
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 20212023
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from: _____________to______________
Commission File Number: 001-39199

TRxADE HEALTH, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 46-3673928 | |
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification No.) |
| 2420 Brunello | ||
| Lutz, Florida | 33558 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (800) 261-0281
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
Common Stock, $0.00001 Par Value Per Share | MEDS | The NASDAQ Stock Market LLC (The NASDAQ Capital Market) |
Securities registered pursuant to Section 12(g) of the Act:
None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☒ | Smaller reporting company ☒ |
| Emerging growth company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No No☒ ☒
The aggregate market value of the voting and non-voting common stock held by non-affiliates of the registrant as of the last business day of the registrant’s most recently completed second fiscal quarter was approximately $16,921,89011,457,013. For purposes of calculating the aggregate market value of shares held by non-affiliates, we have assumed that all outstanding shares are held by non-affiliates, except for shares held by each of our executive officers, directors and 5% or greater stockholders. In the case of 5% or greater stockholders, we have not deemed such stockholders to be affiliates unless there are facts and circumstances which would indicate that such stockholders exercise any control over our company, or unless they hold 10% or more of our outstanding common stock. These assumptions should not be deemed to constitute an admission that all executive officers, directors and 5% or greater stockholders are, in fact, affiliates of our company, or that there are no other persons who may be deemed to be affiliates of our company. Further information concerning shareholdings of our officers, directors and principal stockholders is included or incorporated by reference in Part III, Item 12 of this Annual Report on Form 10-K.
As of March 28, 2022,April 22, 2024, there were 1,406,348 shares of common stock issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement relating to its 20222024 annual meeting of stockholders (the “2022“2024 Proxy Statement”Statement”) are incorporated by reference into Part III of this Annual Report on Form 10-K where indicated. The 20222024 Proxy Statement will be filed with the U.S. Securities and Exchange Commission within 120 days after the end of the fiscal year to which this report relates.
TABLE OF CONTENTS
| 2 |
GLOSSARY
The following are abbreviations and definitions of certain terms used in this Report, which are commonly used in the pharmaceutical industry:
“ACA” means the Patient Protection and Affordable Care Act, often shortened to the Affordable Care Act, nicknamed Obamacare, which is a U.S. federal statute which provides numerous rights and protections that make health coverage fairer and easier to understand, along with subsidies (through “premium tax credits” and “cost-sharing reductions”) to make it more affordable. The law also expands the Medicaid program to cover more people with low incomes.
“ADR” means Authorized Distributor of Record. Under current federal law, an ADR means a distributor with whom a manufacturer has established an ongoing relationship to distribute such manufacturer’s products.
“ANDA” means an abbreviated new drug application which contains data which is submitted to the FDA for the review and potential approval of a generic drug product.
“CMS” means the Centers for Medicare & Medicaid Services, which is a federal agency within the HHS that administers the Medicare program and works in partnership with state governments to administer Medicaid.
“CSA” means the Controlled Substances Act, the statute establishing federal U.S. drug policy under which the manufacture, importation, possession, use, and distribution of certain substances is regulated.
“DEA” means the Drug Enforcement Administration, a United States federal law enforcement agency under the United States Department of Justice, tasked with combating drug trafficking and distribution within the United States.
“DQSA” means the Drug Quality and Security Act which is a law that amended the FFDCA to grant the FDA more authority to regulate and monitor the manufacturing of compounded drugs.
“EUA” means an Emergency Use Authorization filed with the FDA. Under section 564 of the FFDCA, the FDA Commissioner may allow unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or when there are no adequate, approved, and available alternatives.
“FDA” means U.S. The Food and Drug Administration, which is a federal agency of the United States Department of Health and Human Services. The FDA is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and by ensuring the safety of U.S. food supply, cosmetics, and products that emit radiation.
“FDAAA” means the Food and Drug Administration Amendments Act of 2007 which reviewed, expanded, and reaffirmed several existing pieces of legislation regulating the FDA.
“FFDCA” means the Federal Food, Drug and Cosmetic Act, which is a set of U.S. laws passed by Congress in 1938 giving authority to the FDA to oversee the safety of food, drugs, medical devices, and cosmetics.
“Generic drugs” are copies of brand-name drugs that have exactly the same dosage, intended use, effects, side effects, route of administration, risks, safety, and strength as the original drug.
“Health plan” means health insurance coverage provided by an individual or group that provides or pays the cost of medical care. Health plans can be provided by public (Medicaid) or private (an employer) entities.
“HHS”, the U.S. Department of Health and Human Services also known as the Health Department, is a cabinet-level department of the U.S. federal government with the goal of protecting the health of all Americans and providing essential human services.
| 3 |
“HIPAA” means the Health Insurance Portability and Accountability Act of 1996, which has the goal of making it easier for people to keep health insurance, protect the confidentiality and security of healthcare information and help the healthcare industry control administrative costs.
“Individually identifiable health information” is defined by HIPPA to mean information that is a subset of health information, including demographic information collected from an individual, and: (1) is created or received by a health care provider, health plan, employer, or health care clearinghouse; and (2) relates to the past, present, or future physical or mental health or condition of an individual; the provision of health care to an individual; or the past, present, or future payment for the provision of health care to an individual; and (a) that identifies the individual; or (b) with respect to which there is reasonable basis to believe the information can be used to identify the individual.
“Medicaid” is a federal and state health insurance program in the U.S. that helps with medical costs for some people with limited income and resources. Medicaid also offers benefits not normally covered by Medicare, including nursing home care and personal care services.
“Medicare” is a national health insurance program in the U.S. It primarily provides health insurance for Americans aged 65 and older, but also for some younger people with disability status as determined by the Social Security Administration, as well as people with end stage renal disease and amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease).
“NDC” means a National Drug Code, a unique 10-digit, 3-segment number. It is a universal product identifier for human drugs in the United States. The code is present on all non-prescription (OTC) and prescription medication packages and inserts in the U.S. The 3 segments of the NDC identify the labeler, the product, and the commercial package size.
“PBM” means a Pharmacy Benefits Manager. In the United States, a PBM is a third-party administrator of prescription drug programs for commercial health plans, self-insured employer plans, Medicare Part D plans (prescription drug plans), the Federal Employees Health Benefits Program, and state government employee plans.
“PDMA” means the Prescription Drug Marketing Act of 1987. The PDMA establishes legal safeguards for prescription drug distribution to ensure safe and effective pharmaceuticals and is designed to discourage the sale of counterfeit, adulterated, misbranded, subpotent, and expired prescription drugs.
“Pedigree tracking laws” mean laws which help ensure the integrity of the U.S. drug supply chain through the use of drug pedigrees, verifiable written or electronic documents that track each move in a drug’s journey from manufacturer to patient.
“PPE” means personal protective equipment, which is worn to minimize exposure to hazards that cause serious workplace injuries and illnesses. When used below, PPE typically refers to protective equipment used by medical personnel, including masks, sanitizers and gloves.
“Rebates” these are provided by manufacturers and are typically based on the ability of a payer to move market share for the manufacturer’s product. Rebates are confidential.
“SNI” means Serialized Numerical Identifier. Pursuant to FDA requirements, a product’s SNI has to include the item’s NDC and unique Serial Number (SN).
“Wholesaler” typically, the wholesaler is the first purchaser of a drug product – direct from the manufacturer. Wholesalers buy large quantities and then resell either direct to provider-purchasers (like a large health system, pharmacy or pharmacy chain), or resell to smaller, regional distributors for regional or local distribution to retail pharmacies and hospitals.
| 4 |
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING INFORMATION
This Annual Report on Form 10-K (this “Report”) contains statements that constitute forward-looking statements withinwhich are subject to the meaningsafe-harbor provisions of the federal securities laws, including the Private Securities Litigation Reform Act of 1995. Statements that are not historical are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Some of the statements in this Annual Report constitute forward-looking statements because they relate to future events or our future performance or future financial condition. These forward-looking statements are not historical facts, but rather are based on current expectations, estimates and projections about our company, our industry, our beliefs and our assumptions. Our forward-looking statements include, but are not limited to, statements regarding our or our management team’s expectations, hopes, beliefs, intentions or strategies regarding the future. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. In some cases, you can identify forward-looking statements by the following words: “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” or the negative of these terms or other comparable terminology, although not allsimilar expressions may identify forward-looking statements, containbut the absence of these words. Forward-looking statements arewords does not mean that a guarantee of future performance or results, and willstatement is not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. Forward-looking statements are based on information available at the time the statements are made and involve known and unknown risks, uncertainties and other factors that may cause our results, levels of activity, performance or achievements to be materially different from the information expressed or implied by the forward-looking statements in this Report.forward-looking. These factors include those set forth below and those disclosed under “Risk Factors”, below. These factorsForward-looking statements in this Annual Report may include, but are not limited to:for example, statements about:
| ● | Risks related to our history of operating losses and that our operations may not | |
| ● | Claims relating to alleged violations of intellectual property rights of others; | |
| ● | Technical problems with our websites; | |
| ● | Risks relating to implementing our acquisition | |
| ● | Our ability to manage our growth; | |
| ● | Negative effects on our operations associated with the opioid pain medication health crisis; | |
| ● | Regulatory and licensing requirement risks; | |
| ● | Risks related to changes in the U.S. healthcare environment; | |
| ● | The status of our information systems, facilities and distribution networks; | |
| ● | Risks associated with the operations of our more established competitors; | |
| ● | Regulatory changes; | |
| ● | Healthcare fraud; |
| 5 |
| ● | ||
| ● | Changes in laws or regulations relating to our operations; | |
| ● | Privacy laws; | |
| ● | System errors; | |
| ● | Dependence on current management; | |
| ● | Our growth strategy; and | |
| ● | Other risks disclosed below under, and incorporated by reference in, |
You should read the matters described and incorporated by reference in “Risk Factors” and the other cautionaryThe forward-looking statements madecontained in this Annual Report are based on our current expectations and incorporated by reference herein, as being applicable to all relatedbeliefs concerning future developments and their potential effects on us. There can be no assurance that future developments affecting us will be those that we have anticipated. These forward-looking statements wherever they appear in this Report. We cannot assure youinvolve a number of risks, uncertainties (some of which are beyond our control) or other assumptions that the forward-looking statements in this Report will provemay cause actual results or performance to be accurate and therefore prospective investors are encouraged not to place undue reliance onmaterially different from those expressed or implied by these forward-looking statements.
Forward-lookingThese risks and uncertainties include, but are not limited to, those factors described under the section of this Annual Report entitled “Risk Factors”. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. We undertake no obligation to update or revise any forward-looking statements, speak onlywhether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
We use words such as “anticipates,” “believes,” “expects,” “intends,” “seeks,” “plans,” “estimates,” “targets” and similar expressions to identify forward-looking statements. The forward-looking statements contained in this Annual Report involve risks and uncertainties. Our actual results could differ materially from those implied or expressed in the forward-looking statements for any reason, including the factors set forth in “Part I — Item 1A. Risk Factors” in this Annual Report.
Although we believe that the assumptions on which these forward-looking statements are based are reasonable, any of those assumptions could prove to be inaccurate, and as a result, the forward-looking statements based on those assumptions also could be inaccurate. In light of these and other uncertainties, the inclusion of a projection or forward-looking statements in this Annual Report should not be regarded as a representation by us that our plans and objectives will be achieved.
We have based the forward-looking statements included in this Annual Report on information available to us on the date of this Annual Report, or the date of any document incorporated by reference in this Report, as applicable. Except to the extent required by applicable law or regulation,and we do not undertake anyassume no obligation to update any such forward-looking statements. Although we undertake no obligation to revise or update any forward-looking statements to reflectin this Annual Report, whether as a result of new information, future events or circumstances afterotherwise, you are advised to consult any additional disclosures that we may make directly to you or through reports that we may file in the date of this Report or to reflectfuture with the occurrence of unanticipated events.Securities and Exchange Commission (the “SEC”), including Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K.
PART I
| ITEM 1. | BUSINESS |
INTRODUCTION
This information included in this Annual Report on Form 10-K should be read in conjunction with the consolidated financial statements and related notes in “Item 8. Financial Statements and Supplemental Data” of this Report.
Please see the “Glossary” above for a list of abbreviations and definitions used throughout this Report.
Our logo and some of our trademarks and tradenames are used in this Report. This Report may also includesinclude trademarks, tradenames and service marks that are the property of others. Solely for convenience, trademarks, tradenames and service marks referred to in this Report may appear without the ®, ™ and SM symbols. References to our trademarks, tradenames and service marks are not intended to indicate in any way that we will not assert to the fullest extent under applicable law our rights or the rights of the applicable licensors if any, nor that respective owners to other intellectual property rights will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
The market data and certain other statistical information used throughout this Report are based on independent industry publications, reports by market research firms or other independent sources that we believe to be reliable sources. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We are responsible for all of the disclosures contained in this Report, and we believe these industry publications and third-party research, surveys and studies are reliable. While we are not aware of any misstatements regarding any third-party information presented in this Report, their estimates, in particular, as they relate to projections, involve numerous assumptions, are subject to risks and uncertainties, and are subject to change based on various factors, including those discussed under the section entitled “Risk Factors” beginning on page 1917 of this Report. These and other factors could cause our future performance to differ materially from our assumptions and estimates. Some market and other data included herein, as well as the data of competitors as they relate to TRxADE HEALTH, INC., is also based on our good faith estimates.
Our fiscal year ends on December 31st. Interim results are presented on a quarterly basis for the quarters ended March 31st, June 30th, and September 30th, the first quarter, second quarter and third quarter, respectively, with the quarter ending December 31st being referenced herein as our fourth quarter. “Fiscal 2023” means the Fiscal 2021year ended December 31, 2023, whereas “Fiscal 2022” means the year ended December 31, 2021, whereas fiscal 2020 means the year ended December 31, 2020.2022.
Unless the context requires otherwise, references to the “Company,” “we,” “us,” “our,” “Trxade”, “Trxade Group” and “TRxADE HEALTH, INC.” refer specifically to TRxADE HEALTH, INC. and its consolidated subsidiaries.
In addition, unless the context otherwise requires and for the purposes of this Report only:
| ● | “Exchange Act” refers to the Securities Exchange Act of 1934, as amended; | |
| ● | “SEC” or the “Commission” refers to the United States Securities and Exchange Commission; and | |
| ● | “Securities Act” refers to the Securities Act of 1933, as amended. |
Where You Can Find OtherAvailable Information
We file annual, quarterly, and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov and are available for download, free of charge, soon after such reports are filed with or furnished to the SEC, on the “NASDAQ: MEDS,” “SEC Filings” page of our website at www.rx.trxade.comwww.trxadehealth.com. Copies of documents filed by us with the SEC are also available from us without charge, upon oral or written request to our Secretary, who can be contacted at the address and telephone number set forth on the cover page of this Report. Our website addresses arewww.trxadehealth.comwww.rx.trxade.com www.trxadegroup.com, www.rx.trxade.com, www.bonumhealth.com, www.comsprx.com, and www.rxintegra.com. Information on our websites is not incorporated by reference into this Form 10-K. The information on, or that may be accessed through, our website iswebsites not incorporated by reference into this Report and should not be considered a part of this Report.
CORPORATE AND ORGANIZATIONAL HISTORY
Background of XCEL
Our Company was incorporated in Delaware on July 15, 2005, as “Bluebird Exploration Company” (“Bluebird”). Bluebird was originally formed to engage in the exploitation of mineral properties. In December 2008, Bluebird changed its name to “Xcellink International, Inc.” (“XCEL”), and subsequently announced that its business plan was being expanded to include the development and marketing of platform-independent customer-centric payment systems and methodologies. XCEL was unable to raise the funds necessary to implement its business strategy, and never generated any revenue and was reporting as a “shell” corporation.revenue. On January 9, 2014, Trxade Group, Inc., a then privately held Nevada corporation, merged with and into XCEL, and XCEL changed its name to “Trxade Group, Inc.” On June 1, 2021, the Company changed its name from “Trxade Group, Inc” to “TRxADE HEALTH, INC.”
| 7 |
Background of Trxade
PharmaCycle LLC, a Nevada limited liability company (“PharmaCycle”), was formed in August 2010 by Prashant Patel, our President, to serve as a web-based market platform designed to enable trading among healthcare buyers and sellers of pharmaceuticals, accessories and services. In January 2013, PharmaCycle converted into a Florida corporation and changed its name to Trxade, Inc. (“Trxade Florida”). In May 2013, Trxade Florida created a new wholly-owned subsidiary, Trxade Group, Inc., a Nevada corporation (“Trxade Nevada”). Trxade Nevada acquired Trxade Florida pursuant to a reverse triangular merger, resulting in Trxade Florida becoming a wholly-owned subsidiary of Trxade Nevada (the “Nevada-Florida Merger”). The sole purpose of the Nevada-Florida Merger was to provide for a holding company to own Trxade Florida, the operating company. At all times, up to the Nevada-Florida Merger, Trxade Florida was capitalized exclusively by cash capital contributions from Messrs. Suren Ajjarapu and Patel, our Chief Executive Officer and President, respectively. Immediately following the Nevada-Florida Merger, Messrs. Ajjarapu and Patel collectively owned 99% of Trxade Nevada. After the Nevada-Florida Merger (but prior to the merger with XCEL), Trxade Nevada raised $670,000 through the sale of its preferred stock in private placements made to third party investors.
Reverse Merger with Trxade
On September 26, 2008, Mark Fingarson, the former President, sole Director and controlling shareholder of XCEL, sold 80,000,000 shares of XCEL (prior to the Merger Reverse Split and Reverse Stock SplitSplits (each discussed and defined below)) to XCEL’s then attorney, Ron McIntyre.. On November 22, 2013, Trxade Nevada acquired Mr. McIntyre’s controlling interest of 80,000,000 shares in XCEL pursuant to a Purchase and Sale Agreement dated November 7, 2013. At the time of the sale, XCEL had 104,160,000 shares of common stock issued and outstanding, including the 80,000,000 shares of stock acquired by Trxade Nevada (prior to the Merger Reverse Split and Reverse Stock SplitSplit(s) (each discussed and defined below)).
On December 16, 2013, Trxade Nevada and XCEL entered into a definitive merger agreement (the “Merger Agreement”) providing for the merger (the “Merger”) of Trxade Nevada with and into XCEL, with XCEL continuing as the surviving corporation. The Merger closed on January 8, 2014. Under the terms of the Merger Agreement, we amended our certificate of incorporation and changed our name to “Trxade Group, Inc.,” and changed our trading symbol to “TRXD”.
Recapitalization of Common Stock by a Reverse Split and Increase of Authorized Shares of Stock
We also reversed our issued and outstanding stock at the ratio of one for one thousand (1:1,000) shares effective upon the closing of the Merger (the “Merger Reverse Split”). In connection with the Merger Reverse Split, 104,160,000 outstanding shares of our common stock, including the 80,000,000 shares held by Trxade Nevada, were exchanged for 104,160 post-Merger Reverse Split shares of common stock. As a result of the Merger, Trxade Nevada stockholders holding 28,800,000 shares of common stock and 670,000 shares of Series A Preferred Stock converted their shares on a one-to-one basis into 28,800,000 shares of our common stock and 670,000 shares of our Series A Preferred Stock, for an aggregate total of 29,470,000 shares. Further, 100,000 shares of our common stock (on a post-Reverse Split basis and considering the Reverse Stock SplitSplit(s) (discussed below)) were issued following the Merger in connection with the conversion of our promissory notes. The 80,000,000 pre-Merger shares held by Trxade Nevada, which amounted to 13,334 shares (on a post-Reverse Split basis and taking into account the Reverse Stock Split)Split(s)), reverted to treasury stock of the Company. Except as otherwise disclosed, the share amounts in the paragraph above have not been adjusted for the Merger Reverse Split or the Reverse Stock Split.
February 2020 Reverse Stock Split and NASDAQ Capital Market Listing
On October 9, 2019, our Board of Directors, and on October 15, 2019, stockholders holdingIn February 2020, the Company effected a majority of our outstanding voting shares, approved resolutions authorizing a1-for-6 reverse stock split of the then outstanding shares of our common stock in the range from one-for-two (1-for-2) to one-for-ten (1-for-10), and provided authority to our Board of Directors to select the ratio of the reverse stock split in their discretion (the “Stockholder Authority”). On February 12, 2020, the Board of Directors of the Company approved a stock split ratio of 1-for-6 (“Reverse Stock Split”) in connection with the Stockholder Authority and the Company filed a Certificate of Amendment with the Secretary of Delaware to affect the Reverse Stock Split. The Reverse Stock Split became effective at 12:01 a.m. Eastern Standard Time on February 13, 2020. The Reverse Stock Split was completed in order to allow us to meet the initial listing criteria of The NASDAQ Capital Market.
Our common stock was approved for listing on The NASDAQ Capital Market under the symbol “MEDS”, on February 13, 2020.
| 8 |
June 2023 Reverse Stock Split.
In June 2023 the Company effected a 1-for-15 reverse stock split of its issued and outstanding common stock.
Subsidiaries
We own 100% of Trxade Inc. (a Florida corporation). This subsidiary is included in our attached consolidated financial statements and is engaged in the same line of business as Trxade. Trxade Inc. iswas the subsidiary through which we previously operated a web-based market platform that enablesto enable commerce among healthcare buyers and sellers of pharmaceuticals, accessories and services. On February 16, 2024, we entered into an asset purchase agreement with Trxade, Inc. and Micro Merchant Systems, Inc. (“MMS”), under which MMS purchased for cash substantially all of the assets of Trxade, Inc. in a transaction that closed on February 16, 2024.
We own 100% of Integra Pharma Solutions, LLC (formerly Pinnacle Tek, Inc., a Florida corporation) founded by Mr. Suren Ajjarapu, our CEO, in 2011 (“Integra”). Until the end of 2016, Integra served as our technology consultant provider, but we discontinued that line of business in 2016. Integra now serves as our logistics company for pharmaceutical distribution.
We own 100% of Community Specialty Pharmacy, LLC, an independent retail specialty pharmacy with a focus on specialty medications.Former Subsidiaries
WeIn February of 2022 we entered into an agreement with Exchange Health to own 100%51% of Alliance Pharma Solutions, LLC (d.b.a. DelivMeds), a Florida limited liability company, which was founded in January 2018 (“Alliance”). Alliance previously owned 30% of SyncHealth MSO, LLC (“SyncHealth”) which was part of a joint venture formed in January 2019 with PanOptic Health, LLC (“PanOptic”) with the goal of enabling independent retail pharmacies to better compete with large national pharmacies on pricing, distribution and logistics. We did not realize any income from the joint venture, and we terminated the joint venture agreements pursuant to their terms effective as of January 31, 2020, and assigned the 30% ownership of SyncHealth back to PanOptic. As of February 1, 2020, we own no equity in SyncHealth and only the terms of the agreements relating to confidentiality, non-solicitation and each party’s obligation to cease use of the other party’s intellectual property survive the termination.
We own 100% of Bonum Health,SOSRx, LLC a Delaware limited liability company which owns our “Bonum Health Hub”company. In December of 2022 management determined that the subsidiary did not generate significant revenue and the assets and operations as discussed in further detail below.
We previously ownedwere 100% impaired. In February of MedCheks, LLC, a Delaware limited liability company which was formed in January 2021, had no revenue in 2021 and was dissolved in December 2021.2023 we voluntarily withdrew from the agreement with Exchange Health.
We previously owned 100% of PharmCentrix,Community Specialty Pharmacy, LLC (“CSP”) and Alliance Pharma Solutions, LLC (d.b.a. DelivMeds) (“APS”). Our interests in CSP and APS were sold in September 2023 and have been included in discontinued operations.
As of December 31, 2023 we owned 100% of Superlatus, Inc. (“Superlatus”), and its wholly owned subsidiary Sapientia Technologies, LLC. Superlatus is a Delaware limited liabilitydiversified food technology company which had no revenuewith distribution capabilities and systems to optimize food security and population health via innovative Consumer Packaged Goods products, agritech, foodtech, plant-based proteins and alt-protein and includes, Sapientia, a food tech business. In March 2024 we divested our entire interest in 2020 and was dissolved in December 2020.Superlatus.
Acquisition of Community Specialty Pharmacy, LLC
On October 15, 2018, the Company entered into and consummated the purchase ofWe previously owned 100% of the equity interestsThe Urgent Company, Inc. (“The Urgent Company” or “TUC”), a retail and distribution provider of Community Specialty Pharmacy, LLC, a Florida limited liability company, (“CSP”), pursuant to the terms and conditions of the Membership Interest Purchase Agreement, entered into by and among the Company as the buyer, and CSP, and Nikul Panchal, the equity owner of CSP, a non-executive officer of the Company (collectively, the “Seller”). The purchase price for the 100% equityprepackaged, prepared foods. We divested our interest in CSP was $300,000 in cash, a promissory note issued by theThe Urgent Company in the amount of $300,000, and warrants to purchase 67,585 shares of common stock of the Company (on a post-Reverse Split basis and taking into account the Reverse Stock Split) of which 33% of such warrants were revocable by the Company prior to October 15, 2019 (but were not revoked); 33% were revocable by the Company prior to October 15, 2020 (but were not revoked); and the remaining 33% of such warrants are revocable by the Company prior to October 15, 2021 (which were revoked on September 23, 2021), which are exercisable for eight (8) years from the issuance date at a strike price of $0.06 per share. As of the date of this Report, there are no warrants to purchase shares of common stock remain outstanding in connection with the purchase.our divestiture of Superlatus.
SyncHealth MSO, LLC Joint Venture
On January 17, 2019, the Company and Alliance Pharma Solutions, LLC, a Delaware limited liability company and wholly-owned subsidiary of the Company (hereafter “Alliance,” with Alliance and Trxade referred to collectively herein as the “Trxade Parties”), entered into a transaction effective as of January 17, 2019 with PanOptic Health, LLC, a Delaware limited liability company (“PanOptic”), to create a new entity, SyncHealth MSO, LLC (“SyncHealth”) as part of a joint venture to enable independent retail pharmacies to better compete with large national pharmacies on pricing, distribution and logistics. As part of the transaction Alliance owned 30% of SyncHealth. We did not realize any income from the joint venture, and we terminated the joint venture agreements pursuant to their terms effective as of January 31, 2020, and assigned the 30% ownership of SyncHealth back to PanOptic. As of February 1, 2020, we own no equity in SyncHealth and only the terms of the agreements relating to confidentiality, non-solicitation and each party’s obligation to cease use of the other party’s intellectual property survive the termination.
Bonum Health Asset Acquisition
On October 23, 2019, Bonum Health, LLC, a Delaware limited liability company, and a then newly formed wholly-owned subsidiary of the Company (“Bonum Health”) entered into an Asset Purchase Agreement with Bonum Health, LLC, a Florida limited liability company (“Seller”) and the sole member of the Seller (the “Member”). Pursuant to the Asset Purchase Agreement, the Company (through Bonum Health) acquired from the Seller, certain specified assets and certain specified contracts associated with the assets of the Seller’s operation as a telehealth service provider (the Tele Meds Platform)(the “Assets”). Included with the acquisition of the Assets, were contracts (relating to the Assets), intellectual property for the Bonum Health Tele Medicine software & Technology and personal computers. The Company agreed to provide the Seller consideration equal to 41,667 shares of restricted common stock of the Company at the closing, and the Seller had the right to earn up to an additional 108,334 shares of restricted common stock of the Company in the event certain milestones were met within the first anniversary of the Closing date, none of which were met.
The Asset Purchase Agreement includes a three year non-compete requirement, prohibiting the Seller and the Member from competing against the Assets, customary representations and indemnification obligations, subject to a $25,000 minimal claim amount and certain limitations on liability disclosed in the Asset Purchase Agreement.
Subsequent to the acquisition, the Company determined that the assets were not usable and wrote off the value of the assets amounting to approximately $369,000.
| 9 |
BUSINESS OF TRXADE
Company Overview
We are a health services IT company focused on digitalizing the retail pharmacy experience by optimizing drug procurement, the prescription journey and patient engagement in the U.S. and have designed and developed, and now own and operate, a business-to-business web-based marketplace. Our core service brings the nation’s independent pharmacies, accredited national suppliers, and manufacturers of pharmaceuticals together to provide efficient and transparent buying and selling opportunities.
We began operations as Trxade Group, Inc., a Nevada corporation (“Trxade Nevada”) in August of 2010 and spent over two years creating and enhancing our web-based services. The Company changed its name on June 1, 2021, from “Trxade Group, Inc” to “TRxADE HEALTH, INC.” Our services provideprovided pricing transparency, purchasing capabilities and other value-added services on a single platform focused on serving the nation’s approximately 19,397 independent pharmacies with annual purchasing power of $67.1 billion (according to the National Community of Pharmacists Association’s 2021 Digest). Our national wholesale supply partners and manufacturers are able to fulfill orders on our platform in real-time and provide pharmacies and wholesale suppliers with cost-saving payment terms and next-day delivery capabilities in unrestrictive states. We have expanded significantly since 2015 and now serveserved approximately 13,100+14,400+ registered members on our sales platform.
Our Principal Products and Services and their Markets.Markets
Trxade.com is apreviously operated the Company’s web-based pharmaceutical marketplace engaged in promoting and enabling commerce among independent pharmacies, small chains, hospitals, clinics, and alternate dispensing sites with large pharmaceutical suppliers nationally. OurThat marketplace hashad over 60 national and regional pharmaceutical suppliers providing over 120,000 branded and generic drugs, including over-the-counter drugs (OTCs), and drugs available for purchase by pharmacists. We serveserved approximately 13,100+14,400+ registered members, providing access to Trxade’s proprietary pharmaceutical database and data analytics regarding medication pricing. We generategenerated revenue from these services by charging a transaction fee to the seller of the products for sales conducted via the Trxade platform. The buyers do not bear the cost of transaction fees for the purchases that they make, nor do they pay a fee to join or register with our platform. In February 2024 we divested substantially all of our assets related to our web-based pharmaceutical marketplace previously operated through TRxADE, Inc. Substantially all of our revenues during the years ended December 31,Fiscal 2023, Fiscal 2022, and Fiscal 2021 and 2020, were from platform revenue generated on www.rx.trxade.com, product sales through Integra Pharma Solutions, LLC, and prescription sales through Community Specialty Pharmacy, LLC.
Status of current products and services.services and business plans; Status of former business products and initiatives
We havepreviously had a number of products and services stillfocused on the US market in development,operation and business assets, which are described below. In addition, in 2024 we expect to explore other strategic transactions and acquisitions as a means to monetize and enhance the Company’s current assets and operations, which transactions may involve effecting acquisitions of new businesses in industries that differ from our legacy operations.
Integra Pharma Solutions, LLC. Integra is intended to serve as our logistics company for pharmaceutical distribution.
We currently distribute through our manufacturer and strategic distribution partners prescription medication, medical devices and over the counter medication to over 1,600 pharmacies and medical clinics across 38 states.
Community Specialty Pharmacy, LLC. We acquired Community Specialty Pharmacy, LLC, a Florida limited liability company (“CSP”), on October 15, 2018. CSP is an accredited pharmacy located in St. Petersburg, Florida. CSP has a focus on specialty medications. The company operates with an innovative pharmacy model which offers home delivery services to any patient thereby providing convenience.
| 10 |
Delivmeds.com. Delivmeds.com was launched in late 2018 as a consumer-based app to provide delivery of pharmaceutical products associated with Alliance Pharma Solutions, LLC. We are currently working on reformulating the application from a prescription delivery portal to a fully integrated, interoperable, end-to-end prescription delivery and medication adherence tool. The new product has been rebranded and is targeted for consumer re-release and use in the near future. To date, we have not generated any revenue from this product.
Trxade Prime. Trxade Prime allows previously allowed pharmacy members on the Trxade platform to process, consolidate and ship purchase orders that are placed directly with Trxade suppliers via the Trxade Prime service.Prime. This isservice was provided at no cost, with the goal of offering a single tool with one low order minimum, one invoice, one package and one delivery from multiple quality wholesalers and distributors. Revenue hashad been generated from this service thoughthrough our Integra subsidiary, which provides the consolidation of the orders.
Bonum Health Hub and Application. The “Bonum Health Hub”, a self-enclosed, free standing virtual examination room, was launched by the Company’s wholly-owned Bonum Health, LLC subsidiary, in November 2019 and was expected to be operational in April 2020; however, due to the COVID-19 pandemic, the Company does not anticipate installations moving forward, and has taken a write off of the hubs purchased at June 30, 2021 in the amount of $143,891, which is included under loss on inventory investments in the statement of operations for the year ended December 31, 2021, in Note 8 - Other Receivables” to the Notes to Consolidated Financial Statements included herein under “Item 8. Financial Statements and Supplemental Data”.
The “Bonum Health app,”, which provides previously provided an overall healthcare experience comparable to a Primary Care practitioner, and an online portal as a personal electronic medical record and scheduling system iswas available on a subscription basis, primarily as a stand-alone telehealth software application that can be licensed on a business-to-business (B2B) model to clients as an employment health benefit for the clients’ employees. Revenue has beenwas generated from this service through our Bonum subsidiary.
Bonum+ Business to Business (B2B). Bonum+ bundlespreviously bundled telehealth, a COVID-19 risk assessment tool and a Personal Protective Equipment (PPE) purchasing tool, through a secure mobile dashboard for corporate clients. The B2B platform easeseased pressure on employees who arewere required to report any relevant health issues daily, centralizing communication and contact tracing to deliver risk scores. This allowsallowed employers to monitor employee COVID-19 risk profiles and streamlinesstreamlined the ordering of new PPE as needed. An integrated artificial intelligence (AI) tool offersoffered health recommendations and connects employees with board certified physicians, as needed. To date, we have notNo revenue was generated any revenue from this product.
MedCheks Health Passport. The Health Passport is a patient-centered, digital, precision healthcare platform that lets patients consolidate and control their health data via a digital Health Passport and allows them to share their health profile, tests and vaccinations simply and safely. Secured in a blockchain, the Health Passport includes health and vaccination status verification via a QR code, which is available for travel, entry into stadiums, concert venues, events, offices, industrial plants, warehouses, and other physical access points. The Passport stores all of a user’s health records securely in one place. We have not generated any revenue from this product to date and the product was discontinued at the end of December 2021. We previously owned 100% of MedCheks, LLC, a Delaware limited liability company which was formed in January 2021, had no revenue in 2021 and was dissolved in December 2021.
SOSRx, LLC. On February 15, 2022, the Company entered into a relationship with Exchange Health, LLC, a technology company providing an online platform for manufacturers and suppliers to sell and purchase pharmaceuticals (“Exchange Health”). SOSRx LLC, a Delaware limited liability company (“SOSRx”), was formed, which is owned 51% by the Company and 49% by Exchange Health. SOSRx did not generate material revenue and in February of 2023, the Company voluntarily withdrew from the joint venture agreement. The asset impairment is reflected in the statement of operations for Fiscal 2022 as impairment of intangible asset. Additionally, the Company contributed a cash investment of $275,000 in February of 2022 when the joint venture was formed. The Company did not recover this investment as part of the withdrawal settlement.
Superlatus. As of December 31, 2023, Superlatus was a wholly owned subsidiary of the Company as a result of a merger transaction that closed in July 2023. Superlatus is a diversified food technology company with distribution capabilities and systems to optimize food security and population health via innovative Consumer Packaged Goods products, agritech, foodtech, plant-based proteins and alt-protein and includes wholly-owned subsidiary, Sapientia, Inc., a food tech business. Subsequent to December 31, 2023, the Company divested its entire interest in Superlatus.
All of our product offerings are focused on the United States markets. Some products are restricted just to certain states, depending upon the various applicable state regulations and guidelines pertaining to pharmaceuticals, particularly, and drug businesses, generally. Our services are distributed through our online platform
| 11 |
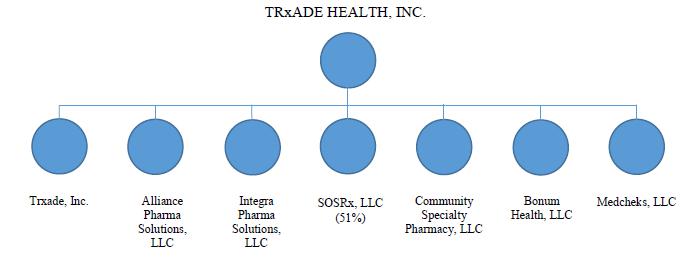
Organizational Structure
The diagram below depicts our current organizational structure:
The Pharmaceutical Industry
According to the NCPA 2020 Digest Report, United States pharmaceutical companies comprise a burgeoning estimated $685 billion industry by 2023, consisting of over 65,000 pharmacy facilities. Management believes that few platforms are currently in place to bring these participants together to share market knowledge, product pricing transparency and product availability. According to this, theThe pharmaceutical market is comprised primarily of three wholesalers that control an estimated approximately 92% of the market. Our management believes that this concentration has, over the years, led to a lack of price and cost transparency, thereby resulting in severe limitations on the purchasing choices of industry participants. These market dynamics have enabled these large wholesalers (McKesson, Cardinal Health and AmerisourceBergen), known as ADR distributors, to dominate the industry with respect to both generic and brand pharmaceuticals.
To fuel this change, insurance companies (Pharmacy Benefits Management (“PBM”) and private health payers) and the federal government have initiated lower medication reimbursement payments to healthcare providers. We believe that pharmacies face increasing pressure to source medications as inexpensively as possible and improve operational efficiency. Trxade seeksHealth aims to be in the forefront of solvingaddress these transparency and pricing concerns by providing independent, retail pharmacies with real-time, pharmacy acquisition cost (“PAC”) benchmarks to the National Drug Code (the “NDC”) standard. The NDC mark is a unique product identifierexceptional service and pricing on their most commonly used in the United States for drugs intended for human use.pharmaceuticals by partnering with strategic manufacturers and other authorized suppliers.
Competitive Business Conditions, Our competitive positionCompetitive Position in our Industry, and our Methods of Competition.Competition
We expect to face competition from the three large ADR distributors (McKesson,(including McKesson, Cardinal Health and AmerisourceBergen), other pharmaceutical distributors, buying groups, software products, and other start-up companies. Most of our competitors’ operations have substantially greater financial- and manufacturer-backed resources, longer operating histories, greater name recognition, and more established relationships in the industry.
Other Start-up Companies Which Provide Competitive Services.Services
We have identified start-ups that provide for supplier-pharmacyThere are currently several smaller regional and national secondary distributors of pharmaceuticals such as (Anda Pharmaceutical, Masters Pharmaceutical etc)as well as other innovative trading platforms such as PharmaBid, RxCherrypick, PharmSaver, MatchRx and GenericBid, and provide web-based services similar to ours, allowingthat allow pharmacies to buy from several suppliers. TrxadeIntegra differentiates itself from these exchangesdistributors by providing our pharmacies with bothunique specialty, brand and generic pharmaceutical products. Additional companies target “direct-to-consumer” pharmacy deliveries, including Amazon.com’s PillPack, Capsule, Costplusdrugs, products that are in short supply and GetRoman.com.offered via limited distribution channels.
Buying Groups.Groups
Buying Groups provide discounted prices to their members by negotiating better pricing with one primary wholesaler, while charging administrative fees generally ranging from 3 to 5 percent. Some Buying Groups are structured like co-operatives (such as Independent Pharmacy Cooperative (IPC) and American Pharmacy Cooperative, Inc. (APCI)) and offer their members monthly or quarterly rebates. Although they can function well to bring pricing competition to the industry, they often offer rebates only after the purchase. Management does not believe Buying Groups will provide long-term savings to customers with this model given the increased transparency and competition in the industry.
| 12 |
Pharmaceutical Software.Software
Some pharmaceutical software companies compete with us to varying degrees at different levels. SureCost, for example, provides inventory management software enabling pharmacies to comply with primary supplier contracts. This software is fee-based and requires training.
Pharmacies may be reluctant to buy pharmaceuticals on the internet due to the historical negativity and uncertainty with respect to the origin and purity of drugs purchased off the web. Trxade management believes that as we continue to develop our brand, our customer base, and our vast product offerings, we will gain the trust of the market and overcome the negativity associated with purchasing via a pharmaceutical online marketplace.from multiple distributors outside of their primary vendor agreements.
One advantage that we believe we have over our competition is our ability to be flexible and fast moving in adjusting our business model to address the needs of our customer base. Trxade started by offering pharmacies a reverse auction model to enhance savings on the purchase of their pharmaceuticals. Customer feedback suggested that pharmacies prefer a more “buy now” format, which we implemented. This resulted in a “one-stop-one-search” platform to buy quality pharmaceuticals for less and a data-rich platform to help pharmacies overcome the complexities related to supply chain purchasing.
Telehealth Providers
We also anticipatepreviously anticipated facing competition in the telehealth industry (in connection with “Bonum Health”) from current and future health care companies in the telehealth market including, Teladoc Health, Inc., MDLive, Inc., American Well Corporation and Grand Rounds, Inc., among other smaller industry participants.
Sources and Availability of Raw Materials; Principal Suppliers.Suppliers
Trxade is a web-based technology platform. Because we are not a manufacturing company, we do not need any raw materials. Our module on the platform is drug supplier-to-retailer. We bring buyers and sellers together on this platform. Our suppliers include National Apothecary Solutions, Integral RX, and South Pointe Wholesale, Inc.
Dependence on One or More Major Customers.Customers
As of the date of this filing, we have approximately 13,100+1600 registered members and over 3010 pharmaceutical suppliers as customers, with an estimated market potential of approximately 19,39720,000+ independent pharmacies and 1,500 regional and local suppliers. We have a working relationship with over 25 wholesalers and the nation’s largest buying group. Although we believe those entities are satisfied with their business relationship with Trxade, if our buying group and two or three of the largest wholesalers decided no longer to do business with Trxade, the resulting supplier void would materially and adversely affect our competitiveness in the marketplace.pharmacies.
Intellectual Property.Property
Although we believe that our name and brand are protected by applicable state common law trademark laws, we do not currently have any patents, concessions, licenses, royalty agreements, or franchises, provided that we do currently maintain a number of registered trademarks and our pharmaceutical pricing benchmarks, PAC. Our business operates under a proprietary software system which includes trade secrets within our database, business practices and pricing model. We also maintain a number of websites.franchises.
We believe that we have taken all necessary steps to protect our proprietary rights, but no assurance can be given that we will be able to successfully enforce or protect our rights in the event that they are infringed upon by a third party.
Need for Government Approval of Products and Services.Services
We are required to hold state pharmaceutical business licenses and to follow applicable state and federal government regulations detailed herein. In October 2018, we acquired Community Specialty Pharmacy, LLC, an accredited independent retail pharmacy with a focus on specialty medications, which requires state approval, which have been obtained in 36 states.
Effect of Existing or Probable Government Regulations on the Business.
Federal Drug Administration Guidelines
On April 12, 1988, President Ronald Reagan signed into law the Prescription Drug Marketing Act of 1987 (PDMA), setting the baseline for wholesale distribution regulations. The final regulations were published in 1999, establishing the minimum wholesale distribution requirements for state licensure. With the intent to prevent the introduction and retail sale of substandard, ineffective, or counterfeit drugs into the distribution system, state licensing systems moved to update their standards to match those provided federally as guided under FDA’s Guidelines for State Licensing of Wholesale Prescription Drug Distributors (21 CFR 205). PDMA established minimum federal pedigree requirements to trace the ownership of prescription drugs through the supply chain. The principal goal of the PDMA was to further secure the nation’s drug supply from counterfeit and substandard prescription drugs. The law establishes two types of distributors: “Authorized distributor[s] of record” or ADRs; and “Unauthorized distributor[s],” such as wholesalers. The pedigree requirement was to require each person engaged in the wholesale distribution of a prescription drug in interstate commerce, who is not the manufacturer or an authorized distributor of record for that drug, to provide a pedigree to the recipient. After meeting resistance from various stakeholders, the FDA delayed the effective date of the regulations several times, until final implementation in December 2006.
At the federal level the implementation of the track and trace legislation which went into effect in 2018, requires the use of pharmaceutical pedigree to track the movement of pharmaceuticals along the supply chain. The costs of complying with this new legislation may be too burdensome for many of the smaller suppliers.
State Drug Administration Guidelines
There are a number of national and state-wide regulations that have an effect on our business. All drug wholesalers must be licensed under state licensing systems, which must in turn meet the FDA guidelines under State Licensing of Wholesale Prescription Drug Distributors (21 CFR Part 205). The regulations set forth minimum requirements for prescription drug storage and security as well as for the treatment of returned, damaged, and outdated prescription drugs. Further, wholesale drug distributors must establish and maintain inventories and records of all transactions regarding the receipt and distribution of prescription drugs and make these available for inspection and copying by authorized federal, state, or local law enforcement officials. In most states, wholesale distributor licenses are issued by the State Boards of Pharmacy and require periodic renewal. Approximately 40 states also require out-of-state wholesalers that distribute drugs within their borders to be licensed as well.
| 13 |
On February 4, 2022, the FDA published a proposed rule to set national standards for the licensing of prescription drug wholesale distributors and third-party logistics providers. The comment period was open until June 6, 2022. New regulations and requirements for wholesale distributors and third-party logistics providers could be too burdensome and could impact our registered suppliers on the Trxade platform.
California, Florida, Nevada, New Mexico and Indiana define the normal distribution channel to not include the lateral sales of pharmaceuticals between wholesalers. The Supply Chain Act, part of the Quality Drug Act, which was signed into federal law in December 2013, precludes all states from restricting, investigating or inspecting the distribution channel and transactional history. Until the federal government provides guidelines for the new federal law, no state regulation or guideline exists.
The warehousing of pharmaceuticals is also restricted and requires additional state licenses. Some licenses require bonds and written exams and may take some time to approve. Currently, Integra Pharma Solutions, LLC, our wholesale distributor, asks for formal pedigrees from the ADR wholesalers and provides pedigrees to those entities they sell to in the marketplace. This requirement limits liability and provides assurance if a recall is warranted that Trxade and its participants will receive value for the commodity.
Our national wholesale supply partners are able to fulfill orders on our platform in real-time and provide pharmacies with cost-saving payment terms and next-day delivery capabilities in unrestrictive states under the Model State Pharmacy Act and Model Rules of the National Association of Boards of Pharmacy (Model Act).
Potential New Regulations; Price Gouging Rules
In addition to the above, regulatory mandates in response to certain unexpected events, such as viral outbreaks, could negatively impact sales. For example, in December 2019 an outbreak of a coronavirus surfaced in China and resulted in governments around the world adopting restrictions on public gatherings, travel and restrictions on companies’ (including our) ability to conduct normal business operations.
Price gouging may be an issue in the coming months due to the continued effects of the coronavirus and responses thereto and supply chain issues associated therewith and separately; as of the date of this Report, 42 states have enacted price gouging laws of one kind or another. The laws vary from state to state, but one constant throughout is a prohibition to charge “excessive” or “unconscionable” prices for consumer goods. Some states define “excessive” or “unconscionable” while others define what makes a prima faciafacie case for price gouging and what constitutes a prima faciafacie defense, shifting the burden of proof to the accuser. In almost all of the 42 states with price gouging laws on the books, a price is excessive or unconscionable if the price of a good has increased, in some states by a certain percentage, over the price of the good prior to the onset of the abnormal disruption of the market. Some states have clearly excepted from the price gouging definition a rise in prices caused by an increase in the merchant’s cost of delivering that good for sale – whether it be increased shipping costs, gasoline prices or simply the cost of the good itself. Other states have less defined exceptions – Virginia for example only treats the fact of increased input costs as a merchant’s prima faciafacie defense to an accusation of price gouging. Several states except from the price gouging definition prices that do not exceed a normal margin (i.e., the merchant’s margin immediately prior to the market disruption) PLUS 10%. In general, while the lawlaw may not specifically define what constitutes an “unconscionably excessive price,” the statutes typically provide that a price may be “unconscionably excessive” if: the amount charged represents a “gross disparity” from the price such goods or services were sold or offered for sale immediately prior to the onset of the abnormal disruption of the market. Merchants may provide evidence that justifies their higher prices were justified by increased costs beyond their control. We will need to comply with the excessive price statutes; as of the date of this Report, we believe we were in compliance with all 42 states’ price gouging laws.
| 14 |
U.S. Federal and State Fraud and Abuse Laws
Federal Stark Law
We are subject to the federal self-referral prohibitions, commonly known as the Stark Law. Where applicable, this law prohibits a physician from referring Medicare patients to an entity providing “designated health services” if the physician or a member of such physician’s immediate family has a “financial relationship” with the entity, unless an exception applies. The penalties for violating the Stark Law include the denial of payment for services ordered in violation of the statute, mandatory refunds of any sums paid for such services, civil penalties, disgorgement and possible exclusion from future participation in the federally funded healthcare programs. A person who engages in a scheme to circumvent the Stark Law’s prohibitions may be subject to fines for each applicable arrangement or scheme. The Stark Law is a strict liability statute, which means proof of specific intent to violate the law is not required. In addition, the government and some courts have taken the position that claims presented in violation of the various statutes, including the Stark Law can be considered a violation of the federal False Claims Act (described below) based on the contention that a provider impliedly certifies compliance with all applicable laws, regulations and other rules when submitting claims for reimbursement. A determination of liability under the Stark Law could have a material adverse effect on our business, financial condition and results of operations.
Federal Anti-Kickback Statute
We are also subject to the federal Anti-Kickback Statute. The Anti-Kickback Statute is broadly worded and prohibits the knowing and willful offer, payment, solicitation or receipt of any form of remuneration in return for, or to induce, (i) the referral of a person covered by Medicare, Medicaid or other governmental programs, (ii) the furnishing or arranging for the furnishing of items or services reimbursable under Medicare, Medicaid or other governmental programs or (iii) the purchasing, leasing or ordering or arranging or recommending purchasing, leasing or ordering of any item or service reimbursable under Medicare, Medicaid or other governmental programs. In addition, a person or entity does not need to have actual knowledge of this statute or specific intent to violate it to have committed a violation. Moreover, the government may assert that a claim including items or services resulting from a violation of the Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act, as discussed below. Violations of the Anti-Kickback Statute can result in exclusion from Medicare, Medicaid or other governmental programs as well as civil and criminal penalties and fines. Imposition of any of these remedies could have a material adverse effect on our business, financial condition and results of operations.
Federal False Claims Act & HIPAA
BothThe federal and stateFalse Claims Act provides, in part, that the federal government agenciesmay bring a lawsuit against any person whom it believes has knowingly presented, or caused to be presented, a false or fraudulent request for payment from the federal government, or who has made a false statement or used a false record to get a claim approved. In addition, amendments in 1986 to the federal False Claims Act have continued civil and criminal enforcement efforts as part of numerous ongoing investigations of healthcaremade it easier for private parties to bring “qui tam” whistleblower lawsuits against companies and their executives and managers. Although there are a number of civil and criminal statutes that can be applied to healthcare providers, a significant number of these investigations involveunder the federal False Claims Act. These investigations can be initiated not only by the government but also by a private party asserting direct knowledge of fraud. Penalties include significant civil monetary penalties for False Claims Act violations include fines,each false claim, plus up to three times the amount of damages sustained bythat the federal government.government sustained because of the act of that person. Qui tam actions have increased significantly in recent years, causing greater numbers of healthcare companies to have to defend a false claim action, pay fines, be excluded from Medicare, Medicaid or other federal or state healthcare programs, or be subject to integrity oversight and reporting obligations to resolve allegations of non-compliance, as a result of an investigation arising out of such action.
There are other federal anti-fraud laws that that prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services.
Additionally, HIPAA established two federal crimes for healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A False Claims Act violation of either of these statutes is a felony and may provide the basis forresult in fines, imprisonment, exclusion from the federally fundedMedicare, Medicaid or other federal or state healthcare programs. In addition, some states have adopted similar fraud, whistleblowerprograms, or integrity oversight and false claims provisions.reporting obligations to resolve allegations of non-compliance.
State Fraud and Abuse Laws
Several states in which we operate have also adopted similar fraud and abuse laws as described above. The scope of these laws and the interpretations of them vary from state to state and are enforced by state courts and regulatory authorities, each with broad discretion. Some state fraud and abuse laws apply to items or services reimbursed by any payor, including patients and commercial insurers, not just those reimbursed by a federally funded healthcare program. A determination of liability under such state fraud and abuse laws could result in fines and penalties and restrictions on our ability to operate in these jurisdictions.
Other Healthcare Laws
The federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their implementing regulations, which we collectively refer to as HIPAA, established several separate criminal penalties for making false or fraudulent claims to insurance companies and other non-governmental payors of healthcare services. Under HIPAA, these two additional federal crimes are: “Healthcare Fraud” and “False Statements Relating to Healthcare Matters.” The Healthcare Fraud statute prohibits knowingly and recklessly executing a scheme or artifice to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government sponsored programs. The False Statements Relating to Healthcare Matters statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact by any trick, scheme or device or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. These provisions are intended to punish some of the same conduct in the submission of claims to private payors as the federal False Claims Act covers in connection with governmental health programs.
In addition, the Civil Monetary Penalties Law imposes civil administrative sanctions for, among other violations, inappropriate billing of services to federally funded healthcare programs and employing or contracting with individuals or entities who are excluded from participation in federally funded healthcare programs. Moreover, a person who offers or transfers to a Medicare or Medicaid beneficiary any remuneration, including waivers of copayments and deductible amounts (or any part thereof), that the person knows or should know is likely to influence the beneficiary’s selection of a particular provider, practitioner or supplier of Medicare or Medicaid payable items or services may be liable for civil monetary penalties for each wrongful act. Moreover, in certain cases, providers who routinely waive copayments and deductibles for Medicare and Medicaid beneficiaries can also be held liable under the Anti-Kickback Statute and civil False Claims Act, which can impose additional penalties associated with the wrongful act. One of the statutory exceptions to the prohibition is non-routine, unadvertised waivers of copayments or deductible amounts based on individualized determinations of financial need or exhaustion of reasonable collection efforts. Although this prohibition applies only to federal healthcare program beneficiaries, the routine waivers of copayments and deductibles offered to patients covered by commercial payers may implicate applicable state laws related to, among other things, unlawful schemes to defraud, excessive fees for services, tortious interference with patient contracts and statutory or common law fraud.
Climate Change Regulation
The U.S. government and foreign governments are currently in the process of considering new or expanded laws to address climate change. Such laws, if adopted, may include limitations on greenhouse gas (“GHG”) emissions, mandates that companies implement processes to monitor and disclose climate-related matters, additional taxes or offset charges on specified energy sources, and other requirements. Compliance with climate-related laws may be further complicated by different regulatory approaches and requirements in the various jurisdictions in which we operate. New or expanded climate-related laws could impose substantial costs on us. Until the timing and extent of climate-related laws are clarified, we cannot predict their potential effect on our capital expenditures or our results of operations.Status as a Public Company
Environmental Regulations
Our operations are subject to regulations under various federal, state, local and foreign laws concerning the environment, including laws addressing the discharge of pollutants into the air and water, the management and disposal of hazardous substances and wastes, and the cleanup of contaminated sites. We could incur substantial costs, including cleanup costs, fines and civil or criminal sanctions and third-party damage or personal injury claims, if in the future we were to violate or become liable under environmental laws. We are not awarean “emerging growth company,” as defined in Section 2(a) of any costs or effects of our compliance with environmental laws.
Jumpstart Our Business Startupsthe Securities Act,
In April 2012, as modified by the Jumpstart Our Business Startups Act (“JOBSof 2012 (the “JOBS Act”). As such, we are eligible to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act,”) was enacted into law. The JOBS Act provides, among other things: reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. If some investors find our securities less attractive as a result, there may be a less active trading market for our securities and the prices of our securities may be more volatile.
In general, under the JOBS Act a company is an “emerging growth company” if its initial public offering (“IPO”) of common equity securities was affected after December 8, 2011, and the company had less than $1.07 billion of total annual gross revenues during its last completed fiscal year. A company will no longer qualify as an “emerging growth company” after the earliest of
The JOBS Act provides additional new guidelines and exemptions for non-reporting companies and for non-public offerings. Those exemptions that impact the Company are discussed below.
Financial Disclosure. The financial disclosure in a registration statement filed by an “emerging growth company” pursuant to the Securities Act, will differ from registration statements filed by other companies as follows:
However, the requirements for financial disclosure provided by Regulation S-K promulgated by the Rules and Regulations of the SEC already provide certain of these exemptions for smaller reporting companies. The Company is a smaller reporting company. Currently a smaller reporting company is not required to file as part of its registration statement selected financial data and only needs to include audited financial statements for its two most current fiscal years with no required tabular disclosure of contractual obligations.
The JOBS Act also exempts the Company’s independent registered public accounting firm from having to comply with any rules adopted by the Public Company Accounting Oversight Board (“PCAOB”) after the date of the JOBS Act’s enactment, except as otherwise required by SEC rule.
The JOBS Act further exempts an “emerging growth company” from any requirement adopted by the PCAOB for mandatory rotation of the Company’s accounting firm or for a supplemental auditor report about the audit.
Internal Control Attestation. The JOBS Act also provides an exemption from the requirement of the Company’s independent registered public accounting firm to file a report on the Company’s internal control over financial reporting, although management of the Company is still required to file its report on the adequacy of the Company’s internal control over financial reporting.
Section 102(a) of the JOBS Act exempts “emerging growth companies” from the requirements in §14A(e) of the Exchange Act for companies with a class of securities registered under the Exchange Act to hold stockholder votes for executive compensation and golden parachutes.
Other Items of the JOBS Act. The JOBS Act also provides that an “emerging growth company” can communicatetake advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with potential investors that are qualified institutional buyersnew or institutions that are accredited to determine interest in a contemplated offering either prior to or after the date of filing the respective registration statement. The JOBS Act also permits research reports by a broker or dealer aboutrevised accounting standards. In other words, an “emerging growth company” regardlesscan delay the adoption of whether such report provides sufficient information for an investment decision. In addition, the JOBS Act precludes the SEC and FINRA from adopting certain restrictive rules or regulations regarding brokers, dealers and potential investors, communications with management and distribution of research reports on the “emerging growth company’s” initial public offerings (IPOs).
Section 106 of the JOBS Act permits “emerging growth companies” to submit registration statements under the Securities Act on a confidential basis provided that the registration statement and all amendments thereto are publicly filed at least 21 days before the issuer conducts any road show (which time period has since been reduced to 15 days). This is intended to allow “emerging growth companies” to explore the IPO option without disclosing to the market the fact that it is seeking to go public or disclosing the information contained in its registration statement until the company is ready to conduct a roadshow.
Election to Opt Out of Transition Period. Section 102(b)(1) of the JOBS Act exempts “emerging growth companies” from being required to comply with new or revised financial accounting standards until those standards would otherwise apply to private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are requiredcompanies. We intend to comply with the new or revised financial accounting standard.
The JOBS Act provides that a company can elect to opt outtake advantage of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt outbenefits of thethis extended transition period.
Status as Emerging Growth Company. Our first sale of common equity securities pursuant to an effective registration statement under the Securities Act occurred on or around May 2019. As such, weWe will remain an emerging growth company until no later than December 31, 2024, the completionearlier of (1) the last day of the fiscal year of(a) following the fifth anniversary of the Company’s IPO.completion of our initial public offering, (b) in which we have total annual gross revenue of at least $1.235 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our Class A ordinary shares that are held by non-affiliates equals or exceeds $700 million as of the last business day of the preceding second fiscal quarter, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period.
Additionally, we are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company until the last day of the fiscal year in which (1) the market value of our ordinary shares held by non-affiliates exceeds $250 million as of the last business day of that year’s second fiscal quarter, or (2) our annual revenues exceeded $100 million during such completed fiscal year and the market value of our ordinary shares held by non-affiliates equals or exceeds $700 million as of the last business day of that year’s second fiscal quarter.
Research and Development.Development
During the last two fiscal years, Trxade.com, DelivMeds, MedCheks Health Passport and Bonum HealthCurrently we do not have been developed as proprietary software. For the years ended December 31, 2021, and 2020, $509,210 and $662,726, respectively, was spent by the Company inany product research and development activities, which were includedefforts underway. In future we may opt to engage in general and administrative expenses. Nonefurther development of these expenses were borne directly by customers.our logistics model depending on customer feedback.
Employees
Currently, we have approximately 478 full-time employees. Our compensation programs are designed to align the compensation of our employees with performance and to provide the proper incentives to attract, retain and motivate employees to achieve superior results. The structure of our compensation programs balances incentives earnings for both short-term and long-term performance such as health insurance, paid time off and flexibility schedules. To empower employees to unleash their potential, we provide onboarding training, development mentorship with C-suite executives, and one on one coaching. The Company believes that its rich culture of inclusion and diversity enables it to create, develop and fully leverage the strength of its workforce to exceed customer expectation and meet its growth objectives. The Company places a high value on diversity and inclusion.
We also utilize numerous outside consultants. Our future success will depend partially on our ability to attract, retain and motivate qualified personnel. We are not a party to any collective bargaining agreements and have not experienced any strikes or work stoppages. We consider our relations with our employees and consultants to be satisfactory.
Seasonality
Our business is not directly affected by seasonal fluctuations but is affected indirectly by the fall and winter flu season, to the extent it leads to an increased demand for certain generic pharmaceuticals.
| 16 |
| ITEM 1A. | RISK FACTORS |
Summary Risk Factors
Our business is subject to numerous risks and uncertainties, many of which are beyond our control, including those highlighted in the section titled “Risk Factors” immediately following this summary. These risks include, among others, the following:
| ● | We | |
| ● | We may need additional financing in the future, which may not be available on favorable terms, if at all; | |
| ● | We may not be able to manage our future growth; |
| ● | Risks relating to implementing our acquisition strategies, and the risk that acquisitions will likely be dilutive to our stockholders and we may not realize the anticipated benefits of certain strategic transactions that we pursue or effect; |
| ● | Many of our competitors are better established and have resources significantly greater than | |
| ours; | ||
| ● | We face risks associated with our operations within the pharmaceutical distribution market; | |
| ● | We are dependent on our current management; | |
| ● | We rely on third party contracts, which may not be renewed or may be terminated; | |
| ● | We |
| ● | We have in the past, and may in the future, not be able to sell our inventory, at or above the price we acquired such inventory for, | |
| ● | We have in the past, and may in the future, not receive products or receive refunds for deposited amounts and have | |
| ● | We may be subject to claims that we violated intellectual property rights of others, which are extremely costly to defend and could require us to pay significant damages and limit our ability to operate; | |
| ● | Our business and operations depend on the proper functioning of information systems, critical facilities and distribution networks and a disruption, cyber-attack, failure or destruction of such networks, systems, or technologies may disrupt our business or result in liability; | |
| ● | There may be losses or unauthorized access to or releases of confidential information, including personally identifiable information, that could subject the Company to significant reputational, financial, legal and operational consequences; | |
| ● | We face numerous risks, | |
| risks from the Office of Inspector General, U.S. Department of Health and Human Services (OIG) and the United States Department of Justice (DOJ) and state regulators around pharmaceutical distribution now and in the future. | ||
| ● | Our certificate of incorporation limits the liability of our officers and directors and provides for indemnification rights, mandatory forum selection provisions and limits the ability of stockholders to call special meetings of stockholders; | |
| ● | We incur significant costs to ensure compliance with U.S. and NASDAQ Capital Market reporting and corporate governance requirements; | |
| ● | ||
| of our common stock on the NASDAQ Capital Market; | ||
| ● | Regulatory changes that affect our distribution channels could harm our business; | |
| ● | Healthcare fraud laws are often vague and uncertain, exposing us to potential liability; | |
| ● | New and expanded laws or regulations could have a material adverse effect on our business operations, cash flows or future prospects; |
| ● | The public health crisis involving the abuse of prescription opioid pain medication could have a material negative effect on our | |
| business operations and timely reporting to new state regulations. | ||
| ● | Consolidation in the U.S. healthcare industry may negatively impact our results of operations; | |
| ● | We have identified material weaknesses in our internal control over financial reporting and controls and procedures; | |
| ● | There may not be sufficient liquidity in the market for our securities in order for investors to sell their shares. The market price of our common stock may continue to be volatile; | |
| ● | Stockholders may experience dilution to future equity sales, the exercise or conversion of outstanding convertible securities or future transactions; | |
| ● | Our results of operations are subject to rising inflation, rising interest rates, governmental responses thereto and possible recessions caused thereby; | |
| ● | Our Chief Executive Officer and President are our two largest stockholders and, as a result, they can exert significant control over us and have actual or potential interests that may differ from yours; | |
| ● | Cyber security attacks and website problems; | |
| ● | ||
| ● | Claims, litigation, government investigations, and other proceedings that may adversely affect our business and results of |
| 17 |
Risk Factors
You should be aware that there are substantial risks for an investment in our common stock. You should carefully consider these risk factors before you decide to invest in our common stock.The reader should not consider this list to be a complete statement of all risks and uncertainties.
If any of the following risks were to occur, such as our business, financial condition, results of operations or other prospects, any of these could materially affect our likelihood of success. If that happens, the market price of our common stock, if any, could decline, and prospective investors would lose all or part of their investment in our common stock.
Risks Related to Our Liquidity and Business Operations and Plans
Our business, financial condition and results of operations are subject to various risks and uncertainties, including those described below. This section discusses factors that, individually or in the aggregate, could cause our actual results to differ materially from expected and historical results. Our business, financial condition or results of operations could be materially adversely affected by any of these risks. It is not possible to predict or identify all such factors. Consequently, the following description of Risk Factors is not a complete discussion of all potential risks or uncertainties applicable to our business.
We have been, and may in the future be, adversely affected by the global COVID-19 pandemic, the duration and economic, governmental and social impact of which is difficult to predict, which may significantly harm our business, prospects, financial condition and operating results.
During 2020 and continuing into 2022, there has been a widespread worldwide impact from the COVID-19 pandemic, and we have been, and may in the future be, adversely affected as a result. The outbreak of the COVID-19 coronavirus, the global response to such coronavirus, including travel restrictions and quarantines that governments instituted during 2020 and 2021, adversely affected our operations, and future restrictions or governmental requirements may have an adverse effect on our operations in the future, and/or may have a significant negative impact on our results of operations, the production of pharmaceuticals and our ability to timely obtain pharmaceuticals for resale. Currently, we are experiencing reductions to, and interruptions in, the delivery of supply chain pharmaceuticals that are having a negative impact on our wholesalers and certain technology outsourcing in India and the Philippines and we are also having a hard time finding qualified staff, due to the pandemic. Notwithstanding the above disruptions, our results of operations have not, to date, been materially adversely affected by the pandemic. However, if we continue to experience production difficulties, quality control problems or further shortages in supply of pharmaceuticals or personnel in the future, this could harm our business and results of operations, any of which could have a material adverse effect on our operations and the value of our securities. In addition, employee sicknesses and remote working environments, and the potential negative effect thereof on productivity and internal controls, related to the coronavirus and the federal, state and local responses to such virus, could materially impact our consolidated results for the year 2022 and beyond. The COVID-19 outbreak could also restrict our access to capital such as credit facilities and lead to material nonrecurring charges, write-downs, impairments and expenses. The Company is actively and continually monitoring the pandemic’s effect on our businesses and endeavoring to adapt quickly in real time to meet the rapidly-changing demands of our Customers and Suppliers.
To mitigate the spread of COVID-19, we implemented sanitation and personal protection measures. The Company’s corporate office reopened on January 3, 2022, in accordance to Center for Disease Control and Prevention (CDC) guidance, allowing only management and certain key operational employees to return to the office, while hourly employees remain working remotely until further notice. These measures might not fully mitigate COVID-19 risks to our workforce, and we could experience unusual levels of absenteeism that might impair operations and delay delivery of products. The COVID-19 pandemic affects product manufacturing, supply and transport availability and cost. The pandemic has in the past reduced demand for some products due to delays or cancellations of elective medical procedures, consumer self-isolation and business closures, among other reasons, which may become issues again in the future if the number of persons infected does not continue to decline. The COVID-19 pandemic also influences shortages of some products, with product allocation resulting in delivery delays for customers. Additionally, as a result of the coronavirus outbreak, various states have adopted price gouging laws. Our failure to comply with such laws and regulations could subject us to claims, penalties, fines or lawsuits.
We have been impacted and may be further impacted by COVID-19 as follows:
| ||
| ||
|
COVID-19 may cause further disruptions to our business, including, but not limited to:
The ongoing impacts of the pandemic may cause, or make more likely, a general economic slowdown or recession in one or more markets, disruptions and volatility in global capital markets and other broad and adverse effects on the economy, business conditions, commercial activity and the healthcare industry. The pandemic might impact our business operations, financial position and results of operation in unpredictable ways that depend on highly-uncertain future developments, such as determining the effectiveness of current or future government actions to address the public health or economic impacts of the pandemic. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
We were recently unprofitable, we have recently generated net losses, and we may incur losses in the future.
Revenues generated from our consolidated operations for the years ended December 31, 20212023 and 20202022 were $9,889,433$8,272,214 and $17,122,520,$10,250,168, respectively.
We incurred a net loss of $5,315,883$13,720,546 for during the year ended December 31, 2023, compared to a net loss of $2,403,442 for the year ended December 31, 2021, compared to a net loss of $2,536,051 for the year ended December 31, 2020.2022. We may incur other losses in the foreseeable future due to the significant costs associated with our business development,operations, including costs associated with maintaining industry regulatory and licensure compliance. We also incur significant compliance undercosts associated with maintaining SEC regulatory and financial reporting standards.requirements; as well as costs to maintain minimum listing requirements of Nasdaq. We cannot assure you that our operations will annually generate sufficient revenues to fund our continuing operations or to fully implement our business plan, and thereafter sustain profitability in any future period.
The likelihood of our success must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered in connection with the start and growth of a business, the implementation and execution of our business plan, and the regulatory environment affecting the distribution of pharmaceuticals in which we operate.
We need additional capital which may not be available on commercially acceptable terms, if at all, which creates substantial doubt about our ability to continue as a going concern.
Our historical financial statements have been prepared under the assumption that we will continue as a going concern. As of December 31, 2023, the Company had an accumulated deficit of $33,245,940. We have limited financial resources, as of December 31, 2023, we had a working capital deficit of approximately $8,803,000 and a cash balance of approximately $152,000. We will need to raise additional capital or secure debt funding to support on-going operations. The sources of this capital are expected to be the sale of equity and debt, which may not be available on favorable terms, if at all, and may, if sold, cause significant dilution to existing stockholders. If we are unable to access additional capital moving forward, it may hurt our ability to grow and to generate future revenues, our financial position, and liquidity. These matters, when considered in the aggregate, raise substantial doubt about the Company’s ability to continue as a going concern for a reasonable period of time, which is defined as within one year after the date that our condensed financial statements are issued. The financial statements incorporated by reference herein do not contain any adjustments to reflect the possible future effects on the classification of assets or the amounts and classification of liabilities that might result from the outcome of this uncertainty. The doubt regarding our potential ability to continue as a going concern may adversely affect our ability to obtain new financing on reasonable terms or at all. Additionally, if we are unable to continue as a going concern, our stockholders may lose some or all of their investment in the Company.
Additional financing may not be available to us when needed or, if available, it may not be obtained on commercially reasonable terms. If we are not able to obtain the necessary additional financing on a timely or commercially reasonable basis, we will be forced to delay or scale down some or all of our development activities (or perhaps even cease the operation of our business). Our access to additional capital may be negatively affected by future recessions, downturns in the economy or the markets as a whole, or inflation.
| 18 |
If we do not obtain additional financing, our business, prospects, financial condition and results of operations will be adversely affected.
Management anticipates that we will require additional working capital in the future to pursue continued development of products, services, and marketing operations. We cannot accurately predict the timing and amount of such capital requirements. Additional financing may not be available to us when needed or, if available, it may not be obtained on commercially reasonable terms. If we are not able to obtain the necessary additional financing on a timely or commercially reasonable basis, we will be forced to delay or scale down some or all of our development activities (or perhaps even cease the operation of our business). Our access to additional capital may be negatively affected by future recessions, downturns in the economy or the markets as a whole, or inflation.
We have no commitments for any additional financing, and such commitments may not be obtained on favorable terms, if at all. Any additional equity financing will be dilutive to our stockholders, and debt financing, if available, may involve restrictive covenants with respect to dividends, raising future capital, and other financial and operational matters. If we are unable to obtain additional financing as needed, we may be required to reduce the scope of our operations or our anticipated expansion, which could have a material adverse effect on us.
We have attempted to expand, and may further explore, the expansion of our business beyond our legacy healthcare and pharmacy focused business model, and those efforts may not prove successful.
We expect to attempt to broaden our current assets and operations through additional business combinations and acquisition transactions. Certain of these transactions may involve companies involved in industries that are outside and different from our legacy operations, which focused on the healthcare and pharmaceutical industries. For example, during the year ended December 31, 2023 we acquired Superlatus a diversified food technology company. These transactions, if successful, may result in a change of the Company’s focus, a change in the composition of its management, and otherwise result in the Company entering new businesses in which it does not have substantial prior experience. As a result, these transactions may not prove successful or may result potential negative effects that prevent us from realizing the benefits of such transaction and, in turn, have a material adverse impact on our stock price, financial condition, results of operations and liquidity.
It is likely that any efforts we may make to acquire a business will result in substantial additional dilution to our stockholders.
Our existing resources will likely be insufficient to support business operations for a significant period of time. Furthermore, with any business combination or acquisition in which we engage, we will likely issue shares of our common stock rather than paying cash for the business. Moreover, if we raise capital for any operations in the future or issue stock for a business combination or acquisition, such action will require the issuance of equity or debt securities which will likely result in substantial dilution to our existing stockholders. Although we will attempt to minimize the dilutive impact of any future business acquisition or capital-raising activities, we cannot offer any assurance that we will be able to do so.
Our acquisitions and investments in new businesses and new products, services, and technologies is inherently risky, and could disrupt our ongoing businesses.
We have invested and expect to continue to invest in new businesses, products, services, and technologies. Such endeavors may involve significant risks and uncertainties, including insufficient revenues from such investments to offset any new liabilities assumed and expenses associated with these new investments, inadequate return of capital on our investments, distraction of management from current operations, and unidentified issues not discovered in our due diligence of such strategies and offerings that could cause us to fail to realize the anticipated benefits of such investments and incur unanticipated liabilities. Because these new ventures are inherently risky, no assurance can be given that such strategies and offerings will be successful and will not adversely affect our reputation, financial condition, and operating results. To date we have taken losses and/or write-downs on several businesses, products, services, and technologies. For example:
| a) | We had $725,973 of loss on impairment of goodwill for the fiscal year ended December 31, 2020, in connection with the acquisition of Community Specialty Pharmacy, LLC; | |
| b) | We designed and invested resources into the “Bonum Health Hub”, a self-enclosed, free standing virtual examination room, which was launched by the Company’s wholly-owned Bonum Health, LLC, in November 2019 and was expected to be operational in April 2020; however, the Company does not anticipate installations moving forward, and took a write off of the hubs purchased at June 30, 2021 in the amount of $143,891, which was included under loss on inventory investments in the statement of operations for the year ended December 31, 2021; | |
| c) | We also used resources and funding to create a Health Passport application during 2020 and 2021, which was planned to store a user’s health and vaccination status and allow confirmation thereof via a QR code; however, we did not generate any revenue from this product and the product was discontinued at the end of December 2021; | |
| d) | We had $792,500 of loss on impairment of intangible assets related to our investment in the joint venture SOSRx, LLC formed in February of 2022. The subsidiary did not generate material revenue and in February of 2023 the Company voluntarily withdrew from the joint venture agreement. The asset impairment is reflected in the statement of operations for the year ended December 31, 2022 as impairment of intangible asset. Additionally, the Company contributed a cash investment of $275,000 in February of 2022 when the joint venture was formed, the Company did not recover this investment as part of the withdrawal settlement; | |
| e) | We recorded a loss of $875,250 in connection with CSP Test Kits purchased for our Community Specialty Pharmacy that were later deemed inappropriate for distribution by the FDA. The inventory was written down and was recorded as loss on inventory investment in the statement of operations during the year ended December 31, 2022; and | |
| d) | During the year ended December 31, 2023 we acquired Superlatus through a merger transaction, however, due to various complications with the post-closing integration we elected to divest Superlatus in March 2024. |
The use of resources for new businesses and new products, services, and technologies, to the extent such new businesses and new products, services, and technologies do not generate revenues or profits may take management’s focus and time away from more profitable endeavors, may require the Company to take significant write-downs or write-offs, may take funding away from the Company’s other operations or growth opportunities, which may ultimately be more profitable, and may have a material adverse effect on the Company’s cash flows, liquidity and revenues, any or all of which may cause the value of the Company’s securities to decline in value or become worthless.
Failure to adequately manage our planned aggressive growth strategy may harm our business or increase our risk of failure.
For the foreseeable future, we intend to pursue an aggressive growth strategy for the expansion of our operations through increased product development and marketing (or acquisitions of business operations and assets outside of our legacy operations). Our ability to rapidly expand our operations will depend upon many factors, including our ability to work in a regulated environment, market value-added products effectively to independent pharmacies, establish and maintain strategic relationships with suppliers, and obtain adequate capital resources on acceptable terms. Any restrictions on our ability to expand may have a materially adverse effect on our business, results of operations, and financial condition. Accordingly, we may be unable to achieve our targets for sales growth, and our operations may not be successful or achieve anticipated operating results.
Additionally, our growth may place a significant strain on our managerial, administrative, operational, and financial resources and our infrastructure. Our future success will depend, in part, upon the ability of our senior management to manage growth effectively. This will require us to, among other things:
| ● | implement additional management information systems; | |
| ● | further develop our operating, administrative, legal, financial, and accounting systems and controls; | |
| ● | hire additional personnel; | |
| ● | develop additional levels of management within our company; | |
| ● | locate additional office space; | |
| ● | maintain close coordination among our engineering, operations, legal, finance, sales and marketing, and client service and support organizations; and | |
| ● | manage our expanding international operations. |
| 20 |
U.S.As a result, we may lack the resources to deploy our services on a timely and global economic conditionscost-effective basis. Failure to accomplish any of these requirements could materially adversely affect the Company’s business, results of operations, financial conditionimpair our ability to deliver services in a timely fashion or attract and growth.retain new customers.
Adverse macroeconomic conditions, including inflation, slowerFuture business combinations and acquisition transactions, if any, as well as recently closed business combinations and acquisition transactions, may not succeed in generating the intended benefits and may adversely affect our business.
Part of our growth strategy is to evaluate strategic acquisitions or recession,relationships from time to time. The inability of our management to successfully integrate acquired businesses, assets or technologies, and any related diversion of management’s attention, could have a material adverse effect on our business, operating results and financial condition. Business combinations and other acquisition transactions may have a direct adverse effect on our financial condition, results of operations, liquidity or stock price. To complete acquisitions or other business combinations, we may have to use cash, issue new equity securities with dilutive effects on existing stockholders, take on new debt, assume contingent liabilities or increased tariffs, changes to fiscalamortize assets or expenses in a manner that might have a material adverse effect on our balance sheet, results of operations or liquidity. These and monetary policy, tighter credit, higher interest rates, high unemploymentother potential negative effects of an acquisition transaction could prevent us from realizing the benefits of such transaction and currency fluctuations could have a material adverse impact on demand for the Company’s productsour stock price, financial condition, results of operations and services. In addition, consumer confidence and spending could be adversely affected in response to financial market volatility, negative financial news, conditions in the real estate and mortgage markets, declines in income or asset values, changes to fuel and other energy costs, labor and healthcare costs and other economic factors.liquidity.
In addition to an adverse impact on demand for the Company’s products, uncertainty about, or a decline in, U.S. or global economic conditionsIf we do not successfully implement any acquisition strategies, our operating results and prospects could have a significant impact on the Company’s suppliers, the pharmacy industry as a whole, the Company’s network of independent pharmacies and other partners. Potential effects include financial instability; inability to obtain credit to finance operations and purchases of the Company’s products, payment defaults and insolvency.be harmed.
A downturnWe face competition within our industry for acquisitions of businesses, technologies and assets, and, in the economic environmentfuture, such competition may become more intense. As such, even if we are able to identify an acquisition that we would like to consummate, we may not be able to complete the acquisition on commercially reasonable terms or at all because of such competition. Furthermore, if we enter into negotiations that are not ultimately consummated, those negotiations could also leadresult in diversion of management time and significant out-of-pocket costs. Even if we are able to increased creditcomplete such acquisitions, we may additionally expend significant amounts of cash or incur substantial debt to finance them, which indebtedness could result in restrictions on our business and collectability riskuse of available cash. In addition, we may finance or otherwise complete acquisitions by issuing equity or convertible debt securities, which could result in dilution of our existing stockholders. If we fail to evaluate and execute acquisitions successfully, we may not be able to realize their benefits. If we are unable to successfully address any of these risks, our business, financial condition or operating results could be harmed.
If we make any acquisitions, they may disrupt or have a negative impact on our business.
If we make acquisitions in the future, funding permitting, which may not be available on favorable terms, if at all, we could have difficulty integrating the acquired company’s assets, personnel and operations with our own. We do not anticipate that any acquisitions or mergers we may enter into in the future would result in a change of control of the Company. In addition, the key personnel of the acquired business may not be willing to work for us. We cannot predict the effect expansion may have on our core business. Regardless of whether we are successful in acquiring, the negotiations could disrupt our ongoing business, distract our management and employees and increase our expenses. In addition to the risks described above, acquisitions are accompanied by a number of inherent risks, including, without limitation, the following:
| ● | the difficulty of integrating acquired products, services or operations; | |
| ● | the potential disruption of the ongoing businesses and distraction of our management and the management of acquired companies; | |
| ● | difficulties in maintaining uniform standards, controls, procedures and policies; | |
| ● | the potential impairment of relationships with employees and customers as a result of any integration of new management personnel; |
| ● | the potential inability or failure to achieve additional sales and enhance our customer base through cross-marketing of the products to new and existing customers; | |
| ● | the effect of any government regulations which relate to the business acquired; | |
| ● | potential unknown liabilities associated with acquired businesses or product lines, or the need to spend significant amounts to retool, reposition or modify the marketing and sales of acquired products or operations, or the defense of any litigation, whether or not successful, resulting from actions of the acquired company prior to our acquisition; and | |
| ● | potential expenses under the labor, environmental and other laws of various jurisdictions. |
| 21 |
Our business could be severely impaired if and to the extent that we are unable to succeed in addressing any of these risks or other problems encountered in connection with an acquisition, many of which cannot be presently identified. These risks and problems could disrupt our ongoing business, distract our management and employees, increase our expenses and adversely affect our results of operations.
If we do not maintain a current and effective prospectus relating to the common stock issuable upon exercise of the Private Placement Warrants, holders may exercise such Private Placement Warrants on a “cashless basis.”
On October 4, 2022 the Company entered into a securities purchase agreement (the “Purchase Agreement”) with a certain institutional investor. The Purchase Agreement provided for the sale and issuance by the Company of an aggregate of: (i) 61,334 shares of the Company’s common stock, (ii) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 40,116 shares of common stock and (iii) warrants (the “Private Placement Warrants”“) to purchase up to 177,537 shares of common stock.
If we do not maintain a current and effective prospectus relating to the shares of common stock issuable upon exercise of the Private Placement Warrants at the time that holders wish to exercise such warrants, they will be able to exercise them on a “cashless basis”. As a result, the number of shares of common stock that holders will receive upon exercise of the Private Placement Warrants will be fewer than it would have been had such holders exercised their Private Placement Warrants for cash. Pursuant to the terms of the Purchase Agreement, we filed a registration statement to register the shares of common stock issuable upon the exercise of the Private Placement Warrants (the “Private Placement Warrant Shares”). We have agreed to keep such registration statement effective at all times until the investor holds no Private Placement Warrants or Private Placement Warrant Shares issuable upon exercise thereof. However, we cannot assure you that we will be able to do so. If the Private Placement Warrants are exercised on a “cashless” basis, we will not receive any consideration from such exercises.
Provisions of the Private Placement Warrants and our outstanding Series C Preferred Stock could discourage an acquisition of us by a third party.
Certain provisions of the Private Placement Warrants and our outstanding Series C Preferred Stock could make it more difficult or expensive for a third party to acquire us. The securities prohibit us from engaging in certain transactions constituting “fundamental transactions” unless, among other things, the surviving entity assumes our obligations under the Private Placement Warrants and the Series C Preferred Stock. Further, the Private Placement Warrants provide that, in the event of certain transactions constituting “fundamental transactions,” with some exception, holders of such warrants will have the right, at their option, to require us to repurchase such warrants at a price described in such warrants. These and other provisions of the Private Placement Warrants could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to you.
The Private Placement Warrants have certain anti-dilutive rights.
The Private Placement Warrants include full ratchet anti-dilutive rights in the event any shares of common stock or other equity or equity equivalent securities payable in common stock are granted, issued or sold (or the Company enters into any agreement to grant, issue or sell), or in accordance with the terms of the warrant agreement evidencing the Private Placement Warrants, are deemed to have granted, issued or sold, in each case, at a price less than the exercise price, which automatically decreases the exercise price of the Warrants upon the occurrence of such event, as described in greater detail in the warrant agreement, subject to a defined minimum exercise price. Such anti-dilution rights, if triggered, could result in a significant decrease in the exercise price of the Private Placement Warrants, which could result in significant dilution to existing shareholders.
| 22 |
The Private Placement Warrants are accounted for as liabilities and the changes in value of such Private Placement Warrants may have a material effect on our financial results.
Private Placement Warrants, with certain terms as included in the Purchase Agreement should be accounted for as liability instruments. As a result, the Company recorded warrant liability on the balance sheet as of December 31, 2022. Under the liability accounting treatment, the Company is required to measure the fair value of these instruments at the end of each reporting period and recognize changes in the fair value from the prior period in the Company’s receivables; limitationsoperating results for the current period. As a result of the recurring fair value measurement, our financial statements and results of operations may fluctuate quarterly based on factors which are outside our control. In the event the Private Placement Warrants are required to be accounted for under liability accounting treatment, we will recognize noncash gains or losses due to the quarterly fair valuation of these warrants which could be material. The impact of changes in fair value on our earnings may have an adverse effect on the Company’smarket price of our common stock and/or our stockholders’ equity, which may make it harder for us to, or prevent us from, meeting the continued listing standards of The Nasdaq Capital Market.
The issuance and sale of common stock upon exercise of the Private Placement Warrants may cause substantial dilution to existing stockholders and may also depress the market price of our common stock.
The Private Placement Warrants are exercisable for up to 177,537 shares of common stock, provided that the Private Placement Warrants contain a provision limiting each holder’s ability to raise new funding throughexercise the salewarrants if such exercise would cause the holder’s (or any affiliate of debtany such holder) holdings in the Company to exceed 4.99% of the Company’s issued and outstanding shares of common stock (which may be increased or equity; reduced liquidity;decreased with 61 days prior written notice from the holder, to up to 9.99% of the Company’s issued and declinesoutstanding shares of common stock). The ownership limitation does not prevent such holder from exercising some of the warrants, selling those shares, and then exercising the rest of the warrants, while still staying below the 4.99% limit. In this way, the holder of the warrants could sell more than this limit while never actually holding more shares than this limit allows. If the holder of the warrants chooses to do this, it will cause substantial dilution to the then holders of our common stock.
If exercises of the warrants and sales of such shares issuable upon exercise thereof take place, the price of our common stock may decline. In addition, the common stock issuable upon exercise of the warrants may represent overhang that may also adversely affect the market price of our common stock. Overhang occurs when there is a greater supply of a company’s stock in the market than there is demand for that stock. When this happens the price of the company’s stock will decrease, and any additional shares which shareholders attempt to sell in the market will only further decrease the share price. If the share volume of our common stock cannot absorb shares sold by the warrant holders, then the value of the Company’s securities. These and other economic factors could materially adversely affect the Company’s business, results of operations, financial condition and growth.our common stock will likely decrease.
Our business is subject to rigorous regulatory and licensing requirements.
As described in greater detail in “Item 1. Business”, above, our business is highly regulated in the United States, at both the federal and state level, and in foreign countries. If we fail to comply with regulatory requirements, or if allegations are made that we fail to comply, our results of operations and financial condition could be adversely affected.
To lawfully operate our businesses, we are required to obtain, and hold permits, product registrations, licenses and other regulatory approvals from, and to comply with operating and security standards of, numerous governmental bodies. For example, as a wholesale distributor of controlled substances, we must hold valid DEA registrations and state-level licenses, meet various security and operating standards, and comply with the Controlled Substances Act (CSA).bodies.. Failure to maintain or renew necessary permits, product registrations, licenses or approvals, or to comply with required standards, could have an adverse effect on our results of operations and financial condition. We are also required to comply with various state pricing gouging laws. Products that we source and distribute must also comply with regulatory requirements.
Noncompliance or concerns over noncompliance may result in suspension of our ability to distribute or import products, product bans, recalls or seizures, or criminal or civil sanctions, which, in turn, could result in product liability claims and lawsuits, including class actions.
Many of our competitors are better established and have resources significantly greater than we have, which may make it difficult to fend off competition.
We expect to compete with the three largestlarge ADR distributors (McKesson,(such as McKesson, Cardinal Health and AmerisourceBergen), in addition to other pharmaceutical distributors, buying groups, software products, and various start-up drug companies. Many of these companies have substantially greater financial and manufacturer-backed resources, longer operating histories, greater name recognition and more established relationships in the industry than us. In addition, a number of these competitors may combine or form strategic partnerships. As a result, our competitors may establish a more favorable footing in the pharmaceutical industry with respect to pricing or other factors. Our failure to compete successfully with any of these companies would have a material adverse effect on our business and the trading price of our common stock.
The three distributors listed above have a strong control over our industry, as they have contracts with approximately 24,000 independent, retail pharmacies that limit the participants’ ability to purchase pharmaceuticals outside of those primary distributors. Additional restrictive elements exist within the pharmaceutical channels of distribution. For example, a number of the inventory management systems, either developed by the distributors or third-party vendors, have been developed to require compliance to these restrictive purchasing agreements. Management anticipates that other existing and prospective competitors will adopt technologies or business plans similar to ours or seek other means to develop operations competitive with ours, particularly if our development of large-scale production progresses as scheduled.
We will need to expand our member base or our profit margins to attain profitability.
Currently, we are paid an administrative feeaware of up to 6 percent of the buying price on the generic pharmaceuticals sold to pharmacies and up to 1 percent on brand pharmaceuticals that pass through our pharmaceutical exchanges. Our management is aware that the competitiveness of the group of suppliers that participate inwithin our systemindustry and intend to price products on our exchange is a key factor in determining how many purchasing pharmacies and wholesalers will purchase products through our platforms.accordingly. However, price is not the only factor that influences where retail pharmacies will obtain their product. Quality fulfillment services are also important, and retail pharmacies have historically received quality fulfillment services from the three major ADR distributors. In order to be more competitive, we must improve our customer service and wholesaler fulfillment efforts, because the independent retail pharmacy has for years considered this element of the fulfillment process as important as price. Other factors influencing the pharmacies purchasing behavior in the future will be changes brought upon by the ACA, which regulates some aspects of pharmaceutical spending and pricing. Management believes that we should benefit substantially from our pricing and product knowledge that is offered by our platform.
Profitability may be further increased as a result of lower cost of goods, should the Company build stronger relationships with manufacturers and other larger buying groups that serve wholesalers and distributors. On a larger scale, those margins are expected to drop depending upon the breadth of products provided in the market and the sale turn rates required. We are currently undertaking a significant effort to increase our membership base through attendance at annual conferences and other strategies. Trxade has an expandedWe intend to expand our e-mail marketing strategy based on our competitive price advantages and price trend analysis tools.unique distribution services.
There are inherent risks associated with our operations within the Pharmaceutical Distribution Market.
There are inherent risks involved with doing business within the pharmaceutical distribution market, including:
| ● | Improperly manufactured products may prove dangerous to the end consumer. | |
| ● | Products may become adulterated by improper warehousing methods or modes of shipment. | |
| ● | Counterfeit products or products with fake pedigree papers. | |
| ● | Unlicensed or unlawful participants in the distribution channel. | |
| ● | Risk with default and the assumption of credit loss. | |
| ● | Regulatory risks. | |
| ● | Risk related to the loss of supply, or the loss of a number of suppliers, or in the delay of obtaining the supply of drugs. |
Although all of our end-user agreements require our customers to indemnify us and for any and all liabilities resulting from our participation in the pharmaceutical distribution industry, we cannot assure you that the parties required to provide such indemnification will have the financial resources to do so. Additionally, although we have evaluated appropriate state statutes and federal laws pertaining to pharmaceutical distribution in an effort to diminish our risks, the Board of Pharmacy for each state is responsible for interpreting their state laws, and their interpretations may not comport with our analysis. It is also possible that any third-party logistics arrangements may disrupt service, create a loss of income, or other unforeseen disruptions should the service provider experience any legal, financial or other difficulties of their own.
We do not have a traditional credit facility with a financial institution, which may adversely impact our operations.
We do not have a traditional credit facility with a financial institution, such as a working line of credit. The absence of such a facility could adversely impact our operations, as it may constrain our ability to have available the working capital for equipment purchases or other operational requirements. If adequate funds are not otherwise available, we may be required to delay, scale back or eliminate portions of our business development efforts. Without credit facilities, we could be forced to cease operations and investors in our securities could lose their entire investment.
| 24 |
We offer limited credit to the pharmacies which limits the amount of the orders that they place and may result in us losing business and a reduction in our revenues.
We currently offer a limited amount of credit to our members. Such limited credit reduces the risk that such members do not pay for products; however, it also limits the amount of revenue we generate per member. We believe that if we were to increase the amount of credit we provide to members we would generate more revenues, but bear more risk of non-payment. We are currently exploring increasing the amount of credit we provide to members, which may in turn result in an increase in receivables and write-offs.
We are dependent upon our current management, who may have conflicts of interest.
We are dependent upon the efforts of our current management. All of our officers and directors have duties and affiliations with other companies. Even though these companies are not competitors or involved in pharmaceutical distribution, involvement of our officers and directors in other businesses may still present a conflict of interest regarding decisions they make for Trxade or with respect to the amount of time available for Trxade. The loss of any of our officers or directors and, in particular, Mr. Prashant Patel, our President or Mr. Suren Ajjarapu, our Chief Executive Officer and Chairman of the Company, could have a materially adverse effect upon our business and future prospects.
The Company holds, on behalf of and for the benefit of Mr. Suren Ajjarapu, a personal disability insurance policy providing for a $1,500,000 lump sum benefit, payable to Mr. Ajjarapu, in the event of Mr. Ajjarapu’s disability. The premiums on such policy will be paid by the Company for so long as Mr. Ajjarapu is employed by the Company.
The Company also holds a $4,000,000 key-man life insurance policy on the life of Mr. Suren Ajjarapu, and a $1,500,000 lump sum disability insurance policy on Mr. Ajjarapu, providing for the Company as beneficiary of such policies.
While our management team has considerable information technology and entrepreneurial experience, none of our management was involved in pharmaceutical distribution prior to joining the Company and, as such, did not have any technical experience in pharmaceutical distribution prior to joining us. In the event of the loss of Mr. Ajjarapu’s services, we will seek to hire and retain a qualified professional. In the event of the loss of his services in connection with his death, upon obtaining funding from the key-man life insurance, management intends to hire qualified and experienced personnel. We may be unable to find a suitable or qualified replacement for Mr. Ajjarapu and as such our operations and/or prospects may suffer.
We rely on third party contracts.
We depend on others to provide products and services to us. We do not manufacture pharmaceuticals and we do not sell pharmaceuticals to the end consumer. We do not control these wholesalers, suppliers and purchasers, and although our arrangements with them will be terminable or of limited length, a change may be difficult to implement. At this time, we have a working relationship with over 50 wholesalers10 manufacturers and the nation’s largest buying group.other suppliers. Although we believe that those entities are satisfied with their business relationship with Trxade, if our buying group pharmacies and two or threeseveral of the wholesalersour vendors decided no longer to do business with us, that suppliervendor void would materially and adversely affect our competitiveness in the marketplace.
We depend on suppliers to make their drugs and other medical products available to us for resale and are subject to risks associated with the availability of these drugs and other medical products.
We do not directly manufacture any of the products we sell and instead we rely on third parties to manufacture and/or procure such drugs and other medical products for us to resell. Supply chain constraints have, and may in the future have, a negative impact on the availability of drugs and medical products that we sell. Our supplier relationships could be interrupted, become less favorable to us or be terminated and the supply of these drugs or products could be interrupted or become insufficient. Supply interruptions or other disruptions in manufacturing processes could be caused by events beyond our control, including natural disasters, supplier facility shut-downs, defective raw materials, the impact of epidemics or pandemics, such as COVID-19, and actions by U.S. or international governments, including export restrictions or tariffs. A sustained supply reduction or interruption, and an inability to develop alternative and additional sources for such supply, could result in lost sales, increased cost, damage to our reputation, and may have an adverse effect on our business.
| 25 |
We may have difficulties in sourcing or selling products due to a variety of causes.
We might experience difficulties and delays in sourcing and selling products due to a variety of causes, such as: difficulties in complying with the legal requirements for export or import of pharmaceuticals or supplies; suppliers’ failure to satisfy production demand; manufacturing or supply problems such as inadequate resources; and real or perceived quality issues. Difficulties in product manufacturing or access to raw materials could result in supplier production shutdowns, product shortages and other supply disruptions. The COVID-19 pandemic has adversely affected the availability of some products, resulting in product allocation and delivery delays. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
Rapid technological change in our industry presents us with significant risks and challenges.
Our industry is characterized by rapid technological change, changing consumer requirements, short product lifecycles and evolving industry standards. Our success will depend on our ability to develop or to acquire and market new services. There is no guarantee that we will possess the resources, either financial or personnel, for the research, design and development of new applications or services, or that we will be able to utilize these resources successfully and avoid technological or market obsolescence. Further, there can be no assurance that technological advances by one or more of our competitors or future competitors will not result in our present or future applications and services becoming uncompetitive or obsolete.
We are currently facing and may in the future face difficulties in sourcing products and inventory due to a variety of causes.
Due to the continued effects of the COVID-19 pandemic, the governmental responses to contain the spread of such virus,At times, we have to date experienced issues with the availability of certain products, resulting in product allocation and delivery delays, which has not to date, had a material adverse effect on our results of operations. We might also experience difficulties and delays in sourcing products and inventory due to a variety of causes in the future, such as: difficulties in complying with the legal requirements for export or import of pharmaceuticals or components; suppliers’ failures to satisfy production demand; manufacturing or supply problems such as inadequate resources; real or perceived quality issues; and advanced deposits which are at risk of return if product is not delivered. Difficulties in product manufacturing or access to raw materials could result in supplier production shutdowns, product shortages and other supply disruptions. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
We have in the past, and may in the future, not be able to sell our inventory, at or above the price we acquired such inventory for, and have in the past, and may in the future, be forced to write-down inventory and certain of our other assets which may have a material adverse effect on our balance sheet.
Due to the supply and demand nature of our pharmaceutical business and the personal protective equipment (PPE) business, especially in connection with the rapidly changing regulations, recommendations and guidance surrounding COVID-19,varying demand of certain medications the inventory of products we have acquired, or may acquire in the future, has been/may be, acquired at a cost higher than the price at which we may be able to resell such products. As a result, in the past we have, and in the future we may not be able to, make a profit on such sales and have in the past and may in the future, have to write-downwrite down a significant portion of our inventory. During the years ended December 31, 20212023 and 2020, write-down2022, write down to market value was $376,348$4,265,399 and $1,220,269,$0 respectively. A significant write-downwrite down of assets may have a material adverse effect on our balance sheet and results of operations.
We may not receive products or receive refunds for deposited amounts and may experience losses in connection with such deposits.
We might not receive products or the return of funds on deposits that have been provided. We have two deposits outstanding as of the date of this report in an aggregate amount of approximately $1,081,250. In the event we do not receive the return of our deposits (through litigation or otherwise), this will cause us financial harm and as a result the Company has taken a significant charge on our financial statements by taking a loss in the amount of such deposit amount. Additionally, in the future we may provide additional deposits for products which may be material, which deposits may not be refunded timely, if at all, and which products may not be delivered, or may be defective or unusable. Any significant losses of deposited funds could have a material adverse effect on our financial condition, results of operations and the value of our securities.
In July 2020, the Company’s wholly-owned subsidiary, Integra, entered into an agreementpast we (or our subsidiaries) have been involved in litigation with suppliers and disputes regarding deposits made with third parties, including litigation involving Studebaker Defense Group, LLC (“Studebaker”) wherein Integra would pay Studebaker a down payment of $500,000 and Studebaker would deliver 180,000 boxes of nitrile gloves by August 14, 2020. Integra wired the $500,000 to Studebaker, but to date, Studebaker has not delivered the gloves or provided a refund of the deposit. On December 31, 2020, we filed a complaint against Studebaker in Florida state court, Case No. 20-CA-010118 in the Circuit Court for the Thirteenth Judicial Circuit in Hillsborough County, for among other things, breach of contract. On January 29, 2021, Integra Pharma Solutions filed a motion for clerk’s default against Studebaker. On February 2, 2021, the clerk of court issued default against Studebaker. On March 4, 2021, Integra Pharma Solutions filed a motion for final default judgment against Studebaker. On March 22, 2021, counsel for Studebaker filed a notice of appearance in the case. On March 24, Studebaker filed a response in opposition to the motion for final judgment, and on March 25, 2021, Studebaker filed a motion to dismiss the case. On May 14, 2021, the Court denied Integra’s motion for final default judgment, granted Studebaker’s motion to set aside the clerk’s default, and denied Studebaker’s motion to dismiss. An amended answer and affirmative defenses were filed by Studebaker on October 14, 2021. Integra’s motion to strike the affirmative defenses, or in the alternative, motion for more definite statement is scheduled for hearing on April 27, 2022. We have also scheduled the deposition of Studebaker’s corporate representative on April 12, 2022, and moved to compel better answers to outstanding discovery. The litigation remains pending and is in the discovery phase. Integra remains confident it can successfully prosecute its claims against Studebaker on the merit. On June 30, 2021, the $500,000 was recorded as Loss on Inventory Investment.
In August 2020, Integra, entered into an agreement with Sandwave Group Dsn Bhd (“Sandwave”), wherein Integra would pay Sandwave a down payment of $581,250 and Sandwave’s supplier, Crecom Burj Group SDN BHD (“Crecom”), would deliver 150,000 boxes of nitrile gloves within 45 days. Integra wired the $581,250 to Sandwave, which in turn wired the purchase price to Crecom, which Crecom accepted; however, to date, Crecom has not delivered the nitrile gloves. Integra demanded return of its $581,250 and Crecom has acknowledged that Integra is entitled to a refund, but to date Crecom has failed to return Integra’s money. In February 2021, Integra filed a complaint against Crecom in Malaysia: Case No. WA-22NCC-55-02/2021Bhd. These disputes previously resulted in the High Court of Malaysia at Kuala Lumpur in the Federal Territory, Malaysia for the Malaysian equivalent of breach of contract. Crecom filed an appearanceCompany recording a loss on March 1, 2021. In April 2021, an Application for Summary Judgment was filed with the court, and on May 25, 2021, the Court extracted the sealed application, and a copy thereof was served on Crecom’s attorneys and Crecom, 14 days later, filed an Affidavit in Reply with the court alleging that there are issues to be tried and that this case must go to a full trial. On June 28, 2021, the court directed both parties to file their written submissions/arguments in relation to the application for summary judgment on or before July 12, 2021, and scheduled a hearing thereon for August 26, 2021. At the final hearing on October 18, 2021, the ruling for the summary judgment was denied and a trial date is pending. The Company believes that it will prevail in the lawsuit filed; but the steps to enforce a judgment in Malaysia, if any, may be cumbersome, time consuming or costly. The Company cannot determine the timing of the judgment, nor the amount ultimately collected. At June 30, 2021, the $581,250 was recorded as Loss on Inventory Investment.inventory investments.
Our quarterly results have in the past, and may in the future, fluctuate significantly due to certain non-recurring sales of products.
Our quarterly revenues have in the past and may in the future fluctuate significantly due to certain non-recurring sales of personal protective equipment (PPE) and other products and associated costs of revenues therewith, which may be compounded in our year over year financial results. As such, we believe that quarter-to-quarter comparisons of our revenues, operating results and cash flows may not be meaningful and should not be relied upon as an indication of future performance.
Our investments in new businesses and new products, services, and technologies is inherently risky, and could disrupt our ongoing businesses.
We have invested and expect to continue to invest in new businesses, products, services, and technologies. Such endeavors may involve significant risks and uncertainties, including insufficient revenues from such investments to offset any new liabilities assumed and expenses associated with these new investments, inadequate return of capital on our investments, distraction of management from current operations, and unidentified issues not discovered in our due diligence of such strategies and offerings that could cause us to fail to realize the anticipated benefits of such investments and incur unanticipated liabilities. Because these new ventures are inherently risky, no assurance can be given that such strategies and offerings will be successful and will not adversely affect our reputation, financial condition, and operating results. To date we have taken losses and/or write-downs on several businesses, products, services, and technologies. For example, (a) we had $725,973 of loss on impairment of goodwill for the year ended December 31, 2020, in connection with the acquisition of Community Specialty Pharmacy, LLC; (b) we designed and invested resources into the “Bonum Health Hub”, a self-enclosed, free standing virtual examination room, which was launched by the Company’s wholly-owned Bonum Health, LLC, in November 2019 and was expected to be operational in April 2020; however, due to the COVID-19 pandemic, the Company does not anticipate installations moving forward, and has taken a write off of the hubs purchased at June 30, 2021 in the amount of $143,891, which is included under loss on inventory investments in the statement of operations for the year ended December 31, 2021; and (c) we also used resources and funding to create a Health Passport application during 2020 and 2021, which was planned to store a user’s health and vaccination status and allow confirmation thereof via a QR code; however, we did not generate any revenue from this product and the product was discontinued at the end of December 2021. The use of resources for new businesses and new products, services, and technologies, to the extent such new businesses and new products, services, and technologies do not generate revenues or profits may take management’s focus and time away from more profitable endeavors, may require the Company to take significant write-downs or write-offs, may take funding away from the Company’s other operations or growth opportunities, which may ultimately be more profitable, and may have a material adverse effect on the Company’s cash flows, liquidity and revenues, any or all of which may cause the value of the Company’s securities to decline in value or become worthless.
Risks Relating to Our Information Systems; Technology and Intellectual Property
We may be subject to claims that we violated intellectual property rights of others, which are extremely costly to defend and could require us to pay significant damages and limit our ability to operate.
Companies primarily conducting their business on the Internet andinternet, in the technology industries,sector, and other patent and trademark holders seeking to profit from royalties in connection with grants of licenses, own large numbers of patents, copyrights, trademarks and trade secrets and frequently enter into litigation based on allegations of infringement or other violations of intellectual property rights. There may be intellectual property rights held by others, including issued or pending patents and trademarks, that cover significant aspects of our technologies, content, branding or business methods. Any intellectual property claims against us, regardless of merit, could be time-consuming and expensive to settle or litigate and could divert our management’s attention and other resources. These claims also could subject us to significant liability for damages and could result in our having to stop using technology, content, branding or business methods found to be in violation of another party’s rights. We might be required or may opt to seek a license for rights to intellectual property held by others, which may not be available on commercially reasonable terms, or at all. If we cannot license or develop technology, content, branding or business methods for any allegedly infringing aspect of our business, we may be unable to compete effectively. Even if a license is available, we could be required to pay significant royalties, which could increase our operating expenses. We may also be required to develop alternative non-infringing technology, content, branding or business methods, which could require significant effort and expense and be inferior. Any of these results could harm our operating results.
Our business and operations depend on the proper functioning of information systems, critical facilities and distribution networks.
We rely on our manufacturer, vendors and other third-party service providers’ information systems for a wide variety of critical operations, including to obtain, rapidly process, analyze and manage data to:
| ● | facilitate the purchase and distribution of inventory items | |
| ● | receive, process and ship orders on a timely basis; | |
| ● | manage accurate billing and collections for thousands of customers; | |
| ● | process payments to suppliers; and | |
| ● | generate financial information. |
Our business also depends on the proper functioning of our critical facilities and our distribution networks. Our results of operations could be adversely affected if our or a service provider’s information systems, critical facilities or distribution networks are disrupted (including disruption of access), are damaged or fail, whether due to physical disruptions, such as fire, natural disaster, pandemic or power outage, or due to cyber-security incidents, ransomware or other actions of third parties, including labor strikes, political unrest and terrorist attacks. Manufacturing disruptions also can occur due to regulatory action, production quality deviations, safety issues or raw material shortages or defects, or because a key product or component is manufactured at a single manufacturing facility with limited alternate facilities.
We rely on network and information systems and other technologies and a disruption, cyber-attack, failure or destruction of such networks, systems, or technologies may disrupt our business or result in liability.liability.
Network and information systems and other technologies, including those related to our computer, data back-up and processing systems, network management, customer service operations and programming delivery, are critical to our business activities. Network and information systems-related events, such as computer hackings, cyber-attacks, computer viruses, worms or other destructive or disruptive software, process breakdowns, denial of service attacks, malicious social engineering or other malicious activities, or any combination of the foregoing, or power outages, natural disasters, terrorist attacks or other similar events, could result in a degradation or disruption of our services or damage to our properties, equipment and data. These events also could result in large expenditures to repair or replace the damaged properties, networks or information systems or to protect them from similar events in the future.
The risk of these systems-related events and security breaches occurring has intensified, in part because we maintain certain information necessary to conduct our businesses in digital form stored on cloud servers. While we develop and maintain systems seeking to prevent systems-related events and security breaches from occurring, the development and maintenance of these systems isare costly and requires ongoing monitoring and updating as technologies change and efforts to overcome security measures become more sophisticated. Despite these efforts, there can be no assurance that these events and security breaches will not occur in the future. Moreover, we may provide certain confidential, proprietary and personal information to third parties in connection with our businesses, and while we obtain assurances that these third parties will protect this information, there is a risk that this information could be compromised.
If any of our systems are damaged, fail to function properly or otherwise become unavailable, we may incur substantial costs to repair or replace them, and may experience loss or corruption of critical data and interruptions or delays in our ability to perform critical functions, which could adversely affect our business and results of operations. In addition, we are currently making, and expect to continue to make, substantial investments in our information technology systems and infrastructure, some of which are significant. Upgrades involve replacing existing systems with successor systems, making changes to existing systems, or cost-effectively acquiring new systems with new functionality. Implementing new systems carries significant potential risks, including failure to operate as designed, potential loss or corruption of data or information, cost overruns, implementation delays, disruption of operations, and the potential inability to meet business and reporting requirements. While we are aware of inherent risks associated with replacing these systems and believe we are taking reasonable action to mitigate known risks, these technology initiatives may not be deployed as planned or may not be timely implemented without disruption to our operations.
In the past, we had an incident with an email account being compromised and an attempt was made to get the us to wire outgoing money. We did not fall victim to the attempt, conducted a thorough investigation, performed cleanup procedures, and instituted additional security measuremeasures to mitigate the risk of this incident from occurring in the future. Risk mitigation includes the board of directors inquiring with the information technology department on the status of cyber risks management, on a quarterly basis.
There may be losses or unauthorized access to or releases of confidential information, including personally identifiable information, that could subject the Company to significant reputational, financial, legal and operational consequences.
The Company’s business requires it to use, transmit and store confidential information including, among other things, personally identifiable information (“PII”) with respect to the Company’s customers and employees. The Company devotes significant resources to network and data security, including through the use of encryption and other security measures intended to protect its systems and data. But these measures cannot provide absolute security, and losses or unauthorized access to or releases of confidential information occur and could materially adversely affect the Company’s reputation, financial condition and operating results. The Company’s business also requires it to share confidential information with third parties. Although the Company takes steps to secure confidential information that is provided to third parties, such measures are not always effective and losses or unauthorized access to or releases of confidential information occur and could materially adversely affect the Company’s reputation, financial condition and operating results.
For example, the Company may experience a security breach impacting the Company’s information technology systems that compromises the confidentiality, integrity or availability of confidential information. Such an incident could, among other things, impair the Company’s ability to attract and retain customers for its products and services, impact the Company’s stock price, materially damage supplier relationships, and expose the Company to litigation or government investigations, which could result in penalties, fines or judgments against the Company.
The Company has implemented systems and processes intended to secure its information technology systems and prevent unauthorized access to or loss of sensitive data. As with all companies, these security measures may not be sufficient for all eventualities and may be vulnerable to hacking, employee error, malfeasance, system error, faulty password management or other irregularities. In addition to the risks relating to general confidential information described above, the Company is also subject to specific obligations relating to health data and payment card data. Health data is subject to additional privacy, security and breach notification requirements, and the Company can be subject to audit by governmental authorities regarding the Company’s compliance with these obligations. If the Company fails to adequately comply with these rules and requirements, or if health data is handled in a manner not permitted by law or under the Company’s agreements with healthcare institutions, the Company could be subject to litigation or government investigations, may be liable for associated investigatory expenses, and could also incur significant fees or fines.
Under payment card rules and obligations, if cardholder information is potentially compromised, the Company could be liable for associated investigatory expenses and could also incur significant fees or fines if the Company fails to follow payment card industry data security standards. The Company could also experience a significant increase in payment card transaction costs or lose the ability to process payment cards if it fails to follow payment card industry data security standards, which would materially adversely affect the Company’s reputation, financial condition and operating results.
| 28 |
System errors or failures of our platform or services to conform to specifications could cause unforeseen liabilities or injury, harm our reputation and have a material adverse impact on our results of operations.
The software and technology services that we operate are complex. As with complex systems offered by others, our software and technology services may contain errors, especially when first introduced. Failure of a customer’s system to perform in accordance with our documentation could constitute a breach of warranty and could require us to incur additional expenseexpenses in order to make the system comply with the documentation. If such failure is not remedied in a timely manner, it could constitute a material breach under a contract, allowing the client to cancel the contract, obtain refunds of amounts previously paid, or assert claims for significant damages.
Risks Associated with Bonum Health Telemedicine Services
The telehealth market is immature and volatile.
The telehealth market is relatively new and unproven, and it is uncertain whether it will achieve and sustain high levels of demand, consumer acceptance and market adoption. Our success will depend to a substantial extent on the willingness of our clients’ members or patients to use, and to increase the frequency and extent of their utilization of, our services, as well as on our ability to demonstrate the value of telehealth to employers, health plans, government agencies and other purchasers of healthcare for beneficiaries. Negative publicity concerning our services or the telehealth market as a whole could limit market acceptance of our services. If our clients, or their members or patients, do not perceive the benefits of our services, or if our services are not competitive, then our market may not develop at all, or it may develop more slowly than we expect. Similarly, individual and healthcare industry concerns or negative publicity regarding patient confidentiality and privacy in the context of telehealth could limit market acceptance of our healthcare services. If any of these events occurs, it could have a material adverse effect on our business, financial condition or results of operations.
Our telehealth business could be adversely affected by legal challenges to our business model or by actions restricting our ability to provide services in certain jurisdictions.
Our ability to conduct telehealth services in a particular U.S. state is dependent upon the applicable laws governing remote healthcare and the practice of medicine and healthcare delivery in general in such location which are subject to changing political, regulatory and other influences. With respect to telehealth services, such services and our ability to offer such services are subject to rules established or interpreted by state medical boards and whether such boards consider such services to be the practice of medicine. The definition of practicing medicine is subject to change and open to evolving interpretations by medical boards and state attorneys’ generals, among others. Accordingly, we must monitor our compliance with laws in the jurisdictions in which we operate, on an ongoing basis, and we cannot provide assurance that our activities and arrangements, if challenged, will be found to be in compliance with the law. Additionally, it is possible that the laws and rules governing the practice of medicine, including remote healthcare, in one or more jurisdictions may change in a manner which negatively effects our ability to operate. If a successful legal challenge or an adverse change in the relevant laws were to occur, and we were unable to adapt our business model accordingly, our operations in the affected jurisdictions would be disrupted, which could have a material adverse effect on our business, financial condition and results of operations.
In our telehealth business, we will be dependent on our relationships with affiliated professions and our business would be adversely affected if those relationships were disrupted.
There is a risk that state authorities in some jurisdictions may find that contractual relationships with physicians providing telehealth violate laws prohibiting the corporate practice of medicine. State corporate practice of medicine doctrines also often impose penalties on physicians themselves for aiding the corporate practice of medicine, which could discourage physicians from participating in our network of providers. A material change in our relationship with our healthcare providers, whether resulting from a dispute among the entities, a change in government regulation, or the loss of these affiliations, could impair our ability to provide services and could have a material adverse effect on our business, financial condition and results of operations.
Our “Bonum Health” telehealth business will depend on our ability to maintain and expand a network of qualified providers.
The success of our “Bonum Health” telehealth services is dependent upon our ability to maintain a network of qualified telehealth providers. If we are unable to recruit and retain board-certified physicians and other healthcare professionals, it would have a material adverse effect on our “Bonum Health” business and ability to grow such operations. We may not be willing to pay the costs demanded by such services providers and/or changes in Medicare and/or Medicaid reimbursement levels and other pressures on healthcare providers and consolidation activity among hospitals, physician groups and healthcare providers may make such providers harder or more expensive to find and contract with. The result of the above may be that our “Bonum Health” telehealth services are unsuccessful, which may result in a material adverse effect to our operations.
Rapid technological change in the telehealth industry presents us with significant risks and challenges.
The telehealth market is characterized by rapid technological change, changing consumer requirements, short product lifecycles and evolving industry standards. Our success will depend on our ability to enhance our offerings with next-generation technologies and to develop or to acquire and market new services. There is no guarantee that we will possess the resources, either financial or personnel, for the research, design and development of new applications or services, or that we will be able to utilize these resources successfully and avoid technological or market obsolescence. Further, there can be no assurance that technological advances by one or more of our competitors or future competitors will not result in our present or future software-based products and services becoming uncompetitive or obsolete.
The telehealth industry is competitive, and if we are not able to compete effectively, our business, financial condition and results of operations will be harmed.
While the telehealth market is in an early stage of development, it is competitive and we expect it to attract increased competition, which could make it difficult for us to succeed. We currently face competition in the telehealth industry from a range of companies, including specialized software and solution providers that offer similar solutions, often at substantially lower prices, and that are continuing to develop additional products and becoming more sophisticated and effective. These competitors include Doctor On Demand, MDLive, Teladoc and others. In addition, large, well-financed health systems have in some cases developed their own telehealth tools and provide these solutions to their customers at discounted prices. The surge in interest in telehealth, and in particular the relaxation of HIPAA privacy and security requirements, has also attracted new competition from providers who utilize consumer-grade video conferencing platforms such as Zoom, Microsoft Teams, Google Meet and Twilio. Competition from large software companies or other specialized solution providers, communication tools and other parties could result in continued pricing pressures, which is likely to lead to price declines in certain product segments, which could negatively impact our future market, sales, profitability and market share (if any). If we are unable to successfully compete in the telehealth market, our business, financial condition and results of operations could be materially adversely affected.
The emergence of new technologies may render our telehealth solution obsolete or require us to expend significant resources in order to remain competitive.
The U.S. healthcare industry is massive, with a number of large market participants with conflicting agendas, and it is subject to significant government regulation and is currently undergoing significant change. Changes in the telehealth industry, for example, such as the emergence of new technologies as more competitors enter our market, could result in our telehealth solution being less desirable or relevant. If healthcare benefits trends shift or entirely new technologies are developed that replace existing solutions, our existing or future products could be rendered obsolete, and our business could be adversely affected. In addition, we may experience difficulties with industry standards, design or marketing that could delay or prevent our development, introduction or implementation of new applications and enhancements.
If we fail to develop widespread brand awareness cost-effectively, our business may suffer.
We believe that developing and maintaining widespread awareness of our brand in a cost-effective manner is critical to achieving widespread adoption of our products and attracting new clients. Our brand promotion activities may not generate client awareness or increase revenue, and even if they do, any increase in revenue may not offset the expenses we incur in building our brand. If we fail to successfully promote and maintain our brand, or incur substantial expenses in doing so, we may fail to attract or retain clients necessary to realize a sufficient return on our brand-building efforts or to achieve the widespread brand awareness that is critical for broad client adoption of our solution.
Risks Associated with Our Governing Documents and Delaware Law
Our certificate of incorporation provides for indemnification of officers and directors at our expense and limits their liability, which may result in a major cost to us and hurt the interests of our stockholders because corporate resources may be expended for the benefit of officers or directors.
Our Certificatecertificate of Incorporationincorporation provides for indemnification as follows: “To the fullest extent permitted by applicable law, the Corporation is authorized to provide indemnification of, and advancement of expenses to, such agents of the Corporation (and any other persons to which Delaware law permits the Corporation to provide indemnification) through Bylaw provisions, agreements with such agents or other persons, vote of stockholders or disinterested directors or otherwise, in excess of the indemnification and advancement otherwise permitted by Section 145 of the Delaware General Corporation Law (the “DGCL”), subject only to limits created by applicable Delaware law (statutory or non-statutory), with respect to actions for breach of duty to the Corporation, its stockholders and others.” Our obligation to indemnify our officers and directors may discourage stockholders from bringing a lawsuit against our officers or directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against our officers and directors, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against our officers and directors pursuant to these indemnification provisions.
We have been advised that, in the opinion of the SEC, indemnification for liabilities arising under federal securities laws is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification for liabilities arising under federal securities laws, other than the payment by us of expenses incurred or paid by a director, officer or controlling person in the successful defense of any action, suit or proceeding, is asserted by a director, officer or controlling person in connection with our activities, we will (unless in the opinion of our counsel, the matter has been settled by controlling precedent) submit to a court of appropriate jurisdiction, the question whether indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. The legal process relating to this matter if it were to occur is likely to be very costly and may result in us receiving negative publicity, either of which factors is likely to materially reduce the market and price for our shares.
| 29 |
Our certificate of incorporation contains a specific provision that limits the liability of our directors for monetary damages to the Company and the Company’s stockholders and requires us, under certain circumstances, to indemnify officers, directors and employees.
The limitation of monetary liability against our directors, officers and employees under Delaware law and the existence of indemnification rights to them may result in substantial expenditures by us and may discourage lawsuits against our directors, officers and employees.
Our certificate of incorporation contains a specific provision that limits the liability of our directors for monetary damages to the Company and the Company’s stockholders, including as a result of a breach of their fiduciary duties, except to the extent such exception from liability is not permitted under Delaware General Corporation Law.the DGCL. We also have contractual indemnification obligations under our employment and engagement agreements with our executive officers and directors, as well as pursuant to indemnification agreements. The foregoing indemnification obligations could result in us incurring substantial expenditures to cover the cost of settlement or damage awards against our directors and officers, which the Company may be unable to recoup. These provisions and resultant costs may also discourage us from bringing a lawsuit against our directors and officers for breaches of their fiduciary duties and may similarly discourage the filing of derivative litigation by our stockholders against our directors and officers, even though such actions, if successful, might otherwise benefit us and our stockholders.
Our directors have the right to authorize the issuance of shares of preferred stock and additional shares of our common stock.
Our directors, within the limitations and restrictions contained in our certificate of incorporation and without further action by our stockholders, have the authority to issue shares of preferred stock from time to time in one or more series and to fix the number of shares and the relative rights, conversion rights, voting rights, and terms of redemption, liquidation preferences and any other preferences, special rights and qualifications of any such series. Any issuance of shares of preferred stock could adversely affect the rights of holders of our common stock. Should we issue additional shares of our common stock at a later time, each investor’s ownership interest in our stock would be proportionally reduced.
Anti-takeover provisions may impede the acquisition of the Company.
Certain provisions of the Delaware General Corporation Law (DGCL)DGCL have anti-takeover effects and may inhibit a non-negotiated merger or other business combination, notwithstanding the fact that our certificate of incorporation provides that we are not subject to Section 203 of Delaware General Corporation Law,the DGCL, which relates to certain restrictions on business combinations with interested stockholders. These provisions are intended to encourage any person interested in acquiring the Company to negotiate with, and to obtain the approval of, our directors, in connection with such a transaction. As a result, certain of these provisions may discourage a future acquisition of the Company, including an acquisition in which the stockholders might otherwise receive a premium for their shares. In addition, we can also authorize “blank check” preferred stock, which could be issued by our Board of Directors without stockholder approval and may contain voting, liquidation, dividend and other rights superior to our common stock.
Compliance, Reporting and Listing Risks
We incur significant costs to ensure compliance with U.S. and NASDAQ Capital Market reporting and corporate governance requirements.
We incur significant costs associated with our public company reporting requirements and with applicable U.S. and NASDAQ Capital Market corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 and other rules implemented by the SEC and The NASDAQ Capital Market. The rules of The NASDAQ Capital Market include requiring us to maintain independent directors, comply with other corporate governance requirements and pay annual listing and stock issuance fees. All of such SEC and NASDAQ obligations require a commitment of additional resources including, but not limited to, additional expenses, and may result in the diversion of our senior management’s time and attention from our day-to-day operations. We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consumingtime-consuming and costly. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our Board of Directors or as executive officers.
We will continue to incur increased costs as a result of being a reporting company, and given our limited capital resources, such additional costs may have an adverse impact on our profitability.
We are an SEC-reporting company. The rules and regulations under the Exchange Act require reporting companies to provide periodic reports with interactive data files, which require that we engage legal, accounting and auditing professionals, and inline eXtensible Business Reporting Language (iXBRL) and EDGAR (Electronic Data Gathering, Analysis, and Retrieval) service providers. The engagement of such services can be costly, and we may continue to incur additional losses, which may adversely affect our ability to continue as a going concern. In addition, the Sarbanes-Oxley Act of 2002, as well as a variety of related rules implemented by the SEC, have required changes in corporate governance practices and generally increased the disclosure requirements of public companies. For example, as a result of being a reporting company, we are required to file periodic and current reports and other information with the SEC, and we have adopted policies regarding disclosure controls and procedures and regularly evaluate those controls and procedures.
The additional costs we continue to incur in connection with becoming a reporting company (expected to be several hundred thousand dollars per year) will continue to further stretch our limited capital resources. Due to our limited resources, we have to allocate resources away from other productive uses in order to continue to comply with our obligations as an SEC reporting company. Further, there is no guarantee that we will have sufficient resources to continue to meet our reporting and filing obligations with the SEC as they come due.
We may not be able to comply with NASDAQ’s continued listing standards.
Our common stock was approved for listing on The NASDAQ Capital Market under the symbol “MEDS”, in February 2020. Notwithstanding such listing, there can be no assurance any broker will be interested in trading our stock. Therefore, it may be difficult to sell your shares of common stock if you desire or need to sell them. Our former underwriters are not obligated to make a market in our securities, and even if they do make a market, they can discontinue market makingmarket-making at any time without notice. Neither we nor the underwriters canWe cannot provide any assurance that an active and liquid trading market in our securities will develop or, if developed, that such a market will continue.
There is also no guarantee that we will be able to maintain our listing on The NASDAQ Capital Market for any period of time by perpetually satisfying NASDAQ’s continued listing requirements. Our failure to continue to meet these requirements may result in our securities being delisted from NASDAQ.
Among At times, including during our 2023 fiscal year we have received deficiency notices from Nasdaq regarding our inability, at times, to comply with various of the conditions required foron-going listing rules of NASDAQ’S (including stockholders’ equity requirements, publicly held share requirements, and timely filing requirements). In the past we have taken steps to attempt to regain compliance with these listing rules, however, in the future we may be unable to remain in compliance with NASDAQ’s continued listing on The NASDAQ Capital Market, NASDAQ requires us to maintain at least $2.5 million in stockholders’ equityrequirements or $500,000 in net income over the prior two years or two of the prior three years, to have a majority of independent directors, an audit committee of at least three independent directors (subject to certain limited exceptions), and to maintain a stock price over $1.00 per share. Our stockholders’ equity may not remain above NASDAQ’s $2.5 million minimum, we may not generate over $500,000 of yearly net income, we may not be able to maintain independent directors or an audit committee of at least three independent directors (subject to certain limited exceptions), and we may not be able to maintain a stock price over $1.00 per share.remedy any deficiencies. If we fail to timely comply with the applicable requirements, our stock may be delisted. In addition, even if we demonstrate compliance with the requirements above, we will have to continue to meet other objective and subjective listing requirements to continue to be listed on The NASDAQ Capital Market. Delisting from The NASDAQ Capital Market could make trading our common stock more difficult for investors, potentially leadingwere to declines in our share price and liquidity. Without abe delisted from NASDAQ Capital Market listing, stockholders may have a difficult time getting a quote for the sale or purchase of our stock, the sale or purchase of our stockit would likely be made more difficult, andreduce the trading volume and liquidity of our common stock, could decline. Delisting from The NASDAQ Capital Market could also result in negative publicity and, could alsoamong other things, may decrease the attractiveness of our common stock to the investment community, and make it more difficult for us to raise additional capital. The absenceissue equity securities for capital raising purposes or for acquisitions.
We are currently prohibited from filing any new registration statements on Form S-3 and effective upon the date that our Annual Report on Form 10-K for the year ended December 31, 2022 is filed with the Commission, we will be prohibited from using our Shelf Form S-3 until at least December 2024.
Due to our failure to timely file a Quarterly Report on Form 10-Q, we are currently prohibited from using Form S-3 to register securities with the Commission. Separately, our ability to use our previously effective shelf Form S-3, is suspended until at least December 2024. As a result, we will be required to use Form S-1, a longer-form registration statement for future offerings, and are prohibited, until at least December 2024, from undertaking at-the-market offerings. Furthermore, in the event that the Pre-Funded Warrants have not been exercised in full by such date, the shares of such a listing may adversely affect the acceptance of our common stock as currency orissuable upon exercise of the value accorded by other parties. Further, if we are delisted, we would also incur additional costsPre-Funded Warrants will need to be registered on Form S-1 in order to continue to be registered under state blue sky laws in connection with any sales of our securities. These requirements could severely limit the market liquidity of our common stock and the ability of our stockholders to sell our common stock in the secondary market. If our common stock is delisted by NASDAQ, our common stock may be eligible to trade on an over-the-counter quotation system, such as the OTCQB Market or the OTC Pink market, where an investor may find it more difficult to sell our stock or obtain accurate quotations as to the market value of our common stock. In the event our common stock is delisted from The NASDAQ Capital Market, we may not be able to list our common stock on another national securities exchange or obtain quotation on an over-the counter quotation system.Securities Act.
Regulatory Risks
Regulatory changes that affect our distribution channels could harm our business.
At the federal level, track and trace legislation requiring the use of pharmaceutical pedigree may restrict and disrupt the movement of pharmaceuticals along the supply chain should the cost of complying with this legislation be too burdensome for smaller suppliers. Changes in the United States healthcare industry and regulatory environment could have a material adverse impact on our results of operations.
Many of our products and services are intended to function within the structure of the healthcare financing and reimbursement system currently being used in the United States. In recent years, the healthcare industry in the United States has changed significantly in an effort to enhance efficiencies, reduce costs and improve patient outcomes. These changes have included cuts in Medicare and Medicaid reimbursement levels, changes in the basis for payments, shifting away from fee-for-service and towards value-based payments and risk-sharing models, increases in the use of managed care, and consolidation in the healthcare industry generally. We expect that the healthcare industry in the United States shall continue to change and evolve in the near future. Changes in the healthcare industry’s (or our pharmaceutical suppliers’) pricing, selling, inventory, distribution or supply policies or practices could significantly reduce our revenues and net income. Additionally, if we experience disruptions in our supply of generic drugs, our margins could be adversely affected.
We distribute generic pharmaceuticals, which can be subject to both price deflation and price inflation. Continued volatility in the availability, pricing trends or reimbursement of these generic drugs, or significant fluctuations in the nature, frequency and magnitude of generic pharmaceutical launches, could have a material adverse impact on our results of operations. Additionally, any future changes in branded and genericsgeneric drug pricing could be significantly different than our projections. Generic drug manufacturers are increasingly challenging the validity or enforceability of patents on branded pharmaceutical products. During the pendency of these legal challenges, a genericsgeneric drugs manufacturer may begin manufacturing and selling a generic version of the branded product prior to the final resolution of its legal challenge over the branded product’s patent. To the extent we source, contract manufacture, and distribute such generic products, the brand-name company could assert infringement claims against us. While we generally obtain indemnification against such claims from generic manufacturers as a condition of distributing their products, these rights may not be adequate or sufficient to protect us.
We are also required to comply with various state pricing gouging laws.
The healthcare industry is highly regulated, and further regulation of our distribution businesses and technology products and services could impose increased costs, negatively impact our profit margins and the profit margins of our customers, delay the introduction or implementation of our new products, or otherwise negatively impact our business and expose us to litigation and regulatory investigations.
Healthcare fraud laws are often vague and uncertain, exposing us to potential liability.
We are subject to extensive, and frequently changing, local, state and federal laws and regulations relating to healthcare fraud, waste and abuse. Local, state and federal governments continue to strengthen their position and scrutiny over practices involving fraud, waste and abuse affecting Medicare, Medicaid and other government healthcare programs. Many of the regulations applicable to us, including those relating to marketing incentives, are vague or indefinite and have not been interpreted by the courts. The regulations may be interpreted or applied by a prosecutorial, regulatory, or judicial authority in a manner that could require us to make changes in our operations. If we fail to comply with applicable laws and regulations, we could become liable for damages and suffer civil and criminal penalties, including the loss of licenses or our ability to participate in Medicare, Medicaid and other federal and state healthcare programs.
Laws reducing reimbursements for pharmaceuticals could negatively affect our industry.
Both our profit margins and the profit margins of our customers may be adversely affected by laws and regulations reducing reimbursement rates for pharmaceuticals, medical treatments and related services, or changing the methodology by which reimbursement levels are determined. The federal government may adopt measures that could reduce Medicare or Medicaid spending, or impose additional requirements on healthcare entities. We cannot predict what alternative or additional deficit reduction initiatives or Medicare payment reductions, if any, will ultimately be enacted into law, or the timing or affecteffect any such initiatives or reductions would have on us. Any of the changes discussed above may have a material adverse impact on our results of operations, cash flows, prospects and/or the value of our securities.
Operating, security and licensure standards of federal agencies challenge our ability to comply with applicable laws and regulations.
We are subject to the operating and security standards of the Drug Enforcement Administration (the DEA), the U.S. Food and Drug Administration (the FDA), various state boards of pharmacy, state health departments, the U.S. Department of Health and Human Services (HHS), the Centers for Medicare & Medicaid Services (CMS), and other comparable agencies. We are also subject to certain state laws relating to price gouging. Although we have enhanced our procedures to ensure compliance, a regulatory agency or tribunal may conclude that our operations are not compliant with applicable laws and regulations. In addition, we may be unable to maintain or renew existing permits, licenses or any other regulatory approvals or obtain without significant delay, future permits, licenses or other approvals needed for the operation of our businesses. Any noncompliance by us with applicable laws and regulations or the failure to maintain, renew or obtain necessary permits and licenses could lead to litigation and have a material adverse impact on our results of operations.
Pedigree tracking laws and regulations could increase our regulatory burdens.
Congress and state and federal agencies, including state boards of pharmacy and departments of health and the FDA, have made increased efforts in the past year to regulate the pharmaceutical distribution system in order to prevent the introduction of counterfeit, adulterated or mislabeled drugs into the pharmaceutical distribution system (otherwise known as “pedigree tracking”). In November 2013, Congress passed (and President Barack Obama signed into law) the Drug Quality and Security Act (the “DQSA”). The DQSA establishes federal standards requiring supply-chain stakeholders to participate in an electronic, interoperable, lot-level prescription drug track-and-trace system. The law also preempts state drug pedigree requirements and establishes new requirements for drug wholesale distributors and third-party logistics providers, including licensing requirements in states that had not previously licensed such entities.
In addition, the Food and Drug Administration Amendments Act of 2007 requires the FDA to establish standards and identify and validate effective technologies for the purpose of securing the pharmaceutical supply chain against counterfeit drugs. These standards may include track-and-trace or authentication technologies, such as radio frequency identification devices, 2D data matrix barcodes, and other similar technologies. On March 26, 2010, the FDA released the Serialized Numerical Identifier (the “SNI”) guidance for manufacturers who serialize pharmaceutical packaging. To date we have been able to accommodate these SNI regulations in our distribution operations. The DQSA and other pedigree tracking laws and regulations have increased the overall regulatory burden and costs associated with our pharmaceutical distribution business and have had a material adverse impact on our results of operations.
We are uncertain how new privacy laws shall be interpreted.
There are numerous federal and state laws and regulations related to the privacy and security of personal information. In particular, regulations promulgated pursuant to the Health Insurance Portability and Accountability Act of 1996 (HIPAA) establish privacy and security standards that limit the use and disclosure of individually identifiable health information (known as “protected health information”) and require the implementation of administrative, physical and technological safeguards to protect the privacy of protected health information and ensure the confidentiality, integrity and availability of electronic protected health information. We are directly subject to certain provisions of the regulations as a “Business Associate” through our relationships with customers. We are also directly subject to the HIPAA privacy and security regulations as a “Covered Entity” with respect to our operations as a healthcare clearinghouse, specialty pharmacy and medical surgical supply business. If we are unable to properly protect the privacy and security of protected health information entrusted to us, we could be found to have breached our contracts with our customers. Further, if we fail to comply with applicable HIPAA privacy and security standards, we could face civil and criminal penalties. Although we have implemented and continue to maintain policies and processes to assist us in complying with these regulations and our contractual obligations, we cannot provide assurances regarding how these regulations will be interpreted, enforced or applied by the government and regulators to our operations. In addition to the risks associated with enforcement activities and potential contractual liabilities, our ongoing efforts to comply with evolving laws and regulations at the federal and state level might also require us to make costly system purchases /or modifications from time to time.
We might be adversely impacted by healthcare reform such as changes in pricing and reimbursement models.
Many of our products and services are designed and intended to function within the structure of current healthcare financing and reimbursement systems. The healthcare industry and related government programs are changing. Some of these changes increase our risks and create uncertainties for our business.
For example, some changes in reimbursement methodologies (including government rates) for pharmaceuticals, medical treatments and related service reduces profit margins for us and our customers and impose new legal requirements on healthcare providers. Those changes have included cuts in Medicare and Medicaid reimbursement levels, changes in the basis for payments, shifting away from fee-for-service and toward value-based payment and risk-sharing models, and increases in the use of managed care.
In the U.S., the Patient Protection and Affordable Care Act (“ACA”) significantly expanded health insurance coveredcoverage to uninsured Americans and changed the way healthcare is financed by both governmental and private payers. There are continued efforts to challenge the ACA. There are also efforts to broaden healthcare coverage. U.S. lawmakers also have explored proposals to reduce drug prices, including requiring price transparency and drug importation measures. These proposals might result in significant changes in the pharmaceutical value chain as manufacturers, PBM, managed care organizations and other industry stakeholders look to implement new transactional flows and adapt their business models.
| 33 |
Provincial governments in Canada that provide partial funding for the purchase of pharmaceuticals and independently regulate the sale and reimbursement of drugs have sought to reduce the costs of publicly funded health programs. For example, provincial governments have taken steps to reduce consumer prices for generic pharmaceuticals and, in some provinces, change professional allowances paid to pharmacists by generic manufacturers.
Many European governments provide or subsidize healthcare to consumers and regulate pharmaceutical prices, patient eligibility and reimbursement levels in order to control government healthcare system costs. Some European governments have implemented or are considering austerity measures to reduce healthcare spending. These measures exert pressure on the pricing and reimbursement timelines for pharmaceuticals and may cause our customers to purchase fewer of our products and services or influence us to reduce prices.
Medical billing and coding laws may subject us to fines and investigations.
Medical billing, coding and collection activities are governed by numerous federal and state civil and criminal laws. In connection with these laws, we may be subjected to federal, or state government investigations and possible penalties may be imposed upon us, false claims actions may have to be defended, private payers may file claims against us, and we may be excluded from Medicare, Medicaid or other government-funded healthcare programs. Any such proceeding or investigation could have a material adverse impact on our results of operations.
It may be difficult and costly for us to comply with the extensive government regulations to which our business is subject.
Our operations are subject to extensive regulation by the U.S. federal and state governments. In addition, as we expand our operations, we may also become subject to the regulations of foreign jurisdictions, as well as additional regulations relating to environmental matters, transportation of pharmaceutical products, shipping restrictions, and import and export restrictions. We are also required to comply with various state pricing gouging laws.
Further, the enactment of new rules and regulations could adversely affect our business. Depending on future enforcement or additional rules and regulations created around it, pharmaceutical pricing controls could be established, resulting in substantially reduced margins and limited reimbursement for pharmacies and all other healthcare provider bases. In turn, this may adversely affect our cash flow, profitability, and growth.
Risks Relating to Our Industry in General
The public health crisis involving the abuse of prescription opioid pain medication could have a material negative effect on our business.
Our Pharmaceutical segment distributes prescription opioid pain medications. In recent years, the abuse of prescription opioid pain medication has become a public health crisis.
A significant number of counties, municipalities and other plaintiffs, including a number of state attorney generals, have filed lawsuits against pharmaceutical manufacturers, pharmaceutical wholesale distributors, retail chains and others relating to the manufacturing, marketing or distribution of certain prescription opioid pain medications. The defense and resolution of future lawsuits and events relating to these lawsuits could have a material adverse effect on our results of operations, financial condition, cash flows or liquidity or have adverse reputational or operational effects on our business.
Other legislative, regulatory or industry measures related to the public health crisis involving the abuse of prescription opioid pain medication and the distribution of these medications could affect our business in ways that we may not be able to predict. For example, several states have now adopted taxes or other fees on the sale of opioids, and several other states have proposed similar legislative initiatives. These laws and proposals vary in the tax amounts imposed and the means of calculation. Liabilities for taxes or assessments under any such laws could have an adverse impact on our results of operations unless we are able to mitigate them through operational changes or commercial arrangements where permitted.
| 34 |
Changes to the U.S. healthcare environment may not be favorable to us.
Over a number of years, the U.S. healthcare industry has undergone significant changes designed to increase access to medical care, improve safety and patient outcomes, contain costs and increase efficiencies. These changes include adoption of the Patient Protection and Affordable Care Act (ACA), a general decline in Medicare and Medicaid reimbursement levels, efforts by healthcare insurance companies to limit or reduce payments to pharmacies and providers, the basis for payments beginning to transition from a fee-for-service model to value-based payments and risk-sharing models, and the industry shifting away from traditional healthcare venues like hospitals and into clinics, physician offices and patients’ homes.
We expect the U.S. healthcare industry to continue to change significantly in the future. Possible changes include repeal and replacement of major parts of the Patient Protection and Affordable Care Act, further reduction or limitations on governmental funding at the state or federal level, efforts by healthcare insurance companies to further limit payments for products and services or changes in legislation or regulations governing prescription pharmaceutical pricing, healthcare services or mandated benefits. These possible changes, and the uncertainty surrounding these possible changes, may cause healthcare industry participants to reduce the number of products and services they purchase from us or the price they are willing to pay for our products and services, which could adversely affect us.
Consolidation in the U.S. healthcare industry may negatively impact our results of operations.
In recent years, U.S. healthcare industry participants, including distributors, manufacturers, suppliers, healthcare providers, insurers and pharmacy chains, have consolidated or formed strategic alliances. Consolidations create larger enterprises with greater negotiating power, and also could result in the possible loss of a customer where the combined enterprise selects one distributor from two incumbents. If this consolidation trend continues, it could adversely affect our results of operations.
Accounting Risks
We have identified material weaknesses in our internal control over financial reporting and controls and procedures which could, if not remediated, adversely affect our ability to report our financial condition, cash flows and results of operations in a timely and accurate manner and/or increase the risk of future misstatements, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our shares of common stock and/or debt securities to decline.
Maintaining effective internal control over financial reporting and effective disclosure controls and procedures are necessary for us to produce reliable financial statements. As reported under “Item 9A. Controls and Procedures”, as of December 31, 2021,2023, our CEO and CFO have determined that our disclosure controls and procedures were not effective. Additionally, our management is responsible for establishing and maintaining adequate internal control over our financial reporting, as defined in Rule 13a-15(f) under the Exchange Act. As disclosed below under “Item 9A. Controls and Procedures”, based on reviews conducted by management, we have concluded that a material weakness exists and has existed since approximately 2014 in the Company’s internal controls over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal controls over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. Such internal control over financial reporting and disclosure controls and procedures have been ineffective since approximately June 30, 2014 and December 31, 2015, respectively.
The material weaknesses identified in our controls and procedures as of December 31, 2021,2023, included the fact that (1) The Company did not maintain a fully integrated financial consolidation and reporting system throughout the period and as a result, extensive manual analysis, reconciliation and adjustments were required in order to produce financial statements for external reporting purposes. and (2) the Company does not currently have a sufficient complement of technical accounting and external reporting personnel commensurate to support standalone external financial reporting under public company or SEC requirements. Specifically, the Company did not effectively segregate certain accounting duties due to the small size of its accounting staff and maintain a sufficient number of adequately trained personnel necessary to anticipate and identify risks critical to financial reporting and the closing process. In addition, there were inadequate reviews and approvals by the Company’s personnel of certain reconciliations and other processes in day-to-day operations due to the lack of a full complement of accounting staff.
The material weaknesses identified in our internal control over financial reporting include the fact that: the Company did not maintain a fully integrated financial consolidation and reporting system throughout the period and as a result, extensive manual analysis, reconciliation and adjustments were required in order to produce financial statements for external reporting purposes; and the Company does not currently have a sufficient complement of technical accounting and external reporting personnel commensurate to support standalone external financial reporting under public company or SEC requirements. Specifically, the Company did not effectively segregate certain accounting duties due to the small size of its accounting staff and maintain a sufficient number of adequately trained personnel necessary to anticipate and identify risks critical to financial reporting and the closing process. In addition, there were inadequate reviews and approvals by the Company’s personnel of certain reconciliations and other processes in day-to-day operations due to the lack of a full complement of accounting staff.
| 35 |
Maintaining effective disclosure controls and procedures and effective internal control over financial reporting are necessary for us to produce reliable financial statements and the Company is committed to remediating its material weaknesses in such controls as promptly as possible.
TheSince Fiscal 2014 when the material weakness became effective, the Company has identified certain remediation actions and is in the process of implementing them, but suchhas implemented many efforts are not complete and remain ongoing.in process. If we do not complete our remediation in a timely manner or if our remedial measures are insufficient to address the material weaknesses, or if additional material weaknesses in our internal controls and/or controls and procedures are discovered or occur in the future, it may materially adversely affect our ability to report our financial condition and results of operations in a timely and accurate manner and there will continue to be an increased risk of future misstatements. Although we regularly review and evaluate internal controls systems to allow management to report on the effectiveness of our internal controls over financial reporting and controls and procedures, we may discover additional weaknesses in our internal controls over financial reporting or disclosure controls and procedures. The next time we evaluate our internal controls over financial reporting and disclosure controls and procedures, if we identify one or more new material weaknesses or have been unable to timely remediate our existing material weaknesses, we would be unable to conclude that our internal controls over financial reporting or disclosure controls and procedures are effective. If we are unable in the future to conclude that our internal controls over financial reporting or our disclosure controls and procedures are effective, we may not be able to report our financial condition and results of operations in a timely and accurate manner, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our shares of common stock to decline. In addition, any potential future restatements could subject us to additional adverse consequences, including sanctions by the SEC, stockholder litigation and other adverse actions. Moreover, we may be the subject of further negative publicity focusing on such financial statement adjustments and resulting restatement and negative reactions from our stockholders, creditors or others with whom we do business. The occurrence of any of the foregoing could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our shares of common stock to decline.
We may experience adverse impacts on our reported results of operations as a result of adopting new accounting standards or interpretations.
Our implementation of and compliance with changes in accounting rules, including new accounting rules and interpretations, have not affected our reported financial position or operating results to date or cause unanticipated fluctuations in our reported operating results in future periods.
A significant amount of our revenues has historically been due to only a small number of customers and we depend on a small number of major wholesalers, and if we were to lose any of those customers or suppliers, our results of operations would be adversely affected.
During the years ended December 31, 20212023 and 2020,2022, no sales to customersany specific customer represented greater than 10% of revenue in 2021 and sales to two customers represented 25% and 15% of revenue, respectively, in 2020.revenue. In the event our customers do not pay us amounts owed, sales to such customers cease or we are unable to find new customers moving forward, it could have a materially adverse effect on our results of operations. We have a working relationship with over 25 wholesalers and the nation’s largest buying group. Although we believe those entities are satisfied with their business relationship with Trxade, if our buying group and two or three of the largest wholesalers decidedsupply chain vendors decide to no longer to do business with Trxade, and we wereare unable to find additional entities to step into their shoes, the resulting supplier void would materially and adversely affect our competitiveness in the marketplace, and could cause a material adverse effect on our results of operations.
We might be harmed by changes in our relationships or contracts with suppliers.
We attempt to structure our agreements with wholesalers to ensure that we are appropriately and predictably compensated for the services we provide. We cannot control the frequency or magnitude of pharmaceutical price changes. We might be unable to renew agreements with wholesalers in a timely and favorable manner. Any of these risks might have a materially adverse impact on our business operations and our financial positions or results of operations.
| 36 |
Risks Related to Our Common Stock and Organizational Documents
Our common stock has in the past been a “penny stock” under SEC rules, and may be subject to the “penny stock” rules in the future. It may be more difficult to resell securities classified as “penny stock.”
In the past (including immediately prior to our common stock being listed on The NASDAQ Capital Market in February 2020), our common stock was a “penny stock” under applicable SEC rules (generally defined as non-exchange traded stock with a per-share price below $5.00). While our common stock is not now considered a “penny stock” because it is listed on The NASDAQ Capital Market, if we are unable to maintain that listing, unless we maintain a per-share price above $5.00, our common stock will become “penny stock.” These rules impose additional sales practice requirements on broker-dealers that recommend the purchase or sale of penny stocks to persons other than those who qualify as “established customers” or “accredited investors.” For example, broker-dealers must determine the appropriateness for non-qualifying persons of investments in penny stocks. Broker-dealers must also provide, prior to a transaction in a penny stock not otherwise exempt from the rules, a standardized risk disclosure document that provides information about penny stocks and the risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, disclose the compensation of the broker-dealer and its salesperson in the transaction, furnish monthly account statements showing the market value of each penny stock held in the customer’s account, provide a special written determination that the penny stock is a suitable investment for the purchaser, and receive the purchaser’s written agreement to the transaction.
Legal remedies available to an investor in “penny stocks” may include the following:
| ● | If a “penny stock” is sold to the investor in violation of the requirements listed above, or other federal or states securities laws, the investor may be able to cancel the purchase and receive a refund of the investment. | |
| ● | If a “penny stock” is sold to the investor in a fraudulent manner, the investor may be able to sue the persons and firms that committed the fraud for damages. |
These requirements may have the effect of reducing the level of trading activity, if any, in the secondary market for a security that becomes subject to the penny stock rules. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from effecting transactions in our securities, which could severely limit the market price and liquidity of our securities. These requirements may restrict the ability of broker-dealers to sell our common stock and may affect your ability to resell our common stock.
Many brokerage firms will discourage or refrain from recommending investments in penny stocks. Most institutional investors will not invest in penny stocks. In addition, many individual investors will not invest in penny stocks due, among other reasons, to the increased financial risk generally associated with these investments.
| 37 |
For these reasons, penny stocks may have a limited market and, consequently, limited liquidity. We can give no assurance at what time, if ever, our common stock will not be classified as a “penny stock” in the future.
A significant numberPenny stocks are generally considered to be high-risk investments. There are several factors that contribute to the high-risk nature of our shares are eligible for sale and their sale or potential sale may depress the market price of our common stock.penny stocks, including:
| ● | Volatility: Penny stocks are known for their extreme price fluctuations. This volatility can be caused by a number of factors, including changes in the overall stock market, news about the company or industry, and changes in investor sentiment. | |
| ● | Lack of liquidity: Penny stocks are often traded on over-the-counter markets, which can make them more difficult to buy and sell. This lack of liquidity can increase the risk of large price swings and can make it difficult to exit a position if needed. | |
| ● | Lack of information: Many penny stock companies are not required to file regular reports with the Securities and Exchange Commission (SEC), which means there may be limited information available to investors. This can make it difficult to evaluate the financial health of the company and to make informed investment decisions. | |
| ● | Manipulation: Because of their low trading volumes and lack of regulatory oversight, penny stocks can be vulnerable to market manipulation. This can include practices such as “pump and dump” schemes, where investors artificially inflate the price of a stock before selling their shares for a profit. |
Sales of a significant number of shares of our common stock in the public market could harm the market price of our common stock. Most of our common stock is available for resale in the public market, and if sold would increase the supply of our common stock, thereby causing a decrease in its price. Some or all of our shares of common stock may be offered from time to time in the open market pursuant to effective registration statements and/or compliance with Rule 144, which sales could have a depressive effect on the market for our shares of common stock. Subject to certain restrictions, a person who has held restricted shares for a period of six months may generally sell common stock into the market. The sale of a significant portion of such shares when such shares are eligible for public sale may cause the value of our common stock to decline in value.
Overall, it’s important to approach penny stocks with caution and to thoroughly research any investment before making a decision. It’s also a good idea to diversify your portfolio and to limit your exposure to any one stock or sector.
There may not be sufficient liquidity in the market for our securities in order for investors to sell their shares. The market price of our common stock may continue to be volatile.
The market price of our common stock will likely continue to be highly volatile. Some of the factors that may materially affect the market price of our common stock are beyond our control, such as conditions or trends in the industry in which we operate or sales of our common stock. This situation is attributable to a number of factors, including the fact that we are a small company which is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable.
As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a mature issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. It is possible that a broader or more active public trading market for our common stock will not develop or be sustained, or that trading levels will not continue. These factors may materially adversely affect the market price of our common stock, regardless of our performance. In addition, the public stock markets have experienced extreme price and trading volume volatility. This volatility has significantly affected the market prices of securities of many companies for reasons frequently unrelated to the operating performance of the specific companies. These broad market fluctuations may adversely affect the market price of our common stock.
The exercise of outstanding warrants, options and shares issued in connection with a joint venture and acquisition will be dilutive to our existing stockholders.stockholders.
As of the date of this Report, we had 8,181,041 shares of our commonoutstanding various warrants, stock issuedoptions and outstanding and the followingother securities whichthat are exercisable into shares of our common stock:
● 44,535 shares of our common stock issuable upon the exercise of warrants with exercise prices ranging from $0.06 to $9.00 per share, with a weighted average price of $0.32; and
● 410,964 shares of our common stock issuable upon the exercise of options with exercise prices ranging from $2.46 per share to $9.60 per share, with a weighted average price of $4.88.
For the life of the options and warrants, the holders have the opportunity to profit from a rise in the market price of our common stock without assuming the risk of ownership. The issuance of shares upon the exercise of outstanding securities will also dilute the ownership interests of our existing stockholders.
The availability of these shares for public resale, as well as any actual resales of these shares, could adversely affect the trading price of our common stock. Certain of the shares of common stock underlying outstanding options will be available for resale immediately in the public market without restriction.
We cannot predict the size of future issuances of our common stock pursuant to the exercise of outstanding options or warrants, or the effect, if any, that future issuances and sales of shares of our common stock may have on the market price of our common stock. Sales or distributions of substantial amounts of our common stock (including shares issued in connection with an acquisition), or the perception that such sales could occur, may cause the market price of our common stock to decline.
| 38 |
We have nevernot historically paid or declared any dividends on our common stock.stock and do not expect to pay or declare cash dividends in the future on a regular basis, if at all.
We have neverAlthough we declared and paid a special cash dividend in March 2024 that dividend was paid as the result of a sale various business assets and not paid from cash generated in our operations, the Company has not historically paid or declared any dividends on our common stock or preferred stock. Likewise, we do not anticipate paying, in the near future, dividends or distributions on our common stock. Any future dividends on common stock will be declared at the discretion of our Board of Directors and will depend, among other things, on our earnings, our financial requirements for future operations and growth, and other facts as we may then deem appropriate. Since we do not anticipate paying cash dividends on our common stock,As such, the return on your investment, if any, will dependhas historically been dependent solely on an increase, if any, in the market value of our common stock.
Our common stock price is likely to be highly volatile because of several factors, including a limited public float.
The market price of our common stock has been volatile in the past and the market price of our common stock is likely to be highly volatile in the future. You may not be able to resell shares of our common stock following periods of volatility because of the market’s adverse reaction to volatility.
Other factors that could cause such volatility may include, among other things:
| ● | actual or anticipated fluctuations in our operating results; | |
| ● | the absence of securities analysts covering us and distributing research and recommendations about us; | |
| ● | we may have a low trading volume for a number of reasons, including that a large portion of our stock is closely held; | |
| ● | overall stock market fluctuations; |
| ● | announcements concerning our business or those of our competitors; | |
| ● | actual or perceived limitations on our ability to raise capital when we require it, and to raise such capital on favorable terms; | |
| ● | conditions or trends in our industry; | |
| ● | litigation; | |
| ● | changes in market valuations of other similar companies; | |
| ● | future sales of common stock; | |
| ● | departure of key personnel or failure to hire key personnel; and | |
| ● | general market conditions. |
Any of these factors could have a significant and adverse impact on the market price of our common stock. In addition, the stock market in general has at times experienced extreme volatility and rapid decline that has often been unrelated or disproportionate to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock, regardless of our actual operating performance.
Our Chief Executive Officer and President are two of our two largest stockholders and, as a result, they can exert controlinfluence over us and have actual or potential interests that may differ from yours.
Mr. Suren Ajjarapu, our CEO, and Mr. Prashant Patel, our President, beneficially own, in the aggregate, over 53% of our common stock. As a result, these stockholders, acting together, willmay be able to influence many matters requiring stockholder approval, including the election of directors and approval of mergers and other significant corporate transactions. This concentration of ownership may have the effect of delaying, preventing or deterring a change in control, and could deprive our stockholders of an opportunity to receive a premium for their shares of common stock as part of a sale of our company and may affect the market price of our stock.
| 39 |
Further, Mr. Ajjarapu and Mr. Patel may have interests that differ from those of other holders of our common stock. As a result, Mr. Ajjarapu and Mr. Patel may vote the shares they own or control or otherwise cause us to take actions that may conflict with your best interests as a stockholder, which could adversely affect our results of operations and the trading price of our common stock.
Through this control,influence, Mr. Ajjarapu and Mr. Patel can controlinfluence our management, affairs and all matters requiring stockholder approval, including the approval of significant corporate transactions, a sale of our company, decisions about our capital structure and the composition of our Board of Directors.
Our common stock may continue to be followed by only a limited number of analysts and there may continue to be a limited number of institutions acting as market makers for our common stock.
For the foreseeable future, our common stock is unlikely to be followed by a significant number of market analysts, and there may be few institutions acting as market makers for our common stock. Either of these factors could adversely affect the liquidity and trading price of our common stock. Until our common stock is fully distributed, and an orderly market develops in our common stock, if ever, the price at which it trades is likely to fluctuate significantly. Prices for our common stock are determined in the marketplace and may be influenced by many factors, including the depth and liquidity of the market for shares of our common stock, developments affecting our business, including the impact of the factors referred to elsewhere in these Risk Factors, investor perception of us and general economic and market conditions. No assurances can be given that an orderly or liquid market will ever develop for the shares of our common stock.
Our bylaws require, to the fullest extent permitted by law, that derivative actions brought in our name, actions against our directors, officers, other employees or stockholders for breach of fiduciary duty and certain other actions may be brought only in the Court of Chancery in the State of Delaware, and if brought outside of Delaware, the stockholder bringing the suit will, subject to certain exceptions, be deemed to have consented to service of process on such stockholder’s counsel, which may have the effect of discouraging lawsuits against our directors, officers, other employees or stockholders.
Our bylaws require that unless the Company consents in writing to an alternative forum, the Court of Chancery of the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for (a) any derivative action or proceeding brought on behalf of the Company; (b) any action asserting a claim of breach of fiduciary duty owed by, or other wrongdoing by, any director, officer, employee or agent of the Company to the Company or the Company’s stockholders; (c) any action asserting a claim arising pursuant to any provision of Delaware General Corporation Law or the certificate of incorporation or bylaws of the Company; (d) any action to interpret, apply, enforce or determine the validity of the certificate of incorporation or bylaws of the Company; or (e) any action asserting a claim governed by the internal affairs doctrine, in each case subject to said Court of Chancery having personal jurisdiction over the indispensable parties named as defendants therein (or such indispensable parties consenting to the personal jurisdiction of the Court of Chancery within 10 days following any determination by the Court of Chancery that an indispensable party is not subject to such personal jurisdiction); provided that, if the Court of Chancery of the State of Delaware dismisses any action for lack of subject matter jurisdiction, such action may be brought in another state or federal court sitting in the State of Delaware. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and consented to the forum provisions in our bylaws. This choice of forum provision may limit or make more costly a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or any of our directors, officers, other employees or stockholders, which may discourage lawsuits with respect to such claims. Alternatively, if a court were to find the choice of forum provision contained in our bylaws to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, operating results and financial condition.
As described above, our bylaws provide that the exclusive forum provision will be applicable to the fullest extent permitted by applicable law, subject to certain exceptions. However, Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As a result, the exclusive forum provision will not apply to suits brought to enforce any duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. We also note that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Section 22 of the Securities Act, creates concurrent jurisdiction for state and federal courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder.
| 40 |
Our stockholders have no right to call special meetings of stockholders.
Our bylaws provide that special meetings of our stockholders may be called only by the chairperson of the board of directors, the chief executive officer or president (in the absence of a chief executive officer). Because our stockholders do not have the right to call a special meeting, a stockholder could not force stockholder consideration of a proposal over the opposition of our board of directors by calling a special meeting of stockholders prior to such time as the chairperson of the board of directors, the chief executive officer or president (in the absence of a chief executive officer) believed the matter should be considered or until the next annual meeting provided that the requestor met the notice requirements. The restriction on the ability of stockholders to call a special meeting means that a proposal to replace our board of directors also could be delayed until the next annual meeting.
Provisions in our certificate of incorporation and bylaws may inhibit a takeover of us, which could limit the value of our securities and could entrench management.
Our certificate of incorporation and bylaws contain provisions that may discourage unsolicited takeover proposals that stockholders may consider to be in their best interests. These provisions include the ability of the board of directors to designate the terms of and issue new series of preferred shares and the requirement to receive the affirmative vote of holders of at least two-thirds of the outstanding capital stock of the Company to amend any provision of the bylaws of the Company, without Board of Directors approval (which Board of Directors approved amendments may be affected solely by the Board of Directors, without stockholder approval, subject to certain exceptions, without stockholder approval), which may make the removal of management more difficult and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities. These provisions may make the removal of management more difficult and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities.
General Risk Factors
U.S. and global economic conditions could materially adversely affect the Company’s business, results of operations, financial condition and growth.
Adverse macroeconomic conditions, including inflation, slower growth or recession, new or increased tariffs, changes to fiscal and monetary policy, tighter credit, higher interest rates, high unemployment and currency fluctuations could have a material adverse impact on demand for the Company’s products and services. In addition, consumer confidence and spending could be adversely affected in response to financial market volatility, negative financial news, conditions in the real estate and mortgage markets, declines in income or asset values, changes to fuel and other energy costs, labor and healthcare costs and other economic factors.
In addition to an adverse impact on demand for the Company’s products, uncertainty about, or a decline in, U.S. or global economic conditions could have a significant impact on the Company’s suppliers, the pharmacy industry as a whole, the Company’s network of independent pharmacies and other partners. Potential effects include financial instability, inability to obtain credit to finance operations and purchases of the Company’s products, payment defaults and insolvency.
A downturn in the economic environment could also lead to increased credit and collectability risk on the Company’s receivables; limitations on the Company’s ability to raise new funding through the sale of debt or equity; reduced liquidity; and declines in the value of the Company’s securities. These and other economic factors could materially adversely affect the Company’s business, results of operations, financial condition and growth.
Risks Relating to The JOBS Act
The JOBS Act allows us to postpone the date by which we must comply with certain laws and regulations and to reduce the amount of information provided in reports filed with the SEC. We cannot be certain if the reduced disclosure requirements applicable to “emerging growth companies” will make our common stock less attractive to investors.
We are and we will remain an “emerging growth company” until the earliest to occur of (i) the last day of the fiscal year during which our total annual revenues equal or exceed $1.07 billion (subject to adjustment for inflation), (ii) the last day of the end of our 2024 fiscal year (5 years from our first public offering), (iii) the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt, or (iv) the date on which we are deemed a “large accelerated filer” (with at least $700 million in public float) under the Exchange Act. For so long as we remain an “emerging growth company” as defined in the JOBS Act, we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” as described in further detail in the risk factors below. We cannot predict if investors will find our common stock less attractive because we will rely on some or all of these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. If we avail ourselves of certain exemptions from various reporting requirements, as is currently our plan, our reduced disclosure may make it more difficult for investors and securities analysts to evaluate us and may result in less investor confidence.
Our election not to opt out of the JOBS Act extended accounting transition period may not make our financial statements easily comparable to other companies.
Pursuant to the JOBS Act, as an “emerging growth company”, we can elect to opt out of the extended transition period for any new or revised accounting standards that may be issued by the Public Company Accounting Oversight Board (PCAOB) or the SEC. We have elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an “emerging growth company”, can adopt the standard for the private company. This may make a comparison of our financial statements with any other public company which is not either an “emerging growth company” nor an “emerging growth company” which has opted out of using the extended transition period, more difficult or impossible as possible different or revised standards may be used.
The JOBS Act also allows us to postpone the date by which we must comply with certain laws and regulations intended to protect investors and to reduce the amount of information provided in reports filed with the SEC.
The JOBS Act is intended to reduce the regulatory burden on “emerging growth companies”. The Company meets the definition of an “emerging growth company” and so long as it qualifies as an “emerging growth company,” it will, among other things:
The Company has and intends to continue to take advantage of all of the reduced regulatory and reporting requirements that will be available to it so long as it qualifies as an “emerging growth company”. The Company has elected not to opt out of the extension of time to comply with new or revised financial accounting standards available under Section 102(b)(1) of the JOBS Act. Among other things, this means that the Company’s independent registered public accounting firm will not be required to provide an attestation report on the effectiveness of the Company’s internal control over financial reporting so long as it qualifies as an “emerging growth company”, which may increase the risk that weaknesses or deficiencies in the internal control over financial reporting go undetected. Likewise, so long as it qualifies as an “emerging growth company”, the Company may elect not to provide certain information, including certain financial information and certain information regarding compensation of executive officers, which it would otherwise have been required to provide in filings with the SEC, which may make it more difficult for investors and securities analysts to evaluate the Company. As a result, investor confidence in the Company and the market price of its common stock may be adversely affected.
Notwithstanding the above, we are also currently a “smaller reporting company”, meaning that we are not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company and have a public float of less than $700 million and less than $100 million annual revenues or a public float of less than $250 million, during the most recently completed fiscal year. In the event that we are still considered a “smaller reporting company”, at such time are we cease being an “emerging growth company”, the disclosure we will be required to provide in our SEC filings will increase, but will still be less than it would be if we were not considered either an “emerging growth company” or a “smaller reporting company”. Specifically, similar to “emerging growth companies”, “smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings; are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting; except from the requirement to include the detailed compensation discussion and analysis disclosures and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. Decreased disclosures in our SEC filings due to our status as an “emerging growth company” or “smaller reporting company” may make it harder for investors to analyze the Company’s results of operations and financial prospects.
General Risk Factors
Failure to adequately manage our planned aggressive growth strategy may harm our business or increase our risk of failure.
For the foreseeable future, we intend to pursue an aggressive growth strategy for the expansion of our operations through increased product development and marketing. Our ability to rapidly expand our operations will depend upon many factors, including our ability to work in a regulated environment, market value-added products effectively to independent pharmacies, establish and maintain strategic relationships with suppliers, and obtain adequate capital resources on acceptable terms. Any restrictions on our ability to expand may have a materially adverse effect on our business, results of operations, and financial condition. Accordingly, we may be unable to achieve our targets for sales growth, and our operations may not be successful or achieve anticipated operating results.
Additionally, our growth may place a significant strain on our managerial, administrative, operational, and financial resources and our infrastructure. Our future success will depend, in part, upon the ability of our senior management to manage growth effectively. This will require us to, among other things:
As a result, we may lack the resources to deploy our services on a timely and cost-effective basis. Failure to accomplish any of these requirements could impair our ability to deliver services in a timely fashion or attract and retain new customers.
If we do not successfully implement any acquisition strategies, our operating results and prospects could be harmed.
We face competition within our industry for acquisitions of businesses, technologies and assets, and, in the future, such competition may become more intense. As such, even if we are able to identify an acquisition that we would like to consummate, we may not be able to complete the acquisition on commercially reasonable terms or at all because of such competition. Furthermore, if we enter into negotiations that are not ultimately consummated, those negotiations could result in diversion of management time and significant out-of-pocket costs. Even if we are able to complete such acquisitions, we may additionally expend significant amounts of cash or incur substantial debt to finance them, which indebtedness could result in restrictions on our business and use of available cash. In addition, we may finance or otherwise complete acquisitions by issuing equity or convertible debt securities, which could result in dilution of our existing stockholders. If we fail to evaluate and execute acquisitions successfully, we may not be able to realize their benefits. If we are unable to successfully address any of these risks, our business, financial condition or operating results could be harmed.
If we make any acquisitions, they may disrupt or have a negative impact on our business.
If we make acquisitions in the future, funding permitting, which may not be available on favorable terms, if at all, we could have difficulty integrating the acquired company’s assets, personnel and operations with our own. We do not anticipate that any acquisitions or mergers we may enter into in the future would result in a change of control of the Company. In addition, the key personnel of the acquired business may not be willing to work for us. We cannot predict the effect expansion may have on our core business. Regardless of whether we are successful in acquiring, the negotiations could disrupt our ongoing business, distract our management and employees and increase our expenses. In addition to the risks described above, acquisitions are accompanied by a number of inherent risks, including, without limitation, the following:
Our business could be severely impaired if and to the extent that we are unable to succeed in addressing any of these risks or other problems encountered in connection with an acquisition, many of which cannot be presently identified. These risks and problems could disrupt our ongoing business, distract our management and employees, increase our expenses and adversely affect our results of operations.
We may apply working capital and future funding to uses that ultimately do not improve our operating results or increase the value of our securities.
In general, we have complete discretion over the use of our working capital and any new investment capital we may obtain in the future. Because of the number and variety of factors that could determine our use of funds, our ultimate expenditure of funds (and their uses) may vary substantially from our current intended operating plan for such funds.
We intend to use existing working capital and future funding to support the development of our products and services, product purchases in our wholesale distribution division, the expansion of our marketing, or the support of operations to educate our customers. We will also use capital for market and network expansion, acquisitions, and general working capital purposes. However, we do not have more specific plans for the use and expenditure of our capital. Our management has broad discretion to use any or all of our available capital reserves. Our capital could be applied in ways that do not improve our operating results or otherwise increase the value of a stockholder’s investment.
Our websites may encounter technical problems and service interruptions.
Our websites may, in the future, experience slower response times or interruptions as a result of increased traffic or other reasons. These delays and interruptions resulting from failure to maintain Internet service connections to our site could frustrate visitors and reduce our future web site traffic, which could have a material adverse effect on our business.
The sale of shares by our directors and officers may adversely affect the market price for our shares.
Sales of significant amounts of shares held by our officers and directors, or the prospect of these sales, could adversely affect the market price of our common stock. Management’s stock ownership may discourage a potential acquirer from making a tender offer or otherwise attempting to obtain control of us, which in turn could reduce our stock price or prevent our stockholders from realizing a premium over our stock price.
Stockholders may be diluted significantly through our efforts to obtain financing and satisfy obligations through the issuance of additional shares of our common stock.
Wherever possible, our Board of Directors will attempt to use non-cash consideration to satisfy obligations. In many instances, we believe that the non-cash consideration will consist of restricted shares of our common stock or where shares are to be issued to our officers, directors and applicable consultants. Our Board of Directors has authority, without action or vote of the stockholders, but subject to NASDAQ rules and regulations (which generally require shareholder approval for any transactions which would result in the issuance of more than 20% of our then outstanding shares of common stock or voting rights representing over 20% of our then outstanding shares of stock), to issue all or part of the authorized but unissued shares of common stock. In addition, we may attempt to raise capital by selling shares of our common stock, possibly at a discount to market. These actions will result in dilution of the ownership interests of existing stockholders, which may further dilute common stock book value, and that dilution may be material. Such issuances may also serve to enhance existing management’s ability to maintain control of the Company because the shares may be issued to parties or entities committed to supporting existing management.
Levels or types of insurance may not be adequate to cover claims.
Although we maintain current and active insurance policies, we cannot guarantee that all levels and types of insurance will be adequate to cover claims brought against the Company.
Our growth depends in part on the success of our strategic relationships with third parties.
In order to grow our business, we anticipate that we will need to continue to depend on our relationships with third parties, including our technology providers. Identifying partners, and negotiating and documenting relationships with them, requires significant time and resources. Our competitors may be effective in providing incentives to third parties to favor their products or services, or utilization of, our products and services. In addition, acquisitions of our partners by our competitors could result in a decrease in the number of our current and potential customers. If we are unsuccessful in establishing or maintaining our relationships with third parties, our ability to compete in the marketplace or to grow our revenue could be impaired and our results of operations may suffer. Even if we are successful, we cannot assure you that these relationships will result in increased customer use of our products or increased revenue.
Claims, litigation, government investigations, and other proceedings may adversely affect our business and results of operations.
As a company offering a wide range of products and services, we are regularly subject to actual and threatened claims, litigation, reviews, investigations, and other proceedings, including proceedings relating to goods and services offered by us and by third parties, and other matters. Any of these types of proceedings, including currently pending proceedings as discussed herein, may have an adverse effect on us because of legal costs, disruption of our operations, diversion of management resources, negative publicity, and other factors. The outcomes of these matters are inherently unpredictable and subject to significant uncertainties. Determining legal reserves and possible losses from such matters involves judgment and may not reflect the full range of uncertainties and unpredictable outcomes. Until the final resolution of such matters, we may be exposed to losses in excess of the amount recorded, and such amounts could be material. Should any of our estimates and assumptions change or prove to have been incorrect, it could have a material effect on our business, consolidated financial position, results of operations, or cash flows. In addition, it is possible that a resolution of one or more such proceedings, including as a result of a settlement, could require us to make substantial future payments, prevent us from offering certain products or services, require us to change our business practices in a manner materially adverse to our business, requiring development of non-infringing or otherwise altered products or technologies, damaging our reputation, or otherwise having a material effect on our operations.
We may be adversely affected by climate change or by legal, regulatory or market responses to such change.
The long-term effects of climate change are difficult to predict; however, such effects may be widespread. Impacts from climate change may include physical risks (such as rising sea levels or frequency and severity of extreme weather conditions—which may affect our current operations due to among other things, the fact that we are based in Florida, which is only on average 6 feet higher than current sea level), social and human effects (such as population dislocations or harm to health and well-being), compliance costs and transition risks (such as regulatory or technology changes) and other adverse effects. The effects of climate change could increase the cost of certain products, commodities and energy (including utilities), which in turn may impact our ability to procure goods or services required for the operation of our business. Climate change could also lead to increased costs as a result of physical damage to or destruction of our facilities, loss of inventory, and business interruption due to weather events that may be attributable to climate change. These events and impacts could materially adversely affect our business operations, financial position or results of operation.
We might be adversely impacted by changes in accounting standards.
Our consolidated financial statements are subject to the application of U.S. GAAP, which periodically is revised or reinterpreted. From time to time, we are required to adopt new or revised accounting standards issued by recognized authoritative bodies, including the Financial Accounting Standards Board (“FASB”) and the SEC. It is possible that future accounting standards may require changes to the accounting treatment in our consolidated financial statements and may require us to make significant changes to our financial systems. Such changes might have a materially adverse impact on our financial position or results of operations.
For all of the foregoing reasons and others set forth herein, an investment in our securities involves a high degree of risk.
| 43 |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 1C. | CYBERSECURITY |
The Company has not adopted any formal cybersecurity risk management program or formal processes for assessing, identifying, and managing material risks from cybersecurity threats. The full board of directors has oversight responsibility for the Company’s overall risk management, including cybersecurity risk, and has not delegated oversight authority for cybersecurity risks to any committee. During the year ended December 31, 2023, we did not identify any cybersecurity threats that have materially affected or are reasonably likely to materially affect our business strategy, results of operations, or financial condition.
| ITEM 2. | PROPERTIES |
We do not own any real property. We moved out of our previous corporate facility located at 3480 Land O’Lakes Blvd, Land O Lakes, Florida 33556 on December 31, 2021 and entered into a lease for our current corporate office space at 2420 Brunello Trace, Lutz, Florida 33558 on November 8, 2021. The lease hasincluded a five-year term, beginning January 1, 2022, and ending December 31, 2026. OurThe office space occupies approximately 9,850 square feet. Pursuant to the lease, the Company will also beis responsible for water/sewer costs ($140 per month) and its proportionate share of the building’s operating expenses, including property taxes. We paid a security deposit of $38,500 in connection with our entry into the agreement.
Our rental cost under the agreement, during the term of the lease, is as follows:
| Lease Period | Monthly Rent | |||
| January 1, 2022 to December 31, 2022 | $ | 18,469 | ||
| January 1, 2023 to December 31, 2023 | $ | 19,023 | ||
| January 1, 2024 to December 31, 2024 | $ | 19,594 | ||
| January 1, 2025 to December 31, 2025 | $ | 20,181 | ||
| January 1, 2026 to December 31, 2026 | $ | 20,787 | ||
Pursuant to the lease, we have an option to renew the lease for two additional five-year terms with a mutually agreed increase in rental cost.
The Company’s obligations under the lease are guaranteed by Suren Ajjarapu, the Company’s Chief Executive Officer and Chairman but has not been formally documented to date.
The Company also has, pursuant to the terms of the lease, a right to match any offer for purchase on 4.12 acres of Sienna Village I, parcel 26-26-18-0000-04800-000 and 4 buildings totaling 23,048 square feet of office space in its entirety, for a 2-year period commencing with the execution of the lease. We also have a right of first refusal to lease available vacant buildings at the then current market rate only after the landlord has made such space available for leasing.
In connection with our entry into the lease, we purchased certain furniture and assets of the landlord located at the leased premises for (a) $60,000, payable in 12 installments of $5,000 each, with title to such assets transferring on the date of the last payment, December 1, 2022; and (b) $37,500, which was payablesurrendered upon our entry into the lease agreement, upon which payment, title to such purchased assets transferred.
The lease contains customary indemnification and termination provisions. In addition, the lease contains customary events of default, including payment defaults, breaches of covenants and/or certain representations and warranties, bankruptcy or insolvency proceedings and other events of default customary for this type of transaction. The lease also contains remedies for such events of default, including the landlord’s right to cure a default (together with our requirement to pay a 15% administrative fee in connection therewith), interest and other amounts, the right to accelerate all amounts due during the remaining term of the lease, termination of the lease and other remedies customary for this type of transaction.in November 2023.
We entered into a lease for Integra Pharma Solutions, LLC at 6308 Benjamin Road, Tampa, Florida 33634 for approximately $43,000 per year ($3,583 per month) under a five-year lease agreement, effective October 17, 2018, occupying approximately 6,300 square feet.
We entered into a lease for Superlatus Food Service Holding Company, LLC at 20 Sarine Road, Wurtsboro, New York 12790 for approximately $132,000 per year ($11,000 per month) under a three-year lease agreement, effective October 1, 2023. As a result of our divestiture of Superlatus in March 2024 we no longer have obligations related to this lease.
We believe our current and future facilities are adequate for our current and near-term needs. Additional space may be required as we expand our activities. We do not currently foresee any significant difficulties in obtaining any required additional facilities.
| 44 |
| ITEM 3. | LEGAL PROCEEDINGS |
In the ordinary course of business, we may become a party to lawsuits involving various matters. The impact and outcome of litigation, if any, is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business. We believe the ultimate resolution of any such current proceeding will not have a material adverse effect on our continued financial position, results of operations or cash flows, except as otherwise set forth below. However, assessment of the current litigation or other legal claims could change in light of the discovery of facts not presently known to the Company or by judges, juries or other finders of fact, which are not in accord with management’s evaluation of the possible liability or outcome of such litigation or claims.
For a description of our material pending legal proceedings, please see “Note 8 - Other Receivables” and “Note 9“Note 16 – Contingencies”Contingencies”. to the Notes to Consolidated Financial Statements included herein under “Item“Item 8. Financial Statements and Supplemental DataData.”.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
PART II
| ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market for Common Stock
Our common stock was approved for listing on The NASDAQ Capital Market under the symbol “MEDS”, on February 13, 2020. Prior to that, it traded on the OTCQB Market under the symbol “TRXD”. At present, there is a limited market for our common stock.
Common Stock and Preferred Stock Outstanding and Holders of Record
As of March 28, 2022,April 22, 2024, we had 8,181,0411,406,348 shares of common stock outstanding, held by 3952 stockholders of record, not including holders who hold their shares in street name, and noalso shares of Series C Preferred Stock issued or outstanding.
Dividend Policy
WeAlthough we paid a special cash dividend in the first quarter of 2024, we have nevernot historically paid or declared any cash dividends on our common stock and do not anticipate paying cash dividends in the foreseeable future. We anticipate that we will retain all of our future earnings for use in the operation of our business and for general corporate purposes.stock. Any determination to pay dividends in the future will be at the discretion of our board of directors. Accordingly, investors must relyhave historically relied on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investments.
Recent Sales of Unregistered Securities
The disclosures below include information on recentThere were no sales of unregistered securities during the three months ended December 31, 2021,2023, and from the period from January 1, 2022,2024, to the filing date of this report, and dothat were not include information which has previously been includeddisclosed in a Quarterly Report on Form 10-Q or in a Current Report on Form 8-K:8-K.
In January 2022, warrants to purchase 14,584 shares of common stock were exercised with an exercise price of $0.06 per share; the Company issued 14,584 shares of common stock, and $875 in proceeds were received in connection with such exercise.
We claim an exemption from registration pursuant to Section 4(a)(2) and/or Rule 506 of Regulation D of the Securities Act, since the foregoing issuances did not involve a public offering, the recipients were (a) “accredited investors”; and/or (b) had access to similar documentation and information as would be required in a Registration Statement under the Securities Act. The securities are subject to transfer restrictions, and the certificates evidencing the securities contain an appropriate legend stating that such securities have not been registered under the Securities Act and may not be offered or sold absent registration or pursuant to an exemption therefrom.
Issuer Purchases of Equity Securities
The following table sets forth share repurchase activity forIn May and December of 2021, the respective periods:
| Period | Total Number of Shares Purchased | Average Price Paid Per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Maximum Approximate Dollar Value of Shares that May Yet Be Purchased Under the Plans or Programs(1) | Maximum Number of Shares that May Yet Be Purchased Under the Plans or Programs(2) | |||||||||||||||
| October 1, 2021 – October 31, 2021 | — | $ | — | — | $ | 1,000,000 | — | |||||||||||||
| November 1, 2021 – November 30, 2021 | — | $ | — | — | $ | 1,000,000 | — | |||||||||||||
| December 1, 2021 – December 31, 2021 | — | $ | — | — | $ | 1,000,000 | 100,000 | |||||||||||||
| Total | — | $ | — | — | ||||||||||||||||
(1) On May 27, 2021, ourCompany’s Board of Directors authorized the repurchase of up to $1 million of the currently outstanding shares of the Company’s common stock. Under the stock repurchase program, shares may be repurchased from time to time in the open market or through negotiated transactions at prevailing market rates, or by other means in accordance with federal securities laws. Repurchases will be made at management’s discretion at prices management considers to be attractive and in the best interests of both the Company and its stockholders, subject to the availability of stock, general market conditions, the trading price of the stock, alternative uses for capital, and the Company’s financial performance. OpenAny open market purchases will be conducted in accordance with the limitations set forth in Rule 10b-18 of Exchange Act and other applicable legal requirements. Repurchases may also be made under a Rule 10b5-1 plan. There was no time frame or expiration date for the repurchase program, and such program was to remain in place until a maximum of $1.0 million of the Company’s common stock had been repurchased or until such program was suspended or discontinued by the Board of Directors.
On July 18, 2021, our Board of Directors approved an “at-the-market” offering and paused During Fiscal 2023 the Stock Repurchase Program until the offering is complete.
Company did not
On July 22, 2021, our Board of Directors delayed the “at-the-market” offering and reactivated the Stock Repurchase Program.
On August 5, 2021, our Board of Directors paused the Stock Repurchase Program until a planned “at-the-market” offering was complete, which “at-the-market” offering was terminated effective on December 5, 2021.
Currently no dollar amount of shares may be purchased pursuant to the terms of the Stock Repurchase Program, which as discussed in footnote (2) below, has been modified to allow for the repurchase of 100,000 shares of common stock instead of a dollar amount.
(2) On December 10, 2021, the Board of Directors authorized and approved the resumption of the Company’s prior share repurchase program (as modified). The share repurchase program as approved by the Board of Directors on December 10, 2021, modified the prior repurchase program to allow for the repurchase of up to 100,000 of the currently outstanding shares of the Company’s common stock. There is no time frame for the repurchase program, and such program will remain in place until a maximum of 100,000or any shares of the Company’s common stock have been repurchased or until such program is discontinued byunder the Board of Directors.repurchase program.
| 45 |
| ITEM 6. | [RESERVED] |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion of the Company’s historical performance and financial condition should be read together with the consolidated financial statements and related notes in “Item“Item 8. Financial Statements and Supplemental Data”Data” of this Report. This discussion contains forward-looking statements based on the views and beliefs of our management, as well as assumptions and estimates made by our management. See “Cautionary“Cautionary Statement Regarding Forward-Looking Information”Information” above. These statements by their nature are subject to risks and uncertainties and are influenced by various factors. As a consequence, actual results may differ materially from those in the forward-looking statements. See “Item“Item 1A. Risk Factors”Factors” of this report for the discussion of risk factors. For all periods presented, the consolidated statements of income and consolidated balance sheet data have been adjusted for the reclassification of discontinued operations information, unless otherwise noted. All references to years relate to the calendar year ended December 31 of the particular year.
Our Management’s Discussion and Analysis of Financial Condition and Results of Operations (the “MD&A”) is provided in addition to the accompanying consolidated financial statements and notes to assist readers in understanding our results of operations, financial condition, and cash flows. MD&A is organized as follows:
| ● | Plan of Operations. Summary of the Company’s plan of operations for the next 12 months. | |
| ● | Sources of Revenue. Summary of the main sources of Company revenue during the reported periods. | |
| ● | Results of Operations. An analysis of our financial results comparing the | |
| ● | Liquidity and Capital Resources. An analysis of changes in our balance sheets and cash flows and discussion of our financial condition. | |
| ● | Critical Accounting Policies and Estimates. Accounting policies and estimates that we believe are important to understanding the assumptions and judgments incorporated in our reported financial results and forecasts. | |
| ● | Recently Issued Accounting Standards. A summary of recently issued accounting standards affecting the Company, if any. |
Plan of Operations
We had a working capital deficit of $3,448,218$8,803,293 as of December 31, 2021,2023, compared to $8,379,060working capital deficit of $53,668 as of December 31, 2020.2022. The decrease in working capital of $4,930,842 was$8,749,625 is related to write off of other receivables of $1,087,675decreases in cash and inventory write-offs of $376,348, and spend on research and development expenses of $1,367,895. increases in liabilities. Below are reasons for the decrease in working capital.
| ● | Cash decreased approximately $943,000 from December 31, 2022 to December 31, 2023. The reasons for the decrease are as follows: |
| ○ | $250,000 repayments of debt, net of new debt issuances; and | |
| ○ | $733,694 paid for interest expense related to the sale of future accounts receivable. |
| ● | Increases in current liabilities of approximately $9,409,564 from December 31, 2022 to December 31, 2023 were driven by the following main factors: |
| ○ | $1,554,070 increase in accounts payable balance as of December 31, 2023 compared to the comparable period; | |
| ○ | $1,138,310 increase in the balance due on the sale of future accounts receivable as of December 31, 2023 compared to the comparable period; | |
| ○ | $6,363,333 increase in the current portion of notes payable balance; and a | |
| ○ | $350,000 increase in purchase price payable balance as of December 31, 2023 compared to the comparable period. |
With our current cash on hand, expected revenues, and based on our current average monthly expenses, we do not anticipate the need for additional funding in order to continue our operations at their current levels, and to pay the costs associated with being a public company, for the next 12 months. We may require additional funding in the future to expand or complete acquisitions. The sources of this capital are expected to be equity investments and notes payable. Our plan for the next twelve months is to continue development of the information technology used in the Company subsidiaries, which it is anticipated that current cash on hand is able to fund and continue providing a quality product with excellent customer service while also seeking to expand our operations organically or through acquisitions as funding and opportunities arise.subsidiaries. As our business continues to grow, customer feedback will be integral in making small adjustments to improve the product and overall customer experience. In the event weWe will require additional funding, we planmay seek to raise that through the sale of debt or equity, which may not be available on favorable terms, if at all, and may, if sold, cause significant dilution to existing stockholders. If we are unable to access additional capital moving forward, it may hurt our ability to grow and to generate future revenues.
Novel Coronavirus (COVID-19)
In December 2019, a novel strain of coronavirus, which causes the infectious disease known as COVID-19, was reported in Wuhan, China. The World Health Organization declared COVID-19 a “Public Health Emergency of International Concern” on January 30, 2020, and a global pandemic on March 11, 2020. In March and April 2020, many U.S. states and local jurisdictions began issuing ‘stay-at-home’ orders. For example, the state of Florida, where the Company’s principal business operations are, issued a ‘stay-at-home’ order effective on April 1, 2020, which remained in place, subject to certain exceptions, through June 2020, when the order was gradually lifted until September 2020, when the order was completely lifted. The U.S. in general and Florida specifically, has recently seen decreases in total new COVID-19 infections (after sharp increases in infections in mid-to-late January 2022), as vaccines and boosters are now widely available and the number of individuals who have received vaccines has increased, and the pool of persons who do not have natural or vaccine immunity have declined; however, it is unknown whether such decreases will continue, new strains of the virus will cause current vaccines to be less effective or whether infection numbers will increase, and/or whether the state of Florida, or other jurisdictions in which we operate, will issue new or expanded ‘stay-at-home’ orders, or how those orders, or others, may affect our operations or whether such locations will see increases in infection rates, hospitalizations and deaths.
To date, we have been deemed an essential healthcare technology provider under applicable governmental orders based on the critical nature of the products we offer and the community we serve. As such, our business operations were not materially impacted by the prior restrictions put in place by the State of Florida to slow the spread of COVID-19, which have since expired. Additionally, as shown in our results of operations below, we have to date, not experienced any significant material negative impact to our operations, revenues or gross profit due to COVID-19. We have however been adversely affected by reductions to, and interruptions in, the delivery of supply chain pharmaceuticals that have had a negative impact on our wholesalers, certain technology outsourcing in India and the Philippines and finding qualified staff due to the pandemic, which may become more frequent or material in the future. We are carefully managing our inventory supply network while we work to overcome these hopefully temporary challenges. As a result of the above, the full extent of the impact of COVID-19 on our business and operations currently cannot be estimated and will depend on a number of factors including the continued scope and duration of the global pandemic.
Since the start of the pandemic, we have taken steps to prioritize the health and safety of our employees. The Company’s employees started working remotely around March 17, 2020, and our corporate office was closed through December 31, 2021. The office reopened for our management team on January 3, 2022, while our remaining employees will continue to work remotely until further notice.
Currently we believe that we have sufficient cash on hand and will generate sufficient cash through operations and potential future equity sales, to support our operations for the foreseeable future; however, we will continue to evaluate our business operations based on new information as it becomes available and will make changes that we consider necessary in light of any new developments regarding the ongoing pandemic. We may also raise additional funding in the future through sales of debt or equity.
Sources of Revenue
We currently have threeDuring 2023 we had four main revenue streams:
(1) Trxade, Inc., our wholly-owned subsidiary, provides an online web-based buying and selling platform for licensed pharmaceutical wholesalers (“Suppliers”) to sell products and services to licensed pharmacies (“Customers”). The Company charges Suppliers a transaction fee, a percentage of the purchase price of the prescription drugs and other products sold through its website service. The Company holds no inventory and assumes no responsibility for the shipment or delivery of any products or services from our website. The Company considers itself an agent for this revenue stream and as such, reports revenue as net. Subsequent to December 31, 2023, we divested substantially all of our assets previously owned and operated by Trxade, Inc.
(2) Integra Pharma Solutions, LLC, our wholly-owned subsidiary, is a licensed wholesaler of brand, generic and non-drug products to Customers. The Company takes orders for products, creates invoices for each order and recognizes revenue at the time the Customer receives the product. Customer returns, to date, have not been material.
(3) Community Specialty Pharmacy, LLC, our wholly-owned subsidiary, is a licensed retail pharmacy. The Company fills prescriptions for drugs written by a doctor and recognizes revenue at the time the patient confirms delivery of the prescription. Customer returns, to date, have not been material. In August 2023 we sold our entire interest in Community Specialty Pharmacy, LLC.
(4) The Urgent Company, Inc., our wholly-owned subsidiary, is a retail and distribution provider of prepackaged, prepared foods. Subsequent to December 31, 2023, we divested our interest in The Urgent Company, LLC.
Results of Operations
For the Year Ended December 31, 2021,2023, compared to the Year Ended December 31, 20202022
The following selected consolidated financial data should be read in conjunction with the consolidated financial statements and the notes to these statements included in “Item“Item 8. Financial Statements and Supplemental Data”Data” of this Report. For all years presented, the consolidated statements of income and consolidated balance sheet data set forth in this Form 10-K have been adjusted for the reclassification of discontinued operations information, unless otherwise noted.
| Fiscal Year Ended | Percentage | |||||||||||||||
| December 31, 2021 | December 31, 2020 | Change | Change | |||||||||||||
| Revenues | $ | 9,889,433 | $ | 17,122,520 | (7,233,087 | ) | (42.2 | )% | ||||||||
| Cost of Sales | 5,143,468 | 11,415,198 | (6,271,730 | ) | (54.9 | )% | ||||||||||
| Gross Profit | 4,745,965 | 5,707,322 | (961,357 | ) | (16.8 | )% | ||||||||||
| Operating Expenses: | ||||||||||||||||
| Loss on Inventory Investment | 1,226,426 | - | 1,226,426 | 100.0 | % | |||||||||||
| Technology, Research & Development | 1,367,895 | 662,726 | 705,169 | 106.4 | % | |||||||||||
| Loss on Impairment of Goodwill | - | 725,973 | (725,973 | ) | (100.0 | )% | ||||||||||
| Other General and Administrative | 7,053,861 | 4,962,237 | 2,091,624 | 42.2 | % | |||||||||||
| Warrants and Options Expense | 390,076 | 1,863,048 | (1,472,972 | ) | (79.1 | )% | ||||||||||
| Total Operating Expense | 10,038,258 | 8,213,984 | 1,824,274 | 22.2 | % | |||||||||||
| Interest Expense | (23,590 | ) | (29,389 | ) | 5,799 | (19.7 | )% | |||||||||
| Income (Loss) from Operations | $ | (5,315,883 | ) | $ | (2,536,051 | ) | (2,779,832 | ) | (109.6 | )% | ||||||
| Fiscal Year Ended December 31, | Percentage | |||||||||||||||
| 2023 | 2022 | Change | Change | |||||||||||||
| Revenues | $ | 8,272,214 | $ | 10,250,168 | (1,977,954 | ) | (19.3 | %) | ||||||||
| Cost of sales | 5,673,957 | 4,730,897 | 943,060 | 19.9 | % | |||||||||||
| Gross profit | 2,598,257 | 5,519,271 | (2,921,014 | ) | (52.9 | %) | ||||||||||
| Operating expenses: | ||||||||||||||||
| Loss on inventory investment | - | 875,250 | (875,250 | ) | (100 | %) | ||||||||||
| Technology, research & development | 1,376,908 | 993,185 | 383,723 | 38.6 | % | |||||||||||
| Wages and salary | 2,698,178 | 3,581,089 | (882,911 | ) | (24.7 | %) | ||||||||||
| Accounting and legal | 1,534,377 | 829,751 | 704,626 | 84.9 | % | |||||||||||
| Professional fees | 1,466,567 | 466,735 | 999,832 | 214.2 | % | |||||||||||
| Other general and administrative (less stock-based compensation expense) | 2,498,123 | 1,355,946 | 1,142,177 | 84.2 | % | |||||||||||
| Warrants and options expense | 287,510 | 333,284 | (45,774 | ) | (13.7 | %) | ||||||||||
| Total operating expenses | 9,861,663 | 8,435,240 | 1,426,423 | 16.9 | % | |||||||||||
| Change in fair value of warrant liability | (148,420 | ) | 825,544 | (973,964 | ) | (118.0 | %) | |||||||||
| Interest, net | (1,194,148 | ) | (315,217 | ) | (878,931 | ) | 278.8 | % | ||||||||
| Goodwill impairment | (5,129,115 | ) | - | (5,129,115 | ) | (100.0 | %) | |||||||||
| Gain on disposal of asset | - | 2,200 | (2,200 | ) | (100.0 | %) | ||||||||||
| Other income | 14,543 | - | 14,543 | 100.0 | % | |||||||||||
| Net loss from operations | $ | (13,720,546 | ) | $ | (2,403,442 | ) | $ | (11,317,104 | ) | 470.9 | % | |||||
| Loss on discontinued operations | (4,123,028 | ) | (1,506,426 | ) | (2,616,602 | ) | 173.7 | % | ||||||||
| Net loss attributable to TRxADE Health, Inc. | (17,843,574 | ) | (3,472,099 | ) | (14,371,475 | ) | 413.9 | % | ||||||||
| Net loss attributable to non-controlling interests | - | (437,769 | ) | 437,769 | (100.0 | %) | ||||||||||
| 47 |
Operations
Our revenues during the years ended December 31, 2021,2023, and 20202022 were mainly from the Trxade Inc. platform, Community Specialty Pharmacy and Integra Pharma Solutions.Solutions, and The Urgent Company. Revenues decreased by $7,233,087$1,977,954 for the 2021fiscal year 2023, compared to the prior year’s period. Inrevenue of $10,250,168. Trxade, Inc., revenue decreasedincreased by $622,731$852,933 or 11%16% to $4,924,015,$6,200,334, compared to $5,546,746,$5,347,401, for the years ended December 31, 2021,2023, and 2020,2022, which is attributable to larger amounts of personal protective equipment (PPE) items being solda 16% increase in sales volume on the platform in 2020 than in 2021 as a result of the COVID-19 Pandemic and more brand pharmaceutical product being sold through the platform at a lower transaction fee than generic pharmaceutical products with a higher transaction fee.2023. Integra Pharma SolutionsSolutions’ revenue decreased by $6,626,506,$3,390,237, or 71%, which is attributable to non-recurringdecreased sales of personal protective equipment (PPE) items that were needed in large quantities in 2020 as a result of the COVID-19 Pandemic,volume and which line of products the Company did not continue in during 2021.pricing changes. The Trxade, Inc. platform is a secondary marketplace for pharmaceuticals and medical supplies with consistent growth year over year. We see a trend that whenever there is a supply shortage on the primary market, the platform being a secondary market, will see increase in traffic or sales. Therefore, extraordinary events such as COVID-19 will results in larger increases in addition to the normal growth year over year.
Cost of Salessales was 5,143,468$5,673,957 and gross profit was $4,745,965,$2,598,257, for the year ended December 31, 2021,2023, compared to $11,415,198$4,730,897 and $5,707,322,$5,519,271, respectively, for the year ended December 31, 2020. As sales for PPE decreased2022. The increase in 2021, the cost of sales decreased.is attributed to the inventory costs and inventory write-downs associated with The Urgent Company.
Gross profit as a percentage of sales was 48%31.4% for the year ended December 31, 2021,2023, compared to 33%53.8% for the year ended December 31, 2020.2022. The reason for the increasedecrease in gross profit as a percentage of sales was a result of a larger percentage of our revenue being from the Trxade Platform, which carries noincreased inventory and cost of sales in 2021, while in 2020, a larger percentage of our revenue was related to orders of PPE related product, which include a relatively high cost of sales.associated with The Urgent Company.
Technology, research and development expenditures increased to $1,367,895$1,376,908 for 2021,the year ended December 31, 2023, compared to $662,726$993,185 for 2020,the year ended December 31, 2022, as the Company continued to develop apps for customers.customers and make improvements to our platform technology.
Professional fees increased for the year ended December 31, 2023 by $999,832 to $1,466,567 compared to $466,735 for the year ended December 31, 2022. The increase in professional fees for the year ended December 31, 2023 related to the merger with Superlatus and purchase of The Urgent Company.
General and administrative expenses (less stock-based compensation expense, technology, research and development, loss on inventory investments)expense) increased for the year ended December 31, 2021,2023 to $7,053,861,$2,498,123 compared to $4,962,237$1,355,946 for the comparable period in 2020.year ended December 31, 2022. The increase was mainly due to increases in employee compensation in order to complete in the current challenging labor market, legal expensesis largely driven by amortization expense related to historically disclosed lawsuits, and research and development expenses as a result of expanding and developingintangible assets acquired through the newer business units.Sapientia Technologies acquisition.
Total stock-based compensation expense decreased by 79%13.7% or $45,774 to $287,510 from $333,284 for the year ended December 31, 2021,2023, compared to the prior year’s periodperiod. The decrease was due to less common stock issued for services during the Company not granting warrants and bonus sharesyear ended December 31, 2023 compared to executives in 2021, as described in greater detail under “Item 8. Financial Statements and Supplemental Data”– “Note 4 – Stockholders’ Equity”.the year ended December 31, 2022.
We had $1,226,426 ofThe Company recognized a loss on inventory investment of $875,520 for the year ended December 31, 2021,2022, in connection with our write-down of our the Bonum Health Hubs after we determinedCOVID-19 test kits that the Hubswere purchased and could not be assembled and placed into serviceresold due to generate revenue without requiring further investments and our write-down of other receivables related to inventory deposits we made to suppliers that were not refunded to us whenissues with the suppliers could not fulfill our purchase order as described in greater detail under NOTE - 8 OTHER RECEIVABLES.FDA.
| 48 |
We
The Company had $725,973interest expense, net, of loss on impairment of goodwill$1,194,148 for the year ended December 31, 2020, in connection with the acquisition of Community Specialty Pharmacy, LLC. In 2020, we performed a qualitative and quantitative assessment2023, compared to determine the impairment of goodwill and found that due to the decrease in patient prescription post acquisition and COVID-19 uncertainties that the company may have likely overpaid for the acquisition and impaired goodwill to zero.
We had interest expense of $23,590$315,217 for the year ended December 31, 2021, compared to2022. The increased interest expense is driven by the increases in the contingent funding liability due to additional accounts receivable advances during the year ended December 31, 2023.
During the year ended December 31, 2023, the Company recognized a loss from the change in the fair value of $29,389warrants of $148,420. During the year ended December 31, 2022, the Company recognized a gain from the change in the fair value of warrants of $825,544.
The Company recognized a goodwill impairment loss of $5,129,115 for the year ended December 31, 2020, which decreased due2023. The goodwill resulted from the acquisition of Superlatus and was subsequently determined to decreases inbe impaired based on the amountfacts and circumstances surrounding the sale of outstanding debtSuperlatus on March 5, 2024.
Net loss from operations increased $11,317,104 to a net loss of $13,720,546 for the Company had to $0 from $225,000 at the yearsyear ended December 31, 2021,2023, compared to a net loss of $2,403,442 for the year ended December 31, 2022. The increase in net loss is mainly due to the write-down of inventory due to spoilage, increases in spending related to the merger transaction with Superlatus and 2020, respectively.purchase of TUC and goodwill impairment charges.
Net loss from discontinued operations increased by $2,779,832,$2,616,602 to a net loss of $5,315,883$4,123,028 for the year ended December 31, 2021,2023, compared to a net loss from discontinued operations of $2,536,051$1,506,426 for the year ended December 31, 2020, mainly due to the increase in general and administrative expenses associated with research and development cost for our new business units and write-off of other receivables related to inventory deposits as explained above (see NOTE - 8 OTHER RECEIVABLES).2022.
Liquidity and Capital Resources
Cash and Cash Equivalents
Cash and cash equivalents were $3,122,578 at$151,908 as of December 31, 2021.2023. We expect that our future available capital resources will consist primarily of cash generated from operations, remaining cash balances, proceeds from potential asset divestitures or strategic transactions, borrowings, and any additional funds raised through sales of debt and/or equity.
Liquidity
Cash, and cash equivalents, current assets,, current liabilities, short term debt and working capital at the end of each period were as follows:
| December 31, 2021 | December 31, 2020 | |||||||
| Cash | $ | 3,122,578 | $ | 5,919,578 | ||||
| Current assets (excluding cash) | 1,251,666 | 3,301,720 | ||||||
| Current liabilities (excluding short term debt) | 926,026 | 617,238 | ||||||
| Short term debt* | - | 225,000 | ||||||
| Working Capital | 3,448,218 | 8,379,060 | ||||||
* Short term notes payable – related parties.
| December 31, 2023 | December 31, 2022 | |||||||
| Cash | $ | 151,908 | $ | 1,094,894 | ||||
| Current assets (excluding cash) | 2,601,154 | 998,229 | ||||||
| Current liabilities (excluding short term debt) | 5,026,355 | 1,980,124 | ||||||
| Short term debt | 6,530,000 | 166,667 | ||||||
| Working deficit | (8,803,293 | ) | (53,668 | ) | ||||
Our principal sources of liquidity during the years ended December 31, 2021,2023 and 20202022 have been cash provided by operations (internal source), and during 2020,. During the year ended December 31, 2023, sales of future receivables provided a principal source of liquidity. During the year ended December 31, 2022, equity capital and borrowings under various debt arrangements (external source). and a stock placement deal of 920,000 shares. Our principal uses of cash have been for operating expenses and research and development of our newer business units. We anticipate these uses will continue to be our principal uses of cash in the future in addition to any necessary business acquisitions. We currently do not have any material unused sources of liquid assets.
Cash decreased by $942,986 and other current assets decreasedincreased by $2,797,000 and $2,050,054 respectively.$1,602,925. The decrease in cash and cash equivalents was primarily due to amounts spent on researchinterest expense associated with the sale of future receivables and developmentnet repayments of debt as well as the professional fees and accounting and legal expenses related to our newer business units.associated with the merger with Superlatus and the acquisition of The decreaseUrgent Company. The increase in ourother current assets was primarily due to the write offa note receivable and other receivables related to inventory deposits to suppliers that we did not get refunded whenfrom the suppliers could not fulfill our purchase order (see NOTE - 8 OTHER RECEIVABLES).sale of APS and CSP.
| 49 |
Current liabilities (excluding short term debt) increased by $83,788.$3,046,231 from $1,980,124 to $5,026,355 for the year ended December 31, 2023. The increase is primarily due to an increase in operating accounts payable not being paid until January 4, 2022, afterand contingent funding liabilities from the account payable balance was recorded for the year ended December 31, 2021.sale of future receivables.
Liquidity Outlook cash explanation
Cash Requirements
Our primary objectives for 20222024 are to continue the development of the Trxade Platform, DelivMeds and Bonum Health and work to increase our client base and operational revenue. As a resultexpansion of Integra Pharma Solutions and to explore strategic transactions, relationships or acquisitions to grow or operations whether in our cash generated through operations and cash on hand, we believe we have sufficient cash to support our operations for the foreseeable future.legacy industry or outside of that general industry. There can be no assurance that our operations will generate significant positive cash flow, or that additional funds will be available to us, through borrowings or otherwise, on favorable terms if required in the future, or at all.
We estimate our operating expenses and working capital requirements for the next 12 months to be approximately as follows:
| Projected Expenses for 2022 | Amount | |||||||
| Projected Expenses for 2024 | Amount | |||||||
| General and administrative (1) | $ | 7,100,000 | $ | 4,800,000 | ||||
| Total | $ | 7,100,000 | $ | 4,800,000 | ||||
| (1) | Includes wages and payroll, legal and accounting, marketing, rent and technology development. |
We have historically funded our operations primarily through debt and equity capital raises and operational revenue. In 2021, common stock was sold for net proceeds of $16,822 in connection with the exercise of warrants and stock options previously awarded. In 2020, common stock was sold for net proceeds of $5,262,068.
We may require additional funding in the future to expand or complete acquisitions. The sources of this capital are expected to be equity investments and notes payable. Our plan for the next twelve months is to continue using the same marketing and management strategies to promote our Integra Pharma Solutions assets and continue providing a quality product with excellent customer serviceoperations, exploring strategic transactions involving our corporate assets, while also seeking to expand our operations organically or through acquisitions, as funding and opportunities arise. As our business continues to grow, customer feedback will be integral in making small adjustments to improve our products and overall customer experience. In the event we require additional funding, we plan to raise that through the sale of debt or equity, which may not be available on favorable terms, if at all, and may, if sold, cause significant dilution to existing stockholders. If we are unable to access additional capital moving forward, it may hurt our ability to grow and to generate future revenues.
We believe that we have adequate cash to implement our plan to operate a business-to-business web-based marketplace focused on the United States pharmaceutical industry. Our core service is designed to bring the nation’s independent pharmacies and accredited national suppliers of pharmaceuticals together to provide efficient and transparent buying and selling opportunities.
Cash Flows
The following table summarizes our Consolidated Statements of Cash Flows for the fiscal years ended December 31, 2021,2023, and 2020:2022:
| December 31, 2021 | December 31, 2020 | Change | Percent Change | |||||||||||||
| Net Loss | $ | (5,315,883 | ) | $ | (2,536,051 | ) | $ | (2,716,347 | ) | (107.11 | )% | |||||
| Net Cash Provided by (used in): | ||||||||||||||||
| Operating Activities | (2,566,226 | ) | (2,214,786 | ) | (409,958 | ) | (18.51 | )% | ||||||||
| Investing Activities | (22,596 | ) | (37,505 | ) | 294,747 | 195.78 | % | |||||||||
| Financing Activities | (208,178 | ) | 5,300,175 | (5,508,353 | ) | (103.93 | )% | |||||||||
| Net increase (decrease) in cash | $ | (2,797,000 | ) | $ | 3,047,884 | $ | (5,844,885 | ) | $ | (191.77 | )% | |||||
| December 31, 2023 | December 31, 2022 | Change | Percent Change | |||||||||||||
| Net loss from continuing operations | $ | (13,720,546 | ) | $ | (2,403,442 | ) | $ | (11,317,104 | ) | 470.9 | % | |||||
| Net cash provided by (used in): | ||||||||||||||||
| Net cash (used in) operating activities from continuing operations | (1,592,424 | ) | (199,020 | ) | (1,393,404 | ) | 700.1 | % | ||||||||
| Net cash (used in) operating activities from discontinued operations | (481,177 | ) | (1,365,648 | ) | 884,471 | (64.8 | %) | |||||||||
| Operating activities | (2,073,601 | ) | (1,564,668 | ) | (508,933 | ) | 32.5 | % | ||||||||
| Net cash (used in) investing activities from continuing operations | (344,454 | ) | (427,845 | ) | 83,391 | (19.5 | %) | |||||||||
| Net cash provided by (used in) investing activities from discontinued operations | 68,737 | - | 68,737 | 100.0 | % | |||||||||||
| Investing activities | (275,717 | ) | (427,845 | ) | 152,128 | (35.6 | %) | |||||||||
| Net cash provided by (used in) financing activities from continuing operations | 1,906,332 | (35,171 | ) | 1,941,503 | (5,520.2 | %) | ||||||||||
| Net cash provided by (used in) financing activities from discontinued operations | (500,000 | ) | - | (500,000 | ) | 100.0 | % | |||||||||
| Financing activities | 1,406,332 | (35,171 | ) | 1,441,503 | (4,098.6 | %) | ||||||||||
| Net change in cash | $ | (942,986 | ) | $ | (2,027,684 | ) | $ | 1,084,698 | (53.5 | %) | ||||||
| 50 |
Cash used byin operations for the fiscal year ended December 31, 2021,2023 was $2,566,226.$2,073,601. This compared to $2,214,786$1,564,668 of cash used byin operating activities for the fiscal year ended December 31, 2020.2022. The increase in cash used in operations was primarilymainly due to spendingincreased professional fees and accounting and legal expense for research & development relatedthe comparable period as a result of the merger with Superlatus and the purchase of The Urgent Company, partially offset by decreased wages and salary expense due to the development MedCheks Health Passport Application, the developmentdeparture of DelivMeds Application, legal expenses related to outstanding lawsuits, repaymenttwo members of related party loan, and employee payroll. For additional information refer to Notes to Consolidated Financial Statements.
management during 2023.
Cash used byin investing activities for the fiscal year ended December 31, 2021,2023 was $22,596.$275,717. This compared to $37,505$427,845 of cash used in investing activities for the fiscal year ended 2020.December 31, 2022. In 2021,2023, the net cash used mainly related to net cash exchanged in acquisition and disposals. During the year ended December 31, 2022, the cash was used to purchase a single delivery vehicle for Community Specialty Pharmacy, LLC. In 2020, the cash was used to purchase a single forklift to the Integra Pharmacy Solution, LLC warehouse.an investment in capitalized software for Delivmeds.
Cash usedprovided by financing activities for the fiscal year ended December 31, 2021,2023 was $208,178, which $225,000 was$1,406,332 and cash used to repay a related party loan and $16,822 was received from the exercise of warrants and options. This compared to $5,994,424 of proceeds and $5,300,175 of cash to the Company after expenses, and the exercise of warrants and options which generated cash of $38,107in financing activities for the fiscal year ended December 31, 2020.2022, was $35,171. The increase was mainly due to proceeds from the sale of future receivables.
Known Contractual and Other Obligations & Commitments
In addition to our long-term debt obligations to our various lenders, we have certain other known contractual working capital obligations, including contractual purchase obligations related to various supply contracts, lease obligations, and other liabilities.
The following table summarizes our contractual obligations as of December 31, 2021:2023:
| Payments due by Period | Payments due by Period | |||||||||||||||||||||||||||||||||||||||
| Contractual Obligations | Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||||||||||||||||||
| Operating lease obligations | 1,629,125 | 294,932 | 907,746 | 320,916 | 105,531 | 651,528 | 187,935 | 356,634 | 106,959 | - | ||||||||||||||||||||||||||||||
| Total Contractual obligations | $ | 1,629,125 | 294,932 | 907,746 | 320,916 | 105,531 | $ | 651,528 | 187,935 | 356,634 | 106,959 | - | ||||||||||||||||||||||||||||
Off-Balance Sheet Arrangements
We had no outstanding off-balance sheet arrangements as of December 31, 2021.2023.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of net sales and expenses for each period. We consider an accounting estimate to be critical if the estimate requires us to make assumptions about matters that were uncertain at the time the accounting estimate was made and if different estimates that we reasonablereasonably could have used in the current period, or changes in the accounting estimate that are reasonably likely to occur from period to period, could have a material impact on our financial condition or results from operations. Below are the estimates that we believe are critical to the understanding of our operation results and financial condition. Other accounting policies are described in Financial NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES.POLICIES. Because of the uncertainty inherent in such estimates, actual results may differ from these estimates.
Allowance for Doubtful Accounts
We provide short-term credit and other customer financing arrangements to customers who purchase our products. We estimate the receivables for which we do not expect full collection based on historical collection rates and specific knowledge regarding the current creditworthiness of our customers and record an allowance in our consolidated financial statements for these amounts.
We consider historical experience, the current economic environment, customer credit ratings or bankruptcies, and reasonable and supportable forecasts to develop its allowance for doubtful accounts. Management reviews these factors quarterly to determine if any adjustments are needed to the allowance.
| 51 |
Reserve methodologies are assessed annually based on historical losses and economic, business and market trends. In addition, reserves are reviewed quarterly and updated if unusual circumstances or trends are present. We believe the reserves maintained and expenses recorded in 2021during the year ended December 31, 2023 are appropriate and consistent in the context of historical methodologies employed, as well as assessment of trends currently available.
Allowance for Doubtful AccountsInventory Costs
In determining whether an inventory valuation allowance is required, we consider various factors including estimated quantities of slow-moving inventory by reviewing on-hand quantities, outstanding purchase obligations and forecasted sales. Shifts in market trends and conditions, changes in customer preferences due to the introduction of generic drugs or new pharmaceutical products or the loss of one or more significant customers are factors that could affect the value of our inventories. We write down inventories which are considered excess and obsolete as a resultsresult of these revies.reviews. These factors could make our estimate of inventory valuation differ from actual results.
Business Combinations
We account for business combinations using the acquisition method of accounting whereby the identifiable assets and liabilities of the acquired business, as well as any noncontrolling interest in the acquired business, are recorded at their estimated fair value as the date that we obtain control of the acquired business. Any purchase consideration in excess of the fair values of the net assets acquired is recorded as goodwill. Acquisition-related expenses and related restructuring costs are expensed as incurred.
Several valuation methods may be used to determine the fair value of the assets acquired and liabilities assumed. For intangible assets, we typically use a method that is a form of variation of the income approach, whereby a forecast of future cash flows attributable to the asset are discounted to present value using a risk-adjusted discount rate. Some of the more significant estimates and assumptions inherent in the income approach include the amount and timing of projected future cash flows, the discount rate selected to measure the risks inherent in the future cash flows and the assessment of the asset’s expected useful life.
Goodwill
We perform an impairment test on goodwill balances annually in the third quarter and more frequently if indicators for potential impairment exist. Indicators that are considered include significant declines in performance relative to expected operating results, significant changes in the use of the assets, significant negative industry or economic trends, or a significant decline in the Company’s stock price and/or market capitalization for a sustained period of time.
Goodwill impairment testing is conducted at the reporting unit level, which is generally defined as an operating segment or a component, one level below our operating segment, for which discrete financial information is available and segment management regularly reviews the operating results of the reporting unit.
To estimate the fair value of our reporting units, we generally use a combination of the market approach and the income approach. Under the market approach, we estimate fair value by comparing the business to similar business, or guideline companies whose securities are actively traded in public markets. Under the income approach, we use a discounted cash flow (“DCF”DCF”) model in which cash flows anticipated over several periods, plus a terminal value at the end of that time horizon, are discounted to their present value using an appropriate rate that is commensurate with the risk inherent within the reporting unit. In addition, we compare the aggregate of the reporting units’ fair values to our market capitalization as further corroboration of the fair values.
Estimates of fair value result from a complex series of judgements about future events and uncertainties and rely heavily on estimates and assumptions at a point in time. Judgements made in determining an estimate of fair value may materially impact our results of operations. The valuations are based on information available as of the impairment testing date and are based on expectations and assumptions that have been deemed reasonablyreasonable by management. Any material changes in key assumptions, including failure to meet business plans, negative changes in government reimbursement rates, deterioration in the U.S. and global financial markets, an increase in interest rates or an increase in the cost of equity financing by market participants within the industry or other unanticipated events and circumstances, may decrease the projected cash flows or increase the discount rate and could potentially result in an impairment charge. Under the market approach, significant estimates and assumptions also include the selection of appropriate guideline companies and the determination of appropriate valuation multiples to apply to the reporting unit. Under the income approach, significant estimates and assumptions also include the determination of discount rates. The discount rates represent the weight-average cost of capital measuring the reporting unit’s cost of debt and equity financing, which are weighted by the percentage of debt and percentage of equity in a company’s target capital structure. Included in the estimate of the weight-average cost of capital is the assumption of an unsystematic risk premium to address the incremental uncertainty related to the reporting units’ future cash flow projections. An increase in the unsystematic risk premium increases the discount rate.
| 52 |
Valuation of Equity Method Investments
We evaluate our investments for other-than-temporary impairments when circumstances indicate those assets may be impaired. When the decline in value is deemed to be other than temporary, an impairment is recognized to the extent that the fair value is less than the carrying value of the investment. We consider various factors in determining whether a loss in value of investment is other than temporary including: the length of time and the extent to which the fair value has been below the cost, the financial condition of the investees, and our intent and ability to retain the investment for a period of time sufficient to allow for recovery of value. Management makes certain judgments and estimates in its assessment including but not limited to: identifying if circumstances indicate a decline in value is other than temporary, expectations about the business operations of investees, as well as industry, financial, and market factors. Any significant changes in assumptions or judgments in assessing impairments could result in an impairment charge.
Income Taxes
Our income tax expenses, and deferred tax assets and liabilities reflect management’s best assessment of estimated current and future taxes to be paid. We are subject to income taxes in the U.S. Significant judgments and estimates are required in determining the consolidated income tax provision and in evaluating income tax uncertainties. We review our tax positions at the end of each quarter and adjust the balances as new information becomes available.
Deferred income taxes arise from temporary differences between the tax and financial statement recognition of revenue and expense. In evaluating our ability to recover our deferred tax assets, we consider all available positive and negative evidence including our past operating results, the existence of cumulative net operating losses in the most recent years, and our forecast of future taxable income. In estimating the future taxable income, we develop assumptions including the amount of future federal operating income, the reversal of temporary differences, and the implementation of feasible and prudent tax planning strategies. These assumptions require significant judgment about the forecasts of future taxable income and are consistent with the plans and estimate we use to manage the underlying businesses.
Changes in tax laws and rates could also affect recorded deferred tax assets and liabilities in the future. Should tax laws change, our tax expense and cash flows could be materially impacted.
Loss Contingencies
We may be subject to various claims, including claims with customers and vendors, pending and potential legal actions for damages, investigations relating to laws and regulations and other matters arising out of the normal conduct of our business. When a loss is considered probably and reasonably estimable, we record a liability in the amount of our best estimate for the ultimate loss. However, the likelihood of a loss with respect to a particular contingency is often difficult to predict and determining a meaningful estimate of the loss or a range of loss may not be practicable based on the information available and the potential effect of future events and decisions by third party that will determine the ultimate resolution of the contingency. Moreover, it is not uncommon for such matters to be resolved over many years, during which time relevant developments and new information must be reevaluated at least quarterly to determine both the likelihood of potential loss and whether it is possible to reasonably estimate a range of possible loss. When a material loss is reasonably possible or probably, but a reasonable estimate cannot be made, disclosure of the proceeding is provided. Legal fees are recognized as incurred when the legal services are provided.
We review all contingencies at least quarterly to determine whether the likelihood of loss has changed and to assess whether a reasonable estimate of the potential loss or range of the loss can be made. As discussed above, development of a meaningful estimate of loss or a range of potential loss is complex when the outcome is directly dependent on future negotiations with our decision by third parties, such as regulatory agencies, the court system and other interest parties.
Stock-Based Compensation
We account for stock-based compensation to employees in accordance with ASC 718, “Compensation-Stock Compensation”. ASC 718 requires companies to measure the cost of employee services received in exchange for an award of equity instruments, including stock options, based on the grant date fair value of the award and to recognize it as compensation expense over the period the employee is required to provide service in exchange for the award, usually the vesting period. Stock option forfeitures are recognized at the date of employee termination. Effective January 1, 2019, the Company adopted ASU 2018-07 for the accounting of share-based payments granted to non-employees for goods and services.
Recently Issued Accounting Standards
For more information on recently issued accounting standards, see “Note 2 - Summary of Significant Accounting Policies”, to the Notes to Consolidated Financial Statements included herein under “Item 8. Financial Statements and Supplemental Data”.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Pursuant to Item 305(e) of Regulation S-K (§ 229.305(e)), the Company is not required to provide the information required by this Item as it is a “smaller reporting company,” as defined by Rule 229.10(f)(1).
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTAL DATA |
TABLE OF CONTENTS TO FINANCIAL STATEMENTS
Consolidated Financial Statements
Table of Contents
| Report of Independent Registered Public Accounting Firm (Firm ID: 6866) | |
| Report of the Independent Registered Public Accounting Firm (MaloneBailey, LLP, Houston, Texas, Firm ID: 00206) | 57 |
| Consolidated Balance Sheets | |
| Consolidated Statements of Operations | |
| Consolidated Statements of Changes in Stockholders’ Equity | |
| Consolidated Statements of Cash Flows | |
| Notes to Consolidated Financial Statements |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors ofand
Stockholders of TRxADE HEALTH INC.Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheetssheet of TRxADE HEALTH, INC. and its subsidiaries (collectively, the “Company”)Health, Inc. (the Company) as of December 31, 2021, and 2020,2023, and the related consolidated statements of operations, changes in stockholders’ equity, and cash flows for the yearsyear then ended, and the related notes (collectively referred to as the “financial statements”)financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021, and 2020,2023, and the results of theirits operations and theirits cash flows for the yearsyear ended then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits.audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”)(PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our auditsaudit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits,audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our auditsaudit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our auditsaudit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provideaudit provides a reasonable basis for our opinion.
Emphasis of a matter – Going concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Fair value of acquired intangible assets
Description of the matter
As discussed in Note 1 and Note 3 to the consolidated financial statements, on July 31, 2023, the Company acquired Superlatus, Inc. in a transaction accounted for as a business combination. As a result of the transaction, the Company recognized acquired technology associated with the generation of future income. The acquisition-date fair value of the acquired technology was $9.8 million.
| 55 |
We identified the evaluation of the acquisition-date fair value of the acquired technology as a critical audit matter. A high degree of subjective auditor judgment was required to evaluate the key assumptions within the discounted cash flows model used to estimate the acquisition-date fair value of the acquired technology, specifically the revenue growth rate, margin, and discount rate. There was limited observable market information related to these assumptions and the estimated acquisition-date fair value of the acquired technology was sensitive to minor changes in such assumptions.
How We Addressed the Matter in our Audit
The following are the primary procedures we performed to address this critical audit matter.
| ● | We evaluated the Company’s revenue growth rate and margin assumptions by comparing them to the pre-acquisition budget and the Company’s historical financial results. | |
| ● | We evaluated the discount rate used by comparing it to a discount rate that was developed using publicly available market data for comparable entities. | |
| ● | We compared the revenue growth rate, margin, to those of comparable entities | |
| ● | We validated the mathematical accuracy of the management’s calculations. |
Impairment of Goodwill
Description of the Matter
As reflected in the Company’s consolidated financial statements at December 31, 2023, the Company impaired all goodwill as of December 31, 2023. As disclosed in Notes 1 to the consolidated financial statements, goodwill is tested for impairment at least annually or more frequently if indicators of impairment require the performance of an interim impairment assessment. As a result of these assessments, management concluded that there was an impairment to goodwill for the year ended December 31, 2023, in the amount of $5.1 million.
Auditing management’s impairment tests of goodwill is complex and highly judgmental due to the significant measurement uncertainty in determining the fair values of the reporting units. In particular, the fair value estimates of the reporting units were sensitive to changes in significant assumptions such as discount rates, revenue growth rates, operating margins, estimated spending on capital expenditures, terminal growth rates, and comparable company specific information. These assumptions are affected by current and expected future market or economic conditions.
How We Addressed the Matter in our Audit
Our audit procedures related to the selection of the discount rates used and forecasts of future net sales, operating margins, operating expenses, and other market and economic data of the reporting units, involved:
| ● | Obtaining an understanding of the Company’s process and related controls to evaluate goodwill for impairment. | |
| ● | Evaluating the reasonableness of managements forecasts of future net sales, operating margins, and operating expenses by comparing the forecasts to historical results, marketing plans relevant economic factors, and other comparable company and industry information. |
/s/ CM3 Advisory
We have served as the Company’s auditor since 2023
San Diego, California
April 22, 2024
| 56 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
TRxADE HEALTH, INC.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of TRxADE HEALTH, INC. (the “Company”) as of December 31, 2022, and the related statements of operations, stockholders’ equity, and cash flows for the year then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Matter
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ MaloneBailey, LLP
www.malonebailey.com
We have served as the Company’s auditor since 2013.from 2013 to 2023.
Houston, Texas
March 28, 202227, 2023
(PCAOB ID: 00206)
TRxADE HEALTH, INC.
Consolidated Balance Sheets
December 31, 20212023 and 20202022
| December 31, 2021 | December 31, 2020 | |||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash | $ | 3,122,578 | $ | 5,919,578 | ||||
| Accounts Receivable, net | 978,973 | 805,043 | ||||||
| Inventory | 56,279 | 1,257,754 | ||||||
| Prepaid Assets | 216,414 | 151,248 | ||||||
| Other Receivables | - | 1,087,675 | ||||||
| Total Current Assets | 4,374,244 | 9,221,298 | ||||||
| Property Plant and Equipment, Net | 98,751 | 162,397 | ||||||
| Other Assets | ||||||||
| Deposits | 60,136 | 21,636 | ||||||
| Right of use leased assets | 1,233,033 | 387,371 | ||||||
| Total Assets | $ | 5,766,164 | $ | 9,792,702 | ||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current Liabilities | ||||||||
| Accounts Payable | $ | 477,028 | $ | 256,829 | ||||
| Accrued Liabilities | 270,437 | 219,256 | ||||||
| Current Portion - Operating Lease Liabilities | 178,561 | 131,153 | ||||||
| Customer Deposits | - | 10,000 | ||||||
| Notes Payable – Related Party | 0 | 225,000 | ||||||
| Total Current Liabilities | 926,026 | 842,238 | ||||||
| Long Term Liabilities | ||||||||
| Operating Lease Liabilities, net of current portion | 1,069,965 | 271,306 | ||||||
| Total Liabilities | 1,995,991 | 1,113,544 | ||||||
| Stockholders’ Equity | ||||||||
| Series A Preferred Stock, $ par value; shares authorized; issued and outstanding as of December 31, 2021, and December 31, 2020, respectively | - | - | ||||||
| Common Stock, $ par value; shares authorized; and shares issued and outstanding as of December 31, 2021 and 2020, respectively | 82 | 81 | ||||||
| Additional Paid-in Capital | 20,017,528 | 19,610,631 | ||||||
| Retained Deficit | (16,247,437 | ) | (10,931,554 | ) | ||||
| Total Stockholders’ Equity | 3,770,173 | 8,679,158 | ||||||
| Total Liabilities and Stockholders’ Equity | $ | 5,766,164 | $ | 9,792,702 | ||||
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash | $ | 151,908 | $ | 1,094,894 | ||||
| Accounts receivable, net | 821,804 | 629,921 | ||||||
| Inventory | 968 | 65,523 | ||||||
| Prepaid assets | 107,774 | 104,461 | ||||||
| Notes receivable | 1,300,000 | - | ||||||
| Other receivables | 370,608 | - | ||||||
| Current assets of discontinued operations | - | 198,324 | ||||||
| Total Current Assets | 2,753,062 | 2,093,123 | ||||||
| Property, plant and equipment, net | 277,009 | 65,214 | ||||||
| Intangible assets and capitalized software, net | 8,962,688 | - | ||||||
| Security deposits | 10,531 | 49,029 | ||||||
| Operating lease right-of-use assets | 529,623 | 1,051,815 | ||||||
| Noncurrent assets of discontinued operations | - | 450,845 | ||||||
| Total Assets | $ | 12,532,913 | $ | 3,710,026 | ||||
| Liabilities and Shareholders’ Equity | ||||||||
| Current Liabilities | ||||||||
| Accounts payable | 2,082,054 | 527,984 | ||||||
| Accrued liabilities | 400,987 | 271,230 | ||||||
| Other current liabilities | 70,310 | 67,517 | ||||||
| Contingent funding liabilities | 1,246,346 | 108,036 | ||||||
| Lease liabilities – current portion | 139,705 | 196,872 | ||||||
| Notes payable – current portion | 6,530,000 | 166,667 | ||||||
| Warrant liability | 736,953 | 588,533 | ||||||
| Purchase price payable | 350,000 | - | ||||||
| Current liabilities of discontinued operations | - | 219,952 | ||||||
| Total Current liabilities | 11,556,355 | 2,146,791 | ||||||
| Long Term Liabilities | ||||||||
| Lease liabilities – net of current portion | 409,205 | 887,035 | ||||||
| Notes payable | 25,000 | 333,333 | ||||||
| Total Liabilities | 11,990,560 | 3,367,159 | ||||||
| Stockholders’ Equity | ||||||||
| Series A preferred stock, $ par value; shares authorized; issued and outstanding as of December 31, 2023 and December 31, 2022 | - | - | ||||||
| Series B preferred stock, $ par value; shares authorized; outstanding as of December 31, 2023, and as December 31, 2022 | - | - | ||||||
| Series C preferred stock, $ par value; shares authorized; issued and outstanding as of December 31, 2023, and as of December 31, 2022 | - | - | ||||||
| Preferred stock value | ||||||||
| Common stock, $ par value; shares authorized; , and shares issued and outstanding as of December 31, 2023 and December 31, 2022, respectively | 9 | 6 | ||||||
| Additional paid-in capital | 33,788,284 | 20,482,666 | ||||||
| Retained deficit | (33,245,940 | ) | (19,719,536 | ) | ||||
| Total TRxADE Health, Inc stockholders’ equity | 542,353 | 763,136 | ||||||
| Non-controlling interest in subsidiary | - | (420,269 | ) | |||||
| Total stockholders’ equity | 542,353 | 342,867 | ||||||
| Total Liabilities and Stockholders’ Equity | $ | 12,532,913 | $ | 3,710,026 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
TRxADE HEALTH, INC.
Consolidated Statements of Operations
Years Ended December 31, 20212023 and 20202022
| 2021 | 2020 | |||||||
| Revenues, net | $ | 9,889,433 | $ | 17,122,520 | ||||
| Cost of Sales | 5,143,468 | 11,415,198 | ||||||
| Gross Profit | 4,745,965 | 5,707,322 | ||||||
| Operating Expenses | ||||||||
| Loss on Inventory Investment | 1,226,426 | - | ||||||
| Loss on Impairment of Goodwill | 0 | 725,973 | ||||||
| General and Administrative | 8,811,832 | 7,488,011 | ||||||
| Total Operating Expenses | 10,038,258 | 8,213,984 | ||||||
| Operating Loss | (5,292,293 | ) | (2,506,662 | ) | ||||
| Interest Expense | (23,590 | ) | (29,389 | ) | ||||
| Net Loss | $ | (5,315,883 | ) | $ | (2,536,051 | ) | ||
| Net Loss per Common Share – Basic and Diluted | $ | (0.65 | ) | $ | (0.33 | ) | ||
| Weighted average Common Shares Outstanding – Basic and Diluted | 8,136,740 | 7,705,620 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
TRxADE HEALTH, INC.
Consolidated Statements of Changes in Stockholders’ Equity
Years Ended December 31, 2021 and 2020
| Shares | Amount | Shares | Amount | Capital | Deficit | Equity | ||||||||||||||||||||||
| Preferred Stock | Common Stock | Additional Paid-in- | Accumulated | Total Stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Equity | ||||||||||||||||||||||
| Balance at December 31, 2019 | - | $ | - | 6,539,415 | $ | 65 | $ | 12,535,655 | $ | (8,395,503 | ) | $ | 4,140,217 | |||||||||||||||
| Common Stock Issued from Offering | - | - | 922,219 | 10 | 5,994,414 | - | 5,994,424 | |||||||||||||||||||||
| Fractional Common Stock Issued due to reverse split | - | - | 40 | - | - | - | - | |||||||||||||||||||||
| Stock Issuance Costs | - | - | - | - | (820,587 | ) | - | (820,587 | ) | |||||||||||||||||||
| Common Stock Issued for Services | - | - | 217,965 | 2 | 1,357,757 | - | 1,357,759 | |||||||||||||||||||||
| Options Exercised for Cash | - | - | 167 | - | 501 | - | 501 | |||||||||||||||||||||
| Warrants Exercised for Cash | - | - | 413,393 | 4 | 37,602 | - | 37,606 | |||||||||||||||||||||
| Warrants Expense | - | - | - | - | 56,885 | - | 56,885 | |||||||||||||||||||||
| Options Expense | - | - | - | - | 448,404 | - | 448,404 | |||||||||||||||||||||
| Net Loss | - | - | - | - | - | (2,536,051 | ) | (2,536,051 | ) | |||||||||||||||||||
| Balance at December 31, 2020 | - | $ | - | 8,093,199 | $ | 81 | $ | 19,610,631 | $ | (10,931,554 | ) | $ | 8,679,158 | |||||||||||||||
| Common Stock Issued for Services | - | - | 37,905 | - | 181,163 | - | 181,163 | |||||||||||||||||||||
| Options Exercised for Cash | - | - | 30,353 | - | 1,821 | - | 1,821 | |||||||||||||||||||||
| Warrants Exercised for Cash | - | - | 5,000 | 1 | 15,000 | - | 15,001 | |||||||||||||||||||||
| Warrants Expense | - | - | - | - | 21,640 | - | 21,640 | |||||||||||||||||||||
| Options Expense | - | - | - | - | 187,273 | - | 187,273 | |||||||||||||||||||||
| Net Loss | - | - | - | - | - | (5,315,883 | ) | (5,315,883 | ) | |||||||||||||||||||
| Balance at December 31, 2021 | - | - | 8,166,457 | 82 | 20,017,528 | (16,247,437 | ) | 3,770,173 | ||||||||||||||||||||
| 2023 | 2022 | |||||||
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Revenues | $ | 8,272,214 | $ | 10,250,168 | ||||
| Cost of sales | 5,673,957 | 4,730,897 | ||||||
| Gross Profit | 2,598,257 | 5,519,271 | ||||||
| Operating Expenses: | ||||||||
| Loss on inventory investment | - | 875,250 | ||||||
| Wage and salary expense | 2,698,178 | 3,581,089 | ||||||
| Professional fees | 1,466,567 | 466,735 | ||||||
| Accounting and legal expense | 1,534,377 | 829,751 | ||||||
| Technology expense | 1,376,908 | 993,185 | ||||||
| General and administrative | 2,785,633 | 1,689,230 | ||||||
| Total operating expenses | 9,861,663 | 8,435,240 | ||||||
| Operating Loss | (7,263,406 | ) | (2,915,969 | ) | ||||
| Nonoperating Income (Expense) | ||||||||
| Change in fair value of warrant liability | (148,420 | ) | 825,544 | |||||
| Interest income | 4,198 | 20,989 | ||||||
| Goodwill impairment | (5,129,115 | ) | - | |||||
| Gain on disposal of asset | - | 2,200 | ||||||
| Other income | 14,543 | - | ||||||
| Interest expense | (1,198,346 | ) | (336,206 | ) | ||||
| Total nonoperating income (expense) | (6,457,140 | ) | 512,527 | |||||
| Net loss from continuing operations | (13,720,546 | ) | (2,403,442 | ) | ||||
| Net loss on discontinued operations | (4,123,028 | ) | (1,506,426 | ) | ||||
| Net Loss | (17,843,574 | ) | (3,909,868 | ) | ||||
| Net loss attributable to TRxADE Health, Inc. | (17,843,574 | ) | (3,472,099 | ) | ||||
| Net loss attributable to non-controlling interests | - | (437,769 | ) | |||||
| Net loss per common share from continuing operations | ||||||||
| Basic | $ | (17.96 | ) | $ | (3.48 | ) | ||
| Diluted | $ | (5.76 | ) | $ | (3.47 | ) | ||
| Net loss per common share from discontinued operations | ||||||||
| Basic | $ | (5.40 | ) | $ | (2.67 | ) | ||
| Diluted | $ | (1.73 | ) | $ | (2.66 | ) | ||
| Net loss attributable to common stockholders | ||||||||
| Basic | $ | (23.35 | ) | $ | (6.15 | ) | ||
| Diluted | $ | (7.49 | ) | $ | (6.13 | ) | ||
| Weighted average common shares outstanding | ||||||||
| Basic | 764,058 | 564,862 | ||||||
| Diluted | 2,381,443 | 566,609 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
TRxADE HEALTH, INC.
Consolidated Statements of Cash FlowsChanges in Stockholders’ Equity
Years endedEnded December 31, 20212023 and 20202022
| 2021 | 2020 | |||||||
| Operating Activities: | ||||||||
| Net loss | $ | (5,315,883 | ) | $ | (2,536,051 | ) | ||
| Adjustments to reconcile net income (loss) to net cash provided by operating activities: | ||||||||
| Depreciation Expense | 7,351 | 5,500 | ||||||
| Options Expense | 187,273 | 448,404 | ||||||
| Warrant Expense | 21,640 | 56,885 | ||||||
| Common Stock Issued for Services | 181,163 | 1,357,759 | ||||||
| Bad Debt Expense | 615,657 | 10,539 | ||||||
| Loss on Inventory Investment | 143,891 | - | ||||||
| Loss on Impairment of Goodwill | 0 | 725,973 | ||||||
| Loss on write-down of Inventory | 376,348 | 1,218,020 | ||||||
| Amortization of Right-of-Use Asset | 131,558 | 97,020 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts Receivable | (789,587 | ) | (23,532 | ) | ||||
| Prepaid Assets and Other Current Assets | (103,666 | ) | (68,796 | ) | ||||
| Inventory | 825,127 | (2,419,013 | ) | |||||
| Deposits for Inventory Purchases | - | (1,087,675 | ) | |||||
| Other Receivables | 1,087,675 | - | ||||||
| Lease Liability | (131,153 | ) | (97,033 | ) | ||||
| Accounts Payable | 220,199 | (33,190 | ) | |||||
| Accrued Liabilities and Other Liabilities | (13,819 | ) | 120,404 | |||||
| Customer Deposits | (10,000 | ) | 10,000 | |||||
| Net cash used in operating activities | (2,566,226 | ) | (2,214,786 | ) | ||||
| Investing Activities: | ||||||||
| Purchase of Fixed Assets | (22,596 | ) | (37,505 | ) | ||||
| Net cash used in Investing Activities | (22,596 | ) | (37,505 | ) | ||||
| Financing Activities: | ||||||||
| Repayments of Short-Term Promissory Notes – Related Parties | (225,000 | ) | - | |||||
| Payment of Stock Issuance Costs | - | (732,356 | ) | |||||
| Proceeds from Exercise of Warrants | 15,001 | 37,606 | ||||||
| Proceeds from Exercise of Stock Options | 1,821 | 501 | ||||||
| Proceeds from Issuance of Common Stock | - | 5,994,424 | ||||||
| Net Cash provided by (used in) financing activities | (208,178 | ) | 5,300,175 | |||||
| Net increase (decrease) in Cash | (2,797,000 | ) | 3,047,884 | |||||
| Cash at Beginning of the Year | 5,919,578 | 2,871,694 | ||||||
| Cash at End of the Year | $ | 3,122,578 | $ | 5,919,578 | ||||
| Supplemental Cash Flow Information | ||||||||
| Cash Paid for Interest | $ | 28,337 | $ | 29,442 | ||||
| Cash Paid for Income Taxes | $ | - | $ | - | ||||
| Non-Cash Transactions | ||||||||
| Remeasurement of ROU Assets and Lease Liability for Nonrenewal of Lease | $ | - | $ | 273,319 | ||||
Series B Preferred Stock | Series C Preferred Stock | Common Stock | Additional | Non-Controlling | Total | |||||||||||||||||||||||||||||||||||
| Shares | $ Amount | Shares | $ Amount | Shares | $ Amount | Paid-in Capital | Accumulated Deficit | Interest in Subsidiary | Stockholders’ Equity | |||||||||||||||||||||||||||||||
| Balance at December 31, 2021 | - | $ | - | - | $ | - | 544,430 | $ | 5 | $ | 20,017,605 | $ | (16,247,437 | ) | $ | - | $ | 3,770,173 | ||||||||||||||||||||||
| Capital Contributions | - | - | - | - | - | - | - | - | 792,500 | 792,500 | ||||||||||||||||||||||||||||||
| Capital Distribution | - | - | - | - | - | - | - | - | (775,000 | ) | (775,000 | ) | ||||||||||||||||||||||||||||
| Common stock issued for services | - | - | - | - | 19,511 | - | 254,106 | - | - | 254,106 | ||||||||||||||||||||||||||||||
| Common stock issued for placement, net issuance costs | - | - | - | 61,334 | 1 | 130,917 | - | - | 130,918 | |||||||||||||||||||||||||||||||
| Warrants exercised for cash | - | - | - | - | 972 | - | 875 | - | - | 875 | ||||||||||||||||||||||||||||||
| Options expense | - | - | - | - | - | - | 79,163 | - | - | 79,163 | ||||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (3,472,099 | ) | (437,769 | ) | (3,909,868 | ) | |||||||||||||||||||||||||||
| Balance at December 31, 2022 | - | $ | - | - | $ | - | 626,247 | $ | 6 | $ | 20,482,666 | $ | (19,719,536 | ) | $ | (420,269 | ) | $ | 342,867 | |||||||||||||||||||||
| Balance | - | $ | - | 626,247 | $ | 6 | $ | 20,482,666 | $ | (19,719,536 | ) | $ | (420,269 | ) | $ | 342,867 | ||||||||||||||||||||||||
| Common stock issued for services | - | - | - | - | 38,480 | - | 257,772 | - | - | 257,772 | ||||||||||||||||||||||||||||||
| Warrants exercised for cash | - | - | - | - | 41,911 | 1 | 1,621 | - | - | 1,622 | ||||||||||||||||||||||||||||||
| Options expense | - | - | - | - | - | - | 29,738 | - | - | 29,738 | ||||||||||||||||||||||||||||||
| Reverse split rounding adjustment | - | - | - | - | 21,929 | - | - | - | - | - | ||||||||||||||||||||||||||||||
| Disposition of assets | - | - | - | - | - | - | - | 4,317,170 | 420,269 | 4,737,439 | ||||||||||||||||||||||||||||||
| Shares issued pursuant to merger agreement | 15,759 | - | - | - | 136,441 | 1 | 12,500,088 | - | - | 12,500,089 | ||||||||||||||||||||||||||||||
| Shares issued pursuant to securities purchase agreement | - | - | 290 | - | 40,000 | 1 | 516,399 | - | - | 516,400 | ||||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | (17,843,574 | ) | - | (17,843,574 | ) | ||||||||||||||||||||||||||||
| Balance at December 31, 2023 | 15,759 | $ | - | 290 | $ | - | 905,008 | $ | 9 | $ | 33,788,284 | $ | (33,245,940 | ) | $ | - | $ | 542,353 | ||||||||||||||||||||||
| Balance | 15,759 | $ | - | 290 | - | 905,008 | $ | 9 | $ | 33,788,284 | $ | (33,245,940 | ) | $ | - | $ | 542,353 | |||||||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
TRxADE HEALTH, INC.
Consolidated Statements of Cash Flows
Years ended December 31, 2023 and 2022
| 2023 | 2022 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (13,720,546 | ) | $ | (2,403,442 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation expense | 19,375 | 13,486 | ||||||
| Options expense | 29,738 | 79,163 | ||||||
| Common stock issued for services | 257,772 | 254,106 | ||||||
| Bad debt expense | - | (246,683 | ) | |||||
| Loss on write-off of intangible asset | - | 792,500 | ||||||
| Loss on inventory investment | - | 875,250 | ||||||
| Goodwill impairment | 5,129,115 | - | ||||||
| Loss on inventory investments | - | - | ||||||
| Gain on sale of asset | - | (2,200 | ) | |||||
| Amortization of right-of-use assets | 215,665 | 181,218 | ||||||
| Amortization of intangible assets | 814,790 | - | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable, net | (293,784 | ) | 369,932 | |||||
| Prepaid assets and deposits | 38,367 | 335,066 | ||||||
| Inventory | 4,232,947 | (51,737 | ) | |||||
| Other receivables | (254,924 | ) | (875,250 | ) | ||||
| Right-of-use assets | 306,527 | - | ||||||
| Lease liability | (534,997 | ) | (164,618 | ) | ||||
| Accounts payable | 1,607,625 | 199,833 | ||||||
| Accrued liabilities | 58,692 | (211,694 | ) | |||||
| Purchase price payable | 350,000 | - | ||||||
| Current liabilities | 2,794 | 67,517 | ||||||
| Warrant liability | 148,420 | 588,533 | ||||||
| Net cash used in operating activities from continuing operations | (1,592,424 | ) | (199,020 | ) | ||||
| Net cash used in operating activities from discontinued operations | (481,177 | ) | (1,365,648 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Funds acquired through acquisitions | (344,454 | ) | - | |||||
| Proceeds from sale of fixed assets | - | 749 | ||||||
| Investment in capitalized software | - | - | ||||||
| Net cash (used in) investing activities from continuing operations | (344,454 | ) | 749 | |||||
| Net cash provided by investing activities from discontinued operations | 68,737 | (428,594 | ) | |||||
| Cash flows from financing activities: | ||||||||
| Proceeds from the issuance of debt | 400,000 | - | ||||||
| Repayment of debt | (150,000 | ) | - | |||||
| Repayment of contingent liability | (1,043,107 | ) | (716,964 | ) | ||||
| Proceeds from sale of future revenue | 2,181,417 | 825,000 | ||||||
| Proceeds from exercise of stock options | - | - | ||||||
| Proceeds from exercise of warrants | 1,622 | 875 | ||||||
| Proceeds from securities purchase agreement | 516,400 | - | ||||||
| Proceeds from issuance of common stock, net of issuance costs | - | 130,918 | ||||||
| Net cash provided by (used in) financing activities from continuing operations | 1,906,332 | 239,829 | ||||||
| Net cash (used in) financing activities from discontinued operations | (500,000 | ) | (275,000 | ) | ||||
| Net decrease in cash | (942,986 | ) | (2,027,684 | ) | ||||
| Cash at beginning of the year | 1,094,894 | 3,122,578 | ||||||
| Cash at end of the period | $ | 151,908 | $ | 1,094,894 | ||||
| Supplemental disclosure of cash flow information | ||||||||
| Cash paid for interest, net | $ | 733,694 | $ | 336,206 | ||||
| Cash paid for income taxes | $ | - | $ | - | ||||
| Non-Cash Transactions | ||||||||
| Insurance premium financed | $ | 306,152 | $ | 220,354 | ||||
| Note issued as SOSRx contribution | - | $ | 500,000 | |||||
| Not cancelled from SORx agreement termination | $ | 500,000 | - | |||||
| Intangible asset contribution from non-controlling interest | $ | - | $ | 792,500 | ||||
| Disposition of assets, related party | $ | 492,030 | - | |||||
| Issuance of note receivable | $ | 1,300,000 | $ | - | ||||
The accompanying notes are an integral part of the consolidated financial statements.
| 61 |
TRxADE HEALTH, INC.
Notes to Consolidated Financial Statements
For the years ended December 31, 20212023 and 20202022
NOTE 1 – ORGANIZATION AND BASIS OF PRESENTATION
Overview
TRxADE HEALTH, INC. (“we”, “our”, “Trxade”, and the “Company”) owns as of December 31, 2023, 100% of Trxade, Inc., and Integra Pharma Solutions, LLC, Community Specialty Pharmacy, LLC, Alliance Pharma Solutions, LLC, Bonum Health, LLC, and MedCheks, LLC (from January 2021 to December 2021, when it was dissolved). The merger of Trxade,Superlatus, Inc. and TRxADE HEALTH, INC. occurredits wholly-owned subsidiaries, Sapientia Technologies, LLC (“Sapientia”), Superlatus Food Service Holding Company, Superlatus PD Holding Company, and The Urgent Company, Inc. On July 31, 2023, the Company completed a merger transaction that resulted in May 2013. Community Specialty Pharmacy waswith Superlatus, Inc. becoming a wholly owned subsidiary of the Company (see “Merger”, below). On September 27, 2023, the Company acquired in October 2018.The Urgent Company, Inc. and its related subsidiaries (see Note 3).
During the year ended December 31, 2023, Trxade, Inc. operates, operated a web-based market platform that enables commerce among healthcare buyers and sellers of pharmaceuticals, accessories and services.
Integra Pharma Solutions, LLC (“IPS”, d.b.a. Trxade Prime), is a licensed pharmaceutical wholesaler and sells brand, generic and non-drug products.products to customers. IPS customers include all healthcare markets including government organizations, hospitals, clinics and independent pharmacies nationwide.
Community Specialty Pharmacy, LLC, (“CSP”) is an accredited independent retail pharmacy with a focus on specialty medications and a community-based model offering home delivery services to patients.
Alliance Pharma Solutions, LLC (d.b.a.(“APS”, d.b.a. DelivMeds) is currently being rebranded and the consumer-based app is still being developed. To date, the Company has developednot generated any revenue from this product.
On January 20, 2023, the Company entered into Membership Interest Purchase Agreements to sell 100% of the outstanding membership interests of the Company’s subsidiaries, CSP and APS. The Company will receive consideration in the amount of $125,000 for APS and $100,000 for CSP. The Company also agreed to enter into a same day Pharma delivery software – Delivmeds.comMaster Service Agreement to operate the businesses prior to closing. Additional amounts owed to the Company as a result of this Master Service Agreement totaled $1,075,000 as of the closing date of August 22, 2023 (see Note 3 and invested in SyncHealth MSO, LLC a managed services organization in January 2019, which investment was divested in February 2020.Note 7).
Bonum Health, LLC (“Bonum Health”), was formed to hold certain telehealth assets acquired in October 2019. The “Bonum“Bonum Health Hub”Hub” was launched in November 2019 and was expected to be operational in AprilFebruary 2020; however, due to the COVID-19 pandemic, the Company does not anticipate installations moving forward, and has taken a write off of the hubs purchased at June 30, 2021, in Loss on Inventory Investments of $143,891 for the year ended December 31, 2021.forward. The Bonum Health mobile application is available on a subscription basis, primarily as a stand-alone telehealth software application that can be licensed on a business-to-business (B2B) model to clients as an employment health benefit for the clients’ employees.
MedCheks,SOSRx, LLC (“SOSRx”) was formed on February 15, 2022. The Company entered into a relationship with Exchange Health, LLC (“Exchange Health”), a technology company providing an online platform for manufacturers and suppliers to sell and purchase pharmaceuticals. SOSRx, a Delaware limited liability company, was formed, which was owned 51% by the Company and 49% by Exchange Health. SOSRx did not generate material revenue and in January 2021February of 2023, the Company voluntarily withdrew from the joint venture agreement. As part of the voluntary withdrawal the Company has recorded a loss of $352,244 from disposal of assets, which is included in net loss on discontinued operations in the audited consolidated statement of operations in the amount of for the year ended December 31, 2023.
Merger
On July 14, 2023, the Company entered into an Amended and Restated Agreement and Plan of Merger (the “Merger Agreement”) with Superlatus, Inc., a U.S.-based holding company of food products and distribution capabilities (“Superlatus”) and Foods Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub”).
Superlatus is a patient-centered, digital, precision healthcare platform that lets patients consolidatediversified food technology company with distribution capabilities and control theirsystems to optimize food security and population health data via innovative Consumer Packaged Goods (“CPG”) products, agritech, foodtech, plant-based proteins and alt-protein and includes wholly-owned subsidiary, Sapientia, Inc. (“Sapientia”), a digital Health Passport. This product has been discontinued and MedCheks, LLC was subsequently dissolved in December 2021.food tech business.
On October 9, 2019,July 31, 2023 (the “Closing Date”), the Company’s BoardCompany completed its acquisition of Directors,Superlatus in accordance with the terms and on October 15, 2019, stockholders holding a majorityconditions of the Company’s outstanding voting shares, approved resolutions authorizingMerger Agreement (the “Merger”), pursuant to which the Company acquired Superlatus by way of a reverse stock splitmerger of the Merger Sub with and into Superlatus, with Superlatus being a wholly owned subsidiary of the Company and the surviving entity in the Merger.
Under the terms of the Merger Agreement, at the closing of the Merger (the “Closing”), shareholders of Superlatus received in aggregate shares of common stock of the Company, representing 19.99% of the then total issued and outstanding common stock of the Company after the consummation of the Merger and shares of Company’s Series B Preferred Stock, par value $ per share (the “Series B Preferred Stock”), with a conversion ratio of shares of Series B Preferred Stock to one share of common stock. At Closing, the value of the common stock was $ per share, resulting in a total value of $225,000,169. Upon consummation of the Merger, the Company continued to trade under the current ticker symbol “MEDS.”
| 62 |
As a condition and inducement to Superlatus’ willingness to enter into the Merger Agreement, on June 28, 2023, Suren Ajjarapu and Prashant Patel (the “Principal Stockholders”) entered into an agreement with TRxADE (the “Stock Swap Agreement”), pursuant to which, TRxADE was to transfer all of the shares or membership interest of the operating subsidiaries currently owned by TRxADE to Principal Stockholders, in exchange for Suran Ajjarapu to surrender share of common stock of TRxADE and Prashant Patel to surrender shares of the common stock of TRxADE (the “Stock Swap Transaction”). The closing of the Stock Swap Transaction was to take place simultaneously with the approval of TRxADE stockholders of the conversion of the Series B preferred stock into common stock. As of the date of this filing, TRxADE stockholders have not approved the conversion.
In connection with the Merger, effective one (1) business day immediately prior to the Closing Date (the “MEDS Rights Record Date”), the Company issued to the shareholders of the Company as of the MEDS Rights Record Date, including the independent directors who are entitled to certain amount of common stock of the Company in connection with their 2023 annual compensation and regardless of whether the common stock has been issued or vest before the MEDS Rights Records Date (collectively, the “MEDS Rights Shareholders”) a non-transferrable right to receive one share of common stock of the Company at no cost (the “MEDS Rights”), with seven (7) MEDS Rights issued per share of common stock of the Company held as of the MEDS Rights Record Date, conditioned upon their execution of a Registration Rights Agreement. Such issuances will be made in reliance on the exemption from registration pursuant to Section 3(a)(9) or Section 4(a)(2) of the Securities Act, Regulation D under the Securities Act promulgated thereunder, and corresponding provisions of state securities or “blue sky” laws. The MEDS Rights are not actionable or transferable until registration; provided they become transferable one year after the date of the Merger if no registration has occurred. As of the date of this filing, no MEDS Rights shares have been issued.
Not all of the closing conditions of the Merger Agreement were met. As a result, the Company entered into Amendment No. 1 to the Amended and Restated Agreement and Plan of Merger (the “Amendment”) on January 8, 2024. Under the terms of the Amendment, the merger consideration to the shareholders of Superlatus was adjusted to the aggregate of shares of common stock of the Company, representing 19.99% of the total issued and outstanding common stock of the Company after the consummation of the Merger and shares of Company’s Series B Preferred Stock, par value $ per share (the “Series B Preferred Stock”), with a conversion ratio of shares of Series B Preferred Stock to one share of common stock. At Closing, the value of the common stock was $ per share, resulting in a total value of $12,500,089. Additionally, the shareholders of Superlatus agreed to surrender back to the Company shares of the Company’s common stock in the range from one-for-two (1-for-2) to one-for-ten (1-for-10) and provided authority to the Company’s Board of Directors to select the ratio of the reverse stock split in their discretion (the “Stockholder Authority”). On February 12, 2020, the Board of Directors ofSeries B Preferred Stock. In March 2024 the Company approved a stock split ratiodivested of 1-for-6 (“Reverseits interest in Superlatus and, among other things, the Stock Split”)Swap Transaction in connection with the Stockholder Authority and the Company filed a Certificate of Amendment with the Secretary of Delawarenot expected to affect the Reverse Stock Split.
Proportional adjustments were made to the conversion and exercise prices of the Company’s outstanding warrants and stock options, and to the number of shares issued and issuable under the Company’s stock incentive plans in connection with the Reverse Stock Split. The Reverse Stock Split did not affect any stockholder’s ownership percentage of the Company’s common stock, except to the limited extent that the Reverse Stock Split resulted in any stockholder owning a fractional share. Fractional shares of common stock were rounded up to the nearest whole share based on each holder’s aggregate ownership of the Company. All issued and outstanding shares of common stock, options and warrants to purchase common stock and per share amounts contained in the financial statements, have been retroactively adjusted to reflect the Reverse Stock Split for all periods presented.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) in all material respects and have been consistently applied in preparing the accompanying financial statements.
The summary of significant accounting policies presented below is designed to assist in understanding the Company’s financial statements. Such financial statements and accompanying notes are the representations of the Company’s management, who are responsible for their integrity and objectivity.occur.
LiquidityBasis of Presentation and Principles of Consolidation– Historically, operations have been funded primarily through the sale of equity or debt securities and operating activities. In 2020, the Company raised approximately $5.99 million in capital (See Note 4 – Stockholders’ Equity). The Company has the ability to maintain the current level of spending or reduce expenditures to maintain operations if funding is not available.
Use of Estimates – In preparing these financial statements, management is required to make estimates and assumptions that effect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amount of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
Reclassification– Certain prior year amounts have been reclassified to conform to the current year presentation.
Principle of Consolidation – The Company’s consolidated financial statements include the accounts of TRxADE HEALTH, INC., Trxade, Inc., Integra Pharma Solutions, Inc., Alliance Pharma Solutions, LLC, Community Specialty Pharmacy, LLC, Bonum Health, LLC, Superlatus, Inc., Sapientia Technologies, LLC and MedCheks, LLC.The Urgent Company, Inc. The accompanying consolidated financial statements of TRxADE HEALTH, Inc. have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and the rules of the SEC. All significant intercompany accounts and transactions have been eliminated.
Use of Estimates
Cash
The preparation of condensed consolidated financial statements in accordance with U.S. GAAP requires management to make estimates and Cash Equivalents – Cashassumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses in bank accountsthe reporting period. The Company bases its estimates and assumptions on current facts, historical experience and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are at risk tonot readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from its estimates. To the extent that they exceed U.S. Federal Deposit Insurance Corporation insured amounts. All investments purchased with a maturitythere are material differences between estimates and the actual results, future results of three months or less areoperations will be affected. Significant estimates for the years ended December 31, 2023 and 2022 include the valuation of intangible assets, including goodwill.
| 63 |
Fair value of financial instruments
The carrying amounts for cash, equivalents. Cashaccounts receivable, accounts payable, accrued liabilities, and cash equivalents are available on demand and are generally within FDIC insurance limits for 2021.other current liabilities approximate their fair value because of their short-term maturity.
Stock Split
Effective June 21, 2023, the Company executed a 1:15 reverse stock split for stockholders of record on that date. This was executed to comply with the Nasdaq Listing Rule 5550(a)(2) to have the price of the stock above $.
Recently Issued Accounting Pronouncements
In June 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-13, “Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments” (“ASU 2016-13”). ASU 2016-13 requires companies to measure credit losses utilizing a methodology that reflects expected credit losses and requires a consideration of a broader range of reasonable and supportable information to inform credit loss estimates. ASU 2016-13 is effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. The Company adopted ASU 2016-13 effective January 1, 2023. The Company determined that the update applied to trade receivables, but that there was no material impact to the consolidated financial statements from the adoption of ASU 2016-13.
In August 2020, the FASB issued ASU 2020-06, “Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40)”. This ASU reduces the number of accounting models for convertible debt instruments and convertible preferred stock and amends the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. In addition, this ASU improves and amends the related earnings per share guidance. This standard is effective for us on January 1, 2022, including interim periods within those fiscal years. Adoption is either a modified retrospective method or a fully retrospective method of transition. The adoption of ASU 2020-06 did not have a material impact on the consolidated financial statements.
Accounts Receivable, net –
On January 1, 2023, the Company adopted ASU 2016-13 “Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments” and its related amendments using the prospective method. The new standard requires the use of a current expected credit loss impairment model to develop and recognize credit losses for financial instruments at amortized cost when the asset is first originated or acquired, and each subsequent reporting period.
The Company’s receivables are from customers and are collectibletypically collected within 90 days. The Company determines the allowance based on known troubled accounts, historical experience, and other currently available evidence. During the years ended December 31, 2021, and 2020, $615,657 and $10,539 of bad debt expense, respectively and $0 of recovery of bad debt, was recognized.
Inventory– Inventories are stated at the lower of cost or net realizable value. Cost is determined on a first in first out basis. These are merchandise inventories at Community Specialty Pharmacy, LLC and Integra Pharma Solutions, LLC. On a quarterly basis, we evaluate inventory for net realizable value using estimates based on historical experience, current or projected pricing trends, specific categories of inventory, age and expiration dates of on-hand inventory and manufacturer return policies. If actual conditions are less favorable than our assumptions, additional inventory write-downs may be required, and no reserve is maintained as obsolete or expired inventories are written off. We believe that the inventory valuation provides a reasonable approximation of the current value of inventory. There is 0 reserve for inventory obsolescence and inventory is not pledged during the periods presented. During the years ended December 31, 2021 and 2020, included in cost of sales were write-downs to reduce inventory to net realizable value of $376,348 and $1,218,020, respectively.
Beneficial Conversion Features – The intrinsic value of a beneficial conversion feature inherent to a convertible note payable, which is not bifurcated and accounted for separately from the convertible note payable and may not be settled in cash upon conversion, is treated as a discount to the convertible note payable. This discount is amortized over the period from the date of issuance to the date the note is due using the effective interest method. If the note payable is retired prior to the end of its contractual term, the unamortized discount is expensed in the period of retirement to interest expense. In general, the beneficial conversion feature is measured by comparing the effective conversion price, after considering the relative value of detachable instruments included in the financing transaction, if any, to the fair value of the common shares at the commitment date to be received upon conversion.
Fair Value of Financial Instruments – The Company measures its financial assets and liabilities in accordance with the requirements of Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) 820, “Fair Value Measurements and Disclosures”. ASC 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1 – Quoted prices are available in active markets for identical assets or liabilities as of the reporting date. Active markets are those in which transactions for the asset or liability occur in sufficient frequency and volume to provide pricing information on an ongoing basis. Level 1 primarily consists of financial instruments such as exchange-traded derivatives, marketable securities and listed equities.
Level 2 – Pricing inputs are other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reported date and includes those financial instruments that are valued using models or other valuation methodologies. These models are primarily industry-standard models that consider various assumptions, including quoted forward prices for commodities, time value, volatility factors, and current market and contractual prices for the underlying instruments, as well as other relevant economic measures. Substantially all of these assumptions are observable in the marketplace throughout the full term of the instrument, can be derived from observable data or are supported by observable levels at which transactions are executed in the marketplace. Instruments in this category generally include non-exchange-traded derivatives such as commodity swaps, interest rate swaps, options and collars.
Level 3 – Pricing inputs include significant inputs that are generally less observable from objective sources. These inputs may be used with internally developed methodologies that result in management’s best estimate of fair value.
The Company does not have any assets or liabilities that are required to be measured and recorded at fair value on a recurring basis.
The carrying amounts of cash, accounts receivable, accounts payable, accrued liabilities and short-term debt approximate fair value because of the short-term nature of these instruments. The carrying amount of long-term debt approximates fair value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Goodwill– The Company accounts for goodwill and intangible assets in accordance with ASC 350 “Intangibles Goodwill and Other”. ASC 350 requires that goodwill and other intangibles with indefinite lives be tested for impairment annually or on an interim basis if events or circumstances indicate that the fair value of an asset is more likely than not has decreased below its carrying value. The Company performed impairment analysis using the quantitative analysis under ASC 350-20 and because of declining revenues and operating losses an impairment of goodwill was recognized as of December 31, 2021 and 2020, was $0 and $725,973, respectively.
Revenue Recognition – In May 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update No. 2014-09 (Topic 606) “Revenue from Contracts with Customers.” Topic 606 supersedes the revenue recognition requirements in Accounting Standards Codification Topic 605, “Revenue Recognition”, and requires entities to recognize revenue when they transfer control of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods or services. The Company adopted ASU 2014-09 using the modified retrospective approach effective January 1, 2018, under which prior periods were not retrospectively adjusted. The adoption of Topic 606 did not have a material impact on the Company’s consolidated financial statements, including the presentation of revenues in the Company’s Consolidated Statements of Operations.
Trxade, Inc. provides an online website service, a buying and selling marketplace for licensed Pharmaceutical Wholesalers to sell products and services to licensed Pharmacies. The Company charges Suppliers a transaction fee, a percentage of the purchase price of the Prescription Drugs and other products sold through its website service. The fulfillment of confirmed orders, including delivery and shipment of Prescription Drugs and other products, is the responsibility of the Supplier and not of the Company. The Company holds no inventory and assumes no responsibility for the shipment or delivery of any products or services from the Company’s website. The Company considers itself an agent for this revenue stream and as such, reports revenue as net. Step One: Identify the contract with the customer – Trxade, Inc.’s Terms and Use Agreement is acknowledged between the Wholesaler and Trxade, Inc. which outlines the terms and conditions. The collection is probable based on the credit evaluation of the Wholesaler. Step Two: Identify the performance obligations in the contract – The Company provides to the Supplier access to the online website, uploading of catalogs of products and Dashboard access to review status of inventory posted and processed orders. The Agreement requires the supplier to provide a catalog of pharmaceuticals for posting on the platform, deliver the pharmaceuticals and upon shipment remit the stated platform fee. Step Three: Determine the transaction price – The Fee Agreement outlines the fee based on the type of product, generic, brand or non-drug. There are no discounts for volume of transactions or early payment of invoices. Step Four: Allocate the transaction price – The Fee Agreement outlines the fee. There is no difference between contract price and “stand-alone selling price”. Step Five: Recognize revenue when or as the entity satisfies a performance obligation – Revenue is recognized the day the order has been processed by the Supplier.
Integra Pharma Solutions, LLC is a licensed wholesaler and sells to licensed pharmacies brand, generic and non-drug products. The Company takes orders for product and creates invoices for each order and recognizes revenue at the time the Customer receives the product. Customer returns are not material. Step One: Identify the contract with the customer – The Company requires that an application and a credit card for payment is completed by the Customer prior to the first order. Each transaction is evidenced by an order form sent by the customer and an invoice for the product is sent by the Company. The collection is probable based on the application and credit card information provided prior to the first order. Step Two: Identify the performance obligations in the contract – Each order is distinct and evidenced by the shipping order and invoice. Step Three: Determine the transaction price – The consideration is variable if product is returned. The variability is determined based on the return policy of the product manufacturer. There are no sales or volume discounts. The transaction price is determined at the time of the order evidenced by the invoice. Step Four: Allocate the transaction price – There is no difference between contract price and “stand-alone selling price”. Step Five: Recognize revenue when or as the entity satisfies a performance obligation - The Revenue is recognized when the Customer receives the product.
Community Specialty Pharmacy, LLC is in the retail pharmacy business. The Company fills prescriptions for drugs written by a doctor and recognizes revenue at the time the patient confirms delivery of the prescription. Customer returns are not material. Step One: Identify the contract with the customer – The prescription is written by a doctor for a customer and delivered to the Company. The prescription identifies the performance obligations in the contract. The Company fills the prescription and delivers to the Customer the prescription, fulfilling the contract. The collection is probable because there is confirmation that the customer has insurance for the reimbursement to the Company prior to filling of the prescription. Step Two: Identify the performance obligations in the contract – Each prescription is distinct to the Customer. Step Three: Determine the transaction price – The consideration is not variable. The transaction price is determined to be the price of the prescription at the time of delivery which considers the expected reimbursements from third party payors (e.g., pharmacy benefit managers, insurance companies and government agencies). Step Four: Allocate the transaction price – The price of the prescription invoiced represents the expected amount of reimbursement from third party payors. There is no difference between contract price and “stand-alone selling price”. Step Five: Recognize revenue when or as the entity satisfies a performance obligation – Revenue is recognized upon the delivery of the prescription.
Cost of Goods Sold – The Company recognized cost of goods sold from activities in Integra Pharma Solutions, LLC and Community Specialty Pharmacy, LLC.
Income Taxes – The Company accounts for income taxes utilizing ASC 740, “Income Taxes” (SFAS No. 109). ASC 740 requires the measurement of deferred tax assets for deductible temporary differences and operating loss carry forwards, and of deferred tax liabilities for taxable temporary differences. Measurement of current and deferred tax liabilities and assets is based on provisions of enacted tax law. The effects of future changes in tax rates are not included in the measurement. The Company recognizes the amount of taxes payable or refundable for the current year and recognizes deferred tax liabilities and assets for the expected future tax consequences of events and transactions that have been recognized in the Company’s financial statements or tax returns. The Company currently has substantial net operating loss carry forwards. The Company has recorded a 100% valuation allowance against net deferred tax assets due to uncertainty of their ultimate realization. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Tax years from 2018 forward are open to examination by the Internal Revenue Service.
Equity Investments – If the investments are less than 50% owned and more than 20% owned, the entities use the equity method of accounting in accordance with ASC 323-10 Investments – Equity Method and Joint Ventures.
The share of income (loss) of such entities is recorded as a single amount as share in equity income (loss) of investments. Dividends, if any, are recorded as a reduction of the investment.
The Company had an account receivable with a single customer, GSG PPE, LLC (“GSG”), for the amount of $630,000, which was past due. The Company had obtained a Note Receivable which was due on September 30, 2021 and remained unpaid. The Company did not believe the amount to be collectible without legal actions, and therefore, recorded bad debt expense reflected on the consolidated statement of operations during the year ended December 31, 2021. The note was not paid pursuant to its terms and the Company had filed a suit to collect on the note and the personal guaranty securing the note. The Company settled the lawsuit in June of 2022. During the years ended December 31, 2023, and 2022, there was a bad debt recovery from the GSG lawsuit of $32,074 and $98,841 respectively.
Other Receivables, net
The Company’s other receivables balance is from one vendor. On May 20, 2022, effective as of May 18, 2022, Community Specialty Pharmacy, LLC (“CSP”) entered into an agreement to acquire COVID-19 testing kits from a third-party vendor for an aggregate of $1,200,000, of which $875,000 was paid on May 23, 2022. The Company received the COVID-19 testing kits in July 2022. On August 18, 2022, the Company was informed by the vendor that the vendor had received a letter from the U.S. Food and Drug Administration (“FDA”) that the COVID-19 test kits were misbranded under Section 502(o) of the Federal Food, Drug, and Cosmetic Act (“FDC Act”) (21 USC 352(o)) and adulterated under Section 501(f) of the FDC Act (21 USC 351(f)). Furthermore, the vendor informed the Company that the letter from the FDA also stated that because of the FDA’s prohibition on the distribution of adulterated and/or misbranded devices applies to all parties along the distribution chain, the FDA was advising the vendor against furthering the distribution of the COVID-19 test kits in interstate commerce. The company wrote the amount off as a loss of inventory as of December 31, 2022. As of December 31, 2023, and December 31, 2022, the balance of this receivable was $0 equity.
| 64 |
On August 22, 2023, the Company completed the sale of CSP and APS (see Note 3). The net balance due to the Company from these entities, in excess of the Note Receivable (see Note 6), was $370,608 as of December 31, 2023.
Acquisitions
The Company accounts for acquisitions and investments in businesses as business combinations if the target meets the definition of a business and (a) the target is a variable interest entity (“VIE”) and the Company is the target’s primary beneficiary, and therefore the Company must consolidate its financial statements, or (b) the Company acquires more than 50% of the voting interest of the target and it was not previously consolidated. The Company records business combinations using the acquisition method of accounting, which requires all the assets acquired and liabilities assumed to be recorded at fair value as of the acquisition date. The excess of the purchase price over the estimated fair values of the net tangible and intangible assets acquired is recorded as goodwill.
The application of the acquisition method of accounting for business combinations requires management to make significant estimates and assumptions in the determination of the fair value of assets acquired and liabilities assumed in order to properly allocate purchase price consideration between assets that are depreciated and amortized from goodwill. The fair value assigned to tangible and intangible assets acquired and liabilities assumed are based on management’s estimates and assumptions, as well as other information compiled by management, including valuations that utilize customary valuation procedures and techniques. Significant assumptions and estimates include, but are not limited to, the cash flows that an asset is expected to generate in the future, the appropriate weighted-average cost of capital, and the cost savings expected to be derived from acquiring an asset, if applicable.
If the actual results differ from the estimates and judgments used in these estimates, the amounts recorded in the Company’s financial statements may be exposed to potential impairment of the intangible assets and goodwill.
If the Company’s investment involves the acquisition of an asset or group of assets that does not meet the definition of a business, the transaction is accounted for as an asset acquisition. An asset acquisition is recorded at cost, which includes capitalizing transaction costs, and does not result in the recognition of goodwill.
Intangible Assets and Goodwill
The Company tests indefinite-lived intangible assets for impairment on an annual basis or whenever events or changes occur that would more-likely-than not reduce the fair value of the indefinite-lived intangible asset below its carrying value between annual impairment tests. Any indefinite-lived intangible asset assessment is performed at the Company level.
The Company recognized a goodwill impairment loss of $5,129,115 for the year ended December 31, 2021.2023. The goodwill resulted from the acquisition of Superlatus and was subsequently determined to be impaired based on the facts and circumstances surrounding the sale of Superlatus on March 5, 2024. See Note 20.
Income (loss) Per Common Share –
Basic net income (loss) per common share is computed by dividing net lossincome available to common stockholders by the weighted average number of common shares outstanding. Diluted net lossincome per common share is computed similar to basic net lossincome per common share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. The dilutive effect of the Company’s options and warrants is computed using the treasury stock method. As of December 31, 2021,2023, we had 44,535218,729 outstanding warrants to purchase shares of common stock and options to purchase shares of common stock. As part of the termination of the White Lion deal, White Lion was issued shares of stock per the agreement on March 1, 2023. Armistice Capital executed its pre-funded warrants on January 4, 2023, and purchased 601,740 shares (40,116 shares after the effect of the 1:15 reverse stock split on June 21, 2023, see Note 13) of stock with a purchase price of $6.02.
| 65 |
SCHEDULE OF BASIC AND DILUTIVE INCOME (LOSS) PER COMMON SHARE
| December 31, 2021 | December 31, 2020 | |||||||
| Numerator: | ||||||||
| Net Income (Loss) | $ | (5,315,883 | ) | $ | (2,536,051 | ) | ||
| Numerator for basic and diluted EPS - income (loss) available to common Shareholders | $ | (5,315,883 | ) | $ | (2,536,051 | ) | ||
| Denominator: | ||||||||
| Denominator for basic and diluted EPS – Weighted average shares | 8,136,740 | 7,705,620 | ||||||
| Basic Income (Loss) per common share | $ | (0.65 | ) | $ | (0.33 | ) | ||
| For the Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Numerator: | ||||||||
| Net loss from continuing operations | $ | (13,720,546 | ) | $ | (2,403,442 | ) | ||
| Net loss attributable to noncontrolling interest | - | (437,769 | ) | |||||
| Net loss from continuing operations available to common stockholders | (13,720,546 | ) | (1,965,673 | ) | ||||
| Net loss from discontinued operations | (4,123,028 | ) | $ | (1,506,426 | ) | |||
| Numerator for basic and diluted EPS - income available to common stockholders | (17,843,574 | ) | $ | (3,472,099 | ) | |||
| Denominator: | ||||||||
| Denominator for EPS – weighted average shares | ||||||||
| Basic | 764,058 | 564,862 | ||||||
| Diluted | 2,381,443 | 566,609 | ||||||
| Net loss per common share attributable to common stockholders | ||||||||
| Basic | $ | (23.35 | ) | $ | (6.15 | ) | ||
| Diluted | $ | (7.49 | ) | $ | (6.13 | ) | ||
| Net loss per common share from continuing operations | ||||||||
| Basic | $ | (17.96 | ) | $ | (3.48 | ) | ||
| Diluted | $ | (5.76 | ) | $ | (3.47 | ) | ||
| Net loss per common share from discontinued operations | ||||||||
| Basic | $ | (5.40 | ) | $ | (2.67 | ) | ||
| Diluted | $ | (1.73 | ) | $ | (2.66 | ) | ||
Concentration of Credit Risks and Major CustomersIncome taxes - Financial instruments that potentially subject the Company to credit risk consist principally of cash and cash equivalents and receivables. The Company places its cash and cash equivalents with financial institutions. Deposits are insured to Federal Deposit Insurance Corp limits. The amount of cash not insured by the FDIC as of December 31, 2021, is $2,332,137.
During the years ended December 31, 2021, no sales to customers represented greater than 10% of revenue.
Recent Accounting Pronouncements – The Company has implemented all new relevant accounting pronouncements that are in effect through the date of these financial statements. The pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its consolidated financial position or results of operations.
Effective January 1, 2019, the Company adopted ASU No. 2016-02, Leases (Topic 842) (“ASU 2016-02”) using the required modified retrospective approach. The most significant changes under the new guidance include clarification of the definition of a lease, and the requirements for lessees to recognize a Right of Use (“ROU”) asset and a lease liability for all qualifying leases with terms longer than twelve months in the consolidated balance sheet. In addition, under Topic 842, additional disclosures are required to meet the objective of enabling users of financial statements to assess the amount, timing and uncertainty of cash flows arising from leases. See Note 10 – Leases, below for more detail on the Company’s accounting with respect to leases.
Effective January 1, 2019, the Company adopted ASU No. 2018-07, Compensation – Stock Based Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting (“ASU 2018-7”), which aligns accounting for share-based payments issued to nonemployees to that of employees under the existing guidance of Topic 718, with certain exceptions. This update supersedes previous guidance for equity-based payments to nonemployees under Subtopic 505-50, Equity – Equity-Based Payments to Non-Employees. The adoption of ASU 2018-07 did not have a material impact on the Company’s consolidated financial statements.
Recently Issued Accounting Pronouncements Not Yet Adopted - In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments” (“ASU 2016-13”). ASU 2016-13 requires financial assets measured at amortized cost to be presented at the net amount expected to be collected. The measurement of expected credit losses is based on relevant information about past events, including historical experience, current conditions, and reasonable and supportable forecasts that affect the collectability of the reported amounts. An entity must use judgment in determining the relevant information and estimation methods that are appropriate in its circumstances. ASU 2016-13 is effective for annual reporting periods beginning after December 15, 2019, including interim periods within those fiscal years, and a modified retrospective approach is required, with a cumulative-effect adjustment to retained earnings as of the beginning of the first reporting period in which the guidance is effective. In November of 2019, the FASB issued ASU 2019-10, which delayed the implementation of ASU 2016-13 to fiscal years beginning after December 15, 2022, for smaller reporting companies.
The Company’s provision for income taxes was $0 for the year ended December 31, 2023, and $0 for the year ended December 31, 2022, respectively. The income tax provisions for the twelve-month periods are based upon estimates of annual income (loss), annual permanent differences and statutory tax rates in the various jurisdictions in which the Company does not expectoperates. For all periods presented, the adoptionCompany utilized net operating loss carryforwards to offset the impact of any taxable income. The Company’s tax rate differs from the applicable statutory rates due primarily to the establishment of a valuation allowance, utilization of deferred and the effect of permanent differences and adjustments.
NOTE 2 – GOING CONCERN
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates realization of assets and the satisfaction of liabilities in the normal course of business within one year after the date the consolidated financial statements are issued. In accordance with Financial Accounting Standards Board, or the FASB, Accounting Standards Update No. 2014-15, Presentation of Financial Statements - Going Concern (Subtopic 205-40), our management evaluates whether there are conditions or events, considered in aggregate, that raise substantial doubt about our ability to continue as a going concern within one year after the date that the financial statements are issued.
As of December 31, 2023, the Company had an accumulated deficit of $33,245,940. The Company has limited financial resources. As of December 31, 2023, the Company had a working capital deficit of $8,803,293 and a cash balance of $151,908. The Company will need to raise additional capital or secure debt funding to support on-going operations. The sources of this new accounting guidancecapital are expected to have a material impactbe the sale of equity and debt, which may not be available on itsfavorable terms, if at all, and may, if sold, cause significant dilution to existing stockholders. If the Company is unable to access additional capital moving forward, it may hurt the Company’s ability to grow and to generate future revenues, financial position, resultsand liquidity. These factors raise substantial doubt about the ability of operations, or cash flows.the Company to continue as a going concern. Unless Management is able to obtain additional financing, it is unlikely that the Company will be able to meet its funding requirements during the next 12 months. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
NOTE 3 – SHORT-TERM DEBTACQUISITIONS AND RELATED PARTIES DEBTDISPOSITIONS
Related Party Promissory NotesAcquisitions
Superlatus, Inc.
On July 31, 2023, the Company entered into the Merger Agreement (see Note 1) with Superlatus (“Seller”) whereby the Company acquired 100% of the stock of the Seller (the “Acquisition”). Superlatus includes a wholly-owned subsidiary, Sapientia. Consideration for the Acquisition consisted of (i) shares of the Company’s common stock at a fair value of $ per share, representing 19.99% of the total issued and outstanding share of the Company’s common stock at Closing, and (ii) shares of the Company’s Series B Preferred Stock, a new class of the Company’s non-voting convertible preferred stock with a conversion ratio of 100 to one. The total fair value of the common stock and Series B Preferred Stock on the Closing Date was $225,000,169 (“Purchase Price”). On January 8, 2024, the Company entered into Amendment No. 1 to the Agreement and Plan of Merger (the “Amendment”). Under the terms of the Amendment, the merger consideration to the shareholders of Superlatus was adjusted to an aggregate of shares of common stock of the Company, representing 19.99% of the total issued and outstanding common stock of the Company after the consummation of the Merger and shares of Company’s Series B Preferred Stock, par value $ per share, with a conversion ratio of shares of Series B Preferred Stock to one share of common stock. The total fair value of the common stock and Series B Preferred Stock on the Closing Date was adjusted to $12,500,089 (“Amended Purchase Price”). Additionally, the shareholders of Superlatus agreed to surrender back to the Company shares of the Company’s Series B Preferred Stock previously received before the Amendment.
| 66 |
The acquisition of Superlatus was accounted for as a business combination using the acquisition method pursuant to FASB ASC Topic 805. As the acquirer for accounting purposes, the Company had estimated the Purchase Price, assets acquired and liabilities assumed as of the acquisition date, with the excess of the Purchase Price over the fair value of net assets acquired recognized as goodwill. An independent valuation expert assisted the Company in determining these fair values.
The Amended Purchase Price allocation as of the acquisition date is presented as follows:
SCHEDULE OF PURCHASE PRICE ALLOCATION
| July 31, 2023 | ||||
| Purchase consideration: | ||||
| Common Stock, at fair value | $ | 996,019 | ||
| Series B Preferred Stock, at fair value | 11,504,070 | |||
| Total purchase consideration | $ | 12,500,089 | ||
| Purchase price allocation: | ||||
| Cash | $ | 5,546 | ||
| Prepaid expenses | 3,705 | |||
| Inventory | 122,792 | |||
| Intangible assets, net | 9,777,479 | |||
| Goodwill | 5,129,115 | |||
| Assets acquired | 15,038,637 | |||
| Accounts payable and other current liabilities | (283,548 | ) | ||
| Purchase price payable | (350,000 | ) | ||
| Notes payable | (1,905,000 | ) | ||
| Liabilities assumed | (2,538,548 | ) | ||
| Net assets acquired | $ | 12,500,089 | ||
The Urgent Company, Inc.
On September 27, 2023, the Company entered into an Asset Purchase Agreement (“APA”) with The Urgent Company, Inc. (“TUC”) and its wholly owned subsidiaries, pursuant to which, the Company was assigned certain inventory and property and equipment and assumed certain operating leases for consideration of $4,400,000 in promissory notes (“Purchase Price”, see Note 11). This acquisition is expected to enhance the Company’s production of sustainable food products and enable the expansion of market share.
| 67 |
The transaction was accounted for as an asset acquisition pursuant to FASB ASC Topic 805. As the acquirer for accounting purposes, the Company allocated the cost of the asset acquisition to the assets acquired and liabilities assumed as of the acquisition date based on their respective relative fair value as of the date of the transaction.
The following summarizes the relative fair values of the assets acquired as of the acquisition date based on the allocation of the cost of the asset acquisition:
SCHEDULE OF FAIR VALUES OF ASSETS ACQUIRED
| September 27, 2023 | ||||
| Purchase consideration: | ||||
| Promissory note | $ | 4,400,000 | ||
| Total purchase consideration | $ | 4,400,000 | ||
| Allocation of cost of assets acquired: | ||||
| Inventory | $ | 4,168,830 | ||
| Property and equipment | 231,170 | |||
| Assets acquired | 4,400,000 | |||
| Net assets acquired | $ | 4,400,000 | ||
Dispositions and Divestitures
SOSRx, LLC
Effective on, February 1, 2023, the Company, Exchange Health and SOSRx, entered into a Voluntary Withdrawal and Release Agreement, which was replaced in its entirety, corrected, and became effective on February 4, 2023 (as replaced and corrected, the “Release Agreement”).
As part of the Release Agreement, a note payable to Exchange Health was forgiven in the amount of $500,000 and $15,000 in accounts payable was waived. Effective February 4, 2023, the operations of SOSRx were discontinued and operations were shut down. As a result of this, the assets and liabilities of SOSRx have been reflected as assets and liabilities of discontinued operations in the Company’s consolidated balance sheets. As of December 31, 2023 and December 31, 2022 as follows:
SCHEDULE OF FINANCIAL STATEMENTS OF DISCONTINUED OPERATIONS
| December 31, 2023 | December 31, 2022 | |||||||
| Cash | $ | - | $ | 22,474 | ||||
| Accounts receivable | - | 363 | ||||||
| Total assets of discontinued operations | $ | - | $ | 22,837 | ||||
| Accounts payable | $ | - | $ | 46,500 | ||||
| Total liabilities of discontinued operations | $ | - | $ | 46,500 | ||||
The terms of the Release Agreement qualify the transaction as a discontinued operation in accordance with U.S. GAAP. As a result, operating results and cash flows related to the SOSRx operations have been reflected as discontinued operations in the Company’s consolidated statements of operations, consolidated statements of cash flows and consolidated statements of shareholders’ equity.
| 68 |
Alliance Pharma Solutions, LLC and Community Specialty Pharmacy, LLC
On August 22, 2023, the Company and Wood Sage, LCC (“Wood Sage”) entered into a Membership Interest Purchase Agreement, pursuant to which the Company sold 100% of the membership interest in Alliance Pharma Solutions, LLC (“ASP MIPA”) for consideration of a $125,000 promissory note (“ASP Sale Price”) and a Membership Interest Purchase Agreement, pursuant to which the Company sold 100% of the membership interest in Community Specialty Pharmacy, LLC (“CSP MIPA”) in exchange for a $100,000 promissory note (“CSP Sale Price”).
The divestiture of APS and CSP represented an intended strategic shift in the Company’s operations and will allow the Company to become focused on food technology As a result, the results of APS and CSP were classified as discontinued operations in our condensed statements of operations and excluded from both continuing operations and segment results for the years ended December 31, 2023 and 2022.
As part of recognizing the business as held for sale in accordance with U.S. GAAP, the Company was required to measure APS and CSP at the lower of its carrying amount or fair value less cost to sell. As a result of this analysis, during the year ended December 31, 2023, the Company recognized a non-cash, pre-tax loss on disposal of $3,300,225.42. The loss is included in “Net loss from discontinued operations” in the consolidated statements of operations. The loss was determined by comparing the fair value of the consideration received for the sale of a 100% interest in APS and CSP with the net assets of APS and CSP, respectively, immediately prior to the transaction.
As a result of the transactions, the following assets and liabilities of APS and CSP were transferred to Wood Sage as of August 22, 2023:
SCHEDULE OF ASSETS AND LIABILITIES
| Alliance Pharma Solutions, LLC | Community Specialty Pharmacy, LLC | |||||||
| Cash | $ | 1,050 | $ | 61,988 | ||||
| Accounts receivable, net | - | 101,901 | ||||||
| Inventory | - | 123,230 | ||||||
| Prepaid assets | - | 525 | ||||||
| Intangible assets and capitalized software, net | 739,337 | - | ||||||
| Accounts payable | (23,982 | ) | (231,876 | ) | ||||
| Accrued liabilities | - | (10,182 | ) | |||||
| Net assets sold | $ | 716,405 | $ | 45,586 | ||||
Discontinued Operations
The results of operations from discontinued operations for the years ended December 31, 2023 and 2022, have been reflected as discontinued operations in the consolidated statements of operations and consist of the following:
SCHEDULE OF DISCONTINUED OPERATIONS
| 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | |||||||||||||||||||||||||
| SOSRx | APS | CPS | Total | |||||||||||||||||||||||||||||
| Years ended December 31, | Years ended December 31, | Years ended December 31, | Years ended December 31, | |||||||||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | |||||||||||||||||||||||||
| Revenue | $ | - | $ | 22,623 | $ | - | $ | - | $ | 851,755 | $ | 1,175,474 | $ | 851,755 | $ | 1,198,097 | ||||||||||||||||
| Cost of sales | - | - | - | - | 705,206 | 1,266,152 | 705,206 | 1,266,152 | ||||||||||||||||||||||||
| Gross Profit | - | 22,623 | - | - | 146,549 | (90,678 | ) | 146,549 | (68,055 | ) | ||||||||||||||||||||||
| Operating Expenses | ||||||||||||||||||||||||||||||||
| Impairment of intangible asset | 792,500 | - | - | - | 792,500 | |||||||||||||||||||||||||||
| Wage and salary expense | - | 55,439 | - | - | 456,297 | 304,947 | 456,297 | 360,386 | ||||||||||||||||||||||||
| Professional fees | - | - | 3,125 | 46,787 | 20,246 | 6,120 | 23,371 | 52,907 | ||||||||||||||||||||||||
| Accounting and legal expense | - | - | 7,773 | 104 | 63,000 | 500 | 70,773 | 604 | ||||||||||||||||||||||||
| Technology expense | - | 63,160 | 20,611 | 86,688 | 9,464 | 17,823 | 30,075 | 167,671 | ||||||||||||||||||||||||
| General and Administrative | - | 4,931 | 3,762 | 11,562 | 32,830 | 49,710 | 36,592 | 66,203 | ||||||||||||||||||||||||
| Total operating expense | - | 916,030 | 35,271 | 145,141 | 581,837 | 379,100 | 617,108 | 1,440,271 | ||||||||||||||||||||||||
| Operating income (loss) from discontinued operations | - | (893,407 | ) | (35,271 | ) | (145,141 | ) | (435,288 | ) | (469,778 | ) | (470,559 | ) | (1,508,326 | ) | |||||||||||||||||
| Other income (expense) | - | |||||||||||||||||||||||||||||||
| Gain (loss) on asset sale | - | - | - | 1,900 | - | - | - | 1,900 | ||||||||||||||||||||||||
| Total other income (expense) | - | - | - | 1,900 | - | - | - | 1,900 | ||||||||||||||||||||||||
| Net income (loss) from discontinued operations | $ | - | $ | (893,407 | ) | $ | (35,271 | ) | $ | (143,241 | ) | $ | (435,288 | ) | $ | (469,778 | ) | $ | (470,559 | ) | $ | (1,506,426 | ) | |||||||||
| 69 |
NOTE 4 - RELATED PARTY TRANSACTIONS
On April 1, 2023 and July 1, 2023 the Company entered into a relationship with Scietech, LLC (“Scietech”) in an independent contractor agreement to consult on increasing sales on the IPS and Trxade Inc. platforms. The agreement was for an annual fee of $400,000 to be split equally between IPS and Trxade Inc. A 31% investor in Scietech is the spouse of the interim CFO, Prashant Patel, which qualifies as a related party. The company was chosen because they were the most qualified to perform the desired qualifications.
On February 15, 2022, the Company entered into a relationship with Exchange Health, a technology company providing an online platform for manufacturers and suppliers to sell and purchase pharmaceuticals. In October 2018,connection therewith, SOSRx was formed in connection withFebruary 2022, which is owned 51% by the acquisitionCompany and 49% by Exchange Health. On February 15, 2022, the Company contributed cash to SOSRx in the amount of Community Specialty Pharmacy, LLC,$325,000, issued a $300,000 promissory note to SOSRx in the amount of $500,000, which was issuedimmediately assigned to Nikul Panchal, a non-executive officerExchange Health (the “Promissory Note”), and agreed to make an earn out payment of up to $400,000, payable, at the Company’s discretion, in cash or common stock of the Company, accruing simple interest atbased on SOSRx achieving certain revenue targets of SOSRx (the “Earn Out Payments”); and entered into a Distribution Services Agreement with SOSRx (the “Distribution Agreement”). Exchange Health contributed $792,000 in software and contracts which was recorded as an intangible asset on the ratebalance sheet of 10% per annum, payable annually,SOSRx. The intangible asset was determined to be impaired and having a maturity datewas written off on October 15, 2021. In October 2019, $75,000 of the note was converted into common shares at $3.00 per share, leaving $225,000 of principal owed under the promissory note. There was a loss recognized on this conversion of $76,500. In September 2021, the promissory note was paid in full.December 31, 2022.
At December 31, 2021 and 2020,2023, total related party debt was $0.
On and effective on, February 1, 2023, the Company, Exchange Health and SOSRx, entered into a Voluntary Withdrawal and Release Agreement, which was replaced in its entirety and corrected on February 4, 2023, and effective February 4, 2023 (as replaced and corrected, the “Release Agreement”). Pursuant to the Release Agreement, the Company voluntarily withdrew as a member of SOSRx pursuant to the terms of the Operating Agreement of SOSRx, which provided that the Company would withdraw from SOSRx if certain revenue targets were not met, which targets have not been met.
Also pursuant to the Release Agreement, (a) the Company agreed to the termination of its interests in SOSRx and its withdrawal as a member thereof for no consideration (the “Withdrawal”); (b) the Promissory Note, and all of the Company’s obligations under such Promissory Note were terminated; and (c) the parties agreed that no Earn Out Payments will be due. The Release Agreement also (i) provides that all accumulated losses of SOSRx through December 20, 2022, will be allocated 51% to the Company and 49% to Exchange Health; (ii) provides for a total of approximately $15,000 in outstanding invoices owed by the Company to SOSRx to be waived; (iii) includes certain indemnification obligations of SOSRx and Exchange Health; (iv) requires SOSRx to pay certain pre-agreed outstanding invoices of SOSRx; (v) includes mutual releases of the Company and SOSRx and Exchange Health; and (vi) includes customary representations and warranties of the parties.
NOTE 5 – REVENUE RECOGNITION
The Company derives revenue from two primary sources—product revenue and service revenue.
Product revenue consists of shipments of:
| ● | Resale of pharmaceutical products to pharmacies; and | |
| ● | Revenues for our products are recognized and invoiced when the product is shipped to the customer. |
Service revenue consists primarily of:
| ● | Transaction fees from the facilitation of buyer generated purchase orders to suppliers, billed monthly; | |
| ● | Data service fees associated with providing vendors of pharmaceutical products with data analysis of their catalogues and branding of their products or company to the Company’s registered buyers, billed monthly or as a one-time fee; and | |
| ● | Software-as-a-Service (“SaaS”) fees for a platform for virtual healthcare provider visits, billed monthly. |
Revenues for the Company’s services that are billed monthly are recognized and invoiced when the at the beginning of the month. Revenues for one-time services are recognized at the point in time when services are rendered.
Payment terms for products and services are generally 0 to 60 days and the Company has no contract assets or liabilities.
The following table presents disaggregated revenue by major product and service categories during the years ended December 31, 2023, and 2022:
SCHEDULE OF DISAGGREGATED REVENUE
| Years ended December 31, | 2023 | 2022 | ||||||
| Product revenues | ||||||||
| Pharmaceutical product resale | $ | 1,363,830 | $ | 4,754,067 | ||||
| Packaged food resale | 487,021 | - | ||||||
| Total product revenue | $ | 1,850,851 | $ | 4,754,067 | ||||
| Service revenues | ||||||||
| Transaction fee income | $ | 6,200,334 | $ | 5,347,401 | ||||
| Data service fee income | 201,825 | 88,413 | ||||||
| SaaS fee income | 19,204 | 60,287 | ||||||
| Total service revenue | $ | 6,421,363 | 5,496,101 | |||||
| Total revenues | $ | 8,272,214 | $ | 10,250,168 | ||||
| 70 |
NOTE 6 – INVENTORY
Inventory value is determined using the weighted average cost method and is stated at the lower cost or net realizable value. As of December 31, 2023, and 2022, inventory was comprised of the following:
SCHEDULE OF INVENTORY
| As of December 31, | 2023 | 2022 | ||||||
| Raw materials | $ | - | $ | 65,523 | ||||
| Finished goods | 968 | - | ||||||
| Inventory | $ | 968 | $ | 65,523 | ||||
NOTE 7 – NOTES RECEIVABLE
On August 22, 2023, the Company received a Promissory Note (the “Wood Sage Note”) in the amount of $1,300,000 from Wood Sage, LLC and entered into the APS MIPA and CSP MIPA for the Company to sell APS and CSP and entered into a Master Service Agreement (“Wood Sage MSA”). The Wood Sage Note bears no interest and is due and payable within thirty days of a change in control, as defined by the Wood Sage Note, of the borrower. As of December 31, 2023, the outstanding balance of the Wood Sage Note was $1,300,000.
NOTE 8 – INTANGIBLE ASSETS
As of December 31, 2023, intangible assets, net consisted of the following:
SCHEDULE OF INTANGIBLE ASSETS NET
| Weighted Average | ||||||||||||||||
| Useful Life | Accumulated | |||||||||||||||
| (years) | Cost | Amortization | Net | |||||||||||||
| Developed technology | 5.0 | $ | 9,777,478 | $ | (814,790 | ) | $ | 8,962,688 | ||||||||
| December 31, 2023 | December 31, 2022 | |||||||
| Amortization expense | $ | 814,790 | $ | - | ||||
| Total Amortization Expense | $ | 814,790 | $ | - | ||||
NOTE 9 – OTHER CURRENT LIABILITIES
As of December 31, 2023 and December 31, 2022, other current liabilities consisted of the following:
SCHEDULE OF OTHER CURRENT LIABILITIES
| December 31, 2023 | December 31, 2022 | |||||||
| Insurance refunds payable | $ | 62,390 | $ | 62,390 | ||||
| Deferred revenue | - | 5,127 | ||||||
| Other payables | 7,920 | - | ||||||
| Other current liabilities | $ | 70,310 | $ | 67,517 | ||||
| 71 |
NOTE 10 – CONTINGENT FUNDING LIABILITIES
On December 13, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables (the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $150,000 to purchase $214,500 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables of Trxade Inc. The Company also paid $7,500 as a one-time origination fee in connection with the Receivables Agreement. The Receivables Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events of default. As of December 31, 2023, the balance of the payable balance is $144,231.
On November 22, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables (the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $275,000 to purchase $393,250 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables of Trxade Inc. The Company also paid $13,750 as a one-time origination fee in connection with the Receivables Agreement. The Receivables Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events of default. As of December 31, 2023, the balance of the payable balance is $222,115.
On October 25, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables (the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $1,200,000 to purchase $1,728,000 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables of Trxade Inc. The Company also paid $60,000 as a one-time origination fee in connection with the Receivables Agreement. The Receivables Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events of default. As of December 31, 2023, the balance of the payable balance is $880,000.
On June 27, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables (the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $1,250,000 to purchase $1,800,000 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables of Trxade Inc. The Company also paid $62,500 as a one-time origination fee in connection with the Receivables Agreement. The Receivables Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events of default. This agreement was fully paid off in October 2023.
On March 14, 2023, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables (the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $875,000 to purchase $1,224,000 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables of Trxade Inc. The Company also paid $42,500 as a one-time origination fee in connection with the Receivables Agreement. The Receivables Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events of default. This agreement was fully paid off in June 2023.
On September 14, 2022, the Company entered into a non-recourse funding agreement with a third-party for the purchase and sale of future receivables (the “Receivables Agreement”). Pursuant to the Receivables Agreement, the third party agreed to fund the Company $275,000 to purchase $396,000 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables of Trxade Inc. The Company also paid $15,000 as a one-time origination fee in connection with the Receivables Agreement. The Receivables Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events of default. This agreement was fully paid off in January 2023.
On June 27, 2022, the Company entered into a non-recourse funding agreement with a third-party funder for the purchase and sale of future receivables. Pursuant to the Receivables Agreement, the third party agreed to fund the Company $550,000 to purchase $792,000 of future receivables. Under the funding agreement, the third-party receives a priority interest in the receivables of Trxade Inc. The Company also paid $27,500 as a one-time origination fee in connection with the Receivables Agreement. The Receivables Agreement also allows for the third-party funder to file UCCs securing their interest in the receivables and includes customary events of default. This agreement was fully paid off in January 2023.
| 72 |
The Company’s relationship with the funding source meets the criteria in ASC 470-10-25 – Sales of Future Revenues or Various Other Measures of Income (“ASC 470”), which relates to cash received from a funding source in exchange for a specified percentage or amount of revenue or other measure of income of a particular product line, business segment, trademark, patent or contractual right for a defined period. Under this guidance, the Company recognized the fair value of its contingent obligation to the funding source, as of the acquisition date, as a current liability in its consolidated balance sheet.
Under ASC 470, amounts recorded as debt are to be amortized under the interest method. The Company made an accounting policy election to utilize the prospective method when there is a change in the estimated future cash flows, whereby a new effective interest rate is determined based on the revised estimate of remaining cash flows. The new rate is the discount rate that equates the present value of the revised estimate of remaining cash flows with the carrying amount of the debt, and it will be used to recognize interest expense for the remaining period. Under this method, the effective interest rate is not constant, and any change in expected cash flows is recognized prospectively as an adjustment to the effective yield. As of December 31, 2023, and December 31, 2022, the total contingent funding liability was $1,246,346 and $225,000108,036, respectively, and the effective interest rate was approximately 31% and 31%, respectively. This rate represents the discount rate that equates the estimated future cash flows with the fair value of the debt and is used to compute the amount of interest to be recognized each period. Any future payments made to the funding source will decrease the contingent funding liability balance accordingly.
NOTE 11 – NOTES PAYABLE
On November 17, 2023, the Company issued promissory notes to Moku Foods, Inc. (the “Moku Foods November 2023 Note”) in the amount of $50,000. The promissory note accrues interest at 11.5% per annum, compounded monthly and is payable upon demand at any time after November 30, 2023. As of December 31, 2023, the balance of the Moku Foods October 2023 Note is $50,000. The Company has accrued interest of $945 as of December 31, 2023.
On October 16, 2023, the Company issued promissory notes to Moku Foods, Inc. (the “Moku Foods October 2023 Note”) in the amount of $150,000. The promissory note accrues interest at 11.5% per annum, compounded monthly and is payable upon demand at any time after October 31, 2023. As of December 31, 2023, the balance of the Moku Foods October 2023 Note is $150,000. The Company has accrued interest of $4,300 as of December 31, 2023.
On September 27, 2023, the Company issued promissory notes to Perfect Day, Inc. (the “Perfect Day Note”) in the amount of $4,400,000 as consideration for the TUC APA (see Note 3). The promissory notes do not accrue interest and are payable upon demand at any time after October 31, 2023. The entire aggregate, unpaid principal sum of the note is immediately due and payable upon the occurrence of a change in control, as defined in the agreement.
On September 14, 2023, the Company issued a promissory note to Danam Health, Inc. (the “Danam Note”) in the amount of $300,000. The Company received a deposit of $200,000 on September 14, 2023, and an additional deposit of $100,000 on October 13, 2023. The Danam Note accrues interest at 0% per annum and is due and payable no later than 30 days after a change in control of borrower, as defined in the note agreement. As of December 31, 2023, the balance of the Danam Note is $50,000.
On June 16, 2023, the Company issued a secured debenture to Eat Well Investment Group, Inc. (the “Eat Well June 2023 Note”) in the amount of $1,150,000 for the purchase of Sapientia, a wholly-owned subsidiary of Superlatus. The Eat Well June 2023 Note is secured by 100% of the membership interests in Sapientia. The Eat Well June 2023 Note began accruing interest at 12% per annum, compounded monthly, as of October 31, 2023. The Eat Well June 2023 matured on December 31, 2023. As of December 31, 2023, the balance of the Eat Well June 2023 Note is $1,150,000. The Company has accrued interest of $23,063 as of December 31, 2023. As of the date of this filing, the parties are working on an amendment for an extension.
| 73 |
On February 8, 2023, Sapientia, a wholly-owned subsidiary of Superlatus, entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well February 2023 Note”) in the amount of $25,000. The Eat Well February 2023 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures February 7, 2025. As of December 31, 2023, the balance of the Eat Well February 2023 Note is $25,000. The Company has accrued interest of $418 as of December 31, 2023.
On September 14, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well September 2022 Note”) in the amount of $50,000. The Eat Well September 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures September 13, 2024. As of December 31, 2023, the balance of the Eat Well September 2022 Note is $50,000. The Company has accrued interest of $1,212 as of December 31, 2023.
On July 26, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well July 26, 2022 Note”) in the amount of $35,000. The Eat Well July 26, 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures July 25, 2024. As of December 31, 2023, the balance of the Eat Well July 26, 2022 Note is $35,000. The Company has accrued interest of $938 as of December 31, 2023.
On July 12, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well July 12, 2022 Note”) in the amount of $25,000. The Eat Well July 12, 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures July 11, 2024. As of December 31, 2023, the balance of the Eat Well July 12, 2022 Note is $25,000. The Company has accrued interest of $688 as of December 31, 2023.
On March 15, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well March 2022 Note”) in the amount of $100,000. The Eat Well March 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures March 14, 2024. As of December 31, 2023, the balance of the Eat Well March 2022 Note is $100,000. The Company has accrued interest of $3,361 as of December 31, 2023.
On February 1, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well February 2022 Note”) in the amount of $100,000. The Eat Well February 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures February 1, 2024. As of December 31, 2023, the balance of the Eat Well February 2022 Note is $100,000. The Company has accrued interest of $3,576 as of December 31, 2023.
On January 20, 2022, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well January 2022 Note”) in the amount of $20,000. The Eat Well January 2022 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matures January 20, 2024. As of December 31, 2023, the balance of the Eat Well January 2022 Note is $20,000. The Company has accrued interest of $728 as of December 31, 2023.
On December 24, 2021, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well December 2021 Note”) in the amount of $100,000. The Eat Well December 2021 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matured December 24, 2023. As of December 31, 2023, the balance of the Eat Well December 2021 Note is $100,000. The Company has accrued interest of $3,776 as of December 31, 2023. As of the date of this filing, the parties are working on an amendment for an extension.
On November 10, 2021, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well November 2021 Note”) in the amount of $50,000. The Eat Well November 2021 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matured November 10, 2023. As of December 31, 2023, the balance of the Eat Well November 2021 Note is $50,000. The Company has accrued interest of $2,001 as of December 31, 2023. As of the date of this filing, the parties are working on an amendment for an extension.
On August 18, 2021, Sapientia entered into a Loan Agreement with Eat Well Investment Group, Inc. (the “Eat Well August 2021 Note”) in the amount of $250,000. The Eat Well August 2021 Note is unsecured, accrues interest at a rate of 1.87% per annum, and matured August 18, 2023. As of December 31, the balance of the Eat Well August 2021 Note is $250,000. The Company has accrued interest of $11,079 as of December 31, 2023. As of the date of this filing, the parties are working on an amendment for an extension.
The following table summarizes notes payable balances as of December 31, 2023:
SCHEDULE OF NOTES PAYABLE BALANCES
| Current | Noncurrent | Accrued | ||||||||||||||
| Portion | Portion | Total | Interest | |||||||||||||
| Current Portion | Non current Portion | Note Payable Total | Accrued Interest | |||||||||||||
| Perfect Day Notes | $ | 4,400,000 | $ | - | $ | 4,400,000 | $ | - | ||||||||
| Danam Note | 50,000 | - | 50,000 | - | ||||||||||||
| Moku Foods November 2023 Note | 50,000 | - | 50,000 | 945 | ||||||||||||
| Moku Foods October 2023 Note | 150,000 | - | 150,000 | 4,300 | ||||||||||||
| Eat Well June 2023 Note | 1,150,000 | - | 1,150,000 | 57,847 | ||||||||||||
| Eat Well February 2023 Note | - | 25,000 | 25,000 | 418 | ||||||||||||
| Eat Well September 2022 Note | 50,000 | - | 50,000 | 1,212 | ||||||||||||
| Eat Well July 26, 2022 Note | 35,000 | - | 35,000 | 938 | ||||||||||||
| Eat Well July 12, 2022 Note | 25,000 | - | 25,000 | 688 | ||||||||||||
| Eat Well March 2022 Note | 100,000 | - | 100,000 | 3,361 | ||||||||||||
| Eat Well February 2022 Note | 100,000 | - | 100,000 | 3,576 | ||||||||||||
| Eat Well January 2022 Note | 20,000 | - | 20,000 | 728 | ||||||||||||
| Eat Well December 2021 Note | 100,000 | - | 100,000 | 3,776 | ||||||||||||
| Eat Well November 2021 Note | 50,000 | - | 50,000 | 2,001 | ||||||||||||
| Eat Well August 2021 Note | 250,000 | - | 250,000 | 11,079 | ||||||||||||
| $ | 6,530,000 | $ | 25,000 | $ | 6,555,000 | $ | 90,869 | |||||||||
| 74 |
NOTE 12 – INCOME TAXES
The provision for income taxes on income from operations for fiscal 2023 and 2022 consists of the following:
SCHEDULE OF PROVISION FOR INCOME TAXES
| 2023 | 2022 | |||||||
| Federal: | ||||||||
| Current | - | - | ||||||
| Deferred | - | - | ||||||
| State | ||||||||
| Current | - | - | ||||||
| Deferred | - | - | ||||||
| Total | - | - | ||||||
Income (loss) before income taxes for the years ended December 31, 2023 and 2022 consisted of the following:
SCHEDULE OF INCOME (LOSS) BEFORE INCOME TAXES
| For the year ended December 31, | 2023 | 2022 | ||||||
| US | (17,843,574 | ) | (3,909,868 | ) | ||||
As a result of the full net valuation allowance position, the Company did not recognize any U.S. federal income tax expense or tax benefit on any components of continuing or discontinued operations.
SCHEDULE OF DEFERRED TAX ASSETS AND LIABILITIES
| 2023 | 2022 | |||||||
| Deferred Tax Assets | ||||||||
| Net operating Losses | 5,800,214 | 4,030,755 | ||||||
| Purchased Intangibles | 151,877 | - | ||||||
| Lease Liability | 127,896 | - | ||||||
| Total Deferred Tax Assets | 6,079,987 | 4,030,755 | ||||||
| Deferred Tax Liabilities | ||||||||
| Purchased Goodwill | (15,534 | ) | - | |||||
| Right to Use Assets | (127,896 | ) | - | |||||
| Total Deferred Tax Liabilities | (143,430 | ) | - | |||||
| Valuation Allowance | (5,936,557 | ) | (4,030,755 | ) | ||||
| Net Deferred Taxes | - | - | ||||||
The Company has established a valuation allowance equal to the full amount of the deferred tax asset primarily due to uncertainty in the utilization of the net operating loss carry forwards.
The estimated net operating loss carry forwards of approximately $24,893,624 will be available based on the new carryover rules in section 172(a) passed with the Tax Cuts and Jobs Acts.
NOTE 413 – STOCKHOLDERS’ EQUITY
Designation of Series C Preferred Stock
Effective October 4, 2023, the Company filed a Certificate of Designation, Preferences, Rights and Limitations of the Series C Preferred Stock with the Secretary of the State of Delaware which designatedshares of the Company’s authorized and unissued preferred stock as convertible Series C Preferred Stock at a par value of $ per share.
Hudson Global Ventures Stock Purchase Agreement
On October 4, 2023, the Company entered into a Securities Purchase Agreement (“Agreement”, or “SPA”) with Hudson Global Ventures, LLC (“Hudson”). Under the terms of the Agreement, the Company agreed to sell, and Hudson agreed to purchase, Two Hundred Ninety () shares of Series C Preferred Stock (the “Purchased Shares”) at a price of $ per share and a Warrant to purchase up to shares of Common Stock. Additionally, pursuant to the Agreement, shares of Common Stock were issued to Hudson upon closing for a commitment fee. The Company received $250,000 in exchange for the Purchased Shares, Common Stock, and Warrants, net of issuance costs.
Designation of Series B Preferred Stock
Effective June 26, 2023, the Company filed a Certificate of Designation, Preferences, Rights and Limitations of the Series B Preferred Stock with the Secretary of the State of Delaware which designated shares of the Company’s authorized and unissued preferred stock as convertible Series B Preferred Stock at a par value of $ per share.
2023 1:15 Stock Split
Effective June 21, 2023, the Company executed a 1:15 reverse stock split for stockholders of record on that date. This was executed to comply with the Nasdaq Listing Rule 5550(a)(2) to have the price of the stock above $.
2022 Equity Compensation Awards
Effective September 1, 2022, the Board of Directors and Compensation Committee of the Company, with the approval of each of the following officers, agreed to reduce the annual cash compensation payable to Suren Ajjarapu, the Company’s Chief Executive Officer; Prashant Patel, the Company’s President and Chief Operating Officer and Janet Huffman, the Company’s former Chief Financial Officer, in an effort to conserve cash.
In lieu of the reduced cash salary payable to each officer, the Board and Compensation Committee agreed to issue such officers shares of the Company’s common stock equal to the amount of reduced cash salary, divided by the closing sales price of the Company’s common stock on the NASDAQ Capital Market on August 31, 2022, the date approved by the Board of Directors. The total amount of shares of common stock issued on August 31, 2022, to the officers was .
| 75 |
The shares of common stock issuable to the officers vested at the rate of 1/4th of such shares on each of September 30, 2022, October 31, 2022, November 30, 2022, and December 31, 2022, subject to each applicable Officer’s continued service to the Company on such dates and subject to the restricted stock award agreements entered into as evidence of such awards.
Separately, certain employees of the Company agreed to reduce their cash salaries by an aggregate of $ in consideration for an aggregate of shares of the Company’s restricted common stock, with the same vesting terms as the officer shares discussed above.
Effective on August 31, 2022, the Board of Directors approved the issuance of shares of common stock of the Company to each independent member of the Board of Directors, for services rendered to the Company during fiscal 2022, which shares were granted with an exercisevalued at $63,250, based on the closing sales price of $3.00 per share, and were exercised at $3.00 per share; the Company issued shares ofCompany’s common stock and $15,000 in proceeds were received in connection with such exercise.
2020 Equity Compensation Awards
On April 14, 2020,on the Compensation Committeedate approved by the grantBoard of (a) shares of restricted common stock to the Company’s legal counsel; and (b) shares of restricted common stock to Howard A. Doss, the Company’s Chief Financial Officer, whichDirectors. The shares vested at the rate of ¼th1/4th of such shares immediately on the grant date, and 1/4th of such shares on July 1 andeach of October 1, 2020, and2022, January 1, 2023, and April 1, 2021. The shares have a fair value of $107,100 and the Company recognized stock-based compensation expense of $ for the twelve months ended December 31, 2021.
On April 14, 2020, the then three independent members of the Board of Directors (Mr. Donald G. Fell, Dr. Pamela Tenaerts, and Mr. Michael L. Peterson), were each awarded shares of restricted stock, which vested at the rate of ¼th of such shares on July 1 and October 1, 2020, and January 1 and April 1, 2021. The shares have a fair value of $165,000 and the Company recognized stock-based compensation expense of $ for the twelve months ended December 31, 2021.
2021 Equity Compensation Awards
On April 15, 2021, the Board of Directors, with the recommendation of the Compensation Committee, approved the grant of options to purchase an aggregate of shares of our common stock to certain employees of the Company, in consideration for services to be rendered by such individuals through 2025. The options vest at the rate of ¼th of such options per year, on the first, second, third and fourth anniversaries of the grant date,2023, subject to such option holders continuing to provide serviceseach applicable independent director’s continued service to the Company on such dates, subject todates.
All of the terms ofawards discussed above were issued under the Company’s Second Amended and Restated 2019 Equity Incentive Plan (the “Plan”) and all restricted stock awards discussed above were evidenced by Restricted Stock Grant Agreements.
NOTE 14 – PREFUNDED AND PRIVATE PLACEMENT WARRANTS
On October 4, 2022 the option agreementsCompany entered into evidence such grants.a securities purchase agreement (the “Purchase Agreement”) with a certain institutional investor (the “Purchaser”) which provided for the sale and issuance by the Company of (i) the Company’s common stock (the “Common Stock”), (ii) pre-funded warrants (the “Pre-Funded Warrants”) and (iii) warrants (the “Private Placement Warrants” and, together with the Shares and the Pre-Funded Warrants, the “Securities”). The optionsPrivate Placement Warrants were granted pursuant to, andsold in a concurrent private placement (the “Private Placement”).
Simultaneously with the closing of the stock placement, the investor pre-purchased 40,116 Private Warrants at a purchase price of $17.25 per warrant. The Pre-Funded Warrants are subject to, the Plan, and have a termimmediately exercisable into one share of from the grant date. The optionscommon stock per warrant, have an exercise price of $4.760.00015 per share, and may be exercised at any time until all of the closingPre-Funded Warrants are exercised in full. On January 4, 2023, the investor exercised the 40,116 warrants for a purchase price of $6.02. The investor was issued the Company’s common stockshares on this date. Each Private Warrant has an exercise price of $22.50 per share, will be exercisable following Stockholder Approval, which was obtained in December 2022, and will expire on the datefifth anniversary of the grantdate on which the Private Warrants become exercisable. The Private Warrants contain standard adjustments to the exercise price including for stock splits, stock dividend, rights offerings and pro rata distributions, and include full ratchet anti-dilutive rights in the event the Company issues shares of Common Stock or Common Stock equivalents within fifteen months of the initial exercise date, with a value less than the then exercise price of such options.
In connection withPrivate Warrants, subject to certain customary exceptions, and pursuantfurther subject to the independent director compensation policy previously adopted by the Boarda minimum exercise price of Directors, on April 15, 2021, the then three independent members of the Board of Directors (Mr. Donald G. Fell, Dr. Pamela Tenaerts, and Mr. Michael L. Peterson), were each awarded shares of restricted stock, valued at $55,000 ($ per share) based onshare. The Private Warrants also include certain rights upon ‘fundamental transactions’ as described in the closing sales price ofPrivate Warrants, including allowing the Company’s common stock onholders thereof to require that the Nasdaq Capital Market on the effective date of the grant, April 1, 2021, which vestCompany re-purchase such Private Warrants at the rate of ¼th Black Scholes Value of such shares on July 1 and October 1, 2021 and January 1 and April 1, 2022, subject to such persons continuing to provide services to the Company on such dates, subject to the terms of the Plan and the Restricted Stock Grant Agreements entered into as evidence of such awards. The shares have a fair value of $165,000 and the Company recognized stock-based compensation expense of $ for the twelve months ended December 31, 2021. Common Shares totaling were cancelled on May 27, 2021, when the director services of Mr. Peterson and Ms. Tenaerts were terminated.
The Board of Directors of the Company, on May 27, 2021, confirmed the vesting of shares of common stock previously issued to each of Michael L. Peterson and Dr. Pamela Tenaerts on July 1, 2021, which were subject to forfeiture subject to such persons continued service on the Board of Directors prior to the vesting date.
In connection with and pursuant to the independent director compensation policy previously adopted by the Board of Directors, on May 27, 2021, the Board of Directors awarded Charles L. Pope, and Christine L. Jennings, each independent members of the Board of Directors appointed to the Board of Directors on May 27, 2021, shares of restricted stock each, valued at $41,250 each ($ per share) based on the closing sales price of the Company’s common stock on the Nasdaq Capital Market on the effective date of the grant, May 27, 2021, which vested at the rate of 1/3rd of such shares on October 1, 2021 and January 1, with the last tranche thereof vesting on April 1, 2022, subject to such persons continuing to provide services to the Company on such date. The Company recognized stock-based compensation expense of $ for the twelve months ended December 31, 2021.
Employment Agreement with Suren Ajjarapu, Chief Executive Officer
In connection with our employment agreement with Mr. Suren Ajjarapu, our Chief Executive Officer, stock or other equity compensation was granted for the year ended December 31, 2021.
Stock Repurchase Program
On May 27, 2021, the Board of Directors of the Company authorized and approved a stock repurchase program for up to $1 million of the currently outstanding shares of the Company’s common stock. There is no time frame for the repurchase program, and such program will remain in place until a maximum of $1.0 million of the Company’s common stock has been repurchased or until such program is suspended or discontinued by the Board of Directors.
At the Market Offering
On August 5, 2021, our Board of Directors paused the Stock Repurchase Program until the “at-the-market” offering (discussed below) was complete.
On August 6, 2021, the Company entered into an Equity Distribution Agreement, relating to an “at-the-market” offering for the sale of up to $9 million in shares of the common stock under which EF Hutton, division of Benchmark Investments, LLC, the distribution agent, could sell the offering shares in public market transactions reported on the consolidated tape or privately negotiated transactions which could include block trades pursuant to and in connection with the Company’s previously filed Form S-3 Shelf Registration Statement filed with the Securities and Exchange Commission on August 28, 2020 and declared effective by the Commission on September 3, 2020 (File Number: 333-248473) and the Prospectus Supplement was filed with the Commission under Rule 424(b)(5) dated August 6, 2021 (the “ATM Program”).
Effective on November 30, 2021, the Company provided the distribution agent notice of the termination of the Equity Distribution Agreement and the ATM Program (each of which were terminated effective December 5, 2021, pursuant to the terms of the Equity Distribution Agreement), and as a result, $128,000 of deferring offering costs were recognized.
shares of common stock were sold pursuant to the “at-the-market” offering prior to the termination date.
Continuation of the Stock Repurchase Program
On December 10, 2021, the Board of Directors authorized and approved the resumption of the Company’s prior share repurchase program. The share repurchase program as approved by the Board of Directors on December 10, 2021, modified the prior repurchase program to allow for the repurchase of up to of the currently outstanding shares of the Company’s common stock. There is no time frame for the repurchase program, and such program will remain in place until a maximum of 100,000 shares of the Company’s common stock has been repurchased or until such program is discontinued by the Board of Directors.
As of December 31, 2021, shares have been repurchased.securities.
NOTE 5 -15 – WARRANTS
In 2021,During the year ended December 31, 2023, warrants were granted, and expired. During the year ended December 31, 2023, prefunded warrants and granted warrants to purchase shares of common stock were granted, were exercised, and warrants to purchase 38,216 shares of common stock expired and were forfeited. See Note 4 – Stockholders’ Equity.
For the twelve-month period ended December 31, 2021, warrants to purchase shares of common stock were exercised resulting in proceedsfor a total purchase price of $15,0001,621. See Note 13 for further description.
The Company uses the Black-Scholes pricing model to estimate the fair value of stock-based awards on the date of the grant. The
There was compensation cost related to the warrants granted was $ and $for the yearyears ended December 31, 2021,2023, and 2020,2022, respectively.
| 76 |
The following table summarizes the assumptions used to estimate the fair value of the outstanding warrants granted during the years ended December 31, 20212023, and 2020.2022.
SUMMARY OF ASSUMPTIONS USED TO ESTIMATE FAIR VALUE OF WARRANTS GRANTED
| 2021 | 2020 | 2023 | 2022 | |||||||||||||
| Expected dividend yield | 0 | % | 0 | % | 0 | % | 0 | % | ||||||||
| Weighted-average expected volatility | 217 | % | 217 | % | 165 | % | 86 | % | ||||||||
| Weighted-average risk-free interest rate | 2.75 | % | 2.75 | % | 3.9 | % | 4.3 | % | ||||||||
| Warrants, measurement input | 3.9 | % | 4.3 | % | ||||||||||||
| Expected life of warrants | 5 years | 5 years | 3.8 years | 5 years | ||||||||||||
The Company’s outstanding and exercisable warrants as of December 31, 20212023 and 20202022 are presented below:
SCHEDULE OF OUTSTANDING AND EXERCISABLE WARRANTS
Number Outstanding | Weighted Average Exercise Price | Contractual Life in Years | Intrinsic Value | |||||||||||||
| Warrants Outstanding as of December 31, 2019 | $ | 0.42 | $ | |||||||||||||
| Warrants granted | 5,000 | $ | 0.06 | - | ||||||||||||
| Warrants forfeited | (33,336 | ) | $ | 2.30 | - | - | ||||||||||
| Warrants exercised | (413,393 | ) | $ | 0.09 | - | - | ||||||||||
| Warrants Outstanding as of December 31, 2020 | $ | 1.33 | $ | |||||||||||||
| Warrants granted | 5,000 | $ | 3.00 | - | ||||||||||||
| Warrants forfeited | (38,216 | ) | $ | 2.51 | - | - | ||||||||||
| Warrants exercised | (5,000 | ) | $ | 3.00 | - | - | ||||||||||
| Warrants Outstanding as of December 31, 2021 | 44,535 | $ | 0.32 | $ | ||||||||||||
| Warrants Exercisable as of December 31, 2021 | 44,535 | $ | 0.32 | $ | ||||||||||||
| Number Outstanding | Weighted Average Exercise Price | Contractual Life In Years | Intrinsic Value | |||||||||||||
| Warrants outstanding as of December 31, 2021 | 2,969 | $ | $ | 11,135 | ||||||||||||
| Warrants granted | 177,536 | - | ||||||||||||||
| Warrants forfeited, expired, cancelled | (202 | ) | - | - | ||||||||||||
| Warrants exercised | (972 | ) | - | - | ||||||||||||
| Warrants outstanding as of December 31, 2022 | 179,331 | 6,731 | ||||||||||||||
| Warrants granted | 41,193 | - | ||||||||||||||
| Warrants forfeited, expired, cancelled | - | - | - | - | ||||||||||||
| Warrants exercised | (1,795 | ) | - | - | ||||||||||||
| Warrants outstanding as of December 31, 2023 | 218,729 | $ | - | |||||||||||||
| Warrants exercisable as of December 31, 2023 | 218,279 | $ | - | |||||||||||||
The Company maintains stock option plans under which certain employees are awarded option grants based on a combination of performance and tenure. The stock option plans provide for the grant of up to shares, and the Company’s Second Amended and Restated 2019 Equity Incentive Plan provides for automatic increases in the number of shares available under such plan (currently shares) on April 1st of each calendar year,
For 2021, options to purchase shares of common stock were granted, were exercised, were forfeited, and expired. The options granted during the period vest over a period, the average exercise price was $ per share and the options have a term of years.
For the twelve-month periodyear ended December 31, 2021,2023, options to purchase shares were granted, options to purchase shares were forfeited and options expired. For the year ended December 31, 2023, options to purchase shares of common stock were exercised, resulting in proceeds of $1,821.exercised.
Under the Black-Scholes option price model, fair value of the options granted in 2021 and 2020 were $ and $, respectively.
SCHEDULE OF ESTIMATE FAIR VALUE OF STOCK OPTIONS
| 2021 | 2020 | |||||||
| Expected dividend yield | 0 | % | 0 | % | ||||
| Weighted-average expected volatility | - | % | - | % | ||||
| Weighted-average risk-free interest rate | % | % | ||||||
| Expected life of options | years | - years | ||||||
Total compensation cost related to stock options granted was $ and $ for the years ended December 31, 20212023, and 2020,2022, respectively. As of December 31, 2021, there was $
135,118 of unrecognized compensation costs related to stock options, which is expected to be recognized over a weighted average period of
SCHEDULE OF STOCK OPTION ACTIVITY
| Number Outstanding | Weighted-Average Exercise Price | Weighted-Average Contractual Life in Years | Intrinsic Value | |||||||||||||
| Options outstanding as of December 31, 2021 | 27,398 | $ | 4.78 | $ | 368,417 | |||||||||||
| Options exercisable as of December 31, 2021 | 20,146 | 4.88 | 257,186 | |||||||||||||
| Options granted | - | - | - | - | ||||||||||||
| Options forfeited | (1,234 | ) | 87.37 | - | ||||||||||||
| Options expired | (6,456 | ) | 86.03 | - | ||||||||||||
| Options exercised | - | - | - | - | ||||||||||||
| Options outstanding as of December 31, 2022 | 19,708 | 66.00 | - | |||||||||||||
| Options exercisable as of December 31, 2022 | 17,167 | 66.30 | - | |||||||||||||
| Options granted | 9,053 | 6.08 | - | |||||||||||||
| Options forfeited | (140 | ) | 82.33 | - | ||||||||||||
| Options expired | (2,392 | ) | 89.89 | - | ||||||||||||
| Options exercised | - | - | - | - | ||||||||||||
| Options outstanding as of December 31, 2023 | 26,229 | $ | 43.04 | $ | - | |||||||||||
| Options exercisable as of December 31, 2023 | 16,141 | $ | 60.75 | $ | - | |||||||||||
Number Outstanding | Weighted Average Exercise Price | Contractual Life in Years | Intrinsic Value | |||||||||||||
| Options Outstanding as of December 31, 2019 | $ | $ | 817,220 | |||||||||||||
| Options Exercisable as of December 31, 2019 | $ | 314,338 | ||||||||||||||
| Options granted | 4.42 | 3.97 | ||||||||||||||
| Options forfeited | (15,168 | ) | 3.18 | 7.12 | ||||||||||||
| Options expired | - | - | - | - | ||||||||||||
| Options exercised | (167 | ) | 3.00 | - | - | |||||||||||
| Options Outstanding as of December 31, 2020 | $ | $ | 597,322 | |||||||||||||
| Options Exercisable as of December 31, 2020 | $ | $ | 384,226 | |||||||||||||
| Options granted | 36,700 | 5.74 | 4.19 | - | ||||||||||||
| Options forfeited | (21,200 | ) | 6.45 | 4.11 | - | |||||||||||
| Options expired | - | - | - | - | ||||||||||||
| Options exercised | (30,353 | ) | 0.06 | - | - | |||||||||||
| Options Outstanding as of December 31, 2021 | $ | $ | 368,417 | |||||||||||||
| Options Exercisable as of December 31, 2021 | $ | $ | 257,186 | |||||||||||||
NOTE 717 – INCOME TAXESCONTINGENCIES
On December 22, 2017, H.R. 1, originally known as the Tax Cuts and Jobs Act, (the “Tax Act”) was enacted. Among the significant changes to the U.S. Internal Revenue Code, the Tax Act lowers the U.S. federal corporate income tax rate (“Federal Tax Rate”) from 35% to 21% effective January 1, 2018.
Studebaker Defense Group, LLC
The statutory tax rate is the percentage imposed by law; the effective tax rate is the percentage of income actually paid by a company after considering tax deductions, exemptions, credits and operating loss carry forwards.
At December 31, 2021 and 2020 deferred tax assets consist of the following:
SCHEDULE OF DEFERRED TAX ASSETS
| December 31, 2021 | December 31, 2020 | |||||||
| Federal loss carryforwards | $ | 2,347,266 | $ | 1,309,534 | ||||
| Less: valuation allowance | (2,347,266 | ) | (1,309,534 | ) | ||||
| Deferred tax assets | $ | - | $ | - | ||||
The Company has established a valuation allowance equal to the full amount of the deferred tax asset primarily due to uncertainty in the utilization of the net operating loss carry forwards.
The estimated net operating loss carry forwards of approximately $10,462,828 will be available based on the new carryover rules in section 172(a) passed with the Tax Cuts and Jobs Acts.
NOTE 8 – OTHER RECEIVABLES
In July 2020, the Company’s wholly-owned subsidiary, Integra,IPS, entered into an agreement with Studebaker Defense Group, LLC (“Studebaker”) wherein IntegraIPS would pay Studebaker a down payment of $500,000 and Studebaker would deliver 180,000 boxes of nitrile gloves by August 14, 2020. IntegraIPS wired the $500,000 to Studebaker, but to date, Studebaker has not delivered the gloves or provided a refund of the deposit. OnIn December 31, 2020, wethe Company filed a complaint against Studebaker in Florida state court, Case No. 20-CA-010118 in the Circuit Court for the Thirteenth Judicial Circuit in Hillsborough County, for among other things, breach of contract. On January 29, 2021, Integra Pharma Solutions filed a motionStudebaker did not answer the complaint, nor did counsel for clerk’s default against Studebaker. OnStudebaker file an appearance. Accordingly, in February 2, 2021, the clerk of court issuedCompany filed for a default against Studebaker. On March 4, 2021, Integra Pharma Solutions filed a motion for final default judgment against Studebaker. Onjudgment; however, on March 22, 2021, counsel for Studebaker filed a notice ofan appearance in the case. On March 24, Studebaker filed a response in opposition to the motion for final judgment, and on March 25, 2021, Studebakershortly thereafter filed a motion to vacate the default judgment and dismiss the case. On May 14, 2021, the Court denied Integra’s motion for final default judgment,complaint on jurisdictional grounds. The court granted Studebaker’s motion to set aside the clerk’s default andjudgment but denied Studebaker’sthe motion to dismiss. An amended answer and affirmative defenses were filed by Studebaker on October 14, 2021. Integra’s motion to strike the affirmative defenses, or in the alternative, motion for more definite statement is scheduled for hearing on April 27, 2022. We have also scheduled the deposition of Studebaker’s corporate representative on April 12, 2022, and moved to compel better answers to outstanding discovery. The litigation remains pending and is in the discovery phase. Integra remains confident it can successfully prosecute its claims against Studebaker on the merit. OnAt June 30, 2021, the $500,000 was recorded as Loss on Inventory Investment. The Company won this case but has not collected any settlement yet, another lawsuit was filed to collect.
On April 13, 2023, a settlement was reached in the Studebaker and IPS legal case. The court found in favor of IPS and ordered Studebaker to pay $550,000 to IPS. The payments were to commence on May 1, 2023 and continue monthly in 17 installments until the full amount is paid in full but as of the filing date, no payment has been received by IPS.
Sandwave Group Dsn Bhd and Crecom Burj Group SDN BHD
In August 2020, Integra,IPS entered into an agreement with Sandwave Group Dsn Bhd (“Sandwave”), wherein IntegraIPS would pay Sandwave a down payment of $581,250 and Sandwave’s supplier, Crecom Burj Group SDN BHD (“Crecom”), would deliver 150,000 boxes of nitrile gloves within 45 days. IntegraIPS wired the $581,250 to Sandwave, which in turn wired the purchase price to Crecom, which Crecom accepted; however, to date, Crecom has not delivered the nitrile gloves. IntegraIPS demanded return of its $581,250 and Crecom has acknowledged that Integra isIPS was entitled to a refund, but to date Crecom has failed to return Integra’s money. Inrefund. As of February 2021, IntegraCrecom had not returned any funds and IPS filed a complaint against Crecom in Malaysia: Case No. WA-22NCC-55-02/2021 in the High Court of Malaysia at Kuala Lumpur in the Federal Territory, Malaysia for the Malaysian equivalent of breach of contract. On September 1, 2022 counsel for Crecom filed an appearance on March 1, 2021. In April 2021, an Application for Summary Judgment was filed withinformed the court andthat Crecom had been wound up on May 25, 2021,August 23, 2022; under Section 471 of the Court extractedMalaysian Companies Act 2016, the sealed application, and a copy thereofsuit filed by IPS was served on Crecom’s attorneys and Crecom, 14 days later, filed an Affidavit in Reply withstayed until leave of the court alleging that there are issuesis obtained to be tried and thatproceed. Given this case must gonew information regarding Crecom the Company has decided at this time to a full trial. On June 28, 2021, the court directed both parties to file their written submissions/arguments in relation to the applicationstop its pursuit of this lawsuit until or unless additional information is obtained by counsel for summary judgment on or before July 12, 2021, and scheduled a hearing thereon for August 26, 2021. At the final hearing on October 18th, the ruling for the summary judgment was denied and a trial date is pending. The Company believes that it will prevail in the lawsuit filed; but the steps to enforce a judgment in Malaysia, if any, may be cumbersome, time consuming or costly. The Company cannot determine the timing of the judgment, nor the amount ultimately collected.IPS. At June 30, 2021, the $581,250 was recorded as Loss on Inventory Investment.
GSG PPE, LLC
On November 19, 2021, IntegraIPS filed a complaint against GSG PPE, LLC (“GSG”) and Gary Waxman (“Waxman”), the owner, alleging three counts of breach of contract for a purchase agreement, a promissory note, and a personal guaranty. Collectively, the company alleges that GSG and Waxman have materially breached all three contracts. In late 2020, GSG and IntegraIPS executed a valid initial contract setting the terms of a business transaction. GSG failed to pay IntegraIPS approximately 75% of the amount owed to Integra.IPS. GSG acknowledged it owed the money and executed a promissory note in favor of IntegraIPS in the amount of $630,000 which matured on September 30, 2021. The note provides for attorney fees and interest in addition to the $630,000. Waxman’s personal guaranty confirmed that GSG owed IntegraIPS $630,000. Integra has propounded discovery and plans to file a motion for summary judgment on all three counts of breach of contract shortly after this filing. The company believes that the facts of the case are favorable to Integra, but the outcome of the summary judgment hearing is unknown. On September 30, 2021, the $630,000was recorded as Bad Debt Expense. A settlement was entered into between the parties in June 2022, whereby GSG and Waxman agreed to pay $743,000 which included attorney fees and interest, which is required to be paid to the Company in monthly installments over 17 months. The Company received additional monthly installment payments as part of the agreement through January 2023. As of December 31, 2023, and through the date of this filing, the Company has not received the monthly installment payments due to the Company from GSG since January of 2023.
NOTE 9 - CONTINGENCIES
Jain, et al., v. Memantine, et al.
In January 2020, we became aware of a complaint filed by Jitendra Jain, Manish Arora, Scariy Kumaramangalam, Harsh Datta and Balvant Arora (collectively, plaintiffs), against our wholly-owned subsidiary, Trxade, Inc. and our Chief Executive Officer, Suren Ajjarapu as well as certain unrelated persons, Annapurna Gundlapalli, Gajan Mahendiran and Nexgen Memantine (collectively, defendants), in the Circuit Court of Madison County, Alabama (Case:47-CV-2019-902216.00). The complaint alleged causes of actions against the defendants including fraud in the inducement, relating to certain investments alleged to have been made by plaintiffs in Nexgen Memantine, breach of fiduciary duty, conversion and voidable transactions. The complaint related to certain investments alleged made by the plaintiffs in Nexgen Memantine and certain alleged fraudulent transfers of assets and funds alleged to have been taken by the defendants which are unrelated to the Company.
On May 14, 2021, Plaintiffs filed a second amended complaint against the defendants. The second amended complaint alleges causes of action against the defendants including securities fraud, breach of fiduciary duty, violation of the Florida RICO Act, and breach of contract. The operative complaint relates to certain investments alleged to have been made by the plaintiffs in Nexgen Memantine and certain alleged transfers of assets and funds alleged to have been taken by the defendants which are unrelated to the Company. The amended complaint seeks injunctive relief, $425,000 in compensatory damages, treble damages, punitive damages, and fees and costs
In February 2022, A settlement as to Suren Ajjarapu, Annapurna Gundlapalli and Trxade Group has been reached and signed. This settlement involves no admission of liability and a full and complete release of all actions after a lump-sum payment of $225,000 is made. Because the complaint purports to be a derivative action, court approval is required. A hearing was held on the request to approve the settlement, and changes were made at the instruction of the court which should lead to it being approved by the court. The settlement has been fully funded and the money transferred to the attorneys for the $225,000.
A settlement has also been reached regarding defendant Nexgen Memantine, Inc., to which defendant Gajan Mahendiran has objected because of some of the factual recitations. This dispute is before a court-appointed mediator and should not prevent the Ajjarapu/Trxade settlement from being approved, but this is causing some delay. Mahendiran, Ajjarapu, Gundlapalli and Trxade have agreed to move the Court to dismiss all counter and crossclaims that were filed between the defendants in this matter and will do so once the Court approves the settlement. Because the suit against Gajan Mahendiran remains active, it is possible that Trxade may incur future expenses related to its employees being called as witnesses by either or both of the sides. However, it is expected that all liability issues will be resolved once the settlement is finally approved.
NOTE 1018 – LEASES
The Company elected the practical expedient under ASU 2018-11 “Leases: Targeted Improvements” which allows the Company to apply the transition provision for Topic 842 at the Company’s adoption date instead of at the earliest comparative period presented in the financial statements. Therefore, the Company recognized and measured leases existing at January 1, 2019, but without retrospective application. In addition, the Company elected the optional practical expedient permitted under the transition guidance which allows the Company to carry forward the historical accounting treatment for existing leases upon adoption. No impact was recorded to the beginning retained earnings for Topic 842. The Company has two operating leases for corporate offices.offices as of December 31, 2023. The following table outlines the details of suchthe leases:
SCHEDULE OF OPERATING LEASES
| Lease 1 | Lease 2 | |||||||
| Initial Lease Term | January 2021 to December 2021 | November 2018 to November 2023 | ||||||
| Renewal Lease Term | - | November 2023 to November 2028 | ||||||
| New Initial Lease Term | January 2022 to December 2026 | - | ||||||
| New Renewal Lease Term | January 2027 to December 2031 | - | ||||||
| Initial Recognition of Right to use assets at January 1, 2019 | $ | 534,140 | $ | 313,301 | ||||
| New Initial Recognition of Right to use Assets at December 31, 2021 | $ | 977,220 | $ | - | ||||
| Incremental Borrowing Rate | 10 | % | 10 | % | ||||
| Lease 1 | Lease 2 | Lease 3 | ||||||||||
| Initial Lease Term | January 2021 to December 2021 | October 2018 to November 2023 | October 2023 to September 2026 | |||||||||
| New Initial Lease Term | January 2022 to December 2026 | November 2023 to October 2028 | - | |||||||||
| Initial Recognition of Right of use assets at January 1, 2019 | $ | 534,140 | $ | 313,301 | - | |||||||
| New Initial Recognition of Right of use Assets at December 31, 2021 | $ | 977,220 | $ | - | - | |||||||
| New Initial Recognition of Right of use Assets at December 31, 2023 | 351,581 | |||||||||||
| Incremental Borrowing Rate | 10 | % | 10 | % | 10 | % | ||||||
| 79 |
The Company entered into a new corporate office lease (Lease 1) onin January 2022. TheAt inception, the Company determined that entering into the new lease required remeasurement of the lease liability resulting in the increase of the right-of-use asset and the associated lease liability by $977,220. The Company and the Lessor agreed to terminate the lease and vacate the premises in November 2023. The termination resulted in the surrender of the Company’s security deposit of $38,500. The related right-of-use assets of $642,887 and lease liabilities of $664,992 were removed from the balance sheet as of December 31, 2023.
The Company entered into a lease agreement (Lease 2) for the period of October 2018 to November 2023. At inception, management had included the renewal period from November 2023 to November 2028 within the initial recognition of the related right of use assets and lease liabilities, as it was reasonably expected, at the time, that the renewal option would be exercised. The Company determined that the new lease required measurement and recognition of the lease liability and right-of-use assets of $313,301. The lease is still classified as an operating lease. No incentives were included in the lease.
The Company entered into a new warehouse lease (Lease 3) October 2023. The Company determined that the new lease required measurement and recognition of the lease liability and right-of-use assets of $351,581. The lease is classified as an operating lease. No incentives were included in the lease.
The table below reconciles the fixed component of the undiscounted cash flows for each of the first five years and the total remaining years to the operating lease liabilities recorded in the Consolidated Balance Sheet as of December 31, 2021.2023.
SCHEDULE OF FUTURE MINIMUM PAYMENTS FOR OPERATING LEASE LIABILITIES
| Amounts due within twelve months of December 31 | ||||||||
| 2022 | $ | 294,932 | ||||||
| 2023 | 293,683 | |||||||
| Future lease obligations | ||||||||
| 2024 | 302,494 | 187,935 | ||||||
| 2025 | 311,569 | 193,487 | ||||||
| 2026 | 320,916 | 163,146 | ||||||
| 2027 | 58,347 | |||||||
| 2028 | 48,612 | |||||||
| Thereafter | 105,531 | - | ||||||
| Total minimum lease payments | 1,629,125 | 651,527 | ||||||
| Less: effect of discounting | (380,599 | ) | (102,617 | ) | ||||
| Present value of future minimum lease payments | 1,248,526 | 548,910 | ||||||
| Less: current obligations under leases | 178,561 | 139,705 | ||||||
| Long-term lease obligations | $ | 1,069,965 | $ | 409,205 | ||||
| Weighted Average Discount Rate | 10 | % | ||
| Weighted Average Term Remaining | 3.6 Years | |||
| Short-Term Lease Expense Remaining | $ | 187,361 |
For the years ended December 31, 2021,2023, and 2020, amortization of assets2022, total lease expense was $131,558385,977 and $97,020344,525, respectively.
For the years ended December 31, 2021,2023, and 2020, operating lease liabilities paid2022, amortization of right-of-use assets was $131,153215,665 and $97,033181,218, respectively.
For the years ended December 31, 2023, and 2022, net operating lease liabilities settled was $195,475 and $164,618, respectively.
NOTE 1119 – SEGMENT REPORTING
The Company classifies its business interests into reportable segments which are Trxade, Inc., Community Specialty Pharmacy, LLC, Integra Pharma, LLC and Other (Unallocated). Operating segments are defined as the components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision makers in deciding how to allocate resources and in assessing performance. The Company’s chief operating decision makers direct the allocation of resources to operating segments based on the profitability, cash flows, and growth opportunities of each respective segment.
The Company classifies its business interests into reportable segments which are:
| ● | Trxade, Inc. - Web based pharmaceutical marketplace platform – B2B sales |
| 80 |
| ● | IPS - Integra Pharma, LLC - Licensed wholesaler of brand, generic and non-drug products – B2B sales | |
| ● | Superlatus – holds Sapientia’s intellectual property for advanced food extrusion technology and The Urgent Company – Manufacturer of ice cream that is animal product-free, vegan, lactose-free, and made with plants – B2B sales | |
| ● | Unallocated - Other – corporate overhead expense, discontinued operations and Bonum Health, LLC. |
SCHEDULE OF BUSINESS INTERESTS INTO REPORTABLE SEGMENTS
Year Ended December 31, 2021 | Trxade, Inc. | Community Specialty Pharmacy, LLC | Integra Pharma, LLC | Unallocated | Total | |||||||||||||||||||||||||||||||||||
| Years Ended December 31, 2023 | Trxade, Inc. | Integra | Superlatus | Unallocated | Total | |||||||||||||||||||||||||||||||||||
| Revenue | $ | 4,924,015 | $ | 1,652,841 | $ | 3,250,561 | $ | 62,016 | $ | 9,889,433 | 6,402,159 | 1,363,830 | 487,021 | 19,204 | 8,272,214 | |||||||||||||||||||||||||
| Gross Profit | $ | 4,921,084 | $ | 156,785 | $ | (393,582 | ) | $ | 61,678 | $ | 4,745,965 | 6,402,159 | 49,030 | (3,872,136 | ) | 19,204 | 2,598,257 | |||||||||||||||||||||||
| Segment Assets | $ | 2,273,330 | $ | (431,593 | ) | $ | 565,619 | $ | 3,358,808 | $ | 5,766,164 | 1,375,109 | 220,634 | 9,663,310 | 1,273,860 | 12,532,913 | ||||||||||||||||||||||||
| Segment Profit/Loss | $ | 1,977,938 | $ | (128,563 | ) | $ | (2,749,028 | ) | $ | (4,416,230 | ) | $ | (5,315,883 | ) | 2,325,175 | (668,625 | ) | (10,416,347 | ) | (9,083,777 | ) | (17,843,574 | ) | |||||||||||||||||
| Cost of Sales | - | 1,314,800 | 4,359,157 | - | 5,673,957 | |||||||||||||||||||||||||||||||||||
Year Ended December 31, 2020 | Trxade, Inc. | Community Specialty Pharmacy, LLC | Integra Pharma, LLC | Unallocated | Total | |||||||||||||||||||||||||||||||||||
| Years Ended December 31, 2022 | Trxade, Inc. | Integra | Superlatus | Unallocated | Total | |||||||||||||||||||||||||||||||||||
| Revenue | $ | 5,546,746 | $ | 1,653,924 | $ | 9,877,067 | $ | 44,783 | $ | 17,122,520 | 5,435,814 | 4,754,067 | - | 60,287 | 10,250,168 | |||||||||||||||||||||||||
| Gross Profit | $ | 5,546,746 | 107,771 | 8,374 | $ | 44,431 | $ | 5,707,322 | 5,433,641 | 25,343 | - | 60,287 | 5,519,271 | |||||||||||||||||||||||||||
| Segment Assets | $ | 2,076,934 | $ | (457,784 | ) | 2,698,357 | $ | 5,475,195 | $ | 9,792,702 | 1,877,881 | 445,264 | - | 1,386,881 | 3,710,026 | |||||||||||||||||||||||||
| Segment Profit/Loss | $ | 3,309,128 | $ | (900,427 | ) | $ | (531,092 | ) | $ | (4,413,660 | ) | $ | (2,536,051 | ) | ||||||||||||||||||||||||||
| Segment Profit (Loss) | 1,924,355 | (545,557 | ) | - | (5,288,666 | ) | (3,909,868 | ) | ||||||||||||||||||||||||||||||||
| Cost of Sales | 2,173 | 4,728,724 | - | - | 4,730,897 | |||||||||||||||||||||||||||||||||||
NOTE 1220 – SUBSEQUENT EVENTS
STOCKHOLDERS’ EQUITY
In January 2022, warrants to purchase 14,584 shares of common stock were exercised with an exercise price of $0.06 per share; the Company issued shares of common stock, and $875 in proceeds were received in connection with such exercise.
ENTRY INTO A MATERIAL DEFINITIVE AGREEMENT – EXCHANGE HEALTH, LLCAsset Purchase Agreement
On February 15, 2022,16, 2024, the Company, entered intotogether with Trxade, Inc., a relationship with Exchange Health, LLC, a technology company providing an online platform for manufacturers and suppliers to sell and purchase pharmaceuticals (“Exchange Health”). SOSRx LLC, a Delaware limited liability company (“SOSRx”), was formed, which iswholly owned 51% bysubsidiary of the Company, and 49%Micro Merchant Systems, Inc. (“MMS”) entered into an asset purchase agreement (the “APA”) under which MMS agreed to purchase for cash substantially all of the assets of Trxade, Inc. On February 16, 2024, the parties consummated the closing of the transactions contemplated by Exchange Health.the APA. Trxade, Inc. operated a web-based market platform designed to enable trading among healthcare buyers and sellers of pharmaceuticals, accessories and services. The purchase price paid at closing was $22.5 million, subject to customary adjustments for cash, indebtedness, working capital and transaction expenses. Subject to the terms and conditions of the APA, if, during the period beginning on the closing date and ending on the four-month anniversary of the closing date, MMS receives $1.6 million or greater in certain collections from third parties resulting from any products or services sold, or provided, by the business assets and operations acquired from Trxade, Inc., Trxade, Inc. will be due an additional $7.5 million payment from MMS.
Subscription Agreement
On February 15, 2022,29, 2024, the Company contributed cashCompany’s wholly owned subsidiary Trxade, Inc. entered into a Subscription Agreement (the “Subscription Agreement”) with Lafayette Energy Corp., a Delaware corporation (“Lafayette”). Pursuant to SOSRxthe Subscription Agreement, Trxade, Inc. will, in the amount of $325,000, issuedtwo equal tranches, invest a promissory note to SOSRx in the amount of $500,000, which was immediately assigned to Exchange Health (the “Promissory Note”), and agreed to make an earn out paymenttotal of up to $400,0005.0, million in Lafayette in exchange for up to shares of Lafayette’s newly created Series A Convertible Preferred Stock, with the second tranche becoming payable only upon Trxade, Inc.’s receipt of notice that Lafayette has successfully drilled its first oil and gas well and produced at the Company’s discretion, in cash or common stockleast one hundred (100) barrels of oil. Mr. Michael Peterson is a director of the Company based on SOSRx achieving certain revenue targetsas well as the CEO of SOSRxLafayette and a member of Lafayette’s board of directors. This relationship was disclosed to the Company’s Board of Directors and the audit committee of the Board of Directors prior to, and at the time that the terms of the Subscription Agreement and the transaction effected thereby were approved by the Board of Directors as discussed below (the “Earn Out Payments”);a whole and entered into a Distribution Services Agreement with SOSRx (the “Distribution Agreement”).the members of the audit committee.
The Earn Out Payments require the Company to pay (a) $25,000 to Exchange Health if total revenue for SOSRx are over $0.7 million, and $25,000 to Exchange Health if total EBITDA is over $0.5 million, for fiscal year ending 2022; (b) $87,500 to Exchange Health if total revenue for SOSRx is over $3.3 million, and $87,500 to Exchange Health if total EBITDA is over $2.95 million, for fiscal year ending 2023; and (c) $87,500 to Exchange Health if total revenue for SOSRx is over $5.7 million, and $87,500 to Exchange Health if total EBITDA is over $4.9 million, for fiscal year ending 2024, provided that certain amounts will be payable in the event at least 95% of such milestones are met, and such payments will be grossed up or down by up to 5% of such amounts, if such milestone amounts are between 95% and 105% of the required thresholds. At the Company’s option, the Earn Out Payments may be paid in cash or shares of common stock, valued at the then current trading price of the Company’s common stock. If one year’s milestones are not achieved, no earnout will be payable for that year and those earn out payments will not be eligible to be earned in any other year.
Exchange Health contributed certain property, contracts and licenses to SOSRx, having an agreed value of $792,500, in exchange for its 49% membership interest in SOSRx and received a cash payment of $275,000 from SOSRx, LLC, pursuant to a Member Asset ContributionStock Purchase Agreement (the “Asset Contribution Agreement”), also entered into on February 15, 2022.
Promissory Note
The Promissory Note, which was immediately assigned to Exchange Health, and represents amounts currently due to Exchange Health, bears interest at the rate of the prime rate, plus 2% per annum (currently 5.25% per annum), with (i) one-third of the principal ($166,666.67) and interest payable after one year (on February 15, 2023) and (ii) the remaining two-thirds of principal payable quarterly over the next two years in eight equal installments of $41,666.67, together with any unpaid accrued interest thereupon, at the end of every full fiscal quarter, beginning, June 20, 2023. The Promissory Note may be prepaid by the Company, at its discretion, in whole or in part at any time, without premium or penalty.
Notwithstanding the foregoing, if the Company effectuates a Voluntary Withdrawal (defined below) under the Company Agreement (as discussed below) prior to February 15, 2024 (the “Earn Out Period”), and SOSRx has failed to meet any of the revenue targets required by the Earn Out Payments prior to the expiration of the Earn Out Period, then all remaining amounts of interest and principal not yet due and payable under the Promissory Note shall immediately terminate and all related indebtedness evidenced hereby shall be deemed canceled.
Amounts owed under the Promissory Note are secured by the Company’s membership interests in the SOSRx and are a non-recourse obligation of the Company, secured solely by such membership interests.
In the event that the Company is delinquent to pay when due (whether at maturity, by reason of acceleration or otherwise) any principal of or interest on the Promissory Note, then if such payment is not made within fifteen days of the due date, then Exchange Health may declare an additional interest fee of 2% of the delinquent amount to be due. If the delinquency is thirty days or more late from the due date, then Exchange Health may declare another additional interest fee of 3%, to make a total of 5%, for the delinquent payment.
In the event that we fail to pay when due (whether at maturity, by reason of acceleration or otherwise) any principal of or interest on Promissory Note, then if such payment is not made within sixty days of the due date, then Exchange Health may declare all obligations (including without limitation, outstanding principal and accrued and unpaid interest thereon) under the Promissory Note to be immediately due and payable.
SOSRx Operating Agreement
The rights of the Company and Exchange Health in connection with SOSRx are set forth in the Operating Agreement of SOSRx (the “Operating Agreement”), effective February 15, 2022. Pursuant to the Operating Agreement, SOSRx is to be managed by a management committee consisting of three members, two of which are nominated by the Company, who currently include Suren Ajjarapu, the Company’s Chief Executive Officer and Chairman and Prashant Patel, the Company’s President and director, and one person nominated by Exchange Health. If either the Company or Exchange Health shall ever hold less than 25% of the membership interests of SOSRx, such entity shall forfeit its management appointment rights, and such appointment rights shall be held by such other member which holds over 50% of the membership interests.
The Operating Agreement includes customary transfer restrictions on the SOSRx membership interests, right of first refusal rights upon receipt of a bona fide third party offer for purchase of a member’s membership interest (exercisable first by SOSRx and then the other members), preemptive rights (subject to certain exceptions), tag-along rights, and drag-along rights (applying if any greater than 50% owner desires to transfer their ownership in SOSRx).
Any member of SOSRx has the right to effect a voluntary withdrawal from the Company (a “Voluntary Withdrawal”), provided that such member must give ninety days prior written notice to all other members. Any member who effectuates a Voluntary Withdrawal is not permitted to receive the fair value or any value of the member’s membership interest as of the date of the Voluntary Withdrawal, and may instead effect a Voluntary Withdrawal by forfeiture of its membership interests in SOSRx without compensation or consideration; provided however, that if the Company (a) effectuates a Voluntary Withdrawal prior to February 15, 2024, and (b) SOSRx has failed to meet any of the revenue targets required by the Earn Out Payments prior to the date of withdrawal, then all obligations of the Company under the Earn Out Payments and the Promissory Note shall terminate.
The Company or its assigns may at any time by written notice to any other member, offer to purchase all (but not less than all) of such other member’s membership interests, which shall be calculated and payable pursuant to a discounted cash flow model. If the buyout is paid to Exchange Health or its successors or assigns, any remaining amounts payable under the Promissory Note become immediately due and payable upon such payment.
The Operating Agreement also provides, that without the prior written approval of the unanimous consent of the management committee, a manager or member may not, directly or indirectly, (a) enter into a business relationship with any other person that is materially adverse to the business of SOSRx or an affiliate of SOSRx, or (b) cause any person to reduce or terminate its relationship with SOSRx or any affiliate of SOSRx. The foregoing covenants apply to each member, and each manager during the period in which each manager is a member.
Distribution Agreement
On February 15, 2022, SOSRxMarch 5, 2024, the Company entered into the Distributionin a Stock Purchase Agreement (“SPA”) with Integra Pharma Solutions LLC, the Company’s wholly-owned subsidiary (“Integra”Superlatus Foods Inc. (the “Buyer”). Pursuant to the Distribution Agreement, Integra appoints each SOSRx member an active account for Manufacturer Non-Control (Schedule 2-5 as classified bySPA, the US Drug Enforcement Agency) products bought on the SOSRx platform. The agreement remains in effect until December 31, 2023, and renews thereafter on a yearly basis until terminated; which agreement can only be terminated by the non-breaching party, upon the breachCompany sold all of the agreement byissued and outstanding stock (the “Stock”) of Superlatus Inc., a party thereto, with a 30-day cure right. PursuantDelaware corporation and wholly-owned subsidiary of the Company (“Superlatus”), to the Distribution Agreement,Buyer. The purchase price for each calendar quarter (or portion thereof) during the term, SOSRx agreedStock was $1.00 which was delivered to pay Integra a fee equal to 2%the Company at the closing, which occurred simultaneously with the execution of the net priceSPA. As a result of all purchasesthe transaction Superlatus is no longer a subsidiary of products during such period. Integra also agreedthe Company, and the rights and assets of Superlatus together with various liabilities and obligations that were specific to participate in SOSRx’s annual trade show, once established. Integra made certain representationsSuperlatus became rights and warranties in the Distribution Services Agreement, and agreed to indemnify SOSRx against certain damages and losses. The Distribution Services Agreement included customary confidentiality obligations.obligations of Buyer.
Asset Contribution AgreementSpecial Cash Dividend
On February 15, 2022, Exchange Health entered intoMarch 6, 2024, the Company announced the declaration of a Member Asset Contribution Agreementspecial cash dividend of eight dollars ($8.00) per share of common stock, payable to stockholders of record as of March 18, 2024, with SOSRx, pursuant to which it contributed certain assets and assigned certain contracts, relating to software, manufacturers and members, to SOSRx, in consideration for its 49% membership interest in SOSRx. SOSRx did not assume anythe dividend being paid on or about March 22, 2024. The special dividend was paid using a portion of Exchange Health’s liabilities or obligations other than the obligations and commitmentsproceeds from the closing of Exchange Health arising under the assumed contracts.sale of the Company’s web-based market platform assets.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Disclosure controls and procedures are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported, within the time period specified in the SEC’s rules and forms and is accumulated and communicated to the Company’s management, as appropriate, in order to allow timely decisions in connection with required disclosure.
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Executive Officer and our interim Chief Financial Officer, Mr. Ajjarapu and Mr. Doss,Patel, respectively, we conducted an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, as of the end of the period covered by this Annual Report (December 31, 2021)2023). Based on this evaluation, our Chief Executive Officer and our interim Chief Financial Officer concluded that as of December 31, 2021,2023, our disclosure controls and procedures were not effective to provide reasonable assurance that information required to be disclosed in our reports filed with the SEC pursuant to the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC and that such information is accumulated and communicated to our management, including our CEO and interim CFO, as appropriate, to allow timely decisions regarding required disclosures.
As a result of the formative stage of our development, the Company has not fully implemented the necessary internal controls. The matters involving internal controls and procedures that the Company’s management considered to be material weaknesses under the standards of the Committee of Sponsoring Organizations of the Treadway Commission (COSO) were: (1) The Company did not maintain a fully integrated financial consolidation and reporting system throughout the period and as a result, extensive manual analysis, reconciliation and adjustments were required in order to produce financial statements for external reporting purposes.purposes, and (2) The Company does not currently have a sufficient complement of technical accounting and external reporting personnel commensurate to support standalone external financial reporting under public company or SEC requirements. Specifically, the Company did not effectively segregate certain accounting duties due to the small size of its accounting staff and maintain a sufficient number of adequately trained personnel necessary to anticipate and identify risks critical to financial reporting and the closing process. In addition, there were inadequate reviews and approvals by the Company’s personnel of certain reconciliations and other processes in day-to-day operations due to the lack of a full complement of accounting staff.
Management believes that the material weaknesses set forth above did not have an effect on the Company’s financial results reported herein. We are committed to improving our financial organization. As part of this commitment, we have increased our personnel resources and technical accounting expertise as we develop the internal and financial resources of the Company. In addition, the Company has prepared and implemented sufficient written policies and checklists which will set forth procedures for accounting and financial reporting with respect to the requirements and application of GAAP and SEC disclosure requirements.
Management has prepared and is in the process of implementing sufficient written policies and checklists to remedy the following material weaknesses (i) insufficient written policies and procedures for accounting and financial reporting with respect to the requirements and application of GAAP and SEC disclosure requirements; and (ii) ineffective controls over period end financial close and reporting processes.
We have improved our financial organization as we have increased our personnel resources and technical accounting expertise. We will continue to monitor and evaluate the effectiveness of our internal controls and procedures and our internal controls over financial reporting on an ongoing basis.
Management’s Report on Internal Control Over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP, but because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. The Company’s internal control over financial reporting includes those policies and procedures that are designed to:
| ● | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and |
| ● | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. |
| 82 |
Management conducted an assessment of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2021.2023. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control — Integrated Framework (2013). Based on our assessment, management concluded that the Company’s internal controls over financial reporting were not effective as of December 31, 2021,2023, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with GAAP. Specifically, management’s determination was based on the following material weaknesses which existed as of December 31, 2021:2023:
| ● | Financial Reporting Systems: The Company did not maintain a fully integrated financial consolidation and reporting system throughout the period and as a result, extensive manual analysis, reconciliation and adjustments were required in order to produce financial statements for external reporting purposes. |
| ● | Segregation of Duties: The Company does not currently have a sufficient complement of technical accounting and external reporting personnel commensurate to support standalone external financial reporting under public company or SEC requirements. Specifically, the Company did not effectively segregate certain accounting duties due to the small size of its accounting staff and maintain a sufficient number of adequately trained personnel necessary to anticipate and identify risks critical to financial reporting and the closing process. In addition, there were inadequate reviews and approvals by the Company’s personnel of certain reconciliations and other processes in day-to-day operations due to the lack of a full complement of accounting staff. |
Limitations on the Effectiveness of Controls
Management of the Company, including its Chief Executive Officer and its Chief Financial Officer, does not expect that the Company’s disclosure controls and procedures or its internal control over financial reporting will prevent or detect all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Furthermore, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons or by the collusion of two or more persons. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of the effectiveness of controls to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Changes in Internal Control Over Financial Reporting.
There have not been any changes in our internal control over financial reporting during the quarter ended December 31, 2021,2023, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
As a result of COVID-19, ourOur workforce operated primarily in a work from home environment for the year ended December 31, 2021.2023. While pre-existing controls were not specifically designed to operate in our current work from homework-from-home operating environment, we do not believe that such work from homework-from-home actions have had a material adverse effect on our internal controls over financial reporting. We have continued to re-evaluate and refine our financial reporting process to provide reasonable assurance that we could report our financial results accurately and timely.
| ITEM 9B. | OTHER INFORMATION |
None.Securities Trading Plans of Directors and Officers
During the three months ended December 31, 2023, no director or officer of the Company adopted or terminated a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408(a) of Regulation S-K.
Resignation of Director
Effective April 10, 2024, Candice Beaumont voluntarily resigned as a director of the Company. Ms. Beaumont’s decision to resign was not the result of any dispute or disagreement with the Company or any matter relating to the Company’s operations, policies or practices.
Nasdaq Notification
On April 2, 2024 the Company filed with the SEC a Notification of Late Filing on Form 12b-25 reporting that it required additional time to complete its Annual Report on Form 10-K for the period ending December 31, 2023 (the “Form 10-K”).
On April 17, 2024, the Company received a notice (the “Notice”) from the Nasdaq Listing Qualifications Department indicating that the Company was not compliant with the timely filing requirement for continued listing under Nasdaq Listing Rule 5250(c)(1) (the “Listing Rule”), which requires listed companies to timely file all required periodic reports with the SEC.
The Notice had no immediate effect on the listing or trading of the Company’s common stock. The Notice indicated that the Company must, no later than June 17, 2024, submit a plan to regain compliance with respect to the filing requirement. However, as a result of filing this Form 10-K on April 22, 2024, the Company believes it has fully regained compliance with the Nasdaq Listing Rule.
| ITEM 9C. | DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
Not applicable.
PART III
Information required by Items 10, 11, 12, 13 and 14 of Part III is omitted from this Annual Report and will be filed in a definitive proxy statement or by an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report (subject to any extension provided by Exchange Act Rule 0-3).
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this Item will be set forth in the Company’s 20222024 Proxy Statement to be filed with the SEC within 120 days after December 31, 20212023 (subject to any extension provided by Exchange Act Rule 0-3) in connection with the solicitation of proxies for the Company’s 20222024 annual meeting of stockholders including under the headings “Election of Directors”, “Information about our Executive Officers”, “Corporate Governance”, “Code of Ethics”, “Committees of the Board”, and “Delinquent Section 16(a) Reports” (to the extent applicable and warranted), and is incorporated herein by reference.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this Item will be set forth in the Company’s 20222024 Proxy Statement to be filed with the SEC within 120 days after December 31, 2021 (subject2023 (subject to any extension provided by Exchange Act Rule 0-3), including under the headings “Executive Compensation”, “Directors Compensation”, “Outstanding Equity Awards at Fiscal Year-End”, “Compensation Committee Interlocks and Insider Participation” and “Compensation Committee Report” (to the extent required), and is incorporated herein by reference.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this Item will be set forth under the heading “Voting Rights and Principal Stockholders” and “Equity Compensation Plan Information” in the Company’s 20222024 Proxy Statement to be filed with the SEC within 120 days after December 31, 2021 (subject2023 (subject to any extension provided by Exchange Act Rule 0-3), and is incorporated herein by reference.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this Item will be set forth in the Company’s 20222024 Proxy Statement to be filed with the SEC within 120 days after December 31, 2021 (subject2023 (subject to any extension provided by Exchange Act Rule 0-3), including under the headings “Certain Relationships and Related Transactions” and “Committees of the Board” - “Director Independence”, and is incorporated herein by reference.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this Item will be set forth under the heading “Ratification of Appointment of Auditors” - “Audit Fees” in the Company’s 20222024 Proxy Statement to be filed with the SEC within 120 days after December 31, 2021 (subject2023 (subject to any extension provided by Exchange Act Rule 0-3), and is incorporated herein by reference.
PART IV
| ITEM 15. | EXHIBITS, FINANCIAL STATEMENTS AND SCHEDULES |
(a) Documents filed as part of this Annual Report:
The following is an index of the financial statements, schedules and exhibits included in this Form 10-K or incorporated herein by reference.
| (1) | All Financial Statements |
| Index to Consolidated Financial Statements | |
| Report of Independent Registered Public Accounting Firm | |
| Consolidated Balance Sheets | |
| Consolidated Statements of Operations | |
| Consolidated Statements of Changes in Stockholders’ Equity | |
| Consolidated Statements of Cash Flows | |
| Notes to Consolidated Financial Statements |
| (2) | Consolidated Financial Statement Schedules |
Except as provided above, all financial statement schedules have been omitted, since the required information is not applicable or is not present in amounts sufficient to require submission of the schedule, or because the information required is included in the consolidated financial statements and notes thereto included in this Form 10-K.
| (3) | Exhibits |
| 86 |
| * | Filed herewith. |
| ** | Furnished herewith. |
| *** | Indicates management contract or compensatory plan or arrangement. |
| ITEM 16. | FORM 10–K SUMMARY |
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| TRxADE HEALTH, INC. | ||
| Date: | /s/ Suren Ajjarapu | |
| By: | Suren Ajjarapu, Chief Executive Officer (Principal Executive Officer) | |
| Date: | /s/ | |
| By: |
(Principal Financial and Accounting Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Suren Ajjarapu | Chairman of the Board, Chief Executive Officer and Secretary | |||
| Suren Ajjarapu | (Principal Executive Officer) | |||
| /s/ | ||||
| Director, President, Principal Accounting Officer and Chief Operating Officer | ||||
| Prashant Patel | ||||
| /s/ Donald G. Fell | Director | |||
| Donald G. Fell | ||||
| /s/ | Director | |||
| /s/ | Director | |||