
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
[X] (Mark One)
☒ANNUAL REPORT UNDERPURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE FISCAL YEAR ENDED - OCTOBERFor the fiscal year ended December 31 2017, 2023
ORor
[ ]☐TRANSITION REPORT UNDERPURSUANT TO SECTION 13 OR 15 (d)
15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE TRANSITION PERIOD FROM ______ TO ______For the transition period from ________to________
COMMISSION FILE NUMBER 000-28489Commission File Number 001-36138
ADVAXIS,AYALA PHARMACEUTICALS, INC.
(NameExact name of Registrantregistrant as specified in Its Charter)its charter)
| Delaware | 02-0563870 | |
| (State or | (I.R.S. Employer | |
| Identification No.) |
| 9 Deer Park Drive, Suite K-1, Monmouth Junction, NJ | 08852 | |
| (Address of | (Zip Code) |
(609)452-9813
(Issuer’s Telephone Number)Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None.
Securities registered pursuant to Section 12(g) of the Act.
Common Stock, par value $0.001 per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes [ ] ☐ No [X] ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act.
Yes [ ] ☐ No [X] ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes [X] ☒ No [ ]☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes [X] ☒ No [ ]☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer | Accelerated Filer | |||
| Non-accelerated Filer | ||||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. [ ]☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes [ ]☐ No [X]☒
As of AprilJune 30, 2017,2023, the aggregate market value of the voting common equity held by non-affiliates was approximately $336,054,000$3.7 million based on the closing bid price of the registrant’s Common Stockcommon stock on the NASDAQ Capital Market. (For- OTCQX ® Best Market (for purposes of determining this amount, only directors, executive officers, and 10% or greater shareholders and their respective affiliates have been deemed affiliates). [X]☒
The registrant had 41,303,988 shares of Common Stock,common stock, par value $0.001 per share, outstanding as of December 15, 2017.April 8, 2024.
DOCUMENTS INCORPORATED BY REFERENCEDocuments Incorporated by Reference
Portions of the Proxy Statementdefinitive proxy statement for the registrant’s 20182024 Annual Meeting of Stockholders (the “Proxy“2024 Proxy Statement”) to be filed within 120 days of the end of the fiscal year ended October 31, 2017 are incorporated by reference into Items 10, 11, 12, 13 and 14 in Part III hereof. Except with respect to information specifically incorporated by reference inof this Annual Report on Form 10-K,10-K. If the 2024 Proxy Statement is not deemedfiled by April 29, 2024 (the day that is 120 days after the last day of the registrant’s 2023 fiscal year), an amendment to this annual report on Form 10-K setting forth this information will be duly filed as a part hereof.with the Securities and Exchange Commission.
Table of Contents
Form 10-K Index
| Page | ||
| PART I | ||
| Item | 5 | |
| Item 1A. | ||
| Item | ||
| Item | 18 | |
| Item 2. | 19 | |
| Item 3. | ||
| Item | ||
| PART II | ||
| Item | ||
| Item | 20 | |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | |
| Item | ||
| Item | ||
| Item | Changes in and Disagreements with Accountants on Accounting and Financial | |
| Item | ||
| Item | ||
Disclosure Regarding Foreign Jurisdictions That Prevent Inspections | 32 | |
| PART III | ||
| Item | ||
| Item | ||
| Item | ||
| Item | Certain Relationships and Related Transactions, and Director Independence | |
| Item | ||
| Item | ||
| 38 |
| 2 |
INTRODUCTORY NOTE
Merger of Advaxis, Inc. with Ayala Pharmaceuticals, Inc.
On October 18, 2022, the Registrant entered into an Agreement and Plan of Merger (the “Merger Agreement”), by and among the Registrant, Old Ayala, Inc. (f/k/a Ayala Pharmaceuticals, Inc.), a Delaware corporation (“Old Ayala”), and Doe Merger Sub, Inc. (“Merger Sub”), a Delaware corporation and a wholly-owned subsidiary of the Registrant. On January 19, 2023 (the “Closing Date”), pursuant to the Merger Agreement, Merger Sub merged with and into Old Ayala, with Old Ayala continuing as the surviving company and a wholly-owned subsidiary of the Registrant (the “Merger”).
The Merger Agreement and additional information on the details of the Merger may be found in the Current Report on Form 8-K filed with the Securities and Exchange Commission (“SEC”) by the Registrant on October 19, 2022.
At the effective time of the Merger (the “Effective Time”), which occurred on January 19, 2023, (i) each share of the common stock, par value $0.01 per share, of Old Ayala (the “Old Ayala Common Stock”) issued and outstanding immediately prior to the Merger was automatically converted into the right to receive 0.1874 shares (the “Exchange Ratio”) of the common stock, par value $0.001 per share, of the Registrant (the “Common Stock”), (ii) each outstanding option to purchase shares of the Old Ayala Common Stock (each, an “Old Ayala Option”) was substituted and converted automatically into an option (each, an “New Ayala Replacement Option”) to purchase the number of shares of New Ayala Common Stock equal to the product obtained by multiplying (a) the number of shares of Old Ayala Common Stock subject to such Old Ayala Option immediately prior to the effective time of the Merger, by (b) the Exchange Ratio, with any fractional shares rounded down to the nearest whole share, with each such New Ayala Replacement Option to have an exercise price per share of Common Stock equal to (x) the per share exercise price for the shares of Old Ayala Common Stock subject to the corresponding Old Ayala Option immediately prior to the effective time of the Merger, divided by (y) the Exchange Ratio, rounded up to the nearest whole cent, and (iii) each restricted stock unit of Old Ayala (each, an “Old Ayala RSU”) outstanding immediately prior to the effective time of the Merger, whether or not vested or issuable, was substituted and converted automatically into a restricted stock unit award of New Ayala with respect to a number of shares of Common Stock equal to the product obtained by multiplying (a) the total number of shares of Old Ayala Common Stock subject to such Old Ayala RSU immediately prior to the effective time of the Merger by (b) the Exchange Ratio, with any fractional shares rounded down to the nearest whole share.
The issuance of Common Stock in connection with the Merger Agreement was registered under the Securities Act of 1933, as amended (the “Securities Act”), pursuant to The Registrant’s registration statement on Form S-4 (Registration No. 333-268586) declared effective by the SEC on December 12, 2022 (the “Registration Statement”). The proxy statement/prospectus in the Registration Statement contains additional information about the Merger.
As a result of the consummation of the Merger, Old Ayala, the surviving entity in the Merger, became a wholly-owned subsidiary of the Registrant. For accounting purposes, the Merger was treated as a “reverse acquisition” and Old Ayala was considered the accounting acquirer. Accordingly, Old Ayala will be reflected as the predecessor and acquirer in the financial statements of the Registrant (the legal acquirer) for periods ending after December 31, 2022. The Registrant’s historical financial condition and results of operations shown for comparative purposes in periodic filings for periods ending after December 31, 2022 reflect Old Ayala’s historical results.
Merger with Biosight Ltd.
FORWARD LOOKING STATEMENTSOn July 26, 2023, the Registrant, Advaxis Israel Ltd., a company organized under the laws of the State of Israel and a wholly owned subsidiary of the Registrant (“ADXS Merger Sub”) and Biosight, Ltd., a company organized under the laws of the State of Israel (“Biosight”) entered into an Agreement and Plan of Merger and Reorganization (the “Biosight Merger Agreement”). On October 18, 2023 (the “Closing Date”), pursuant to the Merger Agreement, ADXS Merger Sub consummated the merger with and into Biosight, with Biosight continuing as the surviving company and a wholly-owned subsidiary of the Registrant (the “Biosight Merger”).
The Biosight Merger Agreement and additional information on the details of the Biosight Merger may be found in the Current Report on Form 8-K filed with the SEC by the Registrant on October 20, 2023.
The Biosight Merger Agreement provided, among other things, that on the terms and subject to the conditions set forth therein, each share of Biosight issued and outstanding immediately prior to the effective time of the Merger (excluding any shares held by any of Biosight’s subsidiaries, the Registrant, ADXS Merger Sub or any of their respective subsidiaries, which will remain outstanding, and certain dormant shares under Israeli law, which will be cancelled, retired and cease to exist) was automatically deemed to have been transferred to the Registrant in exchange for the right to receive 1.82285 shares (the “Exchange Ratio”) of Common Stock. The Exchange Ratio was subject to equitable adjustment pursuant to the terms of the Biosight Merger Agreement. Each outstanding option or other right to purchase ordinary or preferred shares of Biosight was cancelled as of the Effective Time and will have no further force or effect. In connection with the closing of the Biosight Merger, the Registrant issued approximately 5,913,480 shares of the Common Stock to former holders of Biosight shares.
The issuance of the shares of Common Stock in the Biosight Merger was made pursuant to exemptions from the registration requirements of the Securities Act pursuant to Regulations D and S thereunder.
| 3 |
Sale of Assets to Immunome, Inc.
On February 5, 2024, the Registrant and Immunome, Inc. (“Immunome”), entered into an Asset Purchase Agreement (the “Asset Purchase Agreement”) pursuant to which Immunome agreed to acquire certain of the Registrant’s assets and liabilities related to its AL101 and AL102 programs (the “Asset Sale”), which constitute substantially all of the Registrant’s assets.
On March 25, 2024, the Registrant and Immunome consummated the Asset Sale pursuant to the Asset Purchase Agreement. Immunome paid to the Registrant an aggregate purchase price of $20,000,000 in cash (of which $4,000,000 had been paid upon entering into the Asset Purchase Agreement), subject to certain adjustments and issued to the Registrant 2,175,489 shares (the “Shares”) of Immunome’s common stock, $0.0001 par value. The Asset Purchase Agreement further provides that Immunome may pay the Registrant up to $37,500,000 in cash due upon the achievement of certain development and commercial milestone events set forth in the Asset Purchase Agreement. At the March 25, 2023 closing, Immunome paid the remaining $16,000,000 of the cash consideration to the Company, at the Company’s direction, to certain vendors of the Company.
The Asset Purchase Agreement contains customary representations, warranties, conditions and covenants, including covenants (i) concerning the conduct of business by the Registrant prior to the closing of the Asset Sale, (ii) prohibiting the Registrant and its representatives from soliciting, initiating or knowingly inducing, encouraging or facilitating any competing acquisition proposal, (iii) prohibiting the Registrant and its controlled affiliates from competing with Immunome for five years following the closing of the Asset Sale in certain fields, and (iv) restricting the Registrant’s ability to make distributions to stockholders, dissolve or wind up its business or file for bankruptcy for six months following the closing of the Asset Sale.
The Asset Purchase Agreement and additional information on the details of the Asset Sale may be found in the Current Report on Form 8-K filed with the SEC by the Registrant on February 6, 2024.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This annual report on Form 10-K for the year ended December 31, 2023 (“Form 10-K”) includes statements that are, or may be deemed to be, “forward-looking statements.” In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this Form 10-K and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drug candidates, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, our available cash, liquidity, prospects, growth and strategies, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, the degree of clinical utility of our product candidates, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growthimpacts of the ongoing conflicts in the Ukraine and strategies,the Middle East, inflation and the Federal Reserve’s responses thereto, including increasing interest rates, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect theour industry or us.
By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this Form 10-K, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this Form 10-K. In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this Form 10-K, they may not be predictive of results or developments in future periods.
Some of the factors that we believe could cause actual results to differ from those anticipated or predicted include:
| ||
Any forward-looking statements that we make in this Form 10-K speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this Form 10-K. You should also read carefully the factors described in the “Risk Factors” section of this Form 10-K to better understand the risks and uncertainties inherent in our business and underlying any forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Form 10-K will prove to be accurate.
This Form 10-K includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third-parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe these industry publications and third-party research, surveys and studies are reliable, we have not independently verified such data.
In this Form 10-K, unless otherwise stated or the context otherwise indicates, references to “New Ayala,” “Advaxis,” the “Company,” “we,” “us,” “our” and similar references refer to Ayala Pharmaceuticals, Inc., a Delaware corporation, which prior to the change of its name effected on January 19, 2023 was known as Advaxis, Inc.
We qualify all of our forward-looking statements by these cautionary statements. In addition, with respect to all of our forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
| 4 |
PART I
Item 1. Business.
General
Advaxis, Inc. (“Advaxis” or the “Company”) is a late-stage biotechnology Company focused on the discovery, development and commercialization of proprietaryLm Technology antigen delivery products based on a platform technology that utilizes live attenuatedListeria monocytogenes (“Lm”) bioengineered to secrete antigen/adjuvant fusion proteins. TheseLm-based strains are believed to be a significant advancement in immunotherapy as they integrate multiple functions into a single immunotherapy by accessing and directing antigen presenting cells to stimulate anti-tumor T cell immunity, stimulate and activate the immune system with the equivalent of multiple adjuvants, and simultaneously reduce tumor protection in the Tumor Microenvironment (“TME”) to enable the T cells to eliminate tumors. The Company believes thatLm Technology immunotherapies can complement and address significant unmet needs in the current oncology treatment landscape. Specifically, their product candidates have the potential to optimize checkpoint performance, while having a generally well-tolerated safety profile, and most of their product candidates are immediately available for treatment with a low cost of goods. The Company’s passion for the clinical potential ofLm Technology is balanced by focus and fiscal discipline and driven towards increasing shareholder value.
Advaxis is focused on four franchises in various stages of clinical and pre-clinical development, which they believe will provide the greatest opportunity to have a significant impact on patients and their families:
| ||
All four clinical franchises are anchored in the Company’sLm TechnologyTM, a unique platform designed for its ability to safely and effectively target various cancers in multiple ways. As an intracellular bacterium,Lm is an effective vector for the presentation of antigens through both the Major Histocompatibility Complex (“MHC”) I and II pathways, due to its active phagocytosis by Antigen Presenting Cells (“APCs”). Within the APCs,Lm produces virulence factors which allow survival in the host cytosol and potently stimulate the immune system.
Through a license from the University of Pennsylvania, Advaxis has exclusive access to a proprietary formulation of attenuatedLmcalledLmTechnology.Lm Technology optimizes this natural system, and one of the keys to the enhanced immunogenicity ofLm Technology is thetLLO – fusion protein, which is made up of tumor associated antigen (“TAA”) fused to a highly immunogenic bacterial protein that triggers potent cellular immunity. ThetLLO-fusion protein also helps to reduce immune tolerance in the TME and promotes antigen spreading, thereby improving activity in the TME. Multiple copies of thetLLO-fusion protein within each construct may increase antigen presentation and TME impact.
As the field of immunotherapy continues to evolve, the flexibility of theLm Technology platform has allowed Advaxis to develop highly innovative products. To date,Lm Technology has demonstrated preclinical synergy with multiple checkpoint inhibitors, co-stimulatory agents and radiation therapy, with clinical trials currently underway or planned in combination with Merck & Co., Inc. (“Merck”), AstraZeneca PLC (“AstraZeneca”), and Bristol-Myers Squibb Company’s (“BMS”) PD-1/PDL-1 inhibitors. The safety profile of allLm Technology constructs seen to date has been predictable and manageable, consisting mostly of mild to moderate flu-like symptoms that have been transient and associated with infusion.
The Advaxis Corporate Strategy
The Advaxis corporate strategy is to advance theLm Technology platform and leverage its unique capabilities to design and develop an array of cancer treatments. The Company and its collaborators are currently conducting or planning clinical studies ofLm Technology immunotherapies in HPV-associated cancers (including cervical and head and neck), prostate cancer, non-small cell lung cancers, and microsatellite stable colorectal cancer. Advaxis’ partners and collaborators include Amgen, Inc. (“Amgen”), BMS, Merck, AstraZeneca, the Gynecological Oncology Group (“GOG”) Foundation, Inc. (now a member of NRG Oncology), the European Network for Gynaecological Oncological Trial groups (“ENGOT”), the Parker Institute for Cancer Immunotherapy, Baylor College of Medicine and the Prostate Cancer Foundation.
Moving forward, the Company will continue to invest in its core clinical franchises and will also remain opportunistic in evaluating Investigator Sponsored Trials (“ISTs”) as well as licensing opportunities. TheLmTechnology platform is protected by a range of patents, covering both product and process, some of which the Company believes can be maintained into 2037.
Lm Technology and the Immunotherapy Landscape
The challenge of cancer immunotherapy has been to find the best overall balance between efficacy and side effects when mobilizing the body’s immune system to fight against cancer. The development of immune checkpoint inhibitors was a significant step forward, and brought with it impressive clinical activity in many different types of cancers, including melanoma and lung cancer. However, a literature review published inPharmacy and Therapeuticsin 2016 noted that checkpoint inhibitor monotherapy response rates are only in the 15-20% range, and rise to around 50% higher only in selected groups of patients with melanoma or lung cancer. Therefore, for most cancer patients, there is room for improvement. Checkpoint inhibitors can expand existing cancer fighting cells that may already be present in low numbers and support their activity against cancer cells, but if the right cancer-fighting cells are not present, checkpoint inhibitors may not provide clinical benefit. Similarly, there are many mechanisms of immune tolerance that are distinct from the checkpoints which, may also be blocking the immune system from fighting cancer. Based on both pre-clinical and early clinical data, Advaxis believes that checkpoint inhibitors, when combined with treatments such asLm Technology, can have an amplified anti-tumor effect.LmTechnology incorporates several complementary elements that include innate immune stimulation, potent generation of cancer-targeted T cells, ability to boost immunity through multiple treatments, enhancing lymphocyte infiltration into tumors, reduction of non-checkpoint mediated immune tolerance within the tumor microenvironment, and promotion of antigen spreading which may amplify the effects of treatment. These results provide rationale for further testing ofLm Technology agents in combination with checkpoint inhibitors.
Traditional cancer vaccines were another development within immunotherapy and have a history beginning over 30 years ago. Unfortunately, these vaccines have largely been unsuccessful for a variety of potential reasons. These include poor selection of targets, imbalanced antigen presentation by inclusion of certain immune enhancing agents (adjuvants), failure to consider the blocking actions of immune tolerance, and choice of vaccine vectors. In some cases, patients may develop neutralizing antibodies, preventing further treatments. In contrast to traditional cancer vaccines,Lm Technology takes advantage of a natural pathway in the immune system that evolved to protect us againstListeria infections, that also happens to generate the same type of immunity that is required when fighting cancer. The live but weakened (attenuated) bacteria stimulate a balanced concert of innate immune triggers and present the tumor antigen target precisely where it needs to be to generate potent cancer fighting cells from within the immune system itself. The multitude of accompanying signals serves to broadly mobilize most of the immune system in support of fighting what seems to be aListeriainfection, and is then “re-directed” against cancer cell targets. Additionally, the unique intracellular lifecycle ofListeria avoids the creation of neutralizing antibodies, thereby allowing for repeat administration as a chronic therapy with a sustained enhancing of tumor antigen-specific T cell immunity.
More recently, a new category of immunotherapies called Adoptive Cell Transfer (“CAR-Ts”, “TCRs”, “TILs”) has provided further evidence of the benefits of providing an enhanced T cell presence to fight cancer. As published in theJournal of the American Medical Association in November 2017, CAR-T has achieved dramatic results in liquid tumors, and can provoke clinical responses when other treatments stop working. These cells are artificially engineered outside the body and early versions have suffered from unanticipated side effects. The therapies are also limited in activity against a specific target, and have a limited persistence within the patient. To date, CAR-Ts activity has been limited to liquid tumors and have shown potentially meaningful toxicity concerns. Moreover, CAR-Ts are complex therapeutic products that are primarily manufactured and released for each patient, leading to costly manufacturing and a number of treatments.
Looking back on the last two decades, there have been promising technology advancements to harness and activate killer T cells against cancers and every day more is learned about the interplay between immunity and cancer that can lead to improved treatments. However there are still significant unmet needs in the immunotherapy landscape that Advaxis feelsLm Technology can address and complement. Specifically,Lm Technology has the potential to optimize and expand checkpoint inhibitor activity in combination. It also avoids many of the limitations of previous cancer vaccine attempts by tapping into the pathway reserved for defense againstListeriainfection while incorporating the best cancer-targets science can identify, including neoantigens that result from mutations in the cancer. To date,Lm Technology products have a generally well-tolerated safety profile, do not generate neutralizing antibodies lending themselves to retreatments, and most of the products are immediately available for treatment without the complication and expense of modifying a patient’s own cells in a laboratory.
Lm Technology: An optimizedListeria-based antigen delivery system
Advaxis’Listeria-based immunotherapies are optimized for antigen delivery through a process of insertion of multiple copies of the proprietarytLLO-fusion protein into each extrachromosomal protein expression and secretion plasmid that makes and secretes the target protein right inside the patient’s antigen presenting cells to initiate and/or boost their immune response. ThetLLO-fusion protein approach was developed at the University of Pennsylvania as an improvement over insertion of a single copy of the target gene, as an ACT-A (or otherLmpeptide) fusion, within the bacterial genome for four key reasons:
Through a license from the University of Pennsylvania, Advaxis has exclusive access to this proprietary formulation of attenuatedLmwhich further enhances this nearly ideal natural system. Clinical application in the Company’s four franchise areas is the core focus of current development efforts.
Clinical PipelineRecent Developments
Advaxis is focused on the discovery, development and commercialization of proprietaryLmRecent Strategic TransactionsTechnology antigen delivery products, with the lead program for cervical cancer in Phase 3 development. The Company and its collaborators are currently conducting or planning clinical studies ofLm Technology immunotherapies in four franchises:
As a late stage biotechnology company with no commercial products, Advaxis is aware of the need for fiscal responsibility, and is focusing our investment into the four franchises listed above. Additionally, the company will continue to be opportunistic by exploring ISTs, licensing and other external opportunities.
In October of 2015, the Company received notification from the FDA that the INDs for axalimogene filolisbac were putSee “Introductory Note” beginning on clinical hold in response to its submission of a safety report to the FDA. The clinical hold also included the INDs for ADXS-PSA and ADXS-HER2. Following discussions with the FDA and in accordance with their recommendations, the Company agreed to implement certain risk mitigation measures, including revised trial protocol inclusion / exclusion criteria, post administration antibiotic treatment and patient surveillance and monitoring measures. In December 2015, the FDA notified the Company that the hold had been lifted with respect to its INDs.
Advaxis Pipeline of Product Candidates

HPV Related Cancers: Proof of Concept ofLmTechnology
The Company is developing a franchise in HPV-related cancers including both axalimogene filolisbac and ADXS-DUAL. Axalimogene filolisbac is anLm–based antigen delivery product directed against HPV and designed to target cells expressing HPV. ADXS-DUAL, the Company’s second immunotherapy targeting HPV-associated cancers, builds on the learnings from the clinical development of axalimogene filolisbac and incorporates an additional HPV target antigen into theLm Technology vector. The company’s HPV-related products are currently under investigation in two HPV-associated cancers: cervical cancer and head and neck cancer, either as a monotherapy or in combination. Advaxis has also completed treatment in two Phase 2 studies of axalimogene filolisbac for the treatment of anal cancer, and is evaluating the potential for collaborative external opportunities to further develop this program.
Cervical Cancer: axalimogene filolisbac and ADXS-DUAL
HPV is the most common viral infection of the reproductive tract and is the cause of a range of conditions in both females and males. In women, persistent infection with specific oncogenic types of HPV (most frequently alpha7 and alpha9 families) may lead to precancerous lesions which, if untreated, may progress to cervical cancer. There are approximately 527,000 new cases of cervical cancer caused by HPV worldwide every year, and 12,000 new cases in the U.S. alone, according to the World Health Organization (“WHO”) Human Papillomavirus and Related Cancers in the World Summary Report 2017. There are approximately 4,250 deaths from cervical cancer each year according to the National Institutes of Health. Current preventative HPV vaccines such as Gardasil®and Cervarix® cannot treat or protect the large population of adults already infected with the virus, leaving several generations of women vulnerable. Furthermore, challenges with acceptance, accessibility, and compliance have resulted in suboptimal vaccination rates, with approximately 50% of young women and 38% of young men being fully vaccinated in the United States, according to statistics published by the Centers for Disease Control in 2017. Vaccination rates are even lower in other countries around the world.
Current treatments for metastatic cervical cancer can only extend life for about 6-8 months. Axalimogene filolisbac and ADXS-DUAL areLm Technology immunotherapies designed to secrete thetLLO-E7 fusion protein to target HPV-positive tumors.
Axalimogene filolisbac has received FDA orphan drug designation for invasive International Federation of Gynecology and Obstetrics (“FIGO”) Stage II-IV cervical cancer, and has received Fast Track designation from the FDA for the treatment of high-risk locally advanced cervical cancer. Axalimogene filolisbac has also been classified as an advanced-therapy medicinal product (“ATMP”) for the treatment of cervical cancer by the European Medicines Agency’s (“EMA”) Committee for Advanced Therapies (“CAT”). The CAT is the EMA’s committee responsible for assessing the quality, safety and efficacy of ATMPs. The Company has completed the CAT certification procedure and the CAT has certified that the preclinical and quality information have met the scientific and technical standards for a MAA.
Phase 2 Trial Results – axalimogene filolisbac
In 2014, the company completed a randomized Phase 2 clinical trial (Lm-LLO-E7-15) in 109 women with recurrent/refractory cervical cancer. The final results were presented at the 2014 American Society of Clinical Oncology (“ASCO”) Annual Meeting, and showed that 32% (35/109) of patients were alive at 12 months, 22% (24/109) of patients were Long-term Survivors (“LTS”) alive greater than 18 months, and 18% (16/91) evaluable with adequate follow-up of patients were alive for more than 24 months. Of the 109 patients treated in the trial, LTS included not only patients with tumor shrinkage but also patients who had experienced stable disease or increased tumor burden. 17% (19/109) of the patients in the trial had recurrence of disease after at least two prior treatments for their cervical cancer; these patients comprised 8% (2/24) of LTS. Among the LTS, 25% (3/12) of patients had a baseline Eastern Cooperative Oncology Group (“ECOG”) performance status of 2, a patient population that is often excluded from clinical trials. Furthermore, a 10% objective response rate (including 5 complete responses and 6 partial responses) and a disease control rate of 38% (42/109) were observed. The addition of cisplatin chemotherapy to axalimogene filolisbac in this trial did not significantly improve overall survival or objective tumor response (p =0.9981).
In this trial, 109 patients received 254 doses of axalimogene filolisbac. Axalimogene filolisbac was found to be well tolerated with 38% (41/109) of patients experiencing mild to moderate Grade 1 or 2 transient adverse events associated with infusion; 1 patient experienced a Gradepage 3 Serious Adverse Events (“SAE”). All observed treatment-related adverse events either self-resolved or responded readily to symptomatic treatment.
Based on these results, the Gynecological Oncology Group (“GOG”) Foundation, Inc. (now a member of NRG Oncology), under the sponsorship of the Cancer Therapy Evaluation Program (“CTEP”) of the National Cancer Institute (“NCI”), conducted GOG-0265, an open-label, single arm Phase 2 trial of axalimogene filolisbac in persistent or recurrent cervical cancer (patients must have received at least 1 prior chemotherapy regimen for the treatment of their recurrent/metastatic disease, not including that administered as a component of primary treatment) at numerous clinical sites in the U.S. The trial was a Simon 2-stage design, with the primary efficacy endpoint being 12-month survival, with the objective of the secondary efficacy endpoints to evaluate progression-free survival, overall survival and objective tumor response. The primary safety endpoints were to evaluate the number of patients with dose-limiting toxicities and the frequency and severity of adverse effects.
To evaluate the trial’s primary endpoint of 12-month overall survival rate, the GOG’s protocol featured a prospectively-defined logistic model-based calculation of the expected 12-month survival rate using key predictive factors significantly related to survival and derived from 17 serially conducted GOG/NRG 2-stage studies of inactive agents in persistent/recurrent metastatic cervical cancer (“PRmCC”) involving approximately 500 patients. This accumulated data by GOG used a consistent protocol design and a similar data collection methodology resulting in a robust and homogeneous patient dataset for the per protocol analysis of the primary endpoint. Per the trial protocol, this logistic model-based calculation was then used as a comparator for evaluating the 12-month survival rate of axalimogene filolisbac observed in GOG-0265.
The first stage of enrollment in GOG-0265 was successfully completed with 26 patients treated and met the predetermined safety and efficacy criteria required to proceed into the second stage of patient enrollment. Clinical data from the first stage of GOG-0265 was presented at the American Gynecological & Obstetrical Society (“AGOS”) annual meeting on September 17, 2015. Overall survival at 12 months was 38.5% (10/26) (the predefined criteria for 12-month survival in order to progress to Stage 2 was ≥20%), and, among patients who had received the full treatment regimen of 3 doses of axalimogene filolisbac, the 12-month survival rate was 55.6% (10/18). The adverse events observed in the first stage of the trial have been consistent with those reported in other clinical studies with axalimogene filolisbac.
Stage 2 of the trial began enrollment in February 2015 which included a protocol amendment to allow patients to continue to receive repeat cycles of therapy until disease progression. Stage 2 enrollment was temporarily suspended with a clinical hold in October 2015 that resolved in mid-December 2015. Prior to re-initiating enrollment of a new cohort of Stage 2 patients, Advaxis and the GOG Foundation/NRG Oncology examined the 12-month survival rate and safety data obtained from the 24 patients who had previously enrolled in Stage 2. The Stage 2 population demonstrated that treatment with axalimogene filolisbac resulted in a 37.5% (9/24) 12-month survival rate. This data was consistent with the findings in Stage 1 that showed a 38.5% 12-month survival rate, despite a greater proportion of Stage 2 patients having failed bevacizumab treatment. Taken together, the available data from both stages of GOG-0265 comprise a Phase 2 clinical trial in 50 subjects with a 12 month survival rate of 38% (19/50). The protocol defined logistic model-based calculation of the expected 12-month milestone survival rate was calculated to be 24.5% using the key predictors from the patients enrolled in the trial. The 12 month survival rate of 38% for patients receiving axalimogene filolisbac in the trial represented a 52% improvement over the expected 12-month milestone survival rate of 24.5%.
Overall, 28 out of 50 (56%) patients experienced a Grade 1 or Grade 2 TRAE associated with axalimogene filolisbac infusion. The most common (>30%) Grade 1 or Grade 2 TRAEs were fatigue, chills, anemia, nausea and fever. Eighteen (36%) patients experienced a Grade 3 TRAE and two patients experienced a Grade 4 AE, including a Klebsiella lung infection in one patient, and hypotension/cytokine related symptoms in another patient, which were considered possibly related to treatment.
In October 2016, upon review of these encouraging findings, the Company announced early closure of GOG-0265. Results from the GOG-0265 trial were presented as an oral late-breaker presentation at the Society of Gynecologic Oncology (“SGO”) annual meeting on March 14, 2017. Based on these data, the Company has made a strategic decision to submit for conditional Marketing Authorization (“MA”) in Europe.
Advaxis is also conducting an ongoing clinical trial with axalimogene filolisbac through a collaboration agreement with MedImmune, the global biologics research and development arm of AstraZeneca. This is a Phase 1/2, open-label, multicenter, dose determination and expansion cohort trial to determine the recommended Phase 2 dose (“RP2D”) and evaluate the safety and efficacy of axalimogene filolisbac, in combination with MedImmune’s investigational anti-PD-L1 immune checkpoint inhibitor, IMFINZITM(“durvalumab”), as a treatment for patients with metastatic squamous or non-squamous carcinoma of the cervix and metastatic HPV-associated squamous cell carcinoma of the head and neck (“SCCHN”). The dose determination phase of the trial is complete. Two dose cohorts were enrolled. Cohort 1 enrolled 5 patients with metastatic cervical cancer who received 1 x 109 cfu of axalimogene filolisbac + 3 mg/mg durvalumab. Cohort 2 enrolled 3 patients with metastatic cervical cancer and 2 patients with SCCHN who received 1 x 109cfu of axalimogene filolisbac + 10 mg/mg of durvalumab. No dose-limiting toxicities were observed in either cohort. The RP2D was determined to be 1 x 109 cfu for axalimogene filolisbac + 10 mg/mg for durvalumab. Preliminary data showed that two patients with metastatic cervical cancer achieved a durable complete response (with confirmation) and a partial response (without confirmation), respectively.
Treatment-related adverse events (“TRAE”) were reported in 91% of patients; the majority were either Grade 1 (64%) or Grade 2 (55%) events. The most commonly reported TRAEs were chills, fever, nausea and hypotension. Three patients reported Grade 3 TRAEs (1 x 109+ 3 mg/kg rigor/chills; 1 x 109 + 10 mg/kg - rigor/chills; and 1 x 109 + 10 mg/kg - diarrhea and shortness of breath). One patient experienced a grade 4 TRAE of hypotension. The safety profile of the combination of axalimogene filolisbac + durvalumab was consistent with previous reported findings for both axalimogene filolisbac and durvalumab administered as monotherapy.
Enrollment in the Part A expansion phase (N = 20 patients with SCCHN) and Part B expansion phase (N=90 patients with metastatic cervical cancer) are open to enrollment. As of the latest data cut-off date of October 18, 2017, 7 patients receiving 1 x 109 axalimogene filolisbac + 10 mg/kg durvalumab have been enrolled. In the Part B expansion, a total of 32 patients with metastatic cervical cancer were randomized to receive either 10 mg/kg durvalumab alone (N=16 patients) or 1 x 109 axalimogene filolisbac + 10 mg/kg durvalumab (N = 16 patients) expansion phases. The Company expects an interim readout of the safety and efficacy data from the Part B expansion in 2019.
Ongoing Registrational and Phase 3 Studies: ADXS-DUAL and axalimogene filolisbac
In evaluating the data from GOG-0265, Advaxis observed that there was a significantly higher representation of alpha7 family viruses in the PRmCC patient population than are typically seen at an initial diagnosis of cervical cancer. Although axalimogene filolisbac had already been shown to improve survival in both alpha7 and alpha9 family cancers, the Company made the decision that late stage, metastatic patients need a potent therapy that presents antigens to both families, in order to give them the best chance to impede their disease progression. As a result, ADXS-DUAL was developed to have the potential to be even more potent against the alpha7 family strains found in metastatic cervical cancer. ADXS-DUAL incorporates additional peptides to increase potency against these alpha7 strains. ADXS-DUAL may give patients the best chance of benefit in the PRmCC setting, especially if combined with a checkpoint inhibitor.
To this end, the Company has entered into a clinical development collaboration agreement with BMS to evaluate their PD-1 immune checkpoint inhibitor, OPDIVO®(“nivolumab”), in combination with ADXS-DUAL as a potential treatment option for women with PRmCC. Advaxis plans to file an IND application for ADXS-DUAL in early 2018. Pending FDA feedback, we plan to initiate a global, randomized, registrational quality trial in 1H 2018 and will evaluate this combination regimen in women with persistent, recurrent or metastatic (squamous or non-squamous cell) carcinoma of the cervix who have failed at least one prior line of systemic chemotherapy (“ADVANCE” or “ADVAxis Immunotherapy withNivolumab to Treat Recurrent or MetastaticCErvical Cancer”). Under the terms of the agreement, each party will bear its own internal costs and provide its immunotherapy agents. Advaxis will sponsor the trial and pay third-party costs. Subject to the positive outcome of this trial, the Company plans to file a Biologics License Application (“BLA”) for approval of ADXS-DUAL for metastatic cervical cancer in combination treatment.
Women who are diagnosed with high risk, locally advanced carcinoma of the cervix (“HRLACC”) face a higher chance that their cancer may recur following initial treatment when compared to earlier stages of the disease. When cervical cancer recurs, there are very few treatment options and the prognosis is dire. To address this unmet need, in 2016 the Company reached an agreement with the FDA, under the Special Protocol Assessment (“SPA”) process, for a Phase 3 trial evaluating axalimogene filolisbac in patients with HRLACC (“AIM2CERV” or “AdvaxisImmunotherapy2 PreventCervical Recurrence”) to be conducted in collaboration with the GOG/NRG Oncology.
AIM2CERV is a double-blind, randomized, placebo-controlled, Phase 3 trial of adjuvant axalimogene filolisbac following primary chemoradiation treatment of women with HRLACC. The primary objective of AIM2CERV is to compare the disease free survival of axalimogene filolisbac to placebo administered in the adjuvant setting following standard concurrent chemotherapy and radiotherapy (“CCRT”) administered with curative intent to patients with HRLACC. Secondary endpoints include examining overall survival and safety. The company’s goal is to develop a treatment to prevent or reduce the risk of cervical cancer recurrence after primary, standard of care treatment in women who are at high risk of recurrence. The AIM2CERV trial is expected to enroll 450 patients globally and, subject to the positive outcome, is designed to support a BLA submission in the U.S. and in other territories around the world.
The trial is active in ten countries and is currently enrolling.
Axalimogene filolisbac EU conditional approval Filing
BasedAnnual Report on scientific advice provided by the Paul Ehrlich Institute in Germany and the Medical Products Agency in Sweden, the Company has made a strategic decision to submit for conditional Marketing Authorization (“MA”) in Europe. The company plans to file for conditional MA under the requirements in the EMA’s Commission Regulation and Committee for Medicinal Products for Human Use (“CHMP”) Guideline on MAs, which includes criteria such as a positive risk/benefit balance, unmet medical needs being fulfilled, benefits to public health outweighing the additional data required, and a high likelihood that comprehensive clinical data will be provided in the future.
Progress to date towards submission of the filing includes axalimogene filolisbac’s designation as an ATMP, certification of quality and non-clinical data by the CAT, CHMP confirmation of eligibility for Union Marketing Authorization, assignment of EU rapporteurs, and pre-submission meetings held with the rapporteurs. Feedback from the rapporteurs advising the provision of additional clinical information in the submission package led to a delay of the filing which is now planned for Q1 2018. Once the package is submitted, the CHMP review process is expected to take approximately 13 months, and the Company plans to provide an update at the end of the review.
HPV Franchise Licensing Agreements
Biocon Limited (“Biocon”), our co-development and commercialization partner for axalimogene filolisbac in India and key emerging markets, filed a Marketing Authorization Application (“MAA”) for licensure of this immunotherapy in India. The companies are currently evaluating next steps.
Especificos Stendhal SA de CV (“Stendhal”), the Company’s co-development and commercialization partner for axalimogene filolisbac in Mexico, Brazil, Colombia and other Latin American countries, will pay $10 million in support payments towards the expense of AIM2CERV over the duration of the trial, contingent upon Advaxis achieving annual project milestones.
Knight Therapeutics Inc. (“Knight”), holds an exclusive license to commercialize axalimogene filolisbac in Canada, as well as our other product candidates.
Advaxis granted Global BioPharma (“GBP”) an exclusive license for the development and commercialization of axalimogene filolisbac for the treatment of cervical cancer in Asia. GBP is responsible for all development and commercial costs and activities associated with the development in their territories.
Head and Neck Cancer
Squamous Cell Carcinoma of the Head and Neck (“SCCHN”) is the most frequently occurring malignant tumor of the head and neck and is a major cause of morbidity and mortality worldwide. More than 90% of SCCHNs originate from the mucosal linings of the oral cavity, pharynx, or larynx and 70% of these cancers are caused by HPV. According to the American Cancer Society, head and neck cancer accounts for about 3% of all cancers in the United States. But while the Pap smear and other HPV tests have reduced rates of cervical cancer, rates of oral cancer are growing, with 12,000 new cases projected to be diagnosed in the United Stated in 2016 according to the Surveillance, Epidemiology, and End Results (“SEER”) database.
A study published in the Annals of Internal Medicine found that 11.5% percent of U.S. men and 3% of women were actively infected with oral HPV between 2011 and 2014. That adds up to 11 million men and 3 million women who are at risk for developing SCCHN. SCCHN is typically asymptomatic until it has metastasized, and screening options do not exist. The only way to prevent infection is the HPV vaccine, but compliance has been low to date. Another challenge is that preventative vaccines cannot protect those already infected or older than 26, leaving several generations of Americans vulnerable to SCCHN with no way of knowing if cancer is silently growing.
The safety and immunogenicity of axalimogene filolisbac is being evaluated in a Phase 2 IST at Mount Sinai and Baylor College of Medicine in a pre-surgery “window of opportunity” trial in patients with HPV-positive head and neck cancer. This clinical trial is the first to evaluate the immunologic and pathologic effects of axalimogene filolisbac in patients at the time of initial diagnosis of HPV-associated head and neck cancer. The trial is designed to show that axalimogene filolisbac is highly immunogenic and worth further investigation if the overall rate of vaccine-induced T-cell responses is 75 percent or more. Preliminary clinical data from this trial was presented at the American Association of Cancer Research (“AACR”) annual meeting on April 18, 2016. The data from eight of the nine patients enrolled in Stage 1 who were treated with axalimogene filolisbac confirmed that the trial met the target for the overall rate of vaccine-induced T-cell response. The results demonstrated that HPV E7- and/or E6-specific T cell responses increased in the peripheral blood in five of the trial patients. Increased infiltration of both CD4+ and CD8+ T cells were observed in the TME of four patients, with a reduction of FOXP3+ regulatory T cells within the tumors of 3/6 patients. Increased T cell responses to HPV E6 supports enhanced immune activity against additional tumor targets. Changes to the TME included cytotoxic T cell infiltration into the post-resection tumor, increased immune activation, a reduction of regulatory T cells, infiltration of cytotoxic T cells, and increased expression of inflammatory activation markers. In addition, fluctuations of circulating serum cytokine (IL-15, IL-9, TNFa, IL-2 and MIP-1b) levels were observed potentially suggesting consumption by activated T cells and migration of T cells to the TME. These results confirmed that the trial met its Stage 1 primary objective which allowed accrual to proceed in the second stage of the trial which is intended. The Stage 2 objective is consistent with Stage 1 and enrollment is ongoing.
As stated above, we have entered into a clinical trial collaboration agreement with MedImmune to collaborate on a Phase 1/2, open-label, multicenter, two part trial to evaluate safety and efficacy of axalimogene filolisbac, in combination with durvalumab (MEDI4736), for patients with metastatic squamous or non-squamous carcinoma of the cervix and metastatic HPV-associated SCCHN. Part 1 of this trial is complete, and the Company has commenced enrollment in the Part A (20 patients with SCCHN) and B (90 patients with cervical cancer) expansion phases.
We will continue to be opportunistic towards alternative funding approaches for continued development in HPV-positive head and neck cancer, and plan to announce an IST with a prestigious academic institution in 2018. Axalimogene filolisbac has received FDA orphan drug designation for HPV-associated head and neck cancer.
Anal Cancer
According to the American Cancer Society, nearly all squamous cell anal cancers are linked to infection by HPV, the same virus that causes cervical cancer. According to the SEER database, approximately 7,500 new cases will be diagnosed in the United States in 2016.
The safety and efficacy of axalimogene filolisbac is being evaluated in a Phase 2 IST by Brown University in patients with high-risk locally advanced anal cancer. As of December 2017, no further enrollment in this trial is planned. 10 patients were treated including one with N2 and four with N3 disease. Two patients had grade 3 acute toxicities following the initial dose of axalimogene filolisbac including chills/rigors (n = 2), back pain (n = 1), and hyponatremia (n = 1). All toxicities occurred within 24 hours of administration. There was no apparent increase in chemoradiation toxicities or myelosuppression. One patient had a Grade 5 cardiopulmonary event shortly after beginning 5-FU treatment. This patient did not receive a dose of axalimogene filolisbac. All 9 assessable patients had complete clinical responses by sigmoidoscopy. Eight of the 9 patients (89%) are progression-free at a median follow-up of 42 months. These data were accepted and published in the International Journal of Radiation Oncology.
We conducted a Phase 2 multi-center, open-label, Simon two-stage trial (“FAWCETT” or “FightingAnal-CancerwithCTLEnhancingTumorTherapy”), testing axalimogene filolisbac in patients with persistent or recurrent metastatic anal cancer. FAWCETT is designed to evaluate the efficacy and safety of axalimogene filolisbac as a monotherapy in patients with HPV-associated metastatic anal cancer who have received at least one prior treatment regimen for the advanced disease. Patients will receive intravenous axalimogene filolisbac monotherapy (1x109 CFU) every 3 weeks for ≤ 2 years or until a discontinuation criterion is met. Stage 1 of the trial targeted enrollment of 36 patients with anal cancer whose disease recurred after receiving treatment, with an interim analysis planned on enrollment of 31 evaluable pts (≥ 1 post-baseline scan) and met the predetermined safety and efficacy criteria required to proceed into the second stage of patient enrollment.
Preliminary Stage 1 results from 29 of the planned evaluable patients showed 1 (3.5%) patient had a durable partial response lasting > 6 months (after progression on prior anti-PD-1 therapy) and 7 had stable disease (24%). Disease control rate was 28%. The current Kaplan Meier 6-month PFS estimate is 22%, indicating the trial met the criteria to proceed to Stage 2 of enrollment. Common (≥ 30%) TRAEs were grade 1-2 chills/rigors, fever, hypotension and vomiting. Grade 3 TRAEs of cytokine related symptoms (n=1; SAE), infusion related reactions (n=2; 1 SAE) and hypotension (n=2; 1 SAE) were reported. These results were reported at the annual meeting of the European Society for Medical Oncology (“ESMO”) in September 2017.Form 10-K.
The Company has decidedincurred recurring losses since inception as a research and development organization. For the year ended December 31, 2023, the Company used approximately $29.5 million of cash in operations and incurred a net loss of $48.1 million. As of December 31, 2023, the Company had $4.9 million in cash and cash equivalents, $25.0 million in current liabilities and an accumulated deficit of $197.2 million.
Upon closing of the Asset Sale, on March 25, 2024, the Company received $13.0 million in cash, and Immunome paid $3.0 million of the Company’s liabilities directly to vendors of the Company. In addition, under the Asset Sale Agreement the Company received 2,175,489 shares of Immunome’s common stock (the “Immunome Shares”). The Asset Sale Agreement prohibits the Company from selling more than 50% of the Immunome Shares in the first six months following the closing of the Asset Sale. In addition, on March 1, 2024, the Company issued additional convertible notes and warrants in exchange for $2.0 million in funding from the Convertible Notes investors. As of the date of this filing, the cash proceeds received from the Asset Sale and the convertible notes sold on March 1, 2024 were not sufficient to initiatepay the Stage 2Company’s existing liabilities. Therefore, the Company has limited available cash resources and requires additional financing, through the sale of a portion of the trialImmunome Shares or otherwise, in order to focuscontinue to fund its resourcescurrent operations beyond May 2024.
Raising additional funds or the satisfactory sale of a portion of the Immunome Shares prior to the end of May 2024 is essential to provide sufficient cash flow to meet future liabilities and other obligations, such as tax payments arising from the Asset Sale. Furthermore, even if the Company is successful in selling a portion of the Immunome Shares or raising additional funds through other means, the Company cannot give any assurance that it will, in the future, be able to achieve a level of profitability from the sale of its products to sustain its operations.
If the Company is unable to obtain funding, or able to receive sufficient funds from the sale of a portion of the Immunome Shares, the Company would be forced to delay, reduce, or eliminate its research and development programs, or the Company may be unable to continue operations. As such, those factors raise substantial doubt about the Company’s ability to continue as a going concern.
As part of a cost reduction plan, during the year ended December 31, 2023, the Company had a reduction in workforce in which the employment of approximately 50% of the Company’s employees was terminated. During the first quarter of 2024, the Company gave notice of termination to 18 additional employees and two officers (including the Chief Financial Officer, whose employment will terminate on June 25, 2024). After the effectiveness of such terminations, the Chief Executive Officer will be the only employee of the Company. The Company expects to be able to meet its financial obligations to its employees.
The Company can give no assurances that it will be successful in raising funds through the sale of a portion of the Immunome Shares or any other clinical priorities at this time. Wealternative. Any inability to so obtain additional financing or funding will likely cause the Company to cease business operations. The consolidated financial statements have been prepared assuming that the Company will continue as a going concern. Therefore, the consolidated financial statements for the year ended December 31, 2023, do not include any adjustments to evaluate alternative funding sourcesreflect the possible future effects on the recoverability and collaborationsclassification of assets or the amounts and classification of liabilities that may result from uncertainty related to further develop this program. Axalimogene filolisbac has received FDA and EMA orphan drug designation for anal cancer.the Company’s ability to continue as a going concern.
Neoantigen Therapy (ADXS-NEO)Overview
LmTechnology is an effective vector for immunotherapy, andFollowing the completion of the Asset Sale on March 25, 2024, the Company madeis a clinical-stage oncology company whose primary clinical assets currently include aspacytarabine (BST-236), a novel proprietary anti-metabolite for first line treatment in unfit acute myeloid leukemia (AML). BST-236 is a novel prodrug of cytarabine, enabling delivery of high cytarabine doses with reduced systemic toxicity, creating a potential new backbone for AML combination regimens. We believe that our novel product candidates, if approved, have the decisionpotential to branch into the growing field of individualized cancer treatments with ADXS-NEO. ADXS-NEO is designedtransform treatment outcomes for patients suffering from solid and hematological cancers. We also continue to create individualized therapies by activating the patient’s immune systemconduct certain operations relating to respond against multiple mutations, or neoantigens, identified from an individual patient’s tumor through DNA sequencing. In August 2016, Advaxis entered into a global agreement with Amgen Inc. (“Amgen”) forformer Advaxis’ operations as clinical-stage biotechnology company focused on the development and commercialization of ADXS-NEO.proprietary Listeria monocytogenes (“Lm”)-based antigen delivery products. These efforts are primarily focused on the development of ADXS-504, an Lm-based therapy for early-stage prostate cancer.
ADXS-NEO is an individualizedLm Technology antigen delivery product developed using whole-exome sequencing of a patient’s tumor to identify neoantigens. ADXS-NEO is designed to work by presenting a large payload of neoantigens directly into dendritic cells within the patient’s immune system and stimulating a T cell response against cancerous cells.
The FDA has cleared the initial IND application of ADXS-NEO and we will file an IND amendment in early 2018. This amendment is largely driven by enhancements to the manufacturing and antigen selection processes. We do not expect this to impact our project timelines, and we plan to initiate a Phase 1 trial in first half of 2018. The initial tumor types for the Phase 1 are non-small cell lung cancer, microsatellite stable colorectal cancer, and head and neck cancer. The Company has entered into various research collaborations, including the Parker Institute for Cancer Immunotherapy, to advance the trial of neoepitope-based, personalized cancer therapy as part of the Tumor neoantigEn SeLection Alliance (“TESLA”) initiative.
Disease focused hotspot / cancer antigen therapies (ADXS-HOT)
Advaxis is creating a new group of immunotherapy constructs for major cancers that combines our optimizedLmTechnology vector with promising targets to generate potent anti-cancer immunity. The ADXS-HOT franchise is a series of novel cancer immunotherapies that will target somatic mutations (“hotspots”), cancer testis antigens (“CTAs”) and oncofetal antigens (“OFAs”). These three types of targets form the basis of the ADXS-HOT program because they are more capable of generating potent, tumor specific, and high strength killer T cells, versus more traditional over-expressed native sequence TAAs. Most hotspot mutations and OFA/CTA proteins play critical roles in oncogenesis; targeting both at once could significantly impair cancer proliferation. The ADXS-HOT products will combine many of the potential high avidity targets that are expressed in all patients with the target disease into one “off-the-shelf”, ready to administer treatment. The ADXS-HOT technology has a strong Intellectual Property (“IP”) position, with potential protection into 2037, and an IP filing strategy providing for broad coverage opportunities across multiple disease platforms and combination therapies.
The Company is currently prioritizing product development in the most prevalent cancers, with the first indication planned to be Non Small Cell Lung Cancer (“NSCLC”). Advaxis plans to file multiple ADXS-HOT INDs in 2018, including NSCLC, with our first in human trial in one of the ADXS-HOT products to commence in 2018.
| 5 |
Prostate Cancer (ADXS-PSA)
We have incurred significant net operating losses in every year since our inception and expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. Our net losses may fluctuate significantly from quarter to quarter and year to year and could be substantial. Our net losses were approximately $48.1 million and $38.0 million for the years ended December 31, 2023 and 2022, respectively. As of December 31, 2023, we had an accumulated deficit of $197.2 million.
According to the American Cancer Society, prostate cancer is the second most common type of cancer found in American men, and is the second leading cause of cancer death in men, behind only lung cancer. More than 161,000 men are estimated to be diagnosed with prostate cancer in 2017, with over 26,000 deaths each year. Unfortunately, in about 10 – 20% of cases, men with prostate cancer will go on to develop castration-resistant prostate cancer (“CRPC”), which refers to prostate cancer that progresses despite androgen deprivation therapy. Metastatic CRPC (“mCRPC”) occurs when the cancer spreads to other partsFollowing closing of the bodysale of AL102 and there isAL101 to Immunome, we anticipate that our expenses will decrease as we:
| ● | Continue to conduct certain operations related to advancing the development of BST-236 | |
| ● | Satisfy certain debts and obligations remaining from our operations prior to the consummation of the Asset Sale |
We do not expect to generate revenue from product sales unless and until we successfully complete clinical development and obtain regulatory approval for a rising prostate-specific antigen (“PSA”) level. This stage of prostate cancer has an average survival of 9-13 months, isproduct candidate. Additionally, we currently use contract research organizations, or CROs, to carry out our clinical development activities. Furthermore, we incur additional costs associated with deterioration in quality of life, and has few therapeutic options available.operating as a public company.
The Company has incurred recurring losses since inception as a research and development organization. For the year ended December 31, 2023, the Company used approximately $29.5 million of cash in operations and incurred a net loss of $48.1 million. As of December 31, 2023, the Company had $4.9 million in cash and cash equivalents, $25.0 million in current liabilities and an accumulated deficit of $197.2 million.
According to a data review published by MD Anderson Cancer Center in 2016, checkpoint inhibitor monotherapy has not shown significant activity in mCRPC to date. The authors hypothesize that may be due to the inability
Upon closing of the checkpoint inhibitorAsset Sale, on March 25, 2024, the Company received $13.0 million in cash, and Immunome paid $3.0 million of the Company’s liabilities directly to infiltratevendors of the tumor microenvironment, and that combination therapy with agents that induce T cell infiltration withinCompany. In addition, under the tumor may improve performanceAsset Sale Agreement the Company received 2,175,489 shares of checkpoints in prostate cancer.
Lm Technology constructs have been shown by multiple labs to reduce number and suppressive functionImmunome’s common stock (the “Immunome Shares”). The Asset Sale Agreement prohibits the Company from selling more than 50% of Tregs and MDSCsthe Immunome Shares in the tumor microenvironment and causefirst six months following the destruction of Tregs in the TME as soon as 5 days after dosing in models. This reduction of immune suppression in the tumors has been attributed to our proprietarytLLO-fusion peptides expressed by multiple copiesclosing of the plasmidsAsset Sale. In addition, on March 1, 2024, the Company issued additional convertible notes and warrants in each bacteria. Advaxis feels thatexchange for $2.0 million in funding from the combinationConvertible Notes investors. As of ADXS-PSA, our immunotherapy designedthe date of this filing, the cash proceeds received from the Asset Sale and the convertible notes sold on March 1, 2024 were not sufficient to targetpay the PSA antigen, withCompany’s existing liabilities. Therefore, the Company has limited available cash resources and requires additional financing, through the sale of a checkpoint inhibitor may provide an alternative treatment option for patients with mCRPC. Clinical benefit in prostate cancer could be a significant value creator to expand theLmTechnology platform into the prostate cancer market.
Advaxis has entered into a clinical trial collaboration and supply agreement with Merck to evaluate the safety and efficacy of ADXS-PSA as monotherapy and in combination with KEYTRUDA® (“pembrolizumab”), Merck’s anti PD-1 antibody, in a Phase 1/2, open-label, multicenter, dose determination and expansion trial in patients with previously treated metastatic, castration-resistant prostate cancer (Keynote-046). For the ADXS-PSA monotherapy dose escalation and determination portion of the trial, cohorts were started at a dose of 1 x 109 cfu (n=7) and successfully escalatedImmunome Shares or otherwise, in order to higher dose levels of 5x109 cfu (n=3) and 1x1010 cfu (n=3) without achieving a maximum tolerated dose. Treatment emergent adverse events noted at these higher dose levels were generally consistent with those observed atcontinue to fund its current operations beyond May 2024.
Raising additional funds or the lower dose level (1 x 109 cfu) other than a higher occurrence rate of Grade 2/3 hypotension. The ADXS-PSA monotherapy dose-determination phasesatisfactory completion of the trial has been completed. The RP2Dsell of ADXS-PSA monotherapy was determined to be 1x 109cfu based on a review of the totality of the clinical data. This dose was used in combination with 200 mg of pembrolizumab in a cohort of 6 patients to evaluate the safety of the combination before moving into an expanded cohort of patients (N=30 patients). The safety of the combination was confirmed and enrollment in the expansion cohort phase was initiated. Enrollment in this phase of the trial was completed in January 2017.
Preliminary data correlating clinical observations with immune data in mCRPC patients treated with ADXS-PSA monotherapy were presented at the third annual CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference in September 2017. By profiling and quantifying immune-related gene expression in patients before and after ADXS-PSA monotherapy, differences were noted between the pre- and post-treatment immune status of stable disease (“SD”) and non-stable disease patients. 100% of the patients who achieved SD (31%; 4/13) showed expansion of pre-existing, as well as generation of new T cells. There were significantly higher expression levels of genes indicative of CD4+ and CD8+ T cell activation and genes supporting M1 macrophages, and lower expression levels of immunosuppressive (protumor) genes. Patients with non-stable disease (69%; 9/13) exhibited gene expression profiles suggesting a more immunosuppressive myeloid profile with asynchronous and unsustained clonal T cell expansions, and increased expression of prostate cancer tumor markers in peripheral circulation. Together, these findings suggest that SD patients treated with ADXS-PSA may have a more pro-inflammatory immunologic picture, fewer circulating tumor cells, and potentially less tumor burden.
In November 2017 at the Society for Immunotherapy of Cancer (“SITC”), the Company presented additional preliminary data from this monotherapy arm that shows that after administration of ADXS-PSA, all 9 patients who received all three doses saw increasing numbers and strength of T cells targeting PSA, using an ELISpot data analysis. In 56% (5/9) patients, this increase was 3-fold. This data shows, for the first time clinically, that anLm Technology construct has shown specific T cell generation against a specific target. In addition, the Company evaluated four other markers expressed by prostate cancers and 100% (9/9) patients had an increased frequency of T cells to at least one of these targets, demonstrating that antigen spreading after administration of ADXS-PSA does occur in the clinic and can expand activity.
Preliminary safety data shows the most common side effects include chills, fever, rigors, hypertension, fatigue, hypotension and vomiting which are consistent with cytokine release in response to ADXS-PSA administration.
Viewed collectively, the data presented at CRI-CIMT-EATI-AACR 2017 and SITC 2017 is an encouraging signal that ADXS-PSA allows for generation of sustained, strengthened T cells against prostate cancer, while weakening the TME and allowing T cells accessImmunome Shares prior to the tumor. The Company looks forwardend of May 2024 is essential to providing additional data in 2018provide sufficient cash flow to meet future liabilities and other obligations, such as tax payments resulted from the combination arm of this trial with pembrolizumab.
In addition,Asset Sale. Furthermore, even if the Company is actively developing ansuccessful in selling the Immunome Shares or raising additional product candidate for prostate cancer, currentlyfunds, the Company cannot give any assurance that it will, in preclinical testing, which could complement ADXS-PSA.the future, be able to achieve a level of profitability from the sale of its products to sustain its operations.
Other Lm Technology ProductsIf the Company is unable to obtain funding, or able to receive sufficient funds from sale of Immunome Shares the Company would be forced to delay, reduce, or eliminate its research and development programs, or the Company may be unable to continue operations. As such, those factors raise substantial doubt about the Company’s ability to continue as a going concern.
HER2 Expressing Solid Tumors
HER2 is overexpressedAs part of a cost reduction plan, during the year ended December 31, 2023, the Company had a reduction in a percentageworkforce in which the employment of solid tumors including osteosarcoma. Accordingapproximately 50% of the Company’s employees was terminated. During the first quarter of 2024, the Company gave notice of termination to published literature, up18 additional employees and two officers (including the Chief Financial Officer, whose employment will terminate on June 25, 2024). After the effectiveness of such terminations, the Chief Executive Officer will be the only employee of the Company. The Company expects to 60% of osteosarcomas are HER2 positive, and this overexpression is associated with poor outcomes for patients.be able to meet its financial obligations to its employees.
ADXS-HER2 is anLm Technology antigen delivery product candidate designed to target HER2 expressing solid tumors including human and canine osteosarcoma. ADXS-HER2 has received FDA and EMA orphan drug designation for osteosarcoma and has received Fast Track designation from the FDA for patients with newly-diagnosed, non-metastatic, surgically-resectable osteosarcoma.
The dose finding phase of a Phase 1b trial in HER2 expressing tumors has been completed. Based on priorities and focus, the Company has decided not to proceed to the expansion phase of the trial. The Company remains open to investigator-initiated research or licensing proposals for ADXS-HER2.
Canine Osteosarcoma
On March 19, 2014, we entered into a definitive Exclusive License Agreement (the “Aratana Agreement”) with Aratana Therapeutics Inc. (“Aratana”), where we granted Aratana an exclusive, worldwide, royalty-bearing license, with the right to sublicense, certain of our proprietary technologycan give no assurances that enables Aratana to develop and commercialize animal health products thatit will be targeted for treatment of osteosarcoma and other cancer indicationssuccessful in animals. A product license request was filed by Aratana for ADXS-HER2 (also known as AT-014 by Aratana) for the treatment of canine osteosarcoma with the United States Department of Agriculture (“USDA”). Aratana received communication in December 2017 that the USDA granted Aratana conditional licensure for AT-014 for the treatment of dogs diagnosed with osteosarcoma, one year of age or older. Aratana plans to conduct an extended field study in a clinical setting and anticipates initiating the study in early 2018, which will be fully funded by Aratana. Initially, Aratana plans to make the therapeutic available for purchase at approximately two dozen veterinary oncology practice groups across the United States who participate in the study.
Aratana has been granted exclusive worldwide rights by us to develop and commercialize ADXS-HER2 in animals. Aratana is further responsible for the conduct of clinical research with ADXS-Survivin in canine/feline lymphoma, as well as pending investigations of two additional Advaxis constructs in animals.
Under the terms of the Aratana Agreement, Aratana paid an upfront payment to us in the amount of $1,000,000 upon signing of the Aratana Agreement. Aratana will also pay us (a) up to $36.5 million based on the achievement of milestone relating to the advancement of productsraising funds through the approval process with the USDA in the United States and the relevant regulatory authorities in the European Union (“E.U.”) in all four therapeutic areas and up to an additional $15 million in cumulative sales milestones based on achievement of gross sales revenue targets for sales of any and all products for use in non-human animal health applications (the “Aratana Field”) (regardless of therapeutic area), and (b) tiered royalties starting at 5% and going up to 10%, which will be paid based on net sales of any and all products (regardless of therapeutic area) in the Aratana Field in the United States. Royalties for sales of products outside of the United States will be paid at a rate equal to half of the royalty rate payable by Aratana on net sales of products in the United States (starting at 2.5% and going up to 5%). Royalties will be payable on a product-by-product and country-by-country basis from first commercial sale of a product in a country until the later of (a) the 10th anniversary of first commercial sale of such product by Aratana, its affiliates or sub licensees in such country or (b) the expirationportion of the last-to-expire valid claimImmunome Shares or any other alternative. Any inability to so obtain additional financing or funding will likely cause the Company to cease business operations. The consolidated financial statements have been prepared assuming that the Company will continue as a going concern. Therefore, the consolidated financial statements for the year ended December 31, 2023, do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern. See “— Liquidity and Capital Resources.” Substantial doubt about our ability to continue as a going concern may materially and adversely affect the price per share of our patentscommon stock, and it may be more difficult for us to obtain financing. If potential collaborators decline to do business with us or joint patents claiming or coveringpotential investors decline to participate in any future financings due to such concerns, our ability to increase our cash position may be limited. We will need to generate significant revenues to achieve profitability, and we may never do so. Because of the compositionnumerous risks and uncertainties associated with the development of matter, formulation or methodour current and any future product candidates and because the extent to which we may enter into collaborations with third parties for the development of useany of suchour product in such country. Aratana will also pay us 50%candidates is unknown, we are unable to estimate the amounts of all sublicense royalties received by Aratanaincreased capital outlays and its affiliates.operating expenses required for completing the research and development of our product candidates.
Corporate Information
We were originally incorporated in the State of Colorado on June 5, 1987 under the name Great Expectations, Inc. We were a publicly-traded “shell” company without any business until November 12, 2004 when we acquired Advaxis, Inc.,New Ayala, a Delaware corporation, through a Share Exchange and Reorganization Agreement, dated as of August 25, 2004, which we refer to as the Share Exchange, by and among Advaxis,New Ayala, the stockholders of AdvaxisNew Ayala and us. As a result of the Share Exchange, AdvaxisNew Ayala became our wholly-owned subsidiary and our sole operating company. On December 23, 2004, we amended and restated our articles of incorporation and changed our name to Advaxis, Inc.New Ayala. On June 6, 2006, our stockholders approved the reincorporation of our company from Colorado to Delaware by merging the Colorado entity into our wholly-owned Delaware subsidiary. Our date of inception, for financial statement purposes, is March 1, 2002 and the Company was uplisted to NASDAQlisted on The Nasdaq Capital Market (“Nasdaq”) in 2014. In December 2021, the Company was delisted from Nasdaq and accepted onto the OTCQX. Shares of New Ayala’s common stock are currently quoted on the OTCQX under the symbol “ADXS.”
Our principal executive offices and manufacturing facility isare located at 305 College Road East, Princeton,9 Deer Park Drive, Suite K-1, Monmouth Junction, New Jersey 0854008852, and our telephone number is (609) 452-9813. We maintain a corporate website at www.advaxis.comwww.ayalapharma.com which contains descriptions of our technology, our product candidates and the development status of each drug. We make available free of charge through our Internetinternet website our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reportsCurrent Reports on Form 8-K, and any amendments to these reports, as soon as reasonably practicable after we electronically file such material with, or furnish such material to, the SEC. We are not including the information on our website as a part of, nor incorporating it by reference into, this report. You may read and copy any materials we file at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549 on official business days during the hours of 10:00 a.m. to 3:00 p.m. Please call the SEC at 1-800-SEC-0330 for information on the Public Reference Room. Additionally, theThe SEC maintains a website that contains annual, quarterly, and current reports, proxy statements, and other information that issuers (including us) file electronically with the SEC. The SEC’s website address is http://www.sec.gov.
Intellectual Property
Protection of our intellectual property is important to our business. We have a robust and extensive patent portfolio that protects our product candidates andLm -based Lm-based immunotherapy technology. Currently, we own or have rights to approximately 433numerous patents and applications, which are owned, licensed from, or co-owned with University of Pennsylvania, (“Penn”) , Merck,or Penn, National Institute of Health, (“NIH”),or NIH, and/or Augusta University. We continuously grow this portfolio by filing new applications to protect our ongoing research and development efforts. We aggressively prosecute and defend our patents and proprietary technology. Our patents and applications are directed to the compositions of matter, use, and methods thereof, of ourLm -LLO Lm-LLO immunotherapies for our product candidates, including axalimogene filolisbac, ADXS-DUAL,AXAL, ADXS-PSA, ADXS-NEO, ADXS-HOT, ADXS-HER2. We have and may continue to abandon prosecuting certain patents that are not strategically aligned with the direction of the Company.
Our approach to the intellectual property portfolio is to create, maintain, protect, enforce and defend our proprietary rights for the products we develop from our immunotherapy technology platform. We endeavor to maintain a coherent and aggressive strategic approach to building our patent portfolio with an emphasis in the field of cancer vaccines.
Issued patents which are directed to axalimogene filolisbac,AXAL, ADXS-PSA, and ADXS-HER2 in the United States, will expire between 20172024 and 2032. Issued patents directed to our product candidates axalimogene filolisbac,AXAL, ADXS-PSA, and ADXS-HER2 outside of the United States, will expire in 2032. Issued patents directed to ourLm -based immunotherapy platform in the United States, will expire between 20172024 and 2031. Issued patents directed to ourLm -based Lm-based immunotherapy platform outside of the United States, will expire between 20182024 and 2033.
| 6 |
We have pending patent applications directed to our product candidates axalimogene filolisbac,AXAL, ADXS-PSA, ADXS-HER2, ADXS-NEO, ADXS-DUAL and ADXS-HOT that, if issued would expire in the United States and in countries outside of the United States between 20202024 and 2037. We have pending patent applications directed to methods of using of our product candidates axalimogene filolisbac, ADXS-DUAL,AXAL, ADXS-PSA, ADXS-NEO, ADXS-HOT, ADXS-HER2 directed to the following indications and others: HPV-related cervical cancer, prostate cancer and her2/neu-expressing cancer, that, if issued would expire in the United States and in countries outside of the United States between 20202024 and 2037, depending on the specific indications.
We will be able to protect our technology from unauthorized use by third parties only to the extent it is covered by valid and enforceable patents or is effectively maintained as trade secrets. Patents and other proprietary rights are an essential element of our business.
BST-236
Our commercial success will dependdepends, in part, on our ability to obtain and maintain proprietary protection for our product candidate, BST-236, as well any future product candidates, technology,novel discoveries, product development technologies and know-how,know-how. We own all of our intellectual property relating to operate without infringing on the proprietaryBST-236 and have a patent portfolio that includes several patent families that cover composition, methods and use. We protect these intellectual property rights, of others, and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions, and improvements thatwhich are importantcritical to the development, formulation and marketing of our business. We alsoproduct candidate, by filing patents or patent applications with the U.S. Patent & Trademark Office and the comparable patent offices of Israel, Europe and other countries. In addition, we rely on trade secrets, know-how, continuing technological innovation, orphan drug exclusivity and potential in-licensing opportunities to develop and maintain our proprietary position.
Any patent applications which As of April 4, 2024, we have filed or will file orpatent applications relating to which we have or will have license rights may not issue,BST-236 in multiple jurisdictions, including Australia, Brazil, Canada, China, Europe, India, Japan, Israel, Russia, Korea, Hong Kong and patents that do issue may not contain commercially valuable claims. In addition, any patents issued to us or our licensors may not afford meaningful protection for our products or technology, or may be subsequently circumvented, invalidated, narrowed, or found unenforceable. Our processes and potential products may also conflict with patents which have been or may be granted to competitors, academic institutions or others. As the pharmaceutical industry expands and more patents are issued, the risk increases that our processes and potential products may give rise to interferences filed by others in the U.S. Patent and Trademark Office, or to claims of patent infringement by other companies, institutions or individuals. These entities or persons could bring legal actions against us claiming damages and seeking to enjoin clinical testing, manufacturing and marketing of the related product or process. In recent years, several companies have been extremely aggressive in challenging patents covering pharmaceutical products, and the challenges have often been successful. If any of these actions are successful, in addition to any potential liability for damages, we could be required to cease the infringing activity or obtain a license in order to continue to manufacture or market the relevant product or process. We may not prevail in any such action and any license required under any such patent may not be made available on acceptable terms, if at all. Our failure to successfully defend a patent challenge or to obtain a license to any technology that we may require to commercialize our technologies or potential products could have a materially adverse effect on our business. In addition, changes in either patent laws or in interpretations of patent laws in the United States, and other countries may materially diminish the value of our intellectual property or narrow the scope of our patent protection.
We also rely upon unpatented proprietary technology, and in the future may determine in some cases that our interests would be better served by reliance on trade secrets or confidentiality agreements rather than patents or licenses. We may not be able to protect our rights to such unpatented proprietary technology and others may independently develop substantially equivalent technologies. If we are unable to obtain strong proprietary rights to our processes or products after obtaining regulatory clearance, competitors may be able to market competing processes and products.
Others may obtain patents having claims which cover aspects of our products or processes which are necessary for, or useful to, the development, use or manufacture of our services or products. Should any other group obtain patent protection with respect to our discoveries, our commercialization of potential therapeutic products and methods could be limited or prohibited.
The Drug Development Process
The product candidates in our pipeline are at various stages of preclinical and clinical development. The path to regulatory approval includes multiple phases of clinical trials in which we collect data that will ultimately support an application to regulatory authorities to allow us to market a product for the treatment, of a specific type of cancer. There are many difficulties and uncertainties inherent in research and development of new products, resulting in high costs and variable success rates. Bringing a drug from discovery to regulatory approval, and ultimately to market, takes many years and significant costs.
Clinical testing, known as clinical trials or clinical studies, is either conducted internally by pharmaceutical or biotechnology companies or is managed on behalf of these companies by Clinical Research Organizations (“CRO”). The process of conducting clinical studies is highly regulated by the FDA, as well as by other governmentalunder the Patent Cooperation Treaty. We have been issued five patents in United States, two in Europe two in each of Australia, Israel, Canada, Japan and professional bodies. In a clinical trial, participants receive specific interventions accordingRussia and one in each India and Macau. Issued patents and currently pending patent applications are summarized below:
| ● | BIOST/002 patent family. We have two issued US patents (US 8,993,278, US 7,989,188) and one European patent (validated in France, United Kingdom and Germany) to cover BST-236, composition and use. |
| ● | BIOST/004. We have one granted patent in the US, two granted Australia, One granted in each of Russia, Canada, China, Monaco, Israel and India and one under examination in each of US, EP, Brazil,, Japan (two applications)to cover BST-236 pharmaceutically acceptable salts and use. |
| ● | BIOST/005 patent family. We have one granted patent and one allowed patent in US, one granted in each of Australia, Russia, Israel, Japan, Canada and one European patent (validated in Germany, France and United Kingdom), and one under examination in each of , Brazil, China, Japan and Israel to cover use. |
| ● | BIOST/007. We have one granted patent in Israel and one granted patent in Japan, and one under examination in each of US, Europe, Brazil, Japan, Canada, China, Korea and Hong Kong, to cover combination therapy. |
| ● | BIOST/008. We have patents under examination in US, Europe, Israel, Brazil, Canada, China, Hong Kong, Japan and Korea to cover use. |
| ● | BIOST/009. We have patents under examination in US, Europe, Israel, Brazil, Canada, China, Hong Kong, Japan and Korea to cover formulation. |
| ● | BIOST/010. We have patents under examination in US, Europe, Israel, Australia, Brazil, Canada, China, Hong Kong, India, Japan and Korea to cover polymorph. |
| ● | BIOST/011. We have one patent under the PCT to cover Dimers. |
| ● | BIOST/012. We have one patent under the PCT to cover Crystalline forms. |
| ● | BIOST/013. We have one patent under the PCT to cover Intermediates. |
BIOST/011. We have one patent under the PCT to the research plan or protocol created by the study sponsor and implemented by study investigators. Clinical trials may compare a new medical approach to a standard one that is already available or to a placebo that contains no active ingredients or to no intervention. Some clinical trials compare interventions that are already available to each other. When a new product or approach is being studied, it is not usually known whether it will be helpful, harmful, or no different than available alternatives. The investigators try to determine the safety and efficacy of the intervention by measuring certain clinical outcomes in the participants.cover Impurities.
| 7 |
Phase 1. Phase 1 clinical trials begin when regulatory agencies allow initiation of clinical investigation of a new drug or product candidate. They typically involve testing an investigational new drug on a limited number of patients. Phase 1 studies determine a drug’s basic safety, maximum tolerated dose and how the drug is absorbed by, and eliminated from, the body. Typically, cancer therapies are initially tested on late-stage cancer patients.
Phase 2. Phase 2 clinical trials involve larger numbers of patients that have been diagnosed with the targeted disease or condition. Phase 2 clinical trials gather preliminary data on effectiveness (where the drug works in people who have a certain disease or condition) and to determine the common short-term side effects and risks associated with the drug. If Phase 2 clinical trials show that an investigational new drug has an acceptable range of safety risks and probable effectiveness, a company will continue to evaluate the investigational new drug in Phase 3 studies.
Phase 3. Phase 3 clinical trials are typically controlled multi-center trials that involve a larger number of patients to ensure the study results are statistically significant. The purpose is to confirm effectiveness and safety on a large scale and to provide an adequate basis for physician labeling. These trials are generally global in nature and are designed to generate clinical data necessary to submit an application for marketing approval to regulatory agencies.
U.S. Drug Development Process
Biologic License Application (BLA). The results of the clinical trials using biologics are submitted to the FDA as part of a BLA. Following the completion of Phase 3 studies, if the sponsor of a potential product in
In the United States, believes it has sufficient information to support the safety and effectiveness of the investigational new drug, the sponsor submits a BLA to the FDA requesting thatregulates drugs under the investigational new drug be approved for marketing.federal Food, Drug, and Cosmetic Act, or the FDCA, and its implementing regulations. The application is a comprehensive filing that includes the resultsprocess of all preclinical and clinical studies, information about the drug’s composition,obtaining regulatory approvals and the sponsor’s plans for manufacturing, packaging, labelingsubsequent compliance with appropriate federal, state, local and testingforeign statutes and regulations require the investigational new drug. expenditure of substantial time and financial resources.
The FDA’s review of an application is designated either as a standard review with a target review time of 10 months or a priority review with a target of 6 months. Depending upon the completeness of the application and the number and complexity of follow-up requests and responses betweenprocess required by the FDA and the sponsor, the review time can take months to many years. Once approved through this process,before a drug may be marketed in the United States generally involves the following:
● completion of preclinical laboratory tests, animal studies and formulation studies in accordance with FDA’s good laboratory practice requirements and other applicable regulations;
● submission to the FDA of an investigational new drug application, or IND, which must become effective before human clinical trials may begin;
● approval by an independent institutional review board, or IRB, or ethics committee at each clinical site before each trial may be initiated;
● performance of adequate and well-controlled human clinical trials in accordance with good clinical practice requirements, or GCPs to establish the safety and efficacy of the proposed drug for its intended use;
● submission to the FDA of an NDA after completion of all pivotal trials;
● satisfactory completion of an FDA advisory committee review, if applicable;
● satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with cGMP requirements to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity, and of selected clinical investigation sites to assess compliance with GCPs; and
● FDA review and approval of the NDA to permit commercial marketing of the product for particular indications for use in the United States.
Prior to beginning the first clinical trial with a product candidate in the United States, we must submit an IND to the FDA. An IND is a request for authorization from the FDA to administer an investigational new drug product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for clinical studies. The IND also includes results of animal and in vitro studies assessing the toxicology, pharmacokinetics, pharmacology, and pharmacodynamics characteristics of the product; chemistry, manufacturing, and controls information; and any available human data or literature to support the use of the investigational product. An IND must become effective before human clinical trials may begin. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises safety concerns or questions about the proposed clinical trial. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before the clinical trial can begin. Submission of an IND therefore may or may not result in FDA authorization to begin a clinical trial.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCPs, which include the requirement that all research subjects provide their informed consent for their participation in any clinical study. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development and for any subsequent protocol amendments. While the IND is active, progress reports summarizing the results of the clinical trials and nonclinical studies performed since the last progress report, among other information, must be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and investigators for serious and unexpected suspected adverse events, findings from other studies suggesting a significant risk to humans exposed to the drug, findings from animal or in vitro testing suggesting a significant risk to humans exposed to the drug, and any clinically important increased rate of a serious suspected adverse reaction compared to that listed in the protocol or investigator brochure.
Furthermore, an independent IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial and its informed consent form before the clinical trial begins at that site and must monitor the study until completed. Regulatory authorities, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk or that the trial is unlikely to meet its stated objectives. Some studies also include oversight by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain data from the study and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. There are also requirements governing the reporting of ongoing clinical studies and clinical study results to public registries.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
● Phase 1: The product candidate is initially introduced into healthy human subjects or patients with the target disease or condition. These studies are designed to test the safety, dosage tolerance, absorption, metabolism and distribution of the investigational product in humans, the side effects associated with increasing doses, and, if possible, to gain early evidence on effectiveness. In the case of some products for severe or life-threatening diseases, such as cancer, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients.
| 8 |
● Phase 2: The product candidate is administered to a limited patient population with a specified disease or condition to evaluate the preliminary efficacy, optimal dosages and dosing schedule and to identify possible adverse side effects and safety risks. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase 3 clinical trials.
● Phase 3: The product candidate is administered to an expanded patient population to further evaluate dosage, to provide statistically significant evidence of clinical efficacy and to further test for safety, generally at multiple geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the investigational product and to provide an adequate basis for product approval.
Post-approval trials, sometimes referred to as Phase 4 studies, may be conducted after initial marketing approval. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA.
During the development of a new drug, sponsors are given opportunities to meet with the FDA at certain points. These points may be prior to submission of an IND, at the end of Phase 2, and before an NDA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and the FDA to reach consensus on the next phase of development. Sponsors typically use the meetings at the end of the Phase 2 trial to discuss Phase 2 clinical results and present plans for the pivotal Phase 3 clinical trials that they believe will support approval of the new drug.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug. In addition, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
U.S. Review and Approval Process
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, preclinical and other non-clinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. The submission of an NDA is subject to any conditions imposedthe payment of substantial user fees; a waiver of such fees may be obtained under certain limited circumstances. Additionally, no user fees are assessed on NDAs for products designated as orphan drugs, unless the product also includes a non-orphan indication. The FDA conducts a preliminary review of all NDAs within the first 60 days after submission, before accepting them for filing, to determine whether they are sufficiently complete to permit substantive review The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once filed, the FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality and purity. Under the Prescription Drug User Fee Act, or PDUFA, guidelines that are currently in effect, the FDA has a goal of ten months from the date of “filing” of a standard NDA for a new molecular entity to review and act on the submission. This review typically takes twelve months from the date the NDA is submitted to FDA because the FDA has approximately two months to make a “filing” decision after it the application is submitted.
The FDA may refer an application for a novel drug to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the FDA.recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA will typically inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP and adequate to assure consistent production of the product within required specifications. Additionally, before approving a NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCPs.
| 9 |
After the FDA evaluates an NDA, it will issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the drug with prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete, and the application will not be approved in its present form. A Complete Response Letter usually describes the specific deficiencies in the NDA identified by the FDA and may require additional clinical data, such as an additional clinical or other significant and time-consuming requirements related to clinical trials, nonclinical studies or manufacturing. If a Complete Response Letter is issued, the sponsor must resubmit the NDA or, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information are submitted, the FDA may decide that the NDA does not satisfy the criteria for approval.
If regulatory approval of a product is granted, such approval will be granted for particular indications and may entail limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the NDA with a Risk Evaluation and Mitigation Strategy, or REMS, to ensure the benefits of the product outweigh its risks. A REMS is a safety strategy to manage a known or potential serious risk associated with a medicine and to enable patients to have continued access to such medicines by managing their safe use, and could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing requirements is not maintained or if problems occur after the product reaches the marketplace. The FDA may also require one or more Phase 4 post-market studies and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization, and may limit further marketing of the product based on the results of these post-marketing studies.
In addition, the Pediatric Research Equity Act, or PREA, requires a sponsor to conduct pediatric clinical trials for most drugs, for a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. Under PREA, original NDAs and supplements must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must evaluate the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The sponsor or FDA may request a deferral of pediatric clinical trials for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that the drug is ready for approval for use in adults before pediatric clinical trials are complete or that additional safety or effectiveness data need to be collected before the pediatric clinical trials begin. The FDA must send a non-compliance letter to any sponsor that fails to submit the required assessment, keep a deferral current or fails to submit a request for approval of a pediatric formulation.
Government Regulations
General
Government authorities in the United States and other countries extensively regulate, among other things, the preclinical and clinical testing, manufacturing, labeling, storage, record-keeping, advertising, promotion, import, export, marketing and distribution of drugs.biopharmaceutical and drug products. In the United States, the FDA subjects drugs to rigorous review under the Federal Food, Drug and Cosmetic Act, or FDCA, the Public Health Service Act, or PHSA, and other federal statutes andimplementing regulations.
| 10 |
Orphan Drug Designation
Under the Orphan Drug Act (“ODA”),ODA, the FDA may grant Orphan Drug Designation (“ODD”)ODD, to a drug or biological product intended to treat a rare disease or condition, which means a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States, but for which there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States will be recovered from domestic sales of the product. Additionally, sponsors must present a plausible hypothesis for clinical superiority to obtain ODD if there is a product already approved by the FDA that that is considered by the FDA to be the same as the already approved product and is intended for the same indication. This hypothesis must be demonstrated to obtain orphan exclusivity.
The benefits of ODD can be substantial, including research and development tax credits, grants and exemption from user fees, enhanced access to advice fromfees. The tax advantages, however, were limited in the 2017 Tax Cuts and Jobs Act. Moreover, if there is no other product that the FDA whileconsiders to be the drugsame product that is being developed, andapprove for the orphan indication, the orphan designated product is eligible for 7 years of orphan market exclusivity once the product reaches approval and begins sales, providedis approved. During that period, the new product is first to market and that this new product hasFDA generally may not been previously approvedapprove any other application for the same product for the same indication, although there are exceptions, most notably when the later product is shown to be clinically superior to the product with exclusivity. Other applicants, however, may receive approval of different products for the orphan diseaseindication or condition, with or withoutthe same product for a different indication during the orphan drug designation.exclusivity period. In order to qualify for these incentives, a company must apply for designation of its product as an “Orphan Drug” and obtain approval from the FDA. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process; however,process.
In the EU, orphan drug designation provides up to 10 years of market exclusivity in Europe, in addition to the 7-year exclusivity in the U.S. as a result of the FDA’s orphan drug designation in 2019. EMA designation will also provide us with other benefits and incentives such as clinical protocol assistance, access to a centralized marketing authorization procedure that is valid in all European Union (“EU”) member states and reduced regulatory fees.
The Company has an ODD product may be eligibleBST-236 (. As well as EMA ODD for priority review. A drug that is approved for the ODD indication is granted seven years of orphan drug exclusivity. During that period, the FDA generally may not approve any other application for the same product for the same indication, although there are exceptions, most notably when the later product is shown to be clinically superior to the product with exclusivity.BST-236.
We currently have ODD with the FDA for axalimogene filolisbacAXAL for treatment of anal cancer (granted August 2013), HPV-associated head and neck cancer (granted November 2013); and treatment of Stage II-IV invasive cervical cancer (granted May 2014). We also have ODD with the FDA for ADXS-HER2 for the treatment of osteosarcoma (granted May 2014).
In Europe, the Committee for Orphan Medicinal Products, (“COMP”)COMP, has issued a positive opinion on the application for ODD of axalimogene filolisbacAXAL for the treatment of anal cancer (December 2015) and on the application for ODD of ADXS-HER2 for osteosarcoma (November 2015).
Expedited Review and Approval Programs for Serious Conditions
Four core FDA programs are intended to facilitate and expedite the development and review of new drugsbiologics to address unmet medical need in the treatment of serious or life-threatening conditions: fast trackFast Track designation, breakthrough therapy designation, accelerated approval, and priority review designation.review. We intend to avail ourselves of any and all of these programs as applicable to our products.
FDA is required to facilitate the development, and expedite the review, of products that are intended for the treatment of a serious or life-threatening disease or condition, and which demonstrate the potential to address unmet medical needs for the condition. Under the Fast Track program, the sponsor of a new biologic product candidate may request that the FDA designate the drug candidate for a specific indication as a Fast Track drug concurrent with, or after, the filing of the IND for the product candidate. FDA must determine if the product candidate qualifies for Fast Track designation within 60 days of receipt of the sponsor’s request. If Fast Track designation is obtained, sponsors may be eligible for more frequent development meetings and correspondence with the FDA. FDA may also initiate review of sections of a Fast Track product’s BLA before the application is complete. This rolling review is available if the applicant provides, and FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA’s time period goal for reviewing an application does not begin until the last section of the BLA is submitted.
Under FDA’s accelerated approval programs, FDA may approve a product for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments.
In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions or survives. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. A product candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, will allow the FDA to withdraw the product from the market on an expedited basis. All promotional materials for product candidates approved under accelerated regulations are subject to prior review by the FDA.
Under the provisions of the FDA Safety and Innovation Act, or FDASIA, enacted in 2012, a sponsor can request the designation of a product candidate as a “breakthrough therapy.” A breakthrough therapy is defined as a product that is intended, alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Products designated as breakthrough therapies are eligible for intensive guidance on an efficient development program beginning as early as Phase 1 trials, a commitment from the FDA to involve senior managers and experienced review staff in a proactive collaborative and cross-disciplinary review, rolling review, and the facilitation of cross-disciplinary review.
| 11 |
Another expedited pathway is the Regenerative Medicine Advanced Therapy, or RMAT, designation. Qualifying products must be a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or a combination of such products, and not a product solely regulated as a human cell and tissue product. The product must be intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition, and preliminary clinical evidence must indicate that the product has the potential to address an unmet need for such disease or condition. Advantages of the RMAT designation include all the benefits of the Fast Track and breakthrough therapy designation programs, including early interactions with the FDA. These early interactions may be used to discuss potential surrogate or intermediate endpoints to support accelerated approval.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA regulated products, including biologics, are required to register and submit certain clinical trial information within specific timeframes to the National Institutes of Health, or NIH, for public dissemination on their clinicaltrials.gov website. Information related to the product, patient population, phase of the investigation, Trial sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed in certain circumstances for up to two years, depending on the circumstances, after the date of completion of the trial. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
Coverage, Pricing and Reimbursement
Successful commercialization of new drug products depends in part on the extent to which reimbursement for those drug products will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drug products they will pay for and establish reimbursement levels. The availability and extent of reimbursement by governmental and private payors is essential for most patients to be able to afford a drug product. Sales of drug products depend substantially, both domestically and abroad, on the extent to which the costs of drugs products are paid for by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by government health administration authorities, private health coverage insurers and other third-party payors.
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and other third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular drug products. In many countries, the prices of drug products are subject to varying price control mechanisms as part of national health systems. In general, the prices of drug products under such systems are substantially lower than in the United States. Other countries allow companies to fix their own prices for drug products, but monitor and control company profits. Accordingly, in markets outside the United States, the reimbursement for drug products may be reduced compared with the United States. In the United States, the principal decisions about reimbursement for new drug products are typically made by CMS an agency within HHS. CMS decides whether and to what extent a new drug product will be covered and reimbursed under certain federal governmental healthcare programs, such as Medicare, and private payors tend to follow CMS to a substantial degree. However, no uniform policy of coverage and reimbursement for drug products exists among third-party payors and coverage and reimbursement levels for drug products can differ significantly from payor to payor. In the United States, the process for determining whether a third-party payor will provide coverage for a biological product typically is separate from the process for setting the price of such product or for establishing the reimbursement rate that the payor will pay for the product once coverage is approved. With respect to biologics, third-party payors may limit coverage to specific products on an approved list, also known as a formulary, which might not include all of the FDA-approved products for a particular indication, or place products at certain formulary levels that result in lower reimbursement levels and higher cost sharing obligation imposed on patients. A decision by a third-party payor not to cover our product candidates could reduce physician utilization of a product. Moreover, a third-party payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable a manufacturer to maintain price levels sufficient to realize an appropriate return on its investment in product development. Additionally, coverage and reimbursement for products can differ significantly from payor to payor. One third-party payor’s decision to cover a particular medical product does not ensure that other payors will also provide coverage for the medical product, or will provide coverage at an adequate reimbursement rate. As a result, the coverage determination process usually requires manufacturers to provide scientific and clinical support for the use of their products to each payor separately and is a time-consuming process.
Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future. Third-party payors are increasingly challenging the prices charged for medical products and services, examining the medical necessity and reviewing the cost-effectiveness of pharmaceutical products, in addition to questioning safety and efficacy. If third-party payors do not consider a product to be cost-effective compared to other available therapies, they may not cover that product after FDA approval or, if they do, the level of payment may not be sufficient to allow a manufacturer to sell its product at a profit.
| 12 |
In addition, in many foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing and reimbursement vary widely from country to country. In the European Union, governments influence the price of products through their pricing and reimbursement rules and control of national healthcare systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed to by the government. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost effectiveness of a particular product to currently available therapies. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. The downward pressure on healthcare costs in general, particularly prescription products, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross border imports from low-priced markets exert a commercial pressure on pricing within a country (particularly in the EEA where it is illegal to impede such imports from elsewhere within the EEA).
Other Healthcare Laws
Manufacturing, sales, promotion and other activities following product approval are also subject to regulation by numerous regulatory authorities in the United States in addition to the FDA, including CMS, the HHS Office of Inspector General and HHS Office for Civil Rights, other divisions of the HHS and the Department of Justice.
Healthcare providers, physicians, and third-party payors will play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Our current and future arrangements with third-party payors, healthcare providers and physicians may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any drugs for which we obtain marketing approval. In the United States, these laws include, without limitation, state and federal anti-kickback, false claims, physician transparency, and patient data privacy and security laws and regulations, including but not limited to those described below.
The AKS prohibits, among other things, any person or entity from knowingly and willfully offering, paying, soliciting, receiving or providing any remuneration, directly or indirectly, overtly or covertly, to induce or in return for purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any good, facility, item or service reimbursable, in whole or in part, under Medicare, Medicaid or other federal healthcare programs. The term “remuneration” has been broadly interpreted to include anything of value. The AKS has been interpreted to apply to arrangements between pharmaceutical and medical device manufacturers on the one hand and prescribers, purchasers, formulary managers and beneficiaries on the other hand. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the AKS. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. In addition, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Moreover, a claim including items or services resulting from a violation of the AKS constitutes a false or fraudulent claim for purposes of the FCA.
Although we would not submit claims directly to payors, drug manufacturers can be held liable under the FCA, which imposes civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities (including manufacturers) for, among other things, knowingly presenting, or causing to be presented to federal programs (including Medicare and Medicaid) claims for items or services, including drugs, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. The government may deem manufacturers to have “caused” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. Several biopharmaceutical, medical device and other healthcare companies have been prosecuted under the FCA and civil monetary penalty laws for, among other things, allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of products for unapproved (e.g., or off-label), and thus non-covered, uses. In addition, the civil monetary penalties statute imposes penalties against any person who is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. Claims which include items or services resulting from a violation of the federal AKS are false or fraudulent claims for purposes of the FCA.
Our future marketing and activities relating to the reporting of wholesaler or estimated retail prices for our products, if approved, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state and third-party reimbursement for our products, and the sale and marketing of our product candidates, are subject to scrutiny under these laws.
| 13 |
HIPAA, created additional federal criminal statutes that prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud or to obtain, by means of false or fraudulent pretenses, representations or promises, any money or property owned by, or under the control or custody of, any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians and certain other healthcare providers. The Affordable Care Act, or the ACA, imposed, among other things, new annual reporting requirements through the Physician Payments Sunshine Act for covered manufacturers for certain payments and “transfers of value” provided to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately and completely the required information for all payments, transfers of value and ownership or investment interests may result in civil monetary penalties. Covered manufacturers must submit reports by the 90th day of each subsequent calendar year and the reported information is publicly made available on a searchable website.
We may also be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA, as amended by HITECH and their respective implementing regulations, including the Final HIPAA Omnibus Rule published on January 25, 2013, impose specified requirements relating to the privacy, security and transmission of individually identifiable health information held by covered entities and their business associates. Among other things, HITECH made HIPAA’s security standards directly applicable to “business associates,” defined as independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity, although it is unclear that we would be considered a “business associate” in the normal course of our business. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same requirements, thus complicating compliance efforts.
Similar state and foreign fraud and abuse laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services. Such laws are generally broad and are enforced by various state agencies and private actions. Also, many states have similar fraud and abuse statutes or regulations that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs. Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant federal government compliance guidance, and require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, marketing expenditures or drug pricing.
In order to distribute products commercially, we must comply with state laws that require the registration of manufacturers and wholesale distributors of drug and biological products in a state, including, in certain states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical and biotechnology companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities, and/or register their sales representatives, as well as to prohibit pharmacies and other healthcare entities from providing certain physician prescribing data to pharmaceutical and biotechnology companies for use in sales and marketing, and to prohibit certain other sales and marketing practices. All of our activities are potentially subject to federal and state consumer protection and unfair competition laws.
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, imprisonment, exclusion of drugs from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations, as well as additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, any of which could adversely affect our ability to operate our business and our financial results. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to significant criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs. Ensuring business arrangements comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time- and resource-consuming and can divert a company’s attention from the business.
| 14 |
Current and Future Legislation
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval. We expect that current laws, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and additional downward pressure on the price that we, or any collaborators, may receive for any approved products.
The ACA, for example, contains provisions that subject biological products to potential competition by lower-cost biosimilars and may reduce the profitability of drug products through increased rebates for drugs reimbursed by Medicaid programs, extend Medicaid rebates to Medicaid managed care plans, provide for mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies’ share of sales to federal healthcare programs. With the current Congress, there will likely be additional administrative or legislative changes, including modification, repeal or replacement of all, or certain provisions of the ACA, which may impact reimbursement for drugs and biologics. On January 20, 2017, former President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. On October 13, 2017, former President Trump signed an Executive Order terminating the cost-sharing subsidies that reimburse insurers under the ACA. Several state Attorneys General filed suit to stop the administration from terminating the subsidies, but their lawsuit was dismissed by a federal judge in California on July 18, 2018. In addition, CMS has recently finalized regulations that would give states greater flexibility in setting benchmarks for insurers in the individual and small group marketplaces, which may have the effect of relaxing the essential health benefits required under the ACA for plans sold through such marketplaces. Further, each chamber of Congress has put forth multiple bills, and may do so again in the future, designed to repeal or repeal and replace portions of the ACA.
While Congress has not passed repeal legislation, the Tax Reform Act includes a provision that repealed, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” Further, the Bipartisan Budget Act of 2018, or the BBA, among other things, amended the ACA, effective January 1, 2019, to increase from 50 percent to 70 percent the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D and to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole.” Congress may consider other legislation to repeal and replace elements of the ACA. On December 14, 2018, a U.S. District Court judge in the Northern District of Texas ruled that the individual mandate portion of the ACA is an essential and inseverable feature of the ACA, and therefore because the mandate was repealed as part of the Tax Cuts and Jobs Act, the remaining provisions of the ACA are invalid as well. The Trump administration and CMS have both stated that the ruling will have no immediate effect, and on December 30, 2018 the same judge issued an order staying the judgment pending appeal. A Fifth Circuit U.S. Court of Appeals hearing to determine whether certain states and the House of Representatives have standing to appeal the lower court decision was held on July 9, 2019, but it is unclear when a Court will render its decision on this hearing, and what effect it will have on the status of the ACA. Litigation and legislation over the ACA are likely to continue, with unpredictable and uncertain results.
Additionally, other federal health reform measures have been proposed and adopted in the United States since the ACA was enacted:
| ● | The Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013 and, due to subsequent legislative amendments to the statute, including the BBA, will remain in effect through 2027, unless additional Congressional action is taken. | |
| ● | The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. | |
| ● | The Middle Class Tax Relief and Job Creation Act of 2012 required that CMS reduce the Medicare clinical laboratory fee schedule by 2% in 2013, which served as a base for 2014 and subsequent years. In addition, effective January 1, 2014, CMS also began bundling the Medicare payments for certain laboratory tests ordered while a patient received services in a hospital outpatient setting. |
| 15 |
Further, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which have resulted in several recent Congressional inquiries and proposed and enacted bills designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. In addition, the U.S. government, state legislatures, and foreign governments have shown significant interest in implementing cost containment programs, including price-controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs to limit the growth of government paid healthcare costs. For example, the U.S. government has passed legislation requiring pharmaceutical manufacturers to provide rebates and discounts to certain entities and governmental payors to participate in federal healthcare programs. Further, Congress and the current administration have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs, and the current administration recently released a “Blueprint”, or plan, to reduce the cost of drugs. The Blueprint contains certain measures that HHS is already working to implement. For example, in May 2019, CMS issued a final rule to allow Medicare Advantage Plans the option of using step therapy for Part B drugs beginning January 1, 2020. This final rule codified CMS’s policy change that was effective January 1, 2019. Congress has indicated that it will continue to seek new legislative and/or administrative measures to control drug costs. Individual states in the United States have also been increasingly passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Non-U.S. Regulation
Before our products can be marketed outside the United States, they are subject to regulatory approval of the respective authorities in the country in which the product should be marketed. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary widely from country to country. No action can be taken to market any product in a country until an appropriate application has been approved by the regulatory authorities in that country. The time spent in gaining approval varies from that required for FDA approval, and in certain countries, the sales price of a product must also be approved. The pricing review period often begins after market approval is granted. Even if a product is approved by a regulatory authority, satisfactory prices might not be approved for such product.
Collaborations, Partnerships and Agreements
Collaborations, partnerships and agreements are a key component of Advaxis’New Ayala’s corporate strategy. As a lateclinical stage biotechnology company without sales revenue, partnerships are an essential part of the ongoing strategy. Additionally, the evolution of the field of immunotherapy has resulted in combination treatments becoming ubiquitous; ongoing clinical studies and agreements with many of the leading, large oncology pharmaceutical companies and teaching institutions ensureshelps validate thatLmTechnology will bemay play a key component ofrole in the cancer treatment protocols of the future.
Our collaborators and partners include Amgen, Aratana, AstraZeneca, BMS, Biocon, GBP, Knight, Merck, Sellas Life Science Group, Stendhal, and others. For more information, see Footnote 9. Collaboration and Licensing Agreements with this Form 10-K and is incorporated herein by reference.
We entered into an exclusive worldwide license agreement with Penn, on July 1, 2002 with respect to the innovative work of Yvonne Paterson, Ph.D., Associate Dean for Research at the School of Nursing at Penn, and former Professor of Microbiology at Penn, in the area of innate immunity, or the immune response attributed to immune cells, including dendritic cells, macrophages and natural killer cells, that respond to pathogens non-specifically (subject to certain U.S. government rights). This agreement was amended and restated as of February 13, 2007, and, thereafter, has been amended from time to time.
This license, unless sooner terminated in accordance with its terms, terminates upon the latter of (a) the expiration of the last to expire of the Penn patent rights; or (b) twenty years after the effective date of the license. Penn may terminate the license agreement early upon the occurrence of certain defaults by us, including, but not limited to, a material breach by us of the Penn license agreement that is not cured within 60 days after notice of the breach is provided to us.
The license provides us with the exclusive commercial rights to the patent portfolio developed by Penn as of the effective date of the license, in connection with Dr. Paterson and requires us to pay various milestone, legal, filing and licensing payments to commercialize the technology. In exchange for the license, Penn received shares of our Common Stock. However, as of October 31, 2016, Penn does not own shares of our Common Stock. In addition, Penn is entitled to receive a non-refundable initial license fee, royalty payments and milestone payments based on net sales and percentages of sublicense fees and certain commercial milestones. Under the amended licensing agreement, Penn is entitled to receive 2.5% of net sales in the territory. Should annual net sales exceed $250 million, the royalty rate will increase to 2.75%, but only with respect to those annual net sales in excess of $250 million. Additionally, Penn will receive tiered sales milestone payments upon the achievement of cumulative global sales ranging between $250 million and $2 billion, with the maximum aggregate amounts payable to Penn in the event that maximum sales milestones are achieved is $40 million. Notwithstanding these royalty rates, upon first in-human commercial sale (U.S. & E.U.), we have agreed to pay Penn a total of $775,000 over a four-year period as an advance minimum royalty, which shall serve as an advance royalty in conjunction with the above terms. In addition, under the license, we are obligated to pay an annual maintenance fee of $100,000 commencing on December 31, 2010, and each December 31st thereafter for the remainder of the term of the agreement until the first commercial sale of a Penn licensed product. We are responsible for filing new patents and maintaining and defending the existing patents licensed to us and we are obligated to reimburse Penn for all attorney’s fees, expenses, official fees and other charges incurred in the preparation, prosecution and maintenance of the patents licensed from Penn.
Upon first regulatory approval in humans (US or EU), Penn will be entitled to a milestone payment of $600,000. Furthermore, upon the achievement of the first sale of a product in certain fields, Penn will be entitled to certain milestone payments, as follows: $2.5 million will be due upon the first in-human commercial sale (US or EU) of the first product in the cancer field and $1.0 million will be due upon the date of first in-human commercial sale (US or EU) of a product in each of the secondary strategic fields sold.
| 16 |
Manufacturing
Current Good Manufacturing Practices (“cGMPs”)
cGMPs, are the standards identified to conform to requirements by governmental agencies that control authorization and licensure for manufacture and distribution of drugbiologic products for either clinical investigations or commercial sale. GMPs identify the requirements for procurement, manufacturing, testing, storage, distribution and the supporting quality systems to ensure that a drug product is safe for its intended application. cGMPs are enforced in the United States by the FDA, under the authorities of the Federal Food, Drug and Cosmetic Act and its implementing regulations and use the phrase “current good manufacturing practices” (“cGMP”) to describe these standards.
Each of Advaxis’our wholly owned product candidates is manufactured using a platform process, with uniform methods and testing procedures. This allows for an acceleratedexpedited pathway from construct discovery to clinical product delivery, while keepinghelping to keep cost of goods low. The Company intends to add new constructs to this standardized manufacturing process as their pipeline evolves.
AdvaxisNew Ayala has entered into agreements with multiple third-party organizations, (“CMOs”)or CMOs, to handle the manufacturing, testing, and distribution of product candidates. These organizations have extensive experience within the biologics space and with the production of clinical and commercial GMP supplies. In 2017, the Company expanded and standardized their external clinical manufacturing network in order to access additional manufacture capacity as needed, and reduce their external manufacturing cost structure.
In parallel, the Company has also continued to invest in internal process/analytical development, quality systems, manufacturing, and quality control infrastructure with the goal of accelerating and advancing our pipeline. Advaxis has constructed a state-of-the-art manufacturing facility and laboratory to develop and manufacture clinical-grade products, supporting the clinical trials and future commercialization of the Company’s therapeutics. Increased manufacturing capability and capacity allows Advaxis to manufacture its own material and reduce reliance on CMOs, and improve supply flexibility, scalability, lead times, and costs of goods. The Company’s long-term manufacturing strategy is to leverage both their partners’ capabilities and their internal capabilities in order to build a supply chain that is reliable, flexible, and cost competitive.Competition
In support of future conditional regulatory filing and future launch of axalimogene filolisbac in Europe, the Company has completed a full mapping of supply chain requirements and has established and validated a commercial process and testing in Europe. The Company plans to continue to refine end-to-end product delivery during the ongoing regulatory review process.
Competition
The biotechnology and biopharmaceutical industries are characterized by rapid technological developments and a high degree of competition. As a result, our actual or proposed immunotherapies could become obsolete before we recoup any portion of our related research and development expenses. While we believe that our product candidates, technology, knowledge and experience provide us with competitive advantages, we face competition from established and emerging pharmaceutical and biotechnology companies, among others. The biotechnology and biopharmaceutical industries are highly competitive, and this competition comes from both biotechnology firms and from major pharmaceutical companies, including: Aduro Biotech, Agenus Inc.,BioNtech, Moderna, Gritstone, BMS, Celldex Therapeutics, Inovio Pharmaceutical Inc., ISA Pharmaceuticals, AstraZeneca, Merck, Neon Therapeutics, Oncolytics Biotech Inc., Oncothyreon Inc., et al., each of which is pursuing cancer vaccines and/or immunotherapies.
Many of these companies have substantially greater financial, marketing, and human resources than we do (including, in some cases, substantially greater experience in clinical testing, manufacturing, and marketing of pharmaceutical products). We also experience competition in the development of our immunotherapies from universities and other research institutions and compete with others in acquiring technology from such universities and institutions. In addition, certain of our immunotherapies may be subject to competition from investigational new drugs and/or products developed using other technologies, some of which have completed numerous clinical trials.
Our competition will be determined in part by the potential indications for which drugs are developed and ultimately approved by regulatory authorities. Additionally, the timing of market introduction of some of our potential immunotherapies or of competitors’ products may be an important competitive factor. Accordingly, the speed with which we can develop immunotherapies, complete preclinical testing, clinical trials and approval processes and supply commercial quantities to market are expected to be important competitive factors. We expect that competition among products approved for sale will be based on various factors, including product efficacy, safety, administration, reliability, acceptance, availability, price and patent position.
The pharmaceutical industry is characterized by rapid evolution of technologies and intense competition. While we believe that our product candidates, technology, knowledge, experience and scientific resources provide us with competitive advantages, we face competition from major pharmaceutical and biotechnology companies, academic institutions, governmental agencies and public and private research institutions, among others. Any product candidates that we successfully develop and commercialize will compete with approved treatment options, if any, including off-label therapies, and new therapies that may become available in the future. Key considerations that would impact our ability to effectively compete with other therapies include the efficacy, safety, method of administration, cost, level of promotional activity and intellectual property protection of our products. Many of the companies against which we may compete have significantly greater financial resources and expertise than we do in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products.
| 17 |
With respect to MM, we are aware that other companies are, or may be, developing product candidates with GSI as anti-BCMA agents, including, but not limited to, Springworks Therapeutics, Inc. in collaboration with GlaxoSmithKline plc, Janssen, Allogene, Pfizer, Precision Biosciences and Celgene Corporation, recently acquired by BMS.
With respect to BST-236, our competitors include companies such as, but not limited to, AbbVie, Aprea, Astellas, BMS, Gilead, Jazz Pharmaceuticals, Novartis and Pfizer.
Smaller or early-stage companies, including oncology-focused therapeutics companies, may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These companies may also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites, enrolling patients in clinical trials and acquiring technologies complementary to, or necessary for, our programs.
The availability of reimbursement from government and private payors will also significantly impact the pricing and competitiveness of our products. Our competitors may obtain FDA or other regulatory approvals for their products more rapidly than we may obtain approvals for our product candidates, if any, which could result in our competitors establishing a strong market position before we are able to commercialize our product candidates.
Experience and Expertise
Our management team has extensive experience in oncology development, including contract research, development, manufacturing and commercialization across a board range of science, technologies, and process operations. We have built internal capabilities supporting research, clinical, medical, manufacturing and compliance operations and have extended our expertise with collaborations.
Employees
As of OctoberDecember 31, 2017,2023, we had 10821 employees, all20 of which were full time employees, 87 of whom were engaged in research and development activities and 21 of whom were engaged in finance, business development, facilities, human resources and administrative support.employees. Of our full-time employees, 28seven hold a M.D. or Ph.D. degrees.degree. None of our employees are represented by a labor union, and we consider our relationship with our employees to be good.
As part of a cost reduction plan, during the year ended December 31, 2023, we had a reduction in workforce in which the employment of approximately 50% of our employees was terminated. During the first quarter of 2024, we gave notice of termination to 18 additional employees and two officers (including the Chief Financial Officer, whose employment will terminate on June 25, 2024). After the effectiveness of such terminations, the Chief Executive Officer will be the only employee of the Company.
Properties
Our principal office is located at Oppenheimer 4, Rehovot 7670104, Israel. Our principal U.S. office is located at 9 Deer Park Drive, Suite K-1, Monmouth Junction, New Jersey 08852. We will continue to rent necessary offices and laboratories to support our growing business. We believe that our facilities are sufficient to meet our current needs and that suitable additional space will be available as and when needed.
Item 1A: Risk Factors.Legal Proceedings
You should carefully consider the risks described below as well as other information providedThe Company is from time to youtime involved in this annual report, including informationlegal proceedings in the sectionordinary course of this document entitled “Forward-Looking Statements.”our business. The risks and uncertainties described below areCompany does not the only ones facing us. Additional risks and uncertainties not presently known to us orbelieve that we currently believe are immaterial may also impair our business operations. If any of these claims or proceedings against us is likely to have, individually or in the following risks actually occur, our business,aggregate, a material adverse effect on the financial condition or results of operations. For more information regarding legal proceedings involving the Company, please see Note 7 – Commitments and Contingencies to our consolidated financial statements.
Item 1A. Risk Factors.
Omitted.
Item 1B. Unresolved Staff Comments.
None.
Item 1C. Cybersecurity.
As a regular part of our ordinary business operations, we collect and store data, including information necessary for our operations, information from our customers, employees, and our business partners. We recognize these networks and systems may be subject to increasing and continually evolving cybersecurity risks. Our Audit Committee is responsible for overseeing risk management and cybersecurity is an integral part of the Company’s overall risk management program. Our risk management process is designed to identify, prioritize, and monitor risks that could beaffect our ability to execute our corporate strategy and fulfill our business objectives and to appropriately mitigate such risks.
Our management team is involved in assessing and managing the Company’s material risks from cybersecurity threats, including by hiring appropriate personnel, considering cybersecurity risk in our enterprise risk management strategy, helping prepare for cybersecurity incidents, and participating in the cybersecurity incident response and remediation process for incidents escalated to it including determining materiality. Our management that is involved in these processes includes our Chief Financial Officer, our VP of regulatory affairs as well as an external IT company, who collectively share risk management, compliance and technical experience. Management also escalates, as appropriate, reports relating to cybersecurity incidents or threats to our Board or to its Audit Committee.
| 18 |
While we have not yet experienced any material impacts from a cyber-attack, any one or more future cyber-attacks could materially adversely affected,impact the valueCompany, including a loss of trust among our customers, departures of key employees, general diminishment of our Common Stock could decline,reputation and youfinancial losses from remediation actions, loss of business or potential litigation or regulatory liability. Further, evolving market dynamics are increasingly driving heightened cybersecurity protections and mandating cybersecurity standards for our products, and we may lose all or partincur additional costs to address these increased risks and to comply with such demands.
Item 2. Properties.
Our principal office is located at Oppenheimer 4, Rehovot 7670104, Israel, where we lease office and laboratory space under a lease agreement that terminates in 2025. Our principal U.S. office is located at 9 Deer Park Drive, Suite K-1, Monmouth Junction, New Jersey 08852.
We believe that our facilities are sufficient to meet our current needs and that suitable additional space will be available as and when needed.
Item 3. Legal Proceedings.
See the matters set forth in note 7, “Commitment and Contingent Liabilities – Purported Stockholder Claims” in the Notes to Consolidated Financial Statements included herein.
Item 4. Mine Safety Disclosures.
Not applicable.
INFORMATION ABOUT OUR EXECUTIVE OFFICERS AND DIRECTORS
The following table sets forth information regarding our executive officers and directors as of your investment.the filling date of this Annual Report on Form 10-K.
| Name | Age | Position | ||
| Executive Officers | ||||
| Kenneth A. Berlin | 59 | President and Chief Executive Officer, Director | ||
| Roy Golan, CPA, LLM | 50 | Chief Financial Officer |
Executive Officers
Kenneth Berlin has served as our President and Chief Executive Officer and a member of our Board of Directors since April 2018. Mr. Berlin served as our Interim Chief Financial Officer from September 2020 to May 2022. Prior to joining the Company, Mr. Berlin served as President and Chief Executive Officer of Rosetta Genomics from November 2009 until April 2018. Prior to Rosetta Genomics, Mr. Berlin was Worldwide General Manager at cellular and molecular cancer diagnostics developer Veridex, LLC, a Johnson & Johnson company. At Veridex he grew the organization to over 100 employees, launched three cancer diagnostic products, led the acquisition of its cellular diagnostics partner, and delivered significant growth in sales as Veridex transitioned from an R&D entity to a commercial provider of oncology diagnostic products and services. Mr. Berlin joined Johnson & Johnson in 1994 and served as corporate counsel for six years. From 2001 until 2004 he served as Vice President, Licensing and New Business Development in the pharmaceuticals group, and from 2004 until 2007 served as Worldwide Vice President, Franchise Development, Ortho-Clinical Diagnostics. Mr. Berlin holds an A.B. degree from Princeton University and a J.D. from the University of California Los Angeles School of Law. Mr. Berlin’s experience in life science companies, as well as his business experience in general, qualify him to service as our director.
| 19 |
Roy Golan, CPA, LLM, has served as our Chief Financial Officer since October 2023. Mr. Golan is a registered CPA with a broad experience in aspects of Nasdaq, IPOs and M&As. Prior to joining Ayala, Mr. Golan served in several financial management positions in the biotech industry, including as the EVP and CFO of Biosight from 2019 until the merger with Ayala in October 2023. From 2014 to 2018 Mr. Golan served as CFO of Neuroderm, where he had a pivotal role in their successful Nasdaq IPO, two Follow-On Offerings, and Neuroderm’s acquisition by Mitsubishi Tanabe Pharmaceutical Corporation for a total of $1.1 billion. Mr. Golan started his career at PriceWaterhouseCoopers (PWC). Mr. Golan is a registered CPA, holds a B.A. in Accounting and Business from the Israeli College of Management School of Business and an LL.M. in Law from Bar Ilan University.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information and Holders
Our common stock is quoted on the OTCQX under the symbol “ADXS”.
As of December 31, 2023 there were approximately 117 holders of record of our common stock. The actual number of holders of our common stock is greater than this number of record holders, and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers or held by other nominees.
We have not declared or paid any cash dividends on our common stock, and we do not anticipate declaring or paying cash dividends for the foreseeable future.
Recent Sales of Unregistered Securities
All information required by Item Furnish the information required by Item 201 of Regulation S-K and Item 701 of Regulation S-K as to all equity securities that we sold during the period covered by this Annual Report on Form 10-K that were not registered under the Securities Act has been included in our previously filed Quarterly Reports on Form 10-Q or Current Reports on Form 8-K and is thus not furnished here.
Item 6. [Reserved].
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes included elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
A discussion of the fiscal year ended December 31, 2022 compared to the fiscal year ended December 31, 2021, which constitute our financial statements for such periods, has been reported previously in Old Ayala’s Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 31, 2023, under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| 20 |
Risks Related toOverview
See “Introductory Note” beginning on page 3 of this Annual Report on Form 10-K for a description of certain transactions that have occurred since the beginning of our Business and Industryfiscal year ended December 31, 2023.
Because the Asset Sale was consummated following the end of our fiscal year ended December 31, 2023, the financial results discussed in this Management’s Discussion and Analysis of Financial Condition and Results of Operations describe our business as it existed during that period. For a description of this business, please refer to:
| ● | the description of the business of Old Ayala set forth in pages 233-273 of the Definitive Proxy Statement of Old Ayala for the Special Meeting of Stockholders of Old Ayala held on January 13, 2023, which was filed with the Commission on December 12, 2022 and which pages are set forth on Exhibit 99.1 to this Annual Report on Form 10-K, and are, in accordance with Rule 12b-23 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), incorporated by reference herein. |
Following the completion of the Asset Sale on March 25, 2024, the Company is a clinical-stage oncology company whose primary clinical assets currently include aspacytarabine (BST-236), a novel proprietary anti-metabolite for first line treatment in unfit acute myeloid leukemia (AML). BST-236 is a novel prodrug of cytarabine, enabling delivery of high cytarabine doses with reduced systemic toxicity, creating a potential new backbone for AML combination regimens. We believe that our novel product candidates, if approved, have the potential to transform treatment outcomes for patients suffering from solid and hematological cancers. We also continue to conduct certain operations relating to former Advaxis’ operations as clinical-stage biotechnology company focused on the development and commercialization of proprietary Listeria monocytogenes (“Lm”)-based antigen delivery products. These efforts are a clinical stage company.primarily focused on the development of ADXS-504, an Lm-based therapy for early-stage prostate cancer.
We arewere originally incorporated in the State of Colorado on June 5, 1987 under the name Great Expectations, Inc. We were a clinical stage biotechnologypublicly-traded “shell” company withwithout any business until November 12, 2004 when we acquired New Ayala, a historyDelaware corporation, through a Share Exchange and Reorganization Agreement, dated as of lossesAugust 25, 2004, which we refer to as the Share Exchange, by and can provide no assurance as to future operating results.among New Ayala, the stockholders of New Ayala and us. As a result of losses that will continue throughoutthe Share Exchange, New Ayala became our clinical stage,wholly-owned subsidiary and our sole operating company. On December 23, 2004, we may exhaustamended and restated our financial resourcesarticles of incorporation and be unablechanged our name to completeNew Ayala. On June 6, 2006, our stockholders approved the developmentreincorporation of our products. We anticipate thatcompany from Colorado to Delaware by merging the Colorado entity into our ongoing operational costs will increase significantly aswholly-owned Delaware subsidiary. Our date of inception, for financial statement purposes, is March 1, 2002 and the Company was listed on The Nasdaq Capital Market (“Nasdaq”) in 2014. In December 2021, the Company was delisted from Nasdaq and accepted onto the OTCQX. Shares of New Ayala’s common stock are currently quoted on the OTCQX under the symbol “ADXS.” Our operations to date have been limited to organizing and staffing our company, business planning, raising capital and conducting research and development activities for our product candidates. To date, we continue conductinghave funded our clinical development program. Our deficit will continue to grow during our drug development period.operations primarily through the sales of common stock convertible notes, warrants and convertible preferred stock.
| 21 |
We have sustainedincurred significant net operating losses from operations in each fiscalevery year since our inception and we expect losses to continue to incur significant expenses and increasing operating losses for the indefinite future dueforeseeable future. Our net losses may fluctuate significantly from quarter to quarter and year to year and could be substantial. Our net losses were $48.1 million and $38.0 million for the substantial investment in researchyears ended December 31, 2023 and development.2022, respectively. As of OctoberDecember 31, 2017,2023, we had an accumulated deficit of $301,142,227 and shareholders’ equity of $54,260,167.$197.2 million. We anticipate that our expenses will increase significantly as we:
| ● | Continue to conduct certain operations related to advancing the development of BST-236 | |
| ● | Satisfy certain debts and obligations remaining from our operations prior to the consummation of the Asset Sale |
We do not expect to spendgenerate revenue from product sales unless and until we successfully complete clinical development and obtain regulatory approval for a product candidate. Additionally, we currently use contract research organizations, or CROs, to carry out our clinical development activities. Furthermore, we incur additional costs associated with operating as a public company. As a result, we will need substantial additional sums on the continued administrationfunding to support our continuing operations, pursue our growth strategy and research and development of proprietary products and technologies with no certainty that our immunotherapies will become commercially viable or profitablecontinue as a resultgoing concern. Until such time as we can generate significant revenue from product sales, if ever, we expect to fund our operations through public or equity offerings or debt financings, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements or other sources. We may, however, be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. Our failure to raise capital or enter into such other arrangements as and when needed would have a negative impact on our financial condition and our ability to develop our current or any future product candidates.
Because of these expenditures.the numerous risks and uncertainties associated with therapeutics product development, we are unable to predict accurately the timing or amount of increased expenses or when or if we will be able to achieve or maintain profitability. Even if we are able to generate revenue from product sales, we may not become profitable. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce or terminate our operations.
The Company has incurred recurring losses since inception as a research and development organization. For the year ended December 31, 2023, the Company used approximately $29.5 million of cash in operations and incurred a net loss of $48.1 million. As of December 31, 2023, the Company had $4.9 million in cash and cash equivalents, $25.0 million in current liabilities and an accumulated deficit of $197.2 million.
Upon closing of the Asset Sale, on March 25, 2024, the Company received $13.0 million in cash, and Immunome paid $3.0 million of the Company’s liabilities directly to vendors of the Company. In addition, under the Asset Sale Agreement the Company received 2,175,489 shares of Immunome’s common stock (the “Immunome Shares”). The Asset Sale Agreement prohibits the Company from selling more than 50% of the Immunome Shares in the first six months following the closing of the Asset Sale. In addition, on March 1, 2024, the Company issued additional convertible notes and warrants in exchange for $2.0 million in funding from the Convertible Notes investors. As of the date of this filing, the cash proceeds received from the Asset Sale and the convertible notes sold on March 1, 2024 were not sufficient to pay the Company’s existing liabilities. Therefore, the Company has limited available cash resources and requires additional financing, through the sale of a portion of the Immunome Shares or otherwise, in order to continue to fund its current operations beyond May 2024.
Raising additional funds or the satisfactory sale of a portion of the Immunome Shares prior to the end of May 2024 is essential to provide sufficient cash flow to meet future liabilities and other obligations, such as tax payments arising from the Asset Sale. Furthermore, even if the Company is successful in selling a portion of the Immunome Shares or raising additional funds through other means, the Company cannot give any assurance that it will, in the future, be able to achieve a level of profitability from the sale of its products to sustain its operations.
If the Company is unable to obtain funding, or able to receive sufficient funds from the sale of a portion of the Immunome Shares, the Company would be forced to delay, reduce, or eliminate its research and development programs, or the Company may be unable to continue operations. As such, those factors raise substantial doubt about the Company’s ability to continue as a significantgoing concern.
As part of a cost reduction plan, during the year ended December 31, 2023, the Company had a reduction in workforce in which the employment of approximately 50% of the Company’s employees was terminated. During the first quarter of 2024, the Company gave notice of termination to 18 additional employees and two officers (including the Chief Financial Officer, whose employment will terminate on June 25, 2024). After the effectiveness of such terminations, the Chief Executive Officer will be the only employee of the Company. The Company expects to be able to meet its financial obligations to its employees.
The Company can give no assurances that it will be successful in raising funds through the sale of a portion of the Immunome Shares or any other alternative. Any inability to so obtain additional financing or funding will likely cause the Company to cease business operations. The consolidated financial statements have been prepared assuming that the Company will continue as a going concern. Therefore, the consolidated financial statements for the year ended December 31, 2023, do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern.
Due to the uncertainty in securing additional funding, and the insufficient amount of capital,cash and cash equivalent resources at December 31, 2023, we have concluded that substantial doubt exists with respect to our ability to continue as a going concern through May 2024. See “—Liquidity and Capital Resources.” Substantial doubt about our ability to continue as a going concern may materially and adversely affect the price per share of our common stock, and it may be more difficult for us to obtain financing. If potential collaborators decline to do business with us or potential investors decline to participate in any future financings due to such concerns, our ability to increase our cash position may be limited. We will need to significantly curtail operations or cease operations in the near future. If any of our product candidates fail in clinical trials or does not gain regulatory approval,generate significant revenues to achieve profitability, and we may never become profitable. Even if we achieve profitability indo so. Because of the numerous risks and uncertainties associated with the development of our current and any future product candidates, and because the extent to which we may not be able to sustain profitability in subsequent periods.
Drug discovery andenter into collaborations with third parties for the development is a complex, time-consuming and expensive process that is fraught with risk and a high rate of failure.
Product candidates are subject to extensive pre-clinical testing and clinical trials to demonstrate their safety and efficacy in humans. Conducting pre-clinical testing and clinical trials is a lengthy, time-consuming and expensive process that takes many years. We cannot be sure that pre-clinical testing or clinical trials of any of our product candidates will demonstrate the safety, efficacy and benefit-to-risk profile necessary to obtain marketing approvals. In addition, product candidates that experience success in pre-clinical testing and early-stage clinical trials will not necessarily experience the same success in larger or late-stage clinical trials, which are required for marketing approval.
Even ifis unknown, we are successful in advancing a product candidate intounable to estimate the clinical development stage, before obtaining regulatory and marketing approvals, we must demonstrate through extensive human clinical trials that the product candidate is safe and effective for its intended use. Human clinical trials must be carried out under protocols that are acceptable to regulatory authorities and to the independent committees responsible for the ethical review of clinical studies. There may be delays in preparing protocols or receiving approval for them that may delay the start or completion of the clinical trials. In addition, clinical practices vary globally, and there is a lack of harmonization among the guidance provided by various regulatory bodies of different regions and countries with respect to the data that is required to receive marketing approval, which makes designing global trials increasingly complex. There are a number of additional factors that may cause our clinical trials to be delayed, prematurely terminated or deemed inadequate to support regulatory approval, such as:
Any deficiency in the design, implementation or oversight of our development programs could cause us to incur significant additional costs, conduct additional trials, experience significant delays, prevent us from obtaining marketing approval for any product candidate or abandon development of certain product candidates, any of which could harm our business and cause our stock price to decline.
Our operating history does not afford investors a sufficient history on which to base an investment decision.
We commenced ourLm -LLO based immunotherapy development business in February 2002 and today exist as a clinical stage company. We have no approved products and therefore have not derived any significant revenue from the sales of products and have not yet demonstrated ability to obtain regulatory approval, formulate and manufacture commercial scale products, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, there is limited information for investors to use as basis for assessing our future viability. Investors must consider the risks and difficulties we have encountered in the rapidly evolving vaccine and immunotherapy industry. Such risks include the following:
We cannot be certain that our strategy will be successful or that we will successfully address these risks. In the event that we do not successfully address these risks, our business, prospects, financial condition and results of operations could be materially and adversely affected. We may be required to reduce our staff, discontinue certain research or development programs of our future products and cease to operate.
We may face legal claims; litigation is expensive and we may not be able to afford the costs.
We may face legal claims involving stockholders, consumers, competitors, regulators and other parties. As described in “Legal Proceedings” in Part I Item 3 of this Form 10-K, we are engaged in a number of legal proceedings. Litigation and other legal proceedings are inherently uncertain, and adverse rulings could occur, including monetary damages, or an injunction stopping us from engaging in business practices, or requiring other remedies, such as compulsory licensing of patents.
The costs of litigation or any proceeding relating to our intellectual property or contractual rights could be substantial, even if resolved in our favor. Some of our competitors or financial funding sources have far greater resources than we do and may be better able to afford the costs of complex litigation. Also, a lawsuit, even if frivolous, will require considerable time commitments on the part of management, our attorneys and consultants. Defending these types of proceedings or legal actions involve considerable expense and could negatively affect our financial results.
We can provide no assurance of the successful and timely development of new products.
Our immunotherapies are at various stages of research and development. Further development and extensive testing will be required to determine their technical feasibility and commercial viability. We will need to complete significant additional clinical trials demonstrating that our product candidates are safe and effective to the satisfaction of the FDA and other non-U.S. regulatory authorities. The drug approval process is time-consuming, involves substantial expenditures of resources, and depends upon a number of factors, including the severity of the illness in question, the availability of alternative treatments, and the risks and benefits demonstrated in the clinical trials. Our success will depend on our ability to achieve scientific and technological advances and to translate such advances into licensable, FDA-approvable, commercially competitive products on a timely basis. Failure can occur at any stage of the process. If such programs are not successful, we may invest substantial amounts of timeincreased capital outlays and money without developing revenue-producing products. As we enter a more extensive clinical programoperating expenses required for our product candidates, the data generated in these studies may not be as compelling as the earlier results.
The proposed development schedules for our immunotherapies may be affected by a variety of factors, including technological difficulties, clinical trial failures, regulatory hurdles, clinical holds, competitive products, intellectual property challenges and/or changes in governmental regulation, many of which will not be within our control. Any delay in the development, introduction or marketing of our products could result either in such products being marketed at a time when their cost and performance characteristics would not be competitive in the marketplace or in the shortening of their commercial lives. In light of the long-term nature of our projects, the unproven technology involved and the other factors described elsewhere in this section, there can be no assurance that we will be able to successfully complete the development or marketing of any new products which could materially harm our business, results of operations and prospects.
Our research and development expenses are subject to uncertainty.
Factors affecting our research and development expenses include, but are not limited to:
There can be no guarantee that our research and development expenses will be consistent from period to period. We may be required to accelerate or delay incurring certain expenses depending on the results of our studies and the availability of adequate funding.
We are subject to numerous risks inherent in conducting clinical trials.
We outsource the management of our clinical trials to third parties. Agreements with clinical research organizations, clinical investigators and medical institutions for clinical testing and data management services, place substantial responsibilities on these parties that, if unmet, could result in delays in, or termination of, our clinical trials. For example, if any of our clinical trial sites fail to comply with FDA-approved good clinical practices, we may be unable to use the data gathered at those sites. If these clinical investigators, medical institutions or other third parties do not carry out their contractual duties or regulatory obligations or fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to their failure to adhere to our clinical protocols or for other reasons, our clinical trials may be extended, delayed or terminated, and we may be unable to obtain regulatory approval for, or successfully commercialize, our agents. We are not certain that we will successfully recruit enough patients to complete our clinical trials nor that we will reach our primary endpoints. Delays in recruitment, lack of clinical benefit or unacceptable side effects would delay or prevent the initiation of future development of our agents.
We or our regulators may suspend or terminate our clinical trials for a number of reasons. We may voluntarily suspend or terminate our clinical trials if at any time we believe they present an unacceptable risk to the patients enrolled in our clinical trials or do not demonstrate clinical benefit. In addition, regulatory agencies may order the temporary or permanent discontinuation of our clinical trials, or place our products on temporary or permanent hold, at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to the patients enrolled in our clinical trials.
Our clinical trial operations are subject to regulatory inspections at any time. If regulatory inspectors conclude that we or our clinical trial sites are not in compliance with applicable regulatory requirements for conducting clinical trials, we may receive reports of observations or warning letters detailing deficiencies, and we will be required to implement corrective actions. If regulatory agencies deem our responses to be inadequate, or are dissatisfied with the corrective actions we or our clinical trial sites have implemented, our clinical trials may be temporarily or permanently discontinued, we may be fined, we or our investigators may be precluded from conducting any ongoing or any future clinical trials, the government may refuse to approve our marketing applications or allow us to manufacture or market our products, and we may be criminally prosecuted.
The lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval for our product candidates, which would materially harm our business, results of operations and prospects.
The successful development of immunotherapies is highly uncertain.
Successful development of biopharmaceuticals is highly uncertain and is dependent on numerous factors, many of which are beyond our control. Immunotherapies that appear promising in the early phases of development may fail to reach the market for several reasons including:
Success in preclinical and early clinical studies does not ensure that large-scale clinical studies will be successful. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. The length of time necessary to complete clinical studies and to submit an application for marketing approval for a final decision by a regulatory authority varies significantly from one immunotherapy to the next, and may be difficult to predict.
Even if we are successful in getting market approval, commercial success of any of our product candidates will also depend in large part on the availability of coverage and adequate reimbursement from third-party payers, including government payers such as the Medicare and Medicaid programs and managed care organizations, which may be affected by existing and future health care reform measures designed to reduce the cost of health care. Third-party payers could require us to conduct additional studies, including post-marketing studies related to the cost effectiveness of a product, to qualify for reimbursement, which could be costly and divert our resources. If government and other health care payers were not to provide adequate coverage and reimbursement levels for one any of our products once approved, market acceptance and commercial success would be reduced.
In addition, if one of our products is approved for marketing, we will be subject to significant regulatory obligations regarding product promotion, the submission of safety and other post-marketing information and reports and registration, and will need to continue to comply (or ensure that our third party providers) comply with cGMPs, and Good Clinical Practices (“GCP”), for any clinical trials that we conduct post-approval. In addition, there is always the risk that we or a regulatory authority might identify previously unknown problems with a product post-approval, such as adverse events of unanticipated severity or frequency. Compliance with these requirements is costly, and any failure to comply or other issues with our product candidates’ post-market approval could have a material adverse effect on our business, financial condition and results of operations.
We must comply with significant government regulations.
The research and development, manufacturing and marketing of human therapeutic and diagnostic products are subject to regulation, primarily by the FDA in the United States and by comparable authorities in other countries. These national agencies and other federal, state, local and foreign entities regulate, among other things, research and development activities (including testing in animals and in humans) and the testing, manufacturing, handling, labeling, storage, record keeping, approval, advertising and promotion of the products that we are developing. If we obtain approval for any of our product candidates, our operations will be directly or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statue and the federal False Claims Act, and privacy laws. Noncompliance with applicable laws and requirements can result in various adverse consequences, including delay in approving or refusal to approve product licenses or other applications, suspension or termination of clinical investigations, revocation of approvals previously granted, fines, criminal prosecution, civil and criminal penalties, recall or seizure of products, exclusion from having our products reimbursed by federal health care programs, the curtailment or restructuring of our operations, injunctions against shipping products and total or partial suspension of production and/or refusal to allow a company to enter into governmental supply contracts.
The process of obtaining requisite FDA approval has historically been costly and time-consuming. Current FDA requirements for a new human biological product to be marketed in the United States include: (1) the successful conclusion of preclinical laboratory and animal tests, if appropriate, to gain preliminary information on the product’s safety; (2) filing with the FDA of an IND to conduct human clinical trials for drugs or biologics; (3) the successful completion of adequate and well-controlled human clinical trials to establish the safety and efficacy of the investigational new drug for its recommended use; and (4) filing by a company and acceptance and approval by the FDA of a BLA for marketing approval of a biologic, to allow commercial distribution of a biologic product. The FDA also requires that any drug or formulation to be tested in humans be manufactured in accordance with its cGMP regulations. This has been extended to include any drug that will be tested for safety in animals in support of human testing. The cGMPs set certain minimum requirements for procedures, record-keeping and the physical characteristics of the laboratories used in the production of these drugs. A delay in one or more of the procedural steps outlined above could be harmful to us in terms of getting our immunotherapies through clinical testing and to market.
We can provide no assurance that our clinical product candidates will obtain regulatory approval or that the results of clinical studies will be favorable.
We are currently evaluating the safety and efficacy of several of our candidates in a number of ongoing pre-clinical and clinical trials. However, even though the initiation and conduct of the clinical trials is in accordance with the governing regulatory authorities in each country, as with any investigational new drug (under an IND in the United States, or the equivalent in countries outside of the United States), we are at risk of a clinical hold at any time based on the evaluation of the data and information submitted to the governing regulatory authorities.
There can be delays in obtaining FDA (U.S.) and/or other necessary regulatory approvals in the United States and in countries outside the United States for any investigational new drug and failure to receive such approvals would have an adverse effect on the investigational new drug’s potential commercial success and on our business, prospects, financial condition and results of operations. The time required to obtain approval by the FDA and non-U.S. regulatory authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. For example, the FDA or non-U.S. regulatory authorities may disagree with the design or implementation of our clinical trials or study endpoints; or we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks. In addition, the FDA or non-U.S. regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials or the data collected from clinical trials of our product candidates may not be sufficient to support the submission of a New Drug Application (“NDA”) or other submission or to obtain regulatory approval in the United States or elsewhere. The FDA or non-U.S. regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and the approval policies or regulations of the FDA or non-U.S. regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval.
In addition to the foregoing, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not submitted for nor obtained regulatory approval for any product candidate in-humans (US & EU) and it is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain regulatory approval.
We may not obtain or maintain the benefits associated with orphan drug designation, including market exclusivity.
Although we have been granted FDA orphan drug designation for axalimogene filolisbac for use in the treatment of anal cancer, HPV-associated head and neck cancer, Stage II-IV invasive cervical cancer and for ADXS-HER2 for the treatment of osteosarcoma in the United States, as well as EMA orphan drug designation for axalimogene filolisbac for the treatment of anal cancer and for ADXS-HER2 for the treatment of osteosarcoma in the EU, and intend to continue to expand our designation for these uses where applicable , we may not receive the benefits associated with orphan drug designation. This may result from a failure to maintain orphan drug status, or result from a competing product reaching the market that has an orphan designation for the same disease indication. Under U.S. rules for orphan drugs, if such a competing product reaches the market before ours does, the competing product could potentially obtain a scope of market exclusivity that limits or precludes our product from being sold in the United States for seven years. Even if we obtain exclusivity, the FDA could subsequently approve the same drug for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. A competitor also may receive approval of different products for the same indication for which our orphan product has exclusivity, or obtain approval for the same product but for a different indication for which the orphan product has exclusivity.
In addition, if and when we request orphan drug designation in Europe, the European exclusivity period is ten years but can be reduced to six years if the drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan drug exclusivity may be lost if the FDA or EMEA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
We rely upon patents to protect our technology. We may be unable to protect our intellectual property rights and we may be liable for infringing the intellectual property rights of others.
Our ability to compete effectively will depend on our ability to maintain the proprietary nature of our technologies, including theLm -LLO based immunotherapy platform technology, and the proprietary technology of others with whom we have entered into collaboration and licensing agreements.
Currently, we own or have rights to approximately 433 patents and applications, which are owned, licensed from, or co-owned with Penn, Merck, NIH, and/or Augusta University. We have obtained the rights to all future patent applications in this field originating in the laboratories of Dr. Yvonne Paterson and Dr. Fred Frankel, at the University of Pennsylvania.
We own or hold licenses to a number of issued patents and U.S. pending patent applications, as well as foreign patents and foreign counterparts. Our success depends in part on our ability to obtain patent protection both in the United States and in other countries for our product candidates, as well as the methods for treating patients in the product indications using these product candidates. Such patent protection is costly to obtain and maintain, and we cannot guarantee that sufficient funds will be available. Our ability to protect our product candidates from unauthorized or infringing use by third parties depends in substantial part on our ability to obtain and maintain valid and enforceable patents. Due to evolving legal standards relating to the patentability, validity and enforceability of patents covering pharmaceutical inventions and the scope of claims made under these patents, our ability to obtain, maintain and enforce patents is uncertain and involves complex legal and factual questions. Even if our product candidates, as well as methods for treating patients for prescribed indications using these product candidates are covered by valid and enforceable patents and have claims with sufficient scope, disclosure and support in the specification, the patents will provide protection only for a limited amount of time. Accordingly, rights under any issued patents may not provide us with sufficient protection for our product candidates or provide sufficient protection to afford us a commercial advantage against competitive products or processes.
In addition, we cannot guarantee that any patents will issue from any pending or future patent applications owned by or licensed to us. Even if patents have issued or will issue, we cannot guarantee that the claims of these patents are or will be valid or enforceable or will provide us with any significant protection against competitive products or otherwise be commercially valuable to us. The laws of some foreign jurisdictions do not protect intellectual property rights to the same extent as in the United States and many companies have encountered significant difficulties in protecting and defending such rights in foreign jurisdictions. Furthermore, different countries have different procedures for obtaining patents, and patents issued in different countries offer different degrees of protection against use of the patented invention by others. If we encounter such difficulties in protecting or are otherwise precluded from effectively protecting our intellectual property rights in foreign jurisdictions, our business prospects could be substantially harmed.
The patent positions of biotechnology and pharmaceutical companies, including our patent position, involve complex legal and factual questions, and, therefore, validity and enforceability cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated, or circumvented as a result of laws, rules and guidelines that are changed due to legislative, judicial or administrative actions, or review, which render our patents unenforceable or invalid. Our patents can be challenged by our competitors who can argue that our patents are invalid, unenforceable, lack utility, sufficient written description or enablement, or that the claims of the issued patents should be limited or narrowly construed. Patents also will not protect our product candidates if competitors devise ways of making or using these product candidates without infringing our patents.
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our technologies, methods of treatment, product candidates, and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets and we have the funds to enforce our rights, if necessary.
The expiration of our owned or licensed patents before completing the research and development of our product candidates.
License Agreements
Bristol-Myers Squibb
Our financial results for the periods ended December 31, 2023 and 2022, as discussed in this Management’s Discussion and Analysis of Financial Condition and Results of Operations, largely related to our AL101 and AL102 programs, which we sold to Immunome on March 25, 2024. We had licensed those assets pursuant to an exclusive worldwide license agreement with Bristol-Myers Squibb Company, or BMS, which we describe herein. We transferred this license agreement to Immunome as part of the Asset Sale.
We entered into the license agreement with BMS in November 2017. Under the terms of the license agreement, we licensed the exclusive worldwide development and commercialization rights for AL101 (previously known as BMS-906024) and AL102 (previously known as BMS-986115).
| 22 |
Under the license agreement, we were responsible for all future development and commercialization of AL101 and AL102. In consideration for the rights granted under the agreement, we paid BMS a payment of $6 million and issued to BMS 1,125,929 shares of Series A preferred stock valued at approximately $7.3 million. We were obligated under the license agreement to pay BMS up to approximately $142 million in the aggregate upon the achievement of certain clinical development or regulatory milestones and up to $50 million in the aggregate upon the achievement of certain commercial milestones by each product containing the licensed BMS compounds. In addition, we were obligated under the license agreement to pay BMS tiered royalties ranging from a high single-digit to a low teen percentage on worldwide net sales of all products containing the licensed BMS compounds. Both we and BMS had the right to terminate the BMS License Agreement in its entirety upon written notice to the licensee under certain circumstances described therein.
Components of Results of Operations
Revenue Recognition
We recognize revenue in accordance with ASC Topic 606, Revenue from Contracts with Customers, which applies to all contracts with customers. Under Topic 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration that the entity expects to receive in exchange for those goods or services.
In December 2018, we entered into an agreement with Novartis Agreement for which we paid for its research and development costs. This agreement was terminated in 2022.
We concluded that there is one distinct performance obligation under the Novartis Agreement: Research and development services, an obligation which is satisfied over time.
Revenue associated with the research and development services in the amount of $13 thousand was recognized in the year ended December 31, 2023, compared to $0.7 million in fiscal year 2022.
We concluded that progress towards completion of the research and development performance obligation related to the Novartis Agreement is best measured in an amount proportional to the expenses incurred from the total estimated expenses. We periodically review and update our estimates, when appropriate, which may adjust revenue recognized for the period. The transaction price to be recognized as revenue under the Novartis Agreement consists of the reimbursable research and development costs.
Operating Expenses
Our operating expenses since inception have consisted solely of research and development expenses and general and administrative expenses.
Research and Development Expenses
Research and development expenses consist primarily of costs incurred for our research activities, including the development of and pursuit of regulatory approval of our lead product candidates, including AL101 and AL102 (which were disposed of in early 2024), Lm-based products and BST-236, which include:
| ● | employee-related expenses, including salaries, benefits and stock-based compensation expense for personnel engaged in research and development functions; | |
| ● | expenses incurred in connection with the preclinical and clinical development of our product candidates, including under agreements with CROs, investigative sites and consultants; | |
| ● | costs of manufacturing our product candidates for use in our preclinical studies and clinical trials, as well as manufacturers that provide components of our product candidates for use in our preclinical and current and potential future clinical trials; | |
| ● | costs associated with our bioinformatics platform; | |
| ● | consulting and professional fees related to research and development activities; | |
| ● | costs related to compliance with clinical regulatory requirements; and | |
| ● | facility costs and other allocated expenses, which include expenses for rent and maintenance of our facility, utilities, depreciation and other supplies. |
| 23 |
We expense research and development costs as incurred. Our external research and development expenses consist primarily of costs such as fees paid to consultants, contractors and CROs in connection with our preclinical and clinical development activities. We typically use our employee and infrastructure resources across our development programs and we do not allocate personnel costs and other internal costs to specific product candidates or development programs with the exception of the costs to manufacture our product candidates.
The following table summarizes our research and development expenses by product candidate or development program for the years ended December 31, 2023 and 2022:
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Program-specific costs: | ||||||||
| AL101 | ||||||||
| ACC | $ | 395 | $ | 3,601 | ||||
| TNBC | 509 | 3,747 | ||||||
| General Expenses | 322 | 2,533 | ||||||
| AL102 | ||||||||
| General Expenses | 282 | 295 | ||||||
| Desmoid | 20,458 | 17,675 | ||||||
| Other R&D expenses (Mainly Lm -based products and BST-236) | 2,115 | - | ||||||
| Total research and development expenses | $ | 24,081 | $ | 27,851 | ||||
Research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect that our research and development expenses will continue to be significant in the future to the extent that we initiate additional clinical trials, scale our manufacturing processes, continue to develop additional product candidates and receiving all required approvalshire additional clinical and scientific personnel.
The successful development of Lm-based products and BST-236 and any future product candidate is highly uncertain. Accordingly, at this time, we cannot reasonably estimate or know the nature, timing and costs of the efforts that will be necessary to complete the development of these product candidates. We are also unable to predict when, if ever, we will generate revenue and material net cash inflows from the commercialization and sale of any of our product candidates for which we may obtain marketing approval. We may never succeed in order to sellachieving regulatory approval for any of our product candidates. The duration, costs and distribute the productstiming of preclinical studies, clinical trials and development of our product candidates will depend on a commercial scale can adversely affectvariety of factors, including:
| ● | successful completion of clinical trials with adequate safety, tolerability and efficacy profiles for any potential future product candidates that are satisfactory to the FDA or any comparable foreign regulatory authority; | |
| ● | approval of INDs for Lm-based products and BST-236 and any potential future product candidate to commence planned or future clinical trials in the United States or foreign countries; | |
| ● | significant and changing government regulation and regulatory guidance; | |
| ● | timing and receipt of marketing approvals from applicable regulatory authorities; | |
| ● | establishing arrangements with contract manufacturing organizations, or CMOs, for third-party clinical and commercial manufacturing to obtain sufficient supply of our product candidates; | |
| ● | obtaining, maintaining, protecting and enforcing patent and other intellectual property rights and regulatory exclusivity for our product candidates; | |
| ● | commercializing the product candidates, if and when approved, whether alone or in collaboration with other organizations; | |
| ● | acceptance of the product, if and when approved, by patients, the medical community and third-party payors; | |
| ● | competition with other therapies; and | |
| ● | maintenance of a continued acceptable safety profile of the products following approval |
| 24 |
A change in the outcome of any of these variables with respect to the development, manufacture or commercialization enabling activities of any of our businessproduct candidates would significantly change the costs, timing and viability associated with the development of that product candidate. For example, if the FDA or another regulatory authority were to require us to conduct clinical trials beyond those that we anticipate will be required for the completion of clinical development of a product candidate, or if we experience significant delays in our clinical trials due to patient enrollment or other reasons, we would be required to expend significant additional financial resources and time on the completion of clinical development.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and other related costs, including stock-based compensation, for personnel in executive, finance and administrative functions. General and administrative expenses also include direct and allocated facility-related costs as well as professional fees for legal, patent, consulting, investor and public relations, accounting, auditing, tax services and insurance costs.
We expect to continue to incur expenses associated with being a public company, including the costs of personnel, accounting, audit, legal, regulatory and tax-related services associated with maintaining compliance with Securities and Exchange Commission, or SEC, requirements, director and officer insurance costs, and investor and public relations costs.
Financial Income, Net
Financial income, net primarily consists of an immediate loss and a revaluation of Convertible Note and Warrant Liability offset by interest income earned on our cash and cash equivalents and restricted bank deposits.
Critical Accounting Policies and Use of Estimates
Our management’s discussion and analysis of financial condition and results of operations.operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities in our consolidated financial statements during the reporting periods. These items are monitored and analyzed by us for changes in facts and circumstances, and material changes in these estimates could occur in the future. We base our estimates on historical experience, known trends and events, and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known. Actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in the notes to our consolidated financial statements appearing elsewhere in this Annual Report on Form 10-K, we believe the following accounting policies used in the preparation of our consolidated financial statements require the most significant judgments and estimates.
| 25 |
Warrant Liabilities
The Company analyzes warrants issued to determine whether they meet the classification as liabilities or equity under US GAAP. Warrant liabilities are adjusted to reflect fair value at each reporting period, with any increase or decrease in the fair value recorded as finance expenses (income) in the statement of operations. The Company uses a fair valuation specialist to estimate the value of these instruments using numerous iterations using the Black–Scholes option price model.
The key assumptions used in the models are the expected future volatility in the price of the Company’s shares and the expected life of the warrants.
Convertible Notes
The Company analyzes convertible notes issued to determine whether they meet the classification as liabilities or equity under US GAAP. Convertible notes are adjusted to reflect fair value at each reporting period, with any increase or decrease in the fair value recorded as finance expenses (income) in the statement of operations. The Company uses a fair valuation specialist to estimate the value of these instruments using numerous iterations using the Monte Carlo Simulation.
The key assumptions used in the models are the expected future outcome as well as volatility in the price of the Company’s shares and the expected life of the notes.
Litigation regarding patents, patent applicationsBusiness Combination
We account for business combinations in accordance with ASC 805, “Business Combinations”. ASC 805 requires recognition of assets acquired and liabilities assumed at the acquisition date, measured at their fair values as of that date. The Company determines the recognition of intangible assets based on the following criteria: (i) the intangible asset arises from contractual or other rights; or (ii) the intangible asset is separable or divisible from the acquired entity and capable of being sold, transferred, licensed, returned or exchanged. The excess of the fair value of the purchase price over the fair values of the identifiable assets and liabilities is recorded as goodwill. Determining the fair value of the identifiable assets and liabilities requires management to use significant judgment and estimates including the forecasted revenue and revenues growth rates, discount rates, customer contract renewal rates and customer attrition rates.
The process of estimating the fair values requires significant estimates, especially with respect to intangible assets. Critical estimates in valuing certain intangible assets include, but are not limited to, future expected cash flows and discount rates. The Company estimates fair value based upon assumptions that are believed to be reasonable, but which are inherently uncertain and unpredictable and, as a result, actual results may differ from estimates.
Impairment of long-lived assets
Long-lived assets, including property and equipment and finite-lived intangible assets, are reviewed for impairment whenever facts or circumstances either internally or externally may indicate that the carrying value of an asset may not be recoverable. If there are indications of an impairment, the Company tests for recoverability by comparing the estimated undiscounted future cash flows expected to result from the use of the asset to the carrying amount of the asset or asset group. If the asset or asset group is determined to be impaired, any excess of the carrying value of the asset or asset group over its estimated fair value is recognized as an impairment loss.
Goodwill and intangible assets
Goodwill and acquired intangible assets have been recorded in the Company’s financial statements resulting from the a business combinations. Goodwill represents the excess of the purchase price in a business combination over the fair value of identifiable tangible and intangible assets acquired and liabilities assumed. Goodwill is subject to an annual impairment test.
Reporting units are evaluated when changes in the Company’s operating structure occur, and if necessary, goodwill would be reassigned using a relative fair value allocation approach. The Company operates in one operating segment, and this segment is the only reporting unit.
ASC 350, Intangibles—Goodwill and Other (“ASC 350”) requires goodwill to be tested for impairment at least annually and, in certain circumstances, between annual tests. The accounting guidance gives the option to perform a qualitative assessment to determine whether further impairment testing is necessary. The qualitative assessment considers events and circumstances that might indicate that a reporting unit’s fair value is less than its carrying amount. If it is determined, as a result of the qualitative assessment, that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, a quantitative test is performed.
The Company elects to perform an annual impairment test of goodwill as of December 31 of each year, or more frequently if impairment indicators are present. The Company performed its annual goodwill impairment test on December 31, 2023, and the Company concluded that no additional goodwill impairment should be recorded. There were no impairments of goodwill during the year ended December 31, 2023.
Separately acquired intangible assets are measured on initial recognition at cost including directly attributable costs. Intangible assets acquired in a business combination are measured at fair value at the acquisition date.
In process technology are amortized on a straight-line basis over the estimated useful life of the assets: approximately four years from the start of revenue which is estimated to start in 2030. Amortization of in process technology is presented within Research and Development in the consolidated statement of operations.
| 26 |
Income Taxes
We calculate income tax provisions based on our results in each jurisdiction in which we operate. The calculation is based on estimated tax consequences and on assumptions as to our entitlement to various benefits under the applicable local tax laws.
Significant judgment is required in evaluating our uncertain tax positions. We establish reserves for uncertain tax positions based on the evaluation of whether or not our uncertain tax position is “more likely than not” to be sustained upon examination based on our technical merits. We record estimated interest pertaining to our uncertain tax positions in the financial statements as income tax expense.
Deferred tax assets are recognized for unused tax losses, unused tax credits, and deductible temporary differences to the extent that it is probable that future taxable profits will be available, against which they can be used. Deferred taxes for each jurisdiction are presented as a net asset or liability, net of any valuation allowances. We estimate the need for any valuation allowance by applying significant judgment and considering all available evidence including past results and future projections. We reassess our estimates periodically and record a partial or full valuation allowance release if needed.
We cannot assure that future final tax outcomes will not be different than our tax provisions and reserves for uncertain tax positions. To the extent that the final tax outcome of these matters is different than the amounts recorded, such differences will impact the provision for income taxes in the period in which such determination is made.
Stock-Based Compensation
We measure stock options and other proprietary rights maystock-based awards granted to employees, directors, consultants or advisors of the company or its affiliates based on their fair value on the date of the grant and recognize compensation expense of those awards, over the requisite service period, which is generally the vesting period of the respective award. We apply the accelerated method of expense recognition to all awards with only service-based vesting conditions.
For stock-based awards granted to non-employees, compensation expense is recognized over the period during which services are rendered by such non-employees until completed.
We estimate the fair value of each stock option grant on the date of grant using the Black-Scholes option-pricing model, which uses as inputs the fair value of our common stock and assumptions we make for the volatility of our common stock, the expected term of our stock options, the risk-free interest rate for a period that approximates the expected term of our stock options and our expected dividend yield.
Previously, as a private company with no active public market for our common stock, our board of directors historically determined the fair value of our common stock on each date of grant, with input from management. Our board of directors periodically determined the estimated per share fair value of our common stock at various dates using valuations performed by third parties. All options to purchase shares of our common stock were intended to be expensivegranted with an exercise price per share no less than the fair value per share of our common stock underlying those options on the date of grant, based on the information known to us on the date of grant. Our determinations of the fair value of our common stock were made using methodologies, approaches and time consuming. If we are involved in such litigation, it could cause delays in bringing product candidates to marketassumptions consistent with the American Institute of Certified Public Accountants Audit and harm our ability to operate.Accounting Practice Aid Series: Valuation of Privately Held Company Equity Securities Issued as Compensation, or the Practice Guide.
| 27 |
Our success will depend in part on our abilityboard of directors considered various objective and subjective factors, along with input from management, to operate without infringingdetermine the proprietary rights of third parties. The pharmaceutical industry is characterized by extensive litigation regarding patents and other intellectual property rights. Other parties may obtain patents in the future and allege that the products or usefair value of our technologies infringe these patent claims or that we are employing their proprietary technology without authorization.common stock, including:
In addition, third parties may challenge or infringe upon our existing or future patents. Proceedings involving our patents or patent applications or those of others could result in adverse decisions regarding:
| ● | the | |
| ● | our results of operations and financial condition, including cash on hand; | |
| ● | the material risks related to our business; | |
| ● | our stage of development and business strategy; | |
| ● | the composition of, and changes to, our management team and board of directors; | |
| ● | the market performance of publicly traded companies in the life sciences and biotechnology sectors, as well as recently completed initial public offerings, or IPOs, of companies in the life sciences and biotechnology sectors; and | |
| ● | the likelihood of achieving a liquidity event given prevailing market conditions. |
There are significant judgments and estimates inherent in the determination of the fair value of our common stock. These judgments and estimates are management’s best estimates and include assumptions regarding our future operating performance, the time to completing an initial public offering or other liquidity event, the related company valuations associated with such events and the determinations of the appropriate valuation methods. If we had made different assumptions, our stock-based compensation expense, net loss and net loss per common share could have been different.
Results of Operations
Comparison of the year ended December 31, 2023 and 2022
The following table summarizes our results of operations for the year ended December 31, 2023 and 2022 (in thousands):
| Years Ended December 31, | ||||||||||||
| 2023 | 2022 | Change | ||||||||||
| Revenue from license agreement | $ | 13 | $ | 692 | (679 | ) | ||||||
| Cost of revenue | (13 | ) | (602 | ) | 589 | |||||||
| Gross profit | - | 90 | (90 | ) | ||||||||
| Operating expenses: | ||||||||||||
| Research and development | 24,081 | 27,851 | (3,770 | ) | ||||||||
| General and administrative | 12,185 | 9,742 | 2,443 | |||||||||
| Operating loss | (36,266 | ) | (37,503 | ) | (1,327 | ) | ||||||
| Financial income (expense), net | (15,718 | ) | 74 | (15,792 | ) | |||||||
| Loss before income tax | (51,984 | ) | (37,429 | ) | (14,555 | ) | ||||||
| Taxes on income | 3,912 | (584 | ) | 4,496 | ||||||||
| Net loss | $ | (48,072 | ) | $ | (38,013 | ) | (10,059 | ) | ||||
Revenue
Revenue associated with the research and development services mainly under the Novartis Agreement in the amount of $13 thousand was recognized in the year ended December 31, 2023, compared to $0.7 million recognized in fiscal year 2022.
Research and Development Expenses
Research and development expenses were $24.1 million for the year ended December 31, 2023 compared to $27.9 million for the year ended December 31, 2022, a decrease of $3.8 million. The decrease was due to the termination of the TENACITY trial and winding down of the ACCURACY trial offset by expenses incurred by the programs of former Advaxis and Biosight.
General and Administrative Expenses
General and administrative expenses were $12.2 million for the year ended December 31, 2023 compared to $9.7 million for the year ended December 31, 2022, an increase of $2.4 million. The increase was mainly due to severance agreement obligations recognized in the period to former executives of $0.9 million in salary compensation and $0.9 million in stock-based compensation due to acceleration of options as part of severance agreement.
Financial Income (expense), net
Financial expense, net was $15.7 million for the year ended December 31, 2023 compared to financial income, net of $74 thousand for the year ended December 31, 2022. The increase in financial expenses was mainly due to revaluation of convertible notes side letter agreements and warrants issued.
| 28 |
Liquidity and Capital Resources
Sources of Liquidity
The Company has incurred recurring losses since inception as a research and development organization. For the year ended December 31, 2023, the Company used approximately $29.5 million of cash in operations and incurred a net loss of $48.1 million. As of December 31, 2023, the Company had $4.9 million in cash and cash equivalents, $25.0 million in current liabilities and an accumulated deficit of $197.2 million.
Upon closing of the Asset Sale, on March 25, 2024, the Company received $13.0 million in cash, and Immunome paid $3.0 million of the Company’s liabilities directly to vendors of the Company. In addition, under the Asset Sale Agreement the Company received 2,175,489 shares of Immunome’s common stock (the “Immunome Shares”). The Asset Sale Agreement prohibits the Company from selling more than 50% of the Immunome Shares in the first six months following the closing of the Asset Sale. In addition, on March 1, 2024, the Company issued additional convertible notes and warrants in exchange for $2.0 million in funding from the Convertible Notes investors. As of the date of this filing, the cash proceeds received from the Asset Sale and the convertible notes sold on March 1, 2024 were not sufficient to pay the Company’s existing liabilities. Therefore, the Company has limited available cash resources and requires additional financing, through the sale of a portion of the Immunome Shares or otherwise, in order to continue to fund its current operations beyond May 2024.
Raising additional funds or the satisfactory sale of a portion of the Immunome Shares prior to the end of May 2024 is essential to provide sufficient cash flow to meet future liabilities and other obligations, such as tax payments arising from the Asset Sale. Furthermore, even if the Company is successful in selling a portion of the Immunome Shares or raising additional funds through other means, the Company cannot give any assurance that it will, in the future, be able to achieve a level of profitability from the sale of its products to sustain its operations.
If the Company is unable to obtain funding, or able to receive sufficient funds from the sale of a portion of the Immunome Shares, the Company would be forced to delay, reduce, or eliminate its research and development programs, or the Company may be unable to continue operations. As such, those factors raise substantial doubt about the Company’s ability to continue as a going concern.
As part of a cost reduction plan, during the year ended December 31, 2023, the Company had a reduction in workforce in which the employment of approximately 50% of the Company’s employees was terminated. During the first quarter of 2024, the Company gave notice of termination to 18 additional employees and two officers (including the Chief Financial Officer, whose employment will terminate on June 25, 2024). After the effectiveness of such terminations, the Chief Executive Officer will be the only employee of the Company. The Company expects to be able to meet its financial obligations to its employees.
The Company can give no assurances that it will be successful in raising funds through the sale of a portion of the Immunome Shares or any other alternative. Any inability to so obtain additional financing or funding will likely cause the Company to cease business operations. The consolidated financial statements have been prepared assuming that the Company will continue as a going concern. Therefore, the consolidated financial statements for the year ended December 31, 2023, do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern.
On February 19, 2021, Old Ayala entered into a Securities Purchase Agreement (the “2021 Purchase Agreement”) with the purchasers named therein (the “Investors”). Pursuant to the 2021 Purchase Agreement, Old Ayala agreed to sell (i) an aggregate of 62,467 shares of Old Ayala’s common stock (the “Private Placement Shares”), par value $0.01 per share, together with warrants to purchase an aggregate of 21,863 shares of Old Ayala’s common stock with an exercise price of $96.58 per share (the “Common Warrants”), for an aggregate purchase price of $4,999,995.00 and (ii) pre-funded warrants to purchase an aggregate of 249,867 shares of our common stock with an exercise price of $0.05 per share (the “Pre-Funded Warrants” and collectively with the Common Warrants, the “Private Placement Warrants”), together with an aggregate of 87,453 Common Warrants, for an aggregate purchase price of $19,986,661.67 (collectively, the “Private Placement”). The Private Placement closed on February 23, 2021.
The exercise price and the number of shares of common stock issuable upon exercise of each Private Placement Warrant are subject to adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting the common stock. In addition, in certain circumstances, upon a fundamental transaction, a holder of Common Warrants will be entitled to receive, upon exercise of the Common Warrants, the kind and amount of securities, cash or other property that such holder would have received had they exercised the Private Placement Warrants immediately prior to the fundamental transaction. The Pre-Funded Warrants will be automatically exercised on cashless basis upon the occurrence of a fundamental transaction. Each Common Warrant is exercisable from the date of issuance and has a term of three years and each Pre-Funded Warrant is exercisable from the date of issuance and has a term of ten years. Pursuant to the 2021 Purchase Agreement, Old Ayala registered the Private Placement Shares and Private Placement Warrants for resale by the Investors on a registration statement on Form S-3 (the “Private Placement Registration Statement”).
In June 2021, Old Ayala entered into an Open Market Sales Agreement, or the Sales Agreement, with Jefferies LLC, or Jefferies, as sales agent, pursuant to which Old Ayala was able to, from time to time, issue and sell common stock with an aggregate value of up to $200.0 million in “at-the-market” offerings (the “ATM”), under a registration statement on Form S-3 filed with the SEC. Sales of common stock, if any, pursuant to the Sales Agreement, could be made in sales deemed to be an “at the market offering” as defined in Rule 415(a) of the Securities Act, including sales made directly through The Nasdaq Global Market or on any other existing trading market for our common stock. During the year ended December 31, 2022, Old Ayala sold a total of 310,417 shares of its common stock for total gross proceeds of approximately $526 thousand. The Sales Agreement was terminated in January 2023.
On October 18, 2022, the Company, which at the time was named Advaxis, Inc., entered into a Merger Agreement (the “Merger Agreement”), with an entity then known as Ayala Pharmaceuticals, Inc. (which shortly prior to the closing of the merger in January 2023 changed its name to Old Ayala, Inc., (“Old Ayala”) and Doe Merger Sub, Inc. (“Merger Sub”), a direct, wholly-owned subsidiary of the Company. Under the terms of the Merger Agreement, Merger Sub merged with and into Old Ayala, with Old Ayala continuing as the surviving company and a wholly-owned subsidiary of the Company (the “January 2023 Merger”). Immediately after the January 2023 Merger, former Advaxis stockholders as of immediately prior to the Merger own approximately 37.5% of the outstanding shares of the combined Company and former Old Ayala shareholders own approximately 62.5% of the outstanding shares of the combined Company.
At the effective time of the January 2023 Merger (the “Effective Time”), each share of share capital of Old Ayala issued and outstanding immediately prior to the Effective Time was converted into the right to receive a number of shares of the Company’s common stock, par value $0.001 per share, equal to the exchange ratio, 0.1874 shares of the Company’s common stock per Old Ayala share.
On August 7, 2023, the Company entered into an agreement for the issuance of Senior Secured Convertible Promissory Notes (the “Secured Notes”) to Israel Biotech Fund I, L.P. The Secured Notes provided for the borrowing by the Company of up to $2.0 million dollars, which borrowings which the Company received on September 1, 2023.
On November 17, 2023, the Company issued Senior Convertible Promissory Notes (collectively, the “Senior Convertible Notes”), in an aggregate amount of $4.0 million, to several existing lenders and investors in the Company, including Israel Biotech Fund I, L.P., Israel Biotech Fund II, L.P., Arkin Bio Ventures L.P. and Biotel Limited. The amounts borrowed by the Company under the Senior Convertible Notes were funded to the Company on November 20, 2023. The Senior Convertible Notes were convertible into shares of the Company’s common stock, par value $0.001 per share (the “Common Stock”) at any time at the option of the noteholders, and were subject to mandatory conversion upon certain events, including a change of control transaction and certain financing transactions involving the Company, at a conversion price equal to the lower of (i) 50% of the Common Stock’s price per share as of market close on November 16, 2023 and (ii) 50% of the Common Stock’s price per share as of the close of market on the Trading Day immediately prior to the date of the Notice of Conversion, subject to certain adjustments. In connection with the issuance of the Senior Convertible Notes, the Company issued to the noteholders warrants to purchase an aggregate of 15,000,000 shares of the Common Stock with an exercise price equal to the lower of (A) 50% of the Common Stock’s price per share as of market close on November 16, 2023 and (ii) 50% of the Common Stock’s price per share as of the close of market on the Trading Day immediately prior to the date of the Notice of Exercise of the warrant, subject to adjustment, which exercise may be on a cashless basis.
The noteholders also had the right, pursuant to a Side Letter Agreement between the noteholders and the Company, to lend an additional $4.0 million dollars to the Company on the same terms.
The Company and Israel Biotech Fund I, L.P. and Israel Biotech Fund II, L.P. also agreed to amend and restate the terms of Secured Notes to conform to the terms of the Senior Convertible Notes, and issued to the holders of the Secured Notes warrants to purchase an aggregate of 7,500,000 shares of the Common Stock on the terms of the above-described warrants.
In February 2024, the Secured Notes and the Senior Convertible Notes, and the warrants issued in connection with all of them, were converted into shares of the Company’s common stock under the terms described in such notes and warrants.
| 29 |
Cash Flows
The following table provides information regarding our total cash and cash equivalents and restricted bank cash at December 31, 2023 and 2022 (in thousands):
| As of December 31, | ||||||||
| 2023 | 2022 | |||||||
| Cash and cash equivalents and restricted cash | $ | 5,212 | $ | 2,724 | ||||
Cash Flows
The following table provides information regarding our cash flows for the years ended December 31, 2023 and 2022 (in thousands):
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Net cash used in operating activities | $ | (29,485 | ) | $ | (34,510 | ) | ||
| Net cash provided by (used in) investing activities | 5,946 | (2 | ) | |||||
| Net cash provided by financing activities | 26,001 | 103 | ||||||
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | 26 | - | ||||||
| Net increase (decrease) in cash and cash equivalents and restricted cash | $ | 2,488 | $ | (34,615 | ) | |||
Net Cash Used in Operating Activities
The cash used in operating activities resulted primarily from expenses associated with our clinical development programs and early-stage research and general and administrative expenses.
Net cash used in operating activities during the year ended December 31, 2023 of $29.5 million was primarily attributable to our net loss of $48.1 million, adjusted for non-cash expenses of $17.5 million, which includes revaluation of warrants and convertible notes of $15.7 and stock-based compensation of $1.2 million, and a net increase in working capital of $1.1 million.
Net cash used in operating activities during the year ended December 31, 2022 of $34.5 million was primarily attributable to our net loss of $38.0 million, adjusted for non-cash expenses of $2.7 million, which includes stock-based compensation of $2.2 million and a net increase in working capital of $0.8 million.
Net Cash Provided by (Used in) Investing Activities
Net cash provided by investing activities of $5.9 million during the year ended December 31, 2023 was mainly attributable to $4.0 million in proceeds from the sale of our AL 101 and AL 102 assets and $1.9 million of proceeds received in connection with the merger with Biosight.
Net cash used in investing activities during the year ended December 31, 2022, of $2 thousand was attributable to purchases of property and equipment.
Net Cash Provided by Financing Activities
Net cash provided by financing activities during the year ended December 31, 2023 of $26.0 million was primarily attributable to the January 2023 Merger and the Convertible Loans.
Net cash provided by financing activities during the year ended December 31, 2022, of $0.1 million was primarily attributable to our issuance of shares at market, net of issuance costs.
| 30 |
Funding Requirements and Going Concern
Our future capital requirements are difficult to forecast and will depend on many factors, including our ability to raise additional funding. To the extent that we continue the research and development for, initiate later-stage clinical trials for, and seek marketing approval for, our product candidates, we expect our expenses would increase. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing, and distribution. Furthermore, we incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our research and development programs or future commercialization efforts.
The Company has incurred recurring losses since inception as a research and development organization. For the year ended December 31, 2023, the Company used approximately $29.5 million of cash in operations and incurred a net loss of $48.1 million. As of December 31, 2023, the Company had $4.9 million in cash and cash equivalents, $25.0 million in current liabilities and an accumulated deficit of $197.2 million.
Upon closing of the Asset Sale, on March 25, 2024, the Company received $13.0 million in cash, and Immunome paid $3.0 million of the Company’s liabilities directly to vendors of the Company. In addition, under the Asset Sale Agreement the Company received 2,175,489 shares of Immunome’s common stock (the “Immunome Shares”). The Asset Sale Agreement prohibits the Company from selling more than 50% of the Immunome Shares in the first six months following the closing of the Asset Sale. In addition, on March 1, 2024, the Company issued additional convertible notes and warrants in exchange for $2.0 million in funding from the Convertible Notes investors. As of the date of this filing, the cash proceeds received from the Asset Sale and the convertible notes sold on March 1, 2024 were not sufficient to pay the Company’s existing liabilities. The Company does not believe it has sufficient capital to fund its current obligations, as they become due, as the Company has limited available cash resources and requires additional funds in order to continue to fund its current operations , and to pay existing and future liabilities and other obligations. Our future capital requirements will depend on many factors, including:
| ● | the ability to successfully sale a portion of the Immunome Shares received in the Asset Sale;. | |
| ● | The cost of conducting and completing clinical trials of BST-236; | |
| ● | the scope, progress, results and costs of discovery, preclinical development, laboratory testing and clinical trials for other potential product candidates we may develop or acquire, if any; | |
| ● | the costs, timing and outcome of regulatory review of our | |
| ● | the achievement of milestones or occurrence of other developments that trigger payments under any current or future license, collaboration or other agreements; | |
| ● | the costs and timing of future commercialization activities, including product sales, marketing, manufacturing and distribution, for any of our product | |
| ● | the amount of revenue, if any, received from commercial sales of our product candidates, should any of our product candidates receive marketing approval; | |
| ● | the costs of preparing, filing and prosecuting patent applications, obtaining, maintaining, protecting and enforcing our intellectual property rights and defending intellectual property-related claims; | |
| ● | the costs of operating as a public company. |
Conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of products that we do not expect to be commercially available for many years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances and licensing arrangements. We do not have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interests may be diluted.. Any debt financing, if available, may involve agreements that include restrictive covenants that limit our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends, that could adversely impact our ability to conduct our business.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Omitted.
| 31 |
Item 8. Financial Statements and Supplementary Data.
AYALA PHARMACEUTICALS, INC.
INDEX TO FINANCIAL STATEMENTS
| F-1 |
 | Kost Forer Gabbay & Kasierer | Tel: +972-3-6232525 |
| 144 Menachem Begin Road, Building A | Fax: +972-3-5622555 | |
| Tel-Aviv 6492102, Israel | ey.com |
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of
AYALA PHARMACEUTICALS, INC.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Ayala Pharmaceuticals, Inc. (the Company) as of December 31, 2023 and 2022, the related consolidated statements of operations, changes in stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2023, in conformity with U.S. generally accepted accounting principles.
The Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations, has a negative cash flows from operating activities, and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management’s evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatements, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
KOST FORER GABBAY & KASIERER
A Member of EY Global
We have served as the Company’s auditor since 2017.
Tel-Aviv, Israel
April 16, 2024
| F-2 |
AYALA PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
U.S. dollars in thousands (except share and per share data)
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Assets | ||||||||
| Current Assets: | ||||||||
| Cash and Cash Equivalents | $ | 4,882 | $ | 2,408 | ||||
| Trade Receivables | - | 234 | ||||||
| Prepaid Expenses and Other Current Assets | 2,646 | 546 | ||||||
| Total Current Assets | 7,528 | 3,188 | ||||||
| Long-Term Assets: | ||||||||
| Deferred issuance costs | - | 1,953 | ||||||
| Intangible assets, net | 3,898 | - | ||||||
| Goodwill | 4,500 | - | ||||||
| Operating lease right of use asset | 102 | 1,462 | ||||||
| Property and Equipment, Net | 540 | 960 | ||||||
| Other Assets | 11 | 206 | ||||||
| Total Long-Term Assets | 9,051 | 4,581 | ||||||
| Total Assets | $ | 16,579 | $ | 7,769 | ||||
| Liabilities and Stockholders’ Equity: | ||||||||
| Current Liabilities: | ||||||||
| Trade Payables | $ | 6,076 | $ | 4,080 | ||||
| Operating lease liabilities | 166 | 419 | ||||||
| Accrued expenses and other payables | 5,554 | 708 | ||||||
| Side Agreement and reinvestment rights** | 8,436 | |||||||
| Accrued payroll and employee benefits | 786 | 994 | ||||||
| Proceed from Asset Sale | 4,000 | - | ||||||
| Total Current Liabilities | 25,018 | 6,201 | ||||||
| Long-Term Liabilities: | ||||||||
| Long-term warrant liability** | 6,057 | - | ||||||
| Convertible Note** | 8,141 | - | ||||||
| Uncertain tax position | 1,771 | 1,335 | ||||||
| Long-term operating lease liabilities | 9 | 1,332 | ||||||
| Total Long-Term Liabilities | $ | 15,978 | $ | 2,667 | ||||
| Stockholders’ Equity: | ||||||||
| Common Stock of $ par value per share; and * shares authorized at December 31, 2023 and 2022, respectively; and * shares issued at December 31, 2023 and 2022, respectively; and * shares outstanding at December 31, 2023 and 2022, Respectively. | $ | 12 | $ | 3 | ||||
| Additional Paid-in Capital | 172,797 | 148,052 | ||||||
| Accumulated Deficit | (197,226 | ) | (149,154 | ) | ||||
| Total Stockholders’ Equity | (24,417 | ) | (1,099 | ) | ||||
| Total Liabilities and Stockholders’ Equity | $ | 16,579 | $ | 7,769 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
| * | All of the Common Stock, additional paid-in capital and per share data have been retroactively adjusted for the impact of the January 2023 merger between Old Ayala, Inc. (f/k/a Ayala Pharmaceuticals, Inc.) and Ayala Pharmaceutical, Inc.(f/k/a Advaxis, Inc.). See note 1. |
| ** | Received from related party see note 14. |
| F-3 |
AYALA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
U.S. dollars in thousands (except shares and per shares data)
| Year ended | Year ended | |||||||
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Revenue | $ | 13 | $ | 692 | ||||
| Cost of Revenue | (13 | ) | (602 | ) | ||||
| Gross Profit | - | 90 | ||||||
| Research and Development | $ | 24,081 | $ | 27,851 | ||||
| General and Administrative | 12,185 | 9,742 | ||||||
| Operating Loss | (36,266 | ) | (37,503 | ) | ||||
| Financial income (expenses), net | (15,718 | ) | 74 | |||||
| Loss before taxes on income | (51,984 | ) | (37,429 | ) | ||||
| Taxes on Income | 3,912 | (584 | ) | |||||
| Net Loss | $ | (48,072 | ) | $ | (38,013 | ) | ||
| Net Loss per Share attributable to Common Stockholders, Basic and Diluted | $ | ) | $ | ) | ||||
| Weighted Average Shares Used to Compute Net Loss per Share, Basic and Diluted | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-4 |
AYALA PHARMACEUTICALS, INC.
STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
U.S. dollars in thousands (except share amounts)
| ** | ** | |||||||||||||||||||
| Common Stock** | Additional paid-in | Accumulated | Total Stockholders’ | |||||||||||||||||
| Number | Amount | Capital** | Deficit | Equity | ||||||||||||||||
| Balance as of December 31, 2021 | 2,615,360 | $ | 3 | $ | 145,296 | $ | (111,141 | ) | $ | 34,158 | ||||||||||
| Proceeds from Issuance of common stock, net of issuance cost of $14 | 58,172 | -* | 512 | — | 512 | |||||||||||||||
| Stock Based Compensation | 21,535 | -* | 2,244 | — | 2,244 | |||||||||||||||
| Net Loss | — | — | — | (38,013 | ) | (38,013 | ) | |||||||||||||
| Balance as of December 31, 2022 | 2,695,067 | $ | 3 | $ | 148,052 | $ | (149,154 | ) | $ | (1,099 | ) | |||||||||
| Balance | 2,695,067 | $ | 3 | $ | 148,052 | $ | (149,154 | ) | $ | (1,099 | ) | |||||||||
| exercise of warrants | 246,192 | -* | - | - | - | |||||||||||||||
| Issuance of shares upon January 2023 Merger, net of issuance costs of $3,153 | 1,815,951 | 2 | 16,947 | - | 16,949 | |||||||||||||||
| Issuance of shares upon Bio sight Merger | 5,913,480 | 6 | 5,508 | - | 5,514 | |||||||||||||||
| Conversion of SAFE | 1,145,053 | 1 | 1,067 | - | 1,068 | |||||||||||||||
| Share based compensation | 41,650 | - | 1,223 | - | 1,223 | |||||||||||||||
| Net Loss | - | - | - | (48,072 | ) | (48,072 | ) | |||||||||||||
| Balance as of December 31, 2023 | 11,857,393 | 12 | 172,797 | (197,226 | ) | (24,417 | ) | |||||||||||||
| Balance | 11,857,393 | 12 | 172,797 | (197,226 | ) | (24,417 | ) | |||||||||||||
| * | Represents an amount lower than $1. |
** | All of the Common Stock and per share data have been retroactively adjusted and Additional paid in Capital to adjust for common stock amount, for the impact of the January 2023 merger between Old Ayala, Inc. (f/k/a Ayala Pharmaceuticals, Inc.) and Ayala Pharmaceutical, Inc.(f/k/a Advaxis, Inc.). See note 1 |
The accompanying notes are an integral part of the consolidated financial statements.
| F-5 |
AYALA PHARMACEUTICALS, INC.
STATEMENTS OF CONSOLIDATED CASH FLOWS
U.S. dollars in thousands
| Year Ended | Year Ended | |||||||
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net Loss | $ | (48,072 | ) | $ | (38,013 | ) | ||
| Adjustments to reconcile Net Loss to Net Cash used in Operating Activities: | ||||||||
| Shared Based Compensation | 1,223 | 2,244 | ||||||
| Depreciation and Amortization | 369 | 162 | ||||||
| Asset write-downs | 145 | - | ||||||
| Remeasurement of long-term warrant liability | 5,854 | - | ||||||
| Remeasurement of Side Letter Agreements | 7,751 | - | ||||||
| Remeasurement of convertible note | 2,141 | - | ||||||
| (Increase) Decrease in Prepaid Expenses and other Assets | (1,491 | ) | 2,232 | |||||
| Decrease (Increase) in Trade Receivables | 234 | (234 | ) | |||||
| Decrease in Trade Payables | (724 | ) | (472 | ) | ||||
| Decrease in operating lease right-of-use assets | 1,429 | 288 | ||||||
| Decrease in operating lease liabilities | (1,641 | ) | (536 | ) | ||||
| Increase (decrease) in accrued expenses and other payables | 3,123 | 121 | ||||||
| Increase (decrease) in accrued payroll and employee benefits | (248 | ) | (767 | ) | ||||
| Changes in uncertain tax position | 448 | 465 | ||||||
| Other | (26 | ) | - | |||||
| Net Cash used in Operating Activities | (29,485 | ) | (34,510 | ) | ||||
| Cash Flows from Investing Activities: | ||||||||
| Proceed from Asset Sale | 4,000 | - | ||||||
| Cash acquired from the Biosight Merger | 1,909 | - | ||||||
Other | 37 | (2 | ) | |||||
| Net Cash used in Investing Activities | 5,946 | (2 | ) | |||||
| Cash Flows from Financing Activities: | ||||||||
| Proceeds from issuance of convertible note from related party | 6,000 | - | ||||||
| Proceeds from issuance of shares, net of issuance cost of $14 | - | 512 | ||||||
| Issuance of shares upon January 2023 Merger, net of issuance costs | 20,001 | (615 | ) | |||||
| Net Cash used in Financing Activities | 26,001 | (103 | ) | |||||
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | 26 | |||||||
| Increase (decrease)in Cash and Cash Equivalents and Restricted Cash | 2,488 | (34,615 | ) | |||||
| Cash and Cash Equivalents and Restricted Cash at Beginning of the Year | 2,724 | 37,339 | ||||||
| Cash and Cash Equivalents and Restricted Cash at End of the Year | $ | 5,212 | $ | 2,724 | ||||
| Supplemental Disclosure of investing and financing Activities | ||||||||
| Lease liabilities arising from new right-of-use assets | $ | - | $ | 537 | ||||
| Deferred issuance costs accrued but not yet paid | $ | - | $ | 1,338 | ||||
| Supplemental Disclosures of Cash Flow Information | ||||||||
| Cash Paid for Income Taxes | $ | 371 | $ | 244 | ||||
| Year Ended | Year Ended | |||||||
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Cash and Cash Equivalents | $ | 4,882 | $ | 2,408 | ||||
| Restricted Cash in Prepaid Expenses and Other Current Assets | 330 | 110 | ||||||
| Restricted Cash in Other Assets | - | 206 | ||||||
| Cash and Cash Equivalents and Restricted Cash at End of the Year | $ | 5,212 | $ | 2,724 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-6 |
AYALA PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. General
In these financial statements, unless otherwise stated or the context otherwise indicates, references to “New Ayala” and the “Company,” refers to Ayala Pharmaceuticals, Inc., a Delaware corporation, which prior to the change of its name effected on January 19, 2023, was known as Advaxis, Inc. The name change was affected in connection with the January 2023 Merger, as described below. References to “former Advaxis” refer to the Company solely in the period prior to the January 2023 Merger.
Prior to the January 2023 Merger, the Company was a clinical-stage biotechnology company focused on the development and commercialization of proprietary Listeria monocytogenes (“Lm”)-based antigen delivery products. These efforts utilized the Company Lm platform directed against tumor-specific targets in order to engage the patient’s immune system to destroy tumor cells. Through a license from the University of Pennsylvania, the Company has exclusive access to this proprietary formulation of attenuated Lm called Lm TechnologyTM.
Following the January 2023 Merger, the Company became primarily a clinical-stage oncology company focused on developing and commercializing small molecule therapeutics for patients suffering from rare and aggressive cancers, primarily in genetically defined patient populations. The Company differentiated development approach is predicated on identifying and addressing tumorigenic drivers of cancer, through a combination of the Company’s bioinformatics platform and next-generation sequencing to deliver targeted therapies to underserved patient populations. The Company’s portfolio of product candidates following the January 2023 Merger, AL101 and AL102, targets the aberrant activation of the Notch pathway using gamma secretase inhibitors. All of the Company’s assets relating to AL 101 and AL 102 were sold on March 25, 2024 in the Asset Sale (as defined below). Following the January 2023 Merger, the Company also continued to conduct certain operations relating to former Advaxis’ operations as a clinical-stage biotechnology company focused on the development and commercialization of proprietary Listeria monocytogenes (“Lm”)-based antigen delivery products. These efforts are primarily focused on the development of ADXS-504, a Lm-based therapy for early-stage prostate cancer. See note 3.
On July 26, 2023, the Company and its wholly owned subsidiary organized under the laws of the State of Israel, Advaxis Israel Ltd. (“Biosight Merger Sub”), entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) with Biosight Ltd. (“Biosight”), a privately-held Israeli pharmaceutical company developing innovative therapeutics for hematological malignancies and disorders. Under the terms of the Merger Agreement, on October 18, 2023, Merger Sub merged with and into Biosight, which is now a wholly owned subsidiary of the Company (the “Biosight Merger”). See note 3.
Based on the agreement, Ayala Pharmaceuticals, Inc. was the legal acquirer in the Biosight Merger. In addition, the Company considered ASC 805-10-55 to determine the accounting acquirer in the Biosight Merger. As the Company holds a majority of the members of the governing body of the combined Company and the Company’s former management dominates the majority of the senior management of the combined Company, and after considering all other factors according to ASC 805-10-55, the Company was identified as the accounting acquirer in the Biosight Merger. The Company has accounted for the Biosight Merger as a business combination according to ASC 805 “Business Combinations”.
On February 5, 2024, the Company and Immunome, Inc. (“Immunome”), entered into an Asset Purchase Agreement (the “Asset Purchase Agreement”) pursuant to which Immunome agreed to acquire certain of the Company’s assets and liabilities related to its AL101 and AL102 programs (the “Asset Sale”), which constitute substantially all of the Company’s assets.
On March 25, 2024, the Company and Immunome consummated the Asset Sale pursuant to the Asset Purchase Agreement. Immunome paid to the Company an aggregate purchase price of $20.0 in cash (of which $4.0 had been paid upon entering into the Asset Purchase Agreement), subject to certain adjustments, and issued to the Company shares (the “Shares”) of Immunome’s common stock, $par value. The Asset Purchase Agreement further provides that Immunome may pay the Company up to $37.5 million in cash due upon the Immunome’s achievement of certain development and commercial milestone events set forth in the Asset Purchase Agreement. At the March 25, 2023 closing of the Asset Sale, Immunome paid the remaining $16.0 million of cash consideration to the Company and, at the Company’s direction, to certain vendors of the Company.
The Asset Purchase Agreement contains customary representations, warranties, conditions and covenants, including covenants (i) concerning the conduct of business by the Company prior to the closing of the Asset Sale, (ii) prohibiting the Company and its representatives from soliciting, initiating or knowingly inducing, encouraging or facilitating any competing acquisition proposal, (iii) prohibiting the Company and its controlled affiliates from competing with Immunome for five years following the closing of the Asset Sale in certain fields, and (iv) restricting the Company’s ability to make distributions to stockholders, dissolve or wind up its business or file for bankruptcy for six months following the closing of the Asset Sale.
In the Asset Sale, the Company disposed of the assets relating to AL102, an oral gamma secretase inhibitor in Phase 3 clinical development, and AL 101, and as such, the Company’s clinical assets currently include aspacytarabine (BST-236), a novel proprietary anti-metabolite for first line treatment in unfit acute myeloid leukemia (AML).
During the year ended December 31, 2023, the Company had a reduction in workforce in which the employment of approximately 50% of the Company’s employees was terminated. This reduction in workforce has not yet required the Company to cease any major development efforts. Following the reduction in workforce, the Company had 21 employees. See note 17 for information regarding the additional reduction of 18 employees and one officer during the first quarter of 2024, with the employment of a second officer to terminate on June 25, 2024.
Going Concern
The Company has incurred recurring losses since inception as a research and development organization. For the year ended December 31, 2023, the Company used approximately $29.5 million of cash in operations and incurred a net loss of $48.1 million. As of December 31, 2023, the Company had $4.9 million in cash and cash equivalents, $25.0 million in current liabilities and an accumulated deficit of $197.2 million.
| F-7 |
Upon closing of the Asset Sale, on March 25, 2024, the Company received $13.0 million in cash, and Immunome paid $3.0 million of the Company’s liabilities directly to vendors of the Company. In addition, under the Asset Sale Agreement the Company received shares of Immunome’s common stock (the “Immunome Shares”). The Asset Sale Agreement prohibits the Company from selling more than 50% of the Immunome Shares in the first six months following the closing of the Asset Sale. In addition, on March 1, 2024, the Company issued additional convertible notes and warrants in exchange for $2.0 million in funding from the Convertible Notes investors. As of the date of this filing, the cash proceeds received from the Asset Sale and the convertible notes sold on March 1, 2024 were not sufficient to pay the Company’s existing liabilities. Therefore, the Company has limited available cash resources and requires additional financing, through the sale of a portion of the Immunome Shares or otherwise, in order to continue to fund its current operations beyond May 2024.
Raising additional funds or the satisfactory sale of a portion of the Immunome Shares prior to the end of May 2024 is essential to provide sufficient cash flow to meet future liabilities and other obligations, such as tax payments arising from the Asset Sale. Furthermore, even if the Company is successful in selling a portion of the Immunome Shares or raising additional funds through other means, the Company cannot give any assurance that it will, in the future, be able to achieve a level of profitability from the sale of its products to sustain its operations.
If the Company is unable to obtain funding, or able to receive sufficient funds from the sale of a portion of the Immunome Shares, the Company would be forced to delay, reduce, or eliminate its research and development programs, or the Company may be unable to continue operations. As such, those factors raise substantial doubt about the Company’s ability to continue as a going concern.
As part of a cost reduction plan, during the year ended December 31, 2023, the Company had a reduction in workforce in which the employment of approximately 50% of the Company’s employees was terminated. During the first quarter of 2024, the Company gave notice of termination to 18 additional employees and two officers (including the Chief Financial Officer, whose employment will terminate on June 25, 2024). After the effectiveness of such terminations, the Chief Executive Officer will be the only employee of the Company. The Company expects to be able to meet its financial obligations to its employees.
The Company can give no assurances that it will be successful in raising funds through the sale of a portion of the Immunome Shares or any other alternative. Any inability to so obtain additional financing or funding will likely cause the Company to cease business operations. The consolidated financial statements have been prepared assuming that the Company will continue as a going concern. Therefore, the consolidated financial statements for the year ended December 31, 2023, do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern.
2.Significant Accounting Policies
Basis of Presentation
The consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). The significant accounting policies followed in the preparation of the consolidated financial statements, are as follows:
Use of Estimates:
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. The Company’s management believes that the estimates, judgment and assumptions used are reasonable based upon information available at the time they are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the consolidated financial statements. Actual results could differ from those estimates.
Consolidated Financial Statements in U.S. Dollars
A substantial portion of the Company’s financing activities, including equity transactions and cash investments, are incurred in U.S. dollars. The Company’s management believes that the U.S. dollar is the currency of the primary economic environment in which the Company operates. Thus, the functional and reporting currency of the Company is the U.S. dollar.
A subsidiary’s functional currency is the currency of the primary economic environment in which the subsidiary operates; normally, that is the currency of the environment in which a subsidiary primarily generates and expends cash. In making the determination of the appropriate functional currency for a subsidiary, the Company considers cash flow indicators, local market indicators, financing indicators and the subsidiary’s relationship with both the parent company and other subsidiaries. For subsidiaries that are primarily a direct and integral component or extension of the parent entity’s operations, the U.S. dollar is the functional currency.
| F-8 |
The Company has determined the functional currency of its foreign subsidiary is the U.S. Dollar. The foreign operation is considered a direct and integral part or extension of the Company’s operations. The day-to-day operations of the foreign subsidiary are dependent on the economic environment of the U.S. Dollar.
Accordingly, monetary accounts maintained in currencies other than the U.S. dollar are remeasured into U.S. dollars in accordance with Statement of the Accounting Standard Codification (“ASC”) No. 830 “Foreign Currency Matters” (“ASC No. 830”). All transaction gains and losses of the remeasured monetary balance sheet items are reflected in the statements of operations as financial income or expenses as appropriate.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and the Subsidiary. Intercompany balances and transactions have been eliminated upon consolidation.
Cash and Cash Equivalents and Short-term restricted Cash and Cash Equivalents
The Company considers all highly liquid certificates of deposits with original maturities of three months or less from the purchase date to be cash equivalents. Cash equivalents consist primarily of amounts invested in money market accounts in the United States and are stated at carrying value which approximate their fair values. Restricted Cash and Cash Equivalents consist of a bank deposit accounts that serves as collateral for a credit card agreement and lease agreements at one of the Company’s financial institutions.
Property and Equipment, Net
Property and equipment are stated at cost, less accumulated depreciation. Depreciation is calculated on a straight-line basis over the estimated useful lives of the related assets, at the following annual rates:
Schedule of Property and Equipment Estimated Useful Lives
| Computers and Software | 33 | % | ||
| Lab Equipment | 15 | % | ||
| Office Furniture and Equipment | 7-9 | % |
Leasehold improvements are amortized on a straight-line basis over the shorter of the assets’ estimated useful life (10%) or the remaining term of the lease (10-50%).
Maintenance and repair costs are expensed as incurred.
Impairment of Long-Lived Assets
The Company’s long-lived assets are reviewed for impairment in accordance with ASC No. 360, “Property, Plant and Equipment,” whenever events or changes in circumstances indicate that the carrying amount of an asset (assets group) may not be recoverable. If indicators of impairment exist and the undiscounted future cash flows that the assets (assets group) are expected to generate are less than the carrying value of the assets (assets group), the Company reduces the carrying amount of the assets through an impairment charge, to their estimated fair values. During the years ended December 31, 2023, and 2022, no impairment loss have been recorded.
Accrued Post-Employment Benefit
Under Israeli employment laws, employees of the Company are included under Section 14 of the Severance Compensation Act, 1963 (“Section 14”) for a portion of their salaries. According to Section 14, these employees are entitled to monthly payments made by the Company on their behalf with insurance companies.
Payments in accordance with Section 14 release the Company from any future severance payments with respect to those employees. The obligation to make the monthly deposits is expensed as incurred. In addition, the aforementioned deposits are not recorded as an asset in the consolidated balance sheet, and there is no liability recorded as the Company does not have a future obligation to make any additional payments. Severance costs amounted to approximately $0.2 million and $0.3 million for the years ended December 31, 2023 and 2022, respectively.
The Company maintains a 401(k) retirement savings plan for its U.S. employees. Each eligible employee may elect to contribute a portion of the employee’s compensation to the plan. As of December 31, 2023, and 2022, the Company has matched 100% of all employee contributions, up to 6% of the employee’s base salary.
| F-9 |
Leases
The Company’s leases include offices for its facilities, as well as car leases, which are all classified as operating leases. Short-term leases with a term of 12 months or less are not recorded on the balance sheet. The Company does not separate lease components from non-lease components.
At the inception of an arrangement, the Company determines whether the arrangement is or contains a lease. Operating lease liabilities and their corresponding right-of-use assets are recorded at commencement date. The Company records lease liabilities based on the present value of lease payments over the lease term. The ROU asset also includes any lease payments made and excludes lease incentives. The Company generally uses an incremental borrowing rate to discount its lease liabilities, as the rate implicit in the lease is typically not readily determinable. Certain lease agreements include renewal options that are under the Company’s control. The Company includes optional renewal periods in the lease term only when it is reasonably certain that The Company will exercise its option.
Certain lease agreements contain variable payments, which are expensed as incurred and not included in the operating lease right-of-use (“ROU”) assets and liabilities.
Fair Value of Financial Instruments:
The Company measures and discloses the fair value of financial assets and liabilities in accordance with ASC Topic 820, “Fair Value Measurement.” Fair value is based on the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs.
The accounting standard establishes a fair value hierarchy that prioritizes observable and unobservable inputs used to measure fair value into three broad levels, which are described below:
Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs.
Level 2: Observable inputs that are based on inputs not quoted on active markets but corroborated by market data.
Level 3: Unobservable inputs are used when little or no market data are available.
Restricted Cash and Cash Equivalent, trade receivables, trade payables are stated at their carrying value, which approximates fair value due to the short time to the expected receipt or payment date.
Research and Development
Research and development costs are expensed as incurred. Research and development costs include payroll and personnel expenses, consulting costs, external contract research and development expenses, raw materials, drug product manufacturing costs, and allocated overhead including depreciation, rent, and utilities. Research and development costs that are paid in advance of performance are classified as a prepaid expense and amortized over the service period as the services are provided.
Clinical Trial Costs
Clinical trial costs are a component of research and development expenses. The Company bases its expenses related to Clinical Research Organization (“CRO”) on the services received, and efforts expanded pursuant to agreements with them. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. For reoccurring services fees, the Company records the services over the time period which services will are based on inputs received from CRO. If the actual timing of the performance of services varies from the calculation, the Company adjusts the accrual or amount of prepaid expenses accordingly to adjust for such changes in time.
| F-10 |
Patent Costs
Legal and related patent costs are expensed as incurred as their realization is uncertain. Costs related to patent registration are classified as general and administrative expenses, and costs related to acquired patents are classified as research and development expenses in the accompanying consolidated statements of operations.
Contingent Liabilities
The Company accounts for its contingent liabilities in accordance with ASC No. 450, “Contingencies”. A provision is recorded when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated. With respect to legal matters, provisions are reviewed and adjusted to reflect the impact of negotiations, estimated settlements, legal rulings, advice of legal counsel and other information and events pertaining to a particular matter. See Note 7.
Income Taxes
The Company accounts for income taxes in accordance with ASC 740, “Income Taxes”. This standard prescribes the use of the liability method whereby deferred tax asset and liability account balances are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance, if necessary, to reduce deferred tax assets to their estimated realizable value, if it is more likely than not that some portion of the entire deferred tax asset will not be realized.
The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740-10, “Income Taxes”. Accounting guidance addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the consolidated financial statements, under which a Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position.
The tax benefits recognized in the consolidated financial statements from such a position should be measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. Although the Company believes that it has adequately reserved for its uncertain tax positions (including net interest and penalties), it can provide no assurance that the final tax outcome of these matters will not be materially different. The Company makes adjustments to these reserves when facts and circumstances change, such as the closing of a tax audit or the refinement of an estimate. To the extent that the final tax outcome of these matters is different from the amounts recorded, such differences will affect the income tax expense in the period in which such determination is made.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash and cash equivalents. Bank deposits are held by accredited financial institutions and these deposits may at times be in excess of insured limits. Money Market funds are of Prime A and only invested in government issued securities. The Company limits its credit risk associated with cash and cash equivalents by placing them with financial institutions that it believes are of high-quality credit rating. The Company has not experienced any losses on its deposits of cash or cash equivalents.
The Company did not have any customers as of December 31, 2023. The Company’s trade receivables as of December 31, 2022 were from one customer. In addition, the potential risk of loss with any one counterparty resulting from this type of credit risk is monitored on an ongoing basis.
Stock-Based Compensation
The Company measures its stock-based payment awards made to employees, directors, and non-employee service providers based on estimated fair values. The fair value of each option award is estimated on the grant date using the Black-Scholes option pricing model. The Company recognizes compensation expenses based on the accelerated method over the requisite service period. The Company recognizes forfeitures of awards as they occur.
The Black-Scholes option pricing model requires a number of assumptions, of which the most significant are share price, expected volatility, expected option term (the time from the grant date until the options are exercised or expire), risk-free rate, and expected divided rate. After the IPO, the fair value of each ordinary share was based on the closing price of the Company’s publicly traded ordinary shares as reported on the date of the grant.
| F-11 |
Expected volatility
As the Company has a short trading history for its ordinary shares, the expected volatility is derived from the average historical share volatilities of several unrelated public companies within the Company’s industry that the Company considers to be comparable to its own business over a period equivalent to the option’s expected term.
Expected Dividend Yield
The Company has historically not paid dividends and has no foreseeable plans to pay dividends, and therefore the Company uses an expected dividend yield of %.
Risk-Free Interest Rate
The risk-free interest rate is based on the yield from U.S. Treasury zero-coupon bonds with an equivalent expected term.
Expected TermThe expected option term is calculated for options granted to employees and directors using the “simplified” method. Under this approach, the expected term is presumed to be the midpoint between the weighted average vesting term and the contractual term of the option. The simplified method makes the assumption that the employee will exercise share options evenly over the period when the share options are vested and ending on the date when the share options expire. The expected option term for options granted to non-employees is based on the contractual term. Changes in the determination of each of the inputs can affect the fair value of the share options granted and the results of operations of the Company.
Restricted shares are valued at the fair value of shares on the date of grant.
Basic loss per share is computed by dividing the net loss by the weighted average number of shares of Common Stock outstanding during the period. Diluted loss per share is computed by dividing the net loss by the weighted average number of shares of Common Stock outstanding together with the number of additional shares of Common Stock that would have been outstanding if all potentially dilutive shares of Common Stock had been issued. Diluted net loss per share is the same as basic net loss per share in periods when the effects of potentially dilutive shares of Common Stock are anti-dilutive.
Business combinations
The Company accounts for business combinations by applying the provisions of ASC 805, Business Combination (“ASC 805”) and allocates the fair value of the purchase consideration to the tangible and intangible assets acquired and liabilities assumed based on their estimated fair values. The excess of the fair value of purchase consideration over the fair value of these identifiable assets and liabilities is recorded as goodwill. When determining the fair value of assets acquired and liabilities assumed, management makes significant estimates and assumptions, especially with respect to intangible assets.
Acquisition-related expenses are expensed as incurred.
Goodwill and acquired intangible assets
Goodwill and acquired intangible assets recorded in the Company’s financial statements result from both business combinations. Goodwill represents the excess of the purchase price in a business combination over the fair value of identifiable tangible and intangible assets acquired and liabilities assumed. Goodwill is not amortized as it is estimated to have an indefinite life. As such, goodwill is subject to an annual impairment test.
The Company allocates goodwill to reporting units based on the expected benefit from the business combination. Reporting units are evaluated when changes in the Company’s operating structure occur, and if necessary, goodwill is reassigned using a relative fair value allocation approach. The Company operates in one operating segment, and this segment is the only reporting unit.
ASC 350, Intangibles—Goodwill and Other (“ASC 350”) requires goodwill to be tested for impairment at least annually or more frequently if events or changes in circumstances indicate that goodwill may be impaired. The Company elects to perform an annual impairment test of goodwill as of December 31 of each year. The accounting guidance gives the option to perform a qualitative assessment to determine whether further impairment testing is necessary. The qualitative assessment considers events and circumstances that might indicate that a reporting unit’s fair value is less than its carrying amount. If it is determined, as a result of the qualitative assessment, that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, a quantitative test is performed.
During the last quarter of 2023, the Company experienced a significant decline in the stock price, which might suggest the fair value of the reporting unit is less than its carrying amount. However, when the Company performed its annual goodwill impairment test on December 31, 2023, it was noted that the reporting unit have a negative carrying amount. The Company concluded that no goodwill impairment should be recorded since after performing a quantitative test, the reporting unit’s fair value is not less than its carrying amount.
Separately acquired intangible assets are measured on initial recognition at cost including directly attributable costs. Intangible assets acquired in a business combination are measured at fair value at the acquisition date. Acquired identifiable finite-lived intangible assets are amortized on a straight-line basis over the estimated useful life of the respective asset. Each period the Company evaluates the estimated remaining useful lives of its intangible assets and whether events or changes in circumstances warrant a revision to the remaining period of amortization. Acquired indefinite-lived intangible assets are not amortized but are tested for impairment at least annually or more frequently if events or changes in circumstances indicate that the intangible asset may be impaired.
| F-12 |
Segment Information
Financial information is available for evaluation by the chief operating decision maker, the Company’s Chief Executive Officer, in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business in one operating segment. Operating segments are defined as components of an enterprise in which separate financial information is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and assessing performance.
The geographical regions of the Company’s intangible assets are as follows:
Schedule of Geographical Region of Long-Lived Assets
| 2023 | 2022 | |||||||
| Year ended | ||||||||
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Israel | 3,800 | - | ||||||
| United States | 98 | - | ||||||
| Total | 3,898 | - | ||||||
| Total intangible assets | 3,898 | - | ||||||
The geographical regions of the Company’s long lived asset including rights of use are as follows:
| Year ended | Year ended | |||||||
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Israel | 600 | 2,392 | ||||||
| United States | 42 | 30 | ||||||
| Total | 642 | 2,422 | ||||||
| Total rights of use of assets and property and equipment | 642 | 2,422 | ||||||
All of the Company’s revenues are generated in the United States.
Schedule of Geographical Region of Revenues
Revenue Recognition
The Company recognizes revenue in accordance with ASC Topic 606, Revenue from Contracts with Customers, which applies to all contracts with customers. Under Topic 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration that the entity expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that an entity determines are within the scope of Topic 606, the entity performs the following five steps:
| (i) | identify the contract(s) with a customer; |
| (ii) | identify the performance obligations in the contract; |
| (iii) | determine the transaction price; |
| (iv) | allocate the transaction price to the performance obligations in the contract; and |
| (v) | recognize revenue when (or as) the entity satisfies a performance obligation. |
At contract inception, once the contract is determined to be within the scope of Topic 606, the Company assesses the goods or services promised within the contract and determines those that are performance obligations and assesses whether each promised good or service is distinct.
Customer option to acquire additional goods or services gives rise to a performance obligation in the contract only if the option provides a material right to the customer that it would not receive without entering into that contract.
In a contract with multiple performance obligations, the Company develop estimates and assumptions that require judgment to determine the underlying stand-alone selling price for each performance obligation, which determines how the transaction price is allocated among the performance obligations.
The Company evaluates each performance obligation to determine if it can be satisfied at a point in time or over time.
Revenue is recognized when control of the promised goods or services is transferred to the customers, in an amount that reflects the consideration the Company expect to be entitled to receive in exchange for those goods or services.
| F-13 |
Recently Adopted Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13 (Topic 326), Financial Instruments—Credit Losses: Measurement of Credit Losses on Financial Instruments, which replaces the existing incurred loss impairment model with an expected credit loss model and requires a financial asset measured at amortized cost to be presented at the net amount expected to be collected. The new guidance was effective for the Company on January 1, 2023 and the adoption did not have a material impact on the Company’s consolidated financial statements.
Recently Issued Accounting Pronouncements Not Yet Adopted
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires public entities to disclose information about their reportable segments’ significant expenses and other segment items on an interim and annual basis. Public entities with a single reportable segment are required to apply the disclosure requirements in ASU 2023-07, as well as all existing segment disclosures and reconciliation requirements in ASC 280 on an interim and annual basis. ASU 2023-07 is effective for fiscal years beginning after December 15, 2023, and for interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. The Company is currently evaluating the impact of adopting ASU 2023-07.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which requires public entities, on an annual basis, to provide disclosure of specific categories in the rate reconciliation, as well as disclosure of income taxes paid disaggregated by jurisdiction. ASU 2023-09 is effective for fiscal years beginning after December 15, 2024, with early adoption permitted. The Company is currently evaluating the impact of adopting ASU 2023-09.
3. Mergers
Merger with Ayala Pharmaceuticals, Inc.
On October 18, 2022, the Company, which at the time was named Advaxis, Inc., entered into a Merger Agreement (the “Merger Agreement”), with an entity then known as Ayala Pharmaceuticals, Inc. (which shortly prior to the closing of the merger in January 2023 changed its name to Old Ayala, Inc., (“Old Ayala”) and Doe Merger Sub, Inc. (“Merger Sub”), a direct, wholly-owned subsidiary of the Company. Under the terms of the Merger Agreement, Merger Sub merged with and into Old Ayala, with Old Ayala continuing as the surviving company and a wholly-owned subsidiary of the Company (the “January 2023 Merger”). Immediately after the January 2023 Merger, former Advaxis stockholders as of immediately prior to the Merger own approximately 37.5% of the outstanding shares of the combined Company and former Old Ayala shareholders own approximately 62.5% of the outstanding shares of the combined Company.
At the effective time of the January 2023 Merger (the “Effective Time”), each share of share capital of Old Ayala issued and outstanding immediately prior to the Effective Time was converted into the right to receive a number of shares of the Company’s common stock, par value $ per share, equal to the exchange ratio, shares of the Company’s common stock per Old Ayala share.
The January 2023 Merger has been accounted for as a reverse merger with Old Ayala as the accounting acquirer and former Advaxis as the accounting acquiree. In identifying Old Ayala as the accounting acquirer, the companies considered ASC 805-10-55 including the structure of the January 2023 Merger, relative outstanding share ownership at closing and the composition of the combined Company’s board of directors and senior management. The financial reporting reflects the accounting from the perspective of Old Ayala (“accounting acquirer”), except for the legal capital, which has been retroactively adjusted to reflect the capital of former Advaxis (“accounting acquiree”) in accordance with ASC 805-40-45. As such, the historical financial information presented is that of Old Ayala as the accounting acquirer in the January 2023 Merger.
Because most of the value of the assets of former Advaxis was in cash and cash equivalents, the January 2023 Merger is treated primarily as a financing transaction for accounting purposes with a small component as a business acquisition. Therefore, no gain or loss is recorded as a result of the January 2023 Merger. Old Ayala’s transaction costs were capitalized and offset against the shareholder’s equity upon the January 2023 Merger, and former Advaxis’ transaction costs were expensed as merger costs. The consolidated financial statements from the closing date of the January 2023 Merger include the assets, liabilities, and results of operations of the combined company.
| F-14 |
Fair Value Allocation
The following sets forth the fair value of acquired identifiable assets and assumed liabilities of former Advaxis which includes adjustments to reflect the fair value of intangible assets acquired (in thousands) as of January 19, 2023:
Schedule of fair value of Intangible Assets Acquired
| Amounts | ||||
| Cash and cash equivalents | $ | 22,539 | ||
| Prepaid expenses and other current assets | 300 | |||
| Property and equipment, net | 34 | |||
| Intangible assets | 130 | |||
| Operating right-of-use asset | 5 | |||
| Other assets | 11 | |||
| Goodwill | ||||
| Total assets | 23,019 | |||
| SAFE liability | ||||
| Common stock warrant liability | (203 | ) | ||
| Other current liabilities and trade payables | (2,714 | ) | ||
| Total liabilities | (2,917 | ) | ||
| Net assets acquired | $ | 20,102 | ||
The fair value estimate for all identifiable assets and liabilities assumed is preliminary and is based on assumptions that market participants would use in pricing an asset, based on the most advantageous market for the asset (i.e., its highest and best use). This fair value estimate could include assets that are not intended to be used, may be sold, or are intended to be used in a manner other than their best use.
The Company recognized intangible assets related to the January 2023 Merger, which consist of the Patents and License agreements valued at $130 thousand with an estimated useful life of four years. Acquired identifiable finite-lived intangible assets are amortized on a straight-line basis over the estimated useful lives of the assets. The basis of amortization approximates the pattern in which the assets are utilized, over their estimated useful lives. The Company routinely reviews the remaining estimated useful lives of finite-lived intangible assets. In case the Company reduces the estimated useful life for any asset, the remaining unamortized balance is amortized or depreciated over the revised estimated useful life.
The results of operations of Advaxis have been included in the consolidated financial statements since the acquisition date of January 19, 2023.There is no practical way to determine net income attributable to the former Advaxis due to integration.
Merger with Biosight Ltd.
On July 26, 2023, the Company and its wholly owned subsidiary organized under the laws of the State of Israel, Advaxis Israel Ltd. (“Biosight Merger Sub”), entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) with Biosight Ltd. (“Biosight”), a privately-held Israeli pharmaceutical company developing innovative therapeutics for hematological malignancies and disorders. Under the terms of the Merger Agreement, on October 18, 2023, Merger Sub merged with and into Biosight, which is now a wholly owned subsidiary of the Company (the “Biosight Merger”). At completion of the Biosight Merger, Ayala’s then-current equity holders own approximately 45% and the former Biosight equity holders own approximately 55% of Ayala’s common stock.
Based on the agreement, the Company was the legal acquirer in the Biosight Merger. In addition, the Company considered ASC 805-10-55 to determine the accounting acquirer in the Biosight Merger. As the Company holds a majority of the members of the governing body of the combined Company and the Company’s former management dominates the majority of the senior management of the combined Company, and after considering all other factors according to ASC 805-10-55, the Company was identified as the accounting acquirer in the Biosight Merger. The Company has accounted for the Biosight Merger as a business combination according to ASC 805 “Business Combinations”.
| F-15 |
Fair Value Allocation
The following sets forth the fair value of acquired identifiable assets and assumed liabilities of Biosight which includes to reflect the fair value of intangible assets acquired (in thousands) as of October 18, 2023:
Schedule of fair value of Intangible Assets Acquired
| Amounts | ||||
| Cash and cash equivalents | $ | 1,909 | ||
Fixed Assets, net | 64 | |||
| Prepaid expenses and other current assets | 89 | |||
| Intangible assets | 3,800 | |||
| Goodwill | 4,500 | |||
| Total assets | 10,363 | |||
| Side Letter Agreement Liability | (685 | ) | ||
| SAFE liability | (1,068 | ) | ||
| Other current liabilities and trade payables | (3,096 | ) | ||
| Total liabilities | (4,849 | ) | ||
| Net assets acquired | $ | 5,514 | ||
The fair value estimate for all identifiable assets and liabilities assumed and is based on assumptions that market participants would use in pricing an asset, based on the most advantageous market for the asset (i.e., its highest and best use). This preliminary fair value estimate could include assets that are not intended to be used, may be sold, or are intended to be used in a manner other than their best use. Such estimates are subject to change during the measurement period, which is not expected to exceed one year. Any adjustments identified during the measurement period will be recognized in the period in which the adjustments are determined.
The Company recognized intangible assets related to the Biosight Merger, consisting of certain patents and license agreements (“In Process Technology”) valued at $3.8 million. Such assets were fair valued using the discounted cash flow method. Acquired indefinite-lived intangible assets will remain indefinite-lived assets until the completion or abandonment of these assets. The Company also recognized goodwill assets related to the Biosight Merger, valued at $4.5 million with an estimated indefinite useful life. The Company did not recognize any changes as of December 31, 2023, in the value of the Biosight that suggested impairment to both intangible asset and goodwill. The goodwill is not deductible for tax purposes
Acquisition-related transaction costs are not included as components of consideration transferred but are accounted for as expenses in the period in which the costs are incurred. The Company incurred transaction costs of $1.0 million during the year ended December 31, 2023 which were included in general and administrative expenses in the consolidated statements of operations (loss).
The results of operations of Biosight have been included in the consolidated financial statements since the acquisition date of October 18, 2023. There is no practical way to determine net income attributable to Biosight due to integration.
The following unaudited table provides certain pro forma financial information for the Company as if the January 2023 Merger and the Biosight Merger occurred on January 1, 2022 (in thousands except per share amounts):
Schedule of Pro Forma Financial Information
| Year | Year | |||||||
| ended | ended | |||||||
| December 31, | December 31, | |||||||
| 2023 | 2022* | |||||||
| Unaudited | Unaudited | |||||||
| Revenue | $ | 13 | $ | 942 | ||||
| Net loss | $ | (57,344 | ) | $ | (63,011 | ) | ||
| * | The pro forma amounts above are derived from historical numbers of the Company, Old Ayala and Biosight. The results of operations for the year ended December 31, 2022 include the operations of the Company for the period from November 1, 2022 to October 31, 2023 which was the fiscal year 2022 prior to the change in the Company’s fiscal year end from October 31 to December 31, which change was effected in January 2023. There are no such adjustments for Biosight as the fiscal year ended on December 31, 2023. |
The unaudited pro forma results have been prepared based on estimates and assumptions, which we believe are reasonable; however, they are not necessarily indicative of the consolidated results of operations had the acquisition occurred on January 1, 2022, or of future results of operations.
| F-16 |
4. Property and Equipment, net
Property and Equipment, net consists of the following:
Schedule of Property and Equipment, Net
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| (in thousands) | ||||||||
| Cost: | ||||||||
| Computers and Software | $ | 60 | $ | 73 | ||||
| Lab Equipment | 95 | 296 | ||||||
| Office Furniture and Equipment | 92 | 146 | ||||||
| Leasehold Improvements | 1,105 | 1,105 | ||||||
| Property and Equipment, Gross | 1,352 | 1,620 | ||||||
| Less: Accumulated Depreciation | 812 | 660 | ||||||
| Property and Equipment, Net | $ | 540 | $ | 960 | ||||
Depreciation expenses for the years ended December 31, 2023, and 2022 was approximately $334 thousand and $162 thousand, respectively.
During the year ended December 31, 2023 the Company recorded a write down to assets due to inactivity of lab equipment and intention to dispose or sell all of them of $145 thousand.
5. Leases
The Company adopted ASC 842, relating to lease accounting, in the first quarter of 2022 using the modified retrospective method. Results for reporting periods beginning after December 31, 2021, have been presented in accordance with the standard. The cumulative effect of initially applying the new leases standard was recognized as an adjustment to the opening consolidated balance sheet as of January 1, 2022.
The Company elected a package of practical expedients for leases that commenced prior to January 1, 2022, and did not reassess historical conclusions on: (i) whether any expired or existing contracts are or contain leases; (ii) lease classification for any expired or existing leases; and (iii) initial direct costs capitalization for any existing leases.
This standard had a significant impact on the Company’s consolidated balance sheet but did not have a significant impact on the Company’s consolidated statements of operations. The most significant effects relate to the recognition on the consolidated balance sheet of ROU assets and lease liabilities for offices and for car operating leases.
Upon adoption, the Company recognized lease liabilities and corresponding ROU assets, adjusted for the accrued rent and remaining lease incentives received on the adoption date, as follows:
Schedule of Lease Liabilities and ROU Assets
| ROU assets | Lease liabilities | |||||||
| January 1, 2022 | ||||||||
| ROU assets | Lease liabilities | |||||||
| Offices | $ | 1,448 | $ | 2,020 | ||||
| Cars | 302 | 267 | ||||||
| Total operating leases | $ | 1,750 | $ | 2,287 | ||||
In January 2019, the Company signed a new lease agreement. The term of the lease is for 63 months and includes an option to extend the lease for an additional 60 months. As part of the agreement, the lessor also provided the Company an amount of approximately $0.5 million paid in arrears for of leasehold improvements. The amount was recorded as an incentive and is taken into account when computing the ROU asset.
A subsidiary of the Company obtained a bank guarantee in the amount of approximately $0.2 million for the office lease agreement.
On March 25, 2021, the Company entered into a one-year lease agreement for its corporate office/lab with base rent of approximately $29 thousand per year, plus other expenses. In September 2021, the Company exercised its option to renew the lease, extending the lease term until March 31, 2023. On March 25, 2023 the Company signed an extension up to March 31, 2025, with base rent of approximately $36 thousand per year. The Company recorded an ROU asset and liability of approximately $65 thousand.
The Company did not extend the lease for an additional five years, and as such the Company recognized a gain of $238 thousand in the consolidated income statement due to early termination of the lease liabilities.
| F-17 |
The Company has the following operating ROU assets and lease liabilities:
Schedule of Operating Right of Use Assets and Lease Liabilities
| December 31, 2023 | ||||||||
ROU assets | Lease liabilities | |||||||
| Offices | $ | 42 | $ | 134 | ||||
| Cars | 60 | 41 | ||||||
| Total operating leases | $ | 102 | $ | 175 | ||||
| December 31, 2022 | ||||||||
| ROU assets | Lease liabilities | |||||||
| Offices | $ | 1,273 | $ | 1,612 | ||||
| Cars | 189 | 139 | ||||||
| Total operating leases | $ | 1,462 | $ | 1,751 | ||||
December 31, 2023 | December 31, 2022 | |||||||
Lease liabilities | Lease liabilities | |||||||
| Current lease liabilities | $ | 166 | $ | 419 | ||||
| Non-current lease liabilities | 9 | 1,332 | ||||||
| Total lease liabilities | $ | 175 | $ | 1,751 | ||||
The following table summarizes the lease costs recognized in the consolidated statement of operations:
Schedule of Lease Costs
December 31, 2023 | December 31, 2022 | |||||||
| Operating lease cost | $ | 457 | $ | 442 | ||||
| Variable lease cost | 19 | 10 | ||||||
| Total lease cost | $ | 476 | $ | 452 | ||||
As of December 31, 2023, the weighted-average remaining lease term and weighted-average discount rate for operating leases are 0.7 years and 14.16%, respectively.
The following table summarizes the future payments of the Company for its operating lease liabilities:
Schedule of Future Payments Operating Lease Liabilities
December 31, 2023 | ||||
| 2024 | 171 | |||
| 2025 | 9 | |||
| Total undiscounted lease payments | $ | 180 | ||
| Less: Interest | (5 | ) | ||
| Total lease liabilities - operating | $ | 175 | ||
| F-18 |
6. Goodwill and intangible assets, net
Goodwill
The following table represents the changes in the carrying amounts of the Company’s total goodwill:
Schedule of Goodwill
| Carrying | ||||
| Amount | ||||
Even if we are successful in these proceedings, we may incur substantial costs and divert management time and attention in pursuing these proceedings, which could have a material adverse effect on us. If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, develop or obtain non-infringing technology, fail to defend an infringement action successfully or have infringed patents declared valid, we may:
| - | ||||
| Addition from acquisitions | ||||
| 4,500 | ||||
| Balance as of December 31, 2023 | 4,500 |
Intangible assets, net
Schedule of Intangible Assets
December 31, 2023 | December 31, 2023 | |||||||
| Unamortized | Amortized | |||||||
| Cost: | ||||||||
| In Process Technology | $ | 3,800 | $ | 130 | ||||
| Less - accumulated amortization | - | 32 | ||||||
| Intangible assets, net | $ | 3,800 | $ | 98 | ||||
As of December 31, 2023, the Company did not have any acquired intangible assets.
Estimated amortization expense for the years ended:
Schedule of Amortization Expenses
| 2024 | $ | 33 | ||
| 2025 | 33 | |||
| 2026 | 32 | |||
| 2027 | - | |||
| Thereafter | 3,800 | |||
| Total | $ | 3,898 |
Amortization expense of $32 thousand related to intangible assets, net was included in the consolidated statements of operations as Research and Development cost.
For the year ended December 31, 2022, the Company did not have acquired intangible assets.
7. Commitments and Contingent Liabilities
WeAsset Transfer and License Agreement with Bristol-Myers Squibb Company.
In November 2017, the Company entered into a license agreement, or the BMS License Agreement, with Bristol-Myers Squibb Company, or BMS, under which BMS granted the Company a worldwide, non-transferable, exclusive, sublicensable license under certain patent rights and know-how controlled by BMS to research, discover, develop, make, have made, use, sell, offer to sell, export, import and commercialize AL101 and AL102, or the BMS Licensed Compounds, and products containing AL101 or AL102, or the BMS Licensed Products, for all uses including the prevention, treatment or control of any human or animal disease, disorder or condition. On March 25, 2024, we sold our assets and liabilities related to AL101 and AL102 programs to Immunome, and the Company transferred the BMS License Agreement to Immunome as part of such transaction.
Under the BMS License Agreement, we were obligated to use commercially reasonable efforts to develop at least one BMS Licensed Product, and had sole responsibility for, and bear the cost of, conducting research and development and preparing all regulatory filings and related submissions with respect to the BMS Licensed Compounds and/or BMS Licensed Products. BMS has assigned and transferred all INDs for the BMS Licensed Compounds to the Company. The Company is also required to use commercially reasonable efforts to obtain regulatory approvals in certain major market countries for at least one BMS Licensed Product, as well as to effect the first commercial sale of and commercialize each BMS Licensed Product after obtaining such regulatory approval. Under the BMS License Agreement, we had sole responsibility for, and bear the cost of, commercializing BMS Licensed Products. For a limited period of time, the Company may not, engage directly or indirectly in the clinical development or commercialization of a Notch inhibitor molecule that is not a BMS Licensed Compound.
The Company was required to pay BMS payments upon the achievement of certain development or regulatory milestone events of up to $95 million in the aggregate with respect to the first BMS Licensed Compound to achieve each such event and up to $47 million in the aggregate with respect to each additional BMS Licensed Compound to achieve each such event. The Company was also obligated to pay BMS payments of up to $50 million in the aggregate for each BMS Licensed Product that achieves certain sales-based milestone events and tiered royalties on net sales of each BMS Licensed Product by the Company or its affiliates or sublicensees at rates ranging from a high single-digit to low teen percentage, depending on the total annual worldwide net sales of each such Licensed Product. If the Company sublicenses or assigns any rights to the licensed patents, the BMS Licensed Compounds and/or the BMS Licensed Products, the Company is required to share with BMS a portion of all consideration received from such sublicense or assignment, ranging from a mid-teen to mid-double-digit percentage, depending on the development stage of the most advanced BMS Licensed Compound or BMS Licensed Product that is subject to the applicable sublicense or assignment, but such portion may be unablereduced based on the milestone or royalty payments that are payable by the Company to adequately prevent disclosureBMS under the BMS License Agreement.
| F-19 |
The Company accounted for the acquisition of trade secretsthe rights granted by BMS as an asset acquisition because it did not meet the definition of a business. The Company recorded the total consideration transferred and other proprietary information.value of shares issued to BMS as research and development expense in the consolidated statement of operations as incurred since the acquired the rights granted by BMS represented in-process research and development and had no alternative future use.
The Company accounts for contingent consideration payable upon achievement of sales milestones in such asset acquisitions when the underlying contingency is resolved.
Both we and BMS had the right to terminate the BMS License Agreement in its entirety upon written notice to the licensee under certain circumstances described therein.
We also rely on trade secrets
On March 25, 2024, the Company sold the assets and liabilities related to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficultthe AL101 and AL102 programs to protect. We rely inImmunome, and the Company transferred the BMS License Agreement to Immunome as part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.such transaction.
We are dependent upon ourExclusive worldwide license agreement with Penn; if we breach the license agreement and/or fail to make payments due and owing to Penn under our license agreement, our business will be materially and adversely affected.Penn.
Pursuant to the terms of ourThe Company entered into an exclusive worldwide license agreement with Penn, whichon July 1, 2002 with respect to the innovative work of Yvonne Paterson, Ph.D., Associate Dean for Research at the School of Nursing at Penn, and former Professor of Microbiology at Penn, in the area of innate immunity, or the immune response attributed to immune cells, including dendritic cells, macrophages and natural killer cells, that respond to pathogens non-specifically (subject to certain U.S. government rights). This agreement was amended and restated as of February 13, 2007, and, thereafter, has been amended from time to time, we have acquired exclusive worldwide licenses for patents andtime.
This license, unless sooner terminated in accordance with its terms, terminates upon the latter of (a) the expiration of the last to expire of the Penn patent applications relatedrights; or (b) twenty years after the effective date of the license. Penn may terminate the license agreement early upon the occurrence of certain defaults by the Company, including, but not limited to, our proprietary Listeria vaccine technology. a material breach by the Company of the Penn license agreement that is not cured within 60 days after notice of the breach is provided to the company.
The license provides usthe Company with the exclusive commercial rights to the patent portfolio developed atby Penn as of the effective date of the license, in connection with Dr. Paterson and requires usthe Company to pay various milestone, legal, filing and licensing payments to commercialize the technology. AsIn exchange for the license, Penn received shares of Octoberour Common Stock. In addition, Penn is entitled to receive a non-refundable initial license fee, royalty payments and milestone payments based on net sales and percentages of sublicense fees and certain commercial milestones. Under the amended licensing agreement, Penn is entitled to receive 2.5% of net sales in the territory. Should annual net sales exceed $250 million, the royalty rate will increase to 2.75%, but only with respect to those annual net sales in excess of $250 million. Additionally, Penn will receive tiered sales milestone payments upon the achievement of cumulative global sales ranging between $250 million and $2 billion, with the maximum aggregate amounts payable to Penn in the event that maximum sales milestones are achieved is $40 million. Notwithstanding these royalty rates, upon first in-human commercial sale (U.S. & E.U.), we have agreed to pay Penn a total of $775 thousand over a four-year period as an advance minimum royalty, which shall serve as an advance royalty in conjunction with the above terms. In addition, under the license, we are obligated to pay an annual maintenance fee of $100 thousand commencing on December 31, 2017, we had no outstanding payments2010, and each December 31st thereafter for the remainder of the term of the agreement until the first commercial sale of a Penn licensed product. We are responsible for filing new patents and maintaining and defending the existing patents licensed to the Company are obligated to reimburse Penn for all attorney’s fees, expenses, official fees and other charges incurred in the preparation, prosecution and maintenance of the patents licensed from Penn. We can provide no assurance that we
Upon first regulatory approval in humans (US or EU), Penn will be ableentitled to make all future payments due and owing thereunder, that such licenses will not be terminated or expire during critical periods, that wea milestone payment of $600,000. Furthermore, upon the achievement of the first sale of a product in certain fields, Penn will be ableentitled to obtain licenses from Penn for other rights that may be important to us, or, if obtained, that such licensescertain milestone payments, as follows: $2.5 million will be obtained on commercially reasonable terms. The lossdue upon the first in-human commercial sale (US or EU) of any currentthe first product in the cancer field and $1.0 million will be due upon the date of first in-human commercial sale (US or future licenses from Penn orEU) of a product in each of the exclusivity rights provided therein could materially harm our business, financial condition and operating results.secondary strategic fields sold.
If we are unable to obtain licenses neededOS Therapies LLC
On September 4, 2018, the Company entered into a development, license and supply agreement with OS Therapies (“OST”) for the use of ADXS31-164, also known as ADXS-HER2, for evaluation in the treatment of osteosarcoma in humans. Under the terms of the license agreement, as amended, OST is responsible for the conduct and funding of a clinical study evaluating ADXS-HER2 in recurrent, completely resected osteosarcoma. Under the most recent amendment to the licensing agreement, the Company will initiate the transfer of the intellectual property and licensing rights of ADXS31-164, which were licensed pursuant to the Penn Agreement, back to the University of Pennsylvania. Contemporaneously, OST will enter negotiations with the University of Pennsylvania to establish a licensing agreement for ADXS31-164 to OST for clinical and commercial development of our product candidates, or if we breach any of the agreements under which we license rights to patents or other intellectual property from third parties, we could lose license rights that are important to our business.ADXS31-164 technology.
If we are unable to maintain and/or obtain licenses needed for the development of our product candidates in the future, we may have to develop alternatives to avoid infringing on the patents of others, potentially causing increased costs and delays in drug development and introduction or precluding the development, manufacture, or sale of planned products. Some of our licenses provide for limited periods of exclusivity that require minimum license fees and payments and/or may be extended only with the consent of the licensor. We can provide no assurance that we will be able to meet these minimum license fees in the future or that these third parties will grant extensions on any or all such licenses. This same restriction may be contained in licenses obtained in the future.
| F-20 |
Additionally, we can provide no assurancePurported Stockholder Claims
Purported Stockholder Claims Related to January 2023 Merger with Old Ayala
On December 15, 2022, a purported stockholder of Old Ayala filed a complaint in the U.S. District Court for the Southern District of New York against Old Ayala and the members of its Board, captioned Stephen Bushansky v. Ayala Pharmaceuticals, Inc., Case No.1:22-cv-10621 (S.D.N.Y.) (the “Complaint”).
The Complaint asserts claims against all defendants under Section 14(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and Rule 14a-9 promulgated thereunder for omitting or misrepresenting material information from Old Ayala’s Proxy Statement and against the individual defendants under Section 20(a) of the Exchange Act for alleged “control person” liability with respect to such alleged omissions and misrepresentations. The allegations in the Complaint include that the patents underlying any licenses will be validProxy Statement omitted material information regarding Old Ayala’s financial projections and enforceable. To the extent any products developed by us are based on licensed technology, royalty paymentsfinancial analyses of Old Ayala’s financial advisor for the January 2023 Merger. The Complaint seeks, among other relief, (1) to enjoin defendants from consummating the January 2023 Merger; (2) to enjoin a vote on the licenses will reduce our gross profit from such product salesJanuary 2023 Merger; (3) to rescind the January 2023 Merger Agreement or recover damages, if the Merger is completed; (4) a declaration that defendants violated Sections 14(a) or 20(a) and may renderRule 14a-9 of the sales of such products uneconomical. In addition, the loss of any current or future licenses or the exclusivity rights provided therein could materially harm our business financial conditionExchange Act; and our operations.(5) attorneys’ fees and costs. The complaint was never served on all defendants.
We have limitedIn addition, approximately nine purported stockholders of Old Ayala sent letters to no manufacturing, sales, marketing or distribution capabilitythose noted in the above-referenced Complaint alleging similar deficiencies in Old Ayala’s Proxy Statement (collectively, the “Demand Letters”).
A final settlement agreement relating to these claims was entered into on March 21, 2024, and we must rely upon third parties for such.in connection therewith, the Company has accrued $200 thousand of expense.
We currently have agreementsStockholder letter with various third party manufacturing facilities for productionregards to breaches of manyfiduciary duty
On March 6, 2024, a stockholder of our immunotherapies for researchAyala Pharmaceuticals, Inc. (the “Company”), submitted a letter (the “Letter”) threatening legal action against the Company and developmentalleging breaches of fiduciary duty in connection with the Company’s January 9, 2023 merger with Advaxis, Inc., the Company’s October 19, 2023 merger with Biosight, Ltd., the Company’s November 17, 2023 issuance of Senior Convertible Promissory Notes and testing purposes. While we have built our own manufacturing facility onsite in Princeton to manufacture clinical materials for some of our products, included ADXS-NEO, we depend on third-party manufacturers to supply most of our preclinical and clinical materials and will be reliant on a third-party manufacturer to produce axalimogene filolisbac on a commercial scale, should that product receive regulatory approval. Third-party manufacturers must be able to meet our deadlines as well as adhere to quality standards and specifications. Our predominant reliance on third partieswarrants for the manufacturepurchase of our drug substance, investigational new drugs15,000,000 shares of the Company’s stock, and the Company’s February 5, 2024 Asset Purchase Agreement with Immunone, Inc. The attorney representing the stockholder thereafter made a monetary demand in exchange for a release of the claims asserted in the future, any approved products, creates a dependency that could severely disrupt our research and development, our clinical testing, and ultimately our sales and marketing efforts ifLetter. Settlement negotiations are ongoing.
At this time, the sourceCompany is unable to predict the likelihood of such supply provesan unfavorable outcome with respect to be unreliable or unavailable. If our own manufacturing operation or any contracted manufacturing operation is unreliable or unavailable, we may not be able to manufacture clinical drug supplies of our immunotherapies, and our preclinical and clinical testing programs may not be able to move forward and our entire business plan could fail. If we are able to commercialize our products in the future, there is no assurance that our own manufacturing operation or any third-party manufacturers will be able to meet commercialized scale production requirements in a timely manner or in accordance with applicable standards or current GMP.Demand Letter.
8. Common Stock Purchase Warrants and Warrant Liability
If we are unable to establish or manage strategic collaborations inCommon Stock Rights
The Common Stock Rights confer upon the future, our revenue and drug development may be limited.
Our strategy includes eventual substantial reliance upon strategic collaborations for marketing and commercialization of our clinical product candidates, and we may rely even more on strategic collaborations for research, development, marketing and commercialization for some of our immunotherapies. To date, we have been heavily reliant upon third party outsourcing for our clinical trials execution and production of drug supplies for use in clinical trials. Establishing strategic collaborations is difficult and time-consuming. Our discussions with potential collaborators may not lead to the establishment of collaborations on favorable terms, if at all. For example, potential collaborators may reject collaborations based upon their assessment of our financial, clinical, regulatory or intellectual property position. Our current collaborations, as well as any future new collaborations, may never result in the successful development or commercialization of our immunotherapies or the generation of sales revenue. To the extent that we have entered or will enter into co-promotion or other collaborative arrangements, our product revenues are likely to be lower than if we directly marketed and sold any products that we may develop.
Management of our relationships with our collaborators will require:
If we continue to enter into research and development collaborations at the early phases of drug development, our success will in part depend on the performance of our corporate collaborators. We will not directly control the amount or timing of resources devoted by our corporate collaborators to activities related to our immunotherapies. Our corporate collaborators may not commit sufficient resources to our research and development programs or the commercialization, marketing or distribution of our immunotherapies. If any corporate collaborator fails to commit sufficient resources, our preclinical or clinical development programs related to this collaboration could be delayed or terminated. Also, our collaborators may pursue existing or other development-stage products or alternative technologies in preference to those being developed in collaboration with us. Finally, if we fail to make required milestone or royalty payments to our collaborators or to observe other obligations in our agreements with them, our collaborators may haveholders the right to terminate those agreements.vote in annual and special meetings of the Company, and to participate in the distribution of the surplus assets of the Company upon liquidation of the Company.
We may incur substantial liabilities from any product liability claims if our insurance coverage for those claims is inadequate.Warrants
We face an inherent riskAs of product liability exposure relatedDecember 31, 2023 there were 22,965,771 warrants outstanding of which 22,790,706 were exercisable warrants to the testingpurchase shares of our immunotherapies in human clinical trials,common stock, with exercise prices ranging from $to $per share. As of December 31, 2022, there were outstanding and will face an even greater risk ifexercisable warrants to purchase 337,320 shares of our common stock with exercise prices ranging from $ to $per share. Information on the approved products are sold commercially. An individual may bring a liability claim against us if oneoutstanding warrants as of the immunotherapies causes, or merely appears to have caused, an injury. If we cannot successfully defend ourselves against the product liability claim, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:December 31, 2023 is as follows:
Schedule Of Warrants Outstanding
| Number of | ||||||||||
| Shares | ||||||||||
| Underlying | ||||||||||
| Exercise Price | Warrants | Expiration Date | Type of Financing | |||||||
| $ | 2.79 | 879 | September 2024 | September 2018 Public Offering | ||||||
| $ | 224.00 | 4,092 | July 2024 | July 2019 Public Offering | ||||||
| $ | 28.00 | 57,230 | November 2025 | November 2020 Public Offering | ||||||
| $ | 56.00 | 140,552 | April 2026 | April 2021 Registered Direct Offering (Accompanying Warrants) | ||||||
| $ | 56.00 | 175,065 | 5 years after the date such warrants become exercisable, if ever | April 2021 Private Placement (Private Placement Warrants) | ||||||
| $ | 96.58 | 87,453 | * | February 2024 | February 2021 Private Placement (issued by Old Ayala) | |||||
| $ | 0.34 | 22,500,500 | ** | November 2028 | November 17, 2023 Financing | |||||
| Grand Total | 22,965,771 | |||||||||
| F-21 |
We have Product Liability and Clinical Trial Liability insurance coverage
On November 17, 2023, the Company issued securities convertible into or exercisable for each clinical trial. We do not have product liability insurance for sold commercial products because we do not have products on shares of common stock as part of the market. We currently are in the process of obtaining insurance coverage and plan to expand such coverage to include the sale of commercial products if marketing approval is obtained for any of our immunotherapies. However, insurance coverage is increasingly expensive and we may not be able to maintain insurance coverage at a reasonable cost and we may not be able to obtain insurance coverage that will be adequate to satisfy any liability that may arise.Convertible Loans. See Convertible Loan bellow.
We may incur significant costs complying with environmental laws and regulations.
We and our contracted third parties use hazardous materials, including chemicals and biological agents and compounds that could be dangerous to human health and safety or the environment. As appropriate, we store these materials and wastes resulting from their use at our or our outsourced laboratory facility pending their ultimate use or disposal. We contract with a third party to properly dispose of these materials and wastes. We are subject to a variety of federal, state and local laws and regulations governing the use, generation, manufacture, storage, handling and disposal of these materials and wastes. Compliance with such laws and regulations may be costly.
If we use biological materials in a manner that causes injury, we may be liable for damages.
Our research and development activities involve the use of biological and hazardous materials. Although we believe our safety procedures for handling and disposing of these materials complies with federal, state and local laws and regulations, we cannot entirely eliminate the risk of accidental injury or contamination from the use, storage, handling or disposal of these materials. We do not carry specific biological waste or pollution liability or remediation insurance coverage, nor do our workers’ compensation, general liability, and property and casualty insurance policies provide coverage for damages and fines/penalties arising from biological exposure or contamination. Accordingly, in the event of contamination or injury, we could be held liable for damages or penalized with fines in an amount exceeding our resources, and our clinical trials or regulatory approvals could be suspended or terminated.
We need to attract and retain highly skilled personnel; we may be unable to effectively manage growth with our limited resources.
As of December 15, 2017, we31, 2023, the Company had 108 employees,289,327 of its total 22,965,771 outstanding warrants classified as equity. As of December 31, 2022, all outstanding warrants were classified as equity. At issuance, equity warrants are recorded in additional paid-In capital.
A summary of whichwarrant activity for the year ended December 31, 2023 was as follows (in thousands, except share and per share data):
| Shares | Weighted Avg. Exercise Price | Weighted Avg. Remaining Contractual Life in Years | Aggregate Intrinsic Value | |||||||||||||
| Outstanding and Exercisable Warrants at December. 31 2022 | 337,320 | $ | 25.08 | $ | 144,083 | |||||||||||
| Merged Warrants | 377,818 | $ | 53.45 | |||||||||||||
| Issued as part of November 17 financing | 22,500,500 | $ | 0.34 | |||||||||||||
| Exercised | (249,867 | ) | $ | 0.05 | ||||||||||||
| Outstanding Warrants at December 31, 2023 | 22,965,771 | $ | 1.58 | $ | 7,537,668 | |||||||||||
| Exercisable Warrants at December 31, 2023 | 22,790,706 | $ | 1.15 | $ | 7,537,668 | |||||||||||
| * | Exercise price and warrant numbers have been retroactively adjusted for the impact of the January 2023 Merger, see note 1. | |
| ** | See details under Warrant Liability Senior Convertible Notes for information on the exercise price |
Shares Issued for Warrants Exercises
During the year ended December 31, 2023, warrants were full time employees. Our ability to attract and retain highly skilled personnel is critical to our operations and expansion. We face competitionexercised in exchange for these types shares of personnel from other technology companies and more established organizations, many of which have significantly larger operations and greater financial, technical, human and other resources than we have. We may not be successful in attracting and retaining qualified personnelthe Company’s common stock on a timely basis, on competitive terms, or at all. If we are not successful in attracting and retaining these personnel, or integrating them into our operations, our business, prospects, financial condition and results of operations will be materially adversely affected. In such circumstances we may be unable to conduct certain research and development programs, unable to adequately manage our clinical trials and other products, unable to commercialize any products, and unable to adequately address our management needs.cash less exercise basis.
We depend upon our senior management and key consultants and their loss or unavailability could put us at a competitive disadvantage.Convertible Note
We depend uponFollowing the efforts and abilities of our senior executives, as well as the services of several key consultants. The loss or unavailabilityconsummation of the servicesJanuary 2023 Merger, management of anythe Company, in consultation with the Board, determined that the Company would require additional financing to further the development of these individuals for any significant period of time could have a material adverse effect on our business, prospects, financial condition and results of operations. We have not obtained, do not own, nor are we the beneficiary of, key-person life insurance.
The biotechnology and immunotherapy industries are characterized by rapid technological developments and a high degree of competition. We may be unable to compete with more substantial enterprises.
The biotechnology and biopharmaceutical industries are characterized by rapid technological developments and a high degree of competition.Old Ayala’s late-stage program. As a result, our actual or proposed immunotherapies could become obsolete before we recoup any portionthe Company continued to pursue potential financing alternatives. However, despite significant efforts in this regard over a number of our related researchmonths, the Company was not able to find such feasible financing alternatives.
On August 7, 2023, having concluded that there were at that time no other readily available alternatives, and developmentin order to obtain temporary financing as it pursued its ongoing efforts to achieve longer-term financing, the Company entered into an agreement for the issuance of Senior Secured Convertible Promissory Notes (the “Secured Notes”) to Israel Biotech Fund I, L.P. The Secured Notes provided for the borrowing by the Company of up to $2.0 million dollars, which borrowings which the Company received on September 1, 2023.
On November 17, 2023, having once again concluded that there were at that time no other readily available alternatives, and commercialization expenses. Competitionin order to obtain temporary financing as it pursued its ongoing efforts to achieve longer-term financing, and having concluded that the Company would not able to survive financially without additional funds the Company issued Senior Convertible Promissory Notes (collectively, the “Senior Convertible Notes”), in an aggregate amount of $4.0 million, to several existing lenders and investors in the biopharmaceutical industry is based significantlyCompany, including Israel Biotech Fund I, L.P., Israel Biotech Fund II, L.P., Arkin Bio Ventures L.P. and Biotel Limited. The amounts borrowed by the Company under the Senior Convertible Notes were funded to the Company on scientificNovember 20, 2023. The Senior Convertible Notes were convertible into shares of the Company’s common stock, par value $ per share (the “Common Stock”) at any time at the option of the noteholders, and technological factors. These factors includewere subject to mandatory conversion upon certain events, including a change of control transaction and certain financing transactions involving the availabilityCompany, at a conversion price equal to the lower of patent(i) 50% of the Common Stock’s price per share as of market close on November 16, 2023 and other protection for technology(ii) 50% of the Common Stock’s price per share as of the close of market on the Trading Day immediately prior to the date of the Notice of Conversion, subject to certain adjustments. In connection with the issuance of the Senior Convertible Notes, the Company issued to the noteholders warrants to purchase an aggregate of 15,000,000 shares of the Common Stock with an exercise price equal to the lower of (A) 50% of the Common Stock’s price per share as of market close on November 16, 2023 and products,(ii) 50% of the abilityCommon Stock’s price per share as of the close of market on the Trading Day immediately prior to commercialize technological developmentsthe date of the Notice of Exercise of the warrant, subject to adjustment, which exercise may be on a cashless basis.
The noteholders also obtained the right, pursuant to a Side Letter Agreement between the noteholders and the abilityCompany, to obtain governmental approval for testing, manufacturing and marketing. We compete with specialized biopharmaceutical firms inlend an additional $4.0 million dollars to the United States, Europe and elsewhere, as well as a growing number of large pharmaceutical companies that are applying biotechnology to their operations. Many biopharmaceutical companies have focused their development efforts in the human therapeutics area, including cancer. Many major pharmaceutical companies have developed or acquired internal biotechnology capabilities or made commercial arrangements with other biopharmaceutical companies. These companies, as well as academic institutions and governmental agencies and private research organizations, also compete with us in recruiting and retaining highly qualified scientific personnel and consultants. Our ability to compete successfully with other companies in the pharmaceutical field will also depend to a considerable degreeCompany on the continuing availability of capital to us.same terms.
| F-22 |
We
The Company has elected the fair value option to measure the Secured Notes and the Senior Convertible Notes upon issuance and conversion, in accordance with ASC 825-10. Under the fair value option, the Secured Notes and the Senior Convertible Notes are awaremeasured at fair value each period with changes in fair value reported in the consolidated statements of certain investigational new drugs under development or approved products by competitorsoperations. According to ASC 825-10, changes in fair value that are used for the prevention, diagnosis, or treatment of certain diseases we have targeted for drug development. Various companies are developing biopharmaceutical products that have the potential to directly compete with our immunotherapies even though their approach to may be different. The biotechnology and biopharmaceutical industries are highly competitive, and this competition comes from both biotechnology firms and from major pharmaceutical companies, including companies like: Aduro Biotech, Agenus Inc., Celldex Therapeutics, Inovio Pharmaceutical Inc., ISA Pharmaceuticals, MedImmune LLC, Neon Therapeutics, Oncolytics Biotech Inc. and Oncothyreon Inc., each of which is pursuing cancer vaccines and/or immunotherapies. Many of these companies have substantially greater financial, marketing, and human resources than we do (including, in some cases, substantially greater experience in clinical testing, manufacturing, and marketing of pharmaceutical products). We also experience competitioncaused by changes in the development of our immunotherapies from universities and other research institutions and compete with others in acquiring technology from such universities and institutions.
In addition, certain of our immunotherapies may be subject to competition from investigational new drugs and/or products developed using other technologies, some of which have completed numerous clinical trials.
We may not obtain or maintain the benefits associated with breakthrough therapy designation.
If we apply for Breakthrough Therapy Designation (“BTD”), we may not be granted BTD, or even if granted, we may not receive the benefits associated with BTD. This may result from a failure to maintain breakthrough therapy status if it is no longer considered to be a breakthrough therapy. For example, a drug’s development program may be granted BTD using early clinical testing that shows a much higher response rate than available therapies. However, subsequent interim data derived from a larger study may show a response that is substantially smaller than the response seen in early clinical testing. Another example is where BTD is granted to two drugs that are being developed for the same use. If one of the two drugs gains traditional approval, the other would not retain its designation unless its sponsor provided evidence that the drug may demonstrate substantial improvement over the recently approved drug. When BTD is no longer supported by emerging data or the designated drug development program is no longer being pursued, the FDA may choose to send a letter notifying the sponsor that the program is no longer designated as a breakthrough therapy development program.
We believe that our immunotherapies under development and in clinical trials will address unmet medical needs in the treatment of cancer. Our competitioninstrument-specific credit risk will be determined in part by the potential indications for which drugs are developed and ultimately approved by regulatory authorities. Additionally, the timing of market introduction of some of our potential products or of competitors’ products may be an important competitive factor. Accordingly, the relative speed with which we can develop immunotherapies, complete preclinical testing, clinical trials and approval processes and supply commercial quantities to market is expected to be important competitive factors. We expect that competition among products approved for sale will be based on various factors, including product efficacy, safety, reliability, availability, price and patent position.
Approval of our product candidates does not ensure successful commercialization and reimbursement.
We are not currently marketing our product candidates, however we are seeking commercial opportunities for axalimogene filolisbac. We cannot assure you that we will be able to commercialize it or any other candidate ourselves or find a commercialization partner or that we will be able to agree to acceptable terms with any partner to launch and commercialize our products.
The commercial success of our product candidates is subject to risks in both the United States and European countries. In addition, in European countries, pricing and payment of prescription pharmaceuticals is subject to more extensive governmental control than in the United States. Pricing negotiations with European governmental authorities can take six to 12 months or longer after the receipt of regulatory approval and product launch. If reimbursement is unavailable in any country in which reimbursement is sought, limited in scope or amount, or if pricing is set at or reduced to unsatisfactory levels, our ability or any potential partner’s ability to successfully commercialize in such a country would be impacted negatively. Furthermore, if these measures prevent us or any potential partner from selling on a profitable basis in a particular country, they could prevent the commercial launch or continued sale in that country and could adversely impact the commercialization market opportunitypresented separately in other countries.
Moreover, as a condition of approval, the regulatory authorities may require that we conduct post-approval studies. Those studies may reveal new safety or efficacy findings regarding our drug that could adversely impact the continued commercialization or future market opportunity in other countries.
In addition, Advaxis predominantly relies on a network of suppliers and vendors to manufacture its products. Should a regulatory authority make any significant findings on an inspection of Advaxis’ own operations or the operations of those companies, the ability of Advaxis to continue producing its products could be adversely impacted and further production could cease. Regulatory GMP requirements are extensive and can present a risk of injury or recall, among other risks, if not manufactured or labeled properly under GMPs.
Our potential revenues from the commercialization of our product candidates are subject to these and other factors, and therefore we may never reach or maintain profitability.
Risks Related to our Securities
The price of our Common Stock and warrants may be volatile.
The trading price of our Common Stock and warrants may fluctuate substantially. The price of our Common Stock and warrants that will prevail in the market may be higher or lower than the price you have paid, depending on many factors, some of which are beyond our control and may not be related to our operating performance. These fluctuations could cause you to lose part or all of your investment in our Common Stock and warrants. Those factors that could cause fluctuations include, but are not limitedcomprehensive income (loss). Prior to the following:
In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been brought against that company. Due to the potential volatility of our stock price, we may therefore be the target of securities litigation in the future. Securities litigation could result in substantial costs and divert management’s attention and resources from our business.
A limited public trading market may cause volatility in the price of our Common Stock.
The quotation of our Common Stock on the NASDAQ does not assure that a meaningful, consistent and liquid trading market currently exists, and in recent years such market has experienced extreme price and volume fluctuations that have particularly affected the market prices of many smaller companies like us. Our Common Stock is thus subject to this volatility. Sales of substantial amounts of Common Stock, or the perception that such sales might occur, could adversely affect prevailing market prices of our Common Stock and our stock price may decline substantially in a short time and our shareholders could suffer losses or be unable to liquidate their holdings.
The market prices for our Common Stock may be adversely impacted by future events.
Our Common Stock began trading on the over-the-counter-markets on July 28, 2005 and is currently quoted on the NASDAQ Stock Market under the symbol ADXS. Market prices for our Common Stock and warrants will be influenced by a number of factors, including:
If we fail to remain current with our listing requirements, we could be removed from the NASDAQ Capital Market, which would limit the ability of broker-dealers to sell our securities and the ability of shareholders to sell their securities in the secondary market.
Companies trading on the NASDAQ Marketplace, such as our Company, must be reporting issuers under Section 12 of the Exchange Act, as amended, and must meet the listing requirements in order to maintain the listing of our Common Stock on the NASDAQ Capital Market. If we do not meet these requirements, the market liquidity for our securities could be severely adversely affected by limiting the ability of broker-dealers to sell our securities and the ability of shareholders to sell their securities in the secondary market.
Sales of additional equity securities may adversely affect the market price of our Common Stock and your rights may be reduced.
We expect to continue to incur drug development and selling, general and administrative costs, and to satisfy our funding requirements, we will need to sell additional equity securities, which may be subject to registration rights and warrants with anti-dilutive protective provisions. The sale or the proposed sale of substantial amounts of our Common Stock or other equity securities in the public markets may adversely affect the market price of our Common Stock and our stock price may decline substantially. Our shareholders may experience substantial dilution and a reduction in the price that they are able to obtain upon sale of their shares. Also, new equity securities issued may have greater rights, preferences or privileges than our existing Common Stock.
Additional authorized shares of Common Stock available for issuance may adversely affect the market price of our securities.
We are currently authorized to issue 65,000,000 shares of our Common Stock. As of December 15, 2017, we had 41,303,988 shares of our Common Stock issued and outstanding, excluding shares issuable upon exercise of our outstanding warrants, options, convertible promissory notes and shares of Common Stock earned but not yet issued under our director compensation program. Under our 2011 Employee Stock Purchase Plan, or ESPP, our employees can buy our Common Stock at a discounted price. To the extent the shares of Common Stock are issued, options and warrants are exercised or convertible promissory notes are converted, holders of our Common Stock will experience dilution. In the event of any future financing of equity securities or securities convertible into or exchangeable for, Common Stock, holders of our Common Stock may experience dilution. In addition, as of December 15, 2017, we had outstanding options to purchase 4,380,557 shares of our Common Stock at a weighted average exercise price of approximately $11.47 per share and outstanding warrants to purchase 3,092,395 shares of our Common Stock (including the above warrants subject to weighted-average anti-dilution protection); and zero shares of our Common Stock are available for grant under the ESPP.
We do not intend to pay cash dividends.
We have not declared or paid any cash dividends on our Common Stock, and we do not anticipate declaring or paying cash dividends for the foreseeable future. Any future determination as to the payment of cash dividends on our Common Stock will be at our Board of Directors’ discretion and will depend on our financial condition, operating results, capital requirements and other factors that our Board of Directors considers to be relevant.
Our certificate of incorporation, bylaws and Delaware law have anti-takeover provisions that could discourage, delay or prevent a change in control, which may cause our stock price to decline.
Our certificate of incorporation, Bylaws and Delaware law contain provisions which could make it more difficult for a third party to acquire us, even if closing such a transaction would be beneficial to our shareholders. To date, we have not issued shares of preferred stock, however, we are authorized to issue up to 5,000,000 shares of preferred stock. This preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our Board of Directors without further action by shareholders.Secured Notes, the Company has elected the fair value option to measure the Secured Notes. The terms of any series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. The issuance of any preferred stock could materially adversely affect the rights of the holders of our Common Stock, and therefore, reduce the value of our Common Stock. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Provisions of our certificate of incorporation, Bylaws and Delaware law also could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a shareholder might consider favorable. Such provisions may also prevent or frustrate attempts by our shareholders to replace or remove our management. In particular, the certificate of incorporation, Bylaws and Delaware law, as applicable, among other things; provide the Board of Directors with the ability to alter the Bylaws without shareholder approval, and provide that vacancies on the Board of Directors may be filled by a majority of directors in office, although less than a quorum.
We are also subject to Section 203 of the Delaware General Corporation Law, which, subject to certain exceptions, prohibits “business combinations” between a publicly-held Delaware corporation and an “interested shareholder,” which is generally defined as a shareholder who becomes a beneficial owner of 15% or more of a Delaware corporation’s voting stock for a three-year period following the date that such shareholder became an interested shareholder.
These provisions are expected to discourage certain types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of our company to first negotiate with its board. These provisions may delay or prevent someone from acquiring or merging with us, which may cause the market price of our Common Stock to decline.
Item 1B: Unresolved Staff Comments.
None.
Our corporate offices and manufacturing facility are located in approximately 48,500 square feet of office space at 305 College Road East, Princeton, New Jersey 08540 which is occupied pursuant to a lease which expires on November 30, 2025.
The information required under this item is set forth in Footnote 10. Commitments and Contingencies – Legal Proceedings with this Form 10-K and is incorporated herein by reference.
Item 4. Mine Safety Disclosures.
None.
Item 5. Market for Our Common Stock and Related Shareholder Matters.
Our common stock is listed on the NASDAQ Global Select Market under the symbol “ADXS”. The following table sets forth for the periods indicated the high and low sales prices per share of our common stock as reported on the NASDAQ Stock Market:
| Fiscal 2017 | High | Low | ||||||
| Fourth Quarter | $ | 7.41 | $ | 3.09 | ||||
| Third Quarter | $ | 9.16 | $ | 5.94 | ||||
| Second Quarter | $ | 9.60 | $ | 7.70 | ||||
| First Quarter | $ | 10.30 | $ | 7.13 | ||||
| Fiscal 2016 | High | Low | ||||||
| Fourth Quarter | $ | 15.98 | $ | 7.87 | ||||
| Third Quarter | $ | 9.66 | $ | 7.01 | ||||
| Second Quarter | $ | 9.99 | $ | 5.46 | ||||
| First Quarter | $ | 14.45 | $ | 6.64 | ||||
As of December 15, 2017, there were approximately 98 shareholders of record. Because shares of our Common Stock are held by depositaries, brokers and other nominees, the number of beneficial holders of our shares is substantially larger than the number of shareholders of record. On December 15, 2017, the last reported sale price per share for our Common Stock as reported by NASDAQ was $3.14.
We have not declared or paid any cash dividends on our Common Stock, and we do not anticipate declaring or paying cash dividends for the foreseeable future.
Recent Sales of Unregistered Securities
On September 29, 2017, the registrant issued 1,439 shares of Common Stock to its Executive Officers, pursuant to their Employment Agreements.
On October 24, 2017, the registrant issued 10,000 shares of Common Stock to accredited investors as payment for consulting services.
On October 31, 2017 the registrant issued 1,322 shares of Common Stock to its Executive Officers, pursuant to their Employment Agreements.
On November 30, 2017 the registrant issued 2,919 shares of Common Stock to its Executive Officers, pursuant to their Employment Agreements.
Equity Compensation Plan Information
The following table provides information regarding the status of our existing equity compensation plans at October 31, 2017:
| Plan category | Number of shares of Common Stock to be issued on exercise of outstanding options, warrants and rights | Weighted- average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in the previous columns) | |||||||||
| Equity compensation plans approved by security holders | ||||||||||||
| Options | 3,893,558 | $ | 12.51 | N/A | ||||||||
| Restricted stock | 1,363,119 | N/A | N/A | |||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 5,256,677 | N/A | 710,853 | * | ||||||||
* Number of securities remaining can be utilized for either options or restricted stock.
Treasury Share Repurchases
The following table represents treasury share repurchases during the year ended October 31, 2017:
| Period | (a) Total Number of Shares Purchased (1) | (b) Average Price Paid Per Share | (c) Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | (d) Maximum Dollar Value of Shares that May Yet Be Purchased Under the Program | ||||||||
| February 1, 2017 – February 29, 2017 | 9,485 | $ | 8.74 | N/A | N/A | |||||||
| July 1, 2017 – July 31, 2017 | 119,128 | 6.70 | N/A | N/A | ||||||||
| Total | 128,613 | $ | 6.85 | N/A | N/A | |||||||
(1)Consists of shares repurchased by the Company for certain employees’ restricted stock units that vested to satisfy minimum tax withholding obligations that arose on the vesting of the restricted stock units.
Common Stock Performance Graph
The following graph compares the cumulative total stockholder return on our common stock for the period from October 17, 2013 through October 31, 2017, with the cumulative total return over such period on (i) the U.S. Index of The NASDAQ Stock Market and (ii) the Biotechnology Index of The NASDAQ Stock Market. The graph assumes an investment of $100 on October 17, 2013, in our common stock (at the closing market price) and in each of the indices listed above, and assumes the reinvestment of dividends.
COMPARISON OF CUMULATIVE TOTAL RETURN*
Among Advaxis, Inc., the NASDAQ Composite Index
and the NASDAQ Biotechnology Index
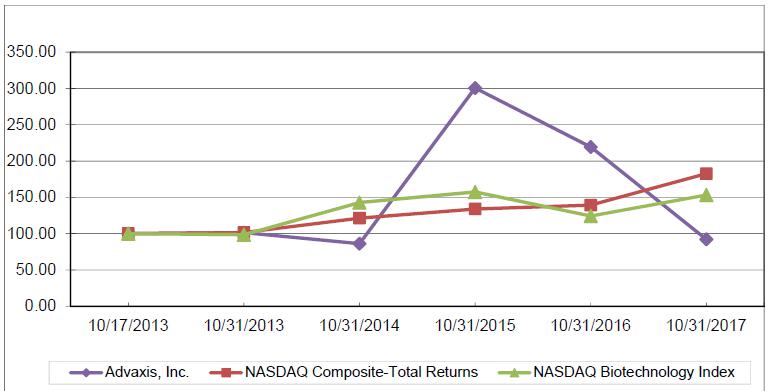
* $100 invested on October 17, 2013 in stock or index, including reinvestment of dividends.
Fiscal year ending October 31.
ITEM 6. Selected Financial Data.
The selected financial data included in this section are not intended to replace the consolidated financial statements included elsewhere in this Annual Report on Form 10-K. We derived the selected statements of operations data for the years ended October 31, 2017, 2016 and 2015 and the selected balance sheet data at October 31, 2017 and 2016 from our audited financial statements included elsewhere in this report. We derived the selected statements of operations data for the years ended October 31, 2014 and 2013 and the selected balance sheet data at October 31, 2015, 2014 and 2013 from our audited financial statements which are not included in this Annual Report on Form 10-K. Our historical results are not necessarily indicative of the results that may be expected in the future. You should read the selected historical consolidated financial data below in conjunction with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the audited consolidated financial statements included elsewhere in this report.
| Year Ended October 31, | ||||||||||||||||||||
| 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||||
| Statements of Operations Data: | ||||||||||||||||||||
| Revenue | $ | 12,031,050 | $ | 3,994,856 | $ | - | $ | 1,000,000 | $ | - | ||||||||||
| Operating Expenses: | ||||||||||||||||||||
| Research and Development Expenses | 71,900,462 | 48,774,589 | 24,455,447 | 8,862,854 | 5,621,989 | |||||||||||||||
| General and Administrative Expenses | 38,658,464 | 31,712,505 | 24,243,690 | 11,675,724 | 9,071,613 | |||||||||||||||
| Total Operating Expenses | 110,558,926 | 80,487,094 | 48,699,137 | 20,538,578 | 14,693,602 | |||||||||||||||
| Loss from Operations | (98,527,876 | ) | (76,492,238 | ) | (48,699,137 | ) | (19,538,578 | ) | (14,693,602 | ) | ||||||||||
| Other Income (Expense): | ||||||||||||||||||||
| Interest Income | 669,759 | 331,529 | 114,219 | 36,305 | (987,746 | ) | ||||||||||||||
| Net Changes in Fair Value of Derivative Liabilities | 20,156 | 69,055 | (48,950 | ) | 619,089 | (1,504,465 | ) | |||||||||||||
| (Loss) on Note Retirement | - | - | - | - | (3,455,327 | ) | ||||||||||||||
| Other Income (Expense), Net | (123 | ) | (201 | ) | (6,599 | ) | 990 | (70,876 | ) | |||||||||||
| Net Loss Before Income Tax Benefit | (97,838,084 | ) | (76,091,855 | ) | (48,640,467 | ) | (18,882,194 | ) | (20,712,016 | ) | ||||||||||
| Income Tax Benefit | 4,402,682 | 2,535,625 | 1,609,349 | 2,356,880 | 725,190 | |||||||||||||||
| Dividends Attributable to Preferred Shares | - | - | - | - | (555,000 | ) | ||||||||||||||
| Net Loss Applicable to Common Stock | $ | (93,435,402 | ) | $ | (73,556,230 | ) | $ | (47,031,118 | ) | $ | (16,525,314 | ) | $ | (20,541,826 | ) | |||||
| Net Loss | $ | (93,435,402 | ) | $ | (73,556,230 | ) | $ | (47,031,118 | ) | $ | (16,525,314 | ) | $ | (19,986,826 | ) | |||||
| Net Loss per Common Share, Basic and Diluted | $ | (2.31 | ) | $ | (2.08 | ) | $ | (1.68 | ) | $ | (0.97 | ) | $ | (4.10 | ) | |||||
| Weighted Average Number of Common Shares Outstanding, Basic and Diluted | 40,527,844 | 35,400,980 | 28,026,197 | 17,106,577 | 5,012,105 | |||||||||||||||
| October 31, | ||||||||||||||||||||
| 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||||
| Balance Sheet Data: | ||||||||||||||||||||
| Cash and Cash Equivalents and Investments(a) | $ | 70,885,113 | $ | 152,087,528 | $ | 112,156,178 | $ | 17,606,860 | $ | 20,552,062 | ||||||||||
| Working capital | 60,378,526 | 132,168,809 | 111,096,966 | 17,778,325 | 15,872,461 | |||||||||||||||
| Total Assets | 93,641,778 | 169,044,060 | 119,605,693 | 23,377,813 | 23,585,921 | |||||||||||||||
| Common Stock Warrant Liability | - | 20,156 | 89,211 | 32,091 | 646,734 | |||||||||||||||
| Accumulated Deficit | (301,142,227 | ) | (207,706,825 | ) | (134,054,259 | ) | (86,991,137 | ) | (70,465,823 | ) | ||||||||||
| Total Shareholders’ Equity | 54,260,167 | 119,302,194 | 115,598,875 | 20,629,986 | 18,002,142 | |||||||||||||||
(a) Includes restricted cash of $587,000 at October 31, 2017. See Note 2. Summary of Significant Accounting Policies with this Form 10-K.
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
This Management’s Discussion and Analysis of Financial Conditions and Results of Operations and other portions of this report contain forward-looking information that involves risks and uncertainties. Our actual results could differ materially from those anticipated by the forward-looking information. Factors that may cause such differences include, but are not limited to, availability and cost of financial resources, product demand, market acceptance and other factors discussed in this report under the heading “Risk Factors”. This Management’s Discussion and Analysis of Financial Conditions and Results of Operations should be read in conjunction with our financial statements and the related notes included elsewhere in this report.
Overview
Advaxis, Inc. (“Advaxis” or the “Company”) is a late-stage biotechnology Company focused on the discovery, development and commercialization of proprietaryLm Technology antigen delivery products based on a platform technology that utilizes live attenuatedListeria monocytogenes (“Lm”) bioengineered to secrete antigen/adjuvant fusion proteins. TheseLm-based strains are believed to be a significant advancement in immunotherapy as they integrate multiple functions into a single immunotherapy by accessing and directing antigen presenting cells to stimulate anti-tumor T cell immunity, stimulate and activate the immune system with the equivalent of multiple adjuvants, and simultaneously reduce tumor protection in the Tumor Microenvironment (“TME”) to enable the T cells to eliminate tumors. The Company believes thatLm Technology immunotherapies can complement and address significant unmet needs in the current oncology treatment landscape. Specifically, their product candidates have the potential to optimize checkpoint performance, while having a generally well-tolerated safety profile, and most of their product candidates are immediately available for treatment with a low cost of goods. The Company’s passion for the clinical potential ofLmTechnology is balanced by focus and fiscal discipline and driven towards increasing shareholder value.
Advaxis is focused on four franchises in various stages of clinical and pre-clinical development, which they believe will provide the greatest opportunity to have a significant impact on patients and their families:
All four clinical franchises are anchored in the Company’sLm TechnologyTM, a unique platform designed for its ability to safely and effectively target various cancers in multiple ways. As an intracellular bacterium,Lm is an effective vector for the presentation of antigens through both the Major Histocompatibility Complex (“MHC”) I and II pathways, due to its active phagocytosis by Antigen Presenting Cells (“APCs”). Within the APCs,Lm produces virulence factors which allow survival in the host cytosol and potently stimulate the immune system.
Results of Operations
Fiscal Year 2017 Compared to Fiscal Year 2016
Revenue
Revenue increased $8,036,194 to $12,031,050 for the year ended October 31, 2017 compared to $3,994,856 for the year ended October 31, 2016. The increase was primarily due to a full year recognition of upfront fees received from Amgen in conjunction with the collaboration agreement signed in August 2016.
Research and Development Expenses
We make significant investments in research and development to support our pre-clinical and clinical development programs. Research and development expenses for the years ended October 31, 2017 and 2016 were categorized as follows:
| Year ended October 31, | ||||||||
| 2017 | 2016 | |||||||
| HPV-associated cancers | $ | 26,650,340 | $ | 12,875,876 | ||||
| Prostate cancer | 3,879,894 | 2,293,549 | ||||||
| Neoantigen therapy | 2,286,701 | 1,812,086 | ||||||
| Personnel expenses | 22,113,902 | 18,584,165 | ||||||
| Professional fees | 14,453,151 | 7,800,517 | ||||||
| Laboratory costs | 8,610,682 | 2,751,557 | ||||||
| Other clinical trial expenses | 1,771,549 | 1,795,355 | ||||||
| Other expenses | 2,634,243 | 861,484 | ||||||
| Partner reimbursements | (10,500,000 | ) | - | |||||
| Total research & development expense | $ | 71,900,462 | $ | 48,774,589 | ||||
HPV-Associated Cancer Therapy
HPV-associated expenses increased $13,774,464 to $26,650,340 for the year ended October 31, 2017 compared to $12,875,876 for the year ended October 31, 2016. The increase resulted primarily from startup activities for additional countries in the Phase 3 AIM2CERV trial, as well as pre-clinical support on ADXS-DUAL.
Prostate Cancer
Prostate cancer expenses increased $1,586,345 to $3,879,894 for the year ended October 31, 2017 compared to $2,293,549 for the year ended October 31, 2016. The increase resulted primarily from higher costs incurred due to the active enrollment of the expansion cohort on the Phase 1/2 trial in combination with Merck’s KEYTRUDA® (pembrolizumab).
Neoantigen Therapy
Neoantigen therapyexpenses increased $474,615 to $2,286,701 for the year ended October 31, 2017 compared to $1,812,086 for the year ended October 31, 2016. The increase was a result of Phase 1 startup costs incurred during the year ended October 31, 2017.
Personnel Expenses
Personnel expenses increased $3,529,737 to $22,113,902 for the year ended October 31, 2017 compared to $18,584,165 for the year ended October 31, 2016. The increase relates primarily to a 33% increase in R&D headcount.
Professional Fees
Professional fees increased $6,652,634 to $14,453,151 for the year ended October 31, 2017 compared to $7,800,517 for the year ended October 31, 2016. The increase is primarily attributable to an increase in drug manufacturing process validation costs and drug stability studies in support of the MAA.
Laboratory Costs
Laboratory costs increased $5,859,125 to $8,610,682 for the year ended October 31, 2017 compared to $2,751,557 for the year ended October 31, 2016. The increase is primarily attributable to an increase in headcount and laboratory space, as well as support of the MAA.
Other Clinical Trial Expenses
Clinical trial expenses decreased $23,806 to $1,771,549 for the year ended October 31, 2017 compared to $1,795,355 for the year ended October 31, 2016 primarilyfair value as a result of the Company decision notmodification was recorded in the consolidated statement of operation.
The Convertible Notes were valued at the end of the year using a probability-weighted expected return model, which incorporated significant unobservable inputs such as the likelihood of a voluntary note conversion (60% likelihood), the notes being held to proceedmaturity (20% likelihood) and the mandatory conversion of the notes in a PIPE (20% likelihood). This resulted in an implied borrowing rate of 50% was used as an input to the expansion phasefair value measurement. None of the HER2 trial.change in fair value was deemed to be attributable to instrument-specific credit risk and thus the full amount of such change was recognized in the statements of operations.
Other Expenses
Other expenses increased $1,772,759As discussed above, the Company had no financial alternatives at that time of the issuance of the Senior Convertible Notes and the Company issued the notes to $2,634,243 forits principal stockholders, which are also related parties of the year ended October 31, 2017 compared to $861,484 for the year ended October 31, 2016. The increase was due to additional infrastructure costs incurred to support the increased headcount and laboratory expansion.
Partner Reimbursements
Partner reimbursements for the year ended October 31, 2017 were $10,500,000.Company. The Company received clinical development payments from Amgen for ADXS-NEO totaling $7,500,000$4.0 million, while at the time the Senior Convertible Notes were issued with fair value of $6.6 million. In addition, as noted above, in connection with the issuance of the Senior Convertible Notes, the Company modified certain terms of the Secured Notes to be consistent with the terms of the Senior Convertible Notes, which resulted in a revaluation of the Secured Notes resulting in expense of $0.6 million to the Company. Furthermore, the Company issued warrants to the recipients of the Senior Convertible Notes, which at time of grant had fair value of $7.1 million. When determining whether fair value at initial recognition equals the transaction price, the Company considered the specific factors of the transaction and noted that the transaction took place under duress, the Company was forced to accept the price in the transaction and the transaction was with the Company’s related parties. In addition, the Company noted that the transaction does not represent a pro-rata distribution. Since the estimated fair value of the items above exceeded the proceed received and after considering the transaction specific factors, the excess of the estimated fair value over the proceed received described above were recorded on November 17, 2023 in financial expenses, net in the Company’s consolidated statements of operations.
The Company used the following significant inputs in measuring the Secured Notes and Senior Convertible Notes:
Schedule Of Assumptions Used In Warrant Liability
| December 31, 2023 | November 17, 2023 | August 7, 2023 | ||||||||||
| Stock price | $ | 0.67 | $ | 0.77 | $ | 1.15 | ||||||
| Interest rate | 12.4 | 7.2 | % | 7.3 | % | |||||||
| Implied discount | 47.7 | % | 75.2 | % | (32.4 | )% | ||||||
| Risk Free Rate | 5.60 | % | 4.50 | % | 4.20 | % | ||||||
Warrant Liability Senior Convertible Notes
On November 17, 2023, in connection with the issuance of the Senior Convertible Notes, the Company amended the Secured Notes to match the same terms described above, and issued to the holders of the Secured Notes and Senior Convertible Notes, collectively, warrants to purchase an additional paymentaggregate of $3,000,000 from Stendhal22,500,500 shares of the Common Stock with an exercise price equal to support the AIM2CERV program.lower of (A) 50% of the Common Stock’s price per share as of market close on November 16, 2023 and (ii) 50% of the Common Stock’s price per share as of the close of market on the Trading Day immediately prior to the date of the Notice of Exercise of the warrant, subject to adjustment, which exercise may be on a cashless basis. The warrants require liability classification as the warrants contains an unpermitted adjustment to the exercise price, which precludes an equity classification. The Company used the Black Scholes model to calculate the fair value of these warrants at the issuance and at each reporting date
General and Administrative Expenses
General and administrative expenses primarily include salary and benefit costs for employees included in our finance, legal and administrative organizations, outside legal and professional services, and facilities costs. General and administrative expenses increased $6,945,960 to $38,658,464In measuring the warrant liability for the year ended Octoberwarrants issued on November 17, 2023 at December 31, 2017, compared with $31,712,505 for2023, the year ended October 31, 2016. The increase is primarily attributable to an increaseCompany used the following inputs in stock based compensation expense and severance associated with the resignation of the Company’s Chief Executive Officer in July, 2017 and increased facility costs associated with the facility expansion. These increases were offset by a decrease in consulting fees resulting primarily from non-recurring costs associated with the collaboration agreement with Amgen which closed in August 2016.its Black Scholes model:
December 31, 2023 | November 17, 2023 | |||||||
| Assumed Exercise Price | $ | 0.04 | $ | 0.05 | ||||
| Diluted Stock Price | $ | 0.26 | $ | 0.31 | ||||
| Expected Term | 4.9 years | 5.0 years | ||||||
| Volatility % | 95.7 | % | 96.3 | % | ||||
| Risk Free Rate | 3.85 | % | 4.45 | % | ||||
We anticipate general and administrative expenses in the near term to remain comparable to current levels, exclusive of the impact of future stock awards, severance and facility expansion expenses.
Interest Income
Interest income was $669,759 for the year ended October 31, 2017, compared with $331,529 for the year ended October 31, 2016. The increase in interest income earned was driven primarily by an increase in interest rates as well as a higher investable balance resulting from cash received in fiscal 2016 from sales of the Company’s common shares and an up-front cash payment received in conjunction with the collaboration agreement with Amgen.
Changes in Fair Values
For the year ended OctoberDecember 31, 2017,2023, the Company recorded non-cash income fromreported a loss of approximately $6.0 million due to changes in the fair value of the warrant liability of $20,156 dueliability.
Side Agreement and reinvestment rights
On September 11, 2023, the Company entered into the Side Letter Agreement for Conversion (“September Side Agreement”) in reference to the expirationSimple Agreement for Future Equity (“SAFE”) by and between Biosight Ltd. and various investors. The September Side Agreement provided that amounts invested in Biosight Ltd under the SAFE shall convert to shares of the Common Stock upon the closing of the Biosight Merger. In addition the uninvested amount of $1.8 million as of the close of the Merger shall convert to shares of the Common Stock (at a % discount to the lowest effective price per share at which stock is purchased) upon the closing of the private investment in public equity (“PIPE)”. Should a PIPE transaction not close within six months, the SAFE may be converted into shares of Common Stock at a % discount to the Company’s stock price (based on the average closing price of the Company’s stock on the five trading days immediately preceding the date of exercise) for a period of 30 days.
The September Side Agreement is a freestanding equity contract which is considered issued for accounting purposes. As the September Side Agreement does not meet all the conditions to be classified as equity pursuant to ASC 815-40-25-10, the Company classified the September Side Agreement as a liability with changes in fair value recorded in the consolidated statements of operations.
On November 17, 2023, as part of the Senior Convertible Notes (with the same terms as the Senior Convertible Notes described above) an additional side letter agreement was signed (“November Side Agreement”), allowing a portion ($1,458 thousand) of the September Side Agreement investors to instead convert the SAFE uninvested amount into Convertible Promissory and warrants under the same terms as the Senior Convertible Notes, resulting in $349 thousand remaining under the September Side Agreement as of December 31, 2023.
In addition to conversion of the first September Side Agreement, and as part of the Senior Convertible Notes, the November Side Agreement also allowed investors of the Senior Convertible Notes to re-invest up to their original investment (up to $4.0 million collectively) in the same terms as the Senior Convertible Notes. The November Side Agreement is a freestanding equity contract which is considered issued for accounting purposes. As the November Side Agreement does not meet all the conditions to be classified as equity pursuant to ASC 815-40-25-10, the Company classified the November Side Agreement as a liability warrants.with changes in fair value recorded in the consolidated statements of operations. The portion of the modified September Side Agreement resulted in expense of $2.2 million on the modification date which was recorded in the consolidated statements of operations.
For the year ended OctoberDecember 31, 2016,2023, the Company recorded non-cash incomereported a loss of approximately $7.2 million due to changes in the fair value of the September and November Side Agreements liability.
Warrant Liability April 2021 Private Placement and The September 2018 Public Placement
The warrants issued in the April 2021 Private Placement will become exercisable only on such day, if ever, that is 14 days after the Company files an amendment to the Company’s Amended and Restated Certificate of Incorporation to increase the number of authorized shares of common stock, $ par value per share from shares to shares. These warrants expire five years after the date they become exercisable. As of December 31, 2023, such an amendment has not been filed, and thus the warrants have not become exercisable. Accordingly, based on certain indemnification provisions of the securities purchase agreement, the Company concluded that liability classification is warranted. The Company utilized the Black Scholes model to calculate the fair value of these warrants at the merger and reporting date.
| F-23 |
The September 2018 Public Offering warrants contain a down round feature, except for exempt issuances as defined in the warrant agreement, in which the exercise price would immediately be reduced to match a dilutive issuance of common stock, options, convertible securities and changes in option price or rate of conversion. As of December 31, 2023, the down round feature was triggered five times and the exercise price of the warrants were reduced from $ to $. The warrants require liability classification as the warrant agreement requires the Company to maintain an effective registration statement and does not specify any circumstances under which settlement in other than cash would be permitted or required. In addition, the contract contains an unpermitted adjustment to the exercise price, and therefore precludes an equity classification. As a result, net cash settlement is assumed, and liability classification is warranted. The Company utilized the Black Scholes model to calculate the fair value of these warrants at the merger and reporting date.
In measuring the warrant liability for the warrants issued in the April 2021 Private Placement and September 2018 Public Offering at December 31, 2023, the Company used the following inputs in its Black Scholes model:
December 31, 2023 | January 19, 2023 | |||||||
| Exercise Price | $ | 55.73 | $ | 55.73 | ||||
| Stock Price | $ | 0.67 | $ | 2.95 | ||||
| Expected Term | 4.98 years | 4.98 years | ||||||
| Volatility % | 127 | % | 117 | % | ||||
| Risk Free Rate | 3.85 | % | 3.60 | % | ||||
For the year ended December 31, 2023, the Company reported a gain of approximately $161 due to changes in the fair value of the warrant liabilityliability.
9. Fair Value Measurements
As of $69,055 due to a decrease inDecember 31, 2022 the Company did not have any assets or liabilities carried at fair value of liability warrants ason a smaller range of share prices were used inrecurring basis. The following table provide the calculation of the BSM volatility input as well as a decrease in our share price from $11.09liabilities carried at October 31, 2015 to $8.09 at October 31, 2016.
Income Tax Benefit
We may be eligible, from time to time, to receive cash from the sale of our Net Operating Losses (“NOLs”) under the State of New Jersey NOL Transfer Program.
During the year ended October 31, 2017, the Company recorded Income Tax Receivable of $4,452,682 from the sale of its state NOLs and research and development tax credits for the period ended October 31, 2016. We paid $50,000 in Taiwanese withholding taxes in connection with the revenue generated from an annual exclusive license fee from GBP.
During the year ended October 31, 2016, the Company recorded Income Tax Receivable of $2,549,862 from the sale of its state NOLs and research and development tax credits for the period ended October 31, 2015. In addition, the Company received a net cash amount of $35,764 in excess of what was recorded as Income Tax Receivable at October 31, 2015. We paid $50,000 in Taiwanese withholding taxes in connection with the revenue generated from an annual exclusive license fee from GBP.
Fiscal Year 2016 Compared to Fiscal Year 2015
Revenue
During the year ended October 31, 2016, the Company recorded revenue of $3,994,856. The Company recognized $3,744,856 of revenue from the collaboration agreement with Amgen related to amortization of the upfront fees received. In addition, $250,000 of revenue was due to the receipt of an annual exclusive license fee from GBP for the development and commercialization of axalimogene filolisbac.
We did not record any revenue for the year ended October 31, 2015.
Research and Development Expenses
We make significant investments in research and development in support of our development programs both clinically and pre-clinically. Research and development costs are expensed as incurred and primarily include salary and benefit costs, third-party grants, fees paid to clinical research organizations, and supply costs. Research and development expenses for the years ended October 31, 2016 and 2015 were categorized as follows:
| Year ended October 31, | ||||||||
| 2016 | 2015 | |||||||
| HPV-associated cancers | $ | 12,875,876 | $ | 7,358,020 | ||||
| Prostate cancer | 2,293,549 | 1,826,978 | ||||||
| Neoantigen therapy | 1,812,086 | 172,541 | ||||||
| Personnel expenses | 18,584,165 | 8,492,511 | ||||||
| Professional fees | 7,800,517 | 3,464,321 | ||||||
| Laboratory costs | 2,751,557 | 508,002 | ||||||
| Other clinical trial expenses | 1,795,355 | 1,867,831 | ||||||
| Other expenses | 861,484 | 765,243 | ||||||
| Total research & development expense | $ | 48,774,589 | $ | 24,455,447 | ||||
HPV-Associated Cancer Therapy
HPV-associated expenses increased $5,517,856 to $12,875,876 for the year ended October 31, 2016 compared to $7,358,020 for the year ended October 31, 2015. The increase primarily resulted from the startup costs related to the initiation of the Phase 3 AIMZCERV trial.
Prostate Cancer
Prostate cancer expenses increased $466,571 to $2,293,549 for the year ended October 31, 2016 compared to $1,826,978 for the year ended October 31, 2015. The increase primarily resulted from higher costs incurred due to an increase in enrollment and maintenance costs associated with the Phase 1/2 trial in combination with Merck’s KEYTRUDA® (pembrolizumab).
Neoantigen Therapy
Neoantigen therapy expenses increased $1,639,545 to $1,812,086 for the year ended October 31, 2016 compared to $172,541 for the year ended October 31, 2015. The increase was primarily a result of pre-IND activities and startup costs incurred during the year ended October 31, 2016.
Personnel Expenses
Personnel expenses increased $10,091,654 to $18,584,165 for the year ended October 31, 2016 compared to $8,492,511 for the year ended October 31, 2015. The increase primarily relates to a 53% increase in headcount as well as non-cash stock based compensation for past employees.
Professional Fees
Professional fees increased $4,336,196 to $7,800,517 for the year ended October 31, 2016 compared to $3,464,321 for the year ended October 31, 2015. The increase is primarily attributed to an increase in drug manufacturing process validation costs and drug stability studies.
Laboratory Costs
Laboratory costs increased $2,243,555 to $2,751,557 for the year ended October 31, 2016 compared to $508,002 for the year ended October 31, 2015. The increase is primarily attributable to an increase in headcount as well as an expansion of laboratory space.
Other Clinical Trial Expenses
Clinical trial expenses decreased $72,476 to $1,795,355 for the year ended October 31, 2016 compared to $1,867,831 for the year ended October 31, 2015. These expenses were related to the HER2 study and were consistent with the comparable prior period.
Other Expenses
Other expenses increased $96,241 to $861,484 for the year ended October 31, 2016 compared to $765,243 for the year ended October 31, 2015. The increase was primarily due to additional infrastructure costs incurred to support the increased headcount and laboratory expansion.
General and Administrative Expenses
General and administrative expenses primarily include salary and benefit costs for employees included in our finance, legal and administrative organizations, outside legal and professional services, and facilities costs. General and administrative expense increased $7,468,815 to $31,712,505 for the year ended October 31, 2016, compared with $24,243,690 for the year ended October 31, 2015. There was an increase of approximately $6,925,600 in compensation related expense, including a non-cash increase in stock based compensation costs of approximately $2,600,000, attributable to increases in our employees, the grant date fair value measured on a recurring basis as of stock awardsDecember 31, 2023:
Schedule of Fair Value, Assets and the number of awards. Costs pertaining to the Company’s infrastructure expansion, including leased space and information technology related costs, increased by approximately $1,564,900. Business development costs increased by approximately $1,491,900. This was partially offset by a decrease in non-cash investor relations costs of approximately $2,200,000.Liabilities Measured on Recurring Basis
Fair Value Measured at December 31, 2023
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Financial liabilities at fair value: | ||||||||||||||||
| Convertible note | $ | - | - | $ | 8,141 | $ | 8,141 | |||||||||
| Side Letter Agreements | - | - | 8,436 | 8,436 | ||||||||||||
| Long term warrant liability | - | - | 6,057 | 6,057 | ||||||||||||
| Total financial liabilities at fair value | $ | - | $ | - | $ | 22,634 | $ | 22,634 | ||||||||
We anticipate general and administrative expenses in the near term to remain comparable to current levels, exclusive of the impact of future stock awards and one-time expenses.
Interest Income
Interest income was $331,529 for the year ended October 31, 2016, compared with $114,219 for the year ended October 31, 2015. The increase in interest income earned was attributable to an increase in cash resulting from sales of the Company’s common shares. The cash was invested in held-to-maturity investments and a savings account.
Changes in Fair Values
For the year ended October 31, 2016, the Company recorded non-cash income from changes in the fair value of the warrant liabilityCompany’s Level 3 financial liabilities, which are measured on a recurring basis are as follows (in thousands):
Schedule of $69,055 dueFair Value Liabilities Measured on Recurring Basis
For the year ended December 31, 2023 | ||||
| December 31, 2022 | - | |||
| Long term warrant assumed from January 2023 Merger | 203 | |||
| Proceed from issuance of Convertible Notes | $ | 2,000 | ||
| Side Letter Agreement in connection with Biosight Merger | 685 | |||
| SAFE assumed from Biosight Merger | 1,068 | |||
| Conversion of of shares to SAFE | (1,068 | ) | ||
| Proceed from issuance of Senior Convertible Notes | 4,000 | |||
| Remeasurement recorded in financial loss, net | 15,746 | |||
| December 31, 2023 | 22,634 | |||
10. Common Stock
The Common Stock confer upon the holders the right vote in annual and special meetings of the Company, and to a decreaseparticipate in the fair value of liability warrants as a smaller range of share prices were used in the calculationdistribution of the BSM volatility input as well as a decrease in our share price from $11.09 at October 31, 2015 to $8.09 at October 31, 2016.
For the year ended October 31, 2015,surplus assets of the Company recorded non-cash expense from changes in the fair valueupon liquidation of the warrant liability of $48,950 due to an increase inCompany, after the fair value of liability warrants primarily resulting from a larger range of share prices used in the calculationdistribution of the Black-Scholes Model (“BSM”) volatility input, as well as a significant increase in our share price from $3.18 at October 31, 2014 to $11.09 at October 31, 2015. This was partially offset by the expiration of some warrants.
Income Tax Benefit
We may be eligible, from time to time, to receive cash from the sale of our Net Operating Losses (“NOLs”) under the State of New Jersey NOL Transfer Program.
During the year ended October 31, 2016, the Company recorded Income Tax Receivable of $2,549,862 from the sale of its state NOLs and research and development tax credits for the period ended October 31, 2015. In addition, the Company received a net cash amount of $35,764 in excess of what was recorded as Income Tax Receivable at October 31, 2015. We paid $50,000 in Taiwanese withholding taxes in connection with the revenue generated from an annual exclusive license fee from GBP.
During the year ended October 31, 2015, the Company recorded Income Tax Receivable of $1,609,349 from the sale of its state NOLs and research and development tax credits for the period ended October 31, 2014.
Liquidity and Capital Resources
Our major sources of cashpreferred stock liquidation preference. No dividends have been proceeds from various publicdeclared as of December 31, 2023 and private offerings of our common stock, option and warrant exercises, and interest income. From October 2013 through October 2017, we raised approximately $222.5 million in gross proceeds from various public and private offerings of our common stock. We have not yet commercialized any drug, and we may not become profitable. Our ability to achieve profitability depends on a number of factors, including our ability to complete our development efforts, obtain regulatory approvals for our drug, successfully complete any post-approval regulatory obligations, successfully compete with other available treatment options in the marketplace, overcome any clinical holds that the FDA may impose and successfully manufacture and commercialize our drug alone or in partnership. We may continue to incur substantial operating losses even after we begin to generate revenues from our drug candidates. As of October 31, 2017, the Company had approximately $70.9 million in cash, restricted cash, cash equivalents and investments on its balance sheet. We believe our current cash position is sufficient to fund our business plan approximately into fiscal 2019. The actual amount of cash that we will need to operate is subject to many factors.2022.
| * | Does not include and shares of restricted Common Stock issued but not outstanding in 2023 and 2022, respectively. |
| ** | All of the Common Stock and per share data have been retroactively adjusted for the impact of the January 2023 merger between Old Ayala, Inc. (f/k/a Ayala Pharmaceuticals, Inc.) and Ayala Pharmaceutical, Inc.(f/k/a Advaxis, Inc.). See note 1 |
Since our inception through October 31, 2017, we reported accumulated net losses of approximately $301.1 million and recurring negative cash flows from operations. We anticipate that we will continue to generate significant losses from operations for the foreseeable future.
| F-24 |
Operating ActivitiesThe. 2015 Incentive Plan (the “2015 Plan”) was originally ratified and approved by the Company’s stockholders on May 27, 2015. Subject to proportionate adjustment in the event of stock splits and similar events, the aggregate number of shares of common stock that may be issued under the 2015 Plan is shares.
Cash
As of December 31, 2023, there were shares available for issuance under the 2015 Plan.
Pursuant to the January 2023 merger, the Company assumed 117,360 restricted shares, all of which are issued and outstanding, and outstanding options.
Schedule of Fair Value of Stock Options Granted
| Year ended | ||||||||
| December 31, | ||||||||
| 2023* | 2022 | |||||||
| Expected volatility | % | % | ||||||
| Expected dividends | % | % | ||||||
| Expected term (in years) | - | |||||||
| Risk free rate | % | %- | % | |||||
| * | no shares have been granted |
Schedule of Share Based Compensation Expense
| Year ended | Year ended | |||||||
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Research and Development | $ | 119 | $ | 717 | ||||
| General and Administrative | 1,104 | 1,527 | ||||||
| Total Stock-Based Compensation | $ | 1,223 | $ | 2,244 | ||||
Schedule of Changes in Stock Option Plan
| Year ended December 31, 2023 | ||||||||||||||||
| Weighted | ||||||||||||||||
| average | ||||||||||||||||
| Weighted | remaining | |||||||||||||||
| average | contractual | Aggregate | ||||||||||||||
| Number of | exercise | term | intrinsic | |||||||||||||
| options | price | (in years) | value | |||||||||||||
| Outstanding at Beginning of Year | 197,897 | $ | 38.34 | $ | - | |||||||||||
| Assumed in advaxis merger | 9,815 | 1,243.88 | ||||||||||||||
| Granted | - | - | - | - | ||||||||||||
| Forfeited | (6,539 | ) | 38.34 | |||||||||||||
| Expired | (98,281 | ) | 38.91 | |||||||||||||
| Outstanding, December 31, 2023 | 102,892 | $ | 151.86 | $ | - | |||||||||||
| Exercisable Options, December 31, 2023 | 90,596 | $ | 131.77 | $ | - | |||||||||||
| F-25 |
The Company did not grant any options during 2023. The weighted-average grant date per-share fair value of stock options granted during 2022 was $. No stock options were exercised during the years ended December 31, 2023 and 2022. As of December 31, 2023, there was approximately $ million of total unrecognized compensation cost related to non-vested stock-based compensation arrangements granted under the Plan. That cost is expected to be recognized over a weighted-average period of years.
Company’s restricted shares:
In May 2022, the Company granted restricted shares to officers and employees of the Company. The restricted shares vest over starting May 15, 2022.
In August 2022, the Company granted restricted shares to employees of the Company. The restricted shares vest over starting Aug 14, 2022.
Schedule of Summarizes Information Relating to Restricted Shares
| Year ended December 31, | Weighted average Fair value on grant date | Year ended December 31, | Weighted average Fair value on grant date | |||||||||||||
| 2022 | 2023 | |||||||||||||||
| Opening balance | 23,303 | $ | 58.09 | 80,840 | 18.78 | |||||||||||
| Forfeited | (5,926 | ) | 16.67 | (5,176 | ) | 10.67 | ||||||||||
| Granted | 84,997 | 10.42 | - | - | ||||||||||||
| Vested | (21,534 | ) | 28.92 | (58,607 | ) | 21.37 | ||||||||||
| 80,840 | $ | 18.78 | 17,057 | - | ||||||||||||
The weighted average fair value at grant date of restricted shares granted for the year ended OctoberDecember 31, 2022 was $, per share. There were grants during 2023.
Restricted shares are subject to a repurchase right by the Company on certain occasions. Under the repurchase right, the Company may reacquire restricted shares, for no consideration, if certain conditions occur including the employees’ end of service with the Company.
12. Taxes on Income
The Company records income tax expense related to profits realized in the United States and realized by its subsidiary in Israel.
United States:
On December 22, 2017, the United States enacted the Tax Cuts and Jobs Act (the “U.S. Tax Reform”); a comprehensive tax legislation that includes significant changes to the taxation of business entities. These changes, most of which are effective for tax years beginning after December 31, 2017, was approximately $76.9 million (including proceedsinclude several key tax provisions that might impact the Company, among others: (i) a permanent reduction to the statutory federal corporate income tax rate from the sale of our state NOLs and Research and Development (R&D)35% (top rate) to 21% (flat rate) effective for tax credits of approximately $2.5 million) primarily from spending associated with our clinical trial programs and general and administrative spending.
Cash used in operating activities for the year ended October 31, 2016 was approximately $9.1 million. Spending associated with our clinical trial programs and general and administrative spending was partially offset by a $40 million upfront payment received from Amgen in connection with the collaboration agreement as well as proceeds from the sale of our state NOLs and Research and Development (R&D) tax credits of approximately $1.6 million.
Cash used in operating activities for the year ended October 31, 2015 was approximately $24.1 million (including proceeds from the sale of our state NOLs and R&D tax credits of approximately $1.7 million) primarily from spending associated with our clinical trial programs and general and administrative spending.
Investing Activities
Cash used in investing activities for the year ended Octoberyears beginning after December 31, 2017 was approximately $12.5 million resulting from restricted cash established with letter of credit agreement, held-to-maturity investments, purchases of property and equipment, legal cost spending(ii) a new tax deduction in support of our intangible assets (patents) and costs paid to Penn for patents.
Cash provided by investing activities for the year ended October 31, 2016 was approximately $1.6 million resulting from net proceeds from investments in held-to-maturity investments, purchases of property and equipment, construction of cleanroom and laboratory facilities, legal cost spending in support of our intangible assets (patents) and costs paid to Penn for patents.
Cash used in investing activities for the year ended October 31, 2015 was approximately $47.4 million resulting from investments in held-to-maturity investments, purchases of property and equipment to support expansion, legal cost spending in support of our intangible assets (patents) and costs paid to Penn for patents.
Financing Activities
Cash provided by financing activities for the year ended October 31, 2017 was approximately $528,000, resulting from the sale of 92,145 shares of our Common Stock in two at-the-market transactions resulting in net proceeds of approximately $656,000 and proceed from the issuance of stock under an employee stock purchase plan of approximately $251,000. This was partially offset by approximately $357,000 of taxes paid related to the net share settlement of equity awards.
Cash provided by financing activities for the year ended October 31, 2016 was approximately $53.7 million, resulting from the sale of 3,047,446 shares of our Common Stock to Amgen resulting in net proceeds of approximately $25 million and a registered direct offering of 2,244,443 shares of our Common Stock resulting in net proceeds of approximately $28.2 million. In addition, approximately $614,000 in proceeds was received on option and warrant exercises. This was partially offset by approximately $36,000 of taxes paid related to the net share settlement of equity awards.
Cash provided by financing activities for the year ended October 31, 2015 was approximately $120.5 million, resulting primarily from registered direct offerings of 8,806,165 shares of our Common Stock resulting in net proceeds of approximately $63.1 million and a public offering of 2,800,000 shares of Common Stock resulting in net proceeds of approximately $56.7 million. In addition, the Company received approximately $2.4 million from the proceeds received on option and warrant exercises. This was partially offset by approximately $1.6 million of taxes paid related to the net share settlement of equity awards.
Our capital resources and operations to date have been funded primarily with the proceeds from public, private equity and debt financings, NOL tax sales and income earned on investments and grants. We have sustained losses from operations in each fiscal year since our inception, and we expect losses to continue for the indefinite future, due to the substantial investment in research and development. As of October 31, 2017 and October 31, 2016, we had an accumulated deficit of $301,142,227 and $207,706,825, respectively, and shareholders’ equity of $54,260,167 and $119,302,194, respectively.
The Company believes its current cash position is sufficient to fund its business plan approximately into fiscal 2019. We have based this estimate on assumptions that may prove to be wrong, and we could use available capital resources sooner than currently expected. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amount of increased capital outlays37.5% of “foreign derived intangible income” that effectively reduces the federal corporate tax on certain qualified foreign derived sales/licenses/leases and operating expenses associated with completing the developmentservice income in excess of our current product candidates.
The Company recognizes it may needa base amount to raise additional capital in order to continue to execute its business plan. There is no assurance that additional financing will be available when needed or that management will be able to obtain financing on terms acceptable13.125% (as compared to the Company or whetherregular corporate income tax rate of 21%); (iii) stricter limitation on the Company will become profitabletax deductibility of business interest expense; (iv) a shift of the U.S. taxation of multinational corporations from a tax on worldwide income to a territorial system (along with certain rules designed to prevent erosion of the U.S. income tax base) (v) a one-time deemed repatriation tax on accumulated offshore earnings held in cash and generate positive operating cash flow. Ifilliquid assets, with the Company is unablelatter taxed at a lower rate and (vi) an expansion of the U.S. controlled foreign corporation (“CFC”) anti deferral starting with the CFC’s first tax year beginning in 2018 intended to raise sufficient additional funds, it will have to scale back its business plan, extend payables and reduce overhead until sufficient additional capital is raised to support further operations. There can be no assurance that such a plan will be successful.tax in the U.S. “global intangible low-taxed income” (“GILTI”).
| F-26 |
The Company recorded loss from continuing operations, before taxes on income for the period indicated as follows (in thousands):
Schedule of Income Before Income Tax
| Year ended | Year ended | |||||||
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| United States | $ | (49,912 | ) | $ | (36,674 | ) | ||
| Israel | (2,072 | ) | (755 | ) | ||||
| Net loss before tax | $ | (51,984 | ) | $ | (37,429 | ) | ||
Income tax expense is summarized as follows (in thousands):
Schedule of Income Tax Expense
| Year ended | Year ended | |||||||
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Current: | ||||||||
| Domestic | $ | (4,335 | ) | $ | 57 | |||
| Foreign | 423 | 527 | ||||||
| Current Income tax expense | $ | (3,912 | ) | $ | 584 | |||
| Income tax expense | $ | (3,912 | ) | $ | 584 | |||
The effective income tax rate differed from the amount computed by applying the federal statutory rate to our loss before income taxes as follows:
Schedule of Tax Federal Statutory Rate
| Year ended | Year ended | |||||||
| December, 31 | December, 31 | |||||||
| 2023 | 2022 | |||||||
| U.S. federal tax provision at statutory rate | 21.00 | % | 21.00 | % | ||||
| State and local tax, net of federal benefit | 37.96 | 0.72 | ||||||
| Foreign rate differences | 0.03 | (0.06 | ) | |||||
| Non-deductible stock compensation | (0.49 | ) | (1.26 | ) | ||||
| Section 951A GILTI | 0.00 | 0.00 | ||||||
| Effect of other permanent differences | (0.05 | ) | (0.01 | ) | ||||
| Uncertain tax positions | (0.81 | ) | (1.15 | ) | ||||
| Change in valuation allowance | (48.44 | ) | (25.51 | ) | ||||
| Federal Tax Reform Rate Change | 0.00 | 0.00 | ||||||
| Tax Credits | - | 4.14 | ||||||
| Provision to Return | (1.68 | ) | 1.67 | |||||
| Other adjustments | (0.01 | ) | (1.10 | ) | ||||
| Effective tax rate | 7.51 | % | (1.56 | )% | ||||
| F-27 |
Tabular Disclosure of Contractual Obligations
| Payments Due by Period | ||||||||||||||||||||
| Contractual Obligations | Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | |||||||||||||||
| Operating Leases | $ | 10,447,167 | $ | 1,041,895 | $ | 2,340,292 | $ | 2,686,459 | $ | 4,378,521 | ||||||||||
| Employment Agreements | $ | 1,978,225 | $ | 1,978,225 | ||||||||||||||||
| Consulting and other Services | $ | 2,173,388 | $ | 1,877,492 | $ | 262,896 | $ | 33,000 | ||||||||||||
We enter into agreements in the normal course of business with contract research organizations for clinical trials and with vendors for preclinical studies and other services and products for operating purposes which are cancelable at any time by us, generally upon 30 days prior written notice. These payments are not included in this table of contractual obligations.
We are obligated to make future payments to third parties under in-license agreements, including sublicense fees, royalties and payments that become due and payable on the achievement of certain development and commercialization milestones. As the amount and timing of sublicense fees and the achievement and timing of these milestones are not probable and estimable, such commitments have not been included on our consolidated balance sheets or in the contractual obligations table above.
Off-Balance Sheet Arrangements
As of October 31, 2017, we had no off-balance sheet arrangements.
Critical Accounting Estimates
The preparation of financial statements in accordance with GAAP accepted in the U.S. requires management to make estimates and assumptions that affect the reported amounts and related disclosures in the financial statements. Management considers an accounting estimate to be critical if:
While we base our estimates and judgments on our experience and on various other factors that we believe to be reasonable under the circumstances, actual results could differ from those estimates and the differences could be material. The most significant estimates impact the following transactions or account balances: stock compensation, warrant liability valuation and impairment of intangibles.
Revenue Recognition
The Company is expected to derive the majority of its revenue from patent licensing. In general, these revenue arrangements provide for the payment of contractually determined fees in consideration for the grant of certain intellectual property rights for patented technologies owned or controlled by the Company. The intellectual property rights granted may be perpetual in nature, or upon the final milestones being met, or can be granted for a defined, relatively short period of time, with the licensee possessing the right to renew the agreement at the end of each contractual term for an additional minimum upfront payment. The Company recognizes licensing fees when there is persuasive evidence of a licensing arrangement, fees are fixed or determinable, delivery has occurred and collectability is reasonably assured.
Revenue associated with nonrefundable upfront license fees under arrangements where the license fees and research and development activities cannot be accounted for as separate units of accounting is deferred and recognized as revenue on a straight-line basis over the expected period of performance.
Revenues from the achievement of research and development milestones, if deemed substantive, are recognized as revenue when the milestones are achieved and the milestone payments are due and collectible. If not deemed substantive, the Company recognizes such milestones as revenue on a straight-line basis over the remaining expected performance period under the arrangement. All such recognized revenues are included in collaborative licensing and development revenue in the Company’s statements of operations.
Milestones are considered substantive if all of the following conditions are met: (1) the milestone is nonrefundable; (2) achievement of the milestone was not reasonably assured at the inception of the arrangement; (3) substantive effort is involved to achieve the milestone; and (4) the amount of the milestone appears reasonable in relation to the effort expended, and the other milestones in the arrangement and the related risk associated with the achievement of the milestone and any ongoing research and development or other services are priced at fair value.
If product development is successful, the Company will recognize revenue from royalties based on licensees’ sales of its products or products using its technologies. Royalties are recognized as earned in accordance with the contract terms when royalties from licensees can be reasonably estimated and collectability is reasonably assured. If royalties cannot be reasonably estimated or collectability of a royalty amount is not reasonably assured, royalties are recognized as revenue when the cash is received.
Deferred revenue represents the portion of payments received for which the earnings process has not been completed. Deferred revenue expected to be recognized within the next 12 months is classified as a current liability.
An allowance for doubtful accounts is established based on the Company’s best estimate of the amount of probable credit losses in the Company’s existing license fee receivables, using historical experience. The Company reviews its allowance for doubtful accounts periodically. Past due accounts are reviewed individually for collectability. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. To date, this is yet to occur.
Stock Based Compensation
We account for stock-based compensation using fair value recognition and record stock-based compensation as a charge to earnings net of the estimated impact of forfeited awards. As such, we recognize stock-based compensation cost only for those stock-based awards that are estimated to ultimately vest over their requisite service period, based on the vesting provisions of the individual grants.
The process of estimating the fair value of stock-based compensation awards and recognizing stock-based compensation cost over their requisite service period involves significant assumptions and judgments. We estimate the fair value of stock option awards on the date of grant using the Black-Scholes option-valuation model for the remaining awards, which requires that we make certain assumptions regarding: (i) the expected volatility in the market price of our Common Stock; (ii) dividend yield; (iii) risk-free interest rates; and (iv) the period of time employees are expected to hold the award prior to exercise (referred to as the expected holding period). As a result, if we revise our assumptions and estimates, our stock-based compensation expense could change materially for future grants.
Stock-based compensation for employees, executives and directors is measured based on the fair value of the shares issued on the date of grant and is to be recognized over the requisite service period in both research and development expenses and general and administrative expenses on the statement of operations. For non-employees, the fair value of the award is generally measured based on contractual terms.
Derivative Financial instruments
We do not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks. We evaluate all of our financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the statements of operations. The determination of fair value requires the use of judgment and estimates by management. For stock-based derivative financial instruments, we used the BSM which approximated the binomial lattice options pricing model to value the derivative instruments at inception and on subsequent valuation dates. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period. Derivative liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the instrument could be required within 12 months of the balance sheet date. The variables used in the model are projected based on our historical data, experience, and other factors. Changes in any of these variables could result in material adjustments to the expense recognized for changes in the valuation of the warrant derivative liability.
Intangible Assets
Intangible assets primarily consist of legal and filing costs associated with obtaining patents and licenses and are amortized on a straight-line basis over their remaining useful lives which are estimated to be twenty years from the effective dates of the University of Pennsylvania (Penn) License Agreements, beginning in July 1, 2002. These legal and filing costs are invoiced to the Company through Penn and its patent attorneys.
Management has reviewed its long-lived assets for impairment whenever events and circumstances indicate that the carrying value of an asset might not be recoverable and its carrying amount exceeds its fair value, which is based upon estimated undiscounted future cash flows. Net assets are recorded on the balance sheet for patents and licenses related to axalimogene filolisbac, ADXS-PSA and ADXS-HER2 and other products that are in development. However, if a competitor were to gain FDA approval for a treatment before us or if future clinical trials fail to meet the targeted endpoints, the Company would likely record an impairment related to these assets. In addition, if an application is rejected or fails to be issued, the Company would record an impairment of its estimated book value.
Income Taxes
The Company uses the asset and liability method of accounting forDeferred income taxes in accordance with ASC Topic 740, “Income Taxes.” Under this method, incomereflect the net tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year and (ii) deferred tax consequenceseffects of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred taxbetween the carrying amounts of assets and liabilities are measured using enactedfor financial reporting purposes and the amounts used for income tax rates expected to apply to taxable income inpurposes. Significant components of the years in which those temporary differences are expected to be recovered or settled. The effect onCompany’s deferred tax assets are as follows (in thousands):
Schedule of Deferred Tax Assets
| 2023 | 2022 | |||||||
| ($ in thousands) | ||||||||
| As of December 31, | ||||||||
| 2023 | 2022 | |||||||
| Deferred tax assets: | ||||||||
| Federal net operating loss carry forwards | $ | 69,040 | $ | 27,592 | ||||
| Tax credit carry forwards | 16,668 | 4,862 | ||||||
| Intangible and other related assets | 3,095 | 2,990 | ||||||
| Research and Development Costs | 36,632 | 3,074 | ||||||
| Accrued expenses | 169 | 72 | ||||||
| Warrant and other fair value Liabilities | 5,142 | - | ||||||
Stock Based Compensation | 4,782 | - | ||||||
| Lease Liability | 460 | 410 | ||||||
| Total deferred tax assets before valuation allowance | 135,988 | 39,000 | ||||||
| Valuation allowance | (135,570 | ) | (38,652 | ) | ||||
| Total deferred tax assets | 418 | 348 | ||||||
| Deferred tax liabilities: | ||||||||
| Right of Use Asset | 418 | 348 | ||||||
| Total deferred tax liabilities | 418 | 348 | ||||||
| Net deferred tax assets | $ | - | $ | - | ||||
As of December 31, 2023, the Company has provided a valuation allowance of approximately $135.6 million in respect of the Company’s deferred tax assets resulting from tax loss carryforwards, tax credits and liabilitiesother temporary differences. Realization of a changedeferred tax assets is dependent upon future earnings, if any, the time and amount of which are uncertain. As the Company is still in its development stage, it is more likely than not that sufficient taxable income will not be available for the tax rates is recognizedlosses to be utilized in the results of operations in the period that includes the enactment date. Afuture. Therefore, a valuation allowance is providedwas recorded to reduce the deferred tax assets reported if based onto their recoverable amounts.
Available Carryforward Tax Losses
At December 31, 2023, the weightCompany had federal net operation loss (NOL) carryforwards of approximately $214.9 million. At December 31, 2023, the Company had federal research and development credit carryforwards of approximately $11.4 million. The federal net operating loss carryforwards begin to expire in 2028, losses generated in 2018 or later of $190.5 million will carry forward indefinitely. The federal credit carryforwards begin to expire in 2032. Sections 382 and 383 of the available positiveInternal Revenue Code of 1986 subject the future utilization of net operating losses and negative evidence,certain other tax attributes, such as research and experimental tax credits, to an annual limitation in the event of certain ownership changes, as defined. The Company may be subject to the net operating loss utilization provision of Section 382 of the Internal Revenue Code. The effect of an ownership change would be the imposition of an annual limitation of the use of NOL carryforwards attributable to periods before the change. The amount of the annual limitation depends upon the value of the Company immediately before the change, changes to the Company’s capital during a specified period prior to the change, and the federal published interest rate. Although the Company has not completed an analysis under Section 382 of the Code, it is more likely than not some portion or allthat the utilization of the deferredNOLs will be limited.
Uncertain Tax Positions
The Company has reviewed the tax assets willpositions taken, or to be taken, in our tax returns for all tax years currently open to examination by a taxing authority. As of December 31, 2023, and 2022, the Company has recorded an uncertain tax position liability exclusive of interest and penalties of approximately $1.8 million, and $1.3 million, respectively. As of December 31, 2023, the Company has not be realized.accrued penalties for uncertain tax positions. A reconciliation of the Company’s unrecognized tax benefits is below:
Schedule of Reconciliation of Company’s Unrecognized Tax Benefits
| 2023 | 2022 | |||||||
| (in thousands) | (in thousands) | |||||||
| Uncertain tax position at the beginning of year | $ | 1,335 | $ | 858 | ||||
| Additions for uncertain tax position of prior years (foreign exchange and interest) | 13 | 36 | ||||||
| Additions for tax positions of current year | 423 | 441 | ||||||
| Uncertain tax position at the end of the year | $ | 1,771 | $ | 1,335 | ||||
| F-28 |
The Company remains subject to examination until the statute of limitations expires for each respective tax jurisdiction. The statute of limitations is currently open for 2018, 2019, 2020, 2021 and 2022 for all tax jurisdictions.
Israel:
The Israeli corporate income tax rate was 23% in 2023 and 2022. Income not eligible for Preferred Enterprise benefits is taxed at the regular corporate tax rates as described above.
The following table sets forth the computation of the loss per share for the period presented (in thousands, except for share data):
Schedule of Diluted Net Loss Per Share
| 2023 | 2022 | |||||||
| For the year Ended | ||||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Net loss attributable to common stockholders, basic and diluted | (48,072 | ) | (38,013 | ) | ||||
| Weighted average common shares outstanding, basic and diluted* | ||||||||
| Warrants | 22,965,771 | 87,453 | ||||||
| Stock options | 102,892 | 197,897 | ||||||
| Anti dilutive shares outstanding which were not included in the diluted calculation | 23,068,663 | 285,350 | ||||||
| Net loss per share attributable to common stockholders, basic and diluted | $ | ) | $ | ) | ||||
14. Related parties
| a. | Israel Biotech Fund I, L.P., Israel Biotech Fund II, L.P., Arkin Bio Ventures L.P. hold each 20% of the company, in addition after converting the Convertible Loan in 2024, they collectively hold 75% of the Company’s shares, in addition they have three affiliated directors in the Company’s Board of Directors. |
Schedule of Related Party Transactions
| b. | The following related party balances are included in the consolidated balance sheets: |
| 2023 | 2022 | |||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Convertible Loan (1) | $ | 8,007 | $ | - | ||||
| Long-term warrant liability (1) | $ | 5,915 | - | |||||
| Side Letter Agreement liabilities | $ | 8,175 | $ | - | ||||
| Accrued expenses (2) | $ | 118 | $ | 69 | ||||
| c. | The following related party transactions are included in the consolidated statements of income (loss): |
| 2023 | 2022 | |||||||
| Year ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Financial expenses (income), net (1) | $ | 8,351 | $ | - | ||||
| General and Administrative (2) | $ | 128 | $ | 65 | ||||
| (1) | In August and November 2023, the Company entered into Convertible Note agreement. See note 8. | |
| (2) | For director fees to members of Israel Biotech Fund I, L.P., Israel Biotech Fund II, L.P., Arkin Bio Ventures L.P. | |
| (3) | In November 2023, the Company entered into the Side Letter Agreement. See note 8. |
15. Prepaid Expenses and Other Current Assets
Schedule of Prepaid Expenses and Other Current Assets
| 2023 | 2022 | |||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Prepaid Insurances | $ | 2,144 | $ | 431 | ||||
| Short-term restricted bank deposits | 330 | 110 | ||||||
| Other Assets | 172 | 5 | ||||||
| Total | $ | 2,646 | $ | 546 | ||||
16. Finance expenses net
Schedule of Finance Expenses Net
| 2023 | 2022 | |||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Remeasurement of long-term warrant liability | $ | 5,854 | $ | - | ||||
| Remeasurement of Side Letter Agreements | 7,751 | - | ||||||
| Remeasurement of convertible note | 2,141 | - | ||||||
| Others | (28 | ) | (74 | ) | ||||
| Total | $ | 15,718 | $ | (74 | ) | |||
ASC Topic 740-10-30 clarifies
17. Subsequent Events
On February 5, 2024, the accountingCompany and Immunome, Inc. (“Immunome”), entered into an Asset Purchase Agreement (the “Asset Purchase Agreement”) pursuant to which Immunome agreed to acquire certain of the Company’s assets and liabilities related to its AL101 and AL102 programs (the “Asset Sale”), which constitute substantially all of the Company’s assets. See Note 1 for uncertaintyadditional information.
On February 7, 2024, the Secured Notes issued in income taxes recognizedAugust 2023 and the Senior Convertible Notes issued in November 2023 were converted into shares of Common Stock. In addition, the holders exercised warrants to purchase shares of the Common Stock issued in connection with the November Financing, on a cashless basis, for shares of the Common Stock.
On March 1, 2024, the Company issued an enterprise’s financial statementsadditional $2.0 million of convertible notes pursuant to the rights of the investors under the November 2023 side agreement, and prescribesreceived the proceeds of such loans.
On March 6, 2024, the Company received a recognition thresholdletter from a stockholder threatening legal action against the Company and measurement attributealleging breaches of fiduciary duty in connection with the Company’s January 9, 2023 merger with Advaxis, Inc., the Company’s October 19, 2023 merger with Biosight, Ltd., the Company’s November 17, 2023 issuance of Senior Convertible Promissory Notes and warrants for the financial statement recognitionpurchase of 15,000,000 shares of the Company’s stock, and measurementthe Company’s February 5, 2024 Asset Purchase Agreement with Immunone, Inc.
On March 21, 2024 the Company settled the Purported Stockholder Claims related to the January 2023 Merger with Old Ayala (see Note 7) for a cash settlement payment of $200 thousand.
As part of a tax position taken or expected to be taken in a tax return. ASC Topic 740-10-40 provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The Company will classify as income tax expense any interest and penalties. The Company has no material uncertain tax positions for any of the reporting periods presented. The Company files tax returns in U.S. federal and state jurisdictions, including New Jersey, and is subject to audit by tax authorities beginning withcost reduction plan, during the year ended OctoberDecember 31, 2013.
New Accounting Pronouncements
See Note 2 to our financial statements that discusses new accounting pronouncements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
At October 31, 2017,2023, the Company had a reduction in workforce in which the employment of approximately $70.9 million in cash, restricted cash, cash equivalents and investments, which consisted primarily50% of bank deposits, restricted cash, money market funds and short term investments such as certificates of deposit, domestic governmental agency loans and U.S treasury notes. The Company’s investment policy and strategy are focused on preservation of capital and supporting the Company’s liquidity requirements.employees was terminated. During the first quarter of 2024, the Company gave notice of termination to 18 additional employees and two officers (including the Chief Financial Officer, whose employment will terminate on June 25, 2024). After the effectiveness of such terminations, the Chief Executive Officer will be the only employee of the Company. The Company uses a combination of internal and external management to execute its investment strategy and achieve its investment objectives. The Company typically invests in highly-rated securities, and its investment policy generally limits the amount of credit exposure to any one issuer. The policy requires investments generallyexpects to be investment grade, with the primary objective of minimizing the potential risk of principal loss. Such interest-earning instruments carry a degree of interest rate risk; however, historical fluctuations of interest income have not been significant.able to meet its financial obligations to its employees.
We have not been exposed nor do we anticipate being exposed to material risks due to changes in interest rates. A hypothetical 10% change in interest rates during any of the periods presented would not have had a material impact on our consolidated financial statements.
| F-29 |
Item 8: Financial Statements and Supplementary Data.
The index to Financial Statements appears on the page immediately prior to page F-1, the Report of the Independent Registered Public Accounting Firms appears on page F-1, and the Financial Statements and Notes to Financial Statements appear on pages F-2 to F-26.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
Not applicable.None.
Item 9A:9A. Controls and Procedures.
Assessment of
Inherent Limitations on the Effectiveness of Internal Controls over Financial Reporting
Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an evaluation of the
The effectiveness of ourany system of internal control over financial reporting, usingincluding ours, is subject to inherent limitations, including the criteria set forth byexercise of judgment in designing, implementing, operating, and evaluating the Committeecontrols and procedures, and the inability to eliminate misconduct completely. Accordingly, in designing and evaluating the disclosure controls and procedures, management recognizes that any system of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework published in 2013. Based on its evaluation, our management concluded that our internal control over financial reporting, was effective as of the end of the period covered by this Annual Report on Form 10-K.
(a) Evaluation of Disclosure Controls and Procedures
An evaluation was performed under the supervision and with the participation of our management, including our chief executive officer and our chief financial officer as to the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report. Any controls and procedures,ours, no matter how well designed and operated, can only provide only reasonable, not absolute, assurance of achieving the desired control objectives. Based on that evaluation,In addition, the chief executive officer and the chief financial officerdesign of the Company have concluded that, as of the end of the period covered by this report, our disclosure controls and procedures are effective.
(b) Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. Internal control over financial reporting includes those policies and procedures that:
(i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
(ii) provide reasonable assurance that the transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with the authorization of management and/or our Board of Directors; and
(iii) provide reasonable assurance regarding the prevention or timely detection of any unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Due to its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate due to changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Marcum LLP, an independent registered public accounting firm, has audited the Consolidated Financial Statements included in this Annual Report on Form l0-K and, as part of the audit, has issued an attestation report, included herein, on the effectiveness of our internal control over financial reporting. See “Reports of Independent Registered Public Accounting Firm” included in this filling.
(c) Changes in Internal Control over Financial Reporting
During the quarter ended October 31, 2017, there were no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on the Effectiveness of Controls. Our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls must be consideredand procedures relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our company have been detected.

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM ON INTERNAL CONTROL OVER FINANCIAL REPORTING
To the Audit Committee of the
Board of Directors and Shareholders of
Advaxis, Inc.
We have audited Advaxis, Inc.’s (the “Company”) internal control over financial reporting as of October 31, 2017, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013. The Company’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Management Annual Report on Internal Control over Financial Reporting”. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also,Moreover, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Advaxis, Inc. maintained,
Material Weaknesses
A material weakness is a deficiency, or a combination of deficiencies, in all material aspects, effective internal control over financial reporting, such that a reasonable possibility exists that a material misstatement of our annual or interim financial statements could not be prevented or detected on a timely basis. We have identified material weaknesses in our controls to the aggregation of open control deficiencies across the Company’s financial reporting processes, a lack of a formal review process over preparation of financial information and an inadequate number of personnel to properly implement control procedures because the controls were not fully designed and operating effectively.
As such, management determined that it did not fully implement components of the COSO framework, including elements of the control environment, information and communication, control activities and monitoring activities components, relating to: (i) providing sufficient and timely management oversight and ownership over the internal control evaluation process; (ii) hiring and training sufficient personnel to timely support the Company’s internal control objectives; (iii) performing timely monitoring and oversight to ascertain whether the components of internal control are present and functioning effectively.
These deficiencies aggregated with other business process level control deficiencies could result in material misstatement in the financial statements and therefore constitute a material weakness. Based on this material weakness, the Company’s management concluded that as of OctoberDecember 31, 2017,2023, the Company’s internal control over financial reporting was not effective.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated, as of the end of the period covered by this Annual Report on Form 10-K, the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based on that evaluation, our principal executive officer and principal financial officer concluded that, as of December 31, 2023, our disclosure controls and procedures were not designed and operating effectively.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act. Our internal control over financial reporting is a process designed to provide reasonable assurance to our management and board of directors regarding the reliability of financial reporting and the preparation of the financial statements for external purposes in accordance with U.S. GAAP. Our management conducted an assessment of the effectiveness of our internal control over financial reporting based on the criteria establishedset forth in Internal“Internal Control –- Integrated Framework (2013)” issued by the Committee of Sponsoring Organizations of the Treadway CommissionCommission. Based on this assessment, our management concluded that, as of December 31, 2023, our internal control over financial reporting was not effective, management has identified a material weakness in 2013.internal controls related to the to the aggregation of open control deficiencies across the Company’s financial reporting processes, a lack of a formal review process over preparation of financial information and an inadequate number of personnel to properly implement control procedures.
Our financial conditions has prevented us from being able to employ sufficient resources to enable us to have an adequate level of financial reporting and review processes within our system of internal control, including to employ sufficient resources to properly implement control procedures regarding the Company’s financial reporting and review processes.
Therefore, it is difficult to ensure effective segregation of accounting and financial reporting duties or effective controls over financial reporting processes.
While we strive to remedy these controls as much as practicable, there is an insufficient volume of transactions at this point in time to justify additional full-time staff following the Company’s financial conditions. We believe that this is typical in many development companies. We may not be able to fully remediate the material weakness until we commence operations at which time, we would expect to hire more staff. We will continue to monitor and assess the costs and benefits of additional staffing.
Notwithstanding the results of this evaluation, management believes that the consolidated financial statements in this Annual Report on Form 10-K present, in all material aspects, the Company’s financial condition as reported in conformity with U.S. Generally Accepted Accounting Principles (“US GAAP”).
This Annual Report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to the SEC rules that require us to provide only management’s report in this Annual Report.
Changes in Internal control over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the quarter ended December 31, 2023 that have also audited, in accordancematerially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
On April 15, 2024, the Company’s Compensation Committee approved the payment to Roy Golan, the Company’s Chief Financial Officer, on or promptly after June 25, 2024, and conditioned upon his employment with the standardsCompany through such date, of a cash retention bonus in the Public Company Accounting Oversight Board (United States), the balance sheets asamount of October 31, 2017 and 2016, and the related statements of operations, shareholders’ equity, and cash flows for the years ended October 31, 2017, 2016 and 2015 of the Company and our report dated December 20, 2017 expressed an unqualified opinion on those financial statements.$350,000, subject to any applicable withholding requirements.
|

Not applicable.
| 32 |
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information with respect to this item is incorporated by reference from the definitive Proxy Statement for our 2024 Annual Meeting of Stockholders (the “2024 Proxy Statement”), which is to be filed with the Securities and Exchange Commission no later than April 29, 2024 (the day that is 120 days after the last day of our 2023 fiscal year). If the 2024 Proxy Statement is not filed by April 29, 2024 an amendment to this annual report on Form 10-K setting forth this information will be duly filed with the Securities and Exchange Commission.
Information regarding our executive officers is included at the end of Item 9B: Other Information.1 in Part I of this report.
None.ITEM 11. EXECUTIVE COMPENSATION
Information with respect to this item is incorporated by reference from the 2024 Proxy Statement. If the 2024 Proxy Statement is not filed by April 29, 2024 an amendment to this annual report on Form 10-K setting forth this information will be duly filed with the Securities and Exchange Commission.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Information with respect to this item is incorporated by reference from the 2024 Proxy Statement. If the 2024 Proxy Statement is not filed by April 29, 2024 an amendment to this annual report on Form 10-K setting forth this information will be duly filed with the Securities and Exchange Commission.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Information with respect to this item is incorporated by reference from the 2024 Proxy Statement. If the 2024 Proxy Statement is not filed by April 29, 2024 an amendment to this annual report on Form 10-K setting forth this information will be duly filed with the Securities and Exchange Commission.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Information with respect to this item is incorporated by reference from the 2024 Proxy Statement. If the 2024 Proxy Statement is not filed by April 29, 2024 an amendment to this annual report on Form 10-K setting forth this information will be duly filed with the Securities and Exchange Commission.
| 33 |
PART IV
Item 15. Exhibits and Financial Statement Schedules.
(a)(1) Financial Statements.
The financial statements required by this item are listed in Item 8, “Financial Statements and Supplementary Data” herein.
(a)(2) Financial Statement Schedules.
All financial statement schedules have been omitted because they are not applicable, not required or the information required is shown in the financial statements or the notes thereto.
(a)(3) Exhibits.
The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
Exhibit Index
See the Exhibit Index in Item 15(b) below.
| 34 |
Item 10: Directors, Executive Officers and Corporate Governance.
The information required by this Item is incorporated herein by reference from our Proxy Statement for our 2018 Annual Meeting of Stockholders.
Item 11: Executive Compensation.
The information required by this Item is incorporated herein by reference from our Proxy Statement for our 2018 Annual Meeting of Stockholders.
Item 12: Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters.
The information required by this Item is incorporated herein by reference from our Proxy Statement for our 2018 Annual Meeting of Stockholders.
Item 13: Certain Relationships and Related Transactions, and Director Independence.
The information required by this Item is incorporated herein by reference from our Proxy Statement for our 2018 Annual Meeting of Stockholders.
Item 14: Principal Accountant Fees and Services.
The information required by this Item is incorporated herein by reference from our Proxy Statement for our 2018 Annual Meeting of Stockholders.
Item 15: Exhibits and Financial Statements Schedules.
See Index of Exhibits below. The Exhibits are filed with or incorporated by reference in this report.
(a)Exhibits. The following exhibits are included herein or incorporated herein by reference.
| 35 |
| 36 |
| 37 |
| 99.1 | Definitive Proxy Statement of Old Ayala (f/k/a Ayala Pharmaceuticals, Inc.). Incorporated by reference to the description of the business of Old Ayala set forth in pages 233-273 of the Definitive Proxy Statement of Old Ayala for the Special Meeting of Stockholders of Old Ayala held on January 13, 2023, filed with the SEC on December 12, 2022 (File No. 001-39279). | |
| 101.INS** | Inline XBRL Instance Document | |
| 101.SCH** | Inline XBRL Taxonomy Extension Schema Document | |
| 101.CAL** | Inline XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF** | Inline XBRL Taxonomy Extension Definitions Linkbase Document | |
| 101.LAB** | Inline XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE** | Inline XBRL Taxonomy Extension Presentation Linkbase Document | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
| * | Filed herewith. |
| ** | Furnished herewith. |
| ‡ | Denotes management contract or compensatory plan or arrangement. |
| | |
| + | Certain schedules and exhibits to this agreement have been omitted in accordance with Item 601(a)(5) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the Securities and Exchange Commission on request. |
| † | Certain confidential information contained in this document, marked by ***, has been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K because it is both (i) not material and (ii) the type of information that the registrant treats as private or confidential. |
| (1) | Pursuant to Instruction 2 to Item 601 of Regulation S-K, the Company has filed a copy of one Common Stock Purchase Warrant (Convertible Note), and has set forth as follows the other documents omitted. The Company acknowledges that the Commission may at any time in its discretion require filing of copies of any documents so omitted. 1. Common Stock Purchase Warrant (Convertible Note), dated March 1, 2024, issued by Ayala Pharmaceuticals, Inc. to Israel Biotech Fund II, L.P. 2. Common Stock Purchase Warrant (Convertible Note), dated March 1, 2024, issued by Ayala Pharmaceuticals, Inc. to Arkin Bio Ventures L.P. 3. Common Stock Purchase Warrant (Convertible Note), dated March 1, 2024, issued by Ayala Pharmaceuticals, Inc. to Biotel Limited. |
Item 16. Form 10-K Summary
None.
| 38 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrantRegistrant has duly caused this Annual Report to be signed on its behalf by the undersigned, thereunto duly authorized in Princeton, Mercer County, State of New Jersey, on this 20thday of December 2017..
| Date: April 16, 2024 | By: | /s/ | |
| President and Chief Executive Officer | |||
| Date: April 16, 2024 | By: | /s/ Roy Golan. | |
| Roy Golan | |||
| Chief Financial Officer | |||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Daniel J. O’Connor and Sara M. Bonstein (with full power to act alone), as his true and lawful attorneys-in-fact and agents, with full powers of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitute or substitutes, lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this reportReport has been signed below by the following persons on behalf of the registrant andRegistrant in the capacities and on the dates indicated:indicated.
| Title | ||||
| /s/ Kenneth Berlin | President, Chief Executive Officer and Director | April 16, 2024 | ||
| Kenneth Berlin | (principal executive officer) | |||
| /s/ Roy Golan. | Chief Financial Officer | April 16, 2024 | ||
| Roy Golan | (principal financial and accounting officer) | |||
| /s/ David Sidransky | ||||
| Chairman of the Board of Directors | ||||
| David Sidransky | ||||
| /s/ Roni Appel | Director | April 16, 2024 | ||
| Roni Appel | ||||
| /s/ | Director | April 16, 2024 | ||
ADVAXIS, INC.
FINANCIAL STATEMENTS
INDEX
| April 16, 2024 | ||||
| Vered Bisker-Leib | ||||
| /s/ Yuval Cabilly | Director | April 16, 2024 | ||
| April 16, 2024 | ||||
| Murray A. Goldberg | ||||
| /s/ Bridget Martell | Director | April 16, 2024 | ||
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee of the
Board of Directors and Shareholders of
Advaxis, Inc.
We have audited the accompanying balance sheets of Advaxis, Inc. (the “Company”) as of October 31, 2017 and 2016, and the related statements of operations, shareholders’ equity and cash flows for the years ended October 31, 2017, 2016, and 2015. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Advaxis, Inc. as of October 31, 2017 and 2016, and the results of its operations and its cash flows for the years ended October 31, 2017, 2016, and 2015 in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Advaxis Inc.’s internal control over financial reporting as of October 31,2017, based on the criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated December 20, 2017 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
| Director | April 16, 2024 |
ADVAXIS, INC.
| October 31, | ||||||||
| 2017 | 2016 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 23,899,809 | $ | 112,750,980 | ||||
| Restricted cash | 587,000 | - | ||||||
| Investments | 46,398,304 | 39,336,548 | ||||||
| Income tax receivable | 4,452,682 | 2,549,862 | ||||||
| Deferred expenses | 2,986,385 | 4,291,385 | ||||||
| Prepaid expenses and other current assets | 2,918,644 | 946,423 | ||||||
| Total current assets | 81,242,824 | 159,875,198 | ||||||
| Property and equipment (net of accumulated depreciation) | 7,111,081 | 4,389,074 | ||||||
| Intangible assets (net of accumulated amortization) | 4,856,775 | 4,329,121 | ||||||
| Other assets | 431,098 | 450,667 | ||||||
| Total assets | $ | 93,641,778 | $ | 169,044,060 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 5,121,406 | $ | 1,720,428 | ||||
| Accrued expenses | 8,700,036 | 10,905,003 | ||||||
| Deferred revenue | 6,995,336 | 15,020,576 | ||||||
| Other current liabilities | 47,520 | 60,382 | ||||||
| Total current liabilities | 20,864,298 | 27,706,389 | ||||||
| Deferred revenue | 17,478,758 | 21,234,568 | ||||||
| Other liabilities | 1,038,555 | 800,909 | ||||||
| Total liabilities | 39,381,611 | 49,741,866 | ||||||
| Commitments and contingencies – Note 10 | ||||||||
| Shareholders’ equity: | ||||||||
| Preferred stock, $0.001 par value; 5,000,000 shares authorized; Series B Preferred Stock; 0 shares issued and outstanding at October 31, 2017 and 2016. Liquidation preference of $0 at October 31, 2017 and 2016. | - | - | ||||||
| Common stock - $0.001 par value; 65,000,000 shares authorized, 41,206,538 shares issued and outstanding at October 31, 2017 and 40,057,067 shares issued and 40,041,047 shares outstanding at October 31, 2016. | 41,207 | 40,057 | ||||||
| Additional paid-in capital | 355,361,187 | 327,098,749 | ||||||
| Treasury stock, at cost, 0 shares at October 31, 2017 and 16,020 shares October 31, 2016 | - | (129,787 | ) | |||||
| Accumulated deficit | (301,142,227 | ) | (207,706,825 | ) | ||||
| Total shareholders’ equity | 54,260,167 | 119,302,194 | ||||||
| Total liabilities and shareholders’ equity | $ | 93,641,778 | $ | 169,044,060 | ||||
The accompanying notes should be read in conjunction with the financial statements.
ADVAXIS, INC.
| Year Ended October 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Revenue | $ | 12,031,050 | $ | 3,994,856 | $ | - | ||||||
| Operating expenses: | ||||||||||||
| Research and development expenses | 71,900,462 | 48,774,589 | 24,455,447 | |||||||||
| General and administrative expenses | 38,658,464 | 31,712,505 | 24,243,690 | |||||||||
| Total operating expenses | 110,558,926 | 80,487,094 | 48,699,137 | |||||||||
| Loss from operations | (98,527,876 | ) | (76,492,238 | ) | (48,699,137 | ) | ||||||
| Other income (expense): | ||||||||||||
| Interest income | 669,759 | 331,529 | 114,219 | |||||||||
| Net changes in fair value of derivative liabilities | 20,156 | 69,055 | (48,950 | ) | ||||||||
| Other income (expense), net | (123 | ) | (201 | ) | (6,599 | ) | ||||||
| Net loss before income tax benefit | (97,838,084 | ) | (76,091,855 | ) | (48,640,467 | ) | ||||||
| Income tax benefit | 4,402,682 | 2,535,625 | 1,609,349 | |||||||||
| Net loss | $ | (93,435,402 | ) | $ | (73,556,230 | ) | $ | (47,031,118 | ) | |||
| Net loss per common share, basic and diluted | $ | (2.31 | ) | $ | (2.08 | ) | $ | (1.68 | ) | |||
| Weighted average number of common shares outstanding, basic and diluted | 40,527,844 | 35,400,980 | 28,026,197 | |||||||||
The accompanying notes should be read in conjunction with the financial statements.
ADVAXIS, INC.
STATEMENTS OF SHAREHOLDERS’ EQUITY
| Preferred Stock | Common Stock | Additional Paid-In | Treasury Stock | Accumulated | Total Shareholders’ | |||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Shares | Amount | Deficit | Equity | ||||||||||||||||||||||||||||
| Balance at October 31, 2014 | - | $ | - | 19,630,139 | $ | 19,630 | $ | 107,601,493 | - | $ | - | $ | (86,991,137 | ) | $ | 20,629,986 | ||||||||||||||||||||
| Stock based compensation | 1,167,976 | 1,169 | 21,374,861 | 21,376,030 | ||||||||||||||||||||||||||||||||
| Tax withholdings paid related to net share settlement of equity awards | (1,375,979 | ) | (1,375,979 | ) | ||||||||||||||||||||||||||||||||
| Tax withholdings paid on equity awards | (114,445 | ) | (1,388,086 | ) | (1,388,086 | ) | ||||||||||||||||||||||||||||||
| Tax shares sold to pay for tax withholdings on equity awards | 16,273 | 97,526 | 1,200,325 | (32,004 | ) | 1,184,594 | ||||||||||||||||||||||||||||||
| Common Stock issued upon exercise of options | 65,167 | 65 | 58,335 | 58,400 | ||||||||||||||||||||||||||||||||
| Common Stock issued upon exercise of warrants | 691,268 | 691 | 2,341,758 | 2,342,449 | ||||||||||||||||||||||||||||||||
| Conversion of notes payable into common stock | 4,104 | 4 | 39,928 | 39,932 | ||||||||||||||||||||||||||||||||
| Issuance of shares to employees under ESPP Plan | 7,063 | 7 | 28,784 | 28,791 | ||||||||||||||||||||||||||||||||
| Advaxis registered direct offerings | 8,806,165 | 8,806 | 63,046,722 | 63,055,528 | ||||||||||||||||||||||||||||||||
| Advaxis Public Offering | 3,220,000 | 3,220 | 56,675,128 | 56,678,348 | ||||||||||||||||||||||||||||||||
| Net Loss | (47,031,118 | ) | (47,031,118 | ) | ||||||||||||||||||||||||||||||||
| Balance at October 31, 2015 | - | $ | - | 33,591,882 | $ | 33,592 | $ | 249,807,303 | (16,919 | ) | $ | (187,761 | ) | $ | (134,054,259 | ) | $ | 115,598,875 | ||||||||||||||||||
| Stock based compensation | 1,042,527 | 1,043 | 23,451,904 | 23,452,947 | ||||||||||||||||||||||||||||||||
| Tax withholdings paid related to net share settlement of equity awards | (61,350 | ) | (61,350 | ) | ||||||||||||||||||||||||||||||||
| Tax withholdings paid on equity awards | (332,537 | ) | (2,729,230 | ) | (2,729,230 | ) | ||||||||||||||||||||||||||||||
| Tax shares sold to pay for tax withholdings on equity awards | 64,110 | 333,436 | 2,787,204 | (96,336 | ) | 2,754,978 | ||||||||||||||||||||||||||||||
| Common stock issued upon exercise of warrants | 122,661 | 123 | 614,245 | 614,368 | ||||||||||||||||||||||||||||||||
| Conversion of notes payable into common stock | 1,481 | 1 | 29,548 | 29,549 | ||||||||||||||||||||||||||||||||
| Issuance of shares to employees under ESPP Plan | 6,627 | 7 | 73,237 | 73,244 | ||||||||||||||||||||||||||||||||
| Advaxis registered direct offerings | 2,244,443 | 2,244 | 28,154,163 | 28,156,407 | ||||||||||||||||||||||||||||||||
| Sale of common shares to Amgen | 3,047,446 | 3,047 | 24,965,589 | 24,968,636 | ||||||||||||||||||||||||||||||||
| Net Loss | (73,556,230 | ) | (73,556,230 | ) | ||||||||||||||||||||||||||||||||
| Balance at October 31, 2016 | - | $ | - | 40,057,067 | $ | 40,057 | $ | 327,098,749 | (16,020 | ) | $ | (129,787 | ) | $ | (207,706,825 | ) | $ | 119,302,194 | ||||||||||||||||||
| Stock based compensation | 1,030,507 | 1,031 | 27,864,642 | 27,865,673 | ||||||||||||||||||||||||||||||||
| Tax withholdings paid related to net share settlement of equity awards | (356,949 | ) | (356,949 | ) | ||||||||||||||||||||||||||||||||
| Tax withholdings paid on equity awards | (997,029 | ) | (128,613 | ) | (881,055 | ) | (1,878,084 | ) | ||||||||||||||||||||||||||||
| Tax shares sold to pay for tax withholdings on equity awards | 843,526 | 144,633 | 1,010,842 | 1,854,368 | ||||||||||||||||||||||||||||||||
| Common stock issued upon exercise of warrants | 225 | 1,125 | 1,125 | |||||||||||||||||||||||||||||||||
| Issuance of shares to employees under ESPP Plan | 26,594 | 27 | 251,347 | 251,374 | ||||||||||||||||||||||||||||||||
| Advaxis at-the-market sales | 92,145 | 92 | 655,776 | 655,868 | ||||||||||||||||||||||||||||||||
| Net Loss | (93,435,402 | ) | (93,435,402 | ) | ||||||||||||||||||||||||||||||||
| Balance at October 31, 2017 | - | $ | - | 41,206,538 | $ | 41,207 | $ | 355,361,187 | - | $ | - | $ | (301,142,227 | ) | $ | 54,260,167 | ||||||||||||||||||||
The accompanying notes should be read in conjunction with the financial statements.
ADVAXIS, INC.
| Year ended October 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| OPERATING ACTIVITIES | ||||||||||||
| Net loss | $ | (93,435,402 | ) | $ | (73,556,230 | ) | $ | (47,031,118 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Stock compensation | 27,835,673 | 23,472,947 | 21,431,030 | |||||||||
| Loss (gain) on change in value of warrants and embedded derivative | (20,156 | ) | (69,055 | ) | 48,950 | |||||||
| Loss (gain) on disposal of property and equipment | 3,187 | - | (10,000 | ) | ||||||||
| Warrant expense | - | - | 8,170 | |||||||||
| Write-offof intangible assets | 315,394 | - | 28,480 | |||||||||
| Depreciation expense | 790,554 | 283,538 | 59,033 | |||||||||
| Amortization expense of intangible assets | 329,866 | 252,654 | 206,357 | |||||||||
| Amortization of premium on held to maturity investments | 183,947 | 252,730 | 60,608 | |||||||||
| Debt conversion expense | - | - | 6,599 | |||||||||
| Change in operating assets and liabilities: | ||||||||||||
| Prepaid expenses and other current assets | (1,972,221 | ) | (447,167 | ) | (293,096 | ) | ||||||
| Income taxes receivable | (1,902,820 | ) | (940,513 | ) | 121,968 | |||||||
| Other assets | 1,324,569 | (3,843,419 | ) | 104,529 | ||||||||
| Accounts payable and accrued expenses | 1,159,534 | 8,354,447 | 1,094,155 | |||||||||
| Deferred revenue | (11,781,050 | ) | 36,255,144 | - | ||||||||
| Other liabilities | 244,940 | 841,135 | ||||||||||
| Net cash used in operating activities | (76, 923,985 | ) | (9,143,789 | ) | (24,164,335 | ) | ||||||
| INVESTING ACTIVITIES | ||||||||||||
Restricted cash established with letter of credit agreement | (587,000 | ) | - | - | ||||||||
| Purchases of investments | (71,175,703 | ) | (44,524,783 | ) | (45,655,103 | ) | ||||||
| Proceeds from maturities and redemptions of investments | 63,930,000 | 50,530,000 | - | |||||||||
| Purchase of property and equipment | (3,449,271 | ) | (3,222,442 | ) | (972,859 | ) | ||||||
| Cost of intangible assets | (1,172,914 | ) | (1,226,742 | ) | (821,925 | ) | ||||||
| Net cash provided by (used in) investing activities | (12,454,888 | ) | 1,556,033 | (47,449,887 | ) | |||||||
| FINANCING ACTIVITIES | ||||||||||||
| Net proceeds of issuance of common stock | 655,868 | 53,125,043 | 119,733,876 | |||||||||
| Proceeds from employee stock purchase plan | 251,374 | 73,244 | 28,791 | |||||||||
| Proceeds from exercise of options | - | - | 58,400 | |||||||||
| Proceeds from the exercise of warrants | 1,125 | 614,368 | 2,342,449 | |||||||||
| Tax withholdings paid related to net share settlement of equity awards | (356,949 | ) | (61,350 | ) | (1,375,979 | ) | ||||||
| Employee tax withholdings paid on equity awards | (1,878,084 | ) | (2,729,230 | ) | (1,388,086 | ) | ||||||
| Tax shares sold to pay for employee tax withholdings on equity awards | 1,854,368 |
| 2,754,978 | 1,169,594 | ||||||||
| Net cash provided by financing activities | 527,702 | 53,777,053 | 120,569,045 | |||||||||
| Net increase (decrease) in cash and cash equivalents | (88,851,171 | ) | 46,189,297 | 48,954,823 | ||||||||
| Cash and cash equivalents at beginning of year | 112,750,980 | 66,561,683 | 17,606,860 | |||||||||
| Cash and cash equivalents at end of year | $ | 23,899,809 | $ | 112,750,980 | $ | 66,561,683 | ||||||
The accompanying notes should be read in conjunction with the financial statements.
Supplemental Disclosures of Cash Flow Information
| Year Ended October 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Cash paid for interest | $ | - | $ | - | $ | - | ||||||
| Cash paid for taxes | $ | 50,000 | $ | 50,000 | $ | - | ||||||
Supplemental Schedule of Noncash Investing and Financing Activities
| Year Ended October 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Accounts payable and accrued expenses settled with common stock | $ | 75,000 | $ | 55,000 | $ | - | ||||||
| Conversion of notes payable into common stock | $ | - | $ | 29,549 | $ | 39,932 | ||||||
| Sale of treasury shares pending settlement | $ | - | $ | - | $ | 15,000 | ||||||
| Property and equipment included in accounts payable and accrued expenses | $ | 66,477 | $ | 362,926 | $ | - | ||||||
The accompanying notes should be read in conjunction with the financial statements.
ADVAXIS, INC.
1. NATURE OF OPERATIONS AND BASIS OF PRESENTATION
Advaxis, Inc. (“Advaxis” or the “Company”) is a late-stage biotechnology Company focused on the discovery, development and commercialization of proprietaryLm Technology antigen delivery products based on a platform technology that utilizes live attenuatedListeria monocytogenes (“Lm”) bioengineered to secrete antigen/adjuvant fusion proteins. TheseLm-based strains are believed to be a significant advancement in immunotherapy as they integrate multiple functions into a single immunotherapy by accessing and directing antigen presenting cells to stimulate anti-tumor T cell immunity, stimulate and activate the immune system with the equivalent of multiple adjuvants, and simultaneously reduce tumor protection in the Tumor Microenvironment (“TME”) to enable the T cells to eliminate tumors. The Company believes thatLm Technology immunotherapies can complement and address significant unmet needs in the current oncology treatment landscape. Specifically, their product candidates have the potential to optimize checkpoint performance, while having a generally well-tolerated safety profile, and most of their product candidates are immediately available for treatment with a low cost of goods. The Company’s passion for the clinical potential ofLmTechnology is balanced by focus and fiscal discipline and driven towards increasing shareholder value.
Advaxis is focused on four franchises in various stages of clinical and pre-clinical development, which they believe will provide the greatest opportunity to have a significant impact on patients and their families:
| Pini Orbach |
All four clinical franchises are anchored in the Company’sLm TechnologyTM, a unique platform designed for its ability to safely and effectively target various cancers in multiple ways. As an intracellular bacterium,Lm is an effective vector for the presentation of antigens through both the Major Histocompatibility Complex (“MHC”) I and II pathways, due to its active phagocytosis by Antigen Presenting Cells (“APCs”). Within the APCs,Lm produces virulence factors which allow survival in the host cytosol and potently stimulate the immune system.
Liquidity and Financial Condition
The Company’s products that are being developed have not generated significant revenue. As a result, the Company has suffered recurring losses. These losses are expected to continue for an extended period of time. Our major sources of cash have been proceeds from various public and private offerings of our common stock, option and warrant exercises, and interest income. From October 2013 through October 2017, we raised approximately $222.5 million in gross proceeds from various public and private offerings of our common stock.
As of October 31, 2017, the Company had approximately $70.9 million in cash, restricted cash, cash equivalents and investments on its balance sheet. The Company plans to continue to be disciplined in regards to its utilization of its capital and anticipates its cash burn will decrease from fiscal 2017. This decrease will largely be due to several one-time items in fiscal 2017 related to the preparation of our MAA filing of axalimogene filolisbac and other one-time costs that the Company does not anticipate to recur. We believe our current cash position is sufficient to fund our business plan into fiscal 2019. The actual amount of cash that we will need to operate is subject to many factors.
The Company also recognizes it may need to raise additional capital in order to continue to execute its business plan. There is no assurance that additional financing will be available when needed or that management will be able to obtain financing on terms acceptable to the Company or whether the Company will become profitable and generate positive operating cash flow. If the Company is unable to raise sufficient additional funds, it will have to scale back its operations.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Estimates are used when accounting for such items as the fair value and recoverability of the carrying value of property and equipment and intangible assets (patents and licenses), the fair value of options, the fair value of embedded conversion features, warrants and related disclosure of contingent assets and liabilities. On an on-going basis, the Company evaluates its estimates, based on historical experience and on various other assumptions that it believes to be reasonable under the circumstances. Actual results may or may not differ from estimates.
Reclassification
Certain amounts in the prior period financial statements have been reclassified to conform to the presentation of the current period financial statements. These reclassifications had no effect on the previously reported net loss.
Collaboration Agreements
The Company evaluates whether an arrangement is a collaborative arrangement under the Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”) Topic 808, Collaborative Arrangements, at its inception based on the facts and circumstances specific to the arrangement. The Company also reevaluates whether an arrangement qualifies or continues to qualify as a collaborative arrangement whenever there is a change in either the roles of the participants or the participants’ exposure to significant risks and rewards dependent on the ultimate commercial success of the endeavor. For those collaborative arrangements where it is determined that the Company is the principal participant, costs incurred and revenue generated from third parties are recorded on a gross basis in the financial statements.
From time to time, the Company enters into collaborative arrangements for the research and development, manufacture and/or commercialization of products and product candidates. These collaborations generally provide for non-refundable, upfront license fees, research and development and commercial performance milestone payments, cost sharing, royalty payments and/or profit sharing. The Company’s collaboration agreements with third parties are performed on a ‘‘best efforts’’ basis with no guarantee of either technological or commercial success.
Revenue Recognition
The Company is expected to derive the majority of its revenue from patent licensing and research and development services associated with patent licensing. In general, these revenue arrangements provide for the payment of contractually determined fees in consideration for the grant of certain intellectual property rights for patented technologies owned or controlled by the Company. The intellectual property rights granted may be perpetual in nature, or upon the final milestones being met, or can be granted for a defined, relatively short period of time, with the licensee possessing the right to renew the agreement at the end of each contractual term for an additional minimum upfront payment. The Company recognizes licensing fees when there is persuasive evidence of a licensing arrangement, fees are fixed or determinable, delivery has occurred and collectability is reasonably assured.
Revenue associated with nonrefundable upfront license fees under arrangements where the license fees and research and development activities cannot be accounted for as separate units of accounting is deferred and recognized as revenue on a straight-line basis over the expected period of performance.
Revenues from the achievement of research and development milestones, if deemed substantive, are recognized as revenue when the milestones are achieved and the milestone payments are due and collectible. If not deemed substantive, the Company recognizes such milestones as revenue on a straight-line basis over the remaining expected performance period under the arrangement. All such recognized revenues are included in collaborative licensing and development revenue in the Company’s statements of operations.
Milestones are considered substantive if all of the following conditions are met: (1) the milestone is nonrefundable; (2) achievement of the milestone was not reasonably assured at the inception of the arrangement; (3) substantive effort is involved to achieve the milestone; and (4) the amount of the milestone appears reasonable in relation to the effort expended, and the other milestones in the arrangement and the related risk associated with the achievement of the milestone and any ongoing research and development or other services are priced at fair value.
If product development is successful, the Company will recognize revenue from royalties based on licensees’ sales of its products or products using its technologies. Royalties are recognized as earned in accordance with the contract terms when royalties from licensees can be reasonably estimated and collectability is reasonably assured. If royalties cannot be reasonably estimated or collectability of a royalty amount is not reasonably assured, royalties are recognized as revenue when the cash is received.
Deferred revenue represents the portion of payments received for which the earnings process has not been completed. Deferred revenue expected to be recognized within the next 12 months is classified as a current liability.
An allowance for doubtful accounts is established based on the Company’s best estimate of the amount of probable credit losses in the Company’s existing license fee receivables, using historical experience. The Company reviews its allowance for doubtful accounts periodically. Past due accounts are reviewed individually for collectability. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. To date, this is yet to occur.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash equivalents.
Restricted Cash and Letter of Credit
During 2017, the Company established a letter of credit with a financial institution as security for the purchase of custom equipment. The letter of credit is collateralized by cash which is unavailable for withdrawal or for usage for general obligations. No amount is outstanding under the letter of credit as of October 31, 2017.
Concentration of Credit Risk
The Company maintains its cash in bank deposit accounts (checking) that at times exceed federally insured limits. Approximately $23.1 million is subject to credit risk at October 31, 2017. However, these cash balances are maintained at creditworthy financial institutions. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant credit risk.
Investments
Investment securities consist of certificates of deposit, domestic governmental agency loans, and U.S. treasury notes. The Company classifies these securities as held-to-maturity. Held-to-maturity securities are those securities in which the Company has the ability and intent to hold until maturity. Held-to-maturity securities are recorded at amortized cost, adjusted for the amortization or accretion of premiums or discounts. Premiums and discounts are amortized or accreted over the life of the related held-to-maturity security as an adjustment to yield using the effective interest method.
A decline in the market value of any investment security below cost, that is deemed to be other than temporary, results in a reduction in the carrying amount to fair value. The impairment is charged to operations and a new cost basis for the security is established. Other-than-temporary impairment charges are included in Other Income (Expense), net. The Company did not recognize any impairment charges during the years ended October 31, 2017, 2016 or 2015. Interest income is recognized when earned.
Deferred Expenses
Deferred expenses consist of advanced payments made on research and development projects. Expense is recognized in the Statement of Operations as the research and development activity is performed.
Property and Equipment
Property and equipment is stated at cost. Additions and improvements that extend the lives of the assets are capitalized, while expenditures for repairs and maintenance are expensed as incurred. Leasehold improvements are amortized on a straight-line basis over the shorter of the asset’s estimated useful life or the remaining lease term. Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets ranging from three to ten years.
When depreciable assets are retired or sold the cost and related accumulated depreciation are removed from the accounts and any resulting gain or loss is recognized in operations.
Intangible Assets
Intangible assets are recorded at cost and include patents and patent application costs, licenses and software. Intangible assets are amortized on a straight-line basis over their estimated useful lives ranging from 3 to 20 years. Patent application costs are written-off if the application is rejected, withdrawn or abandoned.
Impairment of Long-Lived Assets
The company reviews its long-lived assets, including property and equipment and intangible assets, for impairment whenever events and circumstances indicate that the carrying value of an asset might not be recoverable. Recoverability of assets held and used is measured by comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated from the use of the asset and its eventual disposition.If the total of the undiscounted future cash flows is less than the carrying amount of those assets, an impairment loss is recognized in the Statement of Operations based on the excess of the carrying amount over the fair value of the asset.
Net Income (Loss) per Share
Basic net income or loss per common share is computed by dividing net income or loss available to common shareholders by the weighted average number of common shares outstanding during the period. Diluted earnings per share give effect to dilutive options, warrants, convertible debt and other potential Common Stock outstanding during the period. In the case of a net loss the impact of the potential Common Stock resulting from warrants, outstanding stock options and convertible debt are not included in the computation of diluted loss per share, as the effect would be anti-dilutive. In the case of net income, the impact of the potential Common Stock resulting from these instruments that have intrinsic value are included in the diluted earnings per share. The table sets forth the number of potential shares of Common Stock that have been excluded from diluted net loss per share.
| As of October 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Warrants | 3,092,935 | 3,110,575 | 3,241,466 | |||||||||
| Stock options | 3,893,558 | 3,351,795 | 1,981,939 | |||||||||
| Restricted stock units | 1,363,119 | 719,448 | 1,069,335 | |||||||||
| Convertible debt (using the if-converted method) | - | - | 1,576 | |||||||||
| Total | 8,349,612 | 7,181,818 | 6,294,316 | |||||||||
Research and Development Expenses
Research and development costs are expensed as incurred and include but are not limited to clinical trial and related manufacturing costs, payroll and personnel expenses, lab expenses, and related overhead costs.
Stock Based Compensation
The Company has an equity plan which allows for the granting of stock options to its employees, directors and consultants for a fixed number of shares with an exercise price equal to the fair value of the shares at date of grant. The Company measures the cost of services received in exchange for an award of equity instruments based on the fair value of the award. For employees and directors, the fair value of the award is measured on the grant date and for non-employees, the fair value of the award is generally measured based on contractual terms. The fair value amount is then recognized over the requisite service period, usually the vesting period, in both research and development expenses and general and administrative expenses on the statement of operations, depending on the nature of the services provided by the employees or consultants.
The process of estimating the fair value of stock-based compensation awards and recognizing stock-based compensation cost over their requisite service period involves significant assumptions and judgments. The Company estimates the fair value of stock option awards on the date of grant using the Black Scholes Model (“BSM”) for the remaining awards, which requires that the Company makes certain assumptions regarding: (i) the expected volatility in the market price of its Common Stock; (ii) dividend yield; (iii) risk-free interest rates; and (iv) the period of time employees are expected to hold the award prior to exercise (referred to as the expected holding period). As a result, if the Company revises its assumptions and estimates, stock-based compensation expense could change materially for future grants.
The Company accounts for stock-based compensation using fair value recognition and records forfeitures as they occur. As such, the Company recognizes stock-based compensation cost only for those stock-based awards that vest over their requisite service period, based on the vesting provisions of the individual grants.
Treasury Stock
The Company accounts for repurchases of common stock and shares withheld in lieu of taxes when restricted stock vests using the cost method with common stock in treasury classified in the balance sheet as a reduction in shareholders’ equity.
Fair Value of Financial Instruments
The carrying value of financial instruments, including cash and cash equivalents, restricted cash and accounts payable approximated fair value as of the balance sheet date presented, due to their short maturities. The carrying amounts of financing arrangements issued approximate fair value as of the balance sheet date presented, because interest rates on these instruments approximate market interest rates after consideration of stated interest rates, anti-dilution protection and associated warrants.
Derivative Financial Instruments
The Company does not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks. The Company evaluates all of its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the statements of operations. For stock-based derivative financial instruments, the Company used the Black Scholes valuation model which approximated the binomial lattice options pricing model to value the derivative instruments at inception and on subsequent valuation dates. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period. Derivative liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the instrument could be required within 12 months of the balance sheet date.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes in accordance with ASC Topic 740, “Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year and (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if based on the weight of the available positive and negative evidence, it is more likely than not some portion or all of the deferred tax assets will not be realized.
Recent Accounting Pronouncements
In May 2014, as part of its ongoing efforts to assist in the convergence of GAAP and International Financial Reporting Standards, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers, which is a new standard related to revenue recognition. Under the new standard, recognition of revenue occurs when a customer obtains control of promised services or goods in an amount that reflects the consideration to which the entity expects to receive in exchange for those goods or services. In addition, the standard requires disclosure of the nature, amount, timing, and uncertainty of revenue and cash flows arising from customer contracts. The standard must be adopted using either a full retrospective approach for all periods presented in the period of adoption or a modified retrospective approach. In July 2015, the FASB issued ASU 2015-14, Revenue from Contracts with Customers - Deferral of the Effective Date, which defers the implementation of this new standard to be effective for fiscal years beginning after December 15, 2017. Early adoption is permitted effective January 1, 2017. In March 2016, the FASB issued ASU 2016-08, Principal versus Agent Considerations, which clarifies the implementation guidance on principal versus agent considerations in the new revenue recognition standard pursuant to ASU 2014-09. In April 2016, the FASB issued ASU 2016-10, Identifying Performance Obligations and Licensing, and in May 2016, the FASB issued ASU 2016-12, Narrow-Scope Improvements and Practical Expedients, which amend certain aspects of the new revenue recognition standard pursuant to ASU 2014-09. In December 2016, the FASB issued ASU 2016-20, Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers to clarify the codification or to correct unintended application of guidance. In September and November 2017 , the FASB issued ASU 2017-13 , Revenue Recognition (Topic 605), Revenue from Contracts with Customers (Topic 606), Leases (Topic 840), and Leases (Topic 842) and ASU 2017-14, Income Statement—Reporting Comprehensive Income (Topic 220), Revenue Recognition (Topic 605), and Revenue from Contracts with Customers (Topic 606) which amends certain aspects of the new revenue recognition standard The Company is currently evaluating which transition approach we will utilize and the impact of adopting this accounting standard on the Company’s financial statements.
In August 2014, the FASB issued ASU 2014-15, Disclosures of Uncertainties About an Entity’s Ability to Continue as a Going Concern. The new standard provides guidance around management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures. The new standard is effective for fiscal years, and interim periods within those fiscal years, ending after December 15, 2016. The Company has adopted this standard effective for the year ending October 31, 2017. There was no impact on the Company’s financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases (“ASU 2016-02”). The standard amends the existing accounting standards for lease accounting, including requiring lessees to recognize most leases on their balance sheets and making targeted changes to lessor accounting. ASU 2016-02 will be effective beginning in the first quarter of fiscal 2020. Early adoption of ASU 2016-02 is permitted. The new leases standard requires a modified retrospective transition approach for all leases existing at, or entered into after, the date of initial application, with an option to use certain transition relief. In September, the FASB issued ASU 2017-13, Revenue Recognition (Topic 605), Revenue from Contracts with Customers (Topic 606), Leases (Topic 840), and Leases (Topic 842) which amends certain aspects of the new lease standard. The Company is currently evaluating the impact of adopting ASU 2016-02 on the Company’s financial statements.
In November 2016, the FASB issued ASU 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash. The new standard changes the presentation of restricted cash and cash equivalents on the statement of cash flows. Restricted cash and restricted cash equivalents will be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. The new standard is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017. Early adoption is permitted. This ASU is not expected to have a material impact on the Company’s financial statements.
In January 2017, the FASB issued ASU No. 2017-01, “Business Combinations (Topic 805): Clarifying the Definition of a Business.” The amendments in this Update clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions (or disposals) of businesses. The amendments in this Update provide a screen to determine when a set is not a business. If the screen is not met, it (1) requires that to be considered a business, a set must include, at a minimum, an input and a substantive process that together significantly contribute to the ability to create output and (2) removes the evaluation of whether a market participant could replace the missing elements. This Update is the final version of Proposed ASU 2015-330 Business Combinations (Topic 805) – Clarifying The Definition of a Business, which has been deleted. The amendments in this Update are effective for all entities for annual periods, and interim periods within those annual periods, beginning after December 15, 2017. Early adoption is permitted. This ASU is not expected to have a material impact on the Company’s financial statements.
In May 2017, the FASB issued ASU 2017-09, “Compensation—Stock Compensation (Topic 718): Scope of Modification Accounting” to provide clarity and reduce both (1) diversity in practice and (2) cost and complexity when applying the guidance in Topic 718, Compensation—Stock Compensation, to a change to the terms or conditions of a share-based payment award. The amendments in this Update provide guidance about which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting in Topic 718. This Update is the final version of Proposed ASU 2016-360—Compensation—Stock Compensation (Topic 718)—Scope of Modification Accounting, which has been deleted. The amendments in this Update are effective for all entities for annual periods, and interim periods within those annual periods, beginning after December 15, 2017. Early adoption is permitted. This ASU is not expected to have a material impact on the Company’s financial statements.
Management does not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material impact on the accompanying consolidated financial statements.
3. INVESTMENTS
The following table summarizes the Company’s investment securities at amortized cost as of October 31, 2017 and 2016:
| October 31, 2017 | ||||||||||||||||
| Amortized Cost, as Adjusted | Gross Unrealized Holding Gains | Gross Unrealized Holding Losses | Estimated Fair Value | |||||||||||||
| Short-term investments: | ||||||||||||||||
| Certificates of Deposit | $ | 11,391,147 | $ | - | $ | - | $ | 11,391,147 | ||||||||
| Domestic Governmental Agency Loans | 499,957 | - | 162 | 499,795 | ||||||||||||
| U.S Treasury Notes | 34,507,200 | - | 25,351 | 34,481,849 | ||||||||||||
| Total short-term investment securities | $ | 46,398,304 | $ | - | $ | 25,513 | $ | 46,372,791 | ||||||||
| October 31, 2016 | ||||||||||||||||
| Amortized Cost, as Adjusted | Gross Unrealized Holding Gains | Gross Unrealized Holding Losses | Estimated Fair Value | |||||||||||||
| Short-term investments: | ||||||||||||||||
| Certificates of Deposit | $ | 10,737,563 | $ | - | $ | - | $ | 10,737,563 | ||||||||
| Domestic Governmental Agency Loans | 2,500,000 | - | 250 | 2,499,750 | ||||||||||||
| U.S Treasury Notes | 26,098,985 | 2,404 | 7,556 | 26,093,833 | ||||||||||||
| Total short-term investment securities | $ | 39,336,548 | $ | 2,404 | $ | 7,806 | $ | 39,331,146 | ||||||||
All of the Company’s investments mature within the next 12 months.
4. PROPERTY AND EQUIPMENT
Property and equipment consists of the following:
| October 31, | ||||||||
| 2017 | 2016 | |||||||
| Leasehold improvements | $ | 2,167,990 | $ | 1,835,602 | ||||
| Laboratory equipment | 4,381,428 | 2,038,704 | ||||||
| Furniture and fixtures | 728,725 | 549,025 | ||||||
| Computer equipment | 394,523 | 240,910 | ||||||
| Construction in progress | 645,000 | 151,368 | ||||||
| Total property and equipment | 8,317,666 | 4,815,609 | ||||||
| Accumulated depreciation and amortization | (1,206,585 | ) | (426,535 | ) | ||||
| Net property and equipment | $ | 7,111,081 | $ | 4,389,074 | ||||
Depreciation expense for the years ended October 31, 2017, 2016 and 2015 was $790,554, $283,538 and $59,033, respectively.
5. INTANGIBLE ASSETS
Intangible assets consist of the following:
| October 31, | ||||||||
| 2017 | 2016 | |||||||
| Patents | $ | 5,727,298 | $ | 4,980,610 | ||||
| License | 776,992 | 776,992 | ||||||
| Software | 108,604 | 19,625 | ||||||
| Total intangibles | 6,612,894 | 5,777,227 | ||||||
| Accumulated amortization | (1,756,119 | ) | (1,448,106 | ) | ||||
| Net intangible assets | $ | 4,856,775 | $ | 4,329,121 | ||||
The expirations of the existing patents range from 2017 to 2038 but the expirations can be extended based on market approval if granted and/or based on existing laws and regulations. Capitalized costs associated with patent applications that are abandoned without future value are charged to expense when the determination is made not to pursue the application. Patent applications having a net book value of $315,394, $0 and $28,480 and were abandoned and were charged to research and development expenses in the Statement of Operations for the years ended October 31, 2017, 2016 and 2015, respectively. Amortization expense for intangible assets is included in general and administrative expenses and aggregated $329,866, $252,654 and $206,357 for the years ended October 31, 2017, 2016 and 2015, respectively.
At October 31, 2017, the estimated amortization expense by fiscal year based on the current carrying value of intangible assets is as follows:
| 2018 | $ | 365,848 | ||
| 2019 | 363,448 | |||
| 2020 | 346,593 | |||
| 2021 | 329,647 | |||
| 2022 | 329,647 | |||
| Thereafter | 3,121,592 | |||
| Total | $ | 4,856,775 |
6. ACCRUED EXPENSES:
The following table represents the major components of accrued expenses:
| October 31, | ||||||||
| 2017 | 2016 | |||||||
| Salaries and other compensation | $ | 2,652,583 | $ | 2,467,650 | ||||
| Vendors | 2,811,956 | 2,098,792 | ||||||
| Professional fees | 3,235,497 | 6,338,561 | ||||||
| Total accrued expenses | $ | 8,700,036 | $ | 10,905,003 | ||||
7. COMMON STOCK PURCHASE WARRANTS AND WARRANT LIABILITY
Warrants
A summary of warrant activity was as follows:
| Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life In Years | Aggregate Intrinsic Value | |||||||||||||
| Outstanding and exercisable warrants at October 31, 2014 | 4,158,092 | $ | 5.43 | 3.94 | $ | 9,518 | ||||||||||
| Issued | 2,361 | 7.20 | ||||||||||||||
| Exercised * | (769,349 | ) | 5.12 | |||||||||||||
| Expired | (149,638 | ) | 14.61 | |||||||||||||
| Outstanding and exercisable warrants at October 31, 2015 | 3,241,466 | $ | 5.07 | 2.90 | $ | 19,588,099 | ||||||||||
| Exercised | (122,661 | ) | 5.01 | |||||||||||||
| Expired | (8,230 | ) | 18.75 | |||||||||||||
| Outstanding and exercisable warrants at October 31, 2016 | 3,110,575 | $ | 5.04 | 1.91 | $ | 9,558,159 | ||||||||||
| Exercised | (225 | ) | 5.00 | |||||||||||||
| Expired | (17,955 | ) | 11.43 | |||||||||||||
| Outstanding and exercisable warrants at October 31, 2017 | 3,092,395 | $ | 5.00 | 0.92 | $ | - | ||||||||||
* Includes the cashless exercise of 300,376 warrants that resulted in the issuance of 222,295 shares of common stock.
At October 31, 2017, the Company had all of its total 3.09 million outstanding warrants classified as equity (equity warrants). At October 31, 2016, the Company had approximately 3.09 million of its total 3.11 million outstanding warrants classified as equity. At October 31, 2015, the Company had approximately 3.22 million of its total 3.24 million outstanding warrants classified as equity. At issuance, equity warrants are recorded at their relative fair values, using the Relative Fair Value Method, in the shareholders equity section of the balance sheet. The Company’s equity warrants can only be settled through the issuance of shares and are not subject to anti-dilution provisions.
Warrant Liability
At October 31, 2017, the Company had no warrants classified as liabilities (liability warrants), as all of the liability warrants expired. At October 31, 2016, the Company had approximately 18,000 of its total 3.11 million outstanding warrants classified as liabilities. At October 31, 2015, the Company had approximately 18,000 of its total 3.24 million outstanding warrants classified as liabilities. The Company utilizes the BSM to calculate the fair value of these warrants at issuance and at each subsequent reporting date. For those warrants with exercise price reset features (anti-dilution provisions), the Company computes multiple valuations, each quarter, using an adjusted BSM, to account for the various possibilities that could occur due to changes in the inputs to the BSM as a result of contractually-obligated changes (for example, changes in strike price to account for down-round provisions). The Company effectively weights each calculation based on the likelihood of occurrence to determine the value of the warrants at the reporting date. As of October 31, 2015, all of the liability warrants that were subject to weighted-average anti-dilution provisions had expired. The remaining liability warrants contain a cash settlement provision in the event of a fundamental transaction (as defined in the Common Stock purchase warrant). Any changes in the fair value of the warrant liability (i.e. - the total fair value of all outstanding liability warrants at the balance sheet date) between reporting periods will be reported on the statement of operations.
At October 31, 2017 and October 31, 2016, the fair value of the warrant liability was $0 and $20,156, respectively and is reflected in other current liabilities in the Balance Sheet. For the years ended October 31, 2017, 2016 and 2015, the Company reported income of $20,156, income of $69,055 and a loss of $48,950, respectively, due to changes in the fair value of the warrant liability.
In fair valuing the warrant liability, at October 31, 2016 and 2015, the Company used the following inputs in its BSM:
| 10/31/2016 | 10/31/2015 | |||||||
| Exercise price | $ | 10.63-18.75 | $ | 10.63-18.75 | ||||
| Stock price | $ | 8.09 | $ | 11.09 | ||||
| Expected term | 0.55-0.75 years | 1.52-1.76 years | ||||||
| Volatility % | 81.84%-87.09 | % | 93.87%-95.00 | % | ||||
| Risk free rate | 0.51%-0.66 | % | .075 | % | ||||
Warrants with anti-dilution provisions
Some of the Company’s warrants contained anti-dilution provisions originally set at an exercise price of $25.00 with a term of five years. As of October 31, 2015, all of these warrants had expired. If the Company had issued any Common Stock, except for exempt issuances as defined in the warrant agreement, for consideration less than the exercise price, then the exercise price and the amount of warrant shares available would have been adjusted to a new price and amount of shares per the “weighted average” formula included in the warrant agreement. For the year ended October 31, 2015, this anti-dilution provision required the Company to issue approximately 2,400 additional warrant shares, and the exercise price to be lowered to $7.20.
For those warrants with exercise price reset features (anti-dilution provisions), the Company computed multiple valuations, each quarter, using an adjusted BSM, to account for the various possibilities that could occur due to changes in the inputs to the BSM as a result of contractually-obligated changes (for example, changes in strike price to account for down-round provisions). The Company utilized different exercise prices of $7.20 and $6.00, weighting the possibility of warrants being exercised at $7.20 between 40% and 50% and warrants being exercised at $6.00 between 50% and 60%.
8. SHARE BASED COMPENSATION
The following table summarizes share-based compensation expense included in the Statement of Operations by expense category for the years ended October 31, 2017, 2016 and 2015, respectively:
| Year Ended October 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Research and development | $ | 5,647,913 | $ | 7,985,651 | $ | 6,293,791 | ||||||
| General and administrative | 22,187,760 | 15,487,296 | 15,137,239 | |||||||||
| Total | $ | 27,835,673 | $ | 23,472,947 | $ | 21,431,030 | ||||||
Amendments
The Advaxis, Inc. 2015 Incentive Plan (the “2015 Plan”) was originally ratified and approved by the Company’s stockholders on May 27, 2015. Subject to proportionate adjustment in the event of stock splits and similar events, the aggregate number of shares of Common Stock that may be issued under the 2015 Plan is 3,600,000 shares, plus a number of additional shares (not to exceed 650,000) underlying awards outstanding as of the effective date of the 2015 Plan under the prior plan that thereafter terminate or expire unexercised, or are cancelled, forfeited or lapse for any reason.
At the Annual Meeting of Stockholders of the Company held on March 10, 2016, the stockholders ratified and approved an amendment to the 2015 Plan to increase the aggregate number of shares of common stock authorized for issuance under such plan from 3,600,000 shares to 4,600,000 shares. Furthermore, the stockholders approved an amendment to the Company’s Certificate of Incorporation to increase the total number of authorized shares of common stock from 45,000,000 shares of common stock to 65,000,000 shares of common stock.
At the Annual Meeting of Stockholders of the Company held on April 5, 2017, the stockholders ratified and approved an amendment to the 2015 Plan to increase the aggregate number of shares of common stock authorized for issuance under such plan from 4,600,000 shares to 6,100,000 shares. The amendment also included a provision that provides for pre-defined annual increases in the number of shares available for issuance under the Plan equal to the lesser of: (i) 5% of the total number of shares of Common Stock outstanding, (ii) 2,500,000, or (iii) a lesser number determined by the Board of Directors. As of October 31, 2017, there were 710,853 shares available for issuance under the 2015 Plan.
Restricted Stock Units (RSUs)
A summary of the Company’s RSU activity and related information for the year ended October 31, 2017, 2016 and 2015 is as follows:
| Number of RSU’s | Weighted-Average Grant Date Fair Value | |||||||
| Balance at October 31, 2014: | 791,879 | $ | 3.81 | |||||
| Granted | 864,192 | 15.14 | ||||||
| Vested | (583,403 | ) | 7.58 | |||||
| Cancelled | (3,333 | ) | 11.76 | |||||
| Balance at October 31, 2015: | 1,069,335 | $ | 10.89 | |||||
| Granted | 695,040 | 9.31 | ||||||
| Vested | (824,317 | ) | 8.35 | |||||
| Cancelled | (220,610 | ) | 15.81 | |||||
| Balance at October 31, 2016 | 719,448 | $ | 10.77 | |||||
| Granted | 1,632,134 | 7.90 | ||||||
| Vested | (877,383 | ) | 9.15 | |||||
| Cancelled | (111,080 | ) | 8.74 | |||||
| Balance at October 31, 2017 | 1,363,119 | $ | 8.54 | |||||
The fair value as of the respective vesting dates of RSUs was approximately $6,045,000, $6,643,000 and $7,771,000 for the years ended October 31, 2017, 2016 and 2015, respectively.
As of October 31, 2017, there was approximately $9,434,000 of unrecognized compensation cost related to non-vested RSUs, which is expected to be recognized over a remaining weighted average vesting period of approximately 1.99 years.
As of October 31, 2017, the aggregate intrinsic value of non-vested RSUs was approximately $4,635,000.
Employee Stock Awards
Common Stock issued to executives and employees related to vested incentive retention awards, employment inducements, management purchases and employee excellence awards totaled 878,948 shares (834,600 shares on a net basis after employee taxes), 719,610 shares (712,106 shares on a net basis after employee taxes), and 506,736 shares (422,781 shares on a net basis after employee taxes) during the years ended October 31, 2017, 2016 and 2015, respectively. Total stock compensation expense associated with these awards for the years ended October 31, 2017, 2016 and 2015 was $8,883,123, $5,458,823 and $5,432,494, respectively.
Furthermore, during the year ended October 31, 2015, non-executive employees were entitled to receive a performance-based year-end cash bonus. Several non-executive employees voluntarily requested to be paid all or a portion of their cash bonus in the Company’s common stock instead of cash. During the year ended October 31, 2015, the total fair value of these equity purchases were $102,022, or 9,150 shares of the Company’s Common Stock.
Director Stock Awards
During the years ended October 31, 2017, 2016 and 2015, common stock issued to the Directors for compensation related to board and committee membership was 30,000 shares, 152,386 shares and 267,186, respectively. During the years ended October 31, 2017, 2016 and 2015, total stock compensation expense to the Directors was $403,200, $1,184,780 and $1,223,118, respectively.
Stock Options
A summary of changes in the stock option plan for the years ended October 31, 2017, 2016 and 2015 is as follows:
| Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life In Years | Aggregate Intrinsic Value | |||||||||||||
| Outstanding as of October 31, 2014 | 467,968 | $ | 15.51 | 6.34 | $ | - | ||||||||||
| Granted | 1,668,995 | 13.41 | ||||||||||||||
| Exercised * | (137,667 | ) | 12.29 | |||||||||||||
| Cancelled or expired | (17,357 | ) | 36.24 | |||||||||||||
| Outstanding as of October 31, 2015 | 1,981,939 | $ | 13.78 | 8.72 | $ | 285,330 | ||||||||||
| Granted | 1,385,000 | 12.81 | ||||||||||||||
| Cancelled or expired | (15,144 | ) | 29.69 | |||||||||||||
| Outstanding as of October 31, 2016 | 3,351,795 | $ | 13.31 | 7.82 | $ | 61,980 | ||||||||||
| Granted | 556,952 | 7.71 | ||||||||||||||
| Cancelled or expired | (15,189 | ) | 14.07 | |||||||||||||
| Outstanding as of October 31, 2017 | 3,893,558 | $ | 12.51 | 5.72 | $ | - | ||||||||||
| Vested and exercisable at October 31, 2017 | 2,795,826 | $ | 13.05 | 4.80 | $ | - | ||||||||||
* Includes the cashless exercise of 117,667 options that resulted in the issuance of 45,167 shares of common stock.
The following table summarizes information about the outstanding and exercisable options at October 31, 2017;
| Options Outstanding | Options Exercisable | |||||||||||||||||||||||||||||||
| Weighted | Weighted | Weighted | Weighted | |||||||||||||||||||||||||||||
| Average | Average | Average | Average | |||||||||||||||||||||||||||||
| Exercise | Number | Remaining | Exercise | Intrinsic | Number | Remaining | Exercise | Intrinsic | ||||||||||||||||||||||||
| Price Range | Outstanding | Contractual | Price | Value | Exercisable | Contractual | Price | Value | ||||||||||||||||||||||||
| $3.63 - $9.99 | 672,672 | 6.58 | $ | 7.91 | $ | 61,980 | 285,954 | 4.02 | $ | 8.19 | $ | - | ||||||||||||||||||||
| $10.00 - $14.99 | 3,006,606 | 5.63 | $ | 13.14 | $ | - | 2,295,592 | 4.95 | $ | 13.18 | $ | - | ||||||||||||||||||||
| $15.01 - $19.99 | 213,480 | 4.29 | $ | 18.07 | $ | - | 213.480 | 4.29 | $ | 18.07 | $ | - | ||||||||||||||||||||
| $20.00 - $21.25 | 800 | 2.60 | $ | 21.25 | $ | - | 800 | 2.60 | $ | 21.25 | $ | - | ||||||||||||||||||||
The fair value of each option granted from the Company’s stock option plans during the years ended October 31, 2017, 2016 and 2015 was estimated on the date of grant using the Black-Scholes option-pricing model. Using this model, fair value is calculated based on assumptions with respect to (i) expected volatility of the Company’s Common Stock price, (ii) the periods of time over which employees and Board Directors are expected to hold their options prior to exercise (expected lives), (iii) expected dividend yield on the Company’s Common Stock, and (iv) risk-free interest rates, which are based on quoted U.S. Treasury rates for securities with maturities approximating expected lives of the options. The Company used their own historical volatility in determining the volatility to be used. The expected term of the stock option grants was calculated using the “simplified” method in accordance with the SEC Staff Accounting Bulletin 107. The “simplified” method was used since the Company believes its historical data does not provide a reasonable basis upon which to estimate expected term and the Company does not have enough option exercise data from its grants issued to support its own estimate as a result of vesting terms and changes in the stock price. The expected dividend yield is zero as the Company has never paid dividends to common shareholders and does not currently anticipate paying any in the foreseeable future.
The following table provides the weighted average fair value of options granted to directors and employees and the related assumptions used in the Black-Scholes model:
| Year Ended | ||||||||||||
| October 31, 2017 | October 31, 2016 | October 31, 2015 | ||||||||||
| Weighted average fair value of options granted | $ | 6.36 | $ | 10.71 | $ | 17.38 | ||||||
| Expected term | 5.50-6.50 years | 5.51-6.51 years | 5-10 years | |||||||||
| Expected volatility | 107.07%-110.93 | % | 109.23%-115.25 | % | 109.26%-154.54 | % | ||||||
| Expected dividends | 0 | % | 0 | % | 0 | % | ||||||
| Risk free interest rate | 1.26%-1.58 | % | 1.65-2.00 | % | 1.41%-2.27 | % | ||||||
Total compensation cost related to the Company’s outstanding stock options, recognized in the statement of operations for the years ended October 31, 2017, 2016 and 2015 was approximately $17,195,000, $15,223,000 and $9,521,000, respectively. Included in compensation expense is $1,641,000 recognized as a result of the modification of certain option agreements associated with the resignation of the Company’s Chief Executive Officer in July 2017. Pursuant to the separation agreement all the outstanding options vested immediately and the expiration date was extended until July 5, 2021.
During the year ended October 31, 2017, 556,952 options were granted with a total grant date fair value of approximately $3,542,000. During the year ended October 31, 2016, 1,385,000 options were granted with a total grant date fair value of approximately $14,838,000. During the year ended October 31, 2015, 1,668,995 options were granted with a total grant date fair value of approximately $29,014,000.
As of October 31, 2017, there was approximately $4,680,000 of unrecognized compensation cost related to non-vested stock option awards, which is expected to be recognized over a remaining weighted average vesting period of approximately 1.11 years.
Shares Issued to Consultants
During the year ended October 31, 2017, 165,907 shares of Common Stock valued at $1,384,350 were issued to consultants for services. The Company recorded a liability on its balance sheet for $45,000 for shares earned pursuant to consulting agreements but not delivered. The common stock share values were based on the dates the shares vested.
During the year ended October 31, 2016, 168,885 shares of Common Stock valued at $1,565,888 were issued to consultants for services. The Company recorded a liability on its balance sheet for $75,000 for shares earned pursuant to consulting agreements but not delivered. The common stock share values were based on the dates the shares vested.
During the year ended October 31, 2015, 378,538 shares of Common Stock valued at $4,707,440 were issued to consultants for services. The Company recorded a liability on its balance sheet for $55,000 for shares earned pursuant to consulting agreements but not delivered. The common stock share values were based on the dates the shares vested.
2011 Employee Stock Purchase Plan
The Advaxis, Inc. 2011 Employee Stock Purchase Plan (“ESPP”) was approved by the Company’s shareholders in September 2011. The ESPP allows employees to purchase Common Stock of the Company at a 15% discount to the market price on designated exercise dates. Employees were eligible to participate in the ESPP beginning December 30, 2011. 40,000 shares of the Company’s Common Stock are reserved for issuance under the ESPP.
During the year ended October 31, 2017, 2016 and 2015 shares purchased under the ESPP were 26,594, 6,627 and 7,063 and the Company recorded expense of $251,374, $73,244 and $28,791 respectively. As of October 31, 2017, 0 shares of Company’s Common Stock remain available for issuance under the ESPP.
9. COLLABORATION AND LICENSING AGREEMENTS
BMS
On May 30, 2017, the Company announced a clinical development collaboration with BMS to evaluate ADXS-DUAL, its second investigational immunotherapy targeting HPV-associated cancers, and BMS’ PD-1 immune checkpoint inhibitor, Opdivo ® (nivolumab), as a potential combination treatment option for women with metastatic cervical cancer.
Under the terms of the agreement, each party will bear their own internal costs and provide its immunotherapy agent. The Company will sponsor the trial and pay third-party costs.
Sellas Life Science Group
On February 27, 2017, the Company entered into a license agreement with Sellas Life Science Group (“Sellas”) to develop a novel cancer immunotherapy agent using Advaxis’ proprietaryLm-based antigen delivery product with SELLAS’ patented WT1 targeted heteroclitic peptide antigen mixture (galinpepimut-S)). Pursuant to the agreement, Advaxis will conduct all pre-clinical activities required for an IND filing and Sellas will be responsible for all clinical development and commercial activities. Advaxis will receive future payments of up to $358 million from SELLAS if certain development, regulatory, and commercial milestones are met. SELLAS has agreed to pay Advaxis single-digit to low double-digit royalties based on worldwide net sales upon commercialization. If SELLAS sublicenses its rights, Advaxis will receive a percentage of applicable sublicense revenue paid.
Amgen
On August 1, 2016, the Company entered into a global agreement (the “Amgen Agreement”) with Amgen for the development and commercialization of the Company’s ADXS-NEO, a novel, preclinical investigational immunotherapy, using the Company’s proprietary Listeria monocytogenes attenuated bacterial vector which activates a patient’s immune system to respond against unique mutations, or neoepitopes, contained in and identified from an individual patient’s tumor. Under the terms of the Amgen Agreement, Amgen receives an exclusive worldwide license to develop and commercialize ADXS-NEO. Amgen made an upfront payment to Advaxis of $40 million and purchased $25 million of Advaxis common stock. Amgen will fund the clinical development and commercialization of ADXS-NEO and Advaxis will retain manufacturing responsibilities. Advaxis and Amgen will collaborate through a joint steering committee for the development and commercialization of ADXS-NEO. Advaxis will also receive development, regulatory and sales milestone payments of up to $475 million and high single digit to double digit royalty payments based on worldwide sales.
The Company identified the following performance obligations under the agreement: 1) the license, 2) the obligation to provide research activities, 3) the obligation to provide clinical supplies, 4) the obligation to perform regulatory functions and 5) the obligation to participate on a Joint Research Committee.
The Company considered the provisions of the multiple-element arrangement guidance in determining how to recognize the total consideration of the agreement. The Company determined that none of the deliverables have standalone value; all of these obligations will be delivered throughout the estimated period of performance and therefore are accounted for as a single unit of accounting. Accordingly, the Company recorded the $40 million upfront payment as deferred revenue on the balance sheet and will recognize revenue on a straight-line basis over the estimated period of performance under the Amgen Agreement. Changes in the estimated period of performance will be accounted for prospectively as a change in estimate. During the years ended October 31, 2017 and 2016, the Company recognized revenue from the Amgen Agreement of approximately $11,781,000 and $3,745,000 related to amortization of the upfront fees.
In connection with the Amgen Agreement, Amgen purchased directly from Advaxis 3,047,446 shares of the Company’s Common Stock, at approximately $8.20 per share (representing a purchase at market using a 20 day VWAP methodology). The gross proceeds to Advaxis from the sale of the shares was approximately $25 million.
The Company considered the provisions of the research and development and collaboration guidance in determining how to recognize the clinical development payments to be received from Amgen. The Company determined the clinical development payments should be accounted for within the scope of collaboration arrangement accounting guidance. As a result, the Company will account for the clinical development payments as a reduction of research and development expenses in the Statement of Operations. During the year ended October 31, 2017, the Company received clinical development payments from Amgen totaling $6,000,000. In addition, the Company recorded an expected clinical development payment of $1,500,000 as prepaid expenses and other current assets on the balance sheet. In November 2017, the Company received the $1,500,000 expected clinical development payment from Amgen.
Especificos Stendhal SA de CV
On February 3, 2016, the Company entered into a Co-Development and Commercialization Agreement (the “Stendhal Agreement”) with Especificos Stendhal SA de CV (“Stendhal”), for Advaxis’ leadLm Technology™ immunotherapy, axalimogene filolisbac, in HPV-associated cancers. Under the terms of the Stendhal Agreement, Stendhal will pay $10 million (“Support Payments”) towards the expense of AIM2CERV. The Support Payments will be made over the duration of the trial. Stendhal will also work with the Company to complete the clinical trial of axalimogene filolisbac in Mexico, Brazil, Colombia and other investigational sites in Latin American countries. Stendhal will manage and is responsible for the costs associated with the regulatory approval process, promotion, commercialization and market access for axalimogene filolisbac in these markets. Upon approval and commercialization of axalimogene filolisbac, Advaxis and Stendhal will share profits on a pre-determined basis.
The Company considered the provisions of the research and development and collaboration guidance in determining how to recognize the Support Payments to be received from Stendhal. The Company determined the Stendhal Agreement should be accounted for within the scope of collaboration arrangement accounting guidance. As a result, the Company will account for the support payments as a reduction of research and development expenses in the Statement of Operations. During the year ended October 31, 2017, the Company reached the annual project milestones and received a $3,000,000 Support Payment from Stendhal.
Knight Therapeutics
On August 26, 2015, the Company entered into a licensing agreement with Knight Therapeutics Inc. (“Knight”), a Canadian-based specialty pharmaceutical company focused on acquiring, in-licensing, selling and marketing innovative prescription and over-the-counter pharmaceutical products, to commercialize in Canada the Company’s product candidates. Under the terms of the licensing agreement, Knight will be responsible to conduct and fund all regulatory and commercial activities in Canada. The Company is eligible to receive royalty and sales. In connection with the licensing agreement, the Company sold directly to Knight 359,454 shares of the common stock at $13.91 per share
Under the terms of the agreement, Knight will be responsible to conduct and fund all regulatory and commercial activities in Canada. We are eligible to receive double digit royalty as well as approximately $33 million in cumulative sales milestones.
Merck & Co., Inc.
On August 22, 2014, the Company entered into a Clinical Trial Collaboration and Supply Agreement (the “Merck Agreement”) with Merck, pursuant to which the parties are collaborating on a Phase 1/2 dose-determination and safety trial. The Phase 1 portion of the trial evaluated the safety of ourLm -LLO based immunotherapy for prostate cancer, ADXS-PSA (the “Advaxis Compound”) as monotherapy and in combination with KEYTRUDA® (pembrolizumab), Merck’s humanized monoclonal antibody against PD-1, (the “Merck Compound”) and has determined a recommended Phase 2 combination dose. The Phase 2 portion is evaluating the safety and efficacy of the Advaxis Compound in combination with the Merck Compound. Both phases of the trial are in patients with previously treated metastatic castration-resistant prostate cancer. A joint development committee, comprised of equal representatives from both parties, is responsible for coordinating all regulatory and other activities under, and pursuant to, the Merck Agreement.
Each party is responsible for their own internal costs and expenses to support the trial, while the Company will be responsible for all third party costs of conducting the trial. Merck is responsible for manufacturing and supplying the Merck Compound. The Company is responsible for manufacturing and supplying the Advaxis Compound. The Company is the sponsor of the trial and hold the IND related to the trial.
All data and results generated under the trial (“Collaboration Data”) will be jointly owned by the parties, except that ownership of data and information generated from sample analysis to be performed by each party on its respective compound will be owned by the party conducting such testing. All rights to all inventions and discoveries, which claim or cover the combined use of the Advaxis Compound and the Merck Compound shall belong jointly to the parties. Inventions and discoveries relating solely to the Advaxis Compound, or a live attenuated bacterial vaccine, shall be the exclusive property of us. Inventions and discoveries relating solely to the Merck Compound, or a PD-1 antagonist, shall be the exclusive property of Merck.
The Merck Agreement shall continue in full force and effect until completion of all of the obligations of the parties or a permitted termination.
During the years ended October 31, 2017, 2016 and 2015, the Company incurred approximately $2,925,000, $1,587,000 and $1,723,000, respectively, in expenses pertaining to the Merck agreement, and such expenses were a component of research and development expenses in the statement of operations.
MedImmune/AstraZeneca
On July 21, 2014, the Company entered into a Clinical Trial Collaboration Agreement (the “MedImmune Agreement”) with MedImmune, the global biologics research and development arm of AstraZeneca, pursuant to which the parties initiated a Phase 1/2 clinical trial in the United States to evaluate the safety and efficacy of MedImmune’s investigational anti-PD-L1 immune checkpoint inhibitor, MEDI4736, in combination with our investigationalLm -LLO cancer immunotherapy, axalimogene filolisbac , as a combination treatment for patients with advanced, recurrent or refractory cervical cancer and HPV-associated head and neck cancer. A joint steering committee, composed of equal representatives from both parties, is responsible for various matters associated with the collaboration, including protocol approval, as well as reviewing and monitoring the progress of the trial.
MedImmune is responsible for providing MEDI4736 at no cost, as well as costs related to the proprietary assays performed by MedImmune or a third party on behalf of MedImmune. The Company is the sponsor of the trial and is responsible for the submission of all regulatory filings to support the trial, the negotiation and execution of the clinical trial agreements associated with each trial site, and the packaging and labelling of the Advaxis and MedImmune product candidates used in the trial and the costs associated therewith. For a period beginning upon the completion of the trial and the receipt by MedImmune of the last final report for the trial and ending one hundred twenty (120) days thereafter (unless extended), MedImmune will be granted first right to negotiate in good faith in an attempt to enter into an agreement with us with respect to the development, regulatory approval and commercialization of axalimogene filolisbac and MEDI4736 to be used in combination with each other for the treatment or prevention of cancer. Neither party is obligated to enter into such an agreement. In the event the parties do not enter an agreement and we obtain regulatory approval for axalimogene filolisbac in combination with any PD-1 antibody or PD-L1 antibody, we shall pay MedImmune a royalty obligation and one-time payment.
All intellectual property rights made, conceived or generated through the clinical trials that relate solely to a MedImmune development product shall be owned solely by MedImmune. All intellectual property rights made, conceived or generated through the clinical trials that relate solely to an Advaxis development product shall be owned solely by us. All intellectual property rights made, conceived or generated through the clinical trials that relate to the combination of one or more MedImmune development product and one or more Advaxis development product shall be jointly owned by both parties; provided, however that in the event the parties do not enter into a clinical development and commercialization agreement, we will not exploit, commercialize or license the joint inventions, except for the performance of its obligations under the MedImmune Agreement. MedImmune has the sole right to prosecute and enforce all patents and other intellectual property rights covering all joint inventions and all associated costs will be shared by the parties.
The MedImmune Agreement shall remain in effect until the earlier of (i) permitted termination, (ii) the parties entering into a clinical development and commercialization agreement or expiration of the negotiation period (unless extended), except with respect to rights that survive termination. Either party may terminate the MedImmune Agreement upon thirty (30) days written notice upon material breach of the other party, unless the breach is cured in such period or reasonable actions to cure the breach are initiated and pursued (if the breach is not capable of being cured during the 30-day notice period). In addition, either party may terminate the MedImmune Agreement immediately if the party determines in good faith that the trials may unreasonably affect the safety of trial subjects.
During the years ended October 31 2017, 2016 and 2015, the Company incurred approximately $2,787,000, $1,978,000 and $1,888,000, respectively, in expenses pertaining to the MedImmune agreement, and such expenses were a component of research and development expenses in the Statement of Operations.
Aratana Therapeutics
On March 19, 2014, the Company and Aratana entered into a definitive Exclusive License Agreement (the “Aratana Agreement”). Pursuant to the Agreement, Advaxis granted Aratana an exclusive, worldwide, royalty-bearing, license, with the right to sublicense, certain Advaxis proprietary technology that enables Aratana to develop and commercialize animal health products that will be targeted for treatment of osteosarcoma and other cancer indications in animals. Under the terms of the Aratana Agreement, Aratana paid an upfront payment to the Company, of $1 million. As this license has stand-alone value to Aratana (who has the ability to sublicense) and was delivered to Aratana, upon execution of the Aratana Agreement, the Company recorded the $1 million payment as licensing revenue during the 12 months ended October 31, 2014. Aratana will also pay the Company up to an additional $36.5 million based on the achievement of certain milestones with respect to the advancement of products pursuant to the terms of the Aratana Agreement. In addition, Aratana may pay the Company an additional $15 million in cumulative sales milestones pursuant to the terms of the Aratana Agreement.
Advaxis (i) issued and sold 306,122 shares of Advaxis’ Common Stock to Aratana at a price of $4.90 per share, which was equal to the closing price of the Common Stock on the NASDAQ Capital Market on March 19, 2014, and (ii) issued a ten-year warrant to Aratana giving Aratana the right to purchase up to 153,061 additional shares of Advaxis’ Common Stock at an exercise price of $4.90 per share. In connection with the sale of the Common Stock and warrants, Advaxis received aggregate net proceeds of $1,500,000.
Biocon Limited
On January 20, 2014, we entered into a Distribution and Supply Agreement (“Biocon Agreement”) with Biocon Limited, a company incorporated under the laws of India.
Pursuant to the Biocon Agreement, we granted Biocon an exclusive license (with a right to sublicense) to (i) use our data from clinical development activities, regulatory filings, technical, manufacturing and other information and know-how to enable Biocon to submit regulatory filings for axalimogene filolisbac in the following territories: India, Malaysia, Bangladesh, Bhutan, Maldives, Myanmar, Nepal, Pakistan, Sri Lanka, Bahrain, Jordan, Kuwait, Oman, Saudi Arabia, Qatar, United Arab Emirates, Algeria, Armenia, Egypt, Eritrea, Iran, Iraq, Lebanon, Libya, Sudan, Syria, Tunisia and Yemen (collectively, the “Territory”) and (ii) import, promote, market, distribute and sell pharmaceutical products containing axalimogene filolisbac .
Global BioPharma Inc.
On December 9, 2013, the Company entered into an exclusive licensing agreement for the development and commercialization of axalimogene filolisbac with Global BioPharma, Inc. (“GBP”), a Taiwanese based biotech company funded by a group of investors led by Taiwan Biotech Co., Ltd (TBC).
GBP is planning to conduct a randomized Phase 2, open-label, controlled trial in HPV-associated NSCLC in patients following first-line induction chemotherapy. GBP has obtained Taiwanese regulatory approval for this trial and plans to initiate this trial in 2018. This trial will be fully funded exclusively by GBP. GBP will continue to explore the use of our lead product candidate in several other indications including head and neck, and anal cancer. GBP also plans to conduct registration trials with axalimogene filolisbac for the treatment of advanced cervical cancer.
GBP will pay Advaxis event-based financial milestones, an annual license fee, and annual net sales royalty payments in the high single to double digits. In addition, as an upfront payment, GBP made an investment in Advaxis of $400,000 by purchasing from the Company 108,724 shares of its Common Stock at a price of $3.68 per share, GBP also received 100,000 warrants at an exercise price of $5.52 which expire in December 2018.
GBP will be responsible for all clinical development and commercialization costs in the GBP territory. GBP will also reimburse Advaxis $2.25 million toward AIM2CERV. GBP is committed to establishing manufacturing capabilities for its own. Under the terms of the agreement, we will exclusively license the rights of axalimogene filolisbac to GBP for the Asia, Africa, and former USSR territory, exclusive of India and certain other countries, for all HPV-associated indications. Advaxis will retain exclusive rights to axalimogene filolisbac for the rest of the world.
During each of the years ended October 31, 2017 and 2016, the Company recognized revenue of $250,000 for annual license fees.
10. COMMITMENTS AND CONTINGENCIES
Legal Proceedings
Knoll
On August 21, 2015, Knoll Capital Management L.P. (“KCM”) filed a complaint against the Company in the Delaware Court of Chancery. The complaint alleged the existence of an oral agreement for the purchase by Knoll from the Company of 1,666,666.67 shares of Company stock at a price of $3.00 per share. KCM alleged that the Company breached this alleged agreement and sought specific performance or, alternatively, money damages for breach of contract. KCM served the Company with the complaint on August 31, 2015, and then served an amended complaint on October 16, 2015. The Company moved to dismiss the amended complaint on October 26, 2015 and that motion was denied on January 29, 2016. The Company filed an answer to the amended complaint on February 12, 2016.
In lieu of continuing to unnecessarily incur litigation expenses, on April 27, 2017, the Company settled the matter for a non-material amount, predominately reimbursed by the Company’s insurance, and the parties entered into a definitive confidential settlement agreement. The Company expressly denies any admission or wrongdoing and the settlement was entered into solely for the purpose of avoiding the burden, inconvenience, and expense of further litigation. On May 11, 2017, following resolution of the matter by the parties, the Court granted a Stipulation Of Dismissal With Prejudice.
Bono
On August 20, 2015, a derivative complaint was filed by a purported Company shareholder in the United States District Court for the District of New Jersey styled David Bono v. O’Connor, et al., Case No. 3:15-CV-006326-FLW-DEA (D.N.J. Aug. 20, 2015) (the “Bono Action”). The complaint is based on general allegations related to certain stock options granted to the individual defendants and generally alleges counts for breaches of fiduciary duty and unjust enrichment. The complaint also alleges additional claims for violation of Section 14(a) of the Securities Exchange Act of 1934 and for waste of corporate assets. The complaint seeks damages and costs of an unspecified amount, disgorgement of compensation obtained by the individual defendants, and injunctive relief.
Defendants filed a motion to dismiss the Bono Action. On May 23, 2016, the Court issued an opinion and order granting in part and denying in part defendants’ motion to dismiss. On October 5, 2016, the Court denied plaintiff’s motion for reconsideration of its May 23 order. On April 13, 2017, the parties advised the Court that they had reached a tentative agreement in principle to settle the action, subject to negotiating an award of attorneys’ fees and expenses to plaintiff’s counsel and a stipulation of settlement, and, ultimately, Court approval. The parties subsequently executed the stipulation of settlement on October 2, 2017. The Court entered an order preliminarily approving the settlement on November 7, 2017. The final fairness hearing with the Court is presently scheduled for January 29, 2018.
Corporate Office & Manufacturing Facility Lease
The Company leases its corporate office and manufacturing facility under an operating lease expiring in November, 2025.
Future minimum payments under the Company’s operating leases are as follows:
| Year ended October 31, | ||||
| 2018 | $ | 1,041,895 | ||
| 2019 | 1,107,385 | |||
| 2020 | 1,232,907 | |||
| 2021 | 1,317,640 | |||
| 2022 | 1,368,819 | |||
| Thereafter | 4,378,521 | |||
| Total | $ | 10,447,167 | ||
Rent expense for the years ended October 31, 2017, 2016 and 2015 was $1,188,005, $935,281 and $150,000, respectively.
11. INCOME TAXES:
The income tax provision (benefit) consists of the following:
| October 31, 2017 | October 31, 2016 | October 31, 2015 | ||||||||||
| Federal | ||||||||||||
| Current | $ | - | $ | - | $ | - | ||||||
| Deferred | (34,296,121 | ) | (18,152,484 | ) | (14,513,684 | ) | ||||||
| State and Local | ||||||||||||
| Current | (4,452,682 | ) | (2,535,625 | ) | (1,609,349 | ) | ||||||
| Deferred | (1,123,593 | ) | (3,698,506 | ) | (1,840,276 | ) | ||||||
| Change in valuation allowance | 35,419,714 | 21,850,990 | 16,353,960 | |||||||||
| Income tax provision (benefit) | $ | (4,452,682 | ) | $ | (2,535,625 | ) | $ | (1,609,349 | ) | |||
The Company has U.S. federal net operating loss carryovers (“NOLs”) of approximately $187,254,000, $137,082,000 and $100,662,000 at October 31, 2017, 2016 and 2015, respectively, available to offset taxable income which expire beginning in 2023. If not used, these NOLs may be subject to limitation under Internal Revenue Code Section 382 should there be a greater than 50% ownership change as determined under the regulations. In fiscal years 2017 and 2016, the Company performed a detailed analysis of any historical and/or current Section 382 ownership changes that may limit the utilization of the net operating loss carryovers. From the entire federal NOL of $187,254,000 as of October 31, 2017, approximately $155,930,000 is available for immediate use based on Internal Revenue Code Section 382 analysis. The NOL and the deferred tax asset table below does not include approximately $24,824,000 of NOL’s that may expire unused. The Company also has New Jersey State Net Operating Loss carryovers of approximately $50,745,000, $66,029,000 and $26,245,000 as of October 31, 2017, 2016 and 2015, respectively, available to offset future taxable income through 2037.
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon future generation for taxable income during the periods in which temporary differences representing net future deductible amounts become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. After consideration of all the information available, management believes that significant uncertainty exists with respect to future realization of the deferred tax assets and has therefore established a full valuation allowance.
The Company evaluated the provisions of ASC 740 related to the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements. ASC 740 prescribes a comprehensive model for how a company should recognize, present, and disclose uncertain positions that the company has taken or expects to take in its tax return. For those benefits to be recognized, a tax position must be more-likely-than-not to be sustained upon examination by taxing authorities. Differences between tax positions taken or expected to be taken in a tax return and the net benefit recognized and measured pursuant to the interpretation are referred to as “unrecognized benefits.” A liability is recognized (or amount of net operating loss carry forward or amount of tax refundable is reduced) for unrecognized tax benefit because it represents an enterprise’s potential future obligation to the taxing authority for a tax position that was not recognized as a result of applying the provisions of ASC 740.
If applicable, interest costs related to the unrecognized tax benefits are required to be calculated and would be classified as “Other Income (Expense)” in the statement of operations. Penalties would be recognized as a component of “General and Administrative Expenses” in the statement of operations.
No interest or penalties on unpaid tax were recorded during the years ended October 31, 2017, 2016 and 2015, respectively. As of October 31, 2017 and 2016, no liability for unrecognized tax benefits was required to be reported. The Company does not expect any significant changes in its unrecognized tax benefits in the next year.
The Company files tax returns in the U.S. federal and state jurisdictions and is subject to examination by tax authorities beginning with the year ended October 31, 2013.
The Company’s deferred tax assets (liabilities) consisted of the effects of temporary differences attributable to the following:
| Years Ended | ||||||||
| October 31, 2017 | October 31, 2016 | |||||||
| Deferred Tax Assets | ||||||||
| Net operating loss carryovers | $ | 66,681,000 | $ | 51,701,000 | ||||
| Stock-based compensation | 21,921,000 | 15,239,000 | ||||||
| Research and development credits | 7,293,000 | 5,672,000 | ||||||
| Deferred revenue | 9,775,000 | - | ||||||
| Other deferred tax assets | 1,515,000 | - | ||||||
| Total deferred tax assets | $ | 107,185,000 | $ | 72,612,000 | ||||
| Valuation allowance | (104,738,000 | ) | (69,317,000 | ) | ||||
| Deferred tax asset, net of valuation allowance | $ | 2,447,000 | $ | 3,295,000 | ||||
| Deferred Tax Liabilities | ||||||||
| Other deferred tax liabilities | (2,447,000 | ) | (3,295,000 | ) | ||||
| Total deferred tax liabilities | $ | (2,447,000 | ) | $ | (3,295,000 | ) | ||
| Net deferred tax asset (liability) | $ | - | $ | - | ||||
The expected tax (expense) benefit based on the statutory rate is reconciled with actual tax expense benefit as follows:
| Years Ended | ||||||||||||
| October 31, 2017 | October 31, 2016 | October 31, 2015 | ||||||||||
| US Federal statutory rate | 34.00 | % | 34.00 | % | 34.00 | % | ||||||
| State income tax, net of federal benefit | 1.15 | 4.86 | 3.78 | |||||||||
| Permanent differences | (2.30 | ) | (2.00 | ) | (1.91 | ) | ||||||
| Research and development credits | 2.36 | 2.16 | 2.06 | |||||||||
| Income tax benefit from sale of New Jersey NOL carryovers | 4.55 | 3.33 | 3.31 | |||||||||
| Change in valuation allowance | (36.20 | ) | (28.72 | ) | (33.62 | ) | ||||||
| Other | 0.99 | (10.30 | ) | (4.31 | ) | |||||||
| Income tax (provision) benefit | 4.55 | % | 3.33 | % | 3.31 | % | ||||||
Sale of Net Operating Losses (NOLs)
The Company may be eligible, from time to time, to receive cash from the sale of its Net Operating Losses under the State of New Jersey NOL Transfer Program. In fiscal 1Q 2018, the Company plans to receive a net cash amount of $4,452,682 from the sale of its state NOLs and research and development tax credits for the period ended October 31, 2016. In November 2016, the Company received a net cash amount of $2,549,862 from the sale of its state NOLs and research and development tax credits for the period ended October 31, 2015. In December 2015, the Company received a net cash amount of $1,609,349 from the sale of its state NOLs and research and development tax credits for the period ended October 31, 2014. Following the receipt of the NOL and research and development tax credit for the period ending October 31, 2016, the Company will have reached the limit under the NJ NOL program and will no longer be able to participate in future sales.
12. SHAREHOLDERS’ EQUITY:
Registered Direct Offerings
On August 19, 2016, the Company sold 2,244,443 shares of common stock in a registered direct offering at a per share price of $13.50 for gross proceeds of approximately $30.3 million. The net proceeds to the Company, after deducting the Placement Agents’ fees and other estimated offering expenses payable by the Company, were approximately $28.2 million.
On February 18, 2015, the Company priced a registered direct offering of 3,068,095 shares of its Common Stock at $7.50 per share. The transaction closed on February 19, 2015, and the Company received gross proceeds of approximately $23.0 million from the offering. After deducting offering expenses, the net proceeds from the offering were approximately $22.3 million.
On December 19, 2014, the Company priced a registered direct offering of 3,940,801 shares of its Common Stock at $4.25 per share. The transaction closed on December 22, 2014, and the Company received gross proceeds of approximately $16.7 million from the offering. After deducting offering expenses, the net proceeds from the offering were approximately $15.8 million.
Public Offerings
On May 5, 2015, the Company closed on an underwritten public offering of 2,800,000 shares of Common Stock at a public offering price of $19.00 per share. On May 20, 2015, the Company closed the underwriters’ overallotment option to purchase 420,000 shares of its Common Stock at a public offering price of $19.00 per share. The Company received gross proceeds of approximately $61.2 million from the May 2015 public offerings. After deducting offering expenses, the net proceeds from the May 2015 public offerings were approximately $56.7 million.
13. EMPLOYEE BENEFIT PLAN
The Company sponsors a 401(k) Plan. Employees become eligible for participation upon the start of employment. Participants may elect to have a portion of their salary deferred and contributed to the 401(k) plan up to the limit allowed under the Internal Revenue Code. The Company makes a matching contribution to the plan for each participant who has elected to make tax-deferred contributions for the plan year. The Company made matching contributions which amounted to $449,086, $172,276 and $51,403 for the years ended October 31, 2017, 2016 and 2015, respectively. These amounts were charged to the Statement of Operations. The Employer contributions vest immediately.
14. SELECTED QUARTERLY FINANCIAL DATA (UNAUDITED)
The following interim financial information presents the Company’s 2017, 2016 and 2015 results of operations on a quarterly basis (in thousands, except per share amounts):
| Quarter Ended | ||||||||||||||||
| January 31, 2017 | April 30, 2017 | July 31, 2017 | October 31, 2017 | |||||||||||||
| Revenue | $ | 3,790,842 | $ | 3,425,380 | $ | 3,051,620 | $ | 1,763,208 | ||||||||
| Net loss | (17,081,003 | ) | (20,467,655 | ) | (32,625,595 | ) | (23,261,249 | ) | ||||||||
| Net loss income per common share, basic and diluted | (0.43 | ) | (0.51 | ) | (0.80 | ) | (0.57 | ) | ||||||||
| Quarter Ended | ||||||||||||||||
| January 31, 2016 | April 30, 2016 | July 31, 2016 | October 31, 2016 | |||||||||||||
| Revenue | $ | 250,000 | $ | - | $ | - | $ | 3,744,856 | ||||||||
| Net loss | (19,844,935 | ) | (15,522,450 | ) | (16,486,008 | ) | (21,702,837 | ) | ||||||||
| Net loss income per common share, basic and diluted | (0.59 | ) | (0.45 | ) | (0.48 | ) | (0.55 | ) | ||||||||
| Quarter Ended | ||||||||||||||||
| January 31, 2015 | April 30, 2015 | July 31, 2015 | October 31, 2015 | |||||||||||||
| Revenue | $ | - | $ | - | $ | - | $ | - | ||||||||
| Net loss | (7,033,870 | ) | (13,855,259 | ) | (13,562,026 | ) | (12,579,963 | ) | ||||||||
| Net loss income per common share, basic and diluted | (0.33 | ) | (0.52 | ) | (0.44 | ) | (0.38 | ) | ||||||||
15. SUBSEQUENT EVENTS
On November 2, 2017, the Company granted to executives 300,000 options with an exercise price of $3.19 and 84,000 performance-based RSU’s (“PRSU’s). The options and PRSU’s vest annually in three equal installments beginning on the first anniversaries of the grant date.
On November 2, 2017, the Company granted to Directors 180,000 options with an exercise price of $3.19. The options shall vest in one installment on the first anniversary of the grant date.