

UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2022
Or
| ☐ | |
For the transition period from ___________ to ___________
Commission file number:001-34932


VYCOR MEDICAL, INC.
(Exact name of registrant as specified in charter)
| Delaware | 20-3369218 | |
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification No.) |
951 Broken Sound Boulevard, Suite, 320 Boca Raton, FL33487
(Address of principal executive offices) (Zip Code)
Registrant’s telephone Number: (561) 558-2000(561) 558-2020
Securities registered pursuant to section 12(g)Section 12(b) of the Act:
Common Stock par value $.0001
| Title of each class | Trading Symbol | Name of each exchange on which registered | ||
| Common Stock | VYCO | OTCQB |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. [ ]☐ Yes [ ] ☒ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. [ ]☐ Yes [ ] ☒ No
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. [ ]☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ☐ Yes [ ] ☒ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ]☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer”, “non-accelerated filer”, and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large Accelerated Filer | Accelerated Filer | |
| Non-accelerated Filer | Smaller Reporting Company | |
| Emerging Growth Company ☐ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). [ ]☐ Yes [X] ☒ No
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter. $3,140,851$1,667,743 (assuming $0.30$0.14 per share)
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date. 20,071,988date shares of common stock par value $0.0001 as of March 23, 2018April 14, 2023
DOCUMENTS INCORPORATED BY REFERENCE: NONE
TABLE OF CONTENTS
| 2 |
PART I
This Form 10-K contains some forward-looking statements. Forward-looking statements give our current expectations or forecasts of future events. You can identify these statements by the fact that they do not relate strictly to historical or current facts. Forward-looking statements involve risks and uncertainties. Forward-looking statements include statements regarding, among other things, (a) our projected sales, profitability, and cash flows, (b) our growth strategies, (c) anticipated trends in our industries, (d) our future financing plans and (e) our anticipated needs for working capital. They are generally identifiable by use of the words “may,” “will,” “should,” “anticipate,” “estimate,” “plans,” “potential,” “projects,” “continuing,” “ongoing,” “expects,” “management believes,” “we believe,” “we intend” or the negative of these words or other variations on these words or comparable terminology. These statements may be found under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” as well as in this Form 10-K generally. In particular, these include statements relating to future actions, prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results.
Any or all of our forward-looking statements in this report may turn out to be inaccurate. They can be affected by inaccurate assumptions we might make or by known or unknown risks or uncertainties. Consequently, no forward-looking statement can be guaranteed. Actual future results may vary materially as a result of various factors, including, without limitation, the risks outlined under “Risk Factors” and matters described in this Form 10-K generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this filing will in fact occur. You should not place undue reliance on these forward-looking statements.
The forward-looking statements speak only as of the date on which they are made, and, except to the extent required by federal securities laws, we undertake no obligation to publicly update any forward-looking statements, whether as the result of new information, future events, or otherwise.
ITEM 1. DESCRIPTION OF BUSINESS.
1. Organizational History
The Company was formed as a limited liability company under the laws of the State of New York on June 17, 2005 as “Vycor Medical LLC”. On August 14, 2007, we converted into a Delaware corporation and changed our name to “Vycor Medical, Inc.” (“Vycor”). The Company’s listing went effective on February 2009 and on November 29, 2010 Vycor completed the acquisition of substantially all of the assets of NovaVision, Inc. (“NovaVision”) and on January 4, 2012 Vycor, through its wholly-owned NovaVision subsidiary, completed the acquisition of all the shares of Sight Science Limited (“Sight Science”), a previous competitor to NovaVision.
2. Overview of Business
Vycor is dedicated to providing the medical community with innovative and superior surgical and therapeutic solutions and operates two distinct business units within the medical device industry. Vycor Medical designs, develops and markets medical devices for use in neurosurgery. NovaVision provides non-invasive rehabilitation therapies for those who have vision disorders resulting from neurological brain damage such as that caused by a stroke. Both businesses adopt a minimally or non-invasive approach. Both technologies have strong sales growth potential, address large potential markets and have the requisite regulatory approvals. The Company has 6661 issued or allowed patents and a further 1311 pending. The Company leverages joint resources across the divisions to operate in a cost-efficient manner.
| 3 |
The Company periodically engages in discussions with potential strategic partners for or purchasers of each or both of our operating divisions. In April 2020, the board of Vycor took the decision to close the German operations of NovaVision, including the German office and NovaVision GmbH, and instead migrate to a licensed business model, entering into a license agreement with HelferApp GmbH, a cognitive therapy specialist. Under the agreement, HelferApp is licensed to provide NovaVision’s products and therapies in Germany, Austria and Switzerland to patients and professionals. The NovaVision German office was closed effective June 30, 2020.
Vycor Medical
Vycor Medical designs, develops and markets medical devices for use in neurosurgery. Vycor Medical’s ViewSite Brain Access System (“VBAS”) is a next generation retraction and access system that was fully commercialized in early 2010 and is the first significant technological change to brain tissue retraction in over 50 years in contrast to significant development in most other neuro-surgical technologies.system. Vycor Medical is ISO 13485:2003 compliant,2016 and MDSAP (Medical Device Single Audit Program) certified, and VBAS has U.S. FDA 510(k) clearance and CE Marking for Europe (Class III) for brain and spine surgeries, and regulatory approvals in Australia, Brazil, Canada, China, Korea, Japan, Russiaa number of other international markets. Vycor Medical has 30 granted and Taiwan.11 pending patents.
 | 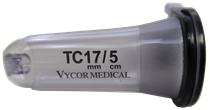 |  |
We believe VBAS offers several advantages over other brain retractor systems, commonly known as ribbon or blade retractors that are metallic, including having the potential to significantly reduce brain tissue trauma that arises from excessive pressure at the edges of the blade. The design of VBAS can minimize the size of the brain entry access necessary for surgical procedures and is believed to significantly reduce the pressure and hence trauma on the surrounding brain tissue.
NovaVision
NovaVision provides non-invasive, computer-based rehabilitation therapies targeted at a substantial and largely un-addressed market of people who have lost their sightimpaired vision as a result of stroke or other brain injury.injury and has 31 granted patents.
NovaVision hasStrategy
The Company is continuing to execute on a family of therapies that both restoreplan to achieve revenue growth and compensate for lost vision:
VRT and NeuroEyeCoach are therefore highly complementary and are provideda reduction in an Internet-delivered suite to ensure broad benefits to NovaVision’s patients. NeET is provided on its ownannual cash operating losses1. For Vycor Medical this plan includes: increasing market penetration in the UK for those just seeking restoration therapy.
NovaVision also has professional models of VRTUS; increasing international growth in territories where we are not represented or under-represented and NeuroEyeCoach for physicianscontinued new product development in response to market demands and a clinic-based model comprising NeuroEyeCoach and VIDIT, a diagnostic program that enables therapists to perform high-resolution visual field testsdemonstrating applicability in less than ten minutes.
NovaVision’s VRT is the only medical device aimed at the restoration of vision lost as a result of neurological damage which has FDA 510(k) clearance to be marketed in the U.S; and NeuroEyeCoach is registered in the US as a Class I 510(k) exempt device. VRT, NEC and NeET have CE Marking for the EU. NovaVision has 45 granted and 1 pending patents worldwide.
Competition
The VBAS device is both a brain access system and a retractor and is therefore unique with no direct competitors. Competitive manufacturers of brain retractors include Cardinal Health (V. Mueller line), Aesculap, Integra Life Science and Codman (Division of Johnson & Johnson). Nico Corporation has a brain access device specifically designed to work with its Myriad resection and suction product.
NovaVision provides restoration therapies (VRT and NeET) and compensation or saccadic therapies (NeuroEyeCoach) for those suffering vision loss as a result of neurological trauma. The other therapy type for this condition is substitution (optical aids such as prisms) and is not considered by NovaVision as competition.
In restoration, competition has been reduced through NovaVision’s acquisition of Sight Science and there are a few very small companies or entities offering some form of vision rehabilitation product in Germany. Within compensation there are no real direct competitors. Other companies in the general rehabilitation space include RevitalVision, PositScience and Dynavision. In the professional market, NovaVision competes with aggregator products or those that provide a range of non-specific therapies, such a Rehacom, Sanet Vision Integrator and Bioness BITS. NovaVision’s products are dedicated to vision.
The Market For the Company’s Products And Therapies
VBAS is used for craniotomy procedures. Based on statistics from the American Association of Neurological Surgeons (AANS), management estimates 700,000 such procedures are performed in the US annually. Of this, management believe approximately 225,000 (32 percent) are addressable by the current VBAS range. Management estimates, for the global market, there exists a current addressable market of approximately 1,100,000 procedures.
The market for NovaVision’s therapies comprises those suffering from vision loss resulting from neurological trauma such as stroke or other brain injury. Based on American Heart Association estimates, there are approximately 8 million Americans who have previously had a stroke incident, with 795,000 additional strokes occurring annually; adjusting for repeat strokes and deaths, there are 481,000 new stroke survivors each year. Additionally, approximately 5.3 million Americans live with the long-term effects of a TBI, with a further 275,000 hospitalizations each year. The most recent scientific research estimates that approximately 28.5% experience some visual impediment and 20.5% of these patients experience a permanent visual field deficit, reducing mobility and other activities of daily living. The target market for VRT and NeET is this subset of patients who have suffered a permanent visual field deficit; NeuroEyeCoach was developed to address up to the 28.5% of patients who experience visual impediments. Management estimates that the addressable target market for its therapies on the basis of these statistics is approximately 2.9 million people in the US and a further 2.8 million people in Europe.
Our Growth Strategy
Vycor Medical
Vycor Medical’s growth strategy includes:
1. Increasing U.S. market penetration through broader hospital coverage and targeted direct physician marketing. Vycor Medical’s sales and marketing strategy is to penetrate a well-defined US target market of 4,500 neurosurgeons. Vycor markets direct to surgeons as well as marketing and distributing through independent distributors, with a focus both on adding new hospitals and expanding to additional surgeons in hospitals where VBAS is already approved, and to expand usage to a broader range of procedures. Vycorpathologies. In the US the Company is pursuing a policy of continually evaluatingfocused on increasing market penetration through targeting neurosurgeons systematically, both through its distribution network and upgrading its distributors as well as adding additional distributors in regionsalso directly by leveraging existing key opinion leader (“KOL”) neurosurgeon VBAS supporters to access new neurosurgeon users.
The Company continues to target key international territories including Europe where it has littleintends to no presence.drive adoption of its VBAS product through selected key KOL neurosurgeon VBAS users in each territory to identify both new potential users and also high-quality distribution partners to bolster our existing network.
2. Provision of more Clinical and Scientific Data supporting the products superiority over the current standard-of-care blade retractors and to demonstrate VBAS’ potential for cost savings. Clinical and scientific data (in the form of peer reviewed articles, clinical studies and other reports and case studies) are critical in driving adoption, and in turn revenues, further and faster by demonstrating VBAS’ superiority as a minimally invasive access system that helps VBAS move further up the hospital cost/benefit curve. To date theThe Company has already had 12 Peer Reviewed studies and 6 other clinical papers published or presented.
3. International Market Growth
Vycor Medical utilizes select medical device distributorsfor some time been working to better integrate its VBAS with experience in neurosurgical devices in their countries or regions. VBAS has regulatory approvals in Australia, Brazil, Canada, China, Europe (EU – Class III), Korea, Mexico, Japan, Russia and Taiwan. Vycor Medical is actively pursuing new distribution agreements inneuronavigation. The first phase of the countries where it does not have any market presence.
4. New Product Development
New Product Development is targeted at both drivingmodification of the use of its existing VBAS product range through ancillary products and modalities that will facilitate the product’s use and through new product extensions to broaden VBAS applicability to procedures currently not addressed by the existing product line.
Vycor is modifying its existing VBAS product suite to make it easier to integrate with Image Guidance Systems (IGS) by re-engineering its VBAS product range so that the entire range of 12 devices, excluding the VBASmini, will be able to more easily accommodate pointers from the leading IGS system providers. The first phase of this was completed in September 2017 and was well received by surgeons. The second phase involves the final phaseintroduction of an optional Alignment Clip accessory that snaps onto the VBAS and allows for a neuronavigation pointer to be fully integrated into the body of the VBAS; this new model range, known as the VBAS AC, was launched in September 2022. The Company will continue to work with neuronavigation companies to seek ways to further integrate the VBAS with neuronavigation and with other companies with complementary technologies used in neurosurgery. We will also be completed during 2018exploring with neurosurgeons and then subjectfocus groups additional selected development work targeted at increasing the ease and applicability of our products to regulatory approval inadditional common procedures.
For NovaVision, given the US and internationally. Increasingly, all major neuro centers have image guidance systems, and where this is in place management believes over 90% of surgeries are carried out using IGS and management strongly believes that the existing VBAS rigid structure lends itself well to being incorporated into this increasing trend.
NovaVision
While speech, physical, and occupational therapies are the long-standing treatment standards for stroke and brain injury survivors, vision has been largely unaddressed. Increasingly the healthcare community, partly driven by strong lobbying by stroke associations worldwide, are recognizing that vision is not only a significant issue post stroke or brain injury, but that visual field loss can have a significant impact on the success of other rehabilitation modalitiescompany’s resources, and the patient’s quality of life. VRT is the first and only FDA-cleared clinical-supported vision restoration therapy that can physically enhance the visual field after a stroke or brain injury.
NovaVision is now able to provide a clinically supported, cost-effective and scalable visual therapy solutions offering broad benefits to patients suffering from visual impairments following neurological brain damage; and a highly flexible range of products and licensing options for medical professionals.
NovaVisions’s potential customers often tend to have other medical issues associated with their neurological condition making this a hard target universe. Rehabilitation therapies of this type are a time and effort commitment for patients, who therefore need to be in a condition to carry them out and to be motivated to rehabilitate their vision disorder. The market is also very diverse; stroke patients pass through the rehabilitation system in the first weeks after stroke, and unless they are acute patients are then sent home for recovery. At this point they may see physiotherapists, occupational therapists, eye-care professionals and other physicians, as well as stroke support groups. NovaVision’s challenge, has been to continue to drive awareness amongst patients understanding that they are subject to a diverse range of other influencers.
NovaVision taking the aforementioned issue into consideration has chosen to target four routes-to-market aimed at patients and professionals, comprising: direct-to-patient; optometrists and other eye-care professionals, rehabilitation centers and clinics; stroke associations and support groups; and other physicians. NovaVision has initially focused on direct-to-patient, with a website lead-driven inbound and outbound marketing strategy targeted at prospective patients and relatives. Given the company’s resources,large size and diversity of its end markets, we believe that the most efficient way to tackle the distribution of its broad range of patient and professional market, NovaVision believes the best route to market hereproducts is by partnering with entities who alreadyin selected geographies that have the capability of deliveryeither direct access to the various professional target markets.end users or a desire and financial wherewithal to leverage the NovaVision therapy platform, including into new areas. As a result, the Company closed the NovaVision German office and entered into a license agreement with HelferApp, a cognitive therapy specialist, for Germany, Austria and Switzerland. Management is identifying and is in dialogue with such qualified entities; thealso open to a broad range of alternatives for NovaVision as a whole, which could comprise distribution and marketing partnerships, licensing, merger sale and/or restructuring of its activities.sale.
Manufacturing1 Operating Income or Loss before Depreciation, Amortization and non-cash Stock Compensation
Vycor Medical uses a sub-contract manufacturer to manufacture, package, label and sterilize its VBAS products. The Company has migrated all its VBAS manufacturing to Life Science Outsourcing, Inc. in Brea, California that is FDA-registered and meets ISO standards and certifications.
Intellectual Property
Patents
Vycor Medical maintains a portfolio of patent protection on its methods and apparatus for its Brain and Spine products and technology in the form of issued patents and applications, both domestically and internationally, with a total of 21 granted/allowed and 12 pending patents.
NovaVision maintains a portfolio of patent protection on its methods and apparatus in the form of issued patents and applications, both domestically and internationally, with a total of 45 granted and 1 pending patents (including Sight Science).
Trademarks
VYCOR MEDICAL is a registered trademark andVIEWSITE is a common law trademark.
NovaVision maintains a portfolio of registered trademarks forNOVAVISION, NOVAVISION VRT,VRT VISION RESTORATION THERAPY and NEUROEYECOACH, amongst others, along with relevant logos, both in the US and internationally.
3. Other Matters
Product Liability Insurance
We presently have Product Liability insurance for both Vycor Medical and Professional Liability insurance for NovaVision.
Government Regulations
We are committed to an integrated total quality management system. We believe that we have completed the necessary procedures and Vycor Medical is certified to the ISO standards expected of medical device manufacturers as follows:
| 4 |
ISO 13485:20032016 Medical Devices — Quality Management Systems
The certification of a quality management system to ISO 13485,1348:2016, specifically for medical devices, is advantageous and often essential for medical companies to export their products to the global market, as well as maintain and enter into certain agreements and business growth opportunities within the U.S. For example, Canada requires that medical device manufacturers marketing their products in Canada must have a quality system certified to ISO 13485:2003.2016 and from January 1, 2019 also be certified to MDSAP. The certification is also required for placement of branded devices into the European Union.
Vycor Medical has the following certification/licensing:
| ● | ISO 13485.2016 | |
| ● | MDSAP (Medical Device Single Audit Program) | |
| ● | Fully Quality Assurance System Directive 93/42/EEC for Medical Devices, Annex II (3) | |
| ● | EC Design-Examination Certificate Directive 93/42/EEC for Medical Devices, Annex II (4) | |
Continuing Regulatory Requirements
Vycor Medical’s productsVBAS and VBAS AC have been classified as Class II products by the FDA and cleared for marketing through the 510(k) process. NovaVision’s VRT product has been cleared as a Class U product through the 510(k) while its NeuroEyeCoach is registered as an exempt Class 1 device.
After a device is placed on the market, numerous regulatory requirements apply. Failure to comply with applicable regulatory requirements, and failure to respond to requested corrective actions on an ongoing basis, can result in enforcement action by the FDA.
Medical device laws are also in effect in many of the countries outside of the United States in which we do business. These laws range from comprehensive device approval and quality system requirements for some or all of our medical device products to simple requests for product data or certifications. The number and scope of these requirements are increasing. In June 1998, the European Union Medical Device Directive became effective, and all medical devices must meet the Medical Device Directive standards and receive CE mark certification. CE mark certification involves a comprehensive Quality System program, and submission of data on a product to the Notified Body in Europe.
Vycor Medical has obtained the CE marking approval to allow for distribution of its VBAS products in Europe as a Class III device and has received HPB licensing approvala Health Canada Medical Device License (MDL) for distribution in Canada as a Class II device. VBAS also has full regulatory or partial approvals in Australia, Brazil, China, Korea, Japan and Russia.a number of other international markets. NovaVison’s VRT, NeuroEyeCoach and Sight Science’s NeET have CE mark registrations as Class I devices in Europe.
Employees
We currently have 116 employees.
Website.
The Company operates websites at www.vycormedical.com, www.vycorvbas.com, www.novavision.com and www.sightscience.com. NovaVision’s German partner, HelferApp, maintains the www.novavision.de and www.sightscience.comwebsite.
Smaller reporting companies are not required to provide the information required by this item.
ITEM 1B. UNRESOLVED STAFF COMMENTS
N/A
The Company leased office space located at 6401 Congress Ave., Suite 140, Boca Raton, FL 33487 from Catexor Limited Partnership for a gross rent of $14,260 plus sales tax per month. The term of the lease was 5 years and 6 months and terminated July 30, 2017. Effective August 1, 2017 the Company leasedleases office space located at 951 Broken Sound Parkway, Suite 320, Boca Raton, FL 33487 from WPT Land 2L.P.2 L.P., for a gross rent of approximately $5,700$4,000 per month, plus sales taxother charges of approximately $2,500 per month. The lease terminatesterminated September 30, 2020. The Company’s subsidiaries in Germany2020 and the UK occupy properties on short-term lease agreements.was extended for a further three years to August 31, 2023.
We are subject from time to time to litigation, claims and suits arising in the ordinary course of business. As of the date of this Annual Report, we were not a party to any material litigation, claim or suit whose outcome could have a material effect on our financial statements.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable
| 5 |
Not applicable
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
MARKET INFORMATIONMarket Information
Beginning on July 20, 2009, our Common Stock was quoted on the OTC Bulletin Board under the symbol “VYCO”.
The following table shows the high and low prices of our common shares on the OTC Bulletin Board for each quarter for fiscal years 2016 and 2017. The following quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions:
| Period | High | Low | ||||||
| January 1, 2016-March 31, 2016 | $ | 0.94 | $ | 0.53 | ||||
| April 1, 2016-June 30, 2016 | $ | 0.70 | $ | 0.36 | ||||
| July 1, 2016-September 30, 2016 | $ | 0.59 | $ | 0.26 | ||||
| October 1, 2016-December 31, 2016 | $ | 0.45 | $ | 0.24 | ||||
| January 1, 2017-March 31, 2017 | $ | 0.34 | $ | 0.12 | ||||
| April 1, 2017-June 30, 2017 | $ | 0.30 | $ | 0.12 | ||||
| July 1, 2017-September 30, 2017 | $ | 0.35 | $ | 0.15 | ||||
| October 1, 2017-December 31, 2017 | $ | 0.60 | $ | 0.19 | ||||
The market price of our common stock, like that of other early stageearly-stage medical device companies, is highly volatile and is subject illiquidity and to fluctuations in response to variations in operating results, announcements of technological innovations or new products, or other events or factors. Our stock price may also be affected by broader market trends unrelated to our performance.
Holders
As of March 23, 2018April 14, 2023 and April 12, 2022 there were 20,071,98832,628,835 and 31,455,744 shares of common stock issued and outstanding, respectively, and approximately 154129 stockholders of record.
Transfer Agent and Registrar
Our transfer agent is Corporate Stock Transfer, 3200 Cherry Creek Dr. SouthEQ by Equiniti, 1110 Centre Pointe Curve, Suite 430 Denver, CO 80209; telephone (303) 282-4800.101, Mendota Heights, MV 55120
Dividend Policy
We have never paid any cash dividends on our Common Stock and do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. We intend to retain future earnings to fund ongoing operations and future capital requirements of our business. Any future determination to pay cash dividends will be at the discretion of the Board of Directors and will be dependent upon our financial condition, results of operations, capital requirements and such other factors as the Board of Directors deems relevant. The Company’s Series D Convertible Preferred Stock bears a 7%12% per annum dividend payable in cash or Series D Preferred Stock at the option of the Company.
RECENT SALES OF UNREGISTERED SECURITIES
Below is a list of securities sold by us from January 1, 20172022 through March 23, 2018April 14, 2023 which were not registered under the Securities Act
Common Stock:
| Issuance Type | Security | Security | ||||||
| FHC Management Fees (1) | Common | 1,607,142 | ||||||
| Common | 203,326 | |||||||
1) Per Agreement between the Company and Fountainhead Capital Management Limited
2) Consulting fees paid in connection with a Consulting Agreement between the Company and the holder
The securities issued in the above mentionedabove-mentioned transactions were issued in connection with private placements exempt from the registration requirements of Section 5 of the Securities Act of 1933, as amended, pursuant to the terms of Section 4(2) of that Act and Rule 506 of Regulation D.
ITEM 6. SELECTED FINANCIAL DATA
Not applicable
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION.
RESULTS OF OPERATIONS
Comparison of the Year Ended December 31, 20172022 to the Year Ended December 31, 20162021
Revenue and Gross Margin:
Vycor Medical recorded revenue of $1,178,063$1,130,915 from the sale of its products for the year ended December 31, 2017,2022, a decrease of $82,886$142,687 (or 11%) over 2021. The 2021 period had an unusually high level of activity as Vycor’s markets, particularly the US, recovered from Covid and hospitals re-stocked their inventories and recommenced surgeries and procedures that had been deferred or 7% over 2016. Vycor has been in the process, during 2017, of modifying its existing VBAS product suite to make it easier to integrate with Imaging Guided Systems. The Company experienced manufacturing delays in connection with this re-engineeringpostponed, particularly during the first half2nd and 3rd quarters of 2017 and as a result was unable to fulfill shipments of certain models, particularly for some large international orders. These delays were resolved and back orders were filled during the third and fourth quarters.
Gross2021.Gross margin of 85%88% was recorded for the year ended December 31, 20172022 compared to 87%90% in 2016. Following the release of the enhanced VBAS model at the end of September, Vycor took the decision in February 2018, based on surgeon feedback, to accelerate the roll-out of the enhanced model and cease shipment of the previous model. As a result, the Company took a special provision against obsolete inventory of $39,675, in addition to the monthly provision totaling $5,088, for theyear ended December 31, 2017. Excluding this provision, gross margin would have been 86% for the year2021.
NovaVision recorded revenues of $206,908$92,074 for the year ended December 31, 2017, an increase2022, a decrease of $15,143$27,311 from 2016,2021, and gross margin of 89%92%, compared to 91%93% for 2016.2021.
| 6 |
Research and& Development:
Research & Development Expense:
Research and development expenses were $3,015 in 2017 compared to $4,739 for 2016. Capitalized software development costs in NovaVision$0 for the year ended December 31, 2017 and 2016 were $2,745 and $12,679, respectively.2022 compared to $15,159 for the year ended December 31, 2021, reflecting early-stage product development for the Vycor division.
Selling, General and Administrative Expenses:
Selling, General and administrativeAdministrative expenses decreased by $405,117$282,357 to $2,191,969$1,330,627 in 20172022 from $2,597,086$1,612,984 in 2016.2021. Included within Selling, General and Administrative Expenses are non-cash charges for share-basedstock-based compensation as the result of amortizing employee and non-employee shares warrants and options which have been issued by the Company over various periods. The charge for 20172022 was $559,972,$185,610, a decrease of $169,637$155,488 from $729,609$341,098 in 2016, primarily as a result2021, following an amendment to the Fountainhead Consulting Agreement and the departure from the Board of the changed compensation structure for managementMessrs. Girgenti and Fountainhead.Rush. Also included within Selling, General and Administrative Expenses are Sales Commissions, which increaseddecreased by $892$47,599 to $196,332. $212,910 reflecting decreased level of sales in the US.
The remaining Selling, General and Administrative expenses decreased by $235,480$79,270 from $1,672,038$1,011,378 to $1,436,558. $932,106. Patent costs decreased by $44,137 due to lower patent activity during the period and lower costs of NovaVision patents; software development and associated scientific and clinical costs related to additional development in NovaVision decreased by $57,218; marketing costs increased by $19,358 primarily from the launch of the VBAS AC product and payroll costs increased by $31,137 due to the addition of employees.
An analysis of the change in cash and non-cash G&A is shown in the table below:
| Cash G&A | Non-Cash G&A | |||||||
| Payroll | (122,653 | ) | (3,288 | ) | ||||
| Other (travel/regulatory/premises) | (54,454 | ) | - | |||||
| Investor relations and road show costs | (50,437 | ) | (40,700 | ) | ||||
| Legal, professional and other consulting | (28,446 | ) | - | |||||
| Commission expense | 892 | - | ||||||
| Sales, marketing and travel | 8,059 | - | ||||||
| Board, financial and scientific advisory | 11,559 | (125,649 | ) | |||||
| Total change | (235,480 | ) | (169,637 | ) | ||||
| 2022 G&A Change | Cash G&A | Non-Cash G&A | ||||||
| Board and financial | - | (155,488 | ) | |||||
| Scientific, clinical and software development | (57,218 | ) | - | |||||
| Legal, patent, audit/accounting and regulatory | (48,195 | ) | - | |||||
| Commissions | (47,599 | ) | - | |||||
| Premises, insurances, other | (24,552 | ) | - | |||||
| Sales and marketing | 19,558 | - | ||||||
| Payroll | 31,137 | - | ||||||
| Total change | (126,869 | ) | (155,488 | ) | ||||
| 7 |
Interest Expense:
Interest comprises expense on the Company’s debt and insurance policy financing. Related Party Interest expense for 2017 decreased2022 increased $7,722 following the conversionissuance of Related Party debt into the Offering in January 2017.related party notes during 2022 and 2021 to $39,563 from $31,841 for 2021. Other Interest expense for 20172022 decreased by $362$3,306 to $48,523$52,664 from $48,885$55,970 for 2016.2021.
Other Income:
Other Income of $117,200 was recorded during the year ended December 31, 2021 from the forgiveness of Paycheck Protection Program loans
Operating loss from Discontinued Operations:
Operating loss from Discontinued Operations decreased by $22,201 to $5,212 in 2022 from $27,413 in 2021 as the business is wound down.
Liquidity and Capital Resources
Liquidity
The following table shows cash flow and liquidity data for the periodsyears ended December 31, 20172022 and December 31, 2016:2021:
| December 31, 2017 | December 31, 2016 | $ Change | December 31, 2022 | December 31, 2021 | $ Change | |||||||||||||||||||
| Cash | $ | 206,213 | $ | 56,859 | $ | 149,354 | $ | 37,035 | $ | 90,941 | $ | (53,906 | ) | |||||||||||
| Accounts receivable, inventory and other current assets | $ | 402,295 | $ | 480,230 | $ | (77,935 | ) | $ | 480,728 | $ | 396,470 | $ | 84,258 | |||||||||||
| Total current liabilities | $ | (1,368,015 | ) | $ | (1,511,688 | ) | $ | 143,673 | $ | (3,654,796 | ) | $ | (3,149,997 | ) | $ | (504,799 | ) | |||||||
| Working capital | $ | (759,507 | ) | $ | (974,599 | ) | $ | 215,092 | $ | (3,137,033 | ) | $ | (2,662,586 | ) | $ | (474,447 | ) | |||||||
| Cash provided by financing activities | $ | 863,851 | $ | 266,710 | $ | 597,141 | $ | 165,889 | $ | 61,945 | $ | 103,944 | ||||||||||||
Private Placement.
On January 11, and February 23, 2017 (the “Closings”) the Company completed the sale of $1,274,717 in shares of Common Stock and Warrants to accredited investors. Included in these gross proceeds is the conversion of $248,000 of debt on the balance sheet at December 31, 2016, so that proceeds net of debt conversion were $1,026,717. The Closings raised net cash proceeds, after debt conversion and expenses, of $943,873, of which $842,873 was received during 2017.
Operating Activities. Cash used inprovided by (used in) operating activities comprises net loss adjusted for non-cash items and the effect of changes in working capital and other activities. The net repayment of normal insurance financing should also be taken into account when considering cash used inprovided by (used in) operating activities.
The following table shows the principal components of cash used inprovided by (used in) operating activities during the yearyears ended December 31, 20172022 and 2016,2021, with a commentary of changes during the periodsyears and known or anticipated changes:
| December 31, 2017 | December 31, 2016 | $ Change | December 31, 2022 | December 31, 2021 | $ Change | |||||||||||||||||||
| Net loss | $ | (1,477,045 | ) | $ | (1,652,280 | ) | $ | 175,235 | $ | (404,917 | ) | $ | (435,662 | ) | $ | 30,745 | ||||||||
| Adjustments to reconcile net loss to cash used in operating activities: | ||||||||||||||||||||||||
| Adjustments to reconcile net loss to cash provided by (used in) operating activities: | ||||||||||||||||||||||||
| Amortization and depreciation of assets | $ | 289,975 | $ | 267,496 | $ | 22,479 | $ | 61,403 | $ | 68,418 | $ | (7,015 | ) | |||||||||||
| Share based compensation | $ | 334,972 | $ | 482,110 | $ | (147,138 | ) | |||||||||||||||||
| Accrued share based compensation | $ | 225,000 | $ | 247,500 | $ | (22,500 | ) | |||||||||||||||||
| Warrant issuance expense | $ | 120,788 | - | $ | 120,788 | |||||||||||||||||||
| Gain or loss on foreign exchange | $ | (1,477 | ) | $ | 144 | $ | (1,621 | ) | ||||||||||||||||
| Stock based compensation | $ | 185,610 | $ | 341,098 | $ | (155,488 | ) | |||||||||||||||||
| Forgiveness of PPP loan | $ | - | $ | (117,200 | ) | $ | 117,200 | |||||||||||||||||
| Other | $ | 44,760 | $ | 10,174 | $ | 34,586 | $ | 15,515 | $ | 12,360 | $ | 3,155 | ||||||||||||
| $ | 1,014,018 | $ | 1,007,424 | $ | 6,594 | $ | 262,528 | $ | 304,676 | $ | (42,148 | ) | ||||||||||||
| Net loss adjusted for non-cash items | $ | (463,027 | ) | $ | (644,856 | ) | $ | 181,829 | $ | (142,389 | ) | $ | (130,986 | ) | $ | (11,403 | ) | |||||||
| Changes in working capital | ||||||||||||||||||||||||
| Accounts receivable, accounts payable and accrued liabilities | $ | (108,397 | ) | $ | (65,664 | ) | $ | (42,733 | ) | |||||||||||||||
| Accounts receivable | $ | (30,108 | ) | $ | 33,142 | $ | (63,250 | ) | ||||||||||||||||
| Accounts payable and accrued liabilities | $ | (63,283 | ) | $ | 58,521 | $ | (121,804 | ) | ||||||||||||||||
| Inventory | $ | (54,573 | ) | $ | 78,292 | $ | (132,865 | ) | $ | (56,868 | ) | $ | (38,785 | ) | $ | (18,083 | ) | |||||||
| Prepaid expenses and net insurance financing repayments | $ | 18,162 | $ | (13,939 | ) | $ | 32,101 | $ | (21,547 | ) | $ | 13,379 | $ | (34,926 | ) | |||||||||
| Accrued interest (not paid in cash) | $ | 48,680 | $ | 60,293 | $ | (11,613 | ) | $ | 92,227 | $ | 83,423 | $ | 8,804 | |||||||||||
| Other | $ | 33,254 | $ | 6,666 | $ | 26,588 | ||||||||||||||||||
| Changes in discontinued operations, net | $ | (1,659 | ) | $ | (3,980 | ) | $ | 2,321 | ||||||||||||||||
| $ | (62,874 | ) | $ | 65,648 | $ | (128,522 | ) | $ | (81,238 | ) | $ | 145,700 | $ | (226,938 | ) | |||||||||
| Cash used in operating activities, adjusted for net insurance repayments | $ | (525,901 | ) | $ | (579,208 | ) | $ | 53,307 | ||||||||||||||||
| Cash provided by (used in) operating activities, adjusted for net insurance repayments | $ | (223,627 | ) | $ | 14,714 | $ | (238,341 | ) | ||||||||||||||||
| 8 |
The adjustments to reconcile net loss to cash used of $1,014,018$262,528 in the period have no impact on Liquidity.liquidity. The reduction in net loss (as adjusted for non-cash items) by $181,829 to $463,027 was primarily due to a reduction in cash operating expenses during the period. The net change in accounts receivable, accounts payable and accrued liabilities is primarilyof $121,804 between the result2022 and 2021 periods was mainly due to the settlement of expenses during the 2021 period incurred during the final quarter of 2020.
Additional inventory of $162,750 was purchased during the year ended December 31, 2022 as part of normal production and for the launch of the payment during the first quarter of $69,314 of accounts payable deferred at the end of December 2016, offset by collections of accounts receivable. The Company is in the process of modifying the VBAS product suite to make it easier to integrate with IGS. During the periodAC, and the Company completed the first phase of this project and as a result purchased VBAS inventory for $85,748 and will beanticipates purchasing additional inventory in the first quarter of 2018 of approximately $11,000. The Company anticipates completing the second phase of this project during 2018 and as a result will purchase additional new inventory of approximately $45,000.$75,000 during the next twelve months for VBAS and VBAS AC.
Investing Activities.Cash used in investing activities of continuing operations for the year ended December 31, 20172022 was $183,258, of$2,780 compared to $38,375 in 2021, which $166,154primarily reflected the expenditure on the first phaseVBAS AC during 2021.
Financing Activities. During the year ended December 31, 2022 the Company received funds of modifying the VBAS product suite to make it easier to integrate with IGS.$172,500 in respect of loans from Fountainhead. The Company anticipates expenditure forhad received a second loan of $58,600 during 2021 pursuant to the second phasePaycheck Protection Program (the “PPP”) under Division A, Title I of this project during 2018 of approximately $100,000.
Financing Activities.On January 11,the CARES Act. Both loans received under the Paycheck Protection Program were forgiven in August 2021 and February 23, 2017 the Company completed the sale of $1,274,717resulted in shares of Common Stock and Warrants to accredited investors (the “Private Placement”). Included in these gross proceeds is the conversion of $248,000an extinguishment of debt for $117,200, during 2021, which is included in the adjustment to reconcile net loss to cash provided by (used in) operating activities section on the balance sheet at December 31, 2016, so that proceeds net of debt conversion were $1,026,717. The Private Placement raised net cash proceeds, after debt conversion and expenses, of $943,873. $101,000 of these proceeds were reflected in the in the balance sheet at December 31, 2016 and so the net increase in liquidity during the period from the Private Placement was $842,873.flow statement.
Liquidity and Plan of Operations, Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. The Company has incurred losses since its inception, including a net loss of $1,477,045$404,917 and $1,652,280$435,662 for the years ending December 31, 20172022 and 20162021 respectively and has not generated sufficient cash flows from operations. As at December 31, 20172022 the Company had stockholder’s equity of $81,442,cash of $206,213 and a working capital deficiency of $197,297,$551,433 excluding related party liabilitiesof $562,210.$2,585,600. As a result these conditions, among others, raise substantial doubt regarding our ability to continue as a going concern. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
As described earlier in this ITEM 21 “Our Growth Strategy”, the Company is executing on a plan to achieve growth in revenues for both the Vycor Medical and NovaVision divisions as well asa reduction in costs, thereby further reducing its cash operating usage. For Vycor Medicallosses2. Included within the working capital deficiency above is a term note for $300,000 to EuroAmerican Investment Corp. (“EuroAmerican”), together with accrued interest of $424,897, which has a maturity date of December 31, 2023, having been extended on a number of occasions from its initial due date of June 11, 2011. At this includes in particular: increasing penetration in the US market through targeted marketing and a potential new medical education program; increased international market growth; and new product development centered around the modification of its existing VBAS product range to maketime, it more easy to use with the most common IGS systems, the first phase of which was completed in September 2017. For NovaVision, after a prolonged and now complete period of re-development, the Company is focusing its resources on direct-to-patient marketing through a website lead-driven inbound and outbound marketing strategy. Given the company’s resources, and the size and diversitynot known whether any further extension of the professional market, NovaVision believes the best route to the professional market for its products is by partnering with entities who already have established distribution channels and expertise in the various professional target markets. The Company is identifying and is in dialogue with such qualified entities; the range of alternatives for NovaVision could comprise distribution partnerships, merger, sale and/or restructuring of its activities.
note beyond December 31, 2023 will be available. However, the Company believes it may not have sufficient cash to meet its various cash needs through March 31, 20192024 unless the Company is able to obtain additional cash from the issuance of debt or equity securities. Fountainhead, the Company’s largest shareholder, is currently providinghas provided working capital funding to the Company on an as-needed basis, although there is no guarantee that this will continue to be the case. The Company may consider seeking additional equity or debt funding, although there is no assurance that this would be available on acceptable terms or at all. If adequate funds are not available, the Company may have to delay or curtail development or commercialization of products or cease some of its operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability
2 Operating Income or Loss before Depreciation, Amortization and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.non-cash Stock Compensation
Off-Balance Sheet Arrangements
As of December 31, 2017,2022, we had no off-balance sheet arrangements.
| 9 |
Seasonality
Our operating results are not affected by seasonality.
Inflation
Our business and operating results are not affected in any material way by inflation.inflation, although rising raw material and labor costs will result in an increase in the cost of sales.
Critical Accounting Policies and Estimates
Uses of estimates in the preparation of financial statements
The preparation of consolidated financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from those estimated. To the extent management’s estimates prove to be incorrect, financial results for future periods may be adversely affected. Significant estimates and assumptions contained in the accompanying consolidated financial statements include management’s estimate of the allowance for uncollectible accounts receivable, amortizationprovision for inventory obsolescence, useful life of intangible assets, and the fair values of options and warrant included in the determination of debt discounts and sharestock based compensation.
Cash and cash equivalents
The Company maintains cash balances at various financial institutions. Accounts at each institution are insured by the Federal Deposit Insurance Corporation up to $250,000. Cash balances may at times exceed the FDIC insured limits. Cash also includes a US investment account in a money market backed by government securities up to 105% of the account balance. The Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents. Included within cash are deposits paid by patients, held by the Company until the patient returns the VRT device or chinrest at the end of therapy. At December 31, 2017 and 2016 patient deposits amounted to $41,172 and $33,351, respectively, and are included in other current liabilities.
Fixed assets
The Company records fixed assets at cost and calculates depreciation using the straight-line method over the estimated useful life of the assets, which is estimated to be between three and seven years. Maintenance, repairs and minor renewals are charged to expense when incurred. Replacements and major renewals are capitalized.
Income taxes
We use the asset and liability method of accounting for income taxes in accordance with ASC Topic 740, “Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year and (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if based on the weight of the available positive and negative evidence, it is more likely than not some portion or all of the deferred tax assets will not be realized.
ASC Topic 740.10.30 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740.10.40 provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. We have no material uncertain tax positions for any of the reporting periods presented.
Patents and Other Intangible Assets
The Company capitalizes legal and related costs associated with the establishment and enhancement of patents for its products once patents have been applied for. Costs associated with the development of the patented item or processes are charged to research and development costs as incurred. The capitalized costs are amortized over the life of the patent. The Company reviews intangible assets on an annual in accordance with the authoritative guidance. Trademarks have an indefinite life and are reviewed annually by management for impairment in accordance with the authoritative guidance.
Software Development Costs
The authoritative accounting guidance requires software development costs to be capitalized upon completion of the preliminary project stage. Accordingly, direct internal and external costs associated with the development of the features and functionality of the Company’s software, incurred during the application development stage, are capitalized and amortized using the straight-line method over the estimated life of five years.
Revenue Recognition
On January 1, 2018, the Company adopted the new accounting standard, ASC 606, Revenue from Contracts with Customers and all the related amendments (new revenue standard) to all contracts. The adoption of the new accounting standard had no impact on company’s consolidated financial statements.
Vycor Medical generates revenue from the sale of its surgical access system to hospitals and other medical professionals. Vycor Medical records revenue from product sales when obligations under the terms of a completed contract forwith customers are satisfied. Generally, this occurs with the sale exists,transfer of control of the product is invoiced and shippedgoods to the customer.customers. Vycor Medical does not provide for product returns or warranty costs.
Vycor determines revenue recognition through the following steps:
| ● | Identification of the contract, or contracts, with a customer | |
| ● | Identification of the performance obligations in the contract | |
| ● | Determination of the transaction price | |
| ● | Allocation of the transaction price to the performance obligations in the contract | |
| ● | Recognition of revenue when Vycor satisfy a performance obligation |
NovaVision generates revenues from various programs, therapy services and other sources such as software license sales. Therapy services revenues represent fees from NovaVision’s vision restoration therapy software, eye movement training software, diagnostic software, clinic set up and training fees, and the professional and support services associated with the therapy. NovaVision provides vision restoration therapy directly to patients. The typical vision restoration therapy program consists of NeuroEyeCoach, performed over 2-4 weeks, and six modules of Vision Restoration Therapy, performed on average over 6 months in the U.S. and U.K. and 10 months in Germany.months. A patient contract comprises set-up fees and monthly therapy fees. Set-up fees are recognized at the outset of the contract and therapy revenue is recognized ratably over the therapy period. Patient therapy is restricted to being completed by a patient within a specified time frame. NovaVision’s saccadic training software is generally completed within 2-4 weeks and revenue is therefore recognized fully at commencement.
Deferred revenue results from patients paying for the therapy in advance of receiving the therapy.
Accounts Receivable
The Company’s accounts receivable are due from the hospitals and distributors in the case of Vycor Medical, and from patients directly for therapy or physicians for diagnostic products in the case of NovaVision. Accounts receivable are due once products have been delivered or at the time the therapy is initiated; however, for NovaVision therapy patients sometimes credit is extended through various payment plans based on individual financial conditions, generally not to exceed the 9 or 10 month therapy period. The outstanding balances are stated net of an allowance for doubtful accounts. The Company determines its allowance by considering a number of factors, including the length of time accounts receivable are past due, and the customer’s ability to pay its obligations. The Company writes off accounts receivable when they become uncollectible.
| 10 |
Inventory
Inventories are stated at the weighted average cost method. Net realizable value is the estimated selling price, in the ordinary course of business, less estimated costs to complete and dispose of the product. If the Company identifies excess, obsolete or unsalable items, its inventories are written down to their realizable value in the period in which the impairment is first identified. The provision charge for inventory for the years ended December 31, 20172022 and 20162021 was $44,760$13,160 and $10,174, respectively, of which $39,672 is a special provision related to the introduction of the enhanced VBAS discussed above.$12,360, respectively. Shipping and handling costs incurred for inventory purchases and product shipments are recorded in cost of sales.
Foreign CurrencyDiscontinued Operations
The Euro is the local currencyIn accordance with ASU No. 2014-08, Reporting Discontinued Operations and Disclosures of the country in which NovaVision GmbH conducts its operations and is considered the functional currencyDisposals of this entity; the GB Pound is the local currencyComponents of the country in which Sight Science Limited conducts its operations and is considered the functional currencyan Entity, a disposal of this entity. All balance sheet amounts are translated to U.S. dollars using the U.S. exchange rate at the balance sheet date except for the equity section which is translated at historical rates. Operating statement amounts are translated using an average exchange rate for the period of operations. Foreign currency translation effects are accumulated as part of the accumulated other comprehensive income (loss) and included in shareholders’ (deficit) in the accompanying Consolidated Balance Sheet.
Educational marketing and advertising expenses
The Company may incur costs for the education of customers on the uses and benefits of its products. The Company will include education, marketing and advertising expense as a component of selling, generalan entity or a group of components of an entity is required to be reported as discontinued operations if the disposal represents a strategic shift that has (or will have) a major effect on an entity’s operations and administrative costsfinancial results when the components of an entity meets the criteria in paragraph 205-20-45-1E to be classified as such costsheld for sale. When all of the criteria to be classified as held for sale are incurred.met, including management, having the authority to approve the action, commits to a plan to sell the entity, the major current assets, other assets, current liabilities, and noncurrent liabilities shall be reported as components of total assets and liabilities separate from those balances of the continuing operations. At the same time, the results of all discontinued operations (which we presented as operations to be disposed and operations disposed), less applicable income taxes (benefit), shall be reported as components of net income (loss) separate from the net income (loss) of continuing operations in accordance with ASC 205-20-45.
Contractual Obligations
As a “smaller reporting company” as defined by Item 10 of Regulation S-K, the Company is not required to provide this information.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a “smaller reporting company” as defined by Item 10 of Regulation S-K, the Company is not required to provide this information.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
TheSet forth below are the audited consolidated financial information required by Item 8 begins onstatements for the following page.Company for the fiscal years ended December 31, 2022 and 2021 and the report thereon of Prager Metis CPAs, LLC (PCAOB ID No. 273),
| 11 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors andStockholders of
Vycor Medical, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Vycor Medical, Inc (the Company)Inc. (“the Company”) as of December 31, 20172022 and 2016,2021, and the related consolidated statements of comprehensive loss, stockholders’ equity (deficiency),deficiency, and cash flows for each of the years in the two-year period ended December 31, 2017,2022 and 2021, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 20172022 and 2016,2021, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2017,2022 and 2021, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred net losses since inception, including a net loss of $404,917 and $435,662 for the years ended December 31, 2022 and 2021 respectively, and has not generated sufficient cash flows from its operations. As of December 31, 2022, the Company had working capital deficiency of $551,433, excluding related party liabilities of $2,585,600. These factors, among others, raise substantial doubt regarding the Company’s ability to continue as a going concern. Management’s plans in regards to these matters are described in Note 1 to the financial statements. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The accompanyingcritical audit matters are matters arising from the current period audit of the financial statements have been prepared assuming that were communicated or required to be communicated to the Company will continue as a going concern. As discussed in note 2audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements the Company has incurred a net loss since inception, including a net loss of $1,477,045 and $1,652,280(2) involved our especially challenging, subjective or complex judgments. We determined that there is no critical audit matters for the year ended December 31, 2017 and 2016, respectively, and has not generated cash flows from operations. As of December 31, 2017, the Company had working capital deficiency of $197,297, excluding related party liabilities of $562,210. These factors, among others, raise substantial doubt regarding the Company’s ability to continue as a going concern. Management’s plans in regards to these matters are described in Note 1 to the financial statements. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertaintycurrent audit period.
| /s/ Prager Metis CPAs, LLC | |
| We have served as the Company’s auditor since 2018. | |
| Hackensack, New Jersey | |
| April 14, 2023 |
We have served as the Company’s auditor since 2007
Hackensack, New Jersey
March 29, 2018
| 12 |
VYCOR MEDICAL, INC.
Consolidated Balance Sheets
| December 31, 2017 | December 31, 2016 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | 206,213 | $ | 56,859 | ||||
| Trade accounts receivable | 110,422 | 148,784 | ||||||
| Inventory | 213,883 | 204,071 | ||||||
| Prepaid expenses and other current assets | 77,990 | 127,375 | ||||||
| Total Current Assets | 608,508 | 537,089 | ||||||
| Fixed assets, net | 489,170 | 401,051 | ||||||
| Intangible and Other assets: | ||||||||
| Trademarks | 251,157 | 251,157 | ||||||
| Patents, net of accumulated amortization | 81,064 | 238,571 | ||||||
| Website, net of accumulated amortization | 10,389 | 14,958 | ||||||
| Security deposits | 9,169 | 42,424 | ||||||
| Total Intangible and Other assets | 351,779 | 547,110 | ||||||
| TOTAL ASSETS | $ | 1,449,457 | $ | 1,485,250 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIENCY) | ||||||||
| Current Liabilities | ||||||||
| Accounts payable | $ | 141,319 | $ | 249,949 | ||||
| Accrued interest: Other | 184,765 | 136,765 | ||||||
| Accrued interest: Related party | 12,840 | 12,161 | ||||||
| Accrued liabilities - Other | 161,328 | 199,457 | ||||||
| Accrued liabilities - Related Party | 549,370 | 247,500 | ||||||
| Monies in Escrow Related Party - Offering | - | 101,000 | ||||||
| Notes payable: Related Party | - | 248,000 | ||||||
| Notes payable: Other | 318,393 | 316,856 | ||||||
| Total Current Liabilities | 1,368,015 | 1,511,688 | ||||||
| STOCKHOLDERS’ EQUITY (DEFICIENCY) | ||||||||
| Preferred stock, $0.0001 par value, 10,000,000 shares authorized, 270,306 and 270,306 issued and outstanding as at December 31, 2017 and December 31, 2016 respectively | $ | 27 | $ | 27 | ||||
| Common Stock, $0.0001 par value, 25,000,000 shares authorized at December 31, 2017 and 2016, 19,925,322 and 11,439,357 shares issued and 19,821,988 and 11,336,023 outstanding at December 31, 2017 and 2016 respectively | 1,993 | 1,144 | ||||||
| Additional Paid-in Capital | 26,921,574 | 25,007,850 | ||||||
| Treasury Stock (103,334 shares of Common Stock as at December 31, 2017 and 2016 respectively, at cost) | (1,033 | ) | (1,033 | ) | ||||
| Accumulated Deficit | (26,965,960 | ) | (25,164,545 | ) | ||||
| Accumulated Other Comprehensive Income | 124,841 | 130,119 | ||||||
| Total Stockholders’ Equity (Deficiency) | 81,442 | (26,438 | ) | |||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIENCY) | $ | 1,449,457 | $ | 1,485,250 | ||||
| December 31, | December 31, | |||||||
| 2022 | 2021 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | 37,035 | $ | 90,941 | ||||
| Trade accounts receivable | 156,204 | 126,096 | ||||||
| Inventory | 248,874 | 207,521 | ||||||
| Prepaid expenses and other current assets | 74,438 | 62,473 | ||||||
| Current assets of discontinued operations | 1,212 | 380 | ||||||
| Total Current Assets | 517,763 | 487,411 | ||||||
| Fixed assets, net | 303,770 | 362,393 | ||||||
| Intangible and Other assets: | ||||||||
| Security deposits | 6,000 | 6,000 | ||||||
| Operating lease - right of use assets | 32,645 | 79,560 | ||||||
| Total Intangible and Other assets | 38,645 | 85,560 | ||||||
| TOTAL ASSETS | $ | 860,178 | $ | 935,364 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | ||||||||
| Current Liabilities | ||||||||
| Accounts payable | $ | 200,044 | $ | 227,720 | ||||
| Accrued interest: Other | 424,897 | 380,479 | ||||||
| Accrued interest: Related party | 146,007 | 106,444 | ||||||
| Accrued liabilities - Other | 91,352 | 126,959 | ||||||
| Accrued liabilities - Related Party | 1,946,220 | 1,621,850 | ||||||
| Notes payable: Other | 324,711 | 319,329 | ||||||
| Notes payable: Related Party | 493,373 | 320,873 | ||||||
| Current operating lease liabilities | 29,591 | 46,915 | ||||||
| Current liabilities of discontinued operations | (1,399 | ) | (572 | ) | ||||
| Total Current Liabilities | 3,654,796 | 3,149,997 | ||||||
| Loan Payable - SBA EIDL | 146,253 | 150,000 | ||||||
| Operating lease liability - Long term | - | 30,580 | ||||||
| Total Long-term Liabilities | 146,253 | 180,580 | ||||||
| Total Liabilities | 3,801,049 | 3,330,577 | ||||||
| STOCKHOLDERS’ DEFICIENCY | ||||||||
| Preferred stock, $ par value, shares authorized, and issued and outstanding as at December 31, 2022 and December 31, 2021 respectively | 27 | 27 | ||||||
| Common Stock, $ par value, shares authorized at December 31, 2022 and December 31, 2021, and shares issued and and outstanding at December. 31, 2022 and December 31, 2021 respectively | 3,263 | 3,092 | ||||||
| Additional Paid-in Capital | 29,355,626 | 29,172,169 | ||||||
| Treasury Stock ( shares of Common Stock as at December 31, 2022 and December 31, 2021 respectively, at cost) | (1,033 | ) | (1,033 | ) | ||||
| Accumulated Deficit | (32,426,429 | ) | (31,697,142 | ) | ||||
| Accumulated Other Comprehensive Income (Loss) | 127,675 | 127,674 | ||||||
| Total Stockholders’ Deficiency | (2,940,871 | ) | (2,395,213 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | $ | 860,178 | $ | 935,364 | ||||
See accompanying notes to consolidated financial statements
| 13 |
VYCOR MEDICAL, INC.
Consolidated StatementStatements of Comprehensive Loss
| 2022 | 2021 | |||||||||||||||
| For the year ended December 31, | For the year ended December 31, | |||||||||||||||
| 2017 | 2016 | 2022 | 2021 | |||||||||||||
| Revenue | $ | 1,384,971 | $ | 1,452,714 | $ | 1,222,989 | $ | 1,392,987 | ||||||||
| Cost of Goods Sold | 227,134 | 184,685 | 140,644 | 134,728 | ||||||||||||
| Gross Profit | 1,157,837 | 1,268,029 | 1,082,345 | 1,258,259 | ||||||||||||
| Operating expenses: | ||||||||||||||||
| Operating Expenses: | ||||||||||||||||
| Research and development | 3,015 | 4,739 | - | 15,159 | ||||||||||||
| Depreciation and Amortization | 275,416 | 257,582 | ||||||||||||||
| Depreciation and amortization | 58,232 | 66,070 | ||||||||||||||
| Selling, general and administrative | 2,191,969 | 2,597,086 | 1,330,627 | 1,612,984 | ||||||||||||
| Total Operating expenses | 2,470,400 | 2,859,407 | ||||||||||||||
| Total Operating Expenses | 1,388,859 | 1,694,213 | ||||||||||||||
| Operating loss | (1,312,563 | ) | (1,591,378 | ) | (306,514 | ) | (435,954 | ) | ||||||||
| Other income (expense) | ||||||||||||||||
| Other Income (Expense) | �� | |||||||||||||||
| Interest expense: Related Party | (39,563 | ) | (31,841 | ) | ||||||||||||
| Interest expense: Other | (48,522 | ) | (48,885 | ) | (52,664 | ) | (55,970 | ) | ||||||||
| Interest expense: Related Party | (680 | ) | (12,161 | ) | ||||||||||||
| Gain (loss) on foreign currency exchange | 1,477 | 144 | ||||||||||||||
Otherincome and expense | 4,031 | - | ||||||||||||||
| Warrant issuance expense | (120,788 | ) | - | |||||||||||||
| Total Other Income (expense) | (164,482 | ) | (60,902 | ) | ||||||||||||
| Loss on foreign currency exchange | (964 | ) | (1,684 | ) | ||||||||||||
| Forgiveness of debt: Paycheck Protection Program | - | 117,200 | ||||||||||||||
| Total Other Income (Expense) | (93,191 | ) | 27,705 | |||||||||||||
| Loss Before Credit for Income Taxes | (1,477,045 | ) | (1,652,280 | ) | ||||||||||||
| Credit for income taxes | - | - | ||||||||||||||
| Loss Before Provision for Income Taxes | (399,705 | ) | (408,249 | ) | ||||||||||||
| Provision for income taxes | - | - | ||||||||||||||
| Net Loss from continuing operations | (399,705 | ) | (408,249 | ) | ||||||||||||
| Loss from discontinued operations | (5,212 | ) | (27,413 | ) | ||||||||||||
| Net Loss | (1,477,045 | ) | (1,652,280 | ) | (404,917 | ) | (435,662 | ) | ||||||||
| Preferred stock dividends | (324,370 | ) | (179,727 | ) | (324,370 | ) | (324,370 | ) | ||||||||
Net Loss available to common stockholders | (1,801,415 | ) | (1,832,007 | ) | ||||||||||||
| Comprehensive Loss | ||||||||||||||||
| Net Loss Available to Common Stockholders | $ | (729,287 | ) | $ | (760,032 | ) | ||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||
| Foreign Currency Translation Adjustment | 5,278 | 1,989 | 1 | 5 | ||||||||||||
| Comprehensive Loss | (1,471,766 | ) | (1,650,291 | ) | $ | (404,916 | ) | $ | (435,657 | ) | ||||||
| Net Loss Per Share | ||||||||||||||||
| Basic and diluted | $ | (0.10 | ) | $ | (0.17 | ) | ||||||||||
| Net Loss Per Share – basic and diluted | ||||||||||||||||
| Net Loss from continuing operations | $ | (0.01 | ) | $ | (0.01 | ) | ||||||||||
| Loss from discontinued operations | $ | (0.00 | ) | $ | (0.00 | ) | ||||||||||
| Net Loss available to common stockholders | $ | (0.02 | ) | $ | (0.03 | ) | ||||||||||
| Weighted Average Number of Shares Outstanding – Basic and Diluted | 18,373,355 | 11,066,217 | 31,708,076 | 29,627,527 | ||||||||||||
See accompanying notes to consolidated financial statements
| 14 |
VYCOR MEDICAL, INC.
Consolidated Statement of Stockholders’ Equity (Deficiency)Deficiency
| Common Stock | Preferred C | Preferred D | Treasury Stock | Additional Paid-in | Accumulated | Accum OCI | ||||||||||||||||||||||||||||||||||||||||||
| Number | Amount | Number | Amount | Number | Amount | Number | Amount | Capital | Deficit | (Loss) | Total | |||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2015 | 11,032,560 | $ | 1,103 | 1 | $ | 0 | 252,336 | $ | 25 | (103,334 | ) | $ | (1,033 | ) | $ | 24,346,057 | $ | (23,332,538 | ) | $ | 132,108 | $ | 1,145,722 | |||||||||||||||||||||||||
| Issuance of stock for board and consulting fees | 291,393 | 29 | - | - | - | - | - | - | 156,847 | - | - | 156,876 | ||||||||||||||||||||||||||||||||||||
| Share-based compensation for consulting service | 115,404 | 12 | - | - | - | - | - | - | 49,022 | - | - | 49,034 | ||||||||||||||||||||||||||||||||||||
| Deferred compensation for board | 78,000 | 78,000 | ||||||||||||||||||||||||||||||||||||||||||||||
| Share based compensation issued to management/employees | - | - | - | - | - | - | 198,200 | - | - | 198,200 | ||||||||||||||||||||||||||||||||||||||
| Preferred stock dividends | - | - | 17,970 | 2 | 179,724 | - | - | 179,726 | ||||||||||||||||||||||||||||||||||||||||
| Accumulated Comprehensive Loss | - | - | - | - | - | - | - | - | - | - | (1,989 | ) | (1,989 | ) | ||||||||||||||||||||||||||||||||||
| Net loss for year ended December 31, 2016 | (1,832,007 | ) | (1,832,007 | ) | ||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2016 | 11,439,357 | $ | 1,144 | 1 | $ | 0 | 270,306 | $ | 27 | (103,334 | ) | $ | (1,033 | ) | $ | 25,007,850 | $ | (25,164,545 | ) | $ | 130,119 | $ | (26,438 | ) | ||||||||||||||||||||||||
| Issuance of stock for board and consulting fees | 2,343,885 | 235 | 496,637 | 496,872 | ||||||||||||||||||||||||||||||||||||||||||||
| Issuance of warrants related to 2014 Debt Preferred exchange agreement | - | - | 120,788 | 120,788 | ||||||||||||||||||||||||||||||||||||||||||||
| Directors deferred compensation granted | 84,000 | 84,000 | ||||||||||||||||||||||||||||||||||||||||||||||
| Share based compensation issued to management/employees | 1,609 | 1,609 | ||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of shares and warrants pursuant to offering, net | 6,070,078 | 607 | 1,191,256 | 1,191,863 | ||||||||||||||||||||||||||||||||||||||||||||
| Issuance of shares pursuant to exercise of warrants | 72,002 | 7 | 19,434 | 19,441 | ||||||||||||||||||||||||||||||||||||||||||||
| Accumulated Comprehensive Loss | (5,278 | ) | (5,278 | ) | ||||||||||||||||||||||||||||||||||||||||||||
| Net loss for year ended December 31, 2017 | (1,801,415 | ) | (1,801,415 | ) | ||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2017 | 19,925,322 | $ | 1,993 | 1 | $ | 0 | 270,306 | $ | 27 | (103,334 | ) | $ | (1,033 | ) | $ | 26,921,574 | $ | (26,965,960 | ) | $ | 124,841 | $ | 81,442 | |||||||||||||||||||||||||
| Additional | Accum | |||||||||||||||||||||||||||||||||||||||||||||||
| Common Stock | Preferred C | Preferred D | Treasury Stock | Paid-in | Accumulated | OCI | ||||||||||||||||||||||||||||||||||||||||||
| Number | Amount | Number | Amount | Number | Amount | Number | Amount | Capital | Deficit | (Loss) | Total | |||||||||||||||||||||||||||||||||||||
| Accumulated Comprehensive Loss | 27,534,740 | $ | 2,753 | 1 | $ | 0 | 270,306 | $ | 27 | (103,334 | ) | $ | (1,033 | ) | $ | 28,826,378 | $ | (30,937,110 | ) | $ | 127,669 | $ | (1,981,316 | ) | ||||||||||||||||||||||||
| Issuance of stock for board and consulting fees | 3,386,961 | 339 | - | - | 324,791 | 325,131 | ||||||||||||||||||||||||||||||||||||||||||
| Directors deferred compensation granted | - | - | - | - | 21,000 | 21,000 | ||||||||||||||||||||||||||||||||||||||||||
| Comprehensive Income | - | - | - | - | 5 | 5 | ||||||||||||||||||||||||||||||||||||||||||
| Net loss for year ended December 31, 2021 | (760,032 | ) | (760,032 | ) | ||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2021 | 30,921,701 | 3,092 | 1 | 0 | 270,306 | 27 | (103,334 | ) | (1,033 | ) | 29,172,169 | (31,697,142 | ) | 127,674 | (2,395,213 | ) | ||||||||||||||||||||||||||||||||
| Balance | 30,921,701 | 3,092 | 1 | 0 | 270,306 | 27 | (103,334 | ) | (1,033) | 29,172,169 | (31,697,142 | ) | 127,674 | (2,395,213 | ) | |||||||||||||||||||||||||||||||||
| Issuance of stock for board and consulting fees | 1,708,805 | 171 | - | - | - | - | 183,457 | 183,628 | ||||||||||||||||||||||||||||||||||||||||
| Comprehensive Income | - | - | - | - | 1 | 1 | ||||||||||||||||||||||||||||||||||||||||||
| Net loss for year ended December 31, 2022 | (729,287 | ) | (729,287 | ) | ||||||||||||||||||||||||||||||||||||||||||||
| Net loss for period | (729,287 | ) | (729,287 | ) | ||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2022 | 32,630,506 | $ | 3,263 | 1 | $ | 0 | 270,306 | $ | 27 | (103,334 | ) | $ | (1,033 | ) | $ | 29,355,626 | $ | (32,426,429 | ) | $ | 127,675 | $ | (2,940,871 | ) | ||||||||||||||||||||||||
| Balance | 32,630,506 | $ | 3,263 | 1 | $ | 0 | 270,306 | $ | 27 | (103,334 | ) | $ | (1,033 | ) | $ | 29,355,626 | $ | (32,426,429 | ) | $ | 127,675 | $ | (2,940,871 | ) | ||||||||||||||||||||||||
See accompanying notes to consolidated financial statements
| 15 |
VYCOR MEDICAL, INC.
Consolidated StatementStatements of Cash Flows
| For the year ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (1,477,045 | ) | $ | (1,652,280 | ) | ||
| Adjustments to reconcile net loss to cash used in operating activities: | ||||||||
| Amortization of intangible assets | 162,076 | 89,157 | ||||||
| Depreciation of fixed assets | 127,899 | 178,339 | ||||||
| Inventory provision and write-off | 44,760 | 10,174 | ||||||
| Share based compensation | 334,972 | 482,110 | ||||||
| (Gain) loss on foreign exchange | (1,477 | ) | 144 | |||||
| Warrant issuance expense | 120,788 | - | ||||||
| Accrued liabilities - Related party | 225,000 | 247,500 | ||||||
| Changes in assets and liabilities: | ||||||||
| Accounts receivable | 38,362 | (42,445 | ) | |||||
| Inventory | (54,573 | ) | 78,292 | |||||
| Prepaid expenses | 16,625 | 68,351 | ||||||
| Accrued interest to related party | 680 | 12,161 | ||||||
| Accrued interest other | 48,000 | 48,132 | ||||||
| Accounts payable | (108,630 | ) | (418 | ) | ||||
Accrued liabilities – Other | (38,129 | ) | (22,801 | ) | ||||
| Security Deposit | 33,254 | 6,666 | ||||||
| Cash used in operating activities | (527,438 | ) | (496,918 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchase of fixed assets | (183,258 | ) | (57,451 | ) | ||||
| Cash used in investing activities | (183,258 | ) | (57,451 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Net proceeds from issuance of common stock, net | 842,873 | - | ||||||
| Issuance of shares pursuant to exercise of warrants | 19,441 | - | ||||||
| Proceeds from issuance of Notes Payable: Related Party | - | 248,000 | ||||||
| Monies in Escrow Related Party - Offering | - | 101,000 | ||||||
| Proceeds, net of repayment of Notes Payable - Other | 1,537 | (82,290 | ) | |||||
| Cash provided by financing activities | 863,851 | (266,710 | ) | |||||
| Effect of exchange rate changes on cash | (3,801 | ) | (2,959 | ) | ||||
| Net decrease in cash | 149,354 | (290,618 | ) | |||||
| Cash at beginning of year | 56,859 | 347,477 | ||||||
| Cash at end of year | $ | 206,213 | $ | 56,859 | ||||
| Supplemental Disclosures of Cash Flow information: | ||||||||
| Non-Cash Transactions: | ||||||||
| Preferred stock dividends satisfied in new preferred stock | $ | - | $ | 179,727 | ||||
| Conversion into Common Stock of Notes Payable: Related Party | $ | (248,000 | ) | $ | - | |||
| Proceeds from monies in Escrow Related Party - Offering | $ | (101,000 | ) | $ | - | |||
| 2022 | 2021 | |||||||
| For the twelve months ended | ||||||||
| December 31, | December 31, | |||||||
| 2022 | 2021 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (404,917 | ) | $ | (435,662 | ) | ||
| Adjustments to reconcile net loss to cash provided by (used in) operating activities: | ||||||||
| Amortization of intangible assets | - | 11,349 | ||||||
| Depreciation of fixed assets | 61,403 | 57,069 | ||||||
| Inventory provision | 15,515 | 12,360 | ||||||
| Stock based compensation | 185,610 | 341,098 | ||||||
| Forgiveness of debt Paycheck Protection Program | - | (117,200 | ) | |||||
| Changes in assets and liabilities: | ||||||||
| Accounts receivable | (30,108 | ) | 33,142 | |||||
| Inventory | (56,868 | ) | (38,785 | ) | ||||
| Prepaid expenses | (14,936 | ) | 20,034 | |||||
| Accrued interest - Related Party | 39,563 | 31,841 | ||||||
| Accrued interest - Other | 52,664 | 51,582 | ||||||
| Accounts payable | (27,676 | ) | 48,611 | |||||
| Accrued liabilities - Other | (35,607 | ) | 9,911 | |||||
| Changes in discontinued operations, net | (1,659 | ) | (3,980 | ) | ||||
| Cash provided by (used in) operating activities | (217,016 | ) | 21,369 | |||||
| Cash flows from investing activities: | ||||||||
| Purchase of fixed assets | (2,780 | ) | (38,375 | ) | ||||
| Cash used in investing activities | (2,780 | ) | (38,375 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds from Notes Payable - Related Party | 172,500 | 10,000 | ||||||
| Proceeds from Paycheck Protection Program and EIDL | - | 58,600 | ||||||
| Repayments net of Proceeds - Notes Payable - Other | (6,611 | ) | (6,655 | ) | ||||
| Cash provided by financing activities | 165,889 | 61,945 | ||||||
| Effect of exchange rate changes on cash | 1 | - | ||||||
| Net increase (decrease) in cash | (53,906 | ) | 44,939 | |||||
| Cash at beginning of year | 90,941 | 46,002 | ||||||
| Cash at end of year | $ | 37,035 | $ | 90,941 | ||||
| Supplemental Disclosures of Cash Flow information: | ||||||||
| Cash paid for interest | $ | 8,246 | $ | 4,386 | ||||
| Cash paid for income tax | $ | 0 | $ | 0 | ||||
| Non-cash activities: | ||||||||
| Unamortized stock compensation | $ | 3,050 | $ | 5,032 | ||||
See accompanying notes to consolidated financial statements
| 16 |
VYCOR MEDICAL, INC.
Notes to Consolidated Financial Statements
1. BUSINESS OF THE COMPANY AND GOING CONCERN
Business Description
Vycor Medical, Inc. (the “Company”) designs, develops and markets neurological medical devices and therapies through two operating divisions: Vycor Medical and NovaVision. Vycor Medical focuses on brain and cervical surgical access systems for sale to hospitals and medical professionals; NovaVision focuses on neuro-stimulation therapies and diagnostic devices for the treatment and screening of vision field loss resulting from neurological damage.
Ability to continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. The Company has incurred losses since its inception, including a net loss of $1,477,045$404,917 and $1,652,280$435,662 for the years ending December 31, 20172022 and 20162021 respectively and has not generated sufficient cash flows from operations. As at December 31, 20172022 the Company had a working capital deficiency of $197,297,$551,433 excluding related party liabilities of $562,210.$2,585,600. As a result these conditions, among others, raise substantial doubt regarding our ability to continue as a going concern. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty
The Company is executing on a plan to achieve growth in revenues for both the Vycor Medical and NovaVision divisions as well as a reduction in costs, thereby reducing its cash operating usage.losses. Included within the working capital deficiency above is a term note for $300,000 to EuroAmerican Investment Corp. (“EuroAmerican”), together with accrued interest of $424,897, which has a maturity date of December 31, 2023, having been extended on a number of occasions from its initial due date of June 11, 2011. At this time, it is not known whether any further extension of the note beyond December 31, 2023 will be available. However, the Company believes it may not have sufficient cash to meet its various cash needs through March 31, 20192024 unless the Company is able to obtain additional cash from the issuance of debt or equity securities. Fountainhead, the Company’s largest shareholder, is currently providinghas provided working capital funding to the Company on an as-needed basis, although there is no guarantee that this will continue to be the case. The Company may consider seeking additional equity or debt funding, although there is no assurance that this would be available on acceptable terms or at all. If adequate funds are not available, the Company may have to delay or curtail development or commercialization of products or cease some of its operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
2. SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation and Basis of Presentation
The consolidated financial statements include the accounts of Vycor Medical, Inc., and its wholly-owned subsidiaries, NovaVision, Inc. (a Delaware corporation), NovaVision GmbH (a German corporation) and Sight Science Limited (a UK corporation), both wholly owned subsidiaries of NovaVision, Inc. The Company is headquartered in Boca Raton, FL. All material inter-company accounts, transactions, and balances have been eliminated in consolidation. Certain reclassifications and format changesFollowing the decision in April 2020 to close the German office of NovaVision, the activities of NovaVision GmbH have been madeaccounted for as discontinued operations.
Revenue Recognition
On January 1, 2018, the Company adopted ASC 606, Revenue from Contracts with Customers and all the related amendments (new revenue standard) to prior year amounts to conform toall contracts. The adoption of the current year presentation.new accounting standard had no impact on company’s consolidated financial statements.
Revenue Recognition
Vycor Medical generates revenue from the sale of its surgical access system to hospitals and other medical professionals. Vycor Medical records revenue from product sales when obligations under the terms of a completed contract forwith customers are satisfied. Generally, this occurs with the sale exists, price is fixed or determinable,transfer of control of the product is invoiced and shippedgoods to the customer and collectability is reasonably assured.customers. Vycor Medical does not provide for product returns or warranty costs.
| 17 |
Vycor determines revenue recognition through the following steps:
| ● | Identification of the contract, or contracts, with a customer | |
| ● | Identification of the performance obligations in the contract | |
| ● | Determination of the transaction price | |
| ● | Allocation of the transaction price to the performance obligations in the contract | |
| ● | Recognition of revenue when Vycor satisfy a performance obligation |
NovaVision generates revenues from various programs, therapy services and other sources such as software license sales. Therapy services revenues represent fees from NovaVision’s vision restoration therapy software, eye movement training software, diagnostic software, clinic set up and training fees, and the professional and support services associated with the therapy. NovaVision provides vision restoration therapy directly to patients. The typical vision restoration therapy program consists of NeuroEyeCoach, performed over 2-4 weeks, and six modules of Vision Restoration Therapy, performed on average over 6 months in the U.S. and U.K. and 10 months in Germany.months. A patient contract comprises set-up fees and monthly therapy fees. Set-up fees are recognized at the outset of the contract and therapy revenue is recognized ratably over the therapy period. Patient therapy is restricted to being completed by a patient within a specified time frame. NovaVision’s saccadic training software is generally completed within 2-4 weeks and revenue is therefore recognized fully at commencement.
Deferred revenue results from patients paying for the therapy in advance of receiving the therapy.
As part of the adoption of ASC 606, see Note 7 to the Consolidated Financial Statements for further disaggregation of revenue.
Cash and cash equivalents
The Company maintains cash balances at various financial institutions. Accounts at each institution in the U.S. are insured by the Federal Deposit Insurance Corporation up to $250,000.$250,000. Cash balances may at times exceed the FDIC insured limits. Cash also includes a US investment account in a money market backed by government securities up to 105% of the account balance. The Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents. Included within cash are deposits paid by patients, held by the Company until the patient returns the VRT device or chinrest at the end of therapy. At December 31, 20172022 and 20162021 patient deposits amounted to $41,172$19,154 and $33,351,$30,944, respectively, and are included in Accrued Liabilities.
Accounts Receivable and Allowance for Doubtful Accounts Receivable
The Company’s accounts receivable are due from the hospitals and distributors in the case of Vycor Medical, and from patients directly for therapy or physicians for diagnostic products in the case of NovaVision. Accounts receivable are due once products have been delivered or at the time the therapy is initiated; however, for NovaVision therapy patients sometimes credit is extended through various payment plans based on individual financial conditions, generally not to exceed the therapy period. The outstanding balances are stated net of an allowance for doubtful accounts.
We have a policy of reserving for uncollectible accounts based on our best estimate of the amount of probable credit losses in our existing accounts receivable. We extend credit to our customers based on an evaluation of their financial condition and other factors. We generally do not require collateral or other security to support accounts receivable. We perform ongoing credit evaluations of our customers and maintain an allowance for potential bad debts if required. We determine whether an allowance for doubtful accounts is required by evaluating specific accounts where information indicates the customers may have an inability to meet financial obligations. In these cases, we use assumptions and judgment, based on the best available facts and circumstances, to record a specific allowance for those customers against amounts due to reduce the receivable to the amount expected to be collected. These specific allowances are re-evaluated and adjusted as additional information is received. The amounts calculated are analyzed to determine the total amount of the allowance. We may also record a general allowance as necessary. Direct write-offs are taken in the period when we have exhausted our efforts to collect overdue and unpaid receivables or otherwise evaluate other circumstances that indicate that we should abandon such efforts.
| 18 |
Inventories
Inventories consist of raw materials, work in process and finished goods that are stated at the lower of cost determined using the weighted average cost method, or market.net realizable value. Net realizable value is the estimated selling price, in the ordinary course of business, less estimated costs to complete and dispose of the product. If the Company identifies excess, obsolete or unsalable items, its inventories are written down to their realizable value in the period in which the impairment is first identified. The charge provision for inventory obsolescence for the years ended December 31, 20172022 and 20162021 was $44,760$13,160 and $10,174, respectively, of which $39,672 is a special provision related to the introduction of the enhanced VBAS.$12,360, respectively. Shipping and handling costs incurred for inventory purchases and product shipments are recorded in cost of sales in the Company’s consolidated statements of operations.comprehensive loss.
Leases
The Company has one leased building in Boca Raton, Florida that is classified as operating lease right-of use (“ROU”) assets and operating lease liabilities in the Company’s consolidated balance sheet as per ASC 842. ROU assets and lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at the commencement date for leases exceeding 12 months. Minimum lease payments include only the fixed lease component of the agreement. Operating lease expense is recognized on a straight-line basis over the lease term and is included in cost of Selling, General and Administrative expenses.
The standard was effective for us beginning January 1, 2019. The Company elected the available practical expedients on adoption. The adoption had a material impact on our consolidated balance sheets but did not have a material impact on our consolidated statements of comprehensive loss. The most significant impact was the recognition of ROU assets and lease liabilities for operating leases.
Discontinued Operations
In accordance with ASU No. 2014-08, Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity, a disposal of a component of an entity or a group of components of an entity is required to be reported as discontinued operations if the disposal represents a strategic shift that has (or will have) a major effect on an entity’s operations and financial results when the components of an entity meets the criteria in paragraph 205-20-45-1E to be classified as held for sale. When all of the criteria to be classified as held for sale are met, including management, having the authority to approve the action, commits to a plan to sell the entity, the major current assets, other assets, current liabilities, and noncurrent liabilities shall be reported as components of total assets and liabilities separate from those balances of the continuing operations. At the same time, the results of all discontinued operations (which we presented as operations to be disposed and operations disposed), less applicable income taxes (benefit), shall be reported as components of net income (loss) separate from the net income (loss) of continuing operations in accordance with ASC 205-20-45.
Foreign Currency
The Euro is the local currency of the country in which the discontinued operations of NovaVision GmbH conducts its operations and is considered the functional currency of this entity; the GB Pound is the local currency of the country in which Sight Science Limited conducts its operations and is considered the functional currency of this entity. All balance sheet amounts are translated to U.S. dollars using the U.S. exchange rate at the balance sheet date except for the equity section which is translated at historical rates. Operating statement amounts are translated using an average exchange rate for the period of operations. Foreign currency translation effects are accumulated as part of the accumulated other comprehensive income (loss) and included in stockholders’ equitydeficiency in the accompanying Consolidated Balance Sheet.Sheets.
| 19 |
Educational marketing and advertising expenses
The Company may incur costs for the education of customers on the uses and benefits of its products. The Company will include education, marketing and advertising expense as a component of selling, general and administrative costs as such costs are incurred. There were no such expenses for the years ended December 31, 2022 and 2021.
Income taxes
We use the asset and liability method of accounting for income taxes in accordance with ASC Topic 740, “Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year and (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if based on the weight of the available positive and negative evidence, it is more likely than not some portion or all of the deferred tax assets will not be realized.
ASC Topic 740.10.30 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740.10.40 provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. We have no material uncertain tax positions for any of the reporting periods presented.
Accounting for forgivable loan received under the Small Business Administration Paycheck Protection Program
During the year ended December 31, 2020, the Company received a loan of $58,600 (“First Draw Loan”), pursuant to the Paycheck Protection Program (the “PPP”) under Division A, Title I of the CARES Act. During the year ended December 31, 2021, the Company received an additional PPP loan of $58,600 (“Second Draw Loan”). Under the terms of the PPP, both the First Draw Loan and Second Draw Loan were forgiven during the year ended 2021 as they were used for qualifying expenses as described in the CARES Act.
The Company accounted for the loans as a financial liability in accordance with FASB ASC 470 and accrued interest in accordance with the interest method under FASB ASC 835-30. For purposes of derecognition of the liability, FASB ASC 470-50-15-4 refers to guidance in FASB ASC 405-20. Based on this guidance, the proceeds of the loans were recorded as a liability until either (1) the loans are, in part or wholly, forgiven and the Company has been “legally released”, or (2) the Company pays off the loans. The Company has accordingly reduced the liability by the amount forgiven and recorded a gain on the extinguishment.
Fixed assets
Fixed assets are stated at cost less accumulated depreciation. Depreciation is provided for on a straight-line basis over the useful lives of the assets. Expenditures for additions and improvements are capitalized; repairs and maintenance are expensed as incurred.
Patents and Other Intangible Assets
The Company capitalizes legal and related costs associated with the establishment and enhancement of patents for its products once patents have been applied for. Costs associated with the development of the patented item or processes are charged to research and development costs as incurred. The capitalized costs are amortized over the life of the patent. The Company reviews intangible assets on an annual in accordance with the authoritative guidance. Trademarks have an indefinite life and are reviewed annually by management for impairment in accordance with the authoritative guidance.
| 20 |
Impairment of long-lived assets
Long-lived assets are reviewed for impairment when circumstances indicate the carrying value of an asset may not be recoverable. For assets that are to be held and used, impairment is recognized when the estimated undiscounted cash flows associated with the asset or group of assets is less than their carrying value. If impairment exists, an adjustment is made to write the asset down to its fair value, and a loss is recorded as the difference between the carrying value and fair value. Fair values are determined based on quoted market values, discounted cash flows or internal and external appraisals, as applicable. Assets to be disposed of are carried at the lower of carrying value or estimated net realizable value.
Research and Development
The Company expenses all research and development costs as incurred. For the years ended December 31, 20172022 and 2016,2021, the amounts charged to research and development expenses were $3,015$0 and $4,739,$15,159, respectively.
Software Development Costs
The Company accounts for software development costs in accordance with ASC 350-40, whereby all costs incurred during the preliminary stage of a development project should be charged to expense as incurred. Capitalization of costs begins after the preliminary stage has been completed, management commits to funding the project, it is probable that the project will be completed, and the software will be used for its intended function. All post-implementation costs are charged to expense as incurred. Accordingly, direct internal and external costs associated with the development of the features and functionality of the Company’s software, incurred during the application development stage, are capitalized and amortized using the straight-line method of the estimated life of five years. Foryears. There was no capitalized for software development during the years ended December 31, 20172022 and 2016, the amounts capitalized for software development were $2,745 and $12,679 respectively, for the Company’s VRT 7.0 and NeuroEyeCoach programs.2021.
Uses of estimates in the preparation of financial statements
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from those estimated. To the extent management’s estimates prove to be incorrect, financial results for future periods may be adversely affected. Significant estimates and assumptions contained in the accompanying consolidated financial statements include management’s estimate of the allowance for uncollectible accounts receivable and provision for inventory obsolescence, useful life of intangible assets, andobsolescence.
Stock Option Plan
Under ASC Topic 718, the Company estimates the fair valuesvalue of optionsoption awards on the date of grant using an option-pricing model. The grant date fair value is recognized over the option-vesting period, the period during which an employee is required to provide service in exchange for the award. No compensation cost is recognized for equity instruments for which employees do not render the requisite service. Under these standards, compensation cost for employee cost for employee stock-based awards is based on the estimated grant-date fair value and warrants included inrecognized over the determinationvesting period of debt discounts and share based compensation.the applicable award on a straight-line basis.
The Company recognizes the cost of all share-basedstock -based payments under the relevant authoritative accounting guidance. Share-basedStock-based payments include any remuneration paid by the Company in shares of the Company’s common stock or financial instruments that grant the recipient the right to acquire shares of the Company’s common stock. For share-basedstock-based payments to employees, which consist only of awards made under the stock option plan described below, the Company accounts for the payments in accordance with the provisions of ASC Topic 718, “Stock Compensation”. Share-basedStock-based payments to consultants, service providers and other non-employees are accounted for under in accordance with ASC Topic 718, ASC Topic 505, “Equity Payments to Non-Employees” or other applicable authoritative guidance.
| 21 |
Convertible Instruments
We evaluate and account for conversion options embedded in convertible instruments in accordance with ASC 815 “Derivatives and Hedging Activities”.
Applicable GAAP requires companies to bifurcate conversion options from their host instruments and account for them as free standingfree-standing derivative financial instruments according to certain criteria. The criteria include circumstances in which (a) the economic characteristics and risks of the embedded derivative instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b) the hybrid instrument that embodies both the embedded derivative instrument and the host contract is not re-measured at fair value under other GAAP with changes in fair value reported in earnings as they occur and (c) a separate instrument with the same terms as the embedded derivative instrument would be considered a derivative instrument.
We account for convertible instruments (when we have determined that the embedded conversion options should not be bifurcated from their host instruments) as follows: We record when necessary, discounts to convertible notes for the intrinsic value of conversion options embedded in debt instruments based upon the differences between the fair value of the underlying common stock at the commitment date of the note transaction and the effective conversion price embedded in the note. Debt discounts under these arrangements are amortized over the term of the related debt to their stated date of redemption. The embedded conversion option in connection with our convertible debt could not be exercised unless and until we completed a Qualifying Financing transaction. Accordingly, we determined based on authoritative guidance that the embedded conversion option is deemed to be a contingent conversion rather than active conversion option that did not require accounting recognition at the commitment dates of the issuances of the Notes.
Common Stock Purchase Warrants and Other Derivative Financial Instruments
We classify as equity any contracts that require physical settlement or net-share settlement or provide us a choice of net-cash settlement or settlement in our own shares (physical settlement or net-share settlement) provided that such contracts are indexed to our own stock as defined in ASC 815-40 (“Contracts in Entity’s Own Equity”). We classify as assets or liabilities any contracts that require net-cash settlement (including a requirement to net cash settle the contract if an event occurs and if that event is outside our control) or give the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement). We assess classification of our common stock purchase warrants and other free-standing derivatives at each reporting date to determine whether a change in classification between assets and liabilities is required.
Fair Value Measurements
We adopted the provisions of ASC Topic 820, “Fair Value Measurements and Disclosures”, which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring fair value and expands disclosure of fair value measurements.
The estimated fair value of certain financial instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued expenses are carried at historical cost basis, which approximates their fair values because of the short-term nature of these instruments. The carrying amounts of our short and long-term credit obligations approximate fair value because the effective yields on these obligations, which include contractual interest rates taken together with other features such as concurrent issuances of warrants and/or embedded conversion options, are comparable to rates of returns for instruments of similar credit risk.
ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 describes three levels of inputs that may be used to measure fair value:
Level 1 — quoted prices in active markets for identical assets or liabilities
Level 2 — quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 — inputs that are unobservable (for example cash flow modeling inputs based on assumptions)
The Company has no Financialfinancial instruments measured at Fairfair value on a recurring basis.
| 22 |
Net Loss Per Share
Basic net loss per share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. Diluted net loss per share is computed giving effect to all dilutive potential common shares that were outstanding during the period. Dilutive potential common shares consist of incremental shares issuable upon exercise of stock options and warrants and conversion of preferred stock and convertible debt. Such potentially dilutive shares are excluded when the effect would be to reduce a net loss per share. No dilution adjustment has been made to the weighted average outstanding common shares in the periods presented because the assumed exercise of outstanding options and warrants and the conversion of preferred stock and debt would be anti-dilutive.
SCHEDULE OF COMMON STOCK NOT INCLUDED IN CALCULATION OF DILUTED NET LOSS PER SHARE
| December 31, | December 31, | December 31, 2022 | December 31, 2021 | |||||||||||||
| 2017 | 2016 | |||||||||||||||
| Stock options outstanding | 725,557 | 705,557 | ||||||||||||||
| Warrants to purchase common stock | 6,929,386 | 6,007,048 | ||||||||||||||
| Debentures convertible into common stock | 2,308,405 | 242,647 | 3,451,889 | 3,223,317 | ||||||||||||
| Preferred shares convertible into common stock | 1,272,052 | 1,272,052 | 1,272,052 | 1,272,052 | ||||||||||||
| Directors Deferred Compensation Plan | 510,527 | 176,479 | ||||||||||||||
| Total | 11,745,927 | 8,403,783 | 4,723,941 | 4,495,369 | ||||||||||||
Recent Accounting Pronouncements
In August 2014, the FASB issued ASU No. 2014-15 —Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. The ASU requires management to evaluate whether there are conditions and events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the financial statements are issued and if management’s plans will alleviate that doubt. Management will be required to make this evaluation for both annual and interim reporting periods. The Company adopted this guidance for the fiscal year ended December 31, 2017. This adoption did not have a material impact on the Company’s consolidated financial statements.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers. This ASU supersedes most of the existing guidance on revenue recognition in Accounting Standards Codification ("ASC") Topic 605, Revenue Recognition and establishes a broad principle that would require an entity to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve this principle, an entity identifies the contract with a customer, identifies the separate performance obligations in the contract, determines the transaction price, allocates the transaction price to the separate performance obligations and recognizes revenue when each separate performance obligation is satisfied. The FASB has subsequently issued additional ASUs to clarify certain elements of the new revenue recognition guidance. The guidance is effective for fiscal years beginning after December 15, 2017, and is to be applied retrospectively using one of two transition methods at the entity's election. The full retrospective method requires companies to recast each prior reporting period presented as if the new guidance had always existed. Under the modified retrospective method, companies would recognize the cumulative effect of initially applying the standard as an adjustment to opening retained earnings at the date of initial application. Early adoption is permitted for fiscal years beginning after December 15, 2016.
In May 2016, FASB issued ASU 2016-12, "Revenue from Contracts with Customers (Topic 606) - Narrow-Scope Improvements and Practical Expedients". The update is to address certain issues identified by the FASB/IASB Joint Transition Resource Group for Revenue Recognition (TRG) in the guidance on assessing collectability, presentation of sales taxes, noncash consideration, and completed contracts and contract modifications at transition, the Board decided to add a project to its technical agenda to improve Topic 606, Revenue from Contracts with Customers, by reducing: 1) The potential for diversity in practice at initial application 2) The cost and complexity of applying Topic 606 both at transition and on an ongoing basis. The amendments in this Update affect entities with transactions included within the scope of Topic 606. The scope of that Topic includes entities that enter into contracts with customers to transfer goods or services (that are an output of the entity's ordinary activities) in exchange for consideration. The amendments to the recognition and measurement provisions of Topic 606 also affect entities with transactions included within the scope of Topic 610, Other Income. The amendments in this Update affect the guidance in Accounting Standards Update No. 2014-09, Revenue from Contracts with Customers (Topic 606), which is not yet effective. The effective date and transition requirements for the amendments in this Update are the same as the effective date and transition requirements for Topic 606 (and any other Topic amended by Update 2014-09). Accounting Standards Update No. 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date, defers the effective date of Update 2014-09 by one year. The Company is currently evaluating the potential impact of adopting this new standard on its consolidated statements and related disclosures.
In December 2017, the Securities and Exchange Commission (“SEC”) released Staff Accounting Bulletin No. 118 (the “Bulletin”), which provides accounting guidance regarding accounting for income taxes for the reporting period that includes the enactment of the Tax Act. The Bulletin provides guidance in those situations where the accounting for certain income tax effects of the Tax Act will be incomplete by the time financial statements are issued for the reporting period that includes the enactment date. For those elements of the Tax Act that cannot be reasonably estimated, no effect will be recorded.
The SEC has provided in the Bulletin that in situations where the accounting is incomplete for certain effects of the Tax Act, a measurement period which begins in the reporting period that includes the enactment of the Tax Act and ends when the entity has obtained, prepared and analyzed the information is needed in order to complete the accounting requirements. The measurement period shall not exceed one year from enactment.
In February 2018, the FASB issued ASU 2018-02, “Income Statement - Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income,” which allows a reclassification from accumulated other comprehensive income to retained earnings for stranded tax effects resulting from the Tax Cuts and Jobs Act. This guidance is effective for all entities for fiscal years, and interim periods within those years, beginning after December 15, 2018, with early adoption permitted. The amendments in ASU 2018-02 should be applied either in the period of adoption or retrospectively to each period in which the effect of the change in the U.S. federal corporate income tax rate in the Tax Cuts and Jobs Act is recognized. The Company is currently evaluating the potential impact of adopting this new standard on its consolidated statements and related disclosures.
From time to time new accounting pronouncements are issued by the Financial Accounting Standards Board or other standard setting bodies that may have an impact on the Company’s accounting and reporting. The Company believes that suchother recently issued accounting pronouncements and other authoritative guidance for which the effective date is in the future will not have an impact on its accounting or reporting or that such impact will not be material to its financial position, results of operations and cash flows when implemented.
3. DISCONTINUED OPERATIONS
In April 2020, the board of Vycor took the decision to close the German operations of NovaVision, including the German office and NovaVision GmbH, and instead migrate to a licensed business model; effective July 1, 2020 Vycor entered into a license agreement and transition agreement (the “Agreements”) with HelferApp GmbH, a cognitive therapy specialist. Under the Agreements, HelferApp is licensed to provide NovaVision’s products and therapies in Germany, Austria and Switzerland to patients and professionals; and assumed responsibility for the current patients of NovaVision in the territory. The NovaVision German office was closed effective June 30, 2020. The Company will continue to fund the remaining expenses of the German operations, which are non-material, until such a time as NovaVision GmbH will be formally wound up.
Reconciliation of the major line items from discontinued operations that are presented in the consolidated balance sheets and consolidated statements of comprehensive loss are as follows:
SCHEDULE OF DISCONTINUED OPERATIONS
Major line items constituting assets and liabilities in the consolidated balance sheets
| December 31, | December 31, | |||||||
| 2022 | 2021 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | 1,212 | $ | 380 | ||||
| Total Current Assets | 1,212 | 380 | ||||||
| TOTAL ASSETS | $ | 1,212 | $ | 380 | ||||
| LIABILITIES | ||||||||
| Current Liabilities | ||||||||
| Accounts payable | $ | 693 | $ | 4 | ||||
| Other current liabilities | (2,092 | ) | (576 | |||||
| Total Current Liabilities | $ | (1,399 | ) | $ | (572 | |||
| 23 |
3. INVENTORYMajor line items constituting loss from discontinued operations
| December 31, 2017 | December 31, 2016 | |||||||
| Raw materials and work in process | 80,139 | 67,936 | ||||||
| Finished goods | 133,744 | 136,135 | ||||||
| Total Inventory | 213,883 | 204,071 | ||||||
| 2022 | 2021 | |||||||
| For years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Revenue | $ | - | $ | - | ||||
| Cost of Goods Sold | - | - | ||||||
| Gross Profit | - | - | ||||||
| Operating Expenses: | ||||||||
| Selling, general and administrative | 5,062 | 26,545 | ||||||
| Total Operating Expenses | 5,062 | 26,545 | ||||||
| Operating Loss | (5,062 | ) | (26,545 | ) | ||||
| Other Income (Expense) | ||||||||
| Loss on foreign currency exchange | (150 | ) | (868 | ) | ||||
| Total Other Income (Expense) | (150 | ) | (868 | ) | ||||
| Loss Before Provision for Income Taxes | (5,212 | ) | (27,413 | ) | ||||
| Provision for income taxes | - | - | ||||||
| Loss from discontinued operations, net of tax | $ | (5,212 | ) | $ | (27,413 | ) | ||
4. NOTES PAYABLEINVENTORY
SCHEDULE OF INVENTORY
December 31, 2022 | December 31, 2021 | |||||||
| Raw materials and work in process | $ | 109,894 | $ | 77,166 | ||||
| Finished goods | 138,980 | 130,355 | ||||||
| Total Inventory | $ | 248,874 | $ | 207,521 | ||||
Other Notes Payable5. LEASE
Other Notes Payable consists of:The Company recognized the following related to a lease in its consolidated balance sheets:
SCHEDULE OF SUPPLEMENTAL BALANCE SHEET INFORMATION RELATED TO LEASES
| December 31, 2017 | December 31, 2016 | |||||||
| On March 25, 2011 the Company issued a term note for $300,000 to EuroAmerican Investment Corp. (“EuroAmerican”). The term note bears interest at 16% per annum and was due June 25, 2011, and has been extended on a number of occasions. On the note’s most recent due date, the note was amended and extended to December 31, 2018. See further note below. | 300,000 | 300,000 | ||||||
| Insurance policy finance agreements. | 18,393 | 16,856 | ||||||
| Total Other Notes Payable | $ | 318,393 | $ | 316,856 | ||||
| 2022 | 2021 | |||||||
| Year Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Operating Lease ROU Assets | $ | 32,645 | $ | 79,560 | ||||
| Operating Lease Liabilities | ||||||||
| Current portion | $ | 29,591 | $ | 46,915 | ||||
| Long- term portion | - | 30,580 | ||||||
| Operating Lease Liabilities | $ | 29,591 | $ | 77,495 | ||||
| 24 |
6. NOTES PAYABLE
Related Party Notes Payable consists of:
SUMMARY OF NOTES PAYABLE
| December 31, 2022 | December 31, 2021 | |||||||
| On June 25, 2018 the Company issued promissory notes to Peter Zachariou for $30,000. The notes bear interest at 10% per annum and are payable on the earlier of one year or five days following the delivery of written demand for payment by the Payee. The note was extended for another twelve months on its due date to June 25, 2022 or on demand by the Payee. | $ | 30,000 | $ | 30,000 | ||||
| On June 25, 2018 the Company issued promissory notes to Peter Zachariou for $30,000. The notes bear interest at 10% per annum and are payable on the earlier of one year or five days following the delivery of written demand for payment by the Payee. The note was extended for another twelve months on its due date to June 25, 2023 or on demand by the Payee. | $ | 30,000 | $ | 30,000 | ||||
| Between March 26, 2018 and November 17, 2022 the Company issued fifteen promissory notes to Fountainhead Capital Management Limited for $463,873. The notes bear interest at 10% per annum and are payable on the earlier of one year or five days following the delivery of written demand for payment by the Payee. Twelve of the notes were extended on their due dates for another twelve months. The Notes will be due between April 2023 and March 2024 or on demand by the Payee. | 463,373 | 290,873 | ||||||
| Total Related Party Notes Payable | $ | 493,373 | $ | 320,873 | ||||
Other Notes Payable consists of:
| December 31, 2022 | December 31, 2021 | |||||||
| On March 25, 2011 the Company issued a term note for $300,000 to EuroAmerican Investment Corp. (“EuroAmerican”). The term note bears interest at 16% per annum and was due June 25, 2011, and has been extended on a number of occasions. On the note’s most recent due date, the note was amended and extended to December 31, 2023. See further note below. | $ | 300,000 | $ | 300,000 | ||||
| Insurance policy finance agreements and current portion of EIDL Loan (see Long-Term Notes Payable below) | 24,711 | 19,329 | ||||||
| Total Notes Payable: | $ | 324,711 | $ | 319,329 | ||||
Long-Term Notes Payable consists of:
| December 31, 2022 | December 31, 2021 | |||||||
| On July 7, 2020, the Company was granted a $150,000 loan under the Economic Injury Disaster Loan Program pursuant to the Coronavirus Aid, Relief and Economic Security (CARES) Act (“Loan”). The Loan, evidenced by a promissory note dated July 7, 2020, has a term of thirty (30) years, bears interest at a fixed rate of three and three-quarters percent (3.75%) per annum, with monthly payments in the amount of $731.00 per month commencing July 7, 2021 and is secured by essentially all of the assets of the Company. The proceeds of the Loan have been used for general working capital purposes to alleviate economic injury caused by disaster occurring in the month of January 2020 and continuing thereafter. | $ | 146,253 | $ | 150,000 | ||||
| Total Long-term Notes Payable: | $ | 146,253 | $ | 150,000 | ||||
| 25 |
| December 31, 2017 | December 31, 2016 | |||||||
| The Company issued promissory notes to Fountainhead Capital Management Limited for $248,000. The notes bear interest at 10% per annum and are payable on the earlier of one year or five days following the delivery of written demand for payment by the Payee. The notes were converted into 1,180,953 shares of common stock and 1,180,953 warrants in connection with the Private Placement in January 2017. | - | 248,000 | ||||||
| Total Related Party Notes Payable | $ | - | $ | 248,000 | ||||
On January 24, 2018 the Company entered into an amendment agreement (the “Amendment”) with EuroAmerican Investments (“EuroAmerican”) regarding its $300,000$300,000 loan note (the “Note”). Under the Amendment, the Note was extended until December 31, 2018 and the conversion terms of the Note reduced to $0.21,$0.21, the same as the offering price of the 2018 Offering. Conversion of the Note and accrued interest would result in the issuance of 2,308,405 shares of Common Stock. Notwithstanding, EuroAmerican agreed that the Note could not be converted without first offering the Company the right to redeem the Note at principal and accrued interest, and secondly Fountainhead the right to purchase the Note, which cannot be converted prior to such offer and the failure of the Company and Fountainhead to exercise such option in accordance with the amendment terms. In addition, the Company agreed to issue warrants to purchase the same number of shares of Common Stock at $0.27, the same terms as the 2018 Offering, exercisable for three years from January 1, 2018, if and when the conversion option is exercised. The amendment was recognized as a modification, based on the guidance in ASC 470-50. No other term was amended on the Note.
The Company routinely finances all their insurance policies through a third partythird-party finance company which requires a down payment and subsequent monthly payments, the time periods vary from 10 months to 12 equal monthly payments.
5. 7. SEGMENT REPORTING, GEOGRAPHICAL INFORMATION
(a) Business segments
The Company operates in two business segments: Vycor Medical, which focuses on devices for neurosurgery; and NovaVision, which focuses on neuro stimulation therapies and diagnostic devices for the treatment and screening of vision field loss. Discontinued operations were part of NovaVision and revenues and assets were in Europe; see Note 3. Set out below are the revenues, gross profits and total assets for each segment.
SCHEDULE OF BUSINESS SEGMENTS INFORMATION
| Twelve Months Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Revenue: | ||||||||
| Vycor Medical | $ | 1,178,063 | $ | 1,260,949 | ||||
| NovaVision | $ | 206,908 | $ | 191,765 | ||||
| $ | 1,384,971 | $ | 1,452,714 | |||||
| Gross Profit | ||||||||
| Vycor Medical | $ | 973,883 | $ | 1,093,055 | ||||
| NovaVision | $ | 183,954 | $ | 174,974 | ||||
| $ | 1,157,837 | $ | 1,268,029 | |||||
| 2022 | 2021 | |||||||
| Year Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Revenue: | ||||||||
| Vycor Medical | $ | 1,130,915 | $ | 1,273,602 | ||||
| NovaVision | $ | 92,074 | $ | 119,385 | ||||
| Revenue | $ | 1,222,989 | $ | 1,392,987 | ||||
| Gross Profit | ||||||||
| Vycor Medical | $ | 997,667 | $ | 1,147,574 | ||||
| NovaVision | $ | 84,678 | $ | 110,685 | ||||
| Gross Profit | $ | 1,082,345 | $ | 1,258,259 | ||||
| December 31, | December 31, | |||||||
| 2022 | 2021 | |||||||
| Total Assets: | ||||||||
| Vycor Medical | $ | 822,174 | $ | 901,930 | ||||
| NovaVision | 36,792 | 33,054 | ||||||
| Discontinued operations | 1,212 | 380 | ||||||
| Total Assets | $ | 860,178 | $ | 935,364 | ||||
| 26 |
| December 31, 2017 | December 31, 2016 | |||||||
| Total Assets: | ||||||||
| Vycor Medical | $ | 1,263,197 | $ | 805,716 | ||||
| NovaVision | 186,260 | 679,534 | ||||||
| Total Assets | $ | 1,449,457 | $ | 1,485,250 | ||||
(b) Geographic information. The Company operates in two geographic segments, the United States and Europe. Discontinued operations were part of NovaVision and revenues and assets were in Europe; see Note 3. Set out below are the revenues, gross profits and total assets for each segment.
SUMMARY OF GEOGRAPHIC INFORMATION
| 2022 | 2021 | |||||||||||||||
| Twelve Months Ended December 31, | Year Ended December 31, | |||||||||||||||
| 2017 | 2016 | 2022 | 2021 | |||||||||||||
| Revenue: | ||||||||||||||||
| United States | $ | 1,282,527 | $ | 1,355,439 | $ | 1,214,723 | $ | 1,374,770 | ||||||||
| Europe | $ | 102,444 | $ | 97,275 | $ | 8,266 | $ | 18,217 | ||||||||
| $ | 1,384,971 | $ | 1,452,714 | |||||||||||||
| Revenue | $ | 1,222,989 | $ | 1,392,987 | ||||||||||||
| Gross Profit | ||||||||||||||||
| United States | $ | 1,067,243 | $ | 1,176,448 | $ | 1,074,143 | $ | 1,240,305 | ||||||||
| Europe | $ | 90,594 | $ | 91,581 | $ | 8,202 | $ | 17,954 | ||||||||
| $ | 1,157,837 | $ | 1,268,029 | |||||||||||||
| Gross Profit | $ | 1,082,345 | $ | 1,258,259 | ||||||||||||
| December 31, | December 31, | |||||||||||||||
| December 31, 2017 | December 31,2016 | 2022 | 2021 | |||||||||||||
| Total Assets: | ||||||||||||||||
| United States | $ | 977,145 | $ | 1,258,624 | $ | 854,236 | $ | 928,761 | ||||||||
| Europe | 472,312 | 226,626 | 4,730 | 6,223 | ||||||||||||
| Discontinued operations | 1,212 | 380 | ||||||||||||||
| Total Assets | $ | 1,449,457 | $ | 1,485,250 | $ | 860,178 | $ | 935,364 | ||||||||
6. 8. FIXED ASSETS
As of December 31, 2017 and 2016, Fixed Assets and the estimated lives used in the computation of depreciation are as follows:
SCHEDULE OF FIXED ASSETS
| Estimated | December 31, | December 31, | ||||||||||||||||||
| Estimated Useful Lives | December 31, 2017 | December 31, 2016 | Useful Lives | 2022 | 2021 | |||||||||||||||
| Machinery and equipment | 3 years | $ | 138,258 | $ | 137,453 | 3 years | $ | 64,762 | $ | 64,762 | ||||||||||
| Leasehold Improvements | 3years | 8,881 | 6,206 | 3 years | 8,881 | 8,881 | ||||||||||||||
| Purchased Software | 3 years | 27,706 | 27,706 | 3 years | 27,706 | 27,706 | ||||||||||||||
| Molds and Tooling | 5 years | 608,699 | 409,784 | 5 years | 808,611 | 808,545 | ||||||||||||||
| Furniture and fixtures | 7 years | 26,120 | 25,844 | 7 years | 11,152 | 11,152 | ||||||||||||||
| Therapy Devices | 3 years | 123,739 | 112,857 | 3 years | 104,627 | 101,913 | ||||||||||||||
| Internally Developed Software | 5 years | 1,190,336 | 1,187,591 | 5 years | 363,472 | 363,472 | ||||||||||||||
| 2,123,739 | 1,907,441 | |||||||||||||||||||
| Property, and Equipment, Gross | 1,389,211 | 1,386,431 | ||||||||||||||||||
| Less: Accumulated depreciation and amortization | (1,634,569 | ) | (1,506,390 | ) | (1,085,441 | ) | (1,024,038 | ) | ||||||||||||
| Property and Equipment, net | $ | 489,170 | $ | 401,051 | $ | 303,770 | $ | 362,393 | ||||||||||||
Depreciation expense for the years ended December 31, 20172022 and 20162021 was $127,899$61,403 and $178,339$57,069 respectively, including $14,558$3,171 and $9,914$2,348 respectively for Therapy Devices which is allocated to Cost of Sales.
7. 9. INTANGIBLE ASSETS
Intangible Assets consists of:
SCHEDULE OF INTANGIBLE ASSETS
| December 31, | 2022 | 2021 | ||||||||||||||
| 2017 | 2016 | December 31, | ||||||||||||||
| Amortized intangible assets: Patent (8 years useful life) | ||||||||||||||||
| 2022 | 2021 | |||||||||||||||
| Gross carrying Amount | $ | 865,639 | $ | 865,639 | ||||||||||||
| Amortized intangible assets: Patent (8 years useful life) | ||||||||||||||||
| Gross carrying Amount | $ | 865,639 | $ | 865,639 | $ | 865,639 | $ | 865,639 | ||||||||
| Accumulated Amortization | (784,575 | ) | (627,068 | ) | (865,639 | ) | (865,639 | ) | ||||||||
| $ | 81,064 | $ | 238,571 | |||||||||||||
| Amortized intangible assets: Website (5 years useful life) | ||||||||||||||||
| Finite lived intangible assets net | $ | - | $ | - | ||||||||||||
| Amortized intangible assets: Website (5 years useful life) | ||||||||||||||||
| Gross carrying Amount | $ | 50,760 | $ | 50,760 | $ | 20,382 | $ | 20,382 | ||||||||
| Accumulated Amortization | (40,371 | ) | (35,802 | ) | (20,382 | ) | (20,382 | ) | ||||||||
| $ | 10,389 | $ | 14,958 | |||||||||||||
| Intangible assets not subject to amortization | ||||||||||||||||
| Trademarks | $ | 251,157 | $ | 251,157 | ||||||||||||
| Finite lived intangible assets net | $ | - | $ | - | ||||||||||||
Intangible asset amortization expense for the periodsyears ended December 31, 20172022 and 20162021 was $162,076$0 and $89,157,$11,349, respectively.
| 27 |
8. EQUITY
10. EQUITY
Equity Transactions
From JanuaryOn April 1, 2022 and 2021 the Company issued shares of Common Stock to Ricardo Komotar (RJK Consulting), a consultant, in accordance with the terms of a consulting agreement (see Note 14).
During each of the years ended December 2017,31, 2022 and 2021, under the terms of the Consultancy Agreement referred to in Note 14, the Company issued and shares of Common Stock to Fountainhead for fees of $171,428 and $305,001, respectively.
During the year ended December 31, 2021, the Company granted 334,048 shares of Common Stock (valued at $84,000)$) to non-employee Directors. Under the terms of the Directors Deferred Compensation Plan, the receipt of these shares is deferred until January 15th of the year following termination of their services as a director. As of December 31, 2017 these shares have yet to be issued.
From January to December 2017, the Company issued 106,451 shares of Common Stock (valued at $25,314) to members of the NovaVision, Inc. Scientific Advisory Board as compensation for their services.
From January to December 2017, the Company issued 644,286 shares of Common Stock (valued at $135,300) to Fountainhead under the terms of a Consulting Agreement and 1,571,429 shares of Common Stock (valued at $330,000) to Fountainhead following the achievement of certain enumerated milestones.
From January to December 2017, the Company issued 21,718 shares of Common Stock (valued at $6,251) to Techmed, Inc. in accordance with the terms of a consulting agreement.
During January to December 2016, the Company granted 176,479 shares of Common Stock (valued at $78,000) to non-employee Directors. Under the terms of the Directors Deferred Compensation Plan, the receipt of these shares iswas deferred until the January 15thfollowing the termination of their services as a director. Asdirector or following the termination of December 30, 2016 these shares have yet to be issued.
During January to December 2016,the Plan. The Plan was terminated on April 1, 2021 and the Company issued 78,915 shares of Common Stock (valued at $36,875) to members of the NovaVision, Inc. Scientific Advisory Board in respect of their services.
During January to December 2016, the Company issued 212,478 shares of Common Stock (valued at $120,000) to Fountainhead in accordance with the terms of a Consulting Agreement.
During April to December 2016, the Company issued 15,404 shares of Common Stock (valued at $8,334) to Techmed, Inc. in accordance with the terms of a consulting agreement.
During May to December 2016, the Company issued 100,000 share of Common Stock (valued at $40,700) to Valeo Consulting in accordance with the terms of a consulting agreement.
During January to December 31, 2016, the Company paid an aggregate of 17,971 shares of Preferred D Stock, valued at $179,727, representing preferred stock dividends. The Preferred D shares are convertible on their terms at $2.15 per share of Common Stock into 83,586 shares. An aggregate of 17,142 shares of Preferred D Stock dividends were in respect of related parties.
From January to December the Company issued 72,002 and shares of common stock (valued at $19,441) pursuant to exercise of warrants related toSteve Girgenti and Lowell Rush, respectively, for their Deferred Compensation shares following their resignations from the offering in 2017.board.
Private PlacementPreferred Stock.
On January 11 and February 23, 2017, the Company completed the sale of $1,274,717 in shares of Vycor Common Stock (each a “Share”) and Warrants (together with the Shares, the “Securities”) to accredited investors (the “Investors”). The Shares were issued in a private placement (the “Private Placement”) pursuant to the terms of Stock Purchase Agreements between the Company andDuring each of the Investors,years ended December 31, 2022 and was limited to current shareholders of2021, the Company asaccrued an aggregate of November 9, 2016 (the “Record Date”).
Included$324,370 of dividends in these gross proceeds is the conversion of $248,000 of debt on the balance sheet at December 31, 2016 and $101,000 funds held in escrow on the balance sheet at December 31, 2016. The Private Placement raised net cash proceeds, after debt conversion and expenses, of $943,207, of which $842,207 was received during the period.
The Securities comprised one Share at a purchase price $0.21 per share and a Warrant to purchase one Share at an exercise price of $0.27, exercisable over a period of three (3) years. A total of 6,070,078 Shares and Warrants to purchase 6,070,078 Shares were issued in the Private Placement.
Warrants and Options
In August 2014, Fountainhead, Peter Zachariou (“the Related Party Noteholders”) and Craig Kirsch (collectively, the “Noteholders”) agreed, pursuant to a Securities Exchange Agreement (“Exchange Agreement”), to exchange their outstanding debt from the Company into sharesrespect of Company Series D Convertible Preferred Stock. Pursuant to the Exchange Agreement, on August 5, 2017 (the 3rd anniversaryshares (“Preferred D Stock”). The Preferred D Stock carry a cumulative preferred dividend of the exchange) the Noteholders were issued warrants exercisable12% per annum. The Preferred D Stock is convertible into 628,619 shares ofCompany Common Stock,Shares at a price of $0.30 per share, of which$2.15 and the Related Party Noteholders were issued warrants exercisable into 599,651 shares of Common Stock. The warrants expire on August 4, 2020 and were valuedCompany is able to redeem the Preferred D Stock at $120,788 (of which $115,222 related to the Related Party Noteholders) and included in Other Income/Expense, Warrant Issuance Expense for the three months ending September 30, 2017.par at any time, at its sole option.
Stock Options
SCHEDULE OF STOCK OPTIONS
| Weighted average | ||||||||
| exercise price | ||||||||
| Number of shares | per share | |||||||
| Outstanding at December 31, 2020 | 680,000 | $ | 0.28 | |||||
| Granted | - | - | ||||||
| Exercised | - | - | ||||||
| Cancelled or expired | (680,000 | ) | 0.28 | |||||
| Outstanding at December 31, 2021 | - | $ | - | |||||
| Granted | - | - | ||||||
| Exercised | - | - | ||||||
| Cancelled or expired | - | - | ||||||
| Outstanding at December 31, 2022 | - | $ | - | |||||
STOCK WARRANTS:
| Weighted average | ||||||||
| exercise price | ||||||||
| Number of shares | per share | |||||||
| Outstanding at December 31, 2015 | 6,007,048 | $ | 2.57 | |||||
| Granted | - | - | ||||||
| Exercised | - | - | ||||||
| Cancelled or expired | - | - | ||||||
| Outstanding at December 31, 2016 | 6,007,048 | $ | 2.57 | |||||
| Granted | 6,901,388 | $ | 0.27 | |||||
| Exercised | (72,002 | ) | $ | 0.27 | ||||
| Cancelled or expired | (5,907,048 | ) | $ | 1.88 | ||||
| Outstanding at December 31, 2017 | 6,929,386 | $ | 0.31 | |||||
| 28 |
STOCK OPTIONS:
| Weighted average | ||||||||
| exercise price | ||||||||
| Number of shares | per share | |||||||
| Outstanding at December 31, 2015 | 25,557 | $ | 20.25 | |||||
| Granted (see Note 9) | 680,000 | $ | 0.79 | |||||
| Exercised | - | - | ||||||
| Cancelled or expired | - | - | ||||||
| Outstanding at December 31, 2016 | 705,557 | $ | 0.97 | |||||
| Granted (see Note 9) | 20,000 | 0.27 | ||||||
| Exercised | - | - | ||||||
| Cancelled or expired | - | - | ||||||
| Outstanding at December 31, 2017 | 725,557 | $ | 0.95 | |||||
As of December 31, 2017, the weighted-average remaining contractual life of outstanding warrants and options is 2.11 and 1.21 years, respectively. Options to purchase 5,557 shares of Company Common Stock expired on February 12, 2018.
9. SHARE-BASED COMPENSATION
The Company from time to timetime-to-time issues common stock, stock options or common stock warrants to acquire services or goods from non-employees. Common stock, stock options and common stock warrants issued to other than employees or directors are recorded on the basis of their fair value, which is measured as of the “measurement date” using an option pricing model.model, or their contractual value if different in the case of common stock. The “measurement date” for options and warrants related to contracts that have substantial disincentives to non-performance is the date of the contract, and for all other contracts is the vesting date. Expense related to the options and warrants is recognized on a straight-line basis over the shorter of the period over which services are to be received or the life of the option or warrant.
Non-Employee Stock Option PlanCompensation
Under ASC Topic 718, the Company estimates the fair valueAggregate stock-based compensation for shares of option awards on the date of grant using an option pricing model. The grant date fair value is recognized over the option vesting period, the period during which an employee is requiredcommon stock granted to provide service in exchangenon-employees for the award. No compensation cost is recognized for equity instruments for which employees do not render the requisite service. Under these standards, compensation cost for employee cost for employee stock-based awards is based on the estimated grant-date fair value and recognized over the vesting period of the applicable award on a straight-line basis.
For the years ended December 31, 20172022 and 2016,2021 was $ and $. $171,428 was in respect of the Company recognized share-based compensation of $1,609Fountainhead Consulting Agreement (see Notes 14 and $198,200, respectively for the issuance of stock options to management and employees.
Stock appreciation rights may be granted either on a stand-alone basis or in conjunction with all or part of any other stock options granted under the plan.15). As of December 31, 2017 there were no awards of any stock appreciation rights.
Non-Employee Stock Compensation
Aggregate stock-based compensation expense charged to operations for stock and warrants granted to non-employees for the twelve months ended December 31, 2017 and 2016 was $558,363 and $531,409, respectively, of which $249,363 and $205,909, respectively, related to stock issued during the periods. The expense related to stock not issued during the periods comprise: $84,000 and $78,000, respectively, related to stock granted but not issued to directors under the Directors Deferred Compensation Plan; and $225,000 and $247,500, respectively of fees payable in stock to Fountainhead that were accrued but not issued during the period. As of December 31, 2017,2022, there was $0$ of total unrecognized compensation costs related to warrant and stock awards and non-vested options.
During the twelve months ended December 31, 2017 options with a value of $86,754 were granted to Fountainhead with performance vesting conditions (see Note 12); the value of these options will not be recognized as share-based compensation unless or until the Company concludes that it is probable the performance conditions will be achieved.
Stock-based Compensation Valuation Methodology
Stock-based compensation resulting from the issuance of Common Stock is calculated by reference to the valuation of the Stock on the date of issuance, the expense being recognized as the compensation is earned. Stock-based compensation expenses related to employee options and warrants granted to non-employees are recognized as the stock options and warrants are earned. The fair value of the stock options or warrants granted is estimated at the grant date, using the Black-Scholes option pricing model, and the expense is recognized on a straight-line basis over the shorter of the period over which services are to be received or the life of the option or warrant. The grant date fair value of employee share options and similar instruments is estimated using the Black-Scholes option pricing model on the basis of the fair value of the underlying common stock on the measurement date, adjusted for the unique characteristics of those equity instruments, using the assumptions noted in the table below. The fair value of the common stock is determined by the then-prevailing private placement purchase price. Expected volatility was based on the historical volatility of a peer group of publicly traded companies. The expected term of options and warrants was based upon the life of the option, and the risk-free rate used was based on the U.S. Treasury Constant Maturity rate.
The stock compensation expensed during the year ended December 31, 2017 resulted from the issuance of Common Stock valued on the date of issuance and options. The following assumptions were used in calculations of the Black-Scholes option pricing model for option-based stock compensation in year ended December 31, 2016:
| Year ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Risk-free interest rates | 1.50 | % | 0.91 | % | ||||
| Expected life | 1.5 – 3.0 years | 1.5 years | ||||||
| Expected dividends | 0 | % | 0 | % | ||||
| Expected volatility | 104 | % | 95 | % | ||||
| Vycor Common Stock fair value | $ | 0.20- .30 | $ | 0.71 | ||||
10. 12. INCOME TAXES
Loss Before Taxes
SUMMARY OF LOSS BEFORE TAXES
| December 31, 2017 | December 31, 2016 | December 31, 2022 | December 31, 2021 | |||||||||||||
| Domestic | $ | 1,304,136 | $ | 1,351,693 | $ | 382,553 | $ | 398,536 | ||||||||
| Foreign | 172,909 | 300,587 | 22,364 | 37,126 | ||||||||||||
| $ | 1,477,045 | $ | 1,652,280 | |||||||||||||
| Loss Before Taxes | $ | 404,917 | $ | 435,662 | ||||||||||||
The reconciliation of income tax expense (credit) at the U.S. statutory rate of 35%21%, to the Company’s effective tax rate is as follows:
SCHEDULE OF RECONCILIATION OF INCOME TAX EXPENSE STATUTORY RATE
| 2022 | 2021 | |||||||||||||||
| Year Ended December 31, | Year Ended December 31, | |||||||||||||||
| 2017 | 2016 | 2022 | 2021 | |||||||||||||
| US statutory rate | $ | (516,966 | ) | $ | (578,298 | ) | $ | (85,033 | ) | $ | (91,489 | ) | ||||
| Tax difference between foreign and U.S. | 10,248 | 25,275 | (204 | ) | (2,684 | ) | ||||||||||
| Change in Valuation Allowance | (506,718 | ) | (553,023 | ) | (85,237 | ) | (94,173 | ) | ||||||||
| Tax Provision | $ | - | $ | - | $ | - | $ | - | ||||||||
Deferred Income Taxes
The Company has operations in the US, Germany and the UK and incurred net operating losses since inception. The Company has not reflected any tax benefit related to such net operating losses in the financial statements. Prior to August 15, 2007 the CompanyCompany’s US operating entity was a limited liability company and losses were passed through to the individual members, therefore the Company only has potential tax benefits from the date it became a ‘C’ corporation.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company and its subsidiaries’ deferred tax assets at December 31, 20172022 and December 31, 20162021 are as follows:
SCHEDULE OF DEFERRED TAX ASSETS
December 31, 2022 | December 31, 2021 | |||||||
| Operating loss carry-forward – US | $ | 19,881,000 | $ | 19,058,000 | ||||
| Operating loss carry-forward – Foreign | $ | 1,656,000 | $ | 1,710,000 | ||||
| Operating loss carry-forward | ||||||||
| Deferred tax asset before Valuation allowance – US | 4,175,000 | 4,002,000 | ||||||
| Deferred tax asset before Valuation allowance – Foreign | 497,000 | 509,000 | ||||||
| Deferred tax asset before Valuation allowance | ||||||||
| Valuation allowance | (4,672,000 | ) | (4,511,000 | ) | ||||
| Net deferred tax asset | $ | — | $ | — | ||||
| 29 |
| December 31, 2017 | December 31, 2016 | |||||||
| Operating loss carry-forward | $ | 6,195,000 | $ | 6,050,000 | ||||
| Deferred tax asset before Valuation allowance | 6,195,000 | 6,050,000 | ||||||
| Valuation allowance | (6,195,000 | ) | (6,050,000 | ) | ||||
| Net deferred tax asset | $ | — | $ | — | ||||
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income.
The authoritative guidance requires a valuation allowance to reduce the deferred tax assets reportedrecorded if, based on the weight of the evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Based on the level of historical taxable losses and projections of future taxable income (losses) over the periodsFurthermore, in circumstances in which management has determined that existing conditions or events raise substantial doubt about its ability to continue as a going concern the authoritative guidance requires that a valuation allowance should be recorded for all deferred tax assets can be realized, management currently believes that it is more likely than not that the Company will not realize the benefits of these deductible differences.assets. Accordingly, management has determined that a 100%100% valuation allowance is appropriate at December 31, 20172022 and December 31, 2016.2021.
The U.S. Tax Cuts and Jobs Act (Tax Act) was enacted on December 22, 2017 and introduces significant changes to U.S. income tax law. Effective in 2018, the Tax Act reduces the U.S. statutory tax rate from 35% to 21% and creates new taxes on certain foreign-sourced earnings and certain related-party payments, which are referred to as the global intangible low-taxed income tax and the base erosion tax, respectively. The Tax Act requires us to pay U.S. income taxes on accumulated foreign subsidiary earnings not previously subject to U.S. income tax at a rate of 15.5%15.5% to the extent of foreign cash and certain other net current assets and 8%8% on the remaining earnings. Due toSince the timing of the enactmentCompany’s foreign subsidiaries have not generated taxable income and the complexity involved in applying the provisions of the Tax Act,have no accumulated earnings, the Company has not recorded any adjustments according to Tax Act. As we collect and prepare necessary data, and interpret the Tax Act and any additional guidance issued by the U.S. Treasury Department, the IRS, and other standard-setting bodies, we may make adjustments to the provisional amounts. Those adjustments may materially impact our provision for income taxes and effective tax rate in the period in which the adjustments are made. The accounting for the tax effects ofbelieves that the Tax Act will be completed in 2018.not have a significant impact on the Company’s consolidated financial statements.
Net Operating Loss Carry-Forwards
As of December 31, 20172022 and 2016,2021, the Company had U.S. accumulated losses for tax purposes of approximately $17,700,000$19,881,000 and $17,300,000$19,058,000 respectively, which may be carried forward and offset against U.S. taxable income, and which expire during the tax years 20272029 through 2032.2041.
Federal tax laws impose significant restrictions on the utilization of net operating loss carry-forwards and in the event of a change in ownership of the Company, as defined by the Internal Revenue Code Section 382. The Company’s net operating loss carry-forwards may be subject to the above limitations.
As of December 31, 20172022 and 2016,2021, the Company had German accumulated losses for tax purposes of approximately $1,633,000$1,450,000 and $1,012,000$1,498,000 respectively, which may be carried forward and offset against German taxable income subject to certain restrictions and limitations. Such carry-forwards are subject to certain restrictions and limitations in the event of changes in the NovaVision GmbH’s ownership. As disclosed in Note 3, the Company is in the process of winding down the entity, following which these tax losses will no longer be available.
As of December 31, 20172022 and 2016,2021, the Company had UK accumulated losses for tax purposes of approximately $249,000$206,000 and $198,000$212,000 respectively, which may be carried forward and offset against UK taxable income subject to certain restrictions and limitations.
Tax Rates
The applicable US income tax rate for the Company for both of the years ended December 31, 20172022 and 20162021 was 35%21%. Non-US subsidiaries are taxed according to the tax laws in their respective country of residence. The German applicable rate for both of the years ended December 31, 20172022 and 20162021 was 31.58%31.58%; the UK applicable rate for both the years ended December 31, 20172022 and 20162021 was 20%19%.
US income taxes and foreign withholding taxes were not provided for on undistributed earnings of the Company’s foreign subsidiaries. The Company intends to reinvest these earnings indefinitely in its foreign subsidiaries. If theseany earnings were distributed to US in the form of dividends or otherwise, after the repayment of intercompany debt, the Company would be subject to additional US income taxes (subject to an adjustment for foreign tax credits) and foreign withholding taxes.
| 30 |
Uncertain Tax Position
The Company has recorded no liability for income taxes associated with unrecognized tax benefits at the date of adoption and has not recorded any liability associated with unrecognized tax benefits during 20172022 and 2016.2021. Accordingly, the Company has notnot recorded any interest or penalty in regard to any unrecognized benefit.
11. 13. COMMITMENTS AND CONTINGENCIES
Lease
The Company leased office space located at 6401 Congress Ave., Suite 140, Boca Raton, FL 33487 from Catexor Limited Partnership for a gross rent of approximately $14,260 plus sales tax per month. The term of the lease was 5 years and 6 months and terminated July 30, 2017. Effective August 1, 2017 the Company leased office space located at 951 Broken Sound Parkway, Suite 320, Boca Raton, FL 33487 from WPT Land 2L.P., for a gross rent of approximately $5,700$4,000 per month, plus sales taxother charges of approximately $2,500 per month. The lease terminateswas renewed in September 30, 2020. The Company’s subsidiary in Germany occupies premises on a short-term lease agreement.2020 for an additional 3 years. Rent expense for the year ended December 31, 20172022 and 20162021 was $158,278$79,314 and $212,054$79,663 respectively.
The table below provides the future lease payments each year until the lease expiration date:
SCHEDULE OF FUTURE LEASE PAYMENTS
Future Lease Payments
| Year | Liability | |||
| 2023 | $ | 34,384 | ||
Potential German tax liability
In June 2012 the Company’s NovaVision German subsidiary received a preliminary assessment for Magdeburg City trade tax of approximately €75,000€75,000 (approximately $94,000)$82,000), with an additional interest charge of €12,000 (approximately $13,200). This assessment is for the 2010 fiscal year and relates to the Company’s acquisition of the assets of the former NovaVision, Inc. An initial assessment for corporate tax for the same period has beenwas preliminarily reduced to zero. The Company hasdid not acceptedaccept this trade tax assessment and is in discussion withappealed against it to the relevant tax authorities with a view to its reduction. The relevant tax authorities agreed to suspend the assessment pending the outcome of certain court hearings and proposed legal amendments,tax legislation, and the Company agreed to make limited monthly payments on account totaling €75,000 (approximately $82,000) which were completed in October 2016.2016 and fully expensed. At that time the Company appealed against the interest charge of €12,000 (approximately $13,200) which the tax authorities did not accept but also agreed to suspend pending the outcome of the hearings and proposed legislation outlined above. Accordingly, the Company has made no provision for this liability for yearsin the year ended December 31, 20172022 and 2016 respectively, other than recording2021 respectively. The Company is in the monthly paymentsprocess of winding down the entity, as an expense.disclosed in Note 3.
12. 14. CONSULTING AND OTHER AGREEMENTS
The following agreements were entered into or remained in force during the yearyears ended December 31, 2017:2022 and 2021:
Consulting Agreement with Fountainhead
During the year ended December 31, 2017, following the achievement of certain milestones established in the March 2016 Compensation Plan, the Company accrued deferred compensation of $82,500. This together with the balance of the deferred compensation accrued during the year ended December 31, 2016, was paid to Fountainhead by the issuance of 1,571,429 shares of Common Stock (valued at $330,000) during the year ended December 31, 2017.
In March 2017 and effective April 1, 2017, as part of a streamlining of compensation arrangements with executive management, the Company established the March 2017 Compensation Plan. Under this Plan, the Company amended the Fountainhead Consulting Agreement. Under the Amended Agreement, (“the Amendment”) to increase the annual fees of $450,000 are payable to Fountainhead, by $330,000 to a total of $37,500 per month. Concurrently, annual compensation payable to executive management under the March 2016 Compensation Plan was reduced by $330,000 to $0. These changes had no financial impact on the Company. The other terms of the Consulting Agreement remained the same, including the ability of Fountainhead at itswith an option to receive $5,000$5,000 per month in cash and the remainder payable in Company Common Stock issued at the higher of the Private Placement price ($0.21)$ and the average price for the 30 days prior to issuance, and deliverable at the end of each fiscal quarter. TheThis was amended slightly effective January 1, 2021 (“the Amended Agreement”). Under the Amended Agreement, fees are payable to Fountainhead in Company Common Stock (“Shares”) as follows: 1) Shares on the last day of each quarter; or 2) if the average closing price of the Shares for the 30 trading days prior to issuance is above $, a number of Shares calculated by dividing $112,500 by the average closing price of the Shares for the 30 trading days prior to issuance. Under the terms of the Amended Agreement, Fountainhead continued to provide the executive management team of the Company, including the positions of CEO, President and CFO, whose employment agreements with the Company stipulate they receive no remuneration from the Company.
Effective October 1, 2022 the Amended Agreement was terminated by Fountainhead and the Company by mutual agreement. Effective the same date the Company entered into revised employment agreements with Peter Zachariou, David Cantor and Adrian Liddell under which they would continue as CEO, President and CFO respectively as individuals and not as representatives of Fountainhead; there is no compensation payable under the employment agreements.
During each of the years ended December 31, 2022 and 2021 the Company issued and shares of Common Stock to Fountainhead for fees of $171,428 and $305,001, respectively.
| 31 |
Other Agreements
On March 30, 2021, Vycor entered into a Consulting Agreement also contains provisions for Fountainheadwith Ricardo J. Komotar, M.D. (the “Agreement”) to receive a higher proportionprovide certain specified services over the three-year term of its fees in cash subject to certain future liquidity events and Board approval.the Agreement. Under the Amendment, Fountainhead was granted options pursuantAgreement, Dr. Komotar will provide general scientific advisory consultancy services, and will also provide scientific advisory services based around certain specific pre-determined milestones. In consideration of the Consultant’s services, the Company agreed to deliver to the Vycor Medical, Inc. 2008 Stock Option Plan, to purchase 660,000Consultant over the course of the three-year term, a total of shares of Company Common Stock at the same $0.27 exercise price as thatin respect of the warrants issued in the Private Placement. Vesting of these options is subjectgeneral consultancy, and up to the achievement of certain milestones by March 31, 2018. In March 2018 the Company extended the time period for the achievement of these milestones to June 30, 2018. These options are consistent with the options granted to executive management under the March 2016 Compensation Plan.
During the year ended December 31, 2017, under the terms of this amended Consulting Agreement, Fountainhead received total fees of $367,500, of which $135,300 was paid through the issuance of 644,286 shares of Company Common Stock duringin respect of the period, $7,200 was paid in cashmilestones, the actual number of shares to be delivered being determined by the achievement of the pre-determined milestones. On April 1, 2022 and $225,000 was accrued and recorded as payable, of which $92,500 was paid after the year end through the issuance of 250,000 shares of Company Common Stock.
In March 2018 Fountainhead was granted options pursuant to the Vycor Medical, Inc. 2018 Stock Option Plan, to purchase 660,0002021 shares of Company Common Stock (valued at an exercise price$12,200 and $20,129, respectively) were issued under the terms of $0.46 (the average closing price for the 5 trading days before the grant). Vesting of these optionsAgreement, which is subject to the achievement of certain milestones by March 31, 2019.being amortized over twelve months.
13. 15. RELATED PARTY TRANSACTIONS AND BALANCES
Peter Zachariou and David Cantor, directors of the Company, are investment managers of Fountainhead which owned, at December 31, 2017, 46%2022, 62.5% of the Company’s Common Stock and 95%69.7% of the Company’s Series D Preferred D Stock. Adrian Liddell, Chairman, is a consultant forto Fountainhead. Peter Zachariou owns 0.15% of the Company’s Common Stock and 25.7% of the Company’s Series D Preferred Stock.
As referred to in Note 4, on January 24, 2018During each of the Company entered into the Amendment with EuroAmerican. regarding its $300,000 Note. Under the Amendment, EuroAmerican granted a right of first refusal prior to converting or selling or the Note a) first to Vycor to redeem the Note and accrued interest at face value and b) if not exercised second to Fountainhead to purchase the Note and accrued interest at face value on the same terms.
During the yearyears ended December 31, 2017, following the achievement of certain milestones established in the March 2016 Compensation Plan, the Company accrued deferred compensation of $82,500. This together with the balance of the deferred compensation accrued during the year ended December 31, 2016, was paid to Fountainhead through the issuance 1,571,429 shares of Common Stock (valued at $330,000) during the period ended September 30, 2017.
During the year ended December 31, 2017,2022 and 2021, under the terms of this amended Consultingthe Consultancy Agreement referred to in Note 12, Fountainhead received total fees of $367,500, of which $135,300 was paid through the issuance of 644,286 shares of Company Common Stock, $7,200 was paid in cash and $225,000 was accrued but not yet paid.
On January 11, and February 23, 201714, the Company completed the sale of $1,274,717 inissued and shares of Common Stock to Fountainhead for fees of $171,428and Warrants to accredited investors (the “Private Placement”). Fountainhead purchased a total of $477,939 of shares in the Private Placement of which approximately $248,000 represented amounts that Fountainhead had already advanced to the Company and was held by the Company in the form of notes. As a result, Fountainhead was issued 2,275,901 shares of Common Stock and three-year Warrants to purchase 2,275,901 shares of Common Stock at an exercise price of $0.27.$305,001, respectively.
During the yearyears ended December 31, 2017 $225,000 of fees payable in stock were accrued; however these shares were not issued.
Pursuant to the Exchange Agreement, on August 5, 2017 (the 3rd anniversary of the exchange) the Related Party Noteholders were issued warrants exercisable into 599,651 shares of Common Stock, at a price of $0.30 per share. The warrants expire on August 2, 20202022 and were valued at $115,222 (see Note 8).
During the year ended December 31, 2016, in accordance with the terms of the Consulting Agreement, the Company issued 212,478 shares of Common Stock (valued at $120,000) to Fountainhead.
During the year ended December 31, 2016, the Company paid an aggregate of 17,971 shares of Preferred D Stock, valued at $179,727, representing preferred stock dividends. The Preferred D shares are convertible on their terms at $2.15 per share of Common Stock into 83,586 shares. An aggregate of 17,142 shares of Preferred D Stock dividends were in respect of related parties.
During the year ended December 31, 2016, 2016,2021, respectively, the Company issued unsecured loan notes to Fountainhead for a total of $248,000.$172,500 and $10,000, respectively. The loan notes borebear interest at a rate of 10%10% and were are due on demand or by their one-year anniversary. The notes were converted into Common Stock in the Offering during 2017.anniversary (see Note 6).
At December 31, 2016 the Company was holding $101,000 cash in escrow from Fountainhead in respect of the Private Placement Initial Closing that occurred on January 11, 2017.
There were no other related party transactions duringDuring the years ended December 31, 20172022 and 2016.2021, respectively, the Company accrued interest on related party loans of $39,563 and $31,841, respectively.
14. CONCENTRATIONDuring each of the years ended December 31, 2022 and 2021, the Company accrued an aggregate of $324,370 of Preferred D Stock dividends, of which $226,037 was regarding Fountainhead and $83,386 was regarding Peter Zachariou. Total accrued Preferred D Stock dividends at December 31, 2022 and 2021 was $1,946,220 and $1,621,850, respectively, of which $1,356,224 and $1,130,186, respectively, was regarding Fountainhead and $500,315 and $416,930, respectively, was regarding Peter Zachariou.
16. CONCENTRATION
Vycor Medical sells its neurosurgical devices in the US primarily direct to hospitals, and internationally through distributors who in turn sell to hospitals. The sales to one international distributor represented 16% and 14%, respectively,
SCHEDULE OF CONCENTRATION
| Sales Concentration | Year ended December 31, | |||||||
| 2022 | 2021 | |||||||
| Number of customers over 10% | 0 | 0 | ||||||
| Percentage of sales | 0 | % | 0 | % | ||||
| 32 |
Accounts Receivable Concentration
| At December 31, | ||||||||
| 2022 | 2021 | |||||||
| Number of customers over 10% | 1 | 1 | ||||||
| Percentage of accounts receivable | 13 | % | 11 | % | ||||
We have two sub-contract manufacturers who each represent over 10% of total sales for the years ended December 31, 2017 and 2016.purchases on an annual basis.
15. 17. SUBSEQUENT EVENTS
On February 09, 2018, the Board of Directors of the Company unanimously adopted a resolution seeking stockholder approval to (a) amend the Company’s Certificate of Incorporation to increase the number of authorized Company Common Shares from 25,000,000 to 55,000,000 and (b) to adopt the Company’s 2018 Stock Incentive Plan. Thereafter, on February 09, 2018, pursuant to the By-Laws of the Company and applicable Delaware law, stockholders holding in excess of fifty percent (50%) of the votes entitled to be cast on the aforementioned two matters (identified in the section entitled “Voting Securities and Principal Holders Thereof”) adopted a resolution to authorize the Board of Directors, in its sole discretion, to increase the number of authorized shares of Company Common Stock from 25,000,000 to 55,000,000 and adopt the Company’s 2018 Stock Incentive Plan . As of the date of this Report, the increase in authorized capital the Company’s 2018 Stock Incentive Plan have yet to become effective and the Company expects that they will become effective during the Company’s second fiscal quarter of 2018.
During March 2018April 1, 2023 the Company issued unsecured loan notes101,663 shares of Common Stock to Fountainhead forRicardo Komotar (RJK Consulting), a totalconsultant, in accordance with the terms of $30,000. The loan notes bear interest at a rate of 10% and are due on demand or by their one-year anniversary.
consulting agreement (see Note 14).
Other than the foregoing, theThe Company has evaluated the existence of events and transactions subsequent to the balance sheet date through the date the consolidated financial statements were issued and has determined that, other than that disclosed above, there were no significant subsequent events or transactions whichthat would require recognition or disclosure in the financial statements.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
a)Disclosure Controls and Procedures
We are required to maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports filed under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms, and that such information is accumulated and communicated to our management, including our chief executive officer (also our principal executive officer) and our chief financial officer (also our principal financial and accounting officer) to allow for timely decisions regarding required disclosure.
Pursuant to Rule 13a-15(b) under the Securities Exchange Act of 1934 (“Exchange Act”), the Company’s management, including the Company’s Chief Executive Officer (“CEO”) (the Company’s principal executive officer) and Chief Financial Officer (“CFO”) (the Company’s principal financial and accounting officer), has evaluated the effectiveness of the Company’s disclosure controls and procedures (as defined under Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report. Based uponon such evaluation, our CEO and our CFO have concluded that evaluationa material weakness occurred as of April 1, 2021 with the resignation of the independent members of the Company’s CEO and CFO concluded that the Company’s disclosure controls and procedures were effectiveAudit Committee as of December 31, 2017that date. Effective that date, our disclosure and controls were no longer effective to ensure that information required to be disclosed by the Company in the reports that the Companyit files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to the Company’s management, including the Company’sits CEO and its CFO, as appropriate, to allow timely decisions regarding required disclosure.
The matter involving internal controls and procedures that our management considered to be a material weakness under the standards of the Public Company Accounting Oversight Board was a lack of a functioning audit committee with independent members, resulting in ineffective oversight in the establishment and monitoring of required internal controls and procedures. This weakness occurred as of April 1, 2021 due to the resignation of the independent members of the Audit Committee from the Board of Directors effective as of April 1, 2021.
Management believes that the material weakness set forth did not have an effect on our financial results. However, management believes that the lack of a functioning audit committee and the lack of a majority of outside directors on our board of directors, results in ineffective oversight in the establishment and monitoring of required internal controls and procedures, which could result in a material misstatement in our financial statements in future periods.
| 33 |
b)Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of, the company’s principal executive and principal financial officers and effected by the company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America and includes those policies and procedures that:
| ● | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the company; |
| ● | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and |
| ● | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Because of the inherent limitations of internal control, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
We carried out an assessment, under the supervision and with the participation of our management, including our CEO and CFO, of the effectiveness of the design and operation of our internal controls over financial reporting, as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as of December 31, 2017.2022. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission inInternal Control — Integrated Framework (2013). Based on that assessment and on those criteria, our CEO and CFO concluded that our internal control over financial reporting was not effective as of December 31, 2017.2022 due to the a lack of a functioning audit committee with independent members, resulting in ineffective oversight in the establishment and monitoring of required internal controls and procedures.
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to temporary rules of the SEC that permit us to provide only the management’s report in this annual report.
c)Changes in Internal Controls
Effective April 1, 2021, the independent members of the Audit Committee resigned from the Board of Directors. There have not been anyno other changes in the Company’s internal control over financial reporting (as such term is defined in Rules 13a-15(f) under the Exchange Act) during the fiscal period to which this report relates that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
The Company’s management, including the Company’s CEO and CFO, doesdo not expect that the Company’s internal control over financial reporting will prevent all errors and all fraud. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree or compliance with the policies or procedures may deteriorate.
ITEM 9B. OTHER INFORMATION.
None
| 34 |
On February 09, 2018, the Board of Directors of the Company unanimously adopted a resolution seeking stockholder approval to (a) amend the Company’s Certificate of Incorporation to increase the number of authorized Company Common Shares from 25,000,000 to 55,000,000 and (b) to adopt the Company’s 2018 Stock Incentive Plan. Thereafter, on February 09, 2018, pursuant to the By-Laws of the Company and applicable Delaware law, stockholders holding in excess of fifty percent (50%) of the votes entitled to be cast on the aforementioned two matters (identified in the section entitled “Voting Securities and Principal Holders Thereof”) adopted a resolution to authorize the Board of Directors, in its sole discretion, to increase the number of authorized shares of Company Common Stock from 25,000,000 to 55,000,000 and adopt the Company’s 2018 Stock Incentive Plan . As of the date of this Report, the increase in authorized capital the Company’s 2018 Stock Incentive Plan have yet to become effective and the Company expects that they will become effective during the Company’s second fiscal quarter of 2018.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Our Directors and Executive Officers
Set forth below is certain biographical information concerning our current executive officers and directors. We currently have two executive officers as described below.
| Directors and Executive Officers | Position/Title | Age | ||
| Peter C. Zachariou | Chief Executive Officer and a Director | |||
| David Marc Cantor | President and a Director | |||
| Adrian Christopher Liddell | Chairman of the Board, Chief Financial Officer and a Director | |||
Peter C. Zachariou was appointed a Director of the Company in May 2010, Executive Vice President in September 2010 and Chief Executive Officer on January 2, 2014. As CEO, he has responsibility for the sales, marketing and customer functions of both the Vycor and NovaVision divisions. For the past 30 years, Mr. Zachariou has been an active investor in and proprietor of a variety of companies and industries, predominantly in U.S. emerging and growth companies across a broad range of industry sectors. He is an investment manager for Fountainhead Capital Management Limited, an investment company based in Jersey, Channel Islands, which invests in, raises capital for and provides strategic advice to growth companies in healthcare and other sectors. For the past 20 years, Mr. Zachariou has been an active investor in a variety of companies and industries, both public and private, specializing in workouts and capital formation. Mr. Zachariou’s investments and activities have predominantly been in U.S. emerging and growth companies across a broad range of industry sectors. He has also been proprietor and operator of several businesses in the U.K. and U.S. in the manufacturing, retail and leisure industries.Islands.
David Marc Cantor has been President of the Company since September 2010 and a Director since January 2010. He is an investment managerAs President he has responsibility for the clinical, program and product development and manufacturing functions of Fountainhead Capital Management Limited, an investment company based in Jersey, Channel Islands, which invests in, raises capital forboth the Vycor and provides strategic advice to growth companies across a broad range of sectors.NovaVision divisions, as well as their patent and trademark portfolios. Mr. Cantor has over 22 years of experience in Investment Banking with a focus on Mergers and Acquisitions and Equity Capital Raisings. Prior to Fountainhead fromFrom 2001 – 2005 he was at Citigroup Capital Markets where he was Co-head of its M&A European Business Development Group and subsequently European Head of its Diversified Industrials and Aerospace activities. Prior to Citigroup he was a Managing Director in M&A at Donaldson Lufkin & Jenrette and worked at Lehman Brothers both in New York and London in both the Equity Capital and M&A groups. He is an investment manager of Fountainhead Capital Management Limited, an investment company based in Jersey, Channel Islands. Mr. Cantor has a BSc with Honours from City Business School, London.London
Adrian Christopher Liddell has been Chairman of the Board and a Director of the Company since January 2010, and serves as the Company’s CFO. HeAs CFO he is an advisor to Fountainhead Capital Management Limited, an investmentresponsible for the financial operation of the company, based in Jersey, Channel Islands, which invests in, raises capital foras well as regulatory and provides strategic advice to growth companies in healthcare and other sectors.legal matters. Mr. Liddell has over 3035 years of strategic, corporate and financial advisory and company investment experience. From 2003-2006,2003 to 2006, Mr. Liddell was an investment advisor at Phoenix Equity Partners, a European private equity fund. From 1998 to 2003 Mr. Liddell served as Managing Director, Mergers & Acquisitions, at Donaldson Lufkin & Jenrette and then Citigroup in London. From 1984 to 1998 Mr. Liddell held various positions in corporate finance and mergers & acquisitions at Lehman Brothers and Samuel Montagu & Co, and Lehman Brothers in London. He is also an advisor to Fountainhead Capital Management Limited, an investment company based in Jersey, Channel Islands, Mr. Liddell qualified as a Chartered Accountant in 1984 with Arthur Young (now Ernst & Young) and holds an MA, Hons. from Christ’s College, University of Cambridge.
Steven Girgenti has been a Vycor Medical director since November 2008 and is Chairman of the Nominating and Governance Committee and of the Compensation Committee. He is presently the Managing Partner of Medi-Pharm Consulting, LLC providing strategic services to a number of medical device, pharmaceutical and diagnostic businesses. Steve was formerly CEO and co-founder of DermWorx Incorporated a specialty pharmaceutical company dedicated to solutions for dermatological conditions. Steve was also the Worldwide Chairman and CEO of Ogilvy Healthworld, a leading global healthcare communications network with 55 offices in 36 countries. The network has more than 1,000 brand assignments from nearly 200 clients worldwide, providing strategic marketing and communications services to many of the world’s leading healthcare companies. Mr. Girgenti founded Healthworld in 1986 and, under his leadership, the company made numerous acquisitions to expand and diversify the business. Healthworld went public in 1997. In 1998, and again in 1999, Business Week named Healthworld one of the “Best Small Corporations in America”. In 1999, Forbes listed Healthworld as one of the “200 Best Small Companies”. Mr. Girgenti was recognized as “Entrepreneur of the Year” by NASDAQ in 1999, and was named Med Ad News’ first “Medical Advertising Man of the Year” in 2000. In 2010 he was inducted into the Medical Advertising Hall of Fame. In addition, Mr. Girgenti is presently Executive Chairman of BioAffinity Technologies, Inc. and serves as a Director of Hancock Jaffe Laboratories, Inc. (NASDAQ:HJLI). He is also Vice Chairman of the Board of Governors for the Mt. Sinai Hospital Prostate Disease and Research Center in New York City, and is on the Board of Directors for Jack Martin Fund, a Mt. Sinai Hospital affiliated charitable organization devoted to pediatric oncology research. He graduated from Columbia University and has worked in the pharmaceutical industry since 1968 for companies such as Bristol-Myers Squibb, Carter Wallace and DuPont, as well as advertising agencies that specialize in healthcare. During his career, Steve has held positions in marketing research, product management, new product planning and commercial development.
Oscar Bronsther, M.D., F.A.C.S has been a director since November 2011. Dr. Bronsther is Chief Executive Officer of ERAD Therapeutics, LLC, a pre-clinical Orphan Drug company focusing on treating Cystic Fibrosis and Gaucher disease since 2016. He was formerly Chief Executive Officer of MetaStat, Inc. (OTCBB: MTST), a development stage life sciences company that develops and commercializes diagnostic products for the early and reliable prediction and treatment of systemic metastasisis. Dr Bronsther is also currently Clinical Professor at George Washington University, Washington, DC and has served in that capacity since 2002. He had previously served as an Associate Professor at the University of Rochester, Rochester, NY (1994-2001), University of Pittsburgh, Pittsburgh, PA (1989-1994) and University of California San Diego (1984), and Chairman, Section of General Surgery at Inova Fairfax Hospital. Since 2002, he has served as a Board Member, National Board Member and Director of Transplant Services of Kaiser Permanente Medical Group. Dr. Bronsther is a graduate of the University of Rochester (B.A. 1973) and Downstate Medical Center, Brooklyn, N.Y. (M.D. 1978). He did post-graduate work at Downstate Medical Center (Research Assistant 1975; Kidney Transplant Fellowship 1983-1984), Mount Sinai Medical Center, New York, N.Y (Residency 1978-1983) and Children’s Hospital Medical Center, Boston, MA (Research Fellowship 1980-1981). He resides in Potomac, MD.
Lowell Rush was appointed a Director in April 2013 and is Chairman of the Audit Committee. Mr. Rush has extensive experience in financial management and operational development, and since April 2017 has been Chief Financial Officer of Coral Gables - based The Revenue Optimization Companies (T-ROC), a leading provider of retail services. Previously, he was Chief Financial Officer of Ft. Lauderdale-based Paybox Corp. (OTCQB:PBOX) (2013-2017), and held positions as: Chief Operating Officer of Miami-based Cosmetic Dermatology, Inc. , CFO of Bijoux Terner, LLC (2008-2010); CFO of Little Switzerland, Inc. (2006-2008); and VP Sales Operations of Rewards Network, Inc. He is a CPA with an MBA in International Business, who has also held financial management roles at multi-national companies Sunglass Hut International, Burger King Corporation and Knight-Ridder, Inc. He began his career with the accounting firms Ernst & Young and Deloitte & Touche.
All of our directors hold office until the next annual meeting of stockholders and until their respective successors have been elected or qualified. Officers serve at the discretion of the board of directors. There are no family relationships among our directors or executive officers. There is no arrangement or understanding between or among our officers and directors pursuant to which any director or officer was or is to be selected as a director or officer, and there is no arrangement, plan or understanding as to whether non-management stockholders will exercise their voting rights to continue to elect the current board of directors.
| 35 |
None of our directors and executive officers have during the past five years:
| ● | had any bankruptcy petition filed by or against any business of which he was a general partner or executive officer, either at the time of the bankruptcy or within two years prior to that time; |
| ● | been convicted in a criminal proceeding and is not subject to a pending criminal proceeding; |
| ● | been subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities, futures, commodities or banking activities; |
| ● | or been found by a court of competent jurisdiction (in a civil action), the Securities Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacated. |
Board Committees
Given that the Company has only three incumbent directors, none of whom are independent, the Board of Directors
Our Company has three committees does not maintain any Board Committees as of its Board of Directors—(a) a Nominating and Governance Committee (b) a Management Compensation Committee and (c) an Audit Committee. The Board of Directors has approved charters for each committee. Steven Girgenti is Chairman of the Nominating and Governance Committee and the Management Compensation Committee, and Lowell Rush is a member. Lowell Rush is Chairman ofthis date, with the Audit Committee and Steven Girgenti and Adrian Liddell are members. The Board has determined that Lowell Rush qualifies as an Audit Committee financial expert, as that term is defined in applicable regulations ofcomprising the SEC. The Committees are intended to operate consistent with applicable NASDAQ governance requirements.whole Board.
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves as a member of the Board of Directors or compensation committee of any other entity that has one or more of its executive officers serving as a member of our Board of Directors.
Section 16(a) Beneficial Ownership Reporting Compliance
Pursuant to Section 16(a) of the Exchange Act and the rules thereunder, the Company’s executive officers and directors and persons who own more than 10% of a registered class of the Company’s equity securities are required to file with the SEC reports of their ownership of, and transactions in, the Company’s common stock.
ITEM 11. EXECUTIVE COMPENSATION.
The following is a summary of the compensation we paid for each of the last two years ended December 31, 20172022 and 2016,2021, respectively (i) to the persons who acted as our principal executive officer during our fiscal year ended December 31, 20172022 and (ii) to the person who acted as our next most highly compensated executive officer other than our principal executive officer who was serving as an executive officer as of the end of our last fiscal year.
| Name and Principal Position | Year | Salary($) | Bonus($) | Stock Awards($) | Option Awards($) | Non- Equity Incentive Plan Compensation | Non-Qualified Deferred Compensation Earnings($) | All other Compensation($) | Total($) | |||||||||||||||||||||||||
| Peter Zachariou | 2016 | $ | — | — | — | $ | 64,435 | — | — | — | $ | 64,435 | ||||||||||||||||||||||
| CEO | 2017 | $ | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| David Cantor | 2016 | $ | — | — | — | $ | 64,435 | — | — | — | $ | 64,435 | ||||||||||||||||||||||
| President | 2017 | $ | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||
Name and Principal Position | Year | Salary($) | Bonus($) | Stock Awards($) | Option Awards($) | Non- Equity Incentive Plan Compensation | Non- Qualified Deferred Compensation Earnings ($) | All other Compensation($) | Total($) | |||||||||||||||||||||||||||
| Peter Zachariou | 2022 | $ | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||
| CEO | 2021 | $ | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||
| David Cantor | 2022 | $ | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||
| President | 2021 | $ | — | — | — | — | — | — | — | — |
OUTSTANDING EQUITY AWARDS
Grants of Plan-Based Awards
| Equity Compensation Plan Information | ||||||||||||
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (1) | Weighted- average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (1) | |||||||||
| Equity compensation plans approved by security holders | 3,380,000 | $ | - | 3,380,000 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 3,380,000 | $ | - | 3,380,000 | ||||||||
| (1) | As of December 31, 2022. |
| 36 |
OUTSTANDING EQUITY AWARDS
Grants of Plan-Based Awards
| Option Awards | ||||||||||||||||
| Name | Grant Date | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Number of Securities Underlying Unexercised Options (#) Unexercisable (1) | Option Exercise Price($) | Option Expiration Date | |||||||||||
| Adrian Liddell | 3/23/2016 | - | 220,000 | $ | 0.78 | 3/22/2019 | ||||||||||
| David Cantor | 3/23/2016 | - | 220,000 | $ | 0.78 | 3/22/2019 | ||||||||||
| Peter C. Zachariou | 3/23/2016 | - | 220,000 | $ | 0.78 | 3/22/2019 | ||||||||||
| Equity Compensation Plan Information | ||||||||||||
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights(1) | Weighted- average exercise price of outstanding options,warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (1) | |||||||||
| Equity compensation plans approved by security holders | 720,000 | $ | $0.81 | 1,978,532 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 720,000 | $ | $0.81 | 1,978,532 | ||||||||
(1) As of December 31, 2017. Excludes 5,557 Options which expired February 13, 2018
Warrants Issued to Management
| Name | Grant Date | Number of Securities Underlying Unexercised Exercisable Warrants | Number of Securities Underlying Unexercised Exercisable Warrants | Warrant Exercise Price($) | Warrant Expiration Date | |||||||||||||||
| None | ||||||||||||||||||||
| Total | ||||||||||||||||||||
Employment Agreements
Effective September 30, 2010, the Company entered into virtually identical employment agreements with David Cantor to serve as the Company’s Interim President and Peter C. Zachariou to serve as the Company’s Interim Chief Executive Officer. Each employment agreement continued until August 30, 2011 and was then automatically extended unless terminated by either party, and provided that the executives receive no compensation for services rendered under the agreements.
Effective January 2, 2014, the Company entered into:into amended employment agreements with Peter Zachariou and David Cantor to serve as the Company’s Chief Executive Officer and President respectively;respectively as appointees of Fountainhead; and an employment agreement with Adrian Liddell to serve as the Company’s Chief Financial Officer.Officer as appointee of Fountainhead; these agreements were further amended in March 2017. Each agreement continues for a period of twelve months and is then automatically extended unless terminated by either party. The agreements provided for no monthly compensation to be payable, but a deferred compensation payableAs appointees of $110,000 for each individual, subject to certain conditions and milestones being met. The compensation was payable in cash or Restricted Shares ofFountainhead under the Company’s Common Stock. In March 2017 the Company amended the employment agreement such thatFountainhead Consulting Agreement no compensation would beis payable under the employment agreements. The same level of compensation would instead be payable toEffective October 1, 2022, the Fountainhead under an amended consulting agreement. These changes had no financial impact onConsulting Agreement was terminated by Fountainhead and the Company but streamlinesby mutual agreement and the shareholding structure.
In March 2016 the Board of Directors approved the issuance of options to purchase 220,000 shares of Company Common Stock pursuant to the Vycor Medical, Inc. 2008 Stock Option Plan to each ofentered into revised employment agreements with Peter C. Zachariou, David Cantor and Adrian Liddell such optionsunder which they would continue as CEO, President and CFO respectively as individuals and not as representatives of Fountainhead; there continues to vest immediately upon issuance. The options are exercisable for a period of three years from issuance at an exercise price of $0.78.be no compensation payable under the employment agreements.
Compensation of Directors
Not applicable
| 37 |
During the period January 1, 2017 through December 31 2017, we granted Steven Girgenti, Oscar Bronsther, M.D. and Lowell Rush a total of 112,878, 110,585 and 110,585 shares of the Company’s Common Stock respectively for their service to the Board of Directors under the Company’s Deferred Compensation Plan. Under this Plan, the directors may defer their director’s compensation to the January 15th following the termination of their service as a director. All of the above-mentioned Stock was granted under the Plan. Each of Mr. Girgenti, Dr. Bronsther and Mr. Rush are entitled to receive $7,000 in cash or stock at the option of the company per quarter. No other directors of the Company receive compensation for their service to the Company other than as disclosed underEmployment Agreementsabove.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The following table sets forth certain information with respect to the beneficial ownership of our voting securities by (i) any person or group owning more than 5% of any class of voting securities, (ii) each director, (iii) our chief executive officer and president and (iv) all executive officers and directors as a group as of April 4, 2014.14, 2023. Unless noted, the address for the following beneficial owners and management is 951 Broken Sound Parkway, Suite 320, Boca Raton, FL 33487.
| Title of Class | Name and Address of Beneficial Owner | Amount and Nature of Beneficial Owner (1) | Percent of Class (2) | Name and Address of Beneficial Owner | Amount and Nature of Beneficial Owner (1) | Percent of Class (2) | ||||||||||||||
| Common Stock | Steven Girgenti | 232,186 | 1.15 | % | ||||||||||||||||
| Common Stock | Oscar Bronsther, M.D | 213,685 | 1.06 | % | ||||||||||||||||
| Common Stock | Lowell Rush | 201,722 | 1.00 | % | ||||||||||||||||
| Common Stock | Adrian Christopher Liddell | 220,000 | 1.08 | % | ||||||||||||||||
| Common Stock | Marc David Cantor | 220,000 | 1.08 | % | ||||||||||||||||
| Common Stock | Peter C. Zachariou | 381,598 | 1.87 | % | Peter C. Zachariou | 48,856 | 0.15 | % | ||||||||||||
| Series D Preferred Stock | Peter C. Zachariou | 69,487 | 25.71 | % | Peter C. Zachariou | 69,487 | 25.71 | % | ||||||||||||
| Common Stock | All executive officers and directors as a group | 1,469,191 | 6.86 | % | All executive officers and directors as a group | 48,856 | 0.15 | % | ||||||||||||
| Series D Preferred Stock | All executive officers and directors as a group | 69,487 | 25.71 | % | All executive officers and directors as a group | 69,487 | 25.71 | % | ||||||||||||
| Common Stock | Fountainhead Capital Management Limited 17 Bond Street, St. Helier, Jersey JE2 3NP | 9,120,757 | 53.04 | % | Fountainhead Capital Management Limited 13 Castle Street, St. Helier, Jersey JE2 3BT | 20,340,520 | 62.34 | % | ||||||||||||
| Series D Preferred Stock | Fountainhead Capital Management Limited 17 Bond Street, St. Helier, Jersey JE2 3NP | 188,363 | 95.4 | % | Fountainhead Capital Management Limited 13 Castle Street, St. Helier, Jersey JE2 3BT | 188,363 | 69.69 | % | ||||||||||||
* Less than 1%
(1) In determining beneficial ownership of our Common Stock and Series D Preferred Stock, the number of shares shown includes shares which the beneficial owner may acquire upon exercise of debentures, warrants and options which may be acquired within 60 days. In the case of directors, the number of shares includes shares granted but not issued under the director’s Deferred Compensation Plan. In determining the percent of Common Stock or Series D Preferred Stock owned by a person or entity on March 23, 2017,30, 2023, (a) the numerator is the number of shares of the class beneficially owned by such person or entity, including shares which the beneficial ownership may acquire within 60 days of exercise of debentures, warrants and options, and the issuance of shares granted but not issued under the director’s Deferred Compensation Plan; and (b) the denominator is the sum of (i) the total shares of that class outstanding on March 23, 2017 (20,071,988April 14, 2023 (32,628,835 shares of Common Stock and 270,307270,306 shares of Series D Preferred Stock) and (ii) the total number of shares that the beneficial owner may acquire upon exercise of the debentures, warrants and options or that can be issued under the director’s Deferred Compensation Plan. Unless otherwise stated, each beneficial owner has sole power to vote and dispose of its sharesshares.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Related Party Transactions
During each of the years ended December 31, 2022 and 2021, under the terms of the Consultancy Agreement referred to in Note 14, the Company issued 1,607,142 and 2,142,856 shares of Common Stock to Fountainhead for fees of $171,428 and $305,001, respectively.
During the years ended December 31, 2022 and 2021, respectively, the Company issued unsecured loan notes to Fountainhead for a total of $172,500 and $10,000, respectively. The loan notes bear interest at a rate of 10% and are due on demand or by their one-year anniversary (see Note 6).
During the years ended December 31, 2022 and 2021, respectively, the Company accrued interest on related party loans of $39,563 and $31,841, respectively.
Please refer to Financial Statement, Note 12,During each of the years ended December 31, 2022 and 2021, the Company accrued an aggregate of $324,370 of Preferred D Stock dividends, of which is incorporated in its entirety by this reference.$226,037 was regarding Fountainhead and $83,386 was regarding Peter Zachariou. Total accrued Preferred D Stock dividends at December 31, 2022 and 2021 was $1,946,220 and $1,621,850, respectively, of which $1,356,224 and $1,130,130, respectively, was regarding Fountainhead and $500,315 and $416,929, respectively, was regarding Peter Zachariou.
Director Independence
As of March 30, 2018, ofApril 14, 2023, our six (6) directors, Steven Girgenti, Oscar Bronsther and Lowell Rush are considered “independent” in accordance with Rule 4200(a)(15) of the NASDAQ Marketplace Rules. The remaining three (3) directors are not considered “independent”.
| 38 |
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
Audit Fees
The aggregate fees billed by our principal accountant for the audit of our annual financial statements, review of financial statements included in the quarterly reports and other fees that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for the fiscal years ended December 31, 20172022 and December 31, 2016,2021, respectively, were approximately $49,500$65,000 and $48,500.$59,000.
Tax Fees
The fees billed for professional services rendered by our principal accountant for tax compliance, tax advice and tax planning for the fiscal years ended December 31, 20172022 and 20162021 were $1,500$4,000 and $2,000$3,500 respectively.
All Other Fees
There were no fees billed for other products or services provided by our principal accountant for 20172022 or 2016.2021.
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
The following documents are filed as part of this 10-K:
1. FINANCIAL STATEMENTS
The following documents are filed in Part II, Item 8 of this annual report on Form 10-K:
2. FINANCIAL STATEMENT SCHEDULES
All financial statement schedules have been omitted as they are not required, not applicable, or the required information is otherwise included.
| 39 |
3. EXHIBITS
The exhibits listed below are filed as part of or incorporated by reference in this report.
| 101.INS# | Inline XBRL Instance Document. | |
| 101.SCH# | Inline XBRL Taxonomy Extension Schema Document. | |
| 101.CAL# | Inline XBRL Taxonomy Extension Calculation Linkbase Document. | |
| 101.DEF# | Inline XBRL Taxonomy Extension Definition Linkbase Document. | |
| 101.LAB# | Inline XBRL Taxonomy Extension Label Linkbase Document. | |
| 101.PRE# | Inline XBRL Taxonomy Extension Presentation Linkbase Document. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
| 40 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Vycor Medical, Inc. | ||
| (Registrant) | ||
| By: | /s/ Peter C. Zachariou | |
| Peter C. Zacharion | ||
| Chief Executive Officer and Director (Principal Executive Officer) | ||
| Date | ||
| By: | /s/ Adrian Liddell | |
| Adrian Liddell | ||
| Chairman of the Board and Director | ||
| (Principal Financial and Accounting Officer) | ||
| Date | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following person on behalf of the registrant and in the capacity and on the date indicated.
| By: | /s/ Peter C. Zachariou | |
| Peter C. Zachariou | ||
| Chief Executive Officer and Director | ||
| Date | ||
| By: | /s/ David Marc Cantor | |
| David Marc Cantor | ||
| President and Director (Principal Executive Officer) | ||
| Date |
| By: | /s/ Adrian Christopher Liddell | |
| Adrian Christopher Liddell | ||
| Chairman of the Board and Director (Principal Financial and Accounting Officer) | ||
| Date | ||
| 41 |