
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31 2018, 2023
OR
☐TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from___________from ___________ to _____________________
Commission file number 1-38519
AgeX Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 82-1436829 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
1010 Atlantic Avenue, 1101 Marina Village Parkway, Suite 102201
Alameda, California94501
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area codecode: (510) 871-4190(510)671-8370
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol | Name of exchange on which registered | ||
| Common Stock, par value $0.0001 per share | AGE | NYSE American |
Securities registered pursuant to Section 12(g) of the Act:None
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐No☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐No☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes☒No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes☒No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer☐ | Accelerated filer☐ |
| Non-accelerated filer☒ | Smaller reporting company☒ |
| Emerging growth company☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act): Yes ☐No☒
The Registrant’s common stock did not have a market price as of the last day of the Registrant’s second fiscal quarter, therefore theapproximate aggregate market value of shares of voting common stock held by non-affiliates computed by reference to the outstandingprice at which shares of common stock were last sold as of June 30, 2023 was $18.7 million. Shares held by each executive officer and director and by each person who beneficially owns more than 5% of the outstanding common stock have been excluded in that such date cannotpersons may under certain circumstances be calculated.deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 19, 2019,14, 2024, there were outstanding 37,630,000 shares of common stock, par value $0.0001 per share.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement for its 2019 Annual Meeting of Stockholders are incorporated by reference in Part IIINone
AgeX Therapeutics, Inc.
Table of Contents
| 1 |
IMPORTANT PRELIMINARY NOTE
Planned Merger with Serina Therapeutics, Inc. and Related Transactions
On August 29, 2023, AgeX entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) with Serina Therapeutics, Inc. (“Serina”), and Canaria Transaction Corporation, a wholly owned subsidiary of AgeX (“Merger Sub”). Upon the terms and subject to the satisfaction of the conditions described in the Merger Agreement, Merger Sub will be merged with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX (the “Merger”). At a special meeting of AgeX stockholders on March 14, 2024 (the “Special Meeting”), AgeX stockholders approved certain proposals required for consummation of the Merger pursuant to the terms of the Merger Agreement. Serina stockholders have also approved the Merger. There is no assurance that all conditions to the Merger will be met or waiver and that the Merger will be consummated. AgeX stockholders will face a number of risks related to the terms of the Merger Agreement and the Merger, some of which risks are described in this Annual Report on Form 10-K (“Report”). References to the “Combined Company” in this Report mean AgeX after the Merger through which AgeX will have acquired Serina.
On March 14, 2024 AgeX effected a 1 for 35.17 reverse stock split of its common stock (the “Reverse Stock Split”) by filing an amendment to its certificate of incorporation, as approved by AgeX stockholders at the Special Meeting. The Reverse Stock Split resulted in approximately 2,500,000 shares of AgeX common stock being outstanding immediately upon the filing of the amendment to the certificate of incorporation. Except for references to authorized but unissued shares of AgeX common stock, and except as may be otherwise stated in the notes to financial statements, numbers of shares of AgeX common stock issued and outstanding, or issuable upon the exercise of options or warrants or upon conversion of convertible indebtedness, and AgeX common stock prices, referenced in this Report reflect the effect of the Reverse Stock Split, and such amounts shown in the case of historical information, including amounts shown in the consolidated financial statements and notes thereto, have been retroactively adjusted to reflect the effect of the Reverse Stock Split.
On March 19, 2024, AgeX issued to each holder of AgeX common stock as of the dividend record date, March 18, 2024, three warrants (“Post-Merger Warrants”) for each five shares of AgeX common stock held by such stockholder. Each Post-Merger Warrant will be exercisable for one unit of AgeX (“AgeX Unit”) at a price equal to $13.20 per unit and will expire on July 31, 2025. Each AgeX Unit will consist of (i) one share of AgeX common stock and (ii) one warrant (“Incentive Warrant”). Each Incentive Warrant will be exercisable for one share of AgeX common stock at a price equal to $18.00 per warrant and will expire on the four-year anniversary of the closing date of the Merger.
Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of the Combined Company, and stockholders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of the Combined Company, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions, including the Actual Closing Price (as defined in the Merger Agreement) of AgeX common stock being equal to or greater than $12.00 per share, giving effect to the Reverse Stock Split and excluding the impact of any Post-Merger Warrant, Incentive Warrant or the issuance of any share of AgeX common stock upon exercise of any Post-Merger Warrant or Incentive Warrant.
Concurrently with the execution of the Merger Agreement, AgeX, Serina, and AgeX’s controlling stockholder Juvenescence Limited (“Juvenescence”) entered into a Side Letter, which will become effective immediately prior to the closing of the Merger. The Side Letter provides, among other things, that (i) effective immediately before the consummation of the Merger, Juvenescence will cancel all out of the money AgeX warrants held by Juvenescence; (ii) Juvenescence will exercise all Post-Merger Warrants it holds to provide the Combined Company an additional $15 million in capital according to the following schedule: (x) at least one-third on or before May 31, 2024, (y) at least one-third on or before November 30, 2024, and (z) at least one-third on or before June 30, 2025; (iii) Juvenescence will not sell any shares of AgeX Series A Preferred Stock or AgeX Series B Preferred Stock and will take all actions necessary to convert all of such Preferred Stock into AgeX common stock before a Reverse Stock Split that will occur before the Merger; (iv) Juvenescence will release all security interests, guarantees, pledges, assignments and other forms of collateral that it may have in AgeX’s assets pursuant to the terms of Juvenescence loans to AgeX; and (v) Juvenescence will consent to a newly formed subsidiary of AgeX assuming AgeX’s obligations with respect to loan agreements and promissory notes governing loans payable to Juvenescence, including obligations for amounts currently owed and future advances of loan funds, and Juvenescence shall release AgeX from those loan obligations. Juvenescence’s covenant regarding retaining ownership of and converting the Preferred Stock into AgeX common stock has been satisfied through the conversion of the Preferred Stock into AgeX common stock on February 1, 2024.
Prior to the closing of the Merger, all assets of AgeX other than certain “Legacy Assets” will be transferred into a recently formed subsidiary of AgeX “UniverXome Bioengineering, Inc. (“UniverXome”). In consideration of the transfer of such assets, UniverXome will assume (i) all indebtedness of AgeX issued to Juvenescence that has not been previously converted into AgeX Series A Preferred Stock or AgeX Series B Preferred Stock, which will be secured by the Legacy Assets and (ii) all other liabilities of AgeX in existence as of the effective time of the Merger (other than certain transaction expenses related to the Merger).
| 2 |
Serina currently has a pipeline of small molecule candidates targeting central nervous system (“CNS”) indications, enabled by the company’s proprietary POZ PlatformTM delivery technology. In addition to advancing Serina’s wholly owned pipeline assets, Serina is working with pharma partners currently advancing pre-clinical studies exploring POZ polymer lipid-nanoparticles (“LNPs”) in next generation LNP delivered RNA vaccines. In addition, Serina is advancing a lead drug candidate, SER-252 (POZ-apomorphine) for the treatment of advanced Parkinson’s Disease through pre-clinical studies towards the goal of an investigational new drug submission or “IND” to the Food and Drug Administration for the initiation of a Phase I clinical trial during the fourth quarter of 2024. Serina has two other pipeline assets that are positioned to enter IND enabling studies, SER-227 (POZ-buprenorphine) for certain post-operative pain indications, and SER-228 (POZ-cannabidiol) for treatment refractory epilepsy indications. Serina is also focused on expanding its LNP and anti-body drug conjugate partnering collaborations.
If the Merger is completed, the Combined Company will primarily focus on developing Serina’s product candidates and it is anticipated that the Combined Company will not continue to develop AgeX product candidates, other than potentially the development program of NeuroAirmid Therapeutics, Inc. described elsewhere in this Report. If the Merger is not completed, we expect AgeX to continue to execute on its current business strategies described under the section titled “Pre-Merger Business Strategy” below while seeking out and evaluating potential strategic alternatives with respect to our assets and development programs, which may include a merger, business combination, investment into AgeX, sale or other disposition of assets or other strategic transaction. In such case, we may not be successful in executing such strategies or identifying or implementing any such strategic alternatives, and there is a risk that Juvenescence may decide to stop funding our operations, which would likely result in our delisting and dissolution.
Summary of Risk Factors
Below is a summary of the material factors that make an investment in our common shares speculative or risky. This summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below under the heading “Risk Factors” in Item 1A of Part I of this Report and should be carefully considered, together with other information in this Report and our other filings with the Securities and Exchange Commission (the “SEC”) before making investment decisions regarding our common shares.
Risks Related to Our Financial Condition and Capital Resources
| ● | AgeX needs additional financing to execute its operating plan and continue to operate; | |
| ● | As a discovery-stage development company with incurred operating losses and limited capital resources, AgeX anticipates that it will incur continued losses for the foreseeable future and will need to continue to raise capital to finance our operations and is unable to predict whether it will achieve or sustain profitability; | |
| ● | AgeX is highly leveraged, carrying a significant amount of indebtedness, including indebtedness secured by its assets, that will become due and payable over the next three years and there is no assurance that AgeX will be able to refinance those obligations as they become due; | |
| ● | The terms of our loans from Juvenescence and a related Security Agreement could make it more difficult for us to raise additional capital from other sources; | |
| ● | Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates; | |
| ● | Unless AgeX common stock continues to be listed on a national securities exchange, it will become subject to “penny stock” rules that impose restrictive sales practice requirements; | |
| ● | Delays in, or failing to complete the Merger could materially and adversely affect AgeX’s results of operations, business, financial condition or stock price; | |
| ● | If the Merger is not approved or does not occur, AgeX may not be successful in the execution of its current business strategies or identifying and implementing any strategic alternatives with respect to its assets and development programs, and any future strategic alternatives could have negative consequences; | |
| ● | As a major stockholder and creditor of AgeX, Juvenescence, will be able to substantially influence AgeX and exert control over matters subject to stockholder approval; and |
| 3 |
| ● | We are a discovery-stage development company with limited capital resources and have incurred operating losses since our inception. We anticipate that we will incur continued losses for the foreseeable future and will need to continue to raise capital to finance our operations, and we do not know if we will ever attain profitability. |
| ● | The report of our independent registered public accounting firm expresses substantial doubt about our ability to continue as a going concern. We need additional financing to execute our operating plan and continue to operate as a going concern. |
| ● | We are highly leveraged, carrying a significant amount of indebtedness, including indebtedness secured by our assets, that will become due and payable over the next three years and there is no assurance that we will be able to refinance those obligations as they become due. |
| ● | The terms of our outstanding loans from Juvenescence and a related Amended and Restated Security Agreement with Juvenescence could make it more difficult for us to raise additional capital from other sources. |
| ● | Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates. |
PART IRisks Related to Our Relationship with Juvenescence
| ● | Conflicts of interest may arise from our relationship with Juvenescence, which owns approximately 75.6% of our common stock and is a significant creditor and will be able to substantially influence us and exert control over matters subject to stockholder approval and the election of directors prior to the Merger. |
Risks Related to the Merger
| ● | The relative proportion of the Combined Company that AgeX stockholders will own immediately following the closing of the Merger is not adjustable based on the market price of AgeX common stock unless the Actual Closing Price of AgeX common stock, determined in the manner provided in the Merger Agreement, is less than $12.00 per share. If the Actual Closing Price of AgeX common stock is less than $12.00 per share, AgeX has the option to issue additional AgeX common stock to Serina stockholders in the amount necessary to equal the Target Merger Consideration Minimum, determined in the manner provided in the Meger Agreement, which, in such circumstances, could proportionally decrease the amount of the Combined Company that AgeX stockholders would own immediately following the closing of the Merger. Accordingly, the Merger consideration at the closing of the Merger may have a greater or lesser value than at the time the Merger Agreement was signed; |
| ● | Failure to complete the Merger may result in AgeX or Serina paying a termination fee or reimbursement of expenses to the other party and could harm the common stock price of AgeX and future business and operations of each company; |
| ● | If the conditions to the closing of the Merger are not satisfied or waived, the Merger may not occur or the closing of the Merger could be delayed; |
| ● | The Merger may be completed even though material adverse changes may result from the announcement of the Merger, industry-wide changes and/or other causes; |
| ● | Some executive officers and directors of AgeX and Serina have interests in the Merger that are different from the respective stockholders of AgeX and Serina and that may influence them to support or approve the Merger without regard to the interests of the respective stockholders of AgeX and Serina; |
| ● | Juvenescence owns a significant majority of AgeX capital stock and will be able to substantially influence AgeX and exert control over the AgeX Proposals; |
| ● | AgeX stockholders may not realize a benefit from the Merger commensurate with the ownership interest dilution they will experience in connection with the Merger; |
| ● | If the Merger is not completed, the market price of AgeX common stock may decline significantly; |
| ● | The market price of the Combined Company’s common stock following the Merger may decline as a result of the Merger; |
| ● | AgeX stockholder will have a reduced ownership and voting interest in, and will exercise less influence over the management of, the Combined Company following the closing of the Merger as compared to their current ownership and voting interest in AgeX before the Merger; |
| ● | During the pendency of the Merger Agreement, AgeX may be limited in its ability to enter into a business combination with another party on more favorable terms because of restrictions in the Merger Agreement, which could adversely affect AgeX’s business prospects; |
| ● | Certain provisions of the Merger Agreement may discourage third parties from submitting competing proposals, including proposals that may be superior to the transactions contemplated by the Merger Agreement; |
| 4 |
| ● | Because the lack of a public market for Serina’s capital stock makes it difficult to evaluate the fair market value of Serina’s capital stock, the value of the AgeX common stock to be issued to Serina stockholders may be more or less than the fair market value of Serina common stock; |
| ● | Litigation has been filed and additional litigation could in the future arise in connection with the Merger, against AgeX, the AgeX Board, Serina or the Serina Board, which could be costly, prevent the consummation of the Merger, divert management’s attention and otherwise materially harm AgeX’s, Serina’s or the Combined Company’s business. |
| ● | If the Merger is not completed we would not be able to pursue the development and commercialization of Serina’s technologies and product pipeline. |
| ● | If the Merger is not approved or does not occur, we may not be successful in the execution of our current business strategies or identifying and implementing any strategic alternatives with respect to our assets and development programs, and any future strategic alternatives could have negative consequences. |
Risks Related to the Reverse Stock Split
| ● | The Reverse Stock Split may not increase the Combined Company’s stock price over the long-term. |
| ● | The Reverse Stock Split may decrease the liquidity of AgeX common stock or the Combined Company’s common stock. |
| ● | The Reverse Stock Split may lead to a decrease in the Combined Company’s overall market capitalization. |
Risks Related to Our Business Operations
| ● | Due to our limited financial resources, we have reduced our staffing, eliminated our research laboratory facilities, and eliminated in-house research and product development work. We will seek opportunities to outsource or license product development and commercialization but there is no assurance that we will be able to do so successfully. |
| ● | We may expend our limited resources to pursue one or more particular product candidates or indications and fail to pursue product candidates or indications that may be more profitable or for which there is a greater likelihood of success. |
| ● | We have not tested any of our product candidates in clinical trials. Success in early development and preclinical studies or clinical trials may not be indicative of results obtained in later preclinical studies and clinical trials. |
| ● | Our choice of product candidates and our development plans for our product candidates are subject to change based on a variety of factors, and if we abandon development of a product candidate we may not be able to develop or acquire a replacement product candidate. |
| ● | We may determine to expand our organization and obtain laboratory facilities if we are able to raise sufficient capital to do so, and we may experience difficulties in managing this growth, which could disrupt our operations. |
| ● | The commercial success of any of our current or future product candidates will depend upon the degree of market acceptance by physicians, patients, third-party payors, and others in the medical community. |
| ● | If the market opportunities for our product candidates are smaller than we believe they are, we may not meet our revenue expectations and, even assuming approval of a product candidate, our business may suffer. |
| ● | We will face risks related to the manufacture of medical products for any product candidates that we develop. |
| ● | Any cell-based products that receive regulatory approval may be difficult and expensive to manufacture on a commercial scale. |
| ● | If we fail to meet our obligations under license agreements, we may lose our rights to key technologies on which our business depends. |
| ● | The development and commercialization of new drugs to address obesity and type 2 diabetes may substantially limit or eliminate the prospects for AgeX’s prospective AGEX-BAT1 product. |
| 5 |
Risks Related to Our Industry
| ● | We face significant competition in an environment of rapid technological change and the possibility that our competitors may achieve regulatory approval before us or develop therapies that are more advanced or effective than ours, which may harm our business and financial condition, and our ability to successfully market or commercialize our product candidates. |
| ● | The regulatory approval processes of the United States Food and Drug Administration (the “FDA”) and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed. |
| ● | If we encounter difficulties enrolling patients in clinical trials, our clinical development activities could be delayed or otherwise adversely affected. |
| ● | Even if we obtain FDA approval for any of our product candidates in the United States, we may never obtain approval for or commercialize it in any other jurisdiction, which would limit our ability to realize its full market potential. |
| ● | Even if a product candidate receives regulatory approval, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our product candidates. |
| ● | Our product candidates may cause serious adverse events or undesirable side effects or have other properties which may delay or prevent their regulatory approval, limit the commercial profile of an approved label, or, result in significant negative consequences following marketing approval, if any. |
| ● | We face potential product liability, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use or misuse of our product candidates harm patients or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals could be revoked or could otherwise be negatively impacted, and we could be subject to costly and damaging product liability claims. |
| ● | Our insurance policies are expensive and protect us only from some business risks, which leaves us exposed to significant uninsured liabilities. |
| ● | The price and sale of any product candidates that be marketed may be limited by health insurance coverage and government regulation. |
| ● | Enacted and future healthcare legislation, including the Affordable Care Act or ACA, may increase the difficulty and cost to obtain marketing approval of and commercialize our product candidates and may affect the prices we may set. |
Risks Related to our Dependence on Third Parties
| ● | We may become dependent on future collaborations to develop and commercialize our product candidates and to provide the regulatory compliance, sales, marketing, and distribution capabilities required for the success of our business. |
| ● | We have no marketing, sales, or distribution resources for the commercialization of any products or technologies that we might successfully develop. |
| ● | We do not have the ability to independently conduct clinical trials required to obtain regulatory approvals for our product candidates and intend to rely on third parties to conduct, supervise and monitor our clinical trials. |
Risks Related to Intellectual Property
| ● | If we are unable to obtain and enforce patents and to protect our trade secrets, others could use our technology to compete with us, which could limit opportunities for us to generate revenues by licensing our technology and selling our products. |
| ● | There is no certainty that our pending or future patent applications will result in the issuance of patents. |
| ● | The process of applying for and obtaining patents can be expensive and slow. |
| ● | Our patents may not protect our technologies or products from competition. |
| ● | We may not be able to enforce our intellectual property rights throughout the world. |
| ● | We may be subject to patent infringement claims that could be costly to defend, which may limit our ability to use disputed technologies, and which could prevent us from pursuing research and development or commercialization of some of our technologies or products, require us to pay licensing fees to have freedom to operate and/or result in monetary damages or other liability for us. |
| 6 |
Risks Pertaining to Our Common Stock
| ● | There is a limited history to the public trading of our common stock and there is no assurance that a market for our common stock will be sustained. |
| ● | Because we are engaged in the development of pharmaceutical and cell therapy products, the price of shares of our common stock may rise and fall rapidly. |
| ● | Because we do not pay dividends, our stock may not be a suitable investment for anyone who needs to earn dividend income. |
| ● | Securities analysts may not initiate coverage or continue to cover our common stock, and this may have a negative impact on the market price of our shares. |
| ● | You may experience dilution of your ownership interests if we issue additional shares of common stock or preferred stock. |
| ● | Unless our common stock continues to be listed on a national securities exchange, it will become subject to the so-called “penny stock” rules that impose restrictive sales practice requirements. |
Risks Related to the Combined Company
| ● | The Combined Company will need to raise additional financing in the future to fund its operations, which may not be available to it on favorable terms or at all; | |
| ● | The market price of the Combined Company’s common stock is expected to be volatile, and the market price of the common stock may drop following the Merger; |
| ● | The Combined Company will incur costs and demands upon management as a result of complying with the laws, rules and regulations affecting public companies; | |
| ● | Anti-takeover provisions in the Combined Company’s governance documents and under Delaware law could make an acquisition of the Combined Company more difficult and may prevent attempts by the Combined Company stockholders to replace or remove the Combined Company management; and | |
| ● | If the Combined Company fails to attract and retain management and other key personnel, it may be unable to continue to successfully develop or commercialize its product candidates or otherwise implement its business plan. | |
| ● | If the Merger is consummated, the Combined Company will face other risks that are substantially the same as or similar risks faced by AgeX. |
Special Note Regarding Forward-Looking Statements
Certain statements contained herein are forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for AgeX, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management constitute forward-looking statements.1995. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,“anticipates,” “believes,” “plans,“could,” “anticipates,“seeks,” “estimates,” “expects,” “estimates”)“intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “pro forma,” “should,” “would” should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements and as such should be evaluated together with the many uncertainties that affect the businesses of AgeX, particularly those mentioned in the cautionary statements found in AgeX’s filings with the Securities and Exchange Commission.SEC. AgeX disclaims any intent or obligation to update these forward-looking statements.
| 7 |
References to “ AgeX,” “our” or “us” mean AgeX Therapeutics, Inc.
The description or discussion,forward-looking statements in this Form 10-K, of any contract or agreementReport include, among other things, statements about:
| ● | the strategies, prospects, plans, operations, expectations and objectives of management of AgeX if the Merger is not consummated or of the Combined Company following the closing of the Merger; | |
| ● | the progress, scope or duration of the development of product candidates or programs; | |
| ● | the benefits that may be derived from, or the commercial or market opportunity of, the product candidates of AgeX, Serina and the Combined Company; | |
| ● | the ability of AgeX and the Combined Company to protect intellectual property rights; | |
| ● | the ability of AgeX and the Combined Company to maintain compliance with NYSE American listing standards; | |
| ● | the anticipated operations, financial position, losses, costs or expenses of AgeX if the Merger is not consummated or the Combined Company following the closing of the Merger; | |
| ● | statements regarding future economic conditions or performance; | |
| ● | statements concerning proposed products or product candidates; | |
| ● | the approval and closing of the Merger, including the timing of the Merger, whether conditions to the completion of the Merger will be met or waived, the exchange ratio, and relative ownership levels of the Combined Company as of the closing of the Merger; | |
| ● | the expected benefits of and potential value created by the Merger for the stockholders of AgeX; and | |
| ● | statements of belief and any statement of assumptions underlying any of the foregoing. |
For a summary only and is qualified in all respects by reference to the full textdiscussion of the applicable contractfactors that may cause AgeX, Serina or agreement.
PRELIMINARY NOTE ABOUT OWNERSHIP OF OUR COMMON STOCK
On November 28, 2018 (the “Distribution Date”) BioTime, Inc. (“BioTime”) owned 14,416,000 shares of our common stock, par value $0.0001 per share, representing approximately 40.2%the Combined Company’s actual results, performance or achievements following closing of the sharesMerger to differ materially from any future results, performance or achievements expressed or implied in such forward-looking statements, and for a discussion of risk associated with the ability of AgeX and Serina to complete the Merger and the effect of the common stock issued and outstandingMerger on the Distribution Date. Onbusiness of AgeX, Serina and the Distribution Date, BioTime distributed to its shareholders, on a pro rata basis, 12,697,028 sharesCombined Company following the completion of the Merger, see “Risk Factors.” Additional factors that could cause actual results to differ materially from those expressed in the forward-looking statements are discussed in reports filed with the SEC by AgeX.
If any of these risks or uncertainties materializes or any of these assumptions proves incorrect, the results of AgeX, common stock it then held (the “Distribution”). Immediatelyor the Combined Company following completion of the Merger, could differ materially from the forward-looking statements. All forward-looking statements in this Report are current only as of the date on which the statements were made. AgeX does not undertake any obligation (and expressly disclaim any such obligation) to publicly update any forward-looking statement to reflect events or circumstances after the Distribution, BioTime retained 1,718,972 sharesdate on which any statement is made or to reflect the occurrence of unanticipated events, except as required by applicable law.
In addition, statements that “AgeX believes” believes” and similar statements reflect AgeX’s beliefs and opinions on the relevant subject. These statements are based upon information available to AgeX common stock, representing approximately 4.8%as of the common stock then issueddate of this Report, and outstanding. Following the Distribution, our common stock began publicly trading on the NYSE American under the symbol “AGE”.while AgeX believes such information forms a reasonable basis for such statements, such information may be limited or incomplete, and such statements should not be read to indicate that AgeX has conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
INDUSTRY AND MARKET DATAIndustry and Market Data
This Annual Report (“Report”) on Form 10-K contains market data and industry forecasts that were obtained from industry publications, third partythird-party market research and publicly available information. These publications generally state that the information contained therein has been obtained from sources believed to be reliable. While we believe that the information from these publications is reliable, we have not independently verified such information.
This Report also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. We obtained the industry and market data in this Report from our own research as well as from industry and general publications, surveys and studies conducted by third parties, some of which may not be publicly available. Such data involves a number of assumptions and limitations and contains projections and estimates of the future performance of the industries in which we operate that are subject to a high degree of uncertainty. We caution you not to give undue weight to such projections, assumptions and estimates.
PART I
References to “ AgeX,” “our” or “us” mean AgeX Therapeutics, Inc.
In this Annual Report on Form 10-K, the description or discussion of any contract or agreement is a summary only and is qualified in all respects by reference to the full text of the applicable contract or agreement.
Item 1.Business
Development of Our Business
During the twelve months ended December 31, 2023, the following significant developments related to our business have occurred:
On August 29, 2023, we entered into the Merger Agreement with Serina, and a wholly owned subsidiary of AgeX (“Merger Sub”). Upon the terms and subject to the satisfaction of the conditions described in the Merger Agreement, the Merger will be consummated through the merger of Merger Sub with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX. There is no assurance the conditions to the Merger will be met and that the Merger will be consummated. See “IMPORANT PRELIMINARY NOTE — Planned Merger with Serina Therapeutics, Inc. and Related Transactions,” “Risk Factors,” and “Directors, Executive Officers, and Corporate Governance” in this Report for additional information about the Merger.
Serina currently has a pipeline of small molecule candidates targeting central nervous system (“CNS”) indications, enabled by the company’s proprietary POZ PlatformTM delivery technology. In addition to advancing Serina’s wholly owned pipeline assets, Serina is working with pharma partners currently advancing pre-clinical studies exploring POZ polymer lipid-nanoparticles (“LNPs”) in next generation LNP delivered RNA vaccines. In addition, Serina is advancing a lead drug candidate, SER-252 (POZ-apomorphine) for the treatment of advanced Parkinson’s Disease through pre-clinical studies towards the goal of an investigational new drug submission or “IND” to the Food and Drug Administration for the initiation of a Phase I clinical trial during the fourth quarter of 2024. Serina has two other pipeline assets that are positioned to enter IND enabling studies, SER-227 (POZ-buprenorphine) for certain post-operative pain indications, and SER-228 (POZ-cannabidiol) for treatment refractory epilepsy indications. Serina is also focused on expanding its LNP and anti-body drug conjugate partnering collaborations.
If the Merger is completed, the Combined Company consisting of AgeX and Serina as a subsidiary after the Meger will primarily focus on developing Serina’s product candidates and it is anticipated that the Combined Company will not continue to develop the AgeX product candidates and technologies, and will not pursue the business strategy, discussed below in this Report, other than potentially the neural stem cell development program of NeuroAirmid Therapeutics, Inc. (“NeuroAirmid”).
In connection with our sponsored Huntington’s Disease research program at the University of California at Irvine (“UCI”), we and certain researchers who contributed to the Huntington’s Disease research work formed NeuroAirmid to pursue clinical studies of the use of derived neural stem cell to treat that disease. The new subsidiary is still in the organizational stage and commencement of clinical study work will depend NeuroAirmid obtaining a license from UCI to use a UCI patent and on NeuroAirmid’s ability to obtain financing through grants or third-party investment. We presently own 50% of the issued and outstanding shares of NeuroAirmid,
Overview of AgeX’s Current, Pre-Merger Business
We are a biotechnology company focused on the development and commercialization of novel therapeutics targeting human aging.aging and degenerative diseases. Our mission is to apply our comprehensive experience in fundamental biological processes of human aging to a broad range of age-associated medical conditions. We believe that demand for therapeutics addressing such conditions is on the rise, commensurate with the demographic shift of aging in the United States and many other industrialized countries.
Our proprietary technology, based on telomerase-mediated cellular immortality and regenerative biology, allows us to utilize telomerase-expressing regenerative pluripotent stem cellcells (“PSCs”hES cells” or “PSCs”) for the manufacture of cell-based therapies to regenerate tissues afflicted with age-related chronic degenerative disease. We own or have licenses to a number of patents and patent applications used in the generation of these product candidates, including intellectual property related to PSC-derived clonal embryonic progenitorPureStem® technology. Our technology platform also includes UniverCyte™ which uses the HLA-G gene to potentially confer low immune observability to cells, so as to suppress rejection of transplanted cells and tissues. AgeX plans to use or license the use of this patented technology to produce genetically-modified master cell lines (PureStem® technology) andHyStem®delivery matrices.banks of pluripotent stem cells that can then be differentiated into any young cell type of the human body that now express the immune tolerogenic molecule.
Our product candidates are in the discovery stage: Theystage include two cell-based therapies derived from telomerase-positive PSCs and onetwo product candidatecandidates derived from our proprietary induced Tissue Regenerationtissue regeneration (iTRTM) technology. We have also sponsored a research program to derive neural stem cells from PSCs to treat degenerative diseases such as Huntington’s Disease. We will need to conduct or sponsor research and development work, as part ofor license our plantechnology to other biotechnology or pharma companies interested in furthering research and development in order to develop these cell- and drug-based therapies, each targeting large unmet needs in age-related medicine.
| 9 |
Additional Information
AgeX is incorporated in the State of Delaware. Our common shares trade on the NYSE American Stock Exchange under the symbol “AGE.” Our principal executive offices are located at 1010 Atlantic Avenue, Suite 102, Alameda, CA 94501, and our phone number at that address is (510) 871-4190. Our website address is www.AgeXinc.com. The information on, or that can be accessed through our website is not part of this Report. We also make available, free of charge through our website, our most recent annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and any amendments to those reports, as soon as reasonably practicable after the reports are electronically filed with or furnished to the Securities and Exchange Commission (the “SEC”).
RenelonTM, iTRTM, and UniverCyteTM, are trademarks of AgeX Therapeutics, Inc.HyStem®andPureStem®are registered trademarks of BioTime, Inc.GeneCards®is a registered trademark of Yeda Research and Development Co. Ltd.
Emerging Growth Company
We are an “emerging growth company” under theJumpstart our Business Startups Act of 2012 or theJOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable, in general, to public companies that are not emerging growth companies. These provisions include:
We will remain an “emerging growth company” until the earliest of: (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of thedate of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act of 1933,as amended; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt securities during the previous three years; or (iv) the date on which we are deemed to be a “large accelerated filer” under the Securities Exchange Act of 1934, as amended.
The JOBS Act permits an emerging growth company to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. However, we have elected to comply with newly adopted or revised accounting standards when they become applicable to public companies because our financial statements were previously consolidated with those of our former parent company BioTime, Inc. which is not an emerging growth company under the JOBS Act and is therefore not permitted to delay the adoption of new or revised accounting standards that become applicable to public companies. This election under the JOBS Act to not delay the adoption of new or revised accounting standards is irrevocable.
Overview of Our Opportunity in Age-Related Diseases
Aging is one of the most significant demographic trends of our time. As shown in Figure 1, the U.S. Census Bureau projects a sharp rise in the number of Americans over 80 years of age, with a marked inflection point occurring between the years 2020 and 2030.

Figure 1. Projected increase in the numbers of the U.S. population over 80 years of age (U.S. Census Bureau)
This demographic shift associated with 76 million aging baby boomers poses a significant challenge to our healthcare system and our economy as a whole. The unsolved problem relates to the fact that chronic conditions account for some 80% of total health care expenditures in the United States and the elderly have a higher prevalence of chronic degenerative disease than the young. Approximately 80% of older adults have one chronic disease, and 68% have two or more.
Our technology platforms reflect over 25 years of research and development in cell immortality and regenerative medicine. It is designed to address some of the largest unmet needs of an aging population by translating state-of-the-art laboratory science relating to aging into therapeutic biologicals, drugs, and devices.
Overview of Our Product Candidates
Our Pipeline
Our product pipeline includes two cell-based and one drug-based therapeutictwo iTR-based product candidates in development. It also includes currently-marketed online database products and research products outlined in Figure 2.
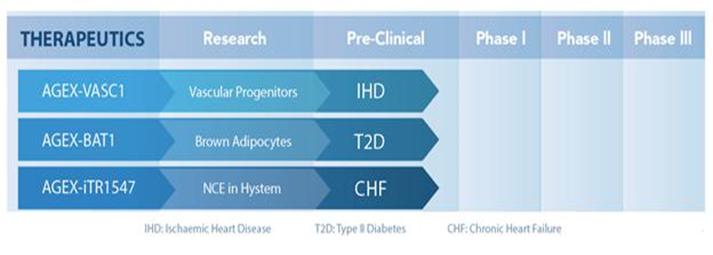
Figure 2. The AgeX product pipeline. IHD (Ischemic Heart Disease), T2D (Type II Diabetes), CHF (Congestive Heart Failure).
Our lead cell-based therapeutic candidates in development are AGEX-BAT1 and AGEX-VASC1:
| ● | AGEX-BAT1 is our lead cell therapy product candidate in the discovery stage of development utilizing PSC-derived brown adipocytes for the treatment of certain age-related metabolic disorders such as Type II (adult-onset) | |
| ● | AGEX-VASC1 is a cell-based therapy in the discovery stage of development comprised of young regenerative vascular-forming cells. AGEX-VASC1 may restore vascular support in aged ischemic tissues such as |
Our lead small molecule drug-based therapeutic candidate for iTRTM in discovery is AGEX-iTR1547: and our lead biologic candidate for iTR is AGEX-iTR1550:
| ● | AGEX-iTR1547 is a drug-based formulation and AGEX-iTR1550 (also known as Renelon™) is a gene delivery technology, both of which are in the discovery stage of |
Our research related to the reprogramming of aging has also led to novel insights into cancer. We have filed patent applications on inventions that relate to these discoveries. These technologies may provide novel targets for cancer therapy and diagnosis. One such cancer therapeutic in the early stages of development is designated “EPROTM” (embryonic promoter-regulated oncolysis). EPRO is an oncolytic gene therapy strategy that may provide a novel means of selectively destroying an array of different types of cancer cells. Successful development of EPRO will be dependent, in part, on the availability of financing and licensing or joint development opportunities.
Our currently marketed research and database products include cGMP ES Cells (humanhuman embryonic stem or “hES”)hES cells produced under current good manufacturing practices (or “cGMP”), PSC-derivedGood Manufacturing Practice (“cGMP” and hES-derived cells for research, and ourGeneCardsDatabase Suite:research:
Overview of Our Technology Platforms
The technology underlying our product development programs is based on telomerase-mediated cellular immortality and regenerative biology. By “telomerase-mediated cellular immortality” we refer to the fact that cells that express sufficient levels of a protein called telomerase are capable of replicating without limit. By “regenerative biology,” we refer to novel methods to regenerate tissues afflicted with age-related chronic degenerative disease such as coronaryperipheral vascular disease and ischemic heart failure, anddisease as well as age-related metabolic disorders such as those associated with Type II diabetes osteoarthritis, or Parkinson’s disease,and obesity, as well as others. We utilize telomerase-expressing regenerative Pluripotent Stem Cells,pluripotent stem cells, or PSCs, for the manufacture of cell-based therapies. We own or have licensed numerous patents and patent applications covering methods and compositions relating to this technology platform.
BackgroundWe believe our core technology platforms provide us with a strong foundation for successfully addressing many of Human Agingthe diseases of ageing by focusing on broad therapeutic applicability and commercially scalable technologies:
Cell Immortality
There is a growing consensus in the scientific community that1. PureStem®: AgeX’s allogeneic cell derivation and manufacturing platform, based on human aging is due in large part to the aging of individualembryonic progenitors, which are cells in state of development between embryonic stem cells and adult cells. We believe PureStem has the various tissues ofpotential to solve several major challenges faced by the body (somatic cells). In contrast, the reproductive lineage of cells (germ-line) perpetuate the human species from generation-to-generation without limit and continue to generate new people over the millennia.cell therapy industry by generating cellular therapeutics which would:
| ● | be commercialized as “off-the-shelf” products; |
| ● | be pure and industrially scalable; |
| ● | have lower cost of goods per unit; |
| ● | be amenable to traditional pharmaceutical supply chain logistics; |
| ● | have the potential for acceptable reimbursement prices, unlike the relatively expensive autologous products; and |
| ● | have higher clinical adoption from expected cost savings and more simplified processes. |
| 10 |
In 1961, Dr. Leonard Hayflick first reported that normal humanaddition, we believe PureStem cells may have advantages over mesenchymal stem cells (MSCs), which may only survive transiently in the body (unlikeand exert any short-term benefit by releasing paracrine factors, which may limit their potential of MSCx.
MSCs neither engraft nor become specialized cells. On the germ-line)other hand, cells derived from PureStem progenitors will be engineered to be young, not prone to the disadvantages associated with older cells, and are expected to become permanently engrafted in the body to deliver a true regenerative outcome. To date, AgeX has isolated more than 200 cell types from PureStem.
2. UniverCyte™: AgeX’s pioneering technology designed to genetically modify allogeneic donor cells to potentially become hypoimmunogenic/universal, so they can proliferatepotentially be transplanted into all patients in an off-the- shelf manner, without the normal need for onlyhuman leukocyte antigen (HLA) matching between donor and receipt or immunosuppression. UniverCyte utilizes a finite number of times (typically fewer than 100 times). This phenomenon, known as the “Hayflick Limit”, “cell mortality”, or “cellular aging”,potent molecule called HLA-G. HLA is a normal propertygroup of somatic cells. Inrelated proteins that helps the 1990s, our CEO Dr. Michael D. West founded a biotechnology company called Geron Corporation where his team isolated forimmune system distinguish the first time the human gene called “Telomerase Reverse Transcriptase” or “telomerase.” In 1998, Geron scientists in collaboration with scientists at the University of Texas Southwestern Medical Center at Dallas, published the result that telomerase could stop the aging of human cells, or could “immortalize” them.
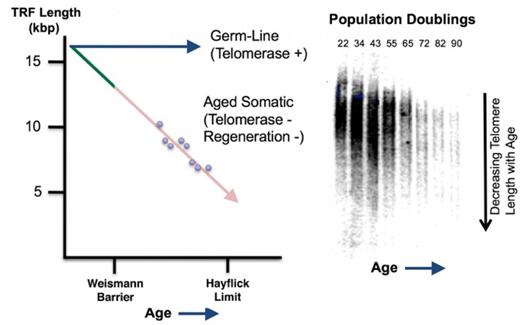
Figure 3. The Germ-line/soma dichotomy wherein germ-line cells express telomerase, maintain telomere length, and exhibit replicative immortality, while body (somatic) cells lack telomerase, showing progressive telomere shortening until they reach the Hayflick limit.
In 1994, Dr. West’s group demonstrated through an assay for measuring telomerase activity that nearly 90% of cancer cell types cultured in the laboratory or tumors surgically removedbody’s own proteins from patients abnormally express telomerase. This broke the then dogma that there was no common mechanism at work in cancer. Scientists have concluded that cell mortality, while being detrimental in old age, benefits us early in lifeproteins made by helping to repress cancer cell growth. Figure 3 illustrates this dichotomy wherein immortal cellsforeign invaders such as viruses and bacteria. HLA-G’s only known physiological role in nature is to prevent destruction of a semi-allogeneic fetus by the germ-linematernal immune system. We believe that UniverCyte could potentially avoid immune rejection of transplanted cells, that perpetuatesolving a major challenge facing the species are immortalallogeneic cell therapy industry. In addition to utilizing UniverCyte™ for its own future cell therapy products, AgeX may make UniverCyte™ available to other cell therapy companies through telomerase activity while body (somatic)licensing arrangements.
3. Induced Tissue Regeneration (iTRTM): The aim of iTR is to return aged cells lack telomerase expression,back to a youthful state, thereby inducing a capacity for scarless regeneration characteristic of early developing tissues, without reverting cells to pluripotency. This technology is sometimes referred to as “partial reprogramming” or “epigenetic reprogramming of aging.” We believe this novel approach may trigger complete regeneration of cells, and as a result show progressive telomere shortening and a finite lifespan (are mortal).
The Weismann Barrier
Early in the evolution of life, primitive unicellular andpotentially even multicellular organisms may have lacked programmed agingcomplex tissues, damaged as a result of the potentialage-related degenerative processes or trauma. The premise behind iTR is that aging, and in turn degenerative diseases of their cells having the potential for both replicative immortality and regeneration. However, in more complex animals such as mammals, somatic cells lose not only replicative immortality, but after most organ systemsold age, are formed during embryonic development, they also lose full regenerative potential. This repression of both telomerase-mediated cell immortality and regeneration potential is called the “Weismann Barrier” (see Figure 4).

Figure 4. The Weismann Barrier coincides with the loss of both replicative immortality and regeneration. Levels of expression of the geneCOX7A1 provide a useful markerresult of the loss of regenerative potential.
PSCs represent the earliest stagestwo characteristics of human development and are the first normal human cells cultured in the laboratory that display both telomerase-mediatedcells; namely, replicative immortality and regenerative potential.capacity. These two characteristics are present in embryonic cells but are lost at the embryonic to fetal transition (EFT). With this loss, humans can no longer generate new cells or repair damaged cells scarlessly and in sufficient numbers to maintain health. We discovered that cells begin expressing the gene COX7A1 at the EFT when regeneration is commonly lost. Therefore, our scientists utilized these cellswe believe the gene may be a key inhibitor of cellular regeneration. For example, we have discovered that restoring a regenerative pattern of COX7A1 gene expression may facilitate hair regeneration in mouse models. In addition, we have invented multiple platforms for delivering iTR using small molecules as well as biologic strategies such as those using gene therapy to transiently express reprogramming factors. We have filed patent applications on the primitive regenerative cells derived from them, called “PureStem®”use of iTR in a wide array of degenerative conditions including cancer.
4. ESI Cell Lines: AgeX has six clinical-grade human embryonic stem cell lines, they are distinguished as the first clinical-grade human pluripotent stem cell lines created under current Good Manufacturing Practice as described in Cell Stem Cell (2007;1:490-4). They are listed on the NIH Stem Cell Registry in the USA and are among the best characterized and documented stem cell lines in the world. ESI-053 is among only a few pluripotent stem cell lines from which a derived cell therapy product candidate has been granted FDA IND clearance for human studies. The FDA cleared an IND application from ImStem Biotechnology, one of our sublicensees, for a MSC product derived from ESI-053 for multiple sclerosis. This was believed to be the first MSC product derived from a pluripotent stem line to be accepted for a human trial by the FDA. The ESI cell lines are available as research where they were comparedor clinical grade product, and have been offered since 2006.
Pre-Merger Business Strategy
We believe our four proprietary platform technologies, PureStem® for cell derivation and manufacturing, UniverCyte™ for generation of hypoimmunogenic cells and iTRTM for reversing the age of cells already in the body present AgeX with a multiplicity of attractive opportunities which we may pursue. Given these platform technologies may be highly desirable to diverse adultmultiple academic and biopharma companies due to their broad applicability and potentially important clinical and commercial benefits, AgeX plans to pursue different business models for these platforms:
| ● | Co-Development and Licensing: Our PureStem® and UniverCyte™ technologies as well as our ESI cell lines may have applications in the development of a broad range of cell therapy products. We will seek opportunities to license these AgeX technologies to other cell therapy or biopharma companies to bring in early revenue streams, especially for therapies that AgeX does not presently intend to develop. |
| ● | Cellular Therapy: AgeX presently does not have the laboratory and research staff required to conduct in-house research and development for its product candidates, including AGEX-BAT1 and AGEX-VASC1. Instead, AgeX may conduct research and development of those product candidates through a variety of alternative strategies, including but not limited to, sponsoring research and development work at research laboratories at universities or other educational institutions, entering into co-development and marketing arrangements with researchers or other companies in the cell therapy or biopharma industry, and engaging contract service providers to conduct research and development and manufacturing for AgeX for particular product candidates. Our sponsored Huntington’s Disease research program at UCI has led to the organization of an AgeX subsidiary, NeuroAirmid, of which we equally owned with certain UCI researchers, to pursue clinical studies of the use of derived neural stem cell to treat that disease. The new subsidiary is still in the organizational stage and commencement of clinical study work will depend on its ability to obtain financing through grants or third-party investment. |
| 11 |
| ● | Reverse Bioengineering, Inc. (Reverse Bio): Partial cellular reprogramming using our iTRTM technology may one day allow us to revert aged or diseased cells inside the body back to a more youthful, healthy and functional state. We incorporated Reverse Bio as an AgeX subsidiary to develop our iTRTM platform. Reverse Bio is intended to allow for a dedicated focus on iTRTM in terms of equity financing and advancing our iTRTM technology to proof-of-concept in an animal model. We have assigned to Reverse Bio our patent portfolio for iTR development, but the future operations of Reverse Bio will depend in large measure on its ability to raise its own capital. |
Each of these models may provide particular benefits to AgeX in terms of financing and efficiency of operations. However, each alternative has potential disadvantages as well. If AgeX out-licenses its technology it will avoid the costs and risks of research and development, clinical trials, regulatory approval, manufacturing, and commercialization of product candidates, but the revenues AgeX would receive from commercialization of products developed under those arrangements would likely be limited to royalties on the mortal sideproduct sales and potentially licensing fees and milestone payments representing a relatively small portion of the Weismann barriertotal product revenues. Similarly, co-development and marketing or similar arrangements would permit AgeX to uncover the mechanisms regulating the loss of regenerative potential. Artificial intelligence algorithms were usedshare costs and risks but would also require AgeX to parse millions of gene expression data points and the results were published in late 2017. Figure 4 shows the Weismann Barrier and the associated rise of a gene expression marker of the non-regenerative state designatedCOX7A1. This proprietary marker, along with other insights obtainedshare revenues from the product candidates that may be successfully developed and commercialized. See elsewhere in this Report for information about certain risks associated with reliance on arrangements with third parties for research, provides us with a window into this biologyproduct development, clinical trials, manufacturing, and a meanscommercializing product candidates.
We plan to finance our iTRTM and AGEX-BAT1 research and development through Reverse Bio. To the extent that such financing is obtained through the sale of screening for agents capablecapital stock or other equity securities to investors or other biopharma companies by Reverse Bio, or the sale of restoring a regenerative state to old nonregenerative cells. ItReverse Bio shares held by AgeX, our equity interest in Reverse Bio and our iTRTM and AGEX-BAT1 business would be diluted.
However, if the Merger is completed, the Combined Company will primarily focus on developing Serina’s product candidates and it is anticipated that such agents maythe Combined Company will not only reset the pattern of gene expression in adult cells backcontinue to that their regenerative counterparts but may also induce tissue regeneration when appliedin vivo in the context of age-related degenerative disease. Since the previously mentioned 2017 publication described the re-emergence of the regenerative phenotype in the majority of cancer cell lines, the discoveries mayopen the door todevelop our product candidates, other than potentially important diagnostic and therapeutic implications as well.
Pluripotent Stem Cells (PSCs)

Figure 5. Pluripotent Stem Cells (PSCs) possess both telomerase-mediated replicative immortality and regenerative potential, capable of producing all human cell types.
In an effort to utilize telomerase-mediated immortality and regenerative biology in the development program of novel therapeutics, in the mid-1990s, Dr. West, organized a collaboration with Drs. James Thomson, John Gearhart,NeuroAirmid, which is described below under “Other AgeX Products and Roger Pedersen that led to the first isolation of PSCs. In contrast to other types of cells, PSCs are unique by at least two important criteria. The first criterion relates to the ability of pluripotent cells to proliferate, or make more copies of themselves, indefinitely, that is to say, they are “immortal”. The second relates to the ability of PSCs to differentiate into any of the hundreds of specialized cell types in the body. This replicative immortality of PSCs facilitates the industrial scalability of product. We believe that many of these cell types have potential for regenerating function in tissues damaged by degenerative diseases when transplanted. A small sampling of these cell types is shown in Figure 5. Unlike PSCs, adult stem cells typically have severely-reduced scale-up potential (are mortal unlike immortal PSCs), and have passed the Weismann Barrier, and are therefore limited in their ability to regenerate normal tissue when transplantedin vivoProduct Candidates — Neural Stem Cells.”. Therefore, we believe that PSC-based cellular therapeutics have significant competitive advantages over cell-based therapeutics being developed by many adult stem cell companies.
OurAgeX Technology Platforms
PureStem®Technology
Regulatory approval of cell- and tissue-based products require high standards of quality control. In the case of stem cell-derived products, there is a high standard for insuringensuring the known identity, purity, and reproducibility of the cells to be administered. PSCs provide certain advantages over adult stem cell products when used in the manufacture of cell-based therapeutics for the treatment of age-related disease. These advantages include:
| ● | The replicative immortality of the PSCs which facilitates the indefinite scale-up of PSC master cell banks for the manufacture of uniform product, as well as an immortal substrate for targeted genetic modifications. |
| ● | ||
| Since most PSCs maintain long and stable telomere lengths, the replicative capacity of derived differentiated cell types is typically longer (younger) than adult or even fetal-derived cells. |
| ● | ||
| Using |
PureStem®technology is based on the observation that embryonic anlagen of many tissues in the human body are naturally comprised of highly proliferative cells with relatively long telomere length. Therefore, it is possible to generate clonal lineages of these cellsin vitro.vitro. Cells derived from adult tissues commonly permanently cease to divide after a certain number of doublings, a condition known as senescence. In addition, adult and even fetal tissues largely contain differentiated cells often with limited or no capacity of replicationin vitro.vitro. As a result, the clonal expansion of human embryonic progenitor cell types allows not only a novel and more facile point of scalability but also generates populations of cells that are multipotent instead of pluripotent, and therefore markedly easier to define identity, purity, and potency.
We have studied the fate of over 200 diversePureStem cell lines in thousands of differentiation conditions. This was accomplished by thawing individual cryopreservedPureStem cell lines, culturing them in the laboratory, and then exposing the cells to factors that differentiate cells such as protein growth and differentiation factors, hormones, and small molecules implicated in causing cells to change from one type of cell into another (differentiation). Using individual cells from the over 200 diversePureStem cell lines previously isolated and cryopreserved, we treated the diverse cells with thousands of differentiation conditions, prepared RNA, and determined the gene expression pattern of the cells using gene expression microarrays. These experiments have shown that thePureStem cell lines display site-specific markers that identify not only the type of cells, but also where in the body the cells would normally reside. Therefore, in the example of cartilage cells, it was possible to produce diverse types of cartilage in this manner. We have licensed from BioTimeour former parent company Lineage PureStemapplications outside of orthopedics, medical aesthetics, and certain ophthalmological applications.
| 12 |
We have chosen twoPureStemapplications for our initial product development based on unmet medical need along with other factors. The first product candidates are Brown Adipose Tissue (BAT)AGEX-BAT1, brown adipose tissue or BAT cells for the treatment of metabolic disorders such as obesity or Type II diabetes, and AGEX-VASC1, vascular endothelial progenitors for the treatment of age-related ischemic disease such as that leading to myocardial ischemiaperipheral vascular disease and infarction. Theseischemic heart disease.
UniverCyte™
Our UniverCyte™ technology uses a proprietary, novel, modified form of HLA-G and is intended to permit donor cells willto be formulated in a delivery matrix designatedHyStem®transplanted into patients without donor-patient tissue matching and without administering immunosuppressant medication. Immunosuppressive drugs can reduce patient resistance to promote viability of the graftinfectious diseases and cancers as well as cause organ and other toxicities. Reducing or eliminating the need for immunosuppressants after cell transplantation by use of hypoimmunogenic cells may make therapies universally available. We plan to localizeuse or license the use of this patented technology to produce genetically-modified master cell banks of pluripotent stem cells to the intended site in the body. See “—Overview” and “—Our Target Market.”
HyStem®Delivery Technology
HyStem®is a patented biomaterial that mimics the extracellular matrix that is the structural network of macromolecules surrounding cells in the body. The extracellular matrix is essential for normal cellular function and survival of transplanted cells. Many tissue engineering and regenerative cell-based therapies are expected to benefit from the delivery of therapeutic cells in a matrix for precise localized delivery and survival.HyStem is a unique hydrogel that has been shown to support cellular attachmentin vivo. Current research at medical institutions has shown thatHyStem is compatible with a wide variety of cells and tissue types including thosecan then be differentiated into any young cell type of the brain, bone, skin, cartilage, vascular system and heart. The technology underlyingHyStem hydrogels was developed athuman body that now express the University of Utah and was been exclusively licensed to BioTime for human therapeutic applications and sublicensed to immune tolerogenic molecule.
AgeX for certain fields. TheHyStem technology is based on a unique thiol cross-linking chemistry to prepare hyaluronan-based matrices as hydrogels. Since the first published report in 2002, there have been numerous academic scientific publications supporting the biocompatibility of thiol cross-linked hyaluronan-based matrices and their applications as medical devices and in cell culture, tissue engineering, and animal models of cell-based therapies.
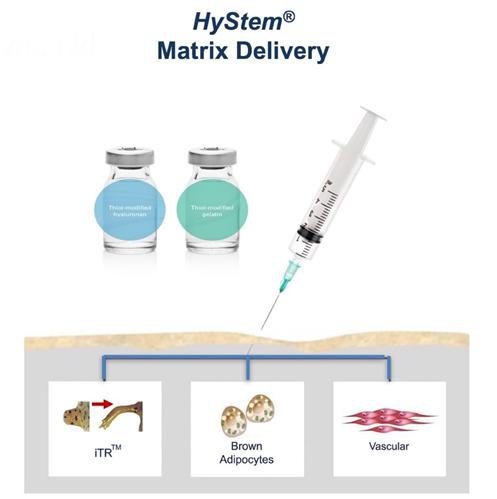
Figure 6. AgeX plans to utilize theHyStem technology for the delivery of cell-based therapeutics.
Due to the unique cross-linking chemistry,HyStem matrices have the ability to be safely combined with living cells and subsequently injected or applied locally as a hydrogel which allows the gel to conform to the three-dimensional contour of a tissue. Building upon this platform, we initially plan to useHyStem for cell-based therapy.
The building blocks forHyStem hydrogels may vary with the application but typically include combinations of hyaluronan, gelatin, or heparin, each of which has been thiol-modified. Hydrogels are formed by cross-linking mixtures of these thiolated macromolecules with polyethylene glycol diacrylate (PEGDA). The rate of gelation and the hydrogel stiffness can be controlled by varying the amount of cross-linker. An important attribute ofHyStemhydrogels is their large water content, over 98%. As a result, these hydrogels have a high permeability for oxygen, nutrients, and other water-soluble metabolites.
Products and Product Candidates
OurAgeX Therapeutic Product Candidates
AGEX-BAT1 - Brown Adipose Tissue (BAT) Progenitors
Brown Adipose Tissueadipose tissue (BAT) is abundant early in life but lost precipitously with age. This tissue is believed to generate heat through expression of a gene calledUCP1.UCP1. In addition, the high levels of glucose and lipid uptake by the tissue is believed to balance metabolism in young people. In contrast, central obesity and Type II diabetes has been correlated with low levels of BAT.

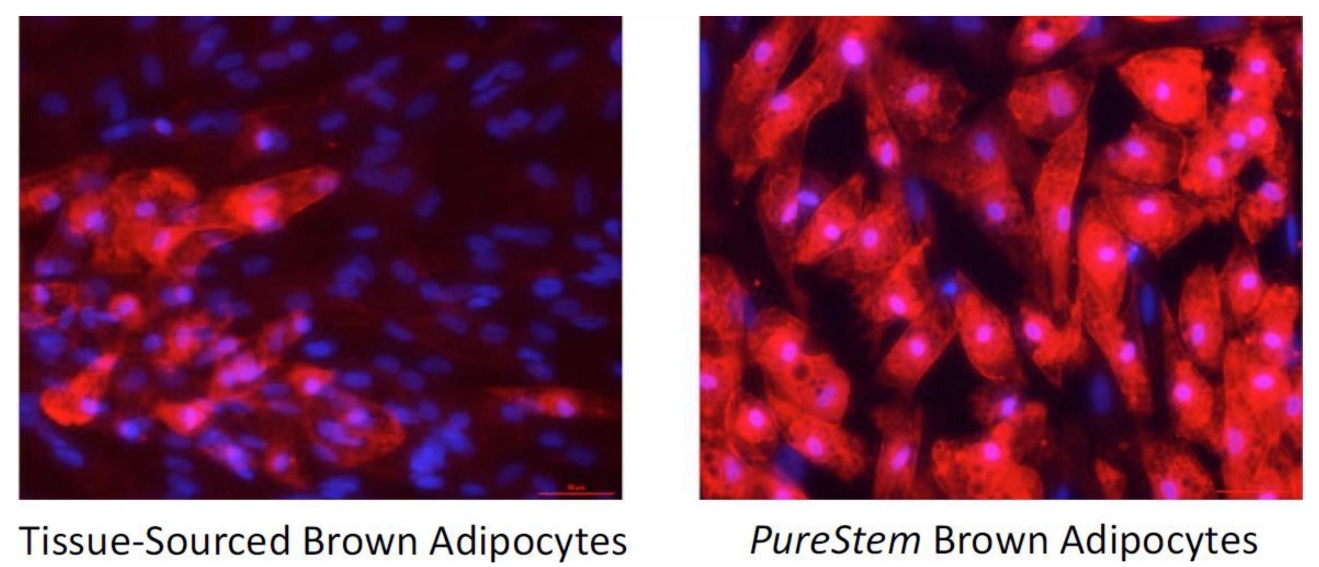
Figure 7.6. Human tissue-derived BAT cells (left) stained red for the presence of UCP1 show a minority of cells being true BAT cells.PureStem-derived PureStem-derived AGEX-BAT1 cells are uniformly UCP1 positive.
The demonstration in published literature in the public domain that the transplantation of BAT from young mice to obese diabetic mice resulted in weight loss and increased insulin sensitivity has led to a search for a source of industrially-scalable clinical grade BAT cells as well as an appropriate matrix for lipotransfer. There currently is no FDA-approved matrix for cell transplantation. However, BioTime has completed a pivotal clinical trial ofHyStembeing developed as a replacement for whole adipose tissue in cell-assisted lipotransfer procedures. Therefore, we believeHyStem can be used for the delivery of BAT cells produced usingPureStemtechnology. As shown in Figure 7,6, theAGEX-BAT1 progenitors strongly express the BAT marker UCP1 when induced to differentiate and show a relatively high degree of purity compared to human tissue-derived BAT.
We entered into a Sponsored Research Agreement with Ohio State University using AGEX-BAT1 in mice to determine whether transplantation of AgeX-BAT1 cells may lead to improvements in diet-induced obesity, metabolic health including glucose metabolism, and cardiac function. For purposes of this proof of concept work, two different cell transplant matrices were tested, HyStem® and a 3-D silk scaffold. We consider this work to be an early stage study and expect to conduct or sponsor additional research on the potential therapeutic benefits of AGEX-BAT1.
| 13 |
A number of new GLP-1 receptor agonist drugs, including Mounjaro, Ozempic, Rybelsus, and Trulicity for treating type 2 diabetes, and Wegovy and Zepbound for weight management, have entered the market. Ozempic is also being used off label for weight loss. The attention and acceptance that these new drugs have attained in the medical field for the treatment of type 2 diabetes and chronic weight management may substantially limit or eliminate the prospects for developing and commercializing any product based on AGEX-BAT1, brown adipose tissue, for those uses. Although the GLP-1 receptor agonist drugs may in certain patients be contraindicated, carry unacceptable medical risks, lead to intolerable side effects, or may not be satisfactorily effective, it is not clear whether those patients would constitute a large enough market for an alternative therapy to warrant the time and expense of developing AGEX-BAT1 for the uses addressed by the products currently on the market. Further, it is likely that the administration of a AGEX-BAT1 cell therapy product would entail a surgical implant procedure which would be expensive and would pose risks to the patient related to the surgical procedure that are not faced by users of the injectable or pill GLP-1 receptor agonist drugs currently on the market.
AGEX-VASC1 - Vascular Progenitors
PureStem® technology can also yield highly purified embryonic vascular components. As shown below, select clonal lines express markers such as VE-Cadherin (CDH5) and PECAM1, as well as VWF and other markers of venous, arterial, and lymphatic endothelium. Flow cytometry shows purity indistinguishable from 100%.
In addition to vascular endothelial cells, we have characterized vascular smooth muscle cell progenitors. This makes it possible for us to construct two of the key cellular components of arterial vessels, such as those compromised in coronary artery disease.
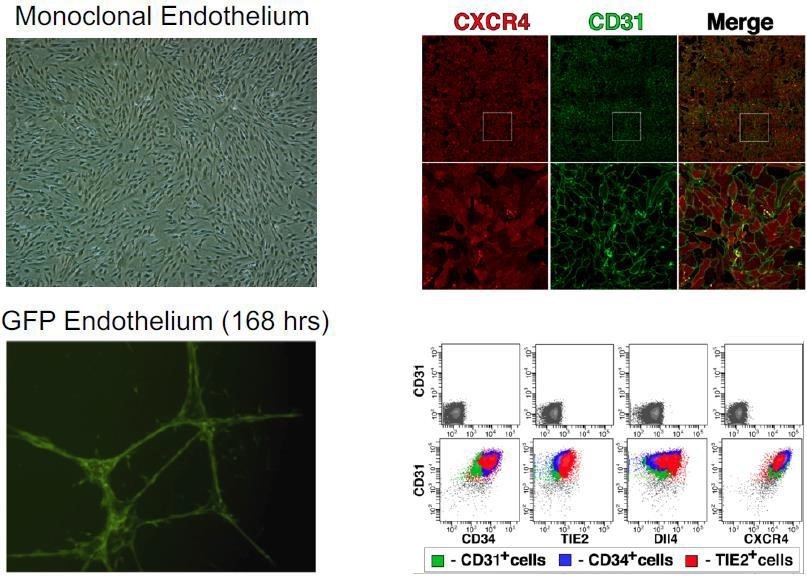
Figure 7. PureStem-derived vascular endothelial cell lines are capable of regenerating young vasculature (bottom left) and appear to have essentially 100% purity by fluorescence activated cell sorting analysis.
Leveraging our assets in pluripotency and bioinformatics, we have performed research manipulating cellular immortality and regenerative biology in human cells. In 2010, our scientists while at Lineage demonstrated the reversal of the developmental aging of human cells using transcriptional reprogramming technology. In 2017, we published certain markers of the Weismann barrier, and the high prevalence of a reversion back before the Weismann barrier in diverse cancer cell types cultured in vitro.
| 14 |
We extended this research to determine whether reprogramming can be modified to only reverse the aging of cells back before the Weismann Barrier, not back to pluripotency. We have utilized for example the gene COX7A1 as a marker of cells that have lost regenerative potential (crossed the Weismann Barrier). As shown in Figure 8, our proprietary formulation AGEX-iTR1547 has demonstrated initial capability of reducing the expression of the marker gene COX7A1 back to before the Weismann Barrier without reverting the cells to pluripotency. When implemented in vivo, this partial reprogramming, or iTR, would be expected to induce tissue regeneration, and when combined with telomerase, may be able to modulate both cellular immortality and regenerative biology for therapeutic effect. In addition to the small molecule product candidate designated iTR1547, we have invented biological interventions based, for example, on gene therapy. Our inventions relating to iTR biologics disclose both DNA and RNA-based strategies. Our gene delivery iTR product candidate is designated iTR1550. We are performing research to optimize AGEX-iTR1547 and in parallel a gene delivery formulation designated AGEX-iTR1550 in order to initiate preclinical studies of one or both of the agents on the scarless regeneration of the skin.

Figure 8. PSCs such as ES Cells and PureStem EP Cells display a regenerative capacity like cells that have not crossed the Weismann Barrier. During pre- and post-natal development, skin cells become increasingly incapable of scarless regeneration as reflected in increasing COX7A1 expression. iPS cell reprograming reverts cells back to pluripotency, while AgeX-iTR1547 reverts cells back only to a point prior to the Weismann Barrier (regenerative state).
Status and Development Plan
The product candidates we have chosen are in the discovery stage of development. Prior to filing an investigational new drug application (IND) for the initiation of clinical trials of our initial product candidates, AGEX-BAT1, AGEX-VASC1, and AGEX-ITR1547/AGEX-iTR1550, a number of important research and development goals will need to be achieved, including discovery-level research for the qualification of reagents used in the manufacture of the product, completion of the standard operating procedures (SOPs) to be used, completion of the methods and documentation for characterization of the product; and producing and testing the genetic modifications in the master cell banks of the pluripotent stem cells under cGMP in order to produce product that will not illicit immune rejection following transplantation. In addition, we will be required to expand the numbers of the pluripotent stem cell master cell banks for future use; produce working cell banks from which the product will be manufactured for clinical trials; produce the relevant product under cGMP conditions; and expand the number of relevant cells and cryopreserve them under cGMP conditions. In addition, we will be required to design the pre-clinical studies including the study endpoints, perform biosafety testing and release the first clinical batch based on preliminary characterization results, and complete full product characterization. Biosafety testing will necessarily include pilot testing in animals such as (NOD/SCID) mice, dosing spiking studies at early and later endpoints, tumorgenicity and biodistribution studies to determine whether the cells form undesired tumors or migrate to inappropriate sites respectively in the animal. Lastly, we will need to define the clinical trial and regulatory strategy and hold various meetings with the U.S. Food and Drug Administration (FDA), as well as successfully submit an IND to the FDA and receive clearance to begin trials. Thereafter, we will need to demonstrate safety and efficacy of the product in human clinical trials in Phase I and II trials, and continued safety and efficacy for achieving the desired endpoint in Phase III trials, potentially then leading to product registration. See “Risk Factors—Risks Related to Our Business Operations” for discussion of risks relating to product development and clinical trials. These include, but are not limited to, failure to successfully complete the aforementioned studies due to the failure of the product, processes, or skills of our employees, unforeseen delays in the development process, failure to raise requisite financing, or failure to receive permission from the FDA to advance product development. To the extent we license development of one or more product candidates to third parties or enter into collaboration arrangements for product development, our licensees or collaborators would need to undertake and achieve the foregoing goals.
| 15 |
Because our product candidates are still in the discovery stage, our choice of product candidates and development plans are subject to change based on a variety of factors. We may determine to abandon the development of one or more of our product candidates, or we may prioritize the development of one or more product candidates, or we may select or acquire and prioritize the development of new product candidates. Our choice and prioritization of product candidates for development will be influenced by a variety of factors, including but not limited to:
| ● | Results of our laboratory research and any animal and clinical trials that we or any licensees or collaborators may conduct; |
| ● | Our ability to enter into licensing or collaborative arrangements with other biotechnology or biopharma companies or universities with their own laboratory facilities and research staffs to conduct research and development of one or more product candidates; |
| ● | Our analysis of third-party competitive and alternative technology that may lead us to conclude that our product candidates or technologies may be non-competitive or obsolete; |
| ● | Our analysis of market demand and market prices for the products we plan to develop could lead us to conclude that market conditions are not favorable for receiving an adequate return on our investment in product development and commercialization; |
| ● | The amount of capital that we will have for our development programs and our projected costs for those programs; |
| ● | The issuance of patents to third parties that might block our use of the same or similar technology to develop a product candidate; and |
| ● | The views of the FDA and comparable foreign regulatory agencies on the pre-clinical product characterization studies required to file an IND in order to initiate human clinical testing of a therapeutic product candidate or to attain marketing approval for that product candidate, or to obtain an investigational device exemption for clinical trials, or clearance for a 510(k) application to market a medical device. |
Other AgeX Products and Product Candidates
Neural Stem Cells
AgeX has sponsored a research and development program at UCI for the manufacture of neural stem cells for use in the treatment of Huntington’s Disease and potentially other neurological diseases and disorders. AgeX has also collaborated with a research group at UCI studying the potential use of exosomes and other extracellular vesicles for the treatment of adverse neurocognitive effects of cancer chemotherapy and radiation therapy on brain function. The neural stem cell sponsored research and development program led to the creation of NeuroAirmid, which is a subsidiary of AgeX co-owned with certain of the UCI researchers and in which UCI will also receive an equity interest as partial consideration for granting to NeuroAirmid a license to use a UCI patent and certain specified technical information, materials, or data (“Associated Technology”) created in the laboratory of the inventors of the licensed patent.
UCI has made an IND submission to the FDA for the use of neural stem cells in a clinical trial for the treatment of Huntington’s Disease. The FDA has removed a clinical hold on the IND permitting a clinical trial to proceed. UCI and NeuroAirmid will apply to the California Institute for Regenerative Medicine (CIRM) for a $12,000,000 CLIN2 grant to fund the proposed clinical trial.
There can be no assurance that: (i) NeuroAirmid and UCI will successfully conclude negotiations and enter into a license agreement providing NeuroAirmid with a license to use a UCI patent needed for NeuroAirmid’s development and production of its planned therapeutic neural stem cell product, (ii) CIRM will provide a grant to finance a clinical trial or that the amount of any grant that may be awarded will ultimately be sufficient to complete the initial phase of the clinical trial; (iii) NeuroAirmid will be able to raise capital needed to finance its operations that will not be funded by a CIRM clinical trial grant or to finance any further clinical trials after any initial CIMR grant funding is exhausted; and (iv) if a clinical trial is commenced, it will lead to the successful clinical development and subsequent FDA marketing approval of a therapeutic cellular product for the treatment of Huntington’s Disease or any other neurological disease or disorder.
| 16 |
ESI BIO Research Products
We, through our ESI BIO research product division, market a number of products related to pluripotent stem cells including research-grade as well as cGMP-grade human PSC lines. We plan to contract with third parties where the third parties to allow them to utilize cGMP PSC lines in defined fields of application in exchange for certain compensation including the payment of royalties to us if they are successful in developing and commercializing a product.
Subsidiaries
AgeX has five subsidiaries, Reverse Bio, ReCyte Therapeutics, Inc. (“ReCyte”), NeuroAirmid, UniverXome Bioengineering, Inc. (“UniverXome”), and Merger Sub. Reverse Bio, ReCyte and NeuroAirmid are early stage pre-clinical research and development companies. UniverXome was organized to hold AgeX assets related to our current core technologies and research and development programs other than NeuroAirmid if the Meger is consummated.
AgeX intends to develop its iTRTM platform and AGEX-BAT1 through Reverse Bio. Reverse Bio will allow for a dedicated focus on iTRTM in terms of equity financing and advancing the iTRTM technology to proof-of-concept in an animal model. AgeX’s patent portfolio for iTR and AGEX-BAT1 development have been assigned to Reverse Bio, but the future operations of Reverse Bio will depend in large measure on its ability to raise its own capital. ReCyte is involved in stem cell-derived endothelial and cardiovascular related progenitor cells for the treatment of vascular disorders and ischemic conditions. AgeX owns 100%, 94.8%, and 50% of the outstanding capital stock of Reverse Bio, ReCyte, and NeuroAirmid, respectively. We expect that our ownership interests in Reverse Bio and NeuroAirmid will be diluted if those subsidiaries are successful in obtaining financing from investors or product development collaborators. All material intercompany accounts and transactions have been eliminated in consolidation.
Manufacturing
We presently do not have any manufacturing facilities and we will need to rely on third party contract manufacturers for the production of our cell lines and product candidates and to comply with quality manufacturing processes and controls.
Facilities
Our principal place of business is located at 1101 Marina Village Parkway, Suite 201, Alameda, California, which we use for office purposes only. We do not have our own research laboratory facilities.
Commercialization Plan
With the exception of our research product sales which generate a trivial amount of revenues, we currently have no commercialized or marketed products such as FDA-approved drugs in our portfolio. As a result, we have not yet assembled an infrastructure for sales and marketing. At the point in time, if ever, that our product candidates approach clearance or approval, we plan to develop a commercial plan that may initially include strategic marketing partnerships.
Intellectual Property
Patents and Trade Secrets
We rely primarily on patents and contractual obligations with employees and third parties to protect our proprietary rights. We have sought, and intend to continue to seek, appropriate patent protection for important and strategic components of our proprietary technologies by filing patent applications in the U.S. and certain foreign countries. There are no assurances that any of our intellectual property rights will guarantee protection or market exclusivity for our products and product candidates. We also use license agreements both to access technologies developed by other companies and universities and to convey certain intellectual property rights to others. Our financial success will be dependent, in part, on our ability to obtain commercially valuable patent claims, to protect and enforce our intellectual property rights, and to operate without infringing upon the proprietary rights of others if we are unable to obtain enabling licenses.
The patents for our core programs are summarized below.
AGEX-BAT1progenitors strongly express the BAT marker UCP1 when induced to differentiate and show a relatively high degree of purity compared to human tissue-derived BAT.
AgeX is currently optimizing process development for the initiation of preclinical development of the use of AGEX-BAT1.
AGEX-VASC1 - Vascular Progenitors
PureStemtechnology can also yield highly purified embryonic vascular components. As shown below, select clonal lines express markers such as VE-Cadherin (CDH5) and PECAM1, as well as VWF and other markers of venous, arterial, and lymphatic endothelium. Flow cytometry shows purity indistinguishable from 100%.
In addition to vascular endothelial cells, we have characterized vascular smooth muscle cell progenitors. This makes it possible for us to construct two of the key cellular components of arterial vessels, such as those compromised in coronary artery disease.

Figure 8.PureStem-derived vascular endothelial cell lines are capable of regenerating young vasculature (bottom left) and appear to have essentially 100% purity by FACs analysis.
HyStemhydrogels have been successfully used as a cell delivery matrix for endothelial progenitor cells to re-establish vasculature in hind limb ischemia models. Therefore, AgeX is currently optimizing process development for planned animal preclinical testing of AgeX-VASC1 formulated inHyStem for delivery into ischemic heart tissue to regenerate collateral circulation.
AGEX-iTR1547— Induced Tissue Regeneration (iTRTM)
Leveraging our assets in pluripotency and bioinformatics, we have performed research manipulating cellular immortality and regenerative biology in human cells. In 2010, BioTime demonstrated the reversal of the developmental aging of human cells using transcriptional reprogramming technology. In 2017, we published certain markers of the Weismann barrier, and the high prevalence of a reversion back before the Weismann barrier in diverse cancer cell types culturedin vitro.
We extended this research to determine whether reprogramming can be modified to only reverse the aging of cells back before the Weismann Barrier, not back to pluripotency or transforming the cells into malignant counterparts. We have utilized for example the geneCOX7A1 as a marker of cells that have lost regenerative potential (crossed the Weismann Barrier).
As shown in Figure 9, our proprietary formulation AGEX-iTR1547 has demonstrated initial capability of reducing the expression of the marker geneCOX7A1back to before the Weismann Barrier without reverting the cells to pluripotency. When implementedin vivo, this partial reprogramming, or iTR, would be expected to induce tissue regeneration, and when combined with telomerase, could modulate both cellular immortality and regenerative biology for therapeutic effect. We are performing research to optimize AGEX-iTR1547 in order to initiate preclinical studies of the agent on the scarless regeneration of the heart during congestive heart failure.

Figure 9. PSCs such as ES Cells andPureStem EP Cells display a regenerative capacity like cells that have not cross the Weismann Barrier. During pre- and post-natal development, skin cells become increasingly incapable of scarless regeneration as reflected in increasingCOX7A1 expression. iPS cell reprograming reverts cells back to pluripotency, while AgeX-iTR1547 reverts cells back only to a point prior to the Weismann Barrier (regenerative state).
Status and Development Plan
The product candidates we are developing are in the discovery stage of development. Prior to filing an Investigational New Drug (IND) application for the initiation of clinical trials of our initial product candidates, AGEX-BAT1, AGEX-VASC1, and AGEX-ITR1547, we will need to complete discovery-level research for the qualification of reagents used in the manufacture of the product, complete the standard operating procedures to be used (SOPs), complete the methods and documentation for characterization of the product, produce and test the genetic modifications in the master cell banks of the pluripotent stem cells under current Good Manufacturing Practices (cGMP) in order to produce product that will not illicit immune rejection following transplantation. In addition, we will be required to expand the numbers of the pluripotent stem cell master cell banks for future use, as well as produce working cell banks from which the product will be manufactured for clinical trials, produce the relevant product under cGMP conditions, expand the number of relevant cells and cryopreserve them under cGMP conditions. In addition, we will be required to design the pre-clinical studies including the study endpoints, perform biosafety testing and release the first clinical batch based on preliminary characterization results, and complete full product characterization. Biosafety testing will necessarily include pilot testing in animals such as (NOD/SCID) mice, dosing spiking studies at early and later endpoints, tumorgenicity and biodistribution studies to determine whether the cells form undesired tumors or migrate to inappropriate sites respectively in the animal. Lastly, we will need to define the clinical trial and regulatory strategy and hold Pre-Pre-IND and Pre-IND meetings with the Food and Drug Administration (FDA), as well as successfully submit an IND to the FDA and receive clearance to begin trials. Thereafter, we will need to demonstrate safety and efficacy of the product in human clinical trials in Phase I and II trials, and continued safety and efficacy for achieving the desired endpoint in Phase III trials, potentially then leading to product registration. See “Risk Factors — Risks Related to Our Business Operations” for discussion of risks relating to our preclinical development and clinical trials. These include, but are not limited to, failure to successfully complete the aforementioned studies due to the failure of the product, processes, or skills of our employees, unforeseen delays in the development process, failure to raise requisite financing, or failure to receive permission from the FDA to advance product development.
Because our product candidates are still in the discovery stage, our choice of product candidates and development plans are subject to change based on a variety of factors. We may determine to abandon the development of one or more of our product candidates, or we may prioritize the development of one or more product candidates, or we may select or acquire and prioritize the development of new product candidates. Our choice and prioritization of product candidates for development will be influenced by a variety of factors, including but not limited to:
Other Products
Other Potential iTR Applications
An additional first generation iTR product candidate that we may develop isRenelonTM, which utilizes a repurposed drug, valproic acid, formatted in a hyaluronic acid based medium.Renelon would not be capable of fully transporting cells back to a regenerative state. However, our gene expression analysis of valproic acid on adult-derived human skin cells and published reports in the scientific literature on the anti-fibrotic effects of valproic acid provide scientific support of the potential use ofRenelon to impart scarless tissue repair in the treatment of wounds or in surgical uses. Although valproic acid has been approved for medical use and is available as a generic drug for certain uses, it has not been approved for the uses we may explore andRenelonhas not been used in clinical trials for the treatment of wounds or in surgical or other tissue repair applications. It is possible thatRenelon, if developed, could be regulated as a medical device rather than as a drug for the use we contemplate, but there is no certainty that the FDA will considerRenelonto be a device.
We believe that iTR may also have applications in the diagnosis and treatment of cancer. We have filed patents on methods of both inducing iTR in cells and maturing them and some of these methods may provide novel diagnostic and therapeutic strategies for cancer.
Online Database Products
We, through our subsidiaries LifeMap Sciences and LifeMap Sciences Ltd, which are collectively referred to as LifeMap Sciences, conduct operations in the U.S. and Israel to commercialize theGeneCards Database Suite, which includes the relational databasesGeneCards®andMalaCardsTM licensed from the YedaResearch and Development Company Ltd., the technology transfer company of theWeizmannInstitute of Science in Rehovot, Israel. TheGeneCards Database Suite had approximately 3.5 million unique users in 2017 from diverse academic and commercial institutions. LifeMap Sciences obtains revenues from advertising as well as subscriptions from commercial entities. LifeMap Sciences also is building a product designatedTGexTM, which provides reports generated by theGeneCards knowledgebase intended for use by health care institutions and containing condensed information on particular genomic profiles of patients.
ESI BIO Research Products
We, through our ESI BIO research product division, market a number of products related to pluripotent stem cells including, research-grade as well as cGMP-grade human PSC lines. We plan to contract with third parties where the third parties to allow them to utilize cGMP PSCl lines in defined fields of application in exchange for certain compensation including the payment of royalties to us if they are successful in developing and commercializing a product.
Subsidiaries
As of, and for the year ended December 31, 2018, AgeX consolidated the following subsidiaries:
| |||||||||||
Manufacturing
Our success will depend in part on our ability to manufacture high quality cells, matrices, and small molecules. Unlike drug manufacturing, this quality needs to be performed at the beginning of the process of using PSCs. Therefore, we have acquired from BioTime cGMP-compatible stem cell lines. We currently operate under a shared facilities agreement with BioTime, but we plan to sublease a facility at which we can establish a cGMP laboratory suitable for manufacturing cell lines and our cell based product candidates. We also will require additional personnel and contracted services to comply with quality manufacturing processes and controls.
Facilities
On March 21, 2019, we entered into a sublease of an office and research facility (the “New Facility”) comprising approximately 23,911 square feet of space in a building in an office and research park at 965 Atlantic Avenue, Alameda, California. We plan to operate our principal offices and research laboratory at the New Facility. The commencement of the sublease and our obligation to pay rent is subject to the conditions that the master landlord approves the sublease, our plans for constructing certain laboratory improvements, and our use of certain reagents in our laboratory in the New Facility (the “Preconditions”).
If the Preconditions to the effectiveness of our sublease are satisfied, the New Facility will replace our use of the laboratory and office facilities that have been provided by BioTime. BioTime has a lease of approximately30,795square feet of rentable space in two buildings located in an office park setting in Alameda, California. Under a Shared Facilities and Services Agreement (the “Shared Facilities Agreement”) with BioTime, we have had use of approximately 2,239 square feet of allocated laboratory and office space at BioTime’s Alameda facility and use of approximately 18,000 square feet of common areas which we share with BioTime and its subsidiaries and affiliates in the same facility. BioTime’s facilities do not provide us with laboratory space for the manufacture of cell lines or our cell based product candidates under cGMP conditions.
If the Preconditions to the sublease of the New Facility are not satisfied and our sublease does not go into effect, we will need to find an alternative facility to lease for the manufacture of cell lines and our cell based product candidates under cGMP conditions and there can be no assurance that we will be able to lease suitable facilities on acceptable terms. In the alternative, we may seek to enter into manufacturing agreements with third parties that have suitable facilities and know-how to manufacture cell lines and product candidate lots for us under cGMP conditions. However, there is no assurance that we will be able to enter into contract manufacturing agreements on acceptable terms.
Commercialization Plan
With the exception of our research product sales which generate a trivial amount of revenues, we currently have no commercialized or marketed products such as FDA-approved drugs in our portfolio. As a result, we have not yet assembled an infrastructure for sales and marketing. At the point in time, if ever, that our product candidates approach clearance or approval, we plan to develop a commercial plan that may initially include strategic marketing partnerships.
Intellectual Property
Patents and Trade Secrets
We rely primarily on patents and contractual obligations with employees and third parties to protect our proprietary rights. We have sought, and intend to continue to seek, appropriate patent protection for important and strategic components of our proprietary technologies by filing patent applications in the U.S. and certain foreign countries. There are no assurances that any of our intellectual property rights will guarantee protection or market exclusivity for our products and product candidates. We also use license agreements both to access technologies developed by other companies and universities and to convey certain intellectual property rights to others. Our financial success will be dependent, in part, on our ability to obtain commercially valuable patent claims, to protect and enforce our intellectual property rights, and to operate without infringing upon the proprietary rights of others if we are unable to obtain enabling licenses.
The patents for our core programs are summarized below.
AGEX-BAT1
Brown Adipose Tissue (BAT) Progenitor Cells: The pending patent applications related to BAT progenitor cells, which are owned by AgeX, include U.S. and international patent applications. The applications are directed to the differentiation of pluripotent stem cells (including hES cells) into progenitor cell types capable of making the cellular components of brown fat. The patents also describe culture and purification methods. The approximate expiration dates of the BAT patents, if issued, will range from 2034 to 2036. The AGEX-BAT1 product may also rely on theHyStem patents, which are described in detail below under the heading “HyStem® Technology”.
AGEX-VASC1
| 17 |
AGEX-VASC1
Vascular Progenitors:Progenitors: The pending patent application pertaining to purified vascular progenitor cells and embryonic vascular components are owned by AgeX or an AgeX subsidiary or licensed from BioTime.Lineage. The patents include U.S. patent applications and are directed to methods to enhance vascular tube networks, compositions of pericyte progenitor cells, compositions of exosomes containing angiogenic molecules, compositions of vascular and lymphatic cells, and methods to culture and purify the cells or components thereof. The approximate expiration dates of the vascular progenitor patents, if issued, range from 2032 to 2038. We plan to file an international patent application claiming priority from a pending US provisional application by the filing deadline, which could lead to a patent that if issued would expire in 2039. The AGEX-VASC1 product may also rely on theHyStem patents, which are described in detail below, under the heading “HyStem® Technology”.
AGEX-iTR1547 and AGEX-iTR1550
Induced Tissue Regeneration (iTRTM): The pending patent applications related to the iTR programs, which are owned by AgeX, include applications pending, for example, in the United States, Australia, Canada, China, Europe, Japan and a pending international patent application. These patents and patent applications are directed to compositions and methods for healing damaged tissue using the iTR treatment methods. The patent applications are also directed to treatment methods by regenerating aging tissue by modulating genes involved in tissue regeneration, including reprogramming cells and tissues back to a regenerative state. The approximate expiration dates of the iTR patents, if issued, will range from 2034 to 2039.2041.
Other AGEX Licensed and Sublicensed Patents
PureStem®Progenitor Cells:Cells: The patents and pending applications related to ourPureStem® technology include patents and applications in the United States, Canada, Europe and Australia. These patents are directed to methods for generating diverse isolated progenitor cell lines which generally do not expressCOX7A1 and combinations of other methods for employing pluripotent stem cell lines suitable for clinical use. The pending applications are directed to clonally purified human embryonic progenitor cell lines and methods for reproducible, large scale production of clonally purified human embryonic progenitor cells, compositions and methods for generating diverse cell types, and assays useful in identifying hES cell lines and pluripotent cells resulting from the transcriptional reprogramming of somatic cells that have embryonic telomere length. The approximate expiration date of thePureStem® issued patents is 2031 and the approximate date of expiration of the pending patents, if issued, will range from 2029 to 2032.
ThePureStem® patent portfolio includes patents and pending applications licensed from Advanced Cell Technology, Inc., which later became Ocata Therapeutics, Inc. (“Ocata”)(Ocata). The Ocata issued patents cover methods for reprogramming animal differentiated somatic cells to undifferentiated cells and methods for producing differentiated progenitor cells using morula-derived or inner cell mass cells from a blastocyst and expire from approximately 20202023 to 2026. The Ocata pending applications relate to methods for the derivation of cells that have a reduced differentiation potential using PSCs, methods for reprogramming animal differentiated somatic cells to undifferentiated cells and methods for producing differentiated progenitor cells using morula-derived or inner cell mass cells from a blastocyst. The Ocata pending patents, if issued, will expire between 20202023 and 2026.
HyStem®Technology: AgeX has a sublicense to theHyStem technology from BioTime and the technology was originally developed by the University of Utah Research Foundation with patents issued in the United States, Canada, Switzerland, Germany, Spain, France, UK, Ireland, Italy, Luxembourg, Monaco, Japan, Australia, and South Africa. The patents have claims covering compositions, pharmaceutical compositions with living cells methods of crosslinking, methods of making, methods of administering the compositions, and the use of the synthetic extracellular matrix in both research and clinical applications. The expiration dates of theHyStem®patents range from 2023 to 2027.
ESI Human Embryonic Stem Cell (hES) (hES) Cell Lines:Lines: AgeX licenses rights to the ES Cell International Pte. Ltd. patent portfolio with patents issued in the United States, Australia, Israel, UK, Singapore, Japan, and applications pending in the US and Europe. The patents are directed to methods for the differentiation of or enhancing the differentiation of stem cells into cardiomyocytes, neural cells, and pancreatic endoderm cells, compositions of pancreatic progenitor cells, methods of promoting the attachment, survival and/or proliferation of substantially undifferentiated stem cells in culture, methods for identifying and selecting cardiomyocytes, methods of freezing stem cells or progenitor cells, methods for identifying cardiogenic factors, compositions and methods for modulating spontaneous differentiation of a stem cell, methods of modulating the differentiation of undifferentiated, pluripotent human embryonic stem cells in culture, isolated endodermal progenitor cells, methods for transducing human embryonic stem cells, cell culture systems. The pending applications are directed to methods for the differentiation of hES cells into the three cell lineages, including for example cardiomyocytes, skeletal muscle cells, vascular endothelial cells, and pancreatic endoderm cells, as well as various culture and purification methods and compositions and methods of treatment. The ESI issued patents will expire from 20192023 to 2027, and the approximate date of expiration of the pending patents, if issued, will range from 20222023 to 2027.
UniverCyteTM Human Leukocyte Antigen-G (HLA-G) Technology:Technology: In August 2018, we acquired from Escape Therapeutics patents and patent applications related to HLA-G-modifiedcells and methods of generating allogeneic cells with reduced risk of being rejected by patients regardless of the HLA class I haplotype. The patents and pending application related to our HLA-G modified cells technology include patents issued in the United States, Australia and Japan and applications are pending in the United States, Australia, Canada, China, Europe, Japan, Korea, and Singapore. The patents are directed to cells which are genetically modified to express a Human Leukocyte Antigen-G (HLA-G)HLA-G and have reduced immunogenicity, and improved immunosuppression, and nucleic acid compositions useful for generating the genetically modified cells. The pending applications are directed to compositions and methods for generating cells which are genetically modified to express HLA-G having reduced immunogenicity, and improved immunosuppression, nucleic acid compositions useful for generating the genetically modified cells, and methods of producing artificial tissues using the genetically modified cells. The approximate expiration date of the UniverCyte™ (HLA-G)HLA-G issued patents is 2033 and the approximate date of expiration of the pending patents, if issued, will also be 2033. We intend to use the UniverCyte™ technology in the development of our two lead product candidates, AGEX-BAT1 and AGEX-VASC1 for the treatment of Type II diabetes and cardiovascular aging,peripheral vascular disease and ischemic heart disease, respectively. In addition, we may seek to license out or form collaborations for the use of our UniverCyte™ technology.
| 18 |
General Risks Related to Obtaining and Enforcing Patent Protection
There is a risk that any patent applications that we file and any patents that we hold or later obtain could be challenged by third parties and be declared invalid or infringing on third partythird-party claims. Litigation, interferences, oppositions, inter partes reviews or other proceedings are, have been and may in the future be necessary in some instances to determine the validity and scope of certain of our proprietary rights, and in other instances to determine the validity, scope or non-infringement of certain patent rights claimed by third parties to be pertinent to the manufacture, use or sale of our products. We may also face challenges to our patent and regulatory protections covering our products by third parties, including manufacturers of generics and biosimilars that may choose to launch or attempt to launch their products before the expiration of our patent or regulatory exclusivity. Litigation, interference, oppositions, inter partes reviews, administrative challenges or other similar types of proceedings are unpredictable and may be protracted, expensive and distracting to management. The outcome of such proceedings could adversely affect the validity and scope of our patent or other proprietary rights, hinder our ability to manufacture and market our products, require us to seek a license for the infringed product or technology or result in the assessment of significant monetary damages against us that may exceed any amounts that we may accrue on our consolidated financial statements as a reserve for contingent liabilities. An adverse determination in a judicial or administrative proceeding or a failure to obtain necessary licenses could prevent us from manufacturing or selling our products. Furthermore, payments under any licenses that we are able to obtain would reduce our profits derived from the covered products and services.
The enforcement of patent rights often requires litigation against third-party infringers, and such litigation can be costly to pursue. Even if we succeed in having new patents issued or in defending any challenge to issued patents, there is no assurance that our patents will be comprehensive enough to provide us with meaningful patent protection against our competitors.
In addition to relying on patents, we rely on trade secrets, know-how, and continuing technological advancement to maintain our competitive position. We have entered into intellectual property, invention, and non-disclosure agreements with our employees, and it is our practice to enter into confidentiality agreements with our consultants. There can be no assurance, however, that these measures will prevent the unauthorized disclosure or use of our trade secrets and know-how, or that others may not independently develop similar trade secrets and know-how or obtain access to our trade secrets, know-how, or proprietary technology.
Our Licensing Arrangements
License Agreement with BioTime:Lineage: iTRTM, PureStem®and Telomere Length
Concurrently with the contribution of assets to us by BioTimeLineage under an Asset Contribution and Separation Agreement, we entered into a License Agreement with BioTimeLineage pursuant to which BioTimeLineage has licensed to us, with rights to sublicense, certain intellectual property, including patents and patent applications and know-how for use in the development, manufacture and commercialization of products or services for the prevention, treatment, amelioration, diagnosis or monitoring of all human and non-human animal diseases and conditions except for the field of medical products, devices and services for the reserved BioTime fieldsLineage Exclusive Field of orthopedic, ophthalmic, and medical aesthetic uses (the “BioTime Exclusive Field”).uses. In addition, BioTimeLineage retains an option right, on terms to be negotiated, to license iTR patents in research, development, manufacturing and commercialization of treatments based on iTR in the BioTimeLineage Exclusive Field. The licensed patents and know-how relate generally to (a) BioTime’sLineage’s PureStem®human embryonic progenitor cell lines, and (b) telomere length and DNA quality control analysis in pluripotent stem cells.
The BioTimeLineage patent rights licensed to us are exclusive and worldwide except for existing third partythird-party licenses, and for medical products, devices, and services related to tendon. We additionally received an option to license certain BioTimeLineage retained rights outside of orthopedic indications unless a license grant would compete with a BioTimeLineage program or products in the BioTimeLineage Exclusive Field.
The License Agreement contains customary provisions pertaining to patent maintenance, enforcement, and defense and related cost allocations, insurance, indemnification, and termination of the license in the event of a breach or default by a party, or the bankruptcy or other insolvency event with respect to a party.
Additional License and Sublicense Agreements
BioTimeLineage and certain BioTimeLineage subsidiaries also entered into agreements pursuant to which they have licensed or sublicense to us, on a non-exclusive, world-wide, royalty bearing basis, certain additional patents and patent rights and know-how relating to BioTimeHyStem®hydrogel technology, human embryonic progenitor cell technology and human pluripotent stem cell lines and technology for use outside the BioTimeLineage Exclusive Fields, or in the case of certain sublicense rights, fields previously licensed to third parties.
| 19 |
Hydrogel Patent License and Sublicense
Sublicense of Certain Progenitor Patents
BioTime
Lineage has granted to us a sublicense of certain patents licensed to BioTime by the University of Utah Research Foundation (the “Utah Sublicense”), and has granted to us a direct license of certain patents held by BioTime (the “HyStem License”), related toHyStem® hydrogel technology for use outside of the BioTime Exclusive Field for products that include cells and that are covered by certain other patents contributed, licensed, or sublicensed to us by BioTime. We may only develop, sell, and otherwise commercialize a product under the Utah Sublicense and HyStem License if we spend at least a low seven figure amount on research with respect to the product. BioTime will agree to provide us with a reasonable amount of the hydrogel product for the purpose of our research for we will pay BioTime’s cost of manufacturing and supplying the hydrogel.
The Utah Sublicense and the HyStem License will not permit sublicensing and will be non-exclusive for medical products, devices, and services related to human tendon, and will be exclusive for all other licensed fields. The Utah Sublicense and HyStem License will expire upon the latest expiration date of a sublicensed or licensed patent, unless terminated earlier pursuant to the respective agreements. We will pay BioTime a royalty, in an amount not exceeding 10 percent, on “net sales” as defined in the Utah Sublicense and HyStem License. Commencing June 30, 2019, and for each 12-month period thereafter, we will pay BioTime a minimum royalty in the low five figures regardless of the actual amount of net sales for the applicable period.
The foregoing description of the HyStem License and the Utah Sublicense is qualified in its entirety by reference to the HyStem License Agreement and the Utah Sublicense Agreement, copies of which are filed as Exhibits to our Registration Statement on Form 10 and are incorporated herein by reference.
Sublicense of Certain Progenitor Patents
BioTime has granted to us a sublicense of certain patents licensed to BioTimeLineage that pertain to the derivation of human embryonic progenitor cell lines. The sublicense will permit us to use the sublicensed patents for the treatment, palliation, diagnosis, or prevention of any disease, disorder or health condition outside of the BioTimeLineage Exclusive Field. The sublicense expires the later of July 10, 2028 or the latest expiration date of a sublicensed patent, unless terminated earlier pursuant to the terms of the sublicense.
We will pay BioTimeLineage a royalty on “net sales,” as defined in the sublicense agreement, until the royalty payments to BioTime’sLineage’s licensor by BioTimeLineage total $1.2 million and thereafter will pay to BioTimeLineage a low single digit royalty on its own net sales and a low double digit royalty on sublicensing consideration.
If we grant a sublicense to use the patents, we will pay BioTimeLineage a portion of any consideration received for a sublicense, including but not limited to, upfront payments and milestones, and non-cash exchanges or considerations, but not payments for developing a product, service or process. If we become obligated to pay royalties to one or more affiliates of BioTimeLineage for the use of patent rights related to this sublicense and as a result, the royalties payable to BioTimeLineage with respect to royalties under the sublicense plus the royalties payable to the affiliates would exceed a designated amount of net sales, the royalties due to BioTimeLineage may be reduced but not less than the designated amount. In addition, we will pay to BioTimeLineage a royalty on “net sales,” as defined in the sublicense agreement, by the sublicensee. If we become obligated to pay royalties to one or more affiliates of BioTimeLineage for the use of patent rights related to this sublicense and as a result, the royalties payable to BioTimeLineage with respect to sales by a sublicensee plus the royalties payable to the affiliates would exceed a designated amount of net sales, the royalty due on net sales by the sublicensee may be reduced but not less than the designated amount.
The sublicense agreement includes reciprocal cross-licenses between BioTimeLineage and us with respect to any new patents that may be issued based on the use of the sublicensed patents. Any such license to BioTimeLineage will be exclusive in the BioTimeLineage Exclusive Field and nonexclusive in all other licensed fields. Any such license from BioTimeLineage to us will be for use outside the BioTimeLineage Exclusive Field and for medical products or services involving tendon. Each license will be for a term of 10 years.
The foregoing description of the sublicense agreement is qualified in its entirety by reference to the sublicense agreement, a copy of which is filed as an exhibit to our Registration Statement on Form 10 and is incorporated herein by reference.
ESI License
BioTime’sLineage’s subsidiary ES Cell International Pte or ESI,(ESI) has granted to us non-exclusive rights to certain ESI patents and human pluripotent stem cell lines, or ESI Cell Lines, for use outside of the BioTimeESI Exclusive Field of orthopedic, ophthalmic, medical aesthetic, and spinal cord injury uses, and outside certain other fields for which ESI has previously granted licenses. We will pay ESI a 2% royalty in an amount not exceeding 10 percent, on “net sales,” as defined in the license agreement. If we become obligated to pay royalties to one or more third party or to BioTimeLineage for the use of patent rights related to this license and as a result the royalties payable to ESI with respect to this license agreement plus the royalties payable to such third party or BioTimeLineage would exceed a designated amount of net sales, the royalty due on net sales by the sublicensee may be reduced. The patent license expires upon the latest expiration date of a licensed patent, unless terminated earlier pursuant to the terms of the license. All other rights under the license are terminable by either party under the conditions specified in the license.
If we grant rights to any third party to use ESI Cell Lines derived under cGMP, we will pay ESI a share of all consideration that we receive as consideration for the grant of those rights, including all cash and non-cash consideration but not royalties. We are not permitted to grant sublicenses to the licensed ESI patents but may sublicense the use of ESI Cell Lines.
The foregoing descriptionAgeX also will pay ESI 5% of the ESI License Agreement is qualified in its entirety by referenceany fees that AgeX may receive for providing third parties with a “drug master file” for submission to the ESI License Agreement, a copyFDA or similar regulatory agencies in other jurisdictions that may be used to provide confidential detailed information about facilities, processes or articles used in the manufacturing, processing, packaging and storing of which is filed as an exhibitone or more human drugs, including but not limited to our Registration Statement on Form 10biologics, cell lines and is incorporated herein by reference.cell products.
| 20 |
Competition
Competition
The biotechnology industry is highly competitive and characterized by rapid change (even disruptive advances) that challenge the ability of any one company to maintain leadership. Therefore, we face competition on multiple fronts, including from other biotechnology companies, large pharmaceutical companies, academic institutions and government research entities. We believe the competitive advantages of our technology platform and resulting product candidates arise from the large market opportunities addressed by our product candidates, their anticipated safety profile, the expected cost of manufacture of off-the-shelf products, our intellectual property, as well the fundamental and widespread role of cell aging and regeneration in human age-related degenerative disease.
There are numerous biotechnology companies developing therapeutics for human aging, with each company often focusing on a specific molecular pathway within cells. For example, ResTORbio, Inc. is developing modulators of themechanistic target of rapamycin (mTOR) pathway to treat immunological and cardiovascular disorders. Calico Life Sciences LLC is a Google-founded research and development company aimed at identifying molecular pathways that control animal lifespan and translating these insights into novel therapeutics designed to increase human healthspan. Calico has not disclosed its lead product development plans. Unity Biotechnology, Inc. focuses on cellular senescence, in particular, the use of agents that can target senescent cells for selective ablation (senolysis). Unity’s stated targeted age-related diseases include osteoarthritis as well as other ophthalmological and pulmonary diseases. In addition, Altos Labs, Inc. (Altos) has reportedly received funding commitments in excess of $3 billion for research and development of products relating to age-reprogramming. The initial technology focus disclosed by Altos may compete with the iTR program within AgeX and its subsidiary Reverse Bio.
Our therapeutic product candidates in development are likely to face competition from a large number of companies and technological strategies including therapeutics intended to address our lead indications, including:
| ● | Type II diabetes: current standard of care treatments (though not necessarily focused on the root cause of the disease) include dieting and exercise programs to reduce weight, or pharmacological interventions with a wide array of medications, including: Metformin (Glucophage, Glumetza, or others); |
| ● | ||
| Vascular | ||
Many of our competitors have greater financial, collaborative, technical, regulatory, and human resources as well as products more advanced in development than our product pipeline, including products already marketed for our target indications. As a result, these competitors may have great success in obtaining regulatory approvals, reimbursement, or market acceptance. Our competitors, may have greater success in attracting qualified personnel, recruiting clinical trial sites, or in establishing strategic partnerships with larger pharmaceutical companies to fund large late-stage clinical trials or product marketing. In addition, our future business could be limited should our competitors commercialize products demonstrated to be more effective, safer, or less expensive than our comparable products.
| 21 |
Government Regulation and Product Approval
Government authorities at the federal, state, and local level, and in other countries, extensively regulate among other things, the development, testing, manufacture, quality, approval, safety, efficacy, distribution, labeling, packaging, storage, record keeping, marketing, import/export, and promotion of drugs, biologics, and medical devices. Authorities also heavily regulate many of these activities for human cells, tissues, and cellular and tissue-based products (“HCT/Ps”)(HCT/Ps).
FDA and Foreign Regulation of Therapeutic Products
The FDA and foreign regulatory authorities will regulate our proposed products as drugs, biologicals, or medical devices, depending upon such factors as: the use to which the product will be put, the chemical composition of the product, and the interaction of the product with the human body. In the United States, the FDA regulates drugs and biologicals under the Federal Food, Drug and Cosmetic Act (“FDCA”)(FDCA), the Public Health Service Act (“PHSA”)(PHSA), and implementing regulations. In addition, establishments that manufacture human cells, tissues, and cellular and tissue-based products are subject to additional registration and listing requirements, including current good tissue practice regulations. To the extent AgeX develops cellular and tissue-based products or therapies, its products will be subject to review by the FDA staff in its Center for Biologics Evaluation and Research (“CBER”)(CBER) Office of Cellular, Tissue, and Gene Therapies. In some instances, AgeX’s clinical study protocol for a cell therapy product must be reviewed by the National Institute of Health through its Recombinant DNA Advisory Committee.
Any human drug and biological products that we may develop for testing, marketing, or use in the United States will be subject to rigorous FDA review and approval procedures. After testing in animals to evaluate the potential efficacy and safety of the product candidate, an investigational new drug (“IND”)IND submission must be made to the FDA to obtain authorization for human testing. Extensive clinical testing, which is generally done in three phases, must then be undertaken at a hospital or medical center to demonstrate optimal use, safety, and efficacy of each product in humans. Each clinical study is conducted under the auspices of an independent Institutional Review Board (“IRB”)institutional review board (IRB). The IRB will consider, among other things, ethical factors, the safety of human subjects, and the possible liability of the institution.
Clinical trials are generally conducted in three “phases.” Phase I clinical trials are conducted in a small number of healthy volunteers or volunteers with the target disease or condition to assess safety. Phase II clinical trials are conducted with groups of patients afflicted with the target disease or condition in order to determine preliminary efficacy, optimal dosages and expanded evidence of safety. In some cases, an initial trial is conducted in diseased patients to assess both preliminary efficacy and preliminary safety, in which case it is referred to as a Phase I/II trial. Phase III trials are large-scale, multi-center, comparative trials and are conducted with patients afflicted with the target disease or condition in order to provide enough data to demonstrate the efficacy and safety required by the FDA. The FDA closely monitors the progress of each of the three phases of clinical testing and may, at its discretion, re-evaluate, alter, suspend, or terminate the clinical trial based upon the data which have been accumulated to that point and its assessment of the risk/benefit ratio to the intended patient population. All adverse events must be reported to the FDA. Monitoring of all aspects of the study to minimize risks is a continuing process. The time and expense required to perform this clinical testing can far exceed the time and expense of the research and development initially required to create the product.
No action can be taken to market any therapeutic product in the U.S. until an appropriate New Drug Application (“NDA”)(NDA) or Biologics License Application (“BLA”)BLA has been approved by the FDA. Submission of the application is no guarantee that the FDA will find it complete and accept it for filing. If an application is accepted for filing, following the FDA’s review, the FDA may grant marketing approval, request additional information, or deny the application if it determines that the application does not provide an adequate basis for approval. FDA regulations also restrict the export of therapeutic products for clinical use prior to FDA approval. To date, the FDA has not granted marketing approval to any pluripotent stem-based therapeutic products and it is possible that the FDA or foreign regulatory agencies may subject our product candidates to additional or more stringent review than drugs or biologicals derived from other technologies.
The FDA offers several programs to expedite development of products that treat serious or life-threatening illnesses and that provide meaningful therapeutic benefits to patients over existing treatments. A product may be eligible for breakthrough therapy designation if it treats a serious or life-threatening disease or condition and preliminary clinical evidence indicates it may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. In 2017, FDA established a new regenerative medicine advanced therapy (“RMAT”)(RMAT) designation as part of its implementation of the 21st Century Cures Act. An RMAT is defined as a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products, with limited exceptions that is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition; and preliminary clinical evidence indicates that it has the potential to address unmet medical needs for such a disease or condition. RMAT designation provides potential benefits that include more frequent meetings with FDA to discuss the development plan for the product candidate and eligibility for rolling review and priority review. Products granted RMAT designation may also be eligible for accelerated approval on the basis of a surrogate or intermediate endpoint reasonably likely to predict long-term clinical benefit, or reliance upon data obtained from a meaningful number of sites, including through expansion to additional sites. Once approved, when appropriate, the FDA can permit fulfillment of post-approval requirements under accelerated approval through the submission of clinical evidence, clinical studies, patient registries, or other sources of real world evidence such as electronic health records; through the collection of larger confirmatory datasets; or through post-approval monitoring of all patients treated with the therapy prior to approval.
| 22 |
Some of our future products may be eligible for RMAT designation. There is no assurance that the FDA will grant breakthrough therapy, accelerated approval or RMAT status to any of our product candidates.
In addition to regulations in the United States, we are subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a drug candidate, we must obtain approval by the comparable regulatory authorities of foreign countries or economic areas, such as the European Union, before we can commence clinical trials or market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
Combination Products
If we develop any products that are used with medical devices, they may be considered combination products, which are defined by the FDA to include products comprised of two or more regulated components or parts such as a biologic and a device. For example, we may useHyStem® hydrogels to administer one or more pluripotent stem cell-based therapy products. When regulated independently, biologics and devices each have their own regulatory requirements. However, regulatory requirements for a combination product comprised of a biologic administered with a delivery device can be more complex, because in addition to the individual regulatory requirements for each component, additional combination product regulatory requirements may apply.
510(k) Medical Devices & Notification
Product marketing in the U.S. for most Class II and limited Class I devices typically follows a 510(k) pathway. To obtain 510(k) clearance, a manufacturer must submit a premarket notification demonstrating that the proposed device is substantially equivalent to a legally marketed device, referred to as the predicate device. A predicate device may be a previously 510(k) cleared device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for submission of PMA applications, or a product classification created by FDA when it granted de novo authorization. The manufacturer must show that the proposed device has the same intended use as the predicate device, and it either has the same technological characteristics, or it is shown to be equally safe and effective and does not raise different questions of safety and effectiveness as compared to the predicate device.
There are three types of 510(k)s: traditional; special, for devices that are modified and the modification needs a new 510(k) but the modification does not affect the intended use or alter the fundamental scientific technology of the device; and abbreviated, for devices that conform to a recognized standard. The special and abbreviated 510(k)s are intended to streamline review. The FDA intends to process special 510(k)s within 30 days of receipt and abbreviated 510(k)s within 90 days of receipt. Though statutorily required to clear a traditional 510(k) within 90 days of receipt, the clearance pathway for traditional 510(k)s can take substantially longer.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require a PMA approval. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If the FDA disagrees with a manufacturer’s decision not to seek a new 510(k) clearance, the agency may retroactively require the manufacturer to seek 510(k) clearance or PMA approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or PMA approval is obtained.
Post-Approval Matters
Even after initial FDA approval has been obtained, further studies may be required to provide additional data on safety or to gain approval for the use of a product as a treatment for clinical indications other than those initially targeted. Data resulting from these clinical trials may result in expansions or restrictions to the labeled indications for which a product has already been approved.
FDA Regulation of Manufacturing
The FDA regulates the manufacturing process of pharmaceutical products, human tissue and cell products, and medical devices, requiring that they be produced in compliance with cGMP. The FDA regulates and inspects equipment, facilities, laboratories, and processes used in the manufacturing and testing of products prior to providing approval to market products. If after receiving approval from the FDA, a material change is made to manufacturing equipment or to the location or manufacturing process, additional regulatory review may be required. The FDA also conducts regular, periodic visits to re-inspect the equipment, facilities, laboratories and processes of manufacturers following an initial approval. If, as a result of those inspections, the FDA determines that that equipment, facilities, laboratories or processes do not comply with applicable FDA regulations and conditions of product approval, the FDA may seek civil, criminal or administrative sanctions and/or remedies against the manufacturer, including suspension of manufacturing operations. Issues pertaining to manufacturing equipment, facilities or processes may also delay the approval of new products undergoing FDA review.
| 23 |
Federal Funding of Research
Effective July 7, 2009, the National Institutes of Health (“NIH”)The NIH has adopted guidelines on the use of hES cells in federally funded research, consistent with President Obama’s Executive Order which rescinded President Bush’s Executive Orders that permitted federal funding of research on hES cells using only the limited number of hES cell lines. The central focus of the guidelines is to assure that hES cells used in federally funded research are derived from human embryos that were created for reproductive purposes, are no longer needed for this purpose, and are voluntarily donated for research purposes with the informed written consent of the donors. Those hES cells that were derived from embryos created for research purposes rather than reproductive purposes, and other hES cells that were not derived in compliance with the guidelines, are not eligible for use in federally funded research.
California State Regulations
The state of California has adopted legislation and regulations that require institutions that conduct stem cell research to notify, and in certain cases obtain approval from, a Stem Cell Research Oversight Committee (“SCRO Committee”)(SCRO Committee) before conducting the research. Under certain California regulations, all hES cell lines that will be used in our research must be acceptably derived. California regulations further require certain records to be maintained with respect to stem cell research and the materials used. AgeX programs that involve the use of stem cells will be reviewed by a SCRO Committee to confirm compliance with federal and state guidelines. The hES cell lines that we use are all on the NIH registry of lines that have been reviewed and meet standards for federal funding grants.
Health Insurance Portability and Accountability Act
Under the Health Insurance Portability and Accountability Act (“HIPAA”), the Department of Health and Human Services (“HHS”)HIPAA, HHS has issued regulations to protect the privacy and security of protected health information used or disclosed by health care providers. HIPAA also regulates standardization of data content, codes, and formats used in health care transactions and standardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include civil and criminal penalties.
The requirements under these regulations may periodically change and could have an effect on our business operations if compliance becomes substantially more costly than under current requirements. New laws governing privacy may also be adopted in the future. We can provide no assurance that we will remain in compliance with diverse privacy requirements in all of the jurisdictions in which we do business. Failure to comply with privacy requirements could result in civil or criminal penalties, which could have a materially adverse effect on our business.
Federal and State Fraud and Abuse Laws
We are also subject to various laws pertaining to healthcare “fraud and abuse,” including anti-kickback laws and false claims laws. Anti-kickback laws make it illegal to solicit, offer, receive or pay any remuneration in exchange for or to induce the referral of business, including the purchase or prescription of a particular drug that is reimbursed by a state or federal program. False claims laws prohibit knowinglyThe term “remuneration” has been broadly interpreted to include anything of value. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the Anti-Kickback Statute has been violated. The Patient Protection and willingly presentingAffordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act, collectively the Affordable Care Act (ACA), among other things, amended the intent requirement of the federal Anti-Kickback Statute such that a person or causingentity no longer needs to be presented for paymenthave actual knowledge of the statute or specific intent to third-party payers (including Medicare and Medicaid) any claims for reimbursed drugs or services that are false or fraudulent, claims for items or services not provided as claimed or claims for medically unnecessary items or services.violate, in order to commit a violation. Violations of fraud and abuse laws may be punishable by criminal and/or civil sanctions, including fines and civil monetary penalties, as well as by the possibility of exclusion from federal healthcare programs (including Medicare and Medicaid). Liability under the false claims laws may also arise when a violation of certain laws or regulations related to the underlying products (e.g., violations regarding improper promotional activity or unlawful payments) contributes to the submission of a false claim.
Additionally, the U.S. Foreign Corrupt Practices Act (“FCPA”)FCPA prohibits U.S. corporations and their representatives from offering, promising, authorizing or making payments to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business abroad. The scope of the FCPA includes interactions with certain healthcare professionals in many countries. Other countries have enacted similar anti-corruption laws and/or regulations.
Healthcare Reform
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes to the healthcare system that could affect our future results of operations. There have been and continue to be a number of initiatives at the United States federal and state levels that seek to reduce healthcare costs.
| 24 |
In particular, the Affordable Care Act (“ACA”)ACA has had, and is expected to continue to have, a significant impact on the healthcare industry. The ACA was designed to expand coverage for the uninsured while at the same time containing overall healthcare costs. Substantial new provisions affecting compliance have also been enacted, which may require us to modify our business practices with healthcare providers and entities, and a significant number of provisions are not yet, or have only recently become, effective.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the ACA, as well as efforts by the current administration to repeal or replace certain aspects of the ACA. For example, since January 2017, the President has signed two Executive Orders and other directives designed to delay, circumvent, or loosen certain requirements mandated by the ACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the ACA. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under the ACA were signed into law. The Tax Cuts and Jobs Act of 2017, or the Tax Act, includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” Additionally, on January 22, 2018, the President signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain ACA-mandated fees, including the so-called “Cadillac” tax on certain high cost employer-sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share, and the medical device excise tax on non-exempt medical devices. Further, the Bipartisan Budget Act of 2018, or the BBA, among other things, amends the ACA, effective January 1, 2019, to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole,” and increase from 50% to 70% the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in the Medicare Part D program. Therethere may be additional challenges and amendments to the ACA in the future. The ACA is likelyfuture, including efforts to continueimplement changes to the downward pressure on pharmaceutical pricinglaw that may impact reimbursement for drugs and may also increase our regulatory burdens and operating costs.biologics.
Further, there has been heightened government scrutiny over the manner in which manufacturers set prices for their marketed pharmaceutical products. Such scrutiny has resulted in several recent Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to pharmaceutical product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. At the federal level, the current administration’s budget proposal for fiscal year 2019 contains further drug price control measures that could be enacted during the 2019 budget process or in other future legislation, including,Such proposals have included, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B and to allow some states to negotiate drug prices under Medicaid, and to eliminate cost sharing for generic drugs for low-income patients. Additionally, on May 11, 2018, President Trump laid out his administration’s “Blueprint to Lower Drug Prices and Reduce Out-of-Pocket Costs” to reduce the cost of prescription drugs while preserving innovation and cures.Medicaid. The Department of Health and Human Services has already started the process of soliciting feedback on some of these measures and, at the same, is immediately implementing others under its existing authority. Although some of these and other proposals will require authorization through additional legislation to become effective, Congress and the Trump administration have each indicated that it willPresident are likely to continue to seek new legislative and/or administrative measures to control drug costs. At the state level, legislatures have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
It is uncertain whether and how future legislation, whether domestic or foreign, could affect prospects for our product candidates or what actions foreign, federal, state, or private payors for health care treatment and services may take in response to any such health care reform proposals or legislation. Adoption of price controls and other cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures reforms may prevent or limit our ability to generate revenue, attain profitability or commercialize our product candidates.
Moreover, the Drug Supply Chain Security ActDSCSA imposes new obligations on manufacturers of pharmaceutical products, among others, related to product tracking and tracing. While some requirements of the DSCSA began in November 2014, many key requirements, development of standards, and the system for product tracing which is beingwill continue to be phased in over several years beginning in 2015.until 2023. Among the requirements of this new legislation,the DSCSA, manufacturers will be required to provide certain information regarding the drug product to individuals and entities to which product ownership is transferred, label drug product with a product identifier, and keep certain records regarding the drug product. The transfer of information to subsequent product owners by manufacturers will eventually be required to be done electronically. Manufacturers will also be required to verify that purchasers of the manufacturers’ products are appropriately licensed. Further, under this new legislation, manufacturers will have drug product investigation, quarantine, disposition, and notification responsibilities related to counterfeit, diverted, stolen, and intentionally adulterated products, as well as products that are the subject of fraudulent transactions or whichthat are otherwise unfit for distribution such that they would be reasonably likely to result in serious health consequences or death.
Reimbursement
Medicare, Medicaid, and Third-Party Reimbursement Programs
Sales of the therapeutic products and medical devices that we and our subsidiaries may develop will depend, in part, on the extent to which the costs of those products will be covered by third-party payors, such as government health programs, commercial insurance, and managed healthcare organizations.
The containment of healthcare costs has become a priority of federal and state governments and the prices of drugs have been a focus in this effort. In the United States, the federal and many state governments have adopted or proposed initiatives relating to Medicaid and other health programs that may limit reimbursement or increase rebates that providers are required to pay to the state. The ACA increased many of the mandatory discounts and rebates and imposed a new Branded Prescription Pharmaceutical Manufacturers and Importers fee payable by manufacturers. Provisions of the Inflation Reduction Act of 2022 may impact the prices of drug products that are sold in the United States, particularly through Medicare programs. Starting in 2023, a manufacturer of a drug or biological product covered by Medicare Parts B or D must pay a rebate to the federal government if the product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by Medicare Parts B or D. Additionally, starting in payment year 2026, CMS will negotiate drug prices annually for a select number of single source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such drug will decrease.
| 25 |
In addition to government regulation, managed care organizations in the United States, which include medical insurance companies, medical plan administrators, health-maintenance organizations, hospital and physician alliances and pharmacy benefit managers, continue to put pressure on the price and usage of healthcare products. Managed care organizations and third-party payers seek to contain healthcare expenditures, and their purchasing strength has been increasing due to their consolidation into fewer, larger organizations and a growing number of enrolled patients. Adoption of price controls, cost-containment measures, and more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. If third-party payors do not consider the products we develop to be cost-effective compared to other therapies, they may not cover our products as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
Efforts by government agencies
Other legislative and state legislatures inregulatory actions that would have a significant impact include: changes to coverage and payment for biosimilars, including the United States could affect uscurrent Medicare biosimilar coverage and our industry.payment policies intended to encourage biosimilar adoption, or other policies that provide easier substitution or reimbursement advantages. The ACA increased many of the mandatory discounts and rebates and imposed a new Branded Prescription Pharmaceutical Manufacturers and Importers fee payable by manufacturers. The new U.S. presidential administration has identified repealing and replacing the ACA as a priority. The timing and method of the full or partial repeal or amendment of the ACA or the adoption of new healthcare legislation remains uncertain, but impending changes will likely impact the number of patient lives covered, the quality of the insurance, Medicaid eligibility and the level of patient protections provided.
Other legislative and regulatory actions that would have a significant impact include: changes to how the Medicare program covers and reimburses current and future drugs, changes in the Federal payment rate or new rebate requirements for covered drugs and policies for payment in Medicare or Medicaid; and changes to coverage and payment for biosimilars, including the current Medicare biosimilar coverage and payment policies intended to encourage biosimilar adoption, or other policies that provide easier substitution or reimbursement advantages.
We face similar issues outside of the United States. In some non-U.S. jurisdictions, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the EU provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for aa medicinal product, or it may instead adopt a system of direct or indirect controls on the profitability of placing a medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the EU do not follow price structures of the United States and generally tend to be significantly lower.
Employees
As of December 31, 2018,2023, we employed 12four persons on a full-time basis and four persons on a part-time basis. Two of which seventhese employees hold a Ph.D.’s
Additional Information
AgeX was incorporated in onethe State of Delaware on January 6, 2017. Our common stock began trading on the NYSE American on November 29, 2018 and trade under the symbol “AGE.” Our mailing address is 1101 Marina Village Parkway, Suite 201, Alameda, CA 94501 and our phone number is (510) 671-8370. Our website address is www.agexinc.com. The information on, or that can be accessed through our website is not part of this Report. We make available, free of charge through our website, our most recent annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and any amendments to those reports, as soon as reasonably practicable after the reports are electronically filed with or furnished to the SEC.
iTRTM, UniverCyteTM, Renelon™, and EPRO™ are trademarks of AgeX. PureStem® is a registered trademark of Lineage Cell Therapeutics, Inc.
Emerging Growth Company
We are an “emerging growth company” under the Jumpstart our Business Startups Act of 2012 or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable, in general, to public companies that are not emerging growth companies. These provisions include:
| ● | reduced disclosure about our executive compensation arrangements; |
| ● | no non-binding stockholder advisory votes on executive compensation or golden parachute arrangements; and |
| ● | exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We will remain an “emerging growth company” until the earliest of: (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act; (iii) the date on which we have issued more fieldsthan $1.0 billion in nonconvertible debt securities during the previous three years; or (iv) the date on which we are deemed to be a “large accelerated filer” under the Securities Exchange Act of science.1934, as amended (the Exchange Act).
| 26 |
The JOBS Act permits an emerging growth company to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. However, we have elected to comply with newly adopted or revised accounting standards when they become applicable to public companies because our financial statements were previously consolidated with those of our former parent company Lineage which is not an emerging growth company under the JOBS Act and is therefore not permitted to delay the adoption of new or revised accounting standards that become applicable to public companies. This election under the JOBS Act to not delay the adoption of new or revised accounting standards is irrevocable.
Item 1A.Risk Factors
Our business is subject to various risks, including those described below. You should consider the following risk factors, together with all of the other information included in this Report, which could materially adversely affect our proposed operations, our business prospects, and financial condition, and the value of an investment in our business. There may be other factors that are not mentioned here or of which we are not presently aware that could also affect our business operations and prospects.
Risks Related to the Merger
The relative proportion of the Combined Company that AgeX stockholders will own immediately following the closing of the Merger is not adjustable based on the market price of AgeX common stock unless the “Actual Closing Price” of AgeX common stock (determined in accordance with the Merger Agreement) is less than $12.00 per share. If the Actual Closing Price of AgeX common stock is less than $12.00 per share, AgeX has the option to issue additional AgeX common stock to Serina stockholders in the amount necessary to equal the Target Merger Consideration Minimum (as defined in the Merger Agreement), which, in such circumstances, could proportionally decrease the amount of the Combined Company that AgeX stockholders would own immediately following the closing of the Merger. Accordingly, the Merger consideration at the closing of the Merger may have a greater or lesser value than at the time the Merger Agreement was signed.
The relative proportion of the Combined Company that AgeX stockholders will own immediately following the closing of the Merger is based on the relative valuations of AgeX and Serina as negotiated by the parties and as specified in the Merger Agreement. At the effective time of the Merger, outstanding shares of Serina common stock will be converted into shares of AgeX common stock. Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of the common stock of the Combined Company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of the Combined Company, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions. It is a closing condition of the Merger that the Actual Closing Price of a share of AgeX common stock is not less than $12.00 per share. However, if the Actual Closing Price of a share of AgeX common stock is less than $12.00 per share, AgeX has the option to satisfy such closing condition by issuing additional AgeX common stock to Serina stockholders in the amount necessary to equal the Target Merger Consideration Minimum (as defined below), which is equal to $12.00 multiplied by the anticipated aggregate number of shares of AgeX common stock to be issued (approximately 7,500,000 shares prior to any such adjustment), and, in such circumstances, AgeX stockholders immediately prior to the closing of the Merger could own a smaller percentage, and Serina stockholders immediately prior to the closing of the Merger could own a larger percentage, of the Combined Company following the closing of the Merger. The exercise of this option would also affect the exchange ratio.
In addition, changes in the market price of AgeX common stock before the completion of the Merger may not affect the number of shares Serina stockholders will be entitled to receive pursuant to the Merger Agreement so long as the Actual Closing Price of AgeX common stock is equal to or greater than $12.00 per share. If before the completion of the Merger, the market price of AgeX common stock increases to be greater than $12.00 per share, then Serina stockholders could receive merger consideration with substantially more value for their shares of Serina capital stock than the parties had negotiated when they established the relative ownership proportions of the Combined Company immediately following the closing of the Merger and the exchange ratio.
Assessing the value of AgeX and Serina through a relative valuation as opposed to an absolute valuation carries potential risk.
The AgeX Board of Directors did not use a dollar or absolute value in determining the relative ownership of AgeX and Serina in the Combined Company. The relative valuations calculated for AgeX and Serina were established based on, among other factors, AgeX’s market capitalization and Serina’s most recent valuation in connection with a capital raise prior to the Merger, AgeX’s and Serina’s operations prior to the Merger, AgeX’s debt accumulated prior to the Merger and consideration of general market conditions for early-stage life sciences companies. Assessing the value of AgeX and Serina through a relative valuation as opposed to an absolute valuation (i.e., a valuation expressed in currency) carries potential risk. If the public markets determine that the relative valuation ascribed to AgeX or Serina is too high or too low, the price of the Combined Company’s common stock may decline following the Merger.
| 27 |
Failure to complete the Merger may result in AgeX or Serina paying a termination fee or reimbursement of expenses to the other party and could harm the common stock price of AgeX and AgeX’s future business and operations.
If the Merger is not completed, each of AgeX is subject to the following risks:
| ● | upon termination of the Merger Agreement, AgeX may be required to pay Serina a termination fee of $1,000,000 or up to $1,000,000 in expense reimbursements; provided that AgeX will not be responsible for payment of more than $1,000,000 in the aggregate; | |
| ● | AgeX has incurred significant expenses related to the Merger, such as legal and accounting fees, which must be paid even if the Merger is not completed; | |
| ● | the price of AgeX’s common stock may decline and remain volatile; and | |
| ● | the reputation of AgeX may be adversely impacted. |
In addition, if the Merger Agreement is terminated and AgeX determines to seek another business combination, there can be no assurance that AgeX will be able to find a partner willing to provide equivalent or more attractive consideration than the consideration to be provided by Serina in the Merger, or any partner at all.
If the conditions to the closing of the Merger are not satisfied or waived, the Merger may not occur or the closing of the Merger could be delayed.
Even though AgeX and Serina have obtained the approvals of their respective stockholders required for the Merger and the related transactions, specified conditions set forth in the Merger Agreement must be satisfied or, to the extent permitted by applicable law, waived to complete the Merger. There is no assurance that all of the conditions will be satisfied or waived. One of the conditions is that at the time of the Merger AgeX must have on hand $500,000 of immediately spendable nonrestricted cash, net of all payables and other “Liabilities” as defined in the Merger Agreement. In order to meet that condition, AgeX will need to obtain additional cash through a loan or other financing the availability and terms of which cannot be assured. If the conditions are not satisfied or waived, the Merger may not occur or could be delayed, and AgeX may lose some or all of the intended benefits of the Merger.
The Merger may be completed even though material adverse changes may result from the announcement of the Merger, industry-wide changes and/or other causes.
In general, either AgeX or Serina can refuse to complete the Merger if there is a material adverse effect affecting the other party between August 29, 2023, the date of the Merger Agreement, and the closing of the Merger. However, certain types of effects are excluded from the concept of a “material adverse effect” and do not permit the parties to refuse to complete the Merger, even if such change could be said to have a material adverse effect on AgeX or Serina. Such exclusions included, but are not limited to:
| ● | general business, political or economic conditions generally affecting the industry in which Serina or AgeX operate; | |
| ● | acts of war, the outbreak or escalation of armed hostilities, acts of terrorism, earthquakes, wildfires, hurricanes or other natural disasters, health emergencies, including pandemics (including COVID-19 and any evolutions or mutations thereof) and related or associated epidemics, disease outbreaks or quarantine restrictions; | |
| ● | changes in financial, banking or securities markets; | |
| ● | any change in the stock price or trading volume of AgeX common stock; | |
| ● | any failure by AgeX to meet internal or analysts’ expectations or projections or the results of operations of AgeX; | |
| ● | any change in or affecting clinical trial programs or studies conducted by or on behalf of AgeX or its subsidiaries; | |
| ● | any change from continued losses from operations or decreases in cash balances of Serina or any of its subsidiaries or on a consolidated basis among Serina and its subsidiaries; | |
| ● | any change in, or any compliance with or action taken for the purpose of complying with, any law or GAAP (or interpretations of any law or GAAP); or | |
| ● | any change resulting from the announcement of the Merger Agreement or the pendency of the transactions related to the Merger; | |
| ● | any change resulting from any disposition of assets permitted by the Merger Agreement; | |
| ● | the taking of any action required to be taken by the Merger Agreement; or | |
| ● | any reduction in the amount of AgeX’s cash and cash equivalents as a result of winding down its activities associated with the termination of its research and development activities. |
If adverse changes occur and AgeX and Serina still complete the Merger, the stock price of the Combined Company following the closing of the Merger may suffer. This in turn may reduce the value of the Merger to the stockholders of AgeX.
| 28 |
Some executive officers and directors of AgeX and Serina have interests in the Merger that are different from the respective stockholders of AgeX and Serina and that may influence them to support or approve the Merger without regard to the interests of the respective stockholders of AgeX and Serina.
Directors and executive officers of AgeX and Serina may have interests in the Merger that are different from, or in addition to, the interests of other stockholders of AgeX and Serina, respectively. These interests with respect to AgeX’s directors and executive officers include, but are not limited to, the expected continued service of one of the directors of AgeX as a director of the Combined Company following the closing of the Merger, the expected continued employment of one of the executive officers of AgeX as an executive officer of the Combined Company following the closing of the Merger, severance benefits if employment is terminated in a qualifying termination in connection with the Merger and rights to continued indemnification, expense advancement and insurance coverage. These interests with respect to Serina’s directors and executive officers include, but are not limited to, the expected continued service of two of the directors of Serina as directors of the Combined Company following the closing of the Merger, the expected continued employment of three of the executive officers of Serina as executive officers of the Combined Company following the closing of the Merger and rights to continued indemnification and expense advancement.
The AgeX Board and Serina Board were aware of and considered such interests, among other matters, in reaching their decisions to approve and adopt the Merger Agreement, approve the Merger and recommend the approval of the Merger Agreement to AgeX and Serina stockholders. These interests, among other factors, may have influenced the directors and executive officers of AgeX and Serina to support or approve the Merger.
AgeX stockholders may not realize a benefit from the Merger commensurate with the ownership interest dilution they will experience in connection with the Merger.
If the Combined Company is unable to realize the full strategic and financial benefits currently anticipated from the Merger, AgeX stockholders will have experienced substantial dilution of their ownership interests without receiving any commensurate benefit, or only receiving part of the commensurate benefit to the extent the Combined Company is able to realize only part of the strategic and financial benefits currently anticipated from the Merger.
If the Merger is not completed, the market price of AgeX common stock may decline significantly.
The market price of AgeX common stock is subject to significant fluctuations. Market prices for securities of pharmaceutical, biotechnology and other life science companies have historically been particularly volatile. In addition, the market price of AgeX common stock will likely be volatile based on whether stockholders and other investors believe that AgeX can complete the Merger. The volatility of the market price of AgeX common stock has been and may be exacerbated by low trading volume. Additional factors that may cause the market price of AgeX common stock to fluctuate include, but are not limited to:
| ● | the entry into, or termination of, key agreements, including commercial partner agreements; | |
| ● | announcements by commercial partners or competitors of new commercial products, clinical progress or lack thereof, significant contracts, commercial relationships or capital commitments; | |
| ● | the loss of key employees; | |
| ● | future sales of its common stock; | |
| ● | general and industry-specific economic conditions that may affect its research and development expenditures; | |
| ● | the failure to meet industry analyst expectations; and | |
| ● | period-to-period fluctuations in financial results. |
Moreover, the stock markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may also adversely affect the trading price of AgeX common stock. In the past, following periods of volatility in the market price of a company’s securities, stockholders have often instituted class action securities litigation against such companies.
The market price of the Combined Company’s common stock following the Merger may decline as a result of the Merger.
The market price of the Combined Company’s common stock may decline as a result of the Merger for a number of reasons, including if:
| ● | investors react negatively to the prospects of the Combined Company’s product candidates, business and financial condition following the closing of the Merger; | |
| ● | the effect of the Merger on the Combined Company’s business and prospects following the closing of the Merger is not consistent with the expectations of financial or industry analysts; or | |
| ● | the Combined Company does not achieve the perceived benefits of the Merger as rapidly or to the extent anticipated by stockholders or financial or industry analysts. |
| 29 |
AgeX stockholders will have a reduced ownership and voting interest in, and will exercise less influence over the management of, the Combined Company following the closing of the Merger as compared to their current ownership and voting interest in AgeX.
Immediately following the closing of the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of the Combined Company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own, excluding the impact of the exercise of any post-merger warrant or incentive warrant, approximately 25% of the outstanding shares of common stock of the Combined Company, in each case, on a pro forma fully diluted basis, subject to certain assumptions. The exchange ratio formula is based upon a fixed percentage of the post-Merger outstanding shares of the Combined Company common stock, expressed on a fully-diluted and as-converted basis, subject to certain adjustments and exclusions.
In addition, the seven-member board of directors of the Combined Company will initially be comprised of (a) Remy Gross, designated by both AgeX and Serina, (b) J. Milton Harris and Steve Ledger, each as a Serina designee, (c) Gregory H. Bailey and Richard Marshall, each as an AgeX designee, and (d) Steven Mintz, as an independent director, until their respective successors are duly elected or appointed and qualified or their earlier death, resignation or removal. The remaining vacancy on the Combined Company Board will be filled by an individual that qualifies as an independent director at a later date. The Chief Financial Officer, the Chief Executive Officer and the Chief Operating Officer of Serina will serve as the Interim Chief Executive Officer, Chief Scientific Officer and Chief Operating Officer and Secretary of the Combined Company, respectively, following the closing of the Merger. The Chief Financial Officer of AgeX will serve as the Interim Chief Financial Officer and Chief Accounting Officer of the Combined Company following the closing of the Merger.
Consequently, following the closing of the Merger, the pre-Merger equity holders of AgeX will own a smaller percentage of the Combined Company than their ownership of AgeX prior to the Merger and will be able to exercise less influence over the management and policies of the Combined Company than they currently exercise over the management and policies of AgeX.
During the pendency of the Merger Agreement, AgeX may be limited in its ability to enter into a business combination with another party on more favorable terms because of restrictions in the Merger Agreement, which could adversely affect AgeX’s business prospects.
Covenants in the Merger Agreement impede the ability of AgeX and Serina to make acquisitions during the pendency of the Merger, subject to specified exceptions. As a result, if the Merger is not completed, AgeX as well as Serina may be at a disadvantage to their competitors during that period. In addition, while the Merger Agreement is in effect, each party is generally prohibited from soliciting, initiating or knowingly encouraging, inducing or facilitating the communication, making, submission or announcement of any acquisition proposal or acquisition inquiry or taking any action that could reasonably be expected to lead to an acquisition proposal or acquisition inquiry regarding transactions involving a third party, including a merger, sale of assets or other business combination, subject to specified exceptions. Any such transactions could be favorable to such party’s stockholders, but the parties may be unable to pursue them.
Certain provisions of the Merger Agreement may discourage third parties from submitting competing proposals, including proposals that may be superior to the transactions contemplated by the Merger Agreement.
The terms of the Merger Agreement prohibit each of AgeX and Serina from soliciting competing proposals or cooperating with persons making unsolicited takeover proposals, except in limited circumstances. In addition, if AgeX or Serina terminate the Merger Agreement under specified circumstances, AgeX may be required to pay Serina a termination fee of $1,000,000 or up to $1,000,000 in expense reimbursements or Serina may be required to pay AgeX a termination fee of $1,000,000 or up to $1,000,000 in expense reimbursements; provided that neither party will be responsible for payment of more than $1,000,000 in the aggregate. The termination fee or expense reimbursements may discourage third parties from submitting competing proposals to AgeX or AgeX stockholders and may cause the AgeX Board to be less inclined to recommend a competing proposal.
Because the lack of a public market for Serina’s capital stock makes it difficult to evaluate the fair market value of Serina’s capital stock, the value of the AgeX common stock to be issued to Serina stockholders may be more or less than the fair market value of Serina common stock.
The outstanding capital stock of Serina is privately held and is not traded in any public market. The lack of a public market makes it difficult to determine the fair market value of Serina’s capital stock. Because the percentage of AgeX equity to be issued to Serina stockholders was determined based on negotiations between the parties, it is possible that the value of the AgeX common stock to be received by Serina stockholders will be more or less than the fair market value of Serina’s capital stock.
| 30 |
Litigation has been filed but dismissed but and additional litigation could in the future arise in connection with the Merger, against AgeX, the AgeX Board, Serina or the Serina Board, which could be costly, prevent the consummation of the Merger, divert management’s attention and otherwise materially harm AgeX’s, Serina’s or the Combined Company’s business.
In the past, securities class action or shareholder derivative litigation has often followed certain significant business transactions, such as the sale of a company or announcement of a merger or any other strategic transaction. AgeX and the AgeX Board has been, and Serina, the Serina Board or the Combined Company may be exposed to such litigation in connection with the Merger and may in the future be exposed to additional litigation even if no wrongdoing occurred. Litigation is usually expensive and diverts management’s attention and resources, which could adversely affect the business and cash resources or either party and the ability of either party to consummate the Merger or the ultimate value the AgeX stockholders receive in from the Merger.
If the Merger is not consummated for any reason, litigation could be filed in connection with the failure to consummate the Merger. Any litigation related to the Merger may result in negative publicity or an unfavorable impression of AgeX, which could adversely affect the price of AgeX common stock, impair the ability of AgeX to recruit or retain employees, damage relationships with customers, suppliers, and other business partners, or otherwise materially harm of AgeX’s operations and financial performance.
Risks Related to the Reverse Stock Split
The Reverse Stock Split may not increase the Combined Company’s stock price over the long-term.
While it is expected that the reduction in the number of outstanding shares of common stock as a result of the Reverse Stock Split will proportionally increase the market price of AgeX common stock, it cannot be assured that the Reverse Stock Split will increase the market price of AgeX common stock by a multiple of the Reverse Stock Split ratio or result in any other permanent or sustained increase in the market price of the Combined Company’s common stock, which is dependent upon many factors, including the Combined Company’s business and financial performance, general market conditions and prospects for future success. Thus, it cannot be assured that the Reverse Stock Split will accomplish any increase in the per-share market price of the Combined Company’s common stock for any meaningful period of time.
The Reverse Stock Split may decrease the liquidity of AgeX common stock or the Combined Company’s common stock.
Although the AgeX Board believes that the anticipated increase in the market price of the Combined Company’s common stock resulting from the proposed Reverse Stock Split could encourage interest in its common stock and possibly promote greater liquidity for its stockholders, such liquidity could also be adversely affected by the reduced number of shares outstanding after the Reverse Stock Split. The reduction in the number of outstanding shares may lead to reduced trading in and a smaller number of market makers for the Combined Company’s common stock.
The Reverse Stock Split may lead to a decrease in the Combined Company’s overall market capitalization.
Should the market price of the Combined Company’s common stock decline after the Reverse Stock Split, the percentage decline may be greater, due to the smaller number of shares outstanding, than it would have been prior to the Reverse Stock Split. A Reverse Stock Split is often viewed negatively by the market and, consequently, can lead to a decrease in the Combined Company’s overall market capitalization. If the per-share market price does not increase in proportion to the Reverse Stock Split ratio, then the value of the Combined Company, as measured by its stock capitalization, will be reduced. In some cases, the per-share stock price of companies that have effected Reverse Stock Splits subsequently declined back to pre-reverse split levels, and accordingly, it cannot be assured that the total market value of the Combined Company’s common stock will remain the same after the Reverse Stock Split is effected, or that the Reverse Stock Split will not have an adverse effect on the Combined Company’s stock price due to the reduced number of shares outstanding after the Reverse Stock Split.
| 31 |
Risks Related to AgeX
If the Merger is not completed, we expect to continue to execute on our current business strategies while seeking out and evaluating potential strategic alternatives with respect to our assets and development programs, and, accordingly, our business will be subject to the risks described in this subsection titled “Risks Related to AgeX.” If the Merger is completed, the Combined Company would face many of the same risks as AgeX described below, including but not limited to: compliance with domestic and foreign laws and government regulation, including regulation by the FDA and comparable foreign regulatory agencies; risks pertaining to conducting clinical trials; competition; staffing; product liability risks; obtaining Medicare reimbursement approval for any medical products that may be commercialized; patent and other intellectual property protection matters; obtaining sufficient financing for operations; risks related to Juvenescence’s stock ownership, which is expected to be significant immediately after the Merger though less than 50% of the outstanding common stock, and secured loans from Juvenescence, reliance on relationships with third parties; system security matters and internal control over financial reporting; and matters pertaining to the trading of the common stock.
Risks Related to Our Current Business, OperationsFinancial Condition and Capital Resources
We need additional financing to execute our operating plan and continue to operate as a going concern.
As required under Accounting Standards Update 2014-15, Presentation of Financial Statements-Going Concern (ASC 205-40), we have the responsibility to evaluate whether conditions and/or events raise substantial doubt about our ability to meet our future financial obligations as they become due within one year after the date the financial statements are issued. Based on our most recent projected cash flows, we believe that our cash and cash equivalents and the amount of credit remaining available from Juvenescence under an Amended and Restated Secured Convertible Promissory Note, dated February 14, 2022, as amended on February 9, 2023, November 9, 2023 and February 9, 2024 (the 2022 Secured Note), would not be sufficient to satisfy our anticipated operating and other funding requirements for the next twelve months from the date of filing of this Report. These factors raise substantial doubt regarding our ability to continue as a going concern and the report of our independent registered public accountants accompanying our audited consolidated financial statements included elsewhere in this Report contains a qualification to such effect.
We have incurred operating losses and negative cash flows since inception and had an accumulated deficit of $131.0 million as of December 31, 2023. We expect to continue to incur operating losses and negative cash flows. Because we will continue to experience operating losses, our ability to continue as a going concern is subject to our ability to obtain necessary capital from outside sources, including obtaining additional capital from the sale of AgeX common stock or other equity securities or assets, obtaining additional loans from financial institutions or investors, and entering into collaborative research and development arrangements or licensing some or all of our patents and know-how to third parties while retaining a royalty and other contingent payment rights related to the development and commercialization of products covered by the licenses. Our continued operating losses, the amount of our debt obligations to Juvenescence and the provisions of our indebtedness agreements with them, including restrictions on the use of loan funds and the security interest they hold in our assets and assets of certain of our subsidiaries, Juvenescence’s ownership of shares of AgeX Preferred Stock, the risks associated with the development of our product candidates and technologies, and our deferral of in-house development of our product candidates and technologies in connection with our reductions in staffing and the closing of our research laboratory facilities, will increase the difficulty in obtaining such capital, and there can be no assurances that we will be able to obtain such capital on favorable terms or at all. If we are unable to raise capital when needed, we may be forced to delay, reduce or eliminate our research and development activities, or ultimately not be able to continue as a going concern.
We are a discovery-stage development company with limited capital resources and have incurred operating losses since our inception. We anticipate that we will incur continued losses for the foreseeable future and will need to continue to raise capital to finance our operations, and we do not know if we will ever attain profitability.
We are a discovery-stage therapeutics company with a limited operating history.history and limited capital resources. Since our inception in August 2017, we have incurred operating losses and negative cash flows and we expect to continue to incur losses and negative cash flow in the future. Our operating lossesnet loss from operations were $11.2$14.8 million and $6.7$10.5 million for the years ended December 31, 20182023 and 2017,2022, respectively, and we had an accumulated deficit of approximately $74.1$131.0 million as of December 31, 2018. We have devoted most of our financial resources to research and development, including our preclinical development activities.2023.
Since inception, we have financed our operations through contributions and advances from our former parent company, BioTime, and the sale of our common stock and warrants to our current stockholders. Although BioTime may continue to provide administrative support to us on a reimbursable basis, BioTime currently owns less than 5% of our outstanding common stock and we do not expect BioTime to provide future financing. Additionally, although Juvenescence is now our largest stockholder, it has no obligation to provide us with financing. There is no assurance that we will be able to obtain any additional financing that we may need, or that any such financing that may become available will be on terms that are favorable to us and our stockholders. Ultimately, our ability to generate sufficient operating revenue to earn a profit depends upon our success in developing and marketing or licensing our products and technology.
We expect to continue to incur significant additional operating losses for the foreseeable future as we seek to advance product candidates through preclinical and clinical development, expand our research and development activities, develop new product candidates, complete clinical trials, seek regulatory approval and, if we receive FDA approval, commercialize our products. Furthermore, the costs of advancing product candidates into each succeeding clinical phase tend to increase substantially over time. The total costs to advance any of our product candidates to marketing approval in even a single jurisdiction would be substantial. Because of the numerous risks and uncertainties associated with development of cell-based and drug-based therapeutics, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to begin generating revenue from the commercialization of products (other than through our LifeMap Sciences subsidiary) or achieve or maintain profitability. Our expenses will also increase substantially if and as we:
Furthermore, our ability to successfully develop, commercialize and license our products and generate product revenue is subject to substantial additional risks and uncertainties. Each of our programs and product candidates will require additional preclinical and clinical development, potential regulatory approval in multiple jurisdictions, securing manufacturing supply, capacity and expertise, building of a commercial organization, substantial investment and significant marketing efforts before we generate any operating income from product sales. As a result, we expect to continue to incur net losses and negative cash flows for the foreseeable future. These net losses and negative cash flows have had, and will continue to have, an adverse effect on our stockholders’ equity and working capital. We expect to continue to incur significant additional operating losses for the foreseeable future and will need to continuously raise additional capital to fund our operations. The amount of our expenses and anticipated losses will depend on our capital resources and whether we license out product development to third parties or participate ourselves directly or financially with collaborators in research, development and commercialization efforts. Although we do not expect to advance our product candidates through clinical trials, seek regulatory approval, or commercialize our product candidates ourselves, our capital needs would increase greatly if we were to change plans and determine to do so.
The amount of our future net losses will depend, in part, on the rate of future growth of our expenses, our ability to raise the capital needed to continue our operations, and our ability to generate revenues. If we or any licensees or collaborators are unable to develop and commercialize one or more of our product candidates, either alone or with collaborators, or if revenues from any product candidatecandidates that receivesreceive marketing approval are insufficient, we will not achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability. If we are unable to achieve and then maintain profitability, the value of our equity securities will be materially and adversely affected.
| 32 |
We will spendare highly leveraged, carrying a substantialsignificant amount of indebtedness, including indebtedness secured by our capital on discoveryassets, that will become due and preclinical researchpayable over the next three years and development, butthere is no assurance that we might not succeed in developing products and technologies that are useful in medicine. If we fail to obtain necessary financing, we may notwill be able to completerefinance those obligations as they become due.
As of March 20, 2024, our borrowings from Juvenescence under various loans and accrued origination fees outstanding totaled approximately $8.1 million. The outstanding balances of those loans will become due and payable as follows: approximately $7.4 million on May 9, 2024 under the development2022 Secured Note and commercialization$692,800 on March 13, 2026 under a $10 million principal amount Secured Convertible Promissory Note, dated March 13, 2023 (the “2023 Secured Note”). During November 2023, we entered into an amendment of the 2022 Secured Note pursuant to which we borrowed an additional $4,400,000 from Juvenescence. Our obligations under the 2022 Secured Note and the 2023 Secured Note are collateralized by all of our product candidates.
We expectassets, including the shares of common stock we hold in subsidiaries ReCyte, Reverse Bio, and UniverXome under the terms of an Amended and Restated Security Agreement (the “Security Agreement”), and those subsidiaries have guaranteed our obligations under the Secured Note, as amended, and have granted a security interest in their respective assets to spend substantial amountsJuvenescence pursuant to complete the developmentSecurity Agreement to secure those obligations. If an Event of seek regulatory approvals forDefault, as defined in the 2022 Secured Note or the 2023 Secured Note, were to occur Juvenescence could foreclose on its security interest and commercializesell our product candidates. We will require additional capital beyondassets and the proceedsassets of this offering, whichthose subsidiaries to satisfy the unpaid principal balances of those loans plus certain loan origination fees and costs incurred in connection with the Event of Default and the foreclosure and sale of the assets. As a result, we may raise through equity offerings, debt financings, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangementsthose subsidiaries could lose some or other sources to enable us to complete the development and potential commercializationall of our product candidates. In addition, we may not be able to enter intorespective assets, leaving few if any collaborations that will generate significant cash. Adequate additional financing may not beassets available to us on acceptable terms, or at all. Our failure to raise capital as and when needed would have a negative effect on our financial condition and our ability to pursuefor the operation of our business strategy. In addition, attempting to secure additional financing may divertor the time and attentionbusinesses of our management from day-to-day activities and harm our product candidate development efforts.
Becausesubsidiaries, or for sale for the length of time and activities associated with successful developmentbenefit of our product candidates is highly uncertain,stockholders through a winding up of our affairs and liquidation of our assets. Although we are unableplan to estimatetransfer most of our assets to UniverXome and to restructure our indebtedness with Juvenescence so that UniverXome will replace AgeX as the actual fundsborrower and will assume AgeX’s obligations under the 2022 Secured Note and 2023 Secured Note and the Security Agreement, Juvenescence will retain its security interest in the assets we will requiretransfer to UniverXome and would be entitled to foreclose on its security interest under the Security Agreement if an Event of Default were to occur after UniverXome assumes AgeX’s obligations.
The terms of our loans from Juvenescence and the Security Agreement could make it more difficult for development and any approved marketing and commercialization activities. Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to:
We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts or onfrom other sources.
The terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of our product candidatesloans from Juvenescence include certain covenants that among other matters such as financial reporting: (i) impose financial restrictions on AgeX while the loans remain unpaid, including restrictions on the incurrence of additional indebtedness by AgeX and its subsidiaries, except that Reverse Bio will be permitted to incur debt convertible into equity not guaranteed or potentially discontinue operations.secured by the assets of AgeX or any other AgeX subsidiary; (ii) require that AgeX use loan proceeds and funds that may be raised through certain equity offerings only for research and development work, professional and administrative expenses, for general working capital, and for repayment of all or a portion of AgeX’s indebtedness to Juvenescence; and (iii) prohibit AgeX from making additional investments in subsidiaries, unless AgeX obtains the written consent of Juvenescence to a transaction that otherwise would be prohibited or restricted. Further, our obligations under the 2022 Secured Note and 2023 Secured Note are collateralized by all of our assets under the terms of the Security Agreement. Accordingly, the terms of our loans from Juvenescence, and the grant of a security interest in our assets pursuant to the Security Agreement to secure our obligations for the repayment of the 2022 Secured Note and 2023 Secured Note with applicable loan fees that become due, could make AgeX less attractive to new equity investors and could impair our ability to finance our operations or the operations of our subsidiaries from sources other than Juvenescence. Although we plan to transfer most of our assets to UniverXome and to restructure our indebtedness with Juvenescence so that UniverXome will replace AgeX as the borrower and will assume AgeX’s obligations under the 2022 Secured Note and 2023 Secured Note and the Security Agreement, the amount and terms of the indebtedness owed to Juvenescence by UniverXome, and Juvenescence’s continuing security interest in the assets held by UniverXome to secure its obligations to Juvenescence could still make AgeX less attractive to new equity investors and could impair our ability to finance our operations or the operations of UniverXome and our other subsidiaries from sources other than Juvenescence.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial revenue, we may finance our cash needs through a combination of equity offerings, debt financings, strategic alliances and licensing arrangements. We do not currently have any committed external source of funds. In addition, we mayWe will need to seek additional capital due to favorableregardless of market conditions or strategic considerations, even if we believeand the terms of any financings that we have sufficient funds for our current or future operating plans.may be available to us.
To the extent that we raise additional capital through the sale of shares of AgeX common stock or other equity or convertible debt securities, AgeX your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Similarly, if any of our subsidiaries raise capital through the sale of equity securities or convertible debt securities AgeX’s interest in those subsidiaries will be diluted and the terms of equity securities issued by those subsidiaries may include liquidation or other preferences that adversely affect our rights as a common stockholder of the subsidiary.
Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we or any of our subsidiaries raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we or our subsidiaries may be required to relinquish valuable rights to ourkey technologies, future revenue streams, or product candidates, or grantand any such licenses may be granted on terms that may not be favorable to us. If we or our subsidiaries are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
| 33 |
Unless AgeX common stock continues to be listed on a national securities exchange, AgeX will become subject to the so-called “penny stock” rules that impose restrictive sales practice requirements.
On November 17, 2021, we received a letter (the Deficiency Letter) from the staff of the NYSE American indicating that AgeX did not meet certain of the NYSE American continued listing standards as set forth in Section 1003(a)(i) of the NYSE American Company Guide in that we had stockholders’ equity of less than $2,000,000 and had incurred losses from continuing operations and/or net losses during our two most recent fiscal years. Pursuant to Section 1009 of the NYSE American Company Guide and as provided in the Deficiency Letter provided, we provided the NYSE American staff with a plan (the Compliance Plan) advising the NYSE American staff of action we had taken and will take that would bring AgeX into compliance with the NYSE American continued listing standards. The NYSE American staff accepted our Compliance Plan but later required us to revise the Compliance Plan as we remained out of listing compliance. On November 22, 2022, we received a notification from the staff of the NYSE American indicating that the NYSE American accepted our revised listing Compliance Plan and granted us an extension of time to regain compliance with the NYSE American continued listing standards as set forth in Section 1003(a)(i) and (ii) of the NYSE American Company Guide by increasing our stockholders equity to not less than $4,000,000. On May 17, 2023, we received a notice from the staff of the NYSE American indicating that they intend to commence proceedings to delist AgeX common stock from the NYSE American based upon AgeX’s non-compliance with the stockholders’ equity requirements set forth in Sections 1003(a)(i), (ii) and (iii) of the NYSE American Company Guide by the end of a compliance plan period that expired on May 17, 2023. Specifically, AgeX did not meet the continued listing standards because it had stockholders equity of less than (A) $2,000,000 and has incurred losses from continuing operations and/or net losses during its two most recent fiscal years, (B) $4,000,000 and had incurred losses from continuing operations and/or net losses during three out of four of its most recent fiscal years, and (C) $6,000,000 or more and had reported losses from continuing operations and/or net losses in its five most recent fiscal years. On May 24, 2023, AgeX filed a request for a review of the delisting determination by a committee of the board of directors of the NYSE American. On May 31, 2023, AgeX received a notice from the staff of the NYSE American which scheduled a hearing for July 25, 2023. On July 24, 2023, AgeX issued shares of AgeX Series A preferred stock and AgeX Series B preferred stock to Juvenescence in exchange for the extinguishment of $36 million of indebtedness owed to Juvenescence with the intent of adding $36 million to stockholders equity and returning AgeX to compliance with the NYSE American continued listing standards. However, due to the incurrence of subsequent losses from operations, AgeX’s stockholders equity declined below $6,000,000 as of December 31, 2023 which could result in the NYSE American initiating delisting proceedings again unless the Merger is consummated and the NYSE American approves listing of the common stock of the Combined Company or unless AgeX raises additional equity capital sufficient to bring its stockholders equity into compliance with the continued listing standards.
If we are unable to maintain the listing of AgeX common stock on the NYSE American or another national securities exchange, AgeX common stock could become subject to the so-called “penny stock” rules if the shares have a market value of less than $5.00 per share. The SEC has adopted regulations that define a penny stock to include any stock that has a market price of less than $5.00 per share, subject to certain exceptions, including an exception for stock traded on a national securities exchange. The SEC regulations impose restrictive sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors. An accredited investor generally is a person whose individual annual income exceeded $200,000, or whose joint annual income with a spouse exceeded $300,000 during the past two years and who expects their annual income to exceed the applicable level during the current year, or a person with net worth in excess of $1.0 million, not including the value of the investor’s principal residence and excluding mortgage debt secured by the investor’s principal residence up to the estimated fair market value of the home, except that any mortgage debt incurred by the investor within 60 days prior to the date of the transaction shall not be excluded from the determination of the investor’s net worth unless the mortgage debt was incurred to acquire the residence. For transactions covered by this rule, the broker-dealer must make a special suitability determination for the purchaser and must have received the purchaser’s written consent to the transaction prior to sale. This means that if we are unable maintain the listing of AgeX common stock on a national securities exchange, the ability of stockholders to sell their AgeX common stock in the secondary market could be adversely affected.
If a transaction involving a penny stock is not exempt from the SEC’s rule, a broker-dealer must deliver a disclosure schedule relating to the penny stock market to each investor prior to a transaction. The broker-dealer also must disclose the commissions payable to both the broker-dealer and its registered representative, current quotations for the penny stock, and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the customer’s account and information on the limited market in penny stocks.
| 34 |
Risks Related to Our Planned Restructuring
As part of our corporate restructuring plans, we are pursuing the Merger with Serina through which AgeX would be able to enter the field of drug delivery polymer technology, but there is no assurance that the Merger will be consummated. Failure to complete, or delays in completing, the Merger with Serina could materially and adversely affect our results of operations, business, financial condition or stock price.
We have been formulating corporate restructuring plans that include the Merger through which AgeX would be able to enter the field of drug delivery polymer technology. If the Merger is completed, the Combined Company will primarily focus on developing Serina’s product candidates and it is anticipated that the Combined Company will not continue to develop our product candidates, other than potentially the development program of NeuroAirmid. Any failure to satisfy a required condition to closing may prevent, delay or otherwise materially and adversely affect the completion of the Merger, which could materially and adversely affect AgeX’s ability to enter the field of drug delivery polymer technology and could materially and adversely affect AgeX’s results of operations, business, financial condition or stock price. AgeX cannot predict with certainty whether or when any of the required closing conditions will be satisfied and whether AgeX will be able to successfully consummate the Merger as currently contemplated under the Merger Agreement or at all. Accordingly, there is no assurance that the Merger will be consummated.
AgeX’s efforts to complete the Merger could cause substantial disruptions in, and create uncertainty surrounding, AgeX’s business, which may materially adversely affect AgeX’s results of operations and business. Uncertainty as to whether the Merger will be completed may affect AgeX’s ability to retain and motivate existing employees or recruit prospective employees if vacancies in staffing need to be filled. Employee retention may be particularly challenging while the Merger is pending because employees may experience uncertainty about their roles following the transaction. A substantial amount of AgeX’s management’s and employees’ attention is being directed toward the completion of the transaction and thus is being diverted from AgeX’s day-to-day operations.
Risks related to the failure to consummate, or delay in consummating, the Merger include, but are not limited to, the following:
| ● | AgeX would not realize any or all of the potential benefits of the Merger, which could have a negative effect on AgeX’s results of operations, business, financial condition or stock price; | |
| ● | under some circumstances, AgeX may be required to pay a termination fee to Serina of $1,000,000 or expense reimbursement of up to $1,000,000, but not both; | |
| ● | AgeX would remain liable for significant transaction costs, including legal, accounting and other costs relating to the merger regardless of whether the Merger is consummated; | |
| ● | the trading price of AgeX’s common stock may decline to the extent that the current market price for AgeX’s stock reflects a market assumption that the Merger will be completed; | |
| ● | the attention of AgeX’s management and employees may have been diverted to the Merger rather than to AgeX’s operations and the pursuit of other opportunities that could have been beneficial to AgeX; | |
| ● | AgeX could be subject to litigation related to any failure to complete the Merger; | |
| ● | AgeX could potentially lose key personnel during the pendency of the Merger as employees may experience uncertainty about their future roles with AgeX following completion of the Merger and seek employment opportunities elsewhere; and | |
| ● | under the Merger Agreement, AgeX is subject to certain customary restrictions on the conduct of AgeX’s business prior to completing the Merger, which restrictions could adversely affect AgeX’s ability to conduct AgeX’s business as AgeX otherwise would have done if AgeX was not subject to these restrictions. |
The occurrence of any of these events individually or in combination could materially and adversely affect AgeX’s results of operations, business, financial condition and stock price.
If the Merger is not approved or does not occur, we may not be successful in the execution of our current business strategies or identifying and implementing any strategic alternatives with respect to our assets and development programs, and any future strategic alternatives could have negative consequences.
If the Merger does not occur, we expect to continue to execute on our current business strategies while seeking out and evaluating potential strategic alternatives with respect to our assets and development programs, which may include a merger, business combination, investment into AgeX, sale or other disposition of assets or other strategic transaction. In such case, we may not be successful in executing such strategies or identifying or implementing any such strategic alternatives, and there is a risk that Juvenescence may decide to stop funding our operations, which would likely result in our delisting and dissolution.
In addition, the process of seeking out and evaluating a potential strategic alternative with respect to our assets and development programs is costly, time-consuming and complex, and we have incurred, and may in the future incur, significant costs related to the Merger and our other ongoing restructuring plans, such as legal and accounting fees and expenses and other related charges. We may also incur additional unanticipated expenses in connection with this process. A considerable portion of these costs will be incurred regardless of whether any such course of action is implemented or transaction is completed, decreasing the remaining cash available for use in our business.
| 35 |
The development and any potential commercialization of our product candidates will require substantial additional cash to fund the costs associated with conducting the necessary preclinical and clinical testing and obtaining regulatory approval. Consequently, any potential counterparty in a strategic transaction involving AgeX may be reluctant to spend additional resources required to pursue development of AgeX’s product candidates and may attribute little or no value to AgeX product assets in such a transaction.
There can be no assurance that any particular course of action, business arrangement or transaction, or series of transactions, will be pursued, successfully consummated, lead to increased stockholder value, or achieve the anticipated results.
Risks Related to Our Relationship with Juvenescence
Conflicts of interest may arise from our relationship with Juvenescence, which owns a significant percentage of AgeX common stock and is a significant creditor and will be able to substantially influence us and exert control over matters subject to stockholder approval.
As of March 5, 2024, Juvenescence beneficially owned approximately 81.1% of the outstanding shares of AgeX common stock, as Juvenescence indicated in its amendment of the Schedule 13D filed with the SEC on March 7, 2024, include both outstanding shares owned and shares that may be acquired upon the exercise of certain warrants and conversion of convertible debt. A member of the AgeX Board is also the Executive Chairman and Co-Founder of Juvenescence, and based on Juvenescence’s ownership of shares of AgeX common stock, if the Merger is not consummated, Juvenescence will continue to substantially influence us and exert control over matters subject to stockholder approval, the elections of directors, approval of our equity incentive plans, amendments to our organizational documents, or approval of any merger, amalgamation, sale of assets or other major corporate transaction. Furthermore, because Juvenescence holds more than 50% of the outstanding AgeX common stock, AgeX qualifies as a “controlled company” as defined by the NYSE American Company Guide. Being a “controlled company” entitles AgeX to exempt itself from the requirement that a majority of its directors be “independent” directors as defined in the NYSE American Company Guide, and that the Compensation Committee and the Nominating & Corporate Governance Committee be comprised entirely of independent directors.
On November 9, 2023, we entered into an amendment of the 2022 Secured Note to increase the amount of the line of credit available to AgeX from Juvenescence by $4,400,000, which has already been drawn down in full. As of March 20, 2024, the outstanding principal balance plus accrued loan origination fees of the 2022 Secured Note was approximately $7,400,000. Juvenescence also has controlling stakes and minority investments in several other companies engaged in various aspects of the aging industry, which companies may propose collaborations with AgeX.
Juvenescence’s interests may not always coincide with our corporate interests or the interests of other stockholders, and it may exercise its voting and other rights, including rights as a creditor, in a manner with which other stockholders may not agree or that may not be in the best interests of AgeX or stockholders other than Juvenescence. So long as Juvenescence continues to own a significant amount of our equity and remains a significant creditor, it will continue to be able to strongly influence and effectively control our decisions. While the directors elected by Juvenescence will be obligated to act in accordance with their fiduciary duty, they may have equity or other interests in Juvenescence and, accordingly, their interests may be aligned with Juvenescence’s interests, which may not always coincide with our corporate interests or the interests of our other stockholders.
| 36 |
Risks Related to Our Business Operations
Due to our limited financial resources, we have reduced our staffing, eliminated our research laboratory facilities, and eliminated in-house research and product development work. We will seek opportunities to outsource or license product development and commercialization but there is no assurance that we will be able to do so successfully.
During April 2020, we implemented a plan to reduce spending on employee salaries and consulting fees that resulted in large staff reductions, including the elimination of most of our research personnel and certain management and administrative personnel. We then subleased most of our former laboratory facility space and we did not renew the laboratory facility lease, or lease other laboratory facility space, after our lease expired at the end of 2020. As a result, we do not have a research laboratory facility or a research staff, and we have curtailed development of our product candidates and technologies except for certain research and development work that is being conducted under sponsored research agreements with certain universities and a limited amount of work contracted out to third-party service providers. We also may license technologies or product development to, or enter into collaborative arrangements with, other companies in the cell therapy or biopharma industry to conduct research and development, manufacturing, and marketing for AgeX for particular product candidates, but there is no assurance that we will be able to enter into any such agreements on terms acceptable to us.
We may expend our limited resources to pursue one or more particular product candidates or indications and fail to pursue product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we may focus on research programs and product candidates that we identify for specific indications and we may seek to develop those product candidates through out-sourcing or out-licensing to third parties if we are able to make such arrangements. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that may later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to timely capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
We have not tested any of our product candidates in clinical trials. Success in early development and preclinical studies or clinical trials may not be indicative of results obtained in later preclinical studies and clinical trials.
Ourproduct candidates have never been evaluated in human clinical trials, and we may experience unexpected or adverse results in the future. Wefuture if any human clinical trials of any of our product candidates are conducted. Typically, it takes about six to ten years to develop a new drug from the time it enters Phase 1 clinical trials to when it is approved for treating patients, but in many cases it may take longer, and the costs of advancing product candidates through clinical trials will be requiredsubstantial and will tend to demonstrate through adequateincrease significantly with each successive clinical trial phase.
Adequate and well-controlled clinical trials will need to demonstrate that our product candidates are safe and effective, with a favorable benefit-risk profile, for use in their target indications before weregulatory approvals can seek regulatory approvalsbe sought for their commercial sale. Any positive results that have beenmay be observed for product candidates similar to ours in preclinical animal models may not be predictive of future clinical trials in humans. Our product candidates may also fail to show the desired safety and efficacy in later stages of clinical development even if they successfully advance through initial clinical trials. Further, some or all of our cell-based therapies under development may require the genetic modification of the pluripotent master cell banks such that the resulting cells can escape immune rejection by the intended patient. There is no certainty that saida genetic modification will provide a long-term solution to transplant rejection, or that saidthe modified cells will not cause unanticipated health risks to the patient that could delay or even halt the development of the products.
Many companies in the biotechnology industry have suffered significant setbacks in late-stage clinical trials after achieving positive results in early-stage development and there is a high failure rate for product candidates proceeding through clinical trials. Data obtained from preclinical and clinical activities are subject to varying interpretations, which may delay, limit or prevent regulatory approval. Even if we demonstratea clinical trial demonstrates statistical significance, regulatory agencies may not accept the use of the historical control. Regulatory delays or rejections may be encountered as a result of many factors, including changes in regulatory policy during the period of product development. We cannot be certain that weany clinical trials and applications for marketing approval will not face similar setbacks.
We do not currently have any products onThe development and commercialization of new drugs to address obesity and type 2 diabetes may substantially limit or eliminate the marketprospects for AgeX’s prospective AGEX-BAT1 product.
A number of new GLP-1 receptor agonist drugs, including Mounjaro, Ozempic, Rybelsus, and have not yet generated any substantial revenues from operations.
We were established and began operations in 2017. Our operations to date have been limited to the preliminary financing and staffing of our company, developing our technology and identifying and developing our product candidates. We have not yet demonstrated an ability to successfully commence or complete any clinical trials, including large-scale, pivotal clinical trials, obtain marketing approval, manufacture a research or commercial scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Typically, it takes about six to ten years to develop a new drug from the time it enters Phase 1 clinical trials to when it is approvedTrulicity for treating type 2 diabetes, and Wegovy and Zepbound for weight management, have entered the market. Ozempic is also being used off label for weight loss. The attention and acceptance that these new drugs have attained in the medical field for the treatment of type 2 diabetes and chronic weight management may substantially limit or eliminate the prospects for developing and commercializing any product based on AGEX-BAT1, brown adipose tissue, for those uses. Although the GLP-1 receptor agonist drugs may in certain patients but in many cases it may take longer. Consequently, predictions about our future successbe contraindicated, carry unacceptable medical risks, lead to intolerable side effects, or viability may not be as accurate as they couldsatisfactorily effective, it is not clear whether those patients would constitute a large enough market for an alternative therapy to warrant the time and expense of developing AGEX-BAT1 for the uses addressed by the products currently on the market. Further, it is likely that the administration of a AGEX-BAT1 cell therapy product would entail a surgical implant procedure which would be if we had a longer operating historyexpensive and would pose risks to the patient related to the surgical procedure that are not faced by users of the injectable or a history of successfully developing and commercializing genetic medicine products. In addition, as a business with a limited operating history, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will eventually need to transition from a company with a research focus to a company capable of supporting commercial activities. We may not be successful in such a transition.pill GLP-1 receptor agonist drugs currently on the market.
| 37 |
We need to successfully develop and market or license therapeutic products or technologies in order to earn revenues in sufficient amounts to meet our operating expenses. Without significant product sales or licensing fee revenues, we will not be able to operate at a profit, and we will not be able to cover our operating expenses without raising additional capital. Should we be able to successfully develop and market any therapeutic products we may not be able to receive reimbursement for them from payers, such as health insurance companies, health maintenance organizations and Medicare, or any reimbursement that we receive may be lower than we anticipate.
As we continue to attempt to build our business, we expect our financial condition and operating results may fluctuate significantly from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. Accordingly, you should not rely upon the results of any particular quarterly or annual period as indications of future operating performance.
Our choice of product candidates and our development plans for our product candidates are subject to change based on a variety of factors, and if we abandon development of a product candidate we may not be able to develop or acquire a replacement product candidate.
We may determine to abandon the development of one or more of our product candidates, or we may change the prioritization of the development of certain product candidates, or we may select or acquire and prioritize the development of new product candidates. Our choice and prioritization of product candidates for development will be influenced by a variety of factors, including but not limited to:
| ● | the amount of capital that we will have for our development programs and our projected costs for those programs; | |
| ● | ability to enter into licensing or collaborative arrangements with other biotechnology or biopharma companies or universities with their own laboratory facilities and research staffs to conduct research and development of one or more product candidates; | |
| ● | competitors may develop alternatives that render our potential product candidates obsolete or less attractive; | |
| ● | potential product candidates we develop may nevertheless be covered by third parties’ patents or other exclusive rights; | |
| ● | potential product candidates may not be effective in treating their targeted diseases; | |
| ● | potential product candidates may, on further study, be shown to have harmful side effects, toxicities or other characteristics that indicate that they are unlikely to be products that will receive marketing approval and achieve market acceptance; | |
| ● | ||
our analysis of market demand and market prices for the products we plan to develop could lead us to conclude that market conditions are not favorable for receiving an adequate return on our investment in product development and commercialization; | ||
| ● | a potential product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; or | |
| ● | the regulatory pathway for a potential product candidate is too complex and difficult to navigate successfully or economically. |
In addition, we may choose to focus our efforts and resources on a potential product candidate that ultimately proves to be unsuccessful. As a result, we may fail to capitalize on viable commercial products or profitable market opportunities, be required to forego or delay pursuit of opportunities with other product candidates or other diseases that may later prove to have greater commercial potential, or relinquish valuable rights to such product candidates through collaboration, licensing or other royalty arrangements in cases in which it would have been advantageous for us to retain sole development and commercialization rights. If we are unable to identify additional suitable product candidates for clinical development, this would adversely impact our business strategy and our financial position and share price and could potentially cause us to cease operations.
We will needmay determine to expand our organization and obtain laboratory facilities if we are able to raise sufficient capital to do so, and we may experience difficulties in managing this growth, which could disrupt our operations.
As of December 31, 2018,2023, we had 12eight employees. We will needIf we are able to significantly expandobtain sufficient capital and determine to reinstitute our organizationinternal research and development efforts, we may have difficulty locating, leasing, and equipping a new laboratory facility and identifying, hiring and integrating new scientific and laboratory personnel. Many of the biotechnology companies that we compete against for qualified personnel and consultants have greater financial and other resources, different risk profiles and a longer history in the industry than we do and are better positioned to attract and retain personnel and consultants. If we are unable to continue to attract and retain high-quality personnel and consultants, the rate and success at which we can discover and develop product candidates and operate our business will be limited.
Future growth would impose significant additional responsibilities on our management, including the need to identify, recruit, maintain, motivate and integrate additional employees, consultants and contractors. Also, our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expectedAny growth of administrative resources could require significant capital expenditures and may divert financial resources from other projects, such as the development of product candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenues could be reduced, and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize our product candidates and compete effectively will depend, in part, on our ability to effectively manage any future growth.
Many of the biotechnology companies that we compete against for qualified personnel and consultants have greater financial and other resources, different risk profiles and a longer history in the industry than we do. If we are unable to continue to attract and retain high-quality personnel and consultants, the rate and success at which we can discover and develop product candidates and operate our business will be limited.
The commercial success of any of our current or future product candidates will depend upon the degree of market acceptance by physicians, patients, third-party payors, and others in the medical community.
Even with the approvals from the FDA and comparable foreign regulatory authorities, the commercial success of our products will depend in part on the health care providers, patients, and third-party payors accepting our product candidates as medically useful, cost-effective, and safe. Any product that we bring to the market may not gain market acceptance by physicians, patients, third-party payors and other health care providers. The clinical development, commercialization, and marketing of cell therapies are at an early-stage, substantially research-oriented, and financially speculative. To date, very few companies have been successful in their efforts to develop and commercialize cell therapies. In general, cell therapies may be susceptible to various risks, including undesirable and unintended side effects, unintended immune system responses, inadequate therapeutic efficacy, potentially prohibitive costs or other characteristics that may prevent or limit their approval or commercial use. Furthermore, the number of people who may use cell- or tissue-based therapies is difficult to forecast with accuracy. Our future success is dependent on the establishment of a large global market for cell therapies and our ability to capture a share of this market with our product candidates.
| 38 |
Even if we, a collaborator, or a licensee of our technology successfully develop and obtain regulatory approval for our product candidates, the market may not understand or accept them. Our product candidates represent novel treatments and are expected to compete with a number of more conventional products and therapies manufactured and marketed by others, including major pharmaceutical and biotechnology companies. The degree of market acceptance of any of our products will depend on a number of factors, including without limitation:
| ● | the efficacy of the product as demonstrated in clinical studies and potential advantages over competing treatments; | |
| ● | the prevalence and severity of the disease and any side effects; | |
| ● | the clinical indications for which approval is granted, including any limitations or warnings contained in a product’s approved labeling; | |
| ● | the convenience and ease of administration; | |
| ● | the cost of treatment, particular as additive to existing treatments; | |
| ● | the willingness of the patients and physicians to accept and use these therapies and the perception of efficacy and safety of our approved products by such parties; | |
| ● | the marketing, sales and distribution support for the products; |
| ● | the publicity and ethical, social and legal concerns regarding the use of embryonic stem cells for our products or competing products and treatments; | |
| ● | government regulations restricting or prohibiting our research or manufacturing processes for stem cells due to ethical, social and legal concerns regarding their use in medical research and treatment; and | |
| ● | the pricing and availability of third-party insurance coverage and reimbursement. |
Even if a product displays a favorable efficacy and safety profile upon approval, market acceptance of the product will initially remain uncertain. Efforts to educate the medical community and third-party payors on the benefits of the products may require significant investment and resources and may never be successful. If our products fail to achieve an adequate level of acceptance by physicians, patients, third-party payors, and other health care providers, we will not be able to generate sufficient revenue to become or remain profitable.
If the market opportunities for our product candidates are smaller than we believe they are, we may not meet our revenue expectations and, even assuming approval of a product candidate, our business may suffer.
Our projections of the number of potential users of our product candidates in the markets we are attempting to address are based on our beliefs and estimates and include several key assumptions based on our industry knowledge, industry publications, third-party research reports and other surveys. You should bear in mind the following:
| ● | Our estimates have been derived from a variety of sources, including publications and scientific literature or market research estimating the total number of patients and currently approved or used therapies, as well as certain assumptions regarding the potential size of the market assuming broad regulatory approval or potential usage by physicians beyond the approved label, any of which may prove to be incorrect. | |
| ● | The scope of approval and potential use may be significantly narrower, and the number of patients may turn out to be lower than expected. | |
| ● | Competitive products or approaches may be approved or come into use by medical providers and the potentially addressable patient population for each of our product candidates may be limited or may not be amenable to treatment with our product candidates, and new patients may become increasingly difficult to identify or gain access to, any which could adversely affect our results of operations and our business. |
If the actual market for any of our product candidates is smaller than we expect, our revenue may be limited and it may be more difficult for us to achieve or maintain profitability.
We will face risks related to the manufacture of medical products for any product candidates that we developdevelop..
The manufacture of medical products, and in particular biologics, is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls, none of which we presently have. Unless we are able to raise the capital required to construct our own manufacturing facilities and are able to develop the expertise to manage and operate a manufacturing facility of our own, which we maydo not expect to undertake, we would need to rely on third-party manufacturers to manufacture any products that we develop. There is no assurance that we will be able to identify manufacturers on acceptable terms or at all. Regardless of whether we do our own manufacturing or rely on third parties to manufacture products for us, we will face all risks related to the manufacture of therapeutic products for use in medicine including the following risks:
| ● | We or any third-party manufacturers might be unable to timely formulate and manufacture our products or produce the quantity and quality required to meet our clinical and commercial needs, if any. | |
| ● | We or any third-party manufacturers may not be able to execute our manufacturing procedures appropriately. |
| 39 |
| ● | ||
| Any third-party manufacturers we engage may not perform as agreed or may not remain in the contract manufacturing business for the time required to supply our clinical trials or to successfully produce, store and distribute our products on a commercial scale. | ||
| ● | We or any third-party manufacturers will be subject to ongoing periodic unannounced inspection by the |
| ● | We may not own, or may have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process for our product candidates. | |
| ● | Third-party manufacturers could breach or terminate their agreements with us. | |
| ● | We or third-party manufacturers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable political environments. |
In addition, we may rely on third parties to perform release testing on our product candidates prior to delivery to patients. If these tests are not appropriately conducted and test data are not reliable, patients could be put at risk of serious harm which could result in product liability suits.
If we or any third-party manufacturers that we may engage were to encounter any of these difficulties, our ability to provide our product candidates to patients in clinical trials or to the medical market placemarketplace would be jeopardized. Any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, could require us to either commence new clinical trials at additional expense or terminate clinical trials completely.
The regulatory authorities also may, at any time following approval of a product for sale, audit our manufacturing facilities or those of our third-party contractors. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time-consuming for us or a third party to implement and that may include the temporary or permanent suspension of a clinical study or commercial sales or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business.
Additionally, if supply from one approved manufacturer is interrupted, there could be a significant disruption in commercial supply. The regulatory agencies may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines.
Further, our product candidates are manufactured by starting with established master cell banks of human embryonic cells and other cells that are cryopreserved. We will be required to expand the numbers of the pluripotent stem cell master cell banks for future use, as well as produce working cell banks from which the product will be manufactured for clinical trials, produce the relevant product under cGMP conditions, expand the number of relevant cells and cryopreserve them under cGMP conditions. We may not be able to expand the numbers of the pluripotent stem cell master cell banks to provide sufficient cells for clinical trial or for commercial scale production. We may not be able to manufacture product that meets release criteria due to sterility, identity or potency issues. We may not have access or be able to make the reagents necessary to manufacture the cells and we may not have access to an adequate supply channels to transport and distribute the products. There are also risks that the cells may be destroyed by interruption in their cryopreservation by means of natural disasters such as earthquakes, power outages, or other unexpected events, or the cells may be determined to be unacceptable as a source of human cellular therapies for reasons we cannot envision. We cannot assure you that any stability or other issues relating to the manufacture of any of our product candidates or products will not occur in the future. If any of our master cell banks are lost or destroyed, including due to systems failure in the cryopreservation processes, our planned clinical trials would be severely delayed, and we would incur significant costs associated with obtaining new supply of cell banks. Accordingly, failures or difficulties faced at any level of our supply chain could adversely affect our business and delay or impede the development and commercialization of any of our product candidates or products and could have an adverse effect on our business, prospects, financial condition and results of operations.
Any therapies that we may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing cell-based products for us. Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval.
Each of these risks could delay our clinical trials, any approval of our product candidates by the FDA, or the commercialization of our product candidates, and could result in higher costs or deprive us of potential product revenue.
Any cell-based products that receive regulatory approval may be difficult and expensive to manufacture on a commercial scale.
Pluripotent stem cell and progenitor cell derived therapeutic cells have only been produced on a small scale and not in quantities and at levels of purity and viability that will be needed for wide scale commercialization. If we are successful in developing products that consist of cells or compounds derived from pluripotent stem cells or progenitor cells, we will need to develop facilities, processes, and technology for the commercial production of those products. Pluripotent stem cell or progenitor cell based products are likely to be more expensive to manufacture on a commercial scale than most other drugs on the market today. The highhigher cost of manufacturing a product will require that we charge our customers a highhigher price for the product in order to cover our costs and earn a profit. If the price of our products is too high, hospitals and physicians may be reluctant to purchase our products and we may not be able to sell our products in sufficient volumes to recover our costs or to earn a profit.
If we fail to meet our obligations under license agreements, we may lose our rights to key technologies on which our business depends.
Our business will depend on several critical technologies that we have licensed or sublicensed from BioTimeLineage Cell Therapeutics, Inc. (Lineage) or certain BioTimeLineage subsidiaries. The license and sublicense agreements impose obligations on us, including payment obligations and obligations to pursue development and commercialization of products and technologies under the licensed patents or technology. If the licensor or sublicensor believes that we have failed to meet our obligations under a license or sublicense agreement, they could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, our loss of the licensed rights. In addition, certain of our licensing counterparties may terminate without cause, including Yeda Research in connection with the relational databases that we in-license from Yeda for LifeMap Sciences. During the period of any such litigation our ability to carry out the development and commercialization of potential new products or technologies, and our ability to raise any capital that we might then need, could be significantly and negatively affected. If our license rights were restricted or ultimately lost, we would not be able to continue to use the licensed or sublicensed technology in our business.
Our future success depends on our ability to retain our key personnel and to attract, retain and motivate qualified personnel.
Our industry has experienced a high rate of turnover of management personnel in recent years. We are highly dependent on the development, regulatory, commercialization and business development expertise of Michael West, Ph.D., our executive officers, including our Interim Chief Executive Officer as well as the other principal members ofand our management, scientific and clinical teams.Chief Financial Officer. Although we have employment agreementsa consulting agreement with our executive officers,Interim Chief Executive Officer and an employment agreement with our Chief Financial Officer, these agreements do not prevent them from terminating their consulting arrangement or employment with us at any time. In addition, because we will rely on BioTime and Juvenescence Limited (“Juvenescence”) to provide the services of certain administrative and management personnel, we will not have the benefit of the full time and effort of those BioTime and Juvenescence employees in the management and development of our business.
If we lose one or more of our executive officers or key employees, our ability to implement the Merger and the other transactions contemplated by the Merger Agreement or, if the Merger is not completed, our current business strategystrategies successfully could be seriously harmed. Furthermore, replacing executive officers and key employees may be difficult, in particular during the pendency of the Merger, and may take an extended period of time because of the uncertainty about such persons roles following the Merger and the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain regulatory approval of and commercialize product candidates successfully. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these additional key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also will experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we will rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be engaged by entities other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high quality personnel, our ability to developimplement the Merger and commercialize product candidatesthe other transactions contemplated by the Merger Agreement or, if the Merger is not completed, to continue to execute on our current business strategies while seeking out and evaluating potential strategic alternatives with respect to our assets and development programs will be limited.
| 41 |
Our business and operations could suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters including earthquakes and tsunamis, terrorism, war, and telecommunication and electrical failures. Some of our data related to the development of our product candidates resides on BioTime’s computer servers and will be subject to the same risks described above. Further, while we are working to transfer our data from BioTime’s servers to our own servers, there is a risk that data could be lost or corrupted while in the process of being transferred, or could otherwise not be transferred to us. A loss of or damage to our data, a disruption in access to our data, or inappropriate disclosure of confidential or proprietary information, could disrupt our operations, delay or otherwise adversely affect the development of our product candidates, significantly increase our costs, or result in delays in any future regulatory filings we may make .make.
In addition, our product candidates are manufactured by starting withusing cells that are stored in a cryopreserved master cell bank. While we believe we have adequate backup should any cell bank be lost in a catastrophic event, it is possible that we or our third-party suppliers and manufacturers could lose multiple cell banks and have our manufacturing severely impacted by the need to replace the cell banks. See “—We will face risks related to the manufacture of medical products for any product candidates that we develop.” We cannot assure you that any stability or other issues relating to the manufacture of any of our product candidates or products will not occur in the future. Any delay or interruption in the supply of clinical trial supplies could delay the completion of planned clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require us to commence new clinical trials at additional expense or terminate clinical trials completely. Any adverse developments affecting clinical or commercial manufacturing of our product candidates or products may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls or other interruptions in the supply of our product candidates or products. Accordingly, failures or difficulties faced at any level of our supply chain could adversely affect our business and delay or impede the development and commercialization of any of our product candidates or products and could have an adverse effect on our business, prospects, financial condition and results of operations.
Security breaches and other disruptions could compromise our information and expose us to liability, and could cause our business and reputation to suffersuffer..
In the ordinary course of business, we collect and store sensitive data, including intellectual property, our proprietary business information and that of the licensors and licensees of the patents and other intellectual property we use, and personally identifiable information of employees and consultants. The secure processing, maintenance, and transmission of this information is critical to our operations and business strategy. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance, or other disruptions. Any such breach could compromise our networks and the information stored there could be accessed, publicly disclosed, lost, or stolen. Any such access, disclosure, theft, or other loss of information could result in legal claims or proceedings or liability under laws that protect the privacy of personal information, and could disrupt our operations and damage our reputation. Even if we do not incur an interruption of or our operations, fines, penalties, or financial liability to third parties from a security breach, we could suffer a loss of confidence in our services, which could adversely affect our business and competitive position.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs and product candidates that we identify for specific indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to timely capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
Failure of our internal control over financial reporting could harm our business and financial results.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting for external purposes in accordance with accounting principles generally accepted in the U.S. GAAP. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our consolidated financial statements; providing reasonable assurance that receipts and expenditures of our assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use, or disposition of our assets that could have a material effect on the consolidated financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our consolidated financial statements would be prevented or detected. Because we are an emerging growth company and a smaller reporting issuer, we are exempt from the requirement of having our internal controls over financial reporting audited by our independent registered public accountants, which means that material weaknesses or significant deficiencies in our internal controls that might be detected by an audit may not be detected and remedied. If we are successful in developing new medical products and technologies, the commercialization of those products and technologies will place significant additional pressure on our system of internal control over financial reporting. Any failure to maintain an effective system of internal control over financial reporting could limit our ability to report our financial results accurately and timely or to detect and prevent fraud. See “—Risks Pertaining to Our Common Stock—Our accounting and other management systems and resources may not be adequately prepared to meet the financial reporting and other requirements to which we will be subject, and failure to achieve and maintain effective internal controls could have a material adverse effect on our business.
We are subject to laws and regulations governing corruption, which will require us to develop, maintain, and implement costly compliance programs.
We must comply with a wide range of laws and regulations to prevent corruption, bribery, and other unethical business practices, including the Foreign Corrupt Practices Act (FCPA), anti-bribery and the priceanti-corruption laws in other countries. The creation and implementation of international business practices compliance programs is costly and such programs are difficult to enforce, particularly where reliance on third parties is required.
Anti-bribery laws prohibit us, our employees, and some of our common stock.”
We will initially relyagents or representatives from offering or providing any personal benefit to covered government officials to influence their performance of their duties or induce them to serve interests other than the missions of the public organizations in part on financial systems maintained by BioTimewhich they serve. Certain commercial bribery rules also prohibit offering or providing any personal benefit to employees and upon services provided by BioTime personnel. BioTime will allocaterepresentatives of commercial companies to influence their performance of their duties or induce them to serve interests other than their employers. The FCPA also obligates companies whose securities are listed in the U.S. to comply with certain expenses among itself,accounting provisions requiring us to maintain books and BioTime’s otherrecords that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and affiliates, which creates a risk thatdevise and maintain an adequate system of internal accounting controls for international operations. The anti-bribery provisions of the allocations may not accurately reflect the benefit of an expenditure or use of financial or other resourcesFCPA are enforced primarily by us, BioTime, and the BioTime subsidiaries and affiliates among which the allocations are made.
Recent changes in U.S. federal income tax law may have an adverse effect on our cash flows, results of operations or financial condition.
On December 22, 2017, the United States enacted major federal tax reform legislation, Public Law No. 115-97, commonly referredDepartment of Justice. The SEC is involved with enforcement of the books and records provisions of the FCPA.
| 42 |
Compliance with these anti-bribery laws is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, the anti-bribery laws present particular challenges in the medical industry because in many countries including China, hospitals are state-owned or operated by the government, and doctors and other hospital employees are considered foreign government officials. Furthermore, in certain countries (China in particular), hospitals and clinics are permitted to assell pharmaceuticals to their patients and are primary or significant distributors of pharmaceuticals. Certain payments to hospitals in connection with clinical studies, procurement of pharmaceuticals and other work have been deemed to be improper payments to government officials that have led to vigorous anti-bribery law enforcement actions and heavy fines in multiple jurisdictions, particularly in the 2017 Tax CutsU.S. and Jobs Act (“2017 Tax Act”),China.
It is not always possible to identify and deter violations, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations.
In the medical industry, corrupt practices include, among others, acceptance of kickbacks, bribes or other illegal gains or benefits by the hospitals and medical practitioners from manufacturers of pharmaceutical or other products, distributors or their third party agents in connection with the prescription of certain pharmaceuticals or sale of products. If our employees, affiliates, distributors or third party marketing firms violate these laws or otherwise engage in illegal practices with respect to their sales or marketing of our products or other activities involving our products, we could be required to pay damages or heavy fines by multiple jurisdictions where we operate, which enacted a broad rangecould materially and adversely affect our financial condition and results of changesoperations. There have been recent occurrences in which certain hospitals have denied access to sales representatives from pharmaceutical companies because the hospitals wanted to avoid the perception of corruption. If this attitude becomes widespread among our potential customers, our ability to promote our products to hospitals may be adversely affected.
If we and our subsidiaries expand operations internationally, we will need to increase the scope of our compliance programs to address the risks relating to the Internal Revenue Code. Changes to taxes on corporations impacted by the 2017 Tax Act include, but not limited to, changing the U.S. federal tax rate on corporations to a 21 percent flat tax rate, eliminating the corporate alternative minimum tax (“AMT”), imposing additional limitations on the deductibility of interest and net operating losses, allowing any net operating loss (“NOLs”) generated in tax years ending after December 31, 2017 to be carried forward indefinitely and generally repealing NOL carrybacks, reducing the maximum deductionpotential for NOL carryforwards arising in tax years beginning after 2017 to a percentageviolations of the taxpayer’s taxable income,FCPA and allowingother anti-bribery and anti-corruption laws. Our compliance programs will need to include policies addressing not only the FCPA, but also the provisions of a variety of anti-bribery and anti-corruption laws in multiple foreign jurisdictions, provisions relating to books and records that apply to us as a public company, and include effective training for additional expensingour personnel throughout our organization. The creation and implementation of certain capital expenditures. The 2017 Tax Act also puts into effect a number of changes impacting operations outsideanti-corruption compliance programs is costly and such programs are difficult to enforce, particularly where reliance on third parties is required. Violation of the United StatesFCPA and other anti-corruption laws can result in significant administrative and criminal penalties for us and our employees, including butsubstantial fines, suspension or debarment from government contracting, prison sentences, or even the death penalty in extremely serious cases in certain countries. The SEC also may suspend or bar us from trading securities on U.S. exchanges for violation of the FCPA’s accounting provisions. Even if we are not limitedultimately punished by government authorities, the costs of investigation and review, distraction of our personnel, legal defense costs, and harm to our reputation could be substantial and could limit our profitability or our ability to develop or commercialize our product candidates. In addition, if any of our competitors are not subject to the impositionFCPA, they may engage in practices that will lead to their receipt of a one-time tax “deemed repatriation” on accumulated offshore earnings not previously subjectpreferential treatment from foreign hospitals and enable them to U.S. tax, and shifts the U.S taxation of multinational corporationssecure business from a worldwide system of taxationforeign hospitals in ways that are unavailable to a territorial system. Notwithstanding the reduction in the corporate income tax rate, the overall impact of the new federal tax law is uncertain and our business and financial condition could be adversely affected. In addition, it is uncertain if and to what extent various states will conform to the newly enacted federal tax law.us.
Risks Related to Our Industry
We face significant competition in an environment of rapid technological change and the possibility that our competitors may achieve regulatory approval before us or develop therapies that are more advanced or effective than ours, which may harm our business and financial condition, and our ability to successfully market or commercialize our product candidates.
The biotechnology and pharmaceutical industries are characterized by rapidly changing technologies, competition and a strong emphasis on intellectual property. We may face competition from other companies focused on therapeutics for age-related disease, which is a highly competitive environment. There are numerous biotechnology companies developing therapeutics for human aging, with each company often focusing on a specific molecular pathway within cells. For example, ResTORbio, Inc. is developing modulators of the mechanistic target of rapamycin (mTOR) pathway to treat immunological and cardiovascular disorders. Calico Life Sciences LLC is a Google-founded research and development company aimed at identifying molecular pathways that control animal lifespan and translating these insights into novel therapeutics designed to increase human healthspan. Unity Biotechnology, Inc. focuses on cellular senescence, in particular, the use of agents that can target senescent cells for selective ablation (senolysis). Unity’s stated targeted age-related diseases include osteoarthritis as well as other ophthalmological and pulmonary diseases. Our therapeutic products in development are likely to face competition from a large number of companies and technological strategies including therapeutics intended to address our lead indications. See “Business – Competition.“Business—Competition.”
| 43 |
We may also face competition from large and specialty pharmaceutical and biotechnology companies, academic research institutions, government agencies and public and private research institutions. Many of our current or potential competitors, either alone or with their collaboration partners, have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals, and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical, biotechnology, and gene therapy industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive than any products that we may develop or that would render any products that we may develop obsolete or non-competitive. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In particular, the Ministry of Labor Health and Welfare in Japan may grant SAKIGAKE designation to a competing product candidate, which is designed to provide for faster review and approval for any such product candidate as compared to the conventional process. If any competing product candidate receives SAKIGAKE designation in Japan, it may be commercialized more quickly in Japan than any of our product candidates. Additionally, technologies developed by our competitors may render our potential product candidates uneconomic or obsolete, and we may not be successful in marketing any product candidates we may develop against competitors.
Any product candidates for which we intend to seek approval as biologic products may face competition sooner than anticipated.
The Patient Protection and Affordable Care Act, signed into law on March 23,as amended by the Health Care and Education Reconciliation Act of 2010 (“ACA”),(collectively, the ACA) includes a subtitle called the Biologics Price Competition and Innovation Act of 2009, or BPCIA, which created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first licensed. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLAa biologics license application (BLA) for the competing product containing the sponsor’s own pre-clinicalpreclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty. While it is uncertain when such processes intended to implement BPCIA may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for our biological products.
There is a risk that any of our product candidates approved as a biological product under a BLA would not qualify for the 12-year period of exclusivity or that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate and it is possible that any other product candidates we may seek to develop in the future will ever obtain regulatory approval. Neither we nor any future collaborator or licensee is permitted to market any of our product candidates in the United States until we receive regulatory approval of a biologic license application, or BLA from the FDA. It is possible that the FDA may refuse to accept for substantive review any BLAs that we submit for our product candidates or may conclude after review of our data that our application is insufficient to obtain marketing approval of our product candidates.
| 44 |
Prior to obtaining approval to commercialize a product candidate in the United States or abroad, we or our collaborators mustor licensees will need to demonstrate with substantial evidence from well-controlled clinical trials, and to the satisfaction of the FDA or foreign regulatory agencies, that suchthe product candidates arecandidate is safe and effective for theirthe intended uses. Results from nonclinical studies and clinical trials can be interpreted in different ways. Even if we believe the nonclinical or clinical data for our product candidates are promising, such data may not be sufficient to support approval by the FDA and other regulatory authorities. The FDA may also require us to conduct additional preclinical studies or clinical trials for our product candidates either prior to or post-approval, or it may object to elements of our clinical development program. Depending on the extent of these or any other FDA-required studies, approval of any BLA or application that we submit may be delayed by several years or may require us to expend significantly more resources than we have available.
Any therapeutic products that we and our subsidiaries may develop cannot be sold until the FDA and corresponding foreign regulatory authorities approve the products for medical use. The need to obtain regulatory approval to market a new product means that:
| ● | ||
| ● | Clinical trials and the regulatory approval process for a pharmaceutical or cell-based product can take several years to complete. As a result, we will | |
| ● | Data obtained from preclinical and clinical studies is susceptible to varying interpretations and regulatory changes that could delay, limit, or prevent regulatory agency approvals. |
| ● | Because the therapeutic products we plan to develop with pluripotent stem cell technology or progenitor cell technology involve the application of new technologies and approaches to medicine, the FDA or foreign regulatory agencies may subject those products to additional or more stringent review than drugs or biologicals derived from other technologies. | |
| ● | A product that is approved may be subject to restrictions on use. | |
| ● | The FDA can recall or withdraw approval of a product if it deems necessary. | |
| ● | We will face similar regulatory issues in foreign countries. |
Approval of our product candidates may be delayed or refused for many reasons, including the following:
| ● | ||
| ● | ||
| ● | ||
| ● | ||
| ● | ||
| ● | ||
| ● | ||
| ● |
Of the large number of potential products in development, only a small percentage successfully complete the FDA or foreign regulatory approval processes and are commercialized. The lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval to market our product candidates, which would significantly harm our business, results of operations and prospects.
Ethical, social and legal concerns about research regarding stem cells, could result in regulations restricting or prohibiting the processes we may use. Federal and state agencies, congressional committees and foreign governments have expressed interest in further regulating biotechnology. More restrictive regulations or claims that our products are unsafe or pose a hazard could prevent us from commercializing any products. New government requirements may be established that could delay or prevent regulatory approval of our product candidates under development. It is impossible to predict whether legislative changes will be enacted, regulations, policies or guidance changed, or interpretations by agencies or courts changed, or what the impact of such changes, if any, may be.
| 45 |
Regulatory requirements governing gene and cell therapy products have changed frequently and may continue to change in the future. The FDA has established the Office of Tissues and Advanced Therapies within its Center for Biologics Evaluation and Research, or CBER, to consolidate the review of gene therapy and related products, and has established the Cellular, Tissue and Gene Therapies Advisory Committee to advise the CBER in its review. Gene therapy clinical trials conducted at institutions that receive funding for recombinant DNA research from the NIH,National Institute of Health (NIH) also are potentially subject to review by the NIH Office of Science Policy’s Recombinant DNA Advisory Committee, or the RAC, in limited circumstances. Although the FDA decides whether individual gene therapy protocols may proceed, the RAC public review process, if undertaken, can delay the initiation of a clinical trial, even if the FDA has reviewed the trial design and details and authorized its initiation. Conversely, the FDA can put an investigational new drug application, or IND, on clinical hold even if the RAC has provided a favorable review or an exemption from in-depth, public review. If we were to engage an NIH-funded institution to conduct a clinical trial, that institution’s institutional biosafety committee, or IBC, as well as its institutional review board, or IRB, would need to review the proposed clinical trial to assess the safety of the trial and may determine that RAC review is needed. In addition, adverse developments in clinical trials of gene therapy products conducted by others may cause the FDA or other oversight bodies to change the requirements for approval of any of our product candidates. Similarly, foreign regulatory authorities may issue new guidelines concerning the development and marketing authorization for gene therapy medicinal products and require that we comply with these new guidelines.
Some of our future products may be viewed by the FDA as combination products and the review of combination products is often more complex and more time consuming than the review of other types of products.
Our future products may be regulated by the FDA as combination products. For a combination product, the FDA must determine which center or centers within the FDA will review the product candidate and under what legal authority the product candidate will be reviewed. The process of obtaining FDA marketing clearance or approval is lengthy, expensive, and uncertain, and we cannot be sure that any of our combination products, or any other products, will be cleared or approved in a timely fashion, or at all. In addition, the review of combination products is often more complex and more time consuming than the review of a product candidate under the jurisdiction of only one center within the FDA. We cannot be sure that the FDA will not select to have our combination products reviewed and regulated by only one FDA center and/or different legal authority, in which case the path to regulatory approval would be different and could be more lengthy and costly. If the FDA does not approve or clear our products in a timely fashion, or at all, our business and financial condition will be adversely affected.
If we encounter difficulties enrolling patients in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the study until its conclusion. We may encounter delays in enrolling, or be unable to enroll, a sufficient number of patients to complete any of our clinical trials of our product candidates, and even once enrolled we may be unable to retain a sufficient number of patients to complete any of ourthe trials. The enrollment of patients depends on many factors, including:
| ● | the patient eligibility criteria defined in the protocol; | |
| ● | the size of the patient population required for analysis of the trial’s primary endpoints; | |
| ● | the proximity of patients to study sites; | |
| ● | the design of the trial; | |
| ● | our ability to recruit clinical trial investigators with the appropriate competencies and experience; | |
| ● | clinicians’ and patients’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new products that may be approved for the indications we are investigating; | |
| ● | our ability to obtain and maintain patient consents; and | |
| ● | the risk that patients enrolled in clinical trials will drop out of the trials before completion. |
In addition, our clinical trials of our product candidates will compete with other clinical trials for product candidates of other companies that are in the same therapeutic areas as our product candidates, and this competition will reduce the number and types of patients available to us, because some patients who might have opted to enroll in trials of our trialsproduct candidates may instead opt to enroll in a trial being conducted by one of our competitors. Since the number of qualified clinical investigators is limited, we expect to conduct some ofthat clinical trials or our clinical trialsproduct candidates may be conducted at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials in such clinical trial site.
Delays or failures in planned patient enrollment or retention may result in increased costs, program delays or both, which could have a harmful effect on our ability to develop our product candidates or could render further development impossible.
Even if we obtain FDA approval for any of our product candidates in the United States, we may never obtain approval for or commercialize it in any other jurisdiction, which would limit our ability to realize its full market potential.
In order to market any products in any particular jurisdiction, we mustor a licensee or collaborator will need to establish and comply with numerous and varying regulatory requirements on a country-by-country basis regarding safety and efficacy. Approval by the FDA in the United States does not ensure approval by regulatory authorities in other countries or jurisdictions. However, the failure to obtain approval in one jurisdiction may negatively impact our ability to obtain approval elsewhere. In addition, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not guarantee regulatory approval in any other country.
Approval processes vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking foreign regulatory approval could result in difficulties and increased costs for us and require additional preclinical studies or clinical trials which could be costly and time consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of our products in those countries. We do not have any product candidates approved for sale in any jurisdiction, including in international markets, and we do not have experience in obtaining regulatory approval in international markets. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approvals in international markets are delayed, our target market will be reduced and our ability to realize the full market potential of any product we develop will be unrealized.
Clinical studies are costly, time consuming and inherently risky, and we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authoritiesauthorities.
Clinical development is expensive, time consuming and involves significant risk. We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical studies can occur at any stage of development. Events that may prevent successful or timely completion of clinical development include but are not limited to:
| ● | inability to generate satisfactory preclinical, toxicology, or otherin vivo orin vitro data to support the initiation or continuation of clinical studies necessary for product approval; | |
| ● | delays in reaching agreement on acceptable terms with clinical research organizations | |
| ● | delays in obtaining required Institutional Review Board, or IRB, approval at each clinical study site; | |
| ● | failure to permit the conduct of a study by regulatory authorities, after review of an investigational new drug, or IND, or equivalent foreign application or amendment; | |
| ● | delays in recruiting qualified patients in our clinical studies; | |
| ● | failure by clinical sites or our CROs or other third parties to adhere to clinical study requirements or report complete findings; | |
| ● | failure to perform the clinical studies in accordance with the FDA’s good clinical practices requirements, or applicable foreign regulatory guidelines; | |
| ● | patients dropping out of our clinical studies; | |
| ● | occurrence of adverse events associated with our product candidates; | |
| ● | inability to use clinical trial results from foreign jurisdictions in support of U.S. regulatory approval; | |
| ● | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; | |
| ● | the cost of clinical studies of our product candidates; | |
| ● | negative or inconclusive results from our clinical trials which may result in our deciding, or regulators requiring us, to conduct additional clinical studies or abandon development programs for a product candidate; and | |
| ● | delays in reaching agreement on acceptable terms with third-party manufacturers, or delays in the manufacture of sufficient quantities of our product candidates for use in clinical studies. |
Any inability to successfully complete clinical development and obtain regulatory approval could result in additional costs to us or impair our ability to generate revenue. Clinical study delays could also shorten any periods during which our products have patent protection and may allow competitors to develop and bring products to market before we do and may harm our business and results of operations.
Even if we receivea product candidate receives regulatory approval, of our product candidates, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our product candidates.
Any product candidate for which we obtainthat receives marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, packaging, distribution, adverse event reporting, storage, recordkeeping, export, import, advertising and promotional activities for such product, among other things, will be subject to extensive and ongoing requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, establishment registration and drug listing requirements, continued compliance with cGMP requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping and Good Clinical Practice or GCP,(GCP) requirements for any clinical trials that we conduct post-approval.
| 47 |
The FDA closely regulates the post-approval marketing and promotion of genetic medicines to ensure they are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we market our products for uses beyond their approved indications, we may be subject to enforcement action for off-label marketing. Violations of the U.S. federal Food, Drug, and Cosmetic Act, or FDCA, relating to the promotion of prescription drugs may lead to FDA enforcement actions and investigations alleging violations of federal and state health care fraud and abuse laws, as well as state consumer protection laws.
In addition, later discovery of previously unknown adverse events or other problems with our products, manufacturers or manufacturing processes, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| ● | restrictions on manufacturing such products; | |
| ● | restrictions on the labeling or marketing of a product; | |
| ● | restrictions on product distribution or use; | |
| ● | requirements to conduct post-marketing studies or clinical trials; | |
| ● | warning letters or holds on clinical trials; | |
| ● | withdrawal of the products from the market; | |
| ● | refusal to approve pending applications or supplements to approved applications that we submit; | |
| ● | recall of products; | |
| ● | fines, restitution or disgorgement of profits or revenues; | |
| ● | suspension or withdrawal of marketing approvals; | |
| ● | refusal to permit the import or export of our products; | |
| ● | product seizure or detention; or | |
| ● | injunctions or the imposition of civil or criminal penalties. |
The FDA’s policies may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of any of our product candidates. For example, in December 2016, the 21st Century Cures Act, or Cures Act, was signed into law. The Cures Act, among other things, is intended to modernize the regulation of drugs and biologics and spur innovation, but its ultimate implementation is unclear. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained which would adversely affect our business, prospects and ability to achieve or sustain profitability.
We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. For example, certain policies of the Trump administration may impact our business and industry. Namely, the Trump administration has taken several executive actions, including the issuance of a number of Executive Orders, that could impose significant burdens on, or otherwise materially delay, FDA’s ability to engage in routine regulatory and oversight activities such as implementing statutes through rulemaking, issuance of guidance, and review and approval of marketing applications. Notably, on January 30, 2017, President Trump issued an Executive Order, applicable to all executive agencies, including the FDA, that requires that for each notice of proposed rulemaking or final regulation to be issued in fiscal year 2017, the agency shall identify at least two existing regulations to be repealed, unless prohibited by law. These requirements are referred to as the “two-for-one” provisions. This Executive Order includes a budget neutrality provision that requires the total incremental cost of all new regulations in the 2017 fiscal year, including repealed regulations, to be no greater than zero, except in limited circumstances. For fiscal years 2018 and beyond, the Executive Order requires agencies to identify regulations to offset any incremental cost of a new regulation and approximate the total costs or savings associated with each new regulation or repealed regulation. In interim guidance issued by the Office of Information and Regulatory Affairs within the Office of Management and Budget on February 2, 2017, the Trump administration indicates that the “two-for-one” provisions may apply not only to agency regulations, but also to significant agency guidance documents. Further, on February 24, 2017, President Trump issued an Executive Order requiring each agency to designate a regulatory reform officer and create a regulatory reform task force to evaluate existing regulations and make recommendations regarding their repeal, replacement or modification. It is difficult to predict how these requirements will be implemented, and the extent to which they will impact the FDA’s ability to exercise its regulatory authority. If these executive actions impose constraints on FDA’s ability to engage in oversight and implementation activities in the normal course, our business may be negatively impacted.
Our product candidates may cause serious adverse events or undesirable side effects or have other properties which may delay or prevent their regulatory approval, limit the commercial profile of an approved label, or, result in significant negative consequences following marketing approval, if any.
Serious adverse events or undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt,an interruption, delay or halt of clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign authorities. Results of our clinical trials could reveal a high and unacceptable severity and prevalence of side effects, toxicities or unexpected characteristics, including death.
For example, there have been significant adverse side effects in cell therapy treatments in the past, including reported cases of certain cancers. In addition to side effects that may be caused by our product candidates, the conditioning, administration process or related procedures also can cause adverse side effects, including compromise of a patient’s immune system. If unacceptable side effects arise in the development of our product candidates, we, the FDA, the IRBs at the institutions in which our studies are conducted or Data Safety Monitoring Board, or DSMB, could suspend or terminate our clinical trials or the FDA or comparable foreign regulatory authorities could order us to cease clinical trials or deny approval of our product candidates for any or all targeted indications. Treatment-related side effects could also affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff. We expect to have to train medical personnel using our product candidates to understand the side effect profiles for our clinical trials and upon any commercialization of any of our product candidates. Inadequate training in recognizing or managing the potential side effects of our product candidates could result in patient injury or death. Any of these occurrences may harm our business, financial condition and prospects significantly.
| 48 |
If any of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by any such product, including during any long-term follow-up observation period recommended or required for patients who receive treatment using our products, a number of potentially significant negative consequences could result, including:
| ● | regulatory authorities may withdraw approvals of such product; | |
| ● | we may be required to recall a product or change the way such product is administered to patients; | |
| ● | additional restrictions may be imposed on the marketing of the particular product or the manufacturing processes for the product; |
| ● | regulatory authorities may require additional warnings on the label, such as a “black box” warning or contraindication; | |
| ● | we may be required to implement a Risk Evaluation and Mitigation Strategy, or REMS, or create a medication guide outlining the risks of such side effects for distribution to patients; | |
| ● | the product could become less competitive; | |
| ● | we could be sued and held liable for harm caused to patients; and | |
| ● | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, results of operations and prospects.
We face potential product liability, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use or misuse of our product candidates harm patients or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals could be revoked or could otherwise be negatively impacted, and we could be subject to costly and damaging product liability claims.
The use or misuse of any product candidates in clinical trials and the sale of any products for which we obtain marketing approval exposes us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies, or others selling or otherwise coming into contact with our products. There is a risk that our product candidates may induce adverse events. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| ● | impairment of our business reputation; | |
| ● | initiation of investigations by regulators; | |
| ● | withdrawal of clinical trial participants; | |
| ● | costs due to related litigation; | |
| ● | distraction of management’s attention from our primary business; | |
| ● | substantial monetary awards to patients or other claimants; | |
| ● | the inability to commercialize our product candidates; | |
| ● | product recalls, withdrawals or labeling, and marketing or promotional restrictions; | |
| ● | loss of revenue; and | |
| ● | decreased demand for our product candidates, if approved for commercial sale. |
We may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If and when we commence clinical trials or obtain marketing approval for any product candidates, we intend to increase our insurance coverage to include clinical use or the sale of commercial products, as applicable; however, we may be unable to obtain product liability insurance on commercially reasonable terms or in adequate amounts. On occasion, large judgments have been awarded in class action lawsuits based on drugs or medical treatments that had unanticipated adverse effects. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operations and business.
Our insurance policies are expensive and protect us only from some business risks, which leaves us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risk that our business may encounter. Some of the policies we currently maintain include general liability, employment practices liability, property, auto, workers’ compensation, umbrella, and directors’ and officers’ insurance.
Any additional product liability insurance coverage we acquire in the future, may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If we obtain marketing approval for any of our product candidates, we intend to acquire insurance coverage to include the sale of commercial products; however, we may be unable to obtain product liability insurance on commercially reasonable terms or in adequate amounts. A successful product liability claim or series of claims brought against us could cause our share price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operations and business, including preventing or limiting the commercialization of any product candidates we develop.
| 49 |
As a public company, it can be difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified people to serve on ourthe AgeX Board, of Directors, our board committees or as executive officers. We do not know, however, if we will be able to maintain existing insurance with adequate levels of coverage. Any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our cash position and results of operations.
Our employees and independent contractors, including principal investigators, CROs, consultants, vendors, and any third parties we may engage in connection with development and commercialization may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
Misconduct by our employees and independent contractors, including principal investigators, contract research organizations, or CROs, consultants, vendors, and any third parties we may engage in connection with development and commercialization, could include intentional, reckless or negligent conduct or unauthorized activities that violate: (i) the laws and regulations of the FDA, EMAEuropean Medical Agency (EMA) rules and regulations and other similar regulatory requirements, including those laws that require the reporting of true, complete and accurate information to such authorities; (ii) manufacturing standards; (iii) data privacy, security, fraud and abuse and other healthcare laws and regulations; or (iv) laws that require the reporting of true, complete and accurate financial information and data. Specifically, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Activities subject to these laws could also involve the improper use or misrepresentation of information obtained in the course of clinical trials, creation of fraudulent data in pre-clinicalpreclinical studies or clinical trials or illegal misappropriation of drug product, which could result in regulatory sanctions and cause serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, disgorgements, possible exclusion from participation in Medicare, Medicaid, other U.S. federal healthcare programs or healthcare programs in other jurisdictions, individual imprisonment, other sanctions, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations.
Government-imposed bans or restrictions and religious, moral, and ethical concerns about the use of human embryonic stem cells could prevent us from developing and successfully marketing stem cell products.
Government-imposed bans or restrictions on the use of embryos or human embryonic stem cells (“hES cells”)(hES cells), in research and development in the United States and abroad could generally constrain stem cell research, thereby limiting the market and demand for our products.
California law requires that stem cell research be conducted under the oversight of a SCROStem Cell Research Oversight (SCRO) Committee. Many kinds of stem cell research, including the derivation of new hES cell lines, may only be conducted in California with the prior written approval of the SCRO Committee. A SCRO Committee could prohibit or impose restrictions on the research that we plan to do. An adverse decision by a SCRO Committee, or their imposition of restrictions on a research program could adversely affect our ability to enter into co-development or licensing arrangements for the development of a product candidate.
The use of hES cells may give rise to religious, moral, and ethical issues. These considerations could lead to more restrictive government regulations or could generally constrain stem cell research, thereby limiting the market and demand for our products.
Adverse publicity regarding cell-based therapies could impact our business.
Adverse publicity due to the ethical and social controversies surrounding the use of embryonic stem cells or any adverse reported side effects from any stem cell or other cell therapy clinical trials or to the failure of such trials to demonstrate that these therapies are efficacious could materially and adversely affect our ability to raise capital, conduct and complete clinical trials and achieve market acceptance of such products, if approved. For example, research institutions, including those who may be our collaborators, may from time to time publish findings or studies regarding the human genome (such as the Human Genome Project) that adversely implicate our product candidates, including findings of cancer dependencies in cell lines used in our cell-based therapies.
| 50 |
The price and sale of any productsproduct candidates that we may develop may be limited by health insurance coverage and government regulation.
Success in selling ourany pharmaceutical and cell-based products and medical devices that we may develop may depend in part on the extent to which health insurance companies, HMOs,health maintenance organizations (HMOs), and government health administration authorities such as Medicare and Medicaid will pay for the cost of the products and related treatment. Until we introduce a new product is introduced into the medical marketplace, we will not know with certainty whether adequate health insurance, HMO, and government coverage will be available to permit the product to be sold at a price high enough for us to generate a profit. In some foreign countries, pricing or profitability of health care products is subject to government control, which may result in low prices for our products. InProvisions of the Inflation Reduction Act of 2022 may impact the prices of drug products that are sold in the United States, thereparticularly through Medicare programs. Starting in 2023, a manufacturer of a drug or biological product covered by Medicare Parts B or D must pay a rebate to the federal government if the product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by Medicare Parts B or D. Additionally, starting in payment year 2026, CMS will negotiate drug prices annually for a select number of single source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such drug will decrease. There have been a number of other federal and state proposals to implement similar government controls, and new proposals are likely to be made in the future. We cannot be sure that coverage and reimbursement in the United States, the EU or elsewhere will be available for our product candidates or any product that we may develop, and any reimbursement that may become available may be decreased or eliminated in the future.
Third-party payors increasingly are challenging prices charged for pharmaceutical products and services, and many third-party payors may refuse to provide coverage and reimbursement for particular drugs or biologics when an equivalent generic drug, biosimilar or a less expensive therapy is available. It is possible that a third-party payor may consider our product candidates as substitutable and only offer to reimburse patients for the less expensive product. Even if we show improved efficacy or improved convenience of administration with our product candidates, pricing of existing third-party therapeutics may limit the amount we will be able to charge for our product candidates. These payors may deny or revoke the reimbursement status of a given product or establish prices for new or existing marketed products at levels that are too low to enable us to realize an appropriate return on our investment in our product candidates. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our product candidates and may not be able to obtain a satisfactory financial return on our product candidates.
There is significant uncertainty related to the insurance coverage and reimbursement of newly-approved products. In the United States, third-party payors, including private and governmental payors, such as the Medicare and Medicaid programs, play an important role in determining the extent to which new drugs and biologics will be covered. The Medicare and Medicaid programs increasingly are used as models in the United States for how private payors and other governmental payors develop their coverage and reimbursement policies for drugs and biologics. Some third-party payors may require pre-approval of coverage for new or innovative devices or drug therapies before they will reimburse healthcare providers who use such therapies. We cannot predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our product candidates.
No uniform policy for coverage and reimbursement for products exists among third-party payors in the United States. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our product candidates to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Furthermore, rules and regulations regarding reimbursement change frequently, in some cases on short notice, and we believe that changes in these rules and regulations are likely.
Outside the United States, international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe and other countries have and will continue to put pressure on the pricing and usage of our product candidates. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medical products but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for our product candidates may be reduced compared with the United States and may be insufficient to generate commercially-reasonable revenue and profits.
Moreover, increasing efforts by governmental and third-party payors in the United States and abroad to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates. While it is not possible to predict or model the insurance landscape at the time any of our product candidates may receive regulatory approval, we expect to experience pricing pressures in connection with the sale of our product candidates due to the trend toward managed health care, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and biologics and surgical procedures and other treatments, has become intense. As a result, increasingly high barriers are being erected to the entry of new products.
| 51 |
Enacted and future healthcare legislation, including the ACA, may increase the difficulty and cost for us to obtainof obtaining marketing approval of and commercializecommercializing our product candidates and may affect the prices we may set.
In the United States, the EU and other jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes and proposed changes to the healthcare system that could affect our future results of operations. As a result of the adoption of the ACA in the United States, substantial changes have been made to the system for paying for healthcare in the United States. Certain provisions related to cost-savings and reimbursement measures could adversely affect our future financial performance. For example, among the provisions of the ACA, those of greatest importance to the biopharmaceutical industry includes the following:
| ● | an annual, non-deductible fee payable by any entity that manufactures or imports certain branded prescription drugs and biologic agents (other than those designated as orphan drugs), which is apportioned among these entities according to their market share in certain government healthcare programs; | |
| ● | new requirements to report certain financial arrangements with physicians and teaching hospitals, including reporting “transfers of value” made or distributed to prescribers and other healthcare providers and reporting investment interests held by physicians and their immediate family members; | |
| ● | a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected; | |
| ● | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing a manufacturer’s Medicaid rebate liability; | |
| ● | a licensure framework for follow on biologic products; | |
| ● | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and | |
| ● | establishment of a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services, or CMS, to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. |
Since its enactment, some of the provisions of theThe ACA have yet to be fully implemented, while certain provisions havehas been subject to revision and to judicial, congressional, and executive challenges. As a result there have been delaysof tax reform legislation passed in the implementation of, and action taken to repeal or replace, certain aspects of the ACA. The U.S. Supreme Court has upheld certain key aspects of the legislation, including a tax-based shared responsibility payment imposed on certain individuals who fail to maintain qualifying health coverage for all or part of a year, which is commonly known asDecember 2017, the requirement that all individuals maintain health insurance coverage or pay a penalty, referred to as the “individual mandate.” However, as a result of tax reform legislation passed in December 2017, the individual mandate has beenmandate” was eliminated effective January 1, 2019. According to the Congressional Budget Office, the repeal of the individual mandate will cause 13 million fewer Americans to be insured in 2027 and premiums in insurance markets may rise.
Since January 2017, President Trump has signed two Executive Orders designed to delay the implementation of certain provisions of the ACA or otherwise circumvent some of the requirements for health insurance mandated by the ACA. One Executive Order directs federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. The Order requires that for each notice of proposed rulemaking or final regulation to be issued in fiscal year 2017, the applicable agency shall identify at least two existing regulations to be repealed, unless prohibited by law. These requirements are referred to as the “two-for-one” provisions. This Executive Order includes a budget neutrality provision that requires the total incremental cost of all new regulations in the 2017 fiscal year, including repealed regulations, to be no greater than zero, except in limited circumstances. For fiscal years 2018 and beyond, the Executive Order requires agencies to identify regulations to offset any incremental cost of a new regulation and approximate the total costs or savings associated with each new regulation or repealed regulation. In interim guidance issued by the Office of Information and Regulatory Affairs within the Office of Management and Budget on February 2, 2017, the Trump administration indicates that the “two-for-one” provisions may apply not only to agency regulations, but also to significant agency guidance documents.
The second Executive Order terminates the cost-sharing subsidies that reimburse insurers under the ACA. Several state Attorneys General filed suit to stop the administration from terminating the subsidies, but their request for a restraining order was denied by a federal judge in California on October 25, 2017. The loss of the cost share reduction payments is expected to increase premiums on certain policies issued by qualified health plans under the ACA. In addition, the Centers for Medicare & Medicaid Services, or CMS, has recently proposed regulations that would give states greater flexibility in setting benchmarks for insurers in the individual and small group marketplaces, which may have the effect of relaxing the essential health benefits required under the ACA for plans sold through such marketplaces. Litigation and legislation over the ACA are likely to continue, with unpredictable and uncertain results. We continue to evaluate the effect that the ACA and its possible repeal and replacement has on our business.
Further, on February 24, 2017, President Trump issued an Executive Order requiring each agency to designate a regulatory reform officer and create a regulatory reform task force to evaluate existing regulations and make recommendations regarding their repeal, replacement or modification. It is difficult to predict how these requirements will be implemented, and the extent to which they will impact the FDA’s ability to exercise its regulatory authority.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers of 2% per fiscal year. These reductions went into effect in April 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2025 unless additional action is taken by Congress. In January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several types of providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws or any other similar laws introduced in the future may result in additional reductions in Medicare and other health care funding, which could negatively affect our customers and accordingly, our financial operations.
The costs of prescription pharmaceuticals in the United States hashave also been the subject of considerable debate, and members of Congress and the Trump Administration have indicated that each will address such costs through new legislative and administrative measures.measures could be implemented to address such costs. To date, there have been several recent U.S. congressional inquiries and proposed state and federal legislation designed to, among other things, improve transparency in drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the costs of drugs under Medicare, and reform government program reimbursement methodologies for drug products. Under recent legislation, starting in 2023 a manufacturer of a drug or biological product covered by Medicare Parts B or D must pay a rebate to the federal government if the product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by Medicare Parts B or D. Additionally, starting in payment year 2026, CMS will negotiate drug prices annually for a select number of single source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such drug will decrease. The pricing of prescription pharmaceuticals is also subject to governmental control outside the United States. In these other countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost effectiveness of our product candidates to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our ability to generate revenues and become profitable could be impaired.
| 52 |
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for approved products. In addition, there have been several recent Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the cost of drugs under Medicare and reform government program reimbursement methodologies for drugs. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent labeling and post-marketing testing and other requirements.
Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. In addition, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products. We expect that additional U.S. federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
Individual states in the United States have also become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Legally-mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce the ultimate demand for our product candidates or put pressure on our product pricing.
In the EU, similar political, economic and regulatory developments may affect our ability to profitably commercialize our product candidates, if approved. In addition to continuing pressure on prices and cost containment measures, legislative developments at the EU or member state level may result in significant additional requirements or obstacles that may increase our operating costs. The delivery of healthcare in the EU, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of health care and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most EU member states have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to commercialize our product candidates, if approved.
In markets outside of the United States and EU, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action in the United States, the EU or any other jurisdiction. If we or any third parties we may engage are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we or such third parties are not able to maintain regulatory compliance, our product candidates may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability.
If we fail to comply with the extensive legal and regulatory requirements affecting the health care industry, we could face increased costs, penalties and a loss of business.
Our activities, and the activities of any collaborators, distributors and other third-party providers that we may engage in the future, will be subject to extensive government regulation and oversight both in the U.S. and in foreign jurisdictions. The FDA and comparable agencies in other jurisdictions will directly regulate many of our most critical business activities, including the conduct of preclinical and clinical studies, product manufacturing, advertising and promotion, product distribution, adverse event reporting, and product risk management. Our interactions in the U.S.United States or abroad with physicians and other health care providers that prescribe or purchase our products will also be subject to government regulation designed to prevent fraud and abuse in the sale and use of the products and place greater restrictions on the marketing practices of health care companies. Health care companies such as ours are facing heightened scrutiny of their relationships with health care providers from anti-corruption enforcement officials. In addition, health care companies such as ours have been the target of lawsuits and investigations alleging violations of government regulation, including claims asserting submission of incorrect pricing information, impermissible off-label promotion of pharmaceutical products, payments intended to influence the referral of health care business, submission of false claims for government reimbursement, antitrust violations, and violations related to environmental matters. Risks relating to compliance with laws and regulations may be heightened if we operate globally.
Regulations governing the health care industry are subject to change, with possibly retroactive effect, including:
| ● | new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to health care availability, pricing or marketing practices, compliance with wage and hour laws and other employment practices, method of delivery, payment for health care products and services, compliance with health information and data privacy and security laws and regulations, tracking and reporting payments and other transfers of value made to physicians and teaching hospitals, extensive anti-bribery and anti-corruption prohibitions, product serialization and labeling requirements and used product take-back requirements; |
| 53 |
| ● | ||
| changes in the FDA and foreign regulatory approval processes that may delay or prevent the approval of new products and result in lost market opportunity; | ||
| ● | requirements that provide for increased transparency of clinical trial results and quality data, such as the EMA’s clinical transparency policy, which could impact our ability to protect trade secrets and competitively-sensitive information contained in approval applications or could be misinterpreted leading to reputational damage, misperception, or legal action which could harm our business; and | |
| ● | changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on product distribution or use, or other measures after the introduction of our products to market, which could increase our costs of doing business, adversely affect the future permitted uses of approved products, or otherwise adversely affect the market for our products. |
Violations of governmental regulation may be punishable by criminal and civil sanctions against us, including fines and civil monetary penalties and exclusion from participation in government programs, including Medicare and Medicaid, as well as sanctions against executives overseeing our business. In addition to penalties for violation of laws and regulations, we could be required to repay amounts we received from government payors or pay additional rebates and interest if we are found to have miscalculated the pricing information we have submitted to the government. We cannot ensure that our compliance controls, policies and procedures will in every instance protect us from acts committed by our employees, collaborators, partners or third-party providers that would violate the laws or regulations of the jurisdictions in which we operate. Whether or not we have complied with the law, an investigation into alleged unlawful conduct could increase our expenses, damage our reputation, divert management time and attention, and adversely affect our business.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, and health information privacy and security laws, and if we are unable to comply with such laws, we could face substantial penalties.
If we obtainthe FDA grants marketing approval for any of our product candidates or technologies and begin commercializing those products or technologies begins in the United States, our operations may be subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, and physician sunshine laws and regulations. These laws may impact, among other things, our proposedproduct sales, marketing, and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| ● | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; | |
| ● | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent; | |
| ● | the federal Health Insurance Portability and Accountability Act of 1996 | |
| ● | HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and implementing regulations, which impose certain requirements relating to the privacy, security, and transmission of individually identifiable health information; | |
| ● | the Physician Payments Sunshine Act which requires manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; | |
| ● | the FDCA, which prohibits, among other things, the adulteration or misbranding of drugs, biologics and medical devices; | |
| ● | the U.S. Public Health Service Act, which prohibits, among other things, the introduction into interstate commerce of a biological product unless a biologics license is in effect for that product; and | |
| ● | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payors, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or that otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of health information in certain circumstances. |
| 54 |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent health care reform legislation has strengthened these laws. Further, state laws differ from each other and from federal law in significant ways, thus complicating compliance efforts.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Risks Related to our Dependence on Third Parties
We may become dependent on future collaborations to develop and commercialize our product candidates and to provide the regulatory compliance, sales, marketing, and distribution capabilities required for the success of our business.
We may enter into various kinds of collaborative research and development and product marketing agreements to develop and commercialize our products. The expected future milestone payments and cost reimbursements from collaboration agreements could provide an important source of financing for our research and development programs, thereby facilitating the application of our technology to the development and commercialization of our products, but there are risks associated with entering into collaboration arrangements.
The process of establishing and maintaining collaborative relationships is difficult, time-consuming and involves significant uncertainty, such as:
| ● | a collaboration partner may shift its priorities and resources away from our product candidates due to a change in business strategies, or a merger, acquisition, sale or downsizing; | |
| ● | a collaboration partner may seek to renegotiate or terminate their relationships with us due to unsatisfactory clinical results, manufacturing issues, a change in business strategy, a change of control or other reasons; | |
| ● | a collaboration partner may cease development in therapeutic areas which are the subject of our strategic collaboration; | |
| ● | a collaboration partner may not devote sufficient capital or resources towards our product candidates; | |
| ● | a collaboration partner may change the success criteria for a product candidate thereby delaying or ceasing development of such candidate; | |
| ● | a significant delay in initiation of certain development activities by a collaboration partner will also delay payment of milestones tied to such activities, thereby impacting our ability to fund our own activities; | |
| ● | a collaboration partner could develop a product that competes, either directly or indirectly, with our product candidate; | |
| ● | a collaboration partner with commercialization obligations may not commit sufficient financial or human resources to the marketing, distribution or sale of a product; | |
| ● | a collaboration partner with manufacturing responsibilities may encounter regulatory, resource or quality issues and be unable to meet demand requirements; | |
| ● | a collaboration partner may terminate a strategic alliance; | |
| ● | a dispute may arise between us and a partner concerning the research, development or commercialization of a product candidate resulting in a delay in milestones, royalty payments or termination of an alliance and possibly resulting in costly litigation or arbitration which may divert management attention and resources; and | |
| ● | a partner may use our products or technology in such a way as to invite litigation from a third party. |
There is a risk that a collaboration partner might fail to perform its obligations under the collaborative arrangements or may be slow in performing its obligations. In addition, a collaboration partner may experience financial difficulties at any time that could prevent it from having available funds to contribute to the collaboration. If a collaboration partner fails to conduct its product development, commercialization, regulatory compliance, sales and marketing or distribution activities successfully and in a timely manner, or if it terminates or materially modifies its agreements with us, the development and commercialization of one or more product candidates could be delayed, curtailed, or terminated because we may not have sufficient financial resources or capabilities to continue such development and commercialization on our own.
| 55 |
We have no marketing, sales, or distribution resources for the commercialization of any products or technologies that we might successfully develop.
We do not have any infrastructure for the sales, marketing or distribution of our products, and the cost of establishing and maintaining such an organization may exceed the cost-effectiveness of doing so. There are significant expenses and risks involved with establishing our own sales, marketing and distribution capabilities, including our ability to hire, retain and appropriately incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel, and effectively manage a geographically dispersed sales and marketing team. Any failure or delay in the development of our internal sales, marketing and distribution capabilities could delay any product launch, which would adversely impact the commercialization of any approved product candidate.
If we market products through arrangements with third parties, we may pay sales commissions to sales representatives or we may sell or consign products to distributors at wholesale prices. As a result, our gross profit from product sales may be lower than it would be if we were to sell our products directly to end users at retail prices through our own sales force. There can be no assurance we will be able to negotiate distribution or sales agreements with third parties on favorable terms to justify our investment in our products or achieve sufficient revenues to support our operations.
If we are unable to build our own sales force or negotiate a collaborative relationship for the commercialization of our product candidates, we may be forced to delay the potential commercialization of such candidates or reduce the scope of our sales or marketing activities for them. If we elect to increase our expenditures to fund commercialization activities ourselves, we will need to obtain additional capital, which may not be available to us on acceptable terms, or at all. We could enter into arrangements with collaborative partners at an earlier stage than otherwise would be ideal and we may be required to relinquish rights to our product candidates or otherwise agree to terms unfavorable to us, any of which may have an adverse effect on our business, operating results and prospects.
If we are unable to establish adequate sales, marketing and distribution capabilities, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates and may not become profitable and may incur significant additional losses. We will be competing with many companies that currently have extensive and well-funded marketing and sales operations. Without an internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
We do not have the ability to independently conduct clinical trials required to obtain regulatory approvals for our product candidates and intend to rely on third parties to conduct, supervise and monitor our clinical trials.
We will need to rely on third parties, such as contract research organizations, data management companies, contract clinical research associates, medical institutions, clinical investigators and contract laboratories to conduct any clinical trials that we may undertake for our product candidates. We may also rely on third parties to assist with our preclinical development of product candidates.
If we outsource clinical trials, we may be unable to directly control the timing, conduct and expense of our clinical trials. However, we will remain responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities.
We and our third partythird-party contractors will be required to comply with the GLPsgood laboratory practices (GLPs) and GCPs, which are regulations and guidelines enforced by the FDA and are also required by the Competent Authoritiescompetent authorities of the Member Statesmember states of the European Economic Area (EEA) and comparable foreign regulatory authorities in the form of International Conference on Harmonization guidelines for any of our product candidates that are in preclinical and clinical development. The Regulatoryregulatory authorities enforce GCPs through periodic inspections of trial sponsors, principal investigators and clinical trial sites. If we or our third partythird-party contractors fail to comply with GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP regulations. In addition, our clinical trials must be conducted with product produced under cGMP regulations. Accordingly, if our third partythird-party contractors fail to comply with these regulations or fail to recruit a sufficient number of subjects, we may be required to repeat clinical trials, which would delay the regulatory approval process.
Our third partythird-party contractors will not be our employees, and we will not control whether or not they devote sufficient time and resources to our future clinical and nonclinical programs. These third partythird-party contractors may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials, or other product development activities which could harm our competitive position. We face the risk of potential unauthorized disclosure or misappropriation of our intellectual property by third partythird-party contractors, which may reduce our trade secret protection and allow our potential competitors to access and exploit our proprietary technology. If our third partythird-party contractors do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for any other reasons, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize any product candidate that we develop. As a result, our financial results and the commercial prospects for any product candidate that we develop would be harmed, our costs could increase, and our ability to generate revenues could be delayed.
| 56 |
If our relationship with any third partythird-party contractors terminate,terminates, we may not be able to enter into arrangements with alternative third partythird-party contractors or do so on commercially reasonable terms. Switching or adding additional third partythird-party contractors involves substantial cost and requires management time and focus. In addition, there is a natural transition period when a new third party commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines. Though we intend to carefully manage our relationships with our third partythird-party contractors, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have an adverse impact on our business, financial condition and prospects.
Risks Related to Intellectual Property
If we are unable to obtain and enforce patents and to protect our trade secrets, others could use our technology to compete with us, which could limit opportunities for us to generate revenues by licensing our technology and selling our products.
| ● | Our success will depend in part on our ability to obtain and enforce patents and maintain trade secrets in the United States and in other countries. If we are unsuccessful in obtaining and enforcing patents, our competitors could use our technology and create products or technologies that compete with our products and technologies, without paying license fees or royalties to us. | |
| ● | The preparation, filing, and prosecution of patent applications can be costly and time consuming. Our limited financial resources may not permit us to pursue patent protection of all of our technology and products throughout the world. | |
| ● | Even if we are able to obtain issued patents covering our technology or products, we may have to incur substantial legal fees and other expenses to enforce our patent rights in order to protect our technology and products from infringing uses. We may not have the financial resources to finance the litigation required to preserve our patent and trade secret rights. |
There is no certainty that our pending or future patent applications will result in the issuance of patents.
We acquired rights to patent applications for technology that BioTimeLineage has developed, and we may file additional new patent applications in the future seeking patent protection for new technology or products that we develop ourselves or jointly with others. However, there is no assurance that any of our licensed patent applications, or any patent applications that we may file in the future in the United States or abroad, will result in the issuance of patents.
The process of applying for and obtaining patents can be expensive and slow.
| ● | The preparation and filing of patent applications, and the maintenance of patents that are issued, may require substantial time and money. | |
| ● | A patent interference proceeding may be instituted with the U.S. Patent and Trademark Office (the | |
| ● | A derivation proceeding may be instituted by the USPTO or an inventor alleging that a patent or application was derived from the work of another inventor. |
| ● | Post Grant Review under the new Leahy Smith America Invents Act (America Invents Act) will make available opposition-like proceedings in the United States. As with the USPTO interference proceedings, Post Grant Review proceedings will be very expensive to contest and can result in significant delays in obtaining patent protection or can result in a denial of a patent application. | |
| ● | Oppositions to the issuance of patents may be filed under European patent law and the patent laws of certain other countries. As with USPTO interference proceedings, these foreign proceedings can be very expensive to contest and can result in significant delays in obtaining a patent or can result in a denial of a patent application. |
Intellectual property we may develop using grants received from the federal government are subject to rights maintained by the government.
Research and development we perform that is funded by grants from the federal government, and any intellectual property that we create using those grants, is subject to the rights maintained by the federal government.
| 57 |
Our patents may not protect our technologies or products from competition.
| ● | We might not be able to obtain any patents beyond those we already own or have licensed or sublicensed, and any patents that we do obtain might not be comprehensive enough to provide us with meaningful patent protection. | |
| ● | There will always be a risk that our competitors might be able to successfully challenge the validity or enforceability of any patent issued to us. | |
| ● | In addition to interference proceedings, the USPTO can reexamine issued patents at the request of a third party. Our patents may be subject to inter partes review (replacing the reexamination proceeding), a proceeding in which a third party can challenge the validity of one of our patents to have the patent invalidated. This means that patents owned or licensed by us may be subject to reexamination and may be lost if the outcome of the reexamination is unfavorable to us. | |
| ● | The patents to which we have licenses to |
We may not be able to enforce our intellectual property rights throughout the world.
Filing, prosecuting and defending patents, if issued, on our technology and product candidates in all countries throughout the world would be prohibitively expensive. The requirements for patentability may differ in certain countries, particularly in developing countries. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we may obtain patent protection, but where patent enforcement is not as strong as that in the United States. The products offered by foreign competitors may compete with our products in jurisdictions where we do not have any issued or licensed patents or where any future patent claims or other intellectual property rights may not be effective or sufficient to prevent them from competing with us.
Moreover, our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in foreign intellectual property laws. Additionally, laws of some countries outside of the United States and the EU do not afford intellectual property protection to the same extent as the laws of the United States and the EU. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, including India, China and certain developing countries, do not favor the enforcement of patents and other intellectual property rights. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. Consequently, we may not be able to prevent third parties from practicing our inventions in certain countries outside the United States and the EU. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, if our ability to enforce our patents to stop infringing activities is inadequate. These products may compete with our products, and our patents, if issued, or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and resources from other aspects of our business. Furthermore, while we intend to protect our intellectual property rights in major markets, we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market products or license our patented technologies. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate.
We may be subject to patent infringement claims that could be costly to defend, which may limit our ability to use disputed technologies, and which could prevent us from pursuing research and development or commercialization of some of our technologies or products, require us to pay licensing fees to have freedom to operate and/or result in monetary damages or other liability for us.
The success of our business depends significantly on our ability to operate without infringing patents and other proprietary rights of others. If the technology that we use infringes a patent held by others, we could be sued for monetary damages by the patent holder or its licensee, or we could be prevented from continuing research, development, and commercialization of technologies and products that rely on that technology, unless we are able to obtain a license to use the patent. The cost and availability of a license to a patent cannot be predicted, and the likelihood of obtaining a license at an acceptable cost would be lower if the patent holder or any of its licensees is using the patent to develop or market a technology or product with which our technologies or products would compete. If we could not obtain a necessary license, we would need to develop or obtain rights to alternative technologies, which could prove costly and could cause delays in developing our technologies or products, or we could be forced to discontinue the development or marketing of any technologies and products that were developed using the technology covered by the patent.
| 58 |
Risks Related to Our Relationship with BioTime and Juvenescence
We will initially rely upon BioTime for certain services and resources
Although we have signed a sublease that will allow us to have our own research facilities in the near future if the Preconditions to the sublease are met, we will continue to rely on the use of a portion of BioTime’s office and laboratory facilities until the New Facility under the sublease becomes available to us or we locate and lease an alternative facility. We also are relying on BioTime to provide certain management and administrative services, including patent prosecution, certain legal services, human resources management, accounting, financial management, and controls over financial accounting and reporting, although we plan to have our own management and administrative personnel in the future. We have entered into the Shared Facilities Agreement with BioTime under which we have agreed to bear costs allocated to us by BioTime for our use of BioTime’s office and laboratory facilities and human resources, and for services and materials provided for our benefit by BioTime. We will pay BioTime 105% of its costs of providing personnel and services to us, and for our use of its facilities, including an allocation of general overhead based on that use. We may also share the services of some research personnel with BioTime. Either party to the Shared Facilities Agreement may terminate the agreement for any reason with six months written notice to the other party, except that BioTime may not give us a notice of termination prior to September 1, 2020.
If BioTime’s human resources are not sufficient to serve both BioTime’s needs and ours, or if the Shared Facilities Agreement is terminated, we will have to hire additional personnel of our own, either on a full-time or part-time basis, as employees or as consultants, and the cost of doing so could be greater than the costs that would be allocated to us by BioTime. Also, any new personnel that we may need to hire may not be as familiar with our business or operations as BioTime’s personnel, which means that we would incur the expense and inefficiencies related to training new employees or consultants.
Our Chief Financial Officer and Chief Operating Officer are not fulltime AgeX employees.
Our Chief Financial Officer is the former Chief Financial Officer of BioTime and is providing services to us on a part-time basis as a consultant. Because he is not a full-time employee, we may compete for his time and attention with other companies for whom he may provide services. Our Chief Operating Officer is an employee of Juvenescence and is expected to devote 85% of his time to our affairs and the balance of his time to the affairs of Juvenescence and accordingly we may compete with Juvenescence for his time and attention.
Conflicts of interest may arise from our relationship with BioTime.
As of March 18, 2019, BioTime beneficially owned approximately 4.6% of the voting power of our outstanding common stock, and we have also entered into a Shared Services Agreement with BioTime. Our relationship with BioTime could give rise to certain conflicts of interest that could have an impact on our research and development programs, business opportunities, and operations generally.
Conflicts of interest may arise from our relationship with Juvenescence, which owns a significant percentage of our common stock and will be able to substantially influence us and exert control over matters subject to stockholder approval and the election of directors.
As of March 18, 2019, Juvenescence beneficially owned approximately 43.6% of the voting power of our outstanding common stock, which will be enable them to substantially influence us and exert control through this ownership position. For example, Juvenescence will be able to exert control over or substantially influence elections of directors, approval of our equity incentive plans, amendments to our organizational documents, or approval of any merger, amalgamation, sale of assets or other major corporate transaction. Juvenescence has controlling stakes and minority investments in several other companies engaged in various aspects of the aging industry, which companies may propose collaborations with AgeX. Juvenescence’s interests may not always coincide with our corporate interests or the interests of other stockholders, and it may exercise its voting and other rights in a manner with which you may not agree or that may not be in the best interests of our other stockholders. So long as Juvenescence continues to own a significant amount of our equity, it will continue to be able to strongly influence and effectively control our decisions. While the directors elected by Juvenescence will be obligated to act in accordance with their fiduciary duty, they may have equity or other interests in Juvenescence and, accordingly, their interests may be aligned with Juvenescence’s interests, which may not always coincide with our corporate interests or the interests of our other stockholders.
Our ability to meet our capital needs may be harmed by the loss of financial support from BioTime.
The loss of financial support from BioTime could harm our ability to meet our capital needs. BioTime historically has provided financing to us at rates that we believe are not representative of the cost of financing that we will incur as a stand-alone company. As a public company, we expect to obtain any funds needed in excess of the amounts generated by our operating activities through the capital markets or bank financing, and not from BioTime. As public company, the cost of our financing also will depend on other factors such as our performance and financial market conditions generally. Further, we cannot guarantee that we will be able to obtain capital market financing or credit on favorable terms, or at all, in the future. We cannot be certain that our ability to meet our capital needs, including servicing our own debt, will not be harmed by the loss of financial support from BioTime.
Our accounting and other management systems and resources may not be adequately prepared to meet the financial reporting and other requirements to which we will be subject as a public company, and failure to achieve and maintain effective internal controls could have a material adverse effect on our business and the price of our common stock.
Our financial results previously were included within the consolidated results of BioTime, and we believe that our financial reporting and internal controls were appropriate for a subsidiary of a public company. However, we were not directly subject to the reporting and other requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). As a result of the Distribution, we are now directly subject to reporting and other obligations under the Exchange Act. We will be required to comply with Section 404 of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”) which will require annual management assessments of the effectiveness of our internal controls over financial reporting and after our status as an emerging growth company expires, we will be required to obtain a report by our independent registered public accounting firm as to whether we maintained, in all material respects, effective internal controls over financial reporting as of the last day of the year. These reporting and other obligations may place significant demands on our management, administrative and operational resources, including accounting systems and resources.
The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition. Under the Sarbanes-Oxley Act, we are required to maintain effective disclosure controls and procedures and internal controls over financial reporting. To comply with these requirements, we may need to establish our own systems; implement additional financial and management controls, reporting systems and procedures; and hire additional accounting and finance staff to replace or supplement the systems and services provided to us by BioTime under the Shared Facilities Agreement. We expect to incur additional annual expenses for the purpose of addressing these requirements, and those expenses may be significant. If we are unable to establish our financial and management controls, reporting systems, information technology systems and procedures in a timely and effective fashion, our ability to comply with our financial reporting requirements and other rules that apply to reporting companies under the Exchange Act could be impaired.
If, during periods we are required to assess the effectiveness of our internal controls, we are unable to conclude that we have effective internal controls over financial reporting, we may be unable to report our financial information on a timely basis, investors may lose confidence in our operating results, the price of our common stock could decline and we may be subject to litigation or regulatory enforcement actions, which would require additional financial and management resources. This could have a material adverse effect on our business and lead to a decline in the price of our common stock.
Risks Pertaining to Our Common Stock
There is a limited history to the public trading of ourAgeX common stock and there is no assurance that a market for ourAgeX common stock will be sustained.
Public trading of ourAgeX common stock on the NYSE American began on November 29, 2018. Accordingly, there is only a limited history of the public trading of ourAgeX common stock and there can be no assurance that an active market for ourAgeX common stock will be sustained.
We cannot predict the prices at which ourAgeX common stock may trade. The market price of ourAgeX common stock may fluctuate significantly, depending upon many factors, some of which may be beyond our control, including, but not limited to:
| ● | a shift in our investor base; | |
| ● | the impact of the Reverse Stock Split; | |
| ● | our quarterly or annual earnings, or those of comparable companies; | |
| ● | actual or anticipated fluctuations in our operating results; | |
| ● | our ability to obtain financing as needed; | |
| ● | changes in laws and regulations affecting our business; | |
| ● | changes in accounting standards, policies, guidance, interpretations or principles; | |
| ● | announcements by us or our competitors of significant investments, acquisitions or dispositions; | |
| ● | the failure of securities analysts to cover | |
| ● | changes in earnings estimates by securities analysts or our ability to meet those estimates; | |
| ● | the operating performance and stock price of comparable companies; | |
| ● | overall market fluctuations; and | |
| ● | general economic conditions and other external factors. |
Additional shares of our common stock will become eligible for public sale, and sales of those shares could create downward pressure on the trading price of our common stock.
Shares of our common stock issued before the Distribution, including shares held by Juvenescence, and shares issued upon the exercise of common stock purchase warrants during March 2019, are or will become eligible to be publicly sold without registration in compliance with the provisions of Rule 144 under the Securities Act of 1933, as amended (the “Securities Act”) after the shares have been beneficially owned for at least six months if we have filed all reports required under the Exchange Act, other than any Current Report on Form 8-K. Even if we fail to file those Exchange Act reports, shares that a stockholder has beneficially owned for more than one year may be sold by the stockholder under Rule 144 if at the stockholder has not been an “affiliate” of AgeX within the meaning of Rule 144 at any time during the 90 days immediately before the sale. Certain holders of shares that we issued before the Distribution or through the exercise of warrants also have certain contractual rights to have their shares registered for sale under the Securities Act. Sales of AgeX common stock under Rule 144 or through a Securities Act registration statement could create downward pressure on the trading price of our common stock.
Because we are engaged in the development of pharmaceutical and cell therapy products, the price of shares of ourAgeX common stock may rise and fall rapidly.
The price of ourAgeX common stock may rise rapidly in response to certain events, such as the commencement of clinical trials of an experimental new therapy, even though the outcome of those trials and the likelihood of ultimate FDA approval of a therapeutic product remain uncertain. Similarly, prices of ourAgeX common stock may fall rapidly in response to certain events such as unfavorable results of clinical trials or a delay or failure to obtain FDA approval. Further, the failure of our earnings to meet analysts’ expectations could result in a significant rapid decline in the market price of ourAgeX common stockstock.
Because we do not pay dividends, ourAgeX common stock may not be a suitable investment for anyone who needs to earn dividend income.
We do not have current plans to pay any cash dividends on ourAgeX common stock. The declaration, amount and payment of any future dividends on shares of common stock will be at the sole discretion of ourthe AgeX Board. The AgeX Board of Directors. Our Board of Directors may take into account general and economic conditions, our financial condition and results of operations, our available cash and current and anticipated cash needs, capital requirements, contractual, legal, tax and regulatory restrictions and implications on the payment of dividends by us to our stockholders or by our subsidiaries to us and such other factors as ourthe AgeX Board of Directors may deem relevant. For the foreseeable future we anticipate that any earnings generated in our business will be used to finance the growth of our business and will not be paid out as dividends to our stockholders. This means that our stock may not be a suitable investment for anyone who needs to earn income from their investments.
Securities analysts may not initiate coverage or continue to cover ourAgeX common stock, and this may have a negative impact on the market price of our shares.
The market price and liquidity of ourAgeX common stock will depend, in part, on the research and reports that securities analysts publish about our business and ourAgeX common stock. We do not have any control over these analysts. There is no guarantee that securities analysts will cover ourAgeX common stock. If securities analysts do not cover ourAgeX common stock, the lack of research coverage may adversely affect the market price of those shares. If securities analysts do cover our shares, they could issue reports or recommendations that are unfavorable to the price of our shares, and they could downgrade a previously favorable report or recommendation, and in either case our share price could decline as a result of the report. If one or more of these analysts ceases to cover our shares or fails to publish regular reports on our business, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
| 59 |
You may experience dilution of your ownership interests if we issue additional shares of common stock or preferred stock.
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present stockholders. We are currently authorized to issue an aggregate of 105,000,000205,000,000 shares of capital stock consisting of 100,000,000200,000,000 shares of common stock and 5,000,000 “blank check” shares of preferred stock. As of March 19, 201914, 2024, there were 37,630,0002,500,664 shares of AgeX common stock issued and outstanding, and 2,268,50082,206 shares of AgeX common stock reserved for issuance upon the exercise of outstanding stock options or other stock-based awards under our 2017 Equity Incentive Plan. NoPlan, 1,500,284 shares reserved for issuance upon the exercise of Post-Merger Warrants, 1,500,284 shares reserved for issuance upon the exercise of Incentive Warrants, and 303,255 shares of preferredAgeX common stock are presently outstanding.reserved for issuance upon the exercise of outstanding warrants held by Juvenescence.
We may issue additional AgeX common stock or other securities that are convertible into or exercisable for AgeX common stock in order to raise additional capital, or in connection with hiring or retaining employees or consultants, or in connection with future acquisitions of licenses to technology or medical products or for other business purposes. The future issuance of any additional shares of AgeX common stock or other securities may create downward pressure on the trading price of ourAgeX common stock.
We may also issue additional preferred stock, havingand such preferred stock may have rights, preferences, and privileges senior to the rights of ourAgeX common stock with respect to dividends, rights to share in distributions of our assets if we liquidate our company, or voting rights. Any preferred stock may also be convertible into common stock on terms that would be dilutive to holders of common stock.
UnlessOur subsidiaries may finance a portion of their operations by selling shares of their capital stock or debt securities convertible into shares of their capital stock to private investors. Sales of subsidiary shares would reduce our ownership interest in the subsidiaries, and correspondingly dilute our shareholder’s ownership interests in our consolidated enterprise. Reverse Bio has, and other AgeX subsidiaries could also have, their own stock option plans and the exercise of subsidiary stock options or the sale of restricted stock under those plans would also reduce our ownership interest in the subsidiaries, with a resulting dilutive effect on the ownership interest of our shareholders in our consolidated enterprise. Subsidiaries might also issue preferred stock having rights, preferences, and privileges senior to the rights of the subsidiary common stock continueswe hold with respect to be listed on a national securities exchange it will become subjectdividends, rights to the so-called “penny stock” rules that impose restrictive sales practice requirements.
If we are unable to maintain the listingshare in distributions of our assets if the subsidiary is liquidated, or voting rights. Any subsidiary preferred stock may also be convertible into common stock on the NYSE American or another national securities exchange, ourterms that would be dilutive to us as a holder of subsidiary common stock could become subject to the so-called “penny stock” rules if the shares have a market value of less than $5.00 per share. The SEC has adopted regulations that define a penny stock to include any stock that has a market price of less than $5.00 per share, subject to certain exceptions, including an exception for stock traded on a national securities exchange. The SEC regulations impose restrictive sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors. An accredited investor generally is a person whose individual annual income exceeded $200,000, or whose joint annual income with a spouse exceeded $300,000 during the past two years and who expects their annual income to exceed the applicable level during the current year, or a person with net worth in excess of $1.0 million, not including the value of the investor’s principal residence and excluding mortgage debt secured by the investor’s principal residence up to the estimated fair market value of the home, except that any mortgage debt incurred by the investor within 60 days prior to the date of the transaction shall not be excluded from the determination of the investor’s net worth unless the mortgage debt was incurred to acquire the residence. For transactions covered by this rule, the broker-dealer must make a special suitability determination for the purchaser and must have received the purchaser’s written consent to the transaction prior to sale. This means that if we are unable maintain the listing of our common stock on a national securities exchange, the ability of stockholders to sell their AgeX common stock in the secondary market could be adversely affected.stock.
If a transaction involving a penny stock is not exempt from the SEC’s rule, a broker-dealer must deliver a disclosure schedule relating to the penny stock market to each investor prior to a transaction. The broker-dealer also must disclose the commissions payable to both the broker-dealer and its registered representative, current quotations for the penny stock, and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the customer’s account and information on the limited market in penny stocks.
We are an “emerging growth company,” and may elect to comply with reduced public company reporting requirements applicable to emerging growth companies, which could make ourAgeX common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find ourAgeX common stock less attractive because we may rely on these exemptions. If some investors find ourAgeX common stock less attractive as a result, there may be a less active trading market for ourAgeX common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.” We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act;Act of 1933, as amended (the Securities Act); (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a “large accelerated filer” under the Securities Exchange Act.Act of 1934, as amended (the Exchange Act).
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biopharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
The implementation of a new FASB accounting standard could increase the risk that our future consolidated financial statements could be qualified by going concern uncertainty.
We are subject to ASU No. 2014-15, “Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” which defines management’s responsibility to assess an entity’s ability to continue as a going concern, and to provide related footnote disclosures. In connection with preparing consolidated financial statements for each annual and interim reporting period, ASU No. 2014-15 requires that an entity’s management evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the consolidated financial statements are issued (or within one year after the date that the consolidated financial statements are available to be issued when applicable). As a result of the implementation of ASU No. 2014-15, we will be required to have more cash, cash equivalents, and liquid investments on hand on the date we issue or file our consolidated financial statements than had been the case during prior years in order to avoid a going concern qualification in our auditor’s report and in the footnotes to our consolidated financial statements. If our consolidated financial statements were to become subject to a going concern qualification or uncertainty or if we are unable to alleviate substantial doubt as part of our going concern assessment, or both, the market price of our common stock could decline.
Provisions in our certificateAgeX’s Certificate of incorporationIncorporation (the “AgeX Charter”) and bylawsAgeX’s Bylaws and under Delaware law could make an acquisition of our company, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our certificate of incorporationthe AgeX Charter and our bylawsAgeX’s Bylaws may discourage, delay or prevent a merger, acquisition or other change in control of our company that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of ourAgeX common stock, thereby depressing the market price of ourAgeX common stock. In addition, because ourthe AgeX Board of Directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our Board of Directors.the AgeX Board. Among other things, these provisions include those establishing:
| ● | no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
| 60 |
| the ability of | ||
| ● | the ability of |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the General Corporation Law of the State of Delaware,DGCL, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
Item 1B.1B. Unresolved Staff Comments
None.Not Applicable.
Item 2.Properties1C. Cybersecurity
On March 21 2019,We have implemented cybersecurity measures and processes to address and mitigate material risks from cybersecurity threats. We utilize the services of third party providers to develop, maintain, and implement cybersecurity systems and measures designed to protect our information systems from unauthorized access and damage. AgeX security measures are periodically assessed, tested, and updated. We do not have employees with information technology or cybersecurity expertise and accordingly we entered intoobtain an assessment of our cybersecurity systems and measures from a subleasethird party provider different from the provider that is primarily responsible for installation, update, and maintenance of information technology and cyber security systems. Our information technology and cybersecurity service providers primarily interface with members of our accounting and finance group. These processes are an integral part of our internal controls and risk management and the results of the New Facility comprising approximately 23,911 square feetthird party assessment are reported annually to the Audit Committee along with a report from management on the effectiveness of space in a building in an office and research park at 965 Atlantic Avenue, Alameda, California. internal controls.
We planare not aware of the occurrence of any cybersecurity incidents that have materially affected or are reasonably likely to operatematerially affect our principal offices and research laboratorybusiness strategy, results of operations, or financial condition. However, there can be no assurance that material cybersecurity incidents will not arise or be discovered in the New Facilityfuture.
Item 2. Properties
Our principal place of business is located at 1101 Marina Village Parkway, Suite 201, Alameda, California which we use for office purposes.
Item 3. Legal Proceedings
AgeX may from time to time be a party to litigation and subject to constructclaims incident to the ordinary course of business. In the future, AgeX may become a cGMP laboratory forparty to an increasing number of litigation matters and claims, including in connection with Merger Agreement and the manufacturetransactions contemplated thereby. The outcome of our cell lineslitigation and cell based product candidates. The availabilityclaims cannot be predicted with certainty, and the resolution of these matters could materially affect AgeX’s future results of operations, cash flows or financial position.
On December 11, 2023, a purported stockholder of AgeX, filed a putative shareholder class action and derivative lawsuit in the Superior Court of the New FacilityState of California, County of Alameda, captioned Buttner, et al. v. AgeX Therapeutics, Inc., et al., Case No. 23CV057083 (the Buttner Complaint). The Buttner Complaint names AgeX, the AgeX Board, an officer of AgeX, Juvenescence Limited and Juvenescence US Corp. as defendants. The Buttner Complaint alleges direct claims against the individual defendants for our use and our obligation to pay rent under the sublease is subject to the satisfactionbreaches of fiduciary duty in connection with their approval of the Preconditions. If the Preconditions are met, base monthly rent will be $35,866.50 for the initial 12 months of the sublease termMerger and then will increase to $36,942.50. In addition, we will pay real property taxes, insurance and operating expenses pertaining to the buildingdisclosures made by AgeX in which the New Facility is located. The sublease term will expire on December 31, 2020.
We will be responsible for the maintenance and repair of the leased New Facility, including electrical, plumbing, HVAC and other systems serving the New Facility but excluding structural and other external portions of the building in which the New Facility is located, and other external areas such as parking, landscaping and walkways associated with the building.
We will be in default under the sublease, and the sublandlord may terminate our sublease and may exercise other remedies against us for losses and damages under the sublease and applicable law, if any one or more of the following events occurs: (a) we fail to pay any rent or any other sum required to be paid under the sublease for a period of ten (10) days after written notice of delinquency is delivered by the sublandlord; provided, however, that if we fail to pay rent or other sums due within ten (10) days of the date due three or more times during any twelve month period, then our subsequent failure to pay any rent or other sum when due shall constitute a default without the requirement of any written notice; (b) a material default by us in the performance of any other terms, covenants or conditions of the sublease where the failure continues for thirty (30) days after written notice from the sublandlord; provided that if we default in the performance of the same obligation three or more times in any twelve month period and notice from the sublandlord was given in each instance, no cure period shall thereafter be applicable; (c) we become bankrupt or insolvent, make an assignment for the benefit of creditors, bankruptcy or reorganization proceedings are commenced by or against us,connection therewith and, in the casealternative, alleges derivative claims, purportedly on behalf of an involuntary proceeding are not discharged within 60 days,AgeX, against the appointmentindividual defendants for such alleged breaches of a receiver for a substantial part of our assets, or the levy upon the sublease or our estate in the sublease by attachment or execution, or (d) we abandon the New Facility.
We have agreed to indemnify the sublandlordfiduciary duty. The Buttner Complaint also alleges direct and derivative claims against certain liabilities arising under laws pertaining to hazardous materials. Our indemnityJuvenescence Limited, Juvenescence US Corp., and one member of the sublandlord will pertain to any deposit, spill, discharge or releaseAgeX Board for breaches of hazardous materials that occurs duringfiduciary duty as alleged controlling stockholders of AgeX. On February 29, 2024, the termplaintiff filed a request for dismissal of the sublease or from our failure to comply with requirements of governmental authorities.action without prejudice and on March 5, 2024 the court entered an order dismissing the action per the plaintiff’s request.
The sublease requires us to maintain certain liability and other insurance and contains customary provisions pertaining to matters such as damage or destruction of the New Facility, taking by eminent domain or similar process, restrictions on subletting and assignment, and other matters.
If the Preconditions are not satisfied, we will continue to use a portion of BioTime’s laboratory and office space under the Shared Facilities Agreement until such time as we are able to lease an alternative office and laboratory facility suitable for the manufacture of cell lines and our product candidates under cGMP conditions. BioTime has a lease of approximately30,795square feet of rentable space in two buildings located in an office park setting in Alameda, California. Under the Shared Facilities Agreement, we have use of approximately 2,239 square feet of allocated laboratory and office space and use of approximately 18,000 square feet of common areas which we share with BioTime and its subsidiaries and affiliates in the same facility. BioTime’s facilities do not include a laboratory for the manufacture of cell lines or our product candidates under cGMP conditions.BioTime’s lease expires on January 31, 2023.
From time to time, we may be involved in routine litigation incidental to the conduct of our business. We are not presently involved in any material litigation or proceedings, and to our knowledge no such litigation or proceedings are contemplated.
Item 4.Mine Safety Disclosures
Not applicable.
| 61 |
Not applicable.
PART II
Item 5.Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities
Our common stock has been traded on the NYSE American under the symbol “AGE” since November 29, 2018.
As of March 25, 2019,12, 2024, we had 270228 holders of record of our common stock. This number does not include stockholders whose shares of AgeX common stock are held in “street name” in accounts with securities broker-dealers or other financial institutions or fiduciaries.
The following table shows certain information concerning the stock options outstanding and available for issuance under all of our compensation plans and agreements as of December 31, 20182023 (in thousands, except weighted average exercise price):
| Plan Category | Number of Shares to be Issued upon Exercise of Outstanding Options, Warrants, and Rights | Weighted Average Exercise Price of the Outstanding Options, Warrants, and Rights | Number of Shares Remaining Available for Future Issuance under Equity Compensation Plans | Number of Shares to be Issued upon Exercise of Outstanding Options and Rights | Weighted Average Exercise Price of the Outstanding Options and Rights | Number of Shares Remaining Available for Future Issuance under Equity Compensation Plans | ||||||||||||||||||
| AgeX Stock Option Plans Approved by Stockholders | 2,269 | $ | 2.42 | 1,731 | ||||||||||||||||||||
| AgeX Stock Option Plans Approved by Stockholders (1) | 83 | $ | 80.28 | 156 | ||||||||||||||||||||
| (1) | This information pertains to our 2017 Equity Incentive Plan. Additional information concerning our 2017 Equity Incentive Plan and the stock options may be found in Note 8, Stock-Based Awards to the consolidated financial statements included elsewhere in this Report. |
Additional information concerning our Employee Stock Option Plan and the stock options may be found in Note 6 to the Financial Statements.
Dividend Policy
We have never declared or paid any cash dividends on our common stock. For the foreseeable future, we anticipate that all available funds and any earnings generated in our business will be used to finance the growth of our business and will not be paid out as dividends to our stockholders. Any future determination related to our dividend policy will be made at the discretion of our Board of Directors and will depend upon, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors our Board of Directors may deem relevant.
Item 6. Selected Financial DataReserved
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations is intended to provide information necessary to understand our audited consolidated financial statements for the years ended December 31, 20182023 and 2017,2022, and highlight certain other information which, in the opinion of management, will enhance a reader’s understanding of our financial condition, changes in financial condition and results of operations. These historical financial statements may not be indicative of our future performance. In particular, our future business focus and operations will be substantially different than our historical business focus and operations if the Merger is consummated. See “IMPORTANT PRELIMINARY NOTE — Planned Merger with Serina Therapeutics, Inc. and Related Transactions.” This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains a number of forward-looking statements, all of which are based on our current expectations not taking the Merger into account and couldwill be affected by the Merger if it is consummated and by uncertainties and risks described throughout this filing, particularly in “Risk Factors.”
Emerging Growth Company Status
The Jumpstart ourOur Business Startups Act of 2012 (“JOBS Act”) permits an “emerging growth company” such as AgeX to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. However, we elected to comply with newly adopted or revised accounting standards when they become applicable to public companies because our financial statements were consolidated with those of BioTime, which is not an emerging growth company under the JOBS Act and is therefore not permitted to delay the adoption of new or revised accounting standards that become applicable to public companies.public. This election under the JOBS Act to not delay the adoption of new or revised accounting standards is irrevocable.
Overview
We were incorporated in January 2017 in the state of Delaware as a subsidiary of BioTime, a publicly traded, clinical-stage biotechnology company targeting degenerative diseases. BioTime common stock trades on the NYSE American and Tel Aviv Stock Exchange under the symbol “BTX”.
We are a biotechnology company focused on the development and commercialization of novel therapeutics targeting human aging.aging and degenerative diseases. Our initial discovery and preclinical programs focus on utilizing brown adipose tissue in targeting diabetes, obesity, and heart disease; and induced tissue regeneration in utilizing the human body’s own abilities to scarlessly regenerate tissues damaged from age or trauma.trauma. We may also pursue other early-stage pre-clinical programs.
On August 17, 2017, we completed an asset acquisition and stock sale pursuant to which we received certain assets from BioTime for use in our research and development programs and raised $10.0 million in cash from investors to finance our operations.
From February 28, 2018 to July 10, 2018, we sold warrants to purchase 2,000,000 shares of AgeX common stock (the “Warrants”) for $0.50 per warrant for aggregate cash proceeds of $1,000,000. The Warrants were exercisable at $2.50 per share. Pursuant to the Warrant Agreement governing the Warrants, we set March 18, 2019 as the expiration date of the Warrants and by that date Warrant holders purchased 1.8 million shares of common stock through the exercise of the Warrants for $4.5 million in the aggregate. The Warrants were classified as equity since, among other factors, they were not redeemable, could not be settled in cash or other assets and required settlement by issuing a fixed number of shares of AgeX common stock. The Warrants were sold at fair value determined using the Binomial Lattice option pricing model on the issuance date, with certain management assumptions, which included the timing of an initial public offering of AgeX common stock, peer-group volatility, term to maturity, price cap and AgeX current and future stock prices.
On June 7, 2018, we sold 2.0 million shares of common stock for $2.50 per share to Juvenescence for aggregate cash proceeds to us of $5.0 million.These financings have allowed usto focus our resources on our pre-clinical programs.
On August 30, 2018, BioTime consummated the secondary sale of 14.4 million of its shares of AgeX common stock to Juvenescence pursuant to a stock purchase agreement. Upon completion of the transaction, BioTime’s ownership in us was reduced from 80.4% to 40.2% of our issued and outstanding shares of common stock, and Juvenescence’s ownership in AgeX was increased from 5.6% to 45.8% of our issued and outstanding shares of common stock. As a result, beginning on August 30, 2018, we are no longer considered a subsidiary of BioTime because, on that date, BioTime experienced a “loss of control” of a subsidiary, as defined by generally accepted accounting principles in the United States.Loss of control is deemed to have occurred when, among other things, a parent company owns less than a majority of the outstanding common stock in a subsidiary, lacks a controlling financial interest in the subsidiary, and is unable to unilaterally control the subsidiary through other means such as having, or being able to obtain, the power to elect a majority of the subsidiary’s Board of Directors based solely on contractual rights or ownership of shares holding a majority of the voting power of the subsidiary’s voting securities. All of these loss-of-control factors were present with respect to BioTime’s ownership interest in us as of August 30, 2018.Accordingly, BioTime deconsolidated our consolidated financial statements and results from its consolidated financial statements and results beginning on August 30, 2018.
On November 28, 2018, BioTime owned 14,416,000 shares of our common stock, representing approximately 40.2% of the shares of the common stock issued and outstanding on the Distribution Date. On the Distribution Date, BioTime distributed to its shareholders, on a pro rata basis, 12,697,028 shares of the AgeX common stock it then held. Immediately after the Distribution, BioTime retained 1,718,972 shares of AgeX common stock, representing approximately 4.8% of the common stock then issued and outstanding. Following the Distribution, our common stock began publicly trading on the NYSE American under the symbol “AGE”.
Since inception, our operations have focused on building our technology platform, identifying potential product candidates, establishing and protecting our intellectual property and raising capital. Our revenues have been principally derived from subscription and advertising revenue from LifeMap Sciences’ online databases based upon applicable subscription or advertising periods. We do not have any products approved for sale and have not generated any revenue from product sales.
We believeSince inception, we have sufficient capital to carry out our current researchincurred significant operating losses and development programs and other operationsthrough at least twelve months from the issuance date of our consolidated financial statements included elsewhere in this Report. Wewe will need to obtain additional financing in order to continue our operations, including our research and development programs after that date.programs. See “Liquidity and Capital Resources” for a discussion of our available capital resources and our need for future financing.
Since inception, we have incurred significant operating losses. Our operating lossesnet loss from operations before interest and other expenses were $11.2$9.9 million and $6.7$7.0 million for the years ended December 31, 20182023 and 2017,2022, respectively. As of December 31, 2018,2023, we had an accumulated deficit of $74.1$131.0 million. We expect to continue to incur significant expenses and operating losses overand negative cash flows for the next several years. We anticipateforeseeable future.
The following discussion and analysis of AgeX’s financial condition and results of operations and liquidity and capital resources does not reflect material changes to AgeX’s business, assets, liabilities, financial condition, operations, management, liquidity, capital resources, and prospects that our expenses will increase significantly in connection with our ongoing activities,occur if we:the Merger is consummated.
| 62 |
Critical Accounting PoliciesEstimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“US GAAP”), requires management to make estimates and assumptions that affect the reported amounts in our consolidated financial statements and related notes. Our significant accounting policies are described in Note 2,Summary of Significant Accounting Policies to our consolidated financial statements included elsewhere in this Report. We have identified below our critical accounting policies and estimates that we believe require the greatest amount of judgment. On an ongoing basis, we evaluate our estimates that are subject to significant judgment, including those related to going concern assessment of consolidated financial statements, allocations and adjustments necessary for carve-out basis of presentation, including the separate return method for income taxes, useful lives associated with long-lived assets, including evaluation of asset impairment, allowances for uncollectible accounts receivables, loss contingencies, deferred income taxes and tax reserves, including valuation allowances related to deferred income taxes, and assumptions used to value stock-based awards or other equity instruments.instruments and liability classified warrants. Actual results could differ materially from those estimates. On an ongoing basis, we evaluate our estimates compared to historical experience and trends, which form the basis for making judgments about the carrying value of assets and liabilities. To the extent that there are material differences between our estimates and our actual results, our future consolidated financial statement presentation, financial condition, results of operations and cash flows will be affected.
We believe the assumptions and estimates associated with the following have the greatest potential impact on our consolidated financial statements.
Principles of consolidation – AgeX’s consolidated financial statements include There were no significant changes to the accounts of its subsidiaries and certain research and development departments, including former BioTime personnel, transferred from BioTime to AgeX in connectiondisclosures with the Asset Contribution Agreement described in Note 4respect to our consolidated financial statements. AgeX consolidated its direct and indirect wholly-owned or majority-owned subsidiaries because AgeX has the ability to control their operating and financial decisions andcritical accounting policies through its ownership, and the noncontrolling interest is reflected as a separate element of stockholders’ equity on AgeX’s consolidated balance sheets.
As of, and for the yearyears ended December 31, 2018,AgeX consolidated ReCyte Therapeutics, LifeMap Sciences, Inc. (“LifeMap Sciences”),2023 and LifeMap Sciences, Ltd. (Israel) and included the historical expenses of certain former BioTime research and development departments (see Note 4 to our consolidated financial statements). For periods prior to the year ended December 31, 2018, AgeX also consolidated LifeMap Solutions, Inc., a subsidiary of LifeMap Sciences that was transferred to BioTime during June 2017 and subsequently ceased operations and was dissolved.2022.
Going concern assessment –Concern Assessment
We assess going concern uncertainty for our consolidated financial statements to determine if we have sufficient cash and cash equivalents on hand and working capital to operate for a period of at least one year from the date our consolidated financial statements are issued or are available to be issued, which is referred to as the “look-forward period” as defined by FASB’s ASU No. 2014-15. As part of this assessment, based on conditions that are known and reasonably knowable to us, we consider various scenarios, forecasts, projections, and estimates, and we make certain key assumptions, including the timing and nature of projected cash expenditures or programs, among other factors, and our ability to delay or curtail those expenditures or programs within the look-forward period in accordance with ASU No. 2014-15, if necessary.
Related party transactions - Shared FacilitiesPrinciples of Consolidation
The consolidated financial statements include the accounts of AgeX and Services Agreement -As more fullyits subsidiaries in which AgeX has a controlling financial interest. The consolidated financial statements also include certain variable interest entities in which AgeX is the primary beneficiary (as described in more detail below). For consolidated entities where AgeX has less than 100% of ownership, AgeX records net loss attributable to noncontrolling interest on the consolidated statement of operations equal to the percentage of the ownership interest retained in such entities by the respective noncontrolling parties. The noncontrolling interest is reflected as a separate element of stockholders’ equity/(deficit) on AgeX’s consolidated balance sheets. Any material intercompany transactions and balances have been eliminated upon consolidation.
AgeX assesses whether it is the primary beneficiary of a variable interest entity (“VIE”) at the inception of the arrangement and at each reporting date. This assessment is based on its power to direct the activities of the VIE that most significantly impact the VIE’s economic performance and AgeX’s obligation to absorb losses or the right to receive benefits from the VIE that could potentially be significant to the VIE. If the entity is within the scope of the variable interest model and meets the definition of a VIE, AgeX considers whether it must consolidate the VIE or provide additional disclosures regarding its involvement with the VIE. If AgeX determines that it is the primary beneficiary of the VIE, AgeX will consolidate the VIE. This analysis is performed at the initial investment in the entity or upon any reconsideration event. For entities AgeX holds as an equity investment that are not consolidated under the VIE model, AgeX will consider whether its investment constitutes a controlling financial interest in the entity and therefore should be considered for consolidation under the voting interest model.
AgeX has four subsidiaries, Reverse Bio, ReCyte Therapeutics, Inc. (“ReCyte”), NeuroAirmid Therapeutics, Inc. (“NeuroAirmid”), Canaria Transaction Corporation (“Merger Sub”), and has incorporated but not yet capitalized a fifth subsidiary UniverXome Bioengineering, Inc.(“UniverXome”). Reverse Bio is a wholly owned subsidiary of AgeX through which AgeX plans to finance its iTRTM research and development efforts. AgeX has been seeking equity financing for Reverse Bio and to the extent that such Reverse Bio financing is obtained through the sale of capital stock or other equity securities by Reverse Bio, AgeX’s equity interest in Reverse Bio and its iTRTM business would be diluted. ReCyte is an early stage pre-clinical research and development company involved in stem cell-derived endothelial and cardiovascular related progenitor cells for the treatment of vascular disorders and ischemic conditions. AgeX owns 94.8% of the outstanding capital stock of ReCyte. NeuroAirmid is jointly owned by AgeX with the University of California – Irvine and certain researchers and was recently organized to pursue clinical development and commercialization of cell therapies, focusing initially on Huntington’s Disease. AgeX owns 50% of the outstanding capital stock of NeuroAirmid. AgeX consolidates NeuroAirmid despite not having majority ownership interest as it has the ability to influence decision making and financial results through contractual rights and obligations as per Accounting Standards Codification (“ASC”) 810, Consolidation. Merger Sub was incorporated for the purpose of merging with Serina to implement the Merger. UniverXome is expected, in connection with the planned Merger, to hold certain AgeX assets and assume AgeX indebtedness obligations to Juvenescence.
| 63 |
Long-Lived Intangible Assets
Long-lived intangible assets, consisting primarily of acquired patents, acquired in-process research and development (“IPR&D”) with alternative future uses, patent applications, and licenses to use certain patents, are stated at acquired cost, less accumulated amortization. Amortization expense is computed using the straight-line method over the estimated useful lives of the assets, generally over 10 years.
Impairment of Long-Lived Assets
Long-lived assets, including long-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be fully recoverable. If an impairment indicator is present, we evaluate recoverability by a comparison of the carrying amount of the assets to future undiscounted net cash flows expected to be generated by the assets. If the assets are impaired, the impairment recognized is measured by the amount by which the carrying amount exceeds the estimated fair value of the assets. As of December 31, 2023, there have been no such impairment losses.
Accounting for Warrants
We determine the accounting classification of warrants we issue, as either liability or equity, by first assessing whether the warrants meet liability classification in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity, then in accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock. Under ASC 480, warrants are considered liability classified if the warrants are mandatorily redeemable, obligate us to settle the warrants or the underlying shares by paying cash or other assets, and warrants that must or may require settlement by issuing a variable number of shares. If warrants do not meet the liability classification under ASC 480-10, we assess the requirements under ASC 815-40, which states that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the warrants do not require liability classification under ASC 815-40, in order to conclude equity classification, we also assess whether the warrants are indexed to our common stock and whether the warrants are classified as equity under ASC 815-40 or other U.S. GAAP. After all such assessments, we conclude whether the warrants are classified as liability or equity. Liability classified warrants require fair value accounting at issuance and subsequent to initial issuance with all changes in fair value after the issuance date recorded in the statements of operations. Equity classified warrants only require fair value accounting at issuance with no changes recognized subsequent to the issuance date. We do not have any liability classified warrants as of any period presented. See Note 45, Related Party Transactions to our consolidated financial statements included elsewhere in this Report tofor additional information regarding warrants.
Stock-Based Compensation
We recognize compensation expense related to employee stock option grants and other equity based awards, if any, in accordance with FASB ASC 718, Compensation – Stock Compensation (“ASC 718”).
We use the extent we do not haveBlack-Scholes option pricing model for estimating the fair value of options granted under our own employees, human resources2017 Equity Incentive Plan (the “Incentive Plan”). The fair value of each restricted stock or facilities for our operations, BioTime provides certain employees for administrative or operational services, including laboratory space and administrative facilities, as necessary, for our benefit, under the Shared Facilities Agreement. Accordingly, BioTime allocates expenses such as salaries and payroll related expenses incurred and paid on our behalfrestricted stock unit grant, if any, is determined based on the amountvalue of timethe common stock granted or sold. We have elected to treat stock-based awards with time-based service conditions as a single award and recognize stock-based compensation on a straight-line basis over the requisite service period.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718. Stock option awards issued to non-employees, principally consultants or outside contractors, as applicable, are accounted for at fair value using the Black-Scholes option pricing model. Management believes that particular employees devotethe fair value of the stock options can more reliably be measured than the fair value of services received. We record compensation expense based on the then-current fair values of the stock options at the grant date in accordance with ASU 2018-07, Compensation – Stock Compensation (Topic 718): Improvements to AgeX affairs. Other expenses suchNonemployee Share-Based Payment Accounting, which simplifies the accounting for non-employee share-based payment transactions. We adopted ASU 2018-07 on January 1, 2019. As we had one stock option grant issued to a nonemployee as legal, accountingof the adoption date and financial reporting, marketing, and travel expenses are allocated to usone additional stock option grant during 2019 to the extent that those expenses are incurred by or on behalf of AgeX. BioTime also allocates certain overhead expenses such as rent and utilities, property taxes, insurance, laboratory expenses and supplies, telecommunications and other indirect expenses. These allocations are made based upon activity-based allocation drivers such as time spent, percentage of square feet of office or laboratory space used, headcount and percentage of personnel devoted to our operations or management. Management evaluatessame nonemployee, the appropriatenessapplication of the allocationsnew standard did not have a material impact on a periodic basis and believes that this basis for allocation is reasonable.
Related party transactions - allocated expenses from BioTime -Consistent with the principles of carve-out financial statements and presentation discussed in Note 2 to our consolidated financial statements, certain expenses have been allocated by BioTime and included in our consolidated statements of operations and consolidated statements of stockholders’ equity asstatements. Compensation expense for non-employee grants is recorded on a contribution by BioTime. Research and development expenses include allocations from BioTime primarily attributable to certain former BioTime research departments contributed to us. Those expenses are primarily comprised of former BioTime personnel and related expenses, including stock-based compensation, and other outside expenses relevant to the nature of the research projects that were contributed to us pursuant to the Asset Contribution Agreement discussed in Note 4 to our consolidated financial statements included elsewhere in this Report. Management considers the allocation methodologies used to allocate expenses as reasonable and appropriate based on historical BioTime expenses attributable to us and our operations for purposes of the standalone, carve-out consolidated financial statements included elsewhere in this Report. The expenses reflected in the consolidated financial statements may not be indicative of expenses that we will incur as an independent, publicly-traded companyand should not be relied upon as an indicator of our future results.
Research and development –Research and development expenses include both direct expenses incurred by us or our subsidiaries and indirect overhead costs allocated by BioTime that benefit or support our research and development functions. Direct research and development expenses consist primarily of personnel costs and related benefits, including stock-based compensation, amortization of intangible assets, outside consultants and suppliers, and license fees paid to third parties to acquire patents or licenses to use patents and other technology. Direct research and development expenses also include allocations for carve-out presentation purposes from certain former BioTime research departments discussed above underRelated party transactions - allocated expenses from BioTime. Indirect research and development expenses include overhead expenses allocated to us by BioTime discussed underRelated party transactions - Shared Facilities and Services Agreement above. Research and development costs are expensed as incurred. Research and development expenses incurred and reimbursed by grants from third parties or governmental agencies, including service revenues from co-development projects with customers, if any and as applicable, approximate the respective revenues recognizedstraight-line basis in the consolidated statements of operations.
| 64 |
General
The Black-Scholes option pricing model requires us to make certain assumptions including the fair value of the underlying common stock, the expected term, the expected volatility, the risk-free interest rate and administrative – Generalthe dividend yield.
The fair value of the shares of common stock underlying the stock options has historically been determined by our Board of Directors. Because there was no public market for our common stock prior to November 29, 2018, our Board of Directors determined the fair value of the common stock at the time of the grant of options prior to that date by considering a number of objective and administrative expenses include both direct expenses incurred by ussubjective factors including contemporaneous sales of our common stock to investors, valuation of comparable companies, operating and indirect overhead costs allocated by BioTime that benefit or support ourfinancial performance and general and administrative functions. Direct general and administrative expenses consist primarily of compensation and related benefits, including stock-based compensation, for executive and corporate personnel, and professional and consulting fees. Direct general and administrative expenses also include allocations for carve-out presentation purposes discussed above underRelated party transactions - allocated expenses from BioTime. Indirect general and administrative expenses include overhead expenses allocated to us by BioTime discussed underRelated party transactions - Shared Facilities and Services Agreement above.
Income taxes –For Federal and California purposes, AgeX’s activity through August 30, 2018 and for 2017 will be orindustry-specific economic outlook, amongst other factors. The fair value was included in BioTime’s federal consolidated and California combined tax returns. For those periods, the income tax provision was prepareddetermined in accordance with applicable elements of the practice aid issued by the American Institute of Certified Public Accountants titled Valuation of Privately Held Company Equity Securities Issued as Compensation. Since our common stock began publicly trading on the NYSE American, the fair value of our common stock underlying stock options has been valued based on prevailing market prices.
The expected term of employee stock options represents the weighted-average period that the stock options are expected to remain outstanding. We estimate the expected term of options granted based upon the “simplified method” provided under Staff Accounting Bulletin, Topic 14, or SAB Topic 14.
Because our common stock had no publicly traded history prior to November 29, 2018, for the years ended December 31, 2023 and 2022, we estimated the expected volatility using our own stock price volatility to the extent applicable or a combination of our stock price volatility and the stock price volatility of peer companies, for a period equal to the expected term of the options. The peer companies used include selected public companies within the biotechnology industry with comparable characteristics to AgeX, including similarity in size, lines of business, market capitalization, revenue and financial leverage.
The risk-free interest rate assumption is based upon observed interest rates on the United States government securities appropriate for the expected term of our stock options.
The dividend yield assumption is based on our history and expectation of dividend payouts. We have never declared or paid any cash dividends on our common stock, and we do not anticipate paying any cash dividends in the foreseeable future.
All excess tax benefits and tax deficiencies from stock-based compensation awards accounted for under ASC 740,Income Taxes,using718 are recognized as an income tax benefit or expense, respectively, in the separate return method to determineconsolidated statements of operations. An excess income tax benefit arises when the tax provisiondeduction of AgeXa share-based award for carve-out presentationincome tax purposes exceeds the compensation cost recognized for financial reporting purposes, and a tax deficiency arises when the compensation cost exceeds the tax deduction.
Stock-based compensation expense for the years ended December 31, 2023 and 2022 consists of itsstock-based compensation under the Incentive Plan, and stock-based compensation of AgeX’s subsidiaries that have their own stock option plans.
None of our consolidated financial statements. The separate return method, amongstsubsidiaries have granted stock options or other things, requires thatequity awards for the years ended December 31, 2023 and 2022.
Although the fair value of stock options is determined in accordance with FASB guidance, changes in the assumptions and allocations can materially affect the estimated value and therefore the amount of current and deferred taxcompensation expense for a group that files a consolidated income tax return be allocated among the members of that group as if each group member were a separate taxpayer. As a result, the provision for income taxes has been presented as if AgeX had filed a separate federal consolidated tax return and a California combined tax return for the periods presented. In using the separate return method, the sum of the amounts allocated to the members of the income tax return group may not equalrecognized in the consolidated amount. If tax attributes recorded in the carve-out consolidated financial statements are materially different from the actual tax attributes pertaining to us or our legal entities and our subsidiaries, or to BioTime and its subsidiaries, those differences are identified and disclosed in Note 7 to our consolidated financial statements included elsewhere in the Report. Accordingly, depending on our future legal structure and related tax elections that may be taken by us, our effective tax rate in future years could vary materially from our historical effective tax rates. The historical deferred tax assets, including the operating losses and credit carryforwards generated by certain research and development departments that operated within BioTime and were transferred to us on August 17, 2017, have been presented as our tax attributes consistent with the principles of the separate return method described above.statements.
Income Taxes
As of December 31, 2018,2023, the deferred tax assets and liabilities presented in Note 79, Income Taxes included elsewhere in this Report, including net operating loss carryforwards and research and development credits, represent the tax attributes of AgeX and its subsidiaries. However, the net operating losses and research and development credits generated before August 17, 2017 with respect to BioTime research departments that were transferred to us on that date will remain as tax attributes of BioTime.
In general, net operating losses and other tax credit carryforwards generated by legal entities in a consolidated federal tax group or a combined state tax group, collectively “the tax group”, are available to other members of the tax group depending on the nature of the transaction that a member may enter into while still in the tax group. However, under the Tax Matters Agreement between BioTime and AgeX entered into on August 17, 2017, any use of a member’s net operating loss and other tax credit carryforwards by the other member is subject to reimbursement by the benefiting member for the actual tax benefit realized. Since the August 30, 2018 deconsolidation of AgeX and to date, neither BioTime nor AgeX has used the tax attributes of the other.
Weaccount for income taxes in accordance with ASC 740, which prescribes the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and enacted rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. Our judgments, estimates and projections regarding future taxable income may change over time due to changes, among other factors, in market conditions, changes in tax laws, and tax planning strategies. If our assumptions and consequently our estimates change in the future, the valuation allowance may be increased or decreased, which may have a material impact on our consolidated financial statements.
| 65 |
The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. We recognizeprovided a reserve against our federal and California research and development credits generated. The carryforward amounts for these credits have been reported net of these reserves. Accordingly, no accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penaltieshave been recorded as of December 31, 20182023 and 2017.2022. We aredo not awareexpect that the total amount of any uncertainunrecognized tax positions that could result in significant additional payments, accruals, or other material adjustments forbenefits will materially change over the years ended December 31, 2018 and 2017.next twelve months. We are currently unaware of any tax issues under review.
On December 22, 2017, the United States enacted major federal tax reform legislation, Public Law No. 115-97, commonly referred to as the 2017 Tax Cuts and Jobs Act (“2017 Tax Act”), which enacted a broad range of changes to the Internal Revenue Code. Changes to taxes on corporations impacted by the 2017 Tax Act include, but not limited to, lowering the U.S. federal tax rates to a 21 percent flat tax rate, eliminating the corporate alternative minimum tax (“AMT”), imposing additional limitations on the deductibility of interest and net operating losses, allowing any net operating loss (“NOLs”) generated in tax years ending after December 31, 2017 to be carried forward indefinitely and generally repealing carrybacks, reducing the maximum deduction for NOL carryforwards arising in tax years beginning after 2017 to a percentage of the taxpayer’s taxable income, and allowing for additional expensing of certain capital expenditures. The 2017 Tax Act also puts into effect a number of changes impacting operations outside of the United States including, but not limited to, the imposition of a one-time tax “deemed repatriation” on accumulated offshore earnings not previously subject to U.S. tax, and shifts the U.S taxation of multinational corporations from a worldwide system of taxation to a territorial system. ASC 740 requires the effects of changes in tax rates and laws on deferred tax balances (including the effects of the one-time transition tax) to be recognized in the period in which the legislation is enacted.
On December 22, 2017, the SEC staff issued Staff Accounting Bulletin No. 118 (“SAB 118”) to provide guidance for companies that are not able to complete their accounting for the income tax effects of the 2017 Tax Act in the period of enactment. SAB 118 allows us to record provisional amounts during a measurement period not to extend beyond one year of the enactment date. We applied the guidance in SAB 118 when accounting for the enactment-date effects of the 2017 Tax Act during the years ended December 31, 2018 and 2017. As of December 31, 2018, we completed the accounting for all the enactment-date income tax effects of the 2017 Tax Act as further discussed in Note 7 to our consolidated financial statements included elsewhere in this Report.
Stock-based compensation – We recognize compensation expense related to employee stock option grants and other equity based awards, if any, in accordance with FASB ASC 718,Compensation – Stock Compensation (“ASC 718”).
We estimate the fair value of employee stock-based payment awards on the grant-date and recognize the resulting fair value, net of estimated forfeitures for grants prior to 2017, over the requisite service period. Upon adoption of Accounting Standards Update (“ASU”) 2016-09 on January 1, 2017, forfeitures are accounted for as they occur instead of based on the number of awards that were expected to vest prior to adoption of ASU 2016-09.
We use the Black-Scholes option pricing model for estimating the fair value of options granted under our 2017 Equity Incentive Plan (the “Plan”). The fair value of each restricted stock or restricted stock unit grant, if any, is determined based on the value of the common stock granted or sold. We have elected to treat stock-based awards with time-based service conditions as a single award and recognize stock-based compensation on a straight-line basis over the requisite service period.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718 and FASB ASC 505-50,Equity-Based Payments to Non-Employees. Stock option awards issued to non-employees, principally consultants or outside contractors, as applicable, are accounted for at fair value using the Black-Scholes option pricing model. Management believes that the fair value of the stock options can more reliably be measured than the fair value of services received. We record compensation expense based on the then-current fair values of the stock options at each financial reporting date. Compensation expense recorded during the service period is adjusted in subsequent periods for changes in the fair value of the stock options until the earlier of the date at which the non-employee’s performance is complete or a performance commitment is reached, which is generally when the stock option award vests. Compensation expense for non-employee grants is recorded on a straight-line basis in the consolidated statements of operations.
The Black-Scholes option pricing model requires us to make certain assumptions including the fair value of the underlying common stock, the expected term, the expected volatility, the risk-free interest rate and the dividend yield.
The fair value of the shares of common stock underlying the stock options has historically been determined by our Board of Directors. Because there was no public market for our common stock prior to November 29, 2018, our Board of Directors determined the fair value of the common stock at the time of the grant of options by considering a number of objective and subjective factors including contemporaneous sales of our common stock to investors, valuation of comparable companies, operating and financial performance and general and industry-specific economic outlook, amongst other factors. The fair value was determined in accordance with applicable elements of the practice aid issued by the American Institute of Certified Public Accountants titledValuation of Privately Held Company Equity Securities Issued as Compensation. Since our common stock began publicly trading on the NYSE American, the fair value of our common stock underlying stock options has been valued based on prevailing market prices.
The expected term of employee stock options represents the weighted-average period that the stock options are expected to remain outstanding. We estimate the expected term of options granted based upon the “simplified method” provided underStaff Accounting Bulletin, Topic 14, or SAB Topic 14.
Because our common stock has no publicly traded history prior to November 29, 2018, for the year ended December 31, 2017 we estimated the expected volatility of the awards from the historical volatility of selected public companies within the biotechnology industry with comparable characteristics to us, including similarity in size, lines of business, market capitalization, revenue and financial leverage. We determined the expected volatility assumption using the frequency of daily historical prices of comparable public company’s common stock for a period equal to the expected term of the options. For the year ended December 31, 2018, we estimated the expected volatility using its own stock price volatility to the extent applicable or a combination of our stock price volatility and the stock price volatility of stock of peer companies, for a period equal to the expected term of the options.
The risk-free interest rate assumption is based upon observed interest rates on the United States government securities appropriate for the expected term of our stock options.
The dividend yield assumption is based on our history and expectation of dividend payouts. We have never declared or paid any cash dividends on our common stock, and we do not anticipate paying any cash dividends in the foreseeable future.
All excess tax benefits and tax deficiencies from stock-based compensation awards accounted for under ASC 718 are recognized as an income tax benefit or expense, respectively, in the consolidated statements of operations. Prior to the adoption of ASU 2016-09, AgeX recognized excess tax benefits, if any, in additional paid-in capital only if the tax deduction reduced cash income taxes payable, and excess tax deficiencies were recognized as an offset to accumulated excess tax benefits, if any, on AgeX’s consolidated statements of operations. An excess income tax benefit arises when the tax deduction of a share-based award for income tax purposes exceeds the compensation cost recognized for financial reporting purposes, and a tax deficiency arises when the compensation cost exceeds the tax deduction. Because AgeX had no stock option exercises during the years ended December 31, 2018 and 2017, and because of AgeX’s full valuation allowance as of December 31, 2018 and 2017, there was no impact to AgeX’s consolidated financial statements from the adoption of 2016-09.
Stock-based compensation expense for the years ended December 31, 2018 and 2017 consists of stock-based compensation under the AgeX 2017 Equity Incentive Plan, stock-based compensation allocated from BioTime, and stock-based compensation of AgeX’s subsidiaries that have their own stock option plans.
As discussed above, certain of our consolidated subsidiaries have their own share-based compensation plans. For share-based compensation awards granted by those privately-held consolidated subsidiaries under their respective equity plans, we determine the fair value of the options granted under those plans using similar methodologies and assumptions we used for our stock options discussed above.
Although the fair value of stock options is determined in accordance with FASB guidance, changes in the assumptions and allocations can materially affect the estimated value and therefore the amount of compensation expense recognized in the consolidated financial statements.
Long-lived intangible assets – Long-lived intangible assets, consisting primarily of acquired patents, acquired in-process research and development (“IPR&D”) with alternative future uses, patent applications, and licenses to use certain patents, are stated at acquired cost, less accumulated amortization. Amortization expense is computed using the straight-line method over the estimated useful lives of the assets, generally over 10 years.
Impairment of long-lived assets – Long-lived assets, including long-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be fully recoverable. If an impairment indicator is present, we evaluate recoverability by a comparison of the carrying amount of the assets to future undiscounted net cash flows expected to be generated by the assets. If the assets are impaired, the impairment recognized is measured by the amount by which the carrying amount exceeds the estimated fair value of the assets. Through December 31, 2018, there have been no such impairment losses.
Adoption of ASU 2014-09, Revenues from Contracts with Customers (Topic 606). During May 2014, the FASB issued ASU 2014-09 (“Topic 606”)Revenue from Contracts with Customers which supersedes the revenue recognition requirements in Topic 605Revenue Recognition (“Topic 605”). Topic 606 describes principles an entity must apply to measure and recognize revenue and the related cash flows, using the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligation(s) in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. Topic 606 core principle is that it requires entities to recognize revenue when control of the promised goods or services is transferred to customers at an amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods or services.
We adopted Topic 606 as of January 1, 2018 using the modified retrospective transition method applied to those contracts which were not completed as of the adoption date. Results for reporting periods beginning on January 1, 2018 and thereafter are presented under Topic 606, while prior period amounts are not adjusted and continue to be reported in accordance with our historic revenue recognition accounting under Topic 605.
On January 1, 2018, the impact of the adoption and application of Topic 606 was immaterial, and no cumulative effect adjustment was made as of that date. In the applicable paragraphs below, we have summarized our revenue recognition policies for various revenue sources in accordance with Topic 606.
Subscription and advertisement revenues. LifeMap Sciences, our direct majority-owned subsidiary, sells subscription-based products, including research databases and software tools, for biomedical, gene, and disease research. LifeMap Sciences sells these subscriptions primarily through the internet to biotech and pharmaceutical companies worldwide. LifeMap Sciences’ principal subscription product is theGeneCards® Suite, which includes theGeneCards® human gene database, and theMalaCards human disease database.
LifeMap Sciences’ performance obligations for subscriptions include a license of intellectual property related to its genetic information packages and premium genetic information tools. These licenses are deemed functional licenses that provide customers with a “right to access” to LifeMap Sciences’ intellectual property during the subscription period and, accordingly, revenue is recognized over a period of time, which is generally the subscription period. Payments are typically received at the beginning of a subscription period and revenue is recognized according to the type of subscription sold.
For subscription contracts in which the subscription term commences before a payment is due, LifeMap Sciences records an accounts receivable as the subscription is earned over time and bills the customer according to the contract terms. LifeMap Sciences continuously monitors collections and payments from customers and maintains a provision for estimated credit losses and uncollectible accounts based upon its historical experience and any specific customer collection issues that have been identified. Amounts determined to be uncollectible are written off against the allowance for doubtful accounts. LifeMap Sciences has not historically provided significant discounts, credits, concessions, or other incentives from the stated price in the contract as the prices are offered on a fixed fee basis for the type of subscription package being purchased. LifeMap Sciences may issue refunds only if the packages cease to be available for reasons beyond its control. In such an event, the customer will get a refund on a pro-rata basis. Both the customer and LifeMap Sciences expect the subscription packages to be available during the entire subscription period, and LifeMap Sciences has not experienced any significant issues with the availability of the product and has not issued any material refunds. Using the most likely amount method for estimating refunds under Topic 606, including historical experience, LifeMap Sciences determined that the single most likely amount of variable consideration for refunds is immaterial as LifeMap Sciences does not expect to pay any refunds.
LifeMap Sciences performance obligations for advertising are overall advertising services and represent a series of distinct services. Contracts are typically less than a year in duration and the fees charged may include a combination of fixed and variable fees with the variable fees tied to click throughs to the customer’s products on their website. LifeMap Sciences allocates the variable consideration to each month the click through services occur and allocates the annual fee to the performance obligation period of the initial term of the contract because those amounts correspond to the value provided to the customer each month. For click-through advertising services, at the time the variable compensation is known and determinable, the service has been rendered and revenue is recognized at that time.
LifeMap Sciences deferred subscription revenues primarily represent subscriptions for which cash payment has been received for the subscription term, but the subscription term has not been completed as of the balance sheet date reported. For the years ended December 31, 2018 and 2017, LifeMap Sciences recognized $1.2 million and $1.4 million in subscription and advertisement revenues. As of December 31, 2018, there was $0.3 million included in deferred revenues in the consolidated balance sheets which is expected to be recognized as subscription revenue over the next twelve months.
LifeMap Sciences has licensed from third parties the databases and software it commercializes and has a contractual obligation to pay royalties to the licensor on subscriptions sold. These costs are included in cost of sales on the consolidated statements of operations when the cash is received and the royalty obligation is incurred as the royalty payments do not qualify for capitalization of costs to fulfill a contract under ASC 340-40,Other Assets and Deferred Costs - Contracts with Customers.
Grant revenues. In applying the provisions of Topic 606, we have determined that government grants are out of the scope of Topic 606 because the government entities do not meet the definition of a “customer”, as defined by Topic 606, as there is not considered to be a transfer of control of good or services to the government entities funding the grant. We have, and will continue to, account for grants received to perform research and development services in accordance with ASC 730-20,Research and Development Arrangements, which requires an assessment, at the inception of the grant, of whether the grant is a liability or a contract to perform research and development services for others. If we or a subsidiary receiving the grant are obligated to repay the grant funds to the grantor regardless of the outcome of the research and development activities, then we are required to estimate and recognize that liability. Alternatively, if we or a subsidiary receiving the grant are not required to repay, or if we are required to repay the grant funds only if the research and development activities are successful, then the grant agreement is accounted for as a contract to perform research and development services for others, in which case, grant revenue is recognized when the related research and development expenses are incurred.
In September 2018, we were awarded a grant of up to approximately $225,000 from the NIH.The NIH grant provides funding for continued development of our technologies for treating osteoporosis. The grant funds will be made available to AgeX by the NIH as allowable expenses are incurred. For the year ended December 31, 2018, we incurred approximately $20,000 of allowable expenses under the NIH grant and recognized corresponding grant revenues for that period.
On April 5, 2018, our subsidiary ReCyte Therapeutics was awarded a grant of up to approximately $386,000 from the NIH. The NIH grant provides funding for continued development of our technologies for treating stroke. The grant funds will be made available to ReCyte Therapeutics by the NIH as allowable expenses are incurred. As of December 31, 2018, ReCyte Therapeutics had not incurred any allowable expenses or recognized any grant revenues under the NIH grant.
Arrangements with multiple performance obligations. Future contracts with customers may include multiple performance obligations. For such arrangements, we will allocate revenue to each performance obligation based on its relative standalone selling price. We will generally determine or estimate standalone selling prices based on the prices charged, or that would be charged, to customers for that product or service. As of, and for the year ended, December 31, 2018, we did not have significant arrangements with multiple performance obligations.
Financial Operations Overview
To date, our revenues have been principally derived from subscription and advertising revenue from LifeMap Sciences’ online databases based upon applicable subscription or advertising periods. We do not have any therapeutic products approved for sale and have not generated any revenueinsignificant revenues from commercialized product sales, and we do not expect to generate any revenuesignificant revenues from product sales for the foreseeable future.
Our operating expenses consist of research and development expenses primarily from our pre-clinical programs and general and administrative expenses, including a significant amountexpenses. Since the layoffs of operating expenses allocatedmostly research personnel in April 2020, research and development work have been scaled back and contracted out to third party service providers within the newly imposed budgetary constraints under our loan agreements and promissory notes for loans advanced to us from BioTime, either incurred directly for our benefit or indirect overhead costs, as described under “—Critical Accounting Policies.”by Juvenescence. Accordingly, the historical amounts of expense presented and discussed in this Report are likely not going to be indicative of expenses during future periods.
We expect to continue to incur significant expenses and operating losses over the next several years. We anticipate that our expenses will increase significantly in connection with our ongoing activities, if we:
Results of Operations
Comparison of Years Ended December 31, 20182023 and 20172022
Revenues and Cost of Sales
The amounts in the table below show our consolidated revenues by source and cost of sales for the years ended December 31, 20182023 and 20172022 (in thousands).
| Year Ended December 31, | $ Increase/ | % Increase | ||||||||||||||
| 2018 | 2017 | (Decrease) | (Decrease) | |||||||||||||
| Subscription and advertising revenues | $ | 1,227 | $ | 1,399 | $ | (172 | ) | (12.3 | %) | |||||||
| Service and other revenues | 149 | 5 | 144 | * | % | |||||||||||
| Grant revenues | 20 | - | 20 | * | % | |||||||||||
| Total revenues | 1,396 | 1,404 | (8 | ) | (0.6 | %) | ||||||||||
| Cost of sales | (364 | ) | (168 | ) | 196 | 116.7 | % | |||||||||
| Gross profit | $ | 1,032 | $ | 1,236 | $ | (204 | ) | (16.5 | %) | |||||||
| Year Ended December 31, | $ +Increase/ | % +Increase/ | ||||||||||||||
| 2023 | 2022 | -Decrease | -Decrease | |||||||||||||
| Grant revenues | $ | 77 | $ | - | $ | +77 | * | % | ||||||||
| Other revenues | 65 | 34 | +31 | * | % | |||||||||||
| Total revenues | 142 | 34 | +108 | * | % | |||||||||||
| Cost of sales | (40 | ) | (13 | ) | +27 | * | % | |||||||||
| Gross profit | $ | 102 | $ | 21 | $ | +81 | * | % | ||||||||
Our revenues were primarily generated by LifeMap Sciences, as subscription and advertising revenues from itsGeneCards®online database. Subscription and advertising revenues amounted to $1.2 million and $1.4 million for the years ended December 31, 2018 and 2017.
*% fluctuation is not meaningful.
During the year ended December 31, 2018, LifeMap Sciences also generated $115,000 of revenues from performing services under contracts, which we do not expect will be recurring revenues for LifeMap Sciences, while service revenues were insignificant during 2017.
During 20182023, we recognized income ofapproximately $20,000$77,000 from agrant fromawarded by the NIH. We had no in August 2023 with a one year grant revenue in 2017.
Cost of sales forperiod that commenced on September 1, 2023. During theyear ended December 31, 2018as compared to 2017 increased primarily due to an increase2022, we did not recognize any grant revenues.
During the years ended December 31, 2023 and 2022, we recognized $65,000 and $34,000, respectively, from the sale of research products. Revenues from the sale of research products are included in the royalty payments made or incurred by LifeMap Sciences due to a long-term increase in the royalty rate for its subscriptions products and due to the timing of cash received and the related royalty obligation incurred.other revenues.
| 66 |
Operating Expenses
We have maintained a minimal workforce since the May 1, 2020 reduction in force which resulted in the layoff of most of our research and development personnel and certain administrative personnel. The following table shows our consolidated operating expenses for the years ended December 31, 20182023 and 20172022 (in thousands).
| Year Ended December 31, | $ Increase/ | % Increase/ | Year Ended December 31, | $ +Increase/ | % +Increase/ | |||||||||||||||||||||||||||
| 2018 | 2017 | (Decrease) | (Decrease) | 2023 | 2022 | -Decrease | -Decrease | |||||||||||||||||||||||||
| Research and development expenses | $ | 5,830 | $ | 5,784 | $ | 46 | 0.8 | % | $ | 734 | $ | 1,025 | $ | -291 | -28.4 | % | ||||||||||||||||
| Acquired in-process research and development | 800 | - | 800 | * | % | |||||||||||||||||||||||||||
| General and administrative expenses | 5,647 | 3,869 | 1,778 | 46.0 | % | 9,328 | 5,971 | +3,357 | +56.2% | |||||||||||||||||||||||
Research and development expensesDevelopment Expenses
Research and development expenses and acquired IPR&D increasedfor the year ended December 31, 2023 decreased by $0.8approximately $0.3 million to $6.6$0.7 million in 2018 as compared to $5.8approximately $1.0 million in 2017.2022. The increasenet decrease was primarily attributable to an increasereductions of $0.5$0.2 million for programs utilizingPureStem®cell lines and iTR technology. Additionally, on March 23, 2018, we purchased certain in-processin outside research and development assets, primarily relatedservices allocable to stem cell derived cardiomyocytes (heart muscle cells) to be developed by us, for a total cash consideration of $0.8 million. The transaction was considered an asset acquisition rather than a business combination. Accordingly, the $0.8 million was expensed on the acquisition date as acquired in-process research and development as those assets have no alternative future uses. These increases were partially offset by the LifeMap Sciences’ disposition of its ownership of LifeMap Solutions which accounted for $0.5 million of the decrease in research and development expenses as shownand $0.1 million in the table below.
The following table shows the amountssalaries and percentages of our totalpayroll related expenses allocated to research and development expenses of $6.6 million and $5.8 million, including acquired in-process research and development incurred during 2018, allocated to our primary research and development programs, during the years ended December 31, 2018 and 2017, respectively (amounts in thousands).expenses.
| Year Ended December 31, | ||||||||||||||||||
| Amount(1) | Percent of Total | |||||||||||||||||
| Company | Program | 2018 | 2017 | 2018 | 2017 | |||||||||||||
| AgeX including ReCyte Therapeutics(2) | PureStem®progenitor cell lines, brown adipose fat, iTR technology, and pre-clinical cardiovascular therapy research and development | $ | 4,343 | $ | 3,763 | 65.5 | % | 65.0 | % | |||||||||
| AgeX | Acquired in-process research and development | 800 | - | 12.1 | % | - | % | |||||||||||
| LifeMap Sciences(3) | Biomedical, gene, and disease databases and tools | 1,487 | 1,548 | 22.4 | % | 26.8 | % | |||||||||||
| LifeMap Solutions(4) | Mobile health co-developed software application | - | 473 | - | % | 8.2 | % | |||||||||||
| Total research and development expenses and acquired IPR&D | $ | 6,630 | $ | 5,784 | 100.0 | % | 100.0 | % | ||||||||||
Year Ended December 31, | $ +Increase/ | % +Increase/ | ||||||||||||||
| 2023 | 2022 | -Decrease | -Decrease | |||||||||||||
| iTRTM technology: AGEX-iTR1547 and AGEX-iTR1550 | $ | 166 | $ | 344 | $ | -178 | -51.7 | % | ||||||||
| PureStem® progenitor cell lines, brown adipose fat, neural stem cells (NSC), exosomes, and related research and development: AGEX-BAT1 and cGMP PSC-derived cells including NSC | 568 | 681 | -113 | -16.6 | % | |||||||||||
| Total | $ | 734 | $ | 1,025 | ||||||||||||
General and administrative expensesAdministrative Expenses
The following table shows the amount and percentages of our consolidated general and administrative expenses of $5.6 million and $3.9 million incurred during the years ended December 31, 2018 and 2017, respectively (amounts in thousands).
| Year Ended December 31, | ||||||||||||||||
| Amount(1) | Percent of Total | |||||||||||||||
| Company | 2018 | 2017 | 2018 | 2017 | ||||||||||||
| AgeX including ReCyte Therapeutics | $ | 4,803 | $ | 2,819 | 85.1 | % | 72.9 | % | ||||||||
| LifeMap Sciences(2) | 844 | 569 | 14.9 | % | 14.7 | % | ||||||||||
| LifeMap Solutions(3) | - | 481 | - | % | 12.4 | % | ||||||||||
| Total general and administrative expenses | $ | 5,647 | $ | 3,869 | 100.0 | % | 100.0 | % | ||||||||
General and administrative expenses for the year ended December 31, 20182023 increased by $1.7$3.3 million to $5.6$9.3 million as compared to $3.9$6.0 million in 2017. This2022. The net increase was primarilyis attributable to the following: $0.7increases of $2.5 million in financial reporting, compliance,professional fees for legal services, professional fees for tax and accounting services, and consulting expenses incurred in connection with due diligence and other expenses related to our preparation and filingthe planned Merger, $0.4 million for the write off of prepaid expenses incurred in prior periods related to a shelf registration statement on Form 10 to become a public company;for an at-the-market offering of AgeX common stock that expired in January 2024, $0.4 million estimated litigation fees, $0.2 million in salaries, consulting travelfees, and payroll related expenses;expenses, including severance related expenses arising under the Transition Services and $1.0Separation Agreement with our former Chief Executive Officer, $0.1 million increase in noncash stock-based compensation expense. Since our Equity Incentive Plan was establishedinvestor relations related expenses, and $0.1 million in July 2017insurance expense, allocated to general and based on the timing of our stock option grants occurring during the latter half of 2017, our stock-based compensation expense was significantly higher in 2018.administrative expenses. These increases were partiallyoffset to some extent by a $0.2 million decrease in minimum royalty fees resulting from the termination of certain license and sub-license agreements, $0.1 million net decrease in non-cash stock-based compensation to employees, consultants and directors, and a $0.1 million decrease in patent and license maintenance related fees.
General and administrative expenses include employee and director compensation allocated to general and administrative expenses, consulting fees other than those paid for science-related consulting, facilities and equipment rent and maintenance related expenses, insurance costs allocated to general and administrative expenses, stock exchange-related costs, depreciation expense, marketing costs, legal and accounting costs, and other miscellaneous expenses which are allocated to general and administrative expense.
Other Expense, Net
Other expense, net in 2023 consists primarily of $5.4 million amortization of deferred debt issuance costs to interest expense, write off of deferred debt cost upon $36 million debt exchanged for preferred stock in July 2023, and other debt related expenses included in interest expense offset by approximately $0.5 million net interest income primarily earned from the Serina Note. Other expense, net in 2022 consists primarily of cost savings after June$3.3 million of amortization of deferred debt issuance costs on loans from Juvenescence to interest expense, and $0.2 million change in fair value of warrants issued to Juvenescence in connection with borrowings under the 2022 Secured Note. See Notes 4, Convertible Note Receivable, 5, Related Party Transactions, 6, 2017, resulting from LifeMap Sciences’ disposition of its ownership of LifeMap Solutions as notedWarrant Liability, and 7, Stockholders’ Equity/(Deficit) to our consolidated financial statements included elsewhere in the table above.this Report for additional information about our loan agreement with Serina, loan agreements with Juvenescence, liability classified warrants, and debt exchanged for preferred stock.
| 67 |
Gain on sale of equity method investment in Ascendance
Income Taxes
On March 23, 2018 Ascendance Biotechnology, Inc. (“Ascendance”), a company in which we held shares of common stock accounted for on the equity method, was acquired by a third party in a merger and we received $3.2 million in cash for our Ascendance common stock. We recognized a gain on sale for the same amount included in other income and expenses, net, during
For the year ended December 31, 2018.
Gain on sale of assets
Loss from operations2023, we experienced a loss; therefore, no income tax provision was recorded for the year ended December 31, 2017 includes a $1.8 million gain we recognized on the sale of certain co-developed assets by LifeMap Solutions to its customer prior to the transfer of LifeMap Solutions to BioTime on June 6, 2017.2023.
Income taxes
For Federal and California purposes, our activity through August 30, 2018 and for 2017 will be or was included in BioTime’s federal consolidated and California combined tax returns. For these periods, the provision for income taxes has been presented as if we had filed a separate federal consolidated tax return and a California combined tax return using the separate return method.
As of December 31, 2018,2023, we had net operating loss carryforwards of approximately $31.5$59.7 million for U.S. federal income tax purposes. Of this amount, $8.7 million is attributable to LifeMap Sciences, which includes $2.1 million inNOLs generated while it was included in the consolidated BioTime tax group and would be available to offset income of AgeX in the future. The remaining LifeMap Sciences NOLs of $6.6 million are attributable to NOLs generated for the tax years during which LifeMap Sciences filed a separate federal income tax return and, accordingly, those NOLs are available only to LifeMap Sciences’ taxable income within AgeX in future years.In general, NOLs and other tax credit carryforwards generated by legal entities in a consolidated federal tax group are available to other members of the tax group depending on the nature of the transaction that a member may enter into while still in the consolidated federal tax group. However, under the Tax Matters Agreement between BioTime and AgeX, any use of a member’s NOLs and other tax credit carryforwards by the other member is subject to reimbursement by the benefiting member for the actual tax benefit realized. Since the August 30, 2018 deconsolidation of AgeX, and to date, neither BioTime nor AgeX has used the tax attributes of the other.
On June 6, 2017, BioTime and LifeMap Sciences entered into a Debt Conversion Agreement whereby BioTime acquired additional stock in LifeMap Sciences and other assets, including intellectual property and direct ownership of LifeMap Solutions in exchange for settlement of related party indebtedness of approximately $8.8 million owed to BioTime. These transactions occurred between commonly controlled entities, including noncontrolling interests, and the financial reporting impact is discussed in Note 4 to our consolidated financial statements included elsewhere in this Report. For income tax purposes, the purchase by BioTime of LifeMap Sciences’ intellectual property and other assets resulted in a taxable gain to LifeMap Sciences of $3.7 million for the year ended December 31, 2017. Although LifeMap Sciences had sufficient current year operating losses and regular net operating loss carryforwards to offset the entire gain, it incurred a federal alternative minimum tax payable (“AMT”) of $22,000 as of December 31, 2017. As LifeMap Sciences will ultimately receive a full refund of the current AMT payable, fully offsetting the current provision, there is no tax provision or benefit recorded for the year ended December 31, 2017.
As of December 31, 2018,2023, we had net operating losses of approximately $32.5$19.8 million for California purposes. As we and our subsidiaries have been included in the combined California tax return with BioTime, up to the date of deconsolidation on August 30, 2018, those state net operating losses will remain with AgeX.
Federal net operating losses generated on or prior to December 31, 2017, expire in varying amounts between 20302028 and 2037, while federal net operating losses generated after December 31, 2017, carryforward indefinitely. The state net operating losses expire in varying amounts between 2028 and 2038.2043.
As of December 31, 2018, AgeX2023, we had research and development tax credit carryforwards for federal and state tax purposes of $903,000$0.7 million and $831,000,$0.5 million, respectively. Although this LifeMap Sciences credit has been included as part of the AgeX credit carryforwards, LifeMap Sciences filed a separate federal income tax return prior to January 1, 2018 and its prior research credit carryforwards may not be used to offset federal taxable income of AgeX. As AgeX and its subsidiaries were included in the California combined return with BioTime, these credits noted above will remain with AgeX. The federal tax credits expire between2028 and 2038,2043, while the state tax credits have no expiration date.
As of December 31, 2023, we had capital loss carryforwards for federal and state tax purposes of $12.4 million and $5.9 million, respectively. The federal and California capital loss carryforwards will expire in 2026.
A valuation allowance is provided when it is more likely than not that all or some or allportion of the deferred tax assets will not be realized. We established a full valuation allowance for all periods presented due to the uncertainty of realizing future tax benefits from ourits net operating loss carryforwards and other deferred tax assets. Accordingly, due to losses incurred for all periods presented, we did not record any provision or benefit for income taxes.
Liquidity and Capital Resources
Since inception, we have financed our operations through contributionsOperating Losses and advances from our former parent company, BioTime, the sale of our common stock, the sale and exercise of warrants, and research grants. BioTime has also provided us with the use of BioTime facilities and services under the Shared Facilities Agreement. Although BioTime may continue to provide administrative support to us on a reimbursable basis, we do not expect BioTime will provide us future financing.Going Concern Considerations
Wehave incurred operating losses and negative cash flows since inception and had an accumulated deficit of $74.1$131.0 million as of December 31, 2018.2023. Weexpect to continue to incur operating losses and negative cash flows.
We expecthave been funding our expensesoperations with loans from Juvenescence. As of March 20, 2024, we had drawn down the entire $4,400,000 line of credit made available to increase in the near term in connection with our ongoing activities, including costs related to our planned move to a new office and laboratory facility in Alameda, California under a sublease from a third party that will replace our use of BioTime’s facilities. We will also incur additional costs if we hire our internal administrative personnel and rely less on services providedus by BioTimeJuvenescence under the terms of the Shared Facilities Agreement. Furthermore, now that2022 Secured Note. We do not have any other commitments for additional financing.
We have made certain adjustments to our operating plans and budgets to reduce our projected cash expenditures in order to extend the period over which we are a public company, we will incur additional costs associated with operating as a public company. In the longer term, we expect our expenses to increase as wecan continue our pre-clinicaloperations with our available cash resources. These adjustments entailed down-sizing of our leased office space effective January 1, 2021, a staff force reduction during 2020 primarily impacting research and development activitiespersonnel, and if we initiate clinical trials and seek marketing approval forthe elimination of our product candidates. In addition, if we obtain marketing approval for anyleased laboratory facility. These down-sizing adjustments to our operations will require the deferral of certain work on the development of our product candidates we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution to the extent that such sales, marketing and distribution are not the responsibility of potential collaborators. Accordingly, we will need to obtain substantial additional funding in connection withtechnologies. However, notwithstanding those adjustments, based on our continuing operations.
We have evaluated ourmost recent projected cash flows, and believe that our cash and cash equivalents, the amount of $6.7 million asthe 2022 Secured Note line of December 31, 2018, pluscredit available to us from Juvenescence would not be sufficient to satisfy our anticipated operating and other funding requirements for the $4.5 million we received from the exercise of Warrants during March 2019, providesufficient cash, cash equivalents, and liquidity to carry out our current operations through at leastnext twelve months from the issuance date of thefiling of this Report. These factors raise substantial doubt regarding our ability to continue as a going concern. See Note 5, Related Party Transactions, to our consolidated financial statements included elsewhere in this Report. However, it is likely that weReport for additional information about our loan agreements with Juvenescence. We will need to raise additional capital in the near term to be able to meet our operating expenses beyondexpenses.
The loans from Juvenescence that twelve month period.remain outstanding prohibit us and our subsidiaries ReCyte and Reverse Bio from borrowing funds from other lenders or engaging in certain other transactions without the consent of Juvenescence unless we repay all amounts owed to Juvenescence. AgeX and three of its subsidiaries have granted Juvenescence a security interest and lien on substantially all of their assets to secure AgeX’s obligations for the loans from Juvenescence that remain outstanding and any additional loans that may be advanced under the 2022 Secured Note. The outstanding loan balances, and any future loans advanced, plus accrued loan fees, under the 2022 Secured Note will become due and payable upon maturity on May 9, 2024. Certain accrued loan fees under the 2023 Secured Note, the principal amount of which was converted into preferred stock, also remain payable to Juvenescence.
During July 2023, AgeX and Juvenescence entered into an Exchange Agreement pursuant to which AgeX issued to Juvenescence 211,600 shares of a newly authorized AgeX Series A Preferred Stock and 148,400 shares of a newly authorized AgeX Series B Preferred Stock in exchange for the cancellation of a total of $36,000,000 of indebtedness consisting of the outstanding principal amount of certain loans made by Juvenescence to AgeX and loan origination fees accrued with respect to those loans. On February 1, 2024, the shares of Series A Preferred Stock and Series B Preferred Stock automatically converted into a total of 1,421,666 shares of AgeX common stock in accordance with their terms and after that conversion no shares of AgeX Preferred Stock remained outstanding. Those shares of common stock issued to Juvenescence increased Juvenescence’s direct and indirect holding of outstanding shares of AgeX common stock to 1,889,323 shares, or approximately 75.6% of the shares of common stock outstanding on March 14, 2024, including shares of AgeX common stock held by Juvenescence’s subsidiaries but not taking into account any additional shares of AgeX common stock that Juvenescence may acquire through the conversion of loan balances and the exercise of AgeX common stock purchase warrants or the exercise of Post-Merger Warrants that were distributed by AgeX on March 19.
| 68 |
Although we have been able to reduce our operating expenses by eliminating internal research and development activities and focusing instead on out-sourcing research and development and seeking licensing arrangements for our technologies, this approach has also made it more difficult for us to make progress in developing our target product candidates and technologies, which in turn may make it more difficult for us to raise capital. Further, the extent of Juvenescence’s voting control over AgeX along with the amount and terms of AgeX’s indebtedness to Juvenescence and the impact of potential dilution through the issuance of shares of our common stock upon the conversion of the Juvenescence loans into common stock and the exercise of common stock purchase warrants issued to Juvenescence in connection with certain Juvenescence loans could make AgeX unattractive to new equity investors and could impair our ability to finance our operations or the operations of our subsidiaries unless Juvenescence agrees, in its discretion, to lend us additional funds. The unavailability or inadequacy of financing to meet future capital requirements will depend on many factors. In the near term these factors will include:needs could force us to modify, curtail, delay, or suspend some or all aspects of our planned operations.
We do not have any committed sources of funds for additional financing and we cannot assure that we will be able to raise additional financing on favorable terms or at all. To the extent that we are able to raise additional capital through the sale of AgeX equity or convertible debt securities or the sale of equity or convertible debt securities of any of our subsidiaries, the ownership interest of our present stockholders will be diluted, and the terms of any securities we or our subsidiaries issue may include liquidation or other preferences that adversely affect theirthe rights asof our common stockholders. DebtAdditional debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends, and may involve the issuance of convertible debt or stock purchase warrants that would dilute the equity interests of our stockholders. If we raise funds through additional strategic partnerships or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our researchSummary of Cash Flows
The following table summarizes the major sources and development programs.uses of cash for the periods set forth below (in thousands):
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Net cash provided by (used in): | ||||||||
| Operating activities | $ | (7,800 | ) | $ | (5,939 | ) | ||
| Investing activities | (10,000 | ) | - | |||||
| Financing activities | 17,500 | 6,000 | ||||||
| Net change in cash, cash equivalents, and restricted cash | $ | (300 | ) | $ | 61 | |||
Cash used in operating activitiesOperating Activities
For the year ended December 31, 2018, our total research and development expenses, including acquired in-process research and development expenses were $6.6 million and our general and administrative expenditures were $5.6 million. Net loss attributable to us for the yearsyear ended December 31, 20182023 amounted to $7.5$14.8 million. Net cash used in operating activities during this period amounted to $8.0$7.8 million. The difference between the net loss attributable to us and net cash used in operating activities during the year ended December 31, 20182023 was primarily attributable to the following noncash items: $3.2 million gain on the disposition of our ownership of Ascendance common stock offset to some extent by $0.8 million for acquired in-process research and development expense, $1.5 million in stock-based compensation expense, $0.5$5.4 million in amortization of intangible assets and depreciationdeferred debt issuance costs, $1.5 million in prepaid expenses and other current assets which included a $0.4 million write off of prepaid expenses incurred in prior periods with respect to a shelf registration statement that expired in January 2024, $1.1 million in accounts payables and accrued liabilities, $0.6 million in stock-based compensation expense, and $0.2$0.1 million in net loss attributablerelated party payables. The impact of these non-cash items was offset to noncontrolling interest. Changessome extent by a $1.1 million payment of a financed insurance premium liability and $0.6 million in working capital impactedaccrued interest on a convertible note receivable. See Notes 4, Convertible Note Receivable and 5, Related Party Transactions, to our cash usedconsolidated financial statements included elsewhere in operations by $0.2 million as a net source of cash.this Report for additional information about our loan agreement with Serina and our loan agreements with Juvenescence.
Cash provided by investing activitiesInvesting Activities
During the year ended December 31, 2018,2023, net cash providedused by investing activities was $1.3 million. The primary componentis entirely comprised of the $10 million loan made to Serina. See Note 4, Convertible Note Receivable, to our consolidated financial statements included elsewhere in this amount was $3.2 million in proceeds fromReport for additional information about the disposition of our ownership of Ascendance common stock, offset by a $1.1 million payment to Escape Therapeutics and a $0.8 million payment to Ascendance for the acquisition of in-process research and development assets.Serina Note.
Cash provided by financing activitiesFinancing Activities
During the year ended December 31, 2018,2023, net cash provided by financing activities amounted to $6.0$17.5 million including $5.0 million of proceeds from the issuance of common stock and $1.0 million of proceeds from the issuance of the AgeX Warrants.
Contractual obligations
We had no contractual obligations as of December 31, 2018, with the exception of the Shared Facilities Agreement. Under the terms of the Shared Facilities Agreement, BioTime will allow uswhich was entirely attributable to use its premises and equipment located at Alameda, California for the purpose of conducting our business. BioTime may also provide accounting, billing, bookkeeping, payroll, treasury, payment of accounts payable, and other similar administrative services. BioTime may also provide the services of attorneys, accountants, and other professionals who may also provide professional services to BioTime and its other subsidiaries. BioTime may also provide us with the services of its laboratory and research personnel, including BioTime employees and contractors, for the performance of our research and development work at the premises.
BioTime charges us a “Use Fee” for services received and usage of facilities, equipment, and supplies. For each billing period, BioTime prorates and allocates costs incurred, as applicable, to us, such costs include services of BioTime employees, equipment, insurance, lease, professional, software, supplies and utilities. Allocation depends on key cost drivers including actual documented use, square footage of facilities used, time spent, costs incurred by or for our benefit, or upon proportionate usage by BioTime and us, as reasonably estimated by BioTime (collectively “Use Fees”). BioTime, at its discretion, has the right to charge us a 5% markup on such allocated costs and BioTime has charged this markup since the inception of the Shared Facilities Agreement. The allocated cost of BioTime employees and contractors who provide services is based upon records maintained of the number of hours or percentage of time of such personnel devoted to the performance of services.
The Shared Facilities Agreement will remain in effect from year to year, unless (a) either party gives the other party written six months’ notice to terminate, which BioTime may not give to AgeX prior to September 1, 2020, or (b) the agreement is otherwise terminated under another provision of the agreement.BioTime’s lease expires on January 31, 2023.
The minimum fixed payments dueamounts drawn under the Shared Facilities Agreement are approximately $150,000 per month.credit facilities from Juvenescence. See Notes 5, Related Party Transactions and 7, Stockholders’ Equity (Deficit), to our consolidated financial statements included elsewhere in this Report for additional information about our loan agreements with Juvenescence.
We plan to terminate our use of BioTime’s office and laboratory facilities under the Shared Facilities Agreement if the Preconditions to the sublease of our proposed new New Facility are met, or if we locate and lease an alternative office and laboratory facility.
Off-Balance Sheet Arrangements
As of December 31, 2018,2023, we did not have any off-balance sheet arrangements, as defined in Item 303(a) (4) (ii) of SEC Regulation S-K.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Not applicable.
Item 8. Financial Statements and Supplementary Data
AgeX Therapeutics, Inc. and Subsidiaries
Index to Consolidated Financial Statements
Page Number | |
| Report of Independent Registered Public Accounting Firm (PCAOB ID: 100) | 72 |
| Audited Consolidated Financial Statements: | |
| Consolidated Balance Sheets | 74 |
| Consolidated Statements of Operations | 75 |
| Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity/(Deficit) | 76 |
| Consolidated Statements of Cash Flows | 77 |
| Notes to Consolidated Financial Statements | 78 |
| 70 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Report of Independent Registered Public Accounting Firm
Stockholders and
Board of Directors and Shareholders
AgeX Therapeutics, Inc.
Alameda, California
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of AgeX Therapeutics, Inc. (theand Subsidiaries (collectively, the “Company”) as of December 31, 20182023 and 2017,2022, the related consolidated statements of operations, comprehensive loss,convertible preferred stock and stockholders’ equity equity/(deficit), and cash flows for each of the two years in the period ended December 31, 2018,2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 20182023 and 2017,2022, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2018,2023, in conformity with accounting principles generally accepted in the United States of America.
The Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1, the Company has had recurring losses and negative operating cash flows since inception, an accumulated deficit at December 31, 2023, and insufficient cash and cash equivalents and loan proceeds at December 31, 2023 to fund operations for twelve months from the date of issuance. All of these matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’sthese consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the auditaudits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
| /s/ WithumSmith+Brown, PC | |
| We have served as the Company’s auditor since 2017. | |
| San Francisco, California | |
| March 22, 2024 | |
| PCAOB ID Number 100 |
/s/ OUM & CO. LLP
| 71 |
San Francisco, CaliforniaAGEX THERAPEUTICS, INC. AND SUBSIDIARIES
April 1, 2019CONSOLIDATED BALANCE SHEETS
We have served as(In thousands, except par value amounts)
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 345 | $ | 645 | ||||
| Accounts and grants receivable, net | 57 | 4 | ||||||
| Prepaid expenses and other current assets | 352 | 1,804 | ||||||
| Total current assets | 754 | 2,453 | ||||||
| Restricted cash | 50 | 50 | ||||||
| Intangible assets, net | 607 | 738 | ||||||
| Convertible note receivable | 10,554 | - | ||||||
| TOTAL ASSETS | $ | 11,965 | $ | 3,241 | ||||
| LIABILITIES, CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY/(DEFICIT) | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued liabilities | $ | 2,176 | $ | 1,034 | ||||
| Loans due to Juvenescence, net of debt issuance costs, current portion | 3,672 | 7,646 | ||||||
| Related party payables, net | 66 | 141 | ||||||
| Warrant liability | - | 180 | ||||||
| Insurance premium liability and other current liabilities | - | 1,077 | ||||||
| Total current liabilities | 5,914 | 10,078 | ||||||
| Loans due to Juvenescence, net of debt issuance costs, net of current portion | 693 | 10,478 | ||||||
| TOTAL LIABILITIES | 6,607 | 20,556 | ||||||
| Commitments and contingencies (Note 10) | - | - | ||||||
| Stockholders’ equity/(deficit): | ||||||||
| Preferred stock, $ par value, shares authorized: |
- | - | ||||||
| Series A preferred stock; no par value; stated value $ per share; and shares issued and outstanding, respectively | - | - | ||||||
| Series B preferred stock; no par value; stated value $ per share; and shares issued and outstanding, respectively | - | - | ||||||
| Preferred stock value | - | - | ||||||
| Common stock, $ par value, shares authorized, shares issued and outstanding | - | - | ||||||
| Additional paid-in capital | 136,482 | 98,998 | ||||||
| Accumulated deficit | (131,013 | ) | (116,210 | ) | ||||
| Total AgeX Therapeutics, Inc. stockholders’ equity/(deficit) | 5,469 | (17,212 | ) | |||||
| Noncontrolling interest | (111 | ) | (103 | ) | ||||
| Total stockholders’ equity/(deficit) | 5,358 | (17,315 | ) | |||||
| TOTAL LIABILITIES, CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY/(DEFICIT) | $ | 11,965 | $ | 3,241 | ||||
See accompanying notes to the Company’s auditor since 2017.consolidated financial statements.
| 72 |
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| REVENUES | ||||||||
| Grant revenues | $ | 77 | $ | - | ||||
| Other revenues | 65 | 34 | ||||||
| Total revenues | 142 | 34 | ||||||
| Cost of sales | (40 | ) | (13 | ) | ||||
| Gross profit | 102 | 21 | ||||||
| OPERATING EXPENSES | ||||||||
| Research and development | 734 | 1,025 | ||||||
| General and administrative | 9,328 | 5,971 | ||||||
| Total operating expenses | 10,062 | 6,996 | ||||||
| Gain on disposition of fixed assets | 73 | - | ||||||
| Loss from operations | (9,887 | ) | (6,975 | ) | ||||
| OTHER EXPENSE, NET | ||||||||
| Interest expense, net | (4,900 | ) | (3,335 | ) | ||||
| Change in fair value of warrants | (35 | ) | (225 | ) | ||||
| Other income, net | 11 | 13 | ||||||
| Total other expense, net | (4,924 | ) | (3,547 | ) | ||||
| NET LOSS | (14,811 | ) | (10,522 | ) | ||||
| Net loss attributable to noncontrolling interest | 8 | 60 | ||||||
| NET LOSS ATTRIBUTABLE TO AGEX | $ | (14,803 | ) | $ | (10,462 | ) | ||
| NET LOSS PER COMMON SHARE: | ||||||||
| BASIC AND DILUTED | $ | ) | $ | ) | ||||
| WEIGHTED-AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: | ||||||||
| BASIC AND DILUTED | ||||||||
See accompanying notes to the consolidated financial statements.
| 73 |
Item 8. Financial Statements and Supplementary Data
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETSSTATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY/(DEFICIT)
(In thousands, except par value amountsthousands))
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 6,707 | $ | 7,375 | ||||
| Accounts and grants receivable, net | 131 | 107 | ||||||
| Prepaid expenses and other current assets | 1,015 | 111 | ||||||
| Total current assets | 7,853 | 7,593 | ||||||
| Equipment and furniture, net | 90 | 129 | ||||||
| Deposits and other long-term assets | 19 | 35 | ||||||
| Intangible assets, net | 2,709 | 1,874 | ||||||
| TOTAL ASSETS | $ | 10,671 | $ | 9,631 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued liabilities | $ | 1,416 | $ | 768 | ||||
| Related party payables to BioTime and Juvenescence | 82 | 210 | ||||||
| Deferred revenues | 317 | 180 | ||||||
| Other current liabilities | 625 | 154 | ||||||
| TOTAL LIABILITIES | 2,440 | 1,312 | ||||||
| Commitments and contingencies (Note 8) | ||||||||
| STOCKHOLDERS’ EQUITY | ||||||||
| Preferred stock, $0.0001 par value, authorized 5,000 shares; none issued and outstanding as of December 31, 2018 and 2017 | - | - | ||||||
| Common stock, $0.0001 par value, 100,000 shares authorized; 35,830 and 33,750 shares issued and outstanding as of December 31, 2018 and 2017, respectively | 4 | 3 | ||||||
| Additional paid-in capital | 81,499 | 73,761 | ||||||
| Accumulated other comprehensive income (loss) | (2 | ) | 68 | |||||
| Accumulated deficit | (74,054 | ) | (66,552 | ) | ||||
| AgeX Therapeutics, Inc. stockholders’ equity | 7,447 | 7,280 | ||||||
| Noncontrolling interest | 784 | 1,039 | ||||||
| Total stockholders’ equity | 8,231 | 8,319 | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | $ | 10,671 | $ | 9,631 | ||||
| Number of Shares | Amount | Number of Shares | Amount | Number of Shares | Par Value | Additional Paid-In Capital | Accumulated Deficit | Noncontrolling Interest | Stockholders’ | |||||||||||||||||||||||||||||||
| Preferred Stock | ||||||||||||||||||||||||||||||||||||||||
| Series A | Series B | Common Stock | Total | |||||||||||||||||||||||||||||||||||||
| Number of Shares | Amount | Number of Shares | Amount | Number of Shares | Par Value | Additional Paid-In Capital | Accumulated Deficit | Noncontrolling Interest | Stockholders’ | |||||||||||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2021 | - | $ | - | - | $ | - | 1,079 | $ | - | $ | 93,916 | $ | (105,748 | ) | $ | (43 | ) | $ | (11,875 | ) | ||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units, net of shares retired to pay employee’s taxes | - | - | - | - | - | - | (4 | ) | - | - | (4 | ) | ||||||||||||||||||||||||||||
| Issuance of warrants | - | - | - | - | 178 | - | - | 178 | ||||||||||||||||||||||||||||||||
| Fair value of liability classified warrants issued | - | - | - | - | - | - | 4,148 | - | - | 4,148 | ||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | - | - | - | 760 | - | - | 760 | ||||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (10,462 | ) | (60 | ) | (10,522 | ) | |||||||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2022 | - | - | - | - | 1,079 | - | 98,998 | (116,210 | ) | (103 | ) | (17,315 | ) | |||||||||||||||||||||||||||
| Balance | - | - | - | - | 1,079 | - | 98,998 | (116,210 | ) | (103 | ) | (17,315 | ) | |||||||||||||||||||||||||||
| Issuance of preferred stock, net of issuance costs | 212 | - | 148 | - | - | - | 35,958 | - | - | 35,958 | ||||||||||||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units, net of shares retired to pay employee’s taxes | - | - | - | - | - | - | (1 | ) | - | - | (1 | ) | ||||||||||||||||||||||||||||
| Fair value of liability classified warrants issued | - | - | - | - | - | - | 879 | - | - | 879 | ||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | - | - | - | 648 | - | - | 648 | ||||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (14,803 | ) | (8 | ) | (14,811 | ) | |||||||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2023 | 212 | $ | - | 148 | $ | - | 1,079 | $ | - | $ | 136,482 | $ | (131,013 | ) | $ | (111 | ) | $ | 5,358 | |||||||||||||||||||||
| Balance | 212 | $ | - | 148 | $ | - | 1,079 | $ | - | $ | 136,482 | $ | (131,013 | ) | $ | (111 | ) | $ | 5,358 | |||||||||||||||||||||
See accompanying notes to the consolidated financial statements.
| 74 |
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONSCASH FLOWS
(In thousands, except per share data)thousands)
| Year Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| REVENUES: | ||||||||
| Subscription and advertisement revenues | $ | 1,227 | $ | 1,399 | ||||
| Service and other revenues | 149 | 5 | ||||||
| Grant revenues | 20 | - | ||||||
| Total revenues | 1,396 | 1,404 | ||||||
| Cost of sales | (364 | ) | (168 | ) | ||||
| Gross profit | 1,032 | 1,236 | ||||||
| OPERATING EXPENSES: | ||||||||
| Research and development | (5,830 | ) | (5,784 | ) | ||||
| Acquired in-process research and development | (800 | ) | - | |||||
| General and administrative | (5,647 | ) | (3,869 | ) | ||||
| Total operating expenses | (12,277 | ) | (9,653 | ) | ||||
| Gain on sale of assets | - | 1,754 | ||||||
| Loss from operations | (11,245 | ) | (6,663 | ) | ||||
| OTHER INCOME/(EXPENSES): | ||||||||
| Interest income (expense), net | 116 | (12 | ) | |||||
| Gain on sale of equity method investment in Ascendance | 3,215 | - | ||||||
| Other income, net | 183 | 38 | ||||||
| Total other income, net | 3,514 | 26 | ||||||
| NET LOSS | (7,731 | ) | (6,637 | ) | ||||
| Net loss attributable to noncontrolling interest | 229 | 57 | ||||||
| NET LOSS ATTRIBUTABLE TO AGEX | $ | (7,502 | ) | $ | (6,580 | ) | ||
| NET LOSS PER COMMON SHARE: BASIC AND DILUTED | $ | (0.21 | ) | $ | (0.21 | ) | ||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: BASIC AND DILUTED | 34,914 | 30,644 | ||||||
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| OPERATING ACTIVITIES: | ||||||||
| Net loss attributable to AgeX | $ | (14,803 | ) | $ | (10,462 | ) | ||
| Net loss attributable to noncontrolling interest | (8 | ) | (60 | ) | ||||
| Adjustments to reconcile net loss attributable to AgeX to net cash used in operating activities: | ||||||||
| Change in fair value of warrants | 35 | 225 | ||||||
| Amortization of intangible assets | 131 | 132 | ||||||
| Amortization of debt issuance costs | 5,285 | 3,137 | ||||||
| Stock-based compensation | 648 | 760 | ||||||
| Gain on disposition of fixed assets | (73 | ) | - | |||||
| Write off of prepaid shelf registration statement related expenses | 360 | - | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts and grants receivable | (53 | ) | 21 | |||||
| Prepaid expenses and other current assets | 1,092 | 896 | ||||||
| Interest on convertible note receivable | (554 | ) | - | |||||
| Accounts payable and accrued liabilities | 1,150 | 144 | ||||||
| Related party payables | 69 | 255 | ||||||
| Insurance premium liability | (1,075 | ) | (983 | ) | ||||
| Other current liabilities | (4 | ) | (4 | ) | ||||
| Net cash used in operating activities | (7,800 | ) | (5,939 | ) | ||||
| INVESTING ACTIVITIES: | ||||||||
| Cash advanced on convertible note receivable | (10,000 | ) | - | |||||
| Net cash used in investing activities | (10,000 | ) | - | |||||
| FINANCING ACTIVITIES: | ||||||||
| Draw down on loan facilities from Juvenescence | 17,500 | 6,000 | ||||||
| Net cash provided by financing activities | 17,500 | 6,000 | ||||||
| NET CHANGE IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH | (300 | ) | 61 | |||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH: | ||||||||
| At beginning of the year | 695 | 634 | ||||||
| At end of the year | $ | 395 | $ | 695 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid during the year for interest | $ | 27 | $ | 14 | ||||
| SUPPLEMENTAL SCHEDULE OF NONCASH FINANCING AND INVESTING ACTIVITIES: | ||||||||
| Issuance of preferred stock in exchange for debt | $ | 36,000 | $ | - | ||||
| Issuance of common stock upon vesting of restricted stock units (Note 8) | $ | 2 | $ | 8 | ||||
| Issuance of warrants for debt issuance under the 2020 Loan Agreement | $ | - | $ | 178 | ||||
| Fair value of liability classified warrants at debt inception date (Note 6) | $ | 663 | $ | 4,148 | ||||
| Debt refinanced with new debt (Note 5) | $ | - | $ | 7,160 | ||||
See accompanying notes to the consolidated financial statements.
| 75 |
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
| Year Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| NET LOSS | $ | (7,731 | ) | $ | (6,637 | ) | ||
| Other comprehensive expense, net of tax: | ||||||||
| Foreign currency translation adjustments | (70 | ) | 10 | |||||
| COMPREHENSIVE LOSS | (7,801 | ) | (6,627 | ) | ||||
| Less: Comprehensive loss attributable to noncontrolling interest | 229 | 57 | ||||||
| COMPREHENSIVE LOSS ATTRIBUTABLE TO AGEX | $ | (7,572 | ) | $ | (6,570 | ) | ||
See accompanying notes to the consolidated financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(In thousands)
| Common Stock | Additional | Accumulated Other Comprehensive | Total Stockholders’ | |||||||||||||||||||||||||
| Number of Shares | Par Value | Paid-In Capital | Accumulated Deficit | Noncontrolling Interest | Income/ (Loss) | Equity/ (Deficit) | ||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2016 | 28,800 | $ | 3 | $ | 36,492 | $ | (59,972 | ) | $ | 1,044 | $ | 58 | $ | (22,375 | ) | |||||||||||||
| Cancellation of related party payable to BioTime by ReCyte Therapeutics | - | - | 11,177 | - | - | - | 11,177 | |||||||||||||||||||||
| Issuance of AgeX option for BioTime common stock | - | - | 100 | - | - | - | 100 | |||||||||||||||||||||
| Issuance of common stock to investors other than BioTime | 4,934 | - | 9,868 | - | - | - | 9,868 | |||||||||||||||||||||
| Issuance of common stock to BioTime | 16 | - | 32 | - | - | - | 32 | |||||||||||||||||||||
| Settlement of related party payables to BioTime by LifeMap Sciences and LifeMap Solutions for common stock and certain assets, including $4.4 million gain on transfer of LifeMap Solutions to BioTime and proportional equity transfer from noncontrolling interest | - | - | 13,398 | - | (175 | ) | - | 13,223 | ||||||||||||||||||||
| Contributions from BioTime | - | - | 2,169 | - | - | - | 2,169 | |||||||||||||||||||||
| Stock-based compensation allocated from BioTime | - | - | 411 | - | - | - | 411 | |||||||||||||||||||||
| Stock-based compensation | - | - | 114 | - | - | - | 114 | |||||||||||||||||||||
| Stock-based compensation in subsidiaries | - | - | - | - | 227 | - | 227 | |||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | 10 | 10 | |||||||||||||||||||||
| Net loss | - | - | - | (6,580 | ) | (57 | ) | - | (6,637 | ) | ||||||||||||||||||
| BALANCE AT DECEMBER 31, 2017 | 33,750 | 3 | 73,761 | (66,552 | ) | 1,039 | 68 | 8,319 | ||||||||||||||||||||
| Sale of shares of common stock | 2,000 | 1 | 4,999 | - | - | - | 5,000 | |||||||||||||||||||||
| Issuance of shares to acquire in-process research and development | 80 | - | 240 | - | - | - | 240 | |||||||||||||||||||||
| Sale of warrants | - | - | 1,000 | - | - | - | 1,000 | |||||||||||||||||||||
| Stock-based compensation allocated from BioTime | - | - | 184 | - | - | - | 184 | |||||||||||||||||||||
| Stock-based compensation | - | - | 1,285 | - | - | - | 1,285 | |||||||||||||||||||||
| Stock-based compensation in subsidiaries | - | - | - | - | 4 | - | 4 | |||||||||||||||||||||
| Transactions with noncontrolling interests | - | - | 30 | - | (30 | ) | - | - | ||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | (70 | ) | (70 | ) | |||||||||||||||||||
| Net loss | - | - | - | (7,502 | ) | (229 | ) | - | (7,731 | ) | ||||||||||||||||||
| BALANCE AT DECEMBER 31, 2018 | 35,830 | $ | 4 | $ | 81,499 | $ | (74,054 | ) | $ | 784 | $ | (2 | ) | $ | 8,231 | |||||||||||||
See accompanying notes to the consolidated financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss attributable to AgeX | $ | (7,502 | ) | $ | (6,580 | ) | ||
| Net loss attributable to noncontrolling interest | (229 | ) | (57 | ) | ||||
| Adjustments to reconcile net loss attributable to AgeX to net cash used in operating activities: | ||||||||
| Gain on sale of equity method investment in Ascendance | (3,215 | ) | - | |||||
| Acquired in-process research and development | 800 | |||||||
| Depreciation expense | 58 | 165 | ||||||
| Amortization of intangible assets | 477 | 517 | ||||||
| Amortization of deferred license fees | - | 17 | ||||||
| Gain on sale of assets | - | (1,754 | ) | |||||
| Stock-based compensation | 1,285 | 114 | ||||||
| Stock-based compensation allocated from BioTime | 184 | 411 | ||||||
| Subsidiary stock-based compensation | 4 | 227 | ||||||
| Bad debt expense | - | (121 | ) | |||||
| Foreign currency remeasurement gain and other | (68 | ) | - | |||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable and other receivables | (24 | ) | 322 | |||||
| Prepaid expenses and other current assets | (293 | ) | 10 | |||||
| Accounts payable and accrued liabilities | 648 | 267 | ||||||
| Related party payable to BioTime | (128 | ) | - | |||||
| Deferred revenues | 137 | (84 | ) | |||||
| Other | (129 | ) | 261 | |||||
| Net cash used in operating activities | (7,995 | ) | (6,285 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Proceeds from the sale of equity method investment in Ascendance | 3,215 | - | ||||||
| Purchase of in-process research and development | (1,872 | ) | - | |||||
| Purchase of equipment and other assets | (21 | ) | (1 | ) | ||||
| Removal of cash upon deconsolidation of LifeMap Solutions | - | (3 | ) | |||||
| Security deposit received and other, net | 5 | 9 | ||||||
| Net cash provided by investing activities | 1,327 | 5 | ||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Contributions from BioTime | - | 1,971 | ||||||
| Advances from BioTime | - | 1,304 | ||||||
| Proceeds from sale of common stock | 5,000 | 10,000 | ||||||
| Proceeds from sale of warrants | 1,000 | - | ||||||
| Net cash provided by financing activities | 6,000 | 13,275 | ||||||
| Effect of exchange rate changes on cash and cash equivalents | - | 121 | ||||||
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | (668 | ) | 7,116 | |||||
| CASH AND CASH EQUIVALENTS: | ||||||||
| Beginning of year | 7,375 | 259 | ||||||
| End of year | $ | 6,707 | $ | 7,375 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid during year for interest | $ | 11 | $ | 9 | ||||
| SUPPLEMENTAL SCHEDULE OF NONCASH FINANCING AND INVESTING ACTIVITIES: | ||||||||
| Issuance of common stock for acquired in progress research and development with alternative future uses | $ | 240 | $ | - | ||||
| Settlement of related party payable due to BioTime by LifeMap Sciences and LifeMap Solutions in exchange for common stock, certain assets, and transfer of LifeMap Solutions to BioTime, including proportional equity transfer from noncontrolling interest (see Notes 1 and 4) | - | 13,223 | ||||||
| Cancellation of related party payable due to BioTime by ReCyte Therapeutics (see Note 4) | - | 11,177 | ||||||
See accompanying notes to the consolidated financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization, Basis of Presentation and Liquidity
AgeX Therapeutics, Inc. (“AgeX”AgeX,” “we,” “our,” or “us”) was incorporated in January 2017 in the state of Delaware as a subsidiary of BioTime, Inc. (“BioTime”), a publicly traded, clinical-stage biotechnology company developing new cellular therapies for degenerative retinal diseases, neurological conditions associated with demyelination, and aiding the body in detecting and combating cancer.
Delaware. AgeXis a biotechnology company focused on the development and commercialization of novel therapeutics targeting human aging. and degenerative diseases. AgeX’s initial discoverymission is to apply its comprehensive experience in fundamental biological processes of human aging to a broad range of age-associated medical conditions.
AgeX’s proprietary technology, based on telomerase-mediated cellular immortality and pre-clinical programs focus on utilizing brown adipose tissueregenerative biology, allows AgeX to utilize telomerase-expressing regenerative pluripotent stem cells (“brown fat”PSCs”) in targeting diabetes, obesity,for the manufacture of cell-based therapies to regenerate tissues afflicted with age-related chronic degenerative disease. AgeX’s main technology platforms and heart disease; and induced tissue regeneration (“iTR”) in utilizing the human body’s own abilities to scarlessly regenerate tissue damaged from age or trauma. AgeX may also pursue other early-stage pre-clinical programs. product candidates are as follows:
| ● | PureStem® PSC-derived clonal embryonic progenitor cell lines that may be capable of generating a broad range of cell types for use in cell-based therapies; | |
| ● | UniverCyte™ which uses the HLA-G gene to suppress rejection of transplanted cells and tissues to confer low immune observability to cells; | |
| ● | AGEX-BAT1 using adipose brown fat cells for metabolic diseases such as Type II diabetes; | |
| ● | AGEX-VASC1 using vascular progenitor cells to treat tissue ischemia; and | |
| ● | Induced tissue regeneration or iTR technology to regenerate or rejuvenate cells to treat a variety of degenerative diseases including those associated with aging, as well as other potential tissue regeneration applications such as scarless wound repair. |
AgeX is an “emerging growth company” as defined in the Jumpstart ourOur Business Startups Act of 2012.
Reverse Stock Split
On August 17, 2017,March 14, 2024 AgeX completed an asset acquisition andeffected a reverse stock sale pursuant to which it received certain assets from BioTimesplit of its common stock at a ratio of 1 for use35.17 (the “Reverse Stock Split”) resulting in its research and development programs and raised $10.0 million in cash from investors to finance its operations. This capitalization of AgeXhas allowed it to focus its resources on its pre-clinical programs (see Notes 4 and 9).
On February 28, 2018, AgeX sold warrants to purchase 1,473,600approximately shares of AgeX common stock (the “Warrants”)being outstanding immediately upon the Reverse Stock Split. Except for $0.50 per warrant for aggregate cash proceeds to AgeXthe number of $736,800. On July 10, 2018, AgeX sold additional Warrants to purchase 526,400authorized but unissued shares of AgeX common stock, for $0.50 per warrant for aggregate net cash proceedsand except as may be otherwise stated in these notes to financial statements, numbers of shares of AgeX of $263,200. The Warrants were exercisable at $2.50 per share (see Note 9 concerningcommon stock issued and outstanding, or issuable upon the exercise of options or warrants or upon conversion of convertible indebtedness, and expirationAgeX common stock prices, shown in the consolidated financial statements and these notes thereto have been retroactively adjusted to reflect the effect of the Warrants).Reverse Stock Split. See Note 11, Subsequent Events.
On June 7, 2018,Merger Agreement and Certain Transactions with Serina Therapeutics, Inc.
During March 2023, AgeX sold 2.0 million shares of common stock toborrowed $10,000,000 from Juvenescence Limited (“Juvenescence”) for $2.50 per share for aggregate cashunder the terms of a Secured Convertible Promissory Note (the “2023 Secured Note”) and used the loan proceeds to make a $10,000,000 loan to Serina Therapeutics, Inc. (“Serina”) under the terms of a Convertible Promissory Note to Serina (the “Serina Note”), in order to provide Serina with financing in advance of corporate restructuring plans that include a merger through which AgeX of $5.0 million.would acquire Serina.
BioTime’s sale of significant ownership interest in AgeX to Juvenescence – On August 30, 2018, BioTime consummated29, 2023, AgeX entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) with Serina Therapeutics, Inc. (“Serina”), and Canaria Transaction Corporation, a wholly owned subsidiary of AgeX (“Merger Sub”). Upon the saleterms and subject to the satisfaction of 14,400,000the conditions described in the Merger Agreement, Merger Sub will be merged with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX (the “Merger”). At a special meeting of AgeX stockholders on March 14, 2024 (the “Special Meeting”), AgeX stockholders approved certain proposals required for consummation of the Merger pursuant to the terms of the Merger Agreement. Serina stockholders have also approved the Merger. There is no assurance that all conditions to the Merger will be met or waiver and that the Merger will be consummated. See Note 11, Subsequent Events.
On March 19, 2024, AgeX issued to each holder of AgeX common stock as of the dividend record date, March 18, 2024, three warrants (“Post-Merger Warrants”) for each five shares of AgeX common stock held by such stockholder. Each Post-Merger Warrant will be exercisable for one unit of AgeX (“AgeX Unit”) at a price equal to $13.20 per unit and will expire on July 31, 2025. Each AgeX Unit will consist of (i) one share of AgeX common stock and (ii) one warrant (“Incentive Warrant”). Each Incentive Warrant will be exercisable for one share of AgeX common stock at a price equal to $18.00 per warrant and will expire on the four-year anniversary of the closing date of the Merger. See Note 11, Subsequent Events.
| 76 |
On August 30, 2018, AgeX ceased to be a subsidiary of BioTime because on that date, BioTime experienced a “loss of control” of a subsidiary, asActual Closing Price (as defined by generally accepted accounting principles in the U.S. (“GAAP”).LossMerger Agreement) of control is deemed to have occurred when, among other things, a parent company owns less than a majority of the outstandingAgeX common stock inbeing equal to or greater than $12.00 per share excluding the subsidiary, lacks a controlling financial interest inimpact of any Post-Merger Warrant, Incentive Warrant or the subsidiary and, is unable to unilaterally control the subsidiary through other means such as having, or being able to obtain, the power to elect a majorityissuance of the subsidiary’s Board of Directors based solely on contractual rights or ownership of shares holding a majority of the voting power of the subsidiary’s voting securities. All of these loss-of-control factors were present with respect to BioTime’s ownership interest in AgeX as of August 30, 2018.Accordingly, BioTime deconsolidated AgeX’s consolidated financial statements and results from its consolidated financial statements and results beginning on August 30, 2018.
On November 28, 2018 (the “Distribution Date”), BioTime owned 14,416,000any shares of AgeX common stock representing approximately 40.2%upon exercise of any Post-Merger Warrants or Incentive Warrants.
Concurrently with the execution of the Merger Agreement, AgeX, Serina, and AgeX’s controlling stockholder Juvenescence entered into a Side Letter, which will become effective immediately prior to the closing of the Merger. The Side Letter provides, among other things, that (i) effective immediately before the consummation of the Merger, Juvenescence will cancel all out of the money AgeX warrants held by Juvenescence; (ii) Juvenescence will exercise all Post-Merger Warrants it holds to provide the Combined Company an additional $15 million in capital according to the following schedule: (x) at least one-third on or before May 31, 2024, (y) at least one-third on or before November 30, 2024, and (z) at least one-third on or before June 30, 2025; (iii) Juvenescence will not sell any shares of the common stock issuedAgeX Series A Preferred Stock or AgeX Series B Preferred Stock and outstanding on the Distribution Date. On the Distribution Date, BioTime distributedwill take all actions necessary to its shareholders, on a pro rata basis, 12,697,028 sharesconvert all of thesuch Preferred Stock into AgeX common stock before a Reverse Stock Split that will occur before the Merger; (iv) Juvenescence will release all security interests, guarantees, pledges, assignments and other forms of collateral that it then held (the “Distribution”may have in AgeX’s assets pursuant to the terms of Juvenescence loans to AgeX; and (v) Juvenescence will consent to a newly formed subsidiary of AgeX assuming AgeX’s obligations with respect to loan agreements and promissory notes governing loans payable to Juvenescence, including obligations for amounts currently owed and future advances of loan funds, and Juvenescence shall release AgeX from those loan obligations. Juvenescence’s covenant regarding retaining ownership of and converting the Series A Preferred Stock and Series B Preferred Stock into AgeX common stock has been satisfied through the conversion of such preferred stock into AgeX common stock on February 1, 2024.
Prior to the closing of the Merger, any assets of AgeX other than certain “Legacy Assets” will be transferred into a recently formed subsidiary of AgeX “UniverXome Bioengineering, Inc. (“UniverXome”). Immediately afterUniverXome will assume (i) any indebtedness of AgeX issued to Juvenescence that has not been previously converted into AgeX Series A Preferred Stock or AgeX Series B Preferred Stock, which will be secured by the Distribution, BioTime retained 1,718,972Legacy Assets (ii) most of AgeX’s contracts with third parties, other than certain designated contracts and any contracts that are terminated before the Merger, and (iii) all other liabilities of AgeX in existence as of the effective time of the Merger (other than certain transaction expenses related to the Merger). See Note 11, Subsequent Events.
Serina currently has a pipeline of small molecule candidates targeting central nervous system (“CNS”) indications, enabled by the company’s proprietary POZ PlatformTM delivery technology. In addition to advancing Serina’s wholly owned pipeline assets, Serina is working with pharma partners currently advancing pre-clinical studies exploring POZ polymer lipid-nanoparticles (“LNPs”) in next generation LNP delivered RNA vaccines. In addition, Serina is advancing a lead drug candidate, SER-252 (POZ-apomorphine) for the treatment of advanced Parkinson’s Disease through pre-clinical studies toward the goal of an investigational new drug submission or “IND” to the Food and Drug Administration for the initiation of a Phase I clinical trial during the fourth quarter of 2024. Serina has two other pipeline assets that are positioned to enter IND enabling studies, SER-227 (POZ-buprenorphine) for certain post-operative pain indications, and SER-228 (POZ-cannabidiol) for treatment refractory epilepsy indications. Serina is also focused on expanding its LNP and anti-body drug conjugate partnering collaborations.
If the Merger is completed, the Combined Company will primarily focus on developing Serina’s product candidates and it is anticipated that the Combined Company will not continue to develop AgeX product candidates, other than potentially the development program of NeuroAirmid. If the Merger is not completed, AgeX expects to continue to execute on its current business strategies below while seeking out and evaluating potential strategic alternatives with respect to its assets and development programs, which may include a merger, business combination, investment into AgeX, sale or other disposition of assets or other strategic transaction. In such case, AgeX may not be successful in executing such strategies or identifying or implementing any such strategic alternatives, and there is a risk that Juvenescence may decide to stop funding AgeX’s operations, which would likely result in the delisting of AgeX common stock from the NYSE America and the dissolution of AgeX.
| 77 |
Liquidity and Going Concern
In addition to general economic and capital market trends and conditions, AgeX’s ability to raise sufficient additional capital to finance its operations from time to time will depend on a number of factors specific to AgeX’s operations such as operating expenses and progress in out-licensing its technologies and development of its product candidates. Although AgeX has been able to reduce its operating expenses, with the exception of certain non-recurring expenses incurred related to the possible Merger between AgeX and Serina, by eliminating internal research and development activities and focusing instead on outsourcing research and development and seeking licensing arrangements for AgeX technologies, this approach has also made it more difficult for AgeX to make progress in developing its target product candidates and technologies, which in turn, along with the amount of indebtedness to Juvenescence and Juvenescence’s ownership of approximately 75.6% of the outstanding shares of AgeX common stock, representing approximately 4.8%may make it more difficult for AgeX to raise capital. The unavailability or inadequacy of financing to meet future capital needs could force AgeX to modify, curtail, delay, or suspend some or all aspects of planned operations. Sales of additional equity securities could result in the dilution of the common stock then issued and outstanding. Following the Distribution,interests of its stockholders. AgeX common stock began publicly tradingcannot assure that adequate financing will be available on the NYSE American under the symbol “AGE” (see Notes 4, 7 and 9).favorable terms, if at all.
Liquidity –Since inception, AgeX has financedprimarily finances its operations through contributions and advancesloans from its former parent company, BioTime,Juvenescence. A subsidiary of Juvenescence is the salelargest stockholder of its common stock and warrants, exercises of warrants (see Notes 4, 5 and 9), and research grants. BioTime has also provided AgeX with the use of BioTime facilities and services under a Shared Facilities and Services Agreement as described in Note 4. Although BioTime may continue to provide administrative support to AgeX on a reimbursable basis, AgeX does not expect BioTime to provide future financing.AgeX. AgeX has incurred operating losses and negative cash flows since inception and had an accumulated deficit of $74.1$131.0 million as of December 31, 2018.2023. AgeX expects to continue to incur operating losses and negative cash flows.
Based on a strategic review of its operations, giving consideration to the status of its product development programs, human resources, capital needs and resources, and current conditions in the capital markets, AgeX’s board of directors and management have adopted operating plans and budgets to extend the period over which AgeX has evaluatedcan continue its operations with its available cash resources. Notwithstanding those operating plans and budgets, based on AgeX’s most recent projected cash flows andAgeX believes that its cash and cash equivalents of $6.7 $0.3million as of December 31, 2018,2023, plus cash on hand remaining from the $4.5 drawdown of the $4.4million in proceedsloan facility from exercise of Warrants in March 2019 (see Note 9), provideJuvenescence, would not be sufficient cash, cash equivalents,to satisfy AgeX’s anticipated operating and liquidity to carry out AgeX’s current operations through at leastother funding requirements for the next twelve months from the issuance date of thethese consolidated financial statements included herein.statements. These conditions raise substantial doubt about AgeX’s ability to continue as a going concern. AgeX will need to obtain substantial additional funding in connection with its continuing operations after that date. If AgeX is unable to raise capital when needed or on attractive terms, AgeX would be forced to delay, reduce or eliminate its research and development programs.
Basis of presentation -operations. The accompanying consolidated financial statements presented herein have been prepared on a separate, standalone basis, referreddo not include any adjustments to as “carve-out” basis financial statements. Carve-out financial statements are based on ageneral principlethe amount and classification of assets and liabilities that the historical financial statements of a registrantmay be necessary should reflect, in all material respects, all of the registrant’s costs of doing business, including certain expenses incurred by the parent on its behalf.Prior to August 2017, AgeX’s current subsidiaries and certain research and development departments within AgeX operated as subsidiaries and research and development departments of BioTime (see Note 4). Beginning with the August 17, 2017 capitalization of AgeX and theAsset Contribution and Separation Agreement(“Asset Contribution Agreement”) with BioTime discussed in Note 4, the former subsidiaries and research and development departments of BioTime, including shares of Ascendance Biotechnology, Inc. (“Ascendance”), held by BioTime as an equity method investment, were contributed to AgeX. Although the AgeX legal entity commenced operations in 2017, its subsidiaries, research and development departments and investments, as applicable, operated prior to 2017. Accordingly, the accompanying consolidated financial statements were prepared on a carve-out basis for purposes of presenting what AgeX’s consolidated financial position, results of operations and cash flows would have been had AgeX operated the businessnot continue as a standalone entity for the periods presented. going concern.
Principles of Consolidation
The consolidated financial statements are not necessarily indicative of AgeX’s future performance and do not reflect what AgeX’s financial performance or results would have been had the company operated as an independent, publicly traded company during the periods presented, and should not be relied upon as an indicator of AgeX’s future results.
For periods prior to August 30, 2018,BioTime consolidated the results of AgeX and AgeX’s subsidiaries into BioTime’s consolidated results based on BioTime’s ability to control AgeX’s operating and financial decisions and policies through the majority ownership of AgeX common stock throughout the periods presented. As discussed above, beginning on August 30, 2018, BioTime deconsolidated AgeX’s consolidated financial statements and results from its consolidated financial statements and results.
The consolidated financial statements are presented in accordance withU.S. accounting principles generally accepted accounting principlesin the United States of American (“U.S. GAAP”).
To the extent AgeX does not have its own employees, human resources or facilities for its operations, BioTime or BioTime commonly controlled and consolidated subsidiaries provide certain employees for administrative or operational services, including laboratory space and administrative facilities, as necessary, for the benefit of AgeX, under a Shared Facilities and Services Agreement (the “Shared Facilities Agreement”) with BioTime (see Note 4). Accordingly, BioTime allocates expenses such as salaries and payroll related expenses incurred and paid on behalf of AgeX based on the amount of time that particular employees devote to AgeX affairs. Other expenses such as legal, accounting and financial reporting, marketing, and travel expenses are allocated to AgeX to the extent that those expenses are incurred by or on behalf of AgeX. BioTime also allocates certain overhead expenses such as rent and utilities, property taxes, insurance, laboratory expenses and supplies, telecommunications and other indirect expenses. These allocations are made based upon activity-based allocation drivers such as time spent, percentage of square feet of office or laboratory space used, headcount and percentage of personnel devoted to AgeX’s operations or management. Management evaluates the appropriateness of the allocations on a periodic basis and believes that this basis for allocation is reasonable.
Principles of consolidation – AgeX’s consolidated financial statements include the accounts of AgeX and its subsidiaries and certain research and development departments, including former BioTime personnel, transferred from BioTime to AgeX in connection with the Asset Contribution Agreement (see Note 4). AgeX consolidated its direct and indirect wholly-owned or majority-owned subsidiaries becausewhich AgeX has a controlling financial interest. The consolidated financial statements also include certain variable interest entities in which AgeX is the abilityprimary beneficiary (as described in more detail below). For consolidated entities where AgeX has less than 100% of ownership, AgeX records net loss attributable to control their operating and financial decisions and policies through itsnoncontrolling interest on the consolidated statement of operations equal to the percentage of the ownership andinterest retained in such entities by the respective noncontrolling parties. The noncontrolling interest is reflected as a separate element of stockholders’ equityequity/(deficit) on AgeX’s consolidated balance sheets.
AsAgeX assesses whether it is the primary beneficiary of a variable interest entity (“VIE”) at the inception of the arrangement and at each reporting date. This assessment is based on its power to direct the activities of the VIE that most significantly impact the VIE’s economic performance and AgeX’s obligation to absorb losses or the right to receive benefits from the VIE that could potentially be significant to the VIE. If the entity is within the scope of the variable interest model and meets the definition of a VIE, AgeX considers whether it must consolidate the VIE or provide additional disclosures regarding its involvement with the VIE. If AgeX determines that it is the primary beneficiary of the VIE, AgeX will consolidate the VIE. This analysis is performed at the initial investment in the entity or upon any reconsideration event. For entities AgeX holds as an equity investment that are not consolidated under the VIE model, AgeX will consider whether its investment constitutes a controlling financial interest in the entity and therefore should be considered for consolidation under the year ended, December 31, 2018,voting interest model.
AgeX consolidatedhas four subsidiaries, Reverse Bioengineering, Inc. (“Reverse Bio”), ReCyte Therapeutics, Inc. (“ReCyte Therapeutics”ReCyte”), LifeMap Sciences,NeuroAirmid Therapeutics, Inc. (“LifeMap Sciences”NeuroAirmid”), and LifeMap Sciences, Ltd. (Israel)Canaria Transaction Corporation (“Merger Sub”), and included the historical expenseshas incorporated but not yet capitalized a fifth subsidiary UniverXome. See Note 11, Subsequent Events. Reverse Bio is a wholly owned subsidiary of certain former BioTimeAgeX through which AgeX plans to finance its iTRTM research and development departments (see Note 4). For periods priorefforts. AgeX has been seeking equity financing for Reverse Bio and to the year ended December 31, 2018, AgeX also consolidated, LifeMap Solutions, Inc. (“LifeMap Solutions”), a subsidiaryextent that such Reverse Bio Financing is obtained through the sale of LifeMap Sciences, that was transferred to BioTime during June 2017.
On June 6, 2017, BioTimecapital stock or other equity securities by Reverse Bio, AgeX’s equity interest in Reverse Bio and LifeMap Sciences entered into a Debt Conversion Agreement whereby BioTime acquired: (1) a direct 100% ownership of LifeMap Solutions, a wholly-owned subsidiary of LifeMap Sciences, (2) additional shares of common stock in LifeMap Sciences and (3) certain other assets and intellectual property of LifeMap Sciences. These items were acquired in exchange for the settlement of related party payables due to BioTime by LifeMap Sciences of $8.8 million as of that date (see Note 4).
On August 17, 2017, BioTime contributed all of its then-existing ownership interests in LifeMap Sciences (approximately 82% ownership),iTRTM business would be diluted. ReCyte Therapeutics (approximately 95% ownership), equity method investment in Ascendance (approximately 44% ownership), and certain BioTime generalis an early stage pre-clinical research and development departments, including personnel, tocompany involved in stem cell-derived endothelial and cardiovascular related progenitor cells for the treatment of vascular disorders and ischemic conditions. AgeX in exchange for 28,800,000 shares of AgeX common stock pursuant to the Asset Contribution Agreement discussed in Note 4.
On June 6, 2017, becauseowns 94.8% of the transferoutstanding capital stock of LifeMap SolutionsReCyte. NeuroAirmid is jointly owned by AgeX with the University of California – Irvine and certain researchers and was recently organized to BioTime, LifeMap Sciences deconsolidated LifeMap Solutions from itspursue clinical development and commercialization of cell therapies, focusing initially on Huntington’s Disease. AgeX owns 50% of the outstanding capital stock of NeuroAirmid. AgeX consolidates NeuroAirmid despite not having majority ownership interest as it has the ability to influence decision making and financial statements. The exchange of LifeMap Sciences assets, including LifeMap Solutions,results through contractual rights and obligations as per Accounting Standards Codification (“ASC”) 810, Consolidation. Merger Sub was incorporated for the settlementpurpose of related party payables duemerging with Serina to BioTime, andimplement the subsequent contributionMerger. UniverXome is expected, in August 2017 by BioTime of assetsconnection with the planned Merger, to hold certain AgeX under the Asset Contribution Agreement are transactions between entities under common control. Accordingly, the contributed assets and assume AgeX liabilities, are recorded at historical carrying values, with the resulting gain recorded in AgeX’s additional paid-in capital included in the consolidated statements of stockholders’ equity for the year ended December 31, 2017, in accordance with ASC 805-50,including but not limited to indebtedness obligations to Juvenescence. See Note 11, Transactions Between Entities Under Common Control.Subsequent Events.
The LifeMap Solutions deconsolidation is not considered a discontinued operation in accordance with ASC 205-20,Presentation of Financial Statements Discontinued Operations, because the disposition of LifeMap Solutions does not represent a strategic shift in AgeX’ s operations, as defined by ASC 205-20, and the criteria for discontinued operations under ASC 205-20 are not met.
In accordance with the accounting guidance related to carve-out entities, LifeMap Solutions’ historical financial statements and operating results have been included in the AgeX consolidated financial statements for reporting periods prior to June 6, 2017, the transfer date of LifeMap Solutions to Bio Time.Loss from operations for the year ended December 31, 2017 includes a $1.8 million gain AgeX recognized on the sale of certain co-developed assets by LifeMap Solutions to its customer prior to the transfer of LifeMap Solutions to BioTime on June 6, 2017.
Subsequent to June 6, 2017, LifeMap Solutions’ financial statements and operating results are no longer included in AgeX’s consolidated financial statements.
As of, and for the year ended December 31, 2018, AgeX consolidated the following subsidiaries:
| ||||||||
All material intercompany accounts and transactions between AgeX and its subsidiaries have been eliminated in consolidation.
| 78 |
2. Summary of Significant Accounting Policies
Going Concern Assessment
AgeX assesses going concern uncertainty for its consolidated financial statements to determine if AgeX has sufficient cash and cash equivalents on hand and working capital to operate for a period of at least one year from the date the consolidated financial statements are issued or are available to be issued, which is referred to as the “look-forward period” as defined by Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) No. 2014-15. As part of this assessment, based on conditions that are known and reasonably knowable to AgeX, AgeX will consider various scenarios, forecasts, projections, and estimates, and AgeX will make certain key assumptions, including the timing and nature of projected cash expenditures or programs, and its ability to delay or curtail those expenditures or programs, if necessary, among other factors. Based on this assessment, as necessary or applicable, AgeX makes certain assumptions concerning its ability to curtail or delay research and development programs and expenditures within the look-forward period in accordance with ASU No. 2014-15 (see Note 1, Organization, Basis of Presentation and Liquidity).
Use of estimates -Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect (i) the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and (ii) the reported amounts of revenues and expenses during the reporting period with consideration given to materiality. Significant estimates and assumptions which are subject to significant judgment include those related to going concern assessment of consolidated financial statements, allocations and adjustments necessary for carve-out basis of presentation, including the separate return method for income taxes, useful lives associated with long-lived assets, including evaluation of asset impairment, allowances for uncollectible accounts receivables, loss contingencies, deferred income taxes and tax reserves, including valuation allowances related to deferred income taxes, and assumptions used to value stock-based awards or other equity instruments.instruments and liability classified warrants. Actual results could differ materially from those estimates. To the extent there are material differences between the estimates and actual results, AgeX’s future results of operations will be affected.
2. Summary of Significant Accounting Policies
See Note 6, Going concern assessment –Warrant LiabilityAgeX assesses going concern uncertainty, for its consolidated financial statements to determine if AgeX has sufficient cash and cash equivalentsdiscussion on hand and working capital to operate for a period of at least one year from the date the consolidated financial statements are issued or are available to be issued, which is referred to as the “look-forward period” as defined by Financial Accounting Standard Board’s (“FASB”) ASU No. 2014-15. As part of this assessment, based on conditions that are known and reasonably knowable to AgeX, AgeX will consider various scenarios, forecasts, projections, and estimates, and AgeX will make certain key assumptions, including the timing and nature of projected cash expenditures or programs, and its ability to delay or curtail those expenditures or programs, if necessary, among other factors. Based on this assessment, as necessary or applicable, AgeX makes certain assumptions concerning its ability to curtail or delay research and development programs and expenditures within the look-forward periodestimated change in accordance with ASU No. 2014-15.
Cash and cash equivalents – AgeX considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. As of December 31, 2018 and 2017, AgeX’s cash balances totaled $6.7 million and $7.4 million, respectively, and consist entirely of bank account deposits and amounts held in money market funds.
Concentrations of credit risk – Financial instruments that potentially subject AgeX to significant concentrations of credit risk consist primarily of cash and cash equivalents. AgeX limits the amount of credit exposure of cash balances by maintaining its accounts in high credit quality financial institutions. Cash equivalent deposits with financial institutions may occasionally exceed the limits of insurance on bank deposits; however, AgeX has not experienced any losses on such accounts.
Fair value measurements – Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. To increase the comparability of fair value measures, the following hierarchy prioritizes the inputs to valuation methodologies used to measure fair value (ASC 820-10-50),Fair Value Measurements and Disclosures:
In determining fair value, AgeX utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible, and also considers counterparty credit risk in its assessment of fair value. For the periods presented, AgeX has no financial assets or liabilities recorded at fair value on a recurring basis, except for cash and cash equivalents primarily consisting of money market funds. These assets are measured at fair value using the period-end quoted market prices as a Level 1 input.
The carrying amounts of accounts receivable, net, prepaid expenses and other current assets, related party amounts due to BioTime and other affiliates, accounts payable, accrued liabilities and other current liabilities approximate fair values because of the short-term nature of these items.
Accounts receivable, net –AgeX establishes an allowance for doubtful accounts based on the evaluation of the collectability of its receivables after considering a variety of factors, including the length of time receivables are past due, significant events that may impair the customer’s ability to pay, such as a bankruptcy filing or deterioration in the customer’s operating results or financial position, and historical experience. If circumstances related to customers change, estimates of the recoverability of receivables would be further adjusted. For subscription contracts in which the subscription term commences before a payment is due, LifeMap Sciences records an accounts receivable as the subscription is earned over time and bills the customer according to the contract terms. LifeMap Sciences continuously monitors collections and payments from customers and maintains a provision for estimated credit losses and uncollectible accounts based upon its historical experience and any specific customer collection issues that have been identified. Amounts determined to be uncollectible are written off against the allowance for doubtful accounts. Accounts receivable, net, include allowance for doubtful accounts of approximately $321,000 as of December 31, 2018 and 2017, for those amounts deemed uncollectible by AgeX or LifeMap Sciences.
Equipment and furniture, net – Equipment and furniture is stated at cost and is being depreciated using the straight-line method over their estimated useful lives ranging from 3 to 10 years. Maintenance and repairs are expensed as incurred whereas significant renewals and betterments are capitalized. When assets are retired or otherwise disposed of, the cost and the related accumulated depreciation are removed from the respective accounts and any resulting gain or loss is reflected in AgeX’s results of operations.
Long-lived intangible assets – Long-lived intangible assets, consisting primarily of acquired patents, patent applications, and licenses to use certain patents, includingacquired in-process research and development (“IPR&D”) with alternative future uses, are stated at acquired cost, less accumulated amortization (see Note 3). Amortization expense is computed using the straight-line method over the estimated useful lives of the assets, generally over 10 years.
Impairment of long-lived assets – Long-lived assets, including long-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be fully recoverable. If an impairment indicator is present, AgeX evaluates recoverability by a comparison of the carrying amount of the assets to future undiscounted net cash flows expected to be generated by the assets. If the assets are impaired, the impairment recognized is measured by the amount by which the carrying amount exceeds the estimated fair value of the assets. Through 2018, there have been no impairment losses.warrant liability.
See Note 6, Warrant Liability, for discussion on estimated change in fair value of warrant liability.
Transactions with noncontrolling interestsNoncontrolling Interests of subsidiaries –Subsidiaries
AgeX accounts for a change in ownership interests in its subsidiaries that does not result in a change of control of the subsidiary under the provisions of ASC810-10-45-23,Consolidation –Other Presentation Matters,which prescribes the accounting for changes in ownership interest that do not result in a change in control of the subsidiary, as defined by U.S. GAAP, before and after the transaction.transaction. Under this guidance, changes in a controlling stockholder’s ownership interest that do not result in a change of control, as defined by U.S. GAAP, in the subsidiary are accounted for as equity transactions. Accordingly, if the controlling stockholder retains control, no gain or loss is recognized in the statements of operations of the controlling stockholder. Similarly, the controlling stockholder will not record any additional acquisition adjustments to reflect its subsequent purchases of additional shares in the subsidiary if there is no change of control. Only a proportional and immediate transfer of carrying value between the controlling and the noncontrolling stockholders occurs based on the respective ownership percentages.
ResearchFair Value Measurements of Financial Instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Fair value estimates discussed herein are based upon certain market assumptions and development –Researchpertinent information available to management as of the financial statement presentation date.
The carrying values of cash equivalents, accounts receivable and developmentaccounts payable are carried at, or approximate, fair value as of the reporting date because of their short-term nature. Fair values for AgeX’s warrant liabilities are estimated by utilizing valuation models that consider current and expected stock prices, volatility, dividends, market interest rates, forward yield curves and discount rates. Such amounts and the recognition of such amounts are subject to significant estimates that may change in the future.
| 79 |
To increase the comparability of fair value measures, the following hierarchy prioritizes the inputs to valuation methodologies used to measure fair value (ASC 820-10-50, Fair Value Measurements and Disclosures):
| ● | Level 1 – Inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. | |
| ● | Level 2 – Inputs to the valuation methodology include quoted prices for similar assets or liabilities in active markets, and inputs that are observable for the assets or liabilities, either directly or indirectly, for substantially the full term of the financial instruments. | |
| ● | Level 3 – Inputs to the valuation methodology are unobservable; that reflect management’s own assumptions about the assumptions market participants would make and significant to the fair value. |
In determining fair value, AgeX utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible, and also considers counterparty credit risk in its assessment of fair value. For the periods presented, AgeX has no financial assets recorded at fair value on a recurring basis, except for cash and cash equivalents primarily consisting of money market funds. These assets are measured at fair value using the period-end quoted market prices as a Level 1 input. The carrying amounts of accounts receivable, net, prepaid expenses includeand other current assets, related party amounts due to affiliates, accounts payable, accrued liabilities and other current liabilities approximate fair values because of the short-term nature of these items. The discounted conversion prices triggered by certain qualified events in the Serina Note and the 2023 Secured Note are Level 3 on the fair value hierarchy and subject to fair valuation at inception and remeasurement at each reporting period. The fair value of the discounted conversion prices under both direct expenses incurred bynotes were determined to have an immaterial value at inception and life to date of the notes, as the probability of a future qualifying event is remote. The likelihood of the future qualifying event will be evaluated at the end of each reporting period. For additional information regarding the convertible notes and derivatives, see Notes 4, Convertible Note Receivable, and 5, Related Party Transactions.
The accounting guidance establishes a hierarchy which requires an entity to maximize the use of quoted market prices and minimize the use of unobservable inputs. An asset or liability’s level is based on the lowest level of input that is significant to the fair value measurement. Fair value estimates are reviewed at the origination date and again at each applicable measurement date and interim or annual financial reporting dates, as applicable for the financial instrument, and are based upon certain market assumptions and pertinent information available to management at those times.
The methods and significant inputs and assumptions utilized in estimating the fair value of the warrant liabilities, as well as the respective hierarchy designations are discussed further in Note 6, Warrant Liability. The warrant liability measurement is considered a Level 3 measurement based on the availability of market data and inputs and the significance of any unobservable inputs as of the measurement date. As of December 31, 2023, AgeX has utilized the full credit subject to warrants, and accordingly, the warrants were fully issued for each of the advances of loan funds under the 2022 Secured Note.
See Note 6, Warrant Liability, for additional information on accounting for liability classified warrants and certain Level 3 warrant valuation tables.
Cash and Cash Equivalents
AgeX considers all highly liquid investments purchased with an original maturity of three months or its subsidiariesless to be cash equivalents. As of December 31, 2023 and indirect overhead costs allocated by BioTime2022, AgeX’s cash balances totaled $0.3 million and $0.6 million, respectively, and consist entirely of bank account deposits and amounts held in money market funds.
Concentrations of Credit Risk
Financial instruments that benefit or support AgeX’s research and development functions. Direct research and development expensespotentially subject AgeX to significant concentrations of credit risk consist primarily of personnel costscash and related benefits, including stock-based compensation, amortizationcash equivalents. AgeX limits the amount of intangible assets, outside consultantscredit exposure of cash balances by maintaining its accounts in high credit quality financial institutions. Cash equivalent deposits with financial institutions may occasionally exceed the limits of insurance on bank deposits; however, AgeX has not experienced any losses on such accounts.
Restricted Cash
In accordance with ASU 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash, a reconciliation of AgeX’s cash and suppliers,cash equivalents in the consolidated balance sheets to cash, cash equivalents and license fees paid to third parties to acquire patents or licenses to use patents and other technology. Direct research and development expenses also include allocations for carve-out presentation purposes from certain former BioTime general research departments contributed to AgeX primarily comprised of former BioTime personnel and related expenses, including stock-based compensation, transferred to AgeX, and other outside expenses relevant to the nature of the research projects being conducted that were contributed to AgeX pursuant to the Asset Contribution Agreement discussed in Note 4. Indirect research and development expenses allocated by BioTime to AgeX under the Shared Facilities Agreement (see Note 4), are primarily based on headcount or space occupied, as applicable, and include laboratory supplies, laboratory expenses, rent and utilities, common area maintenance, telecommunications, property taxes and insurance. Research and development expenses incurred and reimbursed by grants from third parties or governmental agencies, including service revenues from co-development projects with customers, if any and as applicable, approximate the respective revenues recognizedrestricted cash in the consolidated statements of operations.cash flows for all periods presented is as follows (in thousands):
Schedule of Cash, Cash Equivalents and Restricted Cash
| 2023 | 2022 | |||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Cash and cash equivalents | $ | 345 | $ | 645 | ||||
| Restricted cash (1) | 50 | 50 | ||||||
| Cash, cash equivalents, and restricted cash as shown in the consolidated statements of cash flows | $ | 395 | $ | 695 | ||||
| (1) | Restricted cash entirely represents the deposit required to maintain AgeX’s corporate credit card program. |
| 80 |
General and administrativeAccounts Receivable, Net – General and administrative expenses include both direct expenses incurred by
AgeX and indirect overhead costs allocated by BioTime that benefit or support AgeX’s general and administrative functions. Direct general and administrative expenses consist primarily of compensation and related benefits, including stock-based compensation,establishes an allowance for executive and corporate personnel, and professional and consulting fees. Direct general and administrative expenses also include allocations from certain BioTime general and administrative expenses, including allocated stock-based compensation, for carve-out presentation purposes of the AgeX consolidated statements of operations. These allocations are principallydoubtful accounts based on the evaluation of the collectability of its receivables after considering a variety of factors, including the length of time receivables are past due, significant events that may impair the customer’s ability to pay, such as a bankruptcy filing or deterioration in the customer’s operating results or financial position, and historical generalexperience. If circumstances related to customers change, estimates of the recoverability of receivables would be further adjusted. There were no amounts reserved for doubtful accounts as of December 31, 2023 and administrative functions2022.
Long-Lived Intangible Assets, Net
Long-lived intangible assets, consisting primarily of acquired in-process research and expenses relevant to BioTimedevelopment (“IPR&D”) and patents, are stated at acquired cost, less accumulated amortization. Amortization expense is computed using the straight-line method over the estimated useful life of 10 years. See Note 3, Selected Balance Sheet Components.
Impairment of Long-Lived Assets
AgeX assesses the impairment of long-lived assets whenever events or changes in circumstances indicate that such assets might be impaired and the AgeX subsidiariescarrying value may not be recoverable. AgeX’s long-lived assets consist entirely of intangible assets. If events or changes in circumstances indicate that operated priorthe carrying amount of an asset may not be recoverable and the expected undiscounted future cash flows attributable to and after AgeX formation during the periods presented. Indirect general and administrative expenses allocated by BioTime to AgeX under the Shared Facilities Agreement (see Note 4)asset are primarily based on headcount or space occupied, as applicable, and include costs for financial reporting and compliance, rent and utilities, common area maintenance, telecommunications, property taxes and insurance.
Foreign currency translation and other comprehensive income or loss, foreign currency transaction gains and losses –In countries in which AgeX operates where the functional currency is otherless than the U.S. dollar, assetscarrying amount of the asset, an impairment loss, equal to the excess of the carrying value of the asset over its fair value, is recorded. As of December 31, 2023, there has been no impairment of long-lived assets.
Leases
AgeX accounts for leases in accordance with ASU 2016-02, Leases (Topic 842) (“ASC 842”), and liabilitiesits subsequent amendments affecting AgeX: (i) ASU 2018-10, Codification Improvements to Topic 842, Leases, and (ii) ASU 2018-11, Leases (Topic 842): Targeted improvements, using the modified retrospective method. AgeX management determines if an arrangement is a lease at inception. Leases are translated using published exchange rates in effect atclassified as either financing or operating, with classification affecting the consolidated balance sheet date. Revenues and expenses and cash flows are translated using an approximate weighted average exchange rate for the period.Resulting foreign currency translation adjustments are recorded as other comprehensive income or loss, netpattern of tax,expense recognition in the consolidated statements of comprehensive incomeoperations. When determining whether a lease is a financing lease or lossan operating lease, ASC 842 does not specifically define criteria to determine “major part of remaining economic life of the underlying asset” and included“substantially all of the fair value of the underlying asset.” For lease classification determination, AgeX continues to use (i) 75% or greater to determine whether the lease term is a major part of the remaining economic life of the underlying asset and (ii) 90% or greater to determine whether the present value of the sum of lease payments is substantially all of the fair value of the underlying asset. Under the available practical expedients, and as applicable, AgeX accounts for the lease and non-lease components as a component of accumulated other comprehensive income or loss onsingle lease component. AgeX recognizes right-of-use (“ROU”) assets and lease liabilities for leases with terms greater than twelve months in the consolidated balance sheetssheets.
ROU assets represent an entity’s right to use an underlying asset during the lease term and lease liabilities represent an entity’s obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. If the lease agreement does not provide an implicit rate in the contract, an entity uses its incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. The operating lease ROU asset also includes any lease payments made and excludes lease incentives. The lease terms may include options to extend or terminate the lease when it is reasonably certain that the entity will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term. AgeX does not capitalize leases that have terms of twelve months or less.
AgeX leased office space in Alameda, California. For 2022 base monthly rent was $1,074 and for 2023 base monthly rent is $844 for slightly less space at the same building. AgeX has elected to not apply the recognition requirements under ASC 842 for the lease agreements and instead recognizes the lease payments as lease cost on a straight-line basis over the lease term as lease payments are not deemed material.
| 81 |
Accounting for Warrants
AgeX determines the accounting classification of warrants it issues, as either liability or equity, by first assessing whether the warrants meet liability classification in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity, then in accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock. Foreign currency translation adjustmentsUnder ASC 480-10, warrants are immaterialconsidered liability classified if the warrants are mandatorily redeemable, obligate AgeX to settle the warrants or the underlying shares by paying cash or other assets, or warrants that must or may require settlement by issuing a variable number of shares. If warrants do not meet liability classification under ASC 480-10, AgeX assesses the requirements under ASC 815-40, which states that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the warrants do not require liability classification under ASC 815-40, and in order to conclude equity classification, AgeX also assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable provisions of U.S. GAAP. After all periods presented.
For transactions denominatedrelevant assessments, AgeX concludes whether the warrants are classified as liability or equity. Liability classified warrants require fair value accounting at issuance and subsequent to initial issuance with all changes in other thanfair value after the functional currency of AgeX or its subsidiaries, AgeX recognizes transaction gains and lossesissuance date recorded in the consolidated statements of operationsoperations. Equity classified warrants only require fair value accounting at issuance with no changes recognized subsequent to the issuance date. AgeX has liability classified warrants as of December 31, 2023. See Notes 5, Related Party Transactions, and classifies the gain or loss based on the nature of the item that generated it. The majority of AgeX’s foreign currency transaction gains and losses are generated by LifeMap Sciences Ltd.’s intercompany payable due to LifeMap Sciences,Inc.6, Warrant Liability, which are U.S. dollar-denominated, while LifeMap Sciences Ltd.’s functional currency is the Israeli New Shekel (“NIS”). Accordingly, foreign currency remeasurement gains and lossesfor additional information regarding AgeX warrants.
AgeX recognizes compensation expense related to this intercompany payable are included in other incomeemployee option grants and expenses, net.
Income taxes –For Federal and California purposes, AgeX’s activity through August 30, 2018 and for 2017 will be or was included in BioTime’s federal consolidated and California combined tax returns. For those periods, the income tax provision was preparedrestricted stock grants, if any, in accordance with ASC 740,718, Income TaxesCompensation – Stock Compensation, (“ASC 718”). AgeX estimates the fair value of employee stock-based payment awards on the grant-date and recognizes the resulting fair value, net of estimated forfeitures for grants prior to 2017, over the requisite service period. Upon adoption of ASU 2016-09 on January 1, 2017 as further discussed below, forfeitures are accounted for as they occur instead of based on the number of awards that were expected to vest prior to adoption of ASU 2016-09.
AgeX uses the Black-Scholes option pricing model for estimating the fair value of options granted under AgeX’s 2017 Equity Incentive Plan (the “Incentive Plan”). The fair value of each restricted stock grant, if any, is determined based on the value of the common stock granted or sold. AgeX has elected to treat stock-based payment awards with time-based service conditions as a single award and recognizes stock-based compensation on a straight-line basis over the requisite service period.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718. Stock option awards issued to non-employees, principally consultants or outside contractors, as applicable, are accounted for at fair value using the separate return methodBlack-Scholes option pricing model. Management believes that the fair value of the stock options and restricted stock units can more reliably be measured than the fair value of services received. AgeX records compensation expense based on the then-current fair values of the stock options and restricted stock units at the grant date. Compensation expense for non-employee grants is recorded on a straight-line basis in the consolidated statements of operations.
The Black-Scholes option pricing model requires AgeX to determinemake certain assumptions including the fair value of the underlying common stock, the expected term, the expected volatility, the risk-free interest rate and the dividend yield (see Note 8, Stock-Based Awards).
The fair value of the shares of common stock underlying the stock options is determined in accordance with the Incentive Plan and is based on prevailing market prices on the NYSE American where AgeX common stock is traded.
The expected term of employee stock options represents the weighted-average period that the stock options are expected to remain outstanding. AgeX estimates the expected term of options granted using the “simplified method” provided under Staff Accounting Bulletin Topic 14, or SAB Topic 14.
Because AgeX’s common stock has public trading history of fewer than five years, AgeX has estimated the expected volatility using its own stock price volatility to the extent applicable or a combination of its stock price volatility and the stock price volatility of peer companies, for a period equal to the expected term of the options, which may exceed five years. The peer companies used include selected public companies within the biotechnology industry with comparable characteristics to AgeX, including similarity in size, lines of business, market capitalization, revenue and financial leverage.
The risk-free interest rate assumption is based upon observed interest rates on the United States government securities appropriate for the expected term of AgeX’s stock options.
The dividend yield assumption is based on AgeX’s history and expectation of dividend payouts. AgeX has never declared or paid any cash dividends on its common stock, and AgeX does not anticipate paying any cash dividends in the foreseeable future.
All excess tax benefits and tax deficiencies from stock-based compensation awards accounted for under ASC 718 are recognized as an income tax benefit or expense, respectively, in the consolidated statements of operations. An excess income tax benefit arises when the tax provisiondeduction of a share-based award for income tax purposes exceeds the compensation cost recognized for financial reporting purposes and, a tax deficiency arises when the compensation cost exceeds the tax deduction.
| 82 |
Stock-based compensation expense for the years ended December 31, 2023 and 2022 consists of stock-based compensation under the AgeX Incentive Plan (see Note 8, Stock-Based Awards).
Certain of AgeX’s consolidated subsidiaries have had their own share-based compensation plans; however, there are no awards granted and outstanding under those plans as of December 31, 2023 and 2022. For share-based compensation awards granted by privately held consolidated subsidiaries under their respective equity plans, AgeX determines the fair value of the options granted under those plans using similar methodologies and assumptions AgeX used for carve-out presentation purposesits stock options discussed above.
Although the fair value of itsstock options and restricted stock units is determined in accordance with FASB guidance, changes in the assumptions and allocations can materially affect the estimated value and therefore the amount of compensation expense recognized in the consolidated financial statements. The separate return method, among other items, requires that the amount of current and deferred tax expense for a group that files a consolidated income tax return be allocated among the members of that group as if each group member were a separate taxpayer. As a result, the provision for income taxes has been presented as if
Income Taxes
AgeX had filed a separate federal consolidated tax return and a California combined tax return for those periods. In using the separate return method, the sum of the amounts allocated to the members of the income tax return group may not equal the consolidated amount. If tax attributes recorded in the carve-out consolidated financial statements are materially different from the actual tax attributes pertaining to the legal entities of AgeX and its subsidiaries, or to BioTime and its subsidiaries, those differences are identified and disclosed in Note 7. Accordingly, depending on the future legal structure of AgeX and related tax elections that may be taken by AgeX, the effective tax rate of AgeX in future years could vary materially from its historical effective tax rates. The historical deferred tax assets, including the operating losses and credit carryforwards generated by certain research and development departments that operated within BioTime and were transferred to AgeX on August 17, 2017 (Note 4), have been presented as tax attributes of AgeX consistent with the principles of the separate return method described above. As of December 31, 2018, the deferred tax assets and liabilities presented in Note 7, including net operating loss carryforwards and research and development credits, represent the tax attributes of the AgeX and its subsidiaries.
However, the net operating losses and research and development credits generated before August 17, 2017, the contribution date to AgeX, will remain as tax attributes of BioTime (see Note 7). In general, net operating losses and other tax credit carryforwards generated by legal entities in a consolidated federal tax group or a combined state tax group, collectively “the tax group”, are available to other members of the tax group depending on the nature of the transaction that a member may enter into while still in the tax group. However, under the Tax Matters Agreement between BioTime and AgeX entered into on August 17, 2017, any use of a member’s net operating loss and other tax credit carryforwards by the other member is subject to reimbursement by the benefiting member for the actual tax benefit realized. Since the August 30, 2018 deconsolidation of AgeX and to date, neither BioTime nor AgeX has used the tax attributes of the other.
AgeXaccounts for income taxes in accordance with ASC 740, which prescribes the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and enacted rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. AgeX’s judgments, estimates and projections regarding future taxable income may change over time due to changes, among other factors, in market conditions, changes in tax laws, and tax planning strategies. If AgeX’s assumptions and consequently its estimates change in the future, the valuation allowance may be increased or decreased, which may have a material impact on AgeX’s consolidated financial statements.
The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. AgeX recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. No unrecognized tax benefits have been recorded and no amounts were accrued for the payment of interest and penalties as of December 31, 20182023 and 2017.2022. AgeX isdoes not awareexpect that the total amount of any uncertainunrecognized tax positions that could result in significant additional payments, accruals, or other material adjustments forbenefits will materially change over the years ended December 31, 2018 and 2017.next twelve months. AgeX is currently unaware of any tax issues under review.
On December 22, 2017,Revenue Recognition
AgeX recognizes revenue in a manner that depicts the United States enacted major federal tax reform legislation, Public Law No. 115-97, commonly referred to as the 2017 Tax Cuts and Jobs Act (“2017 Tax Act”), which enactedtransfer of control of a broad range of changes to the Internal Revenue Code. Changes to taxes on corporations impacted by the 2017 Tax Act include, but not limited to, lowering the U.S. federal tax ratesproduct or a service to a 21% flat tax rate, eliminating the corporate alternative minimum tax (“AMT”), imposing additional limitations on the deductibility of interestcustomer and net operating losses, allowing any net operating loss (“NOLs”) generated in tax years ending after December 31, 2017 to be carried forward indefinitely and generally repealing carrybacks, reducing the maximum deduction for NOL carryforwards arising in tax years beginning after 2017 to a percentage of the taxpayer’s taxable income, and allowing for additional expensing of certain capital expenditures. The 2017 Tax Act also puts into effect a number of changes impacting operations outside of the United States including, but not limited to, the imposition of a one-time tax “deemed repatriation” on accumulated offshore earnings not previously subject to U.S. tax, and shifts the U.S taxation of multinational corporations from a worldwide system of taxation to a territorial system. ASC 740 requires the effects of changes in tax rates and laws on deferred tax balances (including the effects of the one-time transition tax) to be recognized in the period in which the legislation is enacted (see Note 7).
On December 22, 2017, the SEC staff issued Staff Accounting Bulletin No. 118 (“SAB 118”) to provide guidance for companies that are not able to complete their accounting for the income tax effects of the 2017 Tax Act in the period of enactment. SAB 118 allows AgeX to record provisional amounts during a measurement period not to extend beyond one year of the enactment date (see Note 7). AgeX applied the guidance in SAB 118 when accounting for the enactment-date effects of the 2017 Tax Act during the years ended December 31, 2018 and 2017. As of December 31, 2018, AgeX completed its accounting for all the enactment-date income tax effects of the 2017 Tax Act further discussed in Note 7.
For 2017, LifeMap Sciences included a deemed repatriation of $227,000 in accumulated foreign earnings not previously subject to U.S. tax in federal income from LifeMap Sciences Ltd. The federal taxable income was offset by the LifeMap Sciences’ net operating loss carryforwards resulting in no federal income tax due.
Beginning in 2018, the 2017 Tax Act subjects a U.S. stockholder to tax on Global Intangible Low Tax Income (GILTI) earned by certain foreign subsidiaries. In general, GILTI is the excess of a U.S. shareholder’s total net foreign income over a deemed return on tangible assets. The provision further allows a deduction of 50% of GILTI, however this deduction is limited by the company’s pre-GILTI U.S. income. For 2018, AgeX included an immaterial amount of GILTI in U.S. gross income related to LifeMap Sciences, Ltd., which was fully offset by current year operating losses. Current interpretations under ASC 740 state that an entity can make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as GILTI in future years or to provide for the tax expense related to GILTI in the year the tax is incurred as a period expense. AgeX has elected to account for GILTI as a current period expense when incurred.
Stock-based compensation – AgeX recognizes compensation expense related to employee option grants and restricted stock grants, if any, in accordance with FASB ASC 718,Compensation – Stock Compensation (“ASC 718”).
AgeX estimates the fair value of employee stock-based payment awards on the grant-date and recognizes the resulting fair value, net of estimated forfeitures for grants prior to 2017, over the requisite service period. Upon adoption of Accounting Standards Update (“ASU”) 2016-09 on January 1, 2017 as further discussed below, forfeitures are accounted for as they occur instead of based on the number of awards that were expected to vest prior to adoption of ASU 2016-09.
AgeX uses the Black-Scholes option pricing model for estimating the fair value of options granted under AgeX’s 2017 Equity Incentive Plan (the “Plan”). The fair value of each restricted stock grant, if any, is determined based on the value of the common stock granted or sold. AgeX has elected to treat stock-based payment awards with time-based service conditions as a single award and recognizes stock-based compensation on a straight-line basis over the requisite service period.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718 and FASB ASC 505-50,Equity-Based Payments to Non-Employees(seeRecently issued accounting pronouncements not yet adopted below). Stock option awards issued to non-employees, principally consultants or outside contractors, as applicable, are accounted for at fair value using the Black-Scholes option pricing model. Management believes that the fair value of the stock options can more reliably be measured than the fair value of services received. AgeX records compensation expense based on the then-current fair values of the stock options at each financial reporting date. Compensation expense recorded during the service period is adjusted in subsequent periods for changes in the fair value of the stock options until the earlier of the date at which the non-employee’s performance is complete or a performance commitment is reached, which is generally when the stock option award vests. Compensation expense for non-employee grants is recorded on a straight-line basis in the consolidated statements of operations.
The Black-Scholes option pricing model requires AgeX to make certain assumptions including the fair value of the underlying common stock, the expected term, the expected volatility, the risk-free interest rate and the dividend yield (see Note 6).
The fair value of the shares of common stock underlying the stock options has historically been determined by the Board of Directors. Because there was no public market for AgeX’s common stock prior to November 29, 2018, the Board of Directors has determined the fair value of the common stock at the time of the grant of options by considering a number of objective and subjective factors including contemporaneous sales of common stock to investors, valuation of comparable companies, operating and financial performance and general and industry-specific economic outlook, amongst other factors. The fair value was determined in accordance with applicable elements of the practice aid issued by the American Institute of Certified Public Accountants titledValuation of Privately Held Company Equity Securities Issued as Compensation. Since our common stock began publicly trading on the NYSE American, the fair value of our common stock underlying stock options has been valued based on prevailing market prices.
The expected term of employee stock options represents the weighted-average period that the stock options are expected to remain outstanding. AgeX estimates the expected term of options granted using the “simplified method” provided underStaff Accounting Bulletin, Topic 14, or SAB Topic 14.
Because AgeX’s common stock had no publicly traded history prior to November 29, 2018, AgeX estimated the expected volatility of the awards from the historical volatility of selected public companies within the biotechnology industry with comparable characteristics to AgeX, including similarity in size, lines of business, market capitalization, revenue and financial leverage. AgeX determined the expected volatility assumption using the frequency of daily historical prices of comparable public company’s common stock for a period equal to the expected term of the options. For the year ended December 31, 2018, AgeX estimated the expected volatility using its own stock price volatility to the extent applicable or a combination of its stock price volatility and the stock price volatility of stock of peer companies, for a period equal to the expected term of the options.
The risk-free interest rate assumption is based upon observed interest rates on the United States government securities appropriate for the expected term of AgeX’s stock options.
The dividend yield assumption is based on AgeX’s history and expectation of dividend payouts. AgeX has never declared or paid any cash dividends on its common stock, and AgeX does not anticipate paying any cash dividends in the foreseeable future.
All excess tax benefits and tax deficiencies from stock-based compensation awards accounted for under ASC 718 are recognized as an income tax benefit or expense, respectively, in the consolidated statements of operations. An excess income tax benefit arises when the tax deduction of a share-based award for income tax purposes exceeds the compensation cost recognized for financial reporting purposes and, a tax deficiency arises when the compensation cost exceeds the tax deduction. Because AgeX had no stock option exercises during the years ended December 31, 2018 and 2017, and because of AgeX’s full valuation allowance as of December 31, 2018 and 2017, there was no impact to AgeX’s consolidated financial statements from the adoption of 2016-09 (see Note 7).
Since AgeX did not have an equity incentive plan until July 2017 (see Note 6), consolidated stock-based compensation expense for the applicable period prior to that date consisted of stock-based compensation allocated from BioTime, principally related to stock-based compensation of former BioTime employees transferred to AgeX, and stock-based compensation of AgeX’s subsidiaries that have their own equity plans. Stock-based compensation expense for the years ended December 31, 2018 and 2017 consists of stock-based compensation under the AgeX 2017 Equity Incentive Plan (Note 6), stock-based compensation allocated from BioTime and stock-based compensation of AgeX’s subsidiaries that have their own stock option plans.
As discussed above, certain of AgeX’s consolidated subsidiaries have their own share-based compensation plans. For share-based compensation awards granted by those privately-held consolidated subsidiaries under their respective equity plans, AgeX determines the fair value of the options granted under those plans using similar methodologies and assumptions AgeX used for its stock options discussed above.
Although the fair value of stock options is determined in accordance with FASB guidance, changes in the assumptions and allocations can materially affect the estimated value and thereforereflects the amount of compensation expense recognizedthe consideration it expects to receive in the consolidated financial statements.
Segments- AgeX’s executive management team, asexchange for such product or service. In doing so, AgeX follows a group, represents the entity’s chief operating decision makers. To date, AgeX’s executive management team has viewed AgeX’s operations as one segment that includes the research and development ofregenerative medicine technologies targeting the diseases of aging and metabolic disorders,oncology, and neurological diseases and disorders, blood and vascular system diseases and disorders, and pluripotent cell technologies.As a result, the financial information disclosed materially represents all of the financial information related to AgeX’s sole operating segment.
Basic and diluted net income or loss per share attributable to common stockholders – Basic income or loss per share is calculated by dividing net income or loss attributable to AgeX common stockholders by the weighted average number of shares of common shares outstanding, net of unvested restricted stock or restricted stock units, subject to repurchase by AgeX, if any, during the period. Diluted income per share is calculated by dividing the net income attributable to AgeX common stockholders by the weighted average number of common shares outstanding, adjusted for the effects of potentially dilutive common shares issuable under outstanding stock options and warrants, using the treasury-stock method, and convertible preferred stock, if any, using the if-converted method.
On August 17, 2017, in connection with the Asset Contribution Agreement discussed in Note 4, AgeX issued 28.8 million shares of common stock to its then parent company, BioTime. For carve-out presentation purposes and financial reporting purposes of the reported basic and diluted net loss per share attributable to AgeX common stockholders in AgeX’s consolidated statements of operations, the 28.8 million shares of AgeX common stock issued to BioTime are assumed to be issued and outstanding from the beginning of the earliest period presented.
For the years ended December 31, 2018 and 2017, because AgeX reported a net loss attributable to common stockholders, all potentially dilutive common stock, comprised of stock options and warrants, is antidilutive.
The following common stock equivalents were excluded from the computation of diluted net loss per common share for the period presented because including them would have been antidilutive (in thousands):
| Year Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| Stock options | 2,269 | 1,239 | ||||||
| Warrants | 2,000 | - | ||||||
Recently adopted accounting pronouncements
Adoption of ASU 2014-09, Revenues from Contracts with Customers (Topic 606). During May 2014, the FASB issued ASU 2014-09 (“Topic 606”)Revenue from Contracts with Customers which supersedes the revenue recognition requirements in Topic 605Revenue Recognition (“Topic 605”). Topic 606 describes principles an entity must apply to measure and recognize revenue and the related cash flows, using the following five steps:five-step approach: (i) identify the contract(s)contract with a customer;customer, (ii) identify the performance obligations in the contract;contract, (iii) determine the transaction price;price, (iv) allocate the transaction price to the performance obligation(s) in the contract;obligations, and (v) recognize revenue when (or as) the entity satisfies a performance obligation. Topic 606 core principle is that it requires entities to recognize revenue whencustomer obtains control of the promised goodsproduct or services is transferred to customers at an amount that reflectsservice. AgeX considers the consideration to whichterms of a contract and all relevant facts and circumstances when applying the entity expects to be entitled to in exchange for those goods or services.
AgeX adopted Topic 606 as of January 1, 2018 using the modified retrospective transition method applied to those contracts which were not completed as of the adoption date. Results for reporting periods beginning on January 1, 2018 and thereafter are presented under Topic 606, while prior period amounts are not adjusted and continue to be reported in accordance with AgeX’s historic revenue recognition accounting under Topic 605.standard. AgeX applies the revenue recognition standard, including the use of any practical expedients, consistently to contracts with similar characteristics and in similar circumstances.
On January 1, 2018, the impact of the adoption and application of Topic 606 was immaterial, and no cumulative effect adjustment was made as of that date. In the applicable paragraphs below, AgeX has summarized its revenue recognition policies for its various revenue sources in accordance with Topic 606.
Revenue recognition by source and geography.– Revenues are recognized when control of the promised goods or services is transferred to customers, or in the case of governmental entities funding a grant, when allowable expenses are incurred, in an amount that reflects the consideration AgeX or a subsidiary, depending on which company has the customer or the grant, expects to be entitled to in exchange for those goods or services.
The following table presents AgeX’s consolidated revenues disaggregated by source for operations (in thousands).:
Schedule of Disaggregated of Revenues
| REVENUES: | 2023 | 2022 | ||||||||||||||
| Year Ended December 31, | Year Ended December 31, | |||||||||||||||
| REVENUES: | 2018 | 2017(1) | 2023 | 2022 | ||||||||||||
| Subscription and advertisement revenues | $ | 1,227 | $ | 1,399 | ||||||||||||
| Service and other revenues | 149 | 5 | ||||||||||||||
| Grant revenues | 20 | - | $ | 77 | $ | - | ||||||||||
| Other revenues | 65 | 34 | ||||||||||||||
| Total revenues | $ | 1,396 | $ | 1,404 | $ | 142 | $ | 34 | ||||||||
| 83 |
(1)Amounts recognized prior to adoption of Topic 606 have not been adjusted under the Topic 606 modified retrospective transition method.
The following table presents consolidated revenues for operations (in thousands), disaggregated by geography, based on the billing addresses of customers.customers:
Schedule of Disaggregated Geographical Revenue
| REVENUES: | 2023 | 2022 | ||||||||||||||
| Year Ended December 31, | Year Ended December 31, | |||||||||||||||
| REVENUES: | 2018 | 2017(1) | 2023 | 2022 | ||||||||||||
| United States | $ | 813 | $ | 808 | $ | 90 | $ | 10 | ||||||||
| Foreign | 583 | 596 | 52 | 24 | ||||||||||||
| Total revenues | $ | 1,396 | $ | 1,404 | $ | 142 | $ | 34 | ||||||||
(1) Amounts recognized prior to adoption of Topic 606 have not been adjusted under the Topic 606 modified retrospective transition method.
Subscription and advertisement revenues. LifeMap Sciences, a direct majority-owned subsidiary of AgeX, sells subscription-based products, including research databases and software tools, for biomedical, gene, and disease research. LifeMap Sciences sells these subscriptions primarily through the internet to biotech and pharmaceutical companies worldwide. LifeMap Sciences’ principal subscription product is theGeneCards® Suite, which includes theGeneCards® human gene database, and theMalaCards human disease database.
LifeMap Sciences’ performance obligations for subscriptions include a license of intellectual property related to its genetic information packages and premium genetic information tools. These licenses are deemed functional licenses that provide customers with a “right to access” to LifeMap Sciences’ intellectual property during the subscription period and, accordingly, revenue is recognized over a period of time, which is generally the subscription period. Payments are typically received at the beginning of a subscription period and revenue is recognized according to the type of subscription sold.
For subscription contracts in which the subscription term commences before a payment is due, LifeMap Sciences records an accounts receivable as the subscription is earned over time and bills the customer according to the contract terms. LifeMap Sciences continuously monitors collections and payments from customers and maintains a provision for estimated credit losses and uncollectible accounts based upon its historical experience and any specific customer collection issues that have been identified. Amounts determined to be uncollectible are written off against the allowance for doubtful accounts. LifeMap Sciences has not historically provided significant discounts, credits, concessions, or other incentives from the stated price in the contract as the prices are offered on a fixed fee basis for the type of subscription package being purchased. LifeMap Sciences may issue refunds only if the packages cease to be available for reasons beyond its control. In such an event, the customer will get a refund on a pro-rata basis. Both the customer and LifeMap Sciences expect the subscription packages to be available during the entire subscription period, and LifeMap Sciences has not experienced any significant issues with the availability of the product and has not issued any material refunds. Using the most likely amount method for estimating refunds under Topic 606, including historical experience, LifeMap Sciences determined that the single most likely amount of variable consideration for refunds is immaterial as LifeMap Sciences does not expect to pay any refunds.
LifeMap Sciences performance obligations for advertising are overall advertising services and represent a series of distinct services. Contracts are typically less than a year in duration and the fees charged may include a combination of fixed and variable fees with the variable fees tied to click throughs to the customer’s products on their website. LifeMap Sciences allocates the variable consideration to each month the click through services occur and allocates the annual fee to the performance obligation period of the initial term of the contract because those amounts correspond to the value provided to the customer each month. For click-through advertising services, at the time the variable compensation is known and determinable, the service has been rendered. Revenue is recognized at that time. The annual fee is recognized over the initial subscription period because this is a service and the customer simultaneously receives and consumes the benefit of LifeMap Sciences’ performance.
LifeMap Sciences deferred subscription revenues primarily represent subscriptions for which cash payment has been received for the subscription term, but the subscription term has not been completed as of the balance sheet date reported. For the years ended December 31, 2018 and 2017, LifeMap Sciences recognized $1.2 million and $1.4 million, respectively, in subscription and advertisement revenues. As of December 31, 2018 and 2017, there was $0.3 million included in deferred revenues in the consolidated balance sheets which is expected to be recognized as subscription revenue over the next twelve months.
LifeMap Sciences has licensed from third parties the databases and software it commercializes and has a contractual obligation to pay royalties to the licensor on subscriptions sold. These costs are included in cost of sales on the consolidated statements of operations when the cash is received and the royalty obligation is incurred as the royalty payments do not qualify for capitalization of costs to fulfill a contract under ASC 340-40,Other Assets and Deferred Costs - Contracts with Customers.
Grant revenues –. In applying the provisions of Topic 606, AgeX has determined that government grants are out of the scope of Topic 606 because the government entities do not meet the definition of a “customer”, as defined by Topic 606, as there is not considered to be a transfer of control of good or services to the government entities funding the grant. AgeX has, and will continue to, accountaccounts for grants received to perform research and development services in accordance with ASC 730-20,Research and Development ArrangementsArrangements., which requires an assessment, at At the inception of the grant, ofwe perform an assessment as to whether the grant is a liability or a contract to perform research and development services for others. If AgeX or a subsidiary receiving the grant is obligated to repay the grant funds to the grantor regardless of the outcome of the research and development activities, then AgeX is required to estimate and recognize that liability. Alternatively, if AgeX or a subsidiary receiving the grant is not required to repay, or if it is required to repay the grant funds only if the research and development activities are successful, then the grant agreement is accounted for as a contract to perform research and development services for others, in which case, grant revenue is recognized when the related research and development expenses are incurred.
In applying the provisions of Topic 606, AgeX had nohas determined that government grants are out of the scope of Topic 606 because the government entities do not meet the definition of a “customer”, as defined by Topic 606, as there is not considered to be a transfer of control of good or services to the government entities funding the grant. In the absence of applicable guidance under U.S. GAAP, our policy is to recognize grant revenues during 2017.revenue when the related costs are incurred, provided that the applicable conditions under the government contracts have been met. Only costs that are allowable under the grant award, certain government regulations and the National Institutes of Health’s supplemental policy and procedure manual may be claimed for reimbursement, and the reimbursements are subject to routine audits from governmental agencies from time to time. Costs incurred are recorded in research and development expenses on the accompanying consolidated statements of operations.
In September 2018,applying the provisions of Topic 606, AgeX has determined that government grants are out of the scope of Topic 606 because the government entities do not meet the definition of a “customer”, as defined by Topic 606, as there is not considered to be a transfer of control of good or services to the government entities funding the grant. In the absence of applicable guidance under U.S. GAAP, our policy is to recognize grant revenue when the related costs are incurred, provided that the applicable conditions under the government contracts have been met. Only costs that are allowable under the grant award, certain government regulations and the National Institutes of Health (“NIH”) supplemental policy and procedure manual may be claimed for reimbursement, and the reimbursements are subject to routine audits from governmental agencies from time to time. Costs incurred are recorded in research and development expenses on the accompanying consolidated statements of operations.
AgeX believes the recognition of revenue as costs are incurred and amounts become realizable is analogous to the concept of transfer of control of a service over time under ASC 606.
In August 2023, AgeX was awarded a grant of up to approximately $225,000$341,000 from the NIH, National Institutes of Health (NIH).Heart, Lung and Blood Institute. The NIH grant provideswill provide funding for continued development of AgeXAgeX’s technologies toward treating cardiovascular disease over a one year period starting September 1, 2023. Based on our evaluation under the accounting guidance aforementioned, this grant agreement is accounted for treating osteoporosis. Theas a contract to perform research and development services for others, in which case, grant revenue is recognized when the related research and development expenses are incurred. Accordingly, grant funds will beare made available by the NIH as allowable expenses are incurred. For the year ended December 31, 2018,2023, AgeX incurred approximately $20,000$77,000 of allowable expenses under the NIH grant and recognized a corresponding amount ofgrant revenues.revenues.
OnApril 5, 2018, ReCyte Therapeutics was awardedESI BIO research products – AgeX, through its ESI BIO research product division, markets a grantnumber of upproducts related to approximately $386,000human pluripotent stem cells (“PSC lines”), including research-grade PSC lines and PSC lines produced under current good manufacturing practices or “cGMP.” AgeX offers cells from PSC lines to customers under contracts that permit the customers to utilize PSC lines for the research, development, and commercialization of cell-based therapies or other products in defined fields of application. The compensation to AgeX for providing the PSC line cells under such contracts may include up-front payments, milestone payments related to product development, regulatory matters, and commercialization, and the payment of royalties on sales of products developed from AgeX PSC lines. Revenues from the NIH. The NIH grant provides funding for continued developmentsale of ReCyte Therapeutic’s technologies for treating stroke. The grant funds will be made available by the NIH to ReCyte Therapeutics as allowable expensesresearch products are incurred. As of December 31, 2018, no allowable expenses were incurred under the NIH grant.included in other revenues.
Arrangements with multiple performance obligations –. AgeX’s AgeX may enter into contracts with customers maythat include multiple performance obligations. For such arrangements, AgeX allocateswill allocate revenue to each performance obligation based on its relative standalone selling price. AgeX generally determineswill determine or estimatesestimate standalone selling prices based on the prices charged, or that would be charged, to customers for that product or service. As of and for the yearyears ended December 31, 2018,2023 and 2022, AgeX did not have significant arrangements with multiple performance obligations.
| 84 |
Recently issued accounting pronouncements not yet adoptedResearch and Development –
Research and development expenses consist primarily of personnel costs and related benefits, including stock-based compensation, amortization of intangible assets, outside consultants and contractors, sponsored research agreements with certain universities, and suppliers, and license fees paid to third parties to acquire patents or licenses to use patents and other technology. Research and development expenses incurred and reimbursed by grants from third parties or governmental agencies if any and as applicable, approximate the respective revenues recognized in the consolidated statements of operations.
General and Administrative
General and administrative expenses consist primarily of compensation and related benefits, including stock-based compensation, for executive and corporate personnel, and professional and consulting fees.
Segments
AgeX’s executive management team, as a group, represents the entity’s chief operating decision makers. To date, AgeX’s executive management team has viewed AgeX’s operations as one segment that includes the research and development of regenerative medicine technologies targeting the diseases of aging and metabolic disorders, oncology, and neurological diseases and disorders, blood and vascular system diseases and disorders, and pluripotent cell technologies. As a result, the financial information disclosed materially represents all of the financial information related to AgeX’s sole operating segment.
Basic loss per share is calculated by dividing net loss attributable to AgeX common stockholders by the weighted average number of shares of common stock outstanding, net of unvested restricted stock or restricted stock units, subject to repurchase by AgeX, if any, during the period. Diluted loss per share is calculated by dividing the net income attributable to AgeX common stockholders, if any, by the weighted average number of shares of common stock outstanding, adjusted for the effects of potentially dilutive common stock issuable under outstanding stock options, warrants, and restricted stock units, using the treasury-stock method, and convertible preferred stock, if any, using the if-converted method, and treasury stock held by subsidiaries, if any.
For the years ended December 31, 2023 and 2022, because AgeX reported a net loss attributable to common stockholders, all potentially dilutive common stock, comprised of stock options, restricted stock units and warrants, is antidilutive.
Schedule of Antidilutive Securities Excluded from Computation of Earnings Per Share
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Stock options | 91 | 94 | ||||||
| Warrants (1) | 352 | 272 | ||||||
| Restricted stock units | - | - | ||||||
| (1) | As of December 31, 2023 and 2022, warrants to purchase 320,115 and 344,875 shares, respectively, of AgeX common stock were issued and outstanding and held by Juvenescence as consideration for certain loan agreements discussed in Note 5, Related Party Transactions. Amounts shown in the table are weighted average amounts and reflect the fact that the warrants were issued on various dates during applicable periods presented. |
Recently Adopted Accounting Pronouncements
In June 2018,2016, the FASB issued ASU 2018-07,2016-13, Compensation - Stock CompensationFinancial Instruments – Credit Losses (Topic 718)326): Improvements to Nonemployee Share-Based Payment AccountingMeasurement of Credit Losses on Financial Instruments, and subsequent amendments to the initial guidance under ASU 2018-19, ASU 2019-04, ASU 2019-05 and ASU 2019-10, which simplifiesamends the current approach to estimate credit losses on certain financial assets. This ASU requires immediate recognition of management’s estimates of current expected credit losses. Under the prior model, losses were recognized only as they were incurred, which FASB has noted delayed recognition of expected losses that might not yet have met the threshold of being probable. The standard is applicable to all financial assets (and net investment in leases) that are not accounted for at fair value through net income, such as trade receivables, loans, debt securities, and net investment in leases, thereby bringing consistency in accounting for non-employee share-based payment transactions. The newtreatment across different types of financial instruments and requiring consideration of a broader range of variables when forming loss estimates. Subsequent changes in the valuation allowance are recorded in current earnings and reversal of previous losses are permitted. AgeX adopted this standard expands the scopeas of Topic 718 to include share-based payment transactions for acquiring goodsJanuary 1, 2023, and services from non-employees. ASU 2018-07 is effective for fiscal years beginning after December 15, 2018 (including interim periods within that fiscal year), with early adoption permitted. As AgeX doesit did not have a significant number of nonemployee share-based awards, AgeX does not believe that the application of the new standard will have a material impact on itsthe consolidated financial statements.
| 85 |
In February 2016,March 2022, the FASB issued ASU 2016-02,2022-01, LeasesDerivatives and Hedging (Topic 842)815): Fair Value Hedging – Portfolio Layer Method, which requires lessees to recognize assets and liabilitiesclarifies the guidance in ASC 815 on fair value hedge accounting of interest rate risk for leases with lease terms greater than twelve months in the statementportfolios of financial position. Leases will be classifiedassets. The ASU amends the guidance in ASU 2017-12 (released on August 28, 2017) that, among other things, established the “last-of-layer” method for making the fair value hedge accounting for these portfolios more accessible. ASU 2022-01 renames that method the “portfolio layer” method and addresses feedback from stakeholders regarding its application. AgeX adopted this standard as either finance or operating, with classification affectingof January 1, 2023, and it did not have a material impact on the pattern of expense recognition in the income statement. ASU 2016-02 also requires improved disclosures to help users ofconsolidated financial statements better understand the amount, timing and uncertainty of cash flows arising from leases. The update is effective for fiscal years beginning after December 15, 2018, including interim reporting periods within that reporting period. Early adoption is permitted.statements.
In July 2018,March 2022, the FASB issued ASU 2018-102022-02, Financial Instruments – Credit Losses (Topic 326): Troubled Debt Restructurings and ASU 2018-11. ASU 2018-10 provides certain areasVintage Disclosures, which amends the accounting for improvementcredit losses on financial instruments. This amendment eliminates the recognition and measurement guidance on troubled debt restructurings for creditors that have adopted the new credit losses guidance in ASU 2016-02ASC 326 and ASU 2018-11 provides an additional optional transition method by allowingrequires enhanced disclosures about loan modifications for borrowers experiencing financial difficulty. The new guidance also requires public business entities to initiallypresent gross write-offs by year of origination in their vintage disclosures. The guidance became effective for AgeX on January 1, 2023 and includes interim periods. Entities can elect to adopt the guidance on troubled debt restructurings using either a prospective or modified retrospective transition. If an entity elects to apply the new lease standard at the adoption date and recognize a cumulative-effectmodified retrospective transition, it will record a cumulative effect adjustment to the opening balance of retained earnings in the period of adoption. AgeXis completing its assessment of the impact the adoption ofThis ASU 2016-02 will have on its financial statements, but because AgeX doesdid not have significant operating leases as of December 31, 2018, AgeX does not expect the adoption of ASU 2016-02on January 1, 2019, including the use of the optional transition method allowed by ASU 2018-11, will have a material impact on itsthe consolidated financial statements. IfAgeX’sstatements.
On July 14, 2023, the FASB issued ASU 2023-03, Presentation of Financial Statements (Topic 205), Income Statement – Reporting Comprehensive Income (Topic 220), Distinguishing Liabilities from Equity (Topic 480), Equity (Topic 505), and Compensation – Stock Compensation, which amends or supersedes various Securities and Exchange Commission (“SEC”) paragraphs within the codification to conform to past announcements and guidance issued by the SEC. Specifically, the ASU responds to (1) the issuance of SEC Staff Accounting Bulletin (SAB) 120; (2) the SEC staff announcement at the March 24, 2022, EITF meeting; and (3) SAB Topic 6.B, “Accounting Series Release No. 280 — General Revision of Regulation S-X: Income or Loss Applicable to Common Stock.” This ASU is effective immediately and did not have a material impact on AgeX’s consolidated financial statements.
Recently Issued Accounting Pronouncements Not Yet Adopted
In August 2023, the FASB issued ASU 2023-05, Business Combinations—Joint Venture Formations (Subtopic 805-60): Recognition and Initial Measurement, under which an entity that qualifies as either a joint venture or a corporate joint venture as defined in the FASB ASC master glossary is required to apply a new sublease discussedbasis of accounting upon the formation of the joint venture. Specifically, the ASU provides that a joint venture or a corporate joint venture (collectively, “joint ventures”) must initially measure its assets and liabilities at fair value on the formation date. This ASU is effective for AgeX beginning January 1, 2025 and is not expected to have a material impact on the consolidated financial statements.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to income Tax Disclosures, under which entities must consistently categorize and provide greater disaggregation of information in Note 9the rate reconciliation. They must also further disaggregate income taxes paid. ASU 2023-09 enhances annual income tax disclosures to address investor requests for more information about the tax risks and opportunities present in an entity’s worldwide operations. This ASU is consummated,effective for AgeX expects thatbeginning January 1, 2025 and is not expected to have a material impact on the sublease will be subject to the new standard and, upon consummation, will be recognized as a right-of-use asset and operating lease liability in accordance with ASU 2016-02.consolidated financial statements.
3. Selected Balance Sheet Components
Accounts payable and accrued liabilities
At December 31, 2018 and 2017, accounts payable and accrued liabilities were comprised of the following (in thousands):
| December 31, | ||||||||
| 2018 | 2017(1) | |||||||
| Accounts payable | $ | 150 | $ | 75 | ||||
| Accrued compensation | 254 | 257 | ||||||
| Accrued vendors and other expenses | 1,012 | 436 | ||||||
| Accounts payable and accrued liabilities | $ | 1,416 | $ | 768 | ||||
(1) Reflects the effect of the LifeMap Solutions transfer to BioTime on June 6, 2017 discussed in Notes 1 and 4.
Equipment and furniture, net
At December 31, 2018 and 2017, equipment and furniture were comprised of the following (in thousands):
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| Equipment and furniture | $ | 245 | $ | 274 | ||||
| Accumulated depreciation | (155 | ) | (145 | ) | ||||
| Equipment and furniture, net | $ | 90 | $ | 129 | ||||
Depreciation expense amounted to $58,000 and $165,000 for the years ended December 31, 2018 and 2017, respectively. Depreciation expense in 2017 includes amounts allocated from BioTime to AgeX for carve-out basis of presentation (see Note 2).
Intangible assets, netAssets, Net
Intangible assets are primarily comprised of acquired licenses and other rights by LifeMap Sciencesfrom a third party for certain databases it commercializes, which includes theGeneCards® human gene database, and theMalaCards™ human disease database.These databases are available primarily through the internet and sold as subscriptions or on a fee per use basis for use by researchers at pharmaceutical and biotechnology companies and other institutions.
On August 13, 2018, AgeX entered into an Asset Purchase Agreement (the “Purchase Agreement”) with Escape Therapeutics, Inc. (“Escape”) pursuant to which AgeX acquired certain patents and patent applications related primarily to methods of modifying cells and tissues and certain pluripotent stem cell lines so as to reduce their risk of being rejected when transplanted. This technology is called “UniverCyte™.”. AgeX paid Escape $1,072,436$1,072,436 in cash and issued 80,000 shares of AgeX common stock, with an approximate value of $240,000,$240,000, for aggregate acquisition cost of $1.3$1.3 million for the UniverCyte™ assets.The Purchase Agreement was considered an asset acquisition rather than a business combination in accordance with ASC 805-50,Business Combinations.
ASC 730-10-25(c),Research and Development – Intangible Assets Purchased from Others, provides guidance for acquisition and capitalization of the cost of intangible assets purchased from others in an asset acquisition that have alternative future uses in other research and development projects. These intangible assets are referred to as acquired in-process research and developmentIPR&D with alternative future uses and are accounted for as intangible assets and amortized to research and development over their useful life. Acquired IPR&D in an asset acquisition that does not have any alternative future uses is expensed under the same guidance. As an initial focus, AgeX intends to use the UniverCyte™ technology in the development of its two lead products, AGEX-BAT1 and AGEX-VASC1 for the treatment of Type II diabetes and cardiovascular aging, respectively. Accordingly, AgeX recorded the UniverCyte™ technology acquired from Escape as IPR&D intangible assets with alternative future uses in accordance with ASC 730-10-25(c) and is amortizing those assets to research and development expense over their estimated 10 year-year useful life.
| 86 |
Pursuant to the Purchase Agreement, if AgeX has not expended a certain level of funds by the end of 2019 toward the research and development of pluripotent stem cell or progenitor cell products and processes utilizing the acquired patents and the development or improvement of the acquired patents, AgeX will make an additional annual cash payment to Escape. If total development expenditures have still not reached a predetermined level by the end of 2020, AgeX will pay Escape additional amounts and the royalty rate for net sales of products, processes and services will be tripled until total expenditures reach the required threshold. The aggregate cash payments AgeX may make to Escape for not reaching the predetermined level of expenses can be up to $1 million. AgeX expects to meet this requirement as the acquired technology is planned to be developed and used in AgeX’s two leading programs discussed above and, accordingly, no amounts have been accrued as of December 31, 2018 for this provision of the Purchase Agreement.
In addition to the purchase price, AgeX will pay Escape a royalty of less than 1%1% on net sales of products, processes and services under the acquired patents if the assets are commercialized. Additional shares of AgeX common stock totaling up to $4.3$4.3 million of market value will also be issued to Escape upon the attainment of development and regulatory approval milestones by AgeX for each product covered by the acquired patents. Contingent consideration in an asset acquisition is generally recorded when probable and estimable in accordance with ASC 450,Contingencies. Accordingly, none of the milestone payments have been accrued since the attainment of any milestone in the Purchase Agreement iswas not probable as of December 31, 2018.2023.
Escape has agreedAgeX estimated the future undiscounted cash flows expected to indemnify AgeXbe received from certain liabilities.the assets developed through the use of the UniverCyte™ technology when commercialized. The Purchase Agreement contains representations, warrantiesestimate of the future undiscounted cash flows considered AgeX’s financial condition and agreements customary for a transaction of this nature.the royalties that may become payable to Escape.
AgeX has also agreed to engage Escape’s chief executive officer as a consultant for a period of up to three years to assist AgeX in utilizing the acquired patents. AgeX pays $200,000 per year in consulting fees as services are performed included in research and development expenses.
At December 31, 20182023 and 2017,2022, intangible assets,primarily consisting of acquired in-process researchIPR&D and development and patents, and accumulated amortization were as follows (in thousands):
Schedule of Intangible Assets, Net
| 2023 | 2022 | |||||||||||||||
| December 31, | December 31, | |||||||||||||||
| 2018 | 2017 | 2023 | 2022 | |||||||||||||
| Intangible assets | $ | 5,586 | $ | 4,274 | $ | 1,312 | $ | 1,312 | ||||||||
| Accumulated amortization | (2,877 | ) | (2,400 | ) | (705 | ) | (574 | ) | ||||||||
| Intangible assets, net | $ | 2,709 | $ | 1,874 | ||||||||||||
| Total intangible assets, net | $ | 607 | $ | 738 | ||||||||||||
AmortizationAgeX recognized $131,000 and $132,000 in amortization expense amounted to $477,000of intangible assets, included in research and $517,000development expenses, for the years ended December 31, 20182023 and 2017,2022, respectively.
Amortization expense in 2017 also includes amounts allocated from BioTimeof intangible assets for periods subsequent to December 31, 2023 is as follows (in thousands):
Schedule of Amortization Assets
| Year Ending December 31, | Amortization Expense | |||
| 2024 | $ | 131 | ||
| 2025 | 131 | |||
| 2026 | 132 | |||
| 2027 | 131 | |||
| 2028 | 82 | |||
| Total | $ | 607 | ||
Accounts Payable and Accrued Liabilities
At December 31, 2023 and 2022, accounts payable and accrued liabilities were comprised of the following (in thousands):
Schedule of Accounts Payable and Accrued Liabilities
| 2023 | 2022 | |||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Accounts payable | $ | 1,413 | $ | 568 | ||||
| Accrued vendors and other expenses | 529 | 273 | ||||||
| Accrued compensation and severance expenses | 234 | 193 | ||||||
| Total accounts payable and accrued liabilities | $ | 2,176 | $ | 1,034 | ||||
4. Convertible Note Receivable
On March 15, 2023, AgeX for carve-outand Serina entered into a Convertible Note Purchase Agreement (the “Serina Note Purchase Agreement”), pursuant to which AgeX lent to Serina an aggregate principal amount of $10,000,000 as evidenced by the Serina Note on that date. Interest on the principal amount under the Serina Note accrues on the unpaid principal amount at a simple interest rate equal to 7% per annum, computed on the basis of presentation (seethe 360-day year of twelve 30-day months. The outstanding principal amount and accrued interest of $10,544,000 under the Serina Note 2).will become due and payable on March 15, 2026.
4. In connection with the issuance of the Serina Note, AgeX is entitled to elect one member to the board of directors of Serina and receive certain information and inspection rights as well as participation rights for subsequent equity issuances.
| 87 |
The principal balance of the Serina Note with accrued interest will automatically convert into Serina preferred stock if Serina raises at least $25,000,000 through the sale of shares of Serina preferred stock (“qualifying event”). The conversion price per share shall be the lower of (a) 80% of the lowest price at which the shares of preferred stock were sold, and (b) a “capped price” equal to $105,000,000 divided by Serina’s then fully diluted capitalization. AgeX has the option to convert the Serina Note into Serina preferred stock after a sale of Serina preferred stock regardless of the amount sold by Serina. AgeX evaluated the 20% discounted conversion feature of the Serina Note under ASC 815-15, Derivatives and Hedging—Embedded Derivatives, and concluded that it was an embedded derivative which should be bifurcated from the note and accounted for separately. The 20% discount was determined to have an immaterial value at inception and life to date of the Serina Note, as the probability of a future qualifying event is remote. The likelihood of the future qualifying event will be evaluated at the end of each reporting period and any adjustments will be included in Interest expense, net in the Other expense, net section of the consolidated statements of operations.
AgeX may (i) at its election, upon a change of control (as defined in the Serina Note), convert the Serina Note in whole or in part into either (a) cash in an amount equal to 100% of the outstanding principal amount of the Serina Note, plus interest, or (b) into the highest ranking shares of Serina then issued at a conversion price equal to the lowest price per share at which the most senior series of Serina shares has been sold in a single transaction or a series of related transactions through which Serina raised at least $5,000,000 or (ii) if the Serina Note remains outstanding as of the maturity date, AgeX may convert the Serina Note into the most senior shares of Serina issued at the time of conversion at a conversion price equal to the capped price.
If the Merger is consummated, the Serina Note will be canceled for no consideration.
The outstanding principal balance of the Serina Note with accrued interest may become immediately due and payable prior to the stated maturity date if an Event of Default as defined in the Serina Note occurs. In addition to this and any other remedy, both in equity and in law, upon the occurrence of an Event of Default, an interest rate of 10% per annum and computed on the basis of the 360-day year of twelve 30-day months, shall apply to the Convertible Amount until fully paid. Events of Default under the Serina Note include: (i) the commission of any act of bankruptcy by Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X), (ii) the execution by Serina of a general assignment for the benefit of creditors, (iii) the filing by or against Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X) of a petition in bankruptcy or any petition for relief under the federal bankruptcy act (or, in each case, under any similar insolvency law) or the continuation of such petition without dismissal for a period of 60 calendar days or more, (iv) the appointment of a receiver or trustee to take possession of the property or assets of Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X), (v) failure of Serina to pay any amount due under the Serina Note when due, which failure to pay is not cured by Serina within 5 business days of written notice thereof, (vi) unless waived by AgeX, Serina’s material breach of any representation, warranty or covenant of Serina under the Serina Note Purchase Agreement, Serina Note or other agreements entered in connection therewith, which breach, if curable, is not cured by Serina within 10 business days of written notice by AgeX thereof, (vii) Serina or any subsidiary shall default on any of its obligations under any indebtedness which default causes the indebtedness thereunder to (x) become prematurely due and payable, (y) be placed on demand or (z) become capable of being declared by or on behalf of a creditor thereunder to be prematurely due and payable or being placed on demand, in each case, as a result of such default or any provision having a similar effect (howsoever prescribed), (viii) any monetary judgment, writ or similar final process shall be entered or filed against Serina, any subsidiary or any of their respective property or other assets for more than $250,000, and such judgment, writ or similar final process shall remain unvacated, unbonded or unstayed for a period of 45 calendar days, and (ix) Serina experiences a Material Adverse Effect (as defined in the Serina Note Purchase Agreement).
The Serina Note Purchase Agreement and Serina Note each includes certain covenants that among other matters require financial reporting and impose certain restrictions, including (i) restrictions on the incurrence of additional indebtedness by Serina and its subsidiaries; (ii) requiring that Serina use note proceeds and funds that may be raised through certain equity offerings only for research and development work, professional and administrative expenses, and for general working capital; and (iii) prohibiting Serina from entering into any material sale or transfer transactions outside of the ordinary course of business, other than in a merger between AgeX and Serina, without the consent of AgeX.
Subordination Agreement
In connection with the issuance of the Serina Note, Serina, each other holder of Serina indebtedness (each a “Serina Lender”), and AgeX entered into a Subordination Agreement, dated March 15, 2023, pursuant to which each Serina Lender agreed to subordinate to AgeX’s rights of repayment with respect to the obligations owed under the Serina Note Purchase Agreement and the Serina Note (i) all Serina indebtedness owed to such Serina Lender under certain convertible notes between each Serina Lender and Serina, which aggregate principal amount of all of such convertible notes equals $1,450,000, and (ii) any related security interests.
5. Related Party Transactions
Related Party Payables to BioTime
Since inception, ReCyte Therapeutics, LifeMap Sciences and LifeMap Solutions, former subsidiaries of BioTime, had accumulated related party payables due to BioTime, mainly comprised of working capital advances and Use Fees under the Shared Facilities Agreement described below. BioTime generally did not historically charge interest on Use Fee payable and only commenced charging interest for working capital advances in 2017, which was insignificant for the applicable periods presented.
Prior to January 1, 2017, the aggregate related party payables due to BioTime for these working capital advances and Use Fees amounted to $23.2 million, which was comprised of total $12.9 million owed by LifeMap Sciences and its then subsidiary LifeMap Solutions, and $10.3 million owed by ReCyte Therapeutics, included in current liabilities on the consolidated balance sheets.
On June 6, 2017, in contemplation of capitalizingDuring July 2023, AgeX and Juvenescence entered into an Exchange Agreement pursuant to further incentivize new investorswhich AgeX issued shares of Series A Preferred Stock and Series B Preferred Stock to invest in AgeX as discussed below, BioTime agreed to settle or cancel these related party payable balances with the subsidiaries as follows:
On August 17, 2017, BioTime contributed its ownership in LifeMap Sciences, ReCyte Therapeutics and other assets to AgeXJuvenescence in exchange for 28.8 the extinguishment of a total of $36 million of indebtedness under the 2020 Loan Agreement, the 2022 Secured Note, and the 2023 Secured Note discussed below. The unused portion of the line of credit under the 2022 Secured Note remains available to AgeX subject to the terms and conditions of the 2022 Secured Note. The Series A Preferred Stock and Series B Preferred Stock automatically converted into shares of AgeX common stock on February 1, 2024. See Notes 7, Stockholders’ Equity/(Deficit), and 11, Subsequent Events.
| 88 |
2019 Loan Agreement
On August 13, 2019, AgeX and Juvenescence entered into a Loan Facility Agreement (the “2019 Loan Agreement”) pursuant to which Juvenescence provided to AgeX a $2.0 million line of credit for a period of 18 months. On February 10, 2021, AgeX entered into an amendment (the “First Amendment”) to the 2019 Loan Agreement which extended the maturity date of loans under the 2019 Loan Agreement to February 14, 2022, and increased the amount of the loan facility by $4.0 million. On November 8, 2021, AgeX entered into Amendment No. 2 to the 2019 Loan Agreement which increased the amount of the loan facility by another $1.0 million. As of December 31, 2021, AgeX had borrowed all of the $7.0 million total line of credit under the 2019 Loan Agreement, as amended. On February 14, 2022, AgeX refinanced the $7.0 million outstanding principal amount of the loans and a $160,000 origination fee due under the 2019 Loan Agreement, as amended. See discussion regarding the 2022 Secured Convertible Promissory Note within this Note 5.
2020 Loan Agreement
On March 30, 2020, AgeX and Juvenescence entered into a new Secured Convertible Facility Agreement (the “2020 Loan Agreement”) pursuant to which Juvenescence provided to AgeX an $8.0 million line of credit for a period of 18 months. Through December 31, 2023, AgeX had drawn the full $8.0 million line of credit. AgeX issued to Juvenescence shares of AgeX common stock as further discussed belowan arrangement fee for the loan facility when AgeX borrowed an aggregate of $3.0 million under the 2020 Loan Agreement, and in Note 5.
Asset Contribution Agreement
On August 17, 2017, AgeX received its initial assets and cash from BioTime and certain investors. BioTime contributed certain assets and cashissued to AgeX in exchange for 28,800,000Juvenescence warrants to purchase a total of 104,365 shares of AgeX common stock (see Note 5)(“2020 Warrants”) as determined by the warrant formula described below of which 25,628 are outstanding as of December 31, 2023. On March 13, 2023, the 2020 Loan Agreement was amended to extend the maturity date to March 30, 2024. During July 2023, the full $8.0 million of 2020 Loan Agreement indebtedness was extinguished in exchange for shares of Series A Preferred Stock pursuant to the Asset ContributionExchange Agreement. BioTime
2020 Warrants — Under the terms of the 2020 Loan Agreement, each time AgeX received an advance of funds under the 2020 Loan Agreement, AgeX issued to Juvenescence a number of 2020 Warrants equal to 50% of the number determined by dividing the amount of the advance by the applicable Market Price. The Market Price set for each 2020 Warrant when issued was the closing price per share of AgeX common stock on the NYSE American on the date of the applicable notice from AgeX requesting a draw of funds that triggered the obligation to issue the 2020 Warrant. The 2020 Warrants will expire at 5:00 p.m. New York time three years after the date of issue. AgeX had issued to Juvenescence 2020 Warrants to purchase a total of 104,365 shares of AgeX common stock of which are outstanding as of December 31, 2023. The exercise prices of the 2020 Warrants issued through and that are still outstanding as of December 31, 2023 range from $28.49 per share to $66.65 per share representing the market closing price on the NYSE American of AgeX common stock on the one day prior to delivery of the drawdown notices. The number of shares issuable upon exercise of the warrants and the exercise price per share are subject to adjustment upon the occurrence of certain events such as a stock split or reverse split or combination of the common stock, stock dividend, recapitalization or reclassification of the common stock, and similar events, and have been adjusted to give effect to the Reverse Stock Split.
2022 Secured Convertible Promissory Note and Security Agreement
On February 14, 2022, AgeX and Juvenescence entered into a Secured Convertible Promissory Note (the “2022 Secured Note”) pursuant to which Juvenescence agreed to provide to AgeX a $13,160,000 line of credit for a period of 12 months. AgeX drew an initial $8,160,000 of the line of credit and used $7,160,000 to refinance the outstanding principal and the loan origination fees under the 2019 Loan Agreement with Juvenescence. On February 9, 2023, AgeX and Juvenescence entered into an Amended and Restated Secured Convertible Promissory Note which amends and restates the 2022 Secured Note and added $2 million to the line of credit available to be borrowed by AgeX under the 2022 Secured Note subject to Juvenescence’s discretion to approve each loan draw. On May 9, 2023, AgeX and Juvenescence entered into an Allonge and Second Amendment to Amended and Restated Convertible Promissory Note (the “Second Amendment”) that increased the amount of the line of credit available to AgeX by $4,000,000, subject to the terms of the 2022 Secured Note and Juvenescence’s discretion to approve and fund each of AgeX’s future draws of that additional amount of credit. On June 2, 2023, AgeX and Juvenescence entered into a Third Amendment to Amended and Restated Convertible Promissory Note (the “Third Amendment’), to provide that (i) AgeX may draw on the available portion of the line of credit under the 2022 Secured Note until the earlier of the date a Qualified Offering as defined in the 2022 Secured Note is consummated by AgeX or October 31, 2023 (subject to Juvenescence’s discretion to approve each loan draw as provided in the 2022 Secured Note), (ii) AgeX will not be obligated to issue additional common stock purchase warrants to Juvenescence in connection with the receipt of loan funds made available pursuant to the Second Amendment, and (iii) the definition of Reverse Financing Condition was amended to extend to June 20, 2023 the referenced deadline for fulfillment of the condition to permit borrowing or other incurrence of indebtedness by Reverse Bioengineering, Inc. The date on which the outstanding principal balance of the 2022 Secured Note will become due and payable shall be May 9, 2024.
| 89 |
On July 31, 2023, AgeX and Juvenescence entered into a Fourth Amendment (the “Fourth Amendment”) to the 2022 Secured Note to provide that (i) the definition of Reverse Financing Condition is amended to extend to October 31, 2023 the referenced deadline for fulfillment of the condition to permit borrowing or other incurrence of indebtedness by AgeX’s subsidiary Reverse Bio, and (ii) Juvenescence may convert the outstanding amount of the 2022 Secured Note loans or any portion of such loans into AgeX common stock without restriction by the “19.9% Cap” if Juvenescence elects to convert those amounts at a conversion price or prices equal to the “Drawdown Market Prices” applicable to such loan amounts in lieu of a lower conversion price set with reference to the current market price of AgeX common stock at the time of conversion. The 19.9% Cap is a provision of the 2022 Secured Note that limits the amount of common stock that Juvenescence may acquire through the conversion of Secured Note loans in order to comply with NYSE American requirements pertaining to the amount of shares that a listed company, such as AgeX, may sell at a price less than the market prices prevailing at the time the loans were made (the “Drawdown Market Prices”) without shareholder approval.
On October 3, 2023, AgeX drew $500,000 of its credit available under the 2022 Secured Note. On October 31, 2023 AgeX drew the final $500,000 of the credit line available under the Fourth Amendment.
On November 9, 2023, AgeX and Juvenescence entered into the Allonge and Fifth Amendment to Amended and Restated Convertible Promissory Note (the “Fifth Amendment”) that increases the amount of the line of credit available to AgeX by $4,400,000, subject to the terms of the 2022 Secured Note and Juvenescence’s discretion to approve and fund each of AgeX’s future draws of that additional amount of credit. Concurrently with the execution of the Fifth Amendment, AgeX also entered into an additional Pledge Agreement to add shares of a Licensesubsidiary to the collateral under the Security Agreement, and AgeX’s subsidiaries ReCyte, Reverse Bio, and UniverXome each entered into a Guaranty Agreement and Joinder Agreement pursuant to which BioTime licensed or sublicensedeach of them agreed to guaranty AgeX’s obligations to Juvenescence pursuant to the 2022 Secured Note, as amended by the Fifth Amendment, and to grant Juvenescence a security interest in their respective assets pursuant to the Security Agreement to secure their obligations to Juvenescence.
On November 15, 2023, AgeX and AgeX granted to BioTime an option to license back, certain patent rights. Concurrently with the acquisitiondrew $500,000 of assets from BioTimeits credit available under the Asset Contribution Agreement,2022 Secured Note with Juvenescence. On December 5, 2023, AgeX sold 4,950,000 sharesdrew $500,000 of its common stock for $10.0 million in cash primarily to investors other than BioTime, which included $1.2 million from the Chairman of BioTime’s Board of Directors and $32,000 from BioTime. At the close of the financing, BioTime owned 85.4% of the issued and outstanding shares of AgeX common stock.
Assets Contributed:
Pursuant to the Asset Contribution Agreement, BioTime contributed to AgeX the following assets:
Assumption of Liabilities:
AgeX agreed to assume all third-party obligations and liabilities related to the assets contributed and contracts assigned to AgeX or the operation of the AgeX related business.
Other Matters:
The Asset Contribution Agreement also sets forth other terms that govern certain aspects of BioTime’s ongoing relationship with AgeX if in the future BioTime distributes its AgeX shares to BioTime shareholders.
License Agreement
Concurrently with the contribution of assets to AgeXcredit available under the Asset Contribution Agreement, BioTime and2022 Secured Note with Juvenescence.
As of December 31, 2023, AgeX entered intohad borrowed a License Agreement pursuant tototal of $20,160,000 under the 2022 Secured Note, of which BioTime has licensed to AgeX, with rights to sublicense, certain intellectual property, including patents and patent applications and know-how for use in the development, manufacture and commercialization of products or services for the prevention, treatment, amelioration, diagnosis or monitoring of all human and non-human animal diseases and conditions except for the field of medical products, devices and services for the reserved BioTime fields of orthopedic, ophthalmic and medical aesthetic uses. In addition, BioTime retained an option right to license, on terms to be negotiated, iTR patents in research, development, manufacturing and commercialization of treatments in the reserved BioTime fields. The licensed patents and know-how relate generally to (a) BioTime’sPureStem® human embryonic progenitor cell lines, and (b) telomere length and DNA quality control analysis in pluripotent stem cells.
The BioTime patent rights licensed to AgeX are exclusive and worldwide except for existing third-party licenses, and for medical products, devices, and services related to tendons. AgeX additionally received an option to license certain BioTime retained patent rights outside of orthopedic indications unless a license grant would compete with a BioTime program or products in the retained BioTime field.
The Asset Contribution Agreement and other transactions discussed above were completed between entities under common control. Accordingly, the contributed assets and liabilities are recorded at historical carrying values, with the resulting gain recorded in AgeX’s additional paid-in capital included in the consolidated statements of stockholders’ equity for$7,500,000 was borrowed during the year ended December 31, 2017,2023. During July 2023, $17,992,800 of 2022 Secured Note indebtedness, comprised of $16,660,000 borrowing and $1,332,800 of accrued loan origination fees, was extinguished in exchange for shares of Series A Preferred Stock and Series B Preferred Stock pursuant to the Exchange Agreement. See Note 7, Stockholders’ Equity/(Deficit).
As an arrangement fee for the 2022 Secured Note, AgeX will pay Juvenescence an origination fee in an amount equal to 4% of the amount each draw of loan funds, which will accrue as each draw is funded, and an additional 4% of all the total amount of funds drawn that will accrue following the end of the period during which funds may be drawn from the line of credit. The origination fee will become due and payable on the repayment date or in a pro rata amount with any prepayment of in whole or in part of the outstanding principal balance of the 2022 Secured Note.
2022 Warrants – Upon each drawdown of funds under the 2022 Secured Note prior to June 2, 2023 when the Third Amendment went into effect, AgeX issued to Juvenescence warrants to purchase shares of AgeX common stock (“2022 Warrants”). The 2022 Warrants are governed by the terms of a Warrant Agreement between AgeX and Juvenescence. The number of 2022 Warrants issued with respect to each draw of loan funds was equal to 50% of the number determined by dividing the amount of the applicable loan draw by the applicable Market Price. The Market Price was the last closing price per share of AgeX common stock on the NYSE American preceding the delivery of the notice from AgeX requesting the draw of funds that triggered the obligation to issue 2022 Warrants. The exercise price of the 2022 Warrants is the applicable Market Price used to determine the number of Warrants issued. The 2022 Warrants will expire at 5:00 p.m. New York time three years after the date of issue.
As of December 31, 2023, AgeX had issued to Juvenescence 2022 Warrants to purchase a total of 294,482 shares of AgeX common stock, of which 2022 Warrants to purchase 53,980 shares of AgeX common stock were issued during the year ended December 31, 2023. The exercise prices of the 2022 Warrants issued through December 31, 2023 range from $20.75 per share to $30.94 per share representing the market closing price of AgeX common stock on the NYSE American on the one day prior to delivery of the drawdown notices. The number of shares issuable upon exercise of the warrants and the exercise price per share are subject to adjustment upon the occurrence of certain events such as a stock split or reverse split or combination of the common stock, stock dividend, recapitalization or reclassification of the common stock, and similar events, and have been adjusted to give effect to the Reverse Stock Split..
Conversion of Loan Amounts to Common Stock – In lieu of repayment of funds borrowed, AgeX may convert the loan balance and any accrued but unpaid origination fee into AgeX common stock or “units” if AgeX consummates a sale of common stock (or common stock paired with warrants or other convertible securities in “units”) in which the gross sale proceeds are at least $10,000,000. The conversion price per share or units shall be the lowest price at which shares or units are sold. Juvenescence may convert the loan balance in whole or in part into AgeX common stock at any time at Juvenescence’s election at the closing price per share of AgeX common stock on the NYSE American or other national securities exchange on the date prior to the date Juvenescence gives AgeX notice Juvenescence’s election to convert the loan or a portion thereof into common stock.
| 90 |
Default Provisions – The loan balance and origination fees may become immediately due and payable prior to the mandatory repayment date if an Event of Default occurs. Events of Default under the 2022 Secured Note include the following: (a) AgeX fails to pay any principal amount payable by it in the manner and at the time provided under and in accordance with ASC 805-50.
Allocated Expenses from BioTime
Consistentthe 2022 Secured Note; (b) AgeX fails to pay any other amount payable by it in the manner and at the time provided under and in accordance with the principles2022 Secured Note or the Security Agreement described below or any other agreement executed in connection with the 2022 Secured Note (the “Loan Documents”) and the failure is not remedied within three business days; (c) AgeX fails to perform any of carve-outits covenants or obligations or fail to satisfy any of the conditions under the 2022 Secured Note or any other Loan Document and, such failure (if capable of remedy) remains unremedied to the satisfaction of Juvenescence (in its sole discretion) for 10 business days after the earlier of (i) notice requiring its remedy has been given by Juvenescence to AgeX and (ii) actual knowledge of the failure by senior officers of AgeX; (d) if any indebtedness of AgeX in excess of $100,000 becomes due and payable, or a breach or other circumstance arises thereunder such that Juvenescence is entitled to declare such indebtedness due and payable, prior to its due date, or any indebtedness of AgeX in excess of $25,000 is not paid on its due date; (e) AgeX stops payment of its debts generally or ceases or threatens to cease to carry on its business or is unable to pay its debts as they fall due or is deemed by a court of competent jurisdiction to be unable to pay its debts as they fall due, or enters into any arrangements with its creditors generally; (f) if (i) an involuntary proceeding (other than a proceeding instituted by Juvenescence or an affiliate of Juvenescence) shall be commenced or an involuntary petition shall be filed seeking liquidation, reorganization or other relief in respect of AgeX and any subsidiary, or of all or a substantial part of its assets, under any federal, state or foreign bankruptcy, insolvency, receivership or similar law now or hereafter in effect or (ii) an involuntary appointment of a receiver, trustee, custodian, sequestrator, conservator or similar official for AgeX or a subsidiary or for a substantial part of its assets occurs (other than in a proceeding instituted by Juvenescence or an affiliate of Juvenescence), and, in any such case, such proceeding shall continue undismissed and unstayed for sixty (60) consecutive days without having been dismissed, bonded or discharged or an order of relief is entered in any such proceeding; (g) it becomes unlawful for AgeX to perform all or any of its obligations under the 2022 Secured Note or any authorization, approval, consent, license, exemption, filing, registration or other requirement of any governmental, judicial or public body or authority necessary to enable AgeX to comply with its obligations under the 2022 Secured Note or to carry on its business is not obtained or, having been obtained, is modified in a manner that precludes AgeX or its subsidiaries from conducting their business in any material respect, or is revoked, suspended, withdrawn or withheld or fails to remain in full force and effect; (h) the issuance or levy of any judgment, writ, warrant of attachment or execution or similar process against all or any material part of the property or assets of AgeX or a subsidiary if such process is not released, vacated or fully bonded within 60 calendar days after its issue or levy; (i) any injunction, order, judgment or decision of any court is entered or issued which, in the opinion of Juvenescence, materially and adversely affects, or is reasonably likely so to affect, the ability of AgeX or a subsidiary to carry on its business or to pay amounts owed to Juvenescence under the 2022 Secured Note; (j) AgeX, whether in a single transaction or a series of related transactions, sells, leases, licenses, consigns, transfers or otherwise disposes of any material portion of its assets (with any such disposition with respect to any asset or assets with a fair value of at least $250,000 being deemed material), other than (i) certain permitted investments (ii) sales, transfers and dispositions of inventory in the ordinary course of business, (iii) any termination of a lease of real or personal property that is not necessary in the ordinary course of the AgeX’s business, could not reasonably be expected to have a material adverse effect and does not result from AgeX’s default, and (iv) any sale, lease, license, consignment, transfer or other disposition of assets that are no longer necessary in the ordinary course of business or which has been approved in writing by Juvenescence; (k) any of the following shall occur: (i) the security and/or liens created by the Security Agreement or any other Loan Document shall at any time cease to constitute valid and perfected security and/or liens on any material portion of the collateral intended to be covered thereby; (ii) except for expiration in accordance with its terms, the Security Agreement or any other Loan Document pursuant to which a lien is granted by AgeX in favor of Juvenescence shall for whatever reason be terminated or shall cease to be in full force and effect; (iii) the enforceability of the Security Agreement or any other Loan Document pursuant to which a lien is granted by AgeX in favor of Juvenescence shall be contested by AgeX or a subsidiary; (iv) AgeX shall assert that its obligations under the 2022 Secured Note or any other Loan Document shall be invalid or unenforceable; or (v) a loss, theft, damage or destruction occurs with respect to a material portion of the collateral; (l) there is any change in the financial statementscondition of AgeX and presentation discussedits subsidiaries which, in the opinion of Juvenescence, materially and adversely affects, or is reasonably likely so to affect, the ability of AgeX to perform any of its obligations under the 2022 Secured Note; and (m) any representation, warranty or statement made, repeated or deemed made or repeated by AgeX in the 2022 Secured Note, 1,or pursuant to the Loan Documents, is incomplete, untrue, incorrect or misleading in any material respect when made, repeated or deemed made.
Restrictive Covenants – The 2022 Secured Note includes certain covenants that among other matters such as financial reporting: (i) impose financial restrictions on AgeX while the 2022 Secured Note remains unpaid, including restrictions on the incurrence of additional indebtedness by AgeX and its subsidiaries, except that AgeX’s subsidiary Reverse Bio will be permitted to incur debt convertible into equity not guaranteed or secured by the assets of AgeX or any other AgeX subsidiary, (ii) require that AgeX use loan proceeds and funds that may be raised through certain equity offerings only for research and development work, professional and administrative expenses, for general working capital, and for repayment of all or a portion of AgeX’s indebtedness to Juvenescence; and (iii) prohibit AgeX from making additional investments in subsidiaries, unless AgeX obtains the written consent of Juvenescence to a transaction that otherwise would be prohibited or restricted.
| 91 |
Security Agreement – AgeX has entered into a Security Agreement granting Juvenescence a security interest in substantially all of the assets of AgeX, including a security interest in shares of AgeX subsidiaries that hold certain assets, as collateral for AgeX’s loan obligations. If an Event of Default occurs, Juvenescence will have been allocated by BioTimethe right to foreclose on the assets pledged as collateral.
$10 Million Secured Convertible Promissory Note
On March 13, 2023, AgeX and Juvenescence entered into a $10 Million Secured Convertible Promissory Note (the “2023 Secured Note”) pursuant to which Juvenescence has loaned to AgeX $10,000,000. AgeX used the loan proceeds to finance the $10,000,000 loan to Serina under the Serina Note. See Note 4, Convertible Note Receivable, for further information on the Serina Note and the related Serina Note Purchase Agreement.
On July 31, 2023, AgeX and Juvenescence also entered into an amendment to the 2023 Secured Note that mirrors the amendments of the 2022 Secured Note described above, and also creates an earlier time window, ending October 31, 2023, during which Juvenescence may elect to convert any amount outstanding under the 2023 Secured Note into shares of AgeX common stock. After October 31, 2023, Juvenescence may convert outstanding amounts under the 2023 Secured Note into AgeX common stock on any date more than ninety (90) days after the earlier of (a) the occurrence of a Qualified Merger as defined, and (b) March 13, 2024.
The outstanding principal balance of the 2023 Secured Note was scheduled to become due and payable on March 13, 2026. In lieu of accrued interest, AgeX agreed to pay Juvenescence an origination fee in an amount equal to 7% of the loan funds disbursed to AgeX, which will accrue in two installments. The origination fee will become due and payable on the earliest to occur of (i) conversion of the 2023 Secured Note into shares of AgeX common stock, (ii) repayment of the 2023 Secured Note in whole or in part (provided that the origination fee shall be prorated for the amount of any partial repayment), and (iii) the acceleration of the maturity date of the 2023 Secured Note following an Event of Default as defined in the 2023 Secured Note.
During July 2023, the 2023 Secured Note indebtedness, plus a portion of the accrued loan origination fees, was exchanged for Series B Preferred Stock pursuant to the Exchange Agreement.
The 2023 Secured Note includes a provision allowing AgeX to convert the loan balance and any accrued but unpaid origination fee into AgeX common stock or “units” if AgeX consummates a sale of common stock (or common stock paired with warrants or other convertible securities in “units”) in which the gross sale proceeds are at least $10,000,000. If less than $25,000,000 is raised through the sale of common stock or units, the conversion price per share or units shall be the lowest price at which shares or units are sold. If at least $25,000,000 is raised, the conversion price per share shall be 85% of the “Market Price” of AgeX common stock determined as provided in the 2023 Secured Note. AgeX evaluated the 15% discounted conversion feature of the 2023 Secured Note under ASC 815-15, Derivatives and Hedging—Embedded Derivatives, and concluded that it was an embedded derivative which should be bifurcated from the 2023 Secured Note and accounted for separately. The 15% discount was determined to have an immaterial value at inception and life to date of the 2023 Secured Note, as the probability of a future financing event described above is remote. The likelihood of the future qualifying event will be evaluated at the end of each reporting period and any adjustments will be included in Interest expense, net in the AgeXOther expense, net section of the consolidated statements of operationsoperations.
The 2023 Secured Note includes certain covenants that among other matters require financial reporting and consolidated statementsimpose certain restrictions on AgeX that are substantially the same as those under the 2022 Secured Note.
AgeX has entered into an Amended and Restated Security Agreement that amends the February 14, 2022 Security Agreement between AgeX and Juvenescence and adds the 2023 Secured Note to the obligations secured by the Security Agreement. The Security Agreement grants Juvenescence a security interest in substantially all of stockholders’ equity (deficit)the assets of AgeX, including a security interest in shares of AgeX subsidiaries that hold certain assets, as contributioncollateral for AgeX’s loan obligations. If an Event of Default as defined in the 2023 Secured Note occurs, Juvenescence will have the right to foreclose on the assets pledged as collateral with respect to any accrued loan origination fees remaining unpaid under the 2023 Secured Note.
| 92 |
Registration Rights
AgeX entered into certain Registration Rights Agreements, as amended, pursuant to which AgeX has agreed to register for sale under the Securities Act of 1933, as amended (the “Securities Act”) all shares of AgeX common stock presently held by BioTimeJuvenescence or that may be acquired by Juvenescence through the exercise of common stock purchase warrants that they hold or that they may acquire pursuant to the 2020 Loan Agreement and pursuant to the 2022 Secured Note, and shares that they may acquire through the conversion of those loans into AgeX common stock. AgeX has filed a registration statement on Form S-3, which has become effective under the Securities Act, for offerings on a delayed or continuous basis covering 467,657 shares of AgeX common stock held by Juvenescence and 92,358 shares of AgeX common stock that may be issued upon the exercise of warrants held by Juvenescence. Juvenescence retains the right to require AgeX to register additional shares of common stock that Juvenescence may acquire through the exercise of warrants or the conversion of loans. AgeX is obligated to pay the fees and expenses of each registered offering under such registration rights agreement except for underwriting discounts and commissions. AgeX and Juvenescence will indemnify each other from certain liabilities in connection the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act.
AgeX and Juvenescence have entered into a second Registration Rights Agreement pursuant to which AgeX has agreed to use commercially reasonable efforts to register the for sale under the Securities Act the shares of common stock issuable upon conversion of Preferred Stock. A registration statement must be filed upon request of Juvenescence if Form S-3 is available to AgeX. Juvenescence will also have “piggyback” registration rights if AgeX files a registration statement for the periods presented.
Research and development expenses shown below include allocations from BioTime primarily attributablesale of shares for itself or other stockholders, subject to certain former BioTime general research and development departments contributed to AgeX. Such expenses were primarily comprised of personnel expenses and related expenses, including stock-based compensation, and other outside expenses relevant tocustomary exceptions based on the nature of the research projects being conducted that were contributed toregistration statement. AgeX pursuantwill bear the expenses of the registration statement but not underwriting or broker’s commissions related to the Asset Contribution Agreement discussed above.
Generalsale of the common stock. AgeX and administrative expenses shown below include allocationsJuvenescence will indemnify each other from certain BioTime generalliabilities in connection the registration, offer, and administrative expenses,sale of securities under a registration statement, including allocated stock-based compensation, for carve-out presentation purposesliabilities arising under the Securities Act.
Debt Issuance Costs
In accordance with ASU 2015-03, Simplifying the Presentation of Debt Issuance Costs, all debt issuance costs are recorded as a discount on the debt and amortized to interest expense over the term of the AgeX consolidated statementsapplicable loan agreement using the effective interest method. Direct debt issuance costs include but are not limited to legal fees, debt origination fees, estimated fair market value of operations. These allocations are principally based oncommon stock and warrants issued in connection with the historical generalloan agreement, and administrative functions and expenses relevant to BioTimeNYSE American additional listing fees for the underlying shares of warrants issued with each drawdown of funds.
The following table summarizes the debt issuance costs and the AgeX subsidiaries that operated prior to and after AgeX formation during the periods presented
Management considers the allocation methodologies used to allocate expenses as reasonable and appropriate based on historical BioTime expenses attributable to AgeX and its operations for purposes of the standalone, carve-out financial statements included herein. The expenses reflected in the consolidated financial statements may not be indicative of expenses that will be incurred by AgeX as an independent, publicly traded companyand should not be relied upon as an indicator of AgeX’s future results.
Allocated expenses from BioTime,debt balances net of allocations from AgeX to BioTime, included in the consolidated statements of operations weredebt issuance costs by loan agreement as follows (in thousands):
| Year Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| Research and development | $ | (5 | ) | $ | 1,310 | |||
| General and administrative | 476 | 1,294 | ||||||
| Total allocated expenses from BioTime | $ | 471 | $ | 2,604 | ||||
Shared Facilities and Service Agreement
On August 17, 2017, AgeX and BioTime executed the Shared Facilities Agreement. Under the terms of the Shared Facilities Agreement, BioTime will allow AgeX to use its premises and equipment located at Alameda, California for the purpose of conducting business. BioTime will also provide accounting, billing, bookkeeping, payroll, treasury, payment of accounts payable, and other similar administrative services to AgeX. BioTime may also provide the services of attorneys, accountants, and other professionals who may also provide professional services to BioTime and its other subsidiaries. BioTime may also provide AgeX with the services of its laboratory and research personnel, including BioTime employees and contractors, for the performance of research and development work for AgeX at the premises.
BioTime charges AgeX a “Use Fee” for services received and usage of facilities, equipment, and supplies. For each billing period, BioTime prorates and allocates as a Use Fee costs incurred, as applicable, to AgeX. Such costs generally include: services of BioTime employees, consultants, and contractors; equipment use, insurance, lease expense, fees for services of accountants, lawyers, and other professionals; software; supplies; and utilities. Allocation depends on key cost drivers including actual documented use, square footage of facilities used, time spent, costs incurred by or for AgeX, or upon proportionate usage by BioTime and AgeX, as reasonably estimated by BioTime. BioTime, at its discretion, has the right to charge AgeX a 5% markup on such allocated costs and BioTime has charged this markup since the August 17, 2017 inception of the Shared Facilities Agreement with AgeX. The allocated cost of BioTime employees and contractors who provide services is based upon records maintained of the number of hours or percentage of time of such personnel devoted to the performance of services.
The Use Fee is determined and invoiced to AgeX on a monthly basis for each calendar month of each calendar year. If the Shared Facilities Agreement terminates prior to the last day of a billing period, the Use Fee will be determined for the number of days in the billing period elapsed prior to the termination of the Shared Facilities Agreement. Each invoice will be payable in full by AgeX within 30 days after receipt. Any invoice, or portion thereof, not paid in full when due will bear interest at the rate of 15% per annum until paid, unless the failure to make a payment is due to any inaction or delay in making a payment by BioTime employees from AgeX funds available for such purpose, rather than from the unavailability of sufficient funds legally available for payment or from an act, omission, or delay by any employee or agent of AgeX. To date BioTime has not charged AgeX any interest.
In addition to the Use Fees, AgeX will reimburse BioTime for any out of pocket costs incurred by BioTime for the purchase of office supplies, laboratory supplies, and other goods and materials and services for the account or use of AgeX, provided that invoices documenting such costs are delivered to AgeX with each invoice for the Use Fee. Furthermore, BioTime will have no obligation to purchase or acquire any office supplies or other goods and materials or any services for AgeX, and if any such supplies, goods, materials or services are obtained for AgeX, BioTime may arrange for the suppliers thereof to invoice AgeX directly.
The Shared Facilities Agreement will remain in effect from year to year, unless either party gives the other party written six months’ notice to terminate, which BioTime may not give to AgeX prior to September 1, 2020, or unless the agreement is otherwise terminated under another provision of the agreement.
In aggregate, BioTime charged such Use Fees to AgeX and subsidiaries as follows(in thousands):
| Year Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| Research and development | $ | 1,278 | $ | 1,065 | ||||
| General and administrative | 400 | 615 | ||||||
| Total Use Fees | $ | 1,678 | $ | 1,680 | ||||
As of December 31, 2018,2023 (in thousands):
Schedule of Debt Issuance Costs and 2017, AgeX had $34,000 and $210,000, respectively, in related party payables to BioTime, included in current liabilities on the consolidated balance sheets.Debt Balances
Drawdown of Funds | Origination Fee | Debt Exchanged for Preferred Stock | Total Debt | Debt Issuance Costs | Amortization of Debt Issuance Costs | Total Debt, Net | ||||||||||||||||||||||
| Current | ||||||||||||||||||||||||||||
| 2020 Loan Agreement | $ | 8,000 | $ | - | $ | (8,000 | ) | $ | - | $ | (2,806 | ) | $ | 2,806 | $ | - | ||||||||||||
| 2022 Secured Note | 20,160 | 1,590 | (17,993 | ) | 3,757 | (6,197 | ) | 6,112 | 3,672 | |||||||||||||||||||
| Total current, net | 28,160 | 1,590 | (25,993 | ) | 3,757 | (9,003 | ) | 8,918 | 3,672 | |||||||||||||||||||
| Non-current | ||||||||||||||||||||||||||||
| 2023 Secured Note | 10,000 | 700 | (10,007 | ) | 693 | (666 | ) | 666 | 693 | |||||||||||||||||||
| Total debt, net | $ | 38,160 | $ | 2,290 | $ | (36,000 | ) | $ | 4,450 | $ | (9,669 | ) | $ | 9,584 | $ | 4,365 | ||||||||||||
| Total debt, net | $ | 38,160 | $ | 2,290 | $ | (36,000 | ) | $ | 4,450 | $ | (9,669 | ) | $ | 9,584 | $ | 4,365 | ||||||||||||
Related Party Payables, net
Transactions with Juvenescence
SinceFrom October 2018 through December 2023, AgeX’s Chief Operating Officer, (“COO”), who iswas also an employee of Juvenescence, iswas devoting a majority of his time to AgeX’s operations for whichoperations. AgeX reimburses Juvenescence for his services on an agreed uponagreed-upon fixed annual amount of $272,000.approximately $280,000. AgeX reimburses Juvenescence for services provided by other Juvenescence employees on a work order basis under a shared services agreement effective January 1, 2023. As of December 31, 2018,2023 and 2022, AgeX had approximately $48,000$66,000 and $141,000 payable, respectively, to Juvenescence for COO services rendered included in related party payables, net, on the consolidated balance sheets.
Transactions with Ascendance.Indemnification Agreements
On March 21, 2018,13, 2023, AgeX executed that certain Letter of Indemnification in Lieu of or Supplemental to a Medallion Signature Guarantee (“Letter of Indemnification”), pursuant to which AgeX agreed to indemnify American Stock Transfer & Trust Company, LLC and its affiliates, successors and assigns (the “AST Indemnity”) from and against any and all claims, damages, liabilities or losses arising out of the transfer of all of the AgeX common stock held by Juvenescence to its wholly-owned subsidiary, Juvenescence US Corp. (the “Share Transfer”). In connection with AgeX’s execution of the Letter of Indemnification, AgeX and AscendanceJuvenescence entered into an Asset Purchasethat certain Transfer of Shares of AgeX Therapeutics, Inc. Common Stock – Indemnification Agreement, pursuant to which Juvenescence agreed to indemnify AgeX against any and all claims, damages, liabilities or losses arising out of the Share Transfer or AST Indemnity.
On December 21, 2023, AgeX executed that certain Letter of Indemnification in Lieu of or Supplemental to a Medallion Signature Guarantee (the “Asset Agreement”“ETC Letter of Indemnification”) in, pursuant to which AgeX purchased for $800,000 in cashagreed to indemnify Equiniti Trust Company LLC and its affiliates, successors and assigns (the “ETC Indemnity”) from and against any and all claims, damages, liabilities or losses arising out of the transfer 467,657 shares of AgeX common stock held by Juvenescence US Corp. to JuvVentures (the “JUV US Share Transfer”). In connection with AgeX’s execution of the ETC Letter of Indemnification, AgeX, Juvenescence, the ultimate parent company of Juvenescence US Corp. and JuvVentures, entered into that certain assets consisting in value primarilyTransfer of in-process researchShares of AgeX Therapeutics, Inc. Common Stock – Indemnification Agreement, pursuant to which Juvenescence agreed to indemnify AgeX against any and development assets related to stem cell derived cardiomyocytes (heart muscle cells) to be developedall claims, damages, liabilities or losses arising out of the JUV US Share Transfer or ETC Indemnity.
| 93 |
6. Warrant Liability
AgeX determines the accounting classification of warrants it issues, as either liability or equity, by AgeX. The transaction was considered an asset acquisition rather than a business combinationfirst assessing whether the warrants meet liability classification in accordance with ASC 805-50.480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity, then in accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock. Under ASC 480, Distinguishing Liabilities from Equity, warrants are considered liability classified if the warrants are mandatorily redeemable, obligate AgeX to settle the warrants or the underlying shares by paying cash or other assets, or warrants that must or may require settlement by issuing a variable number of shares. If warrants do not meet liability classification under ASC 480-10, AgeX assesses the requirements under ASC 815-40, which states that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the warrants do not require liability classification under ASC 815-40, and in order to conclude equity classification, AgeX also assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable U.S. GAAP.
As a condition of each amount drawn up to $15,160,000 from the 2022 Secured Note, on receipt of each amount drawn AgeX granted to Juvenescence a number of warrants equal to 50% of the gross value of the relevant advance made. The $800,000 purchasegross value is the quotient of the drawdown amount and the exercise price. The exercise price was expensedbased on the acquisitionmarket closing price of AgeX’s common stock on the NYSE American on the one day preceding the delivery of the relevant drawdown notice. See Note 5, Related Party Transactions.
AgeX has utilized the full credit available under the 2022 Secured Note that is subject to warrants and accordingly the warrants were issued for each of the advances of loan funds under the 2022 Secured Note. After all relevant assessments, AgeX determined that the warrants issued under the 2022 Secured Note require classification as a liability pursuant to ASC 480, Distinguishing Liabilities from Equity. In accordance with the accounting guidance, for each reporting period prior to the full drawdown of the entire $15,160,000 of the 2022 Secured Note line of credit subject to warrants, the amount of warrant liability was determined and recognized on the balance sheet for the applicable reporting period based on the number of warrants that would have been issued if $15,160,000 of the 2022 Secured Note line of credit was drawn. The amount of warrant liability attributed to the expected future issuance of warrants upon subsequent loan draws was subsequently adjusted for the fair value of warrants actually issued upon each loan draw, and the number of warrants that could be issued for the remaining credit available was re-measured for the applicable reporting period with changes being recorded as a component of net other expense in the consolidated statements of operations.
Under the Third Amendment, AgeX is not obligated to issue additional warrants to Juvenescence in connection with the receipt of loan funds up to $4 million made available pursuant to the Second Amendment. See Note 5, Related Party Transactions, for further details of the Second Amendment and the Third Amendment.
The fair value of the warrant liabilities was measured using a Black-Scholes option pricing model. Significant inputs into the model at the inception date, the date when warrants were issued upon receipt of amounts drawn during the period, and as acquired in-process researchof the reporting period end remeasurement dates are as follows:
Schedule of Warrants Liabilities And Stock Option Awards using a Black-Scholes Option-Pricing Model
| Black-Scholes Assumptions | Exercise Price (1) | Warrant Expiration Date (2) | Stock Price (3) | Interest Rate (annual) (4) | Volatility (annual) (5) | Time to Maturity (Years) | Calculated Fair Value per Share | |||||||||||||||||||
| Inception Date: 2/14/2022 | $ | 27.43 | $ | 24.32 | 1.80 | % | 122.99 | % | $ | 17.11 | ||||||||||||||||
| Issuance Date: 2/14/2022 | $ | 27.43 | $ | 24.32 | 1.80 | % | 122.99 | % | $ | 17.11 | ||||||||||||||||
| Issuance Date: 2/15/2022 | $ | 27.43 | $ | 26.28 | 1.80 | % | 123.28 | % | $ | 18.81 | ||||||||||||||||
| Period Ended 3/31/2022 | $ | 33.06 | $ | 30.04 | 2.45 | % | 123.28 | % | $ | 21.36 | ||||||||||||||||
| Issuance Date: 4/4/2022 | $ | 30.94 | $ | 28.79 | 2.61 | % | 123.31 | % | $ | 20.59 | ||||||||||||||||
| Issuance Date: 6/6/2022 | $ | 25.01 | $ | 28.14 | 2.94 | % | 122.62 | % | $ | 20.84 | ||||||||||||||||
| Period Ended 6/30/2022 | $ | 21.10 | $ | 20.27 | 2.99 | % | 122.21 | % | $ | 14.53 | ||||||||||||||||
| Issuance Date: 8/16/2022 | $ | 23.56 | $ | 22.51 | 3.19 | % | 121.37 | % | $ | 16.07 | ||||||||||||||||
| Period Ended 9/30/2022 | $ | 21.45 | $ | 19.75 | 4.25 | % | 121.49 | % | $ | 14.09 | ||||||||||||||||
| Issuance Date: 10/21/2022 | $ | 24.27 | $ | 21.81 | 4.52 | % | 120.51 | % | $ | 15.42 | ||||||||||||||||
| Issuance Date: 12/14/2022 | $ | 20.75 | $ | 18.99 | 3.94 | % | 120.01 | % | $ | 13.40 | ||||||||||||||||
| Period Ended 12/31/2022 | $ | 19.34 | $ | 19.41 | 4.22 | % | 119.31 | % | $ | 13.93 | ||||||||||||||||
| Issuance Date: 1/25/2023 | $ | 25.85 | $ | 26.42 | 3.84 | % | 119.17 | % | $ | 18.98 | ||||||||||||||||
| Inception Date: 2/9/2023 | $ | 24.72 | $ | 23.20 | 4.15 | % | 118.94 | % | $ | 16.38 | ||||||||||||||||
| Issuance Date: 2/15/2023 | $ | 21.93 | $ | 21.10 | 4.35 | % | 118.93 | % | $ | 14.99 | ||||||||||||||||
| Period Ended 3/31/2023 | $ | 23.25 | $ | 23.32 | 3.81 | % | 113.43 | % | $ | 16.15 | ||||||||||||||||
| Issuance Date: 4/4/2023 | $ | 23.25 | $ | 23.67 | 3.60 | % | 113.01 | % | $ | 16.39 | ||||||||||||||||
| (1) | Based on the market closing price of AgeX’s common stock on the NYSE American on the day prior to each debt Inception Date, on each presented period ending date, and one day prior to the delivery of the relevant drawdown notice in accordance with terms of the 2022 Secured Note (with such drawdown notice delivery date being shown as the Issuance Date in the table), and as adjusted to reflect the Reverse Stock Split. For this purpose, the date on which the 2022 Secured Note was amended and restated to increase the line of credit by $2,000,000 was treated as a new Inception Date for that portion of the line of credit. |
| (2) | Warrants are exercisable over a three-year period from each Issuance Date. |
| (3) | Based on the market price of AgeX’s common stock on the NYSE American as of each date presented. |
| (4) | Interest rate for U.S. Treasury Bonds, as of each date presented, as published by the U.S. Federal Reserve. |
| (5) | Based on the historical daily volatility of AgeX common stock as of each date presented. |
| 94 |
The warrants outstanding and developmentfair values at each of the respective valuation dates are summarized below.
Schedule of Warrant Outstanding and Fair Values
| Warrant Liability | Credit Line and Draw Amounts (in thousands) | Warrants | Fair Value per Share | Fair Value (in thousands) | ||||||||||||
| Fair value as of January 1, 2022 | $ | - | - | $ | - | $ | - | |||||||||
| Fair value at initial measurement date of 2/14/2022 | 13,160 | (1) | 239,860 | (2) | 17,11 | 4,103 | ||||||||||
| Fair value of warrants issued on 2/14/2022 | (7,160 | )(3) | (130,501 | )(4) | 17.11 | (2,232 | ) | |||||||||
| Fair value of warrants issued on 2/15/2022 | (1,000 | )(3) | (18,226 | )(4) | 18.81 | (343 | ) | |||||||||
| Fair value of warrants issued on 4/4/2022 | (1,000 | )(3) | (16,162 | )(4) | 20.59 | (333 | ) | |||||||||
| Fair value of warrants issued on 6/6/2022 | (1,000 | )(3) | (19,995 | )(4) | 20.84 | (417 | ) | |||||||||
| Fair value of warrants issued on 8/16/2022 | (1,000 | )(3) | (21,222 | )(4) | 16.07 | (341 | ) | |||||||||
| Fair value of warrants issued on 10/21/2022 | (500 | )(3) | (10,301 | )(4) | 15.42 | (159 | ) | |||||||||
| Fair value of warrants issued on 12/14/2022 | (1,000 | )(3) | (24,096 | )(4) | 13.40 | (323 | ) | |||||||||
| Change in fair value of warrants | - | - | - | 225 | ||||||||||||
| Fair value as of December 31, 2022 | $ | 500 | (1) | 12,924 | (2) | $ | 13.93 | $ | 180 | |||||||
| Fair value of warrants issued on 1/25/2023 | (500 | )(3) | (9,671 | )(4) | 18.98 | (184 | ) | |||||||||
| Fair value at initial measurement date of 2/9/2023 | 2,000 | (1) | 40,457 | (2) | 16.38 | 663 | ||||||||||
| Fair value of warrants issued on 2/15/2023 | (1,000 | )(3) | (22,801 | )(4) | 14.99 | (342 | ) | |||||||||
| Fair value of warrants issued on 4/4/2023 | (1,000 | )(3) | (21,507 | )(4) | 16.39 | (352 | ) | |||||||||
| Change in fair value of warrants | - | - | - | 35 | ||||||||||||
| Fair value as of December 31, 2023 | $ | - | (1) | - | (2) | $ | - | $ | - | |||||||
| (1) | Amount of credit available under the 2022 Secured Note on date of inception and as of each period end date. For this purpose, the date on which the 2022 Secured Note was amended and restated to increase the line of credit by $2,000,000 was treated as a new Inception Date for that portion of the line of credit. |
| (2) | Number of warrants issuable, as applicable, (a) if the amount of credit available was drawn for measurement as of the applicable inception date, or (b) subsequently for remeasurement as of each period end date. |
| (3) | Amount of drawdown as of the date presented. |
| (4) | Number of warrants issued upon receipt of amounts drawn against the 2022 Secured Note as of the date presented. |
During the years ended December 31, 2023 and 2022, AgeX recorded a loss of $35,000 and $225,000, respectively, on changes in the fair value of warrants.
The warrant liabilities are considered Level 3 liabilities on the fair value hierarchy as the determination of fair value includes various assumptions about future activities and AgeX’s stock prices and historical volatility as inputs. None of the warrants issued have been exercised.
| 95 |
7. Stockholders’ Equity/(Deficit)
Preferred Stock
On July 24, 2023, AgeX issued to Juvenescence shares of a newly authorized Series A Preferred Stock and shares of a newly authorized Series B Preferred Stock in exchange for the cancellation of a total of $36 million of indebtedness consisting of the outstanding principal amount of loans then outstanding under the 2020 Loan Agreement, the 2022 Secured Note, and the 2023 Secured Note, plus the loan origination fees accrued with respect to the 2022 Secured Note and a portion of the loan origination fees accrued pursuant to the 2023 Secured Note. The cancellation of indebtedness in exchange for the Series A Preferred Stock and Series B Preferred Stock (collectively referred to as the “Preferred Stock”) was conducted pursuant to the Exchange Agreement between AgeX and Juvenescence. All of the shares of Preferred Stock were automatically converted into a total of shares of AgeX common stock on February 1, 2024 in accordance withASC 730-10-25(c) the terms of the Preferred Stock. See Note 11, Subsequent Events.
Classification of Preferred Stock
On November 7, 2023, certain terms of the Series A Preferred Stock and Series B Preferred Stock was amended (i) to clarify that certain change of control or disposition of asset transactions would be treated as those assets have no alternative future uses.adeemed liquidation if the applicable transaction is approved by the Board of Directors or stockholders of AgeX, and (ii) to provide that in case of such a deemed liquidation transaction holders of Preferred Stock would receive the same type of consideration as that distributed or paid to holders of AgeX common stock. This amendment permits the classification of the Series A Preferred Stock and Series B Preferred Stock as permanent equity effective November 7, 2023.
DispositionThe redemption rights of ownership interestthe Series A Preferred Stock and Series B Preferred Stock in Ascendancecase of a “deemed liquidation” provide that the holders of AgeX preferred stock would always be entitled to receive the same form of consideration as the holders of AgeX common stock. Accordingly, the preferred stock has been presented in permanent equity section of the consolidated balance sheet.
On March 23, 2018, Ascendance was acquired byThe following is a third party in a merger through which AgeX received approximately $3.2 million in cash for itsdescription of certain terms of the Preferred Stock that were applicable to the shares of AscendancePreferred Stock outstanding as of December 31, 2023. However, all of the Preferred Stock was converted into shares of common stock.stock on February 1, 2024. See Note 11, Subsequent Events.
Dividends – The Preferred Stock is not entitled to receive any payment or distribution of cash or other dividends.
Liquidation Preference – In the event of any voluntary or involuntary liquidation, dissolution or other winding up of the affairs of AgeX, recognizedsubject to the preferences and other rights of any senior stock, before any assets of AgeX shall be distributed to holders of common stock or other junior stock, all of the assets of AgeX available for distribution to stockholders shall be distributed among the holders of Series A Preferred Stock and Series B Preferred Stock and any other “parity stock” that may be issued ranking parri passu with those series of Preferred Stock with respect to liquidation rights, in proportion to the number of shares of Series B Preferred Stock and parity stock held by each such holder as of the record date for the determination of holders of Series A Preferred Stock, Series B Preferred Stock, and parity stock entitled to receive such distribution, until AgeX shall have distributed to the holders of those shares an amount of assets having a $3.2 million gain asvalue equal to the subscription price per share. If the assets of AgeX shall be insufficient to pay in full such amounts, then the entire assets to be distributed to the holders of Series A Preferred Stock, Series B Preferred Stock and parity stock shall be ratably distributed among such holders. The (i) acquisition of AgeX by another entity by means of any transaction or series of transactions (including, without limitation, any reorganization, merger or consolidation) in which the stockholders of AgeX immediately before such transaction or series of transactions do not own a majority of the outstanding stock of the surviving or acquiring corporation upon completion of such transaction or series of transactions or (ii) a sale of its equity method investment in Ascendance, which is included in other income and expenses, net, for the year ended December 31, 2018. At the closeall or substantially all of the merger, $955,000assets of cash that otherwise would have been payableAgeX in a single transaction or series of related transactions, shall be deemed a liquidation. On November 7, 2023, certain terms of the liquidation preference were amended.
Conversion of Preferred Stock into Common Stock – Each share of Preferred Stock shall be convertible into a number of shares of AgeX common stock determined by dividing (x) a number equal to the Ascendancenumber of dollars and cents comprising the subscription price, by (y) a number equal to the number of dollars and cents comprising the conversion price. The subscription price per share of Preferred Stock is $ which was paid through the exchange of indebtedness for shares of Preferred Stock. The conversion price per share of Series A Preferred Stock or Series B Preferred Stock is $0.72 which was the closing price of AgeX common stock on the NYSE American on the last trading day immediately preceding the execution of the Exchange Agreement and prior to adjustment to give effect to the Reverse Stock Split.
Optional Conversion – Preferred Stock shall be convertible into common stock at the election of the holder of shares of Preferred Stock at any time and from time to time subject to the limitations on conversion of Series B Preferred Stock discussed below.
| 96 |
Automatic Conversion – The outstanding shares of Series A Preferred Stock and Series B Preferred Stock shall automatically be converted into common stock without any further act of AgeX or its stockholders was deposited(“Automatic Conversion”) upon the earliest of: (x) the date on which AgeX or a subsidiary shall have consummated a merger with Serina, or a subsidiary thereof; and (y) February 1, 2024. Further, if the holders of at least a majority of the outstanding shares of Series A Preferred Stock or Series B Preferred Stock approve or consent to the Automatic Conversion of the shares of that series, then the outstanding shares of Series A Preferred Stock or Series B Preferred Stock, as applicable, shall be converted into an escrow account where it maycommon stock upon such approval or consent.
The outstanding shares of Series B Preferred Stock shall automatically be heldconverted into common stock without any further act of AgeX or its stockholders upon the earliest of: (x) the date on which AgeX or a subsidiary shall have consummated a merger with Serina or a subsidiary thereof; and (y) February 1, 2024.
Adjustment of conversion price and subscription price – If AgeX shall (a) declare a dividend or make a distribution on its common stock in shares of common stock, (b) subdivide or reclassify the outstanding common stock into a greater number of shares, or (c) combine or reclassify the outstanding common stock into a smaller number of shares, the conversion price in effect at the time of the record date for such dividend or distribution or the effective date of such subdivision, combination or reclassification shall be proportionately adjusted. If AgeX shall (i) declare a termdividend or make a distribution on a series of up to fifteen months. Funds heldPreferred Stock in shares of Preferred Stock, (ii) subdivide or reclassify the outstanding shares of a series of Preferred Stock into a greater number of shares, or (iii) combine or reclassify the outstanding shares of a series of Preferred Stock into a smaller number of shares, the subscription price in effect at the time of the record date for such dividend or distribution or the effective date of such subdivision, combination or reclassification shall be proportionately adjusted. Successive adjustments in the escrow account mayconversion price or subscription price, as applicable, shall be paidmade whenever any event specified above shall occur.
No Fractional Shares – No fractional share of common stock or scrip shall be issued upon conversion of Preferred Stock. Instead of any fractional share of common stock which would otherwise be issuable upon conversion of any Preferred Stock, AgeX will pay a cash adjustment in respect of such fractional interest in an amount equal to that fractional interest at the then fair value determined in accordance with the terms of the Preferred Stock.
Voting Rights – The following matters shall require the approval of the holders of a majority of the shares of a series of Preferred Stock then outstanding, voting as a separate class: (i) creation of any Preferred Stock ranking as senior stock to the acquirerseries with respect to cover indemnity payments andliquidation preferences; (ii) repurchase of any shares of common stock or other obligations that may arise afterjunior stock except shares issued pursuant to or in connection with a compensation or incentive plan or agreement approved by the merger. After the expiration of the term of the escrow, any funds remaining in the escrow account will be disbursed, on a pro-rata basis, to the former Ascendance stockholders. As of December 31, 2018, no amounts have been recorded in the AgeX consolidated financial statements for any funds held in the escrow account.
Sale of warrants by AgeX
In February 2018, AgeX sold Warrants to certain investors, including toAlfred D. Kingsley, AgeX’s then Executive Chairman and the Chairman of BioTime’s Board of Directors (see Notes 5for any officers, directors, employees or consultants of AgeX; (iii) any sale, conveyance, or other disposition of all or substantially all AgeX’s property or business, or any liquidation or dissolution of AgeX, or a merger into or consolidation with any other corporation (other than a wholly-owned subsidiary corporation) but only to the extent that the Delaware General Corporation Law requires that such transaction be approved by each class or series of Preferred Stock; (iv) any adverse change in the powers, preferences and 9).
5. Stockholders’ Equity
rights of, and the qualifications, limitations or restrictions on, the series of Preferred Stock; or (v) any amendment of AgeX’s Certificate of Incorporation or Bylaws that results in any adverse change in the powers, preferences and rights of, and the qualifications, limitations or restrictions on, the series of Preferred Stock. However, the terms of the Preferred Stock do not restrict or limit the rights and powers of the Board of Directors to fix by resolution the rights, preferences, and privileges of, and restrictions and limitations on, stock ranking as parity stock or junior stock to a series of Preferred Stock. Except as may otherwise be required by the Delaware General Corporation Law, as the same may be amended from time to time, the Preferred Stock will have no other voting rights.
Governing Law – The powers, designations, preferences, rights, qualifications, limitations, and restrictions of either series of Preferred Stock, the validity, authorization and issuance of such Preferred Stock, and the conversion of such Preferred Stock into common stock shall be governed by and construed and enforced in accordance with the internal laws of the State of Delaware, without regard to the principles of conflict of laws thereof, and all legal proceedings pursuant or with respect to or concerning such matters (a “Proceeding”), whether brought by or against a holder of Preferred Stock or AgeX is authorizedor any of their respective directors, officers, stockholders, employees or agents, shall be commenced in the state and federal courts sitting in the State of Delaware (the “Delaware Courts”). The Preferred Stock provides that (a) AgeX and each holder of Preferred Stock irrevocably submits to issue upthe exclusive jurisdiction of the Delaware Courts for the adjudication of any Proceeding, and irrevocably waives, and agrees not to 5,000,000 sharesassert in any Proceeding any claim that they are not personally subject to the jurisdiction of $0.0001 par value preferred stock. To date, no preferred sharessuch Delaware Courts, or such Delaware Courts are issuedan improper or inconvenient venue for such Proceeding, and outstanding.(b) AgeX and each holder of Preferred Stock irrevocably waives personal service of process and consents to process being served in any such Proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to such party and agrees that such service shall constitute good and sufficient service of process and notice.
Common Stock
AgeX has up to 100,000,000 shares of $0.0001common stock, $ par value common stockper share, authorized. The holders of AgeX’s common stock are entitled to receive ratably dividends when, as, and if declared by the Board of Directors out of funds legally available. Upon liquidation, dissolution, or winding up, the holders of AgeX common stock are entitled to receive ratably the net assets available after the payment of all debts and other liabilities and subject to the prior rights of AgeX outstanding preferred shares, if any.
The holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of AgeX stockholders. The holders of common stock have no preemptive, subscription, or redemption rights. The outstanding shares of common stock are fully paid and non-assessable.
On August 17, 2017, AgeX received its initial assets and cash from BioTime and certain investors. BioTime contributed certain assets and cash to AgeX in exchange for 28,800,000 shares of AgeX common stock pursuant to the Asset Contribution Agreement discussed in Note 4. As discussed in Note 2, these 28,800,000 shares of AgeX common stock have been reflected as outstanding as of the earliest reporting period presented. Concurrently with the acquisition of assets from BioTime under the Asset Contribution Agreement, AgeX sold 4,950,000 shares of its common stock for $10.0 million in cash primarily to investors other than BioTime (see Note 4).
The AgeX shares were offered and sold without registration under the Securities Act of 1933, as amended (the “Securities Act”), in reliance on exemptions from registration under Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D and Regulation S thereunder. AgeX has agreed to use commercially reasonable efforts to register the shares of AgeX common stock issued to the AgeX investors for sale under the Securities Act.
See Note 4 for Related Party Transactions with BioTime that impacted AgeX’s consolidated statements of stockholders’ equity for the years ended December 31, 2018 and 2017.
On June 7, 2018, AgeX sold 2.0 million shares of common stock for $2.50 per share to Juvenescence for aggregate cash proceeds to AgeX of $5.0 million.
On August 13, 2018, AgeX issued 80,000 shares with an approximate value of $240,000 as part of the consideration paid to Escape for the asset acquisition discussed in Note 3.
As of December 31, 20182023 and December 31, 2017,2022, there were 35,830,000 (see Note 9) and 33,750,000 shares of AgeX common stock issued and outstanding, respectively.
| 97 |
Issuance and Sale of Warrants by AgeX
On February 28, 2018,In connection with the $2,500,000 of drawdowns of loan funds from Juvenescence under the 2022 Secured Note during the year ended December 31, 2023, AgeX soldissued to Juvenescence 2022 Warrants to purchase 1,473,600 shares of AgeX common stock for $0.50 per Warrant for aggregate cash proceeds to AgeX of $736,800, which included $124,300 from Alfred D. Kingsley, AgeX’s then Executive Chairman and the Chairman of BioTime’s Board of Directors. On July 10, 2018, AgeX sold additional Warrants to purchase 526,400 shares of common stock for $0.50 per warrant for aggregate net cash proceeds to AgeX of $263,200. The Warrants were exercisable at $2.50 per share. See Note 9 concerning the exercise and expiration of the Warrants. The Warrants were classified as equity since, among other factors, they were not redeemable, could not be settled in cash or other assets and required settlement by issuing a fixed number of53,980 shares of AgeX common stock. The Warrants were sold at fair value determined on the Binomial Lattice option pricing model on the issuance date, with certain management assumptions, which included the timing of an initial public offering of AgeX common stock, peer-group volatility, term to maturity, price cap and AgeX current and future stock prices.See Note 6, Warrant Liability.
6. Stock-based Compensation
Equity Incentive Plan
Under the 2017 Equity Incentive Plan, as amended (the “Plan”“Incentive Plan”), AgeX has reserved 4,000,000 shares of common stock for the grant of stock options or the sale of restricted stock (“Restricted Stock”) or for the settlement of restricted stock units which are hypothetical units issued with reference to common stock (“Restricted Stock Units” or “RSUs”). AgeX may also grant stock appreciation rights (“SARs”) under the Incentive Plan. The Plan also permits AgeX to issue such other securities as its Board of Directors (the “Board”) or the Compensation Committee (the “Committee”) administering the Incentive Plan may determine. Awards of stock options, Restricted Stock, SARs, and Restricted Stock UnitsRSUs (“Awards”) may be granted under the Incentive Plan to AgeX employees, directors, and consultants.
Awards may vest and thereby become exercisable or have restrictions on forfeiture lapse on the date of grant or in periodic installments or upon the attainment of performance goals, or upon the occurrence of specified events.
No Awards may be granted under the Incentive Plan more than ten years after the date upon which the Incentive Plan was adopted by the Board, and no options or SARS granted under the Incentive Plan may be exercised after the expiration of ten years from the date of grant.
Stock Options
Options granted under the Incentive Plan may be either “incentive stock options” within the meaning of Section 422(b) of the Internal Revenue Code of 1986, as amended (the(“IRC” or the “Code”), or “non-qualified” stock options that do not qualify incentive stock options. Incentive stock options may be granted only to AgeX employees and employees of subsidiaries. The exercise price of stock options granted under the Incentive Plan must be equal to the fair market of AgeX common stock on the date the option is granted. The aggregate fair market value of common stock (determined as of the grant date of the option) with respect to which incentive stock options become exercisable for the first time by an optionee in any calendar year may not exceed $100,000.$.
The exercise price of an option may be payable in cash or in common stock having a fair market value equal to the exercise price, or in a combination of cash and common stock, or other legal consideration for the issuance of stock as the Board or Committee may approve.
Generally, options will be exercisable only while the optionee remains an employee, director or consultant, or during a specific period thereafter, but in the case of the termination of an employee, director, or consultant’s services due to death or disability, the period for exercising a vested option shall be extended to the earlier of 12 months after termination or the expiration date of the option.
Restricted Stock and Restricted Stock UnitsRSUs
In lieu of granting options, AgeX may enter into purchase agreements with employees under which Employees or consultants, but not executive officers or directors, who purchase Restricted Stock may be permitted to pay for their shares by delivering a promissory note or an installment payment agreement that may be secured by a pledge of their Restricted Stock. Restricted Stock may also be issued for services actually performed by the recipient prior to the issuance of the Restricted Stock. Unvested Restricted Stock for which AgeX has not received payment may be forfeited, or AgeX may have the right to repurchase unvested shares upon the occurrence of specified events, such as termination of employment.
| 98 |
Subject to the restrictions set with respect to the particular Award, a recipient of Restricted Stock generally shall have the rights and privileges of a stockholder, including the right to vote the Restricted Stock and the right to receive dividends; provided that, any cash dividends and stock dividends with respect to the Restricted Stock shall be withheld for the recipient’s account, and interest may be credited on the amount of the cash dividends withheld. The cash dividends or stock dividends so withheld and attributable to any particular share of Restricted Stock (and earnings thereon, if applicable) shall be distributed to the recipient in cash or, at the discretion of the Board or Committee, in shares of common stock having a fair market value equal to the amount of such dividends, if applicable, upon the release of restrictions on the Restricted Stock and, if the Restricted Stock is forfeited, the recipient shall have no right to the dividends.
The terms and conditions of a grant of Restricted Stock UnitsRSUs shall be determined by the Board or Committee. No shares of common stock shall be issued at the time a Restricted Stock UnitRSU is granted. A recipient of Restricted Stock UnitsRSUs shall have no voting rights with respect to the Restricted Stock Units.RSUs. Upon the expiration of the restrictions applicable to a Restricted Stock Unit,RSU, AgeX will either issue to the recipient, without charge, one share of common stock per Restricted Stock UnitRSU or cash in an amount equal to the fair market value of one share of common stock.
At the discretion of the Board or Committee, each Restricted Stock UnitRSU (representing one share of common stock) may be credited with cash and stock dividends paid in respect of one share (“Dividend Equivalents”). Dividend Equivalents shall be withheld for the recipient’s account, and interest may be credited on the amount of cash Dividend Equivalents withheld. Dividend Equivalents credited to a recipient’s account and attributable to any particular Restricted Stock UnitRSU (and earnings thereon, if applicable) shall be distributed in cash or in shares of common stock having a fair market value equal to the amount of the Dividend Equivalents and earnings, if applicable, upon settlement of the Restricted Stock Unit.RSU. If a Restricted Stock UnitRSU is forfeited, the recipient shall have no right to the related Dividend Equivalents.
SARsStock Appreciation Rights (“SARs”)
AnA SAR is the right to receive, upon exercise, an amount payable in cash or shares, or a combination of shares and cash, equal to the number of shares subject to the SAR that is being exercised, multiplied by the excess of (a) the fair market value of a common sharestock on the date the SAR is exercised, over (b) the exercise price specified in the SAR Award agreement. SARs may be granted either as free standing SARs or in tandem with options. No SAR may be exercised later than years after the date of grant.
The exercise price of a SAR shall not be less than % of the fair market value of one share of common stock on the date of grant. A SAR granted in conjunction with an option shall have the same exercise price as the related option, shall be transferable only upon the same terms and conditions as the related option, and shall be exercisable only to the same extent as the related option; provided, however, that the SAR by its terms shall be exercisable only when the fair market value per share exceeds the exercise price per share of the SAR or related option. Upon any exercise of a SAR granted in tandem with an option, the number of shares for which the related option shall be exercisable shall be reduced by the number of shares for which the SAR has been exercised. The number of shares for which a SAR issued in tandem with an option shall be exercisable shall be reduced by the number of shares for which the related option has been exercised.
Equity Incentive Plan Awards
Summary of Stock Option Activity
Shares Available for Grant | Number of Options Outstanding | Number of RSUs Outstanding | Weighted- Average Exercise Price | |||||||||||||
| Balance at January 1, 2022 | 29 | 96 | - | $ | 81.62 | |||||||||||
| Increase option pool | 114 | - | - | - | ||||||||||||
| Options granted | (3 | ) | 3 | - | 27.76 | |||||||||||
| Options forfeited, cancelled or expired | 6 | (6 | ) | - | 99.12 | |||||||||||
| Restricted stock units vested | - | - | - | - | ||||||||||||
| Balance at December 31, 2022 | 146 | 93 | - | $ | 79.07 | |||||||||||
| Options granted | (1 | ) | 1 | - | 26.73 | |||||||||||
| Options forfeited, cancelled or expired | 11 | (11 | ) | - | 67.30 | |||||||||||
| Restricted stock units vested | - | - | - | - | ||||||||||||
| Balance at December 31, 2023 | 156 | 83 | - | $ | 80.28 | |||||||||||
| Options exercisable at December 31, 2023 | 80 | $ | 81.70 | |||||||||||||
There were exercises of stock options during the years ended December 31, 2023 and 2022. Total proceeds, if all options granted and outstanding as of December 31, 2023 were exercised, would be approximately $6.6 million.
| 99 |
At December 31, 2023, AgeX had approximately $ of total unrecognized compensation costs related to the stock options and unvested RSUs under the Incentive Plan that will be recognized as expense over a weighted-average period of approximately years.
The aggregate intrinsic value of options outstanding and options exercisable were nil as of December 31, 2023.
Stock-Based Compensation Expense
Schedule of Stock Based Compensation Expense
| 2023 | 2022 | |||||||
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Research and development | $ | 9 | $ | 32 | ||||
| General and administrative | 639 | 728 | ||||||
| Total stock-based compensation expense | $ | 648 | $ | 760 | ||||
The weighted-average estimated fair value of stock options granted during the years ended December 31, 2023 and 2022 was $ per share and $ per share, respectively, using the Black-Scholes option pricing model with the following weighted-average assumptions:
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Grant Price | $ | 26.73 | $ | 27.76 | ||||
| Expected life (in years) | ||||||||
| Risk-free interest rates | % | % | ||||||
| Volatility | % | % | ||||||
| Dividend yield | - | % | - | % | ||||
The determination of stock-based compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If AgeX had made different assumptions, its stock-based compensation expense and net loss for the years ended December 31, 2023 and 2022 may have been significantly different. See Note 2, Summary of Significant Accounting Policies, for a discussion of the factors used in determining these assumptions.
AgeX does not recognize deferred income taxes for incentive stock option compensation expense and records a tax deduction only when a disqualified disposition has occurred.
9. Income Taxes
Net loss from operations before income taxes for the years ended December 31, 2023 and 2022 was approximately $14.8 million and $10.5 million, respectively.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
The primary components of the net deferred tax assets and liabilities as of December 31, 2023 and 2022 were as follows (in thousands):
Schedule of Components of Deferred Tax Assets and Liabilities
| December 31, | ||||||||
| Deferred tax assets/(liabilities): | 2023 | 2022 | ||||||
| Net operating loss carryforwards | $ | 14,278 | $ | 12,408 | ||||
| Capital loss carryforwards | 3,120 | 3,120 | ||||||
| Research and development credit carryforwards | 1,178 | 1,138 | ||||||
| Patents and fixed assets | 975 | 879 | ||||||
| Stock-based compensation | 676 | 643 | ||||||
| Capitalized research expenses | 414 | 211 | ||||||
| Other, net | 175 | 62 | ||||||
| Valuation allowance | (20,816) | (18,461 | ) | |||||
| Total net deferred tax assets | $ | - | $ | - | ||||
A valuation allowance is provided when it is more likely than not that all or some portion of the deferred tax assets will not be realized. AgeX established a full valuation allowance for all periods presented due to the uncertainty of realizing future tax benefits from its net operating loss (“NOL”) carryforwards and other deferred tax assets.
| 100 |
Income taxes differed from the amounts computed by applying the U.S. federal income tax rate indicated to pretax losses from operations as a result of the following:
Schedule of Income Tax Rate Reconciliation
| 2023 | 2022 | |||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Computed tax benefit at federal statutory rate | 21 | % | 21 | % | ||||
| Research and development and other credits | - | % | 1 | % | ||||
| State tax benefit, net of effect on federal income taxes | 4 | % | (7 | )% | ||||
| Permanent differences | (4 | )% | - | % | ||||
| Stock-based compensation | (1 | )% | (2 | )% | ||||
| Debt finance equity costs | (5 | )% | (6 | )% | ||||
| Return to provision and other adjustments | - | % | (5 | )% | ||||
| Change in valuation allowance | (15 | )% | (2 | )% | ||||
| Income tax rate | - | % | - | % | ||||
AgeX has established an accrual for uncertain tax positions related to its U.S. research and development credits. As of December 31, 2023 and 2022, there was no accrued interest related to uncertain tax positions. AgeX does not believe it is reasonably possible that its unrecognized tax benefits will significantly change in the next twelve months. A reconciliation of beginning and ending balances for unrecognized tax benefits is as follows (in thousands):
Schedule of Unrecognized Tax Benefits
| 2023 | 2022 | |||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Balance at January 1 | $ | 379 | $ | - | ||||
| Additions for tax positions related to the current year | 14 | 23 | ||||||
| Additions for tax positions related to prior years | - | 356 | ||||||
| Reductions for tax positions related to prior years | - | - | ||||||
| Reductions related to settlements | - | - | ||||||
| Reductions related to a lapse of statute | - | - | ||||||
| Balance at December 31 | $ | 393 | $ | 379 | ||||
AgeX monitors proposed and issued tax law, regulations, and cases to determine the potential impact of uncertain income tax positions. At December 31, 2023, AgeX had not identified any potential subsequent events that would have a material impact on unrecognized income tax benefits within the next twelve months.
As of December 31, 2023, AgeX has net operating loss carryforwards of approximately $59.7 million for U.S. federal income tax purposes. In general, NOLs and other tax credit carryforwards generated by legal entities in a consolidated federal tax group are available to other members of the tax group depending on the nature of the transaction that a member may enter into while still in the consolidated federal tax group.
As of December 31, 2023, AgeX has net operating losses of approximately $19.8 million for California purposes. In general, NOLs and other tax credit carryforwards generated by legal entities in a combined state tax group are available to other members of the tax group depending on the nature of the transaction that a member may enter into while still in the combined state tax group. Federal net operating losses generated on or prior to December 31, 2017, expire in varying amounts between 2028 and 2037, while federal net operating losses generated after December 31, 2017, carryforward indefinitely. The state net operating losses expire in varying amounts between 2028 and 2043.
As of December 31, 2023, AgeX has research and development tax credit carryforwards for federal and state tax purposes $0.7 million and $0.5 million, respectively. The federal tax credits expire between 2028 and 2043, while the state tax credits have no expiration date.
As of December 31, 2023, AgeX has capital loss carryforwards for federal and state tax purposes of $12.4 million and $5.9 million, respectively. The federal and California capital loss carryforwards will expire in 2026.
| 101 |
Effective for tax years beginning after December 31, 2021, taxpayers are required to capitalize any expenses incurred that are considered incidental to research and experimentation (“R&E”) activities under IRC Section 174. While taxpayers historically had the option of deducting these expenses under IRC Section 174, the December 2017 Tax Cuts and Jobs Act mandates capitalization and amortization of R&E expenses for tax years beginning after December 31, 2021. Expenses incurred in connection with R&E activities in the US must be amortized over a 5-year period if incurred, and R&E expenses incurred outside the US must be amortized over a 15-year period. R&E activities are broader in scope than qualified research activities considered under IRC Section 41 (relating to the research tax credit). For the year ended December 31, 2023, the Company performed an analysis based on available guidance and determined that it will continue to be in a loss position even after the required capitalization and amortization of its R&E expenses. We will continue to monitor this issue for future developments, but we do not expect R&E capitalization and amortization to require us to pay cash taxes now or in the near future.
For the year ended December 31, 2023, we experienced a loss; therefore, no income tax provision was recorded for the year ended December 31, 2023.
Other Income Tax Matters
Code Section 382 places a limitation (“Section 382 Limitation”) on the amount of taxable income that can be offset by NOL carryforwards after a change in control (generally greater than 50% change in ownership within a three-year period) of a loss corporation. California has similar rules. Generally, after a control change, a loss corporation cannot deduct NOL carryforwards in excess of the Section 382 Limitation. Due to these “change in ownership” provisions, utilization of the NOL and tax credit carryforwards may be subject to an annual limitation regarding their utilization against taxable income in future periods.
AgeX and its subsidiaries may be subject to potential income tax examination by U.S. federal or states authorities. These potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws. AgeX filed its first consolidated federal tax return in 2018. AgeX and its current subsidiaries are not subject to tax examination by federal tax authorities for tax years beginning before 2020 and for state tax authorities beginning before 2019. However, the tax authorities may still make adjustments to the net operating loss and credit carryforwards used in open years by AgeX or any of its subsidiaries. Any potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws.
10. Commitments and Contingencies
Office Lease Agreement
AgeX leased office space in Alameda, California. For 2022, base monthly rent was $1,074 and for 2023, base monthly rent is $844 for slightly less space at the same building. The lease also includes office furniture rental, janitorial services, utilities, and internet service.
ASC 842
For the office lease, AgeX has elected to not apply the recognition requirements under ASC 842 and instead recognized the lease payments as lease costs on a straight-line basis over the lease term, as the amount of the lease payments is not deemed material.
There were no future minimum lease commitments as of December 31, 2023.
Litigation – General
AgeX is subject to various claims and contingencies in the ordinary course of its business, including those related to litigation, business transactions, employee-related matters, and others. When AgeX is aware of a claim or potential claim, it assesses the likelihood of any loss or exposure. If it is probable that a loss will result and the amount of the loss can be reasonably estimated, AgeX will record a liability for the loss. If the loss is not probable or the amount of the loss cannot be reasonably estimated, AgeX discloses the claim if the likelihood of a potential loss is reasonably possible and the amount involved could be material. AgeX is not aware of any claim for which a liability has not been accrued and which is likely to have a material adverse effect on its financial condition or results of operations.
Tax Filings
AgeX tax filings are subject to audit by taxing authorities in jurisdictions where it conducts business. These audits may result in assessments of additional taxes that are subsequently resolved with the authorities or potentially through the courts. Management believes AgeX has adequately provided for any ultimate amounts that are likely to result from these audits; however, final assessments, if any, could be significantly different than the amounts recorded in the consolidated financial statements.
| 102 |
Employment Contracts
AgeX has entered into employment contracts with certain executive officers. Under the provisions of the contracts, AgeX may be required to incur severance obligations for matters relating to changes in control, as defined, and involuntary terminations.
Indemnifications
In the normal course of business, AgeX may provide indemnifications of varying scope under AgeX’s agreements with other companies or consultants, typically for AgeX’s research and development programs. Pursuant to these agreements, AgeX will generally agree to indemnify, hold harmless, and reimburse the indemnified parties for losses and expenses suffered or incurred by the indemnified parties arising from claims of third parties in connection with AgeX’s research and development. Indemnification provisions could also cover third-party infringement claims with respect to patent rights, copyrights, or other intellectual property licensed from AgeX to third parties. Office and laboratory leases will also generally indemnify the lessor with respect to certain matters that may arise during the term of the lease. A sales agreement between AgeX and Chardan Capital Markets, LLC also included indemnification provisions pursuant to which the parties agreed to indemnify each other from certain liabilities that could arise from the offer and sale of AgeX common stock, including liabilities under the Securities Act. Similarly, the Registration Rights Agreement between Juvenescence and AgeX includes indemnification provisions pursuant to which the parties will indemnify each other from certain liabilities in connection with the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act. AgeX has also agreed to provide the AST Indemnity and ETC Indemnity pursuant to the Letter of Indemnification and ETC Letter of Indemnity described in Note 5, Related Party Transactions. The term of these indemnification obligations will generally continue in effect after the termination or expiration of the particular license, lease, or agreement to which they relate. The potential future payments AgeX could be required to make under these indemnification agreements will generally not be subject to any specified maximum amount. Historically, AgeX has not been subject to any claims or demands for indemnification. AgeX also maintains various liability insurance policies that limit AgeX’s financial exposure and in the case of the AST Indemnity and ETC Indemnity AgeX has received cross-indemnities from Juvenescence against all claims, damages, liabilities or losses arising out of the AST Indemnity and ETC Indemnity. As a result, AgeX believes the fair value of these indemnification agreements is minimal. Accordingly, AgeX has not recorded any liabilities for these agreements as of December 31, 2023 and 2022.
Notice of Delisting
On April 20, 2023, AgeX received a letter (the “2023 Deficiency Letter”) from the staff of the Exchange indicating that AgeX does not meet certain of the Exchange’s continued listing standards as set forth in Sections 1003(a)(i), (ii), and (iii) of the Exchange Company Guide in that AgeX has stockholders equity of less than (A) $2,000,000 and has incurred losses from continuing operations and/or net losses during its two most recent fiscal years, (B) $4,000,000 and has incurred losses from continuing operations and/or net losses during three out of four of its most recent fiscal years, and (C) $6,000,000 or more and has reported losses from continuing operations and/or net losses in its five most recent fiscal years. The 2023 Deficiency Letter states that as AgeX remains subject to the conditions set forth in prior letters from the Exchange with respect to AgeX’s deficiencies in stockholders equity, and if AgeX is not in compliance with all of the Exchange’s stockholders equity standards, or does not make progress consistent with AgeX’s Exchange approved plan to come into compliance with the Exchange’s continued listing standards, by May 17, 2023, the Exchange will initiate delisting proceedings as appropriate.
On May 17, 2023 AgeX received a notice from the staff of the Exchange indicating that they intend to commence proceedings to delist AgeX common stock from the Exchange based upon AgeX’s non-compliance with the stockholders’ equity requirements set forth in Sections 1003(a)(i), (ii) and (iii) of the Exchange’s Company Guide by the end of a compliance plan period that expired on May 17, 2023. Specifically, AgeX did not meet the continued listing standards because it had stockholders equity of less than (A) $2,000,000 and has incurred losses from continuing operations and/or net losses during its two most recent fiscal years, (B) $4,000,000 and has incurred losses from continuing operations and/or net losses during three out of four of its most recent fiscal years, and (C) $6,000,000 or more and has reported losses from continuing operations and/or net losses in its five most recent fiscal years.
On May 24, 2023, AgeX filed a request for a review of the delisting determination by a Committee of the Board of Directors of the Exchange. On May 31, 2023, AgeX received a notice from the staff of the Exchange which scheduled a hearing for July 25, 2023. On July 24, 2023, AgeX issued shares of preferred stock to Juvenescence in exchange for the extinguishment of $36 million of indebtedness owed to Juvenescence for the purpose of remediating the deficiency in stockholders equity, and the hearing at the Exchange scheduled for July 25 was cancelled. See Note 7, Stockholders’ Equity/(Deficit), for further discussion on the classification of preferred stock and amendment to Section 3(b) of the terms of the Series A Preferred Stock and Series B Preferred Stock.
| 103 |
11. Subsequent Events
On February 9, 2024, AgeX and Juvenescence executed a Sixth Amendment to Amended and Restated Convertible Promissory Note (the “Sixth Amendment”) that extends to May 9, 2024 the “Repayment Date” on which the outstanding principal balance and accrued loan origination fees will become due and payable pursuant to the 2022 Secured Note.
From January 1 through March 20, 2024, AgeX drew in the aggregate $5,800,000 of its credit available under the 2022 Secured Note with Juvenescence.
On February 1, 2024, all outstanding shares of Series A Preferred Stock and Series B Preferred Stock automatically converted into a total of shares of AgeX common stock in accordance with their terms and after that conversion no shares of AgeX Preferred Stock remained outstanding. Those shares of common stock issued to Juvenescence increased Juvenescence’s direct and indirect holding of outstanding shares of AgeX common stock to shares, or approximately 75.6% of the shares of common stock outstanding on March 14, 2024, including shares of AgeX common stock held by Juvenescence’s subsidiaries but not taking into account any additional shares of AgeX common stock that Juvenescence may acquire through the conversion of loan balances and the exercise of AgeX common stock purchase warrants or Post-Merger Warrants that were distributed on March 19, 2024.
At the Special Meeting of stockholders on March 14, 2024, AgeX stockholders approved certain matters required for the Merger to be consummated in accordance with the terms of the Merger Agreement. On February 16, 2024, holders of outstanding Serina voting securities approved certain matters required for the Merger to be consummated in accordance with the terms of the Merger Agreement.
On March 14, 2024, AgeX implemented the 1 for 35.17 Reverse Stock Split of its common stock. See Note 1, Organization, Basis of Presentation and Liquidity.
On March 19, 2024, AgeX issued the Post-Merger Warrants to each holder of AgeX common stock as of the dividend record date, March 18, 2024.
| 104 |
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
It is management’s responsibility to establish and maintain adequate internal control over all financial reporting pursuant to Rule 13a-15 under the Exchange Act. Our management, including our principal executive officer and our principal financial officer, have reviewed and evaluated the effectiveness of our disclosure controls and procedures as of the end of our fourth quarter. Following this review and evaluation, management collectively determined that our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act (i) is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms; and (ii) is accumulated and communicated to management, including our chief executive officer and our chief financial officer, as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the fourth quarter of our fiscal year ended December 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f), is a process designed by, or under the supervision of, our principal executive officer, our principal operations officer, and our principal financial officer, and effected by our Board of Directors, management, and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP and includes those policies and procedures that:
| ● | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; | |
| ● | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and | |
| ● | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2023, based on criteria established in the 2013 Internal Control – Integrated Framework issued by COSO. Based on this assessment, management believes that, as of that date, our internal control over financial reporting was effective.
| 105 |
Item 9B. Other Information
(a) Warrant Dividend and Entry into Warrant Agreement
On March 19, 2024, AgeX issued to each stockholder of record as of the close of business on March 18, 2024 (the “Warrant Dividend Record Date”) three warrants (each, a “Post-Merger Warrant”) for each five shares of AgeX common stock issued and outstanding held by a stockholder of record as of the Warrant Dividend Record Date. Each Post-Merger Warrant will be exercisable at an exercise price equal to $13.20 per warrant (such exercise price reflecting the Reverse Stock Split) for (i) one share of AgeX common stock and (ii) one warrant (each, an “Incentive Warrant”) and will expire on July 31, 2025. Each Incentive Warrant will be exercisable at an exercise price equal to $18.00 per warrant (such exercise price reflecting the Reverse Stock Split) for one share of AgeX common stock and will expire on the four-year anniversary of closing of the Merger.
Each Post-Merger Warrant was issued and each Incentive Warrant will be issued pursuant to the terms of the warrant agreement, dated as of March 19, 2024 (the “Warrant Agreement”), by and between AgeX and Equiniti Trust Company, LLC, a New York limited liability company, as warrant agent (the “Warrant Agent”). No fractional warrants were issued. The number of Post-Merger Warrants issued to a stockholder of record were rounded down to the nearest whole number if such holder was entitled to receive a fractional warrant.
Registration Requirements. AgeX agreed to use its commercially reasonable efforts to maintain the effectiveness of the registration statement on Form S-4/S-1 (Registration No. 333-275536), and a current prospectus relating thereto, until the expiration of the warrants in accordance with the provisions of the Warrant Agreement.
In addition, AgeX agreed that as soon as practicable, but in no event later than 30 business days after the closing of the Merger, it will use its best efforts to file with the SEC a registration statement for the registration under the Securities Act of the resale of the shares of AgeX common stock issuable upon the exercise of (i) the Post-Merger Warrants and (ii) the Incentive Warrants. AgeX also agreed to use its best efforts to cause such registration statement to become effective within 60 business days after the closing of the Merger and to use its commercially reasonable efforts to maintain the effectiveness of such registration statement, and a current prospectus relating thereto, until the earliest of the following: (a) when a registration statement covering such shares becomes or has been declared effective by the SEC and such shares have been sold or disposed of pursuant to such effective registration statement; (b) when such shares have been sold or disposed of pursuant to Rule 144 under the Securities Act (or any successor or similar provision adopted by the SEC then in effect) under circumstances in which all of the applicable conditions of Rule 144 (as then in effect) are met; (c) when such shares become eligible for resale without volume or manner-of-sale restrictions pursuant to Rule 144 and without the requirement for AgeX to be in compliance with the current public information requirement under Rule 144, as reasonably determined by counsel to AgeX; (d) when such shares are held by AgeX; or (e) when such shares have been sold or disposed of in a private transaction in which the transferor’s rights under the Warrant Agreement are not assigned to the transferee of such securities.
Warrant Exercise. Each warrant may be exercised by the holder in lawful money of the United States.
Maximum Percentage. A holder of a Post-Merger Warrant or Incentive Warrant will be able to notify AgeX in writing in the event such person elects to be subject to the requirement that such holder will not have the right to exercise such warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates), to the Warrant Agent’s actual knowledge, would beneficially own in excess of 9.999% (or such other amount as a holder may specify) of the shares of AgeX common stock outstanding immediately after giving effect to such exercise.
Stock Dividends. If the number of outstanding shares of AgeX common stock is increased by a stock dividend payable in shares of AgeX common stock to all or substantially all holders of AgeX common stock, or by a split-up of shares of AgeX common stock or other similar event, then, on the effective date of such stock dividend, split-up or similar event, the number of shares of AgeX common stock issuable on exercise of each Post-Merger Warrant and Incentive Warrant will be increased in proportion to such increase in the outstanding shares of AgeX common stock.
Aggregation of Shares. If the number of outstanding shares of AgeX common stock is decreased by a consolidation, combination, reverse stock split or reclassification of shares of AgeX common stock or other similar event, then, on the effective date of such consolidation, combination, reverse stock split, reclassification or similar event, the number of shares of AgeX common stock issuable on exercise of each Post-Merger Warrant or Incentive Warrant will be decreased in proportion to such decrease in outstanding shares of AgeX common stock.
Adjustments in Exercise Price. Whenever the number of shares of AgeX common stock purchasable upon the exercise of a Post-Merger Warrant or Incentive Warrant is adjusted, as described above, the applicable warrant exercise price will be adjusted by multiplying the applicable warrant exercise price immediately prior to such adjustment by a fraction (x) the numerator of which will be the number of shares of AgeX common stock purchasable upon the exercise of such warrant immediately prior to such adjustment, and (y) the denominator of which will be the number of shares of AgeX common stock so purchasable immediately thereafter.
Replacement of Securities upon Reorganization, etc. In case of any reclassification or reorganization of the outstanding shares of AgeX common stock (other than those described under “—Stock Dividends” and “—Aggregation of Shares” above or that solely affects the par value of such shares of AgeX common stock), or in the case of any merger or consolidation of AgeX with or into another corporation (other than a consolidation or merger in which AgeX is the continuing corporation and that does not result in any reclassification or reorganization of the outstanding shares of AgeX common stock), or in the case of any sale or conveyance to another corporation or entity of the assets or other property of AgeX as an entirety or substantially as an entirety in connection with which AgeX is dissolved, a holder of a Post-Merger Warrant or Incentive Warrant will thereafter have the right to purchase and receive, upon the basis and upon the terms and conditions specified in such warrant and in lieu of the shares of AgeX common stock immediately theretofore purchasable and receivable upon the exercise of the rights represented thereby, the kind and amount of shares of stock or other securities or property (including cash) receivable upon such reclassification, reorganization, merger or consolidation, or upon a dissolution following any such sale or transfer, that the holder of such warrant would have received if such holder had exercised such warrant immediately prior to such event. However, if such holder of AgeX common stock was entitled to exercise a right of election as to the kind or amount of securities, cash or other assets receivable upon such consolidation or merger, then the kind and amount of securities, cash or other assets for which each such warrant will become exercisable will be deemed to be the weighted average of the kind and amount received per share by such holder of AgeX common stock in such consolidation or merger that affirmatively make such election, and if a tender, exchange or redemption offer will have been made to and accepted by such holder of AgeX common stock under circumstances in which, upon completion of such tender or exchange offer, the maker thereof, together with members of any group (within the meaning of Rule 13d-5(b)(1) under the Exchange Act) of which such maker is a part, and together with any affiliate or associate of such maker (within the meaning of Rule 12b-2 under the Exchange Act) and any members of any such group of which any such affiliate or associate is a part, own beneficially (within the meaning of Rule 13d-3 under the Exchange Act) more than 50% of the outstanding AgeX common stock, the holder of a Post-Merger Warrant or Incentive Warrant will be entitled to receive the highest amount of cash, securities or other property to which such holder would actually have been entitled as a stockholder if such warrant holder had exercised the Post-Merger Warrant or Incentive Warrant prior to the expiration of such tender or exchange offer, accepted such offer and all of the AgeX common stock held by such holder had been purchased pursuant to such tender or exchange offer, subject to adjustments (from and after the consummation of such tender or exchange offer) as nearly equivalent as possible to the adjustments provided for in the Warrant Agreement. Additionally, if less than 70% of the consideration receivable by the holders of AgeX common stock in such a transaction is payable in the form of AgeX capital stock or shares in the successor entity that is listed for trading on a national securities exchange or is quoted in an established over-the-counter market, or is to be so listed for trading or quoted immediately following such event, and if the registered holder of the Post-Merger Warrant or Incentive Warrant properly exercises such warrant within 30 days following public disclosure of the consummation of such transaction, the applicable exercise price of such warrant will be reduced by an amount (in dollars) equal to the difference of (i) the applicable exercise price in effect prior to such reduction minus (ii) (A) the per share consideration (as defined in the Warrant Agreement) (but in no event less than zero) minus (B) the Black-Scholes Warrant Value (as defined in the Warrant Agreement).
No Fractional Warrants. No fractional warrants will be issued. If the holder of any Post-Merger Warrant would be entitled to receive a fractional Incentive Warrant upon the exercise of a Post-Merger Warrant, AgeX will round down to the nearest whole number the number of Incentive Warrants to be issued to such holder.
No Fractional Shares. Notwithstanding any provision contained in the Warrant Agreement to the contrary, no fractional shares of AgeX common stock will be issued upon the exercise of Post-Merger Warrants or Incentive Warrants. If, by reason of any adjustment under the terms of the Warrant Agreement, the holder of any Post-Merger Warrant or Incentive Warrant would be entitled, upon the exercise of such warrant, to receive a fractional interest in a share, AgeX will, upon such exercise, round down to the nearest whole number the number of shares of AgeX common stock to be issued to such holder.
Other Events. In case any event shall occur affecting AgeX as to which none of the adjustment provisions of the Warrant Agreement are strictly applicable, but which would require an adjustment to the terms of the Post-Merger Warrants or Incentive Warrants in order to (i) avoid an adverse impact on such warrants and (ii) effectuate the intent and purpose of the adjustment provisions of the Warrant Agreement, then, in each such case, AgeX will agree to appoint a firm of independent public accountants, investment banking or other appraisal firm of recognized national standing, which will give its opinion as to whether or not any adjustment to the rights represented by the Post-Merger Warrants or Incentive Warrants is necessary to effectuate the intent and purpose of the adjustment provisions of the Warrant Agreement and, if they determine that an adjustment is necessary, the terms of such adjustment. AgeX will agree to adjust the terms of the Post-Merger Warrants or Incentive Warrants in a manner that is consistent with any adjustment recommended in such opinion.
Transfer of Warrants. The Post-Merger Warrants are and the Incentive Warrants will be non-transferrable, other than through a Permitted Transfer (as defined below). The Post-Merger Warrants are not and the Incentive Warrants will not be listed on any quotation system or traded on any securities exchange. For purposes of the Warrant Agreement, the term “Permitted Transfer” will mean a transfer of a Post-Merger Warrant or Incentive Warrant (a) upon death of the holder by will or intestacy; (b) pursuant to a court order; (c) by operation of law (including by consolidation or merger) or without consideration in connection with the dissolution, liquidation or termination of any corporation, limited liability company, partnership or other entity; (d) in the case of Post-Merger Warrants or Incentive Warrants held by Juvenescence to any affiliate of, or third party nominated by Juvenescence, provided that Juvenescence shall remain responsible for the obligations of Juvenescence under the Side Letter; or (e) in the case of such warrant being held in book-entry or other similar nominee form, from a nominee to a beneficial owner and, if applicable, through an intermediary, to the extent allowable by the depository; provided; however, that the permitted transferee will need to enter into a written agreement with AgeX agreeing to be bound by the transfer restrictions in Warrant Agreement.
The foregoing description of the Post-Merger Warrants, Incentive Warrants and the Warrant Agreement is only a summary and is qualified in its entirety by reference to the full text of the Form of Post-Merger Warrant, Form of Incentive Warrant and the Warrant Agreement, copies of which are attached to this Report as Exhibit 4.4, Exhibit 4.5 and Exhibit 10.49 and each is incorporated herein by reference.
(b) Trading Arrangement Disclosure
During the fourth quarter of the fiscal year ended December 31, 2023, none of AgeX’s officers or directors adopted or terminated a “Rule 10b5–1 trading arrangement” or a “non-Rule 10b5–1 trading arrangement” as such terms are defined in Item 408(a) of SEC Regulation S-K.
(c) 2022 Secured Note Transactions
On March 20, 2024, AgeX drew $900,000 of its credit available under the 2022 Secured Note.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
| 106 |
PART III
Item 10. Directors, Executive Officers, and Corporate Governance
Directors
Our Current Directors
The following table sets forth information regarding our Board of Directors as of March 14, 2024. If the Merger is consummated, the membership of our Board of Directors will change, and we expect that Gregory H. Bailey and Steven Mintz will be the only current AgeX directors who will continue to serve as directors of the Combined Company after the Merger. See “Initial Post-Merger Directors” for information about the persons we expect initially to serve as directors of the Combined Company after consummation of the Merger.
| Committee Membership | ||||||||||
| Name of Director | Age | Director Since | Audit | Compensation | Nominating and Corporate Governance | |||||
| Non-Employee Directors | ||||||||||
| Gregory H. Bailey, M.D. | 68 | August 2018 | Chair | Member | ||||||
| Joanne M. Hackett, Ph.D. | 45 | December 2021 | ||||||||
| Jean-Christophe Renondin, M.D. | 64 | August 2023 | Member | Member | Chair | |||||
| Steven Mintz | 57 | January 2024 | Chair | Member | Member | |||||
Gregory H. Bailey, M.D. joined our Board of Directors in August 2018 and served as the Chairman of our Board of Directors from October 2018 until May 2022. Dr. Bailey is currently Executive Chairman of Juvenescence Limited. From October 2017 until January 2023 Dr. Bailey served as the Chief Executive Officer of Juvenescence Limited, a privately held company focused on the development of therapies for ageing and age-related diseases. Dr. Bailey is also a director of Manx Financial Group, plc, BioHaven Inc, SalvaRx Inc and Portage Biotech. Dr. Bailey has founded and served as a director of a number of private and public companies and previously served as a managing partner of Palantir Group, Inc., a merchant bank involved in a number of biotech company startups and financings. Dr. Bailey practiced emergency medicine for ten years before entering finance. Dr. Bailey received his M.D. from the University of Western Ontario. We believe that Dr. Bailey is qualified to serve on our Board based on his years of experience in medicine and as an executive and in finance for the biotechnology industry.
Joanne M. Hackett, Ph.D. joined our Board of Directors in December 2021 and became the Chairperson of our Board of Directors in May 2022, and Interim Chief Executive Officer in August 2023. Dr. Hackett is currently the Head of Genomic and Precision Medicine at IQVIA. IQVIA is a world leader in using data, technology, advanced analytics, and expertise to help customers drive healthcare forward. From 2017 to 2020 Dr. Hackett served as Chief Commercial Officer of Genomics England, where she engaged industry, academia and the clinical community to achieve the goal of sequencing genomes of patients and families of patients with rare diseases, and patients with common cancers. Genomics England is owned by the Department of Health and Social Care in the United Kingdom. During 2016 and 2017 Dr. Hackett served as Chief Commercial Officer and Interim Chief Executive Officer of Precision Medicine Catapult, which was established in the United Kingdom with the goal of developing, delivering and commercializing precision medicine. Dr. Hackett served as Director of Commercial Development for UCLPartners in London, England from 2013 – 2016. UCLPartners is focused on co-creating, testing and implementing innovative healthcare solutions with its academic and healthcare partners, and fostering the wider spread and adoption of those solutions. Previously, she served as Chief Operating Officer and Research Lead at Cambridge University Health Partners, and she has held other positions in the biomedical industry and in academia, including as a research scientist, and she has served on a number of advisory committees and advisory boards in the biomedical and healthcare fields. Dr. Hackett holds a PhD in Molecular Genetics from the University of New Brunswick. Dr. Hackett’s years of Ie in genomics and regenerative medicine with a focus on commercialization of new therapies and technologies makes her an excellent candidate to serve on our Board of Directors and as Interim Chief Executive Officer.
Jean-Christophe Renondin, M.D. joined our Board of Directors during August 2023. Dr. Renondin is Managing Partner at Vesalius Biocapital, a venture capital firm. From 2015 to 2022, Dr. Renondin served as Senior Healthcare Manager at the Sovereign Fund of Oman where he implemented investment strategy and pursued investment opportunities in North America, Europe and Asia. Dr. Renondin has served in management roles at a number of healthcare and investment firms, including serving for five years as managing director of Bryan Garnier & Co. Dr. Renondin served as a director of Cognate Bioservices Limited, a company in the business of contract development and manufacturing, specializing in cell and cell-mediated gene therapy products, which is now owned by Charles River Laboratories International, as a director of Juvenescence Limited from March 2020 until June 2023, and as a director of Viscogliosi Brothers Acquisition Corp. Dr. Renondin received an MBA degree from the Tuck School of Business at Dartmouth University and an MD degree from Universite Paris Cite. We believe that Dr. Renondin is qualified to serve on our Board based on his years of management experience in healthcare, investment, and finance.
| 107 |
Steven Mintz joined our Board of Directors during January 2024. Mr. Mintz has been a self-employed financial consultant since 1998 serving both private individuals and companies, as well as public companies in a variety of industries including mining, oil and gas, real estate and investment strategies. He is currently President of St. Germain Capital Corp., a private consulting and investment firm. He is also a principal and CFO of the Minkids Group, a family investment and development company. Mr. Mintz is currently a director of Portage Biotech, Inc., a clinical-stage immuno-oncology company advancing multi-targeted therapies for cancer. Mr. Mintz previously served as a director of IM Cannabis (formerly Navasota Resources). Mr. Mintz graduated from the University of Toronto in 1989 and obtained his C.A. designation in June of 1992.
Initial Post-Merger Directors
The following table provides information regarding the expected directors of the Combined Company following the closing of the Merger:
Name | Age | Position | ||
| Steve Ledger | 64 | Interim Chief Executive Officer and Class II Director | ||
| Non-Employee Directors | ||||
| Gregory H. Bailey, M.D. | 68 | Chairman of the Board and Class III Director | ||
| Steven Mintz | 57 | Class I Director | ||
| Remy Gross | 53 | Class I Director | ||
| J. Milton Harris, Ph.D. | 83 | Class II Director | ||
| Richard Marshall, BDE, M.D., Ph.D. | 56 | Class III Director |
Steve Ledger has served as Serina’s Chief Financial Officer since June 2021 and as a member of the Serina Board of Directors since December 2022, and is expected to serve as Interim Chief Executive Officer and as a Class II Director of the Combined Company following consummation of the Merger. Serina has engaged an executive search firm to recruit a permanent Chief Executive Officer for AgeX to serve after the Merger. Mr. Ledger will cease to be the Interim Chief Executive Officer but will remain as a Class II Director upon the hiring of the Chief Executive Officer. Mr. Ledger has more than 35 years of experience as an investor, board member, advisor, and in operational roles with early-stage companies. From 2018 to the present, Mr. Ledger serves as Managing Partner of Form & Fiction Ventures, Inc. (FFV), a venture studio that launches and invests in startup and seed stage companies focused on socially responsible initiatives. Mr. Ledger is a co-founder and board director of Entourage Genomics, Inc., a bioinformatics software company formed by FFV in June 2023. From 2018 to February 2022, Mr. Ledger served as an advisor at Caldwell Sutter Capital, Inc., an SEC registered broker dealer and investment management firm focused on value-based equity and debt securities. From 2002 to 2012, Mr. Ledger was the founder and managing member of Tamalpais Partners, LLC, the general partner to funds focused on special situations in the small capitalization public equity markets. Mr. Ledger received a B.A. in Economics from the University of Connecticut.
Non-Employee Directors
Gregory H. Bailey, M.D. Information concerning Dr. Bailey can found above under “Our Current Directors.”
Steven Mintz. Information concerning Mr. Mintz can found above under “Our Current Directors.”
J. Milton Harris, Ph.D. has served as Chair of the Serina Board of Directors since he co-founded Serina in 2006. Dr. Harris has more than 30 years of experience as a senior life sciences executive. Prior to founding Serina, he was the Founder and Chief Executive Officer of Shearwater Polymers, Inc. (Shearwater). Shearwater was founded by Dr. Harris in 1992 and sold in 2001 to Inhale Therapeutics, Inc. (Inhale Therapeutics, Inc., subsequently changed its name to Nektar Therapeutics, Inc.). Shearwater successfully patented, manufactured, and partnered PEG technology that enabled multiple drug products including Neulasta® (Amgen) and Pegasys® (Roche). Dr. Harris has also served on the board of directors of HudsonAlpha Institute for Biotechnology since its founding in 2004. Dr. Harris earned a B.S. from McGill University, where he also was awarded an honorary Sc.D., and a Ph.D. from the Massachusetts Institute of Technology. Dr. Harris has co-authored more than 200 publications and is a co-inventor on more than 75 patents.
Remy Gross has served as Vice President, Business Development & Technology Advancement at the Buck Institute for Research (Buck) on Aging from 2006. Mr. Gross has advised and helped create multiple new biopharmaceutical startups at Buck, including Unity Biotechnology, Inc., Aeovian Therapeutics, Inc., and BhB Therapeutics, Inc. Prior to Buck, Mr. Gross held increasingly senior roles at Shearwater from 1994 to 2001, and at Nektar after its acquisition of Shearwater from 2001 to 2005, including Vice President of Operations. In 2013, Mr. Gross co-founded RCP Companies, Inc., a boutique real estate company providing acquisition, development, and asset management. Mr. Gross serves as a board director of BhB Therapeutics, Inc., Napa Therapeutics, Inc., and Selah Therapeutics, Inc. Mr. Gross serves on several non-profit boards including MidCity Accelerator Foundation, Hatch HSV and k3innovation. Mr. Gross received a B.S. in Chemistry from Loyola University New Orleans.
| 108 |
Richard Marshall, CBE, M.D., Ph.D. has served as the Chief Executive Officer of Juvenescence from January 2023. Dr. Marshall is a physician scientist and highly experienced executive with a 20-year track record of leadership in pharmaceutical Research & Development. From September 2019 to January 2023, Dr. Marshall was Senior Vice President and Global Head of Respiratory & Immunology Development at AstraZeneca plc, overseeing the development and approval of five new medicines. This included the SARS CoV-2 vaccine, Vaxzevria®, and combination antibody, EvushieldTM. In 2021 he was recognized in the Queen’s Honours List with a CBE for his contribution to UK science and the Covid response. From 2002 to 2018, Dr. Marshall held increasingly senior roles at GlaxoSmithKline plc, including Vice President of Fibrosis R&D. Dr. Marshall received a Bachelor of Science in Neuroscience, a Bachelor of Medicine, a Bachelor of Surgery, and a Doctor of Philosophy in Medical Sciences from University College London and has held visiting professor and honorary consultant roles in thoracic medicine at Newcastle University and the Royal Brompton Hospital. Dr. Marshall has co-authored more than 60 original publications in journals including The Lancet and The New England Journal of Medicine.
Family Relationships
There are no family relationships among any of the Combined Company’s proposed directors and executive officers.
Audit Committee
We have established an Audit Committee of the Board of Directors. The members of the Audit Committee are Steven Mintz and Jean-Christophe Renondin, each of whom qualifies as being “independent” under Section 8.03(A) and 8.03(B) of the NYSE American Company Guide and under Rule 10A-3 of the Exchange Act. Michael May served on the Audit Committee during 2023 until his term as a director expired and Joanne M. Hackett also served on the Audit Committee during 2023 until she was appointed Interim Chief Executive Officer. Steven Mintz is the Chair of the Audit Committee. The purpose of the Audit Committee is to recommend the engagement of our independent registered public accountants, to review their performance and the plan, scope, and results of the audit, and to review and approve the fees we pay to our independent registered public accountants. The Audit Committee also will review our accounting and financial reporting procedures and controls, and all transactions between us and our executive officers, directors, and stockholders who beneficially own 5% or more of any class of our voting securities. We have adopted a written charter for our Audit Committee which we have posted on our website at www.agexinc.com. The Board of Directors has also determined that Mr. Mintz is “financially sophisticated” within the meaning of the rules and regulations of the NYSE American and qualifies as an “audit committee financial expert” as defined under applicable rules and regulations of the SEC and the NYSE American.
Code of Ethics
We have adopted a Code of Business Conduct and Ethics (“Code of Ethics”) that applies to our principal executive officers, our principal financial officer and accounting officer, our other executive officers, and our directors. The purpose of the Code of Ethics is to promote (i) honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; (ii) full, fair, accurate, timely, and understandable disclosure in reports and documents that we file with or submit to the SEC and in our other public communications; (iii) compliance with applicable governmental rules and regulations; (iv) prompt internal reporting of violations of the Code of Ethics to an appropriate person or persons identified in the Code of Ethics; and (v) accountability for adherence to the Code of Ethics. A copy of our Code of Ethics has been posted on our internet website and can be found at www.agexinc.com. We intend to disclose any future amendments to certain provisions of our Code of Ethics, and any waivers of those provisions granted to our principal executive officers, principal financial officer, principal accounting officer or controller or persons performing similar functions, by posting the information on our website within four business days following the date of the amendment or waiver.
Information About Our Executive Officers
The following table sets forth information regarding our executive officers as of March 14, 2024: If the Merger is consummated our executive officers will change except that we expect that Andrea E. Park will continue to serve as an executive officer in the role of Interim Chief Financial Officer and as Chief Accounting Officer. See, “Initial Executive Officers of the Combined Company After the Merger” for information about the persons who we expect to serve as the initial executive officers of the Combined Company after the consummation of the Merger.
| Name | Age | Officer Since | Position | |||
| Joanne M. Hackett, Ph.D. | 45 | August 2023 | Interim Chief Executive Officer | |||
| Andrea E. Park | 52 | May 2020 | Chief Financial Officer | |||
| Hal Sternberg, Ph.D. | 70 | August 2017 | Vice President of Research |
Joanne M. Hackett, Ph.D. was appointed Interim Chief Executive Officer on August 9, 2023. Her biographical information can be found above with information about the other members of our Board of Directors.
Andrea E. Park, CPA (inactive) was appointed as our Chief Financial Officer in May 2020. Ms. Park served as our Vice President of Finance and Controller since October 2019. Ms. Park’s career spans over 24 years of public accounting and finance experience. Before joining AgeX, Ms. Park served as Vice President of Finance and Controller from June 2016 to September 2019 and as Corporate Controller from August 2009 to June 2016 for Lineage Cell Therapeutics, Inc. (formerly BioTime, Inc.). While at Lineage, Ms. Park was directly involved in the accounting and financial reporting of the public spin off and eventually the deconsolidation of three of its then subsidiaries including Asterias Biotherapeutics, Inc., Oncocyte Corporation and AgeX. Earlier in her career she worked in the audit and assurance practice at Deloitte. Ms. Park has a B.A. in Business Economics with Concentration in Accounting from the University of California, Santa Barbara.
| 109 |
Hal Sternberg, Ph.D. was appointed Vice President of Research in August 2017. Prior to serving in that role, Dr. Sternberg was Vice President of Research of Lineage for over 25 years and was one of Lineage co-founders. Prior to co-founding and joining Lineage, Dr. Sternberg held various positions at the University of California at Berkeley from 1982 to 1988, where he supervised a team of researchers studying Alzheimer’s Disease. Dr. Sternberg holds an M.S. in Chemistry and Ph.D. in Biochemistry from the University of Maryland.
Initial Executive Officers of the Combined Company After the Merger
The following table provides information regarding the persons we expect to serve as the initial executive officers of the Combined Company following consummation of the Merger.
Name | Age | Position | ||
| Steve Ledger | 64 | Interim Chief Executive Officer and Class II Director | ||
| Andrea E. Park | 52 | Interim Chief Financial Officer and Chief Accounting Officer | ||
| Randall Moreadith, M.D., Ph.D. | 69 | Chief Scientific Officer | ||
| Tacey Viegas, Ph.D. | 66 | Chief Operating Officer; Secretary |
Steve Ledger. Information concerning Mr. Ledger can be found above under “Initial Post-Merger Directors.”
Andrea E. Park, CPA (inactive) is the Chief Financial Officer of AgeX and will serve as the Interim Chief Financial Officer and Chief Accounting Officer of the Combined Company after the Merger. Upon the hiring of a new Chief Financial Officer, Ms. Park will cease to be the Interim Chief Financial Officer but will remain the Chief Accounting Officer. Further information concerning Ms. Park’s career can be found above under “Information About Our Executive Officers.”
Randall Moreadith, M.D., Ph.D. has served as Serina’s President and Chief Executive Officer and as a member of the Serina Board of Directors since September 2010. Dr. Moreadith will serve as Chief Scientific Officer of the Combined Company. From July 2009 to December 2009, Dr. Moreadith was Chief Development Officer at Nektar Therapeutics (Nektar) where he led clinical and drug development programs that successfully moved several of the Nektar’s PEGylated small molecule drugs into clinical trials for four clinical indications (ovarian, breast, cervical and colorectal cancer) and the out-licensing efforts for the approved product now known as Movantik®. Prior to Nektar, Dr. Moreadith served as the Executive Vice President and Chief Medical Officer of Cardium Therapeutics, Inc. where he led the advancement of novel DNA-based adenoviral therapeutics into Phase IIb and Phase III late-stage development, from 2006 to 2008. Prior to Cardium, Dr. Moreadith served as Chief Medical Officer of Renovis, Inc. where he led the Clinical, Regulatory and Quality Assurance Groups in 2004 to 2005. From 1996 to 2003, Dr. Moreadith was co-Founder, President, and Chief Operating Officer of ThromboGenics, Ltd. (now Oxurion), a leader in the field of thrombosis drug development. During his tenure at ThromboGenics, the company advanced four biologics into mid-stage development, with one product later approved (Ocriplasmin™). Dr. Moreadith received his M.D. from Duke University and is trained clinically in Internal Medicine and Cardiovascular Diseases. Dr. Moreadith received his Ph.D. from Johns Hopkins University and following his Fellowship in Cardiology at Duke University, he joined the laboratory of Professor Philip Leder where he was a Howard Hughes Medical Institute Fellow in Genetics at Harvard Medical School. Dr. Moreadith was a member of the faculty of the University of Texas Southwestern Medical Center.
Tacey Viegas, Ph.D. has served as Serina’s Chief Operating Officer since 2006. Dr. Viegas will serve as Chief Operating Officer and Secretary of the Combined Company. Dr. Viegas has managed the discovery and early development activities for both synthetically- and biologically-derived therapeutic agents in the areas of oncology, neurology, influenza, psoriasis, and wound care. He has numerous patents and publications in the area of polymer therapeutics and pharmaceutics. Prior to joining Serina, Dr. Viegas served as Senior Director of Chemistry Manufacturing and Controls at Nektar. Prior to Nektar, Dr. Viegas was Executive Director in product development at Biocryst Pharmaceuticals, Inc. (BioCryst). During his combined tenures at Nektar and BioCryst, he was a co-inventor of nalexogol (Movantik®), etirinotecan pegol and was involved in the early development of peramivir (Rapivab™). Prior to Biocryst, Dr. Viegas was a Director, Product Development at MDV Technologies, Inc. from 1989 to 1994. Dr. Viegas received his B.S. in Chemistry and Pharmacy from Bangalore University, and his M.S. and Ph.D. in Pharmaceutical Sciences from the University of Mississippi.
Delinquent Section 16(a) Reports
Section 16(a) of Exchange Act requires our directors and executive officers and persons who own more than ten percent (10%) of a registered class of our equity securities (“Reporting Persons”) to file with the SEC initial reports of ownership and reports of changes in ownership of our common stock and other AgeX equity securities. Officers, directors and greater than ten percent beneficial owners are required by SEC regulations to furnish us with copies of all reports they file under Section 16(a).
To our knowledge, based solely on our review of the copies of Forms 3 and 4 and amendments thereto filed during the last fiscal year by the Reporting Persons, or written representation from the Reporting Persons that no Form 5 was required, all Section 16(a) filing requirements applicable to our officers, directors, and greater than ten percent beneficial owners were complied with during the fiscal year ended December 31, 2023, except that two Forms 4 were filed late by Juvenescence which owns more than 10% of the outstanding AgeX common stock.
| 110 |
Item 11. Executive Compensation
Emerging Growth Company and Smaller Reporting Company
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 and a “smaller reporting company” as defined in the rules and regulations of the SEC. As an emerging growth company and as a smaller reporting company we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable, in general, to public companies that are not emerging growth companies or smaller reporting companies. Accordingly, this Report includes reduced disclosure about our executive compensation arrangements.
Summary Compensation Table
The following table sets forth the compensation awarded to, earned by, or paid to AgeX’s Chief Executive Officer during fiscal year 2023 and the two highest paid individuals who were serving as executive officers as of December 31, 2023 (AgeX’s Named Executive Officers) in respect of their service to AgeX for the fiscal years ended December 31, 2023 and 2022.
| Name and principal position | Year | Salary | Option Awards(1) | All Other Compensation(2) | Total | |||||||||||||||
| Joanne M. Hackett(3) | 2023 | $ | 63,227 | $ | - | $ | 39,623 | (4) | $ | 102,850 | ||||||||||
| Interim Chief Executive Officer | 2022 | - | 47,325 | 55,740 | (4) | 103,065 | ||||||||||||||
| Michael D. West(5) | 2023 | 520,945 | 448,824 | 89,500 | (6) | 1,059,269 | ||||||||||||||
| Chief Executive Officer | 2022 | 546,782 | - | 15,250 | 562,032 | |||||||||||||||
| Andrea E. Park | 2023 | 284,339 | - | 14,217 | 298,556 | |||||||||||||||
| Chief Financial Officer | 2022 | 281,228 | - | 14,061 | 295,289 | |||||||||||||||
| Nafees N. Malik(7) | 2023 | 282,272 | 19,879 | - | 302,151 | |||||||||||||||
| Chief Operating Officer | 2022 | 282,272 | - | - | 282,272 | |||||||||||||||
| (1) | Amounts shown in this column do not reflect dollar amounts actually received by AgeX’s Named Executive Officers. Instead, these amounts reflect the aggregate grant date fair value of each stock option granted, computed in accordance with the provisions of FASB ASC Topic 718, Compensation-Stock Compensation. AgeX used the Black-Scholes Pricing Model to compute option fair values based on applicable exercise and stock prices, an expected option term, volatility assumptions, and risk-free interest rates. AgeX’s Named Executive Officers will only realize compensation upon exercise of the stock options and to the extent the trading price of AgeX’s common stock is greater than the exercise price of such stock options at the time of exercise. For Dr. West, the amount in this column also reflects the incremental fair value incurred in connection with the extension of the exercise period of 37,816 of Dr. West’s stock options from 90 days to four years following his termination. For Dr. Malik, the amount in this column also reflects the incremental fair value incurred in connection with the acceleration of vesting and extension of the expiration date of options to purchase 13,807 shares of AgeX common stock. Except as otherwise disclosed below, one fourth of the options will vest upon completion of 12 full months of continuous employment measured from the date of grant, and the balance of the options vest in 36 equal monthly installments commencing on the first anniversary of the date of grant, based on the completion of each month of continuous service as an employee or director of AgeX or its subsidiaries. |
| (2) | Amounts represent 401(k) matching contributions by AgeX for the periods presented unless described otherwise in the footnotes below. |
| (3) | Dr. Hackett joined our Board of Directors on December 29, 2021 as a non-employee director, and was appointed as Interim Chief Executive Officer on August 9, 2023. On February 2, 2022, Dr. Hackett was awarded 1,848 stock options which had a fair value of $47,325 on the grant date for her service as a director. These options became exercisable in four equal calendar quarters and were fully vested as of December 31, 2022. |
| (4) | Represents the amount paid to Dr. Hackett in respect of her service on our Board of Directors prior to becoming our Interim Chief Executive Officer. |
| (5) | Dr. West served as our Chief Executive Officer until August 9, 2023 and as Chief Executive Officer for Reverse Bio until October 31, 2023. Dr. West’s salary includes $67,039 for unused vacation hours. |
| (6) | $73,000 of such amount represents the estimated fair market value of certain laboratory equipment transferred to Dr. West in accordance with the terms of a Transition Services and Separation Agreement. |
| (7) | Dr. Malik served as our Chief Operating Officer through December 27, 2023. Dr. Malik served as a consultant, with his services provided by Juvenescence. Dr. Malik devoted a majority of his time to AgeX’s operations and AgeX reimbursed Juvenescence for his services. |
| 111 |
Compensation Agreements and Change of Control Provisions
Joanne M. Hackett: On August 9, 2023, we entered into a Consulting Agreement with Dr. Hackett for her services as our Interim Chief Executive Officer. Pursuant to that agreement she will receive a fee in the amount of $160,000 per year for services rendered as Interim Chief Executive Officer. Dr. Hackett will not be eligible to participate in any AgeX retirement, pension, life, health, accident and disability insurance, or other similar employee benefit plans for AgeX executive officers or employees other than the Incentive Plan.
Michael D. West: AgeX and Dr. West were party to an employment agreement, effective October 18, 2018 (the West Employment Agreement). Pursuant to the West Employment Agreement, Dr. West’s annual base salary was initially set at $525,000. Under the West Employment Agreement, Dr. West was eligible to earn an annual incentive cash bonus with a target of no less than 50% of annual base salary. Actual bonus amounts were based on Dr. West’s attainment of individual performance goals at target levels set by the AgeX Board for the applicable calendar year. If such performance goals for the applicable year were fully achieved, the AgeX Board could approve a bonus amount exceeding the target bonus level.
Under the West Employment Agreement, Dr. West was granted options to purchase 14,217 shares of AgeX’s common stock with an exercise price of $105.51 per share, with one fourth of the options vesting following 12 full months of continuous service as an employee of AgeX, measured from the date of grant, and the balance vesting in 36 equal monthly installments commencing on the first anniversary of the date of grant, based upon the completion of each month of continuous service as an employee of AgeX.
On August 9, 2023, AgeX and Dr. West entered into a Transition Services and Separation Agreement (the “Transition Agreement”) pursuant to which Dr. West stepped down as Chief Executive Officer of AgeX but agreed to continue to serve as Chief Executive Officer and as a director of AgeX’s subsidiary Reverse Bio during a “Transition Period.” The Transition Period ended on October 31, 2023. Pursuant to the Transition Services and Release Agreement, AgeX agreed to transfer to Dr. West title to certain laboratory and other equipment that AgeX has fully amortized for financial reporting purposes, and Dr. West’s AgeX stock options that were vested as of October 31, 2023 will remain exercisable until October 9, 2027; provided, that Dr. West’s out of the money options were cancelled during March 2024 to comply with certain provisions of the Merger Agreement.
Under the West Employment Agreement, and as affirmed in the Transition Agreement, Dr. West has agreed to certain covenants regarding confidential information and assignment of inventions, as well as a covenant not to solicit AgeX’s employees during Dr. West’s employment with AgeX and for one year thereafter. The West Employment Agreement also includes a covenant not to compete with AgeX during his employment.
Andrea E. Park: We have entered into an employment agreement with our Chief Financial Officer Andrea E. Park, effective May 15, 2020 (the “Park Employment Agreement”). Pursuant to the Park Employment Agreement, Ms. Park’s annual base salary was initially set at $265,000. Under the Park Employment Agreement, Ms. Park is eligible to earn an annual incentive cash bonus with a target of no less than 40% of annual base salary. Actual bonus amounts will be based on Ms. Park’s attainment of individual performance goals at target levels set by the Board of Directors for the applicable calendar year. If such performance goals for the applicable year are fully achieved, the Board of Directors may approve a bonus amount exceeding the target bonus level.
Under the Park Employment Agreement, Ms. Park has been granted options to purchase 8,530 shares of our common stock with an exercise price of $25.96 per share, with one fourth of the options vesting following 12 full months of continuous service as an employee of AgeX, measured from the date of grant, and the balance vesting in 36 equal monthly installments commencing on the first anniversary of the date of grant, based upon the completion of each month of continuous service as an employee of AgeX. Such options expire on the earliest of (1) 10 years from the date of grant, (2) three months after Ms. Park ceases to provide continuous service to us (other than due to death or disability) or (3) one year after Ms. Park ceases to provide continuous service to us due to death or disability.
Severance and Change of Control Arrangements for Ms. Park
Pursuant to the Park Employment Agreement, each officer is entitled to severance benefits under certain circumstances.
Pursuant to the Park Employment Agreement, Ms. Park is entitled to severance benefits under certain circumstances.
If AgeX terminates Ms. Park’s employment without “cause” or she resigns for “good reason” at any time (each as defined in the Park Employment Agreement), she will be entitled to (1) 9 months base salary, (2) all accrued but unpaid salary earned prior to or as of the date of termination or resignation, (3) full payment of Ms. Park’s pro-rated target bonus due for such year and (4) for a period of six months, all benefits under any health insurance plan of AgeX. In addition, if AgeX terminates Ms. Park’s employment without “cause” or she resigns for “good reason,” (1) all of Ms. Park’s outstanding equity awards that would otherwise have vested during the 12 months following termination or resignation will become fully vested and exercisable immediately and (2) with respect to any outstanding vested but unexercised options, the exercise period following termination or resignation will be extended to the earlier of (A) 12 months after termination or (B) the natural expiration date of the applicable option. If AgeX terminates Ms. Park’s employment without “cause,” or she resigns for “good reason,” following a “change of control,” (as defined in the Park Employment Agreement) (1) Ms. Park will be entitled to all of the benefits and payments that she would have been entitled to if her employment had been otherwise terminated without “cause” or if she resigned for “good reason,” as set forth above, and (2) all of Ms. Park’s unvested options and restricted stock units, if any, will become fully vested and exercisable immediately. The severance compensation may be paid in a lump sum or, at AgeX’s election, in installments consistent with the payment of Ms. Park’s salary while employed by AgeX. In order to receive the severance benefits, Ms. Park must execute a general release of all claims against AgeX.
Nafees N. Malik: Dr. Malik did not have an employment agreement with AgeX, but has been provided certain acceleration rights with respect to his equity awards by the Compensation Committee of the AgeX Board. On June 4, 2021, the Compensation Committee of the AgeX Board approved certain acceleration rights of the equity awards held by Dr. Malik. As a result of the termination of Dr. Malik’s service with AgeX without “cause,” all outstanding stock options held by Dr. Malik automatically vested as to the number of unvested shares that would otherwise have vested during the twelve months following termination; and with respect to his outstanding vested but unexercised stock option grants, the post-termination exercise period was extended to the earlier of the date twelve (12) months after termination or the expiration date of the stock option. Dr. Mailk’s out of the money options were cancelled on March 12, 2024 to comply with certain provisions of the Merger Agreement. Dr. Malik did not receive any severance benefits other than the foregoing option vesting and expiration rights.
| 112 |
Equity Awards Outstanding at December 31, 2023
The following table summarizes certain information concerning outstanding stock options granted by AgeX under the AgeX 2017 Equity Incentive Plan (the “Incentive Plan”) and held by AgeX’s Named Executive Officers as of December 31, 2023 and as adjusted to give effect to the Reverse Stock Split.
| Option Awards | ||||||||||||||||
Number of Securities Underlying Unexercised Options | Option Exercise | Option Expiration | ||||||||||||||
| Name | Grant Date | Exercisable(1) | Unexercisable | Price | Date | |||||||||||
| Joanne M. Hackett(2) | 2/2/2022 | 1,848 | (3) | - | $ | 29.32 | 2/1/2032 | |||||||||
| Michael D. West(4) | 6/4/2021 | 1,990 | (3) | - | $ | 51.00 | 10/9/2027 | |||||||||
| 3/11/2019 | 2,843 | (3) | - | $ | 150.53 | 10/9/2027 | ||||||||||
| 10/18/2018 | 14,217 | (3) | - | $ | 105.51 | 10/9/2027 | ||||||||||
| 10/10/2017 | 18,766 | (3) | - | $ | 70.34 | 10/9/2027 | ||||||||||
| Andrea E. Park | 6/4/2021 | 1,333 | (3) | 799 | $ | 51.00 | 6/3/2031 | |||||||||
| 5/21/2020 | 7,641 | 889 | $ | 25.96 | 5/20/2030 | |||||||||||
| 10/1/2019 | 569 | (3) | - | $ | 62.25 | 9/30/2029 | ||||||||||
| Nafees N. Malik(5) | 6/4/2021 | 1,865 | (3) | - | $ | 51.00 | 12/26/2024 | |||||||||
| 3/11/2019 | 1,990 | (3) | - | $ | 150.53 | 12/26/2024 | ||||||||||
| 10/18/2018 | 9,952 | (3) | - | $ | 105.51 | 12/26/2024 | ||||||||||
| (1) | Except as set forth below, vesting of all options is subject to continued service as an employee, director and/or consultant of AgeX or a subsidiary on the applicable vesting date. One fourth of the options vested or will vest on the first anniversary of the date of grant, and the remaining balance of the options vested or will vest in 36 equal monthly installments thereafter. | |
| (2) | These options were awarded to Dr. Hackett during 2022 for service as a non-employee director before she was appointed Interim Chief Executive Officer. These options became exercisable in four equal calendar quarters and were fully vested as of December 31, 2022. On August 9, 2023, Dr. Hackett was appointed as Interim Chief Executive Officer. | |
| (3) | These out of the money options were cancelled on March 12, 2024 to comply with certain provisions of the Merger Agreement. | |
| (4) | Dr. West served as Chief Executive Officer of AgeX and Reverse Bio through August 9, 2023 and October 31, 2023, respectively. | |
| (5) | Dr. Malik served as AgeX’s Chief Operating Officer through December 27, 2023. Dr. Malik served as a consultant, with his services provided by Juvenescence. Dr. Malik devoted a majority of his time to AgeX’s operations and AgeX reimbursed Juvenescence for his services. The options will remain exercisable for a one year period through December 26, 2024. |
Risk Considerations and Recoupment Policies
The Compensation Committee of our Board of Directors considers, in establishing and reviewing the executive compensation program, whether the program encourages unnecessary or excessive risk taking. Most of our executive compensation arrangements include a fixed salary that provides a steady income so that executives do not feel pressured to focus exclusively on stock price performance or short term financial targets to the detriment of our long-term operational and strategic objectives. We supplement fixed salaries with discretionary bonus awards based on the executive’s performance as well as the performance of AgeX. The stock options and RSUs that we have granted to our executive officers under the Incentive Plan vest over four years, assuring that the executives take a long-term perspective in viewing their equity ownership. Although we have not adopted compensation plans, or made incentive awards, based on quantified financial performance measures, we have adopted a Clawback Policy intended to comply with Section 811 of the NYSE American Company Guide. In the event of certain restatements of AgeX financial statements, the Clawback Policy will require AgeX to recoup from its executive officers compensation that is granted, earned or vested based wholly or in part upon the attainment of a financial reporting measure, to the extent such compensation (a) was granted during the three fiscal years preceding a determination that AgeX financial statements must be restated, and (b) exceeds the amount of compensation that would have been granted had the grant been based on the restated financial statement amounts. A copy of the Clawback Policy has been filed as an exhibit to this Report and also is posted on our internet website and can be found at www.agexinc.com.
| 113 |
Incentive Plan
The following summary of the Incentive Plan is a summary only and does not purport to include all of the terms of the Incentive Plan, and is qualified by the full terms of the Incentive Plan. If the Merger is consummated, no further Awards will be granted under the Incentive Plan. The Incentive Plan permits us to grant awards (“Awards”) for up to an aggregate of 241,683 shares of our common stock. Awards may include stock options, the grant or sale of restricted stock (“Restricted Stock”), stock appreciation rights (“SARs”), and restricted stock units or RSUs which are hypothetical units issued with reference to our common stock,. Awards may be granted under the Incentive Plan to employees, directors, and consultants of AgeX and our subsidiaries, including also subsidiaries that we may form or acquire in the future. The Incentive Plan will be administered by our Board of Directors (the “Board”) or by a committee authorized by our Board (“Committee”), who will make all determinations with regard to the grant and terms of Awards, subject to the terms of the Incentive Plan.
Awards may vest and thereby become exercisable or have restrictions on forfeiture lapse on the date of grant or in periodic installments or upon the attainment of performance goals, or upon the occurrence of specified events as determined by the Board or the Committee. The Board or Committee, in its discretion, may accelerate the vesting of an Award after the date of grant. To comply with certain provisions of the Merger Agreement, on March 12, 2024 all outstanding out of the money options were cancelled.
No person shall be granted, during any one year period, options to purchase, or SARs with respect to, more than 28,433 shares in the aggregate, or any Awards of Restricted Stock or RSUs with respect to more than 14,216 shares in the aggregate. If an Award is to be settled in cash, the number of shares on which the Award is based shall not count toward the individual share limit.
No Awards may be granted under the Incentive Plan more than ten years after the date upon which the Incentive Plan was adopted by the Board, and no options or SARS granted under the Incentive Plan may be exercised after the expiration of ten years from the date of grant.
Stock Options
Options granted under the Incentive Plan may be either “incentive stock options” within the meaning of Section 422(b) of the Internal Revenue Code of 1986, as amended, or the Code, or “non-qualified” stock options that do not qualify incentive stock options. Incentive stock options may be granted only to employees of AgeX and its subsidiaries. The exercise price of stock options granted under the Incentive Plan must be equal to the fair market of our common stock on the date the option is granted. In the case of an optionee who, at the time of grant, owns more than 10% of the combined voting power of all classes of our stock, the exercise price of any incentive stock option must be at least 110% of the fair market value of our common stock on the grant date, and the term of the option may be no longer than five years. The aggregate fair market value of common stock (determined as of the grant date of the option) with respect to which incentive stock options become exercisable for the first time by an optionee in any calendar year may not exceed $100,000.
The exercise price of an option may be payable in cash or in shares of our common stock having a fair market value equal to the exercise price, or in a combination of cash and common stock, or other legal consideration for the issuance of stock as the Board or Committee may approve.
Generally, options will be exercisable only while the optionee remains an employee, director or consultant, or during a specific period thereafter as approved by the Board or Committee, which will generally be three months, but in the case of the termination of an employee, director, or consultant’s services due to death or disability, the period for exercising a vested option shall be extended to the earlier of 12 months after termination or the expiration date of the option.
The number of shares covered by the Incentive Plan, and the number of shares and the exercise price per share of each outstanding option, shall be proportionately adjusted for any increase or decrease in the number of issued and outstanding shares of common stock resulting from a subdivision or consolidation of shares or the payment of a stock dividend, or any other increase or decrease in the number of issued and outstanding shares of common stock effected without receipt of consideration by us.
Restricted Stock and RSUs
In lieu of granting options, we may enter into purchase agreements with employees under which they may purchase or otherwise acquire Restricted Stock or RSUs subject to such vesting, transfer, and repurchase terms and restrictions as the Board or Committee may determine. We may permit employees or consultants who purchase Restricted Stock to pay for their shares by delivering a promissory note or an installment payment agreement that may be secured by a pledge of their Restricted Stock. We may also issue Restricted Stock for services actually performed by the recipient prior to the issuance of the Restricted Stock.
The Board or Committee may require that Restricted Stock shall be held by us or in escrow pending the expiration or release of the applicable restrictions. Unvested Restricted Stock for which we have not received payment may be forfeited to us, or we may have the right to repurchase unvested shares upon the occurrence of specified events, such as termination of employment.
Subject to the restrictions set by the Board or Committee, a recipient of Restricted Stock generally shall have the rights and privileges of a stockholder, including the right to vote the Restricted Stock and the right to receive dividends; provided that, any cash dividends and stock dividends with respect to the Restricted Stock shall be withheld by us for the recipient’s account, and interest may be credited on the amount of the cash dividends withheld at a rate and subject to such terms as determined by the Board or Committee. The cash dividends or stock dividends so withheld and attributable to any particular share of Restricted Stock (and earnings thereon, if applicable) shall be distributed to the recipient in cash or, at the discretion of the Board or Committee, in common stock having a fair market value equal to the amount of such dividends, if applicable, upon the release of restrictions on the Restricted Stock and, if the Restricted Stock is forfeited, the recipient shall have no right to the dividends.
The terms and conditions of a grant of RSUs shall be determined by the Board or Committee. No common stock shall be issued at the time a RSU is granted, and we will not be required to set aside a fund for the payment of any such award. A recipient of RSUs shall have no voting rights with respect to the RSUs. Upon the expiration of the restrictions applicable to a RSU, we will either issue to the recipient, without charge, one share of common stock per RSU or cash in an amount equal to the fair market value of one share of common stock.
| 114 |
At the discretion of the Board or Committee, each RSU (representing one share of common stock) may be credited with cash and stock dividends paid in respect of one share (“Dividend Equivalents”). Dividend Equivalents shall be withheld by us for the recipient’s account, and interest may be credited on the amount of cash Dividend Equivalents withheld at a rate and subject to such terms as determined by the Board or Committee. Dividend Equivalents credited to a recipient’s account and attributable to any particular RSU (and earnings thereon, if applicable) shall be distributed in cash or, at the discretion of the Board or Committee, in common stock having a fair market value equal to the amount of the Dividend Equivalents and earnings, if applicable, upon settlement of the RSU. If a RSU is forfeited, the recipient shall have no right to the related Dividend Equivalents.
SARs
An SAR is the right to receive, upon exercise, an amount payable in cash or shares or a combination of shares and cash, as determined by the Board or Committee, equal to the number of shares subject to the SAR that is being exercised, multiplied by the excess of (a) the fair market value of a share of common stock on the date the SAR is exercised, over (b) the exercise price specified in the SAR Award agreement. SARs may be granted either as free standing SARs or in tandem with options, and with such terms and conditions as the Board or Committee may determine. No SAR may be exercised later than 10 years after the date of grant.
The exercise price of an SAR will be determined by the Board or Committee, but shall not be less than 100% of the fair market value of one share of common stock on the date of grant. An SAR granted in conjunction with an option shall have the same exercise price as the related option, shall be transferable only upon the same terms and conditions as the related option, and shall be exercisable only to the same extent as the related option; provided, however, that the SAR by its terms shall be exercisable only when the fair market value per share exceeds the exercise price per share of the SAR or related option. Upon any exercise of an SAR granted in tandem with an option, the number of shares for which the related option shall be exercisable shall be reduced by the number of shares for which the SAR has been exercised. The number of shares for which an SAR issued in tandem with an option shall be exercisable shall be reduced by the number of shares for which the related option has been exercised.
Options GrantedWithholding
A summaryTo the extent provided by the terms of an Award Agreement or as may be approved by the AgeX stock option activity under the Plan and related information follows (in thousands except weighted average exercise price):
| Options | Shares Available for Grant | Number of Options Outstanding | Weighed Average Exercise Price | |||||||||
| Outstanding at January 1, 2017 | - | - | $ | - | ||||||||
| Increase in option pool | 4,000 | |||||||||||
| Granted | (1,239 | ) | 1,239 | 2.00 | ||||||||
| Outstanding at December 31, 2017 | 2,761 | 1,239 | $ | 2.00 | ||||||||
| Granted | (1,038 | ) | 1,038 | 2.91 | ||||||||
| Forfeited | 8 | (8 | ) | 2.00 | ||||||||
| Outstanding at December 31, 2018 | 1,731 | 2,269 | 2.42 | |||||||||
| Exercisable at December 31, 2018 | 739 | $ | 2.02 | |||||||||
There were no exercisesBoard or Committee, an optionee or recipient of stock options during the years ended December 31, 2018 and 2017.
Total proceeds if all options granted and outstanding as of December 31, 2018 were exercised would be approximately $5.5 million.
At December 31, 2018, AgeX had approximately $2.55 million of total unrecognized compensation expense relateda Restricted Stock or RSU Award or SAR may satisfy any federal, state or local tax withholding obligation relating to the Plan that will be recognized over a weighted-average periodAward by any of 3.22 years.
The aggregate intrinsic value of options outstanding was $1.3 million and options exercisable was $0.7 million as of December 31, 2018.
Stock-based Compensation Expense
AgeX recorded stock-based compensation expense in the following categories on the accompanying consolidated statements of operations for the years ended December 31, 2018 and 2017means (in thousands):
| Year Ended December 31, | ||||||||
| 2018 | 2017(1) | |||||||
| Research and development | $ | 148 | $ | 245 | ||||
| General and administrative | 1,325 | 507 | ||||||
| Total stock-based compensation expense | $ | 1,473 | $ | 752 | ||||
The weighted-average estimated fair value of stock options granted during the years ended December 31, 2018 and 2017 was $1.99 per share and $1.31 per share, respectively, using the Black-Scholes option pricing model with the following weighted-average assumptions:
| Year Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| Expected life (in years) | 6.05 | 5.84 | ||||||
| Risk-free interest rates | 2.99 | % | 2.04 | % | ||||
| Volatility | 76.4 | % | 74.9 | % | ||||
| Dividend yield | - | % | - | % | ||||
The determination of stock-basedaddition to our right to withhold from any compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If AgeX had made different assumptions, its stock-based compensation expense and net loss for the years ended December 31, 2018 and 2017 may have been significantly different. See Note 2 for a discussion of the factors used in determining these assumptions.
AgeX does not recognize deferred income taxes for incentive stock option compensation expense and records a tax deduction only when a disqualified disposition has occurred.
7. Income Taxes
On December 22, 2017, in responsepaid to the enactmentAward recipient) or by a combination of such means: (a) tendering a cash payment; (b) authorizing us to withhold shares of common stock from the 2017 Tax Act (see Note 2), the SEC staff issued SAB 118 that allows AgeX to record provisional amounts during a measurement period not to extend beyond one year of the enactment date. The repatriation tax is based primarily on LifeMap Sciences Ltd., an Israeli subsidiary of LifeMap Sciences (see Note 4), accumulated foreign earnings and profits that LifeMap Sciences previously excluded from U.S. income taxes. As a result, LifeMap Sciences included$227,000in foreign earnings in federal income for the year ended December 31, 2017. The federal taxable income was offset by operating losses and resulted in no federal income tax due. AgeX applied the guidance in SAB 118 when accounting for the enactment-date effects of the 2017 Tax Act during the years ended December 31, 2018 and 2017. As of December 31, 2018, AgeX completed its accounting for all the enactment-date income tax effects of the 2017 Tax Act discussed below.
AgeX remeasured certain deferred tax assets and liabilities based on the enacted tax rate at which they are expected to reverse in the future. The estimated, tax effected, amount relatedshares otherwise issuable to the remeasurement of these balances was a reduction of AgeX’s net deferred tax assets of$3.9 millionwith a corresponding decrease in the valuation allowance by the same amount, recognized as of December 31, 2017,
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. As of December 31, 2017, the federal portion of the deferred tax assets and liabilities for 2017 were re-rated from 34% to 21% pursuant to the 2017 Tax Act. Accordingly, the federal portion of the deferred tax assets and liabilities for all periods presented are rated at 21%.
The primary components of the net deferred tax assets and liabilities as of December 31, 2018 and 2017 were as follows (in thousands):
| December 31, | ||||||||
| Deferred tax assets/(liabilities): | 2018 | 2017 | ||||||
| Net operating loss carryforwards | $ | 9,893 | $ | 8,367 | ||||
| Research and development credit carryforwards | 1,734 | 1,658 | ||||||
| Patents and fixed assets | 292 | 9 | ||||||
| Stock-based compensation | 241 | 82 | ||||||
| Equity Investments | 0 | 232 | ||||||
| Other, net | 83 | (302 | ) | |||||
| Valuation allowance | (12,243 | ) | (10,046 | ) | ||||
| Total net deferred tax assets | $ | - | $ | - | ||||
A valuation allowance is provided when it is more likely than not that all or some portion of the deferred tax assets will not be realized. AgeX established a full valuation allowance for all periods presented due to the uncertainty of realizing future tax benefits from its net operating loss carryforwards and other deferred tax assets. Accordingly, no tax provision or benefit was recorded for any period presented.
Income taxes differed from the amounts computed by applying the U.S. federal income tax rate indicated to pretax losses from operationsrecipient as a result of the following:exercise or acquisition of shares under the Award, provided, however, that no shares are withheld with a value exceeding the minimum amount of tax required to be withheld by law; or (c) delivering to us previously owned and unencumbered shares of our common stock.
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| Computed tax benefit at federal statutory rate | 21% | 34% | ||||||
| Research and development and other credits | 1% | 2% | ||||||
| Rerate of federal net deferred tax assets | -% | (58%) | ||||||
| State tax benefit, net of effect on federal income taxes | 3% | 4% | ||||||
| Permanent differences | (1%) | (4%) | ||||||
| Change in valuation allowance | (23%) | 22% | ||||||
| Foreign rate differential | (1%) | -% | ||||||
| -% | -% | |||||||
AsChanges in Shares Under the Incentive Plan
In the event of December 31, 2018, AgeX has net operating loss carryforwards of approximately $31.5 million for U.S. federal income tax purposes. Of this amount, $8.7 million is attributable to LifeMap Sciences, which includes $2.1 million inNOLs generated while it was includedchanges in the consolidated BioTime tax groupoutstanding common stock or in our capital structure by reason of any stock or extraordinary cash dividend, stock split, Reverse Stock Split, an extraordinary corporate transaction such as any recapitalization, reorganization, merger, consolidation, combination, exchange, or other relevant change in capitalization, the terms of Awards granted under the Incentive Plan, and wouldthe maximum number of shares subject to all Awards under the Incentive Plan or with respect to which any one person may be availablegranted Awards during any one year period, will be equitably adjusted or substituted, as to offset incomethe number, price or kind of AgeXshares or other consideration subject to the Awards to the extent necessary to preserve the economic intent of the Awards. In making such adjustments, the Board or Committee shall generally ensure that the adjustments will not constitute a modification, extension or renewal of an incentive stock option within the meaning of Section 424(h)(3) of the Code, and in the future. The remaining LifeMap Sciences’ NOLscase of $6.6 million are attributable to NOLs generated fornon-qualified options, ensure that any adjustments will not constitute a modification of such non-qualified options within the tax years during which LifeMap Sciences filed a separate federal income tax return and, accordingly, those NOLs are available only to LifeMap Sciences’ taxable income within AgeX in future years.In general, NOLs and other tax credit carryforwards generated by legal entities in a consolidated federal tax group are available to other membersmeaning of Section 409A of the tax group dependingCode.
Restrictions on Transfers of Options
Under the Incentive Plan, stock options may be transferred to a limited class of defined “Permitted Transferees,” such as the option holder’s immediate family members, family trusts and family controlled companies. In addition, options may be transferred to a securities broker/dealer to exercise the options on the natureoption holder’s behalf as a means of the transactionoption holder obtaining the funds needed to exercise the option, provided that the fair market value of the shares being acquired exceeded the exercise price of the option at the close of the market on the trading day preceding the exercise date.
Repricing Prohibition
The Plan prohibits any modification of the purchase price or exercise price of an outstanding option or other Award if the change would effect a member may enter into while still“repricing” without stockholder approval. As defined in the consolidated federal tax group. However, underIncentive Plan, “repricing” means a reduction in the Tax Matters Agreement between BioTime and AgeX, any useexercise price of a member’s NOLs and other tax credit carryforwards by the other member is subject to reimbursement by the benefiting member for the actual tax benefit realized. Since the August 30, 2018 deconsolidationan outstanding option or SAR or cancellation of AgeX and to date, neither BioTime nor AgeX has used the tax attributes of the other.
On June 6, 2017, BioTime and LifeMap Sciences entered into a Debt Conversion Agreement whereby BioTime acquired additional stock in LifeMap Sciences (see Note 4) and other assets, including intellectual propertyan “underwater” or “out-of-the-money” Award in exchange for related party payableother Awards or cash. An “underwater” or “out-of-the-money” Award is defined to mean an Award for which the exercise price is less than the “fair market value” of approximately $8.8 million owed to BioTime. Consistent withour common stock. The fair market value will generally be determined by the financial reporting impacts discussed in Note 4, LifeMap Sciences recordedAgeX Board, but if our common stock becomes publicly traded, the tax effectfair market value will be the closing price of the transactions in equity instead of the tax provision in accordance with ASC 740-20-45-11(g), which requires that the tax effects of all changes in tax bases of assets and liabilities caused by transactions among or with stockholders be included in equity. In connection with this transaction, LifeMap Sciences utilized approximately $3.7 million in net operating loss carryforwards with a corresponding release of the valuation allowance recorded through equity in accordance with ASC 740-20-45-11(g).
For income tax purposes, the purchase by BioTime of LifeMap Sciences’ intellectual property and other assets resulted in a taxable gain to LifeMap Sciences of $3.7 million for the year ended December 31, 2017. Although LifeMap Sciences had sufficient net operating loss carryforwards to offset the entire gain, it incurred a federal alternative minimum tax payable of $22,000 as of December 31, 2017. As previously noted under the 2017 Tax Act, corporations are no longer subject to the AMT, effective for taxable years beginning after December 31, 2017. To the extent a company has an AMT credit from a prior year, the company can carry the credit forward to offset regular tax. To the extent the company does not have a federal tax liability, a portion of the AMT credit is refundable each year starting in 2018, with any remaining balance fully refundable in 2021. As LifeMap Sciences will ultimately receive a full refund of the current AMT payable, fully offsetting the current provision, there is no tax provision or benefit recorded for the year ended December 31, 2017.
On March 23, 2018, Ascendance was acquired by a third party in a merger through which AgeX received approximately $3.2 million in cash for its shares of Ascendance common stock. For financial reporting purposes, AgeX recognized a $3.2 million gain as a sale of its equity method investment in Ascendance (see Note 4). The sale was a taxable transaction to AgeX generating a taxable gain of approximately $2.2 million. AgeX has sufficient current year losses from operations to offset the entire gain resulting in no income taxes due.
As further discussed in Note 1, on August 30, 2018, BioTime consummated the sale of 14,400,000 shares of common stock of AgeX owned by BioTime to Juvenescence. AgeX received no proceeds from that transaction. Prior toon a national securities exchange or inter-dealer quotation system on which the transaction, Juvenescence owned 5.6% of AgeX’s issued and outstanding common stock. Upon completion of the transaction, BioTime’s ownership in AgeX was reduced from 80.4% to 40.2% of AgeX’s issued and outstanding shares of common stock and Juvenescence’s ownership in AgeX was increased from 5.6% to 45.8% of AgeX’s issued and outstanding shares of common stock. Accordingly, beginning on August 31, 2018, AgeX will no longer be included in BioTime’s consolidated federal and state income tax returns as AgeX will file its own, standalone returns with its subsidiaries.is traded.
As of December 31, 2018, AgeX has net operating losses of approximately $32.5 million for California purposes. As AgeX and its subsidiaries have been included in the combined California tax return with BioTime, up to the date of deconsolidation on August 30, 2018, those state net operating losses will remain with AgeX. In general, NOLs and other tax credit carryforwards generated by legal entities in a combined state tax group are available to other members of the tax group depending on the nature of the transaction that a member may enter into while still in the combined state tax group. However, under the Tax Matters Agreement between BioTime and AgeX, any use of a member’s NOLs and other tax credit carryforwards by the other member is subject to reimbursement by the benefiting member for the actual tax benefit realized. Federal net operating losses generated on or prior to December 31, 2017, expire in varying amounts between 2030 and 2037, while federal net operating losses generated after December 31, 2017, carryforward indefinitely. The state net operating losses expire in varying amounts between 2028 and 2038.
As of December 31, 2018, AgeX has research and development tax credit carryforwards for federal and state tax purposes of $903,000 and $831,000, respectively. Although this LifeMap Sciences credit has been included as part of the AgeX credit carryforwards, LifeMap Sciences filed a separate federal income tax return prior to January 1, 2018 and its prior research credit carryforwards may not be used to offset federal taxable income of AgeX. As AgeX and its subsidiaries were included in the California combined return with BioTime, these credits noted above will remain with AgeX. The federal tax credits expire between2028 and 2038, while the state tax credits have no expiration date.
A valuation allowance is provided when it is more likely than not that some or all of the deferred tax assets will not be realized. AgeX established a full valuation allowance for all periods presented due to the uncertainty of realizing future tax benefits from its net operating loss carryforwards and other deferred tax assets. The change in the valuation allowance was an increase of $2.2 million from 2017 to 2018.
Other Income Tax Matters
Internal Revenue Code Section 382 places a limitation (“Section 382 Limitation”) on the amount of taxable income that can be offset by net operating loss (“NOL”) carryforwards after a change in control (generally greater than 50% change in ownership within a three-year period) of a loss corporation. California has similar rules. Generally, after a control change, a loss corporation cannot deduct NOL carryforwards in excess of the Section 382 Limitation. Due to these “change in ownership” provisions, utilization of the NOL and tax credit carryforwards may be subject to an annual limitation regarding their utilization against taxable income in future periods.
AgeX and its subsidiaries may be subject to potential income tax examination by U.S. federal or states authorities. These potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws. In general, the AgeX legal entity is not subject to tax examination by major taxing authorities since AgeX has not yet filed any income tax returns as it was formed in 2017. For AgeX subsidiaries that did operate and filed separate tax returns, those entities are not subject to tax examination by major taxing authorities for tax years before 2014. However, the taxing authorities may still make adjustments to the net operating loss and credit carryforwards used in open years by AgeX or any of its subsidiaries. Any potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws.
8. Commitments and Contingencies
AgeX had no commitments other than those under the Shared Facilities and Services Agreement described in Note 4. The minimum fixed payments due under the Shared Facilities Agreement are approximately $150,000 per month (see Note 9).
Litigation – General
AgeX is subject to various claims and contingencies in the ordinary course of its business, including those related to litigation, business transactions, employee-related matters, and others. When AgeX is aware of a claim or potential claim, it assesses the likelihood of any loss or exposure. If it is probable that a loss will result and the amount of the loss can be reasonably estimated, AgeX will record a liability for the loss. If the loss is not probable or the amount of the loss cannot be reasonably estimated, AgeX discloses the claim if the likelihood of a potential loss is reasonably possible and the amount involved could be material. AgeX is not aware of any claims likely to have a material adverse effect on its financial condition or results of operations.
Employment Contracts
Limitation on Share Recycling
AgeX has entered into employment contracts with certain executive officers. Under
Shares subject to an Award shall not again be made available for issuance or delivery under the provisionsIncentive Plan if those shares are (a) shares tendered in payment of an option, (b) shares delivered or withheld by us to satisfy any tax withholding obligation, (c) shares covered by a stock-settled SAR or other Award that were not issued upon the settlement of the contracts, AgeXAward, or (d) shares repurchased by us using the proceeds from option exercises. Only shares subject to an Award that is cancelled or forfeited or expires prior to exercise or realization may be required to incur severance obligations for matters relating to changes in control, as defined, and involuntary terminations.regranted under the Incentive Plan.
Indemnification
In the normal course of business, AgeX may provide indemnifications of varying scope under AgeX’s agreements with other companies or consultants, typically for AgeX’s pre-clinical programs. Pursuant to these agreements, AgeX will generally agree to indemnify, hold harmless, and reimburse the indemnified parties for losses and expenses suffered or incurred by the indemnified parties arising from claims of third parties in connection with AgeX’s pre-clinical programs. Indemnification provisions could also cover third party infringement claims with respect to patent rights, copyrights, or other intellectual property pertaining to AgeX’s pre-clinical programs. The term of these indemnification agreements will generally continue in effect after the termination or expirationforegoing description of the particular research, development, services, or license agreement to which they relate. The potential future payments AgeX could be required to make under these indemnification agreements will generally not be subject to any specified maximum amount. Historically, AgeX has not been subject to any claims or demands for indemnification. AgeX also maintains various liability insurance policies that limit AgeX’s financial exposure. As a result, AgeX believes the fair value of these indemnification agreementsIncentive Plan is minimal. Accordingly, AgeX has not recorded any liabilities for these agreements as of December 31, 2018 and 2017.
9. Subsequent Events
Pursuantqualified in its entirety by reference to the Warrant Agreement governingIncentive Plan, a copy of which is filed as an Exhibit to our Registration Statement on Form 10 and is incorporated herein by reference.
Other Compensation Plans
We do not have any pension plans, defined benefit plans, or non-qualified deferred compensation plans. We may make contributions to 401(k) plan accounts for participating executive officers and other employees.
Non-Employee Director Compensation
Directors and members of committees of the Warrants, AgeX’s Board of Directors set March 18, 2019who are our employees are entitled to receive compensation as the expiration dateemployees but are not compensated for serving as directors or attending meetings of the Warrants and onBoard or before that date, holderscommittees of the Warrants purchased 1.8 million shares of common stock through the exerciseBoard. All directors are entitled to reimbursements for their out-of-pocket expenses incurred in attending meetings of the Warrants for $4.5 million in aggregate proceeds to AgeX. Any Warrants not exercised expired on that date.
On March 21, 2019, AgeX entered into a sublease of an office and research facility (the “New Facility”) comprising approximately 23,911 square feet of space in a building in an office and research park at 965 Atlantic Avenue, Alameda, California. AgeX plans to operate its principal offices and research laboratory at the New Facility. The commencementBoard or committees of the sublease and AgeX’s obligation to pay rent is subject toBoard.
For the conditions that the master landlord approves the sublease, AgeX’s plans for constructing certain laboratory improvements, and AgeX’s use of certain reagents in the laboratory in the New Facility.
Base monthly rent will be $35,866.50 for the initial 12 months of the sublease term and then will increase to $36,942.50. In addition, AgeX will pay real property taxes, insurance and operating expenses pertaining to the building in which the New Facility is located. The sublease term will expire on December 31, 2020.
In connection with the sublease, AgeX will also purchase certain laboratory and other equipment from the sublessor for $40,000.
AgeX will be responsible for the maintenance and repair of the New Facility, including electrical, plumbing, HVAC and other systems serving the New Facility but excluding structural and other external portions of the building in which the New Facility is located, and other external areas such as parking, landscaping and walkways associated with the building.
AgeX will be in default under the sublease, and the sublandlord may terminate the sublease and may exercise other remedies against AgeX for losses and damages under the sublease and applicable law, if any one or more of the following events occurs: (a) AgeX fails to pay any rent or any other sum required to be paid under the sublease for a period of ten (10) days after written notice of delinquency is delivered by the sublandlord; provided, however, that if AgeX fails to pay rent or other sums due within ten (10) days of the date due three or more times during any twelve month period, then any subsequent failure to pay any rent or other sum when due shall constitute a default without the requirement of any written notice; (b) a material default by AgeX in the performance of any other terms, covenants or conditions of the sublease where the failure continues for thirty (30) days after written notice from the sublandlord; provided that if AgeX defaults in the performance of the same obligation three or more times in any twelve month period and notice from the sublandlord was given in each instance, no cure period shall thereafter be applicable; (c) AgeX becomes bankrupt or insolvent, makes an assignment for the benefit of creditors, bankruptcy or reorganization proceedings are commenced by or against AgeX, and in the case of an involuntary proceeding are not discharged within 60 days, the appointment of a receiver for a substantial part of AgeX’s assets, or the levy upon the sublease or AgeX’s estate in the sublease by attachment or execution, or (d) AgeX abandons the New Facility.
AgeX has agreed to indemnify the sublandlord against certain liabilities arising under laws pertaining to hazardous materials. The indemnity of the sublandlord will pertain to any deposit, spill, discharge or release of hazardous materials that occurs during the term of the sublease or from AgeX’s failure to comply with requirements of governmental authorities.
The sublease requires AgeX to maintain certain liability and other insurance and contains customary provisions pertaining to matters such as damage or destruction of the New Facility, taking by eminent domain or similar process, restrictions on subletting and assignment, and other matters.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
It is management’s responsibility to establish and maintain adequate internal control over all financial reporting pursuant to Rule 13a-15 under the Exchange Act. Our management, including our principal executive officer and our principal financial officer, have reviewed and evaluated the effectiveness of our disclosure controls and procedures as of the end of our fourth quarter. Following this review and evaluation, management collectively determined that our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act (i) is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms; and (ii) is accumulated and communicated to management, including our chief executive officer and our chief financial officer, as appropriate to allow timely decisions regarding required disclosure.
This Report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of AgeX’s registered public accounting firm due to a transition period established by rules of the Securities and Exchange Commission for newly public companies.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the fourth quarter of our fiscal year ended December 31, 2018 that have materially affected,2023, non-employee Directors were entitled to receive the following annual cash fees for serving as a Member or are reasonably likelyChair of the Board of Directors or a designated committee.
Member of Board of Directors: $35,000
Chair of Board of Directors: $60,000
Chair of Audit Committee: $10,000
Chair of Compensation Committee: $5,000
Chair of Nominating & Corporate Governance Committee: $5,000
The following table summarizes certain information concerning the compensation paid during the past fiscal year to materially affect,each of the persons who served as directors during the year ended December 31, 2023 and who were not our internal control over financial reporting.employees on the date the compensation was earned. The compensation Dr. Hackett received for her service as a non-employee director prior to becoming our Interim Chief Executive Officer is included in the Summary Compensation Table above.
| Name | Fees Earned or Paid in Cash | Option Awards (1) | Total | |||||||||
| Gregory H. Bailey | $ | 40,000 | $ | - | $ | 40,000 | ||||||
| Michael H. May(2) | $ | 42,873 | $ | - | $ | 42,873 | ||||||
| Jean-Christophe Renondin(3) | $ | 15,890 | $ | 16,451 | $ | 32,341 | ||||||
| (1) | In accordance with SEC rules, the amounts shown reflect the aggregate grant date fair value of stock awards granted to Non-Employee Directors during 2023, computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718 (FASB ASC 718). The grant date fair value for the stock options is measured based on the closing price of AgeX’s common stock on the date of grant. See Note 8, Stock-Based Awards to AgeX’s consolidated financial statements included elsewhere in this Report for details as to the assumptions used to determine the fair value of the awards. As of December 31, 2023, Dr. Bailey held 4,691 stock options, Dr. May held 3,597 stock options, and Dr. Renondin held 734 stock options, all of which were fully vested as of December 31, 2023. Options that were out of the money on March 12, 2024 (other than the options held by Dr. May which expired on March 12, 2024) were cancelled on that date to comply with certain provisions of the Merger Agreement. | |
| (2) | Dr. May’s term as a director expired on December 13, 2023. On that date, 3,597 stock options were vested but expired on March 12, 2024, 90 days from the date his term as a director ended. | |
| (3) | Dr. Renondin was appointed as a director to fill a vacancy on the Board of Directors on August 9, 2023. Dr. Renondin was appointed to serve on the Audit Committee, Compensation Committee, and as Chair of the Nominating and Corporate Governance Committee of the Board of Directors. On August 9, 2023, Dr. Renondin was awarded 734 stock options which had a fair value of $16,451 on the grant date. These options were fully vested as of December 31, 2023 but were cancelled on March 12, 2024 to comply with certain provisions of the Merger Agreement. |
None
Item10. Directors, Executive Officers, and Corporate Governance
Information about our directors and each nominee for election as a director is contained under the caption “Election of Directors” in our Proxy Statement for our 2019 Annual Meeting of Stockholders and is incorporated herein by reference. Information about our executive officers, committees of the Board of Directors, and compensation of directors is reported under the caption “Corporate Governance” in our Proxy Statement for our 2019 Annual Meeting of Stockholders and is incorporated herein by reference.
We have a written Code of Business Conduct and Ethics (the “Code of Ethics”) that applies to our principal executive officer, our principal financial officer and accounting officer, our other executive officers, our other employees, and our directors. The purpose of the Code of Ethics is to promote (i) honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; (ii) full, fair, accurate, timely, and understandable disclosure in reports and documents that we file with or submit to the Securities and Exchange Commission and in our other public communications; (iii) compliance with applicable governmental rules and regulations; (iv) prompt internal reporting of violations of the Code of Ethics to an appropriate person or persons identified in the Code; and (v) accountability for adherence to the Code. A copy of our Code of Ethics has been posted on our internet website and can be found atwww.agexinc.com. If we amend or waive a provision of our Code of Ethics that applies to our chief executive officer or chief financial officer, we will post the amended Code of Ethics or information about the waiver on our internet website.
Information about our compliance with Section 16(a) of Exchange Act reported under the caption “Compliance with Section 16(a) of the Securities Exchange Act of 1934” in our Proxy Statement for our 2019 Annual Meeting of Stockholders is incorporated herein by reference.
Item 11. Executive Compensation
Information on compensation of our executive officers reported under the caption “Executive Compensation” in our Proxy Statement for our 2019 Annual Meeting of Stockholders is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management, and Related Stockholder Matters
Information onSecurity Ownership of Certain Beneficial Owners and Management
The following table sets forth information regarding the numberbeneficial ownership of our common sharesstock as of AgeX beneficially ownedMarch 14, 2024, by (i) each stockholderof our named executive officers, (ii) each of our directors, (iii) all of our directors and executive officers as a group; and (iv) each person, or group of affiliated persons, who is known by us to be the beneficial owner ofbeneficially own more than 5% or more of our common stock. Our calculation of the percentage of beneficial ownership is based on 2,500,664 shares of common stock outstanding as of March 14, 2024. Amounts shown do not take into account shares of common stock issuable upon the exercise of the Post-Merger Warrants that were distributed to AgeX stockholders on March 19, 2024, which are not exercisable unless the Merger is consummated
Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, including options that are currently exercisable or exercisable within 60 days of March 14, 2024, and restricted stock units that will vest within 60 days of March 14, 2024. Shares of our common stock issuable pursuant to stock options and restricted stock units currently exercisable or exercisable within 60 days of March 14, 2024, and restricted stock units that will vest within 60 days of March 14, 2024, are deemed outstanding for computing the percentage of the person holding such equity awards and the percentage of any group of which the person is a member but are not deemed outstanding for computing the percentage of any other person. Except as indicated by each directorthe footnotes below, we believe, based on the information furnished to us, that the persons named in the table below have sole voting and named executive officer, and byinvestment power with respect to all directors and named executive officers as a group, contained undershares they beneficially own, subject to community property laws where applicable. The information does not necessarily indicate beneficial ownership for any other purpose, including for purposes of Section 16 of the caption “Principal Stockholders” in our Proxy Statement for our 2019 Annual Meeting of Stockholders is incorporated herein by reference.Exchange Act.
| Name of Beneficial Owner | Number of Shares Beneficially Owned(2) | Percentage of Shares Beneficially Owned | ||||||
| 5% Stockholders | ||||||||
| Juvenescence Limited and certain affiliates(1) | 2,627,231 | 81.1 | % | |||||
| Named Executive Officers and Directors | ||||||||
| Joanne M. Hackett | - | * | ||||||
| Michael D. West(3) | 1,193 | * | ||||||
| Andrea E. Park(4) | 8,361 | * | ||||||
| Nafees N. Malik(5) | - | * | ||||||
| Gregory H. Bailey | - | * | ||||||
| Jean-Christophe Renondin(6) | 734 | * | ||||||
| Steven Mintz(7) | 3,733 | * | ||||||
| All executive officers and directors as a group (8 persons)(8) | 14,024 | * | % | |||||
| * | Less than 1% | |
| (1) | Includes 1,889,323 shares of AgeX common stock held by JuvVentures (UK) Limited (JuvVentures), a wholly-owned subsidiary of Juvenescence Limited, 303,259 shares of AgeX common stock that may be acquired upon the exercise of common stock purchase warrants, 434,649 shares of AgeX common stock that may be acquired through the conversion of $6,000,000 of certain outstanding loans into shares of AgeX common stock at an assumed conversion price of $13.80 per share based on the closing price of AgeX common stock on the NYSE American on March 4, 2024 as adjusted to reflect the Reverse Stock Split. Dr. Gregory Bailey is the executive chairman of Juvenescence Limited and may be deemed to have shared power to vote or direct the vote of, and/or shared power to dispose or to direct the disposition of, the shares held by JuvVentures. This response is not and shall not be construed as an admission that Dr. Bailey is the beneficial owner of any securities of AgeX other than the securities actually owned by Dr. Bailey (if any). The address of Juvenescence is 1st Floor, Viking House, St Paul’s Square, Ramsey, Isle of Man, British Isles, IM8 1GB. The foregoing information is based solely on a Schedule 13D/A filed with the SEC on March 7, 2024, which provides information only as of March 5, 2024 and consequently, Juvenescence’s beneficial ownership may have changed since that date. | |
| (2) | Pursuant to the Merger Agreement, all out of the money options (meaning those options with an exercise price equal to or greater than $0.7751 on a pre-Reverse Stock Split basis) were canceled. | |
| (3) | Dr. West served as Chief Executive Officer of AgeX and of Reverse Bio through August 9, 2023 and October 31, 2023, respectively. | |
| (4) | Includes 8,352 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are presently exercisable or that will become exercisable within 60 days. Excludes 177 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. | |
| (5) | Dr. Malik served as Chief Operating Officer of AgeX through December 27, 2023. | |
| (6) | Entirely shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are presently exercisable. | |
| (7) | Includes 3,307 shares of AgeX common stock held in joint accounts with adult children, 426 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that will become exercisable within 60 days. Excludes 1,386 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. Mr. Mintz was appointed as a director to the AgeX Board on January 8, 2024. | |
| (8) | Includes 9,512 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are presently exercisable or that will become exercisable within 60 days and 3 shares of AgeX common stock held by one officer who is not a Named Executive Officer. Excludes 1,563 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. | |
| 117 |
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information
Related Person Transactions Policy and Procedures
The AgeX Board has adopted a written Related Person Transaction Policy, which applies to transactions exceeding $120,000 in which any of AgeX’s officers, directors, beneficial owners of more than 5% of the outstanding shares of AgeX common stock, or any member of their immediate family, has a direct or indirect material interest, determined in accordance with the policy. Those transactions are referred to as Related Person Transactions. A Related Person Transaction will be subject to review and approval by the Audit Committee of the AgeX Board prior to effectiveness or consummation, to the extent practical. The Audit Committee will review the relevant information available to it about transactions with related persons; reportedthe Related Person Transaction. The Audit Committee may approve or ratify the Related Person Transaction only if the Audit Committee determines that, under the caption “Principal Stockholders,”circumstances, the transaction is in, or is not in conflict with, AgeX’s best interests.
Certain Related Person Transactions
Compensation of AgeX’s Interim Chief Executive Officer
On August 9, 2023, AgeX entered into a Consulting Agreement with Dr. Hackett pursuant to which she will receive a fee in the amount of $160,000 per year for services rendered as Interim Chief Executive Officer of AgeX. Dr. Hackett will not be eligible to participate in any AgeX retirement, pension, life, health, accident and information about director independence reporteddisability insurance, or other similar employee benefit plans for AgeX executive officers or employees other than the Incentive Plan.
Compensation of AgeX’s Chief Operating Officer
From October 2018 through December 31, 2023, AgeX’s Chief Operating Officer, Dr. Malik, who was an employee of Juvenescence, devoted a majority of his time to AgeX’s operations for which AgeX reimbursed Juvenescence for his services on an agreed upon fixed annual rate of approximately $272,000 from October 18, 2018 through March 10, 2019 and approximately $283,000 from March 11, 2019 through December 31, 2023. Additionally, Dr. Malik received a $50,000 bonus in March 2019. As of December 31, 2023 AgeX had accrued approximately $66,000 payable to Juvenescence for Dr. Malik’s services rendered.
2019 Loan Agreement and Warrant Agreement
On August 13, 2019, AgeX and Juvenescence entered into a Loan Facility Agreement (the “2019 Loan Agreement”) pursuant to which Juvenescence has provided to AgeX a $2.0 million line of credit for a period of 18 months. On February 10, 2021, AgeX entered into an amendment (the First Amendment) to the 2019 Loan Agreement. The First Amendment extended the maturity date of loans under the caption “Election2019 Loan Agreement to February 14, 2022 and increased the amount of Directors,” in our Proxy Statement for ourthe loan facility by $4.0 million. On November 8, 2021, AgeX entered into Amendment No. 2 (the Second Amendment) to the 2019 Annual MeetingLoan Agreement. The Second Amendment increased the amount of Stockholders is incorporated hereinthe loan facility by reference.
Item 14. Principal Accounting Fees and Services
Information about our Audit Committee’s pre-approval policy for audit services, and information on our principal accounting fees and services reportedanother $1.0 million. As of December 31, 2021, AgeX had borrowed all of the $7.0 million total line of credit under the caption “Ratification2019 Loan Agreement. Concurrent with the first draw down of funds under the 2019 Loan Agreement, AgeX issued to Juvenescence 540 shares of AgeX common stock, with an approximate value of $56,000. On February 14, 2022, AgeX refinanced the $7.0 million outstanding principal amount of the Selectionloans and a $160,000 origination fee due under the 2019 Loan Agreement. See the discussion below regarding the 2022 Secured Note and repayment of Our Independent Auditors” in our Proxy Statementthe amounts borrowed under the 2019 Loan Agreement.
As consideration for ourthe line of credit under the 2019 Annual MeetingLoan Agreement, AgeX issued to Juvenescence warrants to purchase 4,265 shares of Stockholders is incorporated herein by reference.AgeX common stock, with an exercise price of $91.44 per share, which was the volume weighted average price on the NYSE American (VWAP) of AgeX common stock over the twenty trading days prior to the date the warrants were issued. The warrants expired on August 12, 2022.
2020 Loan Agreement and New Warrant Agreement
On March 30, 2020, AgeX and Juvenescence entered into a new Secured Convertible Facility Agreement (the “2020 Loan Agreement”), which was amended on March 13, 2023 to extend the maturity date by one year, pursuant to which AgeX borrowed $8.0 million from Juvenescence. During July 2023, the full $8 million of 2020 Loan Agreement indebtedness was extinguished in exchange for shares of AgeX Series A preferred stock pursuant to the 2023 Exchange Agreement between AgeX and Juvenescence described below.
Common Stock and 2020 Warrants – Under the terms of the 2020 Loan Agreement, AgeX issued to Juvenescence 810 shares of AgeX common stock as an arrangement fee for the loan facility when AgeX borrowed an aggregate of $3.0 million, and AgeX issued to Juvenescence warrants to purchase a total of 104,365 shares of AgeX common stock (the “2020 Warrants”). The number of 2020 Warrants issued was determined as follows: each time AgeX received an advance of funds under the 2020 Loan Agreement, AgeX issued to Juvenescence a number of 2020 Warrants equal to 50% of the number determined by dividing the amount of the advance by the applicable market price of AgeX common stock. The market price for each 2020 Warrant when issued was the closing price per share of AgeX common stock on the NYSE American on the date of the applicable notice from AgeX requesting a draw of funds that triggered the obligation to issue the 2020 Warrant. The exercise price of the 2020 Warrants is the applicable market price of AgeX common stock. Each of the 2020 Warrants will expire at 5:00 p.m. New York time three years after the date of its issuance. AgeX had issued to Juvenescence 2020 Warrants to purchase a total of 104,365 shares of AgeX common stock of which 25,628 were outstanding as of December 31, 2023. The exercise prices of the 2020 Warrants that were still outstanding as of December 31, 2023 range from $28.49 per share to $66.65 per share representing the market closing price on the NYSE American of AgeX common stock on the one day prior to delivery of the drawdown notices. The number of shares issuable upon exercise of the 2020 Warrants and the exercise price per share are subject to adjustment upon the occurrence of certain events such as a stock split or reverse split or combination of the common stock, stock dividend, recapitalization or reclassification of the common stock, and similar events.
2022 Secured Convertible Promissory Note and Security Agreement
On February 14, 2022, AgeX and Juvenescence entered into a Secured Convertible Promissory Note (Original 2022 Secured Note) pursuant to which Juvenescence agreed to provide to AgeX a $13,160,000 line of credit for a period of 12 months. AgeX drew an initial $8,160,000 of the line of credit and used $7,160,000 to refinance the outstanding principal and the loan origination fees under the 2019 Loan Agreement with Juvenescence. On February 9, 2023, AgeX and Juvenescence entered into an Amended and Restated Secured Convertible Promissory Note (“2022 Secured Note”) which amended and restated the Original 2022 Secured Note and added $2 million to the line of credit available to be borrowed by AgeX under the Original 2022 Secured Note subject to Juvenescence’s discretion to approve each loan draw. On May 9, 2023, AgeX and Juvenescence entered into an Allonge and Second Amendment to Amended and Restated Convertible Promissory Note (the “2022 Secured Note Second Amendment”) that increased the amount of the line of credit available to AgeX by $4,000,000, subject to the terms of the 2022 Secured Note and Juvenescence’s discretion to approve and fund each of AgeX’s future draws of that additional amount of credit. On June 2, 2023, AgeX and Juvenescence entered into a Third Amendment to Amended and Restated Convertible Promissory Note, to provide that (i) AgeX may draw on the available portion of the line of credit under the 2022 Secured Note until the earlier of the date a Qualified Offering (as defined in the 2022 Secured Note) is consummated by AgeX or October 31, 2023 (subject to Juvenescence’s discretion to approve each loan draw as provided in the 2022 Secured Note), (ii) AgeX will not be obligated to issue additional common stock purchase warrants to Juvenescence in connection with the receipt of loan funds made available pursuant to the 2022 Secured Note Second Amendment, and (iii) the definition of the Reverse Financing Condition as defined in the 2022 Secured Note was amended to extend to June 20, 2023, the referenced deadline for fulfillment of the condition to permit borrowing or other incurrence of indebtedness by Reverse Bio. On July 31, 2023, AgeX and Juvenescence entered into a Fourth Amendment to the 2022 Secured Note to provide that (i) the definition of Reverse Financing Condition was amended to extend to October 31, 2023 the referenced deadline for fulfillment of the condition to permit borrowing or other incurrence of indebtedness by AgeX’s subsidiary Reverse Bio, and (ii) Juvenescence may convert the outstanding amount of the 2022 Secured Note loans or any portion of such loans into AgeX common stock without restriction by the “19.9% Cap” if Juvenescence elects to convert those amounts at a conversion price or prices equal to the “Drawdown Market Prices” applicable to such loan amounts in lieu of a lower conversion price set with reference to the current market price of AgeX common stock at the time of conversion. The 19.9% Cap is a provision of the 2022 Secured Note that limits the amount of common stock that Juvenescence may acquire through the conversion of 2022 Secured Note loans in order to comply with NYSE American requirements pertaining to the amount of shares that a listed company, such as AgeX, may sell at a price less than the market prices prevailing at the time the loans were made (the “Drawdown Market Prices”) without shareholder approval. On November 9, 2023, AgeX and Juvenescence entered into an Allonge and Fifth Amendment to the 2022 Secured Note that increases the amount of the line of credit available to AgeX by $4,400,000, subject to the terms of the 2022 Secured Note and Juvenescence’s discretion to approve and fund each of AgeX’s future draws of that additional amount of credit. On February 9, 2024, AgeX and Juvenescence entered into a 2022 Secured Note Sixth Amendment that extends the repayment date of the 2022 Secured Note to May 9, 2024. As of March 20, 2024, the outstanding principal amount of the 2022 Secured Note was $6,900,000.
As an arrangement fee for the 2022 Secured Note, AgeX will pay Juvenescence an origination fee in an amount equal to 4% of the amount each draw of loan funds, which will accrue as each draw is funded, and an additional 4% of all the total amount of funds drawn that will accrue following the end of the 12 month period during which funds may be drawn from the line of credit. The origination fee will become due and payable on the maturity date of the 2022 Secured Note or in a pro rata amount with any prepayment of in whole or in part of the outstanding principal balance of the 2022 Secured Note.
During July 2023, $17,992,800 of indebtedness and accrued loan origination fees under the 2022 Secured Note was extinguished in exchange for shares of AgeX Series A Preferred Stock and Series B Preferred Stock pursuant to the Exchange Agreement described below.
| 119 |
Conversion of Loan Amounts into Common Stock – In lieu of repayment of funds borrowed, AgeX may convert the loan balance and any accrued but unpaid origination fees into AgeX common stock or “units” if AgeX raises at least $10,000,000 through sale of AgeX common stock (or AgeX common stock paired with warrants or other convertible securities in “units”). The conversion price per share or units shall be the lowest price at which such shares or units are sold. Juvenescence may convert the principal balance and accrued origination fee in whole or in part into AgeX common stock at any time at Juvenescence’s election at the closing price per share of AgeX common stock on the NYSE American or other national securities exchange on the date prior to the date Juvenescence gives AgeX notice of Juvenescence’s election to convert the 2022 Secured Note, in whole or in part, into AgeX common stock.
2022 Warrants – Upon each draw down of funds under the 2022 Secured Note prior to June 2, 2023, AgeX issued to Juvenescence warrants to purchase shares of AgeX common stock (the “2022 Warrants”). The 2022 Warrants are governed by the terms of a Warrant Agreement, as amended by a Reaffirmation and Amendment Agreement, between AgeX and Juvenescence. The number of 2022 Warrants issued is equal to 50% of the number determined by dividing the amount of the applicable loan draw by the applicable market price. The market price was the last closing price per share of AgeX common stock on the NYSE American preceding the delivery of the notice from AgeX requesting a draw of funds that triggered the obligation to issue 2022 Warrants. The exercise price of the 2022 Warrants is the applicable market price. Each of the 2022 Warrants will expire at 5:00 p.m. New York time three years after the date of its issuance.
As of December 31, 2023, AgeX had issued to Juvenescence 2022 Warrants to purchase a total of 294,482 shares of AgeX common stock. The exercise prices of the 2022 Warrants range from $20.75 per share to $30.94 per share representing the market closing price of AgeX common stock on the NYSE American on the one day prior to delivery of the applicable drawdown notices. The number of shares issuable upon exercise of the 2022 Warrants and the exercise price per share are subject to adjustment upon the occurrence of certain events such as a stock split or reverse split or combination of the common stock, stock dividend, recapitalization or reclassification of the common stock, and similar events.
Default Provisions –The loan balance and origination fees may become immediately due and payable prior to the mandatory repayment date if an Event of Default as defined in the 2022 Secured Note occurs. Events of Default under the 2022 Secured Note include the following: (a) AgeX fails to pay any principal amount payable by it in the manner and at the time provided under and in accordance with the 2022 Secured Note; (b) AgeX fails to pay any other amount payable by it in the manner and at the time provided under and in accordance with the 2022 Secured Note or the Security Agreement described below or any other agreement executed in connection with the 2022 Secured Note (the” Loan Documents”) and the failure is not remedied within three business days; (c) AgeX fails to perform any of its covenants or obligations or fails to satisfy any of the conditions under the 2022 Secured Note or any other Loan Document and, and such failure (if capable of remedy) remains unremedied to the satisfaction of Juvenescence (in its sole discretion) for 10 business days after the earlier of (i) notice requiring its remedy has been given by Juvenescence to AgeX and (ii) actual knowledge of the failure by senior officers of AgeX; (d) if any indebtedness of AgeX in excess of $100,000 becomes due and payable, or a breach or other circumstance arises thereunder such that Juvenescence is entitled to declare such indebtedness due and payable, prior to its due date, or any indebtedness of AgeX in excess of $25,000 is not paid on its due date; (e) AgeX stops payment of its debts generally or ceases or threatens to cease to carry on its business or is unable to pay its debts as they fall due or is deemed by a court of competent jurisdiction to be unable to pay its debts as they fall due, or enters into any arrangements with its creditors generally; (f) if (i) an involuntary proceeding (other than a proceeding instituted by Juvenescence or an affiliate of Juvenescence) shall be commenced or an involuntary petition shall be filed seeking liquidation, reorganization or other relief in respect of AgeX and any subsidiary, or of all or a substantial part of its assets, under any federal, state or foreign bankruptcy, insolvency, receivership or similar law now or hereafter in effect or (ii) an involuntary appointment of a receiver, trustee, custodian, sequestrator, conservator or similar official for AgeX or a subsidiary or for a substantial part of its assets occurs (other than in a proceeding instituted by Juvenescence or an affiliate of Juvenescence), and, in any such case, such proceeding shall continue undismissed and unstayed for sixty (60) consecutive days without having been dismissed, bonded or discharged or an order of relief is entered in any such proceeding; (g) it becomes unlawful for AgeX to perform all or any of its obligations under the 2022 Secured Note or any authorization, approval, consent, license, exemption, filing, registration or other requirement of any governmental, judicial or public body or authority necessary to enable AgeX to comply with its obligations under the 2022 Secured Note or to carry on its business is not obtained or, having been obtained, is modified in a manner that precludes AgeX or its subsidiaries from conducting their business in any material respect, or is revoked, suspended, withdrawn or withheld or fails to remain in full force and effect; (h) the issuance or levy of any judgment, writ, warrant of attachment or execution or similar process against all or any material part of the property or assets of AgeX or a subsidiary if such process is not released, vacated or fully bonded within 60 calendar days after its issue or levy; (i) any injunction, order, judgment or decision of any court is entered or issued which, in the opinion of Juvenescence, materially and adversely affects, or is reasonably likely so to affect, the ability of AgeX or a subsidiary to carry on its business or to pay amounts owed to Juvenescence under the 2022 Secured Note; (j) AgeX, whether in a single transaction or a series of related transactions, sells, leases, licenses, consigns, transfers or otherwise disposes of any material portion of its assets (with any such disposition with respect to any asset or assets with a fair value of at least $250,000 being deemed material), other than (i) certain permitted investments (ii) sales, transfers and dispositions of inventory in the ordinary course of business, (iii) any termination of a lease of real or personal property that is not necessary in the ordinary course of AgeX’s business, could not reasonably be expected to have a material adverse effect and does not result from AgeX’s default, and (iv) any sale, lease, license, consignment, transfer or other disposition of assets that are no longer necessary in the ordinary course of business or which has been approved in writing by Juvenescence; (k) any of the following shall occur: (i) the security and/or liens created by the Security Agreement or any other Loan Document shall at any time cease to constitute valid and perfected security and/or liens on any material portion of the collateral intended to be covered thereby; (ii) except for expiration in accordance with its terms, the Security Agreement or any other Loan Document pursuant to which a lien is granted by AgeX in favor of Juvenescence shall for whatever reason be terminated or shall cease to be in full force and effect; (iii) the enforceability of the Security Agreement or any other Loan Document pursuant to which a lien is granted by AgeX in favor of Juvenescence shall be contested by AgeX or a subsidiary; (iv) AgeX shall assert that its obligations under the 2022 Secured Note or any other Loan Document shall be invalid or unenforceable; or (v) a loss, theft, damage or destruction occurs with respect to a material portion of the collateral; (l) there is any change in the financial condition of AgeX and its subsidiaries which, in the opinion of Juvenescence, materially and adversely affects, or is reasonably likely so to affect, the ability of AgeX to perform any of its obligations under the 2022 Secured Note; and (m) any representation, warranty or statement made, repeated or deemed made or repeated by AgeX in the 2022 Secured Note, or pursuant to the Loan Documents, is incomplete, untrue, incorrect or misleading in any material respect when made, repeated or deemed made.
| 120 |
Security Agreement – AgeX has entered into a Security Agreement granting Juvenescence a security interest in substantially all of the assets of AgeX, including a security interest in shares of AgeX subsidiaries that hold certain assets, as collateral for AgeX’s loan obligations. If an Event of Default occurs under the 2022 Secured Note, Juvenescence will have the right to foreclose on the assets pledged as collateral. Concurrently with the execution of the 2022 Secured Note Fifth Amendment, AgeX also entered into an additional Pledge Agreement to add shares of a subsidiary to the collateral under the Security Agreement, and AgeX’s subsidiaries ReCyte, Reverse Bio, and UniverXome each entered into a Guaranty Agreement and Joinder Agreement pursuant to which each of them agreed to guaranty AgeX’s obligations to Juvenescence pursuant to the 2022 Secured Note, as amended by the 2022 Secured Note Fifth Amendment, and to grant Juvenescence a security interest in their respective assets pursuant to the Security Agreement to secure their obligations to Juvenescence.
The 2023 Secured Convertible Promissory Note and Security Agreement
On March 13, 2023, AgeX and Juvenescence entered into a Secured Convertible Promissory Note (the “2023 Secured Note”) pursuant to which Juvenescence loaned to AgeX $10,000,000. AgeX used the loan proceeds to finance a $10,000,000 loan to Serina which is evidenced by a promissory note payable by Serina to AgeX. In lieu of accrued interest, AgeX agreed to pay Juvenescence an origination fee in an amount equal to 7% of the loan funds disbursed to AgeX, which will accrue in two installments. During July 2023, the $10,000,000 principal balance of the 2023 Secured Note indebtedness and a portion of the loan origination fee was extinguished in exchange for shares of AgeX Series B preferred stock pursuant to the Exchange Agreement described below.
Conversion of Loan Amounts into Common Stock – AgeX may convert the any accrued but unpaid origination fee into AgeX common stock or “units” if AgeX consummates a sale of common stock (or common stock paired with warrants or other convertible securities in “units”) in which the gross sale proceeds are at least $10,000,000. If less than $25,000,000 is raised through the sale of AgeX common stock or units, the conversion price per share or units shall be the lowest price at which shares or units are sold. If at least $25,000,000 is raised, the conversion price per share shall be 85% of the “Market Price” of AgeX common stock determined as provided in the 2023 Secured Note. Juvenescence may convert accrued origination fees into AgeX common stock at the market price per share of AgeX common stock.
Amended Security Agreement – AgeX has entered into an Amended and Restated Security Agreement that amended the February 14, 2022 Security Agreement between AgeX and Juvenescence and added the 2023 Secured Note to the obligations secured by the Security Agreement.
| 121 |
Debt Exchanged for Preferred Stock and Remediation of Stock Exchange Listing Deficiency
In order to eliminate a stockholders equity deficiency and to regain compliance with the continued listing requirements of the NYSE American, on July 24, 2023, AgeX issued to Juvenescence 211,600 shares of a newly authorized Series A Preferred Stock and 148,400 shares of a newly authorized Series B Preferred Stock in exchange for the cancellation of a total of $36 million of indebtedness consisting of the outstanding principal amount of loans then outstanding under the 2020 Loan Agreement, the 2022 Secured Note, and the 2023 Secured Note, plus the loan origination fees accrued with respect to the 2022 Secured Note and a portion of the loan origination fees accrued pursuant to the 2023 Secured Note. The cancellation of indebtedness in exchange for the Series A Preferred Stock and Series B Preferred Stock (collectively, the “Preferred Stock”) was conducted pursuant to an Exchange Agreement between AgeX and Juvenescence. The terms of the Preferred Stock are summarized in Note 7, Stockholders’ Equity/(Deficit) in Notes to Consolidated Financial Statements found elsewhere in this Report.
On February 1, 2024, all of the shares of Series A Preferred Stock and Series B Preferred Stock, which were then held by Juvenescence’s subsidiary JuvVentures (UK) Limited (“JuvVentures”), automatically converted into shares of AgeX common stock in accordance with the terms of the Preferred Stock (the “Conversion”). AgeX issued a total of 1,421,666 shares of common stock to JuvVentures in the Conversion.
Based on information reported by Juvenescence in an amendment to its Schedule 13D filed with the SEC on February 5, 2024, as a result of the Conversion, Juvenescence, through JuvVentures, holds of record 1,889,323 issued and outstanding shares of common stock, representing 75.6% of the shares of common stock issued and outstanding as of the close of business on March 14, 2024, without taking into account additional shares of common stock that Juvenescence may acquire through the conversion of certain outstanding indebtedness and the exercise of certain outstanding common stock purchase warrants that Juvenescence holds or Post-Merger Warrants that were distributed to Juvenescence on March 19, 2024. Prior to the Conversion, Juvenescence was already deemed to have beneficially owned the shares of common stock issuable in connection with the Conversion, and Juvenescence reported beneficially owning those shares of common stock in its Schedule 13D prior to the Conversion such that the Conversion did not result in a change in Juvenescence’s beneficial ownership of common stock. However, because the number of issued and outstanding shares of common stock held of record by JuvVentures and beneficially owned by Juvenescence following the Conversion exceeds 50% of all issued and outstanding shares of common stock, Juvenescence now has voting power over a majority of the outstanding shares of the common stock, and a change of control of AgeX may be deemed to have occurred as a result of the Conversion.
As a controlling stockholder holding more than 50% of the outstanding shares of common stock through its subsidiary JuvVentures, Juvenescence has the power to elect all members of the AgeX’ Board and to approve or reject all matters submitted for stockholder approval by the AgeX Board, by Juvenescence as a stockholder, or by other stockholders, including, but not limited to: equity compensation plans for employees, officers, and directors; mergers, acquisitions, and consolidations; sales of AgeX assets; and amendments of AgeX’s certificate of incorporation and bylaws, including all of the Stockholder Matters. If the Merger is consummated in accordance with the terms of the Merger Agreement, immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of AgeX, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of AgeX, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions, including the actual closing price of common stock being equal to or greater than $12.00 per share excluding the impact of the distribution of Post-Merger Warrants to AgeX stockholders. Accordingly, it is expected that the shares of common stock now beneficially owned by Juvenescence would represent substantially less than 50% of the outstanding shares of common stock immediately after the Merger as a result of the issuance of shares of common stock to securities holders of Serina pursuant to the Merger Agreement.
Presently, one member of the AgeX Board, Gregory H. Bailey, is a director of Juvenescence, while a majority of our directors are “independent” directors as defined in the NYSE American Company Guide (the Company Guide), and the Audit Committee, Compensation Committee, and the Nominating & Corporate Governance Committee of our Board are comprised entirely of independent directors. While Juvenescence controls more than 50% of the outstanding common stock, AgeX will qualify as a “controlled company” as defined by the Company Guide. Being a “controlled company” will allow AgeX to exempt itself from the requirements that a majority of its directors be “independent” directors as defined in the Company Guide and that the Compensation Committee and the Nominating & Corporate Governance Committee be comprised entirely of independent directors. The exemption does not apply to the Audit Committee which must be comprised of independent directors. If AgeX were to take advantage of any or all of these exemptions available to controlled companies under the Company Guide, it would be required to disclose doing so in its annual meeting proxy statement or in its Annual Report on Form 10-K.
| 122 |
Registration Rights Agreements
AgeX entered into a Registration Rights Agreement and certain amendments to the original agreement, pursuant to which it has agreed to register for sale under the Securities Act all shares of AgeX common stock presently held by Juvenescence or that may be acquired by Juvenescence through the exercise of common stock purchase warrants that they hold or that they may acquire pursuant to the 2020 Loan Agreement and the 2022 Secured Note, and shares that they may acquire through the conversion of loans under the 2020 Loan Agreement and the 2022 Secured Note, including principal and accrued interest, and the amount of the loan origination fee under the 2022 Secured Note. AgeX has filed a registration statement on Form S-3, which has become effective under the Securities Act, for offerings on a delayed or continuous basis covering 467,657 shares of AgeX common stock held by Juvenescence and 92,358 shares of AgeX common stock that may be issued upon the exercise of a portion of the warrants held by Juvenescence. Juvenescence retains the right to require AgeX to register additional shares of AgeX common stock that Juvenescence may acquire through the exercise of warrants or the conversion of 2020 Loan Agreement loans, 2022 Secured Note loans, and the origination fee under the 2022 Secured Note. AgeX is obligated to pay the fees and expenses of each registered offering under such registration rights agreement except for underwriting discounts and commissions. AgeX and Juvenescence will indemnify each other from certain liabilities in connection the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act.
During July 2023, AgeX and Juvenescence entered into a Registration Rights Agreement pursuant to which AgeX has agreed to use commercially reasonable efforts to register the for sale under the Securities Act the shares of common stock issuable upon conversion of Preferred Stock. A registration statement must be filed upon request of Juvenescence if Form S-3 is available to AgeX. Juvenescence will also have “piggyback” registration rights if AgeX files a registration statement for the sale of shares for itself or other stockholders, subject to certain customary exceptions based on the nature of the registration statement. AgeX will bear the expenses of the registration statement but not underwriting or broker’s commissions related to the sale of the common stock. AgeX and Juvenescence will indemnify each other from certain liabilities in connection the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act.
Stockholder Approval of Certain Matters
2020 Loan Agreement, 2022 Secured Note, 2020 Warrants, and 2022 Warrants
In order to comply with applicable NYSE American listing requirements, the 2020 Loan Agreement and the 2022 Secured Note and the related Warrant Agreements governing the 2020 Warrants and 2022 Warrants placed certain limits on the number of shares of AgeX common stock that may be issued to Juvenescence upon conversion of outstanding loan amounts or exercise of the 2020 Warrants or 2022 Warrants prior to stockholder approval of the issuance of shares to Juvenescence that would result in (a) Juvenescence receiving additional shares in excess of a 19.9% Cap based on the number of shares of AgeX common stock outstanding as of March 30, 2020 in the case of a conversion of the outstanding loan amounts under the 2020 Loan Agreement into AgeX common stock, or outstanding as of February 14, 2022 in the case of a conversion of the outstanding loan amounts under 2022 Secured Note into AgeX common stock, for less than the greater of book value or the applicable tranche market values of AgeX common stock as of March 20, 2020 or February 14, 2022, or (b) Juvenescence owning 50% or more of the outstanding AgeX common stock (the 50% Cap) in the case of a conversion of the outstanding loan amounts under the 2020 Loan Agreement or the 2022 Secured Note or the exercise of the 2020 Warrants or 2022 Warrants. As required by the terms of the 2020 Loan Agreement and the 2022 Secured Note, AgeX sought and obtained the vote of AgeX stockholders approving (i) the ability of AgeX and Juvenescence to convert the loans under the 2020 Loan Agreement and the 2022 Secured Note into shares of AgeX common stock under the applicable loan conversion provisions even if the conversion would result in (a) Juvenescence receiving additional shares in excess of the 19.9% Cap or the 50% Cap limits, and (ii) the ability of Juvenescence to exercise its 2020 Warrants and 2022 Warrants even if the exercise would cause Juvenescence’s ownership of AgeX common stock to equal or exceed the 50% Cap limit.
Series A Preferred Stock and Series B Preferred Stock
At the 2023 annual meeting of stockholders, AgeX sought and obtained the vote of AgeX stockholders approving a proposal eliminating the 19.9% Cap and 50% Cap that limited the number of shares of AgeX Series B Preferred Stock that may be converted into shares of AgeX common stock.
Indemnification Agreements
On March 13, 2023, AgeX executed a Letter of Indemnification in Lieu of or Supplemental to a Medallion Signature Guarantee (the Letter of Indemnification) pursuant to which AgeX agreed to indemnify Equiniti Trust Company, LLC (the “Equiniti Indemnity”) from and against any and all claims, damages, liabilities or losses arising out of the transfer of all of the AgeX common stock held by Juvenescence to its wholly-owned subsidiary, Juvenescence US Corp. (the “Share Transfer”). In connection with the execution of the Letter of Indemnification, AgeX and Juvenescence entered into a Transfer of Shares of AgeX Therapeutics, Inc. Common Stock – Indemnification Agreement, pursuant to which Juvenescence agreed to indemnify AgeX against any and all claims, damages, liabilities or losses arising out of the Share Transfer or the Equiniti Indemnity.
| 123 |
On December 21, 2023, AgeX executed that certain Letter of Indemnification in Lieu of or Supplemental to a Medallion Signature Guarantee (the “ETC Letter of Indemnification”), pursuant to which AgeX agreed to indemnify Equiniti Trust Company LLC and its affiliates, successors and assigns (the “ETC Indemnity”) from and against any and all claims, damages, liabilities or losses arising out of the transfer 467,657 shares of AgeX common stock held by Juvenescence US Corp. to JuvVentures (UK) Limited (the “JUV US Share Transfer”). In connection with AgeX’s execution of the Letter of Indemnification, AgeX and Juvenescence Limited, the ultimate parent company of Juvenescence US Corp. and JuvVentures (UK) Limited, entered into that certain Transfer of Shares of AgeX Therapeutics, Inc. Common Stock – Indemnification Agreement, pursuant to which Juvenescence agreed to indemnify AgeX against any and all claims, damages, liabilities or losses arising out of the JUV US Share Transfer or the ETC Indemnity.
Director Independence
Jean-Christophe Renondin and Steven Mintz qualify as “independent” in accordance with Section 803(A) of the NYSE American Company Guide. Michael May whose term as a director expired at the 2023 annual meeting of stockholders, also qualified as independent under that standard, as did Joanne Hackett until she was appointed Interim Chief Executive Officer. The members of our Audit Committee meet the additional independence standards under Section 803(B)(2) of the NYSE American Company Guide and Rule 10A-3 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The members of our Compensation Committee meet the additional independence standards under Section 805(c)(1) of the NYSE American Company Guide. Our independent directors received no compensation or remuneration during the last fiscal year for serving as directors except as disclosed under “Non-Employee Director Compensation” in Item 11 of this Report. None of the independent directors, nor any of the members of their respective families, have participated in any transaction with us that would disqualify them as “independent” directors under the standards described above.
Gregory H. Bailey does not meet the independence standard because he is a director and former chief executive officer of Juvenescence, which is our largest stockholder that beneficially owns approximately 81.1% of our common stock as reflected in the table included in Item 12 to this Report. Joan Hackett does not meet the independence standard because she is our Interim Chief Executive Officer.
Item 14. Principal Accounting Fees and Services
Audit Fees, Audit Related Fees, Tax Fees and Other Fees
The following table sets forth the aggregate fees billed to us during the fiscal years ended December 31, 2023 and 2022 by WithumSmith+Brown, PC (“Withum”):
| 2023 | 2022 | |||||||
| Audit Fees (1) | $ | 483,000 | $ | 358,000 | ||||
| Audit Related (2) | - | 53,000 | ||||||
| $ | 483,000 | $ | 411,000 | |||||
| (1) | Audit Fees consist of fees billed for professional services rendered for the audit of our annual financial statements included in our Annual Report on Form 10-K, and review of interim financial statements included in our Quarterly Reports on Form 10-Q, and services that are normally provided by our independent registered public accountants in connection with statutory and regulatory filings or engagements. |
| (2) | Audit-Related Fees relate to assurance and related services that are reasonably related to the performance of the audit or review of our consolidated financial statements and are not reported under “Audit Fees.” This category would include fees related to non-routine SEC filings. |
Pre-Approval of Audit and Permissible Non-Audit Services
Our Audit Committee requires pre-approval of all audit and non-audit services. Other than de minimis services incidental to audit services, non-audit services shall generally be limited to tax services such as advice and planning and financial due diligence services. All fees for such non-audit services must be approved by the Audit Committee, except to the extent otherwise permitted by applicable SEC regulations. The Audit Committee may delegate to one or more designated members of the Audit Committee the authority to grant pre-approvals, provided such approvals are presented to the Audit Committee at a subsequent meeting. During 2023 and 2022, 100% of the fees paid to Withum were approved by the Audit Committee.
| 124 |
PART IV
Item 15. Exhibits and Financial Statement and ExhibitsSchedules
(a)(1) Financial Statements.Statements
The following financial statements of AgeX are filed in this Report:
Audited Consolidated Financial Statements
Consolidated Statements of Operations
Consolidated Statements of Stockholders’ Equity (Deficit)
Consolidated Statements of Cash Flows
Notes to Consolidated Financial Statements
(b) Exhibits.(a)(2) Financial Statement Schedules
All other schedules are omitted because they are not required or the required information is included in the financial statements or notes thereto.
(a)(3) Exhibits
The following exhibits are filed herewith or incorporated by reference:
| 125 |
| 126 |
| 127 |
| 128 |
| List of Subsidiaries | ||||||||||
| Consent of | ||||||||||
| Rule 13a-14(a)/15d-14(a) Certification | ||||||||||
| Section 1350 Certification | ||||||||||
| 97.1* | AgeX Therapeutics, Inc. Clawback Policy | |||||||||
| 101.INS* | Inline XBRL Instance Document (the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document) | |||||||||
| 101.SCH* | Inline XBRL Taxonomy Extension Schema | |||||||||
| 101.CAL* | Inline XBRL Taxonomy Extension Calculation Linkbase | |||||||||
| 101.DEF* | Inline XBRL Taxonomy Extension Definition Linkbase | |||||||||
| 101.LAB* | Inline XBRL Taxonomy Extension Label Linkbase | |||||||||
| 101.PRE* | Inline XBRL Taxonomy Extension Presentation Linkbase | |||||||||
| 104 | Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) |
| * | Filed herewith. |
| # | Confidential treatment has been granted with respect to portions of this exhibit (indicated by asterisks) and those portions have been separately filed by |
| Certain schedules and exhibits to this agreement have been omitted in accordance with Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the Securities and Exchange Commission on request. | |
| ‡ | Management contract or compensatory plan. |
None.
SIGNATURES(b) See (a)3 Exhibits
(c)(1) Separate Financial Statements of subsidiaries not consolidated and fifty percent or less owned persons
AgeX consolidates NeuroAirmid Therapeutics, Inc. despite not having majority ownership interest as it has the ability to influence decision making and financial results through contractual rights and obligations as per ASC 810, Consolidation. For further discussion on principles of consolidation, see Note 1, Organization, Basis of Presentation and Liquidity to the consolidated financial statements included elsewhere in this Report.
(c)(2) Financial Statements of affiliates whose securities are pledged as collateral
Presented below are the unaudited financial statements of ReCyte Therapeutics, Inc. of which AgeX holds approximately 94.8% of its shares of common stock issued and outstanding and Reverse Bioengineering, Inc., a wholly owned subsidiary of AgeX whose securities are pledged as collateral to Juvenescence in connection with the debt agreements with Juvenescence. AgeX’s security interests in UniverXome Bioengineering, Inc. (UniverXome), a wholly owned subsidiary of AgeX is pledged as collateral to Juvenescence in connection with the debt agreements with Juvenescence, but UniverXome has no financial statement transactions as of December 31, 2023. For further information on debt agreements with Juvenescence, see Note 5, Related Party Transactions, to the consolidated financial statements included elsewhere in this Report.
RECYTE THERAPEUTICS, INC.
BALANCE SHEETS
(In thousands, except par value amounts)
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued liabilities | $ | 5 | $ | - | ||||
| Amount due to AgeX | 887 | 864 | ||||||
| TOTAL LIABILITIES | 892 | 864 | ||||||
| Stockholders’ deficit: | ||||||||
| Preferred stock, no par value, authorized 5,000 shares; none issued and outstanding | - | - | ||||||
| Common stock, no par value, 50,000 shares authorized; 25,305 shares issued and outstanding | 15,104 | 15,104 | ||||||
| Accumulated deficit | (15,950 | ) | (15,923 | ) | ||||
| Total ReCyte Therapeutics, Inc. stockholders’ deficit | (846 | ) | (819 | ) | ||||
| Noncontrolling interest | (46 | ) | (45 | ) | ||||
| Total stockholders’ deficit | (892 | ) | (864 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | - | $ | - | ||||
RECYTE THERAPEUTICS, INC.
STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| OPERATING EXPENSES | ||||||||
| General and administrative | $ | 28 | $ | 40 | ||||
| NET LOSS | 28 | 40 | ||||||
| Net loss attributable to noncontrolling interest | (1 | ) | (2 | ) | ||||
| NET LOSS ATTRIBUTABLE TO RECYTE | $ | 27 | $ | 38 | ||||
| NET LOSS PER COMMON SHARE: | ||||||||
| BASIC AND DILUTED | $ | - | $ | - | ||||
| WEIGHTED-AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: | ||||||||
| BASIC AND DILUTED | 25,305 | 25,305 | ||||||
| 130 |
RECYTE THERAPEUTICS, INC.
STATEMENTS OF STOCKHOLDERS’ DEFICIT
(In thousands)
| Common Stock | Total | |||||||||||||||||||
| Number of Shares | Amount | Accumulated Deficit | Noncontrolling Interest | Stockholders’ Deficit | ||||||||||||||||
| BALANCE AT DECEMBER 31, 2021 | 25,305 | $ | 15,104 | $ | (15,885 | ) | $ | (43 | ) | $ | (824 | ) | ||||||||
| Net loss | - | - | (38 | ) | (2 | ) | (40 | ) | ||||||||||||
| BALANCE AT DECEMBER 31, 2022 | 25,305 | 15,104 | (15,923 | ) | (45 | ) | (864 | ) | ||||||||||||
| Net loss | - | - | (27 | ) | (1 | ) | (28 | ) | ||||||||||||
| BALANCE AT DECEMBER 31, 2023 | 25,305 | $ | 15,104 | $ | (15,950 | ) | $ | (46 | ) | $ | (892 | ) | ||||||||
RECYTE THERAPEUTICS, INC.
STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| OPERATING ACTIVITIES: | ||||||||
| Net loss attributable to ReCyte | $ | (27 | ) | $ | (38 | ) | ||
| Net loss attributable to noncontrolling interest | (2 | ) | (2 | ) | ||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts payable and accrued liabilities | 5 | (1 | ) | |||||
| Amount due to AgeX | 24 | 41 | ||||||
| Net cash used in operating activities | - | - | ||||||
| NET CHANGE IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH | - | - | ||||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH: | ||||||||
| At beginning of the year | - | - | ||||||
| At end of the year | $ | - | $ | - | ||||
REVERSE BIOTHERAPEUTICS, INC.
BALANCE SHEETS
(In thousands, except par value amounts)
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued liabilities | $ | 25 | $ | 53 | ||||
| Amount due to AgeX | 1,505 | 883 | ||||||
| TOTAL LIABILITIES | 1,530 | 936 | ||||||
| Stockholders’ deficit: | ||||||||
| Preferred stock, $0.0001 par value, authorized 5,000 shares; none issued and outstanding | - | - | ||||||
| Common stock, $0.0001 par value, 100,000 shares authorized; 1 shares issued and outstanding, respectively | - | - | ||||||
| Accumulated deficit | (1,530 | ) | (936 | ) | ||||
| Total stockholders’ deficit | (1,530 | ) | (936 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | - | $ | - | ||||
| 131 |
REVERSE BIOTHERAPEUTICS, INC.
STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| OPERATING AND ORGANIZATION EXPENSES | ||||||||
| Research and development | $ | 166 | $ | 288 | ||||
| General, administrative and organization | 428 | 260 | ||||||
| NET LOSS | $ | 594 | $ | 548 | ||||
| NET LOSS PER COMMON SHARE: | ||||||||
| BASIC AND DILUTED | $ | (594 | ) | $ | (548 | ) | ||
| WEIGHTED-AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: | ||||||||
| BASIC AND DILUTED | 1 | 1 | ||||||
REVERSE BIOTHERAPEUTICS, INC.
STATEMENTS OF STOCKHOLDERS’ DEFICIT
(In thousands)
| Common Stock | Total | |||||||||||||||
Number of Shares | Par Value | Accumulated Deficit | Stockholders’ Deficit | |||||||||||||
| BALANCE AT DECEMBER 31, 2021 | - | $ | - | $ | (388 | ) | $ | (388 | ) | |||||||
| Issuance of common stock to AgeX in connection with Asset Contribution Agreement | 1 | - | - | - | ||||||||||||
| Net loss | - | - | (548 | ) | (548 | ) | ||||||||||
| BALANCE AT DECEMBER 31, 2022 | 1 | - | (936 | ) | (936 | ) | ||||||||||
| Net loss | - | - | (594 | ) | (594 | ) | ||||||||||
| BALANCE AT DECEMBER 31, 2023 | 1 | $ | - | $ | (1,530 | ) | $ | (1,530 | ) | |||||||
REVERSE BIOTHERAPEUTICS, INC.
STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (594 | ) | $ | (548 | ) | ||
| Changes in operating assets and liabilities: | ||||||||
| Accounts payable and accrued liabilities | (28 | ) | 49 | |||||
| Amount due to AgeX | 622 | 499 | ||||||
| Net cash used in operating activities | - | - | ||||||
| NET CHANGE IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH | - | - | ||||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH: | ||||||||
| At beginning of the year | - | - | ||||||
| At end of the year | $ | - | $ | - | ||||
| SUPPLEMENTAL SCHEDULE OF NONCASH FINANCING AND INVESTING ACTIVITIES: | ||||||||
| Issuance of 1 common stock to AgeX in connection with Asset Contribution Agreement in 2022 | $ | - | $ | - | ||||
(c)(3) Schedules
All other schedules are omitted because they are not required or the required information is included in the financial statements.
Item 16. Form 10-K Summary
None.
| 132 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized on the 122stnd day of April 2019.March 2024.
| AGEX THERAPEUTICS, INC. | ||
| By: | /s/ | |
| Interim Chief Executive Officer | ||
| Signature | Title | Date | ||
| /s/ | ||||
| (Principal Executive Officer) | ||||
| /s/ | Chief Financial Officer | |||
| (Principal Financial and Accounting Officer) | ||||
| /s/Gregory H. Bailey | Director | |||
| GREGORY H. BAILEY | ||||
| /s/ | Director | |||
| /s/ | Director | |||
| 133 |