SECURITIES AND EXCHANGE COMMISSION
FORM
10-K(Mark One)
☒ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
| | | | | |
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year
ended ended: December 31,, 2021☐TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
2023
or
| | | | | |
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the
transition periodTransition Period from
toto.Commission File Number 001-40427
|
NKGen Biotech, Inc. |
GRAF ACQUISITION CORP. IV
|
(Exact name of registrant as specified in its charter) |
| | | | | | | | |
| Delaware | | 001-40427
| | 86-2191918 |
(State or other jurisdiction of incorporation or organization) | |
(Commission
File Number)
| | Identification Number) |
3001 Daimler Street Santa Ana, CA, 92705 (Address of Principal Executive Offices) (Zip Code) (949) 396-6830 | | |
1790 Hughes Landing Blvd., Suite 400
The Woodlands, Texas
| |
77380
|
(Address of principal executive offices) | | (Zip Code)
|
Registrant’s telephone number, including area code: (346) 442-0819
Telephone Number, Including Area Code)
(Former
name or former address,Name, Former Address and Former Fiscal Year, if
changed since last report)Changed Since Last Report)
Securities registered pursuant to Section 12(b) of the Act:
Act:
| | | | | | | | |
Title of Each Class:each class | Trading Symbol(s) | Trading Symbols
| | Name of Each Exchange on Which Registered: the principal U.S. market |
Common Stock, $0.0001 par value $0.0001 per share | NKGN | GFOR
| | New York Stock Exchange
Nasdaq Global Market |
Warrants, to purchaseeach whole warrant exercisable for one share of Common Stock at an exercise price of $11.50 per share | NKGNW | GFOR WS
| | New York Stock Exchange
|
Units, each consisting of one share of Common Stock and one-fifth Warrant
| | GFOR.U
| | New York Stock Exchange
Nasdaq Capital Market |
Securities registered pursuant to
Sectionsection 12(g) of the Act:
Nonenone.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.Yes ☐o No ☒x.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐o No ☒x.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☐x No ☒o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒x No ☐o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer,
a smaller reporting company, or an emerging growth company. See
definitionthe definitions of “large accelerated filer,” “accelerated
filer,”filer”, “smaller reporting company”
, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | |
Large accelerated filer | ☐o
| Accelerated filer | ☐o
|
Non-accelerated filer Filer | ☒x
| Smaller reporting company | ☒x
|
Emerging growth company | x☒ | |
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐o Indicate by check mark whether the registrant has filed a report on and attestation to its management’smanagement’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐o If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ⌧o No ☐x.
As of June 30, 2021,April 16, 2024 there were 22,805,643 shares of common stock issued and outstanding, par value $0.0001 per share.
DOCUMENTS INCORPORATED BY REFERENCE
Part III of this report incorporates information by reference from the last business dayCompany's definitive proxy statement, which proxy statement is due to be filed with the Securities and Exchange Commission not later than 120 days after December 31, 2023.
INTRODUCTORY NOTE
Merger
On September 29, 2023 (the “Closing Date”), NKGen Biotech, Inc. (formerly known as Graf Acquisition Corp. IV (“Graf”)), a Delaware corporation (“NKGen” or the “Company”), consummated its previously announced merger transaction in accordance with the terms and conditions of the registrant’s most recently completed second fiscal quarter,Agreement and Plan of Merger, dated as of April 14, 2023 (the “Merger Agreement”), with Austria Merger Sub, Inc., a Delaware corporation and former wholly-owned subsidiary of Graf (“Merger Sub”) and NKGen Operating Biotech, Inc. (formerly known as NKGen Biotech, Inc.), a Delaware corporation (“Legacy NKGen”), whereby such Merger Agreement contemplated Merger Sub merging with and into Legacy NKGen with the aggregate market valueseparate corporate existence of Merger Sub ceasing and Legacy NKGen becoming a wholly-owned subsidiary of ours at the Closing (as defined below) (the “Merger” and, together with the other transactions contemplated by the Merger Agreement, the “Business Combination”). In connection with the consummation of the Merger on the Closing Date, Graf changed its name from Graf Acquisition Corp. IV to NKGen Biotech, Inc. and Legacy NKGen changed its name from NKGen Biotech, Inc. to NKGen Operating Biotech, Inc. The closing of the Business Combination is herein referred to as “Closing.”
In connection with the Business Combination, Graf filed a registration statement on Form S-4 (File No. 333-271929) (as amended, the “Registration Statement”) with the U.S. Securities and Exchange Commission (the “SEC”). On August 14, 2023, the Registration Statement was declared effective by the SEC and on August 14, 2023, Graf filed a Definitive Proxy Statement/Prospectus, which was amended and supplemented by the Supplement No.1 and Supplement No.2 to the Definitive Proxy Statement/Prospectus dated September 21, 2023 and September 22, 2023, respectively (as amended and supplemented, the “Definitive Proxy Statement/Prospectus”).
As a result of the Merger and upon the Closing, among other things, (i) all outstanding shares of Legacy NKGen common stock outstanding, other than shares held by persons who may be deemed affiliatesas of the registrant, computed by referenceimmediately prior to the closing sales price for theClosing, including outstanding Legacy NKGen convertible notes converted into Legacy NKGen common stock on June 30, 2021, as reported onimmediately prior to the New York Stock Closing, were exchanged at an exchange ratio of 0.408 (the “Exchange was $170.76 million.Ratio
As”) for an aggregate of March 30, 2022, there were 21,451,87515,595,260 shares of our common stock, par value $0.0001 per share (“our Common Stock” or “NKGen Common Stock”) and (2) each option to purchase shares of Legacy NKGen common stock, whether vested or unvested, were assumed and converted into an option to purchase shares of our Common Stock (“Assumed Option”), with each Assumed Option subject to the same terms and conditions as were applicable to the original Legacy NKGen option and with the resulting exercise price and number of shares of our Common Stock purchasable based on the Exchange Ratio and other terms contained in the Merger Agreement.
Unless the context otherwise requires, “we,” “us,” “our” and the “Company” refer to our and its consolidated subsidiaries following the Closing and references to “Graf” refer to Graf Acquisition Corp. IV at or prior to the Closing. All references herein to the “NKGen Board” refer to the board of directors of the registrantCompany after giving effect to the Business Combination.
In connection with the special meeting of stockholders of Graf, held on September 25, 2023, and the Business Combination, the holders of 3,386,528 shares of Graf’s common stock, par value $0.0001 per share, exercised their right to redeem their shares for cash at a redemption price of approximately $10.4415 per share, for an aggregate redemption amount of approximately $35.4 million. Upon the Closing, the Company received approximately $21.9 million in gross proceeds, comprising approximately $1.7 million from the Graf trust account and approximately $20.2 million from the transactions in relation to the Warrant Subscription Agreements and Securities Purchase Agreement (each as defined below). In addition, in accordance with the Private Placement Agreements (as defined below), approximately $32.9 million in funds were deposited into escrow accounts, which were not received by the Company in connection with the Closing of the Business Combination. The escrowed funds may be released to the Company, the investors, or a combination of both as set forth in Note 4, Private Placement, of the consolidated financial statements.
At the Closing, Graf instructed its transfer agent to separate Graf’s public units into their component securities and, as a result, following the Closing, Graf’s public units are no longer tradeable as a separate security and were delisted from The New York Stock Exchange. On the business day following the Closing, there were 21,888,976 issued and outstanding.outstanding shares of our Common Stock.
Documents Incorporated
The foregoing description of the Merger Agreement is a summary only and is qualified in its entirety by
Reference: None.the full text of the Merger Agreement, a copy of which is attached hereto as Exhibit 2.1, which is incorporated herein by reference.
Capitalized terms used in this Annual Report on Form 10-K but not otherwise defined herein shall have the meanings ascribed to those terms in the Definitive Proxy Statement/Prospectus.
This report contains references to trademarks belonging to other entities, which are the property of their respective holders. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
CERTAIN DEFINED TERMS
“BusinessCombination”meansthetransactionscontemplatedbytheBusinessCombinationAgreement.
“Closing” means the closing of the BusinessCombination.
“Closing Date” means the date of the Closing.
“Charter”meanstheamendedandrestatedcertificateofincorporationofNKGen,filedwiththe Secretary of State of the State of Delaware on May 20, 2021 and amended on May 20, 2023 and September 29,2023.
“Exchange Act” means the U.S. Securities Exchange Act of 1934, as amended.
“ForwardPurchaseAgreements”meansthosecertainforwardpurchaseagreementsdatedSeptember22, 2023, September 26, 2023 and September 29, 2023, by and among Graf and certaininvestors, as amended on January 19, 2024, January 11, 2024, January 2, 2024 and December 26, 2023.
“GAAP” means U.S. generally accepted accounting principles.
“Graf” means Graf Acquisition Corp. IV, a Delaware corporation (which, after the Closing, became NKGen Biotech, Inc.).
“Graf IPO” means Graf’s initial public offering, consummated on May 25, 2021, through the sale of 17,161,500 Units at $10.00 per Unit.
“Legacy NKGen” means NKGen Operating Biotech, Inc., a Delaware corporation which, pursuant to the Business Combination, became a direct, wholly owned subsidiary of NKGen Biotech, Inc., and unless the context otherwise requires, its consolidated subsidiaries.
“Merger Agreement” means that Agreement and Plan of Merger, dated as of April 14, 2023, by and among Graf, Merger Sub and Legacy NKGen.
“Nasdaq” means the Nasdaq Stock Market LLC.
“NKGen”meanstheDelawarecorporationwhich,priortoconsummationoftheBusinessCombination, was known as Graf Acquisition Corp.IV.
“NKGen Board” means the board of directors of NKGen.
“NKGen Bylaws” or “Bylaws” means the amended and restated bylaws to be adopted by NKGen immediately after the Closing.
“NKGenCommonStock”or“ourCommonStock”meansanissuedandoutstandingshareofcommon stock, par value $0.0001 per share, ofNKGen.
“NKGen Options” means options to acquire NKGen Common Stock.
“NKMAX” means NKMAX Co., Ltd., the largest stockholder of NKGen, a company formed under the laws of the Republic of Korea.
“PIPE Warrants” means the aggregate 10,209,994 Warrants purchased by those warrant subscribers pursuant to the Warrant Subscription Agreements, each of which is exercisable for cash or cashless exercise under certain circumstances in accordance with the respective Warrant Subscription Agreement.
“PrivateWarrants”meansthe4,721,533WarrantspurchasedbytheSponsorconcurrentlywithGraf IPO,eachofwhichisexercisableforcashatanexercisepriceof$11.50orcashlessexerciseundercertain circumstances for one share of NKGen Common Stock.
“Public Warrants” means the 3,432,286 warrants included as a component of the units of Graf sold in the Graf IPO, each of which is exercisable, at an exercise price of $11.50, for one share of NKGen Common Stock, in accordance with its terms.
“SEC” means the U.S. Securities and Exchange Commission.
“SecuritiesPurchaseAgreement”meansthesecuritiespurchaseagreementinrelationtotheSenior Convertible Notes and 1,000,000 Warrants issued in connection with the Senior Convertible Notes, each of which was exercisable, at an exercise price of $11.50, for one share of NKGen Common Stock,by and among Graf and NKMAX, dated September 15,2023.
“SeniorConvertibleNotes”meansthe$10,000,000aggregateprincipalamountof5.0%/8.0%convertible senior notes due 2027 issued to NKMAX in a private placement pursuant to the Securities Purchase Agreement.
“Sponsor” means Graf Acquisition Partners IV LLC, a Delaware limited liability company.
“Warrant Subscription Agreements” means those certain warrant subscription agreements, dated September 26, 2023 and September 27, 2023, by and among Graf and the warrant investors pursuant to, and on the terms and subject to the conditions of which, the warrant investors have collectively subscribed for and agreed to purchase in private placements an aggregate of 10,209,994 shares of common stock at a purchase price of $1.00 per warrant, resulting in an aggregate purchase price of$10,209,994.
“Warrants” means the Private Warrants, the Public Warrants and the Working Capital Warrants.
“WorkingCapitalNote”meanstheconvertiblepromissorynoteissuedbyGraftotheSponsorwithaprincipal amount up to $1.5 million on May 15, 2023.
“Working Capital Warrants” means the 523,140 Warrants issued upon conversion of the then outstanding amount under the Working Capital Note upon the Closing, each of which is exercisable, for cash at an exercise price of $11.50, for one share of NKGen Common Stock, or on cashless exercise, in accordance with its terms.
NKGen Biotech, Inc.
FORM 10-K
December 31, 2023
INDEX
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTSCertain statements in this
This Annual Report on Form 10-K
(this “Form 10-K”) may
constitutecontain “forward-looking statements”
for purposeswithin the meaning of Section 27A of the
federal securities laws. OurSecurities Act and Section 21E of the Securities Exchange Act of 1934, as amended. The Company’s forward-looking statements include, but are not limited to, statements regarding
ourthe Company’s or
ourits management team’s expectations, hopes, beliefs, intentions or strategies regarding the
future.future, including the Company’s expectations regarding the plans and strategy for our business, future financial performance, expense levels and liquidity sources. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,”
“intend,“intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,”
“would”“would,” “goal” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
Forward-looking statements in this Form 10-K may include, for example, statements about: | ● | our ability to select an appropriate target business or businesses; |
| ● | our ability to complete our initial business combination; |
| ● | our expectations around the performance of the prospective target business or businesses; |
| ● | our success in retaining or recruiting, or changes required in, our officers, key employees or directors following our initial business combination; |
| ● | our officers and directors allocating their time to other businesses and potentially having conflicts of interest with our business or in approving our initial business combination; |
| ● | our potential ability to obtain additional financing to complete our initial business combination; |
| ● | our pool of prospective target businesses; |
| ● | the adverse impacts of the COVID-19 outbreak and other events (such as terrorist attacks, natural disasters or a significant outbreak of other infectious diseases) on our ability to consummate an initial business combination; |
| ● | the ability of our officers and directors to generate a number of potential acquisition opportunities; |
| ● | our public securities’ potential liquidity and trading; |
| ● | the lack of a market for our securities; |
| ● | the use of proceeds not held in the trust account (as defined below) or available to us from interest income on the trust account balance; |
| ● | the trust account not being subject to claims of third parties; or |
| ● | our financial performance. |
The forward-looking statements contained in this Annual Report on Form 10-K and in documents incorporated herein are based on ourthe Company’s current expectations and beliefs concerning future developments and their potential effects on us.us taking into account information currently available to the Company. There can be no assurance that future developments affecting usthe Company will be those that we havethe Company has anticipated. These forward-looking statements involve a number of risks, uncertainties (some(many of which are difficult to predict and beyond ourthe Company’s control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. These
As a result of a number of known and unknown risks and uncertainties, include, but are not limited to, those factors described under the section of this Form 10-K entitled “Risk Factors.” Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect,Company’s actual results or performance may vary in material respectsbe materially different from those projected inexpressed or implied by these forward-looking statements. WeSome factors that could cause actual results to differ include:
•the Company’s ability to raise financing in the future;
•the Company’s ability to service its operations and expenses and other liquidity needs and to address its ability to continue as a going concern;
•the ability to recognize the anticipated benefits of the Business Combination;
•the ability to maintain the listing of the NKGen Common Stock on the Nasdaq Global Market and its warrants on the Nasdaq Capital Market, and the potential liquidity and trading of such securities;
•theriskthattheconsummationoftheBusinessCombinationdisruptscurrentplansandoperations of theCompany;
•costs related to the Business Combination and expenses and/or payments due to third parties;
•changes in applicable laws or regulations;
•the Company’s success in retaining or recruiting, or changes required in, our officers, key employees or directors after the Business Combination;
•the Company’s ability to successfully commercialize any product candidates that it successfully develops and that are approved by applicable regulatory authorities;
•the Company’s expectations for the timing and results of data from clinical trials and regulatory approval applications;
•the Company’s business, operations and financial performance including:
othe Company’s history of operating losses and expectations of significant expenses and continuing losses for the foreseeable future;
othe Company’s ability to execute its business strategy;
othe Company’s ability to develop and maintain its brand and reputation;
•the Company’s ability to partner with other companies;
•the size of the addressable markets for the Company’s product candidates;
•the Company’s expectations regarding its ability to obtain and maintain intellectual property protection and not infringe on the rights of others;
•the outcome of any legal proceedings that may be instituted against the Company; and
•unfavorable conditions in the Company’s industry, the global economy or global supply chain, including financial and credit market fluctuations, international trade relations, pandemics, political turmoil, natural catastrophes, warfare (such as the war between Russia and Ukraine and the armed conflict in Israel and the Gaza Strip and Israel’s declaration of war against Hamas), and terrorist attacks.
These forward-looking statements are based on information available as of the date of this Annual Report on Form 10-K, and current expectations, forecasts and assumptions, and involve a number of risks and uncertainties. Accordingly, forward-looking statements in this Annual Report on Form 10-K and in any document incorporated herein by reference should not be relied upon as representing the Company’s views as of any subsequent date, and the Company does not undertake
noany obligation to update
or revise any forward-looking statements
to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
2
You should read this Annual Report on Form 10-K and the documents incorporated herein, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report on Form 10-K and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and such statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and investors are cautioned not to unduly rely upon these statements.
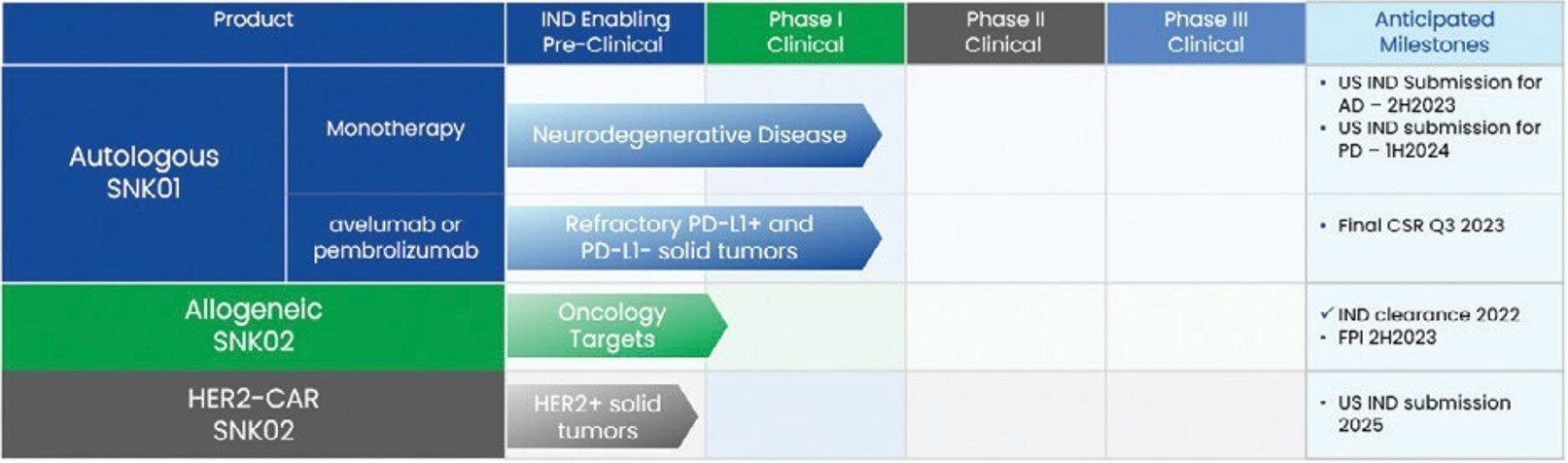
Item 1. Business
Our Mission and Vision
Our mission is to improve patient outcomes in this reportthe areas of neurodegenerative and oncological diseases by developing safe and effective cellular therapies that leverage the power of a patient’s immune system. Our vision is to “we,” “us,” “our” orbecome the “Company” refer to Graf Acquisition Corp. IV. References to our “management” or our “management team” refer to our officers and directors.Introduction
global leader in CAR-natural killer (“
NK”) cell therapies.
Overview
We are a blank checkbiotechnology company incorporateddeveloping cell therapies for neurodegenerative and oncological diseases based on January 28, 2021 as a Delaware corporation for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses (the “business combination”). We have reviewed, and continue to review, a number of opportunities to enter into a business combination, but weactivated NK cells. NK cells are not able to determine at this time whether we will complete a business combination with anypart of the target businesseshuman innate immune response system that wecan selectively identify and destroy abnormal or diseased cells. Our product candidates are based on a proprietary manufacturing and cryopreservation process which produces SuperNKTM (“SNK”) cells that have reviewed or with any other target business. We also have neither engaged in any operations nor generated any revenue to date. Based on our business activities, the Company is a “shell company”shown increased activity as defined under the Exchange Act of 1934 (the “Exchange Act”) because we have no operations and nominal assets consisting almost entirely of cash.Our registration statement for our initial public offering (the “IPO”) was declared effective on May 20, 2021. On May 25, 2021, we consummated our IPO of 15,000,000 units (the “units” and, with respectcompared to the common stock included in the units being offered, the “common stock” or the “public shares”), at $10.00 per unit, with each unit containing one public share and one-fifthstarting population of one redeemable warrant (the “public warrants”), generating gross proceeds of $150.0 million, and incurring offering costs of approximately $8.8 million, of which approximately $5.3 million was for deferred underwriting commissions. We granted the underwriter a 45-day option to purchase up to an additional 2,250,000 units at the IPO price to cover over-allotments. On June 2, 2021, the underwriters partially exercised their over-allotment option and purchased 2,161,500 additional units (the “additional units”), generating gross proceeds of approximately $21.6 million (the “over-allotment”). The Company incurred additional offering costs of approximately $1.2 million in connection with the over-allotment (of which approximately $757,000 was for deferred underwriting fees).
Prior to the consummation of the IPO, on February 13, 2021, Graf Acquisition Partners LLC (“Graf LLC”) paid an aggregate of $25,000 for certain expenses on our behalf in exchange for issuance of 4,312,500 shares of common stock, or approximately $0.006 per share (the “founder shares”). On April 2, 2021, Graf LLC transferred all of its founder shares to Graf Acquisition Partners IV LLC (the “sponsor”). On April 8, 2021, our sponsor transferred 20,000 founder shares to each of our independent directors, resulting in our sponsor holding 4,252,500 founder shares. The number of founder shares issued was determinedNK cells, based on the expectationresults of in vitro experiments performed by NKMAX, as defined by parameters such as cytotoxicity, cytokine production and activating receptor expression. SNK cells can be produced in large quantities and cryopreserved, while maintaining high levels of cytotoxicity and activating receptor expression after thawing and reconstitution. We believe that SNK cells have the potential to deliver transformational benefits to patients with both neurodegenerative diseases, such founder shares would represent 20%as Alzheimer’s Disease (“
AD”) and Parkinson’s disease (“PD”), and oncological diseases. Our initial insights into the potential of SNK01, an autologous cell therapy candidate, in neurodegenerative disease is derived from compassionate use data from three patients with AD and two patients with PD. Compassionate use refers to the outstanding shares afteruse outside of a clinical trial of an investigational, or unapproved, medical product (drug, biologic or medical device) in patients with a chronically or seriously debilitating disease who cannot be treated satisfactorily by an authorized medicinal product. Treatment of these five patients with SNK01 was associated with marked improvement in certain clinical symptoms typically associated with AD and PD, such as cognitive, vocal and motor impairments. Although the IPO. The founder sharesresults from these compassionate use case studies provide no assurance or guarantee that SNK01 will be worthless ifdeemed to be safe or effective for the treatment of AD or PD, and extensive clinical testing and regulatory approval will be required for SNK01, such results led NKGen to initiate formal clinical development of SNK01 as a potential treatment for neurodegenerative diseases. Accordingly, we do not completeare conducting a Phase I trial in Mexico (MX04) to assess the safety and tolerability of SNK01 in AD patients, received clearance in October 2023 of an initial business combination. In addition, as discussed in more detail below, our sponsor purchased an aggregate of 4,433,333 private placement warrants (as defined below)investigational new drug (“IND”) by the FDA, for a purchase price of $6,650,000, or $1.50 per warrant, that will also be worthless if we do not complete an initial business combination.Simultaneously with the closing of the IPO, we consummated the private placement (the “private placement”) of 4,433,333 warrants (each, a “private placement warrant” and collectively, the “private placement warrants”), at a price of $1.50 per private placement warrant to our sponsor, generating proceeds of approximately $6.7 million. Simultaneously with the closing of the over-allotment on June 2, 2021, we consummated the second closing of the private placement, resulting in the purchase of an aggregate of an additional 288,200 private placement warrants at $1.50 per private placement warrant (the “additional private placement warrants”), generating additional gross proceeds of approximately $432,000.
Upon the closing of the IPO, the over-allotment, and private placement, $171.6 million ($10.00 per unit) of the net proceeds of the sale of the units in the IPO, the over-allotment and of the private placement warrants in the private placement were placed in a trust account (the “trust account”)Phase I/IIa trial in the United States, maintainedreceived clearance in December 2023 by Continental Stock Transfer & Trust Company,Health Canada of a CTA for the same trial, and initiated the first patient in the US for this trial in December 2023.
In oncology, SNK01 treatment in Phase I trials has demonstrated certain antitumor activity, tumor shrinkage and stabilization of disease in solid tumors as trustee,monotherapy, in combination with checkpoint inhibitors, and will be invested onlywith targeted therapies. In the monotherapy treatment group with SNK01, six out of nine heavily pre-treated and refractory patients had stopped tumor progression for a period of time. At the highest dose level (which was 4x109 cells in U.S. “government securities” within the meaningPhase I trial), there was a trend towards tumor reduction, but it did not meet response evaluation criteria in solid tumors. In the combination treatment group of Section 2(a)(16)SNK01 and an immune checkpoint inhibitor, which consisted of heavily pre-treated and refractory patients, some patients achieved a partial response or were able to maintain a state of stabilized disease. This Phase I trial was not designed to support statistical significance testing.
The clinical readouts of the Investment Company Actstudies initiated in 2023 for SNK01 (Phase I/IIa trial in the United States for AD) and SNK02 (in solid tumors) are expected to serve as the basis for subsequent combination trials. In 2024 and beyond, if funding permits, we intend to submit an IND to the FDA to conduct a Phase I trial in PD, to evaluate the expansion into other neurodegenerative diseases, accelerate development in oncology through strategic collaborations, and continue investment in our manufacturing technology.
NK cells are components of 1940,the innate immune system, comprising approximately five to fifteen percent of circulating lymphoid cells, or lymphocytes. NK cells have the broad ability to recognize and destroy many types of cells that express markers associated with cellular damage or infection. Target cells for NK cell destruction include, without limitation, cancer cells, damaged neurons and infected cells. Although hundreds of clinical trials have been initiated with NK cells, there have been no FDA approvals of NK cell therapies to date. We believe that a key barrier to improving clinical outcomes is related to how potential NK cell therapies are prepared, and that our proprietary process has the potential to produce NK cells that may be transformational in the treatment of neurodegenerative and oncological diseases.
Our Solution
We have developed an innovative manufacturing process for SNK cells that addresses several factors that we believe have limited the potential of NK cell therapy to date.
•Expandability. We have demonstrated the ability to generate NK cells from both healthy donors and cancer patients, minimizing manufacturing failures that can leave patients without therapy.
•Activity. SNK cells have shown the ability to deliver increased NK cell activity per dose as amended (the ”Investment Company Act”), havingcompared to the starting populations of NK cells, based on the results of in vitro experiments performed by NKMAX, as defined by parameters such as cytotoxicity, cytokine production, and activating receptor expression.
•Cryopreservation. We have developed technologies that facilitate the cryopreservation of NK cells that retain the majority of their cell activity. Because of this capability, we believe we are able to generate product candidates that can be made readily available as off-the-shelf therapies.
•Scalability. We have invested in developing the technology to enable the generation of hundreds of thousands of doses of SNK cells while maintaining high cellular activity and viability. This capability is critical as we seek to address highly prevalent diseases. We own and operate a maturity25,000 sq. ft. drug manufacturing facility in Santa Ana, California, of 185 days or lesswhich approximately half is equipped for good manufacturing practice (“GMP”) production of NK cells.
We have treated approximately 64 oncology patients and 13 patients with AD in clinical trials with SNK01 either as monotherapy or in
money market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act which invest only in direct U.S. government treasury obligations, until the earlier of: (i) the completion of a business combination
with other agents, including chemotherapy, cetuximab, avelumab, pembrolizumab and
(ii) the distribution of the trust account, as described below.AFM24. As of December 31, 2021, there was approximately $1.7 million2023, these patients account for more than 530 infusions of fresh SNK01. The median number of doses administered per patient across all studies is six infusions, with a minimum of one infusion and a maximum of 38 infusions. Five additional patients have been treated for either AD or PD on a compassionate use basis. There have been no reported significant adverse events deemed related to SNK01 and no immune-related AE ≥ Grade 2 attributed to SNK01. These factors have given us confidence to pursue treatment in ourneurodegenerative diseases, where we are assessing the therapeutic potential of SNK01 directly in human patients, rather than in animal models.
For clinical trials sponsored by NKGen initiated after September 30, 2023, SNK01 have/will be using the cryopreserved SNK01. All allogeneic studies will use cryopreserved SNK02.
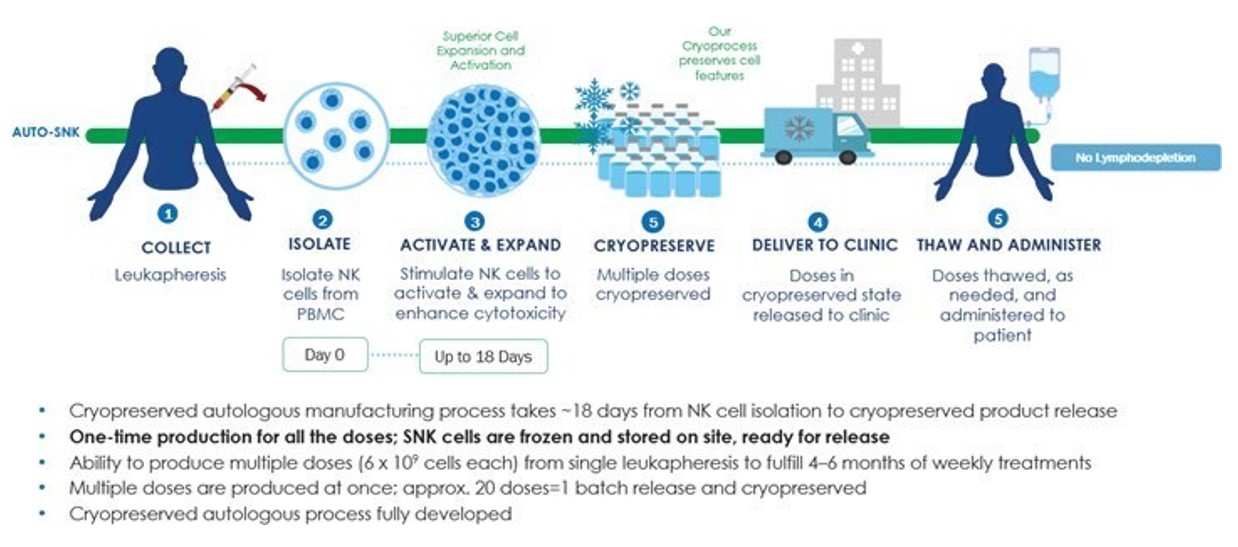
Autologous vs. Allogeneic SNK
Our novel manufacturing technology allows the production of SNK cells for use in either autologous (SNK01) or allogeneic (SNK02) cell therapy. Autologous SNK01 is manufactured using an individual patient’s own NK cells and the generated product is infused back into the same patient. The patient’s NK cells are purified and culture-expanded for up to 18 days, and the harvested cells are washed, packaged, and stored as a cryopreserved product at ≤-130°C. Allogeneic SNK02, on the other hand, is an “off the shelf” product generated from a healthy donor’s NK cells. The donor-derived NK cells are purified and used to establish a working cell bank account(“WCB”). The WCBs are further processed by subjecting the NK cells to long- term culture and working capitalmultiple passages which allow the production of approximately $642,000.
multiple doses of the allogeneic cell therapy product. The manufactured SNK02 are cryopreserved at ≤-130°C and can be used by any patient.
Effecting our initial business combination
SNK01
GeneralWe are not presently engageddeveloping SNK01 for the potential treatment of neurodegenerative diseases, such as AD and PD, based on data from compassionate use cases in five patients. Based on the reported observations from these cases, and despite the caveats associated with limited data from uncontrolled case studies, we believe that SNK01 has the potential to transform the treatment of such neurodegenerative diseases. Patients with severe AD, who could no longer walk, talk or feed themselves, partially regained these abilities after treatment. AD is often assessed using the Mini-Mental State Examination (“MMSE”) score. Patients with early-stage disease typically have MMSE scores between 20 and 25. As patients develop moderate symptoms and exhibit clear impairment, their MMSE scores typically range from 13 to 20. One AD patient who was treated by NKGen on a compassionate use basis and exhibited severe dementia, had a documented pre- treatment MMSE score of 12, but improved to an MMSE score of 23 after six doses of SNK01.
We have opened an IND with the FDA to initiate Phase I/II clinical trials to assess the potential of SNK01, in AD (study initiated in December 2023). In preparation for this trial, we conducted a dose escalation Phase I safety and tolerability trial of SNK01 that, as of the date of this annual report, has been dosed in 11 AD patients in Mexico (MX04) under the hospital’s research ethics committee and Mexico regulatory body’s approval. As part of this trial, we are conducting cognitive function testing collecting exploratory biomarker data to help assess the effects of SNK01 on disease severity in AD. Data that we have obtained to date indicates that intravenously administered SNK01 was well- tolerated by patients and led to stable or improved cognitive functions. Patients were evaluated using the Clinical Dementia Rating Sum of Boxes, the Alzheimer’s Disease Assessment Scale - Cognitive Subscale and the MMSE, each of which are widely used and clinically validated general cognitive measures in clinical trials for AD. The data also indicated that SNK01 dosing was associated with stable or reduced levels of amyloid protein, tau, and neuroinflammation biomarkers in cerebrospinal fluid that are suggestive of altering disease pathology.
We and NKMAX have also conducted several trials with fresh SNK01 that we believe demonstrates the tolerability and therapeutic potential of SNK cells in oncological diseases. These trials fall into three categories: (1) as a monotherapy, SNK01 treatment in highly advanced progressive cancer patients led to a stabilization of disease in six out of nine evaluated patients for at least nine weeks; (2) in combination with checkpoint inhibitors, the addition of SNK01 led to improved overall survival and an increase in progression free survival in refractory lung cancer patients; and (3) NKMAX’s collaboration with Merck KGaA, NKMAX is also conducting a Phase I/IIa trial investigating the combination of SNK01 with a therapeutic antibody, cetuximab, marketed as Erbitux® in advanced epidermal growth factor receptor mutated NSCLC that is refractory to tyrosine kinase inhibitors. Preliminary results from this collaborative trial presented at the annual American Society of Clinical Oncology (“ASCO”) 2023 meeting in June 2023 showed that three of six patients treated with SNK01 in combination with cetuximab achieved partial responses. All other patients treated with SNK01 had stable disease at the time of analysis for the ASCO meeting.
SNK02
Based on the proof-of-concept data generated with SNK01 in oncological diseases, the preference to use an off-the-shelf product and evidence suggesting there may be an improved antitumor response using allogeneic NK cells compared to autologous NK cells, we are transitioning our oncological diseases development program from SNK01, an autologous product, to cryopreserved SNK02, an allogeneic product. As a result, we believe that SNK02 may have greater potential in human clinical trials. Because of our manufacturing expertise, we anticipate that we will not engage in, any operations for an indefinite periodbe able to create hundreds of time. We intendthousands of doses of cryogenically preserved SNK02 which can be made readily available to effectuate our initial business combination using cashpatients, improving upon the current time and resource-intensive process of generating fresh NK cell products on demand. On October 14, 2022, we received IND clearance from the proceedsFDA for SNK02 allogenic NK cell therapy for solid tumors. We have begun dosing in Phase I of the SNK01 clinical trial in refractory solid tumors. Our allogeneic NK cell therapy product candidate will undergo clinical testing without the need for lymphodepletion. We believe this may provide an advantage in terms of antitumor response.
Background
We were founded in the United States in 2017 as a majority-owned subsidiary of NKMAX, a leading biotechnology company in South Korea that specializes in NK cell therapy and the development and manufacture of diagnostic assays, antibodies, and proteins. NKMAX became a publicly traded company on the KOSDAQ in 2015. Shortly thereafter, NKMAX began developing a unique NK cell therapy leading to the creation of a subsidiary in Japan via a collaboration with a Japanese clinic. Through this collaboration, NKMAX obtained early data on its autologous NK cell therapy treatment in human patients. This data served as the basis for NKMAX’s clinical strategy development and provided the basis in 2019 for starting, together with a leading hospital in South Korea, its first clinical trial in non-small cell lung cancer patients using an autologous NK cell therapy, SNK01, combined with an immune checkpoint inhibitor, pembrolizumab. We were founded to further develop SNK01 in oncological and neurodegenerative diseases, such as AD and PD.
After SNK01 was developed, NKMAX proceeded to develop an off-the-shelf cryopreserved allogeneic NK cell therapy, SNK02, to expand its clinical program in oncology. NKMAX plans to initiate its Phase I SNK02 trial in South Korea, received clearance in October 2023 of an IND by the FDA by the South Korean regulatory body, MFDS. We have begun the dosing in Phase I of the clinical trials for SNK02 in the United States in August 2023.
In accordance with the terms of the Intercompany License, we have a license to use all data (non- clinical, clinical, and any other data) that NKMAX controls that would be reasonably useful to develop, manufacture, have manufactured, use or commercialize NK cell pharmaceutical products, processes, services, or therapies in the Licensed Territory. As such, NKMAX’s clinical development in South Korea is expected to continue to provide insight into the potential uses for SNK cells for years to come, for which we will have the right to use the data. See the section titled “Business — Licensing Agreements — NKMAX License” for additional details.
Our goal is to bring transformative NK cell therapies to patients with both neurodegenerative and oncological diseases and thereby to realize the potential of our
IPO, the sale of the private placement warrants, the proceeds of the sale ofteam’s extensive NK cell expertise. We believe our
shares in connection with our initial business combination (including pursuant to forward purchase agreements or backstop agreements we may enter into following the consummation of the IPO or otherwise), shares issued to the owners of the target, debt issued to bank or other lenders or the owners of the target, or a combination of the foregoing. We may seek to complete our initial business combination with a company or business that may be financially unstable or in its early stages of development or growth, which would subjectdifferentiated strategy enables us to
the numerous risks inherent in such companiesleverage our highly integrated platform to develop and
businesses.If our initial business combination is paid for using equity or debt securities, or not all of the funds released from the trust account are used for payment of the consideration in connection with our initial business combination or used for redemptions of our common stock, we may apply the balance of the cash released to us from the trust account for general corporate purposes, including for maintenance ormanufacture NK cell therapies. Our expansion of operations of the post-transaction company, the payment of principal or interest due on indebtedness incurred in completing our initial business combination, to fund the purchase of other companies or for working capital.
Sources of Target Businesses
We anticipate that target business candidates will be brought to our attention from various unaffiliated sources, including investment bankers and investment professionals. Target businesses may be brought to our attention by such unaffiliated sources as a result of being solicited by us by calls or mailings. These sources may also introduce us to target businesses in which they think we may be interested on an unsolicited basis, since many of these sources will have read the registration statement relating to the IPO and know what types of businesses we are targeting. Our officers and directors,into neurodegenerative diseases as well as our sponsorsolid tumor oncology strategy serve as pillars for our unique NK cell therapies. Key highlights of our strategy include, but are not limited to:
•Advance clinical development of SNK01 in AD. The results obtained thus far with SNK01 in advanced AD patients have revealed the possibility of bringing transformational therapeutic benefits to patients. On October 20, 2023, we received IND clearance from the FDA for SNK01 in AD. On December 21, 2023, we received the No Objection Letter from Health Canada for our clinical trial application of SNK01 in AD. On December 28, 2023, we dosed our first participant in the US on the SNK01-AD01 clinical trial. During 2024 and their affiliates, may also bringbeyond, we intend to (i) advance the clinical development of SNK01 and continue enrolling the Phase I/IIa trial in the United States and Canada for AD, and (ii) continue the Phase I trial with SNK02 in refractory solid tumors.
•Advance clinical development of SNK01 in PD. Preliminary results from patients treated with SNK01 in a compassionate use basis suggests that SNK01 is well-tolerated and has the potential to be a disease-modifying agent in PD. In Q1 2024, we submitted an IND to the FDA to conduct a Phase I trial in PD, and if funding permits, we will continue to evaluate the expansion into other neurodegenerative diseases and accelerate development in oncology through strategic collaborations.
•Develop SNK02 as the backbone for multiple oncology therapies. Based on data generated with SNK02, which shows similar characteristics to SNK01, we believe that our SNK02 allogeneic product candidate presents the opportunity for a scalable off-the-shelf alternative to our attentionautologous SNK01 product. We obtained an IND clearance from the FDA in October 2022 for SNK02 in solid tumors and have begun dosing in Phase I of the clinical trial for SNK02 in August 2023. We are also developing chimeric antigen receptor (“CAR”), derivatives of SNK02 to target business candidatescertain high-prevalence solid tumors.
•Accelerate development in oncology through collaboration. We have identified potential opportunities for SNK cell therapy to significantly enhance the antitumor potential of leading cancer therapeutics such as immune checkpoint inhibitors and therapeutic antibodies. We established a collaboration with Merck KGaA (through AresTrading) to evaluate combinations of SNK01 with avelumab, and anticipate establishing similar partnerships with other biotechnology companies in the future.
•Continue to invest in manufacturing technology. Our SNK manufacturing technology has demonstrated the ability to address certain key limitations of other NK cell manufacturing approaches. We believe we are capable of producing hundreds of thousands of potential doses of NK cell therapies from material collected from a single donor. We believe this is critical in unlocking the therapeutic potential of NK cell therapies. We continue to optimize the industrialization of our processes that they become awarewill be required to address the market opportunities presented by our clinical development activities. Finally, we plan to invest in optimizing and developing the automation of through their business contacts asour processes. We own and operate a result25,000 sq. ft. drug manufacturing facility in Santa Ana, California, of formal or informal inquiries or discussions they maywhich approximately half is equipped for GMP production of NK cells.
Pipeline
Challenges in developing NK cell therapies
Although literature and clinical experience have as well as attending trade shows or conventions. We expect to receiveprovided a rationale for the therapeutic use of NK cells, there have been a number of proprietary deal flow opportunitieschallenges that wouldhave limited NK cells’ clinical potential including, but not otherwise necessarily be availablelimited to.
•Expansion limitations. Existing manufacturing processes have not been optimized for robust production of NK cells from all patients or donors and this unpredictable expansion capability often represents a major hurdle in developing a therapeutic product.
•Low activity during expansion. Extended periods of cell culture, intended to usincrease cell numbers, can lead to loss of cell activity as a resultcompared to the starting population of NK cells and induce senescence.
•Loss of activity following cryopreservation. Cryopreserved NK cells have been reported at an effector to target (E:T) ratio of 10:1 to have about 50 percent decrease in cell-killing activity compared to freshly prepared NK cells.
•Difficult to scale commercially. Because of the business relationshipsrelatively short half-life of NK cells, therapy often requires multiple doses, which is difficult to achieve with existing manufacturing processes.
To date, these limitations have often restricted the ability of other NK cell therapy companies to timely generate truly off-the-shelf allogeneic products where hundreds of thousands of doses may be required to meet clinical needs.
NKGen believes that these factors have led to the treatment of patients with NK cells that fail to demonstrate the full potential of NK cell therapy because of the low activity (as compared to the starting population of NK cells) based on in vitro experiments performed by NKMAX and the low numbers of NK cells that can be delivered with each dose. The combination of low activity (as compared to the starting population of NK cells) and low cell numbers also typically imposes the requirement that patients undergo lymphodepletion to enable NK cell therapy to survive due to competition for cytokine support from other immune cells. NKGen believes that lymphodepletion is counterproductive for a therapy that is intended to stimulate an immune response to disease. Lymphodepletion also often limits the ability to administer repeat doses to patients, especially for NK cell product candidates that must be freshly prepared rather than cryopreserved and prepared for use as needed.
Our Solution — SNK cells
We aim to develop NK cell therapies that address the limitations described by others, by focusing on the optimization of parameters that we believe are critical for NK cell therapy to drive clinical and commercial success. These optimization parameters include, but are not limited to:
•improved cell expansion capabilities;
•production of highly active cells compared to the starting population of NK cells;
•process improvements to enable cryopreservation with minimal loss of NK cell activity pre- and post- freezing; and
•the ability to generate cells using GMP processes at a commercial scale.
NK cells typically comprise between approximately five and fifteen percent of circulating lymphocytes. We isolate NK cells from these blood samples and expands them using a proprietary process generating what refer to as “SNK cells”. SNK cells are then delivered to the patient without the need for preconditioning through lymphodepletion.
Figure 1. Overview of SNK01 autologous process of isolating, expanding, and treating patients with cell therapy.
We refer to our officers and directorsautologous NK cell product candidate as SNK01 and our sponsorallogeneic NK cell product candidate as SNK02.
Our Manufacturing Process
Processes for isolating and their affiliates. While we do not presently anticipate engaging the services of professional firms or other individuals that specialize in business acquisitions on any formal basis, we may engage these firms or other individuals in the future, in which event we may pay a finder’s fee, consulting fee, advisory fee or other compensation to be determined in an arm’s length negotiation based on the terms of the transaction. We will engage a finder only to the extent our management determines thatexpanding NK cells involve the use of cytokines such as interleukin-2, or IL-2; interleukin-15, or IL-15; and interleukin-21, or IL-21; often used in combinations. In some cases, other cells, referred to as feeder cells, are used to provide signaling stimuli to NK cells to increase their activation and proliferation. The reported cell expansion efficiencies of these processes vary widely from approximately five-fold in two weeks to over a finder may bring opportunitiesthousand-fold in the same time period.
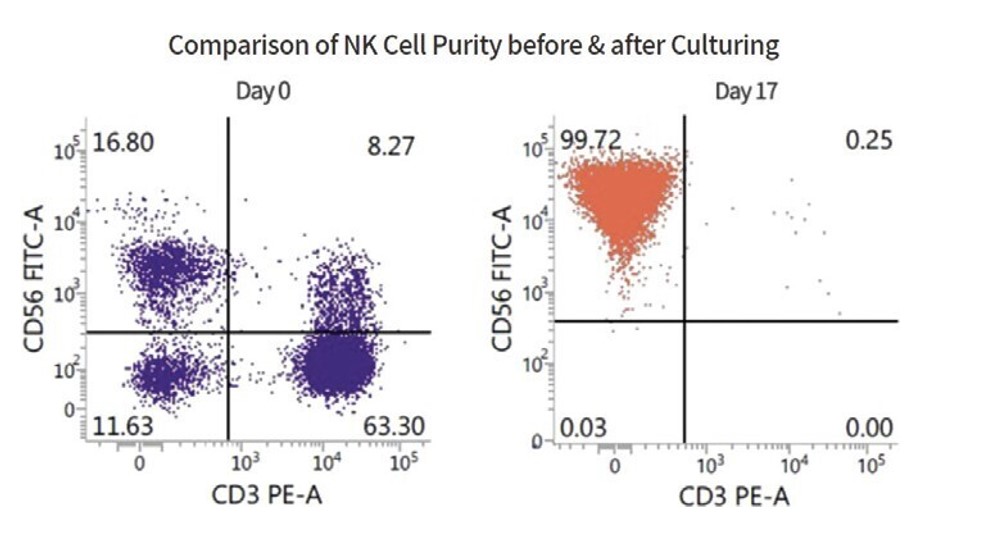
We have developed a proprietary process that combines cytokine stimulation and feeder cell culture that routinely results in expansions over a period of seventeen to useighteen days of several thousand-fold. This process typically yields a population of cells that may not otherwise be available to us or if finders approach us onare greater than 99 percent NK cells as measured by high levels of expression of an unsolicited basis with a potential transaction that our management determines is in our best interest to pursue. Payment of finder’s fees is customarily tied to completionNK cell marker, CD56, and low expression of a transaction,CD3,aT cell marker.
Figure 2. The NKGen manufacturing process results in a highly enriched population of NK cells.
Expandability
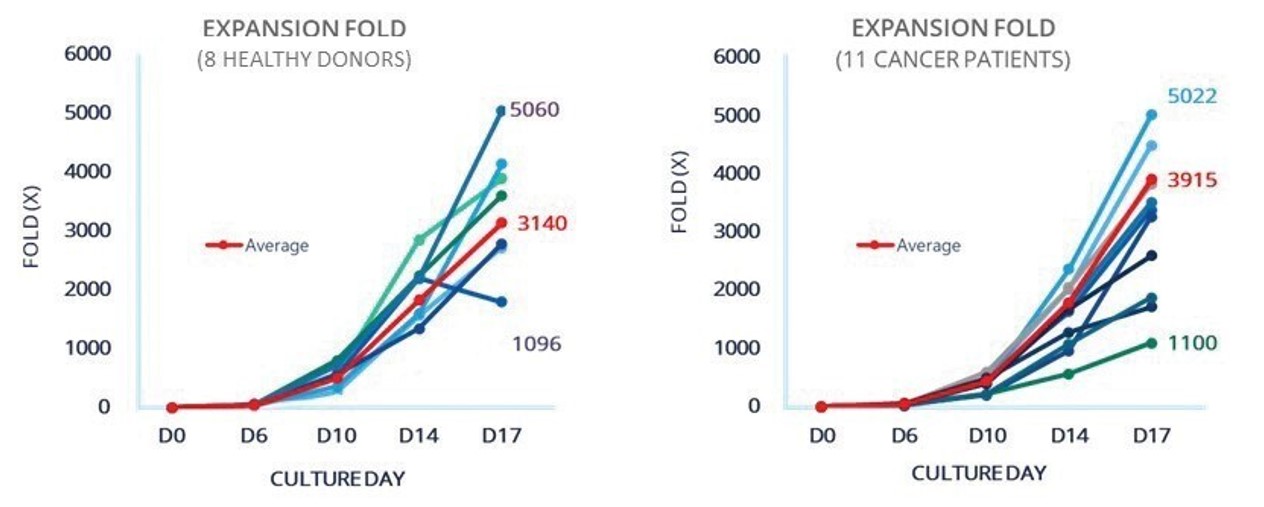
Our process is highly reproducible from patient to patient which is critical for autologous therapies.
Ideally, the goal is for every patient who is recommended for treatment to have access to NK cell therapy on a timely basis, rather than add to their risk of disease progression due to manufacturing failures or delays. We have demonstrated our ability to generate large quantities of SNK01 cells from both healthy donors and cancer patients, the latter being essential for the development of autologous cell therapy for these individuals. This contrasts with traditional methods of autologous NK cell expansion from cancer patients, for which prior cancer treatments negatively affected both the ability to expand NK cells and their activity as compared to the starting population of NK cells, based on in vitro experiments performed by NKMAX.
Figure 3. In vitro experiments performed by NKMAX show NKGen manufacturing process is reproducible in both healthy donors and heavily pretreated cancer patients.
Activity
We designed our manufacturing process to generate NK cells that have increased activity compared to the activity of the initial and expanded NK cells obtained from donors. Importantly for their intended use as therapeutics, our manufacturing process generates SNK01 cells that have similar potencies across donors regardless of the activity of the original donor’s NK cells. The NK cell activity of expanded SNK01 cells from the initial NK cells of three donors was shown in vitro experiments performed by NKMAX to have increased relative to the NK cell activity of donor-matched initial and unexpanded NK cells. This was demonstrated by an increase of cytotoxicity, cytokine expression of IFN-γ and TNF-α, and expression of activating NK cell receptors as described below.
To characterize the cytokine released by expanded NK cells upon short incubations with K562 target cells, expanded NK cells were incubated with target cells and their supernatants that were harvested, and the concentration of 36 different human cytokines and chemokines were determined by a proteome profiler human cytokine array kit by NKMAX. The stimulation of NK cells with K562 cells induced IFN-γ and TNF-α secretion. Moreover, to investigate the ability of cytokine secretion by NK cells, the number of NK cells producing TNF-α and IFN-γ in response to K562 stimulation was analyzed by intracellular staining with initial and unexpanded and expanded NK cells. After stimulation with K562, the expression levels of TNF-α and IFN-γ were increased 68.5-fold and 8.2-fold, respectively, in the expanded NK cells (expressed 7.54% ± 0.10% of TNF-α and 55.30% ± 1.10% of IFN-γ), compared to the initial and unexpanded NK cells (expressed 0.11% ± 0.01% of TNF-α and 6.75% ± 0.60% of IFN-γ). In addition, the expression levels of TNF-α and INF-γ upon stimulation with K562 cells were increased 2.9-fold and 1.6-fold, respectively, in the simulated expanded NK cells (expressed 7.54 ± 0.10% of TNF-α and 55.30 ± 1.10% of IFN-γ), compared to the unstimulated expanded NK cells (expressed 2.60 ± 0.10% of TNF-α and 33.80 ± 0.50% of IFN-γ).
To characterize cytotoxicity of expanded NK cells toward K562 target cells, a comparison of cancer cell killing ability was done before and after culturing of SNK01. The cytotoxicity of SNK01 was shown to have increased several folds (i.e., 2.0- to 10.9-fold) higher than before NK Cell expansion from the same donor sample. Refer to Figure 4 in which
case any such fee will be paid outdonor 1 cytotoxicity went from less than 8.4% to approximately 91.8%, donor 2 cytotoxicity went from approximately 21.60% to approximately 90.1%, and donor 3 cytotoxicity went from approximately 44.70% to approximately 91.0%. In an NK cell cytotoxicity assay, the cancer cell killing ability percentage represents the proportion of
the funds held in the trust account. In no event, however, will our sponsor or any of our existing officers or directors, or any entity with which our sponsor or officers are affiliated, be paid any finder’s fee, reimbursement, consulting fee, monies in respect of any payment of a loan or other compensationtarget (cancer) cells that have been killed by the
company for services rendered prior to, or for any services rendered in order to effectuate, the completion of our initial business combination (regardless of the type of transaction that it is). Some of our officers and directors may enter into employment or consulting agreements with the post-transaction company following our initial business combination. The presence or absence of any such fees or arrangements will not be used as a criterion in our selection process of an initial business combination candidate.We are not prohibited from pursuing an initial business combination with an initial business combination target that is affiliated with our sponsor, officers or directors or making the initial business combination through a joint venture or other form of shared ownership with our sponsor, officers or directors. In the event we seek to complete our initial business combination with an initial business combination target that is affiliated with our sponsor, officers or directors, we, or a committee of independent directors, would obtain an opinion from an independent investment banking firm or another independent valuation or appraisal firm that regularly prepares fairness opinions that such an initial business combination is fair to our company from a financial point of view. We are not required to obtain such an opinion in any other context.
Selection of a target business and structuring of our initial business combination
We may pursue an initial business combination target in any business or industry or geographic region, which may include, without limitation, targets in industries such as mobility, technology, transportation, new energy, software, infrastructure, consumer,
defense and cybersecurity, business and real estate services, financial and data services, healthcare, diversified industrial manufacturing, technology, distribution and services, as well as companies that help to address evolving environmental, social and governance (“ESG”) related issues.
New York Stock Exchange (the “NYSE”) rules require that we must consummate an initial business combination with one or more operating businesses or assets with a fair market value equal to at least 80% of the net assets held in the trust account (net of amounts disbursed to management for working capital purposes, if permitted, and excluding the amount of any deferred underwriting commissions). Our board of directors will make the determination as to the fair market value of our initial business combination. If our board of directors is not able to independently determine the fair market value of our initial business combination, we will obtain an opinion from an independent investment banking firm or another independent valuation or appraisal firm that regularly provides fairness opinions solely with respect to the satisfaction of such criteria. While we consider it unlikely that our board will not be able to make such independent determination of fair market value, it may be unable to do soNK cells. For example, if the board is less familiar or experienced with the target company’s business, there isassay in Figure 4 below shows a significant amount of uncertainty as to the value of the company’s assets or prospects, including if such company is at an early stage of development, operations or growth, or if the anticipated transaction involves a complex financial analysis or other specialized skills and the board determines90% cytotoxicity, it means that outside expertise would be helpful or necessary in conducting such analysis. Since any opinion, if obtained, would merely state that the fair market value meets the 80% of net assets test, unless such opinion includes material information regarding the valuation of a target business or the consideration to be provided, it is not anticipated that copies of such opinion would be distributed to our stockholders. However, if required under applicable law, any proxy statement that we deliver to stockholders and file with the SEC in connection with a proposed transaction will include such opinion.
We anticipate structuring our initial business combination so that the post-transaction company in which our public stockholders own shares will own or acquire 100% of the equity interests or assetsapproximately 90% of the target business or businesses. We may, however, structure our(cancer) cells have been killed by the NK cells, while the remaining 10% are still alive. Higher percentages indicate greater cytotoxic activity and a more effective response by the NK cells.
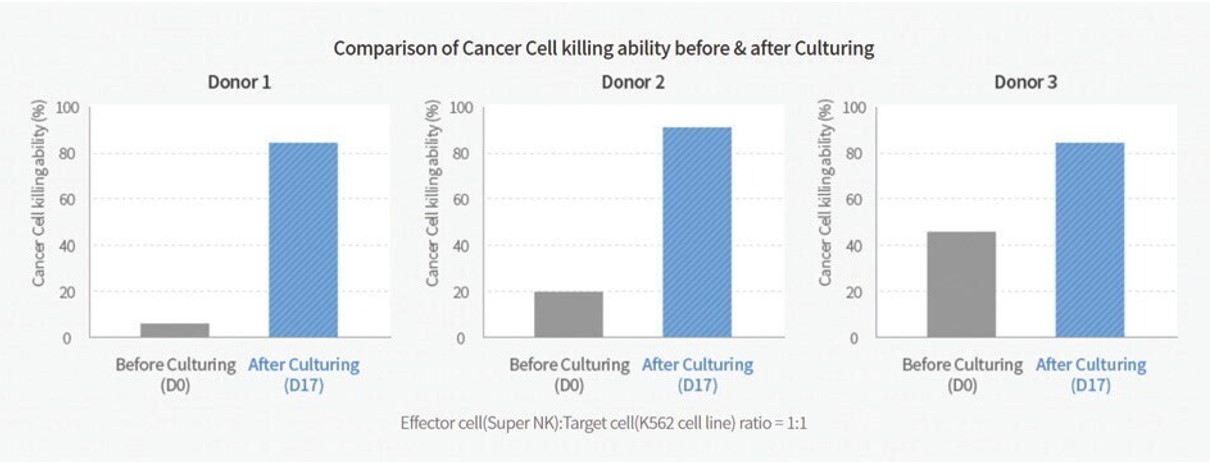
Figure 4. In in vitro experiments performed by NKMAX, SNK01 cells have demonstrated increased activity as compared to the NK cells from which they were derived.
To investigate the change in the percentage expression level of NK cell activating receptor on expanded NK cells, both expanded and initial business combination suchNK cells were stained with antibodies for NK cell activating receptors of CD16, NKp30, NKp46, NKp44, and NKG2D, and then analyzed by flow cytometry. There was an increase of 1.2-fold for CD16 (expressed 97.21 ± 1.76%), 5.8-fold for NKp30 (expressed 97.32 ± 3.58%), 135.1-fold for NKp44 (expressed 62.15 ± 14.88%), 2.3-fold for NKp46 (expressed 93.98 ± 6.59%), and 1.1-fold for NKG2D (expressed 99.88 ± 0.10%) in the expanded NK cells, compared to initial unexpanded NK cells (expressed 80.83 ± 14.63% for CD16, 16.81 ± 13.06% for NKp30, 0.46 ± 0.38% for NKp44, 40.32 ± 23.07 for NKp46, and 91.29 ± 8.18% for NKG2D). Please see Figure 7 and Figure 8 below for more details.
The results of these in vitro experiments show significant increases with p values below 0.05 from the three donors. However, no statistical analyses were performed in a larger population of donors. Accordingly, we cannot guarantee that the post- transaction company ownsresults for every donor or acquires less than 100%the median donor would have been statistically significant in a larger population.
Cryopreservation
We have developed a cryopreservation method that preserves not only the viability of such interests or assetsSNK cells but, more importantly, an increased level of their activity. We have shown that the target business in order to meet certain objectivescell-killing activity of both unmodified SNK cells and genetically modified CAR NK cells are largely preserved after thawing. We believe that the target management team or stockholders or for other reasons, but we will only complete such business combination if the post-transaction company owns or acquires 50% or morehigh activity of the outstanding voting securities of the target or otherwise acquires a controlling interest in the target sufficient for it not to be required to registerSNK cells, as an investment company under the Investment Company Act. Even if the post-transaction company owns or acquires 50% or more of the voting securities of the target, our stockholders priorcompared to the business combination may collectively own a minority intereststarting population of NK cells, combined with the slightly decreased activity observed during cryopreservation enables the company to generate off-the-shelf NK cell product candidates that are more active than many freshly prepared NK cells generated by other methods.
Figure 5. In in vitro experiments performed by NKMAX, our cryopreservation method preserves the post-transaction company, depending on valuations ascribedincreased activity of SNK product candidates (as compared to the target and us in the business combination transaction. For example, we could pursue a transaction in which we issue a substantial numberstarting population of new shares in exchange for allNK cells).
Scaling
We believe our manufacturing process is highly scalable. Cells that are produced show little loss of
the outstanding capital stock of a target. In this case, we would acquire a 100% controlling interest in the target. However,activity as
a result of the issuance of a substantial number of new shares, our stockholders immediately prior to our initial business combination could own less than a majority of our outstanding shares subsequent to our initial business combination. If less than 100% of the equity interests or assets of a target business or businesses are owned or acquired by the post-transaction company, the portion of such business or businesses that is owned or acquired is what will be taken into account for purposes of NYSE’s 80% of net assets test. If the business combination involves more than one target business, the 80% of net assets test will be based on the aggregate value of all of the target businesses.To the extent we effect our initial business combination with a company or business that may be financially unstable or in its early stages of development or growth we may be affected by numerous risks inherent in such company or business. Although our management will endeavor to evaluate the risks inherent in a particular target business, we cannot assure you that we will properly ascertain or assess all significant risk factors.
In evaluating a prospective target business, we expect to conduct a due diligence review which may encompass, among other things, meetings with incumbent management and employees, document reviews, interviews of customers and suppliers, inspections of facilities, as well as reviewing financial and other information made available to us and other reviews as we deem appropriate. We may also retain consultants with expertise relating to a prospective target business.
The time required to select and evaluate a target business and to structure and complete our initial business combination, and the costs associated with this process, are not currently ascertainable with any degree of certainty. Any costs incurred with respectcompared to the identification and evaluationstarting population of a prospective target business with which our initial business combinationNK cells, nor is not ultimately completedthere evidence of senescence even after extended periods of cell culture. This potential scalability will result in our incurring losses and will reduceenable the funds we can usecompany to complete another business combination.
Lackthousands of Business Diversification
For an indefinite perioddoses of time after the completion of our initial business combination, the prospects for our success may depend entirely on the future performance ofallogeneic SNK cells from a single business. Unlike other entities that have the resources to complete business combinations with multiple entities in one or several industries, it is probable that we will not have the resources to diversify our operations and mitigate the risks of being in a single line of business in a single industry. By completing our initial business combination with only a single entity, our lack of diversification may:
| ● | subject us to negative economic, competitive and regulatory developments, any or all of which may have a substantial adverse impact on the particular industry in which we operate after our initial business combination, and |
| ● | cause us to depend on the marketing and sale of a single product or limited number of products or services. |
Limited Ability to Evaluate the Target’s Management Team
Although we intend to closely scrutinize the management of a prospective target business when evaluating the desirability of effecting our initial business combination with that business, our assessment of the target business’ management may not prove to be correct. In addition, the future management may not have the necessary skills, qualifications or abilities to manage a public company. Furthermore, the future role of members of our management team, if any, in the target business cannot presently be stated with any certainty. The determination as to whether any of the members of our management team will remaindonor. Combined with the combined company will be made at the time of our initial business combination. While it is possible that one or more of our directors will remain associated in some capacity with us following our initial business combination, it is unlikely that any of them will devote their full effortsability to our affairs subsequent to our initial business combination.
Moreover,cryopreserve these cells, we cannot assure you that members of our management team will have significant experience or knowledge relating to the operations of the particular target business.
We cannot assure you that any of our key personnel will remain in senior management or advisory positions with the combined company. The determination as to whether any of our key personnel will remain with the combined company will be made at the time of our initial business combination.
Following an initial business combination, we may seek to recruit additional managers to supplement the incumbent management of the target business. We cannot assure youbelieve that we will have the abilitycapacity to recruit additional managers, or that additional managers willoffer off-the-shelf cell therapy solutions to patients. However, we have not yet developed a validated method of manufacturing our product candidates for long- term storage, in large quantities without damage, in a cost-efficient manner and without degradation beyond two years. We own and operate a 25,000-square-foot drug manufacturing facility in Santa Ana, California, of which approximately half is equipped for GMP production of NK cells.
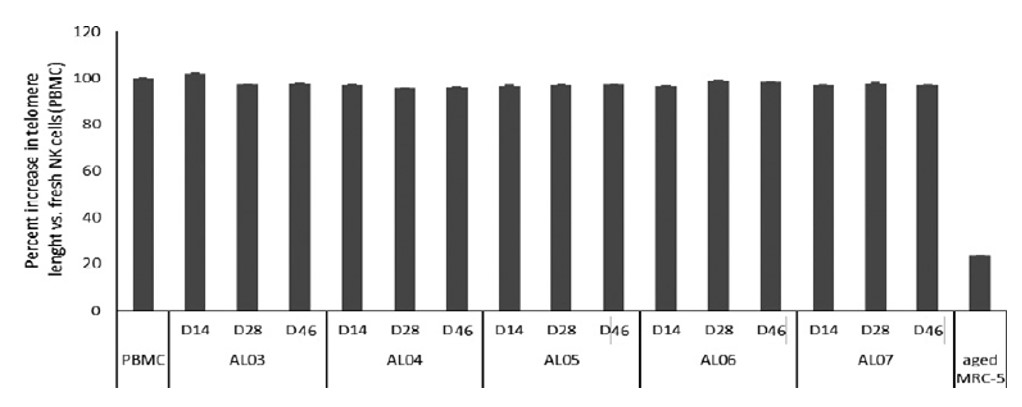
Figure 6. No evidence of senescence, measured by telomere length, with extended expansion of SNK02 cells from multiple donors.
Molecular characteristics of SNK01
The levels of a group of natural cytotoxicity receptors (“NCRs”), including NKp30, NKp46 and NKp44, typically increase during the requisite skills, knowledge or experience necessarymanufacturing of SNK01. These receptors have been shown to enhancebe critical for the incumbent management.Stockholders May Not Haverecognition and elimination of tumor cells. One of the Abilityligands for NKp30, for example, is B7-H6, a common tumor antigen. The binding of NKp30 to Approve Our Initial Business Combination
We may conduct redemptions without a stockholder vote pursuantB7-H6 leads to the tender offer rulessecretion of cytokines such as TNF-alpha and IFN-gamma, and cell lysis perforins and granzymes. Expression of NKp30 has also been shown to correlate with overall improved survival and better prognosis in gastrointestinal stromal tumors. Whereas resting NK cells routinely express low levels of NKp30 and NKp46, NKp44 is only found on activated NK cells. Cell surface receptor expression between the starting (primary) NK cells and the expanded SNK cells have also been shown to increase several fold. In Figures 7 and 8, the cell receptor NKp30 went from approximately 0% to 95%, approximately 20% to 100%, and approximately 30% to 100% in donors 1, 2 and 3, respectively. The cell receptor NKp46 went from approximately 10% to 80%, approximately 40% to 100%, and approximately 60% to 100% in donors 1, 2 and 3, respectively. The cell receptor NKp44 went from approximately 0% to 45%, approximately 0% to 65%, and approximately 0% to 75%, in donors 1, 2 and 3, respectively. The cell receptor NKG2D went from approximately 70% to 100%, approximately 75% to 100%, and approximately 75% to 100% in donors 1, 2 and 3, respectively. Its high level of expression on SNK01 along with the elevated levels of NKp30, NKp44 and NKp46 serves as a confirmation of the SEC. However, we will seek stockholder approval if it is required by law or applicable stock exchange rule, or we may decide to seek stockholder approval for business or other legal reasons. Presented in the table below is a graphic explanationactivation state of the types of initial business combinations we may consider and whether stockholder approval is currently required under Delaware law for each such transaction.
| | |
TYPE OF TRANSACTION
|
| WHETHER STOCKHOLDER APPROVAL IS REQUIRED
|
Purchase of assets
| SNK01.
| No
|
Purchase of stock of target not involving a merger with the company
|
| No
|
Merger of target into a subsidiary of the company
|
| No
|
Merger of the company with a target
|
| Yes
|
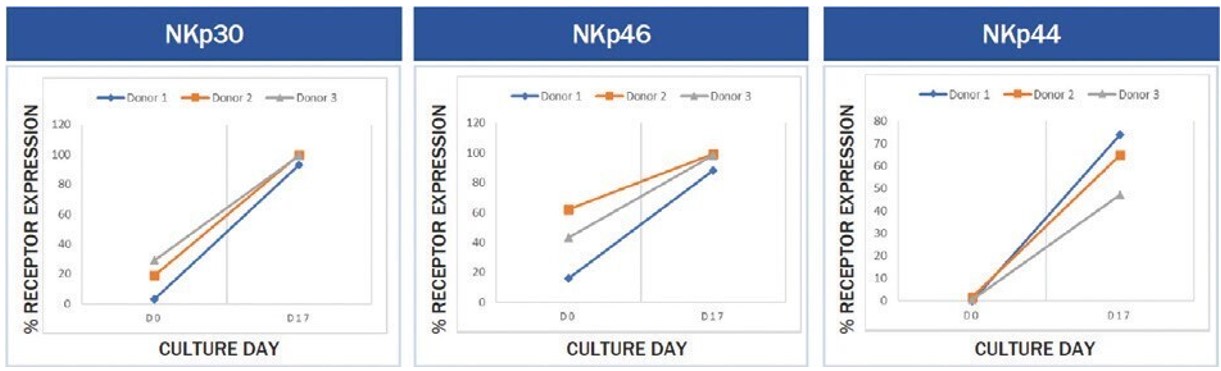
UnderFigure 7. In in vitro experiments performed by NKMAX, increased expression of NCRs during the NYSE’s listing rules, stockholder approval would be requiredmanufacturing of SNK01 was observed across donors.
SNK01 cells also typically have high expression of NKG2D, a master regulator of immune response, and DNAM-1, a receptor that is essential for our initial business combination if,NK-cell mediated lysis of damaged cells such as tumor cells.
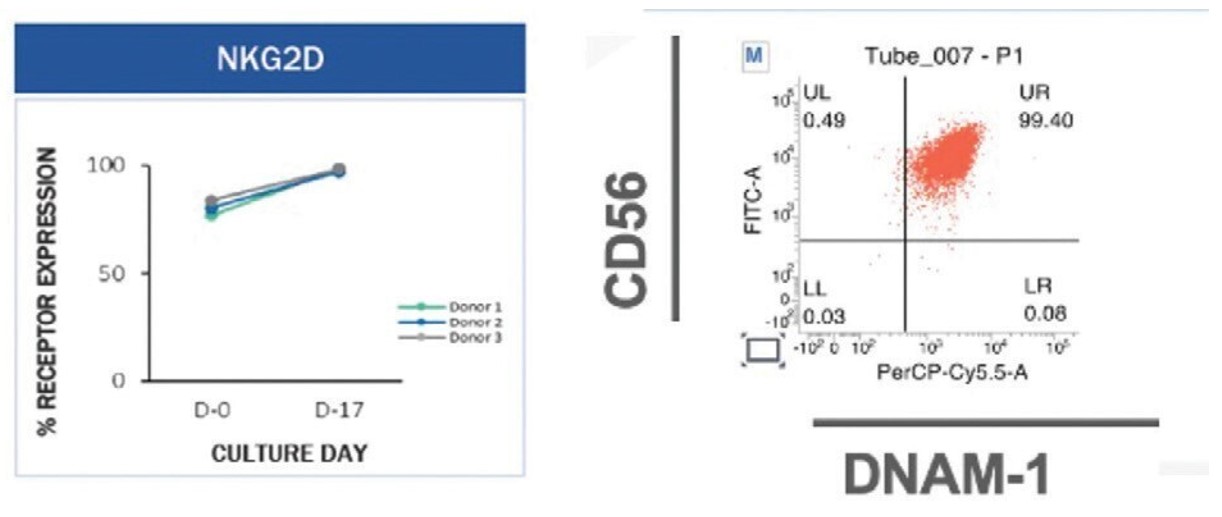
Figure 8. SNK01 cells have high expression of NKG2D and DNAM-1. SNK01 for
example: | ● | we issue (other than in a public offering for cash) shares of common stock that will either (a) be equal to or in excess of 20% of the number of shares of common stock then outstanding or (b) have voting power equal to or in excess of 20% of the voting power then outstanding; |
| ● | any of our directors, officers or substantial security holders (as defined by the NYSE rules) has a 5% or greater interest, directly or indirectly, in the target business or assets to be acquired and if the number of shares of common stock to be issued, or if the number of shares of common stock into which the securities may be convertible or exercisable, exceeds either (a) 1% of the number of shares of common stock or 1% of the voting power outstanding before the issuance in the case of any of our directors and officers or (b) 5% of the number of shares of common stock or 5% of the voting power outstanding before the issuance in the case of any substantial security holders; or |
| ● | the issuance or potential issuance of common stock will result in our undergoing a change of control. |
Permitted Purchasesthe treatment of our Securities
If we seek stockholder approvalneurodegenerative diseases
We are developing SNK01, an autologous NK cell therapy, for the treatment of our initial business combinationneurodegenerative diseases including AD and PD. Results from case studies of SNK01 administered on a compassionate use basis have shown reversal of several symptoms in patients with advanced stages of these diseases. These results stand in contrast to many of those reported for other therapies in neurodegenerative diseases, for which meaningful improvements in parameters such as cognition have rarely been observed. We believe that SNK01 has the potential to transform the treatment of these debilitating diseases and we do not conduct redemptionsare initiating clinical trials to explore these early signals.
Industry and Competition
The biotechnology industry in
connection with our initial business combination pursuant to the tender offer rules, our sponsor, initial stockholders, directors, officers, advisors or their affiliates may purchase shares or public warrants in privately negotiated transactions or in the open market either prior to or following the completion of our initial business combination. There is no limit on the number of shares our initial stockholders, directors, officers, advisors or their affiliates may purchase in such transactions, subject to compliance with applicable law. However, they have no current commitments, plans or intentions to engage in such transactions and have not formulated any terms or conditions for any such transactions. If they engage in such transactions, they will not make any such purchases when they are in possession of any material non-public information not disclosed to the seller or if such purchases are prohibited by Regulation M under the Exchange Act. We do not currently anticipate that such purchases, if any, would constitute a tender offer subject to the tender offer rules under the Exchange Act or a going-private transaction subject to the going-private rules under the Exchange Act; however, if the purchasers determine at the time of any such purchases that the purchases are subject to such rules, the purchasers will comply with such rules. Any such purchases will be reported pursuant to Section 13 and Section 16 of the Exchange Act to the extent such purchasers are subject to such reporting requirements. None of the funds held in the trust account will be used to purchase shares or public warrants in such transactions prior to completion of our initial business combination.The purpose of any such purchases of shares could be to vote such shares in favor of the initial business combination and thereby increase the likelihood of obtaining stockholder approval of the initial business combination or to satisfy a closing condition in an agreement with a target that requires us to have a minimum net worth or a certain amount of cash at the closing of our initial business combination, where it appears that such requirement would otherwise not be met. The purpose of any such purchases of public warrants could be to reduce the number of public warrants outstanding or to vote such warrants on any matters submitted to the warrant holders for approval in connection with our initial business combination. Any such purchases of our securities may result in the completion of our initial business combination that may not otherwise have been possible. In addition, if such purchases are made, the public “float” of our shares of common stock or warrants may be reducedgeneral, and the number of beneficial holders of our securities may be reduced, which may make it difficult to maintain or obtain the quotation, listing or trading of our securitiescell therapy field in particular, is characterized by rapidly advancing and changing technologies, intense competition and a strong emphasis on a national securities exchange.
Our sponsor, officers, directors and/or their affiliates anticipate that they may identify the stockholders with whom our sponsor, officers, directors or their affiliates may pursue privately negotiated purchases by either the stockholders contacting us directly or by our receipt of redemption requests submitted by stockholders following our mailing of proxy materials in connection with our initial business combination. To the extentintellectual property. While we believe that our sponsor, officers, directors, advisors or their affiliates enter into a private purchase, they would identifyapproach, strategy, technology, knowledge, and contact only potential selling stockholders who have expressed their election to redeem their shares for a pro rata share of the trust account or vote against our initial business combination, whether or not such stockholder has already submitted a proxyexperience provide us with competitive advantages, we face substantial competition with respect to our initial business combination. Our sponsor, officers, directors, advisorsproduct candidates currently in development and will face competition with respect to other product candidates that we may seek to develop or commercialize in the future. Sources of competition include major pharmaceutical, specialty pharmaceutical, and existing or emerging biotechnology companies, academic research institutions and governmental agencies and public and private research institutions worldwide. Many of our competitors, either alone or with their affiliates will only purchase shares ifcollaborators, have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. As a result, they may be able to develop and commercialize their product candidates at a faster rate. Mergers and acquisitions in the biotechnology industry may result in even greater resource concentration among a smaller number of competitors. Smaller or early-stage companies may also prove to be significant competitors, either alone or through collaborative arrangements with large and established companies.
We currently face competition from Acepodia, Artiva, Celularity, Century Therapeutics, Cytovia Therapeutics, Fate Therapeutics, Nkarta, and ImmunityBio each of which have clinical-stage allogeneic programs. In addition, other competitors, such
purchases comply with Regulation M under the Exchange Actas Affimed, Innate Pharma, Dragonfly Therapeutics and
the other federal securities laws.Any purchases by our sponsor, officers, directors and/or their affiliates whoGT Biopharma, are affiliated purchasers under Rule 10b-18 under the Exchange Act will only be madeseeking to harness NK biology through cell engagers that direct a patient’s own NK cells to the extent such purchasessite of a tumor. We are not aware of any other NK cell companies that have received FDA approvals of NK cell therapies to-date. However, it is also possible that new competitors, including those developing similar cellular immunotherapy product candidates or alternatives to immunotherapy, may emerge and acquire significant market share.
These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient enrollment in clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. As a result, our competitors may discover, develop, license or commercialize products before or more successfully than we do.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for our products, which could result in our competitors establishing a strong market position before we are able to be madeenter the market. The key competitive factors affecting the success of all of our programs are likely to include their efficacy, safety, convenience, price and degree of reimbursement.
Licensing Agreements
We entered into license agreements in compliance with Rule 10b-18, whichthe ordinary course of its business. We have in licensed certain technology from NKMAX that is a safe
harbor from liability for manipulation under Section 9(a)(2)necessary to research and Rule 10b-5develop its NK cell program. Because of the Exchange Act. Rule 10b-18 has certain technical requirements that must be complied with in order for the safe harbor to be available to the purchaser. Our sponsor, officers, directors and/or their affiliates will not make purchases of common stock if the purchases would violate Section 9(a)(2) or Rule 10b-5 of the Exchange Act. Any such purchases will be reported pursuant to Section 13 and Section 16 of the Exchange Act to the extent such purchases are subject to such reporting requirements.
Redemption Rights for Public Stockholders upon Completionbroad potential applicability of our Initial Business Combination
We will providetherapeutic candidates, we may, but do not currently have plans to, out license our public stockholders with the opportunitytechnology to redeem all or a portion of their shares of common stock upon the completion of our initial business combination at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account as of two business days prior to the consummation of the initial business combination including interest (which interest shall be net of taxes payable), divided by the number of then outstanding public shares, subject to the limitations described herein. The amount in the trust account is initially anticipated to be approximately $10.00 per public share. The per-share amount we will distribute to investors who properly redeem their shares will not be reduced by the deferred underwriting commissions payable to our underwriters. Our sponsor, executive officers and directors have entered into a letter agreement with us, pursuant to which they have agreed to waive their redemption rights with respect to any founder shares and any public shares held by them in connection with the completion of our initial business combination.
Manner of Conducting Redemptions
We will provide our public stockholders with the opportunity to redeem all or a portion of their shares of common stock upon the completion of our initial business combination either (i) in connection with a stockholder meeting called to approve the initial business combination or (ii) by means of a tender offer. The decision as to whether we will seek stockholder approval of a proposed initial business combination or conduct a tender offer will be made by us, solely in our discretion, and will be based on a variety of factors such as the timing of the transaction and whether the terms of the transaction would require us to seek stockholder approval under the law or stock exchange listing requirement. Asset acquisitions and stock purchases would not typically require stockholder approval while direct mergers with our company wherethird parties for development for other purposes that we do not survive and any transactions whereintend to pursue or for certain territories.
NKMAX License
On February 12, 2020, we issue more than 20% of our outstanding common stock or seek to amend ourentered into that certain License Agreement with NKMAX (the “Original License”), which was amended and restated certificateby that certain Amended License Agreement that we entered into with NKMAX on October 14, 2021, April 10, 2023 and August 1, 2023 (“Intercompany License”). Under the Intercompany License, NKMAX granted us an exclusive, royalty-bearing license, with the right to sublicense through multiple tiers, under certain patents and know-how of incorporation would require stockholder approval.If we structure an initial businessNKMAX in any fields of use to (i) research, develop, manufacture, have manufactured, use and commercialize NK cell pharmaceutical product, process, service or therapy or a combination of any of the forgoing with a target companyany other active ingredient, product or service (the “
Licensed Products”) in a manner that requires stockholder approval, we will notall countries excluding the countries and territories in Asia (the “Licensed Territory”) and (ii) research, develop, have discretion asmanufactured and manufacture Licensed Products outside of the Licensed Territory solely to whether to seek a stockholder vote to approvesupport our rights in the proposed initial business combination. We may conduct redemptions without a stockholder vote pursuantLicensed Territory. Pursuant to the
tender offer rulesIntercompany License, we granted NKMAX an exclusive, royalty-free, fully-paid, irrevocable, perpetual license, with very limited exception and the right to sublicense through multiple tiers, under certain of its patents and know-how for all fields of use to research, develop, manufacture, have manufactured, use and commercialize any Licensed Products in Asia. We also granted NKMAX a non-exclusive, royalty-free, fully-paid license, with the right to sublicense through multiple tiers, under certain of its patents and know-how for all fields of use for the purpose of manufacturing and having manufactured the Licensed Products outside of Asia solely for the purpose of development, manufacture, having manufactured and commercialization of the
SEC unless stockholder approval is required by lawLicensed Products in Asia. We reserved the non-exclusive right under these licenses and our interest in any joint inventions or
stock exchange listing requirementsjoint patents to make and have made Licensed Products in Asia solely for the purpose of development, manufacture or
we choose to seek stockholder approval for business or other legal reasons.If a stockholder vote is not requiredhave manufactured, and we do not decide to hold a stockholder vote for business or other legal reasons, we will, pursuant to our amended and restated certificatecommercialization of incorporation:
| ● | conduct the redemptions pursuant to Rule 13e-4 and Regulation 14E of the Exchange Act, which regulate issuer tender offers, and |
| ● | file tender offer documents with the SEC prior to completing our initial business combination which contain substantially the same financial and other information about the initial business combination and the redemption rights as is required under Regulation 14A of the Exchange Act, which regulates the solicitation of proxies. |
Upon the public announcement of our initial business combination, we or our sponsor will terminate any plan established in accordance with Rule 10b5-1 to purchase shares of our common stockLicensed Products in the open market if we electLicensed Territory for all fields of use.
As partial consideration for the rights granted to
redeem our public shares through a tender offer, to comply with Rule 14e-5us under the
Exchange Act.In the eventIntercompany License, we conduct redemptions pursuant to the tender offer rules, our offer to redeem will remain open for at least 20 business days, in accordance with Rule 14e-1(a) under the Exchange Act, and we will not be permitted to complete our initial business combination until the expirationpreviously paid an upfront fee of the tender offer period. In addition, the tender offer will be conditioned on public stockholders not tendering more than a specified number of public shares which are not purchased by our sponsor, which number will be based on the requirement that we may not redeem public shares in an amount that would cause our net tangible assets to be less than $5,000,001 upon
consummation of our initial business combination (so that we are not subject to the SEC’s “penny stock” rules) or any greater net tangible asset or cash requirement which may be contained in the agreement relating to our initial business combination. If public stockholders tender more shares than we have offered to purchase, we will withdraw the tender offer and not complete the initial business combination.
If, however, stockholder approval of the transaction is required by law or stock exchange listing requirement, or we decide to obtain stockholder approval for business or other legal reasons, we will, pursuant to our amended and restated certificate of incorporation:
| ● | conduct the redemptions in conjunction with a proxy solicitation pursuant to Regulation 14A of the Exchange Act, which regulates the solicitation of proxies, and not pursuant to the tender offer rules, and |
| ● | file proxy materials with the SEC. |
In the event that we seek stockholder approval of our initial business combination, we will distribute proxy materials and, in connection therewith, provide our public stockholders with the redemption rights described above upon completion of the initial business combination.
If we seek stockholder approval, we will complete our initial business combination only if a majority of the outstanding shares of common stock voted are voted in favor of the initial business combination. A quorum for such meeting will consist of the holders present in person or by proxy of shares of outstanding capital stock of the company representing a majority of the voting power of all outstanding shares of capital stock of the company entitled to vote at such meeting. Our initial stockholders will count toward this quorum and pursuant to the letter agreement, our sponsor, executive officers and directors have agreed to vote their founder shares and any public shares purchased during or after the IPO (including in open market and privately negotiated transactions) in favor of our initial business combination. For purposes of seeking approval of the majority of our outstanding shares of common stock voted, non-votes will have no effect on the approval of our initial business combination once a quorum is obtained. As a result, in addition to our initial stockholders’ founder shares, we would need only 6,435,563, or 37.5%, of the 17,161,500 public shares sold in the IPO to be voted in favor of an initial business combination (assuming all outstanding shares are voted) in order to have our initial business combination approved. These quorum and voting thresholds, and the voting agreements of our initial stockholders, may make it more likely that we will consummate our initial business combination. Each public stockholder may elect to redeem its public shares irrespective of whether they vote for or against the proposed transaction.
Our amended and restated certificate of incorporation provides that in no event will we redeem our public shares in an amount that would cause our net tangible assets to be less than $5,000,001 upon consummation of our initial business combination (so that we are not subject to the SEC’s “penny stock” rules) or any greater net tangible asset or cash requirement which may be contained in the agreement relating to our initial business combination. For example, the proposed initial business combination may require: (i) cash consideration to be paid to the target or its owners, (ii) cash to be transferred to the target for working capital or other general corporate purposes or (iii) the retention of cash to satisfy other conditions$1.0 million in accordance with the terms of the proposed initial business combination. In the event the aggregate cash consideration we would beOriginal License. We are also required to pay one-time milestone payments for all sharesthe first receipt of common stock thatregulatory approval by us or any of our affiliates for a Licensed Product in the following jurisdictions (and amounts): the United States ($5.0 million), the European Union ($4.0 million), and four other countries ($1.0 million each). To date, we have not paid any milestone payments. We are validly submitted for redemption plus any amountobligated to pay a mid-single digit royalty on net sales of Licensed Products by us, our affiliates or our sublicensees, subject to customary reductions. Our royalty obligations continue on a Licensed Product-by-Licensed Product and country-by-country basis until the expiration of the last-to- expire valid claim of the patents licensed to us under the Intercompany License claiming such Licensed Product in such country of sale. We are also required to satisfy cash conditions pursuantpay a percentage of our sublicensing revenue ranging from a low double-digit percentage to the termsa mid-single digit percentage.
We may require our public stockholders seekingunilaterally terminate the Intercompany License for any reason with a specified prior notice period, and NKMAX may terminate the Intercompany License if we fail to exercise their redemption rights, whether they are record holders or hold their shares in “street name,” to either tender their certificates to our transfer agent up to two business daysmake any required payments under the Intercompany License after a specified cure and prior tonotice period. Either party may terminate the vote on the proposal to approve the initial business combinationIntercompany License in the event of the other party’s insolvency or for the other party’s uncured material breach of the Intercompany License. Absent early termination, the Intercompany License will automatically expire upon the expiration of our obligations to pay royalties unless we distribute proxy materials,mutually agree in writing to extend the term. Upon the expiration but not earlier termination of the Intercompany License, the licenses granted to us by NKMAX and the licenses we granted to NKMAX will survive on a royalty free, fully paid, irrevocable and perpetual basis. However, if we terminate the Intercompany License for the insolvency of NKMAX or an uncured material breach by NKMAX, the licenses we granted to NKMAX will terminate and revert to us. If we voluntarily terminate the Intercompany License, we agree to provide, upon the request of NKMAX all existing data in support of registration of Licensed Products with all regulatory authorities in the Licensed Territories, and NKMAX will have the unrestricted right to provide such data to third parties.
In April 2023, we executed an amendment to the Original License to expand the scope of Licensed Products initially limited to cancer treatment to any field of use.
Manufacturing
Our processes for cellular therapeutic candidates are designed to generate both autologous and allogeneic products.
Autologous process (SNK01)
Our manufacturing processes are designed to generate a consistent quality of activated cells, including cells sourced from cancer patients who are known to be immunocompromised, and to reduce the risk of manufacturing issues that could deprive patients of their desired therapy products. We aim to produce cryopreserved doses of NK cells for eight to 12 months of bi-weekly infusions through a one-time process, without compromising the NK cells’ activity.
The manufacturing process for SNK01 can be summarized as follows: The source material for SNK01 is primary NK cells that are isolated from either peripheral blood or leukapheresis from patients. These primary NK cells are then activated and expanded for up to 18 days using our proprietary methodology that involves two types of feeder cells and cytokines. The result is a cryopreserved product, achieved through the implementation of its proprietary cryopreservation method.
To have a steady clinical supply of SNK01 available, we established our own GMP manufacturing capabilities. This strategic move facilitates clinical product supply and mitigates the risk associated with manufacturing disruptions, while ultimately enabling a more cost-effective supply of SNK01 for commercial purposes. In 2019, we completed the construction of a new 25,000-square-foot clinical GMP facility, situated at our headquarters in Santa Ana, California, approximately half of which is fit for GMP production of NK cells. The implementation of GMP hardware and a robust Quality Management System (“QMS”) was finalized in the same year, encompassing manufacturing equipment, laboratory facilities, warehousing and a dedicated and cryo-storage area. Following a comprehensive qualification process, including multiple test runs, we commenced manufacturing operations for its U.S. oncology clinical trial in 2020. We believe that our clinical GMP facility is capable of producing approximately 12,000 doses of cryopreserved drug product per year, thus adequately meeting the anticipated demands of our clinical trials.
Allogeneic process (SNK02)
We have developed a manufacturing process for our allogeneic off-the-shelf NK cell therapy product, SNK02, building upon our manufacturing process for its autologous product, SNK01. Our primary focus has been on scalability, reproducibility, cost-effectiveness, and maintaining consistent activity of SNK02 post cryopreservation. To achieve these goals, our manufacturing process incorporates, without limitation, the following key elements:
•source cells from healthy donor’s peripheral blood, ensuring they meet the eligibility criteria for allogeneic donation;
•activation and expansion technologies that allow NKGen to generate hundreds of thousands of doses from a single donor, without senescence or exhaustion;
•cryopreservation techniques that enable bulk SNK02 product to be effectively frozen, ensuring its long-term stability; and
•thawing techniques for the frozen NK cell product that are user-friendly and adaptable to different clinical settings.
These techniques are designed to deliver their sharesconsistent cell recovery, viability, and activity. Our overall manufacturing scheme is depicted in the figure below.
Figure 9. Overview of SNK02 allogeneic process of isolating, expanding, and treating patients with cell therapies.
The production of SNK02 begins with the isolation of pure primary NK cells from the peripheral blood of healthy donors, ensuring a reliable and high-quality source material. The NK cells undergo a process of activation and expansion through a proprietary process. Following an initial expansion period of fourteen days, the cells are harvested and cryopreserved, resulting in the generation of several hundred vials comprising NKGen’s WCB. To further activate and expand the product, one vial from the WCB is thawed and subjected to additional activation and expansion. The resulting cells are then harvested and cryopreserved, resulting in a cryopreserved final product. To facilitate off-the-shelf administration, the cryopreserved final product is shipped to the transfer agent electronicallydesignated clinical sites. At the clinical sites, the product is thawed and reconstituted, rendering it ready for administration to patients.
We believe that NKMAX, who will produce SNK02 from its facility in South Korea using The Depository Trust Company’s DWAC (Deposit/Withdrawal At Custodian) System,our processes, has the GMP manufacturing capabilities required to ensure a consistent and reliable source of its SNK02 product for clinical and commercial use. NKMAX has invested heavily in new manufacturing equipment, laboratory infrastructure and cryo-storage areas, all of which are managed under a rigorous QMS. With its extensive experience in clinical trial management and its track record of producing both SNK01 and SNK02, NKMAX is believed to be well-positioned to deliver cost-effective and high-quality products for NKGen’s Phase I study in solid tumors, which has already received IND clearance from the FDA. We estimate that NKMAX’s GMP facility can produce approximately 12,000 doses per year, providing ample supply to meet its anticipated clinical trial needs.
Corporate Information
We were originally known as Graf Acquisition Corp. IV. On September 29, 2023, Legacy NKGen, Graf and Merger Sub consummated the transactions contemplated under the Merger Agreement, following the approval at the holder’s option.special meeting of the stockholders of Graf held on September 25, 2023. In connection with the Closing, we changed our name from Graf Acquisition Corp. IV to NKGen Biotech, Inc.
Our principal executive offices are located at 3001 Daimler St., Santa Ana, California 92705, and our telephone number is (949) 396-6830. Our corporate website address is https://nkgenbiotech.com. Information contained on or accessible through our website is not a part of this Annual Report on Form 10-K, and the inclusion of our website address in this Annual Report on Form 10-K is an inactive textual reference only.
Emerging Growth Company Status
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). As an emerging growth company, we are exempt from certain requirements related to executive compensation, including the requirements to hold a nonbinding advisory vote on executive compensation and to provide information relating to the ratio of total compensation of our President and Chief Executive Officer to the median of the annual total compensation of all of our employees, each as required by the Investor Protection and Securities Reform Act of 2010, which is part of the Dodd-Frank Act.
Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a registration statement under the Securities Act declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The proxy materialsJOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. We have elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of NKGen Biotech’s financial statements with those of another public company that is neither an emerging growth company nor an emerging growth company that has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
We will remain an emerging growth company until the earlier of: (1) the last day of the fiscal year following (a) the fifth anniversary of the Closing of Graf’s IPO, (b) in which we have total annual gross revenue of at least $1.235 billion or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common equity that is held by non-affiliates exceeds $700 million as of the end of the prior fiscal year’s second fiscal quarter; and (2) the date on which we have issued more than $1.00 billion in non-convertible debt securities during the prior three-year period. References herein to “emerging growth company” are to its meaning under the Securities Act, as modified by the JOBS Act.
Liquidity
We do not currently have sufficient funds to service our operations and our expenses and other liquidity needs and require additional capital immediately, and our independent registered public accountants and management have expressed substantial doubt as to our ability to continue as a going concern.
As of December 31, 2023 and 2022, we had cash and cash equivalents of approximately less than $0.1 million and $0.1 million, respectively, and working capital deficits of approximately $37.5 million and $14.4 million, respectively. We have incurred substantial transaction expenses in connection with the Business Combination. Approximately $14.3 million in transaction expenses and deferred underwriter fees were settled upon the consummation of the Business Combination. However, we continue to have substantial transaction expenses accrued and unpaid subsequent to the Closing. As of December 31, 2023, we had incurred approximately $13.4 million in accounts payable and accrued expenses, including transaction expenses from the Business Combination and our ongoing business operations. We continue to have substantial accrued and unpaid transaction expenses and other accrued and unpaid operating expenses subsequent to the Business Combination. Furthermore, we expect to incur additional expenses in connection with transitioning to, and operating as, a public company.
We had approximately $19.9 million in outstanding debts as of December 31, 2023, inclusive of our revolving line of credit with East West Bank, loans with related parties and the Senior Convertible Notes. In addition, our revolving line of credit with East West Bank is secured by all of our assets, and requires us to maintain a minimum cash balance of $15.0 million with the bank as of December 31, 2023 and at all times thereafter as long as there is an outstanding balance under the revolving line of credit. Such cash balance requirement has been contractually waived by East West Bank as of December 31, 2023, and pursuant to an amendment entered into on April 5, 2024, East West Bank has agreed to replace such minimum cash balance requirement with a covenant to use East West Bank as the Company's only commercial bank for cash deposits and extend the maturity date to September 18, 2024. We intend to continue to seek delays on certain payments and explore other ways of potentially reducing expenses with the goal of preserving cash until additional financing is secured. These efforts may not be successful or sufficient in amount or on a timely basis to meet our ongoing capital requirements. We continue to actively seek additional financing. In the absence of additional sources of liquidity, we do not have sufficient existing cash resources to meet operating and liquidity needs. However, there is no assurance that we will furnishbe able to holders of our public sharestimely secure such additional liquidity or be successful in connection with our initial business combination will indicate whether we are requiring public stockholders to satisfyraising additional funds or that such delivery requirements. Accordingly, a public stockholder would have from the time we send out our proxy materials up to two days prior to the vote on the initial business combination to tender its sharesrequired funds, if it wishes to seek to exercise its redemption rights. Given the relatively short exercise period, it is advisable for stockholders to use electronic delivery of their public shares.
There is a nominal cost associated with the above-referenced tendering process and the act of certificating the shares or delivering them through the DWAC System. The transfer agent will typically charge the tendering broker $80.00 and it would be up to the broker whether or not to pass this cost on to the redeeming holder. However, this fee would be incurred regardless of whether or not we require holders seeking to exercise redemption rights to tender their shares. The need to deliver shares is a requirement of exercising redemption rights regardless of the timing of when such delivery must be effectuated.
In addition, if we conduct redemptions in connection with a stockholder vote, a public stockholder seeking redemption of its public shares must also submit a written request for redemption to our transfer agent two business days prior to the vote in which the name of the beneficial owner of such shares is included.
Any request to redeem such shares, once made, may be withdrawn at any time up to the date of the stockholder meeting set forth in our proxy materials. Furthermore, if a holder of a public share delivered its certificate in connection with an election of redemption rights and subsequently decides prior to the applicable date not to elect to exercise such rights, such holder may simply request that the transfer agent return the certificate (physically or electronically). It is anticipated that the funds to be distributed to holders of our public shares electing to redeem their sharesavailable, will be distributed promptly after the completion ofavailable on acceptable terms or that they will not have a significant dilutive effect on our initial business combination.
If our initial business combination is not approved or completed for any reason, then our public stockholders who elected to exercise their redemption rights would not be entitled to redeem their shares for the applicable pro rata share of the trust account.existing stockholders. In such case, we will promptly return any certificates delivered by public holders who elected to redeem their shares.
If our initial proposed initial business combination is not completed, we may continue to try to complete an initial business combination with a different target until 24 months from the closing of the IPO, or by May 25, 2023.
Redemption of Public Shares and Liquidation if no Initial Business Combination
Our amended and restated certificate of incorporation provides that we have only 24 months from the closing of the IPO, or until May 25, 2023, to complete our initial business combination. Ifaddition, we are unable to determine at this time whether any of these potential sources of liquidity will be adequate to support our operations or provide sufficient cash flows to us to meet our obligations as they become due and continue as a going concern. In the event we determine that additional sources of liquidity will not be available to us or will not allow us to meet our obligations as they become due, we may need to file a voluntary petition for relief under the United States Bankruptcy Code in order to implement a restructuring plan or liquidation. In addition, substantial doubt about our ability to continue as a going concern may cause, investors or other financing sources to be unwilling to provide funding to us on commercially reasonable terms, if at all. If sufficient funds are not available, we will have to delay, reduce the scope of, or eliminate some or all of our business activities, which would adversely affect our business prospects and our ability to continue our operations.
In addition, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our financial statements, and/or seek protection under Chapters 7 or 11 of the United States Bankruptcy Code. This could potentially cause us to cease operations and result in a complete or partial loss of your investment in our initialcommon stock. This could potentially cause us to cease operations and result in a total loss of your investment in our common stock. See “Risk Factors — Risks Related to Our Business and Industry — We currently do not have sufficient funds to service our operations and accrued expenses and payables and require additional capital. Our independent registered public accountants and management have expressed substantial doubt as to our ability to continue as a going concern without additional capital” for more details.
Intellectual Property
We strive to protect the proprietary technology that we believe is important to our business, combination within such 24-month period, we will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equalincluding seeking and maintaining patents intended to cover our product candidates and technologies that are important to the aggregate amount thendevelopment of our business, either directly or in collaboration with NKMAX. We also rely on deposittrade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection, as well as know-how, trademarks, continuing technological innovation and in-licensing opportunities to develop and maintain its proprietary position. We have in-licensed the patent portfolio on which we rely for our NK cell therapy program. We have not sought but may in the trust accountfuture seek appropriate patent protection for our product candidates, as well as other proprietary technologies and their uses by filing patent applications in the U.S. and other select countries.
Patents
We strive to protect and enhance the proprietary technology, inventions and improvements that are commercially important to our business, including interest (which interest shall be netseeking, maintaining and defending patent rights, whether developed internally or licensed from our collaborators or other third parties. Our policy is to seek to protect our proprietary position by, among other methods, obtaining licenses to and filing patent applications in the United States and in jurisdictions outside of taxes payablethe United States related to our proprietary technology, inventions, improvements and upproduct candidates that are important to $100,000the development and implementation of interestour business. We also may rely on trade secrets and know-how relating to pay dissolution expenses), dividedour proprietary technology and product candidates, continuing innovation, and in-licensing opportunities to develop, strengthen and maintain our proprietary position in the field of cell therapy. We additionally plan to rely on data exclusivity, market exclusivity and patent term extensions if and when available, and ifappropriate, may seek and rely on regulatory protection afforded through orphan drug designations. Our commercial success may depend in part on our ability to obtain and maintain patent and other proprietary protection for our technology, inventions and improvements; to preserve the confidentiality of our trade secrets; to maintain our licenses to use intellectual property owned by third parties; to defend and enforce our proprietary rights, including our patents; and to operate without infringing on the numbervalid and enforceable patents and other proprietary rights of then outstanding public shares,third parties.
We have in-licensed numerous patents and patent applications, which redemption will completely extinguish public stockholders’ rightsinclude claims directed to methods of making, methods of use, and compositions, and possesses know-how and trade secrets relating to the development of our cell engineering technology platforms and related product candidates, including related manufacturing processes and protocols.
As of December 31, 2023, our in-licensed and owned patent portfolio includes approximately three licensed U.S. issued patents, approximately three licensed U.S. pending non-provisional patent applications, as stockholderswell as approximately one licensed patent issued in jurisdictions outside of the United States, and approximately 15 licensed patent applications (including five PCT applications) pending in jurisdictions outside of the rightUnited States. The licensed patents and patent applications outside of the United States in our portfolio are held in countries including Brazil, Canada, Chile, Egypt, Europe, Mexico, South Africa and Ukraine. Our U.S. issued patents and pending patent applications are utility patents or patent applications, all of which relate to receive further liquidating distributions, if any)our product candidates SNK01 and/or SNK02. The licensed U.S. patents and U.S. patent applications have anticipated expiration dates that fall between May 2033 and November 2040 (or between May 2033 and November 2043, including PCT patent applications), subject to applicable law,changes to patent terms, including, but not limited to, patent term adjustments or extensions. NKGen’s non-U.S. patent and (iii) as promptly as reasonably possible following such redemption,patent applications (including non-provisional and PCT) are also utility patent or patent applications and relate to our product candidates SNK01 and/or SNK02. The non-U.S. patent and patent applications have anticipated expiration dates that fall between January 2039 and November 2043, subject to the approvalchanges to patent terms, including, butnot limited to patent term adjustments, extensions, or supplementary protection certificates.
We intend to develop and commercialize our product candidates and related manufacturing processes. We may pursue, when possible, on our own or in collaboration with our licensor, composition, method of our remaining stockholdersuse, process, dosing and our board of directors, dissolveformulation patent protection. We may also pursue patent protection with respect to manufacturing and liquidate, subject in each case to our obligations under Delaware law to provide for claims of creditorsdrug development processes and the requirements of other applicable law. There will be no redemption rights or liquidating distributionstechnology and with respect to our warrants, which will expire worthless iftechnology platform. When available to expand market exclusivity, we failmay obtain or license additional intellectual property related to complete our initial business combination withincurrent or contemplated development technology platforms, core elements of technology and/or product candidates.
Individual patents extend for varying periods of time, depending upon the 24-month time period.Our sponsor, executive officersdate of filing of the patent application, the date of patent issuance and directors have entered into a letter agreement with us, pursuant tothe legal term of patents in the countries in which they have waived their rights to liquidating distributionsare obtained. Generally, patents issued for applications filed in the United States are effective for 20 years from the trust account with respectearliest nonprovisional filing date. In the United States, a patent’s term may be lengthened by patent term adjustment (“
PTA”), which compensates a patentee for administrative delays by the USPTO in examining and granting a patent, or may be shortened if a patent is terminally disclaimed over an earlier filed patent. In addition, in certain instances, the patent term of a U.S. patent that covers an FDA-approved drug may also be eligible for extension to any founder shares held by them if we fail to complete our initial business combination within 24 months from the closingrecapture a portion of the IPO, or by May 25, 2023, or any extended period of time that we may have to consummate an initial business combinationterm effectively lost as a result of an amendment to our amended and restated certificate of incorporation. However, if our sponsor, officers or directors acquire public shares in or after the IPO, they will be entitled to liquidating distributions from the trust account with respect to such public shares if we fail to complete our initial business combination within the allotted 24-month time period.Our sponsor, executive officers and directors have agreed, pursuant to a written agreement with us, that they will not propose any amendment to our amended and restated certificate of incorporation to modify the substance or timing of our obligation to redeem 100% of our public shares if we do not complete our initial business combination within 24 months from the closing of the IPO, or by May 25, 2023, or to offer redemption in connection with a business combination unless we provide our public stockholders with the opportunity to redeem their shares of common stock upon approval of any such amendment at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account including interest (which interest shall be net of taxes payable) divided by the number of then outstanding public shares. However, we may not redeem our public shares in an amount that would cause our net tangible assets to be less than $5,000,001 upon consummation of our initial business combination (so that we are not subject to the
SEC’s “penny stock” rules). If this optional redemption right is exercised with respect to an excessive number of public shares such that we cannot satisfy the net tangible asset requirement (described above), we would not proceed with the amendment or the related redemption of our public shares at such time.
We expect that all costs and expenses associated with implementing our plan of dissolution, as well as payments to any creditors, will be funded from amounts remaining out of the proceeds held outside the trust account at that time, although we cannot assure you that there will be sufficient funds for such purpose. We will depend on sufficient interest being earned on the proceeds held in the trust account to pay any franchise and income tax obligations we may owe. However, if those funds are not sufficient to cover the costs and expenses associated with implementing our plan of dissolution, to the extent that there is any interest accrued in the trust account not required to pay franchise and income taxes on interest income earned on the trust account balance, we may request the trustee to release to us an additional amount of up to $100,000 of such accrued interest to pay those costs and expenses.
If we were to expend all of the net proceeds from the IPO, the over-allotment,clinical trials and the sale ofFDA regulatory review period. Such extension is referred to as patent term extension (“PTE”). The restoration period cannot be longer than five years and the private placement warrants, other thantotal patent term, including the proceeds deposited in the trust account, and without taking into account interest, if any, earned on the trust account, the per-share redemption amount received by stockholders upon our dissolution would be approximately $10.00. The proceeds deposited in the trust account could,restoration period, must not exceed 14 years following FDA approval, however become subject to the claims of our creditors which would have higher priority than the claims of our public stockholders. We cannot assure you that the actual per-share redemption amount received by stockholders will not be substantially less than $10.00. Under Section 281(b) of the Delaware General Corporation Law (the “DGCL”), our plan of dissolution must provide for all claims against us to be paid in full or make provision for payments to be made in full, as applicable, if there are sufficient assets. These claims must be paid or provided for before we make any distribution of our remaining assets to our stockholders. While we intend to pay such amounts, if any, we cannot assure you that we will have funds sufficient to pay or provide for all creditors’ claims.
Although we will seek to have all vendors, service providers (other than our independent registered public accounting firm), prospective target businesses or other entities with which we do business execute agreements with us waiving any right, title, interest or claim of any kind in or to any monies held in the trust account for the benefit of our public stockholders, there is no guarantee that theythe applicable authorities, including the FDA in the United States, will executeagree with our assessment of whether such agreementsextensions should be granted, and if granted, the length of such extensions. Furthermore, NKGen may not have the right to seek extensions of patents that are in-licensed to it, or even if they execute such agreementslicenses are terminated, NKGen may not have rights to any patents eligible for extension. Similar provisions are available in Europe and certain other foreign jurisdictions to extend the term of a patent that they wouldcovers an approved drug. The duration of patents outside of the United States varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest nonprovisional filing date. The actual protection afforded by a patent varies on a product-by-product basis, from country-to-country, and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
In some instances, we or our licensors may submit patent applications directly to the USPTO as provisional patent applications. Provisional applications for patents were designed to provide a lower-cost first patent filing in the United States. Corresponding nonprovisional patent applications must be preventedfiled not later than 12 months after the provisional application filing date to claim priority to the provisional application. The claims in the corresponding nonprovisional application may or may not be entitled to the benefit of the earlier provisional application filing date(s), and the patent term of the finally issued patent is calculated from bringing claims against the trust account including butlater non-provisional application filing date. This system allows us or our licensors to potentially obtain an early priority date, add material to the patent application(s) during the priority year, obtain a later start to the patent term and to delay prosecution costs. Such delay may be useful in the event that we or our licensors decide not to pursue prosecution of the application. While we may file nonprovisional patent applications relating to our provisional patent applications where appropriate, we cannot predict whether any such nonprovisional patent applications will result in the issuance of patents that provide it with any competitive advantage.
We or our licensors can file U.S. nonprovisional applications and PCT applications that claim the benefit of the priority date of earlier filed provisional applications, when applicable. The PCT system allows a single application to be filed within 12 months of the original priority date of the patent application, and to designate all of the PCT member states in which national or regional patent applications can later be pursued based on the international patent application filed under the PCT. The PCT searching authority performs a patentability search and issues a non-binding patentability opinion. Although a PCT application does not issue as a patent, it allows the applicant to seek protection in any of the member states through national/regional-phase applications. At the end of the period of two and a half years from the first priority date ofthe patent application, separate patent applications can be pursued in any ofthe PCT memberstates either by direct national filing or, in some cases by filing through a regional patent organization, such as the European Patent Organization. The PCT system delays expenses, allows a limited evaluation of the chances of success for national/regional patent applications and enables substantial savings where applications are abandoned within the first two and a half years of filing.
In some cases, patent prosecution of our licensed technology may be controlled solely by our licensors. If our licensors fail to fraudulent inducement, breach of fiduciary responsibilityobtain and maintain patent or other similarprotection for the proprietary intellectual property it in-licenses, then we could lose our rights to the intellectual property or our exclusivity with respect to those rights, and our competitors could market competing products using the intellectual property. In addition, our licensors may not pursue or obtain claims that are in our best interest. For patent applications that we may file on our own behalf, we will determine claiming strategy on a case-by-case basis. Advice of counsel, country-specific patent laws and our business model and needs are always considered. We may file patents containing claims for protection of useful applications of our proprietary technology platforms and any products, as well as new applications and/or uses we discover for existing technology platforms and products, assuming these are strategically valuable. We will continuously reassess the number and type of patent applications, as well as the pending and issued patent claims, challengingto help pursue maximum coverage and value for our processes, and compositions, given existing patent office rules and regulations. Further, claims may be modified during patent prosecution to meet our intellectual property and business needs.
We recognize that the enforceabilityability to obtain patent protection and the degree of such protection depends on a number of factors, including, for example, the extent of the waiver,prior art, the novelty and non- obviousness of the invention and the ability to satisfy the patent eligibility, written description and enablement or support requirement of the patent laws. In addition, the coverage claimed in each casea patent application can be significantly reduced before a patent is issued, and the scope of a patent can be reinterpreted or further altered even after issuance. Consequently, we or our licensors may not ultimately obtain or maintain adequate patent protection for any of their product candidates or for their technology platform. We cannot predict whether the patent applications that NKGen has in-licensed will issue as patents in orderany particular jurisdiction or whether the claims of any issued patents will provide sufficient proprietary protection against competitors. Any patents that NKGen holds or licenses may be challenged, circumvented or invalidated by third parties.
The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. No consistent policy regarding the scope of claims allowable in patents in the field of cell therapy has emerged in the United States. The patent situation outside ofthe United States is alsouncertain. Changes in either the patent laws or their interpretation in the United States and other countries may diminish our or our licensors’ ability to gain an advantageprotect their inventions and enforce their intellectual property rights, and more generally could affect the value of our intellectual property. In particular, our ability to stop third parties from making, using, selling, offering to sell, or importing products that infringe our intellectual property will depend in part on our or our licensors’ success in obtaining and enforcing patent claims that cover their technology, inventions and improvements. With respect to both licensed and company-owned intellectual property, NKGen cannot be sure that patents will be granted with respect to a claim againstany of our assets, including the funds held in the trust account.Ifor our licensors’ pending patent applications or with respect to any third party refuses to execute an agreement waiving such claims to the monies held in the trust account,patent applications filed by us or our management will perform an analysis of the alternatives available to it and will only enter into an agreement with a third party that has not executed a waiver if management believes that such third party’s engagement would be more beneficial to us than any alternative. Examples of possible instances where we may engage a third party that refuses to execute a waiver include the engagement of a third party consultant whose particular expertise or skills are believed by management to be superior to those of other consultants that would agree to execute a waiver or in cases where management is unable to find a service provider willing to execute a waiver.
In addition, there is no guarantee that such entities will agree to waive any claims they may havelicensors in the future, as a resultnor can we be certain that any of our in-licensed existing patents or arising out of, any negotiations, contracts or agreements with us and will not seek recourse against the trust account for any reason. Our sponsor has agreedpatents that it willmay be liable to us if and to the extent any claims by a third party for services rendered or products soldgranted to us or our licensors in the future will be commercially useful in protecting our products, their use and the methods used to manufacture those products. Moreover, even our in-licensed issued patents do not guarantee us the right to practice our technology in relation to the commercialization of our products. The area of patent and other intellectual property rights in biotechnology is an evolving one with many risks and uncertainties, and third parties may have blocking patents that could be used to prevent us from commercializing our patented product candidates and practicing our proprietary technology. It is uncertain whether the issuance of any third-party patent would require us to alter our development or commercial strategies, or our products or processes, obtain licenses or cease certain activities. Our breach of any license agreements or our failure to obtain a prospective target business withlicense to proprietary rights required to develop or commercialize our future products may have a material adverse impact on it. If third parties prepare and file patent applications in the United States that also claim technology to which we have entered into a written letter of intent, confidentialityrights, we or similar agreementour licensors may have to participate in interference or business combination agreement, reduce the amount of fundsderivation proceedings in the trust accountUSPTO to below the lesserdetermine priority of (i) $10.00 per public shareinvention. Our in-licensed issued patents and (ii) the actual amount per public share heldthose that may issue in the trust account asfuture may be challenged, invalidated, or circumvented, which could limit our ability to stop competitors from marketing related products or limit the length of the dateterm of
patent protection that we may have for our product candidates. In addition, the rights granted under any issued patents may not provide us with protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may independently develop similar technologies. For these reasons, we may have competition for our product candidates. Moreover, because of the liquidationextensive time required for development, testing and regulatory review of the trust account, if less than $10.00 per share due to reductions in the value of the trust assets, less taxes payable, provided that such liability will not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to the monies held in the trust account (whether or not such waiver is enforceable) nor will it apply to any claims under our indemnity of the underwriters of the IPO against certain liabilities, including liabilities under the Securities Act. However, we have not asked our sponsor to reserve for such indemnification obligations, nor have we independently verified whether our sponsor has sufficient funds to satisfy its indemnity obligations and believe that our sponsor’s only assets are securities of our company. Therefore, we cannot assure you that our sponsor would be able to satisfy those obligations. None of our officers or directors will indemnify us for claims by third parties including, without limitation, claims by vendors and prospective target businesses.In the event that the proceeds in the trust account are reduced below (i) $10.00 per public share or (ii) such lesser amount per public share held in the trust account as of the date of the liquidation of the trust account, due to reductions in value of the trust assets,
in each case net of the amount of interest which may be withdrawn to pay taxes, and our sponsor asserts that it is unable to satisfy its indemnification obligations or that it has no indemnification obligations related to a particular claim, our independent directors would determine whether to take legal action against our sponsor to enforce its indemnification obligations. While we currently expect that our independent directors would take legal action on our behalf against our sponsor to enforce its indemnification obligations to us,potential product, it is possible that, before any particular product candidate can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent. Our commercial success will also depend in part on not infringing upon the proprietary rights of third parties. Patent disputes are sometimes interwoven into other business disputes.
We may also rely, in some circumstances, on trade secrets to protect our technology. However, trade secrets are difficult to protect. We seek to protect our proprietary information, including our trade secrets and proprietary know-how, in part, by entering into confidentiality agreements with those who have access to our confidential information, including our employees, contractors, consultants, collaborators and advisors. These agreements generally provide that all confidential information developed or made known during the course of the relationship with us be kept confidential and not be disclosed to third parties except in specific circumstances. In the case of our employees, the agreements also typically provide that all inventions resulting from work performed for us, utilizing our property or relating to our business and conceived or completed during employment shall be our exclusive property to the extent permitted by law. Where appropriate, agreements we obtain with our consultants also typically contain similar assignment of invention provisions. Further, we generally require confidentiality agreements from business partners and other third parties that receive our confidential information. We also seek to preserve the integrity and confidentiality of our proprietary technology and processes by maintaining physical security of our premises and physical and electronic security of our information technology systems. Although we have confidence in these individuals, organizations and systems, agreements or security measures may be breached and it may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or may be independently discovered by competitors. To the extent that our employees, contractors, consultants, collaborators and advisors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. For this and more comprehensive risks related to our proprietary technology, inventions, improvements and product candidates, see the subsection of this prospectus entitled “Risk Factors — Risks Related to Our Intellectual Property.”
Trademarks
Our registered trademark portfolio contains approximately 17 registered trademarks and pending trademark applications, consisting of approximately six pending trademark applications in the United States, approximately three foreign pending trademark applications in Canada, and trademark registrations through national filings in the United States, Europe, Canada, and Switzerland.
Government Regulation
We are subject to extensive regulation by the FDA and other federal, state, and local regulatory agencies. The Federal Food, Drug, and Cosmetic Act (the “FD&C Act”) and the FDA’s implementing regulations set forth, among other things, requirements for the testing, development, including clinical trials, manufacture, quality control, safety, effectiveness, approval/clearance, labeling, storage, record-keeping, reporting, distribution, import, export, sale, advertising and promotion of our products and product candidates. Although the discussion below focuses on regulation in the U.S. because that is currently our primary focus, We may seek approval/clearance for, and market, our products in other countries in the future.
Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the U.S., although there can be important differences. We expect the global regulatory environment will continue to evolve, which could impact the cost, the time needed to approve, and ultimately, our ability to maintain existing approvals or obtain future approvals for our products. Regulations of the FDA and other regulatory agencies in and outside the U.S. impose extensive compliance and monitoring obligations on our business. These agencies review our design and manufacturing practices, labeling, record keeping, and manufacturers’ required reports of adverse experiences and other information to identify potential problems with marketed products. Weare also subject toperiodic inspections for compliance with applicable manufacturing and quality system regulations, which govern the methods used in, and the facilities and controls used for, the design, manufacture, packaging, and servicing of finished drugs and medical devices intended for human use. In addition, the FDA and other regulatory bodies, both within and outside the U.S. (including, without limitation, the Federal Trade Commission, the Office of the Inspector General of the Department of Health and Human Services, the U.S. DOJ, and various state attorneys general), monitor the promotion and advertising of our products. Any adverse regulatory action, depending on its magnitude, may limit our ability to effectively market and sell our products, limit our ability to obtain future pre-market approvals or result in a substantial modificationto our business practices and operations.
Drug Development and Approval
In the United States, biological products are subject to regulation under the Federal Food, Drug, and Cosmetic Act, the Public Health Service Act and other federal, state, local and foreign statutes and regulations. The process required by the FDA before biologics may be marketed in the United States generally involves, without limitation, the following:
•completion of preclinical laboratory tests and animal studies performed in accordance with the FDA’s Good Laboratory Practice requirements (“GLP”);
•submission to the FDA of an IND, which must become effective before clinical trials may begin;
•approval by an IRB or ethics committee at each clinical site before the trial is commenced;
•performance of adequate and well-controlled human clinical trials according to the FDA’s regulations commonly referred to as GCP, regulations and any additional requirements for the protection of human research subjects and their health information to establish the safety, purity and potency of the proposed biologic product candidate for its intended purpose;
•preparation of and submission to the FDA of a Biologics License Application (“BLA”), after completion of all pivotal clinical trials;
•satisfactory completion of an FDA Advisory Committee review, if applicable;
•a determination by the FDA within 60 days of its receipt of a BLA to file the application for review;
•satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the proposed product is produced to assess compliance with GMP and to assure that the facilities, methods and controls are adequate to preserve the biological product’s continued safety, purity and potency and, if applicable, to assess compliance with the FDA’s current good tissue practice requirements for the use of human cellular and tissue products, and of selected clinical investigation sites to assess compliance with GCPs;
•potential FDA audit of the nonclinical and clinical trial sites that generated the data in support of the BLA; and
•FDA review and approval of the BLA to permit commercial marketing of the product for particular indications for use in the United States.
Before testing any biological product candidate in humans, the product candidate enters the preclinical testing stage. Preclinical tests, also referred to as nonclinical studies, include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies to assess the potential safety and activity of the product candidate. The conduct of the preclinical tests must comply with federal regulations and requirements including GLPs.
Prior to beginning the first clinical trial with a product candidate in the United States, we must submit an IND to the FDA. An IND is a request for authorization from the FDA to administer an investigational new drug to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for clinical trials. The IND also includes results of animal and in vitro studies assessing the toxicology, pharmacokinetics, pharmacology and pharmacodynamic characteristics of the product; chemistry, manufacturing and controls information; and any available human data or literature to support the use ofthe investigational product. An IND must become effective before human clinical trials may begin. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises safety concerns or questions about the proposed clinical trial. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before the clinical trial can begin. Submission of an IND therefore may or may not result in FDA authorization to begin a clinical trial.
In addition to the submission of an IND to the FDA before initiation of a clinical trial in the United States, certain human clinical trials involving recombinant or synthetic nucleic acid molecules are subject to oversight of IBCs as set forth in the NIH Guidelines for Research Involving Recombinant DNA Molecules (the “NIH Guidelines”). Specifically, under the NIH Guidelines, supervision of human gene transfer trials includes evaluation and assessment by an IBC, a local institutional committee that reviews and oversees research utilizing recombinant or synthetic nucleic acid molecules at that institution. The IBC assesses the safety of the research and identifies any potential risk to public health or the environment, and such review may result in some delay before initiation of a clinical trial. While the NIH Guidelines are not mandatory unless the research in question is being conducted at or sponsored by institutions receiving NIH funding of recombinant or synthetic nucleic acid molecule research, many companies and other institutions not otherwise subject to the NIH Guidelines voluntarily follow them.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCPs, which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development and for any subsequent protocol amendments. Furthermore, an independent directorsIRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial and its informed consent form before the clinical trial begins at that site, and must monitor the study until completed. Regulatory authorities, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk or that the trial is unlikely to meet its stated objectives. Some studies also include oversight by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain data from the study and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. There are also requirements governing the reporting of ongoing clinical trials and clinical trial results to public registries.
For purposes of BLA approval, human clinical trials are typically conducted in exercisingthree sequential phases that may overlap or be combined:
•Phase I — The investigational product is initially introduced into healthy human subjects or patients with the target disease or condition. These trials are designed to test the safety, dosage tolerance, absorption, metabolism and excretion of the investigational product in humans, the side effects associated with increasing doses, and, if possible, to gain early evidence on effectiveness.
•Phase II — The investigational product is administered to a limited patient population with a specified disease or condition to evaluate the preliminary efficacy, optimal dosages and dosing schedule and to identify possible adverse side effects and safety risks. Multiple Phase II clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase 3 clinical trials.
•Phase III — The investigational product is administered to an expanded patient population tofurther evaluate dosage, to provide statistically significant evidence of clinical efficacy and to further test for safety, generally at multiple geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the investigational product and to provide an adequate basis for product approval.
In some cases, the FDA may require, or companies may voluntarily pursue, additional clinical trials after a product is approved to gain more information about the product in the intended therapeutic indication, particularly for long-term safety follow-up. These so-called Phase 4 trials may also be made a condition to approval of the BLA.
Concurrent with clinical trials, companies may complete additional animal studies and develop additional information about the biological characteristics of the product candidate, and must finalize a process for manufacturing the product in commercial quantities in accordance with GMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
BLA Submission and Review by the FDA
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, nonclinical studies and clinical trials are submitted to the FDA as part of a BLA requesting approval to market the product for one or more indications. The BLA must include all relevant data available from preclinical and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company- sponsored clinical trials intended to test the safety and effectiveness of a use of the product, or from a number of alternative sources, including trials initiated by independent investigators. The submission of a BLA requires payment of a substantial application user fee to the FDA, unless a waiver or exemption applies.
Within 60 days following submission of the application, the FDA reviews a BLA submitted to determine if it is substantially complete before the FDA accepts it for filing. The FDA may refuse to file any BLA that it deems incomplete or not properly reviewable at the time of submission and may request additional information. In this event, the BLA must be resubmitted with the additional information. Once a BLA has been accepted for filing, the FDA’s goal is to review standard applications within ten months after the filing date, or, if the application qualifies for priority review, six months after the FDA accepts the application for filing. In both standard and priority reviews, the review process may also be extended by FDA requests for additional information or clarification. The FDA reviews a BLA to determine, among other things, whether a product is safe, pure and potent and the facility in which it is manufactured, processed, packed or held meets standards designed to assure the product’s continued safety, purity and potency. The FDA may also convene an advisory committee to provide clinical insight on application review questions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving a BLA, the FDA will typically inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with GMP and adequate to assure consistent production of the product within required specifications. For a product candidate that is also a human cellular or tissue product, the FDA also will not approve the application if the manufacturer is not in compliance with cGTPs. These are FDA regulations that govern the methods used in, and the facilities and controls used for, the manufacture of human cells, tissues and cellular and tissue-based products, or HCT/Ps, which are human cells or tissue intended for implantation, transplant, infusion, or transfer into a human recipient. The primary intent of the GTP requirements is to ensure that cell and tissue-based products are manufactured in a manner designed to prevent the introduction, transmission and spread of communicable disease. FDA regulations also require tissue establishments to register and list their business judgmentHCT/Ps with the FDA and, when applicable, to evaluate donors through screening and testing.
Additionally, before approving a BLA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may choosedecide that the application does not satisfy the regulatory criteria for approval.
After the FDA evaluates a BLA and conducts inspections of manufacturing facilities where the investigational product and/or its drug substance will be produced, the FDA may issue an approval letter ora Complete Response Letter (“CRL”). An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A CRL will describe all of the deficiencies that the FDA has identified in the BLA, except that where the FDA determines that the data supporting the application are inadequate to support approval, the FDA may issue the CRL without first conducting required inspections, testing submitted product lots, and/or reviewing proposed labeling. In issuing the CRL, the FDA may recommend actions that the applicant might take to place the BLA in condition for approval, including requests for additional information or clarification. The FDA may delay or refuse approval of a BLA if applicable regulatory criteria are not satisfied, require additional testing or information and/or require post-marketing testing and surveillance to monitor safety or efficacy of a product.
If regulatory approval of a product is granted, such approval will be granted for particular indications and may entail limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the BLA with a REMS, to ensure the benefits of the product outweigh its risks, or otherwise limit the scope of any approval. A REMS is a safety strategy implemented to manage a known or potential serious risk associated with a product and to enable patients to have continued access to such medicines by managing their safe use, and could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing requirements is not maintained or if problems occur after the product reaches the marketplace. The FDA may require one or more Phase 4 post- marketing trials and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization, and may limit further marketing of the product based on the results of these post- marketing studies.
Expedited Development and Review Programs
The FDA offers a number of expedited development and review programs for qualifying product candidates. For example, the Fast Track program is intended to expedite or facilitate the process for reviewing new products that are intended to treat a serious or life-threatening disease or condition and demonstrate the potential to address unmet medical needs for the disease or condition. Specifically, new biological products are eligible for Fast Track designation if they are intended to treat a serious or life- threatening disease or condition and demonstrate the potential to address unmet medical needs for the disease or condition. Fast Track designation applies to the combination ofthe product and the specific indication for which it is being studied. The sponsor of a new biologic may request that the FDA designate the biologic as a Fast Track product at any time during the clinical development of the product. The sponsor of a Fast Track product has opportunities for more frequent interactions with the applicable FDA review team during product development and, once a BLA is submitted, the product candidate may be eligible for priority review. A Fast Track product may also be eligible for rolling review, where the FDA may consider for review sections of the BLA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the BLA, the FDA agrees to accept sections of the BLA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the BLA.
A product candidate intended to treat a serious or life-threatening disease or condition may also be eligible for Breakthrough Therapy designation to expedite its development and review. A product candidate can receive Breakthrough Therapy designation if preliminary clinical evidence indicates that the product candidate, alone or in combination with one or more other drugs or biologics, may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The designation includes all of the Fast Track program features, as well as more intensive FDA interaction and guidance beginning as early as Phase I and an organizational commitment to expedite the development and review of the product candidate, including involvement of senior managers.
Any marketing application for a drug or biologic submitted to the FDA for approval, including a product candidate with a Fast Track designation and/or Breakthrough Therapy designation, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. A product candidate is eligible for priority review if it has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attemptto direct additional resources to the evaluation of an application for a new biological product designated for priority review in an effort to facilitate the review. For original BLAs, priority review designation means the FDA’s goal is to take action on the marketing application within six months of the 60-day filing date (as compared to ten months under standard review).
Additionally, product candidates studied for their safety and effectiveness in treating serious or life- threatening diseases or conditions may receive accelerated approval upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. As a condition of accelerated approval, the FDA will generally require the sponsor to perform adequate and well- controlled post-marketing clinical trials to verify and describe the anticipated effect on irreversible morbidity or mortality or other clinical benefit. Products receiving accelerated approval may be subject to expedited withdrawal procedures if the sponsor fails to conduct the required post-marketing studies or if such studies fail to verify the predicted clinical benefit. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product.
In 2017, the FDA established a new RMAT designation, which is intended to facilitate an efficient development program for, and expedite review of, any drug or biologic that meets the following criteria: (i) the drug or biologic qualifies as a RMAT, which is defined as a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products, with limited exceptions; (ii) the drug or biologic is intended to treat, modify, reverse, or cure a serious or life- threatening disease or condition; and (iii) preliminary clinical evidence indicates that the drug or biologic has the potential to address unmet medical needs for such a disease or condition. RMAT designation provides all the benefits of Breakthrough Therapy designation, including more frequent meetings with the FDA to discuss the development plan for the product candidate and eligibility for rolling review and priority review.
Product candidates granted RMAT designation may also be eligible for accelerated approval on the basis of a surrogate or intermediate endpoint reasonably likely to predict long-term clinical benefit, or reliance upon data obtained from a meaningful number of clinical trial sites, including through expansion of trials to additional sites. RMAT-designated products that receive accelerated approval may, as appropriate, fulfill their post-approval requirements through submission of clinical evidence, clinical trials, patient registries, or other sources of real-world evidence (such as electronic health records); through the collection of larger confirmatory data sets; or via post-approval monitoring of all patients treated with such therapy prior to approval of such therapy. Fast track designation, Breakthrough Therapy designation, priority review, accelerated approval, and RMAT designation do sonot change the standards for approval but may expedite the development or approval process. Even if a product candidate qualifies for example,one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide thatthe time period for FDA review or approval will not be shortened.
Orphan Drug Designation and Exclusivity
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, defined as a disease or condition with a patient population of fewer than 200,000 individuals in the United States, or a patient population greater than 200,000 individuals in the United States and when there is no reasonable expectation that the cost of such legal action is deemeddeveloping and making available the drug or biologic in the United States will be recovered from sales in the United States for that drug or biologic. Orphan drug designation must be requested before submitting a BLA. After the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the independent directorsFDA. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
In the United States, orphan drug designation entitles a party to be too high relativefinancial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. In addition, if a product that has orphan drug designation subsequently receives the first FDA approval for a particular drug or biologic for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full BLA, to market the same biologic for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the amount recoverableproduct with orphan drug exclusivity or if the FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated.
Orphan drug exclusivity does not prevent the FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Orphan product exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same biological product as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease.
A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or, as noted above, if a second applicant demonstrates that its product is clinically superior to the approved product with orphan exclusivity or the manufacturer of the approved product is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Post-Approval Requirements
Biologics are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to record-keeping, reporting of adverse experiences, periodic reporting, product sampling and distribution and advertising and promotion of the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are continuing, annual program fees for any marketed products.
Biologic manufacturers and other entities involved in the manufacture and distribution of approved biological products are required to register their establishments with the FDA and certain state agencies,and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with GMP requirements and other laws. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain GMP compliance. Changes to the manufacturing process or facility are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from GMP and impose reporting requirements. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with GMP and other aspects of regulatory compliance.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program.
Other potential consequences include, among other things:
•restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
•fines, warning letters or untitled letters;
•clinical holds on clinical trials;
•refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals;
•product seizure or detention, or refusal to permit the import or export of products;
•consent decrees, corporate integrity agreements, debarment or exclusion from federal healthcare programs;
•mandated modification of promotional materials and labeling and the issuance of corrective information;
•the issuance of safety alerts, Dear Healthcare Provider letters, press releases and other communications containing warnings or other safety information about the product; or
•injunctions or the imposition of civil or criminal penalties.
The FDA closely regulates the marketing, labeling, advertising and promotion of biologics. A company can make only those claims relating to safety and efficacy, purity and potency that are approved by the FDA and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. Failure to comply with these requirements can result in, among other things, adverse publicity, warning letters, corrective advertising and potentialcivil and criminal penalties. FDA sanctions could include refusal to approve pending applications, withdrawal of an approval, clinical hold, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, mandated corrective advertising or communications with doctors, debarment, restitution, disgorgement of profits, or civil or criminal penalties. Physicians may prescribe legally available products for uses that are not described in the product’s labeling and that differ from those tested and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe, in their independent directors determinemedical judgment, that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturer’s communications on the subject of off-label use of their products.
Biosimilars and Reference Product Exclusivity
The Affordable Care Act, signed into law in 2010, includes a subtitle called the Biologics Price Competition and Innovation Act (“BPCIA”), which created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. The FDA has issued several guidance documents outlining an approach to review and approval of biosimilars.
Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity and potency, can be shown through analytical studies, animal studies, and a clinical trial or trials. Interchangeability requires that a favorable outcomeproduct is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product in any given patient and, for products that are administered multiple times to an individual, the biologic and the reference biologic may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. However, complexities associated with the larger, and often more complex, structures of biological products, as well as the processes by which such products are manufactured, pose significant hurdles to implementation of the abbreviated approval pathway that are still being worked out by the FDA.
Under the BPCIA, an application for a biosimilar product may not likely. We havebe submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not asked our sponsorbe made effective by the FDA until 12 years from the date on which the reference product was first licensed. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing that applicant’s own preclinical data and data from adequate and well-controlled clinical trials to reservedemonstrate the safety, purity and potency of its product. The BPCIA also created certain exclusivity periods for biosimilars approved as interchangeable products. At this juncture, it is unclear whether products deemed “interchangeable” by the FDA will, in fact, be readily substituted by pharmacies, which are governed by state pharmacy law.
A biological product can also obtain pediatric market exclusivity in the United States. Pediatric exclusivity, if granted, adds six months to existing exclusivity periods and patent terms. This six-month exclusivity, which runs from the end ofother exclusivity protection or patent term, may be granted based on the voluntary completion of a pediatric study in accordance with an FDA-issued “Written Request” for such indemnification obligationsa study. The BPCIA is complex and we cannot assure you that our sponsor wouldcontinues to be able to satisfy those obligations. Accordingly, we cannot assure you that due to claims of creditorsinterpreted and implemented by the actual value of the per-share redemption price will not be less than $10.00 per public share.We will seekFDA. In addition, government proposals have sought to reduce the possibility12-year reference product exclusivity period.
Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. As a result, the ultimate impact, implementation and impact of the BPCIA is subject to significant uncertainty.
Other Healthcare Laws
Pharmaceutical companies are subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which they conduct their business and may constrain the financial arrangements and relationships through which we research, sell, market and distribute any products for which we obtain marketing approval. Such laws include, without limitation, federal and state anti-kickback, fraud and abuse, false claims, data privacy and security, price reporting and physician and other health care provider transparency laws and regulations. If our operations are found to be in violation of any of such laws or any other governmental regulations that our sponsorapply, we may be subject to penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of operations, integrity oversight and reporting obligations, exclusion from participation in federal and state healthcare programs and imprisonment.
The federal Anti-Kickback Statute prohibits, among other things, any person or entity, from knowingly and willfully offering, paying, soliciting or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any item or service reimbursable under Medicare, Medicaid or other federal healthcare programs. The term remuneration has been interpreted broadly to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution.
The exceptions and safe harbors are drawn narrowly and practices that involve remuneration that may be alleged to be intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor but the exceptions and safe harbors are drawn narrowly and require strict compliance in order to offer protection. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will havebe evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances.
Additionally, the intent standard under the Anti-Kickback Statute and the criminal healthcare fraud statutes under the federal HIPAA was amended by the ACA to indemnify the trust account due to claims of creditors by endeavoringa stricter standard such that a person or entity no longer needs to have all vendors, service providers (other than our independent registered public accounting firm), prospective target businessesactual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the ACA codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act (“FCA”) (discussed below).
The FCA prohibits, among other entitiesthings, any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to, or approval by, the federal government or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the U.S. government.
Pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with which we do businessthe expectation that the customers would bill federal programs for the product and for causing false claims to be submitted because of the companies’ marketing ofthe product for unapproved, and thus non-covered, uses.
HIPAA also created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, agreements with us waivinga scheme to defraud or to obtain, by means of false or fraudulent pretenses, representations or promises, any right, title, interestmoney or claimproperty owned by, or under the control or custody of, any kind inhealthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or to monies held in the trust account. Our sponsor will also not be liable as tocovering up by trick, scheme or device, a material fact or making any claims under our indemnity of the underwriters of the IPO against certain liabilities, including liabilities under the Securities Act. We will have access to up to approximately $1,750,000 from the proceeds of the IPO, less operating expenses incurred to that date, with which to pay any such potential claims (including costs and expenses incurredmaterially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Also, many states have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
Additionally, the federal Physician Payments Sunshine Act within the ACA, and its implementing regulations, require that certain manufacturers of drugs, devices, biological and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) annually report information related to certain payments or other transfers of value made or distributed to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals and certain ownership and investment interests held by these healthcare providers and their immediate family members. Beginning in 2022, applicable manufacturers also will be required to report information regarding its payments and other transfers of value to physician assistants, nurse practitioners, clinical nurse specialists, anesthesiologist assistants, certified registered nurse anesthetists and certified nurse midwives during the previous year.
Wemay also be subject to data privacy and security regulations by both the federal government andthe states in which we conduct our liquidation, currently estimatedbusiness. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”) and its implementing regulations, impose requirements on covered entities, including certain healthcare providers, health plans, healthcare clearinghouses and their respective business associates that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity as well as their covered subcontractors relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to business associates, independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
In order to distribute products commercially, we must comply with state laws that require the registration of manufacturers and wholesale distributors of pharmaceutical products in a state, including, in certain states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, track and report gifts, compensation and other remuneration made to physicians and other healthcare providers, clinical trials and other activities, and/or register their sales representatives, as well as to prohibit pharmacies and other healthcare entities from providing certain physician prescribing data to pharmaceutical companies for use in sales and marketing, and to prohibit certain other sales and marketing practices. All of our activities are potentially subject to federal and state consumer protection and unfair competition laws.
If our operations are found to be no more than approximately $100,000). Inin violation of any of the eventfederal and state healthcare laws described above or any other governmental regulations that we liquidate and it is subsequently determined that the reserve for claims and liabilities is insufficient, stockholders who received funds from our trust account could be liable for claims made by creditors. In the event that our offering expenses including directors and officers insurance premiums exceed our estimate of approximately $1,900,000,apply to us, we may fundbe subject to significant penalties, including without limitation, civil, criminal and/or administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government programs, such excess with funds from the funds not to be heldas Medicare and Medicaid, injunctions, private “qui tam” actions brought by individual whistleblowers in the trust account. Inname of the government, or refusal to allow us to enter into government contracts, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Coverage and Reimbursement
Sales of any product depend, in part, on the extent to which such case,product will be covered by third-party payors, such as federal, state and foreign government healthcare programs, commercial insurance and managed healthcare organizations, and the amountlevel of funds we intend to be held outside the trust account would decreasereimbursement for such product by a corresponding amount. Conversely, in the event that the offering expenses including directors and officers insurance premiums are less than our estimate of approximately $1,900,000, the amount of funds we intend to be held outside the trust account would increase by a corresponding amount.Under the DGCL, stockholders may be held liable for claims by third parties against a corporation tothird-party payors. Decisions regarding the extent of distributions receivedcoverage and amount of reimbursement to be provided are made on a plan- by-plan basis. Reimbursement by thema third-party payor may depend upon a number of factors, including the third-party payor’s determination that a product is safe, effective and medically necessary; appropriate for the specific patient; cost-effective; supported by peer-reviewed medical journals; included in clinical practice guidelines; and neither cosmetic, experimental, nor investigational. A third-party payor could also require that certain lines of therapy be completed or failed prior to reimbursing our therapy. The principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services (“
CMS”), an agency within the U.S. Department of Health and Human Services (“HHS”). CMS decides whether and to what extent products will be covered and reimbursed under Medicare and private payors tend to follow CMS to a substantial degree. Third-party payors determine which products and procedures they will cover and establish reimbursement levels. Even if a third-party payor covers a particular product or procedure, the resulting reimbursement payment rates may not be adequate. These third-party payors are increasingly reducing coverage and reimbursement for medical products, drugs and services. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost- containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit sales of any product. Decreases in third-party reimbursement for any product or a decision by a third-party payor not to cover a product could reduce physician usage and patient demand for the product and also have a material adverse effect on sales.
Healthcare Reform
In the United States, in March 2010, the ACA was enacted, which substantially changed the way healthcare is financed by both governmental and private insurers, and significantly affected the pharmaceutical industry. The ACA contained a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement adjustments and changes to fraud and abuse laws. For example, the ACA:
•increased the minimum level of Medicaid rebates payable by manufacturers of brand name drugs from 15.1% to 23.1% of the average manufacturer price;
•required collection of rebates for drugs paid by Medicaid managed care organizations;
•required manufacturers to participate in a dissolution. The pro rata portioncoverage gap discount program, under which they must agree to offer 70% point-of-sale discounts off negotiated prices of our trust account distributedapplicable brand drugs to our public stockholders uponeligible beneficiaries during their coverage gap period, as a condition for the redemptionmanufacturer’s outpatient drugs to be covered under Medicare Part D;
•imposed a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specified federal government programs;
•imposed a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specified federal government programs;
•expanded the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; and
•created a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research, along with funding for such research.
There have been executive, judicial and Congressional challenges to certain aspects of our public sharesthe ACA. For example, the Tax Act was enacted, which includes a provision repealing, effective January 1, 2019, the tax- based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” On December 14, 2018, a U.S. District Court Judge in the event we do not complete our initial business combination within 24 months fromNorthern District of Texas ruled that the closingindividual mandate is a critical and inseverable feature ofthe ACA, and therefore, because it was repealed as part of the IPO, or by May 25, 2023, may be considered a liquidating distribution under Delaware law. IfTax Act, the corporation complies with certain procedures set forth in Section 280remaining provisions of the DGCL intended to ensureACA are invalid as well. Additionally, on December 18, 2019, the U.S. Court of Appeals for the 5th Circuit ruled that it makes reasonable provision for all claims against it, including a 60-day notice period during which any third-party claims can be brought againstthat the corporation, a 90-day period during whichindividual mandate was unconstitutional and remanded the corporation may reject any claims brought, and an additional 150-day waiting period before any liquidating distributions are made to stockholders, any liability of stockholders with respect to a liquidating distribution is limitedcase back to the lesser of such stockholder’s pro rata shareDistrict Court to determine whether the remaining provisions of the claimACA are invalid as well. The U.S. Supreme Court is currently reviewing this case, but it is unknown when a decision will be reached. Although the U.S. Supreme Court has not yet ruled on the constitutionality of the ACA, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructs certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the amount distributed toACA. It is unclear how the stockholder,Supreme Court ruling, other such litigation and any liabilitythe healthcare reform measures of the stockholder would be barred afterBiden administration will impact the third anniversary of the dissolution.Furthermore, if the pro rata portion ofACA and our trust account distributed to our public stockholders upon the redemption of our public sharesbusiness.
Other legislative changes have also been proposed and adopted in the event we do not complete our initial business combination within 24 monthsUnited States since the ACA was enacted. On August 2, 2011, the Budget Control Act of 2011, among other things, included aggregate reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013. However, COVID-19 relief legislation suspended the 2% Medicare sequester from May 1, 2020 through December 31, 2021. In January 2013, the closingAmerican Taxpayer Relief Act of the IPO, or by May 25, 2023, is not considered a liquidating distribution under Delaware2012 was signed into law, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and such redemption distribution is deemed to be unlawful (potentially due to the imposition of legal proceedings that a party may bring or due to other circumstances that are currently unknown), then pursuant to Section 174 of the DGCL,cancer treatment centers, and increased the statute of limitations period for claimsthe government to recover overpayments to providers from three to five years.
There has been heightened governmental scrutiny recently over the manner in which pharmaceutical companies set prices for their marketed products, which has resulted in several Congressional inquiries and proposed federal legislation, as well as state efforts, designed to, among other things, bring more transparency to product pricing, reduce the cost of creditorsprescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. The likelihood of success of these and other measures initiated by the former Trump administration is uncertain, particularly in light of the new Biden administration. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
We anticipate that these new laws will result in additional downward pressure on coverage and the price that we receive for any approved product, and could thenseriously harm our business. Any reduction in reimbursement from Medicare and other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our products (if approved). In addition, it is possible that there will be six years afterfurther legislation or regulation that could harm our business, financial condition and results of operations. For example, it is possible that additional governmental action is taken in response to address the unlawful redemption distribution, insteadCOVID-19 pandemic.
Coverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of three years,our products and any product candidates for which we may obtain regulatory approval. Sales of any of our products and product candidates, if approved, will depend, in part, on the extent to which the costs ofthe products will be covered by third-party payors, including government healthcare programs such as Medicare and Medicaid, and private payors, such as commercial health insurers and managed care organizations. Third-party payors determine which drugs they will cover and the amount of reimbursement they will provide for a covered drug. In the U.S., there is no uniform system among payors for making coverage and reimbursement decisions. In addition, the process for determining whether a payor will provide coverage for a product may beseparate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the FDA-approved products for a particular indication.
In order to secure coverage and reimbursement for our products we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costly studies required to obtain FDA or other comparable regulatory approvals. Even if we conduct pharmacoeconomic studies, our products and product candidates may not be considered medically necessary or cost-effective by payors. Further, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved.
Furthermore, the healthcare industry in the caseU.S. has experienced a trend toward cost containment as government and private insurers seek to control healthcare costs by imposing lower payment rates and negotiating reduced contract rates with service providers. Therefore, we cannot be certain that the procedures using our products will be reimbursed at a cost-effective level. Nor can we be certain that third-party payors using a methodology that sets amounts based on the type of a liquidating distribution. Ifprocedure performed, such as those utilized by government programs and in many privately managed care systems, will view the cost of our products to be justified so as to incorporate such costs into the overall cost of the procedure. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to achieve profitability. Moreover, we are unable to completepredict what changes will be made to the reimbursement methodologies used by third-party payors in the future.
Additional legislative changes, regulatory changes and judicial challenges related to the Affordable Care Act remain possible, as discussed above under the subheading “U.S. Healthcare Reform.” In addition, there likely will continue to be proposals by legislators at both the federal and state levels, regulators, and third-party payors to contain healthcare costs. Thus, even if we obtain favorable coverage and reimbursement status for our initialproducts and any product candidates for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Healthcare Fraud and Abuse Laws
In addition to FDA restrictions on marketing of pharmaceutical products, our business combination within 24 months fromis subject to healthcare fraud and abuse regulation and enforcement by both the closingfederal government and the states in which we conduct our business. These laws include, but are not limited to, the following:
•The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or in kind, to induce or in return for purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any healthcare item or service reimbursable, in whole or in part, under Medicare, Medicaid or other federally financed healthcare programs. The term “remuneration” has been broadly interpreted to include anything of value. The Affordable Care Act, among other things, amended the intent requirement of the IPO,federal Anti-Kickback Statute such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate in order to commit a violation.
•The federal civil and criminal false claims laws, including the False Claims Act, which can be enforced by May 25, 2023, we will: (i) cease all operations exceptprivate individuals on behalf of the government through civil whistleblower or qui tam actions, and civil monetary penalty laws prohibit individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for the purposepayment of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, atgovernment funds, or knowingly making, using, or causing to be made or used, a per-share price, payable in cash, equalfalse record or statement material to an obligation to pay money to the aggregate amount then on deposit in the trust account including interest (which interest shall be net of taxes payablegovernment or knowingly concealing or knowingly and up to $100,000 of interestimproperly avoiding, decreasing, or concealing an obligation to pay dissolution expenses), dividedmoney to the U.S. federal government.
•HIPAA, prohibits, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors. HIPAA also prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services.
•HIPAA, as amended by the numberHITECH and its implementing regulations, imposes obligations on “covered entities,” including certain healthcare providers, health plans, and healthcare clearinghouses, as well as their respective “business associates” and their subcontractors that create, receive, maintain or transmit individually identifiable health information for or on behalf of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (includinga covered entity, with respect to safeguarding the right to receive further liquidating distributions, if any), subject to applicable law,privacy, security and (iii) as promptly as reasonably possible following such redemption, subject totransmission of individually identifiable health information. HITECH also increased the approval of our remaining stockholderscivil and our board of directors, dissolve and liquidate, subject in each case to our obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. Accordingly, it is our intention to redeem our public shares as soon as reasonably possible following our 24th month and, therefore, we do not intend to comply with those procedures.
As such, our stockholders could potentially be liable for any claims to the extent of distributions received by them and any liability of our stockholders may extend well beyond the third anniversary of such date.
Because we will not be complying with Section 280, Section 281(b) of the DGCL requires us to adopt a plan, based on facts known to us at such time that will provide for our payment of all existing and pending claims or claimscriminal penalties that may be potentially brought against us withinimposed under HIPAA and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce HIPAA.
•The majority of states also have statutes or regulations similar to the subsequent 10 years. However, because we are a blank check company, rather than an operating company,federal anti-kickback and our operations willfalse claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Several states now require pharmaceutical companies to report expenses relating to the marketing and promotion ofpharmaceutical productsin those states and to report gifts and payments to individual health care providers in those states.
•Some of these states also prohibit certain marketing-related activities including the provision of gifts, meals, or other items to certain health care providers. Other states have laws requiring pharmaceutical sales representatives to be limitedregistered or licensed, and still others impose limits on co- pay assistance that pharmaceutical companies can offer to searchingpatients. In addition, several statesrequire pharmaceutical companies to implement compliance programs or marketing codes.
•The Physician Payments Sunshine Act, implemented as the Open Payments program, and its implementing regulations, requires manufacturers of drugs, devices, biologics and medical supplies for prospective target businesseswhich payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to acquire, the only likely claimsreport annually to arise would be from our vendorsCMS information related to direct or indirect payments and other transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), other healthcare professionals (such as lawyers,physician assistants and nurse practitioners) and teaching hospitals, as well as ownership and investment bankers, etc.) or prospective target businesses. As described above, pursuant to the obligation contained in our underwriting agreement, we will seek to have all vendors, service providers, prospective target businesses or other entities with which we do business execute agreements with us waiving any right, title, interest or claim of any kind in or to any moniesinterests held in the trust account. As a resultcompany by physicians and their immediate family members.
Compliance with such laws and regulations requires substantial resources. Because of
this obligation, the
claims that could be made against us are believed to be significantly limitedbreadth of these laws and the
likelihoodnarrowness of available statutory exceptions and regulatory safe harbors, it is possible that
any claim that would result in any liability extending to the trust account is believed to be remote. Further,some of our
sponsor may be liable only to the extent necessary to ensure that the amounts in the trust account are not reduced below (i) $10.00 per public share or (ii) such lesser amount per public share held in the trust account as of the date of the liquidation of the trust account, due to reductions in value of the trust assets, in each case net of the amount of interest withdrawn to pay taxes and will not be liable as to any claims under our indemnity of the underwriters of the IPO against certain liabilities, including liabilities under the Securities Act. In the event that an executed waiver is deemed to be unenforceable against a third party, our sponsor will not be responsible to the extent of any liability for such third-party claims.If we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, the proceeds held in the trust accountbusiness activities could be subject to legal challenge and enforcement actions. In the event governmental authorities conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable bankruptcy law,fraud and abuse or other healthcare laws and regulations, they may impose sanctions under these laws, which are potentially significant and may include civil monetary penalties, damages, exclusion of an entity or individual from participation in government health care programs, criminal fines and imprisonment, additional reporting requirements if we become subject to a corporate integrity agreement or other settlement to resolve allegations of violations of these laws, as well as the potential curtailment or restructuring of our operations. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity.
Foreign Corrupt Practices Act
In addition, the U.S. Foreign Corrupt Practices Act of 1997 prohibits corporations and their intermediaries from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any official of another country, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in that capacity.
NKGen’s Team
As of December 31, 2023, we had approximately 63 full-time employees. Substantially all of our employees are located in California.
None of our employees is represented by a labor union or covered under a collective bargaining agreement. We have not experienced any material work stoppages and we consider our relationship with our employees to be includedgood, healthy and transparent. We actively engage with managers to collect feedback and ideas on how to improve its working environment.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining incentivizing and integrating its existing and new employees, advisors and consultants. The principal purpose of our equity and cash incentive plans is to attract, retain, and reward personnel through the granting of stock-based and cash-based compensation awards, in order to increase stockholder value and our bankruptcy estatesuccess by motivating such individuals to perform to the best of their abilities and achieve our objectives.
Available Information
The Company is subject to the
claimsreporting and information requirements of
third parties with priority over the
claims of our stockholders. To the extent any bankruptcy claims deplete the trust account, we cannot assure you we will be able to return $10.00 per share to our public stockholders. Additionally, if we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, any distributions received by stockholders could be viewed under applicable debtor/creditor and/or bankruptcy lawsExchange Act, and as
either a “preferential transfer” or a “fraudulent conveyance.” As a result,
a bankruptcy court could seek to recover some or all amounts received by our stockholders.Furthermore, our board of directors may be viewed as having breached its fiduciary duty to our creditors and/or may have acted in bad faith, thereby exposing itself and our company to claims of punitive damages, by paying public stockholders from the trust account prior to addressing the claims of creditors. We cannot assure you that claims will not be brought against us for these reasons.
Our public stockholders will be entitled to receive funds from the trust account only upon the earlier to occur of: (i) the completion of our initial business combination, (ii) the redemption of any public shares properly tendered in connection with a stockholder vote to amend any provisions of our amended and restated certificate of incorporation to modify the substance or timing of our obligation to redeem 100% of our public shares if we do not complete our initial business combination within 24 months from the closing of the IPO, or by May 25, 2023, or to provide for redemption in connection with a business combination and (iii) the redemption of all of our public shares if we are unable to complete our business combination within 24 months from the closing of the IPO, or by May 25, 2023, subject to applicable law. In no other circumstances will a stockholder have any right or interest of any kind to or in the trust account. In the event we seek stockholder approval in connection with our initial business combination, a stockholder’s voting in connection with the initial business combination alone will not result in a stockholder’s redeeming its shares to us for an applicable pro rata share of the trust account. Such stockholder must have also exercised its redemption rights as described above. These provisions of our amended and restated certificate of incorporation, like all provisions of our amended and restated certificate of incorporation, may be amended with a stockholder vote.
Competition
In identifying, evaluating and selecting a target business for our initial business combination, we may encounter intense competition from other entities having a business objective similar to ours, including other blank check companies, private equity groups and leveraged buyout funds, and operating businesses seeking strategic business combinations. Many of these entities are well established and have extensive experience identifying and effecting business combinations directly or through affiliates and may have outstanding securities with more attractive terms than ours. Moreover, many of these competitors possess greater financial, technical, human and other resources than we do. Our ability to acquire larger target businesses will be limited by our available financial resources. This inherent limitation gives others an advantage in pursuing the initial business combination of a target business. Notwithstanding the foregoing, the registration statement relating to the IPO, our amended and restated certificate of incorporation and our Code of Business
Conduct and Ethics preclude our executive officers and directors from directly competing with us. Furthermore, our obligation to pay cash in connection with our public stockholders who exercise their redemption rights may reduce the resources available to us for our initial business combination and our outstanding warrants, and the future dilution they potentially represent, may not be viewed favorably by certain target businesses. Either of these or other factors may place us at a competitive disadvantage in successfully negotiating an initial business combination.
Facilities
We lease our executive offices, which are located at 1790 Hughes Landing Blvd., Suite 400, The Woodlands, Texas 77380, from G-SPAC Management LLC, an affiliate of our sponsor, and our telephone numberit is (346) 442-0819. We consider our current office space adequate for our current operations. We pay G-SPAC Management LLC $15,000 per month for office space, secretarial, administrative and support services provided to us and members of our management team. Upon completion of our initial business combination or our liquidation, we will cease paying these monthly fees.
Employees / Officers
We currently have six executive officers: James A. Graf, our Chief Executive Officer; Gus Garcia and Lewis Silberman our Co-Presidents and Directors; Anantha Ramamurti, our Chief Financial Officer and Treasurer; Anthony A. Kuznik, our Executive Vice President, General Counsel and Secretary; and Sabrina McKee, our Executive Vice President, Strategy and no employees or contractors. Our executive officers are not obligated to devote any specific number of hours to our matters but they intend to devote as much of their time as they deem necessary to our affairs until we have completed our initial business combination. The amount of time they will devote in any time period will vary based on whether a target business has been selected for our initial business combinationfile annual, quarterly and the stage of the initial business combination process we are in. All the executive officers will focus substantially all of their professional time on the company, on Graf Acquisition Corp. II (“Graf II”) and Graf Acquisition Corp. III (“Graf III”) (once Graf II and Graf III consummate their respective initial public offerings), and subsequent Graf-affiliated SPACs. We do not intend to have any full time employees or contractors prior to the completion of our initial business combination.
Available Information
We are required to file Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q with the SEC on a regular basis, and are required to disclose certain material events (e.g., changes in corporate control, acquisitions or dispositions of a significant amount of assets other than in the ordinary course of business and bankruptcy) in a Current Report on Form 8-K. The SEC maintains an Internet website that containscurrent reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. The SEC’s InternetCompany makes these filings available free of charge on its website is located at http:(https://www.sec.gov.nkgenbiotech.com/investors/financials-filings/) as soon as reasonably practicable after it electronically files them with, or furnishes them to, the SEC. Information on the Company’s website does not constitute part of this 2023 Annual Report. In addition, we will provide copiesthe SEC maintains a website (http://www.sec.gov) that contains the annual, quarterly and current reports on Form 8-K, proxy and information statements, and other information the Company electronically files with, or furnishes to, the SEC.
Item 1A.Risk Factors
An investment
SUMMARY RISK FACTORS
Investments in our securities involve substantial risk. The following is a summary of select risks and uncertainties that could materially adversely affect us and our business, financial condition and results of operations. You should carefully consider all the information in this Annual Report of Form 10-K, including matters set forth under the section entitled “Risk Factors” for more details. The below summary is qualified in its entirety by that more complete discussion of such risks and uncertainties. These risks include the following, among others:
Risks Related to Our Business and Industry
•We currently do not have sufficient funds to service our operations and accrued expenses and payables and require additional capital. Our independent registered public accountants and management have expressed substantial doubt as to our ability to continue as a going concern without additional capital.
•Our business depends upon the success of our NK cell therapy platform.
•Utilizing NK cells represents a novel approach to the treatment of oncological and neurodegenerative diseases, and we must overcome significant challenges in order to develop, commercialize and manufacture our product candidates.
•Certain aspects of the function and production of NK cells are currently unknown or poorly understood, and may only become known through further preclinical testing and clinical trials. Any potential changes to our process may result in delays and additional expenses.
•Results of any patient who receives our product candidates through the compassionate use access program should not be viewed as representative of how the product candidate will perform in a well- controlled clinical trial, and cannot be used to establish safety or efficacy for regulatory approval.
•Clinical development involves a lengthy and expensive process with an uncertain outcome, and we may encounter substantial delays due to a variety of reasons outside our control.
•Our business is highly dependent on the clinical success of our product candidates, and on the clinical success of SNK01 and SNK02 in particular, and we may fail to develop SNK01, SNK02 and/or our other product candidates successfully or may be unable to obtain regulatory approval for them.
•Even if we obtain regulatory approval for a product candidate, our products will remain subject to continuous subsequent regulatory obligations and scrutiny.
•We have never commercialized a product candidate before, and we may lack the necessary expertise, personnel and resources to successfully commercialize any products, if approved. We may be unable to establish effective marketing and sales capabilities or enter into agreements with third parties or related parties to market and sell our product candidates, if they are approved, and as a result, we may be unable to generate product revenues.
Risks Related to Our Financial Position
•We have a limited operating history, have incurred significant losses since our inception, and we expect to continue to incur significant losses for the foreseeable future.
•We have never generated revenue from product sales and may never achieve or maintain profitability.
•The East West Bank Loan Agreement and Equity and Business Loan Agreement (as defined below) provide each lender with a security interest in all of our assets, and contain financial covenants and other restrictions on our actions that may limit our operational flexibility or otherwise adversely affect our results of operations.
•The terms of our 2023 NKMAX Loan Agreements, the East West Bank Loan Agreement and the Equity and Business Loan Agreement require us to meet certain payment obligations, and may subject us to default.
Risks Related to Government Regulations
•The regulatory approval process of the FDA and comparable foreign regulatory authorities are lengthy, time-consuming and inherently unpredictable, and even if we complete the necessary clinical trials, we cannot predict when, or if, we will obtain regulatory approval for any of our product candidates, and any such regulatory approval may be for a more narrow indication than we seek.
•We are and will be subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We could face criminal and/or civil liability and other serious consequences for violations, which would harm our business.
Risks Related to Manufacturing
•Our manufacturing process is novel and complex, and we may encounter difficulties in production, or difficulties with internal manufacturing, which would delay or prevent our ability to provide a sufficient supply of our product candidates for clinical trials or our products for patients, if approved.
•Delays in commissioning and receiving regulatory approvals for our manufacturing facilities could delay our development plans and thereby limit our ability to develop our product candidates and generate revenues.
Risks Related to Our Intellectual Property
•If our license agreement with NKMAX is terminated, we could lose our rights to key components enabling our NK cell technology platform.
•We may need to license additional intellectual property from third parties, and any such licenses may not be available or may not be available on commercially reasonable terms.
•Our development and commercialization rights to our current and future product candidates and technology are subject, in part, to the terms and conditions of licenses granted to us by others.
Risks Related to Ownership of Our Securities
•Our stock price may be volatile and may decline regardless of its operating performance.
•We may be unable to maintain the listing of our securities on Nasdaq in the future.
•Future sales of shares by existing stockholders could cause our stock price to decline.
•The Warrants and PIPE Warrants may not be exercised at all or may be exercised on a cashless basis and we may not receive any cash proceeds from the exercise of the Warrants or PIPE Warrants.
•We may be required to pay cash or issue shares of common stock to investors with whom we entered into Forward Purchase Agreements, which could reduce the amount of cash available to us or further dilute your ownership in us.
•We may issue additional shares of common stock or other equity securities without your approval, which would dilute your ownership interests and may depress the market price of our common stock.
RISK FACTORS
Investing in our securities involves a high degree of risk. You should
consider carefully
all ofconsider the risks
and uncertainties described below together with
all of the other information contained in this
Annual Report on Form
10-K.10-K, including the risks and uncertainties discussed above under “Special Note Regarding Forward-Looking Statements,” our financial statements and related notes appearing at the end of this Annual Report on Form 10-K and in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before deciding to invest in our securities. If any of the
following events
or developments described below were to occur, our business,
prospects, operating results and financial condition
and operating results may becould suffer materially,
adversely affected. In that event, the trading price of our
securitiescommon stock could decline, and you could lose all or part of your
investment.Summary of Risk Factors
| ● | Our public stockholders may not be afforded an opportunity to vote on our proposed initial business combination, which means we may complete our initial business combination even though a majority of our public stockholders do not support such a combination. |
| ● | If we seek stockholder approval of our initial business combination, our initial stockholders have agreed to vote in favor of such initial business combination, regardless of how our public stockholders vote. |
| ● | Your only opportunity to affect the investment decision regarding a potential business combination will be limited to the exercise of your right to redeem your shares from us for cash, unless we seek stockholder approval of the initial business combination. |
| ● | The ability of our public stockholders to redeem their shares for cash may make our financial condition unattractive to potential business combination targets, which may make it difficult for us to enter into an initial business combination with a target. |
| ● | The ability of our public stockholders to exercise redemption rights with respect to a large number of our shares may not allow us to complete the most desirable business combination or optimize our capital structure. |
| ● | The requirement that we complete our initial business combination within 24 months after the closing of the IPO, or by May 25, 2023, may give potential target businesses leverage over us in negotiating a business combination and may limit the time we have in which to conduct due diligence on potential business combination targets, in particular as we approach our dissolution deadline, which could undermine our ability to complete our initial business combination on terms that would produce value for our stockholders. |
| ● | Our search for a business combination, and any target business with which we ultimately consummate a business combination, may be materially adversely affected by COVID-19 outbreak and other events and the status of debt and equity markets. |
| ● | If we seek stockholder approval of our initial business combination, our sponsor, directors, officers, advisors and their affiliates may elect to purchase shares or warrants from public stockholders, which may influence a vote on a proposed initial business combination and reduce the public “float” of our common stock. |
| ● | You will not be entitled to protections normally afforded to investors of many other blank check companies. |
| ● | Because of our limited resources and the significant competition for business combination opportunities, it may be more difficult for us to complete our initial business combination. If we are unable to complete our initial business combination, our public stockholders may receive only approximately $10.00 per share on our redemption of our public shares, or less than such amount in certain circumstances, and our warrants will expire worthless. |
| ● | If the net proceeds of the IPO, the over-allotment, and the sale of the private placement warrants not being held in the trust account are insufficient to allow us to operate through at least May 25, 2023, we may be unable to complete our initial business combination, in which case our public stockholders may only receive $10.00 per share, or less than such amount in certain circumstances, and our warrants will expire worthless. |
| ● | If our officers and directors allocate time to other businesses , then this may cause conflicts of interest in their determination as to how much time to devote to our affairs. This conflict of interest could have a negative impact on our ability to complete our initial business combination. |
| ● | You will not have any rights or interests in funds from the trust account, except under certain limited circumstances. To liquidate your investment, therefore, you may be forced to sell your public shares or warrants, potentially at a loss. |
| ● | The NYSE may delist our securities from trading on its exchange, which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions. |
| ● | We are a newly formed company with no operating history and no revenues, and you have no basis on which to evaluate our ability to achieve our business objective. |
Risks Relating to our Search for, Consummation of, or Inability to Consummate, a Business Combination
Our public stockholders may not be afforded an opportunity to vote on our proposed initial business combination, which means we may complete our initial business combination even though a majority of our public stockholders do not support such a combination.
We may choose not to hold a stockholder vote to approve our initial business combination unless the initial business combination would require stockholder approval under applicable law or stock exchange listing requirements or if we decide to hold a stockholder vote for business or other legal reasons. Except as required by law, the decision as to whether we will seek stockholder approval of a proposed initial business combination or will allow stockholders to sell their shares to us in a tender offer will be made by us, solely in our discretion,investment. The risks and will be based on a variety of factors, such as the timing of the transaction and whether the terms of the transaction would otherwise require us to seek stockholder approval. Accordingly, we may complete our initial business combination even if holders of a majority of our public shares do not approve of the initial business combination we complete.
If we seek stockholder approval of our initial business combination, our initial stockholders have agreed to vote in favor of such initial business combination, regardless of how our public stockholders vote.
Our initial stockholders own 20% of our outstanding shares of common stock. Pursuant to a letter agreement entered into at the time of the IPO, our sponsor, executive officers and directors have agreed to vote their founder shares, as well as any public shares purchased during or after the IPO (including in open market and privately negotiated transactions), in favor of our initial business
combination. As a result, in addition to our initial stockholders’ founder shares, we would need only 4,290,376 public shares, or 25.0%, of the 17,161,500 public shares sold in the IPO, to be voted in favor of an initial business combination in order to have our initial business combination approved. Our initial stockholders own shares representing 20% of our outstanding shares of common stock immediately following the completion of the IPO. Accordingly, if we seek stockholder approval of our initial business combination, the agreement by our initial stockholders to vote in favor of our initial business combination will increase the likelihood that we will receive the requisite stockholder approval for such initial business combination.
Your only opportunity to affect the investment decision regarding a potential business combination will be limited to the exercise of your right to redeem your shares from us for cash, unless we seek stockholder approval of the initial business combination.
You may not be provided with an opportunity to evaluate the specific merits or risks of our initial business combination. Since our board of directors may complete an initial business combination without seeking stockholder approval, public stockholders may not have the right or opportunity to vote on the initial business combination, unless we seek such stockholder vote. Accordingly, if we do not seek stockholder approval, your only opportunity to affect the investment decision regarding a potential business combination may be limited to exercising your redemption rights within the period of time (which will be at least 20 business days) set forth in our tender offer documents mailed to our public stockholders in which we describe our initial business combination.
The ability of our public stockholders to redeem their shares for cash may make our financial condition unattractive to potential business combination targets, which may make it difficult for us to enter into an initial business combination with a target.
We may seek to enter into an initial business combination agreement with a prospective target that requires, as a closing condition, that we have a minimum net worth or a certain amount of cash. If too many public stockholders exercise their redemption rights, we would not be able to meet such closing condition and, as a result, would not be able to proceed with the initial business combination. Furthermore, in no event will we redeem our public shares in an amount that would cause our net tangible assets to be less than $5,000,001 upon consummation of our initial business combination (so that weuncertainties described below are not subject to the SEC’s “penny stock” rules) or any greater net tangible asset or cash requirement which may be contained in the agreement relating to our initial business combination. Consequently, if accepting all properly submitted redemption requests would cause our net tangible assets to be less than $5,000,001 upon consummation of our initial business combination or such greater amount necessary to satisfy a closing condition as described above,only ones we would not proceed with such redemption and the related business combination and may instead search for an alternate business combination. Prospective targets will be aware of theseface. Additional risks and thus, may be reluctant to enter into an initial business combination with us.
The ability of our public stockholders to exercise redemption rights with respect to a large number of our shares mayuncertainties not allow us to complete the most desirable business combination or optimize our capital structure.
At the time we enter into an agreement for our initial business combination, we will not know how many stockholders may exercise their redemption rights, and therefore will need to structure the transaction based on our expectations as to the number of shares that will be submitted for redemption. If our initial business combination agreement requires us to use a portion of the cash in the trust account to pay the purchase price, or requires us to have a minimum amount of cash at closing, we will need to reserve a portion of the cash in the trust account to meet such requirements, or arrange for third party financing. In addition, if a larger number of shares are submitted for redemption than we initially expected, we may need to restructure the transaction to reserve a greater portion of the cash in the trust account or arrange for third party financing. Raising additional third party financing may involve dilutive equity issuances or the incurrence of indebtedness at higher than desirable levels. The above considerations may limit our ability to complete the most desirable business combination availablepresently known to us or optimize our capital structure. The per-share amount we will distribute to stockholders who properly exercise their redemption rights will not be reduced by the deferred underwriting commissions payable to our underwriters.
The ability of our public stockholders to exercise redemption rights with respect to a large number of our shares could increase the probability that our initial business combination would be unsuccessful and that you would have to wait for liquidation in order to redeem your stock.
If our initial business combination agreement requires us to use a portion of the cash in the trust account to pay the purchase price, or requires us to have a minimum amount of cash at closing, the probability that our initial business combination would be unsuccessful is increased. If our initial business combination is unsuccessful, you would not receive your pro rata portion of the trust account until we liquidate the trust account. If you are in need of immediate liquidity, you could attempt to sell your stock in the open
market; however, at such time our stock may trade at a discount to the pro rata amount per share in the trust account. In either situation, you may suffer a material loss on your investment or lose the benefit of funds expected in connection with our redemption until we liquidate or you are able to sell your stock in the open market.
The requirement that we complete our initial business combination within 24 months after the closing of the IPO, or by May 25, 2023,currently believe to be immaterial may give potential target businesses leverage over us in negotiating a business combination and may limit the time we have in which to conduct due diligence on potential business combination targets, in particular as we approach our dissolution deadline, which could undermine our ability to complete our initial business combination on terms that would produce value for our stockholders.
Any potential target business with which we enter into negotiations concerning a business combination will be aware that we must complete our initial business combination within 24 months from the closing of the IPO, or by May 25, 2023. Consequently, such target business may obtain leverage over us in negotiating a business combination knowing that if we do not complete our initial business combination with that particular target business, we may be unable to complete our initial business combination with any target business. This risk will increase as we get closer to the timeframe described above. In addition, we may have limited time to conduct due diligence and may enter into our initial business combination on terms that we would have rejected upon a more comprehensive investigation.
Our search for a business combination, and any target business with which we ultimately consummate a business combination, may be materially adversely affected by COVID-19 outbreak and other events and the status of debt and equity markets.
In December 2019, a novel strain of coronavirus was reported to have surfaced, which has and is continuing to spread throughout parts of the world, including the United States. On January 30, 2020, the World Health Organization declared the outbreak of COVID-19 a “Public Health Emergency of International Concern.” On January 31, 2020, U.S. Health and Human Services Secretary Alex M. Azar II declared a public health emergency for the United States to aid the U.S. healthcare community in responding to COVID-19 and, on March 11, 2020, the World Health Organization characterized the outbreak as a “pandemic.” The COVID-19 outbreak has adversely affected, and other events (such as terrorist attacks, natural disasters or a significant outbreak of other infectious diseases) couldalso adversely affect economiesour business.
Risks Related to Our Business and
financial markets worldwide, business operations and the conduct of commerce generally, and the business of any potential target business with which we consummate a business combination could be, or may already have been, materially and adversely affected. Furthermore, we may be unable to complete a business combination if concerns relating to COVID-19 continue to restrict travel or limit the ability to have meetings with potential investors, or the target company’s personnel, vendors and services providers are unavailable to negotiate and consummate a transaction in a timely manner. The extent to which COVID-19 impacts our search for a business combination will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others.In addition, if any treatment or vaccine for COVID-19 is ineffective or underutilized, any impact on our business and any potential target business may be prolonged. If the disruptions posed by COVID-19 or other events (such as terrorist attacks, natural disasters or a significant outbreak of other infectious diseases) continue for an extensive period of time, our ability to consummate a business combination, or the operations of a target business with which we ultimately consummate a business combination, may be materially adversely affected. In addition, our ability to consummate a transaction may be dependent on the ability to raise equity and debt financing which may be impacted by COVID-19 and other events (such as terrorist attacks, natural disasters or a significant outbreak of other infectious diseases), including as a result of increased market volatility and decreased market liquidity and third-party financing being unavailable on terms acceptable to us or at all. Finally, the outbreak of COVID-19 may also have the effect of heightening many of the other risks described in this “Risk Factors” section, such as those related to the market for our securities and cross border transactions.
Industry
We
may not be able to complete our initial business combination within 24 months after the closing of the IPO, in which case we would cease all operations except for the purpose of winding up and we would redeem our public shares and liquidate, in which case our public stockholders may only receive $10.00 per share, or less than such amount in certain circumstances, and our warrants will expire worthless.We may not be able to find a suitable target business and complete our initial business combination within 24 months after the closing of the IPO, or by May 25, 2023. Our ability to complete our initial business combination may be negatively impacted by general market conditions, volatility in the capital and debt markets and other risks, including those described herein. For example, the outbreak
of COVID-19 continues to grow both in the U.S. and globally and, while the extent of the impact of the outbreak on us will depend on future developments, it could limit our ability to complete our initial business combination, including as a result of increased market volatility, decreased market liquidity and third-party financing being unavailable on terms acceptable to us or at all. Additionally, the outbreak of COVID-19 may negatively impact businesses we may seek to acquire. If we have not completed our initial business combination within such time period, we will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account including interest (which interest shall be net of taxes payable and up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of our remaining stockholders and our board of directors, dissolve and liquidate, subject in each case to our obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. In such case, our public stockholders may only receive $10.00 per share, and our warrants will expire worthless. In certain circumstances, our public stockholders may receive less than $10.00 per share on the redemption of their shares. See “- If third parties bring claims against us, the proceeds held in the trust account could be reduced and the per-share redemption amount received by stockholders may be less than $10.00 per share” and the other risk factors herein.
If we seek stockholder approval of our initial business combination, our sponsor, directors, officers, advisors and their affiliates may elect to purchase shares or warrants from public stockholders, which may influence a vote on a proposed initial business combination and reduce the public “float” of our common stock.
If we seek stockholder approval of our initial business combination and wecurrently do not conduct redemptions in connection with our initial business combination pursuant to the tender offer rules, our sponsor, directors, officers, advisors or their affiliates may purchase shares or public warrants or a combination thereof in privately negotiated transactions or in the open market either prior to or following the completion of our initial business combination, although they are under no obligation to do so. However, they have no current commitments, plans or intentions to engage in such transactions and have not formulated any terms or conditions for any such transactions. None of the funds in the trust account will be used to purchase shares or public warrants in such transactions.
Such a purchase may include a contractual acknowledgement that such stockholder, although still the record holder of our shares is no longer the beneficial owner thereof and therefore agrees not to exercise its redemption rights. In the event that our sponsor, directors, officers, advisors or their affiliates purchase shares in privately negotiated transactions from public stockholders who have already elected to exercise their redemption rights, such selling stockholders would be required to revoke their prior elections to redeem their shares. The purpose of such purchases could be to vote such shares in favor of the initial business combination and thereby increase the likelihood of obtaining stockholder approval of the initial business combination, or to satisfy a closing condition in an agreement with a target that requires us to have a minimum net worth or a certain amount of cash at the closing of our initial business combination, where it appears that such requirement would otherwise not be met. The purpose of any such purchases of public warrants could be to reduce the number of public warrants outstanding or to vote such warrants on any matters submitted to the warrant holders for approval in connection with our initial business combination. Any such purchases of our securities may result in the completion of our initial business combination that may not otherwise have been possible. Any such purchases will be reported pursuant to Section 13 and Section 16 of the Exchange Act to the extent such purchasers are subject to such reporting requirements.
In addition, if such purchases are made, the public “float” of our common stock or public warrants and the number of beneficial holders of our securities may be reduced, possibly making it difficult to obtain or maintain the quotation, listing or trading of our securities on a national securities exchange.
You will not be entitled to protections normally afforded to investors of many other blank check companies.
Since the net proceeds of the IPO, the over-allotment, and the sale of the private placement warrants are intended to be used to complete an initial business combination with a target business that has not been selected, we may be deemed to be a “blank check” company under the United States securities laws. However, we are exempt from rules promulgated by the SEC to protect investors in blank check companies, such as Rule 419. Accordingly, investors will not be afforded the benefits or protections of those rules. Among other things, this means that our units will be immediately tradable and we will have a longer period of time to complete our initial business combination than do companies subject to Rule 419. Moreover, if the IPO were subject to Rule 419, that rule would prohibit the release of any interest earned on funds held in the trust account to us unless and until the funds in the trust account were released to us in connection with our completion of an initial business combination.
Because of our limited resources and the significant competition for business combination opportunities, it may be more difficult for us to complete our initial business combination. If we are unable to complete our initial business combination, our public stockholders may receive only approximately $10.00 per share on our redemption of our public shares, or less than such amount in certain circumstances, and our warrants will expire worthless.
We expect to encounter intense competition from other entities having a business objective similar to ours, including private investors (which may be individuals or investment partnerships), other blank check companies and other entities competing for the types of businesses we intend to acquire. Many of these individuals and entities are well-established and have extensive experience in identifying and effecting, directly or indirectly, acquisitions of companies operating in or providing services to various industries. Many of these competitors possess greater technical, human and other resources or more industry knowledge than we do, and our financial resources will be relatively limited when contrasted with those of many of these competitors. While we believe there are numerous target businesses we could potentially acquire with the net proceeds of the IPO, the over-allotment, and the sale of the private placement warrants, our ability to compete with respect to the acquisition of certain target businesses that are sizable will be limited by our available financial resources. This inherent competitive limitation gives others an advantage in pursuing the acquisition of certain target businesses. Furthermore, because we are obligated to pay cash for the shares of common stock which our public stockholders redeem in connection with our initial business combination, target companies will be aware that this may reduce the resources available to us for our initial business combination. This may place us at a competitive disadvantage in successfully negotiating an initial business combination. If we are unable to complete our initial business combination, our public stockholders may receive only approximately $10.00 per share on the liquidation of our trust account and our warrants will expire worthless. In certain circumstances, our public stockholders may receive less than $10.00 per share upon our liquidation. See “- If third parties bring claims against us, the proceeds held in the trust account could be reduced and the per-share redemption amount received by stockholders may be less than $10.00 per share” and other risk factors herein.
As the number of special purpose acquisition companies evaluating targets increases, attractive targets may become scarcer and there may be more competition for attractive targets. This could increase the cost of our initial business combination and could even result in our inability to find a target or to consummate an initial business combination.
In recent years, the number of special purpose acquisition companies that have been formed has increased substantially. Many potential targets for special purpose acquisition companies have already entered into an initial business combination, and there are still many special purpose acquisition companies preparing for an initial public offering, as well as many such companies currently in registration. As a result, at times, fewer attractive targets may be available to consummate an initial business combination.
In addition, because there are more special purpose acquisition companies seeking to enter into an initial business combination with available targets, the competition for available targets with attractive fundamentals or business models may increase, which could cause targets companies to demand improved financial terms. Attractive deals could also become scarcer for other reasons, such as economic or industry sector downturns, geopolitical tensions, or increases in the cost of additional capital needed to close business combinations or operate targets post-business combination. This could increase the cost of, delay or otherwise complicate or frustrate our ability to find and consummate an initial business combination, and may result in our inability to consummate an initial business combination on terms favorable to our investors altogether.
If the net proceeds of the IPO, the over-allotment, and the sale of the private placement warrants not being held in the trust account are insufficient to allow us to operate through at least May 25, 2023, we may be unable to complete our initial business combination, in which case our public stockholders may only receive $10.00 per share, or less than such amount in certain circumstances, and our warrants will expire worthless.
The funds available to us outside of the trust account may not be sufficient to allow us to operate through at least May 25, 2023, assuming that our initial business combination is not completed during that time. We believe that the funds available to us from the IPO, the over-allotment, and those held outside of the trust account will be sufficient to allow us to operate through at least May 25, 2023; however, we cannot assure you that our estimate is accurate. Of the funds available to us, we could use a portion of the funds available to us to pay fees to consultants to assist us with our search for a target business. We could also use a portion of the funds as a down payment or to fund a “no-shop” provision (a provision in letters of intent or merger agreements designed to keep target businesses from “shopping” around for transactions with other companies on terms more favorable to such target businesses) with respect to a particular proposed initial business combination, although we do not have any current intention to do so. If we paid fees to consultants or entered into a letter of intent or merger agreement where we paid for the right to receive exclusivity from a target business and were subsequently
required to forfeit such funds (whether as a result of our breach or otherwise), we might not have sufficient funds to service our operations and accrued expenses and payables and require additional capital. Our independent registered public accountants and management have expressed substantial doubt as to our ability to continue searching for, or conduct due diligenceas a going concern without additional capital.
We do not currently have sufficient funds to service our operations and our expenses and other liquidity needs and require additional capital immediately, and our independent registered public accountants and management have expressed substantial doubt as to our ability to continue as a going concern.
As of December 31, 2023 and 2022, we had cash and cash equivalents of approximately less than $0.1 million and $0.1 million, respectively, and working capital deficits of approximately $37.5 million and $14.4 million, respectively. We have incurred substantial transaction expenses in connection with respectthe Business Combination. Approximately $14.3 million in transaction expenses and deferred underwriter fees were settled upon the consummation of the Business Combination. However, we continue to have substantial transaction expenses accrued and unpaid subsequent to the Closing. As of December 31, 2023, we had incurred approximately $13.4 million in accounts payable and accrued expenses, including transaction expenses from the Business Combination and our ongoing business operations. We continue to have substantial accrued and unpaid transaction expenses and other accrued and unpaid operating expenses subsequent to the Business Combination. Furthermore, we expect to incur additional expenses in connection with transitioning to, and operating as, a target business. If we are unable to complete our initial business combination, our public stockholders may receive onlycompany.
We had approximately
$10.00 per share on the liquidation$19.9 million in outstanding debts as of December 31, 2023, inclusive of our
trust account and our warrants will expire worthless. In certain circumstances, our public stockholders may receive less than $10.00 per share upon our liquidation. See “- If thirdrevolving line of credit with East West Bank, loans with related parties bring claims against us, the proceeds held in the trust account could be reduced and the per-share redemptionSenior Convertible Notes. In addition, our revolving line of credit with East West Bank is secured by all of our assets, and requires us to maintain a minimum cash balance of $15.0 million with the bank as of December 31, 2023 and at all times thereafter as long as there is an outstanding balance under the revolving line of credit. Such cash balance requirement has been contractually waived by East West Bank as of December 31, 2023, and pursuant to an amendment entered into on April 5, 2024, East West Bank has agreed to replace such minimum cash balance requirement with a covenant to use East West Bank as the Company's only commercial bank for cash deposits and extend the maturity date to September 18, 2024. We intend to continue to seek delays on certain payments and explore other ways of potentially reducing expenses with the goal of preserving cash until additional financing is secured. These efforts may not be successful or sufficient in amount received by stockholders may be less than $10.00 per share” and other risk factors herein.If the net proceeds of the IPO, the over-allotment, and the sale of the private placement warrants not being held in the trust account are insufficient, it could limit the amount availableor on a timely basis to fundmeet our search for a target business or businesses and complete our initial business combination and we will depend on loans from our sponsor or management team to fund our search for an initial business combination, to pay our franchise and income taxes and to complete our initial business combination. If we are unable to obtain these loans, we may be unable to complete our initial business combination.
Of the net proceeds of the IPO, the over-allotment, and the sale of the private placement warrants, only $1,750,000 were initially available to us outside the trust account to fund our workingongoing capital requirements. We continue to actively seek additional financing. In the event that our offering expenses including directors and officers insurance premiums exceed our estimateabsence of approximately $1,900,000, we may fund such excess with funds not to be held in the trust account. In such case, the amountadditional sources of funds we intend to be held outside the trust account would decrease by a corresponding amount. The amount held in the trust account will not be impacted as a result of such increase or decrease. Conversely, in the event that the IPO expenses, including directors and officers insurance premiums, are less than our initial estimate of approximately $1,900,000, the amount of funds we intend to be held outside the trust account would increase by a corresponding amount. If we are required to seek additional capital, we would need to borrow funds from our sponsor, management team or other third parties to operate or may be forced to liquidate. None of our sponsor, members of our management team nor any of their affiliates is under any obligation to advance funds to us in such circumstances. Any such advances would be repaid only from funds held outside the trust account or from funds released to us upon completion of our initial business combination. Up to $1,500,000 of such working capital loans may be convertible into additional warrants at a price of $1.50 per warrant at the option of the lender. Prior to the completion of our initial business combination, we do not expect to seek loans from parties other than our sponsor or an affiliate of our sponsor as we do not believe third parties will be willing to loan such funds and provide a waiver against any and all rights to seek access to funds in our trust account. If we are unable to obtain these loans, we may be unable to complete our initial business combination. If we are unable to complete our initial business combination becauseliquidity, we do not have sufficient funds availableexisting cash resources to us,meet operating and liquidity needs. However, there is no assurance that we will be forcedable to cease operations and liquidate the trust account. Consequently, our public stockholders may only receive approximately $10.00 per share on our redemption of our public shares, and our warrants will expire worthless. In certain circumstances, our public stockholders may receive less than $10.00 per share on the redemption of their shares. See “- If third parties bring claims against us, the proceeds heldtimely secure such additional liquidity or be successful in the trust account could be reduced and the per-share redemption amount received by stockholders may be less than $10.00 per share” and other risk factors herein.
If third parties bring claims against us, the proceeds held in the trust account could be reduced and the per-share redemption amount received by stockholders may be less than $10.00 per share.
Our placing ofraising additional funds in the trust account may not protect those funds from third-party claims against us. Although we will seek to have all vendors, service providers (other than our independent registered public accounting firm), prospective target businesses and other entities with which we do business execute agreements with us waiving any right, title, interest or claim of any kind in or to any monies held in the trust account for the benefit of our public stockholders, such parties may not execute such agreements, or even if they execute such agreements they may not be prevented from bringing claims against the trust account, including, but not limited to, fraudulent inducement, breach of fiduciary responsibility or other similar claims, as well as claims challenging the enforceability of the waiver, in each case in order to gain advantage with respect to a claim against our assets, including the funds held in the trust account. If any third party refuses to execute an agreement waiving such claims to the monies held in the trust account, our management will perform an analysis of the alternatives available to it and will only enter into an agreement with a third party that has not executed a waiver if management believes that such third party’s engagement would be more beneficial to us than other alternatives.
Examples of possible instances where we may engage a third party that refuses to execute a waiver include the engagement of a third party consultant whose particular expertise or skills are believed by management to be superior to those of other consultants that would agree to execute a waiver or in cases where management is unable to find a service provider willing to execute a waiver. In addition, there is no guarantee that such entities will agree to waive any claims they may have in the future as a result of, or arising out of, any negotiations, contracts or agreements with us and will not seek recourse against the trust account for any reason. Upon redemption
of our public shares,required funds, if we are unable to complete our initial business combination within the prescribed timeframe, or upon the exercise of a redemption right in connection with our initial business combination, we will be required to provide for payment of claims of creditors that were not waived that may be brought against us within the 10 years following redemption. Accordingly, the per-share redemption amount received by public stockholders could be less than the $10.00 per share initially held in the trust account, due to claims of such creditors. Pursuant to letter agreement entered into at the time of the IPO, which is filed as an exhibit to this Form 10-K, our sponsor has agreed that it will be liable to us if and to the extent any claims by a third party for services rendered or products sold to us, or a prospective target business with which we have entered into a written letter of intent, confidentiality or similar agreement or business combination agreement, reduce the amount of funds in the trust account to below the lesser of (i) $10.00 per public share and (ii) the actual amount per public share held in the trust account as of the date of the liquidation of the trust account, if less than $10.00 per share due to reductions in the value of the trust assets, less taxes payable, provided that such liability will not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to the monies held in the trust account (whether or not such waiver is enforceable) nor will it apply to any claims under our indemnity of the underwriters of the IPO against certain liabilities, including liabilities under the Securities Act. However, we have not asked our sponsor to reserve for such indemnification obligations, nor have we independently verified whether our sponsor has sufficient funds to satisfy its indemnity obligations and believe that our sponsor’s only assets are securities of our company.
Therefore, we cannot assure you that our sponsor would be able to satisfy those obligations. None of our officers or directors will indemnify us for claims by third parties including, without limitation, claims by vendors and prospective target businesses.
Our directors may decide not to enforce the indemnification obligations of our sponsor, resulting in a reduction in the amount of funds in the trust account available, for distribution to our public stockholders.
In the event that the proceeds in the trust account are reduced below the lesser of (i) $10.00 per share and (ii) the actual amount per share held in the trust account as of the date of the liquidation of the trust account if less than $10.00 per share due to reductions in the value of the trust assets, in each case net of the interest which may be withdrawn to pay taxes, and our sponsor asserts that it is unable to satisfy its obligations or that it has no indemnification obligations related to a particular claim, our independent directors would determine whether to take legal action against our sponsor to enforce its indemnification obligations.
While we currently expect that our independent directors would take legal action on our behalf against our sponsor to enforce its indemnification obligations to us, it is possible that our independent directors in exercising their business judgment and subject to their fiduciary duties may choose not to do so in any particular instance if, for example, the cost of such legal action is deemed by the independent directors to be too high relative to the amount recoverable or if the independent directors determine that a favorable outcome is not likely. If our independent directors choose not to enforce these indemnification obligations, the amount of funds in the trust account available for distribution to our public stockholders may be reduced below $10.00 per share.
Changes in the market for directors and officers liability insurance could make it more difficult and more expensive for us to negotiate and complete an initial business combination.
In recent months, the market for directors and officers liability insurance for special purpose acquisition companies has changed in ways adverse to us and our management team. Fewer insurance companies are offering quotes for directors and officers liability coverage, the premiums charged for such policies have generally increased and the terms of such policies have generally become less favorable. These trends may continue into the future.
The increased cost and decreased availability of directors and officers liability insurance could make it more difficult and more expensive for us to negotiate an initial business combination. In order to obtain directors and officers liability insurance or modify its coverage as a result of becoming a public company, the post-business combination entity might need to incur greater expense, accept less favorable terms or both. However, any failure to obtain adequate directors and officers liability insurance could have an adverse impact on the post-business combination’s ability to attract and retain qualified officers and directors.
In addition, even after we were to complete an initial business combination, our directors and officers could still be subject to potential liability from claims arising from conduct alleged to have occurred prior to the initial business combination. As a result, in order to protect our directors and officers, the post-business combination entity may need to purchase additional insurance with respect to any such claims (“run-off insurance”). The need for run-off insurance would be an added expense for the post-business combination entity, and could interfere with or frustrate our ability to consummate an initial business combination on terms favorable to our investors.
The securities in which we invest the proceeds held in the trust account could bear a negative rate of interest, which could reduce the interest income available for payment of taxes or reduce the value of the assets held in trust such that the per share redemption amount received by stockholders may be less than $10.00 per share.
The net proceeds of the IPO, the over-allotment, and certain proceeds from the sale of the private placement warrants, in the amount of $171,615,000, will be held in an interest-bearing trust account. The proceeds held in the trust account may only be invested in direct U.S. Treasury obligations having a maturity of 185 days or less, or in certain money market funds which invest only in direct U.S. Treasury obligations. While short-term U.S. Treasury obligations currently yield a positive rate of interest, they have briefly yielded negative interest rates in recent years. Central banks in Europe and Japan pursued interest rates below zero in recent years, and the Open Market Committee of the Federal Reserve has not ruled out the possibility that it may in the future adopt similar policies in the United States. In the event of very low or negative yields, the amount of interest income would be reduced. As described herein, we will be required in certain circumstances to redeem our public shares for their pro-rata share of the proceeds held in the trust account, plus any interest income. If the balance of the trust account is reduced below $171,615,000 as a result of negative interest rates, the amount of funds in the trust account available for distribution to our public stockholders may be reduced below $10.00 per public share.
If, after we distribute the proceeds in the trust account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, a bankruptcy court may seek to recover such proceeds, and we and our board may be exposed to claims of punitive damages.
If, after we distribute the proceeds in the trust account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, any distributions received by stockholders could be viewed under applicable debtor/creditor and/or bankruptcy laws as either a “preferential transfer” or a “fraudulent conveyance.” As a result, a bankruptcy court could seek to recover all amounts received by our stockholders. In addition, our board of directors may be viewed as having breached its fiduciary duty to our creditors and/or having acted in bad faith, thereby exposing itself and us to claims of punitive damages, by paying public stockholders from the trust account prior to addressing the claims of creditors.
If, before distributing the proceeds in the trust account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, the claims of creditors in such proceeding may have priority over the claims of our stockholders and the per-share amount that would otherwise be received by our stockholders in connection with our liquidation may be reduced.
If, before distributing the proceeds in the trust account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, the proceeds held in the trust account could be subject to applicable bankruptcy law, and may be included in our bankruptcy estate and subject to the claims of third parties with priority over the claims of our stockholders. To the extent any bankruptcy claims deplete the trust account, the per-share amount that would otherwise be received by our stockholders in connection with our liquidation may be reduced.
If we are deemed to be an investment company under the Investment Company Act, we may be required to institute burdensome compliance requirements and our activities may be restricted, which may make it difficult for us to complete our initial business combination.
If we are deemed to be an investment company under the Investment Company Act, our activities may be restricted, including:
| ● | restrictions on the nature of our investments; and |
| ● | restrictions on the issuance of securities, each of which may make it difficult for us to complete our initial business combination. |
In addition, we may have imposed upon us burdensome requirements, including:
| ● | registration as an investment company; |
| ● | adoption of a specific form of corporate structure; and |
| ● | reporting, record keeping, voting, proxy and disclosure requirements and other rules and regulations. |
In order not to be regulated as an investment company under the Investment Company Act, unless we can qualify for an exclusion, we must ensure that we are engaged primarily in a business other than investing, reinvesting or trading in securities and that our activities do not include investing, reinvesting, owning, holding or trading “investment securities” constituting more than 40% of our total assets (exclusive of U.S. government securities and cash items) on an unconsolidated basis. Our business will be to identify and complete an initial business combination and thereafter to operate the post-transaction business or assets for the long term. We do not plan to buy businesses or assets with a view to resale or profit from their resale. We do not plan to buy unrelated businesses or assets or to be a passive investor.
We do not believe that our anticipated principal activities will subject us to the Investment Company Act. To this end, the proceeds held in the trust account may only be invested in United States “government securities” within the meaning of Section 2(a)(16) of the Investment Company Act having a maturity of 185 days or less or in money market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act which invest only in direct U.S. government treasury obligations. Pursuant to the trust agreement, the trustee is not permitted to invest in other securities or assets. By restricting the investment of the proceeds to these instruments, and by having a business plan targeted at acquiring and growing businesses for the long term (rather than on buying and selling businesses in the manner of a merchant bank or private equity fund), we intend to avoid being deemed an “investment company” within the meaning of the Investment Company Act. Our securities are not intended for persons who are seeking a return on investments in government securities or investment securities. The trust account is intended as a holding place for funds pending the earliest to occur of: (i) the completion of our initial business combination; (ii) the redemption of any public shares properly submitted in connection with a stockholder vote to amend our amended and restated certificate of incorporation to modify the substance or timing of our obligation to redeem 100% of our public shares if we do not complete our initial business combination within 24 months from the closing of the IPO, or by May 25, 2023, or to provide for redemption in connection with a business combination; or (iii) absent an initial business combination within 24 months from the closing of the IPO, or by May 25, 2023, our return of the funds held in the trust account to our public stockholders as part of our redemption of the public shares. If we do not invest the proceeds as discussed above, we may be deemed to be subject to the Investment Company Act. If we were deemed to be subject to the Investment Company Act, compliance with these additional regulatory burdens would require additional expenses for which we have not allotted funds and may hinder our ability to complete an initial business combination or may result in our liquidation. If we are unable to complete our initial business combination, our public stockholders may receive only approximately $10.00 per share, or less than such amount in certain circumstances, on the liquidation of our trust account and our warrants will expire worthless.
Changes in laws or regulations, or a failure to comply with any laws and regulations, may adversely affect our business, including our ability to negotiate and complete our initial business combination and results of operations.
We are subject to laws and regulations enacted by national, state and local governments. In particular, we will be required to comply with certain SEC and other legal requirements. Compliance with, and monitoring of, applicable laws and regulations may be difficult, time consuming and costly.
Those laws and regulations and their interpretation and application may also change from time to time and those changes could have a material adverse effect on our business, investments and results of operations. In addition, a failure to comply with applicable laws or regulations, as interpreted and applied, could have a material adverse effect on our business, including our ability to negotiate and complete our initial business combination and results of operations.
Our stockholders may be held liable for claims by third parties against us to the extent of distributions received by them upon redemption of their shares.
Under the DGCL, stockholders may be held liable for claims by third parties against a corporation to the extent of distributions received by them in a dissolution. The pro rata portion of our trust account distributed to our public stockholders upon the redemption of our public shares in the event we do not complete our initial business combination within 24 months from the closing of the IPO, or by May 25, 2023, may be considered a liquidating distribution under Delaware law. If a corporation complies with certain procedures set forth in Section 280 of the DGCL intended to ensure that it makes reasonable provision for all claims against it, including a 60-day notice period during which any third-party claims can be brought against the corporation, a 90-day period during which the corporation may reject any claims brought, and an additional 150-day waiting period before any liquidating distributions are made to stockholders, any liability of stockholders with respect to a liquidating distribution is limited to the lesser of such stockholder’s pro rata share of the claim or the amount distributed to the stockholder, and any liability of the stockholder would be barred after the third anniversary of the dissolution. However, it is our intention to redeem our public shares as soon as reasonably possible following the 24th month from the
closing of the IPO in the event we do not complete our initial business combination and, therefore, we do not intend to comply with the foregoing procedures.
Because we will not be complying with Section 280, Section 281(b) of the DGCL requires us to adopt a plan, based on facts known to us at such time that will provide for our payment of all existing and pending claims or claims that may be potentially brought against us within the 10 years following our dissolution. However, because we are a blank check company, rather than an operating company, and our operations will be limited to searching for prospective target businesses to acquire, the only likely claims to arise would be from our vendors (such as lawyers, investment bankers, etc.) or prospective target businesses. If our plan of distribution complies with Section 281(b) of the DGCL, any liability of stockholders with respect to a liquidating distribution is limited to the lesser of such stockholder’s pro rata share of the claim or the amount distributed to the stockholder, and any liability of the stockholder would likely be barred after the third anniversary of the dissolution. We cannot assure you that we will properly assess all claims that may be potentially brought against us. As such, our stockholders could potentially be liable for any claims to the extent of distributions received by them and any liability of our stockholders may extend beyond the third anniversary of such date. Furthermore, if the pro rata portion of our trust account distributed to our public stockholders upon the redemption of our public shares in the event we do not complete our initial business combination within 24 months from the closing of the IPO, or May 25, 2023, is not considered a liquidating distribution under Delaware law and such redemption distribution is deemed to be unlawful (potentially due to the imposition of legal proceedings that a party may bring or due to other circumstances that are currently unknown), then pursuant to Section 174 of the DGCL, the statute of limitations for claims of creditors could then be six years after the unlawful redemption distribution, instead of three years, as in the case of a liquidating distribution.
We may not hold an annual meeting of stockholders until after the consummation of our initial business combination, which could delay the opportunity for our stockholders to elect directors.
In accordance with the NYSE corporate governance requirements, we are not required to hold an annual meeting until no later than one full year after our first fiscal year end following our listing on the NYSE. Under Section 211(b) of the DGCL, we are, however, required to hold an annual meeting of stockholders for the purposes of electing directors in accordance with our bylaws unless such election is made by written consent in lieu of such a meeting. We may not hold an annual meeting of stockholders to elect new directors prior to the consummation of our initial business combination, and thus we may not be in compliance with Section 211(b) of the DGCL, which requires an annual meeting. Therefore, if our stockholders want us to hold an annual meeting prior to the consummation of our initial business combination, they may attempt to force us to hold one by submitting an application to the Delaware Court of Chancery in accordance with Section 211(b) of the DGCL.
Because we are neither limited to evaluating a target business in a particular industry sector nor have we selected any specific target businesses with which to pursue our initial business combination, you will be unable to ascertain the merits or risks of any particular target business’s operations.
We may pursue business combination opportunities in any industry or sector, except that we will not, under our amended and restated certificate of incorporation, be permitted to effectuate our initial business combination with another blank check company or similar company with nominal operations. Because we have not yet selected any specific target business with respect to a business combination, there is no basis to evaluate the possible merits or risks of any particular target business’s operations, results of operations, cash flows, liquidity, financial condition or prospects. To the extent we complete our initial business combination, we may be affected by numerous risks inherent in the business operations with which we combine. For example, if we combine with a financially unstable business or an entity lacking an established record of sales or earnings, we may be affected by the risks inherent in the business and operations of a financially unstable or a development stage entity. Although our officers and directors will endeavor to evaluate the risks inherent in a particular target business, we cannot assure you that we will properly ascertain or assess all of the significant risk factors or that we will have adequate time to complete due diligence.
Furthermore, some of these risks may be outside of our control and leave us with no ability to control or reduce the chances that those risks will adversely impact a target business. We also cannot assure you that an investment in our units will ultimately prove to be more favorable to investors than a direct investment, if such opportunity were available, in a business combination target. Accordingly, any stockholders who choose to remain stockholders following our initial business combination could suffer a reduction in the value of their securities. Such stockholders are unlikely to have a remedy for such reduction in value.
Although we have identified general criteria and guidelines that we believe are important in evaluating prospective target businesses, we may enter into our initial business combination with a target that does not meet such criteria and guidelines, and as a result, the target business with which we enter into our initial business combination may not have attributes entirely consistent with our general criteria and guidelines.
Although we have identified general criteria and guidelines for evaluating prospective target businesses, it is possible that a target business with which we enter into our initial business combination will not have some or all of these attributes. If we complete our initial business combination with a target that does not meet some or all of these guidelines, such combination may not be as successful as a combination with a business that does meet all of our general criteria and guidelines. In addition, if we announce a prospective business combination with a target that does not meet our general criteria and guidelines, a greater number of stockholders may exercise their redemption rights, which may make it difficult for us to meet any closing condition with a target business that requires us to have a minimum net worth or a certain amount of cash. In addition, if stockholder approval of the transaction is required by law, or we decide to obtain stockholder approval for business or other legal reasons, it may be more difficult for us to attain stockholder approval of our initial business combination if the target business does not meet our general criteria and guidelines. If we are unable to complete our initial business combination, our public stockholders may receive only approximately $10.00 per share, or less than such amount in certain circumstances, on the liquidation of our trust account and our warrants will expire worthless. In certain circumstances, our public stockholders may receive less than $10.00 per share on the redemption of their shares. See “- If third parties bring claims against us, the proceeds held in the trust account could be reduced and the per-share redemption amount received by stockholders may be less than $10.00 per share” and other risk factors herein.
We may seek business combination opportunities in industries or sectors which may or may not be outside of our management’s area of expertise.
We may consider an initial business combination outside of our management’s area of expertise if an initial business combination candidate is presented to us and we determine that such candidate offers an attractive business combination opportunity for our company. Although our management will endeavor to evaluate the risks inherent in any particular business combination candidate, we cannot assure you that we will adequately ascertain or assess all of the significant risk factors. We also cannot assure you that an investment in our units will not ultimately prove to be less favorable to investors in the IPO than a direct investment, if an opportunity were available, in an initial business combination candidate. In the event we elect to pursue a business combination outside of the areas of our management’s expertise, our management’s expertise may not be directly applicable to its evaluation or operation, and the information contained in this report regarding the areas of our management’s expertise would not be relevant to an understanding of the business that we elect to acquire. As a result, our management may not be able to adequately ascertain or assess all of the significant risk factors. Accordingly, any stockholders who choose to remain stockholders following our initial business combination could suffer a reduction in the value of their shares. Such stockholders are unlikely to have a remedy for such reduction in value.
We may seek business combination opportunities with a financially unstable business or an entity lacking an established record of revenue, cash flow or earnings, which could subject us to volatile revenues, cash flows or earnings or difficulty in retaining key personnel.
To the extent we complete our initial business combination with a financially unstable business or an entity lacking an established record of revenues or earnings, we may be affected by numerous risks inherent in the operations of the business with which we combine. These risks include volatile revenues or earnings and difficulties in obtaining and retaining key personnel. Although our officers and directors will endeavor to evaluate the risks inherent in a particular target business, we may not be able to properly ascertain or assess all of the significant risk factors and we may not have adequate time to complete due diligence. Furthermore, some of these risks may be outside of our control and leave us with no ability to control or reduce the chances that those risks will adversely impact a target business.
We are not required to obtain an opinion from an independent investment banking firm or from an independent accounting firm, and consequently, you may have no assurance from an independent source that the price we are paying for the business is fair to our company from a financial point of view.
Unless we complete our initial business combination with an affiliated entity, we are not required to obtain an opinion from an independent investment banking firm or from another independent valuation or appraisal firm that regularly prepares fairness opinions that the price we are paying is fair to our company from a financial point of view.
In addition, if our board of directors is not able to determine the fair market value of the target business or businesses, in connection with the NYSE rules that require that our initial business combination must occur with one or more operating businesses or assets with a fair market value equal to at least 80% of the net assets held in the trust account (net of amounts disbursed to management for working capital purposes, if permitted, and excluding the amount of any deferred underwriting commissions), we will obtain an opinion from an independent investment banking firm or from another independent valuation or appraisal firm that regularly prepares fairness opinions solely with respect to the satisfaction of such criteria.
Other than the two circumstances described above, we are not required to obtain an opinion from an independent investment banking firm or from another independent valuation or appraisal firm. If no opinion is obtained, our stockholders will be relying on the judgment of our board of directors, who will determine fair market value based on standards generally accepted by the financial community. Such standards used will be disclosed in our tender offer documents or proxy solicitation materials, as applicable, related to our initial business combination.
We may issue additional common stock or preferred stock to complete our initial business combination or under an employee incentive plan after completion of our initial business combination. Any such issuances would dilute the interest of our stockholders and likely present other risks.
Our amended and restated certificate of incorporation authorizes the issuance of up to 400,000,000 shares of common stock, par value $0.0001 per share and 1,000,000 shares of preferred stock, par value $0.0001 per share. Immediately after the IPO, there were 381,250,000 authorized but unissued shares of common stock available for issuance (assuming that the underwriters have not exercised their over-allotment option), which amount does not take into account the shares of common stock reserved for issuance upon exercise of outstanding warrants. Immediately after the consummation of the IPO, there were no shares of preferred stock issued and outstanding.
We may issue a substantial number of additional shares of common or preferred stock to complete our initial business combination or under an employee incentive plan after completion of our initial business combination (although our amended and restated certificate of incorporation provides that we may not issue securities that can vote with common stockholders on matters related to our pre-initial business combination activity). However, our amended and restated certificate of incorporation provides, among other things, that prior to our initial business combination, we may not issue additional shares of capital stock that would entitle the holders thereof to (i) receive funds from the trust account or (ii) vote on any initial business combination. These provisions of our amended and restated certificate of incorporation, like all provisions of our amended and restated certificate of incorporation, may be amended with the approval of our stockholders. However, our executive officers and directors have agreed, pursuant to a written agreement with us, that they will not propose any amendment to our amended and restated certificate of incorporation to modify the substance or timing of our obligation to redeem 100% of our public shares if we do not complete our initial business combination within 24 months from the closing of the IPO, or by May 25, 2023, or to provide for redemption in connection with a business combination unless we provide our public stockholders with the opportunity to redeem their shares of common stock upon approval of any such amendment at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account, including interest (which interest shall be net of taxes payable), divided by the number of then outstanding public shares. Any such redemptions may be subject to the same procedures and limitations as for redemptions in connection with a business combination.
The issuance of additional shares of common or preferred stock:
| ● | may significantly dilute the equity interest of investors in the IPO; |
may subordinate the rights of holders of common stock if preferred stock is issued with rights senior to those afforded our common stock;
| ● | could cause a change of control if a substantial number of shares of our common stock are issued, which may affect, among other things, our ability to use our net operating loss carry forwards, if any, and could result in the resignation or removal of our present officers and directors; and |
| ● | may adversely affect prevailing market prices for our units, common stock and/or warrants. |
The value of the founder shares following completion of our initial business combination is likely to be substantially higher than the nominal price paid for them, even if the trading price of our common stock at such time is substantially less than $10.00 per share.
Our sponsor have invested in us an aggregate of $6,675,000, comprised of the $25,000 purchase price for the founder shares and the $6,650,000 purchase price for the private placement warrants. Assuming a trading price of $10.00 per share upon consummation of our initial business combination, the 3,750,000 founder shares would have an aggregate implied value of $37,500,000. Even if the trading price of our common stock was as low as $1.78 per share, and the private placement warrants were worthless, the value of the founder shares would be equal to the sponsor’s initial investment in us. As a result, our sponsor is likely to be able to recoup its investment in us and make a substantial profit on that investment, even if our public shares have lost significant value. Accordingly, our management team, which owns interests in our sponsor, may have an economic incentive that differs from that of the public stockholders to pursue and consummate an initial business combination rather than to liquidate and to return all of the cash in the trust to the public stockholders, even if that business combination were with a riskier or less-established target business, than would be the case if our sponsor had paid the same per share price for the founder shares as our public stockholders paid for their public shares. Accordingly, you should consider our management team’s financial incentive to complete an initial business combination when evaluating whether to redeem your shares prior to or in connection with the initial business combination.
Resources could be wasted in researching business combinations that are not completed, which could materially adversely affect subsequent attempts to locate and acquire or merge with another business. If we are unable to complete our initial business combination, our public stockholders may receive only approximately $10.00 per share, or less than such amount in certain circumstances, on the liquidation of our trust account and our warrants will expire worthless.
We anticipate that the investigation of each specific target business and the negotiation, drafting and execution of relevant agreements, disclosure documents and other instruments will require substantial management time and attention and substantial costs for accountants, attorneys, consultants and others. If we decide not to complete a specific initial business combination, the costs incurred up to that point for the proposed transaction likely would not be recoverable. Furthermore, if we reach an agreement relating to a specific target business, we may fail to complete our initial business combination for any number of reasons including those beyond our control. Any such event will result in a loss to us of the related costs incurred which could materially adversely affect subsequent attempts to locate and acquire or merge with another business. If we are unable to complete our initial business combination, our public stockholders may receive only approximately $10.00 per share on the liquidation of our trust account and our warrants will expire worthless. In certain circumstances, our public stockholders may receive less than $10.00 per share on the redemption of their shares. See “- If third parties bring claims against us, the proceeds held in the trust account could be reduced and the per-share redemption amount received by stockholders may be less than $10.00 per share” and other risk factors herein.
We may engage in an initial business combination with one or more target businesses that have relationships with entities that may be affiliated with our sponsor, officers, directors or existing holders which may raise potential conflicts of interest.
In light of the involvement of our sponsor, officers and directors with other entities, we may decide to acquire one or more businesses affiliated with our sponsor, officers or directors. Our directors and officers also serve as officers and board members of other entities, including, without limitation, those described under the section of this report entitled “Item 10. Directors, Executive Officers and Corporate Governance.” Such entities may compete with us for business combination opportunities. Our sponsor, officers and directors are not currently aware of any specific opportunities for us to complete our initial business combination with any entities with which they are affiliated, and there have been no preliminary discussions concerning an initial business combination with any such entity or entities. Although we will not be specifically focusing on, or targeting, any transaction with any affiliated entities, we might pursue such a transaction if we determined that such affiliated entity met our criteria for an initial business combination, or does not have some or all of these attributes, and such transaction was approved by all of our directors. Despite our agreement to obtain an opinion from an independent investment banking firm, or from another independent valuation or appraisal firm, regarding the fairness to our stockholders from a financial point of view of an initial business combination with one or more domestic or international businesses affiliated with our officers, directors or existing holders, potential conflicts of interest still may exist and, as a result, the terms of the initial business combination may not be as advantageous to our public stockholders as they would be absent any conflicts of interest.
We may engage one or more of our underwriters or one of their respective affiliates to provide additional services to us after the IPO, which may include acting as financial advisor in connection with an initial business combination or as placement agent in connection with a related financing transaction. Our underwriters are entitled to receive deferred commissions that will released from the trust only on a completion of an initial business combination. These financial incentives may cause them to have potential conflicts of interest in rendering any such additional services to us after the IPO, including, for example, in connection with the sourcing and consummation of an initial business combination.
We may engage one or more of our underwriters or one of their respective affiliates to provide additional services to us after the IPO, including, for example, identifying potential targets, providing financial advisory services, acting as a placement agent in a private offering or arranging debt financing. We may pay such underwriter or its affiliate fair and reasonable fees or other compensation that would be determined at that time in an arm’s length negotiation; provided that no agreement will be entered into with any of the underwriters or their respective affiliates and no fees or other compensation for such services will be paid to any of the underwriters or their respective affiliates prior to the date that is 60 days from the date of the IPO, unless such payment would not be deemed underwriters’ compensation in connection with the IPO. The underwriters are also entitled to receive deferred commissions that are conditioned on the completion of an initial business combination. The underwriters’ or their respective affiliates’ financial interests tied to the consummation of a business combination transaction may give rise to potential conflicts of interest in providing any such additional services to us, including potential conflicts of interest in connection with the sourcing and consummation of an initial business combination.
Since our sponsor, officers and directors will lose their entire investment in us if our initial business combination is not completed (other than with respect to public shares they may acquire during or after the IPO), a conflict of interest may arise in determining whether a particular business combination target is appropriate for our initial business combination.
On February 13, 2021, Graf LLC paid an aggregate of $25,000 for certain expenses on our behalf in exchange for issuance of 4,312,500 founder shares or approximately $0.006 per share. On April 2, 2021, Graf LLC transferred all of its founder shares to our sponsor. On April 8, 2021, our sponsor transferred 20,000 founder shares to each of our independent directors, resulting in our sponsor holding 4,252,500 founder shares. The number of founder shares issued was determined based on the expectation that such founder shares would represent 20% of the outstanding shares after the IPO. The founder shares will be worthless if we do not complete an initial business combination. In addition, our sponsor purchased an aggregate of 4,433,333 private placement warrants for a purchase price of $6,650,000, or $1.50 per warrant, that will also be worthless if we do not complete an initial business combination.
The holders of our founder shares have agreed (A) to vote any shares owned by them in favor of any proposed initial business combination and (B) not to redeem any founder shares in connection with a stockholder vote to approve a proposed initial business combination or in connection with a tender offer. In addition, we may obtain loans from our sponsor, affiliates of our sponsor or an officer or director. Our sponsor is controlled by James A. Graf and the membership units relating to the founder shares are owned by Mr. Graf. The personal and financial interests of our officers and directors may influence their motivation in identifying and selecting a target business combination, completing an initial business combination and influencing the operation of the business following the initial business combination.
We may issue notes or other debt securities, or otherwise incur substantial debt, to complete an initial business combination, which may adversely affect our leverage and financial condition and thus negatively impact the value of our stockholders’ investment in us.
Although we have no commitments to issue any notes or other debt securities, or to otherwise incur outstanding debt following the IPO, we may choose to incur substantial debt to complete our initial business combination. We have agreed that we will not incur any indebtedness, unless we have obtained from the lender a waiver of any right, title, interest or claim of any kind in or to the monies held in the trust account. As such, no issuance of debt will affect the per-share amount available for redemption from the trust account, subject to the waiver being valid and enforceable. Nevertheless, the incurrence of debt could have a variety of negative effects, including:
| ● | default and foreclosure on our assets if our operating revenues after an initial business combination are insufficient to repay our debt obligations; |
| ● | acceleration of our obligations to repay the indebtedness even if we make all principal and interest payments when due if we breach certain covenants that require the maintenance of certain financial ratios or reserves without a waiver or renegotiation of that covenant; |
| ● | our immediate payment of all principal and accrued interest, if any, if the debt security is payable on demand; |
| ● | our inability to obtain necessary additional financing if the debt security contains covenants restricting our ability to obtain such financing while the debt security is outstanding; |
| ● | our inability to pay dividends on our common stock; |
| ● | using a substantial portion of our cash flow to pay principal and interest on our debt, which will reduce the funds available for dividends on our common stock if declared, our ability to pay expenses, make capital expenditures and acquisitions, and fund other general corporate purposes; |
| ● | limitations on our flexibility in planning for and reacting to changes in our business and in the industry in which we operate; |
| ● | increased vulnerability to adverse changes in general economic, industry and competitive conditions and adverse changes in government regulation; |
| ● | limitations on our ability to borrow additional amounts for expenses, capital expenditures, acquisitions, debt service requirements, and execution of our strategy; and |
| ● | other disadvantages compared to our competitors who have less debt. |
We may only be able to complete one business combination with the proceeds of the IPO, the over-allotment, and the sale of the private placement warrants, which will cause us to be solely dependent on a single business which may have a limited number of services and limited operating activities. This lack of diversification may negatively impact our operating results and profitability.
Of the net proceeds from the IPO, the over-allotment, and the sale of the private placement warrants, $171,615,000, excluding cash held outside the trust account, will be available to complete our initial business combination and pay related fees and expenses including, $21,615,000 of the Over-Allotment option exercised, of deferred underwriting commissions.
We may effectuate our initial business combination with a single target business or multiple target businesses simultaneously or within a short period of time. However, we may not be able to effectuate our initial business combination with more than one target business because of various factors, including the existence of complex accounting issues and the requirement that we prepare and file pro forma financial statements with the SEC that present operating results and the financial condition of several target businesses as if they had been operated on a combined basis. By completing our initial business combination with only a single entity, our lack of diversification may subject us to numerous economic, competitive and regulatory developments. Further, we would not be able to diversify our operations or benefit from the possible spreading of risks or offsetting of losses, unlike other entities which may have the resources to complete several business combinations in different industries or different areas of a single industry. Accordingly, the prospects for our success may be:
| ● | solely dependent upon the performance of a single business, property or asset, or |
| ● | dependent upon the development or market acceptance of a single or limited number of products, processes or services. |
This lack of diversification may subject us to numerous economic, competitive and regulatory risks, any or all of which may have a substantial adverse impact upon our operating results and profitability and the particular industry in which we may operate subsequent to our initial business combination.
We may attempt to simultaneously complete business combinations with multiple prospective targets, which may hinder our ability to complete our initial business combination and give rise to increased costs and risks that could negatively impact our operations and profitability.
If we determine to simultaneously acquire several businesses that are owned by different sellers, we will need for each of such sellers to agree that our purchase of its business is contingent on the simultaneous closings of the other business combinations, which may make it more difficult for us, and delay our ability, to complete our initial business combination. We do not, however, intend to purchase multiple businesses in unrelated industries in conjunction with our initial business combination. With multiple business combinations, we could also face additional risks, including additional burdens and costs with respect to possible multiple negotiations and due diligence investigations (if there are multiple sellers) and the additional risks associated with the subsequent assimilation of the operations and services or products of the acquired companies in a single operating business. If we are unable to adequately address these risks, it could negatively impact our profitability and results of operations.
We may attempt to complete our initial business combination with a private company about which little information is available, which may result in an initial business combination with a company that is not as profitable as we suspected, if at all.
In pursuing our initial business combination strategy, we may seek to effectuate our initial business combination with a privately held company. Very little public information generally exists about private companies, and we could be required to make our decision on whether to pursue a potential initial business combination on the basis of limited information, which may result in an initial business combination with a company that is not as profitable as we suspected, if at all.
We do not have a specified maximum redemption threshold. The absence of such a redemption threshold may make it possible for us to complete an initial business combination with which a substantial majority of our stockholders do not agree.
Our amended and restated certificate of incorporation will not provide a specified maximum redemption threshold, except that in no event will we redeem our public shares in an amount that would cause our net tangible assets to be less than $5,000,001 upon consummation of our initial business combination (such that we are not subject to the SEC’s “penny stock” rules) or any greater net tangible asset or cash requirement which may be contained in the agreement relating to our initial business combination. As a result, we may be able to complete our initial business combination even though a substantial majority of our public stockholders do not agree with the transaction and have redeemed their shares or, if we seek stockholder approval of our initial business combination and do not conduct redemptions in connection with our initial business combination pursuant to the tender offer rules, have entered into privately negotiated agreements to sell their shares to our sponsor, officers, directors, advisors or their affiliates. In the event the aggregate cash consideration we would be required to pay for all shares of common stock that are validly submitted for redemption plus any amount required to satisfy cash conditions pursuant to the terms of the proposed initial business combination exceed the aggregate amount of cash available to us, we will not complete the initial business combination or redeem any shares, all shares of common stock submitted for redemption will be returned to the holders thereof, and we instead may search for an alternate business combination.
In order to effectuate an initial business combination, blank check companies have, in the recent past, amended various provisions of their charters and other governing instruments, including their warrant agreements. We cannot assure you that we will not seek to amend our amended and restated certificate of incorporation or governing instruments in a manner that will make it easier for us to complete our initial business combination that our stockholders may not support.
In order to effectuate an initial business combination, blank check companies have, in the recent past, amended various provisions of their charters and modified governing instruments, including their warrant agreements. For example, blank check companies have amended the definition of business combination, increased redemption thresholds and extended the time to consummate an initial business combination and, with respect to their warrants, amended their warrant agreements to require the warrants to be exchanged for cash and/or other securities. Amendments to our amended and restated certificate of incorporation pertaining to pre-business combination activity (including the requirement to deposit proceeds of the IPO, the over-allotment, and the private placement of warrants into the trust account and not release such amounts except in specified circumstances, and to provide redemption rights to public stockholders as described herein) will require the approval of holders of 65% of our common stock, and amending our warrant
agreement will require a vote of holders of at least 50% of the public warrants. In addition, our amended and restated certificate of incorporation requires us to provide our public stockholders with the opportunity to redeem their public shares for cash if we propose an amendment to our amended and restated certificate of incorporation to modify the substance or timing of our obligation to redeem 100% of our public shares if we do not complete our initial business combination within 24 months from the closing of the IPO, or by May 25, 2023, or to offer redemption in connection with a business combination. To the extent any such amendments would be deemed to fundamentally change the nature of any securities offered through this registration statement, we would register, or seek an exemption from registration for, the affected securities. We cannot assure you that we will not seek to amend our charter or governing instruments or extend the time to consummate an initial business combination in order to effectuate our initial business combination.
The provisions of our amended and restated certificate of incorporation that relate to our pre-business combination activity (and corresponding provisions of the agreement governing the release of funds from our trust account), including an amendment to permit us to withdraw funds from the trust account such that the per share amount investors will receive upon any redemption or liquidation is substantially reduced or eliminated, may be amended with the approval of holders of 65% of our common stock, which is a lower amendment threshold than that of some other blank check companies. It may be easier for us, therefore, to amend our amended and restated certificate of incorporation and the trust agreement to facilitate the completion of an initial business combination that some of our stockholders may not support.
Our amended and restated certificate of incorporation provides that any of its provisions related to pre-initial business combination activity (including the requirement to deposit proceeds of the IPO, the over-allotment, and the private placement of warrants into the trust account and not release such amounts except in specified circumstances, and to provide redemption rights to public stockholders as described herein and including to permit us to withdraw funds from the trust account such that the per share amount investors will receive upon any redemption or liquidation is substantially reduced or eliminated) may be amended if approved by holders of 65% of our common stock entitled to vote thereon, and corresponding provisions of the trust agreement governing the release of funds from our trust account may be amended if approved by holders of 65% of our common stock entitled to vote thereon. In all other instances, our amended and restated certificate of incorporation may be amended by holders of a majority of our outstanding common stock entitled to vote thereon, subject to applicable provisions of the DGCL or applicable stock exchange rules. We may not issue additional securities that can vote on amendments to our amended and restated certificate of incorporation. Our initial stockholders, who will collectively beneficially own up to 20% of our common stock upon the closing of the IPO, will participate in any vote to amend our amended and restated certificate of incorporation and/or trust agreement and will have the discretion to vote in any manner they choose. As a result, we may be able to amend the provisions of our amended and restated certificate of incorporation which govern our pre-initial business combination behavior more easily than some other blank check companies, and this may increase our ability to complete an initial business combination with which you do not agree. Our stockholders may pursue remedies against us for any breach of our amended and restated certificate of incorporation.
Our sponsor, executive officers and directors have agreed, pursuant to a written agreement with us, that they will not propose any amendment to our amended and restated certificate of incorporation to modify the substance or timing of our obligation to redeem 100% of our public shares if we do not complete our initial business combination within 24 months from the closing of the IPO, or by May 25, 2023, or to offer redemption in connection with a business combination unless we provide our public stockholders with the opportunity to redeem their shares of common stock upon approval of any such amendment at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account, divided by the number of then outstanding public shares. These agreements are contained in a letter agreement that we have entered into with our sponsor, executive officers and directors. Our stockholders are not parties to, or third-party beneficiaries of, these agreements and, as a result, will not have the ability to pursue remedies against our sponsor, officers or directors for any breach of these agreements. As a result, in the event of a breach, our stockholders would need to pursue an alternative remedy, such as a stockholder derivative action, subject to applicable law.
We may be unable to obtain additional financing to complete our initial business combination or to fund the operations and growth of a target business, which could compel us to restructure or abandon a particular business combination. If we are unable to complete our initial business combination, our public stockholders may only receive their pro rata portion of the funds in the trust account that are available for distribution to public stockholders, and our warrants will expire worthless.
Although we believe that the net proceeds of the IPO, the over-allotment, and the sale of the private placement warrants will be sufficient to allow us to complete our initial business combination, because we have not yet selected any specific target business we cannot ascertain the capital requirements for any particular transaction. If the net proceeds of the IPO, the over-allotment, and the sale of the private placement warrants prove to be insufficient, either because of the size of our initial business combination, the depletion of
the available net proceeds in search of a target business, the obligation to redeem for cash a significant number of shares from stockholders who elect redemption in connection with our initial business combination or the terms of negotiated transactions to purchase shares in connection with our initial business combination, we may be required to seek additional financing or to abandon the proposed business combination. We cannot assure you that such financing will be available on acceptable terms if at all. The current economic environment has made it especially difficult for companies to obtain acquisition financing. To the extentor that additional financing proves to be unavailable when needed to completethey will not have a significant dilutive effect on our initial business combination, we would be compelled to either restructure the transaction or abandon that particular business combination and seek an alternative target business candidate. Ifexisting stockholders. In addition, we are unable to completedetermine at this time whether any of these potential sources of liquidity will be adequate to support our initial business combination,operations or provide sufficient cash flows to us to meet our public stockholders may only receive their pro rata portionobligations as they become due and continue as a going concern. In the event we determine that additional sources of the funds in the trust account that areliquidity will not be available for distribution to public stockholders, andus or will not allow us to meet our warrants will expire worthless. In addition, even if we do not need additional financing to complete our initial business combination,obligations as they become due, we may require such financingneed to fund the operations or growth of the target business. The failure to secure additional financing could havefile a material adverse effect on the continued development or growth of the target business. None of our officers, directors or stockholders is required to provide any financing to us in connection with or after our initial business combination.
Our initial stockholders may exert a substantial influence on actions requiring a stockholder vote, potentially in a manner that you do not support.
Our initial stockholders own shares representing 20% of our issued and outstanding shares of common stock. Accordingly, they may exert a substantial influence on actions requiring a stockholder vote, potentially in a manner that you do not support, including the election of directors, amendments to our amended and restated certificate of incorporation and approval of major corporate transactions. If our initial stockholders purchase any units in the IPO or if our initial stockholders purchase any additional shares of common stock in the aftermarket or in privately negotiated transactions, this would increase their control. Factors that would be considered in making such additional purchases would include consideration of the current trading price of our common stock. In addition, our board of directors, whose members were elected by our initial stockholders, is divided into three classes, each of which, other than the initial term, will generally servevoluntary petition for a term of three years with only one class of directors being elected in each year. We may not hold an annual meeting of stockholders to elect new directors prior to the completion of our initial business combination, in which case all of the current directors will continue in office until at least the completion of the initial business combination. If there is an annual meeting, as a consequence of our “staggered” board of directors, only a minority of the board of directors will be considered for election and our initial stockholders, because of their ownership position, will have considerable influence regarding the outcome. Accordingly, our initial stockholders will continue to exert control at least until the completion of our initial business combination.
Because we must furnish our stockholders with target business financial statements, we may lose the ability to complete an otherwise advantageous initial business combination with some prospective target businesses.
The federal proxy rules require that a proxy statement with respect to a vote on an initial business combination meeting certain financial significance tests include historical and/or pro forma financial statement disclosure in periodic reports. We will include the same financial statement disclosure in connection with our tender offer documents, whether or not they are requiredrelief under the tender offer rules. These financial statements may be required to be prepared in accordance with, or be reconciled to, accounting principles generally accepted in the United States of America (“GAAP”), or international financial reporting standards as issued by the International Accounting Standards Board (“IFRS”), depending on the circumstances and the historical financial statements may be required to be audited in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”). These financial statement requirements may limit the pool of potential target businesses we may acquire because some targets may be unable to provide such financial statements in time for us to disclose such statements in accordance with federal proxy rules and complete our initial business combination within the prescribed time frame.
Compliance obligations under the Sarbanes-Oxley Act may make it more difficult for us to effectuate our initial business combination, require substantial financial and management resources, and increase the time and costs of completing an initial business combination.
Section 404 of the Sarbanes-Oxley Act requires that we evaluate and report on our system of internal controls beginning with our Annual Report on Form 10-K for the year ending December 31, 2022. Only in the event we are deemed to be a large accelerated filer or an accelerated filer, and no longer qualify as an emerging growth company, will we be required to comply with the independent registered public accounting firm attestation requirement on our internal control over financial reporting. Further, for as long as we remain an emerging growth company, we will not be required to comply with the independent registered public accounting firm
attestation requirement on our internal control over financial reporting. The fact that we are a blank check company makes compliance with the requirements of the Sarbanes-Oxley Act particularly burdensome on us as compared to other public companies because a target company with which we seek to complete our initial business combination may not be in compliance with the provisions of the Sarbanes- Oxley Act regarding adequacy of its internal controls. The development of the internal control of any such entity to achieve compliance with the Sarbanes-Oxley Act may increase the time and costs necessary to complete any such business combination.
Risks Relating to the Post-Business Combination
Subsequent to the completion of our initial business combination, we may be required to take write-downs or write-offs, restructuring and impairment or other charges that could have a significant negative effect on our financial condition, results of operations and our stock price, which could cause you to lose some or all of your investment.
Even if we conduct extensive due diligence on a target business with which we combine, we cannot assure you that this diligence will surface all material issues that may be present inside a particular target business, that it would be possible to uncover all material issues through a customary amount of due diligence, or that factors outside of the target business and outside of our control will not later arise. As a result of these factors, we may be forced to later write-down or write-off assets, restructure our operations, or incur impairment or other charges that could result in our reporting losses. Even if our due diligence successfully identifies certain risks, unexpected risks may arise and previously known risks may materialize in a manner not consistent with our preliminary risk analysis. Even though these charges may be non-cash items and not have an immediate impact on our liquidity, the fact that we report charges of this nature could contribute to negative market perceptions about us or our securities. In addition, charges of this nature may cause us to violate net worth or other covenants to which we may be subject as a result of assuming pre-existing debt held by a target business or by virtue of our obtaining debt financing to partially finance the initial business combination. Accordingly, any stockholders who choose to remain stockholders following the initial business combination could suffer a reduction in the value of their shares. Such stockholders are unlikely to have a remedy for such reduction in value.
The key personnel or officers and directors of an acquisition candidate may resign upon completion of our initial business combination. The loss of a business combination target’s key personnel or officers and directors could negatively impact the operations and profitability of our post-combination business.
The role of an acquisition candidate’s key personnel or officers and directors upon the completion of our initial business combination cannot be ascertained at this time. Although we contemplate that certain members of an acquisition candidate’s key personnel or officers and directors will remain associated with the acquisition candidate following our initial business combination, it is possible that certain key personnel or officers and directors of an acquisition candidate will not wish to remain in place.
Our management may not be able to maintain control of a target business after our initial business combination.
We may structure an initial business combination so that the post-transaction company in which our public stockholders own shares will own less than 100% of the equity interests or assets of a target business, but we will only complete such business combination if the post-transaction company owns or acquires 50% or more of the outstanding voting securities of the target or otherwise acquires a controlling interest in the target sufficient for us not to be required to register as an investment company under the Investment Company Act. We will not consider any transaction that does not meet such criteria. Even if the post- transaction company owns 50% or more of the voting securities of the target, our stockholders prior to the initial business combination may collectively own a minority interest in the post business combination company, depending on valuations ascribed to the target and us in the initial business combination. For example, we could pursue a transaction in which we issue a substantial number of new shares of common stock in exchange for all of the outstanding capital stock of a target. In this case, we would acquire a 100% interest in the target. However, as a result of the issuance of a substantial number of new shares of common stock, our stockholders immediately prior to such transaction could own less than a majority of our outstanding shares of common stock subsequent to such transaction. In addition, other minority stockholders may subsequently combine their holdings resulting in a single person or group obtaining a larger share of the company’s stock than we initially acquired. Accordingly, this may make it more likely that our management will not be able to maintain our control of the target business. We cannot provide assurance that, upon loss of control of a target business, new management will possess the skills, qualifications or abilities necessary to profitably operate such business.
We may have a limited ability to assess the management of a prospective target business and, as a result, may effect our initial business combination with a target business whose management may not have the skills, qualifications or abilities to manage a public company, which could, in turn, negatively impact the value of our stockholders’ investment in us.
When evaluating the desirability of effecting our initial business combination with a prospective target business, our ability to assess the target business’s management may be limited due to a lack of time, resources or information. Our assessment of the capabilities of the target’s management, therefore, may prove to be incorrect and such management may lack necessary skills, qualifications or abilities. Should the target’s management not possess the skills, qualifications or abilities necessary to manage a public company, the operations and profitability of the post-combination business may be negatively impacted. Accordingly, any stockholders who choose to remain stockholders following the initial business combination could suffer a reduction in the value of their shares. Such stockholders are unlikely to have a remedy for such reduction in value.
Risks Relating to Our Management Team
We are dependent upon our key personnel and our executive officers and directors, and their departure could adversely affect our ability to operate.
Our operations are dependent upon a relatively small group of individuals and, in particular, our key personnel and our executive officers and directors. We believe that our success depends on the continued service of our key personnel and our executive officers and directors, at least until we have completed our initial business combination. However, the registration statement relating to the IPO, our amended and restated certificate of incorporation and ourBankruptcy Code of Business Conduct and Ethics preclude our executive officers and directors from directly competing with us. In addition, at the time of our IPO, all of our executive officers agreed to focus substantially all of their professional time on us and Graf II and Graf III (once Graf II and Graf III consummate their respective initial public offerings), and subsequent Graf-affiliated SPACs. We do not have an employment agreement with, or key-man insurance on the life of, any of our key personnel or our directors or executive officers. The unexpected loss of the services of one or more of our key personnel or our directors or executive officers could have a detrimental effect on us.
Our ability to successfully effect our initial business combination and to be successful thereafter will be totally dependent upon the efforts of our key personnel, some of whom may join us following our initial business combination. The loss of key personnel could negatively impact our ability to effect our initial business combination or the operations and profitability of our post-combination business.
Our ability to successfully effect our initial business combination is dependent upon the efforts of our key personnel. The role of our key personnel in the target business, however, cannot presently be ascertained. Although some of our key personnel may remain with the target business in senior management or advisory positions following our initial business combination, it is likely that some or all of the management of the target business will remain in place. While we intend to closely scrutinize any individuals we employ after our initial business combination, we cannot assure you that our assessment of these individuals will prove to be correct. These individuals may be unfamiliar with the requirements of operating a company regulated by the SEC, which could cause us to have to expend time and resources helping them become familiar with such requirements. In addition, the officers and directors of an initial business combination candidate may resign upon completion of our initial business combination. The departure of an initial business combination target’s key personnel could negatively impact the operations and profitability of our post- combination business. The role of an initial business combination candidate’s key personnel upon the completion of our initial business combination cannot be ascertained at this time. Although we contemplate that certain members of an initial business combination candidate’s management team will remain associated with the initial business combination candidate following our initial business combination, it is possible that members of the management of an initial business combination candidate will not wish to remain in place. The loss of key personnel could negatively impact the operations and profitability of our post- combination business.
Our key personnel may negotiate employment or consulting agreements with a target business in connection with a particular business combination. These agreements may provide for them to receive compensation following our initial business combination and as a result, may cause them to have conflicts of interest in determining whether a particular business combination is the most advantageous.
Our key personnel may be able to remain with the company after the completion of our initial business combination only if they are able to negotiate employment or consulting agreements in connection with the initial business combination. Such negotiations
would take place simultaneously with the negotiation of the initial business combination and could provide for such individuals to receive compensation in the form of cash payments and/or our securities for services they would render to us after the completion of the initial business combination. The personal and financial interests of such individuals may influence their motivation in identifying and selecting a target business. However, we believe the ability of such individuals to remain with us after the completion of our initial business combination will not be the determining factor in our decision as to whether or not we will proceed with any potential business combination. There is no certainty, however, that any of our key personnel will remain with us after the completion of our initial business combination. We cannot assure you that any of our key personnel will remain in senior management or advisory positions with us. The determination as to whether any of our key personnel will remain with us will be made at the time of our initial business combination.
If our officers and directors allocate time to other businesses ,then this may cause conflicts of interest in their determination as to how much time to devote to our affairs. This conflict of interest could have a negative impact on our ability to complete our initial business combination.
Our officers and directors are not required to, and will not, commit their full time to our affairs, which may result in a conflict of interest in allocating their time between our operations and our search for an initial business combination and their other businesses. Each of our officers are engaged in several other business endeavors and are not obligated to contribute any specific number of hours per week to our affairs. In particular, each of our officers and directors serve as an officer or director of Graf II and Graf III, and may have similar responsibilities in subsequent Graf-affiliated SPACs, each of which are blank check companies sponsored by Graf Acquisition Partners InvestCo LLC (our “sponsor parent entity”), which have not yet consummated their initial public offerings, or of other blank check companies. However, all of our executive officers are expected to focus substantially all of their professional time on our affairs, on Graf II and Graf III (to the extent Graf II and Graf III consummate their respective initial public offerings) and subsequent Graf-affiliated SPACs In addition, our officers and directors may also have conflicts of interest with respect to their other professional activities, including Mr. Graf’s involvement as an independent director of Catcha Investment Corp. and Messrs. Garcia, Silberman and Ramamurti’s service as (a) officers and directors of GSR II Meteora Acquisition Corp. (Nasdaq: GSRM) (“GSR”), a blank check company, and (b) managing partners of SPAC Advisory Partners, LLC (“SAP LLC”), an advisory firm. If our officers’ and directors’ other business affairs require them to devote substantial amounts of time to such affairs (other than time commitments pertaining to us, Graf II, Graf III, and other Graf-affiliated SPACs) in excess of their current commitment levels, it could limit their ability to devote time to our affairs which may have a negative impact on our ability to complete our initial business combination.
Past performance by our management team may not be indicative of future performance of an investment in the Company.
Information regarding performance by, or businesses associated with, our management team or businesses associated with them is presented for informational purposes only. Past performance by our management team, including, without limitation, with respect to Graf Industrial Corp. (“Graf I”) is not a guarantee (i) of success with respect to any business combination we may or may attempt to consummate or (ii) that we will be able to locate a suitable candidate for our initial business combination. You should not rely on the historical record of the performance of our management team or businesses associated with them, including Graf I, as indicative of our future performance of an investment in the company or the returns the company will, or is likely to, generate going forward.
All of our officers and directors are now, and may in the future become, affiliated with entities engaged in business activities similar to those intended to be conducted by us, including another blank check company, and, accordingly, may have conflicts of interest in allocating their time and determining to which entity a particular business opportunity should be presented.
Until we consummate our initial business combination, we intend to engage in the business of identifying and combining with one or more businesses. Our sponsor and officers and directors are, and may in the future become, affiliated with entities (such as operating companies or investment vehicles) that are engaged in a similar business, including another blank check company that may have acquisition objectives that are similar to ours. All of our executive officers are expected to focus substantially all of their professional time on the Company, on Graf II and Graf III (once Graf II and Graf III consummate their respective initial public offerings), and subsequent Graf-affiliated SPACs. However, our officers and directors may have conflicts of interest with respect to their other professional activities, including Mr. Graf’s involvement as an independent director of Catcha Investment Corp. and each of Messrs. Garcia, Silberman and Ramamurti’s service as (a) officers and directors of GSR, a blank check company and (b) managing partners of SAP LLC, an advisory firm. We do not have employment contracts with our officers and directors that will limit their ability to work at other businesses, however, the registration statement relating to our IPO, our amended and restated certificate of incorporation and our Code of Business Conduct and Ethics preclude our executive officers and directors from directly competing with us. In addition to the above, our sponsor, officers and directors may participate in the formation of, or become an officer or director of, other blank check
companies prior to completion of our initial business combination. As a result, our sponsor, officers or directors could have conflicts of interest in determining whether to present business combination opportunities to us or to any other blank check company with which they may become involved. Although we have no formal policy in place for vetting potential conflicts of interest, our board of directors will review any potential conflicts of interest on a case-by-case basis, including, but not limited to, the involvement of each of Messrs. Garcia, Silberman and Ramamurti in the GSR and SAP LLC. In particular, each of our officers and directors serve as an officer or director of Graf II and Graf III, and may have similar responsibilities in subsequent Graf-affiliated SPACs, each of which are blank check companies sponsored by the sponsor parent entity, which have not yet consummated their initial public offerings, or of other blank check companies. Each of Graf II and Graf III may seek to complete a business combination in any location and is not focusing on any particular industry for business combinations and differs only in size. Any such companies, including Graf II, Graf III, Catcha Investment Corp., GSR and SAP LLC, may present additional conflicts of interest in pursuing an acquisition target.
Our officers and directors also may become aware of business opportunities which may be appropriate for presentation to us and the other entities to which they owe certain fiduciary or contractual duties.
Accordingly, they may have conflicts of interest in determining to which entity a particular business opportunity should be presented. These conflicts may not be resolved in our favor and a potential target business may be presented to another entity prior to its presentation to us. Our amended and restated certificate of incorporation provides that we renounce our interest in any corporate opportunity offered to any director or officer unless such opportunity is expressly offered to such person solely in his or her capacity as a director or officer of our company and such opportunity is one we are legally and contractually permitted to undertake and would otherwise be reasonable for us to pursue, and to the extent the director or officer is permitted to refer that opportunity to us without violating another legal obligation. The purpose for the surrender of corporate opportunities is to allow officers, directors or other representatives with multiple business affiliations to continue to serve as an officer of our company or on our board of directors. Our officers and directors may from time to time be presented with opportunities that could benefit both another business affiliation and us. In the absence of the “corporate opportunity” waiver in our charter, certain candidates would not be able to serve as an officer or director. We believe we substantially benefit from having representatives, who bring significant, relevant and valuable experience to our management, and, as a result, the inclusion of the “corporate opportunity” waiver in our amended and restated certificate of incorporation provides us with greater flexibility to attract and retain the officers and directors that we feel are the best candidates.
However, the personal and financial interests of our directors and officers may influence their motivation in timely identifying and selecting a target business and completing a business combination. The different timelines of competing business combinations could cause our directors and officers to prioritize a different business combination over finding a suitable acquisition target for our business combination. Consequently, our directors’ and officers’ discretion in identifying and selecting a suitable target business may result in a conflict of interest when determining whether the terms, conditions and timing of a particular business combination are appropriate and in our stockholders’ best interest, which could negatively impact the timing for a business combination.
Our officers, directors, security holders and their respective affiliates may have competitive pecuniary interests that conflict with our interests.
We have not adopted a policy that expressly prohibits our directors, officers, security holders or affiliates from having a direct or indirect pecuniary or financial interest in any investment to be acquired or disposed of by us or in any transaction to which we are a party or have an interest. In fact, we may enter into an initial business combination with a target business that is affiliated with our sponsor, our sponsor parent entity, our directors or officers, or any of their affiliates, although we do not intend to do so. Accordingly, such persons or entities may have a conflict between their interests and ours.
The personal and financial interests of our directors and officers may influence their motivation in timely identifying and selecting a target business and completing a business combination. Consequently, our directors’ and officers’ discretion in identifying and selecting a suitable target business may result in a conflict of interest including Mr. Graf’s involvement as an independent director of Catcha Investment Corp and Messrs. Garcia, Silberman and Ramamurti’s service as (a) officers and directors of GSR and (b) managing partners of SAP LLC, an advisory firm, when determining whether the terms, conditions and timing of a particular business combination are appropriate and in our stockholders’ best interest. If this were the case, it would be a breach of their fiduciary duties to us as a matter of Delaware law and we or our stockholders might have a claim against such individuals for infringing on our stockholders’ rights. However, we might not ultimately be successful in any claim we may make against them for such or any other reason.
We may not have sufficient funds to satisfy indemnification claims of our directors and executive officers.
We have agreed to indemnify our officers and directors to the fullest extent permitted by law. However, our executive officers and directors have agreed to waive any right, title, interest or claim of any kind in or to any monies in the trust account and to not seek recourse against the trust account for any reason whatsoever.
Accordingly, any indemnification provided will be able to be satisfied by us only if (i) we have sufficient funds outside of the trust account, (ii) proceeds have been received from D&O insurance policies, or (iii) we consummate an initial business combination. Our obligation to indemnify our officers and directors may discourage stockholders from bringing a lawsuit against our officers or directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against our officers and directors, even though such an action, if successful, might otherwise benefit us and our stockholders.
Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against our officers and directors pursuant to these indemnification provisions.
Certain agreements related to the IPO may be amended without stockholder approval.
Each of the agreements related to the IPO to which we are a party, other than the warrant agreement and the investment management trust agreement, may be amended without stockholder approval. Such agreements include: the underwriting agreement; the letter agreement among us and our initial stockholders, sponsor, officers and directors; the registration rights agreement among us and our initial stockholders; the private placement warrants purchase agreement between us and our sponsor; and the administrative service agreement among us and an affiliate of our sponsor. These agreements contain various provisions that our public stockholders might deem to be material. For example, our letter agreement and the underwriting agreement contain certain lock-up provisions with respect to the founder shares, private placement warrants and other securities held by our initial stockholders, sponsor, officers and directors. Amendments to such agreements would require the consent of the applicable parties thereto and would need to be approved by our board of directors, which may do so for a variety of reasons, including to facilitate our initial business combination. While we do not expect our board of directors to approve any amendment to any of these agreements prior to our initial business combination, it may be possible that our board of directors, in exercising its business judgment and subject to its fiduciary duties, chooses to approve one or more amendments to any such agreement. Any amendment entered into in connection with the consummation of our initial business combination will be disclosed in our proxy solicitation or tender offer materials, as applicable, related to such initial business combination, and any other material amendment to any of our material agreements will be disclosed in a filing with the SEC. Any such amendments would not require approval from our stockholders, may result in the completion of our initial business combination that may not otherwise have been possible, and may have an adverse effect on the value of an investment in our securities. For example, amendments to the lock-up provision discussed above may result in our initial stockholders selling their securities earlier than they would otherwise be permitted, which may have an adverse effect on the price of our securities.
Risks Relating to our Securities
You will not have any rights or interests in funds from the trust account, except under certain limited circumstances. To liquidate your investment, therefore, you may be forced to sell your public shares or warrants, potentially at a loss.
Our public stockholders will be entitled to receive funds from the trust account only upon the earliest to occur of: (i) our completion of an initial business combination, and then only in connection with those shares of common stock that such stockholder properly elected to redeem, subject to the limitations described herein, (ii) the redemption of any public shares properly submitted in connection with a stockholder vote to amend our amended and restated certificate of incorporation to modify the substance or timing of our obligation to redeem 100% of our public shares if we do not complete our initial business combination within 24 months from the closing of the IPO, or by May 25, 2023, or to provide for redemption in connection with a business combination and (iii) the redemption of our public shares if we are unable to complete an initial business combination within 24 months from the closing of the IPO, or by May 25, 2023, subject to applicable law and as further described herein. In no other circumstances will a public stockholder have any right or interest of any kind in the trust account. Holders of warrants will not have any right to the proceeds held in the trust account with respect to the warrants. Accordingly, to liquidate your investment, you may be forced to sell your public shares or warrants, potentially at a loss.
The NYSE may delist our securities from trading on its exchange, which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions.
Our units, common stock and warrants are currently listed on the NYSE. Although after giving effect to the IPO we expect to meet, on a pro forma basis, the minimum listing standards set forth in the NYSE listing standards, we cannot assure you that our securities will continue to be listed on the NYSE in the future or prior to our initial business combination. In order to continue listing our securities on the NYSE prior to our initial business combination, we must maintain certain financial, distribution and share price levels. Generally, we must maintain a minimum average global market capitalization and a minimum number of holders of our securities.
Additionally, in connection with our initial business combination, we will be required to demonstrate compliance with the NYSE’s initial listing requirements, which are more rigorous than the NYSE’s continued listing requirements, in order to continue to maintain the listing of our securities on the NYSE. For instance, our share price would generally be required to be at least $4.00 per share, our global market capitalization would be required to be at least $150 million, the aggregate market value of our publicly-held shares would be required to be at least $40 million and we would be required to haveimplement a minimum of 400 round lot holders and 1,100,000 publicly held shares. We cannot assure you that we will be able to meet those initial listing requirements at that time.
If the NYSE delists our securities from trading on its exchange and we are not able to list our securities on another national securities exchange, we expect our securities could be quoted on an over-the-counter market. If this were to occur, we could face significant material adverse consequences, including:
| ● | a limited availability of market quotations for our securities; |
| ● | reduced liquidity for our securities; |
| ● | a determination that our common stock is a “penny stock” which will require brokers trading in our common stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our securities; |
| ● | a limited amount of news and analyst coverage; and |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
The National Securities Markets Improvement Act of 1996, which is a federal statute, preventsrestructuring plan or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” Because our units, common stock and warrants are listed on the NYSE, we expect that our units, common stock and warrants will be covered securities under the statute. Although the states are preempted from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. While we are not aware of a state having used these powers to prohibit or restrict the sale of securities issued by blank check companies, other than the State of Idaho, certain state securities regulators view blank check companies unfavorably and might use these powers, or threaten to use these powers, to hinder the sale of securities of blank check companies in their states. Further, if we were no longer listed on the NYSE, our securities would not be covered securities and we would be subject to regulation in each state in which we offer our securities, including in connection with our initial business combination.
If you exercise your public warrants on a “cashless basis,” you will receive fewer shares of common stock from such exercise than if you were to exercise such warrants for cash.
There are circumstances in which the exercise of the public warrants may be required or permitted to be made on a cashless basis. First, if a registration statement covering the shares of common stock issuable upon exercise of the warrants is not effective by the 60th business day after the closing of our initial business combination, warrant holders may, until such time as there is an effective registration statement, exercise warrants on a cashless basis in accordance with Section 3(a)(9) of the Securities Act or another exemption from registration. Second, if our common stock is at any time of any exercise of a warrant not listed on a national securities exchange such that it satisfies the definition of a “covered security” under Section 18(b)(1) of the Securities Act, we may, at our option, require holders of public warrants who exercise their warrants to do so on a cashless basis in accordance with Section 3(a)(9) of the Securities Act and, in the event we so elect, we will not be required to file or maintain in effect a registration statement, and in the event
we do not so elect, we will use commercially reasonable efforts to register or qualify the shares under applicable blue sky laws to the extent an exemption is not available. Third, if we call the public warrants for redemption, our management will have the option to require all holders that wish to exercise warrants to do so on a cashless basis.liquidation. In the event of an exercise on a cashless basis, a holder would pay the warrant exercise price by surrendering the warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the warrants, multiplied by the excess of the “fair market value” of our common stock (as defined in the next sentence) over the exercise price of the warrants by (y) the fair market value. The “fair market value” is the average reported last sale price of the common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of exercise is received by the warrant agent or on which the notice of redemption is sent to the holders of warrants, as applicable. As a result, you would receive fewer shares of common stock from such exercise than if you were to exercise such warrants for cash.
The grant of registration rights to our initial stockholders may make it more difficult to complete our initial business combination, and the future exercise of such rights may adversely affect the market price of our common stock.
Pursuant to an agreement entered into concurrently with the issuance and sale of the securities in the IPO, our initial stockholders and their permitted transferees can demand that we register the private placement warrants, the shares of common stock issuable upon exercise of the founder shares and the private placement warrants held by them and holders of warrants that may be issued upon conversion of working capital loans may demand that we register such warrants or the common stock issuable upon exercise of such warrants. We will bear the cost of registering these securities. The registration and availability of such a significant number of securities for trading in the public market may have an adverse effect on the market price of our common stock. In addition, the existence of the registration rights may make our initial business combination more costly or difficult to conclude. This is because the stockholders of the target business may increase the equity stake they seek in the combined entity or ask for more cash consideration to offset the negative impact on the market price of our common stock that is expected when the securities owned by our initial stockholders or holders of working capital loans or their respective permitted transferees are registered.
The determination of the IPO price of our units and the size of the IPO is more arbitrary than the pricing of securities and size of an offering of an operating company in a particular industry. You may have less assurance, therefore, that the IPO price of our units properly reflects the value of such units than you would have in a typical offering of an operating company.
Prior to the IPO there has been no public market for any of our securities. The public offering price of the units and the terms of the warrants were negotiated between us and the underwriters. In determining the size of the IPO, management held customary organizational meetings with representatives of the underwriters, both prior to our inception and thereafter, with respect to the state of capital markets, generally, and the amount the underwriters believed they reasonably could raise on our behalf. Factors considered in determining the size of the IPO, prices and terms of the units, including the common stock and warrants underlying the units, include:
| ● | the history and prospects of companies whose principal business is the acquisition of other companies; |
| ● | prior offerings of those companies; |
| ● | our prospects for acquiring an operating business; |
| ● | a review of debt to equity ratios in leveraged transactions; |
| ● | an assessment of our management and their experience in identifying operating companies; |
| ● | general conditions of the securities markets at the time of the IPO; and |
| ● | other factors as were deemed relevant. |
Although these factors were considered, the determination of our offering price is more arbitrary than the pricing of securities of an operating company in a particular industry since we have no historical operations or financial results.
We may amend the terms of the warrants in a manner that may be adverse to holders of public warrants with the approval by the holders of at least 50% of the then outstanding public warrants. As a result, the exercise price of your warrants could be increased, the exercise period could be shortened and the number of shares of our common stock purchasable upon exercise of a warrant could be decreased, all without your approval.
Our warrants will be issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and us. The warrant agreement provides that the terms of the warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, but requires the approval by the holders of at least 50% of the then outstanding public warrants to make any change that adversely affects the interests of the registered holders of public warrants. Accordingly, we may amend the terms of the public warrants in a manner adverse to a holder if holders of at least 50% of the then outstanding public warrants approve of such amendment. Although our ability to amend the terms of the public warrants with the consent of at least 50% of the then outstanding public warrants is unlimited, examples of such amendments could be amendments to, among other things, increase the exercise price of the warrants, convert the warrants into cash or stock, shorten the exercise period or decrease the number of shares of our common stock purchasable upon exercise of a warrant.
Our warrant agreement designates the courts of the State of New York or the United States District Court for the Southern District of New York as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by holders of our warrants, which could limit the ability of warrant holders to obtain a favorable judicial forum for disputes with our company.
Our warrant agreement provides that, subject to applicable law, (i) any action, proceeding or claim against us arising out of or relating in any way to the warrant agreement, including under the Securities Act, will be brought and enforced in the courts of the State of New York or the United States District Court for the Southern District of New York, and (ii) that we irrevocably submit to such jurisdiction, which jurisdiction shall be the exclusive forum for any such action, proceeding or claim. We will waive any objection to such exclusive jurisdiction and that such courts represent an inconvenient forum.
Notwithstanding the foregoing, these provisions of the warrant agreement will not apply to suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal district courts of the United States of America are the sole and exclusive forum. Any person or entity purchasing or otherwise acquiring any interest in any of our warrants shall be deemed to have notice of and to have consented to the forum provisions in our warrant agreement. If any action, the subject matter of which is within the scope the forum provisions of the warrant agreement, is filed in a court other than a court of the State of New York or the United States District Court for the Southern District of New York (a “foreign action”) in the name of any holder of our warrants, such holder shall be deemed to have consented to: (x) the personal jurisdiction of the state and federal courts located in the State of New York in connection with any action brought in any such court to enforce the forum provisions (an “enforcement action”), and (y) having service of process made upon such warrant holder in any such enforcement action by service upon such warrant holder’s counsel in the foreign action as agent for such warrant holder.
This choice-of-forum provision may limit a warrant holder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with our company, which may discourage such lawsuits. Alternatively, if a court were to find this provision of our warrant agreement inapplicable or unenforceable with respect to one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could materially and adversely affect our business, financial condition and results of operations and result in a diversion of the time and resources of our management and board of directors.
Certain of our warrants are expected to be accounted for as a warrant liability and will be recorded at fair value upon issuance with changes in fair value each period reported in earnings, which may have an adverse effect on the market price of our common stock or may make it more difficult for us to consummate an initial business combination.
We account for the 4,721,533 private placement warrants in accordance with the guidance contained in Derivatives and Hedging - Contracts in Entity’s Own Equity (ASC 815-40). Such guidance provides that because the warrants do not meet the criteria for equity treatment thereunder, each warrant must be recorded as a liability. Accordingly, we will classify each warrant as a liability at its fair value. This liability is subject to re-measurement at each balance sheet date. With each such re-measurement, the warrant liability will be adjusted to fair value, with the change in fair value recognized in our statement of operations and therefore our reported earnings. The impact of changes in fair value on earnings may have an adverse effect on the market price of our common stock. In addition, potential targets may seek a SPAC that does not have warrants that are accounted for as a warrant liability, which may make it more difficult for us to consummate an initial business combination with a target business.
We may redeem your unexpired warrants prior to their exercise at a time that is disadvantageous to you, thereby making your warrants worthless.
We have the ability to redeem outstanding warrants at any time after they become exercisable and prior to their expiration, at a price of $0.01 per warrant, provided that the last reported sales price of our common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within a 30 trading-day period ending on the third trading day prior to the date on which we give proper notice of such redemption and provided certain other conditions are met. If and when the warrants become redeemable by us, we may exercise our redemption right even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws. We will use commercially reasonable efforts to register or qualify such shares of common stock under the blue sky laws of the state of residence in those states in which the warrants were offered by us in the IPO. Redemption of the outstanding warrants could force you (i) to exercise your warrants and pay the exercise price therefor at a time when it may be disadvantageous for you to do so, (ii) to sell your warrants at the then-current market price when you might otherwise wish to hold your warrants or (iii) to accept the nominal redemption price which, at the time the outstanding warrants are called for redemption, is likely to be substantially less than the market value of your warrants. None of the private placement warrants will be redeemable by us so long as they are held by the sponsor or its permitted transferees.
Our warrants may have an adverse effect on the market price of our common stock and make it more difficult to effectuate our initial business combination.
We issued warrants to purchase 3,432,300 shares of our common stock as part of the units offered by the IPO and, simultaneously with the closing of the IPO, we issued in a private placement, an aggregate of 4,721,533 private placement warrants, each exercisable to purchase one share of common stock at $11.50 per share, including the partial exercise by the underwriters of their over-allotment option. In addition, if our sponsor or its affiliates, or any of our officers or directors, makes any working capital loans, up to $1,500,000 of such loans may be converted into additional warrants at a price of $1.50 per warrant at the option of the lender. Such warrants would be identical to the private placement warrants, including as to exercisability and exercise price.
To the extent we issue shares of common stock to effectuate an initial business combination, the potential for the issuance of a substantial number of additional shares of common stock upon exercise of these warrants and conversion rights could make us a less attractive business combination vehicle to a target business. Any such issuance will increase the number of issued and outstanding shares of our common stock and reduce the value of the shares of common stock issued to complete the initial business combination. Therefore, our warrants and founder shares may make it more difficult to effectuate an initial business combination or increase the cost of acquiring the target business.
The private placement warrants are identical to the warrants sold as part of the units in the IPO except that, so long as they are held by our sponsor or its permitted transferees, they (including the common stock issuable upon exercise of these warrants) may not, subject to certain limited exceptions, be transferred, assigned or sold by our sponsor until 30 days after the completion of our initial business combination.
Because each unit contains one-fifth of one warrant and only a whole warrant may be exercised, the units may be worth less than units of other blank check companies.
Each unit contains one-fifth of one warrant. Pursuant to the warrant agreement, no fractional warrants will be issued upon separation of the units, and only whole units will trade. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, round down to the nearest whole number the number of shares of common stock to be issued to the warrant holder. This is different from other offerings similar to ours, whose units include one share of common stock and one warrant to purchase one whole share. We have established the components of the units in this way in order to reduce the dilutive effect of the warrants upon completion of a business combination since the warrants will be exercisable in the aggregate for one-fifth of the number of shares compared to units that each contain a whole warrant to purchase one share, thus making us, we believe, a more attractive merger partner for target businesses. Nevertheless, this unit structure may cause our units to be worth less than if it included a warrant to purchase one whole share.
A provision of our warrant agreement may make it more difficult for us to complete an initial business combination.
If (i) we issue additional common stock or equity-linked securities for capital raising purposes in connection with the closing of our initial business combination at a Newly Issued Price of less than $9.20 per share of common stock, (ii) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of our initial business combination on the date of the completion of our initial business combination (net of redemptions), and (iii) the Market Value is below $9.20 per share, then the exercise price of the warrants will be adjusted to be equal to 115% of the higher of the Market Value and the Newly Issued Price, and the $18.00 per share redemption trigger prices will be adjusted (to the nearest cent) to be equal to 180% of the higher of the Market Value and the Newly Issued Price. This may make it more difficult for us to complete an initial business combination with a target business.
A market for our securities may not develop, or if developed, it may not be sustained, which would adversely affect the liquidity and price of our securities.
The price of our securities may vary significantly due to one or more potential business combinations and general market or economic conditions. Furthermore, an active trading market for our securities may never develop or, if developed, it may not be sustained. You may be unable to sell your securities unless a market can be established and sustained.
Provisions in our amended and restated certificate of incorporation and Delaware law may have the effect of discouraging lawsuits against our directors and officers.
Our amended and restated certificate of incorporation require, unless we consent in writing to the selection of an alternative forum, that (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee to us or our stockholders, (iii) any action asserting a claim against us, our directors, officers or employees arising pursuant to any provision of the DGCL or our amended and restated certificate of incorporation or bylaws, or (iv) any action asserting a claim against us, our directors, officers or employees governed by the internal affairs doctrine may be brought only in the Court of Chancery in the State of Delaware, except any claim (A) as to which the Court of Chancery of the State of Delaware determines that there is an indispensable party not subject to the jurisdiction of the Court of Chancery (and the indispensable party does not consent to the personal jurisdiction of the Court of Chancery within ten days following such determination), (B) which is vested in the exclusive jurisdiction of a court or forum other than the Court of Chancery or (C) for which the Court of Chancery does not have subject matter jurisdiction. If an action is brought outside of Delaware, the stockholder bringing the suit will be deemed to have consented to service of process on such stockholder’s counsel. Although we believe this provision benefits us by providing increased consistency in the application of Delaware law in the types of lawsuits to which it applies, a court may determine that this provision is unenforceable, and to the extent it is enforceable, the provision may have the effect of discouraging lawsuits against our directors and officers, although our stockholders will not be deemed to have waived our compliance with federal securities laws and the rules and regulations thereunder.
Notwithstanding the foregoing, our amended and restated certificate of incorporation provides that the exclusive forum provision will not apply to suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. Additionally, unless we consent in writing to the selection of an alternative forum, the federal courts shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act against us or any of our directors, officers, other employees or agents. Section 22 of the Securities Act, however, created concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. Accordingly, there is uncertainty as to whether a court would enforce these exclusive forum provisions, and the enforceability of similar choice of forum provisions in other companies’ charter documents has been challenged in legal proceedings. While the Delaware courts have determined that such exclusive forum provisions are facially valid, a stockholder may nevertheless seek to bring a claim in a venue other than those designated in the exclusive forum provisions, and there can be no assurance that such provisions will be enforced by a court in those other jurisdictions. Any person or entity purchasing or otherwise acquiring any interest in our securities shall be deemed to have notice of and consented to these provisions; however, we note that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Although we believe this provision benefits us by providing increased consistency in the application of Delaware law in the types of lawsuits to which it applies, the provision may limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us and may have the effect of discouraging lawsuits against our directors and officers.
Provisions in our amended and restated certificate of incorporation and Delaware law may inhibit a takeover of us, which could limit the price investors might be willing to pay in the future for our common stock and could entrench management.
Our amended and restated certificate of incorporation contains provisions that may discourage unsolicited takeover proposals that stockholders may consider to be in their best interests. These provisions include a staggered board of directors and the ability of the board of directors to designate the terms of and issue new series of preferred shares, which may make the removal of management more difficult and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities.
We are also subject to anti-takeover provisions under Delaware law, which could delay or prevent a change of control. Together these provisions may make the removal of management more difficult and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities.
General Risk Factors
We are a newly formed company with no operating history and no revenues, and you have no basis on which to evaluate our ability to achieve our business objective.
We are a newly formed company with no operating results, and we will not commence operations until completing an initial business combination. Because we lack an operating history, you have no basis upon which to evaluate our ability to achieve our business objective of completing our initial business combination with one or more target businesses. We have no plans, arrangements or understandings with any prospective target business concerning an initial business combination and may be unable to complete our initial business combination. If we fail to complete our initial business combination, we will never generate any operating revenues.
Our independent registered public accounting firm’s report contains an explanatory paragraph that expresses substantial doubt about our ability to continue as a “going concern”.
Asgoing concern may cause, investors or other financing sources to be unwilling to provide funding to us on commercially reasonable terms, if at all. If sufficient funds are not available, we will have to delay, reduce the scope of, or eliminate some or all of our business activities, which would adversely affect our business prospects and our ability to continue our operations.
In addition, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our financial statements, and/or seek protection under Chapters 7 or 11 of the United States Bankruptcy Code. This could potentially cause us to cease operations and result in a complete or partial loss of your investment in our common stock. This could potentially cause us to cease operations and result in a total loss of your investment in our common stock.
The Report of Independent Registered Public Accounting Firm to our December 31,
2021, we had approximately $1.7 million in our operating bank account and working capital of approximately $642,000. Further, we have incurred and expect to continue to incur significant costs in pursuit of our acquisition plans. We cannot assure you2023, financial statements includes an explanatory paragraph that
our cash reserves will be sufficient to complete our acquisition plans or that our plans to consummate an initial business combination will be successful. These factors, among others, raiseexpressed substantial doubt about our ability to continue as a going concern.
TheOur management has also independently determined that there is substantial doubt about our ability to continue as a going concern because we have incurred significant operating losses and expect to continue incurring losses for the foreseeable future. Our financial statements
contained elsewhere in this annual reportwere prepared assuming that we will continue as a going concern and do not include any adjustments that
mightmay result from
the outcome of this uncertainty. Given the uncertainty regarding our
inabilityfinancial condition, substantial doubt exists about our ability to continue as a going
concern.Cyber incidentsconcern for a reasonable period of time.
Because the proceeds from the Business Combination and our recent financing arrangements as discussed in the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” herein, including the Forward Purchase Agreements, the Warrant Subscription Agreements and the Securities Purchase Agreement, are not adequate to cover our accrued and unpaid expenses and provide the cash and liquidity necessary to operate our business, we continue to seek additional financing, including debt and equity financing, and other sources of financing such as forward purchase arrangements, senior convertible promissory notes and other sources of capital, including with related parties. To the extent that we raise additional capital through the future sale of equity or attacks directeddebt, the ownership interest of our stockholders will be diluted. The terms of these securities may include liquidation or other preferences that adversely affect the rights of our existing common stockholders. If we raise additional funds through collaboration agreements, marketing agreements, or licensing arrangements, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates on terms that may not be favorable to it.
If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms or at all. Further, the perception that we may be unable to continue as a going concern may impede our ability to pursue any potential strategic opportunities or operate our business due to concerns regarding our ability to discharge our contractual obligations. Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and, if approved, commercialize our product candidates. In addition, our ability to raise necessary financing could be impacted by macro-economic conditions, such as an inflationary period or economic slowdown, and market impacts as a result of geopolitical events, including relating to Russia’s invasion of Ukraine and the State of Israel’s war against Hamas. If we are unable to obtain sufficient funding on a timely basis and on acceptable terms and continue as a going concern, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any product candidates or to otherwise reduce or discontinue our operations. If we are ultimately unable to continue as a going concern, we may have to seek the protection of bankruptcy laws or liquidate our assets and may receive less than the value at which those assets are carried on our audited financial statements, and it is likely that our stockholders will lose all or a part of their investment.
Our business depends upon the success of our NK cell therapy platform.
Our success depends on our ability to utilize our NK cell technology platform to generate product candidates, to obtain regulatory approval for such product candidates, and to ultimately commercialize such product candidates. Phase I and Phase I/II clinical trials to evaluate our first NK cell product candidate, SNK01, in humans are ongoing. All of our product candidates developed from our technology platform will require significant additional clinical and non-clinical development, review and approval by the U.S. Food and Drug Administration (the “FDA”) or other regulatory authorities in one or more jurisdictions, substantial investment, access to sufficient commercial manufacturing capacity and significant marketing efforts before they can be successfully commercialized. If any of our product candidates encounter safety or efficacy problems, developmental delays or regulatory issues, or other problems, such problems could impact the development plans for our other product candidates because all of our product candidates are based on the same core NK cell manufacturing technology.
Utilizing NK cells represents a novel approach to the treatment of oncological and neurodegenerative diseases, and we must overcome significant challenges in order to develop, commercialize and manufacture our product candidates.
To date, the FDA has approved only a few cell-based therapies for commercialization and no NK-based cell therapy has been approved for commercial use by any regulatory authority. The processes and requirements imposed by the FDA or other applicable regulatory authorities may cause delays and additional costs in obtaining approvals for marketing authorization for our product candidates. We believe our NK cell platform product candidates are novel, and because cell-based therapies are relatively new, regulatory agencies may lack precedents for evaluating product candidates like our NK product candidates. As the cell-based therapy field develops further, the processes and requirements imposed by the regulatory agencies may evolve in a manner that adversely impacts us. The novelty of our product candidates may also lengthen the regulatory review process, including the time it takes for the FDA to review our IND applications if and when submitted, increase our development costs and delay or prevent approval and commercialization of our NK cell therapy platform product candidates.
Additionally, advancing novel cell-based therapies for the treatment of oncological and neurodegenerative diseases creates significant challenges for us, including, but not limited to:
•enrolling and retaining sufficient numbers of patients in our ongoing and future clinical trials;
•training a sufficient number of medical personnel on how to properly prepare and administer our NK cells;
•training a sufficient number of medical and clinical laboratory personnel in the proper collection and handling of clinical samples in our clinical trials to enable a sufficient understanding of pharmacokinetics and pharmacodynamics for the design of an optimal dosing regimen;
•educating medical personnel regarding the potential side-effect profile of our NK cells and, as the clinical program progresses, on observed side effects with the therapy;
•developing a reliable and safe and an effective means of manufacturing our NK cells;
•manufacturing, cryopreservation, storage, and transport logistics of handling our NK cells on a large scale and in a cost-effective manner;
•sourcing starting material suitable for clinical and commercial manufacturing; and
•establishing sales and marketing capabilities, as well as developing a manufacturing process and distribution network to support the commercialization of any approved products.
We must be able to overcome these challenges in order for us to develop, commercialize and manufacture our product candidates utilizing NK cells.
Certain aspects of the function and production of NK cells are currently unknown or poorly understood, and may only become known through further preclinical testing and clinical trials. Any potential changes to our process may result in delays and additional expenses.
Our current clinical experience with NK cell therapy is predominantly based on cells from both healthy donors and patients. Current industry limitations include difficulty in expanding cell production to commercial levels, low cell cytotoxicity at baseline, loss of cytotoxicity after cryopreservation, low persistence requiring repeated dosing, and poor solid tumor microenvironment penetration. We are conducting Phase I clinical trials for SNK01 and SNK02, and we advance the clinical development of SNK01 and have initiated a Phase I/IIa trial in the United States for AD. There is a risk that the early clinical results or compassionate use results may not be reflective of future clinical trial results which may require us to re-evaluate trial design and other aspects of the testing procedures. There is also a limited history of NK cell manufacturing for clinical use, and our understanding of NK cell biology is continuously expanding. If we find that our current manufacturing processes are inadequate, or should we identify opportunities for material improvement, adaptation of process improvements may require significant time and expense. Process improvements might also necessitate new pre-clinical studies and clinical protocols to establish product comparability. If we are unable to show comparability after a process change, further changes to our manufacturing process and/or clinical trials will be required. For example, if sufficient comparability is not shown, we may be required to repeat one or more clinical trials.
The foregoing processes would require us to redesign the clinical protocols and clinical trials for our product candidates and could require significant additional time and resources to complete, as well as the participation of a significant number of additional clinical trial participants and cell donors, any of which would delay the clinical development of our product candidates and their eventual commercialization.
Results of any patient who receives our product candidates through the compassionate use access program should not be viewed as representative of how the product candidate will perform in a well-controlled clinical trial, and cannot be used to establish safety or efficacy for regulatory approval.
We have received requests for compassionate use access to our investigational drugs by physicians for their patients that do not meet the entry criteria for enrollment into our clinical trials. Generally, physicians requesting compassionate use for their patients have no other treatment alternatives for these serious conditions. We evaluate each compassionate use request on an individual basis, and in some cases grant access to our investigational product candidates outside of our sponsored clinical trials in cases where there is rationale that our investigational product may impact the condition and only after currently approved treatments have been exhausted.
Individual patient results from compassionate use access, including but not limited to, their experiences, testimonials, testing results and related images, may not be used to support submission of a regulatory application, may not support approval of a product candidate, and should not be considered to be indicative of results from any on-going or future well-controlled clinical trial. Before we can seek regulatory approval for any of our product candidates, we must demonstrate in well-controlled clinical trials statistically significant evidence that the product candidate is both safe and effective for the indication for which we are seeking approval. The results of our compassionate use program may not be used to establish safety or efficacy or regulatory approval.
Clinical development involves a lengthy and expensive process with an uncertain outcome, and we may encounter substantial delays due to a variety of reasons outside our control.
Clinical trials are expensive, time consuming and subject to substantial uncertainty. A failure of one or more of our clinical trials can occur at any time during the clinical trial process due to scientific feasibility, safety, efficacy, changing standards of medical care and other variables. Any failure of one or more of our clinical trials could prevent us from obtaining the FDA and other regulatory approvals necessary to commercialize our product candidates. The results from preclinical testing, compassionate use or early clinical trials of a product candidate may not predict the results that will be obtained in later phase clinical trials of the product candidate. The FDA, or other applicable regulatory authorities may suspend or terminate clinical trials of a product candidate at any time for various reasons, including, but not limited to, a belief that subjects participating in such trials are being exposed to unacceptable health risks or adverse side effects, or other adverse initial experiences or findings. The FDA, or other applicable regulatory authorities may also require us to conduct additional preclinical studies or clinical trials due to negative or inconclusive results or other reasons, fail to approve or find deficiencies in the raw materials, manufacturing processes or facilities of third-party manufacturers upon which we rely, and change their approval policies or regulations or their prior guidance to us during clinical development in a manner rendering our clinical data insufficient for approval. In addition, data collected from clinical trials may not be sufficient to support the submission of a BLA or other applicable regulatory filings. We cannot guarantee that any clinical trials that we may plan or initiate will be conducted as planned or completed on schedule, if at all.
Events that may prevent successful initiation, timely completion, or positive outcomes of our clinical development include, but are not limited to:
•delays in obtaining regulatory approval to commence a clinical trial;
•delays in reaching agreement on acceptable terms with prospective clinical trial sites or contract research organizations (“CROs”), the terms of which can be subject to extensive negotiation and may vary significantly among different trial sites and CROs;
•our inability to recruit and maintain sufficient patients for our clinical trials in a timely manner or at all;
•delays in achieving a sufficient number of clinical trial sites or obtaining the required institutional review board (“IRB”) and/or other site-specific review committee(s), approval(s) at each clinical trial site;
•imposition of a temporary or permanent clinical hold by us or by the FDA or other regulatory agencies based on emerging data;
•clinical sites deviating from trial protocol or dropping out of a trial;
•our inability to obtain long-term follow-up data due to patient drop out or in cases where patients elect to receive post-protocol treatment for their disease before it progresses;
•suspension or termination of a clinical trial by the IRB of the institutions in which such trials are being conducted or by a data safety monitoring board (where applicable);
•delays in sufficiently developing, characterizing or controlling a manufacturing process suitable for clinical trials, or production delays, shutdowns or setbacks at any of our contract manufacturers;
•delays due to additional regulatory, site and clinical trial participant approvals required if a product candidate, especially a product candidate custom manufactured for a specific patient, does not meet the required specifications;
•delays in reaching a consensus with regulatory agencies on the design or implementation of our clinical trials;
•changes in regulatory requirements or guidance that may require us to amend or submit new clinical protocols, or such requirements may not be as we anticipate;
•changes in the standard of care or treatment landscape on which a clinical development plan was based, which may require new or additional trials;
•insufficient quantities or inadequate quality of our product candidates or other materials necessary to conduct preclinical studies or clinical trials of our product candidates, including potential limitations to the availability of comparator or combination agents;
•clinical trials of our product candidates producing negative or inconclusive results, which may result in our deciding, or regulators requiring us, to conduct additional clinical trials or abandon product development programs;
•failure of enrolled patients in foreign countries to adhere to clinical protocol as a result of differences in healthcare services or cultural customs, or additional administrative burdens associated with foreign regulatory schemes;
•failure of regulators to accept data from our clinical trials completed in foreign jurisdictions if we do not satisfy certain regulatory requirements;
•failure of ourselves or any third-party manufacturers, contractors or suppliers to comply with regulatory requirements, maintain adequate quality controls, or be able to provide sufficient product supply to conduct and complete preclinical studies or clinical trials of our product candidates;
•failure of obligations by or termination of relationships with our or NKMAX’s collaboration partners, such as Merck KgaA; or
•failure by one of our partners to provide combination drug whether due to shortage, discontinuation of product, termination of collaboration, or for any other reason.
Our business is highly dependent on the clinical success of our product candidates, and on the clinical success of SNK01 and SNK02 in particular, and we may fail to develop SNK01, SNK02 and/or our other product candidates successfully or may be unable to obtain regulatory approval for them.
We cannot guarantee that SNK01, SNK02 (which include allogeneic SNK02 and HER2-CAR SNK02), or any of our future product candidates, will be safe and effective, or will be approved for commercialization, on a timely basis or at all. Although we have employees with prior experience with clinical trials, regulatory approvals, and current GMP, we have completed clinical trials in non-small cell lung cancer using SNK01 but have not submitted a BLA to the FDA, or similar regulatory approval filings to comparable foreign authorities, for any product candidate, and we cannot be certain that SNK01 and SNK02, or any of our other product candidates, will be successful in clinical trials or receive regulatory approval. The FDA, and other comparable global regulatory authorities can delay, limit or deny approval of a product candidate for many reasons.
For further details about such reasons, see “— Clinical development involves a lengthy and expensive process with an uncertain outcome, and we may encounter substantial delays due to a variety of reasons outside our control.” Any delay in obtaining, or inability to obtain, applicable regulatory approval will delay or harm our ability to successfully commercialize SNK01, SNK02, or any of our other product candidates, and could materially adversely affect our business, financial condition, results of operations and growth prospects.
SNK01 is in an early-stage clinical trial and is subject to the risks inherent in drug development. If the ongoing trials of SNK01 or SNK02 encounter concerning safety signals, efficacy concerns, manufacturing problems, enrollment issues, development delays, regulatory issues, or other problems, our development plans for SNK01or SNK02 could be significantly impaired, which could materially adversely affect our business, financial condition, results of operations and growth prospects.
Furthermore, because SNK01 and SNK02 are our lead product candidates, and because our other product candidates are based on similar technology, if our clinical trials of SNK01 or SNK02 experience any of the foregoing issues, our development plans for our other product candidates in our pipeline could also be significantly impaired, which could materially adversely affect our business, financial condition, results of operations and growth prospects.
We may also evaluate our product candidates in combination with one or more other neurodegenerative diseases treatments that have not yet been approved for marketing by the FDA or similar regulatory authorities outside of the United States. If the FDA or similar regulatory authorities outside of the United States do not approve these other drugs or revoke their approval of, or if safety, efficacy, manufacturing, or supply issues arise with, the drugs we choose to evaluate in combination with any product candidate we develop, we may be unable to obtain approval of or market our product candidates.
Even if we obtain regulatory approval for a product candidate, our products will remain subject to continuous subsequent regulatory obligations and scrutiny.
We intend to develop our product candidates to treat neurodegenerative diseases. Even if any product candidate we develop were to receive marketing approval or be commercialized for use in combination with other existing therapies, we would continue to be subject to the risks that the FDA or similar regulatory authorities outside of the United States could revoke approval of the combination therapy used with our product candidate or that safety, efficacy, manufacturing or supply issues could arise with these existing therapies. This could result in our own products being removed from the market or being less successful commercially.
If our product candidates are approved, they will be subject to ongoing regulatory requirements for pharmacovigilance, manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record- keeping, conduct of post-marketing studies (if any) and submission of other post-market information, theft,including both federal and state requirements in the United States and equivalent requirements of comparable regulatory authorities.
Manufacturers and manufacturers’ facilities are required to comply with extensive FDA, and comparable regulatory authority requirements, including ensuring that quality control and manufacturing procedures conform to GMP regulations. As such, we and our contract manufacturers, if any, will be subject to continual review and inspections to assess compliance with GMP and adherence to commitments made in any marketing authorization application. Accordingly, we and others with whom we work must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality control.
Any regulatory approvals that we or our collaboration partners receive for our product candidates may be subject to limitations on the approved conditions of use for which the product may be marketed or to the conditions of approval or may contain requirements for potentially costly additional data corruption, operational disruptiongeneration, including clinical trials. We will be required to report certain adverse reactions and production problems, if any, to the FDA and comparable regulatory authorities, and to conduct surveillance to monitor the safety and efficacy of the product candidate. Any new legislation addressing drug safety could result in delays in product development or commercialization or increased costs to assure compliance.
We will have to comply with requirements concerning advertising and promotion for our products. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions that vary throughout the world and must be consistent with the information in the product’s approved label. As such, we may not promote our products in ways that are not consistent with FDA- approved labeling, e.g., for indications or uses for which they do not have approval.
If our product candidates are approved, we must submit new or supplemental applications and obtain prior approval for certain changes to the licensed products, therapeutic indications, product labeling and manufacturing process. These changes may require submission of substantial data packages that may include clinical data.
If a regulatory authority discovers previously unknown problems with an approved product, such as adverse events of unanticipated severity or frequency, or if there are problems with the facility where the product is manufactured or the regulatory authority disagrees with the advertising, promotion, marketing or labeling of a product, such regulatory agency may impose restrictions on that product or on us. If we fail to comply with applicable regulatory requirements, a regulatory authority such as FDA may, among other things:
•issue warning or untitled letters;
•refer a case to the U.S. Department of Justice (“U.S. DOJ”) to impose civil or criminal penalties;
•begin proceedings to suspend or withdraw regulatory approval;
•issue an import alert;
•suspend our ongoing clinical studies or put our IND on clinical hold;
•refuse to approve pending applications (including supplements to approved applications) submitted by us;
•ask us to initiate a product recall; or
•refer a case to the U.S. DOJ to seize and forfeit products or obtain an injunction imposing restrictions on its operations.
Any government investigation of alleged violations of law or regulations could require us to expend significant time and resources in response and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenue from our products. If regulatory sanctions are applied or if regulatory approval is withdrawn, our value and operating results will be adversely affected.
We have never commercialized a product candidate before, and we may lack the necessary expertise, personnel and resources to successfully commercialize any products, if approved. We may be unable to establish effective marketing and sales capabilities or enter into agreements with third parties or related parties to market and sell our product candidates, if they are approved, and as a result, we may be unable to generate product revenues.
We have little to no prior experience in, and currently have a limited commercial infrastructure for, the marketing, sale and distribution of biopharmaceutical products. To achieve commercial success for the product candidates which we may license to others, we will rely on the assistance and guidance of those collaborators. For product candidates for which we retain commercialization rights and marketing approval, if approved, in order to commercialize our product candidates, we must continue to build out our marketing, sales and distribution capabilities, including a comprehensive healthcare compliance program, or arrange with third parties to perform these services, which will take time and require significant financial expenditures and could delay any product launch and we may not be successful in doing so. There are significant risks involved with building and managing a commercial infrastructure.
We, or our collaborators, will have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train, manage and retain medical affairs, marketing, sales and commercial support personnel. Recruiting, training and retaining a sales force is expensive and time-consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have incurred these commercialization expenses prematurely or unnecessarily. These efforts may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel. In the event we are unable to develop a commercial infrastructure, we may not be able to commercialize our current or future product candidates, which would limit our ability to generate product revenues. Even if we are able to effectively establish a sales force and develop a marketing and sales infrastructure, our sales force and marketing teams may not be successful in commercializing our current or future product candidates. To the extent we rely on third parties to commercialize any products for which we obtain regulatory approval, we would have less control over their sales efforts and could be held liable if they failed to comply with applicable legal or regulatory requirements.
Enrollment and retention of patients in clinical trials is an expensive and time-consuming process and could be delayed, made more difficult or rendered impossible by multiple factors outside our control.
Clinical trials of a new product candidate require the enrollment of a sufficient number of patients, including patients who are suffering from the disease that the product candidate is intended to treat and who meet other eligibility criteria. The rates of patient enrollment, a significant component in the timing of clinical trials, are affected by many factors, including, but not limited to:
•our ability to identify and qualify investigation sites to participate in our clinical trials;
•the size and nature of the patient population;
•the design and eligibility criteria of the clinical trial;
•the proximity of subjects to clinical sites;
•the patient referral practices of physicians;
•staff turnover at the clinical sites;
•changing medical practice patterns or guidelines related to the indications we are investigating;
•competing clinical trials or approved therapies which present an attractive alternative to patients and their physicians;
•perceived risks and benefits of the product candidate under study, including as a result of adverse effects observed in similar or competing therapies;
•our ability to obtain and maintain patient consents due to various reasons;
•the risk that enrolled subjects will drop out or die before completion of the trial;
•patients failing to complete a clinical trial or returning for post-treatment follow-up;
•our ability to manufacture the requisite supply of our product candidates for a patient and clinical trials; and
•any failure or any delay by us or by our clinical sites to obtain sufficient quantities of components and supplies necessary for the conduct of our clinical trials, including potential limitations to the availability of comparator or combination agents.
In addition, we need to compete with many ongoing clinical trials to recruit patients into our expected clinical trials. Our clinical trials may also compete with other clinical trials of product candidates that are in a similar cellular immunotherapy area as our product candidates, and this competition could reduce the number and types of patients available to us, because some patients who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors. Since the number of qualified clinical investigators is limited, we may conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials at such clinical trial site. If we are unable to enroll a sufficient number of patients in our clinical trials in a timely manner, our completion of clinical trials may be delayed or may not be achieved, which would prevent us from further developing or commercializing our product candidates.
The clinical development of our product candidates depends on our ability to manufacture and provide the requisite supply of our product candidates for our clinical trials. Any failure or delays by us to manufacture and provide our product candidates in sufficient quantity and quality for the conduct of our clinical trials, may delay our ability to enroll and treat patients in, or complete, our current or future clinical trials of our product candidates on time, if at all.
The clinical development of our product candidates also depends on the availability of a sufficient supply of certain other materials and agents used in our clinical trials. For example, certain clinical trial protocols require the use of comparator treatments. If any standard of care therapies become unavailable or limited in supply, it would adversely impact our ability to complete the trial. Further, we may develop certain of our product candidates as a combination therapy with other neurodegenerative diseases treatments, which would require the availability and use of those therapeutic agents in certain of our clinical trial protocols.
If we are unable to enroll a sufficient number of patients in our clinical trials in a timely manner, our completion of clinical trials may be delayed or may not be achieved, which would prevent us from further developing or commercializing our product candidates.
Our preclinical programs may experience delays or may never advance to clinical trials, which would adversely affect our ability to obtain regulatory approvals or commercialize these programs on a timely basis or at all.
In order to obtain FDA or other regulatory authority approval to market a new biological product we must demonstrate proof of safety, purity and potency, or efficacy, in humans. To meet these requirements, we will have to conduct adequate and well-controlled clinical trials. On October 14, 2022, we received IND clearance from the FDA for SNK02 allogenic NK cell therapy for solid tumors. On October 20, 2023, we received IND clearance from the FDA for SNK01 in AD. During the remainder of 2023, we intend to (i) advance the clinical development of SNK01 and initiate a Phase I/Iia trial in the United States for AD, and (ii) continue the Phase I trial with SNK02 in refractory solid tumors. Before we can commence clinical trials for additional product candidates, we must complete extensive preclinical testing and studies that support our planned INDs in the United States.
We cannot be certain of the timely completion or outcome of our preclinical testing and studies and cannot predict if the FDA will accept our proposed clinical programs or if the outcome of our preclinical testing and studies will ultimately support the further development of our programs. In addition, we may voluntarily decide to delay, suspend, terminate or partner with third parties in respect of certain product development programs, for example to prioritize other product candidates. As a result, we may not submit INDs or similar applications for our preclinical programs within our anticipated timelines, if at all, and submission of INDs or similar applications may not result in the FDA or other regulatory authorities allowing clinical trials to begin.
Conducting preclinical testing is a lengthy, time-consuming and expensive process. The length of time may vary substantially according to the type, complexity and novelty of the program, and often can be several years or more per program. Any delays in preclinical testing and studies conducted by us or potential future partners may cause us to incur additional operating expenses. The commencement and rate of completion of preclinical studies for a product candidate may be delayed by many factors, including, for example:
•inability to generate sufficient preclinical or other in vivo or in vitro data to support the initiation of clinical trials;
•delays in reaching a consensus with regulatory agencies on study design;
•the FDA (or other regulatory authorities) not allowing us to rely on clinical trials completed in foreign jurisdictions if we do not satisfy certain regulatory requirements; and
•the FDA (or other regulatory authorities) not allowing us to rely on previous findings of safety and efficacy for other similar products and published scientific literature.
Moreover, because standards for pre-clinical assessment are evolving and may change rapidly, even if we reach an agreement with the FDA on a pre-IND proposal, the FDA may not accept the IND submissions as presented, in which case the clinical trial timeline could be delayed.
The results of preclinical studies and early-stage clinical trials may not be predictive of future results. Interim, “topline” and preliminary data from our clinical trials may differ materially from the final data.
The results of preclinical studies may not be predictive of the results of clinical trials, and the results of any early-stage clinical trials we commence may not be predictive of the results of the later-stage clinical trials. For example, preclinical models as applied to cell therapy in oncology do not adequately represent the clinical setting, and thus cannot predict clinical activity nor all potential risks, and may not provide adequate guidance as to the appropriate dose or administration regimen of a given therapy.
From time to time, we may publicly disclose preliminary or “topline” data from our clinical trials, which is based on a preliminary analysis of then-available data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular trial, including as patient enrollment continues and more data on existing patients becomes available. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to evaluate all data fully and carefully. As a result, any topline data from our clinical trials, such as SNK01, may differ from, and may not be indicative of, future results of the same clinical trials, or different conclusions or considerations may qualify such topline results once additional data have been received and fully evaluated. Topline data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, topline data should be viewed with caution until the final data are available and negative differences between preliminary or interim data and final data could materially adversely affect the prospects of any product candidate that is impacted by such data updates.
Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and the value of our company in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is typically a summary of extensive information, and others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure, and any information we determine not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product, product candidate or our business. If the topline data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our product candidates may be harmed.
If any of our product candidates, or any competing product candidates, demonstrate relevant, serious adverse events, we may be required to halt or delay further clinical development.
Undesirable side effects that may be caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label than anticipated or the delay or denial of regulatory approval by the FDA or comparable foreign regulatory authorities. Results of our clinical trials could reveal a high and unacceptable severity and prevalence of side effects or unexpected characteristics.
Current data from the SNK01 clinical trials indicates that SNK01 is generally well-tolerated. To date, there have been a total of four events ≥ Grade 2 reported by two participants as related/possibly related to SNK01 across the clinical trials. One patient experienced a total of three events which were grade 2 chills, grade 3 chills, and grade 2 infusion reaction, all of which resolved. A different patient experienced one grade 2 event of intermittent pain upper central abdomen which also resolved. However, due to the few events that have been reported on the SNK01 development program, there may be additional and unforeseen events that may emerge as we continue to conduct clinical trials.
While the data from our SNK01 Phase I clinical trial investigating the safety and tolerability in AD patients and Phase I/IIa clinical trial investigating the combination of SNK01 with a therapeutic antibody, cetuximab, indicate that NK cell-based therapies may be well-tolerated, there can be no assurance that future patients will not experience adverse effects. If unacceptable side effects arise in the development of our product candidates such that there is no longer a positive benefit-risk profile, we, the FDA, or the IRBs at the institutions in which our trials are conducted could suspend or terminate our clinical trials or the FDA or comparable foreign regulatory authorities could order us to cease clinical trials or deny approval of our product candidates for any or all targeted indications. Treatment-related side effects could also affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff, and inadequate training in recognizing or managing the potential side effects of our product candidates could result in patient injury or death. The occurrence of side effects may also harm our reputation or the reputation of our products, which may have a significant impact on our business and stock price.
If we are not able to maintain or secure agreements with the third parties that conduct the activities related to our clinical trials on acceptable terms, or at all, or if these third parties do not perform their services as contractually required, or if these third parties fail to timely transfer any regulatory information held by them to us, we may not be able to obtain regulatory approval for our product candidates or commercialize any product candidates that may result from our development efforts, or may miss expected deadlines.
We rely on entities outside of our control, which may include academic institutions, CROs, hospitals, clinics and other third-party strategic partners, to monitor, support, conduct and oversee preclinical studies and clinical trials of our current and future product candidates. As a result, we have less control over the timing and cost of these studies and the ability to recruit trial subjects than if we conducted these trials with our own personnel. If we are unable to maintain or enter into agreements with these third parties on acceptable terms, or if any such engagement is terminated prematurely, we may be unable to enroll subjects on a timely basis or otherwise conduct our clinical trials as planned. In addition, there is no guarantee that these third parties will devote adequate time and resources to our clinical trials or perform as required by our contract or in accordance with regulatory requirements, including maintenance of clinical trial information regarding our product candidates. If these third parties fail to meet expected deadlines, fail to transfer to us any regulatory information in a timely manner, fail to adhere to protocols or fail to act in accordance with regulatory requirements or our agreements with them, or if they otherwise perform in a substandard manner or in a way that compromises the quality or accuracy of their activities or the data they obtain, then clinical trials of our product candidates may be extended or delayed with additional costs incurred, or our data may be rejected by the FDA or other regulatory agencies. Ultimately, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on third parties does not relieve us of our regulatory responsibilities.
We and our CROs are required to comply with good clinical practice (“GCP”), regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for products in clinical development. Regulatory authorities enforce these GCP through periodic inspections of clinical trial sponsors, principal investigators and clinical trial sites. If we or any of our CROs fail to comply with applicable GCP, the clinical data generated in our clinical trials may be deemed unreliable and our submission of marketing applications may be delayed, or the FDA or foreign regulatory authority may require us to perform additional clinical trials before approving our marketing applications. Upon inspection, the FDA or comparable foreign regulatory authority could determine that any of our clinical trials fail or have failed to comply with applicable GCP.
Our business also may be implicated if any of our CROs and/or clinical trial sites violates fraud and abuse or false claims laws and regulations or healthcare privacy and security laws.
If any of our third-party clinical trial sites terminate for any reason, we may experience the loss of follow-up information on subjects enrolled in our ongoing clinical trials unless we are able to transfer the care of those subjects to another qualified clinical trial site. Further, our CROs and/or clinical trial sites are not required to work indefinitely or exclusively with us. Our existing agreements with our CROs and/or clinical trial sites may be subject to termination by the counterparty upon the occurrence of certain circumstances. If any CRO and/or clinical trial sites terminates its agreement with us, the research and development of the relevant product candidate would be suspended, and our ability to research, develop and license future product candidates would be impaired. We may be required to devote additional resources to the development of our product candidates or seek a new CRO partner and/or clinical trial sites, and the terms of any additional arrangements that we establish may not be favorable to us. Switching or adding CROs and/or clinical trial sites or other service providers can involve substantial cost and require extensive management time and focus. In addition, there is a natural transition period when a new CRO and/or clinical trial sites or service provider commences work. As a result, delays may occur, which can materially impact our ability to meet our desired clinical development timelines. If we are required to seek alternative arrangements, the resulting delays and potential inability to find suitable replacements could materially and adversely impact our business.
Our approach to the development of product candidates based on our NK cell therapy platform is unproven, and we do not know whether we will be able to develop any products of commercial value, or if competing technological approaches will limit the commercial value of our product candidates or render our platform obsolete.
Our success depends on our ability to develop, obtain regulatory approval for and commercialize our product candidates utilizing our NK cell therapy platform, including manufacturing capabilities, which leverages relatively novel technologies. While we have had favorable preclinical study results based on our platform, we have not yet succeeded and may not succeed in demonstrating efficacy and safety for any product candidates in clinical trials or in obtaining marketing approval thereafter. We initiated Phase I trial of our lead product candidates, SNK01 and SNK02. There is no guarantee that we will be able to timely complete our clinical study and we may experience additional timeline delays or serious adverse events, and our product candidates may never become commercialized. All of our product candidates will require significant additional clinical and non-clinical development, review and approval by the FDA or other regulatory authorities in one or more jurisdictions, substantial investment, and significant marketing efforts before they can be successfully commercialized. Our methodology and novel approach to cellular therapy may be unsuccessful in identifying additional product candidates, and any product candidates based on our platform may be shown to have harmful side effects or may have other characteristics that may necessitate additional clinical testing, or make the product candidates unmarketable or unlikely to receive marketing approval.
Further, because all of our product candidates and development programs are based on our NK cell therapy platform, adverse developments with respect to one of our programs may have a significant adverse impact on the actual or perceived likelihood of success and value of our other programs. For example, if our clinical trials of SNK01 encounter safety, efficacy or manufacturing problems, development delays, regulatory issues or other problems, our development plans for our other product candidates in our pipeline could be significantly impaired.
In addition, from time to time, our competitors may also disclose interim or final data and/or findings from their preclinical studies or trials. Adverse data or findings released by our competitors, whether in relation to efficacy or safety of NK cell therapy, may have an adverse impact on our business and operations, including but not limited to, our ability to enroll patients in our clinical trials and could require additional studies to be conducted to refute the “class effect” interpretation, which would require additional time, resources, and financing.
We may seek special designations by the regulatory authorities to expedite regulatory approvals, but may not be successful in receiving such designations, and even if received, they may not benefit the development and regulatory approval process.
We may seek various expedited programs available through regulatory authorities such as Regenerative Medicine Advanced Therapy (“RMAT”) designation, Breakthrough Therapy designation, Fast Track designation, Priority Review or PRIority Medicine (“PRIME”), from regulatory authorities, for any product candidate that we develop. A product candidate may receive RMAT designation from the FDA if it is a regenerative medicine therapy that is intended to treat, modify, reverse or cure a serious or life-threatening condition, and preliminary clinical evidence on a clinically meaningful endpoint, indicates that the product candidate has the potential to address an unmet medical need for such condition. A Breakthrough Therapy is defined by the FDA as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over currently approved therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. If a product is intended for the treatment of a serious or life-threatening condition and preclinical or clinical data demonstrate the potential to address an unmet medical need for this condition, the product sponsor may apply for Fast Track designation by the FDA. PRIME is a voluntary scheme launched by the European Medicines Agency (“EMA”), to strengthen support for the development of medicines that target an unmet medical need through enhanced interaction and early dialogue with developers of promising medicines in order to optimize development plans and speed up evaluation to help such medicines reach patients earlier.
Seeking and obtaining these designations is dependent upon results of our clinical program and other considerations, and we cannot guarantee whether and when we may have the data from our clinical programs to support an application to obtain any such designation. The FDA and the EMA, as applicable, have broad discretion whether or not to grant any of these designations, so even if we believe a particular product candidate is eligible for one or more of these designations, we cannot assure you that the applicable regulatory authority would decide to grant it. Even if we do receive the designations we may apply for, we may not experience a faster development process, review or approval compared to conventional FDA or EMA procedures, as applicable. The FDA or EMA, as applicable, may rescind any granted designations if it believes that the designation is no longer supported by data from our clinical development program.
Public opinion and scrutiny of cell-based immuno-oncology therapies for treating neurodegenerative diseases may impact public perception of our company and product candidates, or impair our ability to conduct our business.
Our platform utilizes a novel technology involving the isolation of pure primary NK cells from peripheral blood or leukapheresis of patients themselves or from screened healthy adult donors, which is subsequently expanded. Future products may be developed using genetic modifications. To our knowledge, to date, there are no NK cell-based therapies with FDA-approval. Public perception may be negatively influenced by claims that NK cell-based immunotherapy is ineffective, unsafe, unethical, or immoral and, consequently, our approach may not gain the acceptance of the public or the medical community. Negative public reaction to cell-based immunotherapy in general could result in greater government regulation and stricter labeling requirements of cell-based immunotherapy products, including any of our product candidates, and could cause a decrease in the demand for any products we may develop. Adverse public attitudes may adversely impact our ability to enroll clinical trials. More restrictive government regulations or negative public opinion could have an adverse effect on our business or financial loss.condition and may delay or impair the development and commercialization of our product candidates or demand for any products we may develop.
We may not identify or discover other product candidates and may fail to capitalize on programs or product candidates that may present a greater commercial opportunity or for which there is a greater likelihood of success.
Our business depends upon our ability to identify, develop and commercialize product candidates. A key element of our strategy is to discover and develop additional product candidates based upon our NK cell therapy platform. We are seeking to do so through our internal research programs and may also explore strategic collaborations for the discovery of new product candidates. Research programs to identify product candidates require substantial technical, financial and human resources, whether or not any product candidates are ultimately identified. In addition, targets for different neurodegenerative diseases may require changes to our NK manufacturing platform, which may slow down development or make it impossible to manufacture our product candidates. Our research programs may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development for many reasons, including, but not limited to, the following:
•the research methodology or technology platform used may not be successful in identifying potential product candidates;
•competitors may develop alternatives that render our product candidates obsolete or less attractive;
•we may choose to cease development if we determine that clinical results do not show promise;
•product candidates we develop may nevertheless be covered by third parties’ patents or other exclusive rights;
•a product candidate may be shown to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria; and
•a product candidate may not be accepted as safe and effective by patients, the medical community or third-party payors.
Because we have limited resources, we must choose to pursue and fund the development of specific types of treatment, or treatment for a specific type of neurodegenerative disease, and we may forego or delay pursuit of opportunities with certain programs or product candidates or for indications that later prove to have greater commercial potential. Our estimates regarding the potential market for our product candidates could be inaccurate, and if we do not accurately evaluate the commercial potential for a particular product candidate, we may relinquish valuable rights to that product candidate through strategic collaboration, licensing or other arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate. Alternatively, we may allocate internal resources to a product candidate in a therapeutic area in which it would have been more advantageous to enter into a partnering arrangement.
If any of these events occur, we may be forced to abandon or delay our development efforts with respect to a particular product candidate or fail to develop a potentially successful product candidate.
If third parties that we rely on to conduct clinical trials do not successfully carry out their contractual duties, comply with regulatory requirements or meet expected deadlines, we may not be able to obtain marketing approval for or commercialize our product candidates.
We do not have the ability to independently conduct clinical trials. We rely on medical institutions, clinical investigators, contract laboratories, and other third parties, such as CROs to conduct or otherwise support clinical trials for our product candidates. We rely heavily on these parties for execution of clinical trials for our product candidates and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the applicable protocol, legal and regulatory requirements and scientific standards, and our reliance on CROs and other third parties will not relieve us of our regulatory responsibilities. For any violations of laws and regulations during the conduct of our clinical trials, we could be subject to untitled letters, warning letters or enforcement action that may include civil penalties up to and including criminal prosecution.
We and the third parties on which we rely for clinical trials are required to comply with regulations and requirements, including GCPs for conducting, monitoring, recording and reporting the results of clinical trials to ensure that the data and results are scientifically credible and accurate, and that the trial patients are adequately informed of the potential risks of participating in clinical trials and their rights are protected. These regulations are enforced by the FDA and comparable foreign regulatory authorities for any drugs in clinical development. The FDA enforces GCP requirements through periodic inspections of clinical trial sponsors, principal investigators and trial sites. If we or these third parties fail to comply with applicable GCP, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, the FDA will determine that any of our future clinical trials do not deviate from GCP. In addition, our clinical trials must be conducted with product candidates produced under GMP regulations. Our failure or the failure of these third parties to comply with these regulations may require us to repeat clinical trials, which would delay the marketing approval process and could also subject us to enforcement action. We also are required to register certain ongoing clinical trials and provide certain information, including information relating to the trial’s protocol, on a government-sponsored database, ClinicalTrials.gov, within specific timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Although we intend to design the clinical trials for our product candidates, we plan to rely on third parties to conduct our clinical trials. As a result, many important aspects of our clinical development, including their conduct and timing, will be outside of our direct control. Our reliance on third parties to conduct future clinical trials will also result in less direct control over the management of data developed through clinical trials than would be the case if we were relying entirely upon our own staff. Communicating with outside parties can also be challenging, potentially leading to mistakes as well as difficulties in coordinating activities. Outside parties may, without limitation:
•have staffing difficulties;
•fail to comply with contractual obligations;
•experience regulatory compliance issues;
•experience interruption of, or delays in enrolling patients for our clinical trials or manufacture our product candidates;
•undergo changes in priorities or become financially distressed; or
•form relationships with other entities, some of which may be our competitors.
If third parties do not perform our clinical trials in a satisfactory manner, breach their obligations to us or fail to comply with regulatory requirements, we would be unable to rely on clinical data collected by these third parties and may be required to repeat, extend the duration of, or increase the size of any clinical trials we conduct, which could significantly delay commercialization and require significantly greater expenditures.
If any of our relationships with these third parties terminate, we may not be able to enter into arrangements with alternative third parties on commercially reasonable terms, or at all. If third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain are compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, any clinical trials such third parties are associated with may be extended, delayed or terminated, and we may not be able to obtain marketing approval for or successfully commercialize our product candidates. As a result, we believe that our financial results and the commercial prospects for our product candidates in the subject indication would be harmed, our costs could increase and our ability to generate revenue could be delayed.
If we are not able to establish pharmaceutical or biotechnology collaborations on commercially reasonable terms, or at all, we may have to alter our development and commercialization plans.
The advancement of our product candidates and development programs and the potential commercialization of our current and future product candidates will require us to enter into collaborations, partnerships or other agreements with third parties, which may require substantial additional cash to fund expenses related to such relationships. Any of these relationships, may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing stockholders, relinquish valuable rights to our product candidates, or disrupt our management and business.
We face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Whether we reach a definitive agreement for new collaborations will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the progress of our clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications from our competitors that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidate. Further, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for future product candidates because they may be deemed to be at too early of a stage of development for collaborative effort or third parties may not view them as having the requisite potential to demonstrate safety and efficacy. Any delays in entering into new collaborations or strategic partnership agreements related to any product candidate we develop could delay the development and commercialization of our product candidates, which would harm our business prospects, financial condition, and results of operations.
If any of our product candidates are approved for marketing and commercialization and we have not developed or secured marketing, sales and distribution capabilities, either internally or from third parties, we will be unable to successfully commercialize such products and may not be able to generate product revenue.
We currently do not have any commercial sales. We will need to develop internal and external sales, marketing and distribution capabilities and infrastructure to commercialize any product candidate that gains FDA or other regulatory authority approval, which would be expensive and time-consuming, or enter into partnerships with third parties to perform these services. If we decide to market any approved products directly, we will need to commit significant financial and managerial resources to develop a marketing and sales force with technical expertise and supporting distribution, administration and compliance capabilities. If we rely on third parties to market products or decide to co-promote products with partners, we will need to establish and maintain marketing and distribution arrangements with third parties, and there can be no assurance that we will be able to enter into such arrangements on acceptable terms or at all. In entering into third-party marketing or distribution arrangements, any product revenue we receive will depend upon the efforts of the third parties and we cannot assure you that such third parties will establish adequate sales and distribution capabilities or be successful in gaining market acceptance for any approved product. If we are not successful in commercializing any product approved in the future, if any, either on our own or through third parties, our business, financial condition, results of operations and growth prospects could be materially adversely affected.
The market opportunities for our product candidates, if and when approved, may be limited, and if such market opportunities are smaller than we expect, our revenues could be materially adversely affected and our business could suffer.
Our product candidates have not received FDA or other regulatory approval for market sales. We do not know at this time whether either SNK01 or SNK02 or any of our product candidates will be safe for use in humans or whether they will demonstrate any improvement in neurodegenerative diseases. If the activity is sufficient, we may initially seek approval of any product candidates we develop as a therapy for patients who have received one or more prior approved treatments. However, there is no guarantee that product candidates we develop, even if approved for later lines of therapy, would be approved for earlier lines of therapy, and, prior to any such approvals, we will have to conduct additional clinical trials.
The number of patients who have the neurodegenerative diseases we are targeting may turn out to be lower than expected. Additionally, the potentially addressable patient population for our current programs or future product candidates may be limited. Potentially addressable patient populations for our product candidates are only estimates. These estimates could prove to be incorrect, and the estimated number of potential patients in the United States and elsewhere could be lower than expected. It may also be that such patients may not be otherwise amenable to treatment with our product candidates, or patients could become increasingly difficult to identify and access for a variety of reasons including other drugs being approved, any of which could materially adversely affect our business, financial condition, results of operations and growth prospects.
The commercial success of any of our product candidates will depend upon such product candidate’s degree of market acceptance by physicians, patients, third-party payors and others in the medical community.
Our product candidates may not be commercially successful. Even if requisite approvals are obtained from the FDA in the United States and other regulatory authorities internationally, the commercial success of our product candidates will depend, in part, on the acceptance by physicians, patients and healthcare payors of cell therapy products in general, and our product candidates in particular, as medically necessary, cost-effective and safe. Physicians, patients, healthcare payors and others in the medical community may not accept any product that we commercialize. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of cell therapy products and, in particular, our product candidates, if approved for commercial sale, will depend on digital technologies,several factors, including, but not limited to:
•the efficacy and safety of such product candidates as demonstrated in clinical trials;
•the potential and perceived advantages of product candidates over alternative treatments;
•the cost of treatment relative to alternative treatments;
•the clinical indications for which the product candidate is approved by the FDA;
•the willingness of physicians to refer patients and prescribe new therapies;
•the willingness of the target patient population to try new therapies;
•the nature, prevalence and severity of any side effects;
•product labeling or product insert requirements imposed by the FDA or other regulatory authorities, including any limitations or warnings contained in a product’s approved labeling;
•the relative convenience and ease of administration;
•the timing of market introduction of competitive products;
•adverse publicity concerning our product candidates or favorable publicity about competing products and treatments;
•sufficient third-party payor coverage, any limitations in terms of center or personnel training requirement imposed by third parties and adequate reimbursement;
•the willingness of the target patient population to pay out-of-pocket in the absence of coverage and reimbursement by third-party payors and government authorities;
•limitations or warnings contained in the FDA-approved labeling for our product candidates;
•any FDA requirement to undertake a risk evaluation and mitigation strategy (“REMS”);
•the effectiveness of our sales, marketing and distribution efforts; and
•potential product liability claims.
Even if a product candidate displays a favorable efficacy and safety profile in preclinical studies and clinical trials, market acceptance of the product will not be fully known until after such product is launched. Our product candidates may not achieve broad market acceptance.
Furthermore, the FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay marketing approval of a product.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability.
We and/or NKMAX have entered into collaboration agreements with Affimed, Pfizer and Merck KgaA regarding certain product candidates, and we may enter into additional collaborations with third parties to develop or commercialize other product candidates. Our prospects with respect to those product candidates will depend in significant part on the success of those collaborations, and we may not realize the benefits of such collaborations.
We, NKGen Biotech, previously entered into a clinical trial collaboration and supply agreement with AresTrading S.A., Z.I de l’Ouriettaz (“AresTrading”) (which is a subsidiary of Merck KgaA), and Pfizer, Inc. (“Pfizer”) in August 2020 to evaluate the safety and tolerability of SNK01 with avelumab, and a strategic collaboration agreement with Affimed GmbH (“Affimed”) in September 2020 to investigate the potential combination of SNK01 with AFM24 (which study was discontinued by mutual agreement in June 2023). As of July 2023, the collaborative alliance between Merck KgaA (through its subsidiary, AresTrading) and Pfizer was terminated and our collaboration with Merck KgaA with respect to the study on the safety and tolerability of SNK01 with avelumab is still in place. NKMAX, our parent company, entered into a clinical trial collaboration and supply agreement with Merck KgaA in April 2021 to investigate the potential combination of SNK01 with cetuximab. We believe these collaborations help us to further establish our clinical development plans and design and advance our NK cell therapy platform to treat oncologic diseases.
We may form strategic alliances or create joint ventures or collaborations with respect to our product candidates that we believe will complement or augment our existing business. We routinely engage, and are engaged, in partnering discussions with a range of pharmaceutical and biotechnology companies and could enter into new collaborations at any time. If we enter into a collaboration, strategic alliance or license arrangement, there is no guarantee that the collaboration will be successful, or that any future partner will commit sufficient resources to the development, regulatory approval, and commercialization effort for such products, or that such alliances will result in us achieving revenues that justify such transactions.
If we and/or NKMAX terminate any of these collaboration agreements in its entirety or with respect to a particular product candidate, due to a material breach by either party thereto or for other reasons, then our costs may increase as we may need to pay termination fees and shoulder additional costs to continue research, development, and commercialization of the terminated product candidate(s) on our own at our sole expense. We and/or NKMAX may not be able to re-negotiate terms with these partners or negotiate future agreements with terms that are favorable to us. Furthermore, assumption of sole responsibility for further development may increase our expenditures and may mean we would need to limit the size and scope of one or more of our programs, seek additional funding and/or choose to stop work altogether on one or more of the affected product candidates. This could result in a limited potential to generate future revenue from such product candidates, and our business could be adversely affected.
Whenever we enter into collaborations with third parties, we could face, without limitation, the following risks:
•collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations;
•collaborators may not pursue development or may elect not to continue or renew development programs based on clinical trial results, changes in their strategic focus due to the acquisition of competitive drugs, availability of funding or other external factors that diverts resources or creates competing priorities;
•collaborators may delay clinical trials, provide insufficient funding for a clinical trial, stop a clinical trial, abandon a product candidate, or repeat or conduct new clinical trials;
•collaborators could independently develop, or develop with third parties, products and processes that compete directly or indirectly with our products or product candidates;
•collaborators may own or co-own intellectual property that results from our collaborating with them, and in such cases, we could potentially not have the exclusive right to commercialize such intellectual property;
•collaborators may not properly enforce, maintain or defend our intellectual property rights or may use our proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation, or other intellectual property proceedings;
•disputes may arise between a collaborator and us that cause the delay or termination of the research, development or commercialization of the product candidate, or that result in costly litigation or arbitration that diverts management attention and resources;
•if a present or future collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program under such collaboration could be delayed, diminished or terminated; and
•collaboration agreements may restrict our right to independently pursue new product candidates.
If conflicts arise between our collaborators and us, our collaborators may act in a manner adverse to us and could limit our ability to implement our strategies. Affirmed, Pfizer or Merck KgaA or future collaborators may develop, either alone or with others, products in related fields that are competitive with the products or potential products that are the subject of these collaborations. Competing products, either developed by the collaborators or to which the collaborators have rights, may result in the withdrawal of support for our product candidates. Our collaborators may preclude us from entering into collaborations with their competitors, fail to obtain timely regulatory approvals, terminate their agreements with us prematurely or fail to devote sufficient resources to the development and commercialization of products.
Competing product candidates, either developed by the collaborators or strategic partners or to which the collaborators or strategic partners have rights, may result in the withdrawal of our collaborator’s or partner’s support for our product candidates. Any of these developments could harm our product development efforts.
As a result, we may not be able to realize the benefit of new or existing collaboration agreements and strategic partnerships if we are unable to successfully integrate them with our existing operations, which could delay our timelines or otherwise adversely affect our business. We also cannot be certain that, following a strategic transaction or license, we will achieve the revenue or specific net income that justifies such transaction.
If we fail to compete effectively with academic institutions and other biotechnology companies that develop similar or alternatives to cellular immunotherapy product candidates, our business will be materially adversely affected.
The development and commercialization of new cellular immunotherapy products is highly competitive. We face competition from existing and future competitors with respect to each of our product candidates currently in development, and will face competition with respect to other product candidates that we may seek to develop or commercialize in the future. For example, Acepodia, Artiva, Celularity, Century Therapeutics, Cytovia Therapeutics, Fate Therapeutics, Nkarta, and ImmunityBio each have clinical-stage allogeneic programs. In addition, other competitors, such as Affimed, Innate Pharma, Dragonfly Therapeutics and GT Biopharma, are seeking to harness NK biology through cell engagers that direct a patient’s own NK cells to the site of a tumor. A number of academic institutions are also conducting preclinical and clinical research in these areas. It is also possible that new competitors, including those developing similar or alternatives to cellular immunotherapy product candidates, may emerge and acquire significant market share. Such competitors may have an advantage over us due to their greater size, resources or institutional experience, or may develop product candidates that are safer, more effective, more widely accepted, more cost-effective or enable higher patient quality of life than ours. More established biotechnology companies may also develop and commercialize their product candidates at a faster rate, which could render our product candidates obsolete or non-competitive before they are fully developed or commercialized. If we are not able to compete effectively against our existing and potential competitors, our business, financial condition, results of operations and growth prospects may be materially adversely affected.
We will need to increase the size of our organization, and we may experience difficulties in managing growth.
As of December 31, 2023, we had 63 full-time employees. We will need to continue to expand our managerial, operational, clinical, quality, human resources, legal, manufacturing, supply chain, finance, commercial and other resources in order to manage our operations and clinical trials, continue our development activities and eventually commercialize our product candidates. Our management and personnel, systems and facilities currently in place may not be adequate to support this future growth. Our need to effectively execute our growth strategy requires, without limitation, that we:
•discover new product candidates, develop the process and analytical methods for IND-enabling studies and FDA submissions, complete the required IND-enabling studies for each, and receive approval from the FDA and other regulatory authorities to initiate clinical trials for such product candidates;
•manage our vendors and clinical trials effectively;
•identify, recruit, retain, incentivize and integrate additional employees;
•expand into additional office and laboratory space as we grow our employee base; and
•continue to improve our operational, financial and management controls, reports systems and procedures.
If we are unable to attract skilled employees, increase the size of our organization or manage our future growth effectively, it will impair our ability to execute our business strategy and our business, financial condition, results of operations and growth prospects will be materially adversely affected. Moreover, our management may need to divert a disproportionate amount of its attention away from its day-to-day activities and devote a substantial amount of time to managing these growth activities, which may adversely affect our ability to develop and, if approved, commercialize our product candidates.
If we fail to attract and retain senior management, clinical, and key scientific personnel, we may be unable to successfully develop our product candidates, conduct our clinical trials and commercialize our product candidates.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel. In addition, we are highly dependent upon our senior management, particularly our chief executive officer, Dr. Paul Y. Song, as well as other members of our senior management team. The loss of services of any of these individuals could delay or prevent the successful development of our product pipeline, initiation or completion of our planned clinical trials or the commercialization of our future product candidates. We do not have employment agreements with our senior management team.
Competition for qualified personnel in the biotechnology and pharmaceuticals fields is intense due to the limited number of individuals who possess the skills and experience required by our industry. We will need to hire additional personnel as we expand our clinical development and manufacturing activities, or if we initiate commercial activities. We may not be able to attract and retain quality personnel on acceptable terms, or at all. If we are unable to hire and retain the qualified personnel we need to operate our business, our business, financial condition, results of operations and growth prospects would be materially adversely affected. In addition, to the extent we hire personnel from competitors, we may be subject to allegations that they have been improperly solicited or that they have divulged proprietary or other confidential information, or that their former employers own their research output.
Our employees, independent contractors, consultants, commercial partners, principal investigators, CROs, suppliers and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of employee fraud or other illegal activity by our employees, independent contractors, consultants, commercial partners, principal investigators, CROs, suppliers and vendors. Misconduct by these parties could include intentional, reckless and/or negligent conduct that fails to: comply with the laws of the FDA and other similar foreign regulatory bodies, provide true, complete and accurate information to the FDA and other similar foreign regulatory bodies, comply with manufacturing standards we have established, comply with healthcare fraud and abuse laws in the U.S. and similar foreign fraudulent misconduct laws, or report financial information or data accurately or to disclose unauthorized activities to us. If we obtain FDA approval of any of our product candidates and begin commercializing those product candidates in the U.S., our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws and regulations designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials, which could result in regulatory sanctions and serious harm to our reputation.
It is not always possible to identify and deter misconduct or other improper activities by our employees or third parties that we engage for our business operations and the precautions we take to detect and prevent inappropriate conduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a material adverse effect on our business, financial condition, results of operations and prospects, including the imposition of significant fines or other sanctions, including exclusion from government healthcare programs, and serious harm to our reputation. In addition, the approval and commercialization of any of our product candidates outside the U.S. will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws. Efforts to ensure that our business arrangements will comply with applicable healthcare laws may involve substantial costs.
If any of the third parties that we rely on for various operational and administrative aspects of our business fail to provide timely, accurate and ongoing service or if the technology systems and infrastructure suffer outages that we are unable to mitigate, our business may be adversely affected.
We currently rely upon third-party consultants and contractors to provide specific operational and administrative services, including research and clinical consultation and management. The failure of any of these third parties to provide accurate and timely service may adversely impact our business operations.
In addition, if such third-party service providers were to cease operations, temporarily or permanently, face financial distress or other business disruption, increase their fees or if our relationships with these providers deteriorate, we could suffer increased costs until an equivalent provider could be found, if at all, or we could develop internal capabilities, if ever. In addition, if we are unsuccessful in choosing or finding high-quality partners, if we fail to negotiate cost-effective relationships with them, or if we ineffectively manage these relationships, it could have an adverse impact on our business and financial performance.
Further, our operations depend on the continuing and efficient operation of our information technology, communications systems and infrastructure, and cloudon cloud-based platforms. Any of these systems and infrastructure are vulnerable to damage or interruption from earthquakes, vandalism, sabotage, terrorist attacks, floods, fires, power outages, telecommunications failures, computer viruses or other deliberate attempts to harm the systems. The occurrence of a natural or intentional disaster, any decision to close a facility we are using without adequate notice, or particularly an unanticipated problem at a cloud-based virtual server facility, could result in harmful interruptions in our service, resulting in adverse effects to our business.
Product liability lawsuits against us could cause us to incur substantial liabilities and could limit commercialization of any product candidate that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in clinical trials and may face an even greater risk if we commercialize any product candidate that we may develop. If we cannot successfully defend ourselves against claims that any such product candidates caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may, without limitation, result in:
•decreased demand for any product candidate that we may develop;
•loss of revenue;
•substantial monetary awards to trial participants or patients;
•significant time and costs to defend the related litigation;
•withdrawal of clinical trial participants;
•increased insurance costs;
•the inability to commercialize any product candidate that we may develop; and
•injury to our reputation and significant negative media attention.
Any such outcomes could materially adversely affect our business, financial condition, results of operations and growth prospects.
Our insurance policies may be inadequate, may not cover all of our potential liabilities and may potentially expose us to unrecoverable risks.
We do not carry insurance for all categories of risk that our business may encounter. Although we maintain product liability insurance coverage that also covers our clinical trials, such insurance may not be adequate to cover all liabilities that we may incur, and we may be required to increase our product liability insurance coverage. We anticipate that we will need to increase our insurance coverage each time we commence a clinical trial and if we successfully commercialize any product candidate. Insurance availability, coverage terms and pricing continue to vary with market conditions. We endeavor to obtain appropriate insurance coverage for insurable risks that we identify. However, we may fail to correctly anticipate or quantify insurable risks, we may not be able to obtain appropriate insurance coverage and insurers may not respond as we intend to cover insurable events that may occur. Any significant uninsured liability may require us to pay substantial amounts, which would materially adversely affect our business, financial condition, results of operations and growth.
In addition, although we are dependent on certain key personnel, we do not have any key man life insurance policies on any such individuals. Therefore, if any of our chief executive officer or other executive officers die or become disabled, we will not receive any compensation to assist with such individual’s absence. The loss of any such person could materially adversely affect our business, financial condition, results of operations and growth prospects.
Our business involves the use of hazardous materials and we and our third-party manufacturers and suppliers must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our research and development and manufacturing activities and our third-party manufacturers’ and suppliers’ activities involve the controlled storage, use and disposal of hazardous materials owned by us. We and our manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at our manufacturers’ facilities pending their use and disposal.
We cannot eliminate the risk of contamination, which could cause an interruption of our research and development efforts and business operations, including drug supply and inventory, and environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by our third-party manufacturers and suppliers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently and have tended to become more stringent over time. We cannot predict the impact of such changes and cannot be certain of our future compliance. We do not currently carry biological or hazardous waste insurance coverage. Any contamination by such hazardous materials could therefore materially adversely affect our business, financial condition, results of operations and growth prospects.
Risks Related to Our Financial Position
We have a limited operating history, have incurred significant losses since our inception, and we expect to continue to incur significant losses for the foreseeable future.
We are a clinical-stage biotechnology company developing cell therapies for neurodegenerative and oncological diseases with a limited operating history upon which you can evaluate its business and prospects. Since our inception in 2017, we have incurred significant operating losses. Our net losses were $83.0 million and $26.7 million for the years ended December 31, 2023 and 2022, respectively. Our accumulated deficit was $162.1 million as of December 31, 2023. See “— Risks Related to Our Business and Industry — We currently do not have sufficient funds to service our operations and accrued expenses and payables and require additional capital. Our independent registered public accountants and management have expressed substantial doubt as to our ability to continue as a going concern without additional capital” for more details on our current financial and business information and related risks.
We expect to continue to incur increasing operating losses for the foreseeable future as we continue to develop our product candidates. In addition, we anticipate that our expenses will increase substantially if, and as, we:
•continue the clinical development of SNK01 and SNK02;
•advance additional product candidates to clinical trials, including product candidates under the collaboration with Merck KgaA;
•develop our current product candidates for additional disease indications;
•seek to discover and develop additional product candidates;
•maintain our own clinical- and commercial-scale clinical GMP facilities;
•seek regulatory approval of our product candidates in various jurisdictions for commercial sale;
•maintain, expand and protect our intellectual property portfolio;
•acquire or in-license other product candidates and technologies;
•incur additional costs associated with operating as a public company;
•develop or secure marketing, sales and distribution capabilities, either internally or with third parties, to support commercialization; and
•increase our employee headcount and related expenses to support the foregoing activities.
We may find that these efforts are more expensive than we currently anticipate or that these efforts may not result in revenues, which would further increase our losses. In addition, we have limited experience and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in the same industry. If we are unable to achieve and/or sustain profitability, or if we are unable to achieve the growth that we expect from these efforts, it could have a material adverse effect on our business, financial condition or results of operations. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
We have never generated revenue from product sales and may never achieve or maintain profitability.
We are a clinical-stage biotechnology company without any products approved for commercial sale, and have not generated any revenue from product sales. We are focused on developing cell therapies for neurodegenerative and oncological diseases based on activated NK cells and our technologies are relatively new and largely unproven. Since our inception in 2017, we have invested most of our resources in developing our product candidates, building our intellectual property portfolio, conducting clinical trials, developing our in-house manufacturing capability, conducting business planning, raising capital and providing general and administrative support for these operations. Consequently, we have no meaningful operations upon which to evaluate our business, and predictions about our future success or viability may not be as accurate as they could be if we had a longer operating history or a history of successfully developing and commercializing drug products. We have not yet demonstrated an ability to overcome many of the risks and uncertainties frequently encountered by companies in the rapidly evolving biotechnology industry.
We continue to incur significant research and development and other expenses related to ongoing operations and the development of our two lead product candidates, SNK01 and SNK02. All of our product candidates will require substantial additional development time and resources before we would be able to apply for or receive regulatory approvals and begin generating revenue from product sales. Neither the FDA nor any other regulatory authority has approved SNK01, SNK02 or any of our other product candidates, and we do not anticipate generating revenues from product sales unless and until such time as SNK01, SNK02 or another of our product candidates has been approved by the FDA or another regulatory authority, if ever, and we are able to successfully market and sell a product candidate. Our ability to generate revenues from product sales depends on, without limitation, our, or potential future collaborators’ success in:
•completing clinical development of our product candidates;
•seeking and obtaining regulatory approvals for product candidates for which we successfully complete positive clinical trials, if any;
•launching and commercializing product candidates, by establishing a commercial infrastructure or, alternatively, collaborating with a commercialization partner;
•qualifying for adequate coverage and reimbursement by government and third-party payors for our product candidates;
•establishing, maintaining and enhancing a sustainable, scalable, reproducible and transferable manufacturing process for each of our cell therapy product candidates;
•establishing and maintaining supply and manufacturing relationships with third parties that can provide adequate products and services, in both amount and quality, to support clinical development and the market demand for our product candidates, if approved;
•obtaining market acceptance of our product candidates as a viable treatment option;
•addressing any competing technological and market developments;
•implementing additional internal systems and infrastructure, as needed;
•negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter and performing our obligations in such collaborations;
•maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets, know-how, and trademarks;
•avoiding and defending against third-party interference or infringement claims; and
•attracting, hiring and retaining qualified personnel.
We anticipate incurring significant costs associated with commercializing any approved product candidate. Our expenses could increase beyond our current expectations if we are required by the FDA or other global regulatory authorities to perform clinical trials and/or other preclinical studies in addition to, or beyond the scope of, those that we currently anticipate being required to perform.
Even if we are able to generate revenues from the sale of any approved products, we may not become profitable or be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable could decrease the value of our company and impair our ability to raise capital, thereby limiting our research and development programs and efforts to expand our business or continue our operations.
The East West Bank Loan Agreement and Equity and Business Loan Agreement (as defined below) provide each lender with a security interest in all of our assets, and contain financial covenants and other restrictions on our actions that may limit our operational flexibility or otherwise adversely affect our results of operations.
In June 2023, we entered into a $5.0 million revolving line of credit agreement with East West Bank. This revolving line of credit is secured by a first priority lien on all of the assets of NKGen Legacy, including a deed of trust over our owned real property located in Santa Ana, California. We were required to maintain a minimum cash balance of $0.3 million with the bank to secure this revolving line of credit and were required to maintain a minimum cash balance of $15.0 million with the bank as of March 31, 2024 and at all times thereafter as long as there is an outstanding balance under the revolving line of credit. Failure to meet the minimum cash balance requirement would constitute an event of default under the East West Bank Loan Agreement, which would permit East West Bank to accelerate the indebtedness under the East West Loan Agreement and, if NKGen is unable to pay such indebtedness, foreclose on NKGen’s assets, including its owned real property which is subject to a deed of trust in favor of East West Bank. On April 5, 2024, we entered into an amendment which replaces such minimum cash balance requirement with a covenant to use East West Bank as the Company's only commercial bank for cash deposits and extend the maturity date to September 18, 2024. The East West Bank Loan Agreement permits NKGen to terminate the East West Bank Loan Agreement and security interest thereunder at any time by repaying in full the loan provided thereunder (together with all interest and any fees owed thereon). See the section of this Annual Report on Form 10-K titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources — Sources of Liquidity — Subsequent Financing Arrangements” for more details.
In April 2024, we entered into an equity and business loan agreement (the “Equity and Business Loan Agreement”) with NKGen Legacy and BDW Investments LLC ("BDW"). The Equity and Business Loan Agreement provided for a multi draw term loan financing in a principal amount of up to $5 million. These term loans are secured by a first priority lien on all assets of NKGen and a second priority lien on all assets of the NKGen Legacy, including a deed of trust over our owned real property located in Santa Ana, California, subject to an intercreditor agreement with East West Bank. See Note 4, Subsequent Events, of the consolidated financial statements for more details
The terms of our outstanding debt may restrict our current and future operations and could adversely affect our ability to finance our future operations or capital needs or to execute business strategies in the manner desired. In addition, complying with these covenants may make it more difficult for us to successfully execute our business strategy, invest in our growth strategy, and compete against companies who are not subject to such restrictions.
A failure by us to comply with any of the covenants or payment requirements specified in the revolving line of credit agreement or Equity and Business Loan Agreement could result in an event of default under the revolving line of credit agreement or Equity and Business Loan Agreement, which would give the lenders the right to terminate their commitments to provide additional loans and extensions of credit and to declare any and all debt outstanding, together with accrued and unpaid interest and fees, to be immediately due and payable. In addition, the lenders would have the right to proceed against the collateral in which we granted a security interest to them, which consists of substantially all our assets. If our outstanding debt were to be accelerated, we may not have sufficient cash or be able to borrow sufficient funds to refinance the loan or sell sufficient assets to repay the loan, which could materially and adversely affect our cash flows, business, results of operations and financial condition.
The terms of our 2023 NKMAX Loan Agreements, the East West Bank Loan Agreement and the Equity and Business Loan Agreement require us to meet certain payment obligations, and may subject us to default.
We entered into a series of 2023 NKMAX Loan Agreements between January 2023 and April 2023, for an aggregate principal amount of $5.0 million. The proceeds of the loans are used by us for working capital and to fund our general business requirements. The loans carry an interest rate of 4.6% per annum and have a maturity date of December 31, 2024. In June 2023, we also entered into a $5.0 million revolving line of credit agreement with East West Bank, which bears an interest rate based on the higher of (i) the one month secured overnight financing rate plus 2.9% or (ii) 7.5%. In April 2024, we entered into a multi draw term loan financing in a principal amount of up to $5 million with BDW, which bears interest at a rate per annum equal to the interest rate applicable to the East West Bank Loan Agreement for as long as the East West Bank Loan Agreement is outstanding, or if the East West Bank Loan Agreement has been refinanced, the interest rate applicable to such refinancing facility or, on any such date that the East West Bank Loan Agreement or any refinancing facility thereof is no longer outstanding, the term loans will bear interest at a rate equal to 1-month term SOFR plus 2.85%; provided that in no event will the rate per annum be less than 7.50% at any time. If we default under the 2023 NKMAX Loan Agreements, we must pay to NKMAX all costs of collection including applicable attorney’s fees. If we default under the East West Bank Loan Agreement or the Equity and Business Loan Agreement, at the lenders' option, all indebtedness will immediately become due and payable, with very limited exceptions. The occurrence of an event of default under any of these agreements could result in breach of our obligations under other agreements, including the Merger Agreement. Any declaration by any of these lenders of an event of default could materially harm our business and prospects and limit how we conduct our business.
Risks Related to Government Regulations
The regulatory approval process of the FDA and comparable foreign regulatory authorities are lengthy, time- consuming and inherently unpredictable, and even if we complete the necessary clinical trials, we cannot predict when, or if, we will obtain regulatory approval for any of our product candidates, and any such regulatory approval may be for a more narrow indication than we seek.
The research, testing, manufacturing, labeling, approval, selling, import, export, marketing, and distribution of drug products, including biologics, are subject to extensive regulation by the FDA and other regulatory authorities in and outside the United States. We are not permitted to market any biological drug product in the United States until we receive approval of a BLA from the FDA. We have not previously submitted a BLA to the FDA, or similar approval filings to comparable foreign authorities. A BLA must include extensive preclinical and clinical data and supporting information to establish the product candidate’s safety and effectiveness for each desired indication. The BLA must also include significant information regarding the chemistry, manufacturing and controls for the product, including with respect to chain of identity and chain of custody of the product.
Our product candidates could fail to receive regulatory approval from the FDA or a comparable foreign regulatory authority for many reasons, including, but not limited to:
•disagreement with the design or conduct of our clinical trials;
•failure to demonstrate to the satisfaction of regulatory agencies that our product candidates are safe and effective, or have a positive benefit/risk profile for its proposed indication;
•failure of clinical trials to meet the level of statistical significance required for approval;
•failure to conduct clinical trials according to GCP and guidelines as set forth by the International Council for Harmonization;
•disagreement with our interpretation of data from preclinical studies or clinical trials;
•the insufficiency of data collected from clinical trials of our product candidates to support the submission and filing of a BLA or other submission or to obtain regulatory approval;
•failure to obtain approval of our manufacturing processes or facilities of third-party manufacturers with whom we contract for clinical and commercial supplies or our own manufacturing facility; or
•changes in the approval policies or regulations that render our preclinical and clinical data insufficient for approval.
This lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval to market our product candidates, which would significantly harm our business, results of operations and prospects. The FDA or a comparable foreign regulatory authority may require more information, including additional preclinical or clinical data to support approval, which may delay or prevent approval and our commercialization plans, or we may decide to abandon the development program. If we were to obtain approval, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request (including failing to approve the most commercially promising indications), may grant approval contingent on the performance of costly post-marketing clinical studies, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Even if our product candidates meet their safety and efficacy endpoints in clinical trials, the regulatory authorities may not complete their review processes in a timely manner, or we may not be able to obtain regulatory approval.
We expect the novel nature of our product candidates to create further challenges in obtaining regulatory approval. The FDA may also require a panel of experts, referred to as an advisory committee (the “Advisory Committee”), to deliberate on the adequacy of the safety and efficacy data to support marketing authorization. The opinion of the Advisory Committee, although not binding, may have a significant impact on our ability to obtain marketing authorization of the product candidates based on the completed clinical trials, as the FDA often adheres to the Advisory Committee’s recommendations. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory authority policy during the period of product development, clinical trials and the review process. Accordingly, the regulatory approval pathway for our product candidates may be uncertain, complex, expensive and lengthy, and approval may not be obtained. Regulatory authorities also may approve a product candidate for more limited indications than requested or they may impose significant limitations in the form of narrow indications, warnings or a REMS. These regulatory authorities may require labeling that includes precautions or contra-indications with respect to conditions of use, or they may grant approval subject to the performance of costly post-marketing clinical trials. In addition, regulatory authorities may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates. Regulatory authorities may withdraw or suspend their approval of the product or may impose restrictions on its distribution after obtaining marketing approval. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates and materially adversely affect our business, financial condition, results of operations and prospects.
We are and will be subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti- corruption laws and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We could face criminal and/or civil liability and other serious consequences for violations, which would harm our business.
Our product candidates will be subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations and various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls, the U.S. Foreign Corrupt Practices Act of 1977, the U.S. domestic bribery statute contained in 18 U.S.C. §201, the U.S. Travel Act, the USA PATRIOT Act and possibly other state and national anti-bribery and anti-money laundering laws in countries in which we conduct activities. Exports of our product candidates must be made in compliance with export control and sanctions laws and regulations. In some cases, certain licensing, authorization, or reporting requirements may need to be performed. In addition, these laws may restrict or prohibit altogether the supply of certain of our product candidates to certain governments, persons, entities, countries, and territories. Changes in our product candidates or changes in applicable export or import laws and regulations may create delays in the introduction or provision of our product candidates in other jurisdictions, prevent others from using our product candidates or, in some cases, prevent the export or import of our product candidates to certain countries, governments or persons altogether. Any limitation on our ability to export or provide our product candidates could adversely affect our business, financial condition and results of operations.
Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, third-party intermediaries, joint venture partners and collaborators from authorizing, promising, offering or providing, directly or indirectly, improper payments or benefits to recipients in the public or private sector. We may use CROs abroad for clinical trial activities. In addition, we may engage third-party intermediaries to sell our product candidates and solutions abroad once we enter a commercialization phase for our product candidates and/or to obtain necessary permits, licenses, and other regulatory approvals. We or our third-party intermediaries may have direct or indirect interactions with officials and employees of government agencies or state-owned or affiliated entities. We can be held liable for the corrupt or other illegal activities of these third-party intermediaries, our employees, representatives, contractors, partners and agents, even if we do not explicitly authorize or have actual knowledge of such activities. If we fail to comply with these laws and regulations, we and certain of our employees could be subject to substantial civil or criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm and other consequences.
We have adopted an anti-corruption policy, which mandates compliance with the FCPA and other anti- corruption laws applicable to our business throughout the world. However, there can be no assurance that our employees and third-party intermediaries will comply with this policy or such anti-corruption laws. Non-compliance with anti-corruption and anti-money laundering laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, other investigations, or other enforcement actions. If such actions are launched, or governmental or other sanctions are imposed, or if we do not prevail in any possible civil or criminal litigation, our business, results of operations and financial condition could be materially harmed. In addition, responding to any action will likely result in a materially significant diversion of management’s attention and resources and significant defense and compliance costs and other professional fees. In certain cases, enforcement authorities may even cause us to appoint an independent compliance monitor, which can result in added costs and administrative burdens.
Healthcare reform initiatives and other administrative and legislative proposals may harm our business.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action in the United States, Mexico, Japan, the European Union or any other jurisdiction. In the United States, there have been several recent Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. For example, on August 16, 2022, President Biden signed the Inflation Reduction Act of 2022 (“IRA”) into law, which, among other things (i) directs the U.S. Department of Health and Human Services (“HHS”) to negotiate the price of certain high- expenditure, single-source drugs and biologics covered under Medicare and (ii) imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation. These provisions will take effect progressively starting in fiscal year 2023, although they may be subject to legal challenges. HHS has and will continue to issue and update guidance as these programs are implemented. It is currently unclear how the IRA will be implemented but is likely to have a significant impact on the pharmaceutical industry. In addition, in response to the Biden administration’s October 2022 executive order, on February 14, 2023, HHS released a report outlining three new models for testing by the Center for Medicare and Medicaid Innovation which will be evaluated on their ability to lower the cost of drugs, promote accessibility, and improve quality of care. It is unclear whether the models will be utilized in any health reform measures in the future. If we or any third parties we may engage are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we or such third parties are not able to maintain regulatory compliance, our product candidates may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability. Furthermore, future price controls or other changes in pricing regulation or negative publicity related to the pricing of pharmaceutical drugs could restrict the amount that we are able to charge for our drug products, which could render our product candidates, if approved, commercially unviable and materially adversely affect our ability to raise additional capital on acceptable terms.
If third-party payors fail to provide adequate coverage and reimbursement for our product candidates it could have a material adverse effect on our operating results and overall financial condition.
Significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we may obtain regulatory approval. Sales of any of product candidates, if approved, will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, including government healthcare programs such as Medicare and Medicaid, and private payors, such as commercial health insurers and managed care organizations. Third-party payors determine which drugs they will cover and the amount of reimbursement they will provide for a covered drug. In the U.S., there is no uniform system among payors for making coverage and reimbursement decisions. In addition, the process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the FDA-approved products for a particular indication.
In order to secure coverage and reimbursement for our product candidates, once approved, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costly studies required to obtain FDA or other comparable regulatory approvals. Even if we conduct pharmacoeconomic studies, our product candidates, once approved, may not be considered medically necessary or cost-effective by payors. Further, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved.
Furthermore, the healthcare industry in the U.S. has experienced a trend toward cost containment as government and private insurers seek to control healthcare costs by imposing lower payment rates and negotiating reduced contract rates with service providers. Therefore, we cannot be certain that the procedures using our product candidates, once approved, will be reimbursed at a cost-effective level. Nor can we be certain that third-party payors using a methodology that sets amounts based on the type of procedure performed, such as those utilized by government programs and in many privately managed care systems, will view the cost of our product candidates, once approved, to be justified so as to incorporate such costs into the overall cost of the procedure. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to achieve profitability. Moreover, we are unable to predict what changes will be made to the reimbursement methodologies used by third-party payors in the future.
Our inability to promptly obtain coverage and adequate reimbursement from third-party payors for any of our product candidates for which we obtain marketing approval could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Obtaining and maintaining marketing approval or commercialization of our product candidates in one jurisdiction does not mean that we will be successful in obtaining marketing approval of our product candidates in other jurisdictions.
Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials as clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
If we market approved products outside the United States, we expect that we will be subject to additional risks in commercialization, including, but not limited to:
•different regulatory requirements for approval of therapies in foreign countries;
•reduced protection for intellectual property rights;
•unexpected changes in tariffs, trade barriers and regulatory requirements;
•economic weakness, including inflation, or political instability in particular foreign economies and markets;
•compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
•foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country;
•foreign reimbursement, pricing and insurance regimes;
•workforce uncertainty in countries where labor unrest is more common than in the United States;
•production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
•business interruptions resulting from geopolitical actions, including war and terrorism (such as the military conflict between Russia and Ukraine and the State of Israel’s war against Hamas), natural disasters including earthquakes, typhoons, floods and fires, and other public health crises, illnesses, epidemics or pandemics.
We have no prior experience in these areas. In addition, there are complex regulatory, tax, labor and other legal requirements imposed by many of the individual countries in which we may operate, with which we will need to comply. Any of the foregoing difficulties, if encountered, could materially adversely affect our business, financial condition, results of operations and growth prospects.
Our business operations and relationships with investigators, healthcare professionals, consultants, third-party payors, patient organizations and customers will be subject to applicable fraud and abuse and other healthcare laws and regulations, which could expose us to penalties.
These laws may constrain the business or financial arrangements and relationships through which we conduct our operations, including how we research, market, sell and distribute our product candidates, if approved. Such laws include, the U.S. federal Anti-Kickback Statute, the U.S. federal civil and criminal false claims and civil monetary penalties laws, including the civil False Claims Act, the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, the U.S. Physician Payments Sunshine Act and its implementing regulations, U.S. state laws and regulations, including, state anti-kickback and false claims laws, laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the U.S. federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources, laws and regulations that require drug manufacturers to file reports relating to pricing and marketing information, laws requiring the registration of pharmaceutical sales representatives, laws governing the privacy and security of health information in certain circumstances, and similar healthcare laws and regulations in other jurisdictions, including reporting requirements detailing interactions with and payments to healthcare providers.
It is not always possible to identify and deter misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from government investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. Ensuring that our internal operations and future business arrangements with third parties comply with applicable healthcare laws and regulations will also involve substantial costs. If our operations are found to be in violation of any of the laws described above or any other governmental laws and regulations that may apply to us, we may be subject to significant penalties, including civil, criminal and administrative penalties, damages, fines, exclusion from government-funded healthcare programs, such as Medicare and Medicaid or similar programs in other countries or jurisdictions, integrity oversight and reporting obligations to resolve allegations of non-compliance, disgorgement, individual imprisonment, contractual damages, reputational harm, diminished profits and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs and imprisonment, which could affect our ability to operate our business. Further, defending against any such actions can be costly, time- consuming and may require significant personnel resources. Any of the foregoing could significantly harm our business, financial condition, results of operations and growth prospects.
We are subject to stringent and evolving laws, regulations, rules, contractual obligations, policies and other obligations related to data privacy and security. Our actual or perceived failure to comply with such obligations could lead to regulatory investigations or actions, litigation, fines and penalties, disruptions of our business operations, reputational harm, loss of revenue or profits, and other adverse business consequences.
In the ordinary course of business, we collect, receive, store, process, generate, use, transfer, disclose, make accessible, protect, secure, dispose of, transmit, and share (collectively, “processing”) personal data and other sensitive information, including proprietary and confidential business data, trade secrets, intellectual property, data we collect about trial participants in connection with clinical trials sensitive third-party data, business plans, transactions, and financial information (collectively, “sensitive data”).
Our data processing activities may subject us to numerous data privacy and security obligations, such as various laws, regulations, guidance, industry standards, external and internal privacy and security policies, contractual requirements, and other obligations relating to data privacy and security.
In the United States, federal, state, and local governments have enacted numerous data privacy and security laws, including data breach notification laws, personal data privacy laws, consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), and other similar laws (e.g., wiretapping laws). For example, the California Consumer Privacy Act of 2018 (“CCPA”) applies to personal information of consumers, business representatives, and employees, and requires businesses to provide specific disclosures in privacy notices and honor requests of California residents to exercise certain privacy rights. The CCPA provides for civil penalties of up to $7,500 per violation and allows private litigants affected by certain data breaches to recover significant statutory damages. Although the CCPA exempts some data processed in the context of clinical trials, the CCPA increases compliance costs and potential liability with respect to other personal data we maintain about California residents. In addition, the California Privacy Rights Act of 2020 expands the CCPA’s requirements, including by adding a new right for individuals to correct their personal information and establishing a new regulatory agency to implement and enforce the law. Other states, such as Virginia and Colorado, have also passed comprehensive privacy laws, and similar laws are being considered in several other states, as well as at the federal and local levels. While these states, like the CCPA, also exempt some data processed in the context of clinical trials, these developments may further complicate compliance efforts, and increase legal risk and compliance costs for us and the third parties upon whom we rely.
Outside the United States, an increasing number of laws, regulations, and industry standards may govern data privacy and security and may become applicable to us as we expand. For example, the European Union’s General Data Protection Regulation (“EU GDPR”) and the United Kingdom’s GDPR impose strict requirements for processing personal data. For example, under the EU GDPR, companies may face temporary or definitive bans on data processing and other corrective actions; fines of up to 20 million Euros or 4% of annual global revenue, whichever is greater; or private litigation related to processing of personal data brought by classes of data subjects or consumer protection organizations authorized at law to represent their interests.
In addition, data localization requirements or limitations on cross-border data flows may render us unable to transfer personal data from other jurisdictions to the United States or other countries. For example, Europe and other jurisdictions have enacted laws requiring data to be localized or limiting the transfer of personal data to other countries. Other jurisdictions may adopt similarly stringent interpretations of their data localization and cross-border data transfer laws.
In addition to data privacy and security laws, we may become contractually subject to industry standards adopted by industry groups and other such obligations in the future. We are also bound by contractual obligations related to data privacy and security, and our efforts to comply with such obligations may not be successful. We also publish privacy policies, marketing materials, and other statements regarding data privacy and security. If these policies, materials or statements are found to be deficient, lacking in transparency, deceptive, unfair, or misrepresentative of our practices, we may be subject to investigation, enforcement actions by regulators, or other adverse consequences.
Obligations related to data privacy and security are quickly changing, becoming increasingly stringent, and creating regulatory uncertainty. Additionally, these obligations may be subject to differing applications and interpretations, which may be inconsistent or conflict among jurisdictions. Preparing for and complying with these obligations requires us to devote significant resources and may necessitate changes to our services, information technologies, systems, and practices and to those of any third parties that process personal data on our behalf. In addition, these obligations may require us to change our business model.
We may at times fail (or be perceived to have failed) in our efforts to comply with our data privacy and security obligations. Moreover, despite our efforts, our personnel or third parties on whom we rely may fail to comply with such obligations, which could negatively impact our business operations. If we or the third parties on which we rely fail, or are perceived to have failed, to address or comply with applicable data privacy and security obligations, we could face significant consequences, including but not limited to: government enforcement actions (e.g., investigations, fines, penalties, audits, inspections, and similar); litigation (including class-action claims); additional reporting requirements and/or oversight; bans on processing personal data; and orders to destroy or not use personal data. Any of these events could have a material adverse effect on our reputation, business, or financial condition, including but not limited to: loss of customers; inability to process personal data or to operate in certain jurisdictions; limited ability to develop or commercialize our products; expenditure of time and resources to defend any claim or inquiry; adverse publicity; or substantial changes to our business model or operations.
Risks Related to Manufacturing
Our manufacturing process is novel and complex, and we may encounter difficulties in production, or difficulties with internal manufacturing, which would delay or prevent our ability to provide a sufficient supply of our product candidates for clinical trials or our products for patients, if approved.
Our product candidates are engineered human cells, and the process of manufacturing such product candidates, is complex, highly regulated and subject to numerous risks. Manufacturing our product candidates involves harvesting blood cells from a healthy donor or patient, isolating the NK cells from peripheral blood mononuclear cells, activating and expanding the NK cells, cryopreservation, storage and eventually shipment. Our ability to consistently and reliably manufacture cell therapy product candidates is essential to our success, and there are risks associated with scaling to the level required for advanced clinical trials or commercialization, including cost overruns, potential problems with sourcing of materials, quality control, stability issues, consistency and timely availability of raw materials.
Our manufacturing process will be susceptible to product loss or failure, or product variation that may negatively impact patient outcomes, due to logistical issues associated with the collection of starting material from the donor, shipping such material to the manufacturing site, shipping the final product to the clinical trial recipient, preparing the product for administration, manufacturing issues or different product characteristics resulting from the differences in donor starting materials, variations between reagent lots, interruptions in the manufacturing process, contamination, equipment or reagent failure, improper installation or operation of equipment, vendor or operator error, inconsistency in cell growth and variability in product characteristics.
Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our product candidates or in any of the manufacturing facilities in which products or other materials are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. Any failure in the manufacturing processes could render a batch of product unusable, could impact supply and delay the progress of our clinical trials, could affect the regulatory approval of such product candidate, could cause us to incur fines or penalties or could harm our reputation and that of our product candidates.
Our manufactured product candidates may fail to meet the required specifications for any of a variety of reasons, including variability in starting material, deviations from normal manufacturing process, or insufficient optimization of specific process steps. This failure to meet specifications could result in supply shortages, or delays related to obtaining additional regulatory, site and patient approvals to continue dosing the clinical trial. If the required additional approvals cannot be obtained, additional delays may occur as manufacturing would need to be restarted and/or the patient may be unable to remain in the study. Any delay in the clinical development or commercialization of SNK01, SNK02, or our other product candidates could materially adversely affect our business, financial condition, results of operations and growth prospects.
We may make changes to our manufacturing process at various points during development, and even after commercialization, for various reasons, such as to control costs, achieve scale, decrease processing time, increase manufacturing success rate or for other reasons. Changes to our manufacturing process carry the risk that they will not achieve their intended objectives, and any of these changes could cause our product candidates to perform differently and affect the results of our ongoing clinical trials, or the performance of the product once commercialized. Changes to our process made during the course of clinical development could require us to show the comparability of the product candidate used in earlier clinical phases or at earlier portions of a trial to the product candidate used in later clinical phases or later portions of the trial. It is difficult to establish comparability of cell therapy products, and this may complicate efforts to verify process changes during scale up. Other changes to our manufacturing process made before or after commercialization could require us to show the comparability of the resulting product to the product candidate used in the clinical trials using earlier processes. Such showings could require us to collect additional nonclinical or clinical data from any modified process prior to obtaining marketing approval for the product candidate produced with such modified process. If such data are not ultimately comparable to that seen in the earlier trials or earlier in the same trial in terms of safety or efficacy, or if regulatory authorities do not agree that comparability has been established, we may be required to make further changes to our process and/or undertake additional clinical testing, either of which could significantly delay the clinical development or commercialization of the associated product candidate, which would materially adversely affect our business, financial condition, results of operations and growth prospects.
Although we are manufacturing SNK01 in our own internal manufacturing facility for the SNK01 clinical trials, and plan to manufacture other product candidates, including SNK02, in our internal manufacturing facilities in the future, we may encounter problems with the internal production of our product candidates. We believe our current clinical GMP manufacturing facility will supply our anticipated clinical trial needs, but if the dose and number of cycles needed increases, our current manufacturing process may not be able to support the enrollment of trials which could lead to delays until we scale up the manufacturing. While we believe that we have a manufacturing facility with capabilities to meet increased production needs, it would still require an increase in staff and significant internal resources. Our manufacturing facilities will be subject to compliance with regulatory requirements, which we may struggle to meet. We may encounter problems with properly staffing our internal manufacturing facilities due to hiring challenges or other issues. For example, factors such as potential future outbreaks of COVID-19 variants and related restrictions could impact our ability to properly staff production of our product candidates. Current inflationary pressures are negatively affecting and could continue to negatively affect the costs of constructing our commercial-scale manufacturing facility. Global supply chain disruptions, including procurement delays and long lead times on certain materials, have adversely impacted and could continue to adversely impact the scheduled completion and/or costs of constructing our commercial-scale manufacturing facility. We may also encounter problems with training the staff we have to effectively manage and control the complex manufacturing process required to produce our product candidates and comply with all necessary regulations. We may also find it difficult to properly manage supply chain issues critical to the manufacturing process. If we are unable to build, maintain, and properly staff our manufacturing facilities, manage and control the manufacturing process, and comply with regulations, the clinical development or commercialization of our product candidates could be significantly delayed, which would materially adversely affect our business, financial condition, results of operations and growth prospects.
Delays in commissioning and receiving regulatory approvals for our manufacturing facilities could delay our development plans and thereby limit our ability to develop our product candidates and generate revenues.
We believe that internal GMP manufacturing is important to facilitate clinical product supply, lower the risk of manufacturing disruptions and enable more cost-effective manufacturing. We have a GMP facility in Santa Ana, California that allows us to supply the product candidates needed for our early-stage clinical trials.
Furthermore, our manufacturing facilities will be subject to ongoing, periodic inspection by the FDA and other comparable regulatory agencies to ensure continued compliance with GMP. Our failure to follow and document our adherence to these regulations or other regulatory requirements may lead to significant delays in the availability of product candidates for clinical use or may result in the termination of or a hold on a clinical study. Failure to comply with applicable regulations could also result in sanctions being imposed on us, including fines, injunctions, civil penalties, a requirement to suspend or put on hold one or more of our clinical trials, failure of regulatory authorities to grant marketing approval of our drug candidates, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of drug candidates, operating restrictions and criminal prosecutions, any of which could materially adversely affect our business, financial condition, results of operations and growth prospects.
We also may encounter problems with, without limitation, the following:
•complying with regulations regarding evolving donor infectious disease testing, traceability, manufacturing, release of product candidates and other requirements from regulatory authorities outside the United States;
•achieving adequate or clinical-grade materials that meet regulatory agency standards or specifications with consistent and acceptable production yield and costs;
•bacterial, fungal or viral contamination in our manufacturing facilities;
•disruptions due to natural disasters or supply chain interruptions; and
•shortages of qualified personnel, raw materials or key contractors.
Our product candidates, if approved by applicable regulatory authorities, may require significant commercial supply to meet market demand. In these cases, we may need to increase, or “scale up,” the production process by a significant factor over the initial level of production. If we fail to develop sufficient manufacturing capacity and experience, whether internally or with a third party, are delayed in doing so, or fail to manufacture our product candidates economically or on reasonable scale or volumes, or in accordance with GMP, or if the cost of this scale-up is not economically feasible, our development programs and commercialization of any approved products will be materially adversely affected and we may not be able to produce our product candidates in a sufficient quantity to meet future demand and our business, financial condition, results of operations and growth prospects may be materially adversely affected.
Any contamination or interruption in our manufacturing process, shortages of raw materials or failure of our suppliers to deliver necessary components could result in delays in our clinical development or marketing schedules.
Given the nature of cell therapy manufacturing, there is a risk of contamination. If microbial, viral or other contaminants are discovered in our product candidates or in any of the manufacturing facilities in which products or other materials are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. Any contamination could adversely affect our ability to produce product candidates on schedule and could, therefore, delay our clinical trials, harm our results of operations and cause reputational damage. Some of the raw materials required in our manufacturing process are derived from biologic sources. These raw materials are difficult to procure and may be subject to contamination or recall. A material shortage, contamination, recall or restriction on the use of biologically derived substances in the manufacture of our product candidates could adversely impact or disrupt the commercial manufacturing or the production of clinical material, which could adversely affect our development timelines and our business, financial condition, results of operations and prospects.
The optimal donor and manufacturing parameters for our product candidates have not been definitively established, which may hinder our ability to optimize our product candidates or to address any safety or efficacy issues that may arise.
If any of our clinical trials reveal issues with the safety or efficacy of any of our product candidates, modification of the donor selection criteria or the manufacturing process may be necessary to address such issues. Alternatively, we may choose to modify the manufacturing process in an effort to improve the efficiency of the process or efficacy of the product candidates. However, we have not, at present, fully characterized or identified how donor characteristics and manufacturing process parameters affect the optimal potency of function for our engineered NK cell product candidates for in vitro and animal efficacy studies or how such potency differences may translate into efficacy to be seen in human clinical trials, including both the proportion of patients who achieve a meaningful clinical response, and the duration of any such clinical responses. Our ability to improve our manufacturing process or product potency, safety, or efficacy according to such parameters is limited and may require significant trial and error, which may cause us to incur significant costs or could result in significant delays to the clinical development and eventual commercialization of our product candidates.
Dependency on third parties to store our NK cells, viral vector, master and working cell banks, and any damage or loss would cause delays in replacement, and our business could suffer.
The NK cells, the viral vector, and the master and working cell banks are stored in freezers at third-party biorepositories and will also be stored in our freezers at our production facility. If these materials are damaged at these facilities, including by the loss or malfunction of these freezers or our back-up power systems, as well as by damage from fire, power loss or other natural disasters, we would need to establish replacement of NK cells, viral vector, and master and working cell banks, which would impact clinical supply and delay our patients’ treatments. If we are unable to establish replacement materials, we could incur significant additional expenses and liability to patients whose treatment is delayed, and our business could suffer.
We have not yet established a shelf life beyond one to two years for our product candidates, which may have an impact on commercial supply and expenses.
We have not yet developed a validated method of manufacturing our product candidates for long-term storage, in large quantities without damage, in a cost-efficient manner and without degradation beyond one to two years. We may encounter difficulties not only in developing the relevant methodologies but also in obtaining the necessary regulatory approvals for using such methodologies in treatment. If we cannot adequately demonstrate that our product candidates can be safely stored for long-term and to the satisfaction of regulatory authorities, we could face substantial delays in obtaining regulatory approvals to market and further commercialize our products. If we are unable to develop a validated method to store our product candidates for long-term for shipping purposes, our ability to promote the adoption of our product candidates, as well as achieve economies of scale by utilizing our production facility, will be limited. Even if we are able to successfully develop such methodology, we will also need to develop a cost-effective and reliable distribution and logistics network, which we may be unable to accomplish.
In addition, if the product candidates cannot be stored for extended periods of time, then we may need to reduce manufacturing batch size to ensure that the material we produce will be used before it expires. In that case, the scaling of our production processes will not deliver the efficiencies we expect, and the cost per dose of our product candidates will be substantially higher. Furthermore, if our product candidates do not have established long-term stability, then we may incur significant additional expenses, such as costs for conducting more frequent manufacturing runs or potential disputes or issues that may arise in relation to the use of product candidates due to stability issues.
Risks Related to Our Intellectual Property
If our license agreement with NKMAX is terminated, we could lose our rights to key components enabling our NK cell technology platform.
On February 12, 2020, we entered into a license agreement, amended October 2021, April 2023 and August 1, 2023, with NKMAX (the “Intercompany License”). Pursuant to Intercompany License, NKMAX granted to us an exclusive (even to NKMAX and its affiliates), royalty-bearing, sublicensable license under certain patents and know-how related to NK cell therapy in any fields to (i) research, develop, manufacture, have manufactured, use and commercialize any NK cell pharmaceutical product, process, service or therapy or a combination of any of the forgoing with any other active ingredient, product or service (the “Licensed Products”) in all countries excluding the countries and territories in Asia (the “Licensed Territory”) and (ii) research, develop, manufacture and have manufactured Licensed Products outside of the Licensed Territory solely to support our rights in the Licensed Territory. We are reliant upon certain rights and proprietary technology provided to us under the Intercompany License for the production and development of certain of our product candidates, such as SNK01 and SNK02. We previously paid a non-refundable upfront fee of $1.0 million to NKMAX, and we are required to pay certain one-time milestone fees to NKMAX upon the first receipt of regulatory approval of a Licensed Product by us or any of our affiliates, which range from $1.0 million to $5.0 million, depending on the jurisdiction, in addition to a mid-single digit royalty on net sales of Licensed Products by us, our affiliates or our sublicensees, subject to customary reductions. NKMAX may terminate the Intercompany License upon the occurrence of certain events, such as an uncured material breach by us, our failure to make any required payments under the Intercompany License or our insolvency.
If NKMAX terminates the Intercompany License, we could lose the use of intellectual property rights that may be material or necessary to the development, production, or marketing of our product candidates, including SNK01 and SNK02, which could impede or prevent our successful commercialization of such product candidates and materially and adversely affect our business, financial condition, results of operations and growth prospects. If any of the foregoing were to occur, it could delay our development and commercialization of our product candidates, which in turn could materially and adversely affect our business, financial condition, results of operations and growth prospects.
We may need to license additional intellectual property from third parties, and any such licenses may not be available or may not be available on commercially reasonable terms.
The growth of our business may depend in part on our ability to acquire or in-license additional proprietary rights. For example, our programs may involve product candidates that may require the use of additional proprietary rights held by third parties. Our product candidates may also require specific formulations to work effectively and efficiently. These formulations may be covered by intellectual property rights held by others. We may develop products containing our compositions and pre-existing pharmaceutical compositions. These pharmaceutical products may be covered by intellectual property rights held by others. We may be required by the FDA, EMA or other foreign regulatory authorities to provide a companion diagnostic test or tests with our product candidates. These diagnostic test or tests may be covered by intellectual property rights held by others. We may be unable to acquire or in-license any relevant third-party intellectual property rights that we identify as necessary or important to our business operations. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all, which would harm our business. We may need to cease use of the compositions or methods covered by such third-party intellectual property rights, and may need to seek to develop alternative approaches that do not infringe on such intellectual property rights, which may entail additional costs and development delays, even if we were able to develop such alternatives, which may not be feasible. Even if we are able to obtain a license to such intellectual property rights, any such license may be non-exclusive, which may allow our competitors access to the same technologies licensed to us.
Our development and commercialization rights to our current and future product candidates and technology are subject, in part, to the terms and conditions of licenses granted to us by others.
We are a party to a variety of intellectual property license agreements with third parties and expect to enter into additional license agreements in the future. These license agreements provide us with access to certain rights and proprietary technology from third parties for the production and development of our current and future product candidates, including SNK01 and SNK02. However, these licenses may not provide exclusive rights to use such intellectual property and technology in all relevant fields of use and in all territories in which we choose to develop or commercialize our technology and product candidates in the future. As a result, we may not be able to prevent competitors from developing and commercializing competitive products in territories included in all of our licenses.
We also engage in collaborations or advisory partnerships with scientists at academic and non-profit institutions to access technologies and materials that are not otherwise available to us. Although the agreements that govern these collaborations or advisory partnerships may include an option to negotiate licenses to the institution’s rights in any inventions that are created in the course of these collaborations, we may not be able to come to a final agreement for an exclusive license with the institution.
We also have entered, and may in the future enter, into collaboration or license agreements with commercial entities to access technologies and materials that are not otherwise available to us. Our agreements with such entities may provide licenses to technology useful for the discovery, development, or commercialization of our product candidates. These licenses may in some instances, be non-exclusive.
Such licenses and other contracts may be the subject of disagreements with the grantors and/or various third parties regarding the interpretation of such licenses and contracts. The resolution of any such disagreements that may arise could affect the scope of our rights to the relevant technology, or affect financial or other obligations under the relevant agreement, either of which could inhibit our ability to utilize the underlying technology in a cost-effective manner to develop and commercialize our product candidates, which in turn could materially and adversely affect our business, financial condition, results of operations and growth prospects.
Our existing license agreements impose, and we expect that our future license agreements will impose, various diligence, milestone payment, royalty, insurance, indemnification and other obligations on us. Under certain circumstances such as a material breach of terms, our licensors could terminate our license agreements. If these in-licenses are terminated, or if the underlying patents fail to provide the intended exclusivity, competitors could have the freedom to seek regulatory approval of, and to market, products identical or similar to ours. In addition, we may seek to obtain additional licenses from our licensors and, in connection with obtaining such licenses, we may agree to amend our existing licenses in a manner that may be more favorable to the licensors, including by agreeing to terms that could enable third parties (potentially including our competitors) to receive licenses to a portion of the intellectual property that is subject to our existing licenses.
In addition, we may not have the right to control the preparation, filing, prosecution, maintenance, enforcement and defense of patents and patent applications directed to the technology that we license from third parties. Therefore, we cannot be certain that these patents and patent applications will be prepared, filed, prosecuted, maintained, enforced and defended in a manner consistent with our best interests. For example, if we do not have the right to control patent prosecution and maintenance of patents and patent applications directed to the technology that we license from licensors, such licensors could file terminal disclaimers and/or take other actions that could shorten the term of the patents or patent applications. If our licensors fail to prosecute, maintain, enforce and defend such patents, or lose rights to those patents or patent applications, the rights we have licensed may be reduced or eliminated, and our right to develop and commercialize any of our product candidates that are the subject of such licensed rights could be impaired. Additionally, we may be required to reimburse our licensors for all of their expenses related to the prosecution, maintenance, enforcement and defense of patents and patent applications that we in-license from them. Moreover, if these rights are narrowed or not enforced, third parties, including our competitors, may be able to compete with our products and technology.
Furthermore, our licensors may have relied on third-party consultants or collaborators or on funds from third parties such that our licensors are not the sole and exclusive owners of the patents we in- licensed. If other third parties have ownership rights to our in-licensed patents, they may be able to license such patents to our competitors, and our competitors could market competing products and technology. This could harm our competitive position, and our business.
Duration of patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time, and the expiration of our patents may subject us to increased competition.
As of December 31, 2023, the patent portfolio that is assigned to us, jointly owned with others or licensed to us includes issued patents in the United States and Mexico, and pending patent applications in the United States, Brazil, Canada, Chile, Egypt, Europe, Mexico, South Africa and Ukraine across our platform, SNK01, SNK02 and their patent families. Our portfolio of issued patents, excluding pending patent applications, has expected expiration dates between approximately June 2033 and January 2039. Our portfolio, including issued patents, and including pending non-provisional applications (including Patent Cooperation Treaty applications) if they are issued, has expected expiration dates between approximately May 2033 and November 2043. Various events, such as patent term adjustment, patent term extension, or disclaimers, may alter the expiration dates. We may file additional patent applications directed to our SNK01 and SNK02 product candidates. However, we can provide no assurance that we will be able to file or receive additional patent protection for these or other product candidates.
Patent expiration dates may be shortened or lengthened by a number of factors, including terminal disclaimers, patent term adjustments, supplemental protection certificates and patent term extensions. Patent term extensions and supplemental protection certificates, filing prior to the full one-year period for conversion of a provisional, and the like, may be impacted by the regulatory process and may not significantly lengthen patent term. Our patent protection could also be reduced or eliminated for noncompliance with various procedural, document submission, fee payment and other requirements imposed by government patent agencies. In addition, if we or our licensors fail to apply for applicable patent term extensions or adjustments, we will have a more limited time during which we can enforce our or our licensors granted patent rights.
Given the amount of time required for the development, testing and regulatory review of product candidates, patents protecting such candidates might expire before or shortly after such product candidates are commercialized. We may be able to seek extensions of patent terms in the United States and, if available, in other countries where we have or will obtain patent rights. In the United States, the Drug Price Competition and Patent Term Restoration Act of 1984 permits a patent term extension of up to five years beyond the normal expiration of the patent; provided that the patent is not enforceable for more than 14 years from the date of drug approval, which is limited to the approved indication (or any additional indications approved during the period of extension). Furthermore, only one patent per approved product can be extended and only those claims directed to the approved product, a method for using it or a method for manufacturing it may be extended. However, the applicable authorities, including the FDA and the United States Patent and Trademark Office (the “USPTO”) in the United States, and any equivalent regulatory authority in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. We may not have the right to seek extensions of patents that are in-licensed to us, or if such licenses are terminated, we may not have rights to any patents eligible for extension. If we are responsible for patent prosecution and maintenance of patent rights in-licensed to us, we could be exposed to liability to the applicable patent owner. If we or our licensors fail to maintain the patents and patent applications directed to our product candidates and technologies, we may not be able to prevent a competitor from marketing products that are the same as or similar to our product candidates. Further, others commercializing products similar or identical to ours, and our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data and launch their product earlier than might otherwise be the case, which could increase competition for our product candidates and materially and adversely affect our business, financial condition, results of operations and growth prospects.
If any patent protection we or our licensors obtain is not sufficiently robust, our competitors could develop and commercialize products and technology similar or identical to ours.
The market for cell therapy is highly competitive and subject to rapid technological change. Our success depends, in large part, on our ability to maintain a competitive position in the development and protection of technologies and products for use in these fields and to obtain and maintain or license patent protection in the United States and other countries with respect to our product candidates and our technology. We may protect our proprietary position by filing patent applications in the United States and abroad related to our product candidates and our technology that are important to our business. If we are unable to protect our intellectual property, our competitive position could be materially and adversely affected, as third parties may be able to make, use or sell products and technologies that are substantially the same as ours without incurring the sizeable development and licensing costs that we have incurred. This, in turn, would materially and adversely affect our ability to compete in the market.
The patent position of biotechnology and pharmaceutical companies generally is uncertain, involves complex legal and factual questions and has, in recent years, been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our in-licensed pending and future patent applications may not result in patents being issued that protect our technology or product candidates or effectively prevent others from commercializing competitive technologies and product candidates.
The patent prosecution process is expensive, time-consuming and complex, and we may not be able to file, prosecute, maintain, or license all necessary or desirable patent applications at a reasonable cost or in a timely manner. We also may fail to identify patentable aspects of our research and development output, or may identify patentable aspects of our research and development output once it is too late to obtain patent protection.
Claim scope in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Even if the patent applications we license or own do issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors or other third parties from competing with us or otherwise provide us with any competitive advantage. Our competitors or other third parties may be able to circumvent our patents by developing similar or alternative products in a non-infringing manner.
Even after issuance, our in-licensed patents or patents we obtain the future may be subject to challenge, which if successful could require us to obtain licenses from third parties, which may not be available on commercially reasonable terms or at all, or to cease the use of the underlying technology, which could materially and adversely affect our business.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our or our licensors’ patents, even after issuance, may be challenged in the courts or patent offices in the United States and abroad. Third-party challenges may result in a loss of exclusivity or in our or our licensors’ patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to prevent others from using or commercializing similar or identical technology and products, or could limit the duration of the patent protection of our technology and product candidates.
Even if our patents are determined to be valid and enforceable, they may not be interpreted sufficiently broadly to prevent others from marketing products similar to ours or designing around our or our licensors’ patents.
We may not identify relevant third-party patents or may incorrectly interpret the relevance, scope or expiration of a third-party patent, which could materially and adversely affect our ability to develop, manufacture and market our product candidates.
There are many patents issued or applied for in the biotechnology industry, and we may not be aware of patents or patent applications held by others that relate to our business. We cannot guarantee that any of our or our licensors’ patent searches or analyses, including, but not limited to, the identification of relevant patents, analysis of the scope of relevant patent claims or determination of the expiration of relevant patents, are complete or thorough, nor can we be certain that we have identified each and every third-party patent and pending application in the United States and elsewhere that is relevant to or necessary for the development and commercialization of our product candidates in any jurisdiction.
For example, patent applications in the United States and many international jurisdictions are typically not published until 18 months after the filing of certain priority documents (or, in some cases, are not published until they issue as patents) and publications in the scientific literature often lag behind actual discoveries. Thus, we cannot be certain that others have not filed patent applications or made public disclosures relating to our technology or our contemplated technology. A third party may have filed, and may in the future file, patent applications directed to our product candidates or technology similar to ours or that of our licensors. Any such patent application may have an earlier priority date than our patent applications or patents, or those of our licensors, which could further require us to obtain rights to patents directed to such technologies. Under certain circumstances, if third parties have filed such patent applications, an interference proceeding in the United States can be initiated by any such third party, or by the USPTO itself, to determine who was the first to invent any of the subject matter recited by the patent claims of our applications or issued patents.
Furthermore, after issuance, the scope of patent claims remains subject to construction as determined by an interpretation of the law, the written disclosure in a patent and the patent’s prosecution history. Our interpretation of the relevance or the scope of a patent or a pending application may be incorrect, and we may incorrectly determine that our product candidates or technology are not covered by a third party’s patent or may incorrectly predict whether a third party’s pending application will issue with claims of relevant scope. Our determination of the expiration date of any patent in the United States or elsewhere that we consider relevant may also be incorrect. If we fail to correctly identify or interpret relevant patents, we may be subject to infringement claims. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we fail in any such dispute, in addition to being forced to pay monetary damages, we may be temporarily or permanently prohibited from commercializing our product candidates. We may also be forced to attempt to redesign our product candidates or technology in a manner that no longer infringes third-party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to the development and commercialization of our product candidates.
Claims brought against us for infringing, misappropriating or otherwise violating intellectual property rights of third parties or engaging in unfair competition, would be costly and time-consuming and could prevent or delay us from successfully developing or commercializing our product candidates.
Our success depends in part on our ability to develop, manufacture and market our technology and use our technology without infringing the proprietary rights of third parties. We or our collaborators may be subject to third-party claims that could cause us to incur substantial expenses to defend and these claims, if successful, could require us to pay substantial damages and/or limit our ability to commercialize our product candidates if we or our collaborators are found to be infringing a third party’s intellectual property rights.
There are third-party patents and patent applications that may relate to the areas in which we are developing product candidates. Additionally, as our industry expands and more patents are issued, the risk increases that there may be patents issued to third parties that relate to our product candidates and technology of which we are not aware or that we may need to challenge to continue our operations as currently contemplated. As a result, our technology and any future products that we commercialize could be alleged to infringe patent rights or other proprietary rights of third parties, which may require costly litigation and, if we are not successful in defending against such litigation, could cause us to pay substantial damages and/or limit our ability to commercialize our product candidates. Issued patents are entitled to a presumption of validity in many countries, including the United States and many European countries, and issued patents held by others that claim our technology or any of our product candidates may limit our ability to commercialize our product candidates, unless and until these patents expire or are declared invalid or unenforceable in a court of applicable jurisdiction, if we do not obtain a license or other right to practice the claimed inventions.
We employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. While such employees are prohibited from disclosing to us confidential information belonging to their former employers, we may be subject to claims that these employees, or we, have used or disclosed trade secrets or other proprietary information of their former employers.
Third parties could threaten or initiate litigation or other legal proceedings alleging that we have infringed their patents, trade secrets, trademarks or other intellectual property rights. Litigation may make it necessary to defend ourselves by determining the scope, enforceability and validity of third-party proprietary rights, or to establish our proprietary rights. Regardless of whether any such claims that we are infringing patents or other intellectual property rights have merit, such claims can be time-consuming, divert management attention and financial resources and are costly to evaluate and defend.
Results of any such litigation are difficult to predict and may require us to stop treating certain conditions, obtain licenses or modify our product candidates or technology while we develop non-infringing substitutes, or may result in significant settlement costs. Litigation can involve substantial damages for infringement (and if the court finds that the infringement was willful, we could be ordered to pay treble damages and the patent owner’s attorneys’ fees), and the court could prohibit us from selling our product candidates or require us to take a license from a third party, which the third party is not required to do at a commercially reasonable price or at all. If a license is available from a third party, we may have to pay substantial royalties, upfront fees, or milestone fees, or grant cross-licenses to intellectual property rights for our product candidates or technology. We may also have to redesign our product candidates or technology so they do not infringe third-party intellectual property rights, which may not be possible or may require substantial monetary expenditures and time, during which our product candidates may not be available for manufacture, use, or sale.
We may not be able to effectively monitor unauthorized use of our intellectual property and enforce our or our in- licensed intellectual property rights against infringement, and may incur substantial costs as a result of bringing litigation or other proceedings relating to our or our in-licensed intellectual property rights.
Monitoring unauthorized use of our intellectual property is difficult and costly. We may not be able to detect unauthorized use of, or take appropriate steps to enforce, our intellectual property rights. Any inability to meaningfully monitor unauthorized use of our intellectual property could result in competitors offering products that incorporate our product candidates or service features, which could in turn reduce demand for our products.
We may also, from time to time, seek to enforce our intellectual property rights against infringers when we determine that a successful outcome is probable and may lead to an increase in the value of the intellectual property. If we choose to enforce our patent rights against a party, that party could counterclaim that our patent is invalid and/or unenforceable. The defendant may challenge our or our licensors’ patents through proceedings before the Patent Trial and Appeal Board (“PTAB”), including inter partes and post-grant review.
Proceedings to challenge patents are also available internationally, including, for example, opposition proceedings and nullity actions. In patent litigation in the United States, counterclaims alleging invalidity and/or unenforceability and PTAB challenges are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of patentable subject matter, lack of novelty, lack of obviousness, lack of written description, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before the PTAB, even outside the context of litigation. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we, our licensors, and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we may lose at least part, and perhaps all, of the patent protection on our product candidates.
In addition, such lawsuits and proceedings are expensive and would consume time and resources and divert the attention of managerial and scientific personnel even if we were successful in stopping the infringement of such patents. Litigation is inherently unpredictable, and there is a risk that the court will decide that such patents are not valid and that we do not have the right to stop the other party from using the inventions. Furthermore, some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. There is also the risk that, even if the validity of such patents is upheld, the court will refuse to stop the other party on the ground that such other party’s activities do not infringe our intellectual property rights.
Pharmaceutical products are vulnerable to counterfeiting. If our product candidates are approved and commercialized, third parties may illegally produce and distribute counterfeit versions of our products that are below the various manufacturing and testing standards that our products undergo. Counterfeit pharmaceutical products are often unsafe, ineffective and potentially life-threatening. As many counterfeit products may be visually indistinguishable from their authentic versions, the presence of counterfeit products could affect overall consumer confidence in the authentic product. A public loss of confidence in the integrity of pharmaceutical products in general or in any of our products in particular due to counterfeiting could have a material adverse effect on our business, prospects, financial condition and results of operations. In addition, we may also be subject to potential legal disputes and/or regulatory proceedings that may divert our management’s attention and resources, which could have a material adverse impact on our financial position.
There could also be public announcements of the results of hearings, motions or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could materially adversely affect the price of our common stock. Finally, any uncertainties resulting from the initiation and continuation of any litigation could materially and adversely affect our ability to raise the funds necessary to continue our operations.
We and our licensors will not seek to protect our intellectual property rights in all jurisdictions throughout the world and we may not be able to adequately enforce our intellectual property rights even in the jurisdictions where we seek protection.
We in-license a number of international patents and patent applications and expect our licensors to continue to pursue patent protection in many of the significant markets in which we intend to do business. However, filing, prosecuting and defending patents relating to our product candidates and technology, including all of our in-licensed patent rights, in all countries throughout the world would be prohibitively expensive. We and our licensors must ultimately seek patent protection on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we or our licensors may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
Furthermore, the protection offered by intellectual property rights in certain countries outside of the United States may be less extensive than those in the United States. Consequently, we may not be able to prevent third parties from utilizing proprietary technology in all countries outside of the United States, even if we or our licensors pursue and obtain issued patents in particular foreign jurisdictions, or from selling or importing products made using our proprietary technology in and into the United States or other jurisdictions. Such products may compete with our products, and our or in-licensed patent rights or our other intellectual property rights may not be effective or sufficient to prevent them from competing. If such competing products arise in jurisdictions where we are unable to exercise intellectual property rights to combat them, our business, financial condition, results of operations and growth prospects could be materially and adversely affected.
Changes in U.S. patent law or the patent law of other jurisdictions could decrease the certainty of our or our licensors’ ability to obtain patents and diminish the value of patents in general, thereby impairing our ability to protect our current and any future product candidates.
The U.S. Supreme Court and the Court of Appeals for the Federal Circuit have made, and will likely continue to make, changes in how the patent laws of the United States are interpreted. For example, in recent years the U.S. Supreme Court modified some tests used by the USPTO in granting patents over the past 20 years, which may decrease the likelihood that we or our licensors will be able to obtain patents and increase the likelihood of a challenge of any patents we obtain or license. Similarly, international courts have made, and will likely continue to make, changes in how the patent laws in their respective jurisdictions are interpreted. Those changes may materially and adversely affect our patent rights and our or our licensors’ ability to obtain issued patents.
Changes in either the patent laws or interpretation of the patent laws in the United States could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. For instance, the Leahy-Smith America Invents Act (the “America Invents Act”), enacted in 2011, included a number of significant changes to patent law in the United States. Many of the substantive changes to patent law under the America Invents Act came into effect in March 2013. For example, in March 2013, the United States transitioned from a “first-to-invent” patent system to a patent system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application is entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. The America Invents Act also included a number of significant changes that affect the way patent applications are prosecuted and how issued patents may be challenged, such as allowing third-party submission of prior art to the USPTO during patent prosecution and new post-grant administrative proceedings which can be used by third parties to attack the validity of an issued patent, including post-grant review, inter partes review and derivation proceedings. The America Invents Act and its implementation could increase the uncertainties and/or costs surrounding the prosecution of our or our licensors’ patent applications and the enforcement or defense of our in-licensed issued patents, all of which could materially and adversely affect our business, financial condition, results of operations and growth prospects.
In addition, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our or our licensors’ ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on actions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could weaken our ability to obtain new patents or to enforce patents that we have licensed or might obtain in the future.
Similarly, changes in patent law and regulations in other countries or jurisdictions, changes in the governmental bodies that enforce them or changes in how the relevant governmental authority enforces patent laws or regulations may weaken our or our licensors’ ability to obtain new patents or to enforce patents that we have licensed or that we may obtain in the future, which in turn could materially adversely affect our business, financial condition, results of operations and growth prospects. For example, the complexity and uncertainty of European patent laws have also increased in recent years. In Europe, a new unitary patent system will take effect on June 1, 2023, which will significantly impact European patents, including those granted before the introduction of such a system. Under the unitary patent system, European patent applications will have the option, upon grant of a patent, of becoming a Unitary Patent, which will be subject to the jurisdiction of the Unitary Patent Court (the “UPC”). As the UPC is a new court system, there is no precedent for the court or any decisions that it may take, increasing the uncertainty of any litigation. Existing European patents that have not lapsed as of June 1, 2023 and for which no action has been filed before the UPC will have the option of opting out of the jurisdiction of the UPC and remaining as national patents in the UPC countries. Patents under the jurisdiction of the UPC will be potentially vulnerable to a single UPC-based revocation challenge that, if successful, could invalidate the patent in all countries that have ratified the UPC agreement. We cannot predict with certainty the long-term effects of any potential changes.
We may fail to obtain or enforce assignments of intellectual property rights from our employees and contractors.
While it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing an enforceable agreement with each party who in fact conceives or develops intellectual property that we regard as our own. Furthermore, our assignment agreements may not be self-executing or may be breached, and we may be forced to bring or defend claims to determine the ownership of what we regard as our intellectual property, and we may not be successful in such claims. If we fail to obtain agreements assigning intellectual property rights or in bringing or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Such an outcome could materially adversely affect our business, financial condition, results of operations and growth prospects. Even if we are successful in defending against such claims, litigation could result in substantial costs and distraction to management and other employees.
If we are not able to adequately prevent disclosure of trade secrets and other proprietary information, the value of our technology and product candidates could be materially diminished.
Trade secrets are difficult to protect. We may rely on trade secrets to protect our proprietary information and technologies, especially where we do not believe patent protection is appropriate or obtainable, or where such patents would be difficult to enforce. We rely in part on confidentiality agreements with our employees, consultants, contractors, collaboration partners, scientific collaborators, and other advisors to protect our trade secrets and other proprietary information. We cannot guarantee that we have entered into such agreements with each party that may have had access to our proprietary information or technologies, or that such agreements, even if in place, will not be circumvented. These agreements may not effectively prevent disclosure of proprietary information or technology and may not provide an adequate remedy in the event of unauthorized disclosure of such information or technology. In addition, others may independently discover our trade secrets and proprietary information, in which case we may have no right to prevent them from using such trade secrets or proprietary information to compete with us. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could materially adversely affect our business, financial condition, results of operations and growth prospects.
General Risk Factors
Our business is affected by macroeconomic conditions, including rising inflation, interest rates and supply chain constraints.
Various macroeconomic factors could adversely affect our business, results of operations and financial condition, including changes in inflation, interest rates and overall economic conditions and uncertainties, such as those resulting from the current and future conditions in the banking system and the global financial markets. For instance, inflation has negatively impacted us and could continue to negatively impact us by increasing our cost of labor (through higher wages), commercial support, construction, manufacturing and clinical supply expenditures. See above the subsection titled under “— Risks Related to Manufacturing” above for the risks related to the impact of inflation on the construction of our commercial-scale manufacturing facility. Current inflationary pressures, if sustained, could have a negative impact on our operations. In addition, interest rates, the liquidity of the credit markets and the volatility of the capital markets could also affect our ability to raise capital in order to fund our operations, if needed. Financial conditions affecting the banking system and financial markets may threaten our ability to access our cash, as well as our access to letters of credit or other funding necessary to support our business, which may require us to find additional sources of cash or funding on short notice. Similarly, these macroeconomic factors could affect the ability of our third-party manufacturers, contractors or suppliers to manufacture materials required for our product candidates on a cost-effective basis, if at all.
Any acquisitions or strategic collaborations may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities or subject us to other risks.
From time to time, we may evaluate various acquisitions and strategic collaborations, including licensing or acquiring complementary drugs, intellectual property rights, technologies or businesses. Any potential acquisition or strategic partnership may entail numerous risks, including, but not limited to:
•increased operating expenses and cash requirements;
•the assumption of indebtedness or contingent or unknown liabilities;
•assimilation of operations, intellectual property and drugs of an acquired company, including difficulties associated with integrating new personnel;
•adequately prosecuting and maintaining protection of any acquired intellectual property rights;
•the diversion of our management’s attention from our existing drug programs and initiatives in pursuing such a strategic partnership, merger or acquisition;
•retention of key employees, the loss of key personnel, and uncertainties about our ability to maintain key business relationships;
•risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing drugs or product candidates and regulatory approvals; and
•our inability to generate revenue from acquired drugs, intellectual property rights, technologies, and/or businesses sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs.
In addition, if we engage in acquisitions or strategic partnerships, we may issue dilutive securities, assume or incur debt obligations, incur large one-time expenses or acquire intangible assets that could result in significant future amortization expense. Moreover, we may not be able to locate suitable acquisition or strategic partnership opportunities, and this inability could impair our growth or limit access to technology or drugs that may be important to the development of our business.
Changes to, or interpretations of, financial accounting standards may affect our results of operations and could cause us to change our business practices.
We prepare our financial statements in accordance with GAAP. These accounting principles are subject to interpretation by the Financial Accounting Standards Board, the SEC and various bodies formed to interpret and create accounting rules and regulations. Changes in accounting rules can have a significant effect on our reported financial results and may affect our reporting of transactions completed before a change is announced. Changes to those rules or the questioning of current practices may materially adversely affect our financial results, including those contained in this filing, or the way we conduct our business.
If our information technology systems or data, or those of third parties withupon which we rely, are or were compromised, we could experience adverse consequences resulting from such compromise, including but not limited to regulatory investigations or actions, litigation, fines and penalties, disruptions of our business operations, reputational harm, loss of revenue or profits, and other adverse consequences.
In the ordinary course of our business, we and the third parties upon which we rely process sensitive data, and, as a result, we and the third parties upon which we rely face a variety of evolving threats, including but not limited to ransomware attacks, which could cause security incidents. Cyber-attacks, malicious internet-based activity, online and offline fraud, and other similar activities threaten the confidentiality, integrity, and availability of our sensitive data and information technology systems, and those of the third parties upon which we rely. Such threats are prevalent and continue to rise, are increasingly difficult to detect, and come from a variety of sources, including traditional computer “hackers,” threat actors, “hacktivists,” organized criminal threat actors, personnel (such as through theft or misuse), sophisticated nation states, and nation-state-supported actors.
Some actors now engage and are expected to continue to engage in cyber-attacks, including without limitation nation-state actors for geopolitical reasons and in conjunction with military conflicts and defense activities. During times of war and other major conflicts, we and the third parties upon which we rely may deal. Sophisticatedbe vulnerable to a heightened risk of these attacks, including retaliatory cyber-attacks that could materially disrupt our systems and deliberateoperations, supply chain, and ability to develop and commercialize our product candidates.
We and the third parties upon which we rely are subject to a variety of evolving threats, including but not limited to social-engineering attacks on,(including through phishing attacks), malicious code (such as viruses and worms), malware (including as a result of advanced persistent threat intrusions), denial-of-service attacks (such as credential stuffing), credential harvesting, personnel misconduct or security breacheserror, ransomware attacks, supply-chain attacks, software bugs, server malfunctions, software or hardware failures, loss of data or other information technology assets, adware, telecommunications failures, earthquakes, fires, floods, and other similar threats.
In particular, severe ransomware attacks are becoming increasingly prevalent and can lead to significant interruptions in our operations, loss of sensitive data and income, reputational harm, and diversion of funds. Extortion payments may alleviate the negative impact of a ransomware attack, but we may be unwilling or unable to make such payments due to, for example, applicable laws or regulations prohibiting such payments.
Remote work has become more common and has increased risks to our information technology systems and data, as more of our employees utilize network connections, computers, and devices outside our premises or network, including working at home, while in transit and in public locations. Additionally, future or past business transactions (such as acquisitions or integrations) could expose us to additional cybersecurity risks and vulnerabilities, as our systems could be negatively affected by vulnerabilities present in acquired or integrated entities’ systems and technologies. Furthermore, we may discover security issues that were not found during due diligence of such acquired or integrated entities, and it may be difficult to integrate companies into our information technology environment and security program.
In addition, our reliance on third-party service providers could introduce new cybersecurity risks and vulnerabilities, including supply-chain attacks, and other threats to our business operations. We rely on third-party service providers and technologies to operate critical business systems to process sensitive data in a variety of contexts, including, without limitation, cloud-based infrastructure, data center facilities, encryption and authentication technology, employee email, and other functions. We also rely on third-party service providers to provide other products, services, parts, or otherwise to operate our business. Our ability to monitor these third parties’ information security practices is limited, and these third parties may not have adequate information security measures in place. If our third-party service providers experience a security incident or other interruption, we could experience adverse consequences. While we may be entitled to damages if our third-party service providers fail to satisfy their privacy or security-related obligations to us, any award may be insufficient to cover our damages, or we may be unable to recover such award. In addition, supply-chain attacks have increased in frequency and severity, and we cannot guarantee that third parties’ infrastructure in our supply chain or our third-party partners’ supply chains have not been compromised.
Any of the previously identified or similar threats could cause a security incident or other interruption that could result in unauthorized, unlawful, or accidental acquisition, modification, destruction, loss, alteration, encryption, disclosure of, or access to our sensitive data or our information technology systems, or infrastructure,those of the third parties upon whom we rely. A security incident or the systems or infrastructureother interruption could disrupt our ability (and that of third parties upon whom we rely) to develop and commercialize our product candidates and operate our business.
We may expend significant resources or the cloud, could leadmodify our business activities (including our clinical trial activities) to corruptiontry to protect against security incidents. Additionally, certain data privacy and security obligations may require us to implement and maintain specific security measures or misappropriation ofindustry-standard or reasonable security measures to protect our assets, proprietary information technology systems and sensitive or confidential data. As an early stage company without significant investments in data
While we have implemented security protection,measures designed to protect against security incidents, there can be no assurance that these measures will be effective. We take steps to detect and remediate vulnerabilities, but we may not be sufficiently protected againstable to detect and remediate all vulnerabilities because the threats and techniques used to exploit the vulnerability change frequently and are often sophisticated in nature. Therefore, such occurrences. Wevulnerabilities could be exploited but may not be detected until after a security incident has occurred. These vulnerabilities pose material risks to our business. Further, we may experience delays in developing and deploying remedial measures designed to address any such identified vulnerabilities.
Applicable data privacy and security obligations may require us to notify relevant stakeholders of security incidents. Such disclosures are costly, and the disclosure or the failure to comply with such requirements could lead to adverse consequences. If we (or a third party upon whom we rely) experience a security incident or are perceived to have experienced a security incident, we may experience adverse consequences, such as government enforcement actions (for example, investigations, fines, penalties, audits, and inspections); additional reporting requirements and/or oversight; restrictions on processing sensitive data (including personal data); litigation (including class claims); indemnification obligations; negative publicity; reputational harm; monetary fund diversions; interruptions in our operations (including availability of data); financial loss; and other similar harms. Security incidents and attendant consequences may negatively impact our ability to grow and operate our business.
Our contracts may not contain limitations of liability, and even where they do, there can be no assurance that limitations of liability in our contracts are sufficient resources to adequately protect against,us from liabilities, damages, or claims related to our data privacy and security obligations. We cannot be sure that our insurance coverage will be adequate or sufficient to protect us from or to investigatemitigate liabilities arising out of our privacy and remediate any vulnerabilitysecurity practices, that such coverage will continue to cyber incidents. It is possiblebe available on commercially reasonable terms or at all, or that anysuch coverage will pay future claims.
In addition to experiencing a security incident, third parties may gather, collect, or infer sensitive information about us from public sources, data brokers, or other means that reveals competitively sensitive details about our organization and could be used to undermine our competitive advantage or market position.
Risks Related to Ownership of
them, could have adverse consequences on our business and lead to financial loss.Our Securities
We are an
emerging“emerging growth
companycompany” and
a smaller“smaller reporting
companycompany” within the meaning of the Securities Act and if
we takeit takes advantage of certain exemptions from disclosure requirements available to emerging growth companies,
or smaller reporting companies, thisit could make our securities less attractive to investors and may make it more difficult to compare our performance
withto the performance of other public companies.
We are an “emerging growth company” within the meaningas defined in Section 2(a)(19) of the Securities Act, as modified by the JOBS Act,Act. As such, we are eligible for and we mayintends to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies for as long as it continues to be an emerging growth company, including, but not limited to, (a) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes- OxleySarbanes-Oxley Act, (b) reduced disclosure obligations regarding executive compensation in our periodic reports and
proxy statements and (c) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. As a result, our stockholders may not have access to certain information they may deem important. We could bewill remain an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including ifuntil the earliest of (i) the last day of the fiscal year in which the market value of ourshares of common stock that are held by non-affiliates exceeds $700 million as of any June 30 beforeof that time,fiscal year, (ii) the last day of the fiscal year in which case we would no longer be an emerging growth company asit has total annual gross revenue of $1.235 billion or more during such fiscal year (as indexed for inflation), (iii) the date on which it has issued more than $1 billion in non-convertible debt in the prior three-year period or (iv) December 31, 2026, which is the last day of the fiscal year following December 31.the fifth anniversary of the date of the first sale of common stock in Graf’s IPO. We cannot predict whether investors will find our securities less attractive because weit will rely on these exemptions.
If some investors find our securities less attractive as a result of
ourits reliance on these exemptions, the trading prices of our securities may be lower than they otherwise would be, there may be a less active trading market for our securities and the trading prices of our securities may be more volatile.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such
an election to opt out is irrevocable. We have elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of our financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
As an emerging growth company, we may also take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to obtain an assessment of the effectiveness of our internal controls over financial reporting from our independent registered public accounting firm pursuant to Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our shares of common stock less attractive because we will rely on these exemptions. If some investors find our shares of common stock less attractive as a result, there may be a less active market for our shares of common stock and our share price may be more volatile.
Additionally, we
arequalify as a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We
expect that we will remain a smaller reporting company until the last day of
theany fiscal year
in which (1)for so long as either (a) the market value of
our common stockthe NKGen Common Stock held by non-affiliates
equalsdoes not equal or
exceedsexceed $250 million as of the
prior June 30, and (2)end of that year’s second quarter, or (b) our annual revenues
equaleddid not equal or
exceededexceed $100 million during such completed fiscal year and the market value of our common stock held by non-affiliates
equalsdid not equal or
exceedsexceed $700 million as of the
prior June 30th.end of that year’s second quarter. To the extent we take advantage of such reduced disclosure obligations, it may also make comparison of our financial statements with other public companies difficult or impossible.
Our stock price may be volatile and may decline regardless of its operating performance.
The market price of our Common Stock may fluctuate significantly in response to numerous factors and may continue to fluctuate for these and other reasons, many of which are beyond our control, including, but not limited to:
•actual or anticipated fluctuations in our revenue and results of operations;
•any financial projections we may provide to the public in the future, any changes in these projections or its failure to meet these projections;
•failure of securities analysts to initiate and maintain our coverage, changes in financial estimates or ratings by any securities analysts who follow us or its failure to meet these estimates or the expectations of investors;
•announcements by us or our competitors of significant technical innovations, acquisitions, strategic partnerships, joint ventures, results of operations or capital commitments;
•changes in operating performance and stock market valuations of other life sciences companies generally, or those in the biotechnology industry in particular;
•price and volume fluctuations in the overall stock market, including as a result of trends in the economy as a whole;
•trading volume of our common stock;
•the inclusion, exclusion or removal of our common stock from any indices;
•changes in the NKGen Board or management;
•transactions in NKGen Common Stock by directors, officers, affiliates and other major investors;
•lawsuits threatened or filed against us;
•changes in laws or regulations applicable to our business;
•changes in our capital structure, such as future issuances of debt or equity securities;
•short sales, hedging and other derivative transactions involving our capital stock;
•general economic conditions in the United States and other markets in which we operate;
•pandemics or other public health crises, including, but not limited to, the COVID-19 pandemic (including additional variants);
•other events or factors, including those resulting from war, incidents of terrorism or responses to these events; and
•the other factors described in this “Risk Factors” section.
The stock market has recently experienced extreme price and volume fluctuations. The market prices of securities of companies have experienced fluctuations that often have been unrelated or disproportionate to their operating results. In the past, stockholders have sometimes instituted securities class action litigation against companies following periods of volatility in the market price of their securities. Any similar litigation against us could result in substantial costs, divert management’s attention and resources and harm its business, financial condition and results of operations.
We may be unable to maintain the listing of our securities on Nasdaq in the future.
Our Common Stock and Public Warrants are currently listed on Nasdaq. However, we cannot guarantee that our securities will continue to be listed on Nasdaq. If we fail to meet the requirements of the applicable listing rules, such failure may result in a suspension of the trading of our shares or delisting in the future. In the event of a delisting, we can provide no assurance that any action taken by us to restore compliance with listing requirements would allow our securities to become listed again, stabilize the market price or improve the liquidity of our securities, prevent our securities from dropping below the minimum share price requirement or prevent future non-compliance with the listing requirements. This may further result in legal or regulatory proceedings, fines and other penalties, legal liability for us, the inability for our stockholders to trade their shares and negatively impact our share price, reputation, operations and financial position, as well as our ability to conduct future fundraising activities. If Nasdaq delists our securities and we are not able to list our securities on another national securities exchange, we expect that our securities could be quoted on an over-the-counter market. If this were to occur, we could face significant material adverse consequences, including but not limited to:
•a limited availability of market quotations for our securities;
•reduced liquidity for our securities;
•a limited amount of news and analyst coverage for the company; and
•a decreased ability to issue additional securities or obtain additional financing in the future.
An active trading market for our common stock may not be sustained.
Our common stock is listed on The Nasdaq Global Market under the symbol “NKGN” and trades on that market. We cannot assure you that an active trading market for its common stock will be sustained.
Accordingly, we cannot assure you of the liquidity of any trading market, your ability to sell your shares of common stock when desired or the prices that you may obtain for your shares.
Future sales of shares by existing stockholders could cause our stock price to decline.
If our existing stockholders sell or indicate an intention to sell substantial amounts of NKGen Common Stock in the public market, the trading price of the NKGen Common Stock could decline. All the shares of NKGen Common Stock subject to stock options outstanding and reserved for issuance under its equity incentive plans are expected to be registered on Form S-8 under the Securities Act and such shares are eligible for sale in the public markets, subject to Rule 144 under the Securities Act (“Rule 144”) limitations applicable to affiliates. If these additional shares are sold, or if it is perceived that they will be sold in the public market, the trading price of NKGen Common Stock could decline. In addition, NKMAX donated an aggregate of 2,500,000 shares of NKGen Common Stock to eight charitable organizations or entities, including Alzheimer’s Drug Discovery Foundation, Alzheimer’s Research and Prevention Foundation, American Brian Foundation, Korea AI Blockchain Convergence, Korean Brain Research Institute, Korean Institute of Economic and Social Studies, The Earthshine Charity Ltd, and The University of Chicago, for no consideration on December 13, 2023. The charity recipients will continue to be subject to any sale or transfer restrictions on such donated shares until the relevant restrictions end.
Although the Sponsor and certain stockholders may be subject to restrictions regarding the transfer of shares of NKGen Common Stock held by them, these shares may be sold after the expiration of their respective lock-ups. As restrictions on resale end and the registration statements for the resale of our securities are available for use, the market price of NKGen Common Stock could decline if the holders of currently restricted shares sell them or are perceived by the market as intending to sell them.
The Warrantsand PIPE Warrants may not be exercised at all or may be exercised on a cashless basis and we may not receive any cash proceeds from the exercise of the Warrantsor PIPEWarrants.
The exercise price of the Warrantsor PIPE Warrants may be higher than the prevailing market price of the underlying shares of NKGen Common Stock. The exercise price of the Warrantsor PIPE Warrants is subject to market conditions and may not be advantageous if the prevailing market price of the underlying shares of NKGen Common Stock is lower than the exercise price. The cash proceeds associated with the exercise of Warrantsor PIPE Warrants to purchase our Common Stock are contingent upon our stock price. The value of our Common Stock will fluctuate and may not align with the exercise price of the warrants at any given time. We believe that if the Warrantsand PIPE Warrants are“outofthemoney,”meaningtheexercisepriceishigherthanthemarketpriceofourCommonStock,there is a high likelihood that warrant holders may choose not to exercise their warrants. As a result, we may not receive any proceeds from the exercise of the Warrantsor PIPEWarrants.
Furthermore,withregardtothePrivateWarrants,WorkingCapitalWarrantsandthePIPEWarrants, itispossiblethatwemaynotreceivecashupontheirexercise,sincecertain conditions including (i) delayed registration of the shares of NKGen Common Stock underlying these warrants and (ii) the price per share of NKGen Common Stock which could permit certain warrant holders to convert certain warrants to shares on a cashless basis.Acashlessexerciseallowswarrantholderstoconvertthewarrantsintosharesofourcommon stockwithouttheneedforacashpayment.Insteadofpayingcashuponexercise,thewarrantholderwould receive a reduced number of shares based on a predetermined formula. As a result, the number of shares issued through a cashless exercise will be lower than if the warrants were exercised on a cash basis, which could impact the cash proceeds we receive from the exercise of such warrants.
The Public Warrants and the PIPE Warrants may only be exercised for cash provided there is then an effective registration statement registering the shares of NKGen Common Stock issuable upon the exercise of such warrants. If there is not a then-effective registration statement, then such warrants may be exercised on a “cashless basis,” pursuant to an available exemption from registration under the Securities Act.
We may be required to pay cash or issue shares of common stock to investors with whom we entered into Forward Purchase Agreements, which could reduce the amount of cash available to us or further dilute your ownership in us.
In connection with the Closing of the Business Combination, Graf entered into Forward Purchase Agreements with certain investors (“FPA Investors”) on September 22, 2023, September 26, 2023 and September 29, 2023, pursuant to which the FPA Investors agreed to collectively purchase approximately 3.2 million shares of NKGen Common Stock for approximately $32.9 million, which were not paid to us, but deposited into escrow accounts (the “Escrow Accounts”), in accordance with the terms and conditions of the Forward Purchase Agreements. All funds in the escrow accounts will be released to the Company and/or the FPA Investors at or before the one (1) year anniversary of Closing. In addition, all interest earned on the funds in each of the escrow accounts will be released to the respective FPA Investors.
The Forward Purchase Agreements provide that the Reset Price (as defined below), which was initially set at $10.44 per share, could be reduced to a lower sales price if the Company sells, issues or grants any common stock or securities convertible or exchangeable into NKGen Common Stock (excluding any secondary transfers) at a price below the then applicable Reset Price. The Reset Price is used as the settlement share price in the calculations for settlement at maturity and in the case of an Optional Early Termination (as defined below), which are discussed in turn below, and works as a “floor” share price for sales to effect Prepayment Shortfall (as defined below). If the Reset Price is effectively reduced to a lower price, then it could in turn result in less money to be released to us as set out in the Forward Purchase Agreements. See “Management’s Discussions and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources — Sources of Liquidity — Forward Purchase Agreements, FPA Subscription Agreements, and Side Letter” for more information on how the Forward Purchase Agreements and related agreements operate and how the payments from the escrow accounts are calculated. In addition, the Reset Price may influence the FPA Investors’ decision to sell, early terminate or hold part or all their shares, and sales of such shares could result in fluctuations in the trading volume and/or trading price of our initialcommon stock. Such volatility in the trading volume and/or trading price of common stock could adversely affect our ability to raise additional funds.
The amounts to be potentially released to the Company from the Escrow Accounts will be based on the trading price over the Valuation Period (as defined below) and the applicable Reset Price. However, the Company may not receive all the funds in the escrow accounts and may be required to pay the Settlement Amount Adjustment (as defined below) in stock or in cash as discussed above. If our stock price exceeds the applicable Reset Price (as defined below) by more than $2.00, then the FPA Investors may be economically incented to sell their Subscribed Shares (as defined below) and exercise the Optional Early Termination (as defined below) rights as they would potentially more consideration collectively from the Escrow Account and from proceeds from such sales in the open market, less amounts payable to the Company than if they were to hold the Subscribed Shares until the Valuation Date (as defined below). Any such sales could increase the volatility of the trading price and/or result in a decline in the trading price. In addition, if the FPA Investors hold some or all of their Subscribed Shares until the Valuation Date, and the applicable volume weighted average price per share for 20 trading days of our common stock is less than $2.00 per share, then we would be required to pay an amount that equals to $2.00 per the Subscribed Shares held as of the Valuation Date (as defined below) (or the Settlement Amount Adjustment) to the FPA Investors in stock (unless we elect to pay it in cash), which could cause substantial dilution and further depress our stock price. If we are unable to pay such amount in stock, we may be required under certain of the agreements with the FPA Investors to settle any shortfall in the payment of the Settlement Adjustment Amount in cash. In any case, we would not receive any cash proceeds and could face adverse effects on our liquidity or financial position, which could negatively impact our business combinationand results of operations. Such activities could also adversely affect the trading price of our common stock, which may also negatively affect the trading positions of our other security holders.
We may issue additional shares of common stock or other equity securities without your approval, which would dilute your ownership interests and may depress the market price of our Common Stock.
As of December 31, 2023, we had NKGen Options outstanding to purchase up to an aggregate of 2,101,760 shares of NKGen Common Stock and Warrants outstanding to purchase up to 5,246,033 shares of NKGen Common Stock (excluding the shares issuable upon the exercise of the PIPE Warrants or the conversion of the Senior Convertible Notes). NKGen will also have the ability to initially issue such number of shares of NKGen Common Stock equal to up to 12.0% of the fully diluted outstanding shares of NKGen Common Stock as of the Closing under the 2023 equity incentive plan adopted upon consummation of the Business Combination and such number of shares of NKGen Common Stock equal to up to 3.0% of the fully diluted shares of common stock outstanding under the ESPP as of the Closing Date.
We may issue additional shares of common stock or other equity securities of equal or senior rank in the future in connection with, among other things, future acquisitions or repayment of outstanding indebtedness, without stockholder approval, in a companynumber of circumstances.
Our issuance of additional shares of NKGen Common Stock or other equity securities of equal or senior rank could, without limitation, have the following effects:
•our existing stockholders’ proportionate ownership interest in us will decrease;
•the amount of cash available per share, including for payment of dividends (if any) in the future, may decrease;
•the relative voting strength of each previously outstanding share of NKGen Common Stock may be diminished; and
•the market price of shares of our Common Stock may decline.
If securities or industry analysts either do not publish research about us or publish inaccurate or unfavorable research about us, our business, or its market, or if they change their recommendations regarding our common stock adversely, the trading price or trading volume of our Common Stock could decline.
The trading market for our Common Stock is influenced in part by the research and reports that securities or industry analysts may publish about us, our business, market, or competitors. If one or more of the analysts initiate research with operationsan unfavorable rating or opportunities outsidedowngrade the common stock, provide a more favorable recommendation about our competitors, or publish inaccurate or unfavorable research about its business, the trading price of the common stock would likely decline. In addition, we currently expect that securities research analysts will establish and publish their own periodic projections for its business. These projections may vary widely and may not accurately predict the results we actually achieve. Its stock price may decline if its actual results do not match the projections of these securities research analysts. While we expects research analyst coverage, if no analysts commence coverage of it, the trading price and volume for the common stock could be adversely affected. If any analyst who may cover us were to cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the trading price or trading volume of its common stock to decline.
Delaware law and provisions in our Charter and Bylaws could make a merger, tender offer, or proxy contest difficult, thereby depressing the trading price of our common stock.
Our Charter and Bylaws contains provisions that could depress the trading price of the NKGen Common Stock by acting to discourage, delay, or prevent a change of control of us or changes in our management that our stockholders may deem advantageous. These provisions include, without limitation, the following:
•a classified board of directors so that not all members of the NKGen Board are elected at one time;
•the right of the board of directors to establish the number of directors and fill any vacancies and newly created directorships;
•director removal by stockholders solely for cause and with the affirmative vote of at least two-thirds (2/3) of the voting power of our then-outstanding shares of capital stock entitled to vote generally in the election of directors;
•“blank check” preferred stock that the NKGen Board could use to implement a stockholder rights plan;
•the right of the NKGen Board to issue our authorized but unissued common stock and preferred stock without stockholder approval;
•no ability of our stockholders to call special meetings of stockholders;
•no right of our stockholders to act by written consent, which requires all stockholder actions to be taken at a meeting of our stockholders;
•limitations on the liability of and the provision of indemnification to, our director and officers;
•the right of the board of directors to make, alter, or repeal the NKGen Bylaws; and
•advance notice requirements for nominations for election to the NKGen Board or for proposing matters that can be acted upon by stockholders at annual stockholder meetings.
Any provision of our Charter or NKGen Bylaws that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of NKGen Common Stock and could also affect the price that some investors are willing to pay for NKGen Common Stock.
Our Charter provides that the Court of Chancery of the State of Delaware will be the exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our Charter provides that the Court of Chancery of the State of Delaware is the exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a breach of fiduciary duty, any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, as amended, our Charter or NKGen Bylaws or any action asserting a claim against us that is governed by the internal affairs doctrine. These choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees and may discourage these types of lawsuits. This provision would not apply to claims brought to enforce a duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Our Charter provides further that, to the fullest extent permitted by law, the federal district courts of the United States will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. However, Section 22 of the Securities Act provides that federal and state courts have concurrent jurisdiction over lawsuits brought under the Securities Act or the rules and regulations thereunder. To the extent the exclusive forum provision restricts the courts in which claims arising under the Securities Act may be brought, there is uncertainty as to whether a court would enforce such a provision. We note that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Furthermore, the enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings and it is possible that a court could find these types of provisions to be inapplicable or unenforceable. While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring a claim in a venue other than those designated in the exclusive forum provisions and there can be no assurance that such provisions will be enforced by a court in those other jurisdictions. If a court were to find the exclusive-forum provision contained in our Charter to be inapplicable or unenforceable in an action, we wouldmay incur additional costs associated with resolving such action in other jurisdictions, which could harm its business.
We do not intend to pay dividends for the foreseeable future.
We currently intend to retain any future earnings to finance the operation and expansion of its business and we do not expect to declare or pay any dividends in the foreseeable future. Moreover, the terms of any revolving credit facility into which we or any of our subsidiaries enter may restrict our ability to pay dividends and any additional debt we or any of our subsidiaries may incur in the future may include similar restrictions. As a result, stockholders must rely on sales of their common stock after price appreciation as the only way to realize any future gains on their investment.
We will incur increased costs and obligations as a result of being a public company.
As a publicly traded company, we will incur significant legal, accounting and other expenses that we were not required to incur in the recent past, particularly after we are no longer an “emerging growth company” as defined under the JOBS Act. In addition, new and changing laws, regulations and standards relating to corporate governance and public disclosure, including the Dodd Frank Wall Street Reform and Consumer Protection Act and the rules and regulations promulgated and to be subjectpromulgated thereunder, as well as under the Sarbanes-Oxley Act, the JOBS Act and the rules and regulations of the SEC and national securities exchanges have created uncertainty for public companies and increased the costs and the time that the NKGen Board and management must devote to complying with these rules and regulations. We expect these rules and regulations to increase our legal and financial compliance costs and lead to a varietydiversion of additional risks thatmanagement time and attention from revenue generating activities.
Furthermore, the need to establish the corporate infrastructure demanded of a public company may
negatively impactdivert management’s attention from implementing our
operations.If we effectgrowth strategy, which could prevent us from improving our initial business, combination with a company with operations or opportunities outside of the United States, we would be subject to any special considerations or risks associated with companies operating in an international setting, including any of the following:
| ● | higher costs and difficulties inherent in managing cross-border business operations and complying with different commercial and legal requirements of overseas markets; |
| ● | rules and regulations regarding currency redemption; |
| ● | complex corporate withholding taxes on individuals; |
| ● | laws governing the manner in which future business combinations may be effected; |
| ● | tariffs and trade barriers; |
| ● | regulations related to customs and import/export matters; |
| ● | longer payment cycles and challenges in collecting accounts receivable; |
| ● | tax issues, including but not limited to tax law changes and variations in tax laws as compared to the United States; |
| ● | currency fluctuations and exchange controls; |
| ● | cultural and language differences; |
| ● | crime, strikes, riots, civil disturbances, terrorist attacks, natural disasters and wars; |
| ● | deterioration of political relations with the United States; and |
| ● | government appropriations of assets. |
We may not be able to adequately address these additional risks. If we were unable to do so, our operations might suffer, which may adversely impact our results of operations and financial condition.
Our initial business combination We have made and will continue to make, changes to our structure thereafterinternal controls and procedures for financial reporting and accounting systems to meet our reporting obligations as a publicly traded company. However, the measures we take may not be tax-efficientsufficient to satisfy our stockholdersobligations as a publicly traded company.
The requirements of being a public company may strain our resources, divert management’s attention and warrant holders. Asaffect our ability to attract and retain qualified board members.
We are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act and any rules promulgated thereunder, as well as the rules of Nasdaq. The requirements of these rules and regulations increase our legal and financial compliance costs, make some activities more difficult, time- consuming or costly and increase demand on our systems and resources. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal controls for financial reporting. In order to maintain and, if required, improve our disclosure controls and procedures and internal control over financial reporting to meet this standard, significant resources and management oversight are required and, as a result,
of our business combination, our tax obligationsmanagement’s attention may be
diverted from other business concerns. These rules and regulations can also make it more
complex, burdensomedifficult for us to attract and
uncertain.Although we will attemptretain qualified independent members of the board of directors. Additionally, these rules and regulations make it more difficult and more expensive for us to structureobtain director and officer liability insurance. We may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. The increased costs of compliance with public company reporting requirements and our initial business combination in a tax-efficient manner, tax structuring considerations are complex, the relevant facts and law are uncertain and may change, and we may prioritize commercial and other considerations over tax considerations. For example, in connection with our initial business combination and subject to any requisite stockholder approval, we may structure our business combination in a manner that requires stockholders and/or warrant holders to recognize gain or income for tax purposes, effect a business combination with a target company in another jurisdiction, or reincorporate in a different jurisdiction (including, but not limited to, the jurisdiction in which the target company or business is located). We do not intend to make any cash distributions to stockholders or warrant holders to pay taxes in connection with our business combination or thereafter. Accordingly, a stockholder or a warrant holder may needpotential failure to satisfy any liability resulting from our initial business combination with cash from its own funds or by selling all or a portion of the shares received. In addition, stockholders and warrant holders may also be subject to additional income, withholding or other taxes with respect to their ownership of us after our initial business combination.
In addition, we may effect a business combination with a target company that has business operations outside of the United States, and possibly, business operations in multiple jurisdictions. If we effect such a business combination, wethese requirements could be subject to significant income, withholding and other tax obligations in a number of jurisdictions with respect to income, operations and subsidiaries related to those jurisdictions. Due to the complexity of tax obligations and filings in other jurisdictions, we may have a heightened risk related to audits or examinations by U.S. federal, state, local and non-U.S. taxing authorities. This additional complexity and risk could have anmaterial adverse effect on our after-tax profitabilityoperations, business, financial condition or results of operations.
If we fail to establish and maintain proper and effective internal control over financial condition.reporting, as a public company, our ability to produce accurate and timely financial statements could be impaired, investors may lose confidence in our financial reporting and the trading price of our common stock may decline.
Pursuant to Section 404 of the Sarbanes-Oxley Act, the report by management on internal control over financial reporting will be on our financial reporting and internal controls (as accounting acquirer) and, when we are no longer an emerging growth company, an attestation of the independent registered public accounting firm will also be required. The rules governing the standards that must be met for management to assess internal control over financial reporting are complex and require significant documentation, testing and possible remediation. We have not historically had to comply with all of these rules and to comply with the Sarbanes-Oxley Act, the requirements of being a reporting company under the Exchange Act and any complex accounting rules in the future, we may need to upgrade our legacy information technology systems, implement additional financial and management controls, reporting systems and procedures and hire additional accounting and finance staff.
If we are unable to hire the additional accounting and finance staff necessary to comply with these requirements, we may need to retain additional outside consultants. If we or, if required, our independent registered public accounting firm, are unable to conclude that our internal controls over financial reporting are effective, investors may lose confidence in our financial reporting, which could negatively impact the price of our securities.
Changes in laws or regulations or how such laws or regulations are interpreted or applied, or a failure to comply with any laws or regulations, may adversely affect our business and results of operations.
We are subject to laws and regulations enacted by national, regional and local governments. In particular, we are required to comply with certain SEC and other legal requirements. Compliance with and monitoring of, applicable laws and regulations may be difficult, time consuming and costly. A failure to comply with applicable laws or regulations, as interpreted and applied, could have a material adverse effect on our business and results of operations. In addition, those laws and regulations and their interpretation and application may change from time to time, including as a result of changes in economic, political, social and government policies and those changes could have a material adverse effect on our business and results of operations.
Item 1B.Unresolved Staff Comments
Item 1C. Cybersecurity
Prior to the Business Combination, we were a blank check company with no operations and therefore did not have any operations of our own that faced material cybersecurity threats. We plan to develop processes, including those intended to follow an internal Information Technology (“IT”) Security Policy, which seek to assess, identify, and manage material risks from cybersecurity threats to the IT systems and information that we create, use, transmit, receive, and maintain. We also seek to integrate these processes and policies into our overall enterprise risk management system and processes. The processes for assessing, identifying, and managing material risks from cybersecurity threats, including threats associated with our use of third-party service providers, will include our efforts to identify the relevant assets that could be affected, determine possible threat sources and threat events, assess threats based on their potential likelihood and impact, and identify controls that are in place or necessary to manage and/or mitigate such risks. We plan to implement and conduct security assessments of all third-party service providers before engagement and maintain ongoing monitoring to ensure compliance with relevant cybersecurity standards.
In the event of a cybersecurity incident impacting us, the management team will report to the board of directors and provide updates on the management team’s incident response plan for addressing and mitigating any risks associated with such an incident. As an early-stage company without significant investments in data security protection, we may not be sufficiently protected against such occurrences. We also lack sufficient resources to adequately protect against, or to investigate and remediate any vulnerability to, cyber incidents. It is possible that any of these occurrences, or a combination of them, could have material adverse consequences on our business and lead to financial loss.
Item 2. Properties
Our executive officesprincipal office is located in Santa Ana California, where we own approximately 25,000 square feet of office, manufacturing and laboratory space, approximately halfofwhich is fit for GMP production of NK cells. We also lease office facilities in Irvine, California.
We believe our facilities are
located at 1790 Hughes Landing Blvd., The Woodlands, Texas 77380. Our executive offices are providedadequate to
us by an affiliate of our sponsor, andmeet its current needs, although we
have agreedmay seek to
pay such affiliate of our sponsor up to $15,000 per monthnegotiate new leases or evaluate additional or alternate space for
office space, utilities and secretarial and administrative support. We consider our current office space adequate for our currentits operations.
Item 3.Legal Proceedings
There is no
From time to time, we may be subject to legal proceedings. We are not currently a party to or aware of any active legal proceedings that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or results of operations. Regardless of outcome, litigation arbitration or governmental proceeding currently pending againstcan have an adverse impact on us or any membersbecause of ourdefense and settlement costs, diversion of management team in their capacity as such.
resources and other factors.
Item 4.Mine Safety Disclosures
Not applicable.
Item 5.Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity
SecuritiesMarket Information
Our units,
We have our common stock
and warrants are listed on the
NYSENasdaq Global Market under the
symbols “GFOR.U,” “GFOR”symbol “NKGN” and
“GFOR WS”, respectively.our warrants on the Nasdaq Capital Market under the symbol “NKGNW”.
As of
March 10, 2022,April 16, 2024, there were
1 holder of record of our units, 6approximately 53 holders of record of
our common stockNKGen Common Stock and 2 holders of record of our
warrants.Dividends
We have not paid any cash dividends on our common stock to date and do not intend to pay cash dividends prior to the completion of an initial business combination. The payment of cash dividends in the future will be dependent upon our revenues and earnings, if any, capital requirements and general financial conditions subsequent to completion of an initial business combination. The payment of any cash dividends subsequent to an initial business combination will be within the discretion of our board of directors at such time. Further, if we incur any indebtedness, our ability to declare dividends may be limited by restrictive covenants we may agree to in connection therewith.
Securities AuthorizedPublic Warrants, each exercisable for Issuance under Equity Compensation Plans
None.
Recent Sales of Unregistered Securities and Use of Proceeds from Registered Offerings
On May 25, 2021, we consummated our IPO of 15,000,000 units. The units were sold at an offering price of $10.00 per unit, generating total gross proceeds of $150,000,000. J.P. Morgan and Oppenheimer & Co. (the “underwriters”) acted as book-running managers for the IPO. On June 2, 2021, the underwriters partially exercised the over-allotment option and purchased the 2,161,500 additional units, generating gross proceeds of $21,615,000. The securities in the offering were registered under the Securities Act on a registration statement on Form S-1 (Registration No. 333-253411). The SEC declared the registration statement effective on May 20, 2021.
Simultaneous with the consummation of the IPO, we consummated the private placement of an aggregate of 4,433,333 private placement warrants, at a price of $1.50 per private placement warrant, to our sponsor, generating total proceeds of $6,650,000. In connection with the over-allotment, we consummated the private placement of an additional 288,200 private placement warrants at a price of $1.50 per private placement warrant, generating additional proceeds of $432,300. Each whole private placement warrant is exercisable to purchase one share of common stock at an exercise price of $11.50 per share. The issuance wasactual number of stockholders is greater than this number of record holders, and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividend Policy
We have not declared any cash dividends since inception, and we do not anticipate paying any dividends in the foreseeable future. Instead, we anticipate that all of our earnings will be used to provide working capital, to support our operations, and to finance the growth and development of our business. The payment of dividends is within the discretion of the NKGen Board and will depend on our earnings; capital requirements; financial condition; prospects; applicable Texas law, which provides that dividends are only payable out of surplus or current net profits; and other factors the NKGen Board might deem relevant. There are no restrictions that currently limit our ability to pay dividends on our common stock other than those generally imposed by applicable state law.
Recent Sales of Unregistered Securities
Except as previously disclosed in a Current Report on Form 8-K, no unregistered sales of the Company’s equity securities were made pursuantduring the fiscal year ended December 31, 2023.
Issuer Purchases of Equity Securities
We did not purchase any of our common stock or other equity securities during the quarter or year ended December 31, 2023.
Securities Authorized for Issuance Under Equity Compensation Plans.
The information required by this Item regarding equity compensation plans is incorporated by reference to the
exemption from registration containedinformation set forth in
Section 4(a)(2)Item 12 of
the Securities Act.The private placement warrants are identical to the warrants underlying the units sold in the IPO, except that the private placement warrants are not transferable, assignable or salable until 30 days after the completion of an initial business combination, subject to certain limited exceptions.
Of the gross proceeds received from the IPO, the over-allotment, and the private placement warrants, an aggregate of $171,615,000 was placed in the trust account.
this Annual Report on Form 10-K. Item 6. [Reserved]
We paid a total of $3,432,300 in underwriting discounts and commissions and $279,527 for other costs and expenses related to the IPO. In addition, the underwriters agreed to defer $6,006,525 in underwriting discounts and commissions.
Item 6.RESERVED
Item 7.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSReferences to the “Company,” “our,” “us” or “we” refer to Graf Acquisition Corp. IV. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of
the Company’sour financial condition and results of operations should be read in conjunction with the
unaudited condensedconsolidated financial statements and
therelated notes
thereto containedincluded elsewhere in this
Annual Report on Form 10-K.
CertainIn addition to the consolidated financial information,
contained in the
following discussion
and analysis set forth below includescontains forward-looking statements
that reflect our plans, estimates, beliefs, and expectations that involve risks and uncertainties.
Cautionary Note Regarding Forward-Looking Statements
This Our actual results and the timing of events could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this Annual Report on Form 10-K, includes forward-looking statements within the meaning of Section 27A of the Securities Actparticularly in Item 1A. “Risk Factors” and Section 21E of the Exchange Act. We have based these forward- looking statements on our current expectations and projections about future events. These forward-looking statements are subject to known and unknown risks, uncertainties and assumptions about us that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by such forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “should,” “could,” “would,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “continue,” or the negative of such“Special Note Regarding Forward-Looking Statements”. Capitalized terms or other similar expressions. Such statements include, but are not limited to, possible business combinations and the financing thereof, and related matters, as well as all other statements other than statements of historical fact includedused in this Form 10-K. Factors that might cause or contribute to such a discrepancy include,Item 7 but are not limitedotherwise defined herein shall have the meanings ascribed to those describedterms in our other SEC filings.
the in Item 8.
We are a blank checkclinical-stage biotechnology company focused on the development and commercialization of innovative autologous, allogeneic, and CAR-NK cell therapies utilizing a proprietary SNK platform. Our product candidates are based on a proprietary manufacturing and cryopreservation process which produces SNK cells that have increased activity as compared to the starting population of NK cells, based on the results of in vitro experiments performed by NKMAX, as defined by parameters such as cytotoxicity, cytokine production and activating receptor expression. NKGen believes that SNK cells have the potential to deliver transformational benefits to patients with neurodegenerative diseases, such as Alzheimer’s disease (“AD”) and Parkinson’s disease (“PD”), and cancer.
We were originally incorporated in Delaware on January 28, 2021 asunder the name Graf Acquisition Corp. IV, a Delaware corporation and formedspecial-purpose acquisition company for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or engaging in any other similar business combination.combination with one or more businesses or entities.
On April 14, 2023, we entered into the Agreement and Plan of Merger by and among Graf, Merger Sub, and Legacy NKGen. Upon consummation of the transactions under the Merger Agreement on September 29, 2023, Merger Sub merged with and into the Company with Legacy NKGen surviving the merger as a wholly owned subsidiary of Graf. In connection with the consummation of the Business Combination, Graf was renamed to “NKGen Biotech, Inc.” and Legacy NKGen changed its name to “NKGen Operating Biotech, Inc.” The Common Stock and warrants of the combined company began trading on The Nasdaq Stock Market LLC under the symbols “NKGN” and “NKGNW”, respectively, on October 2, 2023.
Throughout the notes to the consolidated financial statements, unless otherwise noted or otherwise suggested by context, the “Company”, “we”, “us”, “our” refers to Legacy NKGen prior to the consummation of the Business Combination, and the Company after the consummation of the Business Combination.
The Business Combination
In connection with the Business Combination, several financial instruments were issued. This included the Senior Convertible Notes, the SPA Warrants, the PIPE Warrants, and the Forward Purchase Agreements. In addition, several pre-existing financial instruments of Graf were deemed issued pursuant to the reverse recapitalization treatment of the Business Combination, including Graf’s remaining public shares, the Private Warrants, the Public Warrants, and the Working Capital Warrants. Furthermore, we incurred transaction costs, certain Founder Shares were terminated or placed under vesting conditions, our Legacy Convertible Notes converted, all assets and liabilities of Graf were combined with the assets and liabilities of NKGen on a historical cost basis, all of Legacy NKGen’s common stock and stock options were exchanged for common stock of the Company based upon the Exchange Ratio, among other material events. Refer to Note 3, Reverse Recapitalization, of the consolidated financial statements for details surrounding the Business Combination.
Business Highlights
Our goal is to bring transformative Natural Killer (“NK”) cell therapies to patients with both neurodegenerative and oncological diseases and thereby realize the potential of our extensive NK cell expertise. On October 14, 2022, we received investigational new drug (“IND”) clearance from the U.S. Food and Drug Administration (“FDA”) for SNK02 allogenic NK cell therapy for solid tumors. On October 20, 2023, we received IND clearance from the FDA for SNK01 in AD. On December 21, 2023, we received the No Objection Letter from Health Canada for our clinical trial application of SNK01 in AD. On December 28, 2023, we dosed our first participant in the US on the SNK01-AD01 clinical trial. During 2024 and beyond, we intend to (i) advance the clinical development of SNK01 and continue enrolling the Phase I/IIa trial in the United States and Canada for AD, and (ii) complete the Phase I trial with SNK02 in refractory solid tumors. We are an emerging growth companyalso intend to conduct a trial in PD (contingent on FDA clearance of the IND, to evaluate the expansion into other neurodegenerative diseases, accelerate development in oncology through strategic collaborations, and continue investment in our manufacturing technology.
NKGen presented its Phase I clinical trial data at the 16th Annual Clinical Trials on Alzheimer’s Disease conference on October 25, 2023. Ten AD patients from the first three cohorts in our ten-week Phase I dose escalation clinical trial were analyzed. NK cells were successfully activated and expanded for 100% of the patients in the trial. No treatment-related adverse events were observed. One week after the last dose (Week 11), 30% of patients showed clinical improvement on the AD composite score (“ADCOMS”) compared to baseline, 60% of patients showed a stable ADCOMS score compared to baseline, and 50–70% of patients were stable or improved on the clinical dementia rating sum of boxes (“CDR-SB”), Alzheimer’s Disease assessment scale-cognitive subscale (“ADAS-Cog”) and/or mini-mental state examination (“MMSE”) scores. One patient’s score showed a switch from a moderate classification on the ADCOMS to a mild classification. Twelve weeks after the last dose (Week 22), 44–89% of patients remained stable or improved in all cognitive scores compared to Week 11, and 50% of patients’ ADCOMS scores remained stable compared to Week 11. Based on the CSF biomarker data, SNK01 given via IV appears to cross the blood-brain barrier to reduce CSF pTau181 levels and neuroinflammation, as measured by GFAP; this effect appears to be persistent at Week 22. Our goal is to utilize our extensive NK cell expertise and bring transformative NK cell therapies to patients with neurodegenerative disease.
Factors Affecting Our Performance
Our operations to date have been limited to business planning, raising capital, developing, and identifying NK cell therapies utilizing our SNK platform, clinical studies, and other research and development activities. We have never been profitable from operations and our net losses were $83.0 million and $26.7 million for the years ended December 31, 2023 and 2022, respectively. As of December 31, 2023, our accumulated deficit was $162.1 million. We expect to continue incurring significant expenses and operating losses for at least the next several years associated with our ongoing activities as we:
•initiate and complete nonclinical studies and clinical trials for our product candidates;
•contract to manufacture and perform additional process development for our product candidates;
•continue research and development efforts to build our pipeline beyond the current product candidates;
•maintain, expand, and protect our intellectual property portfolio;
•hire additional clinical, quality control, scientific, and management personnel;
•add operational and financial personnel to support our product development efforts and planned future commercialization; and
•add operational and administrative capabilities applicable to operating as a public company.
We do not expect to generate any revenues from product sales unless and until we successfully complete development and obtain regulatory approval for one or more product candidates, which will not be for many years, if ever. Accordingly, until such time as we can generate significant revenue from sales of our product candidates, if ever, we expect to finance our cash needs through equity offerings, debt financings or other capital sources, including from related parties, and potentially grants, collaborations, licenses, or other similar arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. Our failure to raise capital or enter into such other arrangements when needed would have a negative impact on our financial condition and could force us to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates or to our platform technologies that we would otherwise prefer to develop and market ourselves.
We do not currently have, and do not currently expect to have, sufficient funds to service our operations and our expenses and other liquidity needs and will require additional capital immediately. In addition, we have expressed substantial doubt as to our ability to continue as a going concern. There can be no assurance that we will be able to timely secure such additional funding on acceptable terms and conditions, or at all. If we are subjectunable to allraise sufficient capital immediately, we will not have sufficient cash and liquidity to finance our business operations and make required payments and may be required to delay, limit, curtail or terminate our product development or may be forced to cease operations or file for bankruptcy protection.
Key Components of Results of Operations
Revenues
We do not currently have any products approved for sale and have not recognized any product revenue to date. In the future, we may generate revenue from a combination of sources, including, without limitation, product sales, payments from licenses, milestone payments or collaboration arrangements. If we fail to achieve clinical success or obtain regulatory approval of any of our product candidates, our ability to generate future revenue will be limited.
We did not have any revenues during the year ended December 31, 2023, and do not expect to generate revenues in connection with COVID-19 (defined below) testing services in future periods as we have ceased providing such services. During the year ended December 31, 2022, we generated revenue in connection with providing testing services for the coronavirus (“COVID-19”).
Costs and Expenses
Cost of Revenues
Cost of revenues historically consisted of test kits and supplements purchased from third parties in connection with providing COVID-19 testing services. We did not have any cost of revenues in the year ended December 31, 2023, and we do not expect to incur such costs in future periods as we have ceased providing COVID-19 testing services.
Research and Development Expenses
We primarily focus our resources on research and development activities, including the conduct of preclinical studies, product development, regulatory support, and clinical trials for our product candidates. Our research and development expenses consist of:
•employee-related expenses, including salaries, benefits, taxes, travel, and stock-based compensation expense, for personnel in research and development functions;
•expenses related to process development and production of product candidates;
•costs associated with preclinical activities and regulatory operations, including the costs of acquiring, developing, and manufacturing research material;
•clinical trials and activities related to regulatory filings for our product candidates; and
•allocation of facilities, overhead, depreciation, and amortization of laboratory equipment and other expenses.
We expect our direct and indirect research and development expenses to increase in the future as we continue to develop our platform and product candidates.
The successful development of our platform and product candidates is highly uncertain. The process of conducting the necessary preclinical and clinical research to obtain regulatory approval is costly and time consuming. At this time, we cannot reasonably estimate the nature, timing, or costs of the
efforts necessary to finish developing any of our product candidates or the period in which material net cash, if any, from these product candidates may commence. This is due to the numerous risks
and uncertainties associated with
emerging growth companies.Our sponsor is Graf Acquisition Partners IV LLC. On May 25, 2021,developing therapeutics and will depend on a variety of factors, including, but not limited to:
•the scope, rate of progress, expense, and results of clinical trials;
•the scope, rate of progress and expense of process development and manufacturing;
•preclinical and other research activities; and
•the timing of regulatory approvals.
Research and development expenses consist of expenses incurred while performing research and development activities to discover and develop our product candidates. Direct research and development costs include external research and development expenses incurred under agreements with contract research organizations, consultants and other vendors that conduct our preclinical and clinical activities, expenses related to manufacturing our product candidates for preclinical and clinical studies, laboratory supplies and license fees. Indirect research and development costs include personnel-related expenses, consisting of employee salaries, payroll taxes, bonuses, benefits, and stock-based compensation charges for those individuals involved in research and development efforts. Costs incurred in our research and development efforts are expensed as incurred.
We typically use employee, consultant, facility, equipment and certain supply resources across our research and development programs. We track outsourced development costs by product candidate or development program, but we consummated our IPOdo not allocate personnel costs, other internal costs or certain external consultant costs to specific product candidates or development programs. These costs are included in indirect research and development expenses. All direct research and development expenses during the years ended December 31, 2023 and 2022 relate to SNK01 and SNK02.
General and Administrative
General and administrative expenses consist primarily of 15,000,000 units, at $10.00 per unit, generating gross proceedspersonnel-related expenses for executives, human resources, finance, and other general and administrative employees, including salary and stock-based compensation, professional services costs and allocation of $150.0 million,facility and incurring offeringoverhead costs.
We anticipate that general and administrative expenses will increase in the future in connection with one-time costs of approximately $8.8 million, of which approximately $5.3 million was for deferred underwriting commissions. On June 2, 2021, the underwriters partially exercised the over-allotment option and purchased the 2,161,500 additional units, generating gross proceeds of approximately $21.6 million. We incurred additional offeringbecoming a public company as well as ongoing costs of approximately $1.2 millionoperating as a public company, including expanding headcount and increased fees for directors and outside advisors. We expect to incur significant costs to comply with corporate governance, internal controls, and similar requirements applicable to public companies. Additionally, we expect to incur increased costs associated with establishing sales, marketing and commercialization functions prior to any potential future regulatory approvals or commercialization of our product candidates.
Interest Expense
For the year ended December 31, 2023, interest expense primarily consists of interest incurred for our Related Party Loans, Short Term Related Party Loan, revolving line of credit, and Senior Convertible Notes.
For the year ended December 31, 2022, interest expense primarily consists of interest incurred associated with our related party loans.
Interest expense associated with the Legacy Convertible Promissory Notes for which we have elected to account for at fair value is included in the change in fair value for such notes.
Change in Fair Value of Convertible Promissory Notes and Convertible Promissory Notes due to Related Parties
Change in fair value of convertible promissory notes and convertible promissory notes due to related parties consists of gains or losses on the change in fair value of the Legacy Convertible Notes for the years ended December 31, 2023 and 2022, previously included within other expenses, net for the years ended December 31, 2022 and 2021. The Senior Convertible Notes are not carried at fair value and thus not included in within this financial statement caption.
Loss on Issuance of Forward Purchase Contract
The loss on issuance of forward purchase contract represents the initial recognition of the forward purchase derivative liability and the Bonus Shares in connection with the
over-allotment (ofPrivate Placement Agreements, which
approximately $0.8 million was for deferred underwriting fees).Simultaneously withwere executed in September 2023, issued upon the closingClosing of the IPO, we consummatedBusiness Combination, and therefore not a component of our results of operations during 2022. We do not receive any additional consideration for the private placement of 4,433,333 private placement warrants atBonus Shares and are a price of $1.50 per private placement warrant to our sponsor, generating proceeds of approximately $6.7 million. We consummated the second closingnon-recurring fair value measurement. The forward purchase derivative liability is a recurring fair value measurement. Accordingly, changes in fair value of the private placementforward purchase derivative liability will be a component of our results of operations in future periods.
Loss on June 2, 2021, simultaneouslyAmendment of Forward Purchase Contract
On December 26, 2023 in exchange for $0.5 million of consideration, the FPA Amendment was executed with an FPA Investor, which impacted the closingcash proceeds we may receive under the forward purchase agreement. As a result of the over-allotment, resultingFPA Amendment, the maximum cash proceeds we could receive under the forward purchase contract, reflected in the subscription receivable balance, was lowered. The reduction in the subscription receivable was caused by (i) the Amended Reset Price, which reduced the maximum price per FPA Share we could receive (initially, $10.44 per share), (ii) the re-designation of 200,000 FPA Shares to Bonus Shares, reducing the total quantity of FPA Shares, and (iii) the Amended Prepayment Shortfall, which increased the prepayment shortfall amount. We do not receive any consideration for sales or settlements of Bonus Shares or Shortfall Sales. The proceeds from the sale of an additional 288,200 private placement warrants, generating additional grossFPA Shares by FPA Investors to third parties are required to be treated as a reduction to the prepayment shortfall until no balance remains, at which point, the Company may start to receive proceeds of approximately $432,000.Upon the closingfrom such sales of the IPO,FPA Shares.
The Company recognized a corresponding reduction to the over-allotment, andforward purchase derivative liability on the private placement, $171.6 million ($10.00 per unit)amendment date as it represents the portion of the net proceedssubscription receivable that may be released to the FPA Investors rather than to us. Change in Fair Value of the saleForward Purchase Derivative Liability
The change in fair value of the unitsforward purchase derivative liability represents the recognition of changes in fair value of the forward purchase derivative liability, which are recognized in net losses on a quarterly basis at each balance sheet date.
Change in Fair Value of Derivative Warrant Liabilities
The change in fair value of derivative warrant liabilities represents the recognition of changes in the IPO andfair value of the private placement warrantsPrivate Warrants, Working Capital Warrants, and PIPE Warrants, which are recognized in net losses on a quarterly basis at each balance sheet date.
Transaction Costs Expensed
Transaction costs expensed represent Legacy NKGen’s transaction costs incurred in connection with the private placementBusiness Combination that were placedallocated to liability-classified instruments issued on a relative fair value basis. The Business Combination occurred in September 2023, and therefore transaction costs expensed were not a component of our results of operations during 2022.
Other Income, Net
Other income, net primarily consists of sublease income and payroll tax credits for the trust account maintainedyear ended December 31, 2023, and sublease income for the year ended December 31, 2022.
Provision for Income Taxes
We are subject to U.S. federal and state income taxes based on enacted rates, as adjusted for allowable credits, deductions, uncertain tax positions, changes in deferred tax assets and liabilities and changes in tax laws.
Provision for income taxes primarily relates to changes in deferred taxes, partially offset by Continental Stock Transfer & Trust Company,valuation allowances.
Results of Operations
Comparison of Years ended December 31, 2023 and 2022
The following tables summarize our results of operations for the years ended December 31, 2023 and 2022 (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Years ended December 31, | | Change |
| 2023 | | 2022 | | $ Change | | % Change |
| Revenues | $ | — | | | $ | 77 | | | (77) | | | * |
| Costs and expenses: |
| | | | | |
|
| Cost of revenues | — | | | 18 | | | (18) | | | * |
| Research and development | 15,668 | | | 16,746 | | | (1,078) | | | (6) | % |
| General and administrative | 14,078 | | | 7,659 | | | 6,419 | | | 84 | % |
| Total expenses | 29,746 | | | 24,423 | | | 5,323 | | | 22 | % |
| Loss from operations | (29,746) | | | (24,346) | | | (5,400) | | | 22 | % |
| Other income (expense): |
| | | | | | |
| Interest expense | (745) | | | (2,306) | | | 1,561 | | | (68) | % |
| Change in fair value of convertible promissory notes due to related parties | (1,043) | | | (177) | | | (866) | | | * |
| Loss on issuance of forward purchase contract | (24,475) | | | — | | | (24,475) | | | * |
| Loss on amendment of forward purchase contract | (442) | | | — | | | (442) | | | * |
| Change in fair value of forward purchase derivative liability | (9,784) | | | — | | | (9,784) | | | * |
| Change in fair value of derivative warrant liabilities | (13,503) | | | — | | | (13,503) | | | * |
| Transaction costs expensed | (3,329) | | | — | | | (3,329) | | | * |
| Other income, net | 120 | | | 82 | | | 38 | | | 46 | % |
| Net loss before provision for income taxes | (82,947) | | | (26,747) | | | (56,200) | | | 210 | % |
| Provision for income taxes | (7) | | | (7) | | | — | | | * |
| Net loss and comprehensive loss | $ | (82,954) | | | $ | (26,754) | | | $ | (56,200) | | | 210 | % |
*Not meaningful
Revenues
There was no revenue recognized during the year ended December 31, 2023. Revenue decreased by $0.1 million for the year ended December 31, 2023 as trustee,compared to the year ended December 31, 2022. This decrease related entirely to the winding down of our COVID-19 testing revenue stream during the year ended December 31, 2023.
Cost of Revenues
There was no cost of revenues incurred during the year ended December 31, 2023. Cost of revenues decreased by less than $0.1 million for the year ended December 31, 2023 as compared to the year ended December 31, 2022. These decreases related entirely to the winding down of NKGen’s COVID-19 testing revenue stream during the year ended December 31, 2023.
Research and will be invested onlyDevelopment Expenses
The following table summarizes the components of our research and development expenses for the year ended December 31, 2023 and 2022 (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Years ended December 31, | | Change |
| 2023 |
| 2022 |
| $ Change | | % Change |
| Total direct research and development expense | $ | 1,485 | |
| $ | 1,394 | |
| $ | 91 | | | 7 | % |
| Indirect research and development expense by type: |
|
|
|
|
| | |
| Personnel-related costs | 8,395 | |
| 8,912 | |
| (517) | | | (6) | % |
| Research and development supplies and services | 4,426 | |
| 4,891 | |
| (465) | | | (10) | % |
| Allocated facility, equipment and other expenses | 1,362 | |
| 1,549 | |
| (187) | | | (12) | % |
| Total indirect research and development expense | 14,183 | |
| 15,352 | |
| (1,169) | | | (8) | % |
| Total research and development expense | $ | 15,668 | |
| $ | 16,746 | |
| $ | (1,078) | | | (6) | % |
Total research and development expenses decreased by $1.1 million, or 6%, for the year ended December 31, 2023 as compared to the year ended December 31, 2022. The decrease was primarily attributable to a decrease in U.S. “government securities” within the meaningtotal indirect research and development expenses of Section 2(a)(16) of the Investment Company Act having a maturity of 185 days$1.2 million, or less or in money market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act which invest only8%.
The increase in direct U.S. government treasury obligations, untilresearch and development expenses for the earlier of: (i)year ended December 31, 2023 as compared to the year ended December 31, 2022 was primarily attributable to the commencement of our SNK02 Phase 1 clinical trials during the third quarter of 2023.
The decrease in total indirect research and development expenses for the year ended December 31, 2023 as compared to the year ended December 31, 2022 was primarily attributable to a $0.5 million, or 10%, decrease in research and development supplies and services, a $0.5 million, or 6%, decrease in personnel-related costs, and a $0.2 million, or 12%, decrease in allocated facility, equipment, and other expenses.
The decrease in research and development supplies and services for the year ended December 31, 2023 as compared to the year ended December 31, 2022 was primarily attributable to a $0.4 million, or 17%, decrease in laboratory supply costs due to decreased purchases of research and development materials during the year ended December 31, 2023 as compared to the year ended December 31, 2022, in addition to a $0.1 million, or 6%, decrease in professional fees due to decreased consulting and regulatory affairs costs.
The decrease in personnel-related costs for the year ended December 31, December 31, 2023 as compared to the year ended December 31, 2022 was primarily attributable to a $1.3 million, or 17%, decrease in compensation costs for research and development personnel associated with the substantial completion of NKGen’s SNK01 sarcoma Phase 1 clinical trials, which occurred during the second half of 2022, partially offset by a business combination$0.9 million increase in stock-based compensation expense as a result of stock option grants made during the first quarter of 2023 that vest over periods ranging from immediately upon grant to four years.
The decrease in allocated facility, equipment, and (ii)other expenses for the distributionyear ended December 31, 2023 as compared to the year ended December 31, 2022 was primarily attributable to a $0.2 million, or 47%, decrease in repair and maintenance costs due to decreased maintenance expenses during the year ended December 31, 2023 as compared to the year ended December 31, 2022, partially offset by a less than $0.1 million increase in utility costs.
General and Administrative Expenses
General and administrative expenses increased by $6.4 million, or 84%, for the year ended December 31, 2023 as compared to the year ended December 31, 2022. The increase was primarily attributable to an increase in stock-option compensation expense of $3.2 million as a result of stock option grants made during the trust accountfirst quarter of 2023 that vest over periods ranging from two to four years, as described below.
well as an increase in professional fees of $2.1 million due to increases in legal, consultant, and accounting costs incurred in relation to becoming a public company.
Our management has broad discretion with respectInterest Expense
Interest expense decreased by $1.6 million, or 68%, for the year ended December 31, 2023 as compared to the specific applicationyear ended December 31, 2022. The decrease was primarily attributable to a decrease of the net proceeds of the IPO, the over-allotment, and the sale of the private placement warrants, although substantially all of the net proceeds are intended to be applied generally toward consummating a business combination.If we have not consummated an initial business combination within 24 months$1.8 million in related party loans interest expense, offset by increase in interest expense from the closingrevolving line of the IPO, or by May 25, 2023, we will (i) cease all operations except for the purposecredit and Senior Convertible Notes. This as a result of winding up, (ii)reductions in outstanding principal on related party loan balances as promptly as reasonably possible, but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account including interest (which interest shall be net of taxes payable and up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the remaining stockholders and the board of directors, dissolve and liquidate, subject in each case to the Company’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law.
Liquidity and Going Concern
As of December 31, 2021, we had approximately $1.7 million in our operating bank account and working capital of approximately $642,000.
Our liquidity needs through2023 as compared to December 31, 2021 were satisfied through a payment2022 due to conversions of $25,000 from our sponsor to purchase the founder shares, thecertain related party loan of approximately $67,000 from the sponsor under a promissory note, and the proceeds from the consummationbalances into equity at Closing as described in Note 7,
Debt, of the private placement not held inconsolidated financial statements as of and for the trust account. We repaidyear ended December 31, 2023. Interest expense associated with the promissory note in full on May 26, 2021. In addition, in orderrevolving line of credit was $0.2 million and was zero for the year ended December 31, 2023 and 2022, respectively, due to finance transaction costs in connection with a business combination, the sponsor or an affiliatetiming of the sponsor, or certainestablishment of our officers and directors may, but are not obligatedthe revolving line of credit facility in June 2023.
Change in Fair Value of Convertible Promissory Notes
The change in fair value of convertible notes for the year ended December 31, 2023, was based upon the fair value of the Legacy Convertible Notes as of December 31, 2022 as compared to
loan us funds, from timethe fair value of the Legacy Convertible Notes upon conversion at Closing (exclusive of increases in fair value due to
time or at any time, in whatever amount they deem necessary in their sole discretion (the “working capital loans”)issuances). As of December 31,
2021, there were no amounts outstanding under any working capital loans.In2022, the probability of conversion was zero. At Closing, the Legacy Convertible Notes converted at their contractual discounts. We recognized a loss for the change in fair value of convertible promissory notes and convertible promissory notes due to related parties totaling $1.0 million for the year ended December 31, 2023 because of changes in expected conversion prices, probabilities of conversion, and the recognition of accrued interest from issuance during such periods until conversion.
Loss on Issuance of Forward Purchase Contract
The Closing of the Business Combination was on September 29, 2023, therefore, loss on issuance of forward purchase contract was not a component of our results of operations during 2022.
The loss on issuance of forward purchase contract of $24.5 million for the year ended December 31, 2023 consisted of the initial recognition of the forward purchase derivative liability and the Bonus Shares issued in connection with the Company’s assessmentPrivate Placement Agreements.
Loss on Amendment of going concern considerationsForward Purchase Contract
The Closing of the Business Combination was on September 29, 2023, therefore, losses on amendment to forward purchase contracts were not a component of our results of operations during 2022. The loss on amendment of forward purchase contract for the year ended December 31, 2023 was $0.4 million.
On December 26, 2023, in accordanceexchange for $0.5 million of consideration, the FPA Amendment was executed with Financial Accounting Standards Board's (“FASB”) accounting Standards Update (“ASU”) 2014-15, “Disclosuresan FPA Investor, which impacted the cash proceeds we may receive under the forward purchase agreement. The loss recognized in connection with the FPA Amendment represents the reduction in the subscription receivable, partially offset by the corresponding reduction in the forward purchase derivative liability and the $0.5 million of Uncertainties about an Entity’s Abilityconsideration received by the Company.
Change in Fair Value of Forward Purchase Derivative Liability
The Closing of the Business Combination was on September 29, 2023, therefore, changes in the fair value of the forward purchase derivative liability were not components of our results of operations during 2022.
The forward purchase derivative liability represents the portion of the subscription receivable that may be released to Continuethe FPA Investors. The change in fair value of forward purchase derivative liability represents the recognition of gains and losses resulting from the remeasurement of the fair value of the forward purchase derivative liability at each balance sheet date. The reduction in fair value of the forward purchase derivative liability was primarily related to the effect of reductions in our share price during the year ended December 31, 2023 as compared to initial issuance, which decreased the potential amounts to be received under the subscription receivable, which has a corresponding reduction in the fair value of the forward purchase derivative liability.
Change in Fair Value of Derivative Warrant Liabilities
The Closing of the Business Combination was on September 29, 2023, therefore, changes in the fair value of the derivative warrant liabilities were not components of our results of operations during 2022.
The change in fair value of derivative warrant liabilities represents the recognition of gains and losses resulting from the remeasurement of the fair value of the Private Warrants, Working Capital Warrants, and PIPE Warrants at each balance sheet date. The Private Warrants and Working Capital Warrants fair value decreased by $1.5 million and $0.2 million, respectively, during the year ended December 31, 2023. The PIPE Warrants fair value increased by $15.1 million during the year ended December 31, 2023. The reduction in the fair value of the Private Warrants and Working Capital Warrants were primarily attributable to decreases in our share price during the year ended December 31, 2023 as compared to initial issuance. The increase in the fair value of the PIPE Warrants was primarily attributable to the impact of certain features of the PIPE Warrants, including the strike price reset and downside protection cash, as well as changes in our stock price volatility during the period between initial issuance and December 31, 2023.
Transaction Costs Expensed
The Closing of the Business Combination was on September 29, 2023, therefore, transaction costs expensed was not a component of our results of operations during 2022.
Transaction costs expensed of $3.3 million for the year ended December 31, 2023 consisted of our transaction costs allocated on a relative fair value basis to liability-classified instruments issued in connection with the Business Combination.
Other Income, Net
Other income, net, increased by less than $0.1 million, or 46%, for the year ended December 31, 2023 as compared to the year ended December 31, 2022. The increase was primarily attributable to $0.1 million in COVID-19 payroll tax credits that were recognized during the year ended December 31, 2023, partially offset by a decrease of $0.1 million in sublease income due to the expiration of a sublease for which NKGen was the lessor. The sublease ended prior to July 2023.
Provision for Income Taxes
Provision for income taxes increased by less than $0.1 million for the year ended December 31, 2023 as compared to the year ended December 31, 2022 primarily due to changes in deferred tax balances partially offset by valuation allowances.
Liquidity and Capital Resources
Funding Requirements and Going Concern” management has determined
We have incurred operating losses and negative cash flows from operations since inception. We are still in our early stages of development and expect to continue to incur significant expenses and operating losses for the foreseeable future as we continue our research and preclinical studies and clinical trials, including our Phase 1 and Phase 1/2 clinical trials and anticipated Phase 2 clinical trials, expand our pipeline or scope of our current studies for our product candidates, initiate additional preclinical or other studies or clinical trials for our product candidates, change or add additional manufacturers or suppliers, seek regulatory and marketing approvals for any of our product candidates that successfully complete clinical studies, if any, acquire or in-license other product candidates and technologies, maintain, protect and expand our intellectual property portfolio, attract and retain skilled personnel, and experience any delays or encounter issues with any of the Companyabove.
Until such time as we can generate substantial product revenue, if ever, we expect to finance our cash needs through a combination of equity and debt financings, or other capital sources, including with related parties. To the extent that we raise additional capital through the future sale of equity or debt, the ownership interest of our stockholders will be diluted. The terms of these securities may include liquidation or other preferences that adversely affect the rights of our existing common stockholders. If we raise additional funds through collaboration agreements, marketing agreements, or licensing arrangements, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates on terms that may not be favorable to us. If we are unable to raise sufficient funds through equity or debt financings, we may be required to delay, limit, curtail or terminate our product development or future commercialization efforts or may be forced to cease operations or file for bankruptcy protection. Additionally, we may never become profitable, or if we do, may not be able to sustain profitability on a recurring basis.
As of December 31, 2023 and December 31, 2022, we had cash and cash equivalents of approximately less than $0.1 million and $0.1 million and working capital deficits of approximately $37.5 million and $14.4 million, respectively.
We have incurred substantial transaction expenses in connection with the Business Combination. As of December 31, 2023, we had accrued approximately $13.4 million in accounts payable and accrued expenses, including the transaction expenses from the Business Combination and our ongoing business operations. However, we continue to have substantial transaction expenses accrued and unpaid subsequent to the Business Combination. Furthermore, we have and expect to incur additional expenses in connection with transitioning to, and operating as, a public company. Additionally, we have $19.9 million in outstanding debts as of December 31, 2023, inclusive of our revolving line of credit with East West Bank, debts with related parties, and debts due within less than one year following December 31, 2023. Moreover, our revolving line of credit, as amended, with East West Bank, which is secured by all of our assets, required us to maintain a minimum cash balance of $15.0 million with the bank as of December 31, 2023 and at all times thereafter as long as there is an outstanding balance under the revolving line of credit. Such cash balance requirement has been contractually waived by East West Bank as of December 31, 2023, and pursuant to an amendment entered into on April 5, 2024, East West Bank has agreed to replace such minimum cash balance requirement with a covenant to use East West Bank as the Company's only commercial bank for cash deposits and extend the maturity date to September 18, 2024. See “Risk Factors — Risks Related to Our Financial Position — The East West Bank Loan Agreement and Equity and Business Loan Agreement (as defined below) provide each lender with a security interest in all of our assets, and contain financial covenants and other restrictions on our actions that may limit our operational flexibility or otherwise adversely affect our results of operations” for more details. We have entered into certain forward purchase arrangements with various investors in order to facilitate the consummation of the Business Combination. However, in accordance with such Forward Purchase Agreements, the funds raised in connection with such transactions were placed into escrow accounts and not received by the Company at the Closing of the Business Combination. There is no guarantee that the Company will receive substantial funds or any in connection with the Forward Purchase Agreements. In addition, we may be required to pay cash or issue additional shares of our common stock to holders of the PIPE Warrants under certain circumstances, which could adversely affect our financial position and results of operations. See “Risk Factors — Risks Related to Ownership of Our Securities — We may not receive any cash proceeds from the exercise of certain outstanding warrants and we may be required to pay cash or issue additional shares of Common Stock under certain circumstances” for more details.
We have considered that our long-term operations anticipate continuing net losses and the need for potential debt or equity financing. However, there can be no assurances that additional funding or other sources of capital will be available on terms acceptable to us, or at all. If additional capital is not secured when required, we may need to delay or curtail our operations until such funding is received. If we cannot expand our operations or otherwise capitalize on our business opportunities because we lack sufficient capital, our business, financial condition, and results of operations could be materially and adversely affected.
We do not currently have, and do not currently expect to have, sufficient funds to service our operations and our expenses and other liquidity needs and will require additional capital immediately. In addition, we have expressed substantial doubt as to our ability to continue as a going concern. There can be no assurance that we will be able to timely secure such additional funding on acceptable terms and conditions, or at all. If we are unable to raise sufficient capital immediately, we will not have sufficient cash and liquidity to finance our business operations and make required payments and may be required to delay, limit, curtail or terminate our product development or may be forced to cease operations or file for bankruptcy protection.
Because the proceeds from our financing arrangements will not be adequate to cover our accrued and unpaid expenses and provide the cash and liquidity necessary to operate our business, we continue to seek opportunities for raising additional funds through potential alternatives, which may include, among other things, the issuance of equity, equity-linked, and/or debt securities, debt financings, forward purchase arrangements or other capital sources. However, we may not be successful in securing additional financing on a timely basis, on acceptable terms and conditions or at all. In addition, substantial doubt about our ability to continue as a going concern may cause investors or other financing sources to be unwilling to provide funding to us on commercially reasonable terms, if at all. If sufficient funds are not available, we will have to delay, reduce the scope of, or eliminate some of our business activities, including related operating expenses, which would adversely affect our business prospects and our ability to continue our operations and would have a negative impact on our financial condition and ability to pursue our business strategies. In addition, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our financial statements, and/or seek protection under Chapters 7 or 11 of the United States Bankruptcy Code which could potentially cause us to cease operations and result in a complete or partial loss for our investors.
As a result of these conditions, we have concluded that there is substantial doubt over our ability to continue as a going concern as conditions and events, considered in the aggregate, indicate that we are currently unable to meet itsour obligations as they become due and expect to be unable to meet our obligations within one year after the date that the financial statements are availableissued. The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and settlement of liabilities and commitments in the normal course of business. The financial information and financial statements do not include any adjustments that might be necessary if the Company is unable to be issued. As such, management has determined that the liquidity condition raises substantial doubt about the Company’scontinue as a going concern. Our ability to continue as a going concern.concern is dependent upon our ability to raise additional funds and financing. We will need to raise additional capital immediately to continue operations based on our current business plan, and expectations and assumptions considering current macroeconomic conditions. There can be no assurance that we will be able to secure such additional funding on acceptable terms and conditions, or at all. If we cannot obtain sufficient capital immediately, we will not have sufficient cash flows and liquidity to finance our business operations as currently contemplated and we may need to substantially alter, or possibly even discontinue, our operations. In the event of a bankruptcy proceeding or insolvency, or restructuring of our capital structure, our stockholders could suffer a total loss of their investment.
Sources of Liquidity
To date, we have funded our operations primarily with the net proceeds from the issuance of senior convertible promissory notes, the issuance of related party loans, draws upon a revolving line of credit, the issuance and sale of equity securities, PIPE warrants, private placements and proceeds from the Business Combination. As of December 31, 2021,2023, we have cash and cash equivalents of less than $0.1 million and restricted cash of $0.3 million. In the future, we expect to finance our cash needs through a combination of equity and debt financings, including with related parties.
Senior Convertible Notes
We entered into convertible note subscription agreements with NKMAX for total proceeds of $10.0 million upon Closing with a four-year term and for which we expect to make interest payments of 8.0% paid in kind rather than 5.0% paid in cash semi-annually.
Legacy Convertible Notes
From November to December 2019, we issued the 2019 Convertible Notes and the 2019 Related Party Convertible Notes, and from March to September 2023, we issued the 2023 Convertible Notes and the 2023 Related Party Convertible Notes, collectively referred to as the Legacy Convertible Notes.
Total proceeds raised from the 2019 Convertible Notes and 2019 Related Party Convertible Notes were $10.8 million and $0.3 million, respectively.
The Closing of the Business combination triggered the conversion of the Legacy Convertible Notes at their contractual discounts. Pursuant to their terms, all of the Legacy Convertible Notes were converted into 5,579,266 shares of Legacy NKGen common stock, which then converted into 2,278,598 shares of common stock at Closing based on the Exchange Ratio.
Revolving Line of Credit
In June 2023, we entered into a $5.0 million revolving line of credit agreement with a commercial bank with a one year term. The revolving line of credit is secured by all of our assets, including a deed of trust over our owned real property located in Santa Ana, California. Additionally, we are required to maintain a restricted cash balance of $0.3 million following the issuance. Through December 31, 2023, we drew down $4.9 million upon the revolving line of credit and no adjustments have been maderepayments of drawdown occurred.
Related Party Loans
Between August 2019 and April 2023, we entered into Related Party Loans with NKMAX.
In December 2022, the then-outstanding aggregate Related Party Loans’ principal and interest of $66.1 million was converted into 6,943,789 shares of common stock (after the application of the Exchange Ratio) which was recognized as a capital contribution for the year ended December 31, 2022.
From January through April 2023, we entered into additional Related Party Loans with NKMAX for aggregate gross proceeds of $5.0 million. These additional Related Party Loans bear an interest rate of 4.6% and mature on December 31, 2024. There are no financial or non-financial covenants associated with the Related Party Loans. The additional Related Party Loans are not convertible into equity.
Short Term Related Party Loan
In September 2023, we raised $0.3 million in proceeds in connection with the Short Term Related Party Loan, which bore a 30-day term and an interest rate of 5.12%. The Short Term Related Party Loan was not convertible into equity and was subsequently repaid on October 5, 2023.
Private Placement
As of December 31, 2023, we had not received cash proceeds from the Private Placement Agreements. During 2024, we received proceeds from the amendments to the carrying amountsPrivate Placement Agreements as set forth below.
On January 2, 2024, we amended our Forward Purchase Agreement with an FPA Investor to increase the prepayment shortfall by $0.5 million in exchange for $0.5 million. All other terms and conditions remained unchanged.
On January 11, 2024, we amended our Forward Purchase Agreement with an FPA Investor to increase the prepayment shortfall by $0.5 million plus a variable amount in exchange for $0.5 million. All other terms and conditions remained unchanged.
On January 19, 2024, we amended our Forward Purchase Agreement with an FPA Investor to increase the prepayment shortfall by $0.3 million plus 20% of
OperationsOur entire activity from January 28, 2021 (inception) through December 31, 2021, wasthe then-current prepayment shortfall balance in preparationexchange for our IPO, and since our IPO, our activity has been limiteda $0.3 million payment to the searchCompany. The agreement also amends the Reset Price such that the Reset Price (i) is adjusted on a rolling basis as based on the weekly trailing VWAP subject to a ceiling of $10.44 per share ("Initial Price"), and (ii) discounts of generally 10% to the VWAP measurement that benefit the FPA Investor.. All other terms and conditions remained unchanged.
On February 21, 2024, we amended our Forward Purchase Agreement with an FPA Investor to increase the prepayment shortfall by $0.2 million and increase the Bonus Shares by 200,000 in exchange for $0.2 million. All other terms and conditions remained unchanged.
SPA Warrants
We did not receive any proceeds from the SPA Warrants upon the issuance at Closing but may receive proceeds upon their exercise.
PIPE Warrants
Prior to the Closing, we entered into warrant subscription agreements with certain investors, which closed on September 29, 2023. Pursuant to the Warrant Subscription Agreements, the Warrant Investors purchased an aggregate of 10,209,994 warrants, at a purchase price of $1.00 per warrant for total proceeds of $10.2 million.
On February 9, 2024, we amended our Warrant Subscription Agreement with a Warrant Investor to, among other things, (i) make all subscription warrants held by the Warrant Investor immediately eligible to accelerate the share conversion provisions of the Warrant Subscription Agreement in exchange for a prospective initial business combination. cash payment of $0.3 million, (ii) a second cash payment of up to $0.3 million based on the trailing 5-day VWAP following the effective registration of the shares, (iii) to grant the Warrant Investor “Most Favored Nation” status with respect to warrant restructuring for so long as any subscription warrants remain outstanding and (iv) to grant certain registration rights to the Warrant Investor. All other terms and conditions remained unchanged.
Working Capital Warrants
We willdid not generatereceive any operating revenues untilproceeds from the closingWorking Capital Warrants upon the issuance at Closing but may receive proceeds upon their exercise.
Public Warrants
We did not receive proceeds from the Public Warrants at Closing but may receive proceeds upon their exercise.
Private Warrants
Concurrently with Graf’s IPO, Graf issued 4,721,533 warrants to Graf Acquisition Partners IV LLC. Concurrently with Graf’s IPO, Graf issued 4,721,533 warrants to Graf Acquisition Partners IV LLC. We did not receive any additional proceeds from the Private Warrants at Closing but may receive proceeds upon their exercise.
Convertible Bridge Loans
On February 7, 2024, we entered into a related party bridge loan agreement for $0.4 million with a 20% premium due at maturity. The related party bridge loan matures at the earlier of (i) 60 days from issuance or (ii) upon a financing event with third parties exceeding $5.0 million. In April 2024 the maturity of the bridge loan was amended to be the earliest of (i) 90 days from issuance, (ii) upon a financing event with third parties exceeding $5.0 million, or (iii) the
occurrence of any event of default. The counterparty to this bridge loan agreement is also entitled to receive 400,000 warrants to purchase 400,000 shares of the Company’s common stock each at a strike price of $2.00 per share.
On February 20, 2024, we entered into a bridge loan agreement for $0.1 million with a 20% premium due at maturity. The bridge loan matures at the earlier of (i) 60 days from issuance or (ii) upon a financing event with third parties exceeding $10.0 million. The counterparty to this bridge loan agreement also received 100,000 warrants to purchase 100,000 shares of the Company’s common stock at a $2.00 strike price per share.
On February 27, 2024 and completionMarch 7, 2024, we entered into a bridge loan agreements each for $0.1 million with a 20% premium due at maturity. The bridge loan matures at the earlier of our initial business combination. We will generate non-operating income in(i) 60 days from their respective issuance or (ii) upon a financing event with third parties exceeding $5.0 million. Each individual counterparty to the formbridge loan agreements also received 3,667 shares of investment income from our investments held in the trust account. We expect to incur increased expenses as a result of being a public company (for legal, financial reporting, accounting and auditing compliance),common stock as well as 375,000 warrants to purchase 375,000 shares of the Company’s common stock at a $1.50 strike price per share.
Bridge Loans
On March 7, 2024, we entered into two bridge loan agreements for $0.1 million each that mature 15 days from issuance, with a 7.5% premium due diligence expenses.For periodat maturity. Both loans were
repaid upon closing of the convertible secured promissory note. Convertible Promissory Notes
On March 21, 2024, we entered into a 12% promissory note agreement for $0.3 million with a one year term, issued at a 10% discount. The lender retains the option to convert any or all outstanding and unpaid principal amount and interest into shares of the Company's common stock from January 28, 2021 (inception) throughthe date of issuance until the maturity date. The convertible promissory note was repaid upon closing of the convertible secured promissory note. Concurrently with this agreement, we issued the lender warrants entitling the lender to acquire up to 330,000 shares of common stock at an initial exercise price of $2.00 per share, subject to adjustment.
On March 26, 2024, we entered into a 12% promissory note agreement with lender who is also a FPA Investor for $0.3 million with a one year term, issued at a 10% discount. The lender retained the option to convert any or all outstanding and unpaid principal amount and interest into shares of the Company's common stock from the date of issuance until the maturity date. Concurrently with this agreement, we issued the lender warrants entitling the lender to acquire up to 330,000 shares of common stock at an initial exercise price of $2.00 per share, subject to adjustment.
On April 1, 2024, the Company entered into a 12% promissory note agreement with lender for $0.2 million with a one year term, issued at a 10% discount. The lender retains the option to convert any or all outstanding and unpaid principal amount and interest into shares of the Company's common stock from the date of issuance until the maturity date. Concurrently with this agreement, we issued the lender warrants entitling the lender to acquire up to 220,000 shares of common stock at an initial exercise price of $2.00 per share, subject to adjustment.
On April 1, 2024, the Company entered into a 12% promissory note agreement with lender who is also a FPA Investor for $0.3 million, issued at a 10% discount. The promissory note matures on April 1, 2025. The lender retains the option to convert any or all outstanding and unpaid principal amount and interest into shares of the Company's common stock from the date of issuance until the maturity date. Concurrently with this agreement, we issued the lender warrants entitling the lender to acquire up to 330,000 shares of common stock at an initial exercise price of $2.00 per share, subject to adjustment.
Convertible Secured Promissory Note
On April 5, 2024, we entered into a convertible secured promissory note agreement for $5.0 million with an interest rate of the one month secured overnight financing rate plus 2.85% payable in cash in arrears on a monthly basis, with payments commencing one month from issuance, which will mature on October 4, 2026. The convertible promissory note was issued in two tranches, the first of which was for $1.0 million and closed on April 8, 2024 and the second tranche was for $4.0 million which closed on April 9, 2024. The convertible secured promissory note is secured by a second lien on the Company’s owned real property located in Santa Ana, California. The convertible secured promissory note is subordinate to the $5.0 million revolving line of credit. The outstanding principal amount is convertible at any time until its maturity at the option of the lender, into common stock at a $2.00 conversion price (subject to customary anti-dilution adjustments for stock splits and the like). Concurrently with this agreement, the lender is entitled to receive 833,333 shares of common stock upon the first closing and an amount of shares equal to $2.5 million divided by a five days VWAP measurement upon the second closing as well as warrants entitling the lender to acquire up to 1,000,000 shares of common stock at an initial exercise price of $2.00 per share, subject to adjustment.
Cash Flows
The following is a summary of our cash flows (in thousands):
| | | | | | | | | | | |
| Years ended December 31, |
| 2023 | | 2022 |
| Net cash used in operating activities | $ | (21,948) | | | $ | (22,557) | |
| Net cash used in investing activities | $ | (48) | | | $ | (163) | |
| Net cash provided by financing activities | $ | 22,155 | | | $ | 22,486 | |
Net cash used in operating activities
The decrease in net cash used in operating activities of $0.6 million for the year ended December 31, 2021, we had a2023 as compared to the year ended December 31, 2022 was primarily attributable to decreased research and development expenditures due to the substantial completion of NKGen’s SNK01 sarcoma Phase 1 clinical trials, which occurred during the second half of 2022.
Net cash used in operating activities of $21.9 million for the year ended December 31, 2023 was primarily attributable to NKGen’s net loss of approximately $991,000$83.0 million, partially offset by changes in operating assets and liabilities of $2.2 million and $58.8 million in non-cash charges, which primarily relate to $24.5 million due to the loss on the issuance of the forward purchase contract, $9.8 million in the change in the fair value of forward purchase derivative liability, and $13.5 million in the change in the fair value of derivative warrant liability.
Net cash used in operating activities of $22.6 million for the year ended December 31, 2022 was primarily attributable to NKGen’s net loss of $26.8 million and changes in operating assets and liabilities of less than $0.1 million, offset by $4.2 million in non-cash charges, which primarily relate to $2.3 million of related party non-cash interest expense, and $1.2 million of depreciation and amortization.
Net cash used in investing activities
The decrease in net cash used in investing activities of $0.1 million for the year ended December 31, 2023 as compared to year ended December 31, 2022 was primarily attributable to decreased purchases of property and equipment.
Net cash used in investing activities was less than $0.1 million for the year ended December 31, 2023, which consisted of less than $0.1 million in purchases of capitalized software.
Net cash used in investing activities was $0.2 million for the year ended December 31, 2022, which consisted of a non-operating loss$0.1 million in purchases of capitalized software and $0.1 million in purchases of property and equipment.
Net cash provided by financing activities
The decrease in net cash provided by financing activities of $0.3 million for the year ended December 31, 2023 as compared to the year ended December 31, 2022 was primarily attributable to issuances of senior convertible promissory notes, draws upon a revolving line of credit, issuances of warrants, offset by payments of transaction costs as well as decrease in related party loans.
Net cash provided by financing activities was $22.2 million for the year ended December 31, 2023, which primarily consisted of proceeds of $10.0 million from the issuance of
private placement warrantsSenior Convertible Notes with SPA Warrants, $10.2 million from the issuance of
approximately $4.2PIPE Warrants, $5.3 million
approximately $2.2from Related Party Loans and Short Term Related Party Loans, $6.2 million
in generalfrom issuances of Legacy Convertible Notes, $5.0 million from draws on revolving line of credit facility and
administrative expenses, approximately $185,000 of franchise tax expenses, and related party administrative expenses of approximately $108,000, approximately $34,000 in offering cost allocated to derivative warrant liability, offset by approximately $5.7$1.7 million
in change in fair value of warrant liability, and approximately $41,000 in income from
investments held in trust account.Contractual Obligations
We do not have any long-term debt obligations, capital lease obligations, operating lease obligations, purchase obligations or long-term liabilities at December 31, 2021.
Registration and Stockholder Rights
The holders of our founder shares, private placement warrants and warrants that may be issued upon conversion of working capital loans (and any sharesthe issuance of common stock, issuable upon the exercise of the private placement warrants and warrants that may be issued upon conversion of working capital loans) were entitled to registration rights pursuant to a registration rights agreement signed upon the effective date of the IPO. These holders are entitled to make up to three demands, excluding short form registration demands, that the Company registered such securities for sale under the Securities Act. In addition, these holders will have “piggy-back” registration rights to include their securities in other registration statements filedoffset by the Company. We will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriting Agreement
We granted the underwriters a 45-day option from the date of the final prospectus relating to the IPO to purchase up to 2,250,000 additional units less the underwriting discounts and commissions. On June 2, 2021, the underwriters partially exercised the over-allotment option.
The underwriters were entitled to an underwriting discount of $0.20 per unit, or $3.4$14.6 million in payments of transaction costs.
Net cash provided by financing activities was $22.5 million for the
aggregate, paid upon the closing of the IPO and over-allotment. In addition, $0.35 per unit, or approximately $6.0 million in the aggregate will be payable to the underwriters for deferred underwriting commissions. The deferred fee will become payable to the underwriters from the amounts held in the trust account solely in the event that we complete a business combination, subject to the terms of the underwriting agreement.Administrative Services Agreement
On May 20, 2021, we entered into an agreement that provided that, commencing on the date that the Company’s securities were first listed on the NYSE through the earlier of consummation of the initial business combination and the liquidation, the Company agreed to pay G-SPAC Management LLC, an affiliate of the sponsor, $15,000 per month for office space, utilities, secretarial, administrative and support services provided to the Company and members of the management team. For the three monthsyear ended December 31, 20212022, which primarily consisted of proceeds of $23.0 million from Related Party Loan and for$0.2 million from exercises of common stock options, partially offset by $1.0 million in repayments on payroll protection program loans.
Contractual Obligations and Commitments
Leases
Our operating leases primarily consist of corporate offices. For additional information, see Note 14 – Commitments and Contingencies in the period from January 28, 2021 (inception) through December 31, 2021, the Company incurred expenses of approximately $45,000 and $108,000, respectively, under this agreement. As of December 31, 2021, the Company had no outstanding balance for services in connection with such agreement on the accompanying balance sheet.In addition, the sponsor, officers and directors, or any of their respective affiliates, will be reimbursed for any out-of-pocket expenses incurred in connection with activities on the Company’s behalf such as identifying potential target businesses and performing due diligence on suitable business combinations. The audit committee will review on a quarterly basis all payments that were made by the Companynotes to the sponsor, officers or directors, or their affiliates. Any such payments priorconsolidated financial statements included in Item 8, “Financial Statements and Supplementary Data,” of this Annual Report on Form 10-K.
Long-Term Debt
We have long-term debt which matures in 2027. For additional information, see Note 6 – Convertible Notes and Note 7 – Debt in the notes to an initial business combination will be made from funds held outside the trust account.consolidated financial statements included in Item 8, “Financial Statements and Supplementary Data,” of this Annual Report on Form 10-K.
Critical Accounting Policies and Estimates
This
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with
generally accepted accounting principles in the United States, or GAAP. The preparation of these
unaudited condensed financial statements requires
usmanagement to make estimates and
judgmentsassumptions that affect the reported amounts of assets
liabilities, revenues and
expensesliabilities and the disclosure of contingent assets and liabilities
in ourat the date of the financial
statements. On an ongoing basis, westatements, as well as the reported revenues and expenses during the reporting periods. We evaluate
ourthese estimates and judgments
including those related to fair value of financial instruments and accrued expenses.on an ongoing basis. We base our estimates on historical experience
known trends and
events andon various other factors that we believe
to beare reasonable under the circumstances, the results of which form the basis for making judgments about the carrying
valuesvalue of assets and liabilities that are not readily apparent from other sources.
ActualOur actual results may differ from these estimates under different assumptions or conditions.
Derivative Warrant LiabilityWhile our significant accounting policies are more fully described in Note 2, Summary of Significant Accounting Policies, to our financial statements, we believe that the following accounting policies are the most critical to fully understanding and evaluating our financial condition and results of operations.
Accrued Clinical and Research and Development Expenses
All research and development costs are expensed in the period incurred. Research and development expenses primarily consist of services provided by contract organizations for clinical development, salaries, and related expenses for personnel, including stock-based compensation expense, outside service providers, facilities costs, fees paid to consultants and other professional services, license fees, depreciation and supplies used in research and development. Payments made prior to the receipt of goods or services to be used in research and development are capitalized until the related goods or services are received.
As part of the process of preparing our financial statements, we are required to estimate our accrued expenses as of each balance sheet date. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. We make estimates of our accrued expenses as of each balance sheet date based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments, if necessary. The significant estimates in our accrued clinical trial and research and development expenses include the costs incurred for services performed by our vendors in connection with clinical trial and research and development activities for which we have not yet been invoiced.
We determine our expenses related to clinical trial and research and development activities on our estimates of the services received and efforts expended pursuant to quotes and contracts with vendors that conduct clinical trials and research and development on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the clinical trial and research and development expense. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or prepaid expense accordingly. Advance payments for goods and services that will be used in future clinical trial or research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made.
Although we do not use derivative instrumentsexpect our estimates to hedge exposuresbe materially different from amounts actually incurred, if our estimates of the status and timing of services performed differ from the actual status and timing of services performed, it could result in our reporting amounts that are too high or too low in any particular period. To date, there have been no material differences between our estimates of such expenses and the amounts actually incurred.
Stock-Based Compensation
Stock-based compensation expense is comprised of stock options awarded to cash flow,employees and consultants. Our stock option awards granted to date contain service based vesting conditions only and do not require the achievement of a market or foreign currency risks.performance condition in order to vest. These share-based awards are accounted for under the fair-value-based method prescribed by ASC 718-10, Stock Compensation. The Companyfair value of stock options is estimated using the Black-Scholes option pricing model on the date of grant. This option pricing model involves a number of estimates, including the per share value of the underlying common stock, exercise price, estimate of future volatility, expected term of the stock option award, risk-free interest rate and expected annual dividend yield.
We recognize the expense for options with graded-vesting schedules on a straight-line basis over the requisite service period, which is generally the vesting period. Forfeitures are recognized as they occur.
Valuation of Common Shares
Given the absence of a public trading market for our common shares prior to October 2, 2023, which was the first day of trading of our common stock following the Closing, and in accordance with the American Institute of Certified Public Accountants Accounting and Valuation Guide, Valuation of Privately- Held-Company Equity Securities Issued as Compensation, our board of directors exercises its reasonable judgment and considered numerous objective and subjective factors to determine the best estimate of fair value of our common shares, including, but not limited to:
•independent third-party valuations of our common shares;
•capital resources and financial condition;
•the likelihood and timing of achieving a liquidity event;
•historical operating and financial performance as well as our estimates of future financial performance;
•valuations of comparable companies;
•the status of our development;
•the relative lack of marketability of our common shares prior to the October 2, 2023;
•industry information such as market growth and volume and macro-economic events;
•additional objective and subjective factors relating to our business; and
•implied fair values upon a merger transaction.
Prior to October 2, 2023, our board of directors determined the fair value of our common shares using both the income and market approach valuation methods. The income approach estimates value based on the expectation of future cash flows that a company will generate. The market approach estimates value based on a comparison of the subject company to comparable public companies in a similar line of business as well as implied fair values upon a merger transaction such as the Business Combination. Under the market approach, based on a comparison of the subject company to comparable public companies in a similar line of business, a discount for lack of marketability (“DLOM”) was applied to arrive at a fair value of common shares. A DLOM was meant to account for the lack of marketability of shares that were not publicly traded. The valuation of common shares underlying common stock options granted during the year ended December 31, 2023 were estimated under the market approach, based upon the implied fair value of common stock agreed upon in the Business Combination, where the fair values of our common shares as of the respective grant dates were determined using a linear interpolation between the previous valuation and the anticipated closing date of the Business Combination based on circumstances existing as of the respective grant dates. It was determined that the straight-line calculation provides the most reasonable basis for the valuation of our common stock because there was no single event that occurred during the period between the valuation dates that would have caused a material change in fair value.
Applying these valuation approaches involves the use of estimates, judgments and assumptions that are highly complex and subjective, including our expected future revenue and expenses, the determination of discount rates, interpolations, valuation multiples, the selection of comparable public companies and the probability of future events. Changes in any or all of these estimates and assumptions impact our valuation as of each valuation date. Such changes may have a material impact on the valuation of our common shares and our share-based awards.
Beginning October 2, 2023 the fair value of our common shares was based upon our publicly listed share price.
Accounting for Select Financial Instruments Issued in Connection with the Business Combination
In connection with the Business Combination, among other instruments, we issued Public Warrants, Private Warrants, PIPE Warrants, SPA Warrants, Working Capital Warrants, Senior Convertible Notes, Deferred Founder Shares, and a forward purchase derivative (collectively, “Select Financial Instruments”). The accounting determinations surrounding the Select Financial Instruments has a significant effect on our reported financial position and results of operations.
We determine the accounting classification of the Select Financial Instruments by first assessing each instrument under Financial Accounting Standards Board (“FASB”) Accounting Standard Codification (“ASC”) 480, Distinguishing Liabilities from Equity, then assessing each instrument under ASC 815, Derivatives and Hedging Activities.Under ASC 480, instruments are considered liability classified if they are mandatorily redeemable, obligate us to settle the warrants or the underlying shares by paying cash or other assets, and instruments that must or may require settlement by issuing variable number of shares. If instruments do not meet the liability classification under ASC 480-10, we assess the requirements under ASC 815-40, which states that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the financial instruments do not require liability classification under ASC 815-40, in order to conclude equity classification, we also assess whether the instruments are indexed to our own common stock and whether the instruments are classified as equity under ASC 815-40 or other GAAP. After all such assessments, we conclude whether the instruments are classified as liability or equity.
In addition, ASC 815 requires companies to bifurcate certain features from their host instruments and account for them as free-standing derivative financial instruments should certain criteria be met. We evaluate itsour financial instruments to determine ifwhether such instruments are derivatives or contain features that qualify as embedded derivatives. Embedded derivatives must be separately measured from the host contract if all the requirements for bifurcation are met. The assessment of the conditions surrounding the bifurcation of embedded derivatives depends on the nature of the host contract and the features of the derivatives. Bifurcated embedded derivatives are recognized at fair value, with changes in accordancefair value recognized in the consolidated statements of operations and comprehensive loss each period. Bifurcated embedded derivatives are classified with FASB ASC Topic 815, “Derivatives and Hedging” (“ASC 815”).the related host contract in our consolidated balance sheets. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period.
For convertible debt instruments that are not considered liabilities under ASC 480 or ASC 815, we apply ASC 470, Debt, for the accounting of such instruments, including any premiums or discounts.Liability classified instruments require fair value accounting at issuance and subsequent to initial issuance with all changes in fair value after the issuance date recorded in the consolidated statements of operations and comprehensive loss. Equity classified instruments only require fair value accounting at issuance with no changes recognized subsequent to the issuance date.
The private placement warrants are recognized asthe foregoing accounting guidance to the terms, features, and circumstances surrounding the Company’s Select Financial Instruments, the Public Warrants, SPA Warrants, and Deferred Founder Shares were determined to be equity classified instruments, and the Senior Convertible Notes, Private Warrants, PIPE Warrants, Working Capital Warrants, Legacy Convertible Notes, and forward purchase derivative liabilities in accordance withwere determined to be liability classified instruments. While the Senior Convertible Notes were determined to be liability-classified, they were determined to be in-scope of ASC 470 and not in-scope of ASC 480 or ASC 815. Accordingly, the Company recognizes the warrant instruments as liabilitiesSenior Convertible Notes will not be measured at fair value on a recurring basis as the fair value measurement of this instrument was for purposes of the relative fair value allocation described below as the Senior Convertible Notes were issued together with the SPA Warrants.
Fair Value of Financial Instruments
We account for the fair value of our financial instruments under the framework established by US GAAP which defines fair value and adjustsexpands disclosures about fair value measurements. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value. These estimates may be subjective in nature and involve uncertainties and matters of significant judgment and therefore cannot be determined with precision.
Level 1 — Quoted prices in active markets for identical assets or liabilities we have the ability to access at the measurement date.
Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of assets or liabilities.
Level 3 — Pricing inputs that are unobservable, supported by little or no market activity and are significant to the fair value of the assets or liabilities.
Transfers to/from Levels 1, 2, and 3 are recognized at the beginning of the reporting period. There were no transfers to/from Levels 1, 2, and 3 during the year ended December 31, 2023 and 2022.
ASC 820, Fair Value Measurement, states that in many cases, the transaction price will equal the fair value (for example, that might be the case when on the transaction date the transaction to buy an asset takes place in the market in which the asset would be sold). In determining whether a transaction price represents the fair value at initial recognition, we consider various factors such as whether the transaction was between related parties, is a forced transaction, or whether the unit of account for the transaction price does not represent the unit of account for the measured instrument.
We do not measure assets at fair value on a recurring basis. The carrying value of the instruments toour related party loans approximates fair value as the stated interest rate approximates market rates for similar loans and due to the short-term nature of such loans, which are due within three years or less from issuance. The carrying value of our cash, restricted cash, accounts payable, accrued expenses, other current liabilities, prepaid expenses and other current assets, capitalized software, related party loans, and revolving line of credit approximates fair value primarily due to the short-term nature of such accounts.
The fair value of equity-classified instruments are determined based on trading prices of identical securities as of the measurement date. Liability-classified instruments measured at each reporting period until theyfair value on a recurring basis include the Private Warrants, Working Capital Warrants, forward purchase derivative liability, PIPE Warrants, and the Legacy Convertible Notes. Determining the fair value of the liability classified instruments requires the use of accounting estimates and assumptions. Liability-classified instruments measured at fair value on a non-recurring basis include the Senior Convertible Notes.
These estimates and assumptions are exercised.judgmental in nature and could have a has a significant effect on our reported financial position and results of operations.
The terms of the Private Warrants and Working Capital Warrants are identical. Accordingly, the methodology and assumptions used to value these instruments is identical. The fair value of the private placement warrants asPrivate Warrants and Working Capital Warrants was measured at fair value using a Black-Scholes model. The estimated fair value of December 31, 2021 isthe Private Warrants and Working Capital Warrants was determined using Level 3 inputs. Inherent in a Black-Scholes option pricing model.model are assumptions related to expected stock-price volatility, expected life, risk-free interest rate and dividend yield. The determinationCompany estimates the volatility ofour Private Warrants and Working Capital Warrants based on implied volatility from the Company’s traded Private Warrants and Working Capital Warrants and from historical volatility of select peer company’s common stock that matches the expected remaining life of the Private Warrants and Working Capital Warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected remaining life of the Private Warrants and Working Capital Warrants. The expected life of the Private Warrants and Working Capital Warrants is assumed to be equivalent to their remaining contractual term. The dividend rate is based on the historical rate, which we anticipate remaining at zero.
We historically determined the carrying amount of the Legacy Convertible Notes using a scenario-based analysis that estimates the fair value of the
warrant liability may be subjectLegacy Convertible Notes based on the probability-weighted present value of expected future investment returns by measuring the fair value of similar debt instruments that do not have the conversion feature. If no similar debt instrument existed, fair value was estimated by using assumptions that market participants would use in pricing a debt instrument, including market interest rates, credit standing, yield curves and volatilities. The fair value of Legacy Convertible Notes immediately prior to
change as more current information becomes available and accordingly the actual results could differ significantly. Derivative warrant liabilities are classified as non-current liabilities as their
liquidation is not reasonably expected to require the use of current assets or require the creation of current liabilities.Common Stock Subject to Possible Redemption
The Company accounts for its common stock subject to possible redemption in accordance with the guidance in ASC 480. Common stock subject to mandatory redemption (if any) are classified as liability instruments and are measuredconversion at fair value. Conditionally redeemable common stock (including shares of common stock that feature redemption rights that are either within the control of the holder or subject to redemptionClosing was based upon the occurrence of uncertain events not solely within the Company’s control) are classified as temporary equity. At all other times, common stock is classified as stockholders’ equity. The Company’s common stock features certain redemption rights that are considered to be outside of the Company’s control and subject to occurrence of uncertain future events. Accordingly, as of IPO (including exercise of the over-allotment option), 17,161,500 shares of common stock subject to possible redemption at the redemption amount were presented at redemption value as temporary equity, outside of the stockholders’ deficit section of the Company’s balance sheet.
We recognize changes in redemption value immediately as they occur and adjust the carryingfair value of the shares of our common stock shares subject to possible redemption to equalissued upon their conversion totaling based upon the redemptionfair value at the end of each reporting period. This method would view the end of the reporting period as if it were also the redemption date for the security. Effective with the closing of the Initial Public Offering (including exercise of the over-allotment option), the Company recognized the accretion from initial book value to redemption amount, which resulted in charges against additional paid-in capital (to the extent available) and accumulated deficit.
Net Income (Loss) Per Share of Common Stock
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share.” Net income (loss) perour common stock is computed by dividing net loss byat Closing, which was the weighted average number of shares of common stock outstanding during the period.
The calculation of diluted net income (loss) per common stock does not consider the effect of the warrants issued in connection with the IPO (including exercise of the over-allotment option) and the private placement to purchase an aggregate of 8,153,833 shares of common stock in the calculation of diluted income (loss) per share, because their exercise is contingent upon future events and their inclusion would be anti-dilutive under the treasury stock method. As a result, diluted net income (loss) per share is the same as basic net income (loss) per share for the period from January 28, 2021 (inception) through December 31, 2021. Accretion associated with the redeemable common stock is excluded from earnings per share as the redemption value approximates fair value.
Recent Accounting Standards
In August 2020, the FASB issued ASU No. 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. The ASU also removes certain settlement conditions that are required for equity-linked contracts to qualify for the derivative scope exception, and it simplifies the diluted earnings per share calculation in certain areas. The Company adopted ASU 2020- 06 on January 28, 2021 using a modified retrospective method for transition. Adoption of the ASU did not impact our financial position, results of operations or cash flows.
Management does not believe that any other recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material effect on our financial statements.
conversion date. Off-Balance Sheet Arrangements
As of December 31, 2021,2023, the fair value of the PIPE warrants was estimated using a Monte Carlo simulation approach. The Company’s common share price was assumed to follow a Geometric Brownian Motion over a period from the Valuation Date to the Expiration Date. The breadth of all possible scenarios was captured in an estimate of volatility, based on comparable companies’ historical equity volatilities, considering differences in their capital structure. For each simulation path, the Test Price and Reset Price were calculated based on the daily stock price during the measurement period. On each Reset Date, the downside protection condition was assessed to see if it was met by comparing the Test Price with the downside protection threshold price. The value of each tranche of warrants was then computed, factoring in any downside protection shares and downside protection cash, if applicable. The average value across this range of possible scenarios, discounted to present using the risk-free rate, was used as the fair value of the PIPE Warrants.
The fair value of the forward purchase derivative liability was estimated using a Monte Carlo simulation approach. Our common share price was simulated with daily time steps for a range of various possible scenarios. The breadth of all possible scenarios was captured in an estimate of volatility, based on comparable companies’ historical equity volatilities, considering differences in their capital structure. The simulated prices were compared against the settlement adjustment features of the Forward Purchase Agreements. Under each simulated scenario of future stock price, we did not have any off-balance sheet arrangementscalculated the value of the forward purchase derivative liability arrangement. The average value across this range of possible scenarios, discounted to present using the risk-free rate, was used as the fair value of the forward purchase derivative liability.
The Senior Convertible Notes were issued together with the SPA Warrants. Each instrument was recorded at its fair value, limited to a relative fair value based upon the percentage of its fair value to the total fair value based on the transaction price at Closing on September 29, 2023. The relative fair value of the SPA Warrants was treated as a discount to the Senior Convertible Notes, which will be amortized to interest expense over the term of the Senior Convertible Notes.
We determined the stand-alone fair value of the Senior Convertible Notes using a binomial lattice model, which generates a distribution of stock prices over the term of the note, calculates the associated payoff for the note, and discounts the probability-weighted values from the lattice back to the valuation date. The fair value was estimated by using assumptions that market participants would use in pricing a convertible debt instrument, including market interest rates, credit rating, yield curves, and volatilities.
Recently Issued and Adopted Accounting Pronouncements
We describe the recently issued accounting pronouncements that apply in Note 2, Summary of Significant Accounting Policies, of the consolidated financial statements as of and for the year ended December 31, 2023.
Emerging Growth Company Status
We qualify as an emerging growth company, as defined in Item 303(a)(4)(ii) of Regulation S-K.JOBS Act
Thethe Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) contains provisions that, among other things, relax certain reporting requirements for qualifying public companies. We qualify as an “emerging growth company” and under the (“
JOBS Act are allowed to comply with new or revised accounting pronouncements based on the effective date for private (not publicly traded) companies. We are electing to delay the adoption of new or revised accounting standards,”) and as a result, we may not comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. As a result, the unaudited condensed financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.Additionally, we are in the process of evaluating the benefits of relying on the other reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, if, asremain an “emergingemerging growth company” we choose for up to rely on such exemptions we may not be required to, among other things, (i) provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404, (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may be adopted by the PCAOB regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis) and (iv) disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the CEO’s compensation to median employee compensation. These exemptions will apply for a period of five years following the completion of our IPO or untilGraf’s initial public offering. For so long as we remain an emerging growth company, we are no longerpermitted and intends to rely on certain exemptions from various public company reporting requirements, including delaying adopting new or revised accounting standards issued until such time as those standards apply to private companies, not being required to have our internal control over financial reporting audited by our independent registered public accounting firm pursuant to Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and any golden parachute payments not previously approved. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
Following the closing of the Business Combination, we are an “emergingemerging growth company until theearliest of (i) the last day of the fiscal year following the fifth anniversary of the consummation of Graf’s initial public offering, (ii) the last day of the fiscal year in which we have total annual gross revenue of at least $1.235 billion, (iii) the last day of the fiscal year in which we are deemed to be a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our common stock held by non-affiliates exceeded $700.0 million as of the last business day of the second fiscal quarter of such year, or (iv) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period.
We qualify as a “smaller reporting company,” whicheveras such term is earlier.defined in Rule 12b-2 of the Exchange Act, meaning that the market value of our common stock held by non-affiliates plus the proposed aggregate amount of gross proceeds to the Company as a result of the Business Combination is less than $700.0 million and our annual revenue is less than $100.0 million during the most recently completed fiscal year. We may continue to be a smaller reporting company following the closing of the Business Combination if either (i) the market value of our common stock held by non-affiliates is less than $250.0 million or (ii) our annual revenue is less than $100.0 million during the most recently completed fiscal year and the market value of our common stock held by non-affiliates is less than $700.0 million. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company, we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation. Item 7A.Quantitative and Qualitative Disclosures about Market
RiskRisk.
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information otherwise required under this item.
Item 8.Financial Statements and Supplementary DataData.
Index to Consolidated Financial Statements
GRAF ACQUISITION CORP. IV
INDEX TO FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
To the
StockholdersShareholders and the Board of Directors of
Graf Acquisition Corp. IV
NKGen Biotech, Inc.
Opinion on the Financial Statements
We have audited the accompanying
consolidated balance
sheetsheets of
Graf Acquisition Corp. IVNKGen Biotech, Inc. (the
“Company”)Company) as of December 31,
2021,2023 and 2022, the related statements of operations
changes in stockholders’ deficitand comprehensive loss, and stockholders' equity (deficit) and cash flows for the
period from January 28, 2021 (inception) through December 31, 2021years then ended, and the related notes (collectively referred to as the
“financial“consolidated financial statements”). In our opinion, the
consolidated financial statements present fairly, in all material respects, the financial position of the Company
as ofat December 31,
2021,2023 and 2022, and the results of its operations and its cash flows for the
period from January 28, 2021 (inception) through December 31, 2021,years then ended, in conformity with
accounting principlesU.S. generally accepted
in the United States of America.Restatement of Previously Issued Financial Statement
As described in Note 2accounting principles.
The Company’s Ability to theContinue as a Going Concern
The financial statements
do not include any adjustments that might result from the
Company’s previously issued May 25, 2021 financial statement has been restated herein to correct a certain misstatement.Going Concern
outcome of this uncertainty. The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, if the Company is unable to raise additional funds to alleviate liquidity needs the Company will cease allhas suffered recurring losses from operations, except for the purpose of liquidating. The liquidity condition raiseshas working capital deficiency, and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management'sManagement’s evaluation of the events and conditions and management’s plans in regard toregarding these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
These financial statements are the responsibility of the
Company’sCompany's management. Our responsibility is to express an opinion on the
Company'sCompany’s financial statements based on our
audit.audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States)
(“PCAOB”)(PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our
auditaudits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our
audit,audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our
auditaudits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our
auditaudits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our
audit providesaudits provide a reasonable basis for our opinion.
/s/ WithumSmith+Brown, PCErnst & Young LLP
We have served as the
Company'sCompany’s auditor since
2021.New York, New York
March 30, 2022
PCAOB No. 100
2020.Irvine, California
April 16, 2024
GRAF ACQUISITION CORP. IV
NKGEN BIOTECH, INC.
CONSOLIDATED BALANCE
SHEETDECEMBER 31, 2021
| | | |
Assets: | | |
Current assets: | | | |
Cash | | $ | 1,722,506 |
Prepaid expenses | | | 812,724 |
Total current assets | | | 2,535,230 |
Investments held in Trust Account | | | 171,656,153 |
Total assets | | $ | 174,191,383 |
| | | |
Liabilities, Common Stock Subject to Possible Redemption and Stockholders' Deficit: | | | |
Current liabilities: | | | |
Accounts payable | | $ | 51,807 |
Accrued expenses | | | 1,656,137 |
Franchise tax payable | | | 185,205 |
Total current liabilities | | | 1,893,149 |
Derivative warrant liability | | | 5,571,410 |
Deferred underwriting commissions in connection with the initial public offering | | | 6,006,525 |
Total Liabilities | | | 13,471,084 |
| | | |
Commitments and Contingencies (Note 5) | | | |
| | | |
Common stock subject to possible redemption; 17,161,500 shares at redemption value of $10.00 per share | | | 171,615,000 |
| | | |
Stockholders' Deficit: | | | |
Preferred stock, $0.0001 par value; 1,000,000 shares authorized; 0 shares issued and outstanding | | | — |
Common stock, $0.0001 par value; 400,000,000 shares authorized; 4,290,375 shares issued and outstanding | | | 429 |
Additional paid-in capital | | | — |
Accumulated deficit | | | (10,895,130) |
Total stockholders' deficit | | | (10,894,701) |
Total Liabilities, Common Stock Subject to Possible Redemption and Stockholders' Deficit | | $ | 174,191,383 |
SHEETS
(In thousands, except share and par value data)
| | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 26 | | | $ | 117 | |
| Accounts receivable | — | | | 29 | |
| Restricted cash | 250 | | | — | |
| Prepaid expenses and other current assets | 1,654 | | | 204 | |
| Total current assets | 1,930 | | | 350 | |
| Property and equipment, net | 14,459 | | | 15,521 | |
| Operating lease right-of-use assets, net | — | | | 362 | |
| Capitalized software, net | 92 | | | 97 | |
| Total assets | $ | 16,481 | | | $ | 16,330 | |
| Liabilities and Stockholders’ Equity (Deficit) | | | |
| Current liabilities: | | | |
| Accounts payable and accrued expenses (including related party amounts of $688 and $81 as of the year ended December 31, 2023 and 2022, respectively) | $ | 13,395 | | | $ | 2,652 | |
| Convertible promissory notes, current | — | | | 11,392 | |
| Convertible promissory notes, due to related parties | — | | | 263 | |
| Revolving line of credit | 4,991 | | | — | |
| Related party loans | 5,000 | | | — | |
| Operating lease liability | — | | | 379 | |
| Other current liabilities (including related party amounts of $202 and zero as of the year ended December 31, 2023 and 2022, respectively) | 262 | | | 55 | |
| Forward purchase derivative liability | 15,804 | | | — | |
| Total current liabilities | 39,452 | | | 14,741 | |
| Deferred tax liability | 33 | | | 26 | |
| Derivative warrant liabilities | 25,759 | | | — | |
| Senior convertible promissory notes, noncurrent, due to related parties | 9,930 | | | — | |
| Total liabilities | 75,174 | | | 14,767 | |
| Commitments and contingencies (Note 14) | | | |
| Stockholders’ equity (deficit): | | | |
| Common stock, $0.0001 par value; 500,000,000 authorized shares as of December 31, 2023 and 2022; 21,888,976 and 13,303,795 shares issued and outstanding as of December 31, 2023 and 2022, respectively | 2 | | | 1 | |
| Additional paid-in capital | 121,727 | | | 80,738 | |
| Subscription receivable | (17,792) | | | — | |
| Receivable from shareholder | (500) | | | — | |
| Accumulated deficit | (162,130) | | | (79,176) | |
| Total stockholders’ equity (deficit) | (58,693) | | | 1,563 | |
| Total liabilities and stockholders’ equity (deficit) | $ | 16,481 | | | $ | 16,330 | |
The accompanying notes are an integral part of these
consolidated financial statements.
GRAF ACQUISITION CORP. IV
STATEMENTNKGEN BIOTECH, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE PERIOD FROM JANUARY 28, 2021 (INCEPTION) THROUGH DECEMBER 31, 2021
| | | |
General and administrative expenses | | $ | 2,214,615 |
General and administrative expenses - related party | | | 108,387 |
Franchise tax expenses | | | 185,205 |
Loss from operations | | | (2,508,207) |
| | | |
Other income (expenses): | | | |
Offering costs - derivative warrant liability | | | (34,474) |
Change in fair value of derivative warrant liability | | | 5,665,840 |
Income from investments held in Trust Account | | | 41,153 |
Loss upon issuance of private placement warrants | | | (4,154,950) |
Total other income | | | 1,517,569 |
Net loss | | $ | (990,638) |
| | | |
Weighted average shares outstanding of common stock, basic and diluted | | | 15,083,469 |
Basic and diluted net loss per share, common stock | | $ | (0.07) |
AND COMPREHENSIVE LOSS
(In thousands, except share and per share data)
| | | | | | | | | | | |
| Years ended December 31, |
| 2023 | | 2022 |
| Revenues | $ | — | | | $ | 77 | |
| Costs and expenses: | | | |
| Cost of revenues | — | | | 18 | |
| Research and development (including related party amounts of $620 and $439 for the year ended December 31, 2023 and 2022, respectively) | 15,668 | | | 16,746 | |
| General and administrative | 14,078 | | | 7,659 | |
| Total expenses | 29,746 | | | 24,423 | |
| Loss from operations | (29,746) | | | (24,346) | |
| Other income (expense): | | | |
| Interest expense (including related party amounts of $431 and $2,271 for the year ended December 31, 2023 and 2022, respectively) | (745) | | | (2,306) | |
| Change in fair value of convertible promissory notes and convertible promissory notes due to related parties (including related party amounts of $12 and $4 for the years ended December 31, 2023 and 2022, respectively) | (1,043) | | | (177) | |
| Loss on issuance of forward purchase contract | (24,475) | | | — | |
| Loss on amendment of forward purchase contract | (442) | | | — | |
| Change in fair value of forward purchase derivative liability | (9,784) | | | — | |
| Change in fair value of derivative warrant liabilities | (13,503) | | | — | |
| Transaction costs expensed | (3,329) | | | — | |
| Other income, net | 120 | | | 82 | |
| Net loss before provision for income taxes | (82,947) | | | (26,747) | |
| Provision for income taxes | (7) | | | (7) | |
| Net loss and comprehensive loss | $ | (82,954) | | | $ | (26,754) | |
| Weighted-average common shares outstanding, basic and diluted | 15,426,908 | | 6,356,348 |
| Net loss per share, basic and diluted | $ | (5.38) | | | $ | (4.21) | |
The accompanying notes are an integral part of these
consolidated financial statements.
GRAF ACQUISITION CORP. IV
STATEMENTNKGEN BIOTECH, INC.
CONSOLIDATED STATEMENTS OF
CHANGES IN STOCKHOLDERS’
DEFICITFOR THE PERIOD FROM JANUARY 28, 2021 (INCEPTION) THROUGH DECEMBEREQUITY (DEFICIT)
(In thousands, except share data)
Years Ended December 31,
2021
| | | | | | | | | | | | | | | |
| | | | | Additional | | | | | Total |
| | Common Stock | | | | | Paid-In | | Accumulated | | Stockholders' |
| | Shares | | Amount | | Capital | | Deficit | | Deficit |
Balance - January 28, 2021 (inception) | | | 0 | | $ | 0 | | $ | 0 | | $ | 0 | | $ | 0 |
Issuance of common stock to Sponsor | | | 4,312,500 | | | 431 | | | 24,569 | | | 0 | | | 25,000 |
Common stock forfeited | | | (22,125) | | | (2) | | | 2 | | | 0 | | | — |
Fair value of Public Warrants included in the Units sold in the Initial Public Offering | | | — | | | — | | | 7,894,290 | | | 0 | | | 7,894,290 |
Offering costs associated with issuance of Public Warrants | | | — | | | — | | | (434,186) | | | 0 | | | (434,186) |
Accretion to common stock subject to possible redemption amount | | | — | | | — | | | (7,484,675) | | | (9,904,492) | | | (17,389,167) |
Net loss | | | — | | | — | | | 0 | | | (990,638) | | | (990,638) |
Balance - December 31, 2021 | | $ | 4,290,375 | | $ | 429 | | $ | 0 | | $ | (10,895,130) | | $ | (10,894,701) |
2023 and 2022
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Legacy Common Stock | | Common Stock | | | | | | | | | | |
| Shares | | Amount | | Shares | | Amount | | Additional
Paid-In
Capital | | Subscription
Receivable | | Receivable
from
Shareholder | | Accumulated
Deficit | | Total
Stockholders'
Equity
(Deficit) |
| Balance as of December 31, 2021 | 14,382,093 | | $ | 14 | | | - | | $ | - | | | $ | 14,356 | | | $ | - | | | $ | - | | | $ | (52,422) | | | $ | (38,052) | |
| Retroactive application of recapitalization | (14,382,093) | | (14) | | | 5,873,711 | | 1 | | | 13 | | | - | | | - | | | - | | | - | |
| Balance as of December 31, 2021, adjusted | - | | - | | | 5,873,711 | | 1 | | | 14,369 | | | - | | | - | | | (52,422) | | | (38,052) | |
| Stock-based compensation | - | | - | | | - | | - | | | 69 | | | - | | | - | | | - | | | 69 | |
| Exercise of common stock options | - | | - | | | 486,295 | | - | | | 161 | | | - | | | - | | | - | | | 161 | |
| Issuance of common stock upon conversion of related party loans (Note 7) | - | | - | | | 6,943,789 | | - | | | 66,139 | | | - | | | - | | | - | | | 66,139 | |
| Net loss | - | | - | | | - | | - | | | - | | | - | | | - | | | (26,754) | | | (26,754) | |
| Balance as of December 31, 2022 | - | | - | | | 13,303,795 | | 1 | | | 80,738 | | | - | | | - | | | (79,176) | | | 1,563 | |
| Stock-based compensation | - | | - | | | - | | - | | | 4,135 | | | - | | | - | | | - | | | 4,135 | |
| Exercise of common stock options | - | | - | | | 12,867 | | - | | | 12 | | | - | | | - | | | - | | | 12 | |
| Reverse recapitalization transactions, net | - | | - | | | 8,572,314 | | 1 | | | 36,842 | | | (32,915) | | | - | | | - | | | 3,928 | |
| Loss on amendment of forward purchase contract | - | | - | | | - | | - | | | - | | | 15,123 | | | (500) | | | - | | | 14,623 | |
| Net loss | - | | - | | | - | | - | | | - | | | - | | | - | | | (82,954) | | | (82,954) | |
| Balance as of December 31, 2023 | - | | $ | - | | | 21,888,976 | | $ | 2 | | | $ | 121,727 | | | $ | (17,792) | | | $ | (500) | | | $ | (162,130) | | | $ | (58,693) | |
The accompanying notes are an integral part of these
consolidated financial statements.
GRAF ACQUISITION CORP. IV
STATEMENTNKGEN BIOTECH, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE PERIOD FROM JANUARY 28, 2021 (INCEPTION) THROUGH DECEMBER 31, 2021
| | | |
Cash Flows from Operating Activities: | | | |
Net loss | | $ | (990,638) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | |
Change in fair value of derivative warrant liability | | | (5,665,840) |
General and administrative expenses paid by related party in exchange for issuance of common stock | | | 1,000 |
General and administrative expenses paid by related party under promissory note | | | 1,981 |
Offering costs - derivative warrant liability | | | 34,474 |
Income from investments held in Trust Account | | | (41,153) |
Loss upon issuance of private placement warrants | | | 4,154,950 |
Change in operating liabilities: | | | |
Prepaid expenses | | | (812,724) |
Accrued expenses | | | 1,557,213 |
Accounts payable | | | 51,807 |
Franchise tax payable | | | 185,205 |
Net cash used in operating activities | | $ | (1,523,725) |
| | | |
Cash Flows from Investing Activities | | | |
Cash deposited in Trust Account | | | (171,615,000) |
Net cash used in investing activities | | | (171,615,000) |
| | | |
Cash Flows from Financing Activities: | | | |
Proceeds from note payable to related party | | | 500 |
Repayment of note payable to related party | | | (69,809) |
Proceeds received from initial public offering, gross | | | 171,615,000 |
Proceeds received from private placement | | | 7,082,300 |
Offering costs paid | | | (3,766,760) |
Net cash provided by financing activities | | | 174,861,231 |
| | | |
Net change in cash | | | 1,722,506 |
| | | |
Cash - beginning of the period | | | 0 |
Cash - end of the period | | $ | 1,722,506 |
| | | |
Supplemental disclosure of noncash activities: | | | |
Offering costs paid by Sponsor in exchange for issuance of common stock | | $ | 24,000 |
Offering costs included in accrued expenses | | $ | 98,924 |
Offering costs paid by related party under promissory note | | $ | 67,327 |
Deferred underwriting commissions in connection with the initial public offering | | $ | 6,006,525 |
(In thousands)
| | | | | | | | | | | |
| Years ended December 31, |
| 2023 | | 2022 |
| Operating Activities | | | |
| Net loss | $ | (82,954) | | | $ | (26,754) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | |
| Depreciation and amortization | 1,203 | | | 1,210 | |
| Stock-based compensation | 4,135 | | | 69 | |
| Noncash lease expense | 362 | | | 440 | |
| Change in fair value of convertible promissory notes and convertible promissory notes due to related parties | 1,043 | | | 177 | |
| Noncash interest expense (including related party amounts of $431 and $2,271 for the years ended December 31, 2023 and 2022, respectively) | 504 | | | 2,271 | |
| Transaction costs expensed | 3,329 | | | — | |
| Loss on issuance of forward purchase contract | 24,475 | | | — | |
| Loss on amendment of forward purchase contract | 442 | | | — | |
| Change in fair value of forward purchase derivative liability | 9,784 | | | — | |
| Change in the fair value of derivative warrant liability | 13,503 | | | — | |
| Changes in operating assets and liabilities: | | | |
| Accounts receivable | 29 | | | (29) | |
| Prepaid expenses and other current assets | (1,403) | | | 57 | |
| Accounts payable and accrued expenses | 3,993 | | | 443 | |
| Operating lease liabilities | (379) | | | (437) | |
| Other, net | (14) | | | (4) | |
| Net cash used in operating activities | (21,948) | | | (22,557) | |
| Investing activities | | | |
| Purchases of property and equipment | — | | | (101) | |
| Purchases of capitalized software | (48) | | | (62) | |
| Net cash used in investing activities | (48) | | | (163) | |
| Financing activities | | | |
| Proceeds from exercise of common stock options | 12 | | | 161 | |
| Proceeds from related party loans | 5,300 | | | 23,000 | |
| Repayments of related party loans | (300) | | | — | |
| Proceeds from issuance of convertible promissory notes and convertible promissory notes due to related parties | 6,215 | | | — | |
| Proceeds from draws on revolving line of credit | 4,991 | | | — | |
| Proceeds from issuance of common stock | 1,668 | | | — | |
| Proceeds from related party issuance of senior convertible promissory notes with warrants | 10,000 | | | — | |
| Proceeds from issuance of PIPE warrants | 10,210 | | | — | |
| Payment of debt issuance costs on revolving line of credit | (100) | | | — | |
| Repayments on paycheck protection loan | — | | | (675) | |
| Payment of deferred underwriting fee | (1,250) | | | — | |
| Payment of transaction costs | (14,591) | | | — | |
| Net cash provided by financing activities | 22,155 | | | 22,486 | |
| Net increase (decrease) in cash, cash equivalents, and restricted cash | 159 | | | (234) | |
| Cash, cash equivalents, and restricted cash at the beginning of period | 117 | | | 351 | |
| Cash, cash equivalents, and restricted cash at the end of period | $ | 276 | | | $ | 117 | |
| Cash and cash equivalents | 26 | | | 117 | |
| Restricted cash | 250 | | | — | |
| Total cash, cash equivalents, and restricted cash | $ | 276 | | | $ | 117 | |
| Supplemental cash flow information | | | |
| Cash paid for interest expense | $ | 241 | | | $ | 35 | |
| Supplemental disclosure of noncash investing and financing activities | | | |
| Related party loans and interest payable converted into common stock | $ | — | | | $ | 66,139 | |
| Issuance of subscription receivable | $ | 32,915 | | | $ | — | |
| Conversion of legacy convertible promissory notes (including accrued interest) | $ | 18,913 | | | $ | — | |
| Unpaid transaction costs included in accounts payable and accrued expenses | $ | 5,802 | | | $ | — | |
| Assumption of derivative warrant liabilities | $ | 2,046 | | | $ | — | |
| Receivable from shareholders in connection with amendment of forward purchase contracts | $ | 500 | | | $ | — | |
| Property and equipment included in accounts payable and accrued expenses | $ | 73 | | | $ | 8 | |
| Capitalized software costs included in accounts payable and accrued expenses | $ | 15 | | | $ | — | |
The accompanying notes are an integral part of these
consolidated financial statements.
NKGEN BIOTECH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1.Company Information
NKGen Biotech, Inc. (“Company” or “NKGen”), a Delaware corporation headquartered in Santa Ana, California, is a clinical-stage biotechnology company focused on the development and commercialization of Organizationinnovative autologous, allogeneic and Business OperationsCAR-NK natural killer cell therapies utilizing their proprietary SNK (Super-Natural-Killer) platform. The Company is majority owned and controlled by NKMAX Co., Ltd. (“
NKMAX”), a company formed under the laws of the Republic of Korea. The Company was originally Incorporated in Delaware on January 28, 2021 under the name Graf Acquisition Corp. IV (the “Company”(“Graf”), isas a newly organized blank checkspecial-purpose acquisition company incorporated in Delaware and formed for the purpose of effecting into a merger, sharecapital stock exchange, asset acquisition, stock purchase, recapitalization, reorganization or engaging in any other similar business combination with 1one or more businesses or entities (the “Business Combination”).As of December 31, 2021,entities.
On April 14, 2023, the Company had not yet commenced operations. All activity forentered into the period from January 28, 2021 (inception) through December 31, 2021, relates to the Company’s formationAgreement and the initial public offering (the “Initial Public Offering”), which is described below,Plan of Merger by and subsequent to the Initial Public Offering, identifying a target company for a Business Combination. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company will generate non-operating income in the form of interest income on the proceeds derived from the Initial Public Offering.The Company’s sponsor isamong Graf, Acquisition Partners IV LLC,Austria Merger Sub, Inc., a Delaware limited liability companycorporation and wholly owned subsidiary of Graf (“
Merger Sub”), and NKGen Biotech, Inc. (“Merger Agreement”). Upon consummation of the transactions under the Merger Agreement on September 29, 2023 (the “Sponsor”“Business Combination”), Merger Sub merged with and into NKGen Biotech, Inc. (“Legacy NKGen”) with Legacy NKGen surviving the merger as a wholly owned subsidiary of Graf (the “Merger”). The registration statement for the Company’s Initial Public Offering was declared effective on May 20, 2021. On May 25, 2021, the Company consummated its Initial Public Offering of 15,000,000 units (the “Units” and, with respect to the common stock included in the Units being offered, the “Public Shares”), at $10.00 per Unit, generating gross proceeds of $150.0 million, and incurring offering costs of approximately $8.8 million, of which approximately $5.3 million was for deferred underwriting commissions (see Note 6). The Company granted the underwriter a 45-day option to purchase up to an additional 2,250,000 Units at the Initial Public Offering price to cover over-allotments. On June 2, 2021, the underwriters partially exercised the over-allotment option and purchased 2,161,500 additional Units (the “Additional Units”), generating gross proceeds of approximately $21.6 million (the “Over-Allotment”). The Company incurred additional offering costs of approximately $1.2 million inIn connection with the Over-Allotment (of which approximately $757,000 was for deferred underwriting fees).Simultaneously with the closingconsummation of the Initial Public Offering,Business Combination (the “
Closing”), Graf was renamed to “NKGen Biotech, Inc.” and Legacy NKGen changed its name to “NKGen Operating Biotech, Inc.” The Common Stock and warrants of the Company consummatedcombined company began trading on The Nasdaq Stock Market LLC under the private placement (“Private Placement”) of 4,433,333 warrants (each, a “Private Placement Warrant”symbols “NKGN” and collectively,“NKGNW”, respectively, on October 2, 2023. Throughout the “Private Placement Warrants”) at a price of $1.50 per Private Placement Warrantnotes to the Sponsor, generating proceedsconsolidated financial statements, unless otherwise noted or otherwise suggested by context, the “Company” refers to Legacy NKGen prior to the consummation of approximately $6.7 million (see Note 5). Simultaneously with the closing of the Over-Allotment on June 2, 2021, the Company consummated the second closing of the Private Placement, resulting in the purchase of an aggregate of an additional 288,200 Private Placement Warrants at $1.50 per Private Placement Warrant (the “Additional Private Placement Warrants”), generating additional gross proceeds of approximately $432,000.Upon the closing of the Initial Public Offering, Over-Allotment, and Private Placement, $171.6 million ($10.00 per Unit) of the net proceeds of the sale of the Units in the Initial Public Offering, Over-Allotment and of the Private Placement Warrants in the Private Placement were placed in a trust account (“Trust Account”) in the United States maintained by Continental Stock Transfer & Trust Company, as trustee, and will be invested only in U.S. “government securities” within the meaning of Section 2(a)(16) of the Investment Company Act of 1940, as amended, or the Investment Company Act, having a maturity of 185 days or less or in money market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act which invest only in direct U.S. government treasury obligations, until the earlier of: (i) the completion of a Business Combination, and (ii) the distributionCompany after the consummation of the Trust Account as described below.
The Company’s management has broad discretion with respect to the specific application of the net proceeds of its Initial Public Offering and the sale of Private Placement Warrants, although substantially all of the net proceeds are intended to be applied generally toward consummating a Business Combination. The Company’s initial Business Combination must be with one or more operating businesses or assets with a fair market value equal to at least 80% of the net assets held in the Trust Account (net of amounts disbursed to management for working capital purposes, if permitted, and excluding the amount of any deferred underwriting commissions) at the time the Company signs a definitive agreement in connection with the initial Business Combination. However, the Company will only complete a Business Combination if the post-transaction company owns or acquires 50% or more of the outstanding voting securities of the target or otherwise acquires a controlling interest in the target sufficient for it not to be required to register as an investment company under the Investment Company Act.
LiquidityThe Company will provide the holders of Public Shares (the “Public Stockholders”) with the opportunity to redeem all or a portion of their Public Shares upon the completion of a Business Combination either (i) in connection with a stockholder meeting called to approve the Business Combination or (ii) by means of a tender offer. The decision as to whether the Company will seek stockholder approval of a Business Combination or conduct a tender offer will be made by the Company, solely in its discretion. The Public Stockholders will be entitled to redeem their Public Shares for a pro rata portion of the amount then in the Trust Account (initially at $10.00 per share, plus any pro rata interest earned on the funds held in the Trust Account and not previously released to the Company to pay its tax obligations). The per-share amount to be distributed to Public Stockholders who redeem their Public Shares will not be reduced by the deferred underwriting commissions the Company will pay to the underwriters (as discussed in Note 6). These Public Shares were recorded at a redemption value and classified as temporary equity, in accordance with thefollows Financial Accounting Standards Board’sBoard (“FASB”FASB”) Accounting Standards Codification (“ASC”ASC”) Topic 480, “Distinguishing Liabilities from Equity" ("ASC 480"). In such case,205-40, Presentation of Financial Statements — Going Concern, which requires that management evaluate whether there are relevant conditions and events that in aggregate raise substantial doubt about the Company will proceed with a Business Combination if the Company has net tangible assets of at least $5,000,001 upon such consummation of a Business Combination and a majority of the shares voted are voted in favor of the Business Combination. If a stockholder vote is not required by law and the Company does not decideentity’s ability to hold a stockholder vote for business or other legal reasons, the Company will, pursuant to the amended and restated certificate of incorporation which will be adopted by the Company upon the consummation of the Initial Public Offering (the “Amended and restated certificate of incorporation”), conduct the redemptions pursuant to the tender offer rules of the U.S. Securities and Exchange Commission (the “SEC”), and file tender offer documents with the SEC prior to completing a Business Combination. If, however, a stockholder approval of the transactions is required by law, or the Company decides to obtain stockholder approval for business or legal reasons, the Company will offer to redeem shares in conjunction with a proxy solicitation pursuant to the proxy rules and not pursuant to the tender offer rules. Additionally, each Public Stockholder may elect to redeem their Public Shares irrespective of whether they vote for or against the proposed transaction. If the Company seeks stockholder approval in connection with a Business Combination, the holders of the Founder Shares prior to this Initial Public Offering (the “Initial Stockholders”) agreed to vote their Founder Shares (as defined in Note 5) and any Public Shares purchased during or after the Initial Public Offering in favor of a Business Combination. In addition, the Initial Stockholders agreed to waive their redemption rights with respect to their Founder Shares and Public Shares in connection with the completion of a Business Combination. In addition, the Company agreed not to enter into a definitive agreement regarding an initial Business Combination without the prior consent of the Sponsor.
Notwithstanding the foregoing, the Company’s Amended and restated certificate of incorporation provides that a Public Stockholder, together with any affiliate of such stockholder or any other person with whom such stockholder is acting in concert orcontinue as a “group” (as defined under Section 13 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), will be restricted from redeeming its shares with respect to more than an aggregate of 20% or more of the shares of common stock sold in the Initial Public Offering, without the prior consent of the Company.
The Company’s Sponsor, executive officers, and directors have agreed not to propose an amendment to the Company’s Amended and restated certificate of incorporation that would affect the substance or timing of the Company’s obligation to provide for the redemption of its Public Shares in connection with a Business Combination or to redeem 100% of its Public Shares if the Company does not complete a Business Combination, unless the Company provides the Public Stockholders with the opportunity to redeem their shares of common stock in conjunction with any such amendment.
If a Business Combination has not been consummated within 24 months from the closing of the Initial Public Offering, or May 25, 2023 (the “Combination Period”), the Company will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the Public Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account including interest (which interest shall be net of taxes payable and up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding Public Shares, which redemption will completely extinguish Public Stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the remaining stockholders and the board of directors, dissolve and liquidate, subject in each case to the Company’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law.
The Initial Stockholders agreed to waive their liquidation rights with respect to the Founder Shares if the Company fails to complete a Business Combination within the Combination Period. However, if the Initial Stockholders should acquire Public Shares in or after the Initial Public Offering, they will be entitled to liquidating distributions from the Trust Account with respect to such Public Shares if the Company fails to complete a Business Combination within the Combination Period. The underwriters agreed to waive their rights to their deferred underwriting commission (see Note 6) held in the Trust Account in the event the Company does not complete a Business Combination within the Combination Period and, in such event, such amounts will be included with the funds held in the Trust Account that will be available to fund the redemption of the Company’s Public Shares. In the event of such distribution, it is possible that the per share value of the residual assets remaining available for distribution (including Trust Account assets) will be only $10.00 per share initially held in the Trust Account.
The Company will seek to have all third parties (except the Company's independent registered public accounting firm) and any prospective target businesses enter into valid and enforceable agreements with the Company waiving any right, title, interest or claim of any kind they may have in or to any monies held in the Trust Account. Nevertheless, there is no guarantee that vendors, service providers and prospective target businesses will execute such agreements. The Company’s insiders agreed that they will be jointly and severally liable to the Company if and to the extent any claims by a vendor for services rendered or products sold to the Company, or a prospective target business with which the Company has discussed entering into a transaction agreement, reduce the amount of funds in the Trust Account to below $10.00 per Public Share, except as to any claims by a third party who executed an agreement with the Company waiving any right, title, interest or claim of any kind they may have in or to any monies held in the Trust Account and except as to any claims under the Company’s indemnity of the underwriters of the Initial Public Offering against certain liabilities, including liabilities under the Securities Act. However, the Company’s insiders may not be able to satisfy their indemnification obligations. Moreover, the Company’s insiders will not be liable to the Public Stockholders and instead will only have liability to the Company.
Liquidity and Going Concern
As of December 31, 2021, we had approximately $1.7 million in our operating bank account and working capital of approximately $642,000.
Our liquidity needs through December 31, 2021 were satisfied through a payment of $25,000 from the Sponsor to purchase the Founder Shares, the loan of approximately $67,000 from the Sponsor under the Note (as defined in Note 5 to the financial statements), and the proceeds from the consummation of the Private Placement not held in the Trust Account. We repaid the Note in full on May 26, 2021. In addition, in order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, provide us Working Capital Loans (as defined in Note 5 to the financial statements). As of December 31, 2021, there were no amounts outstanding under any Working Capital Loans.
In connection with the Company’s assessment of going concern considerations in accordance with Financial Accounting Standards Board's (“FASB”) accounting Standards Update (“ASU”) 2014-15, “Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” management has determined that the Company may not be ableand to meet its obligations as they become due within one year after the date that the consolidated financial statements are availableissued. Under the guidance, the Company must first evaluate whether there are conditions and events that raise substantial doubt about the entity’s ability to be issued.continue as a going concern (step 1). If the Company concludes substantial doubt is raised, management also is required to consider whether its plans alleviate that doubt (step 2).
The Company has a limited operating history, has incurred significant operating losses since its inception, and the revenue and income potential of the Company’s business and market are unproven. The Company’s consolidated financial statements are prepared using the generally accepted accounting principles applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business. As such, managementof December 31, 2023, the Company had an accumulated deficit of $162.1 million and cash and cash equivalents of less than $0.1 million. To date, the Company has determinedfunded its operations primarily with the net proceeds from the issuance of senior convertible promissory notes, the issuance of related party loans, draws upon a revolving line of credit, the issuance and sale of equity securities, PIPE warrants, private placements and proceeds from the Business Combination. The Company expects to incur substantial operating losses for the next several years and will need to obtain additional near-term financing in order to continue its research and development activities, initiate and complete clinical trials and launch and commercialize any product candidates for which it receives regulatory approval. Management has prepared cash flow forecasts which indicate that based on the liquidity condition raisesCompany’s expected operating losses and negative cash flows, there is substantial doubt about the Company’s ability to continue as a going concern. Asconcern for twelve months from the issuance of December 31, 2021, no adjustments have been madethese consolidated financial statements.
The Company plans to the carrying amounts of assetscontinue to fund its losses from operations and capital funding needs through additional debt or liabilities.
Note 2— Restatement of Previously Reported Balance Sheet
In preparation of the Company’s financial statements for the period from January 28, 2021 (inception) through December 31, 2021, the Company concluded it should restate its previously issued Post-IPO Balance Sheet (as defined below) to classify all redeemable common stock subject to redemption in temporary equity. In accordance with Accounting Standards Codification (“ASC”) 480-10-S99, redemption provisions not solely within the control of the Company, require shares subject to redemptionequity financings to be classified outside of permanent equity. The Company had previously classified a portion of its redeemable common stock in permanent equity.
Although the Company did not specify a maximum redemption threshold, its charter currently provides that the Company will not redeem its Public Shares in an amount that would cause its net tangible assets to be less than $5,000,001. Previously, the Company did not consider redeemable shares classified as temporaryreceived from related parties, private equity, as part of net tangible assets. Effective with these financial statements, the Company restated this interpretation to include temporary equity in net tangible assets.
In accordance with SEC Staff Accounting Bulletin No. 99, “Materiality,” and SEC Staff Accounting Bulletin No. 108, “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements,” the Company evaluated the corrections and has determined that the related impact was material to the previously filed financial statement that contained the error, reported in the Company’s Form 8-K for the audited balance sheet as of May 25, 2021 (“Post-IPO Balance Sheet”). Therefore, the Company, in consultation with its Audit Committee, concluded that the Post-IPO Balance Sheet should be restated to present all redeemable common stock subject to possible redemption as temporary equity and to recognize accretion from the initial book value to redemption value at the time of its Initial Public Offering. As such,or other sources. If the Company is reportingnot able to secure adequate additional funding, the restatementsCompany may be forced to make reductions in spending, extend payment terms with suppliers, liquidate assets where possible, suspend or curtail planned programs, or may be forced to cease operations or file for bankruptcy protection. Any of these actions could materially harm the Company’s business, results of operations and future prospects. There can be no assurance that such financing will be available or will be at terms acceptable to the Post-IPO Balance Sheet in this annual report.Company. The previously presented Post-IPO Balance Sheet should no longer be relied upon.
The restatementpreparation of these consolidated financial statements does not have an impact oninclude any adjustments that may result from the Company’s cash position and balanceoutcome of the Trust Account.
The impact of the restatement to the Post-IPO Balance Sheet is the reclassification of 1,771,784 redeemable common stock from permanent equity to temporary equity. The table below presents the financial statement adjustment related to the restatement discussed above of the Company’s previously reported balance sheet as of May 25, 2021:
this uncertainty.
2. | | | | | | | | | |
| | As of May 25, 2021 |
| | As Previously | | | | | | |
| | Reported | | Adjustments | | As Restated |
| | | | | | | | | |
Total assets | | $ | 153,674,699 | | $ | — | | $ | 153,674,699 |
Total liabilities | | $ | 16,392,534 | | $ | — | | $ | 16,392,534 |
Common stock subject to possible redemption | | | 132,282,160 | | | 17,717,840 | | | 150,000,000 |
Stockholders’ equity (deficit): | | | | | | | | | |
Preferred stock | | | — | | | — | | | — |
Common stock | | | 608 | | | (177) | | | 431 |
Additional paid-in-capital | | | 9,001,994 | | | (9,001,994) | | | — |
Accumulated deficit | | | (4,002,597) | | | (8,715,669) | | | (12,718,266) |
Total stockholders' equity (deficit) | | | 5,000,005 | | | (17,717,840) | | | (12,717,835) |
Total Liabilities, Common Stock Subject to Possible Redemption and Stockholders' Equity (Deficit) | | $ | 153,674,699 | | $ | — | | $ | 153,674,699 |
Note 3—Basis of Presentation and Summary of Significant Accounting Policies
The accompanying financial statements are presentedhave been prepared in U.S. dollars in conformityaccordance with the rules and regulations of the Securities and Exchange Commission (“SEC”) and generally accepted accounting principles generally accepted in the United States of America (“US GAAP”). The Company maintains its accounting records under the accrual method of accounting in conformity with US GAAP.
Business Combination
NKMAX held a majority of the voting power of Legacy NKGen before the Business Combination and pursuantcontinued to hold a majority of the voting power of the Company after the Business Combination. Therefore, as there was no change in control, the Business Combination was accounted for as a common control transaction with respect to Legacy NKGen along with a reverse recapitalization of the Company. Accordingly, for accounting purposes, the financial statements of the Company represent a continuation of the financial statements of Legacy NKGen with the Business Combination being treated as the equivalent of Legacy NKGen issuing shares for the net assets of Graf, accompanied by a recapitalization. The net assets of Graf were recognized as of the Closing at historical cost, with no goodwill or other intangible assets recorded. Operations prior to the rulesBusiness Combination are presented as those of Legacy NKGen and regulationsthe accumulated deficit of Legacy NKGen has been carried forward after Closing.
Upon the consummation of the Business Combination, all of Legacy NKGen’s equity was converted into equity of the Company based upon an exchange ratio (“Exchange Ratio”). In addition, all stock options of Legacy NKGen were converted using the Exchange Ratio into options exercisable for shares of the Company with the same terms and vesting conditions. The Exchange Ratio as of September 29, 2023, the date of Closing, was approximately 0.408.
All periods prior to the Business Combination have been retrospectively adjusted using the Exchange Ratio to reflect the reverse recapitalization. In connection with the reverse recapitalization treatment of the Business Combination, all issued and outstanding securities of Graf upon Closing were treated as issuances of the Company upon the consummation of the Business Combination.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its wholly owned subsidiary. All significant intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in accordance with US GAAP requires management to make estimates and assumptions that impact the reported amounts of certain assets and liabilities, certain disclosures at the date of the consolidated financial statements, as well as the reported amounts of revenues and expenses during the reporting period. The most significant estimates in the Company’s consolidated financial statements include, but are not limited to, accrued clinical and research and development expenses, legacy convertible promissory notes, senior convertible promissory notes due to related parties, forward purchase derivative liabilities, derivative warrant liabilities, common stock, and equity awards. These estimates and assumptions are based upon historical experience, knowledge of current events, and various other factors believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of expenses that are not readily apparent from other sources. Actual results could differ materially from those estimates.
Segments
Operating segments are defined as components of an entity for which separate financial information is available and that is regularly reviewed by the Chief Operating Decision Maker (“CODM”) in deciding how to allocate resources to an individual segment and in assessing performance. The Company’s Chief Executive Officer is the Company’s CODM. The CODM reviews financial information presented on an enterprise-wide basis for purposes of making operating decisions, allocating resources, and evaluating financial performance. As such, the Company has determined that it operates in one reportable segment. Additionally, the Company generates all of its revenues, and maintains all of its long-lived assets within the United States SecuritiesStates.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less when purchased to be cash equivalents. The carrying amounts reported in the balance sheets for cash and cash equivalents are valued at cost, which approximate their fair value. The Company has not experienced any losses in such accounts and management believes the Company has no highly liquid investments exposed to credit risk.
Restricted Cash
Restricted cash consists of funds that are contractually restricted due to a revolving line of credit, which was entered into during June 2023. In accordance with the terms of the revolving line of credit, the Company was required to maintain $15.0 million in cash balances with the lender from March 31, 2024 until repayment of all principals and other payables to the lender under the revolving line of credit was made as additional collateral for the borrowings. In April 2024, the lender subsequently waived the minimum cash deposit requirement in exchange for the Company's agreement to use the lender as their primary banking relationship. As of December 31, 2023, $0.3 million in restricted cash was recorded on the consolidated balance sheet. No restricted cash balances were recorded as of December 31, 2022. The Company includes its restricted bank deposits in cash, cash equivalents and restricted cash when reconciling beginning-of-period and end-of-period total amounts shown on the consolidated statements of cash flows for the years ended December 31, 2023 and 2022.
Concentrations of Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist of cash and cash equivalents. The Company minimizes the amount of credit exposure by investing cash that is not required for immediate operating needs in money market funds, government obligations, and/or commercial paper with short maturities. To date, the Company has not experienced any losses associated with this credit risk and continues to believe this exposure is not significant. Cash deposits are insured by the Federal Deposit Insurance Corporations (“FDIC”) up to $250,000. From time to time, the Company may have cash deposits in excess of the FDIC insured limit.
For each of the years ended December 31, 2023 and 2022, no customer accounted for over 10% of total revenue and the Company had no trade accounts receivables outstanding. For each of the years ended December 31, 2023 and 2022, the Company had zero and less than $0.1 million in other receivables, respectively.
Property and Equipment, net
Property and equipment are recorded at cost and depreciated over their estimated useful lives using the straight-line method. Leasehold improvements are depreciated over the shorter of their estimated useful lives or the term of the lease by use of the straight-line method. Repairs and maintenance costs are charged to expense as incurred. When assets are retired or sold, the assets and accumulated depreciation are removed from the respective amounts and any gain or loss is recognized, as applicable, in the accompanying consolidated statements of operations and comprehensive loss.
Capitalized Software, net
Expenditures related to internal use software are capitalized. Such expenditures are amortized over their period of benefit, which are generally three-year period, using the straight-line method.
Transaction Costs
The Company capitalizes deferred transaction costs, which primarily consist of incremental legal fees, accounting fees and other costs directly attributable to anticipated capital-raising transactions. The deferred transaction costs are reclassified upon the occurrence of the associated capital-raising transactions. All deferred transaction costs during the year ended December 31, 2023 were reclassified upon Closing of the Business Combination. No deferred transaction costs were recorded as of December 31, 2022.
Transaction costs not specific to a single instrument are allocated on a relative fair value basis. Transaction costs allocated to equity-classified instruments are recorded to additional paid in capital. Transaction costs allocated to liability-classified instruments with recurring fair value measurements are recorded as transaction costs expenses in the consolidated statements of operations and comprehensive loss.
Deferred Debt Issuance Costs
Costs incurred through the issuance of the revolving line of credit to parties who are providing short-term financing availability are reflected as deferred debt issuance costs. These costs are generally amortized to interest expense over the life of the financing instrument using the effective interest rate method or other methods approximating the effective interest method. As of December 31, 2023, less than $0.1 million in deferred debt issuance costs were recorded to prepaid expenses and other current assets on the consolidated balance sheets. No deferred debt issuance costs were recorded as of December 31, 2022.
Hybrid Instruments
The Company follows Financial Accounting Standards Board (“FASB”) Accounting Standard Codification (“ASC”) 480, Distinguishing Liabilities from Equity, when evaluating the accounting for its hybrid instruments. A financial instrument that embodies an unconditional obligation, or a financial instrument other than an outstanding share that embodies a conditional obligation, that the issuer must or may settle by issuing a variable number of its equity shares shall be classified as a liability (or an asset in some circumstances) if, at inception, the monetary value of the obligation is based solely or predominantly on any one of the following: (a) a fixed monetary amount known at inception; (b) variations in something other than the fair value of the issuer’s equity shares; or (c) variations inversely related to changes in the fair value of the issuer’s equity shares. Hybrid instruments meeting these criteria are not further evaluated for any embedded derivatives and are carried as a liability at fair value at each balance sheet date.
Derivative Instruments
FASB ASC 815, Derivatives and Hedging Activities, requires companies to bifurcate certain features from their host instruments and account for them as free-standing derivative financial instruments should certain criteria be met. The Company does not use derivative instruments to hedge exposures to interest rate, market, or foreign currency risks. The Company evaluates its financial instruments to determine whether such instruments are derivatives or contain features that qualify as embedded derivatives. Embedded derivatives must be separately measured from the host contract if all the requirements for bifurcation are met. The assessment of the conditions surrounding the bifurcation of embedded derivatives depends on the nature of the host contract and the features of the derivatives. Bifurcated embedded derivatives are recognized at fair value, with changes in fair value recognized in the consolidated statements of operations and comprehensive loss each period. Bifurcated embedded derivatives are classified with the related host contract in the Company’s consolidated balance sheet. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period.
Debt
For convertible debt instruments that are not considered liabilities under ASC 480 or ASC 815, the Company applies ASC 470, Debt, for the accounting of such instruments, including any premiums or discounts. The Company’s senior convertible promissory notes are accounted for under ASC 470. Accrued interest paid-in-kind is added to the carrying amount of the Company’s senior convertible promissory notes.
Subscription and Shareholder Receivables
The Company records stock issuances at the effective date. If the amounts are not funded upon issuance, the Company records a subscription receivable or shareholder receivable as an asset on the balance sheet. When subscription receivables or shareholder receivables are not received prior to the balance sheet date in satisfaction of the requirements under ASC 505, Equity, the subscription or shareholder receivable is reclassified as a contra account to stockholder’s equity (deficit) on the balance sheet.
Shareholder receivables represent amounts due from shareholders. If the shareholder does not fund the receivable prior to the balance sheet date, the Company records a receivable that is reclassified as a contra account to stockholder’s equity (deficit) on the balance sheet.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate the carrying amount of an asset, or asset group, may not be recoverable. Recoverability is measured by a comparison of the carrying amount of an asset or asset group to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset or asset group exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset or asset group exceeds the fair value of the asset or asset group. The Company has not recognized any impairment losses for the years ended December 31, 2023 and 2022.
Fair Value Option
In lieu of bifurcation, on an instrument-by-instrument basis, the Company may elect the fair value option for certain financial instruments that meet the required criteria under ASC 825, Financial Instruments. The Company elected the fair value option for its legacy convertible promissory notes, which met the required criteria under ASC 825, Financial Instruments. Interest expense associated with the legacy convertible promissory notes is included in the change in fair value of such instruments.
Fair Value of Financial Instruments
The Company follows ASC 820-10, Fair Value Measurements and Disclosures, issued by the FASB with respect to fair value reporting for financial assets and liabilities. The guidance defines fair value, provides guidance for measuring fair value and requires certain disclosures. The guidance does not apply to measurements related to share-based payments. The guidance discusses valuation techniques such as the market approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to replace the service capacity of an asset or replacement cost). The guidance establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels.
The Company’s financial instruments include cash and cash equivalents, prepaid expenses and other current assets, capitalized software, accounts payable, accrued expenses, convertible promissory notes issued from 2019 through 2022 to investors (“2019 Convertible Notes”), convertible promissory notes due to related parties (“Related Party Convertible Notes”, together with the 2019 Convertible Notes, “Convertible Notes”), debt due to a related party (“Related Party Loans”), and other current liabilities. The carrying amount of cash and cash equivalents, prepaid expenses and other current assets, capitalized software, accounts payable, accrued expenses, revolving line of credit, and related party loans, and other current liabilities are generally considered to be representative of their respective values because of the short-term nature of those instruments.
The Company elects to account for its 2019 Convertible Notes and Related Party Convertible Notes, which meet the required criteria, at fair value at inception and at each subsequent reporting date. Subsequent changes in fair value of the Convertible Notes are recorded within other expenses, net on the accompanying consolidated statements of operations and comprehensive loss. Interest expense associated with the Convertible Notes is included in the change in fair value for the Convertible Notes. These estimates may be subjective in nature and involve uncertainties and matters of significant judgment and therefore cannot be determined with precision. The carrying value of the Company’s Related Party Loans approximates fair value as the stated interest rate approximates market rates for similar loans and due to the short-term nature of such loans.
ASC 820, Fair Value Measurement, states that in many cases, the transaction price will equal the fair value (for example, that might be the case when on the transaction date, the transaction to buy an asset takes place in the market in which the asset is sold). In determining whether a transaction price represents the fair value at initial recognition, the Company considers various factors such as whether the transaction was between related parties, is a forced transaction, or whether the unit of account for the transaction price does not represent the unit of account for the measured instrument. The Company does not measure assets at fair value on a recurring basis. Refer to Note 9, Fair Value of Financial Instruments, for further discussion regarding the Company’s fair value measurements. The carrying value of the Company’s related party loans approximates fair value as the stated interest rate approximates market rates for similar loans and due to the short-term nature of such loans, which are due within one year from December 31, 2023.
Employee Benefit Plan
Effective January 1, 2019, the Company adopted and maintains a defined contribution plan, which qualifies under Section 401(k) of the Internal Revenue Code, on behalf of its eligible employees. Upon consummation of the Business Combination, the Company adopted the 2023 equity incentive plan (“2023 Plan”), at which date NKGen determined it will no longer grant any additional awards under the 2019 Plan. Employee contributions are voluntary and are determined on an individual basis, limited to the maximum amount allowable under federal tax regulations. During the years ended December 31, 2023 and 2022, the Company did not contribute to either plan.
Revenue Recognition
Historically, the Company recognized revenue in connection with Coronavirus Disease of 2019 (“COVID-19”) testing services. During the first quarter of the year ended December 31, 2023, the Company ceased providing COVID-19 testing services.
The Company recognizes revenue in accordance with ASC 606, Revenue from Contracts with Customers, which applies to all contracts with customers, except for contracts within the scope of other standards, such as leases, insurance, collaboration arrangements, and financial instrument. Under ASC 606, revenue is recognized in a manner that depicts the transfer of control of a product or a service to a customer and reflects the amount of the consideration the Company is entitled to receive in exchange for such product or service. In doing so, the Company follows a five-step approach: (i) identify the contract with a customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations, and (v) recognize revenue when (or as) the customer obtains control of the product or service. The Company considers the terms of a contract and all relevant facts and circumstances when applying the revenue recognition standard. The Company applies the revenue recognition standard, including the use of any practical expedients, consistently to contracts with similar characteristics and in similar circumstances.
The transaction price is the amount of consideration the Company is entitled to receive in exchange for the transfer of control of a product or a service to a customer. To determine the transaction price, the Company considers the existence of any significant financing component, the effects of any variable elements, noncash considerations and consideration payable to the customer. If a significant financing component exists, the transaction price is adjusted for the time value of money. If an element of variability exists, the Company must estimate the consideration it expects to receive and uses that amount as the basis for recognizing revenue as the product or the service is transferred to the customer.
If a contract has multiple performance obligations, the Company allocates the transaction price to each distinct performance obligation in an amount that reflects the consideration the Company is entitledto receive in exchange for satisfying each distinct performance obligation. For each distinct performance obligation, revenue is recognized when (or as) the Company transfers control of the product or the service applicable to such performance obligation.
In those instances where the Company first receives consideration in advance of satisfying its performance obligation, the Company classifies such consideration as contract liability until (or as) the Company satisfies such performance obligation. In those instances where the Company first satisfies its performance obligation prior to its receipt of consideration, the consideration is recorded as accounts receivable.
The Company expenses incremental costs of obtaining and fulfilling a contract as and when incurred if the expected amortization period of the asset that would be recognized is one year or less, or if the amount of the asset is immaterial. Otherwise, such costs are capitalized as contract assets if they are incremental to the contract and amortized to expense proportionate to revenue recognition of the underlying contract.
Collaboration Agreement
The Company has entered into a research agreement that falls under the scope of ASC 808, Collaborative Arrangements. Reimbursements from a collaboration partner are recorded as a reduction to research and development expense in the consolidated statements of operations and comprehensive loss. Similarly, amounts that are owed to a collaboration partner are recognized as research and development expense in the consolidated statements of operations and comprehensive loss.
Research and Development Expenses
All research and development costs are expensed in the period incurred. Research and development expenses primarily consist of services provided by contract organizations for clinical development, salaries and related expenses for personnel, including stock-based compensation expense, outside service providers, facilities costs, fees paid to consultants and other professional services, license fees, depreciation and supplies used in research and development. Payments made prior to the receipt of goods or services to be used in research and development are capitalized until the related goods or services are received. Costs are accrued for research performed over the service periods specified in the contracts and estimates are adjusted, if required, based upon an ongoing review of the level of effort and costs actually incurred.
Leases
The Company accounts for its leases under ASC 842, Leases. Operating lease right-of-use (“ROU”) assets represent the Company’s right to use an underlying asset during the lease term, and operating lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating leases are included in ROU assets, current operating lease liabilities, and non-current operating lease liabilities in the accompanying balance sheets. Operating lease ROU assets and lease liabilities are initially recognized based on the present value of the future minimum lease payments over the lease term at commencement date calculated using the Company’s incremental borrowing rate applicable to the lease asset, unless the implicit rate is readily determinable. Operating lease ROU assets also include any lease payments made at or before lease commencement and exclude any lease incentives received. The Company determines the lease term as the noncancelable period of the lease and may include options to extend or terminate the lease when it is reasonably certain the Company will exercise that option. Leases with a term of 12 months or less are not recognized in the balance sheet. The Company’s leases do not contain any residual value guarantees. Lease expense for minimum lease payments is recognized as rent expense on a straight-line basis over the lease term. Variable lease payments include lease operating expenses.
Stock-Based Compensation
Stock-based compensation expense is comprised of stock options awarded to employees and consultants. The Company accounts for share-based awards under the fair value method prescribed by ASC 718-10, Stock Compensation. The fair value of stock options is estimated using the Black-Scholes option pricing model on the date of grant. This option pricing model involves a number of estimates, including the per share value of the underlying common stock, exercise price, estimate of future volatility, expected term of the stock option award, risk-free interest rate and expected annual dividend yield.
The fair value of the shares of common stock underlying the stock options has historically been determined by the Company’s board of directors as there was no public market for the underlying common stock prior to October 2, 2023. The Company’s board of directors determines the fair value of the Company’s common stock by considering a number of objective and subjective factors including contemporaneous third-party valuations of its common stock, the valuation of comparable companies, sales of the Company’s common stock to outside investors in arms-length transactions, the Company’s operating and financial performance, the lack of marketability, and general and industry specific economic outlook, and the implied fair values upon a merger transaction, amongst other factors.
The Company recognizes the expense for options with graded-vesting schedules on a straight-line basis over the requisite service period, which is generally the vesting period. Forfeitures are recognized as they occur.
Income Taxes
The Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined on the basis of the differences between the financial statements and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
The Company recognizes net deferred tax assets to the extent the Company believes these assets are more likely than not to be realized. In making such a determination, management considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and results of recent operations. If management determines the Company would be able to realize its deferred tax assets in the future in excess of their net recorded amount, management would make an adjustment to the deferred tax asset valuation allowance, which would reduce the provision for income taxes.
The Company records uncertain tax positions on the basis of a two-step process whereby (1) management determines whether it is more likely than not the tax positions will be sustained on the basis of the technical merits of the position and (2) for those tax positions that meet the more-likely-than-not recognition threshold, management recognizes the largest amount of tax benefit that is more than 50% likely to be realized upon ultimate settlement with the related tax authority. The Company recognizes interest and penalties related to unrecognized tax benefits within income tax expense. Any accrued interest and penalties are included within the related tax liability. No tax liability, interest and penalties have been recognized in the consolidated financial statements attributed to uncertain tax positions.
Basic and Diluted Net Loss Per Common Share
Basic net loss per common share is computed by dividing net loss for the year by the weighted-average number of common shares outstanding during the year. Diluted net loss per share is computed by dividing the net loss by the weighted-average number of common shares outstanding and potentially dilutive securities outstanding for the period using the treasury stock or if-converted method if their inclusion is dilutive. Diluted net loss per common share is the same as basic net loss per common share because the inclusion of potentially dilutive shares would be anti-dilutive to the calculation of loss and comprehensive loss per common share.
The Company has one class of shares Issued and outstanding. Accordingly, basic and diluted net loss per share is not allocated among multiple classes of shares. Basic and diluted net loss per share for all periods prior to the Closing have been retrospectively adjusted by the Exchange
Commission (“SEC”).Ratio to affect the reverse recapitalization.
Potentially anti-dilutive shares excluded from the calculation of diluted net loss per share for the year ended December 31, 2023 include the following:
| | | | | |
| Private warrants | 4,721,533 |
| Working capital warrants | 523,140 |
| Public warrants | 3,432,286 |
| PIPE warrants | 10,209,994 |
| Stock options | 2,078,986 |
| SPA warrants | 1,000,000 |
| Senior convertible notes’ shares | 1,000,000 |
Deferred founder shares (1) | 1,173,631 |
| Total | 24,139,570 |
(1)As described in Note 8, Related Party Transactions, deferred founder shares do not have voting rights, do not participate in dividends and are not transferrable absent the Company’s consent. Therefore, while deferred founder shares are considered outstanding for legal purposes and are included in the total quantity of outstanding shares on the consolidated statements of stockholders’ equity (deficit), they are not considered outstanding for accounting purposes, including basic and diluted net loss per share purposes.
Potentially anti-dilutive shares excluded from the calculation of diluted net loss per share for the year ended December 31, 2022 includes stock options of 185,231 (after giving effect to the Exchange Ratio), in addition to the shares underlying the Legacy Convertible Notes. The Company is unable to quantify the number of shares underlying the legacy convertible notes for the year ended December 31, 2022 as the quantity of shares issuable upon conversion was not determinable for the period.
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Ourour Business Startups Act of 2012, (the “JOBS Act”“JOBS Act”), and it may take advantage of certain exemptions from various
reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the independent registered public accounting firmauditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholdershareholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that
an emerging growtha company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such
an election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s
consolidated financial statements with another public company
thatwhich is neither an emerging growth company nor an emerging growth company
thatwhich has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Recently Adopted Accounting Pronouncements
UseIn June 2016, the Financial Accounting Standard Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-13, Measurement of EstimatesCredit Losses on Financial Instruments. ASU 2016-13, together with a series of subsequently issued related ASUs, has been codified in Topic 326. Topic 326 establishes new requirements for companies to estimate expected credit losses when measuring certain financial assets, including accounts receivables. The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of income and expenses during the reporting period.
Making estimates requires management to exercise significant judgment. Itnew guidance is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ significantly from those estimates.
Cash and Cash Equivalents
effective for fiscal years beginning after December 15, 2022. The Company considers all short-term investmentsadopted the new guidance with an original maturityits fiscal year beginning January 1, 2023. The adoption of three months or less when purchased to be cash equivalents. The CompanyASC 326 had 0 cash equivalents as of December 31, 2021.
Investments Held in the Trust Account
The Company’s portfolio of investments is comprised of U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less, or investments in money market funds that invest in U.S. government securities and generally have a readily determinable fair value, or a combination thereof. Whenno material impact on the Company’s investments held in the Trust Account are comprised of U.S. government securities, the investments are classified as trading securities. Gains and losses resulting from the change in fair value of these securities is included in income on investments held in the Trust Account in the accompanying statement of operations. The estimated fair values of investments held in the Trust Account are determined using available market information.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash accounts in a financial institution, which, at times, may exceed the Federal Depository Insurance Corporation coverage limit of $250,000. As of December 31,
statements.2021, the Company had not experienced losses on these accounts and management believes the Company is not exposed to significant risks on such accounts.
Fair Value of Financial Instruments
The fair value of the Company’s assets and liabilities which qualify as financial instruments under the FASB Topic ASC 820, “Fair Value Measurements,” equal or approximate the carrying amounts represented in the balance sheet, primarily due to their short-term nature, other than the derivative warrant liabilities.
Fair Value Measurements
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers consist of:
| ● | Level 1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; |
| ● | Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
| ● | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement.
Derivative Warrant Liability
The Company does not use derivative instruments to hedge exposures to cash flow, market, or foreign currency risks. The Company will evaluate its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with FASB ASC Topic 815, “Derivatives and Hedging” (“ASC 815”). The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period.
The Private Placement Warrants are recognized as derivative liabilities in accordance with ASC 815. Accordingly, the Company recognizes the warrant instruments as liabilities at fair value and adjusts the carrying value of the instruments to fair value at each reporting period until they are exercised. The fair value of the Private Placement Warrants as of December 31, 2021 is determined using Black-Scholes option pricing model. The determination of the fair value of the warrant liability may be subject to change as more current information becomes available and accordingly the actual results could differ significantly. Derivative warrant liabilities are classified as non-current liabilities as their liquidation is not reasonably expected to require the use of current assets or require the creation of current liabilities.
Offering Costs Associated with the Initial Public Offering
Deferred offering costs consist of legal, underwriting fees, accounting, and other costs incurred through the balance sheet date that were directly related to the Initial Public Offering. If the Initial Public Offering proved to be unsuccessful, these deferred costs, as well as additional expenses incurred, would be charged to operations. Offering costs are allocated to the separable financial instruments issued in the Initial Public Offering based on a relative fair value basis, compared to total proceeds received. Offering costs associated with derivative warrant liability will be expensed as incurred, presented as non-operating expenses in the statement of operations. Offering costs associated with the Public Shares issued were charged to stockholders’ equity upon the completion of the Initial Public Offering.
Common Stock Subject to Possible Redemption
The Company accounts for its common stock subject to possible redemption in accordance with the guidance in ASC 480. Common stock subject to mandatory redemption (if any) are classified as liability instruments and are measured at fair value. Conditionally redeemable common stock (including shares of common stock that feature redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) are classified as temporary equity. At all other times, common stock is classified as stockholders’ equity. The Company’s common stock features certain redemption rights that are considered to be outside of the Company’s control and subject to occurrence of uncertain future events. Accordingly, as of Initial Public Offering (including exercise of the over-allotment option), 17,161,500 shares of common stock subject to possible redemption at the redemption amount were presented at redemption value as temporary equity, outside of the stockholders’ deficit section of the Company’s balance sheet.
Effective with the closing of the Initial Public Offering (including exercise of the over-allotment option), the Company recognized the accretion from initial book value to redemption amount, which resulted in charges against additional paid-in capital (to the extent available) and accumulated deficit.
Income Taxes
The Company follows the asset and liability method of accounting for income taxes under FASB ASC Topic 740, “Income Taxes” (“ASC 740”). Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that included the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. As of December 31, 2021, the Company had full valuation allowance against the deferred tax assets.
ASC 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. There were 0 unrecognized tax benefits as of December 31, 2021. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. NaN amounts were accrued for the payment of interest and penalties as of December 31, 2021. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
Net Income (Loss) Per Share of Common Stock
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share.” Net income (loss) per common stock is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the period.
The calculation of diluted net income (loss) per common stock does not consider the effect of the warrants issued in connection with the Initial Public Offering (including exercise of the over-allotment option) and the Private Placement to purchase an aggregate of 8,153,833 shares of common stock in the calculation of diluted income (loss) per share, because their exercise is contingent upon future events and their inclusion would be anti-dilutive under the treasury stock method. As a result, diluted net income (loss) per share is the same as basic net income (loss) per share for the three months ended December 31, 2021 and for the period from January 28, 2021 (inception) through December 31, 2021. Accretion associated with the redeemable common stock is excluded from earnings per share as the redemption value approximates fair value.
Recent Accounting Pronouncements
In August 2020, the FASB issued Accounting Standard Update (“ASU”) No.ASU 2020-06, Debt—Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. The ASU alsoaccounting principles and removes certain settlement conditions that are required for equity-linkedequity contracts to qualify for the derivative scope exception, and it simplifiesexception. ASU 2020-06 will be effective for the diluted earnings per share calculation in certain areas.Company for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years, with early adoption permitted. The Company adopted the new guidance during the fiscal year ended December 31, 2023. The adoption of ASU 2020-06 on January 28, 2021 using a modified retrospective method for transition. Adoption of the ASU did nothad no material impact the Company’s financial position, results of operations or cash flows.
Management does not believe that any other recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material effect on the Company’s financial statements.
F-13
Recently Issued Accounting Pronouncements Not Yet AdoptedIn December 2023, the FASB issued ASU No. 2023-09, Improvements to Income Tax Disclosures (Topic 740). The ASU requires disaggregated information about a reporting entity’s effective tax rate reconciliation as well as additional information on income taxes paid. The ASU is effective on a prospective basis for annual periods beginning after December 15, 2024. Early adoption is also permitted for annual financial statements that have not yet been issued or made available for issuance. This ASU will result in the required additional disclosures being included in our consolidated financial statements, once adopted.The Company is currently evaluating the impact of this standard on its financial statements and related disclosures.
3.Reverse Recapitalization
As discussed in Note 4—Initial Public OfferingOn May 25, 2021,1,
Company Information, the Company consummated its Initial Public OfferingClosing of 15,000,000 Units, at $10.00 per Unit, generating gross proceedsthe Business Combination occurred on September 29, 2023. In connection with the Business Combination: •All of $150.0 million,Legacy NKGen’s legacy convertible notes were converted into shares of Legacy NKGen common stock immediately prior to Closing and incurring offering costspursuant to their terms, totaling 5,579,266 shares, which were then cancelled and converted into 2,278,598 shares of approximately $8.8 million,the Company’s common stock after giving effect to the Exchange Ratio;
•All of which approximately $5.3 million was for deferred underwriting commissions. Legacy NKGen’s 38,185,814 issued and outstanding shares were cancelled and converted into 15,595,262 shares of the Company’s common stock after giving effect to the Exchange Ratio (inclusive of shares attributable to the Legacy NKGen convertible notes);
•All of Legacy NKGen’s 5,146,354 issued and outstanding stock options were cancelled and converted into 2,101,760 outstanding stock options of the Company;
•The Company’s amended and restated certificate of incorporation and amended and restated bylaws were adopted;
•The Company granted the underwriters a 45-day option toadopted an employee stock purchase up to 2,250,000 additional Units at the Initial Public Offering price less the underwriting discountsplan; and commissions. On June 2, 2021, the underwriters partially exercised the over-allotment option and purchased 2,161,500 additional Units (the “Additional Units”), generating gross proceeds of approximately $21.6 million (the “Over-Allotment”).
•The Company incurred additional offering costs of approximately $1.2 millionadopted the 2023 equity incentive plan.
The other related events that occurred in connection with the Over-Allotment (ofClosing include the following:
•The execution of the private placement agreements, as described in Note 4, Private Placement;
•The assumption of the public and private warrants, as described in Note 5, Warrants;
•The execution of the warrant subscription agreements, as described in Note 5, Warrants;
•The conversion of Legacy NKGen’s legacy convertible promissory notes, as described in Note 6, Convertible Notes;
•The execution of the securities purchase agreement, as described in Note 6, Convertible Notes; and
•The execution of the amended and restated sponsor support and lockup agreement, as described in Note 8, Related Party Transactions.
Refer to Note 9, Fair Value of Financial Instruments, for the Company’s measurements with respect to the financial instruments issued in connection with the foregoing agreements.
Legacy NKGen incurred $7.5 million of transaction costs in connection with the Business Combination, which approximately $0.8was determined to be a capital-raising transaction for Legacy NKGen. Of the $7.5 million in transaction costs, $4.2 million and $3.3 million was allocated on a relative fair value basis to equity-classified instruments and liability-classified instruments, respectively.
The following tables reconcile elements of the Business Combination to the Company’s consolidated financial statements, and should be read in conjunction with the footnotes referenced above (in thousands, except share amounts):
| | | | | |
| Shares |
| Graf public shares, net of redemptions | 93,962 |
| Private placement investors’ shares | 3,683,010 |
| Graf founder shares | 2,516,744 |
| Total Graf shares outstanding immediately prior to the Business Combination | 6,293,716 |
| Conversion of Legacy NKGen convertible promissory notes (after the application of the Exchange Ratio) | 2,278,598 |
| Legacy NKGen rollover shares (after the application of the Exchange Ratio) | 13,316,662 |
| Total Legacy NKGen shares | 15,595,260 |
| Total Company common stock outstanding immediately following the Business Combination | 21,888,976 |
| | | | | |
| (In thousands) | Recapitalization |
| Closing proceeds | |
| Proceeds from issuance of common stock | $ | 1,667 | |
| Proceeds from issuance of PIPE warrants | 10,210 | |
| Proceeds from issuance of senior convertible promissory notes with warrants | 10,000 | |
| Closing disbursements | |
| Less: Payment of Graf deferred underwriter fees | (1,250) | |
Less: Payment of Graf transaction costs at Closing (1) | (7,456) | |
| Less: Payment of Legacy NKGen transaction costs at Closing | (3,510) | |
| Net cash proceeds from the Business Combination at Closing | $ | 9,661 | |
| Less: Payment of Legacy NKGen transaction costs prior to Closing | (2,089) | |
| Net cash proceeds from the Business Combination | $ | 7,572 | |
| Noncash activity | |
| Conversion of legacy NKGen convertible promissory notes | 18,913 | |
| Less: Operating liabilities assumed from Graf | (860) | |
Less: Unpaid transaction costs – assumed from Graf (1) | (5,400) | |
| Less: Unpaid transaction costs – Legacy NKGen | (1,938) | |
| Liability-classified instruments | |
| Less: Fair value of PIPE warrants | (10,210) | |
| Less: Fair value of forward purchase derivative liability | (20,201) | |
Less: Fair value of senior convertible promissory notes(2) | (9,707) | |
| Less: Fair value of private warrants | (1,841) | |
| Less: Fair value of working capital warrants | (204) | |
| Net equity impact of the Business Combination | $ | (23,876) | |
(1)The Graf transaction costs includes a $4.0 million accrual related to a certain vendor to be paid in cash and common stock of $2.0 million each. At Closing, a cash payment of $1.3 million was for deferred underwriting fees).Each Unit consists of one share of common stock, and one-fifth of 1 redeemable warrant (“Public Warrant”). Each whole Warrant entitles the holderdisbursed to purchase 1 share of common stock atthis vendor. The remaining $2.7 million amount was recognized as a price of $11.50 per share, subject to adjustment (see Note 8).
Note 5—Related Party Transactions
Founder Shares
On February 13, 2021, Graf Acquisition Partners LLC (‘‘Graf LLC’’) paid an aggregate of $25,000 for certain offering costs on behalfcomponent of the unpaid transaction costs assumed from Graf, of which $0.7 million represents a cash settlement obligation, and the remaining $2.0 million represents an obligation to issue a variable number of shares for a fixed monetary amount which was accounted for as a liability under ASC 480,
Distinguishing Liabilities from Equity (“ASC 480”). Under ASC 480, the obligation to issue shares is not subsequently measured at fair value with changes recorded in earnings because the monetary amount is fixed. (2)Represents allocated fair value.
As presented in the consolidated statements of stockholders’ equity (deficit) (in thousands):
| | | | | |
| Net equity impact of the Business Combination | $ | (23,876) | |
| Loss on issuance of forward purchase contract | 24,475 | |
| Transaction costs expensed | 3,329 | |
Total Impact of Business Combination on total stockholders’ deficit (1) | $ | 3,928 | |
| Issuance of subscription receivable | 32,915 | |
| Par value of common stock issued | (1) | |
| Total Impact of Business Combination on additional paid-in capital | $ | 36,842 | |
(1)Excludes impact of the Business Combination on net loss, which is presented separately in the consolidated statements of stockholders’ equity (deficit).
4.Private Placement
Initial Recognition
Background
Prior to the Closing, the Company in exchange for issuanceentered into private agreements (“Private Placement Agreements”) with investors (“FPA Investors”) consisting of 4,312,500forward purchase agreements (“Forward Purchase Agreements”), subscription agreements, a side letter, and escrow agreements. The Private Placement Agreements closed on September 29, 2023. Pursuant to the Private Placement Agreements, the FPA Investors purchased 3,168,121 shares of common stock (the “Founder Shares”(“FPA Shares”) for $32.9 million (“Prepayment Amount”). On April 2, 2021, Graf LLC transferred all of its Founder sharesPursuant to the Sponsor. On April 8, 2021,Private Placement Agreements the Sponsor transferred 20,000 FounderFPA Investors may subscribe for and purchase an additional 767,990 shares of common stock.
The Prepayment Amount was deposited into escrow accounts. The terms of the Private Placement Agreements provide that following a one-year period after the Closing, subject to early termination and settlement with respect to any number of FPA Shares at the election of the FPA Investors (“Measurement Period”), funds in the escrow accounts may be released to eachthe FPA Investors, the Company or a combination of both based on a combination of factors, including the volume weighted average price of the Company’s independent directors, resultingcommon stock (“VWAP”) over a specified valuation period during the Measurement Period (“Reset Price”), the number of shares sold by the FPA Investors during the Measurement Period, and the application of antidilution provisions. The Private Placement Agreements expire at the end of the Measurement Period.
All funds in escrow will be released to the Company, the FPA Investors, or a combination of both, at or before the one year anniversary of the Closing. The maximum and minimum that could be released from escrow is the Prepayment Amount and zero, respectively, for both the FPA Investors and the Company. In addition, all interest earned on the funds in the Sponsor holding 4,252,500 Founder Shares. The holders ofescrow accounts will be released to the Founder Shares agreed to forfeit up to an aggregate of 562,500 Founder Shares, on a pro rata basis,FPA Investors.
During the Measurement Period, to the extent the Company’s share price approaches or exceeds $10.44 per share, the likelihood and amount of escrow funds to be released to the Company increases and the likelihood and amount of escrow funds to be released to the FPA Investors decreases. Conversely, during the Measurement Period, to the extent the Company’s share price decreases below $10.44 per share, the likelihood and amount of escrow funds to be released to the Company decreases and the likelihood and amount of escrow funds to be released to the FPA Investors increases. Other drivers of settlement outcomes include the number of shares sold by FPA Investors to third parties during the Measurement Period, whereby the selling of shares may decrease portions of escrow funds to be released to the FPA Investors by up to $2.00 per share sold, the application of antidilution provisions, the timing of sales and settlements, among other factors. Additionally, a prepayment shortfall of $0.1 million was established in connection with the Private Placement Agreements (“Prepayment Shortfall”). Pursuant to the terms and conditions of the Private Placement Agreements, sales of FPA Shares to third-parties are required to first be applied towards the Prepayment Shortfall prior to the subscription receivable (described further below).
In addition to the FPA Shares, the FPA Investors received 514,889 shares of common stock for no incremental consideration (“Bonus Shares”). The Bonus Shares are not subject to an escrow arrangement.
Accounting
All FPA Shares and Bonus Shares are outstanding shares of the Company that are not held in escrow, are transferrable without restrictions, and have the same voting as well as dividend and liquidation participation rights as other shares of the Company. Accordingly, such shares are equity classified and presented together with other shares of common stock in the consolidated financial statements.
The escrow agreements provide that funds placed into escrow are held in escrow for the benefit of the FPA Investors until they are released to the Company pursuant to the terms of the Private Placement Agreements and the Company’s creditors do not have access to the funds held in escrow in the event of bankruptcy of the Company. Accordingly, the Company accounted for the original Prepayment Amount of $32.9 million as a contra-equity subscription receivable because the funds held in escrow represent receivables from shareholders.
The features of the Private Placement Agreements met the derivatives criteria under ASC 815 because they contained an underlying, notional amount, payment provision, and net settlement. Accordingly, a derivative liability was recognized based on the estimated measurement of the portion of the funds in escrow that could be released to the FPA Investors, based on circumstances existing as of Closing. The net balance of the Prepayment Amount presented as a subscription receivable and the derivative liability when considered together represents the estimated amount of escrow funds the Company expects to receive from the escrow accounts. Subsequent changes in fair value of the derivative liability associated with the Private Placement Agreements will be recognized through earnings on a quarterly basis.
Upon the Closing, in addition to the $32.9 million subscription receivable, a loss on issuance of forward purchase contract totaling $24.5 million was recorded, which consisted of the fair value of the derivative liability of $20.2 million plus the fair value of the Bonus Shares of $4.3 million. The forward purchase derivative liability is treated as a current liability because the Private Placement Agreements mature or otherwise are subject to early termination at or prior to the one year at or before the one year anniversary of the Closing.
December 2023 Amendment
On December 26, 2023, the Company and an FPA Investor entered into an amendment to their Forward Purchase Agreement ("FPA Amendment") for total proceeds of $0.5 million. No other Private Placement agreements were amended during the year ended December 31, 2023. The FPA Amendment provided that, (i) 200,000 FPA Shares were re-designated to Bonus Shares, (ii) the definition of Reset Price was changed (“Amended Reset Price”), (iii) the definition of the prepayment shortfall was amended (“Amended Prepayment Shortfall”), and (iv) the funds in the escrow account were transferred to a separate account held by the FPA Investor. The funds from the FPA Investor in connection with the amendment were not received by the Company until January 2024.
The terms of the Amended Reset Price provide for (i) a rolling ceiling effective as of the FPA Amendment execution date based on a weekly trailing VWAP such that the optionCompany does not benefit from increases in share price during the Measurement Period, and (ii) discounts of generally 10% to the VWAP measurement that benefit the FPA Investor.
Proceeds from the sale of FPA Shares by FPA Investors to third parties are required to be treated as a reduction to the prepayment shortfall until no balance remains in the prepayment shortfall (“Shortfall Sales”), at which point proceeds from such sales of stock may be treated as reductions to the subscription receivable that may result in cash proceeds to the Company. If all FPA Shares are sold without full satisfaction of the prepayment shortfall, the Company is required, at their election, to either pay a cash amount equal to the remaining prepayment shortfall balance or issue additional shares at 90% of the VWAP for the trailing 20 trading days.
The terms of the Amended Prepayment Shortfall provide for a $0.5 million increase to the FPA Investor’s pre-existing prepayment shortfall of $0.1 million.
Upon execution of the FPA Amendment, the Company recognized a loss on amendment to forward purchase additional units is not exercisedcontract as set forth below (in thousands):
| | | | | |
| Loss on Amendment |
| Reduction of subscription receivable | $ | 15,123 |
| Reduction in forward purchase derivative liability | (14,181) |
| Shareholder receivable | (500) |
| Loss on amendment to forward purchase contract | $ | 442 |
The $0.4 million loss recognized in fullconnection with the FPA Amendment represents the reduction in cash proceeds the Company may receive under the forward purchase contract, partially offset by the underwriters, so thatreduction in the Founderforward purchase derivative liability and the shareholder receivable. As a result of the FPA Amendment, the maximum cash proceeds the Company could receive under the forward purchase contract, reflected in the subscription receivable balance, was lowered. The reduction in the subscription receivable of $15.1 million was caused by (i) the Amended Reset Price, which reduced the maximum price per FPA Share the Company could receive (initially, $10.44 per share), (ii) the re-designation of 200,000 FPA Shares will represent 20%to Bonus Shares, reducing the total quantity of FPA Shares, and (iii) the Amended Prepayment Shortfall, which increased the prepayment shortfall amount. The Company does not receive any consideration for sales or settlements of Bonus Shares or Shortfall Sales, described further below. In addition to the reduction in the subscription receivable, the Company recognized a corresponding reduction in the fair value of the forward purchase derivative liability of $14.2 million.
Sales of FPA Shares
Pursuant to the terms and conditions of the Private Placement Agreements, any sales of the Company’s issuedcommon stock associated with the Private Placement Agreements may not be treated as sales of Bonus Shares until all FPA Shares are sold, at which point such sales of stock may be considered sales of Bonus Shares.
Following the FPA Amendment, the FPA Investor sold 139,793 shares of the Company’s common stock to third parties, which were treated as Shortfall Sales of FPA Shares. Accordingly, the balance of the subscription receivable was not impacted. No other sales of FPA Shares occurred during the year ended December 31, 2023.
Change in fair value
As set forth above in this Note 4, the forward purchase derivative liability represents the portion of the reduced subscription receivable, that may be released to the FPA Investors rather than the Company. Gains and losses resulting from the remeasurement of changes in the fair value of the forward purchase derivative liability are recognized in earnings on a quarterly basis. Refer to Note 9, Fair Value of Financial Instruments, for further discussion.
5.Warrants
As of December 31, 2023, all warrants described below remained outstanding shares after the Initial and unexercised.
Public Offering. The underwriters partially exercised their over-allotment option on June 2, 2021, and forfeited the remaining option; and, as a result, an aggregate of 22,125 Founder Shares were forfeited, resulting in 4,290,375 Founder Shares outstanding. On July 14, 2021, the Sponsor transferred 20,000 Founder Shares to Alexandra Lebenthal inWarrants
In connection with her appointmentGraf’s initial public offering (“IPO”), 3,432,286 warrants were issued to Graf’s investors (“Public Warrants”). The Public Warrants, which entitle the registered holder to purchase one share of the Company's boardCompany’s common stock, have an exercise price of directors.The Initial Stockholders agreed not to transfer, assign or sell any of their Founder Shares until the earlier to occur of: (A) one year
$11.50 per warrant, became exercisable 30 days after the completion of the initial Business Combination or (B) subsequent to the initial Business Combination, (x) if the last sale price of the common stock equals or exceeds $12.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 120 days after the initial Business Combination, or (y) the date on which the Company completes a liquidation, merger, capital stock exchange or other similar transaction that results in all of the stockholders having the right to exchange their shares of common stock for cash, securities or other property.Private Placement
Simultaneously with the closing of the Initial Public Offering, the Company consummated the Private Placement of 4,433,333 Private Placement Warrants, at a price of $1.50 per Private Placement Warrant to the Sponsor, generating proceeds of approximately $6.7 million. Simultaneously with the closing of the Over-Allotment on June 2, 2021, the Company consummated the second closing of the Private Placement, resulting in the purchase of an aggregate of an additional 288,200 Private Placement Warrants at $1.50 per Private Placement Warrant (the “Additional Private Placement Warrants”), generating additional gross proceeds of approximately $0.4 million.
Each whole Private Placement Warrant entitles the holder thereof to purchase 1 common stock at an exercise price of $11.50 per full share. A portion of the proceeds from the Private Placement Warrants was added to the proceeds from the Initial Public Offering held in the Trust Account. If the Company does not complete a Business Combination within the Combination Period, the Private Placement Warrants will expire worthless. The Private Placement Warrants will be non-redeemable and exercisable on a cashless basis so long as they are held by the Sponsor or its permitted transferees (see Note 9).
Related Party Loans
On January 29, 2021, the Sponsor agreed to loan the Company up to $150,000 to be used for the payment of costs related to the Initial Public Offering pursuant to a promissory note (the “Note”). The Note was non-interest bearing, unsecured and due upon the consummation of the Initial Public Offering. The Company had borrowed approximately $70,000 under the Note. The Note was paid back in full on May 26, 2021. Subsequent to the repayment, the facility was no longer available to the Company.
In addition, in order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor or any of the Company’s officers or directors may, but are not obligated to, loan the Company funds, from time to time or at any time, in whatever amount they deem reasonable in their sole discretion (the “Working Capital Loans”). Each loan would be evidenced by a promissory note. The notes would either be paid upon consummation of the initial Business Combination, without interest. In the event that the initial Business Combination does not close, the Company may use a portion of the working capital held outside the Trust Account to repay such loaned amounts but no proceeds from the Trust Account would be used for such repayment. Up to $1,500,000 of such Working Capital Loans may be convertible into additional warrants at a price of $1.50 per warrant at the option of the lender. The warrants would be identical to the Private Placement Warrants. Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans. As of December 31, 2021, the Company had 0 borrowings under the Working Capital Loans.
Administrative Services Agreement
On May 20, 2021, the Company entered into an agreement that provided that, commencing on the date that the Company’s securities were first listed on the NYSE through the earlier of consummation of the initial Business Combination, and the liquidation, the Company agreedare set to pay G-SPAC Management LLC, an affiliate of the Sponsor, $15,000 per month for office space, utilities, secretarial, administrative and support services provided to the Company and members of the management team. For the period from January 28, 2021 (inception) through December 31, 2021, the Company incurred expenses of approximately $108,000, respectively, under this agreement. As of December 31, 2021, the Company had 0 outstanding for services in connection with such agreement on the accompanying balance sheet.
In addition, the Sponsor, officers and directors, or any of their respective affiliates will be reimbursed for any out-of-pocket expenses incurred in connection with activities on the Company’s behalf such as identifying potential target businesses and performing due diligence on suitable Business Combinations. The audit committee will review on a quarterly basis all payments that were made by the Company to the Sponsor, officers or directors, or their affiliates. Any such payments prior to an initial Business Combination will be made from funds held outside the Trust Account.
expire five years
Note 6—Commitments and Contingencies
Registration and Stockholder Rights
The holders of the Founder Shares, Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans (and any shares of common stock issuable upon the exercise of the Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans) were entitled to registration rights pursuant to a registration rights agreement signed upon the effective date of the Initial Public Offering. These holders were entitled to make up to 3 demands, excluding short form
registration demands, that the Company registered such securities for sale under the Securities Act. In addition, these holders will have “piggy-back” registration rights to include their securities in other registration statements filed by the Company. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriting Agreement
The Company granted the underwriters a 45-day option from the date of the final prospectus relating to the Initial Public Offering to purchase up to 2,250,000 additional Units less the underwriting discounts and commissions. On June 2, 2021, the underwriters partially exercised the over-allotment option. On June 2, 2021, the Underwriters partially exercised the over-allotment option and purchased an additional 2,161,500 Units.
The underwriters were entitled to an underwriting discount of $0.20 per unit, or $3.4 million in the aggregate, paid upon the closing of the Initial Public Offering ($3.0 million) and Over-Allotment (approximately $0.4 million). In addition, $0.35 per unit, or approximately $6.0 million in the aggregate will be payable to the underwriters for deferred underwriting commissions (approximately $5.25 million related to the Initial Public Offering and $0.8 million related to the Over-Allotment). The deferred fee will become payable to the underwriters from the amounts held in the Trust Account solely in the event that the Company completes a Business Combination, subject to the terms of the underwriting agreement.
Risks and Uncertainties
Management continues to evaluate the impact of the COVID-19 pandemic on the industry and has concluded that while it is reasonably possible that the virus could have a negative effect on the Company’s financial position, results of its operations and/or search for a target company, the specific impact is not readily determinable as of the date of these financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Note 7— Common Stock Subject to Possible Redemption
The Company’s common stock features certain redemption rights that are considered to be outside of the Company’s control and subject to the occurrence of future events. The Company is authorized to issue 400,000,000 shares of common stock with a par value of $0.0001 per share. Holders of the Company’s common stock are entitled to 1 vote for each share. As of December 31, 2021, there were 21,451,875 shares of common stock outstanding, of which 17,161,500 shares were subject to possible redemption and classified outside of permanent equity in the balance sheet.
The common stock subject to possible redemption reflected on the balance sheet is reconciled on the following table:
| | | |
Gross proceeds from Initial Public Offering | | $ | 171,615,000 |
Less: | | | |
Fair value of Public Warrants at issuance | | | (7,894,290) |
Offering costs allocated at common stock subject to possible redemption | | | (9,494,877) |
Plus: | | | |
Accretion on common stock subject to possible redemption | | | 17,389,167 |
Common stock subject to possible redemption | | $ | 171,615,000 |
Note 8—Stockholders’ Deficit
Preferred stock— The Company is authorized to issue 1,000,000 shares of preferred stock, par value $0.0001 per share, with such designations, voting and other rights and preferences as may be determined from time to time by the Company’s board of directors. As of December 31, 2021, there were 0 shares of preferred stock issued or outstanding.
Common Stock — The Company is authorized to issue 400,000,000 shares of common stock, par value $0.0001. Holders of the Company’s common stock are entitled to 1 vote for each share. As of December 31, 2021, there were 4,290,375 shares of common stock issued and outstanding, excluding 17,161,500 shares of common stock subject to possible redemption (see Note 7).
Note 9—Warrants
As of December 31, 2021, the Company has 3,432,300 and 4,721,533 Public Warrants and Private Placement Warrants, respectively, outstanding.
Public Warrants may only be exercised for a whole number of shares. No fractional Public Warrants will be issued upon separation of the Units and only whole Public Warrants will trade. The Public Warrants will become exercisable commencing 30 days after the completion of a Business Combination; provided that the Company has an effective registration statement under the Securities Act covering the shares of common stock issuable upon exercise of the Public Warrants and a current prospectus relating to them is available (or the Company permits holders to exercise their Public Warrants on a cashless basis and such cashless exercise is exempt from registration under the Securities Act). The Company agreed that as soon as practicable, but in no event later than 20 business days after the closing of the initial Business Combination, it will use commercially reasonable efforts to file with the SEC a registration statement covering the shares of common stock issuable upon exercise of the warrants, to cause such registration statement to become effective and to maintain a current prospectus relating to those shares of common stock until the warrants expire or are redeemed, as specified in the warrant agreement. If a registration statement covering the shares of common stock issuable upon exercise of the warrants is not effective by the 60 business day after the closing of the initial Business Combination, warrant holders may, until such time as there is an effective registration statement and during any period when the Company will have failed to maintain an effective registration statement, exercise warrants on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act or another exemption. Notwithstanding the above, if the common stock is at the time of any exercise of a warrant not listed on a national securities exchange such that it satisfies the definition of a “covered security” under Section 18(b)(1) of the Securities Act, the Company may, at its option, require holders of Public Warrants who exercise their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act and, in the event the Company so elects, it will not be required to file or maintain in effect a registration statement, and in the event the Company does not so elect, it will use commercially reasonable efforts to register or qualify the shares under applicable blue sky laws to the extent an exemption is not available.
The warrant has an exercise price of $11.50 per full share and will expire five years after the completion of a Business Combination, or earlier upon redemption. The Public Warrants may be called for redemption or liquidation. In addition, if (x)at the sole discretion of the Company issues additional shares of commonif the Company’s stock price equals or equity-linked securities for capital raising purposes in connection with the closing of the initial Business Combination at an issue price or effective issue price of less than $9.20 per share of common stock (with such issue price or effective issue price to be determined in good faith by the board of directors, and in the case of any such issuance to the Sponsor or its affiliates, without taking into account any Founder Shares held by the Sponsor or such affiliates, as applicable, prior to such issuance) (the “Newly Issued Price”), (y) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of the initial Business Combination on the date of the consummation of the initial Business Combination (net of redemptions), and (z) the volume weighted average trading price of the common stock during the 20 trading day period starting on the trading day after the day on which the Company consummates the initial Business Combination (such price, the “Market Value”) is below $9.20 per share, the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the higher of the Market Value and the Newly Issued Price, and theexceeds $18.00 per share redemption trigger price described below will be adjusted (toand other certain conditions are met. The Public Warrants are equity classified due to terms indexed to the nearest cent)Company’s own stock and the satisfaction of other equity classification criteria.
Private Warrants
Concurrently with Graf’s IPO, Graf issued 4,721,533 warrants to be equal to 180%Graf Acquisition Partners IV LLC (“Private Warrants”). The terms of the higher of the Market Value and the Newly Issued Price.Once the warrants become exercisable, the Company may redeem the outstanding warrants:
| ● | in whole and not in part; |
| ● | at a price of $0.01 per warrant; |
| ● | upon a minimum of 30 days’ prior written notice of redemption; and |
| ● | if, and only if, the last sale price of the common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date on which the Company sends the notice of redemption to the warrant holders. |
If the Company calls the warrants for redemption as described above, it will have the option to require all holders that wish to exercise warrants to do so on a “cashless basis.”
The Private Placement Warrants are identical to the Public Warrants underlying the Units sold in the Initial Public Offering,with an exercise price of $11.50 per warrant, except that none of the Private Placement Warrants will bethey are subject to certain transfer and sale restrictions and are not optionally redeemable by the Company so long as they are held by the initial purchasers or any of their permitted transferees.
Additionally, the Private Warrants are exercisable on a cashless basis. If the Private Warrants are held by a party other than the initial purchasers or their permitted transferees, the Private Warrants will be redeemable by the Company is unableand exercisable by such holders on the same basis as the Public Warrants. The Private Warrants are liability classified due to completeterms not indexed to the Company’s own stock. As described in Note 8, Related Party Transactions, the Private Warrants are a Business Combination withinrelated party financial instrument. Private Warrants are classified to non-current liabilities because their term ends beyond one year from the Combination Periodlatest consolidated balance sheet date.
SPA Warrants
Together with the issuance of the Senior Convertible Notes described in Note 6, Convertible Notes, 1,000,000 warrants were issued to NKMAX at an exercise price of $11.50 per warrant (“SPA Warrants”). The terms of the SPA Warrants are identical to the terms of the Public Warrants with redemption at the sole discretion of the Company if the Company’s stock price equals or exceeds $18.00 per share and other certain conditions are met. The SPA Warrants are equity classified due to terms indexed to the Company’s own stock and the Company liquidates the funds heldsatisfaction of other equity classification criteria, including redemption in the Trust Account, holdersCompany’s control if the Company’s stock price equals or exceeds $18.00 per share. As described in Note 8, Related Party Transactions, the SPA Warrants are a related party financial instrument.
Working Capital Warrants
Prior to the Closing, Graf executed drawdowns upon a working capital loan facility. Upon Closing, the $0.8 million balance of the working capital loan facility was settled through the issuance of 523,140 warrants will(“Working Capital Warrants”). The terms of the Working Capital Warrants are identical to the terms of the Private Warrants with an exercise price of $11.50 per warrant. The Working Capital Warrants are liability classified due to terms not receive anyindexed to the Company’s own stock. As described in Note 8, Related Party Transactions, the Working Capital Warrants are a related party financial instrument. Working Capital Warrants are classified to non-current liabilities because their term ends beyond one year from the latest consolidated balance sheet date.
PIPE Warrants
Prior to the Closing, the Company entered into warrant subscription agreements (the “Warrant Subscription Agreements”) with certain investors (“Warrant Investors”), which closed on September 29, 2023. Pursuant to the Warrant Subscription Agreements, the Warrant Investors purchased an aggregate of such funds with respect10,209,994 warrants, at a purchase price of $1.00 per warrant (“PIPE Warrants”) for total proceeds of $10.2 million. The PIPE Warrants are exercisable for cash (or by “cashless” exercise under certain circumstances) during the five-year period beginning on the Closing. One-third of the PIPE Warrants are exercisable initially at $10.00 per warrant, one-third of the PIPE Warrants are exercisable initially at $12.50 per warrant, and one-third of the PIPE Warrants are exercisable initially at $15.00 per warrant. The initial exercise prices of each tranche are subject to adjustment every 180 days after the Closing based upon declines in trading prices of the Company’s common stock, as well as antidilution adjustments for stock splits, stock dividends, and the like. In addition, the PIPE Warrants contain a downside protection provision, pursuant to which the Warrant Investors may demand a cashless exchange of certain PIPE Warrants and, to the extent the relevant reference price is less than $1.50 per share, a cash payment calculated as the difference between $1.50 per share and the then-current exercise price multiplied by the applicable number of warrant shares shall be paid to the Warrant Investors. The PIPE Warrants are liability classified due to terms not indexed to the Company’s own stock and their cash settlement provisions.
PIPE Warrants are classified to non-current liabilities because their term ends beyond one year from the latest consolidated balance sheet date.
6.Convertible Notes
Legacy Convertible Notes
From November to December 2019, the Company issued convertible promissory notes to investors (“2019 Convertible Notes”) and related parties (“2019 Related Party Convertible Notes”). From March to September 2023, the Company issued additional convertible promissory notes issued to investors (“2023 Convertible Notes”) and to related parties (“2023 Related Party Convertible Notes”), collectively referred to as “Legacy Convertible Notes”.
Total proceeds raised from the 2019 Convertible Notes and 2019 Related Party Convertible Notes were $10.8 million and $0.3 million, respectively, which each bore interest at 1.7% per year and had a maturity date of December 31, 2023. Total proceeds raised from the 2023 Convertible Notes and 2023 Related Party Convertible Notes were $6.1 million and $0.1 million, respectively, which each bore interest at 4.6% per year and had maturity dates of three years from their respective issuance dates. The terms of the Legacy Convertible Notes provided for conversion into common stock upon the occurrence of a qualified financing transaction, including upon the Closing of the Business Combination.
Pursuant to their warrants, nor will they receive any distribution fromterms, immediately prior to Closing, all of the Legacy Convertible Notes were converted into 5,579,266 shares of Legacy NKGen common stock, which then converted into 2,278,598 shares of the Company’s assets held outsideCommon Stock at Closing based on the Exchange Ratio.
Senior Convertible Notes
Prior to the Closing, the Company entered into convertible note subscription agreements (“Securities Purchase Agreement”) with NKMAX for total proceeds of $10.0 million, with a four-year term and an interest rate of 5.0% paid in cash semi-annually or 8.0% paid in kind (“Senior Convertible Notes”), which closed on September 29, 2023. Interest began accruing at Closing and is payable semi-annually in arrears, with interest that is paid in kind (if applicable) increasing the principal amount outstanding on each interest payment date. The Company currently expects to make their interest payments in-kind in lieu of periodic cash payments. The Senior Convertible Notes are convertible at any time, in whole or in part, at NKMAX’s option at a conversion price of $10.00 per share of common stock (subject to anti-dilution adjustments in the event of stock splits and the like). The Senior Convertible Notes have a put option which may be exercised by NKMAX 2.5 years after the issuance of the Trust AccountSenior Convertible Notes. No less than six months after exercise of the put option, the Company will be required to repay all principal and accrued interest of the Senior Convertible Notes. Should the put option remain unexercised, the outstanding principal and accrued interest will be due and payable on September 29, 2027. Additionally, as described in Note 5, Warrants, together with the respectSecurities Purchase Agreement, the SPA Warrants were issued to such warrants. Accordingly,NKMAX, and accordingly, a relative fair value allocation was applied and discount was recognized on the warrants may expire worthless.
Senior Convertible Notes as set forth in Note 10—9,
Fair Value of MeasurementsFinancial Instruments
. There are no financial or non-financial covenants associated with the Senior Convertible Notes. During the year ended December 31, 2023, the Company recorded $0.2 million of interest expense and discount amortization related to the Senior Convertible Notes. As described in Note 8, Related Party Transactions, the Senior Convertible Notes are a related party financial instrument. The following table presents information abouta reconciliation of the Senior Convertible Notes (in thousands):
| | | | | |
| Senior Convertible
Notes |
| Balance as of December 31, 2022 | $ | — |
| Issuance | 9,707 |
| Amortization of discount | 17 |
| Paid-in-kind interest | 206 |
| Balance as of December 31, 2023 | $ | 9,930 |
7.Debt
Revolving Line of Credit
In June 2023, the Company entered into a $5.0 million revolving line of credit agreement (as amended on September 19, 2023, January 30, 2024 and April 5, 2024) with a commercial bank with a one-year term and an interest rate based on the higher of (i) the one month secured overnight financing rate plus 2.9% or (ii) 7.5%. Issuance fees of $0.1 million were incurred in connection with this revolving line of credit. All outstanding balances under the revolving line of credit were due and payable on June 20, 2024. In April 2024, the agreement was amended to extended the maturity date of the revolving line of credit to September 18, 2024. The revolving line of credit is secured by all of the Company’s assets, including a deed of trust over the Company’s owned real property located in Santa Ana, California. The Company was required to maintain deposits with the lender in an amount of at least $15.0 million from a certain period of time as long as there was a debt balance outstanding. The Company was in compliance with our debt covenants as of December 31, 2023. In April 2024, the lender subsequently waived the minimum cash deposit requirement in exchange for the Company's agreement to use the lender as their primary banking relationship. Additionally, the Company is required to maintain a restricted cash balance of $0.3 million following the issuance. As of December 31, 2023, the interest rate for the revolving line of credit was 8.2%.
Through December 31, 2023, the Company drew down $4.9 million upon the revolving line of credit and no repayments of drawdowns occurred. Interest expense of $0.2 million was incurred upon the revolving line of credit, which was paid in cash during the year ended December 31, 2023. No interest expense was incurred for the revolving line of credit during the year ended December 31, 2022.
Related Party Loans
Between August 2019 and April 2023, the Company entered into related party loans with NKMAX (“Related Party Loans”).
In December 2022, the then-outstanding aggregate Related Party Loans’ principal and interest of $66.1 million was converted into 6,943,789 shares of common stock (after the application of the Exchange Ratio) which was recognized as a capital contribution for the year ended December 31, 2022.
From January through April 2023, the Company entered into additional Related Party Loans with NKMAX for aggregate gross proceeds of $5.0 million. These additional Related Party Loans bear an interest rate of 4.6% and mature on December 31, 2024. There are no financial or non-financial covenants associated with the Related Party Loans. The additional Related Party Loans are not convertible into equity.
In connection with the Related Party Loans, interest expenses incurred were $0.2 million and $2.3 million for the year ended December 31, 2023 and 2022, respectively. Related party interest payable amounts recorded to other current liabilities on the consolidated balance sheets were $0.2 million and zero as of December 31, 2023 and December 31, 2022, respectively.
Short Term Related Party Loan
In September 2023, NKGen raised $0.3 million in proceeds in connection with a related party loan with a 30-day term and an interest rate of 5.1% (“Short Term Related Party Loan”). This related party loan was not convertible into equity and was repaid in cash on October 5, 2023. Related party interest expense was less than $0.1 million for the year ended December 31, 2023, and zero for the year ended December 31, 2022.
Paycheck Protection Program Loan
In May 2020, the Company received loan proceeds of $1.1 million pursuant to the Paycheck Protection Program (“PPP”). The PPP, established as part of the CARES Act, provides loans for small businesses to cover qualified payroll costs, rent, utilities, and interest on mortgage and other debt obligations. The loan has an interest rate of 1.0%. The loan was paid off in May 2022. The Company recorded interest expense of $0.1 million related to the PPP loan to interest expense in the consolidated statements of operations and comprehensive loss for the year ended December 31, 2022. No amounts related to the Paycheck Protection Program Loan were recorded as of December 31, 2023.
8.Related Party Transactions
Founder Shares
Contemporaneously with the execution of the Merger Agreement, Graf and NKGen entered into an amended and restated sponsor support and lockup agreement (“Amended and Restated Sponsor Support and Lockup Agreement”). In connection with the Amended and Restated Sponsor Support and Lockup Agreement, of the 4,290,375 shares of Graf formerly held by Graf’s sponsor and insiders (“Founder Shares”): (i) 1,773,631 shares were forfeited, (ii) 1,173,631 shares became restricted shares subject to vesting conditions (“Deferred Founder Shares”), and (iii) the remaining 1,343,113 shares are subject to trading restrictions for up to two years and continued to be outstanding and fully vested shares.
Deferred Founder Shares do not have voting rights, do not participate in dividends and are not transferable. During the vesting period of five years from Closing (“Vesting Period”), if the trading price or price per share consideration upon a change in control for Common Stock is greater than or equal to $14.00 at any 20 trading days in a 30 consecutive trading-day period, then 873,631 Deferred Founder Shares will immediately vest; and if greater than or equal to $20.00 at any 20 trading days in a 30 consecutive trading-day period, then an additional 300,000 Deferred Founder Shares will immediately vest. In the event there is a sale of the Company, then immediately prior to the consummation of such sale, the calculated Acquiror Sale Price, as defined in the agreement, will take into account the number of Deferred Founder Shares that will vest upon a change in control. Upon the expiration of the Vesting Period, unvested Founder Shares will be forfeited and cancelled for no consideration.
All Founder Shares, including Deferred Founder Shares, are equity classified primarily due to terms indexed to the Company’s own stock, including upon a change in control.
Related Party Financial Instruments
The Company’s related party financial instruments include (i) the Founder Shares, including Deferred Founder Shares described above in this Note 8, (ii) the SPA Warrants described in Note 5, Warrants, (iii) the Working Capital Warrants described in Note 5, Warrants, (iv) the Senior Convertible Notes described in Note 6, Convertible Notes, (v) select Legacy Convertible Notes described in Note 6, Convertible Notes, (vi) the Related Party Loans described in Note 7, Debt, (vii) the Short Term Related Party Loan described in Note 7, Debt, and (viii) the Private Warrants described in Note 5, Warrants.
Advisory and research services
The Company was provided professional clinical program advisory services from Paul Song, prior to his hiring as Chief Executive Officer in December 2022. No such services were provided to or incurred by the Company during the year ended December 31, 2023. For the year ended December 31, 2022, $0.4 million in research and development expenses related to these advisory services were recorded. As of December 31, 2022, amounts payable of less than $0.1 million relating to advisory and research services from related parties remained outstanding, which were recorded to accounts payable and accrued expenses on the consolidated balance sheet. As of December 31, 2023, no amounts payable remained outstanding relating to advisory and research services from related parties.
Purchases of laboratory supplies
For the year ended December 31, 2023 and December 31, 2022, the Company recorded research and development expenses of $0.6 million and $0.1 million, respectively, associated with the purchase of laboratory supplies from NKMAX. As of December 31, 2023 and December 31, 2022, $0.6 million and less than $0.1 million, respectively, remained outstanding relating to the purchase of laboratory supplies from NKMAX, which were recorded to accounts payable and accrued expenses on the consolidated balance sheet.
9.Fair Value of Financial Instruments
The Company accounts for the fair value of its financial instruments under the framework established by US GAAP which defines fair value and expands disclosures about fair value measurements. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value.
The Company’s management used the following methods and assumptions to estimate the fair value of its financial instruments:
Level 1 — Quoted prices in active markets for identical assets or liabilities the Company has the ability to access at the measurement date.
Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of assets or liabilities.
Level 3 — Pricing inputs that are unobservable, supported by little or no market activity and are significant to the fair value of the assets or liabilities.
The carrying amounts of the Company’s financial assets and financial liabilities are considered to be representative of their respective fair values because of the short-term nature of those instruments. The Company does not measure assets at fair value on a recurring basis.
Liabilities measured at fair value on a recurring basis as of December 31, 2023 are as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Fair Value Measurements at Reporting Date Using |
| Balance as of
December 31,
2023 | | Level 1 | | Level 2 | | Level 3 |
| Private Warrants | $ | 378 | | | $ | — | | | $ | — | | | $ | 378 | |
| Working Capital Warrants | 42 | | | — | | | — | | | 42 | |
| Forward Purchase Derivative Liability | 15,804 | | | — | | | — | | | 15,804 | |
| PIPE Warrants | 25,339 | | | — | | | — | | | 25,339 | |
| Total | $ | 41,563 | | | $ | — | | | $ | — | | | $ | 41,563 | |
In addition to items that are measured at fair value on a recurring basis, the Company also has liabilities that are measured at fair value on a nonrecurring basis. As these liabilities are not measured at fair value on a recurring basis, they are not included in the tables above. Liabilities that are measured at fair value on a nonrecurring basis as of December 31, 2021 and indicates2023 include the Senior Convertible Notes. The valuation of the Senior Convertible Notes was $8.5 million as of December 31, 2023. The Senior Convertible Notes were determined to be in-scope of ASC 470, Debt. Accordingly, this instrument will not be measured at fair value on a recurring basis as the fair value hierarchymeasurement of this instrument was for purposes of the valuation techniques thatrelative fair value allocation described below as the Company utilized to determine such fair value.
| | | | | | | | | |
| | | | | Significant | | Significant |
| | | | | Other | | Other |
| | Quoted Prices in | | Observable | | Unobservable |
| | Active Markets | | Inputs | | Inputs |
Description | | (Level 1) | | (Level 2) | | (Level 3) |
Assets - Investments held in Trust Account: | | | | | | | | | |
| | | | | | | | | |
U.S. Treasury securities | | $ | 171,656,153 | | $ | — | | $ | — |
Liabilities | | | | | | | | | |
Derivative warrant liability- Private Placement Warrants | | $ | — | | $ | — | | $ | 5,571,410 |
Transfers to/from Levels 1, 2, and 3 are recognized atSenior Convertible Notes were issued together with the beginningSPA Warrants.
Legacy Convertible Notes
The following table presents a reconciliation of the
reporting period. There were 0 transfers to/from Levels 1, 2, and 3 during the period from January 28, 2021 (inception) through December 31, 2021.Level 1 assets include investments in U.S. government securities. Legacy Convertible Notes (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2019
Convertible
Notes | | 2019 Related
Party
Convertible
Notes | | 2023
Convertible
Notes | | 2023 Related
Party
Convertible
Notes | | Total |
| Balance as of December 31, 2021 | $ | 11,219 | | | $ | 259 | | | $ | — | | | $ | — | | | $ | 11,478 | |
| Change in fair value | 173 | | | 4 | | | — | | | — | | | 177 | |
| Balance as of December 31, 2022 | 11,392 | | | 263 | | | — | | | — | | | 11,655 | |
| Issuance | — | | | — | | | 6,090 | | | 125 | | | 6,215 | |
| Change in fair value | 1,083 | | | 13 | | | (52) | | | (1) | | | 1,043 | |
| Conversion and settlement | (12,475) | | | (276) | | | (6,038) | | | (124) | | | (18,913) | |
| Balance as of December 31, 2023 | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
The Company
uses inputs such as actual trade data, quoted market prices from dealers or brokers, and other similar sources to determinehistorically determined the carrying amount of the Legacy Convertible Notes using a scenario-based analysis that estimates the fair value of
its investments.the Legacy Convertible Notes based on the probability-weighted present value of expected future investment returns by measuring the fair value of similar debt instruments that do not have the conversion feature. If no similar debt instrument existed, fair value was estimated by using assumptions that market participants would use in pricing a debt instrument, including market interest rates, credit standing, yield curves and volatilities.
The following unobservable assumptions were used in determining the fair value of the Legacy Convertible Notes as of December 31, 2022:
| | | | | |
| Probability of conversion | — | |
| Probability of holding until maturity without conversion | — | |
| Remaining term until potential conversion trigger date (years) | 0 |
Discount yield (1) | 20.0 | % |
(1)Estimated using a comparable bond analysis and under S&P Global Inc.’s credit rating scale using a multinominal logical regression.
The fair value of Legacy Convertible Notes immediately prior to their conversion at Closing was based upon the fair value of the 2,278,598 shares of the Company’s common stock issued upon their conversion totaling $18.9 million, at a per share value of $8.30 based upon the fair value of the Company’s common stock at Closing, which was the conversion date.
Senior Convertible Notes
The Senior Convertible Notes were recognized at Closing on September 29, 2023. Additionally, as described above in Note 6, Convertible Notes, the Senior Convertible Notes are not measured at fair value on a recurring basis. As such, a reconciliation of the Senior Convertible Notes is not presented.
The Company determined the stand-alone fair value of the Senior Convertible Notes using a binomial lattice model, which generates a distribution of stock prices over the term of the note, calculates the associated payoff for the note, and discounts the probability-weighted values from the lattice back to the valuation date. The fair value was estimated by using assumptions that market participants would use in pricing a convertible debt instrument, including market interest rates, credit rating, yield curves, and volatilities.
The following unobservable assumptions were used in determining the fair value of the Senior Convertible Notes at Closing:
| | | | | |
Credit spread (1) | 12.1 | % |
| Equity volatility | 45.0 | % |
(1)Estimated using a comparable bond analysis and under S&P Global Inc.’s credit rating scale using a multinominal logical regression.
As of December 31, 2023, the Company determined the fair value of the Senior Convertible Notes was $8.5 million.
The following unobservable assumptions were used in determining the fair value of the Senior Convertible Notes at December 31, 2023:
| | | | | |
Credit spread (1) | 12.7 | % |
| Equity volatility | 45.0 | % |
(1)Estimated using a comparable bond analysis and under S&P Global Inc.’s credit rating scale using a multinominal logical regression.
Private Warrants and Working Capital Warrants
The Private Warrants and Working Capital Warrants were recognized at Closing on September 29, 2023. The fair value as of Closing was $1.8 million and $0.2 million, respectively. As of December 31, 2023, the fair value was $0.4 million and less than $0.1 million respectively.
The following table presents a reconciliation of the Private Warrants and Working Capital Warrants (in thousands):
| | | | | | | | | | | | | | | | | |
| Private
Warrants | | Working
Capital
Warrants | | Total |
| Balance as of December 31, 2022 | $ | — | | | $ | — | | | $ | — | |
| Recognition in connection with Business Combination | 1,841 | | | 204 | | | 2,045 | |
| Change in fair value | (1,464) | | | (162) | | | (1,626) | |
| Balance as of December 31, 2023 | $ | 377 | | | $ | 42 | | | $ | 419 | |
The terms of the Private Warrants and Working Capital Warrants are identical. Accordingly, the methodology and assumptions used to value these instruments is identical.
The fair value of the Private Placement Warrants wasand Working Capital Warrants were measured at fair value using a Black-Scholes model. The estimated fair value of the Private Placement Warrants isand Working Capital Warrants was determined using Level 3 inputs. Inherent in a Black-Scholes model isare assumptions related to expected stock-price volatility, expected life, risk-free interest rate and dividend yield. The Company estimates the volatility of its common stock warrantsPrivate Warrants and Working Capital Warrants based on implied volatility from the Company’s traded warrantsPrivate Warrants and Working Capital Warrants and from historical volatility of select peer company’s common stock that matches the expected remaining life of the warrants.Private Warrants and Working Capital Warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected remaining life of the warrants.Private Warrants and Working Capital Warrants. The expected life of the warrantsPrivate Warrants and Working Capital Warrants is assumed to be equivalent to their remaining contractual term. The dividend rate is based on the historical rate, which the Company anticipates remaining at zero.
The following table provides quantitative information regarding Level 3 fair value measurements inputs at their measurement dates:
| | | | | | | |
| | At | | December 31, | |
| | issuance | | 2021 | |
Exercise price | | $ | 11.50 | | $ | 11.50 | |
Share price | | $ | 9.93 | | $ | 9.69 | |
Expected term (years) | | | 6.59 | | | 5.98 | |
Volatility | | | 29.50 | % | | 16.20 | % |
Risk-free rate | | | 1.20 | % | | 1.35 | % |
Dividend yield (per share) | | | 0.00 | % | | 0.00 | % |
The changeunobservable assumptions were used in determining the fair value of the derivative warrant liabilities,Private Warrants and Working Capital Warrants at Closing:
| | | | | |
| Private Warrants’ volatility | 9.6 | % |
| Dividend yield (per share) | — | |
The following unobservable assumptions were used in determining the fair value of the Private Warrants and Working Capital Warrants as of December 31, 2023:
| | | | | |
| Private Warrants’ volatility | 35.3 | % |
| Dividend yield (per share) | — |
PIPE Warrants
The PIPE Warrants were recognized at Closing on September 29, 2023. The fair value as of December 31, 2023 was $25.3 million.
The following table presents a reconciliation of the PIPE Warrants (in thousands):
| | | | | |
| PIPE
Warrants |
| Balance as of December 31, 2022 | $ | — |
| Issuance | 10,210 |
| Change in fair value | 15,129 |
| Balance as of December 31, 2023 | $ | 25,339 |
The fair value of the PIPE Warrants was measured using Level 3its respective transaction price of $10.2 million for 10,209,994 PIPE Warrants at a purchase price of $1.00 per warrant at Closing.
As of December 31, 2023 the fair value of the PIPE Warrants was $25.3 million. The fair value of the PIPE Warrants was valued using level three inputs and was estimated using a Monte Carlo simulation approach. The Company’s common share price was assumed to follow a Geometric Brownian Motion over a period from the Valuation Date to the Expiration Date. The breadth of all possible scenarios was captured in an estimate of volatility, based on comparable companies’ historical equity volatilities, considering differences in their capital structure. For each simulation path, the Test Price and Reset Price were calculated based on the daily stock price during the measurement period. On each Reset Date, the downside protection condition was assessed to see if it was met by comparing the Test Price with the downside protection threshold price. The value of each tranche of warrants was then computed, factoring in any downside protection shares and downside protection cash, if applicable. The average value across this range of possible scenarios, discounted to present using the risk-free rate, was used as the fair value of the PIPE Warrants. The change in fair value of the PIPE Warrants was primarily attributable to select features of the Warrant Subscription Agreements, including strike price resets and downside protection which results in increased value as the Company’s stock price declines and stock price volatility increases.
The following unobservable assumptions were used in determining the fair value of the PIPE Warrants at December 31, 2023:
| | | | | |
| Credit spread | 12.7 | % |
| Equity volatility | 100.0 | % |
Forward Purchase Derivative Liability
The forward purchase derivative liability was recognized at Closing on September 29, 2023. The fair value as of December 31, 2023 was $15.8 million.
The following table presents a reconciliation of the Forward Purchase Derivative Liability (in thousands):
| | | | | |
| Forward
Purchase
Derivative
Liability |
| Forward purchase derivative liability at Close | $ | 20,201 | |
| Change in fair value in connection with the loss on amendment of forward purchase contract | (14,181) | |
| Change in fair value of forward purchase derivative liability | 9,784 | |
| Balance as of December 31, 2023 | $ | 15,804 | |
The fair value of the forward purchase derivative liability was estimated using a Monte Carlo simulation approach. The Company’s common share price was simulated with daily time steps for a range of various possible scenarios. The breadth of all possible scenarios was captured in an estimate of volatility, based on comparable companies’ historical equity volatilities, considering differences in their capital structure. The simulated prices were compared against the settlement adjustment features of the Forward Purchase Agreements. Under each simulated scenario of future stock price, the Company calculated the value of the forward purchase derivative liability arrangement. The average value across this range of possible scenarios, discounted to present using the risk-free rate, was used as the fair value of the forward purchase derivative liability.
The following unobservable assumptions were used in determining the fair value of the forward purchase derivative liability at Closing:
| | | | | |
| Dividend yield | 0.0 | % |
| Equity volatility | 105.0 | % |
The following unobservable assumptions were used in determining the fair value of the forward purchase derivative liability immediately before and after modification at December 26, 2023:
| | | | | |
| Dividend yield | 0.0 | % |
| Equity volatility | 125.0 | % |
The following unobservable assumptions were used in determining the fair value of the forward purchase derivative liability at December 31, 2023:
| | | | | |
| Dividend yield | 0.0 | % |
| Equity volatility | 115.0 | % |
Relative Fair Values
The Senior Convertible Notes were issued together with the SPA Warrants. Each instrument was recorded at its fair value, limited to a relative fair value based upon the percentage of its fair value to the total fair value based on the transaction price of the Securities Purchase Agreement of $10.0 million at Closing on September 29, 2023. The relative fair value of the SPA Warrants was treated as a discount to the Senior Convertible Notes, which will be amortized to interest expense over the term of the Senior Convertible Notes.
The stand-alone fair value at initial recognition for the Senior Convertible Notes and SPA Warrants was $12.9 million and $0.4 million, respectively. The stand-alone fair value of the Senior Convertible Notes and SPA Warrants was $8.5 million and $0.1 million as of December 31, 2023. The relative fair value at initial recognition and as of December 31, 2023 for the Senior Convertible Notes and SPA Warrants was $9.7 million and $0.3 million, respectively.
10.Stockholder’s Equity
Reverse Recapitalization
As described in Note 2, Summary of Significant Accounting Policies, all historical equity data, including stock option data, in these consolidated financial statements has been retrospectively adjusted by the Exchange Ratio to reflect the reverse recapitalization that occurred on September 29, 2023.
Common Stock
As of December 31, 2023, the Company had authorized 500,000,000 shares of common stock, par value $0.0001 per share. As of December 31, 2023, 21,888,976 shares of common stock were issued and outstanding, and 478,111,024 shares of common stock were reserved for future issuance.
Preferred Stock
As of December 31, 2023, the Company had authorized 10,000,000 shares of preferred stock, par value $0.0001. As of December 31, 2023, zero shares of preferred stock were issued or outstanding.
Employee Stock Purchase Plan
Upon consummation of the Business Combination, the Company adopted an employee stock purchase plan (“ESPP”). The maximum number of shares of the Company’s common stock that may be issued under the ESPP is 3.0% of the fully diluted common stock of the Company, determined as of immediately following Closing. Such maximum number of shares is subject to automatic annual increases. The Company’s employees and the employees of any designated affiliates may participate in the ESPP. The purchase price of the ESPP shares is 85% of the lesser of the fair market value of the Company’s common stock on the first day of an offering or on the applicable date of purchase. As of December 31, 2023, there were no transactions with respect to the ESPP.
2023 Plan
Upon consummation of the Business Combination, the Company adopted the 2023 equity incentive plan (“2023 Plan”). The maximum number of shares of common stock that may be issued under the 2023 Plan is 12.0% of the fully diluted common stock of the Company, determined as of immediately following Closing. Such maximum number of shares is subject to automatic annual increases. Under the 2023 Plan, restricted shares and stock options with service or performance based conditions may be granted to employees and nonemployees.
Upon the effective date of the 2023 Plan, the Company may not grant any additional awards under the 2019 Plan. As of December 31, 2023, no awards were granted under the 2023 Plan.
2019 Plan
The Company’s 2019 Plan (“2019 Plan”) became effective on October 23, 2019. The 2019 Plan provides for the grant of incentive stock options, non-qualified stock options, stock appreciation rights, restricted stock unit awards and performance share awards to employees, directors, and consultants of the Company. As of December 31, 2023, the Company has only issued stock options.
Stock options granted under the 2019 Plan expire no later than ten years from the date of grant and generally vest over a four-year period, with vesting occurring at a rate of 25% at the end of the first and thereafter in 36 equal monthly installments, or in the case of awards granted to board members, on a monthly basis over three or four years. In general, vested options expire if not exercised within three months after termination of service.
The fair value of each employee and non-employee stock option grant is estimated on the date of grant using the Black-Scholes option-pricing model. Due to the Company’s limited operating history and a lack of company-specific historical and implied volatility data, the Company estimated expected volatility based on the historical volatility of a group of similar companies that are publicly traded. The historical volatility data was computed using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the stock-based awards. Due to the lack of historical exercise history, the expected term of the Company’s stock options for employees has been determined utilizing the “simplified” method for awards. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. The expected dividend yield is zero since the Company has never paid cash dividends and does not expect to pay any cash dividends in the foreseeable future.
A summary of the Company’s stock option activity for the year ended December 31, 2021 and2023 is as follows:
| | | | | | | | | | | |
| Stock Options
Outstanding | | Weighted
Average
Exercise Price |
| Outstanding as of December 31, 2022 | 185,231 | | $ | 1.37 | |
| Granted | 2,173,693 | | 6.67 | |
| Forfeited | (267,072) | | 6.56 | |
| Exercised | (12,866) | | 0.88 | |
| Outstanding as of December 31, 2023 | 2,078,986 | | $ | 6.25 | |
The weighted average assumptions used in the Black-Scholes option pricing model to determine the fair value of stock option grants for the period from January 28, 2021 (inception) through year ended December 31, 2021, is summarized2023 were as follows: | | | | | |
| Common stock fair value | $ | 9.18 | |
| Risk-free interest rate | 3.5% |
| Expected volatility | 111.0% |
| Expected term (in years) | 6.1 |
| Expected dividend yield | 0.0% |
Stock options outstanding, vested and expected to vest and exercisable as of | | | |
Derivative warrant liability at January 28, 2021 (inception) | | $ | — |
Issuance of Private Placement Warrants | | | 10,551,330 |
Issuance of Private Placement Warrants (over-allotment) | | | 685,920 |
Change in fair value of derivative warrant liability | | | (5,665,840) |
Derivative warrant liability at December 31, 2021 | | $ | 5,571,410 |
December 31, 2023
are as follows:
Note 11—Subsequent Events
| | | | | | | | | | | | | | | | | | | | | | | |
| Number of
Stock
Options | | Weighted
Average
Remaining
Contractual
Life (Years) | | Weighted-
Average
Exercise
Price | | Total
Aggregate
Intrinsic
Value (in
thousands) |
| Outstanding as of December 31, 2022 | 185,231 | | 6.98 | | $ | 1.37 | | | $ | 980 | |
| Outstanding as of December 31, 2023 | 2,078,986 | | 8.86 | | $ | 6.25 | | | $ | 317 | |
| Vested and expected to vest as of December 31, 2023 | 2,078,986 | | 8.86 | | $ | 6.25 | | | $ | 317 | |
| Exercisable as of December 31, 2023 | 302,760 | | 7.46 | | $ | 3.79 | | | $ | 317 | |
Intrinsic value is calculated as the difference between the exercise price of the underlying options and the estimated fair value of the common stock for the options that had exercise prices that were lower than the per share fair value of the common stock on the related measurement date. The aggregate intrinsic value of stock options exercised during the year ended December 31, 2023 was $0.1 million. The aggregate fair value of stock options vested during the year ended December 31, 2023 was $1.2 million.
As of December 31, 2023, the total unrecognized stock-based compensation related to unvested stock option awards granted was $14.1 million, which the Company expects to recognize over a remaining weighted- average period of approximately 3.0 years.
Stock-based compensation expense, recognized in the Company’s consolidated statements of operations and comprehensive loss for the 2019 Plan was recorded as follows (in thousands):
| | | | | | | | | | | |
| Years ended
December 31 |
| 2023 | | 2022 |
| Research and development | $ | 898 | | | $ | 45 | |
| General and administrative | 3,237 | | | 24 | |
| Total stock-based compensation expense | $ | 4,135 | | | $ | 69 | |
11.Property and Equipment, net
Property and equipment, net consist of the following (in thousands):
| | | | | | | | | | | | | | | | | |
| Useful Life | | December 31,
2023 | | December 31,
2022 |
| Land | — | | $ | 5,025 | | | $ | 5,025 | |
| Buildings | 40 years | | 8,325 | | | 8,325 | |
| Furniture and fixtures | 7 years | | 749 | | | 677 | |
| Lab equipment | 5 years | | 4,004 | | | 4,003 | |
| Leasehold improvements | Lesser of estimated useful life or
related lease term | | 52 | | | 52 | |
| Office equipment | 5 years | | 17 | | | 17 | |
| Vehicles | 5 years | | 112 | | | 112 | |
| | | 18,284 | | | 18,211 | |
| Less: Accumulated depreciation | | | (3,825) | | | (2,690) | |
| | | $ | 14,459 | | | $ | 15,521 | |
Depreciation expense related to property and equipment was $1.1 million and $1.2 million for the years ended December 31, 2023 and 2022, respectively. No gains or losses on the disposal of property and equipment have been recorded for each of the years ended December 31, 2023 and 2022.
12.Additional Balance Sheet Information
Prepaid expenses and other current assets consist of the following (in thousands):
| | | | | | | | | | | |
| December 31,
2023 | | December 31,
2022 |
| Prepaid expenses | $ | 1,565 | | | $ | 133 | |
| Other receivables | 26 | | | 67 | |
| Revolving line of credit issuance fees | 47 | | | — | |
| Other | 16 | | | 4 | |
| Prepaid expenses and other current assets | $ | 1,654 | | | $ | 204 | |
Accounts payable and accrued expenses consist of the following (in thousands):
| | | | | | | | | | | |
| December 31,
2023 | | December 31,
2022 |
| Accounts payable | $ | 11,040 | | | $ | 975 | |
| Accrued liabilities | 1,360 | | | 1,359 | |
| Employee compensation | 911 | | | 291 | |
| Other | 84 | | | 27 | |
| Accounts payable and accrued expenses | $ | 13,395 | | | $ | 2,652 | |
13.Collaboration Agreement
On September 17, 2020, the Company entered into a strategic collaboration with Affimed GmbH (“Affimed”) to initiate a Phase 1/2 trial of SNK01 in combination with AFM24, a tetravalent biologic created by Affimed designed to direct NK cell killing of epidermal growth factor receptor (“EGFR”) expressing tumors. Under the collaboration agreement, the Company and Affimed split the development costs of the combination product equally. The study associated with the strategic collaboration with Affimed was discontinued by mutual agreement in June 2023.
Total reductions to research and development expenses for the years ended December 31, 2023 and 2022 were $0.2 million and $0.4 million, respectively.
14.Commitments and Contingencies
Leases
In February 2018, the Company entered into an operating lease agreement for office space located in 10 Pasteur, Irvine with a lease term of approximately five years. Rent payments commenced in February 2018. The lease expired on February 5, 2023. In October 2021, the Company entered into an operating lease agreement for office space located in 19700 Fairchild with a lease term of approximately two years with an option to extend the term for one two-year term, which at the time was not reasonably assured of exercise and therefore, not included in the lease term. Rent payments commenced in December 2021. The lease expired on December 31, 2023.
On November 9, 2023, the Company entered into a new operating lease agreement for office space located in Irvine, California with a lease term of approximately three years and rent payments commencing on January 1, 2024 (“New Office Lease”). The lease commencement date is January 1, 2024.
Future minimum lease payments under the New Office Lease are as follows (in thousands):
| | | | | |
| Minimum lease
payments |
| 2024 | $ | 235 | |
| 2025 | 242 | |
| 2026 | 249 | |
| Total operating lease liability | $ | 726 | |
License Agreements
The Company evaluated subsequent eventshas entered into exclusive license agreements with NKMAX, as amended in October 2021, April 2023 and transactions that occurred upAugust 2023 (“Intercompany License”), pursuant to which the Company acquired certain intellectual property. Pursuant to each license agreement, as consideration for an exclusive license to the date financial statements were issued. Based upon this review,intellectual property, the Company didpaid an upfront fee of $1.0 million (“Licensed Technology”).
As the license has no alternative future use, the Company recognized the upfront fee as research and development expense in the consolidated statements of operations and comprehensive loss during the year ended December 31, 2020. Additionally, the Company is also required to pay one-time milestone payments for the first receipt of regulatory approval by the Company or any of its affiliates for a Licensed Technology in the following jurisdictions (and amounts): the United States ($5.0 million), the European Union (“EU”) ($4.0 million), and four other countries ($1.0 million each). The Company is obligated to pay a mid-single digit royalty on net sales of Licensed Technology by it, its affiliates or its sublicensees, subject to customary reductions. The Company is also required to pay a percentage of its sublicensing revenue ranging from a low double-digit percentage to a mid-single digit percentage. As of December 31, 2023, the Company has not paid any milestone payments and no sales of Licensed Technology have occurred.
Litigation
The Company is subject to legal proceedings and claims, which arise in the ordinary course of business.
The Company is not identifysubject to any subsequent eventscurrently pending legal matters or claims that would have required adjustmenta material adverse effect on its financial position, results of operations or disclosurecash flows.
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future but have not yet been made. The Company accrues a liability for such matters when it is probable that future expenditures will be made, and such expenditures can be reasonably estimated. No amounts were accrued as of December 31, 2023 and December 31, 2022.
15.Income Taxes
The Company is subject to taxation in the U.S. and various state jurisdictions. The Company is not subject to taxation in foreign countries. The provision for income taxes for the years ended December 31, 2023 and 2022 are as follows (in thousands):
| | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 |
| Current: | | | |
| Federal | $ | — | | | $ | — | |
| State | — | | | — | |
| Deferred: | | | |
| Federal | 7 | | | 7 | |
| State | — | | | — | |
| Provision for income taxes | $ | 7 | | | $ | 7 | |
A reconciliation of the income tax computed at federal statutory income tax rate to the reported provision for income taxes is as follows (in thousands):
| | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 |
| Tax benefit at statutory federal rate | $ | (17,419) | | | $ | (5,618) | |
| State tax, net of federal tax benefit | (1,946) | | | (1,694) | |
| Interest expense | 47 | | | 477 | |
| Increase in valuation allowance | 9,189 | | | 7,908 | |
| Permanent items | 12 | | | 37 | |
| Stock compensation | 359 | | | (4) | |
| Unrealized loss FV of notes | 219 | | | — | |
| General business tax credit | (1,278) | | | (1,098) | |
| Loss on issuance of forward purchase contract | 5,140 | | | — | |
| Loss on amendment of the subscription receivable | 93 | | | — | |
| Loss on derivative valuation | 4,890 | | | — | |
| Other | 701 | | | (1) | |
| Provision for income taxes | $ | 7 | | | $ | 7 | |
Significant components of the Company’s deferred income taxes are as follows (inthousands):
| | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 |
| Deferred tax assets: | | | |
| Net operating losses | $ | 22,843 | | | $ | 17,890 | |
| Tax credit carryforwards, net | 4,523 | | | 3,285 | |
| Accrued expenses | 395 | | | 347 | |
| Section 174 R&E capitalization | 4,939 | | | 2,847 | |
| Intangibles | 707 | | | — | |
| Lease liability | — | | | 106 | |
| Stock-based compensation | 699 | | | 20 | |
| Total deferred tax assets | 34,106 | | | 24,495 | |
| Deferred tax liabilities: | | | |
| Operating lease right-of-use asset | — | | | (101) | |
| Property and equipment | (406) | | | (595) | |
| Total deferred tax liabilities | (406) | | | (696) | |
| Net deferred tax assets | 33,700 | | | 23,799 | |
| Less: Valuation allowance | (33,733) | | | (23,825) | |
| Net deferred tax liability | $ | (33) | | | $ | (26) | |
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Due to the lack of earnings history, the deferred tax assets have been offset by a valuation allowance net of reversing deferred tax liabilities that provided for a source of future taxable income. The valuation allowance increased by approximately $9.9 million and $7.9 million for the years ended December 31, 2023 and 2022, respectively.
The Company has net operating loss carryforwards for federal and state income tax purposes of approximately $76.3 million and $96.7 million, respectively, as of December 31, 2023. Under the Tax Act and Jobs Act of 2017, the $76.3 million of federal net operating losses generated after December 31, 2017 will be carried forward indefinitely. The California net operating loss carryforwards will begin to expire in 2037 unless previously utilized.
As of December 31, 2023, the Company also had federal and California research and development tax credit carryforward of approximately $3.2 million and $2.3 million, respectively. The federal research and development credit carryforwards will begin to expire in 2038. The California research and development credit carryforwards are available indefinitely.
Federal and California tax laws imposes significant restrictions on the utilization of net operating loss carryforwards in the event of a change in ownership of the Company, as defined by Internal Revenue Code Sections 382 and 383. The Company has not completed a formal study to determine the limitations on their tax attributes due to change in ownership and may have limitations on the utilization of net operating loss carryforwards, credit carryforwards, or other tax attributes due to ownership changes.
The Inflation Reduction Act of 2022 (“IRA”) which incorporates a Corporate Alternative Minimum Tax (CAMT) was signed on August 16, 2022. The changes will be effective for the tax years beginning after December 31, 2022. The new tax law will require companies to compute two separate calculations for federal income tax purposes and pay the greater of the new minimum tax or their regular tax liability. The IRA is not expected to have a material impact for the Company.
Uncertain Tax Benefits
No liability related to uncertain tax positions is recorded on the financial statements.
In February 2022, The following table summarizes the Russian Federation and Belarus commencedactivity related to the Company’s unrecognized tax benefits for the year ended
December 31 (in thousands): | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 |
| Beginning balance | $ | 403 | | $ | 269 |
| Additions for tax positions related to the current year | 129 | | 131 |
| Reductions for tax positions related to prior years | 19 | | 3 |
| Ending balance | $ | 551 | | $ | 403 |
The reversal of uncertain tax benefits would not affect the effective tax rate to the extent that the Company continues to maintain a military action withvaluation allowance against its deferred tax assets. The Company does not anticipate any significant changes to unrecognized tax benefits over the country of Ukraine. As a result of this action, various nations, includingnext 12 months.
Income tax returns are filed in the United States have instituted economic sanctions againstand California. The Company is not currently under audit by the Russian FederationInternal Revenue Service and Belarus. Further, the impactState of this actionCalifornia. The years 2019 and forward remain open to examination for federal income tax purposes and the years 2018 and forward for California income tax to which the Company is subject. Due to net operating loss carryforwards, all years effectively remain open to income tax examination by the domestic taxing jurisdictions in which the Company files tax returns.
The Company’s practice is to recognize interest and penalties related sanctionsto income tax matters in income tax expense. For the years ended December 31, 2023 and 2022 the Company has not recognized any interest or penalties related to income tax in the Company’s consolidated statements of operations and comprehensive loss.
16.Subsequent Events
Forward Purchase Contract Amendments
On January 2, 2024, the Company amended their Forward Purchase Agreement with an FPA Investor to increase the prepayment shortfall by $0.5 million in exchange for a $0.5 million payment to the Company. All other terms and conditions remained unchanged.
On January 11, 2024, the Company amended their Forward Purchase Agreement with an FPA Investor to increase the prepayment shortfall by $0.5 million in exchange for a $0.5 million payment to the Company. All other terms and conditions remained unchanged.
On January 19, 2024, the Company amended their Forward Purchase Agreement with an FPA Investor to increase the prepayment shortfall by $0.3 million plus 20% of the then-current prepayment shortfall balance in exchange for a $0.3 million payment to the Company. The agreement also amends the Reset Price such that the Reset Price (i) is adjusted on a rolling basis as based on the world economy are not determinableweekly trailing VWAP subject to a ceiling of $10.44 per share ("Initial Price"), and (ii) discounts of generally 10% to the VWAP measurement that benefit the FPA Investor. All other terms and conditions remained unchanged.
On February 21, 2024, the Company amended their Forward Purchase Agreement with an FPA Investor to increase the prepayment shortfall by $0.2 million and increase the Bonus Shares by 200,000 in exchange for a $0.2 million payment to the Company. All other terms and conditions remained unchanged.
PIPE Warrants Amendment
On February 9, 2024, the Company amended their Warrant Subscription Agreement with a Warrant Investor to, among other things, (i) make all subscription warrants held by the Warrant Investor immediately eligible to accelerate the share conversion provisions of the Warrant Subscription Agreement in exchange for a cash payment of $0.3 million, (ii) a second cash payment of up to $0.3 million based on the trailing 5-day VWAP following the effective registration of the shares, (iii) to grant the Warrant Investor “Most Favored Nation” status with respect to warrant restructuring for so long as any subscription warrants remain outstanding and (iv) to grant certain registration rights to the Warrant Investor. All other terms and conditions remained unchanged.
Convertible Bridge Loans
On February 7, 2024, the Company entered into a related party bridge loan agreement for $0.4 million with a 20% premium due at maturity. The related party bridge loan matures at the earlier of (i) 60 days from issuance or (ii) upon a financing event with third parties exceeding $5.0 million. In April 2024 the maturity of the bridge loan was amended to be the earliest of (i) 90 days from issuance, (ii) upon a financing event with third parties exceeding $5.0 million, or (iii) the occurrence of any event of default. The counterparty to this bridge loan agreement is also entitled to receive 400,000 warrants to purchase 400,000 shares of the Company’s common stock each at a strike price of $2.00 per share.
On February 20, 2024, the Company entered into a bridge loan agreement for $0.1 million with a 20% premium due at maturity. The bridge loan matures at the earlier of (i) 60 days from issuance or (ii) upon a financing event with third parties exceeding $10.0 million. The counterparty to this bridge loan agreement also received 100,000 warrants to purchase 100,000 shares of the Company’s common stock at a $2.00 strike price per share.
On February 27, 2024, the Company entered into a bridge loan agreement for $0.1 million with a 20% premium due at maturity. The bridge loan matures at the earlier of (i) 60 days from issuance or (ii) upon a financing event with third parties exceeding $5.0 million. The counterparty to this bridge loan agreement is also entitled to receive 3,667 shares of common stock as well as 375,000 warrants to purchase 375,000 shares of the Company’s common stock at a $1.50 strike price per share.
On March 7, 2024, the Company entered into a bridge loan agreement for $0.1 million with a 20% premium due at maturity. The bridge loan matures at the earlier of (i) 60 days from issuance or (ii) upon a financing event with third parties exceeding $5.0 million. The counterparty to this bridge loan agreement is also entitled to receive 3,667 shares of common stock as well as 375,000 warrants to purchase 375,000 shares of the Company’s common stock at a strike price.
Bridge Loans
On March 7, 2024, the Company entered into two bridge loan agreements for $0.1 million each that matured on March 22, 2024 with a 7.5% premium due at maturity. Both bridge loans were subsequently paid in full on April 10, 2024.
Convertible Promissory Notes
On March 21, 2024, the Company entered into a 12% promissory note agreement for $0.3 million with a one year term, issued at a 10% discount. The lender retained the option to convert any or all outstanding and unpaid principal amount and interest into shares of the Company's common stock from the date of issuance until the maturity date. The promissory note was subsequently paid in full on April 8, 2024. Concurrently with this agreement, the Company issued the lender warrants entitling the lender to acquire up to 330,000 shares of common stock at an initial exercise price of $2.00 per share, subject to adjustment.
On March 26, 2024, the Company entered into a 12% promissory note agreement with lender who is also a FPA Investor for $0.3 million with a one year term, issued at a 10% discount. The lender retains the option to convert any or all outstanding and unpaid principal amount and interest into shares of the Company's common stock from the date of issuance until the maturity date. Concurrently with this agreement, the Company issued the lender warrants entitling the lender to acquire up to 330,000 shares of common stock at an initial exercise price of $2.00 per share, subject to adjustment.
On April 1, 2024, the Company entered into a 12% promissory note agreement for $0.2 million with a one year term, issued at a 10% discount. The lender retains the option to convert any or all outstanding and unpaid principal amount and interest into shares of the Company's common stock from the date of issuance until the maturity date. Concurrently with this agreement, the Company issued the lender warrants entitling the lender to acquire up to 220,000 shares of common stock at an initial exercise price of $2.00 per share, subject to adjustment.
On April 1, 2024, the Company entered into a 12% promissory note agreement with lender who is also a FPA Investor for $0.3 million with a one year term, issued at a 10% discount. The lender retains the option to convert any or all outstanding and unpaid principal amount and interest into shares of the Company's common stock from the date of issuance until the maturity date. Concurrently with this agreement, the Company issued the lender warrants entitling the lender to acquire up to 330,000 shares of common stock at an initial exercise price of $2.00 per share, subject to adjustment.
Convertible Secured Promissory Note
On April 5, 2024, the Company entered into a convertible secured promissory note agreement for $5.0 million with an interest rate of the one month secured overnight financing rate plus 2.85% payable in cash in arrears on a monthly basis, with payments commencing one month from issuance which will mature on October 4, 2026. The convertible promissory note was issued in two tranches, the first of which was for $1.0 million and closed on April 8, 2024 and the second tranche was for $4.0 million which closed on April 9, 2024. The convertible secured promissory note is secured by a second lien on the Company’s owned real property located in Santa Ana, California. The convertible secured promissory note is subordinate to the $5.0 million revolving line of credit. The outstanding principal amount is convertible at any time until its maturity at the option of the lender, into common stock at a $2.00 conversion price (subject to customary anti-dilution adjustments for stock splits and the like).Concurrently with this agreement, the lender is entitled to receive 833,333 shares of common stock upon the first closing and an amount of shares equal to $2.5 million divided by a five days VWAP measurement upon the second closing as well as warrants entitling the lender to acquire up to 1,000,000 shares of common stock at an initial exercise price of $2.00 per share, subject to adjustment.
Stock Option Grants
On February 12, 2024 the Board of Directors approved the grant of 3,233,028 stock options.
Sales of FPA Shares
As of the date of issuance of these financial statements.statements, an aggregate of
1,768,121 FPA Shares were sold,
1,000,000
FPA Shares from initial issuance remained outstanding, and up to an additional
1,167,990 FPA Shares may be issued subject to the Private Placement Agreements.
Item 9.
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.Disclosure
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and
ProceduresUnder the supervision and with the participation of our management, including our principal executive officer and principal financial and accounting officer, we conducted an evaluation of the effectiveness of our disclosureProcedures.
We maintain “disclosure controls and procedures,
” as
of the end of the fiscal quarter ended December 31, 2021, as such term is defined in Rules 13a-15(e) and 15d-15(e) under the
Securities Exchange
Act. Based on this evaluation, our principal executive officer and principal financial officer have concludedAct of 1934, as amended, or the Exchange Act, that
during the period covered by this Form 10-K, our disclosure controls and procedures were not effective as of December 31, 2021, because of a material weakness in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. Specifically, the Company’s management has concluded that our control around the interpretation and accounting for certain complex financial instruments issued by the Company, and the presentation of earnings per share was not effectively designed or maintained. This material weakness resulted in the restatement of the Company’s interim financial statements for the quarter ended June 30, 2021. Additionally, this material weakness could result in a misstatement of the carrying value of complex financial instruments, and related accounts and disclosures, and the presentation of earnings per share that would result in a material misstatement of the financial statements that would not be prevented or detected on a timely basis. As a result, our management performed additional analysis as deemed necessary to ensure that our financial statements were prepared in accordance with GAAP. Accordingly, management believes that the financial statements included in this Form 10-K present fairly, in all material respects, our financial position, results of operations and cash flows of the periods presented. Management understands that the accounting standards applicable to our financial statements are complex and has since the inception of the Company benefited from the support of experienced third-party professionals with whom management has regularly consulted with respect to accounting issues. Management intends to continue to further consult with such professionals in connection with accounting matters.Disclosure controls and procedures are designed to ensure that information required to be disclosed by us in ourthe reports that we file or submit under the Exchange Act reports is (1) recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and that such information is(2) accumulated and communicated to our management, including our principal executive officer and principal financial officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Controls Over Financial Reporting
This Form 10-K does not include a report Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of management’s assessment regarding internal control over financial reporting or an attestation reportachieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Our management, with the participation of our
independent registered public accounting firm due toprincipal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2023. Based upon the evaluation, our principal executive officer and principal financial officer concluded that, as of such date, our disclosure controls and procedures were effective and were operating at a
transition period established by rules of the SEC for newly public companies.reasonable assurance level.
Changes in Internal Control over Financial
ReportingReporting.
There were no changes in our internal control over financial reporting
(as such term is definedidentified in
Rules 13a-15(f)connection with the evaluation required by Rule 13a-15(d) and
15d-15(f)15d-15(d) of the Exchange
Act)Act that occurred during the
most recent fiscal quarterperiod covered by this Annual Report on Form 10-K that
havehas materially affected, or
areis reasonably likely to materially affect, our internal control over financial
reporting, as the circumstances that led to the restatement of our financial statements as noted below.reporting.
Management’s Report on Internal Control Over Financial Reporting
Our principal executive officermanagement is responsible for establishing and principal financial officer performed additional accounting and financial analyses and other post-closing procedures including consulting with subject matter experts related to the accounting for certain complex features of the redeemable common stock and warrants issued by the Company, and the presentation of earnings per share. The Company’s management has expended, and will continue to expend, a substantial amount of effort and resources for the remediation and improvement of ourmaintaining adequate internal control over financial reporting. While we have processes to properly identifyInternal control over financial reporting is defined in Rules 13a-15(f) and evaluate15d-15(f) promulgated under the appropriate accounting technical pronouncementsExchange Act as a process designed by, or under the supervision of, a company's principal executive and principal financial officers and effected by the Company's board of directors, management and other literaturepersonnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for all significant or unusual transactions,external purposes in accordance with U.S. GAAP.
As disclosed elsewhere in this Annual Report on Form 10-K, we have expandedcompleted the Business Combination on September 29, 2023. Prior to the Business Combination, NKGen was a special purpose acquisition corporation. As a result, the design of the Company’s public company internal control over financial reporting post-Business Combination has required and will continue to improve these processesrequire significant time and resources from our management and other personnel. Therefore, management was unable, without deploying an unreasonable level of resources, to ensure that the nuances of such transactions are effectively evaluated in the contextconduct an assessment of the increasingly complex accounting standards.
Company’s internal control over financial reporting as of December 31, 2023. Therefore, the Company is excluding management’s report on internal control over financial reporting pursuant to Section 215.02 of the SEC’s Regulation S-K Compliance and Disclosure Interpretations.
Item 9B. Other Information
Item 9C. Disclosure Regarding Foreign
Jurisdictions thatJurisdiction the Prevent
InspectionsInspection
Not applicable.
Item 10.
Directors, Executive Officers and Corporate Governance Officers and DirectorsOur executive officers and directors are as follows:
| | | | | |
NAME
|
| AGE
|
| POSITION
|
|
James A. Graf
|
| 57
|
| Chief Executive Officer
|
|
Gus Garcia
|
| 41
|
| Co-President and Director
|
|
Lewis Silberman
|
| 43
|
| Co-President and Director
|
|
Anantha Ramamurti
|
| 46
|
| Chief Financial Officer and Treasurer
|
|
Anthony A. Kuznik
|
| 57
|
| Executive Vice President, General Counsel and Secretary
|
|
Sabrina McKee
|
| 54
|
| Executive Vice President, Strategy
|
|
Edwin J. Rigaud
|
| 78
|
| Director
|
|
A.B. Cruz III
|
| 63
|
| Director
|
|
Jeanne L. Manischewitz
|
| 48
|
| Director
|
|
Alexandra Lebenthal
|
| 58
|
| Director
|
|
James A. Graf has been our Chief Executive Officer since January 2021. Currently, Mr. Graf has served as an independent director of Catcha Investment Corp. (NYSE: CHAA) since February 2021, and as the chief executive officer of Graf II and Graf III since July 2020 and January 2021, respectively. Mr. Graf served as the chief executive officer of Graf Industrial Corp., a blank check company, from June 2018 through its business combination with Velodyne Lidar, Inc. in September 2020. Mr. Graf served as a director of Graf Industrial Corp. from June 2018 to September 2019 and served as a director of Velodyne Lidar, Inc. from September 2020 to February 2021. Mr. Graf served as a director of Platinum Eagle Acquisition Corp. from January 2018 through its business combination with Target Logistics Management, LLC and RL Signor Holdings, LLC in March 2019. Mr. Graf served as the vice president, chief financial officer and treasurer of Double Eagle Acquisition Corp. from its inception in June 2015 through its business combination with Williams Scotsman, Inc. in November 2017. He served as vice president, chief financial officer, treasurer and secretary of Silver Eagle Acquisition Corp. from its inception in April 2013 through Silver Eagle’s business combination with Videocon d2h Limited (“VDTH”), and he served as vice president, chief financial officer, treasurer and secretary of Global Eagle Acquisition Corp. (“GEE”) from its inception in February 2011 to its business combination with Row 44, Inc. and Advanced Inflight Alliance AG in January 2013. He was vice chairman of Global Entertainment AG, the German entity holding GEE’s equity in AIA from 2013 to 2014 and special advisor to GEE in 2013. He served as a special advisor to VDTH from 2015 to 2016. From 2008 to 2011 Mr. Graf served as a managing director of TC Capital Ltd., an investment bank, in Singapore. From 2007 to 2008, Mr. Graf was engaged as a consultant to provide financial advisory services to Metro- Goldwyn-Mayer, Inc. In 2001, Mr. Graf founded and became chief executive officer of Praedea Solutions, Inc., an enterprise software company with operations in the United States, Malaysia and Ukraine. The assets of Praedea Solutions, Inc. were sold in 2006 to a Mergent Inc., a wholly-owned subsidiary of Xinhua Finance Ltd., and renamed Mergent Data Technology, Inc., where Mr. Graf continued to serve as Chief Executive Officer from 2006 to 2007. Praedea Solutions Inc. was renamed PSI Capital Inc., and currently serves as an investment holding company for Mr. Graf’s private investments. Mr. Graf continues toinformation required by this Item will be chief executive of PSI Capital Inc. Prior to founding Praedea, Mr. Graf was a managing director at Merrill Lynch, in Singapore from 1998 to 2000 and a consultant to Merrill Lynch in 2001. From 1996 to 1998, Mr. Graf served as a director and then managing director and president of Deutsche Bank’s investment banking entity in Hong Kong, Deutsche Morgan Grenfell (Hong Kong) Ltd. From 1993 to 1996, he was a vice president at Smith Barney in Hong Kong and Los Angeles. From 1987 to 1993, Mr. Graf was an analyst and then associate at Morgan Stanley in New York, Los Angeles, Hong Kong and Singapore. Mr. Graf received a Bachelor of Arts degree from the University of Chicago in 1987.
Gus Garcia has been our Co-President since March 2021 and has served on our board of directors since May 2021. Mr. Garcia has served as the co-chief executive officer of GSR and as a member of GSR’s board of directors since October 2021, as a managing partner of SAP LLC since its inception in 2021, and has also served as a co-president of both Graf II and Graf III since March 2021. Mr. Garcia is the former Head of SPAC M&A for Bank of America, where he was responsible for advising private companies and SPACs on all aspects of mergers involving SPACs. In his last 12 months at Bank of America, Mr. Garcia advised on 13 SPAC transactions with, in the aggregate, approximately $20 billion in negotiated equity value. The five most recently announced SPAC transactions that Mr. Garcia advised on are the business combination between BuzzFeed and 890 5th Avenue Partners, the business combination between Velo3D and Jaws Spitfire Acquisition Corp., the business combination between Spire Global and NavSight Holdings, the business combination between Origin Materials and Artius Acquisition Inc., and the business combination between XOS and NextGen Acquisition Corp. In addition, Mr. Garcia has worked on and overseen transactions with an excess of $150 billion in value across PIPEs, corporate separations and other mergers & acquisitions. Prior to being the Head of SPAC M&A, Mr. Garcia led the separations practice for Bank of America where he focused on complex corporate transactions such as spin-offs (including one of the top 5 largest spin-offs in corporate history), split-offs, reverse morris trusts, carve-out IPOs and structured private capital raises. Prior to the merger with Bank of America, Mr. Garcia worked at Merrill Lynch in the Corporate Finance group where he also focused on complex corporate transactions, as well as structured investments held on the bank’s balance sheet in partnership with Merrill Lynch’s Global Principal Investment group. Prior to Merrill Lynch, Mr. Garcia worked in HSBC’s Healthcare Investment Banking Group and, prior to HSBC, he worked in Wells Fargo Securities’ M&A group. Mr. Garcia also sits on the Board of Directors for New York Cares. Mr. Garcia graduated magna cum laude with a Bachelor of Science in Business Administration and a Master of Science in International Commerce & Finance from Georgetown University in 2003.
Lewis Silberman has been our Co-President since March 2021 and has served on our board of directors since May 2021. Mr. Silberman has served as the co-chief executive officer of GSR and as a member of GSR’s board of directors since October 2021, as a managing partner of SAP LLC since its inception in 2021, and has also served as a co-president of both Graf II and Graf III since March 2021. Mr. Silberman is the former Head of SPAC Equity Capital Markets for Oppenheimer & Co. Inc., where he led financings for the firm’s SPAC IPOs and business combination clients. During his last year at Oppenheimer & Co. Inc., Mr. Silberman has managed SPAC IPOs including Gig4 Acquisition Corp. (GIGGU), Noble Rock Acquisition Corp. (NRACU), MDH Acquisition (MDH/U), Class Acceleration Corp. (CLAS/U), and Rodgers Silicon Valley Acquisition Corp. (RSVAU). Additionally, during this time, Mr. Silberman and his team acted in an advisory or placement agent role on transactions including Ascendent Acquisition Corp.’s combination with financial media and content company Beacon Street Group Holdings, Rodgers Silicon Valley Acquisition Corp.’s combination with next-generation battery manufacturer Enovix, Alpha Healthcare Acquisition Corp.’s combination with bioengineering firm Humacyte, Acies Acquisition Corp.’s combination with mobile gaming and loyalty rewards program company PlayStudios, GigCapital2’s combination with healthcare technology provider UpHealth/Cloudbreak, and Roth CH Acquisition I Co.’s combination with PureCycle Technologies. Prior to his role in Oppenheimer’s Equity Capital Markets group, Mr. Silberman was the Head of Equity Sales for Oppenheimer for five years. Before joining Oppenheimer, Mr. Silberman spent three years at CIBC World Markets Corp., where he worked in a special situations client-coverage group focused on strategies including merger-arbitrage, ADR-arbitrage, and closed-end fund arbitrage. Prior to CIBC World Markets, Mr. Silberman worked at PaineWebber, Inc. Mr. Silberman holds a Bachelor of Science degree from the Leonard N. Stern School of Business at New York University, with a dual major in finance and marketing (2000), as well as a Masters of Business Administration from the Stern School at New York University, with a dual concentration in Financial Markets and Management (2010). Mr. Silberman has completed three New York City Marathons (2017, 2018, and 2019) as a member of Fred’s Team to raise money for Memorial Sloan-Kettering Cancer Center.
Anantha Ramamurti has been our Chief Financial Officer and Treasurer since March 2021. Mr. Ramamurti has served as the president of GSR and as a member of GSR’s board of directors since October 2021, as a managing partner of SAP LLC since its inception in 2021, and has also served as the chief financial officer and treasurer of both Graf II and Graf III since March 2021. Mr. Ramamurti has over 23 years of experience in the Technology sector across engineering, corporate finance and investment banking roles. Mr. Ramamurti was most recently a Managing Director and the Head of Global Mobility Group at Bank of America Securities where he was responsible for the coverage of the AutoTech sector and other emerging technologies since joining the firm in 2017. During his career at Bank of America, Mr. Ramamurti advised on several SPAC merger transaction, including the sale of Spire Global to NavSight Holdings, the acquisition of Lucid Motors by Churchill Capital Corp. IV, the sale of Xos Trucks to NextGen Acquisition Corp., the acquisition of EVgo by Climate Change Crisis Real Impact I Acquisition Corporation, the sale of Proterra to ArcLight Clean Transition Corp., the sale of Lightning eMotors to GigCapital3, the sale of ChargePoint to Switchback Energy Acquisition Corporation, the sale of Canoo to Hennessy Capital Acquisition Corp. IV, and the sale of Velodyne Lidar to Graf Industrial Corp. Prior to Bank of America, Mr. Ramamurti worked at Deutsche Bank since 2010 where he was most recently a Director in the Technology Investment Banking group and covered clients across semiconductor, communications, networking and cleantech sectors. Mr. Ramamurti started
his investment banking career in 2009 at Guggenheim Securities in its Consumer & Retail investment banking division. During his banking career, Mr. Ramamurti had led execution on over 65 transactions totaling over $80 billion in transaction value across all product areas, including equity offerings, debt issuances, SPAC mergers and other M&A advisory. Prior to banking, Mr. Ramamurti served as a Senior Financial Analyst at Taco Bell, a division of Yum! Brands, where he oversaw the operations of company-owned stores in several states in the Midwest. Prior to finance, Mr. Ramamurti spent almost 12 years in various engineering roles at Rockwell Semiconductor Systems, Texas Instruments and several other firms, where he was responsible for the design and development of semiconductor processor chips. Mr. Ramamurti holds four patents in the areas of design and development. Mr. Ramamurti has an MBA with Honors in Finance from the UCLA Anderson School of Management, a Master’s with Honors in Electrical Engineering from the University of Pittsburgh, and a Bachelor’s with Honors in Instrumentation from the Birla Institute of Technology and Science (BITS), Pilani, India.
Anthony A. Kuznik has been our Executive Vice President, General Counsel and Secretary since February 2021. Currently, Mr. Kuznik has served as the executive vice president, general counsel and secretary of both Graf II and Graf III since February 2021. Mr. Kuznik was most recently the vice president and general counsel of two strategic business units of Honeywell International Inc., a Fortune 100 company, where he served as the chief legal counsel responsible for the strategic direction and execution of global legal activities and the management of legal and contracts personnel worldwide. He was vice president and general counsel of Honeywell Building Solutions from 2009 - 2020 and vice president and general counsel of Honeywell Sensing and Control from 2005 - 2009. From 2001 - 2005, Mr. Kuznik was executive vice president, general counsel and director of Praedea Solutions, Inc., an enterprise software company with operations in the United States, Malaysia and Ukraine. From 1999 - 2001, Mr. Kuznik was a director at the investment banking firm Goldsmith Agio Helms (later Lazard Middle Market), where he was a senior member of M&A teams responsible for originating and executing domestic and international, middle-market transactions. From 1996 - 1999, Mr. Kuznik was an associate director at Deutsche Bank’s investment banking entity in Hong Kong, Deutsche Morgan Grenfell (Hong Kong) Ltd. From 1995 - 1996, Mr. Kuznik was the managing attorney at Law Audit Services in St. Paul, a national legal management and auditing firm. From 1990 - 1995, Mr. Kuznik was an attorney at the law firm of Dorsey & Whitney in Minneapolis. Mr. Kuznik received a Juris Doctor degree from the Columbia University School of Law in 1990 and a Bachelor of Arts (Hons) degree from the University of Chicago in 1987. Mr. Kuznik also completed an Executive Management Program at the European Institute of Business Administration (INSEAD) in 1996.
Sabrina McKee has been our Executive Vice President, Strategy since April 2021. Currently, Ms. McKee has served as the executive vice president (Strategy) of both Graf II and Graf III since April 2021. Ms. McKee was most recently Director, Head of North America Strategy at Ford Motor Company until March 2021. Ms. McKee was previously Global Head of Mobility Strategy from February 2019 to January 2020 and Director of Investor Relations at Ford from February 2017 to February 2019. Ms. McKee served as a Director of Graf Industrial Corp. from October 2018 to September 2020. Ms. McKee a capital markets professional with over 20 years of experience in all aspects of the investment process, has a unique blend of sellside, buyside and corporate experience offering a comprehensive understanding of the investment process. From April 2014 to June 2016, Ms. McKee was Managing Director, Head of Equity Capital Markets at Sterne Agee CRT LLC, where she worked with public and private companies on all aspects of the capital-raising process, including SPACs. From 2011 to 2014, Ms. McKee worked as a Director of the Corporate Access businesses at Guggenheim Securities LLC and during 2010 she worked as an Executive Director at Morgan Stanley where she connected public and private companies to the investment community. From 2007 to 2010, Ms. McKee was Vice President at Two Sigma Investments LP, where she helped build out a successful, global, Alpha Capture business, enabling quantitative investment managers to integrate fundamental factors into quantitative stock selection models. In addition, from 2000 to 2007, Ms. McKee worked for UBS Investment Bank as an Executive Director of Equity Research Sales and Equities, from 1999 to 2000 for Schroders plc as a Senior Vice President and from 1991 to 1998 for Tucker Anthony as a Senior Vice President of Institutional Research Sales, where she covered a diverse range of large institutional investors. Ms. McKee received a Bachelor of Arts degree from William Smith College in 1989.
Edwin J. Rigaud has served on our board of directors since May 2021. Mr. Rigaud has more than 50 years of business experience across a multitude of operating and leadership roles. He served as Chairman of the Board of Directors and Chief Executive Officer of Legacy Acquisition Corp., a SPAC, since its inception in November 2017 until the closing of its business combination with PARTS iD, Inc. in November 2020. He continues to serve as a member of the board of directors of PARTS iD, Inc. In 2007, Mr. Rigaud founded EnovaPremier and commenced operations through the acquisition of the assets of T&WA, Inc. from entrepreneur Tommie Burns and his partner, the Goodyear Tire & Rubber Co. Since that time, he has served as owner and the President and Chief Executive Officer of EnovaPremier (2007-2018) and as Chairman (2019) while guiding that company to a position as one of the leading providers of automotive tire & wheel pre-assembly services in the United States. Prior to founding EnovaPremier, Mr. Rigaud served in numerous operating and management capacities at Procter & Gamble from 1965 to 2001. Mr. Rigaud’s notable leadership positions at Procter &
Gamble included his role as a Vice President of Food & Beverage Products and as a Vice President of Government Relations in North America. Adding to his experience as a senior manager, Mr. Rigaud developed significant expertise in product development and brand management having been the first Technical Brand Manager in the exploratory phase of Pringles, and ultimately the Product Development Group Leader during the execution of Pringles’ national launch. Mr. Rigaud also led the product development efforts of Secret Deodorant & Antiperspirant improvements, including key active ingredient technology and perfume upgrades, while having direct participation with the Leo Burnett Agency in the creation of the famous advertising slogan, “strong enough for a man, but made for a woman.” Mr. Rigaud’s leadership in these efforts helped to facilitate a major relaunch of the Secret brand. He was ultimately named a Director in Product Development. Outside of his corporate leadership experience, Mr. Rigaud has served on the Board of the Federal Reserve Bank of Cleveland and the Board of the local affiliate of Fifth Third Bank of Cincinnati. Mr. Rigaud has also held appointments by Governor Bob Taft to the Ohio Board of Regents, and by President George W. Bush to the national Institute of Museum and Library Services. In 1997, Mr. Rigaud became the first CEO of the National Underground Railroad Freedom Center, located in Cincinnati, Ohio. This 9-year development program included raising $110 million while working closely with John Pepper, former Chairman and CEO of Procter & Gamble, who served as the national building Campaign Chairman. Mr. Rigaud is also the head of one of the first African American co-ownership groups of a Major League Baseball franchise, the Cincinnati Reds.
Rear Admiral Anatolio Benedicto (A.B.) Cruz III has served on our board of directors since May 2021. Since April 2020, Admiral Cruz has been a Senior Advisor at BarkerGilmore LLC, where he provides executive coaching, leadership development and executive search services to legal leaders, CEOs and corporate boards. Since November 2019, he has served as the President of the Board of Governors for the National Asian Pacific American Bar Association. From September 2016 to October 2018, he served as Senior Vice President & Divisional General Counsel at USAA, a Fortune 100 financial services company, where he led the legal teams that supported the Company’s C-suite members and their organizations. He spearheaded the optimization of the chief legal office’s operating and interaction models, and reviewed and approved new products and services to foster USAA’s future growth, competitiveness, and technology transformation. From 2013-2016, Admiral Cruz was Executive Vice President, General Counsel, Corporate Secretary, Chief Compliance & Ethics Officer, and PAC chairman for Emergent BioSolutions, Inc. (NYSE: EBS). From 2008 to 2012, Admiral Cruz served as Executive Vice President, Chief Legal Officer & Corporate Secretary for Scripps Networks Interactive, Inc. (Nasdaq: SNI), and from 2004-2008 was Executive Vice President & General Counsel for The E.W. Scripps Company (Nasdaq: SSP). He was Vice President and Deputy General Counsel of BET Networks, playing a leading role in BET’s $3 billion merger with Viacom (Nasdaq: VIA). Other private sector roles include serving as an attorney at Wiley Rein LLP, Roberts & Eckard, Gardner Carton & Douglas, and Leventhal Senter & Lerman. Admiral Cruz served the United States in the US Navy for 33 years, including the following command positions: Reserve Deputy Commander, Maritime Operations at US Fleet Forces Command; Deputy Commander at US Naval Forces Command / US Fourth Fleet; Deputy Commander, Navy Region Southwest; Commanding Officer, Naval Expeditionary Combat Command; Commanding Officer, Naval Inspector General; Commanding Officer, Harbor Defense Command 207; Commanding Officer, Naval Special Warfare Unit 2; and Commanding Officer, Mobile Inshore Undersea Warfare 206. Admiral Cruz holds a JD from The Catholic University of America, an MA in Marketing from The University of Maryland, and a BS in General Engineering & Physical Sciences from U.S. Naval Academy. Admiral Cruz has served on numerous boards and previously served as the board chair for the Minority Corporate Counsel Association. He currently serves as an advisory board member for Bellatorum Resources as well as VetStoreUSA. He is also a board director and committee chair for the Yellow Ribbon Fund, a board director and governance committee chair for the World Affairs Council of San Antonio, and a board director for the Down Syndrome Association of South Texas. He also serves as a board trustee, executive committee member and finance committee chair for Saint Mary’s Hall, and as a board trustee and governance committee member for the U.S. Naval Academy Alumni Association & Foundation.
Jeanne L. Manischewitz has served on our board of directors since May 2021. Ms. Manischewitz is an experienced fiduciary and investment professional with over 25 years in the financial services industry. She currently serves as an independent director of Healthcare Services Acquisition Corp., a SPAC. Most recently she spent 15 years at York Capital Management where she was a portfolio manager and a partner of the firm until September 2020. During that time, she also served on the firm’s ESG committee and was a steering committee member of the Women’s Network. Prior to her time at York Capital Management, Ms. Manischewitz spent a total of seven years as a senior credit analyst at Moore Capital Management and Halcyon Capital Management. Ms. Manischewitz started her career on Wall Street as an investment banker at Salomon Smith Barney. Ms. Manischewitz received her undergraduate degree from Princeton University.
Alexandra Lebenthal has served on our board of directors since July 2021. Ms. Lebenthal is a senior advisor in the Financial Sponsors Group at Houlihan Lokey, Inc. (NYSE: HLI) where she leads an initiative focused on female led and founded companies. Ms. Lebenthal previously was the chief executive officer of Lebenthal Holdings, a diversified financial services firm that included one of the nation’s largest woman-owned broker dealers. She was president of Lebenthal Funds, overseeing the firm’s mutual and money
market funds. Ms. Lebenthal engineered the sale of Lebenthal Funds in 2001 to The Advest Group, remaining at the firm until it was subsequently sold to Merrill Lynch in 2005. During that time, in addition to managing Lebenthal Funds, she also ran marketing and municipal capital markets for the parent company. Lebenthal Funds was the number one woman-owned firm in debt and equity capital markets from 2012-2016. Ms. Lebenthal was named one of the top 50 Women in Wealth Management by Wealth Manager Magazine, as well as Private Asset Management. She has also been named to the Crain’s New York Top Women-Owned Businesses and the Crain’s Fastest 50 Growing Businesses in New York. She currently is an advisory board member for Interprice Technologies, a former board member and Treasurer of the Securities Industry Financial Markets Association (SIFMA), and a member of C200, the leading organization for female businesswomen. Ms. Lebenthal is the co-founder of “The Women’s Executive Circle,” and “Women On Wall Street”, which cultivates high-profile Jewish women under the auspices of United Jewish Appeal. Ms. Lebenthal’s first novel, “The Recessionistas,” a thriller set in Manhattan during the financial crisis, was published in August 2010. Ms. Lebenthal is a graduate of Princeton University with an A.B. in history.
Number and Terms of Office of Officers and Directors
Our board of directors consists of six members, including four independent members. Our board is divided into three classes, with only one class of directors being elected in each year, and with each class (except for those directors appointed prior to our first annual meeting of stockholders) serving a three-year term. The term of office of the first class of directors, consisting of Gus Garcia and Lewis Silberman, will expire at our first annual meeting of stockholders. The term of office of the second class of directors, consisting of Edwin J. Rigaud and Anatolio Benedicto (A.B.) Cruz III, will expire at our second annual meeting of stockholders. The term of office of the third class of directors, consisting of Jeanne L. Manischewitz and Alexandra Lebenthal will expire at our third annual meeting of stockholders. We may not hold an annual meeting of stockholders until after we consummate our initial business combination. Subject to any other special rights applicable to the stockholders, any vacancies on our board of directors may be filled by the affirmative vote of a majority of the directors present and voting at the meeting of our board, or by a majority of the holders of our common stock. Our executive officers are appointed by the board of directors and serve at the discretion of the board of directors, rather than for specific terms of office. Our board of directors is authorized to appoint persons to the offices set forth in our bylaws as it deems appropriate. Our bylaws provide thatdefinitive proxy statement for our officers may consist2024 Annual Meeting of a Chairman of the Board, Chief Executive Officer, Chief Financial Officer, President, Vice Presidents, Secretary, Treasurer, Assistant SecretariesStockholders (the “Proxy Statement”) and such other offices as may be determinedis incorporated herein by the board of directors.
Committees of the Board of Directors
Our board of directors has three standing committees: an audit committee, a compensation committee and a nominating and corporate governance committee. Each of our audit committee, compensation committee and nominating and corporate governance committee are comprised solely of independent directors.
Audit Committeereference.
The members of our audit committee are Jeanne L. Manischewitz, Alexandra Lebenthal, Edwin J. Rigaud and Anatolio Benedicto (A.B.) Cruz III. Jeanne L. Manischewitz serves as chairman of the audit committee.
Each member of the audit committee is financially literate and our board of directors has determined that Jeanne L. Manischewitz qualifies as an “audit committee financial expert” as defined in applicable SEC rules and has accounting or related financial management expertise.
We have adopted an audit committee charter, which details the principal functions of the audit committee, including:
| ● | assisting board oversight of (1) the integrity of our financial statements, (2) our compliance with legal and regulatory requirements, (3) our independent auditor’s qualifications and independence, and (4) the performance of our internal audit function and independent auditors; the appointment, compensation, retention, replacement, and oversight of the work of the independent auditors and any other independent registered public accounting firm engaged by us; |
| ● | pre-approving all audit and non-audit services to be provided by the independent auditors or any other registered public accounting firm engaged by us, and establishing pre-approval policies and procedures; reviewing and discussing with the independent auditors all relationships the auditors have with us in order to evaluate their continued independence; |
| ● | setting clear policies for audit partner rotation in compliance with applicable laws and regulations; obtaining and reviewing a report, at least annually, from the independent auditors describing (1) the independent auditor’s internal quality-control procedures and (2) any material issues raised by the most recent internal quality-control review, or peer review, of the audit firm, or by any inquiry or investigation by governmental or professional authorities, within the preceding five years respecting one or more independent audits carried out by the firm and any steps taken to deal with such issues; |
| ● | meeting to review and discuss our annual audited financial statements and quarterly financial statements with management and the independent auditor, including reviewing our specific disclosures under “Management’s Discussion and Analysis of Financial Condition and Results of Operations”; reviewing and approving any related party transaction required to be disclosed pursuant to Item 404 of Regulation S-K promulgated by the SEC prior to us entering into such transaction; and |
| ● | reviewing with management, the independent auditors, and our legal advisors, as appropriate, any legal, regulatory or compliance matters, including any correspondence with regulators or government agencies and any employee complaints or published reports that raise material issues regarding our financial statements or accounting policies and any significant changes in accounting standards or rules promulgated by the Financial Accounting Standards Board, the SEC or other regulatory authorities. |
Compensation Committee
The members of our Compensation Committee are Edwin J. Rigaud, Alexandra Lebenthal, Anatolio Benedicto (A.B.) Cruz III and Jeanne L. Manischewitz. Edwin J. Rigaud serves as chairman of the compensation committee.
We have adopted a compensation committee charter, which details the principal functions of the compensation committee, including:
| ● | reviewing and approving on an annual basis the corporate goals and objectives relevant to our Chief Executive Officer’s compensation, evaluating our Chief Executive Officer’s performance in light of such goals and objectives and determining and approving the remuneration (if any) of our Chief Executive Officer based on such evaluation; |
| ● | reviewing and making recommendations to our board of directors with respect to the compensation, and any incentive-compensation and equity-based plans that are subject to board approval of all of our other officers; |
| ● | reviewing our executive compensation policies and plans; |
| ● | implementing and administering our incentive compensation equity-based remuneration plans; assisting management in complying with our proxy statement and annual report disclosure requirements; |
| ● | approving all special perquisites, special cash payments and other special compensation and benefit arrangements for our officers and employees; |
| ● | producing a report on executive compensation to be included in our annual proxy statement; and |
| ● | reviewing, evaluating and recommending changes, if appropriate, to the remuneration for directors. |
Accordingly, it is likely that prior to the consummation of an initial business combination, the compensation committee will only be responsible for the review and recommendation of any compensation arrangements to be entered into in connection with such initial business combination.
The charter also provides that the compensation committee may, in its sole discretion, retain or obtain the advice of a compensation consultant, independent legal counsel or other adviser and will be directly responsible for the appointment, compensation and oversight of the work of any such adviser. However, before engaging or receiving advice from a compensation consultant, external
legal counsel or any other adviser, the compensation committee will consider the independence of each such adviser, including the factors required by the NYSE and the SEC.
Nominating and Corporate Governance Committee
The members of our nominating and corporate governance are Edwin J. Rigaud, Alexandra Lebenthal, Anatolio Benedicto (A.B.) Cruz III and Jeanne L. Manischewitz. Anatolio Benedicto (A.B.) Cruz III serves as chair of the nominating and corporate governance committee.
We have adopted a nominating and corporate governance committee charter, which details the purpose and responsibilities of the nominating and corporate governance committee, including:
| ● | identifying, screening and reviewing individuals qualified to serve as directors, consistent with criteria approved by the board, and recommending to the board of directors candidates for nomination for election at the annual meeting of stockholders or to fill vacancies on the board of directors; |
| ● | developing and recommending to the board of directors and overseeing implementation of our corporate governance guidelines; |
| ● | coordinating and overseeing the annual self-evaluation of the board of directors, its committees, individual directors and management in the governance of the company; and |
| ● | reviewing on a regular basis our overall corporate governance and recommending improvements as and when necessary. |
The charter also provides that the nominating and corporate governance committee may, in its sole discretion, retain or obtain the advice of, and terminate, any search firm to be used to identify director candidates, and will be directly responsible for approving the search firm’s fees and other retention terms.
We have not formally established any specific, minimum qualifications that must be met or skills that are necessary for directors to possess. In general, in identifying and evaluating nominees for director, the board of directors considers educational background, diversity of professional experience, knowledge of our business, integrity, professional reputation, independence, wisdom, and the ability to represent the best interests of our stockholders.
Compensation Committee Interlocks and Insider Participation
None of our officers currently serves, or in the past year has served, as a member of the compensation committee of any entity that has one or more officers serving on our board of directors.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics applicable to our directors, officers and employees. We filed a copy of our Code of Business Conduct and Ethics as an exhibit to the registration statement in connection with our IPO. You are able to review this document by accessing our public filings at the SEC’s web site at www.sec.gov. In addition, a copy of the Code of Business Conduct and Ethics and the charters of the committees of our board of directors can be provided without charge upon request from us. If we make any amendments to our Code of Business Conduct and Ethics other than technical, administrative or other non-substantive amendments, or grant any waiver, including any implicit waiver, from a provision of the Code of Business Conduct and Ethics applicable to our principal executive officer, principal financial officer principal accounting officer or controller or persons performing similar functions requiring disclosure under applicable SEC or NYSE rules, we will disclose the nature of such amendment or waiver on our website. The information included on our website is not incorporated by reference into this Form 10-K or in any other report or document we file with the SEC, and any references to our website are intended to be inactive textual references only.
Limitation on Liability and Indemnification of Officers and Directors
Our amended and restated certificate of incorporation provides that our officers and directors will be indemnified by us to the fullest extent authorized by Delaware law, as it now exists or may in the future be amended. In addition, our amended and restated certificate of incorporation provides that our directors will not be personally liable for monetary damages to us or our stockholders for breaches of their fiduciary duty as directors, unless they violated their duty of loyalty to us or our stockholders, acted in bad faith, knowingly or intentionally violated the law, authorized unlawful payments of dividends, unlawful stock purchases or unlawful redemptions, or derived an improper personal benefit from their actions as directors.
We have entered into agreements with our executive officers and directors to provide contractual indemnification in addition to the indemnification provided for in our amended and restated certificate of incorporation. Our bylaws also will permit us to secure insurance on behalf of any officer, director or employee for any liability arising out of his or her actions, regardless of whether Delaware law would permit such indemnification. We will purchase a policy of directors’ and officers’ liability insurance that insures our officers and directors against the cost of defense, settlement or payment of a judgment in some circumstances and insures us against our obligations to indemnify our officers and directors.
These provisions may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against officers and directors, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against officers and directors pursuant to these indemnification provisions.
We believe that these provisions, the directors’ and officers’ liability insurance and the indemnity agreements are necessary to attract and retain talented and experienced officers and directors.
Item 11.
Executive Compensation.None of our executive officers or directors has received any cash compensation for services rendered. WeCompensation
The information required by this Item will
reimburse G-SPAC Management LLC, an affiliate of our sponsor, for office space, secretarial and administrative services provided to members of our management team in an amount not to exceed $15,000 per monthbe set forth in the
event such space and/or services are utilized and we do not pay directly for such services. No compensation of any kind, including any finder’s fee, reimbursement, consulting fee or monies in respect of any payment of a loan, will be paidProxy Statement is incorporated herein by
us to our sponsor, officers and directors, or any affiliate of theirs, for services rendered prior to, or for any services rendered in order to effectuate, the consummation of our initial business combination (regardless of the type of transaction that it is). However, these individuals will be entitled to certain payments including, but not limited to, reimbursement for any out-of-pocket expenses incurred in connection with activities on our behalf such as identifying potential target businesses and performing due diligence on suitable business combinations. Additionally, these individuals will be eligible to receive a transfer or reallocation of founder shares for any extraordinary services rendered in order to identify or effectuate the consummation of our initial business combination. Our audit committee will review on a quarterly basis all payments that were made to our sponsor, officers or directors, or our or their affiliates. Any such payments prior to an initial business combination will be made using funds held outside the trust account. Other than quarterly audit committee review of such payments, we do not expect to have any additional controls in place governing our reimbursement payments to our directors and executive officers for their out-of-pocket expenses incurred in connection with identifying and consummating an initial business combination.After the completion of our initial business combination, directors or members of our management team who remain with us may be paid consulting or management fees from the combined company. All of these fees will be fully disclosed to stockholders, to the extent then known, in the tender offer materials or proxy solicitation materials furnished to our stockholders in connection with a proposed initial business combination. We have not established any limit on the amount of such fees that may be paid by the combined company to our directors or members of management. It is unlikely the amount of such compensation will be known at the time of the proposed initial business combination, because the directors of the post-combination business will be responsible for determining officer and director compensation. Any compensation to be paid to our officers will be determined, or recommended to the board of directors for determination, either by a compensation committee constituted solely by independent directors or by a majority of the independent directors on our board of directors.
reference.
We do not intend to take any action to ensure that members of our management team maintain their positions with us after the consummation of our initial business combination, although it is possible that some or all of our officers and directors may negotiate employment or consulting arrangements to remain with us after our initial business combination. The existence or terms of any such employment or consulting arrangements to retain their positions with us may influence our management’s motivation in identifying or selecting a target business but we do not believe that the ability of our management to remain with us after the consummation of our initial business combination will be a determining factor in our decision to proceed with any potential business combination. We are not party to any agreements with our officers and directors that provide for benefits upon termination of employment.
Item 12.
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.Stockholders Matters
The
following table setsinformation required by this Item will be set forth
information regarding the beneficial ownership of our common stock as of March 30, 2022, by: | ● | each person known by us to be the beneficial owner of more than 5% of our outstanding shares of common stock; |
| ● | each of our officers and directors; and |
| ● | all our officers and directors as a group. |
Unless otherwise indicated, we believe that all persons named in the table below have sole voting and investment power with respect to all shares of common stock beneficially ownedProxy Statement is incorporated herein by them. The following table does not reflect beneficial ownership of the public warrants or private placement warrants as these warrants are not exercisable within 60 days of the date of this report.
We have based our calculation of the percentage of beneficial ownership on 21,451,875 shares of our common stock issued and outstanding as of December 31, 2021.
| | | | | |
| | NUMBER OF | | APPROXIMATE | |
| | SHARES | | PERCENTAGE OF | |
| | BENEFICIALLY | | OUTSTANDING | |
NAME AND ADDRESS OF BENEFICIAL OWNER(1) | | OWNED(2) | | COMMON STOCK | |
Directors, Executive Officers and Founders | | 4,210,375 | | 19.6 | % |
James A. Graf(3) | | 4,210,375 | | 19.6 | % |
Gus Garcia | | — | | — | |
Lewis Silberman | | — | | — | |
Anantha Ramamurti | | — | | — | |
Anthony A. Kuznik | | — | | — | |
Sabrina McKee | | — | | — | |
Edwin J. Rigaud | | 20,000 | | * | |
A.B. Cruz III | | 20,000 | | * | |
Jeanne L. Manischewitz | | 20,000 | | * | |
Alexandra Lebenthal | | 20,000 | | * | |
All executive officers, directors and director as a group (10 individuals) | | 4,290,375 | | 20.0 | % |
Five Percent Holders | | — | | — | |
Graf Acquisition Partners IV LLC(3) | | 4,210,375 | | 19.6 | % |
Adage Capital Partners(4) | | 1,350,000 | | 6.29 | % |
Highbridge Capital Management, LLC(5) | | 1,124,431 | | 5.24 | % |
*Less than 1%
| (1) | Unless otherwise noted, the business address of each of the following entities or individuals is c/o Graf Acquisition Corp. IV, 1790 Hughes Landing Blvd., Suite 400, The Woodlands, Texas 77380. |
| (2) | Interests shown consist solely of founder shares. |
| (3) | Represents shares of our common stock held by Graf Acquisition Partners IV LLC, our sponsor. James A. Graf, our Chief Executive Officer, is the managing member of our sponsor and sponsor parent entity and has sole voting and investment discretion with respect to the founder shares held by our sponsor. Certain of our officers and directors are members of our |
| | sponsor and own an aggregate of approximately 25% to 70% of the membership interests of our sponsor, depending on financial returns. The remaining membership interests are held by third-party investors that are not affiliated with members of our management. Item 13.Certain Relationships and Related Transactions, and Director Independence. |
| (4) | According to a Schedule 13G filed with the SEC on May 25, 2021 on behalf of Adage Capital Partners, L.P. (“ACP L.P.”), Adage Capital Partners GP, L.L.C. (“ACP GP”), Adage Capital Advisors, L.L.C. (“ACA”), Robert Atchinson and Phillip Gross. Each of ACA L.P., ACP GP, ACA, Mr. Atchinson and Mr. Gross may be deemed to beneficially own 1,350,000 shares of the Company’s common stock. The business address of each of ACA L.P., ACA GP, ACA, Mr. Atchinson and Mr. Gross is 200 Clarendon Street, 52nd Floor, Boston, Massachusetts 02116. |
| (5) | According to Amendment No. 1 to Schedule 13G filed with the SEC on December 31, 2021 on behalf of Highbridge Capital Management, LLC (“Highbridge”). Highbridge may be deemed to beneficially own 1,124,431 shares of the Company’s common stock. The business address for Highbridge is 277 Park Avenue, 23rd Floor, New York, New York 10172. |
Our initial stockholders beneficially own 20% of the issued and outstanding shares of our common stock. Because of this ownership block, our initial stockholders may be able to effectively influence the outcome of all matters requiring approval by our stockholders, including the election of directors, amendments to our amended and restated certificate of incorporation and approval of significant corporate transactions, including approval of our initial business combination.
The holders of our founder shares have agreed (A) to vote any shares owned by them in favor of any proposed initial business combination and (B) not to redeem any shares in connection with a stockholder vote to approve a proposed initial business combination or in connection with a tender offer.
Our sponsor is deemed to be our “promoter” as such term is defined under the federal securities laws.
Item 13. Certain Relationships and Related Transactions, and Director Independence
On February 13, 2021, we issued an aggregate of 4,312,500 founder shares to Graf LLC for an aggregate purchase price of $25,000, or approximately $0.006 per share. On April 2, 2021, Graf LLC transferred all of its founder shares to our sponsor. On April 8, 2021, our sponsor transferred 20,000 founder shares to each of our independent directors, resulting in our sponsor holding 4,252,500 founder shares.
The
number of founder shares issued was determined based on the expectation that such founder shares would represent 20% of the outstanding shares upon completion of the IPO. Up to 562,500 founder shares are subject to forfeitureinformation required by
our sponsor depending on the extent to which the underwriters’ over-allotment option is exercised. The founder shares may not, subject to certain limited exceptions,this Item will be
transferred, assigned or sold by the holder.Our sponsor purchased an aggregate of 4,433,333 private placement warrants at a price of $1.50 per warrant for an aggregate purchase price of $6,650,000 in a private placement that occurred simultaneously with the closing of the IPO. Each private placement warrant entitles the holder to purchase one share of common stock at $11.50 per share. The private placement warrants (including the shares of common stock issuable upon exercise thereof) may not, subject to certain limited exceptions, be transferred, assigned or sold by the holder.
On June 2, 2021, the underwriters partially exercised the over-allotment option and purchased an additional 2,161,500 units, generating gross proceeds of $21,615,000. A total of $171,615,000 of the net proceeds from the sale of the units, the over-allotment units and the private placement warrants was placedset forth in the trust account.
As more fully discussedProxy Statement is incorporated herein if anyby reference.
Item 14. Principle Accountant Fees and Services
The information required by this Item will be set forth in the Proxy Statement is incorporated herein by reference.
PART IV
Item 15. Exhibits and Financial Statements.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Incorporated by Reference |
| | | | |
| Exhibit No. | | Description | | Schedule/
Form | | File No. | | Exhibit | | Filing Date |
| 2.1+ | | | | 8-K | | 001-40427 | | 2.1 | | April 17, 2023 |
| 3.1 | | | | 8-K | | 001-40427 | | 3.1 | | October 5, 2023 |
| 3.2 | | | | 8-K | | 001-40427 | | 3.2 | | October 5, 2023 |
| 4.1 | | | | 8-K | | 001-40427 | | 4.1 | | March 27, 2024 |
| 4.2 | | | | 8-K | | 001-40427 | | 4.2 | | March 27, 2024 |
| 4.3 | | | | 8-K | | 001-40427 | | 4.1 | | April 5, 2024 |
| 4.3 | | | | 8-K | | 001-40427 | | 4.2 | | April 5, 2024 |
| 4.4 | | | | 8-K | | 001-40427 | | 4.1 | | April 11, 2024 |
| 10.1.1 | | | | 8-K | | 001-40427 | | 10.1 | | September 22, 2023 |
| 10.1.2 | | | | 8-K | | 001-40427 | | 10.2 | | September 22, 2023 |
| 10.1.3 | | | | 8-K | | 001-40427 | | 10.1.3 | | October 5, 2023 |
| 10.2 | | | | 8-K | | 001-40427 | | 10.3 | | September 29, 2023 |
| 10.3.1 | | | | 8-K | | 001-40427 | | 10.4 | | September 29, 2023 |
| 10.3.2 | | | | 8-K | | 001-40427 | | 10.5 | | September 29, 2023 |
| 10.4.1 | | | | 8-K | | 001-40427 | | 10.1 | | September 19, 2023 |
| 10.4.2 | | | | 8-K | | 001-40427 | | 10.2 | | September 29, 2023 |
| 10.4.3 | | | | 8-K | | 001-40427 | | 10.1 | | September 29, 2023 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10.5 | | | | 8-K | | 001-40427 | | 10.1 | | September 18, 2023 |
| 10.6# | | | | 8-K | | 001-40427 | | 10.6 | | October 5, 2023 |
| 10.7.1 | | | | 8-K | | 001-40427 | | 10.1 | | April 17, 2023 |
| 10.7.2 | | | | 8-K | | 001-40427 | | 10.1 | | September 22, 2023 |
| 10.7.3 | | Third Amended and Restated Sponsor Support and Lockup Agreement, dated September 29, 2023, by and among Graf Acquisition Corp. IV, NKGen Biotech, Inc., Graf Acquisition Partners IV LLC and certain officers and directors of Graf Acquisition Corp. IV named as parties thereto. | | 8-K | | 001-40427 | | 10.7.3 | | October 5, 2023 |
| 10.7.4 | | | | 8-K | | 001-40427 | | 10.7.4 | | October 5, 2023 |
| 10.8 | | | | S-4 | | 001-40427 | | 10.4 | | May 15, 2023 |
| 10.9 | | | | S-4 | | 001-40427 | | 10.6 | | May 15, 2023 |
| 10.10# | | | | 8-K | | 001-40427 | | 10.10 | | October 5, 2023 |
| 10.11.1* | | | | S-4/A | | 333-271929 | | 10.15.1 | | August 4, 2023 |
| 10.11.2* | | | | S-4/A | | 333-271929 | | 10.15.2 | | August 4, 2023 |
| 10.12.1* | | | | S-4/A | | 333-271929 | | 10.13 | | June 26, 2023 |
| 10.12.2* | | | | S-4/A | | 333-271929 | | 10.14.1 | | June 26, 2023 |
| 10.12.3* | | | | S-4/A | | 333-271929 | | 10.14.2 | | June 26, 2023 |
| 10.13.1#+ | | | | S-4/A | | 333-271929 | | 10.16 | | August 4, 2023 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10.13.2# | | | | 8-K | | 001-40427 | | 10.13.2 | | October 5, 2023 |
| 10.14 | | | | S-4/A | | 333-271929 | | 10.17.1 | | August 4, 2023 |
| 10.15 | | | | S-4/A | | 333-271929 | | 10.17.2 | | August 4, 2023 |
| 10.16 | | | | S-4/A | | 333-271929 | | 10.17.3 | | August 4, 2023 |
| 10.17 | | | | S-4/A | | 333-271929 | | 10.17.4 | | August 4, 2023 |
| 10.18 | | | | S-4/A | | 333-271929 | | 10.17.5 | | August 4, 2023 |
| 10.19.1* | | | | S-4/A | | 333-271929 | | 10.19.1 | | August 4, 2023 |
| 10.19.2* | | | | S-4/A | | 333-271929 | | 10.19.2 | | August 4, 2023 |
| 10.20*# | | | | S-4/A | | 333-271929 | | 10.18 | | August 4, 2023 |
| 10.21*# | | | | S-4/A | | 333-271929 | | 10.21 | | August 4, 2023 |
| 10.22*# | | | | S-4/A | | 333-271929 | | 10.20 | | August 4, 2023 |
| 10.23*# | | | | 8-K | | 001-40427 | | 10.23 | | October 5, 2023 |
| 10.24.1* | | | | 8-K | | 001-40427 | | 10.24.1 | | October 5, 2023 |
| 10.24.2* | | | | 8-K | | 001-40427 | | 10.24.2 | | October 5, 2023 |
| 10.24.3* | | | | 8-K | | 001-40427 | | 10.24.3 | | October 5, 2023 |
| 10.25* | | | | 8-K | | 001-40427 | | 10.25 | | October 5, 2023 |
| 10.26* | | | | 8-K | | 001-40427 | | 10.26 | | October 5, 2023 |
| 10.27 | | | | 8-K | | 001-40427 | | 10.1 | | December 27, 2023 |
| 10.28 | | | | 8-K | | 001-40427 | | 10.1 | | January 8, 2024 |
| 10.29 | | | | 8-K | | 001-40427 | | 10.1 | | January 11, 2024 |
| 10.30 | | | | 8-K | | 001-40427 | | 10.1 | | January 22, 2024 |
| 10.31 | | | | 8-K | | 001-40427 | | 10.1 | | February 12, 2024 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10.32 | | | | 8-K | | 001-40427 | | 10.1 | | February 22, 2024 |
| 10.33 | | | | 8-K | | 001-40427 | | 10.1 | | March 27, 2024 |
| 10.34+ | | | | 8-K | | 001-40427 | | 10.2 | | March 27, 2024 |
| 10.35 | | | | 8-K | | 001-40427 | | 10.3 | | March 27, 2024 |
| 10.36+ | | | | 8-K | | 001-40427 | | 10.4 | | March 27, 2024 |
| 10.37 | | | | 8-K | | 001-40427 | | 10.1 | | April 5, 2024 |
| 10.38+ | | | | 8-K | | 001-40427 | | 10.2 | | April 5, 2024 |
| 10.39 | | | | 8-K | | 001-40427 | | 10.3 | | April 5, 2024 |
| 10.40+ | | | | 8-K | | 001-40427 | | 10.4 | | April 5, 2024 |
| 10.41 | | | | 8-K | | 001-40427 | | 10.1 | | April 11, 2024 |
| 10.42+ | | | | 8-K | | 001-40427 | | 10.2 | | April 11, 2024 |
| 10.43 | | | | 8-K | | 001-40427 | | 10.3 | | April 11, 2024 |
| 10.44 | | | | 8-K | | 001-40427 | | 10.4 | | April 11, 2024 |
| 14.1** | | | | | | | | | | |
| 19.1** | | | | | | | | | | |
| 21.1** | | | | | | | | | | |
| 24.1** | | | | | | | | | | |
| 31.1** | | | | | | | | | | |
| 31.2** | | | | | | | | | | |
32.1^ | | | | | | | | | | |
| 101.INS | | Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | | |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document. | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document. | | |
| 101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document. | | |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document. | | |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document | | |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document). | | |
| | | | | | | | | | |
+The schedules and exhibits to this agreement have been omitted pursuant to Item 601(a)(5) of Regulation S-K. A copy of any entity to which he omitted schedule and/or she has then-current fiduciary or contractual obligations, he or she will honor his or her fiduciary or contractual obligations to present such business combination opportunity to such other entity. Our officers and directors currently have certain relevant fiduciary duties or contractual obligations that may take priority over their duties to us.No cash finder’s fee, reimbursement, consulting fee or monies in respect of any payment of a loan,exhibit will be paid by usfurnished to our sponsor, officersthe SEC upon request.
#Certain portions of this Exhibit have been omitted in accordance with Regulation S-K Item 601(b)(10)(iv) because they are not material and directors, or any affiliate of our sponsor or officers for services rendered, prior to, or for any services rendered in order to effectuate, the consummation of an initial business combination (regardless ofare the type of transaction that it is). However, as more fully discussed above, these individuals will be entitled to certain payments including, but not limited to, reimbursement for any out-of-pocket expenses incurred in connection with activities on our behalf such as identifying potential target businesses and performing
due diligence on suitable business combinations. Additionally, these individuals will be eligible to receive a transfer or reallocation of founder shares for any extraordinary services rendered in order to identify or effectuate the consummation of our initial business combination. Our audit committee will review on a quarterly basis all payments that were made to our sponsor, officers, directors or our or their affiliates and will determine which expenses and the amount of expenses that will be reimbursed. There is no cap or ceiling on the reimbursement of out-of-pocket expenses incurred by such persons in connection with activities on our behalf.
Related Party Loans
In addition, in order to finance transaction costs in connection with an intended initial business combination, our sponsor or an affiliate of our sponsor or certain of our officers and directors may, but are not obligated to, loan us funds as may be required. If we complete an initial business combination, we would repay such loaned amounts. In the eventinformation that the initial business combination does not close, we may use a portionRegistrant treats as private or confidential. The Registrant agrees to furnish supplementally an unredacted copy of the working capital held outsideExhibit, or any section thereof, to the trust accountSEC upon request.
*Indicates management contract or compensatory plan or arrangement.
^Furnished herewith and not deemed to repay such loaned amounts but no proceeds from our trust account would be used“filed” for such repayment. Up to $1.5 millionpurposes of such working capital loans may be convertible into additional warrants at a price of $1.50 per warrant at the optionSection 18 of the lender. Such warrants wouldExchange Act and shall not be identical to the private placement warrants, including as to exercisability and exercise price. The terms of such working capital loans by our sponsor or its affiliates, or our officers and directors, if any, have not been determined and no written agreements exist with respect to such loans. We do not expect to seek loans from parties other than our sponsor or an affiliate of our sponsor as we do not believe third parties will be willing to loan such funds and provide a waiver against any and all rights to seek access to funds in our trust account.Administrative Support Agreement and Officer and Director Compensation
We have agreed to pay G-SPAC Management LLC, an affiliate of our sponsor, $15,000 per month for office space, utilities, secretarial, administrative and support services provided to us and members of our management team. We will cease paying these monthly fees upon the earlier of the consummation of a business combination or our liquidation.
Item 14.Principal Accounting Fees and Services.
The following is a summary of fees paid ordeemed to be paid to WithumSmith+Brown, PC (“Withum”), for services rendered.
Audit Fees: Audit fees consist of fees for professional services rendered forincorporated by reference into any filing under the audit of our year ended December 31, 2021 financial statements and services that are normally provided by Withum in connection with regulatory filings. The aggregate fees for Withum for professional services rendered forSecurities Act or the audit of our annual financial statements, review ofExchange Act (whether made before or after the financial information and other required filings with the SEC for the period from January 28, 2021 (inception) through December 31, 2021 totaled approximately $102,000. The above amounts include interim procedures and audit fees, as well as attendance at audit committee meetings.
Audit-Related Fees: Audit-related services consist of fees billed for assurance and related services that are reasonably related to performance of the audit or review of our financial statements and are not reported under “Audit Fees.” These services include attest services that are not required by statute or regulation and consultations concerning financial accounting and reporting standards. We did not pay Withum for consultations concerning financial accounting and reporting standards for the period from January 28, 2021 (inception) through December 31, 2021.
Tax Fees: We did not pay Withum for tax planning and tax advice for the period from January 28, 2021 (inception) through December 31, 2021.
All Other Fees: We did not pay Withum for other services for the period from January 28, 2021 (inception) through December 31, 2021.
PART IV
Item 15.Exhibits, Financial Statement Schedules.
The following documents are filed as partdate of this Annual Report on Form 10-K:
(a) | Financial Statements: See “Index to Financial Statements” as “Item 8. Financial Statements and Supplementary Data” herein.10-K), irrespective of any general incorporation language contained in such filing. |
(b) | Financial Statement Schedules. All schedules are omitted for the reason that the information is included in the financial statements or the notes thereto or that they are not required or are not applicable. |
(c) | Exhibits: The exhibits listed in the accompanying index to exhibits are filed or incorporated by reference as part of this Annual Report on Form 10-K. |
Exhibit Index
| | |
Number
|
| Description
|
3.1
|
| Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K (File No. 001-40427), filed with the SEC on May 25, 2021).
|
3.2
|
| Bylaws (incorporated by reference to Exhibit 3.2 to the Registrant’s Registration Statement on Form S-1 (File No. 333-253411), filed with the SEC on April 12, 2021).
|
4.1
|
| Specimen Unit Certificate (incorporated by reference to Exhibit 4.1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-253411), filed with the SEC on May 5, 2021).
|
4.2
|
| Specimen Common Stock Certificate (incorporated by reference to Exhibit 4.2 to the Registrant’s Registration Statement on Form S-1 (File No. 333-253411), filed with the SEC on May 5, 2021).
|
4.3
|
| Specimen Warrant Certificate (incorporated by reference to Exhibit 4.3 to the Registrant’s Registration Statement on Form S-1 (File No. 333-253411), filed with the SEC on May 5, 2021).
|
4.4
|
| Warrant Agreement, dated as of May 20, 2021, by and between the Registrant and Continental Stock Transfer & Trust Company, as warrant agent (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K (File No. 001-40427), filed with the SEC on May 25, 2021).
|
4.5*
|
| Description of Securities.
|
10.1
|
| Letter Agreement, dated as of May 20, 2021, by and among the Registrant, its executive officers, its directors and Graf Acquisition Partners IV LLC (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K (File No. 001-40427), filed with the SEC on May 25, 2021).
|
10.2
|
| Investment Management Trust Agreement, dated as of May 20, 2021, by and among the Registrant and Continental Stock Transfer & Trust Company, as trustee (incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K (File No. 001-40427), filed with the SEC on May 25, 2021).
|
10.3
|
| Registration Rights Agreement, dated as of May 20, 2021, by and among the Registrant, Graf Acquisition Partners IV LLC and the other holders party thereto (incorporated by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K (File No. 001-40427), filed with the SEC on May 25, 2021).
|
10.4
|
| Securities Subscription Agreement, dated as of February 13, 2021, by and between the Registrant and Graf Acquisition Partners LLC (incorporated by reference to Exhibit 10.5 to the Registrant’s Registration Statement on Form S-1 (File No. 333-253411), filed with the SEC on April 12, 2021).
|
10.5
|
| Private Placement Warrants Purchase Agreement, dated as of May 20, 2021, by and between the Registrant and Graf Acquisition Partners IV LLC (incorporated by reference to Exhibit 10.4 to the Registrant’s Current Report on Form 8-K (File No. 001-40427), filed with the SEC on May 25, 2021).
|
10.6
|
| Form of Indemnity Agreement (incorporated by reference to Exhibit 10.7 to the Registrant’s Registration Statement on Form S-1 (File No. 333-253411), filed with the SEC on May 5, 2021).
|
10.7
|
| Administrative Services Agreement, dated as of May 20, 2021, by and between the Company and the G-SPAC Management LLC (incorporated by reference to Exhibit 10.5 to the Registrant’s Current Report on Form 8-K (File No. 001-40427), filed with the SEC on May 25, 2021).
|
24.1*
|
| Power of Attorney (included on the signature pages herein).
|
Item 16. Form 10-K Summary
None.
SIGNATURES
SIGNATURES
Pursuant to the requirements ofIn accordance with Section 13 or 15(d) of the Securities Exchange Act, of 1934, as amended, the Registrant has dulyregistrant caused this Annual Report on Form 10-Kreport to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| NKGen Biotech, Inc. | | |
|
| |
| Date: April 16, 2024 | GRAF ACQUISITION CORP. IV
By: | /s/ Paul Y. Song |
| |
Paul Y. Song |
|
|
|
| By:
|
|
| /s/ James A. Graf
|
| Name:
| James A. Graf
|
| Title:
| Chief Executive Officer |
| (Principal executive officer) |
|
|
Dated: March 30, 2022
|
Executive Officer) |
POWER OF ATTORNEY
Each of the undersigned,
KNOW BY ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below
hereby constitutes and appoints
Paul Song and James
A. Graf
and each of them, his or her true and lawful attorney-in-fact
and agents with full
and several power of substitution,
and resubstitution for him
or her and
in his
or her name, place and stead, in any and all capacities, to sign any and all amendments
including post-effective amendments to this
Annual Report on Form 10-K,report, and to file the same, with all exhibits thereto, and
otherall documents in connection therewith, with the
SEC,Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorney-in-fact
and agents or
his substitute, each acting alone,any of them, or their substitutes, may lawfully do or cause to be
done by virtue thereof.done.
Pursuant to the requirements of the Securities
Exchange Act of
1933,1934, as amended, this
Annual Report
on Form 10-K has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
| Name
| | | | | | | | | | | | | |
| Signature | |
Title | | Title
| Date |
| | | | |
| /s/ Paul Y. Song | | Chief Executive Officer and Director | | April 16, 2024 |
| Paul Y. Song | | (principal executive officer) | | |
| | | | |
/s/ James A. Graf | |
Chief Executive Officer
|
| March 30, 2022
|
James A. Graf
|
| (Principal Executive Officer)
|
|
|
|
|
|
|
|
/s/ Gus Garcia
|
| Co-President and Director
|
| March 30, 2022
|
Gus Garcia
|
|
|
|
|
|
|
|
|
|
/s/ Lewis Silberman
|
| Co-President and Director
|
| March 30, 2022
|
Lewis Silberman
|
|
|
|
|
|
|
|
|
|
/s/ Anantha Ramamurti
|
| Interim Chief Financial Officer and Treasurer | |
March 30, 2022
April 16, 2024 |
Anantha Ramamurti
James Graf | |
(principal financial and accounting officer) | | (Principal Financial and Accounting Officer)
|
| | | | |
| /s/ Sangwoo Park | |
| | |
| Sangwoo Park | |
Director | | April 16, 2024 |
|
| | | |
| /s/ Michael Klowden | |
| | |
| Michael Klowden | |
Director | |
April 16, 2024 |
| | | | |
/s/ Anthony A. KuznikKathleen Scott | |
| | |
| Kathleen Scott | | Executive Vice President, General Counsel and Secretary
Director | |
March 30, 2022
April 16, 2024 |
| Anthony A. Kuznik
|
|
|
|
|
|
|
|
|
/s/ Sabrina McKee
|
| Executive Vice President, Strategy
|
| March 30, 2022
|
Sabrina McKee
|
|
|
|
|
|
|
|
|
|
/s/ Edwin J. Rigaud
|
| Director
|
| March 30, 2022
|
Edwin J. Rigaud
|
|
|
|
|
|
|
|
|
|
/s/ A.B. Cruz III
|
| Director
|
| March 30, 2022
|
A.B. Cruz III
|
|
|
|
|
|
|
|
|
|
/s/ Jeanne L. Manischewitz
|
| Director
|
| March 30, 2022
|
Jeanne L. Manischewitz
|
|
|
|
|
|
|
|
|
|
/s/ Alexandra Lebenthal
|
| Director
|
| March 30, 2022
|
Alexandra Lebenthal
|
|
|
|
|