
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
FORM 10-K
(Mark One)
[X]☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31 2020, 2023
[ ]☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ____________ to ____________
Commission file number: 001-36268
Akers Biosciences,MyMD Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| 22-2983783 | ||
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) |
| ||
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (856)848-8698
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class: | Trading Symbol(s) | Name of Each Exchange on Which Registered: | ||
| Shares of | The |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] ☐ No [X] ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes [ ] ☐ No [X] ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [X] ☒ No [ ]☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [X] ☒ No [ ]☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definition of “large accelerated filer,” “accelerated filer, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ||||
| Non-accelerated filer | ☒ | Smaller reporting company | ||||
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. [ ]☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal controlscontrol over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. [ ]☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes [ ]☐ No [X]☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant on June 30, 2020,2023, based on a closing price of $3.48$45.00 was $21,254,305.$63 million.
As of February 26, 2021,March 29, 2024, the registrant had 16,652,829 shares of its common stock, noCommon Stock, par value $0.001 per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.None.
EXPLANATORY NOTE
Reincorporation
On March 4, 2024 (the “Effective Date”), MyMD Pharmaceuticals, Inc., a New Jersey corporation (“MyMD New Jersey” or, prior to the Reincorporation (as defined below), the “Company”) merged with and into its wholly-owned subsidiary, MyMD Pharmaceuticals, Inc., a Delaware corporation (“MyMD Delaware” or, following the Reincorporation, the “Company”), with MyMD Delaware being the surviving corporation, pursuant to that certain Agreement and Plan of Merger, dated as of March 4, 2024, by and between MyMD New Jersey and MyMD Delaware (the “Plan of Merger”), for the purpose of changing the Company’s state of incorporation from New Jersey to Delaware (the “Reincorporation”). The Plan of Merger and the Reincorporation were approved by the Company’s stockholders at the 2023 annual meeting of stockholders, held on July 31, 2023 (the “2023 Annual Meeting”).
MyMD Delaware is deemed to be the successor issuer of MyMD New Jersey under Rule 12g-3 of the Securities Exchange Act of 1934, as amended.
The Reincorporation did not result in any change in the Company’s name, business, management, fiscal year, accounting, location of the principal executive offices, assets or liabilities. In addition, the Company’s common stock retains the same CUSIP number and continues to trade on the Nasdaq Capital Market under the symbol “MYMD.”
As of the Effective Date of the Reincorporation, the rights of the Company’s stockholders are governed by the Delaware General Corporation Law, the MyMD Delaware Certificate of Incorporation, and the Bylaws of MyMD Delaware.
See Note 1 of the Consolidated Financial Statements for additional information.
Reverse Stock Split
Effective as of 4:05 p.m. Eastern Standard Time on February 14, 2024, we effected a one-for-thirty reverse stock split of our common stock (the “Reverse Stock Split”). Simultaneously with the Reverse Stock Split, number of shares of our common stock authorized for issuance was reduced from 500,000,000 shares to 16,666,666 shares, and our authorized capital stock was reduced from 550,000,000 shares to 66,666,666 shares. All share and per share information in this report have been retroactively adjusted to reflect the Reverse Stock Split.
TABLE OF CONTENTS
| 2 |
CAUTIONARY NOTE REGARDING FORWARD LOOKING STATEMENTS
This Annual Report on Form 10-K (the “Annual Report”) and the documents we have filed with the Securities and Exchange Commission (which we refer to herein as the “SEC”) that are incorporated by reference herein contain “forward-looking statements” within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of forward-looking terms such as “anticipates,” “assumes,” “believes,” “can,” “could,” “estimates,” “expects,” “forecasts,” “future,” “guides,” “intends,” “is confident that,”, “may,” “plans,” “seeks,” “projects,” “targets,” and “would” or the negative of such terms or other variations on such terms or comparable terminology. These statements relate to future events or our future financial performance or condition and involve known and unknown risks, uncertainties and other factors that could cause our actual results, levels of activity, performance or achievement to differ materially from those expressed or implied by these forward-looking statements.
Examples of forward-looking statements in this Annual Report and our other SEC filings include, but are not limited to, our expectations regarding our business strategy, business prospects, operating results, operating expenses, working capital, liquidity and capital expenditure requirements. These statements are based on our management’s expectations, beliefs and assumptions concerning future events affecting us, which in turn are based on currently available information and are subject to significant risks and uncertainties that could cause actual outcomes and results to differ materially. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, without limitation, the risks and uncertainties set forth under “Risk Factors” in Item 1A of this Annual Report on Form 10-K, which discussions are incorporated herein by reference.
These risks and uncertainties include, but are not limited to:
| ● |
| ||
| ● | the impact of dilution on our | ||
| ● |
| ||
| |||
| |||
| ● | the impact of our ability to realize the anticipated tax impact of the Merger; | ||
| ● | delisting of our Common Stock from the Nasdaq Capital Market; | ||
| ● | the availability of and our ability to continue to obtain sufficient funding to conduct planned research and development efforts and realize | ||
| ● |
| ||
| ● | the impact of the complexity of the regulatory landscape on our ability to seek and obtain regulatory approval for our product candidates, both within and outside of the U.S.; | ||
| ● | the required investment of substantial time, resources and effort for successful clinical development and marketization of our product candidates; | ||
| ● | challenges we may face with maintaining regulatory approval, if achieved; | ||
| ● | the potential impact of changes in the legal and regulatory landscape, both within and outside of the U.S.; | ||
| ● | the impact of public health emergencies such as the COVID-19 pandemic on the administration, funding and policies of regulatory authorities, both within and outside of the U.S.; | ||
| ● | our dependence on third parties to conduct pre-clinical and clinical trials and manufacture its product candidates; | ||
| 3 |
| ● | the impact of public health emergencies such as the COVID-19 pandemic on our results of operations, business plan and the global economy; | ||
| ● | challenges we may face with respect to our product candidates achieving market acceptance by providers, patients, patient advocacy groups, third party payors and the general medical community; | ||
| ● | the impact of pricing, insurance coverage and reimbursement status of our product candidates; | ||
| ● | emerging competition and rapidly advancing technology in our industry; | ||
| ● | our ability to obtain, maintain and protect our trade secrets or other proprietary rights, operate without infringing upon the proprietary rights of others and prevent others from infringing on its proprietary rights; | ||
| ● | our ability to maintain adequate cyber security and information systems; | ||
| ● | our ability to achieve the expected benefits and costs of the transactions related to the acquisition of | ||
| ● | |||
| |||
our ability to effectively execute and deliver our plans related to commercialization, marketing and manufacturing capabilities and strategy; | ||
| ● | emerging competition and rapidly advancing technology in our industry; | |
| ||
| ● | challenges we may face in identifying, acquiring and operating new business opportunities; | |
| ● | our ability to retain and attract senior management and other key employees; | |
| ● | our ability to quickly and effectively respond to new technological developments; | |
| ● | the outcome of litigation or other proceedings to which are subject as described in the “Legal Proceedings” section of this Annual Report on Form 10-K, or to we may become subject to in the future; | |
| ● | increased levels of competition; | |
| ● | changes in political, economic or regulatory conditions generally and in the markets in which we operate; | |
| ||
| ● | changes in the market acceptance of our products and services; | |
| ● |
| |
| our compliance with all laws, rules, and regulations applicable to our business and | ||
| ● | risks of mergers and acquisitions including the time and cost of implementing transactions and the potential failure to achieve expected gains, revenue growth or expense savings; | |
| ||
| ● | other risks, including those described in the “Risk Factors” section of this Annual |
We operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for us to predict all of those risks, nor can we assess the impact of all of those risks on our business or the extent to which any factor may cause actual results to differ materially from those contained in any forward-looking statement. The forward-looking statements in this Annual Report on Form 10-K and our other filings with the SEC are based on assumptions management believes are reasonable. However, due to the uncertainties associated with forward-looking statements, you should not place undue reliance on any forward-looking statements. Further, forward-looking statements speak only as of the date they are made, and unless required by law, we expressly disclaim any obligation or undertaking to publicly update any of them in light of new information, future events, or otherwise. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements contained throughout this Annual Report and the documents we have filed with the SEC.
| 4 |
Unless the context provides otherwise, all references in this Annual Report to “Akers”, the “Company”, “we”, “our” and “us” refer to Akers Biosciences, Inc.
WeMyMD is a clinical stage pharmaceutical company committed to extending healthy lifespan. MyMD is focused on developing and commercializing two therapeutic platforms based on well-defined therapeutic targets, MYMD-1 and Supera-CBD:
| ● | MYMD-1 is a clinical stage small molecule that regulates the immunometabolic system to treat autoimmune disease, including (but not limited to) rheumatoid arthritis, and inflammatory bowel disease. MYMD-1 is being developed to treat age-related illnesses such as frailty and sarcopenia. MYMD-1 works by regulating the release of numerous pro-inflammatory cytokines, such as TNF-α, interleukin 6 (“IL-6”) and interleukin 17 (“IL-17”) | |
| ● | Supera-CBD is a synthetic analog of CBD being developed to treat various conditions, including, but not limited to, epilepsy, pain and anxiety/depression, through its effects on the CB2 receptor, opioid receptors and monoamine oxidase enzyme (“MAO”) type B. |
The rights to Supera-CBDTM were incorporatedpreviously owned by Supera and were acquired by MyMD Florida (as defined below) immediately prior to the closing of the Merger (as defined below) that occurred in 1989 in the state of New Jersey2021.
MyMD Background and Corporate History
MyMD was organized under the name “A.R.C. Enterprises, Inc,” which was changed to “Akers Research Corporation” on September 28, 1990 and “Akers Laboratories, Inc.” on February 24, 1996. Pursuant to the Amended and Restated Certificate of Incorporation filed on March 26, 2002, the corporation’s name was changed to “Akers Biosciences, Inc.”
We were historically a developer of rapid health information technologies. On March 23, 2020, we entered into that certain membership interest purchase agreement (the “Original MIPA” and, as subsequently amended by Amendment No, 1 on May 14, 2020, the “MIPA”) with the members of Cystron (the “Cystron Sellers”), pursuant to which we acquired 100%laws of the membership interestsState of Cystron (the “Cystron Membership Interests”). Cystron was incorporated on March 10, 2020 and is a party to a license agreement with Premas whereby Premas granted Cystron, among other things, an exclusive license with respect to Premas’ genetically engineered yeast (S. cerevisiae)-based vaccine platform, D-Crypt™,Florida in November 2014 for the developmentpurpose of developing and commercializing certain technology and patent rights relating to MYMD-1 that were developed and/or held by the company’s founder, Jonnie R. Williams, Sr. The company’s sole initial stockholder was The Starwood Trust, a vaccine against COVID-19 and other coronavirus infections. Since our entry intotrust for which Mr. Williams is settlor/grantor. During the MIPA, we have beenperiod from November 2014 through November 2016, MyMD was primarily focused on drug discovery and establishing its patent position through SRQ Patent Holdings, an entity affiliated with Mr. Williams. In November 2016, SRQ Patent Holdings assigned to MyMD all of the rapid developmentpatent rights and manufacturingother intellectual property relating to MYMD-1 pursuant to an agreement under which MyMD granted to SRQ Patent Holdings a royalty based on product sales and other revenue arising from the assigned intellectual property (as further described below).
During the period 2016 through October of 2020, MyMD’s principal business activities consisted of the execution and completion of in vitro assays, in vivo pre-clinical animal studies, and genotoxicity and toxicology studies relating to MYMD-1 (as further described below). On June 25, 2019, MyMD commenced a Phase 1 trial in healthy volunteers for pharmacokinetics and tolerability studies, and in December of 2019 MyMD filed an IND for MYMD-1 for treatment of Hashimoto thyroiditis. The Phase 1 trial was completed on January 30, 2020, after which MyMD commenced preparation of a COVID-19 vaccine candidate (the “COVID-19 Vaccine Candidate”),Phase 2 clinical trial for MYMD-1 The company has also commenced a Phase 2 clinical trial for patients with sarcopenia, with dosing begin in collaborationthe first quarter of 2022. The last patient visit took place on June 6, 2023. The clinical safety report is currently under development.
Additionally, MyMD is working with Premas.Charles River Laboratories to conduct a study titled “A 13 Week Electroencephalogram Safety Study of MYMD-1 by Oral Gavage Administration in Beagle Dog.” Dosing began on December 19, 2023.
Proposed
2021 Merger and Corporate Transactions
On April 16, 2021, pursuant to an Agreement and Plan of Merger and Reorganization, dated November 11, 2020 we entered into(as subsequently amended, the “Merger Agreement”), by and among the Company, previously known as Akers Biosciences, Inc., XYZ Merger Agreement, pursuant to which, among other things, subject to the satisfaction or waiverSub, Inc., a wholly-owned subsidiary of the conditions set forth in the Company (“Merger Agreement,Sub”), and MyMD Pharmaceuticals (Florida), Inc., a Florida corporation previously known as MyMD Pharmaceuticals, Inc. (“MyMD Florida”), Merger Sub will mergewas merged with and into MYMD,MyMD Florida, with MYMD beingMyMD Florida continuing after the merger as the surviving corporationentity and becoming a wholly owned subsidiary of the Company (the “Merger”). The Merger is intended to qualify for federal income tax purposes as a tax-free reorganization under the provisions of Section 368(a) of the Internal Revenue Code of 1986, as amended (the “Code”). In addition, in connection with the execution of the Merger Agreement, Akers agreed to advance a bridge loan of up to $3,000,000 to MYMD pursuant to a secured promissory note (the “Note”). Upon completion of the merger, the combined company is expected to be renamed MyMD Pharmaceuticals, Inc.
Pursuant to the Merger Agreement, upon the effectiveness of the Merger, (i) holders of outstanding shares of MYMD common stock (“ MYMD stockholders”) will be entitled to receive (x) the number of shares of Akers common stock equal to an exchange ratio as described in the Merger Agreement (the “Exchange Ratio”) per share of MYMD common stock they hold, prior to giving effect to the proposed reverse stock split discussed below, (y) an amount in cash, on a pro rata basis, equal to the aggregate cash proceeds received by Akers from the exercise of any options to purchase shares of MYMD common stock assumed by Akers upon closing of the merger prior to the second-year anniversary of the closing of the merger (the “Option Exercise Period”), such payment (the “Additional Consideration”) to occur not later than 30 days after the last day of the Option Exercise Period, up to the maximum amount of cash consideration that may be received by MYMD stockholders without affecting the intended tax consequences of the merger, and (z)included potential milestone payments (“Milestone Shares”) of up to the number of shares of Akers common stock issued to MYMDpre-Merger MyMD Florida stockholders at closing of the Merger (“Milestone(the “Milestone Payments”) payable in shares of the Company’s Common Stock upon the achievement of certain market capitalization milestone events during the 36-month period immediately following the closing of the merger (the “Milestone Period”); and (ii) each outstanding option to purchase MYMD common stock granted under the Second Amendment to Amended & Restated 2016 Stock Incentive PlanMerger.
On November 11, 2020, in connection with an effective date of July 1, 2019, as established and maintained by MYMD (and, as amended and restated from time to time, the “MyMD Incentive Plan”) that has not previously been exercised prior to the closing of the Merger, whether or not vested, will be assumed by Akers subject to certain terms contained inentering into the Merger Agreement, and become an option to purchase a number of shares ofMyMD Florida entered into the Akers common stock equal to the number of shares of MYMD common stock underlying such option multiplied by the Exchange Ratio, which options to purchase MYMD common stock shall be amended to expire on the second-year anniversary of the closing of the Merger, and the exercise price for each share of Akers common stock underlying an assumed option to purchase MYMD common stock will be equal to the exercise price per share of the option to purchase MYMD common stock in effect immediately prior to the completion of the merger divided by the Exchange Ratio. Assuming the exercise in full of the outstanding pre-funded warrants to purchase 1,040,540 shares of our common stock (“Pre-Funded Warrants”) issued in connection with the private placement between Akers and certain institutional and accredited investors that closed on November 17, 2020 (the “Private Placement”) and the exercise in full of additional pre-funded warrants to purchase 932,432 shares of common stock issued certain investors in February 2021 upon cancellation of shares of common stock purchased in the Private Placement and including 9,979,664 shares of combined company common stock underlying options to purchase shares of MYMD common stock to be assumed at the closing of the Merger, (i) MYMD stockholders and optionholders will own approximately 80% of the equity of the combined company; and (ii) our current stockholders, holders of certain outstanding of our options and warrants (excluding shares issuable upon exercise of options and warrants having an exercise price in excess of $1.72, prior to giving effect to any such stock splits, combinations, reorganizations and the like with respect to the Akers common stock between the announcement of the Merger and the closing of the Merger) and holders of our outstanding restricted stock units (“RSUs”) immediately prior to the Merger will own approximately 20% of the equity of the combined company.
Pursuant to the Merger Agreement, on January 15, 2020, we and MYMD filed an initial Registration Statement on Form S-4 (Registration No. 333-252181) (together with the joint proxy and consent solicitation/prospectus included therein, the “S-4 Registration Statement”) describing the Merger and other related matters. Consummation of the Merger is conditioned upon, among other things, approval of the Merger by the stockholders of Akers (including (i) approval, for purposes of complying with Nasdaq Listing rule 5635(a), the issuance of shares of Akers common stock to MYMD stockholders and other parties in connection with the Merger, the Merger Agreement, and the transactions contemplated thereby or in connection therewith (the “Share Issuance Proposal”), (ii) approval of an amendment to the amended and restated certificate of incorporation of the combined company, which will be in effect at the effective time of the merger (the “A&R Charter”) to effect a reverse stock split, if applicable, at a reverse stock split ratio mutually agreed by the Company and MYMD and within the range approved by our stockholders immediately prior to the Effective Time (as defined in the Merger Agreement), which range shall be sufficient to cause the price of our common stock on the Nasdaq Capital Market following the reverse stock split and the Effective Time to be no less than $5.00 per share with respect to the issued and outstanding common stock of the combined company immediately following the merger (the “Reverse Stock Split Proposal”), and (iii) approval of the amended and restated certificate of incorporation of Akers which will be in effect upon consummation of the Merger (the “A&R Charter Proposal”), among others), approval of the Merger by the stockholders of MYMD, the continued listing of Akers’ common stock on The Nasdaq Capital Market after the Merger and satisfaction of a minimum cash threshold by Akers. In addition, the Merger Agreement requires that MYMD consummate the purchase of substantially all of the assets and certain liabilities of Supera Pharmaceuticals, Inc., a Florida corporation (“Supera”) pursuant to an Asset Purchase Agreement pursuant to which MYMDMyMD Florida agreed to acquire from Supera immediately prior to the completion of the Merger, substantially all of its assets (the “Supera Purchase”). After closing of the Merger, the operations of MYMD’s business will comprise substantially all of the combined company’s operations. There is no assurance when or if the Merger will be completed. Any delayassets (including all rights to Supera-CBD) and certain obligations of Supera in completing the Merger may substantially reduce the potential benefits that we expect to obtain from the Merger. Furthermore, the intended benefitsconsideration of the Merger may not be realized.
Coronavirus and COVID-19 Pandemic
In December 2019, SARS-CoV-2 was reportedissuance to have surfaced in Wuhan, China, and on March 12, 2020, the World Health Organization (“WHO”) declared the global outbreakSupera of COVID-19, the disease caused by SARS-CoV-2,an aggregate of 13,096,640 shares of MyMD Florida Common Stock. As partial consideration for such assignment, Supera has granted to beSRQ Patent Holdings II, LLC a pandemic. In an effort to contain and mitigate the spread of COVID-19, many countries, including the United States, Canada, China, and India, have imposed unprecedented restrictions on travel, quarantines, and other public health safety measures. According to the WHO situation report, dated as of February 16, 2021, approximately 108.2 million cases were reported globally and 2.4 million of these were deadly, making the development of effective vaccines to prevent this disease a major global priority. Multiple vaccine candidates against SARS-CoV-2 are under development, and in December 2020, certain large, multinational pharmaceutical companies were granted authorizations for emergency use by the FDA. Widespread distribution of the currently-available vaccines has begun pursuant to Operation Warp Speed, a partnership among components of the U.S. Department of Health and Human Services, the Centers for Disease Control and Prevention, the National Institutes of Health, the Biomedical Advanced Research and Development Authority, and the Department of Defense, as well as certain private firms and other federal agencies. The treatments for COVID-19, including symptomatic and supportive therapies, among other things, continue to be updated on a rolling basis by healthcare authorities and agencies.
Impact of the COVID-19 Pandemic on Our Business
The ultimate impact of the global COVID-19 pandemic or a similar health epidemic is highly uncertain and subject to future developments. These include but are not limited to the duration of the COVID-19 pandemic, new information which may emerge concerning the severity of the COVID-19 pandemic, and any additional preventative and protective actions that regulators, or our board of directors or management, may determine are needed. We do not yet know the full extent of potential delays or impacts on our business, our vaccine development efforts, healthcare systems or the global economy as a whole. However, the effects are likely to have a material impact on our operations, liquidity and capital resources, and we will continue to monitor the COVID-19 situation closely.
In response to public health directives and orders, we have implemented work-from-home policies for many of our employees and temporarily modified our operations to comply with applicable social distancing recommendations. The effects of the orders and our related adjustments in our business are likely to negatively impact productivity, disrupt our business and delay our timelines, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course. Similar health directives and orders are affecting third parties with whom we do business, including Premas, whose operations are located in India. Further, restrictions on our ability to travel, stay-at-home orders and other similar restrictions on our business have limited our ability to support our operations.
Severe and/or long-term disruptions in our operations will negatively impact our business, operating results and financial condition in other ways, as well. Specifically, we anticipate that the stress of COVID-19 on healthcare systems generally around the globe will negatively impact regulatory authorities and the third parties that we and Premas may engage in connection with the development and testing of our COVID-19 Vaccine Candidate.
In addition, while the potential economic impact brought by, and the duration of, COVID-19 may be difficult to assess or predict, it has significantly disrupted global financial markets, and may limit our ability to access capital, which could in the future negatively affect our liquidity. A recession or market correction resulting from the continuation of the COVID-19 pandemic could materially affect our business and the value of our common stock.
Coronavirus Vaccine Development
We have partnered with Premas on the development of the COVID-19 Vaccine Candidate as we seek to advance such candidate through the regulatory process, both with the U.S. Food and Drug Administration (“FDA”) and the office of the drug controller in India. Premas is primarily responsible for the development of the COVID-19 Vaccine Candidate through proof of concept and is entitled to receive milestone payments upon achievement of certain development milestones through proof of concept.
Premas’ D-Crypt platform has been developed to express proteins that are difficult to clone, express and manufacture and are a key component in vaccine development. Premas has identified three major structural proteins of SARS-CoV-2 as antigens for potential vaccine candidates for COVID-19: spike protein or S protein, envelope protein or E protein, and membrane protein or M protein. In April 2020, Premas used its D-Crypt platform to recombinantly express all three of such antigens, which we considered a significant milestone for development of a triple antigen vaccine. We believe including a combination of all three antigens will provide advantages against the likelihood of protein mutation, in which case a single-protein vaccine can be rendered non-efficacious, and therefore, enhance efficacy of our vaccine candidates. We believe the D-Crypt provides us advantages in vaccine production and manufacturing, as the technology platform is highly scalable with a robust process, which we expect will ultimately result in significant cost savings compared to other similar vaccine platforms. Based on genetically engineered baker’s yeast S. cerevisiae, the platform is highly scalable into commercial production quantities and has been previously utilized for the production of multiple human and animal health vaccines candidates during its 10-year development track record. Yeast has a large endoplasmic reticulum, or (“ER”), which is a desirable attribute for expressing membrane protein. In complex cells, ER is where the protein is formed. The larger the surface, the more membrane protein that can attach to the ER inside the cell. Yeast is also generally believed to be easily manipulated and allow for results to be gathered quickly. Yeast multiplies faster than mammalian cells and is cheaper to work with than mammalian systems, which are much more complex and slower to grow comparatively. Yeast has received “Generally Recommended as Safe” status from the FDA.
As of May 14, 2020, Premas successfully completed its vaccine prototype and obtained transmission electron microscopic (“TEM”) images of the recombinant virus like particle (“VLP”) assembled in yeast. A manufacturing protocol has also been established and large-scale production studies have been initiated for our COVID-19 Vaccine Candidate. Though the prototype is complete, the COVID-19 Vaccine Candidate is still in early stages of development, and, accordingly, must undergo pre-clinical testing and all phases of clinical trials before we can submit a marketing application (in this case, a Biologics License Application, or “BLA”) to the FDA. The BLA must be approved by the FDA before any biological product, including vaccines, may be lawfully marketed in the United States. We believe the most pivotal, yet difficult, stage in our anticipated development of the contemplated COVID-19 Vaccine Candidate is the requisite conduct of extensive clinical trials to demonstrate the safety and efficacy of our COVID-19 Vaccine Candidate. Additionally, after we complete the necessary pre-clinical testing, but before we may begin any clinical studies in the United States, we must submit an Investigational New Drug (“IND”) application to the FDA, as this is required before any clinical studies may be conducted in the United States. In some cases, clinical studies may be conducted in other countries; however, the FDA may not accept data from foreign clinical studies in connection with a BLA (or other marketing application) submission.
In July 2020, animal studies for our COVID-19 Vaccine Candidate were initiated in India. In addition, we announced that Premas has successfully completed the manufacturing process for the VLP vaccine candidate. On August 27, 2020, we announced with Premas positive proof of concept results from the animal studies conducted during a four-week test of the COVID-19 Vaccine Candidate in mice. The test had two primary endpoints, safety and immune responses, both of which were met. The study consisted of 50 mice, divided into 10 cohorts dosed with 5, 10 and 20 micrograms of the COVID-19 Vaccine Candidate. The COVID-19 Vaccine Candidate was generally well tolerated and safe at all doses, with no adverse events reported. The COVID-19 Vaccine Candidate was safe even at higher doses and generated a robust immune response against the three SARS-Cov2 antigens, S, E, and M. The COVID-19 Vaccine Candidate elicited neutralizing antibody titers levels in all the dose cohorts starting from 5 microgram to 20 microgram dose regimens. After three doses in mice, all the groups’ cohorts showed binding antibody levels similar to convalescent patients’ levels. Clinical testing is expensive, time consuming, and uncertain as to outcome. We cannot guarantee that any clinical trials will be conducted as planned or completed in a timely manner, if at all. Failures in connection with one or more clinical trials can occur at any stage of testing.
Premas owns, and has exclusively licensed rights to us to, two provisional Indian patent applications filed in January and March 2020. The scope of these Indian provisional patent applications is directed, respectively, to (i) a platform for the expression of difficult to express proteins (“DTE-Ps”), which might provide coverage for a method of making the to-be-developed vaccine; and (ii) an expression platform for SARS-CoV-2-like virus proteins, methods relevant thereto, and a relevant vaccine. If non-provisional patent rights are pursued claiming priority to each of these two provisional applications, any resulting patent rights that issue might not expire until approximately January 20, 2041 and March 4, 2041, if all annuities and maintenance fees are timely paid. The expiration dates may be extendable beyond these dates depending on the jurisdiction and the vaccine development process. As we do not own the patents or patent applications that we license, we may need to rely upon Premas to properly prosecute and maintain those patent applications and prevent infringement of those patents.
Competition
We face, and will continue to face, intense competition from large pharmaceutical companies, specialty pharmaceutical and biotechnology companies as well as academic and research institutions pursing research and development of technologies, drugs or other therapies that would compete with our products or product candidates. The pharmaceutical market is highly competitive, subject to rapid technological change and significantly affected by existing rival drugs and medical procedures, new product introductions and the market activities of other participants. Our competitors may develop products more rapidly or more effectively than us. If our competitors are more successful in commercializing their products than us, their success could adversely affect our competitive position and harm our business prospects.
Specifically, the competitive landscape of potential COVID-19 vaccines and treatment therapies has been rapidly developing since the beginning of the COVID-19 pandemic, with several hundreds of companies claiming to be investigating possible candidates and approximately 4,800 studies registered worldwide as investigating COVID-19 (source: clinicaltrials.gov). Given the global footprint and the widespread media attention on the COVID-19 pandemic, there are efforts by public and private entities to develop a COVID-19 vaccine as soon as possible, including large, multinational pharmaceutical companies such as AstraZeneca, GlaxoSmithKline, Johnson & Johnson, Moderna, Pfizer, and Sanofi. In December 2020, the FDA issued emergency use authorizations for vaccines developed by certain of these large, multinational pharmaceutical companies and it is possible that additional vaccines developed by such large, multinational pharmaceutical companies may receive further approvals and authorizations in the near term. Those other entities have vaccine candidates that are currently at a more advanced stage of development than our COVID-19 Vaccine Candidate and may develop COVID-19 vaccines that are more effective than any vaccine we may develop, may develop a COVID-19 vaccine that becomes the standard of care, may develop a COVID-19 vaccine at a lower cost or earlier than we are able to jointly develop any COVID-19 vaccine, or may be more successful at commercializing a COVID-19 vaccine. Many of these other organizations are much larger than we are and have access to larger pools of capital, and as such, are able to fund and carry on larger research and development initiatives. Such other entities may have greater development capabilities than we do and have substantially greater experience in undertaking nonclinical and clinical testing of vaccine candidates, obtaining regulatory approvals and manufacturing and marketing pharmaceutical products. Our competitors may also have greater name recognition and better access to customers. In addition, based on the competitive landscape, additional COVID-19 vaccines or therapeutics may continue be approved to be marketed. Should another party be successful in producing a more efficacious vaccine for COVID-19, such success could reduce the commercial opportunity for our COVID-19 Vaccine Candidate and could have a material adverse effect on our business, financial condition, results of operations and future prospects. Moreover, if we experience delayed regulatory approvals or disputed clinical claims, we may not have the commercial or clinical advantage over competitors’ products that we believe we currently possess. The success or failure of other entities, or perceived success or failure, may adversely impact our ability to obtain any future funding for our vaccine development efforts or for us to ultimately commercialize and market any vaccine candidate, if approved. In addition, we may not be able to compete effectively if our product candidates do not satisfy government procurement requirementsroyalty with respect to biodefense products.
Acquisitionproduct sales and License Agreements
On March 23, 2020, we acquired Cystron pursuant to the MIPA. Asother consideration for the Cystron Membership Interests, we delivered to the Cystron Sellers: (1) that number of newly issued shares of our common stock equal to 19.9% of the issued and outstanding shares of our common stock and pre-funded warrants as of the date of the MIPA, but, to the extent that the issuance of our common stock would have resulted in any Seller owning in excess of 4.9% of our outstanding common stock, then, at such Seller’s election, such Seller received “common stock equivalent” preferred shares with a customary 4.9% blocker (with such common stock and preferred stock collectively referred to as “Common Stock Consideration”), and (2) $1,000,000 in cash. On March 24, 2020, we paid $1,000,000 to the Cystron Sellers and delivered 411,403 shares of common stock and 211,353 shares of Series D Convertible Preferred Stock with a customary 4.9% blocker, with an aggregate fair market value of $1,233,057.
Additionally, we are required to (A) make an initial payment to the Cystron Sellers of up to $1,000,000 upon its receipt of cumulative gross proceedsarising from the consummation of an initial equity offering after the date of the MIPA of $8,000,000, and (B) pay to the Cystron Sellers an amount in cash equal to 10% of the gross proceeds in excess of $8,000,000 raised from future equity offerings after the date of the MIPA until the Cystron Sellers have received an aggregate additional cash consideration equal to $10,000,000 (collectively, the “Equity Offering Payments”). On May 14, 2020, we entered into an Amendment No. 1 to the MIPA with the Cystron Sellers, which provided that any Equity Offering Payments in respect of an equity offering that is consummated prior to September 23, 2020, shall be accrued, but shall not be due and payable until September 24, 2020. The other provisions of the MIPA remained unmodified and in full force and effect. Upon the achievement of certain milestones, including the completion of a Phase 2 study for a COVID-19 Vaccine Candidate that meets its primary endpoints, the Cystron Sellers are entitled to receive an additional 750,000 shares of our common stock or, in the event we are unable to obtain stockholder approval for the issuance of such shares, 750,000 shares of non-voting preferred stock that are valued following the achievement of such milestones and shall bear a 10% annual dividend (the “Cystron Milestone Shares”). At the 2020 annual meeting of our stockholders, held on August 27, 2020, pursuant to Nasdaq listing rule 5635(a), our stockholders approved of the issuance of Common Stock Consideration (as defined in the MIPA) and the potential future issuance of Cystron Milestone Shares in excess of 20% of our common stock outstanding prior to the closing of the Cystron acquisition.assigned intellectual property.
Pursuant to the MIPA, the Company shall make contingent payments for the achievement of certain development and commercial milestones as follows; (i) $250,000 upon the dosing of the first patient in a Phase I Clinical Trial, (ii) $500,000 upon the dosing of the first patient in a Phase II Clinical Trial, (iii) $5,000,000 upon the dosing of the first patient in a Phase III Clinical Trial, and (iv) $15,000,000 upon approval by the FDA of the NDA for the COVID-19 vaccine.
Pursuant to the Original MIPA, upon our consummation of the registered direct equity offering closed on April 8, 2020, we paid the Cystron Sellers $250,000 on April 20, 2020 (the “April Payment”). On April 30, 2020, Premas, one of the Cystron Sellers, returned to us $83,334, representing their portion of the $250,000 amount paid to the Cystron Sellers on April 20, 2020. Premas advised us that these funds were returned temporarily for Premas to meet certain regulatory requirements in India. We recorded liabilities of $892,500 (the “May Payment”) and $684,790 (the “August Payment”) to the Cystron Sellers upon the consummation of the registered direct equity offerings that closed on May 18, 2020 and August 13, 2020, respectively. These funds (including funds of $299,074 representing Premas’ portion of the cash purchase price and $83,334 representing Premas’ portion of the April Payment temporarily returned to us in April 2020) due the sellers under the MIPA were disbursed on September 25, 2020. On October 13, 2020, Premas returned $908,117 representing Premas’ portion of the initial cash component for the purchase of Cystron and Premas’ portion of the April Payment, May Payment and August Payment under the MIPA. Premas is working with the Reserve Bank of India to comply with regulations related to its ownership in a foreign entity and its ability to receive funds for the sale of that entity. The Company believes that (i) Premas will be successful inpreviously owned, through its efforts to resolve such regulatory matters with the Reserve Bank of India, (ii) the Company will disburse the amounts due to Premas under the MIPA, and (iii) the Company maintains a 100% membership interest in Cystron.
Upon the consummation of the Private Placement, the Company paid $1,204,525 of the proceeds from the Private Placement to three of the four former members ofsubsidiary Cystron and recorded a liability of $602,172 to the fourth former member of Cystron pursuant to the MIPA.
We shall also make quarterly royalty payments to the Cystron Sellers equal to 5% of the net sales of a COVID-19 vaccine or combination product by us for a period of five (5) years following the first commercial sale of the COVID-19 vaccine; provided, that such payment shall be reduced to 3% for any net sales of the COVID-19 vaccine above $500 million.
In addition, the Cystron Sellers shall be entitled to receive 12.5% of the transaction value, as defined in the MIPA, of any change of control transaction, as defined in the MIPA, that occurs prior to the fifth (5th) anniversary of the closing date of the MIPA, provided that we are still developing the COVID-19 Vaccine Candidate at that time. Following the consummation of any change of control transaction, the Cystron Sellers shall not be entitled to any payments as described above under the MIPA.
Support Agreement
On March 23, 2020, as an inducement to enter into the MIPA, and as one of the conditions to the consummation of the transactions contemplated by the MIPA, the Cystron Sellers entered into a shareholder voting agreement with us, pursuant to which each Cystron Seller agreed to vote their shares of our common stock or preferred stock in favor of each matter proposed and recommended for approval by our management at every meeting of the stockholders and on any action or approval by written consent of the stockholders.
Registration Rights Agreement
To induce the Cystron Sellers to enter into the MIPA, on March 23, 2020, we entered into a registration rights agreement with the Cystron Sellers, pursuant to which we filed with the SEC a Registration Statement on Form S-3, as amended, covering resale of the Common Stock Consideration, which was declared effective on June 12, 2020. We also agreed to subsequently register Cystron Milestone Shares, if such securities are issued in the future.
License Agreement
Cystron is a party to the License Agreement with Premas. As a condition to our entry into the MIPA, Cystron amended and restated the Initial License Agreement on March 19, 2020. Pursuant to the License Agreement, Premas granted Cystron, amongst other things,Biotech, LLC (“Cystron”), an exclusive license from Premas Biotech PVT Ltd. (“Premas”) with respect to Premas’ vaccine platform for the development of a vaccine against COVID-19 and other coronavirus infections.
Upon the achievement of certain developmental milestones by Cystron, Cystron shall pay to Premas a total of up to $2,000,000. On April 16, 2020, we paid Premas $500,0002021, pursuant to the Contribution and Assignment Agreement, dated March 18, 2021 (the “Contribution Agreement”) by and among the Company, Cystron, Oravax Medical, Inc. (“Oravax”) and, for the achievementlimited purpose set forth therein, Premas, the Company caused Cystron to contribute substantially all of the firstassets associated with its business of developing and manufacturing Cystron’s COVID-19 vaccine candidate to Oravax. Oravax is pursuing the development of the COVID-19 vaccine candidate. MyMD’s interest in Oravax consists of 13% of Oravax’s outstanding shares of capital stock and the rights to a 2.5% royalty on all future net sales. MyMD has evaluated several options with respect to its interest in Oravax, including a potential distribution of Oravax shares to the MyMD shareholders. This would make Oravax a publicly held company. In addition, MyMD currently has the right to designate a member of the board of directors of Oravax, pursuant to which Mr. Joshua Silverman, our Chairman of the Board, has been designated to serve as a director of Oravax.
| 5 |
Status of MyMD Florida
On April 8, 2022, the MyMD Florida subsidiary was dissolved and merged into the New Jersey corporation MyMD Pharmaceuticals, Inc. pursuant to an Agreement and Plan of Merger dated April 8, 2022.
Reincorporation
On March 4, 2024, the Reincorporation was effected, and the Company changed its state of incorporation from New Jersey to Delaware.
Drug Development
MyMD is developing two platform drugs targeting numerous disease indications. Below is MyMD’s development milestones. On May 18,pipeline:

| 6 |
Strategy
MyMD’s strategy is to focus on extending healthy life span through the development and commercialization of novel drug platforms based on well-defined therapeutic targets. Below are MyMD’s key clinical strategies:
| ● | Completed Phase 2 clinical trial in sarcopenia (i.e., age-related muscle loss) in the second quarter of 2023; In the process of completing the Clinical Safety Report (CSR); | |
| ● | Advance MYMD-1 into Phase 2 clinical trials for rheumatoid arthritis and Hashimoto’s Thyroiditis; | |
| ● | Execute on IND-enabling studies of Supera-CBD to enable submission of an IND for a Phase 1 clinical trial in healthy volunteers followed by Phase 2 clinical trials in epilepsy, addiction and anxiety disorders; | |
| ● | Identify and validate additional novel targets and utilize translational platforms to develop a pipeline of product candidates for aging and other autoimmune disease; | |
| ● | Maintain broad commercial rights to MyMD’s product candidates; and | |
| ● | Continue to strengthen and expand MyMD’s intellectual property portfolio. |
MYMD-1
Overview
MYMD-1 is a clinical stage drug that targets the immune system by inhibiting the release of pro-inflammatory cytokines, such as TNF-α. Cytokines are a broad category of molecules involved in immune system coordination. Immunometabolic regulation is the system of regulating the immune system and its pro-inflammatory cytokines in order to prevent and treat autoimmune diseases and age-related illnesses. By affecting the initial triggers that drive autoimmunity, MYMD-1 targets the underlying cause of these diseases rather than just their symptoms. Based on MYMD-1’s Phase 1 clinical trial, completed in January 2020, we paid Premas $500,000MyMD has completed a Phase 2 clinical trial for sarcopenia (age-related muscle loss) and is planning a Phase 2 clinical trial for rheumatoid arthritis. MyMD has an active IND with the Endocrinology Division at the FDA for other autoimmune diseases. Studies have been completed on the mechanisms of action and efficacy of MYMD-1 in several pre-clinical models of autoimmune diseases (i.e., experimental autoimmune encephalomyelitis (“EAE”) that models multiple sclerosis and autoimmune thyroiditis), and these studies have been published in peer reviewed journals. MyMD plans to pursue these indications.
MYMD-1: An Immunometabolic Regulator
Inflammation, activated through the release of TNF-α and other cytokines, is the body’s normal physiological defense against infections and pathogens, and under normal circumstances such inflammation quickly resolves once the intruder is neutralized. However, elevated levels of pro-inflammatory cytokines, including TNF-α, can lead to prolonged, chronic inflammation, which is closely linked to autoimmune diseases (such as multiple sclerosis, diabetes, rheumatoid arthritis) and aging (i.e., inflamm-aging) as well as cardiovascular disease and cancers, all of which may result in reduced health span (the period of life spent in good health).
The goal of immunometabolic regulatory drugs such as MYMD-1 is to target immune cells that overproduce pro-inflammatory cytokines, such as TNF-α, without preventing normal immune cell function. TNF-α is a cytokine that is released by immune cells that plays a key role in acute and chronic inflammation, autoimmune diseases and aging. Examples of currently approved immunometabolic regulating drugs include Dimethyl Fumarate (“DMF”) (approved for the achievementtreatment of multiple sclerosis) and Rapamycin (used in kidney transplants and being studied in aging).
MYMD-1 is a novel immunometabolic regulator that has demonstrated in vitro and in vivo ability to regulate the release of multiple cytokines from immune cells, including TNF-α. MYMD-1 is being developed to treat chronic inflammatory diseases, such as multiple sclerosis, diabetes, inflammatory bowel disease, rheumatoid arthritis, and aging.
MYMD-1 Regulates Multiple Cytokines
MyMD conducted an in vitro study to demonstrate that MYMD-1 regulates a broad range of cytokines, including TNF-α, interferon gamma (INFγ) and interleukins, including interleukin 2 (“IL-2”) and IL-17A. By blocking these cytokines that have been shown to play key roles in the development and maintenance of autoimmune diseases, MYMD-1 treats the causes—and not just the symptoms—of this class of illnesses.

Figure 1. MYMD-1 modulates the release of a broad spectrum of cytokines.
| 7 |
An additional in vitro study demonstrates that MYMD-1 has broad cytokine inhibiting activity including inhibition of TNF-α, IL-16 and IL-17a. The study also suggested MYMD-1 has limited toxicity, even at high doses, and none up to 2,000 micromoles.

In an in vivo study (NOD.H2 mouse model), MYMD-1 decreased serum levels of TNF-α and INFγ.
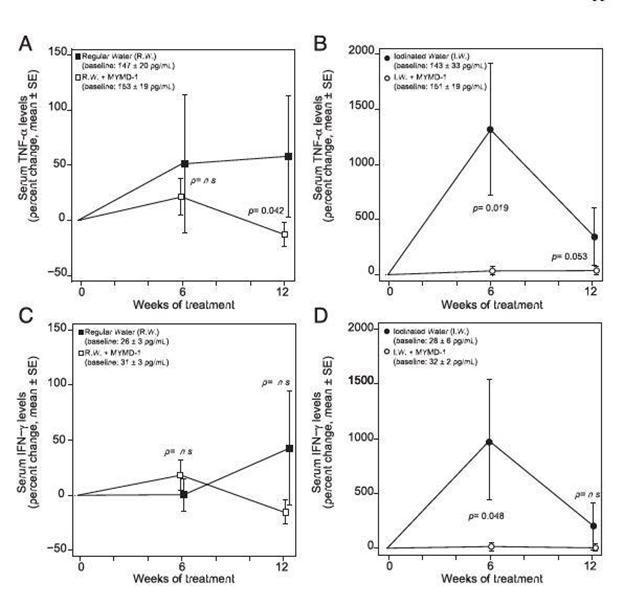
Figure 2. MYMD-1 decreases the serum levels TNF-α and IFN-g in NOD.H-2h4 mice. NOD.H-2h4 mice were treated with either regular water or iodinated water (500 mg/l of sodium iodide), and each group was treated or not treated with MYMD-1 (185 mg/l). Cytokines were measured at baseline and after 6 and 12 weeks of treatment using a multiplex magnetic bead array. (A and B) MYMD-1 significantly decreased serum TNF-α levels in the regular water group and tended to decrease it in the iodinated water group. (C and D) MYMD-1 showed a modest effect on serum IFN-g in the iodinated water group. Results are from three independent experiments. Statistical comparisons were made by longitudinal data analysis with generalized estimating equations.
| 8 |
MYMD-1 Targets Autoimmune Diseases
MYMD-1 is designed to regulate the immunometabolic system and intended for development as a potential treatment for certain autoimmune diseases, including (but not limited to) multiple sclerosis, diabetes, rheumatoid arthritis, and/or inflammatory bowel disease. MYMD-1 is also being developed to treat age-related illnesses such as frailty and sarcopenia. Autoimmune diseases are a broad category of diseases that result from an overactive immune response, where immunometabolic system dysregulation is believed to play an important role. A healthy immune system defends the body against disease and infection. If the immune system malfunctions, it can mistakenly attack healthy cells, tissues, and organs. In response to an often-unknown trigger, the immune system starts producing antibodies that attack the body’s own cells instead of fighting infections.
TNF-α, produced primarily by specific white blood cells, belongs to a category of proteins called cytokines that act as chemical messengers throughout the body to regulate many aspects of the third development milestone. On July 7,immune system. Other key cytokines include IL-6, IL-17A, interleukin 10 (“IL-10”) and Interferon gamma (“INFγ”). Cytokines are essential to mounting an inflammatory response. However, chronic or excessive production of cytokines has been implicated in a number of acute and chronic inflammatory diseases.
A number of drugs target the immunometabolic system to treat autoimmune diseases, including DMF (approved for the treatment of multiple sclerosis) and Rapamycin (being studied in aging, rheumatoid arthritis, and other autoimmune diseases). Additional therapies for autoimmune diseases include anti-inflammatory drugs and immunosuppressive agents including drugs that non-selectively inhibit or block TNF-α (generally referred to as “TNF-α blocking drugs”). Currently available TNF-α blocking drugs must be injected or infused to work. In some instances, the efficacy of a given dosage of TNF-α blockers declines with repeated administration, and side effects can also be a concern. These non-selective TNF-α blockers can cause serious bacterial, fungal, and viral infections. MYMD-1 is a selective, oral TNF-α inhibitor that might provide a safer alternative to existing products on the market. The global market for TNF-α blockers was estimated at $41.6 billion in 2020 we agreedand is projected to reach $45.5 billion by 2027.
An in vitro study involving human blood cells analyzed the cytokine inhibitory effects of MYMD-1 together with Premas thatleading approved TNF-α blockers (monoclonal antibodies).

Figure 3. Comparison of inhibitory effect of MYMD-1 with other TNF-α blockers. MYMD-1 exhibits a dose-dependent reduction in release of several cytokine more effectively than Humira, Enbrel and Remicade.
We believe MYMD-1 is distinguishable from currently marketed TNF-α blockers because it selectively blocks TNF-α production related to adaptive immunity (involved in autoimmunity) but spares the fourth milestone under the License Agreement had been satisfied. Due to the achievementrole of this milestonecytokine in innate immunity (which plays a primary protective role in fighting off invading organisms). Because of the crucial role that TNF-α plays in front line protection by the innate immune system (e.g., from bacterial, fungal, and viral infections), the indiscriminate blockade of TNF-α by TNF-α blocking agents can cause serious and even fatal infections, which is one of the primary limiting factors in the use of this class of drugs. Based on July 7, 2020, Premas was paid $1,000,000our belief regarding the selectivity of MYMD-1 in blocking TNF-α, therefore, we intend to explore the extent to which MYMD-1 may be a safer alternative to treat infectious, inflammatory, and autoimmune conditions, as well as its potential to ameliorate immune mediated depression in such illnesses.
| 9 |
Pre-Clinical Study of MYMD-1 in Multiple Sclerosis Study (EAE Mouse Model)
Multiple sclerosis is an autoimmune disease in which T cells lead an attack on August 4, 2020.
Intellectual Property
We have exclusive rights in-licensed from Premas (as discussed above) to certain know-howoligodendrocytes and two provisional Indian patent applications filedneurons. Multiple sclerosis is the leading neurological cause of disability in Januaryadults aged 30–50, and March 2020. The following table summarizes the two provisional Indian patent applications.
As we do not own the patent applications that we in-license, we may need to rely upon Premas to properly prosecute and maintain those and additional related patent applications, and to prevent infringement of any resulting patents.
We have two U.S. registered trademarks for “Akers Bio.”
Government Regulation and Product Approval
Federal, state, and local government authoritiesapproximately one million people in the United States are affected with this debilitating disease. T cells are one of the major components of the adaptive immune system. Their roles include directly killing infected host cells, activating other immune cells, producing cytokines and regulating the immune response. When naïve, undifferentiated T cells become activated, they differentiate and acquire effector functions that can be delineated by the cytokines they secrete.
Preliminary in vivo studies of the therapeutic efficacy of MYMD-1 in the animal model for multiple sclerosis, known as EAE, indicate that MYMD-1 modulates autoreactive T cell activation in a dose-dependent manner, suppresses T cell activation and ameliorates the course of EAE. Further EAE mouse studies suggest that MYMD-1 suppresses the influx of CD4+ T cells into the brain.

Figure 4. Effects of MYMD-1 on the influx of T cells into the CNS early in EAE. To assess the effects of MYMD-1 on the infiltration of T cells into the CNS, mice were immunized and treated with either vehicle control or 25 mg/mouse/day MYMD-1. Ten to 14 days later, mice were perfused and brains collected for analysis. Infiltration was determined by flow cytometry. Analysis of Th1 and Th17 subsets are shown; data compiled from 2 to 3 experiments, n > 3/group per experiment). Student’s t-test was conducted for statistics.
MYMD-1 In Vivo Study of Autoimmune Thyroiditis (NODH.2 Mouse Model)
Thyroiditis or Hashimoto thyroiditis is an autoimmune disease characterized by lymphocytic infiltration of the thyroid gland. It has been shown that tobacco smoking has a protective effect against Hashimoto thyroiditis as tobacco smokers have a lower prevalence of thyroid autoantibodies than non-smokers.
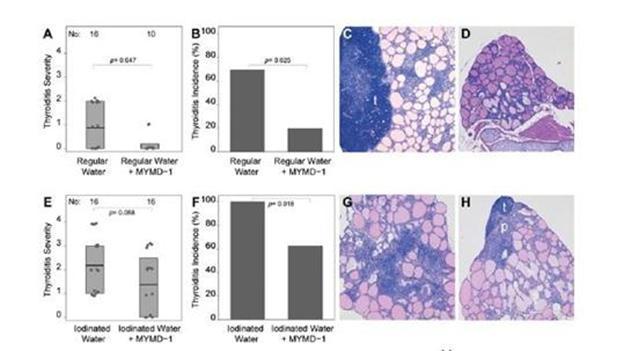
MyMD conducted an in vivo study of autoimmune thyroiditis in a spontaneous thyroiditis (NODH.2) mouse model. We believe the results of this study show MYMD-1’s ability to suppress TNF-α production by CD-4+ T cells in a dose dependent manner. Additionally, the study reported that MYMD-1 statistically decreases the incidence and severity (p <0.001) of thyroiditis in this mouse model. Pre-clinical studies have demonstrated that MYMD-1 ameliorated autoimmune thyroiditis in the thyroiditis mouse model.
| 10 |
Figure 5. MYMD-1 decreases the incidence and severity of autoimmune thyroiditis in NOD.H-2h4 mice, as assessed by H&E histopathology. At 8 weeks old, 58 NOD.H-2h4 mice were divided into regular water and iodinated water groups. In the regular water group, 10 mice (7 M, 3 F) drank water that contained MYMD-1 (185 mg/l), and 16 mice (10 M, 6 F) drank water without it. In the iodinated water group, the water was supplemented with 500 mg/l of sodium iodide and contained (16 mice: 10 M, 6 F) or did not contain (16 mice: 10 M, 6 F) MYMD-1 (185 mg/l). After 12 weeks of treatment, thyroids were removed and divided in half. (A and B) Thyroiditis severity and incidence assessed by histopathology in the regular water group. (C) A representative thyroid from a mouse in the regular water group, showing a severity score of 2. (D) A representative thyroid from a mouse in the regular water group treated with MYMD-1, showing thyroid follicle preservation and an overall normal glandular size (severity score of 0). (E and F) Thyroiditis incidence and severity scores assessed by histopathology in the iodinated water group. (G) A representative thyroid from a mouse in the iodine group, showing marked lymphocytic infiltration, follicular enlargement, and architectural disruption (severity score of 4). (H) A representative thyroid from a mouse in the iodine plus MYMD-1 group (severity score of 2). Results represent the summary of 10 independent experiments, each analyzing 4 to 6 mice, for a total of 58 mice.
MYMD-1 Targets Inflamm-Aging and Related Disorders
Aging is associated with a loss of tight regulation of the immune system. This leads to increased inflammatory activity in the body, including increased circulating levels of TNF-α. Chronic inflammation is a hallmark of aging, referred to as inflamm-aging. Inflamm-aging and chronic inflammation are closely linked to a number of disorders such as obesity, insulin resistance/type 2 diabetes, cardiovascular diseases, and cancers. TNF-α is a multifunctional pro-inflammatory cytokine which may play a part in the pathogenesis of certain age-related disorders such as atherosclerosis. A multi-year pre-clinical, proof of concept in vivo study in aging and longevity confirmed our belief regarding MYMD-1’s potential therapeutic effect on inflamm-aging and other age-related disorders, which we intend to explore further in clinical trials, pending our submission, and the corresponding acceptance, of the requisite regulatory and other relevant submissions.
Bascom Palmer Eye Institute Collaboration
On July 12, 2022, we announced a new collaboration with Bascom Palmer Eye Institute of Miami, Florida (“Bascom Palmer”) to collaborate on a pre-clinical study using MYMD-1 as a potential treatment for traumatic optic neuropathy (TON). To date, our collaboration with Bascom Palmer has included pre-clinical and clinical investigations.
Pre-Clinical
In July 2022 we entered into a Material Transfer Agreement with Bascom Palmer. Our collaboration was announced in a press release and in an article in Ophthalmology Times. Bascom Palmer confirmed in August 2022 that it had received a quantity of our MYMD-1 product candidate and MYMD provided a material safety datasheet and certification of analysis. In August 2022, Bascom Palmer researchers conducted a preliminary introductory study of TON in mice. Investigators ran a crush injury of the mice’s optic nerves with and without MYMD-1. The study drug was given once per day via oral gavage at a dosage of 30 mg/kg of body weight. The mice were treated for five days, untreated for two days, and then sacrificed, and their TNF-α levels were measured. The crush injury raised levels of TNF-α. After being dosed with MYMD, TNF-α levels were brought down in crush injury compared to controls, but the decrease did not meet statistical significance (p=0.095). Likely cause of the result not reaching p<0.05 may be attributed to rebound (e.g. TNF-α levels would have gone up when MYMD-1 stopped; daily dosing may need to me adjusted, crush injury may have been too severe for the medication, and/or possible contribution of ketamine which is an anti-inflammatory. Additional studies are not planned for now.
Clinical
In addition to the pre-clinical study described above, we collaborated with Bascom Palmer to plan a clinical study. In August 2022, Bascom Palmer researchers executed a confidentiality and non-disclosure agreement and Bascom Palmer produced a draft protocol synopsis entitled, Assessment of the Anti-Inflammatory Effects of MYMD-1 in Non-Infectious Anterior Uveitis: A Randomized Controlled, Double Blind Clinical Study. This program is not active.
MYMD-1 Commercialization Targets
MYMD-1 is being developed to address serious and debilitating autoimmune and inflammatory diseases, including sarcopenia, frailty resulting from aging process, and rheumatoid arthritis (RA). According to the U.S. Census Bureau, in 2020, there were approximately 54 million U.S. residents over 65 years of age, representing 16% of the U.S. population. This figure is expected to increase to nearly 22% by the year 2040.1 The Arthritis Foundation estimates that approximately 1.5 million people in the U.S. have RA.2
Supera-CBD
Supera-CBD is a synthetic small molecule that is an analog of naturally grown CBD derived from the Cannabis sativa plant. Supera-CBD is being developed to treat conditions with which CBD is often anecdotally associated but for which no natural or synthetic CBD-containing drugs have been approved by the FDA, such as pain, anxiety/depression and seizures from epilepsy. While naturally grown CBD is a constituent of Cannabis sativa, Supera-CBD is a synthetic analog of CBD, thus eliminating potential complications associated with the psychoactive effects of Tetrahydrocannabinol (“THC”), which is also a constituent of the Cannabis sativa plant. Studies have suggested that CBD may have broad therapeutic properties, including the treatment of neuropsychiatric disorders.
1 U.S. Department of Health and Human Services. 2020 Profile of Older Americans. May 2021 Page 3.
2 The Arthritis Foundation. Rheumatoid Arthritis: Causes, Symptoms, Treatments and More.
| 11 |
Overview
General Pharmacology and Therapeutic Profile
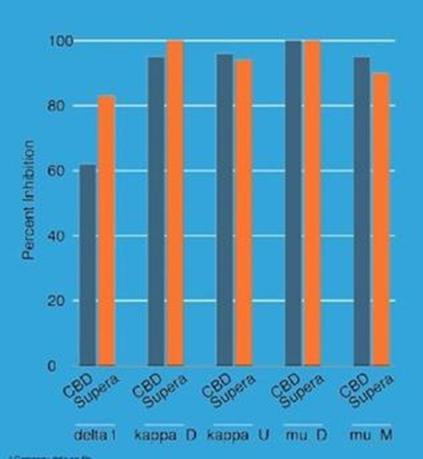
CBD inhibits a number of important receptors, including the CB2 receptor and opioid receptors, and can also inhibit MAO enzymes. In the immune system, one of the important functions of the CB2 receptor is in the regulation of cytokine release from immune cells. Antagonists targeting the CB2 receptor have been proposed for the treatment or management of a range of painful conditions as well as for treating several neurological diseases. The Company conducted an in vitro binding assay study to analyze the CB2 inhibition of Supera-CBD together with that of CBD derived from naturally grown plants.

Opioid receptors are widely expressed in the brain, spinal cord, peripheral nerves and digestive tract. MyMD conducted an in vitro binding analysis of Supera-CBD with the three types of opioid receptors. The profile suggests that Supera-CBD could possibly play a role in treating opioid addiction.
| 12 |
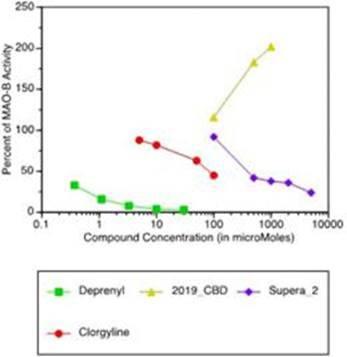
MAOs are enzymes involved in the catabolism, or digestion, of certain neurotransmitters. MyMD conducted an in vitro MAO inhibition study. In this study, Supera-CBD and commercial CBD were analyzed against positive and negative controls. In this study, Supera-CBD far exceeded CBD in dose-dependent inhibition of MAOs, particularly MAO-B. Drugs that inhibit MAOs have been commercially used for decades to treat depression, and more recent studies have suggested MAO-B inhibiting drugs might have a role to play in treating cognitive decline in aging.
Supera-CBD Early-Stage Plans for Development and Potential Commercialization Targets
Supera-CBD is in early-stage development for pain, anxiety, and sleep disorders. There are currently a number of over-the-counter CBD products marketed with unapproved therapeutic claims relating to these conditions, among other conditions. While there are a substantial number of such products on the market that have not been subject to regulatory enforcement action, the FDA has consistently reiterated, in guidance and warning letters against a number of the companies marketing such CBD products for such uses, that CBD products may not be lawfully marketed for therapeutic uses in the United States without first-obtaining FDA approval via the NDA process. CBD product sales in the US reportedly reached $5.3 billion in 2021, 15% growth over 2020 sales, and are projected to reach $16 billion by 2026.3 MyMD believes that if Supera-CBD is approved by the FDA, it may have competitive advantages over currently marketed CBD products that have not been approved by FDA as drug products, as approved drugs must undergo rigorous premarket study and generate results sufficient to support a finding that they are safe and effective for their intended use(s) and remain subject to ongoing FDA postmarket regulation, which provides additional assurances relating to quality, consistency and safety.
Currently, there is one FDA-approved drug with plant-derived CBD as an active ingredient. FDA subsequently approved three other cannabinoid-containing drugs, two of which utilize synthetic cannabinoids analogous or similar to THC as the active ingredient and the other, a combination of synthetic CBD and THC. Epidiolex is being commercialized by GW Pharmaceuticals, plc (“GWPH”) to treat seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients two years of age and older. The reported revenues from Epidiolex in fiscal year 2019 were approximately $296 million. MYMD believes that, by utilizing synthetic, rather than naturally derived, CBD in Supera-CBD may mitigate a number of obstacles generally associated with growing and processing an active drug ingredient produced from naturally grown plant extracts.
On March 2, 2023, we announced that the U.S. Drug Enforcement Administration (DEA) has conducted a scientific review and determined that it would not Supera-CBD a controlled substance or listed chemical under the Controlled Substances Act (CSA) and its governing regulations. We believe that this decision will expedite future research involving Supera-CBD by relieving us or our research partners from having to comply with regulations relating to controlled substances.
Sales and Marketing
MyMD does not currently have sales and marketing infrastructure to support the launch of its products. MyMD intends to build such capabilities in North America prior to launch the commercial MYMD-1, if successfully developed and granted the requisite FDA approval. Outside of North America, MyMD may rely on licensing, co-sale and co-promotion agreements with strategic partners for commercialization of its products. If MyMD builds a commercial infrastructure to support marketing in North America, such commercial infrastructure could be expected to include a targeted sales force supported by sales management, internal sales support, an internal marketing group and distribution support. To develop the appropriate commercial infrastructure internally, MyMD would have to invest financial and management resources, some of which would have to be deployed prior to any confirmation that MYMD-1 or Supera-CBD will be approved, which cannot be guaranteed.
Competition
The biotechnology and biopharmaceutical industries are characterized by rapid evolution of technologies, fierce competition and vigorous defense of intellectual property. Any product candidates that MyMD successfully develops and commercializes will have to compete with existing and future new therapies. While MyMD believes that its drug candidates, development experience and scientific knowledge may provide it with certain competitive advantages, MyMD faces potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions, governmental agencies, and public and private research institutions.
Existing therapies for autoimmune diseases include anti-inflammatory drugs and immunosuppressive agents, including drugs that seek to selectively inhibit or block TNF-α (generally referred to as “TNF-α blocking drugs”). TNF-α blocking drugs are large molecules that are generally injected or infused. In some instances, the period of efficacy of a given dosage of TNF-α blockers can decline with repeated administration and side effects can be a concern. Leading TNF-α blocking drugs include Etanercept (Enbrel), Infliximab (Remicade), and Adalimumab (Humira). The total TNF-α market collectively represented approximately $41 billion in global sales in 2022.4 All of these existing TNF-α blocking drugs require injection, whereas MYMD-1 is being developed to be orally bioavailable. Our management believes patients and providers would view the fact that MYMD-1 can be administered orally as a significant advantage.
Unlike currently marketed TNF-α blockers, MYMD-1 is designed to selectively block TNF-α production related to adaptive immunity (involved in autoimmunity) but to spare the role of this cytokine in innate immunity (which plays the primary initial role in fighting off invading organisms). Because of the crucial role that TNF-α plays in front line protection by the innate immune system from bacterial, fungal, and viral infections, the indiscriminate blockade of TNF-α by TNF-α blocking agents can cause serious and even fatal infections, which is the primary limiting factor in the use of this class of drugs. MyMD thus believes that, if MYMD-1 is approved for marketing, the potential selectivity of MYMD-1 in blocking TNF-α might make it a preferrable alternative to some existing treatments for infectious, inflammatory, and autoimmune conditions, as well as simultaneously resulting in amelioration of immune mediated depression in such illnesses if it is also approved for such indication.
3 Benzinga: US Hemp CBD Market To Hit $5.3B In Sales In 2021.
4 https://www.thebusinessresearchcompany.com/report/tnf-alpha-inhibitor-global-market-report
| 13 |
Intellectual Property
MyMD’s policy is to develop and maintain MyMD’s proprietary position by, among other methods, filing or in-licensing U.S. and foreign patents and applications related to MyMD’s drug candidates and methods of treatment that are material to the development and implementation of MyMD’s business. MyMD also relies on trademarks, know-how, confidentiality agreements and invention assignment agreements to develop and maintain MyMD’s proprietary position.
MyMD’s patent portfolio includes protection for MYMD’s lead product candidates, MYMD-1 and Supera-CBD. Currently, there are multiple patent families relating to (i) age reversal and treatments of age-related disorders including sarcopenia; (ii) reduction of TNF-α levels and treatments of autoimmune disorders; (iii) addiction treatments; and (iv) methods of increasing hair growth. As of the date of this document, MyMD has 16 issued U.S. patents, three pending U.S. patent applications, 64 issued foreign patents, and 10 foreign patent applications pending in such jurisdictions as Australia, Canada, China, European Union, Israel, Japan and South Korea, which, if issued, are expected to expire between 2036 and 2041.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which MyMD files, the patent term is 20 years from the date of filing of the first non-provisional application in which priority is claimed. In the U.S. patent term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the USPTO in granting a patent or may be shortened if a patent is terminally disclaimed over an earlier-filed patent. In the U.S., the term of a patent that covers an FDA-approved drug may also be eligible for a patent term extension of up to five years under the Hatch-Waxman Act, which is designed to compensate for the patent term lost during the FDA regulatory review process. The length of the patent term extension involves a complex calculation based on the length of time it takes for regulatory review. A patent term extension under the Hatch-Waxman Act cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Moreover, a patent can only be extended once, and thus, if a single patent is applicable to multiple products, it can only be extended based on one product. Similar provisions are available in Europe and certain other foreign jurisdictions to extend the term of a patent that covers an approved drug.
MyMD’s commercial success depends in part on its ability to obtain and maintain proprietary protection for MyMD’s product candidates, as well as novel discoveries, core technologies, and know-how, as well as its ability to operate without infringing on the proprietary rights of others and to prevent others from infringing its proprietary rights.
Assignment and Royalty Agreements
MyMD is a party to two Amended and Restated Confirmatory Patent Assignment and Royalty Agreements, both dated November 11, 2020, with SRQ Patent Holdings and SRQ Patent Holdings II, under which MyMD (or its successor) will be obligated to pay to SRQ Patent Holdings or SRQ Patent Holdings II (or its designees) certain royalties on product sales or other revenue received on products that incorporate or are covered by the intellectual property that was assigned to MyMD. The royalty is equal to 8% of the net sales price on product sales and, without duplication, 8% of milestone revenue or sublicense compensation. SRQ Patent Holdings and SRQ Patent Holdings II are affiliates of Mr. Williams.
Government Regulation
Government authorities in the U.S. at the federal, state, and local level and in other countries extensively regulate, among other things, the research, development, testing, manufacturing,manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of drugs and biological products. Generally, before a new drug can be marketed, considerable data demonstrating its quality, safety, and pharmaceutical products such as those we are developing. Our prospective vaccine candidate(s)efficacy in connection with the target indication(s) for use must be obtained, organized into a format specific for each regulatory authority, submitted for review and approved by the regulatory authority.
FDA before they may be legally marketed inApproval Process
In the United States and by the appropriate foreign regulatory agency before they may be legally marketed in foreign countries. Generally, our activities in other countries will beU.S., pharmaceutical products are subject to extensive regulation that is similar in nature and scope as that imposed inunder the United States. The process for obtaining regulatory marketing approvalsFD&C Act and the subsequent compliance with appropriateFDA’s implementing regulations and other federal state, local, and foreignstate statutes and regulations requiregoverning, among other things, the expenditureresearch, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling and import and export of substantial time and financial resources.
U.S. Product Development Process
In the United States, the FDA regulates pharmaceutical and biological products under the Federal Food, Drug, and Cosmetic Act (“FD&C Act”), the Public Health Service Act (“PHSA”), and their respective implementing regulations. Products are also subject to other federal, state, and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources.products. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicanta company to a variety enforcement actions and/or administrative or judicial sanctions.sanctions, including, but not limited to clinical holds, FDA sanctions could include, among other actions, refusal to approve pending applications, withdrawalNDA submissions and/or revocation or limitation of an approval, a clinical hold,existing NDAs for approved products, warning or untitled letters, product recalls, or withdrawals from the market, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgementcivil penalties and criminal prosecution.
| 14 |
Pharmaceutical product development for a new drug product or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us. The process required bycertain changes to an approved product in the U.S. typically requires pre-clinical laboratory and animal tests, the submission to the FDA of an IND, which must become effective before aclinical testing on human subjects may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements are inherently uncertain, expensive, and it typically takes many years to generate sufficient data to apply for approval, even when such approval is not ultimately granted, and the actual time required may vary substantially based upon the type, complexity and novelty of the product or biological product may be marketed in the United States generally involves the following:disease.
Before testing any biological vaccine candidate in humans, the vaccine candidate enters the pre-clinical testing stage. Pre-clinical tests also referred to as nonclinical studies, include laboratory evaluationsevaluation of product chemistry, toxicityformulation and formulation,toxicity, as well as animal studiestrials to assess the characteristics and potential safety and activityefficacy of the vaccine candidate.product. The conduct of the pre-clinical tests must comply with federal regulations and requirements, including GLPs.good laboratory practices. The clinical trial sponsor must submit the results of the pre-clinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol,testing are submitted to the FDA as part of the IND. Somean IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long-term pre-clinical testingtests, such as animal tests of reproductive toxicity and carcinogenicity, may continue even after the IND is submitted. TheA 30-day waiting period after the submission of each IND automatically becomes effective 30 days after receipt byis required prior to the commencement of clinical testing in humans. If the FDA unless the FDA raises concerns or questions regarding the proposed clinical trials and places the trialhas neither commented on a clinical hold within that 30-day time period. In such a case,nor questioned the IND sponsor and the FDA must resolve any outstanding concerns beforewithin this 30-day period, the clinical trial can begin. The FDA may also impose clinical holds on a biological product candidate at any time before or during clinical trials due to safety concerns or non-compliance. If the FDA imposes a clinical hold, trials may not recommence without FDA authorization and then only under terms authorized by the FDA. Accordingly, we cannot be sure that submission of an IND will resultproposed in the FDA allowing clinical trials to begin, or that, once begun, issues will not arise that suspend or terminate such trials.
IND may begin. Clinical trials involve the administration of the biological product candidateinvestigational new drug to healthy volunteers or patients under the supervision of a qualified investigators, generally physicians not employed by or under the trial sponsor’s control.investigator. Clinical trials are conductedmust be conducted: (i) in compliance with federal regulations; (ii) in compliance with GCP, an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors; and (iii) under protocols detailing among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, and the parameters to be used in monitoring safety and the effectiveness criteria to monitor subject safety, including stopping rules that assure a clinical trial will be stopped if certain adverse events should occur.evaluated. Each protocol involving testing on U.S. patients and anysubsequent protocol amendments to the protocol must be submitted to the FDA as part of the IND. Clinical trials must be
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted and monitored in accordance with the FDA’s regulations composing the Good Clinical Practice (“GCP”)FDA requirements including the requirement that all research subjects provide informed consent. Further, each clinical trial must be reviewed and approved byor presents an independent institutional review board, (“IRB”), at or servicing each institution at whichunacceptable risk to the clinical trial will be conducted. An IRB is charged with protecting the welfarepatients. The study protocol and rights of trial participants and considers such items as whether the risks to individuals participatinginformed consent information for patients in the clinical trials are minimizedmust also be submitted to an IRB and are reasonable in relation to anticipated benefits.ethics committee for approval. The IRB will also approves the form and content of the informed consent that must be signed by each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. HumanAn IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether a trial may move forward at designated checkpoints based on access to certain data from the trial.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, thatbut the phases may overlapoverlap. In Phase 1, the initial introduction of the drug into healthy human subjects or be combined:
Post-approvalpatients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence of effectiveness. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the drug for a particular indication, dosage tolerance and optimum dosage, and to identify common adverse effects and safety risks. If a drug demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. In most cases the FDA requires two adequate and well-controlled Phase 3 clinical trials sometimes referred to asdemonstrate the efficacy of the drug. A single Phase 4 clinical trials,3 trial may be conducted after initial marketing approval. These clinical trials are used to gain additional experience fromsufficient in rare instances, including (1) where the treatmenttrial is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of patientsa clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in the intended therapeutic indication, particularly for long-term safety follow-up.a second trial would be practically or ethically impossible or (2) when in conjunction with other confirmatory evidence.
During all phasesThe manufacturer of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical data, andan investigational drug in a Phase 2 or 3 clinical trial investigators. Annual progress reports detailing the resultsfor a serious or life-threatening disease is required to make available, such as by posting on its website, its policy on evaluating and responding to requests for expanded access.
After completion of the required clinical trials must betesting, an NDA is prepared and submitted to the FDA. Written IND safety reports must be promptly submitted to the FDA and the investigators for serious and unexpected adverse events, any findings from other studies, tests in laboratory animals or in vitro testing that suggest a significant risk for human subjects, or any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must submit an IND safety report within 15 calendar days after the sponsor determines that the information qualifies for reporting. The sponsor also must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction within seven calendar days after the sponsor’s initial receiptapproval of the information. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, if at all. The FDA or the sponsor or its data safety monitoring board may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trialNDA is not being conducted in accordance with the IRB’s requirements or if the biological product has been associated with unexpected serious harm to subjects.
Concurrently with clinical trials, companies usually complete additional studies and must also develop additional information about the physical characteristics of the biological product as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. To help reduce the risk of the introduction of adventitious agents with use of biological products, the PHSA emphasizes the importance of manufacturing control for products whose attributes cannot be precisely defined. The manufacturing process must be capable of consistently producing quality batchesrequired before marketing of the product candidate and, among other criteria,may begin in the sponsor must develop methods for testing the identity, strength, quality, potency and purity of the final biological product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the biological product candidate does not undergo unacceptable deterioration over its shelf life.
U.S. Review and Approval Processes
After the completion of clinical trials of a biological product, FDA approval of a BLA must be obtained before commercial marketing of the biological product. The BLANDA must include the results of product development, laboratoryall pre-clinical, clinical and animal studies, human trials, information onother testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture and compositioncontrols.
| 15 |
The cost of the product, proposed labelingpreparing and other relevant information.submitting an NDA is substantial. The FDA may grant deferrals for submission of data, or full or partial waivers. The testingmost NDAs is additionally subject to a substantial application user fee, currently exceeding $4 million for fiscal year 2024 and approval processes require substantial time and effort and there can be no assurance that the FDA will accept the BLA$3.2 million for filing and, even if filed, that any approval will be granted on a timely basis, if at all.
Under the Prescription Drug User Fee Act, as amended (“PDUFA”)fiscal year 2023 (for applications containing clinical data), each BLA must be accompanied by a significant user fee. The FDA adjusts the PDUFA user fees on an annual basis. PDUFA also imposes an annual program feewhich increased from $3.1 million for biological products.fiscal year 2022. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business. Additionally, no user fees are assessed on NDAs for products designated as orphan drugs, unless the product also includes a non-orphan indication. The applicant under an approved NDA is also subject to annual program fees, currently $393,933 for fiscal year 2023 for each prescription product and $416,734 for fiscal year 2024. for each prescription product. The FDA adjusts the user fees on an annual basis, and the fees typically increase annually.
Within 60 days following submission of the application, theThe FDA reviews a BLAeach submitted to determine ifNDA before it is substantially complete before the agency accepts it for filing. The FDA may refusedetermines whether to file any BLA that it deems incomplete or not properly reviewable at the time of submission and may request additional information. In this event,The FDA must make a decision on whether to file an NDA within 60 days of receipt, and such decision could include a refusal to file by the BLA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing.FDA. Once the submission is accepted for filing,filed, the FDA begins an in-depth substantive review of the BLA.NDA. The FDA reviewshas agreed to certain performance goals in the BLAreview of NDAs. Most applications for standard review drug products are reviewed within ten to determine, among other things, whethertwelve months; most applications for priority review drugs are reviewed in six to eight months. Priority review can be applied to drugs that the proposed product is safe, potent, and/FDA determines may offer significant improvement in safety or effectiveeffectiveness compared to marketed products or where no adequate therapy exists. The review process for itsboth standard and priority review may be extended by the FDA for three additional months to consider certain late-submitted information, or information intended use, and has an acceptable purity profile, and whetherto clarify information already provided in the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, safety, strength, quality, potency and purity.submission. The FDA does not always meet its goal dates for standard and priority NDAs, and the review process can be extended by FDA requests for additional information or clarification.
The FDA may also refer applications for novel biologicaldrug products, or biologicaldrug products that present difficult questions of safety or efficacy, to an outside advisory committee, committee—typically a panel that includes clinicians and other experts, experts—for review, evaluation and a recommendation as to whether the application should be approved and under what conditions.conditions, if any. The FDA is not bound by the recommendationsrecommendation of an advisory committee, but it considersgenerally follows such recommendations carefully when making decisions. During the biological product approval process, the FDA also will determine whether a REMS, is necessary to assure the safe use of the biological product. If the FDA concludes a REMS is needed, the sponsor of the BLA must submit a proposed REMS. The FDA will not approve a BLA without a REMS, if required.recommendations.
Before approving a BLA,an NDA, the FDA will inspectconduct a pre-approval inspection of the manufacturing facilities at whichfor the new product is manufactured.to determine whether they comply with cGMP requirements. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and are adequate to assure consistent production of the product within required specifications. Additionally, before approving a BLA,The FDA also typically inspects clinical trial sites to ensure compliance with GCP requirements and the integrity of the data supporting safety and efficacy.
After the FDA will typically inspect one or more clinical sites to assure thatevaluates the clinical trials were conducted in compliance with IND trial requirements and GCP requirements. To assure cGMP and GCP compliance, an applicant must incur significant expenditure of time, money and effort in the areas of training, record keeping, production, and quality control.
Notwithstanding the submission of relevant data and information, the FDA may ultimately decide that the BLA does not satisfy its regulatory criteria for approval and deny approval. Data obtained from clinical trials are not always conclusiveNDA and the FDA may interpret data differently than we interpret the same data. If the agency decides not to approve the BLA in its present form, the FDA will issuemanufacturing facilities, it issues either an approval letter or a complete response letter that describes all of(“CRL”). A CRL generally outlines the specific deficiencies in the BLA identified by the FDA. The deficiencies identifiedsubmission, which may be minor for example, requiring labeling changes,and more technical, or major and more substantive and, in the latter case may require substantial additional testing or data to be eligible for example, requiringsubstantive review by FDA upon resubmission, such as additional clinical trials. Additionally, the complete response letter may include recommended actions that the applicant might takedata, additional pivotal clinical trial(s), and/or other significant and time-consuming requirements related to place the application in a condition for approval.clinical trials, pre-clinical studies or manufacturing. If a complete response letterCRL is issued, the applicant may either resubmit the BLA, seeingNDA addressing all of the deficiencies identified in the letter, or withdraw the application.
Ifapplication, engage in formal dispute resolution or request an opportunity for a product receives regulatory approval,hearing. The FDA has committed to reviewing resubmissions in two to six months depending on the approval may be significantly limited to specific diseasestype of information included. Even if such data and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product.
Further,information are submitted, the FDA may requiredecide that certain contraindications, warnings or precautions be includedthe NDA does not satisfy the criteria for approval.
If the deficiencies identified in the product labeling. TheCRL are addressed to FDA’s satisfaction in a resubmission of the NDA (and FDA may impose restrictions and conditions on product distribution, prescription,does not identify any other issues that need to be corrected prior to approval or dispensation inthat, otherwise, cause the form of a risk management plan, or otherwise limitagency to determine that approval is not appropriate at the scope of any approval. In addition,given time), the FDA may require postwill issue an approval letter. An approval letter authorizes commercial marketing clinical trials, sometimes referred to as Phase 4 clinical trials, designed to further assess a biological product’s safety and effectiveness, and testing and surveillance programs to monitorof the safety of approved products that have been commercialized.
drug with specific prescribing information for specific indications. In addition, under the Pediatric Research Equity Act a BLAof 2003 (“PREA”), as amended and reauthorized, certain NDAs or supplementsupplements to a BLAan NDA must contain data that are adequate to assess the safety and effectiveness of the productdrug for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers.waivers from the pediatric data requirements.
Post-Approval RequirementsAs a condition of NDA approval, the FDA may also require a REMS, to help ensure that the benefits of the drug outweigh the potential risks to patients. A REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use (“ETASU”). ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
AnyChanges to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of an NDA supplement or, in some case, a new NDA, before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
| 16 |
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA regulated products, including drugs, are required to register and disclose certain clinical trial information to the U.S. public by publishing such information on clinicaltrials.gov. Information related to the product, patient population, phase of investigation, study sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed in certain circumstances for up to two years after the date of completion of the trial. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
Expedited Development and Review Programs
The FDA is authorized to designate certain products for expedited review if they are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. These programs are fast track designation, breakthrough therapy designation, and priority review designation. MyMD has not applied for expedited approval under any of these pathways to-date but intends to explore the extent to which we receiveany of its current or future product candidates may be eligible for one or more such pathways. There is no guarantee that FDA approvalswill grant any of MyMD’s products candidates the expedited designation(s) for which it is submitted, if any, or that MyMD will secure any of the applicable benefits associated with any of any expedited designations that may be granted to its current or future product candidates, if applicable.
Fast-Track Designation
Fast track designation may be granted for a product that is intended to treat a serious or life-threatening disease or condition for which pre-clinical or clinical data demonstrate the potential to address unmet medical needs for the condition. The sponsor of an investigational drug product may request that the FDA designate the drug candidate for a specific indication as a fast-track drug concurrent with, or after, the submission of the IND for the drug candidate. The FDA must determine if the drug candidate qualifies for fast-track designation within 60 days of receipt of the sponsor’s request. For fast-track products, sponsors may have greater interactions with the FDA and the FDA may initiate review of sections of a fast-track product’s NDA before the application is complete. This rolling review is available if the FDA determines, after preliminary evaluation of clinical data submitted by the sponsor, that a fast-track product may be effective. The sponsor must also provide, and the FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees. At the time of NDA filing, the FDA will determine whether to grant priority review designation. Additionally, fast track designation may be withdrawn if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
| 17 |
Breakthrough Therapy Designation
In 2012, Congress enacted the Food and Drug Administration Safety and Innovation Act, or FDASIA. This law established a new regulatory scheme allowing for expedited review of products designated as “breakthrough therapies.” A product may be designated as a breakthrough therapy if it is intended, either alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to breakthrough therapies, including holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross-disciplinary project lead for the review team; and taking other steps to design the clinical trials in an efficient manner.
Priority Review Designation
The FDA may designate a product for priority review if it is a drug that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines, on a case- by-case basis, whether the proposed drug represents a significant improvement when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting drug reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes, and evidence of safety and effectiveness in a new subpopulation. A priority designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s goal for taking action on a marketing application from ten months to six months.
Accelerated Approval
Accelerated approval may be granted for a product that is intended to treat a serious or life-threatening condition and that generally provides a meaningful therapeutic advantage to patients over existing treatments. A product eligible for accelerated approval may be approved on the basis of either a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. The accelerated approval pathway is most often used in settings in which the course of a disease is long, and an extended period of time is required to measure the intended clinical benefit of a product, even if the effect on the surrogate or intermediate clinical endpoint occurs rapidly. The accelerated approval pathway is contingent on a sponsor’s agreement to conduct additional post-approval confirmatory studies to verify and describe the product’s clinical benefit. These confirmatory trials must be completed with due diligence and, in some cases, the FDA may require that the trial be designed, initiated, and/or fully enrolled prior to approval. Failure to conduct required post-approval studies, or to confirm a clinical benefit during post-marketing studies, would allow the FDA to withdraw the product from the market on an expedited basis. All promotional materials for product candidates approved under accelerated regulations are subject to prior review by the FDA.
| 18 |
Post-marketing Requirements
Following approval of a new product, the manufacturer and the approved product are subject to continuing regulation by the FDA. Drug manufacturers’ and/or sponsors’ post-marketing FDA including,obligations, include, among other things, monitoring and record-keeping requirements,activities, reporting of adverse experiences, with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, and complying with FDA promotion and advertising requirements, which include among others, standards for direct-to-consumer advertising, restrictions on promoting products for unapproved uses or in patient populations that are not described in the product’s approved uses, known(known as “off-label” use,“off-label use”) and limitations on industry-sponsored scientific and educational activities, and a number of other specific requirements for promotional activities involving the internet.prescription-drug advertising. Although physicians may prescribe legally available products for off-label uses, if the physicians deem to be appropriate in their professional medical judgment, manufacturers may not market or promote suchtheir approved drug products for off-label uses.
In addition, quality control and manufacturing procedures must continue to conform to applicable manufacturing requirements after approval to ensure the long-term stability of the product. cGMP regulations require among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation and the obligation to investigate and correct any deviations from cGMP. Manufacturers and other entities involved in the manufacture and distribution of approved products are required to register their establishments Product approvals may be withdrawn for non-compliance with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance. Discovery ofregulatory standards or if problems with a product after approval may result in restrictions on a product, manufacturer, or holder of an approved BLA, including, among other things, recall or withdrawal of the product from the market. In addition, changes to the manufacturing process are strictly regulated, and depending on the significance of the change, may require prior FDA approval before being implemented. Other types of changes to the approved product, such as adding new indications and claims, are also subject to further FDA review and approval.
Discovery of previously unknown problems with a product or the failure to comply with applicable FDA requirements can have negative consequences, including adverse publicity, judicial or administrative enforcement, warning letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties, among others.occur following initial marketing. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and may also may require the implementation of other risk management measures. Also, new government requirements,measures, including those resulting from new legislation, may be established,a REMS, or the FDA’s policies may change, which could delay or prevent regulatory approvalconduct of our prospective vaccine candidate(s).post-marketing studies to assess a newly discovered safety issue.
Other U.S. Healthcare LawsFDA regulations require that drug products be manufactured in registered drug-manufacturing facilities and Compliance Requirements
Inin accordance with cGMP regulations. MYMD currently relies on third parties to produce clinical quantities of its drug candidates under development in accordance with applicable GCPs and GLPs, and expects to continue to rely, on third parties to produce clinical and commercial quantities of MYMD’s products that are approved for marketing in the United States, ourif any, in accordance with cGMP regulations. These manufacturers must comply with cGMP regulations that require, among other things, quality control and quality assurance, the maintenance of records and documentation and the obligation to investigate and correct any deviations from cGMP. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance. The discovery of violative conditions, including failure to conform to cGMP regulations, could result in a wide range of enforcement actions against the manufacturer, including, but not limited to, recalls, warning letters, “dear doctor” letters, civil lawsuits, fines, and criminal prosecution. And the discovery of previously unknown safety or efficacy problems with a product after approval may result in restrictions on, revocation of, or the addition of conditions to the product’s approval, among other potential adverse actions.
In addition to the requirements applicable to approved drug products, sponsors may also be subject to enforcement action in connection with any promotion of any investigational new drug. A sponsor or investigator, or any person acting on behalf of a sponsor or investigator, may not represent in a promotional context that an investigational new drug is safe or effective for the purposes for which it is under investigation or otherwise promote or market the product.
Other Regulatory Matters
Manufacturing, sales, promotion and other activities following product approval are potentiallyalso subject to regulation by various federal, state and localnumerous regulatory authorities in the U.S. in addition to the FDA, including the CMS, other divisions of the HHS, the DOJ, the Drug Enforcement Administration, the Consumer Product Safety Commission, the Federal Trade Commission, the Occupational Safety & Health Administration, the Environmental Protection Agency and state and local governments and governmental agencies.
Other Healthcare Laws
Healthcare providers, physicians, and third-party payors will play a primary role in the recommendation and prescription of any products for which MyMD may obtain marketing approval. MyMD’s current and future arrangements with third-party payors, healthcare providers and physicians may expose MyMD to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which MyMD markets, sells and distributes any drugs for which MYMD obtains marketing approval. In the U.S., these laws include, without limitation, state and federal anti-kickback, false claims, physician transparency, and patient data privacy and security laws and regulations, including but not limited to those described below. MYMD’s business operations, including its research, marketing, and activities relating to the Centersreporting of wholesale or estimated retail prices for MedicareMyMD’s products, the reporting of prices used to calculate Medicaid rebate information and Medicaid Services (“CMS”), other divisions of the U.S. Department of Health and Human Services (“HHS”), for instance the Office of Inspector General, the U.S. Department of Justice, or (“DOJ”), and individual U.S. Attorney offices within the DOJ, andinformation affecting federal, state and local governments. For example, sales,third-party reimbursement for MyMD’s products, and the sale and marketing of MyMD’s product and scientific/educational grant programs must comply with the anti-fraud and abuse provisions of the Social Security Act, the false claims laws, the physician payment transparency laws, the privacy and security provisions of the federal Health Insurance Portability and Accountability Act (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health or “HITECH” Act, and similar state laws, each as amended.
The federal Anti-Kickback Statute (“AKS”) prohibits, among other things, any person or entity, from knowingly and willfully offering, paying, soliciting or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any item or service reimbursable under Medicare, Medicaid or other federal healthcare programs. The term remuneration has been interpreted broadly to include anything of value. The AKS has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. Therefuture product candidates, are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution. The exceptions and safe harbors are drawn narrowly and practices that involve remuneration that may be alleged to be intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. Our practices may not in all cases meet all of the criteria for protection under a statutory exception or regulatory safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor, however, does not make the conduct per se illegal under the AKS. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances.
Additionally, the intent standard under the AKS was amended by the Affordable Care Act (“ACA”) to a stricter standard, such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the ACA codified case law that a claim including items or services resulting from a violation of the AKS constitutes a false or fraudulent claim for purposes of the federal False Claims Act, or (“FCA”), as discussed below.
The civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
The federal FCA prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to, or approval by, the federal government or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the U.S. government. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of the product for unapproved, and thus non-reimbursable, uses.laws.
| 19 |
HIPAA created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud or to obtain, by means of false or fraudulent pretenses, representations or promises, any money or property owned by, or under the control or custody of, any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up by trick, scheme or device, a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Also, many states have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
We may be subject to data privacy and security regulations by both the federal government and the states in which we conduct our business. HIPAA, as amended by the HITECH Act, imposes requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to business associates, independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions.
| ● | The AKS, makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf), to knowingly and willfully solicit, receive, offer or pay any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, that is intended to induce or reward referrals, including the purchase, recommendation, order or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Violations of this law are punishable by imprisonment, criminal fines, administrative civil money penalties and exclusion from participation in federal healthcare programs. In addition, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it. | |
| ● | The federal civil and criminal false claims laws, including the FCA, which can be enforced through civil whistleblower or qui tam actions, which impose penalties against individuals or entities (including manufacturers) for, among other things, knowingly presenting, or causing to be presented false or fraudulent claims for payment by a federal healthcare program or making a false statement or record material to payment of a false claim or avoiding, decreasing or concealing an obligation to pay money to the federal government. The government may deem manufacturers to have “caused” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. Claims that include items or services resulting from a violation of the AKS are false or fraudulent claims for purposes of the FCA. | |
| ● | The federal anti-inducement law, which prohibits, among other things, the offering or giving of remuneration, which includes, without limitation, any transfer of items or services for free or for less than fair market value (with limited exceptions), to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of items or services reimbursable by a federal or state governmental program. | |
| ● | HIPAA imposes criminal and civil liability for knowingly and willfully executing a scheme, or attempting to execute a scheme, to defraud any healthcare benefit program, including private payors, or falsifying, concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the AKS, a person or entity does not need to have actual knowledge of the healthcare fraud statute implemented under HIPAA or specific intent to violate it in order to have committed a violation. | |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and their respective implementing regulations, imposes, among other things, specified requirements on covered entities and their business associates relating to the privacy and security of individually identifiable health information including mandatory contractual terms and required implementation of technical safeguards of such information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. | |
| ● | The PPSA, enacted as part of the ACA, imposed new annual reporting requirements for certain manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, for certain payments and “transfers of value” provided to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Effective January 1, 2022, these reporting obligations extend to include transfers of value made during the previous year to certain non-physician providers such as physician assistants and nurse practitioners. | |
| ● | Analogous state and foreign fraud and abuse laws and regulations, such as state anti-kickback and false claims laws, which may be broader in scope and apply regardless of payor. These laws are enforced by various state agencies and through private actions. Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant federal government compliance guidance, require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, and restrict marketing practices or require disclosure of marketing expenditures. In addition, certain state and local laws require the registration of pharmaceutical sales representatives. |
State and foreign laws also govern the privacy and security of health information in specified circumstances, many of whichsome circumstances. These data privacy and security laws may differ from each other in significant ways thus complicatingand often are not pre-empted by HIPAA, which may complicate compliance efforts. Furthermore, most states in the United States have enacted laws regulating the confidentiality and security of medical information and increased public focus on privacy may result in amendments or changes to these laws in ways that may have an impact on MyMD’s business activities related to the collection and use of health-related information.
| 20 |
The increased attention on privacy in the United States may also impact MyMD’s business activities for the processing of personal information not otherwise governed by HIPAA. The EU General Data Protection Regulation (“GDPR”) imposes significant privacy and cybersecurity requirements related to the handling of all types of personal information, with heightened requirements on sensitive personal information, such as health information. The GDPR imposes significant limitations on the use of this personal information and grants individuals in the EU certain rights associated with the collection and use of personal information. In the U.S., California enacted the CCPA, which creates new individual privacy rights for California consumers (generally defined as any resident of California, including employees and other business relations) and places increased privacy and security obligations on entities handling personal information of consumers or households. The CCPA also greatly extends the obligations of entities that process personal information to include information not traditionally viewed as personal information and regulated by laws, such as Internet Protocol (IP) addresses, unique identifiers for individuals, and information in online cookies and other online technologies. A majority of other states have already proposed or enacted laws similar to the CCPA, each differing in scope of the personal information covered and the rights of individuals. Furthermore, the CCPA has already been amended with the passage of California’s Proposition 24 (the California Privacy Rights Act, “CPRA”), which adds additional rights and obligations. While the CCPA and CPRA currently provide relatively broad exclusions for protected health information regulated by HIPAA and clinical trials and a limited exception for consumer and business to business information, some of the proposed and enacted laws in other states may not contain the same exceptions. Furthermore, there have been a number of competing proposals for federal laws, some of which propose to not preempt other state laws. The uncertainty surrounding proposed new and changes to existing privacy laws may lead to operational challenges for MYMD to comply with multiple, potentially conflicting, privacy and cybersecurity laws related to the collection and use of personal information in each jurisdiction.
Various state and federal laws and regulations also require entities to implement “reasonable” or “adequate” security measures to protect personal information, but generally do not provide any specific sets of security measures that would be considered compliant to avoid liability. Instead, different regulators have adopted inconsistent and evolving standards based on the regulator’s view of what is appropriate given the nature and scope of the personal information and the processing performed, resulting in unclear obligations. This may result in potential liability if a regulator finds that MYMD’s security practices do not meet or exceed the types of security measures that the regulator believes to be adequate or reasonable under the circumstances.
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially considering the lack of applicable precedent and regulations. Federal and state enforcement bodies have continued to increase their scrutiny of interactions between healthcare companies and healthcare providers, which has led to investigations, prosecutions, convictions and settlements in the healthcare industry. It is possible that governmental authorities will conclude that MyMD’s business practices do not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If MyMD’s operations are found to be in violation of any of these laws or any other related governmental regulations that may apply to it, MyMD may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, disgorgement, exclusion of drugs from government funded healthcare programs, such as Medicare and Medicaid, reputational harm, additional oversight and reporting obligations if MyMD becomes subject to a corporate integrity agreement or similar settlement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of MyMD’s operations. If any of the physicians or other healthcare providers or entities with whom MyMD expects to do business is found to be not in compliance with applicable laws, they may be subject to similar actions, penalties and sanctions. Ensuring business arrangements comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time- and resource-consuming and can divert a company’s attention from its business.
Current and Future Healthcare Reform Legislation
On March 23, 2010, President Obama signed the “Patient Protection and Affordable Care Act” (P.L. 111-148) (the “ACA”) and on March 30, 2010, he signed the “Health Care and Education Reconciliation Act” (P.L. 111-152), collectively commonly referred to as the “Healthcare Reform Law.” The Healthcare Reform Law included a number of new rules regarding health insurance, the provision of healthcare, conditions to reimbursement for healthcare services provided to Medicare and Medicaid patients, and other healthcare policy reforms. Through the law-making process, substantial changes have been and continue to be made to the current system for paying for healthcare in the U.S., including changes made to extend medical benefits to certain Americans who lacked insurance coverage and to contain or reduce healthcare costs (such as by reducing or conditioning reimbursement amounts for healthcare services and drugs, and imposing additional taxes, fees, and rebate obligations on pharmaceutical and medical device companies). This legislation was one of the most comprehensive and significant reforms ever experienced by the U.S. in the healthcare industry and has significantly changed the way healthcare is financed by both governmental and private insurers. This legislation has impacted the scope of healthcare insurance and incentives for consumers and insurance companies, among others. Additionally, the Healthcare Reform Law’s provisions were designed to encourage providers to find cost savings in their clinical operations. Pharmaceuticals represent a significant portion of the cost of providing care. This environment has caused changes in the purchasing habits of consumers and providers and resulted in specific attention to the pricing negotiation, product selection and utilization review surrounding pharmaceuticals. This attention may result in our product candidates, to the extent approved for commercialization in the future, being chosen less frequently or the pricing being substantially lowered. At this stage, it is difficult to estimate the full extent of the direct or indirect impact of the Healthcare Reform Law on us.
These structural changes could entail further modifications to the existing system of private payors and government programs (such as Medicare, Medicaid, and the State Children’s Health Insurance Program), creation of government-sponsored healthcare insurance sources, or some combination of both, as well as other changes. Restructuring the coverage of medical care in the U.S. could impact the reimbursement for prescribed drugs and pharmaceuticals, including any products that we may commercialize or promote in the future. If reimbursement for the products we may commercialize or promote in the future is substantially reduced or otherwise adversely affected in the future, or rebate obligations associated with them are substantially increased, it could have a material adverse effect on our reputation, business, financial condition or results of operations.
Extending medical benefits to those who currently lack coverage will likely result in substantial costs to the U.S. federal Physician Payment Sunshine Actgovernment, which may force significant additional changes to the healthcare system in the U.S. Much of 2010 (“PPSA”) underthe funding for expanded healthcare coverage may be sought through cost savings. While some of these savings may come from realizing greater efficiencies in delivering care, improving the effectiveness of preventive care and enhancing the overall quality of care, much of the cost savings may come from reducing the cost of care and increased enforcement activities. Cost of care could be reduced further by decreasing the level of reimbursement for medical services or products or by restricting coverage (and, thereby, utilization) of medical services or products. In either case, a reduction in the utilization of, or reimbursement for any product we may commercialize or promote in the future, could have a material adverse effect on our reputation, business, financial condition or results of operations.
| 21 |
Several states and private entities initially mounted legal challenges to the Healthcare Reform Law, in particular, the ACA, and itsthey continue to litigate various aspects of the legislation. On July 26, 2012, the U.S. Supreme Court generally upheld the provisions of the ACA at issue as constitutional. However, the U.S. Supreme Court held that the legislation improperly required the states to expand their Medicaid programs to cover more individuals. As a result, states have a choice as to whether they will expand the number of individuals covered by their respective state Medicaid programs. Some states have not expanded their Medicaid programs and have chosen to develop other cost-saving and coverage measures to provide care to currently uninsured individuals. Many of these efforts to date have included the institution of Medicaid-managed care programs. The manner in which these cost-saving and coverage measures are implemented could have a material adverse effect on our reputation, business, financial condition or results of operations.
Further, the healthcare regulatory environment has seen significant changes in recent years and is still in flux. Legislative initiatives to modify, limit, replace, or repeal the ACA and judicial challenges have continued. We cannot predict the impact on our business of future legislative and legal challenges to the ACA or other aspects of the Healthcare Reform Law or other changes to the current laws and regulations. The financial impact of U.S. healthcare reform legislation over the next few years will depend on a number of factors, including the policies reflected in implementing regulations requireand guidance and changes in sales volumes for therapeutics affected by the legislation. From time to time, legislation is drafted, introduced and passed in the U.S. Congress that could significantly change the statutory provisions governing coverage, reimbursement, and marketing of pharmaceutical products. In addition, third-party payor coverage and reimbursement policies are often revised or interpreted in ways that may significantly affect our business and our products.
During his time in office, former President Trump supported the repeal of all or portions of the ACA. President Trump also issued an executive order in which he stated that it is his administration’s policy to seek the prompt repeal of the ACA and in which he directed executive departments and federal agencies to waive, defer, grant exemptions from, or delay the implementation of the provisions of the ACA to the maximum extent permitted by law. Congress has enacted legislation that repeals certain manufacturersportions of drugs, devices, biologicalthe ACA, including but not limited to the Tax Cuts and medical suppliesJobs Act, passed in December 2017, which included a provision that eliminates the penalty under the ACA’s individual mandate, effective January 1, 2019, as well as the Bipartisan Budget Act of 2018, passed in February 2018, which, among other things, repealed the Independent Payment Advisory Board (which was established by the ACA and was intended to reduce the rate of growth in Medicare spending).
Additionally, in December 2018, a district court in Texas held that the individual mandate is unconstitutional and that the rest of the ACA is, therefore, invalid. On appeal, the Fifth Circuit Court of Appeals affirmed the holding on the individual mandate but remanded the case back to the lower court to reassess whether and how such holding affects the validity of the rest of the ACA. The Fifth Circuit’s decision on the individual mandate was appealed to the U.S. Supreme Court. On June 17, 2021, the Supreme Court held that the plaintiffs (comprised of the state of Texas, as well as numerous other states and certain individuals) did not have standing to challenge the constitutionality of the ACA’s individual mandate and, accordingly, vacated the Fifth Circuit’s decision and instructed the district court to dismiss the case. As a result, the ACA will remain in-effect in its current form for the foreseeable future; however, we cannot predict what additional challenges may arise in the future, the outcome thereof, or the impact any such actions may have on our business.
The Biden administration also introduced various measures in 2021 focusing on healthcare and drug pricing, in particular. For example, on January 28, 2021, President Biden issued an executive order that initiated a special enrollment period for purposes of obtaining health insurance coverage through the ACA marketplace, which payment is available under Medicare,began on February 15, 2021, and remained open through August 15, 2021. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the Children’s Health Insurance Program,ACA. On the legislative front, the American Rescue Plan Act of 2021 was signed into law on March 11, 2021, which, in relevant part, eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single source drugs and innovator multiple source drugs, beginning January 1, 2024. And, in July 2021, the Biden administration released an executive order entitled, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response, on September 9, 2021, HHS released a “Comprehensive Plan for Addressing High Drug Prices” that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. And, on August 16, 2022, President Biden signed into law the Inflation Reduction Act of 2022, which aims to lower prescription drug pricing by, among other things, allowing Medicare to negotiate prices for certain exceptions,high-cost prescription drugs covered under Medicare Part D and Part B after the drugs have been on the market for a certain number of years and requiring on drug manufacturers to report information relatedpay rebates if they increase drug prices “faster than inflation.” In the coming years, additional legislative and regulatory changes could be made to governmental health programs that could significantly impact pharmaceutical companies and the success of our product candidates. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain paymentsproduct access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
There is uncertainty as to what healthcare programs and regulations may be implemented or other transfers of value made or distributed to physicians and teaching hospitals, or to entities or individualschanged at the requestfederal and/or state level in the United States or the effect of any future legislation or designatedregulation. Furthermore, we cannot predict what actions the Biden administration will implement in connection with the Health Reform Law. However, it is possible that such initiatives could have an adverse effect on behalfour ability to obtain approval and/or successfully commercialize products in the United States in the future. For example, any changes that reduce, or impede the ability to obtain, reimbursement for our product candidates approved for commercialization in the United States, if any, or any other drug products we may commercialize in the future or that reduce medical procedure volumes could adversely affect our operations and/or future business plans.
| 22 |
Packaging and Distribution in the United States
If MyMD’s product candidates that are approved for commercialization in the United States, if any, are made available to authorized users of the physiciansFederal Supply Schedule of the General Services Administration, additional laws and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately, and completelyrequirements may apply. In relevant part, products must meet applicable child-resistant packaging requirements under the required information may result in civil monetary penalties of up to an aggregate of $150,000 per year and up to an aggregate of $1 million per year for “knowing failures”. Certain states also mandate implementation of compliance programs, impose restrictions on pharmaceutical manufacturer marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to healthcare providers and entities.
In order to distribute products commercially, we must also comply with state laws that require the registration of manufacturers and wholesale distributors of drug and biological products in a state, including, in certain states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical and biotechnology companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures onU.S. Poison Prevention Packaging Act. Manufacturing, sales, marketing, pricing, clinical trialspromotion and other activities and/or register their sales representatives, as well as to prohibit pharmacies and other healthcare entities from providing certain physician prescribing data to pharmaceutical and biotechnology companies for use in sales and marketing, and to prohibit certain other sales and marketing practices. All of our activitiesalso are potentially subject to federal and state consumer protection and unfair competition laws.
If our operations are foundThe distribution of pharmaceutical products is subject to be in violationadditional requirements and regulations, including extensive record-keeping, licensing, storage and security requirements intended to prevent the unauthorized sale of pharmaceutical products.
The failure to comply with any of these laws or regulatory requirements subjects firms to possible legal or regulatory action. Depending on the federal and state healthcare laws described abovecircumstances, failure to meet applicable regulatory requirements can result in criminal prosecution, fines or any other governmental regulations that apply to us, we may be subject to penalties, including without limitation, civil, criminal and/or administrative penalties, damages, fines, disgorgement,injunctions, exclusion from participation in governmentfederal healthcare programs, such as Medicare and Medicaid, injunctions, private “qui tam” actions brought by individual whistleblowers in the namerequests for recall, seizure of the government,products, total or partial suspension of production, denial or withdrawal of product approvals, or refusal to allow usa firm to enter into supply contracts, including government contracts, contractual damages, reputational harm, administrative burdens, diminished profitscontracts. Any action against MyMD for violation of these laws, even if MyMD is successful in defending against it, could cause MyMD to incur significant legal expenses and divert MyMD’s management’s attention from the operation of its business. Prohibitions or restrictions on sales or withdrawal of future earnings, andproducts marketed by MyMD could materially affect its business in an adverse way.
Changes in regulations, statutes or the curtailmentinterpretation of existing regulations could impact MyMD’s business in the future by requiring, for example: (i) changes to MyMD’s manufacturing arrangements; (ii) additions or restructuringmodifications to product labeling; (iii) the recall or discontinuation of our operations,MyMD’s products; or (iv) additional record-keeping requirements. If any of whichsuch changes were to be imposed, they could adversely affect our ability to operate our business and our resultsthe operation of operations.MyMD’s business.
U.S. Healthcare ReformReimbursement
We anticipateSales of any of MyMD’s product candidates that current andare approved for marketing in the United States or any other products MyMD may commercialize in the future, U.S. legislative healthcare reforms may resultas applicable, will depend, in additional downward pressurepart, on the priceextent to which MyMD’s products, if approved, will be covered by third-party payors, such as government health programs, commercial insurers and managed healthcare organizations, as well as the level of reimbursement that we receivethose third-party payors provide for MyMD’s products. Patients and providers are unlikely to use MyMD’s products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of MyMD’s products. In the U.S., no uniform policy of coverage and reimbursement for drugs or biological products exists, and one payor’s determination to provide coverage and adequate reimbursement for a product does not assure that other payors will make a similar determination. Accordingly, decisions regarding the extent of coverage and amount of reimbursement to be provided for any of MyMD’s product candidates, if approved, product, ifwill be made on a payor-by-payor basis. As a result, the coverage determination process may be a time-consuming and costly process that will require MyMD to provide scientific and clinical support for the use of MyMD’s products to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained.
The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the HHS as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. The ACA made several changes to the Medicaid Drug Rebate Program, including increasing pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs and adding a new rebate calculation for “line extensions” (i.e., new formulations, such as extended release formulations) of solid oral dosage forms of branded products, creating a new method by which rebates owed by pharmaceutical manufacturers are calculated for drugs that are inhaled, infused, instilled, implanted or injected, as well as potentially impacting their rebate liability by modifying the statutory definition of average manufacturer’s price (“AMP”). The ACA also expanded the universe of Medicaid utilization subject to drug rebates by requiring pharmaceutical manufacturers to pay rebates on Medicaid managed care utilization and by enlarging the population potentially eligible for Medicaid drug benefits. Pricing and rebate programs must also comply with the Medicaid rebate requirements of the U.S. Omnibus Budget Reconciliation Act of 1990.
The Medicare Prescription Drug Improvement and Modernization Act of 2003 (“MMA”) established the Medicare Part D program to provide a voluntary prescription drug benefit to Medicare beneficiaries. Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities that provide coverage of outpatient prescription drugs. Unlike Medicare Part A and B, Part D coverage is not standardized. While all Medicare drug plans must give at least a standard level of coverage set by Medicare, Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and could seriously harm our business.each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, though not necessarily all the drugs in each category or class. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee. Government payment for some of the costs of prescription drugs may increase demand for products for which MyMD receives marketing approval. However, any negotiated prices for MyMD’s products covered by a Part D prescription drug plan likely will be lower than the prices MyMD might otherwise obtain. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in reimbursementpayment that results from Medicare and other government programsthe MMA may result in a similar reduction in payments from privatenon-governmental payors.
| 23 |
For a drug product to receive federal reimbursement under the Medicaid or Medicare Part B programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B drug pricing program. The implementationrequired 340B discount on a given product is calculated based on the AMP, and Medicaid rebate amounts reported by the manufacturer. As of 2010, the ACA expanded the types of entities eligible to receive discounted 340B pricing, although, under the current state of the law, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted 340B pricing on orphan drugs. In addition, as 340B drug pricing is determined based on AMP and Medicaid rebate data, the revisions to the Medicaid rebate formula and AMP definition described above could cause the required 340B discount to increase. The 340B program imposes ceilings on prices that drug manufacturers can charge for medications sold to certain health care facilities. It is unclear how this decision could affect covered hospitals who might purchase MyMD’s products in the future and affect the rates MyMD may charge such facilities for its approved products. In addition, legislation may be introduced that, if passed, would further expand the 340B program to additional covered entities or would require participating manufacturers to agree to provide 340B discounted pricing on drugs used in an inpatient setting.
As noted above, the marketability of any products for which MyMD receives regulatory approval for commercial sale may suffer if the government and other third-party payors fail to provide adequate coverage and reimbursement. An increasing emphasis on cost containment measures in the U.S. has increased and MyMD expects it will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which MyMD receives regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
These laws, and future state and federal healthcare reform measures may be adopted in the future, any of which may result in additional reductions in Medicare and other healthcare funding and otherwise affect the prices MyMD may obtain for any of its product candidates for which MyMD may obtain regulatory approval or the frequency with which any such product candidate is prescribed or used.
In addition, in most foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing and reimbursement vary widely from country to country. For example, the EU provides options for its Member States to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. Reference pricing used by various EU Member States and parallel distribution, or arbitrage between low-priced and high-priced Member States, can further reduce prices. A Member State may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. In some countries, MyMD may be required to conduct a clinical study or other healthcare reformsstudies that compare the cost-effectiveness of any of MyMD’s product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of MyMD’s products. Historically, products launched in the EU do not follow price structures of the U.S. and, generally, prices tend to be significantly lower. Publication of discounts by third-party payors or authorities may prevent uslead to further pressure on the prices or reimbursement levels within the country of publication and other countries.
Employees
As of December 31, 2023, MyMD had six full-time employees and no part-time employees. MyMD has not experienced any work stoppages. None of MyMD’s employees are represented by a labor union or covered by collective bargaining agreements, and MyMD considers its relationship with its employees to be good.
Management Plans for 2024
In November 2022, the company published data from being ablethe Phase 1 dosing study for MYMD-1 as a treatment for aging. There was a statistically significant decrease in TNF-α levels (p-value <0.05) found in one MYMD-1 treated cohort, but no change in the levels in subjects given placebo.
MyMD in collaboration with its CRO is in the final stages of preparing the end of Phase II, “A double-blind, randomized, Phase 2 study to generate revenue, attain profitabilityinvestigate the efficacy, tolerability and pharmacokinetics of MYMD1 in the treatment of participants aged 65 years or commercializeolder with chronic inflammation associated with sarcopenia/frailty” for submission to the FDA. The submission is planned for the beginning of the second quarter of 2024. Exploratory analysis indicates the biomarker sTNFR1 is the most sensitive biomarker for Sarcopenia patients aged 65-75 years old.
A phase II study for rheumatoid arthritis, “A double-blind, randomized, placebo-controlled multicenter Phase II proof-of-concept study to evaluate the efficacy, safety, biological activity, and pharmacokinetics of MYMD-1™ added to methotrexate in patients with moderate-to-severe active rheumatoid arthritis” IND application was reviewed and approved by the FDA to begin clinical trials on August 9, 2023. On November 17, 2023 an Annual Report was submitted to the FDA.
In October 2020 we completed several in vitro studies from human primary cell-based BioMap systems at Eurofins contrasting MYMD-1 with Humira, Enbrel and Remicade.
MYMD-1 Product Candidate
We completed enrollment in the fourth and final cohort of patients in the Phase 2 Aging and Sarcopenia Study (“A Double-Blind, Placebo-controlled, Randomized Study to Investigate the Efficacy, Tolerability and Pharmacokinetics of MYMD-1 in The Treatment of Participants Aged 65 Years or Older with Chronic Inflammation Associated with Sarcopenia/Frailty”). ”). As mentioned above, MyMD is preparing the submission to the FDA in the beginning of the second quarter of 2024. Exploratory analysis indicates the biomarker sTNFR1 is the most sensitive biomarker for Sarcopenia patients aged 65-75 years old. PK analysis indicates that PK/PD strategy is consistent at measurements of biomarkers 2-4 hours post-dose. There were no serious adverse events reported, no subject dropout’s secondary to an adverse event. Additionally, there were no clinically significant cardiovascular, ECG issues, or neurotoxicity issues with any patients during the study.
| 24 |
IND for Autoimmune Diseases
Animal Studies
| ● | 10-month Dog Study – completed on December 20, 2021: A 39-Week Toxicity and Toxicokinetic Study of MYMD-1 by Oral Gavage in Beagle Dogs. | |
| ● | 6-month Rat Study – completed on December 17, 2021: A 26-Week Toxicity and Toxicokinetic Study of MYMD-1 by Oral Gavage in Rats. | |
| ● | 5-Day Mouse Study – Completed May 2020 with results pending: A Preliminary Introductory Traumatic Optic Neuropathy (TON) in a Mouse study | |
| ● | Studies produced guidance on dosing levels and overall safety in the human studies. | |
| ● | MyMD in collaboration with Charles River Laboratories completed “A 90-Day Oral Gavage Electroencephalogram Safety Study of A Test Item In The Beagle Dog”. Dosing began on December 19, 2023, and ended around March 20, 2024. All animals completed the study and there were no treatment related adverse events reported. | |
| ● | Bascom Palmer in collaboration with MyMD completed a study “MyMD in Traumatic Optic Neuropathy (TON) in a Rat Pilot Study Vehicle versus TNFalpha”. The crush injury raised levels of TNF-α. After being dosed with MYMD-1, TNF-α levels were brought down in crush injury compared to controls, but the decrease did not meet statistical significance (p=0.095). Likely cause of the result not reaching p<0.05 may be attributed to rebound (e.g. TNF-α levels would have gone up when MYMD-1 stopped; daily dosing may need to me adjusted, The initial data was promising and will guide a longer study in the future. |
Publications
A scientific journal article on MYMD-1 was published in The Journals of Gerontology in August 2022. This manuscript supports our prospective vaccine candidate(s). continued efforts to conduct a second Phase 2 Trial for Rheumatoid Arthritis, which was approved for clinical trials in August 2023, and additional autoimmune diseases that we may pursue. Additionally, “MyMD-1 Improves Health Span and Prolongs Life Span in Old Mice: A Noninferiority Study to Rapamycin” by Johns Hopkins Medical School. This journal article details a 12-month mouse trial studying aging and longevity with MYMD-1. We also completed several in vitro studies from human primary cell-based BioMap systems at Eurofins contrasting MYMD-1 versus Rapamycin further supporting our transition to Rheumatoid Arthritis.
In addition, it is possibleNovember 2022, MyMD published “A Double-blind, Placebo-controlled, Randomized, Single Ascending, and Multiple Dose Phase 1 Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Oral Dose Isomyosamine Capsules in Healthy Adult Subjects” Authors: Jenna Brager, Chris Chapman, Leonard Dunn, and Adam Kaplin in Drug Research. This became available in print in February 28, 2023. This journal article details the results from the Phase 1 clinical trial.
Later, an abstract was accepted for presentation at the British Society of Immunology, Liverpool, UK in December 2022. “Pharmacology and clinical profile of MYMD-1® (isomyosamine), an oral, selective, next-generation, TNF- α inhibitor that there willcrosses the blood brain barrier” authored by Jenna Brager, Ronald Christopher, Adam Kaplin, and Chris Chapman.
Moving in to the 2023, an abstract was accepted for presentation at the Society of Toxicology to be further legislationpresented in March 2023, entitled, “A Naturally Occurring Novel Therapeutic and Oral Selective Inhibitor of TNFa, MYMD-1 (Isomyosamine), Significantly Reduced the Inflammation and Disease Severity in Murine Model of Collagen Antibody-Induced Arthritis” authored by Chris Chapman and Sonia Edaye.
An abstract for submission of the Phase II study to the Journal of Immunology or regulation that could harm our business, financial condition and resultssimilar upon final development of operations.the clinical safety report.
MyMD with its partner Frontage Laboratories plans to submit an abstract to the 39th Japanese Society for the Study of Xenobiotics (JSSX) and 26th North American Meeting of International Society for the Study of Xenobiotics (ISSX) in Honolulu, Hawaii; September 15-18, 2024. Identification of the Major Circulating Norcotinine and Elucidation of the Mechanism of Clearance of MYMD-1 in Humans. Role of Aldehyde Oxidase and CYP2A6.
All publications and abstracts support the continued development of MYMD-1® across various indications.
IND for Hashimoto’s Thyroiditis
| ● | On February 18, 2022, we submitted an Annual Update to the FDA for the previously opened Hashimoto’s Thyroiditis IND. Another Annual Report was submitted to the FDA on November 22. 2023. Phase 11 study for Hashimoto’s Thyroiditis is open for protocol development and submission to the FDA. | |
| ● | In April 2021, the FDA gave clearance for a Phase 1 dosing study in normal healthy volunteers; Institutional Review Board (IRB) approval was obtained on April 4, 2021. The clinical trial was conducted by The Clinical Research of West Florida Phase 1 unit with a closeout visit taking place on November 22, 2021. | |
| ● | Analyses of laboratory parameters, vital sign, ECG, and physical findings did not reveal any clinically relevant effect of MYMD-1. In one dose group, there was a decrease in TNF-α levels found in MYMD-1 treated subjects, but no change in the levels in subjects given placebo. In one dose group, there was a decrease in TNF-α levels found in MYMD-1 treated subjects, but no change in the levels in subjects given placebo. | |
| ● | The data from the Phase 1 clinical trial was submitted to the FDA on September 14, 2021 as part of the Annual IND update for Hashimoto’s Thyroiditis IND. The FDA responded by providing guidance on moving forward with Phase 2 clinical trials. | |
| ● | This data was also included in a new commercial IND to the FDA on September 22, 2021. |
The company completed CYP in vitro studies which concluded that clinical drug-drug interactions are not expected with MYMD-1. CYP induction is the most commonly studied form of induction in drug metabolism and is required by regulatory authorities.
We had MYMD-1 synthesized in August 2021 to [14C] MYMD-1 radiolabeled product for Mass Balance, Pharmacokinetic, and Metabolism. Analysis of the rat study results demonstrated that MYMD-1 was metabolized extensively throughout the tissues, crosses the blood brain barrier, was cleared in the urine and feces, and there were no nitrosated metabolite biological samples detected.
Lastly, a Metabolite Identification and Quantitation of MYMD-1 in Rat, Dog, and Human Plasma Samples: Metabolites in Safety Testing (MIST) was completed in October 2022. MYMD-1 was extensively metabolized, and was detected at low levels (<5%) in human plasma.
| 25 |
In November 2022, the company published data from the Phase 1 dosing study for MYMD-1 as a treatment for aging. There was a statistically significant decrease in TNF-α levels (p-value <0.05) found in one MYMD-1 treated subjects cohort, but no change in the levels in subjects given placebo.
Final efficacy data from the Phase 2 study is expected in 2024. We anticipate that we will review the safety and efficacy of this study and present the mandatory end of Phase 2 data to the FDA.
On July 27, 2021, Eurofins showed Commonality in a Comparative Study with FDA-Approved Anti-Inflammatory and Anti-Autoimmune Drugs Used for Arthritis, Colitis and Dermatitis. On October 26, 2021, our President and Chief Medical Officer, Chris Chapman, M.D., was named Honoree of the year by the Arthritis Foundation.
On August 5, 2021, our lead product candidate MYMD-1 was shown to suppress cytokines, which are the major cause of death in COVID-19 patients, in a human cell study. The company plans to consult with the FDA on this indication for post COVID-19 immune mediated depression in the second quarter 2024. During this time, MyMD Pharmaceuticals, Inc. also expects to seek additional FDA guidance on depression in MS patients under an Orphan Drug Designation (ODD).
We have an active IND to start a Phase 2 study for the indication Hashimoto’s Thyroiditis, and plan to present the FDA with a protocol for this pilot Phase 2 study in the fourth quarter 2024.
We intend to begin long-term reproductive toxicity studies in the fourth quarter 2022. These will include study of Fertility and Early Embryonic Development to Implantation in Mice, and study for Effects on Embryo Fetal Development in Mice and Rabbits with a toxicokinetic evaluation. These studies will continue to support long-term dosing in humans.
In manufacturing, we will continue to provide GMP MYMD-1 capsules for Phase 2 clinical trials. We plan to continue analytical analysis to provide GMP product other that capsules for long-term human trials.
We have received domestic patent protection for MYMD-1, including its use in methods of extending lifespan and treating arthritis, autoimmune diseases, and inflammatory and age-related disorders including sarcopenia. We will continue to prosecute patents to protect intellectual property for MYMD-1 in the United States and abroad. Patent Issued March 26, 2024: US Application 17/851,862: Method of Treating Diseases of the Visual System.
Supera-CBD Product Candidate
Data from Eurofins studies involving human primary cell-based BioMap system demonstrated that Supera-CBD delivers an extremely potent therapeutic benefit of 8,000 times that of plant-derived CBD at activating CB2 receptors, permitting its delivery at a very low non-toxic dose.
On August 10, 2021 the company was awarded U.S. Patent 11,085,047 B2, titled “Synthetic Cannabinoid Compounds for Treatment of Substance Addiction and Other Disorders,” covering the Super-CBD product candidate and its pharmaceutical formulations. During 2021 and 2022 corresponding foreign patents were awarded in Australia, Canada, Europe, Israel, and South Korea, and patents are pending in China and Japan.
Johns Hopkins Medicine researchers presented Supera-CBD data at the 3rd annual Neuroimmunology Drug Development Summit on April 26, 2021.
The company presented data referencing Super-CBD at the 4th Annual International Cannabinoid Summit on September 9, 2021.
On March 2, 2023, we announced that the U.S. Drug Enforcement Administration (DEA) has conducted a scientific review and determined that it would not Supera-CBD a controlled substance or listed chemical under the Controlled Substances Act (CSA) and its governing regulations. We believe that this decision will expedite future research involving Supera-CBD by relieving us or our research partners from having to comply with regulations relating to controlled substances.
We plan to continue our preclinical program starting genotoxicity studies in Europe. Those studies include:
| ● | Metabolic profiling and Ames test (initiation December 21, 2021; completion January 20, 2022) and | |
| ● | Micronucleus test (initiation December 21, 2021; completion February 20, 2022). |
A study of Behavioral Biology at Johns Hopkins University Supera-CBD vs. CBD Acute Pain and Inflammation begins has been funded for 2022 and 2023.
| 26 |
In manufacturing, we expect to continue providing GMP Supera-CBD materials for the preclinical toxicity programs. We plan to continue analytical analysis to provide GMP materials for long term toxicity and Human trials.
An example of continued efforts include the JHM Research is conducting a study with MYMD-1 and L/R-Supera-CBD for Depression and Anxiety.
| ● | Forced Swim Test | |
| ● | Tail suspension | |
| ● | Elevated Plus Maze and Fear Conditioning | |
| ● | Dose response study. | |
| ● | Supera-CBD open field and Y maze study. | |
| ● | MYMD-1 LPS induced depression. |
Available information
Our website address is www.akersbio.comwww.mymd.com. We do not intend our website address to be an active link or to otherwise incorporate by reference the contents of the website into this Annual Report on Form 10-K. The SEC maintains an Internet website (http://www.sec.gov) that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC.
Employees
We currently employ four (4) full-time equivalent employees, contractors or consultants, all in general and administrative. None of our employees are represented by a labor union or are a party to a collective bargaining agreement. We believe that we have good relations with our employees.
An investment in our common stockCommon Stock involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully the risks described below, together with other information in this Annual Report on Form 10-K and the other information and documents we file with the SEC. Our business, financial condition and operating results can be affected by a number of factors, whether currently known or unknown, including but not limited to those described below, any one or more of which could, directly, or indirectly, cause our actual financial condition and operating results to vary materially from past, or from anticipated future, financial condition and operating results. Any of these factors in whole or in part, could materially and adversely affect our business, financial condition, operating results and stock price.
The following discussion of risk factors contains forward-looking statements. These risk factors may be important to understanding other statements in this Form 10-K. The following information should be read in conjunction with our consolidated financial statements and related notes thereto and with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this Annual Report on Form 10-K.
Risk Factor Summary
Below is a summary of the principal factors that make an investment in our common stockCommon Stock speculative or risky. This summary does not address all of the risks that we face. Additional discussion of risks summarized in this risk factor summary, and other risks that we face, can be found below under the heading “Risk Factors” and should be carefully considered, together with other information in this Annual Report on Form 10-K and our other filings with the SEC before making investment decisions regarding our common stock.Common Stock.
Risks Related to the Proposed Mergerour Business
| ● | Our financial statements have been prepared on a going concern basis; we must raise additional capital to fund our operations in order to continue as a going concern. | |
| ● | The market price of our Common Stock may be subject to significant fluctuations and volatility, and the stockholders of the Company may be unable to resell their shares at a profit and may incur losses. | |
| ● | We may issue additional equity securities in the future, which may result in dilution to existing investors. | |
| ● | The concentration of the capital stock ownership with insiders of the Company will likely limit the ability of our stockholders to influence corporate matters. | |
| ● | We may not be able to adequately protect or enforce our intellectual property rights, which could harm our competitive position. | |
| ● | An active trading market for our Common Stock may not be sustained. | |
| ● | Our business and operations would suffer in the event of computer system failures, cyber-attacks or deficiencies in our cyber-security or those of third-party providers. |
| 27 |
● The ongoing COVID-19 pandemic may pose risksRisks Related to our Product Development and could harmRegulatory Approval
| ● | If we are unable to develop, obtain regulatory approval for and commercialize MYMD-1, Supera-CBD, or other future product candidates, or if we experience significant delays in doing so, our business will be materially harmed. | |
| ● | Success in pre-clinical studies and earlier clinical trials for our product candidates may not be indicative of the results that may be obtained in later clinical trials, including our Phase 2 clinical trial for MYMD-1, which may delay or prevent obtaining regulatory approval. | |
| ● | Even if we complete the necessary pre-clinical studies and clinical trials, we cannot predict when, or if, we will obtain regulatory approval to commercialize a product candidate and the approval may be for a narrower indication than we seek. | |
| ● | Public health crises, such as the COVID-19 pandemic, could have a material adverse impact the execution of our planned clinical trials. | |
| ● | Any product candidate for which we obtain marketing approval will be subject to extensive post-marketing regulatory requirements and could be subject to post-marketing restrictions or withdrawal from the market, and we may be subject to penalties if we fail to comply with regulatory requirements or if it experiences unanticipated problems with our product candidates, when and if any of them are approved. | |
| ● | Our development program for Supera-CBD, a synthetic analog of CBD, is uncertain and may not yield commercial results and is subject to significant regulatory risks. |
Risks Related to Commercialization and results of operations for us and the combined company following the completion of the Merger.Manufacturing
● There is no assurance when or if the Merger will be complete. Any delay in completing the Merger may substantially reduce the potential benefits that we expect
| ● | The commercial success of our product candidates, including MYMD-1 and Supera-CBD, will depend upon their degree of market acceptance by providers, patients, patient advocacy groups, third-party payors, and the general medical community. | |
| ● | The pricing, insurance coverage, and reimbursement status of newly approved products is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for our product candidates, if approved, could limit our ability to market those products and decrease our ability to generate product revenue. | |
| ● | If third parties on which we depend to conduct our planned pre-clinical studies or clinical trials, do not perform as contractually required, fail to satisfy regulatory or legal requirements or miss expected deadlines, our development program could be delayed with adverse effects on our business, financial condition, results of operations and prospects. | |
| ● | We face significant competition in an environment of rapid pharmacological change and it is possible that our competitors may achieve regulatory approval before us or develop therapies that are more advanced or effective than ours, which may harm our business, financial condition and our ability to successfully market or commercialize MYMD-1, Supera-CBD and our other product candidates. | |
| ● | The manufacture of drugs is complex, and our third-party manufacturers may encounter difficulties in production. If any of our third-party manufacturers encounter such difficulties, our ability to provide supply of MYMD-1, Supera-CBD or our other product candidates for clinical trials, our ability to obtain marketing approval, or our ability to provide supply of our product candidates for patients, if approved, could be delayed or stopped. |
Risks Related to obtain from the Merger. Furthermore, the intended benefits of the Merger may not be realized.Government Regulation
● The issuance of shares of our common stock to MYMD stockholders in the Merger will substantially dilute the voting power of current Akers stockholders. Having a minority share position will reduce the influence that current stockholders have on the management of the combined company.
| ● | Enacted and future legislation may increase the difficulty and cost for us to commercialize and obtain marketing approval of our product candidates and may affect the prices we may set. | |
| ● | The FDA’s ability to review and approve new products may be hindered by a variety of factors, including budget and funding levels, ability to hire and retain key personnel, statutory, regulatory and policy changes and global health concerns. | |
| ● | Our operations and relationships with future customers, providers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to penalties including criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings. |
● The issuance, or expected issuance, of our common stock in connection with the Merger, including the Milestone Shares, could decrease the market price of our common stock.
● Because the lack of a public market for MYMD common stock makes it difficult to evaluate the fairness of the Merger, MYMD stockholders may receive consideration in the Merger that is greater than or less than the fair market value of MYMD common stock.
● Our directors and officers may have interests in the Merger that are different from, or in addition to, those of our stockholders generally that may influence them to support or approve the Merger.
● If the Merger is completed, MYMD executive officers and MYMD appointees to the combined company’s board of directors will have the ability to significantly influence the combined company’s management and business affairs, as well as matters submitted to the combined company’s board of directors or stockholders for approval, especially if they decide to act together with the current MYMD stockholders.
● The announcement and pendency of the Merger could have an adverse effect on our business, financial condition, results of operations or business prospects.
● During the pendency of the Merger, we may not be able to enter into a business combination with another party and will be subject to contractual limitations on certain actions because of restrictions in the Merger Agreement.
● Certain provisions of the Merger Agreement may discourage third parties from submitting competing proposals, including proposals that may be superior to the arrangements contemplated by the Merger Agreement.
● The Exchange Ratio is not adjustable based on the market price of our common stock, so the merger consideration at the closing may have a greater or lesser value than at the time the Merger Agreement was signed.
● We are expected to incur substantial expenses related to the Merger.
● Failure to complete the Merger could negatively affect the value of our common stock and our future business and financial results.
● The Merger may be completed even though material adverse changes may result from the announcement of the Merger, industry-wide changes or other causes.
● We may become involved in additional securities litigation or stockholder derivate litigation in connection with the Merger, and this could divert the attention of our management and harm the combined company’s business, and insurance coverage may not be sufficient to cover all related costs and damages.
● The reverse stock split may not increase the combined company’s stock price over the long term.
● The reverse stock split would have the effect of increasing the amount of common stock that the combined company is authorized to issue without further approval by the combined company’s stockholders.
● The reverse stock split may decrease the liquidity of our common stock and lead to a decrease in overall market capitalization of the combined company.
Risks Related to Our Business PriorIntellectual Property
| ● | Our success depends in part on our ability to obtain, maintain and protect our intellectual property. It is difficult and costly to protect our proprietary rights and technology, and we may not be able to ensure their adequate protection. | |
| ● | Our potential strategy of obtaining rights to key technologies through in-licenses may not be successful. | |
| ● | Changes in patent law in the U.S. and in non-U.S. jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our product candidates. |
Risks Related to Consummation of the MergerOur Series F Convertible Preferred Stock
| ● | Our Series F Convertible Preferred Stock (the “Series F Preferred Stock”) provides for the payment of dividends in cash or in shares of our Common Stock. If we pay such dividends in shares of Common Stock, it may result in dilution to existing investors. | |
| ● | Holders of our Series F Preferred Stock are entitled to certain payments under the Certificate of Designation that may be paid in cash or in shares of Common Stock depending on the circumstances. If we make these payments in cash, it may require the expenditure of a substantial portion of our cash resources. If we make these payments in Common Stock, it may result in substantial dilution to the holders of our Common Stock. | |
| ● | The certificate of designation for the Series F Preferred Stock and the warrants issued concurrently contain anti-dilution provisions that may result in the reduction of the conversion price of the Series F Preferred Stock or the exercise price of such warrants in the future. These features may result in an indeterminate number of shares of Common Stock being issued upon conversion of the Series F Preferred Stock or exercise of the warrants. |
● We have a history of operating losses and we cannot guarantee that we can ever achieve sustained profitability.
● We may fail to realize the anticipated benefits related to our acquisition of Cystron and those benefits may take longer to realize than expected.
● Our pursuit of the COVID-19 Vaccine Candidate is at an early stage. We have not previously tested our rapid response capability and may be unable to produce a vaccine that successfully treats the virus in a timely manner, if at all.
● We operate in a highly competitive industry.
● Our business may be materially adversely affected by the COVID-19 pandemic.
● With regard to our COVID-19 Vaccine Candidate, we must conduct pre-clinical testing, prepare and submit an IND to the FDA, and conduct all phases of clinical studies (which may include postmarket or “Phase 4” studies), which will likely take several years and substantial expenses to complete, before we can submit an application for marketing approval to the FDA, and there is no guarantee that we will complete such clinical development in a timely manner or at all or that our BLA will be approved, if submitted.
● We may be unable to advance the COVID-19 Vaccine Candidate successfully through the pre-clinical and clinical development process.
● Governmental involvement may limit the commercial success of the COVID-19 Vaccine Candidate.
● Even if we are able to commercialize our prospective or future product candidates, the products may not receive coverage or adequate reimbursement from third-party payors in the United States or in other countries in which we seek to commercialize such products, which could harm our business.
● We expect to require additional capital in the future in order to develop the COVID-19 Vaccine Candidate. If we do not obtain any such additional financing, it may be difficult to complete development of the COVID-19 Vaccine Candidate or effectively realize our long-term strategic goals and objectives.
● Our failure to meet the continued listing requirements of The Nasdaq Capital Market could result in a delisting of our common stock. The delisting could adversely affect the market liquidity of our common stock and the market price of our common stock could decrease.
In addition, we face other business, financial, operational and legal risks and uncertainties set forth under “Risk Factors” in Item 1A of this Annual Report on Form 10-K.
| 28 |
Risks Related to the Proposed Mergerour Business
The ongoing COVID-19 pandemic may pose risks and could harm business and results ofOur financial statements have been prepared on a going concern basis; we must raise additional capital to fund our operations for each of Akers, MYMD, and the combined company following the completion of the merger.
The global outbreak of COVID-19 has resulted in and is likelyorder to continue as a going concern.
In its report dated April 1, 2024, Morison Cogen LLP, our independent registered public accounting firm, expressed substantial doubt about our ability to result in, substantial disruptionscontinue as a going concern as we have suffered recurring losses from operations and have insufficient liquidity to markets and economies around the world, including the United States.
Given the ongoing and dynamic nature of the circumstances, it is difficult to predict the full impact of the COVID-19 pandemic onfund our businesses, or the business of MYMD, and the combined company following the completion of the Merger, and there is no guarantee that our efforts, or the efforts of MYMD, and the combined company following the completion of the Merger to address the adverse impacts of the COVID-19 pandemic will be effective. The extent of such impact will depend on future developments, which are highly uncertain and cannot be predicted, including the duration of the pandemic, continued travel restrictions, social distancing requirements, and government mandates, among others.
COVID-19 poses a material risk to the business, financial condition and results of operations of both us and MYMD, and potentially could create risks for the combined company following the completion of the merger, including:
These factors, together or in combination with other events or occurrences not yet known or anticipated, could adversely affect the value of the merger consideration or could delay or prevent the completion of the Merger and the related transactions.operations. If we are or MYMD is, unable to recover fromimprove our liquidity position, we may not be able to continue as a business disruption ongoing concern. The accompanying consolidated financial statements do not include any adjustments that might result if we are unable to continue as a timely basis,going concern and, therefore, be required to realize our assets and discharge our liabilities other than in the Merger and the combined company’snormal course of business and financial conditions and results of operations following the completion of the Merger could be adversely affected. The Merger may also be delayed and adversely affected by the COVID-19 pandemic and become more costly. Each of Akers, MYMD, and the combined company may also incur additional costs to remedy damages caused by such disruptions, which could adversely affect eachcause investors to suffer the loss of all or a substantial portion of their financial condition and resultsinvestment. As of operations.
There is no assurance when or if the Merger will be completed. Any delay in completing the Merger may substantially reduce the potential benefits thatDecember 31, 2023, we expecthad approximately $2.7 million of cash. In order to obtain from the Merger.
Completion of the Merger is subjecthave sufficient cash to the satisfaction or waiver of a number of conditions, as set forthfund our operations in the Merger Agreement, including the approval by our stockholders, approval by Nasdaq of our application for the initial listing of our common stockfuture, we will need to be issued in connection with the Merger,raise additional equity or debt capital and other customary closing conditions. There can be nocannot provide any assurance that we and MYMD will be ablesuccessful in doing so. If are unable to satisfy the closing conditions or that closing conditions beyondraise sufficient capital to fund our or MYMD’s control will be satisfied or waived. If the conditions are not satisfied or waived, the Merger may not occur or may not be completed within the expected timeframe, andoperations, we may materiallyneed to delay, reduce or eliminate certain research and adversely losedevelopment programs or other operations, sell some or all of theour assets or merge with another entity.
We expect that we will need to raise additional funding before we can expect to become profitable from any potential benefits thatfuture sales of our product candidates. This additional financing may not be available on acceptable terms or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
We will require substantial future capital in order to complete planned and future pre-clinical and clinical development for MYMD-1 and Supera-CBD and potentially commercialize these product candidates. We expect increased spending levels in connection with our clinical trials of our product candidates. In addition, if we obtain marketing approval for any of our product candidates, we expect to achieveincur significant expenses related to commercial launch, product sales, medical affairs, regulatory, marketing, manufacturing and distribution. Furthermore, we expect to incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations before any commercial revenue may occur.
Any additional capital raised through the sale of equity or equity-backed securities may dilute our stockholders’ ownership percentages and could also result in a decrease in the market value of our equity securities.
The terms of any securities issued by us in future capital transactions may be more favorable to new investors, and may include preferences, superior voting rights and the issuance of warrants or other derivative securities, which may have a further dilutive effect on the holders of any of our securities then outstanding.
In addition, we may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we issue, such as convertible notes and warrants, which may adversely impact our financial condition.
Additional capital might not be available when we need it and our actual cash requirements might be greater than anticipated. If we require additional capital at a time when investment in its industry or in the marketplace in general is limited, we might not be able to raise funding on favorable terms, if at all. If we are not able to obtain financing when needed or on terms favorable to us, we may need to delay, reduce or eliminate certain research and development programs or other operations, sell some or all of our assets or merge with another entity.
The market price of our Common Stock has been and may continue to be subject to significant fluctuations and volatility, and the stockholders of the MergerCompany may be unable to resell their shares at a profit and may incur losses.
The market price of our Common Stock has been and could continue to be subject to significant fluctuation following. Market prices for securities of life sciences and biopharmaceutical companies in particular have historically been volatile and have shown extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. Broad market and industry factors, as well as general economic, political and market conditions such as recessions or interest rate changes, may seriously affect the market price of our Common Stock, regardless of the actual operating performance of the combined company. Some of the factors that may cause the market price of our Common Stock to fluctuate include:
| ● | the announcement of new products, new developments, services or technological innovations by us or our competitors; | |
| ● | actual or anticipated quarterly increases or decreases in revenue, gross margin or earnings, and changes in our business, operations or prospects; | |
| ● | announcements relating to strategic relationships, mergers, acquisitions, partnerships, collaborations, joint ventures, capital commitments, or other events by the us or our competitors; | |
| ● | conditions or trends in the life sciences and biopharmaceutical industries; | |
| ● | changes in the economic performance or market valuations of other life sciences and biopharmaceutical companies; | |
| ● | general market conditions or domestic or international macroeconomic and geopolitical factors unrelated to our performance or financial condition; | |
| ● | sale of our Common Stock by stockholders, including executives and directors; | |
| ● | volatility and limitations in trading volumes of our Common Stock; | |
| ● | volatility in the market prices and trading volumes of the life sciences and biopharmaceutical stocks; | |
| ● | our ability to finance our business; | |
| ● | ability to secure resources and the necessary personnel to pursue our plans; | |
| ● | failure to meet external expectations or management guidance; | |
| ● | changes in our capital structure or dividend policy, future issuances of securities, sales or distributions of large blocks of Common Stock by stockholders; | |
| ● | our cash position; | |
| ● | announcements and events surrounding financing efforts, including debt and equity securities; | |
| ● | analyst research reports, recommendations and changes in recommendations, price targets, and withdrawals of coverage; | |
| ● | departures and additions of key personnel; | |
| ● | disputes and litigation related to intellectual properties, proprietary rights, and contractual obligations; | |
| ● | investigations by regulators into our operations or those of our competitors; | |
| ● | changes in applicable laws, rules, regulations, or accounting practices and other dynamics; and | |
| ● | other events or factors, many of which may be out of our control. |
In the past, following periods of volatility in the overall market and the market prices of particular companies’ securities, securities class action litigation has often been instituted against these companies. Litigation of this type, if instituted against us, could result in substantial costs and a diversion of management’s attention and resources of the Company. Any adverse determination in any such litigation or any amounts paid to settle any such actual or threatened litigation could require that we make significant payments.
Moreover, pandemics, inflation, war and other macroeconomic and geopolitical factors have resulted in significant financial market volatility and uncertainty in recent years. A continuation or worsening of the levels of market disruption and volatility seen in the recent past could have an adverse effect on our ability to access capital, on our business, results of operations and financial condition, and on the market price of our Common Stock.
| 29 |
We have a history of operating losses, and we may not achieve or sustain profitability. We anticipate that we will continue to incur losses for the foreseeable future. If we fails to obtain additional transactionfunding to conduct our planned research and development efforts, we could be forced to delay, reduce or eliminate our product development programs or commercial development efforts.
We are a clinical-stage pharmaceutical company with a limited operating history. Pharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. Our operations to date have been limited primarily to business planning, raising capital and conducting research and development activities for our product candidates. We have never generated any revenue from product sales. We have not obtained regulatory approvals for any of our product candidates and we have funded our operations to date through proceeds from private placements of Common Stock and a line of credit from an affiliate of MyMD’s founder.
We have incurred net losses in each year since our inception. We incurred net losses attributable to shareholders of $8,218,163 and $15,197,336 for the years ended December 31, 2023 and 2022, respectively. As of December 31, 2023, we had an accumulated deficit of $101,977,067. Substantially all our operating losses have resulted from costs or other effectsincurred in connection with our research and development programs and from general and administrative costs associated with uncertainty about the Merger. In addition, pursuant to the Merger Agreement, we may extend the originally scheduled End Date (defined in the Merger Agreement as April 15, 2021) to a later date, but we will have to make additional loans to MYMD or purchase MYMD common stock for such extensions. Moreover, we have incurred andour operations. We expect to continue to incur significant expenses relatedand operating losses over the next several years and for the foreseeable future as we intend to the Merger, such as legalcontinue to conduct research and accounting fees, somedevelopment, clinical testing, regulatory compliance activities, manufacturing activities, and, if any of which must be paid even if the Mergerour product candidates is not completed.
Weapproved, sales and MYMD can agree at any time to terminate the Merger Agreement, even if our stockholders and/or MYMD’s securityholders have already adopted the Merger Agreementmarketing activities that, together with anticipated general and thereby approved the Merger and the other transactions contemplated by the Merger Agreement. We and MYMD can also terminate the Merger Agreement under other specified circumstances.
In addition, if the Merger Agreement is terminated and our board of directors determines to seek another business combination, we may not be able to find a third party willing to provide equivalent or more attractive consideration than the consideration to be providedadministrative expenses, will likely result in the Merger. In such circumstances,company incurring significant losses for the foreseeable future. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our board of directorsshareholders’ equity and working capital.
Our limited operating history may electmake it difficult to among other things, divest all or a portionevaluate the success of our business or taketo date and to assess our future viability.
MyMD’s predecessor, MyMD Florida, was formed in late 2014. Our operations to date have been limited primarily to business planning, raising capital and conducting research and development activities for our product candidates. We have not yet demonstrated the steps necessaryability to liquidate allcomplete clinical trials of our businessproduct candidates, obtain marketing approvals, manufacture a commercial scale product or conduct sales and assets,marketing activities necessary for successful commercialization. Consequently, predictions about our future success or viability are speculative and in either such case, the consideration that we receiveno assurances can be given about our future performance.
Our stockholders may be less attractive than the consideration to be received by us pursuant tonot realize a benefit from the Merger Agreement.
The issuance of shares of our common stock to MYMD stockholders incommensurate with the Merger will substantially dilute the voting power of our current stockholders. Having a minority share position will reduce the influence that current stockholders have on our management.
Pursuant to the Merger Agreement, upon the effectiveness of the Merger, (i) (“ MYMD stockholders”) will be entitled to receive (x) the number of shares of Akers common stock equal to Exchange Ratio per share of MYMD common stockownership dilution they hold, prior to giving effect to the proposed reverse stock split discussed below, (y) an amount in cash, on a pro rata basis, equal to the Additional Consideration, such payment to occur not later than 30 days after the last day of the Option Exercise Period, up to the maximum amount of cash consideration that may be received by MYMD stockholders without affecting the intended tax consequences of the merger, and (z) potential Milestone Shares payable upon achievement of certain market capitalization milestone events during the Milestone Period; and (ii) each outstanding option to purchase MYMD common stock granted under the MyMD Incentive Plan that has not previously been exercised prior to the closing of the Merger, whether or not vested, will be assumed by Akers subject to certain terms contained in the Merger Agreement, and become an option to purchase a number of shares of Akers common stock equal to the number of shares of MYMD common stock underlying such option multiplied by the Exchange Ratio, which options to purchase MYMD common stock shall be amended to expire on the second-year anniversary of the closing of the Merger, and the exercise price for each share of Akers common stock underlying an assumed option to purchase MYMD common stock will be equal to the exercise price per share of the option to purchase MYMD common stock in effect immediately prior to the completion of the Merger divided by the Exchange Ratio. Assuming the exercise in full of the outstanding Pre-Funded Warrants issuedexperienced in connection with the Private Placement and including 9,979,664 shares of combined company common stock underlying options to purchase shares of MYMD common stock to be assumed at the closing of the Merger, (i) MYMD stockholders and optionholders will own approximately 80% of the equity of the combined company; and (ii) our current stockholders, holders of certain outstanding of our options and warrants (excluding shares issuable upon exercise of options and warrants having an exercise price in excess of $1.72, prior to giving effect to any such stock splits, combinations, reorganizations and the like with respect to the Akers common stock between the announcement of the Merger and the closing of the Merger) and holders of our outstanding RSUs immediately prior to the Merger will own approximately 20% of the equity of the combined company. Accordingly, the issuance of the shares of Akers common stock to MYMD stockholders in the Merger will significantly reduce the ownership stake and relative voting power of each share of Akers common stock held by current Akers stockholders. Consequently, following the Merger, the ability of our current stockholders to influence the management of the combined company will be substantially reduced.Merger.
Moreover, under the terms of the Merger Agreement,If we agreed to pay Milestone Payments, payable in shares of Akers common stock to MYMD stockholders upon the achievement of certain market capitalization milestone events during the Milestone Period, up to the number of shares of Akers common stock issuable to the MYMD stockholders upon the closing of the Merger. In the event that such milestone events are achieved and Milestone Payments are made, our current stockholders will experience further reduction in relative voting power.
The issuance, or expected issuance, of our common stock in connection with the Merger could decrease the market price of our common stock.
In connection with the Merger and as part of the merger consideration, we expect to issue shares of our common stock to MYMD stockholders. The anticipated issuance of our common stock in the Merger may result in fluctuations in the market price of our common stock, including a stock price decrease. In addition, issuance of the milestone shares, if any applicable milestone is achieved, and the perception in the market that the holders of a large number of shares of our common stock may intend to sell shares could reduce the market price of our common stock.
The intended benefits of the Merger may not be realized.
The Merger poses risks for our ongoing operations, including, among others:
As a result of the foregoing, the combined company may be unable to realize the full strategic and financial benefits currently anticipated from the Merger, our stockholders will have experienced substantial dilution of their ownership interests in their respective pre-Merger companies without receiving any commensurate benefit, or only while receiving part of the commensurate benefit to the extent the combined organization is able to realize only part of the strategic and we cannot assure you thatfinancial benefits anticipated at the Merger will be accretive to us intime of the near term or at all.Merger. Furthermore, if we fail to realize the intended benefits of the Merger, the market price of our common stockCommon Stock could decline to the extent that the market price reflects those benefits. Our stockholders will have experienced substantial dilution of their ownership interests in the Company without receiving any commensurate benefit, or only receiving part of the commensurate benefit to the extent the combined company is able to realize only part of the strategic and financial benefits currently anticipated from the Merger.
Because the lack of a public market for MYMD common stock makes it difficult to evaluate the fairness of the Merger, MYMD stockholders may receive consideration in the Merger that is greater than or less than the fair market value of MYMD common stock.
The outstanding common stock of MYMD is privately held and is not traded in any public market. The lack of a public market makes it extremely difficult to determine the fair market value of MYMD shares. Since the percentages of Akers common stock to be issued to MYMD stockholders was determined based on negotiations between the parties, it is possible that the value of Akers common stock to be issued in connection with the Merger will be greater than the fair market value of MYMD shares. Alternatively, it is possible that the value of the shares of Akers common stock to be issued in connection with the Merger will be less than the fair market value of MYMD shares.
Our directors and officers may have interests in the Merger that are different from, or in addition to, those of our stockholders generally that may influence them to support or approve the Merger.
Our officers and directors may have interests in the Merger that are different from, or are in addition to, those of our stockholders generally. Effective upon the closing of the Merger, Christopher Schreiber, current President and Chief Executive Officer of Akers, is expected to serve as an executive officer of the Supera line of business. It is expected that four of the current directors of Akers, Messrs. Schreiber, Silverman, White and Schroeder, are to be appointed as directors of the combined company after the completion of the Merger and will receive cash and equity compensation in consideration for such service. The outstanding unvested RSUs held by our current executive officers and directors will vest in connection with the Merger. In addition, our directors and executive officers also have certain rights to indemnification or to directors’ and officers’ liability insurance that will survive the completion of the Merger. These interests may have influenced our directors and executive officers to support or recommend the proposals that will be presented to our stockholders.
If the Merger is completed, MYMD executive officers and MYMD appointees to the combined company’s board of directors will have the ability to significantly influence the combined company’s management and business affairs, as well as matters submitted to the combined company’s board of directors or stockholders for approval, especially if they decide to act together with the current MYMD stockholders.
Upon completion of the merger, the former MYMD stockholders will own approximately 80% of the combined company on a partially diluted basis, excluding the effect of warrants issued in the Private Placement. If the Merger is completed, the combined company is expected to be led by MYMD executive officers. Furthermore, the combined company’s anticipated board of directors will consist of seven members, three of which will be appointed by MYMD pursuant to the terms of the Merger Agreement. As a result, such persons, if they choose to act together, will have the ability to significantly influence the combined company’s management and business affairs, as well as matters submitted to the combined company’s board of directors or stockholders for approval.
The announcement and pendency of the Merger could have an adverse effect on our business, financial condition, results of operations or business prospects.
The announcement and pendency of the Merger could disrupt Akers’ businesses in the following ways, among others:
Should they occur, any of these matters could adversely affect our businesses of, or harm our financial condition, results of operations or business prospects.
During the pendency of the Merger, we may not be able to enter into a business combination with another party and will be subject to contractual limitations on certain actions because of restrictions in the Merger Agreement.
Covenants in the Merger Agreement impede our ability to make dispositions or acquisitions or complete other transactions that are not in the ordinary course of business pending completion of the Merger, other than the Supera Purchase, potential spin-off of all or a portion of our assets prior to the consummation of the Merger, and certain permitted financings as set forth in the Merger Agreement. As a result, if the Merger is not completed, we may be at a disadvantage to our competitors. In addition, while the Merger Agreement is in effect and subject to limited exceptions, we are prohibited from soliciting, initiating, encouraging or taking actions designed to facilitate any inquiries or the making of any proposal or offer that could lead to entering into certain extraordinary transactions with any third party, such as a sale of assets, an acquisition, a tender offer, a merger or other business combination outside the ordinary course of business. These restrictions may prevent us from pursuing otherwise attractive business opportunities or other capital structure alternatives and making other changes to our business or executing certain of our business strategies prior to the completion of the Merger, which could be favorable to our stockholders.
Certain provisions of the Merger Agreement may discourage third parties from submitting competing proposals, including proposals that may be superior to the arrangements contemplated by the Merger Agreement.
The terms of the Merger Agreement prohibit us from soliciting competing proposals or cooperating with persons making unsolicited takeover proposals, except in limited circumstances if our board of directors determines in good faith, after consultation with its independent financial advisor and outside counsel, that an unsolicited competing proposal constitutes, or would reasonably be expected to result in, a superior competing proposal and that failure to take such action would be reasonably likely to result in a breach of the fiduciary duties of our board of directors. In the event that our board of directors withdraws or modifies its recommendation for the Share Issuance Proposal based on such superior competing proposal, MYMD may terminate the Merger Agreement.
The rights of MYMD stockholders who become Akers stockholders in the Merger and Akers stockholders following the merger will be governed by the A&R Charter and the Akers Bylaws.
Upon consummation of the Merger, outstanding shares of MYMD common stock will be converted into the right to receive shares of Akers common stock. MYMD stockholders who receive shares of Akers common stock in the merger will become Akers stockholders. As a result, MYMD stockholders who become stockholders in Akers will be governed by Akers’ organizational documents and bylaws, rather than being governed by MYMD’s organizational documents and bylaws. Pursuant to the Merger Agreement, the Akers Charter will be amended and restated, subject to Akers stockholders’ approval of the A&R Charter Proposal, immediately prior to the Effective Time.
The Exchange Ratio is not adjustable based on the market price of our common stock, so the merger consideration at the closing may have a greater or lesser value than at the time the Merger Agreement was signed.
The Merger Agreement has set the Exchange Ratio formula for the MYMD common stock, and the Exchange Ratio (as defined in the Merger Agreement) is only adjustable upward or downward to reflect our and MYMD’s equity capitalization as of immediately prior to the Effective Time. Any changes in the market price of common stock before the completion of the Merger will not affect the number of shares MYMD securityholders will be entitled to receive pursuant to the Merger Agreement. Therefore, if before the completion of the Merger, the market price of our common stock declines from the market price on the date of the Merger Agreement, then MYMD securityholders could receive merger consideration with substantially lower value. Similarly, if before the completion of the merger, the market price of our common stock increases from the market price on the date of the Merger Agreement, then MYMD securityholders could receive merger consideration with substantially more value for their shares of MYMD common stock than the parties had negotiated for in the establishment of the Exchange Ratio. In addition, the Exchange Ratio (as defined in the Merger Agreement) does not reflect the potential issuance of the Milestone Shares upon the achievement of certain market capitalization milestone events.
If the merger does not qualify as a reorganization under Section 368(a) of the Internal Revenue Code of 1986, as amended, or is otherwise taxable to United States MYMD stockholders, then such holders may be required to pay United States federal income taxes.
For United States federal income tax purposes, the Merger is intended to constitute a reorganization within the meaning of Section 368(a) of the Code. If the Internal Revenue Service (the “IRS”) or a court determines that the Merger should not be treated as a reorganization, a holder of MYMD common stock would recognize taxable gain or loss upon the exchange of MYMD common stock for our common stock pursuant to the Merger Agreement.
We are expected to incur substantial expenses related to the Merger.
We have incurred, and expect to continue to incur, substantial expenses in connection with the Merger, as well as operating as a public company. We will incur significant fees and expenses relating to legal, accounting, financial advisory and other transaction fees and costs associated with the merger. Actual transaction costs may substantially exceed our estimates and may have an adverse effect on the combined company’s financial condition and operating results.
Failure to complete the Merger could negatively affect the value of our common stock and our future business and financial results.
If the Merger is not completed, our ongoing businesses could be adversely affected and we will be subject to a variety of risks associated with the failure to complete the Merger, including without limitation the following:
If the Merger is not completed, these risks could materially affect the market price of our common stock and our business and financial results (including the cessation of our operations).
The Merger is expected to result in a limitation on the combined company’s ability to utilize its net operating loss carryforward.
Under Section 382 of the Code, use of our net operating loss carryforwards (“NOLs”) will be limited if we experience a cumulative change in ownership of greater than 50% in a moving three-year period. At December 31, 2020, we had approximately $100,615,000 of operating loss carryforwards for federal and approximately $7,548,000 for New Jersey state tax purposes that may be applied against future taxable income. We will experience an ownership change as a result of the Merger and therefore our ability to utilize our NOLs and certain credit carryforwards remaining at the Effective Time will be limited. The limitation will be determined by the fair market value of our common stock outstanding prior to the ownership change, multiplied by the applicable federal rate. It is expected that the Merger will impose a limitation on our NOLs. Limitations imposed on our ability to utilize NOLs could cause United States federal and state income taxes to be paid earlier than would be paid if such limitations were not in effect and could cause such NOLs to expire unused, in each case reducing or eliminating the benefit of such NOLs.
The opinion received by our board of directors from Gemini Valuation Services (“GVS”) has not been, and is not expected to be, updated to reflect changes in circumstances that may have occurred since the date of the opinion.
At a board of directors meeting held on November 11, 2020, our financial advisor, GVS, rendered its opinion as to the fairness, from a financial point of view, of the contribution made and consideration received by the holders of our common stock pursuant to the Merger Agreement and rendered its oral opinion to our board of directors (which was subsequently confirmed in writing as of November 11, 2020) that, as of the date of such opinion and subject to the various assumptions made, procedures followed, matters considered and qualifications and limitations set forth in such opinion, the contribution made and consideration received by the holders of our common stock pursuant to the Merger Agreement was fair to the holders of our common stock from a financial point of view. Such opinion was one of many factors considered by our board of directors in approving the Merger. The opinion does not speak as of the time the Merger will be completed or any date other than the date of such opinion. Subsequent changes in our or MYMD’s operation and prospects, general market and economic conditions and other factors that may be beyond our control, may significantly alter the value of Akers or MYMD or the prices of the shares of our common stock by the time the Merger is to be completed. The opinion does not address the fairness of the merger consideration from a financial point of view to us at the time the Merger is to be completed, or as of any other date other than the date of such opinion, and the Merger Agreement does not require that the opinion be updated, revised or reaffirmed prior to the closing of the Merger to reflect any changes in circumstances between the date of the signing of the Merger Agreement and the completion of the Merger as a condition to closing the Merger.
The Merger may be completed even though material adverse changes may result from the announcement of the Merger, industry-wide changes or other causes.
In general, either party can refuse to complete the Merger if there is a material adverse effect (as defined in the Merger Agreement) affecting the other party between November 11, 2020, the date of the Merger Agreement, and the closing of the Merger. However, some types of changes do not permit either party to refuse to complete the merger, even if such changes would have a material adverse effect on Akers or MYMD, as the case may be:
If adverse changes occur but we must still complete the merger, the market price of our common stock may suffer.
We may become involved in additional securities litigation or stockholder derivative litigation in connection with the merger, and this could divert the attention of our management and harm the combined company’s business, and insurance coverage may not be sufficient to cover all related costs and damages.
Securities litigation or stockholder derivative litigation frequently follows the announcement of certain significant business transactions, such as the sale of a business division or announcement of a business combination transaction. Between January 22, 2021 and February 10, 2021, five alleged Akers stockholders filed separate actions in the state and federal courts of New York and New Jersey against Akers and the members of its board of directors, respectively captioned as follows: (i) Douglas McClain v. Akers Biosciences, Inc., et al., No. 650497/2021 (Sup. Ct., N.Y. Cty.); (ii) Owen Murphy v. Akers Biosciences, Inc., et al., No. 650545/2021 (Sup. Ct., N.Y. Cty.); Sue Gee Cheng v. Akers Biosciences, Inc., et al., No. 1:21-cv-01110 (S.D.N.Y.); Danny Lui v. Akers Biosciences, Inc., et al., No. GLO-C-000006-21 (N.J. Super. Ct., Ch. Div.); and Alan Misenheimer v. Akers Biosciences, Inc., et al., No. 1:21-cv-02310 (D.N.J.) (collectively, the “MYMD Merger Complaints”). The McClain and Lui actions are styled as putative class actions brought on behalf of the plaintiff and other similarly situated stockholders, while the Murphy, Cheng, and Misenheimer actions are brought solely on behalf of the individual stockholders. The MYMD Merger Complaints generally assert that Akers and its board of directors failed to disclose allegedly material information in the joint proxy and consent solicitation statement/prospectus and seek an order enjoining or unwinding the consummation of the Merger Agreement and awarding damages. The defendants believe that the claims asserted in the MYMD Merger Complaints are without merit and intend to appropriately defend themselves against them. Accordingly, we do not expect that these claims will have a material adverse effect on its financial condition or results of operations. We may become involved in more of this type of litigation in connection withAfter the Merger and the combined company may become involved in this type of litigation in the future. Litigation often is expensive and diverts management’s attention and resources, which could adversely affect our business and the business of the combined company.
If the Merger isContribution Transaction were consummated, the business operations, strategies and focus of the combined company willCompany fundamentally change,changed, and these changes may not result in an improvement in the value of its common stock.our Common Stock.
Pending the consummation of the Merger, it is currently anticipated that the combined company would focus its resources on executing MYMD’s current business plan. In addition, prior to the consummation of the Merger, we may, in our discretion, consummate a spin-off of all or a part of our legacy assets. In the event we consummate such spin-off, the stockholders of Akers and MYMD will not participate in the future prospects of such legacy assets.
Following the Merger, it is expected that the combined company’sour primary products will be MYMD’s product candidates: MyMD-1,are MyMD Florida’s therapeutic platforms: MYMD-1, a clinical-stage immunometabolic regulator and Supera-1R,Supera-CBD, a pre-clinical stage patented synthetic cannabidiol derivative. Consequently, if the merger is consummated, an investment in our common stock will primarily represent an investment in the business operations, strategies and focus of MYMD. MYMD expectsCBD analog. We expect to incur losses as it develops itswe develop our product candidates, and MYMD’sour product candidates, may never get approved by the FDA or, even if approved for marketing, may not be profitable. The failure to successfully develop product candidates will significantly diminish the anticipated benefits of the Merger and have a material adverse effect on the business of the combined company.our business. There is no assurance that the combined company’sour business operations, strategies or focus will be successful, following the Merger, and the Mergerwhich could depress the value of our Common Stock.
The Contribution Transaction poses risks for our ongoing operations, including, among others:
| ● | following consummation of the Contribution Transaction, if Oravax is not successful in developing the COVID-19 Vaccine Candidate, we may not realize any value out of its ownership of Oravax shares; | |
| ● | costs and expenses associated with any undisclosed or potential liabilities. |
As a result of the combined company’s common stock.
The reverse stock splitforegoing, we may not increasebe unable to realize the combined company’s stock price overfull strategic and financial benefits originally anticipated from the long term.
If the Reverse Stock Split Proposal is approved, the combined company anticipates effecting a reverse stock split at a reverse stock split ratio as mutually agreed to by AkersContribution Transaction, and MYMD, which range shall be sufficient to cause its stock price to be at least $5.00 immediately following the Merger. While it is expectedwe cannot assure you that the reductionContribution Transaction will be accretive in the number of outstanding shares of common stock will proportionally increasenear term or at all. Furthermore, if we fail to realize the market priceintended benefits of the combined company’s common stock upon effectiveness of the reverse stock split, it cannot be assured that the reverse stock split will result in any sustained proportionate increase in the market price of the combined company’s common stock, which is dependent upon many factors, including the business and financial performance of the combined company, general market conditions, and prospects for future success, which are unrelated to the number of shares of the combined company’s common stock outstanding. Thus, while the stock price of the combined company might meet the initial listing requirements for Nasdaq initially, it cannot be assured that it will continue to do so.
The reverse stock split would have the effect of increasing the amount of common stock that the combined company is authorized to issue without further approval by the combined company’s stockholders.
The proposed A&R Charter for the combined company is anticipated to authorize the combined company to issue 500,000,000 shares of common stock and does not anticipate reducing this amount in connection with the reverse stock split. Except in certain instances, as required by law or by the rules of the securities exchange that lists the combined company’s common stock, these additional shares may be issued by the combined company without further vote of the combined company’s stockholders. If the combined company’s board of directors chooses to issue additional shares of the combined company’s common stock, such issuance could have a dilutive effect on the equity, earnings and voting interests of the combined company’s stockholders.
The reverse stock split may decrease the liquidity of our common stock.
Although our board of directors believes that the anticipated increase inContribution Transaction, the market price of our common stockCommon Stock could encourage interest in our common stock and possibly promote greater liquidity for our stockholders, such liquidity could also be adversely affected bydecline to the reduced number of shares outstanding after the reverse stock split. The reduction in the number of outstanding shares may lead to reduced trading and a smaller number of market makers for our common stock.
The reverse stock split may lead to a decrease in overall market capitalization of the combined company.
Shouldextent that the market price reflects those benefits.
The concentration of the capital stock ownership with insiders of the Company will likely limit the ability of our common stock decline afterstockholders to influence corporate matters.
The executive officers, directors, five percent or greater stockholders, and the reverse stock split,respective affiliated entities of the percentage decline may be greater, due to the smaller number of shares outstanding, than it would have been prior to the reverse stock split. A reverse stock split is often viewed negatively by the market and, consequently, can lead to a decreaseCompany, in the overall market capitalizationaggregate, beneficially owned more than 10% of the combined company. If the per share market price does not increase in proportion to the reverse stock split ratio, then the value of the combined company, as measured by its stock capitalization, will be reduced. In some cases, the per-share stock price of companies that have effected reverse stock splits subsequently declined back to pre-reverse split levels and, accordingly, it cannot be assured that the total market value of our common stock will remain the same after the reverse stock split is effected, or that the reverse stock split will not have an adverse effect on our stock price due to the reduced number of sharesCompany’s outstanding after the reverse stock split.
Risks Related to Our Business Prior to Consummation of the Merger
We have a history of operating losses and we cannot guarantee that we can ever achieve sustained profitability.
We have recorded a net loss attributable to common stockholders in most reporting periods since our inception. We had a net loss of $17,580,609 during the year ended December 31, 2020. Our accumulated deficit at December 31, 2020 was $137,163,739. On account of the unfavorable factors existing within our rapid, point-of-care screening and testing products business, we ceased the production and sale of our screening testing products. We are focusing on the development and manufacturing of the COVID-19 Vaccine Candidate, or combination product candidate in partnership with Premas and expect to incur additional operating losses for the foreseeable future. As part of our efforts to increase shareholder value, on November 11, 2020, Akers entered into the Merger Agreement with MYMD, pursuant to which Merger Sub will merge with and into MYMD, with MYMD becoming our wholly owned subsidiary. For risks related to the merger, please see risk factors set forth under the heading “— Risks Related to the Proposed Merger” herein. However, there can be no assurance of success in reducing our loss, becoming profitable, or having sufficient cash to develop a COVID-19 Vaccine Candidate or to complete the consummation of the Merger.
We may fail to realize the anticipated benefits of our acquisition of Cystron and those benefits may take longer to realize than expected.
On March 23, 2020, we entered into the MIPA with the Cystron Sellers, pursuant to which we acquired the Cystron Membership Interests. Cystron is a party to a License and Development Agreement (the “Initial License Agreement”) with Premas.Common Stock. As a conditionresult, these stockholders, acting together, had, and continue to have, control over matters that require approval by our entry intostockholders, including the MIPA, Cystron amendedelection of directors and restated the Initial License Agreement on March 19, 2020 (as amended and restated, the “License Agreement”). Pursuant to the License Agreement, Premas granted Cystron, amongstapproval of significant corporate transactions. Corporate actions might be taken even if other things, an exclusive license with respect to Premas’ vaccine platform for the developmentstockholders oppose them. This concentration of the COVID-19 Vaccine Candidate. Our ability to realize the anticipated benefits of the acquisition will depend, to a large extent, on our ability to produce an effective vaccine against COVID-19. The development of the COVID-19 Vaccine Candidate is in very early stages and there is no assurance that we will be able to produce an effective vaccine. Moreover, weownership might also have the righteffect of delaying or preventing a corporate transaction that other stockholders may view as beneficial.
| 30 |
Certain stockholders could attempt to terminateinfluence changes within the License Agreement on a country-by-country basis for any reason or for no reason at any time upon sixty (60) days’ prior written notice to Premas, and may decide to cease development of the COVID-19 Vaccine Candidate and terminate the License Agreement. The failure to produce the COVID-19 Vaccine Candidate or termination of the License AgreementCompany, which could adversely affect our business,operations, financial condition and resultsthe value of operations. In addition, we have incurred and expectour Common Stock.
Our stockholders may from time to incur significant expenses relatedtime seek to the acquisition. These expenses include, but are not limited to, the Common Stock Consideration (as definedacquire a controlling stake in the MIPA), a cash considerationCompany, engage in proxy solicitations, advance stockholder proposals or otherwise attempt to effect changes. Campaigns by stockholders to effect changes at publicly traded companies are sometimes led by investors seeking to increase short-term stockholder value through actions such as financial restructuring, increased debt, special dividends, stock repurchases or sales of $1.0 million, related contingent fees, legal feesassets or the entire company. Responding to proxy contests and other related feesactions by activist stockholders can be costly and expenses. Manytime-consuming and could disrupt our operations and divert the attention of these expenses have been paid or will be payable by us regardlessour Board of Directors and senior management. These actions could adversely affect our operations, financial condition, and the value of our Common Stock.
We must attract and retain highly skilled employees to succeed.
To succeed, we must recruit, retain, manage and motivate qualified clinical, scientific, technical and management personnel, and we face significant competition for experienced personnel. If we do not succeed in attracting and retaining qualified personnel, particularly at the management level, it could adversely affect our ability to execute our business plan, harm our results of operations and increase our capabilities to successfully develop the COVID-19 Vaccine Candidate,commercialize MYMD-1, Supera-CBD and we will not be able to recover these expensesour other product candidates. The competition for qualified personnel in the event that we fail to develop the COVID-19 Vaccine Candidate.
Our pursuit of the COVID-19 Vaccine Candidatebiotechnology field is at an early stage. We have not previously tested our rapid response capabilityintense and may be unable to produceas a vaccine that successfully treats the virus in a timely manner, if at all.
In response to the COVID-19 pandemic, we are pursuing the rapid development of the COVID-19 Vaccine Candidate. Our development of the COVID-19 Vaccine Candidate is in early stages, andresult, we may be unable to produce the COVID-19 Vaccine Candidate. Additionally, our abilitycontinue to develop an effective COVID-19 Vaccine Candidate depends on the success of its rapid response capability, which we have not previously testedattract and which will need to be funded by third parties in order to enable us to have sufficient capacity to respond to a global health challenge. If the COVID-19 pandemic is effectively contained or the risk of COVID-19 infection is diminished or eliminated before we can successfully develop and manufacture a COVID-19 Vaccine Candidate, including availabilities of effective vaccines, we may be unable to successfully generate revenue from the manufacturing of the COVID-19 Vaccine Candidate. We are also committing financial resources andretain qualified personnel tonecessary for the development of our business or to recruit suitable replacement personnel.
Many of the COVID-19 Vaccine Candidate which may divert resources from other transactions, despite uncertainties surrounding the longevity and extent of COVID-19 as a global health concern. Our business could be negatively impacted by our allocation of significant resources to a global health threatbiotechnology companies that is unpredictable and could rapidly dissipate orwe compete against which the COVID-19 Vaccine Candidate, if developed, may not be partially or fully effective.
Our acquisition of Cystron could result in additional costs, integration or operating difficulties, dilutionfor qualified personnel have greater financial and other adverse consequences.
In connection withresources, different risk profiles and a longer history in the acquisitionindustry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates than what we have to offer. If we are unable to continue to attract and retain high-quality personnel, the Cystronrate and in pursuit of developing the COVID-19 Vaccine Candidate,success at which we may:
In addition, the process of integrating Cystron’s business may create operating difficulties and expenditures and pose numerous additional risks to our operations, including:
If any of the above events (or more) occur, or if we cannot effectively manage or respond to such events following the acquisition, they may have material adverse effect on our business results of operations and financial condition.will be limited.
Cystron is dependent on technologies that it has licensed, and Cystron may need to license in the future, and if Cystron fails to obtain licenses it needs, or fails to comply with its payment obligations in the agreements under which Cystron in-licenses intellectual property and other rights from third parties, Cystron could lose its ability to develop a COVID-19 Vaccine Candidate.
Cystron currently is dependent on a license from Premas for its key technologies. Any failure to make the payments required by the License Agreement may permit Premas to terminate the license. If Cystron were to lose or otherwise be unable to maintain the license for any reason, it would halt Cystron’s ability to develop a COVID-19 Vaccine Candidate. The foregoing could result in a material adverse effect on Akers’ business or results of operations.
In addition, Cystron does not own the patents or patent applications that it licenses, and as such, Cystron may need to rely upon Premas to properly prosecute and maintain those patent applications and prevent infringement of those patents. If Premas is unable to adequately protect the proprietary intellectual property Cystron licenses from legal challenges, or if Cystron is unable to enforce such licensed intellectual property against infringement or alternative technologies, Akers will not be able to compete effectively in the drug discovery and development business.
We operate in a highly competitive industry.
We face, and will continue to face, intense competition from large pharmaceutical companies, specialty pharmaceutical and biotechnology companies as well as academic and research institutions pursuing research and development of technologies, drugs or other therapies that would compete with our products or product candidates. The pharmaceutical market is highly competitive, subject to rapid technological change and significantly affected by existing rival drugs and medical procedures, new product introductions and the market activities of other participants. Our competitors may develop products more rapidly or more effectively than us. If our competitors are more successful in commercializing their products than us, their success could adversely affect our competitive position and harm our business prospects and may also lead to the diversion of funding away from us and toward other companies.
Specifically, the competitive landscape of potential COVID-19 vaccinesIf we fail to comply with environmental, health, and treatment therapies has been rapidly developing since the beginning of the COVID-19 pandemic, with several hundreds of companies claimingsafety laws and regulations, we could become subject to be investigating possible candidatesfines or penalties or incur costs that could harm our business.
We are subject to numerous environmental, health, and approximately 4,800 studies registered worldwide as investigating COVID-19 (source: clinicaltrials.gov). Given the global footprintsafety laws and regulations, including those governing laboratory procedures and the widespread media attention onhandling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations will involve the COVID-19 pandemic, there are efforts by publicuse of hazardous materials, including chemicals and private entities to develop a vaccine against SARS-CoV-2 as soon as possible, including large, multinational pharmaceutical companies such as AstraZeneca, GlaxoSmithKline, Johnson & Johnson, Moderna, Pfizer, and Sanofi,biological materials. Our operations also may produce hazardous waste products. We generally anticipate contracting with vaccine candidates that are currently at more advanced stage of development than our COVID-19 Vaccine Candidate. In December 2020,third parties for the FDA began to issue emergency use authorizations for vaccines developed by certaindisposal of these large, multinational pharmaceutical companiesmaterials and it is possible that additional vaccines developedwastes. We will not be able to eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from any use by us of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such large, multinational pharmaceutical companieslaws and regulations.
Although we maintain workers’ compensation insurance to cover us for costs and expenses, we may receive further approvalsincur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities.
In addition, we may incur substantial costs in order to comply with current or future environmental, health, and authorizationssafety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
| 31 |
Our business and operations would suffer in the near term. Those other entities may develop COVID-19 vaccines that are more effective than any vaccineevent of computer system failures, cyber-attacks or deficiencies in our cyber-security or those of third-party providers.
In the ordinary course of our business, we may develop, may develop a COVID-19 vaccine that becomes the standard of care, may develop a COVID-19 vaccine at a lower cost or earlier than we are ableand our third-party providers rely on electronic communications and information system to jointly develop any COVID-19 vaccine, or may be more successful at commercializing a COVID-19 vaccine. Many of these other organizations are much larger than we areconduct our operations. We and our third-party providers have access to larger pools of capital,been, and as such, are able to fund and carry on larger research and development initiatives. Such other entities may have greater development capabilities than we do and have substantially greater experience in undertaking nonclinical and clinical testing of vaccine candidates, obtaining regulatory approvals and manufacturing and marketing pharmaceutical products. Our competitors may also have greater name recognition and better access to customers. In addition, based on the competitive landscape, additional COVID-19 vaccines or therapeutics may continue to be, approvedtargeted by parties using fraudulent e-mails and other communications in attempts to misappropriate bank accounting information, passwords, or other personal information or to introduce viruses or other malware to our information systems. Between August and October 2021, we experienced a cybersecurity incident. A third-party forensic technology company’s investigation confirmed that we were a victim of wire fraud due to a compromised electronic mail account. As of the date of this filing, we have identified losses totaling $1,260,864 related to this incident, net of amounts recovered. Following the incident, we have taken measures to enhance our electronic mail security and have modified our internal procedures to ensure the authenticity of payment instructions and we continue to evaluate additional measures for improving cybersecurity. Despite these prophylactic measures, the risk of such cyber-attacks against us or our third-party providers and business partners remains a serious issue. Cybersecurity incidents are pervasive, and the risks of cybercrime are complex and continue to evolve. Although we are making significant efforts to maintain the security and integrity of our information systems and are exploring various measures to manage the risk of a security breach or disruption, there can be marketed. Should another partyno assurance that our security efforts and measures will be effective or that attempted security breaches or disruptions would not be successful in producing a more efficacious vaccine for COVID-19, such success could reduce the commercial opportunity for our COVID-19 Vaccine Candidate and could have a material adverse effect on our business, financial condition, results of operations and future prospects. Moreover, if we experience delayed regulatory approvals or disputed clinical claims, we may not have a commercial or clinical advantage over competitors’ products that we believe we currently possesses. The success or failure of other entities, or perceived success or failure, may adversely impact our ability to obtain any future funding for our vaccine development efforts or for us to ultimately commercialize and market any vaccine candidate, if approved. damaging.
In addition, we may not be ablecollect and store sensitive data, including intellectual property, research data, our proprietary business information and that of our suppliers, technical information about our products, clinical trial plans and employee records. Similarly, our third-party providers possess certain of our sensitive data and confidential information. The secure maintenance of this information is critical to compete effectively if our product candidates do not satisfy government procurement requirementsoperations and business strategy. Despite the implementation of security measures, our internal computer systems, and those of third parties on which we rely, are vulnerable to damage from computer viruses, malware, ransomware, cyber fraud, natural disasters, terrorism, war, telecommunication and electrical failures, cyberattacks or cyberintrusions over the Internet, attachments to emails, persons inside our organization, or persons with respectaccess to biodefense products.
Our business may be materially adversely affectedsystems inside our organization. The risk of a security breach or disruption, particularly through cyberattacks or cyberintrusions, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the COVID-19 pandemic.
In December 2019, a novel strainnumber, intensity and sophistication of coronavirus, COVID-19, was reported to have surfaced in Wuhan, Chinaattempted attacks and has reached multiple other countries, resulting in government-imposed quarantines, travel restrictions and other public health safety measures, including in the United States and India. On March 12, 2020, the WHO COVID-19 to be a global pandemic. The various precautionary measures taken by many governmental authoritiesintrusions from around the world in order to limit the spread of COVID-19 have had and may continue to have an adverse effect on the global markets and global economy. Such government-imposed precautionary measures may have been relaxed in certain countries or states, but there is no assurance that more strict measures will not be put in place again due to a resurgence in COVID-19 cases.
The ultimate impact of the global COVID-19 pandemic or a similar health epidemic is highly uncertain and subject to change. We do not yet know the full extent of potential delays or impacts onincreased. Any such breach could compromise our business, our vaccine development efforts, healthcare systems or the global economy as a whole. However, the effects are likely to have a material impact on our operations, liquidity and capital resources, and we will continue to monitor the COVID-19 situation closely.
In response to public health directives and orders, we implemented and have continued to maintain work-from-home policies for many of our employeesnetworks and the temporary modificationinformation stored there could be accessed, publicly disclosed, encrypted, lost or stolen. Any such access, inappropriate disclosure of confidential or proprietary information or other loss of information, including our data being breached at third-party providers, could result in legal claims or proceedings, liability or financial loss under laws that protect the privacy of personal information, disruption of our operations or our product development programs and damage to comply with applicable social distancing recommendations. The effects of the orders and our related adjustments in our business are likely to negatively impact productivity, disrupt our business and delay our timelines, the magnitude ofreputation, which will depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course. Similar health directives and orders are affecting third parties with whom we do business, including Premas, whose operations are located in India. Further, restrictions on our ability to travel, stay-at-home orders and other similar restrictions on our business have limited, and may continue to limit, our ability to support our operations.
Severe and/or long-term disruptions in our operations will negatively impact our business, operating results and financial condition in other ways as well. Specifically, we anticipate that the stress of COVID-19 on healthcare systems generally around the globe will negatively impact regulatory authorities and the third parties that we and Premas may engage in connection with the development and testing of our COVID-19 Vaccine Candidate.
The anticipated economic consequences of the COVID-19 pandemic have adversely impacted financial markets, resulting in high share price volatility, reduced market liquidity, and substantial declines in the market prices of the shares of most publicly traded companies, including Akers. Volatile or declining markets for equities could adversely affect our ability to raise capital when needed through the sale of shares of common stock or other equity securities. Should these market conditions persist when we need to raise capital, and if we are able to sell shares of our common stock under then prevailing market conditions, we might have to accept lower prices for our shares and issue a larger number of shares than might have been the case under better market conditions, resulting in significant dilution of the interests of our shareholders.business.
Risks Related to Ourour Product Development and Regulatory Approval
With regard to our COVID-19 Vaccine Candidate,Supera-CBD product candidate, we must conduct pre-clinical testing and prepare and submit an IND to the FDA,FDA. With regard to both our MYMD-1 and Supera-CBD product candidates, we must conduct all phases of clinical studies, (which may include postmarket or “Phase 4” studies), which will likely take several years and substantial expenses to complete, before we can submit an application for marketing approval to the FDA, and therewe may be required to complete additional post-market or “Phase 4” studies after application or approval. There is no guarantee that we will complete such clinical development in a timely manner or at all or that our BLAwe will obtain or maintain regulatory approval for either product candidate.
Potential Risks
| ● | FDA – IND review is conducted and feedback is delivered within 30 days of receipt of the initial application. At the time, changes to the study protocol may be requested in order to proceed with the proposed Phase 2 clinical trial. | |
| ● | Institutional Review Board (IRB) – If the FDA requests changes to the protocol included in the initial application, an amendment must be submitted to the IRB for an additional review. This review may include changes to the protocol, informed consent form, surveys, and other assessments planned over the course of the clinical trial. | |
| ● | COVID-19 – Clinical sites must follow specific COVID-19 guidelines. Clinical trial activity must adhere to those guidelines which may change over the course of the study. For example, the protocol may need to be revised to accommodate for in-home visits (if necessary) to maximize patient and research staff safety. | |
| ● | Site Initiation Visit (SIV) – Site initiation visits are scheduled around principal investigator (PI) availability. Due to changing clinic schedules, SIVs may need to be rescheduled to accommodate various PI demands. | |
| ● | Central Lab – Central labs are responsible for creating all the kits (supplies) required for patient visits. Kits are created to execute all aspects of screening through study completion. Kits are developed based on specifications from core labs and third-party vendors (as applicable). All shipping and storing requirements need to be clearly articulated and lab manuals provided to make the kits. The central lab is also responsible for building a database to store all the lab results. | |
| ● | Electronic Database – The overall database used for the study must be built around the schedule of assessments planned for each patient over the course of the clinical trial. This includes every assessment and data element collected. The complexity of the Phase 2 trial also requires development and testing of drug randomization across treatment groups to ensure blinding is maintained. Thorough user-acceptability testing (UAT) is required and is time-intensive. | |
| ● | CoreRx – To maintain adequate blinding across treatment groups, new labels were created and applied to the active drug and placebo bottles. Logistics and manufacturing need to work together to ensure capsules were not only filled appropriately, but also labelled correctly to ensure the electronic database and randomization schemes maintain alignment over the course of the study. |
| 32 |
Clinical drug development is a lengthy, expensive, and inherently uncertain process, and we may experience delays in completing, or ultimately be approved, if submitted.
We expect that a substantial portion of our efforts and expenditures overunable to complete, the next few years will be devoted to our COVID-19 Vaccine Candidate. Accordingly, our business currently depends heavily on the successful development FDA approval, and commercialization of such candidate, which may never receiveour product candidates.
The FDA approval or be successfully commercialized even if FDA approval is received. The research, testing, manufacturing, labeling, approval, sale, marketing, and distribution of the COVID-19 Vaccine Candidate are, and will remain, subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, as applicable. We are not permitted to market our tablet vaccines in the United States until we receive FDA approval of our applicable BLA. To date, we have not-yet begunmust approve any pre-clinical studies for the COVID-19 Vaccine Candidate, nor have we prepared or submitted an IND. Accordingly, we have not submitted a BLA to the FDA or comparable applications to other regulatory authorities and do not expect to be in a position to do so for the foreseeable future, as there are numerous developmental steps that must be completednew drug products before wethey can prepare and submit a BLA.
In the United States, the FDA regulates pharmaceutical and biological products (including vaccines and vaccine candidates, such as the COVID-19 Vaccine Candidate currently in early stages of development) under the FD&C Act and the PHSA, as well as their respective implementing regulations. Such products and product candidates are also subject to other federal, state, and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations requires the expenditure of substantial time and financial resources. The process required by the FDA before a drug or biological product may be marketed in the United States, generally involvesand such approval is contingent upon the following:
Notwithstanding the submissioncollection of relevant datasufficient safety- and information, the FDA may ultimately decide that the BLA does not satisfy its regulatory criteria for approvalefficacy-data from preclinical and deny approval. Data obtained fromclinical studies. We must complete preclinical development and conduct extensive clinical trials is not always conclusiveto demonstrate the safety and the FDA may interpret data differently thanefficacy of our product candidates for their respective targeted indications. With regard to Supera-CBD, we interpret the same data. The COVID-19 Vaccine Candidate isare still in the earliestpre-clinical stage, and we are in relatively early clinical stages of clinical developmentwith regard to certain indications for which MyMD-1 is being developed and therefore, a long way from BLA submission. We cannot predict with any certainty if or when we might submit a BLAin pre-clinical stages for regulatory approval for the COVID-19 Vaccine Candidate or whether any such BLA will be approved by the FDA. Human clinicalothers. Clinical trials are very expensive, and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. For example, the FDA may not agree with our proposed endpoints for any clinical trial we propose, which may delay the commencement of our clinical trials. The clinical trial process is also lengthy and requires substantial time and effort. We estimate that the clinical trials we need to conduct to be in a position to submit a BLA for the COVID-19 Vaccine Candidate willcan take severalmany years to complete. Furthermore, failurecomplete, and their outcomes are inherently uncertain. Failure can occur at any stage oftime during the trials, and we could encounter problems that cause us to abandon or repeat clinical trials. Also, the results of early pre-clinical and clinical testing of the COVID-19 Vaccine Candidate may not be predictive of the results of subsequent clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier studies. Moreover, pre-clinicaltrial process. Nonclinical and clinical data are often susceptible to multiplevarying interpretations and analyses. Manyanalyses, and many companies that have believed their vaccineproduct candidates performed satisfactorily in pre-clinicalnonclinical studies and clinical trials have,and, nonetheless, failed to obtainwere denied marketing approval for such candidates due to insufficient safety or efficacy data and/or other clinical-study deficiencies. It is impossible to predict whether we will be able to prove that either or both of their products. Successour product candidates are safe and effective for any of the indications for which they are, respectively, being developed and, accordingly, when they will be approved for commercialization in the United States for any given indication, if ever.
After completing the requisite preclinical testing, IND submission, internal review board (“IRB”) review, and any other applicable early-development obligations, sponsors must conduct extensive clinical trials to demonstrate the safety and efficacy of the product candidates. We have completed such early-stage preclinical testing and IND-submission for some, but not all, indications for which MyMD-1 is being developed and are currently working towards completion of such pre-IND activities for Supera-CBD. Even if the results of our pre-clinical testing and early clinical trials does not ensure that laterare favorable, we expect our product candidates to remain in clinical development for several years before they may be considered for regulatory approval, and clinical development of either or both candidates for one or more targeted indications may take significantly longer to complete and may never be successful. Failures in connection with one or more clinical trials which involve many more subjects, will becan occur at any stage of testing.
Events that may prevent successful or timely completion of clinical development include:
| ● | delays in reaching a consensus with regulatory authorities on trial design; | |
| ● | delays in reaching agreement on acceptable terms with prospective contract research organization (“CRO”) and clinical trial sites; |
| ● | delays in opening clinical trial sites or obtaining required IRB or independent ethics committee approval at each clinical trial site; | |
| ● | actual or perceived lack of effectiveness of any product candidate during clinical trials; | |
| ● | discovery of serious or unexpected toxicities or side effects experienced by trial participants or other safety issues, such as drug interactions, including those which cause confounding changes to the levels of other concomitant medications; | |
| ● | slower than expected rates of subject recruitment and enrollment rates in clinical trials; | |
| ● | difficulty in retaining subjects for the entire duration of applicable clinical studies (as study subjects may withdraw at any time due to adverse side effects from the therapy, insufficient efficacy, fatigue with the clinical trial process or for any other reason; | |
| ● | delays or inability in manufacturing or obtaining sufficient quantities of materials for use in clinical trials due to regulatory and manufacturing constraints; | |
| ● | inadequacy of or changes in its manufacturing process or product candidate formulation; | |
| ● | delays in obtaining regulatory authorization s, such as INDs and any others that must be obtained, maintained, and/or satisfied to commence a clinical trial, including “clinical holds” or delays requiring suspension or termination of a trial by a regulatory agency, such as the FDA, before or after a trial is commenced; | |
| ● | changes in applicable regulatory policies and regulation, including changes to requirements imposed on the extent, nature or timing of studies; | |
| ● | delays or failure in reaching agreement on acceptable terms in clinical trial contracts or protocols with prospective clinical trial sites; | |
| ● | uncertainty regarding proper dosing; | |
| ● | delay or failure to supply product for use in clinical trials which conforms to regulatory specification; | |
| ● | unfavorable results from ongoing pre-clinical studies and clinical trials; | |
| ● | failure of its CROs, or other third-party contractors to comply with all contractual requirements or to perform their services in a timely or acceptable manner; | |
| ● | Our failure, or the failure of any individuals, entities, or organizations involved in one or more aspects of our clinical development activities, to comply with all applicable FDA or other regulatory requirements relating to the conduct of clinical trials; | |
| ● | scheduling conflicts with participating clinicians and clinical institutions; | |
| ● | failure to design appropriate clinical trial protocols; | |
| ● | regulatory concerns and additional difficulties associated with cannabinoid products, generally; | |
| ● | insufficient data to support regulatory approval; | |
| ● | inability or unwillingness of medical investigators to follow its clinical protocols; or | |
| ● | difficulty in maintaining contact with patients during or after treatment, which may result in incomplete data. |
If any of the results of later clinical trials of any of our current or future therapeutic candidates do not produce favorable results or are found to have been conducted in violation of the FDA’s or other regulatory body’s standards governing such studies, our ability to request and obtain regulatory approval for the therapeutic candidate may not replicate the results of prior clinical trials and pre-clinical testing. Any failure or substantial delay in our vaccine development plans maybe adversely impacted, which could have a material adverse effect on our business.reputation, business, financial condition or results of operations.
| 33 |
We may opt to conduct future clinical studies for the COVID-19 Vaccine Candidate outside the United States, which could heighten the risk of delay and/or failure, as the FDA may not accept data from such studies in support of any BLA we may submit after completing the applicable developmental and regulatory prerequisites, if ever.
We are still in the earliest stages of development with respect to the COVID-19 Vaccine Candidate and may ultimately decide to conduct pre-clinical and/or clinical studies in one or more countries outside the United States. Although the FDA may accept data from clinical trials conducted outside the United States that are not conducted under an IND, the FDA’s acceptance of such data is subject to certain conditions. For example, the clinical trial must be well designed and conducted and performed by qualified investigators in accordance with ethical principles and all applicable FDA regulations. The trial population must also adequately represent the intended United States population, and the data must be applicable to the United States population and United States medical practice in ways that the FDA deems clinically meaningful. In general, the patient population for any clinical trials conducted outside of the United States must be representative of the population for whom we intend to market the COVID-19 Vaccine Candidate in the United States, if approved. In addition, while these clinical trials are subject to the applicable local laws, FDA acceptance of the data will be dependent upon its ability to verify the data and its determination that the trials also complied with all applicable United States laws and regulations. We cannot guarantee that the FDA will accept data from trials we conduct outside of the United States, if any. If the FDA does not accept the data from such clinical trials, it would likely result in the need for additional trials and the completion of additional regulatory steps, which would be costly and time-consuming and could delay or permanently halt our development of the COVID-19 Vaccine Candidate.
If we are successful in producing the COVID-19 Vaccine Candidate, we may need to devote significant resources to our scale-up and development including for use by the United States government.
In the event that the pre-clinical and clinical trials for the COVID-19 Vaccine Candidate are perceived to be successful, we may need to work toward the large scale technical development, manufacturing scale-up and larger scale deployment of this potential vaccine through a variety of United States government mechanisms such as an Expanded Access Program or an Emergency Use Authorization program. In this case, we may need to divert significant resources to this program, which would require diversion of resources from our other businesses. In addition, since the path to licensure of any vaccine against COVID-19 is unclear, if use of the vaccine is mandated by the United States government, we may have a widely used vaccine in circulation in the United States or another country prior to our full validation of the overall long term safety and efficacy profile of its vaccine platform and technology. Unexpected safety issues in these circumstances could lead to significant reputational damage for the Company going forward and other issues, including delays in our other programs, the need for re-design of our clinical trials and the need for significant additional financial resources.
We may be unable to advance the COVID-19 Vaccine Candidate successfully through the pre-clinical and clinical development process.
Our ability to develop, obtain regulatory approval for and ultimately commercialize MYMD-1, Supera-CBD or other future product candidates, or if we experience significant delays in doing so, our business will be materially harmed.
We have invested a substantial amount of effort and financial resources in MYMD-1 and Supera-CBD. We plan to initiate Phase 2 clinical trials for treatment of diabetes, rheumatoid arthritis, aging and multiple sclerosis with MYMD-1 and IND-enabling pre-clinical studies of Supera-CBD to enable submission of an Investigational New Drug (“IND”) application for a Phase 1 in healthy volunteers followed by clinical trials in epilepsy, addiction and anxiety disorders. In order to conduct human clinical trials, we are required obtain approval from Institutional Review Boards (“IRBs”) or Ethics committees. IRBs are independent committee organizations that operate in compliance with U.S. federal regulations (including, but not limited to 21 C.F.R. Parts 50 and 56, and 45 C.F.R. Part 46) in order to help protect the COVID-19 Vaccine Candidate effectivelyrights of research subjects under the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”). IRBs provide expertise in examining research for its ethical implications, including research involving vulnerable populations, such as pediatrics, critically ill, and cognitively impaired participants. There is no guarantee that an IRB will approve our current product candidates for human clinical trials. Without IRB approval, the Company would not be able to perform clinical research on humans and our products would not be able to move through the regulatory approval process.
Our ability to generate product revenue will depend heavily on many factors, including the following:successful development and eventual commercialization of MYMD-1, Supera-CBD and our other product candidates, which may never occur. We currently generate no revenue from sales of any product and we may never be able to develop or commercialize a marketable product.
The COVID-19 Vaccine CandidateEach of our programs and product candidates will require additional non-clinical andfurther clinical and/or pre-clinical development, regulatory reviewapproval in multiple jurisdictions, obtaining pre-clinical, clinical and approval, substantial investment, access to sufficient commercial manufacturing supply, capacity and expertise, building of a commercial organization, substantial investment and significant marketing efforts before we can be in a position to generate any revenue from product sales. We are not permitted to market or promote any vaccine before it receives regulatory approval fromMYMD-1 and Supera-CBD and our other product candidates must be authorized for marketing by the FDA or comparableand certain other foreign regulatory authorities, andagencies before we may never receive such regulatory approval. commercialize any of our product candidates.
The success of our product candidates depends on multiple factors, including:
| ● | successful completion of pre-clinical studies, including those compliant with Good Laboratory Practices (“GLP”) or GLP toxicology studies, biodistribution studies and minimum effective dose studies in animals, and successful enrollment and completion of clinical trials compliant with current Good Clinical Practices (“GCPs”); | |
| ● | effective INDs and Clinical Trial Authorizations (“CTAs”) that allow commencement of our planned clinical trials or future clinical trials for our product candidates in relevant territories; | |
| ● | approval from IRBs or Ethics committees to conduct human clinical trials; | |
| ● | establishing and maintaining relationships with contract research organizations (“CROs”), and clinical sites for the clinical development of our product candidates; | |
| ● | successful clearance of products arriving from foreign countries, needed to perform clinical trials, through U.S. customs; | |
| ● | maintenance of arrangements with third-party contract manufacturing organizations (“CMOs”) for key materials used in our manufacturing processes and to establish backup sources for clinical and large-scale commercial supply; | |
| ● | positive results from our clinical programs that are supportive of safety and efficacy and provide an acceptable risk-benefit profile for our product candidates in the intended patient populations; | |
| ● | receipt of regulatory approvals from applicable regulatory authorities, including those necessary for pricing and reimbursement of our product candidates; | |
| ● | establishment and maintenance of patent and trade secret protection and regulatory exclusivity for our product candidates; | |
| ● | commercial launch of our product candidates, if and when approved, whether alone or in collaboration with others; | |
| ● | acceptance of our product candidates, if and when approved, by patients, patient advocacy groups, third-party payors and the general medical community; | |
| ● | our effective competition against other therapies available in the market; | |
| ● | establishment and maintenance of adequate reimbursement from third-party payors for our product candidates; | |
| ● | our ability to acquire or in-license additional product candidates; | |
| ● | prosecution, maintenance, enforcement and defense of intellectual property rights and claims; | |
| ● | maintenance of a continued acceptable safety profile of our product candidates following approval, including meeting any post-marketing commitments or requirements imposed by or agreed to with applicable regulatory authorities; or | |
| ● | political factors surrounding the approval process, such as government shutdowns, political instability or global pandemics such as the outbreak of the novel strain of coronavirus, COVID-19. |
If we are unable to developdo not succeed in one or receive marketing approvalmore of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize the COVID-19 Vaccine Candidate,our product candidates, which would materially and adversely affectharm our business, financial condition and results of operations.
Government involvement may limit the commercial success of our COVID-19 Vaccine Candidate.
The COVID-19 pandemic has been classified as a pandemic by public health authorities, and it is possible that one or more government entities may take actions that directly or indirectly have the effect of abrogating some of our rights or opportunities.
Various government entities, including the United States government, are offering incentives, grants, and contracts to encourage additional investment by commercial organizations into preventative and therapeutic agents against COVID-19, which may have the effect of increasing the number of competitors and/or providing advantages to known competitors. Accordingly, there can be no assurance thatbusiness. If we will be able to successfully establish a competitive market share, if any,do not receive regulatory approvals for our COVID-19 Vaccine Candidate even if we succeed in developing one.
If we fail to obtain regulatory approval in foreign jurisdictions, then we cannot market our products, including the COVID-19 Vaccine Candidate, in those jurisdictions.
Many foreign countries in which we market or may market our products have regulatory bodies and restrictions similar to those of the FDA. International sales are subject to foreign government regulation, the requirements of which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval and the requirements may differ. We may be required to conduct additional testing or to provide additional information, resulting in additional expenses, to obtain necessary approvals. If we fail to obtain approval in such foreign jurisdictions, we would not be able to market our products, including the COVID-19 Vaccine Candidate, in such jurisdictions, thereby reducing the potential revenue from the sale of our products.
We are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that we have failed to comply, the agency can institute a wide variety of enforcement actions which may materially affect our business operations.
We are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that we have failed to comply, the agency can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions such as:
Our failure to comply with applicable requirements could lead to an enforcement action that may have an adverse effect on our financial condition and results of operations.
Even if we are able to commercialize our prospective or future product candidates, the products may not receive coverage or adequate reimbursement from third-party payors in the United States or in other countries in which we seek to commercialize such products, which could harm our business.
Our ability to commercialize any product successfully will depend, in part, on the extent to which coverage and adequate reimbursement for such products will be available from government health administration authorities, private health insurers, and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, determine which medications they will cover and establish reimbursement levels. A primary trend in the healthcare industry is cost containment.
Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Third-party payors may also seek additional clinical evidence, beyond the data required to obtain regulatory approval, demonstrating clinical benefits and value in specific patient populations before covering our products for those patients. We cannot be sure that coverage and adequate reimbursement will be available for any product that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Coverage and reimbursement may impact the demand for, or the price of, any product candidate for which we obtain regulatory approval. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize any product candidate for which we obtain regulatory approval.continue our operations.
| 34 |
We may not have the resources to conduct clinical protocols sufficient to yield data suitable for publication in peer-reviewed journals and our inability to do so in the future could have an adverse effect on marketing our products effectively.
In order for our products targeted for use by hospital laboratory professionals and healthcare providers to be widely adopted, we would have to conduct clinical protocols that are designed to yield data suitable for publication in peer-reviewed journals. These studies are often time-consuming, labor-intensive and expensive to execute. We have not previously had the resources to effectively implement such clinical programs within our clinical development activities and may not be able to do so in the future. In addition, if a protocol is initiated, the results of such protocol may ultimately not support the anticipated positioning and benefit proposition for the product. Either of these scenarios could hinder our ability to market our products, and revenue may decline.
Success in pre-clinical studies and earlier clinical trials for our product candidates may not be indicative of the results that may be obtained in later clinical trials, including our Phase 2 clinical trial for MYMD-1, which may delay or prevent obtaining regulatory approval.
Clinical development is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. Success in pre-clinical studies and early clinical trials may not be predictive of results in later-stage clinical trials, and successful results from early or small clinical trials may not be replicated or show as favorable an outcome in later-stage or larger clinical trials, even if successful. We will be required to demonstrate through adequate and well-controlled clinical trials that our product candidates are safe and effective for their intended uses before we can seek regulatory approvals for their commercial sale. The conduct of Phase 2 and Phase 3 trials, and the submission of a New Drug Application (“NDA”) is a complicated process. We have not previously conducted any clinical trials, and have limited experience in preparing, submitting and supporting regulatory filings. Consequently, we may experience delaysbe unable to successfully and efficiently execute and complete necessary clinical trials and other requirements in a way that leads to NDA submission and approval of any product candidate we are developing.
Many companies in the pharmaceutical industry have suffered significant setbacks in late-stage clinical trials after achieving positive results in early-stage development, and there is a high failure rate for product candidates proceeding through clinical trials. In addition, different methodologies, assumptions and applications we utilize to assess particular safety or efficacy parameters may yield different statistical results. Even if we believe the data collected from clinical trials of our product candidates are promising, these data may not be sufficient to support approval by the FDA or foreign regulatory authorities. Pre-clinical and clinical data can be interpreted in different ways. Accordingly, the FDA or foreign regulatory authorities could interpret these data in different ways from us or our partners, which could delay, limit or prevent regulatory approval. If our study data do not consistently or sufficiently demonstrate the safety or efficacy of any of our product candidates, including MYMD-1 and Supera-CBD, to the satisfaction of the FDA or foreign regulatory authorities, then the regulatory approvals for such product candidates could be significantly delayed as we work to meet approval requirements, or, if we are not able to meet these requirements, such approvals could be withheld or withdrawn.
Even if we complete the necessary pre-clinical studies and clinical trials, we cannot predict when, or if, we will obtain regulatory approval to commercialize a product candidate and the approval may be for a narrower indication than we seek.
Prior to commercialization in the United States, MYMD-1, Supera-CBD and our other product candidates must be approved by the FDA pursuant to an NDA for their respective target indication(s). The process of obtaining marketing approvals, both in the U.S. and abroad, is expensive and takes many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate. We have not received approval to market MYMD-1, Supera-CBD or any of our other product candidates from regulatory authorities in any phasejurisdiction. We have limited experience in submitting and supporting the applications necessary to gain marketing approvals, and, in the event regulatory authorities indicate that we may submit such applications, we may be unable to do so as quickly and efficiently as desired. Securing marketing approval requires the submission of extensive pre-clinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities. Our product candidates may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use. Regulatory authorities have substantial discretion in the approval process and may refuse to accept or file any application or may decide that our data is insufficient for approval and require additional pre-clinical, clinical or other studies. In addition, varying interpretations of the data obtained from pre-clinical and clinical testing could delay, limit or clinical developmentprevent marketing approval of a product including during its research and development.candidate.
| 35 |
The completionApproval of any of these studiesMYMD-1, Supera-CBD or our other product candidates may be delayed or haltedrefused for numerousmany reasons, including, but not limited to, the following:including:
| ● | the FDA or | |
| ● | we may be unable to demonstrate, to the satisfaction of the FDA or comparable foreign regulatory authorities, that our product candidates are safe and effective for any of their proposed indications; | |
| ● | the populations studied in clinical trials may not | |
| ● | the results of clinical trials may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval; | |
| ● | we may be unable to demonstrate that our product candidates’ clinical and other benefits outweigh their safety risks; | |
| ● | the data collected from clinical trials of our product candidates may not be sufficient to support the submission of an NDA or other comparable submission in foreign jurisdictions or to obtain regulatory approval in the U.S. or elsewhere; | |
| ● | the facilities of third-party manufacturers with which we contract or procure certain service or raw materials, may not be adequate to support approval of our product candidates; and | |
| ● | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical | |
IfEven if our product candidates meet their pre-specified safety and efficacy endpoints in clinical trials, the pre-clinicalregulatory authorities may not complete their review processes in a timely manner and may not consider such the clinical studies that we are requiredtrial results sufficient to conduct to gain regulatory approval are delayedgrant, or unsuccessful, we may not be able to obtain regulatory approval. Additional delays may result if an FDA Advisory Committee or other regulatory authority recommends non-approval or restrictions on approval. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory authority policy during the period of product development, clinical trials and the review process.
Regulatory authorities also may approve a product candidate for more limited indications than requested or they may impose significant limitations in the form of narrow indications, warnings, contraindications or Risk Evaluation and Mitigation Strategies (“REMS”). These regulatory authorities may also grant approval subject to the performance of costly post-marketing clinical trials. In addition, regulatory authorities may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates and adversely affect our business, financial condition, results of operations and prospects.
| 36 |
Any product candidate for which we obtain marketing approval will be subject to extensive post-marketing regulatory requirements and could be subject to post-marketing restrictions or withdrawal from the market, and we may be subject to penalties if we fail to comply with regulatory requirements or if it experiences unanticipated problems with our product candidates, when and if any of them are approved.
Our product candidates and the activities associated with their development and potential commercialization, including their testing, manufacturing, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other U.S. and international regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, requirements relating to manufacturing, including current Good Manufacturing Practices (“cGMPs”), quality control, quality assurance and corresponding maintenance of records and documents, including periodic inspections by the FDA and other regulatory authorities and requirements regarding the distribution of samples to providers and recordkeeping. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic, unannounced inspections by the FDA and other regulatory authorities for compliance with cGMPs.
The FDA may also impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of any approved product.
In addition, later discovery of previously unknown adverse events or other problems with our product candidates, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| ● | restrictions on such product candidates, manufacturers or manufacturing processes; | |
| ● | restrictions on the labeling or marketing of a product; | |
| ● | restrictions on product distribution or use; | |
| ● | requirements to conduct post-marketing studies or clinical trials; | |
| ● | warning or untitled letters; | |
| ● | withdrawal of any approved product from the market; | |
| ● | refusal to approve pending applications or supplements to approved applications that we submit; | |
| ● | recall of product candidates; | |
| ● | fines, restitution or disgorgement of profits or revenues; | |
| ● | suspension or withdrawal of marketing approvals; | |
| ● | refusal to permit the import or export of our product candidates; | |
| ● | product seizure; or | |
| ● | injunctions or the imposition of civil or criminal penalties. |
| 37 |
The FDA also closely regulates the post-approval marketing and promotion of drugs to ensure that they are marketed in a manner consistent with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding use of their products. For example, under applicable FDA marketing regulations, prescription drug promotions must be consistent with and not contrary to approved labeling, present a “fair balance” between the product’s risks and benefits, be truthful and not false or misleading, and be sufficiently substantiated with appropriate documentary evidence, among numerous other requirements. If we promote our products that are approved for marketing in the United States, if any, in a manner inconsistent with FDA-approved labeling or otherwise not in compliance with FDA regulations, we may be subject to enforcement action. Violations of the Federal Food, Drug, and Cosmetic Act (“FD&C Act”) relating to the promotion of prescription drugs may lead to investigation or prosecution by the DOJ or other applicable agencies and could give rise to ancillary violations of federal and state healthcare fraud and abuse laws, as well as state consumer protection laws and similar laws in international jurisdictions. Additionally, our marketing activities relating to any products we may commercialize in the United States in the future may also be subject to enforcement by the FTC and/or state attorneys general, and we may face consumer class-action liability if our marketing practices are actually or allegedly misleading or deceptive.
In addition to the requirements applicable to approved drug products, we may also be subject to enforcement action in connection with any promotion of an investigational new drug. A sponsor or investigator, or any person acting on behalf of a sponsor or investigator, may not represent in a promotional context that an investigational new drug is safe or effective for the purposes for which it is under investigation or otherwise promote the therapeutic candidate. Sponsors must strike the often difficult balance of communicating sufficient information about its product candidates to inform investors and engaging in valid scientific exchanges with the medical community without crossing the often-difficult-to-ascertain line into “promotion,” which is not defined by regulation but is generally interpreted broadly by FDA. Accordingly, if FDA finds any of our communications regarding MyMD-1 or Supera-CBD to be promotional, we may be subject to a wide range of enforcement actions, and our candidates’ prospects for regulatory approval may be adversely affected.
The occurrence of any event or penalty described above could give rise to material reputational harm to our business and our current, and any future, product candidates we may develop and may inhibit our ability to commercialize our product candidates and generate revenue and could require us to expend significant time and resources in response. The FDA’s and other regulatory authorities’ policies may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we develophave obtained, and we may not achieve or sustain profitability.
Our failure to obtain regulatory approval in international jurisdictions would prevent us from marketing our product candidates outside the U.S.
To market and sell MYMD-1, Supera-CBD or our other product candidates in other jurisdictions, we must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time and data required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the U.S. generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the U.S., we must secure product reimbursement approvals before regulatory authorities will approve the product for sale in that country. Failure to obtain foreign regulatory approvals or non-compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our product candidates in certain countries.
If we fail to comply with the regulatory requirements in international markets and receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be harmed and our business will be adversely affected. We may not obtain foreign regulatory approvals on a timely basis, if at all. Our failure to obtain approval of any of our product candidates by regulatory authorities in another country may significantly diminish the commercial prospects of that product candidate and our business prospects could decline.
Our development program for Supera-CBD, a synthetic analog of CBD, is in its infancy and subject to substantial uncertainty and may not yield commercial results and is subject to significant regulatory risks.
We are only in the future. Pre-clinical studiespre-clinical stage of development for Supera-CBD, which is essentially the earliest stage of a candidate’s development process and clinical trials are expensivemust be followed by regulatory submissions (such as, an IND application and difficult to designFDA’s acceptance thereof), IRB approval, as well as the complex, onerous clinical-trial process (which must be conducted in accordance with FDA’s IND regulations), and implement and any delays or prolongment in our pre-clinical and clinical studies will require additional capital.ultimately, NDA submission, the approval of which is not guaranteed. There iscan be no assurance that our development program for Supera-CBD, a synthetic analog of CBD, will be successful, or that any research and development and product testing efforts will result in commercially saleable products, or that the market will accept or respond positively to products based on Supera-CBD.
| 38 |
Federal Regulation of CBD. The market for cannabinoids is heavily regulated. Synthetic cannabinoids may be viewed as qualifying as controlled substances under the federal Controlled Substances Act of 1970 (CSA) and may be subject to a high degree of regulation including, among other things, certain registration, licensing, manufacturing, security, record keeping, reporting, import, export, inspection by DEA clinical and non-clinical studies, insurance and other requirements administered by the U.S. Drug Enforcement Administration (DEA) and/or the FDA.
State Regulation of CBD. Individual states and local jurisdictions have also established controlled substance laws and regulations, which may differ from U.S. federal law. States have also developed CBD-specific laws and regulations that govern a wide range of CBD-related activities, from cultivation to processing to marketing. There is substantial variation among states’ CBD laws, and we will have to devote substantial time, expenses, and resources toward compliance, and such laws are also subject to ongoing evolution and, thus, must be actively monitored. We or our business partners may be required to obtain separate state or country registrations, permits or licenses in order to be able to acquire additional capitaldevelop produce, sell, store and transport cannabinoids.
Compliance is Complex and Costly. Complying with laws and regulations relating to supportcannabinoids is evolving, complex and expensive, and may divert management’s attention and resources from other aspects of our studies. The failurebusiness. Failure to obtain additional capital wouldmaintain compliance with such laws and regulations may result in regulatory action that could have a material adverse effect on our business, results of operations and financial condition. The DEA, FDA or state agencies may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal proceedings.
We anticipateClinical trials. Because synthetic CBD products may be regulated as controlled substances in the U.S., to conduct clinical trials in the U.S., each of our research sites must submit a research protocol to the DEA and obtain and maintain a DEA researcher registration that we will rely completelyallow those sites to handle and dispense products based on third partiesSupera-CBD and to manufacture certain pre-clinical and allobtain product from our manufacturer. If the DEA delays or denies the grant of a research registration to one or more research sites, the clinical drug supplies. Our businesstrial could be harmed if those third parties fail to provide us with sufficient quantitiessignificantly delayed, and we could lose clinical trial sites.
Negative public perception of drug product or fail to do so at acceptable quality levels or prices.
We do not currently have, nor do we plan to acquire,cannabis-related businesses, misconceptions about the infrastructure or capability internally to manufacture our pre-clinical and clinical drug supplies for use in the conductnature of our clinical studies,business or Supera-MD, and we lack the resources and the capability to manufacture any of our product candidates on a clinical or commercial scale. In order to develop products, apply for regulatory approvals and commercialize our products, we will need to develop, contract for, or otherwise arrange for accessuncertainties relating to the necessary manufacturing capabilities. We anticipate that we will rely on CMOs, or contract manufacturing organizations, and other third party contractors, somelegality of whom may have limited cGMP experience, to manufacture formulations and produce larger scale amounts of drug substance and the drug product required for any clinical trials that we initiate.
The manufacturing process for any vaccine candidate is subject to the FDA and foreign regulatory authority approval process, and we will need to contract with manufacturers who can meet all applicable FDA and foreign regulatory authority requirements on an ongoing basis. In addition, if we receive the necessary regulatory approval for any product candidate, we also expect to rely on third parties to produce materials required for commercial supply. We may experience difficulty in obtaining adequate manufacturing capacity for our needs. Furthermore, it is our responsibility to ensure that all of our third-party contractors meet cGMP laws, regulations and guidance. Due to their failure to comply with applicable regulatory requirements, we may face fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval. These actionscannabinoids could each have a material impactadverse effect on the availability of products. If we are unable to obtain or maintain contract manufacturing for these product candidates, or to do so on commercially reasonable terms, we may not be able to successfully develop and commercialize our products.
To the extent that we enter into manufacturing arrangements with third parties, we will depend on these third parties to perform their obligations in a timely manner and consistent with regulatory requirements, including those related to quality control and quality assurance. The failure of a third-party manufacturer to perform its obligations as expected could adversely affect our business in a number of ways, including:
If a third-party manufacturer with whom we contract fails to perform its obligations, we may be forced to seek out one or more other third-party manufacturers to manufacture our pre-clinical and/or clinical trial materials, which could cause delays in the FDA approval process. Further, should the COVID-19 Vaccine Candidate be approved for marketing by the FDA, a change in a third-party manufacturer could cause significant delays to meeting the demand of patients. In some cases, the technical skills required to manufacture our product may be unique to the original manufacturer and we may have difficulty transferring such skills to a back-up or alternate manufacturer, or we may be unable to transfer such skills at all. In addition, if we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines. We will also be required to demonstrate that the newly manufactured material is the same or similar to the previously manufactured material, or we may need to repeat clinical trials with the newly manufactured material. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop product candidates in a timely manner or within budget. Furthermore, a manufacturer may possess technology related to the manufacture of our product candidate that such manufacturer owns independently, which would increase our reliance on such manufacturer or require us to obtain a license from such manufacturer in order to have another third party manufacture our products.
We intend to rely on third parties to conduct our pre-clinical studies and clinical trials and perform other tasks for us. If these third parties do not successfully carry out their contractual duties, meet expected deadlines, or comply with regulatory requirements, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business, financial condition, and results of operationsoperations.
We believe the cannabinoid industry is highly dependent upon consumer perception regarding the safety, efficacy, quality, and legality of cannabinoids, whether naturally derived or synthetic. Consumer perception of cannabinoid products can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention, and other publicity regarding the consumption of CBD products. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention, or other research findings or publicity will be favorable to the CBD market or Supera-CBD, in particular. Our dependence upon consumer perceptions with regard to Supera-CBD, particularly once it is approved for commercialization, if ever, means that adverse scientific research reports, findings, regulatory proceedings, litigation, media attention, or other publicity relating to cannabinoid products, generally, or any particular cannabinoid products or derivatives, in particular, regardless of merit or accuracy, could have a material adverse effect on our business, the development of, or ultimate commercial demand for (if applicable), Supera-CBD. Such adverse publicity or other negative media attention could arise even if the adverse effects reportedly associated with such products resulted from consumers’ failure to consume such products appropriately or as directed. Any adverse publicity or other similar occurrences affecting consumer perception may have a material adverse impact on our reputation, perception of Supera-CBD, and our ability to obtain the necessary regulatory approvals for Supera-CBD and its prospective commercial viability.
Risks Related to Commercialization and Manufacturing
The commercial success of our product candidates, including MYMD-1 and Supera-CBD, will depend upon their degree of market acceptance by providers, patients, patient advocacy groups, third-party payors and the general medical community.
Even with the requisite approvals from the FDA and other regulatory authorities internationally, the commercial success of our product candidates will depend, in part, on the acceptance of providers, patients and third-party payors of our product candidates, as medically necessary, cost-effective and safe. Any product that we commercialize may not gain acceptance by providers, patients, patient advocacy groups, third-party payors and the general medical community. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of MYMD-1, Supera-CBD and our other product candidates, if approved for commercial sale, will depend on several factors, including:
| ● | the efficacy, durability and safety of such product candidates as demonstrated in clinical trials; | |
| ● | the potential and perceived advantages of product candidates over alternative treatments; | |
| ● | the cost of treatment relative to alternative treatments; | |
| ● | the clinical indications for which the product candidate is approved by the FDA or the European Commission; | |
| ● | the willingness of providers to prescribe new therapies; | |
| ● | the willingness of the target patient population to try new therapies; | |
| ● | the prevalence and severity of any side effects; | |
| ● | product labeling or product insert requirements of the FDA or other regulatory authorities, including any limitations or warnings contained in a product’s approved labeling; | |
| ● | the strength of marketing and distribution support; | |
| ● | the timing of market introduction of competitive products; | |
| ● | the quality of our relationships with patient advocacy groups; | |
| ● | publicity concerning our product candidates or competing products and treatments; and | |
| ● | sufficient third-party payor coverage and adequate reimbursement. |
Even if a potential product displays a favorable efficacy and safety profile in pre-clinical studies and clinical trials, market acceptance of the product will not be fully known until after it is launched.
| 39 |
The pricing, insurance coverage and reimbursement status of newly approved products is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for our product candidates, if approved, could limit our ability to market those products and decrease our ability to generate product revenue.
If we are unable to establish or sustain coverage and adequate reimbursement for our product candidates from third-party payors, the adoption of those product candidates and sales revenue will be adversely affected, which, in turn, could adversely affect the ability to market or sell those product candidates, if approved.
We expect that coverage and reimbursement by third-party payors will be essential for most patients to be able to afford these treatments. Accordingly, sales of MYMD-1, Supera-CBD and our other product candidates will depend substantially, harmed.
We planboth domestically and internationally, on the extent to rely upon third-party contract researchwhich the costs of our product candidates will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or CROs,will be reimbursed by government authorities, private health coverage insurers and other third-party payors. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the U.S., third-party payors, including private and governmental payors, such as the Medicare and Medicaid programs, play an important role in determining the extent to which new drugs will be covered and reimbursed. The Medicare program covers certain individuals aged 65 or older, disabled or suffering from end-stage renal disease. The Medicaid program, which varies from state to state, covers certain individuals and families who have limited financial means. The Medicare and Medicaid programs increasingly are used as models for how private payors and other governmental payors develop their coverage and reimbursement policies for drugs. One payor’s determination to provide coverage for a drug product, however, does not assure that other payors will also provide coverage for the drug product. Further, a payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved.
In addition to government and private payors, professional organizations such as the American Medical Association (“AMA”), can influence decisions about coverage and reimbursement for new products by determining standards for care. In addition, many private payors contract with commercial vendors who sell software that provide guidelines that attempt to limit utilization of, and therefore reimbursement for, certain products deemed to provide limited benefit to existing alternatives. Such organizations may set guidelines that limit reimbursement or utilization of our product candidates. Even if favorable coverage and reimbursement status is attained for one or more product candidates for which our collaborators receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Outside the U.S., international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada and other countries has and will continue to put pressure on the pricing and usage of therapeutics such as our product candidates. In many countries, particularly the countries of the European Union, the prices of medical institutions,products are subject to varying price control mechanisms as part of national health systems. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical investigators and contract laboratoriestrial that compares the cost-effectiveness of our product candidate to other available therapies. In general, the prices of products under such systems are substantially lower than in the U.S. Other countries allow companies to fix their own prices for products but monitor and manage datacontrol company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our licensed ongoing pre-clinicalproduct candidates. Accordingly, in markets outside the U.S., the reimbursement for our product candidates may be reduced compared with the U.S. and clinical programs.may be insufficient to generate commercially reasonable revenues and profits.
Moreover, increasing efforts by governmental and third-party payors, in the U.S. and internationally, to cap or reduce healthcare costs may cause such organizations to limit both coverage and level of reimbursement for new products approved and, as a result, they may not cover or provide adequate payment for our product candidates. We expect to continueexperience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, the increasing influence of certain third-party payors, such as health maintenance organizations, and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products into the healthcare market. Recently there have been instances in which third-party payors have refused to reimburse treatments for patients for whom the treatment is indicated in the FDA-approved product labeling. Even if we are successful in obtaining FDA approvals to commercialize our product candidates, we cannot guarantee that we will be able to secure reimbursement for all patients for whom treatment with our product candidates is indicated.
| 40 |
If third parties on which we depend to conduct our planned pre-clinical studies or clinical trials, do not perform as contractually required, fail to satisfy regulatory or legal requirements or miss expected deadlines, our development program could be delayed with adverse effects on our business, financial condition, results of operations and prospects.
We rely on third party CROs, CMOs, consultants and others to design, conduct, supervise and monitor key activities relating to, discovery, manufacturing, pre-clinical studies and clinical trials of our product candidates, and we intend to do the same for future activities relating to existing and future programs. Because we rely on third parties and do not have the ability to conduct all required testing, discovery, manufacturing, preclinical studies or clinical trials independently, we have less control over the timing, quality and other aspects of discovery, manufacturing, pre-clinical studies and clinical trials than we would if we conducted them on our own. These investigators, CROs, CMOs and consultants are not our employees, and we have limited control over the amount of time and resources that they dedicate to our programs. These third parties may have contractual relationships with other entities, some of which may be our competitors, which may draw time and resources from our programs. The third parties we contract with might not be diligent or timely in conducting our discovery, manufacturing, pre-clinical studies or clinical trials, resulting in discovery, manufacturing, pre-clinical studies or clinical trials being delayed or unsuccessful, in whole or in part.
If we cannot contract with acceptable third parties on commercially reasonable terms, or at all, or if these third parties do not carry out their contractual duties, satisfy legal and regulatory requirements for executionthe conduct of pre-clinical studies or clinical trials or meet expected deadlines, our clinical development programs could be delayed and otherwise adversely affected. In all events, we are responsible for ensuring that each of our pre-clinical studies and clinical trials and we control only certain aspects of their activities. Nevertheless, we maintain responsibility for ensuring that each of our clinical trials and pre-clinical studies is conducted in accordance with the general investigational plan and protocols for the trial, as well as in accordance with GLP, GCPs and other applicable protocol, legal, regulatory,laws, regulations and scientific standards and ourstandards. Our reliance on these third parties that we do not control does not relieve us of our regulatory responsibilities. Wethese responsibilities and our CROsrequirements. The FDA and other vendors are required to comply with cGMP, current GCP, and current GLPs, which are a collection of laws and regulations enforced by the FDA or comparable foreign authorities for all of our product candidates in clinical development. Regulatoryregulatory authorities enforce these regulationsGCPs through periodic inspections of manufacturing facilities, pre-clinical study and clinical trial sponsors, principal investigators preclinical study and clinical trial sites, and other contractors.sites. If we or any of our CROs or vendorsthese third parties fails to comply with applicable regulations,GCPs, the clinical data generated in our pre-clinical studies andits clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional pre-clinical studies and clinical trials before approving ourits marketing applications. We cannot assure that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials complyhave complied with GCP regulations.GCPs. In addition, our clinical trials must be conducted with products manufactured consistentlyproduct produced in accordance with cGMP regulations. Failure by us or our third party CROscGMPs. Our failure to comply with these regulations may require us to repeat clinical trials, which wouldcould delay or prevent the developmentreceipt of regulatory approvals. Any such event could have an adverse effect on our business, financial condition, results of operations and prospects.
We face significant competition in an environment of rapid pharmacological change and it is possible that our competitors may achieve regulatory approval processes.before us or develop therapies that are more advanced or effective than ours, which may harm our business, financial condition and our ability to successfully market or commercialize MYMD-1, Supera-CBD and our other product candidates.
If any
The biotechnology and pharmaceutical industries are characterized by rapidly changing technologies, competition and a strong emphasis on intellectual property. We are aware of several companies focused on developing immunometabolic treatments in various indications as well as several companies addressing other treatments for anti-aging, anxiety and depression. We may also face competition from large and specialty pharmaceutical and biotechnology companies, academic research institutions, government agencies and public and private research institutions that conduct research, seek patent protection, and establish collaborative arrangements for research, development, manufacturing and commercialization.
Several companies are focused on developing treatments for immunometabolic dysregulation in treatment of autoimmune disorders.
Many of our relationshipspotential competitors, alone or with these third-party CROs, medical institutions,their strategic partners, may have substantially greater financial, technical and other resources than we do, such as larger research and development, clinical, investigatorsmarketing and manufacturing organizations. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of competitors. Our commercial opportunity could be reduced or contract laboratories terminate,eliminated if competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any product candidates that we may not bedevelop. Competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for our products, which could result in our competitors establishing a strong market position before we are able to enter into arrangements with alternative CROs on commercially reasonable terms,the market, if ever. Additionally, new or at all. In addition,advanced technologies developed by our CROs are notcompetitors may render our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whethercurrent or not they devote sufficient time and resources to our ongoing pre-clinical and clinical programs. If CROs do not successfully carry out their contractual duties,future product candidates uneconomical or comply with current GCP laws, regulations and guidance, or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our protocols, regulatory requirements, or for other reasons, our clinical trials may be extended, delayed or terminatedobsolete, and we may not be ablesuccessful in marketing our product candidates against competitors.
| 41 |
The manufacture of drugs is complex, and our third-party manufacturers may encounter difficulties in production. If any of our third-party manufacturers encounter such difficulties, our ability to provide supply of MYMD-1, Supera-CBD or our other product candidates for clinical trials, our ability to obtain marketing approval, or our ability to provide supply of our product candidates for patients, if approved, could be delayed or stopped.
We intend to establish manufacturing relationships with a limited number of suppliers to manufacture raw materials, the drug substance and finished product of any product candidate for which we are responsible for pre-clinical or clinical development. Each supplier may require licenses to manufacture such components if such processes are not owned by the supplier or in the public domain. As part of any marketing approval, a manufacturer and its processes are required to be qualified by the FDA prior to regulatory approval. If supply from the approved vendor is interrupted, there could be a significant disruption in commercial supply. An alternative vendor would need to be qualified through an NDA supplement which could result in further delay. The FDA or other regulatory agencies outside of the U.S. may also require additional studies if a new supplier is relied upon for commercial production. Switching vendors may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines.
The process of manufacturing drugs is complex, highly regulated and subject to multiple risks. Manufacturing drugs is highly susceptible to product loss due to contamination, equipment failure, improper installation or operation of equipment, vendor or operator error, inconsistency in yields, variability in product characteristics and difficulties in scaling the production process. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered at the facilities of our manufacturers, such facilities may need to be closed for an extended period of time to investigate and remedy the contamination, which could delay clinical trials and adversely harm our business. Moreover, if the FDA determines that our CMOs are not in compliance with FDA laws and regulations, including those governing cGMPs, the FDA may deny NDA approval until the deficiencies are corrected or we replace the manufacturer in our NDA with a manufacturer that is in compliance. In addition, approved products and the facilities at which they are manufactured are required to maintain ongoing compliance with extensive FDA requirements and the requirements of other similar agencies, including ensuring that quality control and manufacturing procedures conform to cGMP requirements. As such, our CMOs are subject to continual review and periodic inspections to assess compliance with cGMPs. Furthermore, although we do not have day-to-day control over the operations of our CMOs, we are responsible for ensuring compliance with applicable laws and regulations, including cGMPs.
In addition, there are risks associated with large scale manufacturing for clinical trials or commercial scale including, among others, cost overruns, potential problems with process scale-up, process reproducibility, stability issues, compliance with good manufacturing practices, lot consistency and timely availability of raw materials. Even if our collaborators obtain regulatory approval for or successfully commercializeany of our product candidates. CROs may also generate higher costs than anticipated. As a result,candidates, there is no assurance that manufacturers will be able to manufacture the approved product to specifications acceptable to the FDA or other regulatory authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product or to meet potential future demand. If our manufacturers are unable to produce sufficient quantities for clinical trials or for commercialization, commercialization efforts would be impaired, which would have an adverse effect on our business, financial condition, and results of operations and the commercial prospects for our product candidates could be materially and adversely affected, our costs could increase, and our ability to generate revenue could be delayed.prospects.
Switching or adding additional CROs, medical institutions, clinical investigators or contract laboratories involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new CRO commences work replacing a previous CRO. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines.
We are opportunistically reviewing strategic transactions and there can be no assurance that any such strategic transaction we pursue will result in additional value for our stockholders. As a result, the makeup of our lines of business may change.
We are assessing alternate ways to generate value for shareholders, including reviewing opportunities that may lead to acquisitions, dispositions, business combinations or other strategic transactions. Strategies we may employ include seeking new or expanding existing specialty market niches, expanding our presence, acquiring businesses complementary to existing strengths and continually evaluating the performance and strategic fit of our existing business units. As a result, the makeup of our lines of business is subject to change. For example, as previously disclosed, in light of the unfavorable factors persistent in our rapid, point-of-care screening and testing product business and the progress we have made in its partnership with Premas, we conducted a strategic review of the screening and testing products business. Following such review, in early July 2020, we ceased the production and sale of our rapid, point-of-care screening and testing products. In connection with the discontinuation of its existing product line, we decided to close the facility located in Thorofare, New Jersey (the “Thorofare Facility”), which previously housed our manufacturing, operations and support personnel, and terminated the lease (the “Thorofare Lease”) on November 30, 2020. Furthermore, on November 11, 2020, we entered into the Merger Agreement with MYMD. For risks related to the Merger, please see risks set forth under the heading “— Risks Related to the Proposed Merger” herein. However, there can be no assurance that our pursuit of such strategic alternatives will result in any transaction or other alternatives.Government Regulation
To the extent we engage in other strategic transactions, the process may be time consuming and disruptive to our business operations and, our business, financial condition and results of operationsWe could be adversely affected. Weaffected if healthcare reform measures substantially change the market for medical care or healthcare coverage in the U.S.
On March 23, 2010, President Obama signed the “Patient Protection and Affordable Care Act” (P.L. 111-148) (the “ACA”) and on March 30, 2010, he signed the “Health Care and Education Reconciliation Act” (P.L. 111-152), collectively commonly referred to as the “Healthcare Reform Law.” The Healthcare Reform Law included a number of new rules regarding health insurance, the provision of healthcare, conditions to reimbursement for healthcare services provided to Medicare and Medicaid patients, and other healthcare policy reforms. Through the law-making process, substantial changes have been and continue to be made to the current system for paying for healthcare in the U.S., including changes made to extend medical benefits to certain Americans who lacked insurance coverage and to contain or reduce healthcare costs (such as by reducing or conditioning reimbursement amounts for healthcare services and drugs, and imposing additional taxes, fees, and rebate obligations on pharmaceutical and medical device companies). This legislation was one of the most comprehensive and significant reforms ever experienced by the U.S. in the healthcare industry and has significantly changed the way healthcare is financed by both governmental and private insurers. This legislation has impacted the scope of healthcare insurance and incentives for consumers and insurance companies, among others. Additionally, the Healthcare Reform Law’s provisions were designed to encourage providers to find cost savings in their clinical operations. Pharmaceuticals represent a significant portion of the cost of providing care. This environment has caused changes in the purchasing habits of consumers and providers and resulted in specific attention to the pricing negotiation, product selection and utilization review surrounding pharmaceuticals. This attention may result in products we may commercialize or promote in the future being chosen less frequently or the pricing being substantially lowered. At this stage, it is difficult to estimate the full extent of the direct or indirect impact of the Healthcare Reform Law on us.
| 42 |
These structural changes could incur substantial expenses associated with evaluatingentail further modifications to the existing system of private payors and negotiating potential strategic alternatives. Furthermore,government programs (such as Medicare, Medicaid, and the State Children’s Health Insurance Program), creation of government-sponsored healthcare insurance sources, or some combination of both, as well as other changes. Restructuring the coverage of medical care in the U.S. could impact the reimbursement for prescribed drugs and pharmaceuticals, including our ability to effectively integrate any future acquisitions or mergers will depend on, among other things, our ability to integrate businesses, the adequacy of our implementation plans, the ability of our management to oversee and operate effectively the combined operationscurrent commercial products, those we and our ability to achieve desired operational efficiencies. If wedevelopment or commercialization partners are unable to successfully integrate the operations of any businessescurrently developing or those that we may acquirecommercialize or promote in the future. If reimbursement for the products we currently commercialize or promote, any product we may commercialize or promote, or approved therapeutic candidates is substantially reduced or otherwise adversely affected in the future, our business, financial position, results of operations or cash flowsrebate obligations associated with them are substantially increased, it could be adversely affected. There can be no assurance that any potential transaction, if consummated, will provide greater value to our stockholders than that reflected in the current price of our common stock.
If we are unable to make acquisitions and investments, or successfully integrate them into our business, our business could be harmed.
As part of our business strategy, we may acquire other companies or businesses. However, we may not be able to find suitable acquisition candidates, and we may not be able to complete acquisitions on favorable terms, if at all. Acquisitions involve numerous risks, any of which could harm our business and negatively affect our operating results, including:
The use of our PIFA products could result in serious injuries, product liability claims, regulatory enforcement action, and/or recalls or market withdrawals, any of which would likely subject us to substantial costs and reputational harm and have a material adverse effect on our business.reputation, business, financial condition or results of operations.
Extending medical benefits to those who currently lack coverage will likely result in substantial costs to the U.S. federal government, which may force significant additional changes to the healthcare system in the U.S. Much of the funding for expanded healthcare coverage may be sought through cost savings. While some of these savings may come from realizing greater efficiencies in delivering care, improving the effectiveness of preventive care and enhancing the overall quality of care, much of the cost savings may come from reducing the cost of care and increased enforcement activities. Cost of care could be reduced further by decreasing the level of reimbursement for medical services or products (including our any product we may commercialize or promote in the future), or by restricting coverage (and, thereby, utilization) of medical services or products. In July 2020,either case, a reduction in the utilization of, or reimbursement for any product which we ceasedreceive marketing approval in the productionfuture, could have a material adverse effect on our reputation, business, financial condition or results of operations.
Several states and sale of its rapid, point-of-care screeningprivate entities initially mounted legal challenges to the Healthcare Reform Law, in particular, the ACA, and testing products. We willthey continue to litigate various aspects of the legislation. On July 26, 2012, the U.S. Supreme Court generally upheld the provisions of the ACA at issue as constitutional. However, the U.S. Supreme Court held that the legislation improperly required the states to expand their Medicaid programs to cover more individuals. As a result, states have a choice as to whether they will expand the number of individuals covered by their respective state Medicaid programs. Some states have not expanded their Medicaid programs and have chosen to develop other cost-saving and coverage measures to provide supportcare to currently uninsured individuals. Many of these efforts to date have included the institution of Medicaid-managed care programs. The manner in which these cost-saving and coverage measures are implemented could have a material adverse effect on our reputation, business, financial condition or results of operations.
Further, the healthcare regulatory environment has seen significant changes in recent years and is still in flux. Legislative initiatives to modify, limit, replace, or repeal the ACA and judicial challenges have continued. We cannot predict the impact on our business of future legislative and legal challenges to the ACA or other aspects of the Healthcare Reform Law or other changes to the current laws and regulations. The financial impact of U.S. healthcare reform legislation over the next few years will depend on a number of factors, including the policies reflected in implementing regulations and guidance and changes in sales volumes for these testing products that remaintherapeutics affected by the legislation. From time to time, legislation is drafted, introduced and passed in the market through their respective product expiration dates. We believeU.S. Congress that could significantly change the statutory provisions governing coverage, reimbursement, and marketing of pharmaceutical products. In addition, third-party payor coverage and reimbursement policies are often revised or interpreted in ways that may significantly affect our business and our products.
During his time in office, former President Trump supported the repeal of all or portions of the ACA. President Trump also issued an executive order in which he stated that it is his administration’s policy to seek the prompt repeal of the ACA and in which he directed executive departments and federal agencies to waive, defer, grant exemptions from, or delay the implementation of the provisions of the ACA to the maximum extent permitted by law. Congress has enacted legislation that repeals certain portions of the ACA, including but not limited to the Tax Cuts and Jobs Act, passed in December 2017, which included a provision that eliminates the penalty under the ACA’s individual mandate, effective January 1, 2019, as well as the Bipartisan Budget Act of 2018, passed in February 2018, which, among other things, repealed the Independent Payment Advisory Board (which was established by the ACA and was intended to reduce the rate of growth in Medicare spending).
Additionally, in December 2018, a district court in Texas held that the users of our PIFA products are likely to be particularly sensitive to test defectsindividual mandate is unconstitutional and errors, as the conditions that the PIFA products are designedrest of the ACA is, therefore, invalid. On appeal, the Fifth Circuit Court of Appeals affirmed the holding on the individual mandate but remanded the case back to identify may cause limb-the lower court to reassess whether and life-threatening complications ifhow such holding affects the validity of the rest of the ACA. The Fifth Circuit’s decision on the individual mandate was appealed to the U.S. Supreme Court. On June 17, 2021, the Supreme Court held that the plaintiffs (comprised of the state of Texas, as well as numerous other states and certain individuals) did not accurately diagnosed in a timely manner.have standing to challenge the constitutionality of the ACA’s individual mandate and, accordingly, vacated the Fifth Circuit’s decision and instructed the district court to dismiss the case. As a result, the failureACA will remain in-effect in its current form for the foreseeable future; however, we cannot predict what additional challenges may arise in the future, the outcome thereof, or the impact any such actions may have on our business.
| 43 |
The Biden administration also introduced various measures in 2021 focusing on healthcare and drug pricing, in particular. For example, on January 28, 2021, President Biden issued an executive order that initiated a special enrollment period for purposes of our testsobtaining health insurance coverage through the ACA marketplace, which began on February 15, 2021, and remained open through August 15, 2021. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or servicesthe ACA. On the legislative front, the American Rescue Plan Act of 2021 was signed into law on March 11, 2021, which, in relevant part, eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single source drugs and innovator multiple source drugs, beginning January 1, 2024. And, in July 2021, the Biden administration released an executive order entitled, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response, on September 9, 2021, HHS released a “Comprehensive Plan for Addressing High Drug Prices” that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to performadvance these principles. And, in August 2022, the Inflation Reduction Act (“IRA”) was signed into law, which will, among other things, allow U.S. Department of Health and Human Services (“HHS”) to negotiate the selling price of certain drugs and biologics that the Centers for Medicare & Medicaid Services (“CMS”) reimburses under Medicare Part B and Part D, although only high-expenditure single-source drugs that have been approved for at least 7 years (11 years for biologics) can be selected by CMS for negotiation, with the negotiated price taking effect two years after the selection year. The negotiated prices, which will first become effective in 2026, will be capped at a statutory ceiling price. Beginning in October 2023, the IRA will also penalize drug manufacturers that increase prices of Medicare Part B and Part D drugs at a rate greater than the rate of inflation. The IRA permits the Secretary of HHS to implement many of these provisions through guidance, as expected could subject usopposed to legal claims arising from any defects or errors.
The use of our PIFA products and our other products could leadregulation, for the initial years. Manufacturers that fail to product liability (and other similar) claims against us if someone were to allege that one of our tests failed to perform as it was designed or as claimed in our promotional materials, was performed pursuant to incorrect or inadequate laboratory procedures, if we delivered incorrect or incomplete test results, or if someone were to misinterpret test results. In addition, wecomply with the IRA may be subject to liabilityvarious penalties, including civil monetary penalties. The IRA also extends enhanced subsidies for errorsindividuals purchasing health insurance coverage in a misunderstandingACA marketplaces through plan year 2025.
There is uncertainty as to what healthcare programs and regulations may be implemented or changed at the federal and/or state level in the U.S. or the effect of any future legislation or inappropriate reliance upon,regulation. Furthermore, we cannot predict what actions the information we provide, or for failure to provide such information,Biden administration will implement in connection with the results generated by our products. A product liability or professional liability claimHealth Reform Law. However, it is possible that such initiatives could result in substantial damages and be costly and time-consuming for us to defend.
Our PIFA products are not 100% accurate and may generate erroneous results that could cause patient harm. For example, PIFA could provide a so-called “false negative” result upon which a patient or physician may rely to make a conclusion about how to proceed with the patient’s treatment. If the false negative causes, or exacerbates, a patient injury or condition, the patient (and/or the patient’s family) may file a lawsuit against us based on product liability.
Any product liability or professional liability claim brought against us, with or without merit, could increase our insurance rates, cause our insurance coverage to be terminated or prevent us from securing insurance coverage in the future.
Further, under the FDA’s Medical Device Regulations, we are required to report to the FDA any incident in which its product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. Repeated product malfunctions may result in a voluntary or involuntary product recall, which could divert managerial and financial resources and have an adverse effect on our reputation,ability to obtain approval and/or successfully commercialize products in the U.S. in the future, as applicable.
We are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that we have failed to comply, the agency can institute a wide variety of enforcement actions which may materially affect our business operations.
We are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that we have failed to comply, with one or more applicable requirements the agency can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions such as:
| ● | fines, injunctions and civil penalties; | |
| ● | recall, detention or seizure of our products; | |
| ● | the issuance of public notices or warnings; | |
| ● | operating restrictions, partial suspension or total shutdown of production; | |
| ● | refusing MyMD’s requests for a 510(k) clearance of new products; | |
| ● | withdrawing a 510(k) clearance already granted; and | |
| ● | criminal prosecution. |
Our failure to comply with applicable requirements could lead to an enforcement action that may have an adverse effect on our financial condition and operating results.results of operations.
Any adverse event involvingThe FDA’s ability to review and approve new products may be hindered by a variety of factors, including budget and funding levels, ability to hire and retain key personnel, statutory, regulatory and policy changes and global health concerns.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, statutory, regulatory and policy changes, the FDA’s ability to hire and retain key personnel and accept the payment of user fees, and other events that may otherwise affect the FDA’s ability to perform routine functions. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable. Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our products could result in future voluntary corrective actions,business. For example, over the last several years, including for 35 days beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory agencies, such as recallsthe FDA, have had to furlough critical employees and stop critical activities.
The ability of the FDA and other government agencies to properly administer their functions is highly dependent on the levels of government funding and the ability to fill key leadership appointments, among various factors. Delays in filling or customer notifications, or regulatory agency action, whichreplacing key positions could include inspection, mandatory recall orsignificantly impact the ability of the FDA and other enforcement action. Any corrective action, whether voluntary or involuntary,agencies to fulfill their functions and could greatly impact healthcare and the pharmaceutical industry.
| 44 |
Our operations and relationships with future customers, providers and third-party payors will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
If we market products or interact with health care practitioners in a manner that violates healthcare fraud or abuse laws, we may be subject to civil or criminalapplicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to penalties including exclusion from participationcriminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers and third-party payors will play a primary role in governmentthe recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with providers, third-party payors and customers will subject us to broadly applicable fraud and abuse and other healthcare programs.laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any product candidates for which we obtain marketing approval.
If we receive payments directly from or bill directly to Medicare, Medicaid or other national or third-party payers for its products, United StatesRestrictions under applicable U.S. federal and state healthcare laws and regulations pertaininginclude the following:
| ● | the federal Anti-Kickback Statute (“AKS”) prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the AKS or specific intent to violate it in order to have committed a violation; | |
| ● | federal false claims laws, including the federal False Claims Act, imposes criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. In addition, the government may assert that a claim including items or services resulting from a violation of the AKS constitutes a false or fraudulent claim for purposes of the civil False Claims Act; | |
| ● | HIPAA imposes criminal and civil liability for, among other things, knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters. Similar to the AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; | |
| ● | the federal Physician Payment Sunshine Act of 2010 (“PPSA”) requires applicable manufacturers of covered drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, with specific exceptions, to report payments and other transfers of value provided during the previous year to physicians, as defined by such law, certain other healthcare providers starting in 2022 (for payments made in 2021), and teaching hospitals, as well as certain ownership and investment interests held by such physicians and their immediate family, which includes annual data collection and reporting obligations; | |
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; and | |
| ● | some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. |
Efforts to fraudensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or abuse will befuture statutes, regulations or case law involving applicable to our business. We are subject to healthcare fraud and abuse regulation by the United States federal governmentor other healthcare laws and the statesregulations. If our operations are found to be in which we conduct our business.
Theviolation of any of these laws or any other governmental regulations that may affect our abilityapply to operate include the AKS, which prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration to induce, or in return for, the purchase, lease or order, or arrangement for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute applies to arrangements between pharmaceutical manufacturers and prescribers, purchasers and formulary managers. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain common activities, the exceptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendationsus, we may be subject to scrutiny if they do not qualify for an exception or safe harbor.
Federal false claims laws prohibit any personsignificant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of product candidates from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Pharmaceutical companies have been prosecuted under these laws for a variety of alleged promotional and marketing activities,government-funded healthcare programs, such as providing free product to customers with the expectation that the customers would bill federal programs for the product, reporting to pricing services inflated average wholesale prices that were then used by federal programs to set reimbursement rates, engaging in off-label promotion that caused claims to be submitted to Medicaid for non-covered off-label uses and submitting inflated best price information to the Medicaid Drug Rebate Program.
HIPAA also created prohibitions against healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payers. The false statements statute immediately noted above prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services.
In addition, there has been a trend of increased federal and state regulation of payments made to physicians. The ACA, through the PPSA, imposed new requirements on manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the Centers for Medicare and Medicaid, Services (“CMS”) information related to paymentsdisgorgement, contractual damages, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of our operations. If any of the physicians or other “transfers of value” madehealthcare providers or entities with whom we expect to physicians (defineddo business is found to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, and applicable manufacturers and group purchasing organizations to report annually to CMS ownership and investment interests held by physicians (as defined above) and their immediate family members and payments or other “transfers of value” to such physician owners and their immediate family members. Manufacturers are required to report such data to the government by the 90th calendar day of each year.
The majority of states also have statutes or regulations similar to these federal laws, which apply to items and services reimbursed under Medicaid and other state programs, or,be not in several states, apply regardless of the payer. In addition, some states have laws that require pharmaceutical companies to adopt comprehensive compliance programs. For example, under California law, pharmaceutical companies must comply with both the April 2003 Office of Inspector General Compliance Program Guidance for Pharmaceutical Manufacturers and the PhRMA Code on Interactions with Healthcare Professionals, as amended. Moreover, certain states mandate the tracking and reporting of gifts, compensation and other remuneration paid by us to physicians and other healthcare providers.
Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, cause reputational harm and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable U.S. federal and state laws, they may prove costly.be subject to criminal, civil or administrative sanctions, including exclusions from government-funded healthcare programs.
| 45 |
Our internal computer systems, or those of its third-party vendors, collaborators, or other contractors may be subject to various federal and state confidentiality and privacy laws in the United States and abroad and could sustain system failures, security breaches, or other disruptions, any of which could have a material adverse effect on our business.
Numerous international, national, federal, provincial and state laws, including state privacy laws (such as the California Consumer Privacy Act), state security breach notification and information security laws, and federal and state consumer protection laws govern the collection, use, and disclosure of personal information. In addition, most healthcare providers who may, in the future, prescribe and dispense our products in the United States and research institutions in the United States with whom we may collaborate in the future are “covered entities” subject to privacy and security requirements under HIPAA. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to business associates, independent contractors, or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. We, or the covered entities we engage with, could be subject to a wide range of penalties and sanctions under HIPAA, including criminal penalties if we, our affiliates, or our agents knowingly obtain or disclosethe individually identifiable health information maintained by a covered entity is disclosed in a manner that is not authorized or permitted by HIPAA. Failure to comply with applicable HIPAA requirements or other current and future privacy laws and regulations could result in governmental enforcement actions (including the imposition of significant penalties), criminal and civil liability, and/or adverse publicity that negatively affects our business.
Moreover, we rely on our internal and third-party provided information technology systems and applications to support our operations and to maintain and process company information including personal information, confidential business information and proprietary information. If these information technology systems are subject to cybersecurity attacks, or are otherwise compromised, due to cyberattacks, human error or malfeasance, system errors or otherwise, it may adversely impact our business, disrupt our operations, or lead to the loss, theft, destruction, corruption, or compromise of our information or that of our collaborators, study subjects, or other third-party contractors, as applicable. Such information technology or security events could also lead to legal liability, regulatory investigations or enforcement actions, loss of business, negative media coverage, and reputational damage. While we seek to protect our information technology systems from these types of incidents, the healthcare sector continues to see a high frequency of cyberattacks and increasingly sophisticated threat actors, and our systems and the information maintained within those systems remain potentially vulnerable to data security incidents.
Any of the above-described cyber or other security-related incidents may trigger notification obligations to affected individuals and government agencies, legal claims or proceedings, and liability under foreign, federal, provincial and state laws that protect the privacy and security of personal information. Our proprietary and confidential information may also be accessed. Any one of these events could cause our business to be materially harmed and our results of operations may be adversely impacted. Finally, as cyber threats continue to evolve, and privacy and cybersecurity laws and regulations continue to develop, we may need to invest additional resources to implement new compliance measures, strengthen our information security posture, or respond to cyber threats and incidents.
Risks Related to Our Intellectual Property
We may fail to retain qualified personnel.
We have substantially reduced the number of our employees in order to reduce our costs. Accordingly, retaining our remaining personnel in the future will be critical to our success. If we fail to retain and motivate these highly skilled personnel, we may be unable to continue our operating activities, and this could have a material adverse effect or our business, financial condition, results of operations and future prospects.
We rely on the key executive officers of the management team.
We are dependentOur success largely depends on our management teamability to execute against our business plan. Failure could result in delays in product development, loss of customersobtain, maintain and sales and diversion of management resources, which could adversely affect our operating results.
Expenses incurred with respect to monitoring, protecting, and defendingprotect our intellectual property rights could adversely affect our business.
Competitors and others may infringe on our intellectual property rights, or may allege that we have infringed on theirs. Monitoring infringement and misappropriation of intellectual property can beproperty. It is difficult and expensive,costly to protect our proprietary rights and technology, and we may not be able to detect infringement or misappropriationensure their adequate protection.
Our commercial success will depend in large part on obtaining and maintaining patent, trademark, trade secret and other intellectual property protection of our proprietary rights.
We may incur substantial coststechnologies and product candidates, which include MYMD-1, Supera-CBD and the other product candidates we have in development, their respective components, formulations, combination therapies, methods used to manufacture them and methods of treatment, as a result of litigation or other proceedings relating to patentwell as successfully defending our patents and other intellectual property rights against third-party challenges. Our ability to stop unauthorized third parties from making, using, selling, offering to sell, importing or otherwise commercializing our product candidates is dependent upon the extent to which we have rights under valid and we may be unable to protect our rights to, or use of, our technology.
Some or all of our patent applications may not result in the issue ofenforceable patents or the claims of any issued patents may not afford meaningful protection for our technologies or products. In addition, patents issued to us or our licensors, if any, may be challenged and subsequently narrowed, invalidated, found unenforceable or circumvented. Patent litigation is widespread in the biotechnology industry and could harm our business. Litigation might be necessary to protect our patent position. Patentability, invalidity, freedom-to-operate or other opinions may be required to determine the scope and validity of third-party proprietary rights. If we choose to go to court to stop a third party from using the inventions protected by our patent,trade secrets that third party would have the right to ask the court to rule that such patents are invalid and/or should not be enforced against that third party. These lawsuits are expensive and we may not have the required resources to pursue such litigation or to protect our patent rights. In addition, there is a risk that the court will decide that our patents are not valid or that we cannot stop the other party from using their inventions. There is also the risk that, even if the validity ofcover these patents is upheld, the court will find that the third party’s activities do not infringe our rights in these patents.
Furthermore, a third party may claim that we are infringing the third party’s patent rights and may go to court to stop us from engaging in its normal operations and activities, including making or selling our products or product candidates. These lawsuits are costly and could affect our results of operations and divert the attention of managerial and technical personnel. There is a risk that a court would decide that we are infringing the third party’s patents and would order us to stop the activities covered by the patents. In addition, there is a risk that a court will order us to pay the other party’s treble damages or attorneys’ fees for having violated the other party’s patents. The biotechnology industry has produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform.activities. If we are suedunable to secure and maintain patent protection for patent infringement,any product or technology we would need to demonstrate that our productsdevelop, or methods of use either do not infringeif the claimsscope of the relevant patent and/protection secured is not sufficiently broad, our competitors could develop and commercialize products and technology similar or that the third-party patent claims are invalid,identical to ours, and our ability to commercialize any product candidates we may develop may be adversely affected.
| 46 |
The patenting process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. In addition, we may not pursue or obtain patent protection in all relevant markets. It is also possible that we will fail to identify patentable aspects of our research and development activities before it is too late to obtain patent protection. Moreover, in some circumstances, we may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology that we may license from or license to third parties and may be reliant on our licensors or licensees to do this. Proving invalidityso. Our pending and future patent applications may not result in issued patents. Even if patent applications we license or own currently or in the United Sates is difficult since it requiresfuture issue as patents, they may not issue in a showingform that will provide us with adequate protection, prevent competitors or other third parties from competing with us, or otherwise provide us with any competitive advantage. Any patents that we hold or in-license may be challenged, narrowed, circumvented or invalidated by third parties. Consequently, we do not know whether any of clearour platform advances and convincing evidence to overcome the presumption of validity enjoyedproduct candidates will be protectable or remain protected by issuedvalid and enforceable patents.
In addition, changesour existing patents and any future patents we obtain may not provide an adequate scope of protection or otherwise may not be enforceable to prevent others from using our technology or from developing competing products and technologies.
We may not be able to adequately protect or enforce our intellectual property rights, which could harm our competitive position.
Our success and future revenue growth will depend, in eitherpart, on our ability to protect our intellectual property. We will primarily rely on patent, copyright, trademark and trade secret laws, as well as nondisclosure agreements and other methods, to protect our proprietary technologies or processes. It is possible that competitors or other unauthorized third parties may obtain, copy, use or disclose proprietary technologies and processes, despite efforts by us to protect our proprietary technologies and processes. While we hold rights in interpretations ofseveral patents, there can be no assurances that any additional patents will be issued, or additional rights will be granted, to us. Even if new patents are issued, the claims allowed may not be sufficiently broad to adequately protect our technology and processes. Our competitors may also be able to develop similar technology independently or design around the patents to which we have rights.
Currently, MyMD has 16 issued U.S. patents, 63 foreign patents, three pending U.S. patent lawsapplications, and 10 foreign patent applications pending in such jurisdictions as Australia, Canada, China, European Union, Israel, Japan and South Korea, which if issued are expected to expire between 2036 and 2041. Although we expect to obtain additional patents and in-licenses in the United Statesfuture, there is no guarantee that we will be able to successfully obtain such patents or in-licenses in a timely manner or at all. Further, any of our rights to existing patents, and otherany future patents issued to us, may be challenged, invalidated or circumvented. As such, any rights granted under these patents may not provide us with meaningful protection. Even if foreign patents are granted, effective enforcement in foreign countries may materially diminishnot be available. If our patents or rights to patents do not adequately protect our technology or processes, competitors may be able to offer products similar to our products.
Our potential strategy of obtaining rights to key technologies through in-licenses may not be successful.
The future growth of our business may depend in part on our ability to in-license or otherwise acquire the valuerights to additional product candidates and technologies. We cannot assure that we will be able to in-license or acquire the rights to any product candidates or technologies from third parties on acceptable terms or at all.
For example, our agreements with certain of our third-party research partners provide that improvements developed in the course of our relationship with a given partner may be owned solely by either us or our third-party research partner, or jointly between us and the third party. If we determine that exclusive rights to such improvements owned solely by a research partner or other third party with whom we collaborate are necessary to commercialize our drug candidates or maintain our competitive advantage, we may need to obtain an exclusive license from such third party in order to use the improvements and continue developing, manufacturing or marketing our drug candidates. We may not be able to obtain such a license on an exclusive basis, on commercially reasonable terms, or at all, which could prevent us from commercializing our drug candidates or allow our competitors or others the opportunity to access technology that is important to our business. We also may need the cooperation of any co-owners of our intellectual property in order to enforce such intellectual property against third parties, and such cooperation may not be provided to us.
In addition, the in-licensing and acquisition of these technologies is a highly competitive area, and a number of more established companies are also pursuing strategies to license or narrowacquire product candidates or technologies that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to license rights to us. Furthermore, we may be unable to identify suitable product candidates or technologies within our area of focus. If we are unable to successfully obtain rights to suitable product candidates or technologies, our business and prospects could be materially and adversely affected.
| 47 |
If we are unable to protect the scopeconfidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to patent protection.protection, we rely upon know-how and trade secret protection, as well as non-disclosure agreements and invention assignment agreements with our employees, consultants and third-parties, to protect our confidential and proprietary information, especially where we do not believe patent protection is appropriate or obtainable.
It is our policy to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information concerning our business or financial affairs developed or made known to the individual or entity during the course of the party’s relationship with us is to be kept confidential and not disclosed to third parties, except in certain specified circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual, and that are related to our current or planned business or research and development or made during normal working hours, on our premises or using our equipment or proprietary information (or as otherwise permitted by applicable law), are our exclusive property. In the case of consultants and other third parties, the agreements provide that all inventions conceived in connection with the services provided are our exclusive property. However, we cannot guarantee that we have entered into such agreements with each party that may have or have had access to our trade secrets or proprietary technology and processes. We have also adopted policies and conduct training that provides guidance on our expectations, and our advice for best practices, in protecting our trade secrets. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches.
In addition to contractual measures, we try to protect the confidential nature of our proprietary information through other appropriate precautions, such as physical and technological security measures. However, trade secrets and know-how can be difficult to protect. These measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and any recourse we might take against this type of misconduct may not provide an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, trade secrets may be independently developed by others in a manner that could prevent us from receiving legal recourse. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, such as through a data breach, or if any of that information was independently developed by a competitor, our competitive position could be harmed. Additionally, certain trade secret and proprietary information may be required to be disclosed in submissions to regulatory authorities. If such authorities do not maintain the confidential basis of such information or disclose it as part of the basis of regulatory approval, our competitive position could be adversely affected.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we have no knowledge of any claims against us, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. To date, none of our employees have been subject to such claims.
Third-party claims of intellectual property infringement may prevent, delay or otherwise interfere with our product discovery and development efforts.
Our commercial success depends in part on our ability to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing, misappropriating or otherwise violating the intellectual property or other proprietary rights of third parties. There is a substantial amount of litigation involving patents and other intellectual property rights in the biotechnology and pharmaceutical industries, as well as administrative proceedings for challenging patents, including interference, derivation, inter partes review, post grant review, and reexamination proceedings before the United States Patent and Trademark Office (“USPTO”) or oppositions and other comparable proceedings in foreign jurisdictions. We may be exposed to, or threatened with, future litigation by third parties having patent or other intellectual property rights alleging that our product candidates and/or proprietary technologies infringe, misappropriate or otherwise violate their intellectual property rights. Numerous U.S. and foreign issued patents and pending patent applications that are owned by third parties exist in the fields in which we are developing our product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may give rise to claims of infringement of the patent rights of others. Moreover, it is not always clear to industry participants, including us, which patents cover various types of drugs, products or their methods of use or manufacture. Thus, because of the large number of patents issued and patent applications filed in our field, third parties may allege they have patent rights encompassing our product candidates, technologies or methods.
| 48 |
If a third party claims that we infringe, misappropriate or otherwise violate its intellectual property rights, we may face a number of issues, including, but not limited to:
| ● | infringement and other intellectual property claims that, regardless of merit, may be expensive and time-consuming to litigate and may divert our management’s attention from our core business; | |
| ● | substantial damages for infringement, which we may have to pay if a court decides that the product candidate or technology at issue infringes on or violates the third party’s rights, and, if the court finds that the infringement was willful, we could be ordered to pay treble damages plus the patent owner’s attorneys’ fees; | |
| ● | a court prohibiting us from developing, manufacturing, marketing, selling or importing our product candidates, or from using our proprietary technologies, unless the third-party licenses its product rights or proprietary technology to us, which it is not required to do in the U.S. and certain other countries, on commercially reasonable terms or at all; | |
| ● | if a license is available from a third party, we may have to pay substantial royalties, upfront fees and other amounts, and/or grant cross-licenses to intellectual property rights for our product candidates; | |
| ● | the requirement that we redesign our product candidates or processes so they do not infringe, which may not be possible or may require substantial monetary expenditures and time; and | |
| ● | there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our Common Stock. |
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations or could otherwise have a material adverse effect on our business, financial condition, results of operations and prospects.
Third parties may assert that we are employing their proprietary technology without authorization, including by enforcing its patents against us by filing a patent infringement lawsuit against us. In this regard, patents issued in the U.S. by law enjoy a presumption of validity that can be rebutted only with evidence that is “clear and convincing,” a heightened standard of proof.
We may not have identified all patents, published applications or published literature that affect our business by blocking our ability to commercialize our products, by preventing the patentability of one or more aspects of our products to us or our licensors, or by covering the same or similar technologies that may affect our ability to market our products. For example, we (or the licensor of a product to us) may not have conducted a patent clearance search sufficient to identify potentially obstructing third party patent rights. Moreover, patent applications in the United States are maintained in confidence for up to 18 months after their filing. In some cases, however, patent applications remain confidential in the U.S. Patent and Trademark Office (the “USPTO”), for the entire time prior to issuance as a U.S. patent. Patent applications filed in countries outside of the United States are not typically published until at least 18 months from their first filing date. Similarly, publication of discoveries in the scientific or patent literature often lags behind actual discoveries. We cannot be certain that we or our licensors were the first to invent, or the first to file, patent applications covering our products. We also may not know if our competitors filed patent applications for technology covered by our pending applications or if we were the first to invent the technology that is the subject of our patent applications. Competitors may have filed patent applications or received patents and may obtain additional patents and proprietary rights that block or compete with our patents.
Therefore, there may be third-party patents of which we are currently unaware with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our product candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents.
If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of our product candidates, or materials used in or formed during the manufacturing process, or any final product itself, the holders of those patents may be able to block our ability to commercialize our product candidates unless we obtain a license under the applicable patents, or until those patents were to expire or those patents are finally determined to be invalid or unenforceable. Similarly, if any third-party patent were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, including combination therapy or patient selection methods, the holders of that patent may be able to block our ability to develop and commercialize a product candidate unless we obtain a license or until such patent expires or is finally determined to be invalid or unenforceable. In either case, a license may not be available on commercially reasonable terms, or at all, particularly if such patent is owned or controlled by one of our primary competitors. If we are unable to obtain a necessary license to a third-party patent on commercially reasonable terms, or at all, our ability to commercialize our product candidates may be impaired or delayed, which could significantly harm our business. Even if we obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Parties making claims against us may seek and obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee time and resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure. We cannot predict whether any license of this nature would be available at all or whether it would be available on commercially reasonable terms. Furthermore, even in the absence of litigation, we may need to obtain licenses from third parties to advance our research or allow commercialization of our product candidates and we may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize our product candidates, which could significantly harm our business.
| 49 |
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful and could result in a finding that such patents are unenforceable or invalid.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that one or more of our patents is not valid, is unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question.
In patent litigation in the U.S., defendant counterclaims alleging invalidity and/or unenforceability are commonplace, and there are numerous grounds upon which a third party can assert invalidity or unenforceability of a patent. Third parties may also raise similar claims before administrative bodies in the U.S. or abroad, even outside the context of litigation. These types of mechanisms include re-examination, post-grant review, inter partes review, interference proceedings, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). These types of proceedings could result in revocation or amendment to our patents such that they no longer cover our product candidates. The outcome for any particular patent following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we, our patent counsel and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, or if we are otherwise unable to adequately protect our rights, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Defense of these types of claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business.
Conversely, we may choose to challenge the patentability of claims in a third party’s U.S. patent by requesting that the USPTO review the patent claims in re-examination, post-grant review, inter partes review, interference proceedings, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings), or we may choose to challenge a third party’s patent in patent opposition proceedings in the Canadian Intellectual Property Office (“CIPO”) the European Patent Office (“EPO”) or another foreign patent office. Even if successful, the costs of these opposition proceedings could be substantial, and may consume our time or other resources. If we fail to obtain a favorable result at the USPTO, CIPO, EPO or other patent office then we may be exposed to litigation by a third party alleging that the patent may be infringed by our product candidates or proprietary technologies.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our former employees may wrongfully use or disclose our trade secrets.
confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to patent protection, we rely heavily upon know-howbe negative, that perception could have a substantial adverse effect on the price of our Common Stock. Any of the foregoing could have a material adverse effect on our business financial condition, results of operations and trade secret protection, as well as non-disclosure agreementsprospects.
We have limited foreign intellectual property rights and invention assignment agreements with our employees, consultants, and third parties,may not be able to protect our confidentialintellectual property rights throughout the world.
We currently have limited intellectual property rights outside the U.S. Filing, prosecuting and proprietary information, especiallydefending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the U.S. can be less extensive than those in the U.S. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the U.S. For example, patents covering therapeutic methods of treating humans are not available in many foreign countries. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the U.S., or from selling or importing products made using our inventions in and into the U.S. or other jurisdictions. Competitors may use our technologies in jurisdictions where we do not believehave or have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection but where enforcement is appropriatenot as strong as that in the U.S. These products may compete with our product candidates in jurisdictions where we do not have any issued patents and our patent claims or obtainable. In additionother intellectual property rights may not be effective or sufficient to contractual measures, we tryprevent them from competing.
| 50 |
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal and political systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to protectbiopharmaceutical products, which could make it difficult for us to stop the confidential natureinfringement of our patents or marketing of competing products against third parties in violation of our proprietary information using physicalrights generally. The initiation of proceedings by third parties to challenge the scope or validity of our patent rights in foreign jurisdictions could result in substantial cost and technological security measures. Such measuresdivert our efforts and attention from other aspects of our business. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could be impossible or impractical due to sanctions or trade disputes between countries, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign patent agencies also require compliance with a number of procedural, documentary, fee payment and other provisions during the patent application process and following the issuance of a patent. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable laws and rules, there are situations in which noncompliance can result in irrevocable abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. Were a noncompliance event to occur, our competitors might be able to enter the market, which would have a material adverse effect on our business financial condition, results of operations and prospects.
Changes in patent law in the U.S. and in non-U.S. jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other pharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the pharmaceutical industry involves both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain.
Past or future patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. For example, in March 2013, under the caseLeahy-Smith America Invents Act (“America Invents Act”), the U.S. moved from a “first to invent” to a “first-inventor-to-file” patent system. Under our “first-inventor-to-file” system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to a patent on the invention regardless of misappropriationwhether another inventor had made the invention earlier. The America Invents Act includes a number of other significant changes to U.S. patent law, including provisions that affect the way patent applications are prosecuted, redefine prior art and establish a new post-grant review system. The effects of these changes continue to evolve as the USPTO continues to promulgate new regulations and procedures in connection with the America Invents Act and many of the substantive changes to patent law, including the “first-inventor-to-file” provisions, only became effective in March 2013. In addition, the courts have yet to address many of these provisions and the applicability of the act and new regulations on the specific patents discussed in this filing have not been determined and would need to be reviewed. Moreover, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Recent cases by the U.S. Supreme Court have held that certain methods of treatment or diagnosis are not patent-eligible. U.S. law regarding patent-eligibility continues to evolve. While we do not believe that any of our patents will be found invalid based on these changes to US patent law, we cannot predict how future decisions by the courts, the U.S. Congress or the USPTO may impact the value of our patents. Any similar adverse changes in the patent laws of other jurisdictions could also have a material adverse effect on our business, financial condition, results of operations and prospects.
| 51 |
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the U.S., if all maintenance fees are timely paid, the natural expiration of a trade secret by an employee, former employee, consultant, former consultantpatent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting our product candidates might expire before or third party with authorized access, provide adequate protection forshortly after our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secretspartners commercialize those candidates. As a result, our owned and providing them to a competitor, and recourse we take against such misconductlicensed patent portfolio may not provide an adequate remedyus with sufficient rights to protectexclude others from commercializing products similar or identical to ours.
If we do not obtain patent term extension for any product candidates we may develop, our interests fully. Enforcingbusiness may be materially harmed.
Depending upon the timing, duration and specifics of any FDA marketing approval of any product candidates we may develop, one or more of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, (the “Hatch-Waxman Amendments”). The Hatch-Waxman Amendments permit a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming,patent extension term of up to five years as compensation for patent term lost during clinical trials and the outcome is unpredictable. In addition, trade secretsFDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent per product may be independently developedextended and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. U.S. and ex-U.S. law concerning patent term extensions and foreign equivalents continue to evolve. Even if we were to seek a patent term extension, it may not be granted because of, for example, the failure to exercise due diligence during the testing phase or regulatory review process, the failure to apply within applicable deadlines, the failure to apply prior to expiration of relevant patents, or any other failure to satisfy applicable requirements. Moreover, the applicable time period of extension or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration sooner than expected, and our business, financial condition, results of operations and prospects could be materially harmed.
Risks Related to Our Series F Preferred Stock
Holders of our Series F Preferred Stock are entitled to certain payments under the Certificate of Designation that may be paid in cash or in shares of Common Stock depending on the circumstances. If we make these payments in cash, it may require the expenditure of a substantial portion of our cash resources. If we make these payments in Common Stock, it may result in substantial dilution to the holders of our Common Stock.
Under the Certificate of Designations (the “Certificate of Designation”) of our Series F Convertible Preferred Stock (“Series F Preferred Stock”), we are required to redeem the shares of Series F Preferred Stock in 12 equal monthly installments, commencing on July 1, 2023. Holders of our Series F Preferred Stock are also entitled to receive dividends, payable in arrears monthly, and dividends payable on installment dates shall be paid as part of the applicable installment amount. Installment amounts are payable, at the company’s election, in shares of Common Stock or, subject to certain limitations, in cash. Installment amounts paid in cash must be paid in the amount of 105% of the applicable payment amount due. For an installment amounts paid in shares of Common Stock, the number of shares of Common Stock shall be calculated by othersdividing the applicable payment amount due by the “installment conversion price.” The installment conversion price shall be equal to the lower of (i) the Conversion Price (as defined in the Certificate of Designation) in effect as of the applicable payment date and (ii) the greater of (A) 80% of the average of the three lowest closing prices of our Common Stock during the thirty trading day period immediately prior to the date the payment is due or (B) $6.60 (subject to adjustment for stock splits, stock dividends, stock combinations, recapitalizations or other similar events) or, in any case, such lower amount as permitted, from time to time, by the Nasdaq Stock Market.
| 52 |
Our ability to make payments due to the holders of our Series F Preferred Stock using shares of Common Stock is subject to certain limitations set forth in the Certificate of Designation. If we are unable to make installment payments in shares of Common Stock, we may be forced to make such payments in cash. If we do not have sufficient cash resources to make these payments, we may need to raise additional equity or debt capital, and we cannot provide any assurance that we will be successful in doing so. If are unable to raise sufficient capital to meet our payment obligations, we may need to delay, reduce or eliminate certain research and development programs or other operations, sell some or all of our assets or merge with another entity.
Our ability to make payments due to the holders of our Series F Preferred Stock using cash is also limited by the amount of cash we have on hand at the time such payments are due as well as certain provisions of the Delaware General Corporation Law (the “DGCL”). Further, we intend to make the installment payments due to holders of Series F Preferred Stock in the form of Common Stock to the extent allowed under the Certificate of Designation and applicable law in order to preserve our cash resources. The issuance of shares of Common Stock to the holders of our Series F Preferred Stock with increase the number of shares of Common Stock outstanding and could result in substantial dilution to the existing holders of our Common Stock.
The Certificate of Designation for the Series F Preferred Stock and the warrants issued concurrently therewith contain anti-dilution provisions that may result in the reduction of the conversion price of the Series F Preferred Stock or the exercise price of such warrants in the future. These features may increase the number of shares of Common Stock being issuable upon conversion of the Series F Preferred Stock or upon the exercise of the warrants.
The Certificate of Designation and the warrants issued concurrently with the Series F Preferred Stock (the “February 2023 Warrants”) contain anti-dilution provisions, which provisions require the lowering of the applicable conversion price or exercise, as then in effect, to the purchase price of equity or equity-linked securities issued in subsequent offerings. If in the future, while any of our Series F Preferred Stock or February 2023 Warrants are outstanding, we issue securities for a consideration per share of Common Stock (the “New Issuance Price”) that is less than the Conversion Price of our Series F Preferred Stock or the exercise price of the February 2023 Warrants, as then in effect, we will be required, subject to certain limitations and adjustments as provided in the Certificate of Designation or the February 2023 Warrants, to reduce the Conversion Price or the exercise price to be equal to the New Issuance Price, which will result in a mannergreater number of shares of Common Stock being issuable upon conversion or exercise, as applicable, which in turn will increase the dilutive effect of such conversion or exercise on existing holders of our Common Stock. It is possible that could prevent legal recoursewe will not have a sufficient number of shares available to satisfy the conversion of the Series F Preferred Stock or the exercise of the February 2023 Warrants if we enter into a future transaction that reduces the applicable Conversion Price or exercise price. If we do not have a sufficient number of available shares for any Series F Preferred Stock conversions or February 2023 Warrant exercises, we may need to seek shareholder approval to increase the number of authorized shares of our Common Stock, which may not be possible and will be time consuming and expensive. The potential for such additional issuances may depress the price of our Common Stock regardless of our business performance and may make it difficult for us to raise additional equity capital while any of our Series F Preferred Stock or February 2023 Warrants are outstanding.
Under the February 2023 Securities Purchase Agreement we are subject to certain restrictive covenants that may make it difficult to procure additional financing.
The Securities Purchase Agreement pursuant to which we issued the Series F Preferred Stock (“February 2023 SPA”) contains the following restrictive covenants: (i) until all of the February 2023 Warrants are exercised, we agreed not to enter into any variable rate transactions; (ii) for approximately ten months after the execution of the February 2023 SPA, we agreed not to issue or sell any equity security or convertible security, subject to certain exceptions; and (iii) we agreed to offer to the investors party to the February 2023 SPA, until the later of no Series F Preferred Shares being outstanding and the maturity date of the Series F Preferred Shares, the opportunity to participate in any subsequent securities offerings by us. If anywe require additional funding while these restrictive covenants remain in effect, we may be unable to effect a financing transaction while remaining in compliance with the terms of the February 2023 SPA, or we may be forced to seek a waiver from the investors party to the February 2023 SPA.
| 53 |
General Risk Factors
Offers or availability for sale of a substantial number of shares of our confidentialCommon Stock may cause the price of our Common Stock to decline.
Sales of a significant number of shares of our Common Stock in the public market could harm the market prices of our Common Stock and make it more difficult for us to raise funds through future offerings of Common Stock or proprietary information,other securities. Our stockholders and the holders of our options and warrants may sell substantial amounts of our Common Stock in the public market. In addition, we may be required to issue shares of Common Stock to the holders of our Series F Preferred Stock upon conversion of shares of our Series F Preferred Stock and the payment of the dividends thereunder in Common Stock as a result of the full ratchet anti-dilution price protection in the Certificate of Designation if the effective Common Stock purchase price in a subsequent offering is less than the then current Series F Preferred Stock conversion price, which in turn will increase the number of shares of Common Stock available for sale. See “Risk Factors—Risks Related to Our Series F Preferred Stock—The Certificate of Designation for the Series F Preferred Stock and the warrants issued concurrently contain anti-dilution provisions that may result in the reduction of the conversion price of the Series F Preferred Stock or the exercise price of such aswarrants in the future. These features may increase the number of shares of Common Stock being issuable upon conversion of the Series F Preferred Stock or upon the exercise of the warrants.”
In addition, the fact that our trade secrets, werestockholders can sell substantial amounts of our Common Stock in the public market, whether or not sales have occurred or are occurring, could make it more difficult for us to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate, or at all.
An active trading market for our Common Stock may not be disclosedsustained.
The listing of our Common Stock on The Nasdaq Capital Market (“Nasdaq”) does not assure that a meaningful, consistent and liquid trading market exists. An active trading market for shares of our Common Stock may not be sustained. If an active market for our Common Stock is not sustained, it may be difficult for investors to sell their shares either without depressing the market price for the shares or misappropriated, or if any such information was independently developed by a competitor, our competitive position could be harmed.at all.
We are subject to various internal control reporting requirements under the Sarbanes-Oxley Act. We can provide no assurance that we will at all times in the future be able to report that our internal controls over financial reporting are effective.
As a public company, we are required to comply with Section 404.404 (“Section 404”) of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”). In any given year, we cannot be certain as to the time of completion of our internal control evaluation, testing and remediation actions or of their impact on our operations. Upon completion of this process, we may identify control deficiencies of varying degrees of severity under applicable SEC and Public Company Accounting Oversight Board (United States)(U.S.) rules and regulations. Our management, including our chiefprincipal executive officer and chiefprincipal financial officer, does not expect that our internal controls and disclosure controls will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. In addition, the design of a control system must reflect the fact that there are resource constraints and the benefit of controls must be relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, in usour company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple errors or mistakes. Further, controls can be circumvented by individual acts of some persons, by collusion of two or more persons, or by management override of the controls. The design of any system of controls is also based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving our stated goals under all potential future conditions. Over time, a control may be inadequate because of changes in conditions, such as growth of the company or increased transaction volume, or the degree of compliance with the policies or procedures may deteriorate. Because of inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
| 54 |
In addition, as a public company, we are required to report, among other things, control deficiencies that constitute material weaknesses or changes in internal controls that, or that are reasonably likely to, materially affect internal controls over financial reporting. A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual consolidated financial statements will not be prevented or detected on a timely basis. If we fail to comply with the requirements of Section 404 or if we report a material weakness, we might be subject to regulatory sanction and investors may lose confidence in our consolidated financial statements, which may be inaccurate if we fail to remedy such material weakness.
We incur increased costs and demands on management as a result of compliance with laws and regulations applicable to public companies, which could harm our operating results.
As a public company, we incur significant legal, accounting and other expenses that we did not incur as a private company, including costs associated with public company reporting requirements. In addition, the Sarbanes-Oxley Act and the Dodd-Frank Act, as well as rules implemented by the SEC and Nasdaq, impose a number of requirements on public companies, including with respect to corporate governance practices. Our management and other personnel need to devote a substantial amount of time to these compliance and disclosure obligations. Moreover, compliance with these rules and regulations has increased our legal, accounting and financial compliance costs and has made some activities more time-consuming and costly. It is also more expensive for us to obtain director and officer liability insurance.
Risks Related to Our Financial Position and Need for Additional Capital
We expect to require additional capital in the future in order to develop the COVID-19 Vaccine Candidate. If we do not obtain any such additional financing, it may be difficultfail to complete developmentcomply with the continued listing requirements of the COVID-19 Vaccine Candidate or effectively realize our long-term strategic goals and objectives.
Our current cash resources will not be sufficient to fund the development of the COVID-19 Vaccine Candidate through all of the required clinical trials to receive regulatory approval and commercialization. While we do not currently have an estimate of all of the costs that we will incur in the development of the COVID-19 Vaccine Candidate, we anticipate that we will need to raise significant additional funds in order to continue the development of the COVID-19 Vaccine Candidate during the next 12-months. If we cannot secure this additional funding when such funds are required, we may fail to develop a COVID-19 Vaccine Candidate or be forced to forego certain strategic opportunities.
Any additional capital raised through the sale of equity or equity-backed securities may dilute our stockholders’ ownership percentages and could also result in a decrease in the market value of our equity securities.
The terms of any securities issued by us in future capital transactions may be more favorable to new investors, and may include preferences, superior voting rights and the issuance of warrants or other derivative securities, which may have a further dilutive effect on the holders of any of our securities then outstanding.
In addition, we may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we issue, such as convertible notes and warrants, which may adversely impact our financial condition.
General Risk Factors
The market price forNasdaq Capital Market, our common stock may be volatile,delisted and your investment in our common stock could decline in value.
The stock market in general has experienced extreme price and volume fluctuations. The market prices of the securities of biotechnology and specialty pharmaceutical companies, particularly companies like ours without product revenues and earnings, have been highly volatile and may continue to be highly volatile in the future. This volatility has often been unrelated to the operating performance of particular companies. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock:stock and our ability to access the capital markets could be negatively impacted.
Moreover, the COVID-19 pandemic has resulted in significant financial market volatilityOur common stock is currently listed for trading on The Nasdaq Capital Market. We must satisfy Nasdaq’s continued listing requirements, including, among other things, a minimum stockholders’ equity of $2.5 million and uncertainty in recent weeks. A continuationa minimum closing bid price of $1.00 per share or worsening of the levels of market disruption and volatility seen in the recent past couldrisk delisting, which would have ana material adverse effect on our ability to access capital, on our business, results of operations and financial condition, and on the market price of our common stock.
In the past, securities class action litigation has often been brought against companies that experience volatility in the market price of their securities. Whether or not meritorious, litigation brought against us could result in substantial costs and a diversion of management’s attention and resources, which could adversely affect our business, operating results and financial condition.
Our failure to meet the continued listing requirements of Nasdaq could result in abusiness. A delisting of our common stock.stock from The delistingNasdaq Capital Market could adversely affectmaterially reduce the market liquidity of our common stock and result in a corresponding material reduction in the price of our common stock. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, suppliers, customers and employees and fewer business development opportunities.
As previously disclosed, on October 11, 2023, we received a written notice (the “Notice”) from the Listing Qualifications Department of the Nasdaq Stock Market indicating that for the last 30 consecutive business days, the bid price for our Common Stock had closed below the minimum $1.00 per share requirement for continued listing on Nasdaq pursuant to Nasdaq Listing Rule 5550(a)(2) (the “Minimum Bid Price Requirement”). The letter also indicated that the Company will be provided with a compliance period until April 8, 2024 (the “Compliance Period”), in which to regain compliance pursuant to Nasdaq Listing Rule 5810(c)(3)(A).
Effective as of 4:05 p.m. Eastern Standard Time on February 14, 2024, we effected the Reverse Stock Split of our common stock at a ratio of one-for-thirty. Simultaneously with the Reverse Stock Split, number of shares of our common stock authorized for issuance was reduced from 500,000,000 shares to 16,666,666 shares, and our authorized capital stock was reduced from 550,000,000 shares to 66,666,666 shares. Our common stock continued to be traded on the Nasdaq Capital Market under the symbol MyMD and began trading on a split-adjusted basis at market open on February 15, 2024. On March 4, 2024, we were notified by Nasdaq that we had regained compliance with all Nasdaq listing requirements and the matter was closed.
There is no assurance that we will maintain compliance with such minimum listing requirements. If our common stock were delisted from Nasdaq, trading of our common stock would most likely take place on an over-the-counter market established for unlisted securities, such as the OTCQB or the Pink Market maintained by OTC Markets Group Inc. An investor would likely find it less convenient to sell, or to obtain accurate quotations in seeking to buy, our common stock on an over-the-counter market, and many investors would likely not buy or sell our common stock due to difficulty in accessing over-the-counter markets, policies preventing them from trading in securities not listed on a national exchange or other reasons. In addition, as a delisted security, our common stock would be subject to SEC rules as a “penny stock,” which impose additional disclosure requirements on broker-dealers. The regulations relating to penny stocks, coupled with the typically higher cost per trade to the investor of penny stocks due to factors such as broker commissions generally representing a higher percentage of the price of a penny stock than of a higher-priced stock, would further limit the ability of investors to trade in our common stock. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, suppliers, customers and employees and fewer business development opportunities. For these reasons and others, delisting would adversely affect the liquidity, trading volume and price of our common stock, causing the value of an investment in us to decrease and having an adverse effect on our business, financial condition and results of operations, including our ability to attract and retain qualified employees and to raise capital.
We may issue additional equity securities in the future, which may result in dilution to existing investors.
To the extent we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. The combined Company may, from time to time, sell additional equity securities in one or more transactions at prices and in a manner it determines. If we sell additional equity securities, existing stockholders may be materially diluted. In addition, new investors could gain rights superior to existing stockholders, such as liquidation and other preferences. In addition, the number of shares available for future grant under our equity compensation plans may be increased in the future. In addition, the exercise or conversion of outstanding options or warrants to purchase shares of capital stock may result in dilution to our stockholders upon any such exercise or conversion.
All of our outstanding shares of Common Stock are, and any shares of our Common Stock that may be issued in the future in respect of potential milestone payments, will be, freely tradable without restrictions or further registration under the Securities Act of 1933, as amended (the “Securities Act”), except for shares subject to lock-up agreements, and any shares held by affiliates, as defined in Rule 144 under the Securities Act. Rule 144 defines an affiliate as a person who directly, or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, the Company and would include persons such as our directors and executive officers and large shareholders. In turn, resales, or the perception by the market that a substantial number of resales could occur, could have the effect of depressing the market price of our common stock could decrease.Common Stock.
In addition, we may be required to issue an indeterminate number of shares of Common Stock to the holders of our Series F Preferred Stock and the February 2023 Warrants upon the conversion or exercise of either, as applicable. See “Risk Factors—Risks Related to Our common stock is listedSeries F Preferred Stock— Holders of our Series F Preferred Stock are entitled to certain payments under the Certificate of Designation that may be paid in cash or in shares of Common Stock depending on the circumstances. If we make these payments in cash, it may require the expenditure of a substantial portion of our cash resources. If we make these payments in Common Stock, it may result in substantial dilution to the holders of our Common Stock.” and “Risk Factors—Risks Related to Our Series F Preferred Stock—The Nasdaq Capital Market. In order to maintain our listing, we must meet minimum financialCertificate of Designation for the Series F Preferred Stock and other requirements, including requirements for a minimum amount of capital and a minimum price per share. We cannot assure youthe warrants issued concurrently contain anti-dilution provisions that we will continue to meet the continued listing requirementsmay result in the reduction of the conversion price of the Series F Preferred Stock or the exercise price of such warrants in the future.
If Nasdaq delists our common stock from trading on its exchange, due to failure to meet its continued listing requirements, and we are not able to list our common stock on another national securities exchange, we expect our securities could be quoted on an over-the-counter market. If this were to occur, we could face significant material adverse consequences, including:
If we sell shares of our common stock in future financings, stockholders may experience immediate dilution and, as a result, our stock price may decline.
We may from time to time issue additional sharesCommon Stock issuable upon conversion of common stock at a discount from the current market price of our common stock. As a result, our stockholders would experience immediate dilutionSeries F Preferred Stock or upon the purchaseexercise of any shares of our common stock sold at such discount. As opportunities present themselves, we may enter into financing or similar arrangements in the future, including the issuance of debt securities, preferred stock or common stock. If we issue common stock or securities convertible or exercisable into common stock, our common stockholders would experience additional dilution and, as a result, our stock price may decline.warrants.”
| 55 |
We do not anticipate paying cash dividends on our common stockCommon Stock and, accordingly, stockholders must rely on stock appreciation for any return on their investment.
We have never declared or paid cash dividends on our common stockCommon Stock and do not expect to do so in the foreseeable future. So long as any shares of Series F Preferred Stock are outstanding, as they are at this time, we are not able to declare or pay any cash dividend or distribution on any of our capital stock (other than as required by the Certificate of Designation) without the prior written consent of the Required Holders (as defined in the Certificate of Designation). The declaration of dividends is further subject to the discretion of our board of directors and limitations under applicable law, and will depend on various factors, including our operating results, financial condition, future prospects and any other factors deemed relevant our board of directors. You should not rely on an investment in us if you require dividend income from your investment in us. The success of your investment will likely depend entirely upon any future appreciation of the market price of our common stock,Common Stock, which is uncertain and unpredictable. There is no guarantee that our common stockCommon Stock will appreciate in value.
Future sales of our common stock, or the perception that future sales may occur, may cause the market price of our common stock to decline, even if our business is doing well.
Sales by our stockholders of a substantial number of shares of our common stock in the public market could occur in the future. Pursuant to the Securities Purchase Agreement for the Private Placement (the “Private Placement SPA”), we are required to file a registration statement for the resale of 9,765,933 shares of common stock issued at an offering price of $1.85 per share or, at the election of each investor, Pre-Funded Warrants, and up to 9,765,933 shares of our common stock issuable upon exercise of the Pre-Funded Warrants shortly after we file a proxy statement with the SEC in connection with the Merger. Following their registration and resale under a registration statement, such shares would become freely tradable. Sales by our stockholders of a substantial number or resales by the purchasers of such shares and shares issuable upon exercise of such warrants pursuant to a registration statement, or the perception in the market that the holders of a large number of shares of common stock may or intend to sell their shares, could reduce the market price of our common stock and make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise desire.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business, or our market, or if they change their recommendations regardingpublish negative evaluations, the price of our stock adversely, our stock price and trading volumeCommon Stock could decline.
The trading market for our common stock will be influenced byCommon Stock relies in part on the availability of research and reports that third-party industry or securitiesfinancial analysts may publish about us, our business, our marketus. There are many large, publicly traded companies active in the life sciences and biopharmaceutical industries, which may mean it will be less likely that we receive widespread analyst coverage. Furthermore, if one or our competitors. If anymore of the analysts who maydo cover us change their recommendation regardingthe Company (if any) downgrades our stock, adversely, or provide more favorable relative recommendations about our competitors, our stock price would likely decline. If any analyst who may cover us were toone or more of these analysts cease coverage of us or fail to regularly publish reports on us,the Company, we could lose visibility in the financial markets,market, which in turn could cause our stock price or trading volume to decline. Additionally, if securities analysts publish negative evaluations of competitors in the life sciences and biopharmaceutical industries, the comparative effect could cause our stock price to decline.
Anti-takeover provisions of our certificate of incorporation, our bylaws and Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove the current members of our board and management.
Certain provisions of our certificate of incorporation and bylaws could discourage, delay or prevent a merger, acquisition or other change of control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove members of our board of directors. These provisions also could limit the price that investors might be willing to pay in the future for our securities, thereby depressing the market price of our securities. Stockholders who wish to participate in these transactions may not have the opportunity to do so. These provisions, among other things:
| ● | allow the authorized number of directors to be changed only by resolution of our board of directors; | |
| ● | authorize our board of directors to issue, without stockholder approval, preferred stock, the rights of which will be determined at the discretion of the board of directors and that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that our board of directors does not approve; | |
| ● | establish advance notice requirements for stockholder nominations to our board of directors or for stockholder proposals that can be acted on at stockholder meetings; and | |
| ● | limit who may call a stockholder meeting. |
In addition, we are governed by the provisions of Section 203 of the Delaware General Corporation Law that may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of the voting rights on our common stock, from merging or combining with us for a prescribed period of time.
We are currentlyhave been subject to a number of securities litigations, and we may be subject to similar or other litigation in the future.
We are currentlyhave been subject to a number of litigations as described elsewhere in these “Risk Factors”this Annual Report on Form 10-K and in Note 108 to our consolidated financial statements. In connection with certain of these litigations, we have entered into settlements of claims for significant monetary damages. We may also be subject to judgements or enter into additional settlements of claims for significant monetary damages for the securities litigations that we have yet to enter into settlement agreements. Defending against the current litigations is or can be time-consuming, expensive and cause diversion of our management’s attention.
Companies that have experienced volatility in the market price of their stock have frequently been the objects of securities class action litigation. We may be the target of this type of litigation in the future. Class action and derivative lawsuits could result in substantial costs to us and cause a diversion of our management’s attention and resources, which could materially harm our financial condition and results of operations.
With respect to any litigation, our insurance may not reimburse us, or may not be sufficient to reimburse us, for the expenses or losses we may suffer in contesting and concluding such lawsuit. Substantial litigation costs, including the substantial self-insured retention that we are required to satisfy before any insurance applies to a claim, unreimbursed legal fees or an adverse result in any litigation may adversely impact our business, operating results or financial condition. We believe that our directors’ and officers’ liability insurance will cover our potential liability with respect to any securities class-action lawsuit; however, the insurer has reserved its rights to contest the applicability of the insurance to such claims and the limits of the insurance may be insufficient to cover any eventual liability.
| 56 |
Item 1B. Unresolved Staff CommentsComments.
Not applicable.
We operate in the biotechnology sector, which is subject to various cybersecurity risks that could adversely affect our business, financial condition, and results of operations, including intellectual property theft; fraud; extortion; harm to employees or customers; violation of privacy laws and other litigation and legal risk; and reputational risk. We recognize the importance of assessing, identifying, and managing material risks associated with cybersecurity threats. Both our executive management team and our board of directors are involved in the assessment, identification, and management of such risks, including prevention, mitigation, detection, and remediation of cybersecurity incidents.
Our executive management team is responsible for day-to-day assessment, identification and management of material risks from cybersecurity threats, including the prevention, mitigation, detection, and remediation of cybersecurity incidents. The executive management team monitors current events in order to remain aware of current cybersecurity threats and is informed of cybersecurity incidents as they arise by our frontline personnel.
Our board of directors is responsible for oversight of risks from cybersecurity threats in conjunction with our executive management team. Our board of directors receives updates from our management team with respect to risks from cybersecurity threats and are notified of any new significant cybersecurity threats or incidents as they arise. Additionally, our board of directors considers risks from cybersecurity threats as part of its overall assessment of risk management, including its general oversight of the Company’s business strategy, risk management policies, and financials.
To date, no cybersecurity incident (or aggregation of incidents) or cybersecurity threat has materially affected our business strategy, results of operations or financial condition, and we are not aware of any cybersecurity incidents that are reasonably likely to materially affect the Company, including our business strategy, results of operations, or financial condition. For further information regarding the risks associated with cybersecurity incidents, see “Risk Factors—Our business and operations would suffer in the event of computer system failures, cyber-attacks or deficiencies in our cyber-security or those of third-party providers” in Item 1A of this Annual Report on Form 10-K.
Item 2. PropertyProperties.
The company utilizes a virtualleases as its corporate headquarters an office facility as our corporate headquarters which is located at 1185 Avenue of the Americas, 3rd Floor, New York, New York 10036.855 North Wolfe Street, Suite 601, Baltimore, Maryland 20215. The current lease term is effective foras amended has a six-monthtwelve-month term beginning on January 13, 2021,December 1, 2022, which term shall automatically renew monthly thereafter until termination by either party with aupon 60 days’ notice. The monthly rent is approximately $4,532 and will increase 3% on each anniversary of approximately $125.00.the December 1, 2022 effective date.
We believe our current facilities are sufficient and adequate for our current needs.
From time to time we are a party to litigation and subject to claims incident to the ordinary course of business. Future litigation may be necessary to defend ourselves and our customers by determining the scope, enforceability, and validity of third-party proprietary rights or to establish our proprietary rights. For a discussion of material legal proceedings affecting us as of December 31, 2020,2023, please read Note 108 to the consolidated financial statements under “Litigation and Settlements,” which information is incorporated herein by reference.
Item 4. Mine Safety DisclosuresDisclosures.
Not Applicable.
Not Applicable.
| 57 |
17 Akers to confirm whether this document was filed and resolution was reached.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stockCommon Stock began trading on the NASDAQNasdaq Capital Market under the symbol “AKER” on January 23, 2014. On April 19, 2021, the symbol for our Common Stock changed to “MYMD.”
Holders
As of February 26, 2020,March 29, 2024, there were approximately 755700 holders of record of our common stock.Common Stock.
Dividends
Except as described herein, we have never paid any cash or other dividends to our stockholders and we do not plan to declare or pay any cash or other dividends in the foreseeable future. On or around September 9, 2020, our Board declared a dividend of one preferred share purchase right for each share of our common stockCommon Stock outstanding held by stockholders of record on September 21, 2020. We currently intend to retain earnings, if any, for use in the operation and expansion of our business. Subject to the foregoing, the payment of cash dividends in the future, if any, will be at the discretion of our Board and will depend on such factors as earning levels, contractual restrictions, capital requirements, our overall financial condition and any other factors deemed relevant by the Board.
Unregistered Sales of Securities
None.
Issuer Purchases of Equity Securities
We did not repurchase any of our equity securities during the fourth quarter of the fiscal year ended December 31, 2020.2023.
Item 6. Selected Financial Data[Reserved]
Not Applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The information set forth below should be read in conjunction with our consolidated financial statements and related notes thereto included elsewhere in this Annual Report on Form 10-K. This discussion and analysis contains forward-looking statements based on our current expectations, assumptions, estimates and projections. These forward-looking statements involve risks and uncertainties. Our actual results could differ materially from those indicated in these forward-looking statements as a result of certain factors, including those discussed in Item 1 of this Annual Report on Form 10-K, entitled “Business,” under “Forward-Looking Statements” and Item 1A of this Annual Report on Form 10-K, entitled “Risk Factors.” References in this discussion and analysis to “us,” “we,” “our,” or “the Company” refer collectively to Akers Biosciences,MyMD Pharmaceuticals, Inc.
Our financial statements are prepared in accordance with GAAP. These accounting principles require us to make certain estimates, judgments and assumptions. We believe that the estimates, judgments and assumptions upon which we rely are reasonable based upon information available to us at the time that these estimates, judgments and assumptions are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities as of the date of the financial statements as well as the reported amounts of revenues and expenses during the periods presented. Our financial statements would be affected to the extent there are material differences between these estimates and actual results. In many cases, the accounting treatment of a particular transaction is specifically dictated by GAAP and does not require management’s judgment in its application. There are also areas in which management’s judgment in selecting any available alternative would not produce a materially different result. The following discussion should be read in conjunction with our financial statements and notes thereto appearing elsewhere in this Annual Report on Form 10-K.
| 58 |
Overview
We were historically a developerThis annual report on Form 10-K and other reports filed by the Company from time to time with the Securities and Exchange Commission (the “SEC” and such reports, collectively, the “Filings”) contain or may contain forward-looking statements and information that are based upon beliefs of, rapid healthand information technologies but since March 2020, have been primarily focused on the development of a vaccine candidate against COVID-19. In responsecurrently available to, the global pandemic,Company’s management as well as estimates and assumptions made by Company’s management. Readers are cautioned not to place undue reliance on these forward-looking statements, which are only predictions and speak only as of the date hereof. When used in the Filings, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions as they relate to the Company or the Company’s management identify forward-looking statements. Such statements reflect the current view of the Company with respect to future events and are subject to risks, uncertainties, assumptions, and other factors, including the risks relating to the Company’s business, industry, and the Company’s operations and results of operations. Should one or more of these risks or uncertainties materialize, or should the underlying assumptions prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended, or planned.
Although we believe that the expectations reflected in the forward-looking statements are pursuing rapid developmentreasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results.
Important factors that could cause actual results to differ materially from the results and manufacturing of our COVID-19 Vaccine Candidate, in collaboration with Premas.events anticipated or implied by such forward-looking statements include, but are not limited to:
| ● | fluctuation and volatility in market price of our Common Stock due to market and industry factors, as well as general economic, political and market conditions; | |
| ● | the impact of dilution on our shareholders; | |
| ● | our ability to realize the intended benefits of the Merger (as defined below) and the Contribution Transaction (as defined below); | |
| ● | the impact of our ability to realize the anticipated tax impact of the Merger; | |
| ● | the outcome of litigation or other proceedings we may become subject to in the future; | |
| ● | delisting of our Common Stock from the Nasdaq; | |
| ● | our availability and ability to continue to obtain sufficient funding to conduct planned research and development efforts and realize potential profits; | |
| ● | our ability to develop and commercialize our product candidates, including MYMD-1, Supera-CBD and other future product candidates; | |
| ● | the impact of the complexity of the regulatory landscape on our ability to seek and obtain regulatory approval for our product candidates, both within and outside of the U.S.; | |
| ● | the required investment of substantial time, resources and effort for successful clinical development and marketization of our product candidates; | |
| ● | challenges we may face with maintaining regulatory approval, if achieved; | |
| ● | the potential impact of changes in the legal and regulatory landscape, both within and outside of the U.S.; | |
| ● | the impact of pandemics, such as COVID-19, on the administration, funding and policies of regulatory authorities, both within and outside of the U.S.; | |
| ● | our dependence on third parties to conduct pre-clinical and clinical trials and manufacture its product candidates; | |
| ● | the impact of the pandemics, such as COVID-19, on our results of operations, business plan and the global economy; | |
| ● | challenges we may face with respect to our product candidates achieving market acceptance by providers, patients, patient advocacy groups, third party payors and the general medical community; | |
| ● | the impact of pricing, insurance coverage and reimbursement status of our product candidates; | |
| ● | emerging competition and rapidly advancing technology in our industry; | |
| ● | our ability to obtain, maintain and protect our trade secrets or other proprietary rights, operate without infringing upon the proprietary rights of others and prevent others from infringing on its proprietary rights; | |
| ● | our ability to maintain adequate cyber security and information systems; | |
| ● | our ability to achieve the expected benefits and costs of the transactions related to the acquisition of Supera Pharmaceuticals, Inc. (“Supera”); | |
| ● | our ability to effectively execute and deliver our plans related to commercialization, marketing and manufacturing capabilities and strategy; | |
| ● | emerging competition and rapidly advancing technology in our industry; | |
| ● | our ability to obtain adequate financing in the future on reasonable terms, as and when we need it; | |
| ● | challenges we may face in identifying, acquiring and operating new business opportunities; | |
| ● | our ability to retain and attract senior management and other key employees; | |
| ● | our ability to quickly and effectively respond to new technological developments; | |
| ● | changes in political, economic or regulatory conditions generally and in the markets in which we operate; and | |
| ● | our compliance with all laws, rules, and regulations applicable to our business. |
| 59 |
Proposed MergerOverview
On November 11, 2020, we entered intoFollowing the Merger Agreement, pursuant to which we will acquire MYMD as a wholly owned subsidiary. Upon completionclosing of the Merger and the combined company is expectedContribution Transaction described below that occurred on April 16, 2021, we have been focused on developing and commercializing two therapeutic platforms based on well-defined therapeutic targets, MYMD-1 and Supera-CBD:
| ● | MYMD-1 is a clinical stage small molecule that regulates the immunometabolic system to treat autoimmune disease, including (but not limited to) multiple sclerosis, diabetes, rheumatoid arthritis, and inflammatory bowel disease. MYMD-1 is being developed to treat age-related illnesses such as frailty and sarcopenia. MYMD-1 works by regulating the release of numerous pro-inflammatory cytokines, such as TNF-α, interleukin 6 (“IL-6”) and interleukin 17 (“IL-17”). MYMD-1 currently is being evaluated in patients with sarcopenia (age-related muscle loss). The company has significant intellectual property coverage to protect these autoimmune indications, as well as therapy as an anti-aging product; MyMD in collaboration with its CRO is in the final stages of preparing the end of Phase II, “A double-blind, randomized, Phase 2 study to investigate the efficacy, tolerability and pharmacokinetics of MYMD1 in the treatment of participants aged 65 years or older with chronic inflammation associated with sarcopenia/frailty” for submission to the FDA. The submission is planned for the beginning of the second quarter of 2024. Exploratory analysis indicates the biomarker sTNFR1 is the most sensitive biomarker for Sarcopenia patients aged 65-75 years old. A phase II study for rheumatoid arthritis, “A double-blind, randomized, placebo-controlled multicenter Phase II proof-of-concept study to evaluate the efficacy, safety, biological activity, and pharmacokinetics of MYMD-1™ added to methotrexate in patients with moderate-to-severe active rheumatoid arthritis” IND application was reviewed and approved by the FDA to begin clinical trials on August 9, 2023. On November 17, 2023 an Annual Report was submitted to the FDA.
| |
| ● | Supera-CBD is a synthetic analog of cannabidiol (“CBD”) being developed to treat various conditions, including, but not limited to, epilepsy, pain, and anxiety/depression, through its effects on the CB2 receptor, and a monoamine oxidase enzyme (“MAO”) type B. Supera-CBD has shown tremendous promise in treating neuroinflammatory and neurodegenerative diseases, and will be a major focus as the Company moves forward. |
The rights to be renamed “MyMD Pharmaceuticals, Inc.”.
Pursuant to the Merger Agreement, upon the effectiveness of the Merger, each share of MYMD common stock issuedSupera-CBD were previously owned by Supera and outstandingwere acquired by MyMD Florida (as defined below) immediately prior to the Effective Time will convert into and become exchangeable for the number of pre-reverse stock split shares of our common stock equal to the number of shares of MYMD common stock multiplied by the Exchange Ratio. As a result of the issuance of the merger consideration and the merger, MYMD stockholders will receive an aggregate of approximately 68,035,360 shares of Akers common stock, without giving effect to the proposed reverse stock split contemplated by the Reverse Stock Split Proposal. Additionally, MYMD stockholders will be entitled to receive (i) an amount in cash, on a pro rata basis, equal to the aggregate cash proceeds received by Akers from the exercise of any options to purchase MYMD common stock assumed by Akers upon closing of the merger duringMerger.
2021 Merger and Milestone Payments
On April 16, 2021, pursuant to the Option Exercise Period, such payment to occur no later than 30 dayspreviously announced Agreement and Plan of Merger and Reorganization, dated November 11, 2020 (as subsequently amended, the “Merger Agreement”), by and among the Company, previously known as Akers Biosciences, Inc., XYZ Merger Sub, Inc., a wholly-owned subsidiary of the Company (“Merger Sub”), and MyMD Pharmaceuticals (Florida), Inc., a Florida corporation previously known as MyMD Pharmaceuticals, Inc. (“MyMD Florida”), Merger Sub was merged with and into MyMD Florida, with MyMD Florida continuing after the last daymerger as the surviving entity and a wholly owned subsidiary of the Option Exercise Period, and (ii)Company (the “Merger”). The Merger consideration included potential Milestone Paymentsmilestone payments to the pre-Merger MyMD Florida stockholders (the “Milestone Payments”) payable in shares of up to an aggregate of 68,035,360 Milestone Shares payablethe Company’s Common Stock upon the achievement of certain market capitalization milestone events (the “Milestone Events”) during the Milestone Period.
Also pursuant to36-month period immediately following the Merger Agreement, on January 15, 2020, we and MYMD filed the S-4 Registration Statement describing the Merger and other related matters. Consummation of the Merger is conditioned upon, among other things, approval of the Merger by the stockholders of Akers (including (i) approval of the Share Issuance Proposal, (ii) approval of the Reverse Stock Split Proposal, and (iii) approval of the A&R Charter Proposal, including, among other things, changing the name of the combined company to MyMD Pharmaceuticals, Inc., among others), approval of the Merger by the stockholders of MYMD, the continued listing of Akers’ common stock on The Nasdaq Capital Market after the Merger and satisfaction of a minimum cash threshold by Akers. In addition, the Merger Agreement requires that MYMD consummate the Supera Purchase. After closing of the Merger the operations of MYMD’s business will comprise substantially all of the combined company’s operations. There is no assurance when or if the Merger will be completed. Any delay in completing the Merger may substantially reduce the potential benefits that we expect to obtain from the Merger. Furthermore, the intended benefits of the Merger may not be realized.
Coronavirus(the “Milestone Period”). The Milestone Events and COVID-19 Pandemic
In December 2019, SARS-CoV-2 was reported to have surfaced in Wuhan, China, and on March 12, 2020, the WHO declared the global outbreak of COVID-19, the disease caused by SARS-CoV-2, to be a pandemic. In an effort to contain and mitigate the spread of COVID-19, many countries, including the United States, Canada, China, and India, have imposed unprecedented restrictions on travel, quarantines, and other public health safety measures. According to the WHO situation report, dated as of February 16, 2021, approximately 108.2 million cases were reported globally and 2.4 million of these were deadly, making the development of effective vaccines to prevent this disease a major global priority. Multiple vaccine candidates against SARS-CoV-2corresponding Milestone Payments are under development, and most recently, certain large, multinational pharmaceutical companies have been granted authorizations for emergency use by the FDA; however, widespread distribution of the vaccines remains limited, with the primary treatment being symptomatic and supportive therapies.
Recent Developments
Agreement and Plan of Merger and Reorganization
On November 11, 2020, the Company, Merger Sub, and MYMD, entered the Merger Agreement, pursuant to which, among other things, subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub will merge with and into MYMD, with MYMD being the surviving corporation and becoming a wholly owned subsidiary of the Company. The Merger is intended to qualify for federal income tax purposes as a tax-free reorganization under the provisions of Section 368(a) of the Internal Revenue Code of 1986, as amended. In addition, in connection with the execution of the Merger Agreement, Akers agreed to advance a bridge loan of up to $3,000,000 to MYMD pursuant to the Note.table below.
Subject to the terms and conditions of the Merger Agreement, at the Effective Time (i) each outstanding share of MYMD common stock, will be converted into the right to receive the number of shares of the Akers common stock equal to the Exchange Ratio; and (ii) each outstanding stock option of MYMD (collectively, “MYMD options”) that has not previously been exercised prior to the Effective Time, whether or not vested, will be assumed by the Company subject to certain terms contained in the Merger Agreement (including, but not limited to, the amendment of such stock option to extend the term of such stock option for a period expiring on the second-year anniversary of the Effective Time). In connection with the Merger, each holder of options is required to enter into a Lock-Up Agreement/Leak-Out Agreement with respect to the shares of Akers common stock issued upon the exercise of such option. Also, not later than 30 days after the second-year anniversary of the Effective Date, the Company will pay stockholders of MYMD on a pro rata basis an amount in cash equal to the aggregate cash proceeds received by Akers from the exercise of any MYMD options assumed by the Company prior to the second-year anniversary of the Effective Time; provided, however, the amount of such payment will not exceed the maximum amount of cash consideration that may be received by stockholders of MYMD without affecting the intended tax consequences of the Merger.
Additionally, under the terms of the Merger Agreement, the Company has agreed to pay contingent consideration to MYMD stockholders in the form of Milestone Payments. The Milestone Payments are payable in the dollar amounts set forth in the chart below upon the achievement of the milestone events set forth opposite such dollar amount during the Milestone Period as follows:
| Milestone Event | Milestone Payment | |
| Market capitalization of | $ | |
| For every | $ | |
| Market | $ | |
| For every | $ |
Each Milestone Payment will be payable in
For purposes of the table above, “market capitalization” means, with respect to any trading day, the product of (i) the total outstanding shares of common stock of Akers,the combined company Common Stock and (ii) the volume weighted average trading price for the combined company Common Stock for such trading day.
The Company previously owned, through its subsidiary Cystron Biotech, LLC (“Cystron”), an exclusive license from Premas Biotech PVT Ltd. (“Premas”) with respect to Premas’ vaccine platform for the number of Milestone Shares to be issued determined by dividing the applicable Milestone Payment amount by the volume-weighted average pricedevelopment of a share of Akers’ common stock duringvaccine against COVID-19 and other coronavirus infections. On April 16, 2021, pursuant to the 10 trading days immediately precedingContribution and Assignment Agreement, dated March 18, 2021 (the “Contribution Agreement”) by and among the achievementCompany, Cystron, Oravax Medical, Inc. (“Oravax”) and, for the limited purpose set forth therein, Premas, the Company caused Cystron to contribute substantially all of the milestone event; provided, however, thatassets associated with its business of developing and manufacturing Cystron’s COVID-19 vaccine candidate to Oravax. Oravax is pursuing the development of the COVID-19 vaccine candidate. MyMD’s interest in no event shall the priceOravax consists of a share13% of Akers commonOravax’s outstanding shares of capital stock used to determine the number of Milestone Shares to be issued be deemed to be less than $5.00 per share (as adjusted for stock splits, stock dividends, reverse stock splits, and the like occurring after the closing date).
Notwithstanding the above, the numberrights to a 2.5% royalty on all future net sales. MyMD has evaluated several options with respect to its interest in Oravax, including a potential distribution of Milestone Shares payable by Akers shall not exceed the number ofOravax shares of Akers common stock to be issued to MyMD stockholders at the Effective Time in connection with the Merger (as described in the following paragraph).
Under the exchange ratio formula in the Merger Agreement, and immediately upon the closing of the Merger, the former MYMD securityholders are expected to own approximately 80% of the aggregate number of shares of Akers common stock issued and outstanding immediately following the consummation of the Merger (the “Post-Closing Shares”), and the stockholders of the Company as of immediately prior to the Merger are expectedMyMD shareholders. This would make Oravax a publicly held company. In addition, MyMD currently has the right to own approximately 20%designate a member of the aggregate number of Post-Closing Shares.
Immediately prior to the Effective Time, the name of the Company will be changed from “Akers Biosciences, Inc.” to “MyMD Pharmaceuticals, Inc.” At the Effective Time, the Merger Agreement contemplates that the board of directors of Oravax, pursuant to which Mr. Joshua Silverman, our Chairman of the Board, has been designated to serve as a director of Oravax.
Reduction in Workforce
During October 2023, the Company will consist of seven directors, with (i) Akers having the right to designate up to four members and (ii) MYMD having the right to designate up toimplemented a reduction in workforce, eliminating three members. The officers of the Company immediately after the Effective Time will be elected by the boardCompany’s ten employees. Separated employees were granted a severance package equal to one-quarter of directors of Akers.their annual salary.
The Merger Agreement contains customary representations, warranties and covenants made byOn June 7, 2023, the Company granted the three separated employees’ options to purchase an aggregate of 7,668 shares of Common Stock with an exercise price of $47.10 per share. As consideration for a waiver and MYMD, including covenants relatingrelease in their separation agreements, the Company amended the employees’ respective June 7, 2023 option agreements to obtaining the requisite approvalsaccelerate vesting of the stockholdersportion of optioned shares that otherwise would have vested upon the Companyfirst and MYMD, indemnificationsecond anniversaries of directors and officers, and the Company’s and MYMD’s conduct of their respective businesses between the date of signinggrant. The options have an exercise period of twelve months from the Merger Agreementdate of separation.
| 60 |
Going Concern
As of December 31, 2023, the Company’s cash on hand was $2,681,010 and marketable securities were $2,242,106. The Company has incurred a net loss attributable to shareholders of $8,218,163 for the year ended December 31, 2023. As of December 31, 2023, the Company had working capital of $828,253 and stockholders’ equity of $12,369,572 including an accumulated deficit of $101,977,067. During the year ended December 31, 2023, cash flows used in operating activities were $12,980,625. The Company does not currently have sufficient available liquidity to fund its operations for at least the next 12 months. Such factors raise substantial doubt about our ability to sustain operations for at least one year from the issuance of the audited financial statements included in this Annual Report. The accompanying financial statements do not include any adjustments related to the recoverability and classification of asset amounts or the classification of liabilities that might be necessary should we be unable to continue as a going concern.
In response to these conditions and events, we are evaluating various financing strategies to obtain sufficient additional liquidity to meet our operating and capital requirements for the next twelve months following the date of this Annual Report. The potential sources of financing that we are evaluating include one or any combination of secured or unsecured debt, convertible debt and equity in both public and private offerings. We also plan to finance near-term operations with our cash on hand, as well as by exploring additional ways to raise capital. There is no assurance we will manage to raise additional capital or otherwise increase cash flows, if required. The sources of financing described above that could be available to us and the closingtiming and probability of obtaining sufficient capital depend, in part, on our further developing and commercializing our product candidates and on future capital market conditions. If our current assumptions regarding the pace of such development are incorrect, or if there are any other changes or differences in our current assumptions that negatively impact our financing strategy, we may have to reduce expenditures or significantly delay, scale back or discontinue the development or commercialization of our product candidates.
Nasdaq Deficiency
As previously disclosed, on October 11, 2023, we received a written notice (the “Notice”) from the Listing Qualifications Department of the Merger. Consummation ofNasdaq Stock Market indicating that for the Merger is subjectlast 30 consecutive business days, the bid price for our Common Stock had closed below the minimum $1.00 per share requirement for continued listing on Nasdaq pursuant to certain closing conditions, including, among other things, approval byNasdaq Listing Rule 5550(a)(2) (the “Minimum Bid Price Requirement”). The letter also indicated that the stockholders of Akers and MYMD.Company would be provided with a compliance period until April 8, 2024 (the “Compliance Period”), in which to regain compliance pursuant to Nasdaq Listing Rule 5810(c)(3)(A).
The Merger Agreement contains certain termination rights for bothEffective as of 4:05 p.m. Eastern Standard Time on February 14, 2024, we effected the Company and MYMD, including, among other things, (a) Akers may, upon written notice, extend the originally scheduled End Date to May 15, 2021 (the “Extended Date”) so long as (i) Akers and Merger Sub are not then in material breachReverse Stock Split of any provision of the Merger Agreement and (ii) within three calendar days of the written request by MYMD, Akers makes an additional loan to MYMD of up to $600,000, which will have the same terms and conditions of the Note (such additional note “Second Note”) and (b) Akers may, upon written notice, extend the Extended Date to June 30, 2021, so long as (i) Akers and Merger Sub are not then in material breach of any provision of the Merger Agreement, (ii) on the effective date of such extension, the loan amount evidenced by the Note and the Second Note may, at the sole option of MYMD upon written notice to Akers, be converted into shares of MYMDour common stock at a conversion priceratio of $2.00 per share, subject to certain adjustments and (iii) Akers will, at MYMD’s request, either (atone-for-thirty. Simultaneously with the optionReverse Stock Split, number of MYMD); (A) subscribe for 300,000 shares of MYMDour common stock at a subscription price of $2.00 per share, subjectauthorized for issuance was reduced from 500,000,000 shares to certain adjustments as set forth in the Merger Agreement, or (B) make an additional loan16,666,666 shares, and our authorized capital stock was reduced from 550,000,000 shares to MYMD of up to $600,000, which will have the same terms and conditions of the Note (the “Third Note,” and all amounts outstanding under the Note, the Second Note and the Third Note, the “Loan Amount”). In addition, if Akers terminates the Merger Agreement under certain circumstances specified therein, the Loan Amount, if any, at the sole discretion of MYMD, will be convertible into shares of66,666,666 shares. Our common stock of MYMD at a conversion price of $2.00 per share upon delivery of written notice by MYMDcontinued to Akers within 30 calendar days after the effective date of termination of the Merger Agreement.
The Merger Agreement also contemplates that the Company will seek approval from its stockholders to effect a reverse stock split, if applicable, at a reverse stock split ratio mutually agreed to by the Company and MYMD and within the range approved by the Company’s stockholders immediately prior to the Effective Time, which range shall be sufficient to cause the price of Akers common stocktraded on the Nasdaq Capital Market following such reverse stock splitunder the symbol MyMD and began trading on a split-adjusted basis at market open on February 15, 2024. On March 4, 2024, we were notified by Nasdaq that we had regained compliance with all Nasdaq listing requirements and the Effective Timematter was closed.
Financial Operations Overview
We will not generate revenue from product sales unless and until we successfully complete clinical development, obtain regulatory approval for, and successfully commercialize our MYMD-1 and Supera-CBD product candidates. The lengthy process of securing marketing approvals for new drugs requires the expenditure of substantial resources. Any significant delay or failure to be no less than $5.00 per share.obtain regulatory approvals would materially adversely affect our product candidate’s development efforts and our business overall. In addition, underif we obtain regulatory approval for MYMD-1 and/or Supera-CBD, we expect to incur significant expenses related to developing our commercialization capability to support product sales, marketing, manufacturing and distribution activities.
We anticipate that our expenses will increase significantly as we:
| ● | advance the development of our MYMD-1 and Supera-CBD; | |
| ● | initiate and continue research and preclinical and clinical development of potential new product candidates; | |
| ● | maintain, expand and protect our intellectual property as it pertains to MYMD-1 and Supera-CBD; | |
| ● | expand our infrastructure and facilities to accommodate our growing employee base and ongoing development activities; | |
| ● | establish agreements with contract research organizations, or CROs, and third-party contract manufacturing organizations, or CMOs, in connection with our Supera-CBD preclinical studies, MYMD-1 ongoing and planned clinical trials, Supera-CBD clinical trials and the development of our manufacturing capabilities for MYMD-1 and Supera-CBD; | |
| ● | develop the large-scale manufacturing processes and capabilities for the commercialization of our MYMD-1 and Supera-CBD drug products; | |
| ● | seek marketing approvals for our MYMD-1 and Supera-CBD product candidates that successfully complete clinical trials and | |
| ● | establish a sales, marketing and distribution infrastructure to commercialize MYMD-1 and Supera-CBD should we obtain marketing approval |
As a result of these anticipated expenditures, we will need substantial additional funding to support our continuing operations and pursue our growth strategy.
Components of our Results of Operations
Revenue
We have not generated any revenue from product sales and do not expect to generate any revenue from the Merger Agreement, Akers may, in its discretion, consummate a spin-offsale of all or a part of its pre-closing assets and liabilities (the “Spin-Off”).
In connection with the Merger, the Company will seek the approval of its stockholders of (a) the transactions contemplatedproducts in the Merger Agreement,near future. If our research and development efforts with MYMD-1 and Supera-CBD are successful, we may generate revenue from product sales or through license agreements with third parties.
Operating Expenses
Our operating expenses are broken into several components, including research and development and general and administrative costs.
We expect operating expenses to increase as we progress through the issuance of Akers common stock pursuant to the Merger and (b) the amendment of its certificate of incorporation, including for purposes of (i) effectuating a reverse split of Akers common stock at a ratio to be determined by a split ratio to be mutually agreed to by Akers and MYMD within the range approved by the Company’s stockholders immediately prior to the Effective Time and on certain terms as specifically described herein, (ii) change Akers’ name to “MyMD Pharmaceuticals, Inc.,” and (c) to the extent necessary, the Spin-Off.
In accordance with the terms of the Merger Agreement, (i) the officers and directors of Akers have each entered into a voting agreement with MYMD (the “Akers Voting Agreements”), and (ii) the officers, directors and certain affiliated stockholders of MYMD have each entered into a voting agreement with Akers (the “MYMD Voting Agreements,” together with the Akers Voting Agreements, the “Voting Agreements”). The Voting Agreements place certain restrictions on the transfer of the shares of Akers and MYMD held by the respective signatories thereto and include covenants as to the voting of such shares in favor of approving the transactions contemplated by the Merger Agreement and against any actions that could adversely affect the consummation of the Merger.
Concurrently with the execution of the Merger Agreement or prior to the closing, the officers and directors of Akers, and the officers, directors and certain stockholders of MYMD, each entered into lock-up/leak-out agreements (the “Lock-Up/Leak-Out Agreements”) pursuant to which they have agreed, among other things, not to sell or dispose of (subject to certain exceptions specified therein) any shares of Akers common stock which are or will be beneficially owned by them at the Effective Time or which are acquired thereafter, with such shares being released from such restrictions 180 days after the Effective Time. After the expiration of such initial 180-day period, such stockholders will be subject to a 180-day leak-out period during which they may not sell shares in excess of the amount permitted by the Rule 144 volume limitations (even if such stockholder is not currently subject to such provisions of Rule 144), which leak-out period shall be extended for an additional 180 days for any shares of Akers common stock issued upon the exercise of existing options or warrants.
Secured Promissory Note
As set forth above, in connection with the execution of the Merger Agreement, Akers will advance a bridge loan to MYMD in an amount of up to $3,000,000 pursuant to the Note. Advances under the Note will be made in accordance with MYMD’s cash needs pursuant to a pre-agreed operating budget for MYMD. The Note accrues interest on the outstanding principal amount at the rate of 5% per annum and matures on the earliest of (i) April 15, 2022, (ii) upon demand of Akersvarious clinical trials in the event the Merger is consummated, or (iii) the date on which MYMD’s obligations under the Note are accelerated in accordance with the termsdevelopment of the Note. As set forth above, in the event the Merger Agreement is terminated by MYMD upon a change in Akers’ board of directors’ recommendations to the Akers stockholders in connection with the Merger AgreementMYMD-1 and certain other circumstances specified in the Merger Agreement, the principal amount of the Note, and all accrued and unpaid interest thereon, shall be converted into shares of MYMD common stock at a conversion price of $2.00 per share. MYMD may prepay the Note in whole or in part at any time or from time to time at its sole discretion. Under the terms of the Note, if, at any time after the termination or expiration of the Merger Agreement, MYMD (i) incurs any debt other than Permitted Debt (as defined in the Note), (ii) issues any equity interests, or (iii) consummates any Asset Sale or Recovery Event (each as defined in the Note) then, in each case, no later than two business days after MYMD receives the net cash proceeds of such incurrence, issuance or other action, then MYMD shall be required to prepay an amount under the Note equal to the net cash proceeds received, up to the total amount of the advances made under the Note at such time, including all accrued and unpaid interest thereon, of the Note. The payment and performance of all obligations under the Note are secured by a first priority security interest in all of MYMD’s right, title and interest in and to its assets as collateral.Supera-CBD.
As of December 31, 2020, the Company had advanced MYMD $1,200,000 under the Note, which is classified as Other Receivables on the Consolidated Balance Sheets. The Company advanced two additional draws of $600,000, or $1,200,000 cumulatively, on January 21, 2021 and February 25, 2021 to MYMD under this secured promissory note.
Private PlacementResearch and Development
ConcurrentlyOur research and development expenses primarily consist of costs associated with the Merger Agreement, on November 11, 2020, Akers entered intodevelopment of MYMD-1 and Supera-CBD. These costs include, but are not limited to:
| ● | Salaries, wages and benefits of the research and development staff; | |
| ● | Contractual agreements with third parties including contract research organizations, preclinical activities and clinical trials; | |
| ● | Outside consultants including fees and expenses; | |
| ● | Laboratory supplies and equipment; | |
| ● | Regulatory compliance; and | |
| ● | Patent application and maintenance costs to protect our intellectual property. |
Four of our six employees are principally involved in research and development activities for either MYMD-1 or Supera-CBD. Their salaries, wages and benefits are captured as a component of research and development but not allocated to specific projects.
We utilize third party contractors and consultants with expertise in specific research or development activities to perform work under the Private Placement SPAsupervision of our researchers. We believe this allows us to control costs and to progress through the development cycle and to utilize our staff more efficiently.
It is difficult to project with certain institutionalabsolute accuracy the duration or final cost of the development of MYMD-1 and accredited investors (the “SPA Purchasers”), pursuantSuper-CBD or if revenue will be generated from the commercialization of these components. The process of achieving regulatory approval is very costly and time consuming. A few of the many factors that contribute to which Akers agreed to issuecosts of duration include:
| ● | Size and scope of pre-clinical trials; | |
| ● | The phases of clinical development and the stage of our product candidates in the cycle; | |
| ● | Per subject trial costs; | |
| ● | The number of sites required for the trials and the availability of appropriate sites to perform the trials; | |
| ● | The time that is required to enroll the appropriate number of trial participants; and | |
| ● | The time required to achieve the approval of regulatory agencies. |
General and sell to the SPA Purchasers (i) an aggregateAdministrative
General and administrative expenses primarily consist of 9,765,933 shares of Akers common stock, at an offering price of $1.85 per share or, at the election of each investor, Pre-Funded Warrants,salaries, wages and (ii)benefits for each share of Akers common stock (or for each Pre-Funded Warrant, as applicable) purchasedour employees in the Private Placement, a common warrant (the “Investor Warrants”executive, legal and together withaccounting functions and third-party costs for legal, accounting, insurance, investor relations, stock market and board expenses.
Although treated as components of general and administrative expenses, we have chosen to disclose the Pre-Funded Warrants,following significant items separately:
Stock Based Compensation
Stock based compensation includes the “Warrants”)fair market value, as determined using the Black-Scholes option pricing model, of stock options issued to purchase one share of Akers common stock, for gross proceeds of approximately $18.1 million before the deduction of placement agent feeskey staff and expenses and estimated offering expenses. In addition, Akers also issued the Placement Agent a warrant to purchase up to 390,368 shares of Akers common stock at an exercise price of $1.85 (the “Placement Agent Warrant”). The Placement Agent Warrant will be exercisable at any time and from time to time, in whole or in part, for a term of five and a half years. The Private Placement closed on November 17, 2020, and Akers issued an aggregate of 8,725,393 shares of Akers common stock, Pre-Funded Warrants to purchase 1,040,540 shares of Akers common stock, and Investor Warrants to purchase 9,765,933 shares of Akers common stock. In February 2021, an investor exchanged 932,432 shares of common stock purchased in the Private Placement into Pre-Funded Warrants to purchase 932,432 shares of common stock.consultants.
In the Private Placement SPA, Akers agreed not to (i) issue, enter into any agreement to issue or announce the issuance or proposed issuance of, any shares of Akers common stock or any securities convertible into or exercisable or exchangeable for shares of Akers common stock at an effective price less than the exercise price of the Investor Warrants or (ii) file any registration statement or any amendment or supplement thereto, other than as contemplated under the Private Placement SPA, for a period of 90 days following the later of (x) the date the Registration Statement (as defined below) is declared effective by the SEC and (y) the record date for the Akers stockholder meeting called to approve the Merger. In addition, Akers agreed not to effect or enter into an agreement to effect any issuance of Akers common stock or common stock equivalents involving a variable rate transaction (as defined in the Private Placement SPA) from the date of the Private Placement SPA until such time as no SPA Purchaser holds any of the Investor Warrants, subject to certain exceptions (including the issuance of any of Akers common stock pursuant to the Merger Agreement).
The Private Placement SPA provides that (i) within 10 days following the date that Akers first files a proxy statement with the SEC in connection with the merger (including by means of a registration statement on Form S-4), Akers shall file a registration statement (the “Registration Statement”) under the Securities Act of 1933, as amended (the “Securities Act”) for the resale of all of the shares of Akers common stock issued in the private placement and the shares of Akers common stock issuable upon exercise of the Warrants (the “Warrant Shares”) by the SPA Purchasers and (ii) Akers shall use commercially reasonable efforts to cause such Registration Statement to be declared effective within 60 days of the filing thereof (or 90 days in the event of a full review); provided, however, that Akers shall not be required to register any shares of Akers common stock issued in the private placement or Warrant Shares that are eligible for resale pursuant to Rule 144 under the Securities Act (assuming cashless exercise of the Warrants).
We currently intend to use the proceeds from the Private Placement in order to satisfy the closing conditions set forth in the Merger Agreement that requires the Company to have a minimum parent net cash amount equal to $25 million, less certain amounts advanced to MyMD, which shall also include any amounts to be used to payoff The Starwood Trust to repay in full the Starwood Line of Credit at the closing of the Merger, and for general working capital purposes. In addition, the Company paid $1,204,525 of the proceeds from the Private Placement to three of the former members of Cystron and recorded a liability of $602,172 to the fourth former member of Cystron pursuant to the MIPA.
In addition, we paid a cash fee of $501,500 and issued warrants to purchase an aggregate of 255,135 shares of common stock to the designees of H.C. Wainwright & Co., LLC (“HCW”), pursuant to a side letter by and between Akers and HCW, dated November 23, 2020, regarding certain tail fees provided in two engagement letters (one dated October 18, 2019 and the other dated April 7, 2020) entered into in connection with prior offerings by and between Akers and HCW. Such warrants issued were in the same form as the Investor Warrants except that the HCW Warrants have an exercise price of $2.3125 per share.
The Investor WarrantsOther Income (Expense), net
Each Investor Warrant issued in the Private Placement has an initial exercise price equal to $2.06 per shareOther income (expense), net consists of common stock. The Investor Warrants are immediately exercisableinterest and will terminate fivedividends earned on our cash, cash equivalents, and a half years following issuance. The exercise price and number of shares of Akers common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting Akers common stock and the exercise price.
If, at any time following the six-month anniversary of November 17, 2020, there is no effective registration statement registering, or the prospectus contained therein is not available for the issuance of the shares underlying the Investor Warrants (the “Investor Warrant Shares”) to the holder, then the Investor Warrants may also be exercised, in whole or in part, at such time by means of a “cashless exercise” in which the holder shall be entitled to receive a number of Investor Warrant Shares according to a formula set forth in the Investor Warrants.
A holder (together with its affiliates) may not exercise any portion of the Investor Warrant to the extent that the holder would own more than 4.99% (or, at the election of a holder prior to the date of issuance, 9.99%) of the outstanding Akers common stock immediately after exercise; provided, however, that upon notice to Akers, the holder may increase or decrease the beneficial ownership limitation, provided that in no event shall the beneficial ownership limitation exceed 9.99% and any increase in the beneficial ownership limitation will not be effective until 61 days following notice of such increase from the holder to Akers.
In the event of a fundamental transaction, as described in the Investor Warrants and generally including any reorganization, recapitalization or reclassification of Akers common stock,investments, gains on the sale transfer or other dispositionmarketable securities, losses on equity investments, gains on the forgiveness of all or substantially all of Akers’ properties or assets, Akers’ consolidation or merger with or into another person, the acquisition of more than 50% of Akers outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by Akers’ outstanding common stock, the holders of the Investor Warrants will be entitled to receive upon exercise of such warrants the kinddebt and amount of securities, cash or other property that the holders would have received had they exercised the Investor Warrants immediately prior to such fundamental transaction. The Merger shall not be deemed a fundamental transaction as defined in the Investor Warrants.an uninsured casualty loss.
The Pre-Funded Warrants
At the request of an investor, in lieu of Akers common stock, certain investors received Pre-Funded Warrants. The Pre-Funded Warrants are exercisable at any time immediately upon issuance and until such warrant is exercised in full. The exercise price of the Pre-Funded Warrants is $0.001 per share of Akers common stock, and, in lieu of making the cash payment otherwise contemplated to be made to Akers upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of Akers common stock determined according to a formula set forth in the Pre-Funded Warrants.
A holder (together with its affiliates) may not exercise any portion of the Pre-Funded Warrants to the extent that the holder would own more than 4.99% (or, at the election of a holder prior to the date of issuance, 9.99%) of the outstanding Akers common stock immediately after exercise; provided, however, that upon notice to Akers, the holder may increase or decrease the beneficial ownership limitation, provided that in no event shall the beneficial ownership limitation exceed 9.99% and any increase in the beneficial ownership limitation will not be effective until 61 days following notice of such increase from the holder to Akers.
Lock-Up and Support Agreement
On November 11, 2020, Akers entered into a Lock-Up and Support Agreement (the “Support Agreement”) with substantially all of the SPA Purchasers, pursuant to which, from the date of the Support Agreement until May 31, 2021, such SPA Purchasers agreed to vote their respective shares of Akers common stock in favor of each matter proposed and recommended for approval by the Akers board of directors or management at every shareholders’ meeting. Pursuant to the Support Agreement, such SPA Purchasers also agreed to, until the earlier of (a) the termination of the Merger Agreement or (b) the date that the SPA Purchasers vote their respective shares of Akers common stock in support of the merger and all matters related to the merger, will not, directly or indirectly, without Akers’ prior written consent, transfer, assign or dispose of their rights to vote the shares of Akers common stock issued in the private placement or otherwise take any act that could restrict or otherwise affect their legal power, authority or right to vote all of their shares of Akers common stock issued in the private placement in the manner required by the Support Agreement.
Katalyst Securities LLC Engagement Letter
On October 31, 2020, Akers entered into an engagement letter with Katalyst Securities LLC (the “Placement Agent” or “Katalyst”), pursuant to which the Placement Agent agreed to serve as the non-exclusive placement agent for Akers, on a reasonable best efforts basis, in connection with the Private Placement. Akers agreed to pay the Placement Agent an aggregate cash fee equal to 6.5% of the gross proceeds received in the Private Placement and reimburse the Placement Agent’s expenses in the Private Placement up to $25,000. In addition, Akers agreed to grant to Katalyst the Placement Agent Warrant, which was issued upon closing of the Private Placement. The Placement Agent Warrant is exercisable at any time and from time to time, in whole or in part, following the date of issuance and for a term of five and a half years.
Results of Operations
As discussed in Note 3 and Note 6 of the Notes to the Consolidated Financial Statements, the results of operations presented below exclude our screening and testing products business due to its classification as discontinued operations.
Summary of Statements of Operations for the Fiscal Years Ended December 31, 20202023 and 20192022
As previously disclosed, in lightWe are focused on developing and commercializing two therapeutic platforms based on well-defined therapeutic targets, MYMD-1 and Supera-CBD. The following table summarized the results of the unfavorable factors persistent in our rapid, point-of-care screening and testing product business and the progress the Company has made in its partnership with Premas, the Company conducted a strategic review of the screening and testing products business. Following such review, in early July 2020, the Company ceased the production and sale of its rapid, point-of-care screening and testing products. The Company will continue to provide support for these testing products that remain in the market through their respective product expiration dates. The Company had been experiencing declining sales revenue and production backlogs for these products and, as it previously reported, had eliminated its sales force for such products. The Company intends to devote its attention to its partnership with Premasoperations for the development of its COVID-19 Vaccine Candidateyears ended December 31, 2023 and transactions that the Company believes will increase shareholder value. In connection with the ceasing production and sale of its existing product line, on July 16, 2020, the Company decided to close the Thorofare Facility and exercised the early termination option under the Thorofare Lease, which provided for a 150-day notice to terminate the lease. Pursuant to the early termination option, the Thorofare Lease matured on December 13, 2020. The lease terminated on November 30, 2020, at the lessor’s request, and the property was handed over to the property manager on November 30, 2020.2022.
| For the Year Ended December 31, | Percent | |||||||||||
| Description | 2023 | 2022 | Change | |||||||||
| Operating Expenses | ||||||||||||
| General and Administrative | 5,442,886 | 5,520,150 | (1.4 | ) | ||||||||
| Research and Development | $ | 7,867,795 | 9,067,422 | (13.2 | ) | |||||||
| Stock Based Compensation | 3,049,537 | 695,191 | 338.7 | |||||||||
| Warrant Issuance Expenses | 762,834 | - | 100.0 | |||||||||
| Total Operating Expenses | $ | 17,123,052 | $ | 15,282,763 | 12.0 | |||||||
| Loss from Operations | (17,123,052 | ) | (15,282,763 | ) | (12.0 | ) | ||||||
| Other Income (Expense), net | 13,123,102 | 85,427 | 15,261.8 | |||||||||
| Net Loss | $ | (3,999,950 | ) | $ | (15,197,336 | ) | 73.7 | |||||
| Preferred Stock Dividends | 4,218,213 | - | 100.0 | |||||||||
| Net Loss Attributable to Common Shareholders | $ | (8,218,163 | ) | $ | (15,197,336 | ) | 45.9 | |||||
The Company determined that the discontinuation of the production and distribution of the Company’s screening and testing products constituted a strategic shift in the Company’s business and as a result the elimination of the product lines should be presented as discontinued operations under FASB ASC 205-20 Presentation of Financial Statements, Discontinued Operations.Revenue
Revenue
We had no revenue from continuing operations during the years ended December 31, 20202023 and December 31, 2019.2022.
| 63 |
Administrative Expenses
Administrative expenses for the year ended December 31, 2020, totaled $4,299,062 which was a 27% increase as compared to $3,372,103 for the year ended December 31, 2019.
The table below summarizes our administrative expenses for the years ended December 31, 20202023 and 20192022 as well as the percentage of change year-over-year:
| For the Years Ended December 31, | Percent | For the Years Ended December 31, | Percent | |||||||||||||||||||||
| Description | 2020 | 2019 | Change | 2023 | 2022 | Change | ||||||||||||||||||
| Personnel Costs | $ | 1,086,529 | $ | 694,197 | 57 | % | $ | 1,410,950 | $ | 1,169,180 | 20.7 | |||||||||||||
| Professional Service Costs | 1,427,158 | 663,131 | 115 | % | 1,043,247 | 1,609,513 | (35.2 | ) | ||||||||||||||||
| Stock Market & Investor Relations Costs | 263,912 | 415,637 | (37 | )% | 901,079 | 961,540 | (6.3 | ) | ||||||||||||||||
| Other Administrative Costs | 1,521,463 | 1,599,138 | (5 | )% | 2,087,610 | 1,779,917 | 17.3 | |||||||||||||||||
| Total Administrative Expense | $ | 4,299,062 | $ | 3,372,103 | 27 | % | $ | 5,442,886 | $ | 5,520,150 | (1.4 | ) | ||||||||||||
Personnel expensescosts increased by 57% for$241,770 during the year ended December 31, 2020 as compared2023. The increase is attributable to changes in the same period of 2019 on account ofbase salary for two executives, bonuses, and the addition of an executive staff member.accumulated personal time off and severance paid to separated employees in October and November 2023.
Professional serviceservices costs increased 115% fordecreased $566,266 during the year ended December 31, 2020 as compared2023. These costs included legal, accounting and specialized consulting services related to the same periodinitial and quarterly calculation of 2019, principally duethe fair market value of the Series F Convertible Preferred Stock and its’ components. The decrease is primarily related to increased accountinga reduction in the usage of general consultants and audit, legalthe allocation of consulting services between the administrative and general consulting fees.research and development functions.
Stock market and investor feesrelations costs decreased 37% for$60,461 during the year ended December 31, 2020. The decrease in these2023. These costs include the annual Nasdaq listing fees, was principally dueactivities related to our delisting fromkeeping the London Stock Exchange during the first half of 2019shareholder base informed through press releases, presentations and other communication efforts, transfer agent fees, and the avoidancecosts of the costs associated with a presence on the London Stock Exchange.annual shareholder meetings.
Other administrative expenses decreased by 5%, principally due to a decrease in bad debt expense, decreases in legal settlements, license and permit fees and travel expenses which were offset by increases in board, building, business insurance and computer expenses.
Sales and Marketing Expenses
Sales and marketing expenses forincreased $307,693 during the year ended December 31, 2020 totaled $22,963 which was2023. These costs include Board expenses, business insurance, corporate travel, and other general business expenses. The increase is attributable to an 8% decrease compared to $25,000increase in corporate travel expenses, offset by small reductions in most other general business expense categories.
Research and Development Expenses
The table below summarizes our research and development expenses for the years ended December 31, 2023 and 2022 as well as the percentage of change year-over-year:
| For the Year Ended December 31, | Percent | |||||||||||
| Description | 2023 | 2022 | Change | |||||||||
| Salaries and Wages | $ | 1,765,488 | $ | 1,087,574 | 62.3 | |||||||
| Development Programs | 5,593,041 | 3,728,568 | 50.0 | |||||||||
| Professional Services | 329,271 | 119,809 | 174.8 | |||||||||
| Regulatory Expenses | 21,574 | 4,121,848 | (89.5 | ) | ||||||||
| Other Research and Development Expenses | 158,421 | 9,623 | 1,546.3 | |||||||||
| Total Research and Development Expenses | $ | 7,867,795 | $ | 9,067,422 | (13.2 | ) | ||||||
Salaries and wages increased $677,914 during the year ended December 31, 2019.2023. The increase is attributable to changes in the base salary for an executive, bonuses, and the accumulated personal time off and severance paid to separated employees in October 2023.
ResearchDevelopment program costs include those associated with pre-clinical development, clinical trials and Development Expenses
Researchother material and development expenses forprograms. Costs increased $1,864,473 during the year ended December 31, 2020 totaled $7,963,678 as compared to $02023, a result of the completion of pre-clinical toxicology studies and the Phase 2 Sarcopenia clinical trial, the analysis of the Phase 2 Sarcopenia study results, and the acquisition of base compounds for use in on-going studies.
Professional services costs increased $209,462 during the year ended December 31, 2019, principally reflecting2023. These costs are primarily related to legal and patent related fees associated with the protection of our current focus onintellectual property and the allocation of consulting services between the research and development of the COVID-19 Vaccine Candidate.and administrative functions.
Other Income and Expense
Other income, net ofRegulatory expenses fordecreased $4,100,274 during the year ended December 31, 2020 totaled $133,489 as compared to other income, net2023. Regulatory expenses include clinical research organizations (CRO) and regulatory consulting fees associated with Phase 2 clinical study designs, protocol preparations and the maintenance of the investigator brochures. These non-recurring regulatory services were completed in 2022.
Other research and development expenses of $90,808 forincreased $148,798 during the year ended December 31, 2019.2022. These expenses include laboratory supplies, training and travel for department personnel while working with third-party trial sites. The increase is attributable to specialized freight costs for materials and travel in support of the studies and data analysis of the Phase 2 Sarcopenia trial results.
| 64 |
Stock-Based Compensation
During the year ended December 31, 2023, stock-based compensation totaled $3,049,536. These expenses include stock options issued to directors, staff, and service providers. During the year ended December 31, 2022, stock-based compensation totaled $695,191 for stock options issued to staff and service providers, restricted stock units and Common Stock warrants issued for services.
Other Income and Expense
The table below summarizes our other income and expenses for the years ended December 31, 20202023 and 20192022 as well as the percentage of change year-over-year:
| For the Years Ended December 31, | ||||||||||||
| Description | 2020 | 2019 | Percent Change | |||||||||
| Loss on Disposal of Property and Equipment | $ | 3,042 | $ | 9,576 | (68 | )% | ||||||
| Foreign Currency Transaction (Gain)/Loss | (93 | ) | 5,501 | (102 | )% | |||||||
| Gain on FMV of Equity Investments | (54,100 | ) | - | NM | ||||||||
| (Gain)/Loss on Investments | 36,714 | (3,952 | ) | (1,029 | )% | |||||||
| Interest and Dividend Income | (119,052 | ) | (101,483 | ) | 17 | % | ||||||
| Total Other (Income)/Expense | $ | (133,489 | ) | $ | (90,808 | ) | 47 | % | ||||
| For the Years Ended December 31, | Percent | |||||||||||
| Description | 2023 | 2022 | Change | |||||||||
| Interest and Dividend Income | $ | 455,570 | $ | 83,991 | 442.4 | |||||||
| Gain/(Loss) on Sale of Marketable Securities | 416 | (5,964 | ) | 107.0 | ||||||||
| Gain on changes in fair value of Marketable Securities | 514 | 2,958 | (82.6 | ) | ||||||||
| Gain on changes in fair value of Derivative Liabilities | 3,088,800 | - | 100.0 | |||||||||
| Gain on changes in fair value of Warrant Liabilities | 9,756,000 | - | 100.0 | |||||||||
| Uninsured Casualty Gain/(Loss) | (178,198 | ) | 4,442 | (4,111.7 | ) | |||||||
| Total Other Income/(Expense) | $ | 13,123,102 | $ | 85,427 | 15,261.8 | |||||||
Equity investment gains increased to $54,100
Other income, net of expenses, totaled $13,123,102 for the year ended December 31, 2020 as compared to $0 for the same period in 2019. The increase was due to an increase in the fair market value2023, and other income, net of the equity investments.
Realized loss on investments was $36,714expenses, totaled $85,427 for the year ended December 31, 2020 as compared to a gain of $3,952 for the same period in 2019. The decrease is principally due to the impact of the COVID-19 pandemic on the financial markets.2022.
Interest and dividend income increased to $119,052 forDuring the year ended December 31, 2020 compared to $101,483 for2023 interest and dividend income, the changes in fair value of our investments and realized gains from the sale of investments are primarily the result of rising interest rates.
During the year ended December 31, 2019.2023, we recorded a gain of $3,088,800 related to the change in fair value of the derivative liabilities. We estimated the $61,000 fair value of the bifurcated embedded derivative at December 31, 2023 using a Monte Carlo simulation model, with the following inputs: the fair value of our common stock of $0.26 ($7.80 post reverse split) on the valuation date, estimated equity volatility of 140.0%, estimated traded volume volatility of 150.0%, the time to maturity of 0.5 year, a discounted market interest rate of 6.40%, dividend rate of 10.0%, a penalty dividend rate of 15.0%, and probability of default of 3.90%.
During the year ended December 31, 2023, we recorded a gain of $9,756,000 related to the change in fair value of the warrant liabilities. The increasefair value of the Warrants of approximately $867,000 was principallyestimated at December 31, 2023 utilizing the Black Scholes Model using the following weighted average assumptions: dividend yield 0%; remaining term of 4.15 years; equity volatility of 120.0%; and a risk-free interest rate of 3.91%.
For the year ended December 31, 2023, we identified a casualty loss of $178,198 related to wire fraud due to a compromised electronic mail account. This incident occurred on May 17, 2023 and was discovered on July 20, 2023 when the increasevendor notified us of a delinquent invoice. An investigation determined that the original invoice from the vendor, sent to our consultant on this project, was intercepted and resent with altered wring instructions from a domain name that varied from the actual vendor’s domain by one character. We notified our cyber insurance carrier on November 9, 2023.During the year ended December 31, 2022, we recovered $4,442 from a financial institution involved in funds available for investment.an October, 2021 incident.
Income Taxes
As of December 31, 2020,2023, and 2019, the Company2022, we had FederalU.S. federal net operating loss carry forwards of approximately $100,615,000$113.1 million and $79,678,000, respectively, expiring through$107.1 million, respectively. Approximately $51.5 million of the year ending December 31, 2037 forU.S. federal net operating losses originatingloss generated in tax years beginning before January 1, 2018. Net2018 expire beginning with the year ending December 31, 2024 through 2037. The remaining U.S. federal net operating losses recorded in tax years beginning January 1, 2018 and after are allowed for an indefinite carryforward period butloss of approximately $61.6 million does not expire, however it is limited to 80% of each subsequent year’s net income. As of December 31, 2020,2023, and 2019, the Company2022, we had New JerseyU.S. state net operating loss carry forwards of approximately $7,548,000$45.2 million and $28,855,000,$41.0 million, respectively, throughsome of which expire beginning with the year ending December 31, 2040.2024 through 2043.
Under Section 382 of the Code, use of our NOLs will benet operating loss carryforwards is limited if we experience a cumulative change in ownership of greater than 50% in a moving three-year period. We will experienceexperienced an ownership change as a result of the Merger and therefore our ability to utilize our NOLsnet operating loss carryforwards and certain credit carryforwards remaining at the Effective Time will beare limited. The limitation will beis determined by the fair market value of our common stock outstanding immediately prior to the ownership change, multiplied by the applicable federal rate. It is expected that the Merger will impose acaused our net operating loss carryforwards to be limited. However, the limitation had no impact on our NOLs. The Company hasfinancial statements since we recorded a full valuation allowance for itsour deferred tax assets as of December 31, 20202023 and 2019.2022 (See Note 97 to the Consolidated Financial Statements).
Liquidity and Capital Resources
As of December 31, 2020,2023, the Company’s cash and cash equivalents on hand was $18,617,955$2,681,010 and its marketable securities were $16,718,452.$2,242,106. The Company has incurred a net lossesloss attributable to shareholders of $17,580,609 and $3,888,249$8,218,163 for the yearsyear ended December 31, 2020 and 2019, respectfully.2023. As of December 31, 2020,2023, the Company had working capital of $34,579,466$828,253 and a stockholders’ equity of $12,369,572 including an accumulated deficit of $137,163,739.$101,977,067. During the year ended December 31, 2020,2023, cash flows used in operating activities were $11,924,941, consisting primarily of a net loss from ongoing operations of $12,152,214 and net loss from discontinued operations of $5,248,395.$12,980,625. Since inception, the Company has met its liquidity requirements principally through the sale of its common and preferred stock in public and private placements.
Development and commercialization ofplacements; however, there is no assurance that management will be able to obtain additional financing in the future. These factors raise substantial doubt about the Company’s COVID-19 Vaccine Candidate will requireability to continue as a going concern. For more information, see the Company to raise significant additional funds as the project proceeds through clinical trials, the attainment of the required regulatory approvals and the commercialization of the vaccine. The timing of these events is difficult to estimate and are unlikely to be fully completed within the next twelve-months.section above titled “Going Concern.”
The Company evaluated the current cash requirements for operations in conjunction with management’s strategic plan and believes that the Company’s current financial resources as of the date of the issuance of these consolidated financial statements, are sufficient to fund its current operating budget and contractual obligations as of December 31, 2020 as they fall due within the next twelve-month period, alleviating any substantial doubt raised by the Company’s historical operating results and satisfying its estimated liquidity needs for twelve months from the issuance of these consolidated financial statements.
Capital expenditures for the years ended December 31, 2020 and December 31, 2019 were $0.
Operating Activities
Our net cash consumedused by operating activities totaled $11,924,941 during the year ended December 31, 2020. Cash was consumed by the net loss from continuing operations2023, were $12,980,625, consisting primarily of $12,152,214 and a net loss from discontinued operations of $5,428,395 reduced$3,999,950 and fair value adjustments of $3,088,800 for derivatives and $9,756,000 for warrants related to the Preferred Shares offset by non-cash adjustments principally consistingshare-based compensation of $4,154,964 for stock-based compensation, $291,442 for impairment$3,049,537, an increase in trade and other payables of $1,042,997 and a decrease in prepaid royalties, $152,822 for impairmentexpenses of intangible assets and $197,723 for inventory adjustment for$327,439.
Our net realizable value. Forcash used by operating activities totaled $12,270,068 during the year ended December 31, 2020, within changes2022. Net cash used consisted principally of assets and liabilities, cash was principally providedthe net loss from operations of $15,197,336 partially offset by an increase in trade and other payables of $733,530 and decreases$1,686,595, a decrease in trade receivables of 42,881 and prepaid expenses of $41,452.$540,560 and non-cash stock compensation expenses of $695,191.
| 65 |
Investing Activities
Our net cash consumedprovided by operatinginvesting activities totaled $3,074,283$1,845,726 for the year ended December 31, 2023 as compared to cash provided by investing activities totaling $6,913,163 during the year ended December 31, 2019. Cash was consumed by the net loss from continuing operations of $3,381,295 and a net loss from discontinued operations of $506,954 reduced by non-cash adjustments principally consisting of $74,064 for depreciation and amortization of non-current assets, $32,980 for impairment of intangible assets, $371,997 for charge for obsolescence inventory, $105,325 for the allowance of doubtful accounts and other receivables and $400,174 for share-based compensation. For2022. During the year ended December 31, 2019, within changes of assets2023 we purchased securities totaling $13,454,304 and liabilities, cash provided consisted principally of a decrease in trade receivables of $128,120, and a decrease in prepaid expenses of $103,152 off-set by a decrease in trade and other payables of $443,735.
Investing Activities
The Company’s net cash used in investing totaled $8,757,469, as compared to $3,940,627 during the years ended December 31, 2020 and 2019, respectively. Net cash used in investing activities forsold securities totaling $15,300,000. During the year ended December 31, 20202022 we purchased securities totaling $4,836,837 and sold securities totaling $11,750,000.
Financing Activities
Net cash provided by financing activities during the year ended December 31, 2023 was $13,066,819 which consisted of 14,685,689 for the net proceeds from the sale of marketable securities of $2,314,374Preferred Stock offset by $9,871,843 consumed by$89,635 for the purchaseredemption of marketable securities.Preferred Stock, $1,452,145 for dividends and $77,090 for premiums related to the Preferred Stock. Net cash used in investingprovided by financing activities forduring the year ended December 31, 20192022 was $5,550,028 which consisted of the net proceeds from the sale of marketableCommon Stock.
August 2022 Offering
On August 15, 2022, we entered into a securities purchase agreement (the “August 2022 SPA”) with certain accredited and institutional investors pursuant to which we agreed to issue 47,059 shares of $2,857,960Common Stock (the “August 2022 Shares”) in a registered direct offering and unregistered warrants to purchase up to an aggregate of 47,063 shares of Common Stock in a concurrent private placement (the “August 2022 Warrants”). The August 2022 Warrants have an exercise price of $157.50 per share, became exercisable six months following the saledate of equipmentissuance and have a term of $6,250 offset by $6,704,837 consumed byexercise equal to five years from the purchase of marketable securities and $100,000 for the issuance of a short-term note receivable.
Financing Activities
The Company’s net cash provided by financing activities in 2020 was $38,667,827 (2019: $6,965,693). Net cash provided during the 2020 period consisted of $29,184,244 ofinitial exercise date. We received net proceeds from the issuancesale of commonthe August 2022 Shares and the August 2022 Warrants, after deducting fees and other estimated offering expenses payable by the Company, of approximately $5.5 million. As of December 31, 2023, none of the August 2022 Warrants have been exercised and 47,063 of the August 2022 Warrants remain outstanding.
February 2023 Offering
On February 21, 2023, we entered into a Securities Purchase Agreement (the “February 2023 SPA”) with certain accredited investors, pursuant to which we agreed to sell in a registered direct offering (the “February 2023 Offering”) (i) an aggregate of 15,000 shares $1,743,503(the “Series F Preferred Shares”) of net proceedsour newly-designated Series F Convertible Preferred Stock, with a stated value of $1,000 per Preferred Share (the “Series F Preferred Stock”), convertible into shares of Common Stock (the “Series F Conversion Shares”) pursuant to the terms of the Certificate of Designations of the Series F Preferred Stock (the “Certificate of Designation”), and (ii) warrants (the “February 2023 Warrants”) to acquire up to an aggregate of 6,651,885 shares of Common Stock (pre-split), subject to adjustment (the “February 2023 Warrant Shares”). The Conversion Price (as defined below) is subject to customary adjustments for stock dividends, stock splits, reclassifications and the issuancelike, and subject to price-based adjustment in the event of prepaid equity forward contractsany issuances of Common Stock, or securities convertible, exercisable or exchangeable for Common Stock, at a price below the purchasethen-applicable Conversion Price (subject to certain exceptions). Following the Reverse Stock Split, (i) the Conversion Price was adjusted to $3.18 per share pursuant to the terms of commonthe Certificate of Designations, and (ii) the Exercise Price was adjusted to $3.18 per share and the number of February 2023 Warrant Shares was adjusted proportionately to 4,716,904 shares and $7,740,000pursuant to the terms of the February 2023 Warrants.
| 66 |
At closing, we received net proceeds from the exerciseFebruary 2023 Offering of warrants for common stock. Net cash provided duringapproximately $14.1 million, after deducting various fees and expenses. We intend to use the 2019 period consisted of $2,147,778 of net proceeds from this offering for general corporate purposes.
As of December 31, 2023, there were 6,833 Series F Preferred Shares outstanding and February 2023 Warrants outstanding to purchase up to 4,716,904 shares of Common Stock.
Series F Preferred Shares
The terms of the Series F Preferred Shares are as set forth in the form of Certificate of Designation. The Series F Preferred Shares became convertible upon issuance into the Conversion Shares at the election of commonthe holder at any time at an initial conversion price of $2.255 (pre-split) (the “Conversion Price”). The Conversion Price is subject to customary adjustments for stock dividends, stock splits, reclassifications and $4,817,857the like, and subject to price-based adjustment in the event of net proceeds from issuanceany issuances of prepaid equity forward contractsCommon Stock, or securities convertible, exercisable or exchangeable for Common Stock, at a price below the then-applicable Conversion Price (subject to certain exceptions). Following the Reverse Stock Split, the Conversion Price for the purchasePreferred Shares was adjusted to $3.18 per share pursuant to the terms of common stock.the Certificate of Designations. The Company is required to redeem the Series F Preferred Shares in 12 equal monthly installments, commencing on July 1, 2023. The amortization payments due upon such redemption are payable, at the company’s election, in cash, or subject to certain limitations, in shares of Common Stock valued at the lower of (i) the Conversion Price then in effect and (ii) the greater of (A) 80% of the average of the three lowest closing prices of the Company’s Common Stock during the thirty trading day period immediately prior to the date the amortization payment is due or (B) the Floor Price (as defined below). For purposes of the Certificate of Designation, the “Floor Price” means $6.60 (subject to adjustment for stock splits, stock dividends, stock combinations, recapitalizations or other similar events) or, in any case, such lower amount as permitted, from time to time, by the Nasdaq Stock Market. The Company may require holders to convert their Series F Preferred Shares into Conversion Shares if the closing price of the Common Stock exceeds $202.95 per share (subject to adjustment for stock splits, stock dividends, stock combinations, recapitalizations or other similar events) for 20 consecutive trading days and the daily dollar trading volume of the Common Stock exceeds $3,000,000 per day during the same period and certain equity conditions described in the Certificate of Designation are satisfied.
The holders of the Series F Preferred Shares are entitled to dividends of 10% per annum, compounded monthly, which are payable in cash or shares of Common Stock at the Company’s option, in accordance with the terms of the Certificate of Designation. Upon the occurrence and during the continuance of a Triggering Event (as defined in the Certificate of Designation), the Series F Preferred Shares accrue dividends at the rate of 15% per annum. In connection with a Triggering Event, each holder of Series F Preferred Shares is able to require the Company to redeem in cash any or all of the holder’s Series F Preferred Shares at a premium set forth in the Certificate of Designation. Upon conversion or redemption, the holders of the Series F Preferred Shares are also entitled to receive a dividend make-whole payment. The holders of Series F Preferred Shares have no voting rights on account of the Series F Preferred Shares, other than with respect to certain matters affecting the rights of the Series F Preferred Shares.
The Company is subject to certain affirmative and negative covenants regarding the incurrence of indebtedness, acquisition and investment transactions, the existence of liens, the repayment of indebtedness, the payment of cash in respect of dividends (other than dividends pursuant to the Certificate of Designation), distributions or redemptions, and the transfer of assets, among other matters. There is no established public trading market for the Series F Preferred Shares and the Company does not intend to list the Series F Preferred Shares on any national securities exchange or nationally recognized trading system.
February 2023 Warrants
The February 2023 Warrants became exercisable immediately upon issuance, have an exercise price of $2.255 per share (pre-split) (as adjusted, the “Exercise Price”) and expire five years from the date of issuance. The Exercise Price is subject to customary adjustments for stock dividends, stock splits, reclassifications and the like, and subject to price-based adjustment, on a “full ratchet” basis, in the event of any issuances of Common Stock, or securities convertible, exercisable or exchangeable for Common Stock, at a price below the then-applicable Exercise Price (subject to certain exceptions). Upon any such price-based adjustment to the Exercise Price, the number of Warrant Shares issuable upon exercise of the Warrants will be increased proportionately. The Warrants were issued with an initial Exercise Price of $2.255 per share (pre-split). Following the Reverse Stock Split, the Exercise Price for the Warrants was adjusted to $3.18 per share and the number of February 2023 Warrant Shares was adjusted to 4,716,904 shares pursuant to the terms of the Warrants. There is no established public trading market for the February 2023 Warrants and the Company does not intend to list the February 2023 Warrants on any national securities exchange or nationally recognized trading system.
| 67 |
Critical Accounting PoliciesEstimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America (“US GAAP”) requires management to make estimates and assumptions about future events that affect the amounts reported in the financial statements and accompanying notes. Future events and their effects cannot be determined with absolute certainty. Therefore, the determination of estimates requires the exercise of judgment. Actual results inevitably will differ from those estimates, and such differences may be material to the financial statements. The most significant accounting estimates inherent in the preparation of our financial statements include estimates associated with revenue recognition, impairment analysis of intangibles and stock-based compensation.
Our financial position, results of operations and cash flows are impacted by the accounting policies we have adopted. In order to get a full understanding of our financial statements, one must have a clear understanding of the accounting policies employed. A summary of our critical accounting policies is presented within the notes to our consolidated financial statements appearing elsewhere in this Annual Report on Form 10-K.
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of our financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, costs and expenses, and the disclosure of contingent assets and liabilities in our financial statements. These items are monitored and analyzed by us for changes in facts and circumstances, and material changes in these estimates could occur in the future. We base our estimates on historical experience, known trends and events, and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may materially differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in the notes to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K, we believe that the following accounting policies are those most significant to the judgments and estimates used in the preparation of our consolidated financial statements.
Income Taxes
The Company utilizes an asset and liability approach for financial accounting and reporting for income taxes. The provision for income taxes is based upon income or loss after adjustment for those permanent items that are not considered in the determination of taxable income. Deferred income taxes represent the tax effects of differences between the financial reporting and tax basis of the Company’s assets and liabilities at the enacted tax rates in effect for the years in which the differences are expected to reverse.
The Company evaluates the recoverability of deferred tax assets and establishes a valuation allowance when it is more likely than not that some portion or all the deferred tax assets will not be realized. Management makes judgments as to the interpretation of the tax laws that might be challenged upon an audit and cause changes to previous estimates of tax liability. In management’s opinion, adequate provisions for income taxes have been made. If actual taxable income by tax jurisdiction varies from estimates, additional allowances or reversals of reserves may be necessary.
Tax benefits are recognized only for tax positions that are more likely than not to be sustained upon examination by tax authorities. The amount recognized is measured as the largest amount of benefit that is greater than 50 percent likely to be realized upon settlement. A liability for “unrecognized tax benefits” is recorded for any tax benefits claimed in the Company’s tax returns that do not meet these recognition and measurement standards. For the years ended December 31, 2023 and 2022, no liability for unrecognized tax benefits was required to be reported.
There was no income tax benefit recorded for the losses for the years ended December 31, 2023 and 2022 since management determined that the realization of the net deferred tax assets is not more likely than not to be realized and has recorded a full valuation allowance on the net deferred tax assets.
The Company’s policy for recording interest and penalties associated with tax audits is to record such items as a component of general and administrative expense. There were no amounts accrued for penalties and interest for the years ended December 31, 2023 and 2022. The Company does not expect its uncertain tax position to change during the next twelve months. Management is currently unaware of any issues under review that could result in significant payments, accruals or material deviations from its position.
Tax years from 2019 through 2023 remain subject to examination by federal and state jurisdictions.
| 68 |
Share-based Compensation
We account for share-based payments by recognizing compensation expense based upon the estimated fair value of the share-based payments on the date of grant. We determine the estimated fair value of the share-based payments granted using the fair market value of the stock in the case of restricted stock awards or Black-Scholes option pricing model in the case of stock options and recognize compensation costs ratably over the requisite service period which approximates the vesting period using the graded method. To calculate the fair value of the options, certain assumptions are made regarding components of the model, including the fair value of the underlying Common Stock, risk-free interest rate, volatility, expected dividend yield and expected option life. Changes to the assumptions could cause significant adjustments to the valuation. We calculate our volatility assumptions using the actual changes in the market value of our stock. Forfeitures are recognized as they occur. Our historical option exercises do not provide a reasonable basis to estimate an expected term due to the lack of sufficient data. Therefore, we estimate the expected term by using the simplified method. The simplified method calculates the expected term as the average of the vesting term plus the contractual life of the options. The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of the grant for treasury securities of similar maturity. The assumptions used in determining the fair value of share-based awards represent our best estimates, but the estimates involve inherent uncertainties and the application of our judgment. As a result, if factors change and we use significantly different assumptions or estimates, our share-based compensation expense could be materially different in the future.
Off-Balance Sheet Arrangements
We have no significant known off balance sheet arrangements.
Recent Developments
Reverse Stock Split
Effective as of 4:05 p.m. Eastern Standard Time on February 14, 2024, we effected the Reverse Stock Split of our common stock at a ratio of one-for-thirty. Simultaneously with the Reverse Stock Split, number of shares of our common stock authorized for issuance was reduced from 500,000,000 shares to 16,666,666 shares, and our authorized capital stock was reduced from 550,000,000 shares to 66,666,666 shares. All share and per share information in this report have been retroactively adjusted to reflect the Reverse Stock Split.
Delaware Reincorporation
On March 4, 2024, MyMD New Jersey merged with and into its wholly owned subsidiary, MyMD Delaware, with MyMD Delaware being the surviving corporation, pursuant to the Plan of Merger for the purpose of changing the Company’s state of incorporation from New Jersey to Delaware. MyMD Delaware is deemed to be the successor issuer of MyMD New Jersey under Rule 12g-3 of the Securities Exchange Act of 1934, as amended.
The Reincorporation did not result in any change in the Company’s name, business, management, fiscal year, accounting, location of the principal executive offices, assets or liabilities. In addition, the Company’s common stock retains the same CUSIP number and continues to trade on the Nasdaq Capital Market under the symbol “MYMD.” As of the Effective Date of the Reincorporation, the rights of the Company’s stockholders are governed by the Delaware General Corporation Law, the MyMD Delaware Certificate of Incorporation, and the Bylaws of MyMD Delaware.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Not applicable.
Item 8. Financial Statements and Supplementary Data.
The information required by this Item 8 is included at the end of this Annual Report on Form 10-K beginning on page F-1.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
Not applicable.
Item 9A. Controls and Procedures.
Disclosure Controls and Procedures
Our principal executive officer and principal financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in the Securities Exchange Act of 1934, as amended (the “Exchange Act”) Rule 13a-15(e) and 15d-15(e)) as of the end of the period covered by this Annual Report on Form 10-K, have concluded that, based on such evaluation, our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in the reports that we filed or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and is accumulated and communicated to our management, including our principal executive officer and principal financial officers as appropriate to allow timely decisions regarding required disclosure.
| 69 |
Internal Control over Financial Reporting
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) under the Exchange Act. Internal control over financial reporting refers to the process designed by, or under the supervision of, our principal executive officer and principal financial officer, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP, including those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and the disposition of our assets, (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with GAAP and that receipts and expenditures are being made only in accordance with authorizations of our management and board of directors, and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies and procedures may deterioratedeteriorate.
Management evaluated the effectiveness of our internal control over financial reporting based on the 2013 framework in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation management concluded that our internal control over financial reporting was effective as of December 31, 2020.2023.
This Annual Report on Form 10-K does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act, which permits us to provide only management’s report in this Annual Report on Form 10-K.
Changes in Internal Controls over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during our last fiscal quarter ended December 31, 20202023 that have materially affected, or are reasonably likely to affect, our internal control over financial reporting.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
| 70 |
PART III
Item 10. Directors, Executive Officers, and Corporate Governance.
Directors and Executive Officers4
The following table sets forth the names, ages and positions of all of our directors and executive officers and the positions they hold as of the date hereof. Our directors serve until their successors are elected and shall qualify. Executive officers are elected by our board of directors (the “Board”) and serve at the discretion of the directors.
| Name | Age | Position with the Company | |||
| 71 | |||||
| Director, President and Chief | |||||
| Adam Kaplin, M.D., Ph.D. | 57 | Chief Scientific Officer | |||
| Ian Rhodes | 51 | Interim Chief Financial Officer | |||
| Craig Eagle, M.D. | 57 | Director | |||
| Christopher Schreiber | 59 | Director | |||
| Joshua Silverman | 53 | ||||
| Jude Uzonwanne | 49 | Director | |||
| Bill J. White | 63 | ||||
Set forth below is a brief description of the background and business experience of each of our executive officers and directors.
Ian RhodesChris Chapman, M.D., has been our interimdirector since April 16, 2021 and currently serves as our President and Chief Medical Officer. Dr. Chapman previously served as President and Chief Medical Officer of MyMD Pharmaceuticals (Florida), Inc., a Florida corporation previously known as MyMD Pharmaceuticals, Inc. (“MyMD Florida”) effective as of November 1, 2020. He also serves as the Chairman and CEO of Telomir Pharmaceuticals, Inc., since November 2022. From April 2023 through March 2024, Dr. Chapman was the Executive Chairman of MIRA Pharmaceuticals, Inc. Since 1999, Dr. Chapman has also served as the Chief Executive Officer of Chapman Pharmaceutical Consulting, Inc., a consulting organization that provides support to pharmaceutical and biotech companies in North America, Europe, Japan, India and Africa on issues such as product safety, pharmacovigilance, medical devices, clinical trials and regulatory issues. In addition, from 2003-2004, Dr. Chapman served as the Associate Director of Drug Safety, Pharmacovigilance, and Clinical Operations for Organon Pharmaceuticals. Prior to his time at Organon, Dr. Chapman served as Director, Medical Affairs, Drug Safety and Medical Writing Departments at Quintiles (currently known as IQVIA), from 1995-2003. Dr. Chapman has also served on the board of directors of Rock Creek Pharmaceuticals, Inc. (f/k/a Star Scientific, Inc.) from 2007-2016, including as a member of the Audit Committee from 2007-2014, chairperson of the Compensation Committee from 2007-2014, and chairperson of the Executive Search Committee from 2007 to 2014. Dr. Chapman is an experienced executive and global medical expert and has extensive experience in providing monitoring and oversight for ongoing clinical trials including both adult and pediatric subjects. Dr. Chapman is also the founder of the Chapman Pharmaceutical Health Foundation, an IRS Section 501(c)(3) nonprofit organization established to solicit public funds and to support healthcare needs. which was organized in 2006. Dr. Chapman received an executive certificate from the Harvard Kennedy School of Cambridge, Massachusetts for financial management in 2020. Dr. Chapman received his M.D. degree from Georgetown University in Washington, D.C. in 1987, and completed his internship in Internal Medicine, a residency in Anesthesiology and a fellowship in Cardiovascular and Obstetric Anesthesiology at Georgetown. Dr. Chapman’s qualifications to sit on the Board include his extensive experience and leadership roles within the pharmaceutical industry.
Adam Kaplin, M.D., Ph.D., has been our Chief Scientific Officer since April 16, 2021. He previously served as Chief Scientific Officer of MYMD Florida effective as of December 18, 2020. Since June 20, 2022, Dr. Kaplin has served as the President and Chief Scientific Officer of Mira Pharmaceuticals, which is developing novel synthetic cannabinoid analogs for a range of neuropsychiatric conditions. He has been an adjunct faculty member at Johns Hopkins since December 18, 2020, and he served as the Chief Psychiatric Consultant to the Johns Hopkins Multiple Sclerosis and Transverse Myelitis Centers from July 1, 2004 to December 18, 2020. Dr. Kaplin completed his undergraduate training at Yale University and his M.D. and Ph.D. training at the Johns Hopkins School of Medicine. His research training experience includes having trained in the labs of two Nobel Laureates and completed his Ph.D. and postdoctoral training in the Lab of Solomon Snyder, M.D., who was the 2005 recipient of the National Medal of Science (the highest science honor in the United States). Dr. Kaplin investigated the biological basis of the effects of the immune system on mood regulation and cognition, and he provided neuropsychiatric care to patients afflicted with such comorbidities. His research is focused on understanding the biological basis of depression and dementia and discovering new ways to diagnose prognosticate and treat these diseases.
| 71 |
Ian Rhodes has been our Interim Chief Financial Officer since February 1, 2021. Mr. Rhodes joined Brio Financial Group (“Brio”) in January 29, 2021. From March 2020 to December 2020, Mr. Rhodes served as the Interim CFO of Roadway Moving and Storage. From November 2018 to July 2019, he served as Interim CFO of Greyston Bakery and Foundation. From December 2016 to September 2018, Mr. Rhodes served as President, CEO and Director of GlyEco, Inc., and served as CFO of GlyEco, Inc. from February 2016 to December 2016. From May 2014 to January 2016, he served as CFO of Calmare Therapeutics. Mr. Rhodes began his career at PricewaterhouseCoopers, where he worked for 15 years. Mr. Rhodes holds a Bachelor of Science degree in Business Administration with a concentration in Accounting from Seton Hall University and is a licensed CPA in New York.
Christopher C. SchreiberCraig Eagle, M.D. has been our director since April 16, 2021. Dr. Eagle is currently the Chief Medical Officer of Guardant Health, Inc. since 2021. Previously, Dr. Eagle was Vice President of Oncology for Genentech, where he oversaw the medical programs across Genentech’s oncology portfolio. Prior to his current role, Dr. Eagle worked in several positions at Pfizer from 2009 to 2019, including as the oncology business lead in the United Kingdom and Canada, the global lead for Oncology Strategic Alliances and Partnerships based in New York, and as the head of the Oncology Therapeutic Area Global Medical and Outcomes Group, including the U.S. oncology medical business. Through his multiple roles at Pfizer, Dr. Eagle delivered significant business growth and was involved in multiple strategic acquisitions and divestitures. In addition, while at Pfizer, Dr. Eagle oversaw extensive oncology clinical trial programs, multiple regulatory and payer approvals across Pfizer’s oncology portfolio, health outcomes assessments and scientific collaborations with key global research organizations like the National Cancer Institute (NCI), has servedand the European Organization for Research and Treatment of Cancer (EORTC), and led worldwide development of several compounds including celecoxib, aromasin, irinotecan, dalteparin and ozagomicin. Dr. Eagle currently serves as a member of the board of directors and chair of the Science and Policy Committee of Pierian Biosciences, a privately held life sciences company. Dr. Eagle attended Medical School at the University of New South Wales, Sydney, Australia and received his general internist training at Royal North Shore Hospital in Sydney. He completed his hemato-oncology and laboratory hematology training at Royal Prince Alfred Hospital in Sydney and was granted Fellowship in the Royal Australasian College of Physicians (FRACP) and the Royal College of Pathologists Australasia (FRCPA). After his training, Dr. Eagle performed basic research at the Royal Prince of Wales hospital to develop a new monoclonal antibody to inhibit platelets before moving into the pharmaceutical industry. Dr. Eagle’s qualifications to sit on the Board include his long and successful career in the international pharmaceutical industry, his senior executive experience in areas such as business growth, strategic alliances and mergers and acquisition transactions, his experience as a member of both public and private company boards in the healthcare and life science industries, and his wealth of oncology experience, including leading and participating in scientific research, regulatory, pricing & re-imbursement negotiations for compounds in therapeutic areas.
Christopher C. Schreiber has been our Boarddirector since August 8, 2017 and currently serveshe previously at various times as our Chief Executive Officer, President, and President. Prior to his time as our Chief Executive Officer, Mr. Schreiber served as our Executive Chairman an executive officer position, and served as our principal executive officer since November 1, 2019. Mr. Schreiber has been our President since July 21, 2020.of the Board. Mr. Schreiber combines over 30 years of experience in the securities industry. AsMr. Schrieber retired in 2023 from his position as the managing directorManaging Director of capital marketsCapital Markets at Taglich Brothers, Inc. (“Taglich Brothers”), where Mr. Schreiber buildsbuilt upon his extensive background in capital markets, deal structures, and syndications. Prior to his time at Taglich Brothers, Inc., he was a member of the board of directors of Paulson Investment Company, a 40-year-old full-service investment banking firm. In 2023, Mr. Schrieber joined the Board of Directors of Sonon Group, a German based company that focuses on providing solar-powered mobility applications. In addition, Mr. Schreiber serves as a director and partner of Long Island Express North, an elite lacrosse training organization for teams and individuals. He also volunteers on the board of directors for Fox Lane Youth Lacrosse, a community youth program. Mr. Schreiber is a graduate of Johns Hopkins University, where he received a Bachelor’s Degreebachelor’s degree in Political Science.political science. Mr. Schreiber was selectedSchreiber’s qualifications to servesit on the Board in part because ofinclude his significantfinancial expertise and his experience in capital markets and knowledge ofwith the Company.
| 72 |
Joshua Silverman has been our company.
Joshua Silverman, has served as a member of our Boarddirector since September 6, 2018 and currently serves as the Board’s lead independent director and as Chairman of the Board. Prior to the completion of the Merger, Mr. Silverman was also the lead independent director. Mr. Silverman currently serves as the managing member of Parkfield Funding LLC. Mr. Silverman was the co-founder, and a principal and managing partner of Iroquois Capital Management, LLC (“Iroquois”), an investment advisory firm. Since its inception in 2003 until July 2016, Mr. Silverman served as co-chief investment officer of Iroquois. While at Iroquois, he designed and executed complex transactions, structuring and negotiating investments in both public and private companies and has often been called upon by the companies solve inefficiencies as they relate to corporate structure, cash flow, and management. From 2000 to 2003, Mr. Silverman served as co-chief investment officer of Vertical Ventures, LLC, a merchant bank. Prior to forming Iroquois, Mr. Silverman was a director of Joele Frank, a boutique consulting firm specializing in mergers and acquisitions. Previously, Mr. Silverman served as assistant press secretary to the president of the United States. Mr. Silverman currently serves as a director of AYRO Inc., Protagenic Therapeutics,Petros Pharmaceuticals, Inc., Synaptogenix Inc., Femasys Inc., and Neurotrope,Pharmacyte Biotech, Inc., all of which are public companies. He previously served as a director of National Holdings Corporation from July 2014 through August 2016 and as a director of Marker Therapeutics, Inc. from August 2016 until October 2018. Mr. Silverman received his B.A. from Lehigh University in 1992. Mr. Silverman’s qualifications to sit on the Board include his experience as an investment banker,professional, management consultant and as a director of numerous public companies.
Jude Uzonwanne has been our director since April 16, 2021. From June 2022 until April 2023, Mr. Uzonwanne served as the Chief Executive Officer for Mira Pharmaceuticals Inc., a US based biopharmaceutical company focused on developing an oral FDA approved marijuana analog. Prior to Mira, he was the Chief Business Officer at a genetics-based healthcare company, 54gene from March 2021 to June 2022. Prior to 54gene, he was a Principal with ZS Associates, Inc., a consulting and professional services firm, a position he held from January 2021 to March 2021. Prior to joining ZS Associates, Mr. Uzonwanne was a Principal at IQVIA, Inc. from 2018 to 2020, where he served as the head of the firm’s US Financial Investors Consulting practice and as management consulting lead for IQVIA’s service to a top-6 global pharmaceutical company and select emerging biopharmaceutical companies. Prior to joining IQVIA, Mr. Uzonwanne served as Vice President (Associate Partner) at EY-Parthenon LLP from 2016 to 2018, where he managed teams advising corporate and private equity investors on a range of commercial due diligence targets in healthcare strategies and advised clients on growth accelerating strategies and investments. Prior to this role, Mr. Uzonwanne has worked for several other companies including Bain & Company, Dalberg Global Development Advisers, the Bill and Melinda Gates Foundation, and Monitor Group. Mr. Uzonwanne is a graduate of Swarthmore College (double Honors B.A in Economics and Political Science). Mr. Uzonwanne’s qualifications to sit on the Board include his experience as a corporate strategy and transaction services adviser in the healthcare markets globally.
Bill J. White, has served as a member of the Boardbeen our director since August 8, 2017. Mr. White has more than 30 years of experience in financial management, operations and business development. He currently serves Most recently he has served as Chief Financial Officer of Sidus Space, Inc (Nasdaq SIDU), as the chief financial officer for ProPhase Labs Inc. (Nasdaq: PRPH), and the chief financial officer, chief operating officer, treasurer and secretary of Intellicheck, Mobilisa, Inc., a technology company listed on the NYSE MKT.(Nasdaq: IDN). Prior to working at Intellicheck, Mobilisa, Inc., he served 11 years as the chief financial officer, chief operating officer, secretary and treasurer of FocusMicro, Inc. (“FM”). As co-founder of FM, Mr. White played an integral role in growing the business from the company’s inception to over $36 million in annual revenue in a five-year period.leading its international expansion into Dubai, UAE. Mr. White has broad domestic and international experience including managing rapid and significant growth, import/export, implementing tough cost management initiatives, exploiting new growth opportunities, merger and acquisitions, strategic planning, resource allocation, tax compliance and organization development. Prior to co-founding FM, he served 15 years in various financial leadership positions in the government sector. Mr. White started his career in Public Accounting. Mr. White holds a Bachelor of Arts in Business Administration from Washington State University and is a Certified Fraud Examiner. Mr. White was selected to serve on the Board of Directors in part because of his significant financial and accounting experience with public companies.
Robert C. Schroeder, has served as a member of the Board since November 1, 2019. Mr. Schroeder is currently the vice president of investment banking at Taglich Brothers, a brokerage firm, and specializes in advisory services and capital raising for small public and private companies. Prior to his time at Taglich Brothers, Mr. Schroeder served as a Senior Equity Analyst publishing sell-side research on publicly traded companies and served in various other positions in the brokerage and public accounting industry. Mr. Schroeder currently serves on the board of directors of publicly traded Intellinetics, Inc., a document solutions software development, sales and marketing company, Air Industries Group (NYSE:AIRI), a manufacturer of aerospace parts and assemblies, and Decisionpoint Systems, Inc., a leading provider and integrator of Enterprise Mobility, Wireless Applications and RFID solutions. Mr. Schroeder received a B.S. degree in accounting and economics from New York University. He is a Chartered Financial Analyst and a member of the CFA Institute and CFA Society of New York. Mr. Schroeder was selected to serve on the Board because of his leadership skills, capital markets expertise, and extensive experience as a director of the board for other public companies.
Family Relationships
There are no family relationships between any of our officers or directors.
Code of Ethics
We have adopted a Code of Ethics, which applies to our Board of Directors, our executive officers and our employees, outlines the broad principles of ethical business conduct we adopted, covering subject areas such as:
A copy of our Code of Ethics is available without charge, to any person desiring a copy of the Code of Ethics, by written request to us at our principal offices at c/o Akers Biosciences, Inc., 1185 Avenue of the Americas, 3rd Floor, New York, New York 10036.
Board Composition and Committees
On August 27, 2020, our shareholders reelected Christopher C. Schreiber, Joshua Silverman, Bill J. White and Robert C. Schroeder as members of the Board. Mr. Silverman, Mr. Schroeder, and Mr. White comprise the Board’s Audit Committee and Risk and Disclosure Committee. Mr. Silverman and Mr. White comprise the Board’s Compensation Committee, and Nominating and Corporate Governance Committee. Mr. White acts as Chairman of the Audit Committee, and Mr. Silverman acts as Chairman of the Compensation Committee. The directors will serve until our next annual meeting and until their successors are duly elected and qualified.Reforms
On May 28, 2020, the United States District Court for the District of New Jersey approved that certain Amended Stipulation and Agreement of Settlement, dated October 1, 2019 (the “Settlement”) among the settling parties in connection with a consolidated shareholder derivative action, Case No.: 2:18-cv-15992. Pursuant to the Settlement, effective as of July 21, 2020, we made various modifications to our corporate governance and business ethics practices as further discussed below.
Code of Ethics
We have adopted a Code of Business Ethics and Conduct, which applies to our Board, our executive officers and our employees, outlines the broad principles of ethical business conduct we adopted, covering subject areas such as, compliance with applicable laws and regulations, handling of books and records, public disclosure reporting, insider trading, conflicts of interest, competition and fair dealing, and other violations. Our Code of Business Ethics and Conduct is available on our website at www.mymd.com in the “Corporate Governance” section found under the “Investors” tab. Pursuant to the Settlement, we will conduct a review of our Code of Business Ethics and Conduct on an annual basis and to monitor compliance. We intend to disclose any amendments to, or waivers from, our Code of Business Ethics and Conduct at the same website address provided above.
In addition, pursuant to the Settlement, we adopted a Whistleblower Policy to encourage employees, officers and directors to bring forward ethical and legal violations. We have disclosed a copy of the Whistleblower Policy and intend to disclose any amendments to the Whistleblower Policy at the same website address provided above.
Pursuant to the Settlement, we formed a Risk and Disclosure Committee, which is served by the members of the Audit Committee, which reviews our ethics and risk program and internal controls over compliance and identifies and recommends to the Board any changes that it deemed necessary. The Risk and Disclosure Committee also monitors compliance with our Code of Business Ethics and Conduct, reviews and evaluates our public disclosures and disclosure controls and procedures and handle any whistleblower complaints.
Board Composition and Committees
Our Amended and Restated Certificate of Incorporation, as amended (the “Charter”), and our Amended and Restated Bylaws (“Bylaws”) provide that our Board will consist of a number of directors to be determined from time to time solely by resolution of the Board, which is currently set at seven directors. Vacancies or newly created directorships resulting from an increase in the authorized number of directors elected by all of the stockholders having the right to vote as a single class may be filled by a majority of the directors then in office, although less than a quorum, or by a sole remaining director.
We have no formal policy regarding Board diversity. Our Board believes that each director should have a basic understanding of the principal operational and financial objectives and plans and strategies of the Company, our results of operations and financial condition and relative standing in relation to our competitors. We take into consideration the overall composition and diversity of the Board and areas of expertise that director nominees may be able to offer, including business experience, knowledge, abilities and customer relationships. Generally, we will strive to assemble a Board that brings to us a variety of perspectives and skills derived from business and professional experience as we may deem are in our and our stockholders’ best interests. In doing so, we will also consider candidates with appropriate non-business backgrounds.
Director Independence
We are currently listed on the NASDAQNasdaq Capital Market and therefore rely on the definition of independence set forth in the NASDAQNasdaq Listing Rules (“NASDAQNasdaq Rules”). Under the NASDAQNasdaq Rules, a director will only qualify as an “independent director” if, in the opinion of our Board, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Based upon information requested from and provided by each director concerning his background, employment, share ownership, and affiliations with other board members, shareholders, business, contractor and family relationships, as well as the amount of the compensation we pay to each director, we have determined that Mr. Silverman, Mr. White, Dr. Eagle, and Mr. SchroederUzonwanne have no material relationships with us that would interfere with the exercise of independent judgment and are “independent directors” as that term is defined in the NASDAQNasdaq Listing Rules.
Pursuant to the Settlement, we also adopted amendments to our Bylaws to require that at least 50% of the Board will qualify as “independent directors” under the NASDAQNasdaq Rules and that the Chairman of the Board will be an independent director. Currently, more than 50% of the Board qualify as “independent directors” under the NASDAQ Rules,Nasdaq Rules. We are currently in compliance with these requirements.
| 74 |
Board Committees
The Board delegates various responsibilities and authority to different Board committees. Committees regularly report on their activities and actions to the Chairman offull Board. Currently, the Board is an independent director.
Board Committees
We havehas established an Audit Committee, a Compensation Committee, a Nominating and Corporate Governance Committee and a Risk and Disclosure Committee. Committee assignments are re-evaluated annually. Each committeeof these committees operates under a charter that has its ownbeen approved by our Board. The current charter whichof each of these committees is available on our website at www.akersbio.com/investor-center/corporate-governance. Information contained on ourwww.mymd.com in the “Corporate Governance” section under “Investors.” Pursuant to the Settlement, we adopted several amendments to the committee charters. We disclosed these amendments and intend to disclose any future amendments to the charters of these committees at the same website is not incorporated herein by reference.address provided above.
The following table sets forth the membership of each of the Board committees listed above.
| Name | Audit Committee | Compensation Committee | Nomination Corporate Governance Committee | Risk and Disclosure Committee | ||||
| Chris Chapman, M.D. | ||||||||
| Craig Eagle, M.D. | Member | |||||||
| Christopher C. Schreiber | ||||||||
| Joshua Silverman | Member | Chair | Member | Member | ||||
| Jude Uzonwanne | Member | Member | Chair | Member | ||||
| Bill J. White | Chair | Member | Chair |
Audit Committee
Our Audit Committee is responsible for, among other matters:
| ● | monitoring the integrity of our financial reporting process, including critical accounting policies and estimates, and systems of internal controls regarding finance, accounting, legal and regulatory compliance; | |
| ● | monitoring the independence and performance of our independent auditors and our accounting personnel; | |
| ● | providing an avenue of communication among the independent auditors, management, our accounting personnel, and the Board; | |
| ● | appointing and providing oversight for the independent auditors engaged to perform the audit of the financial statements; | |
| ● | discussing the scope of the independent auditors’ examination; | |
| ● | reviewing the financial statements and the independent auditors’ report; | |
| ● | reviewing areas of potential significant financial risk and exposure to us, to the extent that there are any, and assess the steps management has taken to monitor such risks; | |
| ● | monitoring compliance with legal and regulatory requirements; | |
| ● | soliciting recommendations from the independent auditors regarding internal controls and other matters; | |
| ● | making recommendations to the Board; | |
| ● | resolving any disagreements between management and the auditors regarding financial reporting; | |
| ● | preparing the report required by Item 407(d) of Regulation S-K, as required by the rules of the SEC; | |
| ● | reviewing issues regarding accounting principles and financial statement presentation (including any significant changes in our selection or application of accounting principles); and | |
| ● | reviewing the effectiveness of any special accounting steps adopted in light of identified significant and/or material control deficiencies. |
Our Audit Committee is composed of Mr.Bill J. White (chairman)(Chair), Mr.Joshua Silverman, and Mr. Schroeder.Jude Uzonwanne. Our Board has determined that each of the current members of the Audit Committee is independent in accordance with NASDAQNasdaq Rules and Rule 10A-3 under the Securities Exchange Act.Act of 1934, as amended (the “Exchange Act”). Our Board has also reviewed the education, experience and other qualifications of each member of the Audit Committee. Based upon that review, our Board has determined that Mr. White qualifies as an “audit committee financial expert,” as defined by the rules of the SEC.
| 75 |
Compensation Committee
Our AuditCompensation Committee is responsible for, among other matters:
| ● | ||
Compensation Committee
The members of our Compensation Committee are Mr. Joshua Silverman (chairman) and Mr. Bill White. Each such member is “independent” within the meaning of the Nasdaq Stock Market Rules. In addition, each member of our Compensation Committee qualifies as a “non-employee director” under Rule 16b-3 of the Exchange Act. Our Compensation Committee assists the Board of Directors in the discharge of its responsibilities relating to the compensation of the Board of Directors and our executive officers. Mr. Silverman will serve as Chairman of our Compensation Committee.
The Committee’s compensation-related responsibilities include, but are not limited to:
| ● | reviewing and approving on an annual basis the compensation of our executive officers other than our Chief Executive Officer; | |
| ● | reviewing | |
| ● | selecting, retaining and terminating any compensation consultant to be used by the Compensation Committee or us to assist in the evaluation of the compensation of non-employee directors, the | |
| ● | reviewing, approving and, when appropriate, making recommendations to the Board | |
| ● | ||
| ● | determining and approving the options and other equity-based compensation to be granted to executive officers, | |
| ● | in conjunction with the |
Our Compensation Committee is composed of Joshua Silverman (Chair), Craig Eagle, M.D., and Jude Uzonwanne. Our Board has determined that each of the current members of the Compensation Committee is independent in accordance with Nasdaq Rules. The Compensation Committee hasmay delegate the authoritydetermination with respect to directly engage, at our expense, any compensation consultants orpersons other advisers as it deems necessarythan officers to carry out its responsibilities in determining the Chief Executive Officer but will approve the aggregate amount granted to all employees and form of employee, executive and director compensation.all new hire grants.
Nominating and Corporate Governance Committee
The members of our Nominating and Corporate Governance Committee are Mr. Josh Silverman and Mr. Bill White. Each such member is “independent” within the meaning of the Nasdaq Stock Market Rules. The purpose of theOur Nominating and Corporate Governance Committee is to recommend to the board nomineesresponsible for, election as directors and persons to be elected to fill any vacancies on the board, develop and recommend a set of corporate governance principles and oversee the performance of the board.among other matters:
The Committee’s responsibilities include:
| ● | overseeing the administration of our Code of Business Ethics and Conduct and related policies; | |
| ● | leading the search for and recommending individuals qualified to become members of the Board, and selecting director nominees to be presented for election by the shareholders at each annual meeting; | |
| ● | ensuring, in cooperation with the Compensation Committee, that no agreements or arrangements are made with directors or relatives of directors for providing professional or consulting services to us or our affiliate or individual officer or one of their affiliated, without appropriate review and evaluation for conflicts of interest; | |
| ● | ensuring that Board members do not serve on more than | |
| ● | reviewing the Board’s committee structure and to recommend to the Board for its approval; | |
| ● | reviewing recommendations received from shareholders for persons to be considered for nomination to the Board; |
| ● | monitoring compliance with our corporate governance guidelines; | |
| ● | developing and implementing an annual self-evaluation of the Board, both individually and as a Board, and of its committees; | |
| ● | reviewing and recommending changes to procedures whereby shareholders may communicate with the Board; | |
| ● | assessing the independence of directors annually and report to the Board; | |
| ● | recommending to the Board for its approval, the leadership structure of the Board, including whether the Board should have an executive or non-executive Chairman, whether the roles of Chairman and Chief Executive Officer should combine, and whether a Lead Director of the Board should be appointed; provided that such structure shall be subject to the bylaws of the Company then in effect. |
| 76 |
The Nominating and Corporate Governance Committee may delegate any of its responsibilities to subcommittees as it deems appropriate. TheOur Nominating and Corporate Governance Committee is authorized to retain independent legalcomposed of Jude Uzonwanne (Chair), Bill J. White, and other advisors, and conduct or authorize investigations into any matter within the scope of its duties.
Risk and Disclosure Committee
Pursuant to the Settlement, we formed a Risk and Disclosure Committee, which is served by the membersJoshua Silverman. Each of the Auditcurrent appointed Nominating and Corporate Governance Committee which reviews our ethics and risk program and internal controls over compliance and identifies and recommends to the Board any changes that it deems necessary. The Risk and Disclosure Committee also monitors compliance with our Code of Business Ethics and Conduct, reviews and evaluates our public disclosures and procedures and handles any whistleblower complaints. Each member of the Risk and Disclosure Committeemembers is “independent” within the meaning of the NASDAQNasdaq Stock Market Rules. The purpose of the
Risk and Disclosure Committee
Our Risk and Disclosure Committee is to (1) assist the Board in fulfilling its oversight responsibilities relating to (a) the compliance by the Company with the Company’s Code of Ethics and the Whistleblower Policy, (b) the design, implementation and execution of the Company’s Code of Ethics and ethics and risk program and evaluation of the internal controls over compliance; and (c) matters relating to the Company’s Whistleblower Policy and the Code of Ethics; and (2) assist the Board and Company management in establishing an appropriate “tone at the top” and promoting a strong “culture of compliance” throughout the Company, while also recognizing thatresponsible for, among other Board committees assist the Board in fulfilling its oversight responsibilities relating to various areas of legal and regulatory compliance.matters:
The Risk and Disclosure Committee’s responsibilities include:
| ● | reviewing the effectiveness of our Code of Ethics annually, including our ethics and risk program, and recommending to the Board any changes to our policies and internal controls as necessary; | |
| ● | monitoring compliance with our Code of Ethics, and specifically reviewing and evaluating our public disclosures and annually reviewing and evaluating our disclosure controls and procedures; | |
| ● | reviewing and approving any waivers of provisions of the Code of Ethics; | |
| ● | addressing any whistleblower complaints and ensuring that all whistleblower complaints are appropriately reviewed by the Risk and Disclosure Committee and that any appropriate remedial action if necessary is taken based on the results of its review; and | |
| ● | ensuring that non-retaliation policies are instituted and strictly complied with in order to protect any Company employee who reports a whistleblower complaint. |
TheOur Risk and Disclosure Committee is empowered to conduct or cause to be conducted any investigation appropriate to fulfilling its responsibilities,composed of Bill J. White (Chair), Joshua Silverman and shall have direct access to the external auditors, the internal auditor and Company employees as necessary. The Committee shall have the authority to (a) retain, at the expenseJude Uzonwanne. Our Board has determined that each of the Company, the advice and assistance of outside advisors, including independent compliance consultants and independent legal advisors, as it may deem necessary or appropriate to fulfill its responsibilities, (b) conduct or authorize investigations into or studies of matters within the Committee’s responsibilities and (c) perform all acts necessary to fulfill its responsibilities and achieve its objectives under its charter and as otherwise directed by the Board, provided that such acts are not in violationcurrent members of the Certificate of Incorporation or Bylaws of the Company, the Company’s Code of Ethics or the Whistleblower Policy or any laws or regulations applicable to the Company.Risk and Disclosure Committee is independent in accordance with Nasdaq Rules.
Involvement in Certain Legal Proceedings
There have been no material legal proceedings that would require disclosure under the federal securities laws that are material to an evaluation of the ability or integrity of our directors or executive officers, or in which any director, officer, nominee or principal stockholder, or any affiliate thereof, is a party adverse to us or has a material interest adverse to us.
Compliance with Section 16(A)16(a) of the Exchange Act
Section 16(a) of the Exchange Act requires our directors executiveand officers, and persons who beneficially own 10% or more than ten percent of a class of securities registered under Section 12 of the Exchange Actour Common Stock, to file with the SEC initial reports of beneficial ownership and reports of changes in beneficial ownership with the SEC. Directors, executive officers and greater than 10% shareholders are required by the rules and regulations of the SEC to furnish us with copies of all reports filed by them in compliance with Section 16(a).our Common Stock.
Based solely upon a review of copies of Section 16(a) reports and representations received by us from reporting persons, and without conducting any independent investigation of our own, in fiscal year 2020,2023, all Forms 3, 4 and 5 were timely filed with the SEC by such reporting persons.
Item 11. Executive Compensation.
The following is a discussion of the material components of the executive compensation providedarrangements of our named executive officers, comprised of (i) our principal executive officer, (ii) the two most highly compensated executive officers other than the principal executive officer who were serving as executive officers at the end of the 2023 fiscal year and whose salary, as determined by Regulation S-K, Item 402, exceeded $100,000 and (iii) up to ourtwo most highly compensated former executive officers who were no longer serving as an executive officer at the end of the 2023 fiscal year (the individuals falling within categories (i), (ii) and (iii) are collectively referred to as the “named executive officers” for 2020 and 2019 is set forth in detail in the Summary Compensation Table and other tables and the accompanying footnotes and narrative that follow this section.).
Our named executive officers who appear in the 2020 Summary Compensation Table are:for 2023 were as follows:
| ● | Chris Chapman, M.D., President and Chief Medical Officer | |
| ● | Adam Kaplin, M.D., Ph.D., Chief Scientific Officer | |
| ● | Ian Rhodes, CPA, Interim Chief Financial Officer | |
| ● | Paul Rivard, Esq., Former Chief Legal Officer and Former Executive Vice President of Operations and General Counsel |
| 77 |
Effective as of 4:05 pm Eastern Time on February 14, 2024 we filed an amendment to our Amended and Restated Certificate of Incorporation to effect a Reverse Stock Split of the issued and outstanding shares of our Common Stock, at a ratio of 1 for 30. The stock awards listed below have been adjusted to give effect to the Reverse Stock Split.
Summary Compensation Table
| Name and Principal Position | Notes | Year | Salary | Bonus | Stock Awards | Option Awards(1) | All Other Compensation(2) | Total | ||||||||||||||||||||||
| Christopher Chapman, M.D.(3) | 2023 | $ | 464,703 | $ | 200,000 | - | 2,218,565 | (7) | 1,058 | $ | 2,884,326 | |||||||||||||||||||
| President, Chief Medical Officer | 2022 | 161,827 | 100,000 | - | - | - | 261,827 | |||||||||||||||||||||||
| Adam Kaplin, M.D., PhD(4) | 2023 | 250,000 | 100,000 | - | 235,245 | (8) | 8,750 | 593,995 | ||||||||||||||||||||||
| Chief Scientific Officer | 2022 | 245,153 | 100,000 | - | 8,580 | 353,733 | ||||||||||||||||||||||||
| Ian Rhodes, CPA.(5) | 2023 | 162,000 | - | - | - | - | 162,000 | |||||||||||||||||||||||
| Interim Chief Financial Officer | 2022 | 162,000 | - | - | - | - | 162,000 | |||||||||||||||||||||||
| Paul Rivard, Esq.(6) | 2023 | 242,209 | - | - | 235,245 | (9) | 100,819 | 578,273 | ||||||||||||||||||||||
| Former Chief Legal Officer | 2022 | 161,827 | 20,000 | - | - | 6,412 | 188,239 | |||||||||||||||||||||||
| (1) | In accordance with SEC rules, this column reflects the aggregate fair value of option awards granted during the fiscal year ended December 31, 2023, computed as of their respective grant dates in accordance with FASB ASC Topic 718 for share-based compensation transactions. |
| (2) | This column reflects the matching contribution paid to participants of the MyMD Pharmaceuticals 401(k) PS Plan (the “401(k) Plan”) and amounts paid for personal time off and severance of separated employees. |
| (3) | Dr. Chapman was appointed President and Chief |
Summary Compensation Table
The following table summarizes information regarding the compensation awarded of MyMD effective April 16, 2021. Prior to earned by or paid to, our Chief Executive Officer, and our other most highly compensated executive officers who earned in excess of $100,000 during 2020 and 2019.
| Name and | Salary | Bonus | Stock Awards | All Other Compensation | Total | |||||||||||||||||
| Principal Position | Year | $ | $ | $(2) | $ | $ | ||||||||||||||||
| Howard R. Yeaton (1) | ||||||||||||||||||||||
| Former Interim | 2020 | 228,386 | - | - | 12,000 | 240,386 | ||||||||||||||||
| Chief Financial Officer | 2019 | 300,000 | - | 26,302 | 12,000 | 338,302 | ||||||||||||||||
| Christopher C. Schreiber (2) | ||||||||||||||||||||||
| President and Chief Executive Officer | 2020 | 300,000 | 150,000 | 590,240 | (4) | 57,618 | 1,097,858 | |||||||||||||||
| 2019 | 50,000 | - | - | 4,637 | 54,637 | |||||||||||||||||
| |
| (4) | Dr. Kaplin was appointed Chief Scientific Officer of MyMD effective April 16, 2021. Prior to the Merger, Dr. Kaplin served as Chief Scientific Officer of MyMD Florida effective December 18, 2020. For further information regarding the terms of Dr. Kaplin’s employment, see the section below titled “Narrative Disclosure to Summary Compensation Table—Employment of Adam Kaplin, M.D., Ph.D.” |
| (5) | Ian Rhodes serves as our interim Chief Financial Officer |
| (6) | On April 16, 2021, Mr. Rivard entered into an employment agreement, under which he received an annual salary of $165,000. On March 22, 2023, Mr. Rivard was appointed as Chief Legal Officer and his annual salary was increased to $275,000, retroactively to January 1, 2023. Prior to the Merger, Mr. Rivard served as Executive Vice President of Operations and General Counsel of MyMD Florida effective September 21, 2020. Effective as of |
| On April 4, 2023, the Company granted 25,000 non-qualified stock options, on June 7, 2023, the Company granted 10,000 non-qualified stock options, and on September 6, 2023 the Company granted 33,334 non-qualified stock options to Dr. Chapman. | |
| (8) | On June 7, 2023, the Company granted 5,000 non-qualified stock options to Dr. Kaplin. |
| (9) | On June 7, 2023, the Company granted 5,000 non-qualified stock options to Mr. Rivard. |
| 78 |
Narrative Disclosure to Summary Compensation Table
We have entered into employment agreements with each of our Named Executive Officers.
Employment of Chris Chapman, M.D.
Pre-Merger Employment Agreement
Effective November 1, 2020, MyMD Florida and Dr. Chapman entered into an employment agreement, which was subsequently amended by that certain First Amendment to Employment Agreement, dated December 18, 2020, that certain Second Amendment to Employment Agreement dated January 8, 2021, and that certain Third Amendment to Employment Agreement dated February 11, 2021 (such agreement, as amended, the “Chapman Employment Agreement”), pursuant to which Dr. Chapman was appointed President and Chief Medical Officer of MyMD Florida. Under the Chapman Employment Agreement, Dr. Chapman is entitled to an annual base salary of $165,000, payable monthly. Dr. Chapman is also eligible to receive bonus compensation in the form of lump-sum cash payments made within 30 days following the completion of certain specified “Bonus Events” (as defined in the Chapman Employment Agreement). The aggregate amount of bonus compensation payable to Dr. Chapman upon achievement of all specified Bonus Events is $800,000. In addition, Dr. Chapman is eligible to receive additional bonus compensation in connection with his annual performance, determined in the sole discretion of MyMD Florida’s board of directors. Pursuant to and on the effective date of the Chapman Employment Agreement, Dr. Chapman was also granted options to purchase 250,000 shares of MyMD Florida Common Stock, at an exercise price of $1.00 per share. (After giving effect to the Exchange Ratio and the Reverse Stock Split, such MyMD Florida options became options to purchase 3,215 shares of the Company’s Common Stock at an exercise price of $77.10.) Such options all vested immediately upon grant. The options had an original term of lasting until the earlier of (i) ten years from the date of grant or (ii) the second-year anniversary of the effective date of a “Reorganization Event” as defined in the MyMD Pharmaceuticals, Inc. Amended and Restated 2016 Equity Incentive Plan (as amended, the “MyMD Florida Incentive Plan”) (the practical effect of which makes the term of such options expire on the second-year anniversary of the effective date of the merger, which occurred on April 16, 2021). MyMD Florida also agreed to provide and cover the cost of health insurance and disability policies for Dr. Chapman during the term of employment under the Chapman Employment Agreement.
Dr. Chapman’s employment with MyMD Florida pursuant to the Chapman Employment Agreement commenced as of the effective date of the Chapman Employment Agreement and was to continue for a period of two years, unless earlier terminated by either party, with such termination effective upon the provision of written notice to the other party. In the event of termination of Dr. Chapman’s employment with MyMD Florida for cause, MyMD Florida was to pay to Dr. Chapman his monthly base salary for a period of three months following the date that notice of termination of employment is provided, which would be the full extent of MyMD Florida’s obligations with respect to severance payments to Dr. Chapman under the Chapman Employment Agreement.
The Chapman Employment Agreement also contains certain standard confidentiality, work for hire and assignment of inventions provisions.
On August 2, 2020, Dr. Chapman received a discretionary grant of options to purchase 200,000 shares of MyMD Florida Common Stock, at an exercise price of $1.00 per share. All such options vested immediately upon grant. The options had an original term of ten years from the date of grant, subject to certain events described in the applicable award agreement, including Dr. Chapman’s, death, disability, retirement or an “Event of Cause” (as defined in the applicable award agreement). In connection with the Merger Agreement, certain terms of such options were amended. After giving effect to the Exchange Ratio and the Reverse Stock Split, such MyMD Florida options became options to purchase 2,572 shares of the Company’s Common Stock at an exercise price of $77.10. These options expired on April 16, 2023.
Post-Merger Employment Agreement
Immediately following the effective time of the Merger, the Board appointed Dr. Chapman to the offices of President and Chief Medical Officer on the terms of the Chapman Employment Agreement.
On November 24, 2021, the Company and Dr. Chapman entered into a Fourth Amendment to Employment Agreement. This agreement provided that certain performance criteria applicable to Dr. Chapman’s bonus compensation under the Chapman Employment Agreement would be waived and deemed to have been achieved, and that Dr. Chapman would be entitled to a bonus payment of $100,000 as a result. On August 30, 2022, the Company and Dr. Chapman entered into a Fifth Amendment to amend one of the performance criteria under the Chapman Employment Agreement, upon the achievement of which by the Company Dr. Chapman would be entitled to an additional bonus payment of $100,000. On February 1, 2023, the Company and Dr. Chapman entered into a Sixth Amendment providing for Dr. Chapman’s annual base salary to be set at $310,000, effective retroactively to January 1, 2023, and on September 8, 2023, the Company and Dr. Chapman entered into a Seventh Amendment providing for Dr. Chapman’s annual base salary to be set at $500,000, effective retroactively to January 1, 2023.
November 2023 Amendment
Effective November 13, 2023, the Company entered the Eighth Amendment to the employment agreement of Dr. Chapman providing for Dr. Chapman’s annual base salary to be adjusted from five hundred thousand dollars ($500,000) (the “Full Base Salary”) to two hundred fifty thousand dollars ($250,000) in cash per annum, until payment of his Full Base Salary would no longer jeopardize the Company’s ability to continue as a going concern, as determined by the Company in its sole discretion. The amendment further provides that the remaining $250,000 of base salary per annum (the “Deferral Amount”) shall be deferred until payment of the Deferral Amount would no longer jeopardize the Company’s ability to continue as a going concern, as determined by the Company in its sole discretion, at which time the Deferral Amount may be paid, at Dr. Chapman’s election, in shares of Common Stock or in cash.
| 79 |
Employment of Adam Kaplin, M.D., Ph.D.
Pre-Merger Employment Agreement
Effective December 18, 2020, MyMD Florida and Dr. Kaplin entered into an employment agreement, which was subsequently amended by that certain First Amendment to Employment Agreement, dated February 11, 2021 (such agreement, as amended, the “Kaplin Employment Agreement”), pursuant to which Dr. Kaplin was appointed Chief Scientific Officer of MyMD Florida. Under the Kaplin Employment Agreement, Dr. Kaplin is entitled to an annual base salary of $250,000, payable monthly. Dr. Kaplin is also eligible to receive bonus compensation in the form of lump-sum cash payments made within 30 days following the completion of certain specified “Bonus Events” (as defined in the Kaplin Employment Agreement). The aggregate amount of bonus compensation payable to Dr. Kaplin upon achievement of all specified Bonus Events is $800,000. In addition, Dr. Kaplin is eligible to receive additional bonus compensation in connection with his annual performance, determined in the sole discretion of MyMD Florida’s board of directors. On the effective date of the Kaplin Employment Agreement, Dr. Kaplin received a signing bonus in the form of a lump-sum cash payment in the amount of $100,000 and was also granted options to purchase 400,000 shares of MyMD Florida Common Stock, at an exercise price of $1.00 per share. (After giving effect to the Exchange Ratio and the Reverse Stock Split, such MyMD Florida options became options to purchase 5,145 shares of the Company’s Common Stock at an exercise price of $77.10.) Such options all vested immediately upon grant. The options had an original term of lasting until the earlier of (i) ten years from the date of grant or (ii) the second-year anniversary of the effective date of a “Reorganization Event” as defined in the MyMD Florida Incentive Plan (the practical effect of which makes the term of such options expire on the second-year anniversary of the effective date of the merger, which occurred on April 16, 2021). MyMD Florida also agreed to provide and cover the cost of health insurance and disability policies for Dr. Kaplin during the term of employment under the Kaplin Employment Agreement.
Dr. Kaplin’s employment with MyMD Florida pursuant to the Kaplin Employment Agreement commenced on December 18, 2020 and was to continue for a term of two years unless earlier terminated by either party, with such termination effective upon the provision of written notice to the other party. In the event of termination of Dr. Kaplin’s employment with MyMD Florida for cause, MyMD Florida was to pay to Dr. Kaplin his monthly base salary for a period of three months following the date that notice of termination of employment is provided, which would be the full extent of MyMD Florida’s obligations with respect to severance payments to Dr. Kaplin under the Kaplin Employment Agreement.
The Kaplin Employment Agreement also contained certain standard confidentiality, work for hire and assignment of inventions provisions.
Post-Merger Employment Agreement
Immediately following the effective time of the Merger, the Board appointed Dr. Kaplin to the office of Chief Scientific Officer on the terms of the Kaplin Employment Agreement.
On November 24, 2021, the Company and Dr. Kaplin entered into a Second Amendment to Employment Agreement. This agreement provided that certain performance criteria applicable to Dr. Kaplin’s bonus compensation under the Kaplin Employment Agreement would be waived and deemed to have been achieved, and that Dr. Kaplin would be entitled to a bonus payment of $100,000 as a result. On August 30, 2022, the Company and Dr. Kaplin entered into a Third Amendment to amend one of the performance criteria under the Kaplin Employment Agreement, upon the achievement of which by the Company Dr. Kaplin would be entitled to an additional bonus payment of $100,000.
November 2023 Amendment
Effective November 13, 2023, the Company entered into an amendment to the employment agreement of Dr. Adam Kaplin providing that Dr. Kaplin’s employment shall have an initial term of four months, which the parties may mutually agree to extend for additional consecutive terms of one month each. The amendment further provided that, in the event of termination without cause by the Company prior to the end of the initial term, Dr. Kaplin would receive his monthly base salary through the end of the initial term. The amendment further provided that all outstanding and unvested shares granted pursuant to the Nonqualified Stock Option Agreement, dated June 7, 2023, between the Company and Dr. Kaplin shall accelerate upon the termination of Dr. Kaplin’s employment. Dr. Kaplin’s amendment further provided that, in the event of a termination for any reason prior to the end of the first renewal term following the end of the initial term, the Company would continue to cover the costs of Dr. Kaplin’s health insurance coverage through the end of the first renewal term, subject to the execution and timely return of a release. The initial term ended on March 12, 2024, and the term of Dr. Kaplin’s employment agreement was not extended. Dr. Kaplin serves as the Company’s Chief Scientific Officer and receives a salary of $125,000 per annum and benefits without an employment agreement.
| 80 |
Employment of Ian Rhodes
On July 21, 2020, the Company entered into a CFO Consulting Agreement (the “Consulting Agreement”) with Brio Financial Group (“Brio”). Effective as of January 29, 2021, the Company appointed Ian Rhodes as its interim Chief Financial Officer. Pursuant to the Consulting Agreement, the Company paid Brio an initial retainer fee of $7,500 and paid a fixed monthly payment of $13,500. The Consulting Agreement also provided that the Company would be billed for travel and other out-of-pocket costs, such as report production, postage, etc. The Consulting Agreement expired on June 30, 2021. Since that time, Mr. Rhodes has continued to serve as the Company’s interim Chief Financial Officer under the same terms set forth in the Consulting Agreement.
Employment of Paul Rivard, Esq.
Pre-Merger Employment Agreement
Effective September 21, 2020, MyMD Florida and Mr. Rivard entered into an employment agreement (such agreement, as amended, the “Rivard Employment Agreement”), pursuant to which Mr. Rivard was appointed Executive Vice President of Operations and General Counsel of MyMD Florida. Under the Rivard Employment Agreement, Mr. Rivard was entitled to an annual base salary of $165,000, payable monthly. Mr. Rivard was also eligible to receive bonus compensation in the form of lump-sum cash payments made within 30 days following the completion of certain specified “Bonus Events” (as defined in the Rivard Employment Agreement). The aggregate amount of bonus compensation payable to Mr. Rivard upon achievement of all specified Bonus Events was $160,000. In addition, Mr. Rivard was eligible to receive additional bonus compensation in connection with his annual performance, determined in the sole discretion of MyMD Florida’s board of directors. On the effective date of the Rivard Employment Agreement, Mr. Rivard was granted options to purchase 200,000 shares of MyMD Florida Common Stock, at an exercise price of $1.00 per share. (After giving effect to the Exchange Ratio and the Reverse Stock Split, such MyMD Florida options became options to purchase 2,572 shares of the Company’s Common Stock at an exercise price of $77.70.) Such options all vested immediately upon grant. The options had an original term of lasting until the earlier of (i) ten years from the date of grant or (ii) the second-year anniversary of the effective date of a “Reorganization Event” as defined in the MyMD Florida Incentive Plan (the practical effect of which makes the term of such options expire on the second-year anniversary of the effective date of the merger, which occurred on April 16, 2021). MyMD Florida also agreed to provide and cover the cost of health insurance and disability policies for Mr. Rivard during the term of employment under the Rivard Employment Agreement.
Mr. Rivard’s employment with MyMD Florida pursuant to the Rivard Employment Agreement commenced on September 21, 2020 and was to continue until terminated by either party, with such termination effective upon the provision of written notice to the other party. In the event of termination of Mr. Rivard employment with MyMD Florida, MyMD Florida was to pay to Mr. Rivard his monthly base salary for a period of three months following the date that notice of termination of employment is provided.
The Rivard Employment Agreement also contained certain standard confidentiality, work for hire and assignment of inventions provisions.
Post-Merger Employment Agreement
Immediately following the effective time of the Merger, the Board appointed Mr. Rivard to the office of Executive Vice President of Operations and General Counsel on the terms of the Rivard Employment Agreement.
On March 22, 2023, Mr. Rivard was appointed Chief Legal Officer and his annual salary was increased to $275,000, retroactively to January 1, 2023.
Separation
Effective November 13, 2023, the Company entered into a mutual employment separation agreement with Paul M. Rivard, its Chief Legal Officer. The separation agreement provided for a lump-sum severance payment equal to three months of his normal base salary in exchange for a waiver and release. The separation agreement further provided that Mr. Rivard will be deemed a contractor providing services to the Company for purposes of any awards previously granted to him under the 2021 Plan if at the relevant time(s) he is providing services to the Company while under the employ of a law firm representing the Company.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information concerning the outstanding equity awards that have been previously awarded to each of our Named Executive Officers and which remain outstanding as of December 31, 2023:
| Named Executive Officer | Number of securities underlying unexercised options exercisable | Number of securities underlying unexercised options unexercisable | Option exercise price | Option expiration date(1) | Number of shares or units of stock that have not vested | Market value of shares or units of stock that have not vested | ||||||||||||||||
| Christopher Chapman, M.D. | 8,333 | (1) | 16,667 | $ | 46.50 | 4/4/2028 | - | $ | - | |||||||||||||
| President, Chief Medical Officer | 1,333 | (2) | 6,667 | 49.80 | 6/7/2033 | - | - | |||||||||||||||
| - | (3) | 33,334 | 24.30 | 9/6/2033 | - | - | ||||||||||||||||
| - | - | - | n/a | 20,000 | (4) | 4,854,000 | ||||||||||||||||
| Adam Kaplin, M.D., PhD | 1,667 | (2) | 3,333 | 49.80 | 6/7/2033 | - | - | |||||||||||||||
| Chief Scientific Officer | - | - | - | n/a | 20,000 | (4) | 4,854,000 | |||||||||||||||
| Ian Rhodes | - | - | - | - | - | - | ||||||||||||||||
| Interim Chief Financial Officer | - | - | - | - | - | - | ||||||||||||||||
| Paul Rivard, Esq | 1,667 | (2) | 3,333 | 49.80 | 6/7/2033 | - | - | |||||||||||||||
| Former Chief Legal Officer | - | - | - | n/a | 6,667 | (4) | 1,618,000 | |||||||||||||||
| (1) | Granted April 4, 2023. One third of the options awarded on such date vest immediately, one third vest on the first anniversary date of the grant date, and one third vest on the second anniversary of the grant date. |
| (2) | Granted June 7, 2023. One third of the options awarded on such date vest immediately, one third vest on the first anniversary date of the grant date, and one third vest on the second anniversary of the grant date. |
| (3) | Granted on September 6, 2023. These options vest at various times based upon the achievement of various performance milestones. |
| (4) | Granted on October 14, 2021. These RSUs vest at various times based upon the market capitalization of the company. |
| 81 |
Director Compensation
The following table presents the total compensation for each person who served as a member of our Board during 2023. All compensation paid to Dr. Chapman during 2023 is reported under the Summary Compensation Table. Other than as set forth in the table and described more fully below, we did not pay any compensation, reimburse any expense of, make any equity awards or non-equity awards to, or pay any other compensation to any of the other members of our Board in such period.
| Name | Fees earned or paid in cash | Stock Awards(1) | All Other Compensation(2) | Total | ||||||||||||
| Josh Silverman | $ | 216,000 | $ | - | - | $ | 216,000 | |||||||||
| Bill J. White | 96,000 | - | - | 96,000 | ||||||||||||
| Craig Eagle, M.D | 96,000 | - | - | 96,000 | ||||||||||||
| Jude Uzonwanne | 96,000 | - | - | 96,000 | ||||||||||||
| Christopher Schreiber(3) | - | - | 310,892 | 310,892 | ||||||||||||
| (1) | In accordance with SEC rules, this column reflects the aggregate fair value of stock awards granted during the fiscal year ended December 31, |
| |
Narrative Disclosure to Summary Compensation Table
We have entered into employment agreements with each of our named executive officers.
Employment of Christopher C. Schreiber
On January 24, 2020, our Board independently reviewed and approved entering into an executive chairman agreement with Christopher C. Schreiber (the “Executive Chairman Agreement”). Pursuant to the Executive Chairman Agreement, Mr. Schreiber agreed to serve as the Executive Chairman of our Board, as long as he is a member of the Board, or until termination of the Executive Chairman Agreement (as described below) or upon his earlier death, incapacity, removal, or resignation. On November 20, 2020, Mr. Schreiber resigned from his position as Executive Chairman of the Board and was appointed as our Chief Executive Officer, effective November 20, 2020, with Mr. Schreiber to continue serving as our principal executive officer and president. Mr. Schreiber’s Executive Chairman Agreement remains in effect, except for the title of his position. Pursuant to the Executive Chairman Agreement, Mr. Schreiber is entitled to receive: (i) an annual base salary of $300,000, payable monthly in equal installments, paid retroactively as of November 1, 2019 (it being agreed that such fee shall be inclusive of any fees associated with Schreiber’s services as both a director of Akers and in the capacity of Executive Chairman), (ii) employee benefits including, health insurance, dental insurance, basic life and accidental death and dismemberment insurance, long and short term disability insurance and participation in our 401(k) Plan, (iii) annual or other bonuses in cash and/or in securities of Akers and/or otherwise, which bonuses, if any, shall be awarded in the complete discretion of the Board or a designated committee thereof and (iv) reimbursements for pre-approved reasonable business-related expenses incurred in good faith in the performance of Mr. Schreiber’s duties for the Company.
The Executive Chairman Agreement established an “at will” employment relationship pursuant to which Mr. Schreiber served as Executive Chairman. We may terminate the Executive Chairman Agreement for any reason or no reason, and Mr. Schreiber may voluntarily resign for any reason or no reason with sixty (60) days’ notice. The Executive Chairman Agreement also provides that Mr. Schreiber may not compete against us or solicit our employees or customers for a period of one (1) year after termination of the Executive Chairman Agreement or his association with us for any reason.
Employment of Howard R. Yeaton
Effective on October 5, 2018, the Board of Directors appointed Howard R. Yeaton, who through FCS served previously as a consultant to us, to serve as our Chief Executive Officer and Interim Chief Financial Officer. Mr. Yeaton is the managing principal of FCS and our relationship with FCS shall continue, with FCS continuing to provide accounting services to us. During the year ended December 31, 2020, we paid a total of $32,823 to FCS in connection with these services, and during the year ended December 31, 2019, we paid a total of $49,972 to FCS in connection with these services. In connection with his appointment as our Chief Executive Officer and interim Chief Financial Officer, we and Mr. Yeaton entered into an offer of employment, dated October 5, 2018 which terminated December 31, 2019, after which date Mr. Yeaton stopped serving as our Chief Executive Officer. The employment agreement provided for the following compensation for Mr. Yeaton: (i) twenty-five thousand dollars ($25,000) per month in base salary, (ii) a monthly grant of one hundred fifty six (156) unrestricted shares of the our common stock pursuant to the Akers Biosciences, Inc. 2017 Stock Incentive Plan, (iii) Mr. Yeaton will be afforded other employee benefits including, health insurance, dental insurance, basic life and accidental death and dismemberment insurance, long and short term disability insurance and participation in our 401(k) Plan, and (iv) will be reimbursed for reasonable and necessary travel and business expenses including the expenses of travel and hotel stays in or near Thorofare, New Jersey.
On January 6, 2020, the Board appointed Mr. Yeaton as our interim Chief Financial Officer. In connection with his appointment as our interim Chief Financial Officer, we and Mr. Yeaton entered into a new offer of employment, dated January 6, 2020, which was scheduled to terminate on August 19, 2020. Pursuant to such agreement, Mr. Yeaton received: (i) twenty-five thousand dollars ($25,000) per month in base salary, (ii) employee benefits including health insurance, dental insurance, basic life and accidental death and dismemberment insurance, long and short term disability insurance and participation in our 401(k) Plan, and (iii) reimbursement of reasonable and necessary travel and business expenses including the expenses of travel and hotel stays in or near Thorofare, New Jersey. Pursuant to a mutual understanding between Akers and Mr. Yeaton, Mr. Yeaton’s employment as interim Chief Financial Officer ceased as of August 19, 2020.
On July 21, 2020, we entered into a CFO Consulting Agreement (the “Consulting Agreement”) with Brio Financial Group (“Brio”), pursuant to which we appointed Mr. Stuart Benson as Interim Chief Financial Officer, effective August 19, 2020, with a term ending June 30, 2021. Pursuant to the Consulting Agreement, the Company will pay Brio an initial retainer fee of $7,500 and a fixed monthly payment of $13,500, commencing August 15, 2020. On January 28, 2021, Stuart Benson notified us that his employment as Interim Chief Financial Officer of the Company would cease effective as of January 29, 2021, as Mr. Benson’s employment with Brio would come to an end on the same date. Effective as of February 1, 2021, we appointed Ian Rhodes as our new Interim Chief Financial Officer, pursuant to the same Consulting Agreement, with a term ending June 30, 2021.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information concerning the outstanding equity awards that have been previously awarded to Mr. Schreiber and which remained outstanding as of December 31, 2020.
| Name | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | ||||||
| Christopher C. Schreiber President and Chief Executive Officer | 263,500 | (1) | 590,240 | |||||
(1) Granted on September 11, 2020.
On January 23, 2014, we adopted the 2013 Stock Incentive Plan (“2013 Plan”). The 2013 Plan was amended by the our Board on January 9, 2015 and September 30, 2016, and such amendments were ratified by stockholders on December 7, 2018. The 2013 Plan provides for the issuance of up to 4,323 shares of Akers common stock, and 1,510 shares of common stock remain available for grants under the 2013 Plan.
On August 7, 2017, the stockholders approved, and Akers adopted the 2017 Stock Incentive Plan (“2017 Plan”). The 2017 Plan provides for the issuance of up to 7,031 shares of Akers common stock. The purpose of the 2017 Plan is to provide additional incentive to those of our officers, employees, consultants and non-employee directors and our parents, subsidiaries and affiliates whose contributions are essential to the growth and success of our business. As of December 31, 2020, grants of restricted stock and options to purchase totaling 3,064 shares of common stock have been issued pursuant to the 2017 Plan and 3,967 shares of common stock remain available for grants under the 2017 Plan. The 2017 Plan provides for the issuance of shares of Akers common stock through the grant of non-qualified options, incentive options, restricted stock and unrestricted stock to directors, officers, consultants, attorneys, advisors and employees.
On December 7, 2018, the stockholders approved, and we adopted the 2018 Plan and on August 27, 2020, the stockholders approved, and we adopted an amendment to the plan to increase the number of shares of common stock available for issuance pursuant to awards under the 2018 Plan by an additional 1,042,000 shares. The 2018 Plan, as amended, provides for the issuance of up to 1,120,125 shares of Akers common stock. The purpose of the 2018 Plan is to provide additional incentive to those of our officers, employees, consultants and non-employee directors and to promote the success of our business. As of December 31, 2020, grants of RSUs to purchase 804,963 shares of common stock have been issued pursuant to the 2018 Plan, and 315,162 shares of common stock remain available for issuance. The 2018 Plan provides for the issuance of shares of Akers common stock through the grant of options, restricted stock, stock appreciation rights, other stock-based awards, performance compensation awards to directors, officers, consultants, advisors and employees. In addition, the 2018 Plan provides the Compensation Committee of the Board with discretion to accelerate the vesting and exercisability of outstanding awards upon the occurrence of a change of control (as defined in the 2018 Plan).
On March 29, 2019, the Compensation Committee of the Board approved the grant of 5,201 RSUs to Mr. Schreiber. Each RSU had a grant date fair value of $23.28 which shall be amortized on a straight-line basis over the vesting period into administrative expenses within our Consolidated Statement of Comprehensive Loss. Such RSUs were granted under the 2018 Plan, and vested on January 1, 2020.
On August 27, 2020, we held our 2020 annual meeting of stockholders. At the annual meeting, the stockholders approved an amendment to the 2018 Plan to increase the number of shares of common stock available for issuance pursuant to awards under the 2018 Plan by an additional 1,042,000 shares, to a total of 1,120,125 shares of Akers common stock.
On September 11, 2020, the Compensation Committee of our Board approved the grant of 263,500 RSUs to Mr. Schreiber. Each RSU had a grant date fair value of $2.24 which shall be amortized on a straight-line basis over the vesting period into administrative expenses within our Consolidated Statement of Comprehensive Loss. Such RSUs were granted under the 2018 Plan, with 50% to vest on the first anniversary of the date of grant, and the remaining 50% to vest on the second anniversary of the date of grant, provided that the RSUs shall vest immediately upon the occurrence of (i) a change in control, provided that Mr. Schreiber is employed or providing services to us and our affiliates on the closing date of such change in control, (ii) Mr. Schreiber’s termination of employment or services to us and our affiliates by reason of death or disability, or (iii) Mr. Schreiber’s termination of employment or services by us without cause. At our election, the vested RSUs may be settled for cash.
Director Compensation
The following table sets forth summary information concerning the total compensation earned for each non-employee member of the Board during the year ended December 31, 2020 and is contemplated to continue serving as a director of the combined company. All compensation paid to Mr. Schreiber is reported under the Summary Compensation Table.
| Name | Fees earned or paid in cash ($) | Stock Awards ($) (1) | Total ($) | |||||||||
| Josh Silverman (2) | 176,000 | 490,560 | (5) | 666,560 | ||||||||
| Bill J. White (3) | 96,000 | 490,560 | (5) | 586,560 | ||||||||
| Robert Schroeder (4) | 96,000 | 196,806 | (5) | 292,806 | ||||||||
| (2) | |
| (3) | On January 24, 2020, Mr. |
Narrative Disclosure to Director Compensation Table
As approved by the Compensation Committee of the Board on March 29, 2019, beginning in April 2019, each serving director who is not also holding a position as an executive officer is paid $8,000 per month. On or around May 2020, the Compensation Committee of the Board approved payments to Mr. Silverman of $18,000 per month, beginning in May 2020. All director fees were paid on a monthly basis. There was no other compensation for directors during the year ended December 31, 2020.2022.
On September 11, 2020,November 13, 2023, the Board approved certain adjustments to the director fees. Mr. Silverman’s fees were decreased from $216,000 to $60,000 annually, with payment of the excess amount of $156,000 deferred until the date that payment of such amount would no longer jeopardize the Company’s ability to continue as a going concern, as determined by the Company in its sole discretion, at which time such amount may be paid, at Mr. Silverman’s election, in shares of Common Stock or in cash. Messrs. Eagle’s, Uzonwanne’s, and White’s fees were decreased from $96,000 to $60,000 annually, with payment of the excess amounts of $36,000 per director deferred until the date that payment of such amounts would no longer jeopardize the Company’s ability to continue as a going concern, as determined by the Company in its sole discretion, at which time such amounts may be paid, at each director’s election, in shares of Common Stock or in cash.
| 82 |
On October 14, 2021, the Compensation Committee of the Board approvedauthorized the grantissuance of 263,500 RSUs to Mr. Schreiber, 219,000 RSUs to each of Mr. Silverman and Mr. White; and 87,860 RSUs to Mr. Schroeder. Each RSU had93,166 restricted stock units with a grant date fair market value of $2.24 which shall$242.70 per RSU to the directors and key employees of the Company. These RSUs will vest in thirds when certain market capitalization milestones are met and maintained for twenty consecutive trading sessions. Upon achievement of a vesting milestone, the expenses related to the vested RSUs will be amortizedrecorded at the fair market value of the Company’s Common Stock on a straight-line basis over the vesting period into administrative expenses within our Consolidated Statementdate of Comprehensive Loss. Such RSUs were granted undervesting.
On June 5, 2023, the 2018 Plan,Compensation Committee of the Board authorized the issuance, effective as of June 7, 2023, of options to purchase an aggregate of 66,498 shares of Common Stock with 50%an exercise price of $49.80 per share to vestthe directors and key employees of the Company. These options vested (i) one third on the date of grant; (ii) one third on the first anniversary of the date of grant,grant; and the remaining 50% to vest(iii) one third on the second anniversary of the date of grant, provided that the holder remains employed by the Company or a subsidiary on the applicable vesting date.
Equity Compensation Plans
2021 Equity Incentive Plan
Pursuant to the Merger Agreement, at the effective time of the Merger, the Company adopted the 2021 Equity Incentive Plan (the “2021 Plan”), which was approved by the Company’s stockholders on April 15, 2021. The 2021 Plan provides for the granting of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock, restricted stock units, performance awards, and other awards which may be granted singly, in combination or in tandem, and which may be paid in cash or shares of Common Stock. At the effective time of the Merger, the number of shares of Common Stock that were reserved for issuance pursuant to awards under the 2021 Plan was 240,940 shares. As of December 31, 2023, 10,622 shares remain available for issuance under the 2021 Plan.
Purpose. The purpose of the 2021 Plan is to enable the Company to remain competitive and innovative in its ability to attract and retain the services of key employees, key contractors, and non-employee directors of the Company or any of its subsidiaries. The 2021 Plan provides for the granting of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock, restricted stock units, performance awards, and other awards, which may be granted singly, in combination, or in tandem, and which may be paid in cash or shares of the Company’s Common Stock. The 2021 Plan is expected to provide flexibility to the Company’s compensation methods in order to adapt the compensation of key employees, key contractors, and non-employee directors to a changing business environment, after giving due consideration to competitive conditions and the impact of applicable tax laws.
Effective Date and Expiration. The 2021 Plan was approved by the Company’s Board of Directors on March 18, 2021 (the “Plan Effective Date”) and approved by the Company’s stockholders on April 15, 2021. The 2021 Plan will terminate on the tenth anniversary of the Plan Effective Date, unless sooner terminated by the Company’s Board of Directors. No awards may be made under the 2021 Plan after its termination date, but awards made prior to the termination date may extend beyond that date in accordance with their terms.
Share Authorization. At the effective time of the Merger, the number of shares of Common Stock that were reserved for issuance pursuant to awards under the 2021 Plan was 240,940 shares, 100% of which may be delivered as incentive stock options. Shares to be issued may be made available from authorized but unissued shares of the Company’s Common Stock, shares held by the Company in its treasury, or shares purchased by the Company on the open market or otherwise. During the term of the 2021 Plan, the Company will at all times reserve and keep enough shares available to satisfy the requirements of the 2021 Plan. If an award under the 2021 Plan is cancelled, forfeited, or expires, in whole or in part, the shares subject to such forfeited, expired, or cancelled award may again be awarded under the 2021 Plan. Awards that may be satisfied either by the issuance of Common Stock or by cash or other consideration shall be counted against the maximum number of shares that may be issued under the 2021 Plan only during the period that the award is outstanding or to the extent the award is ultimately satisfied by the issuance of shares. An award will not reduce the number of shares that may be issued pursuant to the 2021 Plan if the settlement of the award will not require the issuance of shares, as, for example, a stock appreciation right that can be satisfied only by the payment of cash. Shares of Common Stock that are otherwise deliverable pursuant to an award under the 2021 Plan that are withheld in payment of the option price of an option or for payment of applicable employment taxes and/or withholding obligations resulting from the award shall be treated as delivered to the award recipient and shall be counted against the maximum number of shares of our Common Stock that may be issued under the 2021 Plan. Only shares forfeited back to the Company or cancelled on account of termination, expiration, or lapse of an award shall again be available for grant of incentive stock options under the 2021 Plan but shall not increase the maximum number of shares described above as the maximum number of shares of the Company’s Common Stock that may be delivered pursuant to incentive stock options.
Administration. The 2021 Plan is administered by the compensation committee of the Board or such other committee of the board as is designated by it to administer the 2021 Plan (the “2021 Plan Administration Committee”). If necessary to satisfy the requirements of Rule 16b-3 promulgated under the Exchange Act, membership on the 2021 Plan Administration Committee shall be limited to those members of the Board who are “non-employee directors” as defined in Rule 16b-3 promulgated under the Exchange Act. At any time there is no 2021 Plan Administration Committee to administer the 2021 Plan, any reference to the 2021 Plan Administration Committee is a reference to the Board.
The 2021 Plan Administration Committee will determine the persons to whom awards are to be made; determine the type, size, and terms of awards; interpret the 2021 Plan; establish and revise rules and regulations relating to the 2021 Plan as well as any sub-plans for awards to be made to eligible award recipients who are not resident in the United States; establish performance goals for awards and certify the extent of their achievement; and make any other determinations that it believes are necessary for the administration of the 2021 Plan. The 2021 Plan Administration Committee may delegate certain of its duties to one or more of the Company’s officers as provided in the 2021 Plan. Notwithstanding the foregoing, to the extent necessary to satisfy the requirements of Rule 16b-3 promulgated under the Exchange Act, any function relating to an award recipient subject to the reporting requirements of Section 16 of the Exchange Act shall be performed solely by the 2021 Plan Administration Committee.
Upon the adoption of the 2021 Plan, awards granted under the 2018 Plan (as defined below) remained in full force and effect under the terms and conditions of the 2018 Plan and in accordance with each award’s respective terms.
Eligibility. Employees (including any employee who is also a director or an officer), contractors, and non-employee directors of the Company or any of its subsidiaries, whose judgment, initiative, and efforts contributed to or may be expected to contribute to the Company’s successful performance, are eligible to participate in the 2021 Plan. As of the December 31, 2023, the Company had 5 employees, 1 contractor, and 4 non-employee directors who would be eligible for awards under the 2021 Plan.
| 83 |
Stock Options. The 2021 Plan Administration Committee may grant either incentive stock options (“ISOs”) qualifying under Section 422 of the Code, or nonqualified stock options, provided that only employees of the Company and its subsidiaries (excluding subsidiaries that are not corporations) are eligible to receive ISOs. Stock options may not be granted with an option price less than 100% of the fair market value of a share of Common Stock on the date the stock option is granted. If an ISO is granted to an employee who owns or is deemed to own more than 10% of the combined voting power of all classes of the Company’s stock (or of any parent or subsidiary), the option price shall be at least 110% of the fair market value of a share of Common Stock on the date of grant. The 2021 Plan Administration Committee will determine the terms of each stock option at the time of grant, including, without limitation, the methods by or forms in which shares will be delivered to participants or registered in their names. The maximum term of each option, the times at which each option will be exercisable, and provisions requiring forfeiture of unexercised options at or following termination of employment or service generally are fixed by the 2021 Plan Administration Committee, except that the 2021 Plan Administration Committee may not grant stock options with a term exceeding 10 years or, in the case of an ISO granted to an employee who owns or is deemed to own more than 10% of the combined voting power of all classes of our stock (or of any parent or subsidiary), a term exceeding five years.
Recipients of stock options may pay the option price (i) in cash, check, bank draft, or money order payable to the order of the Company; (ii) by delivering to the Company shares of the Company’s Common Stock (including restricted stock) already owned by the participant having a fair market value equal to the aggregate option price and that the participant has not acquired from the Company within six months prior to the exercise date; (iii) by delivering to the Company or its designated agent an executed irrevocable option exercise form, together with irrevocable instructions from the participant to a broker or dealer, reasonably acceptable to the Company, to sell certain of the shares purchased upon the exercise of the option or to pledge such shares to the broker as collateral for a loan from the broker and to deliver to the Company the amount of sale or loan proceeds necessary to pay the purchase price; (iv) by requesting that Company withhold the number of shares otherwise deliverable upon exercise of the stock option by the number of shares having an aggregate fair market value equal to the aggregate option price at the time of exercise (i.e., a cashless net exercise); and (v) by any other form of valid consideration that is acceptable to the 2021 Plan Administration Committee in its sole discretion. No dividends or dividend equivalent rights may be paid or granted with respect to any stock options granted under the 2021 Plan.
Stock Appreciation Rights. The 2021 Plan Administration Committee is authorized to grant stock appreciation rights (“SARs”) as a stand-alone award, or freestanding SARs, or in conjunction with options granted under the 2021 Plan, or tandem SARs. SARs entitle a participant to receive an amount equal to the excess of the fair market value of a share of Common Stock on the date of exercise over the fair market value of a share of our Common Stock on the date of grant. The exercise price of a SAR cannot be less than 100% of the fair market value of a share of the Company’s Common Stock on the date of grant. The 2021 Plan Administration Committee will determine the terms of each SAR at the time of the grant, including, without limitation, the methods by or forms in which shares will be delivered to participants or registered in their names. The maximum term of each SAR, the times at which each SAR will be exercisable, and provisions requiring forfeiture of unexercised SARs at or following termination of employment or service generally are fixed by the 2021 Plan Administration Committee, except that no freestanding SAR may have a term exceeding 10 years and no tandem SAR may have a term exceeding the term of the option granted in conjunction with the tandem SAR. Distributions to the recipient may be made in Common Stock, cash, or a combination of both as determined by the 2021 Plan Administration Committee. No dividends or dividend equivalent rights may be paid or granted with respect to any SARs granted under the 2021 Plan.
Restricted Stock and Restricted Stock Units. The 2021 Plan Administration Committee is authorized to grant restricted stock and restricted stock units. Restricted stock consists of shares of our Common Stock that may not be sold, assigned, transferred, pledged, hypothecated, encumbered, or otherwise disposed of, and that may be forfeited in the event of certain terminations of employment or service, prior to the end of a restricted period as specified by the 2021 Plan Administration Committee. Restricted stock units are the right to receive shares of Common Stock at a future date in accordance with the terms of such grant upon the attainment of certain conditions specified by the 2021 Plan Administration Committee, which include a substantial risk of forfeiture and restrictions on their sale or other transfer by the participant. The 2021 Plan Administration Committee determines the eligible participants to whom, and the time or times at which, grants of restricted stock or restricted stock units will be made; the number of shares or units to be granted; the price to be paid, if any; the time or times within which the shares covered by such grants will be subject to forfeiture; the time or times at which the restrictions will terminate; and all other terms and conditions of the grants. Restrictions or conditions could include, but are not limited to, the attainment of performance goals (as described below), continuous service with the Company, the passage of time, or other restrictions and conditions. Except as otherwise provided in the 2021 Plan or the applicable award agreement, a participant shall have, with respect to shares of restricted stock, all of the rights of a shareholder of the Company holding the class of Common Stock that is the subject of the restricted stock, including, if applicable, the right to vote the Common Stock and the right to receive any dividends thereon, provided that (i) any dividends with respect to such a restricted stock award may be withheld by the Company for the participant’s account until such award is vested, subject to such terms as determined by the 2021 Plan Administration Committee, and (ii) any dividends so withheld by the Company and attributable to any particular restricted stock award shall be distributed to such participant in cash or, at the discretion of the 2021 Plan Administration Committee, in shares of the Company’s Common Stock having a fair market value equal to the amount of such dividends, if applicable, upon vesting of the award. If, however, such restricted stock award is forfeited, the participant’s rights as to such dividends will also be forfeited.
| 84 |
Performance Awards. The 2021 Plan Administration Committee may grant performance awards payable at the end of a specified performance period in cash, shares of Common Stock, units, or other rights based upon, payable in, or otherwise related to the Company’s Common Stock. Payment will be contingent upon achieving pre-established performance goals (as discussed below) by the end of the applicable performance period. The 2021 Plan Administration Committee will determine the length of the performance period, the maximum payment value of an award, and the minimum performance goals required before payment will be made, so long as such provisions are not inconsistent with the terms of the 2021 Plan and, to the extent an award is subject to Section 409A of the Code, are in compliance with the applicable requirements of Section 409A of the Code and any applicable regulations or guidance. In certain circumstances, the 2021 Plan Administration Committee may, in its discretion, determine that the amount payable with respect to certain performance awards will be reduced from the maximum amount of any potential awards. If the 2021 Plan Administration Committee determines, in its sole discretion, that the established performance measures or objectives are no longer suitable because of a change in the Company’s business, operations, corporate structure, or for other reasons that the 2021 Plan Administration Committee deems satisfactory, the 2021 Plan Administration Committee may modify the performance measures or objectives and/or the performance period.
Performance Goals. Awards of restricted stock, restricted stock units, performance awards, and other awards under the 2021 Plan may be made subject to the attainment of performance goals relating to one or more business criteria which shall consist of one or more or any combination of the following criteria (“Performance Criteria”): cash (cash flow, cash generation or other cash measures); cost; revenues; sales; ratio of debt to debt plus equity; net borrowing, credit quality or debt ratings; profit before tax; economic profit; earnings before interest and taxes; earnings before interest, taxes, depreciation and amortization; gross margin; earnings per share (whether on a pre-tax, after-tax, operational or other basis); operating earnings; capital expenditures; improvements in capital structure; expenses (expense management, expense ratio, expense efficiency ratios, expense levels or other expense measures); economic value added; ratio of operating earnings to capital spending or any other operating ratios; free cash flow; profit (net profit, gross profit, operating profit, economic profit, profit margin or other corporate profit measures); net income (before or after taxes, operating income or other income measures); net sales; net asset value per share; business expansion or consolidation (the accomplishment of mergers, acquisitions, dispositions, public offerings or similar extraordinary business transactions); sales growth; price of the Company’s Common Stock; return measures (including, without limitation, return on assets, capital, equity, investments or sales, and cash flow return on assets, capital, equity, or sales); market share; inventory levels, inventory management, inventory turn or shrinkage; stock price or performance; internal rate of return or increase in net present value; working capital targets relating to inventory and/or accounts receivable; service or product delivery or quality; customer satisfaction; employee retention; safety standards; productivity measures; cost reduction measures; strategic plan development and implementation; or total return to shareholders. Any Performance Criteria may be used to measure our performance as a whole or of any of our business units and may be measured relative to a peer group or index. Any Performance Criteria may include or exclude (i) events that are of an unusual nature or indicate infrequency of occurrence, (ii) gains or losses on the disposition of a business; (iii) changes in tax or accounting regulations or laws; (iv) the effect of a merger or acquisition, as identified in the Company’s quarterly and annual earnings releases; or (v) other similar occurrences. In all other respects, Performance Criteria shall be calculated in accordance with the Company’s financial statements, under generally accepted accounting principles, or under a methodology established by the 2021 Plan Administration Committee prior to the issuance of an award, which is consistently applied and identified in the Company’s audited financial statements, including in footnotes, or the Compensation Discussion and Analysis sections of the Company’s annual report and definitive proxy statement, as applicable.
Other Awards. The 2021 Plan Administration Committee may grant other forms of awards, based upon, payable in, or that otherwise relate to, in whole or in part, shares of the Company’s Common Stock, if the 2021 Plan Administration Committee determines that such other form of award is consistent with the purpose and restrictions of the 2021 Plan. The terms and conditions of such other form of award shall be specified in the grant. Such other awards may be granted for no cash consideration, for such minimum consideration as may be required by applicable law, or for such other consideration as may be specified in the grant.
| 85 |
Vesting, Forfeiture and Recoupment, Assignment. The 2021 Plan Administration Committee, in its sole discretion, may determine that an award will be immediately vested, in whole or in part, or that all or any portion may not be vested until a date, or dates, subsequent to its date of grant, or until the occurrence of one or more specified events, subject in any case to the terms of the 2021 Plan. If the 2021 Plan Administration Committee imposes conditions upon vesting, then, subsequent to the date of grant, the 2021 Plan Administration Committee may, in its sole discretion, accelerate the date on which all or any portion of the award may be vested.
The 2021 Plan Administration Committee may impose on any award at the time of grant or thereafter, such additional terms and conditions as the 2021 Plan Administration Committee determines, including terms requiring forfeiture of awards in the event of a participant’s termination of employment or service. The 2021 Plan Administration Committee will specify the circumstances on which performance awards may be forfeited in the event of a termination of service by a participant prior to the end of a performance period or settlement of awards. Except as otherwise determined by the 2021 Plan Administration Committee, restricted stock will be forfeited upon a participant’s termination of employment or service during the applicable restriction period. In addition, the Company may recoup all or any portion of any shares or cash paid to a participant in connection with any award in the event of a restatement of the Company’s financial statements as set forth in the Company’s clawback policy, if any, as such policy may be approved or modified by the Board from time to time.
Awards granted under the 2021 Plan generally are not assignable or transferable except by will or by the laws of descent and distribution, except that the 2021 Plan Administration Committee may, in its discretion and pursuant to the terms of an award agreement, permit transfers of nonqualified stock options or SARs to (i) the spouse (or former spouse), children, or grandchildren of the participant (“Immediate Family Members”); (ii) a trust or trusts for the exclusive benefit of such Immediate Family Members; (iii) a partnership in which the only partners are (a) such Immediate Family Members and/or (b) entities which are controlled by the participant and/or his or her Immediate Family Members; (iv) an entity exempt from federal income tax pursuant to Section 501(c)(3) of the Code or any successor provision; or (v) a split interest trust or pooled income fund described in Section 2522(c)(2) of the Code or any successor provision, provided that (x) there shall be no consideration for any such transfer, (y) the applicable award agreement pursuant to which such nonqualified stock options or SARs are granted must be approved by the 2021 Plan Administration Committee and must expressly provide for such transferability, and (z) subsequent transfers of transferred nonqualified stock options or SARs shall be prohibited except those by will or the laws of descent and distribution.
Adjustments Upon Changes in Capitalization. In the event that any dividend or other distribution (whether in the form of cash, shares of the Company’s Common Stock, other securities or other property), recapitalization, stock split, reverse stock split, rights offering, reorganization, merger, consolidation, split-up, spin-off, split-off, combination, subdivision, repurchase, or exchange of shares of Common Stock or other securities of the Company, issuance of warrants or other rights to purchase shares of Common Stock or other securities of the Company, or other similar corporate transaction or event affects the fair value of an award, then the 2021 Plan Administration Committee shall adjust any or all of the following so that the fair value of the award immediately after the transaction or event is equal to the fair value of the award immediately prior to the transaction or event: (i) the number of shares and type of Common Stock (or the securities or property) which thereafter may be made the subject of awards; (ii) the number of shares and type of Common Stock (or other securities or property) subject to outstanding awards; (iii) the number of shares and type of Common Stock (or other securities or property) specified as the annual per-participant limit under the 2021 Plan; (iv) the option price of each outstanding stock option; (v) the amount, if any, the Company pays for forfeited shares in accordance with the terms of the 2021 Plan; and (vi) the number of or exercise price of shares then subject to outstanding SARs previously granted and unexercised under the 2021 Plan, to the end that the same proportion of the Company’s issued and outstanding shares of Common Stock in each instance shall remain subject to exercise at the same aggregate exercise price; provided, however, that the number of shares of Common Stock (or other securities or property) subject to any award shall always be a whole number. Notwithstanding the foregoing, no such adjustment shall be made or authorized to the extent that such adjustment would cause the 2021 Plan or any stock option to violate Section 422 of the Code or Section 409A of the Code. All such adjustments must be made in accordance with the rules of any securities exchange, stock market, or stock quotation system to which the Company is subject.
| 86 |
Amendment or Discontinuance of the 2021 Plan. The Board may, at any time and from time to time, without the consent of participants, alter, amend, revise, suspend, or discontinue the 2021 Plan in whole or in part; provided, however, that (i) no amendment that requires shareholder approval in order for the 2021 Plan and any awards under the 2021 Plan to continue to comply with Sections 421 and 422 of the Code (including any successors to such sections or other applicable law) or any applicable requirements of any securities exchange or inter-dealer quotation system on which our stock is listed or traded, shall be effective unless such amendment is approved by the requisite vote of our shareholders entitled to vote on the amendment; and (ii) unless required by law, no action by the Board regarding amendment or discontinuance of the 2021 Plan may adversely affect any rights of any participants or obligations of the Company to any participants with respect to any outstanding awards under the 2021 Plan without the consent of the affected participant.
No Repricing of Stock Options or SARs. The 2021 Plan Administration Committee may not, without the approval of our shareholders, “reprice” any stock options or SARs. For purposes of the 2021 Plan, “reprice” means any of the following or any other action that has the same effect: (i) amending a stock option or SAR to reduce its option price or exercise price, respectively; (ii) canceling a stock option or SAR at a time when its option price or exercise price, respectively, exceeds the fair market value of a share of our Common Stock in exchange for cash or a stock option, SAR, award of restricted stock, or other equity award with an option price or exercise price that is less than the option price or exercise price of the original stock option or SAR; or (iii) taking any other action that is treated as a repricing under generally accepted accounting principles.
MyMD Florida Pre-Merger Plan
In 2016, pre-Merger MyMD Florida adopted the MyMD Pharmaceuticals, Inc. Amended and Restated 2016 Equity Incentive Plan (the “2016 Plan”). The MyMD Florida Incentive Plan provided for the issuance of up to 50,000,000 shares of pre-Merger MyMD Florida Common Stock. As of December 31, 2023, options to purchase 0 shares of Company Common Stock have been issued pursuant to the plan and 0 shares of Company Common Stock remain available for issuance.
Pursuant to the Merger Agreement, effective as of the effective time of the Merger, the Company assumed pre-Merger MyMD Florida’s Second Amendment to Amended and Restated 2016 Stock Incentive Plan (collectively with the 2016 Plan, the “MyMD Florida Incentive Plan”), assuming all of pre-Merger MyMD Florida’s rights and obligations with respect to the options issued thereunder (except that the term of the option will be amended to expire on the second-year anniversary of the effective time of closing). The assumed pre-Merger MyMD Florida’s options became a number of shares of Company Common Stock equal to the product of (a) the number of shares of MyMD Florida Common Stock subject to such option, multiplied by (b) the Exchange Ratio and rounding the resulting number down to the nearest whole share of Company Common Stock, at an exercise price per share of Company Common Stock equal to the quotient of (i) the exercise price per share of MyMD Florida Common Stock subject to such option immediately prior to the effective time of the merger divided by (ii) the Exchange Ratio and rounding the resulting exercise price up to the nearest whole cent, and then subsequently adjusted for the reverse stock split of the MyMD Florida Common Stock. Upon the closing of the Merger, the Company assumed all of pre-Merger MyMD Florida’s rights and obligations under pre-Merger MyMD Florida stock options that were outstanding immediately prior to the effective time of the Merger, and no additional awards can be issued under the MyMD Florida Incentive Plan.
The MyMD Florida Incentive Plan authorized the grant of incentive stock options, non-qualified stock options, restricted stock, restricted stock units, and other stock-based awards, or a combination of the foregoing. MyMD Florida granted only incentive stock options and non-qualified stock options under the plan.
Authorized Shares. A total of 50,000,000 shares of pre-Merger MyMD Florida Common Stock were authorized for the grant of awards under the MyMD Florida Incentive Plan.
Plan Administration. The MyMD Florida Incentive Plan was administered by the MyMD Florida board of directors. The MyMD Florida board had the authority to grant awards under the plan and to adopt, amend, and repeal such administrative rules, guidelines, and practices relating to the plan as it deemed advisable. The MyMD Florida board had the authority to determine the persons to whom and the dates on which awards will be granted, the number of shares of Common Stock to be subject to each award, the time or times during the term of each award within which all or a portion of such award may be exercised, the exercise price, the type of consideration to be paid, and the other terms and provisions of each award, which need not be identical. The MyMD Florida board had the power to construe and interpret the MyMD Florida Incentive Plan and awards granted under it. All decisions, determinations and interpretations by the MyMD Florida board regarding the plan were to be final, binding and conclusive on all participants or other persons claiming rights under the plan or any award.
| 87 |
Options. Options granted under the MyMD Florida Incentive Plan could (i) either be “incentive stock options” within the meaning of Section 422 of the Code, or “nonqualified stock options,” and (ii) become vested upon such conditions as were determined by the MyMD Florida board. Such vesting could be based on continued service to MyMD Florida over a certain period, the occurrence of certain performance milestones, or other criteria as determined by the MyMD Florida board. Options granted under the MyMD Florida Incentive Plan could be subject to different vesting terms. Options could not have an exercise price per share of less than 100% of the fair market value of a share of MyMD Florida Common Stock on the date of grant or a term longer than 10 years. To the extent provided by the terms of an option, a participant could satisfy any federal, state or local tax withholding obligation relating to the exercise of such option by a cash payment upon exercise, by authorizing MyMD Florida to withhold a portion of the stock otherwise issuable to the participant upon exercise, or by such other method as may be set forth in the option agreement or authorized by the MyMD Florida board. The treatment of options under the MyMD Florida Incentive Plan upon a participant’s termination of employment with or service to MyMD Florida was set forth in the applicable award agreement, which typically provided that the options would terminate 24 months after a termination of employment or service. In connection with the Merger Agreement, on November 10, 2020, MyMD Florida amended each of the option grant award agreements noted above to, among other things, revise the term of exercisability of such option to expire on the earlier of (i) the 10th anniversary of the date of grant or (ii) the second anniversary of the effective date of a “Reorganization Event” as defined in the MyMD Florida Incentive Plan. Accordingly, the term of each such option was amended to expire on the second anniversary of the effective date of the Merger. Incentive stock options are not transferable except by will or by the laws of descent and distribution. Non-qualified stock options are transferable to certain permitted transferees (as provided in the MyMD Florida Incentive Plan) to the extent included in the option award agreement.
Restricted Stock and Restricted Stock Unit Awards. Subject to certain limitations, the MyMD Florida board was authorized to grant awards of restricted stock and restricted stock units, which are rights to receive shares of MyMD Florida Common Stock or cash, as determined by the MyMD Florida board and as set forth in the applicable award agreement, upon the settlement of the restricted stock units at the end of a specified time. The MyMD Florida board could impose any restrictions or conditions upon the vesting of restricted stock or restricted stock unit awards, or that would provide for a delay in the settlement of a restricted stock unit award after it vests, that the committee deemed appropriate and in accordance with the requirements of Section 409A of the Code. Dividend equivalents could be credited in respect of shares covered by a restricted stock or a restricted stock unit award, as determined by the MyMD Florida board. At the discretion of the MyMD Florida board, such dividend equivalents could be converted into additional shares covered by restricted stock or restricted stock units, as applicable. If a restricted stock or restricted stock unit award recipient’s employment or service relationship with MyMD Florida terminated, any unvested portion of the restricted stock or restricted stock unit award would be forfeited, unless the participant’s award agreement provided otherwise. Restricted stock and restricted stock unit awards are generally not transferable except (i) by will or by the laws of descent and distribution or (ii) to certain permitted transferees, to the extent provided in the award agreement.
Other Stock-Based Awards. The MyMD Florida Incentive Plan authorized the grant of other awards that are valued in whole or in part by reference to, or are otherwise based on, shares of MyMD Florida Common Stock or other property, including awards entitling recipients to receive shares of MyMD Florida Common Stock to be delivered in the future.
Certain Adjustments; Reorganization Events. In connection with any stock split, reverse stock split, stock dividend, dividend in property other than cash, recapitalization, share combination, share reclassification, spin-off, or other similar change in capitalization or event, the MyMD Florida board would equitably adjust the type(s), class(es) and number of shares of stock subject to the MyMD Florida Incentive Plan, and any outstanding awards would also be appropriately adjusted as to the type(s), class(es), number of shares and exercise price per share of Common Stock subject to such awards.
| 88 |
In the event of a “Reorganization Event” (as defined in the MyMD Florida Incentive Plan) such as certain mergers or consolidations, the MyMD Florida board could take any one or more of the following actions as to all or any (or any portion of) outstanding awards on such terms as the board determines: (i) provide that awards will be assumed, or substantially equivalent awards will be substituted, by the acquiring or succeeding corporation (or an affiliate thereof), (ii) upon written notice to a participant, provide that all of the participant’s unexercised awards will terminate immediately prior to the consummation of such Reorganization Event unless exercised by the participant (to the extent then exercisable) within a specified period following the date of such notice, (iii) provide that outstanding awards shall become exercisable, realizable, or deliverable, or restrictions applicable to an award shall lapse, in whole or in part prior to or upon such Reorganization Event, (iv) in the event of a Reorganization Event under the terms of which holders of MyMD Florida Common Stock will receive upon consummation thereof a cash payment for each share surrendered in the Reorganization Event, make or provide for a cash payment to participants with respect to each award held by a participant equal to (A) the number of shares of MyMD Florida Common Stock subject to the vested portion of the award (after giving effect to any acceleration of vesting that occurs upon or immediately prior to such Reorganization Event) multiplied by (B) the excess, if any, of (I) the acquisition price in the Reorganization Event over (II) the exercise price of such award and any applicable tax withholdings, in exchange for the termination of such award, (v) provide that, in connection with a liquidation or dissolution of MyMD Florida, awards shall convey into the right to receive liquidation proceeds (if applicable, net of the exercise price thereof and any applicable tax withholdings) and (vi) any combination of the foregoing. In taking any of above actions, the MyMD Florida board would not be obligated by the MyMD Florida Incentive Plan to treat all awards of the same type identically.
Amendment, Termination. The MyMD Florida board could amend, alter, suspend, discontinue, or terminate the MyMD Florida Incentive Plan, provided that no such amendment would adversely affect the rights of any participant without the participant’s consent. The MyMD Florida Incentive Plan will terminate in 2026, unless earlier terminated earlier by the Company.
Company Pre-Merger Plans
On January 23, 2014, we adopted the 2013 Stock Incentive Plan (the “2013 Plan”). The 2013 Plan was amended by the Board on January 9, 2015 and September 30, 2016, and such amendments were ratified by stockholders on December 7, 2018. The 2013 Plan provides for the issuance of up to 73 shares of the Company’s Common Stock, and as of December 31, 2023 19 shares of Common Stock remain available for grants under the 2013 Plan.
On December 21, 2016, the shareholders approved, and the Company adopted the 2016 Stock Incentive Plan (the “2016 Plan”). The 2016 Plan provides for the issuance of up to 1,666,667 shares of the Company’s common stock. As of December 31, 2023, grants of options to purchase 0 shares of Common Stock have been issued pursuant to the 2016 Plan, and 0 shares of Common Stock remain available for issuance.
On August 7, 2017, the stockholders approved, and the Company adopted the 2017 Stock Incentive Plan (“2017 Plan”). The 2017 Plan provides for the issuance of up to 118 shares of the Company’s Common Stock. The purpose of the 2017 Plan is to provide additional incentive to those of our officers, employees, consultants and non-employee directors and our parents, subsidiaries and affiliates whose contributions are essential to the growth and success of our business. As of December 31, 2022, grants of restricted stock and options to purchase totaling 93 shares of Common Stock have been issued pursuant to the 2017 Plan and as of December 31, 2023, 25 shares of Common Stock remain available for grants under the 2017 Plan. The 2017 Plan provides for the issuance of shares of the Company’s Common Stock through the grant of non-qualified options, incentive options, restricted stock and unrestricted stock to directors, officers, consultants, attorneys, advisors, and employees.
On December 7, 2018, the stockholders approved, and we adopted the 2018 Stock Incentive Plan (the “2018 Plan”) and on August 27, 2020, the stockholders approved, and we adopted an amendment to the plan to increase the number of shares of Common Stock available for issuance pursuant to awards under the 2018 Plan by an additional 17,366 shares. The 2018 Plan, as amended, provides for the issuance of up to 18,670 shares of the Company’s Common Stock. The purpose of the 2018 Plan is to provide additional incentive to those of our officers, employees, consultants and non-employee directors and to promote the success of our business. As of December 31, 2023, grants of RSUs shall vest immediatelyto purchase 8,769 shares of Common Stock had been issued pursuant to the 2018 Plan, and 9,901 shares of Common Stock remained available for issuance. The 2018 Plan provides for the issuance of shares of the Company’s Common Stock through the grant of options, restricted stock, stock appreciation rights, other stock-based awards, performance compensation awards to directors, officers, consultants, advisors, and employees. In addition, the 2018 Plan provides the Compensation Committee of the Board with discretion to accelerate the vesting and exercisability of outstanding awards upon the occurrence of (i) a change of control (as defined in control, provided that the grantee is employed or providing services to us and our affiliates on the closing date of such change in control, (ii) the grantee’s termination of employment or services to us and our affiliates by reason of death or disability, or (iii) the grantee’s termination of employment or services to us without cause. At our election, the vested RSUs may be settled for cash.2018 Plan).
On November 23, 2020, we retained Taglich Brothers on a non-exclusive basis as a consultant to render consulting services, assist with review, and analysis of, financial planning and budgeting matters of the Company for a term of 12 months. Pursuant to the Consulting Agreement with Taglich Brothers, we agreed to pay Taglich Brothers $10,000 per month.
Mr. Schreiber is the managing director of capital markets at Taglich Brothers, and Mr. Schroeder is the vice president of investment banking at Taglich Brothers.
Equity Compensation Plan Information
The following table provides information with respectregarding the number of securities to be issued under the Company’s Equity2021 Plan, the 2013 Plan, the 2016 Plan, the 2017 Plan and the 2018 Plan (collectively, the “Equity Compensation PlanPlans”) as of the fiscal year ended December 31, 2020.2023:
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted-average exercise price of outstanding options, warrants and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | |||||||||||||||||||||
| Equity compensation plans approved by security holders(1) | 789,360 | $ | 2.24 | 320,639 | ||||||||||||||||||||
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted-average exercise price of outstanding options (b) | Securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | |||||||||||||||||||||
| Equity compensation plans approved by security holders(1) | 139,840 | $ | 46.09 | 20,567 | ||||||||||||||||||||
| Equity compensation plans not approved by security holders | — | $ | — | — | - | - | - | |||||||||||||||||
| Total | 789,360 | $ | 2.24 | 320,639 | 139,840 | $ | 46.09 | 20,567 | ||||||||||||||||
(1) Represents shares available to issuance under the Equity Compensation Plans.
| (1) | Represents shares available for issuance under the Equity Compensation Plans. |
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters.
The following table sets forth information regarding the beneficial ownership of our voting securities as of February 26, 2021March 29, 2024 by (i) each person known to us to beneficially own five percent (5%) or more of any class of our voting securities; (ii) each of our named executive officers and directors; and (iii) all of our named directors and executive officers as a group. The percentages of voting securities beneficially owned are reported on the basis of regulations of the SEC governing the determination of beneficial ownership of securities. Under the rules of the SEC, a person is deemed to be a beneficial owner of a security if that person has or shares voting power, which includes the power to vote or to direct the voting of the security, or investment power, which includes the power to dispose of or to direct the disposition of the security. Except as indicated in the footnotes to this table, to our knowledge and subject to community property laws where applicable, each beneficial owner named in the table below has sole voting and sole investment power with respect to all shares beneficially owned and each person’s address is c/o Akers Biosciences,MyMD Pharmaceuticals, Inc., 1185 Avenue of the Americas, 3rd Floor, New York, New York 10036.855 N. Wolfe Street, Suite 601, Baltimore, MD 21205. Percentage of common stockCommon Stock ownership is based on 16,652,8292,157,632 shares of common stockCommon Stock issued and outstanding as of February 26, 2021.March 29, 2024. Percentage of Series D Convertible Preferred Stock (the “Series D Preferred Stock”) ownership is based on 72,992 shares of Series D Preferred Stock issued and outstanding as of March 29, 2024.
The number of shares of Akers common stockCommon Stock beneficially owned by the principal stockholders and the percentage of shares outstanding, as set forth below, take into account certain limitations on the conversion of Akers preferred stock or the exercise of warrants to purchase Akers common stock.Common Stock.
Beneficial ownership is determined in accordance with the rules of the SEC. For the purpose of calculating the number of shares beneficially owned by a stockholder and the percentage ownership of that stockholder, shares of common stockCommon Stock subject to options or warrants that are currently exercisable or exercisable within sixty (60) days of February 26, 2021March 29, 2024 by that stockholder are deemed outstanding.
| As of February 26, 2021 | ||||||||
| Name | Number of Shares of Akers Common Stock Beneficially Owned (1) | Percentage Class (1) | ||||||
| 5% Beneficial Owner | ||||||||
| Iroquois Capital Management L.L.C. | 1,709,890 | (2) | 9.99 | % | ||||
| Intracoastal Capital LLC | 1,459,458 | (3) | 8.40 | % | ||||
| Mainfield Enterprises Inc. | 1,081,081 | (4) | 6.49 | % | ||||
| Named Executive Officers and Directors | ||||||||
| Bill J. White(5)(6) | — | — | ||||||
| Joshua Silverman(5)(6) | — | — | ||||||
| Christopher C. Schreiber(5)(6) | — | — | ||||||
| Robert C. Schroeder(5)(6) | — | — | ||||||
| Howard R. Yeaton(7) | 2,345 | — | ||||||
| All NEOs and directors as a group (5 persons) | 2,345 | * | ||||||
| 90 |
| Name | Number of Shares of Common Stock Beneficially Owned(1) | Percentage of Class | Number of Shares of Series D Preferred Stock Beneficially Owned(2) | Percentage of Class | Total Voting Power | |||||||||||||||
| 5% Beneficial Owner | ||||||||||||||||||||
| Richard Abbe / Iroquois Capital Investment Group LLC(3) | 167,058 | 7.22 | % | - | * | 6.46 | % | |||||||||||||
| Caroline Williams / Starwood Trust(4) | 124,815 | 5.78 | % | - | * | 5.78 | % | |||||||||||||
| Premas Biotech PVT Ltd.(5) | 3,459 | * | 72,992 | 100 | % | * | ||||||||||||||
| Named Executive Officers and Directors | ||||||||||||||||||||
| Joshua Silverman(6) | 5,181 | * | - | * | * | |||||||||||||||
| Bill J White(7) | 4,125 | * | - | * | * | |||||||||||||||
| Craig Eagle, M.D.(8) | 5,277 | * | - | * | ||||||||||||||||
| Jude Uzonwanne(9) | 1,666 | * | - | * | * | |||||||||||||||
| Christopher C Schreiber(10) | 4,607 | * | - | * | * | |||||||||||||||
| Christopher Chapman, M.D.(11) | 11,666 | * | - | * | * | |||||||||||||||
| Adam Kaplin, M.D., PhD(12) | 1,666 | * | - | * | * | |||||||||||||||
| Ian Rhodes | - | |||||||||||||||||||
| Paul Rivard(13) | 15,000 | * | - | * | * | |||||||||||||||
| All current executive officers and Directors as a group (8 persons) | 34,188 | 2.24 | % | - | * | 2.24 | % | |||||||||||||
* Less than 1%.
| Percentage of Series D Preferred Stock ownership is based on 72,992 shares of Series D Preferred Stock issued and outstanding as of March 29, 2024. | ||
| (3) | This information is based on a Schedule 13G/A filed with the SEC on February IMF owns (1) 6,248 shares
Mr. Abbe also has voting control and investment discretion over securities held by Iroquois Capital Investment Group LLC (“ICIG”). As such, Mr. Abbe may be deemed to be the beneficial owner (as determined under Section 13(d) of the
|
| 91 |
This information is based on a Schedule | ||
Ms. Williams individually owns 1,272,972 shares of | ||
| (5) | On March 23, 2020, Premas Biotech PVT., Ltd received 103,782 shares of | |
| (6) | Represents (i) 5,000 shares of Common Stock held by Mr. Silverman, (ii) 2,459 restricted stock unit (“RSU”) awards to Mr. Silverman that are vested or scheduled to vest within 60 days of March 29, | |
| (7) | Represents (i) 2,459 RSU awards to | |
| (9) | Represents 1,666 shares of Common Stock issuable upon the exercise of options held by Mr. Uzonwanne exercisable within 60 days of March 29, 2024. | |
| (10) | Represents (i) 2,941 RSU awards to Mr. Schreiber | |
| (12) | Represents 1,666 shares of Common Stock issuable upon the exercise of options held by Dr. Kaplin exercisable within 60 days of March 29, 2024. | |
| (13) | Represents (i) 15,000 shares of Common Stock and Effective |
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Transactions with related persons are governed by our Code of Business Ethics and Conduct, which applies to all of our associates,employees, as well as each of our directors and certain persons performing services for us. This code covers a wide range of potential activities, including, among others, conflicts of interest, self-dealing and related party transactions. Waiver of the policies set forth in this code will only be permitted when circumstances warrant. Such waivers for directors and executive officers, or that provide a benefit to a director or executive officer, may be made only by the Board, as a whole, or the Audit Committee and must be promptly disclosed as required by applicable law or regulation. Absent such a review and approval process in conformity with the applicable guidelines relating to the particular transaction under consideration, such arrangements are not permitted. All related party transactions for which disclosure is required to be provided herein were approved in accordance with our Code of Business Ethics and Conduct and Whistleblower Policy.
| 92 |
Other than as described below, compensation and employment agreements, and other arrangements which are described below and under “Item 11. Executive Compensation” herein, since January 1, 2019,2022, there has not been, and there is not currently proposed, any transaction or series of similar transactions to which we were or will be a party in which the amount involved exceeded or will exceed the lesser of $120,000 or the average of our total assets at year-end for the last two completed fiscal years and in which any director, executive officers,officer, holder of 5% or more of any class of our capital stock, or any member of their immediate family had or will have a direct or indirect material interest.
On August 17, 2022, pursuant to a securities purchase agreement with certain institutional and accredited investors, dated August 15, 2022, the Company issued and sold, in a registered direct offering (the “August RD”), an aggregate of 47,059 shares of its Common Stock at an offering price of $127.50 per share and, in a concurrent private placement (together with the August RD, the “August Offerings”), 47,063 unregistered investor warrants to purchase up to 47,063 shares of its Common Stock at an exercise price of 157.50, for gross and net proceeds of $5,999,997 and $5,550,028, respectively. In connection with the Private Placement, Iroquois Master Fund Ltd. (“IMF”), and its affiliate,August Offering, we issued to Iroquois Capital Investment Group LLC (“ICIG”), received an aggregate of 1,040,540 7,844 shares of Akers common stock, 1,040,540 Pre-Funded WarrantsCommon Stock and 2,081,080 Investor Warrants and Intracoastal Capital, LLC received 729,729warrants to purchase an additional 7,844 shares of Akers common stock, and 729,729 Investor Warrants, and Mainfield Enterprises Inc.Common Stock. ICIG is the beneficial owner of more than five percent of our Common Stock. In connection with the August Offering, we also issued to Iroquois Master Fund Ltd., an affiliate of ICIG (“Mainfield”IMF”) received 1,081,081, 11,765 shares of Akers common stock,Common Stock and 1,081,081 Investor Warrants. warrants to purchase an additional 11,765 shares of Common Stock.
In addition, eachin connection with the February 2023 Offering we issued to ICIG 2,750 shares of IMF, ICIGour Series F Preferred Stock and Mainfield entered into a lock-up and support agreement with Akers,warrants to purchase up to 40,651 shares of Common Stock (adjusted to 864,780 shares of Common Stock pursuant to which such investors agreed, from the dateterms of the lock-up and support agreement until May 31, 2021,Warrants following the Reverse Stock Split). In connection with the February 2023 Offering, we also issued to vote such investors’IMF 5,000 shares of Akers common stockSeries F Preferred Stock and warrants to purchase up to 73,910 shares of Common Stock (adjusted to 1,572,328 shares of Common Stock pursuant to the terms of the Warrants following the Reverse Stock Split).
On April 14, 2023, the Company issued a reimbursement payment to Mr. Jonnie Williams, Sr. in favorthe amount $500,000. The payment represented reimbursement for expenses incurred by Mr. Williams meeting with potential strategic corporate partners on behalf of each matter proposedthe Company as part of the Company’s business development efforts. Mr. Williams is an immediate family member of a stockholder who beneficially holds more than 5% of our Common Stock.
Director Independence
See “Item 10. Directors, Executive Officers, and recommended for approval by the Board or management at every stockholders’ meeting. For more information on the Private Placement, please see “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Recent Developments—Private Placement” included in this Annual Report on Form 10-K.Corporate Governance—Director Independence,” above.
Item 14. Principal AccountingAccountant Fees and Services.
| 2020 | 2019 | 2023 | 2022 | |||||||||||||
| Audit Fees | $ | 146,000 | $ | 139,000 | $ | 150,492 | $ | 141,924 | ||||||||
| Audit-Related Fees | $ | 5,000 | $ | 37,450 | - | - | ||||||||||
| Tax Fees | $ | 10,000 | $ | 10,000 | 14,300 | 26,212 | ||||||||||
| All Other Fees | $ | - | $ | - | - | 1,000 | ||||||||||
| TOTAL | $ | 161,000 | $ | 186,450 | $ | 164,792 | $ | 169,136 | ||||||||
Audit Fees. This category includes the audit of our annual consolidated financial statements, reviews of our financial statements included in our Form 10-Qs and services that are normally provided by our independent registered public accounting firm in connection with its engagements for those years.
Audit-Related Fees. This category consists of assurance and related services by our independent registered public accounting firm that are reasonably related to the performance of the audit or review of our financial statements and are not reported above under “Audit Fees.” The services for the fees disclosed under this category include consents regarding equity issuances.
Tax Fees. This category typically consists of professional services rendered by our independent registered public accounting firm for tax compliance and tax advice.
All Other Fees. This category includes aggregate fees billed in each of the last two fiscal years for products and services provided by the Morison Cogen LLP, other than the services reported in the categories above.
Pre-Approval Policies and Procedures
Under the Audit Committee’s pre-approval policies and procedures, the Audit Committee is required to pre-approve all fees paid to, and all services performed by, our independent registered public accounting firm. At the beginning of each year, the Audit Committee pre-approves the proposed services, including the nature, type and scope of services contemplated and the related fees to be rendered by our independent registered public accounting firm during the year. In addition, Audit Committee pre-approval is also required for those engagements that may arise during the course of the year that are outside the scope of the initial services and fees pre-approved by the Audit Committee.
All of the services rendered by Morison Cogen LLP in 20202023 were pre-approved by the Audit CommitteeCommittee.
Item 15. Exhibits,Exhibit and Financial Statement Schedules.
(a) The following documents are filed as part of this Annual Report on Form 10-K:
| (1) | Financial Statements |
| (2) | Financial Statements Schedule |
None. Financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto.
| (3) | Exhibits |
See “Index to Exhibits” for a description of our exhibits.
Not applicable
INDEX TO EXHIBITS
| 98 |
| 99 |
+ Filed herewith
* Furnished herewith.
# Management contract or compensatory plan or arrangement.
** The schedules and exhibits to the Agreement and Plan of Merger and Reorganization have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the SEC upon request.
SIGNATURES
In accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: | By: | /s/ Christopher C. |
| Name: | Christopher C. | |
| Title: | President and Chief | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Christopher C. | President, Chief | |||
| Christopher C. | (Principal Executive Officer) | |||
| /s/ Ian Rhodes | Interim Chief Financial Officer | |||
| Ian Rhodes | (Principal Financial Officer and Principal Accounting Officer) | |||
| /s/ Joshua Silverman | Chairman of the Board | |||
| Joshua Silverman | ||||
| /s/ Bill J. White | Director | |||
| Bill J. White | ||||
| /s/ | Director | |||
| /s/ Jude Uzonwanne | Director | April 1, 2024 | ||
| Jude Uzonwanne | ||||
| /s/ Craig Eagle | Director | April 1, 2024 | ||
| Craig Eagle, M.D. |
| 101 |
Index to Consolidated Financial Statements
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Akers Biosciences,
MyMD Pharmaceuticals, Inc. and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Akers Biosciences,MyMD Pharmaceuticals, Inc. and Subsidiaries (the Company) as of December 31, 20202023 and 2019,2022 and the related consolidated statements of comprehensive loss, changes in shareholders’stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2020,2023 and the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 20202023 and 2019,2022 and the results of their operations and their cash flows for each of the two years in the period ended December 31, 2020,2023 in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company has experienced net losses and negative cash flows from operations for the years ended December 31, 2023 and 2022, which raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 3. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the auditaudits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit mattermatters communicated below is a matterare matters arising from the current period audit of the consolidated financial statements that waswere communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit mattermatters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit mattermatters below, providing a separate opinion on the critical audit mattermatters or on the accounts or disclosures to which it relates.they relate.
Valuation of bifurcated embedded derivative
As discussed in Note 2 to the consolidated financial statements, on February 21, 2023, the Company sold 15,000 shares of Series F Convertible Preferred Stock (“Preferred Stock”), with various embedded features. The Preferred Stock was determined to be more akin to a debt-like host than an equity-like host. The Company concluded that the embedded features were not clearly and closely related to the debt host instrument and thus were deemed to be bifurcated embedded derivatives (“Embedded Derivative”). The Embedded Derivative liabilities are measured at fair value at inception and then are required to be re-measured and reported at fair value at each reporting period. Management’s estimate of the Embedded Derivative liabilities at inception and as of December 31, 2023 was $3,149,800 and $61,000. Management applies considerable judgment in selecting assumptions used to estimate the Embedded Derivative liabilities and changes in market conditions or variations in certain assumptions could result in significant fluctuations in the estimate. Management estimates the fair value of the Embedded Derivative liabilities using a Monte Carlo simulation model, with the following inputs: the fair value of the Company’s common stock on the issuance date and re-measurement date, estimated equity volatility, estimated traded volume volatility, the time to maturity, a discounted market interest rate, a dividend rate, a penalty dividend rate, and probability of default. The fair value of the bifurcated derivative liabilities was estimated utilizing the with and without method which uses the probability weighted difference between the scenarios with the derivative and the plain vanilla maturity scenario without a derivative.
Given the inherent uncertainty in selecting assumptions and the complexity of the calculations, we have determined that management’s valuation of Embedded Derivative liabilities is a critical audit matter which required a high degree of auditor judgment and an increased extent of effort when performing audit procedures to evaluate the judgments made and the reasonableness of the models and assumptions used in the valuation. The audit effort included the use of professionals with specialized skill and knowledge to assist in performing these procedures and evaluating the audit evidence obtained from these procedures
| F-2 |
To the Board of Directors and Stockholders of
MyMD Pharmaceuticals, Inc. and Subsidiaries
(Continued)
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included the following:
| ● | With the involvement of our fair value specialists, we developed an independent fair value estimate for a sample and compared our estimate to the Company’s estimate and evaluated any differences. We developed our estimate by evaluating the observable and unobservable inputs used by management or developing independent inputs. | |
| ● | With the involvement of our fair value specialists, we evaluated the methods, models, and judgments applied by management in the determination of principal assumptions and the calculation of Embedded Derivative liabilities. | |
| ● | For the re-measurement at December 31, 2023, we evaluated management’s ability to accurately estimate fair value by comparing management’s fair value re-measurements at quarterly reporting dates during 2023 to their fair value re-measurement at December 31, 2023. |
Going Concern Assessment
As discussed in Note 3 to the consolidated financial statements, historically, the Company has incurred net losses. Since its inception, the Company has met its liquidity requirements principally through the sale of its preferred and common stock in public and private placements. The Company believes that its current financial resources as of the date of issuance of the consolidated financial statements are not sufficient to fund its current operating budget and contractual obligations as of December 31, 20202023 as they fall due in the next twelve-month period, and as such have concluded that there are no material uncertainties related to events or conditions that may cast significant doubt upon the Company’s ability to continue as a going concern. In making such a determination, management prepared a short-term cash flow projection. Management used significant assumptions in preparing the short-term cash flow projection, which included operating costs and financing obligations.
The principal considerations for our determination that performing procedures relating to the going concern assessment is a critical audit matter are the significant judgments in management’s plans to fund its operating budget and contractual obligations. This required a high degree of auditor judgment and an increased extent of effort when performing audit procedures to evaluate management’s conclusion that it is probable the Company’s plans will be effectively implemented within twelve months after the date the consolidated financial statements are issued and will provide the necessary cash flows to fund the Company’s operating budget and contractual obligations.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included the following:
| ● | Evaluation of the reasonableness of key assumptions and estimates used by the management in the short-term cash flow projection in the light of its existing operating requirements and plans. | |
| ● | Evaluation of the reasonableness of management’s plans on the cash flow requirements of the operations. | |
| ● | Testing the completeness, accuracy, and relevance of underlying data in the short-term cash flow projection. | |
| ● | Evaluation of the adequacy of the Company’s disclosure of these circumstances in the consolidated financial statements. |
Assessment of Impairment for Investment in Oravax, Inc.
As discussed in Note 2 to the consolidated financial statements, the Company has elected to measure its investment in Oravax Medical, Inc. as an equity security without a readily determinable fair value. Under this election, an equity security without a readily available fair value is reflected at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. At each reporting period, the Company is required to make a qualitative assessment considering impairment indicators to evaluate whether the investment is impaired. If deemed impaired, the Company is required to estimate the fair value of the investment and recognize an impairment loss equal to the difference between the fair value of the investment and its carry amount. As of December 31, 2023, the Company performed a qualitative assessment to evaluate whether the investment is impaired and determined that the investment was not impaired and thus no adjustment to fair market value was required as of December 31, 2023. In making such a determination, management prepared a detailed qualitative analysis considering various impairment indicators. Management used significant judgment in their qualitative assessment.
The principal considerations for our determination that performing procedures relating to the impairment assessment of investments in equity securities without readily determinable fair value is a critical audit matter is the significant judgment by management in making the qualitative assessment of whether investments in equity securities were impaired. This in turn led to significant auditor judgment and effort in performing procedures to evaluate the reasonableness of significant judgments management applied in determining whether events or changes in circumstances indicate that the carrying amount of the investment might not be recoverable.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included the following:
| ● | Analyzing management’s detailed qualitative analysis considering various impairment indicators that may indicate that the carrying amount of the investment might not be recoverable for reasonableness. | |
| ● | Reviewing management’s assessment of events or changes in circumstances for reasonableness. | |
| ● | Evaluating management’s significant accounting policies related to the election to measure its investment in Oravax Medical, Inc. as an equity security without a readily determinable fair value. |
We have served as the Company’s auditor since 2010.
/s/Morison Cogen LLP
We have served as the Company’s auditor since 2010.
Blue Bell, Pennsylvania
MarchApril 1, 20212024
| F-3 |
AKERS BIOSCIENCES,MYMD PHARMACEUTICALS, INC. AND SUBSIDIARIES
December 31, 20202023 and 20192022
| As of | ||||||||
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash and Cash Equivalents | $ | 18,617,955 | $ | 517,444 | ||||
| Marketable Securities | 16,718,452 | 9,164,273 | ||||||
| Other Receivables | 1,200,009 | - | ||||||
| Prepaid expenses | 294,343 | 334,059 | ||||||
| Current assets of discontinued operations | 12,002 | 295,038 | ||||||
| Total Current Assets | 36,842,761 | 10,310,814 | ||||||
| Non-Current Assets | ||||||||
| Restricted Cash | - | 115,094 | ||||||
| Other Assets | - | 2,722 | ||||||
| Non-current assets of discontinued operations | - | 456,305 | ||||||
| Total Non-Current Assets | - | 574,121 | ||||||
| Total Assets | $ | 36,842,761 | $ | 10,884,935 | ||||
| LIABILITIES | ||||||||
| Current Liabilities | ||||||||
| Trade and Other Payables | $ | 2,203,902 | $ | 891,883 | ||||
| Current liabilities of discontinued operations | 59,393 | 637,882 | ||||||
| Total Current Liabilities | 2,263,295 | 1,529,765 | ||||||
| Total Liabilities | $ | 2,263,295 | $ | 1,529,765 | ||||
| Commitments and Contingencies | ||||||||
| SHAREHOLDERS’ EQUITY | ||||||||
| Preferred Stock, No par value, 50,000,000 total preferred shares authorized | - | - | ||||||
| Series A Convertible Preferred Stock, 10,000,000 shares designated, $0.001 par value and a stated value of $0.0725 per share, 0 shares issued and outstanding as of December 31, 2020 and December 31, 2019 | - | - | ||||||
| Series C Convertible Preferred Stock, 1,990,000 shares designated, no par value and a stated value of $4.00 per share, 0 shares issued and outstanding as of December 31, 2020 and December 31, 2019 | - | - | ||||||
| Series D Convertible Preferred Stock, 211,353 shares designated, no par value and a stated value of $0.01 per share, 72,992 and 0 shares issued and outstanding as of December 31, 2020 and December 31, 2019 | 144,524 | - | ||||||
| Series E Junior Participating Preferred Stock, 100,000 shares designated, no par value and a stated value of $0.001 per share, 0 shares issued and outstanding as of December 31, 2020 and December 31, 2019 | - | - | ||||||
| Common stock, No par value, 100,000,000 shares authorized 17,585,261 and 1,738,837 issued and outstanding as of December 31, 2020 and December 31, 2019 | 171,598,681 | 128,920,414 | ||||||
| Accumulated Other Comprehensive Income | - | 17,886 | ||||||
| Accumulated Deficit | (137,163,739 | ) | (119,583,130 | ) | ||||
| Total Shareholders’ Equity | 34,579,466 | 9,355,170 | ||||||
| Total Liabilities and Shareholders’ Equity | $ | 36,842,761 | $ | 10,884,935 | ||||
| December 31, 2023 | December 31, 2022 | |||||||
| As of | ||||||||
| December 31, 2023 | December 31, 2022 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash and Cash Equivalents | $ | 2,681,010 | $ | 749,090 | ||||
| Marketable Securities | 2,242,106 | 4,086,902 | ||||||
| Prepaid Expenses | 893,226 | 565,787 | ||||||
| Total Current Assets | 5,816,342 | 5,401,779 | ||||||
| Non-Current Assets | ||||||||
| Operating Lease Right-of-Use Assets | 47,389 | 139,662 | ||||||
| Goodwill | 10,498,539 | 10,498,539 | ||||||
| Investment in Oravax, Inc. | 1,500,000 | 1,500,000 | ||||||
| Total Non-Current Assets | 12,045,928 | 12,138,201 | ||||||
| Total Assets | $ | 17,862,270 | $ | 17,539,980 | ||||
| LIABILITIES | ||||||||
| Current Liabilities | ||||||||
| Trade and Other Payables | $ | 3,716,218 | $ | 2,673,221 | ||||
| Due to MyMD Florida Shareholders | 29,982 | 29,982 | ||||||
| Operating Lease Liability | 48,870 | 65,780 | ||||||
| Derivative Liabilities | 61,000 | - | ||||||
| Warrant Liabilities | 867,000 | - | ||||||
| Dividends Payable | 265,019 | - | ||||||
| Total Current Liabilities | 4,988,089 | 2,768,983 | ||||||
| Non-Current Liabilities | ||||||||
| Deferred Compensation Payable | 100,538 | - | ||||||
| Operating Lease Liability, net of current portion | - | 75,941 | ||||||
| Total Non-Current Liabilities | 100,538 | 75,941 | ||||||
| Total Liabilities | $ | 5,088,627 | $ | 2,844,924 | ||||
| Commitments and Contingencies | - | - | ||||||
| Series F Convertible Preferred Stock, with par value $0.001 per share and a stated value of $ per share, and shares designated as of December 31, 2023 and December 31, 2022, and shares issued and outstanding as of December 31, 2023 and December 31, 2022. Liquidation preference of $6,833,500 plus dividends at 10% per annum of $265,350 as of December 31, 2023. | 404,071 | - | ||||||
| STOCKHOLDERS’ EQUITY | ||||||||
| Preferred Stock, with par value $ per share, total preferred shares authorized | ||||||||
| Series D Convertible Preferred Stock, shares designated, with par value $ per share and a stated value of $0.01 per share, shares issued and outstanding as of December 31, 2023 and December 31, 2022 | 144,524 | 144,524 | ||||||
| Preferred stock value | 144,524 | 144,524 | ||||||
| Common stock, par value $ per share, shares authorized and issued and outstanding as of December 31, 2023 and December 31, 2022 | 2,019 | 1,316 | ||||||
| Additional Paid In Capital | 114,200,096 | 108,308,120 | ||||||
| Accumulated Deficit | (101,977,067 | ) | (93,758,904 | ) | ||||
| Total Stockholders’ Equity | 12,369,572 | 14,695,056 | ||||||
| Total Liabilities and Stockholders’ Equity | $ | 17,862,270 | $ | 17,539,980 | ||||
The accompanying notes are an integral part of these consolidated financial statements
| F-4 |
MYMD PHARMACEUTICALS, INC. AND SUBSIDIARIES
Consolidated Statements of Comprehensive Loss
| 2023 | 2022 | |||||||
| For the Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Product Revenue | $ | - | $ | - | ||||
| Product Cost of Sales | - | - | ||||||
| Gross Income | - | - | ||||||
| Administrative Expenses | 5,442,886 | 5,520,150 | ||||||
| Research and Development Expenses | 7,867,795 | 9,067,422 | ||||||
| Stock Based Compensation | 3,049,537 | 695,191 | ||||||
| Warrant Issuance Expenses | 762,834 | - | ||||||
| Loss from Operations | (17,123,052 | ) | (15,282,763 | ) | ||||
| Other (Income) Expenses | ||||||||
| Interest and Dividend Income | (455,570 | ) | (83,991 | ) | ||||
| (Gain)/Loss on Sales of Marketable Securities | (416 | ) | 5,964 | |||||
| Unrealized (Gain)/Loss on Marketable Securities | (514 | ) | (2,958 | ) | ||||
| Change in fair value of Derivatives Liabilities | (3,088,800 | ) | - | |||||
| Change in fair value of Warrant Liabilities | (9,756,000 | ) | - | |||||
| Uninsured Casualty Losses | 178,198 | (4,442 | ) | |||||
| Total Other (Income) Expenses | (13,123,102 | ) | (85,427 | ) | ||||
| Loss Before Income Tax | (3,999,950 | ) | (15,197,336 | ) | ||||
| Income Tax Benefit | - | - | ||||||
| Net Loss | $ | (3,999,950 | ) | $ | (15,197,336 | ) | ||
| Preferred Stock Dividends | 4,218,213 | - | ||||||
| Net Income/(Loss) Attributable to Common Stockholders | $ | (8,218,163 | ) | $ | (15,197,336 | ) | ||
| Basic and Dilutive net loss per common share | $ | ) | $ | ) | ||||
| Weighted average basic and diluted common shares outstanding | ||||||||
The accompanying notes are an integral part to these consolidated financial statements.
AKERS BIOSCIENCES,MYMD PHARMACEUTICALS, INC. AND SUBSIDIARIES
Consolidated Statement of Changes in Stockholders’ Equity
For the Years Ended December 31, 2023 and 2022
| Shares | Series F | Shares | Series D | Shares | Common Stock $0.001 Par Per Share | Additional | Accumulated Deficit | Total Equity | |||||||||||||||||||||||||||||
| Series F Convertible | Series D Convertible | ||||||||||||||||||||||||||||||||||||
| Preferred Stock | Preferred Stock | Common Stock | |||||||||||||||||||||||||||||||||||
| Shares | Series F | Shares | Series D | Shares | Common Stock $0.001 Par Per Share | Additional | Accumulated Deficit | Total Equity | |||||||||||||||||||||||||||||
| Balance at December 31, 2022 | - | $ | - | - | 72,992 | $ | 144,524 | 1,315,674 | $ | 1,316 | 108,308,120 | $ | (93,758,904 | ) | $ | 14,695,056 | |||||||||||||||||||||
| Balance at December 31, 2022 | - | $ | - | - | 72,992 | $ | 144,524 | 1,315,674 | $ | 1,316 | $ | 108,308,120 | $ | (93,758,904 | ) | $ | 14,695,056 | ||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | - | (3,999,950 | ) | (3,999,950 | ) | |||||||||||||||||||||||||
| Round-up shares from the 1-for-30 reverse split effective February 23, 2024 | - | - | - | - | - | 65,960 | 66 | (66 | ) | - | - | ||||||||||||||||||||||||||
| Round-up shares from the 1-for-30 reverse split effective February 23, 2024 | - | - | - | - | - | 65,960 | 66 | (66 | ) | - | - | ||||||||||||||||||||||||||
| Issuance of common stock for vested restricted stock units | - | - | - | - | - | 7,861 | 8 | (8 | ) | - | - | ||||||||||||||||||||||||||
| Exercise of prepaid equity forward contract | - | - | - | - | - | 4,505 | 4 | (4 | ) | - | - | ||||||||||||||||||||||||||
| Issuance of shares of Series F Convertible Preferred Stock, net of discount and offering costs of $14,087,111 | 15,000 | 912,889 | 912,889 | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Conversion of shares of Series F Convertible Preferred Stock, July 1, 2023 installment of $1,429,871 paid with common stock | (1,250 | ) | (76,074 | ) | (76,074 | ) | - | - | 39,587 | 40 | 255,906 | - | 255,945 | ||||||||||||||||||||||||
| Conversion of shares of Series F Convertible Preferred Stock | (1,250 | ) | (76,074 | ) | (76,074 | ) | - | - | 39,587 | 40 | 255,906 | - | 255,945 | ||||||||||||||||||||||||
| Conversion of shares of Series F Convertible Preferred Stock, August 1, 2023 installment of $1,429,871 paid with common stock | (1,250 | ) | (76,073 | ) | (76,073 | ) | - | - | 38,688 | 39 | 255,905 | - | 255,944 | ||||||||||||||||||||||||
| Conversion of shares of Series F Convertible Preferred Stock, One | (1,250 | ) | (76,073 | ) | (76,073 | ) | - | - | 38,688 | 39 | 255,905 | - | 255,944 | ||||||||||||||||||||||||
| Conversion of shares of Series F Convertible Preferred Stock, September 1, 2023 installment of $1,429,871 paid with common stock | (1,250 | ) | (76,074 | ) | (76,074 | ) | - | - | 67,732 | 68 | 255,877 | - | 255,945 | ||||||||||||||||||||||||
| Conversion of shares of Series F Convertible Preferred Stock, Two | (1,250 | ) | (76,074 | ) | (76,074 | ) | - | - | 67,732 | 68 | 255,877 | - | 255,945 | ||||||||||||||||||||||||
| Conversion of shares of Series F Convertible Preferred Stock, October 1, 2023 installment of $1,429,871 paid with common stock | (1,187 | ) | (63,659 | ) | (63,659 | ) | - | - | 58,450 | 58 | 214,118 | - | 214,175 | ||||||||||||||||||||||||
| Conversion of shares of Series F Convertible Preferred Stock, Three | (1,187 | ) | (63,659 | ) | (63,659 | ) | - | - | 58,450 | 58 | 214,118 | - | 214,175 | ||||||||||||||||||||||||
| Accelerated Conversion of shares of Series F Convertible Preferred Stock | (204 | ) | (12,416 | ) | (12,416 | ) | - | - | 10,550 | 10 | 41,759 | - | 41,770 | ||||||||||||||||||||||||
| Accelerated Conversion of 204 shares of Series F Convertible Preferred Stock | (204 | ) | (12,416 | ) | (12,416 | ) | - | - | 10,550 | 10 | 41,759 | - | 41,770 | ||||||||||||||||||||||||
| Accelerated Conversion of shares of Series F Convertible Preferred Stock | (416 | ) | (26,249 | ) | (26,249 | ) | - | - | 41,672 | 42 | 88,271 | - | 88,313 | ||||||||||||||||||||||||
| Accelerated Conversion of shares of Series F Convertible Preferred Stock, One | (416 | ) | (26,249 | ) | (26,249 | ) | - | - | 41,672 | 42 | 88,271 | - | 88,313 | ||||||||||||||||||||||||
| Redemption of shares of Series F Convertible Preferred Stock for cash | (772 | ) | (49,824 | ) | (49,824 | ) | - | - | - | - | - | - | |||||||||||||||||||||||||
| Accelerated Conversion of shares of Series F Convertible Preferred Stock | (570 | ) | (36,263 | ) | (36,263 | ) | - | - | 100,007 | 100 | 121,905 | - | 122,005 | ||||||||||||||||||||||||
| Accelerated Conversion of shares of Series F Convertible Preferred Stock,Two | (570 | ) | (36,263 | ) | (36,263 | ) | - | - | 100,007 | 100 | 121,905 | - | 122,005 | ||||||||||||||||||||||||
| Redemption of shares of Series F Convertible Preferred Stock for cash | (617 | ) | (39,811 | ) | (39,811 | ) | - | - | - | - | - | - | - | ||||||||||||||||||||||||
| Accelerated Conversion of shares of Series F Convertible Preferred Stock | (851 | ) | (52,375 | ) | (52,375 | ) | - | - | 182,848 | 183 | 176,030 | - | 176,213 | ||||||||||||||||||||||||
| Accelerated Conversion of shares of Series F Convertible Preferred Stock, Three | (851 | ) | (52,375 | ) | (52,375 | ) | - | - | 182,848 | 183 | 176,030 | - | 176,213 | ||||||||||||||||||||||||
| Deemed Dividend for the true-up of the August 1, 2023 installment for the Series F Convertible Preferred Stock paid with common stock | - | - | - | - | - | 29,045 | 29 | 766,474 | (766,503 | ) | - | ||||||||||||||||||||||||||
| Deemed Dividend for the true-up of the installment for the Series F Convertible Preferred Stock paid with common stock | - | - | - | - | - | 29,045 | 29 | 766,474 | (766,503 | ) | - | ||||||||||||||||||||||||||
| Deemed Dividend for the true-up of the October 1, 2023 installment for the Series F Convertible Preferred Stock paid with common stock | - | - | - | - | - | 56,278 | 56 | 66,273 | (666,329 | ) | - | ||||||||||||||||||||||||||
| Deemed Dividend for the true-up of the installment for the Series F Convertible Preferred Stock paid with common stock, One | - | - | - | - | - | 56,278 | 56 | 66,273 | (666,329 | ) | - | ||||||||||||||||||||||||||
| Series F Convertible Preferred Stock Dividend | - | - | - | - | - | - | - | - | (2,785,381 | ) | (2,785,381 | ) | |||||||||||||||||||||||||
| Stock based compensation - stock options | - | - | - | - | - | - | - | 3,049,537 | - | 3,049,537 | |||||||||||||||||||||||||||
| Balance at December 31, 2023 | 6,633 | $ | 404,071 | 404,071 | 72,992 | $ | 144,524 | 2,018,857 | $ | 2,019 | $ | 114,200,096 | $ | (101,977,067 | ) | $ | 12,369,572 | ||||||||||||||||||||
| Series F Convertible | Series D Convertible | |||||||||||||||||||||||||||||||||||
| Preferred Stock | Preferred Stock | Common Stock | ||||||||||||||||||||||||||||||||||
| Shares | Series F | Shares | Series D | Shares | Common Stock $0.001 Par Per Share | Additional Paid In Capital | Accumulated Deficit | Total Equity | ||||||||||||||||||||||||||||
| Balance at December 31, 2021 | - | $ | - | 72,992 | $ | 144,524 | 1,255,777 | $ | 1,256 | 102,062,962 | $ | (78,561,568 | ) | $ | 23,647,174 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (15,197,336 | ) | (15,197,336 | ) | |||||||||||||||||||||||||
| Net proceeds from private placement of common shares, net of offering costs $ 449,500 | - | - | - | - | 47,059 | 47 | 5,549,981 | - | 5,550,028 | |||||||||||||||||||||||||||
| Exercise of prepaid equity forward contracts for Common Stock | - | - | - | - | 12,838 | 13 | (13 | ) | - | - | ||||||||||||||||||||||||||
| Stock-based compensation – restricted stock units | - | - | - | - | - | - | 165,997 | - | 165,997 | |||||||||||||||||||||||||||
| Stock-based compensation – stock options | - | - | - | - | - | - | 444,342 | - | 444,342 | |||||||||||||||||||||||||||
| Stock-based compensation – warrants | - | - | - | - | - | - | 84,851 | - | 84,851 | |||||||||||||||||||||||||||
| Balance at December 31, 2022 | - | - | 72,992 | $ | 144,524 | 1,315,674 | $ | 1,316 | 108,308,120 | (93,758,904 | ) | 14,695,056 | ||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
8
| F-6 |
MYMD PHARMACEUTICALS, INC. AND SUBSIDIARIES
Consolidated Statements of Comprehensive LossCash Flows
| For the Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Product Revenue | $ | - | $ | - | ||||
| Product Cost of Sales | - | - | ||||||
| Gross Income | - | - | ||||||
| Administrative Expenses | 4,299,062 | 3,372,103 | ||||||
| Sales and Marketing Expenses | 22,963 | 25,000 | ||||||
| Research and Development Expenses | 7,963,678 | - | ||||||
| Litigation Settlement Expenses | - | 75,000 | ||||||
| Loss from Operations | (12,285,703 | ) | (3,472,103 | ) | ||||
| Other (Income) Expenses | ||||||||
| Loss on Disposal of Non-Current Assets | 3,042 | 9,576 | ||||||
| Foreign Currency Transaction (Gain) Loss | (93 | ) | 5,051 | |||||
| Gain on Fair Market Value Change of Equity Investments | (54,100 | ) | - | |||||
| (Gain) Loss on Investments | 36,714 | (3,952 | ) | |||||
| Interest and Dividend Income | (119,052 | ) | (101,483 | ) | ||||
| Total Other Income | (133,489 | ) | (90,808 | ) | ||||
| Loss Before Income Taxes | (12,152,214 | ) | (3,381,295 | ) | ||||
| Income Tax Benefit | - | - | ||||||
| Net Loss from Continuing Operations | (12,152,214 | ) | (3,381,295 | ) | ||||
| Net Loss from Discontinued Operations | (5,428,395 | ) | (506,954 | ) | ||||
| Net Loss | (17,580,609 | ) | (3,888,249 | ) | ||||
| Other Comprehensive Income (Loss) | ||||||||
| Net Unrealized Gain on Marketable Securities | - | 43,799 | ||||||
| Total Other Comprehensive Income | - | 43,799 | ||||||
| Comprehensive Loss | $ | (17,580,609 | ) | $ | (3,844,450 | ) | ||
| Basic and Diluted Loss per Common Share from Continuing Operations | $ | (1.72 | ) | $ | (5.52 | ) | ||
| Basic and Diluted Loss per Common Share from Discontinued Operations | $ | (0.77 | ) | $ | (0.83 | ) | ||
| Basic and Diluted Loss per Common Share | $ | (2.49 | ) | $ | (6.35 | ) | ||
| Weighted average basic and diluted common shares outstanding | 7,052,686 | 612,672 | ||||||
| 2023 | 2022 | |||||||
| For the Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss from ongoing operations | $ | (3,999,950 | ) | $ | (15,197,336 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Gain (loss) on sale of marketable securities | (416 | ) | 5,964 | |||||
| Change in fair value of marketable securities | (514 | ) | (2,958 | ) | ||||
| Change in fair value of derivatives | (3,088,800 | ) | - | |||||
| Change in fair value of warrants | (9,756,000 | ) | - | |||||
| Stock based compensation: | ||||||||
| Options issued to directors | 944,834 | - | ||||||
| Options issued to key employees | 1,962,138 | 338,922 | ||||||
| Options issued to non-employees | 142,565 | 105,420 | ||||||
| Warrants issued for services | - | 84,851 | ||||||
| Restricted stock units to non-employees | - | 165,997 | ||||||
| Change in assets and liabilities | ||||||||
| Prepaid expenses | (327,439 | ) | 540,560 | |||||
| Trade and other payables | 1,042,997 | 1,686,595 | ||||||
| Operating leases | (578 | ) | 1,917 | |||||
| Deferred compensation payable | 100,538 | - | ||||||
| Net cash used by operating activities | (12,980,625 | ) | (12,270,068 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchases of marketable securities | (13,454,304 | ) | (4,836,837 | ) | ||||
| Proceeds from sale of marketable securities | 15,300,030 | 11,750,000 | ||||||
| Net cash provided by investing activities | 1,845,726 | 6,913,163 | ||||||
| Cash flows from financing activities | ||||||||
| Redemption of Series F Convertible Preferred Stock | (89,635 | ) | - | |||||
| Dividends on Series F Convertible Preferred Stock | (1,452,145 | ) | - | |||||
| Premium on Series F Convertible Preferred Stock | (77,090 | ) | - | |||||
| Net proceeds from the issuance of preferred stock | 14,685,689 | - | ||||||
| Net proceeds from issuance of common stock | - | 5,550,028 | ||||||
| Net cash provided by financing activities | 13,066,819 | 5,550,028 | ||||||
| Net increase in cash and cash equivalents | 1,931,920 | 193,123 | ||||||
| Cash and cash equivalents at beginning of year | 749,090 | 555,967 | ||||||
| Cash and cash equivalents at end of year | $ | 2,681,010 | $ | 749,090 | ||||
| Supplemental cash flow information | ||||||||
| Cash paid for: | ||||||||
| Interest | $ | - | $ | 13,322 | ||||
| Income Taxes | $ | - | $ | - | ||||
| Supplemental Schedule of Non-Cash Financing and Investing Activities | ||||||||
| Operating lease right-of-use asset obtained in exchange for lease obligation | $ | - | $ | 53,196 | ||||
| Initial fair value of warrant liabilities pursuant to the issuance of Series F Convertible Preferred Stock and Warrants | $ | 10,623,000 | $ | - | ||||
| Initial fair value of derivative liabilities pursuant to the issuance of Series F Convertible Preferred Stock and Warrants | $ | 3,149,800 | $ | - | ||||
The accompanying notes are an integral part to these consolidated financial statements.
AKERS BIOSCIENCES,
| F-7 |
MYMD PHARMACEUTICALS, INC. AND SUBSIDIARIES
Consolidated Statement of Changes in Shareholders’ Equity
For the Years Ended December 31, 2020 and 2019
| Series D | ||||||||||||||||||||||||||||
| Convertible | ||||||||||||||||||||||||||||
| Preferred | Series D | Common | Accumulated | |||||||||||||||||||||||||
| Shares | Convertible | Shares | Other | Total | ||||||||||||||||||||||||
| Issued and | Preferred | Issued and | Common | Accumulated | Comprehensive | Shareholders’ | ||||||||||||||||||||||
| Outstanding | Stock | Outstanding | Stock | Deficit | Income/(Loss) | Equity | ||||||||||||||||||||||
| Balance at January 1, 2019 | - | $ | - | 540,607 | $ | 121,554,547 | $ | (115,694,881 | ) | $ | (25,913 | ) | $ | 5,833,753 | ||||||||||||||
| Net loss | - | - | - | - | (3,888,249 | ) | - | (3,888,249 | ) | |||||||||||||||||||
| Public offering – common stock, net of offering costs of $306,222 | - | - | 613,500 | 2,147,778 | - | - | 2,147,778 | |||||||||||||||||||||
| Public offering – pre-funded warrants, net of offering costs of $688,005 | - | - | - | 4,817,857 | - | - | 4,817,857 | |||||||||||||||||||||
| Issuance of stock grants to officer | - | - | 1,563 | 27,367 | - | - | 27,367 | |||||||||||||||||||||
| Issuance of common stock to vendor for services | - | - | 1,667 | 10,802 | - | - | 10,802 | |||||||||||||||||||||
| Exercise of prepaid equity forward contracts for common stock | - | - | 581,500 | 58 | - | - | 58 | |||||||||||||||||||||
| Stock-based compensation – restricted stock units | - | - | - | 362,005 | - | - | 362,005 | |||||||||||||||||||||
| Net unrealized gain on marketable securities | - | - | - | - | - | 43,799 | 43,799 | |||||||||||||||||||||
| Balance at December 31, 2019 | - | $ | - | 1,738,837 | $ | 128,920,414 | $ | (119,583,130 | ) | $ | 17,886 | $ | 9,355,170 | |||||||||||||||
| Net loss | - | - | - | - | (17,580,609 | ) | - | (17,580,609 | ) | |||||||||||||||||||
| Exercise of pre-funded warrants for common stock | - | - | 795,000 | 80 | - | - | 80 | |||||||||||||||||||||
| Stock-based compensation – restricted stock units | - | - | - | 404,589 | - | - | 404,589 | |||||||||||||||||||||
| Stock-based compensation – acquisition of license for preferred series “D” stock | 211,353 | 418,479 | - | - | - | - | 418,479 | |||||||||||||||||||||
| Stock-based compensation – acquisition of license for common stock | - | - | 411,403 | 814,578 | - | - | 814,578 | |||||||||||||||||||||
| Stock-based compensation – shares issued to vendors | - | - | - | 7,318 | - | - | 7,318 | |||||||||||||||||||||
| Exercise of Series C Convertible Preferred Warrants for common stock | - | - | 1,935,000 | 7,740,000 | - | - | 7,740,000 | |||||||||||||||||||||
| Exercise of Series D Convertible Preferred Shares for common stock | (138,361 | ) | (273,955 | ) | 138,361 | 273,955 | - | - | - | |||||||||||||||||||
| Registered direct offering of common stock, net of offering costs of $513,795 | - | - | 766,667 | 4,086,207 | - | - | 4,086,207 | |||||||||||||||||||||
| Registered direct offering of common stock, net of offering costs of $504,281 | - | - | 1,366,856 | 4,320,720 | - | - | 4,320,720 | |||||||||||||||||||||
| Registered direct offering of common stock, net of offering costs of $689,874 | - | - | 1,207,744 | 6,158,034 | - | - | 6,158,034 | |||||||||||||||||||||
| Private placement of common stock, net of offering costs of $1,522,694 | - | - | 8,725,393 | 14,619,283 | - | - | 14,619,283 | |||||||||||||||||||||
| Private placement of pre-funded warrants, net of offering costs of $181,496 | - | - | - | 1,743,503 | - | - | 1,743,503 | |||||||||||||||||||||
| Share-based compensation – shares issued for litigation settlements | - | - | 500,000 | 2,510,000 | - | - | 2,510,000 | |||||||||||||||||||||
| Reclassification of unrealized gain on marketable securities | - | - | - | - | - | (17,886 | ) | (17,886 | ) | |||||||||||||||||||
| Balance at December 31, 2020 | 72,992 | $ | 144,524 | 17,585,261 | $ | 171,598,681 | $ | (137,163,739 | ) | $ | - | $ | 34,579,466 | |||||||||||||||
The accompanying notes are an integral part to these consolidated financial statements.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
For the Years Ended December 31, 2020 and 2019
| For the Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Cash flows from operating activities | ||||||||
| Net loss from continuing operations | $ | (12,152,214 | ) | $ | (3,381,295 | ) | ||
| Net loss from discontinued operations | (5,428,395 | ) | (506,954 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Loss (gain) on sale of securities | 36,714 | (3,952 | ) | |||||
| Gain on fair market value of equity investments | (54,100 | ) | - | |||||
| Accrued income - marketable securities | 2,790 | 3,353 | ||||||
| Depreciation and amortization | 29,452 | 74,064 | ||||||
| Loss on disposal of fixed assets | 3,043 | 9,576 | ||||||
| Impairment of prepaid royalties | 291,442 | - | ||||||
| Impairment of production equipment | 18,680 | - | ||||||
| Impairment of intangible assets | 152,822 | 32,980 | ||||||
| Inventory adjustment for net realizable value | 197,723 | - | ||||||
| Reserve for obsolete inventory | - | 371,997 | ||||||
| Reserve for doubtful trade receivables | - | 5,325 | ||||||
| Reserve for doubtful other receivables | - | 100,000 | ||||||
| Stock-based compensation to employees - common stock | - | 27,367 | ||||||
| Stock-based compensation to directors - restricted stock units | 404,589 | 362,005 | ||||||
| Stock-based compensation - shares issued to vendors | 7,318 | 10,802 | ||||||
| Stock-based compensation – shares issued to Chubeworkx | 2,510,000 | - | ||||||
| Stock-based compensation – shares issued to Cystron | 1,233,057 | - | ||||||
| Changes in assets and liabilities: | ||||||||
| Decrease in trade receivables | 42,881 | 128,120 | ||||||
| (Increase)/decrease in deposits and other receivables | (9 | ) | 9,347 | |||||
| Decrease in inventories | 1,262 | 14,285 | ||||||
| Decrease in prepaid expenses | 41,752 | 103,152 | ||||||
| Decrease in other assets | 2,722 | 9,280 | ||||||
| Increase/(decrease) in trade and other payables | 733,530 | (443,735 | ) | |||||
| Net cash used in operating activities | (11,924,941 | ) | (3,074,283 | ) | ||||
| Cash flows from investing activities | ||||||||
| Proceeds from the sale of equipment | - | 6,250 | ||||||
| Short-term note receivable | (1,200,000 | ) | (100,000 | ) | ||||
| Purchases of marketable securities | (9,871,843 | ) | (6,704,837 | ) | ||||
| Proceeds from sale of marketable securities | 2,314,374 | 2,857,960 | ||||||
| Net cash used in investing activities | (8,757,469 | ) | (3,940,627 | ) | ||||
| Cash flows from financing activities | ||||||||
| Net proceeds from issuance of common stock | 29,184,244 | 2,147,778 | ||||||
| Net proceeds from issuance of pre-funded warrants for the purchase of common stock | 1,743,503 | 4,817,857 | ||||||
| Net proceeds from the exercise of pre-funded warrants for the purchase of common stock | 80 | 58 | ||||||
| Net proceeds from exercise of warrants for common stock | 7,740,000 | - | ||||||
| Net cash provided by financing activities | 38,667,827 | 6,965,693 | ||||||
| Net increase/(decrease) in cash and cash equivalents and restricted cash | 17,985,417 | (49,217 | ) | |||||
| Cash and cash equivalents and restricted cash at beginning of year | 632,538 | 681,755 | ||||||
| Cash and cash equivalents and restricted cash at end of year | $ | 18,617,955 | $ | 632,538 | ||||
| Supplemental cash flow information: | ||||||||
| Cash paid for: | ||||||||
| Interest | $ | - | $ | - | ||||
| Income Taxes | $ | - | $ | - | ||||
| Supplemental Schedule of Non-Cash Financing and Investing Activities | ||||||||
| Net unrealized gains on marketable securities | $ | - | $ | 43,799 | ||||
| Exercise of Series D Convertible Preferred Stock for Common Stock | $ | 273,955 | $ | - | ||||
The accompanying notes are an integral part to these consolidated financial statements.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 1 – Organization and Description of Business
Akers Biosciences,MyMD Pharmaceuticals, Inc. (“Akers”), is a Delaware corporation (“MyMD”) that was incorporated in New Jersey corporation.prior to the Reincorporation (as defined below). These condensed consolidated financial statements include threetwo wholly owned subsidiaries Cystron Biotech, LLC (“Cystron”),as of December 31, 2023, Akers Acquisition Sub, Inc. and Bout Time Marketing Corporation, (together, the “Company”). All material intercompany transactions have been eliminated in consolidation.
On April 8, 2022, the MyMD Florida (as defined below) subsidiary was dissolved and merged into the New Jersey corporation MyMD Pharmaceuticals, Inc. pursuant to an Agreement and Plan of Merger dated April 8, 2022.
At the Company’s annual meeting of stockholders held on July 31, 2023, the stockholders approved a plan to merge the Company with and into a newly formed wholly owned subsidiary, MyMD Pharmaceuticals, Inc., a Delaware corporation (“MyMD Delaware”), with MyMD Delaware being the surviving corporation, for the purpose of changing the Company’s state of incorporation from New Jersey to Delaware (the “Reincorporation”). The Reincorporation was effected as of March 4, 2024. In connection with the Reincorporation to Delaware, the par value of the common and preferred stock was changed to $ per share.
MYMD-1 is an oral, next-generation TNF-α inhibitor with the potential to transform the way TNF-α based diseases are treated due to its selectivity and ability to cross the blood brain barrier. Its ease of oral dosing is a significant differentiator compared to currently available TNF-α inhibitors, all of which require delivery by injection or infusion. MYMD-1 has also been shown to selectively block TNF-α action where it is overactivated without preventing it from doing its normal job of responding to routine infection. MYMD-1 is doubly effective at inhibiting inflammation by blocking both TNF-a and IL-6 activity, whereas currently approved anti-TNF and anti-IL-6 treatments for RA can only target one or the other. In addition, in early clinical studies it has not been associated with serious side effects known to occur with traditional immunosuppressive therapies that treat inflammation.
On February 14, 2024, the Company effected a 1-for-30 reverse stock split (the “Reverse Stock Split”). Simultaneously with the Reverse Stock Split, number of shares of our common stock authorized for issuance was reduced from shares to shares, and our authorized capital stock was reduced from shares to shares. The Reverse Stock Split reduced the total number of issued and outstanding shares of Common Stock, including shares held by the Company as treasury shares. All share amounts have been retroactively adjusted for the Reverse Stock Split.
Recent Events
The February 2023 Offering
On February 21, 2023, the Company entered into a Securities Purchase Agreement (the “Purchase Agreement”) with certain accredited investors (the “Investors”), pursuant to which it agreed to sell to the Investors (i) an aggregate of shares of the Company’s newly-designated Series F convertible preferred stock with a stated value of $1,000 per share, initially convertible into up to shares (pre-split) of the Company’s common stock (the “Common Stock”) at an initial conversion price of $2.255 per share (pre-split), subject to adjustment (the “Preferred Shares”), and (ii) warrants to acquire up to an aggregate of 6,651,885 shares (pre-split) of Common Stock, subject to adjustment (the “Warrants”) (collectively, the “February 2023 Offering”). Following the Reverse Stock Split, (i) the conversion price of the Preferred Shares was adjusted to $per share pursuant to the terms of the Certificate of Designations, and (ii) the exercise price of the Warrants was adjusted to $3.18 per share and the number of shares of Common Stock issuable upon exercise of the Warrants was adjusted proportionately to 4,716,904 shares pursuant to the terms of the Warrants.
| F-8 |
Series F Convertible Preferred Stock
The Preferred Shares became convertible upon issuance into Common Stock (the “Conversion Shares”) at the election of the holder at any time at an initial conversion price of $2.255 (pre-split) (as adjusted, the “Conversion Price”). The Conversion Price is subject to customary adjustments for stock dividends, stock splits, reclassifications and the like, and subject to price-based adjustment in the event of any issuances of Common Stock, or securities convertible, exercisable or exchangeable for Common Stock, at a price below the then-applicable Conversion Price (subject to certain exceptions). Following the Reverse Stock Split, the Conversion Price for the Preferred Shares was adjusted to $ per share pursuant to the terms of the Certificate of Designations. The Company was historicallyis required to redeem the Preferred Shares in 12 equal monthly installments, commencing on July 1, 2023. The amortization payments due upon such redemption are payable, at the company’s election, in cash, or subject to certain limitations, in shares of Common Stock valued at the lower of (i) the Conversion Price then in effect and (ii) the greater of (A) 80% of the average of the three lowest closing prices of the Company’s Common Stock during the thirty trading day period immediately prior to the date the amortization payment is due or (B) a developer“Floor Price” of rapid health information technologies, but, since March 2020, has been primarily focused$6.60 (subject to adjustment for stock splits, stock dividends, stock combinations, recapitalizations or other similar events) or, in any case, such lower amount as permitted, from time to time, by the Nasdaq Stock Market. The Company may require holders to convert their Preferred Shares into Conversion Shares if the closing price of the Common Stock exceeds $ per share (subject to adjustment for stock splits, stock dividends, stock combinations, recapitalizations or other similar events) for 20 consecutive trading days and the daily dollar trading volume of the Common Stock exceeds $3,000,000 per day during the same period and certain equity conditions described in the Certificate of Designation are satisfied.
The holders of the Preferred Shares are entitled to dividends of 10% per annum, compounded monthly, which is payable in cash or shares of Common Stock at the Company’s option, in accordance with the terms of the Certificate of Designations. Upon the occurrence and during the continuance of a Triggering Event (as defined in the Certificate of Designations), the Preferred Shares accrue dividends at the rate of 15% per annum. Upon conversion or redemption, the holders of the Preferred Shares are also entitled to receive a dividend make-whole payment. The holders of Preferred Shares have no voting rights on account of the Preferred Shares, other than with respect to certain matters affecting the rights of the Preferred Shares. During the year ended December 31, 2023, the Company recorded dividends totaling $3,451,710, which are reported as Preferred Stock Dividends on the developmentConsolidated Statement of Comprehensive Income (Loss).
Notwithstanding the foregoing, the Company’s ability to settle conversions and make amortization and dividend make-whole payments using shares of Common Stock is subject to certain limitations set forth in the Certificate of Designations. Further, the Certificate of Designations contains a certain beneficial ownership limitation after giving effect to the issuance of shares of Common Stock issuable upon conversion of, or as part of any amortization payment or dividend make-whole payment under, the Certificate of Designations or Warrants.
The Certificate of Designations includes certain Triggering Events (as defined in the Certificate of Designations), including, among other things, the Company’s failure to pay any amounts due to the holders of the Preferred Shares when due. In connection with a Triggering Event, each holder of Preferred Shares will be able to require the Company to redeem in cash any or all the holder’s Preferred Shares at a premium set forth in the Certificate of Designations.
The Preferred Shares were determined to be more akin to a debt-like host than an equity-like host. The Company identified the following embedded features that are not clearly and closely related to the debt host instrument: 1) make-whole interest upon a contingent redemption event, 2) make-whole interest upon a conversion event, 3) an installment redemption upon an Equity Conditions Failure (as defined in the Certificate of Designation), and 4) variable share-settled installment conversion. These features were bundled together, assigned probabilities of being affected and measured at fair value. Subsequent changes in fair value of these features are recognized in the Condensed Consolidated Statement of Comprehensive Income (Loss). The Company estimated at issuance the $3,149,800 fair value of the bifurcated embedded derivative at issuance using a Monte Carlo simulation model, with the following inputs the fair value of our common stock of $ on the issuance date , estimated equity volatility of 120.0%, estimated traded volume volatility of 190.0%, the time to maturity of 1.35 years, a discounted market interest rate of 6.8%, dividend rate of 10.0%, a penalty dividend rate of 15.0%, and probability of default of 0.5%. The fair value of the bifurcated derivative liabilities was estimated utilizing the with and without method which uses the probability weighted difference between the scenarios with the derivative and the plain vanilla maturity scenario without a derivative.
The discount to the fair value is included as a reduction to the carrying value of the Preferred Shares. During the year ended December 31, 2023, the Company recorded a total discount of $14,087,111 upon issuance of the Preferred Shares, which was comprised of the issuance date fair value of the associated embedded derivative of $3,149,800, stock issuance costs of $314,311 and the fair value of the Warrants of $10,623,000.
| F-9 |
During the year ended December 31, 2023, the Company recorded a gain of $3,088,800 related to the change in fair value of the derivative liabilities which is recorded in other income (expense) on the Consolidated Statement of Comprehensive Income (Loss). The Company estimated the $61,000 fair value of the bifurcated embedded derivative at December 31, 2023 using a Monte Carlo simulation model, with the following inputs the fair value of our common stock of $0.26 ($7.80 post reverse split) on the valuation date , estimated equity volatility of %, estimated traded volume volatility of 150.0%, the time to maturity of 0.5 years, a discounted market interest rate of %, dividend rate of 10.0%, a penalty dividend rate of 15.0%, and probability of default of 3.90%.
Common Stock Warrants
Pursuant to the February 2023 Offering, the Company issued to investors Warrants to purchase 4,716,904 shares of Common Stock, with an exercise price of $3.18 per share (subject to adjustment), for a period of five years from the date of issuance. The Exercise Price and the number of shares issuable upon exercise of the Warrants are subject to customary adjustments for stock dividends, stock splits, reclassifications and the like, and subject to price-based adjustment, on a “full ratchet” basis, in the event of any issuances of Common Stock, or securities convertible, exercisable or exchangeable for Common Stock, at a price below the then-applicable Exercise Price (subject to certain exceptions). Upon any such price-based adjustment to the Exercise Price, the number of shares issuable upon exercise of the Warrants will be increased proportionately.
The Warrants were determined to be within the scope of ASC 480-10 as they are puttable to the Company at Holders’ election upon the occurrence of a vaccine candidate against SARS-CoV-2,Fundamental Transaction (as defined in the agreements). As such, the Company recorded the Warrants as a coronavirus currently causingliability at fair value with subsequent changes in fair value recognized in earnings. The Company utilized the Black Scholes Model to calculate the value of these warrants issued during the year ended December 31, 2023. The fair value of the Warrants of $10,623,000 was estimated at the date of issuance using the following weighted average assumptions: dividend yield 0%; expected term of 5.0 years; equity volatility of 125.0%; and a pandemic throughout the world. In responserisk-free interest rate of 4.09%.
Transaction costs incurred attributable to the global pandemic,issuance of the Warrants of $762,834 were immediately expensed in accordance with ASC 480.
During the year ended December 31, 2023, the Company recorded a gain of $9,756,000 related to the change in fair value of the warrant liabilities which is pursuing rapid developmentrecorded in other income (expense) on the Consolidated Statement of Comprehensive Loss. The fair value of the Warrants of $867,000 was estimated at December 31, 2023 utilizing the Black Scholes Model using the following weighted average assumptions: dividend yield 0%; remaining term of 4.15 years; equity volatility of 120.0%; and manufacturinga risk-free interest rate of its COVID-19 vaccine candidate, or combination product candidate (the “COVID-19 Vaccine Candidate”) in collaboration with Premas Biotech PVT Ltd. (“Premas”), an entity incorporated in India.3.91%.
On July 7, 2020,Reduction in Workforce
During October and November 2023, the Company immediately ceasedimplemented a reduction in workforce, eliminating three of the productionCompany’s ten employees. Separated employees were granted a severance package equal to one-quarter of their annual salary.
On June 7, 2023, the Company granted the three employees options to purchase an aggregate of shares of Common Stock with an exercise price of $per share. As consideration for a waiver and salerelease in their separation agreements, the Company amended the employees’ respective June 7, 2023 option agreements to accelerate vesting of the portion of optioned shares that otherwise would have vested upon the first and second anniversaries of the date of grant. The options have an exercise period of twelve months from the date of separation. The Company recognized as compensation expense $168,496 which represented the remaining unamortized fair value of the original grant.
| F-10 |
Executive Officer Contract Amendments and Separations
Effective November 13, 2023, the Company entered into an amendment to the employment agreement of Dr. Chris Chapman, its rapid, point-of-care screeningPresident and testing products.Chief Medical Officer, providing for Dr. Chapman’s annual base salary to be adjusted from five hundred thousand dollars ($500,000) (the “Full Base Salary”) to two hundred fifty thousand dollars ($250,000) in cash per annum, until payment of his Full Base Salary would no longer jeopardize the Company’s ability to continue as a going concern, as determined by the Company in its sole discretion. The amendment further provides that the remaining $250,000 of base salary per annum (the “Deferral Amount”) shall be deferred until payment of the Deferral Amount would no longer jeopardize the Company’s ability to continue as a going concern, as determined by the Company in its sole discretion, at which time the Deferral Amount may be paid, at Dr. Chapman’s election, in shares of Common Stock or in cash. As of December 31, 2023, the Company had recognized a salary deferral of $28,846 which is included in Deferred Compensation Payable on the Consolidated Balance Sheet.
In connection with an overall reduction in compensation paid to the Company’s directors implemented in November 2023, effective November 13, 2023, the Company entered into an amendment to the employment agreement of Christopher C. Schreiber, a Director and the Company’s former Executive Chairman, providing for Mr. Schreiber’s annual fee to be adjusted from three hundred thousand dollars ($300,000) (the “Full Fee”) to sixty thousand dollars ($60,000) in cash per annum, until payment of his Full Fee would no longer jeopardize the Company’s ability to continue as a going concern, as determined by the Company in its sole discretion. The amendment further provides that the remaining $240,000 of the fees per annum (the “Fee Deferral Amount”) shall be deferred until payment of the Fee Deferral Amount would no longer jeopardize the Company’s ability to continue as a going concern, as determined by the Company in its sole discretion, at which time the Fee Deferral Amount may be paid, at Mr. Schreiber’s election, in shares of Common Stock or in cash. The amendment also clarified that Mr. Schreiber’s title is “Director.” As of December 31, 2023, the Company had recognized a salary deferral of $27,692 which is included in Deferred Compensation Payable on the Consolidated Balance Sheet.
Effective November 13, 2023, the Company entered into an amendment to the employment agreement of Dr. Adam Kaplin, its Chief Scientific Officer, providing that Dr. Kaplin’s employment shall have an initial term of four months, which the parties may mutually agree to extend for additional consecutive terms of one month each. The amendment further provides that, in the event of termination without cause by the Company prior to the end of the initial term, Dr. Kaplin shall receive his monthly base salary through the end of the initial term. The amendment further provides that all outstanding and unvested shares granted pursuant to the Nonqualified Stock Option Agreement, dated June 7, 2023, between the Company and Dr. Kaplin shall accelerate upon the termination of Dr. Kaplin’s employment. Dr. Kaplin’s amendment further provides that, in the event of a termination for any reason prior to the end of the first renewal term following the end of the initial term, the Company will continue to provide support for these testing products that remain incover the marketcosts of Dr. Kaplin’s health insurance coverage through respective product expiration dates. For a more detailed discussionthe end of the first renewal term, subject to the execution and timely return of a release.
Effective November 13, 2023, the Company entered into a mutual employment separation agreement with Paul M. Rivard, its Chief Legal Officer. The separation agreement provides for a lump-sum severance payment equal to three months of his normal base salary in exchange for a waiver and release. The separation agreement further provides that Mr. Rivard will be deemed a contractor providing services to the Company for purposes of any awards previously granted to him under the 2021 Plan if at the relevant time(s) he is providing services to the Company while under the employ of a law firm representing the Company.
Director’s Deferral of Board Service Fees
On November 13, 2023, the Board approved certain adjustments to the director fees. Mr. Silverman’s fees were decreased from $216,000 to $60,000 annually, with payment of the excess amount of $156,000 deferred until the date that payment of such amount would no longer jeopardize the Company’s cessationability to continue as a going concern, as determined by the Company in its sole discretion, at which time such amount may be paid, at Mr. Silverman’s election, in shares of Common Stock or in cash. Messrs. Eagle’s, Uzonwanne’s, and White’s fees were decreased from $96,000 to $60,000 annually, with payment of the excess amounts of $36,000 per director deferred until the date that payment of such amounts would no longer jeopardize the Company’s ability to continue as a going concern, as determined by the Company in its screening and testing products, see Note 3 and Note 6 herein.sole discretion, at which time such amounts may be paid, at each director’s election, in shares of Common Stock or in cash.
Note 2 – Significant Accounting Policies
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to(a) Basis of Presentation
The Consolidated Financial Statements of the Company are prepared in U.S. Dollars and in accordance with accounting principles generally accepted in the United States of America (US GAAP).
Note 2 - Significant Accounting Policies, continued(b) Use of Estimates and Judgments
The preparation of financial statements in conformity with US GAAP requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities and expenses. Actual results may differ from these estimates. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected. Information about significant areas of estimation, uncertainty and critical judgments in applying accounting policies that have the most significant effect on the amounts recognized in the financial statements is included in the following notes for recording research and development expenses, impairment of intangible assets and the valuation of share-based payments.
(c) Functional and Presentation Currency
These consolidated financial statements are presented in U.S. Dollars, which is the Company’s functional currency. All financial information has been rounded to the nearest dollar. Foreign Currency Transaction Gains or Losses, resulting from cash balances denominated in Foreign Currencies, are recorded in the Consolidated Statements of Operations and Comprehensive Loss.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes(d) Comprehensive Income (Loss)
The Company follows Financial Accounting Standards Board Accounting Standards Codification (“FASB ASC”) 220 in reporting comprehensive income (loss). Comprehensive income (loss) is a more inclusive financial reporting methodology that includes disclosure of certain financial information that historically has not been recognized in the calculation of net income. Since the Company has no items of other comprehensive income (loss), comprehensive income (loss) is equal to Consolidatednet income (loss).
(e) Cash and Cash Equivalents
The Company considers all highly liquid investments, which include short-term bank deposits (up to three months from date of deposit) that are not restricted as to withdrawal date or use, to be cash equivalents.
(f) Fair Value of Financial StatementsInstruments
Note 2 - Significant Accounting Policies, continuedThe Company’s financial instruments consist of cash and cash equivalents, marketable securities, receivables and trade and other payables. The carrying value of cash and cash equivalents, receivables and trade and other payables approximate their fair value because of their short maturities.
The framework for measuring fair value provides a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). The three levels of the fair value hierarchy under FASB ASC 820 are described as follows:
| Level 1 | Inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets that the Company | |
| Level 2 | Inputs to the valuation methodology include: |
| ● | quoted prices for similar assets or liabilities in active markets; | |
| ● | ||
| quoted prices for identical or similar assets or liabilities in inactive markets; | ||
| ● | ||
| inputs other than quoted prices that are observable for the asset or liability; | ||
| ● | ||
| inputs that are derived principally from or corroborated by observable market data by correlation or other means | ||
If the asset or liability has a specified (contractual) term, the level 2 input must be observable for substantially the full term of the asset or liability.
| Level 3 | Inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
The asset or liability’s fair value measurement level within the fair value hierarchy is based on the lowest level of input that is significant to the fair value measurement. Valuation techniques maximize the use of relevant observable inputs and minimize the use of unobservable inputs.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes(f) Fair Value of Financial Instruments, continued
The following is a description of the valuation methodologies used for assets measured at fair value as of December 31, 2023 and December 31, 2022.
Schedule of Marketable Securities
Marketable Securities: Valued using quoted prices in active markets for identical assets.
| Quoted Prices in Active Markets for Identical Assets or Liabilities (Level 1) | Quoted Prices for Similar Assets or Liabilities in Active Markets (Level 2) | Significant Unobservable Inputs (Level 3) | ||||||||||
| Marketable securities at December 31, 2023 | $ | 2,242,106 | $ | - | $ | - | ||||||
| Marketable securities at December 31, 2022 | $ | 4,086,902 | $ | - | $ | - | ||||||
Marketable securities are classified as available for sale and are valued at fair market value. Maturities of the securities are less than one year.
As of December 31, 2023 and 2022, the Company held certain mutual funds, which, under FASB ASC 321-10, were considered equity investments. As such, the change in fair value in the year ended December 31, 2023 and 2022 was a gain of $514 and a gain of $2,958, respectively.
Gains and losses resulting from the sales of marketable securities were gains of $416 and losses of $5,964 for the years ended December 31, 2023 and 2022, respectively.
Proceeds from the sales of marketable securities were $15,300,030 and $11,750,000 in the years ended December 31, 2023 and 2022, respectively. Purchases of marketable securities were $13,454,304 and $4,836,837 during the years ended December 31, 2023 and 2022, respectively.
Fair Value on a Recurring Basis
The Company follows the guidance in ASC 820 for its financial assets and liabilities that are re-measured and reported at fair value at each reporting period, and non-financial assets and liabilities that are re-measured and reported at fair value at least annually. The estimated fair value of the warrant liabilities and bifurcated embedded derivatives represent Level 3 measurements. The following table presents information about the Company’s liabilities that are measured at fair value on a recurring basis as of December 31, 2023, and indicates the fair value hierarchy of the valuation inputs the Company utilized to Consolidated Financial Statementsdetermine such fair value:
Note 2 - Significant Accounting Policies, continuedSchedule of Fair Value Hierarchy of the Valuation Inputs
| Description | Level | December 31, 2023 | ||||||
| Liabilities: | ||||||||
| Warrant liabilities (Note 3) | 3 | $ | 867,000 | |||||
| Derivative liabilities (Note 3) | 3 | $ | 61,000 | |||||
The following table sets forth a summary of the change in the fair value of the warrant liabilities that is measured at fair value on a recurring basis:
Summary of Change in Fair Value of Warrant Liabilities
| Balance on December 31, 2022 | $ | - | ||
| Issuance of warrants reported at fair value | 10,623,000 | |||
| Change in fair value of warrant liabilities | (1,175,000 | ) | ||
| Balance on March 31, 2023 | 9,448,000 | |||
| Change in fair value of warrant liabilities | (1,635,000 | ) | ||
| Balance on June 30, 2023 | 7,813,000 | |||
| Change in fair value of warrant liabilities | (5,356,000 | ) | ||
| Balance on September 30, 2023 | 2,457,000 | |||
| Change in fair value of warrant liabilities | (1,590,000 | ) | ||
| Balance on December 31, 2023 | $ | 867,000 |
|
Quoted Prices in Active Markets for Identical Assets or Liabilities | Quoted Prices for Similar Assets or Liabilities in Active Markets | Significant Unobservable | ||||||||||
| Marketable securities at December 31, 2020 | $ | 16,718,452 | $ | - | $ | - | ||||||
| Marketable securities at December 31, 2019 | $ | 9,164,273 | $ | - | $ | - | ||||||
|
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
NotesThe following table sets forth a summary of the change in the fair value of the derivative liabilities that is measured at fair value on a recurring basis:
Summary of Change in Fair Value of Derivative Liabilities
| Balance on December 31, 2022 | $ | - | ||
| Issuance of convertible preferred stock with derivative liabilities | 3,149,800 | |||
| Change in fair value of derivative liabilities | 120,700 | |||
| Balance on March 31, 2023 | 3,270,500 | |||
| Change in fair value of derivative liabilities | 194,500 | |||
| Balance on June 30, 2023 | 3,465,000 | |||
| Change in fair value of derivative liabilities | (2,566,900 | ) | ||
| Balance on September 30, 2023 | 898,100 | |||
| Change in fair value of derivative liabilities | (837,100 | ) | ||
| Balance on December 31, 2023 | $ | 61,000 |
(g) Derivative Financial Instruments
The Company evaluates its financial instruments to Consolidated Financial Statementsdetermine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging.” If liability accounting is required, the Company’s derivative instruments are recorded at fair value at the issuance date and re-valued at each reporting date, with changes in the fair value reported in the statements of operations. Derivative assets and liabilities are classified on the balance sheet as current or non-current based on whether or not net-cash settlement or conversion of the instrument could be required within twelve (12) months of the balance sheet date.
Note 2 - Significant Accounting Policies, continuedThe Company has determined that the Series F Convertible Preferred Stock warrants are derivatives that are required to be accounted for as liabilities. The Company has also determined that the following embedded features in the preferred stock are not clearly and closely related to the debt host instrument: 1) make-whole interest upon a contingent redemption event, 2) make-whole interest upon a conversion event, 3) an installment redemption upon an Equity Conditions Failure (as defined in the Certificate of Designation), and 4) variable share-settled installment conversion and as such are bifurcated from the preferred stock and accounted for as liabilities. The fair value of the warrants and embedded features are estimated using internal valuation models. The Company’s valuation models utilize inputs and other assumptions and may not be reflective of the price at which they can be settled.
|
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES(h) Prepaid Expenses
Notes to Consolidated Financial Statements
Note 2 - Significant Accounting Policies, continued
Prepaid expenses represent expenses paid prior to the date that the related services are rendered or used are recorded as prepaid expenses. Prepaid expenses are comprised principally of prepaid insurance.insurance and research and development expenses.
(i) Concentrations
Financial instruments whichthat potentially subject the Company to concentrations of credit risk consist principally of cash on deposit with financial institutions and accounts receivable. At times, the Company’s cash in banks is in excess ofexceeds the FDIC insurance limit. The Company has not experienced any loss as a resultbecause of these cash deposits. These cash balances are maintained with two banks.banks as of December 31, 2023.
(j) Risk Management of Cash and Investments
It is the Company’s policy to minimize the Company’s capital resources to investment risks, prioritizing the preservation of capital over investment returns. Investments are maintained in securities, primarily publicly traded, short-term money market funds based on highly rated federal, state, and corporate bonds, that minimize the risk to the Company’s capital resources and provide ready access to funds.
The Company’s investment portfolios are regularly monitored for risk and are held with twoone brokerage firms.firm.
| F-14 |
(k) Investments
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
NotesInvestments recorded using the cost method will be assessed for any decrease in value that has occurred that is other than temporary and the other than temporary decrease in value shall be recognized. As and when circumstances and facts change, the Company will evaluate the Company’s ability to Consolidated Financial Statementssignificantly influence operational and financial policy to establish a basis for converting the investment accounted for using the cost method to the equity method of valuation in accordance with FASB ASC 323.
Note 2 - Significant Accounting Policies, continuedIn accordance with FASB ASC 323, the Company recognizes investments in joint ventures based upon the Company’s ability to significantly influence the operational or financial policies of the joint venture. An objective judgment of the level of influence is made at the time of the investment based upon several factors including, but not limited to the following:
| b) | Participation in policy-making processes | |
| c) | Material intra-entity transactions | |
| d) | Interchange of management personnel | |
| e) | Technological dependencies | |
| f) | Extent of ownership and |
The Company follows the equity method for valuating investments in joint ventures when the existence of significant influence over operational and financial policy has been established, as determined by management; otherwise, the Company will valuate these investments using the cost method.
In accordance with FASB ASC 321-10-35-2, the Company has elected to measure its investment in Oravax Medical, Inc. (“Oravax”) (Note 3) as an equity security without a readily determinable fair value. Under this election, an equity security without a readily available fair value is reflected at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. At each reporting period, the Company is required to make a qualitative assessment considering impairment indicators to evaluate whether the investment is impaired. If deemed impaired, the Company is required to estimate the fair value of the investment and recognize an impairment loss equal to the difference between the fair value of the investment and its carry amount. As of December 31, 2023, the Company performed a qualitative assessment to evaluate whether the investment is impaired and determined that the investment was not impaired and thus no adjustment to fair market value was required as of December 31, 2023.
(l) Property, Plant and Equipment
Items of property, plant and equipment are measured at cost less accumulated depreciation and accumulated impairment losses. Costs include expenditures that are directly attributable to the acquisition of the asset.
Gains and losses on disposal of an item of property, plant and equipment are determined by comparing the proceeds from disposal with the carrying amount of property, plant and equipment and are recognized within “other (income)/expense” in the Consolidated Statements of Comprehensive Loss.
Depreciation is recognized over the estimated useful lives of the property, plant and equipment. Leased assets are depreciated over the shorter of the lease term or their useful lives.
The estimated useful lives for the current and comparative periods are as follows:
Schedule of Estimated Useful Lives of Property Plant and Equipment
| (in years) | ||
| Plant and equipment | ||
| Furniture and fixtures | ||
| Computer equipment & software | ||
| Leasehold Improvements | Shorter of the remaining lease or estimated useful life |
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial StatementsDepreciation methods, useful lives and residual values are reviewed at each reporting date.
Note 2 - Significant Accounting Policies, continued(m) Intangible Assets
The Company’s long-lived intangible assets, other than goodwill, are assessed for impairment when events or circumstances indicate there may be an impairment. These assets were initially recorded at their estimated fair value at the time of acquisition and assets not acquired in acquisitions were recorded at historical cost. However, if their estimated fair value is less than the carrying amount, other intangible assets with indefinite lives are reduced to their estimated fair value through an impairment charge to ourin the Consolidated Statements of Comprehensive Loss.
| F-15 |
Patents and Trade Secrets
The Company has developed or acquired several diagnostic tests that can detect the presence of various substances in a person’s breath, blood, urine and saliva. Propriety protection for the Company’s products, technology and process is important to its competitive position. As of December 31, 2019,2023, the Company has ten17 issued U.S. patents, from the United States Patent Office64 foreign patents, 2 pending U.S. patent applications and 10 foreign patent applications pending in effect. Other patents are in effect insuch jurisdictions as Australia, through the Design RegistryCanada, China, European Union, Patents, in Hong KongIsrael, Japan and in Japan. PatentsSouth Korea, which if issued are in the national phase of prosecution in many Patent Cooperation Treaty participating countries. Additional proprietary technology consists of numerous different inventions.expected to expire between 2036 and 2041. Management intends to protect all other intellectual property (e.g. copyrights, trademarks, and trade secrets) using all legal remedies available to the Company.
The Company records expenses related to the application for and maintenance of patents as a component of research and development expenses on the Consolidated Statement of Comprehensive Loss.
Patent Costs
Costs associated with applying for patents are capitalized as patent costs. Once the patents are approved, the respective costs are amortized over their estimated useful lives (maximum of 17 years) on a straight-line basis and assessed for impairment when necessary. Patent pending costs for patents that are not approved are charged to the Consolidated Statements of Comprehensive Loss the year the patent is rejected.
In addition, patentsPatents may be purchased from third parties. The costs of acquiring the patent are capitalized as patent costs if it represents a future economic benefit to the Company. Once a patent is acquired it is amortized over its remaining useful life and assessed for impairment when necessary.
Other Intangible Assets
Other intangible assets that are acquired by the Company, which have definite useful lives, are measured at cost less accumulated amortization and accumulated impairment losses.
Amortization
Amortization is recognized on a straight-line basis over the estimated useful lives of intangible assets, other than goodwill, from the date that they are available for use. The estimated useful lives for the current and comparative periods are as follows:
Schedule of Estimated Useful Lives of Intangible Assets
| Useful Life | ||||
| (in years) | ||||
| Patents and trademarks | 12-17 |
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes(n) Goodwill
Goodwill is evaluated annually for impairment or whenever we identify certain triggering events or circumstances that would more likely than not reduce the fair value below its carrying amount. Events or circumstances that might indicate an interim evaluation is warranted include, among other things, unexpected adverse business conditions, economic factors (for example, the loss of key personnel), supply costs, unanticipated competitive activities, and acts by governments and courts. No impairment was recorded for each of the years ended December 31, 2023 and 2022.
(o) Recoverability of Long-Lived Assets
In accordance with FASB ASC 360-10-35 “Impairment or Disposal of Long-lived Assets”, long-lived assets to Consolidated Financial Statementsbe held and used are analyzed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be fully recoverable or that the useful lives of those assets are no longer appropriate. The Company evaluates at each balance sheet date whether events and circumstances have occurred that indicate possible impairment.
Note 2 - Significant Accounting Policies, continued
The Company determines the existence of such impairment by measuring the expected future cash flows (undiscounted and without interest charges) and comparing such amount to the carrying amount of the assets. An impairment loss, if one exists, is then measured as the amount by which the carrying amount of the asset exceeds the discounted estimated future cash flows. Assets to be disposed of are reported at the lower of the carrying amount or fair value of such assets less costs to sell. Asset impairment charges are recorded to reduce the carrying amount of the long-lived asset that will be sold or disposed of to their estimated fair values. Charges for the asset impairment reduce the carrying amount of the long-lived assets to their estimated salvage value in connection with the decision to dispose of such assets.
(o) Right-of-Use Assets
The Company leased itsa facility in West Deptford, New Jersey (the “Thorofare Facility”Tampa, Florida (“Hyde Park”) under an operating lease (“ThorofareHyde Park Lease”) with annual rentals of $132,000$22,048 to $23,320 plus common area maintenance (CAM) charges.certain operating expenses. The Thorofare Facility housesHyde Park facility housed the Company’s office, manufacturing, laboratory and warehouse space.MyMD Florida operations. The ThorofareHyde Park Lease took effect on JanuaryJuly 1, 2008. On January 7, 2013,2019 for a term of 36 months to expire on June 30, 2022. The Company cancelled the Hyde Park lease in March 2022 without penalty.
The Company extended the Thorofareleases a facility in Baltimore, Maryland (“2021 Wolfe St”) under an operating lease (“2021 Baltimore Lease”) with annual rentals of $52,800 to $56,016 plus certain operating expenses. The 2021 Baltimore Lease extending thetook effect on November 17, 2021 for a term to December 31, 2019. On of 12 months with automatic renewals unless a sixty-day notice is provided. The initial term expires on November 11, 2019, the Company entered into another extension of the Thorofare Lease, extending the term to December 31, 2021, effective January 1, 2020, and providing for an early termination option with a 150-day notice period. On July 16, 2020, the Company exercised the early termination option under the lease agreement, with the effect of the post exercise lease maturity date changing to December 13, 2020.30, 2022. The lease terminatedrenewed effective December 1, 2022 for a term of 12 months with automatic renewals unless a sixty-day notice is provided.
The Company leased a facility in Tampa, Florida (“Platt St”) under an operating lease (“Platt Street Lease”) with annual rentals of $22,030 to $23,259 plus certain operating expenses. The Platt Street Lease took effect on November 30, 2020, at the lessor’s request, and the propertyApril 1, 2022 for a term of 36 months. The Platt Street Lease was handed over to the property manager on November 30, 2020.cancelled without penalty effective October 31, 2023.
On January 1, 2020 (“Effective Date”), the Company adoptedIn accordance with FASB ASC, Topic 842, Leases (“ASC 842”), which increases transparency and comparability by recognizing a lessee’s rights and obligations resulting from leases by recording them on the balance sheet as lease assets and lease liabilities. The new guidance requires the recognition of the right-of-use (“ROU”) assets and related operating and finance lease liabilities on the balance sheet.
The Company adopted the new guidance using the modified retrospective approach on January 1, 2020. As a result, the Consolidated Balance Sheet as of December 31, 2019 was not restated and is not comparative.
The adoption of ASC 842 resulted in the recognition of ROU assets of $306,706 and lease liabilities for an operating lease of $306,706 on the Company’s Consolidated Balance Sheet as of January 1, 2020.
The Company electedutilizes the package of practical expedients permitted within the standard, which allows an entity to forgo reassessing (i) whether a contract contains a lease, (ii) classification of leases, and (iii) whether capitalized costs associated with a lease meet the definition of initial direct costs. Also, the Company elected the expedient allowing an entity to use hindsight to determine the lease term and impairment of ROU assets and the expedient to allow the Company to not have to separate lease and non-lease components. The Company has also elected the short-term lease accounting policy under which the Company would not recognize a lease liability or ROU asset for any lease that at the commencement date has a lease term of twelve months or less and does not include a purchase option that the Company is more than reasonably certain to exercise.
| F-17 |
For contracts entered into on or after the Effective Date, at the inception of a contract, the Company will assess whether the contract is, or contains, a lease. The Company’s assessment is based on: (i) whether the contract involves the use of a distinct identified asset, (ii) whether the Company obtained the right to substantially all the economic benefit from the use of the asset throughout the period, and (iii) whether the Company has the right to direct the use of the asset. Leases entered into prior to January 1, 2020, which were accounted for under ASC 840, were not reassessed for classification.
For operating leases, the lease liability is initially and subsequently measured at the present value of the unpaid lease payments. The Company generally uses its incremental borrowing rate as the discount rate for leases, unless an interest rate is implicitly stated in the lease. The present value of the lease payments is calculated using the incremental borrowing rate for operating leases, which was determined using a portfolio approach based on the rate of interest that the Company would have to pay to borrow an amount equal to the lease payments on a collateralized basis over a similar term. The lease term for all of the Company’s leases includes the non-cancellable period of the lease plus any additional periods covered by either a Company option to extend the lease that the Company is reasonably certain to exercise, or an option to extend the lease controlled by the lessor. All ROU assets are reviewed for impairment.
Lease expense for operating leases consists of the lease payments plus any initial direct costs and is recognized on a straight-line basis over the lease term.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
In June 2020, the Company recorded an adjustment to its right-of-use asset and liability in the amounts of $153,709 and $155,737, respectively, to adjust for the effectThe Company’s operating leases are comprised of the Company having elected2021 Baltimore Lease and the Platt Street Lease on the Consolidated Balance Sheet. The information related to exercise the early termination option under the lease agreement, as discussed earlier. these leases are presented below:
Schedule of Condensed Consolidated Balance Sheet Information Related to Operating Lease
| Balance Sheet Location | Lease | Lease | Total | Lease | Lease | Total | ||||||||||||||||||
| As of December 31, 2023 | As of December 31, 2022 | |||||||||||||||||||||||
| Platt Street | 2021 Baltimore | Hyde Park | 2021 Baltimore | |||||||||||||||||||||
| Balance Sheet Location | Lease | Lease | Total | Lease | Lease | Total | ||||||||||||||||||
| Operating Lease | ||||||||||||||||||||||||
| Lease Right of Use | $ | - | $ | 47,389 | $ | 47,389 | $ | 45,353 | $ | 94,309 | $ | 139,662 | ||||||||||||
| Lease Payable, current | - | 48,870 | 48,870 | 18,741 | 47,039 | 65,780 | ||||||||||||||||||
| Lease Payable - net of current | - | - | - | 27,070 | 48,871 | 75,941 | ||||||||||||||||||
The following information reflects the effectprovides details of the adjustments discussed above in connection with the Company’s exercise of the early termination option.
The Company’s lease expense, including CAM charges was $154,362 for the year ended December 31, 2020.expense:
Schedule of Lease Expense
| Lease Expenses | Lease | Lease | Total | Lease | Lease | Lease | Total | |||||||||||||||||||||
| Year Ended December 31, 2023 | Year Ended December 31, 2022 | |||||||||||||||||||||||||||
| Platt Street | 2021 Baltimore | Hyde Park | Platt Street | 2021 Baltimore | ||||||||||||||||||||||||
| Lease Expenses | Lease | Lease | Total | Lease | Lease | Lease | Total | |||||||||||||||||||||
| Operating Leases | ||||||||||||||||||||||||||||
| Lease Costs | $ | 18,868 | $ | 54,400 | $ | 73,268 | $ | 6,251 | $ | 16,981 | $ | 54,400 | $ | 77,632 | ||||||||||||||
| F-18 |
Other information related to leases is presented below:
| Other information | As of December 31, 2020 | |||
| Operating cash used by operating leases | $ | 151,640 | ||
| Weighted-average remaining lease term – operating leases (in months) | - | |||
| Weighted-average discount rate – operating leases | 10 | % | ||
In accordance with FASB ASC 360-10-35 “Impairment or DisposalSchedule of Long-lived Assets”, long-lived assetsOther Information Related to be held and used are analyzed for impairment whenever events or changes in circumstances indicate thatLeases
| As of December 31, 2023 | ||||||||||||
| Platt | 2021 Baltimore | |||||||||||
| Other Information | Street Lease | Lease | Total | |||||||||
| Operating Leases | ||||||||||||
| Operating cash used | $ | 20,048 | $ | 54,520 | $ | 74,568 | ||||||
| Average remaining lease term | - | 11 | 11 | |||||||||
| Average discount rate | 10.0 | % | 10.0 | % | 10.0 | % | ||||||
As of December 31, 2023, the carrying amountannual minimum lease payments of an asset may not be fully recoverable or that the useful livesCompany’s operating lease liabilities were as follows:
Schedule of those assets are no longer appropriate. Operating Lease Minimum Lease Payments
| Street Lease | Lease | Total | ||||||||||
| As of December 31, 2023 | ||||||||||||
| Platt | 2021 Baltimore | |||||||||||
| Street Lease | Lease | Total | ||||||||||
| For Years Ending December 31, | ||||||||||||
| 2024 | $ | - | $ | 49,867 | $ | 49,867 | ||||||
| Total future minimum lease payments, undiscounted | $ | - | $ | 49,867 | $ | 49,867 | ||||||
| Less: Imputed interest | - | 997 | 997 | |||||||||
| Present value of future minimum lease payments | $ | - | $ | 48,870 | $ | 48,870 | ||||||
(q) Revenue Recognition
The Company evaluates at each balance sheet date whether events and circumstances have occurred that indicate possible impairment.
The Company determines the existence of such impairment by measuring the expected future cash flows (undiscounted and without interest charges) and comparing such amount to the carrying amount of the assets. An impairment loss, if one exists, is then measured as the amount by which the carrying amount of the asset exceeds the discounted estimated future cash flows. Assets to be disposed of are reported at the lower of the carrying amount or fair value of such assets less costs to sell. Asset impairment charges are recorded to reduce the carrying amount of the long-lived asset that will be sold or disposed of to their estimated fair values. Charges for the asset impairment reduce the carrying amount of the long-lived assets to their estimated salvage value in connection with the decision to dispose of such assets.
In accordance with FASB ASC 323, the Company recognizes investments in joint ventures based upon the Company’s ability to significantly influence the operational or financial policies of the joint venture. An objective judgment of the level of influence is made at the time of the investment based upon several factors including, but not limited to the following:
The Company follows the equity method for valuating investments in joint ventures when the existence of significant influence over operational and financial policy has been established, as determined by management; otherwise, the Company will valuate these investments using the cost method.
Investments recorded using the cost method will be assessed for any decrease in value that has occurred that is other than temporary and the other than temporary decrease in value shall be recognized. As and when circumstances and facts change, the Company will evaluate the Company’s ability to significantly influence operational and financial policy to establish a basis for converting the investment accounted for using the cost method to the equity method of valuation.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 2 - Significant Accounting Policies, continued
Beginning on January 1, 2019, the Company recognizesrecognize revenue under ASC 606, Revenue from Contracts with Customers. The core principle of the revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The Company only applies the five-step model to contracts when it is probable that the Company will collect the consideration it is entitled to in exchange for the goods and services transferred to the customer. The following five steps are applied to achieve that core principle:
Step 1: Identify the contract with the customer
Step 2: Identify the performance obligations in the contract
Step 3: Determine the transaction price
Step 4: Allocate the transaction price to the performance obligations in the contract
Step 5: Recognize revenue when the company satisfies a performance obligation
The Company does not have any significant contracts with customers requiring performance beyond delivery. Shipping and handling activities are performed before the customer obtains control of the goods and therefore represent a fulfillment activity rather than a promised service to the customer. Revenue and costs of sales are recognized when control of the product transfers to our customer, which generally occurs upon delivery to the customer but can also occur when goods are shipped by the Company, depending on the shipment terms of the contract. The Company’s performance obligations are satisfied at that time. The Company has not historically experienced customer returns of its products.
The Company uses the most likely amount approach to determine the variable consideration of the transaction price in order to account for the contractual rebates and incentives that are estimated and adjusted for over time. The Company provides for rebates to its distributors.
| 2) | Identify the performance obligations in the contract | |
| 3) | Determine the transaction price | |
| 4) | Allocate the transaction price to the performance obligations in the contract | |
| 5) | Recognize revenue when the company satisfies a performance obligation |
(r) Income Taxes
The Company utilizes an asset and liability approach for financial accounting and reporting for income taxes. The provision for income taxes is based upon income or loss after adjustment for those permanent items that are not considered in the determination of taxable income. Deferred income taxes represent the tax effects of differences between the financial reporting and tax basis of the Company’s assets and liabilities at the enacted tax rates in effect for the years in which the differences are expected to reverse.
The Company evaluates the recoverability of deferred tax assets and establishes a valuation allowance when it is more likely than not that some portion or all the deferred tax assets will not be realized. Management makes judgments as to the interpretation of the tax laws that might be challenged upon an audit and cause changes to previous estimates of tax liability. In management’s opinion, adequate provisions for income taxes have been made. If actual taxable income by tax jurisdiction varies from estimates, additional allowances or reversals of reserves may be necessary.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 2 - Significant Accounting Policies, continued
Tax benefits are recognized only for tax positions that are more likely than not to be sustained upon examination by tax authorities. The amount recognized is measured as the largest amount of benefit that is greater than 50 percent likely to be realized upon settlement. A liability for “unrecognized tax benefits” is recorded for any tax benefits claimed in the Company’s tax returns that do not meet these recognition and measurement standards. For the years ended December 31, 20202023 and 2019,2022, no liability for unrecognized tax benefits was required to be reported.
| F-19 |
There is was no income tax benefit recorded for the losses for the years ended December 31, 20202023 and 20192022 since management has determined that the realization of the net deferred tax assets is not assuredmore likely than not to be realized and has createdrecorded a full valuation allowance foron the entire amount of suchnet deferred tax benefits.assets.
The Company’s policy for recording interest and penalties associated with tax audits is to record such items as a component of general and administrative expense. There were no amounts accrued for penalties and interest for the years ended December 31, 20202023 and 2019.2022. The Company does not expect its uncertain tax position to change during the next twelve months. Management is currently unaware of any issues under review that could result in significant payments, accruals or material deviations from its position.
Tax years from 2020 through 2023 remain subject to examination by federal and state jurisdictions.
In accordance with FASB ASC 730, research
Basic earnings per common share is based on the Company’s behalf. These costs included costs incurred to acquireweighted average number of shares outstanding during the periods presented. Diluted earnings per share is computed using the weighted average number of common shares plus dilutive common share equivalents outstanding during the period. Potential common shares that would have the effect of increasing diluted earnings per share are considered anti-dilutive.
Diluted net loss per share is computed using the weighted average number of shares of Common Stock and developdilutive potential Common Stock outstanding during the licenseperiod.
As the Company reported a net loss for the COVID-19 vaccine project (See Note 3).years ended December 31, 2023 and 2022, Common Stock equivalents were anti-dilutive.
Schedule of sales.Anti-dilutive Securities Excluded from Computation of Earnings Per Share
| 2023 | 2022 | |||||||
| For the Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Stock Options | 47,286 | 149,241 | ||||||
| Unvested Restricted Stock Units | 88,668 | 82,001 | ||||||
| Warrants to purchase Common Stock | 4,933,622 | 217,202 | ||||||
| Pre-funded Warrants to purchase Common Stock | - | 4,505 | ||||||
| Series C Preferred Convertible Warrants | 918 | 918 | ||||||
| Series D Preferred Convertible Stock | 1,217 | 1,217 | ||||||
| Series F Preferred Convertible Stock | 3,318,626 | - | ||||||
| Total potentially dilutive shares | 8,482,891, | 466,252 | ||||||
Notes to Consolidated Financial Statements
Note 2 - Significant Accounting Policies, continued
The Company accounts for stock-based compensation under the provisions of Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) 718, “Compensation - Stock Compensation”, which requires the measurement and recognition of compensation expense for all stock-based awards made to employees and directors based on estimated fair values on the grant date. The Company estimates the fair value of stock-based awards on the date of grant using the Black-Scholes model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods using the straight-line method. In June 2018, the FASB issued ASU No. 2018-07, Compensation – Stock Compensation (Topic 718), Improvements to Nonemployee Share-Based Payment Accounting.Accounting (the “2018 Update”). The amendments in thisthe 2018 Update expand the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. Prior to thisthe 2018 Update, Topic 718 applied only to share-based transactions to employees. Consistent with the accounting requirement for employee share-based payment awards, nonemployee share-based payment awards within the scope of Topic 718 are measured at grant-date fair value of the equity instruments that an entity is obligated to issue when the good has been delivered or the service has been rendered and any other conditions necessary to earn the right to benefit from the instruments have been satisfied.
The Company has elected to account for forfeiture of stock-based awards as they occur.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES(u) Research and Development Costs
Notes to Consolidated Financial Statements
Note 2 - Significant Accounting Policies, continued
Basic earnings per common share is based on the weighted average number of shares outstanding during the periods presented. Diluted earnings per share is computed using the weighted average number of common shares plus dilutive common share equivalents outstanding during the period. Potential common shares that would have the effect of increasing diluted earnings per share are considered anti-dilutive.
As the Company reported a net loss for the years ended December 31, 2020 and 2019, respectively, common stock equivalents were anti-dilutive.
Diluted net loss per share is computed using the weighted average number of common and dilutive potential common shares outstanding during the years ended December 31, 2020 and 2019. The following securities are excluded from the calculation of weighted average dilutive common shares because their inclusion would have been anti-dilutive:
| For the Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Stock Options | - | 40 | ||||||
| Restricted Stock Units | 789,360 | 15,603 | ||||||
| Warrants to purchase Common Stock | 10,925,952 | 247,215 | ||||||
| Pre-funded Warrants to purchase Common Stock | 1,040,540 | 795,000 | ||||||
| Warrants to purchase Series C Preferred Stock | 55,000 | 1,990,000 | ||||||
| Series D Convertible Preferred Stock | 72,992 | - | ||||||
| Total potentially dilutive shares | 12,883,844 | 3,047,858 | ||||||
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 2 - Significant Accounting Policies, continued
In accordance with FASB ASC 205, results730, research and development costs are expensed as incurred and consist of operations of a component of an entityfees paid to third parties that has either been disposed of or is held for sale is to be reported as discontinued operations inconduct certain research and development activities on the consolidated financial statements if the disposition or sale represents a strategic shift that has (or will have) a major effect on an entity’s operations and financial results. See Note 6 herein.Company’s behalf.
(v) Recently Issued Accounting Pronouncements
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 2 - Significant Accounting Policies, continued
Recently Issued Accounting Pronouncements Adopted
In February 2016,May 2021, the FASB issued ASU 2016-02—Leases2021-04, Earnings Per Share (Topic 842) (“ASU-2016-02”)260), which requiresDebt - Modifications and Extinguishments (Subtopic 470-50), Compensation - Stock Compensation (Topic 718), and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40), Issuer’s Accounting for Certain Modifications or Exchanges or Freestanding Equity - Classified Written Call Options. The amendments in this Update clarify an issuer’s accounting for modifications or exchanges of freestanding equity - classified written call options (for example, warrants) that remain equity classified after modification or exchange. The amendments are effective for all entities for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. An entity should apply the amendments prospectively to modifications or exchanges occurring on or after the effective date of the amendments. Early adoption is permitted for all entities, including adoption in an interim period. If an entity elects to recognize right-of-use assets and lease liabilities on its balance sheet and disclose key information about leasing arrangements. ASU 2016-02 offers specific accountingearly adopt the amendments in this Update in an interim period, the guidance for a lessee, a lessor, and sale and leaseback transactions. Lessees and lessors are required to disclose qualitative and quantitative information about leasing arrangements to enable a usershould be applied as of the beginning of the fiscal year that includes the interim period. The adoption of this ASU had no material impact on the Company’s consolidated financial statements to assess the amount, timing and uncertainty of cash flows arising from leases. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. The Company has adopted ASU-2016-02, effective January 1, 2020.related disclosure.
Recently Issued Accounting Pronouncements Not Adopted
| F-20 |
In June 2016, the FASB issued ASUAccounting Standards Update (ASU) No. 2016-13, Financial Instruments -– Credit Losses (Topic 326), Measurement of Credit Losses on Financial Instruments, (“ASU-2016-13”).as modified by FASB ASU 2016-13 affectsNo. 2019-10 and other subsequently issued related ASUs. The amendments in this Update affect loans, debt securities, trade receivables, and any other financial assets that have the contractual right to receive cash. The ASU requires an entity to recognize expected credit losses rather than incurred losses for financial assets. ASU 2016-13 isThe amendments in this Update are effective for the fiscal yearyears beginning after December 15, 2022, including interim periods within thatthose fiscal year.years. The Company expects that there would be noadopted this new guidance effective January 1, 2023 utilizing the modified retrospective transition method. The adoption of this standard did not have a material impact on the Company’s financial statements, but did change how the allowance for credit losses is determined.
Recently Issued Accounting Pronouncements Not Adopted
Management does not believe that any recently issued, but not yet effective, accounting standards could have a material effect on the Company’s condensed consolidated financial statements. As new accounting pronouncements are issued, the Company will adopt those that are applicable under the circumstances.
Note 3 – Going Concern
The Company has evaluated whether there are certain conditions and events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the consolidated financial statements upon the adoption of this ASU.
In July 2017, the FASB issued ASU No. 2017-11, Earnings Per Share (Topic 260), Distinguishing Liabilities from Equity (Topic 480), Derivatives and Hedging (Topic 815): (I) Accounting for Certain Financial Instruments with Down Round Features, (II) Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception. The amendments in Part I change the classification analysis of certain equity-linked financial instruments (or embedded features) with down round features. When determining whether certain financial instruments should be classified as liabilities or equity instruments, a down round feature no longer precludes equity classification when assessing whether the instrument is indexed to an entity’s own stock. The amendments also clarify existing disclosure requirements for equity-classified instruments. The amendments in Part II recharacterize the indefinite deferral of certain Topic 480, Distinguishing Liabilities from Equity, provisions that now are presented as pending content in the Codification to a scope exception. Those amendments do not have an accounting effect. The amendments in Part I are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. Early adoption in permitted. As of January 1, 2020, the Company adopted the amendments in Part I which has no impact on the Company’s financial statements
In August 2020, the FASB issued ASU No. 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40), Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. The amendments in this Update affect entities that issue convertible instruments and/or contracts in an entity’s own equity. For convertible instruments, the instruments primarily affected are those issued with beneficial conversion features or cash conversion features because the accounting models for those specific features are removed. However, all entities that issue convertible instruments are affected by the amendments to the disclosure requirements in this Update. For contracts in an entity’s own equity, the contracts primarily affected are freestanding instruments and embedded features that are accounted for as derivatives under the current guidance because of failure to meet the settlement conditions of the derivatives scope exception related to certain requirements of the settlement assessment. The settlement assessment was simplified by removing the requirements (1) to consider whether the contract would be settled in registered shares, (2) to consider whether collateral is required to be posted, and (3) to assess shareholder rights. Those amendments also affect the assessment of whether an embedded conversion feature in a convertible instrument qualifies for the derivatives scope exception. Additionally, the amendments in this Update affect the diluted EPS calculation for instruments that may be settled in cash or shares and for convertible instruments. The amendments in this Update are effective for public business entities that meet the definition of a Securities and Exchange Commission (SEC) filer, excluding entities eligible to be smaller reporting companies as defined by the SEC, for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. An entity should adopt the guidance as of the beginning of its annual fiscal year. Entities are allowed to adopt the guidance through either a modified retrospective method of transition or a fully retrospective method of transition. The Company expects to adopt this standard as of January 1, 2021 and does not anticipate the adoption to have a material impact on its financial statements.
Certain reclassifications were made to the reported amounts in these consolidated financial statements as of December 31, 2019 to conform to the presentation as of December 31, 2020.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 3 – Recent Developments, Liquidity and Management’s Plans
Ceasing Production and Sale of Rapid, Point-Of-Care Screening and Testing Products
As previously disclosed, in light of the unfavorable factors persistent in our rapid, point-of-care screening and testing product business and the progress the Company has made in its partnership with Premas, the Company conducted a strategic review of the screening and testing products business. Following such review, in early July 2020, the Company ceased the production and sale of its rapid, point-of-care screening and testing products. The Company will continue to provide support for these testing products that remain in the market through their respective product expiration dates. The Company had been experiencing declining sales revenue and production backlogs for these products and, as it previously reported, had eliminated its sales force for such products. The Company intends to devote its attention to its partnership with Premas for the development of its COVID-19 Vaccine Candidate and transactions that the Company believes will increase shareholder value. In connection with the ceasing production and sale of its existing product line, on July 16, 2020, the Company decided to close the Thorofare Facility and exercised the early termination option under the Thorofare Lease, which provided for a 150-day notice to terminate the lease. Pursuant to the early termination option, the Thorofare Lease which matured on December 13, 2020. The lease terminated on November 30, 2020, at the lessor’s request, and the property was handed over to the property manager on November 30, 2020.
issued.
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company determined thathas sustained a net loss attributable to common stockholders of $8,218,163 and $15,197,336 and negative cash flows from operations of $12,980,625 and $12,270,068 for the discontinuation of the productionyears ended December 31, 2023 and distribution of2022. These factors raise substantial doubt about the Company’s screening and testing products constituted a strategic shift in the Company’s business andability to continue as a result the elimination of the product lines should be presentedgoing concern. The Company’s ability to continue as discontinued operations under FASB ASC 205-20 Presentation of Financial Statements, Discontinued Operations.
Acquisition of Cystron
On March 23, 2020, the Company acquired Cystron pursuant to that certain Membership Interest Purchase Agreement (the “MIPA”). Cystron was incorporated on March 10, 2020. Upon the Company’s purchase of Cystron, Cystron’s sole asset consisted of an exclusive license with respect to Premas’ vaccine platforma going concern for the development of a vaccine against COVID-19 and other coronavirus infections. Since its formation and throughnext 12 months from the date of this Annual Report is dependent upon its acquisition by the Company, Cystron did not have any employees. The acquisition of Cystron was accounted for as the purchase of an asset.
As consideration for the Membership Interests (as defined in the MIPA), the Company deliveredability to the members of Cystron (the “Sellers”): (1) that number of newly issued shares of its common stock equal to 19.9% of the issued and outstanding shares of its common stock and pre-funded warrants as ofobtain additional capital financing. Through the date of the MIPA, but, to the extent that the issuance of its common stock would have resulted in any Seller owning in excess of 4.9% of the Company’s outstanding common stock, then, at such Seller’s election, such Seller received “common stock equivalent” preferred shares with a customary 4.9% blocker (with such common stock and preferred stock collectively referred to as “Common Stock Consideration”), and (2) $1,000,000 in cash. On March 24, 2020this Annual Report, the Company paid $1,000,000 tohas been primarily financed through the Sellers and delivered 411,403 shares of common stock and 211,353 shares of Series D Convertible Preferred Stock with a customary 4.9% blocker, with an aggregate fair market value of $1,233,057, totaling $2,233,057 (“March Transaction”).
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Additionally, the Company shall (A) make an initial payment to the Sellers of up to $1,000,000 upon its receipt of cumulative gross proceeds from the consummationsale of an initial equity offering after the date of the MIPA of $8,000,000,preferred and (B) pay to Sellers an amount in cash equal to 10% of the gross proceeds in excess of $8,000,000 raised from future equity offerings after the date of the MIPA until the Sellers have received an aggregate additional cash consideration equal to $10,000,000 (collectively, the “Equity Offering Payments”). Upon the achievement of certain milestones, including the completion of a Phase 2 study for a COVID-19 Vaccine Candidate that meets its primary endpoints, Sellers will be entitled to receive an additional 750,000 shares of the Company’s common stock or, instock. In the event the Company does not complete an offering, the Company expects to seek additional funding through private equity or debt financings. The Company may not be able to obtain financing on acceptable terms, or at all. The issuance of additional equity would result in dilution to existing stockholders. If the Company is unable to obtain stockholder approval for the issuance ofadditional funds when they are needed or if such shares, 750,000 shares of non-voting preferred stock that are valued following the achievement of such milestones and shall bear a 10% annual dividend (the “Milestone Shares”).
Pursuantfunds cannot be obtained on terms acceptable to the MIPA,Company, the Company shall make contingent payments for the achievement of certain development and commercial milestones as follows; (i) $250,000would be unable to execute upon the dosingbusiness plan or pay costs and expenses as they are incurred, which would have a material, adverse effect on the business, financial condition and results of the first patient in a Phase I Clinical Trial, (ii) $500,000 upon the dosing of the first patient in a Phase II Clinical Trial, (iii) $5,000,000 upon the dosing of the first patient in a Phase III Clinical Trial, and (iv) $15,000,000 upon approval by the FDA of the NDA for the COVID-19 vaccine.
Pursuant to the MIPA, upon the Company’s consummation of the registered direct equity offering closed on April 8, 2020,operations. No assurance can be given that the Company paid the Sellers $250,000 on April 20, 2020 (the “April Payment”). Upon consummation of the registered direct equity offerings that closed on May 18, 2020 and August 13, 2020, the Company paid $892,500 (the “May Payment”) and $684,790 (the “August Payment”), respectively, on September 25, 2020.
On October 13, 2020, Premas, one of the former members of Cystron, returned $908,117 representing its portion of the initial cash component for the purchase of Cystron (the “March Transaction”) and its portion of the April Payment, May Payment and August Payment under the MIPA, as amended.
Premas is working with the Reserve Bank of India to comply with regulations related to its ownership in a foreign entity and its ability to receive funds for the sale of that entity. The Company believes that (i) Premas will be successful in its efforts to resolve such regulatory matters with the Reserve Bank of India, (ii) the Company will disburse the amounts due to Premas under the MIPA, and (iii) the Company maintains a 100% membership in Cystron.
Upon the Company’s consummation of the Private Placement (as defined below), the Company paid $1,204,525 of the proceeds from the Private Placement to three of the four former members of Cystron on December 1, 2020 (the “November Payment”) and recorded a liability of $602,172 to the fourth former member of Cystron pursuant to the MIPA.
As of December 31, 2020, $1,510,290 is included in Trade and Other Payables for Premas’ portion of the initial cash component, the April Payment, May Payment, August Payment and November Payment.
For the year ended December 31, 2020, $5,867,046 is included in Research and Development Expense within the Consolidated Statement of Comprehensive Loss for the March Payment, April Payment, May Payment, August Payment and November Payment.
The Company shall also make quarterly royalty payments to Sellers equal to 5% of the net sales of a COVID-19 vaccine or combination product by the Company for a period of five (5) years following the first commercial sale of the COVID-19 vaccine; provided, that such payment shall be reduced to 3% for any net sales of the COVID-19 vaccine above $500 million.
In addition, Sellers shall be entitled to receive 12.5% of the transaction value, as defined in the MIPA, of any change of control transaction, as defined in the MIPA, that occurs prior to the fifth (5th) anniversary of the closing date of the MIPA, provided that the Company is still developing the COVID-19 Vaccine Candidate at that time. Following the consummation of any change of control transaction, the Sellers shall not be entitled to any royalty payments as described above under the MIPA.
License Agreement
Cystron is a party to a License and Development Agreement (the “Initial License Agreement”) with Premas. As a condition to the Company’s entry into the MIPA, Cystron amended and restated the Initial License Agreement on March 19, 2020 (as amended and restated, the “License Agreement”). Pursuant to the License Agreement, Premas granted Cystron, amongst other things, an exclusive license with respect to Premas’ vaccine platform for the development of a vaccine against COVID-19 and other coronavirus infections.
Upon the achievement of certain developmental milestones by Cystron, Cystron shall pay to Premas a total of up to $2,000,000. On April 16, 2020, the Company paid Premas $500,000 for the achievement of the first two development milestones. On May 18, 2020, the Company paid Premas $500,000 for the achievement of the third development milestone. On July 7, 2020, the Company and Premas agreed that the fourth milestone under the License Agreement had been satisfied. Due to the achievement of this milestone on July 7, 2020, Premas was paid $1,000,000 on August 4, 2020. Accordingly, for the year ended December 31, 2020, Research and Development Expenses of $2,000,000 were recorded in the Consolidated Statement of Comprehensive Loss.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Cystron Medical Panel
On April 10, 2020, the Company established the Cystron Medical Panel and appointed its first member to the panel. Each member shall be compensated with an initial grant of the Company’s common stock with an aggregate fair market value of $25,000 and a monthly cash stipend in the initial amount of $2,500. During the year ended December 31, 2020, the Company recorded $31,573 as a charge to research and development expense within the Consolidated Statements of Comprehensive Loss. The Cystron Medical Panel was disbanded effective January 31, 2021.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Agreement and Plan of Merger and Reorganization
On November 11, 2020, the Company, XYZ Merger Sub Inc., a Florida corporation and a wholly-owned subsidiary of the Company (“Merger Sub”), and MYMD Pharmaceuticals, Inc., a privately-held Florida corporation (“MYMD”), entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), pursuant to which, among other things, subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub will merge with and into MYMD, with MYMD being the surviving corporation and becoming a wholly-owned subsidiary of the Company (the “Merger”). The Merger is intended to qualify for federal income tax purposes as a tax-free reorganization under the provisions of Section 368(a) of the Internal Revenue Code of 1986, as amended. In addition, in connection with the execution of the Merger Agreement, Akers agreed to advance a bridge loan of up to $3,000,000 to MYMD pursuant to a Secured Promissory Note.
Subject to the terms and conditions of the Merger Agreement, at the effective time of the Merger (the “Effective Time”), (i) each outstanding share of common stock of MYMD (“MYMD common stock”), will be converted into the right to receive the number of shares of the common stock of Akers (the “Akers common stock”) equal to the exchange ratio described below; and (ii) each outstanding stock option of MYMD (collectively, “MYMD options”) that has not previously been exercised prior to the Effective Time, whether or not vested, will be assumed by the Company subject to certain terms contained in the Merger Agreement (including, but not limited to, the amendment of such stock option to extend the term of such stock option for a period expiring on the second-year anniversary of the Effective Time). In connection with the Merger, each holder of options is required to enter into a Lock-Up Agreement/Leak-Out Agreement with respect to the shares of Akers common stock issued upon the exercise of such option. Also, not later than 30 days after the second-year anniversary of the Effective Date, the Company will pay stockholders of MYMD on a pro rata basis an amount in cash equal to the aggregate cash proceeds received by Akers from the exercise of any MYMD options assumed by the Company prior to the second-year anniversary of the Effective Time; provided, however, the amount of such payment will not exceed the maximum amount of cash consideration that may be received by stockholders of MYMD without affecting the intended tax consequences of the Merger.
Additionally, under the terms of the Merger Agreement, the Company has agreed to pay contingent consideration to MYMD stockholders in the form of milestone payments payable in shares of Akers common stock (collectively, the “Milestone Payments”). The Milestone Payments are payable in the dollar amounts set forth in the chart below upon the achievement of the milestone events set forth opposite such dollar amount during the 36-month period immediately following the Effective Date (the “Milestone Period”) as follows:
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Each milestone payment will be payable in shares of common stock of Akers (the “Milestone Shares”), with the number of Milestone Shares to be issued determined by dividing the applicable Milestone Payment amount by the volume-weighted average price of a share of Akers’ common stock during the 10 trading days immediately preceding the achievement of the milestone event; provided, however, that in no event shall the price of a share of Akers common stock used to determine the number of Milestone Shares to be issued be deemed to be less than $5.00 per share (as adjusted for stock splits, stock dividends, reverse stock splits, and the like occurring after the closing date).
Notwithstanding the above, the number of Milestone Shares payable by Akers shall not exceed the number of shares of Akers common stock to be issued to MyMD stockholders at the Effective Time in connection with the Merger (as described in the following paragraph).
Under the exchange ratio formula in the Merger Agreement, and immediately upon the closing of the Merger, the former MYMD securityholders are expected to own approximately 80% of the aggregate number of shares of Akers common stock issued and outstanding immediately following the consummation of the Merger (the “Post-Closing Shares”), and the stockholders of the Company as of immediately prior to the Merger are expected to own approximately 20% of the aggregate number of Post-Closing Shares.
Immediately prior to the Effective Time, the name of the Company will be changed from “Akers Biosciences, Inc.” to “MyMD Pharmaceuticals, Inc.” At the Effective Time, the Merger Agreement contemplates that the board of directors of the Company will consist of seven directors, with (i) Akers having the right to designate up to four members and (ii) MYMD having the right to designate up to three members. The officers of the Company immediately after the Effective Time will be elected by the board of directors of Akers.
The Merger Agreement contains customary representations, warranties and covenants made by the Company and MYMD, including covenants relating to obtaining the requisite approvals of the stockholders of the Company and MYMD, indemnification of directors and officers, and the Company’s and MYMD’s conduct of their respective businesses between the date of signing the Merger Agreement and the closing of the Merger. Consummation of the Merger is subject to certain closing conditions, including, among other things, approval by the stockholders of Akers and MYMD.
The Merger Agreement contains certain termination rights for both the Company and MYMD, including, among other things, (a) Akers may, upon written notice, extend the originally scheduled End Date (defined in the Merger Agreement as April 15, 2021) to May 15, 2021 (the “Extended Date”) so long as (i) Akers and Merger Sub are not then in material breach of any provision of the Merger Agreement and (ii) within three calendar days of the written request by MYMD, Akers makes an additional loan to MYMD of up to $600,000, which will have the same terms and conditions of the Note (as defined below and such additional note “Second Note”) and (b) Akers may, upon written notice, extend the Extended Date to June 30, 2021, so long as (i) Akers and Merger Sub are not then in material breach of any provision of the Merger Agreement, (ii) on the effective date of such extension, the loan amount evidenced by the Note and the Second Note may, at the sole option of MYMD upon written notice to Akers, be converted into shares of MYMD common stock at a conversion price of $2.00 per share, subject to certain adjustments and (iii) Akers will, at MYMD’s request, either (at the option of MYMD); (A) subscribe for 300,000 shares of MYMD common stock at a subscription price of $2.00 per share, subject to certain adjustments as set forth in the Merger Agreement, or (B) make an additional loan to MYMD of up to $600,000, which will have the same terms and conditions of the Note (the “Third Note,” and all amounts outstanding under the Note, the Second Note and the Third Note, the “Loan Amount”). In addition, if Akers terminates the Merger Agreement under certain circumstances specified therein, the Loan Amount, if any, at the sole discretion of MYMD, will be convertible into shares of common stock of MYMD at a conversion price of $2.00 per share upon delivery of written notice by MYMD to Akers within 30 calendar days after the effective date of termination of the Merger Agreement.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
The Merger Agreement also contemplates that the Company will seek approval from its stockholders to effect a reverse stock split, if applicable, at a reverse stock split ratio mutually agreed to by the Company and MYMD and within the range approved by the Company’s stockholders immediately prior to the Effective Time, which range shall be sufficient to cause the price of Akers common stock on the Nasdaq Capital Market following such reverse stock split and the Effective Time to be no less than $5.00 per share. In addition, under the Merger Agreement, Akers may, in its discretion, consummate a spin-off of all or a part of its pre-closing assets and liabilities (the “Spin-Off”).
In connection with the Merger, the Company will seek the approval of its stockholders of (a) the transactions contemplated in the Merger Agreement, including the issuance of Akers common stock pursuant to the Merger and (b) the amendment of its certificate of incorporation, including for purposes of (i) effectuating a reverse split of Akers common stock at a ratio to be determined by a split ratio to be mutually agreed to by Akers and MYMD within the range approved by the Company’s stockholders immediately prior to the Effective Time and on certain terms as specifically described herein, (ii) change Akers’ name to “MyMD Pharmaceuticals, Inc.,” and (c) to the extent necessary, the Spin-Off.
In accordance with the terms of the Merger Agreement, (i) the officers and directors of Akers have each entered into a voting agreement with MYMD (the “Akers Voting Agreements”), and (ii) the officers, directors and certain affiliated stockholders of MYMD have each entered into a voting agreement with Akers (the “MYMD Voting Agreements,” together with the Akers Voting Agreements, the “Voting Agreements”). The Voting Agreements place certain restrictions on the transfer of the shares of Akers and MYMD held by the respective signatories thereto and include covenants as to the voting of such shares in favor of approving the transactions contemplated by the Merger Agreement and against any actions that could adversely affect the consummation of the Merger.
Concurrently with the execution of the Merger Agreement or prior to the closing, the officers and directors of Akers, and the officers, directors and certain stockholders of MYMD, each entered into lock-up/leak-out agreements (the “Lock-Up/Leak-Out Agreements”) pursuant to which they have agreed, among other things, not to sell or dispose of (subject to certain exceptions specified therein) any shares of Akers common stock which are or will be beneficially owned by them at the Effective Time or which are acquired thereafter, with such shares being released from such restrictions 180 days after the Effective Time. After the expiration of such initial 180-day period, such stockholders will be subject to a 180-day leak-out period during which they may not sell shares in excess of the amount permitted by the Rule 144 volume limitations (even if such stockholder is not currently subject to such provisions of Rule 144), which leak- out period shall be extended for an additional 180 days for any shares of Akers common stock issued upon the exercise of existing options or warrants.
Secured Promissory Note
As set forth above, in connection with the execution of the Merger Agreement, Akers will advance a bridge loan to MYMD in an amount of up to $3,000,000 pursuant to a Secured Promissory Note (the “Note”). Advances under the Note will be made in accordance with MYMD’s cash needs pursuant to a pre-agreed operating budget for MYMD. The Note accrues interest on the outstanding principal amount at the rate of 5% per annum and matures on the earliest of (i) April 15, 2022, (ii) upon demand of Akers in the event the Merger is consummated, or (iii) the date on which MYMD’s obligations under the Note are accelerated in accordance with the terms of the Note. As set forth above, in the event the Merger Agreement is terminated by MYMD upon a change in Akers’ board of directors’ recommendations to the Akers stockholders in connection with the Merger Agreement and certain other circumstances specified in the Merger Agreement, the principal amount of the Note, and all accrued and unpaid interest thereon, shall be converted into shares of MYMD common stock at a conversion price of $2.00 per share. MYMD may prepay the Note in whole or in part at any time or from time to time at its sole discretion. Under the terms of the Note, if, at any time after the termination or expiration of the Merger Agreement, MYMD (i) incurs any debt other than Permitted Debt (as defined in the Note), (ii) issues any equity interests, or (iii) consummates any Asset Sale or Recovery Event (each as defined in the Note) then, in each case, no later than two business days after MYMD receives the net cash proceeds of such incurrence, issuance or other action, then MYMD shall be required to prepay an amount under the Note equal to the net cash proceeds received, up to the total amount of the advances made under the Note at such time, including all accrued and unpaid interest thereon, of the Note. The payment and performance of all obligations under the Note are secured by a first priority security interest in all of MYMD’s right, title and interest in and to its assets as collateral.
As of December 31, 2020, the Company had advanced MYMD $1,200,000 under the Note, which is classified as Other Receivables on the Consolidated Balance Sheets. The Company advanced two additional draws of $600,000, or $1,200,000 cumulatively, on January 21, 2021 and February 25, 2021 to MYMD under this secured promissory note (see Note 2(i)).
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Securities Purchase Agreement
Concurrently with the Merger Agreement, on November 11, 2020, the Company entered into a Securities Purchase Agreement (the “Private Placement SPA”) with certain institutional and accredited investors (the “SPA Purchasers”), pursuant to which the Company agreed to issue and sell to the SPA Purchasers in a private placement (the “Private Placement”) (i) an aggregate of 9,765,933 shares of Akers common stock, at an offering price of $1.85 per share or, at the election of each investor, pre-funded warrants (“Pre-Funded Warrants”), and (ii) for each share of Akers common stock (or for each Pre-Funded Warrant, as applicable) purchased in the Private Placement, a common warrant (the “Investor Warrants” and, together with the Pre-Funded Warrants, the “Warrants”) to purchase one share of Akers common stock, for gross proceeds of approximately $18.1 million before the deduction of placement agent fees and expenses and estimated offering expenses. In addition, the Company also issued the Placement Agent a warrant to purchase up to 390,368 shares of its common stock at an exercise price of $1.85 (the “Placement Agent Warrant”). The Placement Agent Warrant will be exercisable at any time and from time to time in whole or in part for a term of five and a half years. The Private Placement closed on November 17, 2020, and the Company issued an aggregate of 8,725,393 shares of the Company’s common stock, Pre-Funded Warrants to purchase 1,040,540 shares of its common stock, and Investor Warrant to purchase 9,765,933 shares of its common stock. In February 2021, an investor exchanged 932,432 shares of common stock purchased in the Private Placement into Pre-Funded Warrants to purchase 932,432 shares of common stock.
In the Private Placement SPA, the Company agreed not to (i) issue, enter into any agreement to issue or announce the issuance or proposed issuance of, any shares of the Company’s common stock or any securities convertible into or exercisable or exchangeable for shares of the Company’s common stock at an effective price less than the exercise price of the Investor Warrants or (ii) file any registration statement or any amendment or supplement thereto, other than as contemplated under the Private Placement SPA, for a period of 90 days following the later of (x) the date the Registration Statement (as defined below) is declared effective by the SEC and (y) the record date for the Company’s stockholder meeting called to approve the Merger. In addition, the Company agreed not to effect or enter into an agreement to effect any issuance of the Company’s common stock or common stock equivalents involving a variable rate transaction (as defined in the Private Placement SPA) from the date of the Private Placement SPA until such time as no SPA Purchaser holds any of the Investor Warrants, subject to certain exceptions (including the issuance of any of the Company’s common stock pursuant to the Merger Agreement).
The Private Placement SPA provides that (i) within 10 days following the date that the Company first files a proxy statement with the SEC in connection with the Merger (including by means of a registration statement on Form S-4), the Company shall file a registration statement (the “Registration Statement”) under the Securities Act of 1933, as amended (the “Securities Act”) for the resale of all of the Shares and the shares of the Company’s common stock issuable upon exercise of the Warrants (the “Warrant Shares”) by the Purchasers and (ii) the Company shall use commercially reasonable efforts to cause such Registration Statement to be declared effective within 60 days of the filing thereof (or 90 days in the event of a full review); provided, however, that the Company shall not be required to register any Shares or Warrant Shares that are eligible for resale pursuant to Rule 144 under the Securities Act (assuming cashless exercise of the Warrants).
The Company currently intends to use the proceeds from the Private Placement in order to satisfy the closing conditions set forth in the Merger Agreement that requires the Company to have a minimum parent net cash amount equal to $25 million, less certain amounts advanced to MyMD, which shall also include any amounts to be used to payoff The Starwood Trust to repay in full the Starwood Line of Credit at the closing of the Merger, and for general working capital purposes. In addition, the Company paid $1,204,525 of the proceeds from the Private Placement to three of the former members of Cystron and recorded a liability of $602,172 to the fourth former member of Cystron pursuant to the MIPA. In addition, the Company paid a cash fee of $501,500 and issued warrants to purchase an aggregate of 255,135 shares of common stock to the designees of H.C. Wainwright & Co., LLC (“HCW”), pursuant to a side letter by and between the Company and HCW, dated November 23, 2020, regarding certain tail fees provided in two engagement letters (one dated October 18, 2019 and the other dated April 7, 2020) entered into in connection with prior offerings by and between Akers and HCW. Such warrants issued were in the same form as the Investor Warrants except that the HCW warrants have an exercise price of $2.3125 per share.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
The Investor Warrants
Each Investor Warrant issued in the Private Placement has an initial exercise price equal to $2.06 per share of common stock. The Investor Warrants are immediately exercisable and will terminate five and a half years following issuance. The exercise price and number of shares of Akers common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting Akers common stock and the exercise price.
If, at any time following the six-month anniversary of November 17, 2020, there is no effective registration statement registering, or the prospectus contained therein is not available for the issuance of the shares underlying the Investor Warrants (the “Investor Warrant Shares”) to the holder, then the Investor Warrants may also be exercised, in whole or in part, at such time by means of a “cashless exercise” in which the holder shall be entitled to receive a number of Investor Warrant Shares according to a formula set forth in the Investor Warrants.
A holder (together with its affiliates) may not exercise any portion of the Investor Warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder prior to the date of issuance, 9.99%) of the outstanding Akers common stock immediately after exercise; provided, however that upon notice to Akers, the holder may increase or decrease the beneficial ownership limitation, provided that in no event shall the beneficial ownership limitation exceed 9.99% and any increase in the beneficial ownership limitation will not be effective until 61 days following notice of such increase from the holder to Akers.
In the event of a fundamental transaction, as described in the Investor Warrants and generally including any reorganization, recapitalization or reclassification of Akers common stock, the sale, transfer or disposition of all or substantially all of Akers’ properties or assets, Akers’ consolidation or merger with or into another person, the acquisition of more than 50% of Akers outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by Akers’ outstanding common stock, the holders of the Investor Warrants will be entitled to receive upon exercise of such warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Investor Warrants immediately prior to such fundamental transaction. The Merger shall not be deemed a fundamental transaction as defined in the Investor Warrants.
The Pre-Funded Warrants
At the request of an investor, in lieu of Akers common stock, certain investors received Pre-Funded Warrants. The Pre-Funded Warrants are exercisable at any time immediately upon issuance and until such warrant is exercised in full. The exercise price of the Pre-Funded Warrants is $0.001 per share of Akers common stock, and, in lieu of making the cash payment otherwise contemplated to be made to Akers upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of Akers common stock determined according to a formula set forth in the Pre-Funded Warrants.
A holder (together with its affiliates) may not exercise any portion of the Pre-Funded Warrants to the extent that the holder would own more than 4.99% (or, at the election of the holder prior to the date of issuance, 9.99%) of the outstanding Akers common stock immediately after exercise; provided, however, that upon notice to the Company, the holder may increase or decrease the beneficial ownership limitation, provided that in no event shall the beneficial ownership limitation exceed 9.99% and any increase in the beneficial ownership limitation will not be effective until 61 days following notice of such increase from the holder to the Company.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Lock-up and Support Agreement
On November 11, 2020, the Company entered into a Lock-Up and Support Agreement (the “Support Agreement”) with substantially all of the SPA Purchasers, pursuant to which, from the date of the Support Agreement until May 31, 2021, such SPA Purchasers agreed to vote their respective shares of Akers common stock in favor of each matter proposed and recommended for approval by the Akers board of directors or management at every shareholders’ meeting. Pursuant to the Support Agreement, such SPA Purchasers also agreed to, until the earlier of (a) the termination of the Merger Agreement or (b) the date that the SPA Purchasers vote their respective shares of Akers common stock in support of the Merger and all matters related to the Merger, will not, directly or indirectly, without the Company’s prior written consent, transfer, assign, or dispose of their rights to vote the shares of Akers common stock issued in the private placement or otherwise take any act that could restrict or otherwise affect their legal power, authority, or right to vote all of their shares of Akers common stock issued in the Private Placement in the manner required by the Support Agreement.
Katalyst Securities LLC Engagement Letter
On October 31, 2020, the Company entered into an engagement letter (the “Engagement Letter”) with Katalyst Securities LLC (the “Placement Agent”), pursuant to which the Placement Agent agreed to serve as the non-exclusive placement agent for the Company, on a reasonable best efforts basis, in connection with the Private Placement. The Company has agreed to pay the Placement Agent an aggregate cash fee equal to 6.5% of the gross proceeds received in the Private Placement and reimburse the Placement Agent’s expenses in the Private Placement up to $25,000. In addition, the Company agreed to grant to Katalyst the Placement Agent Warrant, which was issued upon closing of the Private Placement. The Placement Agent Warrant is exercisable at any time and from time to time, in whole or in part, following the date of issuance and for a term of five years.
Liquidity
As of December 31, 2020, the Company’s cash and cash equivalents on hand were $18,617,955, and marketable securities were $16,718,452. Historically, the Company has incurred net losses and the Company incurred a net loss of $17,580,609 for the year ended December 31, 2020. As of December 31, 2020, the Company had working capital of $34,579,466 and stockholder’s equity of $34,579,466 and an accumulated deficit of $137,163,739. During the year ended December 31, 2020, cash flows used in operating activities were $11,924,941, consisting primarily of a net loss from operations of $12,152,214 and a net loss from discontinued operations of $5,428,395. Since its inception, the Company has met its liquidity requirements principally through the sale of its common stock in public and private placements.
Development and commercialization of the Company’s COVID-19 Vaccine Candidate will require the Company to raise significant additional funds as the project proceeds through clinical trials, the attainment of the required regulatory approvals and the commercialization of the vaccine. The timing of these events is difficult to estimate and are unlikely to be fully completed within the next twelve-months.efforts. The Company’s ability to obtain additional capital may depend on prevailing economic conditions and financial, business and other factors beyond its control. The COVID-19 pandemic has caused an unstable economic environment globally, and the ultimate impact of the COVID-19 pandemic on the Company’s operations is unknown and will depend on future developments, which are highly uncertain and cannot be predicted with confidence. These include but are not limited to the duration of the COVID-19 pandemic, new information which may emerge concerning the severity of the COVID-19 pandemic, and any additional preventative and protective actions that regulators, or the board or management of the Company, may determine are needed. Disruptions in the global financial markets may adversely impact the availability and cost of credit, as well as the Company’s ability to raise money in the capital markets. Current economic conditions have been and continue to be volatile. Continued instability in these market conditions may limit the Company’s ability to access the capital necessary to fund and grow its business.
The Company evaluated the current cash requirements for operations in conjunction with management’s strategic plan and believes that the Company’s current financial resources as of the date of the issuance of these consolidated financial statements are sufficient to fund its current operating budget and contractual obligations as of December 31, 2020 as they fall due within the next twelve-month period, alleviatingdo not include any substantial doubt raised by the Company’s historical operating results and satisfying its estimated liquidity needs for twelve monthsadjustments that might result from the issuanceoutcome of these consolidated financial statements.this uncertainty.
Note 4 – Inventories
Inventories are measured at the lower of cost or net realizable value. The cost of inventories is based on the weighted-average principle, and includes expenditures incurred in acquiring the inventories, production or conversion costs and other costs incurred in bringing them to their existing location and condition. In the case of manufactured inventories and work in progress, costs include an appropriate share of production overhead based on normal operating capacity. As the Company discontinued the production and distribution of all of the Company’s diagnostic tests on July 7, 2020, all inventories amounting to $197,723 was fully impaired and disposed of as of December 31, 2020.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 5 - 4 – Trade and Other Payables
Trade and other payables consist of the following:
December 31, 2020 | December 31, 2019 | |||||||
| Accounts Payable – Trade | $ | 569,999 | $ | 599,306 | ||||
| Accrued Expenses | 123,613 | 232,827 | ||||||
| Deferred Compensation | - | 59,750 | ||||||
| Accounts Payable – Other (Note 3) | 1,510,290 | - | ||||||
| $ | 2,203,902 | $ | 891,883 | |||||
Schedule of Trade and Other Payables
December 31, 2023 | December 31, 2022 | |||||||
| Accounts Payable – Trade | $ | 3,079,080 | $ | 2,356,555 | ||||
| Accrued Expenses | 637,138 | 316,666 | ||||||
| Trade and other payables, Total | $ | 3,716,218 | $ | 2,673,221 | ||||
| F-21 |
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 6 – Discontinued Operations
The Company conducted a strategic review of the screening and testing products business. Following such review, in early July 2020, the Company ceased the production and sale of its rapid, point-of-care screening and testing products. The Company had been experiencing declining sales revenue and production backlogs for these products and, as it previously reported, had eliminated its sales force for such products.
The assets and liabilities of the discontinued operations have been reflected in the Consolidated Balance Sheet as of December 31, 2020 and consist of the following:
| As of | ||||
| December 31, | ||||
| 2020 | ||||
| Current Assets: | ||||
| Prepaid Expenses | $ | 12,002 | ||
| Total Assets | $ | 12,002 | ||
| Current Liabilities: | ||||
| Trade and Other Payables of Discontinued Operations | $ | 59,393 | ||
| Total Current Liabilities | 59,393 | |||
| Non-Current Liabilities | - | |||
| Total Liabilities | $ | 59,393 | ||
| Shareholders’ Equity | $ | - | ||
| Total Liabilities and Shareholders’ Equity | $ | 59,393 | ||
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
The results from the discontinued operations have been reflected in the Consolidated Statement of Comprehensive Loss for the year ended December 31, 2020 and consist of the following:
| For the | ||||
| Year | ||||
| Ended | ||||
| December 31, | ||||
| 2020 | ||||
| Product Revenue | $ | 361,827 | ||
| Product Cost of Sales | (659,405 | ) | ||
| Gross Loss | (297,578 | ) | ||
| Research and Development Expenses | 2,788 | |||
| Administrative Expenses | 417,730 | |||
| Sales and Marketing Expenses | 51,311 | |||
| Regulatory and Compliance Expenses | 197,312 | |||
| Litigation Settlement Expenses | 3,981,131 | |||
| Amortization of Non-Current Assets | 17,601 | |||
| Impairment of Prepaid Royalties | 291,442 | |||
| Impairment of Production Equipment | 18,680 | |||
| Impairment of Intangible Assets | 152,822 | |||
| Loss from Discontinued Operations | $ | (5,428,395 | ) | |
As a result of the discontinued operations, the previously presented 2019 financial statements have been revised to present the consolidated financial statements of the continuing operations separate from the discontinued operations. The effects on the Consolidated Balance Sheet as of December 31, 2019 were as follows:
| December 31, 2019 | ||||||||||||
| As previously | ||||||||||||
| Reported | Adjustment | As Revised | ||||||||||
| ASSETS | ||||||||||||
| Current Assets | ||||||||||||
| Cash | $ | 517,444 | $ | - | $ | 517,444 | ||||||
| Marketable Securities | 9,164,273 | - | 9,164,273 | |||||||||
| Accounts Receivable, net | 42,881 | 42,881 | - | |||||||||
| Deposits and Other Receivables | - | - | - | |||||||||
| Inventories, net | 198,985 | 198,985 | - | |||||||||
| Prepaid Expenses | 387,231 | 53,172 | 334,059 | |||||||||
| Current Assets – discontinued operations | - | (295,038 | ) | 295,038 | ||||||||
| Total Current Assets | 10,310,814 | - | 10,310,814 | |||||||||
| Non-Current Assets | ||||||||||||
| Prepaid Expenses, net of current | 252,308 | 252,308 | - | |||||||||
| Restricted Cash | 115,094 | - | 115,094 | |||||||||
| Plant, Property and Equipment, net | 33,574 | 33,574 | - | |||||||||
| Intangible assets, net | 170,423 | 170,423 | - | |||||||||
| Other assets | 2,722 | - | 2,722 | |||||||||
| Non-current Assets – discontinued operations | (456,305 | ) | 456,305 | |||||||||
| Total Non-Current Assets | 574,121 | - | 574,121 | |||||||||
| Total Assets | $ | 10,884,935 | $ | - | $ | 10,884,935 | ||||||
| LIABILITIES | ||||||||||||
| Current Liabilities | ||||||||||||
| Trade and Other Payables | 1,529,765 | 637,882 | 891,883 | |||||||||
| Current Liabilities – discontinued operations | - | (637,882 | ) | 637,882 | ||||||||
| Total Current Liabilities | 1,529,765 | - | 1,529,765 | |||||||||
| Total Liabilities | 1,529,765 | - | 1,529,765 | |||||||||
| Commitments and Contingencies | ||||||||||||
| SHAREHOLDERS’ EQUITY | ||||||||||||
| Preferred Stock, No par value, 50,000,000 total preferred shares authorized | - | - | - | |||||||||
| Common stock, No par value, 100,000,000 shares authorized 1,738,837 issued and outstanding as of December 31, 2019 | 128,920,414 | - | 128,920,414 | |||||||||
| Accumulated Other Comprehensive Income | 17,886 | - | 17,886 | |||||||||
| Accumulated Deficit | (119,583,130 | ) | - | (119,583,130 | ) | |||||||
| Total Shareholders’ Equity | 9,355,170 | - | 9,355,170 | |||||||||
| Total Liabilities and Shareholders’ Equity | $ | 10,884,935 | $ | - | $ | 10,884,935 | ||||||
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
The effects on the Consolidated Statement of Comprehensive Loss for the year ended December 31, 2019 were as follows:
| For the Year Ended | ||||||||||||
| December 31, 2019 | ||||||||||||
| As Previously Reported | Adjusted | As Revised | ||||||||||
| Product Revenue | $ | 1,577,033 | $ | 1,577,033 | $ | - | ||||||
| Product Cost of Sales | (1,098,286 | ) | (1,098,286 | ) | - | |||||||
| Gross Income | 478,747 | 478,747 | - | |||||||||
| Research and Development Expenses | - | - | - | |||||||||
| Administrative Expenses | 3,728,514 | 356,411 | 3,372,103 | |||||||||
| Sales and Marketing Expenses | 238,036 | 213,036 | 25,000 | |||||||||
| Compliance and Regulatory Expenses | 276,788 | 276,788 | - | |||||||||
| Litigation Settlement Expenses | 141,478 | 66,478 | 75,000 | |||||||||
| Amortization of Non-Current Assets | 40,008 | 40,008 | - | |||||||||
| Impairment of Intangible Assets | 32,980 | 32,980 | - | |||||||||
| Loss from Operations | (3,979,057 | ) | (506,954 | ) | (3,472,103 | ) | ||||||
| Other (Income) Expense | ||||||||||||
| Loss on Disposal of Non-Current Assets | 9,576 | - | 9,576 | |||||||||
| Foreign Currency Transaction (Gain) Loss | 5,051 | - | 5,051 | |||||||||
| Gain on Investments | (3,952 | ) | - | (3,952 | ) | |||||||
| Interest and Dividend Income | (101,483 | ) | - | (101,483 | ) | |||||||
| Total Other Income | (90,808 | ) | - | (90,808 | ) | |||||||
| Loss from Continuing Operations | (3,888,249 | ) | - | (3,381,295 | ) | |||||||
| Loss from Discontinued Operations | - | (506,954 | ) | (506,954 | ) | |||||||
| Loss Before Income Taxes | (3,888,249 | ) | - | (3,888,249 | ) | |||||||
| Income Tax Benefit | - | - | - | |||||||||
| Net Loss | (3,888,249 | ) | - | (3,888,249 | ) | |||||||
| Other Comprehensive Income | ||||||||||||
| Net Unrealized Gain on Marketable Securities | 43,799 | - | 43,799 | |||||||||
| Total Other Comprehensive Income | 43,799 | - | 43,799 | |||||||||
| Comprehensive Loss | $ | (3,844,450 | ) | - | $ | (3,844,450 | ) | |||||
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
The depreciation, amortization and significant operating noncash items of the discontinued operations were as follows:
| For the Year Ended | ||||||||
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Depreciation and amortization | $ | 29,452 | $ | 74,064 | ||||
| Impairment of Prepaid Royalties | 291,442 | - | ||||||
| Impairment of intangible assets | 152,822 | 32,980 | ||||||
| Impairment of production equipment | 18,680 | - | ||||||
| Inventory adjustment for net realizable value | 197,723 | - | ||||||
| Reserve for obsolete inventory | - | 371,997 | ||||||
| Share based compensation - shares issued to Chubeworkx | 2,510,000 | - | ||||||
| $ | 3,200,119 | $ | 479,041 | |||||
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 7 - Share-based Compensation
Equity incentive Plans
2013 Stock Incentive Plan
On January 23, 2014, the Company adopted the 2013 Stock Incentive Plan (“2013 Plan”). The 2013 Plan was amended by the Board on January 9, 2015 and September 30, 2016, and such amendments were ratified by shareholders on December 7, 2018. The 2013 Plan provides for the issuance of up to 4,323 shares of the Company’s common stock.Common Stock. As of December 31, 2020,2023, grants of restricted stock and options to purchase 2,813 shares of Common Stock have been issued pursuant to the 2013 Plan, and 1,510 shares of Common Stock remain available for issuance.
2016 Stock Incentive Plan
On December 21, 2016, the shareholders approved, and the Company adopted the 2016 Stock Incentive Plan (“2016 Plan”). The 2016 Plan provides for the issuance of up to shares of the Company’s Common Stock. As of December 31, 2023, grants of options to purchase shares of Common Stock have been issued pursuant to the 2016 Plan, and shares of Common Stock remain available for issuance.
2017 Stock Incentive Plan
On August 7, 2017, the shareholders approved, and the Company adopted the 2017 Stock Incentive Plan (“2017 Plan”). The 2017 Plan provides for the issuance of up to 7,031 shares of the Company’s common stock.Common Stock. As of December 31, 2020,2023, grants of restricted stock and options to purchase 3,064 shares of Common Stock have been issued pursuant to the 2017 Plan, and 3,967 shares of Common Stock remain available for issuance.
2018 Stock Incentive Plan
On December 7, 2018, the shareholders approved, and the Company adopted the 2018 Stock Incentive Plan (“2018 Plan”). On August 27, 2020, the 2019 Plan was modified to increase the total authorized shares. The 2018 Plan, as amended, provides for the issuance of up to 1,120,125 shares of the Company’s common stock.Common Stock. As of December 31, 2020,2023, grants of RSUs and restricted stock to purchase 804,963 shares of Common Stock have been issued pursuant to the 2018 Plan, and 315,162 shares of Common Stock remain available for issuance.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes
| F-22 |
2021 Stock Incentive Plan
On April 15, 2021, the shareholders approved, and the Company adopted the 2021 Stock Incentive Plan (“2021 Plan”). The 2021 Plan provides for the issuance of up to Consolidated Financial Statements shares of the Company’s Common Stock. As of December 31, 2023, grants of RSUs and stock options to purchase shares of Common Stock have been issued pursuant to the 2021 Plan, and shares of Common Stock remain available for issuance.
Note 7 - Share-based Compensation, continued
Stock Options
| Weighted | ||||||||||||||||||||
| Average | ||||||||||||||||||||
| Weighted | Weighted | Remaining | ||||||||||||||||||
| Number | Average | Average | Contractual | Aggregate | ||||||||||||||||
| of | Exercise | Grant Date | Term | Intrinsic | ||||||||||||||||
| Shares | Price | Fair Value | (years) | Value | ||||||||||||||||
| Balance at December 31, 2019 | 40 | $ | 236.16 | $ | 151.68 | 0.99 | $ | - | ||||||||||||
| Granted | - | - | - | - | - | |||||||||||||||
| Exercised | - | - | - | - | - | |||||||||||||||
| Forfeited | - | - | - | - | ||||||||||||||||
| Canceled/Expired | (40 | ) | $ | 236.16 | $ | 151.68 | 0.24 | - | ||||||||||||
| Balance at December 31, 2020 | - | $ | - | $ | - | - | $ | - | ||||||||||||
| Exercisable as of December 31, 2020 | - | $ | - | $ | - | - | $ | - | ||||||||||||
Summary of Stock Options Activity
| Weighted | ||||||||||||||||||||
| Average | ||||||||||||||||||||
| Weighted | Weighted | Remaining | ||||||||||||||||||
| Number | Average | Average | Contractual | Aggregate | ||||||||||||||||
| of | Exercise | Grant Date | Term | Intrinsic | ||||||||||||||||
| Shares | Price | Fair Value | (years) | Value | ||||||||||||||||
| Balance at December 31, 2022 | 149,241 | $ | 79.34 | $ | 78.64 | $ | - | |||||||||||||
| Granted | 129,838 | 41.77 | 38.53 | $ | - | |||||||||||||||
| Exercised | - | - | - | - | - | |||||||||||||||
| Forfeited | - | - | - | - | - | |||||||||||||||
| Canceled/Expired | (139,239 | ) | 77.70 | 77.70 | - | - | ||||||||||||||
| Balance at December 31, 2023 | 139,840 | 46.09 | 42.34 | $ | - | |||||||||||||||
| Exercisable as of December 31, 2023 | 47,286 | 56.44 | 51.49 | $ | - | |||||||||||||||
The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the closing stock price of $1.99$ for the Company’s common shares on December 31, 2020. As2023 and the closing stock price of $ for the Company’s common shares on December 31, 20202022.
On January 28, 2022, the Company’s Compensation Committee approved the issuance of stock options under the 2021 Stock Incentive Plan. These shares had a grant date fair value of $ per share or a cumulative fair market value of $717,660 as calculated using Black-Scholes (exercise price $ per share, stock price $ per share, volatility of %, discount rate of % and seven-year term). The grant was segmented into four vesting tranches triggered by performance achievements and expire on January 28, 2029. The Company will amortize the expenses over the vesting cycles of the individual tranches when the performance achievement is lower thanprobable.
On June 21, 2022, the exerciseCompany granted stock options under the 2021 Stock Incentive Plan to a third-party consultant in consideration of services rendered. These shares had a grant date fair value of $ per share or a cumulative fair market value of $199,360 as calculated using Black-Scholes (exercise price there$ per share, stock price $ per share, volatility of %, discount rate of % and -year term). The grant vested immediately and expire on June 21, 2027. The Company is no intrinsicamortizing the expense over twelve months, the term of the consulting agreement.
On April 4, 2023, the Company issued options to a key employee. These shares had a grant date fair value of $per share or a cumulative fair market value of $978,675 as calculated using Black-Scholes (exercise price $per share, stock price $per share, volatility of %, discount rate of % and a five-year term). 1/3 of the options vested on the grant date, 1/3 vest on the first anniversary of the grant and 1/3 vest on the second anniversary of the grant. The 1/3rd of the fair-market value of the options was expensed on the grant date and the remaining 2/3rd is amortized over 24 month vesting.
On June 7, 2023, the Company issued options to disclose.the directors and key employees. These shares had a grant date fair value of $per share or a cumulative fair market value of $3,128,759 as calculated using Black-Scholes (exercise price $per share, stock price $per share, volatility of %, discount rate of % and a -year term). 1/3 of the options vested on the grant date, 1/3 vest on the first anniversary of the grant and 1/3 vest on the second anniversary of the grant. The 1/3rd of the fair-market value of the options was expensed on the grant date and the remaining 2/3rd is amortized over 24 month vesting.
On July 19, 2023, the Company issued options to a consultant for services. These shares had a grant date fair value of $per share or a cumulative fair market value of $48,643 as calculated using Black-Scholes (exercise price $per share, stock price $per share, volatility of %, discount rate of % and a -year term). The options vested on the grant date. The fair-market value of the options was recorded immediately for services previously performed.
On September 6, 2023, the Company issued options to a key employee. These shares had a grant date fair value of $ per share or a cumulative fair market value of $769,700 as calculated using Black-Scholes (exercise price $ per share, stock price $ per share, volatility of %, discount rate of % and a -year term). The Company had no outstanding stock options aswill vest upon the achievement of specific performance goals. The fair-market value of the options will be recognized in the period the vesting event is achieved. As of December 31, 2020.2023, none of the vesting events have occurred.
On September 6, 2023, the Company issued options to a key employee. These shares had a grant date fair value of $ per share or a cumulative fair market value of $76,970 as calculated using Black-Scholes (exercise price $ per share, stock price $per share, volatility of %, discount rate of % and a -year term). ½ of the options vested on the grant date, ½ vest on the first anniversary of the grant. The fair-market value of the vested options was amortized upon the issuance of the grant and the remaining options will be amortized over the 12-month vesting cycle.
During the years ended December 31, 20202023 and 2019,2022, the Company incurredrecognized stock option expenses totaling $0$3,049,537 and $0,$444,342, respectively.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial StatementsThe unamortized stock option expenses as of December 31, 2023 and 2022 totaled $ and $, respectively.
Note 7 - Share-based Compensation, continued
Restricted Stock Units
During the year ended December 31, 2023, the Company converted vested RSUs issued in March 2019 and vested RSUs issued in September 2020 to members of the Board of Directors into common shares of the Company. Expenses related to these RSUs had been recognized by pre-merger Akers Biosciences, Inc in 2021 and prior years.
| F-23 |
On March 29, 2019,October 14, 2021, the Compensation Committee of the Board of Directors approved the grant of 5,201grants totaling Restricted Stock Units (“RSU”) to each of the three directors.Company’s six directors and seven key employees. Each RSU had a grant date fair value of $23.28$ which shallwill be amortized on a straight-line basis over theupon vesting period into administrative expenses within the Consolidated Statement of Comprehensive Loss. Such RSUs were granted under the 2018 Plan, and vested on January 1, 2020. Upon vesting, such RSUs shall be settled with the issuance2021 Plan. Vesting of common stock. The Company stock underlying these RSUs are subject to a lock-up/leak-out agreement for aeach RSU is:
| ● | ||
| ● | ||
| ● | . | |
| ● | In the event that (i) a change in control occurs or (ii) the participant incurs a termination of service by the Company without cause or due to the participant’s death or total and permanent disability, then all unvested units shall become vested units immediately upon the occurrence of such event. |
As of 180 days from the effective dateDecember 31, 2023, none of the merger with MyMD (Note 3).vesting milestones have been met.
On September 11, 2020,January 28, 2022, the Compensation Committee of the Board of Directors approved grants totaling 789,360 Restricted Stock Unitsa grant of RSUs to the Company’s four directors. Each RSU hada sub-contractor with a grant date fair value of $2.24 which shall be amortized on a straight-line basis over the vesting period into administrative expenses within the Consolidated Statement of Comprehensive Loss.$ and vested immediately. Such RSUs were granted under the 2018 Plan, as amended. Fifty percent (50%)2021 Plan. The Company recorded expenses of each RSU will vest$ which is included Stock Based Compensation on the first anniversary dateConsolidated Statement of Comprehensive Loss during the year ended December 31, 2022.
On July 7, 2022, the Compensation Committee of the GrantBoard of Directors approved a grant of RSUs to a sub-contractor with a grant date fair value of $ and vested immediately. Such RSUs were granted under the 2021 Plan. The Company recorded expenses of $ which is included Stock Based Compensation on the Consolidated Statement of Comprehensive Loss during the year ended December 31, 2022.
The following is the status of outstanding unvested restricted stock units outstanding as of December 31, 2023 and the remaining fifty percent (50%) will vest onchanges for the second anniversary date; provided that the RSUs shall vest immediately upon the occurrenceyear ended December 31, 2023:
Summary of (i) a change in control, provided that the director is employed by or providing services to the Company and its affiliates on the closing date of such change of control, or (ii) the director’s termination of employment of service by the Company was without cause.Restricted Stock Units Activity
| Weighted | ||||||||
| Average | ||||||||
| Number of | Grant Date | |||||||
| RSUs | Fair Value | |||||||
| Balance at December 31, 2022 | 93,169 | $ | 242.70 | |||||
| Granted | - | - | ||||||
| Vested | - | - | ||||||
| Forfeited | - | - | ||||||
| Canceled/Expired | (4,501 | ) | 242.70 | |||||
| Balance at December 31, 2023 | 88,668 | $ | 242.70 | |||||
As of December 31, 2020,2023 and 2022, the unamortized value of the RSUs was $1,364,879. A summary$ and $, respectively.
Note 6 – Equity
Authorized Capital Stock
As of activity related to the RSUs for the year ended December 31, 2020 is2023, the Company’s authorized capital stock consisted of 66,666,666 shares, of which are shares of Common Stock, $ par value per share (the “Common Stock”), and are shares of preferred stock, $ par value per share, of which have been designated as follows:
| Weighted | ||||||||
| Average | ||||||||
| Number of | Grant Date | |||||||
| RSUs | Fair Value | |||||||
| Balance at December 31, 2019 | 15,603 | $ | 23.28 | |||||
| Granted | 789,360 | 2.24 | ||||||
| Exercised | - | - | ||||||
| Forfeited | - | - | ||||||
| Vested | (15,603 | ) | 23.28 | |||||
| Canceled/Expired | - | - | ||||||
| Balance at December 31, 2020 | $ | 789,360 | $ | 2.24 | ||||
| Exercisable as of December 31, 2020 | $ | - | $ | - | ||||
During the years endedSeries C Convertible Preferred Stock (the “Series C Preferred Stock”), of which have been designated as Series D Convertible Preferred Stock (the “Series D Preferred Stock”), of which have been designated as Series E Junior Participating Preferred Stock and of which have been designated as Series F Convertible Preferred Stock (the “Series F Preferred Stock”). As of December 31, 20202023 and 2019, the Company incurred RSU expenseDecember 31, 2022, there were and shares of $404,589 and $362,005, respectively.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 7 - Share-based Compensation, continued
Common Stock Warrants
The table below summarizes the warrant activity for the year ended December 31, 2020:
| Weighted | Average | |||||||||||||||
| Average | Remaining | Aggregate | ||||||||||||||
| Number of | Exercise | Contractual | Intrinsic | |||||||||||||
| Warrants | Price | Term (years) | Value | |||||||||||||
| Balance at December 31, 2019 | 247,215 | $ | 29.79 | 4.72 | $ | - | ||||||||||
| Granted | 10,678,737 | 2.16 | 5.36 | - | ||||||||||||
| Exercised | - | - | - | - | ||||||||||||
| Forfeited | - | - | - | - | ||||||||||||
| Canceled/Expired | - | - | - | - | ||||||||||||
| Balance at December 31, 2020 | 10,925,952 | $ | 2.78 | 5.31 | $ | - | ||||||||||
| Exercisable as of December 31, 2020 | 10,925,952 | $ | 2.78 | 5.31 | $ | - | ||||||||||
The aggregate intrinsic value is calculated as the difference between the exercise priceissued and outstanding, respectively. There were shares of the underlying awardsSeries D Preferred Stock issued and the closing stock price of $1.99 for the Company’s common shares on December 31, 2020. All warrants were vested on date of grant.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 7 - Share-based Compensation, continued
Pre-funded Common Stock Warrants
The table below summarizes the pre-funded warrant activity for the year ended December 31, 2020:
| Weighted | Average | |||||||||||||||
| Average | Remaining | Aggregate | ||||||||||||||
| Number of | Exercise | Contractual | Intrinsic | |||||||||||||
| Warrants | Price | Term (years) | Value | |||||||||||||
| Balance at December 31, 2019 | 795,000 | $ | 0.0001 | - | $ | 2,543,921 | ||||||||||
| Granted | 1,040,540 | 0.001 | - | - | ||||||||||||
| Exercised | (795,000 | ) | 0.0001 | - | - | |||||||||||
| Forfeited | - | - | - | - | ||||||||||||
| Canceled/Expired | - | - | - | - | ||||||||||||
| Balance at December 31, 2020 | 1,040,540 | $ | 0.001 | - | $ | 2,069,634 | ||||||||||
| Exercisable as of December 31, 2020 | 1,040,540 | $ | 0.001 | - | $ | 2,069,634 | ||||||||||
All pre-funded warrants were vested on date of grantoutstanding and are exercisable at any time. The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying award and the closing stock price of $1.99 for the Company’s common shares on December 31, 2020.
During the year ended December 31, 2020, pre-funded warrants to purchase 795,000 shares of common stock were exercised at an exercise price of $0.0001 per share, yielding net proceeds of $80.
Series C Preferred Series ‘C’ Stock Warrants
The table below summarizes the warrant activity for the year ended December 31, 2020:
| Weighted | Average | |||||||||||||||
| Average | Remaining | Aggregate | ||||||||||||||
| Number of | Exercise | Contractual | Intrinsic | |||||||||||||
| Warrants | Price | Term (years) | Value | |||||||||||||
| Balance at December 31, 2019 | 1,990,000 | $ | 4.00 | 5.00 | $ | - | ||||||||||
| Granted | - | - | - | - | ||||||||||||
| Exercised | (1,935,000 | ) | 4.00 | - | - | |||||||||||
| Forfeited | - | - | - | - | ||||||||||||
| Canceled/Expired | - | - | - | - | ||||||||||||
| Balance at December 31, 2020 | 55,000 | $ | 4.00 | 3.94 | $ | - | ||||||||||
| Exercisable as of December 31, 2020 | 55,000 | $ | 4.00 | 3.94 | $ | - | ||||||||||
The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the closing stock price of $1.99 for the Company’s common shares on December 31, 2020.
All preferred series ‘C’ warrants were vested on date of grant.During the year ended December 31, 2020, 1,935,000 warrants to purchase 1,935,000 shares of the Company’s common stock were exercised yielding net proceeds of $7,740,000.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 8 – Equity
The holders of common shares are entitled to one vote per share at meetings of the Company. On December 30, 2019, the Company’s shareholders approved an increase to 100,000,000 of the number of the authorizedconvertible into shares of Common Stock.Stock issued and outstanding as of December 31, 2023 and December 31, 2022. There were and shares of Series F Preferred Stock issued and outstanding as of December 31, 2023 and December 31, 2022. There were no shares of Series C Convertible Preferred Stock or Series E Junior Participating Preferred Stock issued and outstanding as of December 31, 2023 and December 31, 2023.
Preferred Stock
The holders of preferred shares or preferred warrants are entitled to vote per share, as limited by the Certificatecertificate of Designationdesignation for each class of preferred shares or warrants, at meetings of the Company. As of December 31, 2020, 50,000,000 shares of Preferred Stock were authorized and four classes of Preferred Stock or Warrants are designated as described below.
| F-24 |
Series A Convertible Preferred Stock
On September 14, 2012, the Company designated 10,000,000 Series A Convertible Preferred Shares, $0.001 par value, with a stated value of $0.0725. The Series A Convertible Preferred Shares have the following rights:
Voting Rights: Preferred stockholders have voting rights equal to the number of common shares stockholder would own upon conversion of shares of preferred stock.
Dividends: The holders of the Convertible Preferred Stock are entitled to receive preferential dividends at a rate of $0.00135 per share. Such dividends compound annually and are fully cumulative and have priority to any dividends on common stock.
Liquidation Preferences: The holders of the Convertible Preferred Stock are entitled to receive liquidation preferences for payment of any dividends due the holders. After payment of the liquidation preferences, the remaining assets, if any, are to be distributed to the holders of the Convertible Preferred Stock and common stock on a pro rata basis.
Conversion: One share of the Convertible Preferred Stock is convertible into five shares of the Company’s common stock at the option of the holder.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Series C Convertible Preferred Stock
On December 9, 2019, the Company designated 1,990,000 Series C Convertible Preferred Shares, no par value with a stated value of $4.00. The Series C Preferred Shares have the following rights.
Voting Rights: Except as otherwise expressly provided or otherwise required by law, the holders of shares of Series C Preferred Stock shall have no voting rights. However, as long as any shares of Preferred Stock are outstanding, the Company shall not, without the affirmative vote of the Holders of a majority of the then outstanding shares of Preferred Stock, (a) alter or change adversely the powers, preferences or rights given to the Preferred Stock or alter or amend the Certificate of Designation, (b) increase the number of authorized shares of Preferred Stock, or (c) enter into any agreement with respect to any of the foregoing. with respect to any of the foregoing
Dividends: Except for stock dividends or distributions for which adjustments are to be made, holders shall be entitled to receive, and the Company shall pay, dividends on shares of Series C Preferred Stock equal (on an as-if-converted-to-Common-Stock basis) to and in the same form as dividends actually paid on shares of the Common Stock when, as and if such dividends are paid on shares of the Common Stock. No other dividends shall be paid on shares of Series C Preferred Stock.
Liquidation Preferences: Upon any liquidation, dissolution or winding-up of Company, whether voluntary or involuntary (a “ Liquidation ”), the Holders shall be entitled to participate on an as-converted-to-Common Stock basis with holders of the Common Stock in any distribution of assets of the Company to the holders of the Common Stock.
Conversion: Each share of Series C Preferred Stock shall be convertible, at any time and from time to time from and after the Original Issue Date at the option of the Holder thereof, into that number of shares of Common Stock determined by dividing the Stated Value of such share of Series C Preferred Stock by the Conversion Price then in effect.
Series D Convertible Preferred Stock
On March 24, 2020,The following are the Company designated 211,353principal terms of the Series D Convertible Preferred Shares, no par value with a stated value of $0.01 per share and filed the Certificate of Designation of Preferences, Rights and Limitations ofStock:
Rank
The Series D Convertible Preferred Stock (the “Certificateranks (1) on parity with Common Stock on an “as converted” basis, (2) senior to any series of Designation”)our capital stock hereafter created specifically ranking by its terms junior to the Series D Preferred Stock, (3) on parity with any series of our capital stock hereafter created specifically ranking by its terms on parity with the SecretarySeries D Preferred Stock, and (4) junior to any series of State of the State of New Jersey. Pursuantour capital stock hereafter created specifically ranking by its terms senior to the CertificateSeries D Preferred Stock in each case, as to dividends or distributions of Designation, in the event of the Company’sassets upon our liquidation, dissolution or winding up of its affairs, the holders of its Series D Convertible Preferred Stock (the “Preferred Stock”) will be entitled to receive the same amount that a holder of the Company’s common stock would receive if the Preferred Stock were fully converted (disregarding for such purposes any conversion limitations set forth in the Certificate of Designation) to common stock which amounts shall be paid pari passu with all holders of the Company’s common stock. Each share of Preferred Stock has a stated value equal to $0.01 (the “Stated Value”), subject to increase as set forth in Section 7 of the Certificate of Designation.whether voluntary or involuntary.
Conversion Rights
A holder of Series D Preferred Stock is entitled at any time to convert any whole or partial number of shares of Series D Preferred Stock into shares of the Company’s common stockour Common Stock, determined by dividing the Stated Value of the Preferred Stock being convertedstated value equal to $0.01 by the conversion price of $0.01$0.01 per share.
A holder of Series D Preferred Stock will beis prohibited from converting Series D Preferred Stock into shares of the Company’s common stockCommon Stock if, as a result of such conversion, the holder, together with its affiliates, would own more than 4.99% of the total number of shares of the Company’s common stockour Common Stock then issued and outstanding (with such ownership restriction referred to as the “Beneficial“Series D Beneficial Ownership Limitation”). immediately after giving effect to the issuance of the shares of Common Stock issuable upon conversion of the Series D Preferred Stock. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99%, provided that any increase in such percentage shall not be effective until 61 days after such notice to us. The conversion rate of the Company.Series D Preferred Stock is subject to proportionate adjustments for stock splits, reverse stock splits and similar events, but is not subject to adjustment based on price anti-dilution provisions.
Dividend Rights
In addition to stock dividends or distributions for which proportionate adjustments will be made, holders of Series D Preferred Stock are entitled to receive dividends on shares of Series D Preferred Stock equal, on an as-if-converted-to-common-stock basis, to and in the same form as dividends actually paid on shares of the Common Stock when, as and if such dividends are paid on shares of the Common Stock. No other dividends are payable on shares of Series D Preferred Stock.
Voting Rights
Subject to the Series D Beneficial Ownership Limitation, on any matter presented to the Company’sour stockholders for their action or consideration at any meeting of the Company’sour stockholders (or by written consent of stockholders in lieu of a meeting), each holder, of Preferred Stock willin its capacity as such, shall be entitled to cast the number of votes equal to the number of whole shares of the Company’s common stockour Common Stock into which the shares ofSeries D Preferred Stock beneficially owned by such holder are convertible as of the record date for determining stockholders entitled to vote on or consent to such matter (taking into account all Series D Preferred Stock beneficially owned by such holder). Except as otherwise required by law or by the other provisions of the Company’s certificateCertificate of incorporation,Designation of Series D Convertible Preferred Stock (the “Series D Certificate of Designation”), the holders of Series D Preferred Stock, willin their capacity as such, shall vote together with the holders of the Company’s common stockour Common Stock and any other class or series of stock entitled to vote thereon as a single class.
ALiquidation Rights
Upon any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary, the holders of Series D Preferred Stock are entitled to receive, pari passu with the holders of Common Stock, out of the assets available for distribution to stockholders an amount equal to such amount per share as would have been payable had all shares of Series D Preferred Stock been converted into Common Stock immediately before such liquidation, dissolution or winding up, without giving effect to any limitation on conversion as a result of the Series D Beneficial Ownership Limitation, as described above.
Exchange Listing
Series D Preferred Stock is not listed on the Nasdaq, any national securities exchange or other nationally recognized trading system. Our Common Stock issuable upon conversion of the Series D Preferred Stock is listed on the Nasdaq under the symbol “MYMD”.
Failure to Deliver Conversion Shares
If we fail to timely deliver shares of Common Stock upon conversion of the Series D Preferred Stock (the “Series D Conversion Shares”) within the time period specified in the Series D Certificate of Designation (within two trading days after delivery of the notice of conversion, or any shorter standard settlement period in effect with respect to trading market on the date notice is delivered), then we are obligated to pay to the holder, as liquidated damages, an amount equal to $25 per trading day (increasing to $50 per trading day on the third trading day and $100 per trading day on the sixth trading day) for each $5,000 of stated value of Series D Preferred Stock being converted which are not timely delivered. If we make such liquidated damages payments, we are also not obligated to make Series D Buy-In (as defined below) payments with respect to the same Series D Conversion Shares.
Compensation for Series D Buy-In on Failure to Timely Deliver Shares
If we fail to timely deliver the Series D Conversion Shares to the holder, and if after the required delivery date the holder is required by its broker to purchase (in an open market transaction or otherwise) or the holder or its brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the holder of the Series D Conversion Shares which the holder anticipated receiving upon such conversion or exercise (a “Series D Buy-In”), then we are obligated to (A) pay in cash to such holder (in addition to any other remedies available to or elected by such holder) the amount, if any, by which (x) such holder’s total purchase price (including any brokerage commissions) for the shares of Common Stock so purchased exceeds (y) the product of (1) the aggregate number of Series D Conversion Shares that such holder was entitled to receive from the conversion at issue multiplied by (2) the actual sale price at which the sell order giving rise to such purchase obligation was executed (including any brokerage commissions) and (B) at the option of such holder, either reissue (if surrendered) the shares of Series D Preferred Stock equal to the number of shares of Series D Preferred Stock submitted for conversion (in which case, such conversion shall be deemed rescinded) or deliver to such holder the number of Series D Conversion Shares that would have been issued if we had timely complied with its delivery requirements.
| F-25 |
As of December 31, 2023, the Company had shares of Series D Convertible Preferred Stock outstanding which represent underlying shares of the Company Common Stock.
Series F Convertible Preferred Stock
The following are the principal terms of the Series F Preferred Stock:
Dividends
The holders of the Series F Preferred Stock are entitled to dividends of 10.0% per annum, compounded monthly, which are payable in cash or shares of Common Stock at the Company’s option, in accordance with the terms of the certificate of designation of the Series F Preferred Stock (the “Series F Certificate of Designation”). Upon the occurrence and during the continuance of a Triggering Event (as defined in the Series F Certificate of Designation), shares of Series F Preferred Stock will accrue dividends at the rate of 15.0% per annum. Upon conversion or redemption, the holders of shares of Series F Preferred Stock are also entitled to receive a dividend make-whole payment.
Voting Rights
The Series F Preferred Stock has no voting rights, except as required by law (including without limitation, the Delaware General Corporation Law (the “DGCL”) and as expressly provided in the Series F Certificate of Designation. To the extent that under the DGCL the vote of the holders of shares of Series F Preferred Stock, voting separately as a class or series, as applicable, is required to authorize a given action of the Company, the affirmative vote or consent of a majority of the outstanding shares of Series F Preferred Stock, voting together in the aggregate and not in separate series unless required under the DGCL, represented at a duly held meeting at which a quorum is presented or by written consent of such majority (except as otherwise may be required under the DGCL) shall constitute the approval of such action by both the class or the series, as applicable. To the extent that under the DGCL holders of shares of Series F Preferred Stock are entitled to vote on a matter with holders of shares of Common Stock, voting together as one class, each share of Series F Preferred Stock shall entitle the holder thereof to cast that number of votes per share as is equal to the number of shares of Common Stock into which it is then convertible (subject to certain beneficial ownership limitations) using the record date for determining the stockholders of the Company eligible to vote on such matters as the date as of which the Conversion Price is calculated.
Liquidation
Upon any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary, the each holder shares of the Series F Preferred Stock shall be entitled to receive dividends as and when paid to the holdersout of the Company’s common stock on an as-converted basis.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Series E Junior Participating Preferred Stock (Rights Agreement)
On September 9, 2020assets, whether capital or surplus, of the Company designated 100,000 Series E Junior Participating Preferred Shares, no par value with a stated value of $0.001. The Series E Junior Participating Preferred Shares have the following rights.
The Company’s board of directors (the “Board”) declared a dividend of one preferred share purchase right (a “Right”) for each of the Company’s issued and outstanding shares of common stock. The dividend is payable to the stockholders of record on September 21, 2020 (the “Record Date”). Each Right entitles the registered holder, subject to the terms of the Rights Agreement (as defined below), to purchase from the Company one one-thousandth of aan amount per share of the Company’s Series E Junior ParticipatingF Preferred Stock no par value with a stated value of $0.001 (the “Preferred Stock”) at $15.00 (the “Purchase Price”), subject to certain adjustments. The description and terms of the Rights are set forth in the Rights Agreement dated as of September 9, 2020 (the “Rights Agreement”) between the Company and VStock Transfer, LLC, as Rights Agent (the “Rights Agent”).
The Rights will not be exercisable until the earlier to occur of (i) the tenth business day following a public announcement or filing that a person has, or affiliates or associates of such person have, become an “Acquiring Person,” which is defined as a person, or affiliates or associates of such person, who, at any time after the date of the Rights Agreement, has acquired, or obtained the right to acquire, Beneficial Ownership of 10% or more of the Company’s outstanding shares of common stock, subject to certain exceptions, or (ii) the tenth business day (or such later date as may be determined by action of the Board prior to such time as any person or group of affiliated or associated persons becomes an Acquiring Person) after the commencement of, or announcement of an intention to commence, a tender offer or exchange offer the consummation of which would result in any person becoming an Acquiring Person (the earlier of such dates being called the “Distribution Date”). Beneficial Ownership, as defined in the Rights Agreement, includes certain interests in securities created by derivatives contracts, which are beneficially owned, directly or indirectly, by a counterparty (or any of such counterparty’s affiliates or associates) under any derivatives contract to which such person or any of such person’s affiliates or associates is a receiving party (as such terms are defined in Rights Agreement), subject to certain limitations.
Until the Distribution Date, (i) the Rights will be evidenced by the common stock certificates (or, for uncertificated shares of common stock, by the book-entry account that evidences record ownership of such shares) and will be transferred with, and only with, such Common Stock, and (ii) new common stock certificates issued after the Record Date will contain a legend incorporating the Rights Agreement by reference (for book entry common stock, this legend will be contained in the notations in book entry accounts). Until the earlier of the Distribution Date and the Expiration Date (defined below), the transfer of any shares of common stock outstanding on the Record Date will also constitute the transfer of the Rights associated with such shares of common stock. As soon as practicable after the Distribution Date, the Rights Agent will send by first-class, insured, postage prepaid mail, to each record holder of the common stock as of the close of business on Distribution Date separate rights certificates evidencing the Rights (“Right Certificates”), and such Right Certificates alone will evidence the Rights. The Company may choose book entry in lieu of physical certificates, in which case, references to “Rights Certificates” shall be deemed to mean the uncertificated book entry representing the Rights.
The Rights, which are not exercisable until the Distribution Date, expire upon the earliest to occur of (i) the close of business on September 8, 2021; (ii) the time at which the Rights are redeemed or exchanged pursuant to the Rights Agreement; and (iii) the time at which the Rights are terminated upon the closing of any merger or other acquisition transaction involving the Company pursuant to a merger or other acquisition agreement that has been approved by the Board prior to any person becoming an Acquiring Person (the earliest of (i), (ii), and (iii) is referred to as the “Expiration Date”).
Each share of Preferred Stock will be entitled to a preferential per share dividend rate equal to the greater of (i) $0.001(A) 125% of the stated value of such share of Series F Preferred Stock (plus any applicable make-whole amount, unpaid late charge or other applicable amount) on the date of such payment and (ii)(B) the sum of (1) 1,000 times the aggregateamount per share amountsuch holder would receive if such holder converted such share of all cash dividends, plus (2) 1,000 times the aggregate per share amount (payable in kind) of all non-cash dividends or other distributions other than certain dividends or subdivisions of the outstanding shares of common stock. EachSeries F Preferred Stock will entitle the holder thereof to a number of votes equal to 1,000 on all matters submitted to a vote of the stockholders of the Company. In the event of any merger, consolidation or other transaction in which shares of common stock are exchanged, each Preferred Stock will be entitled to receive 1,000 times the amount received per one share of common stock. Pursuant to the Rights Agreement, the preferential rates noted above may be adjusted in the event that the Company (i) pays dividends in common stock, (ii) subdivides the outstanding common stock or (iii) combines outstandinginto Common Stock into a smaller number of shares.
The Purchase Price payable, and the number of shares of Preferred Stock or other securities or property issuable, upon exercise of the Rights are subject to adjustment from time to time to prevent dilution (i) in the event of a stock dividend, or a subdivision, combination or reclassification of the Preferred Stock, (ii) if the holders of Preferred Stock are granted certain rights, options or warrants to subscribe for the applicable Preferred Stock or securities convertible into the applicable Preferred Stock at less than the current market price of the applicable Preferred Stock, or (iii) upon the distribution to holders of Preferred Stock of evidences of indebtedness, cash (excluding regular quarterly cash dividends), assets (other than dividends payable in Preferred Stock) or subscription rights or warrants (other than those referred to in (ii) immediately above). The number of outstanding Rights and the number of one one-thousandths of a Preferred Stock issuable upon exercise of each Right are also subject to adjustment in the event of a stock split, reverse stock split, stock dividends and other similar transactions.
With some exceptions, no adjustment in the purchase price relating to a Right will be required until cumulative adjustments amount to at least one percent (1%) of the purchase price relating to the Right. No fractional shares of Preferred Stock are required to be issued (other than fractions which are integral multiples of one one-thousandth of a share of Preferred Stock) and, in lieu of the issuance of fractional shares, the Company may make an adjustment in cash based on the market price of the Preferred Stock on the trading date immediately prior to the date of exercise.
In the event that a person or group of affiliated or associated persons becomes an Acquiring Person, each holder of a Right will thereafter have the right to receive, upon exercise, common stock (or, in certain circumstances, other securities, cash or other assets of the Company) having a value equal to two (2) times the exercise price of the Right. Notwithstanding any of the foregoing, following the occurrence of a person becoming an Acquiring Person, all Rights that are, or (under certain circumstances specified in the Rights Agreement) were, Beneficially Owned by any Acquiring Person (or by certain related parties) will be null and void and any holder of such Rights (including any purported transferee or subsequent holder) will be unable to exercise or transfer any such Rights. However, Rights are not exercisable following the occurrence of a person becoming an Acquiring Person until the Distribution Date.
In the event that, after a person or a group of affiliated or associated persons has become an Acquiring Person, the Company is acquired in a merger or other business combination transaction, or 50% or more of the Company’s assets or earning power are sold, proper provision will be made so that each holder of a Right will thereafter have the right to receive, upon the exercise of a Right that number ofpayment. All shares of commoncapital stock of the personCompany shall be junior in rank to all shares of Series F Preferred Stock with whomrespect to the Company has engaged in the foregoing transaction (or its parent) that at the time of such transaction have a market value of two (2) times the exercise price of the Right.
At any time before any person or group of affiliated or associated persons becomes an Acquiring Person, the Board may redeem the Rights in whole, but not in part, at a price of $0.001 per Right (subjectpreferences as to certain adjustments) (the “Redemption Price”). The redemption of the Rights may be made effective at such time, on such basis and with such conditions as the Board in its sole discretion may establish. Immediatelypayments upon the action of the Board electing to redeem or exchange the Rights, the right to exercise the Rights will terminate and the only right of the holders of Rights will be to receive the Redemption Price.liquidation.
Conversion
The Board may, at its option, at any time after the first occurrence of a Flip-in Event (as defined in the Rights Agreement), exchange all or part of the then outstanding and exercisable Rights for shares of common stock at an exchange ratio of one share of common stock per Right, appropriately adjusted to reflect any stock split, stock dividend or similar transaction occurring after the effective date. However, the Board shall not effect such an exchange at any time after any person, together with all affiliates and associates of such person, becomes a beneficial owner of 50% or more of the outstanding shares of common stock. Immediately upon the action of the Board to exchange the Rights, the Rights will terminate and the only right of the holders of Rights will be to receive the number of shares of Common equal to the number of Rights held by such holder multiplied by the exchange ratio.
Until a RightSeries F Preferred Stock is exercised or exchanged, the holder thereof, as such, will have no rights as a stockholder of the Company, including, without limitation, the right to vote or to receive dividends.
The Board may amend or supplement the Rights Agreement without the approval of any holders of Rights at any time so long as the Rights are redeemable. At any time the Rights are no longer redeemable, no such supplement or amendment may (i) adversely affect the interests of the holders of Rights (other than an Acquiring Person or an affiliate or associate of an Acquiring Person), (ii) cause the Rights Agreement to become amendable other than in accordance with Section 27 of the Rights Agreement, or (iii) cause the Rights again to become redeemable.
The Company does not anticipate any material impact on the consolidated financial statements.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 8 – Equity, continued
Equity Transactions
On December 9, 2019, the Company enteredconvertible into that certain “Purchase Agreement” pursuant to which the Company agreed to sell an aggregate of 613,500 shares of Common Stock, 1,376,500 pre-funded warrants (the “Pre-funded Warrants”), Preferred ‘C’ warrants to purchase approximately 1,990,000 shares of Common Stock (the “Preferred ‘C’ Warrants”“Conversion Shares”). The initial conversion price, subject to adjustment as set forth in the Series F Certificate of Designation, was $2.255 (pre-split) (the “Conversion Price”). The Conversion Price can be adjusted as set forth in the Series F Certificate of Designation for stock dividends and Underwriter’s Warrantsstock splits or the occurrence of a fundamental transaction (generally including any reorganization, recapitalization or reclassification of the Common Stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of the outstanding Common Stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by the outstanding Common Stock). The Conversion Price is also subject to purchase approximately 159,200“full ratchet” price-based adjustment in the event of any issuances of Common Stock, or securities convertible, exercisable or exchangeable for Common Stock, at a price below the then-applicable Conversion Price (subject to certain exceptions). Following the Reverse Stock Split, the Conversion Price for the Preferred Shares was adjusted to $3.18 per share pursuant to the terms of the Certificate of Designations. If any shares of Series F Preferred Stock are converted or reacquired by us, such shares shall resume the status of authorized but unissued shares of Series F Preferred Stock of the Company and shall no longer be designated as Series F Preferred Stock.
The Company is required to redeem the shares of Series F Preferred Stock in 12 equal monthly installments, commencing on July 1, 2023. The amortization payments due upon such redemption are payable, at the Company’s election, in cash, or subject to certain limitations, in shares of Common Stock (the “Underwriter’s Warrants”). The combined purchase price for one sharevalued at the lower of Common Stock was $4.00(i) the Conversion Price then in effect and each Pre-funded Warrant was priced at $3.9999 with (the “Offering”). The Purchase Agreement contains customary representations, warranties, and covenants by(ii) the Company. Through the Offering, the Company raised proceedsgreater of $6,965,635, net of offering costs of $994,227. Offering costs were allocated on a pro rata basis to the proceeds from the sale of each(A) 80% of the Common Stock and the pre-funded warrants.
Each Pre-Funded Warrant has an initial exercise price of $0.0001 per share and is exercisable immediately after the date of issuance. Subject to limited exceptions, a holderaverage of the Pre-Funded Warrants will not have the right to exercise any portion of such securities if the holder, together with its affiliates, would beneficially own in excess of 4.99% of the number of sharesthree lowest closing prices of the Company’s Common Stock outstandingduring the thirty trading day period immediately afterprior to the exercise. date the amortization payment is due or (B) a “Floor Price” of $6.60 (subject to adjustment for stock splits, stock dividends, stock combinations, recapitalizations or other similar events) or, in any case, such lower amount as permitted, from time to time, by the Nasdaq Stock Market; provided that if the Floor Price is the lowest effective price, the Company will be required to make the amortization payment in cash.
| F-26 |
Exchange Cap
The exercise priceCompany was initially restricted from issuing shares of Common Stock upon conversion of the Pre-Funded Warrants,Series F Preferred Stock or exercise of the associated warrants in excess of 19.99% of the shares of Common Stock outstanding as of the date immediately prior to the issuance of the shares of Series F Preferred Stock and in some cases the numberassociated warrants (the “Issuable Maximum”) until the Company obtained stockholder approval for the issuance of shares of Common Stock issuable upon exercise of the Pre-Funded Warrants, will be subject to adjustment in the event of stock splits, stock dividends, combinations, rights offerings and similar events affecting the Common Stock. The pre-funded warrants represented prepaid equity forward contracts that were equity classified, as they were not subject to ASC 480 and did not meet the definition of a derivative under ASC 815 due to their requiring a substantial upfront payment.
Each Preferred ‘C’ Warrant has an initial exercise price of $4.00 per share, is exercisable immediately after the date of issuance and will expire five years from December 30, 2019, the date it became exercisable. Subject to limited exceptions, a holder of the Preferred ‘C’ Warrants will not have the right to exercise any portion of such securities if the holder, together with its affiliates, would beneficially own in excess of 4.99% of the number of shares of the Company’s Common Stock outstanding immediately after the exercise. The exercise price of the Preferred ‘C’ Warrants, and in some cases the number of shares of Common Stock issuable upon exercise of the Preferred ‘C’ Warrants, will be subject to adjustment in the event of stock splits, stock dividends, combinations, rights offerings and similar events affecting the Common Stock.
Each Underwriter’s Warrant has an initial exercise price of $5.00 per share, will be exercisable immediately after the date of issuance and will expire five years from December 30, 2019, the date it became exercisable. Subject to limited exceptions, a holder of the Underwriter’s Warrants will not have the right to exercise any portion of such securities if the holder, together with its affiliates, would beneficially own in excess of 4.99% of the number of shares of the Company’s Common Stock outstanding immediately after the exercise. The exercise price of the Underwriter’s Warrants, and in some cases the number of shares of Common Stock issuable upon exercise of the Underwriter’s Warrants, will be subject to adjustment in the event of stock splits, stock dividends, combinations, rights offerings and similar events affecting the Common Stock.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 8 – Equity, continued
Equity Transactions, continued
In addition, the Warrants provide that, in the event of a fundamental transaction (as such term is described in the Warrant), the holder of such Warrant, at the holder’s option, may receive, for each warrant share (as such term is described in the Warrant) that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration receivable as a result of such fundamental transaction by a holder of the number of shares of Common Stock for which the Warrant is exercisable immediately prior to such fundamental transaction. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a fundamental transaction, then the holder shall be given the same choice as to the alternate consideration it receives upon any exercise of the Warrant following such fundamental transaction.Issuable Maximum (“Stockholder Approval”). The Company shall cause any successor entity (as such term is described inreceived the Warrant),Stockholder Approval on July 31, 2023.
Optional Conversion
The Series F Preferred Stock can be converted at the option of the holder at any time and from time to deliver totime after the holder in exchange fororiginal issuance date. Holders shall effect conversions by providing us with the Warrant a securityform of conversion notice (the “Notice of Conversion”) specifying the successor entity evidenced by a written instrument substantially similar in form and substance to the Warrant which is exercisable for a corresponding number of shares of capitalSeries F Preferred Stock to be converted, the number of shares of Series F Preferred Stock owned subsequent to the conversion at issue and the date on which such conversion is to be effected, which date may not be prior to the date the applicable holder delivers by email such Notice of Conversion to us.
Mandatory Conversion
If on any day after the issuance of the shares of Series F Preferred Stock the closing price of the Common Stock has exceeded $202.95 (subject to adjustment for stock splits, stock dividends, stock combinations, recapitalizations or other similar events) for 20 consecutive trading days and the daily dollar trading volume of the Common Stock has exceeded $3,000,000 per trading day during the same period and certain equity conditions described in the Series F Certificate of Designation are satisfied (the “Mandatory Conversion Date”), we shall deliver written notice of the Mandatory Conversion (as defined below) to all holders on the Mandatory Conversion Date and, on such successor entity (or its parent entity) equivalentMandatory Conversion Date, we shall convert all of each holder’s shares of Series F Preferred Stock into Conversion Shares at the then effective Conversion Price (the “Mandatory Conversion”). If any of the Equity Conditions shall cease to be satisfied at any time on or after the Mandatory Conversion Date through and including the actual delivery of all of the Conversion Shares to the holders, the Mandatory Conversion shall be deemed withdrawn and void ab initio.
Beneficial Ownership Limitation
The Series F Preferred Stock cannot be converted to Common Stock if the holder and its affiliates would beneficially own more than 4.99% or 9.99% at the election of the holder of the outstanding Common Stock. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon notice to us, provided that any increase in this limitation will not be effective until 61 days after such notice from the holder to us and such increase or decrease will apply only to the holder providing such notice.
Common Stock
The holders of common shares are entitled to one vote per share at meetings of the Company.
On April 27, 2023, prefunded warrants were exercised in exchange for shares of common stock.
As of December 31, 2023, the Company had shares of Common Stock acquirableissued and receivable upon exercise of the Warrant (without regard to any limitations on the exercise of this Warrant) prior to such fundamental transaction, and with an exercise price which applies the exercise price hereunder to such shares of capital stock.
The Offering was made pursuant to a registration statement on Form S-1 (Files No. 333-234447 and 333-235359 previously filed with the Securities and Exchange Commission on November 1, 2019 and declared effective on December 5, 2019. Such securities are being offered only by means of a prospectus.
outstanding. During the year ended December 31, 2019, pursuant to his October 2018 employment agreement,2023 the Company issued 1,563 shares of common stock as installment conversions and shares of common stock for make-whole adjustments for the Series F Convertible Preferred.
| F-27 |
On February 16, 2022, prefunded warrants were exercised in exchange for shares of Common Stock under the 2017 Plan to Mr. Yeaton, with a fair value on the date of grant, of $27,367.Stock.
On April 8, 2020,August 17, 2022, pursuant to a securities purchase agreement with certain institutional and accredited investors, dated August 15, 2022, the Company issued and sold in a registered direct offering (the “April“August Offering”) an aggregate of 766,66747,059 shares of common stock of the Companyits Common Stock at an offering price of $6.00$ per share and 47,063 unregistered investor warrants to purchase up to shares of its Common Stock at an exercise price of $157.50, for gross and net proceeds of $4,600,002$5,999,997 and $4,086,207,$5,550,028, respectively.
In connection withCommon Stock Warrants
The table below summarizes the April Offering,warrant activity for the year ended December 31, 2023:
Summary of Warrant Activity
| Weighted | Average | |||||||||||||||
| Average | Remaining | Aggregate | ||||||||||||||
| Number of | Exercise | Contractual | Intrinsic | |||||||||||||
| Warrants | Price | Term (years) | Value | |||||||||||||
| Balance at December 31, 2022 | 217,202 | $ | 147.86 | $ | - | |||||||||||
| Issued | 4,716,904 | 3.18 | 21,650,589 | |||||||||||||
| Exercised | - | - | - | - | ||||||||||||
| Forfeited | - | - | - | - | ||||||||||||
| Canceled/Expired | (484 | ) | 5,414.40 | - | - | |||||||||||
| Balance at December 31, 2023 | 4,933,622 | $ | 9.02 | $ | 21,650,589 | |||||||||||
| Exercisable as of December 31, 2023 | 4,933,622 | $ | 9.02 | $ | 21,650,589 | |||||||||||
The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the closing stock price of $ for the Company’s common shares on December 31, 2023 and the closing stock price of $ for the Company’s common shares on December 31, 2022. All warrants were vested on date of grant.
On July 7, 2022, the Company issued to the placement agent or designees warrants to purchase up to 61,333 shares of its common stockCommon Stock at an exercise price of $7.50 (the “April Placement Agent Warrants”) in$164.40 to a private placement.vendor for services. These warrants had a grant date fair value of $ per warrant or a cumulative fair market value of $84,851 as calculated using Black-Scholes (exercise price $179.40 per share, stock price $ per share, volatility of %, discount rate of % and a five- year term). The April Placement Agent Warrantswarrants will be exercisable at any time and from time to time, in whole or in part, following the date of issuance and for a term of five years from the effective datedate. The fair-market value of the April Offering.
On May 18, 2020, pursuant to a securities purchase agreement with certain institutional and accredited investors,warrants was amortized over the life of the service contract. During the year ended December 31, 2022, the Company issued and soldrecognized $ in a registered direct offering (the “May Offering”) an aggregateexpense which is included in Stock-Based Compensation on the Consolidated Statement of 1,366,856 shares of its common stock at an offering price of $3.53 per share, for gross and net proceeds of $4,825,002 and $4,320,720, respectively.Comprehensive Loss.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
InOn August 17, 2022, in connection with the MayAugust Offering, the Company issued to the placement agent or designeesunregistered investor warrants to purchase up to 109,34847,063 shares of its common stockCommon Stock at an exercise price of $4.4125$157.50 (the “May Placement Agent“August Investor Warrants”) in a private placement. The May Placement AgentAugust Investor Warrants will be exercisable at any time and from time to time, in whole or in part, beginning six-months following the date of issuance and for a term of five years from the effective date ofinitial exercise date.
Pursuant to the May Offering.
On August 13, 2020, pursuant to a securities purchase agreement with certain institutional and accredited investors, dated August 11, 2020, the Company issued and sold in a registered direct offering (the “August Offering”) an aggregate of 1,207,744 shares of its common stock at an offering price of $5.67 per share, for gross and net proceeds of $6,847,908 and $6,158,034, respectively.
In connection with the AugustFebruary 2023 Offering, the Company issued to the placement agent or designees warrantsinvestors Warrants to purchase up to 96,6204,716,904 shares of its common stock atCommon Stock (as adjusted, and subject to further adjustment), with an exercise price of $7.0875 (the “August Placement Agent Warrants”)$3.18 per share (as adjusted, and subject to further adjustment), for a period of five years from the date of issuance. The Exercise Price and the number of shares issuable upon exercise of the Warrants are subject to customary adjustments for stock dividends, stock splits, reclassifications and the like, and subject to price-based adjustment, on a “full ratchet” basis, in the event of any issuances of Common Stock, or securities convertible, exercisable or exchangeable for Common Stock, at a private placement. The August Placement Agentprice below the then-applicable Exercise Price (subject to certain exceptions). Upon any such price-based adjustment to the Exercise Price, the number of shares issuable upon exercise of the Warrants will be exercisable at any time and from time to time, in whole or in part, followingincreased proportionately. (Note 3)
Pre-funded Common Stock Warrants
The table below summarizes the date of issuance andpre-funded warrant activity for a term of five years from the effective date of the August Offering.
On November 17, 2020, pursuant to the Private Placement SPA, the Company issued and sold in the Private Placement an aggregate of 8,725,393 shares of its common stock and 1,040,540 Pre-Funded Warrants at an offering price of $1.85 per share, for gross and net proceeds of $18,066,976 and $16,362,786, respectively.
In connection with the Private Placement, the Company issued Investor Warrants to purchase up to 9,765,933 shares of common stock at an exercise price of $2.06. The Investor Warrants are exercisable at any time and from time to time, in whole or in part, following the date of issuance and for a term of five and one-half years from the effective date of the Private Placement.
In connection with the Private Placement, the Company issued to the Placement Agent or designees the Placement Agent Warrants to purchase up to 390,368 shares of its common stock at an exercise price of $1.85 in a private placement. The Placement Agent Warrants are exercisable at any time and from time to time, in whole or in part, following the date of issuance and for a term of five and one-half years from the effective date of the Private Placement.
During the year ended December 31, 2020, 138,3612023:
Summary of Warrant Activity
| Weighted | Average | |||||||||||||||
| Average | Remaining | Aggregate | ||||||||||||||
| Number of | Exercise | Contractual | Intrinsic | |||||||||||||
| Warrants | Price | Term (years) | Value | |||||||||||||
| Balance at December 31, 2022 | 4,505 | $ | 0.06 | - | $ | 155,135 | ||||||||||
| Issued | - | - | - | - | ||||||||||||
| Exercised | (4,505 | ) | 0.06 | - | - | |||||||||||
| Forfeited | - | - | - | - | ||||||||||||
| Canceled/Expired | - | - | - | - | ||||||||||||
| Balance at December 31, 2023 | - | $ | - | - | $ | - | ||||||||||
| Exercisable as of December 31, 2023 | - | $ | - | - | $ | - | ||||||||||
All pre-funded warrants were vested on date of grant and are exercisable at any time. The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying award and the closing stock price of $ for the Company’s common shares of on December 31, 2022
| F-28 |
Series DC Convertible Preferred Stock were converted to 138,361 common shares. As of December 31, 2020, 72,992 shares of Series D Preferred Stock were issued and outstanding.Warrants
DuringThe table below summarizes the warrant activity for the year ended December 31, 2020, warrants to purchase an2023:
Summary of Warrant Activity
| Weighted | Average | |||||||||||||||
| Average | Remaining | Aggregate | ||||||||||||||
| Number of | Exercise | Contractual | Intrinsic | |||||||||||||
| Warrants | Price | Term (years) | Value | |||||||||||||
| Balance at December 31, 2022 | 918 | $ | 240.00 | $ | - | |||||||||||
| Granted | - | - | - | - | ||||||||||||
| Exercised | - | - | - | - | ||||||||||||
| Forfeited | - | - | - | - | ||||||||||||
| Canceled/Expired | - | - | - | - | ||||||||||||
| Balance at December 31, 2023 | 918 | $ | 240.00 | $ | - | |||||||||||
| Exercisable as of December 31, 2023 | 918 | $ | 240.00 | $ | - | |||||||||||
The aggregate intrinsic value is calculated as the difference between the exercise price of 1,935,000the underlying awards and the closing stock price of $ for the Company’s common shares on December 31, 2023 and the closing stock price of $ for the Company’s common shares on December 31, 2022. All Series C Convertible Preferred Stock Warrants were exercised at an exercise pricevested on date of $4.00 per share, yielding proceeds of $7,740,000 and immediately converted to 1,935,000 shares of common stock.grant.
Note 7 – Income Taxes
During the year ended December 31, 2020, Pre-Funded Warrant holders from the December 9, 2019 public offering exercised warrants for the purchase of 795,000 shares of Common Stock, with an exercise price of $0.0001 per common share, raising net proceeds of $80.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 9 – Income Taxes
The Company’s income tax (benefit)/provision is as follows:follows for the years ended December 31, 2023 and 2022:
Schedule of Income Tax (Benefit)/Provision
| Years Ended December 31, | ||||||||||||||||
| 2020 | 2019 | 2023 | 2022 | |||||||||||||
| Current | $ | - | $ | - | $ | - | $ | - | ||||||||
| Deferred | (1,958,000 | ) | (738,000 | ) | 4,129,000 | (5,914,000 | ) | |||||||||
| Change in Valuation Allowance | 1,958,000 | 738,000 | (4,129,000 | ) | 5,914,000 | |||||||||||
| Income Tax Benefit | $ | - | $ | - | $ | - | $ | - | ||||||||
The reconciliation of income taxes using the statutory U.S. income tax rate and the benefit from income taxes for the years ended December 31, 20202023 and 20192022 are as follows:
Schedule of Reconciliation of Income Tax Rate and Benefit from Income Taxes
| Years Ended December 31, | ||||||||||||||||
| 2020 | 2019 | 2023 | 2022 | |||||||||||||
| Statutory U.S. Federal Income Tax Rate | (21.0 | )% | (21.0 | )% | (21.0 | )% | (21.0 | )% | ||||||||
| New Jersey State income taxes, net of U.S. Federal tax effect | (5.1 | )% | (5.1 | )% | 45.5 | % | (14.5 | )% | ||||||||
| True-up for prior year deferred tax assets | 10.2 | % | 5.9 | % | ||||||||||||
| Adjustment to deferred tax assets | 82.8 | % | (4.1 | )% | ||||||||||||
| Other | 4.8 | % | 1.2 | % | - | % | 0.7 | % | ||||||||
| Change in Valuation Allowance | 11.1 | % | 19.0 | % | (107.3 | )% | 38.9 | % | ||||||||
| Net | 0.0 | % | 0.0 | % | 0.0 | % | 0.0 | % | ||||||||
As of December 31, 20202023, and 2019,2022, the Company had FederalU.S. federal net operating loss carry forwards of approximately $100,615,000$113.1 million and $79,678,000, expiring through$107.1 million, respectively. Approximately $51.5 million of the year ending December 31, 2037 forU.S. federal net operating losses originatingloss generated in tax years beginning before January 1, 2018. Net2018 expire beginning with the year ending December 31, 2024 through 2037. The remaining U.S. federal net operating losses recorded in tax years beginning January1, 2018 and after are allowed for an indefinite carryforward period butloss of approximately $61.6 million does not expire, however it is limited to 80%80% of each subsequent year’s net income. As of December 31, 20202023, and 2019,2022, the Company had New JerseyU.S. state net operating loss carry forwards of approximately $7,548,000$45.2 million and $28,855,000, expiring through$41.0 million, respectively, some of which expire beginning with the year ending December 31, 2040.2024 through 2043. U.S. federal net operating losses of approximately $2.3 million expired during 2023. The timing and manner in which the Company can utilize operating loss carryforwards in any year may be limited by provisions of the Internal Revenue Code regarding changes in ownership of corporations. Such limitation may have an impact on the ultimate realization of its carryforwards and future tax deductions.
Under Section 382 of the Code, use of ourthe Company’s net operating loss carryforwards (“NOLs”) will beis limited if we experiencethe Company experiences a cumulative change in ownership of greater than 50%50% in a moving three-year period. We will experienceThe Company experienced an ownership change as a result of the Merger and therefore ourthe Company’s ability to utilize our NOLsits net operating loss and certain credit carryforwards remaining at the Effective Time will beare limited. The limitation will beis determined by the fair market value of ourthe Company’s common stock outstanding immediately prior to the ownership change, multiplied by the applicable federal rate. It is expected that the Merger will imposecaused the Company’s net operating loss carryforwards to be limited. However, the limitation had no impact on the Company’s financial statements since the Company recorded a limitation on our NOLs.full valuation allowance for the deferred tax assets as of December 31, 2023 and 2022.
The principal components of the deferred tax assets and liabilities, and related valuation allowances as of December 31, 20202023 and 20192022 are as follows:
Schedule of Deferred Tax Assets and Related Valuation Allowances
| Years Ended December 31, | ||||||||||||||||
| 2020 | 2019 | 2023 | 2022 | |||||||||||||
| Reserves and other | $ | 148,000 | $ | 508,000 | $ | 796,000 | $ | 745,000 | ||||||||
| Net operating loss carry-forwards | 21,514,000 | 19,196,000 | 26,494,000 | 26,176,000 | ||||||||||||
| Capitalized research and development | 3,946,000 | 2,177,000 | ||||||||||||||
| Research and development tax credit | 455,000 | 455,000 | 1,326,000 | 610,000 | ||||||||||||
| Share-based compensation | 1,108,000 | 4,542,000 | ||||||||||||||
| Warrant liability | (2,860,000 | ) | - | |||||||||||||
| Derivative liability | (688,000 | ) | - | |||||||||||||
| Valuation Allowance | (22,117,000 | ) | (20,159,000 | ) | (30,122,000 | ) | (34,250,000 | ) | ||||||||
| Net | $ | - | $ | - | ||||||||||||
| Net deferred tax asset | $ | - | $ | - | ||||||||||||
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 9 - Income Tax Expense, continued
| F-29 |
The valuation allowance for deferred tax assets as of December 31, 2020 and 2019 was $22,117,000 and $20,159,000. The change in(decreased) by approximately $(4.1) million during the total valuation for the yearsyear ended December 31, 20202023, due mainly to write-offs of the gross deferred tax asset related to share-based compensation, net of increases in the Company’s deferred tax assets related to its net operating loss carryforward and 2019 werecapitalized research expenses. The valuation allowance for deferred tax assets increased by approximately $5.9 million during the year ended December 31, 2022, due mainly to increases of $1,958,000 and $738,000, respectively.in the Company’s deferred tax asset related to its net operating loss carryforward. In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will notmay be realized.
The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which the net operating losses and temporary differences become deductible. Management consideredconsiders projected future taxable income and tax planning strategies in making this assessment. Furthermore, during December 2019, the shares issued to investors in the capital raise resulted in a greater than 50% change in ownership under the Internal Revenue Service regulations. This change in ownership will result in limitations to the amount of net operating loss carryforwards that may be utilized in future years to offset future taxable income. The value of the deferred tax assets was fully offset by a valuation allowance, due to the current uncertainty of the future realization of the deferred tax assets.
The Company’s policy is to recordfor recording interest and penalties associated with unrecognized tax benefitsaudits is to record such items as additional income taxes ina component of general and administrative expense. There were no amounts accrued for penalties and interest for the Consolidated Statement of Comprehensive Loss. As of January 1, 2020, theyears ended December 31, 2023 and 2022. The Company had no unrecognized tax benefits and no charge during 2020, and accordingly, the Company diddoes not recognize any interest or penalties during 2020 related to unrecognized tax benefits. There is no accrual forexpect its uncertain tax positions asposition to change during the next twelve months. Management is currently unaware of December 31, 2020.any issues under review that could result in significant payments, accruals or material deviations from its position.
The Company files U.S. federal income tax returns and state income tax returns. The U.S.Since the Company had losses in the past, all prior years that generated net operating loss carryforwards are open and state income tax returns filed for the tax years ending on December 31, 2017 and thereafter are subject to audit examination byin relation to the relevant taxing authorities.net operating loss generated from those years.
Note 108 – Commitments and Contingencies
Scientific Advisory Board
On December 4, 2019,February 1, 2021, the Company formed an advisory board (the “Advisory Board”) with expertise in the hemp and minor cannabinoid sectors. TheScientific Advisory Board will assistto (i) provide strategic advice and make recommendations to management regarding current and planned research and development programs, (ii) advise management regarding the Boardscientific merit of Directorstechnology or products involved in itslicensing and acquisition opportunities and (iii) provide strategic review including, potentially, the extraction, testing, purificationadvice to management regarding emerging science and formulation of safe cannabinoids within the hemp industry. During December 2019, the Company appointed two members to the Advisory Board. Compensation over the term of service shall consist of an award of shares of the Company’s stock with a value of $25,000 for each advisor.technology issues and trends. During the years ended December 31, 20202023 and 2019,2022, the Company expensed $50,000incurred costs of $0 and $-$148,000, respectively, which isrespectively. These expenses are included in AdministrativeResearch and Development Expenses on the StatementsConsolidated Statement of Comprehensive Loss. The Scientific Advisory Board was disbanded as of December 31, 2020.effective September 30, 2022.
NASDAQ Capital Market Notice of Delisting or Failure to Satisfy a Continued Listing Rule or Standard
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 10 – Commitments and Contingencies, continued
COVID-19
The ultimate impact of the global COVID-19 pandemic or a similar health epidemic is highly uncertain and subject to future developments. These include but are not limited to the duration of the COVID-19 pandemic, new information which may emerge concerning the severity of the COVID-19 pandemic, and any additional preventative and protective actions that regulators, or the Company’s board of directors or management of the Company, may determine are needed. We do not yet know the full extent of potential delays or impacts on the Company’s business, vaccine development efforts, healthcare systems or the global economy as a whole. However, the effects are likely to have a material impact on the Company’s operations, liquidity and capital resources, and the Company will continue to monitor the COVID-19 situation closely.
In response to public health directives and orders, the Company has implemented work-from-home policies for many of the Company’s employees and temporarily modified the Company’s operations to comply with applicable social distancing recommendations. The effects of the orders and the Company’s related adjustments in its business are likely to negatively impact productivity, disrupt its business and delay the Company’s timelines, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on its ability to conduct its business in the ordinary course. Similar health directives and orders are affecting third parties with whom we do business, including Premas, whose operations are located in India. Further, restrictions on the Company’s ability to travel, stay-at-home orders and other similar restrictions on its business have limited its ability to support its operations.
Severe and/or long-term disruptions in the Company’s operations will negatively impact its business, operating results and financial condition in other ways, as well. Specifically, the Company anticipates that the stress of COVID-19 on healthcare systems generally around the globe will negatively impact regulatory authorities and the third parties that the Company and Premas may engage in connection with the development and testing of the Company’s COVID-19 Vaccine Candidate.
In addition, while the potential economic impact brought by, and the duration of, COVID-19 may be difficult to assess or predict, it has significantly disrupted global financial markets, and may limit the Company’s ability to access capital, which could in the future negatively affect its liquidity. A recession or market correction resulting from the continuation of the COVID-19 pandemic could materially affect the Company’s business and the value of its common stock.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 10 – Commitments and Contingencies, continued
Litigation and Settlements
Watts v. Gormally, et al., No. 2:18-15992 (D.N.J.) and Chan v. Gormally, et al., No. 2:19-cv-4989 (D.N.J.)
On November 9, 2018, Cale Watts (“Watts Plaintiff”) filed a verified shareholder derivative complaint alleging violations of the Securities Exchange Act of 1934, breach of fiduciary duty, unjust enrichment, and waste of corporate assets based on alleged material weaknesses in controls, management, and documentation (the “Watts Action”). On January 14, 2019, the parties reached an agreement in principle to settle the Watts Action that included corporate reforms and a payment of attorneys’ fees of $200,000. The parties finalized a Stipulation of Settlement on March 4, 2019. On February 7, 2019, Tiffany Chan, Jasmine Henderson, and Don Danesh (“Chan Plaintiffs”) filed a verified shareholder derivative complaint alleging violations of Section 14(a) of the Exchange Act and SEC Rule 14a-9, breach of fiduciary duty, unjust enrichment, and waste of corporate assets based on the same circumstances as the Watts Action (the “Chan Action”). The Chan Action further alleged that the Company should not have settled the Watts Action because the Watts Action plaintiffs lacked standing and the settlement would cause irreparable harm to the Company and its shareholders. On March 22, 2019, the Watts Plaintiff filed a motion for preliminary approval of the proposed settlement, approving the proposed form and method of providing notice of the settlement, scheduling a hearing for final approval of the settlement (“Watts Motion for Preliminary Approval”). On April 1, 2019, the Chan Plaintiffs filed an Opposition to the Motion for Preliminary Approval and a Motion to Intervene and Stay Proceedings (“Motion to Intervene and Stay”). Subsequently, the Watts Plaintiff, Chan Plaintiffs, and Defendants reached an agreement in principle to settle the Watts and Chan Actions that included corporate reforms and a payment of attorneys’ fees of $325,000. On October 2, 2019, the Watts Plaintiff filed an Unopposed Motion for Preliminary Approval of the Settlement (the “Omnibus Motion for Preliminary Approval”). The Omnibus Motion for Preliminary Approval was granted on January 8, 2020. Plaintiffs filed a motion for final approval of the proposed settlement by May 7, 2020. On May 28, 2020, the Court entered a final order and judgment approving the settlement. The resolution of this matter had no significant impact on the consolidated financial statements of the Company.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 10 – Commitments and Contingencies, continued
Litigation and Settlements, continued
NovoTek Therapeutics Inc. and NovoTek Pharmaceuticals Limited v. Akers Biosciences, Inc.
On June 21, 2019,11, 2023, the Company received a complaint, filed by Novotek Therapeutics Inc., and Novotek Pharmaceuticals Limited (collectively, “Novotek”), Beijing-based entities, inletter from the United States District Court for the District of New Jersey, alleging, among other things, breach of contract. Novotek is seeking, among other things, damages in the amount of $1,551,562, plus interest, disbursements and attorneys’ fees. The Company vigorously disputed the allegations in the complaint and has retained counsel to defend it. On September 16, 2019, the Company filed a partial motion to dismiss the complaint, which was fully submitted as of November 4, 2019. On June 9, 2020, the Court denied the Company’s motion. In anticipationListing Qualifications Department of the case being settled, on October 20, 2020,Nasdaq Stock Market (“Nasdaq”) indicating that, based upon the Court administratively closed the case. On November 13, 2020, the parties entered into a settlement agreement without either party admitting liability, effective as of November 3, 2020. The settlement agreement requires the Company to make a lump sum payment of $1,350,000 to Novotek within 60 days. The Company disbursed the settlement funds on December 31, 2020. The settlement expense is included in Loss from Discontinued Operations on the Consolidated Statements of Comprehensive Loss for the year ended December 31, 2020.
Neelima Varma v. Akers Biosciences, Inc. and St. David’s Healthcare Partnership, L.P., LLP CAUSE NO: D-1-GN-19-004262
On July 25, 2019, the Company was notified that on July 23, 2019, a complaint was filed by Neelima Varma, against the Company and St. David’s Healthcare Partnership, L.P., LLP (“St. David’s”), in the district court of Travis County, Texas, alleging, among other things, negligence gross negligence and strict product liability, breach of express warranty, breach of implied warranty and fraudulent misrepresentation and omission with respect to a medical device which the Company had sold through one of its distributors to St. David’s. Mr. Varna was seeking aggregate monetary relief from the company and St. David’s in excess of $1,000,000. The Company carries product liability insurance. On July 29, 2020, this matter was resolved. The resolution of this matter had no significant impact on the consolidated financial statements of the Company.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 10 – Commitments and Contingencies, continued
Litigation and Settlements, continued
Douglas Carrara v. Akers Biosciences, Inc., John Does 1-10, and XYZ Corp. 1-10, Docket No. ESX-L-5272-19 (N.J. Super. Ct., Essex County):
Douglas Carrara, a former executive, sued the Company for breach of contract in connections with the termination of his employment. In his operative Complaint, filed August 9, 2019, Carrara primarily alleged that the Company breached the terms of his employment by failing to pay “severance” after terminating his employment “without cause.” Based on this alleged breach, Carrara sought compensatory damages and damages for lost wages and benefits. Carrara also sought punitive and/or liquidated damages and attorney’s fees. On August 29, 2019, the Company filed an answer to the operative complaint, denying all substantive allegations of wrongdoing. As of July 23, 2020, the parties have resolved all material disputes. The parties are in the process of preparing the appropriate documentation to effectuate this resolution and expect to file a stipulation of dismissal with prejudice shortly. The resolution of this matter had no significant impact on the consolidated financial statements of the Company.
ChubeWorkx Settlement Agreement and General Release
On August 3, 2020, the Company entered into a Settlement Agreement and General Release (the “SAGR”) with ChubeWorkx. The Company and ChubeWorkx entered into the SAGR to terminate a prior Settlement Agreement, dated August 17, 2016, by and among the Company and ChubeWorkx, (the “Prior Settlement Agreement” and, collectively with all other contracts, agreements and understandings by and between the Company and ChubeWorkx, whether written or oral, the “Prior Agreements”) pursuant to which the Company granted ChubeWorkx a security interest in substantially all of the Company’s assets, and to fully and finally settle and compromise any and all current and future claims and liabilities of any nature arising between the Company and ChubeWorkx in relation to, or otherwise connected with, the Prior Agreements, on the terms set forth in the SAGR.
As consideration for the settlement of claims pursuant to the SAGR, on August 5, 2020, the Company (i) paid to ChubeWorkx an amount equal to $300,000 and (ii) delivered to ChubeWorkx 500,000 sharesclosing bid price of the Company’s common stock (the “Shares”)for the 30 consecutive business days between August 29, 2023, to October 10, 2023, the Company did not meet the minimum bid price of $1.00 per share required for continued listing on The Nasdaq Capital Market pursuant to Nasdaq Listing Rule 5550(a)(2). The letter also indicated that the Company will be provided with a fair market valuecompliance period of $2,510,000. Accordingly,180 calendar days, or until April 8, 2024 (the “Compliance Period”), in which to regain compliance pursuant to Nasdaq Listing Rule 5810(c)(3)(A).
Effective as of 4:05 p.m. Eastern Standard Time on February 14, 2024, we effected the Reverse Stock Split of our common stock at a ratio of one-for-thirty. Simultaneously with the Reverse Stock Split, number of shares of our common stock authorized for the year ended December 31, 2020, litigation settlement expense of $2,810,000issuance was recorded in Discontinued Operationsreduced from shares to shares, and our authorized capital stock was reduced from shares to shares. Our common stock continued to be traded on the Consolidated Statements of Comprehensive Loss.Nasdaq Capital Market under the symbol MyMD and began trading on a split-adjusted basis at market open on February 15, 2024. On March 4, 2024, we were notified by Nasdaq that we had regained compliance with all Nasdaq listing requirements and the matter was closed.
The Company granted ChubeWorkx registration rights with respect to the Shares. The CompanyLitigation and Settlements
Raymond Akers Actions
On April 14, 2021, Raymond F. Akers, Jr., Ph.D. filed a registration statement on Form S-3 with the Securities and Exchange Commission on August 18, 2020, which was declared effected on September 8, 2020, for the resale of such Shares.
As of the September 8, 2020 (the “Release Date”), the Company delivered and completed the full transfer to ChubeWorkx of the Shares in accordance with the SAGR, and, therefore, any and all claims, differences, and disputes of any current and/or future claims and/or liabilities arising between the Company and ChubeWorkx in relation to, or otherwise connected with, the Prior Agreements were fully and finally settled and compromised (with the exception of any claims arising under the SAGR or the Leak-Out and Support Agreement as described below). As of the Release Date, each of the Prior Agreements was terminated, and ChubeWorkx will automatically and irrevocably released all security interests and liens created under the Security Agreement or otherwise as security for the Company obligations under the Prior Agreements.
Litigation Related to the Merger with MYMD
Between January 22, 2021 and February 10, 2021, five alleged Akers stockholders filed separate actions in the state and federal courts of New York and New Jerseylawsuit against Akers and the members of its board of directors, respectively captioned as follows: (i) Douglas McClain v.MyMD Pharmaceuticals, Inc. (p/k/a Akers Biosciences, Inc., et al., No. 650497/2021 (Sup. Ct., N.Y. Cty.); (ii) Owen Murphy v. Akers Biosciences, Inc., et al., No. 650545/2021 (Sup. Ct., N.Y. Cty.); Sue Gee Cheng v. Akers Biosciences, Inc., et al., No. 1:21-cv-01110 (S.D.N.Y.); Danny Lui v. Akers Biosciences, Inc., et al., No. GLO-C-000006-21 (N.J. Super. Ct., Ch. Div.); and Alan Misenheimer v. Akers Biosciences, Inc., et al., No. 1:21-cv-02310 (D.N.J.) (collectively, the “MYMD Merger Complaints”). The McClain and Lui actions are styled as putative class actions brought on behalf of the plaintiff and other similarly situated stockholders, while the Murphy, Cheng, and Misenheimer actions are brought solely on behalf of the individual stockholders. The MYMD Merger Complaints generally assert that Akers and its board of directors failed to disclose allegedly material information in the joint proxySuperior Court of New Jersey, Law Division, Gloucester County (the “First Raymond Akers Action”). Mr. Akers asserts one common law whistleblower retaliation claim against the Company.
On September 23, 2021, the Court granted MyMD Pharmaceutical, Inc.’s (“MyMD’s”) Motion to Dismiss Plaintiff’s Amended Complaint and consent solicitation statement/prospectus and seek an order enjoining dismissed Plaintiff’s Amended Complaint. The Court indicated that Mr. Akers is “free to file another complaint, however, tort-based ‘Pierce’ allegations, and/or unwindingCEPA claims are barred by the consummationstatute of limitations.”
On March 1, 2022, Mr. Akers filed a second action against MyMD in the Merger Agreement and awarding damages.Superior Court of New Jersey, Law Division, Gloucester County (the “Second Raymond Akers Action”) again asserting one common law whistleblower retaliation claim against the Company. The defendants believeCompany believes that the claims asserted in the MYMD Merger Complaints areSecond Raymond Akers Action is without merit and, intend to appropriately defend themselvesmoreover, was filed against them. Accordingly, the CompanyCourt’s specific admonition that Plaintiff does not expectattempt to circumvent the statute of limitations.
On May 27, 2022, the Court granted-in-part and denied-in-part MyMD’s Motion to Dismiss Plaintiff’s Complaint. The Court reaffirmed the ruling in the First Raymond Akers Action that theseany tort-based Pierce claims will have a material adverse effect onare time-barred. However, the Court denied the Motion as it pertained to Plaintiff’s contract-based Pierce claim and “Repayment of Monies Owed” claim. On July 29, 2022, MyMD filed its financial condition or resultsAnswer, which included affirmative defenses. As of operations.December 31, 2023, the Second Raymond Akers Action is in the discovery phase.
All legal fees incurred were expensed as and when incurred.
AKERS BIOSCIENCES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| F-30 |
Note 119 – Related Parties
Interim CFOSRQ Patent Holdings and SRQ Patent Holdings II
EffectiveMyMD is a party to two Amended and Restated Confirmatory Patent Assignment and Royalty Agreements, both dated November 11, 2020, with SRQ Patent Holdings and SRQ Patent Holdings II, under which MyMD (or its successor) will be obligated to pay to SRQ Patent Holdings or SRQ Patent Holdings II (or its designees) certain royalties on October 5, 2018 and through December 31, 2019,product sales or other revenue received on products that incorporate or are covered by the Board appointed Howard R. Yeaton,intellectual property that was assigned to serve as the Chief Executive Officer and interim Chief Financial OfficerMyMD. The royalty is equal to 8% of the Company. Effectivenet sales price on January 1, 2020,product sales and, without duplication, 8% of milestone revenue or sublicense compensation. SRQ Patent Holdings and SRQ Patent Holdings II are affiliates of Mr. Yeaton entered into a new agreement with the Company whereby he served as the Company’s Interim Chief Financial Officer. PursuantJonnie Williams, Sr. No revenue has been received subject to a mutual understanding between the Company and Mr. Yeaton, Mr. Yeaton’s employment as Interim Chief Financial Officer ceased as of August 19, 2020. During his service as the Company’s Interim Chief Financial Officer Mr. Yeaton was the managing principal of Financial Consulting Strategies (“FCS”), and the Company had an ongoing relationship with FCS with FCS continuing to provide accounting services to the Company,these agreements as of December 31, 2020. As2023 and 2022.
MIRA Pharmaceuticals Limited License Agreement
MyMD is a party to an Amended and Restated Limited License Agreement, dated June 27, 2022 and amended on April 20, 2023, with MIRA Pharmaceuticals, Inc. (Nasdaq: MIRA), under which the parties agreed to share technical information and know-how pertaining to the synthetic manufacture and formulation of December 31, 2020, FCSthe parties’ respective Supera-CBD™ and MIRA1a™ product candidates. MyMD, which holds patent rights to MIRA1a™ in 22 foreign countries, was consideredgranted a perpetual, non-exclusive, royalty-free license to beuse improvements to MIRA1a™ made under the agreement, and MIRA was granted a related party. During the year ended December 31, 2020 and 2019, the Company incurred costs of $14,500 and $38,888, respectively with FCS in connection with these services. As of December 31, 2020, and December 31, 2019 the Company had an obligationlimited, perpetual, worldwide, non-exclusive, royalty-free license to FCSuse Supera-CBD™ as a synthetic intermediate in the amountsmanufacture of $0MIRA1a™. MyMD’s President and $18,323, respectively, for these services whichChief Medical Officer, Chris Chapman, M.D., is included in trade and other payables in the Consolidated Balance Sheets.
AsExecutive Chairman of December 31, 2020, included in accounts payable and accrued expenses was an obligation of $3,173, representing an obligation to issue 471 shares of common stock to Mr. Yeaton, earned during 2019, but not issued. The accrual is reflected in trade and other payables on the Consolidated Balance Sheet.MIRA
Taglich Brothers, Inc.
On November 23, 2020, the Companyretained Taglich Brothers, Inc. (“Taglich Brothers”) on a non-exclusive basis as a consultant to render consulting services, assist with review, and analysis of, financial planning and budgeting matters of the Company for a term of 12 months. Pursuant to the Consulting Agreement with Taglich Brothers, the Company agreed to pay Taglich Brothers $10,000 per month.
Mr. Schreiber is the managing director of capital markets at Taglich Brothers, and Mr. Schroeder is the vice president of investment banking at Taglich Brothers.
Note 1210 – Employee Benefit Plan
The Company maintains a defined contribution benefit plan under section 401(k) of the Internal Revenue Code covering substantially all qualified employees of the Company (the “401(k) Plan”). Under the 401(k) Plan, the Company matches 100%100% up to a 3%3% contribution, and 50%50% over a 3%3% contribution, up to a maximum of 5%5%.
During the years ended December 31, 2020 and 2019, theThe Company made matching contributions to the 401(k) Plan during the years ended December 31, 2023 and 2022 of $19,571$44,942 and $20,420,$41,443, respectively.
Note 11—Patent Assignment and Royalty Agreement
In November 2016, the Company entered into an agreement with the holders of certain intellectual property relating to the Company’s current product candidate. Under the terms of the agreement, the counterparty assigned its rights and interest in certain patents to the Company in exchange for future royalty payments based on a fixed percentage of future revenues, as defined. The agreement is effective until the later of (1) the date of expiration of the assigned patents or (2) the date of expiration of the last strategic partnership or licensing agreement including the assigned patents. No revenue has been received subject to these agreements as of December 31, 2023 and 2022.
Note 1312 – Subsequent Events
On February 11, 2021,March 4, 2024 (the “Effective Date”), MyMD Pharmaceuticals, Inc., a subscriberNew Jersey corporation (“MyMD New Jersey” or, prior to the November 17, 2020 Private Placement directedReincorporation (as defined below), the “Company”) merged with and into its wholly-owned subsidiary, MyMD Pharmaceuticals, Inc., a Delaware corporation (“MyMD Delaware” or, following the Reincorporation, the “Company”), with MyMD Delaware being the surviving corporation, pursuant to that certain Agreement and Plan of Merger, dated as of March 4, 2024, by and between MyMD New Jersey and MyMD Delaware (the “Plan of Merger”), for the purpose of changing the Company’s transfer agentstate of incorporation from New Jersey to cancel 932,432 common shares purchasedDelaware (the “Reincorporation”). The Plan of Merger and issue 932,432 pre-funded warrants pursuantthe Reincorporation were approved by the Company’s stockholders at the 2023 annual meeting of stockholders, held on July 31, 2023 (the “2023 Annual Meeting”).
MyMD Delaware is deemed to be the termssuccessor issuer of MyMD New Jersey under Rule 12g-3 of the securities purchase agreement dated November 11, 2020. As aSecurities Exchange Act of 1934, as amended.
The Reincorporation did not result in any change in the Company’s name, business, management, fiscal year, accounting, location of this transaction, Akers’the principal executive offices, assets or liabilities. In addition, the Company’s common shares issuedstock will retain the same CUSIP number and outstanding as of February 26, 2021 was 16,652,829. The conversion had no significant impactcontinue to trade on the consolidated financial statementsNasdaq Capital Market under the symbol “MYMD.” Holders of shares of the Company.Company’s common stock will not have to exchange their existing Company stock certificates for MyMD Delaware stock certificates.
As of the Effective Date of the Reincorporation, the rights of the Company’s stockholders are governed by the Delaware General Corporation Law, the MyMD Delaware Certificate of Incorporation and the Bylaws of MyMD Delaware.