UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON,Washington, D.C. 20549
FORM 10-K
|
|
| |
| |
|
|
| |
☒Annual Report under Section 13 or 15(d) of the Securities Exchange Act of 1934
For the fiscal year ended December 31, 2023
or
☐Transitional Report under Section 13 or 15(d) of the Securities Exchange Act of 1934
Commission File Number 333-184948001-39531
Heatwurx,Processa Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | ||
| 45-1539785 | |
(State or other jurisdiction of
| (IRS Employer Identification No.) |
530 S Lake Avenue #615, Pasadena, CA 911017380 Coca Cola Drive, Suite 106,
(Address of principal executive offices and Zip Code)Hanover, Maryland21076
(443) 776-3133
(626) 364-5342
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Exchange Act:None
| Title of Each Class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, $0.0001 par value per share | PCSA | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark whetherif the registrant is a well-known seasoned issuer, as defined byin Rule 405 of the Securities Act. YES [ ] NO [X]Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act YES [ ] NO [X]Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES [ ] NO [X]Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES [ ] NO [X]Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [X]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company, or emerging growth company. See the definitionsdefinition of “large accelerated filer,” “accelerated filer,” and “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated filer | ☐ | Smaller reporting company | ☒ |
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. [ ]☐
Indicate by check mark whether the registrant has filed a report on and attestation to its managements’ assessment of the effectiveness of its internal controls over financial reporting under Section 404(b) of the Sarbanes Oxley Act (15 U.S.C 7262(b)) by the registered public accounting firm that prepared or issued its audit report ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES [ ] NO [X]Yes ☐ No ☒
The aggregate market value of the voting and non-voting common unitsequity held by non-affiliates of the registrant on June 30, 20162023, the last business day of the most recently completed second quarter, based upon the closing price of Common Stock on such date as reported on Nasdaq Capital Market, was approximately $1,447,232. As$10.2 million.
The number of September 10, 2017, the numberoutstanding shares of the registrant’s common shares outstandingstock as of March 21, 2024 was 11,017,388..
DOCUMENTS INCORPORATED BY REFERENCE:REFERENCE
None.
Heatwurx, Inc.
Portions of the Proxy Statement for the registrant’s 2024 Annual Meeting of Stockholders (the “Proxy Statement”) to be filed within 120 days of the end of the fiscal year ended December 31, 2023 are incorporated by reference into Part III hereof. Except with respect to information specifically incorporated by reference in this Form 10-K, the Proxy Statement is not deemed to be filed as a part hereof.
Table of Contents
| 2 |
GLOSSARY OF CERTAIN SCIENTIFIC TERMS
The medical and scientific terms used in this Annual Report on Form 10-K have the following meanings:
“Active metabolite” means a drug that is processed by the body into an altered form which effects the body.
“Agonist” means a chemical/drug that binds to a receptor in the body and activates that receptor to produce a biological response.
“Analog” means a compound having a structure similar to that of an approved drug but differing from it with respect to a certain component of the molecule which may cause it to have similar or different effects on the body.
“cGCP” means current Good Clinical Practices. The FDA and other regulatory agencies promulgate regulations and standards, commonly referred to as current Good Clinical Practices, for designing, conducting, monitoring, auditing and reporting the results of clinical trials to ensure that the data and results are accurate and that the rights and welfare of trial participants are adequately protected.
“cGMP” means current Good Manufacturing Practices. The FDA and other regulatory agencies promulgate regulations and standards, commonly referred to as current Good Manufacturing Practices, which include requirements relating to quality control and quality assurance, as well as the corresponding maintenance of records and documentation.
“CMO” means Contract Manufacturing Organization.
“CRO” means Contract Research Organization.
“Deuterated analog” means a small molecule in which one or more of the hydrogen atoms are replaced by deuterium.
“EMA” means the European Medicines Agency.
“FDA” means the Food and Drug Administration.
“IND” means an Investigational New Drug Application. Before testing a new drug on human subjects, the company must file an IND with the FDA. Information must be produced on the absorption, distribution, metabolism, and excretion properties of the drug and detailed protocols for testing on human subjects must be submitted.
“Indication” means a condition which makes a particular treatment or procedure advisable.
“Moiety” means an active or functional part of a molecule.
“NDA” means a New Drug Application submitted to the FDA. Under the Food, Drug, and Cosmetic Act of 1938, an NDA is submitted to the FDA enumerating the uses of the drug and providing evidence of its safety.
“NGC” means Next Generation Chemotherapy, referring to the drugs in our pipeline that have active cancer killing metabolites that are the same or have very similar chemical structure to existing FDA-approved chemotherapy treatments, resulting in our NGCs killing cancer cells following the same mechanism as the FDA-approved treatments.
“NL” means Necrobiosis Lipoidica, a rare chronic and granulomatous disorder.
| 3 |
CAUTIONARY
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This reportAnnual Report on Form 10-K contains forward lookingforward-looking statements that involve risks and uncertainties, principally in the sections entitled “Description of Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”uncertainties. All statements other than statements of historical factfacts contained in this report, including statements regarding future events, our future financial performance, business strategy and plans and objectives of management for future operations,Form 10-K are forward-looking statements. We have attempted toIn some cases, you can identify forward-looking statements by terminology including “anticipates,words such as “anticipate,” “believes,“believe,” “can,“contemplate,” “continue,“continue,” “could,“could,” “estimates,“estimate,” “expects,“expect,” “intends,“intend,” “may,“may,” “plans,“plan,” “potential,“potential,” “predicts,“predict,” or “should,“project,” “seek,” “should,” “target,” “will,” “would,” or the negative of these termswords or other comparable terminology. Although we do not make forward lookingWe have based these forward-looking statements unlesson our current expectations and projections about future events and trends that we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy.may affect our financial condition, results of operations, strategy, short- and long-term business operations and objectives, and financial needs. These forward-looking statements are only predictions and involve known and unknownsubject to a number of risks, uncertainties and other factors,assumptions, including the risks outlined under “Risk Factors” orthose described in “Risk Factors” and elsewhere in this report, which may cause our or our industry’s actual results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.Form 10-K. Moreover, we operate in a very competitive and rapidly changing environment. Newenvironment, and new risks emerge from time to time and ittime. It is not possible for usour management to predict all risk factors,risks, nor can we addressassess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause our actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this Form 10-K may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. AllGiven these uncertainties, you should not place undue reliance on these forward-looking statements. These risks are discussed more fully in the “Risk Factors” section of this Annual Report on Form 10-K and are summarized below under the “Summary Risk Factors” section. These risks include, but are not limited to, the following:
| ● | our ability to obtain funding for our future clinical trials, preclinical activities and our operations; | |
| ● | our ability to obtain and maintain regulatory approval of our product candidates; | |
| ● | our ability to contract with third-party suppliers, manufacturers and other service providers and their ability to perform adequately; | |
| ● | our ability to meet obligations under our license agreements; | |
| ● | the potential market size, opportunity and growth potential for our product candidates, if approved; | |
| ● | our ability to build our own sales and marketing capabilities, or seek collaborative partners, to commercialize our product candidates, if approved; | |
| ● | our ability to retain the continued service of our key professionals and to identify, hire and retain additional qualified professionals; | |
| ● | our ability to advance product candidates into, and successfully complete, clinical trials; | |
| ● | our ability to recruit and enroll suitable patients in our clinical trials; | |
| ● | the initiation, timing, progress and results of clinical trials and pre-clinical studies for our NGC drugs; | |
| ● | the timing or likelihood of the accomplishment of various scientific, clinical, regulatory filings and approvals and other product development objectives; | |
| ● | the pricing and reimbursement of our product candidates, if approved; | |
| ● | the rate and degree of market acceptance of our product candidates by physicians, patients, third-party payors and others in the medical community, if approved; | |
| ● | the implementation of our business model, strategic plans for our business, product candidates and technology; | |
| ● | the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and technology; | |
| ● | developments relating to our competitors and our industry; | |
| ● | the accuracy of our estimates regarding expenses, capital requirements and needs for additional financing; | |
| ● | our financial performance; and | |
| ● | other risks and uncertainties, including those described under part I, Item 1A. Risk Factors of this Annual Report. |
You should not rely upon forward-looking statements includedas predictions of future events. Although we believe that the expectations reflected in this documentthe forward-looking statements are based on information available to us on the date hereof, and we assume no obligation to update any such forward-looking statements.
Undue reliance should not be placed on any forward-looking statement, each of which applies onlyreasonable as of the date of this report. Before investing in our securities, investors should be awareForm 10-K, we cannot guarantee that the occurrencefuture results, levels of theactivity, performance or events describedand circumstances reflected in the section entitled “Risk Factors” and elsewhere in this report could negatively affect our business, operating results, financial condition and stock price. Except as required by law, weforward-looking statements will be achieved or occur. We undertake no obligation to update or revise publicly any of the forward-looking statements for any reason after the date of this reportForm 10-K to conform ourthese statements to new information, actual results or changed expectations.to changes in our expectations, except as required by law.
PART I
You should read this Form 10-K and the documents that we reference in this Form 10-K and have filed with the SEC as exhibits with the understanding that our actual future results, levels of activity, performance, and events and circumstances may be materially different from what we expect.
ITEM
In this Form 10-K, “we,” “us”, “our”, “Processa” and “the Company” refer to Processa Pharmaceuticals, Inc. and its subsidiary.
| 4 |
SUMMARY RISK FACTORS
We are providing the following summary of the risk factors contained in our Form 10-K to enhance the readability and accessibility of our risk factor disclosures. We encourage our stockholders to carefully review the full risk factors contained in this Form 10-K in their entirety for additional information regarding the risks and uncertainties that could cause our actual results to vary materially from our recent results or from our anticipated future results.
Risks Related to Our Financial Position and Need for Additional Capital
| ● | We have a history of losses and we may never become profitable. | |
| ● | We have limited cash resources and will require additional financing. | |
| ● | Our financial statements contain a statement regarding a substantial doubt about our ability to continue as a going concern. | |
| ● | Our ability to use our net operating loss carryforwards and other tax attributes may be limited. |
Risks Relating to Clinical Development and Commercialization of Our Product Candidates
| ● | We currently do not have, and may never develop, any FDA-approved, licensed or commercialized products. | |
| ● | Our licenses are subject to termination by the licensor in certain circumstances. | |
| ● | If we fail to comply with our obligations contained in the agreements under which we license intellectual property rights from third parties, we could lose important license rights. | |
| ● | We depend entirely on the successful development of our product candidates, which have not yet demonstrated efficacy for their target indications in clinical trials. We may never be able to demonstrate efficacy for our product candidates, thus preventing us from licensing, obtaining marketing approval by any regulatory agency, and/or commercializing our product(s). | |
| ● | We must successfully complete clinical trials for our product candidates before we can apply for marketing approval. | |
| ● | We have little corporate history of conducting clinical trials. Our planned clinical trials or those of our collaborators may reveal significant adverse events, toxicities or other side effects not seen in our preclinical studies and may result in a safety profile that could inhibit regulatory approval or market acceptance of any of our product candidates. | |
| ● | Even if we receive regulatory approval for any of our product candidates, we may not be able to successfully license or commercialize the product and the revenue that we generate from its sales, if any, may be limited. | |
| ● | We are completely dependent on third parties to manufacture our product candidates, and our commercialization of our product candidates could be halted, delayed or made less profitable if those third parties fail to obtain manufacturing approval from the FDA or comparable foreign regulatory authorities, fail to provide us with sufficient quantities of our product candidates or fail to do so at acceptable quality levels or prices. | |
| ● | Even if we obtain marketing approval for any of our product candidates, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expenses. | |
| ● | Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions. | |
| ● | Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain. | |
| ● | We could face competition from other biotechnology and pharmaceutical companies, and our operating results would suffer if we fail to innovate and compete effectively. | |
| ● | We rely on third parties to conduct clinical trials for our product candidates. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize any of our product candidates and our business would be substantially harmed. | |
| ● | Clinical drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results. | |
| ● | Even though we may apply for orphan drug designation for a product candidate, we may not be able to obtain orphan drug marketing exclusivity. | |
| ● | Although we may pursue expedited regulatory approval pathways for a product candidate, it may not qualify for expedited development or, if it does qualify for expedited development, it may not actually lead to a faster development, regulatory review or approval process. | |
| ● | Third-party coverage and reimbursement, health care cost containment initiatives and treatment guidelines may constrain our future revenues. | |
| ● | Legal, regulatory and legislative changes with respect to reimbursement, pricing and contracting may adversely affect our business and future prospects. | |
| ● | We may face product liability exposure, and if successful claims are brought against us, we may incur substantial liability if our insurance coverage for those claims is inadequate. | |
| ● | If any of our product candidates are approved for marketing and we are found to have improperly promoted off-label uses, or if physicians misuse our products or use our products off-label, we may become subject to prohibitions on the sale or marketing of our products, product liability claims and significant fines, penalties and sanctions, and our brand and reputation could be harmed. | |
| ● | We may choose not to continue developing or commercializing any of our product candidates at any time during development or after approval, which would reduce or eliminate our potential return on investment for those product candidates. |
Risks Relating to Our Intellectual Property Rights
| ● | We depend on rights to certain pharmaceutical compounds that are or will be licensed to us. We do not own the intellectual property rights to these pharmaceutical compounds and any loss of our rights to them could prevent us from selling our products. | |
| ● | We cannot ensure protection of our licensed intellectual property rights. | |
| ● | Our product candidates may infringe the intellectual property rights of others, which could increase our costs and delay or prevent our development and commercialization efforts. | |
| ● | Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements. |
General Company-Related Risks
| ● | We will need to grow the size of our organization, and we may experience difficulties in managing this growth. | |
| ● | If we lose key management personnel, or if we fail to recruit additional highly skilled personnel, our ability to identify and develop new or next generation product candidates will be impaired, could result in loss of markets or market share and could make us less competitive. | |
| ● | If our information technology systems or data, or those of third parties upon which we rely, are or were compromised, we could experience adverse consequences resulting from such compromise, including but not limited to regulatory investigations or actions, interruptions or disruptions to operations or clinical trials, reputational harm, litigation, fines and penalties. | |
| ● | We are exposed to cyber-attacks and data breaches, including the risks and costs associated with protecting our systems and maintaining integrity and security of our business information, as well as personal data of our guests, employees and business partners. |
Risks Related to Ownership of Our Common Stock
| ● | Our failure to maintain compliance with Nasdaq’s continued listing requirements could result in the delisting of our common stock. | |
| ● | Future equity offerings, license transactions or acquisitions may dilute our existing stockholders’ ownership and/or have other adverse effects on our operations. | |
| ● | Our common stock price is expected to be volatile. | |
| ● | We are a “smaller reporting company,” and the reduced disclosure requirements applicable to us as such may make our common stock less attractive to our stockholders and investors. | |
| ● | We do not currently intend to pay dividends to our stockholders in the foreseeable future, and consequently, your ability to achieve a return on your investment will depend on appreciation in our value. | |
| ● | If securities or industry analysts do not publish research or reports about our business, or if they publish negative evaluations of our stock or negative reports about our business, our stock price and trading volume could decline. | |
| ● | Provisions in our corporate documents and Delaware law could have the effect of delaying, deferring, or preventing a change in control of us, even if that change may be considered beneficial by some of our stockholders. | |
| ● | Provisions in our bylaws provide for indemnification of officers and directors, which could require us to direct funds away from our business and the development of our product candidates. |
| 5 |
Part I
Item 1. Business
Overview
Heatwurx,We are a clinical-stage biopharmaceutical company focused on utilizing our “regulatory science” approach, including the principles associated with FDA’s Project Optimus Oncology initiative and the related FDA Draft Guidance, in the development of Next Generation Chemotherapy (“NGC”) oncology drug products. Our mission is to provide better treatment options than those that presently exist by extending a patient’s survival and/or improving a patient’s quality of life. This is achieved by improving upon FDA-approved, widely used oncology drugs or the cancer-killing metabolites of these drugs by altering how they are metabolized and/or distributed in the body, including how they are distributed to the actual cancer cells.
Our regulatory science approach was conceived in the early 1990s when the founders of Processa and other faculty at the University of Maryland worked with the FDA to develop multiple FDA Guidances. Regulatory science is the science of developing new tools, standards, and approaches to assess the safety, efficacy, quality, and performance of all FDA-regulated products. Over the last 30 years, two of our founders, Dr. David Young and Dr. Sian Bigora, have expanded the original regulatory science concept by including the pre-clinical and clinical studies to justify the benefit-risk assessment required for FDA approval when designing the development programs of new drug products.
Our regulatory science approach defines the scientific information that the FDA requires to determine if the benefit outweighs the risk of a drug in a specific population of patients and at a specific dosage regimen for a specific drug product. The studies are designed to obtain the necessary scientific information to support the regulatory decision.
Recently, the FDA has taken steps to define some of the regulatory science required for the FDA approval of oncology products. Through the FDA’s Project Optimus Oncology Initiative and the related Draft Guidance on determining the “optimal” dosage regimen for an oncology drug, the FDA has chosen to make the development of oncology drugs more science-based than in the past. Since the principles of the FDA’s Project Optimus and the related Draft Guidance have been used by our regulatory science approach in a number of non-oncology drugs, our experience with the principles of Project Optimus differentiates us from other biotechnology companies by focusing us not only on the clinical science, but also on the equally important regulatory process. We believe utilizing our regulatory science approach provides us with three distinct advantages:
| ● | greater efficiencies (e.g., the right trial design and trial readouts); | |
| ● | greater possibility of drug approval by the FDA or other regulatory authorities; and | |
| ● | greater ability to evaluate the benefit-risk of a drug compared to existing therapy, which allows prescribers to provide better treatment options for each patient. |
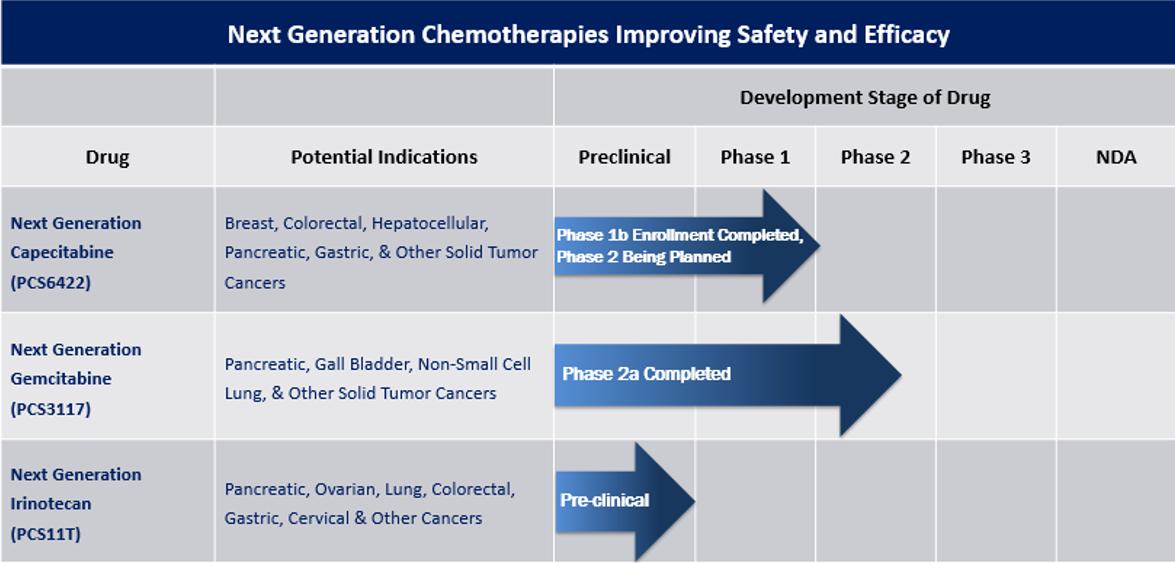
Our strategic prioritization is to advance our pipeline of NGC proprietary small molecule oncology drugs. The NGC products are new chemical entities, but they work by changing the metabolism, distribution and/or elimination of already FDA-approved cancer drugs or their active metabolites while maintaining the mechanism of how the drug kills cancer cells. We believe our NGC treatments will provide improved safety-efficacy profiles when compared to their currently marketed counterparts – capecitabine, gemcitabine, and irinotecan. All future studies of these drugs are subject to availability of capital to conduct the trials.
The three NGC treatments in our pipeline are as follows:
| ● | NGC-Capecitabine (also referred to as NGC-Cap) is a combination of PCS6422 and capecitabine, capecitabine being the oral prodrug of the cancer drug 5-fluorouracil (5-FU). PCS6422, without having any clinically meaningful biological effect itself, alters the metabolism of 5-FU, resulting in more 5-FU distribution to the cancer cells. In clinical trials, NGC-Cap has shown a safety profile different than capecitabine when administered alone. Side effects such as Hand-Foot Syndrome (HFS) and cardiotoxicity typically occur in up to 50-70% of patients treated with capecitabine and are caused by specific capecitabine metabolites. These types of toxicities frequently result in decreased doses, interrupted doses, or discontinuation of treatment with capecitabine. Since a much smaller amount of these metabolites are formed with NGC-Cap, these side effects appear in less patients and are less severe when they do occur. In addition, NGC-Cap has been found to be up to 50 times more potent than capecitabine based on the systemic exposure of the capecitabine metabolite 5-FU, which is metabolized to the cancer-killing metabolites. Like capecitabine, NGC-Cap could potentially be used to treat patients with various cancers, such as metastatic breast, colorectal, gastrointestinal, and pancreatic. On December 11, 2023, we had a successful meeting with the FDA regarding the next Phase 2 study supporting the advancement of NGC-Cap for cancer patients. The meeting with the FDA was supported by the interim results from the ongoing Phase 1B study that was fully enrolled in the first quarter of 2024. Following the meeting with the FDA, we decided the next NGC-Cap trial would be a Phase 2 trial in breast cancer. This decision was supported through discussions with the FDA where we agreed with the FDA that the development of NGC-Cap in breast cancer would be a more efficient development program than metastatic colorectal cancer and improve the likelihood of FDA approval. The FDA has agreed that the data from past and existing studies could be used to directly support the Phase 2 trial in breast cancer. Breast cancer is the most diagnosed cancer, representing approximately 15% of all new cancer patients in 2023. It has a prevalence of more than 3.8 million patients, with nearly 300,000 new diagnoses last year. Over 150,000 women are currently living with advanced or metastatic breast cancer. The NGC-Cap annual newly diagnosed incidence rate for breast, colorectal and other cancers is greater than 250,000 patients per year. |
| 6 |
| ● | PCS3117, also referred to as NGC-Gemcitabine (NGC-Gem), is an oral analog of gemcitabine that is converted to its active metabolite by a different enzyme system than gemcitabine resulting in a positive response in gemcitabine patients as well as some gemcitabine treatment-resistant patients. Like gemcitabine, NGC-Gem could be used to treat patients with various cancers such as pancreatic, biliary tract, lung, ovarian, and breast. We estimate more than 275,000 patients in the United States were newly diagnosed in 2022 with pancreatic, biliary tract, lung, ovarian, and breast cancer. We plan to meet with the FDA to discuss potential study designs including implementation of the Project Optimus initiative as part of the design in 2024. | |
| ● | PCS11T, also referred to as NGC-Irinotecan (NGC-Iri), is a prodrug of the active metabolite of irinotecan (SN-38). The chemical structure of NGC-Iri influences the uptake of the drug into cancer cells, resulting in more NGC-Iri entering cancer cells than normal cells in mice. These levels were significantly greater than those seen with irinotecan, resulting in lower doses of NGC-Iri having greater efficacy than irinotecan and improved safety in animal models. Like irinotecan, NGC-Iri could be used to treat patients with various cancers such as lung, colorectal, gastrointestinal, and pancreatic cancer. We estimate at least 200,000 patients in the United States were newly diagnosed in 2022 with lung, colorectal, gastrointestinal, and pancreatic cancer. We plan to conduct IND-enabling and toxicology studies in 2024-2025. |
We have completed our Phase 2A trial for PCS12852 in gastroparesis patients with positive results. Additionally, in February 2023, due primarily to the inability to identify and enroll patients in our rare disease Phase 2 trial for PCS499 in ulcerative Necrobiosis Lipoidica (uNL), we decided to cease further enrollment in the PCS499 trial and terminated the trial. We did not experience any safety concerns during the conduct of either the PCS12852 or PCS499 trial. We are currently evaluating options to monetize these non-core drug assets, which may include out-licensing or partnering these assets with one or more third parties.
Our shift in prioritization to NGC oncology drugs does not change our mission. We continue to be focused on drug products that improve the survival and/or quality of life for patients by improving the safety and/or efficacy of the drug in a targeted patient population, while providing a more efficient and probable path to FDA approval and differentiating our drugs from those on the market or are currently being developed.
Historically, much of oncology drug development has searched for novel or different ways to treat cancer. Our approach is to take three current FDA-approved cancer drugs and modify and improve how the human body metabolizes and/or distributes these NGC treatments compared to their presently approved counterpart chemotherapy drugs while maintaining the cancer-killing mechanism of action; thus, our reason for calling our drugs Next Generation Chemotherapy (or NGC) treatments. Part of the development includes determining the optimal dosage regimen based on the dose-response relationship as described in the FDA’s Project Optimus Initiative and Draft Optimal Dosage Regimen Oncology Guidance. To date, we have data that we believe suggests our NGC treatments are likely to have a better safety-efficacy profile than the current widely used marketed counterpart drugs, not only potentially making the development and approval process more efficient, but also clearly differentiating our NGC treatments from the existing treatment. We believe our NGC treatments have the potential to extend the survival and/or quality of life for more patients diagnosed with cancer while decreasing the number of patients who are required to dose-adjust or discontinue treatment because of side effects or lack of response.
| 7 |
Our Strategy
Our strategy is to develop our pipeline of NGC proprietary small molecule oncology drugs using our regulatory science approach to determine the optimal dosage regimen of our oncology drugs. By changing either the metabolism, distribution, and/or elimination of already FDA-approved cancer drugs (e.g., capecitabine, gemcitabine, and irinotecan) or their active metabolites, we believe that our three new oncology drugs represent the next generation of chemotherapy with an improved safety profile, improved efficacy profile and/or potentially benefiting more patients while maintaining the mechanism of how the drug kills cancer cells. By combining these modified approved cancer treatments with our regulatory science approach and our experience using the principles of FDA’s Project Optimus initiative, we anticipate that we will be able to increase the probability of FDA approval, improve the safety-efficacy profile over the existing counterparts of our NGC drugs, and more efficiently develop each drug.
Our pipeline of NGCs (i) already has data demonstrating the desired pharmacological activity in humans or appropriate animal models and is able to provide improved safety and/or efficacy by some modification in the formation and/or distribution of the active moieties associated with the drug and (ii) targets cancers for which a single positive pivotal trial demonstrating efficacy might provide enough evidence that the clinical benefits of the drug and its approval outweighs the risks associated with the drug.
Our Team
Our drug development efforts are guided by our knowledge and experience in applying our regulatory science approach to decrease manageable risks, costs, and time toward achieving marketing authorization from regulatory authorities including the FDA. We have assembled a seasoned management team and development team with extensive experience in developing therapies, including advancing product candidates from preclinical research through clinical development and ultimately regulatory approval and commercialization. Our team is led by our President of Research and Development and Founder David Young, Pharm.D., Ph.D. who has extensive experience in research, regulatory approval and business development and who served at Questcor Pharmaceuticals for eight years, initially as an independent director on its Board of Directors and, subsequently, as its Chief Scientific Officer.
To execute our strategy, we assembled an experienced and development team with a successful track record of drug approvals and successful exits. Our team is experienced in developing drug products through all principal regulatory tiers from IND-enabling studies to New Drug Application (NDA) submission. Throughout their careers, the combined scientific, development and regulatory experiences of our team members have resulted in more than 30 drug approvals in indications reviewed by almost every division of the FDA including the oncology divisions, over 100 meetings with the FDA and involvement with more than 50 drug development programs, including drug products targeted to patients who have an unmet medical need and cancer patients. In addition, the FDA Project Optimus Oncology initiative and recent FDA Oncology Guidance applies our regulatory science approach and principles used and refined by our Founders over the last 30 years.
| 8 |
Our Drug Pipeline

Our pipeline currently consists of NGC-Cap, NGC-Gem and NGC-Iri (also identified as PCS6422, PCS3117 and PCS11T, respectively) and two non-oncology drugs (PCS12852 and PCS499). The non-oncology drugs are not included in the pipeline chart above, as we are exploring our options for those drugs, which may include out-licensing or partnership opportunities. A summary of each drug is provided below.
Next Generation Chemotherapy Pipeline
| ● | Next Generation Capecitabine (NGC-Cap) is a combination of PCS6422 and a lower dose of the FDA-approved cancer drug capecitabine. PCS6422 is an orally administered irreversible inhibitor of the enzyme dihydropyrimidine dehydrogenase (DPD). DPD metabolizes 5-Fluorouracil (5-FU), the major metabolite of capecitabine and widely used itself as an intravenous chemotherapeutic agent in many types of cancer, to multiple metabolites classified as catabolites. These catabolites do not have any cancer-killing properties but frequently cause dose-limiting side effects that may require dose adjustments or discontinuation of therapy. |
Capecitabine, as presently prescribed and FDA-approved, forms the cancer drug 5-FU which is then further metabolized to anabolites (which kill both cancer cells and normal duplicating cells) and catabolites (which cause side effects and have no cancer killing properties). When capecitabine is given in combination with PCS6422 in NGC-Cap, PCS6422 significantly changes the metabolism of 5-FU, which results in a change in the distribution of 5-FU within the body. Due to this change in metabolism and the overall metabolite profile of anabolites and catabolites, the side effect and efficacy profile of NGC-Cap has been found to be different from capecitabine given without PCS6422. Since the potency of NGC-Cap is also greater than FDA-approved capecitabine based on the 5-FU systemic exposure per mg of capecitabine administered, the amount of capecitabine anabolites formed from 1 mg of capecitabine administered in NGC-Cap will, therefore, be much greater than formed from the administration of 1 mg of existing capecitabine.
On August 2, 2021, we enrolled the first patient in our Phase 1B dose-escalation maximum tolerated dose trial in patients with advanced refractory gastrointestinal (GI) tract tumors. Our interim analysis of Cohorts 1 and 2A of the ongoing clinical trial found no dose-limiting toxicities (DLTs), no drug-related adverse events greater than Grade 1, and no adverse events associated with the catabolites of 5-FU such as HFS. In this Phase 1B trial, it was demonstrated that the irreversible inhibition of DPD by PCS6422 could alter the metabolism, distribution and elimination of 5-FU, making NGC-Cap significantly (up to 50 times) more potent than capecitabine alone and potentially leading to higher levels of anabolites which can kill replicating cancer and normal cells. By administering NGC-Cap to cancer patients, the balance between anabolites and catabolites changes depending on the dosage regimens of PCS6422 and capecitabine used, making the efficacy-safety profile of NGC-Cap different than that of FDA-approved capecitabine and requiring further evaluation of the PCS6422 and capecitabine regimens to determine the optimal NGC-Cap regimens for patients.
| 9 |
In order for NGC-Cap to provide a safer and more efficacious profile for cancer patients compared to existing chemotherapy, understanding how the different regimens of PCS6422 and capecitabine may affect the systemic and tumor exposure to the anabolites, as well as the systemic exposure to the catabolites, is required. This can be achieved by following the timeline of DPD irreversible inhibition and the formation of new DPD using the plasma concentrations of 5-FU and its catabolites.
In an effort to better estimate the timeline of DPD inhibition and formation of new DPD, we modified the protocol for the Phase 1B trial and began enrolling patients in the amended Phase 1B trial in April 2022. On November 1, 2022, we announced that data from the Phase 1B trial identified multiple dosage regimens with potentially better safety and efficacy profiles than currently existing chemotherapy regimens. Since 5-FU exposure is dependent on both the PCS6422 regimen and the capecitabine regimen, safe regimens were identified as well as regimens that cause DLTs. One of the regimens in the Phase 1B trial did cause DLTs in two patients, one of whom died. The Phase 1B trial is continuing to enroll patients and is expected to complete enrollment in early 2024. The next trial will be a Phase 2 trial to determine which regimens provide an improved efficacy-safety profile over present therapy using the principles of the FDA’s Project Optimus initiative to help guide the design of the trial. This FDA initiative requires us to consider NGC regimens that are not at the maximum tolerated dose or exposure level.
Discussions with the FDA in April 2023 have clarified that the major goal for the next Phase 2 trial will be to evaluate and understand the dose- and exposure-response relationship for anti-tumor activity and safety. The specific dosage regimens for the trial will be defined following the determination of the MTD from our ongoing Phase 1B trial. Cohort 3 in the Phase 1B trial, which dosed patients with PCS6422 in combination with capecitabine at 150 mg BID (twice a day), completed with no dose-limiting toxicities. Enrollment in Cohort 4 was expanded to include six patients to further evaluate the safety at this dose. Enrollment in this cohort is now complete and to date, no DLTs have been observed in this cohort, but safety evaluation for this cohort is still ongoing. Once the cohort and the safety evaluation is complete, the need for any additional cohorts will be further evaluated. Following the FDA meeting on December 11, 2023, we have decided the next NGC-Cap trial would be a Phase 2 trial in breast cancer. This decision was supported through discussions with the FDA where we agreed with the FDA that the development of NGC-Cap in breast cancer would be a more efficient development program than metastatic colorectal cancer and improve the likelihood of FDA approval. The FDA has agreed that the data generated from past and existing studies could be used to directly support the Phase 2 trial in breast cancer. Capecitabine is already approved as both monotherapy and combination therapy in breast cancer, which contributes to the logic and efficiency of our current direction. In addition, the FDA’s agreement that our present data would support a Phase 2 trial in breast cancer makes the expansion seamless. The objective for the Phase 2 trial will be to provide safety-efficacy data to preliminarily demonstrate the benefit of NGC-Cap over capecitabine and other treatment options. Based on this expansion to breast cancer, we expanded our Oncology Advisory Board to include key breast cancer oncologists. We have already determined the Phase 2 study design, which we expect to share with the FDA soon, and plan to use the funding from our January 2024 public offering to begin enrolling patients in the third quarter of 2024.
Our license agreement with Elion Oncology, Inc. (“Elion”) for NGC-Cap requires us to use commercially reasonable efforts, at our sole cost and expense, to research, develop and commercialize products in one or more countries, including meeting specific diligence milestones that include dosing a first patient with a product in a Phase 2 or 3 clinical trial on or before October 2, 2024. We are currently conducting pre-trial activities and planning to dose the first patient in our Phase 2 trial in the third quarter of 2024.
| 10 |
| ● | NGC-Gem is a cytidine analog similar to gemcitabine (Gemzar®), but different enough in chemical structure that some patients are more likely to respond to PCS3117 than gemcitabine. In addition, we believe those patients inherently resistant or who acquire resistance to gemcitabine are likely not to be resistant to NGC-Gem. The difference in response occurs because NGC-Gem is metabolized to its active metabolite through a different enzyme system than gemcitabine. We continue to evaluate the potential use of NGC-Gem in patients with pancreatic and other potential cancers and to evaluate ways to identify patients who are more likely to respond to NGC-Gem than gemcitabine. We plan to meet with the FDA in 2024 to discuss potential trial designs including implementation of the Project Optimus initiative as part of the design. Similar to NGC-Cap, we will need to obtain additional funding before we can begin the Phase 2 trial for NGC-Gem. |
Our license agreement with Ocuphire Pharma, Inc. (“Ocuphire”) for NGC-Gem requires us to use commercially reasonable efforts, at our sole cost and expense to oversee such commercialization efforts, to research, develop and commercialize products in one or more countries, including meeting specific diligence milestones that consist of: (i) dosing a patient in a clinical trial prior to June 16, 2024; and (ii) dosing a patient in a pivotal clinical trial or in a clinical trial for a second indication of the drug prior to June 16, 2026. We are currently in discussions with Ocuphire to extend these deadlines.
| ● | NGC-Iri is an analog of SN38 (SN38 is the active metabolite of irinotecan) and should have an improved safety/efficacy profile in every type of cancer that irinotecan is presently used. The manufacturing process and sites for drug substance and drug product are presently being evaluated and IND-enabling toxicology studies will then be initiated. In addition, we are defining the potential paths to approval, which include defining the targeted patient population and the type of cancer. We plan to conduct IND enabling and toxicology studies in 2024, subject to available funding. |
Non-Oncology Pipeline for Out-licensing or Partnership
| ● | PCS12852 is a highly specific and potent 5HT4 agonist that has already been evaluated in clinical studies in South Korea for gastric emptying and gastrointestinal motility in healthy volunteers and volunteers with a history of constipation. In October 2021, the FDA cleared our IND application to proceed with a Phase 2A trial for the treatment of gastroparesis. We enrolled our first patient on April 5, 2022 and completed enrollment of the trial on September 2, 2022. Results from this Phase 2A trial, which included 25 patients with moderate to severe gastroparesis, demonstrated improvements in gastric emptying in patients receiving 0.5 mg of PCS12852 as compared to placebo. The results indicated that for the patients in the PCS12852 group, the mean time for 50% of the gastric contents to empty (t50) compared to their baseline value (±SD) decreased by -31.90 min (±50.53) (compared to the change seen in the placebo group of only -9.36 min (±42.43). Significant gastric emptying differences were not observed between the placebo and the 0.1 mg dose. Adverse events associated with the administration of PCS12852 were generally mild to moderate as expected, limited in duration, and quickly resolved without any sequelae. There were no cardiovascular safety events or serious adverse events reported during the trial. Additionally, the 0.5 mg of PCS12852 showed a greater improvement than placebo in the gastroparesis symptomology scales used in the trial, including both total scores in the scales, as well as sub-scores such as nausea, vomiting and abdominal pain. With the trial now complete, we have the data necessary to finalize the development plan for the treatment of diabetic gastroparesis patients. We are exploring options for PCS12852, which may include licensing, partnering and/or collaborating opportunities. |
| ● | PCS499 is an oral tablet of the deuterated analog of one of the major metabolites of pentoxifylline (PTX or Trental®). PCS499 is a drug that can be used to treat unmet medical need conditions caused by multiple pathophysiological changes. We completed a Phase 2A trial for PCS499 in patients with ulcerative and non-ulcerative necrobiosis lipoidica (uNL and NL, respectively) in late 2020, and in May 2021, we enrolled the first patient in our Phase 2B trial for the treatment of uNL. Although we initiated several recruitment programs to enroll patients in this trial, we were only able to recruit four patients. We experienced extremely slow enrollment in the trial given the extreme rarity of the condition (rarer than reported in the literature), the impact of COVID-19, and the reluctance of patients to be in a clinical trial. We completed the Phase 2B uNL trial for those currently enrolled but halted further efforts to enroll new patients in the trial and have terminated the trial. There were no safety concerns during the conduct of the trial. Although we believe that PCS499 can be effective in treating uNL, preliminary data indicated that the placebo response was likely to be much greater than the literature and clinical experts believe; thus, a much larger sample size would be required in a pivotal trial for an indication where it was extremely difficult to enroll even four patients. We are exploring options for PCS499 in other indications, which may include licensing, partnering and/or collaborating opportunities. |
| 11 |
Manufacturing and Clinical Supplies
We do not own or operate, and currently have no plans to establish, any manufacturing facilities. We currently rely, and expect to continue to rely, on multiple third-party contract manufacturing organizations (CMOs) for the supply of current Good Manufacturing Practices (cGMP)-grade clinical trial materials and commercial quantities of our product candidates and products, if approved. We require all our CMOs to conduct manufacturing activities in compliance with cGMP. We have assembled a team of experienced employees and consultants to provide the necessary technical, quality and regulatory oversight of our CMOs.
We anticipate that these CMOs will have the capacity to support both clinical supply and commercial-scale production, but we do not have any formal agreements at this time with any of these CMOs to cover commercial production. We also may elect to pursue additional CMOs for manufacturing supplies of drug substance and finished drug product in the future. We believe that our standardized manufacturing process can be transferred to a number of other CMOs for the production of clinical and commercial supplies of our product candidates in the ordinary course of business.
Competition
Many of our potential competitors may have significantly greater financial resources, a more established presence in the market, and more expertise in research and development, manufacturing, pre-clinical and clinical testing, obtaining regulatory approvals and reimbursement, and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These potential competitors may also compete with us in recruiting and retaining top qualified scientific, sales, marketing and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
The key competitive factors affecting each of our products, if approved, are likely to include the efficacy, safety, convenience and price of the products relative to other approved products used on- or off-label for each unmet medical need condition. Although preliminary clinical data exists to support the possibility of improved efficacy and safety profiles for our drugs, more in-depth randomized, controlled studies are required for our products to determine if our preliminary findings will support the approval in the designated unmet medical need indication.
For NGC-Cap, the competitive factors will be related to the efficacy and safety of the product when compared to capecitabine. The market penetration will depend on how much improvement will occur in the efficacy and/or safety profiles when administered in combination with PCS6422. Currently, there are no other reversible or irreversible enzyme inhibitor products approved in the US and no irreversible enzyme inhibitors approved ex-US, which may make PCS6422 the first DPD irreversible inhibitor available.
For NGC-Gem, the competitive factors will include establishing market penetration against other cytidine analogues, such as gemcitabine, which is currently used as first or second line chemotherapy either alone or in combination with other chemotherapy agents. The market penetration will depend on the potential for an improved efficacy profile in patients who have developed tolerance to other agents.
For NGC-Iri, the competitive factors will include establishing marketing penetration against the existing irinotecan product (Camptosar®) and the newer liposomal irinotecan product (Onivyde®). The establishment of that market will be based upon improved efficacy and/or safety of NGC-Iri.
For PCS12852, the competitive factors will include establishing marketing penetration against the metoclopramide products (the only approved drug to treat gastroparesis) and other 5-HT4 receptor agonists used off label. The market penetration will depend on the potential for an improved safety profile due to the very selective 5-HT4 receptor binding by PCS12852 and similar or greater efficacy in the treatment of gastroparesis.
For PCS499, there are currently no FDA-approved drugs for the treatment of patients with NL, and few drugs are used off-label for NL given the lack of efficacy and/or side effect concerns.
| 12 |
Our commercial opportunity for any of our product candidates could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, less expensive, more convenient or easier to administer, or have fewer or less severe side effects, than any products that we may develop. Our competitors also may obtain FDA, EMA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
Intellectual Property
Our success will depend in large part on our ability and that of our licensors to:
| ● | obtain and maintain international and domestic patent and other legal protections for the proprietary technology, inventions and improvements we consider important to our business; | |
| ● | prosecute and defend our existing and future patents, once obtained; | |
| ● | preserve confidentiality of our own and our licensed methods, processes and know-how; and | |
| ● | operate without infringing the patents and proprietary rights of other parties. |
Although we rely extensively on licensing patents from third parties, we intend to seek appropriate patent protection for product candidates in our research and development programs, where applicable, and their uses by filing patent applications in the United States and other selected countries. We intend for these patent applications to cover, where possible, claims for compositions of matter, medical uses, processes for preparation and formulations.
Our current patent portfolio consists of the number of patents related to our drug candidates licensed from each third-party licensor. In addition to the international patents and/or international and U.S. patent applications licensed from our third-party licensors, we have licensed at least the following number of U.S. patents:
| CoNCERT | Yuhan | Aposense | Elion | Ocuphire | Total | ||||||||
| U.S. patents | 9 | 5 | 3 | 2 | 6 | 25 |
A provisional patent for NGC-Cap has been filed.
Besides relying on patents, we may also rely on trade secrets, proprietary know-how and continuing innovation to develop and maintain our competitive position, especially when we do not believe that patent protection is appropriate or can be obtained. In addition, we continuously evaluate opportunities to obtain exclusivity through our regulatory filings with the FDA. We seek protection of these trade secrets, proprietary know-how and any continuing innovation, in part, through confidentiality and proprietary information agreements. However, these agreements may not provide meaningful protection for, or adequate remedies to protect, our technology in the event of unauthorized use or disclosure of information. Furthermore, our trade secrets may otherwise become known to, or be independently developed by, our competitors.
| 13 |
License Agreements
The following descriptions of our license agreements are only summaries. You should also refer to the copies of such agreements which have been filed as exhibits to this Annual Report.
License Agreement with Elion Oncology, Inc.
On August 23, 2020, we entered into a condition precedent License Agreement with Elion Oncology (“Elion License Agreement”), pursuant to which we acquired an exclusive license to develop, manufacture and commercialize PCS6422 globally. The license grant was conditioned on the following being satisfied by October 30, 2020: (i) our closing on an equity financing of at least $15 million in gross proceeds and (ii) successful up-listing to Nasdaq.
On October 6, 2020, all conditions were satisfied, resulting in the addition of PCS6422 to our portfolio, and we paid $100,000 cash and issued 41,250 shares of our common stock to Elion. As part of the Elion License Agreement, we agreed to issue to Elion 5,000 shares of our common stock on each of the first and second anniversary dates of the Elion License Agreement.
As additional consideration, we will pay Elion development and regulatory milestone payments (a portion of which are payable in shares of our common stock and a portion of which are payable in cash) upon the achievement of certain milestones, which include FDA or other regulatory approval and dosing a patient. In addition, we must pay Elion one-time sales milestone payments based on the achievement during a calendar year of one or more thresholds for annual sales for products made and pay royalties based on annual licensing sales. We are also required to split any milestone payments received with Elion based on any sub-license agreement we may enter.
On May 17, 2022, we amended the third Milestone Event of Section 6.4 of our License Agreement with Elion Oncology, Inc. changing the third Milestone Event from “1st Patient in Dose Confirmation Study” to (a) determination of the maximum tolerated dose (MTD) or (b) determination of the recommended Phase 2 Dose. Prior to this amendment, the third milestone was not considered probable since it was unknown when, or if a dose confirmation study was going to be conducted. As a result of the modification, we consider it probable that the recommended Phase 2 dosage regimen could be determined in connection with our current Phase 1B trial for NGC-Cap. We recorded an expense and related liability of $189,000 representing the value of the shares we anticipate issuing to Elion at the fair value on the date of modification. No other terms or conditions of the License Agreement were modified. We determined the dosage for our Phase 2 study on January 25, 2024 and issued 5,000 shares of common stock to Elion for meeting this milestone.
We are required to use commercially reasonable efforts, at our sole cost and expense to research, develop and commercialize products in one or more countries, including dosing a first patient with a product in a Phase 2 or 3 clinical trial by October 2, 2024. We are currently on track to dose our first patient in a Phase 2 clinical trial by the deadline. Either party may terminate the agreement in the event of a material breach of the agreement that has not been cured following written notice and a 90-day opportunity to cure such breach (which is shortened to 15 days for a payment breach).
| 14 |
License Agreement with Ocuphire Pharma, Inc.
On June 16, 2021, we executed a License Agreement with Ocuphire Pharma, Inc. (“Ocuphire Agreement”) under which we received a license to research, develop and commercialize PCS3117 globally, excluding the Republic of Singapore, China, Hong Kong, Macau and Taiwan.
As consideration for the Ocuphire Agreement, we issued 2,235 shares of our common stock to Ocuphire, a cash payment of $200,000 and assumed $66,583 in certain liabilities. Additional consideration includes future development and regulatory milestones payments to Ocuphire upon our achievement of certain defined clinical milestones, such as dosing a patient in pivotal trials and receiving marketing authorization by a regulatory authority in the United States or another country. In addition, we are required to pay Ocuphire one-time sales milestone payments based on the achievement during a calendar year of the highest annual Net Sales for products made and pay royalties based on annual Net Sales, as defined in the Ocuphire Agreement.
We are required to use commercially reasonable efforts, at our sole cost and expense to oversee such commercialization efforts, to research, develop and commercialize products in one or more countries, including meeting specific diligence milestones that consist of: (i) first patient administered drug in a Clinical Trial of a Product prior to June 16, 2024 and (ii) first patient administered drug in a Pivotal Clinical Trial of a Product or first patient administered drug in a Clinical Trial for a Second Indication of a Product prior to June 16, 2026. We are currently in discussions with Ocuphire to extend these deadlines. Although we do not anticipate having any issues in extending the deadlines, there can be no assurance that such negotiations will be successful. Either party may terminate the agreement in the event of a material breach of the agreement that has not been cured following written notice and a 120-day opportunity to cure such breach.
License Agreement with Aposense, Ltd.
On May 24, 2020, we entered into a condition precedent License Agreement with Aposense, Ltd. (“Aposense License Agreement”), pursuant to which we were granted Aposense’s patent rights and Know-How to develop and commercialize their next generation irinotecan cancer drug, PCS11T. The Aposense License Agreement provides us with an exclusive worldwide license (excluding China), to research, develop and commercialize products comprising or containing PCS11T. The license grant was conditioned on the following being satisfied within nine months of May 24, 2020 (or the Aposense License Agreement shall terminate): (i) our closing of an equity financing and successful up-listing to Nasdaq and (ii) Aposense obtaining the approval of the Israel Innovation Authority for the consummation of the transactions contemplated by the Aposense License Agreement.
On October 6, 2020, all conditions were satisfied, resulting in the addition of PCS11T to our portfolio, and we issued 31,250 shares of our common stock to Aposense. As additional consideration, we will pay Aposense development and regulatory milestone payments (up to $3.0 million per milestone) upon the achievement of certain milestones, which primarily consist of having a drug indication approved by a regulatory authority in the United States or another country. In addition, we will pay Aposense one-time sales milestone payments based on the achievement during a calendar year of one or more thresholds for annual sales for products made and pay royalties based on annual licensing sales. We are also required to split any sales milestone payments or royalties we receive with Aposense based on any sub-license agreement we may enter.
License Agreement with Yuhan Corporation
On August 19, 2020, we entered into a License Agreement with Yuhan Corporation (“Yuhan License Agreement”), pursuant to which we acquired an exclusive license to develop, manufacture and commercialize PCS12852 globally, excluding South Korea.
As consideration for the Yuhan License Agreement and related Share Issuance Agreement, we issued to Yuhan 25,000 shares of common stock. As additional consideration, we will pay Yuhan development and regulatory milestone payments (a portion of which are payable in shares of our common stock based on the volume weighted average trading price during the period prior to such achievement and a portion of which are payable in cash) upon the achievement of certain milestones, based on a Yuhan affiliate purchasing 37,500 shares of common stock for $3,000,000 in our October 2020 underwritten public offering. The milestones primarily consist of dosing a patient in pivotal trials or having a drug indication approved by a regulatory authority in the United States or another country. In addition, we must pay Yuhan one-time sales milestone payments based on the achievement during a calendar year of one or more thresholds for annual sales for products made and pay royalties based on annual licensing sales. We are also required to split any milestone payments received with Yuhan based on any sub-license agreement we may enter.
We are required to use commercially reasonable efforts, at our sole cost and expense, in conjunction with a joint Processa-Yuhan Board to oversee such commercialization efforts, to research, develop and commercialize products in one or more countries, including meeting specific diligence milestones that consist of: (i) preparing a first draft of the product development plan within 90 days; (ii) requesting an FDA pre-IND meeting for a product within 6 months; (iii) dosing a first patient in a Phase 2A clinical trial with a product within 24 months; and (iv) dosing a first patient with a product in a Phase 2B clinical trial, Phase 3 clinical trial or other pivotal clinical trial with a product by August 19, 2024. Either party may terminate the agreement in the event of a material breach of the agreement that has not been cured following written notice and a 60-day opportunity to cure such breach (which is shortened to 15 days for a payment breach).
| 15 |
License Agreement with CoNCERT Pharmaceuticals, Inc.
On October 4, 2017, Promet entered into a License Agreement with CoNCERT (“CoNCERT License Agreement”). On March 19, 2018, we, Promet, and CoNCERT entered into an Amended Option Licensing Agreement (“March Amendment”) that, among other things, assigned the CoNCERT Agreement from Promet to us and we exercised the exclusive commercial license option for the PCS499 compound from CoNCERT.
The CoNCERT License Agreement provides us with an exclusive (including as to CoNCERT) royalty-bearing license to CoNCERT’s patent rights and Know-How to develop, manufacture, use, sub-license and commercialize compounds (PCS499 and each metabolite thereof) and pharmaceutical products with such compounds worldwide. We are required to pay CoNCERT royalties, on a product–by-product basis, on future worldwide net sales, or pay a percentage of any sublicense revenue.
We will incur royalty obligations to CoNCERT on a country-by-country and product-by-product basis that expire on a country-by-country and product-by-product basis on the later of (i) expiration or invalidation of the last patent rights covering such product in such country or (ii) the tenth anniversary of the date of the first commercial sale to a non-sublicensee third party of such product in such country.
We are required to use commercially reasonable efforts, at our sole cost and expense, to develop and obtain regulatory approval for one product in the U.S. and at least one other major market and, subject to obtaining regulatory approval in the applicable major market, commercialize one product in the U.S. and at least one other major market. CoNCERT may terminate the agreement if, following written notice and a 60-day opportunity to demonstrate a plan to cure, it believes that we are not using commercially reasonable efforts to develop and obtain regulatory approval for one product in the U.S. and in at least one other major market for any consecutive nine-month period.
The term of the CoNCERT License Agreement continues in full force and effect until the expiration of the last royalty term. On a country-by-country and product-by-product basis, upon the expiration of the royalty term in such country with respect to such product, we shall have a fully paid-up, perpetual, irrevocable license to such intellectual property with respect to such product in such country. In the event of a material breach of the CoNCERT Agreement, either party may terminate the agreement provided such breach is not cured in the 90 days following written notice of the breach (which is shortened to 15 days for a payment breach). In addition, either party may terminate the agreement upon an assignment for the benefit of creditors or the filing of an insolvency proceeding by or against the other party that is not dismissed within 90 days of such filing.
| 16 |
Government Regulation
The FDA and comparable regulatory authorities in state and local jurisdictions and in other countries impose substantial and burdensome requirements upon companies involved in the clinical development, manufacture, marketing and distribution of drugs, such as those we are developing. These agencies and other federal, state and local entities regulate, among other things, the research and development, testing, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion, distribution, post-approval monitoring and reporting, sampling and export and import of our product candidates.
U.S. Government Regulation
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and its implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending NDAs, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
| ● | completion of pre-clinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s good laboratory practice (GLP) regulations; | |
| ● | submission to the FDA of an IND application, which must become effective before human clinical trials may begin; |
| ● | approval by an independent Institutional Review Board (IRB), at each clinical site before each trial may be initiated; | |
| ● | performance of adequate and well-controlled human clinical trials in accordance with good clinical practices (GCP) requirements to establish the safety and efficacy of the proposed drug product for each indication; | |
| ● | submission to the FDA of an NDA; | |
| ● | satisfactory completion of an FDA advisory committee review, if applicable; | |
| ● | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP requirements and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; | |
| ● | FDA review and approval of the NDA, including consideration of the views of any FDA advisory committee, prior to commercial marketing or sale of the drug in the United States; and | |
| ● | compliance with any post-approval requirements, including the potential requirement to implement a Risk Evaluation and Mitigation Strategy (REMS) or to conduct a post-approval study. |
Pre-clinical studies
Before testing any biological product candidate in humans, including our product candidates, the product candidate must undergo rigorous pre-clinical testing. The pre-clinical developmental stage generally involves laboratory evaluations of drug chemistry, formulation and stability, as well as studies to evaluate toxicity in animals, to assess the potential for adverse events and, in some cases, to establish a rationale for therapeutic use. The conduct of pre-clinical studies is subject to federal regulations and requirements, including GLP regulations for safety/toxicology studies. An IND sponsor must submit the results of the pre-clinical studies, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND.
An IND is a request for authorization from the FDA to administer an investigational product to humans and must become effective before human clinical trials may begin. Some long-term pre-clinical testing, such as animal tests of reproductive adverse events and carcinogenicity, may continue after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions before that time related to one or more proposed clinical trials and places the trial on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
| 17 |
Clinical trials
The clinical stage of development involves the administration of the investigational product to healthy volunteers or patients under the supervision of qualified investigators, generally physicians not employed by, or under control of, the trial sponsor, in accordance with GCPs, which include the requirement that all research patients provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria and the parameters to be used to monitor subject safety and assess efficacy. Each protocol, and any subsequent amendments to the protocol, must be submitted to the FDA as part of the IND. Furthermore, each clinical trial must be reviewed and approved by an IRB for each institution at which the clinical trial will be conducted to ensure that the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. There also are requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries. Information about most clinical trials must be submitted within specific timeframes for publication on www.clinicaltrials.gov. Information related to the product, patient population, phase of investigation, study sites and investigators and other aspects of the clinical trial is made public as part of the registration of the clinical trial. Sponsors are also obligated to disclose the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed in some cases for up to two years after the date of completion of the trial. Competitors may use the publicly available information to gain knowledge regarding the progress of development programs.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
| ● | Phase 1 clinical trials generally involve a small number of healthy volunteers or disease-affected patients who are initially exposed to a single dose and then multiple doses of the product candidate. The primary purpose of these clinical trials is to assess the metabolism, pharmacologic action, side effect tolerability and safety of the drug. | |
| ● | Phase 2 clinical trials involve studies in disease-affected patients to determine the dose required to produce the desired benefits. At the same time, safety and further pharmacokinetic and pharmacodynamic information is collected, possible adverse effects and safety risks are identified, and a preliminary evaluation of efficacy is conducted. The goal of the Phase 2 trial is to also determine the “best” dosage regimen(s) to evaluate in the Phase 3 trial. The “best” regimen(s) means the regimen(s) that is(are) most likely to provide a safe-efficacious regimen that appropriately balances the risk-benefit analysis that the FDA is required to evaluate in each drug approval. The ideal way to define this “best” regimen for FDA is to evaluate the adverse event-drug exposure and efficacy-drug exposure relationships, which has also been previously called dose-response relationships or studies, and which has now become the foundation for both the FDA’s Project Optimus Oncology initiative and the draft Guidance to determine the optimal dose for an oncology drug. | |
| ● | Phase 3 clinical trials generally involve a larger number of patients at multiple sites and are designed to provide the data necessary to demonstrate the effectiveness of the product for its intended use, its safety in use and to establish the overall benefit/risk relationship of the product and provide an adequate basis for product approval. These trials may include comparisons with placebo and/or other comparator treatments. The duration of treatment is often extended to mimic the actual use of a product during marketing. |
Post-approval trials, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication, particularly for long-term safety follow up. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of a biologics license application (BLA).
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. The FDA or the sponsor may suspend or terminate a clinical trial at any time, or the FDA may impose other sanctions on various grounds, including a finding that the research patients are being exposed to an unacceptable health risk. Similarly, an IRB can refuse, suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
Concurrently with clinical trials, companies usually complete additional pre-clinical studies and must also develop additional information about the physical characteristics of the biological product as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the sponsor must develop methods for testing the identity, strength, quality, potency and purity of the final biological product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the biological product candidate does not undergo unacceptable deterioration over its shelf life.
| 18 |
Marketing Approval
Assuming successful completion of the required clinical testing, the results of the pre-clinical studies and clinical trials, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA requesting approval to market the product for one or more indications. In most cases, the submission of an NDA is subject to a substantial application user fee.
The review process typically takes twelve months from the date the NDA is submitted to the FDA. The FDA conducts a preliminary review of all NDAs within the first 60 days after submission to determine whether they are sufficiently complete to permit substantive review before accepting them for “filing.” The FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA reviews an NDA to determine, among other things, whether the drug is safe and effective and whether the facility in which it is manufactured, processed, packaged or held meets standards designed to assure the product’s continued safety, quality and purity. Under the current guidelines in effect in the Prescription Drug User Fee Act (PDUFA), the FDA has a goal to review and act on the submission within ten months from the completion of the preliminary review of a standard NDA for a new molecular entity.
In addition, under the Pediatric Research Equity Act of 2003, as amended and reauthorized, certain NDAs or supplements to an NDA must contain data that are adequate to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements.
The FDA also may require submission of a REMS plan to ensure that the benefits of the drug outweigh its risks. The REMS plan could include medication guides, physician communication plans, assessment plans, and/or elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools.
The FDA may refer an application for a novel drug to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA may inspect one or more clinical trial sites to assure compliance with GCP requirements.
After evaluating the NDA and all related information, including the advisory committee recommendation, if any, and inspection reports regarding the manufacturing facilities and clinical trial sites, the FDA may issue an approval letter, or, in some cases, a complete response letter. A complete response letter generally contains a statement of specific conditions that must be met in order to secure final approval of the NDA and may require additional clinical trials or pre-clinical studies in order for FDA to reconsider the application. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications.
| 19 |
Orphan drug designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making the product available in the United States for this type of disease or condition will be recovered from sales of the product in the United States. Orphan drug designation must be requested before submitting a BLA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. Orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers.
If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication for seven years from the date of such approval, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity by means of greater effectiveness, greater safety, by providing a major contribution to patient care or in instances of drug supply issues. Competitors, however, may receive approval of either a different product for the same indication or the same product for a different indication that could be used “off-label” by physicians in the orphan indication, even though the competitor’s product is not approved in the orphan indication. Orphan drug exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval before we do of the same product, as defined by the FDA, for the same indication we are seeking, or if our product candidate is determined to be contained within the scope of the competitor’s product for the same indication or disease. If one of our products designated as an orphan drug receives marketing approval for an indication broader than that which is designated, it may not be entitled to orphan drug exclusivity. Orphan drug status in the European Union, or EU, has similar, but not identical, requirements and benefits.
Expedited review and approval
The FDA has various programs, including fast track designation, accelerated approval, priority review and breakthrough therapy designation, which are intended to expedite or simplify the process for the development and FDA review of drugs that are intended for the treatment of serious or life-threatening diseases or conditions and demonstrate the potential to address unmet medical needs. The purpose of these programs is to provide important new drugs to patients earlier than under standard FDA review procedures.
To be eligible for a fast track designation, the FDA must determine, based on the request of a sponsor, that a product is intended to treat a serious or life-threatening disease or condition and demonstrates the potential to address an unmet medical need. The FDA will determine that a product will fill an unmet medical need if it will provide a therapy where none exists or provide a therapy that may be potentially superior to existing therapy based on efficacy or safety factors. The FDA may review sections of the NDA for a fast track product on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA, the FDA agrees to accept sections of the NDA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA.
The FDA may give a priority review designation to drugs that offer major advances in treatment or provide a treatment where no adequate therapy exists. A priority review means that the goal for the FDA to review an application is six months, rather than the standard review of ten months under current PDUFA guidelines. Under the new PDUFA agreement, these six- and ten-month review periods are measured from the “filing” date rather than the receipt date for NDAs for new molecular entities, which typically adds approximately two months to the timeline for review and decision from the date of submission. Most products that are eligible for fast track designation are also likely to be considered appropriate to receive a priority review.
In addition, products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may be eligible for accelerated approval and may be approved on the basis of adequate and well-controlled clinical trials establishing that the drug product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require a sponsor of a drug receiving accelerated approval to perform post-marketing studies to verify and describe the predicted effect on irreversible morbidity or mortality or other clinical endpoint, and the drug may be subject to accelerated withdrawal procedures.
| 20 |
Moreover, under the provisions of the Food and Drug Administration Safety and Innovation Act, a sponsor can request designation of a product candidate as a “breakthrough therapy.” A breakthrough therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Drugs designated as breakthrough therapies are also eligible for accelerated approval. The FDA must take certain actions, such as holding timely meetings and providing advice, intended to expedite the development and review of an application for approval of a breakthrough therapy.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened. Furthermore, fast track designation, priority review and breakthrough therapy designation do not change the standards for approval, but may expedite the development or approval process. We may explore some of these opportunities for our product candidates as appropriate.
Post-approval requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to prior FDA review and approval. There are also continuing annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
The FDA may impose a number of post-approval requirements as a condition of approval of an NDA. For example, the FDA may require post-marketing testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP requirements and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in mandatory revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| ● | restrictions on the marketing or manufacturing of the product; | |
| ● | complete withdrawal of the product from the market or product recalls; | |
| ● | safety alerts, Dear Healthcare Provider letters, press releases or other communications containing warning or other safety information about the product; | |
| ● | fines, warning letters or holds on post-approval clinical trials; | |
| ● | refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product approvals; product seizure or detention, or refusal to permit the import or export of products; or | |
| ● | injunctions or the imposition of civil or criminal penalties. |
| 21 |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act (PDMA), which regulates the distribution of drugs and drug samples at the federal level and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Other Regulatory Matters
Pharmaceutical companies are subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which they conduct their business. Manufacturing, sales, promotion and other activities following product approval are subject to regulation by numerous regulatory authorities in the United States in addition to the FDA, including Centers for Medicare and Medicaid Services (CMS), other divisions of the Department of Health and Human Services, the Department of Justice, the Drug Enforcement Administration, the Consumer Product Safety Commission, the Federal Trade Commission, the Occupational Safety & Health Administration, the Environmental Protection Agency, and state and local governments.
For example, in the United States, sales, marketing and scientific and educational programs also must comply with state and federal fraud and abuse laws, false claims laws, transparency laws, government price reporting, and health information privacy and security laws. These laws include the following:
| ● | the federal Anti-Kickback Statute, which makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf), to knowingly and willfully solicit, receive, offer or pay any remuneration that is intended to induce or reward referrals, including the purchase, recommendation, order or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Moreover, the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the ACA), provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act; | |
| ● | federal civil and criminal false claims and civil monetary penalties laws, including the civil False Claims Act that can be enforced by private citizens through civil whistleblower or qui tam actions, prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| ● | the Federal Health Insurance Portability and Accountability Act of 1996 (HIPAA) which prohibits, among other things, executing or attempting to execute a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; | |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and their implementing regulations, which also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; | |
| ● | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; |
| 22 |
| ● | the FDCA, which prohibits, among other things, the adulteration or misbranding of drugs, biologics and medical devices; | |
| ● | the federal Physician Payments Sunshine Act, which requires applicable manufacturers of covered drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to annually report to CMS information regarding payments and other transfers of value to physicians and teaching hospitals as well as information regarding ownership and investment interests held by physicians and their immediate family members; and |
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state laws that require biotechnology companies to comply with the biotechnology industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; state laws that require biotechnology companies to report information on the pricing of certain drug products; and state and foreign laws that govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Pricing and rebate programs must also comply with the Medicaid rebate requirements of the U.S. Omnibus Budget Reconciliation Act of 1990 and more recent requirements in the ACA. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. Products must meet applicable child-resistant packaging requirements under the U.S. Poison Prevention Packaging Act. Manufacturing, sales, promotion and other activities also are potentially subject to federal and state consumer protection and unfair competition laws.
The distribution of pharmaceutical products is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage and security requirements intended to prevent the unauthorized sale of pharmaceutical products.
The failure to comply with any of these laws or regulatory requirements subjects firms to possible legal or regulatory action. Depending on the circumstances, failure to meet applicable regulatory requirements can result in significant civil, criminal and administrative penalties, including damages, fines, disgorgement, individual imprisonment, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, integrity oversight and reporting obligations, contractual damages, reputational harm, diminished profits and future earnings, injunctions, requests for recall, seizure of products, total or partial suspension of production, denial or withdrawal of product approvals or refusal to allow a firm to enter into supply contracts, including government contracts.
U.S. Patent-Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of FDA approval of any future product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Act. The Hatch-Waxman Act permits restoration of the patent term of up to five years as compensation for patent term lost during product development and FDA regulatory review process. Patent-term restoration, however, cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent-term restoration period is generally one-half the time between the effective date of an IND or the issue date of the patent, whichever is later, and the submission date of an NDA plus the time between the submission date of an NDA or the issue date of the patent, whichever is later, and the approval of that application, except that the review period is reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved drug is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The United States Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we may apply for restoration of patent term for our currently owned or licensed patents to add patent life beyond its current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant NDA.
| 23 |
Market exclusivity provisions under the FDCA also can delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application (ANDA) or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
European Union Drug Development
Similar to the United States, the various phases of preclinical and clinical research in the European Union are subject to significant regulatory controls. Although the European Union Clinical Trials Directive 2001/20/EC has sought to harmonize the EU clinical trials regulatory framework, setting out common rules for the control and authorization of clinical trials in the EU, the EU Member States have transposed and applied the provisions of the Directive differently. This has led to significant variations in the member state regimes. Under the current regime, before a clinical trial can be initiated, it must be approved in each of the EU countries where the trial is to be conducted by two distinct bodies: the National Competent Authority (NCA) and one or more Ethics Committees (ECs). Under the current regime, all suspected unexpected serious adverse reactions to the investigated drug that occur during the clinical trial have to be reported to the NCA and ECs of the Member State where they occurred.
The EU clinical trials legislation is currently undergoing a transition process mainly aimed at harmonizing and streamlining clinical-trial authorization , simplifying adverse-event reporting procedures, improving the supervision of clinical trials and increasing their transparency. Recently enacted Clinical Trials Regulation EU No 536/2014 ensures that the rules for conducting clinical trials in the EU will be identical. In the meantime, Clinical Trials Directive 2001/20/EC continues to govern all clinical trials performed in the EU.
European Union Drug Review and Approval
In the European Economic Area (EEA), which is comprised of the 26 Member States of the European Union (including Norway and excluding Croatia), Iceland and Liechtenstein, medicinal products can only be commercialized after obtaining a Marketing Authorization (MA). There are two types of marketing authorizations:
| ● | The Community MA is issued by the European Commission through the Centralized Procedure, based on the opinion of the Committee for Medicinal Products for Human Use (CHMP) of the EMA, and is valid throughout the entire territory of the EEA. The Centralized Procedure is mandatory for certain types of products, such as biotechnology medicinal products, orphan medicinal products, advanced-therapy medicines such as gene-therapy, somatic cell-therapy or tissue-engineered medicines and medicinal products containing a new active substance indicated for the treatment of HIV, AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and other immune dysfunctions and viral diseases. The Centralized Procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the European Union. |
| 24 |
| ● | National MAs, which are issued by the competent authorities of the Member States of the EEA and only cover their respective territory, are available for products not falling within the mandatory scope of the Centralized Procedure. Where a product has already been authorized for marketing in a Member State of the European Union, this National MA can be recognized in another Member State through the Mutual Recognition Procedure. If the product has not received a National MA in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralized Procedure. Under the Decentralized Procedure, an identical dossier is submitted to the competent authorities of each of the Member States in which the MA is sought, one of which is selected by the applicant as the Reference Member State (RMS). The competent authority of the RMS prepares a draft assessment report, a draft summary of the product characteristics (SmPC), and a draft of the labeling and package leaflet, which are sent to the other Member States (referred to as the Member States Concerned) for their approval. If the Member States Concerned raise no objections, based on a potential serious risk to public health, to the assessment, SmPC, labeling or packaging proposed by the RMS, the product is subsequently granted a national MA in all the Member States (i.e., in the RMS and the Member States Concerned). |
Under the above-described procedures, before granting the MA, EMA or the competent authorities of the Member States of the European Union make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy. Similar to the U.S. patent term-restoration, Supplementary Protection Certificates (SPCs) serve as an extension to a patent right in Europe for up to five years. SPCs apply to specific pharmaceutical products to offset the loss of patent protection due to the lengthy testing and clinical trials these products require prior to obtaining regulatory marketing approval.
Coverage and Reimbursement
Sales of our products will depend, in part, on the extent to which our products will be covered by third-party payors, such as government health programs, commercial insurance, and managed healthcare organizations. There is significant uncertainty related to third-party payor coverage and reimbursement of newly approved products. In the United States, for example, principal decisions about reimbursement for new products are typically made by CMS. CMS decides whether and to what extent a new product will be covered and reimbursed under Medicare, and private third-party payors often follow CMS’s decisions regarding coverage and reimbursement to a substantial degree. However, no uniform policy of coverage and reimbursement for drug products exists. Accordingly, decisions regarding the extent of coverage and amount of reimbursement to be provided for any of our products will be made on a payor-by-payor basis.
Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Further, such payors are increasingly challenging the price, examining the medical necessity and reviewing the cost effectiveness of medical product candidates. There may be especially significant delays in obtaining coverage and reimbursement for newly approved drugs. Third-party payors may limit coverage to specific product candidates on an approved list, known as a formulary, which might not include all FDA-approved drugs for a particular indication. We may need to conduct expensive pharmaco-economic studies to demonstrate the medical necessity and cost effectiveness of our products. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained.
In addition, in most foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing and reimbursement vary widely from country to country. For example, the European Union provides options for its Member States to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A Member State may approve a specific price for the medicinal product, or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the United States and generally prices tend to be significantly lower.
| 25 |
Healthcare Reform
The United States government, state legislatures, and foreign governments have shown significant interest in implementing cost containment programs to limit the growth of government-paid healthcare costs, including price-controls, restrictions on reimbursement, and requirements for substitution of generic products for branded prescription drugs. For example, the ACA was passed in March 2010 and substantially changed the way healthcare is financed by both the government and private insurers, and significantly impacts the U.S. pharmaceutical industry. The ACA contains provisions that may reduce the profitability of drug products through increased rebates for drugs reimbursed by Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies’ share of sales to federal health care programs.
The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter and have in effect a national rebate agreement with the HHS Secretary as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. The ACA made several changes to the Medicaid Drug Rebate Program, including increasing pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs from 15.1% of average manufacturer price (AMP), to 23.1% of AMP and adding a new rebate calculation for “line extensions” (i.e., new formulations, such as extended release formulations) of solid oral dosage forms of branded products, as well as potentially impacting their rebate liability by modifying the statutory definition of AMP. The ACA also expanded the universe of Medicaid utilization subject to drug rebates by requiring pharmaceutical manufacturers to pay rebates on Medicaid managed care utilization and by enlarging the population potentially eligible for Medicaid drug benefits. Effective April 1, 2020, Medicaid rebate liability will be expanded to include the territories of the United States as well. Additionally, for a drug product to receive federal reimbursement under the Medicaid or Medicare Part B programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B drug pricing program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer.
Some of the provisions of the ACA have yet to be implemented, and there have been judicial, Congressional and executive branch challenges to certain aspects of the ACA. Additionally, there has been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. For example, at the federal level, there is a “Blueprint” to lower prescription drug prices and reduce out-of-pocket costs of drugs that contains additional proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out-of-pocket costs of drug products paid by consumers. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Further, on August 16, 2022, President Biden signed the Inflation Reduction Act of 2022 (“IRA”) into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. It is possible that the Affordable Care Act will be subject to judicial or Congressional challenges in the future. It is unclear how such challenges and the healthcare reform measures of the current administration will impact the Affordable Care Act and our business.
Moreover, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) established the Medicare Part D program to provide a voluntary prescription drug benefit to Medicare beneficiaries. Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities that provide coverage of outpatient prescription drugs. Unlike Medicare Part A and B, Part D coverage is not standardized. While all Medicare drug plans must give at least a standard level of coverage set by Medicare, Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, though not necessarily all the drugs in each category or class. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee. Government payment for some of the costs of prescription drugs may increase demand for products for which we receive marketing approval. However, any negotiated prices for our products covered by a Part D prescription drug plan likely will be lower than the prices we might otherwise obtain. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private third-party payors often follow Medicare coverage policy and payment limitations in setting their own payment rates.
| 26 |
Human Capital
As of March 21, 2024, we had 13 full- and part-time employees. None of our employees is subject to a collective bargaining agreement or represented by a trade or labor union and we believe our relationships with our employees are good.
We are highly dependent upon the principal members of our small management team and staff, including our Chief Executive Officer, George Ng; President of Research and Development, David Young, Pharm.D., Ph.D; and our Chief Development and Regulatory Officer, Sian Bigora, Pharm.D. Despite our efforts to retain valuable employees, members of our management, scientific and development teams may terminate their employment with us on short notice. Although we expect to have employment agreements with our key employees, these employment agreements may still allow these employees to leave our employment at any time, for or without cause. Our success also depends on our ability to continue to attract, retain and motivate highly skilled junior, mid-level and senior managers as well as junior, mid-level and senior scientific and medical and scientific personnel.
Corporate Information
We were incorporated under the laws of the State of Delaware on March 29, 2011 as Heatwurxaq, Inc.2011. Our principal executive office is located at 7380 Coca Cola Drive, Suite 106, Hanover, MD 21076. Our telephone number is (443) 776-3133.
We make available free of charge on or through our Internet website (http://www.processapharmaceuticals.com) our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and, subsequently changed its nameif applicable, amendments to Heatwurx, Inc. (“the Company”those reports filed or “Heatwurx”) on April 15, 2011.
We are an asphalt preservation and repair, equipment company. Our innovative, and eco-friendly hot-in-place recycling process corrects surface distresses within the top 3 inches of existing pavement by heating the surface materialfurnished pursuant to a temperature between 325° and 375° Fahrenheit with our electrically powered infrared heating equipment, mechanically loosening the heated material with our processor/tiller attachment that is optimized for producing a seamless repair, and mixing in additional recycled asphalt pavement and a binder (asphalt-cement), and then compacting repaired area with a vibrating rollerSection 13(a) or compactor. We consider our equipment to be eco-friendly as the Heatwurx process reuses and rejuvenates distressed asphalt, uses recycled asphalt pavement for filler material, eliminates travel to and from asphalt batch plants, and extends the life15(d) of the roadway. We believe our equipment, technology and processes provide savings over other processes that can be more labor and equipment intensive.
Our hot-in-place recycling process and equipment was selected by the Technology Implementation Group of the American Association of State Highway Transportation Officials (“AASHTO TIG”) as an “additionally Selected Technology” for the year 2012. We develop, manufacture and intend to sell our unique and innovative and eco-friendly equipment to federal, state and local agenciesExchange Act, as well as contractors for the repairour Code of Ethics and rehabilitationCode of damaged and deteriorated asphalt surfaces.
In January 2014,Conduct, as soon as reasonably practicable after we acquired Dr. Pave, LLC a service company offering asphalt repair and restoration utilizing the Heatwurx asphalt repair technology. Dr. Pave, offered asphalt restoration services to municipalities and the commercial sector in southern California.
Effective July 22, 2014, we established a new entity named Dr. Pave Worldwide LLC to house our franchise program providing franchiseeselectronically file such material with, the exclusive Heatwurx equipment and processing. We formally launched our franchise sales program throughout the U.S. in the third quarter of 2014; however, to date, no franchises have been sold. The Company has decided not to renew its franchise registrations throughout the U.S. dueor furnish it to, the extensive costs. During 2015, we began offering license agreements, which grants a license of all Heatwurx equipmentSecurities and supplies and the use of the Heatwurx intellectual property within a specified territory. We have one licensee as of December 31, 2016.
During 2016, we did not receive financial support and we are unable to obtain financing from another source. We do not expect a level of revenues adequate to support our cost structure. Do to the slow growth in the service sector and the high cost of the franchise registrations, we discontinued the operations of Dr. Pave, LLC and Dr. Pave Worldwide LLC during 2015. We have significantly scaled back operations to maintain only a minimal level of operations necessary to support our licensee and look for potential merger candidates. It is our intention to move forward as a public entity and to seek a merger candidate. If the Company fails to merge or be acquired by another company, we will be required to terminate all operations.
Section 107 of the JOBS Act provides that an “emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
Heatwurx Products
Heatwurx NEW HWX-30S - Electrically Powered (remote) Infrared Heater
The HEATWURX® HWX-30S Electric Infrared Heater is designed to effectively heat asphalt pavement to a pliable 325° to 375° Fahrenheit without scorching, burning, or oxidizing the existing asphalt with a highly efficient Stainless-Steel reflector. The HWX-30S equipped with supporting legs, lockable swivel Colson castors (6x2) with built-in brakes to support high maneuverability and easy positioning. The HWX-30S can easily attached to a skid steer with standard quick releases that can be used to repair/rejuvenate distressed asphalt. The HEATWURX® HWX-30S Electric Infrared Heater specifications are as follows:
·
Weight 850 lbs. Lightweight for easy maneuverability
·
Heats repair area of 30 square feet
·
Generator requirement 36 kilowatts (minimum) - Fuel consumption approximately 2.8 gallons of fuelper hour
·
Custom industrial heating elements
·
Highly efficient Stainless-Steel heating reflector
·
Cycle times of approximately 20 - 40 minutes depending on depth and weather conditions
·
Heavy duty steel constructed frame
·
Swivel legs with Castors (built in brake) and top wind 7,000 lbs. jacks - superior maneuverability and positioning
·
Heavy duty steel attachment plate for skid steers
·
Electric control panel UL-listed and CSA-certified
Heatwurx HWX-30 - Electrically Powered Infrared Heater
The HEATWURX® HWX-30 Electric Infrared Heater is designed to effectively heat asphalt pavement to a pliable 325° to 375° Fahrenheit without scorching, burning, or oxidizing the existing asphalt. The HWX-30 is easily attached to a skid steer with standard quick releases and is a self-contained mobile infrared heater that can be used to repair/rejuvenate distressed asphalt. The HEATWURX® HWX-30 Electric Infrared Heater specifications are as follows:
·
Weight 3,550 lbs. (with generator mounted)
·
Heats repair area of 30 square feet
·
Generator requirement 36 kilowatts
·
Custom industrial heating elements
·
Cycle times of approximately 20 - 40 minutes depending on depth and weather conditions
·
Fuel consumption approximately 2.8 gallons of fuelper hour
·
Heavy duty steel constructed frame
·
Top wind 7,000 lbs. jacks
·
Six inches of heat resistance insulation
·
Heavy duty high temperature powder coated finish for maximum durability and visibility
·
Heavy duty steel attachment plate for skid steers or forklifts
Heatwurx AP-40 - Asphalt Processor
The HEATWURX® HWX-AP40 Asphalt Processor is powered by an orbital hydraulic motor and has a 40-inch working width. Designed to process and rejuvenate existing asphalt in place, it processes, remixes, and levels the heated, rejuvenated asphalt to the desired depth, ready for compaction. It is designed to easily attach to a skid steer and has custom beveled tines to provide a seamless bond between the repaired area and existing pavement. The HEATWURX® HWX-AP40 Asphalt Processor specifications are as follows:
·
One inch wear plate with ability to adjust to desired depth
·
Orbital hydraulic motor
·
40” working width
·
5/16” processing blades
·
Custom beveled cutting blades tooling to maximize asphalt bonding
·
12 gauge wings to funnel material into desired location
Company History
Heatwurx, Inc. was incorporated under the laws of the State of Delaware on March 29, 2011 as Heatwurxaq, Inc. and subsequently changed its name to Heatwurx, Inc. on April 15, 2011. Our founders were Larry Griffin and David Eastman, the principals of Hunter Capital Group, LLC, an investment banking entity, which acquired our technology, equipment designs, trademarks, and patent applications from Richard Giles, the inventor and a founder of the Company in April 2011.
Competitive Environment
Distressed asphalt occurs when asphalt-surfaced pavements are subjected to a broad spectrum of traffic levels, from two-lane rural routes to multi-lane interstate highways. Any agency responsible for asphalt-surfaced pavements eventually performs pothole patching or repair. Pothole patching or repair is generally performed either as an emergency repair under harsh conditions, or as routine maintenance scheduled for warmer and drier periods. Pothole patching and repair can be performed during various weather conditions.
In most cases, the public likes all potholes to be patched or repaired promptly and forms a negative opinion of the agency when this fails to happen in a timely manner.
Potholes are generally caused by moisture, freeze-thaw cycle, traffic, poor underlying support, or some combination of these factors. Pothole patching or repair is necessary in those situations where potholes compromise safety and cause damage to vehicles.
In general, the competitive environment for asphalt patching and repair is fragmented. Numerous entities including contractors, municipalities and others provide services to repair roads. However, there are a number of generally accepted methods of repairing and patching asphalt that our process competes against which are listed below under, “Examples of Asphalt Repair and Patching Techniques”.
6
Examples of Asphalt Repair and Patching Techniques
The following techniques and asphalt repair methods have been documented by the Transportation Research Board as part the Strategic Highway Research Program (“SHRP”)Exchange Commission (SEC). The Federal Highway Association Long Term Pavement Performance (“LTPP”) program conducted five years of additional research on pothole repair, providing guidelines and recommendationsSEC also maintains a website which provides online access to assist highway maintenance agencies and other related organizations in planning, constructing, and monitoring the performance of pothole repairs in asphalt-surfaced pavements.
Throw-and-Roll
Many maintenance agencies use the “throw-and-roll” method for patching potholes. It is the most commonly used method because of its high rate of production.
The throw-and-roll method consists of the following steps:
1.
Shovel the hot asphalt into a pothole (which may or may not be filled with water or debris).
2.
Drive over the asphalt using the truck tires to compact.
3.
Move on to the next pothole.
One difference between this method and the traditional throw-and-go method is that some effort is made to compact the patches. Compaction provides a tighter patch for traffic than simply leaving loose material. The extra time to compact the patches (generally one to two additional minutes per patch) will not significantly affect productivity.
This is especially true if the areas to be patched are separated by long distances and most of the time is spent traveling between potholes.
Crack Sealing
Crack sealing is utilized by agencies, parking lot owners and homeowners to seal cracks in asphalt pavement to prevent water and other debris from penetrating the asphalt and causing further damage during the freeze and thaw cycles. This method is preventative and not suitable for repairing or patching potholes.
The process for sealing cracks consists of the following steps:
1.
Clean the surface of the area to be sealed and let dry.
2.
Heat the sealing material to 300° to 400° Fahrenheit.
3.
Pour the heated material into the crack.
4.
Let cool and dry.
5.
Place a layer of sand over the sealing material to prevent tracking by vehicle tires.
Spray Injection Patching
The spray injection repair technique is performed by spraying heated aggregate (minerals such as sand, gravel, or crushed stone) into the area to be repaired. This repair method requires a truck to haul the replacement asphalt and specialized machinery to heat and disperse.
The spray-injection procedure consists of the following steps:
1.
Blow water and debris from the pothole with a high-pressure air blower.
2.
Spray a generous layer of binder (asphalt-cement) on the sides and bottom of the pothole.
3.
Blow heated aggregate (minerals such as sand, gravel or crushed stone) and asphalt-cement (binder) into the pothole.
4.
Cover the patched area with a layer of dry aggregate (minerals).
This procedure process does not include compaction of the repaired area.
7
Semi-Permanent Repair (Saw cut)
Many agencies employ semi-permanent repair methods such as saw cutting. This method represents an increased level of effort for repairing potholes. This increased effort increases the performance of the repair by improving the underlying and surrounding support provided for the repair. It also raises the cost, due to the increased labor required and the amount of time the repair takes.
The semi-permanent repair method has traditionally been considered one of the best for repairing potholes, short of full-depth removal and replacement. This procedure includes the following steps:
1.
Remove water and debris from the pothole.
2.
Using a radial saw with a hardened blade, cut the repair area on four sides creating a square or rectangle.
3.
Remove the material inside the section that was cut.
4.
Shovel hot asphalt into the repair area. Spread with an asphalt rake to proper grade.
5.
Compact with a vibrating drum roller or vibrating plate compactor.
This repair procedure results in a tightly compacted repair. However, it requires more workers and equipment and has a lower productivity rate than both the throw-and-roll and the spray-injection procedure.
Intellectual Property
We currently have six issued U.S. patents: five utility patents and one design patent. We have two pending U.S. patent applications and three foreign patent applications.
Three issued utility patents, US Patent Nos. 8,556,536; 8,562,247 and 8,714,871 were issued on Oct. 15, 2013, Oct. 24, 2013, and May 6, 2014, respectively and cover certain unique device and method of use aspects of our asphalt repair equipment. Our design patent, US Patent No. D700,633, was issued on March 4, 2014 and covers the ornamental design of our asphalt processor. U.S. Patent No. 8,801,325 issued August 12, 2014 and covers aspects of our computer-controlled asphalt heater. U.S. Patent No. 9,022,686 was issued May 5, 2015 and covers complementary features of our computer-controlled asphalt heater.
Our patent application entitled, “System and Method for Sending and Managing Pothole Location and Pothole Characteristics” was filed on January 16, 2013; the USPTO rejected the application based on prior art referenced by the examiner. We filed a response which argues that the invention as claimed is patentable over the cited prior art.
Our patent application entitled "System and Method for Roadway Pavement Restoration" was filed on April 7, 2015 in both the U.S. and Canada. The application includes a remotely-operated processor and a heater powered by a truck-mounted generator.
We intend to protect our intellectual property rights in the United States and in a limited number of countries outside of the United States. However, we do not have any assurance that our current pending patent applications will be granted or that we will be able to develop future patentable technologies. We do not believe our ability to operate our business is dependent on the patentability of our technology.
Governmental Regulation
We do not manufacture our own equipment; it will be up to the manufacturer as well as the end-users to comply with any governmental regulations. To the extent that any regulations require changes to our equipment, we will have to comply or risk losing the customers. See “Risk Factors” for a discussion relating to compliance with government regulations.
As part of our sales and service operation, we hired drivers with Commercial Driver’s Licenses (“CDL’s”) to transport our asphalt repair equipment to demonstrate the effectiveness of our equipment to potential clients and utilize our equipment while performing asphalt repairs. As such, we are a motor carrier subject to regulation by the U.S. Department of Transportation (“DOT”) and the Federal Motor Carrier Safety Administration (“FMCSA”), and certain business is also subject to state rules and regulations. The DOT periodically conducts reviews and audits to ensure our compliance with federal safety requirements, and we report certain accidentreports and other information toregarding registrants that file electronically with the DOT.SEC at: www.sec.gov.
We significantly reduced personnel and operations in July 2015, and no longer performed service or demonstrations. We do not anticipate hiring drivers or performing asphalt repair. We will monitor the actions of the FMCSA and other regulatory agencies and evaluate all proposed rules to determine their impactThe information contained on our continuing operations.website and social media channels is not included as a part of, or incorporated by reference into, this report.
Employees
As of September 10, 2017, we had one full-time and no part-time employees.
Competition
According to the 2011 IBIS World Report on US Road and Highway Maintenance, the total spent on road maintenance in the United States is in excess of $30 billion per year. As an emerging company, we were at a competitive disadvantage because we did not have the financial resources of larger, more established competitors, nor did we have a sales force large enough to challenge our competitors. We intended to address this disadvantage by entering into distribution agreements, licensing and franchising opportunities, and provide on-going education and training to our sales partners, customers, and governmental agencies. We have one licensee that we will continue to support. We do not have the funds to create a competitive advantage. We do believe that our equipment and processes are better than what is offered by other companies, is more effective and has a better quality of design, and received recognition from state and federal agencies, however we do not have the financing to support our cost structure. We have significantly reduced our personnel and operations to solely support our licensee. See “Risk Factors” for a discussion of the risks associated with our company.
ITEMItem 1A. Risk Factors
An investment in our common stock involves a high degree of risk. Investors should carefully consider the risks described below, together with all of the other information included in this report, before making an investment decision. If any of the following risks actually occurs,occur, our business, financial condition, and/or results of operations could suffer. In that case,be materially adversely affected, the trading price of our shares of common stock could decline, and investorsyou may lose all or part of theiryour investment. InvestorsYou should readalso refer to the section entitled “Cautionaryother information contained in this Form 10-K, including our consolidated financial statements and the notes to those statements, and the information set forth under the caption “Special Note Regarding Forward-Looking Statements and Risk Factor Summary.” above for a discussion of what types of statementsThe risks described below and contained in our other periodic reports are forward-looking statements, as well asnot the significance of such statements in the context of this report.
Risks Relatingonly ones that we face. Additional risks not presently known to the Company’s Business
We areus or that we currently in default under two separate secured notes and, if either ofdeem immaterial may also adversely affect our creditors chooses, our assets will be foreclosed upon and we will have no business operations.
On September 30, 2016, we failedRisks Related to make a mandatory principalOur Financial Position and interest payments under the senior secured note dated February 16, 2015. The failure to make a mandatory payment constituted an event of default under the note. Upon the occurrence of an event of default under the note, the creditors may foreclose upon all of the assets of our company. The Company has not made any interest payments during 2016.Need for Additional Capital
On June 30, 2016, the Company assumed the revolving line of credit from Dr. Pave, LLC. The revolving line of credit was secured by all the assets of Dr. Pave, LLC. Dr. Pave, LLC was discontinued and there are no assets to secure the liability. There have been no payments under the revolving line of credit.
If any of our creditors who have been issued secured notes elects to foreclose upon our assets, we will no longer have any business operations.
9
We have substantial indebtedness, which could have adverse consequences to us,a history of losses and we may notnever become profitable.
We are a clinical stage biopharmaceutical company. Processa itself as an organization has never had a drug approved by the FDA or any regulatory agency. The likelihood of success of our business plan must be able to generate sufficient cash flow to fund our liquidity needs, including servicing our indebtedness.
We currently haveconsidered in light of the challenges, substantial indebtedness. Our level of indebtedness has important consequences to usexpenses, difficulties, complications and to existing shareholdersdelays frequently encountered in connection with developing and potential investors. For example, our level of indebtedness may:
·
require us to dedicateexpanding early-stage businesses and the regulatory and competitive environment in which we operate. Biopharmaceutical product development is a highly speculative undertaking, involves a substantial portiondegree of risk, and is a capital-intensive business. If we cannot successfully execute our plan to develop our drug pipeline, our business may not succeed.
At December 31, 2023, the accumulated deficit was approximately $75.4 million. We will incur additional losses as we continue our research and development activities, seek regulatory approvals for our product candidates and engage in clinical trials. These losses will cause, among other things, our stockholders’ equity and working capital to decrease. Any future earnings and cash flow from operations to pay interest and principal on our debt, which would reduce the funds available to use for operations future business opportunities and other general corporate purposes;
·
make it more difficult for us to satisfy our debt obligations, and any failure to comply with such obligations, including financial and other restrictive covenants, could result in an event of default or an inability to borrow under the agreements governing such indebtedness;
·
in the case of a default or an event of default, as applicable, lead to, among other things, an acceleration of our indebtedness or foreclosure on the assets securing our indebtedness, which could have a material adverse effect on our business or financial condition;
·
limit our ability to obtain additional financing, or to sell assets to raise funds, if needed, for working capital, capital expenditures, expansion plans and other investments, which may limit our ability to implement our business strategy;
·
place us at a competitive disadvantage relative to others in the industry as it is not common for companies involved in the asphalt repair business to operate with such high leverage;
·
heighten our vulnerability to downturns in our business, the industry or in the general economy and limit our flexibility in planning for or reacting to changes in our business and the retail industry; or
·
reduce our ability to carry out our plans to expand our product offerings and sales channels.
Our ability to service our indebtedness isare dependent on our ability to generate cashfurther develop our products and on revenues and profitability from internal operationssales of products or from additional debt or equity offerings sufficient to make required payments on such indebtedness, which is, to a significant extent, subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control, some of which factors are further described in this “Risk Factors” section. We are permitted by the terms of our indebtedness to incur additional indebtedness; however, we have recently only received funds from a limited number of related parties and those related parties have ceased funding our operations. We have experienced negative cash flows from operating activities since inception, and our business may not generate sufficient cash flow from operations or from debt or equity offerings to enable us to service our indebtedness or to fund our other liquidity needs. We are unable to make required payments on our secured line of credit, we are in default and the creditors could foreclose on all of our assets. Such event would have a material adverse effect on us and we may need to take various actions, including seeking to refinance all or a portion of our indebtedness, seeking additional debt or equity financing or a strategic acquisition, and we may not be able to do so on commercially reasonable terms or at all and be forced to cease all operations.successful joint venture relationships.
| 27 |
We have a single member of management holding the positions of Interim Chief Executive Officer and Interim Chief Financial Officer. We may have a conflict of interest between the best interests of our company and the best interests of our director’s other business interests.
Mr. John McGrain remains our sole officer. Mr. McGrain is also a member of JMW Fund, LLC, San Gabriel Fund, LLC, and Richland Fund, all of which are significant equity and debt holders of the Company. A conflict of interest may arise between the best interests of our company and the best interests of Mr. McGrain's other business interests. In such a situation where a conflict of interest exists any decision by Mr. McGrain which furthers the best interests of his other business interest may be harmful to our business. Additionally, we have no established parameters or understandings regarding the allocation of present or future business opportunities between us and Mr. McGrain’s other business interests.
We rely on consultants and if we are unable to retain these or other similarly qualified individuals, we may not be able to carry out our business operations.
We are dependent upon service providers for accounting and legal matters. Loss of their services would adversely affect our business and our ability to maintain our operations. If we lose the services of these consultants and are unable to find replacement consultants or similarly qualified individuals, our business may fail.
10
Our sole officer does not have experience in public company matters, which could impair our ability to comply with legal and regulatory requirements.
Our sole officer has no public company management experience or responsibilities. This could impair our ability to comply with legal and regulatory requirements such as the Sarbanes-Oxley Act of 2002 and applicable federal securities laws including filing required reports and other information required on a timely basis. There can be no assurance that our management will be able to implement and affect programs and policies in an effective and timely manner that adequately respond to increased legal, regulatory compliance and reporting requirements imposed by such laws and regulations. Our failure to comply with such laws and regulations could lead to the imposition of fines and penalties and further result in the deterioration of our business.
Because our sole officer has no experience with the asphalt restoration industry, we may not generate material sales and we face a high risk of failure.
Our sole officer has no experience operating an asphalt restoration business. As such, there can be no assurance that we will be able to implement planned principal operations or generate any sales, and face a high risk of business failure.
We may be sued by claimants that allege that they were injured due to our equipment. Our business will be negatively impacted if we do not have sufficient insurance to protect us against these claims.
Any business today is at risk of becoming involved in lawsuits. It is extremely difficult to identify all possibly claims that could be made against us based on our business, but to name a few, we may be sued by drivers that claim that roads repaired by our equipment caused them to get into an automobile accident or a worker using our equipment to repair a road may claim that he or she was injured by our equipment. These claims may or may not be meritorious. In any event, we will attempt to protect ourselves against these claims by purchasing general liability insurance. There can be no assurance that we will be able to generate sufficient product revenue to become profitable at all or on a sustained basis. Even if we generate revenues, we expect to have quarter-to-quarter fluctuations in revenues and expenses, some of which could be significant, due to research, development, clinical trial, and marketing and manufacturing expenses and activities. We also expect to incur substantial expenses without corresponding revenues, unless and until we are able to obtain regulatory approval and successfully license or commercialize our product candidates. If our product candidates fail in clinical trials or do not gain regulatory approval, or if our products do not achieve market acceptance, we may never become profitable.
We may never be able to obtain regulatory approval for the marketing of our product candidates in any indication in the United States or internationally. As we commercialize and market products, we will need to incur expenses for product marketing and brand awareness and conduct significant research, development, testing and regulatory compliance activities that, together with general and administrative expenses, could result in substantial operating losses for the foreseeable future. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our stock price may decline, and you may lose all or a substantial part of your investment in us.
We have limited cash resources and will require additional financing.
Since inception, we have not generated any revenue, have incurred net losses, have used net cash in our operations and have funded our business and operations primarily through proceeds from the sale of our securities. We expect to continue to require significant future financing to fund our operating activities and to use cash in operating activities for the foreseeable future as we continue our research and development activities to develop products that can be commercialized to generate revenue. Our ability to obtain additional financing will be subject to many factors, including market conditions, our operating performance and investor sentiment. If we are unable to raise additional capital when required or on acceptable terms, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our product candidates, restrict our operations or obtain funds by entering into agreements on unattractive terms, which would likely have a material adverse effect on our business, stock price and our relationships with third parties with whom we have business relationships, at least until additional funding is obtained. If we do not have sufficient funds to continue operations, we could be required to seek bankruptcy protection or other alternatives that would likely result in our stockholders losing some or all of their investment in us.
We may seek additional capital through a combination of private and public equity offerings, debt financings and strategic collaborations, including sales of our common stock under the existing or a future equity line of credit or through a future at-the-market offering. If we raise additional funds through the issuance of equity or convertible debt securities, the percentage ownership of our stockholders could be significantly diluted, and these newly issued securities may have rights, preferences or privileges senior to those of existing stockholders. Debt, receivables and royalty financings may be coupled with an equity component, such as warrants to purchase stock, which could also result in dilution of our existing stockholders’ ownership. The incurrence of indebtedness would result in increased fixed payment obligations and could also result in certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business and may result in liens being placed on our assets and intellectual property. If we were to default on such indebtedness, we could lose such assets and intellectual property. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our product candidates or grant licenses on terms that are not favorable to us.
| 28 |
Our financial statements contain a statement regarding a substantial doubt about our ability to continue as a going concern.
We had no revenue during the year ended December 31, 2023 or in prior years, and do not have any revenue under contract or any immediate sales prospects. Our primary uses of cash are to fund our planned clinical trials, research and development expenditures and for operating expenses. Cash used to fund operating expenses is impacted by the timing of when we incur and pay these expenses. Our consolidated financial statements have been prepared using U.S. GAAP, and are based on the assumption that we will continue as a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business. We face certain risks and uncertainties that are present in many emerging pharmaceutical companies regarding product development, limited working capital, recurring losses and negative cash flow from operations, future profitability, ability to obtain future capital, protection of patents, technologies and property rights, competition, rapid technological change, navigating the domestic and major foreign markets’ regulatory and clinical environment, recruiting and retaining key personnel, dependence on third party manufacturing organizations, third party collaboration and licensing agreements, lack of sales and marketing activities. We currently have no customers or pharmaceutical products to sell or distribute. These risks and other factors raise substantial doubt about our ability to continue as a going concern.
Our ability to continue as a going concern is dependent on our ability to obtain the insurancenecessary financing to meet our obligations and repay our liabilities arising from the ordinary course of business operations when they become due. The substantial doubt about our ability to continue as a going concern may affect the price of our common stock, may impact our relationship with third parties with whom we do business, may impact our ability to raise additional capital and may impact our ability to comply going forward with covenants in our debt agreements.
Our ability to use our net operating loss carryforwards and other tax attributes may be limited.
As of December 31, 2023, we had net operating loss (NOL) carryforwards of approximately $28.7 million for federal and state income tax available to offset future taxable income, and federal and state research and development tax credits of approximately $1.2 million, prior to consideration of annual limitations that may be imposed under Section 382 of the Internal Revenue Code of 1986, as amended (Section 382). NOL carryforwards prior to 2018 will expire in 2037 if not utilized.
Our NOL and tax credit carryforwards could expire unused and be unavailable to offset future income tax liabilities. Under Section 382, and corresponding provisions of state law, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change, by value, in its equity ownership over a three-year period, the corporation’s ability to use its pre-change NOLs and other pre-change tax attributes, such as research and development tax credits, to offset its post-change income may be limited. We have not completed a Section 382 study and as such our net operating loss carryforwards may be subject to such limitation.
In addition, we may experience additional ownership changes in the future as a result of subsequent shifts in our stock ownership, including through completed or contemplated financings, some of which may be outside of our control. If we determine that a future ownership change has occurred and our ability to use our historical net operating loss and tax credit carryforwards is materially limited, it would harm our future operating results by effectively increasing our future tax obligations.
Risks Relating to Clinical Development and Commercialization of Our Product Candidates
We currently do not have, and may never develop, any FDA-approved, licensed or commercialized products.
We have not yet sought to obtain any regulatory approvals for any product candidates in the United States or in any foreign market. For us to develop any products that might be licensed or commercialized, we will have to invest further time and capital in research and product development, regulatory compliance and market development. Therefore, we and our licensors, prospective business partners and other collaborators may never develop any products that can be licensed or commercialized. All our development efforts will require substantial additional funding, none of which may result in any revenue.
Our licenses are subject to termination by the licensor in certain circumstances.
Our rights to practice the inventions claimed in the licensed patents and patent applications are subject to our licensors abiding by the terms of those licenses and not terminating them. Our licenses may be terminated by the licensor if we are in material breach of certain terms or conditions of the license agreement or in certain other circumstances. Our license agreements each include provisions that allow the licensor to terminate the license if (i) we breach any payment obligation or other material provision under the agreement and fail to cure the breach within a fixed time following written notice of termination; (ii) we or any of our affiliates, licensees or sublicensees directly or indirectly challenge the validity, enforceability, or extension of any of the licensed patents; or (iii) we declare bankruptcy or dissolve. The majority of license agreements require us to satisfy due diligence milestones that relate to the development of new products containing the licensed drug or the agreement may be terminated by such counterparty. Our rights under these licenses are subject to our continued compliance with the terms of the license, including the payment of royalties due under the licenses. Termination of any of these licenses could prevent us from marketing some or all of our products. Because of the complexity of our products and the patents we have licensed, determining the scope of the license and related royalty obligations can be difficult and can lead to disputes between us and the licensor. An unfavorable resolution of such a dispute could lead to an increase in the royalties payable pursuant to the license. If a licensor believed we were not paying the royalties due under the license or were otherwise not in compliance with the terms of the license, the licensor might attempt to revoke the license. If such an attempt were successful, we might be barred from producing and selling some or all of our products.
If we fail to comply with our obligations contained in the agreements under which we license intellectual property rights from third parties, we could lose important license rights.
Our license agreements have various requirements. For example, our license agreement with Elion Oncology, Inc. (“Elion”) for NGC-Cap (also known as PCS6422) requires us to administer the drug to the first patient in a Phase 2 or Phase 3 trial for NGC-Cap on or before October 2, 2024. Our license agreement with Ocuphire Pharma, Inc. (“Ocuphire”) for NGC-Gem (also known as PCS3117) imposes two future obligations on us. The first diligence obligation requires that the first patient must be administered the drug in a clinical trial on or before June 16, 2024, and the second obligation requires that the first patient is administered the drug (i) in a pivotal clinical trial, or (ii) in a clinical trial for a second indication of the drug on or before June 16, 2026. We are in the process of requesting an extension of these obligations with Ocuphire, and there is no assurance that we will be able to obtain such extension.
Should we fail to meet any of the obligations under our license agreements, absent extensions, other arrangements or other extenuating circumstances, the licensor may be able to terminate the license agreement, which would have a material effect on the value of our intellectual property portfolio, our financial condition, future prospects, and results of operations.
| 29 |
We depend entirely on the successful development of our product candidates, which have not yet demonstrated efficacy for their target indications in clinical trials. We may never be able to demonstrate efficacy for our product candidates, thus preventing us from licensing, obtaining marketing approval by any regulatory agency, and/or commercializing our product(s).
Our product candidates are either in the early stages of clinical development or late stages of preclinical development. Significant additional research and development activity and clinical testing are required before we will have a chance to achieve a viable product for licensing or commercialization from such candidates. Our research and development efforts remain subject to all the risks associated with the development of new biopharmaceutical products and treatments. Development of the underlying technology may be affected by unanticipated technical or other problems, among other research and development issues, and the possible insufficiency of funds needed in order to complete development of these product candidates. Safety, regulatory and efficacy issues, clinical hurdles or other challenges may result in delays and cause us to incur additional expenses that would increase our losses. If we and our collaborators cannot complete, or if we experience significant delays in developing, our potential therapeutics or products for use in potential commercial applications, particularly after incurring significant expenditures, our business may fail, and investors may lose the entirety of their investment.
When we submit an IND or foreign equivalent to the FDA or international regulatory authorities seeking approval to initiate clinical trials in the United States and other countries, we may not be successful in obtaining acceptance from the FDA or comparable foreign regulatory authorities to start our clinical trials. If we do not obtain such acceptance, the time in which we expect to commence clinical programs for any product candidate will be extended and such extension will increase our expenses and increase our need for additional capital. Moreover, there is no guarantee that our clinical trials will be successful or that we will continue clinical development in support of an approval from the FDA or comparable foreign regulatory authorities for any indication. We note that most drug candidates never reach the clinical development stage and even those that do commence clinical development have only a small chance of successfully completing clinical development and gaining regulatory approval. Therefore, our business currently depends entirely on the successful development, regulatory approval, and licensing or commercialization of our product candidates, which may never occur.
We must successfully complete clinical trials for our product candidates before we can apply for marketing approval.
Even if we complete our clinical trials, it does not assure marketing approval. Our clinical trials may be unsuccessful, which would materially harm our business. Even if our initial clinical trials are successful, we are required to conduct additional clinical trials to establish our product candidates’ safety and efficacy before submitting an NDA. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to the outcome. Success in early phases of pre-clinical and clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates. The research, testing, manufacturing, labeling, packaging, storage, approval, sale, marketing, advertising and promotion, pricing, export, import and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, which regulations differ from country to country.
We are not permitted to market our product candidates as prescription pharmaceutical products in the United States until we receive approval of an NDA from the FDA, or in any foreign countries until we receive the requisite approval from such countries.
| 30 |
We have little corporate history of conducting clinical trials. Our planned clinical trials or those of our collaborators may reveal significant adverse events, toxicities or other side effects not seen in our preclinical studies and may result in a safety profile that could inhibit regulatory approval or market acceptance of any of our product candidates.
Our operations to date have been limited to financing and staffing, conducting research and developing our core technologies, identifying and optimizing our lead product clinical candidates, performing due diligence on other potential drug in-licensing opportunities and further moving the clinical product candidates through the development programs identified. Some of the activities in the development programs include receiving FDA IND clearance on one indication for two product candidates, completing a Phase 2A trial for PCS12852 in gastroparesis patients, conducting a Phase 1B trial for NGC-Cap in patients with advanced gastrointestinal tumors, completing a Phase 1 healthy human volunteer trial, completing a Phase 2A clinical trial and conducting a Phase 2 clinical trial in patients with NL and receiving FDA orphan designation on PCS499 in NL. Other activities include improving the manufacturing of PCS499, PCS6422 and PCS11T final products and developing regulatory strategy plans for each of the products including expedited review plans, as applicable. Although we have recruited a team that has experience with clinical trials in the United States and outside the United States, as a company, we have only conducted four clinical trials in any jurisdiction and have not had previous experience commercializing product candidates through the FDA or similar submissions to initiate clinical trials or obtain marketing authorization to foreign regulatory authorities. We cannot be certain that other planned clinical trials will begin or be completed on time, if at all; that our development program and studies would be acceptable to the FDA or other regulatory authorities; or that, if regulatory approval is obtained, our product candidates can be successfully commercialized. Clinical trials and commercializing our product candidates will require significant additional financial and management resources, and reliance on third-party clinical investigators, CROs, consultants and collaborators. Relying on third-party clinical investigators, CROs or collaborators may result in delays that are outside of our control.
Furthermore, we may not have the financial resources to continue development of, or to enter into collaborations for, a product candidate if we experience any problems or other unforeseen events that delay or prevent regulatory approval of, or our ability to commercialize, product candidates.
Some preclinical studies and early clinical studies of our product candidates have been completed, but we do not know the predictive value of these studies for our targeted population of patients, and we cannot guarantee that any positive results in these studies will translate successfully to the larger targeted population of patients. It is not uncommon to observe results in human clinical trials that are unexpected based on preclinical testing or early clinical studies, and many product candidates fail in clinical trials despite promising preclinical or early clinical results. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval for their products. Human patients in clinical trials may suffer significant adverse events or other side effects not observed in our preclinical studies, including, but not limited to, immunogenic responses, organ toxicities such as liver, heart or kidney or other tolerability issues or possibly even death. The observed potency and kinetics of our planned product candidates in preclinical studies may not be observed in human clinical trials. If clinical trials of our planned product candidates fail to demonstrate efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our planned product candidates which may result in complete loss of expenditures which we devote to those products.
We may have difficulty recruiting patients to the clinical trial, patients may drop out of our trial, or we may be required to abandon the trial or our development efforts of that product candidate altogether. We, the FDA, an Institutional Review Board (“IRB”), or other applicable regulatory authorities may suspend clinical trials of a product candidate at any time for various reasons, including a belief that subjects in such trials are being exposed to unacceptable health risks or adverse side effects. Some potential therapeutics developed in the biotechnology industry that initially showed therapeutic promise in early-stage studies have later been found to cause side effects that prevented their further development. Even if the side effects do not preclude the drug from obtaining or maintaining marketing approval, undesirable side effects may inhibit market acceptance of the approved product due to its tolerability versus other therapies. Any of these developments could materially harm our business, financial condition, and prospects.
Further, if any of our product candidates obtains marketing approval, toxicities associated with our product candidates may also develop after such approval and lead to a requirement to conduct additional clinical safety trials, additional warnings being added to the labeling, significant restrictions on the use of the product or the withdrawal of the product from the market. We cannot predict whether our product candidates will cause toxicities in humans that would preclude or lead to the revocation of regulatory approval based on preclinical studies or early-stage clinical testing. However, any such event, were it to occur, would cause substantial harm to our business and financial condition and would result in the diversion of our management’s attention.
| 31 |
Even if we receive regulatory approval for any of our product candidates, we may not be able to successfully license or commercialize the product and the revenue that we generate from its sales, if any, may be limited.
If approved for marketing, the commercial success of our product candidates will depend upon each product’s acceptance by the medical community (including physicians, patients and health care payors) and the potential competitive products available to the patients upon commercialization. The degree of market acceptance for any of our product candidates will depend on a number of factors, including:
| ● | demonstration of clinical safety and efficacy; | |
| ● | relative convenience, dosing burden and ease of administration; | |
| ● | the prevalence and severity of any adverse effects; | |
| ● | the willingness of physicians to prescribe our product candidates, and the target patient population to try new therapies; | |
| ● | efficacy of our product candidates compared to competing products; | |
| ● | the introduction of any new products that may in the future become available targeting indications for which our product candidates may be approved; | |
| ● | new procedures or therapies that may reduce the incidences of any of the indications in which our product candidates may show utility; | |
| ● | pricing and cost-effectiveness; | |
| ● | the inclusion or omission of our product candidates in treatment guidelines; | |
| ● | the effectiveness of our own or any future collaborators’ sales and marketing strategies; | |
| ● | limitations or warnings contained in approved labeling from regulatory authorities; | |
| ● | our ability to obtain and maintain sufficient third-party coverage or reimbursement from government health care programs, including Medicare and Medicaid, private health insurers and other third-party payors or to receive the necessary pricing approvals from government bodies regulating the pricing and usage of therapeutics; and | |
| ● | the willingness of patients to pay out-of-pocket in the absence of third-party coverage or reimbursement or government pricing approvals. |
If any of our product candidates are approved, but do not achieve an adequate level of acceptance by physicians, health care payors and patients, we may not generate sufficient revenue and we may not be able to protect us against future claims. Further,achieve or sustain profitability. Our efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may never be successful.
In addition, even if we obtain insurance, someregulatory approvals, the timing or scope of any approvals may prohibit or reduce our ability to commercialize our product candidates successfully. For example, if the litigation claimsapproval process takes too long, we may miss market opportunities and give other companies the ability to develop competing products or establish market dominance. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render our product candidates not commercially viable.
| 32 |
We are completely dependent on third parties to manufacture our product candidates, and our commercialization of our product candidates could be covered underhalted, delayed or made less profitable if those third parties fail to obtain manufacturing approval from the FDA or comparable foreign regulatory authorities, fail to provide us with sufficient quantities of our insurance policies,product candidates or fail to do so at acceptable quality levels or prices.
We do not currently have, nor do we plan to acquire, the capability or infrastructure to manufacture the active pharmaceutical ingredient, or API, in our insurance carriers may seekproduct candidates for use in our clinical trials or for commercial products. In addition, we do not have the capability to deny coverage.formulate any of our product candidates into a finished drug product for commercial distribution. As a result, we will be obligated to rely on contract manufacturers, if and when any of our product candidates are approved for commercialization. We have not entered into an agreement with any contract manufacturers for commercial supply and may not be able to engage a contract manufacturer for commercial supply of any of our product candidates on favorable terms to us, or at all.
The facilities used by our contract manufacturers to manufacture our product candidates must be approved by the FDA or comparable foreign regulatory authorities pursuant to inspections that will be conducted after we submit an NDA or BLA to the FDA or their equivalents to other relevant regulatory authorities. We will not control the manufacturing process of, and will be completely dependent on, our contract manufacturing partners for compliance with cGMPs to manufacture both active drug substances and finished drug products. These cGMP regulations cover all aspects of the manufacturing, testing, quality control and record keeping relating to our product candidates. If our contract manufacturers do not successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or others, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of our product candidates or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved.
Our contract manufacturers will be subject to ongoing periodic unannounced inspections by the FDA and corresponding state and foreign agencies for compliance with cGMPs and similar regulatory requirements. We will not have control over our contract manufacturers’ compliance with these regulations and standards. Failure by any of our contract manufacturers to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure to grant approval to market any of our product candidates, delays, suspensions or withdrawals of approvals, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect our business. In addition, we will not have control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. Failure by our contract manufacturers to comply with or maintain any of these standards could adversely affect our ability to develop, obtain regulatory approval for or market any of our product candidates.
If, for any reason, these third parties are unable or unwilling to perform, we may not be able to terminate our agreements with them, and we may not be able to locate alternative manufacturers or formulators or enter into favorable agreements with them and we cannot be certain that any such third parties will have the manufacturing capacity to meet future requirements. If these manufacturers or any alternate manufacturer of finished drug product experiences any significant difficulties in its respective manufacturing processes for our API or finished products or should cease doing business with us, we could experience significant interruptions in the supply of any of our product candidates or may not be able to create a supply of our product candidates at all. Were we to encounter manufacturing issues, our ability to produce a sufficient supply of any of our product candidates might be negatively affected. Our inability to coordinate the efforts of our third-party manufacturing partners, or the lack of capacity available at our third-party manufacturing partners, could impair our ability to supply any of our product candidates at required levels. Because of the significant regulatory requirements that we would need to satisfy in order to qualify a new bulk or finished product manufacturer, if we face these or other difficulties with our current manufacturing partners, we could experience significant interruptions in the supply of any of our product candidates if we decided to transfer the manufacture of any of our product candidates to one or more alternative manufacturers in an effort to deal with the difficulties.
Any manufacturing problem or the loss of a contract manufacturer could be disruptive to our operations and result in lost sales. Additionally, we rely on third parties to supply the raw materials needed to manufacture our potential products. Any reliance on suppliers may involve several risks, including a potential inability to obtain critical materials and reduced control over production costs, delivery schedules, reliability and quality. Any unanticipated disruption to a future contract manufacturer caused by problems with suppliers could delay shipment of any of our product candidates, increase our cost of goods sold and result in lost sales.
| 33 |
We cannot guarantee that our future manufacturing and supply partners will be able to reduce the costs of commercial scale manufacturing of any of our product candidates over time. If the commercial-scale manufacturing costs of any of our product candidates are higher than expected, these costs may significantly impact our operating results. In order to reduce costs, we may need to develop and implement process improvements. However, in order to do so, we will need, from time to time, to notify or make submissions to regulatory authorities, and the improvements may be subject to approval by such regulatory authorities. We cannot be sure that we will receive these necessary approvals or that these approvals will be granted in a timely fashion. We also cannot guarantee that we will be able to enhance and optimize output in our commercial manufacturing process. If we cannot enhance and optimize output, we may not be able to reduce our costs over time.
Even if we obtain marketing approval for any of our product candidates, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expenses.
Even if we obtain regulatory approval for any of our product candidates for an indication, the FDA or foreign equivalent may still impose significant restrictions on their indicated uses or marketing or the conditions of approval or impose ongoing requirements for potentially costly and time-consuming post-approval studies, including Phase 4 clinical trials, and post-market surveillance to monitor safety and efficacy. Our product candidates will also be requiredsubject to incurongoing regulatory requirements governing the manufacturing, labeling, packaging, storage, distribution, safety surveillance, advertising, promotion, recordkeeping and reporting of adverse events and other post-market information. These requirements include registration with the FDA, as well as continued compliance with current Good Clinical Practices (cGCPs) for any clinical trials that we conduct post-approval. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP regulations, requirements relating to quality control, quality assurance and corresponding maintenance of records and documents. Compliance with such regulations may result in significant legalcosts and expenses.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, but a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from those in the United States, including additional preclinical studies or clinical trials, as clinical studies conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. If we fail to comply with the regulatory requirements in international markets and/or to receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be harmed.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval for our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell our product candidates. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products.
In the United States, the Medicare Modernization Act (MMA) changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for drugs. In addition, this legislation authorized Medicare Part D prescription drug plans to use formularies where they can limit the number of drugs that will be covered in any therapeutic class. As a result of this legislation and the expansion of federal coverage of drug products, we expect that there will be additional pressure to contain and reduce costs. These cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for our product candidates and could seriously harm our business. While the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates, and any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010 or, collectively, the ACA, is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The ACA revised the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. The law also imposed a significant annual fee on companies that manufacture or import branded prescription drug products. Further, on August 16, 2022, President Biden signed the Inflation Reduction Act of 2022 (IRA), into law which, among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. It is unclear how such challenges, and the healthcare reform measures of the Biden administration will impact the ACA and our business.
We do not know whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
| 34 |
We could face competition from other biotechnology and pharmaceutical companies, and our operating results would suffer if we fail to innovate and compete effectively.
Our products are used for indications where we believe that there is an unmet medical need. If existing or newly approved drug products, whether approved by the FDA for the indication or not, are able to successfully treat the same patients, it may be more difficult to perform clinical studies, to develop our product and/or to commercialize our product, adversely affecting our business. Since the biopharmaceutical industry is characterized by intense competition and rapid innovation, our competitors may be able to develop other compounds or drugs that are able to achieve similar or better results than our product candidates. Our competitors may include major multinational pharmaceutical companies, established biotechnology companies, specialty pharmaceutical companies, and universities and other research institutions. Many of our competitors have substantially greater financial, technical and other resources, such as a larger research and development staff and experienced marketing and manufacturing organizations, established relationships with no assuranceCROs and other collaborators, as well as established sales forces. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors. Competition may increase further as a result of outcome,advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors, either alone or with collaborative partners, may succeed in developing, acquiring or licensing on an exclusive basis drug or biologic products that are more effective, safer, more easily commercialized or less costly than our product candidates, or may develop proprietary technologies or secure patent protection and, in turn, exclude us from technologies that we may need for the development of our technologies and potential products.
Even if we obtain regulatory approval of any of our product candidates, we may not be the first to market and that may negatively affect the price or demand for our product candidates. Additionally, we may not be able to implement our business plan if the acceptance of our product candidates is inhibited by price competition or the reluctance of physicians to switch from existing methods of treatment to our product candidates, or if physicians switch to other new drug or biologic products or choose to reserve our product candidates for use in limited circumstances. Furthermore, for drugs that receive orphan drug designation at the FDA, a competitor could obtain orphan product approval from the FDA with respect to such competitor’s drug product. If such competitor drug product is determined to be the same product as one of our product candidates, we may be prevented from obtaining approval from the FDA for such product candidate for the same indication for seven years, except in limited circumstances, and we may be subject to adverse judgmentssimilar restrictions under non-U.S. regulations.
We rely on third parties to conduct clinical trials for our product candidates. If these third parties do not successfully carry out their contractual duties or settlementsmeet expected deadlines, we may not be able to obtain regulatory approval for or commercialize any of our product candidates and our business would be substantially harmed.
We have entered into agreements with third-party CROs to conduct and manage our clinical programs including contracting with clinical sites to perform our clinical studies. We rely heavily on these parties to execute clinical studies for our product candidates and will control only certain aspects of their activities. Nevertheless, we will be responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on CROs and clinical sites will not relieve us of our regulatory responsibilities. We and our CROs will be required to comply with cGCPs, which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area and comparable foreign regulatory authorities for any products in clinical development. The FDA and its foreign equivalents enforce these cGCP regulations through periodic inspections of trial sponsors, principal investigators and trial sites. If we or our CROs fail to comply with applicable cGCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, the FDA or other regulatory authorities will determine that any of our clinical trials comply with cGCPs. In addition, our clinical trials must be conducted with products produced under cGMP regulations and will require a large number of test subjects. Our failure or the failure of our CROs or clinical sites to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process and could also subject us to enforcement action up to and including civil and criminal penalties.
| 35 |
Although we design clinical trials for our product candidates in consultation with CROs, the CROs will manage all of the clinical trials conducted at contracted clinical sites. As a result, many important aspects of our drug development programs would be outside of our direct control. In addition, the CROs and clinical sites may not perform all of their obligations under arrangements with us or in compliance with regulatory requirements. If the CROs or clinical sites do not perform clinical trials in a satisfactory manner, breach their obligations to us or fail to comply with regulatory requirements, the development and commercialization of any of our product candidates for the subject indication may be delayed or our development program materially and irreversibly harmed. We cannot control the amount and timing of resources these CROs and clinical sites will devote to our program or any of our product candidates. If we are unable to rely on clinical data collected by our CROs, we could be required to repeat, extend the duration of, or increase the size of our clinical trials, which could significantly delay commercialization and require significantly greater expenditures.
If any of our relationships with these third-party CROs or clinical sites terminate, we may not be able to enter into arrangements with alternative CROs or clinical sites. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, any such clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our financial results and the commercial prospects for any of our product candidates would be harmed, our costs could increase and our ability to generate revenue could be delayed.
Clinical drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
Clinical testing of drug product candidates is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of pre-clinical studies and early clinical trials may not be predictive of the results of later-stage clinical trials. We cannot assure you that the FDA or comparable foreign regulatory authorities will view the results as we do or that any future trials of any of our product candidates will achieve positive results. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through pre-clinical studies and initial clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials. Any future clinical trial results for our product candidates may not be successful.
In addition, a number of factors could contribute to a lack of favorable safety and efficacy results for any of our product candidates. For example, such trials could result in increased variability due to varying site characteristics, such as local standards of care, differences in evaluation period and surgical technique, and due to varying patient characteristics including demographic factors and health status.
Even though we may apply for orphan drug designation for a product candidate, we may not be able to obtain orphan drug marketing exclusivity.
There is no guarantee that the FDA, EMA or their foreign equivalents will grant any future application for orphan drug designation for any of our product candidates, which would make us ineligible for the additional exclusivity and other benefits of orphan drug designation. Even where orphan drug designation or equivalent status is granted, there is no guarantee of orphan drug marketing exclusivity.
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug available in the Unites States for this type of disease or condition will be recovered from sales of the product. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of regulatory review and approval process.
| 36 |
If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which means the FDA may not approve any other applications to market the same drug for the same indication for seven years, except in limited circumstances, such as (i) the drug’s orphan designation is revoked; (ii) its marketing approval is withdrawn; (iii) the orphan exclusivity holder consents to the approval of another applicant’s product; (iv) the orphan exclusivity holder is unable to assure the availability of a sufficient quantity of drug; or (v) a showing of clinical superiority to the product with orphan exclusivity by a competitor product. If a drug designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan drug exclusivity. While the FDA granted orphan-drug designation to PCS499 for the treatment of NL and to PCS3117 for the treatment of pancreatic cancer, there can be no assurance that we will receive orphan drug designation for any additional product candidates in the indications for which we think they might qualify, if we elect to seek such applications.
Although we may pursue expedited regulatory approval pathways for a product candidate, it may not qualify for expedited development or, if it does qualify for expedited development, it may not actually lead to a faster development, regulatory review or approval process.
Although we believe there may be an opportunity to accelerate the development of certain of our product candidates through one or more of the FDA’s expedited programs, such as fast track, breakthrough therapy, accelerated approval or priority review, we cannot be assured that any of our product candidates will qualify for such programs.
For example, a drug may be eligible for designation as a breakthrough therapy if the drug is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Although breakthrough designation or access to any other expedited program may expedite the development or approval process, it does not change the standards for approval. If we apply for an expedited program for our product candidates, the FDA may determine that our proposed target indication or other aspects of our clinical development plans do not qualify for such expedited program. Even if we are successful in obtaining access to an expedited program, we may not experience faster development timelines or achieve faster review or approval compared to conventional FDA procedures. Access to an expedited program may also be withdrawn by the FDA if it believes that the designation is no longer supported by data from our clinical development program. Additionally, qualification for any expedited review procedure does not ensure that we will ultimately obtain regulatory approval for such product candidate.
Third-party coverage and reimbursement, health care cost containment initiatives and treatment guidelines may constrain our future revenues.
Our ability to successfully market our product candidates will depend in part on the level of reimbursement that government health administration authorities, private health coverage insurers and other organizations provide for the cost of our products and related treatments. Countries in which any of our product candidates may be sold through reimbursement schemes under national health insurance programs frequently require that manufacturers and sellers of pharmaceutical products obtain governmental approval of initial prices and any subsequent price increases. In certain countries, including the United States, government-funded and private medical care plans can exert significant indirect pressure on prices. We may not be able to sell our product candidates profitably if adequate prices are not approved or coverage and reimbursement is unavailable or limited in scope.
Legal, regulatory and legislative changes with respect to reimbursement, pricing and contracting may adversely affect our business and future prospects.
Federal and state governments may adopt policies affecting drug pricing and contracting practices outside of the context of federal programs such as Medicare and Medicaid, which may adversely affect our business. For example, several states have adopted laws that require drug manufacturers to provide advance notice of certain price increases and to report information relating to those price increases. There can be no assurances that future changes to Medicare and/or Medicaid prescription drug reimbursement policies, drug pricing and contracting practices, or government drug price regulation programs such as the Medicaid Drug Rebate Program or 340B Drug Pricing Program will not have an adverse impact on our business and future prospects.
| 37 |
We cannot predict what healthcare reform initiatives may be adopted in the future. Further federal, state and foreign legislative and regulatory developments are likely, and we expect ongoing initiatives to increase pressure on drug pricing. Such reforms could have an adverse effect on anticipated revenues from product candidates and may affect our overall financial condition and ability to develop product candidates.
We may face product liability exposure, and if successful claims are brought against us, we may incur substantial liability if our insurance coverage for those claims is inadequate.
We face an inherent risk of product liability as a result of the clinical testing of our product candidates and will face an even greater risk if we commercialize any products. This risk exists even if a product is approved for commercial sale by the FDA and manufactured in facilities licensed and regulated by the FDA or an applicable foreign regulatory authority. Our products and product candidates are designed to affect important bodily functions and processes. Any side effects, manufacturing defects, misuse or abuse associated with our product candidates could result in injury to a patient or even death. We cannot offer any assurance that we will not face product liability suits in the future, or that our insurance coverage will be sufficient to cover our liability in any such cases.
In addition, a liability claim may be brought against us even if our product candidates merely appear to have caused an injury. Product liability claims may be brought against us by consumers, health care providers, pharmaceutical companies or others selling or otherwise coming into contact with our product candidates, among others. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities and reputational harm. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| ● | withdrawal of clinical trial participants; | |
| ● | termination of clinical trial sites or entire trial programs; | |
| ● | the inability to commercialize our product candidates; | |
| ● | decreased demand for our product candidates; | |
| ● | impairment of our business reputations; | |
| ● | product recall or withdrawal from the market or labeling, marketing or promotional restrictions; | |
| ● | substantial costs of any related litigation or similar disputes; | |
| ● | distractions of management’s attention and other resources from our primary business; | |
| ● | substantial monetary awards to patients or other claimants against us that may not be covered by insurance; or | |
| ● | loss of revenue. |
We have obtained product liability insurance coverage for our clinical trials. However, large judgments have been awarded in class action or individual lawsuits based on drugs that had unanticipated side effects and our insurance coverage may not be sufficient to cover all of our product liability related expenses or losses and may not cover us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost, in sufficient amounts or upon adequate terms to protect us against losses due to product liability. We will need to increase our product liability coverage if any of our product candidates receive regulatory approval, which will be costly, and we may be unable to obtain this increased product liability insurance on commercially reasonable terms, or at all. A successful product liability claim, or series of claims, brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could decrease our cash and could harm our business, financial condition, operating results and prospects.
| 38 |
If any of our product candidates are approved for marketing and we are found to have improperly promoted off-label uses, or if physicians misuse our products or use our products off-label, we may become subject to prohibitions on the sale or marketing of our products, product liability claims and significant fines, penalties and sanctions, and our brand and reputation could be harmed.
The FDA and other regulatory agencies strictly regulate the marketing and promotional claims that are made about drug products. In particular, a product may not be promoted for uses or indications that are not approved by the FDA or such other regulatory agencies as reflected in the product’s approved labeling and comparative safety or efficacy claims cannot be made without direct comparative clinical data. If we are found to have promoted off-label uses of any of our product candidates, we may become subject to significant liability, which would materially harm our business. Both federal and state governments have levied large civil and criminal fines against companies for alleged improper promotion and have enjoined several companies from engaging in off-label promotion. If we become the target of such an investigation or prosecution based on our marketing and promotional practices, we could face similar sanctions, which would materially harm our business. In addition, management’s attention could be diverted from our business operations, significant legal expenses could be incurred, and our brand and reputation could be damaged.
The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. If we are deemed by the FDA to have engaged in the promotion of our products for off-label use, we could be subject to FDA regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our business activities constitute promotion of an off-label use, which could result in significant penalties, including criminal, civil or administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs and the curtailment or restructuring of our operations.
We cannot, however, prevent a physician from using our product candidates outside of those indications for use when the physician’s independent professional medical judgment deems appropriate. Physicians may also misuse our product candidates or use improper techniques, potentially leading to adverse results, side effects or injury, which may lead to product liability claims. If our product candidates are misused or used with improper technique, we may become subject to costly litigation by physicians or their patients. Furthermore, the use of our product candidates for indications other than those cleared by the FDA may not effectively treat such conditions, which could harm our reputation among physicians and patients.
We may choose not to continue developing or commercializing any of our product candidates at any time during development or after approval, which would reduce or eliminate our potential return on investment for those product candidates.
At any time, we may decide to discontinue the development of any of our product candidates or not to continue commercializing one or more of our approved product candidates for a variety of reasons, including changes in our internal product, technology or indication focus, the appearance of new technologies that make our product obsolete, competition from a competing product or changes in or failure to comply with applicable regulatory requirements. If we terminate a program in which we have invested significant resources, we will not receive any return on our investment, and we will have missed the opportunity to have allocated those resources to potentially more productive uses.
| 39 |
Risks Relating to Our Intellectual Property Rights
We depend on rights to certain pharmaceutical compounds that are or will be licensed to us. We do not own the intellectual property rights to these pharmaceutical compounds and any loss of our rights to them could prevent us from selling our products.
Within our present pipeline and potentially future pipeline of drugs, our drugs are in-licensed from other biotech or pharmaceutical companies. We do not currently own any intellectual property rights, including the patents that underlie these licenses. Our rights to use the pharmaceutical compounds we license are subject to the negotiation of, continuation of and compliance with the terms of those licenses. Thus, these patents and patent applications are not written by us or our attorneys, and we did not have control over the drafting and prosecution. The former patent owners and our licensors might not have given the same attention to the drafting and prosecution of these patents and applications as we would have if we had been the owners of the patents and applications and had control over the drafting. Moreover, under certain of our licenses, patent prosecution activities remain under the control of the licensor. We cannot be certain that drafting of the licensed patents and patent applications, or patent prosecution, by the licensors have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights.
Significant additional research and development activity, pre-clinical testing, and/or clinical testing of our drug product candidates are required before we will have a chance to achieve a viable product for licensing or commercialization. Our business currently depends entirely on the successful development, regulatory approval, and licensing or commercialization of our product candidates, which may never occur.
Enforcement of our licensed patents or defense of any claims asserting invalidity of these patents is often subject to the control or cooperation of our licensors. Legal action could be initiated against the owners of the intellectual property that we license and an adverse outcome in such legal action could harm our business because it might prevent such companies or institutions from continuing to license intellectual property that we may need to operate our business. In addition, such licensors may resolve such litigation in a way that benefits them but adversely affects our ability to have freedom to operate to develop and commercialize our product candidates.
We cannot ensure protection of our licensed intellectual property rights.
Our commercial success will depend, in part, on the ability of our licensors to obtain and maintain patent protection for our licensed technologies, products and processes, successfully defend these licensed patents against third-party challenges and successfully enforce these patents against third-party competitors. The patent positions of pharmaceutical companies can be highly uncertain and involve complex legal, scientific and factual questions for which important legal principles remain unresolved. Changes in either the patent laws or in interpretations of patent laws may diminish the value of our licensed intellectual property rights. Accordingly, we cannot predict the breadth of claims that may be allowable or enforceable in our patents. The existing patents and patent applications relating to our drug product candidates may be challenged, invalidated or circumvented by third parties and might not protect us against competitors with similar products or technologies.
The degree of future protection for our proprietary rights is uncertain. We may not be able to adequately protect our rights, gain or keep our competitive advantage, or provide any competitive advantage at all. For example, others have filed, and in the future are likely to file, patent applications covering products and technologies that are similar, identical or competitive to any of our product candidates, or important to our business. We cannot be certain that any patent application owned by a third party will not have priority over patent applications licensed or filed by us, or that our licensed intellectual property or intellectual property that we develop in the future will not be involved in interference, opposition or invalidity proceedings before United States or foreign patent offices.
In the future, we may rely on know-how and trade secrets to protect technology, especially in cases when we believe patent protection is not appropriate or obtainable. However, know-how and trade secrets are difficult to protect. While we intend to require employees, academic collaborators, consultants and other contractors to enter into confidentiality agreements, we may not be able to adequately protect our trade secrets or other proprietary or licensed information. Typically, research collaborators and scientific advisors have rights to publish data and information in which we may also have rights. If we cannot maintain the confidentiality of our licensed or owned proprietary technology and other confidential information, our ability to protect valuable information licensed or owned by us may be imperiled. Enforcing a claim that a third-party entity illegally obtained and is using any of our licensed or owned know-how and trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts are sometimes less willing to protect trade secrets than patents. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how.
| 40 |
If we fail to obtain or maintain patent or trade secret protection for our product candidates or our technologies, third parties could use our licensed or owned intellectual property, which could impair our ability to operate.compete in the market and adversely affect our ability to generate revenues and attain profitability.
We may also rely on the trademarks we may develop to distinguish our products from the products of our competitors. We cannot guarantee that any trademark applications filed by our licensors, us, or our business partners will be approved. Third parties may also oppose such trademark applications, or otherwise challenge our use of the trademarks. In the event that the trademarks we use are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition, and could require us to devote resources to advertising and marketing new brands. Further, we cannot provide assurance that competitors will not infringe the trademarks we use, or that we, our licensors, or business partners will have adequate resources to enforce these trademarks.
Our product candidates may infringe the intellectual property rights of others, which could increase our costs and delay or prevent our development and commercialization efforts.
Our success depends in part on avoiding infringement of the proprietary technologies of others. The pharmaceutical industry has been characterized by frequent litigation regarding patent and other intellectual property rights. Identification of third-party patent rights that may be relevant to our licensed technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. Additionally, because patent applications are maintained in secrecy until the application is published, we may be unaware of third-party patents that may be infringed by commercialization of any of our licensed product candidates or any future product candidate. There may be certain issued patents and patent applications claiming subject matter that we may be required to license in order to research, develop or commercialize any of our product candidates, and we do not know if such patents and patent applications would be available to license on commercially reasonable terms, or at all. Any claims of patent infringement asserted by third parties would be time-consuming and may divert the time and attention of our technical personnel and management.
Third parties may hold proprietary rights that could prevent any of our licensed product candidates from being marketed. Any patent-related legal action against us claiming damages and seeking to enjoin commercial activities relating to any of our product candidates or our processes could subject us to potential liability for damages and require us to obtain a license and pay royalties to continue to manufacture or market any of our product candidates or any future product candidates. We cannot predict whether we would prevail in any such actions or that any license required under any of these patents would be made available on commercially acceptable terms, if at all. In addition, we cannot be sure that we could redesign our product candidates or any future product candidates or processes to avoid infringement, if necessary. Accordingly, an adverse determination in a judicial or administrative proceeding, or the failure to obtain necessary licenses, could prevent us from developing and commercializing any of our product candidates or a future product candidate, which could harm our business, financial condition and operating results.
A number of companies, including several major pharmaceutical companies, have conducted, or are conducting, research within the licensed fields in which we intend to operate, which has resulted, or may result, in the filing of many patent applications related to this research. If we were to challenge the validity of these or any issued United States patent in court, we would need to overcome a statutory presumption of validity that attaches to every issued United States patent. This means that, in order to prevail, we would have to present clear and convincing evidence as to the invalidity of the patent’s claims. If we were to challenge the validity of these or any issued United States patent in an administrative trial before the Patent Trial and Appeal Board in the United States Patent and Trademark Office (USPTO), we would have to prove that the claims are unpatentable by a preponderance of the evidence. There is no assurance that a jury and/or court would find in our favor on questions of infringement, validity or enforceability.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and applications are required to be paid to the USPTO and various governmental patent agencies outside of the U.S. in several stages over the lifetime of the patents and applications. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process and after a patent has issued. There are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction.
| 41 |
General Company-Related Risks
We will need to grow the size of our organization, and we may experience difficulties in managing this growth.
As our development and commercialization plans and strategies develop, we may need to expand the size of our employee and consultant/contractor base. Future growth would impose significant added responsibilities on members of management, including the need to identify, recruit, maintain, motivate and integrate additional employees. In addition, our management may have to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. Our future financial performance and our ability to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to:
| ● | manage all our development efforts effectively, especially our clinical trials; | |
| ● | integrate additional management, administrative, scientific, operation and regulatory personnel; | |
| ● | maintain sufficient administrative, accounting and management information systems and controls; and | |
| ● | hire and train additional qualified personnel. |
We may not be able to accomplish these tasks, and our failure to accomplish any of them could harm our financial results.
If we lose key management personnel, or if we fail to recruit additional highly skilled personnel, our ability to identify and develop new or next generation product candidates will be impaired, could result in loss of markets or market share and could make us less competitive.
We are highly dependent upon the principal members of our small management team and staff, including George Ng, our Chief Executive Officer; David Young, Pharm.D., Ph.D, our President of Research and Development; and Sian Bigora, Pharm.D., our Chief Development and Regulatory Officer. The employment of Drs. Young and Bigora may be terminated at any time by either us or Dr. Young or Dr. Bigora. The loss of any current or future team member could impair our ability to design, identify, and develop new intellectual property and product candidates and new scientific or product ideas. Additionally, if we lose the services of any of these persons, we would likely be forced to expend significant time and money in the pursuit of replacements, which may result in a delay in the development of our product candidates and the implementation of our business plan and plan of operations and diversion of our management’s attention. We can give no assurance that we can find satisfactory replacements for our current and future key scientific and management employees on terms that would not be unduly expensive or burdensome to us.
Despite our efforts to retain valuable employees, members of our management, scientific and development teams may terminate their employment with us on short notice. Although we expect to have employment agreements with our key employees, these employment agreements may still allow these employees to leave our employment at any time, for or without cause. We do not maintain “key man” insurance policies on the lives of these individuals or the lives of any of our other employees. Our success also depends on our ability to continue to attract, retain and motivate highly skilled junior, mid-level and senior managers as well as junior, mid-level and senior scientific and medical and scientific personnel.
If our information technology systems or data, or those of third parties upon which we rely, are or were compromised, we could experience adverse consequences resulting from such compromise, including but not limited to regulatory investigations or actions, interruptions or disruptions to operations or clinical trials, reputational harm, litigation, fines and penalties.
In the ordinary course of our business, we, or the third parties upon which we rely, process, collect, receive, store, use, transmit, transfer, make accessible, protect, secure, dispose of, disclose and share proprietary, confidential, and sensitive data, including personal data (such as health-related data), intellectual property, and trade secrets.
Cyberattacks, malicious internet-based activity, online and offline fraud and other similar activities threaten the confidentiality, integrity, and availability of our sensitive information and information technology systems, and those of the third parties upon which we rely.
We and the third parties upon which we rely are subject to a variety of evolving threats, including but not limited to, social engineering attacks (including through phishing attacks), malicious code (such as viruses and worms), malware (including as a result of advanced persistent threat intrusions), denial-of-service attacks (such as credential stuffing), personnel misconduct or error, ransomware attacks, supply-chain attacks, software bugs, server malfunction, software or hardware failures, loss of data or other information technology assets, adware, telecommunications failures, earthquakes, fire, flood, and other similar threats.
We rely on third-party service providers and technologies to operate critical business systems to process sensitive information in a variety of contexts, including, without limitation, cloud-based infrastructure, drug suppliers, data center facilities, encryption and authentication technology, employee email, content delivery to customers, and other functions. Our ability to monitor these third parties’ information security practices is limited, and these third parties may not have adequate information security measures in place.
Any of the previously identified or similar threats could cause a security incident or other interruption that could result in unauthorized, unlawful, or accidental acquisition, modification, destruction, loss, alteration, encryption, disclosure of, or access to our sensitive information or our information technology systems, or those of the third parties upon whom we rely. A security incident or other interruption could disrupt our ability (and that of third parties upon whom we rely) to perform our research and development activities.
| 42 |
We are a voluntary filerexposed to cyber-attacks and are not required to file Exchange Act reports going forwarddata breaches, including the risks and may cease to file reports at any timecosts associated with protecting our systems and for any reason without notice.maintaining integrity and security of our business information, as well as personal data of our guests, employees and business partners.
On June 5, 2013, our S-1 registration statement became effective which meant we becameWe are subject to certain reporting obligationscyber-attacks. These cyber-attacks can vary in scope and intent from attacks with the objective of compromising our systems, networks and communications for economic gain to attacks with the Exchange Act. Under Section 15(d)objective of disrupting, disabling or otherwise compromising our operations. The attacks can encompass a wide range of methods and intent, including phishing attacks, illegitimate requests for payment, theft of intellectual property, theft of confidential or non-public information, installation of malware, installation of ransomware and theft of personal or business information. The breadth and scope of these attacks, as well as the Exchange Acttechniques and sophistication used to conduct these attacks, have grown over time.
A successful cyber-attack may target us directly, or it may be the result of a third party’s inadequate care. In either scenario, we may suffer damage to our systems and data that could interrupt our operations, adversely impact our reputation and brand and expose us to increased risks of governmental investigation, litigation and other liability, any of which could adversely affect our business. Furthermore, responding to such an issuer isattack and mitigating the risk of future attacks could result in additional operating and capital costs in systems technology, personnel, monitoring and other investments.
In addition, we are also subject to the periodic and current reporting requirements of Section 13(a) of that Act. These reports would include Forms 10-K, 10-Q, and 8-K, but would not include items such as beneficial ownership reports and proxy statements. The reporting obligation for such an issuer is automatically suspended if the class of securities is held by fewer than 300 record holders at the beginning of any fiscal year (other than a year in which the registration statement became effective). Such an issuer then becomes a voluntary filer. A voluntary filer may continue to file periodic reports on a voluntary basis; however, it is not required to do so.
On January 1, 2015, we had fewer than 300 record holders and, thus, our reporting obligations were automatically suspended. As such, we became a voluntary filer. Although we are delinquent at this time in filing our Exchange Act reports for 2016, we have no plans to cease filing Exchange Act reports. We may, however, cease to file our Exchange Act reports at any time and for any reason without notice. As a result, less information may be publicly available than would be otherwise contained in our reports.
11
We may not maintain sufficient insurance coverage for thevarious risks associated with the collection, handling, storage and transmission of sensitive information. In the course of doing business, we collect employee, customer and other third-party data, including personally identifiable information and individual credit data, for various business purposes. These laws continue to develop and may be inconsistent from jurisdiction to jurisdiction. If we fail to comply with the various applicable data collection and privacy laws, we could be exposed to fines, penalties, restrictions, litigation or other expenses, and our business operations. Accordingly,could be adversely impacted.
Any breach, theft, loss, or fraudulent use of employee, third-party or company data, could adversely impact our reputation and expose us to risks of data loss, business disruption, governmental investigation, litigation and other liability, any of which could adversely affect our business. Significant capital investments and other expenditures could be required to remedy the problem and prevent future breaches, including costs associated with additional security technologies, personnel, experts and credit monitoring services for those whose data has been breached. Further, if we or our vendors experience significant data security breaches or fail to detect and appropriately respond to significant data security breaches, we could be exposed to government enforcement actions and private litigation.
Risks Related to Ownership of Our Common Stock
Our failure to maintain compliance with Nasdaq’s continued listing requirements could result in the delisting of our Common Stock.
Our common stock is currently listed for trading on The Nasdaq Capital Market. We must satisfy The Nasdaq Capital Market’s continued listing requirements, including, among other things, a minimum bid price requirement of $1.00 per share or risk delisting, which would have a material adverse effect on our business. A delisting of our common stock from The Nasdaq Capital Market could materially reduce the liquidity of our common stock and result in a corresponding material reduction in the price of our common stock. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may incur significant expensesresult in the potential loss of confidence by investors, suppliers, customers and employees and fewer business development opportunities.
Although we have not been delisted from Nasdaq and currently meet the minimum listing requirements, on March 22, 2023, we received notice from the Listing Qualifications Staff of Nasdaq indicating that, based upon the closing bid price of our common stock for uninsured eventsthe prior 30 consecutive business days, we were not in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing on Nasdaq as set forth in Nasdaq Listing Rule 5550(a)(2). Nasdaq granted us an extension until March 18, 2024 to regain compliance with the minimum closing bid requirement, and we regained compliance with such requirement as of February 2, 2024.
If our common stock were delisted from Nasdaq, trading of our common stock would most likely take place on an over-the-counter market established for unlisted securities, such as the OTCQB or the Pink Market maintained by OTC Markets Group Inc. An investor would likely find it less convenient to sell, or to obtain accurate quotations in seeking to buy, our common stock on an over-the-counter market, and many investors would likely not buy or sell our common stock due to difficulty in accessing over-the-counter markets, policies preventing them from trading in securities not listed on a national exchange or other reasons. In addition, as a delisted security, our common stock would be subject to SEC rules as a “penny stock,” which impose additional disclosure requirements on broker-dealers. The regulations relating to penny stocks, coupled with the typically higher cost per trade to the investor of penny stocks due to factors such as broker commissions generally representing a higher percentage of the price of a penny stock than of a higher-priced stock, would further limit the ability of investors to trade in our common stock. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, suppliers, customers and employees and fewer business development opportunities. For these reasons and others, delisting would adversely affect the liquidity, trading volume and price of our common stock, causing the value of an investment in us to decrease and having an adverse effect on our business, financial condition and results of operations, could be materiallyincluding our ability to attract and adversely affected.
Risks associated with our business and operations include, but are not limited to, claims for wrongful acts committed by our officers, directors,retain qualified employees and to raise capital.
Future equity offerings, license transactions or acquisitions may dilute our existing stockholders’ ownership and/or have other representatives,adverse effects on our operations.
In order to raise additional capital, we may in the lossfuture offer additional shares of intellectual propertyour common stock or other securities convertible into or exchangeable for our common stock at prices that may be higher or lower than what our existing stockholders paid and other securities in the future could have rights the loss of key personnel and risks posed by natural disasters. Any of these risks may result in significant losses. We do not carry business interruption insurance. superior to existing stockholders.
In addition, we cannotmay engage in one or more potential license transactions or acquisitions in the future, which could involve issuing our common stock as some or all of the consideration payable by us to complete such transactions. If we issue common stock or securities linked to our common stock, the newly issued securities may have a dilutive effect on the interests of the holders of our common stock. Additionally, future sales of newly issued shares used to effect a transaction could depress the market price of our common stock and have a dilutive effect on our existing stockholders.
We may also issue equity securities that provide any assurance thatrights, preferences and privileges senior to those of our insurance coverage is sufficientcommon stock. If we raise additional funds by issuing debt securities, these debt securities would have rights senior to cover any losses thatthose of our common stock and the terms of the debt securities issued could impose significant restrictions on our operations, including liens on our assets. If we raise additional funds through collaborations and licensing arrangements, we may sustain,be required to relinquish some rights to our technologies or candidate products, or to grant licenses on terms that we willare not favorable to us.
| 43 |
Our common stock price is expected to be able to successfully claimvolatile.
The market price of our losses under our insurance policies on a timely basis or at all. If we incur any loss not covered by our insurance policies, or the compensated amount is significantly less than our actual loss or is not timely paid, our business, financial condition and results of operations could be materially and adversely affected.
We have raised substantial amounts of capital in private placements and if it is determined that we failed to comply with applicable securities laws, wecommon stock could be subject to rescission claims or lawsuits that could severely damage our financial position.
Wesignificant fluctuations. Market prices for securities of early-stage pharmaceutical, biotechnology and other life sciences companies have offered and sold securities in private placements to investors pursuant to certain exemptions from the registration requirementshistorically been particularly volatile. Some of the Securities Act of 1933, as well as those of various state securities laws. Such exemptions are highly technical in nature andfactors that may cause the basis for relying on such exemptions is factual; that is, the applicability of such exemptions depends upon our conduct and that of those persons contacting prospective investors and making the offering. We have not received a legal opinion to the effect that any of our prior offerings were exempt from registration under any federal or state law. Instead, we have relied upon the operative facts as the basis for such exemptions, including information provided by investors themselves. If any prior offerings did not qualify for such exemption, an investor would have the right to rescind its purchase of the securities if it so desired. If investors were successful in seeking rescission, we would face severe financial demands that could adversely affect our business and operations. Additionally, if we did not in fact qualify for the exemptions upon which it has relied, we may become subject to significant fines and penalties imposed by the Securities and Exchange Commission and state securities agencies.
Risks Related to our Common Stock:
The exercise of our warrants and options may result in a dilution of our current stockholders' voting power and an increase in the number of shares eligible for future resale in the public market which may negatively impact the trading price of our shares of common stock.
The exercise of some or all of our outstanding warrants and options could significantly dilute the ownership interests of our existing stockholders. As of December 31, 2016 we had outstanding warrants to purchase an aggregate of 2,000,304 shares of common stock the issuance of up to 269,500 sharesfluctuate include:
| ● | relatively low trading volume, which can result in significant volatility in the market price of our common stock based on a relatively smaller number of trades and dollar amount of transactions; | |
| ● | changes in estimates or recommendations by securities analysts, if any, who cover our common stock; | |
| ● | the timing and results of our current and any future preclinical or clinical trials of our product candidates; | |
| ● | the entry into or termination of key agreements, including, among others, key collaboration and license agreements; | |
| ● | the results and timing of regulatory reviews relating to the approval of our product candidates; | |
| ● | the initiation of, material developments in, or conclusion of, litigation to enforce or defend any of our intellectual property rights; | |
| ● | failure of any of our product candidates, if approved, to achieve commercial success; | |
| ● | general and industry-specific economic conditions that may affect our research and development expenditures; | |
| ● | the results of clinical trials conducted by others on products that would compete with our product candidates; | |
| ● | issues in manufacturing our product candidates or any approved products; | |
| ● | the introduction of technological innovations or new commercial products by our competitors; | |
| ● | developments or disputes concerning patent applications, issued patents or other proprietary rights; | |
| ● | future sales of our common stock by us, our insiders or our other stockholders; | |
| ● | a negative outcome in any litigation or potential legal proceeding; | |
| ● | additions and departures of key personnel; | |
| ● | negative publicity or announcements regarding regulatory developments relating to our products; | |
| ● | actual or anticipated fluctuations in our financial condition and operating results, including our cash and cash equivalents balance, operating expenses, cash burn rate or revenue levels; | |
| ● | our filing for protection under federal bankruptcy laws; or | |
| ● | the other factors described in this “Risk Factors” section. |
The stock upon exercise of stock options and 40,000 performance stock options. To the extent warrants and/or options are exercised, additional shares of common stock will be issued, and such issuance will dilute existing stockholders.
In additionmarkets in general have experienced substantial volatility that has often been unrelated to the dilutive effects described above, the exerciseoperating performance of those securities would lead to an increase in the number of shares eligible for resale in the public market. Substantial dilution and/or a substantial increase in the number of common shares available for future resaleindividual companies. These broad market fluctuations may negatively impact the trading price of our shares of common stock.
12
An active, liquid and orderly trading market for our common stock may not develop andalso adversely affect the trading price of our common stock may be volatile. If an orderly trading market for our common stock does not develop and/or ifstock. In the trading price for our common stock is volatile, the trading pricepast, following periods of our shares of common stock will likely resultvolatility in higher spreads.
Our common stock is trading in the over-the-counter market and is quoted on the OTC Pink. The over-the-counter market for securities has historically experienced extreme price and volume fluctuations during certain periods. These broad market fluctuations and other factors, such as our ability to implement our business, as well as economic conditions and quarterly variations in our results of operations, may adversely affect the market price of a company’s securities, stockholders have often instituted class action securities litigation against those companies. Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could significantly harm our common stock. In addition, the spreads on stock traded through the over-the-counter market are generally unregulatedprofitability and higher than on stock exchanges, which means that the difference between the price at which shares could be purchased by investors on the over-the-counter market compared to the price at which they could be subsequently sold would be greater than on these exchanges. Significant spreads between the bid and asked prices of the stock could continue during any period in which a sufficient volume of trading is unavailable or if the stock is quoted by an insignificant number of market makers. We cannot insure that our trading volume will be sufficient to significantly reduce this spread, or that we will have sufficient market makers to affect this spread. These higher spreads could adversely affect investors who purchase the shares at the higher price at which the shares are sold, but subsequently sell the shares at the lower bid prices quoted by the brokers. Unless the bid price for the stock increases and exceeds the price paid for the shares by the investor, plus brokerage commissions or charges, the investor could lose money on the sale. For higher spreads such as those on over-the-counter stocks, this is likely a much greater percentage of the price of the stock than for exchange listed stocks. There is no assurance that at the time the investor wishes to sell the shares, the bid price will have sufficiently increased to create a profit on the sale. We do not anticipate that there will be any public trading market for the warrants.reputation.
We are an “emerging growth company”a “smaller reporting company,” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies willus as such may make our common stock less attractive to our stockholders and investors.
We are an “emerging growtha “smaller reporting company” under the federal securities laws and, as such, are subject to scaled disclosure requirements afforded to such companies. For example, as a smaller reporting company,” as defined in the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicablesubject to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 and reduced disclosure obligations regarding executive compensation in our periodic reportsdisclosure requirements. Our stockholders and proxy statements. We cannot predict if investors will find our common stock less attractive if we rely on these exemptions. If some investorsmay find our common stock less attractive as a result of our status as a “smaller reporting company” and our reliance on the reduced disclosure requirements afforded to these companies. If some of our stockholders or investors find our common stock less attractive, there may be a less active trading market for our common stock their respective prices may be more volatile.
Ownership of our common shares is concentrated and investors will have minimal influence on stockholder decisions.
As of December 31, 2016, our executive officer, director, and a small number of investors, beneficially owned an aggregate of 8,240,797 shares of common stock, shares underlying warrants, shares underlying exercisable options and/or preferred stock convertible into common stock, representing approximately 75% of the voting power of our then-outstanding capital stock. As a result, our existing officer, director, and such investors could significantly influence stockholder actions of which other investors disapprove or that are contrary to their interests. This ability to exercise significant influence could prevent or significantly delay another company from acquiring or merging with us and the trading price of our shares of common stock could decline, and, accordingly, investors may lose all or part of their investment.
Securities analysts may not cover our common stock and this may have a negative impact on the market price of our common stock.stock may be more volatile.
| 44 |
We do not currently intend to pay dividends to our stockholders in the foreseeable future, and consequently, your ability to achieve a return on your investment will depend on appreciation in our value.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future. There is no guarantee that our valuation will appreciate in value or even maintain the valuation at which our stockholders have purchased their shares.
If securities or industry analysts do not publish research or reports about our business, or if they publish negative evaluations of our stock or negative reports about our business, our stock price and trading volume could decline.
The trading market for our common stock maywill depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There isWe may never obtain research coverage by industry or financial analysts. If no guaranteeor few analysts commence coverage of us, the trading price of our stock would likely decrease. Even if we do obtain analyst coverage, there can be no assurance that securities analysts will cover us or provide favorable coverage. If one or more of the analysts who cover us downgrades our common stock. If securities analysts do not cover our common stock the lack of research coverage may adversely affect the market priceor changes his or her opinion of our common stock. If we are covered by securities analysts, andstock, our stock is downgraded, the price of our stock would likely decline. If one or more of these analysts ceasescease coverage of our company or fail to cover us or fails toregularly publish regularly reports on us, we could lose or fail to gain visibility in the financial markets, which could cause a decline in our stock price and/or trading volume to decline.
Provisions in our corporate documents and accordingly, investors may lose all or part of their investment.
13
The application of the Securities and Exchange Commission’s “penny stock” rules to our common stockDelaware law could limit trading activity in the market, and our stockholders may find it more difficult to sell their stock.
If our common stock trades at less than $5.00 per share, then it will be subject to the Securities and Exchange Commission’s (“SEC”) penny stock rules. Penny stocks generally are equity securities with a price of less than $5.00. Penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that provides information about penny stocks and the risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s account. The broker-dealer must also make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These requirements may have the effect of reducingdelaying, deferring, or preventing a change in control of us, even if that change may be considered beneficial by some of our stockholders.
The existence of some provisions of our certificate of incorporation or our bylaws or Delaware law could have the leveleffect of trading activity, if any,delaying, deferring, or preventing a change in control of us that a stockholder may consider favorable. These provisions include:
| ● | providing that the number of members of our Board is limited to a range fixed by our bylaws; | |
| ● | establishing advance notice requirements for nominations of candidates for election to our Board of Directors or for proposing matters that can be acted on by stockholders at stockholder meetings; and | |
| ● | authorizing the issuance of “blank check” preferred stock, which could be issued by our Board of Directors to issue securities with voting rights and thwart a takeover attempt. |
| 45 |
As a Delaware corporation, we are also subject to provisions of Delaware law, including Section 203 of the General Corporation Law of the State of Delaware. Section 203 prevents some stockholders holding more than 15% of our voting stock from engaging in certain business combinations unless the business combination or the transaction that resulted in the secondary market for a security that becomes subject tostockholder becoming an interested stockholder was approved in advance by our Board of Directors, results in the penny stock rules. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from effecting transactions in our securities, which could severely limit their market price and liquiditystockholder holding more than 85% of our securities. These requirements may restrictvoting stock (subject to certain restrictions), or is approved at an annual or special meeting of stockholders by the abilityholders of broker-dealersat least 66 2/3% of our voting stock not held by the stockholder engaging in the transaction. Any provision of our certificate of incorporation or our bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to sell our common stock and may affect investors’ ability to resell our common stock which may depress the market pricereceive a premium for their shares of our common stock and accordingly,affect the price that some investors may lose all or part of their investment.
We do not intendare willing to pay dividends onfor our common stockstock.
Provisions in our bylaws provide for indemnification of officers and directors, which could require us to direct funds away from our business and the development of our product candidates.
Our bylaws provide for the foreseeable future,indemnification of our officers and investors must rely on increasesdirectors. We may in the market pricesfuture be required to advance costs incurred by an officer or director and to pay judgments, fines and expenses incurred by an officer or director, including reasonable attorneys’ fees, as a result of actions or proceedings in which our officers and directors are involved by reason of being or having been an officer or director of our common stockcompany. Funds paid in satisfaction of judgments, fines and expenses may be funds we need for returns on their investment.
For the foreseeable future, we intend to retain any earnings to finance the development and expansionoperation of our business and we do not anticipate paying any cash dividends onthe development of our common stock. Accordingly, investors must be preparedproduct candidates, thereby affecting our ability to rely on salesattain profitability.
Item 1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
Cybersecurity Risk Management and Strategy
We have established processes to assess, identify, and manage cybersecurity risks. These processes are integrated into our overall risk management program and are designed to protect our information assets from internal and external cyber threats and include:
| ● | implementing physical, procedural, and technical safeguards; | |
| ● | developing and maintaining comprehensive response plans; | |
| ● | engaging with external cybersecurity experts to enhance our oversight and keep pace with evolving threats; and | |
| ● | considering the cybersecurity capabilities of partners and third-party service providers, both prior to engaging them and on an ongoing basis. |
Cybersecurity Governance and Oversight
Our Board of their common stock after price appreciationDirectors provides direct oversight of cybersecurity risk and has delegated to earn an investment return, which may never occur. Investors seeking cash dividends should not purchaseits audit committee the responsibility of reviewing and discussing with management our common stock. Any determinationrisk exposures relating to pay dividends in the future will be made at the discretion of ourcybersecurity. The Board of Directors and the audit committee will dependreceive regular updates from management on cybersecurity matters and are promptly informed by management about any significant new threats or incidents. In the future, management and our third-party service providers will conduct reviews at least once annually of our cybersecurity readiness to ensure continuous improvement in our cybersecurity strategies.
| 46 |
We have implemented mechanisms to monitor and manage cybersecurity threats and incidents, including utilization of tools for continuous monitoring of our IT environment to detect and mitigate threats, a fundamental plan for responding to cyber incidents and training for employees to recognize and report potential cybersecurity incidents and to foster a culture of cybersecurity awareness and vigilance. Our Chief Administrative Officer, along with a third-party service provider, are responsible for operational oversight of our cybersecurity strategy and policies. Any identified cybersecurity incident is reported to our Chief Administrative Officer who evaluates the severity of the incident. Based on this assessment, further steps are taken involving other members of management and, depending on the severity, the audit committee and the Board of Directors. We believe this structured approach allows us to effectively manage and mitigate cybersecurity risks, safeguarding our systems and data against various digital threats. Additionally, our proactive stance is supported by cybersecurity insurance, which further reinforces our preparedness against potential cyber threats.
Cybersecurity Incident Reporting and Management
During the years ended December 31, 2022 and 2023, we have not identified any risks from cybersecurity threats that have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations, or financial condition, contractual restrictions, restrictions imposed by applicable lawcondition. However, we remain vigilant and other factors our Boardprepared to respond effectively to any incidents, should they arise.
Item 2. Properties.
Our principal executive office is located at 7380 Coca Cola Drive, Suite 106, Hanover, MD 21076. We currently lease approximately 6,500 square feet of Directors deems relevant.office space at this location until September 2025.
ITEM 1B. Unresolved Staff Comments
None.
ITEM 2. Properties
The Company terminated its office and warehouse lease in January 2016. There are currently no properties held by the Company.
ITEMItem 3. Legal ProceedingsProceedings.
The Company faces exposureFrom time to varioustime, we may become involved in litigation or other legal claims in the ordinary course of business. We maintain insurance policies in the amounts and with coverage and deductibles as we believe reasonable and prudent. However, we cannot be assured that the insurance companies will promptly honor their policy obligations or that the coverage or levels of insurance will be adequate to protect us from all material expenses related to future claims.proceedings. We are not currently a party to any litigation or legal proceedings. Regardless of outcome, any litigation that we may become involved in any material legal proceedings, directly or indirectly,can have an adverse impact on us because of defense and we are not awaresettlement costs, diversion of any claims pending or threatened against us or any of the directors that could result in the commencement of material legal proceedings.management resources and other factors.
ITEMItem 4. Mine Safety DisclosureDisclosures.
Not applicable.None.
Part II
PART II
ITEMItem 5. Market for Registrant’s Common Stock andEquity, Related Stockholder Matters and issuer Purchases of Equity Securities.
Our common stock $0.0001 par value, has beenis traded on the Over-the-Counter marketplace (OTCQB) during 2016Nasdaq Capital Market under the symbol “HUWX” since October 7, 2013. Prior“PCSA.”
On June 29, 2023, we amended our Certificate of Incorporation to October 7, 2013, thereincrease the number of authorized shares of our common stock from 50,000,000 to 100,000,000. The number of authorized shares of preferred stock remains unchanged at 1,000,000 shares.
On January 22, 2024, we effected a 1-for-20 reverse stock split, reducing the number of our common shares issued on that date from 24,706,474 shares to 1,291,000 shares. There was no public marketcorresponding reduction in the number of authorized shares of common stock and no change in the par value per share. All share and per share amounts and conversion and exercise prices presented herein have been adjusted retroactively to reflect this change.
Holders
As of March 21, 2024, there were 2,855,981 shares of common stock outstanding and 187 shareholders of record. One of these holders of record is Cede & Co, a nominee for Depository Trust Company (“DTC”). All of the shares of common stock held by brokerage firms and other financial institutions as nominees for beneficial owners are deposited into participant accounts at DTC and are therefore considered to be held of record by Cede & Co. as one stockholder. The actual number of stockholders is greater than the number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock.stock is Continental Stock Transfer & Trust Company.
|
| CLOSING BID |
| CLOSING ASK | ||||
2016 |
| HIGH |
| LOW |
| HIGH |
| LOW |
First Quarter |
| 0.23 |
| 0.16 |
| 0.40 |
| 0.30 |
Second Quarter |
| 0.18 |
| 0.16 |
| 0.33 |
| 0.30 |
Third Quarter |
| 0.16 |
| 0.16 |
| 0.38 |
| 0.30 |
Fourth Quarter |
| 0.16 |
| 0. 14 |
| 0.43 |
| 0.25 |
|
|
|
|
|
|
|
|
|
|
| CLOSING BID |
| CLOSING ASK | ||||
2015 |
| HIGH |
| LOW |
| HIGH |
| LOW |
First Quarter |
| 2.10 |
| 1.10 |
| 2.20 |
| 0.90 |
Second Quarter |
| 1.50 |
| 0.52 |
| 1.70 |
| 0.26 |
Third Quarter |
| 1.01 |
| 0.51 |
| 1.01 |
| 0.55 |
Fourth Quarter |
| 0.51 |
| 0.15 |
| 0.70 |
| 0.15 |
| 47 |
The above quotations, as provided by OTC Markets Group, Inc., reflect inter-dealer prices and do not include retail markup, markdown or commissions. In addition, these quotations may not necessarily represent actual transactions.Dividend Policy
As of September 10, 2017 we had 107 stockholders of record.
Dividends
We have nevernot previously declared or paid any cash dividends on our common stock. Westock and do not anticipate paying any cash dividendsintend to stockholdersdo so in the foreseeablenear future. In addition,We intend to retain any future earnings to fund ongoing operations and future capital requirements of our business. Any future determination to pay cash dividends will be at the discretion of the Board of Directors and will be dependent upon our financial condition, results of operations, capital requirements and such other factors as the Board of Directors deemdeems relevant.
Securities Authorized for Issuance under Equity Compensation Plans
Our Board of Directors and stockholdersapproved the Amended and Restated Heatwurx, Inc. 2011 Equity Incentive Plan (the “Plan”) in October 2012.
Eligibility. Employees, non-employee directors, advisors, and consultants of the Company and its affiliates are eligible to receive grants under the Plan.
Shares Available. In October 2012, the Board of Directors and stockholders increased the number of shares of common stock reserved for issuance under the Plan to a total of 1,800,000 shares. There are currently 269,500 outstanding option grants to officers, directors, employees and consultants under the Plan. If unexercised options expire or are terminated, the underlying shares will again become available for grants under the Plan.
Grants under the Plan. The Plan provides for the grant of options to purchase shares of common stock of the Company. Options may be incentive stock options, designed to satisfy the requirements of Section 422 of the U.S. Internal Revenue Code, or non-statutory stock options, which do not meet those requirements. Incentive stock options may only be granted to employees of the Company and its affiliates. Non-statutory stock options may be granted to employees, non-employee directors, advisors, and consultants of Company and its affiliates.
Outstanding Options. As of December 31, 2016, there were 39,500 outstanding options exercisable at a price per share of $3.00; 85,000 outstanding options exercisable at a price per share of $2.00 and 145,000 outstanding options exercisable at a price per share of $1.50. These options expire five years from the date of issuance. Options issued to directors are fully vested upon grant. Options vest over a period of 2 - 4 years as specified at the time of grant.
Administration of the Plan. The Plan provides that it will be administered by the Board or a Committee designated by the Board. Our Board of Directors appointed a Compensation Committee, which administers the Plan. The Compensation Committee has complete discretion to:
·
determine who should receive an option;
·
determine the type, the number shares, vesting requirements and other terms and conditions of options;
·
interpret the Plan and options granted under the Plan; and
·
make all other decisions relating to the operation and administration of the Plan and the options granted under the Plan.
Terms of Options. The exercise price for non-statutory and incentive stock options granted under the equity compensation plan may not be less than 100% of the fair market value of the common stock on the option grant date or 110% in the case of incentive stock options granted to employees who own stock representing more than 10% of the voting power of all classes of common stock of the Company and its parent and subsidiaries (“10%-Stockholders”). The Compensation Committee has the authority to establishing the vesting, including the terms under which vesting may be accelerated, and other terms and conditions of the options granted. Options can have a term of no more than ten years from the grant date except for incentive stock options granted to 10%-Stockholders which can have a term of no more than five years from the grant date.
The Plan authorizes the Compensation Committee to provide for accelerated vesting of options upon a “Change in Control,” as defined in the Plan. All of the options currently outstanding provide that if there is a Change in Control, (i) immediately prior to the effective date of the Change in Control, an unvested award will become fully exercisable as to all shares subject to the award and (ii) unless the option is assumed by a successor corporation or parent thereof, immediately following the Change in Control any unexercised options will terminate and cease to be outstanding. A Change in Control includes:
·
any Person (as such term is used in Sections 13(b) and 14(b) of the 1934 Act) is or becomes the beneficial owner ("Beneficial Owner") (as defined in Rule 13d-3 promulgated under the 1934 Act), directly or indirectly, of securities representing fifty percent (50%) or more of the combined voting power of the Company’s securities that are then outstanding; provided, however, that an initial public offering shall not constitute a Change in Control for purposes of the Plan;
·
a merger or consolidation after which the Company’s then current stockholders own less than 50% of the surviving corporation; or
·
a sale of all or substantially all of the Company’s assets.
Amendment and Termination. The Board of Directors may amend or terminate the Plan and outstanding options at any time without the consent of option holders provided that such action does not adversely affect outstanding options. Amendments are subject to stockholder approval to the extent required by applicable laws and regulations. Unless terminated sooner, the Plan will automatically terminate on April 15, 2021, the tenth anniversary of April 15, 2011, the date the Plan was adopted by our Board of Directors and approved by our Stockholders.
The table below provides information as to our 2019 Omnibus Incentive Plan as of December 31, 2023.
| Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for issuance under equity compensation plans (excluding securities reflected in column (a)) | ||||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders | 315,159 | (1) | $ | 223.89 | 27,326 | |||||||
| Equity compensation plans not approved by security holders | 2,389 | 397.60 | - | |||||||||
| Total | 317,548 | 27,326 | (2) | |||||||||
| (1) | Includes stock options to purchase 358 shares of our common stock issued under the prior equity compensation plan. | |
| (2) | Consists of shares available for issuance under the 2019 Omnibus Incentive Plan. |
Recent Sales of Unregistered Securities
We had no sales of unregistered securities during 2023 that have not been previously disclosed in a Current Report on Form 8-K or Quarterly Report on Form 10-Q.
Repurchases of Equity Securities
We did not repurchase any shares of our common stock in 2023.
Item 6. [Reserved]
| 48 |
Item 7. Management’s Discussion and Analysis of the Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes and other financial information included elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis contains forward-looking statements that involve risks and uncertainties. You should review the section titled “Risk Factors” in this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described below.
Overview
We are a clinical-stage biopharmaceutical company focused on utilizing our “regulatory science” approach, including the principles associated with FDA’s Project Optimus Oncology initiative and the related FDA Draft Guidance, in the development of Next Generation Chemotherapy (“NGC”) oncology drug products. Our mission is to provide better treatment options than those that presently exist by extending a patient’s survival and/or improving a patient’s quality of life. This is achieved by improving upon FDA-approved, widely used oncology drugs or the cancer-killing metabolites of these drugs by altering how they are metabolized and/or distributed in the body, including how they are distributed to the actual cancer cells.
Our regulatory science approach was conceived in the early 1990s when the founders of Processa and other faculty at the University of Maryland worked with the FDA to develop multiple FDA Guidances. Regulatory science is the science of developing new tools, standards, and approaches to assess the safety, efficacy, quality, and performance of all FDA-regulated products. Over the last 30 years, two of our founders, Dr. David Young and Dr. Sian Bigora, have expanded the original regulatory science concept by including the pre-clinical and clinical studies to justify the benefit-risk assessment required for FDA approval when designing the development programs of new drug products.
Our regulatory science approach defines the scientific information that the FDA requires to determine if the benefit outweighs the risk of a drug in a specific population of patients and at a specific dosage regimen for a specific drug product. The studies are designed to obtain the necessary scientific information to support the regulatory decision.
Recently, the FDA has taken steps to define some of the regulatory science required for the FDA approval of oncology products. Through the FDA’s Project Optimus Oncology Initiative and the related Draft Guidance on determining the “optimal” dosage regimen for an oncology drug, the FDA has chosen to make the development of oncology drugs more science-based than in the past. Since the principles of the FDA’s Project Optimus and the related Draft Guidance have been used by our regulatory science approach in a number of non-oncology drugs in the past, our experience with the principles of Project Optimus differentiates us from other biotechnology companies by focusing us not only on the clinical science, but also on the equally important regulatory process. We believe utilizing our regulatory science approach provides us with three distinct advantages:
| ● | greater efficiencies (e.g., the right trial design and trial readouts); | |
| ● | greater possibility of drug approval by the FDA or other regulatory authorities; and | |
| ● | greater ability to evaluate the benefit-risk of a drug compared to existing therapy, which allows prescribers to provide better treatment options for each patient. |
Our strategic prioritization is to advance our pipeline of NGC proprietary small molecule oncology drugs. The NGC products are new chemical entities, but they work by changing the metabolism, distribution and/or elimination of already FDA-approved cancer drugs or their active metabolites while maintaining the mechanism of how the drug kills cancer cells. We believe our NGC treatments will provide improved safety-efficacy profiles when compared to their currently marketed counterparts – capecitabine, gemcitabine, and irinotecan. All future studies of these drugs are subject to availability of capital to conduct the trials.
| 49 |
The three NGC treatments in our pipeline are as follows:
| ● | NGC-Capecitabine (NGC-Cap) is a combination of PCS6422 and capecitabine, capecitabine being the oral prodrug of the cancer drug 5-fluorouracil (5-FU). PCS6422, without having any clinically meaningful biological effect itself, alters the metabolism of 5-FU, resulting in more 5-FU distribution to the cancer cells. In clinical trials, NGC-Cap has shown a safety profile different than capecitabine when administered alone. Side effects such as Hand-Foot Syndrome (HFS) and cardiotoxicity typically occur in up to 50-70% of patients treated with capecitabine and are caused by specific capecitabine metabolites. These types of toxicities frequently result in decreased doses, interrupted doses, or discontinuation of treatment with capecitabine. Since a much smaller amount of these metabolites are formed with NGC-Cap, these side effects appear in less patients and are less severe when they do occur. In addition, NGC-Cap has been found to be up to 50 times more potent than capecitabine based on the systemic exposure of the capecitabine metabolite 5-FU, which is metabolized to the cancer-killing metabolites. Like capecitabine, NGC-Cap could potentially be used to treat patients with various cancers, such as metastatic breast, colorectal, gastrointestinal, and pancreatic. On December 11, 2023, we had a successful meeting with the FDA regarding our next Phase 2 study supporting the advancement of NGC-Cap for cancer patients. The meeting with the FDA was supported by the interim results from the ongoing Phase 1B study that completed enrollment in the first quarter of 2024. Following our meeting with the FDA, we decided the next NGC-Cap trial would be a Phase 2 trial in breast cancer. This decision was supported through discussions with the FDA where we agreed with the FDA that the development of NGC-Cap in breast cancer would be a more efficient development program than metastatic colorectal cancer and improve the likelihood of FDA approval. The FDA has agreed that the data from past and existing studies could be used to directly support the Phase 2 trial in breast cancer. Breast cancer is the most diagnosed cancer, representing approximately 15% of all new cancer patients in 2023. It has a prevalence of more than 3.8 million patients, with nearly 300,000 new diagnoses last year. Over 150,000 women are currently living with advanced or metastatic breast cancer. The NGC-Cap annual newly diagnosed incidence rate for breast, colorectal and other cancers is greater than 250,000 patients per year. |
| ● | PCS3117, also referred to as NGC-Gemcitabine (NGC-Gem), is an oral analog of gemcitabine that is converted to its active metabolite by a different enzyme system than gemcitabine resulting in a positive response in gemcitabine patients as well as some gemcitabine treatment-resistant patients. Like gemcitabine, NGC-Gem could potentially be used to treat patients with various cancers such as pancreatic, biliary tract, lung, ovarian, and breast. We estimate more than 275,000 patients in the United States were newly diagnosed in 2022 with pancreatic, biliary tract, lung, ovarian, and breast cancer. We plan to meet with the FDA to discuss potential study designs including implementation of the Project Optimus initiative as part of the design in 2024. | |
| ● | PCS11T, also referred to as NGC-Irinotecan (NGC-Iri), is a prodrug of the active metabolite of irinotecan (SN-38). The chemical structure of NGC-Iri influences the uptake of the drug into cancer cells, resulting in more NGC-Iri entering cancer cells than normal cells in mice. These levels were significantly greater than those seen with irinotecan, resulting in lower doses of NGC-Iri having greater efficacy than irinotecan and improved safety in animal models. Like irinotecan, NGC-Iri could be used to treat patients with various cancers such as lung, colorectal, gastrointestinal, and pancreatic cancer. We estimate at least 200,000 patients in the United States were newly diagnosed in 2022 with lung, colorectal, gastrointestinal, and pancreatic cancer. We plan to conduct IND-enabling and toxicology studies in 2024-2025. |
Our shift in prioritization to NGC oncology drugs does not change our mission. We continue to be focused on drug products that improve the survival and/or quality of life for patients by improving the safety and/or efficacy of the drug in a targeted patient population, while providing a more efficient and probable path to FDA approval and differentiating our drugs from those on the market or are currently being developed.
Historically, much of oncology drug development has searched for novel or different ways to treat cancer. Our approach is to take three current FDA-approved cancer drugs, e.g. capecitabine, gemcitabine and irinotecan, and modify and improve how the human body metabolizes and/or distributes these NGC treatments compared to their presently approved counterpart chemotherapy drugs while maintaining the cancer-killing mechanism of action; thus, our reason for calling our drugs Next Generation Chemotherapy (or NGC) treatments. Part of the development includes determining the optimal dosage regimen based on the dose-response relationship as described in the FDA’s Project Optimus Initiative and Draft Optimal Dosage Regimen Oncology Guidance. To date, we have data that we believe suggests our NGC treatments are likely to have a better safety-efficacy profile than the current widely used marketed counterpart drugs, not only potentially making the development and approval process more efficient, but also clearly differentiating our NGC treatments from the existing treatment. We believe our NGC treatments have the potential to extend the survival and/or quality of life for more patients diagnosed with cancer while decreasing the number of patients who are required to dose-adjust or discontinue treatment because of side effects or lack of response.
In 2023, we completed our Phase 2A trial for PCS12852 in gastroparesis patients with positive results. Additionally, in February 2023, due primarily to the inability to identify and enroll patients in our rare disease Phase 2 trial for PCS499 in ulcerative Necrobiosis Lipoidica (uNL), we decided to cease further enrollment in the PCS499 trial and terminated the trial. We did not experience any safety concerns during the conduct of either the PCS12852 or PCS499 trial. We continue to evaluate options outstanding and their weighted average exercise price at December 31, 2016.to monetize these non-core drug assets, which may include out-licensing or partnering these assets with one or more third parties.
| 50 |
EQUITY COMPENSATION PLAN INFORMATIONRecent Developments
Equity compensation plan approved by security holders: | Number of securities to be issued upon exercise of outstanding options | Weighted average exercise price of outstanding options | Number of securities remaining available for future issuance under equity compensation plan |
2011 Equity Incentive Plan | 269,500 (1) | $ 1.88 | 1,530,500 |
(1) Excludes 40,000 performance optionsReverse Stock Split
On January 22, 2024, we effected a 1-for-20 reverse stock split, reducing the number of our common shares issued on that were not issued underdate from 24,706,474 shares to 1,291,000 shares. There is no corresponding reduction in the equity compensation plan.
Unregistered Salesnumber of Equity Securities
During 2015, the Company raised $88,000 and issued 50,284authorized shares of common stock and no change in the par value per share. All share and per share amounts and conversion and exercise prices presented herein have been adjusted retroactively to reflect this change.
Public Offering
On January 30, 2024, we raised gross proceeds of $7.0 million (net proceeds of $6.3 million) from the sale of 476,000 shares of our common stock, pre-funded warrants to purchase up to 1,079,555 shares of our common stock and warrants to purchase 25,1411,555,555 shares of our common stock underin a public offering, as described in Note 13. Simultaneously with the October 1, 2014 non-public equity offering. Each Unit consistsclosing of one common share and one-half warrant, with each whole warrant exercisable at $2.00 per share. The purchase pricethe sale, the pre-funded warrants were exercised in exchange for the Units is payable in either cash, conversion of outstanding Series D preferred shares or certain outstanding promissory notes.
On March 13, 2015, we issued a total of 15,0001,079,555 shares of our common stockstock. We plan to a total of three individualsuse the net proceeds from this financing for services performed. These issuances were made pursuant to Regulation S promulgated by the Securitiescontinued research and Exchange Commission (the “Commission”) under the Securities Act of 1933, as amended (the “Securities Act”). The issuances were made in off-shore transactions as defined in Regulation Sdevelopment for NCG-Cap, and there were no directed selling efforts made in the U.S. by us or any of our respective affiliates, or any person acting on behalf of any of the foregoing. Applicable offering restrictions were also implemented by us in compliance with Rule 903(b)(3) of Regulation S. Those who received the shares also agreed to conform to the restrictions on resale of the securities contained in Regulation S. No selling commissions were paid in connection with the share issuances.working capital and general corporate purposes.
There were no equity securities issued during 2016.
These Units in the above offerings were issued without registration under the Securities Act by reason of the exemption from registration afforded by the provisions of Section 4(a)(2) thereof, and Rule 506(b) promulgated thereunder, as transactions by an issuer not involving any public offering. Each of the investors was an accredited investor as defined in Regulation D. Each investor delivered appropriate investment representations with respect to these issuances and consented to the imposition of restrictive legends upon the stock certificates representing the shares and the warrant certificates. Each investor was afforded the opportunity to ask questions of our management and to receive answers concerning the terms and conditions of the transaction. No underwriting discounts or commissions were paid in connection with the issuances.
ITEM 6. Selected Financial Data
As a smaller reporting company, we have elected not to provide the disclosure required by this item.
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion should be read in conjunction with our consolidated financial statements and related notes thereto as filed with this report.
This Item 7 may contain forward-looking statements that involve substantial risks and uncertainties. When considering these forward-looking statements investors should keep in mind the cautionary statements in this report. Please see the sections entitled “Cautionary Notice Regarding Forward-Looking Statements” and Item 1A. “Risk Factors” elsewhere in the report.
Heatwurx, Inc. was incorporated under the lawsComparison of the State of Delaware on March 29, 2011 as Heatwurxaq, Inc. and subsequently changed its name to Heatwurx, Inc. (“the Company” or “Heatwurx”) on April 15, 2011.
We are an asphalt preservation and repair, equipment company. Our innovative, and eco-friendly hot-in-place recycling process corrects surface distresses within the top 3 inches of existing pavement by heating the surface material to a temperature between 325° and 375° Fahrenheit with our electrically powered infrared heating equipment, mechanically loosening the heated material with our processor/tiller attachment that is optimized for producing a seamless repair, and mixing in additional recycled asphalt pavement and a binder (asphalt-cement), and then compacting repaired area with a vibrating roller or compactor. We consider our equipment to be eco-friendly as the Heatwurx process reuses and rejuvenates distressed asphalt, uses recycled asphalt pavement for filler material, eliminates travel to and from asphalt batch plants, and extends the life of the roadway. We believe our equipment, technology and processes provide savings over other processes that can be more labor and equipment intensive.
17
Our hot-in-place recycling process and equipment was selected by the Technology Implementation Group of the American Association of State Highway Transportation Officials (“AASHTO TIG”) as an “additionally Selected Technology” for the year 2012. We develop, manufacture and intend to sell our unique and innovative and eco-friendly equipment to federal, state and local agencies as well as contractors for the repair and rehabilitation of damaged and deteriorated asphalt surfaces.
During 2014, we acquired Dr. Pave, LLC a service company offering asphalt repair and restoration utilizing the Heatwurx asphalt repair technology and established a new entity Dr. Pave Worldwide LLC to house our franchise program providing franchisees with the exclusive Heatwurx equipment and processing. We launched our franchise sales program throughout the U.S. in the third quarter of 2014; however, to date, no franchises have been sold. The Company decided not to renew its franchise registrations throughout the U.S. do to the extensive costs. In 2015, we offered license agreements, which grants a license of all Heatwurx equipment and supplies and the use of the Heatwurx intellectual property within a specified territory. We have one licensee as of December 31, 2016.
We are no longer receiving financial support and we do not believe we will be able to obtain financing from another source. We do not believe we are able to achieve a level of revenues adequate to support our cost structure. Do to the slow growth in the service sector and the high cost of the franchise registrations, we discontinued the operations of Dr. Pave, LLC and Dr. Pave Worldwide LLC. In addition, we significantly scaled back operations to maintain only a minimal level of operations necessary to support our licensee and look for potential merger candidates. The Company sold the remaining equipment and inventory to the licensee during 2016. Based upon the Company’s current financial position, the Company does not believe it will be able to satisfy the mandatory principal payments. The Company will work with the lenders to explore extension or conversion options. There is no guarantee the lenders will accommodate our requests. As of December 31, 2016; principal in the amount of $962,361 is outstanding and payable under the secured notes. These notes are secured by all the assets of the Company, including intellectual property rights. We are in default on the notes, and as a result the Company’s assets may be foreclosed upon.
The issues described above raise substantial doubt about the Company’s ability to continue as a going concern. It is our intention to move forward as a public entity and to seek potential merger candidates. If the Company fails to merge or be acquired by another company, we will be required to terminate all operations.
Section 107 of the JOBS Act provides that an “emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
Results of operations for the year ended December 31, 2016 compared to year2023 and 2022
The following table summarizes our operations loss during the periods indicated:
| Year Ended December 31, | ||||||||||||
| 2023 | 2022 | Change | ||||||||||
| Operating Expenses | ||||||||||||
| Research and development costs | $ | 5,799,518 | $ | 11,494,230 | $ | (5,694,712 | ) | |||||
| General and administrative expenses | 5,657,543 | 8,763,058 | (3,105,515 | ) | ||||||||
| Impairment of intangible asset | - | 7,268,143 | (7,268,143 | ) | ||||||||
| Operating Loss | (11,457,061 | ) | (27,525,431 | ) | ||||||||
| Other Income (Expense) | ||||||||||||
| Interest income, net | 335,541 | 101,202 | 234,339 | |||||||||
| Net Loss | $ | (11,121,520 | ) | $ | (27,424,229 | ) | ||||||
| 51 |
Revenues.
We had no revenue during the years ended December 31, 2015
For the year ended December 31, 2016, our net loss was approximately $336,000 including income from discontinued operations of approximately $1,000, compared to a net loss of approximately $3,354,000 including loss from discontinued operations of approximately $371,000 for the year ended December 31, 2015. Further description of these losses is provided below.
Revenue
Revenue decreased to $5,000 for the year ended December 31, 2016 from approximately $113,000 for the year ended December 31, 2015 as a result of decreased equipment2023 and consumables sales. The prior year sales of consumables consisted of our proprietary blend of polymer pellets used to strengthen the repair when mixed in to the recycled asphalt product; and RxEHAB rejuvenation strips which is an oil-based product which creates the binding agent for the asphalt repair. The sales in 2015 were offset by significant sales discounts. The Company does not anticipate any future revenue from equipment sales in 2017.
18
Cost of Goods Sold
There was no cost of goods sold during 2016 compared to approximately $126,000 for the year ended December 31, 2015. In 2015 the Company recognized cost of goods sold from consumable sales, and had a negative gross profit as a result of significant discounts.
Selling, General and Administrative
Selling, general and administrative expenses decreased to approximately $70,000 for the year ended December 31, 2016 from approximately $945,000 for the year ended December 31, 2015. The decrease in selling, general and administrative expenses is principally due to the significant reduction in operating activities which include a decrease in employee expenses of approximately $406,000; a decrease in travel and office expenses of approximately $376,000 which includes commercial insurance, rent and other expenses; a decrease in legal and investor relations expenses of $53,000; a decrease in advertising and promotion of approximately $35,000; and a decrease in bad debt expense of $5,000.
Impairment of assets held for sale
As part of the strategy to keep operations running at minimum capacity, in 2015 we chose to sell assets or return collateralized assets to relieve the debt, which were not critical to the continued.2022. We reclassified these non-critical assets for sale from equipment or inventory and recognized an impairment loss when the carrying amount of the assets exceeds its fair value. During the year ended December 31, 2015 we recognized an impairment on assets held for sale in the amount of $186,068.
Impairment of intangible asset
Based on our current financial condition and the inability to obtain financing; we are unable to pursue the necessary commercialization activities to drive us to profitability. We have therefore estimated no future cash flows related to the intangible assets and recognized an impairment of intangible assets in the amount of $1,517,859 for the year ended December 31, 2015.
Research and Development
Research and development decreased to approximately $6,000 for the year ended December 31, 2016 from approximately $29,000 for the year ended December 31, 2015, as a result of fewer patent applications being filed thereby reducing the legal fees associated therewith, a decrease in manufacturing research and development costs, and a decrease in consulting fees.
We currently have six issued U.S. patents: five utility patents and one design patent. We have two pending U.S. patent applications and three foreign patent applications. Three issued utility patents, US Patent Nos. 8,556,536; 8,562,247 and 8,714,871 were issued on Oct. 15, 2013, Oct. 24, 2013, and May 6, 2014, respectively and cover certain unique device and method of use aspects of our asphalt repair equipment. Our design patent, US Patent No. D700,633, was issued on March 4, 2014 and covers the ornamental design of our asphalt processor. U.S. Patent No. 8,801,325 issued August 12, 2014 and covers aspects of our computer-controlled asphalt heater. U.S. Patent No. 9,022,686 was issued May 5, 2015 and covers complementary features of our computer-controlled asphalt heater.
We intend to protect our intellectual property rights in the United States and in a limited number of countries outside of the United States. However, we do not have any assurance that our current pending patent applications will be granted or that we will be able to develop future patentable technologies. We do not believe our ability to operate our business is dependent on the patentability of our technology.
Income Taxes
We have incurred tax losses since we began operations. A tax benefit would have been recorded for losses incurred since March 29, 2011; however, due to the uncertainty of realizing these assets, a valuation allowance was recognized which fully offset the deferred tax assets.
19
Liquidity and capital resources
Overview
We have incurred operating losses, accumulated deficit and negative cash flows from operations since inception. As of December 31, 2016, we had an accumulated deficit of approximately $15,255,000 from operating activities. The Company had total cash on hand of approximately $3,000 as of December 31, 2016. The Company is not able to obtain additional financing adequate to fulfill its commercialization activities, nor achieve a level of revenues adequate to support the Company’s cost structure.
Operating Activities
During 2016, the Company used $30,853 in cash for continuing operations and $12,350 for discontinued operations compared to cash used of $639,550 for continuing operations and $232,356 for discontinued operations during 2015. This decrease in cash used for operating activities was due to the significant reduction in employees and overhead expenses. The Company has had little revenue since inception. The Company does not currently have any revenue under contract nor does it haveor any immediate sales prospects. The Company has significantly scaled back operations to maintain only a minimal level of operations necessary to support our licensee and look for potential merger candidates. For the year ended December 31, 2016, the Company incurred a net loss from continuing operations of approximately $337,000. The operations of Dr. Pave, LLC and Dr. Pave Worldwide, LLC have been discontinued. These business components are included in discontinued operations as of December 31, 2016. It is the Company’s intention to move forward as a public entity and to seek potential merger candidates. If the Company fails to merge or be acquired by another company, we will be required to terminate all operations.
Investing Activities
During 2016 the Company received $17,000 in cash for continuing operations from the sale of assets held for sale. Compared to net cash received of $29,701 from continuing operations and $10,503 from discontinued operations; primarily from the sale of property and equipment in 2015.
Financing Activities
During 2016, the Company generated $15,000 in cash from financing activities compared to $821,098 in 2015. This decrease in cash provided by financing activities was due to the Company no longer receiving financial support for the business and operations from their small group of investors. The Company entered into a Senior secured loan agreement on February 16, 2015, extended on March 23, 2016, with JMW Fund, Richland Fund, and San Gabriel Fund (collectively, the “lenders”) whereby the lenders agreed to loan to the Company up to an aggregate of $2,000,000. The interest rate on the notes is 12% per annum and monthly interest payments are due the first day each month beginning March 1, 2015. The notes mature six months from the date of issuance. If any interest payment remains unpaid in excess of 90 days, and the lender has not declared the entire principal and unpaid accrued interest due and payable, the interest rate on that amount only will be increased to 18% per annum, until the past due interest amount is paid in full. The notes and any future notes under the loan agreement are secured by all of the assets of the Company, including intellectual property rights. Upon the occurrence of an event of default the lenders have the right to foreclose on the assets of the Company. The Company received $15,000 under the Senior secured loan agreement during the year ended December 31, 2016.
During 2015, we closed our non-public equity offering at $1.75 per unit (the “Units”). Each Unit consisted of one common share and one-half warrant, with each whole warrant exercisable at $2.00 per share. The purchase price for the Units was payable in either cash, conversion of outstanding Series D preferred shares or certain outstanding promissory notes. For the year ended December 31, 2015, we raised cash proceeds of $88,000 and issued 50,285 shares of common stock and warrants to purchase 25,141 shares of common stock as part of the private equity offering.
During the year ended December 31, 2015, we issued notes with an aggregate principal amount of $753,000 under the senior secured notes payable under the $2 million loan agreement as described above.
20
We received $80,000 in short-term unsecured notes in January 2015; which were converted on February 23, 2015 into senior secured notes payable under the $2 million loan agreement as described above, and included above in the aggregate principal amount. In addition, on February 23, 2015 we converted the outstanding $20,000 short-term unsecured note, entered into during December 2014, into a senior secured note payable under the $2 million loan agreement, included above in the aggregate principal amount.
In June 2015 we converted $160,000 in secured notes plus accrued interest of $14,361 into senior secured notes payable under the $2 million loan agreement, and is included above in the aggregate principal amount.
Based upon the Company’s current financial position and inability to obtain additional financing, we do not believe it will be able to satisfy the mandatory principal payments under the $2,000,000 senior secured debt. We will continue to work with the lenders to explore additional extension or conversion options as needed. These notes are secured by all the assets of the Company, including intellectual property rights. We are in default on the notes, our assets may be foreclosed upon.
Cash Requirements
The issues described above raise substantial doubt about our ability to continue as a going concern. We have been solely reliant on raising capital or borrowing funds in order to maintain our operations. We were able to raise debt and equity financing through the assistance of a small number of our investors who have been substantial participants in its debt and equity offerings since our formation. These investors have chosen not to assist us with our capital raising initiatives. At this time we are not able to obtain any alternative forms of financing and we will not be able to continue to satisfy our current or long term obligations. We desire to merge or be acquired by another company. If a candidate is not identified we will cease operations all together.
Total Contractual Cash Obligations
A summary of our total contractual cash obligations as of December 31, 2016, is as follows:
Contractual Obligation | Total | Due in 2017 | Due in 2018-2019 | Due in 2020-2021 | Thereafter | |||||
Revolving line of credit (1) | $ | 229,980 | $ | 229,980 | $ | - | $ | - | $ | - |
Unsecured notes payable (2) |
| 420,000 |
| 420,000 |
| - |
| - |
| - |
Senior secured notes payable (3) |
| 962,361 |
| 962,361 |
| - |
| - |
| - |
TOTAL | $ | 1,612,341 | $ | 1,612,341 | $ | - | $ | - | $ | - |
(1) Represents a revolving line of credit entered into by Dr. Pave at its inception in July 2013, assumed by the Company in 2016.
(2) Amount represents outstanding unsecured notes payable under the Company’s loan agreements which matured in January 2016.
(3) Amount represents outstanding balance on senior secured notes payable that matured in September 2016.
Off-Balance Sheet Arrangements
We do not engage in off-balance sheet financing activities.
Critical Accounting Policies and Estimates
The preparation of these financial statements in conformity with GAAP requires management to make estimates, allocations and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, management evaluates its estimates, including those related to impairment of long-lived assets, accrued liabilities and certain expenses. We base our estimates about the carrying values of assets and liabilities that are not readily apparent from other sources on historical experience and on other assumptions believed to be reasonable under the circumstances. Actual results may differ materially from these estimates under different assumptions or conditions. We believe the following critical accounting policies involve significant judgments and estimates used in the preparation of our financial statements. See Note 2 of the accompanying Notes to the Financial Statements included in Item 8 of this Form 10-K for additional information on these policies and estimates, as well as a discussion of additional accounting policies and estimates.
21
Revenue Recognition
Equipment sales revenue is recognized when equipment is shipped to our customer and collection is reasonably assured. We sell our equipment (HWX-30 heater and HWX-AP-40 asphalt processor), as well as certain consumables, such as RxEHAB rejuvenation strips and Polymer pellets, to third parties. Equipment sales revenue is recognized when all of the following criteria are satisfied: (a) persuasive evidence of a sales arrangement exists; (b) price is fixed and determinable; (c) collectability is reasonably assured; and (d) delivery has occurred. Persuasive evidence of an arrangement and a fixed or determinable price exist once we receive an order or contract from a customer. We assess collectability at the time of the sale and if collectability is not reasonably assured, the sale is deferred and not recognized until collectability is probable or payment is received. Typically, title and risk of ownership transfer when the equipment is shipped.
Research and Development Expenses.
ResearchOur research and development costs are expensed as incurredincurred. Research and consist of directdevelopment expenses include (i) program and overhead-related expenses. Expenditurestesting related expenses including external consulting and professional fees related to acquire technologies, including licenses, which are utilized inthe product testing and our development activities and (ii) internal research and development staff related salaries and that have no alternativeother payroll costs including stock-based compensation, payroll taxes and employee benefits. In 2022, we also had expenses related to the amortization of the exclusive PCS499 license intangible asset. Non-refundable advance payments for goods and services to be used in future useresearch and development activities are recorded as prepaid expenses and expensed when incurred. Technologythe research and development activities are performed.
Research and development costs for the years ended December 31, 2023 and 2022 were as follows:
| Year ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Preclinical, clinical trial and other costs | $ | 3,817,669 | $ | 6,269,006 | ||||
| Research and development salaries and benefits | 1,981,849 | 4,436,729 | ||||||
| Amortization of intangible assets | - | 788,495 | ||||||
| Total | $ | 5,799,518 | $ | 11,494,230 | ||||
During the year ended December 31, 2023, our research and development expenses decreased by $5.7 million to $5,799,518 when compared to $11,494,230 for the year ended December 31, 2022. The decrease in preclinical, clinical trial and other costs during 2023 when compared to 2022 was attributable to the completion of our clinical trial for PCS12852 and the early termination of our clinical trial for PCS499. Expenses include costs related to contract research organizations, regulatory filing and maintenance fees, drug product testing and stability, consulting, and other clinical fees. We also did not have any amortization expense in 2023, as we developfully impaired our PCS499 intangible asset at December 31, 2022. In 2022, we had three active clinical trials and amortization expense for useour intangible asset. We also experienced decreases in stock-based compensation of $2.5 million as a result of a reduction in stock awards granted. During 2023, we also experienced a decrease in salaries and related expenses of $29,000. While we have promoted and/or increased salary rates for some personnel, we have fewer full-time R&D employees in 2023 than 2022.
During the year ended December 31, 2022, we amended the third Milestone Event of our products is expensed as incurred until technological feasibility has been established afterLicense Agreement with Elion changing the third Milestone Event. As a result, we recorded research and development expense and a related liability of $189,000, which it is capitalized and depreciated.
Stock-based Compensation
We record equity instrumentsrepresents the value of the 5,000 shares of our common stock we issued to Elion in 2024 in satisfaction of the third Milestone Event (as modified) at theirthe fair value on the measurement date of modification.
On January 30, 2024, we raised net proceeds of $6.3 million, which we intend to use for continued research and development for NCG-Cap and for working capital. We are also incurring costs in preparation for our planned Phase 2 trial for NGC-Cap, including the cost of having drug product manufactured and other pre-trial tasks, as well as costs we may incur in connection with the submission of any Phase 2B protocol for NGC-Gem. In early 2025, we will need to raise additional capital to fund our operations and continue our planned development of our NGC drugs.
| 52 |
The funding necessary to bring a drug candidate to market is subject to numerous uncertainties. Once a drug candidate is identified, the further development of that drug candidate may be halted or abandoned at any time due to a number of factors. These factors include, but are not limited to, funding constraints, safety or a change in market demand. For each of our drug candidate programs, we periodically assess the scientific progress and merits of the programs to determine if continued research and development is economically viable. Some programs may be terminated due to the lack of scientific progress and lack of prospects for ultimate commercialization. We anticipate our research and development costs to increase in the future, subject to obtaining sufficient financing, as we finalize our clinical trials; plan/conduct future clinical trials, including the cost of having drug product manufactured; continue our evaluation of the remaining drugs in our portfolio; and expand our development team.
Our clinical trial cost accruals are based on estimates of patient enrollment and related costs at clinical investigator sites, as well as estimates for the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical trials on our behalf.
In accruing service fees, we estimate the time period over which services will be performed and the level of patient enrollment and activity expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, we will adjust the accrual accordingly. Payments made to third parties under these arrangements in advance of the receipt of the related series are recorded as prepaid expenses until the services are rendered. At December 31, 2023, we have recorded $570,000 in prepaid expenses for advanced payments made to our CRO for our NGC-Cap clinical trial.
General and Administrative Expenses.
Our general and administrative expenses for the year ended December 31, 2023 decreased by utilizing$3.1 million to $5,657,543 when compared to $8,763,058 for the Black-Scholes option-pricing model. Stock Compensationsame period in 2022. The decrease was mostly due to employee stock-based compensation of $4.9 million as a result of a reduction in stock awards granted. We also experienced net decreases in taxes, travel and other miscellaneous office expenses of $38,000. The decreases were primarily offset by increases in professional fees of $1.3 million related to the warrants we issued to Spartan Capital as compensation for all share-based payments, is recognized as an expense overservices provided under a consulting agreement and for salaries and related expenses of $493,000. We share office space with CorLyst, a related party, and during the requisite service period.years ended December 31, 2023 and 2022, they reimbursed us $112,000 and $124,000, respectively, for rent and other costs we incurred on their behalf.
Significant assumptions utilized in determining
Impairment of Intangible Asset.
Our gross intangible assets consisted primarily of costs we capitalized related to the acquisition of license rights to PCS499 from CoNCERT Pharmaceuticals. Inc. (“CoNCERT”) for shares of our common stock. We capitalized $8.0 million, which was the fair value of ourthe common stock options includedwe issued to CoNCERT when we acquired the volatility rate, estimated term of the options, risk-free interest rate and forfeiture rate. In orderlicense rights to estimate the volatility rate at each issuance date, given that the Company has not established a historical volatility rate as it has minimal trading volume since we began trading in October 2013, management reviewed volatility rates for a number of companies with similar manufacturing operations to arrive at an estimated volatility rate for each option grant. The term of the options was assumed to be five years, which is the contractual term of the options. The risk-free interest rate was determined utilizing the treasury rate with a maturity equal to the estimated term of the option grant. Finally, management assumed a 25% forfeiture rate in 2015 due to Company changes and significant reduction in operations and personnel.PCS499 on March 19, 2018.
Non-employee share-based compensation charges generally are immediately vested and have no future performance requirements by the non-employee and the total share-based compensation charge is recorded in the period of the measurement date.
Assets held for sale
When assets are expected to be sold within the next 12 months and meet the other relevant held-for-sale criteria are classified as long-lived assets held-for-sale and measured at their fair value based on the Company’s own judgments about assumptions that market participants would use in pricing the asset and on observable market data, when available. An impairment loss is recorded in the statement of operations for long-lived assets held-for-sale when the carrying amount of the asset exceeds its fair value less cost to sell. A long-lived asset is not depreciated while it is classified as held-for-sale.
Impairment of Long-Lived Assets
We review long-lived assetsamounts previously capitalized for impairment on an annual basis, during the fourth quarterwhenever events or on an interim basis if an event occurschanges in circumstances indicate to us that might reduce the fair value of such assets below their carrying values. An impairment loss would be recognized based on the difference between the carrying value of the assets might not be recoverable. In May 2021, we enrolled our first patient in our Phase 2B trial for the treatment of ulcerative NL with PCS499. Although we initiated a number of recruitment programs to increase the enrollment of patients in this study, we were only able to recruit four patients by December 31, 2022. We experienced extremely slow enrollment in the study given the extreme rarity of the condition (rarer than reported in the literature), the impact of COVID-19, and the reluctance of patients to be in a clinical study. As a result, we terminated the trial early. At December 31, 2022, as a result of our decision to halt future enrollment and terminate our PCS499 clinical trial for ulcerative necrobiosis lipoidica, we recognized an impairment of the remaining book value of the intangible asset of $7.3 million, thereby reducing the value of our intangible asset to zero. Our assessment was based on the uncertainty of determining whether we will be able to out-license PCS499 or enter into a partnering/collaborating arrangement for its future development.
Other Income.
Net other income consisting primarily of interest income was $335,541 and $101,202 for the years ended December 31, 2023 and 2022, respectively.
| 53 |
Income Tax Benefit.
We did not recognize any income tax benefit for the year ended December 31, 2023 or 2022. At December 31, 2023, we recorded deferred tax assets totaling $17,639,071, including $7,685,377 of net operating losses that are fully offset by a valuation allowance.
Liquidity
Sources of Liquidity
At December 31, 2023, we had $4.7 million in cash and cash equivalents and raised an additional $6.3 million net proceeds in January 2024. On January 30, 2024, we sold, pursuant to securities purchase agreements (the “Purchase Agreement”), 476,000 shares of common stock, pre-funded warrants to purchase up to 1,079,555 shares of common stock in lieu of shares of common stock (the “Pre-Funded Warrants”), and warrants to purchase up to 1,555,555 shares of our common stock (the “Common Warrants’) pursuant to a public offering (the “Offering”). The Common Warrants have an exercise price of $4.50, are immediately exercisable and will remain exercisable until the date that is five years after their original issuance. The Shares were offered at a combined public offering price of $4.50 per share and accompanying Common Warrant and $4.4999 per Pre-Funded Warrant and accompanying Common Warrant. The Pre-Funded Warrants had an exercise price of $0.0001 and were exercised in full simultaneously with the closing of the Offering in exchange for 1,079,555 shares of our common stock. Gross proceeds in connection with the Offering were $7.0 million. We received $6.3 million in net proceeds from the Offering, after deducting the fees of the placement agent and other offering-related expenses. We also issued to the placement agent warrants to purchase 62,222 shares of common stock, exercisable at $5.625 per share that expire on February 1, 2027.
We have incurred losses and net cash used in our operating activities during the year ended December 31, 2023, which we expect to continue for the foreseeable future. We have incurred losses since our inception, devoting substantially all of our efforts toward research and development, and have an accumulated deficit of approximately $75.4 million at December 31, 2023. During the year ended December 31, 2023, we generated a net loss of approximately $11.1 million, of which $2.4 million were non-cash expenses. Based on our current business plans, we believe these funds will satisfy our capital needs into early 2025. Our ability to execute our longer-term operating plans, including unplanned future clinical trials for our portfolio of drugs depend on our ability to obtain additional funding from the sale of equity and/or debt securities, a strategic transaction or other funding transactions. We plan to continue to actively pursue financing alternatives, but there can be no assurance that we will obtain the necessary funding in the future when needed.
Our estimate of future cash needs is based on assumptions that may prove to be wrong, and we could utilize our available cash sooner than we currently expect. Our ultimate success depends on the outcome of our planned clinical trials and our research and development activities, as disclosed above. We expect to incur additional losses in the future, and we will need to raise additional capital to fully implement our business plan if the costs of our clinical trials are greater than we expect or they take longer than anticipated. We also expect to incur increased general and administrative expenses in the future. In addition, there may be costs we incur as we develop these drug products that we do not currently anticipate, requiring us to need additional capital sooner than currently expected.
Our future capital requirements will depend on many factors, including:
| ● | the cost of our current and future clinical trials of NGC-Cap and the cost of third-party manufacturing; | |
| ● | the initiation, progress, timing, costs and results of drug manufacturing, pre-clinical studies, and clinical trials of NGC-Gem and NGC-Iri, as well as any other future product candidates; | |
| ● | the number and characteristics of product candidates that we pursue; | |
| ● | the outcome, timing, and costs of seeking regulatory approvals; | |
| ● | the costs associated with hiring additional personnel and consultants for our pre-clinical and clinical activities; | |
| ● | the emergence of competing therapies and other adverse market developments; | |
| ● | the costs involved in preparing, filing, prosecuting, maintaining, expanding, defending, and enforcing patent claims, including litigation costs and the outcome of such litigation; | |
| ● | the extent to which we in-license or acquire other products and technologies; and | |
| ● | the costs of operating as a public company. |
Until such time as we can generate substantial product revenues to support our capital requirements, if ever, we expect to finance our cash needs through a combination of public or private equity offerings, debt financings, collaborations and licensing arrangements or other capital sources. We currently have an effective shelf registration statement on Form S-3 on file with the SEC, which provides us flexibility and optionality to raise capital, including pursuant to a Purchase Agreement with Lincoln Park Capital or a future at-the-market offering, but there can be no assurance that capital will continue to be available to us on acceptable terms, won’t be limited, or be available at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be or could be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders.
| 54 |
Cash Flows
The following table sets forth our sources and uses of cash and cash equivalents for the years ended December 31, 2023 and 2022:
| For the Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Net cash (used in) provided by: | ||||||||
| Operating activities | $ | (8,063,346 | ) | $ | (9,605,143 | ) | ||
| Investing activities | (2,776 | ) | - | |||||
| Financing activities | 6,268,724 | (388,843 | ) | |||||
| Net decrease in cash and cash equivalents | $ | (1,797,398 | ) | $ | (9,993,986 | ) | ||
Net cash used in operating activities
We used net cash in our operating activities of $8,063,346 and $9,605,143 during the years ended December 31, 2023 and 2022, respectively. As we continue our clinical trial for NGC-Cap and evaluate the other NGC drugs in our portfolio, we anticipate our research and development efforts and ongoing general and administrative costs will continue to generate negative cash flows from operating activities for the foreseeable future. Until we begin our Phase 2 clinical trial for NGC-Cap, we anticipate clinical trial costs will decrease when compared to prior periods since we are only conducting one clinical trial for NGC-Cap.
At December 31, 2023, our prepaid expense and other consisted primarily of $570,000 for advanced payments we made to our NGC-Cap CRO that have not yet been applied to our clinical trial and $212,000 in various insurance policies, such as directors’ and officers’ insurance and product liability insurance for conducting our clinical trials. Our prepaid expense and other at December 31, 2022 also included an additional $394,000 for advanced payments made to CROs for our PCS499 and PCS12852 clinical trials; an unamortized non-cash commitment fee we paid to Lincoln Park of approximately $334,000; and unamortized ATM offering related costs of $186,000.
Net cash used in investing activities
We used net cash in our investing activities of $2,776 during year ended December 31, 2023 to purchase property and equipment. We did not have a similar expense during the same period in 2022.
Net cash provided by (used in) financing activities
During the year ended December 31, 2023, we raised net proceeds of $6.4 million from the sale of 421,611 shares of our common stock and used $31,000 in legal expenses related to our January 2024 raise and $53,000 for the settlement of a stock award. During the year ended December 31, 2022, we used net cash in financing activities of $300,000 to purchase 5,000 shares of our common stock from a licensee in early 2022 and $89,000 to pay income taxes owed on vested stock-based compensation.
Contractual Obligations and Commitments
The following table summarizes our contractual obligations at December 31, 2023:
| Payments Due by Period | ||||||||||||||||||||
| Less than | 1-3 | 3-5 | More than | |||||||||||||||||
| Total | 1 Year | Years | Years | 5 Years | ||||||||||||||||
| Operating lease obligations | $ | 160,737 | $ | 90,697 | $ | 70,040 | $ | - | $ | - | ||||||||||
| Total | $ | 160,737 | $ | 90,697 | $ | 70,040 | $ | - | $ | - | ||||||||||
| 55 |
We enter into contracts in the normal course of business with CROs, clinical supply manufacturers and vendors for pre-clinical studies, research supplies and other services and products for operating purposes. These contracts generally provide for termination after a notice period, and, therefore, are cancelable contracts and not included in the table above.
We have also entered into license and collaboration agreements with third parties, which are in the normal course of business. We have not included future payments under these agreements in the table above since obligations under these agreements are contingent upon future events such as our achievement of specified development, regulatory, and commercial milestones, or royalties on net product sales.
Off Balance Sheet Arrangements
During the periods presented we did not have, nor do we currently have, any off-balance sheet arrangements as defined under SEC rules.
Critical Accounting Policies and Use of Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, and expenses and the disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates and judgments, including those related to accrued expenses and stock-based compensation. We base our estimates on historical experience, known trends and events, and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following accounting policies and estimates are most critical to aid in understanding and evaluating our financial results reported in our consolidated financial statements.
Impairment of PCS499 In-process Research and Development Asset
In February 2023, we suspended further enrollment in our PCS499 trial for uNL due to difficulties we encountered in enrolling the trial, along with our limited resources. In May 2021, we enrolled our first patient in our Phase 2B trial for the treatment of ulcerative NL with PCS499. Although we initiated a number of recruitment programs to increase the enrollment of patients in this study, we were only able to recruit four patients by December 31, 2022. We experienced extremely slow enrollment in the study given the extreme rarity of the condition (rarer than reported in the literature), the impact of COVID-19, and the reluctance of patients to be in a clinical study. We completed the study for those currently enrolled and halted further efforts to enroll new patients. As a result, we recognized a non-cash expense of $7.3 million as of December 31, 2022 related to the impairment of the intangible asset for PCS499. We originally recognized this intangible asset in conjunction with its acquisition from CoNCERT Pharmaceuticals, Inc. in 2018.
Clinical Trial Accruals / Research and Development
As part of the process of preparing our consolidated financial statements, we are required to estimate expenses resulting from our obligations under contracts with vendors, CROs and consultants and under clinical site agreements related to conducting our clinical trials. The financial terms of these contracts vary and may result in payment flows that do not match the period over which materials or services are provided under such contracts.
Our clinical trial accruals are based on estimates of patient enrollment and related costs at clinical investigator sites as well as estimates for the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical trials on our behalf. During a clinical trial, we will adjust the clinical expense recognition if actual results differ from estimates. We make estimates of accrued expenses as of each balance sheet date based on the facts and circumstances known at that time. Our clinical trial accruals are partially dependent on the accurate reporting by the CRO and other third-party vendors. Although we do not expect estimates to differ materially from actual amounts, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in reporting amounts that may be too high or too low for any reporting period.
| 56 |
Payments made to third parties under these arrangements in advance of the receipt of the related services are recorded as prepaid expenses until the services are rendered. We expense research and development costs as they are incurred.
Stock-Based Compensation
Stock-based compensation expense is based on the grant-date fair value estimated in accordance with the provisions of ASC 718, Compensation-Stock Compensation. We expense stock-based compensation over the requisite service period based on the estimated grant-date fair value of the awards. Stock-based awards with graded-vesting schedules are recognized on a straight-line basis over the requisite service period for each separately vesting portion of the award. No expense is recognized for stock-based awards with performance-vesting conditions until management believes it is probable the performance-vesting condition will be met. We value restricted stock awards (RSAs) and restricted stock units (RSUs) based on the closing share price on the date of grant. We estimate the fair value of stock option and warrant grants using the Black-Scholes option pricing model, and the assumptions used in calculating the fair value of stock-based awards represent management’s best estimates and involve inherent uncertainties and the application of management’s judgment. We account for forfeitures in the period in which they occur, rather than estimate expected forfeitures.
See Note 3 – Stock-Based Compensation for information concerning certain of the specific assumptions we used in applying the Black-Scholes option pricing model to determine the estimated fair value of warrants granted during the year ended December 31, 2023.
All stock-based compensation costs are recorded in general and administrative or research and development costs in the consolidated statements of operations based upon the underlying individual’s or entity’s role.
Income Taxes
We account for income taxes in accordance with ASC Topic 740, Income Taxes. Deferred income taxes are recorded for the expected tax consequences of temporary differences between the basis of assets and liabilities for financial reporting purposes and amounts recognized for income tax purposes. We have recorded a valuation allowance equal to the full recorded amount of our net deferred tax assets since it was more-likely-than-not that benefits from our deferred tax assets would not be realized. The valuation allowance is reviewed quarterly and is maintained until sufficient positive evidence exists to support its reversal. As part of an evaluation of our tax attributes in 2022, we recharacterized approximately $7.4 million of startup costs previously capitalized as an IRC Section 195 asset as net operating losses. The recharacterization has no impact on total deferred tax assets since we had previously and will continue to provide a full valuation allowance on our unutilized net deferred tax assets. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the year in which wouldthose temporary differences are expected to be determinedrecovered or settled. The effect of changes in tax rates on deferred tax assets and liabilities is recognized in income in the period such changes are enacted. No income tax benefit or expense was recorded for any periods presented nor is expected in the foreseeable future since we expect to generate future taxable net operating losses.
We recognize the impact of an uncertain tax position if the position will more likely than not be sustained upon examination by a taxing authority, based on either discounted future cash flowsthe technical merits of the position. Our policy is to record interest and penalties related to income taxes as part of its income tax provision. At December 31, 2023, we had no unrecognized tax benefits and as such, no liability, interest or other appropriate fair value methods.penalties were required to be recorded. We do not expect this to change significantly in the next twelve months.
Newly Adopted Accounting Standards and RecentRecently Issued Accounting Pronouncements
See Note 2 of Notesour consolidated financial statements for new accounting pronouncements or changes to Consolidated Financial Statements in the recent accounting pronouncements during the year ended December 31, 2023.
Item 8 for7A. Quantitative and Qualitative Disclosures about Market Risk
Item 7A is not applicable to us as a discussion of newly adopted accounting standardssmaller reporting company and recently issued accounting pronouncements.has been omitted.
ITEM
Item 8. Financial Statements and Supplementary Data
Index to Financial Statements
| 57 |
Heatwurx, Inc.INDEX TO FINANCIAL STATEMENTS
| Page | |
|
|
Consolidated Financial Statements | |
| Consolidated Balance Sheets |
|
Consolidated Statements of Operations |
|
| |
Consolidated Statements of Cash Flows |
|
|
| F-1 |
Pritchett, Siler & Hardy, P.C.
Certified Public Accountants
515 South 400 East, Suite 100
Salt Lake City, UT 84111
Office: (801) 328-2727 Fax: (801) 328-11123
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM

To the Board of Directors and
Stockholders of Heatwurx,
Processa Pharmaceuticals, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Heatwurx,Processa Pharmaceuticals, Inc. (the “Company”) as of December 31, 20162023 and 2015, and2022, the related consolidated statements of income,operations, stockholders’ equity, (deficit), and cash flows, for the years then ended. Heatwurx, Inc.’s management is responsible for these consolidatedended, and the related notes (collectively referred to as the “consolidated financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States)statements”). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Heatwurx, Inc.the Company as of December 31, 20162023 and 2015,2022, and the consolidated results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
The Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 21 to the consolidated financial statements, the Company has suffered recurring losses from operations and has minimal working capital which raisesstated that substantial doubt exists about itsthe Company’s ability to continue as a going concern. Management's plan in regard toManagement’s evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 2.1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involve our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing a separate opinion on the critical audit matters or on the account or disclosures to which they relate.
Going Concern
As discussed in Note 1 to the consolidated financial statements, the Company has determined that there is substantial doubt about its ability to continue as a going concern.
We identified the going concern as a critical audit matter due to the difficulty the Company experienced raising funds. Additionally, it has no source of cash beyond equity and debt financing.
Equity Related Matters
As discussed in Notes 2, 3, 4 and 5 to the consolidated financial statements, the Company has substantial equity activity, including financing transactions and stock compensation. The volume and complexity of the activity have resulted in a complex earnings per share calculation.
We identified equity as a critical audit matter due to the materiality of the transactions as well as the complexity of the related calculations. To test the completeness and accuracy of the transactions, we read all relevant agreements and tested management’s calculations.
/s/ Pritchett, Siler and Hardy, P.C.BD & Company, Inc.
Owings Mills, MD
Pritchett, Siler and Hardy, P.C.March 29, 2024
Salt Lake City, UT
September 25, 2017We have served as the Company’s auditor since 2017.
| F-2 |
Processa Pharmaceuticals, Inc.
Consolidated Balance Sheets
| December 31, 2023 | December 31, 2022 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 4,706,197 | $ | 6,503,595 | ||||
| Prepaid expenses and other | 926,300 | 1,883,134 | ||||||
| Total Current Assets | 5,632,497 | 8,386,729 | ||||||
| Property and Equipment, net | 2,554 | - | ||||||
| Other Assets | ||||||||
| Operating lease right-of-use assets, net | 146,057 | 227,587 | ||||||
| Other | 5,535 | 5,535 | ||||||
| Total Other Assets | 151,592 | 233,122 | ||||||
| Total Assets | $ | 5,786,643 | $ | 8,619,851 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current Liabilities | ||||||||
| Current maturities of operating lease liability | $ | 83,649 | $ | 78,896 | ||||
| Accounts payable | 311,617 | 327,548 | ||||||
| Due to licensor | 189,000 | 189,000 | ||||||
| Due to related parties | 39 | 51 | ||||||
| Accrued expenses | 146,274 | 403,061 | ||||||
| Total Current Liabilities | 730,579 | 998,556 | ||||||
| Non-current Liabilities | ||||||||
| Non-current operating lease liability | 66,905 | 150,554 | ||||||
| Total Liabilities | 797,484 | 1,149,110 | ||||||
| Commitments and Contingencies | - | - | ||||||
| Stockholders’ Equity | ||||||||
| Common stock, par value $, shares authorized; issued and outstanding at December 31, 2023 and issued and outstanding at December 31, 2022 | 129 | 80 | ||||||
| Additional paid-in capital | 80,658,111 | 72,018,222 | ||||||
| Treasury stock | (300,000 | ) | (300,000 | ) | ||||
| Accumulated deficit | (75,369,081 | ) | (64,247,561 | ) | ||||
| Total Stockholders’ Equity | 4,989,159 | 7,470,741 | ||||||
| Total Liabilities and Stockholders’ Equity | $ | 5,786,643 | $ | 8,619,851 | ||||
HEATWURX, INC.
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||
| 2016 |
| 2015 | ||
|
|
|
|
|
|
ASSETS |
|
|
|
|
|
CURRENT ASSETS: |
|
|
|
|
|
Cash and cash equivalents | $ | 3,237 |
| $ | 2,090 |
Prepaid expenses and other current assets |
| - |
|
| 47,722 |
Assets held for sale |
| - |
|
| 42,000 |
Current assets from discontinued operations |
| - |
|
| 12,350 |
Total current assets |
| 3,237 |
|
| 104,162 |
EQUIPMENT, net of depreciation |
| - |
|
| 159 |
TOTAL ASSETS | $ | 3,237 |
| $ | 104,321 |
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) |
|
|
|
|
|
CURRENT LIABILITIES: |
|
|
|
|
|
Accounts payable | $ | 166,165 |
| $ | 159,249 |
Accrued liabilities |
| 134,513 |
|
| 95,339 |
Interest payable |
| 108,608 |
|
| 41,990 |
Interest payable, related party |
| 332,566 |
|
| 119,618 |
Income taxes payable |
| 200 |
|
| 200 |
Current portion of senior secured notes payable, related party |
| 962,361 |
|
| 947,361 |
Current portion of unsecured notes payable, net of discount of $0 and $967 at December 31, 2016 and 2015, respectively |
| 420,000 |
|
| 419,033 |
Revolving line of credit |
| 91,980 |
|
| - |
Revolving line of credit, related party |
| 138,000 |
|
| - |
Current liabilities from discontinued operations |
| - |
|
| 300,338 |
Total current liabilities |
| 2,354,393 |
|
| 2,083,128 |
TOTAL LIABILITIES |
| 2,354,393 |
|
| 2,083,128 |
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY (DEFICIT): |
|
|
|
|
|
Series D preferred stock, $0.0001 par value, 178,924 shares issued and outstanding at December 31, 2016 and 2015; liquidation preference of $864,743 at December 31, 2016 and $821,683 at December 31, 2015 |
| 18 |
|
| 18 |
Common stock, $0.0001 par value, 20,000,000 shares authorized; 11,017,388 issued and outstanding at December 31, 2016 and 2015 |
| 1,102 |
|
| 1,102 |
Additional paid-in capital |
| 14,329,057 |
|
| 14,322,366 |
Accumulated deficit |
| (15,254,917) |
|
| (14,874,680) |
Stockholders’ equity (deficit) from discontinued operations |
| (1,426,416) |
|
| (1,427,613) |
Total stockholders’ equity (deficit) |
| (2,351,156) |
|
| (1,978,807) |
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | $ | 3,237 |
| $ | 104,321 |
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
HEATWURX, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
Processa Pharmaceuticals, Inc.
| For the year ended December 31, | ||||
| 2016 |
| 2015 | ||
|
|
|
| ||
REVENUE: |
|
|
| ||
Equipment sales | $ | 5,000 |
| $ | 74,599 |
Other revenue |
| - |
|
| 38,826 |
Total revenues |
| 5,000 |
|
| 113,425 |
|
|
|
|
|
|
COST OF GOODS SOLD |
| - |
|
| 125,876 |
GROSS PROFIT |
| 5,000 |
|
| (12,451) |
|
|
|
|
|
|
EXPENSES: |
|
|
|
|
|
Selling, general and administrative |
| 69,968 |
|
| 945,460 |
Research and development |
| 6,558 |
|
| 29,246 |
Impairment of intangible asset |
| - |
|
| 1,517,859 |
Impairment of assets held for sale |
| - |
|
| 186,068 |
Total expenses |
| 76,526 |
|
| 2,678,633 |
|
|
|
|
|
|
LOSS FROM CONTINUING OPERATIONS, before taxes |
| (71,526) |
|
| (2,691,084) |
|
|
|
|
|
|
OTHER INCOME AND EXPENSE |
|
|
|
|
|
Gain from debt forgiveness |
| 4,731 |
|
| - |
Loss on disposal of assets |
| (25,000) |
|
| (60,743) |
Interest income |
| - |
|
| 4,651 |
Interest expense, including amortization of debt discount |
| (245,384) |
|
| (235,644) |
Total other income and expense |
| (265,653) |
|
| (291,736) |
|
|
|
|
|
|
LOSS BEFORE INCOME TAXES |
| (337,179) |
|
| (2,982,820) |
Income taxes |
| - |
|
| (100) |
LOSS FROM CONTINUED OPERATIONS, net of tax |
| (337,179) |
|
| (2,982,920) |
LOSS FROM DISCONTINUED OPERATIONS, net of tax |
| 1,197 |
|
| (371,307) |
NET LOSS | $ | (335,982) |
| $ | (3,354,227) |
|
|
|
|
|
|
Preferred Stock Cumulative Dividend and Deemed Dividend | $ | (43,058) |
| $ | (42,942) |
Net loss applicable to common stockholders | $ | (379,040) |
| $ | (3,397,169) |
Net loss per common share basic and diluted from continuing operations |
| (0.03) |
|
| (0.27) |
Net loss per common share basic and diluted from discontinued operations |
| 0.00 |
|
| (0.03) |
Net loss per common share basic and diluted | $ | (0.03) |
| $ | (0.31) |
Weighted average shares outstanding used in calculating net loss per common share |
| 11,017,388 |
|
| 11,012,565 |
Consolidated Statements of Operations
| 2023 | 2022 | |||||||
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Operating Expenses | ||||||||
| Research and development expenses | $ | 5,799,518 | $ | 11,494,230 | ||||
| General and administrative expenses | 5,657,543 | 8,763,058 | ||||||
| Impairment of intangible asset | - | 7,268,143 | ||||||
| Operating Loss | (11,457,061 | ) | (27,525,431 | ) | ||||
| Other Income (Expense) | ||||||||
| Interest income, net | 335,541 | 101,202 | ||||||
| Net Operating Loss Before Income Tax Benefit | (11,121,520 | ) | (27,424,229 | ) | ||||
| Income Tax Benefit | - | - | ||||||
| Net Loss | $ | (11,121,520 | ) | $ | (27,424,229 | ) | ||
| Net Loss Per Common Share - Basic and Diluted | $ | ) | $ | ) | ||||
| Weighted Average Common Shares Used to Compute | ||||||||
| Net Loss Per Common Shares - Basic and Diluted | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
HEATWURX, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
Processa Pharmaceuticals, Inc.
| Series D Preferred Stock | Common Stock | Additional Paid-In Capital | Accumulated deficit Disc- ontinued Operations | Accumu- lated Deficit | Total | ||
| Shares | $ | Shares | $ | $ | $ | $ | $ |
|
|
|
|
|
|
|
|
|
Balance at December 31, 2014 | 178,924 | $18 | 10,952,356 | 1,095 | 14,111,944 | (1,056,306) | (11,848,818) | 1,207,933 |
Shares and warrants issued pursuant to private placement dated October 1, 2014 | - | - | 50,285 | 5 | 87,995 | - | - | 88,000 |
Adjustment for shares issued pursuant to private placement dated October 1, 2014 | - | - | (253) | - | - | - | - | - |
Stock issued for consulting services | - | - | 15,000 | 2 | 25,498 | - | - | 25,500 |
Stock-based compensation (options) | - | - | - | - | 96,929 | - | - | 96,929 |
Dividends accrued on Series D shares | - | - | - | - | - | - | (42,942) | (42,942) |
Net loss from continuing operations | - | - | - | - | - | - | (2,982,920) | (2,982,920) |
Net loss from discontinued operations | - | - | - | - | - | (371,307) | - | (371,307) |
Balance at December 31, 2015 | 178,924 | 18 | 11,017,388 | 1,102 | 14,322,366 | (1,427,613) | (14,874,680) | (1,978,807) |
Stock-based compensation (options) | - | - | - | - | 6,691 | - | - | 6,691 |
Dividends accrued on Series D shares | - | - | - | - | - | - | (43,058) | (43,058) |
Net loss from continuing operations | - | - | - | - | - | - | (337,179) | (337,179) |
Net income from discontinued operations | - | - | - | - | - | 1,197 | - | 1,197 |
Balance at December 31, 2016 | 178,924 | 18 | 11,017,388 | 1,102 | 14,329,057 | (1,426,416) | (15,254,917) | (2,351,156) |
Consolidated Statements of Changes in Stockholders’ Equity
Years Ended December 31, 2023 and 2022
| Shares | Amount | Capital | Shares | Amount | Deficit | Total | ||||||||||||||||||||||
| Additional | ||||||||||||||||||||||||||||
| Common Stock | Paid-In | Treasury Stock | Accumulated | |||||||||||||||||||||||||
| Shares | Amount | Capital | Shares | Amount | Deficit | Total | ||||||||||||||||||||||
| Balance at January 1, 2022 | 785,512 | $ | 79 | $ | 62,308,353 | $ | - | $ | - | $ | (36,823,332 | ) | $ | 25,485,100 | ||||||||||||||
| Acquisition of treasury stock | - | - | - | (5,000 | ) | (300,000 | ) | - | (300,000 | ) | ||||||||||||||||||
| Stock-based compensation | 11,890 | 1 | 8,948,712 | - | - | - | 8,948,713 | |||||||||||||||||||||
| Shares issued in connection with the Purchase Agreement with Lincoln Park | 6,181 | 1 | 449,999 | - | - | - | 450,000 | |||||||||||||||||||||
| Shares issued in connection with license agreement | 5,000 | - | 400,000 | - | - | - | 400,000 | |||||||||||||||||||||
| Shares withheld to pay income taxes on stock-based compensation | (1,809 | ) | (1 | ) | (88,842 | ) | - | - | - | (88,843 | ) | |||||||||||||||||
| Net loss | - | - | - | - | - | (27,424,229 | ) | (27,424,229 | ) | |||||||||||||||||||
| Balance, January 1, 2023 | 806,774 | 80 | 72,018,222 | (5,000 | ) | (300,000 | ) | (64,247,561 | ) | 7,470,741 | ||||||||||||||||||
| Balance | 806,774 | 80 | 72,018,222 | (5,000 | ) | (300,000 | ) | (64,247,561 | ) | 7,470,741 | ||||||||||||||||||
| Stock-based compensation | 6,945 | 1 | 1,060,338 | - | - | - | 1,060,339 | |||||||||||||||||||||
| Shares issued in connection with capital raises, net of transaction costs | 421,611 | 42 | 6,352,035 | - | - | - | 6,352,077 | |||||||||||||||||||||
| Warrant granted in connection with a consulting agreement | - | - | 1,310,875 | - | - | - | 1,310,875 | |||||||||||||||||||||
| Settlement of stock award | - | - | (52,746 | ) | - | - | - | (52,746 | ) | |||||||||||||||||||
| Other | 55,670 | 6 | (30,613 | ) | - | - | - | (30,607 | ) | |||||||||||||||||||
| Net loss | - | - | - | - | - | (11,121,520 | ) | (11,121,520 | ) | |||||||||||||||||||
| Balance, December 31, 2023 | 1,291,000 | $ | 129 | $ | 80,658,111 | (5,000 | ) | $ | (300,000 | ) | $ | (75,369,081 | ) | $ | 4,989,159 | |||||||||||||
| Balance | 1,291,000 | $ | 129 | $ | 80,658,111 | (5,000 | ) | $ | (300,000 | ) | $ | (75,369,081 | ) | $ | 4,989,159 | |||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
27Processa Pharmaceuticals, Inc.
HEATWURX, INC.
CONSOLIDATED STATEMENT OF CASH FLOWSConsolidated Statements of Cash Flows
| For the year ended December 31, | ||||
| 2016 |
| 2015 | ||
|
|
|
| ||
OPERATING ACTIVITIES: |
|
|
| ||
Net loss | $ | (335,982) |
| $ | (3,354,227) |
Less: (Income) loss from discontinued operations, net of tax |
| (1,197) |
|
| 371,307 |
Loss from continuing operations |
| (337,179) |
|
| (2,982,920) |
Adjustments to reconcile net loss from continuing operations to net cash used in operating activities: |
|
|
|
|
|
Depreciation expense |
| 159 |
|
| 60,251 |
Gain on debt forgiveness |
| (4,731) |
|
| - |
Amortization of intangible assets |
| - |
|
| 178,571 |
Amortization of discount on note payable |
| 967 |
|
| 58,801 |
Impairment of assets held for sale |
| - |
|
| 186,068 |
Impairment of intangible asset |
| - |
|
| 1,517,859 |
Loss on disposal of equipment |
| 25,000 |
|
| 60,743 |
Bad debt expense |
| - |
|
| 5,148 |
Stock-based compensation |
| 6,691 |
|
| 96,929 |
Shares exchanged for services |
| - |
|
| 25,500 |
Changes in current assets and liabilities: |
|
|
|
|
|
Decrease in receivables |
| - |
|
| (3,838) |
Decrease (increase) in prepaid expenses and other current assets |
| 47,722 |
|
| 105,136 |
Increase in inventory |
| - |
|
| (7,065) |
Increase in income taxes payable |
| - |
|
| 100 |
(Decrease) increase in accounts payable |
| (10,343) |
|
| 5,318 |
(Decrease) increase in accrued liabilities |
| 686 |
|
| (41,774) |
(Decrease) increase in deferred revenue |
| - |
|
| (58,165) |
Increase in interest payable |
| 54,613 |
|
| 31,595 |
Increase in interest payable, related party |
| 185,562 |
|
| 122,193 |
Net cash used in operating activities from continuing operations |
| (30,853) |
|
| (639,550) |
Net cash used in operating activities from discontinued operations |
| (12,350) |
|
| (232,356) |
Net cash used in operating activities |
| (43,203) |
|
| (871,906) |
|
|
|
|
|
|
INVESTING ACTIVITIES: |
|
|
|
|
|
Purchases of property and equipment |
| - |
|
| (1,399) |
Proceeds from the sale of assets held for sale |
| 17,000 |
|
| - |
Proceeds from the sale of property and equipment |
| - |
|
| 31,100 |
Net cash provided by investing activities from continuing operations |
| 17,000 |
|
| 29,701 |
Net cash provided by investing activities from discontinued operations |
| - |
|
| 10,503 |
Net cash provided by investing activities |
| 17,000 |
|
| 40,204 |
|
|
|
|
|
|
FINANCING ACTIVITIES: |
|
|
|
|
|
Proceeds from issuance of senior secured notes payable |
| 15,000 |
|
| 753,000 |
Proceeds from issuance of common shares with warrants |
| - |
|
| 88,000 |
Repayment on equipment loan payable |
| - |
|
| (19,902) |
Net cash provided by financing activities from continuing operations |
| 15,000 |
|
| 821,098 |
Net cash provided by financing activities from discontinued operations |
| - |
|
| - |
Net cash provided by financing activities |
| 15,000 |
|
| 821,098 |
|
|
|
|
|
|
NET CHANGE IN CASH AND CASH EQUIVALENTS |
| (11,203) |
|
| (10,604) |
CASH AND CASH EQUIVALENTS, beginning of period, including discontinued operations |
| 14,440 |
|
| 25,044 |
CASH AND CASH EQUIVALENTS, Continuing Operations, end of period |
| 3,237 |
|
| 2,090 |
CASH AND CASH EQUIVALENTS, Discontinued Operations, end of period | $ | - |
| $ | 12,350 |
| 2023 | 2022 | |||||||
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Cash Flows From Operating Activities | ||||||||
| Net Loss | $ | (11,121,520 | ) | $ | (27,424,229 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 222 | - | ||||||
| Non-cash lease expense for right-of-use assets | 81,530 | 85,518 | ||||||
| Non-cash milestone expense in connection with license agreement | - | 189,000 | ||||||
| Amortization of debt issuance costs | - | 115,613 | ||||||
| Amortization of intangible asset | - | 788,495 | ||||||
| Impairment of intangible asset | - | 7,268,143 | ||||||
| Stock-based compensation | 1,060,339 | 8,828,713 | ||||||
| Warrants issued to purchase 158,007 shares of common stock | 1,310,875 | - | ||||||
| Net changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other | 956,834 | 210,549 | ||||||
| Operating lease liability | (78,896 | ) | (87,937 | ) | ||||
| Accounts payable | (15,931 | ) | 108,643 | |||||
| Due (from) to related parties | (12 | ) | (1,721 | ) | ||||
| Other receivables | - | 70,274 | ||||||
| Accrued expenses | (256,787 | ) | 243,796 | ) | ||||
| Net cash (used in) operating activities | (8,063,346 | ) | (9,605,143 | ) | ||||
| Cash Flows From Investing Activities | ||||||||
| Purchase of property and equipment | (2,776 | ) | - | |||||
| Net cash (used in) provided by investing activities | (2,776 | ) | - | |||||
| Cash Flows From Financing Activities | ||||||||
| Net proceeds from issuance of stock | 6,321,470 | - | ||||||
| Shares withheld to pay taxes on stock-based compensation | - | (88,843 | ) | |||||
| Settlement of stock award | (52,746 | ) | - | |||||
| Acquisition of treasury stock | - | (300,000 | ) | |||||
| Net cash (used in) provided by financing activities | 6,268,724 | (388,843 | ) | |||||
| Net (Decrease) Increase in Cash and Cash Equivalents | (1,797,398 | ) | (9,993,986 | |||||
| Cash and Cash Equivalents - Beginning of Year | 6,503,595 | 16,497,581 | ||||||
| Cash and Cash Equivalents - End of Year | $ | 4,706,197 | $ | 6,503,595 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
Processa Pharmaceuticals, Inc.
Consolidated Statements of Cash Flows (continued)
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Supplemental Cash Flow Information: | ||||||||
| Cash paid for interest | $ | - | $ | - | ||||
| Cash paid for income taxes | - | - | ||||||
| Non-Cash Financing Activities | ||||||||
| Issuance of shares of common stock in satisfaction of accrued director fees | $ | - | $ | 120,000 | ||||
| Issuance of shares of common stock in connection with a licensing agreement which had previously been recorded as a due to licensor | $ | - | $ | 400,000 | ||||
| Issuance of shares of common stock in connection with the Purchase Agreement with Lincoln Park | $ | - | $ | 450,000 | ||||
| - | ||||||||
| Right-of-use asset obtained in exchange for operating lease liability | $ | - | $ | (238,924 | ) | |||
| Operating lease liability | - | 238,924 | ||||||
| Net | $ | - | $ | - | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-7 |
Processa Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
Note 1 – Organization
Organization
HEATWURX, INC.
We are a clinical-stage biopharmaceutical company focused on incorporating our Regulatory Science Approach into the development of our Next Generation Chemotherapy (NGC) drugs to improve the safety and efficacy of cancer treatment. Our NGC drugs are modifications of existing FDA-approved oncology drugs resulting in an alteration of the metabolism and/or distribution while maintaining the well-known and established existing mechanisms of killing the cancer cells. By modifying the NGC drugs in this manner, we believe our three NGC treatments will provide improved safety-efficacy profiles when compared to their currently marketed counterparts.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1.
PRINCIPAL BUSINESS ACTIVITIES:
OrganizationOn January 22, 2024, we filed a Certificate of Amendment to our Certificate of Incorporation, as amended with the Secretary of State of Delaware that effected a 1-for-20 reverse stock split of our common stock, par value $ per share (the “Reverse Stock Split”). Pursuant to the Certificate of Amendment, our issued common stock decreased from shares to shares and Business - Heatwurx, Inc. (“Heatwurx,” the “Company”) is an asphalt repair equipmentour outstanding common stock decreased from to . The Reverse Stock Split did not affect our authorized common stock of shares or our common stock par value. All shares of common stock, including common stock underlying warrants, stock options, and technology company.
2.
BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES:
Basis of Presentation - Theserestricted stock units, as well as exercise prices and per share information in these consolidated financial statements and related notes are presented in accordance withgive retroactive effect to the accounting principles generally accepted in the United States (“U.S. GAAP”) and are expressed in U.S. dollars. The Company’sReverse Stock Split.
Liquidity
Our consolidated financial statements include Dr. Pave, LLC and Dr. Pave Worldwide, LLC; both wholly-owned subsidiaries of the Company, which are represented in the Company’s discontinued operations (Note 6). All intercompany balances and transactions have been eliminated in the consolidated financial statements.
Going Concern and Management’s Plan - The Company’s financial statements are prepared using U.S. GAAP and are subject toon a going concern basis, which contemplates the continuity of operations, realization of assets and liquidationthe satisfaction of liabilities and commitments in the normalordinary course of business. The Company faces certain risksWe have incurred losses since inception, currently devoting substantially all of our efforts toward research and uncertainties that are present in many emerging companies regardingdevelopment of our next generation chemotherapy drug product development, future profitability, ability to obtain future capital, protectioncandidates, including conducting clinical trials and providing general and administrative support for these operations, and have an accumulated deficit of patents and property rights, competition, rapid technological change, government regulations, recruiting and retaining key personnel, and third party manufacturing organizations.
The Company has previously relied exclusively on private placements with a small group of investors to finance its business and operations. The Company has had little revenue since inception. For the year ended$75.4 million at December 31, 2016, the Company incurred a net loss from continuing operations of approximately $337,179 and used approximately $30,853 in net cash from operating activities from continuing operations and approximately $12,350 in net cash from operating activities from discontinued operations. The Company had total cash on hand of approximately $3,237 as of December 31, 2016. The Company is not able to obtain additional financing adequate to fulfill its commercialization activities, nor achieve a level of revenues adequate to support the Company’s cost structure. The Company does not currently have any revenue under contract nor does it have any immediate sales prospects. The Company has significantly reduced employees and overhead. The Company discontinued operations of Dr. Pave, LLC and Dr. Pave Worldwide, LLC on December 31, 2015. These business components are captured within discontinued operations as of December 31, 2016 (Note 6). The Company has significantly scaled back operations to maintain only a minimal level of operations necessary to support our licensee, warehouse the equipment held for the licensee and look for potential merger candidates. It is the Company’s intention to move forward as a public entity and to seek potential merger candidates. If the Company fails to merge or be acquired by another company, we will be required to terminate all operations.
2023. During the year ended December 31, 2016, the Company received2023, we generated a net loss of $11.1 million and used $8.1 million in net cash for operating activities from continuing operations. To date, none of our drug candidates have been approved for sale, and therefore we have not generated any product revenue and do not expect positive cash flow from operations in the aggregateforeseeable future.
We have financed our operations primarily through public equity issuances, including an offering we closed on January 30, 2024 where we sold shares of $15,000 underour common stock, pre-funded warrants to purchase up to 1,079,555 shares of our common stock, and warrants for the $2,000,000 senior securedpurchase of up to 1,555,555 shares of our common stock for net proceeds of $6.3 million, after deducting placement agent fees and offering-related expenses (see Note 13 for additional details). Simultaneously with the closing of the sale, the pre-funded warrants were exercised in exchange for shares of our common stock. We will continue to be dependent upon equity and/or debt offering. Based uponfinancing until we are able to generate positive cash flows from its operations.
At December 31, 2023, we had cash and cash equivalents totaling $4.7 million. Together with the Company’s$6.3 million net proceeds we raised in January 2024, and based on our current financial positionbusiness plans, we believe these funds will satisfy our capital needs into early 2025. Our ability to execute our longer-term operating plans, including future preclinical studies and inabilityclinical trials for our portfolio of drugs depend on our ability to obtain additional funding from the sale of equity and/or debt securities, a strategic transaction or other funding transactions.
We plan to raise additional funds in the future through a combination of public or private equity offerings, debt financings, collaborations, strategic alliances, licensing arrangements and other marketing and distribution arrangements, but will only do so if the terms are acceptable to us. If we are unable to obtain adequate financing when needed, we may have to delay, reduce the Company wasscope of, or suspend our current or planned future clinical trial plans, or research and development programs. This may also cause us to not ablemeet obligations contained in certain of our license agreements and put these assets at risk. To the extent that we raise additional capital through marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to satisfyrelinquish valuable rights to our product candidates, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we raise additional capital through public or private equity offerings, the mandatory principal payments in 2016 underownership interest of our existing stockholders will be diluted, and the $2,000,000 senior secured debt. The Company will continue to work with the lenders to explore extension or conversion options, but there is no guarantee the lenders will agree to modify the repayment terms of these securities may include liquidation or other preferences that adversely affect our stockholders’ rights. If we raise additional capital through debt financing, we may be subject to covenants limiting or restricting our ability to take specific actions, such as incurring additional debt or making capital expenditures. There can be no assurance that future funding will be available when needed.
Absent additional funding, we believe that our cash and cash equivalents will not be sufficient to fund our operations for a period of one year or more after the notes under conditionsdate that will allow the Companythese consolidated financial statements are available to continue to repay the notes, if at all. As these notes are secured by all of the assets of the Company, including intellectual property rights, the Company is in default in regard to interest paymentsbe issued based on the notes,timing and the lenders may call the notesamount of our projected net loss from continuing operations and foreclose on the Company’s assets.
The issues described above raisecash to be used in operating activities during that period of time. As a result, substantial doubt exists about the Company’sour ability to continue as a going concern.concern within one year after the date that these consolidated financial statements are available to be issued. The Company has been solely reliant on raising debt and capital in order to maintain its operations. Previously the Company was able to raise debt and equity financing through the assistance of a small number of investors who have been substantial participants in its debt and equity offerings since the Company’s formation. These investors have chosen not to further assist the Company with its capital raising initiatives and, at this time, the Company is not able to obtain any alternative forms of financing and the Company will not be able to continue to satisfy its current or long term obligations. The Company needs to merge with or be acquired by another company. If a candidate is not identified, the Company will be forced to cease operations all together.
29
The accompanying consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of recorded assets, or the amounts and classification of liabilities that might be different should the Companywe be unable to continue as a going concern.concern based on the outcome of these uncertainties described above.
| F-8 |
Note 2 – Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the rules and regulations of the United States Securities and Exchange Commission (the “SEC”), and reflect all of our activities, including those of our wholly-owned subsidiary. All material intercompany accounts and transactions have been eliminated in consolidation. Operating results for the year ended December 31, 2023 are not necessarily indicative of future results.
Use of Estimates - The preparation of
In preparing our consolidated financial statements and related disclosures in conformity with U.S. GAAP requiresand pursuant to the userules and regulations of the SEC, we make estimates and assumptions by managementjudgments that affect the amounts reported amounts of assets and liabilities and disclosures of contingent assets and liabilities atin the date of theconsolidated financial statements and the reported amounts of revenuesaccompanying notes. Estimates are used for, but not limited to stock-based compensation, intangible assets, future milestone payments and expenses during the reporting period.income taxes. These estimates and assumptions are continuously evaluated and are based on management’s experience and knowledge of the relevant facts and circumstances. While management believeswe believe the estimates to be reasonable, actual results could differ materially from those estimates and could impact future results of operations and cash flows.
Cash and Cash Equivalents - The Company considers
Cash and cash equivalents include cash on hand and money market funds. We consider all highly liquid investments with a maturity atmaturing within three months from the date of purchase as cash equivalents.
Property and Equipment
Property is stated at cost, less accumulated depreciation. Costs of three monthsrenewals and improvements that extend the useful lives of the assets are capitalized. Expenditures for maintenance and routine repairs are charged to expense as incurred. Depreciation is recognized on a straight-line basis over the estimated useful lives of the assets, which generally range from 3 to 5 years. We amortize leasehold improvements over the shorter of the estimated useful life of the asset or lessthe term of the related lease. Upon retirement or disposition of assets, the costs and related accumulated depreciation are removed from the accounts with the resulting net gain or loss, if any, reflected in the consolidated statement of operations.
Intangible Assets
Intangible assets acquired individually or with a group of other assets from others (other than in a business combination) are recognized at cost, including transaction costs, and allocated to the individual assets acquired based on relative fair values and no goodwill is recognized. Cost is measured based on cash consideration paid. If consideration given is in the form of non-cash assets, liabilities incurred, or equity interests issued, measurement of cost is based on either the fair value of the consideration given or the fair value of the assets (or net assets) acquired, whichever is more evident and more reliably measurable. Costs of internally developing, maintaining or restoring intangible assets that are not specifically identifiable, have indeterminate lives or are inherent in a continuing business are expensed as incurred.
| F-9 |
Intangible assets purchased from others for use in research and development activities and that have alternative future uses (in research and development projects or otherwise) are capitalized in accordance with ASC Topic 350, Intangibles – Goodwill and Other. Those that have no alternative future uses (in research and development projects or otherwise), and therefore no separate economic value, are considered research and development costs and are expensed as incurred. Amortization of intangibles used in research and development activities is a research and development cost.
Intangibles with a finite useful life are amortized using the straight-line method unless the pattern in which the economic benefits of the intangible assets are consumed or used up are reliably determinable. The useful life is the best estimate of the period over which the asset is expected to contribute directly or indirectly to our future cash flow. The useful life is based on the duration of the expected use of the asset by us and the legal, regulatory or contractual provisions that constrain the useful life and future cash flows of the asset, including regulatory acceptance and approval, obsolescence, demand, competition and other economic factors. We evaluate the remaining useful life of intangible assets each reporting period to determine whether any revision to the remaining useful life is required. If the remaining useful life is changed, the remaining carrying amount of the intangible asset will be amortized prospectively over the revised remaining useful life. If an income approach is used to measure the fair value of an intangible asset, we consider the period of expected cash flows used to measure the fair value of the intangible asset, adjusted as appropriate for company-specific factors discussed above, to determine the useful life for amortization purposes.
If no regulatory, contractual, competitive, economic or other factors limit the useful life of the intangible to us, the useful life is considered indefinite. Intangibles with an indefinite useful life are not amortized until its useful life is determined to be no longer indefinite. If the useful life is determined to be finite, the intangible is tested for impairment and the carrying amount is amortized over the remaining useful life in accordance with intangibles subject to amortization. Indefinite-lived intangibles are tested for impairment annually and more frequently if events or circumstances indicate that it is more-likely-than-not that the asset is impaired.
Impairment of Long-Lived Assets and Intangibles Other Than Goodwill
We account for the impairment of long-lived assets in accordance with ASC 360, Property, Plant and Equipment and ASC 350, Intangibles – Goodwill and Other, which require that long-lived assets and certain identifiable intangibles be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to its expected future undiscounted net cash equivalents.
Accounts Receivable and Bad Debt Expense - Management reviews individual accounts receivable balances thatflows generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amounts of the assets exceed 90 days from the invoice date.fair value of the assets based on the present value of the expected future cash flows associated with the use of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell. Based on management’s evaluation, we recorded an assessmentimpairment loss of current creditworthiness of the customer, the Company estimates the portion, if any, of the balance that will not be collected. All accounts deemed to be uncollectible are written off to operating expense. The Company recognized no bad debt expense$7.3 million during the year ended December 31, 20162022 (see Note 8).
Fair Value Measurements and $5,148 during the year ended December 31, 2015. There was no allowanceDisclosure
We apply ASC 820, Fair Value Measurements and Disclosures, which expands disclosures for uncollectible accounts for the years ended December 31, 2016assets and 2015.
Equipment - Equipment is statedliabilities that are measured and reported at cost and consists of office and computer equipment depreciatedfair value on a straight-line basis overrecurring basis. Fair value is defined as an estimated useful lifeexit price, representing the amount that would be received upon the sale of three years, and process demonstration equipment (demo equipment) depreciated onan asset or payment to transfer a straight-line basis overliability in an estimated useful life of seven years. Maintenance and repairs are charged to expense as incurred.orderly transaction between market participants.
Assets held for sale - In efforts to streamline the operations and expenses the Company has opted to sell or return certain assets to improve cash flow or settle debt. The assets are expected to be sold within the next 12 months and meet the other relevant held-for-sale criteria are classified as long-lived assets held-for-sale and measured at their fairFair value is a market-based measurement that is determined based on the Company’s own judgments about assumptions that market participants would use in pricing an asset or liability. A three-tier fair value hierarchy is used to prioritize the inputs in measuring fair value as follows:
Level 1 – Quoted market prices (unadjusted) in active markets for identical assets or liabilities that the reporting entity can access at the measurement date.
Level 2 – Quoted market prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable, either directly or indirectly. Fair value determined using models or other valuation methodologies.
Level 3 – Significant unobservable inputs for assets or liabilities that cannot be corroborated by market data. Fair value is determined by the reporting entity’s own assumptions utilizing the best information available and includes situations where there is little market activity for the asset and on observable market data, when available. An impairment loss is recorded in the statement of operations for long-lived assets held-for-sale when the carrying amount of the asset exceeds itsor liability.
| F-10 |
The asset’s or liability’s fair value less cost to sell. A long-lived asset is not depreciated while it is classified as held-for-sale.
Impairment of Long-lived Assets - The Company periodically reviews its long-lived assets to determine potential impairment by comparing the carrying value of the long-lived assets with the estimated future net undiscounted cash flows expected to result from the use of the assets, including cash flows from disposition, at least annually or more frequently if events or changes in circumstances indicate a potential impairment may exist. Should the sum of the expected future net cash flows be less than the carrying value, the Company would recognize an impairment loss at that date. An impairment loss would be measured by comparing the amount by which the carrying value exceedsmeasurement within the fair value (estimated discounted future cash flows)hierarchy is based upon the lowest level of any input that is significant to the fair value measurement. Our policy is to recognize transfers between levels of the long-lived assets. The Company performs its impairment analysisfair value hierarchy in Octoberthe period the event or change in circumstances that caused the transfer. There were no transfers into or out of each year. The Company recognized an impairment of $1,517,859 on intangible assets related to the asset purchase agreementLevel 1, 2, or 3 during the year ended December 31, 2015. (Note 5)periods presented.
We measure compensation expense for stock options and other stock awards in accordance with ASC 718, Compensation—Stock Compensation. Stock-based compensation is measured at fair value on grant date and recognized as part of an acquisition, which was deemed in-process researchcompensation expense over the requisite service period. Generally, we issue stock options and development upon acquisition. During development, in-process research and developmentother stock awards with service-based and/or performance-based vesting conditions. For awards with only service-based vesting conditions, we record compensation cost for these awards using the straight-line method over the service period. For awards that contain performance vesting conditions, we do not recognize compensation expense until achieving the performance condition is not subject to amortization and is tested for impairment. In October 2012, the in-process research and development was reclassified as developed technology. The Company’s developed technology was amortized over its estimated useful life of seven years. Based on the Company’s financial position and substantial doubt about the Company’s ability to continue as a going concern, the Company has chosen toprobable. We estimate future cash flows at zero. The Company recognized an impairment of $1,517,859 during the second quarter of 2015.
Debt Discount - The Company recognizes the fair value of detachable warrants issued in conjunction with a debt instrument as debt discount. The discount is amortizedstock option and warrant grants using the interest method overBlack-Scholes option pricing model, and the lifeassumptions used in calculating the fair value of stock-based awards represent management’s best estimates and involve inherent uncertainties and the application of management’s judgment. We value restricted stock awards (RSAs) and restricted stock units (RSUs) based on the closing share price of our common stock on the date of grant. Stock-based compensation costs are recorded as general and administrative or research and development costs in the statements of operations based upon the underlying individual’s or entity’s role.
Estimates of fair value are not intended to predict actual future events or the value ultimately realized by employees or consultants who receive these awards, and subsequent events are not indicative of the notes. The amortizationreasonableness of our original estimates of fair value. We account for forfeitures in the discount was $967period in which they occur, rather than estimate expected forfeitures.
Basic net loss per share is computed by dividing net loss by the weighted average common stock outstanding (which excludes unvested RSAs) and $58,801vested, but unissued RSUs. Diluted loss per share is computed by dividing our net loss available to common shareholders by the diluted weighted average number of shares of common stock outstanding during the period. Since we have experienced a net loss for all periods presented, basic and diluted net loss per share are the same. As such, diluted loss per share for the years ended December 31, 20162023 and 2015, respectively.2022 excludes the impact of potentially dilutive common shares related to outstanding stock options, unvested restricted stock awards (RSAs), unvested RSUs and purchase warrants.
30
Stock-Based Compensation - The Company accounts for the cost of employee services received in exchange for the award of equity instruments based on the fair value of the award, determined on the date of grant. The expense is to be recognized over the period during which an employee is required to provide services in exchange for the award. The Company estimates forfeitures at the time of grant and makes revisions, if necessary, at each reporting period if actual forfeitures differ from those estimates. The Company estimated future unvested forfeitures at 25% and 0%Our diluted net loss per share for the years ended December 31, 20162023 and 2015, respectively.2022 excluded and of potentially dilutive common shares, respectively, related to outstanding stock options, unvested RSAs, unvested RSUs and warrants since those shares would have had an anti-dilutive effect on loss per share during the years then ended.
| F-11 |
Advertising ExpenseSegments - The Company charges advertising costs to expense as incurred. Advertising costs were ($69)
We operate in one segment. Management uses one measurement of profitability and $25,431 from continuing operationsdoes not segregate its business for internal reporting. Our assets are all located within the year ended December 31, 2016 and 2015, respectively.United States.
Income Taxes Fair Value of Financial Instruments-
The Company accounts for income taxes under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assetscash and liabilitiescash equivalents, accounts receivable and accounts payable approximate their respective tax bases.
The provision for income taxes includes federal and state income taxes currently payable and deferred taxes resulting from temporary differences between the financial statement and tax basis of assets and liabilities. Valuation allowances are recorded to reduce deferred tax assets when it is more-likely-than-not that a tax benefit will not be realized.
With respect to uncertain tax positions, the Company would recognize the tax benefit from an uncertain tax position only if it is more-likely-than-not that the tax position will be sustained upon examination by the taxing authorities, based on the technical meritsfair value because of the position. The Company had no unrecognized tax benefits or uncertain tax positions at December 31, 2016 or 2015.short-term maturity of these instruments.
Compensated absences - For the years ended December 31, 2016 and 2015, the Company recorded a liability for paid time off earned by permanent employees but not taken, in accordance with human resource policies.
Research and development-
Research and development costs are expensed as incurred and consist of direct and overhead-related expenses.expenses related primarily to clinical trials, including development personnel salaries and related costs. Research and development costs totaled $5,799,518 and $11,494,230 for the years ended December 31, 2023 and 2022, respectively. Expenditures to acquire technologies, including licenses, which are utilized in research and development and that have no alternative future use are expensed as the acquisition of in-process research and development when incurred. Technology the Companywe develop for use in itsour products is expensed as incurred until technological feasibility has been established after which it is capitalized and depreciated. No research and development costs were capitalized during the years ended December 31, 2023 and 2022.
Revenue RecognitionIncome Taxes
We account for income taxes in accordance with ASC Topic 740, Income Taxes. Deferred income taxes are recorded for the expected tax consequences of temporary differences between the basis of assets and liabilities for financial reporting purposes and amounts recognized for income tax purposes. As of December 31, 2023 and 2022, we recorded a valuation allowance equal to the full recorded amount of our net deferred tax assets since it is more-likely-than-not that benefits from our deferred tax assets will not be realized. The valuation allowance is reviewed quarterly and is maintained until sufficient positive evidence exists to support its reversal. As part of an evaluation of our tax attributes in 2022, we recharacterized approximately $7.4 million of startup costs previously capitalized as an IRC Section 195 asset as net operating losses. The recharacterization has no impact on total deferred tax assets since we had previously and will continue to provide a full valuation allowance on our unutilized net deferred tax assets.
We recognize the impact of an uncertain tax position if the position will more likely than not be sustained upon examination by a taxing authority, based on the technical merits of the position. Our policy is to record interest and penalties related to income taxes as part of its income tax provision. As of December 31, 2023, we had no unrecognized tax benefits and as such, no liability, interest or penalties were required to be recorded. We do not expect this to change significantly in the next twelve months.
Recent Accounting Pronouncements
From time to time, the Financial Accounting Standards Board (“FASB”) or other standard setting bodies issue new accounting pronouncements. Updates to the FASB Accounting Standards Codification are communicated through issuance of an Accounting Standards Update (“ASU”). We have implemented all new accounting pronouncements that are in effect and that may impact our financial statements. We have considered all recent accounting pronouncements issued since the last audit of our consolidated financial statements. We believe that these recent pronouncements will not have a material effect on our consolidated financial statements.
| F-12 |
The Processa Pharmaceuticals Inc. 2019 Omnibus Equity Incentive Plan (the “2019 Plan”) allows us to make grants of stock options, restricted and unrestricted stock and other stock-based awards to employees, including our executive officers, consultants and directors. The Company sells its equipment (HWX-30 heater, HWX-30S mobile heater2019 Plan originally provided for the aggregate issuance of shares of our common stock. On July 11, 2022, our shareholders approved an increase in the aggregate number of shares of our common stock available for issuance under our 2019 plan by shares to shares in total. As of December 31, 2023, shares were available for future grants.
Stock Compensation Expense
Schedule of Stock-based Compensation Expense
| 2023 | 2022 | |||||||
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Research and development | $ | 363,956 | $ | 2,895,653 | ||||
| General and administrative | 2,007,258 | 5,933,060 | ||||||
| Total | $ | 2,371,214 | $ | 8,828,713 | ||||
No tax benefits were attributed to third parties. Equipment sales revenue is recognized whenthe stock-based compensation expense because a valuation allowance was maintained for all net deferred tax assets relating to this expense.
Stock Options
Schedule of Stock Option Activity
| Total options Outstanding | Weighted average exercise price | Weighted average remaining contractual life (in years) | ||||||||||
| Outstanding as of January 1, 2022 | 8,943 | $ | 341.34 | |||||||||
| Options granted | - | |||||||||||
| Forfeited | - | |||||||||||
| Outstanding as of December 31, 2022 | 8,943 | 341.34 | ||||||||||
| Options granted | - | |||||||||||
| Forfeited or expired | (1,951 | ) | 257.28 | |||||||||
| Outstanding and exercisable as of December 31, 2023 | 6,992 | $ | 364.72 | |||||||||
No forfeiture rate was applied to these stock options. The aggregate intrinsic value of outstanding options, all of which are exercisable, was $ at both December 31, 2023 and 2022. stock options were exercised during the years ended December 31, 2023 or 2022 and there is no unamortized expense at either December 31, 2023 or 2022 since the options are fully vested.
Restricted Stock Awards
Schedule of Restricted Stock Awards (“RSAs”) Activity
| Number of shares | Weighted- average grant-date fair value per share | |||||||
| Unvested as of January 1, 2022 | 4,555 | $ | 157.81 | |||||
| Granted | 9,358 | 74.27 | ||||||
| Forfeited | (1,676 | ) | 110.62 | |||||
| Vested and issued | (9,142 | ) | 100.88 | |||||
| Unvested as of December 31, 2022 | 3,095 | 94.44 | ||||||
| Granted | 10,750 | 14.59 | ||||||
| Forfeited | (1,250 | ) | 133.00 | |||||
| Cancelled | (2,555 | ) | 22.13 | |||||
| Vested and issued | (8,790 | ) | 24.44 | |||||
| Unvested as of December 31, 2023 | 1,250 | $ | 9.26 | |||||
| F-13 |
As of December 31, 2023, unrecognized stock-based compensation expense for RSAs of $ is expected to be fully recognized in 2024.
On January 1, 2023, we granted RSAs totaling shares of common stock to three directors for their service for the six-month period ending June 30, 2023 to align their compensation plan with their service period and changed the annual service period to begin and end on the date of respective Annual Meetings rather than the calendar year. Our directors are satisfied: (a) persuasive evidencecompensated through a combination of cash and equity. On March 8, 2023, the directors increased the cash component and decreased the equity component of their compensation by equal amounts on a sales arrangement exists; (b) priceretroactive basis, to the beginning of their respective service periods. Accordingly, we cancelled RSAs representing shares of previously issued, but unvested common stock.
On July 14, 2023, we granted RSAs totaling shares of common stock to a consultant (of which vested and were issued as of December 31, 2023) for services to be provided through March 18, 2024. RSAs for up to 2,500 shares of common stock are subject to regaining Nasdaq compliance, with RSAs for only 1,250 shares of common stock vesting if we regain Nasdaq compliance through a reverse stock split. Because we effected a reverse stock-split on January 22, 2024 (which we have retroactively applied to all share counts reported in this Annual Report on Form 10-K) and regained Nasdaq compliance on February 2, 2024. Effective December 31, 2023, we cancelled the RSAs for 1,250 shares of common stock that will not vest. On August 31, 2023, in connection with the resignation of our Chief Operating Officer, unvested RSAs representing shares of common stock were forfeited and we reversed previously recognized expense of $72,733.
Restricted Stock Units
Schedule of Restricted Stock Units (“RSUs”) Activity
| Number of shares | Weighted- average grant-date fair value per share | |||||||
| Outstanding at January 1, 2022 | 22,008 | $ | 155.29 | |||||
| Granted | 121,439 | 61.50 | ||||||
| Forfeited | (3,431 | ) | 101.73 | |||||
| Cancelled | (1,876 | ) | 171.58 | |||||
| Issued | (2,399 | ) | 79.07 | |||||
| Outstanding at December 31, 2022 | 135,741 | 73.81 | ||||||
| Granted | 116,078 | 14.18 | ||||||
| Forfeited | (12,296 | ) | 21.69 | |||||
| Cancelled | (16,801 | ) | 71.36 | |||||
| Outstanding at December 31, 2023 | 222,722 | 45.82 | ||||||
| Vested and unissued | (115,145 | ) | 71.90 | |||||
| Unvested at December 31, 2023 | 107,577 | $ | 17.90 | |||||
As of December 31, 2023, unrecognized stock-based compensation expense for RSUs of $ is fixed and determinable; (c) collectability is reasonably assured; and (d) delivery has occurred. Persuasive evidenceexpected to be fully recognized over a weighted average period of an arrangement and years. The unrecognized expense excludes $related to certain RSUs with a fixed or determinable price exist once the Company receives an order or contract from a customer. The Company assesses collectability at the time of the sale and if collectabilityperformance milestone that is not reasonably assured, the sale is deferred and not recognized until collectability iscurrently probable or payment is received. Typically, title and risk of ownership transfer when the equipment is shipped.occurring.
| F-14 |
Other revenue represents consumable revenue and discounts on equipment and consumables sold.
Concentration of Supplier and Customer Risk - During the year ended December 31, 2016, one customer was responsible2023, we granted RSUs related to the future issuance of shares of our common stock to employees and directors, which have service vesting requirements.On August 8, 2023, we also granted RSUs related to the future issuance of shares of our common stock as part of the compensation package to our new Chief Executive Officer, George Ng. Vesting for 100% RSUs occurs ratably over a three-year period. The remaining RSUs will vest upon the achievement of total revenues.certain performance metrics, with the first performance-based RSUs vesting when gross proceeds of $10,000,000 is raised, and the second performance-based RSUs vesting when additional gross proceeds of $10,000,000 is raised.
Reclassifications- Prior year amounts have been adjusted to reflectHolders of our vested RSUs will be issued shares of our common stock upon the current year presentation. These reclassifications had no impactsatisfaction of the distribution restrictions contained in their Restricted Stock Unit Award Agreement. The distribution restrictions are typically different (longer) than the vesting schedule, imposing an additional restriction on the Company’s consolidated balance sheetholder. Unlike RSAs, while employees may hold fully vested RSUs, the individual does not hold any shares or consolidated statementshave any rights of operations or consolidated statementsa shareholder until the distribution restrictions are met. Upon distribution to the employee, each RSU converts into one share of cash flows.our common stock. The RSUs contain dividend equivalent rights.
Recent Accounting Pronouncements - In August 2016,On January 1, 2024, we granted RSUs totaling $1.3 million, contingent upon receiving shareholder approval to increase the FASB issued ASU 2016-15, Classificationnumber of Certain Cash Receipts and Cash Paymentsshares available under our 2019 Omnibus Incentive Plan (“ASU 2016-15”Incentive Plan”), which standardizes cash flow statement classificationwe plan to obtain in June 2024. The number of certain transactions, including cash payments for debt prepayment or extinguishment, proceeds from insurance claim settlements, and distributions received from equity method investments. The new guidance is effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. Early adoption is permitted. We are considering the impact the adoption of ASU 2016-15 may have on our presentation of cash flows.
31
In August 2016, the FASB issued ASU 2016-15, Classification of Certain Cash Receipts and Cash Payments (“ASU 2016-15”), which standardizes cash flow statement classification of certain transactions, including cash payments for debt prepayment or extinguishment, proceeds from insurance claim settlements, and distributions received from equity method investments. The new guidance is effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. Early adoption is permitted. We are considering the impact the adoption of ASU 2016-15 may have on our presentation of cash flows.
From May 2014 through December 2016, the FASB issued several ASUs related to Revenue from Contracts with Customers. These ASUs are intended to provide greater insight into both revenue that has been recognized and revenue that is expectedshares to be recognized in the future from existing contracts. The new guidance is effective for interim and annual periods beginning after December 15, 2017, although entities may adopt one year earlier if they choose. The two permitted transition methodsissued under the new standard are the full retrospective method, in which case the standard wouldRSUs will be applied to each prior reporting period presented and the cumulative effect of applying the standard would be recognized at the earliest period shown, or the modified retrospective method, in which case the cumulative effect of applying the standard would be recognized at the date of initial application. We do not currently anticipate there would be any change to timing or method of recognizing revenue. As such, we do not believe this new standard will have a material impact on our results of operations, financial condition or cash flows.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements - Going Concern (“ASU 2014-15”), which would require disclosure of uncertainties about an entity’s ability to continue as a going concern. The new guidance is effective for the annual period ending after December 15, 2016 and for interim periods thereafter. We adopted ASU 2014-15 as of December 31, 2016, which did not have a significant impact on our financial statement disclosures.
3.
ASSETS HELD FOR SALE:
The Company measures its financial assets and liabilities at fair value. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The authoritative guidance for fair value measurements establishes a three-level hierarchy, which encourages an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The three levels of the hierarchy are defined as follows:
·
Level 1 - inputs to the valuation techniques that are quoted prices in active markets for identical assets or liabilities
·
Level 2 - inputs to the valuation techniques that are other than quoted prices but are observable for the assets or liabilities, either directly or indirectly
·
Level 3 - inputs to the valuation techniques that are unobservable for the assets or liabilities
The Company reviews the carrying amounts of long-lived assets whenever events or changes in circumstances indicate that the carrying amounts may not be recoverable. An impairment loss is recognized when the carrying amount of the long-lived asset is not recoverable and exceeds its fair value. The Company estimated the fair values of long-lived assets based on the Company’s own judgments aboutgreater of: (i) $ per share or (ii) the assumptions that market participants would use inclosing price per share on the day we receive shareholder approval to increase the number of shares available under the Incentive Plan. We will grant a maximum of shares under these RSU grants.
Warrants
Schedule of Warrants Activity
| Total warrants outstanding | Weighted average exercise price | Weighted average remaining contractual life (in years) | ||||||||||
| Outstanding as of January 1, 2022 | 15,190 | $ | 213.22 | |||||||||
| Expired | (907 | ) | 343.28 | |||||||||
| Outstanding as of December 31, 2022 | 14,283 | 205.01 | ||||||||||
| Granted | 173,007 | 19.27 | ||||||||||
| Expired | (6,783 | ) | 266.96 | |||||||||
| Not exercisable | ) | |||||||||||
| Outstanding and exercisable as of December 31, 2023 | 173,007 | $ | 25.41 | |||||||||
In February 2023, we amended our financial consulting agreement with Spartan by extending the term until February 10, 2024. We compensated Spartan for financial consulting services provided under the amendment by granting warrants to purchase 158,007 shares of our common stock on April 17, 2023, with an exercise price of $20.40. The warrants expire on April 17, 2026, and contain both call and cashless exercise provision. We also granted warrants to purchase shares of our common stock to a consultant on November 18, 2023, of which warrants to purchase 7,500 shares of our common stock were exercisable, with an exercise price of $7.40. These warrants expire on November 18, 2025.
Schedule of Stock Option Warrant Valuation Assumption
Assets held for sale |
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) | |||||
|
| Equipment | Inventory | Total | |||
Beginning balance |
| $ | 25,875 | $ | 16,125 | $ | 42,000 |
Disposals |
|
| (25,875) |
| (16,125) |
| (42,000) |
Ending balance |
| $ | -- | $ | -- | $ | -- |
| Average risk-free rate of interest | 4.32-4.88 | % | ||
| Expected term (years) | 2.00–3.00 | |||
| Expected stock price volatility | 82.85-108.47 | % | ||
| Dividend yield | 0 | % |
| F-15 |
Note 4 – Stockholders’ Equity
Preferred Stock
There were issued or outstanding shares of preferred stock at December 31, 2023 or 2022.
Common Stock
Increase in Our Authorized Number of Shares
Subsequent to receiving shareholder approval on June 27, 2023, we amended our Certificate of Incorporation to increase the number of authorized shares of our common stock from to . We believe authorized shares of common stock better aligns our capital structure with our future needs and have shown this retroactively in the consolidated financial statements.
Financings
During the year ended December 31, 20162023, we issued shares of our common stock through several fundraising efforts described below:
| ● | ATM Offering – On February 5, 2023, in connection with our Registered Direct Offering discussed below, we terminated our ATM and suspended the Sales Agreement with Oppenheimer & Co. Inc., but we may reinstate it in the future. During the year ended December 31, 2023, we sold shares at an average price of $per share for aggregate gross proceeds of $693,000 (net proceeds of $672,000) prior to deducting sales commissions. | |
| ● | Lincoln Park Capital Fund, LLC Purchase Agreement– During the year ended December 31, 2023, we sold shares at an average price of $per share for aggregate gross proceeds of $54,000 under the purchase agreement with Lincoln Park. | |
| ● | Registered Direct Offering – On February 14, 2023, we closed a registered direct offering (the “Offering”) for the sale of shares of common stock at a purchase price of $per share for gross proceeds of $6.3 million (net proceeds of $5.6 million). |
We paid the Company recognizedplacement agent, Spartan Capital Securities, LLC, (“Spartan”) a cash fee of 8.0% of the gross proceeds from the Offering, excluding proceeds received from our insiders, and reimbursed Spartan for legal fees of $60,000. The engagement agreement with Spartan required us to indemnify Spartan and certain of its affiliates against certain customary liabilities. On February 14, 2023, we amended the consulting agreement with Spartan originally entered into on August 24, 2022, extending the term of the consulting agreement until February 10, 2024. As compensation for services under the agreement, on April 17, 2023, we granted Spartan warrants to purchase 158,007 shares of our common stock with an exercise price of $20.40. The warrants will expire April 17, 2026 and contain both call and cashless exercise provisions.
During the year ended December 31, 2022, we had the following activity:
| ● | On March 23, 2022, we entered into the Purchase Agreement with Lincoln Park, pursuant to which Lincoln Park has committed to purchase shares of our common stock, subject to the terms and conditions in the Purchase Agreement. We issued shares of common stock (valued at $450,000) to Lincoln Park as a commitment fee in connection with entering into the Purchase Agreement and agreed to reimburse Lincoln Park $25,000 for fees incurred in connection with the Purchase Agreement. Concurrent with entering into the Purchase Agreement, we also entered into a registration rights agreement with Lincoln Park (the “Registration Rights Agreement”), pursuant to which we agreed to take certain actions relating to the registration under the Securities Act of 1933, as amended, of the offer and sale of the shares of common stock available for issuance under the Purchase Agreement. |
| F-16 |
We have the right to present Lincoln Park with a purchase notice (a “Regular Purchase Notice”), directing Lincoln Park to purchase up to shares of our common stock (the “Regular Purchase Amount”) provided that the closing sale price of the common stock on the purchase date is not below a threshold price of $1.00, set forth in the Purchase Agreement (a “Regular Purchase”). The Regular Purchase Amount may be increased to up to shares if the closing sale price of our common stock on the applicable purchase date equals or exceeds certain higher threshold prices set forth in the Purchase Agreement. If the share price of the Regular Purchase Amount is less than $ per share of common stock, we can sell additional shares (defined in the Purchase Agreement as the “Alternate Adjusted Regular Purchase Share Limit”) up to $150,000, subject to the Exchange Cap limitation (defined below). We and Lincoln Park may mutually agree to increase the Regular Purchase Amount with respect to any Regular Purchase under the Purchase Agreement, provided that Lincoln Park’s maximum committed purchase obligation under any single Regular Purchase shall not exceed $1,250,000. The above-referenced share amount limitations and closing sale price thresholds are subject to adjustment for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction as provided in the Purchase Agreement. The purchase price per share for each Regular Purchase will be based on prevailing market prices of the common stock immediately preceding the time of sale as computed in accordance with the terms set forth in the Purchase Agreement. There are no upper limits on the price per share that Lincoln Park must pay for shares of common stock under the Purchase Agreement. Lincoln Park may not assign or transfer its rights and obligations under the Purchase Agreement.
The aggregate number of shares that we can issue to Lincoln Park under the Purchase Agreement may not exceed shares (subject to proportional adjustments for stock splits, reverse stock splits and similar events as described above), which is equal to 19.99% of the outstanding shares of common stock immediately prior to the execution of the Purchase Agreement (the “Exchange Cap”), unless (i) stockholder approval is obtained to issue shares of common stock in excess of the Exchange Cap, in which case the Exchange Cap will no longer apply, or (ii) the average price of all sales of shares of our common stock to Lincoln Park under the Purchase Agreement equals or exceeds the lower of (i) the Nasdaq official closing price immediately preceding the execution of the Purchase Agreement or (ii) the arithmetic average of the five Nasdaq official closing prices for the common stock immediately preceding the execution of the Purchase Agreement, plus an incremental amount to take into account the issuance of the commitment shares to Lincoln Park under the Purchase Agreement, such that the transactions contemplated by the Purchase Agreement are exempt from the Exchange Cap limitation under applicable Nasdaq rules. In all instances, we may not sell shares of our common stock to Lincoln Park under the Purchase Agreement if it would result in Lincoln Park beneficially owning more than 9.99% of the outstanding shares of common stock.
We may terminate the Purchase Agreement at any time, at our sole discretion, without any cost or penalty, by giving one business day notice to Lincoln Park to terminate the Purchase Agreement. Lincoln Park has covenanted not to cause or engage in any manner whatsoever, any direct or indirect short selling or hedging of the common stock.
There are no limitations on use of proceeds, financial or business covenants, restrictions on future financings (other than restrictions on our ability to enter into variable rate transactions described in the Purchase Agreement), rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement. We may deliver Purchase Notices under the Purchase Agreement, subject to market conditions, and in light of our capital needs from time to time and under the limitations contained in the Purchase Agreement. Any proceeds that we receive under the Purchase Agreement are expected to be used for working capital and general corporate purposes.
| ● | We issued shares to Elion for the second milestone payment. | |
| ● | Also, during the year ended December 31, 2022, vested RSAs and RSUs were forfeited to pay federal, state and local income taxes and an additional unvested RSAs were forfeited when one of our directors did not seek reelection. |
Treasury Stock - Repurchase of Shares from Aposense, Ltd.
On March 29, 2022, we purchased shares of our common stock from Aposense Ltd. for $300,000 in a private transactionand are holding these shares as treasury stock until they are reissued or retired at the discretion of our Board of Directors.
| F-17 |
Basic net loss per share is computed by dividing net loss by the weighted average common stock outstanding (which excludes unvested RSAs) and vested, but unissued RSUs. Diluted net loss per share is computed by dividing net loss by the diluted weighted average common stock outstanding, which includes potentially dilutive effect of stock options, unvested RSAs, unvested RSUs and warrants. The treasury-stock method is used to determine the dilutive effect of our stock options and warrants grants. Since we experienced a loss for both periods presented, basic and diluted net loss per share are the same and, as they would have an anti-dilutive impact on disposaldiluted net loss per share, any dilutive common shares outstanding were excluded from the computation shown below.
Schedule of equipmentNet Loss Per Share Basic and inventoryDiluted
| 2023 | 2022 | |||||||
| Basic and diluted net loss per share: | ||||||||
| Net loss available to common shareholders | $ | (11,121,520 | ) | $ | (27,424,229 | ) | ||
| Weighted-average number of common shares-basic and diluted | ||||||||
| Basic and diluted net loss per share | $ | ) | $ | ) | ||||
| 2023 | 2022 | |||||||
| Weighted-average number of common shares outstanding – basic and diluted | ||||||||
| Weighted-average number of vested RSUs– basic and diluted | ||||||||
| Weighted-average number of common shares-basic and diluted | ||||||||
As described in Note 3, we issued various equity instruments during the amountyears ended December 31, 2023 and 2022 which impact our EPS calculation. All granted RSAs are considered issued and outstanding for purposes of $25,000.
4.
PROPERTY AND EQUIPMENT:
A summaryour financial statements. Unvested RSAs are included as dilutive securities, but are excluded from our denominator of basic EPS. At December 31, 2023 and 2022, and RSAs, respectively, were not vested and were excluded from the EPS calculation. Vested RSUs are included in our computation of the costweighted average shares for basic EPS and unvested RSUs are included as dilutive securities. At December 31, 2023 and 2022, and unvested RSUs were excluded from the EPS calculation.
Schedule of Anti-dilutive Securities Excluded from Computation of Earnings Per Share
| 2023 | 2022 | |||||||
| Stock options, unvested RSAs, unvested RSUs and purchase warrants | 296,326 | 128,943 | ||||||
Note 6 – Leases
We lease our office space under an operating lease agreement. This lease does not have significant rent escalation, concessions, leasehold improvement incentives, or other build-out clauses. Further, the lease does not contain contingent rent provisions. Our office space lease includes both lease (e.g., fixed payments including rent, taxes, and insurance costs) and non-lease components (e.g., common-area or other maintenance costs), which are accounted for as a single lease component as we have elected the practical expedient to group lease and non-lease components for all leases. We also lease office equipment by component,under an operating lease. Our leases do not provide an implicit rate and, as such, we have used our incremental borrowing rate of 8% to determine the related accumulated depreciation is as follows:present value of the lease payments based on the information available at the lease commencement date.
| December 31, 2016 |
| December 31, 2015 | ||
Computer equipment & software | $ | 19,150 |
| $ | 19,150 |
Accumulated depreciation |
| (19,150) |
|
| (18,991) |
| $ | -- |
| $ | 159 |
Depreciation expense was $159 and $60,251Lease costs included in our consolidated statements of operations totaled $97,000 for the years ended December 31, 20162023 and 2015, respectively.2022. The Companyweighted average remaining lease terms and discount rate for our operating leases at December 31, 2023 were as follows:
Schedule of Weighted Average Remaining Lease Terms and Discount Rate for Operating Leases
| Remaining lease term (years) for our office lease | 1.8 | |||
| Remaining lease term (years) for our equipment lease | 0.3 | |||
| Weighted average remaining lease term (years) for our facility and equipment leases | 1.7 | |||
| Weighted average discount rate for our facility and equipment leases | 8.0 | % |
| F-18 |
Annual lease liabilities for all operating leases were as follows as of December 31, 2023:
Schedule of Annual Lease Liabilities for all Operating Leases
| 2024 | $ | 92,389 | ||
| 2025 | 70,040 | |||
| Total lease payments | 162,429 | |||
| Less: Interest | (11,875 | ) | ||
| Present value of lease liabilities | 150,554 | |||
| Less: current maturities | (83,649 | ) | ||
| Non-current lease liability | $ | 66,905 |
Note 7 – License Agreements
Elion Oncology, Inc.
On August 23, 2020, we entered into a condition precedent License Agreement with Elion Oncology (“Elion License Agreement”), pursuant to which we acquired an exclusive license to develop, manufacture and commercialize PCS6422 globally. As part of the Elion License Agreement, we agreed to issue to Elion shares of our common stock on each of the first and second anniversary dates of the Elion License Agreement. As additional consideration, we will pay Elion development and regulatory milestone payments (a portion of which are payable in shares of our common stock and a portion of which are payable in cash) upon the achievement of certain milestones, which include FDA or other regulatory approval and dosing a patient. In addition, we must pay Elion one-time sales milestone payments based on the achievement during a calendar year of one or more thresholds for annual sales for products made and pay royalties based on annual licensing sales. We are also required to split any milestone payments received with Elion based on any sub-license agreement we may enter.
On May 17, 2022, we amended the third Milestone Event of Section 6.4 of our License Agreement with Elion Oncology, Inc. changing the third Milestone Event from “1st Patient in Dose Confirmation Study” to (a) determination of the maximum tolerated dose (MTD) or (b) determination of the recommended Phase 2 Dose. Prior to this amendment, the third milestone was not considered probable since it was unknown when, or if a dose confirmation study was going to be conducted. As a result of the modification, we consider it probable that the recommended Phase 2 dosage regimen could be determined in connection with our current Phase 1B trial for NGC-Cap. We recorded an expense and related liability of $189,000 representing the value of the shares we anticipate issuing to Elion at the fair value on the date of modification. No other terms or conditions of the License Agreement were modified. We determined the dosage for our Phase 2 study on January 25, 2024 and issued shares of our common stock for meeting this milestone.
We are required to use commercially reasonable efforts, at our sole cost and expense to research, develop and commercialize products in one or more countries, including dosing a first patient with a product in a Phase 2 or 3 clinical trial within 48 months from when we entered into the License Agreement. We are currently on track to dose our first patient in a Phase 2 clinical trial on or before October 2, 2024. Either party may terminate the agreement in the event of a material breach of the agreement that has not been cured following written notice and a 90-day opportunity to cure such breach (which is shortened to 15 days for a payment breach).
Ocuphire Pharma, Inc.
On June 16, 2021, we executed a License Agreement with Ocuphire Pharma, Inc. (“Ocuphire Agreement”) under which we received a license to research, develop and commercialize PCS3117 globally, excluding the Republic of Singapore, China, Hong Kong, Macau and Taiwan. As consideration for the Ocuphire Agreement, we issued shares of our common stock to Ocuphire, a cash payment of $200,000 and assumed $66,583 in certain liabilities. Additional consideration includes future development and regulatory milestones payments to Ocuphire upon our achievement of certain defined clinical milestones, such as dosing a patient in pivotal trials and receiving marketing authorization by a regulatory authority in the United States or another country. In addition, we are required to pay Ocuphire one-time sales milestone payments based on the achievement during a calendar year of the highest annual Net Sales for products made and pay royalties based on annual Net Sales, as defined in the Ocuphire Agreement.
| F-19 |
We are required to use commercially reasonable efforts, at our sole cost and expense to oversee such commercialization efforts, to research, develop and commercialize products in one or more countries, including meeting specific diligence milestones that consist of: (i) first patient administered drug in a Clinical Trial of a Product prior to June 16, 2024 and (ii) first patient administered drug in a Pivotal Clinical Trial of a Product or first patient administered drug in a Clinical Trial for a Second Indication of a Product prior to June 16, 2026. We are currently in discussions with Ocuphire to extend these deadlines. Either party may terminate the agreement in the event of a material breach of the agreement that has not been cured following written notice and a 120-day opportunity to cure such breach.
Aposense, Ltd.
On May 24, 2020, we entered into a condition precedent License Agreement with Aposense, Ltd. (“Aposense License Agreement”), pursuant to which we were granted Aposense’s patent rights and Know-How to develop and commercialize their next generation irinotecan cancer drug, PCS11T. The Aposense License Agreement provides us with an exclusive worldwide license (excluding China), to research, develop and commercialize products comprising or containing PCS11T. The license grant was conditioned on the following being satisfied within nine months of May 24, 2020 (or the Aposense License Agreement shall terminate): (i) our closing of an equity financing and successful up-listing to Nasdaq and (ii) Aposense obtaining the approval of the Israel Innovation Authority for the consummation of the transactions contemplated by the Aposense License Agreement.
On October 6, 2020, all conditions were satisfied, resulting in the addition of PCS11T to our portfolio, and we issued shares of our common stock to Aposense. As additional consideration, we will pay Aposense development and regulatory milestone payments (up to $3.0 million per milestone) upon the achievement of certain milestones, which primarily consist of having a drug indication approved by a regulatory authority in the United States or another country. In addition, we will pay Aposense one-time sales milestone payments based on the achievement during a calendar year of one or more thresholds for annual sales for products made and pay royalties based on annual licensing sales. We are also required to split any sales milestone payments or royalties we receive with Aposense based on any sub-license agreement we may enter.
Yuhan Corporation
On August 19, 2020, we entered into a License Agreement with Yuhan Corporation (“Yuhan License Agreement”), pursuant to which we acquired an exclusive license to develop, manufacture and commercialize PCS12852 globally, excluding South Korea. As consideration for the Yuhan License Agreement and related Share Issuance Agreement, we issued to Yuhan shares of common stock. As additional consideration, we will pay Yuhan development and regulatory milestone payments (a portion of which are payable in shares of our common stock based on the volume weighted average trading price during the period prior to such achievement and a portion of which are payable in cash) upon the achievement of certain milestones, based on a Yuhan affiliate purchasing 37,500 shares of common stock for $3,000,000 in our October 2020 underwritten public offering. The milestones primarily consist of dosing a patient in pivotal trials or having a drug indication approved by a regulatory authority in the United States or another country. In addition, we must pay Yuhan one-time sales milestone payments based on the achievement during a calendar year of one or more thresholds for annual sales for products made and pay royalties based on annual licensing sales. We are also required to split any milestone payments received with Yuhan based on any sub-license agreement we may enter.
We are required to use commercially reasonable efforts, at our sole cost and expense, in conjunction with a joint Processa-Yuhan Board to oversee such commercialization efforts, to research, develop and commercialize products in one or more countries, including meeting specific diligence milestones that consist of: (i) preparing a first draft of the product development plan within 90 days; (ii) requesting an FDA pre-IND meeting for a product within 6 months; (iii) dosing a first patient in a Phase 2A clinical trial with a product within 24 months; and (iv) dosing a first patient with a product in a Phase 2B clinical trial, Phase 3 clinical trial or other pivotal clinical trial with a product by August 19, 2024. Either party may terminate the agreement in the event of a material breach of the agreement that has not been cured following written notice and a 60-day opportunity to cure such breach (which is shortened to 15 days for a payment breach).
| F-20 |
CoNCERT Pharmaceuticals, Inc.
On March 19, 2018, Promet, Processa and CoNCERT amended the CoNCERT Agreement executed in October 2017. The Amendment assigned the CONCERT Agreement to us and we exercised the exclusive option for the PCS499 compound in exchange for CoNCERT receiving, in part, $8.0 million of our common stock that was held by Promet (shares), for the benefit of Processa in satisfaction of the obligation due for the exclusive license for PCS499 acquired by us. Promet contributed the payment of the obligation due for the exclusive license to us without consideration paid to them. As a result of the transaction, we recognized an exclusive license intangible asset with a lossfair value of $8.0 million and an offsetting increase in additional paid-in capital resulting from the exchange. We fully impaired the intangible asset in 2022.
We are required to pay CoNCERT royalties, on disposala product–by-product basis, on future worldwide net sales, or pay a percentage of fixedany sublicense revenue, as described in the License Agreement with CoNCERT.
Note 8 - Intangible Assets
Our gross intangible assets consisted primarily of $60,743costs we capitalized related to the acquisition of license rights to PCS499 from CoNCERT Pharmaceuticals. Inc. (“CoNCERT”) for shares of our common stock that had an issue date fair value of $8.0 million, $1,782 in transaction costs and $3,037,147 associated with the initial recognition of an offsetting deferred tax liability related to the acquired temporary difference for an asset purchased that is not a business combination and has a tax basis of $1,782 in accordance with ASC 740-10-25-51 Income Taxes. In accordance with ASC Topic 730, Research and Development, we capitalized the costs of acquiring the exclusive license rights to PCS499 from CoNCERT, as the exclusive license rights represented intangible assets to be used in research and development activities that management believed had future alternative uses.
Intangible assets at December 31, 2023 and 2022 consisted of the following:
Summary of Intangible Assets
| 2023 | 2022 | |||||||
| Gross intangible assets | $ | - | $ | 11,059,429 | ||||
| Less: accumulated amortization | - | (3,791,286 | ) | |||||
| Less: impairment of intangible asset | - | (7,268,143 | ) | |||||
| Total intangible assets, net | $ | - | $ | - | ||||
Amortization expense was $788,495for the year ended December 31, 2022 and is included within research and development expense in the accompanying consolidated statements of operations. We did not have a similar expense during the year ended December 31, 2015. The Company reclassified fixed assets with a net2023. At December 31, 2022, following the difficulty we experienced to enroll patients in our Phase 2B clinical trial in PCS499, we terminated the trial for uNL. We recognized an impairment for remaining book value of $186,549the intangible asset of $7.3 million, thereby reducing the value of our intangible asset to assets heldzero. Our assessment was based on the uncertainty of determining whether we will be able to out-license PCS499 or enter a partnering/collaborating arrangement for sale, at fair market valueits future development. We believe the rarity of the disease, along with other factors, makes enrollment not feasible for us due to time and recognized a loss on impairment of assets held for sale during the year endedcost constraints.
| F-21 |
Note 9 - Income Taxes
We have incurred net operating losses since inception. At December 31, 20152023 and 2022, we had available federal and state net operating loss carryforwards of $62,554.
5.
ASSET PURCHASE AGREEMENT:
On April 15, 2011, the Company entered into an Asset Purchase Agreement with an individual who is a founder$28.6 and a current stockholder. Pursuant$24.0 million, respectively. The federal net operating losses generated in 2018 and later of $29.3 million will carry forward indefinitely. Net operating losses generated prior to 2018 will expire 2037. We have not recognized any deferred tax assets related to the agreement, the Company purchased the related business and activities of the design, manufacture and distribution of asphalt repair machinery under the Heatwurx brand. The total purchase price was $2,500,000.
The business essentially consisted of the investment infederal orphan drug or other research and development tax credits as of December 31, 2023 or 2022. The federal research and development tax credits have a 20-year carryforward period.
Pursuant to Code Sec. 382 of the technology, Internal Revenue Code (“the patents applied forCode”), the utilization of our net operating loss carryforwards could be limited as a result of the researcha cumulative change in stock ownership of more than 50% over a three-year period. We have not completed a Sec. 382 study and development activitiesas such our net operating loss carryforwards may be subject to such limitation.
A reconciliation of our effective income tax rate and certain distribution relationships that were in process, but not finalized as of the acquisition date. Collectively, these investments constitute the in-process research and development the Company refers to as the “asphalt preservation and repair solution.” The Company capitalized $2,500,000 of in-process research and development related to this asphalt preservation and repair solution. As of October 1, 2012, in-process research and development was classified as developed technology and amortized over its estimated useful life of seven years. The initial estimated fair value of the in-process research and development was determined using thestatutory income approach. Under the income approach, the expected future cash flows from the asset are estimated and discounted to its net present value at an appropriate risk-adjustedtax rate of return. Based on the Company’s financial position and substantial doubt about the Company’s ability to continue as a going concern, the Company has chosen to estimate future cash flows at zero. The Company recognized an impairment of $1,517,859 during the second quarter of 2015. As of December 31, 2015, the Company’s developed technology intangible asset had no value. Amortization expense prior to the impairment for 2015 was $178,571.
In conjunction with the Asset Purchase Agreement, the Company granted 200,000 performance stock options to a founder of the Company with an exercise price of $0.40 per share and a term of seven years. Following the effectiveness of the seven for one stock split that was completed in October 2011, the 200,000 performance stock options were exchanged for 1,400,000 performance stock options with an exercise price of $0.057 per share. On February 10, 2015, the founder of the Company elected to cancel the 1,400,000 performance stock options.
6.
DISCONTINUED OPERATIONS:
In efforts to streamline operations and expenses the Company elected to discontinue the Dr. Pave and Dr. Pave Worldwide entities during 2015. The financial results of these events are represented in the discontinued operations included in the December 31, 2016 and 2015 financial statements.
The operating results of the discontinued operations of Dr. Pave and Dr. Pave Worldwide for the years ended December 31, 20162023 and 2015 are summarized below:
| 2016 |
| 2015 | ||
Revenue | $ | -- |
| $ | 28,870 |
Expense |
| (1,733) |
|
| 400,177 |
Net Income (loss), before Other income and expense and taxes |
| 1,733 |
|
| (371,307) |
Other income (expense) |
| (536) |
|
|
|
Income tax benefit |
| -- |
|
| -- |
Net Loss, net of tax | $ | 1,197 |
| $ | (371,307) |
The balance sheet items for discontinued operations as of December 31, 2015 and 2014 are summarized below:
| 2016 |
| 2015 | ||
Cash and cash equivalents | $ | -- |
| $ | 12,350 |
Total assets | $ | -- |
| $ | 12,350 |
|
|
|
|
|
|
Payables and accrued liabilities |
| -- |
|
| 70,358 |
Short-term debt |
| -- |
|
| 229,980 |
Total liabilities | $ | -- |
| $ | 300,338 |
The Company’s borrowings included in discontinued operations as of December 31, 2016 and 2015 are2022 is as follows:
Schedule of Effective Income Tax Rate Reconciliation
| 2023 | 2022 | |||||||
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Federal statutory income tax rate | 21.00 | % | 21.00 | % | ||||
| State tax rate, net | 5.77 | % | 5.72 | % | ||||
| Permanent differences | (0.25 | )% | (0.55 | )% | ||||
| Federal orphan drug tax credit | 1.41 | % | 0.93 | % | ||||
| Deferred tax asset valuation allowance | (27.93 | )% | (27.10 | )% | ||||
| Effective income tax rate | 0.00 | % | 0.00 | % | ||||
Revolving lineThe significant components of credit - The Company assumed revolving linesour deferred tax assets and liabilities for Federal and state income taxes consisted of credit through the January 8, 2014 acquisitionfollowing:
Schedule of Dr. PaveDeferred Tax Assets and Liabilities
| 2023 | 2022 | |||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Deferred tax assets: | ||||||||
| Non-current: | ||||||||
| Net operating loss carry forward – Federal | $ | 6,012,941 | $ | 5,048,175 | ||||
| Net operating loss carry forward – State | 1,672,436 | 1,431,839 | ||||||
| Stock compensation expense | 3,453,799 | 2,590,890 | ||||||
| Depreciation and other | 999 | 976 | ||||||
| Purchased in-process R&D | 2,500,562 | 2,494,829 | ||||||
| Federal orphan drug credits | 1,202,955 | 1,046,539 | ||||||
| Capitalized research and development costs | 2,795,379 | 1,929,462 | ||||||
| Start-up expenditures and amortization | - | - | ||||||
| Total non-current deferred tax assets | 17,639,071 | 14,542,710 | ||||||
| Valuation allowance for deferred tax assets | (17,639,071 | ) | (14,542,710 | ) | ||||
| Total deferred tax assets | - | - | ||||||
| Deferred Tax Liabilities: | ||||||||
| Non-current: | ||||||||
| Intangible asset | - | - | ||||||
| Total non-current deferred tax liabilities | - | - | ||||||
| Total deferred tax asset (liability) | $ | - | $ | - | ||||
| F-22 |
Beginning in 2022, the Tax Cuts and Jobs Act of 2017 (TCJA) eliminated the option to deduct research and development expenditures in the amountcurrent year and requires taxpayers to amortize them over five or fifteen years pursuant to IRC Section 174. During 2023 and 2022, for income tax purposes, we capitalized approximately $3.2 million and $7.2 million of $229,980. research and development expenditures, net of amortization of these costs in each year.
In June 2016, Heatwurx, Inc. assumed2022, as part of an evaluation of our tax attributes, we recharacterized approximately $7.4 million of startup costs previously capitalized as an IRC Section 195 asset as net operating losses. The recharacterization has no impact on total deferred tax assets since we had previously and will continue to provide a full valuation allowance on our unutilized net deferred tax assets.
The valuation allowance generally reflects limitations on our ability to use the revolving linestax attributes and reduces the value of credit along withsuch attributes to the accumulated accrued interest frommore-likely-than-not realizable amount. We assessed the discontinued entity Dr. Pave, LLC (see Note 7 below).
Secured Notes Payable - The Company assumed secured notes payable throughavailable positive and negative evidence to estimate if sufficient taxable income will be generated to use the acquisitionexisting net deferred tax assets. Based on a weighing of Dr. Pavethe objectively verifiable negative evidence primarily in the amountform of $160,000.cumulative operating losses, we believe that it is not more-likely-than-not that the deferred tax assets will be realized and, accordingly, a full valuation allowance has been established. The principal amountvaluation allowance increased by $3.1 million and accrued interest in$7.4 million for the amount of $14,361 was converted into the senior secured loan agreement as described below on June 30, 2015.
7.
NOTES PAYABLE:
Unsecured Notes Payable - The Company commenced two non-public offerings of notes and warrants in 2014. The notes bear interest at 12% per annum payable monthly, with principal and unpaid interest due and payable on January 6, 2016. As additional consideration for a lender to enter into the Loan Agreement, the Company agreed to issue to each lender one common stock purchase warrant for each $3.00 loaned to the Company. The Company allocated the fair value of the warrants as a discount on notes payable which is amortized over the term of the notes to interest expense in the income statement. The Warrants expire three years following the date of issuance and may not be offered for sale, sold, transferred or assigned without the consent of the Company. The three-year warrants were exercisable immediately at $3.00 per share. The Company recognized amortization of discount on notes payable in interest expense of $967 and $58,801 during the year ended December 31, 20162023 and 2015,2022, respectively. As
We recognize potential liabilities for uncertain tax positions using a two-step process. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more-likely-than-not that the dateposition will be sustained on audit, including resolution of this filing; these notesrelated appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely to be realized upon settlement. We have not been paid in full and interest continues to accrue.
Secured Notes Payable - On February 16, 2015, the Company entered into a Senior secured loan agreement with JMW Fund, Richland Fund, and San Gabriel Fund (collectively, the “lenders”) whereby the lenders agreed to loan to the Company up to an aggregate of $2,000,000. The interest rate on the notes is 12% per annum and monthly interest payments are due the first day each month beginning March 1, 2015. Ifrecorded any interest payment remains unpaid in excess of 90 days, and the lender has not declared the entire principal and unpaid accrued interest due and payable, the interest rate on that amount only will be increased to 18% per annum, until the past due interest amount is paid in full. The notes and any future notes under the loan agreement are secured by all of the assets of the Company, including intellectual property rights. The Company has not paid the interest on the notes timely and interest is therefore accrued at the 18% interest rate as stated above. On March 23, 2016; all senior secured notes were extended to a maturity date of September 30, 2016. The Company is in default on the senior secured loan agreement. The lenders may call the notes or foreclose upon the assets of the Company.
34
Revolving line of credit - In June 2016, Heatwurx, Inc. assumed the revolving line of credit along with the accumulated accrued interest from the discontinued entity Dr. Pave, LLC. The total available under line of credit is $250,000 with $20,020 unused as of December 31, 2016. The balance on the line of credit bears interest at a rate of 12% per annum. Interest is payable monthly on the first day of each month. The outstanding principal balances became due as of July 1, 2015, and were payable in sixty (60) equally amortized monthly installments of principal and interest due on the fifteenth day of each calendar month until paid in full.uncertain tax positions. As of December 31, 2016 2023 and 2022, we had no principal payments have been made, the Company is in default on the revolving line of credit. The Company is working with the lenders accrued penalties or interest related to explore extension or conversion options.uncertain tax positions.
| Principal Balance | Interest Rate | Accrued Interest | Warrants issued | Warrant Fair Value - Discount | Unamortized Discount | ||||
Unsecured notes payable | $ | 420,000 | 12% | $ | 88,287 | 139,997 | $ | 115,159 | $ | -- |
Secured notes payable | $ | 962,361 | 12% - 18% | $ | 292,703 | -- |
| -- |
| -- |
Revolving line of credit | $ | 229,980 | 12% | $ | 60,184 | -- |
| -- |
| -- |
| $ | 1,612,341 |
| $ | 441,174 | 139,997 | $ | 115,159 | $ | -- |
As of December 31, 2016, the loans are subject to mandatory principal payments as follows:
Year | Payments | |
2016 | $ | 1,612,341 |
2017 |
| -- |
2018 |
| -- |
2019 |
| -- |
2020 |
| -- |
Total principal payments | $ | 1,612,341 |
Less: unamortized debt discount |
| -- |
Total current portion | $ | 1,612,341 |
Based upon the Company’s financial position, the Company does not believe it will be able to satisfy the mandatory principal payments in 2017. The Company will work with the lenders to explore extension or conversion options. There is no guarantee the lenders will accommodate our requests. The Company is in default in regard to interest payments on the notes, the Company’s assets may be foreclosed upon.
8.
INCOME TAXES:
The Company and its predecessorWe file U.S. Federal income tax returns, in the U.S. federal jurisdictionas well as state tax returns for California, Florida and in the states of Colorado, Utah, North Dakota and California.Maryland. There are currently no income tax examinations underway for these jurisdictions. The Company filed its initialHowever, tax returnsyears from and including 2017 remain open for the nine months ended December 31, 2011 with federal and Utah and December 31, 2012 is the initial tax filing period for Colorado, and December 31, 2013 is the initial tax filing period for North Dakota and California.
The Company provides deferred income taxes for differences between the tax reporting bases and the financial reporting bases of assets and liabilities. The Company had no unrecognized income tax benefits. Should the Company incur interest and penalties relating to tax uncertainties, such amounts would be classified as a component of interest expense and operating expense, respectively. Unrecognized tax benefits are not expected to increase or decrease within the next twelve months.
As of December 31, 2016, the Company’s tax year for 2013, 2014 and 2015 are subject to examination by the tax authorities.
Deferred Income Taxes - The Company does not recognize the deferred income tax asset at this time because the realization of the asset is less likely than not. As of December 31, 2016 the Company has net operating losses for federal and state income tax purposes of approximately $13,094,547authorities.
Note 10 – Related Party Transactions
CorLyst, LLC (“CorLyst”) reimburses us for shared costs related to payroll, health insurance and $12,407,648, respectively. As of December 31, 2015, the Company had net operating losses for federal and state income tax purposes of approximately $12,761,702 and $12,704,903, respectively.
35
The net operating lossesrent based on actual costs incurred, which are available for application against future taxable income and which will start expiring in 2031. The benefit associated with the net operating loss carry forward will more likely than not go unrealized unless future operations are successful. Since the success of future operations is indeterminable, the potential benefits resulting from these net operating losses have not been recorded in the financial statements.
| December 31, 2016 |
| December 31, 2015 | ||
Deferred Tax Assets: |
|
|
| ||
Current |
|
|
| ||
Net operating loss carry forward - Federal | $ | 4,452,146 |
| $ | 4,338,979 |
Net operating loss carry forward - State |
| 617,509 |
|
| 598,096 |
Contribution carry forward |
| 199 |
|
| 199 |
Accrued liabilities and deferred rent |
| 723 |
|
| 1,519 |
Total current deferred tax assets |
| 5,070,577 |
|
| 4,938,793 |
|
|
|
|
|
|
Noncurrent |
|
|
|
|
|
Depreciation |
| (135) |
|
| (16,533) |
Amortization |
| -- |
|
| -- |
Total noncurrent deferred tax (liabilities)/assets |
| (135) |
|
| (16,533) |
Total net deferred tax assets |
| 5,070,442 |
|
| 4,992,260 |
Valuation allowance for deferred tax asset |
| (5,070,442) |
|
| (4,922,260) |
Total deferred tax assets | $ | -- |
| $ | -- |
The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, the projected future taxable income and tax planning strategies in making this assessment. Based on management's analysis,recognized as a full reserve has been established against this asset. The change in the valuation allowance in 2016 and 2015 was $148,182 and 1,359,848, respectively.
A reconciliation between the statutory federal income tax rate of 34% and our effective tax rate for the years ended December 31, 2016 and 2015, are as follows:
| Year ended December 31, 2016 | Year ended December 31, 2015 |
Federal statutory income tax rate | 34.0% | 34.0% |
State tax rate (net) | 5.7% | 7.1% |
Permanent differences | (0.9)% | 7.5% |
Deferred tax asset valuation allowance | (38.8)% | (48.6)% |
Effective income tax rate | -- | -- |
9.
STOCKHOLDERS’ EQUITY:
Common Stock - The Company has authorized 20,000,000 common shares with a $0.0001 par value. There were 11,017,388 shares issued and outstanding at December 31, 2016 and 11,017,388 shares issued and outstanding at December 31, 2015.
On October 1, 2014, the Company commenced a non-public equity offering of up to 3,650,807 units at $1.75 per unit (the “Units”). Each Unit consists of one common share and one-half warrant, with each whole warrant exercisable at $2.00 per share. The purchase price for the Units is payable in either cash, conversion of outstanding Series D preferred shares or certain outstanding promissory notes. During the first half of 2015, the Company issued 50,285 shares of common stock and warrants to purchase 25,141 shares of common stock for cash proceeds of $88,000.
On March 13, 2015 the Company issued 15,000 common shares in exchange for consulting services valued at $25,500.
Preferred Stock - The Company has authorized 4,500,000 shares of Preferred Stock with a $0.0001 par value. As holders of any series of preferred stock convert into common shares the preferred shares are no longer outstanding and become available for reissuance.
36
Series D Preferred Stock - As of December 31, 2016 and December 31, 2015 there were 178,924 shares of Series D preferred stock outstanding.
Holders of Series D preferred stock accrue dividends at the rate per annum of $0.24 per share, payable on a quarterly basis. As dividends are accrued and payable quarterly on the Series D preferred stock, the Company paid no dividends during the year ended December 31, 2016 and $31,475 during the year ended December 31, 2015. As of December 31, 2016 the Company has dividends payable in accrued expenses of $89,000.
The holders of the Series D preferred stock have conversion rights equivalent to such number of fully paid and non-assessable shares of common stock as is determined by dividing the Series D original issue price of $3.00 by the then applicable conversion price. Each Series D Share will convert into one sharereduction of our common stock at any time at the optiongeneral and administrative operating expenses in our consolidated statements of the holder of the Series D Shares or will be converted at the option of the Company at any time the trading price of our common stock is at least $4.50 per share for ten consecutive trading days. The conversion ratio is subject to anti-dilution adjustments, includingoperations. We recorded $112,000 and $124,000 in the event that the Company issues equity securities at a price equivalent to or less than the conversion price in effect immediately prior to such issue.
The holders of Series D preferred stock have a liquidation preference over the holders of the Company’s common stock equivalent to the purchase price per share of the Series D preferred stock plus any accrued and unpaid dividends, whether or not declared, on the Series D preferred stock. A liquidation would be deemed to occur upon the happening of customary events, including transfer of all or substantially all of the Company’s common stock or assets or a merger, or consolidation. The Company believes that such liquidation events are within its control and therefore the Company has classified the Series D preferred stock in stockholders’ equity.
The holders of Series D preferred stock vote together as a single class with the holders of the Company’s common stock on all action to be taken by the Company’s stockholders. Each share of Series D preferred stock entitles the holder to the number of votes equal to the number of shares of common stock into which the shares of the Series D preferred stock are convertible as of the record date for determining stockholders entitled to vote on such matter.
Stock Options
| Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Life (Years) |
Balance, December 31, 2014 | 1,246,500 | $ 2.35 | 2.83 |
Granted | 425,000 | $ 1.50 |
|
Exercised | -- | -- |
|
Cancelled | (1,080,333) | $ 2.33 |
|
Balance, December 31, 2015 | 591,167 | $ 1.78 | 3.75 |
Exercisable, December 31, 2015 | 463,000 | $ 1.76 | 3.68 |
Granted | -- | -- |
|
Exercised | -- | -- |
|
Cancelled | (321,667) | $ 1.69 |
|
Balance, December 31, 2016 | 269,500 | $ 1.88 | 2.04 |
Exercisable, December 31, 2016 | 269,500 | $ 1.88 | 2.04 |
On April 30, 2015, the Board of Directors approved the grant of 125,000 options to the former CEO of the Company, David Dworsky, in accordance with the terms of the 2011 Equity Incentive Plan, as amended. The options vested immediately and had an exercise price of $1.50 per share. Mr. Dworsky’s options were cancelled 90 days after his resignation from the Board on August 31, 2015, per the terms of the 2011 Equity Incentive Plan. At the grant of the April 30, 2015 option, Mr. Dworsky forfeited his vested options of 93,750 and unvested options of 206,250 with an exercise price of $3.00 per share.
On April 30, 2015, the Board of Directors approved the grant of 100,000 options to an employee for continued consulting services, in accordance with the terms of the 2011 Equity Incentive Plan, as amended. The options vested immediately and had an exercise price of $1.50 per share. The options cancelled 90 days after the end of the consulting agreement at December 31, 2015. At the grant of the new April 30, 2015 options, the former employee forfeited his vested options of 100,000 and unvested options of 100,000 with an exercise price of $2.00 per share.
37
On April 30, 2015, the Board of Directors approved the grant of 200,000 options to employees of the Company, in accordance with the terms of the 2011 Equity Incentive Plan, as amended. One-half of the options vest immediately, with the remaining vesting on the one year anniversary of the grant date. The options have an exercise price of $1.50 per share, with an expiration date of five years from the grant date. 75,000 options cancelled during 2016 per the terms of the 2011 Equity Incentive Plan.
There were no options granted during the year ended December 31, 2016. The fair value of each stock option granted during the year end December 31, 2015 was estimated on the date of grant using the Black Scholes option pricing model with the following assumptions:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Significant assumptions utilized in determining the fair value of our stock options included the volatility rate, estimated term of the options, risk-free interest rate and forfeiture rate. In order to estimate the volatility rate at each issuance date, given that the Company has not established a historical volatility rate as it has minimal trading volume since we began trading in October 2013, management reviewed volatility rates for a number of companies with similar manufacturing operations to arrive at an estimated volatility rate for each option grant. The term of the options was assumed to be five years, which is the contractual term of the options. The risk-free interest rate was determined utilizing the treasury rate with a maturity equal to the estimated term of the option grant. Finally, management assumed a 25% forfeiture rate in 2015 due to Company changes and significant reduction in operations and personnel.
For the years ended December 31, 2016 and 2015, the Company recorded stock-based compensation expense of $6,691 and $96,929, respectively.
As of December 31, 2016 there was no unrecognized compensation expense and as of December 31, 2015 there was $6,691 of unrecognized compensation expense related to the issuance of the stock options.
Performance Stock Options
There were no performance stock options grantedreimbursements during the years ended December 31, 20162023 and 2015.2022, respectively. No amounts were due from CorLyst at December 31, 2023 or 2022. Our President, Research and Development is the CEO of CorLyst, and CorLyst is a shareholder.
| Number of Options |
| Weighted Average Exercise Price |
Balance, December 31, 2014 | 1,440,000 |
| $ 0.11 |
Granted | -- |
| -- |
Exercised | -- |
| -- |
Cancelled | (1,400,000) |
| $ 0.06 |
Balance, December 31, 2015 | 40,000 |
| $ 2.00 |
Granted | -- |
| -- |
Exercised | -- |
| -- |
Cancelled | -- |
| -- |
Balance, December 31, 2016 | 40,000 |
| $2.00 |
Exercisable, December 31, 2015 and 2016 | 40,000 |
| $ 2.00 |
See Note 511 – Commitments and Contingencies
Purchase Obligations
We enter contracts in the normal course of business with contract research organizations and subcontractors to further develop our products. The contracts are cancellable, with varying provisions regarding termination. If we terminated a cancellable contract with a specific vendor, we would only be obligated for further discussionproducts or services that we received as of the performance options.
Warrants
There were no warrants issued duringeffective date of the year endedtermination and any applicable cancellation fees. As of December 31, 2016.
During 20152023, we are contractually obligated to pay up to approximately $1.4 million for future services under the Company issued 25,141 warrants in connectionagreement with the private equity offering dated October 1, 2014. Each unit consisted of one share of Common stockCRO for our clinical trial in NGC-Cap. Our actual contractual obligations will also vary depending on the progress and one-half warrant, with each whole warrant exercisable at $2.00 per share and grants the right to purchase a shareresults of the Company’s common stock. The warrants expire three years fromremaining clinical trials.
Note 12 – Concentration of Credit Risk
Financial instruments that potentially subject us to significant concentration of credit risk consist primarily of our cash and cash equivalents. We utilize only well-established banks and financial institutions with high credit ratings. Balances on deposit are insured by the date of issuance and are exercisable immediately.Federal Deposit Insurance Corporation (FDIC) up to specified limits. Total cash held by our banks at December 31, 2023, exceeded FDIC limits.
| Number of Warrants | Weighted Average Exercise Price | Weighted Average Remaining Life (Years) |
Balance, December 31, 2014 | 2,424,980 | $ 2.48 | 2.28 |
Granted | 25,141 | $ 2.00 |
|
Exercised | -- | -- |
|
Cancelled | (449,817) | $ 3.00 |
|
Balance, December 31, 2015 | 2,000,304 | $ 2.36 | 1.64 |
Granted | -- | -- |
|
Exercised | -- | -- |
|
Cancelled | -- | -- |
|
Balance, December 31, 2016 | 2,000,304 | $ 2.36 | 0.63 |
10.Note 13 – Subsequent Events
NET LOSS PER COMMON SHARE:
The Company computes loss per shareOn January 30, 2024, we sold, pursuant to securities purchase agreements (the “Purchase Agreement”), shares of common stock, using the two-class method required for participating securities. The Company’s participating securities include all seriespre-funded warrants to purchase up to 1,079,555 shares of its convertible preferred stock. Undistributed earnings allocated to these participating securities are added to net losscommon stock in determining net loss applicable to common stockholders. Basic and Diluted loss per share are computed by dividing net loss applicable to common stockholder by the weighted-average numberlieu of shares of common stock outstanding.
Outstanding options(the “Pre-Funded Warrants”), and warrants underlying 2,309,804to purchase up to 1,555,555 shares do not assume conversion,of our common stock (the “Common Warrants’) pursuant to a public offering (the “Offering”). The Common Warrants have an exercise or contingent exercise inprice of $4.50, are immediately exercisable and will remain exercisable until the computationdate that is five years after their original issuance. The Shares were offered at a combined public offering price of diluted loss$4.50 per share becauseand accompanying Common Warrant and $ per Pre-Funded Warrant and accompanying Common Warrant. The Pre-Funded Warrants had an exercise price of $0.0001 and were exercised in full simultaneously with the effect would be anti-dilutive.
The calculationclosing of the numerator and denominatorOffering in exchange for basic and diluted shares of our common stock. Gross proceeds in connection with the Offering were $7.0 million. We received $6.3 million in net loss per common share is as follows:
| For the year ended December 31, | ||||
| 2016 |
| 2015 | ||
Net loss from continuing operations | $ | (337,179) |
| $ | (2,982,920) |
Net income (loss) from discontinued operations |
| 1,197 |
|
| (371,307) |
Net loss |
| (335,982) |
|
| (3,354,227) |
Basic and diluted: |
|
|
|
|
|
Preferred stock cumulative dividend - Series D |
| (43,058) |
|
| (42,942) |
Income applicable to preferred stockholders |
| (43,058) |
|
| (42,942) |
Net loss applicable to common stockholders | $ | (379,040) |
| $ | (3,397,169) |
11.
COMMITMENTS AND CONTINGENCIES:
Lease Commitments - The Company leased warehouse and office space forproceeds from the equipment and operations located in Gardena, CA. The lease term continued through July 2015. The Company was under a month-to-month agreement August 2015 through January 2016.
Total rent expense forOffering, after deducting the year ended December 31, 2016 and 2015 was $2,250 and $50,035, respectively.
39
Vendors and Debt - The Company has significant liabilities as of December 31, 2016 with limited cash flow generated by the sale of Company assets and revenue. The Company has $300,678 in accounts payable and accrued expenses from continuing operations. In addition, the Company has $2,053,515 in debt and accrued interest from continuing operations. The Company will work with their vendors and lenders to establish payment plans, explore extensions and conversion of debt.
12.
RELATED PARTY TRANSACTIONS:
Justin Yorke is the managerfees of the JMW Fund, LLC,placement agent and other offering-related expenses. We also issued to the San Gabriel Fund, LLC, and the Richland Fund, LLC; and is a director of the Company. Mr. McGrain, our Interim Chief executive officer and Interim Chief financial officer is also a member of the JMW Fund, LLC, the San Gabriel Fund, LLC, and the Richland Fund, LLC. These funds own 4,725,721placement agent warrants to purchase 62,222 shares of common stock, and holds warrants to purchase 1,278,186 common shares in the aggregate.exercisable at $5.625 per share that expire on February 1, 2027.
During the year ended December 31, 2015 Mr. Yorke, converted $20,000 and $160,000 unsecured notes payable into senior secured notes payable. In addition, Mr. Yorke was issued warrants to purchase 2,857 common shares as part of the Private equity offering dated October 1, 2014, during 2015.
| F-23 |
As of December 31, 2016 and 2015, the Company has secured notes payable with Mr. Yorke in the aggregate amount of $962,361 and $947,361, respectively. An outstanding balance of $138,000 on the revolving line of credit as of December 31, 2016 and 2015. Mr. Yorke, as the manager, earned interest from loans payable for the years ended December 31, 2016 and 2015 of $192,728 and $120,565, respectively. Total accrued interest as of December 31, 2016 and 2015 was $332,566 and $139,838, respectively.
During the year ended December 31, 2015, Mr. Gus Blass III, a former member of our board of directors and a stockholder whom resigned September 15, 2015, earned dividends from preferred stock totaling $24,065 and $24,000, for the years ended December 31, 2016 and 2015, respectively. Total accrued dividends as of December 31, 2016 and 2015 was $48,065 and $24,000, respectively.
During the year ended December 31, 2015, Reginald Greenslade, a former member of our board of directors and a stockholder, who resigned August 1, 2015; earned interest totaling $5,533 and $5,519 for the years ended December 31, 2016 and 2015, respectively. Total accrued interest as of December 31, 2016 and 2015 was $10,158 and $4,626, respectively.
David Dworsky, the former Chief Executive Officer and board member of the Company, whom resigned his Officer position effective April 30, 2015 and his board position effective August 31, 2015. Mr. Dworsky earned dividends on 1,500 shares of Series D preferred totaling $361 and $360 for the years ended December 31, 2016 and 2015, respectively. Total accrued dividends as of December 31, 2016 and 2015 was $721 and $360, respectively.
13.
SUPPLEMENTAL CASH FLOW INFORMATION:
| For the year ended December 31, | ||||
| 2016 |
| 2015 | ||
Cash paid for interest | $ | -- |
| $ | 25,356 |
Cash paid for income taxes | $ | -- |
| $ | -- |
|
|
|
|
|
|
Non-Cash investing and financing transactions |
|
|
|
|
|
Assumption of Revolving line of credit | $ | 229,980 |
| $ | -- |
Returned equipment applied to outstanding loan principal | $ | -- |
| $ | 98,098 |
Conversion of unsecured notes to senior secured notes | $ | -- |
| $ | 174,361 |
Shares issued for consulting services | $ | -- |
| $ | 25,500 |
14.
SUBSEQUENT EVENTS:
We have evaluated all events that occurred after the balance sheet date through the date when our financial statements were issued to determine if they must be reported. Management has determined that other than as disclosed below, there were no additional reportable subsequent events to be disclosed.
Debt offerings
The Company entered into Secured notes under the senior secured loan agreement in the aggregate amount of $75,000 on June 9, 2017; $15,000 on July 26, 2017 and $105,000 on August 7, 2017.
Other
On July 17, 2017, the Company issued a press release entitled “Heatwurx Announces Letter of Intent with Promet Therapeutics, LLC Relating to a Reverse Merger” in which the Company disclosed that it has entered into a non-binding letter of intent to engage in a reverse merger with Promet Therapeutics, LLC.
Board of Directors
On August 24, 2017, Mr. Justin Yorke and Mr. Christopher Bragg were appointed to the Board of Directors of the Company.
ITEMItem 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
NoneNone.
ITEMItem 9A. Controls and Procedures
Our management, with the participationEvaluation of our Chief Executive Officer, who also serves as our Chief Financial Officer, has concluded, based on evaluation, as of the end of the period covered by this report, that ourDisclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rule 15d-15(e) under the Exchange Act)that are (1) not effectivedesigned to ensure that material information required to be disclosed by us in our reports filed or submitted by us under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, as our controls are designed to do, and management was required to apply its judgment in evaluating the risks related to controls and procedures.
In connection with the preparation of this Annual Report on Form 10-K, as of December 31, 2023, an evaluation was performed by management, including the Chief Executive Officer and Chief Financial Officer, of the Securitieseffectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Commission dueAct). Our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2023 to the material weakness noted below, and (2) lacking design to ensureprovide reasonable assurance that material information required to be disclosed by us in such reports we file or submit under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the SEC rules and forms and (ii) accumulated organized and communicated to our management, including our principal executive officer and principal financial officer, as appropriated,appropriate to allow timely decisions regarding required disclosure.
Inherent Limitations on Effectiveness of Controls
Our management is responsible for establishing and maintaining adequate internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. Internalprinciples (“GAAP”). Our internal control over financial reporting includes those policies and procedures thatthat: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of our company;assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles,GAAP, and that our receipts and expenditures of the company are being made only in accordance with authorizations of our management and director;directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’sour assets that could have a material effect on the financial statements.
Because of its inherent limitations,Management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our internal control over financial reporting may notcontrols will prevent or detect misstatements.all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of internal controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Also, projections of any evaluation of the effectiveness toof controls in future periods areis subject to the risk that those internal controls may become inadequate because of changes in business conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| 58 |
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of consolidated financial statements for external purposes in accordance with GAAP.
Management assessed our internal control over financial reporting as of December 31, 2016, the end of our fiscal year.2023. Management based its assessment on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO 1992 Criteria)(2013 framework). Management’s assessment included evaluation of elements such elements as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies, and our overall control environment.
Based on ourthis assessment, management has concluded that, as of December 31, 2023, our internal control over financial reporting was not effective as of the end of the fiscal year, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external reporting purposes in accordance with generally acceptedGAAP.
This Annual Report on Form 10-K does not include an attestation report of our independent registered public accounting principles.
In lightfirm, BD & Company, Inc., regarding internal controls over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm as we are a smaller reporting company. We are not currently subject to Section 404(b) of the material weaknesses described below, we performed additional analysis and other post-closing procedures to ensure that our financial statements were preparedSarbanes-Oxley Act of 2002.
Changes in accordance with generally accepted accounting principles. Accordingly, we believe that the financial statements included in this report fairly present, in all material respects, our financial condition, results of operations and cash flows for the periods presented.Internal Control Over Financial Reporting
Material Weakness and Related Remediation Initiatives
Our management concluded that as of December 31, 2016, the following material weaknesses existed: 1. Due to the Company’s budget constraints, the Company’s accounting department does not maintain the number of accounting personnel (either in-house or external) necessary to ensure more complete and effective financial reporting controls. Through the efforts of management, external consultants, and our Audit Committee, we have developed a specific action plan to remediate the material weaknesses. Due to lack of funds we were unable to remediate the material weakness during 2016. Due to the significant reduction in operations, the Company was unable to remediate the material weakness thus far in 2017.
42
There were no changes into our internal control over financial reporting (as definedidentified in Rule 15d - 15(f) underconnection with the evaluation required by Rules 13a-15(d) and 15d-15(d) of the Exchange Act)Act that occurred during the year ended December 31, 20162023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEMItem 9B. Other Information
None
PART III
ITEM 10. Directors, Executive Officers and Corporate Governance
The following information as of September 10, 2017,During the name and age of, and position or positions held by, our executive officer and director and, the employment background, and any directorship held by the current director during the last five years in any company with a class of securities registered pursuant to section 12 of the Exchange Act or subject to the requirements of section 15(d) of the Exchange Act or any company registered as an investment company under the Investment Company Act of 1940 is detailed. The Board consists of a single director, whom was elected by the former Board members prior to resigning in August and September 2015, which believed that the director named below is highly qualified and has the skills and experience required for effective service on the Board. The director’s individual biography follows containing information about his experience, qualifications and skills that led the Board to nominate him.
John P. McGrain (Age 71), CEO, CFO, Director. John McGrain has served as Director since August 31, 2015 and our Interim Chief Executive Officer and Interim Chief Financial Officer since September 14, 2015. Mr. McGrain has served in the following capacities over the last five years: Manager and sole member of the JPG Investments, LLC, and a member of the following funds: JMW Fund, LLC; the Richland Fund, LLC; and the San Gabriel Fund, LLC.
Board of Directors
Our Board of Directors is comprised of three directors. The director will serve a one-year term, or until an earlier resignation, death or removal, or their successors are elected. Directors do not receive cash compensation for service on the Board. The Company reimburses its directors for their out-of-pocket costs, including travel and accommodations, relating to their attendance at any Board of Directors meeting. Directors are entitled to participate in our equity compensation plan.
Biographical information
Justin Yorke (Age 51), Director. Mr. Yorke has over twenty-five years of experience in finance. Mr. Yorke joined San Gabriel Advisors, a private equity investment fund as a partner over 10 years ago. Mr. Yorke is involved in managing day-to-day activities of San Gabriel Advisors’ investment portfolios and is active on the boards of directors of the portfolios investments. Mr. Yorke is also currently a member of the board of directors of Splash Beverage, a private company. Prior to that, Mr. Yorke was based in Hong Kong where he acted as Fund Manager for Darier Henstch, a private Swiss Bank for over 10 years.
Christopher Bragg (Age 34), Director. Mr. Bragg has over fifteen years of experience in the financial industry. Mr. Bragg joined Arroyo Capital Management, an investment fund manager, as a partner in 2014. From 2012 to 2014, Mr. Bragg was the West Coast operations manager at Empire Capital Management, a technology focused hedge fund. Prior to that Mr. Bragg spent six years between client service, trade support and the settlements desk at Camden Asset Management, a convertible arbitrage hedge fund. He currently serves on the board of directors of publicly traded Two Rivers Water and Farming Company, as well as the board of directors of two non-profit organizations.
A conflict of interest may arise between the best interests of our company and the best interests of the directors’ other business interests. In such a situation where a conflict of interest exists any decision by the directors which furthers the best interests of their other business interest may be harmful to our business. Additionally, we have no established parameters or understandings regarding the allocation of present or future business opportunities between us and the directors’ other business interests.
43
Code of Ethics and Business Conduct
We adopted a Code of Ethics and Business Conduct in October 2012, which applies to all of our employees, officers and directors. It establishes standards of conduct for individuals and also individual standards of business conduct and ethics.
ITEM 11. Executive Compensation
The following table provides a summary of annual compensation for our named executive officers for the yearsquarter ended December 31, 20162023, none of our directors or executive officers adopted or terminated a Rule 10b5-1 trading plan or a non-Rule 10b5-1 trading arrangement (as defined in Item 408(c) of Regulation S-K).
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
Part III
Item 10. Directors and December 31, 2015. We do not have an employment agreement with our named executive officers.
SUMMARY COMPENSATION TABLE
Name and principal position | Year | Salary | Option Awards | All other Compensation | Total |
|
| ($) | ($) | ($) | ($) |
John McGrain Interim Chief Executive Officer and Interim Chief Financial Officer(1) | 2016 2015 | 1 1 | - - | - - | 1 1 |
|
|
|
|
|
|
David Dworsky Former Chief Executive Officer (2) | 2016 2015 | - 53,250 | -(3) 32,450(3) | - 16,183(4) | - 101,883 |
(1) John McGrain has served as our Interim Chief Executive Officer and Interim Chief Financial Officer since September 14, 2015.
(2) David Dworsky served as our President, Chief Executive Officer from December 16, 2013 through April 30, 2015.
(3) Upon his resignation from his position as President and Chief Executive Officer Mr. Dworsky forfeited his vested options of 93,750 and unvested options of 206,250 with an exercise price of $3.00 per share and was granted 125,000 options with an exercise price of $1.50 per share, the options vest immediately and expire five years from the date of grant. Mr. Dworsky’s options were cancelled 90 days after his resignation from the Board on August 31, 2015, per the termsOfficers of the 2011 Equity Incentive Plan.Registrant
(4) Mr. Dworsky received a payout
The information required by this Item 10 of his accrued paid-time-offForm 10-K will be in our 2024 Proxy Statement to be filed with the amount of $16,183.
InSEC in connection with his appointment as Interim Chief Executive Officer and Interim Chief Financial Officer, we have agreedthe solicitation of proxies for our 2024 Annual Meeting of Stockholders (“2024 Proxy Statement”) is incorporated herein by reference to pay Mr. McGrain total compensation of $1 annually.
Outstanding Equity Awards at Fiscal Year-End
There are no outstanding stock options held by our named executive officers at December 31, 2016. All options were granted under our 2011 Stock Incentive Plan, per the terms of the plan options, are cancelled 90 days following their last day affiliated2024 Proxy Statement. The 2024 Proxy Statement will be filed with the Company. Our named executive officers did not hold any restricted stock or other stock awards atSEC within 120 days after the end of 2016.the fiscal year to which this report relates.
DirectorItem 11. Executive Compensation
There were no fees earned or paid in cash, no option awards grantedThe information required by this Item 11 of Form 10-K is incorporated herein by reference to our directors (not including our named executive officer) for the year ended December 31, 2016.2024 Proxy Statement.
| 59 |
ITEM
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth information regarding beneficial ownershiprequired by this Item 12 of Form 10-K is incorporated herein by reference to our common stock as of September 10, 2017, by: our named executive officer and directors; all executive officers and directors as a group; and each person who is known by us to beneficially own more than 5% of our outstanding common stock.2024 Proxy Statement.
44
Shares of common stock not outstanding but deemed beneficially owned because an individual has the right to acquire the shares of common stock within 60 days, including shares issuable upon conversion of preferred stock, are treated as outstanding when determining the amount and percentage of common stock owned by that individual and by all directors and executive officers as a group. The address of each named executive officer and director is 530 S. Lake Avenue #165, Pasadena, CA 91101. The address of other beneficial owners is set forth below.
The percentage of shares beneficially owned shown in the table is based upon 11,017,388 shares of common stock outstanding as of September 10, 2017.
Name of beneficial owner |
| Shares beneficially owned |
| % of shares outstanding |
Named executive officers and directors: |
|
|
|
|
John McGrain |
| 137,200 |
| 1.25% |
Justin Yorke (1) 4 Richland Place Pasadena, California 91103 |
| 6,091,401 |
| 49.54% |
|
|
|
|
|
Name and address of beneficial owner |
| Shares beneficially owned |
| % of shares outstanding |
Stockholders owning more than 5%: |
|
|
|
|
JMW Fund LLC (2) 4 Richland Place Pasadena, California 91103 Manager: Justin Yorke |
| 2,777,114 |
| 23.96% |
San Gabriel Fund LLC (3) 4 Richland Place Pasadena, California 91103 Manager: Justin Yorke |
| 2,447,898 |
| 21.42% |
Richland Fund LLC (4) 4 Richland Place Pasadena, California 91103 Manager: Justin Yorke |
| 778,895 |
| 6.90% |
Kirby Enterprise Fund LLC (5) PO Box 3087 Greenwood Village, Colorado 80155 Manager: Charles Kirby |
| 604,000 |
| 5.48% |
Charles F. Kirby (6) PO Box 3087 Greenwood Village, CO 80155 |
| 1,200,737 |
| 10.90% |
(1) Includes ownership of JMW Fund LLC, San Gabriel Fund LLC, and Richland Fund LLC as indicated below and some individually owned by Mr. Yorke. Mr. McGrain is also a member of JMW Fund LLC, San Gabriel Fund LLC, and Richland Fund LLC. Includes a total of 1,278,186 shares underlying warrants.
(2) Justin Yorke is the manager of the JMW Fund and is a director of the Company. Mr. McGrain is also a member of the JMW Fund, LLC. Includes 573,407 shares underlying warrants.
(3) Justin Yorke is the manager of the San Gabriel Fund and is a director of the Company. Mr. McGrain is also a member of the San Gabriel Fund, LLC. Includes 411,279 shares underlying warrants.
(4) Justin Yorke, is the manager of the Richland Fund and is a director of the Company. Mr. McGrain is also a member of the Richland Fund, LLC. Includes 293,500 shares underlying warrants.
(5) Charles Kirby is the manager of the Kirby Enterprise Fund LLC and was a director from April 2011 until October 2011.
(6) Charles Kirby was a director from April 2011 until October 2011. Includes 604,000 shares owned by Kirby Enterprise Fund LLC, of which Mr. Kirby is a manager. Also includes 451,554 shares owned by Charles F. Kirby Roth 401(k), 121,500 shares owned by West Hampton Special Situations Fund LLC, of which Mr. Kirby is a manager, 20,000 shares owned by Kirby Enterprise Capital Management LLC, of which Mr. Kirby is a manager, and 3,683 shares owned by River Bend Fund LLC, of which Mr. Kirby is a manager.
ITEMItem 13. Certain Relationships and Related Transactions
The information required by this Item 13 of Form 10-K is incorporated herein by reference to our 2024 Proxy Statement.
Item 14. Principal Accounting Fees and Director IndependenceServices
Transactions with Related Persons, PromotersThe information required by this Item 14 of Form 10-K is incorporated herein by reference to our 2024 Proxy Statement.
Part IV
Item 15. Exhibits, Financial Statement Schedules
(a)(1) and Certain Control Persons(2) Financial Statements and Schedules:
This section describes the transactions we have engaged in with persons who were our directors or officers at the time of the transaction, and persons or entities known by us to be the beneficial owners of more than 5% of our common stock for the years ended December 31, 2016 and 2015.
Transactions with JMW Fund, LLC
JMW Fund, LLC, managed by Mr. Yorke a director, beneficially owns more than 5% of the outstanding shares of Company common stock. JMW Fund, LLC owns 2,203,707 common shares and warrants to purchase 573,407 shares. During the year ended December 31, 2015 the Company has entered into a $2 Million Loan agreement with the JMW Fund, LLC, and issued senior secured promissory notes payable in the total amount of $390,744. The Company issued additional senior secured promissory notes payable in the amount of $15,000 during the year ended December 31, 2016. As of December 31, 2016, the Company has senior secured notes outstanding in the amount of $405,774 and accrued interest of $122,187 with the JMW Fund, LLC. The Company assumed the Revolving line of credit from Dr. Pave, LLC during 2016, and as of December 31, 2016, had a balance of $46,000 with accrued interest of $13,552 was outstanding with the JMW Fund, LLC. The company is in default on the senior secured loan agreement as of the dateSee Part II, Item 8, of this report.
Transactions with San Gabriel Fund, LLC
San Gabriel Fund, LLC, managed by Mr. Yorke a director, beneficially owns more than 5% of the outstanding shares of Company common stock. San Gabriel Fund, LLC owns 2,036,619 common shares and warrants to purchase 411,279 shares. During the year ended December 31, 2015 the Company has entered into a $2 Million Loan agreement with the San Gabriel Fund, LLC, and issued senior secured promissory notes payable in the total amount of $290,744. As of December 31, 2016, the Company has senior secured notes outstanding in the amount of $290,774 and accrued interest of $89,990 with the San Gabriel Fund, LLC. The Company assumed the Revolving line of credit from Dr. Pave, LLC during 2016, and as of December 31, 2016, had a balance of $46,000 with accrued interest of $13,084 was outstanding with the San Gabriel Fund, LLC. The company is in default on the senior secured loan agreement as of the date of this report.
Transactions with Richland Fund, LLC
Richland Fund, LLC, managed by Mr. Yorke a director, beneficially owns more than 5% of the outstanding shares of Company common stock. The Richland Fund, LLC owns 485,395 common shares and warrants to purchase 293,500 shares. During the year ended December 31, 2015 the Company has entered into a $2 Million Loan agreement with the Richland Fund, LLC, and issued senior secured promissory notes payable in the total amount of $245,872 and converted the December 11, 2014 short-term unsecured note payable into a senior secure promissory note in the amount of $20,000. As of December 31, 2016, the Company has senior secured notes outstanding in the amount of $265,872 and accrued interest of $80,526 with the Richland Fund, LLC. The Company assumed the Revolving line of credit from Dr. Pave, LLC during 2016, and as of December 31, 2016, had a balance of $46,000 with accrued interest of $13,226 was outstanding with the Richland Fund, LLC. The company is in default on the senior secured loan agreement as of the date of this report.
Conflicts of Interest Policies
We have adopted a Code of Ethics and Business Conduct. All our directors, officers, and employees are required to be familiar with the Code of Ethics and comply with its provisions. The Code of Ethics expressly prohibits loans made by the Company to our directors and executive officers. Any other transaction involving an executive officer or director that may create a conflict of interest must receive the prior approval of the Audit Committee. All other conflicts must be reported to the Chief Financial Officer. The Code of Ethics provides that conflicts of interest should be avoided but allows the Audit Committee to approve transactions with executive officers or directors other than loans or guaranty transactions.
Other than as described in this section, there are no material relationships between us and any of our directors, executive officers, or known holders of more than 5% of our common stock.
ITEM 14. Principal Accountant Fees and Services
Hein & Associates LLP (“Hein”) served as the Company’s independent registered public accounting firm from 2011 through September 3, 2015.
Pritchett, Siler & Hardy PC (“PS&H”) has served as the Company’s independent registered public accounting firm since November 2015 and audited its financial statements for the year ended December 31, 2015 and 2016.
For the years ended December 31, 2016 and 2015, the aggregate fees billed by Hein and Pritchett, Siler & Hardy PC to the Company were as follows:
| Year ended December 31, | |||
| (PS&H) 2016 | (Hein) 2016 | (PS&H) 2015 | (Hein) 2015 |
Audit fees (1) | $ 24,683 | $ 14,006 | $ 5,400 | $ 77,000 |
Audit-related fees | - | - | - | 7,385 |
Tax fees | - | - | - | - |
All other fees | - | - | - | - |
Total accounting fees and services | $ 24,683 | $ 14,006 | $ 5,400 | $ 84,385 |
(1) Audit fees includes the aggregate fees billed for professional services for the audit of our annual financial statements for the years ended December 31, 2016 and 2015 and the review of the financial statements included in our quarterly reportsAnnual Report on Form 10-Q filed during the year ended December 31 of the respective years.10-K.
Audit Committee Pre-Approval(3) Exhibits
| 60 |
The Audit Committee reviewed and approved in advance the retention of the independent auditors for the performance of all audit and non-audit services that are not prohibited and the fees for such services. Pre-approval of audit and non-audit services that are not prohibited may be approved pursuant to appropriate policies and procedures established by the Audit Committee for the pre-approval of such services, including through delegation of authority to a member of the Audit Committee or Company management. For the years ended December 31, 2016 and 2015, all audit fees were reviewed and approved in advance of such services.
| + | Indicates a management contract or compensatory plan or arrangement. |
| * | Filed herewith |
PART IV
ITEM 15. ExhibitsItem 16. Form 10-K Summary
The following exhibits are included with this report:None.
Exhibit Number | Exhibit Description | Form | Incorporated by Reference | Filing Date | Filed Here- with | |
File No. | Exhibit | |||||
2.1 | Asset Purchase Agreement dated April 15, 2011 | S-1 | 333-184948 | 2.1 | 11/14/12 |
|
3.1 | Certificate of Incorporation, as amended | 10-Q | 333-184948 | 3.1 | 8/8/13 |
|
3.2 | Bylaws, as amended | 10-K | 333-184948 | 3.2 | 3/27/14 |
|
4.1 | Specimen of Common Stock Certificate | S-1/A | 333-184948 | 4.1 | 5/15/13 |
|
4.2 | Certificate of Designations of Series D Preferred Stock, as amended | 10-K | 333-184948 | 4.2 | 3/27/14 |
|
| 61 |
Exhibit Number | Exhibit Description | Form | Incorporated by Reference | Filing Date | Filed Here- with | |
File No. | Exhibit | |||||
10.1 | Form of Senior Secured Promissory Note (part of a $1.5 million series of notes that were paid off in August 2012) | S-1 | 333-184948 | 10.1 | 11/14/12 |
|
10.2* | Giles Performance Option Grant Notice dated April 15, 2011 | S-1 | 333-184948 | 10.2 | 11/14/12 |
|
10.3 | Form of Investors’ Rights Agreement | S-1 | 333-184948 | 10.3 | 11/14/12 |
|
10.4 | Form of Pledge Agreement | S-1 | 333-184948 | 10.4 | 11/14/12 |
|
10.5* | Giles Consulting Agreement dated April 15, 2011 | S-1 | 333-184948 | 10.5 | 11/14/12 |
|
10.6 | Form of HeatwurxAQ Right of First Refusal and Co-Sale Agreement dated April 15, 2011 | S-1 | 333-184948 | 10.6 | 11/14/12 |
|
10.7 | Form of HeatwurxAQ Right of Voting Agreement dated April 15, 2011 | S-1 | 333-184948 | 10.7 | 11/14/12 |
|
10.8 | HeatwurxAQ Subordinated Security Agreement dated April 15, 2011 | S-1 | 333-184948 | 10.8 | 11/14/12 |
|
10.9 | HeatwurxAQ Subordinated Note dated April 15, 2011 | S-1 | 333-184948 | 10.9 | 11/14/12 |
|
10.10* | Amended and Restated 2011 Equity Incentive Plan | S-1 | 333-184948 | 10.10 | 11/14/12 |
|
10.11* | Form of Stock Option Agreement Under 2011 Equity Incentive Plan | S-1 | 333-184948 | 10.11 | 11/14/12 |
|
10.12* | Form of Grant Notice under 2011 Equity Incentive Plan | S-1 | 333-184948 | 10.12 | 11/14/12 |
|
10.13 | Lease between Heatwurx, Inc. and Syracuse Hill II LLC dated July 18, 2012 | S-1 | 333-184948 | 10.13 | 11/14/12 |
|
10.14 | Conformed Copy of Settlement and Mutual Release Agreement among Heatwurx, Inc. and Larry Griffin and David Eastman | S-1/A | 333-184948 | 10.15 | 1/11/13 |
|
10.15 | Senior Loan Agreement dated May 22, 2013, with forms of Senior Secured Promissory Note and Senior Security Agreement attached | S-1/A | 333-184948 | 10.15 | 5/23/13 |
|
10.16 | Subordination Agreement dated May 22, 2013, with Richard Giles | S-1/A | 333-184948 | 10.16 | 5/23/13 |
|
10.17 | Form of Warrant Agreement - Unit offering (Series D) | 10-K | 333-184948 | 10.17 | 3/27/14 |
|
10.18 | Loan Agreement dated January 6, 2014, including the form of the Promissory Note and the Warrant Agreement | 8-K | 333-184948 | 99.1 | 1/9/14 |
|
10.19 | Agreement and Plan of Reorganization dated January 8, 2014 with Dr. Pave, LLC | 8-K | 333-184948 | 99.2 | 1/9/14 |
|
10.20 | Offer Letter to Alex Kramer dated April 28, 2014 | 10-Q | 333-184948 | 10.1 | 8/19/14 |
|
10.21 | Termination Letter of Consulting Agreement dated April 15, 2011 with Richard Giles | 10-Q | 333-184948 | 10.2 | 8/19/14 |
|
10.22 | Exclusive Distribution Agreement, dated October 23, 2014 | 10-Q | 333-184948 | 10.1 | 10/29/14 |
|
10.23 | Separation and Severance Agreement and Release of Claims, dated October 28, 2014. | 8-K | 333-184948 | 99.1 | 11/3/14 |
|
10.24 | Senior Secured Loan Agreement dated February 16, 2015 | 8-K | 333-184948 | 99.1 | 2/20/15 |
|
10.25 | Promissory Note with San Gabriel Fund, LLC dated February 16, 2015 | 8-K | 333-184948 | 99.2 | 2/20/15 |
|
10.26 | Promissory Note with JMW Fund, LLC dated February 16, 2015 | 8-K | 333-184948 | 99.3 | 2/20/15 |
|
SIGNATURES
Exhibit Number | Exhibit Description | Form | Incorporated by Reference | Filing Date | Filed Here- with | |
File No. | Exhibit | |||||
10.27 | Promissory Note with Richland Fund, LLC dated February 16, 2015 | 8-K | 333-184948 | 99.4 | 2/20/15 |
|
10.28 | Security Agreement dated February 16, 2015 | 8-K | 333-184948 | 99.5 | 2/20/15 |
|
10.29 | Subsidiary Guaranty Agreement dated February 16, 2015 | 8-K | 333-184948 | 99.6 | 2/20/15 |
|
10.30 | Debt Conversion Agreement | 8-K | 333-184948 | 99.1 | 7/7/15 |
|
10.31 | Press Release | 8-K | 333-184948 | 99.2 | 7/7/15 |
|
10.32 | Promissory Note Extension Agreement | 10-Q | 333-184948 | 10.1 | 8/19/15 |
|
14.1 | Code of Ethics and Business Conduct | S-1 | 333-184948 | 14.1 | 11/14/12 |
|
16 | Letter from CPA - Hein | 8-K | 333-184948 | 16 | 9/22/15 |
|
16.1 | Letter from CPA - Cutler & Co | 10-Q | 333-184948 | 16.1 | 12/1/15 |
|
21.1 | List of Subsidiaries | 10-K | 333-184948 | 21.1 | 4/14/15 |
|
23.1 | Consent of Pritchett, Siler & Hardy PC, Independent Registered Public Accounting Firm |
|
|
|
| X |
24.1 | Power of Attorney | S-1 | 333-184948 | 24.1 | 11/14/12 |
|
31.1 | Rule 15d-14a Certification by Principal Executive Officer |
|
|
|
| X |
32.1 | Section 1350 Certification of Principal Executive Officer |
|
|
|
| X |
101.INS | XBRL Instance Document |
|
|
|
| X |
101.SCH | XBRL Taxonomy Extension Schema Document |
|
|
|
| X |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
| X |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
| X |
101.LAB | XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
| X |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
| X |
*Management contract, or compensatory plan or arrangement, required to be filed as an exhibit.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereuntohereunto duly authorized.
HEATWURX, INC.
| PROCESSA PHARMACEUTICALS, INC. | |||
| |||
| By: | /s/ George Ng | ||
| George Ng | |||
|
| ||
| Dated: | March 29, 2024 | ||
| By: | /s/ James Stanker | ||
| James Stanker | |||
(Principal Financial and | |||
| Dated: | March 29, 2024 | ||
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the datedates indicated.
| Name | Capacity | |||
|
|
| ||
/s/ |
|
| ||
| ||||
/s/ James Stanker | Chief Financial Officer | March 29, 2024 | ||
| James Stanker | ||||
| /s/ David Young | President of Research and Development and Director | March 29, 2024 | ||
| David Young | ||||
| /s/ Khoso Baluch | Director | March 29, 2024 | ||
| Khoso Baluch | ||||
| /s/ James Neal | Director | March 29, 2024 | ||
| James Neal | ||||
| /s/ Geraldine Pannu | Director | March 29, 2024 | ||
| Geraldine Pannu | ||||
| /s/ Justin Yorke | Director |
| ||
Justin Yorke | ||||
|
|
| ||
|
Supplemental Information to be Furnished With Reports Filed Pursuant to Section 15(d) of the Act by Registrants Which Have not Registered Securities Pursuant to Section 12 of the Act
| 62 |
No annual report or proxy statement, form of proxy or other proxy soliciting material was sent or provided to shareholders during the year ended December 31, 2016.
50