | 10-Q | | 001-37507 | | 10.4 | | August 6, 2019 |
| | | | | | | | | | |
| 10.57 | | | | 10-Q | | 001-37507 | | 10.1 | | May 11, 2020 |
| | | | | | | | | | |
| 10.58 | | | | 10-Q | | 001-37507 | | 10.1 | | August 15, 2016 |
| | | | | | | | | | |
| 10.59#+ | | | | 10-Q | | 001-37507 | | 10.4 | | November 9, 2020 |
| | | | | | | | | | |
| 10.60#+ | | | | S-4 | | 333-252232 | | 10.31 | | January 19, 2021 |
| | | | | | | | | | |
| 10.61 | | | | S-1 | | 333-205124 | | 10.1 | | June 19, 2015 |
| | | | | | | | | | |
| 10.62# | | | | S-1/A | | 333-205124 | | 10.4 | | July 15, 2015 |
| | | | | | | | | | |
| 10.63# | | | | S-1 | | 333-205124 | | 10.2 | | June 19, 2015 |
| | | | | | | | | | |
| 10.64# | | | | S-8 | | 333-265599 | | 10.1 | | June 14, 2022 |
| | | | | | | | | | |
| 10.65# | | | | S-4 | | 333-252332 | | 10.14 | | January 19, 2021 |
| | | | | | | | | | |
| 21.1* | | | | | | | | | | |
| | | | | | | | | | |
| 23.1* | | | | | | | | | | |
| | | | | | | | | | |
| 24.1* | | Power of Attorney (Contained in Signature Page to this Annual Report). | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | | | Incorporated by Reference Herein |
| Description | | Form | | File No. | | Exhibit No. | | Filing Date |
| | | | | | | | | | |
| 31.1* | | | | | | | | | | |
| | | | | | | | | | |
| 31.2* | | | | | | | | | | |
| | | | | | | | | | |
| 32.1** | | | | | | | | | | |
| | | | | | | | | | |
| 32.2** | | | | | | | | | | |
| | | | | | | | | | |
| 97.1#+* | | | | | | | | | | |
| | | | | | | | | | |
| 101.INS | | Inline XBRL Instance Document (the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document). | | | | | | | | |
| | | | | | | | | | |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document. | | | | | | | | |
| | | | | | | | | | |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document. | | | | | | | | |
| | | | | | | | | | |
| 101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document. | | | | | | | | |
| | | | | | | | | | |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document. | | | | | | | | |
| | | | | | | | | | |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document. | | | | | | | | |
| | | | | | | | | | |
| 104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). | | | | | | | | |
_______________
* Filed herewith.
** The certifications attached as Exhibits 32.1 and 32.2 that accompany this Annual Report are deemed furnished and not filed with the SEC and are not to be incorporated by reference into any filing of ImmunityBio, Inc. under the Securities Act or the Exchange Act, whether made before or after the date of this Annual Report, irrespective of any general incorporation language contained in such filing.
# Indicates a management contract or compensatory plan.
†+ Schedules and exhibits haveSome information has been omittedredacted pursuant to Item 601(b)(2) of601of Regulation S-K. The company agrees to furnish to the SEC a copy of any omitted schedule or exhibitredacted information upon request.
+Schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The company agrees to furnish to the SEC a copy of any omitted schedule or exhibit upon request.
# Indicates a management contract or compensatory plan.
^ Portions of this exhibit have been redacted in compliance with Regulation S-K Item 601(a)(6).
ITEM 16. FORM 10-K SUMMARY.
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Act of 1934, as amended, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| IMMUNITYBIO, INC. |
| Registrant |
| | |
Date: March 1, 202319, 2024 | By: | /s/ Richard Adcock |
| | Richard Adcock |
| | Chief Executive Officer |
| | (Principal Executive Officer) |
| | |
Date: March 1, 202319, 2024 | By: | /s/ David C. Sachs |
| | David C. Sachs |
| | Chief Financial Officer |
| | (Principal Financial Officer) |
| | |
Date: March 1, 202319, 2024 | By: | /s/ Regan J. Lauer |
| | Regan J. Lauer |
| | Chief Accounting Officer |
| | (Principal Accounting Officer) |
POWER OF ATTORNEY
Each person whose signature appears below constitutes and appoints Richard Adcock, David C. Sachs and Jason Liljestrom, and each of them, as his or her true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report, on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitutes, may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Signature | | Title | | Date |
| | | | |
| /s/ Patrick Soon-Shiong | | Global Chief Scientific and Medical Officer, and
Executive Chairman of the Board of Directors | | March 1, 202319, 2024 |
| Patrick Soon-Shiong | | | | |
| | | | |
| /s/ Richard Adcock | | Chief Executive Officer, President and Director (Principal Executive Officer) | | March 1, 202319, 2024 |
| Richard Adcock | | | | |
| | | | |
| /s/ David C. Sachs | | Chief Financial Officer (Principal Financial Officer) | | March 1, 202319, 2024 |
| David C. Sachs | | | | |
| | | | |
| /s/ Regan J. Lauer | | Chief Accounting Officer (Principal Accounting Officer) | | March 1, 202319, 2024 |
| Regan J. Lauer | | | | |
| | | | |
| /s/ Barry J. Simon | | Chief Corporate Affairs Officer and Director | | March 1, 202319, 2024 |
| Barry J. Simon | | | | |
| | | | |
/s/ Michael D. Blaszyk | | Director | | March 1, 202319, 2024 |
| Michael D. Blaszyk | | | | |
| | | | |
| /s/ John Owen Brennan | | Director | | March 1, 202319, 2024 |
| John Owen Brennan | | | | |
| | | | |
| /s/ Wesley Clark | | Director | | March 1, 202319, 2024 |
| Wesley Clark | | | | |
| | | | |
| /s/ Cheryl L. Cohen | | Lead Independent Director | | March 1, 202319, 2024 |
| Cheryl L. Cohen | | | | |
| | | | |
| /s/ Linda Maxwell | | Director | | March 1, 202319, 2024 |
| Linda Maxwell | | | | |
| | | | |
| /s/ Christobel Selecky | | Director | | March 1, 202319, 2024 |
| Christobel Selecky | | | | |